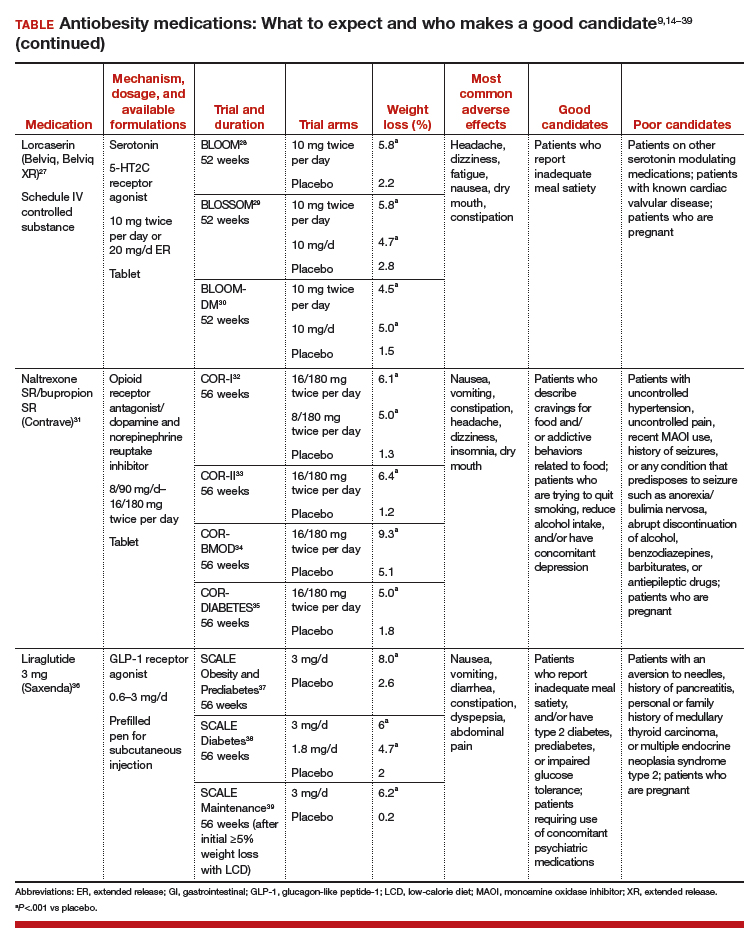
User login
Acute Leukemia of Ambiguous Lineage in Elderly Patients: A SEER-Medicare Database Analysis (FULL)
About Research in Context
In this article, the authors of recent scholarship have been asked to discuss the implications of their research on federal health care providers and specifically the veteran and active-duty service member patient populations. Because the article does not include new research and cannot be blinded, it has undergone an abbreviated peer review process. The original article can be found at Guru Murthy GS, Dhakal I, Lee JY, Mehta P. Acute leukemia of ambiguous lineage in elderly patients - analysis of survival using surveillance epidemiology and end results-Medicare database. Clin Lymphoma Myeloma Leuk. 2017;17(2):100-107.
Acute leukemia of ambiguous lineage (ALAL) is a rare disorder in adults, constituting about 3% to 5% of acute leukemia cases. Unlike acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), ALAL cannot be clearly differentiated into a single subtype based on immunophenotyping. The diagnostic criteria for accurately identifying ALAL has evolved over time. There is paucity of information regarding the outcomes and management of this rare leukemia especially in elderly patients, and it is unclear whether treatment improves survival in these patients.
We performed a retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database to describe the outcomes of ALAL in the elderly population in U.S.1 Patients included in the analysis were aged > 65 years, with a pathologically confirmed diagnosis of ALAL, diagnosed between 1992-2010, and on active follow-up. Information on patient demographics, treatment, chemotherapeutic agents used in treatment, and survival was obtained and analyzed using appropriate statistical methods. A total of 705 patients with a median age of 80 years were included. There was a higher proportion of males than females and a higher proportion of white patients compared with African Americans and other races. We found that the overall survival (OS) declined significantly with increasing age, and treatment with chemotherapy improved the survival. However, factors such as gender, race, or type of chemotherapy received (ALL based, AML based, or other regimens) did not significantly influence the survival.
Even in the current era, the optimal therapy for ALAL is not well established. Although options such as AML-based or ALL-based chemotherapy are available, the best chemotherapy regimen and its sequence is unknown as prior studies have demonstrated varying results.2-5 Among elderly patients, numerous factors such as performance status, comorbidities, and ability to tolerate therapy influence the treatment decision. In light of the poor prognosis in elderly patients, a question often arises in the clinician’s mind about whether chemotherapy would provide any benefit for the patient.
Our study results showed that chemotherapy likely improves survival in these patients. However, due to the smaller number of patients, caution is needed in interpreting the result that there was no significant difference between AML-directed or ALL-directed chemotherapy. Another factor highlighted in the study was that only about 21.5% of patients had been treated with chemotherapy. Due to the inherent nature of the database, we could not identify the factors that may have influenced treatment decisions in these patients. Additionally, patients with stem cell transplantation-related claims could not be included in the analysis due to noncontinuous Medicare coverage during the study period. Hence, the role of stem cell transplantation in these patients could not be determined.
Implications Among Veterans
Actual incidence of ALAL among veterans is not known. Whether the incidence of ALAL relates to exposures to chemicals or toxins during military training and service also is unknown. However, ALAL is likely to be at least as prevalent as it is in the nonveteran population and perhaps more so because of exposures and stresses during military training and service.
It is unclear whether veterans attending VA hospitals receive less or different treatment given the higher comorbidities. Finally, it also is not known whether the outcomes for veterans would be different with or without treatment.
Our findings suggest that treatment should be seriously considered in all patients (veterans or not) who are healthy enough to receive chemotherapy regardless of their age. More research is needed to determine the disease incidence and prevalence among veterans and to evaluate whether there are specific etiologic correlations between ALAL and military exposures, whether the natural history is similar to other populations, and to delineate responsiveness to treatment.
Conclusion
This study suggests a poor survival for elderly patients with ALAL in the U.S. Although treatment is associated with an improvement in survival, only 21.5% of patients have received therapy. The optimal choice of chemotherapy for this disease is still not known and warrants prospective studies.
Click here to read the digital edition.
1. Guru Murthy GS, Dhakal I, Lee JY, Mehta P. Acute leukemia of ambiguous lineage in elderly patients—analysis of survival using surveillance epidemiology and end results—Medicare database. Clin Lymphoma Myeloma Leuk. 2017;17(2):100-107.
2. Rubnitz JE, Onciu M, Pounds S, et al. Acute mixed lineage leukemia in children: the experience of St Jude Children’s Research Hospital. Blood. 2009;113(21):5083-5089.
3. Matutes E, Pickl WF, Van’t Veer M, et al. Mixed phenotype acute leukemia: clinical and laboratory features and out-come in 100 patients defined according to the WHO classification. Blood. 2011;117(11):3163-3171.
4. Wolach O, Stone RM. How I treat mixed-phenotype acute leukemia. Blood. 2015;125(16):2477-2485.
5. Lee JH, Min YH, Chung CW, et al; Korean Society of Hematology AML/MDS Working Party. Prognostic implications of the immunophenotype in biphenotypic acute leukemia. Leuk Lymphoma. 2008;49(4):700-709.
About Research in Context
In this article, the authors of recent scholarship have been asked to discuss the implications of their research on federal health care providers and specifically the veteran and active-duty service member patient populations. Because the article does not include new research and cannot be blinded, it has undergone an abbreviated peer review process. The original article can be found at Guru Murthy GS, Dhakal I, Lee JY, Mehta P. Acute leukemia of ambiguous lineage in elderly patients - analysis of survival using surveillance epidemiology and end results-Medicare database. Clin Lymphoma Myeloma Leuk. 2017;17(2):100-107.
Acute leukemia of ambiguous lineage (ALAL) is a rare disorder in adults, constituting about 3% to 5% of acute leukemia cases. Unlike acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), ALAL cannot be clearly differentiated into a single subtype based on immunophenotyping. The diagnostic criteria for accurately identifying ALAL has evolved over time. There is paucity of information regarding the outcomes and management of this rare leukemia especially in elderly patients, and it is unclear whether treatment improves survival in these patients.
We performed a retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database to describe the outcomes of ALAL in the elderly population in U.S.1 Patients included in the analysis were aged > 65 years, with a pathologically confirmed diagnosis of ALAL, diagnosed between 1992-2010, and on active follow-up. Information on patient demographics, treatment, chemotherapeutic agents used in treatment, and survival was obtained and analyzed using appropriate statistical methods. A total of 705 patients with a median age of 80 years were included. There was a higher proportion of males than females and a higher proportion of white patients compared with African Americans and other races. We found that the overall survival (OS) declined significantly with increasing age, and treatment with chemotherapy improved the survival. However, factors such as gender, race, or type of chemotherapy received (ALL based, AML based, or other regimens) did not significantly influence the survival.
Even in the current era, the optimal therapy for ALAL is not well established. Although options such as AML-based or ALL-based chemotherapy are available, the best chemotherapy regimen and its sequence is unknown as prior studies have demonstrated varying results.2-5 Among elderly patients, numerous factors such as performance status, comorbidities, and ability to tolerate therapy influence the treatment decision. In light of the poor prognosis in elderly patients, a question often arises in the clinician’s mind about whether chemotherapy would provide any benefit for the patient.
Our study results showed that chemotherapy likely improves survival in these patients. However, due to the smaller number of patients, caution is needed in interpreting the result that there was no significant difference between AML-directed or ALL-directed chemotherapy. Another factor highlighted in the study was that only about 21.5% of patients had been treated with chemotherapy. Due to the inherent nature of the database, we could not identify the factors that may have influenced treatment decisions in these patients. Additionally, patients with stem cell transplantation-related claims could not be included in the analysis due to noncontinuous Medicare coverage during the study period. Hence, the role of stem cell transplantation in these patients could not be determined.
Implications Among Veterans
Actual incidence of ALAL among veterans is not known. Whether the incidence of ALAL relates to exposures to chemicals or toxins during military training and service also is unknown. However, ALAL is likely to be at least as prevalent as it is in the nonveteran population and perhaps more so because of exposures and stresses during military training and service.
It is unclear whether veterans attending VA hospitals receive less or different treatment given the higher comorbidities. Finally, it also is not known whether the outcomes for veterans would be different with or without treatment.
Our findings suggest that treatment should be seriously considered in all patients (veterans or not) who are healthy enough to receive chemotherapy regardless of their age. More research is needed to determine the disease incidence and prevalence among veterans and to evaluate whether there are specific etiologic correlations between ALAL and military exposures, whether the natural history is similar to other populations, and to delineate responsiveness to treatment.
Conclusion
This study suggests a poor survival for elderly patients with ALAL in the U.S. Although treatment is associated with an improvement in survival, only 21.5% of patients have received therapy. The optimal choice of chemotherapy for this disease is still not known and warrants prospective studies.
Click here to read the digital edition.
About Research in Context
In this article, the authors of recent scholarship have been asked to discuss the implications of their research on federal health care providers and specifically the veteran and active-duty service member patient populations. Because the article does not include new research and cannot be blinded, it has undergone an abbreviated peer review process. The original article can be found at Guru Murthy GS, Dhakal I, Lee JY, Mehta P. Acute leukemia of ambiguous lineage in elderly patients - analysis of survival using surveillance epidemiology and end results-Medicare database. Clin Lymphoma Myeloma Leuk. 2017;17(2):100-107.
Acute leukemia of ambiguous lineage (ALAL) is a rare disorder in adults, constituting about 3% to 5% of acute leukemia cases. Unlike acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), ALAL cannot be clearly differentiated into a single subtype based on immunophenotyping. The diagnostic criteria for accurately identifying ALAL has evolved over time. There is paucity of information regarding the outcomes and management of this rare leukemia especially in elderly patients, and it is unclear whether treatment improves survival in these patients.
We performed a retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database to describe the outcomes of ALAL in the elderly population in U.S.1 Patients included in the analysis were aged > 65 years, with a pathologically confirmed diagnosis of ALAL, diagnosed between 1992-2010, and on active follow-up. Information on patient demographics, treatment, chemotherapeutic agents used in treatment, and survival was obtained and analyzed using appropriate statistical methods. A total of 705 patients with a median age of 80 years were included. There was a higher proportion of males than females and a higher proportion of white patients compared with African Americans and other races. We found that the overall survival (OS) declined significantly with increasing age, and treatment with chemotherapy improved the survival. However, factors such as gender, race, or type of chemotherapy received (ALL based, AML based, or other regimens) did not significantly influence the survival.
Even in the current era, the optimal therapy for ALAL is not well established. Although options such as AML-based or ALL-based chemotherapy are available, the best chemotherapy regimen and its sequence is unknown as prior studies have demonstrated varying results.2-5 Among elderly patients, numerous factors such as performance status, comorbidities, and ability to tolerate therapy influence the treatment decision. In light of the poor prognosis in elderly patients, a question often arises in the clinician’s mind about whether chemotherapy would provide any benefit for the patient.
Our study results showed that chemotherapy likely improves survival in these patients. However, due to the smaller number of patients, caution is needed in interpreting the result that there was no significant difference between AML-directed or ALL-directed chemotherapy. Another factor highlighted in the study was that only about 21.5% of patients had been treated with chemotherapy. Due to the inherent nature of the database, we could not identify the factors that may have influenced treatment decisions in these patients. Additionally, patients with stem cell transplantation-related claims could not be included in the analysis due to noncontinuous Medicare coverage during the study period. Hence, the role of stem cell transplantation in these patients could not be determined.
Implications Among Veterans
Actual incidence of ALAL among veterans is not known. Whether the incidence of ALAL relates to exposures to chemicals or toxins during military training and service also is unknown. However, ALAL is likely to be at least as prevalent as it is in the nonveteran population and perhaps more so because of exposures and stresses during military training and service.
It is unclear whether veterans attending VA hospitals receive less or different treatment given the higher comorbidities. Finally, it also is not known whether the outcomes for veterans would be different with or without treatment.
Our findings suggest that treatment should be seriously considered in all patients (veterans or not) who are healthy enough to receive chemotherapy regardless of their age. More research is needed to determine the disease incidence and prevalence among veterans and to evaluate whether there are specific etiologic correlations between ALAL and military exposures, whether the natural history is similar to other populations, and to delineate responsiveness to treatment.
Conclusion
This study suggests a poor survival for elderly patients with ALAL in the U.S. Although treatment is associated with an improvement in survival, only 21.5% of patients have received therapy. The optimal choice of chemotherapy for this disease is still not known and warrants prospective studies.
Click here to read the digital edition.
1. Guru Murthy GS, Dhakal I, Lee JY, Mehta P. Acute leukemia of ambiguous lineage in elderly patients—analysis of survival using surveillance epidemiology and end results—Medicare database. Clin Lymphoma Myeloma Leuk. 2017;17(2):100-107.
2. Rubnitz JE, Onciu M, Pounds S, et al. Acute mixed lineage leukemia in children: the experience of St Jude Children’s Research Hospital. Blood. 2009;113(21):5083-5089.
3. Matutes E, Pickl WF, Van’t Veer M, et al. Mixed phenotype acute leukemia: clinical and laboratory features and out-come in 100 patients defined according to the WHO classification. Blood. 2011;117(11):3163-3171.
4. Wolach O, Stone RM. How I treat mixed-phenotype acute leukemia. Blood. 2015;125(16):2477-2485.
5. Lee JH, Min YH, Chung CW, et al; Korean Society of Hematology AML/MDS Working Party. Prognostic implications of the immunophenotype in biphenotypic acute leukemia. Leuk Lymphoma. 2008;49(4):700-709.
1. Guru Murthy GS, Dhakal I, Lee JY, Mehta P. Acute leukemia of ambiguous lineage in elderly patients—analysis of survival using surveillance epidemiology and end results—Medicare database. Clin Lymphoma Myeloma Leuk. 2017;17(2):100-107.
2. Rubnitz JE, Onciu M, Pounds S, et al. Acute mixed lineage leukemia in children: the experience of St Jude Children’s Research Hospital. Blood. 2009;113(21):5083-5089.
3. Matutes E, Pickl WF, Van’t Veer M, et al. Mixed phenotype acute leukemia: clinical and laboratory features and out-come in 100 patients defined according to the WHO classification. Blood. 2011;117(11):3163-3171.
4. Wolach O, Stone RM. How I treat mixed-phenotype acute leukemia. Blood. 2015;125(16):2477-2485.
5. Lee JH, Min YH, Chung CW, et al; Korean Society of Hematology AML/MDS Working Party. Prognostic implications of the immunophenotype in biphenotypic acute leukemia. Leuk Lymphoma. 2008;49(4):700-709.
Open Clinical Trials for Patients With Renal Cell Carcinoma (FULL)
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting from those populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors more than 430 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of July 24, 2017; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for kidney cancer/renal cell carninoma. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
NCI-MATCH: Targeted Therapy Directed by Genetic Testing in Treating Patients With Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma
This phase II trial studies how well treatment that is directed by genetic testing works in patients with solid tumors or lymphomas that have progressed following at least 1 line of standard treatment or for which no agreed upon treatment approach exists. Genetic tests look at the unique genetic material (genes) of patients’ tumor cells. Patients with genetic abnormalities (such as mutations, amplifications, or translocations) may benefit more from treatment that targets their tumor’s particular genetic abnormality. Identifying these genetic abnormalities first may help doctors plan better treatment for patients with solid tumors, lymphomas, or multiple myeloma.
ID: NCT02465060
Sponsor: National Cancer Institute
Locations (contact): Naval Medical Center-San Diego, California (Preston S. Gable); VA Connecticut Healthcare System-West Haven Campus (Herta H. Chao); Durham VAMC, North Carolina (Michael J. Kelley); Walter Reed National Military Medical Center, Bethesda, Maryland (Jeremy G. Perkins)
Bevacizumab, Sorafenib Tosylate, and Temsirolimus in Treating Patients With Metastatic Kidney Cancer
This randomized phase II trial studies different combinations of bevacizumab, temsirolimus, and sorafenib tosylate to see how well they work compared with bevacizumab alone in treating patients with kidney cancer that has spread to other places in the body. Monoclonal antibodies, such as bevacizumab, may interfere with the ability of tumor cells to grow and spread. Bevacizumab and sorafenib tosylate may stop the growth of tumor cells by blocking blood flow to the tumor. Temsirolimus and sorafenib tosylate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving different combinations of bevacizumab, sorafenib tosylate, and temsirolimus may be more effective than bevacizumab alone in treating metastatic kidney cancer.
ID: NCT00378703
Sponsor: National Cancer Institute
Locations: VA San Diego Medical Center, California; Jesse Brown VAMC, Chicago, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; VA New Jersey Health Care System East Orange; Dayton VAMC, Ohio; Michael E. DeBakey VAMC, Houston, Texas
Everolimus in Treating Patients With Kidney Cancer Who Have Undergone Surgery (S0931)
Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth or by blocking blood flow to the tumor. This phase III trial is studying everolimus to see how well it works in treating patients with kidney cancer who have undergone surgery.
ID: NCT01120249
Sponsor: Southwest Oncology Group
Locations: Central Arkansas Veterans Healthcare System, Little Rock; Denver VAMC, Colorado; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC Indianapolis, Indiana; VAMC Baltimore, Maryland; Minneapolis VeteransMedical Center, Minnesota; VA New Jersey Health Care System, East Orange; VA New York Harbor Healthcare System-Brooklyn Campus; Wright-Patterson, Medical Center, Ohio; Michael E.DeBakey VAMC, Houston, Texas; Audie L. Murphy VA Hospital, San Antonio, Texas
Cabozantinib-s-malate or Sunitinib Malate in Treating Patients With Previously Untreated Locally Advanced or Metastatic Kidney Cancer
This randomized phase II trial studies how well cabozantinib-s-malate works compared to sunitinib malate in treating patients with previously untreated kidney cancer that has spread from where it started to nearby tissue or lymph nodes or to other places in the body. Cabozantinib-s-malate and sunitinib malate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. It is not yet known whether cabozantinib-s-malate is more effective than sunitinib malate in treating patients with kidney cancer.
ID: NCT01835158
Sponsor: National Cancer Institute
Locations: VA San Diego Medical Center, California; Minneapolis Veterans Medical Center, Minnesota; VAMC Columbia, Missouri; VA Western New York Health Care System, Buffalo
Everolimus With or Without Bevacizumab in Treating Patients With Advanced Kidney Cancer That Progressed After First-Line Therapy
This randomized phase III trial studies giving everolimus together with bevacizumab to see how well it works compared to everolimus alone in treating patients with advanced kidney cancer that progressed after first-line therapy. Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Monoclonal antibodies, such as bevacizumab, can interfere with tumor growth by blocking the ability of tumor cells to grow and spread. Everolimus and bevacizumab may also stop the growth of kidney cancer by blocking blood flow to the tumor. It is not yet known whether giving everolimus together with bevacizumab is better than everolimus alone in treating patients with advanced kidney cancer that has progressed after first-line therapy.
Sponsor: National Cancer Institute
ID: NCT01198158
Locations: Jesse Brown VAMC, Chicago, Illinois; Walter Reed National Military Medical Center, Bethesda, Maryland; VA Western New York Health Care System, Buffalo
Tivantinib With or Without Erlotinib Hydrochloride in Treating Patients With Metastatic or Locally Advanced Kidney Cancer That Cannot Be Removed by Surgery
This randomized phase II trial studies how well tivantinib with or without erlotinib hydrochloride works in treating patients with metastatic or locally advanced kidney cancer that cannot be removed by surgery. Tivantinib and erlotinib hydrochloride may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth.
Sponsor: National Cancer Institute (NCI)
ID: NCT01688973
Locations: Hines VA Hospital, Illinois; VA New Jersey Health Care System, East Orange; Audie L. Murphy VA Hospital, San Antonio, Texas
Bioequivalence & Food Effect Study in Patients With Solid Tumor or Hematologic Malignancies
This study will enroll approximately 60 subjects in stage I and 60 subjects in stage II with hematologic or solid tumor malignancies, excluding gastrointestinal tumors and tumors that have originated or metastasized to the liver for which no standard treatment exists or have progressed or recurred following prior therapy. Subjects must not be eligible for therapy of higher curative potential where an alternative treatment has been shown to prolong survival in an analogous population. Approximately 23 sites in the U.S. and 2 in Canada will participate in this study.
Sponsor: Celgene
ID: NCT02223052
Location: VAMC Kansas City, Missouri
Gemcitabine Hydrochloride and Cisplatin With or Without Bevacizumab in Treating Patients With Advanced Urinary Tract Cancer
This randomized phase III trial studies gemcitabine hydrochloride, cisplatin, and bevacizumab to see how well they work compared with gemcitabine hydrochloride and cisplatin in treating patients with urinary tract cancer that has spread to other places in the body. Drugs used in chemotherapy, such as gemcitabine hydrochloride and cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Monoclonal antibodies, such as bevacizumab, may interfere with the ability of tumor cells to grow and spread. It is not yet known whether gemcitabine hydrochloride and cisplatin are more effective when given with or without bevacizumab in treating patients with urinary tract cancer.
Sponsor: National Cancer Institute
ID: NCT00942331
Locations: Central Arkansas Veterans Healthcare System, Little Rock; Denver VAMC, Colorado; Baltimore VAMC, Maryland; Columbia VA, Missouri; VA New Jersey Health Care System, East Orange; VA New York Harbor Healthcare System-Brooklyn Campus; VA Western New York Health Care System, Buffalo; Dayton VAMC, Ohio
Eribulin Mesylate in Treating Patients With Locally Advanced or Metastatic Cancer of the Urothelium and Kidney Dysfunction
This phase I/II trial studies the side effects and best dose of eribulin mesylate and to see how well it works in treating patients with cancer of the urothelium that has spread to nearby tissue or to other places in the body and kidney dysfunction. Drugs used in chemotherapy, such as eribulin mesylate, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Chemotherapy drugs may have different effects in patients who have changes in their kidney function.
Sponsor: National Cancer Institute
ID: NCT00365157
Location: VA Hospital-Martinez, California
Click here to read the digital edition.
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting from those populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors more than 430 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of July 24, 2017; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for kidney cancer/renal cell carninoma. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
NCI-MATCH: Targeted Therapy Directed by Genetic Testing in Treating Patients With Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma
This phase II trial studies how well treatment that is directed by genetic testing works in patients with solid tumors or lymphomas that have progressed following at least 1 line of standard treatment or for which no agreed upon treatment approach exists. Genetic tests look at the unique genetic material (genes) of patients’ tumor cells. Patients with genetic abnormalities (such as mutations, amplifications, or translocations) may benefit more from treatment that targets their tumor’s particular genetic abnormality. Identifying these genetic abnormalities first may help doctors plan better treatment for patients with solid tumors, lymphomas, or multiple myeloma.
ID: NCT02465060
Sponsor: National Cancer Institute
Locations (contact): Naval Medical Center-San Diego, California (Preston S. Gable); VA Connecticut Healthcare System-West Haven Campus (Herta H. Chao); Durham VAMC, North Carolina (Michael J. Kelley); Walter Reed National Military Medical Center, Bethesda, Maryland (Jeremy G. Perkins)
Bevacizumab, Sorafenib Tosylate, and Temsirolimus in Treating Patients With Metastatic Kidney Cancer
This randomized phase II trial studies different combinations of bevacizumab, temsirolimus, and sorafenib tosylate to see how well they work compared with bevacizumab alone in treating patients with kidney cancer that has spread to other places in the body. Monoclonal antibodies, such as bevacizumab, may interfere with the ability of tumor cells to grow and spread. Bevacizumab and sorafenib tosylate may stop the growth of tumor cells by blocking blood flow to the tumor. Temsirolimus and sorafenib tosylate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving different combinations of bevacizumab, sorafenib tosylate, and temsirolimus may be more effective than bevacizumab alone in treating metastatic kidney cancer.
ID: NCT00378703
Sponsor: National Cancer Institute
Locations: VA San Diego Medical Center, California; Jesse Brown VAMC, Chicago, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; VA New Jersey Health Care System East Orange; Dayton VAMC, Ohio; Michael E. DeBakey VAMC, Houston, Texas
Everolimus in Treating Patients With Kidney Cancer Who Have Undergone Surgery (S0931)
Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth or by blocking blood flow to the tumor. This phase III trial is studying everolimus to see how well it works in treating patients with kidney cancer who have undergone surgery.
ID: NCT01120249
Sponsor: Southwest Oncology Group
Locations: Central Arkansas Veterans Healthcare System, Little Rock; Denver VAMC, Colorado; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC Indianapolis, Indiana; VAMC Baltimore, Maryland; Minneapolis VeteransMedical Center, Minnesota; VA New Jersey Health Care System, East Orange; VA New York Harbor Healthcare System-Brooklyn Campus; Wright-Patterson, Medical Center, Ohio; Michael E.DeBakey VAMC, Houston, Texas; Audie L. Murphy VA Hospital, San Antonio, Texas
Cabozantinib-s-malate or Sunitinib Malate in Treating Patients With Previously Untreated Locally Advanced or Metastatic Kidney Cancer
This randomized phase II trial studies how well cabozantinib-s-malate works compared to sunitinib malate in treating patients with previously untreated kidney cancer that has spread from where it started to nearby tissue or lymph nodes or to other places in the body. Cabozantinib-s-malate and sunitinib malate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. It is not yet known whether cabozantinib-s-malate is more effective than sunitinib malate in treating patients with kidney cancer.
ID: NCT01835158
Sponsor: National Cancer Institute
Locations: VA San Diego Medical Center, California; Minneapolis Veterans Medical Center, Minnesota; VAMC Columbia, Missouri; VA Western New York Health Care System, Buffalo
Everolimus With or Without Bevacizumab in Treating Patients With Advanced Kidney Cancer That Progressed After First-Line Therapy
This randomized phase III trial studies giving everolimus together with bevacizumab to see how well it works compared to everolimus alone in treating patients with advanced kidney cancer that progressed after first-line therapy. Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Monoclonal antibodies, such as bevacizumab, can interfere with tumor growth by blocking the ability of tumor cells to grow and spread. Everolimus and bevacizumab may also stop the growth of kidney cancer by blocking blood flow to the tumor. It is not yet known whether giving everolimus together with bevacizumab is better than everolimus alone in treating patients with advanced kidney cancer that has progressed after first-line therapy.
Sponsor: National Cancer Institute
ID: NCT01198158
Locations: Jesse Brown VAMC, Chicago, Illinois; Walter Reed National Military Medical Center, Bethesda, Maryland; VA Western New York Health Care System, Buffalo
Tivantinib With or Without Erlotinib Hydrochloride in Treating Patients With Metastatic or Locally Advanced Kidney Cancer That Cannot Be Removed by Surgery
This randomized phase II trial studies how well tivantinib with or without erlotinib hydrochloride works in treating patients with metastatic or locally advanced kidney cancer that cannot be removed by surgery. Tivantinib and erlotinib hydrochloride may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth.
Sponsor: National Cancer Institute (NCI)
ID: NCT01688973
Locations: Hines VA Hospital, Illinois; VA New Jersey Health Care System, East Orange; Audie L. Murphy VA Hospital, San Antonio, Texas
Bioequivalence & Food Effect Study in Patients With Solid Tumor or Hematologic Malignancies
This study will enroll approximately 60 subjects in stage I and 60 subjects in stage II with hematologic or solid tumor malignancies, excluding gastrointestinal tumors and tumors that have originated or metastasized to the liver for which no standard treatment exists or have progressed or recurred following prior therapy. Subjects must not be eligible for therapy of higher curative potential where an alternative treatment has been shown to prolong survival in an analogous population. Approximately 23 sites in the U.S. and 2 in Canada will participate in this study.
Sponsor: Celgene
ID: NCT02223052
Location: VAMC Kansas City, Missouri
Gemcitabine Hydrochloride and Cisplatin With or Without Bevacizumab in Treating Patients With Advanced Urinary Tract Cancer
This randomized phase III trial studies gemcitabine hydrochloride, cisplatin, and bevacizumab to see how well they work compared with gemcitabine hydrochloride and cisplatin in treating patients with urinary tract cancer that has spread to other places in the body. Drugs used in chemotherapy, such as gemcitabine hydrochloride and cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Monoclonal antibodies, such as bevacizumab, may interfere with the ability of tumor cells to grow and spread. It is not yet known whether gemcitabine hydrochloride and cisplatin are more effective when given with or without bevacizumab in treating patients with urinary tract cancer.
Sponsor: National Cancer Institute
ID: NCT00942331
Locations: Central Arkansas Veterans Healthcare System, Little Rock; Denver VAMC, Colorado; Baltimore VAMC, Maryland; Columbia VA, Missouri; VA New Jersey Health Care System, East Orange; VA New York Harbor Healthcare System-Brooklyn Campus; VA Western New York Health Care System, Buffalo; Dayton VAMC, Ohio
Eribulin Mesylate in Treating Patients With Locally Advanced or Metastatic Cancer of the Urothelium and Kidney Dysfunction
This phase I/II trial studies the side effects and best dose of eribulin mesylate and to see how well it works in treating patients with cancer of the urothelium that has spread to nearby tissue or to other places in the body and kidney dysfunction. Drugs used in chemotherapy, such as eribulin mesylate, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Chemotherapy drugs may have different effects in patients who have changes in their kidney function.
Sponsor: National Cancer Institute
ID: NCT00365157
Location: VA Hospital-Martinez, California
Click here to read the digital edition.
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting from those populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors more than 430 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of July 24, 2017; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for kidney cancer/renal cell carninoma. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
NCI-MATCH: Targeted Therapy Directed by Genetic Testing in Treating Patients With Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma
This phase II trial studies how well treatment that is directed by genetic testing works in patients with solid tumors or lymphomas that have progressed following at least 1 line of standard treatment or for which no agreed upon treatment approach exists. Genetic tests look at the unique genetic material (genes) of patients’ tumor cells. Patients with genetic abnormalities (such as mutations, amplifications, or translocations) may benefit more from treatment that targets their tumor’s particular genetic abnormality. Identifying these genetic abnormalities first may help doctors plan better treatment for patients with solid tumors, lymphomas, or multiple myeloma.
ID: NCT02465060
Sponsor: National Cancer Institute
Locations (contact): Naval Medical Center-San Diego, California (Preston S. Gable); VA Connecticut Healthcare System-West Haven Campus (Herta H. Chao); Durham VAMC, North Carolina (Michael J. Kelley); Walter Reed National Military Medical Center, Bethesda, Maryland (Jeremy G. Perkins)
Bevacizumab, Sorafenib Tosylate, and Temsirolimus in Treating Patients With Metastatic Kidney Cancer
This randomized phase II trial studies different combinations of bevacizumab, temsirolimus, and sorafenib tosylate to see how well they work compared with bevacizumab alone in treating patients with kidney cancer that has spread to other places in the body. Monoclonal antibodies, such as bevacizumab, may interfere with the ability of tumor cells to grow and spread. Bevacizumab and sorafenib tosylate may stop the growth of tumor cells by blocking blood flow to the tumor. Temsirolimus and sorafenib tosylate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving different combinations of bevacizumab, sorafenib tosylate, and temsirolimus may be more effective than bevacizumab alone in treating metastatic kidney cancer.
ID: NCT00378703
Sponsor: National Cancer Institute
Locations: VA San Diego Medical Center, California; Jesse Brown VAMC, Chicago, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; VA New Jersey Health Care System East Orange; Dayton VAMC, Ohio; Michael E. DeBakey VAMC, Houston, Texas
Everolimus in Treating Patients With Kidney Cancer Who Have Undergone Surgery (S0931)
Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth or by blocking blood flow to the tumor. This phase III trial is studying everolimus to see how well it works in treating patients with kidney cancer who have undergone surgery.
ID: NCT01120249
Sponsor: Southwest Oncology Group
Locations: Central Arkansas Veterans Healthcare System, Little Rock; Denver VAMC, Colorado; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC Indianapolis, Indiana; VAMC Baltimore, Maryland; Minneapolis VeteransMedical Center, Minnesota; VA New Jersey Health Care System, East Orange; VA New York Harbor Healthcare System-Brooklyn Campus; Wright-Patterson, Medical Center, Ohio; Michael E.DeBakey VAMC, Houston, Texas; Audie L. Murphy VA Hospital, San Antonio, Texas
Cabozantinib-s-malate or Sunitinib Malate in Treating Patients With Previously Untreated Locally Advanced or Metastatic Kidney Cancer
This randomized phase II trial studies how well cabozantinib-s-malate works compared to sunitinib malate in treating patients with previously untreated kidney cancer that has spread from where it started to nearby tissue or lymph nodes or to other places in the body. Cabozantinib-s-malate and sunitinib malate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. It is not yet known whether cabozantinib-s-malate is more effective than sunitinib malate in treating patients with kidney cancer.
ID: NCT01835158
Sponsor: National Cancer Institute
Locations: VA San Diego Medical Center, California; Minneapolis Veterans Medical Center, Minnesota; VAMC Columbia, Missouri; VA Western New York Health Care System, Buffalo
Everolimus With or Without Bevacizumab in Treating Patients With Advanced Kidney Cancer That Progressed After First-Line Therapy
This randomized phase III trial studies giving everolimus together with bevacizumab to see how well it works compared to everolimus alone in treating patients with advanced kidney cancer that progressed after first-line therapy. Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Monoclonal antibodies, such as bevacizumab, can interfere with tumor growth by blocking the ability of tumor cells to grow and spread. Everolimus and bevacizumab may also stop the growth of kidney cancer by blocking blood flow to the tumor. It is not yet known whether giving everolimus together with bevacizumab is better than everolimus alone in treating patients with advanced kidney cancer that has progressed after first-line therapy.
Sponsor: National Cancer Institute
ID: NCT01198158
Locations: Jesse Brown VAMC, Chicago, Illinois; Walter Reed National Military Medical Center, Bethesda, Maryland; VA Western New York Health Care System, Buffalo
Tivantinib With or Without Erlotinib Hydrochloride in Treating Patients With Metastatic or Locally Advanced Kidney Cancer That Cannot Be Removed by Surgery
This randomized phase II trial studies how well tivantinib with or without erlotinib hydrochloride works in treating patients with metastatic or locally advanced kidney cancer that cannot be removed by surgery. Tivantinib and erlotinib hydrochloride may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth.
Sponsor: National Cancer Institute (NCI)
ID: NCT01688973
Locations: Hines VA Hospital, Illinois; VA New Jersey Health Care System, East Orange; Audie L. Murphy VA Hospital, San Antonio, Texas
Bioequivalence & Food Effect Study in Patients With Solid Tumor or Hematologic Malignancies
This study will enroll approximately 60 subjects in stage I and 60 subjects in stage II with hematologic or solid tumor malignancies, excluding gastrointestinal tumors and tumors that have originated or metastasized to the liver for which no standard treatment exists or have progressed or recurred following prior therapy. Subjects must not be eligible for therapy of higher curative potential where an alternative treatment has been shown to prolong survival in an analogous population. Approximately 23 sites in the U.S. and 2 in Canada will participate in this study.
Sponsor: Celgene
ID: NCT02223052
Location: VAMC Kansas City, Missouri
Gemcitabine Hydrochloride and Cisplatin With or Without Bevacizumab in Treating Patients With Advanced Urinary Tract Cancer
This randomized phase III trial studies gemcitabine hydrochloride, cisplatin, and bevacizumab to see how well they work compared with gemcitabine hydrochloride and cisplatin in treating patients with urinary tract cancer that has spread to other places in the body. Drugs used in chemotherapy, such as gemcitabine hydrochloride and cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Monoclonal antibodies, such as bevacizumab, may interfere with the ability of tumor cells to grow and spread. It is not yet known whether gemcitabine hydrochloride and cisplatin are more effective when given with or without bevacizumab in treating patients with urinary tract cancer.
Sponsor: National Cancer Institute
ID: NCT00942331
Locations: Central Arkansas Veterans Healthcare System, Little Rock; Denver VAMC, Colorado; Baltimore VAMC, Maryland; Columbia VA, Missouri; VA New Jersey Health Care System, East Orange; VA New York Harbor Healthcare System-Brooklyn Campus; VA Western New York Health Care System, Buffalo; Dayton VAMC, Ohio
Eribulin Mesylate in Treating Patients With Locally Advanced or Metastatic Cancer of the Urothelium and Kidney Dysfunction
This phase I/II trial studies the side effects and best dose of eribulin mesylate and to see how well it works in treating patients with cancer of the urothelium that has spread to nearby tissue or to other places in the body and kidney dysfunction. Drugs used in chemotherapy, such as eribulin mesylate, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Chemotherapy drugs may have different effects in patients who have changes in their kidney function.
Sponsor: National Cancer Institute
ID: NCT00365157
Location: VA Hospital-Martinez, California
Click here to read the digital edition.
Do Erythropoiesis-Stimulating Agents Have a Risk Evaluation and Mitigation Strategy? (FULL)
Epoetin alfa and darbepoetin alfa are erythropoiesis-stimulating agents (ESAs), approved for the treatment of anemia (low red blood cells [RBCs]) resulting from chronic kidney disease, chemotherapy, and certain treatments for HIV. These ESAs also are used to reduce the number of blood transfusions during and after certain major surgeries. Erythropoiesis-stimulating agents work like the human protein erythropoietin, which stimulates bone marrow to make RBCs. Epoetin alfa (marketed as Procrit and Epogen) and darbepoetin alfa (marketed as Aranesp) are manufactured by Amgen, Inc. (Thousand Oaks, CA).
In 1989 epoetin alfa was approved for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 1993 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy. Epoetin alfa also is indicated for anemia due to zidovudine in patients with HIV and reduction of RBC transfusions during certain surgeries.
Darbepoetin alfa was approved in 2001 for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 2006 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy.
Risk Evaluation and Mitigation Strategies
Both epoetin alfa and darbepoetin alfa increase the risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access and tumor progression or recurrence. Epoetin alfa also can lead to an increase in adverse cardiovascular events, hypertension, seizures, and severe anemia.
In 2008, the FDA determined that Risk Evaluation and Mitigation Strategies (REMS) were necessary for ESAs (darbopoetin alfa and epoetin alfa), to ensure that the benefits for use as treatment for anemia associated with myelosuppressive chemotherapy outweigh the risk of shortened overall survival (OS) and/or the increased risk of tumor progression or recurrence in patients with cancer. The REMS was approved in 2010.
Under the ESA REMS program, referred to as the ESA APPRISE Oncology Program, health care providers (HCPs) that prescribed and/or dispensed darbopoetin alfa to patients with cancer and hospitals that dispensed darbopoetin alfa to patients with cancer were required to enroll and become certified in the ESA REMS. The ESA REMS also required the completion of a Patient and Healthcare Provider Acknowledgement Form for each patient with cancer before the new ESA treatment course to ensure patients were counseled about the benefits and risks of these products.
In April 2017, the FDA determined that the ESA REMS that was limited to the use of epoetin alfa and darbopoetin alfa to treat patients with anemia due to associated myelosuppressive chemotherapy was no longer necessary; the benefits of ESAs outweighed the risks of shortened OS and/or increased risk of tumor progression or recurrence in patients with cancer. 1 The FDA recognized the burden that some REMS can place on HCPs and patients. The agency has authority to modify or remove the REMS to minimize the burden on the health care delivery system of complying with the strategy.
Data
The FDA discontinued the REMS based on an evaluation of the results of the REMS Assessments submitted by Amgen and additional FDA analyses to understand the impact of the various regulatory and other actions on the use of ESAs. The REMS Assessment showed the following:
- The results from surveyed prescribers demonstrated acceptable knowledge of the product risks of decreased survival and/or the increased risk of tumor progression or recurrence and the need to counsel patients about these risks; and
- The drug utilization data indicated appropriate prescribing of ESAs consistent with the intended use as a treatment alternative to RBC transfusion for anemia associated with myelosuppressive chemotherapy.
The FDA also conducted an evaluation of the impact of multiple actions, including the ESA REMS, on the use of the ESAs using sponsor-submitted data from outpatient oncology practices between 2006 and 2014. During 2004 to 2009, the FDA took multiple regulatory actions, including labeling changes. In 2007, the Center for Medicare and Medicaid Services (CMS) made a National Coverage Determination (NCD) to limit coverage of ESAs for nonrenal disease indications. These actions coincided with the following:
- A decrease in the proportion of patients receiving chemotherapy using ESAs;
- An increase in the proportion of patients receiving chemotherapy who initiate ESAs at a hemoglobin level < 10 g/dL; and
- An increase in the proportion of patients who initiate ESAs at a dosage consistent with product prescribing information.
Full implementation of the ESA REMS in 2011 had minimal impact on trends in these 3 ESA utilization metrics beyond the changes observed after the CMS coverage determination and multiple other FDA regulatory actions.
This information led the FDA to conclude that it was no longer necessary to require the certification of prescribers and hospitals that prescribe and/or dispense ESAs to patients with cancer in order to ensure that the benefits outweigh the risks.
The FDA has released the REMS requirements for the epoetin alfa and darbopoetin alfa ESA products, and the risks can be communicated by the current product prescribing information. The appropriate use of ESAs is supported by the CMS NCD, the American Society of Clinical Oncology, and American Society of Hematology clinical guidelines, which are evidence-based guidelines intended to provide a basis for the standard of care in clinical oncology.
Education
While the REMS is no longer necessary to ensure the benefits outweigh the risks, the serious risks of shortened OS and/or increased risk of tumor progression or recurrence associated with these drugs remain. The boxed warning language remains as follows: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE. Health care providers are encouraged to discuss the risks and benefits of using ESAs with each patient before initiating use.
Click here to read the digital edition.
1. U.S. Food & Drug Administration. Information on erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). https://www.fda.gov/Drugs/DrugSafety/ucm109375.htm. Updated April 13, 2017. Accessed July 13, 2017.
Epoetin alfa and darbepoetin alfa are erythropoiesis-stimulating agents (ESAs), approved for the treatment of anemia (low red blood cells [RBCs]) resulting from chronic kidney disease, chemotherapy, and certain treatments for HIV. These ESAs also are used to reduce the number of blood transfusions during and after certain major surgeries. Erythropoiesis-stimulating agents work like the human protein erythropoietin, which stimulates bone marrow to make RBCs. Epoetin alfa (marketed as Procrit and Epogen) and darbepoetin alfa (marketed as Aranesp) are manufactured by Amgen, Inc. (Thousand Oaks, CA).
In 1989 epoetin alfa was approved for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 1993 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy. Epoetin alfa also is indicated for anemia due to zidovudine in patients with HIV and reduction of RBC transfusions during certain surgeries.
Darbepoetin alfa was approved in 2001 for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 2006 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy.
Risk Evaluation and Mitigation Strategies
Both epoetin alfa and darbepoetin alfa increase the risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access and tumor progression or recurrence. Epoetin alfa also can lead to an increase in adverse cardiovascular events, hypertension, seizures, and severe anemia.
In 2008, the FDA determined that Risk Evaluation and Mitigation Strategies (REMS) were necessary for ESAs (darbopoetin alfa and epoetin alfa), to ensure that the benefits for use as treatment for anemia associated with myelosuppressive chemotherapy outweigh the risk of shortened overall survival (OS) and/or the increased risk of tumor progression or recurrence in patients with cancer. The REMS was approved in 2010.
Under the ESA REMS program, referred to as the ESA APPRISE Oncology Program, health care providers (HCPs) that prescribed and/or dispensed darbopoetin alfa to patients with cancer and hospitals that dispensed darbopoetin alfa to patients with cancer were required to enroll and become certified in the ESA REMS. The ESA REMS also required the completion of a Patient and Healthcare Provider Acknowledgement Form for each patient with cancer before the new ESA treatment course to ensure patients were counseled about the benefits and risks of these products.
In April 2017, the FDA determined that the ESA REMS that was limited to the use of epoetin alfa and darbopoetin alfa to treat patients with anemia due to associated myelosuppressive chemotherapy was no longer necessary; the benefits of ESAs outweighed the risks of shortened OS and/or increased risk of tumor progression or recurrence in patients with cancer. 1 The FDA recognized the burden that some REMS can place on HCPs and patients. The agency has authority to modify or remove the REMS to minimize the burden on the health care delivery system of complying with the strategy.
Data
The FDA discontinued the REMS based on an evaluation of the results of the REMS Assessments submitted by Amgen and additional FDA analyses to understand the impact of the various regulatory and other actions on the use of ESAs. The REMS Assessment showed the following:
- The results from surveyed prescribers demonstrated acceptable knowledge of the product risks of decreased survival and/or the increased risk of tumor progression or recurrence and the need to counsel patients about these risks; and
- The drug utilization data indicated appropriate prescribing of ESAs consistent with the intended use as a treatment alternative to RBC transfusion for anemia associated with myelosuppressive chemotherapy.
The FDA also conducted an evaluation of the impact of multiple actions, including the ESA REMS, on the use of the ESAs using sponsor-submitted data from outpatient oncology practices between 2006 and 2014. During 2004 to 2009, the FDA took multiple regulatory actions, including labeling changes. In 2007, the Center for Medicare and Medicaid Services (CMS) made a National Coverage Determination (NCD) to limit coverage of ESAs for nonrenal disease indications. These actions coincided with the following:
- A decrease in the proportion of patients receiving chemotherapy using ESAs;
- An increase in the proportion of patients receiving chemotherapy who initiate ESAs at a hemoglobin level < 10 g/dL; and
- An increase in the proportion of patients who initiate ESAs at a dosage consistent with product prescribing information.
Full implementation of the ESA REMS in 2011 had minimal impact on trends in these 3 ESA utilization metrics beyond the changes observed after the CMS coverage determination and multiple other FDA regulatory actions.
This information led the FDA to conclude that it was no longer necessary to require the certification of prescribers and hospitals that prescribe and/or dispense ESAs to patients with cancer in order to ensure that the benefits outweigh the risks.
The FDA has released the REMS requirements for the epoetin alfa and darbopoetin alfa ESA products, and the risks can be communicated by the current product prescribing information. The appropriate use of ESAs is supported by the CMS NCD, the American Society of Clinical Oncology, and American Society of Hematology clinical guidelines, which are evidence-based guidelines intended to provide a basis for the standard of care in clinical oncology.
Education
While the REMS is no longer necessary to ensure the benefits outweigh the risks, the serious risks of shortened OS and/or increased risk of tumor progression or recurrence associated with these drugs remain. The boxed warning language remains as follows: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE. Health care providers are encouraged to discuss the risks and benefits of using ESAs with each patient before initiating use.
Click here to read the digital edition.
Epoetin alfa and darbepoetin alfa are erythropoiesis-stimulating agents (ESAs), approved for the treatment of anemia (low red blood cells [RBCs]) resulting from chronic kidney disease, chemotherapy, and certain treatments for HIV. These ESAs also are used to reduce the number of blood transfusions during and after certain major surgeries. Erythropoiesis-stimulating agents work like the human protein erythropoietin, which stimulates bone marrow to make RBCs. Epoetin alfa (marketed as Procrit and Epogen) and darbepoetin alfa (marketed as Aranesp) are manufactured by Amgen, Inc. (Thousand Oaks, CA).
In 1989 epoetin alfa was approved for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 1993 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy. Epoetin alfa also is indicated for anemia due to zidovudine in patients with HIV and reduction of RBC transfusions during certain surgeries.
Darbepoetin alfa was approved in 2001 for the treatment of anemia associated with chronic renal failure, including patients on dialysis and patients not on dialysis, and in 2006 for the treatment of anemia due to the effects of concomitant myelosuppressive chemotherapy.
Risk Evaluation and Mitigation Strategies
Both epoetin alfa and darbepoetin alfa increase the risk of death, myocardial infarction, stroke, venous thromboembolism, and thrombosis of vascular access and tumor progression or recurrence. Epoetin alfa also can lead to an increase in adverse cardiovascular events, hypertension, seizures, and severe anemia.
In 2008, the FDA determined that Risk Evaluation and Mitigation Strategies (REMS) were necessary for ESAs (darbopoetin alfa and epoetin alfa), to ensure that the benefits for use as treatment for anemia associated with myelosuppressive chemotherapy outweigh the risk of shortened overall survival (OS) and/or the increased risk of tumor progression or recurrence in patients with cancer. The REMS was approved in 2010.
Under the ESA REMS program, referred to as the ESA APPRISE Oncology Program, health care providers (HCPs) that prescribed and/or dispensed darbopoetin alfa to patients with cancer and hospitals that dispensed darbopoetin alfa to patients with cancer were required to enroll and become certified in the ESA REMS. The ESA REMS also required the completion of a Patient and Healthcare Provider Acknowledgement Form for each patient with cancer before the new ESA treatment course to ensure patients were counseled about the benefits and risks of these products.
In April 2017, the FDA determined that the ESA REMS that was limited to the use of epoetin alfa and darbopoetin alfa to treat patients with anemia due to associated myelosuppressive chemotherapy was no longer necessary; the benefits of ESAs outweighed the risks of shortened OS and/or increased risk of tumor progression or recurrence in patients with cancer. 1 The FDA recognized the burden that some REMS can place on HCPs and patients. The agency has authority to modify or remove the REMS to minimize the burden on the health care delivery system of complying with the strategy.
Data
The FDA discontinued the REMS based on an evaluation of the results of the REMS Assessments submitted by Amgen and additional FDA analyses to understand the impact of the various regulatory and other actions on the use of ESAs. The REMS Assessment showed the following:
- The results from surveyed prescribers demonstrated acceptable knowledge of the product risks of decreased survival and/or the increased risk of tumor progression or recurrence and the need to counsel patients about these risks; and
- The drug utilization data indicated appropriate prescribing of ESAs consistent with the intended use as a treatment alternative to RBC transfusion for anemia associated with myelosuppressive chemotherapy.
The FDA also conducted an evaluation of the impact of multiple actions, including the ESA REMS, on the use of the ESAs using sponsor-submitted data from outpatient oncology practices between 2006 and 2014. During 2004 to 2009, the FDA took multiple regulatory actions, including labeling changes. In 2007, the Center for Medicare and Medicaid Services (CMS) made a National Coverage Determination (NCD) to limit coverage of ESAs for nonrenal disease indications. These actions coincided with the following:
- A decrease in the proportion of patients receiving chemotherapy using ESAs;
- An increase in the proportion of patients receiving chemotherapy who initiate ESAs at a hemoglobin level < 10 g/dL; and
- An increase in the proportion of patients who initiate ESAs at a dosage consistent with product prescribing information.
Full implementation of the ESA REMS in 2011 had minimal impact on trends in these 3 ESA utilization metrics beyond the changes observed after the CMS coverage determination and multiple other FDA regulatory actions.
This information led the FDA to conclude that it was no longer necessary to require the certification of prescribers and hospitals that prescribe and/or dispense ESAs to patients with cancer in order to ensure that the benefits outweigh the risks.
The FDA has released the REMS requirements for the epoetin alfa and darbopoetin alfa ESA products, and the risks can be communicated by the current product prescribing information. The appropriate use of ESAs is supported by the CMS NCD, the American Society of Clinical Oncology, and American Society of Hematology clinical guidelines, which are evidence-based guidelines intended to provide a basis for the standard of care in clinical oncology.
Education
While the REMS is no longer necessary to ensure the benefits outweigh the risks, the serious risks of shortened OS and/or increased risk of tumor progression or recurrence associated with these drugs remain. The boxed warning language remains as follows: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE. Health care providers are encouraged to discuss the risks and benefits of using ESAs with each patient before initiating use.
Click here to read the digital edition.
1. U.S. Food & Drug Administration. Information on erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). https://www.fda.gov/Drugs/DrugSafety/ucm109375.htm. Updated April 13, 2017. Accessed July 13, 2017.
1. U.S. Food & Drug Administration. Information on erythropoiesis-stimulating agents (ESA) epoetin alfa (marketed as Procrit, Epogen), darbepoetin alfa (marketed as Aranesp). https://www.fda.gov/Drugs/DrugSafety/ucm109375.htm. Updated April 13, 2017. Accessed July 13, 2017.
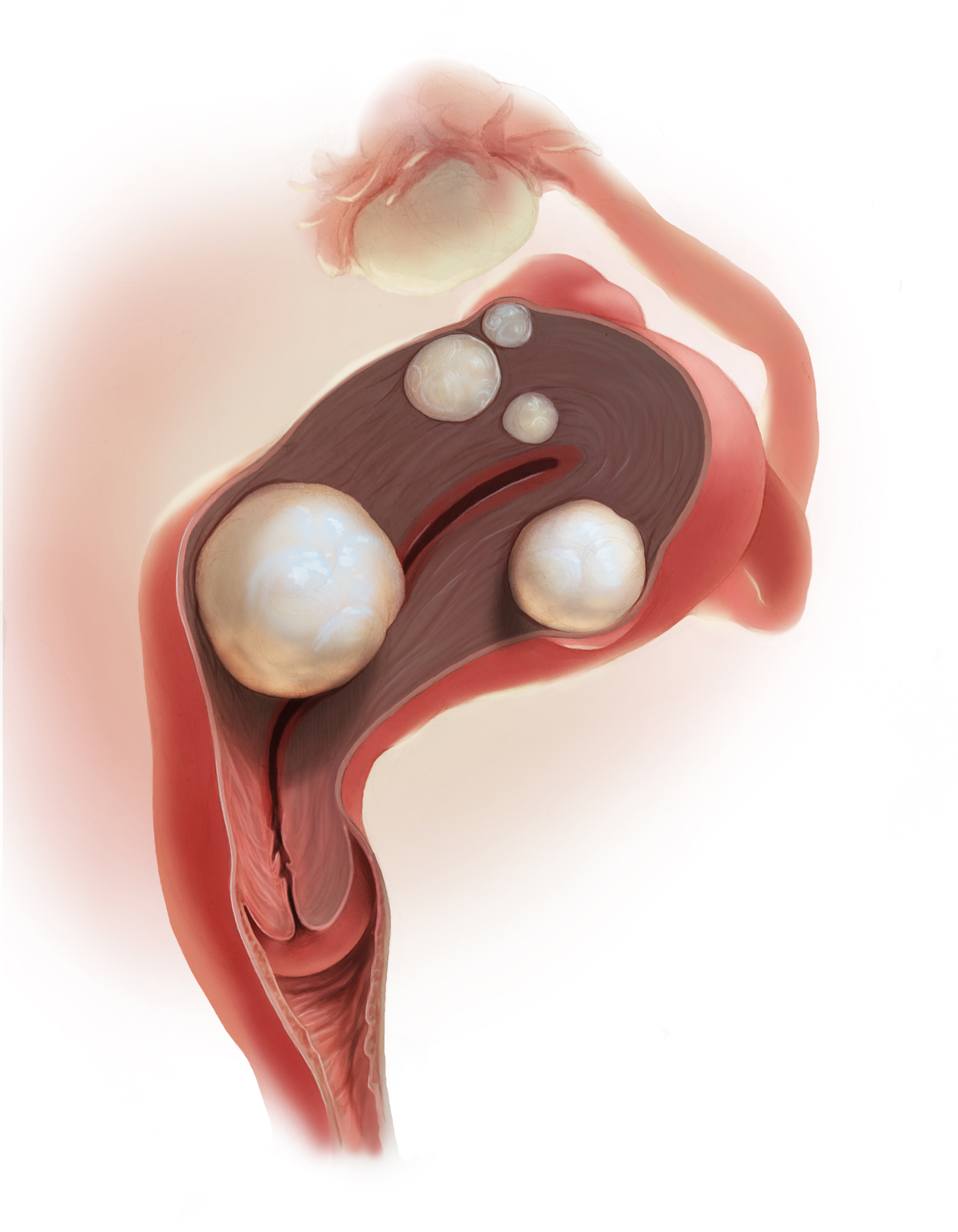
2018 Update on abnormal uterine bleeding
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.
Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.
Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Over the past year, a few gems have been published to help us manage and treat abnormal uterine bleeding (AUB). One study suggests an order of performing hysteroscopy and endometrial biopsy, another emphasizes the continued cost-effectiveness of the levonorgestrel-releasing intrauterine system (LNG-IUS), while a third provides more evidence that ulipristal acetate is effective in the management of leiomyomas.
Optimal order of office hysteroscopy and endometrial biopsy?
Sarkar P, Mikhail E, Schickler R, Plosker S, Imudia AN. Optimal order of successive office hysteroscopy and endometrial biopsy for the evaluation of abnormal uterine bleeding: a randomized controlled trial. Obstet Gynecol. 2017;130(3):565-572.
Office hysteroscopy and endometrial biopsy are frequently used in the evaluation of women presenting with AUB. Sarkar and colleagues conducted a study aimed at estimating the optimal order of office hysteroscopy and endometrial biopsy when performed successively among premenopausal women.
Pain perception, procedure duration, and other outcomes
This prospective single-blind randomized trial included 78 consecutive patients. The primary outcome was detection of any difference in patients' global pain perception based on the order of the procedures. Secondary outcome measures included determining whether the procedure order affected the duration of the procedures, the adequacy of the endometrial biopsy sample, the number of attempts to obtain an adequate tissue sample, and optimal visualization of the endometrial cavity during office hysteroscopy.
Order not important, but other factors may be
Not surprisingly, the results showed that the order in which the procedures were performed had no effect on patients' pain perception or on the overall procedure duration. Assessed using a visual analog scale scored from 1 to 10, global pain perception in the hysteroscopy-first patients (group A, n = 40) compared with the biopsy-first patients (group B, n = 38) was similar (7 vs 7, P = .57; 95% confidence interval [CI], 5.8-7.1). Procedure duration also was similar in group A and group B (3 vs 3, P = .32; 95% CI, 3.3-4.1).
However, when hysteroscopy was performed first, the quality of endometrial cavity images was superior compared with images from patients in whom biopsy was performed first. The number of endometrial biopsy curette passes required to obtain an adequate tissue sample was lower in the biopsy-first patients. The endometrial biopsy specimen was adequate for histologic evaluation regardless of whether hysteroscopy or biopsy was performed first.
Sarkar and colleagues suggested that their study findings emphasize the importance of individualizing the order of successive procedures to achieve the most clinically relevant result with maximum ease and comfort. They proposed that patients who have a high index of suspicion for occult malignancy or endometrial hyperplasia should have a biopsy procedure first so that adequate tissue samples can be obtained with fewer attempts. In patients with underlying uterine anatomic defects, performing hysteroscopy first would be clinically relevant to obtain the best images for optimal surgical planning.
Read next: Which treatment for AUB is most cost-effective?
Which treatment for AUB is most cost-effective?
Spencer JC, Louie M, Moulder JK, et al. Cost-effectiveness of treatments for heavy menstrual bleeding. Am J Obstet Gynecol. 2017;217(5):574.e1-574e.9.
The costs associated with heavy menstrual bleeding are significant. Spencer and colleagues sought to evaluate the relative cost-effectiveness of 4 treatment options for heavy menstrual bleeding: hysterectomy, resectoscopic endometrial ablation, nonresectoscopic endometrial ablation, and the LNG-IUS in a hypothetical cohort of 100,000 premenopausal women. No previous studies have examined the cost-effectiveness of these options in the context of the US health care setting.
Decision tree used for analysis
The authors formulated a decision tree to evaluate private payer costs and quality-adjusted life-years over a 5-year time horizon for premenopausal women with heavy menstrual bleeding and no suspected malignancy. For each treatment option, the authors used probabilities to estimate frequencies of complications and treatment failure leading to additional therapies. They compared the treatments in terms of total average costs, quality-adjusted life years, and incremental cost-effectiveness ratios.
Comparing costs, quality of life, and complications
Quality of life was fairly high for all treatment options; however, the estimated costs and the complications of each treatment were markedly different between treatment options. The LNG-IUS was superior to all alternatives in terms of both cost and quality, making it the dominant strategy. The 5-year cost for the LNG-IUS was $4,500, about half the cost of endometrial ablation ($9,500) and about one-third the cost of hysterectomy ($13,500). When examined over a range of possible values, the LNG-IUS was cost-effective compared with hysterectomy in the large majority of scenarios (90%).
If the LNG-IUS is removed from consideration because of either patient preference or clinical judgment, the decision between hysterectomy and ablation is more complex. Hysterectomy results in better quality of life in the majority of simulations, but it is cost-effective in just more than half of the simulations compared with either resectoscopic or nonresectoscopic ablation. Therefore, consideration of cost, procedure-specific complications, and patient preferences may guide the therapeutic decision between hysterectomy and endometrial ablation.
The 52-mg LNG-IUS was superior to all treatment alternatives in both cost and quality, making it the dominant strategy for the treatment of heavy menstrual bleeding.
Ulipristal may be useful for managing AUB associated with uterine leiomyomas
Simon JA, Catherino W, Segars JH, et al. Ulipristal acetate for treatment of symptomatic uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2018;131(3):431-439.
Managing uterine leiomyomas is a common issue for gynecologists, as up to 70% of white women and more than 80% of black women of reproductive age in the United States have leiomyomas.
Ulipristal acetate is an orally administered selective progesterone-receptor modulator that decreases bleeding and reduces leiomyoma size. Although trials conducted in Europe found ulipristal to be superior to placebo and noninferior to leuprolide acetate in controlling bleeding and reducing leiomyoma size, those initial trials were conducted in a predominantly white population.
Study assessed efficacy and safety
Simon and colleagues recently conducted a randomized double-blind, placebo-controlled trial designed to assess the safety and efficacy of ulipristal in a more diverse population, such as patients in the United States. The 148 participants included in the study were randomly assigned on a 1:1:1 basis to once-daily oral ulipristal 5 mg, ulipristal 10 mg, or placebo for 12 weeks, with a 12-week drug-free follow-up.
Amenorrhea achieved and quality of life improved
The investigators found that ulipristal in 5-mg and 10-mg doses was well tolerated and superior to placebo in both the rate of and the time to amenorrhea (the coprimary end points) in women with symptomatic leiomyomas. In women treated with ulipristal 5 mg, amenorrhea was achieved in 25 of 53 (47.2%; 97.5% CI, 31.6-63.2), and of those treated with the 10-mg dose, 28 of 48 (58.3%; 97.5% CI, 41.2-74.1) achieved amenorrhea (P<.001 for both groups), compared with 1 of 56 (1.8%; 97.5% CI, 0.0-10.9) in the placebo group.
AUB continues to be a significant issue for many women. As women's health care providers, it is important that we deliver care with high value (Quality ÷ Cost). Therefore, consider these takeaway points:
- The LNG-IUS consistently delivers high value by affecting both sides of this equation. We should use it more.
- Although we do not yet know what ulipristal acetate will cost in the United States, effective medical treatments usually affect both sides of the Quality ÷ Cost equation, and new medications on the horizon are worth knowing about.
- Last, efficiency with office-based hysteroscopy is also an opportunity to increase value by improving biopsy and visualization quality.
Ulipristal treatment also was shown to improve health-related quality of life, including physical and social activities. No patient discontinued ulipristal because of lack of efficacy, and 1 patient in the placebo group stopped taking the drug because of an adverse event. Estradiol levels were maintained at midfollicular levels during ulipristal treatment, and endometrial biopsies did not show any atypical or malignant changes. These results are consistent with those of the studies conducted in Europe in a predominantly white, nonobese population.
Results of this study help to define a niche for ulipristal when hysterectomy is not an option for women who wish to preserve fertility. Further, although leuprolide is used for preoperative hematologic improvement of anemia, its use results in hypoestrogenic adverse effects.
The findings from this and other studies suggest that ulipristal may be useful for the medical management of AUB associated with uterine leiomyomas, especially for patients desiring uterine- and fertility-sparing treatment. Hopefully, this treatment will be available soon in the United States.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Obesity: When to consider medication
Modest weight loss of 5% to 10% among patients who are overweight or obese can result in a clinically relevant reduction in cardiovascular (CV) disease risk.1 This amount of weight loss can increase insulin sensitivity in adipose tissue, liver, and muscle, and have a positive impact on blood sugar, blood pressure, triglycerides, and high-density lipoprotein cholesterol.1,2
All patients who are obese or overweight with increased CV risk should be counseled on diet, exercise, and other behavioral interventions.3 Weight loss secondary to lifestyle modification alone, however, leads to adaptive physiologic responses, which increase appetite and reduce energy expenditure.4–6
Pharmacotherapy can counteract this metabolic adaptation and lead to sustained weight loss. Antiobesity medication can be considered if a patient has a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with obesity-related comorbidities such as hypertension, type 2 diabetes, dyslipidemia, or obstructive sleep apnea.3,7
For patients with a body mass index (BMI) ≥30 kg/m2 or BMI ≥27 kg/m2 with weight-related comorbidities:
- Consider antiobesity pharmacotherapy when diet, exercise, and behavior modification do not produce sufficient weight loss. A
- Continue an antiobesity medication if it is deemed effective and well tolerated.A
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Until recently, there were few pharmacologic options approved by the US Food and Drug Administration (FDA) for the management of obesity. The mainstays of treatment were phentermine (Adipex-P, Ionamin, Suprenza) and orlistat (Alli, Xenical). Since 2012, however, 4 agents have been approved as adjuncts to a reduced-calorie diet and increased physical activity for long-term weight management.8,9 Phentermine/topiramate extended-release (ER) (Qsymia) and lorcaserin (Belviq) were approved in 2012,10,11 and naltrexone sustained release (SR)/bupropion SR (Contrave) and liraglutide 3 mg (Saxenda) were approved in 201412,13 (TABLE9,14–39). These medications have the potential to not only limit weight gain but also promote weight loss and, thus, improve blood pressure, cholesterol, glucose, and insulin.40
Despite the growing obesity epidemic and the availability of several additional medications for chronic weight management, use of antiobesity pharmacotherapy has been limited. Barriers to use include inadequate training of health care professionals, poor insurance coverage for new agents, and low reimbursement for office visits to address weight.41
In addition, the number of obesity medicine specialists, while increasing, is still not sufficient. Therefore, it is imperative for other health care professionals—including ObGyns—to be aware of the treatment options available to patients who are overweight or obese and to be adept at using them.
In this review, we present 4 cases that depict patients who could benefit from the addition of antiobesity pharmacotherapy to a comprehen‑sive treatment plan that includes diet, physical activity, and behavioral modification.
CASE 1 Young obese woman is unable to lose weight
A 27-year-old woman with obesity (BMI 33 kg/m2),hyperlipidemia, and migraine headaches, pre‑sents for weight management. Despite a calorie-reduced diet and 200 minutes per week of exercise for the past 6 months, she has been unable to lose weight. The only medications she is taking are oral contraceptive pills and sumatriptan, as needed. She suffers from migraines 3 times a month and has no anxiety. Laboratory test results are normal with the exception of an elevated low-density lipoprotein (LDL) level.
Which medication is an appropriate next step for this patient?
Ask 2 important questions
When considering an antiobesity agent for any patient, there are 2 important questions to ask:
- Are there contraindications, drug-drug interactions, or undesirable adverse effects associated with this medication that could be problematic for the patient?
- Can this medication improve other symptoms or conditions the patient has?
In addition, see “Before prescribing antiobesity medication . . .” below.
Have a frank discussion with the patient and be sure to cover the following points:
The rationale for pharmacologic treatment is to counteract adaptive physiologic responses, which increase appetite and reduce energy expenditure, in response to diet-induced weight loss.
Antiobesity medication is only one component of a comprehensive treatment plan, which also includes diet, physical activity, and behavior modification.
Antiobesity agents are intended for long-term use, as obesity is a chronic disease. If/when you stop the medication, there may be some weight regain, similar to an increase in blood pressure after discontinuing an antihypertensive agent.
Because antiobesity medications improve many parameters including glucose/hemoglobin A1c, lipids, blood pressure, and waist circumference, it is possible that the addition of one antiobesity medication can reduce, or even eliminate, the need for several other medications.
Remember that many patients who present for obesity management have experienced weight bias. It is important to not be judgmental, but rather explain why obesity is a chronic disease. If patients understand the physiology of their condition, they will understand that their limited success with weight loss in the past is not just a matter of willpower. Lifestyle change and weight loss are extremely difficult, so it is important to provide encouragement and support for ongoing behavioral modification.
Phentermine/topiramate ER
Phentermine/topiramate ER is a good first choice for this young patient with class I (BMI 30–34.9 kg/m2) obesity and migraines, as she can likely tolerate a stimulant and her migraines might improve with topiramate. Before starting the medication, ask about insomnia and nephrolithiasis in addition to anxiety and other contraindications (ie, glaucoma, hyperthyroidism, recent monoamine oxidase inhibitor use, or a known hypersensitivity or idiosyncrasy to sympathomimetic amines).23 The most common adverse events reported in phase III trials were dry mouth, paresthesia, and constipation.24–26
Not for pregnant women. Women of childbearing age must have a negative pregnancy test before starting phentermine/topiramate ER and every month while taking the medication. The FDA requires a Risk Evaluation and Mitigation Strategy (REMS) to inform prescribers and patients about the increased risk of congenital malformation, specifically orofacial clefts, in infants exposed to topiramate during the first trimester of pregnancy.42 REMS focuses on the importance of pregnancy prevention, the consistent use of birth control, and the need to discontinue phentermine/topiramate ER immediately if pregnancy occurs.
Flexible dosing. Phentermine/topiramate ER is available in 4 dosages: phentermine 3.75 mg/topiramate 23 mg ER; phentermine 7.5 mg/topiramate 46 mg ER; phentermine 11.25 mg/topiramate 69 mg ER; and phentermine 15 mg/topiramate 92 mg ER. Gradual dose escalation minimizes risks and adverse events.23
Monitor patients frequently to evaluate for adverse effects and ensure adherence to diet, exercise, and lifestyle modifications. If weight loss is slower or less robust than expected, check for dietary indiscretion, as medications have limited efficacy without appropriate behavioral changes.
Discontinue phentermine/topiramate ER if the patient does not achieve 5% weight loss after 12 weeks on the maximum dose, as it is unlikely that she will achieve and sustain clinically meaningful weight loss with continued treatment.23 In this case, consider another agent with a different mechanism of action. Any of the other antiobesity medications could be appropriate for this patient.
CASE 2 Overweight woman with comorbidities
A 52-year-old overweight woman (BMI 29 kg/m2)with type 2 diabetes, hyperlipidemia, osteoarthritis, and glaucoma has recently hit a plateau with her weight loss. She lost 45 lb secondary to diet and exercise, but hasn’t been able to lose any more. She also struggles with constant hunger. Her medications include metformin 1,000 mg twice per day, atorvastatin 10 mg/d, and occasional acetaminophen/oxycodone for knee pain until she undergoes a left knee replacement. Laboratory values are normal except for a hemoglobin A1c of 7.2%.
The patient is afraid of needles and cannot tolerate stimulants due to anxiety. Which medication is an appropriate next step for this patient?
What are good choices for this patient?
Lorcaserin is a good choice for this patient who is overweight and has several weight-related comorbidities. She has worked hard to lose a significant number of pounds and is now at high risk of regaining them. That’s because her appetite has increased with her new exercise regimen, but her energy expenditure has decreased secondary to metabolic adaptation.
Narrowing the field. Naltrexone SR/bupropion SR cannot be used because of her opioid use. Phentermine/topiramate ER is contraindicated for patients with glaucoma, and liraglutide 3 mg is not appropriate given the patient’s fear of needles.
She could try orlistat, especially if she struggles with constipation, but the gastrointestinal adverse effects are difficult for many patients to tolerate. While not an antiobesity medication, a sodium-glucose co-transporter 2 (SGLT2) inhibitor could be prescribed for her diabetes and also may promote weight loss.43
An appealing choice. The glucose-lowering effect of lorcaserin could provide an added benefit for the patient. The BLOOMDM (Behavioral modification and lorcaserin for overweight and obesity management in diabetes mellitus) study reported a mean reduction in hemoglobin A1c of 0.9% in the treatment group compared with a 0.4% reduction in the placebo group,30 and the effect of lorcaserin on A1c appeared to be independent of weight loss.
Mechanism of action: Cause for concern? Although lorcaserin selectively binds to serotonin 5-HT2C receptors, the theoretical risk of cardiac valvulopathy was evaluated in phase III studies, as fenfluramine, a 5-HT2B-receptor agonist, was withdrawn from the US market in 1997 for this reason.44 Both the BLOOM (Behavioral modification and lorcaserin for overweight and obesity management) and BLOSSOM (Behavioral modification and lorcaserin second study for obesity management) studies found that lorcaserin did not increase the incidence of FDA-defined cardiac valvulopathy.28,29
Formulations/adverse effects. Lorcaserin is available in 2 formulations: 10-mg tablets, which are taken twice daily, or 20-mg XR tablets, which are taken once daily. Both are generally well tolerated.27,45 The most common adverse event reported in phase III trials was headache.28,30,43 Discontinue lorcaserin if the patient does not lose 5% of her initial weight after 12 weeks, as weight loss at this stage is a good predictor of longer-term success.46
Some patients don’t respond. Interestingly, a subset of patients do not respond to lorcaserin. The most likely explanation for different responses to the medication is that there are many causes of obesity, only some of which respond to 5-HT2C agonism. Currently, we do not perform pharmacogenomics testing before prescribing lorcaserin, but perhaps an inexpensive test to identify responders will be available in the future.
CASE 3 A preoccupation with food
A 38-year-old woman with obesity (BMI 42 kg/m2),obstructive sleep apnea, gastroesophageal reflux disease, and depression is eager to get better control over her weight. Her medications include lansoprazole 30 mg/d and a multivitamin. She reports constantly thinking about food and not being able to control her impulses to buy large quantities of unhealthy snacks. She is so preoccupied by thoughts of food that she has difficulty concentrating at work.
The patient smokes a quarter of a pack of cigarettes daily, but she is ready to quit. She views bariatric surgery as a “last resort” and has no anxiety, pain, or history of seizures. Which medication is appropriate for this patient?
Discuss all options
This patient with class III obesity (BMI ≥40 kg/m2) is eligible for bariatric surgery; however, she is not interested in pursuing it at this time. It is important to discuss all of her options before deciding on a treatment plan. For patients like this, who would benefit from more than modest weight loss, consider a multidisciplinary approach including lifestyle modifications, pharmacotherapy, devices (eg, an intragastric balloon), and/or surgery. You would need to make clear to the patient that she may still be eligible for insurance coverage for surgery if she changes her mind after pursuing other treatments as long as her BMI remains ≥35 kg/m2 with obesity-related comorbidities.
Naltrexone SR/bupropion SR is a good choice for this patient because she describes debilitating cravings and addictive behavior surrounding food. Patients taking naltrexone SR/bupropion SR in the Contrave Obesity Research (COR)-I and COR-II phase III trials experienced a reduced frequency of food cravings, reduced difficulty in resisting food cravings, and an increased ability to control eating compared with those assigned to placebo.32,33
Added benefits. Bupropion also could help this patient quit smoking and improve her mood, as it is FDA-approved for smoking cessation and depression. She denies anxiety and seizures, so bupropion is not contraindicated. Even if a patient denies a history of seizure, ask about any conditions that predispose to seizures, such as anorexia nervosa or bulimia or the abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs.
Opioid use. Although the patient denies pain, ask about potential opioid use, as naltrexone is an opioid receptor antagonist. Patients should be informed that opioids may be ineffective if they are required unexpectedly (eg, for trauma) and that naltrexone SR/bupropion SR should be withheld for any planned surgical procedure potentially requiring opioid use.
Other options. While naltrexone SR/bupropion SR is the most appropriate choice for this patient because it addresses her problematic eating behaviors while potentially improving mood and assisting with smoking cessation, phentermine/topiramate ER, lorcaserin, and liraglutide 3 mg also could be used and certainly should be tried if naltrexone SR/bupropion SR does not produce the desired weight loss.
Adverse effects. Titrate naltrexone SR/bupropion SR slowly to the treatment dose to minimize risks and adverse events.31 The most common adverse effects reported in phase III trials were nausea, constipation, and headache.34,35,45,46 Discontinue naltrexone SR/bupropion SR if the patient does not achieve 5% weight loss at 16 weeks (after 12 weeks at the maintenance dose).31
CASE 4 Regaining weight after gastric bypass
A 65-year-old woman with obesity (BMI 39 kg/m2)who underwent Roux-en-Y gastric bypass surgery and who has type 2 diabetes, congestive heart failure, coronary artery disease, hypertension, and hyperlipidemia, remains concerned about her weight. She lost 100 lb following surgery and maintained her weight for 3 years, but then regained 30 lb. She comes in for an office visit because she is concerned about her increasing blood sugar and wants to prevent further weight gain. Her medications include metformin 1,000 mg twice per day, lisinopril 5 mg/d, carvedilol 12.5 mg twice per day, simvastatin 20 mg/d, and aspirin 81 mg/d. Laboratory test results are normal except for a hemoglobin A1c of 8%. She denies pancreatitis and a personal or family history of thyroid cancer.
Which medication is an appropriate next step for this patient?
Pharmacotherapy is an option
Pharmacotherapy is a great option for this patient, who is regaining weight following bariatric surgery. Phentermine/topiramate ER is the only medication that would be contraindicated because of her heart disease. Lorcaserin and naltrexone SR/bupropion SR could be considered, but liraglutide 3 mg is the most appropriate option, given her need for further glucose control.
Furthermore, the recent LEADER (Liraglutide effect and action in diabetes: evaluation of CV outcome results) trial reported a significant mortality benefit with liraglutide 1.8 mg/d among patients with type 2 diabetes and high CV risk.47 The study found that liraglutide was superior to placebo in reducing CV events.
Contraindications. Ask patients about a history of pancreatitis before starting liraglutide 3 mg, given the possible increased risk. In addition, liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2. Thyroid C-cell tumors have been found in rodents given supratherapeutic doses of liraglutide48; however, there is no evidence of liraglutide causing C-cell tumors in humans.
For patients taking a medication that can cause hypoglycemia, such as insulin or a sulfonylurea, monitor blood sugar and consider reducing the dose of that medication when starting liraglutide.
Administration and titration. Liraglutide is injected subcutaneously once daily. The dose is titrated up weekly to reduce gastrointestinal symptoms.36 The most common adverse effects reported in phase III trials were nausea, diarrhea, and constipation.37–39Discontinue liraglutide 3 mg if the patient does not lose at least 4% of baseline body weight after 16 weeks.49
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
- Magkos F, Fraterrigo G, Yoshino J. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 pt B):2985-3023.
- Sumithran P, Predergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604.
- Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196.
- Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring). 2016;24:1612-1619.
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
- Saunders KH, Shukla AP, Igel LI, et al. Pharmacotherapy for obesity. Endocrinol Metab Clin North Am. 2016;45:521-538.
- Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
- US Food and Drug Administration. Drug approval package. Qsymia. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000_qsymia_toc.cfm. Accessed August 28, 2017.
- Arena Pharmaceuticals. Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQR (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182. Accessed August 28, 2017.
- Drugs.com. Contrave approval history. https://www.drugs.com/history/contrave.html. Accessed August 28, 2017.
- US Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206321. Accessed August 28, 2017.
- Igel LI, Kumar RB, Saunders KH, et al. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152:1765-1779.
- Adipex-P package insert. http://www.iodine.com/drug/phentermine/fda-package-insert. Accessed August 28, 2017.
- Ionamin package insert. http://druginserts.com/lib/rx/meds/ionamin/. Accessed August 28, 2017.
- Lomaira package insert. https://www.lomaira.com/Prescribing_Information.pdf. Accessed August 28, 2017.
- Suprenza package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202088s001lbl.pdf. Accessed August 28, 2017.
- Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended release in obese adults. Obesity (Silver Spring). 2013;21:2163-2171.
- Alli package labeling. http://druginserts.com/lib/otc/meds/alli-1/. Accessed August 28, 2017.
- Xenical package insert. https://www.gene.com/download/pdf/xenical_prescribing.pdf. Accessed August 28,2017.
- Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161.
- Qsymia package insert. https://www.qsymia.com/pdf/prescribing-information.pdf. Accessed August 28, 2017.
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352.
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308.
- Belviq package insert. https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/Belviq_Prescribing_information-pdf.PDF?la=en. Accessed August 28, 2017.
- Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256.
- Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067-3077.
- O'Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20:1426-1436.
- Contrave package insert. https://contrave.com/wpcontent/uploads/2017/05/Contrave_PI.pdf. Accessed August 28, 2017.
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595-605.
- Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935-943.
- Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110-120.
- Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022-4029.
- Saxenda package insert. http://www.novo-pi.com/saxenda.pdf. Accessed August 28, 2017.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015;314:687-699.
- Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451.
- Saunders KH, Igel LI, Aronne LJ. An update on naltrexone/bupropion extended-release in the treatment of obesity. Expert Opin Pharmacother. 2016. 10.1080/14656566.2016.1244527.
- Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring). 2016;24:1955-1961.
- Qsymia Risk Evaluation and Mitigation Strategy (REMS). VIVUS, Inc. http://www.qsymiarems.com. Accessed January 16, 2017.
- Zinman B, Wanner C, Lachin JM, et al. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
- US Food and Drug Administration. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafety-InformationforPatientsandProviders/ucm179871.htm. Accessed August 28, 2017.
- Belviq XR package insert. https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/belviqxr_prescribing_information-pdf.PDF?la=en. Accessed January 16, 2017.
- Smith SR, O'Neil PM, Astrup A. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity (Silver Spring). 2014;22:2137-2146.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
- Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012;153:1538-1547.
- Fujioka K, O'Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24:2278-2288.
Modest weight loss of 5% to 10% among patients who are overweight or obese can result in a clinically relevant reduction in cardiovascular (CV) disease risk.1 This amount of weight loss can increase insulin sensitivity in adipose tissue, liver, and muscle, and have a positive impact on blood sugar, blood pressure, triglycerides, and high-density lipoprotein cholesterol.1,2
All patients who are obese or overweight with increased CV risk should be counseled on diet, exercise, and other behavioral interventions.3 Weight loss secondary to lifestyle modification alone, however, leads to adaptive physiologic responses, which increase appetite and reduce energy expenditure.4–6
Pharmacotherapy can counteract this metabolic adaptation and lead to sustained weight loss. Antiobesity medication can be considered if a patient has a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with obesity-related comorbidities such as hypertension, type 2 diabetes, dyslipidemia, or obstructive sleep apnea.3,7
For patients with a body mass index (BMI) ≥30 kg/m2 or BMI ≥27 kg/m2 with weight-related comorbidities:
- Consider antiobesity pharmacotherapy when diet, exercise, and behavior modification do not produce sufficient weight loss. A
- Continue an antiobesity medication if it is deemed effective and well tolerated.A
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Until recently, there were few pharmacologic options approved by the US Food and Drug Administration (FDA) for the management of obesity. The mainstays of treatment were phentermine (Adipex-P, Ionamin, Suprenza) and orlistat (Alli, Xenical). Since 2012, however, 4 agents have been approved as adjuncts to a reduced-calorie diet and increased physical activity for long-term weight management.8,9 Phentermine/topiramate extended-release (ER) (Qsymia) and lorcaserin (Belviq) were approved in 2012,10,11 and naltrexone sustained release (SR)/bupropion SR (Contrave) and liraglutide 3 mg (Saxenda) were approved in 201412,13 (TABLE9,14–39). These medications have the potential to not only limit weight gain but also promote weight loss and, thus, improve blood pressure, cholesterol, glucose, and insulin.40
Despite the growing obesity epidemic and the availability of several additional medications for chronic weight management, use of antiobesity pharmacotherapy has been limited. Barriers to use include inadequate training of health care professionals, poor insurance coverage for new agents, and low reimbursement for office visits to address weight.41
In addition, the number of obesity medicine specialists, while increasing, is still not sufficient. Therefore, it is imperative for other health care professionals—including ObGyns—to be aware of the treatment options available to patients who are overweight or obese and to be adept at using them.
In this review, we present 4 cases that depict patients who could benefit from the addition of antiobesity pharmacotherapy to a comprehen‑sive treatment plan that includes diet, physical activity, and behavioral modification.
CASE 1 Young obese woman is unable to lose weight
A 27-year-old woman with obesity (BMI 33 kg/m2),hyperlipidemia, and migraine headaches, pre‑sents for weight management. Despite a calorie-reduced diet and 200 minutes per week of exercise for the past 6 months, she has been unable to lose weight. The only medications she is taking are oral contraceptive pills and sumatriptan, as needed. She suffers from migraines 3 times a month and has no anxiety. Laboratory test results are normal with the exception of an elevated low-density lipoprotein (LDL) level.
Which medication is an appropriate next step for this patient?
Ask 2 important questions
When considering an antiobesity agent for any patient, there are 2 important questions to ask:
- Are there contraindications, drug-drug interactions, or undesirable adverse effects associated with this medication that could be problematic for the patient?
- Can this medication improve other symptoms or conditions the patient has?
In addition, see “Before prescribing antiobesity medication . . .” below.
Have a frank discussion with the patient and be sure to cover the following points:
The rationale for pharmacologic treatment is to counteract adaptive physiologic responses, which increase appetite and reduce energy expenditure, in response to diet-induced weight loss.
Antiobesity medication is only one component of a comprehensive treatment plan, which also includes diet, physical activity, and behavior modification.
Antiobesity agents are intended for long-term use, as obesity is a chronic disease. If/when you stop the medication, there may be some weight regain, similar to an increase in blood pressure after discontinuing an antihypertensive agent.
Because antiobesity medications improve many parameters including glucose/hemoglobin A1c, lipids, blood pressure, and waist circumference, it is possible that the addition of one antiobesity medication can reduce, or even eliminate, the need for several other medications.
Remember that many patients who present for obesity management have experienced weight bias. It is important to not be judgmental, but rather explain why obesity is a chronic disease. If patients understand the physiology of their condition, they will understand that their limited success with weight loss in the past is not just a matter of willpower. Lifestyle change and weight loss are extremely difficult, so it is important to provide encouragement and support for ongoing behavioral modification.
Phentermine/topiramate ER
Phentermine/topiramate ER is a good first choice for this young patient with class I (BMI 30–34.9 kg/m2) obesity and migraines, as she can likely tolerate a stimulant and her migraines might improve with topiramate. Before starting the medication, ask about insomnia and nephrolithiasis in addition to anxiety and other contraindications (ie, glaucoma, hyperthyroidism, recent monoamine oxidase inhibitor use, or a known hypersensitivity or idiosyncrasy to sympathomimetic amines).23 The most common adverse events reported in phase III trials were dry mouth, paresthesia, and constipation.24–26
Not for pregnant women. Women of childbearing age must have a negative pregnancy test before starting phentermine/topiramate ER and every month while taking the medication. The FDA requires a Risk Evaluation and Mitigation Strategy (REMS) to inform prescribers and patients about the increased risk of congenital malformation, specifically orofacial clefts, in infants exposed to topiramate during the first trimester of pregnancy.42 REMS focuses on the importance of pregnancy prevention, the consistent use of birth control, and the need to discontinue phentermine/topiramate ER immediately if pregnancy occurs.
Flexible dosing. Phentermine/topiramate ER is available in 4 dosages: phentermine 3.75 mg/topiramate 23 mg ER; phentermine 7.5 mg/topiramate 46 mg ER; phentermine 11.25 mg/topiramate 69 mg ER; and phentermine 15 mg/topiramate 92 mg ER. Gradual dose escalation minimizes risks and adverse events.23
Monitor patients frequently to evaluate for adverse effects and ensure adherence to diet, exercise, and lifestyle modifications. If weight loss is slower or less robust than expected, check for dietary indiscretion, as medications have limited efficacy without appropriate behavioral changes.
Discontinue phentermine/topiramate ER if the patient does not achieve 5% weight loss after 12 weeks on the maximum dose, as it is unlikely that she will achieve and sustain clinically meaningful weight loss with continued treatment.23 In this case, consider another agent with a different mechanism of action. Any of the other antiobesity medications could be appropriate for this patient.
CASE 2 Overweight woman with comorbidities
A 52-year-old overweight woman (BMI 29 kg/m2)with type 2 diabetes, hyperlipidemia, osteoarthritis, and glaucoma has recently hit a plateau with her weight loss. She lost 45 lb secondary to diet and exercise, but hasn’t been able to lose any more. She also struggles with constant hunger. Her medications include metformin 1,000 mg twice per day, atorvastatin 10 mg/d, and occasional acetaminophen/oxycodone for knee pain until she undergoes a left knee replacement. Laboratory values are normal except for a hemoglobin A1c of 7.2%.
The patient is afraid of needles and cannot tolerate stimulants due to anxiety. Which medication is an appropriate next step for this patient?
What are good choices for this patient?
Lorcaserin is a good choice for this patient who is overweight and has several weight-related comorbidities. She has worked hard to lose a significant number of pounds and is now at high risk of regaining them. That’s because her appetite has increased with her new exercise regimen, but her energy expenditure has decreased secondary to metabolic adaptation.
Narrowing the field. Naltrexone SR/bupropion SR cannot be used because of her opioid use. Phentermine/topiramate ER is contraindicated for patients with glaucoma, and liraglutide 3 mg is not appropriate given the patient’s fear of needles.
She could try orlistat, especially if she struggles with constipation, but the gastrointestinal adverse effects are difficult for many patients to tolerate. While not an antiobesity medication, a sodium-glucose co-transporter 2 (SGLT2) inhibitor could be prescribed for her diabetes and also may promote weight loss.43
An appealing choice. The glucose-lowering effect of lorcaserin could provide an added benefit for the patient. The BLOOMDM (Behavioral modification and lorcaserin for overweight and obesity management in diabetes mellitus) study reported a mean reduction in hemoglobin A1c of 0.9% in the treatment group compared with a 0.4% reduction in the placebo group,30 and the effect of lorcaserin on A1c appeared to be independent of weight loss.
Mechanism of action: Cause for concern? Although lorcaserin selectively binds to serotonin 5-HT2C receptors, the theoretical risk of cardiac valvulopathy was evaluated in phase III studies, as fenfluramine, a 5-HT2B-receptor agonist, was withdrawn from the US market in 1997 for this reason.44 Both the BLOOM (Behavioral modification and lorcaserin for overweight and obesity management) and BLOSSOM (Behavioral modification and lorcaserin second study for obesity management) studies found that lorcaserin did not increase the incidence of FDA-defined cardiac valvulopathy.28,29
Formulations/adverse effects. Lorcaserin is available in 2 formulations: 10-mg tablets, which are taken twice daily, or 20-mg XR tablets, which are taken once daily. Both are generally well tolerated.27,45 The most common adverse event reported in phase III trials was headache.28,30,43 Discontinue lorcaserin if the patient does not lose 5% of her initial weight after 12 weeks, as weight loss at this stage is a good predictor of longer-term success.46
Some patients don’t respond. Interestingly, a subset of patients do not respond to lorcaserin. The most likely explanation for different responses to the medication is that there are many causes of obesity, only some of which respond to 5-HT2C agonism. Currently, we do not perform pharmacogenomics testing before prescribing lorcaserin, but perhaps an inexpensive test to identify responders will be available in the future.
CASE 3 A preoccupation with food
A 38-year-old woman with obesity (BMI 42 kg/m2),obstructive sleep apnea, gastroesophageal reflux disease, and depression is eager to get better control over her weight. Her medications include lansoprazole 30 mg/d and a multivitamin. She reports constantly thinking about food and not being able to control her impulses to buy large quantities of unhealthy snacks. She is so preoccupied by thoughts of food that she has difficulty concentrating at work.
The patient smokes a quarter of a pack of cigarettes daily, but she is ready to quit. She views bariatric surgery as a “last resort” and has no anxiety, pain, or history of seizures. Which medication is appropriate for this patient?
Discuss all options
This patient with class III obesity (BMI ≥40 kg/m2) is eligible for bariatric surgery; however, she is not interested in pursuing it at this time. It is important to discuss all of her options before deciding on a treatment plan. For patients like this, who would benefit from more than modest weight loss, consider a multidisciplinary approach including lifestyle modifications, pharmacotherapy, devices (eg, an intragastric balloon), and/or surgery. You would need to make clear to the patient that she may still be eligible for insurance coverage for surgery if she changes her mind after pursuing other treatments as long as her BMI remains ≥35 kg/m2 with obesity-related comorbidities.
Naltrexone SR/bupropion SR is a good choice for this patient because she describes debilitating cravings and addictive behavior surrounding food. Patients taking naltrexone SR/bupropion SR in the Contrave Obesity Research (COR)-I and COR-II phase III trials experienced a reduced frequency of food cravings, reduced difficulty in resisting food cravings, and an increased ability to control eating compared with those assigned to placebo.32,33
Added benefits. Bupropion also could help this patient quit smoking and improve her mood, as it is FDA-approved for smoking cessation and depression. She denies anxiety and seizures, so bupropion is not contraindicated. Even if a patient denies a history of seizure, ask about any conditions that predispose to seizures, such as anorexia nervosa or bulimia or the abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs.
Opioid use. Although the patient denies pain, ask about potential opioid use, as naltrexone is an opioid receptor antagonist. Patients should be informed that opioids may be ineffective if they are required unexpectedly (eg, for trauma) and that naltrexone SR/bupropion SR should be withheld for any planned surgical procedure potentially requiring opioid use.
Other options. While naltrexone SR/bupropion SR is the most appropriate choice for this patient because it addresses her problematic eating behaviors while potentially improving mood and assisting with smoking cessation, phentermine/topiramate ER, lorcaserin, and liraglutide 3 mg also could be used and certainly should be tried if naltrexone SR/bupropion SR does not produce the desired weight loss.
Adverse effects. Titrate naltrexone SR/bupropion SR slowly to the treatment dose to minimize risks and adverse events.31 The most common adverse effects reported in phase III trials were nausea, constipation, and headache.34,35,45,46 Discontinue naltrexone SR/bupropion SR if the patient does not achieve 5% weight loss at 16 weeks (after 12 weeks at the maintenance dose).31
CASE 4 Regaining weight after gastric bypass
A 65-year-old woman with obesity (BMI 39 kg/m2)who underwent Roux-en-Y gastric bypass surgery and who has type 2 diabetes, congestive heart failure, coronary artery disease, hypertension, and hyperlipidemia, remains concerned about her weight. She lost 100 lb following surgery and maintained her weight for 3 years, but then regained 30 lb. She comes in for an office visit because she is concerned about her increasing blood sugar and wants to prevent further weight gain. Her medications include metformin 1,000 mg twice per day, lisinopril 5 mg/d, carvedilol 12.5 mg twice per day, simvastatin 20 mg/d, and aspirin 81 mg/d. Laboratory test results are normal except for a hemoglobin A1c of 8%. She denies pancreatitis and a personal or family history of thyroid cancer.
Which medication is an appropriate next step for this patient?
Pharmacotherapy is an option
Pharmacotherapy is a great option for this patient, who is regaining weight following bariatric surgery. Phentermine/topiramate ER is the only medication that would be contraindicated because of her heart disease. Lorcaserin and naltrexone SR/bupropion SR could be considered, but liraglutide 3 mg is the most appropriate option, given her need for further glucose control.
Furthermore, the recent LEADER (Liraglutide effect and action in diabetes: evaluation of CV outcome results) trial reported a significant mortality benefit with liraglutide 1.8 mg/d among patients with type 2 diabetes and high CV risk.47 The study found that liraglutide was superior to placebo in reducing CV events.
Contraindications. Ask patients about a history of pancreatitis before starting liraglutide 3 mg, given the possible increased risk. In addition, liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2. Thyroid C-cell tumors have been found in rodents given supratherapeutic doses of liraglutide48; however, there is no evidence of liraglutide causing C-cell tumors in humans.
For patients taking a medication that can cause hypoglycemia, such as insulin or a sulfonylurea, monitor blood sugar and consider reducing the dose of that medication when starting liraglutide.
Administration and titration. Liraglutide is injected subcutaneously once daily. The dose is titrated up weekly to reduce gastrointestinal symptoms.36 The most common adverse effects reported in phase III trials were nausea, diarrhea, and constipation.37–39Discontinue liraglutide 3 mg if the patient does not lose at least 4% of baseline body weight after 16 weeks.49
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Modest weight loss of 5% to 10% among patients who are overweight or obese can result in a clinically relevant reduction in cardiovascular (CV) disease risk.1 This amount of weight loss can increase insulin sensitivity in adipose tissue, liver, and muscle, and have a positive impact on blood sugar, blood pressure, triglycerides, and high-density lipoprotein cholesterol.1,2
All patients who are obese or overweight with increased CV risk should be counseled on diet, exercise, and other behavioral interventions.3 Weight loss secondary to lifestyle modification alone, however, leads to adaptive physiologic responses, which increase appetite and reduce energy expenditure.4–6
Pharmacotherapy can counteract this metabolic adaptation and lead to sustained weight loss. Antiobesity medication can be considered if a patient has a body mass index (BMI) ≥30 kg/m2 or ≥27 kg/m2 with obesity-related comorbidities such as hypertension, type 2 diabetes, dyslipidemia, or obstructive sleep apnea.3,7
For patients with a body mass index (BMI) ≥30 kg/m2 or BMI ≥27 kg/m2 with weight-related comorbidities:
- Consider antiobesity pharmacotherapy when diet, exercise, and behavior modification do not produce sufficient weight loss. A
- Continue an antiobesity medication if it is deemed effective and well tolerated.A
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Until recently, there were few pharmacologic options approved by the US Food and Drug Administration (FDA) for the management of obesity. The mainstays of treatment were phentermine (Adipex-P, Ionamin, Suprenza) and orlistat (Alli, Xenical). Since 2012, however, 4 agents have been approved as adjuncts to a reduced-calorie diet and increased physical activity for long-term weight management.8,9 Phentermine/topiramate extended-release (ER) (Qsymia) and lorcaserin (Belviq) were approved in 2012,10,11 and naltrexone sustained release (SR)/bupropion SR (Contrave) and liraglutide 3 mg (Saxenda) were approved in 201412,13 (TABLE9,14–39). These medications have the potential to not only limit weight gain but also promote weight loss and, thus, improve blood pressure, cholesterol, glucose, and insulin.40
Despite the growing obesity epidemic and the availability of several additional medications for chronic weight management, use of antiobesity pharmacotherapy has been limited. Barriers to use include inadequate training of health care professionals, poor insurance coverage for new agents, and low reimbursement for office visits to address weight.41
In addition, the number of obesity medicine specialists, while increasing, is still not sufficient. Therefore, it is imperative for other health care professionals—including ObGyns—to be aware of the treatment options available to patients who are overweight or obese and to be adept at using them.
In this review, we present 4 cases that depict patients who could benefit from the addition of antiobesity pharmacotherapy to a comprehen‑sive treatment plan that includes diet, physical activity, and behavioral modification.
CASE 1 Young obese woman is unable to lose weight
A 27-year-old woman with obesity (BMI 33 kg/m2),hyperlipidemia, and migraine headaches, pre‑sents for weight management. Despite a calorie-reduced diet and 200 minutes per week of exercise for the past 6 months, she has been unable to lose weight. The only medications she is taking are oral contraceptive pills and sumatriptan, as needed. She suffers from migraines 3 times a month and has no anxiety. Laboratory test results are normal with the exception of an elevated low-density lipoprotein (LDL) level.
Which medication is an appropriate next step for this patient?
Ask 2 important questions
When considering an antiobesity agent for any patient, there are 2 important questions to ask:
- Are there contraindications, drug-drug interactions, or undesirable adverse effects associated with this medication that could be problematic for the patient?
- Can this medication improve other symptoms or conditions the patient has?
In addition, see “Before prescribing antiobesity medication . . .” below.
Have a frank discussion with the patient and be sure to cover the following points:
The rationale for pharmacologic treatment is to counteract adaptive physiologic responses, which increase appetite and reduce energy expenditure, in response to diet-induced weight loss.
Antiobesity medication is only one component of a comprehensive treatment plan, which also includes diet, physical activity, and behavior modification.
Antiobesity agents are intended for long-term use, as obesity is a chronic disease. If/when you stop the medication, there may be some weight regain, similar to an increase in blood pressure after discontinuing an antihypertensive agent.
Because antiobesity medications improve many parameters including glucose/hemoglobin A1c, lipids, blood pressure, and waist circumference, it is possible that the addition of one antiobesity medication can reduce, or even eliminate, the need for several other medications.
Remember that many patients who present for obesity management have experienced weight bias. It is important to not be judgmental, but rather explain why obesity is a chronic disease. If patients understand the physiology of their condition, they will understand that their limited success with weight loss in the past is not just a matter of willpower. Lifestyle change and weight loss are extremely difficult, so it is important to provide encouragement and support for ongoing behavioral modification.
Phentermine/topiramate ER
Phentermine/topiramate ER is a good first choice for this young patient with class I (BMI 30–34.9 kg/m2) obesity and migraines, as she can likely tolerate a stimulant and her migraines might improve with topiramate. Before starting the medication, ask about insomnia and nephrolithiasis in addition to anxiety and other contraindications (ie, glaucoma, hyperthyroidism, recent monoamine oxidase inhibitor use, or a known hypersensitivity or idiosyncrasy to sympathomimetic amines).23 The most common adverse events reported in phase III trials were dry mouth, paresthesia, and constipation.24–26
Not for pregnant women. Women of childbearing age must have a negative pregnancy test before starting phentermine/topiramate ER and every month while taking the medication. The FDA requires a Risk Evaluation and Mitigation Strategy (REMS) to inform prescribers and patients about the increased risk of congenital malformation, specifically orofacial clefts, in infants exposed to topiramate during the first trimester of pregnancy.42 REMS focuses on the importance of pregnancy prevention, the consistent use of birth control, and the need to discontinue phentermine/topiramate ER immediately if pregnancy occurs.
Flexible dosing. Phentermine/topiramate ER is available in 4 dosages: phentermine 3.75 mg/topiramate 23 mg ER; phentermine 7.5 mg/topiramate 46 mg ER; phentermine 11.25 mg/topiramate 69 mg ER; and phentermine 15 mg/topiramate 92 mg ER. Gradual dose escalation minimizes risks and adverse events.23
Monitor patients frequently to evaluate for adverse effects and ensure adherence to diet, exercise, and lifestyle modifications. If weight loss is slower or less robust than expected, check for dietary indiscretion, as medications have limited efficacy without appropriate behavioral changes.
Discontinue phentermine/topiramate ER if the patient does not achieve 5% weight loss after 12 weeks on the maximum dose, as it is unlikely that she will achieve and sustain clinically meaningful weight loss with continued treatment.23 In this case, consider another agent with a different mechanism of action. Any of the other antiobesity medications could be appropriate for this patient.
CASE 2 Overweight woman with comorbidities
A 52-year-old overweight woman (BMI 29 kg/m2)with type 2 diabetes, hyperlipidemia, osteoarthritis, and glaucoma has recently hit a plateau with her weight loss. She lost 45 lb secondary to diet and exercise, but hasn’t been able to lose any more. She also struggles with constant hunger. Her medications include metformin 1,000 mg twice per day, atorvastatin 10 mg/d, and occasional acetaminophen/oxycodone for knee pain until she undergoes a left knee replacement. Laboratory values are normal except for a hemoglobin A1c of 7.2%.
The patient is afraid of needles and cannot tolerate stimulants due to anxiety. Which medication is an appropriate next step for this patient?
What are good choices for this patient?
Lorcaserin is a good choice for this patient who is overweight and has several weight-related comorbidities. She has worked hard to lose a significant number of pounds and is now at high risk of regaining them. That’s because her appetite has increased with her new exercise regimen, but her energy expenditure has decreased secondary to metabolic adaptation.
Narrowing the field. Naltrexone SR/bupropion SR cannot be used because of her opioid use. Phentermine/topiramate ER is contraindicated for patients with glaucoma, and liraglutide 3 mg is not appropriate given the patient’s fear of needles.
She could try orlistat, especially if she struggles with constipation, but the gastrointestinal adverse effects are difficult for many patients to tolerate. While not an antiobesity medication, a sodium-glucose co-transporter 2 (SGLT2) inhibitor could be prescribed for her diabetes and also may promote weight loss.43
An appealing choice. The glucose-lowering effect of lorcaserin could provide an added benefit for the patient. The BLOOMDM (Behavioral modification and lorcaserin for overweight and obesity management in diabetes mellitus) study reported a mean reduction in hemoglobin A1c of 0.9% in the treatment group compared with a 0.4% reduction in the placebo group,30 and the effect of lorcaserin on A1c appeared to be independent of weight loss.
Mechanism of action: Cause for concern? Although lorcaserin selectively binds to serotonin 5-HT2C receptors, the theoretical risk of cardiac valvulopathy was evaluated in phase III studies, as fenfluramine, a 5-HT2B-receptor agonist, was withdrawn from the US market in 1997 for this reason.44 Both the BLOOM (Behavioral modification and lorcaserin for overweight and obesity management) and BLOSSOM (Behavioral modification and lorcaserin second study for obesity management) studies found that lorcaserin did not increase the incidence of FDA-defined cardiac valvulopathy.28,29
Formulations/adverse effects. Lorcaserin is available in 2 formulations: 10-mg tablets, which are taken twice daily, or 20-mg XR tablets, which are taken once daily. Both are generally well tolerated.27,45 The most common adverse event reported in phase III trials was headache.28,30,43 Discontinue lorcaserin if the patient does not lose 5% of her initial weight after 12 weeks, as weight loss at this stage is a good predictor of longer-term success.46
Some patients don’t respond. Interestingly, a subset of patients do not respond to lorcaserin. The most likely explanation for different responses to the medication is that there are many causes of obesity, only some of which respond to 5-HT2C agonism. Currently, we do not perform pharmacogenomics testing before prescribing lorcaserin, but perhaps an inexpensive test to identify responders will be available in the future.
CASE 3 A preoccupation with food
A 38-year-old woman with obesity (BMI 42 kg/m2),obstructive sleep apnea, gastroesophageal reflux disease, and depression is eager to get better control over her weight. Her medications include lansoprazole 30 mg/d and a multivitamin. She reports constantly thinking about food and not being able to control her impulses to buy large quantities of unhealthy snacks. She is so preoccupied by thoughts of food that she has difficulty concentrating at work.
The patient smokes a quarter of a pack of cigarettes daily, but she is ready to quit. She views bariatric surgery as a “last resort” and has no anxiety, pain, or history of seizures. Which medication is appropriate for this patient?
Discuss all options
This patient with class III obesity (BMI ≥40 kg/m2) is eligible for bariatric surgery; however, she is not interested in pursuing it at this time. It is important to discuss all of her options before deciding on a treatment plan. For patients like this, who would benefit from more than modest weight loss, consider a multidisciplinary approach including lifestyle modifications, pharmacotherapy, devices (eg, an intragastric balloon), and/or surgery. You would need to make clear to the patient that she may still be eligible for insurance coverage for surgery if she changes her mind after pursuing other treatments as long as her BMI remains ≥35 kg/m2 with obesity-related comorbidities.
Naltrexone SR/bupropion SR is a good choice for this patient because she describes debilitating cravings and addictive behavior surrounding food. Patients taking naltrexone SR/bupropion SR in the Contrave Obesity Research (COR)-I and COR-II phase III trials experienced a reduced frequency of food cravings, reduced difficulty in resisting food cravings, and an increased ability to control eating compared with those assigned to placebo.32,33
Added benefits. Bupropion also could help this patient quit smoking and improve her mood, as it is FDA-approved for smoking cessation and depression. She denies anxiety and seizures, so bupropion is not contraindicated. Even if a patient denies a history of seizure, ask about any conditions that predispose to seizures, such as anorexia nervosa or bulimia or the abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs.
Opioid use. Although the patient denies pain, ask about potential opioid use, as naltrexone is an opioid receptor antagonist. Patients should be informed that opioids may be ineffective if they are required unexpectedly (eg, for trauma) and that naltrexone SR/bupropion SR should be withheld for any planned surgical procedure potentially requiring opioid use.
Other options. While naltrexone SR/bupropion SR is the most appropriate choice for this patient because it addresses her problematic eating behaviors while potentially improving mood and assisting with smoking cessation, phentermine/topiramate ER, lorcaserin, and liraglutide 3 mg also could be used and certainly should be tried if naltrexone SR/bupropion SR does not produce the desired weight loss.
Adverse effects. Titrate naltrexone SR/bupropion SR slowly to the treatment dose to minimize risks and adverse events.31 The most common adverse effects reported in phase III trials were nausea, constipation, and headache.34,35,45,46 Discontinue naltrexone SR/bupropion SR if the patient does not achieve 5% weight loss at 16 weeks (after 12 weeks at the maintenance dose).31
CASE 4 Regaining weight after gastric bypass
A 65-year-old woman with obesity (BMI 39 kg/m2)who underwent Roux-en-Y gastric bypass surgery and who has type 2 diabetes, congestive heart failure, coronary artery disease, hypertension, and hyperlipidemia, remains concerned about her weight. She lost 100 lb following surgery and maintained her weight for 3 years, but then regained 30 lb. She comes in for an office visit because she is concerned about her increasing blood sugar and wants to prevent further weight gain. Her medications include metformin 1,000 mg twice per day, lisinopril 5 mg/d, carvedilol 12.5 mg twice per day, simvastatin 20 mg/d, and aspirin 81 mg/d. Laboratory test results are normal except for a hemoglobin A1c of 8%. She denies pancreatitis and a personal or family history of thyroid cancer.
Which medication is an appropriate next step for this patient?
Pharmacotherapy is an option
Pharmacotherapy is a great option for this patient, who is regaining weight following bariatric surgery. Phentermine/topiramate ER is the only medication that would be contraindicated because of her heart disease. Lorcaserin and naltrexone SR/bupropion SR could be considered, but liraglutide 3 mg is the most appropriate option, given her need for further glucose control.
Furthermore, the recent LEADER (Liraglutide effect and action in diabetes: evaluation of CV outcome results) trial reported a significant mortality benefit with liraglutide 1.8 mg/d among patients with type 2 diabetes and high CV risk.47 The study found that liraglutide was superior to placebo in reducing CV events.
Contraindications. Ask patients about a history of pancreatitis before starting liraglutide 3 mg, given the possible increased risk. In addition, liraglutide is contraindicated in patients with a personal or family history of medullary thyroid carcinoma or in patients with multiple endocrine neoplasia syndrome type 2. Thyroid C-cell tumors have been found in rodents given supratherapeutic doses of liraglutide48; however, there is no evidence of liraglutide causing C-cell tumors in humans.
For patients taking a medication that can cause hypoglycemia, such as insulin or a sulfonylurea, monitor blood sugar and consider reducing the dose of that medication when starting liraglutide.
Administration and titration. Liraglutide is injected subcutaneously once daily. The dose is titrated up weekly to reduce gastrointestinal symptoms.36 The most common adverse effects reported in phase III trials were nausea, diarrhea, and constipation.37–39Discontinue liraglutide 3 mg if the patient does not lose at least 4% of baseline body weight after 16 weeks.49
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
- Magkos F, Fraterrigo G, Yoshino J. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 pt B):2985-3023.
- Sumithran P, Predergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604.
- Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196.
- Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring). 2016;24:1612-1619.
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
- Saunders KH, Shukla AP, Igel LI, et al. Pharmacotherapy for obesity. Endocrinol Metab Clin North Am. 2016;45:521-538.
- Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
- US Food and Drug Administration. Drug approval package. Qsymia. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000_qsymia_toc.cfm. Accessed August 28, 2017.
- Arena Pharmaceuticals. Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQR (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182. Accessed August 28, 2017.
- Drugs.com. Contrave approval history. https://www.drugs.com/history/contrave.html. Accessed August 28, 2017.
- US Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206321. Accessed August 28, 2017.
- Igel LI, Kumar RB, Saunders KH, et al. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152:1765-1779.
- Adipex-P package insert. http://www.iodine.com/drug/phentermine/fda-package-insert. Accessed August 28, 2017.
- Ionamin package insert. http://druginserts.com/lib/rx/meds/ionamin/. Accessed August 28, 2017.
- Lomaira package insert. https://www.lomaira.com/Prescribing_Information.pdf. Accessed August 28, 2017.
- Suprenza package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202088s001lbl.pdf. Accessed August 28, 2017.
- Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended release in obese adults. Obesity (Silver Spring). 2013;21:2163-2171.
- Alli package labeling. http://druginserts.com/lib/otc/meds/alli-1/. Accessed August 28, 2017.
- Xenical package insert. https://www.gene.com/download/pdf/xenical_prescribing.pdf. Accessed August 28,2017.
- Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161.
- Qsymia package insert. https://www.qsymia.com/pdf/prescribing-information.pdf. Accessed August 28, 2017.
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352.
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308.
- Belviq package insert. https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/Belviq_Prescribing_information-pdf.PDF?la=en. Accessed August 28, 2017.
- Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256.
- Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067-3077.
- O'Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20:1426-1436.
- Contrave package insert. https://contrave.com/wpcontent/uploads/2017/05/Contrave_PI.pdf. Accessed August 28, 2017.
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595-605.
- Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935-943.
- Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110-120.
- Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022-4029.
- Saxenda package insert. http://www.novo-pi.com/saxenda.pdf. Accessed August 28, 2017.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015;314:687-699.
- Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451.
- Saunders KH, Igel LI, Aronne LJ. An update on naltrexone/bupropion extended-release in the treatment of obesity. Expert Opin Pharmacother. 2016. 10.1080/14656566.2016.1244527.
- Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring). 2016;24:1955-1961.
- Qsymia Risk Evaluation and Mitigation Strategy (REMS). VIVUS, Inc. http://www.qsymiarems.com. Accessed January 16, 2017.
- Zinman B, Wanner C, Lachin JM, et al. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
- US Food and Drug Administration. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafety-InformationforPatientsandProviders/ucm179871.htm. Accessed August 28, 2017.
- Belviq XR package insert. https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/belviqxr_prescribing_information-pdf.PDF?la=en. Accessed January 16, 2017.
- Smith SR, O'Neil PM, Astrup A. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity (Silver Spring). 2014;22:2137-2146.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
- Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012;153:1538-1547.
- Fujioka K, O'Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24:2278-2288.
- Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481-1486.
- Magkos F, Fraterrigo G, Yoshino J. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591-601.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2014;63(25 pt B):2985-3023.
- Sumithran P, Predergast LA, Delbridge E, et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365:1597-1604.
- Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes (Lond). 2015;39:1188-1196.
- Fothergill E, Guo J, Howard L, et al. Persistent metabolic adaptation 6 years after "The Biggest Loser" competition. Obesity (Silver Spring). 2016;24:1612-1619.
- Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342-362.
- Saunders KH, Shukla AP, Igel LI, et al. Pharmacotherapy for obesity. Endocrinol Metab Clin North Am. 2016;45:521-538.
- Saunders KH, Kumar RB, Igel LI, et al. Pharmacologic approaches to weight management: recent gains and shortfalls in combating obesity. Curr Atheroscler Rep. 2016;18:36.
- US Food and Drug Administration. Drug approval package. Qsymia. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/022580Orig1s000_qsymia_toc.cfm. Accessed August 28, 2017.
- Arena Pharmaceuticals. Arena Pharmaceuticals and Eisai announce FDA approval of BELVIQR (lorcaserin HCl) for chronic weight management in adults who are overweight with a comorbidity or obese. http://invest.arenapharm.com/releasedetail.cfm?ReleaseID=687182. Accessed August 28, 2017.
- Drugs.com. Contrave approval history. https://www.drugs.com/history/contrave.html. Accessed August 28, 2017.
- US Food and Drug Administration. Drugs@FDA: FDA approved drug products. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206321. Accessed August 28, 2017.
- Igel LI, Kumar RB, Saunders KH, et al. Practical use of pharmacotherapy for obesity. Gastroenterology. 2017;152:1765-1779.
- Adipex-P package insert. http://www.iodine.com/drug/phentermine/fda-package-insert. Accessed August 28, 2017.
- Ionamin package insert. http://druginserts.com/lib/rx/meds/ionamin/. Accessed August 28, 2017.
- Lomaira package insert. https://www.lomaira.com/Prescribing_Information.pdf. Accessed August 28, 2017.
- Suprenza package insert. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202088s001lbl.pdf. Accessed August 28, 2017.
- Aronne LJ, Wadden TA, Peterson C, et al. Evaluation of phentermine and topiramate versus phentermine/topiramate extended release in obese adults. Obesity (Silver Spring). 2013;21:2163-2171.
- Alli package labeling. http://druginserts.com/lib/otc/meds/alli-1/. Accessed August 28, 2017.
- Xenical package insert. https://www.gene.com/download/pdf/xenical_prescribing.pdf. Accessed August 28,2017.
- Torgerson JS, Hauptman J, Boldrin MN, et al. XENical in the prevention of Diabetes in Obese Subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004;27:155-161.
- Qsymia package insert. https://www.qsymia.com/pdf/prescribing-information.pdf. Accessed August 28, 2017.
- Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP). Obesity (Silver Spring). 2012;20:330-342.
- Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341-1352.
- Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297-308.
- Belviq package insert. https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/Belviq_Prescribing_information-pdf.PDF?la=en. Accessed August 28, 2017.
- Smith SR, Weissman NJ, Anderson CM, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245-256.
- Fidler MC, Sanchez M, Raether B, et al. A one-year randomized trial of lorcaserin for weight loss in obese and overweight adults: the BLOSSOM trial. J Clin Endocrinol Metab. 2011;96:3067-3077.
- O'Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring). 2012;20:1426-1436.
- Contrave package insert. https://contrave.com/wpcontent/uploads/2017/05/Contrave_PI.pdf. Accessed August 28, 2017.
- Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376:595-605.
- Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21:935-943.
- Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring). 2011;19:110-120.
- Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013;36:4022-4029.
- Saxenda package insert. http://www.novo-pi.com/saxenda.pdf. Accessed August 28, 2017.
- Pi-Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11-22.
- Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE Diabetes randomized clinical trial. JAMA. 2015;314:687-699.
- Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low-calorie-diet induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443-1451.
- Saunders KH, Igel LI, Aronne LJ. An update on naltrexone/bupropion extended-release in the treatment of obesity. Expert Opin Pharmacother. 2016. 10.1080/14656566.2016.1244527.
- Thomas CE, Mauer EA, Shukla AP, et al. Low adoption of weight loss medications: a comparison of prescribing patterns of antiobesity pharmacotherapies and SGLT2s. Obesity (Silver Spring). 2016;24:1955-1961.
- Qsymia Risk Evaluation and Mitigation Strategy (REMS). VIVUS, Inc. http://www.qsymiarems.com. Accessed January 16, 2017.
- Zinman B, Wanner C, Lachin JM, et al. Empaglifozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117-2128.
- US Food and Drug Administration. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafety-InformationforPatientsandProviders/ucm179871.htm. Accessed August 28, 2017.
- Belviq XR package insert. https://www.belviq.com/-/media/Files/BelviqConsolidation/PDF/belviqxr_prescribing_information-pdf.PDF?la=en. Accessed January 16, 2017.
- Smith SR, O'Neil PM, Astrup A. Early weight loss while on lorcaserin, diet and exercise as a predictor of week 52 weight-loss outcomes. Obesity (Silver Spring). 2014;22:2137-2146.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311-322.
- Madsen LW, Knauf JA, Gotfredsen C, et al. GLP-1 receptor agonists and the thyroid: C-cell effects in mice are mediated via the GLP-1 receptor and not associated with RET activation. Endocrinology. 2012;153:1538-1547.
- Fujioka K, O'Neil PM, Davies M, et al. Early weight loss with liraglutide 3.0 mg predicts 1-year weight loss and is associated with improvements in clinical markers. Obesity (Silver Spring). 2016;24:2278-2288.
Acute Myeloid Leukemia
Introduction
Acute myeloid leukemia (AML) comprises a heterogeneous group of disorders characterized by proliferation of clonal, abnormally differentiated hematopoietic progenitor cells of myeloid lineage that infiltrate the bone marrow, blood, and other tissues.1 In most cases, AML is rapidly fatal if left untreated. Over the past 2 decades, our understanding of the underlying disease biology responsible for the development of AML has improved substantially. We have learned that biological differences drive the various clinical, cytogenetic, and molecular subentities of AML; distinguishing among these subentities helps to identify optimal therapies, while offering improved clinical outcomes for select groups. After years of stagnation in therapeutic advances, 4 new drugs for treating AML were approved by the US Food and Drug Administration (FDA) in 2017. In this article, we review key features of AML diagnosis and management in the context of 2 case presentations.
Epidemiology and Risk Factors
An estimated 21,380 new cases of AML were diagnosed in the United States in 2017, constituting roughly 1.3% of all new cases of cancer.2 Approximately 10,590 patients died of AML in 2017. The median age of patients at the time of diagnosis is 68 years, and the incidence is approximately 4.2 per 100,000 persons per year. The 5-year survival for AML has steadily risen from a meager 6.3% in 1975 to 17.3% in 1995 and 28.1% in 2009.2 The cure rates for AML vary drastically with age. Long-term survival is achieved in approximately 35% to 40% of adults who present at age 60 years or younger, but only 5% to 15% of those older than 60 years at presentation will achieve long-term survival.3
Most cases of AML occur in the absence of any known risk factors. High-dose radiation exposure, chronic benzene exposure, chronic tobacco smoking, and certain chemotherapeutics are known to increase the risk for AML.4 Inconsistent correlations have also been made between exposure to organic solvents, petroleum products, radon, pesticides, and herbicides and the development of AML.4 Obesity may also increase AML risk.4
Two distinct subcategories of therapy-related AML (t-AML) are known. Patients who have been exposed to alkylating chemotherapeutics (eg, melphalan, cyclophosphamide, and nitrogen mustard) can develop t-AML with chromosomal 5 and/or 7 abnormalities after a latency period of approximately 4 to 8 years.5 In contrast, patients exposed to topoisomerase II inhibitors (notably etoposide) develop AML with abnormalities of 11q23 (leading to MLL gene rearrangement) or 21q22 (RUNX1) after a latency period of about 1 to 3 years.6 AML can also arise out of other myeloid disorders such as myelodysplastic syndrome and myeloproliferative neoplasms, and other bone marrow failure syndromes such as aplastic anemia.4 Various inherited or congenital conditions such as Down syndrome, Bloom syndrome, Fanconi anemia, neurofibromatosis 1, and dyskeratosis congenita can also predispose to the development of AML. A more detailed listing of conditions associated with AML can be found elsewhere.4
Molecular Landscape
The first cancer genome sequence was reported in an AML patient in 2008.7 Since then, various elegantly conducted studies have expanded our understanding of the molecular abnormalities in AML. The Cancer Genome Atlas Research Network analyzed the genomes of 200 cases of de novo AML in adults.8 Only 13 mutations were found on average, much fewer than the number of mutations in most adult cancers. Twenty-three genes were commonly mutated, and another 237 were mutated in 2 or more cases. Essentially, all cases had at least 1 nonsynonymous mutation in 1 of 9 categories of genes: transcription-factor fusions (18%), the gene encoding nucleophosmin (NPM1) (27%), tumor-suppressor genes (16%), DNA-methylation–related genes (44%), signaling genes (59%), chromatin-modifying genes (30%), myeloid transcription-factor genes (22%), spliceosome-complex genes (14%), and cohesin-complex genes (13%).
In another study, samples from 1540 patients from 3 prospective trials of intensive chemotherapy were analyzed to understand how genetic diversity defines the pathophysiology of AML.9 The study authors identified 5234 driver mutations from 76 genes or genomic regions, with 2 or more drivers identified in 86% of the samples. Eleven classes of mutational events, each with distinct diagnostic features and clinical outcomes, were identified. Acting as an internal positive control in this analysis, previously recognized mutational and cytogenetic groups emerged as distinct entities, including the groups with biallelic CEBPA mutations, mutations in NPM1, MLL fusions, and the cytogenetic entities t(6;9), inv(3), t(8;21), t(15;17), and inv(16). Three additional categories emerged as distinct entities: AML with mutations in genes encoding chromatin, RNA splicing regulators, or both (18% of patients); AML with TP53 mutations, chromosomal aneuploidies, or both (13%); and, provisionally, AML with IDH2R172 mutations (1%). An additional level of complexity was also revealed within the subgroup of patients with NPM1 mutations, where gene–gene interactions identified co-mutational events associated with both favorable or adverse prognosis.
Further supporting this molecular classification of AML, a study that performed targeted mutational analysis of 194 patients with defined secondary AML (s-AML) or t-AML and 105 unselected AML patients found that the presence of mutations in SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2 (all members of the chromatin or RNA splicing families) was highly specific for the diagnosis of s-AML.10 These findings are particularly clinically useful in those without a known history of antecedent hematologic disorder. These mutations defining the AML ontogeny were found to occur early in leukemogenesis, persist in clonal remissions, and predict worse clinical outcomes. Mutations in genes involved in regulation of DNA modification and of chromatin state (commonly DNMT3A, ASXL1, and TET2) have also been shown to be present in preleukemic stem or progenitor cells and to occur early in leukemogenesis.3 Unsurprisingly, some of these same mutations, including those in epigenetic regulators (DNMT3A, ASXL1, and TET2) and less frequently in splicing factor genes (SF3B1, SRSF2), have been associated with clonal hematopoietic expansion in elderly, seemingly healthy adults, a condition termed clonal hematopoiesis of indeterminate potential (CHIP).3,11,12 The presence of CHIP is associated with increased risk of hematologic neoplasms and all-cause mortality, the latter being possibly driven by a near doubling in the risk of coronary heart disease in humans and by accelerated atherosclerosis in a mouse model.11,13,14
Clinical Presentation and Work-up
Case Patient 1
A 57-year-old woman with a history of hypertension presents to the emergency department with complaints of productive cough and fevers for the previous 3 days. Examination reveals conjunctival pallor, gingival hyperplasia, and decreased breath sounds at the posterior right lung field. Investigations reveal a white blood cell (WBC) count of 51,000/µL with 15% blasts, a hemoglobin of 7.8 g/dL, and a platelet count of 56 × 103/µL. Peripheral blood smear is notable for large myeloblasts with occasional Auer rods. Chest radiograph shows a consolidation in the right lower lobe.
Case Patient 2
A 69-year-old man presents to his primary care physician for evaluation of worsening fatigue for the previous 4 months. Ten years prior to presentation, he had received 6 cycles of RCHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) as treatment for diffuse large B-cell lymphoma. Conjunctival pallor, patches of purpura over the extremities, and mucosal petechiae are noted on examination. Laboratory analyisis reveals a WBC count of 2400/µL with 12% blasts, hemoglobin of 9.0 g/dL, and platelet count of 10 × 103/µL. Peripheral smear shows dysplastic myeloid cells and blasts.
Clinical Features
Patients with AML typically present with features secondary to proliferation of blasts (ie, findings of bone marrow failure and end organ damage).4,5 Fatigue, pallor, dizziness, dyspnea, and headaches occur secondary to anemia. Easy and prolonged bruising, petechiae, epistaxis, gingival bleeding, and conjunctival hemorrhages result from thrombocytopenia. Bleeding from other sites such as the central nervous system and gastrointestinal tract occurs but is uncommon. Patients may also present with infections resulting from unrecognized neutropenia. Constitutional symptoms including anorexia, fevers, and weight loss are frequently reported, while organomegaly (hepatomegaly and/or splenomegaly) is seen in about a quarter of patients.4 Infiltration of blasts into almost every organ has been noted, a condition known as myeloid (or granulocytic) sarcoma.15 This condition is more commonly found in patients with blastic, monoblastic, or myelomonocytic variants of AML, and is known as isolated myeloid sarcoma if no concurrent marrow or blood involvement is identified. In the absence of induction chemotherapy, systemic involvement occurs in a matter of weeks to months following such presentation.16
Laboratory analysis will usually demonstrate derangements in peripheral blood cell lines. At least half of patients have a total WBC count less than 5000/µL, a platelet count less than 50 × 103/µL, or both at the time of diagnosis.4,17 Approximately 10% of patients present with hyperleukocytosis and a WBC count greater than 100,000/µL, which can be associated with leukostasis.5 Additionally, spontaneous electrolyte derangement consistent with tumor lysis syndrome and coagulation abnormalities found in disseminated intravascular coagulation may be noted, even before initiation of therapy.
Work-Up of Suspected AML
Bone marrow biopsy and aspirate, along with touch preparations of the core biopsy sample, are crucial in the workup of suspected AML. At least 200 WBCs on blood smears and 500 nucleated cells on spiculated marrow smears should be counted.3 Reactivity with specific histochemical stains (myeloperoxidase, Sudan black B, or naphthyl AS-D-chloroacetate), presence of Auer rods, and reactivity to monoclonal antibodies against epitopes present on myeloblasts (eg, CD13, CD33, CD117) help distinguish myeloblasts from lymphoblasts.4 Flow cytometric analysis helps in confirming myeloid lineage; blasts generally express CD34 and HLA-DR, markers of immature hematopoietic precursors, and dim CD45 (common leukocyte antigen). One or more lymphoid antigens may be aberrantly expressed as well. Of note, in about 2% to 3% of acute leukemia cases, immunohistochemistry and/or flow cytometry findings demonstrate immature cells with features of both myeloid and lymphoid lineages (biphenotypic) or different populations of myeloid and lymphoid leukemia cells (bilineal). These leukemias are termed mixed-phenotype acute leukemia and are typically treated with either AML or acute lymphoblastic leukemia regimens.18
Cytogenetics, as assessed through conventional karyotype and fluorescence in situ hybridization (FISH), constitutes an essential part of the work-up. Eight balanced translocations and inversions and their variants are included in the World Health Organization (WHO) category “AML with recurrent genetic abnormalities,” while 9 balanced rearrangements and multiple unbalanced abnormalities in the presence of a blast count ≥ 20% are sufficient to establish the diagnosis of “AML with myelodysplasia-related changes.”3,19 Various other gene rearrangements thought to represent disease-initiating events are recognized as well, but these rearrangements do not yet formally define WHO disease categories.3 FISH can help detect RUNX1-RUNX1T1, CBFB-MYH11, KMT2A (MLL), and MECOM (EVI1) gene fusions, as well as chromosomal changes like 5q, 7q, or 17p, especially when fewer than 20 metaphases are assessable (due to failure of culture) by conventional cytogenetic methods.3
As certain molecular markers help with disease prognosis and the selection of personalized therapies, testing for these markers is recommended as part of a complete work-up of AML. The current standard of care is to test for nucleophosmin (NPM1), fms-like tyrosine kinase 3 (FLT3), and CEBPA mutations in all newly diagnosed patients.1RUNX1 mutation analysis should also be considered as its presence defines a provisional WHO subcategory.19 In the case of FLT3, the analysis should include both internal tandem duplications (FLT3-ITD, associated with worse prognosis especially at high allelic ratio) and tyrosine-kinase domain mutations (FLT3-TKD; D835 and I836), especially now that FLT3 inhibitors are regularly used.20 Most academic centers now routinely use next-generation sequencing–based panels to assess multiple mutations.
Diagnosis and Classification
A marrow or blood blast (myeloblasts, monoblasts, megakaryoblasts, or promonocytes [considered blast equivalents]) count of ≥ 20% is required for AML diagnosis.3,19 The presence of t(15;17), t(8;21), inv(16), or t(16;16), however, is considered diagnostic of AML irrespective of blast count.3,19 The previously used French-American-British (FAB) classification scheme has been replaced by the WHO classification (Table 2), which takes into account the morphologic, cytogenetic, genetic, and clinical features of the leukemia.
The category “AML with myelodysplasia-related changes” includes AML that has evolved out of an antecedent myelodysplastic syndrome, has ≥ 50% dysplasia in 2 or more lineages, or has myelodysplasia-related cytogenetic changes (eg, –5/del(5q), –7/del(7q), ≥ 3 cytogenetic abnormalities).19 “Therapy-related myeloid neoplasm,” or therapy-related AML, is diagnosed when the patient has previously received cytotoxic agents or ionizing radiation.19
Cases which do not meet the criteria for 1 of the previously mentioned categories are currently classified as “AML, not otherwise specified.” Further subclassification is pursued as per the older FAB scheme; however, no additional prognostic information is obtained in doing so.3,19 Myeloid sarcoma is strictly not a subcategory of AML. Rather, it is an extramedullary mass of myeloid blasts that effaces the normal tissue architecture.16 Rarely, myeloid sarcoma can be present without systemic disease involvement; it is important to note that management of such cases is identical to management of overt AML.16
Finally, myeloid proliferations related to Down syndrome include 2 entities seen in children with Down syndrome.19 Transient abnormal myelopoiesis, seen in 10% to 30% of newborns with Down syndrome, presents with circulating blasts that resolve in a couple of months. Myeloid leukemia associated with Down syndrome is AML that occurs usually in the first 3 years of life and persists if not treated.19
Case 1 Continued
The presence of 15% blasts in the peripheral blood is concerning for, but not diagnostic of, AML. On the other hand, the presence of Auer rods is virtually pathognomonic for AML. Gingival hyperplasia in this patient may be reflective of extramedullary disease. Cytogenetics from the peripheral blood and marrow aspirate show inv(16) in 20 of 20 cells. Molecular panel is notable for mutation in c-KIT. As such, the patient is diagnosed with core-binding factor AML, which per the ELN classification is considered a favorable-risk AML. The presence of c-KIT mutation, however, confers a relatively worse outcome.
Case 2 Continued
Presence of pancytopenia in a patient who previously received cytotoxic chemotherapy is highly concerning for therapy-related myeloid neoplasm. The presence of 12% blasts in the peripheral blood does not meet the criteria for diagnosis of AML. However, marrow specimens show 40% blasts, thus meeting the criteria for an AML diagnosis. Additionally, cytogenetics are notable for the presence of monosomy 7, while a next-generation sequencing panel shows a mutation in TP53. Put together, this patient meets the criteria for therapy-related AML which is an adverse-risk AML according to the ELN classification.
Management
The 2 most significant factors that must be considered when selecting AML therapies are the patient’s suitability for intensive chemotherapy and the biological characteristics of the AML. The former is a nuanced decision that incorporates age, performance status, and existing comorbidities. Treatment-related mortality calculators can guide physicians when making therapy decisions, especially in older patients (≥ 65 years). Retrospective evidence from various studies suggests that older, medically fit patients may derive clinically comparable benefits from intensive and less intensive induction therapies.25–27 The biological characteristics of the leukemia can be suggested by morphologic findings, cytogenetics, and molecular information, in addition to a history of antecedent myeloid neoplasms. Recently, an AML composite model incorporating an augmented Hematopoietic Cell Transplantation–specific Comorbidity Index (HCT-CI) score, age, and cytogenetic/molecular risks was shown to improve treatment decision-making about AML; this model potentially could be used to guide patient stratification in clinical trials as well.28 The overall treatment model of AML is largely unchanged otherwise. It is generally divided into induction, consolidation, and maintenance therapies.
Induction Therapy
In patients who can tolerate intensive therapies, the role of anthracycline- and cytarabine-based treatment is well established. However, the choice of specific anthracycline is not well established. One study concluded that idarubicin and mitoxantrone led to better outcomes as compared to daunorubicin, while another showed no difference between these agents.29,30 A pooled study of AML trials conducted in patients aged 50 years and older showed that while idarubicin led to a higher complete remission rate (69% versus 61%), the overall survival (OS) did not differ significantly.31 As for dosing, daunorubicin given at 45 mg/m2 daily for 3 days has been shown to have lower complete remission rates and higher relapse rates than a dose of 90 mg/m2 daily for 3 days in younger patients.32–34 However, it is not clear whether the 90 mg/m2 dose is superior to the frequently used dose of 60 mg/m2.35 A French study has shown comparable rates of complete remission, relapse, and OS between the 60 mg/m2 and 90 mg/m2 doses in patients with intermediate or unfavorable cytogenetics.36
If idarubicin is used, a dose of 12 mg/m2 for 3 days is considered the standard. In patients aged 50 to 70 years, there were no statistically significant differences in rates of relapse or OS between daunorubicin 80 mg/m2 for 3 days versus idarubicin 12 mg/m2 for 3 days versus idarubicin 12 mg/m2 for 4 days.37 As for cytarabine, the bulk of the evidence indicates that a dose of 1000 mg/m2 or higher should not be used.38 As such, the typical induction chemotherapy regimen of choice is 3 days of anthracycline (daunorubicin or idarubicin) and 7 days of cytarabine (100–200 mg/m2 continuous infusion), also known as the 7+3 regimen, which was first pioneered in the 1970s. In a recent phase 3 trial, 309 patients aged 60 to 75 years with high-risk AML (AML with myelodysplasia-related changes or t-AML) were randomly assigned to either the 7+3 regimen or CPX-351 (ie, nano-liposomal encapsulation of cytarabine and daunorubicin in a 5:1 molar ratio).39 A higher composite complete response rate (47.7% versus 33.3%; P = 0.016) and improved survival (9.56 months versus 5.95 months; hazard ratio [HR] 0.69, P = 0.005) were seen with CPX-351, leading to its approval by the FDA in patients with high-risk AML.
The 7+3 regimen has served as a backbone onto which other drugs have been added in clinical trials—the majority without any clinical benefits—for patients who can tolerate intensive therapy. In this context, the role of 2 therapies recently approved by the FDA must be discussed. In the RATIFY trial, 717 patients aged 18 to 59 years with AML and a FLT3 mutation were randomly assigned to receive standard chemotherapy (induction and consolidation therapy) plus either midostaurin or placebo; those who were in remission after consolidation therapy received either midostaurin or placebo in the maintenance phase.40 The primary endpoint was met as midostaurin improved OS (HR 0.78, P = 0.009). The benefit of midostaurin was consistent across all FLT3 subtypes and mutant allele burdens, regardless of whether patients proceeded to allogeneic stem cell transplant (allo-SCT). Based on the results of RATIFY, midostaurin was approved by the FDA for treatment of AML patients who are positive for the FLT3 mutation. Whether more potent and selective FLT3 inhibitors like gilteritinib, quizartinib, or crenolanib improve the outcomes is currently under investigation in various clinical trials.20
The development of gemtuzumab ozogamicin (GO) has been more complicated. GO, an antibody-drug conjugate comprised of a CD33-directed humanized monoclonal antibody linked covalently to the cytotoxic agent calicheamicin, binds CD33 present on the surface of myeloid leukemic blasts and immature normal cells of myelomonocytic lineage.41 The drug first received an accelerated approval in 2000 as monotherapy (2 doses of 9 mg/m2 14 days apart) for the treatment of patients 60 years of age and older with CD33-positive AML in first relapse based on the results of 3 open-label multicenter trials.41,42 However, a confirmatory S0106 trial in which GO 6 mg/m2 was added on day 4 in newly diagnosed AML patients was terminated early when an interim analysis showed an increased rate of death in induction (6% versus 1%) and lack of improvement in complete response, disease-free survival, or OS with the addition of GO.43 This study led to the withdrawal of GO from the US market in 2010. However, 2 randomized trials that studied GO using a different dose and schedule suggested that the addition of GO to intensive chemotherapy improved survival outcomes in patients with favorable and intermediate-risk cytogenetics.44,45 The results of the multicenter, open-label phase 3 ALFA-0701 trial, which randomly assigned 271 patients aged 50 to 70 years with newly diagnosed AML to daunorubicin and cytarabine alone or in combination with GO (3 mg/m2 on days 1, 4, and 7 during induction and day 1 of 2 consolidation courses), showed a statistically significant improvement in event-free survival (17.3 months versus 9.5 months; HR 0.56 [95% confidence interval 0.42 to 0.76]).45 Again, the survival benefits were more pronounced in patients with favorable or intermediate-risk cytogenetics than in those with unfavorable cytogenetics. The results of this trial led to the re-approval of GO in newly diagnosed AML patients.
For patients who cannot tolerate intensive therapies, the 2 main therapeutic options are low-dose cytarabine (LDAC) and the hypomethylating agents (HMA) azacitidine and decitabine. A phase 3 trial of decitabine versus mostly LDAC (or best supportive care, BSC) demonstrated favorable survival with decitabine (7.7 months versus 5.0 months).46 In the AZA-AML-001 trial, azacitidine improved median survival (10.4 months versus 6.5 months) in comparison to the control arm (LDAC, 7+3, BSC).47 Emerging data has also suggested that HMAs may be particularly active in patients with unfavorable-risk AML, a group for which LDAC has been shown to be especially useless.48 As such, HMA therapies are generally preferred over LDAC in practice. Finally, it is pertinent to note that GO can also be used as monotherapy based on the results of the open-label phase 3 AML-19 study in which GO demonstrated a survival advantage over BSC (4.9 months versus 3.6 months, P = 0.005).49
Postremission or Consolidation Therapy
There is no standard consolidation therapy for AML at present. In general, for patients who received HMA in the induction phase, the same HMA should be continued indefinitely until disease progression or allo-SCT.3 For those who received intensive chemotherapy in the induction phase, the consensus is to use cytarabine-based consolidation therapies. Cytarabine given as a single agent in high-doses has generally led to similar outcomes as multiagent chemotherapy.50 In this regard, cytarabine regimens, with or without anthracycline, at 3000 mg/m2 have similar efficacy as an intermediate dose of 1000 mg/m2.38 A total of 2 to 4 cycles of post-remission therapy is considered standard.3 Intensified post-remission chemotherapy has not been associated with consistent benefit in older AML patients or those with poor-risk disease. In recent years, measurable residual disease (MRD) assessment has emerged as a potentially useful tool in risk stratification and treatment planning, with various studies suggesting that MRD status in complete remission is one of the most important prognostic factors.51 Prospective studies confirming the significance of MRD as a marker for therapy selection are awaited. Finally, maintenance chemotherapy is not part of standard AML treatment.3
Role of Stem Cell Transplant
AML is the most common indication for allo-SCT. The availability of alternative donor strategies, which include mismatched, unrelated, haplo-identical, and cord blood donor sources, and the development of non-myeloablative and reduced-intensity conditioning (RIC) regimens (which take advantage of graft-versus-leukemia effect while decreasing cytotoxicity from myeloablative regimens) have expanded the possibility of allo-SCT to most patients under the age of 75 years.3 The decision to perform transplant is now largely based upon assessment of the risk (nonrelapse mortality) to benefit (reduction in risk of relapse) ratio, as determined by both disease-related features (cytogenetics, molecular profile) and clinical characteristics of the donor (type, availability, match) and the recipient (comorbidities, performance status).3 In a meta-analysis of 24 prospective trials involving more than 6000 AML patients in first complete remission, allo-SCT was associated with a significant survival benefit in patients with intermediate- and poor-risk AML but not in patients with good-risk AML.52 In line with this, good-risk AML patients are generally not recommended for transplant in first complete remission. For patients with normal karyotype who were said to have de novo AML (historically an intermediate-risk AML group), superior OS was demonstrated with transplant over intensive chemotherapy in those patients with either FLT3-ITD mutations or those with the molecular profile characterized by negativity for mutations in NPM1/CEBPA/FLT3.53 For patients with primary refractory disease and high-risk AML, transplant is probably the only curative option.
The choice of conditioning regimen is guided by several factors, including the subtype of AML, disease status, donor-recipient genetic disparity, graft source, comorbidities in the recipient (ie, tolerability for intensive conditioning regimen), as well as the reliance on graft-versus-leukemia effect as compared to cytotoxic effect of the regimen. The BMT CTN 0901 trial, which randomly assigned 218 patients aged 18 to 65 years to RIC (typically fludarabine/busulfan) or myeloablative regimens, showed an advantage for myeloablative regimens.54 The trial demonstrated a lower risk of relapse (13.5% versus 48.3%, P < 0.01) and higher rates of relapse-free survival (67.7% versus 47.3%, P < 0.01) and OS (67.7% versus. 77.4%, P = 0.07) at 18 months despite higher treatment-related mortality (15.8% versus 4.4%, P = 0.02) and a higher rate of grade 2 to 4 acute graft-versus-host disease (44.7% versus 31.6%, P = 0.024). At present, a RIC regimen is generally recommended for older patients or those with a higher comorbidity burden, while the myeloablative regimen is recommended for younger, fit patients.
Relapsed/Refractory Disease
The treatment of relapsed and refractory AML constitutes a major challenge, with OS estimated around 10% at 3 years.55 Currently, there is no standard salvage therapy in this setting, thus underscoring the need for clinical trials. For younger, fitter patients, the typical approach is to use intensive chemotherapy to achieve a second complete remission followed by a stem cell transplant. In younger patients, a second complete remission is achievable in about 55% of patients, although this rate is lower (~20%–30%) in more unselected patients.56,57 About two thirds of those who achieve complete remission may be able to proceed to transplant.57 For older patients where transplant is not possible, the goal is to use less intensive therapies that help with palliation. HMAs (azacitidine, decitabine) are used and have complete remission rates of 16% to 21% and median survival of 6 to 9 months in older patients.3 LDAC is another option in this setting. The recent approval of GO in this setting has further expanded the options. This approval was based on the outcomes of the phase 2 single-arm MyloFrance-1 study in which single-agent GO administered at 3 mg/m2 on days 1, 4, and 7 led to complete remission in 15 of 57 patients.58
With greater elucidation of the molecular characteristics of AML, the emergence of more effective targeted therapies is possible. Enasidenib, an inhibitor of mutant isocitrate dehydrogenase 2 (IDH2) protein that promotes differentiation of leukemic myeloblasts, recently received regulatory approval based on a single-arm trial. The overall response rate in this study was 38.5%, including a composite complete remission rate of 26.6% at a dose of 100 mg daily.59 IDH differentiation syndrome, akin to the differentiation syndrome seen in acute promyelocytic leukemia, occurred in approximately 12% of the patients, with the most frequent manifestations being dyspnea, fever, pulmonary infiltrates, and hypoxia.60
Survival of patients who relapse following transplant is particularly poor. A recent Center for International Blood and Marrow Transplant Research study found a 3-year OS ranging from a dismal 4% for those who present with early relapses (within 1 to 6 months) post-transplant to a more modest 38% for those who relapsed ≥ 3 years after their first transplant.61 The German Cooperative Transplant Study Group have suggested that azacitidine or chemotherapy followed by donor-lymphocyte infusions might improve responses over chemotherapy alone.62 Ipilimumab-based CTLA-4 blockade was reported to produce responses in a small cohort of patients, which was particularly notable in patients presenting with extramedullary manifestations of relapse.63 In patients who are otherwise fit but have a florid relapse, a second transplant can sometimes be sought, but the value of a different donor for second transplant is unclear.3
Case 1 Conclusion
Given his relatively young age, suitability for intensive therapy, and the presence of a core- binding factor abnormality, the patient is treated with an induction regimen containing daunorubicin, cytarabine, and GO (7+3 + GO). He achieves complete remission. This is followed by consolidation chemotherapy with high-dose cytarabine and GO. Allo-SCT is reserved for later should the AML relapse. Note that dasatinib, a c-KIT inhibitor, can be added to the treatment regimens as per the results of the CALGB 10801 protocol.64 Also, autologous SCT, instead of allo-SCT, can be considered in rare situations with relapsed core-binding factor AML (especially with inv(16) AML, younger patients, longer time in complete remission prior to relapse, and use of GO).
Case 2 Conclusion
The patient is deemed suitable for intensive chemotherapy. As such, CPX-351 is given in induction and consolidation and complete remission is achieved. Because he has adverse-risk AML, an allo-SCT is planned, but the patient relapses before it can be performed. Following 3 courses of decitabine therapy, the patient achieves complete remission once again but declines transplant. He maintains remission for an additional 4 months but then the leukemia progresses. Clinical trials are recommended to the patient, but he decides to pursue hospice care.
Conclusion
AML is the most common acute leukemia in adults. As defined currently, AML represents a group of related but distinct myeloid disorders that are characterized by various chromosomal, genetic, and epigenetic alterations. Early diagnosis and treatment can help prevent the emergence or manage the detrimental effects of its various complications such as leukostasis and tumor lysis syndrome. Improvements in supportive care, incremental treatment advances, and the wide adoption of allo-SCT for less than favorable cases have significantly improved survival of AML patients since the initial design of combinatorial (7+3) induction chemotherapy, particularly in patients presenting at a younger age. HMAs and the emergence of targeted therapies like FLT-3 and IDH2 inhibitors have added to our therapeutic armamentarium. Despite these advances, long-term survival rates in AML patients continue to be only approximately 40% to 50%. Older patients (particularly those over age 65 at the time of diagnosis), those with relapsed disease, and those with AML with certain unfavorable genetic abnormalities continue to have dismal outcomes. The design of newer targeted therapies, epigenetic agents, and immunotherapies will hopefully address this unmet need.
1. Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015;373:1136–52.
2. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts. Leukemia: Acute Myeloid Leukemia (AML). 2018;2018.
3. Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–47.
4. Liesveld JL, Lichtman MA. Acute myelogenous leukemia. In: Kaushansky K, Lichtman MA, Prchal JT, et al, eds. New York: Williams Hematology. 9th ed. New York: McGraw-Hill Education; 2015.
5. Randhawa JK, Khoury J, Ravandi-Kashani F. Adult acute myeloid leukemia. In: Kantarjian HM, Wolff RA, eds. The MD Anderson Manual of Medical Oncology. 3rd ed. New York: McGraw-Hill Medical; 2016.
6. Armstrong SA, Staunton JE, Silverman LB, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet 2002;30:41–7.
7. Graubert TA, Mardis ER. Genomics of acute myeloid leukemia. Cancer J 2011;17:487–91.
8. Cancer Genome Atlas Research Network, Ley TJ, Miller C, Ding L, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013;368:2059–74.
9. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 2016;374:2209–21.
10. Lindsley RC, Mar BG, Mazzola E, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015;125:1367–76.
11. Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014;371:2488–98.
12. Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015;126:9–16.
13. Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017;377:111–21.
14. Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 2014;371:2477–87.
15. Pileri S, Ascani S, Cox M, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 2007;21:340–50.
16. Vachhani P, Bose P. Isolated gastric myeloid sarcoma: a case report and review of the literature. Case Rep Hematol 2014;2014:541807.
17. Rowe JM. Clinical and laboratory features of the myeloid and lymphocytic leukemias. Am J Med Technol 1983;49:103–9.
18. Wolach O, Stone RM. Mixed-phenotype acute leukemia: current challenges in diagnosis and therapy. Curr Opin Hematol 2017;24:139–45.
19. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391–405.
20. Assi R, Ravandi F. FLT3 inhibitors in acute myeloid leukemia: Choosing the best when the optimal does not exist. Am J Hematol 2018;93:553–63.
21. Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 2012;366:1079–89.
22. Ley TJ, Ding L, Walter MJ, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med 2010;363:2424–33.
23. Dores GM, Devesa SS, Curtis RE, et al. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood 2012;119:34–43.
24. Cairoli R, Beghini A, Grillo G, et al. Prognostic impact of c-KIT mutations in core binding factor leukemias: an Italian retrospective study. Blood 2006;107:3463–8.
25. Sorror ML, Storer BE, Elsawy M, et al. Intensive versus non-intensive induction therapy for patients (Pts) with newly diagnosed acute myeloid leukemia (AML) using two different novel prognostic models [abstract]. Blood 2016;128(22):216.
26. Quintás-Cardama A, Ravandi F, Liu-Dumlao T, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 2012;120;4840-5.
27. Gupta N, Miller A, Gandhi Set al. Comparison of epigenetic versus standard induction chemotherapy for newly diagnosed acute myeloid leukemia patients ≥60 years old.Am J Hematol 2015;90:639-46.
28. Sorror ML, Storer BE, Fathi AT, et al. Development and validation of a novel acute myeloid leukemia-composite model to estimate risks of mortality. JAMA Oncol 2017;3:1675–82.
29. Rowe JM, Neuberg D, Friedenberg W, et al. A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: a trial by the Eastern Cooperative Oncology Group. Blood 2004;103:479–85.
30. Mandelli F, Vignetti M, Suciu S, et al. Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: the EORTC and GIMEMA Groups Study AML-10. J Clin Oncol 2009;27:5397–403.
31. Gardin C, Chevret S, Pautas C, et al. Superior long-term outcome with idarubicin compared with high-dose daunorubicin in patients with acute myeloid leukemia age 50 years and older. J Clin Oncol 2013;31:321–7.
32. Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med 2009;361:1249–59.
33. Lee JH, Joo YD, Kim H, et al. A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia. Blood 2011;118:3832–41.
34. Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med 2009;361:1235–48.
35. Burnett AK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood 2015;125:3878–85.
36. Devillier R, Bertoli S, Prebet T, et al. Comparison of 60 or 90 mg/m(2) of daunorubicin in induction therapy for acute myeloid leukemia with intermediate or unfavorable cytogenetics. Am J Hematol 2015;90:E29–30.
37. Pautas C, Merabet F, Thomas X, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol 2010;28:808–14.
38. Lowenberg B. Sense and nonsense of high-dose cytarabine for acute myeloid leukemia. Blood 2013;121:26–8.
39. Lancet JE, Uy GL, Cortes JE, et al. Final results of a phase III randomized trial of CPX-351 versus 7 + 3 in older patients with newly diagnosed high risk (secondary) AML [abstract]. J Clin Oncol 2016;34(15_suppl):7000-7000.
40. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 2017;377:454–64.
41. Jen EY, Ko CW, Lee JE, et al. FDA approval: Gemtuzumab ozogamicin for the treatment of adults with newly-diagnosed CD33-positive acute myeloid leukemia. Clin Cancer Res 2018; doi: 10.1158/1078-0432. CCR-17-3179.
42. Sievers EL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol 2001;19:3244–54.
43. Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013;121:4854–60.
44. Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol 2012;30:3924–31.
45. Castaigne S, Pautas C, Terre C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 2012;379:1508–16.
46. Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012;30:2670–7.
47. Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015;126:291–9.
48. Welch JS, Petti AA, Miller CA, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med 2016;375:2023–36.
49. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol 2016;34:972–9.
50. Miyawaki S, Ohtake S, Fujisawa S, et al. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study. Blood 2011;117:2366–72.
51. Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood 2018;131:1275–91.
52. Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 2009;301:2349–61.
53. Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med 2008;358:1909–18.
54. Pasquini MC, Logan B, Wu J, et al. Results of a phase III randomized, multi-center study of allogeneic stem cell transplantation after high versus reduced intensity conditioning in patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML): Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0901. Blood 2015;126:LBA–8.
55. Bose P, Vachhani P, Cortes JE. Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat Options Oncol 2017;18:17,017-0456-2.
56. Burnett AK, Goldstone A, Hills RK, et al. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol 2013;31:1293–301.
57. Ravandi F, Ritchie EK, Sayar H, et al. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol 2015;16:1025–36.
58. Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia 2007;21:66–71.
59. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017;130:722–31.
60. Fathi AT, DiNardo CD, Kline I, et al. Differentiation syndrome associated with enasidenib, a selective inhibitor of mutant isocitrate dehydrogenase 2: analysis of a phase 1/2 study. JAMA Oncol 2018;doi: 10.1001/jamaoncol.2017.4695.
61. Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant 2015;21:454–9.
62. Schroeder T, Rachlis E, Bug G, et al. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions--a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant 2015;21:653–60.
63. Davids MS, Kim HT, Bachireddy P, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med 2016;375:143–53.
64. Marcucci G, Geyer S, Zhao W, et al. Adding KIT inhibitor dasatinib (DAS) to chemotherapy overcomes the negative impact of KIT mutation/over-expression in core binding factor (CBF) acute myeloid leukemia (AML): results from CALGB 10801 (Alliance) [abstract]. Blood 2014;124:8.
Introduction
Acute myeloid leukemia (AML) comprises a heterogeneous group of disorders characterized by proliferation of clonal, abnormally differentiated hematopoietic progenitor cells of myeloid lineage that infiltrate the bone marrow, blood, and other tissues.1 In most cases, AML is rapidly fatal if left untreated. Over the past 2 decades, our understanding of the underlying disease biology responsible for the development of AML has improved substantially. We have learned that biological differences drive the various clinical, cytogenetic, and molecular subentities of AML; distinguishing among these subentities helps to identify optimal therapies, while offering improved clinical outcomes for select groups. After years of stagnation in therapeutic advances, 4 new drugs for treating AML were approved by the US Food and Drug Administration (FDA) in 2017. In this article, we review key features of AML diagnosis and management in the context of 2 case presentations.
Epidemiology and Risk Factors
An estimated 21,380 new cases of AML were diagnosed in the United States in 2017, constituting roughly 1.3% of all new cases of cancer.2 Approximately 10,590 patients died of AML in 2017. The median age of patients at the time of diagnosis is 68 years, and the incidence is approximately 4.2 per 100,000 persons per year. The 5-year survival for AML has steadily risen from a meager 6.3% in 1975 to 17.3% in 1995 and 28.1% in 2009.2 The cure rates for AML vary drastically with age. Long-term survival is achieved in approximately 35% to 40% of adults who present at age 60 years or younger, but only 5% to 15% of those older than 60 years at presentation will achieve long-term survival.3
Most cases of AML occur in the absence of any known risk factors. High-dose radiation exposure, chronic benzene exposure, chronic tobacco smoking, and certain chemotherapeutics are known to increase the risk for AML.4 Inconsistent correlations have also been made between exposure to organic solvents, petroleum products, radon, pesticides, and herbicides and the development of AML.4 Obesity may also increase AML risk.4
Two distinct subcategories of therapy-related AML (t-AML) are known. Patients who have been exposed to alkylating chemotherapeutics (eg, melphalan, cyclophosphamide, and nitrogen mustard) can develop t-AML with chromosomal 5 and/or 7 abnormalities after a latency period of approximately 4 to 8 years.5 In contrast, patients exposed to topoisomerase II inhibitors (notably etoposide) develop AML with abnormalities of 11q23 (leading to MLL gene rearrangement) or 21q22 (RUNX1) after a latency period of about 1 to 3 years.6 AML can also arise out of other myeloid disorders such as myelodysplastic syndrome and myeloproliferative neoplasms, and other bone marrow failure syndromes such as aplastic anemia.4 Various inherited or congenital conditions such as Down syndrome, Bloom syndrome, Fanconi anemia, neurofibromatosis 1, and dyskeratosis congenita can also predispose to the development of AML. A more detailed listing of conditions associated with AML can be found elsewhere.4
Molecular Landscape
The first cancer genome sequence was reported in an AML patient in 2008.7 Since then, various elegantly conducted studies have expanded our understanding of the molecular abnormalities in AML. The Cancer Genome Atlas Research Network analyzed the genomes of 200 cases of de novo AML in adults.8 Only 13 mutations were found on average, much fewer than the number of mutations in most adult cancers. Twenty-three genes were commonly mutated, and another 237 were mutated in 2 or more cases. Essentially, all cases had at least 1 nonsynonymous mutation in 1 of 9 categories of genes: transcription-factor fusions (18%), the gene encoding nucleophosmin (NPM1) (27%), tumor-suppressor genes (16%), DNA-methylation–related genes (44%), signaling genes (59%), chromatin-modifying genes (30%), myeloid transcription-factor genes (22%), spliceosome-complex genes (14%), and cohesin-complex genes (13%).
In another study, samples from 1540 patients from 3 prospective trials of intensive chemotherapy were analyzed to understand how genetic diversity defines the pathophysiology of AML.9 The study authors identified 5234 driver mutations from 76 genes or genomic regions, with 2 or more drivers identified in 86% of the samples. Eleven classes of mutational events, each with distinct diagnostic features and clinical outcomes, were identified. Acting as an internal positive control in this analysis, previously recognized mutational and cytogenetic groups emerged as distinct entities, including the groups with biallelic CEBPA mutations, mutations in NPM1, MLL fusions, and the cytogenetic entities t(6;9), inv(3), t(8;21), t(15;17), and inv(16). Three additional categories emerged as distinct entities: AML with mutations in genes encoding chromatin, RNA splicing regulators, or both (18% of patients); AML with TP53 mutations, chromosomal aneuploidies, or both (13%); and, provisionally, AML with IDH2R172 mutations (1%). An additional level of complexity was also revealed within the subgroup of patients with NPM1 mutations, where gene–gene interactions identified co-mutational events associated with both favorable or adverse prognosis.
Further supporting this molecular classification of AML, a study that performed targeted mutational analysis of 194 patients with defined secondary AML (s-AML) or t-AML and 105 unselected AML patients found that the presence of mutations in SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2 (all members of the chromatin or RNA splicing families) was highly specific for the diagnosis of s-AML.10 These findings are particularly clinically useful in those without a known history of antecedent hematologic disorder. These mutations defining the AML ontogeny were found to occur early in leukemogenesis, persist in clonal remissions, and predict worse clinical outcomes. Mutations in genes involved in regulation of DNA modification and of chromatin state (commonly DNMT3A, ASXL1, and TET2) have also been shown to be present in preleukemic stem or progenitor cells and to occur early in leukemogenesis.3 Unsurprisingly, some of these same mutations, including those in epigenetic regulators (DNMT3A, ASXL1, and TET2) and less frequently in splicing factor genes (SF3B1, SRSF2), have been associated with clonal hematopoietic expansion in elderly, seemingly healthy adults, a condition termed clonal hematopoiesis of indeterminate potential (CHIP).3,11,12 The presence of CHIP is associated with increased risk of hematologic neoplasms and all-cause mortality, the latter being possibly driven by a near doubling in the risk of coronary heart disease in humans and by accelerated atherosclerosis in a mouse model.11,13,14
Clinical Presentation and Work-up
Case Patient 1
A 57-year-old woman with a history of hypertension presents to the emergency department with complaints of productive cough and fevers for the previous 3 days. Examination reveals conjunctival pallor, gingival hyperplasia, and decreased breath sounds at the posterior right lung field. Investigations reveal a white blood cell (WBC) count of 51,000/µL with 15% blasts, a hemoglobin of 7.8 g/dL, and a platelet count of 56 × 103/µL. Peripheral blood smear is notable for large myeloblasts with occasional Auer rods. Chest radiograph shows a consolidation in the right lower lobe.
Case Patient 2
A 69-year-old man presents to his primary care physician for evaluation of worsening fatigue for the previous 4 months. Ten years prior to presentation, he had received 6 cycles of RCHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) as treatment for diffuse large B-cell lymphoma. Conjunctival pallor, patches of purpura over the extremities, and mucosal petechiae are noted on examination. Laboratory analyisis reveals a WBC count of 2400/µL with 12% blasts, hemoglobin of 9.0 g/dL, and platelet count of 10 × 103/µL. Peripheral smear shows dysplastic myeloid cells and blasts.
Clinical Features
Patients with AML typically present with features secondary to proliferation of blasts (ie, findings of bone marrow failure and end organ damage).4,5 Fatigue, pallor, dizziness, dyspnea, and headaches occur secondary to anemia. Easy and prolonged bruising, petechiae, epistaxis, gingival bleeding, and conjunctival hemorrhages result from thrombocytopenia. Bleeding from other sites such as the central nervous system and gastrointestinal tract occurs but is uncommon. Patients may also present with infections resulting from unrecognized neutropenia. Constitutional symptoms including anorexia, fevers, and weight loss are frequently reported, while organomegaly (hepatomegaly and/or splenomegaly) is seen in about a quarter of patients.4 Infiltration of blasts into almost every organ has been noted, a condition known as myeloid (or granulocytic) sarcoma.15 This condition is more commonly found in patients with blastic, monoblastic, or myelomonocytic variants of AML, and is known as isolated myeloid sarcoma if no concurrent marrow or blood involvement is identified. In the absence of induction chemotherapy, systemic involvement occurs in a matter of weeks to months following such presentation.16
Laboratory analysis will usually demonstrate derangements in peripheral blood cell lines. At least half of patients have a total WBC count less than 5000/µL, a platelet count less than 50 × 103/µL, or both at the time of diagnosis.4,17 Approximately 10% of patients present with hyperleukocytosis and a WBC count greater than 100,000/µL, which can be associated with leukostasis.5 Additionally, spontaneous electrolyte derangement consistent with tumor lysis syndrome and coagulation abnormalities found in disseminated intravascular coagulation may be noted, even before initiation of therapy.
Work-Up of Suspected AML
Bone marrow biopsy and aspirate, along with touch preparations of the core biopsy sample, are crucial in the workup of suspected AML. At least 200 WBCs on blood smears and 500 nucleated cells on spiculated marrow smears should be counted.3 Reactivity with specific histochemical stains (myeloperoxidase, Sudan black B, or naphthyl AS-D-chloroacetate), presence of Auer rods, and reactivity to monoclonal antibodies against epitopes present on myeloblasts (eg, CD13, CD33, CD117) help distinguish myeloblasts from lymphoblasts.4 Flow cytometric analysis helps in confirming myeloid lineage; blasts generally express CD34 and HLA-DR, markers of immature hematopoietic precursors, and dim CD45 (common leukocyte antigen). One or more lymphoid antigens may be aberrantly expressed as well. Of note, in about 2% to 3% of acute leukemia cases, immunohistochemistry and/or flow cytometry findings demonstrate immature cells with features of both myeloid and lymphoid lineages (biphenotypic) or different populations of myeloid and lymphoid leukemia cells (bilineal). These leukemias are termed mixed-phenotype acute leukemia and are typically treated with either AML or acute lymphoblastic leukemia regimens.18
Cytogenetics, as assessed through conventional karyotype and fluorescence in situ hybridization (FISH), constitutes an essential part of the work-up. Eight balanced translocations and inversions and their variants are included in the World Health Organization (WHO) category “AML with recurrent genetic abnormalities,” while 9 balanced rearrangements and multiple unbalanced abnormalities in the presence of a blast count ≥ 20% are sufficient to establish the diagnosis of “AML with myelodysplasia-related changes.”3,19 Various other gene rearrangements thought to represent disease-initiating events are recognized as well, but these rearrangements do not yet formally define WHO disease categories.3 FISH can help detect RUNX1-RUNX1T1, CBFB-MYH11, KMT2A (MLL), and MECOM (EVI1) gene fusions, as well as chromosomal changes like 5q, 7q, or 17p, especially when fewer than 20 metaphases are assessable (due to failure of culture) by conventional cytogenetic methods.3
As certain molecular markers help with disease prognosis and the selection of personalized therapies, testing for these markers is recommended as part of a complete work-up of AML. The current standard of care is to test for nucleophosmin (NPM1), fms-like tyrosine kinase 3 (FLT3), and CEBPA mutations in all newly diagnosed patients.1RUNX1 mutation analysis should also be considered as its presence defines a provisional WHO subcategory.19 In the case of FLT3, the analysis should include both internal tandem duplications (FLT3-ITD, associated with worse prognosis especially at high allelic ratio) and tyrosine-kinase domain mutations (FLT3-TKD; D835 and I836), especially now that FLT3 inhibitors are regularly used.20 Most academic centers now routinely use next-generation sequencing–based panels to assess multiple mutations.
Diagnosis and Classification
A marrow or blood blast (myeloblasts, monoblasts, megakaryoblasts, or promonocytes [considered blast equivalents]) count of ≥ 20% is required for AML diagnosis.3,19 The presence of t(15;17), t(8;21), inv(16), or t(16;16), however, is considered diagnostic of AML irrespective of blast count.3,19 The previously used French-American-British (FAB) classification scheme has been replaced by the WHO classification (Table 2), which takes into account the morphologic, cytogenetic, genetic, and clinical features of the leukemia.
The category “AML with myelodysplasia-related changes” includes AML that has evolved out of an antecedent myelodysplastic syndrome, has ≥ 50% dysplasia in 2 or more lineages, or has myelodysplasia-related cytogenetic changes (eg, –5/del(5q), –7/del(7q), ≥ 3 cytogenetic abnormalities).19 “Therapy-related myeloid neoplasm,” or therapy-related AML, is diagnosed when the patient has previously received cytotoxic agents or ionizing radiation.19
Cases which do not meet the criteria for 1 of the previously mentioned categories are currently classified as “AML, not otherwise specified.” Further subclassification is pursued as per the older FAB scheme; however, no additional prognostic information is obtained in doing so.3,19 Myeloid sarcoma is strictly not a subcategory of AML. Rather, it is an extramedullary mass of myeloid blasts that effaces the normal tissue architecture.16 Rarely, myeloid sarcoma can be present without systemic disease involvement; it is important to note that management of such cases is identical to management of overt AML.16
Finally, myeloid proliferations related to Down syndrome include 2 entities seen in children with Down syndrome.19 Transient abnormal myelopoiesis, seen in 10% to 30% of newborns with Down syndrome, presents with circulating blasts that resolve in a couple of months. Myeloid leukemia associated with Down syndrome is AML that occurs usually in the first 3 years of life and persists if not treated.19
Case 1 Continued
The presence of 15% blasts in the peripheral blood is concerning for, but not diagnostic of, AML. On the other hand, the presence of Auer rods is virtually pathognomonic for AML. Gingival hyperplasia in this patient may be reflective of extramedullary disease. Cytogenetics from the peripheral blood and marrow aspirate show inv(16) in 20 of 20 cells. Molecular panel is notable for mutation in c-KIT. As such, the patient is diagnosed with core-binding factor AML, which per the ELN classification is considered a favorable-risk AML. The presence of c-KIT mutation, however, confers a relatively worse outcome.
Case 2 Continued
Presence of pancytopenia in a patient who previously received cytotoxic chemotherapy is highly concerning for therapy-related myeloid neoplasm. The presence of 12% blasts in the peripheral blood does not meet the criteria for diagnosis of AML. However, marrow specimens show 40% blasts, thus meeting the criteria for an AML diagnosis. Additionally, cytogenetics are notable for the presence of monosomy 7, while a next-generation sequencing panel shows a mutation in TP53. Put together, this patient meets the criteria for therapy-related AML which is an adverse-risk AML according to the ELN classification.
Management
The 2 most significant factors that must be considered when selecting AML therapies are the patient’s suitability for intensive chemotherapy and the biological characteristics of the AML. The former is a nuanced decision that incorporates age, performance status, and existing comorbidities. Treatment-related mortality calculators can guide physicians when making therapy decisions, especially in older patients (≥ 65 years). Retrospective evidence from various studies suggests that older, medically fit patients may derive clinically comparable benefits from intensive and less intensive induction therapies.25–27 The biological characteristics of the leukemia can be suggested by morphologic findings, cytogenetics, and molecular information, in addition to a history of antecedent myeloid neoplasms. Recently, an AML composite model incorporating an augmented Hematopoietic Cell Transplantation–specific Comorbidity Index (HCT-CI) score, age, and cytogenetic/molecular risks was shown to improve treatment decision-making about AML; this model potentially could be used to guide patient stratification in clinical trials as well.28 The overall treatment model of AML is largely unchanged otherwise. It is generally divided into induction, consolidation, and maintenance therapies.
Induction Therapy
In patients who can tolerate intensive therapies, the role of anthracycline- and cytarabine-based treatment is well established. However, the choice of specific anthracycline is not well established. One study concluded that idarubicin and mitoxantrone led to better outcomes as compared to daunorubicin, while another showed no difference between these agents.29,30 A pooled study of AML trials conducted in patients aged 50 years and older showed that while idarubicin led to a higher complete remission rate (69% versus 61%), the overall survival (OS) did not differ significantly.31 As for dosing, daunorubicin given at 45 mg/m2 daily for 3 days has been shown to have lower complete remission rates and higher relapse rates than a dose of 90 mg/m2 daily for 3 days in younger patients.32–34 However, it is not clear whether the 90 mg/m2 dose is superior to the frequently used dose of 60 mg/m2.35 A French study has shown comparable rates of complete remission, relapse, and OS between the 60 mg/m2 and 90 mg/m2 doses in patients with intermediate or unfavorable cytogenetics.36
If idarubicin is used, a dose of 12 mg/m2 for 3 days is considered the standard. In patients aged 50 to 70 years, there were no statistically significant differences in rates of relapse or OS between daunorubicin 80 mg/m2 for 3 days versus idarubicin 12 mg/m2 for 3 days versus idarubicin 12 mg/m2 for 4 days.37 As for cytarabine, the bulk of the evidence indicates that a dose of 1000 mg/m2 or higher should not be used.38 As such, the typical induction chemotherapy regimen of choice is 3 days of anthracycline (daunorubicin or idarubicin) and 7 days of cytarabine (100–200 mg/m2 continuous infusion), also known as the 7+3 regimen, which was first pioneered in the 1970s. In a recent phase 3 trial, 309 patients aged 60 to 75 years with high-risk AML (AML with myelodysplasia-related changes or t-AML) were randomly assigned to either the 7+3 regimen or CPX-351 (ie, nano-liposomal encapsulation of cytarabine and daunorubicin in a 5:1 molar ratio).39 A higher composite complete response rate (47.7% versus 33.3%; P = 0.016) and improved survival (9.56 months versus 5.95 months; hazard ratio [HR] 0.69, P = 0.005) were seen with CPX-351, leading to its approval by the FDA in patients with high-risk AML.
The 7+3 regimen has served as a backbone onto which other drugs have been added in clinical trials—the majority without any clinical benefits—for patients who can tolerate intensive therapy. In this context, the role of 2 therapies recently approved by the FDA must be discussed. In the RATIFY trial, 717 patients aged 18 to 59 years with AML and a FLT3 mutation were randomly assigned to receive standard chemotherapy (induction and consolidation therapy) plus either midostaurin or placebo; those who were in remission after consolidation therapy received either midostaurin or placebo in the maintenance phase.40 The primary endpoint was met as midostaurin improved OS (HR 0.78, P = 0.009). The benefit of midostaurin was consistent across all FLT3 subtypes and mutant allele burdens, regardless of whether patients proceeded to allogeneic stem cell transplant (allo-SCT). Based on the results of RATIFY, midostaurin was approved by the FDA for treatment of AML patients who are positive for the FLT3 mutation. Whether more potent and selective FLT3 inhibitors like gilteritinib, quizartinib, or crenolanib improve the outcomes is currently under investigation in various clinical trials.20
The development of gemtuzumab ozogamicin (GO) has been more complicated. GO, an antibody-drug conjugate comprised of a CD33-directed humanized monoclonal antibody linked covalently to the cytotoxic agent calicheamicin, binds CD33 present on the surface of myeloid leukemic blasts and immature normal cells of myelomonocytic lineage.41 The drug first received an accelerated approval in 2000 as monotherapy (2 doses of 9 mg/m2 14 days apart) for the treatment of patients 60 years of age and older with CD33-positive AML in first relapse based on the results of 3 open-label multicenter trials.41,42 However, a confirmatory S0106 trial in which GO 6 mg/m2 was added on day 4 in newly diagnosed AML patients was terminated early when an interim analysis showed an increased rate of death in induction (6% versus 1%) and lack of improvement in complete response, disease-free survival, or OS with the addition of GO.43 This study led to the withdrawal of GO from the US market in 2010. However, 2 randomized trials that studied GO using a different dose and schedule suggested that the addition of GO to intensive chemotherapy improved survival outcomes in patients with favorable and intermediate-risk cytogenetics.44,45 The results of the multicenter, open-label phase 3 ALFA-0701 trial, which randomly assigned 271 patients aged 50 to 70 years with newly diagnosed AML to daunorubicin and cytarabine alone or in combination with GO (3 mg/m2 on days 1, 4, and 7 during induction and day 1 of 2 consolidation courses), showed a statistically significant improvement in event-free survival (17.3 months versus 9.5 months; HR 0.56 [95% confidence interval 0.42 to 0.76]).45 Again, the survival benefits were more pronounced in patients with favorable or intermediate-risk cytogenetics than in those with unfavorable cytogenetics. The results of this trial led to the re-approval of GO in newly diagnosed AML patients.
For patients who cannot tolerate intensive therapies, the 2 main therapeutic options are low-dose cytarabine (LDAC) and the hypomethylating agents (HMA) azacitidine and decitabine. A phase 3 trial of decitabine versus mostly LDAC (or best supportive care, BSC) demonstrated favorable survival with decitabine (7.7 months versus 5.0 months).46 In the AZA-AML-001 trial, azacitidine improved median survival (10.4 months versus 6.5 months) in comparison to the control arm (LDAC, 7+3, BSC).47 Emerging data has also suggested that HMAs may be particularly active in patients with unfavorable-risk AML, a group for which LDAC has been shown to be especially useless.48 As such, HMA therapies are generally preferred over LDAC in practice. Finally, it is pertinent to note that GO can also be used as monotherapy based on the results of the open-label phase 3 AML-19 study in which GO demonstrated a survival advantage over BSC (4.9 months versus 3.6 months, P = 0.005).49
Postremission or Consolidation Therapy
There is no standard consolidation therapy for AML at present. In general, for patients who received HMA in the induction phase, the same HMA should be continued indefinitely until disease progression or allo-SCT.3 For those who received intensive chemotherapy in the induction phase, the consensus is to use cytarabine-based consolidation therapies. Cytarabine given as a single agent in high-doses has generally led to similar outcomes as multiagent chemotherapy.50 In this regard, cytarabine regimens, with or without anthracycline, at 3000 mg/m2 have similar efficacy as an intermediate dose of 1000 mg/m2.38 A total of 2 to 4 cycles of post-remission therapy is considered standard.3 Intensified post-remission chemotherapy has not been associated with consistent benefit in older AML patients or those with poor-risk disease. In recent years, measurable residual disease (MRD) assessment has emerged as a potentially useful tool in risk stratification and treatment planning, with various studies suggesting that MRD status in complete remission is one of the most important prognostic factors.51 Prospective studies confirming the significance of MRD as a marker for therapy selection are awaited. Finally, maintenance chemotherapy is not part of standard AML treatment.3
Role of Stem Cell Transplant
AML is the most common indication for allo-SCT. The availability of alternative donor strategies, which include mismatched, unrelated, haplo-identical, and cord blood donor sources, and the development of non-myeloablative and reduced-intensity conditioning (RIC) regimens (which take advantage of graft-versus-leukemia effect while decreasing cytotoxicity from myeloablative regimens) have expanded the possibility of allo-SCT to most patients under the age of 75 years.3 The decision to perform transplant is now largely based upon assessment of the risk (nonrelapse mortality) to benefit (reduction in risk of relapse) ratio, as determined by both disease-related features (cytogenetics, molecular profile) and clinical characteristics of the donor (type, availability, match) and the recipient (comorbidities, performance status).3 In a meta-analysis of 24 prospective trials involving more than 6000 AML patients in first complete remission, allo-SCT was associated with a significant survival benefit in patients with intermediate- and poor-risk AML but not in patients with good-risk AML.52 In line with this, good-risk AML patients are generally not recommended for transplant in first complete remission. For patients with normal karyotype who were said to have de novo AML (historically an intermediate-risk AML group), superior OS was demonstrated with transplant over intensive chemotherapy in those patients with either FLT3-ITD mutations or those with the molecular profile characterized by negativity for mutations in NPM1/CEBPA/FLT3.53 For patients with primary refractory disease and high-risk AML, transplant is probably the only curative option.
The choice of conditioning regimen is guided by several factors, including the subtype of AML, disease status, donor-recipient genetic disparity, graft source, comorbidities in the recipient (ie, tolerability for intensive conditioning regimen), as well as the reliance on graft-versus-leukemia effect as compared to cytotoxic effect of the regimen. The BMT CTN 0901 trial, which randomly assigned 218 patients aged 18 to 65 years to RIC (typically fludarabine/busulfan) or myeloablative regimens, showed an advantage for myeloablative regimens.54 The trial demonstrated a lower risk of relapse (13.5% versus 48.3%, P < 0.01) and higher rates of relapse-free survival (67.7% versus 47.3%, P < 0.01) and OS (67.7% versus. 77.4%, P = 0.07) at 18 months despite higher treatment-related mortality (15.8% versus 4.4%, P = 0.02) and a higher rate of grade 2 to 4 acute graft-versus-host disease (44.7% versus 31.6%, P = 0.024). At present, a RIC regimen is generally recommended for older patients or those with a higher comorbidity burden, while the myeloablative regimen is recommended for younger, fit patients.
Relapsed/Refractory Disease
The treatment of relapsed and refractory AML constitutes a major challenge, with OS estimated around 10% at 3 years.55 Currently, there is no standard salvage therapy in this setting, thus underscoring the need for clinical trials. For younger, fitter patients, the typical approach is to use intensive chemotherapy to achieve a second complete remission followed by a stem cell transplant. In younger patients, a second complete remission is achievable in about 55% of patients, although this rate is lower (~20%–30%) in more unselected patients.56,57 About two thirds of those who achieve complete remission may be able to proceed to transplant.57 For older patients where transplant is not possible, the goal is to use less intensive therapies that help with palliation. HMAs (azacitidine, decitabine) are used and have complete remission rates of 16% to 21% and median survival of 6 to 9 months in older patients.3 LDAC is another option in this setting. The recent approval of GO in this setting has further expanded the options. This approval was based on the outcomes of the phase 2 single-arm MyloFrance-1 study in which single-agent GO administered at 3 mg/m2 on days 1, 4, and 7 led to complete remission in 15 of 57 patients.58
With greater elucidation of the molecular characteristics of AML, the emergence of more effective targeted therapies is possible. Enasidenib, an inhibitor of mutant isocitrate dehydrogenase 2 (IDH2) protein that promotes differentiation of leukemic myeloblasts, recently received regulatory approval based on a single-arm trial. The overall response rate in this study was 38.5%, including a composite complete remission rate of 26.6% at a dose of 100 mg daily.59 IDH differentiation syndrome, akin to the differentiation syndrome seen in acute promyelocytic leukemia, occurred in approximately 12% of the patients, with the most frequent manifestations being dyspnea, fever, pulmonary infiltrates, and hypoxia.60
Survival of patients who relapse following transplant is particularly poor. A recent Center for International Blood and Marrow Transplant Research study found a 3-year OS ranging from a dismal 4% for those who present with early relapses (within 1 to 6 months) post-transplant to a more modest 38% for those who relapsed ≥ 3 years after their first transplant.61 The German Cooperative Transplant Study Group have suggested that azacitidine or chemotherapy followed by donor-lymphocyte infusions might improve responses over chemotherapy alone.62 Ipilimumab-based CTLA-4 blockade was reported to produce responses in a small cohort of patients, which was particularly notable in patients presenting with extramedullary manifestations of relapse.63 In patients who are otherwise fit but have a florid relapse, a second transplant can sometimes be sought, but the value of a different donor for second transplant is unclear.3
Case 1 Conclusion
Given his relatively young age, suitability for intensive therapy, and the presence of a core- binding factor abnormality, the patient is treated with an induction regimen containing daunorubicin, cytarabine, and GO (7+3 + GO). He achieves complete remission. This is followed by consolidation chemotherapy with high-dose cytarabine and GO. Allo-SCT is reserved for later should the AML relapse. Note that dasatinib, a c-KIT inhibitor, can be added to the treatment regimens as per the results of the CALGB 10801 protocol.64 Also, autologous SCT, instead of allo-SCT, can be considered in rare situations with relapsed core-binding factor AML (especially with inv(16) AML, younger patients, longer time in complete remission prior to relapse, and use of GO).
Case 2 Conclusion
The patient is deemed suitable for intensive chemotherapy. As such, CPX-351 is given in induction and consolidation and complete remission is achieved. Because he has adverse-risk AML, an allo-SCT is planned, but the patient relapses before it can be performed. Following 3 courses of decitabine therapy, the patient achieves complete remission once again but declines transplant. He maintains remission for an additional 4 months but then the leukemia progresses. Clinical trials are recommended to the patient, but he decides to pursue hospice care.
Conclusion
AML is the most common acute leukemia in adults. As defined currently, AML represents a group of related but distinct myeloid disorders that are characterized by various chromosomal, genetic, and epigenetic alterations. Early diagnosis and treatment can help prevent the emergence or manage the detrimental effects of its various complications such as leukostasis and tumor lysis syndrome. Improvements in supportive care, incremental treatment advances, and the wide adoption of allo-SCT for less than favorable cases have significantly improved survival of AML patients since the initial design of combinatorial (7+3) induction chemotherapy, particularly in patients presenting at a younger age. HMAs and the emergence of targeted therapies like FLT-3 and IDH2 inhibitors have added to our therapeutic armamentarium. Despite these advances, long-term survival rates in AML patients continue to be only approximately 40% to 50%. Older patients (particularly those over age 65 at the time of diagnosis), those with relapsed disease, and those with AML with certain unfavorable genetic abnormalities continue to have dismal outcomes. The design of newer targeted therapies, epigenetic agents, and immunotherapies will hopefully address this unmet need.
Introduction
Acute myeloid leukemia (AML) comprises a heterogeneous group of disorders characterized by proliferation of clonal, abnormally differentiated hematopoietic progenitor cells of myeloid lineage that infiltrate the bone marrow, blood, and other tissues.1 In most cases, AML is rapidly fatal if left untreated. Over the past 2 decades, our understanding of the underlying disease biology responsible for the development of AML has improved substantially. We have learned that biological differences drive the various clinical, cytogenetic, and molecular subentities of AML; distinguishing among these subentities helps to identify optimal therapies, while offering improved clinical outcomes for select groups. After years of stagnation in therapeutic advances, 4 new drugs for treating AML were approved by the US Food and Drug Administration (FDA) in 2017. In this article, we review key features of AML diagnosis and management in the context of 2 case presentations.
Epidemiology and Risk Factors
An estimated 21,380 new cases of AML were diagnosed in the United States in 2017, constituting roughly 1.3% of all new cases of cancer.2 Approximately 10,590 patients died of AML in 2017. The median age of patients at the time of diagnosis is 68 years, and the incidence is approximately 4.2 per 100,000 persons per year. The 5-year survival for AML has steadily risen from a meager 6.3% in 1975 to 17.3% in 1995 and 28.1% in 2009.2 The cure rates for AML vary drastically with age. Long-term survival is achieved in approximately 35% to 40% of adults who present at age 60 years or younger, but only 5% to 15% of those older than 60 years at presentation will achieve long-term survival.3
Most cases of AML occur in the absence of any known risk factors. High-dose radiation exposure, chronic benzene exposure, chronic tobacco smoking, and certain chemotherapeutics are known to increase the risk for AML.4 Inconsistent correlations have also been made between exposure to organic solvents, petroleum products, radon, pesticides, and herbicides and the development of AML.4 Obesity may also increase AML risk.4
Two distinct subcategories of therapy-related AML (t-AML) are known. Patients who have been exposed to alkylating chemotherapeutics (eg, melphalan, cyclophosphamide, and nitrogen mustard) can develop t-AML with chromosomal 5 and/or 7 abnormalities after a latency period of approximately 4 to 8 years.5 In contrast, patients exposed to topoisomerase II inhibitors (notably etoposide) develop AML with abnormalities of 11q23 (leading to MLL gene rearrangement) or 21q22 (RUNX1) after a latency period of about 1 to 3 years.6 AML can also arise out of other myeloid disorders such as myelodysplastic syndrome and myeloproliferative neoplasms, and other bone marrow failure syndromes such as aplastic anemia.4 Various inherited or congenital conditions such as Down syndrome, Bloom syndrome, Fanconi anemia, neurofibromatosis 1, and dyskeratosis congenita can also predispose to the development of AML. A more detailed listing of conditions associated with AML can be found elsewhere.4
Molecular Landscape
The first cancer genome sequence was reported in an AML patient in 2008.7 Since then, various elegantly conducted studies have expanded our understanding of the molecular abnormalities in AML. The Cancer Genome Atlas Research Network analyzed the genomes of 200 cases of de novo AML in adults.8 Only 13 mutations were found on average, much fewer than the number of mutations in most adult cancers. Twenty-three genes were commonly mutated, and another 237 were mutated in 2 or more cases. Essentially, all cases had at least 1 nonsynonymous mutation in 1 of 9 categories of genes: transcription-factor fusions (18%), the gene encoding nucleophosmin (NPM1) (27%), tumor-suppressor genes (16%), DNA-methylation–related genes (44%), signaling genes (59%), chromatin-modifying genes (30%), myeloid transcription-factor genes (22%), spliceosome-complex genes (14%), and cohesin-complex genes (13%).
In another study, samples from 1540 patients from 3 prospective trials of intensive chemotherapy were analyzed to understand how genetic diversity defines the pathophysiology of AML.9 The study authors identified 5234 driver mutations from 76 genes or genomic regions, with 2 or more drivers identified in 86% of the samples. Eleven classes of mutational events, each with distinct diagnostic features and clinical outcomes, were identified. Acting as an internal positive control in this analysis, previously recognized mutational and cytogenetic groups emerged as distinct entities, including the groups with biallelic CEBPA mutations, mutations in NPM1, MLL fusions, and the cytogenetic entities t(6;9), inv(3), t(8;21), t(15;17), and inv(16). Three additional categories emerged as distinct entities: AML with mutations in genes encoding chromatin, RNA splicing regulators, or both (18% of patients); AML with TP53 mutations, chromosomal aneuploidies, or both (13%); and, provisionally, AML with IDH2R172 mutations (1%). An additional level of complexity was also revealed within the subgroup of patients with NPM1 mutations, where gene–gene interactions identified co-mutational events associated with both favorable or adverse prognosis.
Further supporting this molecular classification of AML, a study that performed targeted mutational analysis of 194 patients with defined secondary AML (s-AML) or t-AML and 105 unselected AML patients found that the presence of mutations in SRSF2, SF3B1, U2AF1, ZRSR2, ASXL1, EZH2, BCOR, or STAG2 (all members of the chromatin or RNA splicing families) was highly specific for the diagnosis of s-AML.10 These findings are particularly clinically useful in those without a known history of antecedent hematologic disorder. These mutations defining the AML ontogeny were found to occur early in leukemogenesis, persist in clonal remissions, and predict worse clinical outcomes. Mutations in genes involved in regulation of DNA modification and of chromatin state (commonly DNMT3A, ASXL1, and TET2) have also been shown to be present in preleukemic stem or progenitor cells and to occur early in leukemogenesis.3 Unsurprisingly, some of these same mutations, including those in epigenetic regulators (DNMT3A, ASXL1, and TET2) and less frequently in splicing factor genes (SF3B1, SRSF2), have been associated with clonal hematopoietic expansion in elderly, seemingly healthy adults, a condition termed clonal hematopoiesis of indeterminate potential (CHIP).3,11,12 The presence of CHIP is associated with increased risk of hematologic neoplasms and all-cause mortality, the latter being possibly driven by a near doubling in the risk of coronary heart disease in humans and by accelerated atherosclerosis in a mouse model.11,13,14
Clinical Presentation and Work-up
Case Patient 1
A 57-year-old woman with a history of hypertension presents to the emergency department with complaints of productive cough and fevers for the previous 3 days. Examination reveals conjunctival pallor, gingival hyperplasia, and decreased breath sounds at the posterior right lung field. Investigations reveal a white blood cell (WBC) count of 51,000/µL with 15% blasts, a hemoglobin of 7.8 g/dL, and a platelet count of 56 × 103/µL. Peripheral blood smear is notable for large myeloblasts with occasional Auer rods. Chest radiograph shows a consolidation in the right lower lobe.
Case Patient 2
A 69-year-old man presents to his primary care physician for evaluation of worsening fatigue for the previous 4 months. Ten years prior to presentation, he had received 6 cycles of RCHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) as treatment for diffuse large B-cell lymphoma. Conjunctival pallor, patches of purpura over the extremities, and mucosal petechiae are noted on examination. Laboratory analyisis reveals a WBC count of 2400/µL with 12% blasts, hemoglobin of 9.0 g/dL, and platelet count of 10 × 103/µL. Peripheral smear shows dysplastic myeloid cells and blasts.
Clinical Features
Patients with AML typically present with features secondary to proliferation of blasts (ie, findings of bone marrow failure and end organ damage).4,5 Fatigue, pallor, dizziness, dyspnea, and headaches occur secondary to anemia. Easy and prolonged bruising, petechiae, epistaxis, gingival bleeding, and conjunctival hemorrhages result from thrombocytopenia. Bleeding from other sites such as the central nervous system and gastrointestinal tract occurs but is uncommon. Patients may also present with infections resulting from unrecognized neutropenia. Constitutional symptoms including anorexia, fevers, and weight loss are frequently reported, while organomegaly (hepatomegaly and/or splenomegaly) is seen in about a quarter of patients.4 Infiltration of blasts into almost every organ has been noted, a condition known as myeloid (or granulocytic) sarcoma.15 This condition is more commonly found in patients with blastic, monoblastic, or myelomonocytic variants of AML, and is known as isolated myeloid sarcoma if no concurrent marrow or blood involvement is identified. In the absence of induction chemotherapy, systemic involvement occurs in a matter of weeks to months following such presentation.16
Laboratory analysis will usually demonstrate derangements in peripheral blood cell lines. At least half of patients have a total WBC count less than 5000/µL, a platelet count less than 50 × 103/µL, or both at the time of diagnosis.4,17 Approximately 10% of patients present with hyperleukocytosis and a WBC count greater than 100,000/µL, which can be associated with leukostasis.5 Additionally, spontaneous electrolyte derangement consistent with tumor lysis syndrome and coagulation abnormalities found in disseminated intravascular coagulation may be noted, even before initiation of therapy.
Work-Up of Suspected AML
Bone marrow biopsy and aspirate, along with touch preparations of the core biopsy sample, are crucial in the workup of suspected AML. At least 200 WBCs on blood smears and 500 nucleated cells on spiculated marrow smears should be counted.3 Reactivity with specific histochemical stains (myeloperoxidase, Sudan black B, or naphthyl AS-D-chloroacetate), presence of Auer rods, and reactivity to monoclonal antibodies against epitopes present on myeloblasts (eg, CD13, CD33, CD117) help distinguish myeloblasts from lymphoblasts.4 Flow cytometric analysis helps in confirming myeloid lineage; blasts generally express CD34 and HLA-DR, markers of immature hematopoietic precursors, and dim CD45 (common leukocyte antigen). One or more lymphoid antigens may be aberrantly expressed as well. Of note, in about 2% to 3% of acute leukemia cases, immunohistochemistry and/or flow cytometry findings demonstrate immature cells with features of both myeloid and lymphoid lineages (biphenotypic) or different populations of myeloid and lymphoid leukemia cells (bilineal). These leukemias are termed mixed-phenotype acute leukemia and are typically treated with either AML or acute lymphoblastic leukemia regimens.18
Cytogenetics, as assessed through conventional karyotype and fluorescence in situ hybridization (FISH), constitutes an essential part of the work-up. Eight balanced translocations and inversions and their variants are included in the World Health Organization (WHO) category “AML with recurrent genetic abnormalities,” while 9 balanced rearrangements and multiple unbalanced abnormalities in the presence of a blast count ≥ 20% are sufficient to establish the diagnosis of “AML with myelodysplasia-related changes.”3,19 Various other gene rearrangements thought to represent disease-initiating events are recognized as well, but these rearrangements do not yet formally define WHO disease categories.3 FISH can help detect RUNX1-RUNX1T1, CBFB-MYH11, KMT2A (MLL), and MECOM (EVI1) gene fusions, as well as chromosomal changes like 5q, 7q, or 17p, especially when fewer than 20 metaphases are assessable (due to failure of culture) by conventional cytogenetic methods.3
As certain molecular markers help with disease prognosis and the selection of personalized therapies, testing for these markers is recommended as part of a complete work-up of AML. The current standard of care is to test for nucleophosmin (NPM1), fms-like tyrosine kinase 3 (FLT3), and CEBPA mutations in all newly diagnosed patients.1RUNX1 mutation analysis should also be considered as its presence defines a provisional WHO subcategory.19 In the case of FLT3, the analysis should include both internal tandem duplications (FLT3-ITD, associated with worse prognosis especially at high allelic ratio) and tyrosine-kinase domain mutations (FLT3-TKD; D835 and I836), especially now that FLT3 inhibitors are regularly used.20 Most academic centers now routinely use next-generation sequencing–based panels to assess multiple mutations.
Diagnosis and Classification
A marrow or blood blast (myeloblasts, monoblasts, megakaryoblasts, or promonocytes [considered blast equivalents]) count of ≥ 20% is required for AML diagnosis.3,19 The presence of t(15;17), t(8;21), inv(16), or t(16;16), however, is considered diagnostic of AML irrespective of blast count.3,19 The previously used French-American-British (FAB) classification scheme has been replaced by the WHO classification (Table 2), which takes into account the morphologic, cytogenetic, genetic, and clinical features of the leukemia.
The category “AML with myelodysplasia-related changes” includes AML that has evolved out of an antecedent myelodysplastic syndrome, has ≥ 50% dysplasia in 2 or more lineages, or has myelodysplasia-related cytogenetic changes (eg, –5/del(5q), –7/del(7q), ≥ 3 cytogenetic abnormalities).19 “Therapy-related myeloid neoplasm,” or therapy-related AML, is diagnosed when the patient has previously received cytotoxic agents or ionizing radiation.19
Cases which do not meet the criteria for 1 of the previously mentioned categories are currently classified as “AML, not otherwise specified.” Further subclassification is pursued as per the older FAB scheme; however, no additional prognostic information is obtained in doing so.3,19 Myeloid sarcoma is strictly not a subcategory of AML. Rather, it is an extramedullary mass of myeloid blasts that effaces the normal tissue architecture.16 Rarely, myeloid sarcoma can be present without systemic disease involvement; it is important to note that management of such cases is identical to management of overt AML.16
Finally, myeloid proliferations related to Down syndrome include 2 entities seen in children with Down syndrome.19 Transient abnormal myelopoiesis, seen in 10% to 30% of newborns with Down syndrome, presents with circulating blasts that resolve in a couple of months. Myeloid leukemia associated with Down syndrome is AML that occurs usually in the first 3 years of life and persists if not treated.19
Case 1 Continued
The presence of 15% blasts in the peripheral blood is concerning for, but not diagnostic of, AML. On the other hand, the presence of Auer rods is virtually pathognomonic for AML. Gingival hyperplasia in this patient may be reflective of extramedullary disease. Cytogenetics from the peripheral blood and marrow aspirate show inv(16) in 20 of 20 cells. Molecular panel is notable for mutation in c-KIT. As such, the patient is diagnosed with core-binding factor AML, which per the ELN classification is considered a favorable-risk AML. The presence of c-KIT mutation, however, confers a relatively worse outcome.
Case 2 Continued
Presence of pancytopenia in a patient who previously received cytotoxic chemotherapy is highly concerning for therapy-related myeloid neoplasm. The presence of 12% blasts in the peripheral blood does not meet the criteria for diagnosis of AML. However, marrow specimens show 40% blasts, thus meeting the criteria for an AML diagnosis. Additionally, cytogenetics are notable for the presence of monosomy 7, while a next-generation sequencing panel shows a mutation in TP53. Put together, this patient meets the criteria for therapy-related AML which is an adverse-risk AML according to the ELN classification.
Management
The 2 most significant factors that must be considered when selecting AML therapies are the patient’s suitability for intensive chemotherapy and the biological characteristics of the AML. The former is a nuanced decision that incorporates age, performance status, and existing comorbidities. Treatment-related mortality calculators can guide physicians when making therapy decisions, especially in older patients (≥ 65 years). Retrospective evidence from various studies suggests that older, medically fit patients may derive clinically comparable benefits from intensive and less intensive induction therapies.25–27 The biological characteristics of the leukemia can be suggested by morphologic findings, cytogenetics, and molecular information, in addition to a history of antecedent myeloid neoplasms. Recently, an AML composite model incorporating an augmented Hematopoietic Cell Transplantation–specific Comorbidity Index (HCT-CI) score, age, and cytogenetic/molecular risks was shown to improve treatment decision-making about AML; this model potentially could be used to guide patient stratification in clinical trials as well.28 The overall treatment model of AML is largely unchanged otherwise. It is generally divided into induction, consolidation, and maintenance therapies.
Induction Therapy
In patients who can tolerate intensive therapies, the role of anthracycline- and cytarabine-based treatment is well established. However, the choice of specific anthracycline is not well established. One study concluded that idarubicin and mitoxantrone led to better outcomes as compared to daunorubicin, while another showed no difference between these agents.29,30 A pooled study of AML trials conducted in patients aged 50 years and older showed that while idarubicin led to a higher complete remission rate (69% versus 61%), the overall survival (OS) did not differ significantly.31 As for dosing, daunorubicin given at 45 mg/m2 daily for 3 days has been shown to have lower complete remission rates and higher relapse rates than a dose of 90 mg/m2 daily for 3 days in younger patients.32–34 However, it is not clear whether the 90 mg/m2 dose is superior to the frequently used dose of 60 mg/m2.35 A French study has shown comparable rates of complete remission, relapse, and OS between the 60 mg/m2 and 90 mg/m2 doses in patients with intermediate or unfavorable cytogenetics.36
If idarubicin is used, a dose of 12 mg/m2 for 3 days is considered the standard. In patients aged 50 to 70 years, there were no statistically significant differences in rates of relapse or OS between daunorubicin 80 mg/m2 for 3 days versus idarubicin 12 mg/m2 for 3 days versus idarubicin 12 mg/m2 for 4 days.37 As for cytarabine, the bulk of the evidence indicates that a dose of 1000 mg/m2 or higher should not be used.38 As such, the typical induction chemotherapy regimen of choice is 3 days of anthracycline (daunorubicin or idarubicin) and 7 days of cytarabine (100–200 mg/m2 continuous infusion), also known as the 7+3 regimen, which was first pioneered in the 1970s. In a recent phase 3 trial, 309 patients aged 60 to 75 years with high-risk AML (AML with myelodysplasia-related changes or t-AML) were randomly assigned to either the 7+3 regimen or CPX-351 (ie, nano-liposomal encapsulation of cytarabine and daunorubicin in a 5:1 molar ratio).39 A higher composite complete response rate (47.7% versus 33.3%; P = 0.016) and improved survival (9.56 months versus 5.95 months; hazard ratio [HR] 0.69, P = 0.005) were seen with CPX-351, leading to its approval by the FDA in patients with high-risk AML.
The 7+3 regimen has served as a backbone onto which other drugs have been added in clinical trials—the majority without any clinical benefits—for patients who can tolerate intensive therapy. In this context, the role of 2 therapies recently approved by the FDA must be discussed. In the RATIFY trial, 717 patients aged 18 to 59 years with AML and a FLT3 mutation were randomly assigned to receive standard chemotherapy (induction and consolidation therapy) plus either midostaurin or placebo; those who were in remission after consolidation therapy received either midostaurin or placebo in the maintenance phase.40 The primary endpoint was met as midostaurin improved OS (HR 0.78, P = 0.009). The benefit of midostaurin was consistent across all FLT3 subtypes and mutant allele burdens, regardless of whether patients proceeded to allogeneic stem cell transplant (allo-SCT). Based on the results of RATIFY, midostaurin was approved by the FDA for treatment of AML patients who are positive for the FLT3 mutation. Whether more potent and selective FLT3 inhibitors like gilteritinib, quizartinib, or crenolanib improve the outcomes is currently under investigation in various clinical trials.20
The development of gemtuzumab ozogamicin (GO) has been more complicated. GO, an antibody-drug conjugate comprised of a CD33-directed humanized monoclonal antibody linked covalently to the cytotoxic agent calicheamicin, binds CD33 present on the surface of myeloid leukemic blasts and immature normal cells of myelomonocytic lineage.41 The drug first received an accelerated approval in 2000 as monotherapy (2 doses of 9 mg/m2 14 days apart) for the treatment of patients 60 years of age and older with CD33-positive AML in first relapse based on the results of 3 open-label multicenter trials.41,42 However, a confirmatory S0106 trial in which GO 6 mg/m2 was added on day 4 in newly diagnosed AML patients was terminated early when an interim analysis showed an increased rate of death in induction (6% versus 1%) and lack of improvement in complete response, disease-free survival, or OS with the addition of GO.43 This study led to the withdrawal of GO from the US market in 2010. However, 2 randomized trials that studied GO using a different dose and schedule suggested that the addition of GO to intensive chemotherapy improved survival outcomes in patients with favorable and intermediate-risk cytogenetics.44,45 The results of the multicenter, open-label phase 3 ALFA-0701 trial, which randomly assigned 271 patients aged 50 to 70 years with newly diagnosed AML to daunorubicin and cytarabine alone or in combination with GO (3 mg/m2 on days 1, 4, and 7 during induction and day 1 of 2 consolidation courses), showed a statistically significant improvement in event-free survival (17.3 months versus 9.5 months; HR 0.56 [95% confidence interval 0.42 to 0.76]).45 Again, the survival benefits were more pronounced in patients with favorable or intermediate-risk cytogenetics than in those with unfavorable cytogenetics. The results of this trial led to the re-approval of GO in newly diagnosed AML patients.
For patients who cannot tolerate intensive therapies, the 2 main therapeutic options are low-dose cytarabine (LDAC) and the hypomethylating agents (HMA) azacitidine and decitabine. A phase 3 trial of decitabine versus mostly LDAC (or best supportive care, BSC) demonstrated favorable survival with decitabine (7.7 months versus 5.0 months).46 In the AZA-AML-001 trial, azacitidine improved median survival (10.4 months versus 6.5 months) in comparison to the control arm (LDAC, 7+3, BSC).47 Emerging data has also suggested that HMAs may be particularly active in patients with unfavorable-risk AML, a group for which LDAC has been shown to be especially useless.48 As such, HMA therapies are generally preferred over LDAC in practice. Finally, it is pertinent to note that GO can also be used as monotherapy based on the results of the open-label phase 3 AML-19 study in which GO demonstrated a survival advantage over BSC (4.9 months versus 3.6 months, P = 0.005).49
Postremission or Consolidation Therapy
There is no standard consolidation therapy for AML at present. In general, for patients who received HMA in the induction phase, the same HMA should be continued indefinitely until disease progression or allo-SCT.3 For those who received intensive chemotherapy in the induction phase, the consensus is to use cytarabine-based consolidation therapies. Cytarabine given as a single agent in high-doses has generally led to similar outcomes as multiagent chemotherapy.50 In this regard, cytarabine regimens, with or without anthracycline, at 3000 mg/m2 have similar efficacy as an intermediate dose of 1000 mg/m2.38 A total of 2 to 4 cycles of post-remission therapy is considered standard.3 Intensified post-remission chemotherapy has not been associated with consistent benefit in older AML patients or those with poor-risk disease. In recent years, measurable residual disease (MRD) assessment has emerged as a potentially useful tool in risk stratification and treatment planning, with various studies suggesting that MRD status in complete remission is one of the most important prognostic factors.51 Prospective studies confirming the significance of MRD as a marker for therapy selection are awaited. Finally, maintenance chemotherapy is not part of standard AML treatment.3
Role of Stem Cell Transplant
AML is the most common indication for allo-SCT. The availability of alternative donor strategies, which include mismatched, unrelated, haplo-identical, and cord blood donor sources, and the development of non-myeloablative and reduced-intensity conditioning (RIC) regimens (which take advantage of graft-versus-leukemia effect while decreasing cytotoxicity from myeloablative regimens) have expanded the possibility of allo-SCT to most patients under the age of 75 years.3 The decision to perform transplant is now largely based upon assessment of the risk (nonrelapse mortality) to benefit (reduction in risk of relapse) ratio, as determined by both disease-related features (cytogenetics, molecular profile) and clinical characteristics of the donor (type, availability, match) and the recipient (comorbidities, performance status).3 In a meta-analysis of 24 prospective trials involving more than 6000 AML patients in first complete remission, allo-SCT was associated with a significant survival benefit in patients with intermediate- and poor-risk AML but not in patients with good-risk AML.52 In line with this, good-risk AML patients are generally not recommended for transplant in first complete remission. For patients with normal karyotype who were said to have de novo AML (historically an intermediate-risk AML group), superior OS was demonstrated with transplant over intensive chemotherapy in those patients with either FLT3-ITD mutations or those with the molecular profile characterized by negativity for mutations in NPM1/CEBPA/FLT3.53 For patients with primary refractory disease and high-risk AML, transplant is probably the only curative option.
The choice of conditioning regimen is guided by several factors, including the subtype of AML, disease status, donor-recipient genetic disparity, graft source, comorbidities in the recipient (ie, tolerability for intensive conditioning regimen), as well as the reliance on graft-versus-leukemia effect as compared to cytotoxic effect of the regimen. The BMT CTN 0901 trial, which randomly assigned 218 patients aged 18 to 65 years to RIC (typically fludarabine/busulfan) or myeloablative regimens, showed an advantage for myeloablative regimens.54 The trial demonstrated a lower risk of relapse (13.5% versus 48.3%, P < 0.01) and higher rates of relapse-free survival (67.7% versus 47.3%, P < 0.01) and OS (67.7% versus. 77.4%, P = 0.07) at 18 months despite higher treatment-related mortality (15.8% versus 4.4%, P = 0.02) and a higher rate of grade 2 to 4 acute graft-versus-host disease (44.7% versus 31.6%, P = 0.024). At present, a RIC regimen is generally recommended for older patients or those with a higher comorbidity burden, while the myeloablative regimen is recommended for younger, fit patients.
Relapsed/Refractory Disease
The treatment of relapsed and refractory AML constitutes a major challenge, with OS estimated around 10% at 3 years.55 Currently, there is no standard salvage therapy in this setting, thus underscoring the need for clinical trials. For younger, fitter patients, the typical approach is to use intensive chemotherapy to achieve a second complete remission followed by a stem cell transplant. In younger patients, a second complete remission is achievable in about 55% of patients, although this rate is lower (~20%–30%) in more unselected patients.56,57 About two thirds of those who achieve complete remission may be able to proceed to transplant.57 For older patients where transplant is not possible, the goal is to use less intensive therapies that help with palliation. HMAs (azacitidine, decitabine) are used and have complete remission rates of 16% to 21% and median survival of 6 to 9 months in older patients.3 LDAC is another option in this setting. The recent approval of GO in this setting has further expanded the options. This approval was based on the outcomes of the phase 2 single-arm MyloFrance-1 study in which single-agent GO administered at 3 mg/m2 on days 1, 4, and 7 led to complete remission in 15 of 57 patients.58
With greater elucidation of the molecular characteristics of AML, the emergence of more effective targeted therapies is possible. Enasidenib, an inhibitor of mutant isocitrate dehydrogenase 2 (IDH2) protein that promotes differentiation of leukemic myeloblasts, recently received regulatory approval based on a single-arm trial. The overall response rate in this study was 38.5%, including a composite complete remission rate of 26.6% at a dose of 100 mg daily.59 IDH differentiation syndrome, akin to the differentiation syndrome seen in acute promyelocytic leukemia, occurred in approximately 12% of the patients, with the most frequent manifestations being dyspnea, fever, pulmonary infiltrates, and hypoxia.60
Survival of patients who relapse following transplant is particularly poor. A recent Center for International Blood and Marrow Transplant Research study found a 3-year OS ranging from a dismal 4% for those who present with early relapses (within 1 to 6 months) post-transplant to a more modest 38% for those who relapsed ≥ 3 years after their first transplant.61 The German Cooperative Transplant Study Group have suggested that azacitidine or chemotherapy followed by donor-lymphocyte infusions might improve responses over chemotherapy alone.62 Ipilimumab-based CTLA-4 blockade was reported to produce responses in a small cohort of patients, which was particularly notable in patients presenting with extramedullary manifestations of relapse.63 In patients who are otherwise fit but have a florid relapse, a second transplant can sometimes be sought, but the value of a different donor for second transplant is unclear.3
Case 1 Conclusion
Given his relatively young age, suitability for intensive therapy, and the presence of a core- binding factor abnormality, the patient is treated with an induction regimen containing daunorubicin, cytarabine, and GO (7+3 + GO). He achieves complete remission. This is followed by consolidation chemotherapy with high-dose cytarabine and GO. Allo-SCT is reserved for later should the AML relapse. Note that dasatinib, a c-KIT inhibitor, can be added to the treatment regimens as per the results of the CALGB 10801 protocol.64 Also, autologous SCT, instead of allo-SCT, can be considered in rare situations with relapsed core-binding factor AML (especially with inv(16) AML, younger patients, longer time in complete remission prior to relapse, and use of GO).
Case 2 Conclusion
The patient is deemed suitable for intensive chemotherapy. As such, CPX-351 is given in induction and consolidation and complete remission is achieved. Because he has adverse-risk AML, an allo-SCT is planned, but the patient relapses before it can be performed. Following 3 courses of decitabine therapy, the patient achieves complete remission once again but declines transplant. He maintains remission for an additional 4 months but then the leukemia progresses. Clinical trials are recommended to the patient, but he decides to pursue hospice care.
Conclusion
AML is the most common acute leukemia in adults. As defined currently, AML represents a group of related but distinct myeloid disorders that are characterized by various chromosomal, genetic, and epigenetic alterations. Early diagnosis and treatment can help prevent the emergence or manage the detrimental effects of its various complications such as leukostasis and tumor lysis syndrome. Improvements in supportive care, incremental treatment advances, and the wide adoption of allo-SCT for less than favorable cases have significantly improved survival of AML patients since the initial design of combinatorial (7+3) induction chemotherapy, particularly in patients presenting at a younger age. HMAs and the emergence of targeted therapies like FLT-3 and IDH2 inhibitors have added to our therapeutic armamentarium. Despite these advances, long-term survival rates in AML patients continue to be only approximately 40% to 50%. Older patients (particularly those over age 65 at the time of diagnosis), those with relapsed disease, and those with AML with certain unfavorable genetic abnormalities continue to have dismal outcomes. The design of newer targeted therapies, epigenetic agents, and immunotherapies will hopefully address this unmet need.
1. Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015;373:1136–52.
2. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts. Leukemia: Acute Myeloid Leukemia (AML). 2018;2018.
3. Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–47.
4. Liesveld JL, Lichtman MA. Acute myelogenous leukemia. In: Kaushansky K, Lichtman MA, Prchal JT, et al, eds. New York: Williams Hematology. 9th ed. New York: McGraw-Hill Education; 2015.
5. Randhawa JK, Khoury J, Ravandi-Kashani F. Adult acute myeloid leukemia. In: Kantarjian HM, Wolff RA, eds. The MD Anderson Manual of Medical Oncology. 3rd ed. New York: McGraw-Hill Medical; 2016.
6. Armstrong SA, Staunton JE, Silverman LB, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet 2002;30:41–7.
7. Graubert TA, Mardis ER. Genomics of acute myeloid leukemia. Cancer J 2011;17:487–91.
8. Cancer Genome Atlas Research Network, Ley TJ, Miller C, Ding L, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013;368:2059–74.
9. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 2016;374:2209–21.
10. Lindsley RC, Mar BG, Mazzola E, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015;125:1367–76.
11. Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014;371:2488–98.
12. Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015;126:9–16.
13. Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017;377:111–21.
14. Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 2014;371:2477–87.
15. Pileri S, Ascani S, Cox M, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 2007;21:340–50.
16. Vachhani P, Bose P. Isolated gastric myeloid sarcoma: a case report and review of the literature. Case Rep Hematol 2014;2014:541807.
17. Rowe JM. Clinical and laboratory features of the myeloid and lymphocytic leukemias. Am J Med Technol 1983;49:103–9.
18. Wolach O, Stone RM. Mixed-phenotype acute leukemia: current challenges in diagnosis and therapy. Curr Opin Hematol 2017;24:139–45.
19. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391–405.
20. Assi R, Ravandi F. FLT3 inhibitors in acute myeloid leukemia: Choosing the best when the optimal does not exist. Am J Hematol 2018;93:553–63.
21. Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 2012;366:1079–89.
22. Ley TJ, Ding L, Walter MJ, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med 2010;363:2424–33.
23. Dores GM, Devesa SS, Curtis RE, et al. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood 2012;119:34–43.
24. Cairoli R, Beghini A, Grillo G, et al. Prognostic impact of c-KIT mutations in core binding factor leukemias: an Italian retrospective study. Blood 2006;107:3463–8.
25. Sorror ML, Storer BE, Elsawy M, et al. Intensive versus non-intensive induction therapy for patients (Pts) with newly diagnosed acute myeloid leukemia (AML) using two different novel prognostic models [abstract]. Blood 2016;128(22):216.
26. Quintás-Cardama A, Ravandi F, Liu-Dumlao T, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 2012;120;4840-5.
27. Gupta N, Miller A, Gandhi Set al. Comparison of epigenetic versus standard induction chemotherapy for newly diagnosed acute myeloid leukemia patients ≥60 years old.Am J Hematol 2015;90:639-46.
28. Sorror ML, Storer BE, Fathi AT, et al. Development and validation of a novel acute myeloid leukemia-composite model to estimate risks of mortality. JAMA Oncol 2017;3:1675–82.
29. Rowe JM, Neuberg D, Friedenberg W, et al. A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: a trial by the Eastern Cooperative Oncology Group. Blood 2004;103:479–85.
30. Mandelli F, Vignetti M, Suciu S, et al. Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: the EORTC and GIMEMA Groups Study AML-10. J Clin Oncol 2009;27:5397–403.
31. Gardin C, Chevret S, Pautas C, et al. Superior long-term outcome with idarubicin compared with high-dose daunorubicin in patients with acute myeloid leukemia age 50 years and older. J Clin Oncol 2013;31:321–7.
32. Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med 2009;361:1249–59.
33. Lee JH, Joo YD, Kim H, et al. A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia. Blood 2011;118:3832–41.
34. Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med 2009;361:1235–48.
35. Burnett AK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood 2015;125:3878–85.
36. Devillier R, Bertoli S, Prebet T, et al. Comparison of 60 or 90 mg/m(2) of daunorubicin in induction therapy for acute myeloid leukemia with intermediate or unfavorable cytogenetics. Am J Hematol 2015;90:E29–30.
37. Pautas C, Merabet F, Thomas X, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol 2010;28:808–14.
38. Lowenberg B. Sense and nonsense of high-dose cytarabine for acute myeloid leukemia. Blood 2013;121:26–8.
39. Lancet JE, Uy GL, Cortes JE, et al. Final results of a phase III randomized trial of CPX-351 versus 7 + 3 in older patients with newly diagnosed high risk (secondary) AML [abstract]. J Clin Oncol 2016;34(15_suppl):7000-7000.
40. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 2017;377:454–64.
41. Jen EY, Ko CW, Lee JE, et al. FDA approval: Gemtuzumab ozogamicin for the treatment of adults with newly-diagnosed CD33-positive acute myeloid leukemia. Clin Cancer Res 2018; doi: 10.1158/1078-0432. CCR-17-3179.
42. Sievers EL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol 2001;19:3244–54.
43. Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013;121:4854–60.
44. Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol 2012;30:3924–31.
45. Castaigne S, Pautas C, Terre C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 2012;379:1508–16.
46. Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012;30:2670–7.
47. Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015;126:291–9.
48. Welch JS, Petti AA, Miller CA, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med 2016;375:2023–36.
49. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol 2016;34:972–9.
50. Miyawaki S, Ohtake S, Fujisawa S, et al. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study. Blood 2011;117:2366–72.
51. Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood 2018;131:1275–91.
52. Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 2009;301:2349–61.
53. Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med 2008;358:1909–18.
54. Pasquini MC, Logan B, Wu J, et al. Results of a phase III randomized, multi-center study of allogeneic stem cell transplantation after high versus reduced intensity conditioning in patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML): Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0901. Blood 2015;126:LBA–8.
55. Bose P, Vachhani P, Cortes JE. Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat Options Oncol 2017;18:17,017-0456-2.
56. Burnett AK, Goldstone A, Hills RK, et al. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol 2013;31:1293–301.
57. Ravandi F, Ritchie EK, Sayar H, et al. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol 2015;16:1025–36.
58. Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia 2007;21:66–71.
59. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017;130:722–31.
60. Fathi AT, DiNardo CD, Kline I, et al. Differentiation syndrome associated with enasidenib, a selective inhibitor of mutant isocitrate dehydrogenase 2: analysis of a phase 1/2 study. JAMA Oncol 2018;doi: 10.1001/jamaoncol.2017.4695.
61. Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant 2015;21:454–9.
62. Schroeder T, Rachlis E, Bug G, et al. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions--a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant 2015;21:653–60.
63. Davids MS, Kim HT, Bachireddy P, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med 2016;375:143–53.
64. Marcucci G, Geyer S, Zhao W, et al. Adding KIT inhibitor dasatinib (DAS) to chemotherapy overcomes the negative impact of KIT mutation/over-expression in core binding factor (CBF) acute myeloid leukemia (AML): results from CALGB 10801 (Alliance) [abstract]. Blood 2014;124:8.
1. Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015;373:1136–52.
2. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts. Leukemia: Acute Myeloid Leukemia (AML). 2018;2018.
3. Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129:424–47.
4. Liesveld JL, Lichtman MA. Acute myelogenous leukemia. In: Kaushansky K, Lichtman MA, Prchal JT, et al, eds. New York: Williams Hematology. 9th ed. New York: McGraw-Hill Education; 2015.
5. Randhawa JK, Khoury J, Ravandi-Kashani F. Adult acute myeloid leukemia. In: Kantarjian HM, Wolff RA, eds. The MD Anderson Manual of Medical Oncology. 3rd ed. New York: McGraw-Hill Medical; 2016.
6. Armstrong SA, Staunton JE, Silverman LB, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet 2002;30:41–7.
7. Graubert TA, Mardis ER. Genomics of acute myeloid leukemia. Cancer J 2011;17:487–91.
8. Cancer Genome Atlas Research Network, Ley TJ, Miller C, Ding L, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 2013;368:2059–74.
9. Papaemmanuil E, Gerstung M, Bullinger L, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med 2016;374:2209–21.
10. Lindsley RC, Mar BG, Mazzola E, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015;125:1367–76.
11. Jaiswal S, Fontanillas P, Flannick J, et al. Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 2014;371:2488–98.
12. Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood 2015;126:9–16.
13. Jaiswal S, Natarajan P, Silver AJ, et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. N Engl J Med 2017;377:111–21.
14. Genovese G, Kahler AK, Handsaker RE, et al. Clonal hematopoiesis and blood-cancer risk inferred from blood DNA sequence. N Engl J Med 2014;371:2477–87.
15. Pileri S, Ascani S, Cox M, et al. Myeloid sarcoma: clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia 2007;21:340–50.
16. Vachhani P, Bose P. Isolated gastric myeloid sarcoma: a case report and review of the literature. Case Rep Hematol 2014;2014:541807.
17. Rowe JM. Clinical and laboratory features of the myeloid and lymphocytic leukemias. Am J Med Technol 1983;49:103–9.
18. Wolach O, Stone RM. Mixed-phenotype acute leukemia: current challenges in diagnosis and therapy. Curr Opin Hematol 2017;24:139–45.
19. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016;127:2391–405.
20. Assi R, Ravandi F. FLT3 inhibitors in acute myeloid leukemia: Choosing the best when the optimal does not exist. Am J Hematol 2018;93:553–63.
21. Patel JP, Gonen M, Figueroa ME, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med 2012;366:1079–89.
22. Ley TJ, Ding L, Walter MJ, et al. DNMT3A mutations in acute myeloid leukemia. N Engl J Med 2010;363:2424–33.
23. Dores GM, Devesa SS, Curtis RE, et al. Acute leukemia incidence and patient survival among children and adults in the United States, 2001-2007. Blood 2012;119:34–43.
24. Cairoli R, Beghini A, Grillo G, et al. Prognostic impact of c-KIT mutations in core binding factor leukemias: an Italian retrospective study. Blood 2006;107:3463–8.
25. Sorror ML, Storer BE, Elsawy M, et al. Intensive versus non-intensive induction therapy for patients (Pts) with newly diagnosed acute myeloid leukemia (AML) using two different novel prognostic models [abstract]. Blood 2016;128(22):216.
26. Quintás-Cardama A, Ravandi F, Liu-Dumlao T, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood 2012;120;4840-5.
27. Gupta N, Miller A, Gandhi Set al. Comparison of epigenetic versus standard induction chemotherapy for newly diagnosed acute myeloid leukemia patients ≥60 years old.Am J Hematol 2015;90:639-46.
28. Sorror ML, Storer BE, Fathi AT, et al. Development and validation of a novel acute myeloid leukemia-composite model to estimate risks of mortality. JAMA Oncol 2017;3:1675–82.
29. Rowe JM, Neuberg D, Friedenberg W, et al. A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: a trial by the Eastern Cooperative Oncology Group. Blood 2004;103:479–85.
30. Mandelli F, Vignetti M, Suciu S, et al. Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: the EORTC and GIMEMA Groups Study AML-10. J Clin Oncol 2009;27:5397–403.
31. Gardin C, Chevret S, Pautas C, et al. Superior long-term outcome with idarubicin compared with high-dose daunorubicin in patients with acute myeloid leukemia age 50 years and older. J Clin Oncol 2013;31:321–7.
32. Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med 2009;361:1249–59.
33. Lee JH, Joo YD, Kim H, et al. A randomized trial comparing standard versus high-dose daunorubicin induction in patients with acute myeloid leukemia. Blood 2011;118:3832–41.
34. Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med 2009;361:1235–48.
35. Burnett AK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood 2015;125:3878–85.
36. Devillier R, Bertoli S, Prebet T, et al. Comparison of 60 or 90 mg/m(2) of daunorubicin in induction therapy for acute myeloid leukemia with intermediate or unfavorable cytogenetics. Am J Hematol 2015;90:E29–30.
37. Pautas C, Merabet F, Thomas X, et al. Randomized study of intensified anthracycline doses for induction and recombinant interleukin-2 for maintenance in patients with acute myeloid leukemia age 50 to 70 years: results of the ALFA-9801 study. J Clin Oncol 2010;28:808–14.
38. Lowenberg B. Sense and nonsense of high-dose cytarabine for acute myeloid leukemia. Blood 2013;121:26–8.
39. Lancet JE, Uy GL, Cortes JE, et al. Final results of a phase III randomized trial of CPX-351 versus 7 + 3 in older patients with newly diagnosed high risk (secondary) AML [abstract]. J Clin Oncol 2016;34(15_suppl):7000-7000.
40. Stone RM, Mandrekar SJ, Sanford BL, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med 2017;377:454–64.
41. Jen EY, Ko CW, Lee JE, et al. FDA approval: Gemtuzumab ozogamicin for the treatment of adults with newly-diagnosed CD33-positive acute myeloid leukemia. Clin Cancer Res 2018; doi: 10.1158/1078-0432. CCR-17-3179.
42. Sievers EL, Larson RA, Stadtmauer EA, et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol 2001;19:3244–54.
43. Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013;121:4854–60.
44. Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol 2012;30:3924–31.
45. Castaigne S, Pautas C, Terre C, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 2012;379:1508–16.
46. Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012;30:2670–7.
47. Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015;126:291–9.
48. Welch JS, Petti AA, Miller CA, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med 2016;375:2023–36.
49. Amadori S, Suciu S, Selleslag D, et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J Clin Oncol 2016;34:972–9.
50. Miyawaki S, Ohtake S, Fujisawa S, et al. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study. Blood 2011;117:2366–72.
51. Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood 2018;131:1275–91.
52. Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA 2009;301:2349–61.
53. Schlenk RF, Dohner K, Krauter J, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med 2008;358:1909–18.
54. Pasquini MC, Logan B, Wu J, et al. Results of a phase III randomized, multi-center study of allogeneic stem cell transplantation after high versus reduced intensity conditioning in patients with myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML): Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0901. Blood 2015;126:LBA–8.
55. Bose P, Vachhani P, Cortes JE. Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat Options Oncol 2017;18:17,017-0456-2.
56. Burnett AK, Goldstone A, Hills RK, et al. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol 2013;31:1293–301.
57. Ravandi F, Ritchie EK, Sayar H, et al. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol 2015;16:1025–36.
58. Taksin AL, Legrand O, Raffoux E, et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia 2007;21:66–71.
59. Stein EM, DiNardo CD, Pollyea DA, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 2017;130:722–31.
60. Fathi AT, DiNardo CD, Kline I, et al. Differentiation syndrome associated with enasidenib, a selective inhibitor of mutant isocitrate dehydrogenase 2: analysis of a phase 1/2 study. JAMA Oncol 2018;doi: 10.1001/jamaoncol.2017.4695.
61. Bejanyan N, Weisdorf DJ, Logan BR, et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant 2015;21:454–9.
62. Schroeder T, Rachlis E, Bug G, et al. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions--a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant 2015;21:653–60.
63. Davids MS, Kim HT, Bachireddy P, et al. Ipilimumab for patients with relapse after allogeneic transplantation. N Engl J Med 2016;375:143–53.
64. Marcucci G, Geyer S, Zhao W, et al. Adding KIT inhibitor dasatinib (DAS) to chemotherapy overcomes the negative impact of KIT mutation/over-expression in core binding factor (CBF) acute myeloid leukemia (AML): results from CALGB 10801 (Alliance) [abstract]. Blood 2014;124:8.
Management of Colorectal Cancer in Older Adults
Introduction
Colorectal cancer (CRC) is the fourth most common cancer in the United States and has a high prevalence among the older population.1 In 2017, there were an estimated 135,430 new cases of CRC and 50,260 deaths due to CRC. It is the second leading cause of cancer death in the United States, and the death rate for patients with CRC increases with age (Figure).2
Although elderly persons are more frequently diagnosed with CRC, they are underrepresented in clinical trials. This may be due in part to stringent eligibility criteria in prospective randomized controlled trials that exclude older patients with certain comorbidities and decreased functional status. Hutchins and colleagues compared the proportion of persons aged 65 years and older enrolled in Southwest Oncology Group (SWOG) clinical trials and the proportion of persons in this age group in the US population with the same cancer diagnoses.5 They found that while 72% of the US population with CRC were aged ≥ 65 years, persons in this age group comprised only 40% of patients enrolled in SWOG trials. An update on this study performed after Medicare policy changed in 2000 to include coverage of costs incurred due to clinical trials showed an upward trend in the accrual of older patients in SWOG trials, from 25% during the period 1993–1996 to 38% during the period 2001–2003; however, the percentage of older patients with CRC on clinical trials overall remained stable from 1993 to 2003.6
The underrepresentation of older adults with CRC in clinical trials presents oncologists with a challenging task when practicing evidence-based medicine in this patient population. Analysis of a large claims database demonstrated that the use of multi-agent chemotherapy for the treatment of metastatic CRC in older adults increased over time, while the use of single-agent 5-fluorouracil (5-FU) decreased.7 However, the adoption of combination therapy with irinotecan or oxaliplatin in older adults lagged behind the initial adoption of these agents in younger patients. This data demonstrates that as the field of medical oncology evolves, providers are becoming more comfortable treating older patients with multiple medical problems using standard approved regimens.
Geriatric Assessment
Before treating older patients with cancer, it is necessary to define the patient’s physiological age, ideally through a multidisciplinary team evaluation.
The Eastern Cooperative Oncology Group performance status (ECOG PS) and Karnofsky Performance Status (KPS) are crude measures of functional status.12 Generally, elderly patients with good ECOG PS or KPS scores are considered fit enough to receive standard therapy similar to their younger counterparts. Evaluation of functional status using these performance scores is often suboptimal, resulting in patients with a normal or adequate performance status score who may still experience poor outcomes, including decreased survival and inability to tolerate treatment. A study that explored parameters among older patients that predict for increased risk of chemotherapy-related toxicities found that physician-rated KPS score did not accurately predict the risk for adverse events.13 Therefore, a CGA represents a better way to evaluate functional status and other domains.
Functional status can also be evaluated by self-reported tools such as activities of daily living, which refer to basic self-care, and instrumental activities of daily living (IADLs), which are essential for independent living in the community.14,15 Mobility, gait, and balance can also be measured using the “Timed Get Up and Go” test and gait speed. Klepin et al found that faster gait speed was associated with overall survival (OS) in patients with metastatic cancer.16
Cognitive function is an important component of the geriatric assessment in older patients with cancer, as dementia is a prognostic factor for survival in the overall geriatric population. In a retrospective review, patients with dementia were less likely to have a biopsy-proven diagnosis and were twice as likely to have their CRC diagnosed postmortem.17 In addition, establishing that the patient has intact cognitive function prior to initiating treatment is essential to ensure that the patient can comply with treatment and understands when to report adverse effects. Nutritional status is an important portion of the geriatric assessment because malnutrition is associated with increased mortality and decreased tolerance for chemotherapy.18–20 Evaluating the patient’s psychosocial support is crucial as well because older patients are at greater risk of social isolation and depression.21 While the incidence of depression is lower in older adults with cancer than in younger adults with cancer, clinically significant depression is still noted in 3% to 25% of elderly cancer patients.22 Other critical components of the CGA are review of the patient’s comorbidities and medications to avoid complications of polypharmacy.
Both the Cancer and Aging Research Group (CARG) and Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) toxicity tools are valuable tools, as they predict chemotherapy tolerance in elderly patients.13,23 These tools can help guide discussions between oncologists and patients as well as the formulation of an appropriate treatment plan.24 Although toxicity tools can help to determine which patients are at risk for severe toxicity secondary to treatment, these tools do not replace the CGA. A prospective cohort study that evaluated the impact of CGA on tolerance to chemotherapy in older patients with cancer compared patients aged ≥ 70 years at the start of their treatment with chemotherapy (± radiation therapy) using geriatrician-delivered CGA versus standard care given by oncology.8 Patients who received geriatrician-guided CGA interventions tolerated chemotherapy better and completed treatments as planned (odds ratio 4.14 [95% confidence interval {CI} 1.50 to 11.42], P = 0.006) with fewer treatment modifications.
Unfortunately, the CGA is time-consuming to administer and difficult to incorporate into a busy oncology practice. Therefore, other screening models are used to identify patients who may benefit from a full CGA. The International Society of Geriatric Oncology performed a systematic review of screening tools used to identify older cancer patients in need of geriatric assessment and found that the 3 most studied screening tools are the G8, the Vulnerable Elders Survey-13 (VES-13), and the Flemish version of the Triage Risk Screening Tool.25 Another study found that the G8 was more sensitive than the VES-13 (76.5% versus 68.7%, P = 0.0046), whereas the VES-13 was more specific than the G8 (74.3% versus 64.4%, P < 0.0001).26 In addition to providing guidance to initiate a full geriatric assessment, these screening tools may assist in decision making for older cancer patients, especially those with advanced disease.
Surgery
Early-Stage Disease
When possible, surgical resection of colorectal tumors is the primary treatment in both the curative setting and to avoid complications, such as obstruction or perforation.27 Multiple studies have shown that fit elderly patients benefit from curative surgery similarly to their younger counterparts.27–29 With the growing population of persons aged 65 years or older, surgeons are becoming more comfortable with operating on the elderly.4 However, a large systematic review of 28 independent studies with a total of 34,194 patients showed that older patients were less likely to undergo curative surgery.30 Eligibility for surgery should not be determined by age alone, but rather should be based on a full assessment of the patient’s health, including comorbidities, functional status, nutrition, cognition, social support, and psychological status. The impact of age on short-term outcomes after colorectal surgery in terms of 30-day postoperative morbidity and mortality rates was explored in a study that divided patients into 2 groups: those aged ≥ 80 years (mean age 85) and those aged < 80 years (mean age 55.3).31 There were no statistical differences in 30-day postoperative morbidity and mortality rates between the 2 groups, and preexisting comorbidities and urgent nature of surgery were important predictors of colorectal surgery outcomes in the older adults, results that have been seen in several other studies.28,30 When possible, laparoscopic surgery is preferred as it is associated with less intraoperative blood loss, less postoperative pain, reduced postoperative ileus, a shorter hospital stay, and fewer cardiovascular and pulmonary complications.32 The Preoperative Assessment of Cancer in the Elderly (PACE), which combines surgical risk assessment tools with CGA tools, can assist surgeons in determining candidacy for surgery and help decrease unequal access to surgery in the geriatric population.33
Metastasectomy
A large international multicenter cohort study explored the outcomes of patients aged ≥ 70 years who underwent liver resection of colorectal metastases. The study investigatorsfound that neoadjuvant chemotherapy was used less frequently and less extensive surgery was performed in elderly patients than in younger patients.34 Sixty-day postoperative mortality was slightly higher (3.8% versus 1.6%, P < 0.001) and 3-year OS was slightly lower (57.1% versus 60.2%, P < 0.001) in the elderly group as compared to their younger counterparts, but overall the outcomes after liver surgery were similar. Therefore, the management of liver metastases in oligometastatic disease in elderly patients fit for surgery should be the same as that offered to younger patients. Since outcomes are comparable, older patients should be offered neoadjuvant chemotherapy, as several studies have shown similar response rates and OS in younger and older patients.35,36
Rectal Cancer
The standard of care for locally advanced rectal cancer is combined modality treatment with radiation and chemotherapy followed by total mesorectal excision. However, given conflicting data regarding the ability of elderly patients to tolerate neoadjuvant 5-FU-based chemotherapy and radiation, elderly patients are treated with trimodality therapy less often than their younger counterparts.37,38 A systematic review of 22 randomized trials involving 8507 patients with rectal cancer showed that adjuvant radiation therapy could reduce the risk of local recurrence and death from rectal cancer in patients of all ages.39 However, the risk of noncancer-related death was increased in the older population. The Stockholm II trial showed similar benefits of preoperative radiation overall, but this benefit did not extend to patients older than 68 years because of an increased risk of morbidity and mortality.40 In older patients, mortality from noncancer causes within the first 6 months after surgery was higher in the group that received perioperative radiation than in the group that did not receive radiation. Elderly patients (age > 68 years) accounted for most of the mortality, which was predominantly due to cardiovascular disease.
A retrospective study of 36 patients aged ≥ 70 years with rectal cancer evaluated the toxicity and feasibility of neoadjuvant 5-FU combined with pelvic radiation for treating locally advanced rectal cancer. Patients were classified as healthy and “fit” or “vulnerable” based on the presence of comorbidities.41 This study demonstrated that tolerability and response to neoadjuvant chemotherapy and radiation as well as ability to undergo surgery were similar in “vulnerable” patients and “fit” patients. Conversely, Margalit and colleagues studied the rate of treatment deviations in elderly patients with rectal cancer treated with combined modality therapy and found that most patients required early termination of treatment, treatment interruptions, or dose reductions.42 While trimodality treatment is the standard of care in rectal cancer, there is conflicting data from retrospective studies regarding the tolerability and feasibility of this approach. It is important to proceed with caution but to still consider fit older patients with locally advanced rectal cancer for neoadjuvant chemotherapy and radiation followed by surgery.
In patients who have a complete response (CR) to neoadjuvant chemoradiation, watchful waiting rather than proceeding to surgery may be a reasonable strategy, especially in older patients. A systematic review of 867 patients with locally advanced rectal cancer showed no statistically significant difference in OS between patients who were observed with watchful waiting and those who underwent surgery.43 The International Watch and Wait Database includes 679 patients who were managed with a watch-and-wait regimen because they had a clinical CR after chemoradiation. An outcomes analysis of these patients showed that 25% had local regrowth, with 3-year OS of 91% overall and 87% in patients with local regrowth.44 In most patients (84%), regrowth of the tumor occurred within the first 2 years of follow up.
In frail older adults, for whom longer courses of treatment are not feasible or chemotherapy is contraindicated, short-course radiation therapy can be considered either in the neoadjuvant setting or alone for palliation.45 A randomized trial of short-course radiation versus long-course chemoradiation in patients with T3 rectal cancer found that the difference in 3-year local recurrence rates was not statistically significant.46
Chemotherapy
An expected natural decline in function occurs with age, but given the great variability that exists between patients, it is important to focus on physiologic age rather than chronologic age to determine ability to receive and tolerate anticancer treatment. Decreases in renal and hepatic function, cognitive impairment, changes in gastrointestinal motility, decrements in cardiac and bone marrow reserves, as well as comorbidities and polypharmacy affect a patient’s ability to tolerate chemotherapy.47,48 Toxicity tools such as CARG and CRASH can help to predict severity of toxicity with chemotherapy.13,23 The information provided by these tools can help guide conversations between the oncologist and patient regarding treatment plans.
Adjuvant Chemotherapy for Early-Stage Disease
Stage II Disease
Defining treatment guidelines for older patients with stage II colon cancer is difficult due to lack of data that shows benefit in this population. The QUASAR (Quick and Simple and Reliable) group’s prospective study of adjuvant single-agent 5-FU in stage II colon cancer patients showed an absolute improvement in survival of 3.6% when 5-FU was given after surgery (95% CI 1.0 to 6.0).49 The subgroup analysis of patients aged ≥ 70 years showed a limited benefit of adjuvant 5-FU (hazard ratio [HR] 1.13 [95% CI 0.74 to 1.75]). Given the limited benefit, adjuvant 5-FU for elderly patients with stage II colon cancer should be used judiciously as patients may have competing causes of morbidity or mortality.
The use of oxaliplatin-based therapy in the adjuvant setting for stage II disease was evaluated in a subgroup analysis of the MOSAIC study (Multicenter International Study of Oxaliplatin/5-FU/Leucovorin in the Adjuvant Treatment of Colon Cancer).50 Adjuvant oxaliplatin-based treatment may be offered to patients with stage II colon cancer that carries high-risk features (poorly differentiated histology, lymphovascular invasion, bowel obstruction and/or perforation, < 12 lymph nodes sampled, perineural invasion, or indeterminate or positive margins) due to a trend toward improved disease-free survival (DFS) at 5 years. Patients in this group who received adjuvant FOLFOX (leucovorin, oxaliplatin, 5-FU) versus 5-FU/leucovorin had a DFS of 82.3% versus 74.6%, respectively (HR 0.72 [95% CI 0.50 to 1.02]), a difference that was not statistically significant. A subgroup analysis of 315 patients aged 70 to 75 years with stage II colon cancer enrolled in the MOSAIC study found no statistically significant DFS or OS benefit with the addition of oxaliplatin to 5-FU/leucovorin.51 Therefore, use of this platinum/fluoropyrimidine combination for adjuvant therapy for high-risk stage II disease in older patients remains controversial given its associated risks and the lack of definitive data demonstrating a benefit in this patient group. Decisions regarding this therapy should be made through a shared discussion with patients about its risks and benefits.
Microsatellite status is an important biomarker in the evaluation of stage II CRC. Microsatellite stability is a marker of a functioning DNA mismatch repair system. In patients with colon cancer, tumor microsatellite stability is classified based on the percentage of abnormal microsatellite regions.52 Several studies have shown that patients with tumors that display high microsatellite instability (MSI-H) have an improved prognosis over patients with microsatellite stable tumors.53,54 While patients with stage II MSI-H colon cancer have better outcomes, MSI is associated with a reduced response to treatment with fluoropyrimidines, as demonstrated in a systematic review that found that patients with tumors with MSI obtained no benefit from adjuvant 5-FU (HR 1.24 [95% CI 0.72 to 2.14]).55 Aparicio and colleagues reported an increased prevalence of MSI-H tumors with increasing age.56 Therefore, mismatch repair phenotype should be considered when making adjuvant chemotherapy decisions in the older adult with colon cancer, as it may affect the decision to recommend single-agent 5-FU treatment.
Stage III Disease
The use of single-agent 5-FU for stage III resected CRC has been evaluated in multiple studies. Sargent et al performed a pooled analysis of 3351 patients from 7 randomized phase 3 trials comparing surgery and adjuvant 5-FU-based chemotherapy versus surgery alone in stage II or III colon cancer patients.57 Adjuvant chemotherapy was associated with improvement in both OS and time to tumor recurrence (HR 0.76 and 0.68, respectively). The 5-year OS was 71% for those who received adjuvant treatment and 64% for those who were treated with surgery alone. The benefit of adjuvant treatment was independent of age, and there was no difference in toxicity across age groups, except for 1 study which showed increased rates of leukopenia in the elderly. The oral fluoropyrimidine capecitabine was shown to be an effective alternative to 5-FU plus leucovorin as adjuvant treatment for those with resected stage III colon cancer.58 However, in the subgroup analysis of DFS in the intention-to-treat group, the improvement in DFS was not statistically significant in those aged ≥ 70 years. This study justified the phase 3 Xeloda in Adjuvant Colon Cancer Therapy (X-ACT) trial, which compared capecitabine and 5-FU/leucovorin as adjuvant therapy in patients with resected stage III colon cancer.59 The X-ACT trial showed no significant effect of age on DFS or OS.
The addition of oxaliplatin to 5-FU in the adjuvant setting for stage III tumors has been studied and debated in the elderly population in multiple trials. The MOSAIC trial investigated FOLFOX versus 5-FU/leucovorin in the adjuvant setting.50 The addition of oxaliplatin was associated with a DFS and OS benefit, with a 20% reduction in risk of colon cancer recurrence and 16% reduction in risk of death in all patients. The National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trial then studied 2409 patients with stage II or III colon cancer treated with weekly bolus 5-FU/leucovorin with or without oxaliplatin.60 In this study, OS was significantly improved with the addition of oxaliplatin in patients younger than 70 years, but OS at 5 years was 4.7% worse for patients aged ≥ 70 years treated with weekly 5-FU/leucovorin and oxaliplatin compared with those treated with weekly 5-FU/leucovorin (71.6% versus 76.3%, respectively). In contrast, the XELOXA trial (NO16968), which randomly assigned stage III colon cancer patients to capecitabine and oxaliplatin (XELOX) or bolus 5-FU/leucovorin (standard of care at study start), showed an efficacy benefit, albeit not statistically significant, in patients aged ≥ 70 years (HR 0.87 [95% CI 0.63 to 1.18]).61–63
The Adjuvant Colon Cancer Endpoints (ACCENT) database included 7 randomized trials totaling 14,528 patients with stage II or III colon cancer treated with adjuvant 5-FU with or without oxaliplatin or irinotecan.64 Subgroup analysis of patients aged ≥ 70 years (n = 2575) showed no benefit with an oxaliplatin-based regimen in DFS (HR 0.94 [95% CI 0.78 to 1.13]) or OS (HR 1.04 [95% CI, 0.85 to 1.27]). Based on these studies and the increased toxicity with oxaliplatin, oxaliplatin-based adjuvant chemotherapy is utilized less often than single-agent 5-FU in geriatric patients with early-stage colon cancer.65 Conversely, a recent pooled analysis of individual patient data from 4 randomized trials (NSABP C-08, XELOXA, X-ACT, and AVANT) showed improved DFS and OS with adjuvant XELOX or FOLFOX over single-agent 5-FU in patients aged ≥ 70 years (DFS HR 0.77 [95% CI 0.62 to 0.95], P = 0.014; OS HR 0.78 [95% CI 0.61 to 0.99], P = 0.045).66 This analysis also showed that grade 3 and 4 adverse events related to oxaliplatin were similar across age groups.
These data come from post-hoc analyses, and there is no prospective data to steer decision making in elderly patients with early-stage CRC (Table).
It is well established that patients with stage III colon cancer benefit from oxaliplatin-based adjuvant chemotherapy after curative surgical resection.68 However, older patients are less likely to be referred to oncology as compared with their younger counterparts, due to the conflicting data regarding the benefit of this approach in older adults. Studies have shown that when the referral is placed, the geriatric population is less likely to receive chemotherapy.69 Sanoff et al analyzed 4 data sets (SEER-Medicare, National Comprehensive Cancer Network, New York State Cancer Registry, and Cancer Care Outcomes Research and Surveillance Consortium) to assess the benefit of adjuvant chemotherapy for resected stage III CRC among patients aged ≥ 75 years. Their analysis showed that only 40% of patients evaluated received adjuvant chemotherapy for stage III CRC after surgical resection.70
Summary
Prospective data to guide the treatment of older patients with early-stage CRC in the adjuvant setting is lacking. For fit older patients with stage II disease, limited benefit will be derived from single-agent 5-FU. For those with stage III CRC, the benefit and toxicities of fluoropyrimidines as adjuvant therapy appear to be similar regardless of age. The addition of oxaliplatin to fluoropyrimidines in patients aged ≥ 70 years has not been proven to improve DFS or OS and could result in an incremental toxicity profile. Therefore, treatment plans must be individualized, and decisions should be made through an informed discussion evaluating the overall risk/benefit ratio of each approach.
Metastatic Disease
Palliative Chemotherapy
Approximately 20% of patients with CRC are diagnosed with metastatic disease at presentation, and 35% to 40% develop metastatic disease following surgery and adjuvant therapy.2 The mainstay of treatment in this population is systemic therapy in the form of chemotherapy with or without biologic agents. In this setting, several prospective studies specific to older adults have been completed, providing more evidence-based guidance to oncologists who see these patients. Folprecht et al retrospectively reviewed data from 22 clinical trials evaluating 5-FU-based palliative chemotherapy in 3825 patients with metastatic CRC, including 629 patients aged ≥ 70 years.71 OS in elderly patients (10.8 months [95% CI 9.7 to 11.8]) was equivalent to that in younger patients (11.3 months [95% CI 10.9 to 11.7], P = 0.31). Similarly, relative risk and progression-free survival (PFS) were comparable irrespective of age.
Standard of care for most patients with metastatic colon cancer consists of 5-FU/leucovorin in combination with either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) with a monoclonal antibody.72 A retrospective pooled analysis of patients with metastatic CRC compared the safety and efficacy of FOLFOX4 in patients aged < 70 years versus those aged ≥ 70 years.73 While age ≥ 70 years was associated with an increased rate of grade ≥ 3 hematologic toxicity, it was not associated with increased rates of severe neurologic events, diarrhea, nausea, vomiting, infection, 60-day mortality, or overall incidence of grade ≥ 3 toxicity. The benefit of treatment was consistent across both age groups; therefore, age alone should not exclude an otherwise healthy individual from receiving FOLFOX.
These post-hoc analyses show that fit older patients who were candidates for trial participation tolerated these treatments well; however, these treatments may be more challenging for less fit older adults. The UK Medical Research Council FOCUS2 (Fluorouracil, Oxaliplatin, CPT11 [irinotecan]: Use and Sequencing) study was a prospective phase 3 trial that included 459 patients with metastatic CRC who were deemed too frail or not fit enough for standard-dose chemotherapy by their oncologists.74 In this group, 43% of patients were older than 75 years and 13% were older than 80 years. Patients were randomly assigned to receive infusional 5-FU with levofolinate; oxaliplatin and 5-FU; capecitabine; or oxaliplatin and capecitabine; all regimens were initiated with an empiric 20% dose reduction. The addition of oxaliplatin suggested some improvement in PFS, but this was not significant (5.8 months versus 4.5 months, HR 0.84 [95% CI 0.69 to 1.01], P = 0.07). Oxaliplatin was not associated with increased grade 3 or 4 toxicities. Capecitabine is often viewed as less toxic because it is taken by mouth, but this study found that replacement of 5-FU with capecitabine did not improve quality of life. Grade 3 or 4 toxicities were seen more frequently in those receiving capecitabine than in those receiving 5-FU (40% versus 30%, P = 0.03) in this older and frailer group of patients. As the patients on this study were frail and treatment dose was reduced, this data may not apply to fit older adults who are candidates for standard therapy.
When managing an older patient with metastatic CRC, it is important to tailor therapy based on goals of care, toxicity of proposed treatment, other comorbidities, and the patient’s functional status. One approach to minimizing toxicity in the older population is the stop-and-go strategy. The OPTIMOX1 study showed that stopping oxaliplatin after 6 cycles of FOLFOX7 and continuing maintenance therapy with infusional 5-FU/leucovorin alone for 12 cycles prior to reintroducing FOLFOX7 achieved efficacy similar to continuous FOLFOX4 with decreased toxicity.75 Figer et al studied an exploratory cohort of 37 patients aged 76 to 80 years who were included in the OPTIMOX1 study.76 The overall relative risk, median PFS, and median OS did not differ between the older patients in this cohort and younger patients studied in the original study. Older patients did experience more neutropenia, neurotoxicity, and overall grade 3 to 4 toxicity, but there were no toxic deaths in patients older than 75 years. The approach of giving treatment breaks, as in OPTIMOX2, may also provide patients with better quality of life, but perhaps at the expense of cancer-related survival.77
The combination of irinotecan and 5-FU has also been studied as treatment for patients with metastatic CRC. A pooled analysis of 2691 patients aged ≥ 70 years with metastatic CRC across 4 phase 3 randomized trials investigating irinotecan and 5-FU demonstrated that irinotecan-containing chemotherapy provided similar benefits to both older and younger patients with similar risk of toxicity.78 A phase 2 trial studying FOLFIRI as first-line treatment in older metastatic CRC patients showed this to be a safe and active regimen with manageable toxicity.79 Another randomized phase 3 trial for older patients compared 5-FU/leucovorin with or without irinotecan for first-line treatment of metastatic CRC (FFCD 2001-02).80 The study accrued 282 patients aged ≥ 75 years (median age 80 years), and found that the addition of irinotecan to infusional 5-FU–based chemotherapy did not significantly increase either PFS or OS. Aparicio et al performed a substudy of baseline geriatric evaluation prior to treatment in the FFCD 2001-02 study and assessed the value of geriatric parameters for predicting outcomes (objective response rate [ORR], PFS, and OS).81 Multivariate analysis showed that none of the geriatric parameters were predictive of ORR or PFS but that normal IADL was associated with better OS. This combination may still be appropriate for some older patients with metastatic disease, while single- agent 5-FU may be more appropriate in frail patients.
Biologic Agents
VEGF Inhibitors
Targeted biologic agents have been studied in the treatment of metastatic CRC. Bevacizumab is a recombinant, humanized monoclonal antibody against vascular endothelial growth factor (VEGF) that is approved in the first-line setting for treatment of metastatic CRC. A pooled analysis examined 439 patients 65 years of age and older with metastatic CRC who received bevacizumab plus chemotherapy versus placebo plus chemotherapy.82 In this analysis, the addition of bevacizumab was associated with an improvement in OS (19.3 months versus 14.3 months, HR 0.7 [95% CI 0.55 to 0.90], P = 0.006) and in PFS (9.2 months versus 6.2 months, HR 0.52 [95% CI 0.40 to 0.67], P < 0.0001). Known adverse events associated with bevacizumab were seen in the bevacizumab plus chemotherapy group but not at increased rates in the older population compared to their younger counterparts. Conversely, another pooled analysis found that while there was a PFS and OS benefit in older patients receiving bevacizumab, there was an increased incidence of thrombotic events in patients older than 65 years.83 The BEAT (Bevacizumab Expanded Access Trial) and BRiTE (Bevacizumab Regimens Investigation of Treatment Effects) studies showed similar clinical outcomes across all age groups.84,85 While older patients experienced more arterial thromboembolic events with the addition of bevacizumab, other factors such as ECOG PS, prior anticoagulation, and history of arterial disease were more predictive of these adverse events than age.
The randomized phase 3 AVEX study explored the efficacy and tolerability of capecitabine plus bevacizumab versus capecitabine alone in 280 frail patients aged ≥ 70 years.86 PFS in the capecitabine/bevacizumab arm was 9.1 months versus 5.1 months in the capecitabine alone arm. While the OS difference was not statistically significant, patients in the capecitabine/bevacizumab arm had an OS of 20.7 months versus 16.8 months in the capecitabine alone group. As reported in prior studies, patients in the capecitabine/bevacizumab arm had increased rates of toxic events (40%) compared with those who received capecitabine alone (22%), with reports of hypertension, hand-foot syndrome, bleeding, and thrombotic events. More recently, the phase 2 PRODIGE 20 trial studied the addition of bevacizumab to chemotherapy (5-FU, FOLFOX, or FOLFIRI) based on physician choice in untreated metastatic CRC patients aged ≥ 75 years (median age 80 years).87 They found that the addition of bevacizumab to standard of care chemotherapy was both safe and effective. The adverse events seen with bevacizumab, such as hypertension and thrombotic events, were consistent with prior studies.
A newer antiangiogenic agent, ziv-aflibercept, has been approved for the second-line treatment of metastatic CRC. The VELOUR trial demonstrated that the addition of ziv-aflibercept to FOLFIRI benefited patients across all age groups compared with FOLFIRI plus placebo in patients who had failed prior oxaliplatin-based chemotherapy.88,89 Ramucirumab is a human IgG-1 monoclonal antibody approved in second-line treatment in combination with FOLFIRI. A subgroup analysis of the RAISE study showed that the survival benefit was similar in patients aged ≥ 65 years versus those < 65 years.90 Based on the above data, the use of a VEGF inhibitor in combination with chemotherapy should be considered in older patients with metastatic CRC. Furthermore, based on the conflicting data regarding the benefit of FOLFOX/FOLFIRI over single-agent 5-FU discussed above, the combination of capecitabine plus bevacizumab may be considered a front-line treatment option in older patients based on the AVEX study.
EGFR Inhibitors
Cetuximab and panitumumab are anti-epidermal growth factor receptor (EGFR) antibodies approved for the treatment of RAS wild-type metastatic CRC. Data regarding the use of EGFR inhibitors in the geriatric population is scarce and the data that does exist is conflicting.91,92 The PRIME study demonstrated that panitumumab plus FOLFOX had a PFS benefit compared to FOLFOX alone in KRAS wild-type metastatic CRC patients.92 While the study met its primary endpoint, the benefit did not translate to patients aged ≥ 65 years in subgroup analysis. Conversely, a retrospective study of the efficacy and safety of cetuximab in elderly patients with heavily pretreated metastatic CRC found similar efficacy in older and younger patients as well as no increased adverse events in the older population.91 A phase 2 trial investigating cetuximab as single-agent first-line treatment of metastatic CRC in fit older patients found cetuximab to be safe with moderate activity in this population, but did not support the use of cetuximab as first-line single-agent treatment in fit geriatric patients who may be candidates for combination therapy.93 Our group studied the patterns of use and tolerance of anti-EGFR antibodies in 117 older adults with metastatic CRC with a median age of 73 years.94 The study showed that older age at the time of treatment was associated with administration of anti-EGFR antibody as monotherapy rather than in combination with chemotherapy (P = 0.0009). We found no association between age and presence of grade 3 or higher toxicity. In addition, the toxicity profile seen in older patients was similar to what has been demonstrated in prior studies involving a younger patient population. Given the discordance seen between studies, additional prospective trials are needed to elucidate the efficacy and safety of EGFR inhibitors in the geriatric population.
Other Agents
Two newer agents approved in the treatment of metastatic CRC are regorafenib, a multikinase inhibitor, and trifluridine/tipiracil (TFD/TPI), a nucleoside analog combined with an inhibitor of thymidine phosphorylase. The phase 3 CORRECT trial studied regorafenib as monotherapy in previously treated metastatic CRC and found an OS benefit of 1.4 months and minimal PFS benefit.95 Van Cutsem et al performed a subgroup analysis by age and found similar OS benefit in patients < 65 years of age and ≥ 65 years.96 The most frequent adverse events grade 3 or higher were hand-foot syndrome, fatigue, diarrhea, hypertension, and desquamation/rash, which were seen at similar rates in both age groups. More recently, the phase 2 Regorafenib Dose Optimization Study (ReDOS) found that weekly dose escalation of regorafenib from 80 mg to 160 mg daily over 3 weeks was superior to the standard 160 mg daily dosing in patients with metastatic CRC.97 The dose escalation group had a longer median OS, although this difference was not statistically significant, as well as a more favorable toxicity profile. Therefore, this new dosing strategy may be a reasonable option for older patients with pretreated metastatic CRC. A study of TFD/TPI versus placebo in refractory metastatic CRC found an OS benefit of 7.1 months versus 5.3 months.98 In subgroup analyses, the OS benefit extended to both patients < 65 years and ≥ 65 years. Given the sparse data on these newer agents in the geriatric population and the modest benefit they provide to those with refractory metastatic CRC, more data is needed to determine their utility in elderly patients. The decision to use these agents in the older patients warrants a thorough discussion with the patient regarding risks, benefit, and treatment goals.
Immunotherapy
Between 3.5% and 6.5% of stage IV colorectal cancers are MSI-H and have deficient mismatch repair (dMMR).99–101 A recent phase 2 trial studied the use of pembrolizumab, an IgG4 monoclonal antibody against PD-1 (programmed cell death-1), in heavily pretreated patients with dMMR metastatic CRC, MMR-proficient (pMMR) metastatic CRC, and noncolorectal dMMR metastatic cancer.102 Patients with dMMR metastatic CRC had a 50% ORR and 89% disease control rate (DCR), as compared with an ORR of 0% and DCR of 16% in patients with pMMR metastatic CRC. There was also an OS and PFS benefit seen in the dMMR CRC group as compared with the pMMR CRC group. Another phase 2 study, CheckMate 142, studied the anti-PD-1 monoclonal antibody nivolumab with or without ipilimumab (a monoclonal antibody against cytotoxic T-lymphocyte antigen 4) in patients with dMMR and pMMR metastatic CRC.103 In the interim analysis, nivolumab was found to provide both disease control and durable response in patients with dMMR metastatic CRC.
While these studies led to the FDA approval of pembrolizumab and nivolumab for management of previously treated MSI-H or dMMR metastatic CRC, data on the use of immunotherapy in older adults is scarce. Immunosenescence, or the gradual deterioration of the immune system that comes with aging, may impact the efficacy of immune checkpoint inhibitors (ICI) in older patients with advanced cancer.104 There is conflicting data on the efficacy of PD-1 and programmed death ligand-1) PD-L1 inhibitors in older patients across different cancers. A meta-analysis of immunotherapy in older adults with a variety of malignancies showed overall efficacy comparable to that seen in adults younger than 65 years.105 However, another review found ICIs to be less effective in older patients with head and neck, non-small cell lung cancer, and renal cell carcinoma compared with their younger counterparts.104 Regarding the toxicity profile of ICIs in the elderly, similar rates of grade 3 or higher adverse events in patients younger than 65 years and older than 65 years have been reported.106 However, patients aged ≥ 70 years had increased rates of grade 3 to 5 adverse events as compared to patients younger than 65 years (71.7% versus 58.4%, respectively). Given the scant data on ICIs in older patients with MSI-H or dMMR metastatic CRC, more clinical trials inclusive of this population are needed in order to determine the efficacy and safety of immunotherapy.
Palliative Care
The incorporation of palliative care early following the diagnosis of cancer has been shown to improve quality of life, decrease depression, and help with symptom management.107 The triggers for geriatric patients to initiate palliative care may be different from those of younger patients, as older patients may have different goals of care.108 Older patients will often choose quality over quantity of life when making treatment decisions.109 The ideal medical treatment for the frail patient with colorectal cancer would focus on treating disease while providing palliative measures to help support the patient and improve quality of life. It is paramount that patients maintain functional independence as loss of independence is recognized as a major threat to an older patient’s quality of life.110 The optimal way to achieve these goals is through the efforts of a multidisciplinary care team including not only physicians and nurses, but also social workers, nutritionists, physical therapists, and family who can provide support for the patient’s psychosocial, cognitive, and medical needs.111 Although cancer and noncancer–related death occur more frequently in the geriatric population, data to guide a specific palliative care approach to the elderly population is lacking.108
Conclusion
Colorectal cancer is a disease of older adults with a median age at diagnosis of 67 years.1 With the aging population, oncologists will be faced with treating increasing numbers of older patients, and must adjust their practice to accommodate this population of patients. Treating geriatric patients is challenging given the lack of available data to guide the treatment approach. Although several prospective elderly-specific studies have been conducted evaluating treatments for metastatic CRC, most treatment decisions are made based on the available retrospective studies and pooled analyses. Oncologists must carefully consider and evaluate each patient based on physiologic age rather than chronologic age.112 Overall, older patients should be given the opportunity to receive standard of care treatments in the appropriate setting. The decision to modify treatment plans should be made after a thorough evaluation by a multidisciplinary team and a discussion with the patient regarding their goals and the risks and benefits of the treatment. Geriatric assessment tools can help the care team identify patients with various geriatric syndromes that may not be detected on routine oncology evaluation. This type of evaluation is time consuming and is rarely done in a busy oncology practice. Ongoing studies are aiming to develop a method to incorporate geriatric assessments into the care of older adults.Additional prospective trials targeting older, more frail patients are essential to improve upon our knowledge so we can provide best care for this growing elderly population.
1. National Cancer Institute. SEER cancer stat facts: colorectal cancer. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed March 1, 2018.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
3. Kochanek KD, Murphy S, Xu J, Arias E. Mortality in the United States, 2016. NCHS Data Brief 2017:1-8.
4. Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States, current population reports, P25-1140. Washington, DC: U.S. Census Bureau; 2014.
5. Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061–7.
6. Unger JM, Coltman CA Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol 2006;24:141–4.
7. Vijayvergia N, Li T, Wong YN, et al. Chemotherapy use and adoption of new agents is affected by age and comorbidities in patients with metastatic colorectal cancer. Cancer 2016;122:3191–8.
8. Kalsi T, Babic-Illman G, Ross PJ, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br J Cancer 2015;112:1435–44.
9. National Comprehensive Cancer Network. Older adult oncology (Version 2.2017). Accessed March 1, 2018,
10. Balducci L. Frailty: a common pathway in aging and cancer. Interdiscip Top Gerontol 2013;38:61–72.
11. Baijal P, Periyakoil V. Understanding frailty in cancer patients. Cancer J 2014;20:358–66.
12. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55.
13. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457–65.
14. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86.
15. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970;10:20–30.
16. Klepin HD, Geiger AM, Tooze JA, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc 2010;58:76–82.
17. Gupta SK, Lamont EB. Patterns of presentation, diagnosis, and treatment in older patients with colon cancer and comorbid dementia. J Am Geriatr Soc 2004;52:1681–7.
18. Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–7.
19. Aaldriks AA, van der Geest LG, Giltay EJ, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol 2013;4:218–26.
20. Martucci RB, Barbosa MV, D’Almeida CA, et al. Undernutrition as independent predictor of early mortality in elderly cancer patients. Nutrition 2017;34:65–70.
21. Naeim A, Aapro M, Subbarao R, Balducci L. Supportive care considerations for older adults with cancer. J Clin Oncol 2014;32:2627–34.
22. Kua J. The prevalence of psychological and psychiatric sequelae of cancer in the elderly - how much do we know? Ann Acad Med Singapore 2005;34:250–6.
23. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012;118:3377–86.
24. Kim J, Hurria A. Determining chemotherapy tolerance in older patients with cancer. J Natl Compr Canc Netw 2013;11:1494-502.
25. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol 2015;26:288–300.
26. Soubeyran P, Bellera C, Goyard J, et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One 2014; 9:e115060.
27. Gurevitch AJ, Davidovitch B, Kashtan H. Outcome of right colectomy for cancer in octogenarians. J Gastrointest Surg 2009;13:100–4.
28. Schiffmann L, Ozcan S, Schwarz F, et al. Colorectal cancer in the elderly: surgical treatment and long-term survival. Int J Colorectal Dis 2008;23:601–10.
29. Ong ES, Alassas M, Dunn KB, Rajput A. Colorectal cancer surgery in the elderly: acceptable morbidity? Am J Surg 2008;195:344–8.
30. Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet 2000;356:968–74.
31. Shalaby M, Di Lorenzo N, Franceschilli L, et al. Outcome of colorectal surgery in elderly populations. Ann Coloproctol 2016;32:139–43.
32. Frasson M, Braga M, Vignali A, et al. Benefits of laparoscopic colorectal resection are more pronounced in elderly patients. Dis Colon Rectum 2008;51:296–300.
33. PACE participants, Audisio RA, Pope D, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol 2008;65:156–63.
34. Adam R, Frilling A, Elias D, et al. Liver resection of colorectal metastases in elderly patients. Br J Surg 2010;97:366–76.
35. de Liguori Carino N, van Leeuwen BL, Ghaneh P, et al. Liver resection for colorectal liver metastases in older patients. Crit Rev Oncol Hematol 2008;67:273–8.
36. Tamandl D, Gruenberger B, Herberger B, et al. Surgery after neoadjuvant chemotherapy for colorectal liver metastases is safe and feasible in elderly patients. J Surg Oncol 2009;100:364–71.
37. Shahir MA, Lemmens VE, van de Poll-Franse LV, et al. Elderly patients with rectal cancer have a higher risk of treatment-related complications and a poorer prognosis than younger patients: a population-based study. Eur J Cancer 2006;42:3015–21.
38. Chang GJ, Skibber JM, Feig BW, Rodriguez-Bigas M. Are we undertreating rectal cancer in the elderly? An epidemiologic study. Ann Surg 2007;246:215–21.
39. Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001;358:1291–304.
40. Martling A, Holm T, Johansson H, et al, Stockholm Colorectal Cancer Study Group. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: long-term follow-up of a population-based study. Cancer 2001;92:896–902.
41. Pasetto LM, Friso ML, Pucciarelli S, et al. Rectal cancer neoadjuvant treatment in elderly patients. Anticancer Res 2006;26:3913–23.
42. Margalit DN, Mamon HJ, Ancukiewicz M, et al. Tolerability of combined modality therapy for rectal cancer in elderly patients aged 75 years and older. Int J Radiat Oncol Biol Phys 2011;81:e735–41.
43. Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:501–13.
44. van der Valk M. The International Watch & Wait database (IWWD) for rectal cancer: An update. J Clin Oncol 2017;35 suppl:521.
45. Donato V, Valeriani M, Zurlo A. Short course radiation therapy for elderly cancer patients. Evidences from the literature review. Crit Rev Oncol Hematol 2003;45:305–11.
46. Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827–33.
47. McCleary NJ, Dotan E, Browner I. Refining the chemotherapy approach for older patients with colon cancer. J Clin Oncol 2014;32:2570–80.
48. Millan M, Merino S, Caro A, et al. Treatment of colorectal cancer in the elderly. World J Gastrointest Oncol 2015;7:204–20.
49. Quasar Collaborative Group, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020–9.
50. Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16.
51. Tournigand C, Andre T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353–60.
52. Winder T, Lenz HJ. Molecular predictive and prognostic markers in colon cancer. Cancer Treat Rev 2010;36:550–6.
53. Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–57.
54. Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77.
55. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–18.
56. Aparicio T, Schischmanoff O, Poupardin C, et al. Deficient mismatch repair phenotype is a prognostic factor for colorectal cancer in elderly patients. Dig Liver Dis 2013;45:245–50.
57. Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091–7.
58. Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
59. Twelves C, Scheithauer W, McKendrick J, et al. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol 2012;23:1190–7.
60. Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–74.
61. Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71.
62. Haller DG, Cassidy J, Tabernero J, et al. Efficacy findings from a randomized phase III trial of capecitabine plus oxaliplatin versus bolus 5-FU/LV for stage III colon cancer (NO16968): impact of age on disease-free survival (DFS) [abstract]. J Clin Oncol 2010;28:3521.
63. Schmoll HJ, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 2015;33:3733–40.
64. McCleary NJ, Meyerhardt JA, Green E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol 2013;31:2600–6.
65. Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA 2010;303:1037–45.
66. Haller DG, O’Connell MJ, Cartwright TH, et al. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials. Ann Oncol 2015;26:715-24.
67. Aparicio T, Francois E, Cristol-Dalstein L, et al. PRODIGE 34-FFCD 1402-ADAGE: Adjuvant chemotherapy in elderly patients with resected stage III colon cancer: A randomized phase 3 trial. Dig Liver Dis 2016;48:206–7.
68. Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797–806.
69. Mahoney T, Kuo YH, Topilow A, Davis JM. Stage III colon cancers: why adjuvant chemotherapy is not offered to elderly patients. Arch Surg 2000;135:182–5.
70. Sanoff HK, Carpenter WR, Sturmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol 2012;30:2624–34.
71. Folprecht G, Cunningham D, Ross P, et al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Ann Oncol 2004;15:1330–8.
72. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii1–9.
73. Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 2006;24:4085–91.
74. Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet 2011;377:1749–59.
75. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol 2006;24:394–400.
76. Figer A, Perez-Staub N, Carola E, et al. FOLFOX in patients aged between 76 and 80 years with metastatic colorectal cancer: an exploratory cohort of the OPTIMOX1 study. Cancer 2007;110:2666–71.
77. Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol 2009;27:5727–33.
78. Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol 2008;26:1443–51.
79. Souglakos J, Pallis A, Kakolyris S, et al. Combination of irinotecan (CPT-11) plus 5-fluorouracil and leucovorin (FOLFIRI regimen) as first line treatment for elderly patients with metastatic colorectal cancer: a phase II trial. Oncology 2005;69:384–90.
80. Aparicio T, Lavau-Denes S, Phelip JM, et al. Randomized phase III trial in elderly patients comparing LV5FU2 with or without irinotecan for first-line treatment of metastatic colorectal cancer (FFCD 2001-02). Ann Oncol 2016;27:121–7.
81. Aparicio T, Gargot D, Teillet L, et al. Geriatric factors analyses from FFCD 2001-02 phase III study of first-line chemotherapy for elderly metastatic colorectal cancer patients. Eur J Cancer 2017;74:98–108.
82. Kabbinavar FF, Hurwitz HI, Yi J, et al. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol 2009;27:199–205.
83. Cassidy J, Saltz LB, Giantonio BJ, et al. Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol 2010;136:737–43.
84. Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 2009;20:1842–7.
85. Kozloff MF, Berlin J, Flynn PJ, et al. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: results from the BRiTE observational cohort study. Oncology 2010;78:329–39.
86. Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol 2013;14:1077–85.
87. Aparicio T, Bouche O, Taieb J, et al. Bevacizumab+chemotherapy versus chemotherapy alone in elderly patients with untreated metastatic colorectal cancer: a randomized phase II trial-PRODIGE 20 study results. Ann Oncol 2018;29:133–8.
88. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499–506.
89. Ruff P, Van Cutsem E, Lakomy R, et al. Observed benefit and safety of aflibercept in elderly patients with metastatic colorectal cancer: An age-based analysis from the randomized placebo-controlled phase III VELOUR trial. J Geriatr Oncol 2018;9:32–9.
90. Obermannova R, Van Cutsem E, Yoshino T, et al. Subgroup analysis in RAISE: a randomized, double-blind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression. Ann Oncol 2016;27:2082–90.
91. Bouchahda M, Macarulla T, Spano JP, et al. Cetuximab efficacy and safety in a retrospective cohort of elderly patients with heavily pretreated metastatic colorectal cancer. Crit Rev Oncol Hematol 2008;67:255-62.
92. Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346–55.
93. Sastre J, Gravalos C, Rivera F, et al. First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study. Oncologist 2012;17:339–45.
94. Dotan E, Devarajan K, D’Silva AJ, et al. Patterns of use and tolerance of anti-epidermal growth factor receptor antibodies in older adults with metastatic colorectal cancer. Clin Colorectal Cancer 2014;13:192–8.
95. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12.
96. Van Cutsem E, Sobrero A, Siena S, et al. Regorafenib (REG) in progressive metastatic colorectal cancer (mCRC): Analysis of age subgroups in the phase III CORRECT trial [abstract]. J Clin Oncol 2013;31(15 suppl):3636-3636.
97. Bekaii-Saab TS, Ou FS, Anderson DM, et al. Regorafenib dose optimization study (ReDOS): Randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC): an ACCRU Network study [abstract]. J Clin Oncol 2018;36(4 suppl):611-611.
98. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19.
99. Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer 2009;100:266–73.
100. Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014;20:5322–30.
101. Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst 2013;105:1151–6.
102. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20.
103. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91.
104. Daste A, Domblides C, Gross-Goupil M, et al. Immune checkpoint inhibitors and elderly people: A review. Eur J Cancer 2017;82:155–66.
105. Elias R, Giobbie-Hurder A, McCleary NJ, et al. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer 2018;6:26.
106. Singh H, Kim G, Maher VE, et al. FDA subset analysis of the safety of nivolumab in elderly patients with advanced cancers [abstract]. J Clin Oncol 2016;34(15 suppl):10010-10010.
107. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol 2017;35:834–41.
108. Brighi N, Balducci L, Biasco G. Cancer in the elderly: is it time for palliative care in geriatric oncology? J Geriatr Oncol 2014;5:197–203.
109. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer 2008;113:3459–66.
110. Bagshaw SM, Stelfox HT, Johnson JA, et al. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med 2015;43:973–82.
111. Lynch MP, Marcone D, Kagan SH. Developing a multidisciplinary geriatric oncology program in a community cancer center. Clin J Oncol Nurs 2007;11:929–33.
112. Sheridan J, Walsh P, Kevans D, et al. Determinants of short- and long-term survival from colorectal cancer in very elderly patients. J Geriatr Oncol 2014;5:376–83.
Introduction
Colorectal cancer (CRC) is the fourth most common cancer in the United States and has a high prevalence among the older population.1 In 2017, there were an estimated 135,430 new cases of CRC and 50,260 deaths due to CRC. It is the second leading cause of cancer death in the United States, and the death rate for patients with CRC increases with age (Figure).2
Although elderly persons are more frequently diagnosed with CRC, they are underrepresented in clinical trials. This may be due in part to stringent eligibility criteria in prospective randomized controlled trials that exclude older patients with certain comorbidities and decreased functional status. Hutchins and colleagues compared the proportion of persons aged 65 years and older enrolled in Southwest Oncology Group (SWOG) clinical trials and the proportion of persons in this age group in the US population with the same cancer diagnoses.5 They found that while 72% of the US population with CRC were aged ≥ 65 years, persons in this age group comprised only 40% of patients enrolled in SWOG trials. An update on this study performed after Medicare policy changed in 2000 to include coverage of costs incurred due to clinical trials showed an upward trend in the accrual of older patients in SWOG trials, from 25% during the period 1993–1996 to 38% during the period 2001–2003; however, the percentage of older patients with CRC on clinical trials overall remained stable from 1993 to 2003.6
The underrepresentation of older adults with CRC in clinical trials presents oncologists with a challenging task when practicing evidence-based medicine in this patient population. Analysis of a large claims database demonstrated that the use of multi-agent chemotherapy for the treatment of metastatic CRC in older adults increased over time, while the use of single-agent 5-fluorouracil (5-FU) decreased.7 However, the adoption of combination therapy with irinotecan or oxaliplatin in older adults lagged behind the initial adoption of these agents in younger patients. This data demonstrates that as the field of medical oncology evolves, providers are becoming more comfortable treating older patients with multiple medical problems using standard approved regimens.
Geriatric Assessment
Before treating older patients with cancer, it is necessary to define the patient’s physiological age, ideally through a multidisciplinary team evaluation.
The Eastern Cooperative Oncology Group performance status (ECOG PS) and Karnofsky Performance Status (KPS) are crude measures of functional status.12 Generally, elderly patients with good ECOG PS or KPS scores are considered fit enough to receive standard therapy similar to their younger counterparts. Evaluation of functional status using these performance scores is often suboptimal, resulting in patients with a normal or adequate performance status score who may still experience poor outcomes, including decreased survival and inability to tolerate treatment. A study that explored parameters among older patients that predict for increased risk of chemotherapy-related toxicities found that physician-rated KPS score did not accurately predict the risk for adverse events.13 Therefore, a CGA represents a better way to evaluate functional status and other domains.
Functional status can also be evaluated by self-reported tools such as activities of daily living, which refer to basic self-care, and instrumental activities of daily living (IADLs), which are essential for independent living in the community.14,15 Mobility, gait, and balance can also be measured using the “Timed Get Up and Go” test and gait speed. Klepin et al found that faster gait speed was associated with overall survival (OS) in patients with metastatic cancer.16
Cognitive function is an important component of the geriatric assessment in older patients with cancer, as dementia is a prognostic factor for survival in the overall geriatric population. In a retrospective review, patients with dementia were less likely to have a biopsy-proven diagnosis and were twice as likely to have their CRC diagnosed postmortem.17 In addition, establishing that the patient has intact cognitive function prior to initiating treatment is essential to ensure that the patient can comply with treatment and understands when to report adverse effects. Nutritional status is an important portion of the geriatric assessment because malnutrition is associated with increased mortality and decreased tolerance for chemotherapy.18–20 Evaluating the patient’s psychosocial support is crucial as well because older patients are at greater risk of social isolation and depression.21 While the incidence of depression is lower in older adults with cancer than in younger adults with cancer, clinically significant depression is still noted in 3% to 25% of elderly cancer patients.22 Other critical components of the CGA are review of the patient’s comorbidities and medications to avoid complications of polypharmacy.
Both the Cancer and Aging Research Group (CARG) and Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) toxicity tools are valuable tools, as they predict chemotherapy tolerance in elderly patients.13,23 These tools can help guide discussions between oncologists and patients as well as the formulation of an appropriate treatment plan.24 Although toxicity tools can help to determine which patients are at risk for severe toxicity secondary to treatment, these tools do not replace the CGA. A prospective cohort study that evaluated the impact of CGA on tolerance to chemotherapy in older patients with cancer compared patients aged ≥ 70 years at the start of their treatment with chemotherapy (± radiation therapy) using geriatrician-delivered CGA versus standard care given by oncology.8 Patients who received geriatrician-guided CGA interventions tolerated chemotherapy better and completed treatments as planned (odds ratio 4.14 [95% confidence interval {CI} 1.50 to 11.42], P = 0.006) with fewer treatment modifications.
Unfortunately, the CGA is time-consuming to administer and difficult to incorporate into a busy oncology practice. Therefore, other screening models are used to identify patients who may benefit from a full CGA. The International Society of Geriatric Oncology performed a systematic review of screening tools used to identify older cancer patients in need of geriatric assessment and found that the 3 most studied screening tools are the G8, the Vulnerable Elders Survey-13 (VES-13), and the Flemish version of the Triage Risk Screening Tool.25 Another study found that the G8 was more sensitive than the VES-13 (76.5% versus 68.7%, P = 0.0046), whereas the VES-13 was more specific than the G8 (74.3% versus 64.4%, P < 0.0001).26 In addition to providing guidance to initiate a full geriatric assessment, these screening tools may assist in decision making for older cancer patients, especially those with advanced disease.
Surgery
Early-Stage Disease
When possible, surgical resection of colorectal tumors is the primary treatment in both the curative setting and to avoid complications, such as obstruction or perforation.27 Multiple studies have shown that fit elderly patients benefit from curative surgery similarly to their younger counterparts.27–29 With the growing population of persons aged 65 years or older, surgeons are becoming more comfortable with operating on the elderly.4 However, a large systematic review of 28 independent studies with a total of 34,194 patients showed that older patients were less likely to undergo curative surgery.30 Eligibility for surgery should not be determined by age alone, but rather should be based on a full assessment of the patient’s health, including comorbidities, functional status, nutrition, cognition, social support, and psychological status. The impact of age on short-term outcomes after colorectal surgery in terms of 30-day postoperative morbidity and mortality rates was explored in a study that divided patients into 2 groups: those aged ≥ 80 years (mean age 85) and those aged < 80 years (mean age 55.3).31 There were no statistical differences in 30-day postoperative morbidity and mortality rates between the 2 groups, and preexisting comorbidities and urgent nature of surgery were important predictors of colorectal surgery outcomes in the older adults, results that have been seen in several other studies.28,30 When possible, laparoscopic surgery is preferred as it is associated with less intraoperative blood loss, less postoperative pain, reduced postoperative ileus, a shorter hospital stay, and fewer cardiovascular and pulmonary complications.32 The Preoperative Assessment of Cancer in the Elderly (PACE), which combines surgical risk assessment tools with CGA tools, can assist surgeons in determining candidacy for surgery and help decrease unequal access to surgery in the geriatric population.33
Metastasectomy
A large international multicenter cohort study explored the outcomes of patients aged ≥ 70 years who underwent liver resection of colorectal metastases. The study investigatorsfound that neoadjuvant chemotherapy was used less frequently and less extensive surgery was performed in elderly patients than in younger patients.34 Sixty-day postoperative mortality was slightly higher (3.8% versus 1.6%, P < 0.001) and 3-year OS was slightly lower (57.1% versus 60.2%, P < 0.001) in the elderly group as compared to their younger counterparts, but overall the outcomes after liver surgery were similar. Therefore, the management of liver metastases in oligometastatic disease in elderly patients fit for surgery should be the same as that offered to younger patients. Since outcomes are comparable, older patients should be offered neoadjuvant chemotherapy, as several studies have shown similar response rates and OS in younger and older patients.35,36
Rectal Cancer
The standard of care for locally advanced rectal cancer is combined modality treatment with radiation and chemotherapy followed by total mesorectal excision. However, given conflicting data regarding the ability of elderly patients to tolerate neoadjuvant 5-FU-based chemotherapy and radiation, elderly patients are treated with trimodality therapy less often than their younger counterparts.37,38 A systematic review of 22 randomized trials involving 8507 patients with rectal cancer showed that adjuvant radiation therapy could reduce the risk of local recurrence and death from rectal cancer in patients of all ages.39 However, the risk of noncancer-related death was increased in the older population. The Stockholm II trial showed similar benefits of preoperative radiation overall, but this benefit did not extend to patients older than 68 years because of an increased risk of morbidity and mortality.40 In older patients, mortality from noncancer causes within the first 6 months after surgery was higher in the group that received perioperative radiation than in the group that did not receive radiation. Elderly patients (age > 68 years) accounted for most of the mortality, which was predominantly due to cardiovascular disease.
A retrospective study of 36 patients aged ≥ 70 years with rectal cancer evaluated the toxicity and feasibility of neoadjuvant 5-FU combined with pelvic radiation for treating locally advanced rectal cancer. Patients were classified as healthy and “fit” or “vulnerable” based on the presence of comorbidities.41 This study demonstrated that tolerability and response to neoadjuvant chemotherapy and radiation as well as ability to undergo surgery were similar in “vulnerable” patients and “fit” patients. Conversely, Margalit and colleagues studied the rate of treatment deviations in elderly patients with rectal cancer treated with combined modality therapy and found that most patients required early termination of treatment, treatment interruptions, or dose reductions.42 While trimodality treatment is the standard of care in rectal cancer, there is conflicting data from retrospective studies regarding the tolerability and feasibility of this approach. It is important to proceed with caution but to still consider fit older patients with locally advanced rectal cancer for neoadjuvant chemotherapy and radiation followed by surgery.
In patients who have a complete response (CR) to neoadjuvant chemoradiation, watchful waiting rather than proceeding to surgery may be a reasonable strategy, especially in older patients. A systematic review of 867 patients with locally advanced rectal cancer showed no statistically significant difference in OS between patients who were observed with watchful waiting and those who underwent surgery.43 The International Watch and Wait Database includes 679 patients who were managed with a watch-and-wait regimen because they had a clinical CR after chemoradiation. An outcomes analysis of these patients showed that 25% had local regrowth, with 3-year OS of 91% overall and 87% in patients with local regrowth.44 In most patients (84%), regrowth of the tumor occurred within the first 2 years of follow up.
In frail older adults, for whom longer courses of treatment are not feasible or chemotherapy is contraindicated, short-course radiation therapy can be considered either in the neoadjuvant setting or alone for palliation.45 A randomized trial of short-course radiation versus long-course chemoradiation in patients with T3 rectal cancer found that the difference in 3-year local recurrence rates was not statistically significant.46
Chemotherapy
An expected natural decline in function occurs with age, but given the great variability that exists between patients, it is important to focus on physiologic age rather than chronologic age to determine ability to receive and tolerate anticancer treatment. Decreases in renal and hepatic function, cognitive impairment, changes in gastrointestinal motility, decrements in cardiac and bone marrow reserves, as well as comorbidities and polypharmacy affect a patient’s ability to tolerate chemotherapy.47,48 Toxicity tools such as CARG and CRASH can help to predict severity of toxicity with chemotherapy.13,23 The information provided by these tools can help guide conversations between the oncologist and patient regarding treatment plans.
Adjuvant Chemotherapy for Early-Stage Disease
Stage II Disease
Defining treatment guidelines for older patients with stage II colon cancer is difficult due to lack of data that shows benefit in this population. The QUASAR (Quick and Simple and Reliable) group’s prospective study of adjuvant single-agent 5-FU in stage II colon cancer patients showed an absolute improvement in survival of 3.6% when 5-FU was given after surgery (95% CI 1.0 to 6.0).49 The subgroup analysis of patients aged ≥ 70 years showed a limited benefit of adjuvant 5-FU (hazard ratio [HR] 1.13 [95% CI 0.74 to 1.75]). Given the limited benefit, adjuvant 5-FU for elderly patients with stage II colon cancer should be used judiciously as patients may have competing causes of morbidity or mortality.
The use of oxaliplatin-based therapy in the adjuvant setting for stage II disease was evaluated in a subgroup analysis of the MOSAIC study (Multicenter International Study of Oxaliplatin/5-FU/Leucovorin in the Adjuvant Treatment of Colon Cancer).50 Adjuvant oxaliplatin-based treatment may be offered to patients with stage II colon cancer that carries high-risk features (poorly differentiated histology, lymphovascular invasion, bowel obstruction and/or perforation, < 12 lymph nodes sampled, perineural invasion, or indeterminate or positive margins) due to a trend toward improved disease-free survival (DFS) at 5 years. Patients in this group who received adjuvant FOLFOX (leucovorin, oxaliplatin, 5-FU) versus 5-FU/leucovorin had a DFS of 82.3% versus 74.6%, respectively (HR 0.72 [95% CI 0.50 to 1.02]), a difference that was not statistically significant. A subgroup analysis of 315 patients aged 70 to 75 years with stage II colon cancer enrolled in the MOSAIC study found no statistically significant DFS or OS benefit with the addition of oxaliplatin to 5-FU/leucovorin.51 Therefore, use of this platinum/fluoropyrimidine combination for adjuvant therapy for high-risk stage II disease in older patients remains controversial given its associated risks and the lack of definitive data demonstrating a benefit in this patient group. Decisions regarding this therapy should be made through a shared discussion with patients about its risks and benefits.
Microsatellite status is an important biomarker in the evaluation of stage II CRC. Microsatellite stability is a marker of a functioning DNA mismatch repair system. In patients with colon cancer, tumor microsatellite stability is classified based on the percentage of abnormal microsatellite regions.52 Several studies have shown that patients with tumors that display high microsatellite instability (MSI-H) have an improved prognosis over patients with microsatellite stable tumors.53,54 While patients with stage II MSI-H colon cancer have better outcomes, MSI is associated with a reduced response to treatment with fluoropyrimidines, as demonstrated in a systematic review that found that patients with tumors with MSI obtained no benefit from adjuvant 5-FU (HR 1.24 [95% CI 0.72 to 2.14]).55 Aparicio and colleagues reported an increased prevalence of MSI-H tumors with increasing age.56 Therefore, mismatch repair phenotype should be considered when making adjuvant chemotherapy decisions in the older adult with colon cancer, as it may affect the decision to recommend single-agent 5-FU treatment.
Stage III Disease
The use of single-agent 5-FU for stage III resected CRC has been evaluated in multiple studies. Sargent et al performed a pooled analysis of 3351 patients from 7 randomized phase 3 trials comparing surgery and adjuvant 5-FU-based chemotherapy versus surgery alone in stage II or III colon cancer patients.57 Adjuvant chemotherapy was associated with improvement in both OS and time to tumor recurrence (HR 0.76 and 0.68, respectively). The 5-year OS was 71% for those who received adjuvant treatment and 64% for those who were treated with surgery alone. The benefit of adjuvant treatment was independent of age, and there was no difference in toxicity across age groups, except for 1 study which showed increased rates of leukopenia in the elderly. The oral fluoropyrimidine capecitabine was shown to be an effective alternative to 5-FU plus leucovorin as adjuvant treatment for those with resected stage III colon cancer.58 However, in the subgroup analysis of DFS in the intention-to-treat group, the improvement in DFS was not statistically significant in those aged ≥ 70 years. This study justified the phase 3 Xeloda in Adjuvant Colon Cancer Therapy (X-ACT) trial, which compared capecitabine and 5-FU/leucovorin as adjuvant therapy in patients with resected stage III colon cancer.59 The X-ACT trial showed no significant effect of age on DFS or OS.
The addition of oxaliplatin to 5-FU in the adjuvant setting for stage III tumors has been studied and debated in the elderly population in multiple trials. The MOSAIC trial investigated FOLFOX versus 5-FU/leucovorin in the adjuvant setting.50 The addition of oxaliplatin was associated with a DFS and OS benefit, with a 20% reduction in risk of colon cancer recurrence and 16% reduction in risk of death in all patients. The National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trial then studied 2409 patients with stage II or III colon cancer treated with weekly bolus 5-FU/leucovorin with or without oxaliplatin.60 In this study, OS was significantly improved with the addition of oxaliplatin in patients younger than 70 years, but OS at 5 years was 4.7% worse for patients aged ≥ 70 years treated with weekly 5-FU/leucovorin and oxaliplatin compared with those treated with weekly 5-FU/leucovorin (71.6% versus 76.3%, respectively). In contrast, the XELOXA trial (NO16968), which randomly assigned stage III colon cancer patients to capecitabine and oxaliplatin (XELOX) or bolus 5-FU/leucovorin (standard of care at study start), showed an efficacy benefit, albeit not statistically significant, in patients aged ≥ 70 years (HR 0.87 [95% CI 0.63 to 1.18]).61–63
The Adjuvant Colon Cancer Endpoints (ACCENT) database included 7 randomized trials totaling 14,528 patients with stage II or III colon cancer treated with adjuvant 5-FU with or without oxaliplatin or irinotecan.64 Subgroup analysis of patients aged ≥ 70 years (n = 2575) showed no benefit with an oxaliplatin-based regimen in DFS (HR 0.94 [95% CI 0.78 to 1.13]) or OS (HR 1.04 [95% CI, 0.85 to 1.27]). Based on these studies and the increased toxicity with oxaliplatin, oxaliplatin-based adjuvant chemotherapy is utilized less often than single-agent 5-FU in geriatric patients with early-stage colon cancer.65 Conversely, a recent pooled analysis of individual patient data from 4 randomized trials (NSABP C-08, XELOXA, X-ACT, and AVANT) showed improved DFS and OS with adjuvant XELOX or FOLFOX over single-agent 5-FU in patients aged ≥ 70 years (DFS HR 0.77 [95% CI 0.62 to 0.95], P = 0.014; OS HR 0.78 [95% CI 0.61 to 0.99], P = 0.045).66 This analysis also showed that grade 3 and 4 adverse events related to oxaliplatin were similar across age groups.
These data come from post-hoc analyses, and there is no prospective data to steer decision making in elderly patients with early-stage CRC (Table).
It is well established that patients with stage III colon cancer benefit from oxaliplatin-based adjuvant chemotherapy after curative surgical resection.68 However, older patients are less likely to be referred to oncology as compared with their younger counterparts, due to the conflicting data regarding the benefit of this approach in older adults. Studies have shown that when the referral is placed, the geriatric population is less likely to receive chemotherapy.69 Sanoff et al analyzed 4 data sets (SEER-Medicare, National Comprehensive Cancer Network, New York State Cancer Registry, and Cancer Care Outcomes Research and Surveillance Consortium) to assess the benefit of adjuvant chemotherapy for resected stage III CRC among patients aged ≥ 75 years. Their analysis showed that only 40% of patients evaluated received adjuvant chemotherapy for stage III CRC after surgical resection.70
Summary
Prospective data to guide the treatment of older patients with early-stage CRC in the adjuvant setting is lacking. For fit older patients with stage II disease, limited benefit will be derived from single-agent 5-FU. For those with stage III CRC, the benefit and toxicities of fluoropyrimidines as adjuvant therapy appear to be similar regardless of age. The addition of oxaliplatin to fluoropyrimidines in patients aged ≥ 70 years has not been proven to improve DFS or OS and could result in an incremental toxicity profile. Therefore, treatment plans must be individualized, and decisions should be made through an informed discussion evaluating the overall risk/benefit ratio of each approach.
Metastatic Disease
Palliative Chemotherapy
Approximately 20% of patients with CRC are diagnosed with metastatic disease at presentation, and 35% to 40% develop metastatic disease following surgery and adjuvant therapy.2 The mainstay of treatment in this population is systemic therapy in the form of chemotherapy with or without biologic agents. In this setting, several prospective studies specific to older adults have been completed, providing more evidence-based guidance to oncologists who see these patients. Folprecht et al retrospectively reviewed data from 22 clinical trials evaluating 5-FU-based palliative chemotherapy in 3825 patients with metastatic CRC, including 629 patients aged ≥ 70 years.71 OS in elderly patients (10.8 months [95% CI 9.7 to 11.8]) was equivalent to that in younger patients (11.3 months [95% CI 10.9 to 11.7], P = 0.31). Similarly, relative risk and progression-free survival (PFS) were comparable irrespective of age.
Standard of care for most patients with metastatic colon cancer consists of 5-FU/leucovorin in combination with either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) with a monoclonal antibody.72 A retrospective pooled analysis of patients with metastatic CRC compared the safety and efficacy of FOLFOX4 in patients aged < 70 years versus those aged ≥ 70 years.73 While age ≥ 70 years was associated with an increased rate of grade ≥ 3 hematologic toxicity, it was not associated with increased rates of severe neurologic events, diarrhea, nausea, vomiting, infection, 60-day mortality, or overall incidence of grade ≥ 3 toxicity. The benefit of treatment was consistent across both age groups; therefore, age alone should not exclude an otherwise healthy individual from receiving FOLFOX.
These post-hoc analyses show that fit older patients who were candidates for trial participation tolerated these treatments well; however, these treatments may be more challenging for less fit older adults. The UK Medical Research Council FOCUS2 (Fluorouracil, Oxaliplatin, CPT11 [irinotecan]: Use and Sequencing) study was a prospective phase 3 trial that included 459 patients with metastatic CRC who were deemed too frail or not fit enough for standard-dose chemotherapy by their oncologists.74 In this group, 43% of patients were older than 75 years and 13% were older than 80 years. Patients were randomly assigned to receive infusional 5-FU with levofolinate; oxaliplatin and 5-FU; capecitabine; or oxaliplatin and capecitabine; all regimens were initiated with an empiric 20% dose reduction. The addition of oxaliplatin suggested some improvement in PFS, but this was not significant (5.8 months versus 4.5 months, HR 0.84 [95% CI 0.69 to 1.01], P = 0.07). Oxaliplatin was not associated with increased grade 3 or 4 toxicities. Capecitabine is often viewed as less toxic because it is taken by mouth, but this study found that replacement of 5-FU with capecitabine did not improve quality of life. Grade 3 or 4 toxicities were seen more frequently in those receiving capecitabine than in those receiving 5-FU (40% versus 30%, P = 0.03) in this older and frailer group of patients. As the patients on this study were frail and treatment dose was reduced, this data may not apply to fit older adults who are candidates for standard therapy.
When managing an older patient with metastatic CRC, it is important to tailor therapy based on goals of care, toxicity of proposed treatment, other comorbidities, and the patient’s functional status. One approach to minimizing toxicity in the older population is the stop-and-go strategy. The OPTIMOX1 study showed that stopping oxaliplatin after 6 cycles of FOLFOX7 and continuing maintenance therapy with infusional 5-FU/leucovorin alone for 12 cycles prior to reintroducing FOLFOX7 achieved efficacy similar to continuous FOLFOX4 with decreased toxicity.75 Figer et al studied an exploratory cohort of 37 patients aged 76 to 80 years who were included in the OPTIMOX1 study.76 The overall relative risk, median PFS, and median OS did not differ between the older patients in this cohort and younger patients studied in the original study. Older patients did experience more neutropenia, neurotoxicity, and overall grade 3 to 4 toxicity, but there were no toxic deaths in patients older than 75 years. The approach of giving treatment breaks, as in OPTIMOX2, may also provide patients with better quality of life, but perhaps at the expense of cancer-related survival.77
The combination of irinotecan and 5-FU has also been studied as treatment for patients with metastatic CRC. A pooled analysis of 2691 patients aged ≥ 70 years with metastatic CRC across 4 phase 3 randomized trials investigating irinotecan and 5-FU demonstrated that irinotecan-containing chemotherapy provided similar benefits to both older and younger patients with similar risk of toxicity.78 A phase 2 trial studying FOLFIRI as first-line treatment in older metastatic CRC patients showed this to be a safe and active regimen with manageable toxicity.79 Another randomized phase 3 trial for older patients compared 5-FU/leucovorin with or without irinotecan for first-line treatment of metastatic CRC (FFCD 2001-02).80 The study accrued 282 patients aged ≥ 75 years (median age 80 years), and found that the addition of irinotecan to infusional 5-FU–based chemotherapy did not significantly increase either PFS or OS. Aparicio et al performed a substudy of baseline geriatric evaluation prior to treatment in the FFCD 2001-02 study and assessed the value of geriatric parameters for predicting outcomes (objective response rate [ORR], PFS, and OS).81 Multivariate analysis showed that none of the geriatric parameters were predictive of ORR or PFS but that normal IADL was associated with better OS. This combination may still be appropriate for some older patients with metastatic disease, while single- agent 5-FU may be more appropriate in frail patients.
Biologic Agents
VEGF Inhibitors
Targeted biologic agents have been studied in the treatment of metastatic CRC. Bevacizumab is a recombinant, humanized monoclonal antibody against vascular endothelial growth factor (VEGF) that is approved in the first-line setting for treatment of metastatic CRC. A pooled analysis examined 439 patients 65 years of age and older with metastatic CRC who received bevacizumab plus chemotherapy versus placebo plus chemotherapy.82 In this analysis, the addition of bevacizumab was associated with an improvement in OS (19.3 months versus 14.3 months, HR 0.7 [95% CI 0.55 to 0.90], P = 0.006) and in PFS (9.2 months versus 6.2 months, HR 0.52 [95% CI 0.40 to 0.67], P < 0.0001). Known adverse events associated with bevacizumab were seen in the bevacizumab plus chemotherapy group but not at increased rates in the older population compared to their younger counterparts. Conversely, another pooled analysis found that while there was a PFS and OS benefit in older patients receiving bevacizumab, there was an increased incidence of thrombotic events in patients older than 65 years.83 The BEAT (Bevacizumab Expanded Access Trial) and BRiTE (Bevacizumab Regimens Investigation of Treatment Effects) studies showed similar clinical outcomes across all age groups.84,85 While older patients experienced more arterial thromboembolic events with the addition of bevacizumab, other factors such as ECOG PS, prior anticoagulation, and history of arterial disease were more predictive of these adverse events than age.
The randomized phase 3 AVEX study explored the efficacy and tolerability of capecitabine plus bevacizumab versus capecitabine alone in 280 frail patients aged ≥ 70 years.86 PFS in the capecitabine/bevacizumab arm was 9.1 months versus 5.1 months in the capecitabine alone arm. While the OS difference was not statistically significant, patients in the capecitabine/bevacizumab arm had an OS of 20.7 months versus 16.8 months in the capecitabine alone group. As reported in prior studies, patients in the capecitabine/bevacizumab arm had increased rates of toxic events (40%) compared with those who received capecitabine alone (22%), with reports of hypertension, hand-foot syndrome, bleeding, and thrombotic events. More recently, the phase 2 PRODIGE 20 trial studied the addition of bevacizumab to chemotherapy (5-FU, FOLFOX, or FOLFIRI) based on physician choice in untreated metastatic CRC patients aged ≥ 75 years (median age 80 years).87 They found that the addition of bevacizumab to standard of care chemotherapy was both safe and effective. The adverse events seen with bevacizumab, such as hypertension and thrombotic events, were consistent with prior studies.
A newer antiangiogenic agent, ziv-aflibercept, has been approved for the second-line treatment of metastatic CRC. The VELOUR trial demonstrated that the addition of ziv-aflibercept to FOLFIRI benefited patients across all age groups compared with FOLFIRI plus placebo in patients who had failed prior oxaliplatin-based chemotherapy.88,89 Ramucirumab is a human IgG-1 monoclonal antibody approved in second-line treatment in combination with FOLFIRI. A subgroup analysis of the RAISE study showed that the survival benefit was similar in patients aged ≥ 65 years versus those < 65 years.90 Based on the above data, the use of a VEGF inhibitor in combination with chemotherapy should be considered in older patients with metastatic CRC. Furthermore, based on the conflicting data regarding the benefit of FOLFOX/FOLFIRI over single-agent 5-FU discussed above, the combination of capecitabine plus bevacizumab may be considered a front-line treatment option in older patients based on the AVEX study.
EGFR Inhibitors
Cetuximab and panitumumab are anti-epidermal growth factor receptor (EGFR) antibodies approved for the treatment of RAS wild-type metastatic CRC. Data regarding the use of EGFR inhibitors in the geriatric population is scarce and the data that does exist is conflicting.91,92 The PRIME study demonstrated that panitumumab plus FOLFOX had a PFS benefit compared to FOLFOX alone in KRAS wild-type metastatic CRC patients.92 While the study met its primary endpoint, the benefit did not translate to patients aged ≥ 65 years in subgroup analysis. Conversely, a retrospective study of the efficacy and safety of cetuximab in elderly patients with heavily pretreated metastatic CRC found similar efficacy in older and younger patients as well as no increased adverse events in the older population.91 A phase 2 trial investigating cetuximab as single-agent first-line treatment of metastatic CRC in fit older patients found cetuximab to be safe with moderate activity in this population, but did not support the use of cetuximab as first-line single-agent treatment in fit geriatric patients who may be candidates for combination therapy.93 Our group studied the patterns of use and tolerance of anti-EGFR antibodies in 117 older adults with metastatic CRC with a median age of 73 years.94 The study showed that older age at the time of treatment was associated with administration of anti-EGFR antibody as monotherapy rather than in combination with chemotherapy (P = 0.0009). We found no association between age and presence of grade 3 or higher toxicity. In addition, the toxicity profile seen in older patients was similar to what has been demonstrated in prior studies involving a younger patient population. Given the discordance seen between studies, additional prospective trials are needed to elucidate the efficacy and safety of EGFR inhibitors in the geriatric population.
Other Agents
Two newer agents approved in the treatment of metastatic CRC are regorafenib, a multikinase inhibitor, and trifluridine/tipiracil (TFD/TPI), a nucleoside analog combined with an inhibitor of thymidine phosphorylase. The phase 3 CORRECT trial studied regorafenib as monotherapy in previously treated metastatic CRC and found an OS benefit of 1.4 months and minimal PFS benefit.95 Van Cutsem et al performed a subgroup analysis by age and found similar OS benefit in patients < 65 years of age and ≥ 65 years.96 The most frequent adverse events grade 3 or higher were hand-foot syndrome, fatigue, diarrhea, hypertension, and desquamation/rash, which were seen at similar rates in both age groups. More recently, the phase 2 Regorafenib Dose Optimization Study (ReDOS) found that weekly dose escalation of regorafenib from 80 mg to 160 mg daily over 3 weeks was superior to the standard 160 mg daily dosing in patients with metastatic CRC.97 The dose escalation group had a longer median OS, although this difference was not statistically significant, as well as a more favorable toxicity profile. Therefore, this new dosing strategy may be a reasonable option for older patients with pretreated metastatic CRC. A study of TFD/TPI versus placebo in refractory metastatic CRC found an OS benefit of 7.1 months versus 5.3 months.98 In subgroup analyses, the OS benefit extended to both patients < 65 years and ≥ 65 years. Given the sparse data on these newer agents in the geriatric population and the modest benefit they provide to those with refractory metastatic CRC, more data is needed to determine their utility in elderly patients. The decision to use these agents in the older patients warrants a thorough discussion with the patient regarding risks, benefit, and treatment goals.
Immunotherapy
Between 3.5% and 6.5% of stage IV colorectal cancers are MSI-H and have deficient mismatch repair (dMMR).99–101 A recent phase 2 trial studied the use of pembrolizumab, an IgG4 monoclonal antibody against PD-1 (programmed cell death-1), in heavily pretreated patients with dMMR metastatic CRC, MMR-proficient (pMMR) metastatic CRC, and noncolorectal dMMR metastatic cancer.102 Patients with dMMR metastatic CRC had a 50% ORR and 89% disease control rate (DCR), as compared with an ORR of 0% and DCR of 16% in patients with pMMR metastatic CRC. There was also an OS and PFS benefit seen in the dMMR CRC group as compared with the pMMR CRC group. Another phase 2 study, CheckMate 142, studied the anti-PD-1 monoclonal antibody nivolumab with or without ipilimumab (a monoclonal antibody against cytotoxic T-lymphocyte antigen 4) in patients with dMMR and pMMR metastatic CRC.103 In the interim analysis, nivolumab was found to provide both disease control and durable response in patients with dMMR metastatic CRC.
While these studies led to the FDA approval of pembrolizumab and nivolumab for management of previously treated MSI-H or dMMR metastatic CRC, data on the use of immunotherapy in older adults is scarce. Immunosenescence, or the gradual deterioration of the immune system that comes with aging, may impact the efficacy of immune checkpoint inhibitors (ICI) in older patients with advanced cancer.104 There is conflicting data on the efficacy of PD-1 and programmed death ligand-1) PD-L1 inhibitors in older patients across different cancers. A meta-analysis of immunotherapy in older adults with a variety of malignancies showed overall efficacy comparable to that seen in adults younger than 65 years.105 However, another review found ICIs to be less effective in older patients with head and neck, non-small cell lung cancer, and renal cell carcinoma compared with their younger counterparts.104 Regarding the toxicity profile of ICIs in the elderly, similar rates of grade 3 or higher adverse events in patients younger than 65 years and older than 65 years have been reported.106 However, patients aged ≥ 70 years had increased rates of grade 3 to 5 adverse events as compared to patients younger than 65 years (71.7% versus 58.4%, respectively). Given the scant data on ICIs in older patients with MSI-H or dMMR metastatic CRC, more clinical trials inclusive of this population are needed in order to determine the efficacy and safety of immunotherapy.
Palliative Care
The incorporation of palliative care early following the diagnosis of cancer has been shown to improve quality of life, decrease depression, and help with symptom management.107 The triggers for geriatric patients to initiate palliative care may be different from those of younger patients, as older patients may have different goals of care.108 Older patients will often choose quality over quantity of life when making treatment decisions.109 The ideal medical treatment for the frail patient with colorectal cancer would focus on treating disease while providing palliative measures to help support the patient and improve quality of life. It is paramount that patients maintain functional independence as loss of independence is recognized as a major threat to an older patient’s quality of life.110 The optimal way to achieve these goals is through the efforts of a multidisciplinary care team including not only physicians and nurses, but also social workers, nutritionists, physical therapists, and family who can provide support for the patient’s psychosocial, cognitive, and medical needs.111 Although cancer and noncancer–related death occur more frequently in the geriatric population, data to guide a specific palliative care approach to the elderly population is lacking.108
Conclusion
Colorectal cancer is a disease of older adults with a median age at diagnosis of 67 years.1 With the aging population, oncologists will be faced with treating increasing numbers of older patients, and must adjust their practice to accommodate this population of patients. Treating geriatric patients is challenging given the lack of available data to guide the treatment approach. Although several prospective elderly-specific studies have been conducted evaluating treatments for metastatic CRC, most treatment decisions are made based on the available retrospective studies and pooled analyses. Oncologists must carefully consider and evaluate each patient based on physiologic age rather than chronologic age.112 Overall, older patients should be given the opportunity to receive standard of care treatments in the appropriate setting. The decision to modify treatment plans should be made after a thorough evaluation by a multidisciplinary team and a discussion with the patient regarding their goals and the risks and benefits of the treatment. Geriatric assessment tools can help the care team identify patients with various geriatric syndromes that may not be detected on routine oncology evaluation. This type of evaluation is time consuming and is rarely done in a busy oncology practice. Ongoing studies are aiming to develop a method to incorporate geriatric assessments into the care of older adults.Additional prospective trials targeting older, more frail patients are essential to improve upon our knowledge so we can provide best care for this growing elderly population.
Introduction
Colorectal cancer (CRC) is the fourth most common cancer in the United States and has a high prevalence among the older population.1 In 2017, there were an estimated 135,430 new cases of CRC and 50,260 deaths due to CRC. It is the second leading cause of cancer death in the United States, and the death rate for patients with CRC increases with age (Figure).2
Although elderly persons are more frequently diagnosed with CRC, they are underrepresented in clinical trials. This may be due in part to stringent eligibility criteria in prospective randomized controlled trials that exclude older patients with certain comorbidities and decreased functional status. Hutchins and colleagues compared the proportion of persons aged 65 years and older enrolled in Southwest Oncology Group (SWOG) clinical trials and the proportion of persons in this age group in the US population with the same cancer diagnoses.5 They found that while 72% of the US population with CRC were aged ≥ 65 years, persons in this age group comprised only 40% of patients enrolled in SWOG trials. An update on this study performed after Medicare policy changed in 2000 to include coverage of costs incurred due to clinical trials showed an upward trend in the accrual of older patients in SWOG trials, from 25% during the period 1993–1996 to 38% during the period 2001–2003; however, the percentage of older patients with CRC on clinical trials overall remained stable from 1993 to 2003.6
The underrepresentation of older adults with CRC in clinical trials presents oncologists with a challenging task when practicing evidence-based medicine in this patient population. Analysis of a large claims database demonstrated that the use of multi-agent chemotherapy for the treatment of metastatic CRC in older adults increased over time, while the use of single-agent 5-fluorouracil (5-FU) decreased.7 However, the adoption of combination therapy with irinotecan or oxaliplatin in older adults lagged behind the initial adoption of these agents in younger patients. This data demonstrates that as the field of medical oncology evolves, providers are becoming more comfortable treating older patients with multiple medical problems using standard approved regimens.
Geriatric Assessment
Before treating older patients with cancer, it is necessary to define the patient’s physiological age, ideally through a multidisciplinary team evaluation.
The Eastern Cooperative Oncology Group performance status (ECOG PS) and Karnofsky Performance Status (KPS) are crude measures of functional status.12 Generally, elderly patients with good ECOG PS or KPS scores are considered fit enough to receive standard therapy similar to their younger counterparts. Evaluation of functional status using these performance scores is often suboptimal, resulting in patients with a normal or adequate performance status score who may still experience poor outcomes, including decreased survival and inability to tolerate treatment. A study that explored parameters among older patients that predict for increased risk of chemotherapy-related toxicities found that physician-rated KPS score did not accurately predict the risk for adverse events.13 Therefore, a CGA represents a better way to evaluate functional status and other domains.
Functional status can also be evaluated by self-reported tools such as activities of daily living, which refer to basic self-care, and instrumental activities of daily living (IADLs), which are essential for independent living in the community.14,15 Mobility, gait, and balance can also be measured using the “Timed Get Up and Go” test and gait speed. Klepin et al found that faster gait speed was associated with overall survival (OS) in patients with metastatic cancer.16
Cognitive function is an important component of the geriatric assessment in older patients with cancer, as dementia is a prognostic factor for survival in the overall geriatric population. In a retrospective review, patients with dementia were less likely to have a biopsy-proven diagnosis and were twice as likely to have their CRC diagnosed postmortem.17 In addition, establishing that the patient has intact cognitive function prior to initiating treatment is essential to ensure that the patient can comply with treatment and understands when to report adverse effects. Nutritional status is an important portion of the geriatric assessment because malnutrition is associated with increased mortality and decreased tolerance for chemotherapy.18–20 Evaluating the patient’s psychosocial support is crucial as well because older patients are at greater risk of social isolation and depression.21 While the incidence of depression is lower in older adults with cancer than in younger adults with cancer, clinically significant depression is still noted in 3% to 25% of elderly cancer patients.22 Other critical components of the CGA are review of the patient’s comorbidities and medications to avoid complications of polypharmacy.
Both the Cancer and Aging Research Group (CARG) and Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) toxicity tools are valuable tools, as they predict chemotherapy tolerance in elderly patients.13,23 These tools can help guide discussions between oncologists and patients as well as the formulation of an appropriate treatment plan.24 Although toxicity tools can help to determine which patients are at risk for severe toxicity secondary to treatment, these tools do not replace the CGA. A prospective cohort study that evaluated the impact of CGA on tolerance to chemotherapy in older patients with cancer compared patients aged ≥ 70 years at the start of their treatment with chemotherapy (± radiation therapy) using geriatrician-delivered CGA versus standard care given by oncology.8 Patients who received geriatrician-guided CGA interventions tolerated chemotherapy better and completed treatments as planned (odds ratio 4.14 [95% confidence interval {CI} 1.50 to 11.42], P = 0.006) with fewer treatment modifications.
Unfortunately, the CGA is time-consuming to administer and difficult to incorporate into a busy oncology practice. Therefore, other screening models are used to identify patients who may benefit from a full CGA. The International Society of Geriatric Oncology performed a systematic review of screening tools used to identify older cancer patients in need of geriatric assessment and found that the 3 most studied screening tools are the G8, the Vulnerable Elders Survey-13 (VES-13), and the Flemish version of the Triage Risk Screening Tool.25 Another study found that the G8 was more sensitive than the VES-13 (76.5% versus 68.7%, P = 0.0046), whereas the VES-13 was more specific than the G8 (74.3% versus 64.4%, P < 0.0001).26 In addition to providing guidance to initiate a full geriatric assessment, these screening tools may assist in decision making for older cancer patients, especially those with advanced disease.
Surgery
Early-Stage Disease
When possible, surgical resection of colorectal tumors is the primary treatment in both the curative setting and to avoid complications, such as obstruction or perforation.27 Multiple studies have shown that fit elderly patients benefit from curative surgery similarly to their younger counterparts.27–29 With the growing population of persons aged 65 years or older, surgeons are becoming more comfortable with operating on the elderly.4 However, a large systematic review of 28 independent studies with a total of 34,194 patients showed that older patients were less likely to undergo curative surgery.30 Eligibility for surgery should not be determined by age alone, but rather should be based on a full assessment of the patient’s health, including comorbidities, functional status, nutrition, cognition, social support, and psychological status. The impact of age on short-term outcomes after colorectal surgery in terms of 30-day postoperative morbidity and mortality rates was explored in a study that divided patients into 2 groups: those aged ≥ 80 years (mean age 85) and those aged < 80 years (mean age 55.3).31 There were no statistical differences in 30-day postoperative morbidity and mortality rates between the 2 groups, and preexisting comorbidities and urgent nature of surgery were important predictors of colorectal surgery outcomes in the older adults, results that have been seen in several other studies.28,30 When possible, laparoscopic surgery is preferred as it is associated with less intraoperative blood loss, less postoperative pain, reduced postoperative ileus, a shorter hospital stay, and fewer cardiovascular and pulmonary complications.32 The Preoperative Assessment of Cancer in the Elderly (PACE), which combines surgical risk assessment tools with CGA tools, can assist surgeons in determining candidacy for surgery and help decrease unequal access to surgery in the geriatric population.33
Metastasectomy
A large international multicenter cohort study explored the outcomes of patients aged ≥ 70 years who underwent liver resection of colorectal metastases. The study investigatorsfound that neoadjuvant chemotherapy was used less frequently and less extensive surgery was performed in elderly patients than in younger patients.34 Sixty-day postoperative mortality was slightly higher (3.8% versus 1.6%, P < 0.001) and 3-year OS was slightly lower (57.1% versus 60.2%, P < 0.001) in the elderly group as compared to their younger counterparts, but overall the outcomes after liver surgery were similar. Therefore, the management of liver metastases in oligometastatic disease in elderly patients fit for surgery should be the same as that offered to younger patients. Since outcomes are comparable, older patients should be offered neoadjuvant chemotherapy, as several studies have shown similar response rates and OS in younger and older patients.35,36
Rectal Cancer
The standard of care for locally advanced rectal cancer is combined modality treatment with radiation and chemotherapy followed by total mesorectal excision. However, given conflicting data regarding the ability of elderly patients to tolerate neoadjuvant 5-FU-based chemotherapy and radiation, elderly patients are treated with trimodality therapy less often than their younger counterparts.37,38 A systematic review of 22 randomized trials involving 8507 patients with rectal cancer showed that adjuvant radiation therapy could reduce the risk of local recurrence and death from rectal cancer in patients of all ages.39 However, the risk of noncancer-related death was increased in the older population. The Stockholm II trial showed similar benefits of preoperative radiation overall, but this benefit did not extend to patients older than 68 years because of an increased risk of morbidity and mortality.40 In older patients, mortality from noncancer causes within the first 6 months after surgery was higher in the group that received perioperative radiation than in the group that did not receive radiation. Elderly patients (age > 68 years) accounted for most of the mortality, which was predominantly due to cardiovascular disease.
A retrospective study of 36 patients aged ≥ 70 years with rectal cancer evaluated the toxicity and feasibility of neoadjuvant 5-FU combined with pelvic radiation for treating locally advanced rectal cancer. Patients were classified as healthy and “fit” or “vulnerable” based on the presence of comorbidities.41 This study demonstrated that tolerability and response to neoadjuvant chemotherapy and radiation as well as ability to undergo surgery were similar in “vulnerable” patients and “fit” patients. Conversely, Margalit and colleagues studied the rate of treatment deviations in elderly patients with rectal cancer treated with combined modality therapy and found that most patients required early termination of treatment, treatment interruptions, or dose reductions.42 While trimodality treatment is the standard of care in rectal cancer, there is conflicting data from retrospective studies regarding the tolerability and feasibility of this approach. It is important to proceed with caution but to still consider fit older patients with locally advanced rectal cancer for neoadjuvant chemotherapy and radiation followed by surgery.
In patients who have a complete response (CR) to neoadjuvant chemoradiation, watchful waiting rather than proceeding to surgery may be a reasonable strategy, especially in older patients. A systematic review of 867 patients with locally advanced rectal cancer showed no statistically significant difference in OS between patients who were observed with watchful waiting and those who underwent surgery.43 The International Watch and Wait Database includes 679 patients who were managed with a watch-and-wait regimen because they had a clinical CR after chemoradiation. An outcomes analysis of these patients showed that 25% had local regrowth, with 3-year OS of 91% overall and 87% in patients with local regrowth.44 In most patients (84%), regrowth of the tumor occurred within the first 2 years of follow up.
In frail older adults, for whom longer courses of treatment are not feasible or chemotherapy is contraindicated, short-course radiation therapy can be considered either in the neoadjuvant setting or alone for palliation.45 A randomized trial of short-course radiation versus long-course chemoradiation in patients with T3 rectal cancer found that the difference in 3-year local recurrence rates was not statistically significant.46
Chemotherapy
An expected natural decline in function occurs with age, but given the great variability that exists between patients, it is important to focus on physiologic age rather than chronologic age to determine ability to receive and tolerate anticancer treatment. Decreases in renal and hepatic function, cognitive impairment, changes in gastrointestinal motility, decrements in cardiac and bone marrow reserves, as well as comorbidities and polypharmacy affect a patient’s ability to tolerate chemotherapy.47,48 Toxicity tools such as CARG and CRASH can help to predict severity of toxicity with chemotherapy.13,23 The information provided by these tools can help guide conversations between the oncologist and patient regarding treatment plans.
Adjuvant Chemotherapy for Early-Stage Disease
Stage II Disease
Defining treatment guidelines for older patients with stage II colon cancer is difficult due to lack of data that shows benefit in this population. The QUASAR (Quick and Simple and Reliable) group’s prospective study of adjuvant single-agent 5-FU in stage II colon cancer patients showed an absolute improvement in survival of 3.6% when 5-FU was given after surgery (95% CI 1.0 to 6.0).49 The subgroup analysis of patients aged ≥ 70 years showed a limited benefit of adjuvant 5-FU (hazard ratio [HR] 1.13 [95% CI 0.74 to 1.75]). Given the limited benefit, adjuvant 5-FU for elderly patients with stage II colon cancer should be used judiciously as patients may have competing causes of morbidity or mortality.
The use of oxaliplatin-based therapy in the adjuvant setting for stage II disease was evaluated in a subgroup analysis of the MOSAIC study (Multicenter International Study of Oxaliplatin/5-FU/Leucovorin in the Adjuvant Treatment of Colon Cancer).50 Adjuvant oxaliplatin-based treatment may be offered to patients with stage II colon cancer that carries high-risk features (poorly differentiated histology, lymphovascular invasion, bowel obstruction and/or perforation, < 12 lymph nodes sampled, perineural invasion, or indeterminate or positive margins) due to a trend toward improved disease-free survival (DFS) at 5 years. Patients in this group who received adjuvant FOLFOX (leucovorin, oxaliplatin, 5-FU) versus 5-FU/leucovorin had a DFS of 82.3% versus 74.6%, respectively (HR 0.72 [95% CI 0.50 to 1.02]), a difference that was not statistically significant. A subgroup analysis of 315 patients aged 70 to 75 years with stage II colon cancer enrolled in the MOSAIC study found no statistically significant DFS or OS benefit with the addition of oxaliplatin to 5-FU/leucovorin.51 Therefore, use of this platinum/fluoropyrimidine combination for adjuvant therapy for high-risk stage II disease in older patients remains controversial given its associated risks and the lack of definitive data demonstrating a benefit in this patient group. Decisions regarding this therapy should be made through a shared discussion with patients about its risks and benefits.
Microsatellite status is an important biomarker in the evaluation of stage II CRC. Microsatellite stability is a marker of a functioning DNA mismatch repair system. In patients with colon cancer, tumor microsatellite stability is classified based on the percentage of abnormal microsatellite regions.52 Several studies have shown that patients with tumors that display high microsatellite instability (MSI-H) have an improved prognosis over patients with microsatellite stable tumors.53,54 While patients with stage II MSI-H colon cancer have better outcomes, MSI is associated with a reduced response to treatment with fluoropyrimidines, as demonstrated in a systematic review that found that patients with tumors with MSI obtained no benefit from adjuvant 5-FU (HR 1.24 [95% CI 0.72 to 2.14]).55 Aparicio and colleagues reported an increased prevalence of MSI-H tumors with increasing age.56 Therefore, mismatch repair phenotype should be considered when making adjuvant chemotherapy decisions in the older adult with colon cancer, as it may affect the decision to recommend single-agent 5-FU treatment.
Stage III Disease
The use of single-agent 5-FU for stage III resected CRC has been evaluated in multiple studies. Sargent et al performed a pooled analysis of 3351 patients from 7 randomized phase 3 trials comparing surgery and adjuvant 5-FU-based chemotherapy versus surgery alone in stage II or III colon cancer patients.57 Adjuvant chemotherapy was associated with improvement in both OS and time to tumor recurrence (HR 0.76 and 0.68, respectively). The 5-year OS was 71% for those who received adjuvant treatment and 64% for those who were treated with surgery alone. The benefit of adjuvant treatment was independent of age, and there was no difference in toxicity across age groups, except for 1 study which showed increased rates of leukopenia in the elderly. The oral fluoropyrimidine capecitabine was shown to be an effective alternative to 5-FU plus leucovorin as adjuvant treatment for those with resected stage III colon cancer.58 However, in the subgroup analysis of DFS in the intention-to-treat group, the improvement in DFS was not statistically significant in those aged ≥ 70 years. This study justified the phase 3 Xeloda in Adjuvant Colon Cancer Therapy (X-ACT) trial, which compared capecitabine and 5-FU/leucovorin as adjuvant therapy in patients with resected stage III colon cancer.59 The X-ACT trial showed no significant effect of age on DFS or OS.
The addition of oxaliplatin to 5-FU in the adjuvant setting for stage III tumors has been studied and debated in the elderly population in multiple trials. The MOSAIC trial investigated FOLFOX versus 5-FU/leucovorin in the adjuvant setting.50 The addition of oxaliplatin was associated with a DFS and OS benefit, with a 20% reduction in risk of colon cancer recurrence and 16% reduction in risk of death in all patients. The National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trial then studied 2409 patients with stage II or III colon cancer treated with weekly bolus 5-FU/leucovorin with or without oxaliplatin.60 In this study, OS was significantly improved with the addition of oxaliplatin in patients younger than 70 years, but OS at 5 years was 4.7% worse for patients aged ≥ 70 years treated with weekly 5-FU/leucovorin and oxaliplatin compared with those treated with weekly 5-FU/leucovorin (71.6% versus 76.3%, respectively). In contrast, the XELOXA trial (NO16968), which randomly assigned stage III colon cancer patients to capecitabine and oxaliplatin (XELOX) or bolus 5-FU/leucovorin (standard of care at study start), showed an efficacy benefit, albeit not statistically significant, in patients aged ≥ 70 years (HR 0.87 [95% CI 0.63 to 1.18]).61–63
The Adjuvant Colon Cancer Endpoints (ACCENT) database included 7 randomized trials totaling 14,528 patients with stage II or III colon cancer treated with adjuvant 5-FU with or without oxaliplatin or irinotecan.64 Subgroup analysis of patients aged ≥ 70 years (n = 2575) showed no benefit with an oxaliplatin-based regimen in DFS (HR 0.94 [95% CI 0.78 to 1.13]) or OS (HR 1.04 [95% CI, 0.85 to 1.27]). Based on these studies and the increased toxicity with oxaliplatin, oxaliplatin-based adjuvant chemotherapy is utilized less often than single-agent 5-FU in geriatric patients with early-stage colon cancer.65 Conversely, a recent pooled analysis of individual patient data from 4 randomized trials (NSABP C-08, XELOXA, X-ACT, and AVANT) showed improved DFS and OS with adjuvant XELOX or FOLFOX over single-agent 5-FU in patients aged ≥ 70 years (DFS HR 0.77 [95% CI 0.62 to 0.95], P = 0.014; OS HR 0.78 [95% CI 0.61 to 0.99], P = 0.045).66 This analysis also showed that grade 3 and 4 adverse events related to oxaliplatin were similar across age groups.
These data come from post-hoc analyses, and there is no prospective data to steer decision making in elderly patients with early-stage CRC (Table).
It is well established that patients with stage III colon cancer benefit from oxaliplatin-based adjuvant chemotherapy after curative surgical resection.68 However, older patients are less likely to be referred to oncology as compared with their younger counterparts, due to the conflicting data regarding the benefit of this approach in older adults. Studies have shown that when the referral is placed, the geriatric population is less likely to receive chemotherapy.69 Sanoff et al analyzed 4 data sets (SEER-Medicare, National Comprehensive Cancer Network, New York State Cancer Registry, and Cancer Care Outcomes Research and Surveillance Consortium) to assess the benefit of adjuvant chemotherapy for resected stage III CRC among patients aged ≥ 75 years. Their analysis showed that only 40% of patients evaluated received adjuvant chemotherapy for stage III CRC after surgical resection.70
Summary
Prospective data to guide the treatment of older patients with early-stage CRC in the adjuvant setting is lacking. For fit older patients with stage II disease, limited benefit will be derived from single-agent 5-FU. For those with stage III CRC, the benefit and toxicities of fluoropyrimidines as adjuvant therapy appear to be similar regardless of age. The addition of oxaliplatin to fluoropyrimidines in patients aged ≥ 70 years has not been proven to improve DFS or OS and could result in an incremental toxicity profile. Therefore, treatment plans must be individualized, and decisions should be made through an informed discussion evaluating the overall risk/benefit ratio of each approach.
Metastatic Disease
Palliative Chemotherapy
Approximately 20% of patients with CRC are diagnosed with metastatic disease at presentation, and 35% to 40% develop metastatic disease following surgery and adjuvant therapy.2 The mainstay of treatment in this population is systemic therapy in the form of chemotherapy with or without biologic agents. In this setting, several prospective studies specific to older adults have been completed, providing more evidence-based guidance to oncologists who see these patients. Folprecht et al retrospectively reviewed data from 22 clinical trials evaluating 5-FU-based palliative chemotherapy in 3825 patients with metastatic CRC, including 629 patients aged ≥ 70 years.71 OS in elderly patients (10.8 months [95% CI 9.7 to 11.8]) was equivalent to that in younger patients (11.3 months [95% CI 10.9 to 11.7], P = 0.31). Similarly, relative risk and progression-free survival (PFS) were comparable irrespective of age.
Standard of care for most patients with metastatic colon cancer consists of 5-FU/leucovorin in combination with either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) with a monoclonal antibody.72 A retrospective pooled analysis of patients with metastatic CRC compared the safety and efficacy of FOLFOX4 in patients aged < 70 years versus those aged ≥ 70 years.73 While age ≥ 70 years was associated with an increased rate of grade ≥ 3 hematologic toxicity, it was not associated with increased rates of severe neurologic events, diarrhea, nausea, vomiting, infection, 60-day mortality, or overall incidence of grade ≥ 3 toxicity. The benefit of treatment was consistent across both age groups; therefore, age alone should not exclude an otherwise healthy individual from receiving FOLFOX.
These post-hoc analyses show that fit older patients who were candidates for trial participation tolerated these treatments well; however, these treatments may be more challenging for less fit older adults. The UK Medical Research Council FOCUS2 (Fluorouracil, Oxaliplatin, CPT11 [irinotecan]: Use and Sequencing) study was a prospective phase 3 trial that included 459 patients with metastatic CRC who were deemed too frail or not fit enough for standard-dose chemotherapy by their oncologists.74 In this group, 43% of patients were older than 75 years and 13% were older than 80 years. Patients were randomly assigned to receive infusional 5-FU with levofolinate; oxaliplatin and 5-FU; capecitabine; or oxaliplatin and capecitabine; all regimens were initiated with an empiric 20% dose reduction. The addition of oxaliplatin suggested some improvement in PFS, but this was not significant (5.8 months versus 4.5 months, HR 0.84 [95% CI 0.69 to 1.01], P = 0.07). Oxaliplatin was not associated with increased grade 3 or 4 toxicities. Capecitabine is often viewed as less toxic because it is taken by mouth, but this study found that replacement of 5-FU with capecitabine did not improve quality of life. Grade 3 or 4 toxicities were seen more frequently in those receiving capecitabine than in those receiving 5-FU (40% versus 30%, P = 0.03) in this older and frailer group of patients. As the patients on this study were frail and treatment dose was reduced, this data may not apply to fit older adults who are candidates for standard therapy.
When managing an older patient with metastatic CRC, it is important to tailor therapy based on goals of care, toxicity of proposed treatment, other comorbidities, and the patient’s functional status. One approach to minimizing toxicity in the older population is the stop-and-go strategy. The OPTIMOX1 study showed that stopping oxaliplatin after 6 cycles of FOLFOX7 and continuing maintenance therapy with infusional 5-FU/leucovorin alone for 12 cycles prior to reintroducing FOLFOX7 achieved efficacy similar to continuous FOLFOX4 with decreased toxicity.75 Figer et al studied an exploratory cohort of 37 patients aged 76 to 80 years who were included in the OPTIMOX1 study.76 The overall relative risk, median PFS, and median OS did not differ between the older patients in this cohort and younger patients studied in the original study. Older patients did experience more neutropenia, neurotoxicity, and overall grade 3 to 4 toxicity, but there were no toxic deaths in patients older than 75 years. The approach of giving treatment breaks, as in OPTIMOX2, may also provide patients with better quality of life, but perhaps at the expense of cancer-related survival.77
The combination of irinotecan and 5-FU has also been studied as treatment for patients with metastatic CRC. A pooled analysis of 2691 patients aged ≥ 70 years with metastatic CRC across 4 phase 3 randomized trials investigating irinotecan and 5-FU demonstrated that irinotecan-containing chemotherapy provided similar benefits to both older and younger patients with similar risk of toxicity.78 A phase 2 trial studying FOLFIRI as first-line treatment in older metastatic CRC patients showed this to be a safe and active regimen with manageable toxicity.79 Another randomized phase 3 trial for older patients compared 5-FU/leucovorin with or without irinotecan for first-line treatment of metastatic CRC (FFCD 2001-02).80 The study accrued 282 patients aged ≥ 75 years (median age 80 years), and found that the addition of irinotecan to infusional 5-FU–based chemotherapy did not significantly increase either PFS or OS. Aparicio et al performed a substudy of baseline geriatric evaluation prior to treatment in the FFCD 2001-02 study and assessed the value of geriatric parameters for predicting outcomes (objective response rate [ORR], PFS, and OS).81 Multivariate analysis showed that none of the geriatric parameters were predictive of ORR or PFS but that normal IADL was associated with better OS. This combination may still be appropriate for some older patients with metastatic disease, while single- agent 5-FU may be more appropriate in frail patients.
Biologic Agents
VEGF Inhibitors
Targeted biologic agents have been studied in the treatment of metastatic CRC. Bevacizumab is a recombinant, humanized monoclonal antibody against vascular endothelial growth factor (VEGF) that is approved in the first-line setting for treatment of metastatic CRC. A pooled analysis examined 439 patients 65 years of age and older with metastatic CRC who received bevacizumab plus chemotherapy versus placebo plus chemotherapy.82 In this analysis, the addition of bevacizumab was associated with an improvement in OS (19.3 months versus 14.3 months, HR 0.7 [95% CI 0.55 to 0.90], P = 0.006) and in PFS (9.2 months versus 6.2 months, HR 0.52 [95% CI 0.40 to 0.67], P < 0.0001). Known adverse events associated with bevacizumab were seen in the bevacizumab plus chemotherapy group but not at increased rates in the older population compared to their younger counterparts. Conversely, another pooled analysis found that while there was a PFS and OS benefit in older patients receiving bevacizumab, there was an increased incidence of thrombotic events in patients older than 65 years.83 The BEAT (Bevacizumab Expanded Access Trial) and BRiTE (Bevacizumab Regimens Investigation of Treatment Effects) studies showed similar clinical outcomes across all age groups.84,85 While older patients experienced more arterial thromboembolic events with the addition of bevacizumab, other factors such as ECOG PS, prior anticoagulation, and history of arterial disease were more predictive of these adverse events than age.
The randomized phase 3 AVEX study explored the efficacy and tolerability of capecitabine plus bevacizumab versus capecitabine alone in 280 frail patients aged ≥ 70 years.86 PFS in the capecitabine/bevacizumab arm was 9.1 months versus 5.1 months in the capecitabine alone arm. While the OS difference was not statistically significant, patients in the capecitabine/bevacizumab arm had an OS of 20.7 months versus 16.8 months in the capecitabine alone group. As reported in prior studies, patients in the capecitabine/bevacizumab arm had increased rates of toxic events (40%) compared with those who received capecitabine alone (22%), with reports of hypertension, hand-foot syndrome, bleeding, and thrombotic events. More recently, the phase 2 PRODIGE 20 trial studied the addition of bevacizumab to chemotherapy (5-FU, FOLFOX, or FOLFIRI) based on physician choice in untreated metastatic CRC patients aged ≥ 75 years (median age 80 years).87 They found that the addition of bevacizumab to standard of care chemotherapy was both safe and effective. The adverse events seen with bevacizumab, such as hypertension and thrombotic events, were consistent with prior studies.
A newer antiangiogenic agent, ziv-aflibercept, has been approved for the second-line treatment of metastatic CRC. The VELOUR trial demonstrated that the addition of ziv-aflibercept to FOLFIRI benefited patients across all age groups compared with FOLFIRI plus placebo in patients who had failed prior oxaliplatin-based chemotherapy.88,89 Ramucirumab is a human IgG-1 monoclonal antibody approved in second-line treatment in combination with FOLFIRI. A subgroup analysis of the RAISE study showed that the survival benefit was similar in patients aged ≥ 65 years versus those < 65 years.90 Based on the above data, the use of a VEGF inhibitor in combination with chemotherapy should be considered in older patients with metastatic CRC. Furthermore, based on the conflicting data regarding the benefit of FOLFOX/FOLFIRI over single-agent 5-FU discussed above, the combination of capecitabine plus bevacizumab may be considered a front-line treatment option in older patients based on the AVEX study.
EGFR Inhibitors
Cetuximab and panitumumab are anti-epidermal growth factor receptor (EGFR) antibodies approved for the treatment of RAS wild-type metastatic CRC. Data regarding the use of EGFR inhibitors in the geriatric population is scarce and the data that does exist is conflicting.91,92 The PRIME study demonstrated that panitumumab plus FOLFOX had a PFS benefit compared to FOLFOX alone in KRAS wild-type metastatic CRC patients.92 While the study met its primary endpoint, the benefit did not translate to patients aged ≥ 65 years in subgroup analysis. Conversely, a retrospective study of the efficacy and safety of cetuximab in elderly patients with heavily pretreated metastatic CRC found similar efficacy in older and younger patients as well as no increased adverse events in the older population.91 A phase 2 trial investigating cetuximab as single-agent first-line treatment of metastatic CRC in fit older patients found cetuximab to be safe with moderate activity in this population, but did not support the use of cetuximab as first-line single-agent treatment in fit geriatric patients who may be candidates for combination therapy.93 Our group studied the patterns of use and tolerance of anti-EGFR antibodies in 117 older adults with metastatic CRC with a median age of 73 years.94 The study showed that older age at the time of treatment was associated with administration of anti-EGFR antibody as monotherapy rather than in combination with chemotherapy (P = 0.0009). We found no association between age and presence of grade 3 or higher toxicity. In addition, the toxicity profile seen in older patients was similar to what has been demonstrated in prior studies involving a younger patient population. Given the discordance seen between studies, additional prospective trials are needed to elucidate the efficacy and safety of EGFR inhibitors in the geriatric population.
Other Agents
Two newer agents approved in the treatment of metastatic CRC are regorafenib, a multikinase inhibitor, and trifluridine/tipiracil (TFD/TPI), a nucleoside analog combined with an inhibitor of thymidine phosphorylase. The phase 3 CORRECT trial studied regorafenib as monotherapy in previously treated metastatic CRC and found an OS benefit of 1.4 months and minimal PFS benefit.95 Van Cutsem et al performed a subgroup analysis by age and found similar OS benefit in patients < 65 years of age and ≥ 65 years.96 The most frequent adverse events grade 3 or higher were hand-foot syndrome, fatigue, diarrhea, hypertension, and desquamation/rash, which were seen at similar rates in both age groups. More recently, the phase 2 Regorafenib Dose Optimization Study (ReDOS) found that weekly dose escalation of regorafenib from 80 mg to 160 mg daily over 3 weeks was superior to the standard 160 mg daily dosing in patients with metastatic CRC.97 The dose escalation group had a longer median OS, although this difference was not statistically significant, as well as a more favorable toxicity profile. Therefore, this new dosing strategy may be a reasonable option for older patients with pretreated metastatic CRC. A study of TFD/TPI versus placebo in refractory metastatic CRC found an OS benefit of 7.1 months versus 5.3 months.98 In subgroup analyses, the OS benefit extended to both patients < 65 years and ≥ 65 years. Given the sparse data on these newer agents in the geriatric population and the modest benefit they provide to those with refractory metastatic CRC, more data is needed to determine their utility in elderly patients. The decision to use these agents in the older patients warrants a thorough discussion with the patient regarding risks, benefit, and treatment goals.
Immunotherapy
Between 3.5% and 6.5% of stage IV colorectal cancers are MSI-H and have deficient mismatch repair (dMMR).99–101 A recent phase 2 trial studied the use of pembrolizumab, an IgG4 monoclonal antibody against PD-1 (programmed cell death-1), in heavily pretreated patients with dMMR metastatic CRC, MMR-proficient (pMMR) metastatic CRC, and noncolorectal dMMR metastatic cancer.102 Patients with dMMR metastatic CRC had a 50% ORR and 89% disease control rate (DCR), as compared with an ORR of 0% and DCR of 16% in patients with pMMR metastatic CRC. There was also an OS and PFS benefit seen in the dMMR CRC group as compared with the pMMR CRC group. Another phase 2 study, CheckMate 142, studied the anti-PD-1 monoclonal antibody nivolumab with or without ipilimumab (a monoclonal antibody against cytotoxic T-lymphocyte antigen 4) in patients with dMMR and pMMR metastatic CRC.103 In the interim analysis, nivolumab was found to provide both disease control and durable response in patients with dMMR metastatic CRC.
While these studies led to the FDA approval of pembrolizumab and nivolumab for management of previously treated MSI-H or dMMR metastatic CRC, data on the use of immunotherapy in older adults is scarce. Immunosenescence, or the gradual deterioration of the immune system that comes with aging, may impact the efficacy of immune checkpoint inhibitors (ICI) in older patients with advanced cancer.104 There is conflicting data on the efficacy of PD-1 and programmed death ligand-1) PD-L1 inhibitors in older patients across different cancers. A meta-analysis of immunotherapy in older adults with a variety of malignancies showed overall efficacy comparable to that seen in adults younger than 65 years.105 However, another review found ICIs to be less effective in older patients with head and neck, non-small cell lung cancer, and renal cell carcinoma compared with their younger counterparts.104 Regarding the toxicity profile of ICIs in the elderly, similar rates of grade 3 or higher adverse events in patients younger than 65 years and older than 65 years have been reported.106 However, patients aged ≥ 70 years had increased rates of grade 3 to 5 adverse events as compared to patients younger than 65 years (71.7% versus 58.4%, respectively). Given the scant data on ICIs in older patients with MSI-H or dMMR metastatic CRC, more clinical trials inclusive of this population are needed in order to determine the efficacy and safety of immunotherapy.
Palliative Care
The incorporation of palliative care early following the diagnosis of cancer has been shown to improve quality of life, decrease depression, and help with symptom management.107 The triggers for geriatric patients to initiate palliative care may be different from those of younger patients, as older patients may have different goals of care.108 Older patients will often choose quality over quantity of life when making treatment decisions.109 The ideal medical treatment for the frail patient with colorectal cancer would focus on treating disease while providing palliative measures to help support the patient and improve quality of life. It is paramount that patients maintain functional independence as loss of independence is recognized as a major threat to an older patient’s quality of life.110 The optimal way to achieve these goals is through the efforts of a multidisciplinary care team including not only physicians and nurses, but also social workers, nutritionists, physical therapists, and family who can provide support for the patient’s psychosocial, cognitive, and medical needs.111 Although cancer and noncancer–related death occur more frequently in the geriatric population, data to guide a specific palliative care approach to the elderly population is lacking.108
Conclusion
Colorectal cancer is a disease of older adults with a median age at diagnosis of 67 years.1 With the aging population, oncologists will be faced with treating increasing numbers of older patients, and must adjust their practice to accommodate this population of patients. Treating geriatric patients is challenging given the lack of available data to guide the treatment approach. Although several prospective elderly-specific studies have been conducted evaluating treatments for metastatic CRC, most treatment decisions are made based on the available retrospective studies and pooled analyses. Oncologists must carefully consider and evaluate each patient based on physiologic age rather than chronologic age.112 Overall, older patients should be given the opportunity to receive standard of care treatments in the appropriate setting. The decision to modify treatment plans should be made after a thorough evaluation by a multidisciplinary team and a discussion with the patient regarding their goals and the risks and benefits of the treatment. Geriatric assessment tools can help the care team identify patients with various geriatric syndromes that may not be detected on routine oncology evaluation. This type of evaluation is time consuming and is rarely done in a busy oncology practice. Ongoing studies are aiming to develop a method to incorporate geriatric assessments into the care of older adults.Additional prospective trials targeting older, more frail patients are essential to improve upon our knowledge so we can provide best care for this growing elderly population.
1. National Cancer Institute. SEER cancer stat facts: colorectal cancer. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed March 1, 2018.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
3. Kochanek KD, Murphy S, Xu J, Arias E. Mortality in the United States, 2016. NCHS Data Brief 2017:1-8.
4. Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States, current population reports, P25-1140. Washington, DC: U.S. Census Bureau; 2014.
5. Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061–7.
6. Unger JM, Coltman CA Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol 2006;24:141–4.
7. Vijayvergia N, Li T, Wong YN, et al. Chemotherapy use and adoption of new agents is affected by age and comorbidities in patients with metastatic colorectal cancer. Cancer 2016;122:3191–8.
8. Kalsi T, Babic-Illman G, Ross PJ, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br J Cancer 2015;112:1435–44.
9. National Comprehensive Cancer Network. Older adult oncology (Version 2.2017). Accessed March 1, 2018,
10. Balducci L. Frailty: a common pathway in aging and cancer. Interdiscip Top Gerontol 2013;38:61–72.
11. Baijal P, Periyakoil V. Understanding frailty in cancer patients. Cancer J 2014;20:358–66.
12. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55.
13. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457–65.
14. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86.
15. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970;10:20–30.
16. Klepin HD, Geiger AM, Tooze JA, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc 2010;58:76–82.
17. Gupta SK, Lamont EB. Patterns of presentation, diagnosis, and treatment in older patients with colon cancer and comorbid dementia. J Am Geriatr Soc 2004;52:1681–7.
18. Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–7.
19. Aaldriks AA, van der Geest LG, Giltay EJ, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol 2013;4:218–26.
20. Martucci RB, Barbosa MV, D’Almeida CA, et al. Undernutrition as independent predictor of early mortality in elderly cancer patients. Nutrition 2017;34:65–70.
21. Naeim A, Aapro M, Subbarao R, Balducci L. Supportive care considerations for older adults with cancer. J Clin Oncol 2014;32:2627–34.
22. Kua J. The prevalence of psychological and psychiatric sequelae of cancer in the elderly - how much do we know? Ann Acad Med Singapore 2005;34:250–6.
23. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012;118:3377–86.
24. Kim J, Hurria A. Determining chemotherapy tolerance in older patients with cancer. J Natl Compr Canc Netw 2013;11:1494-502.
25. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol 2015;26:288–300.
26. Soubeyran P, Bellera C, Goyard J, et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One 2014; 9:e115060.
27. Gurevitch AJ, Davidovitch B, Kashtan H. Outcome of right colectomy for cancer in octogenarians. J Gastrointest Surg 2009;13:100–4.
28. Schiffmann L, Ozcan S, Schwarz F, et al. Colorectal cancer in the elderly: surgical treatment and long-term survival. Int J Colorectal Dis 2008;23:601–10.
29. Ong ES, Alassas M, Dunn KB, Rajput A. Colorectal cancer surgery in the elderly: acceptable morbidity? Am J Surg 2008;195:344–8.
30. Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet 2000;356:968–74.
31. Shalaby M, Di Lorenzo N, Franceschilli L, et al. Outcome of colorectal surgery in elderly populations. Ann Coloproctol 2016;32:139–43.
32. Frasson M, Braga M, Vignali A, et al. Benefits of laparoscopic colorectal resection are more pronounced in elderly patients. Dis Colon Rectum 2008;51:296–300.
33. PACE participants, Audisio RA, Pope D, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol 2008;65:156–63.
34. Adam R, Frilling A, Elias D, et al. Liver resection of colorectal metastases in elderly patients. Br J Surg 2010;97:366–76.
35. de Liguori Carino N, van Leeuwen BL, Ghaneh P, et al. Liver resection for colorectal liver metastases in older patients. Crit Rev Oncol Hematol 2008;67:273–8.
36. Tamandl D, Gruenberger B, Herberger B, et al. Surgery after neoadjuvant chemotherapy for colorectal liver metastases is safe and feasible in elderly patients. J Surg Oncol 2009;100:364–71.
37. Shahir MA, Lemmens VE, van de Poll-Franse LV, et al. Elderly patients with rectal cancer have a higher risk of treatment-related complications and a poorer prognosis than younger patients: a population-based study. Eur J Cancer 2006;42:3015–21.
38. Chang GJ, Skibber JM, Feig BW, Rodriguez-Bigas M. Are we undertreating rectal cancer in the elderly? An epidemiologic study. Ann Surg 2007;246:215–21.
39. Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001;358:1291–304.
40. Martling A, Holm T, Johansson H, et al, Stockholm Colorectal Cancer Study Group. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: long-term follow-up of a population-based study. Cancer 2001;92:896–902.
41. Pasetto LM, Friso ML, Pucciarelli S, et al. Rectal cancer neoadjuvant treatment in elderly patients. Anticancer Res 2006;26:3913–23.
42. Margalit DN, Mamon HJ, Ancukiewicz M, et al. Tolerability of combined modality therapy for rectal cancer in elderly patients aged 75 years and older. Int J Radiat Oncol Biol Phys 2011;81:e735–41.
43. Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:501–13.
44. van der Valk M. The International Watch & Wait database (IWWD) for rectal cancer: An update. J Clin Oncol 2017;35 suppl:521.
45. Donato V, Valeriani M, Zurlo A. Short course radiation therapy for elderly cancer patients. Evidences from the literature review. Crit Rev Oncol Hematol 2003;45:305–11.
46. Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827–33.
47. McCleary NJ, Dotan E, Browner I. Refining the chemotherapy approach for older patients with colon cancer. J Clin Oncol 2014;32:2570–80.
48. Millan M, Merino S, Caro A, et al. Treatment of colorectal cancer in the elderly. World J Gastrointest Oncol 2015;7:204–20.
49. Quasar Collaborative Group, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020–9.
50. Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16.
51. Tournigand C, Andre T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353–60.
52. Winder T, Lenz HJ. Molecular predictive and prognostic markers in colon cancer. Cancer Treat Rev 2010;36:550–6.
53. Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–57.
54. Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77.
55. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–18.
56. Aparicio T, Schischmanoff O, Poupardin C, et al. Deficient mismatch repair phenotype is a prognostic factor for colorectal cancer in elderly patients. Dig Liver Dis 2013;45:245–50.
57. Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091–7.
58. Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
59. Twelves C, Scheithauer W, McKendrick J, et al. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol 2012;23:1190–7.
60. Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–74.
61. Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71.
62. Haller DG, Cassidy J, Tabernero J, et al. Efficacy findings from a randomized phase III trial of capecitabine plus oxaliplatin versus bolus 5-FU/LV for stage III colon cancer (NO16968): impact of age on disease-free survival (DFS) [abstract]. J Clin Oncol 2010;28:3521.
63. Schmoll HJ, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 2015;33:3733–40.
64. McCleary NJ, Meyerhardt JA, Green E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol 2013;31:2600–6.
65. Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA 2010;303:1037–45.
66. Haller DG, O’Connell MJ, Cartwright TH, et al. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials. Ann Oncol 2015;26:715-24.
67. Aparicio T, Francois E, Cristol-Dalstein L, et al. PRODIGE 34-FFCD 1402-ADAGE: Adjuvant chemotherapy in elderly patients with resected stage III colon cancer: A randomized phase 3 trial. Dig Liver Dis 2016;48:206–7.
68. Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797–806.
69. Mahoney T, Kuo YH, Topilow A, Davis JM. Stage III colon cancers: why adjuvant chemotherapy is not offered to elderly patients. Arch Surg 2000;135:182–5.
70. Sanoff HK, Carpenter WR, Sturmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol 2012;30:2624–34.
71. Folprecht G, Cunningham D, Ross P, et al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Ann Oncol 2004;15:1330–8.
72. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii1–9.
73. Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 2006;24:4085–91.
74. Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet 2011;377:1749–59.
75. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol 2006;24:394–400.
76. Figer A, Perez-Staub N, Carola E, et al. FOLFOX in patients aged between 76 and 80 years with metastatic colorectal cancer: an exploratory cohort of the OPTIMOX1 study. Cancer 2007;110:2666–71.
77. Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol 2009;27:5727–33.
78. Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol 2008;26:1443–51.
79. Souglakos J, Pallis A, Kakolyris S, et al. Combination of irinotecan (CPT-11) plus 5-fluorouracil and leucovorin (FOLFIRI regimen) as first line treatment for elderly patients with metastatic colorectal cancer: a phase II trial. Oncology 2005;69:384–90.
80. Aparicio T, Lavau-Denes S, Phelip JM, et al. Randomized phase III trial in elderly patients comparing LV5FU2 with or without irinotecan for first-line treatment of metastatic colorectal cancer (FFCD 2001-02). Ann Oncol 2016;27:121–7.
81. Aparicio T, Gargot D, Teillet L, et al. Geriatric factors analyses from FFCD 2001-02 phase III study of first-line chemotherapy for elderly metastatic colorectal cancer patients. Eur J Cancer 2017;74:98–108.
82. Kabbinavar FF, Hurwitz HI, Yi J, et al. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol 2009;27:199–205.
83. Cassidy J, Saltz LB, Giantonio BJ, et al. Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol 2010;136:737–43.
84. Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 2009;20:1842–7.
85. Kozloff MF, Berlin J, Flynn PJ, et al. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: results from the BRiTE observational cohort study. Oncology 2010;78:329–39.
86. Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol 2013;14:1077–85.
87. Aparicio T, Bouche O, Taieb J, et al. Bevacizumab+chemotherapy versus chemotherapy alone in elderly patients with untreated metastatic colorectal cancer: a randomized phase II trial-PRODIGE 20 study results. Ann Oncol 2018;29:133–8.
88. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499–506.
89. Ruff P, Van Cutsem E, Lakomy R, et al. Observed benefit and safety of aflibercept in elderly patients with metastatic colorectal cancer: An age-based analysis from the randomized placebo-controlled phase III VELOUR trial. J Geriatr Oncol 2018;9:32–9.
90. Obermannova R, Van Cutsem E, Yoshino T, et al. Subgroup analysis in RAISE: a randomized, double-blind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression. Ann Oncol 2016;27:2082–90.
91. Bouchahda M, Macarulla T, Spano JP, et al. Cetuximab efficacy and safety in a retrospective cohort of elderly patients with heavily pretreated metastatic colorectal cancer. Crit Rev Oncol Hematol 2008;67:255-62.
92. Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346–55.
93. Sastre J, Gravalos C, Rivera F, et al. First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study. Oncologist 2012;17:339–45.
94. Dotan E, Devarajan K, D’Silva AJ, et al. Patterns of use and tolerance of anti-epidermal growth factor receptor antibodies in older adults with metastatic colorectal cancer. Clin Colorectal Cancer 2014;13:192–8.
95. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12.
96. Van Cutsem E, Sobrero A, Siena S, et al. Regorafenib (REG) in progressive metastatic colorectal cancer (mCRC): Analysis of age subgroups in the phase III CORRECT trial [abstract]. J Clin Oncol 2013;31(15 suppl):3636-3636.
97. Bekaii-Saab TS, Ou FS, Anderson DM, et al. Regorafenib dose optimization study (ReDOS): Randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC): an ACCRU Network study [abstract]. J Clin Oncol 2018;36(4 suppl):611-611.
98. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19.
99. Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer 2009;100:266–73.
100. Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014;20:5322–30.
101. Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst 2013;105:1151–6.
102. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20.
103. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91.
104. Daste A, Domblides C, Gross-Goupil M, et al. Immune checkpoint inhibitors and elderly people: A review. Eur J Cancer 2017;82:155–66.
105. Elias R, Giobbie-Hurder A, McCleary NJ, et al. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer 2018;6:26.
106. Singh H, Kim G, Maher VE, et al. FDA subset analysis of the safety of nivolumab in elderly patients with advanced cancers [abstract]. J Clin Oncol 2016;34(15 suppl):10010-10010.
107. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol 2017;35:834–41.
108. Brighi N, Balducci L, Biasco G. Cancer in the elderly: is it time for palliative care in geriatric oncology? J Geriatr Oncol 2014;5:197–203.
109. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer 2008;113:3459–66.
110. Bagshaw SM, Stelfox HT, Johnson JA, et al. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med 2015;43:973–82.
111. Lynch MP, Marcone D, Kagan SH. Developing a multidisciplinary geriatric oncology program in a community cancer center. Clin J Oncol Nurs 2007;11:929–33.
112. Sheridan J, Walsh P, Kevans D, et al. Determinants of short- and long-term survival from colorectal cancer in very elderly patients. J Geriatr Oncol 2014;5:376–83.
1. National Cancer Institute. SEER cancer stat facts: colorectal cancer. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed March 1, 2018.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
3. Kochanek KD, Murphy S, Xu J, Arias E. Mortality in the United States, 2016. NCHS Data Brief 2017:1-8.
4. Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States, current population reports, P25-1140. Washington, DC: U.S. Census Bureau; 2014.
5. Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061–7.
6. Unger JM, Coltman CA Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol 2006;24:141–4.
7. Vijayvergia N, Li T, Wong YN, et al. Chemotherapy use and adoption of new agents is affected by age and comorbidities in patients with metastatic colorectal cancer. Cancer 2016;122:3191–8.
8. Kalsi T, Babic-Illman G, Ross PJ, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br J Cancer 2015;112:1435–44.
9. National Comprehensive Cancer Network. Older adult oncology (Version 2.2017). Accessed March 1, 2018,
10. Balducci L. Frailty: a common pathway in aging and cancer. Interdiscip Top Gerontol 2013;38:61–72.
11. Baijal P, Periyakoil V. Understanding frailty in cancer patients. Cancer J 2014;20:358–66.
12. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55.
13. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457–65.
14. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86.
15. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970;10:20–30.
16. Klepin HD, Geiger AM, Tooze JA, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc 2010;58:76–82.
17. Gupta SK, Lamont EB. Patterns of presentation, diagnosis, and treatment in older patients with colon cancer and comorbid dementia. J Am Geriatr Soc 2004;52:1681–7.
18. Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–7.
19. Aaldriks AA, van der Geest LG, Giltay EJ, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol 2013;4:218–26.
20. Martucci RB, Barbosa MV, D’Almeida CA, et al. Undernutrition as independent predictor of early mortality in elderly cancer patients. Nutrition 2017;34:65–70.
21. Naeim A, Aapro M, Subbarao R, Balducci L. Supportive care considerations for older adults with cancer. J Clin Oncol 2014;32:2627–34.
22. Kua J. The prevalence of psychological and psychiatric sequelae of cancer in the elderly - how much do we know? Ann Acad Med Singapore 2005;34:250–6.
23. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012;118:3377–86.
24. Kim J, Hurria A. Determining chemotherapy tolerance in older patients with cancer. J Natl Compr Canc Netw 2013;11:1494-502.
25. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol 2015;26:288–300.
26. Soubeyran P, Bellera C, Goyard J, et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One 2014; 9:e115060.
27. Gurevitch AJ, Davidovitch B, Kashtan H. Outcome of right colectomy for cancer in octogenarians. J Gastrointest Surg 2009;13:100–4.
28. Schiffmann L, Ozcan S, Schwarz F, et al. Colorectal cancer in the elderly: surgical treatment and long-term survival. Int J Colorectal Dis 2008;23:601–10.
29. Ong ES, Alassas M, Dunn KB, Rajput A. Colorectal cancer surgery in the elderly: acceptable morbidity? Am J Surg 2008;195:344–8.
30. Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet 2000;356:968–74.
31. Shalaby M, Di Lorenzo N, Franceschilli L, et al. Outcome of colorectal surgery in elderly populations. Ann Coloproctol 2016;32:139–43.
32. Frasson M, Braga M, Vignali A, et al. Benefits of laparoscopic colorectal resection are more pronounced in elderly patients. Dis Colon Rectum 2008;51:296–300.
33. PACE participants, Audisio RA, Pope D, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol 2008;65:156–63.
34. Adam R, Frilling A, Elias D, et al. Liver resection of colorectal metastases in elderly patients. Br J Surg 2010;97:366–76.
35. de Liguori Carino N, van Leeuwen BL, Ghaneh P, et al. Liver resection for colorectal liver metastases in older patients. Crit Rev Oncol Hematol 2008;67:273–8.
36. Tamandl D, Gruenberger B, Herberger B, et al. Surgery after neoadjuvant chemotherapy for colorectal liver metastases is safe and feasible in elderly patients. J Surg Oncol 2009;100:364–71.
37. Shahir MA, Lemmens VE, van de Poll-Franse LV, et al. Elderly patients with rectal cancer have a higher risk of treatment-related complications and a poorer prognosis than younger patients: a population-based study. Eur J Cancer 2006;42:3015–21.
38. Chang GJ, Skibber JM, Feig BW, Rodriguez-Bigas M. Are we undertreating rectal cancer in the elderly? An epidemiologic study. Ann Surg 2007;246:215–21.
39. Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001;358:1291–304.
40. Martling A, Holm T, Johansson H, et al, Stockholm Colorectal Cancer Study Group. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: long-term follow-up of a population-based study. Cancer 2001;92:896–902.
41. Pasetto LM, Friso ML, Pucciarelli S, et al. Rectal cancer neoadjuvant treatment in elderly patients. Anticancer Res 2006;26:3913–23.
42. Margalit DN, Mamon HJ, Ancukiewicz M, et al. Tolerability of combined modality therapy for rectal cancer in elderly patients aged 75 years and older. Int J Radiat Oncol Biol Phys 2011;81:e735–41.
43. Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:501–13.
44. van der Valk M. The International Watch & Wait database (IWWD) for rectal cancer: An update. J Clin Oncol 2017;35 suppl:521.
45. Donato V, Valeriani M, Zurlo A. Short course radiation therapy for elderly cancer patients. Evidences from the literature review. Crit Rev Oncol Hematol 2003;45:305–11.
46. Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827–33.
47. McCleary NJ, Dotan E, Browner I. Refining the chemotherapy approach for older patients with colon cancer. J Clin Oncol 2014;32:2570–80.
48. Millan M, Merino S, Caro A, et al. Treatment of colorectal cancer in the elderly. World J Gastrointest Oncol 2015;7:204–20.
49. Quasar Collaborative Group, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020–9.
50. Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16.
51. Tournigand C, Andre T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353–60.
52. Winder T, Lenz HJ. Molecular predictive and prognostic markers in colon cancer. Cancer Treat Rev 2010;36:550–6.
53. Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–57.
54. Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77.
55. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–18.
56. Aparicio T, Schischmanoff O, Poupardin C, et al. Deficient mismatch repair phenotype is a prognostic factor for colorectal cancer in elderly patients. Dig Liver Dis 2013;45:245–50.
57. Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091–7.
58. Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
59. Twelves C, Scheithauer W, McKendrick J, et al. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol 2012;23:1190–7.
60. Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–74.
61. Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71.
62. Haller DG, Cassidy J, Tabernero J, et al. Efficacy findings from a randomized phase III trial of capecitabine plus oxaliplatin versus bolus 5-FU/LV for stage III colon cancer (NO16968): impact of age on disease-free survival (DFS) [abstract]. J Clin Oncol 2010;28:3521.
63. Schmoll HJ, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 2015;33:3733–40.
64. McCleary NJ, Meyerhardt JA, Green E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol 2013;31:2600–6.
65. Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA 2010;303:1037–45.
66. Haller DG, O’Connell MJ, Cartwright TH, et al. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials. Ann Oncol 2015;26:715-24.
67. Aparicio T, Francois E, Cristol-Dalstein L, et al. PRODIGE 34-FFCD 1402-ADAGE: Adjuvant chemotherapy in elderly patients with resected stage III colon cancer: A randomized phase 3 trial. Dig Liver Dis 2016;48:206–7.
68. Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797–806.
69. Mahoney T, Kuo YH, Topilow A, Davis JM. Stage III colon cancers: why adjuvant chemotherapy is not offered to elderly patients. Arch Surg 2000;135:182–5.
70. Sanoff HK, Carpenter WR, Sturmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol 2012;30:2624–34.
71. Folprecht G, Cunningham D, Ross P, et al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Ann Oncol 2004;15:1330–8.
72. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii1–9.
73. Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 2006;24:4085–91.
74. Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet 2011;377:1749–59.
75. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol 2006;24:394–400.
76. Figer A, Perez-Staub N, Carola E, et al. FOLFOX in patients aged between 76 and 80 years with metastatic colorectal cancer: an exploratory cohort of the OPTIMOX1 study. Cancer 2007;110:2666–71.
77. Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol 2009;27:5727–33.
78. Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol 2008;26:1443–51.
79. Souglakos J, Pallis A, Kakolyris S, et al. Combination of irinotecan (CPT-11) plus 5-fluorouracil and leucovorin (FOLFIRI regimen) as first line treatment for elderly patients with metastatic colorectal cancer: a phase II trial. Oncology 2005;69:384–90.
80. Aparicio T, Lavau-Denes S, Phelip JM, et al. Randomized phase III trial in elderly patients comparing LV5FU2 with or without irinotecan for first-line treatment of metastatic colorectal cancer (FFCD 2001-02). Ann Oncol 2016;27:121–7.
81. Aparicio T, Gargot D, Teillet L, et al. Geriatric factors analyses from FFCD 2001-02 phase III study of first-line chemotherapy for elderly metastatic colorectal cancer patients. Eur J Cancer 2017;74:98–108.
82. Kabbinavar FF, Hurwitz HI, Yi J, et al. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol 2009;27:199–205.
83. Cassidy J, Saltz LB, Giantonio BJ, et al. Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol 2010;136:737–43.
84. Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 2009;20:1842–7.
85. Kozloff MF, Berlin J, Flynn PJ, et al. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: results from the BRiTE observational cohort study. Oncology 2010;78:329–39.
86. Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol 2013;14:1077–85.
87. Aparicio T, Bouche O, Taieb J, et al. Bevacizumab+chemotherapy versus chemotherapy alone in elderly patients with untreated metastatic colorectal cancer: a randomized phase II trial-PRODIGE 20 study results. Ann Oncol 2018;29:133–8.
88. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499–506.
89. Ruff P, Van Cutsem E, Lakomy R, et al. Observed benefit and safety of aflibercept in elderly patients with metastatic colorectal cancer: An age-based analysis from the randomized placebo-controlled phase III VELOUR trial. J Geriatr Oncol 2018;9:32–9.
90. Obermannova R, Van Cutsem E, Yoshino T, et al. Subgroup analysis in RAISE: a randomized, double-blind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression. Ann Oncol 2016;27:2082–90.
91. Bouchahda M, Macarulla T, Spano JP, et al. Cetuximab efficacy and safety in a retrospective cohort of elderly patients with heavily pretreated metastatic colorectal cancer. Crit Rev Oncol Hematol 2008;67:255-62.
92. Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346–55.
93. Sastre J, Gravalos C, Rivera F, et al. First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study. Oncologist 2012;17:339–45.
94. Dotan E, Devarajan K, D’Silva AJ, et al. Patterns of use and tolerance of anti-epidermal growth factor receptor antibodies in older adults with metastatic colorectal cancer. Clin Colorectal Cancer 2014;13:192–8.
95. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12.
96. Van Cutsem E, Sobrero A, Siena S, et al. Regorafenib (REG) in progressive metastatic colorectal cancer (mCRC): Analysis of age subgroups in the phase III CORRECT trial [abstract]. J Clin Oncol 2013;31(15 suppl):3636-3636.
97. Bekaii-Saab TS, Ou FS, Anderson DM, et al. Regorafenib dose optimization study (ReDOS): Randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC): an ACCRU Network study [abstract]. J Clin Oncol 2018;36(4 suppl):611-611.
98. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19.
99. Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer 2009;100:266–73.
100. Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014;20:5322–30.
101. Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst 2013;105:1151–6.
102. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20.
103. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91.
104. Daste A, Domblides C, Gross-Goupil M, et al. Immune checkpoint inhibitors and elderly people: A review. Eur J Cancer 2017;82:155–66.
105. Elias R, Giobbie-Hurder A, McCleary NJ, et al. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer 2018;6:26.
106. Singh H, Kim G, Maher VE, et al. FDA subset analysis of the safety of nivolumab in elderly patients with advanced cancers [abstract]. J Clin Oncol 2016;34(15 suppl):10010-10010.
107. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol 2017;35:834–41.
108. Brighi N, Balducci L, Biasco G. Cancer in the elderly: is it time for palliative care in geriatric oncology? J Geriatr Oncol 2014;5:197–203.
109. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer 2008;113:3459–66.
110. Bagshaw SM, Stelfox HT, Johnson JA, et al. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med 2015;43:973–82.
111. Lynch MP, Marcone D, Kagan SH. Developing a multidisciplinary geriatric oncology program in a community cancer center. Clin J Oncol Nurs 2007;11:929–33.
112. Sheridan J, Walsh P, Kevans D, et al. Determinants of short- and long-term survival from colorectal cancer in very elderly patients. J Geriatr Oncol 2014;5:376–83.
Urothelial Carcinoma: Muscle-Invasive and Metastatic Disease
Introduction
Bladder cancer is by far the most common cancer of the urinary system. Worldwide, approximately 450,000 new cases are diagnosed and 165,000 deaths are caused by bladder cancer each year.1 In the United States and in Europe, the most common type of bladder cancer is urothelial carcinoma (also referred to as transitional cell carcinoma), which accounts for more than 90% of all bladder cancers in these regions of the world. The remainder of bladder cancers are divided among squamous cell carcinomas, adenocarcinomas, small cell carcinomas, and, even more rarely, between various other nonepithelial tumors (eg, sarcoma).
Bladder cancer is classically thought of as a disease of the elderly, with a median age at diagnosis of 69 years in men and 71 years in women.2 The incidence of bladder cancer increases with age: in persons aged 65 to 69 years, incidence is 142 per 100,000 men and 33 per 100,000 women, and in those older than 85 years the rate doubles to 296 per 100,000 men and 74 per 100,000 women.3 The incidence is 3 times greater in men than in women.4
Urothelial carcinoma is traditionally categorized by its degree of invasion into the bladder wall: superficial (non-muscle-invasive), muscle-invasive, or metastatic disease. At the time of diagnosis, most patients have non-muscle-invasive disease (~60%); about 4% of all patients present initially with metastatic disease.5 This article focuses on metastatic bladder cancer, but muscle-invasive disease is discussed as well.
The most important factor contributing to the development of urothelial carcinoma is tobacco smoking. The risk of developing bladder cancer is 4 to 5 times higher in smokers as compared to nonsmokers, with some variation according to sex.6 Quantity of smoking exposure also plays a role, with heavy smokers demonstrating a higher likelihood for high-grade tumors with muscle invasion (or beyond) when compared to light smokers.7 Another important risk factor is occupational exposure to industrial materials, such as carpets, paints, plastics, and industrial chemicals. This type of exposure may be responsible for, or at least contribute to, the development of approximately 20% of urothelial carcinomas. Other risk factors for urothelial carcinoma include but are not limited to prior radiation to the pelvis, prior upper tract urothelial malignancy, human papillomavirus infection, and prior bladder augmentation.
Diagnosis and Staging
Case Presentation
A 63-year-old man with a past medical history of diabetes, deep vein thrombosis, occasional alcohol use, and regular pipe tobacco use presents to his primary care physician with complaints of hematuria. He reports that his urine was a dark red color that morning, which had never happened before. The patient is hemodynamically stable upon evaluation in the office, and a point-of-care urinalysis dipstick is strongly positive for blood. He is referred to a urologist for further evaluation.
In the urology office, urine microscopy is notable for more than 50 red blood cells (RBCs) per high-power field with normal RBC morphology. Flexible cystoscopy performed in the office reveals a single 2-cm, sessile, verrucous, nodular lesion located on the anterior bladder wall. A urine sample and a bladder wash specimen are sent for cytology evaluation. The patient is scheduled to undergo a complete transurethral resection of bladder tumor (TURBT) later that week with samples sent to pathology for evaluation.
- What are the clinical features of bladder cancer?
Hematuria is the most common presentation of bladder cancer, although its specificity is far lower than traditionally thought. In fact, only about 2% to 20% of cases that present with hematuria are found to be caused by malignancy. However, the incidence of genitourinary tract malignancy is much higher in patients presenting with gross hematuria (10%–20%)8–10 than in patients with microscopic hematuria alone.8,10–14 Typically, hematuria associated with malignancy is painless. Multiple studies have shown, however, that hematuria can be a normal variant, with one study demonstrating that up to 61% of patients with hematuria had no identifiable abnormality.8,10,11,13
Abdominal pain, flank pain, dysuria, urinary frequency/urgency, or other irritative voiding symptoms in the absence of hematuria can be presenting symptoms of bladder cancer as well. In these settings, discomfort typically suggests more advanced malignancy with at least local involvement or obstruction. Suprapubic pain may herald invasion into perivesical tissues and nerves, while involvement of the obturator fossa, perirectal fat, urogenital diaphragm, or presacral nerves can often present with perineal or rectal pain. Similarly, lower abdominal pain may represent involvement of lymph nodes, and right upper quadrant pain may signal liver metastasis. Cough or shortness of breath may signify metastatic disease in the lung. Finally, back, rib, or other boney pain may suggest distant metastasis.
- What next steps are required to complete this patient’s staging?
White light cystoscopy remains the gold standard for diagnosis and initial staging of bladder cancer. Additional tools include urine cytology and upper tract studies, including renal computed tomography (CT) urograms. Full urologic evaluation with all 3 modalities (cystourethroscopy, urinary cytology, and upper tract evaluation) is warranted for patients with a high suspicion for malignant etiology of hematuria. CT urograms are particularly useful for upper tract evaluation because they can be used to visualize kidney parenchyma, both renal pelvises and ureters, and pertinent abdominal and pelvic lymph nodes. Initial staging is completed through TURBT, which should ideally contain a segment of muscularis propria to distinguish between Ta (noninvasive), T1, and T2 tumors (Figure 1).
Regarding staging, T1 tumors are distinguished from Ta malignancies by their involvement in the urothelial basement membrane. Tumor invasion into the muscularis propria indicates T2 tumors, while T3 tumors extend through the muscle into the serosa and involve the complete thickness of the bladder wall. Involvement of nearby structures defines T4 bladder cancers, with T4a malignancies involving adjacent organs (prostate, vagina, uterus, or bowel) and T4b tumors involving the abdominal wall, pelvic wall, or other more distant organs. According to the American Joint Committee on Cancer’s most recent TNM staging system (Table 1),16 lymph node involvement in the true pelvis (that is, N1–N3) with T1 to T4a disease is now classified as stage III disease.
Bladder cancer is often broadly categorized as either non-muscle-invasive or muscle-invasive (which can include metastatic disease). This classification has important implications for treatment. As such, all diagnostic biopsies should be performed with the goal of reaching at least the depth of the muscularis propria in order to accurately detect potential muscularis invasion. If no muscle is detected in the initial specimen, re-resection is recommended if safe and feasible. In cases where muscle cannot be obtained, imaging evidence of T3 disease from CT or magnetic resonance imaging may be used as a surrogate indicator. Once muscle-invasive disease is confirmed, CT evaluation of the chest is also recommended, as bladder cancer can metastasize to the lungs; furthermore, patients are often at risk for secondary concomitant lung cancers given that smoking is the most prevalent risk factor for both. However, patients with small, indeterminate lung nodules not amenable to biopsy should not be denied curative intent treatment given the high likelihood that they represent benign findings.17
Pathogenesis
Because non-muscle-invasive and muscle-invasive tumors behave so differently, they are thought to arise from 2 distinct mechanisms. Although there is overlap and non-muscle-invasive cancer can certainly progress to a high-grade, invasive type of malignancy over time, current theory proposes that non-muscle-invasive bladder cancer predominantly develops just from urothelial hyperplasia, which then recruits branching vasculature to grow slowly. More aggressive urothelial carcinomas, including muscle-invasive and metastatic disease, are instead thought to arise directly from flat dysplasia that progresses to carcinoma in situ, and is much more prone to invasive growth and distant spread.18
Regardless of grade and stage, the most commonly identified genomic alterations in urothelial carcinoma are mutations in the promoter region of the telomerase reverse transcriptase (TERT) gene, which have been identified in approximately 70% of cases.19 Mutations in TERT can be readily detected in urine sediments and may ultimately have implications for diagnosis and early detection.20,21 In current practice, however, the clinical relevance of these observations remains under development. Other genomic alterations that may contribute to the development of urothelial carcinoma, and also provide new potential therapeutic targets, include alterations in the TP53 gene, the RB (retinoblastoma) gene, and the FGFR3 (fibroblast growth factor receptor) gene. FGFR3 has particular significance as it appears to be relatively common in non-muscle invasive disease (up to 60%–70%) and is likely an actionable driver mutation that may define a particular molecular subset of urothelial carcinoma; thus, it may have important implications for treatment decisions.22
Treatment
Case Continued
Pathologic evaluation of the specimen reveals a high-grade urothelial carcinoma with tumor invasion into the muscularis propria. A CT urogram is performed and does not reveal any notably enlarged pelvic nodes or suspicious lesions in the upper urinary tract. CT chest does not reveal any evidence of distant metastatic disease. Given the presence of muscle-invasive disease, the patient agrees to proceed with neoadjuvant chemotherapy and radical cystoprostatectomy with pelvic node dissection. He undergoes treatment with dose-dense (accelerated) MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) for 3 cycles, followed by surgery with cystoprostatectomy. Overall, he tolerates the procedure well and recovers quickly. Pathology reveals the presence of disease in 2 regional nodes, consistent with T4a (stage III) disease, and a small degree of residual disease in the bladder. He is followed closely in the oncology clinic, returning for urine cytology, liver and renal function tests, and imaging with CT of chest, abdomen, and pelvis every 3 months.
- What is the first-line approach to management in patients with muscle-invasive disease?
- How would the treatment strategy differ if the patient had presented with metastatic disease (stage IV)?
First-Line Management for Curative Intent: Muscle-Invasive Disease
Muscle-invasive urothelial carcinoma (including T2, T3, or T4 disease) is typically treated in a multidisciplinary fashion with neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy. This approach is recommended over radical cystectomy alone because of high relapse rates following cystectomy alone, even in the setting of bilateral pelvic lymphadenectomy.23 However, because of the associated short- and long-term toxicity of cisplatin-based regimens, this optimal treatment paradigm is reserved for patients deemed cisplatin-eligible.
Medical fitness to receive cisplatin-based chemotherapy is assessed by a number of factors and varies by institution, but most frequently consider functional status (Eastern Cooperative Oncology Group [ECOG] performance status or Karnofsky Performance Status), creatinine clearance, hearing preservation, peripheral neuropathy, and cardiac function.24 Many programs will elect to defer cisplatin-based chemotherapy in patients with low performance status (ie, < 60–70 on Karnofsky scale or > 2 on ECOG scale), creatinine clearance below 60 mL/min, or significant heart failure (NHYA class III or worse). Cisplatin-based chemotherapy may worsen hearing loss in those with hearing loss of 25 dB from baseline at 2 continuous frequencies and also may worsen neuropathy in those with baseline grade 1 peripheral neuropathy. However, these adverse outcomes must be balanced against the curative intent of the multimodality systemic approach.
In patients with renal insufficiency, caution must be taken with regard to cisplatin. Percutaneous nephrostomy placement or ureteral stenting should be attempted to relieve any ureteral outlet obstruction and restore kidney function if a patient’s renal insufficiency has resulted from this obstruction. If medical renal disease or long-term renal insufficiency is present, however, patients should instead be referred for immediate cystectomy or for a bladder-preserving approach. Generally, a creatinine clearance of 60 mL/min is required to safely receive cisplatin-based chemotherapy, although some advocate for treatment with a creatinine clearance as low as 50 mL/min. When this extended criterion is used, the dose of cisplatin may be split over 2 days to minimize renal toxicity and maximize hydration. Analysis of renal function utilizing a 24-hour urine collection should be incorporated whenever possible, as estimates of creatinine clearance have been demonstrated to be inaccurate in some instances.25
For cisplatin-eligible patients, neoadjuvant chemotherapy with a cisplatin base has consistently demonstrated a survival benefit when given prior to surgery.26,27 Historically, several different platinum-based regimens have been studied, with none showing superior effectiveness in a randomized trial over the others in the neoadjuvant setting. These regimens have included classic MVAC, dose-dense MVAC (MVAC with pegfilgrastim), GC (gemcitabine and cisplatin), and CMV (methotrexate, vinblastine, cisplatin, and leucovorin).
While classic MVAC was preferred in the 1990s and early 2000s,28,29 the availability of growth factor, such as pegfilgrastim, has made dose-dense MVAC (otherwise referred to as accelerated MVAC or ddMVAC) widely preferred and universally recommended over classic MVAC. The ddMVAC regimen with the addition of a synthetic granulocyte colony-stimulating factor (G-CSF) is substantially better tolerated than classic MVAC, as the G-CSF support minimizes the severe toxicities of classic MVAC, such as myelosuppression and mucositis, and allows for the administration of drugs in a dose-dense fashion.30,31
Both ddMVAC and GC are considered reasonable options for neoadjuvant chemotherapy and are the predominant choices for cisplatin-eligible patients (Table 2).
Prospective data defining the role of adjuvant chemotherapy for patients after cystectomy has been fraught by a variety of factors, including the known benefit of neoadjuvant chemotherapy, the high complication rate of cystectomy making chemotherapy infeasible, and clinician bias that has hampered accrual in prior trials. Thus, no level 1 evidence exists defining the benefit of adjuvant chemotherapy in patients who did not receive neoadjuvant therapy. In a report of the largest study performed in this setting, there was a statistically significant benefit in PFS but not in OS.36 Criticisms of this trial include its lack of statistical power due to a failure to accrue the targeted goal and the preponderance of node-positive patients. Regardless, for patients who have pT2–4, N1 disease after radical cystectomy and remain cisplatin-eligible after not receiving neoadjuvant chemotherapy, this remains an option.
Despite the established clinical dogma surrounding neoadjuvant chemotherapy followed by surgery, some patients are either not eligible for or decline to receive radical cystectomy, while others are not candidates for neoadjuvant cisplatin-based chemotherapy for the reasons outlined above. For patients who are surgical candidates but unable to receive neoadjuvant chemotherapy due to renal or cardiac function, they may proceed directly to surgery. For patients unable or unwilling to proceed to radical cystectomy regardless, bladder preservation strategies exist. Maximal TURBT may be an option for some patients, but, as outlined above, used alone this would be likely to lead to a high degree of local and distant failure. Combined modality chemoradiotherapy as consolidation after maximal TURBT is an established option for patients unable to undergo surgery or seeking bladder preservation. Several trials have demonstrated encouraging outcomes with this approach and were highlighted in a large meta-analysis.37 Various chemosensitizing chemotherapeutic regimens have been evaluated, including cisplatin alone or as a doublet, gemcitabine alone, and 5-fluouracil plus mitomycin C, but no randomized studies have compared these regimens to each other, nor have they been compared to surgical approaches. However, this strategy remains an option as an alternative to surgery.
First-Line Management: Metastatic Disease
The approach to therapy in patients who present with metastatic urothelial carcinoma is very similar to that used in neoadjuvant perioperative chemotherapy. The consensus first-line treatment in medically appropriate patients is cisplatin-based chemotherapy with either GC or ddMVAC (both category 1 National Comprehensive Cancer Network [NCCN] recommendations; Figure 2).30,31,38–40
Head-to-head studies specifically comparing ddMVAC and GC have been limited. GC has been compared to classic MVAC, with results showing equivalent efficacy but improved tolerability, as expected.38,40 ddMVAC was compared with a modified version of GC (termed “dose-dense GC”) in a phase 3 study from Greece, which demonstrated similar outcomes.41
Surgical intervention with radical cystectomy and regional lymph node dissection is typically deferred for patients who present with distant metastatic disease, unlike those who present with locally advanced disease. Radical cystectomy has traditionally been thought of as overly aggressive without sufficient benefit, although evidence to guide this approach remains sparse.42 As such, most expert recommendations and consensus statements simply recommend against surgical intervention and leave the decision between ddMVAC and GC up to the individual clinician.
In patients who are not eligible for cisplatin therapy, it is reasonable to consider chemotherapy with a combination of gemcitabine and carboplatin. This combination has been shown to be equivalent to MCAVI (methotrexate, carboplatin, vinblastine) in terms of overall survival (OS; 9 months versus 8 months) and progression-free survival (PFS; 6 months versus 4 months) with significantly fewer serious toxicities (9% versus 21%).43
The advent of immunotherapy in recent years has provided several new alternatives for cisplatin-ineligible patients. While immunotherapies such as pembrolizumab or atezolizumab are not yet recommended as first-line therapy for cisplatin-eligible patients, these 2 drugs are approved as options for first-line therapy in cisplatin-ineligible patients with metastatic disease. In a recent phase 2 trial (IMvigor210) involving 119 patients who were given atezolizumab as first-line therapy, median PFS was 2.7 months and median OS was 15.9 months.44 Another trial using data from patients in the KEYNOTE-052 study who received pembrolizumab as first-line therapy demonstrated antitumor activity with pembrolizumab and acceptable tolerability in cisplatin-ineligible patients with advanced urothelial carcinoma.45 The primary endpoint was objective response (either complete or partial response), which was achieved in 24% of the intention-to-treat population. Median PFS was 2 months, and 6-month OS was observed in 67% of patients. Both atezolizumab and pembrolizumab were given accelerated approval based on these single-arm studies in this setting. However, due to inferior outcomes in subsequent trials that included single-agent immunotherapy arms for patients in the first-line setting, the US Food and Drug Administration (FDA) has clarified the approval. In the subsequent trials, patients with a low PD-L1 biomarker based on the individual assay used for each drug did worse on immunotherapy alone (compared to chemotherapy or both combined), and the single-therapy arms were stopped early. Thus, the FDA now recommends that pembrolizumab or atezolizumab be used in the first line only for cisplatin-ineligible patients who have PD-L1 expression on tumor cells above the threshold studied on each individual assay, or are unfit for any platinum-based chemotherapy. Further study regarding the optimal role of biomarkers and chemotherapy-immunotherapy combinations is ongoing.
Case Continued
Ten months after his procedure, the patient is found to have prominent retroperitoneal lymphadenopathy and a 1.0-cm liver nodule suspicious for malignancy is noted on surveillance imaging. CT-guided biopsy of the liver reveals high-grade urothelial carcinoma, consistent with both recurrence and distant metastasis. The patient is informed that he needs to resume systemic therapy for recurrent metastatic disease. The options discussed include salvage single-agent chemotherapy with gemcitabine or immunotherapy with pembrolizumab. He elects to move forward with immunotherapy and is scheduled to begin pembrolizumab.
- What other immunotherapies might this patient consider for second-line therapy?
- Is chemotherapy a second-line option for this patient?
Second-Line Therapies and Management of Progressive Disease
Disease progression is unfortunately seen in the majority of cases of advanced urothelial carcinoma.46 New second-line therapies have recently been approved by the FDA in the form of monoclonal antibodies targeting programmed death 1 (PD-1) and a PD-1 ligand (PD-L1) (Figure 3).
Approval of pembrolizumab, a PD-1 inhibitor, was largely supported by the Keynote-045 trial,47,48 which looked at 542 patients who had progressed or recurred after platinum-based chemotherapy. These patients were randomly assigned to either pembrolizumab or investigator’s choice of chemotherapy (paclitaxel, docetaxel, or vinflunine). Patients treated with pembrolizumab had a significantly improved OS (median of 10.3 months versus 7.4 months), but no statistically significant difference in PFS (2.1 months versus 3.3 months). Interestingly, the rate of responses of 12 months or longer was higher with pembrolizumab than with more traditional second-line chemotherapy (68% versus 35%). The strength of this data has led to a category 1 recommendation in the most recent NCCN guidelines.39
The approval of atezolizumab, a PD-L1 inhibitor, as a second-line therapy for advanced urothelial carcinoma is largely supported by data from IMvigor211, a phase 3 trial that studied 931 patients randomly assigned to atezolizumab or investigator’s choice chemotherapy. OS did not differ significantly between patients in the atezolizumab group who had ≥ 5% expression of PD-L1 on tumor-infiltrating immune cells and patients in the chemotherapy group (11.1 months versus 10.6 months), but mean duration of response was longer (15.9 months versus 8.3 months).49 Therapy with atezolizumab had significantly fewer toxicities than chemotherapy (grade 3 or 4 toxicities of 20% versus 43%).
Phase 3 studies of nivolumab (PD-1 inhibitor), avelumab (PD-L1 inhibitor), and durvalumab (PD-L1 inhibitor) have not yet been published. These agents have received accelerated approval, however, as second-line treatment of advanced urothelial carcinoma based on promising data from phase 1 and phase 2 studies.50–52
Second-line chemotherapy is also an option for patients who do not qualify for immunotherapy or who progress during or after immunotherapy. Although there has been a great deal of excitement about new developments with immunotherapy and the survival benefit seen compared to investigator’s choice chemotherapy, the fact remains that most patients do not respond to immunotherapy. Still, some patients do derive benefit from single-agent chemotherapy in the platinum-refractory setting. Options based on primarily single-arm studies include gemcitabine, paclitaxel, docetaxel, pemetrexed, ifosfamide, oxaliplatin, and eribulin (Figure 2). In a randomized phase 3 trial, vinflunine demonstrated an OS benefit in platinum-refractory patients compared to best supportive care; it subsequently received approval by the European Medicines Agency.53 More recently in the phase 3 RANGE trial, docetaxel plus ramucirumab (a monoclonal antibody targeting vascular endothelial growth factor receptor 2) was compared to docetaxel plus placebo and met its primary endpoint of an improvement in PFS (median 4.07 months versus 2.76 months, P = 0.0118).54 OS has not been reported and this regimen has not yet received regulatory approval, however. Unfortunately, trials comparing these regimens are lacking, and response rates and survival remain modest. Clearly, better therapies and biomarkers to help personalize treatment options are needed.
Further investigations are underway with alternative regimens, including but not limited to targeted therapy in the setting of specific genetic and epigenetic alterations. These include mutations affecting tyrosine kinase receptors (eg, RAS/RAF, PI3K, AKT, and mTOR), cell cycle regulators (eg, TP53 or RB1), FGFR3 mutations, PTEN deletions, gene amplifications (eg, FGFR1, CCND1, and MDM2), or changes in genes responsible for chromatin remodeling (eg, UTX, CHD6, or ARID1A). As noted, there is particular excitement regarding FGFR3 inhibitors, which have shown compelling efficacy in phase 1 and 2 single-arm trials. Several agents are being evaluated in randomized trials and represent a potential path to the first targeted therapeutic class with a role in urothelial malignancies.
Surgical resection of metastases may be considered in very select cases.55 Surgery may have a role in limiting metastatic complications and improving cancer control, but this should be discussed at length with the patient using a multidisciplinary approach with careful restaging prior to surgery.
Case Continued
The patient remains on pembrolizumab every 3 weeks as per protocol with regular surveillance imaging. His disease stabilizes as the nodule in his liver and the retroperitoneal lymph nodes, all representing metastatic disease, became slightly smaller in size without evidence of any new disease. He continues to follow up closely with his genitourinary oncologist, undergoing regular surveillance and imaging every 3 months without evidence of disease progression.
Approximately 12 months into therapy, the patient notices a nonproductive cough with progressive and rapidly worsening shortness of breath. He is noted to be hypoxic with oxygen saturation levels to 79% in clinic and is sent immediately to the emergency department by his oncologist. Diffuse bilateral reticular opacities are noted on chest radiograph. Non-contrast CT scan demonstrates diffuse ground-glass opacities consistent with acute respiratory distress syndrome–pattern pneumonitis. He is admitted to the intensive care unit.
The patient is aggressively treated with high-flow nasal oxygen supplementation, intravenous steroids, and empiric antibiotics. He slowly improves on high-dose steroids (methylprednisolone 1 mg/kg/day) without requiring intubation or infliximab therapy and is discharged home in stable condition after 10 days. Oral steroid therapy is continued with a long taper over 6 weeks. In the setting of his grade 3 pneumonitis, pembrolizumab is discontinued and the patient is scheduled for a follow-up appointment with his oncologist to discuss next steps.
- In addition to pneumonitis, what other toxicities should you monitor for in patients treated with an immune checkpoint inhibitor?
- Is this patient a candidate to receive immunotherapy again in the future?
Treatment Toxicities
As use of immune checkpoint inhibitors has become more prevalent, the medical community has become increasingly aware of various immune-related adverse effects (irAE) associated with these drugs. These toxicities can be seen in virtually any organ system, and even vague complaints that arise years after therapy initiation should be treated with a high level of suspicion. The most commonly affected organ systems include the skin, gastrointestinal (GI) tract, lungs, liver, and endocrine system, although all other organ systems can be involved (Table 3) and toxicities appear to be similar across individual drugs.
The American Society of Clinical Oncology recently published a complete set of recommendations to guide clinicians on appropriate treatment strategies for each manifestation of immunotherapy-related toxicity.56 The details of these recommendations largely fall outside the purview of this article, but the mainstays of management are worth noting. These include high-dose systemic glucocorticoids, along with supportive care and cessation of immunotherapy in grade 3 or 4 toxicities. Infliximab is frequently recommended as an adjunct in severe or refractory cases.
Chemotherapy-related toxicities, on the other hand, are well-described and tend to be more familiar to patients and clinicians (Table 3). Classic MVAC, which has now been largely replaced by ddMVAC, was notoriously difficult to tolerate. It was known for a high rate of serious (grade 3 or 4) myelosuppressive complications as well as frequent GI toxicities. These complications include neutropenia (57%), stomatitis (10%), and nausea and vomiting (6%).23 ddMVAC with growth factor support is much better tolerated than classic MVAC. Prominent complaints with ddMVAC still can include nausea, GI distress, mucositis, and fatigue, but the incidence of myelosuppressive complications in particular has markedly decreased. GC is largely well tolerated, with minimal nausea and manageable myelotoxicity, but it is associated with an increased risk of venous thromboembolism.38
Prognosis
Case Conclusion
After returning home, the patient discusses his complicated medical course with his oncologist. Given his continued high quality of life with good functional status, he requests to continue with therapy for his metastatic bladder cancer and is interested in joining a clinical trial. He is referred to a nearby academic center with openings in a clinical trial for which he would be eligible. In the meantime, his oncologist guides him through filling out an advance directive and recommends that he make an appointment with palliative care services to ensure adequate home support for any future needs he may have.
- What is the estimated 5-year survival rate for patients with metastatic bladder cancer?
Overall, prognosis in patients with metastatic bladder cancer remains poor. Median survival in patients being treated with multi-agent chemotherapy is approximately 15 months,38,40 with an expected 5-year survival of just 15%. This is much improved, however, as prior to the advent of modern chemotherapy estimated survival was just 6 months with metastatic bladder cancer. Importantly, these figures do not take into account the recent advancements with immunotherapy, and thus it is reasonable to assume survival rates may continue to improve. In light of these recent advances, it is strongly recommended that whenever possible patients and clinicians consider participation in clinical trials to continue uncovering new and better therapies moving forward.
A number of tools have been developed to help risk stratify patients based on comorbidity, performance status, and other characteristics, but none have been universally adopted.57–60 As with many other malignancies, performance status is an important predictor of clinical outcomes in these patients.61–63 Sites of metastasis also may serve to suggest the course of disease. Patients with visceral metastases typically exhibit significantly worse disease with a shortened survival. The role of molecular factors as prognostic markers in bladder cancer is still under investigation. Many biomarkers are being considered (including mutations and polymorphisms in p53, ERCC1, and ERCC2), and evidence suggests some may have a role in prognosis; thus far, none have been validated as prognostic or predictive tools in urothelial carcinoma.
Conclusion
Bladder cancer includes an aggressive group of genitourinary tract malignancies, of which urothelial carcinoma is by far the most common in the Western world. Cisplatin-based therapy remains a mainstay of treatment for eligible patients with both localized and metastatic disease, but immunotherapies have provided a new and promising tool to use in the setting of progressing malignancy. The individual impact of these agents on OS is still being examined. Further studies and ongoing participation in clinical trials whenever possible continue to be essential to the discovery of future treatment options for this highly aggressive disease.
1. Stewart BW, Kleihues P. World cancer report. IARCPress. 2003.
2. Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009;115:68–74.
3. Hinotsu S, Akaza H, Miki T, et al. Bladder cancer develops 6 years earlier in current smokers: Analysis of bladder cancer registry data collected by the cancer registration committee of the Japanese Urological Association. Int J Urol 2009;16:64–9.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
5. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: bladder cancer. 2018. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed May 5, 2018.
6. Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45.
7. Pietzak EJ, Mucksavage P, Guzzo TJ, Malkowicz SB. Heavy cigarette smoking and aggressive bladder cancer at initial presentation. Urology 2015;86:968–73.
8. Khadra MH, Pickard RS, Charlton M, et al. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000;163:524–7.
9. Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA 2005;293:810–16.
10. Mariani AJ, Mariani MC, Macchioni C, et al. The significance of adult hematuria: 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. J Urol 1989;141:350–5.
11. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology 2001;57:604–10.
12. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part I: definition, detection, prevalence, and etiology. Urology 2001;57:599–603.
13. Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224–9.
14. Messing EM, Young TB, Hunt VB, et al. Home screening for hematuria: results of a multiclinic study. J Urol 1992;148:289–92.
15. Gray PJ, Lin CC, Jemal A, et al. Clinical–pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the National Cancer Data Base. Int J Radiat Oncol 2014;88:1048–56.
16. Bochner B, Hansel D, Efstathiou J, et al. Urinary bladder. In: Amin M, ed. AJCC cancer staging manual. 8th. New York: Springer; 2017:757.
17. Cahn DB, McGreen B, Lee A, et al. Clinical destiny of indeterminate pulmonary nodules in patients undergoing radical cystectomy for urothelial carcinoma of the bladder [abstract]. J Clin Oncol 2017;35(6 suppl):297-297.
18. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25–41.
19. Kurtis B, Zhuge J, Ojaimi C, et al. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann Diagn Pathol 2016;21:7–11.
20. Ito H, Kyo S, Kanaya T, et al. Detection of human telomerase reverse transcriptase messenger RNA in voided urine samples as a useful diagnostic tool for bladder cancer. Clin Cancer Res 1998;4:2807–10.
21. Utting M, Werner W, Dahse R, et al. Microsatellite analysis of free tumor DNA in urine, serum, and plasma of patients: a minimally invasive method for the detection of bladder cancer. Clin Cancer Res 2002;8:35–40.
22. Sethakorn N, O’Donnell PH. Spectrum of genomic alterations in FGFR3: current appraisal of the potential role of FGFR3 in advanced urothelial carcinoma. BJU Int 2016;118:681–91.
23. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66.
24. Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer ‘unfit’ for cisplatin-based chemotherapy. J Clin Oncol 2011;29:2432–8.
25. Raj GV, Iasonos A, Herr H, Donat SM. Formulas calculating creatinine clearance are inadequate for determining eligibility for cisplatin-based chemotherapy in bladder cancer. J Clin Oncol 2006;24:3095–100.26. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 2003;361:1927–34.
27. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol 2005;48:202–6.
28. Sternberg CN. A critical review of the management of bladder cancer. Crit Rev Oncol Hematol 1999;31:193–207.
29. Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol 1985;133:403–7.
30. Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006;42:50–4.
31. Sternberg CN, de Mulder PHM, Schornagel JH, et al. Randomized phase III trial of high–dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol No. 30924. J Clin Oncol 2001;19:2638–46.
32. Soloway MS, Einstein A, Corder MP, et al. A comparison of cisplatin and the combination of cisplatin and cyclophosphamide in advanced urothelial cancer. A National Bladder Cancer Collaborative Group A Study. Cancer 1983;52:767–72.
33. Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 2014;32:1895–901.
34. Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140–53.
35. Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 2015;68:959–67.
36. Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76–86.
37. Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014;32:3801–9.
38. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068–77.
39. Flaig T, Spiess P, Agarwal N, et al. National Comprehensive Cancer Network. Bladder cancer (version 3.2018). 2018. www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed May 5, 2018.
40. von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602–8.
41. Bamias A, Dafni U, Karadimou A, et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol 2013;24:1011–7.
42. Li R, Metcalfe M, Kukreja J, Navai N. Role of radical cystectomy in non-organ confined bladder cancer: a systematic review. Bladder Cancer 2018;4:31–40.
43. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012;30:191–9.
44. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76.
45. Balar A V, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483–92.
46. Manoharan M, Ayyathurai R, Soloway MS. Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 2009;104:1227–32.
47. Bellmunt J, de Wit R, Vaughn D, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26.
48. Bajorin D, de Wit R, Vaughn D, et al. Planned survival analysis from KEYNOTE-045: Phase 3, open-label study of pembrolizumab (pembro) versus paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC). (Abstract 4501). J Clin Oncol 2017;35(15_suppl):4501-4501.
49. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748–57.
50. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22.
51. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018;19:51–64.
52. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. JAMA Oncol 2017;3:e172411.
53. Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454–61.
54. Petrylak DP, de Wit R, Chi KN, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial. Lancet 2017;390:2266–77.
55. Abufaraj M, Dalbagni G, Daneshmand S, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol 2018;73:543–57.
56. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68.
57. Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–81.
58. Mayr R, May M, Martini T, et al. Comorbidity and performance indices as predictors of cancer-independent mortality but not of cancer-specific mortality after radical cystectomy for urothelial carcinoma of the bladder. Eur Urol 2012;62:662–70.
59. Nakagawa T, Hara T, Kawahara T, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol 2013;189:1275–81.
60. Ploeg M, Kums AC, Aben KK, et al. Prognostic factors for survival in patients with recurrence of muscle invasive bladder cancer after treatment with curative intent. Clin Genitourin Cancer 2011;9:14–21.
61. Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1997;15:2564–9.
62. Lin CC, Hsu CH, Huang CY, et al. Prognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5-fluorouracil-based regimens. Urology 2007;69:479–84.
63. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187–93.
Introduction
Bladder cancer is by far the most common cancer of the urinary system. Worldwide, approximately 450,000 new cases are diagnosed and 165,000 deaths are caused by bladder cancer each year.1 In the United States and in Europe, the most common type of bladder cancer is urothelial carcinoma (also referred to as transitional cell carcinoma), which accounts for more than 90% of all bladder cancers in these regions of the world. The remainder of bladder cancers are divided among squamous cell carcinomas, adenocarcinomas, small cell carcinomas, and, even more rarely, between various other nonepithelial tumors (eg, sarcoma).
Bladder cancer is classically thought of as a disease of the elderly, with a median age at diagnosis of 69 years in men and 71 years in women.2 The incidence of bladder cancer increases with age: in persons aged 65 to 69 years, incidence is 142 per 100,000 men and 33 per 100,000 women, and in those older than 85 years the rate doubles to 296 per 100,000 men and 74 per 100,000 women.3 The incidence is 3 times greater in men than in women.4
Urothelial carcinoma is traditionally categorized by its degree of invasion into the bladder wall: superficial (non-muscle-invasive), muscle-invasive, or metastatic disease. At the time of diagnosis, most patients have non-muscle-invasive disease (~60%); about 4% of all patients present initially with metastatic disease.5 This article focuses on metastatic bladder cancer, but muscle-invasive disease is discussed as well.
The most important factor contributing to the development of urothelial carcinoma is tobacco smoking. The risk of developing bladder cancer is 4 to 5 times higher in smokers as compared to nonsmokers, with some variation according to sex.6 Quantity of smoking exposure also plays a role, with heavy smokers demonstrating a higher likelihood for high-grade tumors with muscle invasion (or beyond) when compared to light smokers.7 Another important risk factor is occupational exposure to industrial materials, such as carpets, paints, plastics, and industrial chemicals. This type of exposure may be responsible for, or at least contribute to, the development of approximately 20% of urothelial carcinomas. Other risk factors for urothelial carcinoma include but are not limited to prior radiation to the pelvis, prior upper tract urothelial malignancy, human papillomavirus infection, and prior bladder augmentation.
Diagnosis and Staging
Case Presentation
A 63-year-old man with a past medical history of diabetes, deep vein thrombosis, occasional alcohol use, and regular pipe tobacco use presents to his primary care physician with complaints of hematuria. He reports that his urine was a dark red color that morning, which had never happened before. The patient is hemodynamically stable upon evaluation in the office, and a point-of-care urinalysis dipstick is strongly positive for blood. He is referred to a urologist for further evaluation.
In the urology office, urine microscopy is notable for more than 50 red blood cells (RBCs) per high-power field with normal RBC morphology. Flexible cystoscopy performed in the office reveals a single 2-cm, sessile, verrucous, nodular lesion located on the anterior bladder wall. A urine sample and a bladder wash specimen are sent for cytology evaluation. The patient is scheduled to undergo a complete transurethral resection of bladder tumor (TURBT) later that week with samples sent to pathology for evaluation.
- What are the clinical features of bladder cancer?
Hematuria is the most common presentation of bladder cancer, although its specificity is far lower than traditionally thought. In fact, only about 2% to 20% of cases that present with hematuria are found to be caused by malignancy. However, the incidence of genitourinary tract malignancy is much higher in patients presenting with gross hematuria (10%–20%)8–10 than in patients with microscopic hematuria alone.8,10–14 Typically, hematuria associated with malignancy is painless. Multiple studies have shown, however, that hematuria can be a normal variant, with one study demonstrating that up to 61% of patients with hematuria had no identifiable abnormality.8,10,11,13
Abdominal pain, flank pain, dysuria, urinary frequency/urgency, or other irritative voiding symptoms in the absence of hematuria can be presenting symptoms of bladder cancer as well. In these settings, discomfort typically suggests more advanced malignancy with at least local involvement or obstruction. Suprapubic pain may herald invasion into perivesical tissues and nerves, while involvement of the obturator fossa, perirectal fat, urogenital diaphragm, or presacral nerves can often present with perineal or rectal pain. Similarly, lower abdominal pain may represent involvement of lymph nodes, and right upper quadrant pain may signal liver metastasis. Cough or shortness of breath may signify metastatic disease in the lung. Finally, back, rib, or other boney pain may suggest distant metastasis.
- What next steps are required to complete this patient’s staging?
White light cystoscopy remains the gold standard for diagnosis and initial staging of bladder cancer. Additional tools include urine cytology and upper tract studies, including renal computed tomography (CT) urograms. Full urologic evaluation with all 3 modalities (cystourethroscopy, urinary cytology, and upper tract evaluation) is warranted for patients with a high suspicion for malignant etiology of hematuria. CT urograms are particularly useful for upper tract evaluation because they can be used to visualize kidney parenchyma, both renal pelvises and ureters, and pertinent abdominal and pelvic lymph nodes. Initial staging is completed through TURBT, which should ideally contain a segment of muscularis propria to distinguish between Ta (noninvasive), T1, and T2 tumors (Figure 1).
Regarding staging, T1 tumors are distinguished from Ta malignancies by their involvement in the urothelial basement membrane. Tumor invasion into the muscularis propria indicates T2 tumors, while T3 tumors extend through the muscle into the serosa and involve the complete thickness of the bladder wall. Involvement of nearby structures defines T4 bladder cancers, with T4a malignancies involving adjacent organs (prostate, vagina, uterus, or bowel) and T4b tumors involving the abdominal wall, pelvic wall, or other more distant organs. According to the American Joint Committee on Cancer’s most recent TNM staging system (Table 1),16 lymph node involvement in the true pelvis (that is, N1–N3) with T1 to T4a disease is now classified as stage III disease.
Bladder cancer is often broadly categorized as either non-muscle-invasive or muscle-invasive (which can include metastatic disease). This classification has important implications for treatment. As such, all diagnostic biopsies should be performed with the goal of reaching at least the depth of the muscularis propria in order to accurately detect potential muscularis invasion. If no muscle is detected in the initial specimen, re-resection is recommended if safe and feasible. In cases where muscle cannot be obtained, imaging evidence of T3 disease from CT or magnetic resonance imaging may be used as a surrogate indicator. Once muscle-invasive disease is confirmed, CT evaluation of the chest is also recommended, as bladder cancer can metastasize to the lungs; furthermore, patients are often at risk for secondary concomitant lung cancers given that smoking is the most prevalent risk factor for both. However, patients with small, indeterminate lung nodules not amenable to biopsy should not be denied curative intent treatment given the high likelihood that they represent benign findings.17
Pathogenesis
Because non-muscle-invasive and muscle-invasive tumors behave so differently, they are thought to arise from 2 distinct mechanisms. Although there is overlap and non-muscle-invasive cancer can certainly progress to a high-grade, invasive type of malignancy over time, current theory proposes that non-muscle-invasive bladder cancer predominantly develops just from urothelial hyperplasia, which then recruits branching vasculature to grow slowly. More aggressive urothelial carcinomas, including muscle-invasive and metastatic disease, are instead thought to arise directly from flat dysplasia that progresses to carcinoma in situ, and is much more prone to invasive growth and distant spread.18
Regardless of grade and stage, the most commonly identified genomic alterations in urothelial carcinoma are mutations in the promoter region of the telomerase reverse transcriptase (TERT) gene, which have been identified in approximately 70% of cases.19 Mutations in TERT can be readily detected in urine sediments and may ultimately have implications for diagnosis and early detection.20,21 In current practice, however, the clinical relevance of these observations remains under development. Other genomic alterations that may contribute to the development of urothelial carcinoma, and also provide new potential therapeutic targets, include alterations in the TP53 gene, the RB (retinoblastoma) gene, and the FGFR3 (fibroblast growth factor receptor) gene. FGFR3 has particular significance as it appears to be relatively common in non-muscle invasive disease (up to 60%–70%) and is likely an actionable driver mutation that may define a particular molecular subset of urothelial carcinoma; thus, it may have important implications for treatment decisions.22
Treatment
Case Continued
Pathologic evaluation of the specimen reveals a high-grade urothelial carcinoma with tumor invasion into the muscularis propria. A CT urogram is performed and does not reveal any notably enlarged pelvic nodes or suspicious lesions in the upper urinary tract. CT chest does not reveal any evidence of distant metastatic disease. Given the presence of muscle-invasive disease, the patient agrees to proceed with neoadjuvant chemotherapy and radical cystoprostatectomy with pelvic node dissection. He undergoes treatment with dose-dense (accelerated) MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) for 3 cycles, followed by surgery with cystoprostatectomy. Overall, he tolerates the procedure well and recovers quickly. Pathology reveals the presence of disease in 2 regional nodes, consistent with T4a (stage III) disease, and a small degree of residual disease in the bladder. He is followed closely in the oncology clinic, returning for urine cytology, liver and renal function tests, and imaging with CT of chest, abdomen, and pelvis every 3 months.
- What is the first-line approach to management in patients with muscle-invasive disease?
- How would the treatment strategy differ if the patient had presented with metastatic disease (stage IV)?
First-Line Management for Curative Intent: Muscle-Invasive Disease
Muscle-invasive urothelial carcinoma (including T2, T3, or T4 disease) is typically treated in a multidisciplinary fashion with neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy. This approach is recommended over radical cystectomy alone because of high relapse rates following cystectomy alone, even in the setting of bilateral pelvic lymphadenectomy.23 However, because of the associated short- and long-term toxicity of cisplatin-based regimens, this optimal treatment paradigm is reserved for patients deemed cisplatin-eligible.
Medical fitness to receive cisplatin-based chemotherapy is assessed by a number of factors and varies by institution, but most frequently consider functional status (Eastern Cooperative Oncology Group [ECOG] performance status or Karnofsky Performance Status), creatinine clearance, hearing preservation, peripheral neuropathy, and cardiac function.24 Many programs will elect to defer cisplatin-based chemotherapy in patients with low performance status (ie, < 60–70 on Karnofsky scale or > 2 on ECOG scale), creatinine clearance below 60 mL/min, or significant heart failure (NHYA class III or worse). Cisplatin-based chemotherapy may worsen hearing loss in those with hearing loss of 25 dB from baseline at 2 continuous frequencies and also may worsen neuropathy in those with baseline grade 1 peripheral neuropathy. However, these adverse outcomes must be balanced against the curative intent of the multimodality systemic approach.
In patients with renal insufficiency, caution must be taken with regard to cisplatin. Percutaneous nephrostomy placement or ureteral stenting should be attempted to relieve any ureteral outlet obstruction and restore kidney function if a patient’s renal insufficiency has resulted from this obstruction. If medical renal disease or long-term renal insufficiency is present, however, patients should instead be referred for immediate cystectomy or for a bladder-preserving approach. Generally, a creatinine clearance of 60 mL/min is required to safely receive cisplatin-based chemotherapy, although some advocate for treatment with a creatinine clearance as low as 50 mL/min. When this extended criterion is used, the dose of cisplatin may be split over 2 days to minimize renal toxicity and maximize hydration. Analysis of renal function utilizing a 24-hour urine collection should be incorporated whenever possible, as estimates of creatinine clearance have been demonstrated to be inaccurate in some instances.25
For cisplatin-eligible patients, neoadjuvant chemotherapy with a cisplatin base has consistently demonstrated a survival benefit when given prior to surgery.26,27 Historically, several different platinum-based regimens have been studied, with none showing superior effectiveness in a randomized trial over the others in the neoadjuvant setting. These regimens have included classic MVAC, dose-dense MVAC (MVAC with pegfilgrastim), GC (gemcitabine and cisplatin), and CMV (methotrexate, vinblastine, cisplatin, and leucovorin).
While classic MVAC was preferred in the 1990s and early 2000s,28,29 the availability of growth factor, such as pegfilgrastim, has made dose-dense MVAC (otherwise referred to as accelerated MVAC or ddMVAC) widely preferred and universally recommended over classic MVAC. The ddMVAC regimen with the addition of a synthetic granulocyte colony-stimulating factor (G-CSF) is substantially better tolerated than classic MVAC, as the G-CSF support minimizes the severe toxicities of classic MVAC, such as myelosuppression and mucositis, and allows for the administration of drugs in a dose-dense fashion.30,31
Both ddMVAC and GC are considered reasonable options for neoadjuvant chemotherapy and are the predominant choices for cisplatin-eligible patients (Table 2).
Prospective data defining the role of adjuvant chemotherapy for patients after cystectomy has been fraught by a variety of factors, including the known benefit of neoadjuvant chemotherapy, the high complication rate of cystectomy making chemotherapy infeasible, and clinician bias that has hampered accrual in prior trials. Thus, no level 1 evidence exists defining the benefit of adjuvant chemotherapy in patients who did not receive neoadjuvant therapy. In a report of the largest study performed in this setting, there was a statistically significant benefit in PFS but not in OS.36 Criticisms of this trial include its lack of statistical power due to a failure to accrue the targeted goal and the preponderance of node-positive patients. Regardless, for patients who have pT2–4, N1 disease after radical cystectomy and remain cisplatin-eligible after not receiving neoadjuvant chemotherapy, this remains an option.
Despite the established clinical dogma surrounding neoadjuvant chemotherapy followed by surgery, some patients are either not eligible for or decline to receive radical cystectomy, while others are not candidates for neoadjuvant cisplatin-based chemotherapy for the reasons outlined above. For patients who are surgical candidates but unable to receive neoadjuvant chemotherapy due to renal or cardiac function, they may proceed directly to surgery. For patients unable or unwilling to proceed to radical cystectomy regardless, bladder preservation strategies exist. Maximal TURBT may be an option for some patients, but, as outlined above, used alone this would be likely to lead to a high degree of local and distant failure. Combined modality chemoradiotherapy as consolidation after maximal TURBT is an established option for patients unable to undergo surgery or seeking bladder preservation. Several trials have demonstrated encouraging outcomes with this approach and were highlighted in a large meta-analysis.37 Various chemosensitizing chemotherapeutic regimens have been evaluated, including cisplatin alone or as a doublet, gemcitabine alone, and 5-fluouracil plus mitomycin C, but no randomized studies have compared these regimens to each other, nor have they been compared to surgical approaches. However, this strategy remains an option as an alternative to surgery.
First-Line Management: Metastatic Disease
The approach to therapy in patients who present with metastatic urothelial carcinoma is very similar to that used in neoadjuvant perioperative chemotherapy. The consensus first-line treatment in medically appropriate patients is cisplatin-based chemotherapy with either GC or ddMVAC (both category 1 National Comprehensive Cancer Network [NCCN] recommendations; Figure 2).30,31,38–40
Head-to-head studies specifically comparing ddMVAC and GC have been limited. GC has been compared to classic MVAC, with results showing equivalent efficacy but improved tolerability, as expected.38,40 ddMVAC was compared with a modified version of GC (termed “dose-dense GC”) in a phase 3 study from Greece, which demonstrated similar outcomes.41
Surgical intervention with radical cystectomy and regional lymph node dissection is typically deferred for patients who present with distant metastatic disease, unlike those who present with locally advanced disease. Radical cystectomy has traditionally been thought of as overly aggressive without sufficient benefit, although evidence to guide this approach remains sparse.42 As such, most expert recommendations and consensus statements simply recommend against surgical intervention and leave the decision between ddMVAC and GC up to the individual clinician.
In patients who are not eligible for cisplatin therapy, it is reasonable to consider chemotherapy with a combination of gemcitabine and carboplatin. This combination has been shown to be equivalent to MCAVI (methotrexate, carboplatin, vinblastine) in terms of overall survival (OS; 9 months versus 8 months) and progression-free survival (PFS; 6 months versus 4 months) with significantly fewer serious toxicities (9% versus 21%).43
The advent of immunotherapy in recent years has provided several new alternatives for cisplatin-ineligible patients. While immunotherapies such as pembrolizumab or atezolizumab are not yet recommended as first-line therapy for cisplatin-eligible patients, these 2 drugs are approved as options for first-line therapy in cisplatin-ineligible patients with metastatic disease. In a recent phase 2 trial (IMvigor210) involving 119 patients who were given atezolizumab as first-line therapy, median PFS was 2.7 months and median OS was 15.9 months.44 Another trial using data from patients in the KEYNOTE-052 study who received pembrolizumab as first-line therapy demonstrated antitumor activity with pembrolizumab and acceptable tolerability in cisplatin-ineligible patients with advanced urothelial carcinoma.45 The primary endpoint was objective response (either complete or partial response), which was achieved in 24% of the intention-to-treat population. Median PFS was 2 months, and 6-month OS was observed in 67% of patients. Both atezolizumab and pembrolizumab were given accelerated approval based on these single-arm studies in this setting. However, due to inferior outcomes in subsequent trials that included single-agent immunotherapy arms for patients in the first-line setting, the US Food and Drug Administration (FDA) has clarified the approval. In the subsequent trials, patients with a low PD-L1 biomarker based on the individual assay used for each drug did worse on immunotherapy alone (compared to chemotherapy or both combined), and the single-therapy arms were stopped early. Thus, the FDA now recommends that pembrolizumab or atezolizumab be used in the first line only for cisplatin-ineligible patients who have PD-L1 expression on tumor cells above the threshold studied on each individual assay, or are unfit for any platinum-based chemotherapy. Further study regarding the optimal role of biomarkers and chemotherapy-immunotherapy combinations is ongoing.
Case Continued
Ten months after his procedure, the patient is found to have prominent retroperitoneal lymphadenopathy and a 1.0-cm liver nodule suspicious for malignancy is noted on surveillance imaging. CT-guided biopsy of the liver reveals high-grade urothelial carcinoma, consistent with both recurrence and distant metastasis. The patient is informed that he needs to resume systemic therapy for recurrent metastatic disease. The options discussed include salvage single-agent chemotherapy with gemcitabine or immunotherapy with pembrolizumab. He elects to move forward with immunotherapy and is scheduled to begin pembrolizumab.
- What other immunotherapies might this patient consider for second-line therapy?
- Is chemotherapy a second-line option for this patient?
Second-Line Therapies and Management of Progressive Disease
Disease progression is unfortunately seen in the majority of cases of advanced urothelial carcinoma.46 New second-line therapies have recently been approved by the FDA in the form of monoclonal antibodies targeting programmed death 1 (PD-1) and a PD-1 ligand (PD-L1) (Figure 3).
Approval of pembrolizumab, a PD-1 inhibitor, was largely supported by the Keynote-045 trial,47,48 which looked at 542 patients who had progressed or recurred after platinum-based chemotherapy. These patients were randomly assigned to either pembrolizumab or investigator’s choice of chemotherapy (paclitaxel, docetaxel, or vinflunine). Patients treated with pembrolizumab had a significantly improved OS (median of 10.3 months versus 7.4 months), but no statistically significant difference in PFS (2.1 months versus 3.3 months). Interestingly, the rate of responses of 12 months or longer was higher with pembrolizumab than with more traditional second-line chemotherapy (68% versus 35%). The strength of this data has led to a category 1 recommendation in the most recent NCCN guidelines.39
The approval of atezolizumab, a PD-L1 inhibitor, as a second-line therapy for advanced urothelial carcinoma is largely supported by data from IMvigor211, a phase 3 trial that studied 931 patients randomly assigned to atezolizumab or investigator’s choice chemotherapy. OS did not differ significantly between patients in the atezolizumab group who had ≥ 5% expression of PD-L1 on tumor-infiltrating immune cells and patients in the chemotherapy group (11.1 months versus 10.6 months), but mean duration of response was longer (15.9 months versus 8.3 months).49 Therapy with atezolizumab had significantly fewer toxicities than chemotherapy (grade 3 or 4 toxicities of 20% versus 43%).
Phase 3 studies of nivolumab (PD-1 inhibitor), avelumab (PD-L1 inhibitor), and durvalumab (PD-L1 inhibitor) have not yet been published. These agents have received accelerated approval, however, as second-line treatment of advanced urothelial carcinoma based on promising data from phase 1 and phase 2 studies.50–52
Second-line chemotherapy is also an option for patients who do not qualify for immunotherapy or who progress during or after immunotherapy. Although there has been a great deal of excitement about new developments with immunotherapy and the survival benefit seen compared to investigator’s choice chemotherapy, the fact remains that most patients do not respond to immunotherapy. Still, some patients do derive benefit from single-agent chemotherapy in the platinum-refractory setting. Options based on primarily single-arm studies include gemcitabine, paclitaxel, docetaxel, pemetrexed, ifosfamide, oxaliplatin, and eribulin (Figure 2). In a randomized phase 3 trial, vinflunine demonstrated an OS benefit in platinum-refractory patients compared to best supportive care; it subsequently received approval by the European Medicines Agency.53 More recently in the phase 3 RANGE trial, docetaxel plus ramucirumab (a monoclonal antibody targeting vascular endothelial growth factor receptor 2) was compared to docetaxel plus placebo and met its primary endpoint of an improvement in PFS (median 4.07 months versus 2.76 months, P = 0.0118).54 OS has not been reported and this regimen has not yet received regulatory approval, however. Unfortunately, trials comparing these regimens are lacking, and response rates and survival remain modest. Clearly, better therapies and biomarkers to help personalize treatment options are needed.
Further investigations are underway with alternative regimens, including but not limited to targeted therapy in the setting of specific genetic and epigenetic alterations. These include mutations affecting tyrosine kinase receptors (eg, RAS/RAF, PI3K, AKT, and mTOR), cell cycle regulators (eg, TP53 or RB1), FGFR3 mutations, PTEN deletions, gene amplifications (eg, FGFR1, CCND1, and MDM2), or changes in genes responsible for chromatin remodeling (eg, UTX, CHD6, or ARID1A). As noted, there is particular excitement regarding FGFR3 inhibitors, which have shown compelling efficacy in phase 1 and 2 single-arm trials. Several agents are being evaluated in randomized trials and represent a potential path to the first targeted therapeutic class with a role in urothelial malignancies.
Surgical resection of metastases may be considered in very select cases.55 Surgery may have a role in limiting metastatic complications and improving cancer control, but this should be discussed at length with the patient using a multidisciplinary approach with careful restaging prior to surgery.
Case Continued
The patient remains on pembrolizumab every 3 weeks as per protocol with regular surveillance imaging. His disease stabilizes as the nodule in his liver and the retroperitoneal lymph nodes, all representing metastatic disease, became slightly smaller in size without evidence of any new disease. He continues to follow up closely with his genitourinary oncologist, undergoing regular surveillance and imaging every 3 months without evidence of disease progression.
Approximately 12 months into therapy, the patient notices a nonproductive cough with progressive and rapidly worsening shortness of breath. He is noted to be hypoxic with oxygen saturation levels to 79% in clinic and is sent immediately to the emergency department by his oncologist. Diffuse bilateral reticular opacities are noted on chest radiograph. Non-contrast CT scan demonstrates diffuse ground-glass opacities consistent with acute respiratory distress syndrome–pattern pneumonitis. He is admitted to the intensive care unit.
The patient is aggressively treated with high-flow nasal oxygen supplementation, intravenous steroids, and empiric antibiotics. He slowly improves on high-dose steroids (methylprednisolone 1 mg/kg/day) without requiring intubation or infliximab therapy and is discharged home in stable condition after 10 days. Oral steroid therapy is continued with a long taper over 6 weeks. In the setting of his grade 3 pneumonitis, pembrolizumab is discontinued and the patient is scheduled for a follow-up appointment with his oncologist to discuss next steps.
- In addition to pneumonitis, what other toxicities should you monitor for in patients treated with an immune checkpoint inhibitor?
- Is this patient a candidate to receive immunotherapy again in the future?
Treatment Toxicities
As use of immune checkpoint inhibitors has become more prevalent, the medical community has become increasingly aware of various immune-related adverse effects (irAE) associated with these drugs. These toxicities can be seen in virtually any organ system, and even vague complaints that arise years after therapy initiation should be treated with a high level of suspicion. The most commonly affected organ systems include the skin, gastrointestinal (GI) tract, lungs, liver, and endocrine system, although all other organ systems can be involved (Table 3) and toxicities appear to be similar across individual drugs.
The American Society of Clinical Oncology recently published a complete set of recommendations to guide clinicians on appropriate treatment strategies for each manifestation of immunotherapy-related toxicity.56 The details of these recommendations largely fall outside the purview of this article, but the mainstays of management are worth noting. These include high-dose systemic glucocorticoids, along with supportive care and cessation of immunotherapy in grade 3 or 4 toxicities. Infliximab is frequently recommended as an adjunct in severe or refractory cases.
Chemotherapy-related toxicities, on the other hand, are well-described and tend to be more familiar to patients and clinicians (Table 3). Classic MVAC, which has now been largely replaced by ddMVAC, was notoriously difficult to tolerate. It was known for a high rate of serious (grade 3 or 4) myelosuppressive complications as well as frequent GI toxicities. These complications include neutropenia (57%), stomatitis (10%), and nausea and vomiting (6%).23 ddMVAC with growth factor support is much better tolerated than classic MVAC. Prominent complaints with ddMVAC still can include nausea, GI distress, mucositis, and fatigue, but the incidence of myelosuppressive complications in particular has markedly decreased. GC is largely well tolerated, with minimal nausea and manageable myelotoxicity, but it is associated with an increased risk of venous thromboembolism.38
Prognosis
Case Conclusion
After returning home, the patient discusses his complicated medical course with his oncologist. Given his continued high quality of life with good functional status, he requests to continue with therapy for his metastatic bladder cancer and is interested in joining a clinical trial. He is referred to a nearby academic center with openings in a clinical trial for which he would be eligible. In the meantime, his oncologist guides him through filling out an advance directive and recommends that he make an appointment with palliative care services to ensure adequate home support for any future needs he may have.
- What is the estimated 5-year survival rate for patients with metastatic bladder cancer?
Overall, prognosis in patients with metastatic bladder cancer remains poor. Median survival in patients being treated with multi-agent chemotherapy is approximately 15 months,38,40 with an expected 5-year survival of just 15%. This is much improved, however, as prior to the advent of modern chemotherapy estimated survival was just 6 months with metastatic bladder cancer. Importantly, these figures do not take into account the recent advancements with immunotherapy, and thus it is reasonable to assume survival rates may continue to improve. In light of these recent advances, it is strongly recommended that whenever possible patients and clinicians consider participation in clinical trials to continue uncovering new and better therapies moving forward.
A number of tools have been developed to help risk stratify patients based on comorbidity, performance status, and other characteristics, but none have been universally adopted.57–60 As with many other malignancies, performance status is an important predictor of clinical outcomes in these patients.61–63 Sites of metastasis also may serve to suggest the course of disease. Patients with visceral metastases typically exhibit significantly worse disease with a shortened survival. The role of molecular factors as prognostic markers in bladder cancer is still under investigation. Many biomarkers are being considered (including mutations and polymorphisms in p53, ERCC1, and ERCC2), and evidence suggests some may have a role in prognosis; thus far, none have been validated as prognostic or predictive tools in urothelial carcinoma.
Conclusion
Bladder cancer includes an aggressive group of genitourinary tract malignancies, of which urothelial carcinoma is by far the most common in the Western world. Cisplatin-based therapy remains a mainstay of treatment for eligible patients with both localized and metastatic disease, but immunotherapies have provided a new and promising tool to use in the setting of progressing malignancy. The individual impact of these agents on OS is still being examined. Further studies and ongoing participation in clinical trials whenever possible continue to be essential to the discovery of future treatment options for this highly aggressive disease.
Introduction
Bladder cancer is by far the most common cancer of the urinary system. Worldwide, approximately 450,000 new cases are diagnosed and 165,000 deaths are caused by bladder cancer each year.1 In the United States and in Europe, the most common type of bladder cancer is urothelial carcinoma (also referred to as transitional cell carcinoma), which accounts for more than 90% of all bladder cancers in these regions of the world. The remainder of bladder cancers are divided among squamous cell carcinomas, adenocarcinomas, small cell carcinomas, and, even more rarely, between various other nonepithelial tumors (eg, sarcoma).
Bladder cancer is classically thought of as a disease of the elderly, with a median age at diagnosis of 69 years in men and 71 years in women.2 The incidence of bladder cancer increases with age: in persons aged 65 to 69 years, incidence is 142 per 100,000 men and 33 per 100,000 women, and in those older than 85 years the rate doubles to 296 per 100,000 men and 74 per 100,000 women.3 The incidence is 3 times greater in men than in women.4
Urothelial carcinoma is traditionally categorized by its degree of invasion into the bladder wall: superficial (non-muscle-invasive), muscle-invasive, or metastatic disease. At the time of diagnosis, most patients have non-muscle-invasive disease (~60%); about 4% of all patients present initially with metastatic disease.5 This article focuses on metastatic bladder cancer, but muscle-invasive disease is discussed as well.
The most important factor contributing to the development of urothelial carcinoma is tobacco smoking. The risk of developing bladder cancer is 4 to 5 times higher in smokers as compared to nonsmokers, with some variation according to sex.6 Quantity of smoking exposure also plays a role, with heavy smokers demonstrating a higher likelihood for high-grade tumors with muscle invasion (or beyond) when compared to light smokers.7 Another important risk factor is occupational exposure to industrial materials, such as carpets, paints, plastics, and industrial chemicals. This type of exposure may be responsible for, or at least contribute to, the development of approximately 20% of urothelial carcinomas. Other risk factors for urothelial carcinoma include but are not limited to prior radiation to the pelvis, prior upper tract urothelial malignancy, human papillomavirus infection, and prior bladder augmentation.
Diagnosis and Staging
Case Presentation
A 63-year-old man with a past medical history of diabetes, deep vein thrombosis, occasional alcohol use, and regular pipe tobacco use presents to his primary care physician with complaints of hematuria. He reports that his urine was a dark red color that morning, which had never happened before. The patient is hemodynamically stable upon evaluation in the office, and a point-of-care urinalysis dipstick is strongly positive for blood. He is referred to a urologist for further evaluation.
In the urology office, urine microscopy is notable for more than 50 red blood cells (RBCs) per high-power field with normal RBC morphology. Flexible cystoscopy performed in the office reveals a single 2-cm, sessile, verrucous, nodular lesion located on the anterior bladder wall. A urine sample and a bladder wash specimen are sent for cytology evaluation. The patient is scheduled to undergo a complete transurethral resection of bladder tumor (TURBT) later that week with samples sent to pathology for evaluation.
- What are the clinical features of bladder cancer?
Hematuria is the most common presentation of bladder cancer, although its specificity is far lower than traditionally thought. In fact, only about 2% to 20% of cases that present with hematuria are found to be caused by malignancy. However, the incidence of genitourinary tract malignancy is much higher in patients presenting with gross hematuria (10%–20%)8–10 than in patients with microscopic hematuria alone.8,10–14 Typically, hematuria associated with malignancy is painless. Multiple studies have shown, however, that hematuria can be a normal variant, with one study demonstrating that up to 61% of patients with hematuria had no identifiable abnormality.8,10,11,13
Abdominal pain, flank pain, dysuria, urinary frequency/urgency, or other irritative voiding symptoms in the absence of hematuria can be presenting symptoms of bladder cancer as well. In these settings, discomfort typically suggests more advanced malignancy with at least local involvement or obstruction. Suprapubic pain may herald invasion into perivesical tissues and nerves, while involvement of the obturator fossa, perirectal fat, urogenital diaphragm, or presacral nerves can often present with perineal or rectal pain. Similarly, lower abdominal pain may represent involvement of lymph nodes, and right upper quadrant pain may signal liver metastasis. Cough or shortness of breath may signify metastatic disease in the lung. Finally, back, rib, or other boney pain may suggest distant metastasis.
- What next steps are required to complete this patient’s staging?
White light cystoscopy remains the gold standard for diagnosis and initial staging of bladder cancer. Additional tools include urine cytology and upper tract studies, including renal computed tomography (CT) urograms. Full urologic evaluation with all 3 modalities (cystourethroscopy, urinary cytology, and upper tract evaluation) is warranted for patients with a high suspicion for malignant etiology of hematuria. CT urograms are particularly useful for upper tract evaluation because they can be used to visualize kidney parenchyma, both renal pelvises and ureters, and pertinent abdominal and pelvic lymph nodes. Initial staging is completed through TURBT, which should ideally contain a segment of muscularis propria to distinguish between Ta (noninvasive), T1, and T2 tumors (Figure 1).
Regarding staging, T1 tumors are distinguished from Ta malignancies by their involvement in the urothelial basement membrane. Tumor invasion into the muscularis propria indicates T2 tumors, while T3 tumors extend through the muscle into the serosa and involve the complete thickness of the bladder wall. Involvement of nearby structures defines T4 bladder cancers, with T4a malignancies involving adjacent organs (prostate, vagina, uterus, or bowel) and T4b tumors involving the abdominal wall, pelvic wall, or other more distant organs. According to the American Joint Committee on Cancer’s most recent TNM staging system (Table 1),16 lymph node involvement in the true pelvis (that is, N1–N3) with T1 to T4a disease is now classified as stage III disease.
Bladder cancer is often broadly categorized as either non-muscle-invasive or muscle-invasive (which can include metastatic disease). This classification has important implications for treatment. As such, all diagnostic biopsies should be performed with the goal of reaching at least the depth of the muscularis propria in order to accurately detect potential muscularis invasion. If no muscle is detected in the initial specimen, re-resection is recommended if safe and feasible. In cases where muscle cannot be obtained, imaging evidence of T3 disease from CT or magnetic resonance imaging may be used as a surrogate indicator. Once muscle-invasive disease is confirmed, CT evaluation of the chest is also recommended, as bladder cancer can metastasize to the lungs; furthermore, patients are often at risk for secondary concomitant lung cancers given that smoking is the most prevalent risk factor for both. However, patients with small, indeterminate lung nodules not amenable to biopsy should not be denied curative intent treatment given the high likelihood that they represent benign findings.17
Pathogenesis
Because non-muscle-invasive and muscle-invasive tumors behave so differently, they are thought to arise from 2 distinct mechanisms. Although there is overlap and non-muscle-invasive cancer can certainly progress to a high-grade, invasive type of malignancy over time, current theory proposes that non-muscle-invasive bladder cancer predominantly develops just from urothelial hyperplasia, which then recruits branching vasculature to grow slowly. More aggressive urothelial carcinomas, including muscle-invasive and metastatic disease, are instead thought to arise directly from flat dysplasia that progresses to carcinoma in situ, and is much more prone to invasive growth and distant spread.18
Regardless of grade and stage, the most commonly identified genomic alterations in urothelial carcinoma are mutations in the promoter region of the telomerase reverse transcriptase (TERT) gene, which have been identified in approximately 70% of cases.19 Mutations in TERT can be readily detected in urine sediments and may ultimately have implications for diagnosis and early detection.20,21 In current practice, however, the clinical relevance of these observations remains under development. Other genomic alterations that may contribute to the development of urothelial carcinoma, and also provide new potential therapeutic targets, include alterations in the TP53 gene, the RB (retinoblastoma) gene, and the FGFR3 (fibroblast growth factor receptor) gene. FGFR3 has particular significance as it appears to be relatively common in non-muscle invasive disease (up to 60%–70%) and is likely an actionable driver mutation that may define a particular molecular subset of urothelial carcinoma; thus, it may have important implications for treatment decisions.22
Treatment
Case Continued
Pathologic evaluation of the specimen reveals a high-grade urothelial carcinoma with tumor invasion into the muscularis propria. A CT urogram is performed and does not reveal any notably enlarged pelvic nodes or suspicious lesions in the upper urinary tract. CT chest does not reveal any evidence of distant metastatic disease. Given the presence of muscle-invasive disease, the patient agrees to proceed with neoadjuvant chemotherapy and radical cystoprostatectomy with pelvic node dissection. He undergoes treatment with dose-dense (accelerated) MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) for 3 cycles, followed by surgery with cystoprostatectomy. Overall, he tolerates the procedure well and recovers quickly. Pathology reveals the presence of disease in 2 regional nodes, consistent with T4a (stage III) disease, and a small degree of residual disease in the bladder. He is followed closely in the oncology clinic, returning for urine cytology, liver and renal function tests, and imaging with CT of chest, abdomen, and pelvis every 3 months.
- What is the first-line approach to management in patients with muscle-invasive disease?
- How would the treatment strategy differ if the patient had presented with metastatic disease (stage IV)?
First-Line Management for Curative Intent: Muscle-Invasive Disease
Muscle-invasive urothelial carcinoma (including T2, T3, or T4 disease) is typically treated in a multidisciplinary fashion with neoadjuvant cisplatin-based chemotherapy followed by radical cystectomy. This approach is recommended over radical cystectomy alone because of high relapse rates following cystectomy alone, even in the setting of bilateral pelvic lymphadenectomy.23 However, because of the associated short- and long-term toxicity of cisplatin-based regimens, this optimal treatment paradigm is reserved for patients deemed cisplatin-eligible.
Medical fitness to receive cisplatin-based chemotherapy is assessed by a number of factors and varies by institution, but most frequently consider functional status (Eastern Cooperative Oncology Group [ECOG] performance status or Karnofsky Performance Status), creatinine clearance, hearing preservation, peripheral neuropathy, and cardiac function.24 Many programs will elect to defer cisplatin-based chemotherapy in patients with low performance status (ie, < 60–70 on Karnofsky scale or > 2 on ECOG scale), creatinine clearance below 60 mL/min, or significant heart failure (NHYA class III or worse). Cisplatin-based chemotherapy may worsen hearing loss in those with hearing loss of 25 dB from baseline at 2 continuous frequencies and also may worsen neuropathy in those with baseline grade 1 peripheral neuropathy. However, these adverse outcomes must be balanced against the curative intent of the multimodality systemic approach.
In patients with renal insufficiency, caution must be taken with regard to cisplatin. Percutaneous nephrostomy placement or ureteral stenting should be attempted to relieve any ureteral outlet obstruction and restore kidney function if a patient’s renal insufficiency has resulted from this obstruction. If medical renal disease or long-term renal insufficiency is present, however, patients should instead be referred for immediate cystectomy or for a bladder-preserving approach. Generally, a creatinine clearance of 60 mL/min is required to safely receive cisplatin-based chemotherapy, although some advocate for treatment with a creatinine clearance as low as 50 mL/min. When this extended criterion is used, the dose of cisplatin may be split over 2 days to minimize renal toxicity and maximize hydration. Analysis of renal function utilizing a 24-hour urine collection should be incorporated whenever possible, as estimates of creatinine clearance have been demonstrated to be inaccurate in some instances.25
For cisplatin-eligible patients, neoadjuvant chemotherapy with a cisplatin base has consistently demonstrated a survival benefit when given prior to surgery.26,27 Historically, several different platinum-based regimens have been studied, with none showing superior effectiveness in a randomized trial over the others in the neoadjuvant setting. These regimens have included classic MVAC, dose-dense MVAC (MVAC with pegfilgrastim), GC (gemcitabine and cisplatin), and CMV (methotrexate, vinblastine, cisplatin, and leucovorin).
While classic MVAC was preferred in the 1990s and early 2000s,28,29 the availability of growth factor, such as pegfilgrastim, has made dose-dense MVAC (otherwise referred to as accelerated MVAC or ddMVAC) widely preferred and universally recommended over classic MVAC. The ddMVAC regimen with the addition of a synthetic granulocyte colony-stimulating factor (G-CSF) is substantially better tolerated than classic MVAC, as the G-CSF support minimizes the severe toxicities of classic MVAC, such as myelosuppression and mucositis, and allows for the administration of drugs in a dose-dense fashion.30,31
Both ddMVAC and GC are considered reasonable options for neoadjuvant chemotherapy and are the predominant choices for cisplatin-eligible patients (Table 2).
Prospective data defining the role of adjuvant chemotherapy for patients after cystectomy has been fraught by a variety of factors, including the known benefit of neoadjuvant chemotherapy, the high complication rate of cystectomy making chemotherapy infeasible, and clinician bias that has hampered accrual in prior trials. Thus, no level 1 evidence exists defining the benefit of adjuvant chemotherapy in patients who did not receive neoadjuvant therapy. In a report of the largest study performed in this setting, there was a statistically significant benefit in PFS but not in OS.36 Criticisms of this trial include its lack of statistical power due to a failure to accrue the targeted goal and the preponderance of node-positive patients. Regardless, for patients who have pT2–4, N1 disease after radical cystectomy and remain cisplatin-eligible after not receiving neoadjuvant chemotherapy, this remains an option.
Despite the established clinical dogma surrounding neoadjuvant chemotherapy followed by surgery, some patients are either not eligible for or decline to receive radical cystectomy, while others are not candidates for neoadjuvant cisplatin-based chemotherapy for the reasons outlined above. For patients who are surgical candidates but unable to receive neoadjuvant chemotherapy due to renal or cardiac function, they may proceed directly to surgery. For patients unable or unwilling to proceed to radical cystectomy regardless, bladder preservation strategies exist. Maximal TURBT may be an option for some patients, but, as outlined above, used alone this would be likely to lead to a high degree of local and distant failure. Combined modality chemoradiotherapy as consolidation after maximal TURBT is an established option for patients unable to undergo surgery or seeking bladder preservation. Several trials have demonstrated encouraging outcomes with this approach and were highlighted in a large meta-analysis.37 Various chemosensitizing chemotherapeutic regimens have been evaluated, including cisplatin alone or as a doublet, gemcitabine alone, and 5-fluouracil plus mitomycin C, but no randomized studies have compared these regimens to each other, nor have they been compared to surgical approaches. However, this strategy remains an option as an alternative to surgery.
First-Line Management: Metastatic Disease
The approach to therapy in patients who present with metastatic urothelial carcinoma is very similar to that used in neoadjuvant perioperative chemotherapy. The consensus first-line treatment in medically appropriate patients is cisplatin-based chemotherapy with either GC or ddMVAC (both category 1 National Comprehensive Cancer Network [NCCN] recommendations; Figure 2).30,31,38–40
Head-to-head studies specifically comparing ddMVAC and GC have been limited. GC has been compared to classic MVAC, with results showing equivalent efficacy but improved tolerability, as expected.38,40 ddMVAC was compared with a modified version of GC (termed “dose-dense GC”) in a phase 3 study from Greece, which demonstrated similar outcomes.41
Surgical intervention with radical cystectomy and regional lymph node dissection is typically deferred for patients who present with distant metastatic disease, unlike those who present with locally advanced disease. Radical cystectomy has traditionally been thought of as overly aggressive without sufficient benefit, although evidence to guide this approach remains sparse.42 As such, most expert recommendations and consensus statements simply recommend against surgical intervention and leave the decision between ddMVAC and GC up to the individual clinician.
In patients who are not eligible for cisplatin therapy, it is reasonable to consider chemotherapy with a combination of gemcitabine and carboplatin. This combination has been shown to be equivalent to MCAVI (methotrexate, carboplatin, vinblastine) in terms of overall survival (OS; 9 months versus 8 months) and progression-free survival (PFS; 6 months versus 4 months) with significantly fewer serious toxicities (9% versus 21%).43
The advent of immunotherapy in recent years has provided several new alternatives for cisplatin-ineligible patients. While immunotherapies such as pembrolizumab or atezolizumab are not yet recommended as first-line therapy for cisplatin-eligible patients, these 2 drugs are approved as options for first-line therapy in cisplatin-ineligible patients with metastatic disease. In a recent phase 2 trial (IMvigor210) involving 119 patients who were given atezolizumab as first-line therapy, median PFS was 2.7 months and median OS was 15.9 months.44 Another trial using data from patients in the KEYNOTE-052 study who received pembrolizumab as first-line therapy demonstrated antitumor activity with pembrolizumab and acceptable tolerability in cisplatin-ineligible patients with advanced urothelial carcinoma.45 The primary endpoint was objective response (either complete or partial response), which was achieved in 24% of the intention-to-treat population. Median PFS was 2 months, and 6-month OS was observed in 67% of patients. Both atezolizumab and pembrolizumab were given accelerated approval based on these single-arm studies in this setting. However, due to inferior outcomes in subsequent trials that included single-agent immunotherapy arms for patients in the first-line setting, the US Food and Drug Administration (FDA) has clarified the approval. In the subsequent trials, patients with a low PD-L1 biomarker based on the individual assay used for each drug did worse on immunotherapy alone (compared to chemotherapy or both combined), and the single-therapy arms were stopped early. Thus, the FDA now recommends that pembrolizumab or atezolizumab be used in the first line only for cisplatin-ineligible patients who have PD-L1 expression on tumor cells above the threshold studied on each individual assay, or are unfit for any platinum-based chemotherapy. Further study regarding the optimal role of biomarkers and chemotherapy-immunotherapy combinations is ongoing.
Case Continued
Ten months after his procedure, the patient is found to have prominent retroperitoneal lymphadenopathy and a 1.0-cm liver nodule suspicious for malignancy is noted on surveillance imaging. CT-guided biopsy of the liver reveals high-grade urothelial carcinoma, consistent with both recurrence and distant metastasis. The patient is informed that he needs to resume systemic therapy for recurrent metastatic disease. The options discussed include salvage single-agent chemotherapy with gemcitabine or immunotherapy with pembrolizumab. He elects to move forward with immunotherapy and is scheduled to begin pembrolizumab.
- What other immunotherapies might this patient consider for second-line therapy?
- Is chemotherapy a second-line option for this patient?
Second-Line Therapies and Management of Progressive Disease
Disease progression is unfortunately seen in the majority of cases of advanced urothelial carcinoma.46 New second-line therapies have recently been approved by the FDA in the form of monoclonal antibodies targeting programmed death 1 (PD-1) and a PD-1 ligand (PD-L1) (Figure 3).
Approval of pembrolizumab, a PD-1 inhibitor, was largely supported by the Keynote-045 trial,47,48 which looked at 542 patients who had progressed or recurred after platinum-based chemotherapy. These patients were randomly assigned to either pembrolizumab or investigator’s choice of chemotherapy (paclitaxel, docetaxel, or vinflunine). Patients treated with pembrolizumab had a significantly improved OS (median of 10.3 months versus 7.4 months), but no statistically significant difference in PFS (2.1 months versus 3.3 months). Interestingly, the rate of responses of 12 months or longer was higher with pembrolizumab than with more traditional second-line chemotherapy (68% versus 35%). The strength of this data has led to a category 1 recommendation in the most recent NCCN guidelines.39
The approval of atezolizumab, a PD-L1 inhibitor, as a second-line therapy for advanced urothelial carcinoma is largely supported by data from IMvigor211, a phase 3 trial that studied 931 patients randomly assigned to atezolizumab or investigator’s choice chemotherapy. OS did not differ significantly between patients in the atezolizumab group who had ≥ 5% expression of PD-L1 on tumor-infiltrating immune cells and patients in the chemotherapy group (11.1 months versus 10.6 months), but mean duration of response was longer (15.9 months versus 8.3 months).49 Therapy with atezolizumab had significantly fewer toxicities than chemotherapy (grade 3 or 4 toxicities of 20% versus 43%).
Phase 3 studies of nivolumab (PD-1 inhibitor), avelumab (PD-L1 inhibitor), and durvalumab (PD-L1 inhibitor) have not yet been published. These agents have received accelerated approval, however, as second-line treatment of advanced urothelial carcinoma based on promising data from phase 1 and phase 2 studies.50–52
Second-line chemotherapy is also an option for patients who do not qualify for immunotherapy or who progress during or after immunotherapy. Although there has been a great deal of excitement about new developments with immunotherapy and the survival benefit seen compared to investigator’s choice chemotherapy, the fact remains that most patients do not respond to immunotherapy. Still, some patients do derive benefit from single-agent chemotherapy in the platinum-refractory setting. Options based on primarily single-arm studies include gemcitabine, paclitaxel, docetaxel, pemetrexed, ifosfamide, oxaliplatin, and eribulin (Figure 2). In a randomized phase 3 trial, vinflunine demonstrated an OS benefit in platinum-refractory patients compared to best supportive care; it subsequently received approval by the European Medicines Agency.53 More recently in the phase 3 RANGE trial, docetaxel plus ramucirumab (a monoclonal antibody targeting vascular endothelial growth factor receptor 2) was compared to docetaxel plus placebo and met its primary endpoint of an improvement in PFS (median 4.07 months versus 2.76 months, P = 0.0118).54 OS has not been reported and this regimen has not yet received regulatory approval, however. Unfortunately, trials comparing these regimens are lacking, and response rates and survival remain modest. Clearly, better therapies and biomarkers to help personalize treatment options are needed.
Further investigations are underway with alternative regimens, including but not limited to targeted therapy in the setting of specific genetic and epigenetic alterations. These include mutations affecting tyrosine kinase receptors (eg, RAS/RAF, PI3K, AKT, and mTOR), cell cycle regulators (eg, TP53 or RB1), FGFR3 mutations, PTEN deletions, gene amplifications (eg, FGFR1, CCND1, and MDM2), or changes in genes responsible for chromatin remodeling (eg, UTX, CHD6, or ARID1A). As noted, there is particular excitement regarding FGFR3 inhibitors, which have shown compelling efficacy in phase 1 and 2 single-arm trials. Several agents are being evaluated in randomized trials and represent a potential path to the first targeted therapeutic class with a role in urothelial malignancies.
Surgical resection of metastases may be considered in very select cases.55 Surgery may have a role in limiting metastatic complications and improving cancer control, but this should be discussed at length with the patient using a multidisciplinary approach with careful restaging prior to surgery.
Case Continued
The patient remains on pembrolizumab every 3 weeks as per protocol with regular surveillance imaging. His disease stabilizes as the nodule in his liver and the retroperitoneal lymph nodes, all representing metastatic disease, became slightly smaller in size without evidence of any new disease. He continues to follow up closely with his genitourinary oncologist, undergoing regular surveillance and imaging every 3 months without evidence of disease progression.
Approximately 12 months into therapy, the patient notices a nonproductive cough with progressive and rapidly worsening shortness of breath. He is noted to be hypoxic with oxygen saturation levels to 79% in clinic and is sent immediately to the emergency department by his oncologist. Diffuse bilateral reticular opacities are noted on chest radiograph. Non-contrast CT scan demonstrates diffuse ground-glass opacities consistent with acute respiratory distress syndrome–pattern pneumonitis. He is admitted to the intensive care unit.
The patient is aggressively treated with high-flow nasal oxygen supplementation, intravenous steroids, and empiric antibiotics. He slowly improves on high-dose steroids (methylprednisolone 1 mg/kg/day) without requiring intubation or infliximab therapy and is discharged home in stable condition after 10 days. Oral steroid therapy is continued with a long taper over 6 weeks. In the setting of his grade 3 pneumonitis, pembrolizumab is discontinued and the patient is scheduled for a follow-up appointment with his oncologist to discuss next steps.
- In addition to pneumonitis, what other toxicities should you monitor for in patients treated with an immune checkpoint inhibitor?
- Is this patient a candidate to receive immunotherapy again in the future?
Treatment Toxicities
As use of immune checkpoint inhibitors has become more prevalent, the medical community has become increasingly aware of various immune-related adverse effects (irAE) associated with these drugs. These toxicities can be seen in virtually any organ system, and even vague complaints that arise years after therapy initiation should be treated with a high level of suspicion. The most commonly affected organ systems include the skin, gastrointestinal (GI) tract, lungs, liver, and endocrine system, although all other organ systems can be involved (Table 3) and toxicities appear to be similar across individual drugs.
The American Society of Clinical Oncology recently published a complete set of recommendations to guide clinicians on appropriate treatment strategies for each manifestation of immunotherapy-related toxicity.56 The details of these recommendations largely fall outside the purview of this article, but the mainstays of management are worth noting. These include high-dose systemic glucocorticoids, along with supportive care and cessation of immunotherapy in grade 3 or 4 toxicities. Infliximab is frequently recommended as an adjunct in severe or refractory cases.
Chemotherapy-related toxicities, on the other hand, are well-described and tend to be more familiar to patients and clinicians (Table 3). Classic MVAC, which has now been largely replaced by ddMVAC, was notoriously difficult to tolerate. It was known for a high rate of serious (grade 3 or 4) myelosuppressive complications as well as frequent GI toxicities. These complications include neutropenia (57%), stomatitis (10%), and nausea and vomiting (6%).23 ddMVAC with growth factor support is much better tolerated than classic MVAC. Prominent complaints with ddMVAC still can include nausea, GI distress, mucositis, and fatigue, but the incidence of myelosuppressive complications in particular has markedly decreased. GC is largely well tolerated, with minimal nausea and manageable myelotoxicity, but it is associated with an increased risk of venous thromboembolism.38
Prognosis
Case Conclusion
After returning home, the patient discusses his complicated medical course with his oncologist. Given his continued high quality of life with good functional status, he requests to continue with therapy for his metastatic bladder cancer and is interested in joining a clinical trial. He is referred to a nearby academic center with openings in a clinical trial for which he would be eligible. In the meantime, his oncologist guides him through filling out an advance directive and recommends that he make an appointment with palliative care services to ensure adequate home support for any future needs he may have.
- What is the estimated 5-year survival rate for patients with metastatic bladder cancer?
Overall, prognosis in patients with metastatic bladder cancer remains poor. Median survival in patients being treated with multi-agent chemotherapy is approximately 15 months,38,40 with an expected 5-year survival of just 15%. This is much improved, however, as prior to the advent of modern chemotherapy estimated survival was just 6 months with metastatic bladder cancer. Importantly, these figures do not take into account the recent advancements with immunotherapy, and thus it is reasonable to assume survival rates may continue to improve. In light of these recent advances, it is strongly recommended that whenever possible patients and clinicians consider participation in clinical trials to continue uncovering new and better therapies moving forward.
A number of tools have been developed to help risk stratify patients based on comorbidity, performance status, and other characteristics, but none have been universally adopted.57–60 As with many other malignancies, performance status is an important predictor of clinical outcomes in these patients.61–63 Sites of metastasis also may serve to suggest the course of disease. Patients with visceral metastases typically exhibit significantly worse disease with a shortened survival. The role of molecular factors as prognostic markers in bladder cancer is still under investigation. Many biomarkers are being considered (including mutations and polymorphisms in p53, ERCC1, and ERCC2), and evidence suggests some may have a role in prognosis; thus far, none have been validated as prognostic or predictive tools in urothelial carcinoma.
Conclusion
Bladder cancer includes an aggressive group of genitourinary tract malignancies, of which urothelial carcinoma is by far the most common in the Western world. Cisplatin-based therapy remains a mainstay of treatment for eligible patients with both localized and metastatic disease, but immunotherapies have provided a new and promising tool to use in the setting of progressing malignancy. The individual impact of these agents on OS is still being examined. Further studies and ongoing participation in clinical trials whenever possible continue to be essential to the discovery of future treatment options for this highly aggressive disease.
1. Stewart BW, Kleihues P. World cancer report. IARCPress. 2003.
2. Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009;115:68–74.
3. Hinotsu S, Akaza H, Miki T, et al. Bladder cancer develops 6 years earlier in current smokers: Analysis of bladder cancer registry data collected by the cancer registration committee of the Japanese Urological Association. Int J Urol 2009;16:64–9.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
5. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: bladder cancer. 2018. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed May 5, 2018.
6. Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45.
7. Pietzak EJ, Mucksavage P, Guzzo TJ, Malkowicz SB. Heavy cigarette smoking and aggressive bladder cancer at initial presentation. Urology 2015;86:968–73.
8. Khadra MH, Pickard RS, Charlton M, et al. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000;163:524–7.
9. Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA 2005;293:810–16.
10. Mariani AJ, Mariani MC, Macchioni C, et al. The significance of adult hematuria: 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. J Urol 1989;141:350–5.
11. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology 2001;57:604–10.
12. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part I: definition, detection, prevalence, and etiology. Urology 2001;57:599–603.
13. Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224–9.
14. Messing EM, Young TB, Hunt VB, et al. Home screening for hematuria: results of a multiclinic study. J Urol 1992;148:289–92.
15. Gray PJ, Lin CC, Jemal A, et al. Clinical–pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the National Cancer Data Base. Int J Radiat Oncol 2014;88:1048–56.
16. Bochner B, Hansel D, Efstathiou J, et al. Urinary bladder. In: Amin M, ed. AJCC cancer staging manual. 8th. New York: Springer; 2017:757.
17. Cahn DB, McGreen B, Lee A, et al. Clinical destiny of indeterminate pulmonary nodules in patients undergoing radical cystectomy for urothelial carcinoma of the bladder [abstract]. J Clin Oncol 2017;35(6 suppl):297-297.
18. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25–41.
19. Kurtis B, Zhuge J, Ojaimi C, et al. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann Diagn Pathol 2016;21:7–11.
20. Ito H, Kyo S, Kanaya T, et al. Detection of human telomerase reverse transcriptase messenger RNA in voided urine samples as a useful diagnostic tool for bladder cancer. Clin Cancer Res 1998;4:2807–10.
21. Utting M, Werner W, Dahse R, et al. Microsatellite analysis of free tumor DNA in urine, serum, and plasma of patients: a minimally invasive method for the detection of bladder cancer. Clin Cancer Res 2002;8:35–40.
22. Sethakorn N, O’Donnell PH. Spectrum of genomic alterations in FGFR3: current appraisal of the potential role of FGFR3 in advanced urothelial carcinoma. BJU Int 2016;118:681–91.
23. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66.
24. Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer ‘unfit’ for cisplatin-based chemotherapy. J Clin Oncol 2011;29:2432–8.
25. Raj GV, Iasonos A, Herr H, Donat SM. Formulas calculating creatinine clearance are inadequate for determining eligibility for cisplatin-based chemotherapy in bladder cancer. J Clin Oncol 2006;24:3095–100.26. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 2003;361:1927–34.
27. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol 2005;48:202–6.
28. Sternberg CN. A critical review of the management of bladder cancer. Crit Rev Oncol Hematol 1999;31:193–207.
29. Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol 1985;133:403–7.
30. Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006;42:50–4.
31. Sternberg CN, de Mulder PHM, Schornagel JH, et al. Randomized phase III trial of high–dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol No. 30924. J Clin Oncol 2001;19:2638–46.
32. Soloway MS, Einstein A, Corder MP, et al. A comparison of cisplatin and the combination of cisplatin and cyclophosphamide in advanced urothelial cancer. A National Bladder Cancer Collaborative Group A Study. Cancer 1983;52:767–72.
33. Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 2014;32:1895–901.
34. Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140–53.
35. Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 2015;68:959–67.
36. Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76–86.
37. Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014;32:3801–9.
38. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068–77.
39. Flaig T, Spiess P, Agarwal N, et al. National Comprehensive Cancer Network. Bladder cancer (version 3.2018). 2018. www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed May 5, 2018.
40. von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602–8.
41. Bamias A, Dafni U, Karadimou A, et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol 2013;24:1011–7.
42. Li R, Metcalfe M, Kukreja J, Navai N. Role of radical cystectomy in non-organ confined bladder cancer: a systematic review. Bladder Cancer 2018;4:31–40.
43. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012;30:191–9.
44. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76.
45. Balar A V, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483–92.
46. Manoharan M, Ayyathurai R, Soloway MS. Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 2009;104:1227–32.
47. Bellmunt J, de Wit R, Vaughn D, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26.
48. Bajorin D, de Wit R, Vaughn D, et al. Planned survival analysis from KEYNOTE-045: Phase 3, open-label study of pembrolizumab (pembro) versus paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC). (Abstract 4501). J Clin Oncol 2017;35(15_suppl):4501-4501.
49. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748–57.
50. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22.
51. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018;19:51–64.
52. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. JAMA Oncol 2017;3:e172411.
53. Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454–61.
54. Petrylak DP, de Wit R, Chi KN, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial. Lancet 2017;390:2266–77.
55. Abufaraj M, Dalbagni G, Daneshmand S, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol 2018;73:543–57.
56. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68.
57. Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–81.
58. Mayr R, May M, Martini T, et al. Comorbidity and performance indices as predictors of cancer-independent mortality but not of cancer-specific mortality after radical cystectomy for urothelial carcinoma of the bladder. Eur Urol 2012;62:662–70.
59. Nakagawa T, Hara T, Kawahara T, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol 2013;189:1275–81.
60. Ploeg M, Kums AC, Aben KK, et al. Prognostic factors for survival in patients with recurrence of muscle invasive bladder cancer after treatment with curative intent. Clin Genitourin Cancer 2011;9:14–21.
61. Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1997;15:2564–9.
62. Lin CC, Hsu CH, Huang CY, et al. Prognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5-fluorouracil-based regimens. Urology 2007;69:479–84.
63. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187–93.
1. Stewart BW, Kleihues P. World cancer report. IARCPress. 2003.
2. Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer 2009;115:68–74.
3. Hinotsu S, Akaza H, Miki T, et al. Bladder cancer develops 6 years earlier in current smokers: Analysis of bladder cancer registry data collected by the cancer registration committee of the Japanese Urological Association. Int J Urol 2009;16:64–9.
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
5. National Cancer Institute Surveillance, Epidemiology, and End Results Program. Cancer stat facts: bladder cancer. 2018. https://seer.cancer.gov/statfacts/html/urinb.html. Accessed May 5, 2018.
6. Freedman ND, Silverman DT, Hollenbeck AR, et al. Association between smoking and risk of bladder cancer among men and women. JAMA 2011;306:737–45.
7. Pietzak EJ, Mucksavage P, Guzzo TJ, Malkowicz SB. Heavy cigarette smoking and aggressive bladder cancer at initial presentation. Urology 2015;86:968–73.
8. Khadra MH, Pickard RS, Charlton M, et al. A prospective analysis of 1,930 patients with hematuria to evaluate current diagnostic practice. J Urol 2000;163:524–7.
9. Grossman HB, Messing E, Soloway M, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA 2005;293:810–16.
10. Mariani AJ, Mariani MC, Macchioni C, et al. The significance of adult hematuria: 1,000 hematuria evaluations including a risk-benefit and cost-effectiveness analysis. J Urol 1989;141:350–5.
11. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology 2001;57:604–10.
12. Grossfeld GD, Litwin MS, Wolf JS, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part I: definition, detection, prevalence, and etiology. Urology 2001;57:599–603.
13. Mohr DN, Offord KP, Owen RA, Melton LJ. Asymptomatic microhematuria and urologic disease. A population-based study. JAMA 1986;256:224–9.
14. Messing EM, Young TB, Hunt VB, et al. Home screening for hematuria: results of a multiclinic study. J Urol 1992;148:289–92.
15. Gray PJ, Lin CC, Jemal A, et al. Clinical–pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the National Cancer Data Base. Int J Radiat Oncol 2014;88:1048–56.
16. Bochner B, Hansel D, Efstathiou J, et al. Urinary bladder. In: Amin M, ed. AJCC cancer staging manual. 8th. New York: Springer; 2017:757.
17. Cahn DB, McGreen B, Lee A, et al. Clinical destiny of indeterminate pulmonary nodules in patients undergoing radical cystectomy for urothelial carcinoma of the bladder [abstract]. J Clin Oncol 2017;35(6 suppl):297-297.
18. Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer 2015;15:25–41.
19. Kurtis B, Zhuge J, Ojaimi C, et al. Recurrent TERT promoter mutations in urothelial carcinoma and potential clinical applications. Ann Diagn Pathol 2016;21:7–11.
20. Ito H, Kyo S, Kanaya T, et al. Detection of human telomerase reverse transcriptase messenger RNA in voided urine samples as a useful diagnostic tool for bladder cancer. Clin Cancer Res 1998;4:2807–10.
21. Utting M, Werner W, Dahse R, et al. Microsatellite analysis of free tumor DNA in urine, serum, and plasma of patients: a minimally invasive method for the detection of bladder cancer. Clin Cancer Res 2002;8:35–40.
22. Sethakorn N, O’Donnell PH. Spectrum of genomic alterations in FGFR3: current appraisal of the potential role of FGFR3 in advanced urothelial carcinoma. BJU Int 2016;118:681–91.
23. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 2003;349:859–66.
24. Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer ‘unfit’ for cisplatin-based chemotherapy. J Clin Oncol 2011;29:2432–8.
25. Raj GV, Iasonos A, Herr H, Donat SM. Formulas calculating creatinine clearance are inadequate for determining eligibility for cisplatin-based chemotherapy in bladder cancer. J Clin Oncol 2006;24:3095–100.26. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis. Lancet 2003;361:1927–34.
27. Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol 2005;48:202–6.
28. Sternberg CN. A critical review of the management of bladder cancer. Crit Rev Oncol Hematol 1999;31:193–207.
29. Sternberg CN, Yagoda A, Scher HI, et al. Preliminary results of M-VAC (methotrexate, vinblastine, doxorubicin and cisplatin) for transitional cell carcinoma of the urothelium. J Urol 1985;133:403–7.
30. Sternberg CN, de Mulder P, Schornagel JH, et al. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 2006;42:50–4.
31. Sternberg CN, de Mulder PHM, Schornagel JH, et al. Randomized phase III trial of high–dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol No. 30924. J Clin Oncol 2001;19:2638–46.
32. Soloway MS, Einstein A, Corder MP, et al. A comparison of cisplatin and the combination of cisplatin and cyclophosphamide in advanced urothelial cancer. A National Bladder Cancer Collaborative Group A Study. Cancer 1983;52:767–72.
33. Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 2014;32:1895–901.
34. Van Allen EM, Mouw KW, Kim P, et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov 2014;4:1140–53.
35. Plimack ER, Dunbrack RL, Brennan TA, et al. Defects in DNA repair genes predict response to neoadjuvant cisplatin-based chemotherapy in muscle-invasive bladder cancer. Eur Urol 2015;68:959–67.
36. Sternberg CN, Skoneczna I, Kerst JM, et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 2015;16:76–86.
37. Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol 2014;32:3801–9.
38. von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000;18:3068–77.
39. Flaig T, Spiess P, Agarwal N, et al. National Comprehensive Cancer Network. Bladder cancer (version 3.2018). 2018. www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. Accessed May 5, 2018.
40. von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 2005;23:4602–8.
41. Bamias A, Dafni U, Karadimou A, et al. Prospective, open-label, randomized, phase III study of two dose-dense regimens MVAC versus gemcitabine/cisplatin in patients with inoperable, metastatic or relapsed urothelial cancer: a Hellenic Cooperative Oncology Group study (HE 16/03). Ann Oncol 2013;24:1011–7.
42. Li R, Metcalfe M, Kukreja J, Navai N. Role of radical cystectomy in non-organ confined bladder cancer: a systematic review. Bladder Cancer 2018;4:31–40.
43. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012;30:191–9.
44. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67–76.
45. Balar A V, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483–92.
46. Manoharan M, Ayyathurai R, Soloway MS. Radical cystectomy for urothelial carcinoma of the bladder: an analysis of perioperative and survival outcome. BJU Int 2009;104:1227–32.
47. Bellmunt J, de Wit R, Vaughn D, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017;376:1015–26.
48. Bajorin D, de Wit R, Vaughn D, et al. Planned survival analysis from KEYNOTE-045: Phase 3, open-label study of pembrolizumab (pembro) versus paclitaxel, docetaxel, or vinflunine in recurrent, advanced urothelial cancer (UC). (Abstract 4501). J Clin Oncol 2017;35(15_suppl):4501-4501.
49. Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018;391:748–57.
50. Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol 2017;18:312–22.
51. Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018;19:51–64.
52. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma. JAMA Oncol 2017;3:e172411.
53. Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454–61.
54. Petrylak DP, de Wit R, Chi KN, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel in patients with locally advanced or metastatic urothelial carcinoma after platinum-based therapy (RANGE): a randomised, double-blind, phase 3 trial. Lancet 2017;390:2266–77.
55. Abufaraj M, Dalbagni G, Daneshmand S, et al. The role of surgery in metastatic bladder cancer: a systematic review. Eur Urol 2018;73:543–57.
56. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68.
57. Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 1999;17:3173–81.
58. Mayr R, May M, Martini T, et al. Comorbidity and performance indices as predictors of cancer-independent mortality but not of cancer-specific mortality after radical cystectomy for urothelial carcinoma of the bladder. Eur Urol 2012;62:662–70.
59. Nakagawa T, Hara T, Kawahara T, et al. Prognostic risk stratification of patients with urothelial carcinoma of the bladder with recurrence after radical cystectomy. J Urol 2013;189:1275–81.
60. Ploeg M, Kums AC, Aben KK, et al. Prognostic factors for survival in patients with recurrence of muscle invasive bladder cancer after treatment with curative intent. Clin Genitourin Cancer 2011;9:14–21.
61. Saxman SB, Propert KJ, Einhorn LH, et al. Long-term follow-up of a phase III intergroup study of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 1997;15:2564–9.
62. Lin CC, Hsu CH, Huang CY, et al. Prognostic factors for metastatic urothelial carcinoma treated with cisplatin and 5-fluorouracil-based regimens. Urology 2007;69:479–84.
63. Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187–93.