User login
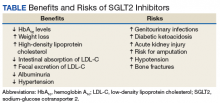
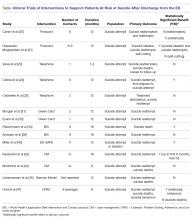
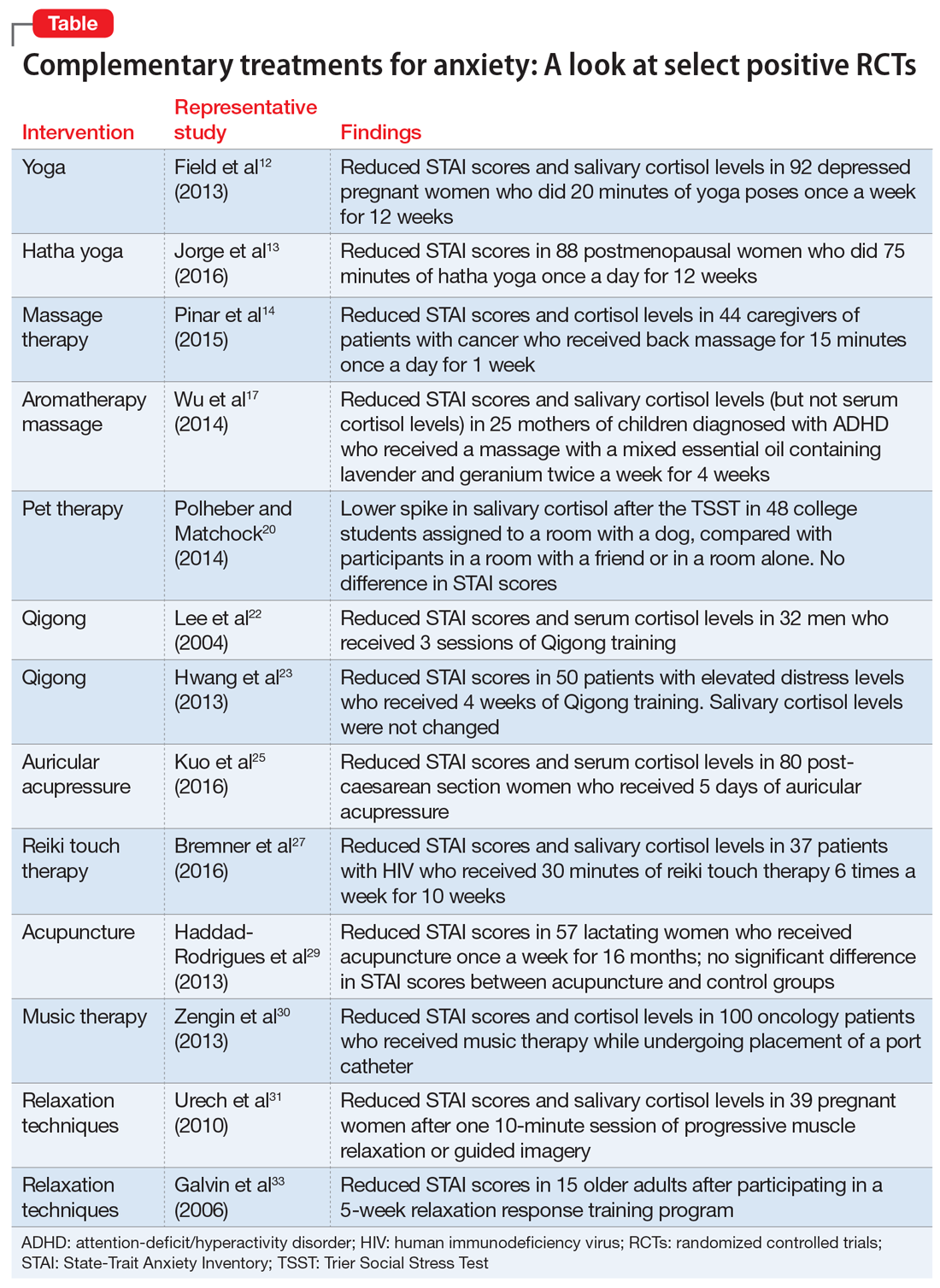
Risks vs Benefits for SGLT2 Inhibitor Medications
Diabetes mellitus (DM) is a metabolic disorder affecting about 5% to 13% of the population in the US.1 Since 1552, the earliest record of a person with DM, many treatment advances have been made.2Sodium-glucose cotransporter 2 (SGLT2) inhibitors are one of the newest antidiabetic pharmaceuticals on the market. The SGLT2 inhibitor drugs include canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, and tofogliflozin; however, only canagliflozin, dapagliflozin, and empagliflozin have been approved by the US Food and Drug Administration (FDA). These pharmaceuticals promote glycosuria via the kidneys and enhance sugar excretion from the body. Along with lifestyle changes and self-care measures, such as healthful eating and increased physical activity, SGLT2 inhibitor pharmaceuticals provide antidiabetic efficacy by facilitating normoglycemia and minimizing vascular pathology.
Although SGLT2 inhibitor pharmaceuticals are newly introduced into the market, their discovery dates to 1835.3 Phlorizin, a nonselective SGLT inhibitor, was first isolated by French chemists from the bark of an apple tree.4 Phlorizin inhibits SGLT1 mostly in small intestinal cells, and SGLT2 similarly affects the kidney.4 Renal SGLT2 is the primary therapeutic target. Canagliflozin was the first pharmaceutical SGLT2 inhibitor approved by the FDA in 2013. Dapagliflozin’s FDA approval followed in 2013 and empagliflozin in 2014.5
Mechanism Of Action
In healthy individuals, tubular glucose is absorbed, resulting in no urinary glucose excretion. Sodium-glucose cotransporters 1 and 2 contribute to the renal absorption of glucose. A SGLT2 is responsible for 90% of the glucose reuptake in the segment 1 of the proximal tubule, while SGLT 1 is accountable for the remaining 10%.3 Unlike other antidiabetic medications, which act by increasing insulin secretion or improving insulin sensitivity for the receptors, SGLT2 inhibitor drugs prevent the reuptake of glucose into the bloodstream. This selective action spares the inhibition of SGLT1 present in other tissues, avoiding gastrointestinal effects.6
Benefits
The SGLT2 inhibitor action is focused on renal excretion of glucose and is independent of insulin action.
Hemoglobin A1c Levels
Canagliflozin, dapagliflozin, and empagliflozin reduce hemoglobin A1c (HbA1c) levels.5 Inagaki and colleagues found significant reductions in HbA1c and weight gain with > 100 mg canagliflozin compared with that of placebo when used for 12 weeks.7 In a study where 2.5-mg, 5-mg, and 10-mg dapagliflozin was compared with placebo, the mean HbA1c change from the baseline was -0.23% with placebo; -0.58% at 2.5 mg; -0.77% at 5 mg; and -0.89% at 10 mg.8 Empagliflozin was more effective in reducing HbA1c levels than was sitagliptin.9 When patients were treated with 10-mg empagliflozin, 25-mg empagliflozin, and sitagliptin, HbA1c levels dropped -1.44%, -1.43%, and -1.04%, respectively.9
Cholesterol
Sodium-glucose cotransporter 2 inhibitors have the beneficial effect of reducing vascular disease risk factors.10,11 A study by Hayashi and colleagues found that dapagliflozin decreases harmful atherogenic small, low-density lipoprotein-cholesterol (LDL-C), increases less atherogenic large, buoyant LDL-C, and increases high-density, lipoprotein-2 cholesterol (HDL-2C).10 Empagliflozin, however, can cause a small dose-dependent increase in HDL-C and LDL-C.11 Although there is an increase in serum LDL-C concentrations, empagliflozin can induce a decrease in intestinal absorption of cholesterol, thus promoting fecal excretion of LDL-C and macrophage-derived cholesterol.11
Weight Loss
A study by Weber and colleagues found that the SGLT2 inhibitor dapagloflozin lead to a reduction in body weight from -1.0 kg to -0.3 kg compared with placebo.12 Cefalu and colleagues found that daily prescribing of 100 mg and/or 300 mg of canagliflozin evidenced dose-dependent loss of weight.13 Neeland and colleagues found that empagliflozin utilization resulted in less adiposity indices in 3,300 subjects.14
Albuminuria
Sodium-glucose cotransporter 2 inhibitors have a reno-protective role in patients with type 2 DM (T2DM). In those receiving renin-angiotensin blockers with T2DM and hypertension, dapagliflozin decreased their albuminuria.15 Canagliflozin has a similar potential.16 Empagliflozin reduced the urine albumin-creatinine ratio in patients with macro- or micro-albuminuria, supporting a direct renal effect by SGLT2 inhibitors.17
Systolic Blood Pressure
Sodium-glucose cotransporter 2 inhibitors can have beneficial effects on physiologic vascular outcomes. In patients with T2DM and hypertension, dapagliflozin reduced mean systolic blood pressure (SBP) compared with placebo: -7.3 mm Hg vs -10.4 mm Hg, respectively.12 Prescribing canagliflozin treatment at 100 mg or 300 mg reduced SBP (-4.3 mm Hg and -5.0 mm Hg, respectively, vs placebo at -0.3 mm Hg).18 Subjects taking empagliflozin 10 mg or 25 mg exhibited an adjusted mean BP change from baseline of -4.60 mm Hg and -5.47mm Hg, respectively, whereas placebo induced a -0.67 mm Hg decline.19
Risks
Nausea, fatigue, polyuria, polydipsia, and xerostomia are common SGLT2 AEs. Use of SGLT2 inhibitors can induce certain other more serious AEs as well.
Increased Risk for Amputations
The Canagliflozin Cardiovascular Assessment Study (CANVAS) and the Canagliflozin Cardiovascular Assessment Study-Renal (CANVAS-R) documented that canagliflozin doubled the incidence of leg and foot amputations in research participants compared with placebo (6.3 vs 3.4 per 1,000 patient-years).16 Therefore, canagliflozin should be prescribed with caution in persons with a prior history of foot ulceration, neuropathy, and/or vascular diseases.20
Acute Renal Injury
The mechanism of kidney damage by SGLT2 inhibitor drugs is not completely understood. About 100 patients experienced renal failure after the intake of SGLT2 inhibitor drugs.21 Among them, more than half reported symptom onset within a month of starting the medication, and their symptoms improved after discontinuing the SGLT2 medication. As a result, the FDA issued a warning to monitor renal function before initiating and during such pharmacotherapy.21
Ketoacidosis
Sodium-glucose cotransporter 2 inhibitors might lead to elevated ketone body levels22 and euglycemic ketoacidosis;23 however, this risk reportedly is negligible.24 Use of SGLT2 inhibitors is not recommended for patients evidencing the presence of precipitating factors like acute gastroenteritis or insulin pump failure.25
Genitourinary Infections
About 10% to 15% of women taking SGLT2 inhibitor medications developed urinary tract infections and vulvovaginitis.26 This could be because of a glycosuria effect caused by SGLT2 inhibitors.27
Hypotension
Sodium-glucose cotransporter 2 inhibitors cause contraction of intravascular volume. Therefore, patients taking SGLT2 inhibitors are at risk for hypotension, leading to dizziness and potentially dangerous falls. Patients already taking volume-depleting medications, such as diuretics, should be advised to use this group of medications with caution and report these AEs.28
Bone Fractures
A clinical trial revealed that SGLT2 inhibitors, such as canagliflozin, decrease bone mineral density possibly leading to bone fractures.29 Bone fractures occurred in about 1.5% of cases of patients taking 100 mg and 300 mg of canagliflozin compared with a 1.1% fracture rate among the placebo group.29
Conclusion
Since the FDA approval of SGLT2 inhibitor medications, their usage has increased. The American Diabetes Association first recommends nonpharmacologic approaches, such as diet modification, exercise, and weight loss for patients diagnosed with DM, followed by a medicinal intervention with metformin if required. Sodium-glucose cotransporter 2 inhibitors are suggested as an additional medication in dual or triple pharmacotherapies when metformin alone fails to achieve normoglycemia.
Prior to starting a patient on SGLT2 inhibitor medication, clinicians should monitor hydration adequacy, check bone density, review the patient’s cardiac profile, and assess hepatic and renal function. Prescribing SGLT2 inhibitors should be restricted if the patient has a history of type 1 DM, ketosisprone T2DM, and in those with a glomerular filtration rate of < 60 mL/min. Considering the preexisting medical conditions of the patient and monitoring the blood glucose levels, renal function, and volume status at every visit should minimize risks and enhance the benefits of prescribing this new medication class.
1. Li C, Balluz LS, Okoro CA, et al; Centers for Disease Control and Prevention. Surveillance of certain health behaviors and condition among states and selected local areas—Behavioral Risk Factor Surveillance System, United States, 2009. MMWR Surveill Summ. 2011;60(9):1-250.
2. Loriaux DL. Diabetes and the ebers papyrus: 1552 BC. Endocrinologist. 2006;16(2):55-56.
3. Malhotra A, Kudyar S, Gupta AK, Kudyar RP, Malhotra P. Sodium glucose cotransporter inhibitors—a new class of old drugs. Int J Appl Basic Med Res. 2015;5(3):161-163.
4. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21(1):31-38.
5. Mosley JF II, Smith L, Everton E, Fellner C. Sodium-glucose linked transporter 2 (SGLT2) inhibitors in the management of type-2 diabetes: a drug class overview. PT. 2015;40(7):451-462.
6. Bays H. Sodium glucose cotransporter type 2 (SGLT2) inhibitors: targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Ther. 2013;4(2):195-220.
7. Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15(12):1136-1145.
8. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217-2224.
9. Roden M, Weng J, Eilbracht J, et al; EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-219.
10. Hayashi T, Fukui T, Nakanishi N, et al. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetology. 2017;16:8.
11. Tsimihodimos V, Filippatos TD, Elisaf MS. Effects of sodium-glucose cotransporter 2 inhibitors on metabolism: unanswered questions and controversies. Expert Opin Drug Metab Toxicol. 2017;13(4):399-408.
12. Weber MA, Mansfield TA, Alessi F, Iqbal N, Parikh S, Ptaszynska A. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin–angiotensin system blockade. Blood Press. 2016;25(2):93-103.
13. Cefalu WT, Stenlöf K, Leiter LA, et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58(6):1183-1187.
14. Neeland IJ, McGuire DK, Chilton R, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2016;13(2):119-126.
15. Heerspink HJ, Johnsson E, Gause-Nilsson I, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18(6):590-597.
16. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7): 644-657.
17. Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59(9):1860-1870.
18. Pfeifer M, Townsend RR, Davies MJ, Vijapurkar U, Ren J. Effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: a post hoc analysis. Cardiovasc Diabetol. 2017;16(1):29.
19. Tikkanen I, Narko K, Zeller C, et al; EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428.
20. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
21. Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol. 2016;12(12):711-712.
22. Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849-2852.
23. Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7(2):135-138.
24. Monami M, Nreu B, Zannoni S, Lualdi C, Mannucci E. Effects of SGLT-2 inhibitors on diabetic ketoacidosis: a meta-analysis of randomised controlled trials. Diabetes Res Clin Pract. 2017;130:53-60.
25. Burke KR, Schumacher CA, Harpe SE. SGLT2 inhibitors: a systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy. 2017;37(2):187-194.
26. Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7(1):2824.
27. Chaplin S. SGLT2 inhibitors and risk of genitourinary infections. Prescriber. 2016;27(12):26-30.
28. Weir MR, Januszewicz A, Gilbert RE, et al. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J Clin Hypertens (Greenwich). 2014;16(12):875-882.
29. Watts NB, Bilezkian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellietus. J Clin Endocrinol Metab. 2016;101(1):157-166.
Diabetes mellitus (DM) is a metabolic disorder affecting about 5% to 13% of the population in the US.1 Since 1552, the earliest record of a person with DM, many treatment advances have been made.2Sodium-glucose cotransporter 2 (SGLT2) inhibitors are one of the newest antidiabetic pharmaceuticals on the market. The SGLT2 inhibitor drugs include canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, and tofogliflozin; however, only canagliflozin, dapagliflozin, and empagliflozin have been approved by the US Food and Drug Administration (FDA). These pharmaceuticals promote glycosuria via the kidneys and enhance sugar excretion from the body. Along with lifestyle changes and self-care measures, such as healthful eating and increased physical activity, SGLT2 inhibitor pharmaceuticals provide antidiabetic efficacy by facilitating normoglycemia and minimizing vascular pathology.
Although SGLT2 inhibitor pharmaceuticals are newly introduced into the market, their discovery dates to 1835.3 Phlorizin, a nonselective SGLT inhibitor, was first isolated by French chemists from the bark of an apple tree.4 Phlorizin inhibits SGLT1 mostly in small intestinal cells, and SGLT2 similarly affects the kidney.4 Renal SGLT2 is the primary therapeutic target. Canagliflozin was the first pharmaceutical SGLT2 inhibitor approved by the FDA in 2013. Dapagliflozin’s FDA approval followed in 2013 and empagliflozin in 2014.5
Mechanism Of Action
In healthy individuals, tubular glucose is absorbed, resulting in no urinary glucose excretion. Sodium-glucose cotransporters 1 and 2 contribute to the renal absorption of glucose. A SGLT2 is responsible for 90% of the glucose reuptake in the segment 1 of the proximal tubule, while SGLT 1 is accountable for the remaining 10%.3 Unlike other antidiabetic medications, which act by increasing insulin secretion or improving insulin sensitivity for the receptors, SGLT2 inhibitor drugs prevent the reuptake of glucose into the bloodstream. This selective action spares the inhibition of SGLT1 present in other tissues, avoiding gastrointestinal effects.6
Benefits
The SGLT2 inhibitor action is focused on renal excretion of glucose and is independent of insulin action.
Hemoglobin A1c Levels
Canagliflozin, dapagliflozin, and empagliflozin reduce hemoglobin A1c (HbA1c) levels.5 Inagaki and colleagues found significant reductions in HbA1c and weight gain with > 100 mg canagliflozin compared with that of placebo when used for 12 weeks.7 In a study where 2.5-mg, 5-mg, and 10-mg dapagliflozin was compared with placebo, the mean HbA1c change from the baseline was -0.23% with placebo; -0.58% at 2.5 mg; -0.77% at 5 mg; and -0.89% at 10 mg.8 Empagliflozin was more effective in reducing HbA1c levels than was sitagliptin.9 When patients were treated with 10-mg empagliflozin, 25-mg empagliflozin, and sitagliptin, HbA1c levels dropped -1.44%, -1.43%, and -1.04%, respectively.9
Cholesterol
Sodium-glucose cotransporter 2 inhibitors have the beneficial effect of reducing vascular disease risk factors.10,11 A study by Hayashi and colleagues found that dapagliflozin decreases harmful atherogenic small, low-density lipoprotein-cholesterol (LDL-C), increases less atherogenic large, buoyant LDL-C, and increases high-density, lipoprotein-2 cholesterol (HDL-2C).10 Empagliflozin, however, can cause a small dose-dependent increase in HDL-C and LDL-C.11 Although there is an increase in serum LDL-C concentrations, empagliflozin can induce a decrease in intestinal absorption of cholesterol, thus promoting fecal excretion of LDL-C and macrophage-derived cholesterol.11
Weight Loss
A study by Weber and colleagues found that the SGLT2 inhibitor dapagloflozin lead to a reduction in body weight from -1.0 kg to -0.3 kg compared with placebo.12 Cefalu and colleagues found that daily prescribing of 100 mg and/or 300 mg of canagliflozin evidenced dose-dependent loss of weight.13 Neeland and colleagues found that empagliflozin utilization resulted in less adiposity indices in 3,300 subjects.14
Albuminuria
Sodium-glucose cotransporter 2 inhibitors have a reno-protective role in patients with type 2 DM (T2DM). In those receiving renin-angiotensin blockers with T2DM and hypertension, dapagliflozin decreased their albuminuria.15 Canagliflozin has a similar potential.16 Empagliflozin reduced the urine albumin-creatinine ratio in patients with macro- or micro-albuminuria, supporting a direct renal effect by SGLT2 inhibitors.17
Systolic Blood Pressure
Sodium-glucose cotransporter 2 inhibitors can have beneficial effects on physiologic vascular outcomes. In patients with T2DM and hypertension, dapagliflozin reduced mean systolic blood pressure (SBP) compared with placebo: -7.3 mm Hg vs -10.4 mm Hg, respectively.12 Prescribing canagliflozin treatment at 100 mg or 300 mg reduced SBP (-4.3 mm Hg and -5.0 mm Hg, respectively, vs placebo at -0.3 mm Hg).18 Subjects taking empagliflozin 10 mg or 25 mg exhibited an adjusted mean BP change from baseline of -4.60 mm Hg and -5.47mm Hg, respectively, whereas placebo induced a -0.67 mm Hg decline.19
Risks
Nausea, fatigue, polyuria, polydipsia, and xerostomia are common SGLT2 AEs. Use of SGLT2 inhibitors can induce certain other more serious AEs as well.
Increased Risk for Amputations
The Canagliflozin Cardiovascular Assessment Study (CANVAS) and the Canagliflozin Cardiovascular Assessment Study-Renal (CANVAS-R) documented that canagliflozin doubled the incidence of leg and foot amputations in research participants compared with placebo (6.3 vs 3.4 per 1,000 patient-years).16 Therefore, canagliflozin should be prescribed with caution in persons with a prior history of foot ulceration, neuropathy, and/or vascular diseases.20
Acute Renal Injury
The mechanism of kidney damage by SGLT2 inhibitor drugs is not completely understood. About 100 patients experienced renal failure after the intake of SGLT2 inhibitor drugs.21 Among them, more than half reported symptom onset within a month of starting the medication, and their symptoms improved after discontinuing the SGLT2 medication. As a result, the FDA issued a warning to monitor renal function before initiating and during such pharmacotherapy.21
Ketoacidosis
Sodium-glucose cotransporter 2 inhibitors might lead to elevated ketone body levels22 and euglycemic ketoacidosis;23 however, this risk reportedly is negligible.24 Use of SGLT2 inhibitors is not recommended for patients evidencing the presence of precipitating factors like acute gastroenteritis or insulin pump failure.25
Genitourinary Infections
About 10% to 15% of women taking SGLT2 inhibitor medications developed urinary tract infections and vulvovaginitis.26 This could be because of a glycosuria effect caused by SGLT2 inhibitors.27
Hypotension
Sodium-glucose cotransporter 2 inhibitors cause contraction of intravascular volume. Therefore, patients taking SGLT2 inhibitors are at risk for hypotension, leading to dizziness and potentially dangerous falls. Patients already taking volume-depleting medications, such as diuretics, should be advised to use this group of medications with caution and report these AEs.28
Bone Fractures
A clinical trial revealed that SGLT2 inhibitors, such as canagliflozin, decrease bone mineral density possibly leading to bone fractures.29 Bone fractures occurred in about 1.5% of cases of patients taking 100 mg and 300 mg of canagliflozin compared with a 1.1% fracture rate among the placebo group.29
Conclusion
Since the FDA approval of SGLT2 inhibitor medications, their usage has increased. The American Diabetes Association first recommends nonpharmacologic approaches, such as diet modification, exercise, and weight loss for patients diagnosed with DM, followed by a medicinal intervention with metformin if required. Sodium-glucose cotransporter 2 inhibitors are suggested as an additional medication in dual or triple pharmacotherapies when metformin alone fails to achieve normoglycemia.
Prior to starting a patient on SGLT2 inhibitor medication, clinicians should monitor hydration adequacy, check bone density, review the patient’s cardiac profile, and assess hepatic and renal function. Prescribing SGLT2 inhibitors should be restricted if the patient has a history of type 1 DM, ketosisprone T2DM, and in those with a glomerular filtration rate of < 60 mL/min. Considering the preexisting medical conditions of the patient and monitoring the blood glucose levels, renal function, and volume status at every visit should minimize risks and enhance the benefits of prescribing this new medication class.
Diabetes mellitus (DM) is a metabolic disorder affecting about 5% to 13% of the population in the US.1 Since 1552, the earliest record of a person with DM, many treatment advances have been made.2Sodium-glucose cotransporter 2 (SGLT2) inhibitors are one of the newest antidiabetic pharmaceuticals on the market. The SGLT2 inhibitor drugs include canagliflozin, dapagliflozin, empagliflozin, ipragliflozin, and tofogliflozin; however, only canagliflozin, dapagliflozin, and empagliflozin have been approved by the US Food and Drug Administration (FDA). These pharmaceuticals promote glycosuria via the kidneys and enhance sugar excretion from the body. Along with lifestyle changes and self-care measures, such as healthful eating and increased physical activity, SGLT2 inhibitor pharmaceuticals provide antidiabetic efficacy by facilitating normoglycemia and minimizing vascular pathology.
Although SGLT2 inhibitor pharmaceuticals are newly introduced into the market, their discovery dates to 1835.3 Phlorizin, a nonselective SGLT inhibitor, was first isolated by French chemists from the bark of an apple tree.4 Phlorizin inhibits SGLT1 mostly in small intestinal cells, and SGLT2 similarly affects the kidney.4 Renal SGLT2 is the primary therapeutic target. Canagliflozin was the first pharmaceutical SGLT2 inhibitor approved by the FDA in 2013. Dapagliflozin’s FDA approval followed in 2013 and empagliflozin in 2014.5
Mechanism Of Action
In healthy individuals, tubular glucose is absorbed, resulting in no urinary glucose excretion. Sodium-glucose cotransporters 1 and 2 contribute to the renal absorption of glucose. A SGLT2 is responsible for 90% of the glucose reuptake in the segment 1 of the proximal tubule, while SGLT 1 is accountable for the remaining 10%.3 Unlike other antidiabetic medications, which act by increasing insulin secretion or improving insulin sensitivity for the receptors, SGLT2 inhibitor drugs prevent the reuptake of glucose into the bloodstream. This selective action spares the inhibition of SGLT1 present in other tissues, avoiding gastrointestinal effects.6
Benefits
The SGLT2 inhibitor action is focused on renal excretion of glucose and is independent of insulin action.
Hemoglobin A1c Levels
Canagliflozin, dapagliflozin, and empagliflozin reduce hemoglobin A1c (HbA1c) levels.5 Inagaki and colleagues found significant reductions in HbA1c and weight gain with > 100 mg canagliflozin compared with that of placebo when used for 12 weeks.7 In a study where 2.5-mg, 5-mg, and 10-mg dapagliflozin was compared with placebo, the mean HbA1c change from the baseline was -0.23% with placebo; -0.58% at 2.5 mg; -0.77% at 5 mg; and -0.89% at 10 mg.8 Empagliflozin was more effective in reducing HbA1c levels than was sitagliptin.9 When patients were treated with 10-mg empagliflozin, 25-mg empagliflozin, and sitagliptin, HbA1c levels dropped -1.44%, -1.43%, and -1.04%, respectively.9
Cholesterol
Sodium-glucose cotransporter 2 inhibitors have the beneficial effect of reducing vascular disease risk factors.10,11 A study by Hayashi and colleagues found that dapagliflozin decreases harmful atherogenic small, low-density lipoprotein-cholesterol (LDL-C), increases less atherogenic large, buoyant LDL-C, and increases high-density, lipoprotein-2 cholesterol (HDL-2C).10 Empagliflozin, however, can cause a small dose-dependent increase in HDL-C and LDL-C.11 Although there is an increase in serum LDL-C concentrations, empagliflozin can induce a decrease in intestinal absorption of cholesterol, thus promoting fecal excretion of LDL-C and macrophage-derived cholesterol.11
Weight Loss
A study by Weber and colleagues found that the SGLT2 inhibitor dapagloflozin lead to a reduction in body weight from -1.0 kg to -0.3 kg compared with placebo.12 Cefalu and colleagues found that daily prescribing of 100 mg and/or 300 mg of canagliflozin evidenced dose-dependent loss of weight.13 Neeland and colleagues found that empagliflozin utilization resulted in less adiposity indices in 3,300 subjects.14
Albuminuria
Sodium-glucose cotransporter 2 inhibitors have a reno-protective role in patients with type 2 DM (T2DM). In those receiving renin-angiotensin blockers with T2DM and hypertension, dapagliflozin decreased their albuminuria.15 Canagliflozin has a similar potential.16 Empagliflozin reduced the urine albumin-creatinine ratio in patients with macro- or micro-albuminuria, supporting a direct renal effect by SGLT2 inhibitors.17
Systolic Blood Pressure
Sodium-glucose cotransporter 2 inhibitors can have beneficial effects on physiologic vascular outcomes. In patients with T2DM and hypertension, dapagliflozin reduced mean systolic blood pressure (SBP) compared with placebo: -7.3 mm Hg vs -10.4 mm Hg, respectively.12 Prescribing canagliflozin treatment at 100 mg or 300 mg reduced SBP (-4.3 mm Hg and -5.0 mm Hg, respectively, vs placebo at -0.3 mm Hg).18 Subjects taking empagliflozin 10 mg or 25 mg exhibited an adjusted mean BP change from baseline of -4.60 mm Hg and -5.47mm Hg, respectively, whereas placebo induced a -0.67 mm Hg decline.19
Risks
Nausea, fatigue, polyuria, polydipsia, and xerostomia are common SGLT2 AEs. Use of SGLT2 inhibitors can induce certain other more serious AEs as well.
Increased Risk for Amputations
The Canagliflozin Cardiovascular Assessment Study (CANVAS) and the Canagliflozin Cardiovascular Assessment Study-Renal (CANVAS-R) documented that canagliflozin doubled the incidence of leg and foot amputations in research participants compared with placebo (6.3 vs 3.4 per 1,000 patient-years).16 Therefore, canagliflozin should be prescribed with caution in persons with a prior history of foot ulceration, neuropathy, and/or vascular diseases.20
Acute Renal Injury
The mechanism of kidney damage by SGLT2 inhibitor drugs is not completely understood. About 100 patients experienced renal failure after the intake of SGLT2 inhibitor drugs.21 Among them, more than half reported symptom onset within a month of starting the medication, and their symptoms improved after discontinuing the SGLT2 medication. As a result, the FDA issued a warning to monitor renal function before initiating and during such pharmacotherapy.21
Ketoacidosis
Sodium-glucose cotransporter 2 inhibitors might lead to elevated ketone body levels22 and euglycemic ketoacidosis;23 however, this risk reportedly is negligible.24 Use of SGLT2 inhibitors is not recommended for patients evidencing the presence of precipitating factors like acute gastroenteritis or insulin pump failure.25
Genitourinary Infections
About 10% to 15% of women taking SGLT2 inhibitor medications developed urinary tract infections and vulvovaginitis.26 This could be because of a glycosuria effect caused by SGLT2 inhibitors.27
Hypotension
Sodium-glucose cotransporter 2 inhibitors cause contraction of intravascular volume. Therefore, patients taking SGLT2 inhibitors are at risk for hypotension, leading to dizziness and potentially dangerous falls. Patients already taking volume-depleting medications, such as diuretics, should be advised to use this group of medications with caution and report these AEs.28
Bone Fractures
A clinical trial revealed that SGLT2 inhibitors, such as canagliflozin, decrease bone mineral density possibly leading to bone fractures.29 Bone fractures occurred in about 1.5% of cases of patients taking 100 mg and 300 mg of canagliflozin compared with a 1.1% fracture rate among the placebo group.29
Conclusion
Since the FDA approval of SGLT2 inhibitor medications, their usage has increased. The American Diabetes Association first recommends nonpharmacologic approaches, such as diet modification, exercise, and weight loss for patients diagnosed with DM, followed by a medicinal intervention with metformin if required. Sodium-glucose cotransporter 2 inhibitors are suggested as an additional medication in dual or triple pharmacotherapies when metformin alone fails to achieve normoglycemia.
Prior to starting a patient on SGLT2 inhibitor medication, clinicians should monitor hydration adequacy, check bone density, review the patient’s cardiac profile, and assess hepatic and renal function. Prescribing SGLT2 inhibitors should be restricted if the patient has a history of type 1 DM, ketosisprone T2DM, and in those with a glomerular filtration rate of < 60 mL/min. Considering the preexisting medical conditions of the patient and monitoring the blood glucose levels, renal function, and volume status at every visit should minimize risks and enhance the benefits of prescribing this new medication class.
1. Li C, Balluz LS, Okoro CA, et al; Centers for Disease Control and Prevention. Surveillance of certain health behaviors and condition among states and selected local areas—Behavioral Risk Factor Surveillance System, United States, 2009. MMWR Surveill Summ. 2011;60(9):1-250.
2. Loriaux DL. Diabetes and the ebers papyrus: 1552 BC. Endocrinologist. 2006;16(2):55-56.
3. Malhotra A, Kudyar S, Gupta AK, Kudyar RP, Malhotra P. Sodium glucose cotransporter inhibitors—a new class of old drugs. Int J Appl Basic Med Res. 2015;5(3):161-163.
4. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21(1):31-38.
5. Mosley JF II, Smith L, Everton E, Fellner C. Sodium-glucose linked transporter 2 (SGLT2) inhibitors in the management of type-2 diabetes: a drug class overview. PT. 2015;40(7):451-462.
6. Bays H. Sodium glucose cotransporter type 2 (SGLT2) inhibitors: targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Ther. 2013;4(2):195-220.
7. Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15(12):1136-1145.
8. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217-2224.
9. Roden M, Weng J, Eilbracht J, et al; EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-219.
10. Hayashi T, Fukui T, Nakanishi N, et al. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetology. 2017;16:8.
11. Tsimihodimos V, Filippatos TD, Elisaf MS. Effects of sodium-glucose cotransporter 2 inhibitors on metabolism: unanswered questions and controversies. Expert Opin Drug Metab Toxicol. 2017;13(4):399-408.
12. Weber MA, Mansfield TA, Alessi F, Iqbal N, Parikh S, Ptaszynska A. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin–angiotensin system blockade. Blood Press. 2016;25(2):93-103.
13. Cefalu WT, Stenlöf K, Leiter LA, et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58(6):1183-1187.
14. Neeland IJ, McGuire DK, Chilton R, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2016;13(2):119-126.
15. Heerspink HJ, Johnsson E, Gause-Nilsson I, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18(6):590-597.
16. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7): 644-657.
17. Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59(9):1860-1870.
18. Pfeifer M, Townsend RR, Davies MJ, Vijapurkar U, Ren J. Effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: a post hoc analysis. Cardiovasc Diabetol. 2017;16(1):29.
19. Tikkanen I, Narko K, Zeller C, et al; EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428.
20. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
21. Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol. 2016;12(12):711-712.
22. Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849-2852.
23. Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7(2):135-138.
24. Monami M, Nreu B, Zannoni S, Lualdi C, Mannucci E. Effects of SGLT-2 inhibitors on diabetic ketoacidosis: a meta-analysis of randomised controlled trials. Diabetes Res Clin Pract. 2017;130:53-60.
25. Burke KR, Schumacher CA, Harpe SE. SGLT2 inhibitors: a systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy. 2017;37(2):187-194.
26. Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7(1):2824.
27. Chaplin S. SGLT2 inhibitors and risk of genitourinary infections. Prescriber. 2016;27(12):26-30.
28. Weir MR, Januszewicz A, Gilbert RE, et al. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J Clin Hypertens (Greenwich). 2014;16(12):875-882.
29. Watts NB, Bilezkian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellietus. J Clin Endocrinol Metab. 2016;101(1):157-166.
1. Li C, Balluz LS, Okoro CA, et al; Centers for Disease Control and Prevention. Surveillance of certain health behaviors and condition among states and selected local areas—Behavioral Risk Factor Surveillance System, United States, 2009. MMWR Surveill Summ. 2011;60(9):1-250.
2. Loriaux DL. Diabetes and the ebers papyrus: 1552 BC. Endocrinologist. 2006;16(2):55-56.
3. Malhotra A, Kudyar S, Gupta AK, Kudyar RP, Malhotra P. Sodium glucose cotransporter inhibitors—a new class of old drugs. Int J Appl Basic Med Res. 2015;5(3):161-163.
4. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005;21(1):31-38.
5. Mosley JF II, Smith L, Everton E, Fellner C. Sodium-glucose linked transporter 2 (SGLT2) inhibitors in the management of type-2 diabetes: a drug class overview. PT. 2015;40(7):451-462.
6. Bays H. Sodium glucose cotransporter type 2 (SGLT2) inhibitors: targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Ther. 2013;4(2):195-220.
7. Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15(12):1136-1145.
8. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33(10):2217-2224.
9. Roden M, Weng J, Eilbracht J, et al; EMPA-REG MONO trial investigators. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208-219.
10. Hayashi T, Fukui T, Nakanishi N, et al. Dapagliflozin decreases small dense low-density lipoprotein-cholesterol and increases high-density lipoprotein 2-cholesterol in patients with type 2 diabetes: comparison with sitagliptin. Cardiovasc Diabetology. 2017;16:8.
11. Tsimihodimos V, Filippatos TD, Elisaf MS. Effects of sodium-glucose cotransporter 2 inhibitors on metabolism: unanswered questions and controversies. Expert Opin Drug Metab Toxicol. 2017;13(4):399-408.
12. Weber MA, Mansfield TA, Alessi F, Iqbal N, Parikh S, Ptaszynska A. Effects of dapagliflozin on blood pressure in hypertensive diabetic patients on renin–angiotensin system blockade. Blood Press. 2016;25(2):93-103.
13. Cefalu WT, Stenlöf K, Leiter LA, et al. Effects of canagliflozin on body weight and relationship to HbA1c and blood pressure changes in patients with type 2 diabetes. Diabetologia. 2015;58(6):1183-1187.
14. Neeland IJ, McGuire DK, Chilton R, et al. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2016;13(2):119-126.
15. Heerspink HJ, Johnsson E, Gause-Nilsson I, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18(6):590-597.
16. Neal B, Perkovic V, Mahaffey KW, et al; CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7): 644-657.
17. Cherney D, Lund SS, Perkins BA, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59(9):1860-1870.
18. Pfeifer M, Townsend RR, Davies MJ, Vijapurkar U, Ren J. Effects of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on blood pressure and markers of arterial stiffness in patients with type 2 diabetes mellitus: a post hoc analysis. Cardiovasc Diabetol. 2017;16(1):29.
19. Tikkanen I, Narko K, Zeller C, et al; EMPA-REG BP Investigators. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428.
20. Boulton AJM, Armstrong DG, Albert SF, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008;31(8):1679-1685.
21. Hahn K, Ejaz AA, Kanbay M, Lanaspa MA, Johnson RJ. Acute kidney injury from SGLT2 inhibitors: potential mechanisms. Nat Rev Nephrol. 2016;12(12):711-712.
22. Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab. 2015;100(8):2849-2852.
23. Ogawa W, Sakaguchi K. Euglycemic diabetic ketoacidosis induced by SGLT2 inhibitors: possible mechanism and contributing factors. J Diabetes Investig. 2016;7(2):135-138.
24. Monami M, Nreu B, Zannoni S, Lualdi C, Mannucci E. Effects of SGLT-2 inhibitors on diabetic ketoacidosis: a meta-analysis of randomised controlled trials. Diabetes Res Clin Pract. 2017;130:53-60.
25. Burke KR, Schumacher CA, Harpe SE. SGLT2 inhibitors: a systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy. 2017;37(2):187-194.
26. Liu J, Li L, Li S, et al. Effects of SGLT2 inhibitors on UTIs and genital infections in type 2 diabetes mellitus: a systematic review and meta-analysis. Sci Rep. 2017;7(1):2824.
27. Chaplin S. SGLT2 inhibitors and risk of genitourinary infections. Prescriber. 2016;27(12):26-30.
28. Weir MR, Januszewicz A, Gilbert RE, et al. Effect of canagliflozin on blood pressure and adverse events related to osmotic diuresis and reduced intravascular volume in patients with type 2 diabetes mellitus. J Clin Hypertens (Greenwich). 2014;16(12):875-882.
29. Watts NB, Bilezkian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellietus. J Clin Endocrinol Metab. 2016;101(1):157-166.
Transgender Care in the Primary Care Setting: A Review of Guidelines and Literature
Lesbian, gay, bisexual, and transgender (LGBT) individuals face significant difficulties in obtaining high-quality,compassionate medical care, much of which has been attributed to inadequate provider knowledge. In this article, the authors present a transgender patient seen in primary care and discuss the knowledge gleaned to inform future care of this patient as well as the care of other similar patients.
The following case discussion and review of the literature also seeks to improve the practice of other primary care providers (PCPs) who are inexperienced in this arena. This article aims to review the basics to permit PCPs to venture into transgender care, including a review of basic terminology; a few interactive tips; and basics in medical and hormonal treatment, follow-up, contraindications, and risk. More details can be obtained through electronic consultation (Transgender eConsult) in the VA.
Case Presentation
A 35-year-old patient who was assigned male sex at birth presented to the primary care clinic to discuss her desire to undergo male-to-female (MTF) transition. The patient stated that she had started taking female estrogen hormones 9 years previously purchased from craigslist without a prescription. She tried oral contraceptives as well as oral and injectable estradiol. While the patient was taking injectable estradiol she had breast growth, decreased anxiety, weight gain, and a feeling of peacefulness. The patient also reported that she had received several laser treatments for whole body hair removal, beginning 8 to 10 years before and more regularly in the past 2 to 3 years. She asked whether transition-related care could be provided, because she could no longer afford the hormones.
The patient wanted to transition because she felt that “Women are beautiful, the way they carry themselves, wear their hair, their nails, I want to be like that.” She also mentioned that when she watched TV, she envisioned herself as a woman. She reported that she enjoyed wearing her mother’s clothing since age 10, which made her feel more like herself. The patient noted that she had desired to remove her body hair since childhood but could not afford to do it until recently. She bought female clothing, shoes and makeup, and did her nails from a young age. The patient also reported that she did not “know what transgender was” until a decade ago.
The patient struggled with her identity growing up; however, she tried not to think about it or talk about it with anyone. She related that she was ashamed of her thoughts and that only recently had made peace with being transgender. Thus, she pursued talking to her medical provider about transitioning. The patient reported that she felt more energetic when taking female hormones and was better able to discuss the issue. Specifically, she noted that if she were not on estrogen now she would not be able to talk about transitioning.
The patient related that she has done extensive research about transitioning, including reading online about other transgender people. She noted that she was aware of “possible backlash with society,” but ultimately, she had decided that transitioning was the right decision for her.
She expressed a desire to have an orchiectomy and continue hormonal therapy to permit her “to have a more feminine face, soft skin, hairless body, big breasts, more fat around the hips, and a high-pitched voice.” She additionally related a desire to be in a stable relationship and be her true self. She also stated that she had not identified herself as a female to anyone yet but would like to soon. The patient reported a history of depression, especially during her military service when she wanted to be a woman but did not feel she understood what was going on or how to manage her feelings. She said that for the past 2 months she felt much happier since beginning to take estradiol 4 mg orally daily, which she had found online. She also tried to purchase anti-androgen medication but could not afford it. In addition, she said that she would like to eventually proceed with gender affirmation surgery.
She was currently having sex with men, primarily via anal receptive intercourse. She had no history of sexually transmitted infections but reported that she did not use condoms regularly. She had no history of physical or sexual abuse. The patient was offered referral to the HIV clinic to receive HIV preexposure prophylaxis therapy (emtricitabine + tenofovir), which she declined, but she was counseled on safe sex practice.
The patient was referred to psychiatry both for supportive mental health care and to clarify that her concomitant mental health issues would not preclude the prescription of gender-affirming hormone treatment. Based on the psychiatric evaluation, the patient was felt to be appropriate for treatment with feminizing hormone therapy. The psychiatric assessment also noted that although the patient had a history of psychosis, she was not exhibiting psychotic symptoms currently, and this would not be a contraindication to treatment.
After discussion of the risks and benefits of cross-sex hormone therapy, the patient was started on estradiol 4 mg orally daily, as well as spironolactone 50 mg daily. She was then switched to estradiol 10 mg intramuscular every 2 weeks with the aim of using a less thrombogenic route of administration.
Treatment Outcomes
The patient remains under care. She has had follow-up visits every 3 months to ensure appropriate signs of feminization and monitoring of adverse effects (AEs). The patient’s testosterone and estradiol levels are being checked every 3 months to ensure total testosterone is 1,2
After 12 months on therapy with estradiol and spironolactone, the patient notes that her mood has improved, she feels more energetic, she has gained some weight, and her skin is softer. Her voice pitch, with the help of speech therapy, is gradually changing to what she perceives as more feminine. Hormone levels and electrolytes are all in an acceptable range, and blood sugar and blood pressure (BP) are within normal range. The patient will be offered age-appropriate cancer screening at the appropriate time.
Discussion
The treatment of gender-nonconforming individuals has come a long way since Lili Elbe, the transgender artist depicted in The Danish Girl, underwent gender-affirmation surgery in the early 20th century. Lili and people like her paved the way for other transgender individuals by doggedly pursuing gender-affirming medical treatment although they faced rejection by society and forged a difficult path. In recent years, an increasing number of transgender individuals have begun to seek mainstream medical care; however, PCPs often lack the knowledge and training to properly interact with and care for transgender patients.3,4
Terminology
Although someone’s sex is typically assigned at birth based on the external appearance of their genitalia, gender identity refers to a person’s internal sense of self and how they fit in to the world. People often use these 2 terms interchangeably in everyday language, but these terms are different.1,2
Transgender refers to a person whose gender identity differs from the sex that was assigned at birth. A transgender man or transman, or female-to-male (FTM) transgender person, is an individual who is assigned female sex at birth but identifies as a male. A transgender woman, or transwoman or a male-to-female (MTF) transgender person, is an individual who is assigned male sex at birth but identifies as female. A nontransgender person may be referred to as cisgender.
Transsexual is a medical term and refers to a transgender individual who sought medical intervention to transition to their identified gender.
Sexual orientation describes sexual attraction only and is not related to gender identity. The sexual orientation of a transgender person is determined by emotional and/or physical attraction and not gender identity.
Gender dysphoria refers to the distress experienced by an individual when one’s gender identity and sex are not completely congruent.
Improving Patient Interaction
Transgender patients might avoid seeking care due to previous negative experiences or a fear of being judged. It is very important to create a safe environment where the patients feel comfortable. Meeting patients “where they are” without judgment will enhance the patient-physician relationship. It is necessary to train all clinic staff about the importance of transgender health issues. All staff should address the patient with the name, pronouns, and gender identity that the patient prefers. For patients with a gender identity that is not strictly male or female (nonbinary patients), gender-neutral pronouns, such as they/them/their, may be chosen. It is helpful to be direct in asking: What is your preferred name? When I speak about you to other providers, what pronouns do you prefer I use, he, she, they? This information can then be documented in the electronic health record (EHR) so that all staff know from visit to visit. Thank the patient for the clarification.
The physical examination can be uncomfortable for both the patient and the physician. Experience and familiarity with the current recommendations can help. The physical examination should be relevant to the anatomy that is present, regardless of the gender presentation. An anatomic survey of the organs currently present in an individual can be useful.1 The physician should be sensitive in examining and obtaining information from the patient, focusing on only those issues relevant to the presenting concern. Chest and genital examinations may be particularly distressing for patients. If a chest or genital examination is indicated, the provider and patient should have a discussion explaining the importance of the examination and how the patient’s comfort can be optimized.
Medical Treatment
Gender-affirmation treatment should be multidisciplinary and include some or all of the following: diagnostic assessment, psychotherapy or counseling, real-life experience (RLE), hormone therapy, and surgical therapy..1,2,5 The World Professional Association for Transgender Health (WPATH) has established internationally accepted Standards of Care (SOC) for the treatment of gender dysphoria that provide detailed expert opinion reviewing the background and guidance for care of transgender individuals. Most commonly, the diagnosis of gender dysphoria is made by a mental health professional (MHP) based on the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) criteria for gender dysphoria.1,2 The involvement of a MHP can be crucial in assessing potential psychological and social risk factors for unfavorable outcomes of medical interventions. In case of severe psychopathology, which can interfere with diagnosis and treatment, the psychopathology should be addressed first.1,2 The MHP also can confirm that the patient has the capacity to make an informed decision.
The 2017 Endocrine Society guidelines for the treatment of gender-dysphoric/gender-incongruent persons emphasize the utility of evaluation of these patients by an expert MHP before starting the treatment.2 However, the guidelines from WPATH and the Center for Transgender Excellence at University of California, San Francisco (UCSF) have stipulated that any provider who feels comfortable assessing the informed decision-making process with a patient can make this determination.
The WPATH SOC states that RLE is essential to transition to the gender role that is congruent with the patient’s gender identity. The RLE is defined as the act of fully adopting a new or evolving gender role or gender presentation in everyday life. In the RLE, the person should fully experience life in the desired gender role before irreversible physical treatment is undertaken. Newer guidelines note that it may be too challenging to adopt the desired gender role without the benefit of feminizing or masculinizing treatment, and therefore, the treatment can be offered at the same time as adopting the new gender role.1
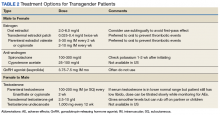
Medical treatment involves administration of masculinizing or feminizing hormone therapy. There are 2 major goals of this hormonal therapy.
For many transgender adults, genital reconstruction surgery and/or gonadectomy is a necessary step toward achieving their goal.
Pretreatment screening and appropriate medical monitoring is recommended for both FTM and MTF transgender patients during the endocrine transition and periodically thereafter.2 The physician should monitor the patient’s weight, BP, directed physical examinations, routine health questions focused on risk factors and medications, complete blood count, renal and liver functions, lipid and blood sugar.2
Physical Changes With Hormone Therapy
Transgender men. Physical changes that are expected to occur during the first 1 to 6 months of testosterone therapy include cessation of menses, increased sexual desire, increased facial and body hair, increased oiliness of skin, increased muscle, and redistribution of fat mass. Changes that occur within the first year of testosterone therapy include deepening of the voice, clitoromegaly, and male pattern hair loss (in some cases). Deepening of the voice, and clitoromegaly are not reversible with discontinuation of hormonal therapy.2
Transgender women. Physical changes that may occur in transgender females in the first 3 to 12 months of estrogen and anti-androgen therapy include decreased sexual desire, decreased spontaneous erections, decreased facial and body hair (usually mild), decreased oiliness of skin, increased breast tissue growth, and redistribution of fat mass. Breast development is generally maximal at 2 years after initiating estrogen, and it is irreversible.2 Effect on fertility may be permanent. Medical therapy has little effect on voice, and most transwomen will require speech therapy to achieve desired pitch.
Routine Health Maintenance
Breast Cancer Screening
Although there are limited data, it is thought that gender-affirming hormone therapy has similar risks as sex hormone replacement therapy in nontransgender males and females. Most AEs arise from use of supraphysiologic doses or inadequate doses.2 Therefore, regular clinical and laboratory monitoring is essential to cross-sex hormone therapy. Treatment with exogenous estrogen and anti-androgens result in transgender women developing breast tissue with ducts, lobules, and acini that is histologically identical to breast tissue in nontransgender females.6
Breast cancer is a concern in transgender women due to prolonged exposure to estrogen. However, the relationship between breast cancer and cross-sex hormone therapy is controversial.
Many factors contribute to breast cancer risk in patients of all genders. Studies of premenopausal and menopausal women taking exogenous estrogen alone have not shown an increase in breast cancer risk. However, the combination of estrogen and progesterone has shown an association with a significant increase in the incidence of breast cancer in postmenopausal women.2,7-10
A study of 5,136 veterans showed a statistically insignificant increased incidence of breast cancer in transgender women compared with data collected from the Surveillance, Epidemiology, and End Results database, although the sample size and duration of the observation were limiting factors.8 A European cohort study found decreased incidence of breast cancer in both MTF and FTM transgender patients, but these patients were an overall younger cohort with decreased risk in general. A cohort of 2,236 MTF individuals in the Netherlands in 1997 showed no increase in all-cause mortality related to hormone therapy at 30-year follow-up. Patients were exposed to exogenous estrogen from 2 months to 41 years.9 A follow-up of this study published in 2013, which included 2,307 MTF individuals taking estrogen for 5 years to > 30 years, revealed only 2 cases of breast cancer, which was the same incidence rate (4.1 per 100,000 person-years) as that of nontransgender women.10
In general, the incidence of breast cancer is rare in nontransgender men, and therefore there have not been a lot of clinical studies to assess risk factors and detection methods. The following risk factors can increase the risk of breast cancer in nontransgender patients: known presence of BRCA mutation, estrogen exposure/androgen insufficiency, Klinefelter syndrome, liver cirrhosis, and obesity.11
Guidelines from the Endocrine Society, WPATH, and UCSF suggest that MTF transgender individuals who have a known increased risk for breast cancer should follow screening guidelines recommended for nontransgender women if they are aged > 50 years and have had more than 5 years of hormone use.2 For FTM patients who have not had chest surgery, screening guidelines should follow those for nontransgender women. For those patients who have had chest reconstruction, small residual amounts of breast tissue may remain. Screening guidelines for these patients do not exist. For these patients, mammography can be technically difficult. Clinical chest wall examination, magnetic resonance imaging (MRI), and/or ultrasound may be helpful modalities. An individual risk vs benefit discussion with the patient is recommended.
Prostate Cancer Screening
Although the prostate gland will undergo atrophy with extended treatment with feminizing hormone therapy, there are case reports of prostate cancer in transgender women.12,13 Usually these patients have started hormone treatment after age 50 years. Therefore, prostate cancer screening is recommended in transgender women as per general guidelines. Because the prostate-specific antigen (PSA) level is expected to be reduced, a PSA > 1.0 should be considered abnormal.1
Cervical Cancer Screening
When a transgender man has a pap smear, it is essential to make it clear to the laboratory that the sample is a cervical pap smear (especially if the gender is marked as male) to avoid the sample being run incorrectly as an anal pap. Also, it is essential to indicate on the pap smear request form that the patient is on testosterone therapy and amenorrhea is present, because the lack of the female hormone can cause atrophy of cervix. This population has a high rate of inadequate specimens. Pretreatment with 1 to 2 weeks of vaginal estrogen can improve the success rate of inadequate specimens. Transgender women who have undergone vaginoplasty do not have a cervix, therefore, cervical cancer screening is not recommended. The anatomy of the neovagina has a more posterior orientation, and an anoscope is a more appropriate tool to examine the neovagina when necessary.
Hematology Health
Transgender women on cross-sex hormone therapy with estrogens may be at increased risk for a venous thromboembolism (VTE). In 2 European studies, patients treated with oral ethinyl estradiol as well as the anti-androgen cyproterone acetate were found to have up to 20 times increased risk of VTE. However, in later studies, oral ethinyl estradiol was changed to either oral conjugated estrogens or transdermal/intramuscular estradiol, and these studies did not show a significant increase in VTE risk.14-16 Tobacco use in combination with estrogen therapy is associated with an increased risk of deep vein thrombosis (DVT).1 All transgender women who smoke should be counseled on tobacco risks and cessation options at every visit.1 The transgender individuals who are not willing to quit smoking may be offered transdermal estrogen, which has lower risk of DVT.14-16
Sexual Health
Clinicians should assess the risks for sexually transmitted infection (STIs) or HIV for transgender patients based on current anatomy and sexual behaviors. Presentations of STIs can be atypical due to varied sexual practices and gender-affirming surgeries. Thus, providers must remain vigilant for symptoms consistent with common STIs and screen for asymptomatic STIs on the basis of behavior history and sexual practices.17 Preexposure prophylaxis for HIV should be considered when appropriate. Serologic screening recommendations for transgender people (eg, HIV, hepatitis B and C, syphilis) do not differ in recommendations from those for nontransgender people.
Cardiovascular Health
The effect of cross-hormone treatment on cardiovascular (CV) health is still unknown. There are no randomized controlled trials that have investigated the relationship between cross-hormone treatment and CV health. Evidence from several studies suggests that CV risk is unchanged among transgender men using testosterone compared with that of nontransgender women.18,19 There is conflicting evidence for transgender women with respect to CV risk and cross-sex hormone treatment.1,18,19 The current American College of Cardiology/American Heart Association guideline advises using the ASCVD risk calculator to determine the need for aspirin and statin treatment based on race, age, gender, and risk factors. There is no guideline on whether to use natal sex or affirmed gender while using the ASCVD calculator. It is reasonable to use the calculator based on natal sex if the cross-hormone treatment has started later in life, but if the cross-sex hormone treatment started at a young age, then one should consider using the affirmed gender to calculate the risk.
As with all patients, life style modifications, including smoking cessation, weight loss, physical activity, and management of BP and blood sugar, are important for CV health. For transgender women with CV risk factors or known CV disease, transdermal route of estrogen is preferred due to lower rates of VTE.18,19
Conclusion
In recent years, an increased number of transgender individuals are seeking mainstream medical care. However, PCPs often lack the knowledge and training to properly interact with and care for transgender patients. It is critical that clinicians understand the difference between sex, gender, and sexuality. For patients who desire transgender care, providers must be able to comfortably ask the patient about their preferred name and prior care, know the basics in cross-sex hormone therapy, including appropriate follow-up of hormonal levels as well as laboratory tests that delineate risk, and know possible complications and AEs. The VA offers significant resources, including electronic transgender care consultation for cases where the provider does not have adequate expertise in the care of these patients.
Both medical schools and residency training programs are starting to incorporate curricula regarding LGBT care. For those who have already completed training, this article serves as a brief guide to terminology, interactive tips, and management of this growing and underserved group of individuals.
1. Deutsch MB. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. http://transhealth.ucsf.edu/protocols. Updated June 17, 2016. Accessed June 13, 2018.
2. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoria/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903.
3. Buchholz L. Transgender care moves into the mainstream. JAMA. 2015;314(17):1785-1787.
4. Sobralske M. Primary care needs of patients who have undergone gender reassignment. J Am Acad Nurse Pract. 2005;17(4):133-138.
5. Unger CA. Hormone therapy for transgender patients. Transl Androl Urol. 2016;5(6):877-884.
6. Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW. Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men. Am J Surg Pathol. 2000;24(1):74-80.
7. Braun H, Nash R, Tangpricha V, Brockman J, Ward K, Goodman M. Cancer in transgender people: evidence and methodological consideration. Epidemiol Rev. 2017;39(1):93-107.
8. Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res Treat. 2015;149(1):191-198.
9. Van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf). 1997;47(3):337-342.
10. Gooren LJ, van Trotsenburg MA, Giltay EJ, van Diest PJ. Breast cancer development in transsexual subjects receiving cross-sex hormone treatment. J Sex Med. 2013;10(12):3129-3134.
11. Johansen Taber KA, Morisy LR, Osbahr AJ III, Dickinson BD. Male breast cancer: risk factors, diagnosis and management (review). Oncol Rep. 2010;24(5):1115-1120.
12. Miksad RA, Bubley G, Church P, et al. Prostate cancer in a transgender woman, 41 years after initiation of feminization. JAMA. 2006;296(19):2316-2317.
13. Turo R, Jallad S, Prescott S, Cross WR. Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy. Can Urol Assoc J. 2013;7(7-8):E544-E546.
14. American College of Obstetricians and Gynecologists. ACOG committee opinion no. 556: postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013;121(4):887-890.
15. Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender treatment. Metabolism. 1989;38(9):869-873.
16. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635-642.
17. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
18. Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol. 2014;170(6):809-819.
19. Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Int Med. 2017;167(4):256-267.
Lesbian, gay, bisexual, and transgender (LGBT) individuals face significant difficulties in obtaining high-quality,compassionate medical care, much of which has been attributed to inadequate provider knowledge. In this article, the authors present a transgender patient seen in primary care and discuss the knowledge gleaned to inform future care of this patient as well as the care of other similar patients.
The following case discussion and review of the literature also seeks to improve the practice of other primary care providers (PCPs) who are inexperienced in this arena. This article aims to review the basics to permit PCPs to venture into transgender care, including a review of basic terminology; a few interactive tips; and basics in medical and hormonal treatment, follow-up, contraindications, and risk. More details can be obtained through electronic consultation (Transgender eConsult) in the VA.
Case Presentation
A 35-year-old patient who was assigned male sex at birth presented to the primary care clinic to discuss her desire to undergo male-to-female (MTF) transition. The patient stated that she had started taking female estrogen hormones 9 years previously purchased from craigslist without a prescription. She tried oral contraceptives as well as oral and injectable estradiol. While the patient was taking injectable estradiol she had breast growth, decreased anxiety, weight gain, and a feeling of peacefulness. The patient also reported that she had received several laser treatments for whole body hair removal, beginning 8 to 10 years before and more regularly in the past 2 to 3 years. She asked whether transition-related care could be provided, because she could no longer afford the hormones.
The patient wanted to transition because she felt that “Women are beautiful, the way they carry themselves, wear their hair, their nails, I want to be like that.” She also mentioned that when she watched TV, she envisioned herself as a woman. She reported that she enjoyed wearing her mother’s clothing since age 10, which made her feel more like herself. The patient noted that she had desired to remove her body hair since childhood but could not afford to do it until recently. She bought female clothing, shoes and makeup, and did her nails from a young age. The patient also reported that she did not “know what transgender was” until a decade ago.
The patient struggled with her identity growing up; however, she tried not to think about it or talk about it with anyone. She related that she was ashamed of her thoughts and that only recently had made peace with being transgender. Thus, she pursued talking to her medical provider about transitioning. The patient reported that she felt more energetic when taking female hormones and was better able to discuss the issue. Specifically, she noted that if she were not on estrogen now she would not be able to talk about transitioning.
The patient related that she has done extensive research about transitioning, including reading online about other transgender people. She noted that she was aware of “possible backlash with society,” but ultimately, she had decided that transitioning was the right decision for her.
She expressed a desire to have an orchiectomy and continue hormonal therapy to permit her “to have a more feminine face, soft skin, hairless body, big breasts, more fat around the hips, and a high-pitched voice.” She additionally related a desire to be in a stable relationship and be her true self. She also stated that she had not identified herself as a female to anyone yet but would like to soon. The patient reported a history of depression, especially during her military service when she wanted to be a woman but did not feel she understood what was going on or how to manage her feelings. She said that for the past 2 months she felt much happier since beginning to take estradiol 4 mg orally daily, which she had found online. She also tried to purchase anti-androgen medication but could not afford it. In addition, she said that she would like to eventually proceed with gender affirmation surgery.
She was currently having sex with men, primarily via anal receptive intercourse. She had no history of sexually transmitted infections but reported that she did not use condoms regularly. She had no history of physical or sexual abuse. The patient was offered referral to the HIV clinic to receive HIV preexposure prophylaxis therapy (emtricitabine + tenofovir), which she declined, but she was counseled on safe sex practice.
The patient was referred to psychiatry both for supportive mental health care and to clarify that her concomitant mental health issues would not preclude the prescription of gender-affirming hormone treatment. Based on the psychiatric evaluation, the patient was felt to be appropriate for treatment with feminizing hormone therapy. The psychiatric assessment also noted that although the patient had a history of psychosis, she was not exhibiting psychotic symptoms currently, and this would not be a contraindication to treatment.
After discussion of the risks and benefits of cross-sex hormone therapy, the patient was started on estradiol 4 mg orally daily, as well as spironolactone 50 mg daily. She was then switched to estradiol 10 mg intramuscular every 2 weeks with the aim of using a less thrombogenic route of administration.
Treatment Outcomes
The patient remains under care. She has had follow-up visits every 3 months to ensure appropriate signs of feminization and monitoring of adverse effects (AEs). The patient’s testosterone and estradiol levels are being checked every 3 months to ensure total testosterone is 1,2
After 12 months on therapy with estradiol and spironolactone, the patient notes that her mood has improved, she feels more energetic, she has gained some weight, and her skin is softer. Her voice pitch, with the help of speech therapy, is gradually changing to what she perceives as more feminine. Hormone levels and electrolytes are all in an acceptable range, and blood sugar and blood pressure (BP) are within normal range. The patient will be offered age-appropriate cancer screening at the appropriate time.
Discussion
The treatment of gender-nonconforming individuals has come a long way since Lili Elbe, the transgender artist depicted in The Danish Girl, underwent gender-affirmation surgery in the early 20th century. Lili and people like her paved the way for other transgender individuals by doggedly pursuing gender-affirming medical treatment although they faced rejection by society and forged a difficult path. In recent years, an increasing number of transgender individuals have begun to seek mainstream medical care; however, PCPs often lack the knowledge and training to properly interact with and care for transgender patients.3,4
Terminology
Although someone’s sex is typically assigned at birth based on the external appearance of their genitalia, gender identity refers to a person’s internal sense of self and how they fit in to the world. People often use these 2 terms interchangeably in everyday language, but these terms are different.1,2
Transgender refers to a person whose gender identity differs from the sex that was assigned at birth. A transgender man or transman, or female-to-male (FTM) transgender person, is an individual who is assigned female sex at birth but identifies as a male. A transgender woman, or transwoman or a male-to-female (MTF) transgender person, is an individual who is assigned male sex at birth but identifies as female. A nontransgender person may be referred to as cisgender.
Transsexual is a medical term and refers to a transgender individual who sought medical intervention to transition to their identified gender.
Sexual orientation describes sexual attraction only and is not related to gender identity. The sexual orientation of a transgender person is determined by emotional and/or physical attraction and not gender identity.
Gender dysphoria refers to the distress experienced by an individual when one’s gender identity and sex are not completely congruent.
Improving Patient Interaction
Transgender patients might avoid seeking care due to previous negative experiences or a fear of being judged. It is very important to create a safe environment where the patients feel comfortable. Meeting patients “where they are” without judgment will enhance the patient-physician relationship. It is necessary to train all clinic staff about the importance of transgender health issues. All staff should address the patient with the name, pronouns, and gender identity that the patient prefers. For patients with a gender identity that is not strictly male or female (nonbinary patients), gender-neutral pronouns, such as they/them/their, may be chosen. It is helpful to be direct in asking: What is your preferred name? When I speak about you to other providers, what pronouns do you prefer I use, he, she, they? This information can then be documented in the electronic health record (EHR) so that all staff know from visit to visit. Thank the patient for the clarification.
The physical examination can be uncomfortable for both the patient and the physician. Experience and familiarity with the current recommendations can help. The physical examination should be relevant to the anatomy that is present, regardless of the gender presentation. An anatomic survey of the organs currently present in an individual can be useful.1 The physician should be sensitive in examining and obtaining information from the patient, focusing on only those issues relevant to the presenting concern. Chest and genital examinations may be particularly distressing for patients. If a chest or genital examination is indicated, the provider and patient should have a discussion explaining the importance of the examination and how the patient’s comfort can be optimized.
Medical Treatment
Gender-affirmation treatment should be multidisciplinary and include some or all of the following: diagnostic assessment, psychotherapy or counseling, real-life experience (RLE), hormone therapy, and surgical therapy..1,2,5 The World Professional Association for Transgender Health (WPATH) has established internationally accepted Standards of Care (SOC) for the treatment of gender dysphoria that provide detailed expert opinion reviewing the background and guidance for care of transgender individuals. Most commonly, the diagnosis of gender dysphoria is made by a mental health professional (MHP) based on the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) criteria for gender dysphoria.1,2 The involvement of a MHP can be crucial in assessing potential psychological and social risk factors for unfavorable outcomes of medical interventions. In case of severe psychopathology, which can interfere with diagnosis and treatment, the psychopathology should be addressed first.1,2 The MHP also can confirm that the patient has the capacity to make an informed decision.
The 2017 Endocrine Society guidelines for the treatment of gender-dysphoric/gender-incongruent persons emphasize the utility of evaluation of these patients by an expert MHP before starting the treatment.2 However, the guidelines from WPATH and the Center for Transgender Excellence at University of California, San Francisco (UCSF) have stipulated that any provider who feels comfortable assessing the informed decision-making process with a patient can make this determination.
The WPATH SOC states that RLE is essential to transition to the gender role that is congruent with the patient’s gender identity. The RLE is defined as the act of fully adopting a new or evolving gender role or gender presentation in everyday life. In the RLE, the person should fully experience life in the desired gender role before irreversible physical treatment is undertaken. Newer guidelines note that it may be too challenging to adopt the desired gender role without the benefit of feminizing or masculinizing treatment, and therefore, the treatment can be offered at the same time as adopting the new gender role.1
Medical treatment involves administration of masculinizing or feminizing hormone therapy. There are 2 major goals of this hormonal therapy.
For many transgender adults, genital reconstruction surgery and/or gonadectomy is a necessary step toward achieving their goal.
Pretreatment screening and appropriate medical monitoring is recommended for both FTM and MTF transgender patients during the endocrine transition and periodically thereafter.2 The physician should monitor the patient’s weight, BP, directed physical examinations, routine health questions focused on risk factors and medications, complete blood count, renal and liver functions, lipid and blood sugar.2
Physical Changes With Hormone Therapy
Transgender men. Physical changes that are expected to occur during the first 1 to 6 months of testosterone therapy include cessation of menses, increased sexual desire, increased facial and body hair, increased oiliness of skin, increased muscle, and redistribution of fat mass. Changes that occur within the first year of testosterone therapy include deepening of the voice, clitoromegaly, and male pattern hair loss (in some cases). Deepening of the voice, and clitoromegaly are not reversible with discontinuation of hormonal therapy.2
Transgender women. Physical changes that may occur in transgender females in the first 3 to 12 months of estrogen and anti-androgen therapy include decreased sexual desire, decreased spontaneous erections, decreased facial and body hair (usually mild), decreased oiliness of skin, increased breast tissue growth, and redistribution of fat mass. Breast development is generally maximal at 2 years after initiating estrogen, and it is irreversible.2 Effect on fertility may be permanent. Medical therapy has little effect on voice, and most transwomen will require speech therapy to achieve desired pitch.
Routine Health Maintenance
Breast Cancer Screening
Although there are limited data, it is thought that gender-affirming hormone therapy has similar risks as sex hormone replacement therapy in nontransgender males and females. Most AEs arise from use of supraphysiologic doses or inadequate doses.2 Therefore, regular clinical and laboratory monitoring is essential to cross-sex hormone therapy. Treatment with exogenous estrogen and anti-androgens result in transgender women developing breast tissue with ducts, lobules, and acini that is histologically identical to breast tissue in nontransgender females.6
Breast cancer is a concern in transgender women due to prolonged exposure to estrogen. However, the relationship between breast cancer and cross-sex hormone therapy is controversial.
Many factors contribute to breast cancer risk in patients of all genders. Studies of premenopausal and menopausal women taking exogenous estrogen alone have not shown an increase in breast cancer risk. However, the combination of estrogen and progesterone has shown an association with a significant increase in the incidence of breast cancer in postmenopausal women.2,7-10
A study of 5,136 veterans showed a statistically insignificant increased incidence of breast cancer in transgender women compared with data collected from the Surveillance, Epidemiology, and End Results database, although the sample size and duration of the observation were limiting factors.8 A European cohort study found decreased incidence of breast cancer in both MTF and FTM transgender patients, but these patients were an overall younger cohort with decreased risk in general. A cohort of 2,236 MTF individuals in the Netherlands in 1997 showed no increase in all-cause mortality related to hormone therapy at 30-year follow-up. Patients were exposed to exogenous estrogen from 2 months to 41 years.9 A follow-up of this study published in 2013, which included 2,307 MTF individuals taking estrogen for 5 years to > 30 years, revealed only 2 cases of breast cancer, which was the same incidence rate (4.1 per 100,000 person-years) as that of nontransgender women.10
In general, the incidence of breast cancer is rare in nontransgender men, and therefore there have not been a lot of clinical studies to assess risk factors and detection methods. The following risk factors can increase the risk of breast cancer in nontransgender patients: known presence of BRCA mutation, estrogen exposure/androgen insufficiency, Klinefelter syndrome, liver cirrhosis, and obesity.11
Guidelines from the Endocrine Society, WPATH, and UCSF suggest that MTF transgender individuals who have a known increased risk for breast cancer should follow screening guidelines recommended for nontransgender women if they are aged > 50 years and have had more than 5 years of hormone use.2 For FTM patients who have not had chest surgery, screening guidelines should follow those for nontransgender women. For those patients who have had chest reconstruction, small residual amounts of breast tissue may remain. Screening guidelines for these patients do not exist. For these patients, mammography can be technically difficult. Clinical chest wall examination, magnetic resonance imaging (MRI), and/or ultrasound may be helpful modalities. An individual risk vs benefit discussion with the patient is recommended.
Prostate Cancer Screening
Although the prostate gland will undergo atrophy with extended treatment with feminizing hormone therapy, there are case reports of prostate cancer in transgender women.12,13 Usually these patients have started hormone treatment after age 50 years. Therefore, prostate cancer screening is recommended in transgender women as per general guidelines. Because the prostate-specific antigen (PSA) level is expected to be reduced, a PSA > 1.0 should be considered abnormal.1
Cervical Cancer Screening
When a transgender man has a pap smear, it is essential to make it clear to the laboratory that the sample is a cervical pap smear (especially if the gender is marked as male) to avoid the sample being run incorrectly as an anal pap. Also, it is essential to indicate on the pap smear request form that the patient is on testosterone therapy and amenorrhea is present, because the lack of the female hormone can cause atrophy of cervix. This population has a high rate of inadequate specimens. Pretreatment with 1 to 2 weeks of vaginal estrogen can improve the success rate of inadequate specimens. Transgender women who have undergone vaginoplasty do not have a cervix, therefore, cervical cancer screening is not recommended. The anatomy of the neovagina has a more posterior orientation, and an anoscope is a more appropriate tool to examine the neovagina when necessary.
Hematology Health
Transgender women on cross-sex hormone therapy with estrogens may be at increased risk for a venous thromboembolism (VTE). In 2 European studies, patients treated with oral ethinyl estradiol as well as the anti-androgen cyproterone acetate were found to have up to 20 times increased risk of VTE. However, in later studies, oral ethinyl estradiol was changed to either oral conjugated estrogens or transdermal/intramuscular estradiol, and these studies did not show a significant increase in VTE risk.14-16 Tobacco use in combination with estrogen therapy is associated with an increased risk of deep vein thrombosis (DVT).1 All transgender women who smoke should be counseled on tobacco risks and cessation options at every visit.1 The transgender individuals who are not willing to quit smoking may be offered transdermal estrogen, which has lower risk of DVT.14-16
Sexual Health
Clinicians should assess the risks for sexually transmitted infection (STIs) or HIV for transgender patients based on current anatomy and sexual behaviors. Presentations of STIs can be atypical due to varied sexual practices and gender-affirming surgeries. Thus, providers must remain vigilant for symptoms consistent with common STIs and screen for asymptomatic STIs on the basis of behavior history and sexual practices.17 Preexposure prophylaxis for HIV should be considered when appropriate. Serologic screening recommendations for transgender people (eg, HIV, hepatitis B and C, syphilis) do not differ in recommendations from those for nontransgender people.
Cardiovascular Health
The effect of cross-hormone treatment on cardiovascular (CV) health is still unknown. There are no randomized controlled trials that have investigated the relationship between cross-hormone treatment and CV health. Evidence from several studies suggests that CV risk is unchanged among transgender men using testosterone compared with that of nontransgender women.18,19 There is conflicting evidence for transgender women with respect to CV risk and cross-sex hormone treatment.1,18,19 The current American College of Cardiology/American Heart Association guideline advises using the ASCVD risk calculator to determine the need for aspirin and statin treatment based on race, age, gender, and risk factors. There is no guideline on whether to use natal sex or affirmed gender while using the ASCVD calculator. It is reasonable to use the calculator based on natal sex if the cross-hormone treatment has started later in life, but if the cross-sex hormone treatment started at a young age, then one should consider using the affirmed gender to calculate the risk.
As with all patients, life style modifications, including smoking cessation, weight loss, physical activity, and management of BP and blood sugar, are important for CV health. For transgender women with CV risk factors or known CV disease, transdermal route of estrogen is preferred due to lower rates of VTE.18,19
Conclusion
In recent years, an increased number of transgender individuals are seeking mainstream medical care. However, PCPs often lack the knowledge and training to properly interact with and care for transgender patients. It is critical that clinicians understand the difference between sex, gender, and sexuality. For patients who desire transgender care, providers must be able to comfortably ask the patient about their preferred name and prior care, know the basics in cross-sex hormone therapy, including appropriate follow-up of hormonal levels as well as laboratory tests that delineate risk, and know possible complications and AEs. The VA offers significant resources, including electronic transgender care consultation for cases where the provider does not have adequate expertise in the care of these patients.
Both medical schools and residency training programs are starting to incorporate curricula regarding LGBT care. For those who have already completed training, this article serves as a brief guide to terminology, interactive tips, and management of this growing and underserved group of individuals.
Lesbian, gay, bisexual, and transgender (LGBT) individuals face significant difficulties in obtaining high-quality,compassionate medical care, much of which has been attributed to inadequate provider knowledge. In this article, the authors present a transgender patient seen in primary care and discuss the knowledge gleaned to inform future care of this patient as well as the care of other similar patients.
The following case discussion and review of the literature also seeks to improve the practice of other primary care providers (PCPs) who are inexperienced in this arena. This article aims to review the basics to permit PCPs to venture into transgender care, including a review of basic terminology; a few interactive tips; and basics in medical and hormonal treatment, follow-up, contraindications, and risk. More details can be obtained through electronic consultation (Transgender eConsult) in the VA.
Case Presentation
A 35-year-old patient who was assigned male sex at birth presented to the primary care clinic to discuss her desire to undergo male-to-female (MTF) transition. The patient stated that she had started taking female estrogen hormones 9 years previously purchased from craigslist without a prescription. She tried oral contraceptives as well as oral and injectable estradiol. While the patient was taking injectable estradiol she had breast growth, decreased anxiety, weight gain, and a feeling of peacefulness. The patient also reported that she had received several laser treatments for whole body hair removal, beginning 8 to 10 years before and more regularly in the past 2 to 3 years. She asked whether transition-related care could be provided, because she could no longer afford the hormones.
The patient wanted to transition because she felt that “Women are beautiful, the way they carry themselves, wear their hair, their nails, I want to be like that.” She also mentioned that when she watched TV, she envisioned herself as a woman. She reported that she enjoyed wearing her mother’s clothing since age 10, which made her feel more like herself. The patient noted that she had desired to remove her body hair since childhood but could not afford to do it until recently. She bought female clothing, shoes and makeup, and did her nails from a young age. The patient also reported that she did not “know what transgender was” until a decade ago.
The patient struggled with her identity growing up; however, she tried not to think about it or talk about it with anyone. She related that she was ashamed of her thoughts and that only recently had made peace with being transgender. Thus, she pursued talking to her medical provider about transitioning. The patient reported that she felt more energetic when taking female hormones and was better able to discuss the issue. Specifically, she noted that if she were not on estrogen now she would not be able to talk about transitioning.
The patient related that she has done extensive research about transitioning, including reading online about other transgender people. She noted that she was aware of “possible backlash with society,” but ultimately, she had decided that transitioning was the right decision for her.
She expressed a desire to have an orchiectomy and continue hormonal therapy to permit her “to have a more feminine face, soft skin, hairless body, big breasts, more fat around the hips, and a high-pitched voice.” She additionally related a desire to be in a stable relationship and be her true self. She also stated that she had not identified herself as a female to anyone yet but would like to soon. The patient reported a history of depression, especially during her military service when she wanted to be a woman but did not feel she understood what was going on or how to manage her feelings. She said that for the past 2 months she felt much happier since beginning to take estradiol 4 mg orally daily, which she had found online. She also tried to purchase anti-androgen medication but could not afford it. In addition, she said that she would like to eventually proceed with gender affirmation surgery.
She was currently having sex with men, primarily via anal receptive intercourse. She had no history of sexually transmitted infections but reported that she did not use condoms regularly. She had no history of physical or sexual abuse. The patient was offered referral to the HIV clinic to receive HIV preexposure prophylaxis therapy (emtricitabine + tenofovir), which she declined, but she was counseled on safe sex practice.
The patient was referred to psychiatry both for supportive mental health care and to clarify that her concomitant mental health issues would not preclude the prescription of gender-affirming hormone treatment. Based on the psychiatric evaluation, the patient was felt to be appropriate for treatment with feminizing hormone therapy. The psychiatric assessment also noted that although the patient had a history of psychosis, she was not exhibiting psychotic symptoms currently, and this would not be a contraindication to treatment.
After discussion of the risks and benefits of cross-sex hormone therapy, the patient was started on estradiol 4 mg orally daily, as well as spironolactone 50 mg daily. She was then switched to estradiol 10 mg intramuscular every 2 weeks with the aim of using a less thrombogenic route of administration.
Treatment Outcomes
The patient remains under care. She has had follow-up visits every 3 months to ensure appropriate signs of feminization and monitoring of adverse effects (AEs). The patient’s testosterone and estradiol levels are being checked every 3 months to ensure total testosterone is 1,2
After 12 months on therapy with estradiol and spironolactone, the patient notes that her mood has improved, she feels more energetic, she has gained some weight, and her skin is softer. Her voice pitch, with the help of speech therapy, is gradually changing to what she perceives as more feminine. Hormone levels and electrolytes are all in an acceptable range, and blood sugar and blood pressure (BP) are within normal range. The patient will be offered age-appropriate cancer screening at the appropriate time.
Discussion
The treatment of gender-nonconforming individuals has come a long way since Lili Elbe, the transgender artist depicted in The Danish Girl, underwent gender-affirmation surgery in the early 20th century. Lili and people like her paved the way for other transgender individuals by doggedly pursuing gender-affirming medical treatment although they faced rejection by society and forged a difficult path. In recent years, an increasing number of transgender individuals have begun to seek mainstream medical care; however, PCPs often lack the knowledge and training to properly interact with and care for transgender patients.3,4
Terminology
Although someone’s sex is typically assigned at birth based on the external appearance of their genitalia, gender identity refers to a person’s internal sense of self and how they fit in to the world. People often use these 2 terms interchangeably in everyday language, but these terms are different.1,2
Transgender refers to a person whose gender identity differs from the sex that was assigned at birth. A transgender man or transman, or female-to-male (FTM) transgender person, is an individual who is assigned female sex at birth but identifies as a male. A transgender woman, or transwoman or a male-to-female (MTF) transgender person, is an individual who is assigned male sex at birth but identifies as female. A nontransgender person may be referred to as cisgender.
Transsexual is a medical term and refers to a transgender individual who sought medical intervention to transition to their identified gender.
Sexual orientation describes sexual attraction only and is not related to gender identity. The sexual orientation of a transgender person is determined by emotional and/or physical attraction and not gender identity.
Gender dysphoria refers to the distress experienced by an individual when one’s gender identity and sex are not completely congruent.
Improving Patient Interaction
Transgender patients might avoid seeking care due to previous negative experiences or a fear of being judged. It is very important to create a safe environment where the patients feel comfortable. Meeting patients “where they are” without judgment will enhance the patient-physician relationship. It is necessary to train all clinic staff about the importance of transgender health issues. All staff should address the patient with the name, pronouns, and gender identity that the patient prefers. For patients with a gender identity that is not strictly male or female (nonbinary patients), gender-neutral pronouns, such as they/them/their, may be chosen. It is helpful to be direct in asking: What is your preferred name? When I speak about you to other providers, what pronouns do you prefer I use, he, she, they? This information can then be documented in the electronic health record (EHR) so that all staff know from visit to visit. Thank the patient for the clarification.
The physical examination can be uncomfortable for both the patient and the physician. Experience and familiarity with the current recommendations can help. The physical examination should be relevant to the anatomy that is present, regardless of the gender presentation. An anatomic survey of the organs currently present in an individual can be useful.1 The physician should be sensitive in examining and obtaining information from the patient, focusing on only those issues relevant to the presenting concern. Chest and genital examinations may be particularly distressing for patients. If a chest or genital examination is indicated, the provider and patient should have a discussion explaining the importance of the examination and how the patient’s comfort can be optimized.
Medical Treatment
Gender-affirmation treatment should be multidisciplinary and include some or all of the following: diagnostic assessment, psychotherapy or counseling, real-life experience (RLE), hormone therapy, and surgical therapy..1,2,5 The World Professional Association for Transgender Health (WPATH) has established internationally accepted Standards of Care (SOC) for the treatment of gender dysphoria that provide detailed expert opinion reviewing the background and guidance for care of transgender individuals. Most commonly, the diagnosis of gender dysphoria is made by a mental health professional (MHP) based on the Diagnostic and Statistical Manual of Mental Disorders (DSM–5) criteria for gender dysphoria.1,2 The involvement of a MHP can be crucial in assessing potential psychological and social risk factors for unfavorable outcomes of medical interventions. In case of severe psychopathology, which can interfere with diagnosis and treatment, the psychopathology should be addressed first.1,2 The MHP also can confirm that the patient has the capacity to make an informed decision.
The 2017 Endocrine Society guidelines for the treatment of gender-dysphoric/gender-incongruent persons emphasize the utility of evaluation of these patients by an expert MHP before starting the treatment.2 However, the guidelines from WPATH and the Center for Transgender Excellence at University of California, San Francisco (UCSF) have stipulated that any provider who feels comfortable assessing the informed decision-making process with a patient can make this determination.
The WPATH SOC states that RLE is essential to transition to the gender role that is congruent with the patient’s gender identity. The RLE is defined as the act of fully adopting a new or evolving gender role or gender presentation in everyday life. In the RLE, the person should fully experience life in the desired gender role before irreversible physical treatment is undertaken. Newer guidelines note that it may be too challenging to adopt the desired gender role without the benefit of feminizing or masculinizing treatment, and therefore, the treatment can be offered at the same time as adopting the new gender role.1
Medical treatment involves administration of masculinizing or feminizing hormone therapy. There are 2 major goals of this hormonal therapy.
For many transgender adults, genital reconstruction surgery and/or gonadectomy is a necessary step toward achieving their goal.
Pretreatment screening and appropriate medical monitoring is recommended for both FTM and MTF transgender patients during the endocrine transition and periodically thereafter.2 The physician should monitor the patient’s weight, BP, directed physical examinations, routine health questions focused on risk factors and medications, complete blood count, renal and liver functions, lipid and blood sugar.2
Physical Changes With Hormone Therapy
Transgender men. Physical changes that are expected to occur during the first 1 to 6 months of testosterone therapy include cessation of menses, increased sexual desire, increased facial and body hair, increased oiliness of skin, increased muscle, and redistribution of fat mass. Changes that occur within the first year of testosterone therapy include deepening of the voice, clitoromegaly, and male pattern hair loss (in some cases). Deepening of the voice, and clitoromegaly are not reversible with discontinuation of hormonal therapy.2
Transgender women. Physical changes that may occur in transgender females in the first 3 to 12 months of estrogen and anti-androgen therapy include decreased sexual desire, decreased spontaneous erections, decreased facial and body hair (usually mild), decreased oiliness of skin, increased breast tissue growth, and redistribution of fat mass. Breast development is generally maximal at 2 years after initiating estrogen, and it is irreversible.2 Effect on fertility may be permanent. Medical therapy has little effect on voice, and most transwomen will require speech therapy to achieve desired pitch.
Routine Health Maintenance
Breast Cancer Screening
Although there are limited data, it is thought that gender-affirming hormone therapy has similar risks as sex hormone replacement therapy in nontransgender males and females. Most AEs arise from use of supraphysiologic doses or inadequate doses.2 Therefore, regular clinical and laboratory monitoring is essential to cross-sex hormone therapy. Treatment with exogenous estrogen and anti-androgens result in transgender women developing breast tissue with ducts, lobules, and acini that is histologically identical to breast tissue in nontransgender females.6
Breast cancer is a concern in transgender women due to prolonged exposure to estrogen. However, the relationship between breast cancer and cross-sex hormone therapy is controversial.
Many factors contribute to breast cancer risk in patients of all genders. Studies of premenopausal and menopausal women taking exogenous estrogen alone have not shown an increase in breast cancer risk. However, the combination of estrogen and progesterone has shown an association with a significant increase in the incidence of breast cancer in postmenopausal women.2,7-10
A study of 5,136 veterans showed a statistically insignificant increased incidence of breast cancer in transgender women compared with data collected from the Surveillance, Epidemiology, and End Results database, although the sample size and duration of the observation were limiting factors.8 A European cohort study found decreased incidence of breast cancer in both MTF and FTM transgender patients, but these patients were an overall younger cohort with decreased risk in general. A cohort of 2,236 MTF individuals in the Netherlands in 1997 showed no increase in all-cause mortality related to hormone therapy at 30-year follow-up. Patients were exposed to exogenous estrogen from 2 months to 41 years.9 A follow-up of this study published in 2013, which included 2,307 MTF individuals taking estrogen for 5 years to > 30 years, revealed only 2 cases of breast cancer, which was the same incidence rate (4.1 per 100,000 person-years) as that of nontransgender women.10
In general, the incidence of breast cancer is rare in nontransgender men, and therefore there have not been a lot of clinical studies to assess risk factors and detection methods. The following risk factors can increase the risk of breast cancer in nontransgender patients: known presence of BRCA mutation, estrogen exposure/androgen insufficiency, Klinefelter syndrome, liver cirrhosis, and obesity.11
Guidelines from the Endocrine Society, WPATH, and UCSF suggest that MTF transgender individuals who have a known increased risk for breast cancer should follow screening guidelines recommended for nontransgender women if they are aged > 50 years and have had more than 5 years of hormone use.2 For FTM patients who have not had chest surgery, screening guidelines should follow those for nontransgender women. For those patients who have had chest reconstruction, small residual amounts of breast tissue may remain. Screening guidelines for these patients do not exist. For these patients, mammography can be technically difficult. Clinical chest wall examination, magnetic resonance imaging (MRI), and/or ultrasound may be helpful modalities. An individual risk vs benefit discussion with the patient is recommended.
Prostate Cancer Screening
Although the prostate gland will undergo atrophy with extended treatment with feminizing hormone therapy, there are case reports of prostate cancer in transgender women.12,13 Usually these patients have started hormone treatment after age 50 years. Therefore, prostate cancer screening is recommended in transgender women as per general guidelines. Because the prostate-specific antigen (PSA) level is expected to be reduced, a PSA > 1.0 should be considered abnormal.1
Cervical Cancer Screening
When a transgender man has a pap smear, it is essential to make it clear to the laboratory that the sample is a cervical pap smear (especially if the gender is marked as male) to avoid the sample being run incorrectly as an anal pap. Also, it is essential to indicate on the pap smear request form that the patient is on testosterone therapy and amenorrhea is present, because the lack of the female hormone can cause atrophy of cervix. This population has a high rate of inadequate specimens. Pretreatment with 1 to 2 weeks of vaginal estrogen can improve the success rate of inadequate specimens. Transgender women who have undergone vaginoplasty do not have a cervix, therefore, cervical cancer screening is not recommended. The anatomy of the neovagina has a more posterior orientation, and an anoscope is a more appropriate tool to examine the neovagina when necessary.
Hematology Health
Transgender women on cross-sex hormone therapy with estrogens may be at increased risk for a venous thromboembolism (VTE). In 2 European studies, patients treated with oral ethinyl estradiol as well as the anti-androgen cyproterone acetate were found to have up to 20 times increased risk of VTE. However, in later studies, oral ethinyl estradiol was changed to either oral conjugated estrogens or transdermal/intramuscular estradiol, and these studies did not show a significant increase in VTE risk.14-16 Tobacco use in combination with estrogen therapy is associated with an increased risk of deep vein thrombosis (DVT).1 All transgender women who smoke should be counseled on tobacco risks and cessation options at every visit.1 The transgender individuals who are not willing to quit smoking may be offered transdermal estrogen, which has lower risk of DVT.14-16
Sexual Health
Clinicians should assess the risks for sexually transmitted infection (STIs) or HIV for transgender patients based on current anatomy and sexual behaviors. Presentations of STIs can be atypical due to varied sexual practices and gender-affirming surgeries. Thus, providers must remain vigilant for symptoms consistent with common STIs and screen for asymptomatic STIs on the basis of behavior history and sexual practices.17 Preexposure prophylaxis for HIV should be considered when appropriate. Serologic screening recommendations for transgender people (eg, HIV, hepatitis B and C, syphilis) do not differ in recommendations from those for nontransgender people.
Cardiovascular Health
The effect of cross-hormone treatment on cardiovascular (CV) health is still unknown. There are no randomized controlled trials that have investigated the relationship between cross-hormone treatment and CV health. Evidence from several studies suggests that CV risk is unchanged among transgender men using testosterone compared with that of nontransgender women.18,19 There is conflicting evidence for transgender women with respect to CV risk and cross-sex hormone treatment.1,18,19 The current American College of Cardiology/American Heart Association guideline advises using the ASCVD risk calculator to determine the need for aspirin and statin treatment based on race, age, gender, and risk factors. There is no guideline on whether to use natal sex or affirmed gender while using the ASCVD calculator. It is reasonable to use the calculator based on natal sex if the cross-hormone treatment has started later in life, but if the cross-sex hormone treatment started at a young age, then one should consider using the affirmed gender to calculate the risk.
As with all patients, life style modifications, including smoking cessation, weight loss, physical activity, and management of BP and blood sugar, are important for CV health. For transgender women with CV risk factors or known CV disease, transdermal route of estrogen is preferred due to lower rates of VTE.18,19
Conclusion
In recent years, an increased number of transgender individuals are seeking mainstream medical care. However, PCPs often lack the knowledge and training to properly interact with and care for transgender patients. It is critical that clinicians understand the difference between sex, gender, and sexuality. For patients who desire transgender care, providers must be able to comfortably ask the patient about their preferred name and prior care, know the basics in cross-sex hormone therapy, including appropriate follow-up of hormonal levels as well as laboratory tests that delineate risk, and know possible complications and AEs. The VA offers significant resources, including electronic transgender care consultation for cases where the provider does not have adequate expertise in the care of these patients.
Both medical schools and residency training programs are starting to incorporate curricula regarding LGBT care. For those who have already completed training, this article serves as a brief guide to terminology, interactive tips, and management of this growing and underserved group of individuals.
1. Deutsch MB. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. http://transhealth.ucsf.edu/protocols. Updated June 17, 2016. Accessed June 13, 2018.
2. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoria/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903.
3. Buchholz L. Transgender care moves into the mainstream. JAMA. 2015;314(17):1785-1787.
4. Sobralske M. Primary care needs of patients who have undergone gender reassignment. J Am Acad Nurse Pract. 2005;17(4):133-138.
5. Unger CA. Hormone therapy for transgender patients. Transl Androl Urol. 2016;5(6):877-884.
6. Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW. Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men. Am J Surg Pathol. 2000;24(1):74-80.
7. Braun H, Nash R, Tangpricha V, Brockman J, Ward K, Goodman M. Cancer in transgender people: evidence and methodological consideration. Epidemiol Rev. 2017;39(1):93-107.
8. Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res Treat. 2015;149(1):191-198.
9. Van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf). 1997;47(3):337-342.
10. Gooren LJ, van Trotsenburg MA, Giltay EJ, van Diest PJ. Breast cancer development in transsexual subjects receiving cross-sex hormone treatment. J Sex Med. 2013;10(12):3129-3134.
11. Johansen Taber KA, Morisy LR, Osbahr AJ III, Dickinson BD. Male breast cancer: risk factors, diagnosis and management (review). Oncol Rep. 2010;24(5):1115-1120.
12. Miksad RA, Bubley G, Church P, et al. Prostate cancer in a transgender woman, 41 years after initiation of feminization. JAMA. 2006;296(19):2316-2317.
13. Turo R, Jallad S, Prescott S, Cross WR. Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy. Can Urol Assoc J. 2013;7(7-8):E544-E546.
14. American College of Obstetricians and Gynecologists. ACOG committee opinion no. 556: postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013;121(4):887-890.
15. Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender treatment. Metabolism. 1989;38(9):869-873.
16. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635-642.
17. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
18. Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol. 2014;170(6):809-819.
19. Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Int Med. 2017;167(4):256-267.
1. Deutsch MB. Guidelines for the primary and gender-affirming care of transgender and gender nonbinary people. http://transhealth.ucsf.edu/protocols. Updated June 17, 2016. Accessed June 13, 2018.
2. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoria/gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903.
3. Buchholz L. Transgender care moves into the mainstream. JAMA. 2015;314(17):1785-1787.
4. Sobralske M. Primary care needs of patients who have undergone gender reassignment. J Am Acad Nurse Pract. 2005;17(4):133-138.
5. Unger CA. Hormone therapy for transgender patients. Transl Androl Urol. 2016;5(6):877-884.
6. Kanhai RC, Hage JJ, van Diest PJ, Bloemena E, Mulder JW. Short-term and long-term histologic effects of castration and estrogen treatment on breast tissue of 14 male-to-female transsexuals in comparison with two chemically castrated men. Am J Surg Pathol. 2000;24(1):74-80.
7. Braun H, Nash R, Tangpricha V, Brockman J, Ward K, Goodman M. Cancer in transgender people: evidence and methodological consideration. Epidemiol Rev. 2017;39(1):93-107.
8. Brown GR, Jones KT. Incidence of breast cancer in a cohort of 5,135 transgender veterans. Breast Cancer Res Treat. 2015;149(1):191-198.
9. Van Kesteren PJ, Asscheman H, Megens JA, Gooren LJ. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf). 1997;47(3):337-342.
10. Gooren LJ, van Trotsenburg MA, Giltay EJ, van Diest PJ. Breast cancer development in transsexual subjects receiving cross-sex hormone treatment. J Sex Med. 2013;10(12):3129-3134.
11. Johansen Taber KA, Morisy LR, Osbahr AJ III, Dickinson BD. Male breast cancer: risk factors, diagnosis and management (review). Oncol Rep. 2010;24(5):1115-1120.
12. Miksad RA, Bubley G, Church P, et al. Prostate cancer in a transgender woman, 41 years after initiation of feminization. JAMA. 2006;296(19):2316-2317.
13. Turo R, Jallad S, Prescott S, Cross WR. Metastatic prostate cancer in transsexual diagnosed after three decades of estrogen therapy. Can Urol Assoc J. 2013;7(7-8):E544-E546.
14. American College of Obstetricians and Gynecologists. ACOG committee opinion no. 556: postmenopausal estrogen therapy: route of administration and risk of venous thromboembolism. Obstet Gynecol. 2013;121(4):887-890.
15. Asscheman H, Gooren LJ, Eklund PL. Mortality and morbidity in transsexual patients with cross-gender treatment. Metabolism. 1989;38(9):869-873.
16. Asscheman H, Giltay EJ, Megens JA, de Ronde WP, van Trotsenburg MA, Gooren LJ. A long-term follow-up study of mortality in transsexuals receiving treatment with cross-sex hormones. Eur J Endocrinol. 2011;164(4):635-642.
17. Workowski KA, Bolan GA; Centers for Disease Control and Prevention. Sexually transmitted disease treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137.
18. Gooren LJ, Wierckx K, Giltay EJ. Cardiovascular disease in transsexual persons treated with cross-sex hormones: reversal of the traditional sex difference in cardiovascular disease pattern. Eur J Endocrinol. 2014;170(6):809-819.
19. Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Int Med. 2017;167(4):256-267.
Diagnosis and Management of Aggressive B-Cell Non-Hodgkin Lymphoma
Abstract
- Objective: To review the diagnosis and management of aggressive B-cell non-Hodgkin lymphoma (NHL).
- Methods: Review of the literature.
- Results: NHL comprises a wide variety of malignant hematologic disorders with varying clinical and biological features. Aggressive NHLs are characterized by rapid clinical progression without therapy. However, a significant proportion of patients are cured with appropriate combination chemotherapy or combined modality regimens. In contrast, the indolent lymphomas have a relatively good prognosis (median survival of 10 years or longer) but usually are not curable in advanced clinical stages. Overall 5-year survival for aggressive NHLs with current treatment is approximately 50% to 60%, with relapses typically occurring within the first 5 years.
- Conclusion: Treatment strategies for relapsed patients offer some potential for cure; however, clinical trial participation should be encouraged whenever possible to investigate new approaches for improving outcomes in this patient population.
Non-Hodgkin lymphoma (NHL) comprises a wide variety of malignant hematologic disorders with varying clinical and biological features. The more than 60 separate NHL subtypes can be classified according to cell of origin (B cell versus T cell), anatomical location (eg, orbital, testicular, bone, central nervous system), clinical behavior (indolent versus aggressive), histological features, or cytogenetic abnormalities. Although various NHL classification schemes have been used over the years, the World Health Organization (WHO) classification is now widely accepted as the definitive pathologic classification system for lymphoproliferative disorders, incorporating morphologic, immunohistochemical, flow cytometric, cytogenetic, and molecular features [1]. While the pathologic and molecular subclassification of NHL has become increasingly refined in recent years, from a management standpoint, classification based on clinical behavior remains very useful. This approach separates NHL subtypes into indolent versus aggressive categories. Whereas indolent NHLs may remain clinically insignificant for months to years, aggressive B-cell NHLs generally become life-threatening within weeks to months without treatment.
Epidemiology
Data from cancer registries show a steady, unexplainable increase in the incidence of NHL during the second half of the 20th century; the incidence has subsequently plateaued. There was a significant increase in NHL incidence between 1970 and 1995, which has been attributed in part to the HIV epidemic. More than 72,000 new cases of NHL were diagnosed in the United States in 2017, compared to just over 8000 cases of Hodgkin lymphoma, making NHL the sixth most common cancer in adult men and the fifth most common in adult women [2]. NHL appears to occur more frequently in Western countries than in Asian populations.
Various factors associated with increased risk for B-cell NHL have been identified over the years, including occupational and environmental exposure to certain pesticides and herbicides [3], immunosuppression associated with HIV infection [4], autoimmune disorders [5], iatrogenically induced immune suppression in the post-transplant and other settings [6], family history of NHL [7], and a personal history of a prior cancer, including Hodgkin lymphoma and prior NHL [8]. In terms of infectious agents associated with aggressive B-cell NHLs, Epstein-Barr virus (EBV) has a clear pathogenic role in Burkitt lymphoma, in many cases of post-transplant lymphoproliferative disorders, and in some cases of HIV-related aggressive B-cell lymphoma [9]. Human herpesvirus-8 viral genomes have been found in virtually all cases of primary effusion lymphomas [10]. Epidemiological studies also have linked hepatitis B and C to increased incidences of certain NHL subtypes [11–13], including primary hepatic diffuse large B-cell lymphoma (DLBCL). Similarly, Helicobacter pylori has been associated with gastric DLBCL.
Staging and Workup
A tissue biopsy is essential in the diagnosis and management of NHL. The most significant disadvantage of fine-needle aspiration cytology is the lack of histologic architecture. The optimal specimen is an excisional biopsy; when this cannot be performed, a core needle biopsy, ideally using a 16-gauge or larger caliber needle, is the next best choice.
The baseline tests appropriate for most cases of newly diagnosed aggressive B-cell NHL are listed in Table 1.
Prior to the initiation of treatment, patients should always undergo a thorough cardiac and pulmonary evaluation, especially if the patient will be treated with an anthracycline or mediastinal irradiation. Central nervous system (CNS) evaluation with magnetic resonance imaging (MRI) and lumbar puncture is essential if there are neurological signs or symptoms. In addition, certain anatomical sites including the testicles, paranasal sinuses, kidney, adrenal glands, and epidural space have been associated with increased involvement of the CNS and may warrant MRI evaluation and lumbar puncture. Certain NHL subtypes like Burkitt lymphoma, high-grade NHL with translocations of MYC and BCL-2 or BCL-6 (double-hit lymphoma), blastoid mantle cell lymphoma, and lymphoblastic lymphoma have a high risk of CNS involvement, and patients with these subtypes need CNS evaluation.
The Lugano classification is used to stage patients with NHL [14]. This classification is based on the Ann Arbor staging system and uses the distribution and number of tumor sites to stage disease. In general, this staging system in isolation is of limited value in predicting survival after treatment. However, the Ann Arbor stage does have prognostic impact when incorporated into risk scoring systems such as the International Prognostic Index (IPI). In clinical practice, the Ann Arbor stage is useful primarily to determine eligibility for localized therapy approaches. The absence or presence of systemic symptoms such as fevers, drenching night sweats, or weight loss (> 10% of baseline over 6 months or less) is designated by A or B, respectively.
Diffuse Large B-Cell Lymphoma
DLBCL is the most common lymphoid neoplasm in adults, accounting for about 25% of all NHL cases [2]. It is increasingly clear that the diagnostic category of DLBCL is quite heterogeneous in terms of morphology, genetics, and biologic behavior. A number of clinicopathologic subtypes of DLBCL exist, such as T cell/histiocyte–rich large B-cell lymphoma, primary mediastinal large B-cell lymphoma, intravascular large B-cell lymphoma, DLBCL associated with chronic inflammation, lymphomatoid granulomatosis, and EBV-positive large B-cell lymphoma, among others. Gene expression profiling (GEP) can distinguish 2 cell of origin DLBCL subtypes: the germinal center B-cell (GCB) and activated B-cell (ABC) subtypes [15].
DLBCL may be primary (de novo) or may arise through the transformation of many different types of low-grade B-cell lymphomas. This latter scenario is referred to as histologic transformation or transformed lymphoma. In some cases, patients may have a previously diagnosed low-grade B-cell NHL; in other cases, both low-grade and aggressive B-cell NHL may be diagnosed concurrently. The presence of elements of both low-grade and aggressive B-cell NHL in the same biopsy specimen is sometimes referred to as a composite lymphoma.
In the United States, incidence varies by ethnicity, with DLBCL being more common in Caucasians than other races [16]. There is a slight male predominance (55%), median age at diagnosis is 65 years [16,17] and the incidence increases with age.
Presentation, Pathology, and Prognostic Factors
The most common presentation of patients with DLBCL is rapidly enlarging lymphadenopathy, usually in the neck or abdomen. Extranodal/extramedullary presentation is seen in approximately 40% of cases, with the gastrointestinal (GI) tract being the most common site. However, extranodal DLBCL can arise in virtually any tissue [18]. Nodal DLBCL presents with symptoms related to the sites of involvement (eg, shortness of breath or chest pain with mediastinal lymphadenopathy), while extranodal DLBCL typically presents with symptoms secondary to dysfunction at the site of origin. Up to one third of patients present with constitutional symptoms (B symptoms) and more than 50% have elevated serum lactate dehydrogenase (LDH) at diagnosis [19].
Approximately 40% of patients present with stage I/II disease. Of these, only a subset present with stage I, or truly localized disease (defined as that which can be contained within 1 irradiation field). About 60% of patients present with advanced (stage III–IV) disease [20]. The bone marrow is involved in about 15% to 30% of cases. DLBCL involvement of the bone marrow is associated with a less favorable prognosis. Patients with DLBCL elsewhere may have low-grade NHL involvement of the bone marrow. Referred to as discordant bone marrow involvement [21], this feature does not carry the same poor prognosis associated with transformed disease [22] or DLBCL involvement of the bone marrow [23].
DLBCL is defined as a neoplasm of large B-lymphoid cells with a diffuse growth pattern. The proliferative fraction of cells, as determined by Ki-67 staining, is usually greater than 40%, and may even exceed 90%. Lymph nodes usually demonstrate complete effacement of the normal architecture by sheets of atypical lymphoid cells. Tumor cells in DLBCL generally express pan B-cell antigens (CD19, CD20, CD22, CD79a, Pax-5) as well as CD45 and surface immunoglobulin. Between 20% and 37% of DLBCL cases express the BCL-2 protein [24], and about 70% express the BCL-6 protein [25]. C-MYC protein expression is seen in a higher percentage (~ 30%–50%) of cases of DLBCL [26].
Many factors are associated with outcome in DLBCL. The IPI score was developed in the pre-rituximab era and is a robust prognostic tool. This simple tool uses 5 easily obtained clinical factors (age > 60 years, impaired performance status, elevated LDH, > 1 extranodal site of disease, and stage III/IV disease). By summing these factors, 4 groups with distinct 5-year overall survival (OS) rates ranging from 26% to 73% were identified (Table 2).
Cytogenetic and molecular factors also predict outcome in DLBCL. The ABC subtype distinguished by GEP has consistently been shown to have inferior outcomes with first-line therapy. As GEP is not routinely available in clinical practice, immunohistochemical (IHC) approaches (eg, the Hans algorithm) have been developed that can approximate the GEP subtypes. These IHC approaches have approximately 80% concordance with GEP [28]. The 3 most common chromosomal translocations in DLBCL involve BCL-2, BCL-6 and MYC. MYC-rearranged DLBCLs have a less favorable prognosis [29,30]. Cases in which a MYC translocation occurs in combination with a BCL-2 or BCL-6 translocation are commonly referred to as double-hit lymphoma (DHL); cases with all 3 translocations are referred to as triple-hit lymphoma (THL). Both DHL and THL have a worse prognosis with standard DLBCL therapy compared to non-DHL/THL cases. In the 2016 revised WHO classification, DHL and THL are an entity technically distinct from DLBCL, referred to as high-grade B-cell lymphoma [1]. In some cases, MYC and BCL-2 protein overexpression occurs in the absence of chromosomal translocations. Cases in which MYC and BCL-2 are overexpressed (by IHC) are referred to as double expressor lymphoma (DEL), and also have inferior outcome compared with non-DEL DLBCL [31,32]. Interestingly, MYC protein expression alone does not confer inferior outcomes, unlike isolated MYC translocation, which is associated with inferior outcomes.
Treatment
First-Line Therapy. DLBCL is an aggressive disease and, in most cases, survival without treatment can be measured in weeks to months. The advent of combination chemotherapy (CHOP [cyclophosphamide, doxorubicin, vincristine, and prednisone] or CHOP-like regimens) led to disease-free survival (DFS) rates of 35% to 40% at 3 to 5 years [33]. The addition of rituximab to CHOP (R-CHOP) has improved both progression-free surivial (PFS) and OS [34,35].
Treatment options vary for patients with localized (stage I/II) and advanced (stage III/IV) disease. Options for limited-stage DLBCL include an abbreviated course of R-CHOP (3 or 4 cycles) with involved-field radiation therapy (IFRT) versus a full course (6–8 cycles) of R-CHOP without radiation therapy (RT). Most studies comparing combined modality therapy (chemotherapy plus RT) versus chemotherapy alone were conducted in the pre-rituximab era. With the introduction of rituximab, Persky and colleagues [36] studied the use of 3 cycles of R-CHOP followed by RT, demonstrating a slightly improved OS of 92% at 4 years as compared to 88% in a historical cohort. The French LYSA/GOELAMS group performed the only direct comparison in the rituximab era (4 cycles of R-CHOP followed by RT versus 4 cycles of R-CHOP followed by 2 additional cycles of R-CHOP) and reported similar outcomes between both arms [37], with OS of 92% in the R-CHOP alone arm and 96% in the R-CHOP + RT arm (nonsignificant difference statistically). IFRT alone is not recommended other than for palliation in patients who cannot tolerate chemotherapy or combined modality therapy. Stage I and II patients with bulky disease (> 10 cm) have a prognosis similar to patients with advanced DLBCL and should be treated aggressively with 6 to 8 cycles of R-CHOP with or without RT [36].
For patients with advanced stage disease, a full course of R-CHOP-21 (6–8 cycles given on a 21-day cycle) is the standard of care. This approach results in OS rates of 70% and 60% at 2 and 5 years, respectively. For older adults unable to tolerate full-dose R-CHOP, attenuated versions of R-CHOP with decreased dose density or decreased dose intensity have been developed [38]. Numerous randomized trials have attempted to improve upon the results of R-CHOP-21 using strategies such as infusional chemotherapy (DA-EPOCH-R [etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, rituximab]) [39]; dose-dense therapy (R-CHOP-14); replacement of rituximab with obinutuzuimab [40]; addition of novel agents such as bortezomib [41], lenalidomide[42], or ibrutinib [43,44] to R-CHOP; and various maintenance strategies such as rituximab, lenalidomide [45], enzastaurin [46], and everolimus [47]. Unfortunately, none of these strategies has been shown to improve OS in DLBCL. In part this appears to be due to the fact that inclusion/exclusion criteria for DLBCL trials have been too strict, such that the most severely ill DLBCL patients are typically not included. As a result, the results in the control arms have ended up better than what was expected based on historical data. Efforts are underway to include all patients in future first-line DLBCL studies.
Currently, autologous hematopoietic cell transplantation (auto-HCT) is not routinely used in the initial treatment of DLBCL. In the pre-rituximab era, numerous trials were conducted in DLBCL patients with high and/or high-intermediate risk disease based on the IPI score to determine if outcomes could be improved with high-dose therapy and auto-HCT as consolidation after patients achieved complete remission with first-line therapy. The results of these trials were conflicting. A 2003 meta-analysis of 11 such trials concluded that the results were very heterogeneous and showed no OS benefit [48]. More recently, the Southwestern Oncology Group published the results of a prospective trial testing the impact of auto-HCT for consolidation of aggressive NHL patients with an IPI score of 3 to 5 who achieved complete remission with first-line therapy with CHOP or R-CHOP. In this study, 75% of the patients had DLBCL and, of the B-cell NHL patients, 47% received R-CHOP. A survival benefit was seen only in the subgroup that had an IPI score of 4 or 5; a subgroup analysis restricted to those receiving R-CHOP as induction was not performed, however [49]. As a result, this area remains controversial, with most institutions not routinely performing auto-HCT for any DLBCL patients in first complete remission and some institutions considering auto-HCT in first complete remission for patients with an IPI score of 4 or 5. These studies all used the IPI score to identify high-risk patients. It is possible that the use of newer biomarkers or minimal-residual disease analysis will lead to a more robust algorithm for identifying high-risk patients and selecting patients who might benefit from consolidation of first complete remission with auto-HCT.
For patients with DHL or THL, long-term PFS with standard R-CHOP therapy is poor (20% to 40%) [50,51]. Treatment with more intensive first-line regimens such as DA-EPOCH-R, R-hyperCVAD (rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone), or CODOX-M/IVAC±R (cyclophosphamide, vincristine, doxorubicin, high‐dose methotrexate/ifosfamide, etoposide, high‐dose cytarabine ± rituximab), along with CNS prophylaxis, however, has been shown to produce superior outcomes [52], with 3-year relapse-free survival rates of 88% compared to 56% for R-CHOP. For patients who achieve a complete response by PET/CT scan after intensive induction, consolidation with auto-HCT has not been shown to improve outcomes based on retrospective analysis. However for DHL/THL patients who achieve complete response after R-CHOP, PFS was improved if auto-HCT was given as consolidation of first remission [53].
Patients with DLBCL have an approximately 5% risk of subsequently developing CNS involvement. Historically (in the pre-rituximab era), patients who presented with multiple sites of extranodal disease and/or extensive bone marrow involvement and/or an elevated LDH had an increased risk (up to 20%–30%) of developing CNS involvement. In addition, patients with involvement of certain anatomical sites (testicular, paranasal sinuses, epidural space) had an increased risk of CNS disease. Several algorithms have been proposed to identify patients who should receive prophylactic CNS therapy. One of the most robust tools for this purpose is the CNS-IPI, which is a 6-point score consisting of the 5 IPI elements, plus 1 additional point if the adrenal glands or kidneys are involved. Importantly, the CNS-IPI was developed and validated in patients treated with R-CHOP-like therapy. Subsequent risk of CNS relapse was 0.6%, 3.4%, and 10.2% for those with low-, intermediate- and high-risk CNS-IPI scores, respectively [54]. A reasonable strategy, therefore, is to perform CNS prophylaxis in those with a CNS-IPI score of 4 to 6. When CNS prophylaxis is used, intrathecal methotrexate or high-dose systemic methotrexate is most frequently given, with high-dose systemic methotrexate favored over intrathecal chemotherapy given that high-dose methotrexate penetrates the brain and spinal cord parenchyma, in addition to treating the cerebrospinal fluid (CSF) [55]. In contrast, intrathecal therapy only treats the CSF and requires repeated lumbar punctures or placement of an Ommaya reservoir. For DLBCL patients who present with active CSF involvement (known as lymphomatous meningitis), intrathecal chemotherapy treatments are typically given 2 or 3 times weekly until the CSF clears, followed by weekly intrathecal treatment for 4 weeks, and then monthly intrathecal treatment for 4 months [56]. For those with concurrent systemic and brain parenchymal DLBCL, a strategy of alternating R-CHOP with mid-cycle high-dose methotrexate can be successful. In addition, consolidation with high-dose therapy and auto-HCT improved survival in such patients in 1 retrospective series [57].
Relapsed/Refractory Disease. Between 30% and 40% of patients with advanced stage DLBCL will either fail to attain a remission with primary therapy (referred to as primary induction failure) or will relapse. In general, for those with progressive or relapsed disease, an updated tissue biopsy is recommended. This is especially true for patients who have had prior complete remission and have new lymph node enlargement, or those who have emergence of new sites of disease at the completion of first-line therapy.
Patients with relapsed disease are treated with systemic second-line platinum-based chemoimmunotherapy, with the usual goal of ultimately proceeding to auto-HCT. A number of platinum-based regimens have been used in this setting such as R-ICE, R-DHAP, R-GDP, R-Gem-Ox, and R-ESHAP. None of these regimens has been shown to be superior in terms of efficacy, and the choice of regimen is typically made based on the anticipated tolerance of the patient in light of comorbidities, laboratory studies, and physician preference. In the CORAL study, R-DHAP (rituximab, dexamethasone, high-dose cytarabine, cisplatin) seemed to show superior PFS in patients with the GCB subtype [58]. However, this was an unplanned subgroup analysis and R-DHAP was associated with higher renal toxicity.
Several studies have demonstrated that long-term PFS can be observed for relapsed/refractory DLBCL patients who respond to second-line therapy and then undergo high-dose therapy with auto-HCT. The Parma trial remains the only published prospective randomized trial performed in relapsed DLBCL comparing a transplant strategy to a non-transplant strategy. This study, performed in the pre-rituximab era, clearly showed a benefit in terms of DFS and OS in favor of auto-HCT versus salvage therapy alone [59]. The benefit of auto-HCT in patients treated in the rituximab era, even in patients who experience early failure (within 1 year of diagnosis), was confirmed in a retrospective analysis by the Center for International Blood and Marrow Transplant Research. In this study, a 44% 3-year PFS was seen in the early failure cohort versus 52% in the late failure cohort [60].
Some DLBCL patients are very unlikely to benefit from auto-HCT. The REFINE study focused on patients with primary induction failure or early relapse within 6 months of completing first-line therapy. Among such patients, primary progressive disease (defined as progression while still receiving first-line therapy), a high NCCN-IPI score at relapse, and MYC rearrangement were risk factors for poor PFS following auto-HCT [61]. Patients with 2 or 3 high-risk features had a 2-year OS of 10.7% compared to 74.3% for those without any high-risk features.
Allogeneic HCT (allo-HCT) is a treatment option for relapsed/refractory DLBCL. This option is more commonly considered for patients in whom an autotransplant has failed to achieve durable remission. For properly selected patients in this setting, a long-term PFS in the 30% to 40% range can be attained [62]. However, in practice, only about 20% of patients who fail auto-HCT end up undergoing allo-HCT due to rapid progression of disease, age, poor performance status, or lack of suitable donor. It has been proposed that in the coming years, allo-HCT will be utilized less commonly in this setting due to the advent of chimeric antigen receptor T-cell (CAR T) therapy.
CAR T-cell therapy genetically modifies the patient’s own T lymphocytes with a gene that encodes an antigen receptor to direct the T cells against lymphoma cells. Typically, the T cells are genetically modified and expanded in a production facility and then infused back into the patient. Axicabtagene ciloleucel is directed against the CD-19 receptor and has been approved by the US Food and Drug Administration (FDA) for treatment of patients with DLBCL who have failed 2 or more lines of systemic therapy. Use of CAR-T therapy in such patients was examined in a multicenter trial (ZUMA-1), which reported a 54% complete response rate and 52% OS rate at 18 months.63 CAR-T therapy is associated with serious side effects such as cytokine release syndrome, neurological toxicities, and prolonged cytopenias. While there are now some patients with ongoing remission 2 or more years after undergoing CAR-T therapy, it remains uncertain what proportion of patients have been truly cured with this modality. Nevertheless, this new treatment option remains a source of optimism for relapsed and refractory DLBCL patients.
Primary Mediastinal Large B-Cell Lymphoma
Primary mediastinal large B-cell lymphoma (PMBCL) is a form of DLBCL arising in the mediastinum from the thymic B cell. It is an uncommon entity and has clinical and pathologic features distinct from systemic DLBCL [64]. PMBCL accounts for 2% of all NHLs and about 7% of all DLBCL [20]. It typically affects women in the third to fourth decade of life.
Presentation and Prognostic Features
PMBCL usually presents as a locally invasive anterior mediastinal mass, often with a superior vena cava syndrome which may or may not be clinically obvious [64]. Other presentations include pericardial tamponade, thrombosis of neck veins, and acute airway obstruction. About 80% of patients present with bulky (> 10 cm) stage I or II disease [65], with distant spread uncommon on presentation. Morphologically and on GEP, PMBL has a profile more similar to classical Hodgkin lymphoma (cHL) than non-mediastinal DLBCL [66]. PMBL is distinguished from cHL by immunophenotyping: unlike cHL, PMBCL has pan B cell markers, rarely expresses CD15, and has weak CD30.
Poor prognostic features in PMBCL are Eastern Cooperative Oncology Group (ECOG) performance status greater than 2, pericardial effusion, bulky disease, and elevated serum LDH. The diagnosis of PMBCL can be difficult because the tumor is often encased with extensive fibrosis and necrosis. As a result, a needle biopsy may not yield sufficient tissue, thus making a surgical biopsy often the only viable way to obtain sufficient tissue.
Treatment
Early series suggested that PMBCL is unusually aggressive, with a poor prognosis [67]. This led to studies using more aggressive chemotherapy regimens (often in combination with mediastinal radiation) as well as upfront auto-HCT [68–70]. The addition of rituximab to treatment regimens significantly improved outcomes in PMBCL. For example, a subgroup analysis of the PMBCL patients in the MinT trial revealed a 3-year event-free survival (EFS) of 78% [71] when rituximab was combined with CHOP. Because of previous reports demonstrating radiosensitivity of PMBL, radiation was traditionally sequenced into treatment regimens for PMBL. However, this is associated with higher long-term toxicities, often a concern in PMBCL patients given that the disease frequently affects younger females, and given that breast tissue will be in the radiation field. For patients with a strong personal or family history of breast cancer or cardiovascular disease, these concerns are even more significant. More recently, the DA-EPOCH-R regimen has been shown to produce very high rates (80%–90%) of long-term DFS, without the need for mediastinal radiation in most cases [72,73]. For patients receiving R-CHOP, consolidation with mediastinal radiation is still commonly given. This approach also leads to high rates of long-term remission and, although utilizing mediastinal radiation, allows for less intensive chemotherapy. Determining which approach is most appropriate for an individual patient requires an assessment of the risks of each treatment option for that patient. A randomized trial by the International Extranodal Lymphoma Study Group (IELSG37) is evaluating whether RT may be safely omitted in PMBCL patients who achieve a complete metabolic response after R-CHOP.
Most relapses of PMBCL occur within the first 1 to 2 years and often present with extranodal disease in various organs. For those with relapsed or refractory disease, high-dose chemotherapy followed by auto-HCT provides 5-year survival rates of 50% to 80% [74–76] In a phase 1b trial evaluating the role of pembrolizumab in relapsed/refractory patients (KEYNOTE-13), 7 of 17 PMBCL patients achieved responses, with an additional 6 demonstrating stable disease [77]. This provides an additional option for patients who might be too weak to undergo auto-HCT or for those who relapse following auto-HCT.
Mantle Cell Lymphoma
The name mantle cell lymphoma (MCL) is based on the presumed normal cell counterpart to MCL, which is believed to be found in the mantle zone surrounding germinal center follicles. It represents approximately 6% of all NHL cases in the United States and Europe [78] MCL occurs at a median age of 63 to 68 years and has a male predominance.
Presentation and Prognostic Features
Patients can present with a broad spectrum of clinical features, and most patients (70%) present with advanced disease [79]. Up to one third of patients have B symptoms, with most demonstrating lymphadenopathy and bone marrow involvement. Approximately 25% present with extranodal disease as the primary presentation (eg, GI tract, pleura, breast, or orbits). MCL can involve any part of the GI tract and often presents as polypoid lesions.
Histologically, the pattern of MCL may be diffuse, nodular, mantle zone, or a combination of the these; morphologically, MCL can range from small, more irregular lymphocytes to lymphoblast-like cells. Blastoid and pleomorphic variants of MCL have a higher proliferation index and a more aggressive clinical course than other variants. MCL is characterized by the expression of pan B cell antigens (CD19+, CD20+) with coexpression of the T-cell antigen CD5, lack of CD23 expression, and nuclear expression of cyclin D1. Nuclear staining for cyclin D1 is present in more than 98% of cases [80]. In rare cases, CD5 or cyclin D1 may be negative [80]. Most MCL cases have a unique translocation that fuses the immunoglobulin heavy chain gene promoter (14q32) to the promoter of the BCL-1 gene (11q13), which encodes the cyclin D1 protein. This translocation is not unique to MCL and can be present in multiple myeloma as well. Interestingly, cyclin D1 is overproduced in cases lacking t(11:14), likely from other point mutations resulting in its overexpression [81]. Cyclin D1–negative tumors overexpress cyclin D2 or D3, with no apparent difference in clinical behavior or outcome [82]. In cyclin D1–negative cases, SOX11 expression may help with diagnosis [83]. A proliferation rate greater than 30% (as measured by Ki-67 staining), low SOX11 expression, and presence of p53 mutations have all been associated with adverse outcome.
In a minority of cases, MCL follows an indolent clinical course. For the remainder, however, MCL is an aggressive disease that generally requires treatment soon after diagnosis. When initially described in the 1980s and 1990s, treatment of MCL was characterized by low complete response rates, short durations of remission, repeated recurrences, and a median survival in the 2- to 5-year range [84]. In recent years, intensive regimens incorporating rituximab and high-dose cytarabine with or without auto-HCT have been developed and are associated with high complete response rates and median duration of first remission in the 6- to 9-year range [85–87]. Several prognostic indices have been applied to patients with MCL, including the IPI, the Follicular Lymphoma International Prognostic Index , and the Mantle Cell Lymphoma International Prognostic Index (MIPI). The MIPI was originally described based on a cohort from the period 1996 to 2004 [88], and subsequently confirmed in a separate cohort of 958 patients with MCL treated on prospective trials between 2004 and 2010 [89]. The MIPI score can identify 3 risk groups with significant survival differences (83%, 63%, and 34% survival at 5 years). A refined version of the MIPI score, the combined MIPI or MIPI-c, incorporates proliferation rate and is better able to stratify patients [90]. The blastoid variant of MCL follows a more aggressive clinical course and is associated with a high proliferation rate, shorter remissions, and a higher rate of CNS involvement [91].
In most patients, MCL is an aggressive disease with a short OS without treatment. A subset of patients may have a more indolent course [92], but unfortunately reliable factors that identify this group at the time of diagnosis are not available. Pretreatment evaluation is as with other lymphomas, with lumbar puncture and MRI of the brain also recommended for patients with the blastoid variant. For those presenting with GI symptoms, endoscopy is recommended as part of the initial evaluation as well.
Treatment
First-line Therapy. For patients under age 65 to 70 years with a good performance status and few comorbidities, an intensive induction regimen (such as R-CHOP/R-DHAP, Maxi-R-CHOP/R-araC, or R-DHAP) followed by consolidation with auto-HCT is commonly given, with a goal of achieving a durable (6–9 year) first remission [87,93,94]. Auto-HCT is now routinely followed by 3 years of maintenance rituximab based on the survival benefit seen in the recent LYSA trial [93]. At many centers, auto-HCT in first remission is a standard of care, with the greatest benefit seen in patients who have achieved a complete remission with no more than 2 lines of chemotherapy [95]. However, there remains some controversy about whether all patients truly benefit from auto-HCT in first remission, and current research efforts are focused on identifying patients most likely to benefit from auto-HCT and incorporation of new agents into first-line regimens. For patients who are not candidates for auto-HCT, bendamustine plus rituximab (BR) or R-CHOP alone or followed by maintenance rituximab is a reasonable approach [96]. Based on the StiL and BRIGHT trials, BR seems to have less toxicity and higher rates of response with no difference in OS when compared to R-CHOP [97,98].
In summary, dose-intense induction chemotherapy with consolidative auto-HCT results in high rates of long-term remission and can be considered in MCL patients who lack significant comorbidities and who understand the risks and benefits of this approach. For other patients, the less aggressive frontline approaches are more appropriate.
Relapsed/Refractory Disease
Despite initial high response rates, most patients with MCL will eventually relapse. For example, most patients given CHOP or R-CHOP alone as first-line therapy will relapse within 2 years [99]. In recent years, a number of therapies have emerged for relapsed/refractory MCL; however, the optimal sequencing of these is unclear. FDA-approved options for relapsed/refractory MCL include the proteasome inhibitor bortezomib [100,101], the BTK inhibitors ibrutinib [102,103] and acalabrutinib [104], and the immunomodulatory agent lenalidomide [105].
Auto-HCT can be considered for patients who did not undergo auto-HCT as part of first-line therapy and who had a reasonably long first remission [95]. Allo-HCT has curative potential in MCL with good evidence of a graft-versus-lymphoma effect. With a matched related or matched unrelated donor, the chance for treatment-related mortality is 15% to 25% at 1 to 2 years, with a 50% to 60% chance for long-term PFS. However, given the risk of treatment-related mortality and graft-versus-host disease, this option is typically reserved for patients with early relapse after auto-HCT, multiple relapses, or relatively chemotherapy-unresponsive disease [95,106]. A number of clinical trials for relapsed/refractory MCL are ongoing, and participation in these is encouraged whenever possible.
Burkitt Lymphoma
Burkitt lymphoma is a rare, aggressive and highly curable subtype of NHL. It can occur at any age, although peak incidence is in the first decade of life. There are 3 distinct clinical forms of Burkitt lymphoma [107]. The endemic form is common in African children and commonly involves the jaw and kidneys. The sporadic (nonendemic) form accounts for 1% to 2% of all lymphomas in the United States and Western Europe and usually has an abdominal presentation. The immunodeficiency-associated form is commonly seen in HIV patients with a relatively preserved CD4 cell count.
Patients typically present with rapidly growing masses and tumor lysis syndrome. CNS and bone marrow involvement are common. Burkitt lymphoma cells are high-grade, rapidly proliferating medium-sized cells with a monomorphic appearance. Biopsies show a classic histological appearance known as a “starry sky pattern” due to benign macrophages engulfing debris resulting from apoptosis. It is derived from a germinal center B cell and has distinct oncogenic pathways. Translocations such as t(8;14), t(2;8) or t(8;22) juxtapose the MYC locus with immunoglobulin heavy or light chain loci and result in MYC overexpression. Burkitt lymphoma is typically CD10-positive and BCL-2-negative, with a MYC translocation and a proliferation rate greater than 95%.
With conventional NHL regimens, Burkitt lymphoma had a poor prognosis, with complete remission in the 30% to 70% range and low rates of long-term remission. With the introduction of short-term, dose-intensive, multiagent chemotherapy regimens (adapted from pediatric acute lymphoblastic leukemia [ALL] regimens), the complete remission rate improved to 60% to 90% [107]. Early stage disease (localized or completely resected intra-abdominal disease) can have complete remission rates of 100%, with 2- to 5-year freedom-from-progression rates of 95%. CNS prophylaxis, including high-dose methotrexate, high-dose cytarabine, and intrathecal chemotherapy, is a standard component of Burkitt lymphoma regimens (CNS relapse rates can reach 50% without prophylactic therapy). Crucially, relapse after 1 to 2 years is very rare following complete response to induction therapy. Classically, several intensive regimens have been used for Burkitt lymphoma. In recent years, the most commonly used regimens have been the modified Magrath regimen of R-CODOX-M/IVAC and R-hyperCVAD. DA-EPOCH-R has also been used, typically for older, more frail, or HIV-positive patients. However, at the American Society of Hematology 2017 annual meeting, results from the NCI 9177 trial were presented which validated, in a prospective multi-center fashion, the use of DA-EPOCH-R in all Burkitt lymphoma patients [108]. In NCI 9177, low-risk patients (defined as normal LDH, ECOG performance score 0 or 1, ≤ stage II, and no tumor lesion > 7 cm) received 2 cycles of DA-EPOCH-R without intrathecal therapy followed by PET. If interim PET was negative, low-risk patients then received 1 more cycle of DA-EPOCH-R. High-risk patients with negative brain MRI and CSF cytology/flow cytometry received 2 cycles of DA-EPOCH-R with intrathecal therapy (2 doses per cycle) followed by PET. Unless interim PET showed progression, high-risk patients received 4 additional cycles of DA-EPOCH-R including methotrexate 12 mg intrathecally on days 1 and 5 (8 total doses). With a median follow-up of 36 months, this regimen resulted in an EFS of 85.7%. As expected, patients with CNS, marrow, or peripheral blood involvement fared worse. For those without CNS, marrow, or peripheral blood involvement, the results were excellent, with an EFS of 94.6% compared to 62.8% for those with CNS, bone marrow, or blood involvement at diagnosis.
Although no standard of care has been defined, patients with relapsed/refractory Burkitt lymphoma are often given standard second-line aggressive NHL regimens (eg, R-ICE); for those with chemosensitive disease, auto- or allo-HCT is often pursued, with long-term remissions possible following HCT [109].
Lymphoblastic Lymphoma
Lymphoblastic lymphoma (LBL) is a rare disease postulated to arise from precursor B or T lymphoblasts at varying stages of differentiation. Accounting for approximately 2% of all NHLs, 85% to 90% of all cases have a T-cell phenotype, while B-cell LBL comprises approximately 10% to 15% of cases. LBL and ALL are thought to represent the same disease entity, but LBL has been arbitrarily defined as cases with lymph node or mediastinal disease. Those with significant (> 25%) bone marrow or peripheral blood involvement are classified as ALL.
Precursor T-cell LBL patients are usually adolescent and young males who commonly present with a mediastinal mass and peripheral lymphadenopathy. Precursor B-cell LBL patients are usually older (median age 39 years) with peripheral lymphadenopathy and extranodal involvement. Mediastinal involvement with B-cell LBL is uncommon, and there is no male predominance. LBL has a propensity for dissemination to the bone marrow and CNS.
Morphologically, the tumor cells are medium sized, with a scant cytoplasm and finely dispersed chromatin. Mitotic features and apoptotic bodies are present since it is a high-grade malignancy. The lymphoblasts are typically positive for CD7 and either surface or cytoplasmic CD3. Terminal deoxynucleotidyl transferase expression is a defining feature. Other markers such as CD19, CD22, CD20, CD79a, CD45, and CD10 are variably expressed. Poor prognostic factors in T-cell LBL are female gender, age greater than 35 years, complex cytogenetics, and lack of a matched sibling donor.
Regimens for LBL are based on dose-dense, multi-agent protocols used in ALL. Most of these regimens are characterized by intensive remission-induction chemotherapy, CNS prophylaxis, a phase of consolidation therapy, and a prolonged maintenance phase, often lasting for 12 to 18 months with long-term DFS rates of 40% to 70% [110,111]. High-dose therapy with auto-HCT or allo-HCT in first complete response has been evaluated in an attempt to reduce the incidence of relapse [112]. However, the intensity of primary chemotherapy appears to be a stronger determinant of long-term survival than the use of HCT as consolidation. As a result, HCT is not routinely applied to patients in first complete remission following modern induction regimens. After relapse, prognosis is poor, with median survival rates of 6 to 9 months with conventional chemotherapy, although long-term survival rates of 30% and 20%, respectively, are reported after HCT in relapsed and primary refractory disease [113].
Treatment options in relapsed disease are limited. Nelarabine can produce responses in up to 40% of relapsed/refractory LBL/ALL patients [114]. For the minority of LBL patients with a B-cell phenotype, emerging options for relapsed/refractory LBL/ALL such as inotuzumab, blinatumomab, or anti-CD19 CAR T-cell therapy should be considered. These are not options for the majority who have a T-cell phenotype, and treatment options for these patients are limited to conventional relapsed/refractory ALL and aggressive NHL regimens.
Summary
Aggressive NHLs are characterized by rapid clinical progression without therapy. However, a significant proportion of patients are cured with appropriate combination chemotherapy or combined modality (chemotherapy + RT) regimens. In contrast, the indolent lymphomas have a relatively good prognosis (median survival of 10 years or longer) but usually are not curable in advanced clinical stages. Overall 5-year survival for aggressive NHLs with current treatment is approximately 50% to 60%, with relapses typically occurring within the first 5 years. Treatment strategies for relapsed patients offer some potential for cure; however, clinical trial participation should be encouraged whenever possible to investigate new approaches for improving outcomes in this patient population.
Corresponding author: Timothy S. Fenske, MD, Division of Hematology & Oncology, Medical College of Wisconsin, 9200 W. Wisconsin Ave., Milwaukee, WI 53226.
1. Swerdlow, SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th edition. Lyon, France: World Health Organization; 2017.
2. Surveillance, Epidemiology, and End Results (SEER) Program. www.seer.cancer.gov. Research Data 2017.
3. Boffetta P, de Vocht F. Occupation and the risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev 2007;16:369–72.
4. Bower M. Acquired immunodeficiency syndrome-related systemic non-Hodgkin’s lymphoma. Br J Haematol 2001;112:863–73.
5. Ekstrom Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood 2008;111:4029–38.
6. Clarke CA, Morton LM, Lynch C, et al. Risk of lymphoma subtypes after solid organ transplantation in the United States. Br J Cancer 2013;109:280–8.
7. Wang SS, Slager SL, Brennan P, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph). Blood 2007;109:3479–88.
8. Dong C, Hemminki K. Second primary neoplasms among 53 159 haematolymphoproliferative malignancy patients in Sweden, 1958–1996: a search for common mechanisms. Br J Cancer 2001;85:997–1005.
9. Hummel M, Anagnostopoulos I, Korbjuhn P, Stein H. Epstein-Barr virus in B-cell non-Hodgkin’s lymphomas: unexpected infection patterns and different infection incidence in low- and high-grade types. J Pathol 1995;175:263–71.
10. Cesarman E, Chang Y, Moore PS, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995;332:1186–91.
11. Viswanatha DS, Dogan A. Hepatitis C virus and lymphoma. J Clin Pathol 2007;60:1378–83.
12. Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol 2010;11:827–34.
13. Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood 2011;117:1792–8.
14. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–68.
15. Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937–47.
16. Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016;66:443–59.
17. Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood 2006;107:265–76.
18. Møller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation--a population-based study of 1575 cases. Br J Haematol 2004;124:151–9.
19. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol 1998;16:2780–95.
20. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 1997;89:3909–18.
21. Sehn LH, Scott DW, Chhanabhai M, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 2011;29:1452–7.
22. Fisher DE, Jacobson JO, Ault KA, Harris NL. Diffuse large cell lymphoma with discordant bone marrow histology. Clinical features and biological implications. Cancer 1989;64:1879–87.
23. Yao Z, Deng L, Xu-Monette ZY, et al. Concordant bone marrow involvement of diffuse large B-cell lymphoma represents a distinct clinical and biological entity in the era of immunotherapy. Leukemia 2018;32:353–63.
24. Gascoyne RD, Adomat SA, Krajewski S, et al. Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin’s lymphoma. Blood 1997;90:244–51.
25. Skinnider BF, Horsman DE, Dupuis B, Gascoyne RD. Bcl-6 and Bcl-2 protein expression in diffuse large B-cell lymphoma and follicular lymphoma: correlation with 3q27 and 18q21 chromosomal abnormalities. Hum Pathol 1999;30:803–8.
26. Chisholm KM, Bangs CD, Bacchi CE, et al. Expression profiles of MYC protein and MYC gene rearrangement in lymphomas. Am J Surg Pathol 2015;39:294–303.
27. Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123:837–42.
28. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–82.
29. Horn H, Ziepert M, Becher C, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 2013;121:2253–63.
30. Barrans S, Crouch S, Smith A, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 2010;28:3360–5.
31. Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 2013;121:4021–31.
32. Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012;30:3460–7.
33. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med 1993;328:1002–6.
34. Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011;12:1013–22.
35. Coiffier B, Lepage E, Brière J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235–42.
36. Persky DO, Unger JM, Spier CM, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol 2008;26:2258–63.
37. Lamy T, Damaj G, Soubeyran P, et al. R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood 2018;131:174–81.
38. Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 2011;12:460–8.
39. Wilson WH, sin-Ho J, Pitcher BN, et al. Phase III randomized study of R-CHOP versus DA-EPOCH-R and molecular analysis of untreated diffuse large B-cell lymphoma: CALGB/Alliance 50303. Blood 2016;128:469 LP-469. 38.
40. Vitolo U, Trne˘ný M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol 2017;35:3529–37.
41. Leonard JP, Kolibaba KS, Reeves JA, et al. Randomized phase II study of R-CHOP with or without bortezomib in previously untreated patients with non-germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol 2017;35:3538–46.
42. Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol 2015;33:251–7.
43. Younes A, Thieblemont C, Morschhauser F, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol 2014;15:1019–26.
44. Younes A, Zinzani PL, Sehn LH, et al. A randomized, double-blind, placebo-controlled phase 3 study of ibrutinib in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in subjects with newly diagnosed nongerminal center B-cell subtype of diffuse large B-cell lymphoma (DLBCL). J Clin Oncol 2014;32(15_suppl):TPS8615.
45. Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol 2013;14:525–33.
46. Leppä S, Fayad LE, Lee J-J, et al. A phase III study of enzastaurin in patients with high-risk diffuse large B cell lymphoma following response to primary treatment: the Prelude trial. Blood 2013;122:371 LP-371.
47. Witzig TE, Tobinai K, Rigacci L, et al. Adjuvant everolimus in high-risk diffuse large B-cell lymphoma: final results from the PILLAR-2 randomized phase III trial. Ann Oncol 2018;29:707–14.
48. Strehl J, Mey U, Glasmacher A, et al. High-dose chemotherapy followed by autologous stem cell transplantation as first-line therapy in aggressive non-Hodgkin’s lymphoma: a meta-analysis. Haematologica 2003;88:1304–15.
49. Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med 2013;369:1681–90.
50. Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol 2014;166:891–901.
51. Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood 2014;124:2354–61.
52. Howlett C, Snedecor SJ, Landsburg DJ, et al. Front-line, dose-escalated immunochemotherapy is associated with a significant progression-free survival advantage in patients with double-hit lymphomas: a systematic review and meta-analysis. Br J Haematol 2015;170:504–14.
53. Landsburg DJ, Falkiewicz MK, Maly J, et al. Outcomes of patients with double-hit lymphoma who achieve first complete remission. J Clin Oncol 2017;35:2260–7.
54. Schmitz N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 2016;34:3150–6.
55. Abramson JS, Hellmann M, Barnes JA, et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer 2010;116:4283–90.
56. Dunleavy K, Roschewski M, Abramson JS, et al. Risk-adapted therapy in adults with Burkitt lymphoma: updated results of a multicenter prospective phase II study of DA-EPOCH-R. Hematol Oncol 2017;35(S2):133–4.
57. Damaj G, Ivanoff S, Coso D, et al. Concomitant systemic and central nervous system non-Hodgkin lymphoma: the role of consolidation in terms of high dose therapy and autologous stem cell transplantation. A 60-case retrospective study from LYSA and the LOC network. Haematologica 2015;100:1199–206.
58. Thieblemont C, Briere J, Mounier N, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol 2011;29:4079–87.
59. Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with dalvage vhemotherapy in relapses of chemotherapy-densitive non-Hodgkin’s lymphoma. N Engl J Med 1995;333:1540–5.
60. Hamadani M, Hari PN, Zhang Y, et al. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant 2014;20:1729–36.
61. Costa LJ, Maddocks K, Epperla N, et al. Diffuse large B-cell lymphoma with primary treatment failure: Ultra-high risk features and benchmarking for experimental therapies. Am J Hematol 2017;92:e24615.
62. Fenske TS, Ahn KW, Graff TM, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol 2016;174:235–48.
63. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44.
64. van Besien K, Kelta M, Bahaguna P. Primary mediastinal B-cell lymphoma: a review of pathology and management. J Clin Oncol 2001;19:1855–64.
65. Savage KJ, Al-Rajhi N, Voss N, et al. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol Off J Eur Soc Med Oncol 2006;17:123–30.
66. Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 2003;198:851–62.
67. Lavabre-Bertrand T, Donadio D, Fegueux N, et al. A study of 15 cases of primary mediastinal lymphoma of B-cell type. Cancer 1992;69:2561–6.
68. Lazzarino M, Orlandi E, Paulli M, et al. Treatment outcome and prognostic factors for primary mediastinal (thymic) B-cell lymphoma: a multicenter study of 106 patients. J Clin Oncol 1997;15:1646–53.
69. Zinzani PL, Martelli M, Magagnoli M, et al. Treatment and clinical management of primary mediastinal large B-cell lymphoma with sclerosis: MACOP-B regimen and mediastinal radiotherapy monitored by (67)Gallium scan in 50 patients. Blood 1999;94:3289–93.
70. Todeschini G, Secchi S, Morra E, et al. Primary mediastinal large B-cell lymphoma (PMLBCL): long-term results from a retrospective multicentre Italian experience in 138 patients treated with CHOP or MACOP-B/VACOP-B. Br J Cancer 2004;90:372–6.
71. Rieger M, Osterborg A, Pettengell R, et al. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol Off J Eur Soc Med Oncol 2011;22:664–70.
72. Shah NN, Szabo A, Huntington SF, et al. R-CHOP versus dose-adjusted R-EPOCH in frontline management of primary mediastinal B-cell lymphoma: a multi-centre analysis. Br J Haematol 2018;180:534–44.
73. Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med 2013;368:1408–16.
74. Aoki T, Shimada K, Suzuki R, et al. High-dose chemotherapy followed by autologous stem cell transplantation for relapsed/refractory primary mediastinal large B-cell lymphoma. Blood Cancer J 2015;5:e372–e372.
75. Sehn LH, Antin JH, Shulman LN, et al. Primary diffuse large B-cell lymphoma of the mediastinum: outcome following high-dose chemotherapy and autologous hematopoietic cell transplantation. Blood 1998;91:717–23.
76. Kuruvilla J, Pintilie M, Tsang R, et al. Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk Lymphoma 2008;49:1329–36.
77. Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood 2017;130:267–70.
78. Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer 2011;105:1684–92.
79. Argatoff LH, Connors JM, Klasa RJ, et al. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood 1997;89:2067–78.
80. Zukerberg LR, Yang WI, Arnold A, Harris NL. Cyclin D1 expression in non-Hodgkin’s lymphomas. Detection by immunohistochemistry. Am J Clin Pathol 1995;103:756–60.
81. Wiestner A, Tehrani M, Chiorazzi M, et al. Point mutations and genomic deletions in CCND1 create stable truncated cyclin D1 mRNAs that are associated with increased proliferation rate and shorter survival. Blood 2007;109:4599–606.
82. Fu K, Weisenburger DD, Greiner TC, et al. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood 2005;106:4315–21.
83. Mozos A, Royo C, Hartmann E, et al. SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1-negative subtype. Haematologica 2009;94:1555–62.
84. Norton AJ, Matthews J, Pappa V, et al. Mantle cell lymphoma: Natural history defined in a serially biopsied population over a 20-year period. Ann Oncol 1995;6:249–56.
85. Chihara D, Cheah CY, Westin JR, et al. Rituximab plus hyper-CVAD alternating with MTX/Ara-C in patients with newly diagnosed mantle cell lymphoma: 15-year follow-up of a phase II study from the MD Anderson Cancer Center. Br J Haematol 2016;172:80–8.
86. Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood 2013;121:48–53.
87. Eskelund CW, Kolstad A, Jerkeman M, et al. 15-year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol 2016;175:410–8.
88. Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008;111:558–65.
89. Hoster E, Klapper W, Hermine O, et al. Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. J Clin Oncol 2014;32:1338–46.
90. Hoster E, Rosenwald A, Berger F, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: Results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol 2016;34:1386–94.
91. Bernard M, Gressin R, Lefrère F, et al. Blastic variant of mantle cell lymphoma: a rare but highly aggressive subtype. Leukemia 2001;15:1785–91.
92. Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol 2009;27:1209–13.
93. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017 Sep 28;377(13):1250–60.
94. Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet 2016;388:565–75.
95. Fenske TS, Zhang M-J, Carreras J, et al. Autologous or reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chemotherapy-sensitive mantle-cell lymphoma: analysis of transplantation timing and modality. J Clin Oncol 2014;32:273–81.
96. Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med 2012;367:520–31.
97. Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014;123:2944–52.
98. Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203–10.
99. Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol 2005;23:1984–92.
100. Belch A, Kouroukis CT, Crump M, et al. A phase II study of bortezomib in mantle cell lymphoma: the National Cancer Institute of Canada Clinical Trials Group trial IND.150. Ann Oncol Off J Eur Soc Med Oncol 2007;18:116–21.
101. Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol 2006;24:4867–74.
102. Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet 2016;387:770–8.
103. Wang ML, Rule S, Martin P, Goy A, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507–16.
104. Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet 2018;391:659–67.
105. Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol 2013;31:3688–95.
106. Khouri IF, Lee M-S, Saliba RM, et al. Nonablative allogeneic stem-cell transplantation for advanced/recurrent mantle-cell lymphoma. J Clin Oncol 2003;21:4407–12.
107. Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood 2004;104:3009–20.
108. Roschewski M, Dunleavy K, Abramson JS, et al. Risk-adapted therapy in adults with Burkitt lymphoma: results of NCI 9177, a multicenter prospective phase II study of DA-EPOCH-R. Blood American Society of Hematology;2017;130(Suppl 1):188.
109. Maramattom L V, Hari PN, Burns LJ, et al. Autologous and allogeneic transplantation for burkitt lymphoma outcomes and changes in utilization: a report from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2013;19:173–9.
110. Zinzani PL, Bendandi M, Visani G, et al. Adult lymphoblastic lymphoma: clinical features and prognostic factors in 53 patients. Leuk Lymphoma 1996;23:577–82.
111. Thomas DA, O’Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood 2004;104:1624–30.
112. Aljurf M, Zaidi SZA. Chemotherapy and hematopoietic stem cell transplantation for adult T-cell lymphoblastic lymphoma: current status and controversies. Biol Blood Marrow Transplant 2005;11:739–54.
113. Sweetenham JW, Santini G, Qian W, et al. High-dose therapy and autologous stem-cell transplantation versus conventional-dose consolidation/maintenance therapy as postremission therapy for adult patients with lymphoblastic lymphoma: results of a randomized trial of the European Group for Blood and Marrow Transplantation and the United Kingdom Lymphoma Group. J Clin Oncol 2001;19:2927–36.
114. Zwaan CM, Kowalczyk J, Schmitt C, et al. Safety and efficacy of nelarabine in children and young adults with relapsed or refractory T-lineage acute lymphoblastic leukaemia or T-lineage lymphoblastic lymphoma: results of a phase 4 study. Br J Haematol 2017;179:284–93.
Abstract
- Objective: To review the diagnosis and management of aggressive B-cell non-Hodgkin lymphoma (NHL).
- Methods: Review of the literature.
- Results: NHL comprises a wide variety of malignant hematologic disorders with varying clinical and biological features. Aggressive NHLs are characterized by rapid clinical progression without therapy. However, a significant proportion of patients are cured with appropriate combination chemotherapy or combined modality regimens. In contrast, the indolent lymphomas have a relatively good prognosis (median survival of 10 years or longer) but usually are not curable in advanced clinical stages. Overall 5-year survival for aggressive NHLs with current treatment is approximately 50% to 60%, with relapses typically occurring within the first 5 years.
- Conclusion: Treatment strategies for relapsed patients offer some potential for cure; however, clinical trial participation should be encouraged whenever possible to investigate new approaches for improving outcomes in this patient population.
Non-Hodgkin lymphoma (NHL) comprises a wide variety of malignant hematologic disorders with varying clinical and biological features. The more than 60 separate NHL subtypes can be classified according to cell of origin (B cell versus T cell), anatomical location (eg, orbital, testicular, bone, central nervous system), clinical behavior (indolent versus aggressive), histological features, or cytogenetic abnormalities. Although various NHL classification schemes have been used over the years, the World Health Organization (WHO) classification is now widely accepted as the definitive pathologic classification system for lymphoproliferative disorders, incorporating morphologic, immunohistochemical, flow cytometric, cytogenetic, and molecular features [1]. While the pathologic and molecular subclassification of NHL has become increasingly refined in recent years, from a management standpoint, classification based on clinical behavior remains very useful. This approach separates NHL subtypes into indolent versus aggressive categories. Whereas indolent NHLs may remain clinically insignificant for months to years, aggressive B-cell NHLs generally become life-threatening within weeks to months without treatment.
Epidemiology
Data from cancer registries show a steady, unexplainable increase in the incidence of NHL during the second half of the 20th century; the incidence has subsequently plateaued. There was a significant increase in NHL incidence between 1970 and 1995, which has been attributed in part to the HIV epidemic. More than 72,000 new cases of NHL were diagnosed in the United States in 2017, compared to just over 8000 cases of Hodgkin lymphoma, making NHL the sixth most common cancer in adult men and the fifth most common in adult women [2]. NHL appears to occur more frequently in Western countries than in Asian populations.
Various factors associated with increased risk for B-cell NHL have been identified over the years, including occupational and environmental exposure to certain pesticides and herbicides [3], immunosuppression associated with HIV infection [4], autoimmune disorders [5], iatrogenically induced immune suppression in the post-transplant and other settings [6], family history of NHL [7], and a personal history of a prior cancer, including Hodgkin lymphoma and prior NHL [8]. In terms of infectious agents associated with aggressive B-cell NHLs, Epstein-Barr virus (EBV) has a clear pathogenic role in Burkitt lymphoma, in many cases of post-transplant lymphoproliferative disorders, and in some cases of HIV-related aggressive B-cell lymphoma [9]. Human herpesvirus-8 viral genomes have been found in virtually all cases of primary effusion lymphomas [10]. Epidemiological studies also have linked hepatitis B and C to increased incidences of certain NHL subtypes [11–13], including primary hepatic diffuse large B-cell lymphoma (DLBCL). Similarly, Helicobacter pylori has been associated with gastric DLBCL.
Staging and Workup
A tissue biopsy is essential in the diagnosis and management of NHL. The most significant disadvantage of fine-needle aspiration cytology is the lack of histologic architecture. The optimal specimen is an excisional biopsy; when this cannot be performed, a core needle biopsy, ideally using a 16-gauge or larger caliber needle, is the next best choice.
The baseline tests appropriate for most cases of newly diagnosed aggressive B-cell NHL are listed in Table 1.
Prior to the initiation of treatment, patients should always undergo a thorough cardiac and pulmonary evaluation, especially if the patient will be treated with an anthracycline or mediastinal irradiation. Central nervous system (CNS) evaluation with magnetic resonance imaging (MRI) and lumbar puncture is essential if there are neurological signs or symptoms. In addition, certain anatomical sites including the testicles, paranasal sinuses, kidney, adrenal glands, and epidural space have been associated with increased involvement of the CNS and may warrant MRI evaluation and lumbar puncture. Certain NHL subtypes like Burkitt lymphoma, high-grade NHL with translocations of MYC and BCL-2 or BCL-6 (double-hit lymphoma), blastoid mantle cell lymphoma, and lymphoblastic lymphoma have a high risk of CNS involvement, and patients with these subtypes need CNS evaluation.
The Lugano classification is used to stage patients with NHL [14]. This classification is based on the Ann Arbor staging system and uses the distribution and number of tumor sites to stage disease. In general, this staging system in isolation is of limited value in predicting survival after treatment. However, the Ann Arbor stage does have prognostic impact when incorporated into risk scoring systems such as the International Prognostic Index (IPI). In clinical practice, the Ann Arbor stage is useful primarily to determine eligibility for localized therapy approaches. The absence or presence of systemic symptoms such as fevers, drenching night sweats, or weight loss (> 10% of baseline over 6 months or less) is designated by A or B, respectively.
Diffuse Large B-Cell Lymphoma
DLBCL is the most common lymphoid neoplasm in adults, accounting for about 25% of all NHL cases [2]. It is increasingly clear that the diagnostic category of DLBCL is quite heterogeneous in terms of morphology, genetics, and biologic behavior. A number of clinicopathologic subtypes of DLBCL exist, such as T cell/histiocyte–rich large B-cell lymphoma, primary mediastinal large B-cell lymphoma, intravascular large B-cell lymphoma, DLBCL associated with chronic inflammation, lymphomatoid granulomatosis, and EBV-positive large B-cell lymphoma, among others. Gene expression profiling (GEP) can distinguish 2 cell of origin DLBCL subtypes: the germinal center B-cell (GCB) and activated B-cell (ABC) subtypes [15].
DLBCL may be primary (de novo) or may arise through the transformation of many different types of low-grade B-cell lymphomas. This latter scenario is referred to as histologic transformation or transformed lymphoma. In some cases, patients may have a previously diagnosed low-grade B-cell NHL; in other cases, both low-grade and aggressive B-cell NHL may be diagnosed concurrently. The presence of elements of both low-grade and aggressive B-cell NHL in the same biopsy specimen is sometimes referred to as a composite lymphoma.
In the United States, incidence varies by ethnicity, with DLBCL being more common in Caucasians than other races [16]. There is a slight male predominance (55%), median age at diagnosis is 65 years [16,17] and the incidence increases with age.
Presentation, Pathology, and Prognostic Factors
The most common presentation of patients with DLBCL is rapidly enlarging lymphadenopathy, usually in the neck or abdomen. Extranodal/extramedullary presentation is seen in approximately 40% of cases, with the gastrointestinal (GI) tract being the most common site. However, extranodal DLBCL can arise in virtually any tissue [18]. Nodal DLBCL presents with symptoms related to the sites of involvement (eg, shortness of breath or chest pain with mediastinal lymphadenopathy), while extranodal DLBCL typically presents with symptoms secondary to dysfunction at the site of origin. Up to one third of patients present with constitutional symptoms (B symptoms) and more than 50% have elevated serum lactate dehydrogenase (LDH) at diagnosis [19].
Approximately 40% of patients present with stage I/II disease. Of these, only a subset present with stage I, or truly localized disease (defined as that which can be contained within 1 irradiation field). About 60% of patients present with advanced (stage III–IV) disease [20]. The bone marrow is involved in about 15% to 30% of cases. DLBCL involvement of the bone marrow is associated with a less favorable prognosis. Patients with DLBCL elsewhere may have low-grade NHL involvement of the bone marrow. Referred to as discordant bone marrow involvement [21], this feature does not carry the same poor prognosis associated with transformed disease [22] or DLBCL involvement of the bone marrow [23].
DLBCL is defined as a neoplasm of large B-lymphoid cells with a diffuse growth pattern. The proliferative fraction of cells, as determined by Ki-67 staining, is usually greater than 40%, and may even exceed 90%. Lymph nodes usually demonstrate complete effacement of the normal architecture by sheets of atypical lymphoid cells. Tumor cells in DLBCL generally express pan B-cell antigens (CD19, CD20, CD22, CD79a, Pax-5) as well as CD45 and surface immunoglobulin. Between 20% and 37% of DLBCL cases express the BCL-2 protein [24], and about 70% express the BCL-6 protein [25]. C-MYC protein expression is seen in a higher percentage (~ 30%–50%) of cases of DLBCL [26].
Many factors are associated with outcome in DLBCL. The IPI score was developed in the pre-rituximab era and is a robust prognostic tool. This simple tool uses 5 easily obtained clinical factors (age > 60 years, impaired performance status, elevated LDH, > 1 extranodal site of disease, and stage III/IV disease). By summing these factors, 4 groups with distinct 5-year overall survival (OS) rates ranging from 26% to 73% were identified (Table 2).
Cytogenetic and molecular factors also predict outcome in DLBCL. The ABC subtype distinguished by GEP has consistently been shown to have inferior outcomes with first-line therapy. As GEP is not routinely available in clinical practice, immunohistochemical (IHC) approaches (eg, the Hans algorithm) have been developed that can approximate the GEP subtypes. These IHC approaches have approximately 80% concordance with GEP [28]. The 3 most common chromosomal translocations in DLBCL involve BCL-2, BCL-6 and MYC. MYC-rearranged DLBCLs have a less favorable prognosis [29,30]. Cases in which a MYC translocation occurs in combination with a BCL-2 or BCL-6 translocation are commonly referred to as double-hit lymphoma (DHL); cases with all 3 translocations are referred to as triple-hit lymphoma (THL). Both DHL and THL have a worse prognosis with standard DLBCL therapy compared to non-DHL/THL cases. In the 2016 revised WHO classification, DHL and THL are an entity technically distinct from DLBCL, referred to as high-grade B-cell lymphoma [1]. In some cases, MYC and BCL-2 protein overexpression occurs in the absence of chromosomal translocations. Cases in which MYC and BCL-2 are overexpressed (by IHC) are referred to as double expressor lymphoma (DEL), and also have inferior outcome compared with non-DEL DLBCL [31,32]. Interestingly, MYC protein expression alone does not confer inferior outcomes, unlike isolated MYC translocation, which is associated with inferior outcomes.
Treatment
First-Line Therapy. DLBCL is an aggressive disease and, in most cases, survival without treatment can be measured in weeks to months. The advent of combination chemotherapy (CHOP [cyclophosphamide, doxorubicin, vincristine, and prednisone] or CHOP-like regimens) led to disease-free survival (DFS) rates of 35% to 40% at 3 to 5 years [33]. The addition of rituximab to CHOP (R-CHOP) has improved both progression-free surivial (PFS) and OS [34,35].
Treatment options vary for patients with localized (stage I/II) and advanced (stage III/IV) disease. Options for limited-stage DLBCL include an abbreviated course of R-CHOP (3 or 4 cycles) with involved-field radiation therapy (IFRT) versus a full course (6–8 cycles) of R-CHOP without radiation therapy (RT). Most studies comparing combined modality therapy (chemotherapy plus RT) versus chemotherapy alone were conducted in the pre-rituximab era. With the introduction of rituximab, Persky and colleagues [36] studied the use of 3 cycles of R-CHOP followed by RT, demonstrating a slightly improved OS of 92% at 4 years as compared to 88% in a historical cohort. The French LYSA/GOELAMS group performed the only direct comparison in the rituximab era (4 cycles of R-CHOP followed by RT versus 4 cycles of R-CHOP followed by 2 additional cycles of R-CHOP) and reported similar outcomes between both arms [37], with OS of 92% in the R-CHOP alone arm and 96% in the R-CHOP + RT arm (nonsignificant difference statistically). IFRT alone is not recommended other than for palliation in patients who cannot tolerate chemotherapy or combined modality therapy. Stage I and II patients with bulky disease (> 10 cm) have a prognosis similar to patients with advanced DLBCL and should be treated aggressively with 6 to 8 cycles of R-CHOP with or without RT [36].
For patients with advanced stage disease, a full course of R-CHOP-21 (6–8 cycles given on a 21-day cycle) is the standard of care. This approach results in OS rates of 70% and 60% at 2 and 5 years, respectively. For older adults unable to tolerate full-dose R-CHOP, attenuated versions of R-CHOP with decreased dose density or decreased dose intensity have been developed [38]. Numerous randomized trials have attempted to improve upon the results of R-CHOP-21 using strategies such as infusional chemotherapy (DA-EPOCH-R [etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, rituximab]) [39]; dose-dense therapy (R-CHOP-14); replacement of rituximab with obinutuzuimab [40]; addition of novel agents such as bortezomib [41], lenalidomide[42], or ibrutinib [43,44] to R-CHOP; and various maintenance strategies such as rituximab, lenalidomide [45], enzastaurin [46], and everolimus [47]. Unfortunately, none of these strategies has been shown to improve OS in DLBCL. In part this appears to be due to the fact that inclusion/exclusion criteria for DLBCL trials have been too strict, such that the most severely ill DLBCL patients are typically not included. As a result, the results in the control arms have ended up better than what was expected based on historical data. Efforts are underway to include all patients in future first-line DLBCL studies.
Currently, autologous hematopoietic cell transplantation (auto-HCT) is not routinely used in the initial treatment of DLBCL. In the pre-rituximab era, numerous trials were conducted in DLBCL patients with high and/or high-intermediate risk disease based on the IPI score to determine if outcomes could be improved with high-dose therapy and auto-HCT as consolidation after patients achieved complete remission with first-line therapy. The results of these trials were conflicting. A 2003 meta-analysis of 11 such trials concluded that the results were very heterogeneous and showed no OS benefit [48]. More recently, the Southwestern Oncology Group published the results of a prospective trial testing the impact of auto-HCT for consolidation of aggressive NHL patients with an IPI score of 3 to 5 who achieved complete remission with first-line therapy with CHOP or R-CHOP. In this study, 75% of the patients had DLBCL and, of the B-cell NHL patients, 47% received R-CHOP. A survival benefit was seen only in the subgroup that had an IPI score of 4 or 5; a subgroup analysis restricted to those receiving R-CHOP as induction was not performed, however [49]. As a result, this area remains controversial, with most institutions not routinely performing auto-HCT for any DLBCL patients in first complete remission and some institutions considering auto-HCT in first complete remission for patients with an IPI score of 4 or 5. These studies all used the IPI score to identify high-risk patients. It is possible that the use of newer biomarkers or minimal-residual disease analysis will lead to a more robust algorithm for identifying high-risk patients and selecting patients who might benefit from consolidation of first complete remission with auto-HCT.
For patients with DHL or THL, long-term PFS with standard R-CHOP therapy is poor (20% to 40%) [50,51]. Treatment with more intensive first-line regimens such as DA-EPOCH-R, R-hyperCVAD (rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone), or CODOX-M/IVAC±R (cyclophosphamide, vincristine, doxorubicin, high‐dose methotrexate/ifosfamide, etoposide, high‐dose cytarabine ± rituximab), along with CNS prophylaxis, however, has been shown to produce superior outcomes [52], with 3-year relapse-free survival rates of 88% compared to 56% for R-CHOP. For patients who achieve a complete response by PET/CT scan after intensive induction, consolidation with auto-HCT has not been shown to improve outcomes based on retrospective analysis. However for DHL/THL patients who achieve complete response after R-CHOP, PFS was improved if auto-HCT was given as consolidation of first remission [53].
Patients with DLBCL have an approximately 5% risk of subsequently developing CNS involvement. Historically (in the pre-rituximab era), patients who presented with multiple sites of extranodal disease and/or extensive bone marrow involvement and/or an elevated LDH had an increased risk (up to 20%–30%) of developing CNS involvement. In addition, patients with involvement of certain anatomical sites (testicular, paranasal sinuses, epidural space) had an increased risk of CNS disease. Several algorithms have been proposed to identify patients who should receive prophylactic CNS therapy. One of the most robust tools for this purpose is the CNS-IPI, which is a 6-point score consisting of the 5 IPI elements, plus 1 additional point if the adrenal glands or kidneys are involved. Importantly, the CNS-IPI was developed and validated in patients treated with R-CHOP-like therapy. Subsequent risk of CNS relapse was 0.6%, 3.4%, and 10.2% for those with low-, intermediate- and high-risk CNS-IPI scores, respectively [54]. A reasonable strategy, therefore, is to perform CNS prophylaxis in those with a CNS-IPI score of 4 to 6. When CNS prophylaxis is used, intrathecal methotrexate or high-dose systemic methotrexate is most frequently given, with high-dose systemic methotrexate favored over intrathecal chemotherapy given that high-dose methotrexate penetrates the brain and spinal cord parenchyma, in addition to treating the cerebrospinal fluid (CSF) [55]. In contrast, intrathecal therapy only treats the CSF and requires repeated lumbar punctures or placement of an Ommaya reservoir. For DLBCL patients who present with active CSF involvement (known as lymphomatous meningitis), intrathecal chemotherapy treatments are typically given 2 or 3 times weekly until the CSF clears, followed by weekly intrathecal treatment for 4 weeks, and then monthly intrathecal treatment for 4 months [56]. For those with concurrent systemic and brain parenchymal DLBCL, a strategy of alternating R-CHOP with mid-cycle high-dose methotrexate can be successful. In addition, consolidation with high-dose therapy and auto-HCT improved survival in such patients in 1 retrospective series [57].
Relapsed/Refractory Disease. Between 30% and 40% of patients with advanced stage DLBCL will either fail to attain a remission with primary therapy (referred to as primary induction failure) or will relapse. In general, for those with progressive or relapsed disease, an updated tissue biopsy is recommended. This is especially true for patients who have had prior complete remission and have new lymph node enlargement, or those who have emergence of new sites of disease at the completion of first-line therapy.
Patients with relapsed disease are treated with systemic second-line platinum-based chemoimmunotherapy, with the usual goal of ultimately proceeding to auto-HCT. A number of platinum-based regimens have been used in this setting such as R-ICE, R-DHAP, R-GDP, R-Gem-Ox, and R-ESHAP. None of these regimens has been shown to be superior in terms of efficacy, and the choice of regimen is typically made based on the anticipated tolerance of the patient in light of comorbidities, laboratory studies, and physician preference. In the CORAL study, R-DHAP (rituximab, dexamethasone, high-dose cytarabine, cisplatin) seemed to show superior PFS in patients with the GCB subtype [58]. However, this was an unplanned subgroup analysis and R-DHAP was associated with higher renal toxicity.
Several studies have demonstrated that long-term PFS can be observed for relapsed/refractory DLBCL patients who respond to second-line therapy and then undergo high-dose therapy with auto-HCT. The Parma trial remains the only published prospective randomized trial performed in relapsed DLBCL comparing a transplant strategy to a non-transplant strategy. This study, performed in the pre-rituximab era, clearly showed a benefit in terms of DFS and OS in favor of auto-HCT versus salvage therapy alone [59]. The benefit of auto-HCT in patients treated in the rituximab era, even in patients who experience early failure (within 1 year of diagnosis), was confirmed in a retrospective analysis by the Center for International Blood and Marrow Transplant Research. In this study, a 44% 3-year PFS was seen in the early failure cohort versus 52% in the late failure cohort [60].
Some DLBCL patients are very unlikely to benefit from auto-HCT. The REFINE study focused on patients with primary induction failure or early relapse within 6 months of completing first-line therapy. Among such patients, primary progressive disease (defined as progression while still receiving first-line therapy), a high NCCN-IPI score at relapse, and MYC rearrangement were risk factors for poor PFS following auto-HCT [61]. Patients with 2 or 3 high-risk features had a 2-year OS of 10.7% compared to 74.3% for those without any high-risk features.
Allogeneic HCT (allo-HCT) is a treatment option for relapsed/refractory DLBCL. This option is more commonly considered for patients in whom an autotransplant has failed to achieve durable remission. For properly selected patients in this setting, a long-term PFS in the 30% to 40% range can be attained [62]. However, in practice, only about 20% of patients who fail auto-HCT end up undergoing allo-HCT due to rapid progression of disease, age, poor performance status, or lack of suitable donor. It has been proposed that in the coming years, allo-HCT will be utilized less commonly in this setting due to the advent of chimeric antigen receptor T-cell (CAR T) therapy.
CAR T-cell therapy genetically modifies the patient’s own T lymphocytes with a gene that encodes an antigen receptor to direct the T cells against lymphoma cells. Typically, the T cells are genetically modified and expanded in a production facility and then infused back into the patient. Axicabtagene ciloleucel is directed against the CD-19 receptor and has been approved by the US Food and Drug Administration (FDA) for treatment of patients with DLBCL who have failed 2 or more lines of systemic therapy. Use of CAR-T therapy in such patients was examined in a multicenter trial (ZUMA-1), which reported a 54% complete response rate and 52% OS rate at 18 months.63 CAR-T therapy is associated with serious side effects such as cytokine release syndrome, neurological toxicities, and prolonged cytopenias. While there are now some patients with ongoing remission 2 or more years after undergoing CAR-T therapy, it remains uncertain what proportion of patients have been truly cured with this modality. Nevertheless, this new treatment option remains a source of optimism for relapsed and refractory DLBCL patients.
Primary Mediastinal Large B-Cell Lymphoma
Primary mediastinal large B-cell lymphoma (PMBCL) is a form of DLBCL arising in the mediastinum from the thymic B cell. It is an uncommon entity and has clinical and pathologic features distinct from systemic DLBCL [64]. PMBCL accounts for 2% of all NHLs and about 7% of all DLBCL [20]. It typically affects women in the third to fourth decade of life.
Presentation and Prognostic Features
PMBCL usually presents as a locally invasive anterior mediastinal mass, often with a superior vena cava syndrome which may or may not be clinically obvious [64]. Other presentations include pericardial tamponade, thrombosis of neck veins, and acute airway obstruction. About 80% of patients present with bulky (> 10 cm) stage I or II disease [65], with distant spread uncommon on presentation. Morphologically and on GEP, PMBL has a profile more similar to classical Hodgkin lymphoma (cHL) than non-mediastinal DLBCL [66]. PMBL is distinguished from cHL by immunophenotyping: unlike cHL, PMBCL has pan B cell markers, rarely expresses CD15, and has weak CD30.
Poor prognostic features in PMBCL are Eastern Cooperative Oncology Group (ECOG) performance status greater than 2, pericardial effusion, bulky disease, and elevated serum LDH. The diagnosis of PMBCL can be difficult because the tumor is often encased with extensive fibrosis and necrosis. As a result, a needle biopsy may not yield sufficient tissue, thus making a surgical biopsy often the only viable way to obtain sufficient tissue.
Treatment
Early series suggested that PMBCL is unusually aggressive, with a poor prognosis [67]. This led to studies using more aggressive chemotherapy regimens (often in combination with mediastinal radiation) as well as upfront auto-HCT [68–70]. The addition of rituximab to treatment regimens significantly improved outcomes in PMBCL. For example, a subgroup analysis of the PMBCL patients in the MinT trial revealed a 3-year event-free survival (EFS) of 78% [71] when rituximab was combined with CHOP. Because of previous reports demonstrating radiosensitivity of PMBL, radiation was traditionally sequenced into treatment regimens for PMBL. However, this is associated with higher long-term toxicities, often a concern in PMBCL patients given that the disease frequently affects younger females, and given that breast tissue will be in the radiation field. For patients with a strong personal or family history of breast cancer or cardiovascular disease, these concerns are even more significant. More recently, the DA-EPOCH-R regimen has been shown to produce very high rates (80%–90%) of long-term DFS, without the need for mediastinal radiation in most cases [72,73]. For patients receiving R-CHOP, consolidation with mediastinal radiation is still commonly given. This approach also leads to high rates of long-term remission and, although utilizing mediastinal radiation, allows for less intensive chemotherapy. Determining which approach is most appropriate for an individual patient requires an assessment of the risks of each treatment option for that patient. A randomized trial by the International Extranodal Lymphoma Study Group (IELSG37) is evaluating whether RT may be safely omitted in PMBCL patients who achieve a complete metabolic response after R-CHOP.
Most relapses of PMBCL occur within the first 1 to 2 years and often present with extranodal disease in various organs. For those with relapsed or refractory disease, high-dose chemotherapy followed by auto-HCT provides 5-year survival rates of 50% to 80% [74–76] In a phase 1b trial evaluating the role of pembrolizumab in relapsed/refractory patients (KEYNOTE-13), 7 of 17 PMBCL patients achieved responses, with an additional 6 demonstrating stable disease [77]. This provides an additional option for patients who might be too weak to undergo auto-HCT or for those who relapse following auto-HCT.
Mantle Cell Lymphoma
The name mantle cell lymphoma (MCL) is based on the presumed normal cell counterpart to MCL, which is believed to be found in the mantle zone surrounding germinal center follicles. It represents approximately 6% of all NHL cases in the United States and Europe [78] MCL occurs at a median age of 63 to 68 years and has a male predominance.
Presentation and Prognostic Features
Patients can present with a broad spectrum of clinical features, and most patients (70%) present with advanced disease [79]. Up to one third of patients have B symptoms, with most demonstrating lymphadenopathy and bone marrow involvement. Approximately 25% present with extranodal disease as the primary presentation (eg, GI tract, pleura, breast, or orbits). MCL can involve any part of the GI tract and often presents as polypoid lesions.
Histologically, the pattern of MCL may be diffuse, nodular, mantle zone, or a combination of the these; morphologically, MCL can range from small, more irregular lymphocytes to lymphoblast-like cells. Blastoid and pleomorphic variants of MCL have a higher proliferation index and a more aggressive clinical course than other variants. MCL is characterized by the expression of pan B cell antigens (CD19+, CD20+) with coexpression of the T-cell antigen CD5, lack of CD23 expression, and nuclear expression of cyclin D1. Nuclear staining for cyclin D1 is present in more than 98% of cases [80]. In rare cases, CD5 or cyclin D1 may be negative [80]. Most MCL cases have a unique translocation that fuses the immunoglobulin heavy chain gene promoter (14q32) to the promoter of the BCL-1 gene (11q13), which encodes the cyclin D1 protein. This translocation is not unique to MCL and can be present in multiple myeloma as well. Interestingly, cyclin D1 is overproduced in cases lacking t(11:14), likely from other point mutations resulting in its overexpression [81]. Cyclin D1–negative tumors overexpress cyclin D2 or D3, with no apparent difference in clinical behavior or outcome [82]. In cyclin D1–negative cases, SOX11 expression may help with diagnosis [83]. A proliferation rate greater than 30% (as measured by Ki-67 staining), low SOX11 expression, and presence of p53 mutations have all been associated with adverse outcome.
In a minority of cases, MCL follows an indolent clinical course. For the remainder, however, MCL is an aggressive disease that generally requires treatment soon after diagnosis. When initially described in the 1980s and 1990s, treatment of MCL was characterized by low complete response rates, short durations of remission, repeated recurrences, and a median survival in the 2- to 5-year range [84]. In recent years, intensive regimens incorporating rituximab and high-dose cytarabine with or without auto-HCT have been developed and are associated with high complete response rates and median duration of first remission in the 6- to 9-year range [85–87]. Several prognostic indices have been applied to patients with MCL, including the IPI, the Follicular Lymphoma International Prognostic Index , and the Mantle Cell Lymphoma International Prognostic Index (MIPI). The MIPI was originally described based on a cohort from the period 1996 to 2004 [88], and subsequently confirmed in a separate cohort of 958 patients with MCL treated on prospective trials between 2004 and 2010 [89]. The MIPI score can identify 3 risk groups with significant survival differences (83%, 63%, and 34% survival at 5 years). A refined version of the MIPI score, the combined MIPI or MIPI-c, incorporates proliferation rate and is better able to stratify patients [90]. The blastoid variant of MCL follows a more aggressive clinical course and is associated with a high proliferation rate, shorter remissions, and a higher rate of CNS involvement [91].
In most patients, MCL is an aggressive disease with a short OS without treatment. A subset of patients may have a more indolent course [92], but unfortunately reliable factors that identify this group at the time of diagnosis are not available. Pretreatment evaluation is as with other lymphomas, with lumbar puncture and MRI of the brain also recommended for patients with the blastoid variant. For those presenting with GI symptoms, endoscopy is recommended as part of the initial evaluation as well.
Treatment
First-line Therapy. For patients under age 65 to 70 years with a good performance status and few comorbidities, an intensive induction regimen (such as R-CHOP/R-DHAP, Maxi-R-CHOP/R-araC, or R-DHAP) followed by consolidation with auto-HCT is commonly given, with a goal of achieving a durable (6–9 year) first remission [87,93,94]. Auto-HCT is now routinely followed by 3 years of maintenance rituximab based on the survival benefit seen in the recent LYSA trial [93]. At many centers, auto-HCT in first remission is a standard of care, with the greatest benefit seen in patients who have achieved a complete remission with no more than 2 lines of chemotherapy [95]. However, there remains some controversy about whether all patients truly benefit from auto-HCT in first remission, and current research efforts are focused on identifying patients most likely to benefit from auto-HCT and incorporation of new agents into first-line regimens. For patients who are not candidates for auto-HCT, bendamustine plus rituximab (BR) or R-CHOP alone or followed by maintenance rituximab is a reasonable approach [96]. Based on the StiL and BRIGHT trials, BR seems to have less toxicity and higher rates of response with no difference in OS when compared to R-CHOP [97,98].
In summary, dose-intense induction chemotherapy with consolidative auto-HCT results in high rates of long-term remission and can be considered in MCL patients who lack significant comorbidities and who understand the risks and benefits of this approach. For other patients, the less aggressive frontline approaches are more appropriate.
Relapsed/Refractory Disease
Despite initial high response rates, most patients with MCL will eventually relapse. For example, most patients given CHOP or R-CHOP alone as first-line therapy will relapse within 2 years [99]. In recent years, a number of therapies have emerged for relapsed/refractory MCL; however, the optimal sequencing of these is unclear. FDA-approved options for relapsed/refractory MCL include the proteasome inhibitor bortezomib [100,101], the BTK inhibitors ibrutinib [102,103] and acalabrutinib [104], and the immunomodulatory agent lenalidomide [105].
Auto-HCT can be considered for patients who did not undergo auto-HCT as part of first-line therapy and who had a reasonably long first remission [95]. Allo-HCT has curative potential in MCL with good evidence of a graft-versus-lymphoma effect. With a matched related or matched unrelated donor, the chance for treatment-related mortality is 15% to 25% at 1 to 2 years, with a 50% to 60% chance for long-term PFS. However, given the risk of treatment-related mortality and graft-versus-host disease, this option is typically reserved for patients with early relapse after auto-HCT, multiple relapses, or relatively chemotherapy-unresponsive disease [95,106]. A number of clinical trials for relapsed/refractory MCL are ongoing, and participation in these is encouraged whenever possible.
Burkitt Lymphoma
Burkitt lymphoma is a rare, aggressive and highly curable subtype of NHL. It can occur at any age, although peak incidence is in the first decade of life. There are 3 distinct clinical forms of Burkitt lymphoma [107]. The endemic form is common in African children and commonly involves the jaw and kidneys. The sporadic (nonendemic) form accounts for 1% to 2% of all lymphomas in the United States and Western Europe and usually has an abdominal presentation. The immunodeficiency-associated form is commonly seen in HIV patients with a relatively preserved CD4 cell count.
Patients typically present with rapidly growing masses and tumor lysis syndrome. CNS and bone marrow involvement are common. Burkitt lymphoma cells are high-grade, rapidly proliferating medium-sized cells with a monomorphic appearance. Biopsies show a classic histological appearance known as a “starry sky pattern” due to benign macrophages engulfing debris resulting from apoptosis. It is derived from a germinal center B cell and has distinct oncogenic pathways. Translocations such as t(8;14), t(2;8) or t(8;22) juxtapose the MYC locus with immunoglobulin heavy or light chain loci and result in MYC overexpression. Burkitt lymphoma is typically CD10-positive and BCL-2-negative, with a MYC translocation and a proliferation rate greater than 95%.
With conventional NHL regimens, Burkitt lymphoma had a poor prognosis, with complete remission in the 30% to 70% range and low rates of long-term remission. With the introduction of short-term, dose-intensive, multiagent chemotherapy regimens (adapted from pediatric acute lymphoblastic leukemia [ALL] regimens), the complete remission rate improved to 60% to 90% [107]. Early stage disease (localized or completely resected intra-abdominal disease) can have complete remission rates of 100%, with 2- to 5-year freedom-from-progression rates of 95%. CNS prophylaxis, including high-dose methotrexate, high-dose cytarabine, and intrathecal chemotherapy, is a standard component of Burkitt lymphoma regimens (CNS relapse rates can reach 50% without prophylactic therapy). Crucially, relapse after 1 to 2 years is very rare following complete response to induction therapy. Classically, several intensive regimens have been used for Burkitt lymphoma. In recent years, the most commonly used regimens have been the modified Magrath regimen of R-CODOX-M/IVAC and R-hyperCVAD. DA-EPOCH-R has also been used, typically for older, more frail, or HIV-positive patients. However, at the American Society of Hematology 2017 annual meeting, results from the NCI 9177 trial were presented which validated, in a prospective multi-center fashion, the use of DA-EPOCH-R in all Burkitt lymphoma patients [108]. In NCI 9177, low-risk patients (defined as normal LDH, ECOG performance score 0 or 1, ≤ stage II, and no tumor lesion > 7 cm) received 2 cycles of DA-EPOCH-R without intrathecal therapy followed by PET. If interim PET was negative, low-risk patients then received 1 more cycle of DA-EPOCH-R. High-risk patients with negative brain MRI and CSF cytology/flow cytometry received 2 cycles of DA-EPOCH-R with intrathecal therapy (2 doses per cycle) followed by PET. Unless interim PET showed progression, high-risk patients received 4 additional cycles of DA-EPOCH-R including methotrexate 12 mg intrathecally on days 1 and 5 (8 total doses). With a median follow-up of 36 months, this regimen resulted in an EFS of 85.7%. As expected, patients with CNS, marrow, or peripheral blood involvement fared worse. For those without CNS, marrow, or peripheral blood involvement, the results were excellent, with an EFS of 94.6% compared to 62.8% for those with CNS, bone marrow, or blood involvement at diagnosis.
Although no standard of care has been defined, patients with relapsed/refractory Burkitt lymphoma are often given standard second-line aggressive NHL regimens (eg, R-ICE); for those with chemosensitive disease, auto- or allo-HCT is often pursued, with long-term remissions possible following HCT [109].
Lymphoblastic Lymphoma
Lymphoblastic lymphoma (LBL) is a rare disease postulated to arise from precursor B or T lymphoblasts at varying stages of differentiation. Accounting for approximately 2% of all NHLs, 85% to 90% of all cases have a T-cell phenotype, while B-cell LBL comprises approximately 10% to 15% of cases. LBL and ALL are thought to represent the same disease entity, but LBL has been arbitrarily defined as cases with lymph node or mediastinal disease. Those with significant (> 25%) bone marrow or peripheral blood involvement are classified as ALL.
Precursor T-cell LBL patients are usually adolescent and young males who commonly present with a mediastinal mass and peripheral lymphadenopathy. Precursor B-cell LBL patients are usually older (median age 39 years) with peripheral lymphadenopathy and extranodal involvement. Mediastinal involvement with B-cell LBL is uncommon, and there is no male predominance. LBL has a propensity for dissemination to the bone marrow and CNS.
Morphologically, the tumor cells are medium sized, with a scant cytoplasm and finely dispersed chromatin. Mitotic features and apoptotic bodies are present since it is a high-grade malignancy. The lymphoblasts are typically positive for CD7 and either surface or cytoplasmic CD3. Terminal deoxynucleotidyl transferase expression is a defining feature. Other markers such as CD19, CD22, CD20, CD79a, CD45, and CD10 are variably expressed. Poor prognostic factors in T-cell LBL are female gender, age greater than 35 years, complex cytogenetics, and lack of a matched sibling donor.
Regimens for LBL are based on dose-dense, multi-agent protocols used in ALL. Most of these regimens are characterized by intensive remission-induction chemotherapy, CNS prophylaxis, a phase of consolidation therapy, and a prolonged maintenance phase, often lasting for 12 to 18 months with long-term DFS rates of 40% to 70% [110,111]. High-dose therapy with auto-HCT or allo-HCT in first complete response has been evaluated in an attempt to reduce the incidence of relapse [112]. However, the intensity of primary chemotherapy appears to be a stronger determinant of long-term survival than the use of HCT as consolidation. As a result, HCT is not routinely applied to patients in first complete remission following modern induction regimens. After relapse, prognosis is poor, with median survival rates of 6 to 9 months with conventional chemotherapy, although long-term survival rates of 30% and 20%, respectively, are reported after HCT in relapsed and primary refractory disease [113].
Treatment options in relapsed disease are limited. Nelarabine can produce responses in up to 40% of relapsed/refractory LBL/ALL patients [114]. For the minority of LBL patients with a B-cell phenotype, emerging options for relapsed/refractory LBL/ALL such as inotuzumab, blinatumomab, or anti-CD19 CAR T-cell therapy should be considered. These are not options for the majority who have a T-cell phenotype, and treatment options for these patients are limited to conventional relapsed/refractory ALL and aggressive NHL regimens.
Summary
Aggressive NHLs are characterized by rapid clinical progression without therapy. However, a significant proportion of patients are cured with appropriate combination chemotherapy or combined modality (chemotherapy + RT) regimens. In contrast, the indolent lymphomas have a relatively good prognosis (median survival of 10 years or longer) but usually are not curable in advanced clinical stages. Overall 5-year survival for aggressive NHLs with current treatment is approximately 50% to 60%, with relapses typically occurring within the first 5 years. Treatment strategies for relapsed patients offer some potential for cure; however, clinical trial participation should be encouraged whenever possible to investigate new approaches for improving outcomes in this patient population.
Corresponding author: Timothy S. Fenske, MD, Division of Hematology & Oncology, Medical College of Wisconsin, 9200 W. Wisconsin Ave., Milwaukee, WI 53226.
Abstract
- Objective: To review the diagnosis and management of aggressive B-cell non-Hodgkin lymphoma (NHL).
- Methods: Review of the literature.
- Results: NHL comprises a wide variety of malignant hematologic disorders with varying clinical and biological features. Aggressive NHLs are characterized by rapid clinical progression without therapy. However, a significant proportion of patients are cured with appropriate combination chemotherapy or combined modality regimens. In contrast, the indolent lymphomas have a relatively good prognosis (median survival of 10 years or longer) but usually are not curable in advanced clinical stages. Overall 5-year survival for aggressive NHLs with current treatment is approximately 50% to 60%, with relapses typically occurring within the first 5 years.
- Conclusion: Treatment strategies for relapsed patients offer some potential for cure; however, clinical trial participation should be encouraged whenever possible to investigate new approaches for improving outcomes in this patient population.
Non-Hodgkin lymphoma (NHL) comprises a wide variety of malignant hematologic disorders with varying clinical and biological features. The more than 60 separate NHL subtypes can be classified according to cell of origin (B cell versus T cell), anatomical location (eg, orbital, testicular, bone, central nervous system), clinical behavior (indolent versus aggressive), histological features, or cytogenetic abnormalities. Although various NHL classification schemes have been used over the years, the World Health Organization (WHO) classification is now widely accepted as the definitive pathologic classification system for lymphoproliferative disorders, incorporating morphologic, immunohistochemical, flow cytometric, cytogenetic, and molecular features [1]. While the pathologic and molecular subclassification of NHL has become increasingly refined in recent years, from a management standpoint, classification based on clinical behavior remains very useful. This approach separates NHL subtypes into indolent versus aggressive categories. Whereas indolent NHLs may remain clinically insignificant for months to years, aggressive B-cell NHLs generally become life-threatening within weeks to months without treatment.
Epidemiology
Data from cancer registries show a steady, unexplainable increase in the incidence of NHL during the second half of the 20th century; the incidence has subsequently plateaued. There was a significant increase in NHL incidence between 1970 and 1995, which has been attributed in part to the HIV epidemic. More than 72,000 new cases of NHL were diagnosed in the United States in 2017, compared to just over 8000 cases of Hodgkin lymphoma, making NHL the sixth most common cancer in adult men and the fifth most common in adult women [2]. NHL appears to occur more frequently in Western countries than in Asian populations.
Various factors associated with increased risk for B-cell NHL have been identified over the years, including occupational and environmental exposure to certain pesticides and herbicides [3], immunosuppression associated with HIV infection [4], autoimmune disorders [5], iatrogenically induced immune suppression in the post-transplant and other settings [6], family history of NHL [7], and a personal history of a prior cancer, including Hodgkin lymphoma and prior NHL [8]. In terms of infectious agents associated with aggressive B-cell NHLs, Epstein-Barr virus (EBV) has a clear pathogenic role in Burkitt lymphoma, in many cases of post-transplant lymphoproliferative disorders, and in some cases of HIV-related aggressive B-cell lymphoma [9]. Human herpesvirus-8 viral genomes have been found in virtually all cases of primary effusion lymphomas [10]. Epidemiological studies also have linked hepatitis B and C to increased incidences of certain NHL subtypes [11–13], including primary hepatic diffuse large B-cell lymphoma (DLBCL). Similarly, Helicobacter pylori has been associated with gastric DLBCL.
Staging and Workup
A tissue biopsy is essential in the diagnosis and management of NHL. The most significant disadvantage of fine-needle aspiration cytology is the lack of histologic architecture. The optimal specimen is an excisional biopsy; when this cannot be performed, a core needle biopsy, ideally using a 16-gauge or larger caliber needle, is the next best choice.
The baseline tests appropriate for most cases of newly diagnosed aggressive B-cell NHL are listed in Table 1.
Prior to the initiation of treatment, patients should always undergo a thorough cardiac and pulmonary evaluation, especially if the patient will be treated with an anthracycline or mediastinal irradiation. Central nervous system (CNS) evaluation with magnetic resonance imaging (MRI) and lumbar puncture is essential if there are neurological signs or symptoms. In addition, certain anatomical sites including the testicles, paranasal sinuses, kidney, adrenal glands, and epidural space have been associated with increased involvement of the CNS and may warrant MRI evaluation and lumbar puncture. Certain NHL subtypes like Burkitt lymphoma, high-grade NHL with translocations of MYC and BCL-2 or BCL-6 (double-hit lymphoma), blastoid mantle cell lymphoma, and lymphoblastic lymphoma have a high risk of CNS involvement, and patients with these subtypes need CNS evaluation.
The Lugano classification is used to stage patients with NHL [14]. This classification is based on the Ann Arbor staging system and uses the distribution and number of tumor sites to stage disease. In general, this staging system in isolation is of limited value in predicting survival after treatment. However, the Ann Arbor stage does have prognostic impact when incorporated into risk scoring systems such as the International Prognostic Index (IPI). In clinical practice, the Ann Arbor stage is useful primarily to determine eligibility for localized therapy approaches. The absence or presence of systemic symptoms such as fevers, drenching night sweats, or weight loss (> 10% of baseline over 6 months or less) is designated by A or B, respectively.
Diffuse Large B-Cell Lymphoma
DLBCL is the most common lymphoid neoplasm in adults, accounting for about 25% of all NHL cases [2]. It is increasingly clear that the diagnostic category of DLBCL is quite heterogeneous in terms of morphology, genetics, and biologic behavior. A number of clinicopathologic subtypes of DLBCL exist, such as T cell/histiocyte–rich large B-cell lymphoma, primary mediastinal large B-cell lymphoma, intravascular large B-cell lymphoma, DLBCL associated with chronic inflammation, lymphomatoid granulomatosis, and EBV-positive large B-cell lymphoma, among others. Gene expression profiling (GEP) can distinguish 2 cell of origin DLBCL subtypes: the germinal center B-cell (GCB) and activated B-cell (ABC) subtypes [15].
DLBCL may be primary (de novo) or may arise through the transformation of many different types of low-grade B-cell lymphomas. This latter scenario is referred to as histologic transformation or transformed lymphoma. In some cases, patients may have a previously diagnosed low-grade B-cell NHL; in other cases, both low-grade and aggressive B-cell NHL may be diagnosed concurrently. The presence of elements of both low-grade and aggressive B-cell NHL in the same biopsy specimen is sometimes referred to as a composite lymphoma.
In the United States, incidence varies by ethnicity, with DLBCL being more common in Caucasians than other races [16]. There is a slight male predominance (55%), median age at diagnosis is 65 years [16,17] and the incidence increases with age.
Presentation, Pathology, and Prognostic Factors
The most common presentation of patients with DLBCL is rapidly enlarging lymphadenopathy, usually in the neck or abdomen. Extranodal/extramedullary presentation is seen in approximately 40% of cases, with the gastrointestinal (GI) tract being the most common site. However, extranodal DLBCL can arise in virtually any tissue [18]. Nodal DLBCL presents with symptoms related to the sites of involvement (eg, shortness of breath or chest pain with mediastinal lymphadenopathy), while extranodal DLBCL typically presents with symptoms secondary to dysfunction at the site of origin. Up to one third of patients present with constitutional symptoms (B symptoms) and more than 50% have elevated serum lactate dehydrogenase (LDH) at diagnosis [19].
Approximately 40% of patients present with stage I/II disease. Of these, only a subset present with stage I, or truly localized disease (defined as that which can be contained within 1 irradiation field). About 60% of patients present with advanced (stage III–IV) disease [20]. The bone marrow is involved in about 15% to 30% of cases. DLBCL involvement of the bone marrow is associated with a less favorable prognosis. Patients with DLBCL elsewhere may have low-grade NHL involvement of the bone marrow. Referred to as discordant bone marrow involvement [21], this feature does not carry the same poor prognosis associated with transformed disease [22] or DLBCL involvement of the bone marrow [23].
DLBCL is defined as a neoplasm of large B-lymphoid cells with a diffuse growth pattern. The proliferative fraction of cells, as determined by Ki-67 staining, is usually greater than 40%, and may even exceed 90%. Lymph nodes usually demonstrate complete effacement of the normal architecture by sheets of atypical lymphoid cells. Tumor cells in DLBCL generally express pan B-cell antigens (CD19, CD20, CD22, CD79a, Pax-5) as well as CD45 and surface immunoglobulin. Between 20% and 37% of DLBCL cases express the BCL-2 protein [24], and about 70% express the BCL-6 protein [25]. C-MYC protein expression is seen in a higher percentage (~ 30%–50%) of cases of DLBCL [26].
Many factors are associated with outcome in DLBCL. The IPI score was developed in the pre-rituximab era and is a robust prognostic tool. This simple tool uses 5 easily obtained clinical factors (age > 60 years, impaired performance status, elevated LDH, > 1 extranodal site of disease, and stage III/IV disease). By summing these factors, 4 groups with distinct 5-year overall survival (OS) rates ranging from 26% to 73% were identified (Table 2).
Cytogenetic and molecular factors also predict outcome in DLBCL. The ABC subtype distinguished by GEP has consistently been shown to have inferior outcomes with first-line therapy. As GEP is not routinely available in clinical practice, immunohistochemical (IHC) approaches (eg, the Hans algorithm) have been developed that can approximate the GEP subtypes. These IHC approaches have approximately 80% concordance with GEP [28]. The 3 most common chromosomal translocations in DLBCL involve BCL-2, BCL-6 and MYC. MYC-rearranged DLBCLs have a less favorable prognosis [29,30]. Cases in which a MYC translocation occurs in combination with a BCL-2 or BCL-6 translocation are commonly referred to as double-hit lymphoma (DHL); cases with all 3 translocations are referred to as triple-hit lymphoma (THL). Both DHL and THL have a worse prognosis with standard DLBCL therapy compared to non-DHL/THL cases. In the 2016 revised WHO classification, DHL and THL are an entity technically distinct from DLBCL, referred to as high-grade B-cell lymphoma [1]. In some cases, MYC and BCL-2 protein overexpression occurs in the absence of chromosomal translocations. Cases in which MYC and BCL-2 are overexpressed (by IHC) are referred to as double expressor lymphoma (DEL), and also have inferior outcome compared with non-DEL DLBCL [31,32]. Interestingly, MYC protein expression alone does not confer inferior outcomes, unlike isolated MYC translocation, which is associated with inferior outcomes.
Treatment
First-Line Therapy. DLBCL is an aggressive disease and, in most cases, survival without treatment can be measured in weeks to months. The advent of combination chemotherapy (CHOP [cyclophosphamide, doxorubicin, vincristine, and prednisone] or CHOP-like regimens) led to disease-free survival (DFS) rates of 35% to 40% at 3 to 5 years [33]. The addition of rituximab to CHOP (R-CHOP) has improved both progression-free surivial (PFS) and OS [34,35].
Treatment options vary for patients with localized (stage I/II) and advanced (stage III/IV) disease. Options for limited-stage DLBCL include an abbreviated course of R-CHOP (3 or 4 cycles) with involved-field radiation therapy (IFRT) versus a full course (6–8 cycles) of R-CHOP without radiation therapy (RT). Most studies comparing combined modality therapy (chemotherapy plus RT) versus chemotherapy alone were conducted in the pre-rituximab era. With the introduction of rituximab, Persky and colleagues [36] studied the use of 3 cycles of R-CHOP followed by RT, demonstrating a slightly improved OS of 92% at 4 years as compared to 88% in a historical cohort. The French LYSA/GOELAMS group performed the only direct comparison in the rituximab era (4 cycles of R-CHOP followed by RT versus 4 cycles of R-CHOP followed by 2 additional cycles of R-CHOP) and reported similar outcomes between both arms [37], with OS of 92% in the R-CHOP alone arm and 96% in the R-CHOP + RT arm (nonsignificant difference statistically). IFRT alone is not recommended other than for palliation in patients who cannot tolerate chemotherapy or combined modality therapy. Stage I and II patients with bulky disease (> 10 cm) have a prognosis similar to patients with advanced DLBCL and should be treated aggressively with 6 to 8 cycles of R-CHOP with or without RT [36].
For patients with advanced stage disease, a full course of R-CHOP-21 (6–8 cycles given on a 21-day cycle) is the standard of care. This approach results in OS rates of 70% and 60% at 2 and 5 years, respectively. For older adults unable to tolerate full-dose R-CHOP, attenuated versions of R-CHOP with decreased dose density or decreased dose intensity have been developed [38]. Numerous randomized trials have attempted to improve upon the results of R-CHOP-21 using strategies such as infusional chemotherapy (DA-EPOCH-R [etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, rituximab]) [39]; dose-dense therapy (R-CHOP-14); replacement of rituximab with obinutuzuimab [40]; addition of novel agents such as bortezomib [41], lenalidomide[42], or ibrutinib [43,44] to R-CHOP; and various maintenance strategies such as rituximab, lenalidomide [45], enzastaurin [46], and everolimus [47]. Unfortunately, none of these strategies has been shown to improve OS in DLBCL. In part this appears to be due to the fact that inclusion/exclusion criteria for DLBCL trials have been too strict, such that the most severely ill DLBCL patients are typically not included. As a result, the results in the control arms have ended up better than what was expected based on historical data. Efforts are underway to include all patients in future first-line DLBCL studies.
Currently, autologous hematopoietic cell transplantation (auto-HCT) is not routinely used in the initial treatment of DLBCL. In the pre-rituximab era, numerous trials were conducted in DLBCL patients with high and/or high-intermediate risk disease based on the IPI score to determine if outcomes could be improved with high-dose therapy and auto-HCT as consolidation after patients achieved complete remission with first-line therapy. The results of these trials were conflicting. A 2003 meta-analysis of 11 such trials concluded that the results were very heterogeneous and showed no OS benefit [48]. More recently, the Southwestern Oncology Group published the results of a prospective trial testing the impact of auto-HCT for consolidation of aggressive NHL patients with an IPI score of 3 to 5 who achieved complete remission with first-line therapy with CHOP or R-CHOP. In this study, 75% of the patients had DLBCL and, of the B-cell NHL patients, 47% received R-CHOP. A survival benefit was seen only in the subgroup that had an IPI score of 4 or 5; a subgroup analysis restricted to those receiving R-CHOP as induction was not performed, however [49]. As a result, this area remains controversial, with most institutions not routinely performing auto-HCT for any DLBCL patients in first complete remission and some institutions considering auto-HCT in first complete remission for patients with an IPI score of 4 or 5. These studies all used the IPI score to identify high-risk patients. It is possible that the use of newer biomarkers or minimal-residual disease analysis will lead to a more robust algorithm for identifying high-risk patients and selecting patients who might benefit from consolidation of first complete remission with auto-HCT.
For patients with DHL or THL, long-term PFS with standard R-CHOP therapy is poor (20% to 40%) [50,51]. Treatment with more intensive first-line regimens such as DA-EPOCH-R, R-hyperCVAD (rituximab plus hyperfractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone), or CODOX-M/IVAC±R (cyclophosphamide, vincristine, doxorubicin, high‐dose methotrexate/ifosfamide, etoposide, high‐dose cytarabine ± rituximab), along with CNS prophylaxis, however, has been shown to produce superior outcomes [52], with 3-year relapse-free survival rates of 88% compared to 56% for R-CHOP. For patients who achieve a complete response by PET/CT scan after intensive induction, consolidation with auto-HCT has not been shown to improve outcomes based on retrospective analysis. However for DHL/THL patients who achieve complete response after R-CHOP, PFS was improved if auto-HCT was given as consolidation of first remission [53].
Patients with DLBCL have an approximately 5% risk of subsequently developing CNS involvement. Historically (in the pre-rituximab era), patients who presented with multiple sites of extranodal disease and/or extensive bone marrow involvement and/or an elevated LDH had an increased risk (up to 20%–30%) of developing CNS involvement. In addition, patients with involvement of certain anatomical sites (testicular, paranasal sinuses, epidural space) had an increased risk of CNS disease. Several algorithms have been proposed to identify patients who should receive prophylactic CNS therapy. One of the most robust tools for this purpose is the CNS-IPI, which is a 6-point score consisting of the 5 IPI elements, plus 1 additional point if the adrenal glands or kidneys are involved. Importantly, the CNS-IPI was developed and validated in patients treated with R-CHOP-like therapy. Subsequent risk of CNS relapse was 0.6%, 3.4%, and 10.2% for those with low-, intermediate- and high-risk CNS-IPI scores, respectively [54]. A reasonable strategy, therefore, is to perform CNS prophylaxis in those with a CNS-IPI score of 4 to 6. When CNS prophylaxis is used, intrathecal methotrexate or high-dose systemic methotrexate is most frequently given, with high-dose systemic methotrexate favored over intrathecal chemotherapy given that high-dose methotrexate penetrates the brain and spinal cord parenchyma, in addition to treating the cerebrospinal fluid (CSF) [55]. In contrast, intrathecal therapy only treats the CSF and requires repeated lumbar punctures or placement of an Ommaya reservoir. For DLBCL patients who present with active CSF involvement (known as lymphomatous meningitis), intrathecal chemotherapy treatments are typically given 2 or 3 times weekly until the CSF clears, followed by weekly intrathecal treatment for 4 weeks, and then monthly intrathecal treatment for 4 months [56]. For those with concurrent systemic and brain parenchymal DLBCL, a strategy of alternating R-CHOP with mid-cycle high-dose methotrexate can be successful. In addition, consolidation with high-dose therapy and auto-HCT improved survival in such patients in 1 retrospective series [57].
Relapsed/Refractory Disease. Between 30% and 40% of patients with advanced stage DLBCL will either fail to attain a remission with primary therapy (referred to as primary induction failure) or will relapse. In general, for those with progressive or relapsed disease, an updated tissue biopsy is recommended. This is especially true for patients who have had prior complete remission and have new lymph node enlargement, or those who have emergence of new sites of disease at the completion of first-line therapy.
Patients with relapsed disease are treated with systemic second-line platinum-based chemoimmunotherapy, with the usual goal of ultimately proceeding to auto-HCT. A number of platinum-based regimens have been used in this setting such as R-ICE, R-DHAP, R-GDP, R-Gem-Ox, and R-ESHAP. None of these regimens has been shown to be superior in terms of efficacy, and the choice of regimen is typically made based on the anticipated tolerance of the patient in light of comorbidities, laboratory studies, and physician preference. In the CORAL study, R-DHAP (rituximab, dexamethasone, high-dose cytarabine, cisplatin) seemed to show superior PFS in patients with the GCB subtype [58]. However, this was an unplanned subgroup analysis and R-DHAP was associated with higher renal toxicity.
Several studies have demonstrated that long-term PFS can be observed for relapsed/refractory DLBCL patients who respond to second-line therapy and then undergo high-dose therapy with auto-HCT. The Parma trial remains the only published prospective randomized trial performed in relapsed DLBCL comparing a transplant strategy to a non-transplant strategy. This study, performed in the pre-rituximab era, clearly showed a benefit in terms of DFS and OS in favor of auto-HCT versus salvage therapy alone [59]. The benefit of auto-HCT in patients treated in the rituximab era, even in patients who experience early failure (within 1 year of diagnosis), was confirmed in a retrospective analysis by the Center for International Blood and Marrow Transplant Research. In this study, a 44% 3-year PFS was seen in the early failure cohort versus 52% in the late failure cohort [60].
Some DLBCL patients are very unlikely to benefit from auto-HCT. The REFINE study focused on patients with primary induction failure or early relapse within 6 months of completing first-line therapy. Among such patients, primary progressive disease (defined as progression while still receiving first-line therapy), a high NCCN-IPI score at relapse, and MYC rearrangement were risk factors for poor PFS following auto-HCT [61]. Patients with 2 or 3 high-risk features had a 2-year OS of 10.7% compared to 74.3% for those without any high-risk features.
Allogeneic HCT (allo-HCT) is a treatment option for relapsed/refractory DLBCL. This option is more commonly considered for patients in whom an autotransplant has failed to achieve durable remission. For properly selected patients in this setting, a long-term PFS in the 30% to 40% range can be attained [62]. However, in practice, only about 20% of patients who fail auto-HCT end up undergoing allo-HCT due to rapid progression of disease, age, poor performance status, or lack of suitable donor. It has been proposed that in the coming years, allo-HCT will be utilized less commonly in this setting due to the advent of chimeric antigen receptor T-cell (CAR T) therapy.
CAR T-cell therapy genetically modifies the patient’s own T lymphocytes with a gene that encodes an antigen receptor to direct the T cells against lymphoma cells. Typically, the T cells are genetically modified and expanded in a production facility and then infused back into the patient. Axicabtagene ciloleucel is directed against the CD-19 receptor and has been approved by the US Food and Drug Administration (FDA) for treatment of patients with DLBCL who have failed 2 or more lines of systemic therapy. Use of CAR-T therapy in such patients was examined in a multicenter trial (ZUMA-1), which reported a 54% complete response rate and 52% OS rate at 18 months.63 CAR-T therapy is associated with serious side effects such as cytokine release syndrome, neurological toxicities, and prolonged cytopenias. While there are now some patients with ongoing remission 2 or more years after undergoing CAR-T therapy, it remains uncertain what proportion of patients have been truly cured with this modality. Nevertheless, this new treatment option remains a source of optimism for relapsed and refractory DLBCL patients.
Primary Mediastinal Large B-Cell Lymphoma
Primary mediastinal large B-cell lymphoma (PMBCL) is a form of DLBCL arising in the mediastinum from the thymic B cell. It is an uncommon entity and has clinical and pathologic features distinct from systemic DLBCL [64]. PMBCL accounts for 2% of all NHLs and about 7% of all DLBCL [20]. It typically affects women in the third to fourth decade of life.
Presentation and Prognostic Features
PMBCL usually presents as a locally invasive anterior mediastinal mass, often with a superior vena cava syndrome which may or may not be clinically obvious [64]. Other presentations include pericardial tamponade, thrombosis of neck veins, and acute airway obstruction. About 80% of patients present with bulky (> 10 cm) stage I or II disease [65], with distant spread uncommon on presentation. Morphologically and on GEP, PMBL has a profile more similar to classical Hodgkin lymphoma (cHL) than non-mediastinal DLBCL [66]. PMBL is distinguished from cHL by immunophenotyping: unlike cHL, PMBCL has pan B cell markers, rarely expresses CD15, and has weak CD30.
Poor prognostic features in PMBCL are Eastern Cooperative Oncology Group (ECOG) performance status greater than 2, pericardial effusion, bulky disease, and elevated serum LDH. The diagnosis of PMBCL can be difficult because the tumor is often encased with extensive fibrosis and necrosis. As a result, a needle biopsy may not yield sufficient tissue, thus making a surgical biopsy often the only viable way to obtain sufficient tissue.
Treatment
Early series suggested that PMBCL is unusually aggressive, with a poor prognosis [67]. This led to studies using more aggressive chemotherapy regimens (often in combination with mediastinal radiation) as well as upfront auto-HCT [68–70]. The addition of rituximab to treatment regimens significantly improved outcomes in PMBCL. For example, a subgroup analysis of the PMBCL patients in the MinT trial revealed a 3-year event-free survival (EFS) of 78% [71] when rituximab was combined with CHOP. Because of previous reports demonstrating radiosensitivity of PMBL, radiation was traditionally sequenced into treatment regimens for PMBL. However, this is associated with higher long-term toxicities, often a concern in PMBCL patients given that the disease frequently affects younger females, and given that breast tissue will be in the radiation field. For patients with a strong personal or family history of breast cancer or cardiovascular disease, these concerns are even more significant. More recently, the DA-EPOCH-R regimen has been shown to produce very high rates (80%–90%) of long-term DFS, without the need for mediastinal radiation in most cases [72,73]. For patients receiving R-CHOP, consolidation with mediastinal radiation is still commonly given. This approach also leads to high rates of long-term remission and, although utilizing mediastinal radiation, allows for less intensive chemotherapy. Determining which approach is most appropriate for an individual patient requires an assessment of the risks of each treatment option for that patient. A randomized trial by the International Extranodal Lymphoma Study Group (IELSG37) is evaluating whether RT may be safely omitted in PMBCL patients who achieve a complete metabolic response after R-CHOP.
Most relapses of PMBCL occur within the first 1 to 2 years and often present with extranodal disease in various organs. For those with relapsed or refractory disease, high-dose chemotherapy followed by auto-HCT provides 5-year survival rates of 50% to 80% [74–76] In a phase 1b trial evaluating the role of pembrolizumab in relapsed/refractory patients (KEYNOTE-13), 7 of 17 PMBCL patients achieved responses, with an additional 6 demonstrating stable disease [77]. This provides an additional option for patients who might be too weak to undergo auto-HCT or for those who relapse following auto-HCT.
Mantle Cell Lymphoma
The name mantle cell lymphoma (MCL) is based on the presumed normal cell counterpart to MCL, which is believed to be found in the mantle zone surrounding germinal center follicles. It represents approximately 6% of all NHL cases in the United States and Europe [78] MCL occurs at a median age of 63 to 68 years and has a male predominance.
Presentation and Prognostic Features
Patients can present with a broad spectrum of clinical features, and most patients (70%) present with advanced disease [79]. Up to one third of patients have B symptoms, with most demonstrating lymphadenopathy and bone marrow involvement. Approximately 25% present with extranodal disease as the primary presentation (eg, GI tract, pleura, breast, or orbits). MCL can involve any part of the GI tract and often presents as polypoid lesions.
Histologically, the pattern of MCL may be diffuse, nodular, mantle zone, or a combination of the these; morphologically, MCL can range from small, more irregular lymphocytes to lymphoblast-like cells. Blastoid and pleomorphic variants of MCL have a higher proliferation index and a more aggressive clinical course than other variants. MCL is characterized by the expression of pan B cell antigens (CD19+, CD20+) with coexpression of the T-cell antigen CD5, lack of CD23 expression, and nuclear expression of cyclin D1. Nuclear staining for cyclin D1 is present in more than 98% of cases [80]. In rare cases, CD5 or cyclin D1 may be negative [80]. Most MCL cases have a unique translocation that fuses the immunoglobulin heavy chain gene promoter (14q32) to the promoter of the BCL-1 gene (11q13), which encodes the cyclin D1 protein. This translocation is not unique to MCL and can be present in multiple myeloma as well. Interestingly, cyclin D1 is overproduced in cases lacking t(11:14), likely from other point mutations resulting in its overexpression [81]. Cyclin D1–negative tumors overexpress cyclin D2 or D3, with no apparent difference in clinical behavior or outcome [82]. In cyclin D1–negative cases, SOX11 expression may help with diagnosis [83]. A proliferation rate greater than 30% (as measured by Ki-67 staining), low SOX11 expression, and presence of p53 mutations have all been associated with adverse outcome.
In a minority of cases, MCL follows an indolent clinical course. For the remainder, however, MCL is an aggressive disease that generally requires treatment soon after diagnosis. When initially described in the 1980s and 1990s, treatment of MCL was characterized by low complete response rates, short durations of remission, repeated recurrences, and a median survival in the 2- to 5-year range [84]. In recent years, intensive regimens incorporating rituximab and high-dose cytarabine with or without auto-HCT have been developed and are associated with high complete response rates and median duration of first remission in the 6- to 9-year range [85–87]. Several prognostic indices have been applied to patients with MCL, including the IPI, the Follicular Lymphoma International Prognostic Index , and the Mantle Cell Lymphoma International Prognostic Index (MIPI). The MIPI was originally described based on a cohort from the period 1996 to 2004 [88], and subsequently confirmed in a separate cohort of 958 patients with MCL treated on prospective trials between 2004 and 2010 [89]. The MIPI score can identify 3 risk groups with significant survival differences (83%, 63%, and 34% survival at 5 years). A refined version of the MIPI score, the combined MIPI or MIPI-c, incorporates proliferation rate and is better able to stratify patients [90]. The blastoid variant of MCL follows a more aggressive clinical course and is associated with a high proliferation rate, shorter remissions, and a higher rate of CNS involvement [91].
In most patients, MCL is an aggressive disease with a short OS without treatment. A subset of patients may have a more indolent course [92], but unfortunately reliable factors that identify this group at the time of diagnosis are not available. Pretreatment evaluation is as with other lymphomas, with lumbar puncture and MRI of the brain also recommended for patients with the blastoid variant. For those presenting with GI symptoms, endoscopy is recommended as part of the initial evaluation as well.
Treatment
First-line Therapy. For patients under age 65 to 70 years with a good performance status and few comorbidities, an intensive induction regimen (such as R-CHOP/R-DHAP, Maxi-R-CHOP/R-araC, or R-DHAP) followed by consolidation with auto-HCT is commonly given, with a goal of achieving a durable (6–9 year) first remission [87,93,94]. Auto-HCT is now routinely followed by 3 years of maintenance rituximab based on the survival benefit seen in the recent LYSA trial [93]. At many centers, auto-HCT in first remission is a standard of care, with the greatest benefit seen in patients who have achieved a complete remission with no more than 2 lines of chemotherapy [95]. However, there remains some controversy about whether all patients truly benefit from auto-HCT in first remission, and current research efforts are focused on identifying patients most likely to benefit from auto-HCT and incorporation of new agents into first-line regimens. For patients who are not candidates for auto-HCT, bendamustine plus rituximab (BR) or R-CHOP alone or followed by maintenance rituximab is a reasonable approach [96]. Based on the StiL and BRIGHT trials, BR seems to have less toxicity and higher rates of response with no difference in OS when compared to R-CHOP [97,98].
In summary, dose-intense induction chemotherapy with consolidative auto-HCT results in high rates of long-term remission and can be considered in MCL patients who lack significant comorbidities and who understand the risks and benefits of this approach. For other patients, the less aggressive frontline approaches are more appropriate.
Relapsed/Refractory Disease
Despite initial high response rates, most patients with MCL will eventually relapse. For example, most patients given CHOP or R-CHOP alone as first-line therapy will relapse within 2 years [99]. In recent years, a number of therapies have emerged for relapsed/refractory MCL; however, the optimal sequencing of these is unclear. FDA-approved options for relapsed/refractory MCL include the proteasome inhibitor bortezomib [100,101], the BTK inhibitors ibrutinib [102,103] and acalabrutinib [104], and the immunomodulatory agent lenalidomide [105].
Auto-HCT can be considered for patients who did not undergo auto-HCT as part of first-line therapy and who had a reasonably long first remission [95]. Allo-HCT has curative potential in MCL with good evidence of a graft-versus-lymphoma effect. With a matched related or matched unrelated donor, the chance for treatment-related mortality is 15% to 25% at 1 to 2 years, with a 50% to 60% chance for long-term PFS. However, given the risk of treatment-related mortality and graft-versus-host disease, this option is typically reserved for patients with early relapse after auto-HCT, multiple relapses, or relatively chemotherapy-unresponsive disease [95,106]. A number of clinical trials for relapsed/refractory MCL are ongoing, and participation in these is encouraged whenever possible.
Burkitt Lymphoma
Burkitt lymphoma is a rare, aggressive and highly curable subtype of NHL. It can occur at any age, although peak incidence is in the first decade of life. There are 3 distinct clinical forms of Burkitt lymphoma [107]. The endemic form is common in African children and commonly involves the jaw and kidneys. The sporadic (nonendemic) form accounts for 1% to 2% of all lymphomas in the United States and Western Europe and usually has an abdominal presentation. The immunodeficiency-associated form is commonly seen in HIV patients with a relatively preserved CD4 cell count.
Patients typically present with rapidly growing masses and tumor lysis syndrome. CNS and bone marrow involvement are common. Burkitt lymphoma cells are high-grade, rapidly proliferating medium-sized cells with a monomorphic appearance. Biopsies show a classic histological appearance known as a “starry sky pattern” due to benign macrophages engulfing debris resulting from apoptosis. It is derived from a germinal center B cell and has distinct oncogenic pathways. Translocations such as t(8;14), t(2;8) or t(8;22) juxtapose the MYC locus with immunoglobulin heavy or light chain loci and result in MYC overexpression. Burkitt lymphoma is typically CD10-positive and BCL-2-negative, with a MYC translocation and a proliferation rate greater than 95%.
With conventional NHL regimens, Burkitt lymphoma had a poor prognosis, with complete remission in the 30% to 70% range and low rates of long-term remission. With the introduction of short-term, dose-intensive, multiagent chemotherapy regimens (adapted from pediatric acute lymphoblastic leukemia [ALL] regimens), the complete remission rate improved to 60% to 90% [107]. Early stage disease (localized or completely resected intra-abdominal disease) can have complete remission rates of 100%, with 2- to 5-year freedom-from-progression rates of 95%. CNS prophylaxis, including high-dose methotrexate, high-dose cytarabine, and intrathecal chemotherapy, is a standard component of Burkitt lymphoma regimens (CNS relapse rates can reach 50% without prophylactic therapy). Crucially, relapse after 1 to 2 years is very rare following complete response to induction therapy. Classically, several intensive regimens have been used for Burkitt lymphoma. In recent years, the most commonly used regimens have been the modified Magrath regimen of R-CODOX-M/IVAC and R-hyperCVAD. DA-EPOCH-R has also been used, typically for older, more frail, or HIV-positive patients. However, at the American Society of Hematology 2017 annual meeting, results from the NCI 9177 trial were presented which validated, in a prospective multi-center fashion, the use of DA-EPOCH-R in all Burkitt lymphoma patients [108]. In NCI 9177, low-risk patients (defined as normal LDH, ECOG performance score 0 or 1, ≤ stage II, and no tumor lesion > 7 cm) received 2 cycles of DA-EPOCH-R without intrathecal therapy followed by PET. If interim PET was negative, low-risk patients then received 1 more cycle of DA-EPOCH-R. High-risk patients with negative brain MRI and CSF cytology/flow cytometry received 2 cycles of DA-EPOCH-R with intrathecal therapy (2 doses per cycle) followed by PET. Unless interim PET showed progression, high-risk patients received 4 additional cycles of DA-EPOCH-R including methotrexate 12 mg intrathecally on days 1 and 5 (8 total doses). With a median follow-up of 36 months, this regimen resulted in an EFS of 85.7%. As expected, patients with CNS, marrow, or peripheral blood involvement fared worse. For those without CNS, marrow, or peripheral blood involvement, the results were excellent, with an EFS of 94.6% compared to 62.8% for those with CNS, bone marrow, or blood involvement at diagnosis.
Although no standard of care has been defined, patients with relapsed/refractory Burkitt lymphoma are often given standard second-line aggressive NHL regimens (eg, R-ICE); for those with chemosensitive disease, auto- or allo-HCT is often pursued, with long-term remissions possible following HCT [109].
Lymphoblastic Lymphoma
Lymphoblastic lymphoma (LBL) is a rare disease postulated to arise from precursor B or T lymphoblasts at varying stages of differentiation. Accounting for approximately 2% of all NHLs, 85% to 90% of all cases have a T-cell phenotype, while B-cell LBL comprises approximately 10% to 15% of cases. LBL and ALL are thought to represent the same disease entity, but LBL has been arbitrarily defined as cases with lymph node or mediastinal disease. Those with significant (> 25%) bone marrow or peripheral blood involvement are classified as ALL.
Precursor T-cell LBL patients are usually adolescent and young males who commonly present with a mediastinal mass and peripheral lymphadenopathy. Precursor B-cell LBL patients are usually older (median age 39 years) with peripheral lymphadenopathy and extranodal involvement. Mediastinal involvement with B-cell LBL is uncommon, and there is no male predominance. LBL has a propensity for dissemination to the bone marrow and CNS.
Morphologically, the tumor cells are medium sized, with a scant cytoplasm and finely dispersed chromatin. Mitotic features and apoptotic bodies are present since it is a high-grade malignancy. The lymphoblasts are typically positive for CD7 and either surface or cytoplasmic CD3. Terminal deoxynucleotidyl transferase expression is a defining feature. Other markers such as CD19, CD22, CD20, CD79a, CD45, and CD10 are variably expressed. Poor prognostic factors in T-cell LBL are female gender, age greater than 35 years, complex cytogenetics, and lack of a matched sibling donor.
Regimens for LBL are based on dose-dense, multi-agent protocols used in ALL. Most of these regimens are characterized by intensive remission-induction chemotherapy, CNS prophylaxis, a phase of consolidation therapy, and a prolonged maintenance phase, often lasting for 12 to 18 months with long-term DFS rates of 40% to 70% [110,111]. High-dose therapy with auto-HCT or allo-HCT in first complete response has been evaluated in an attempt to reduce the incidence of relapse [112]. However, the intensity of primary chemotherapy appears to be a stronger determinant of long-term survival than the use of HCT as consolidation. As a result, HCT is not routinely applied to patients in first complete remission following modern induction regimens. After relapse, prognosis is poor, with median survival rates of 6 to 9 months with conventional chemotherapy, although long-term survival rates of 30% and 20%, respectively, are reported after HCT in relapsed and primary refractory disease [113].
Treatment options in relapsed disease are limited. Nelarabine can produce responses in up to 40% of relapsed/refractory LBL/ALL patients [114]. For the minority of LBL patients with a B-cell phenotype, emerging options for relapsed/refractory LBL/ALL such as inotuzumab, blinatumomab, or anti-CD19 CAR T-cell therapy should be considered. These are not options for the majority who have a T-cell phenotype, and treatment options for these patients are limited to conventional relapsed/refractory ALL and aggressive NHL regimens.
Summary
Aggressive NHLs are characterized by rapid clinical progression without therapy. However, a significant proportion of patients are cured with appropriate combination chemotherapy or combined modality (chemotherapy + RT) regimens. In contrast, the indolent lymphomas have a relatively good prognosis (median survival of 10 years or longer) but usually are not curable in advanced clinical stages. Overall 5-year survival for aggressive NHLs with current treatment is approximately 50% to 60%, with relapses typically occurring within the first 5 years. Treatment strategies for relapsed patients offer some potential for cure; however, clinical trial participation should be encouraged whenever possible to investigate new approaches for improving outcomes in this patient population.
Corresponding author: Timothy S. Fenske, MD, Division of Hematology & Oncology, Medical College of Wisconsin, 9200 W. Wisconsin Ave., Milwaukee, WI 53226.
1. Swerdlow, SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th edition. Lyon, France: World Health Organization; 2017.
2. Surveillance, Epidemiology, and End Results (SEER) Program. www.seer.cancer.gov. Research Data 2017.
3. Boffetta P, de Vocht F. Occupation and the risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev 2007;16:369–72.
4. Bower M. Acquired immunodeficiency syndrome-related systemic non-Hodgkin’s lymphoma. Br J Haematol 2001;112:863–73.
5. Ekstrom Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood 2008;111:4029–38.
6. Clarke CA, Morton LM, Lynch C, et al. Risk of lymphoma subtypes after solid organ transplantation in the United States. Br J Cancer 2013;109:280–8.
7. Wang SS, Slager SL, Brennan P, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph). Blood 2007;109:3479–88.
8. Dong C, Hemminki K. Second primary neoplasms among 53 159 haematolymphoproliferative malignancy patients in Sweden, 1958–1996: a search for common mechanisms. Br J Cancer 2001;85:997–1005.
9. Hummel M, Anagnostopoulos I, Korbjuhn P, Stein H. Epstein-Barr virus in B-cell non-Hodgkin’s lymphomas: unexpected infection patterns and different infection incidence in low- and high-grade types. J Pathol 1995;175:263–71.
10. Cesarman E, Chang Y, Moore PS, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995;332:1186–91.
11. Viswanatha DS, Dogan A. Hepatitis C virus and lymphoma. J Clin Pathol 2007;60:1378–83.
12. Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol 2010;11:827–34.
13. Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood 2011;117:1792–8.
14. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–68.
15. Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937–47.
16. Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016;66:443–59.
17. Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood 2006;107:265–76.
18. Møller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation--a population-based study of 1575 cases. Br J Haematol 2004;124:151–9.
19. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol 1998;16:2780–95.
20. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 1997;89:3909–18.
21. Sehn LH, Scott DW, Chhanabhai M, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 2011;29:1452–7.
22. Fisher DE, Jacobson JO, Ault KA, Harris NL. Diffuse large cell lymphoma with discordant bone marrow histology. Clinical features and biological implications. Cancer 1989;64:1879–87.
23. Yao Z, Deng L, Xu-Monette ZY, et al. Concordant bone marrow involvement of diffuse large B-cell lymphoma represents a distinct clinical and biological entity in the era of immunotherapy. Leukemia 2018;32:353–63.
24. Gascoyne RD, Adomat SA, Krajewski S, et al. Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin’s lymphoma. Blood 1997;90:244–51.
25. Skinnider BF, Horsman DE, Dupuis B, Gascoyne RD. Bcl-6 and Bcl-2 protein expression in diffuse large B-cell lymphoma and follicular lymphoma: correlation with 3q27 and 18q21 chromosomal abnormalities. Hum Pathol 1999;30:803–8.
26. Chisholm KM, Bangs CD, Bacchi CE, et al. Expression profiles of MYC protein and MYC gene rearrangement in lymphomas. Am J Surg Pathol 2015;39:294–303.
27. Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123:837–42.
28. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–82.
29. Horn H, Ziepert M, Becher C, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 2013;121:2253–63.
30. Barrans S, Crouch S, Smith A, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 2010;28:3360–5.
31. Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 2013;121:4021–31.
32. Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012;30:3460–7.
33. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med 1993;328:1002–6.
34. Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011;12:1013–22.
35. Coiffier B, Lepage E, Brière J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235–42.
36. Persky DO, Unger JM, Spier CM, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol 2008;26:2258–63.
37. Lamy T, Damaj G, Soubeyran P, et al. R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood 2018;131:174–81.
38. Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 2011;12:460–8.
39. Wilson WH, sin-Ho J, Pitcher BN, et al. Phase III randomized study of R-CHOP versus DA-EPOCH-R and molecular analysis of untreated diffuse large B-cell lymphoma: CALGB/Alliance 50303. Blood 2016;128:469 LP-469. 38.
40. Vitolo U, Trne˘ný M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol 2017;35:3529–37.
41. Leonard JP, Kolibaba KS, Reeves JA, et al. Randomized phase II study of R-CHOP with or without bortezomib in previously untreated patients with non-germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol 2017;35:3538–46.
42. Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol 2015;33:251–7.
43. Younes A, Thieblemont C, Morschhauser F, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol 2014;15:1019–26.
44. Younes A, Zinzani PL, Sehn LH, et al. A randomized, double-blind, placebo-controlled phase 3 study of ibrutinib in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in subjects with newly diagnosed nongerminal center B-cell subtype of diffuse large B-cell lymphoma (DLBCL). J Clin Oncol 2014;32(15_suppl):TPS8615.
45. Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol 2013;14:525–33.
46. Leppä S, Fayad LE, Lee J-J, et al. A phase III study of enzastaurin in patients with high-risk diffuse large B cell lymphoma following response to primary treatment: the Prelude trial. Blood 2013;122:371 LP-371.
47. Witzig TE, Tobinai K, Rigacci L, et al. Adjuvant everolimus in high-risk diffuse large B-cell lymphoma: final results from the PILLAR-2 randomized phase III trial. Ann Oncol 2018;29:707–14.
48. Strehl J, Mey U, Glasmacher A, et al. High-dose chemotherapy followed by autologous stem cell transplantation as first-line therapy in aggressive non-Hodgkin’s lymphoma: a meta-analysis. Haematologica 2003;88:1304–15.
49. Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med 2013;369:1681–90.
50. Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol 2014;166:891–901.
51. Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood 2014;124:2354–61.
52. Howlett C, Snedecor SJ, Landsburg DJ, et al. Front-line, dose-escalated immunochemotherapy is associated with a significant progression-free survival advantage in patients with double-hit lymphomas: a systematic review and meta-analysis. Br J Haematol 2015;170:504–14.
53. Landsburg DJ, Falkiewicz MK, Maly J, et al. Outcomes of patients with double-hit lymphoma who achieve first complete remission. J Clin Oncol 2017;35:2260–7.
54. Schmitz N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 2016;34:3150–6.
55. Abramson JS, Hellmann M, Barnes JA, et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer 2010;116:4283–90.
56. Dunleavy K, Roschewski M, Abramson JS, et al. Risk-adapted therapy in adults with Burkitt lymphoma: updated results of a multicenter prospective phase II study of DA-EPOCH-R. Hematol Oncol 2017;35(S2):133–4.
57. Damaj G, Ivanoff S, Coso D, et al. Concomitant systemic and central nervous system non-Hodgkin lymphoma: the role of consolidation in terms of high dose therapy and autologous stem cell transplantation. A 60-case retrospective study from LYSA and the LOC network. Haematologica 2015;100:1199–206.
58. Thieblemont C, Briere J, Mounier N, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol 2011;29:4079–87.
59. Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with dalvage vhemotherapy in relapses of chemotherapy-densitive non-Hodgkin’s lymphoma. N Engl J Med 1995;333:1540–5.
60. Hamadani M, Hari PN, Zhang Y, et al. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant 2014;20:1729–36.
61. Costa LJ, Maddocks K, Epperla N, et al. Diffuse large B-cell lymphoma with primary treatment failure: Ultra-high risk features and benchmarking for experimental therapies. Am J Hematol 2017;92:e24615.
62. Fenske TS, Ahn KW, Graff TM, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol 2016;174:235–48.
63. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44.
64. van Besien K, Kelta M, Bahaguna P. Primary mediastinal B-cell lymphoma: a review of pathology and management. J Clin Oncol 2001;19:1855–64.
65. Savage KJ, Al-Rajhi N, Voss N, et al. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol Off J Eur Soc Med Oncol 2006;17:123–30.
66. Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 2003;198:851–62.
67. Lavabre-Bertrand T, Donadio D, Fegueux N, et al. A study of 15 cases of primary mediastinal lymphoma of B-cell type. Cancer 1992;69:2561–6.
68. Lazzarino M, Orlandi E, Paulli M, et al. Treatment outcome and prognostic factors for primary mediastinal (thymic) B-cell lymphoma: a multicenter study of 106 patients. J Clin Oncol 1997;15:1646–53.
69. Zinzani PL, Martelli M, Magagnoli M, et al. Treatment and clinical management of primary mediastinal large B-cell lymphoma with sclerosis: MACOP-B regimen and mediastinal radiotherapy monitored by (67)Gallium scan in 50 patients. Blood 1999;94:3289–93.
70. Todeschini G, Secchi S, Morra E, et al. Primary mediastinal large B-cell lymphoma (PMLBCL): long-term results from a retrospective multicentre Italian experience in 138 patients treated with CHOP or MACOP-B/VACOP-B. Br J Cancer 2004;90:372–6.
71. Rieger M, Osterborg A, Pettengell R, et al. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol Off J Eur Soc Med Oncol 2011;22:664–70.
72. Shah NN, Szabo A, Huntington SF, et al. R-CHOP versus dose-adjusted R-EPOCH in frontline management of primary mediastinal B-cell lymphoma: a multi-centre analysis. Br J Haematol 2018;180:534–44.
73. Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med 2013;368:1408–16.
74. Aoki T, Shimada K, Suzuki R, et al. High-dose chemotherapy followed by autologous stem cell transplantation for relapsed/refractory primary mediastinal large B-cell lymphoma. Blood Cancer J 2015;5:e372–e372.
75. Sehn LH, Antin JH, Shulman LN, et al. Primary diffuse large B-cell lymphoma of the mediastinum: outcome following high-dose chemotherapy and autologous hematopoietic cell transplantation. Blood 1998;91:717–23.
76. Kuruvilla J, Pintilie M, Tsang R, et al. Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk Lymphoma 2008;49:1329–36.
77. Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood 2017;130:267–70.
78. Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer 2011;105:1684–92.
79. Argatoff LH, Connors JM, Klasa RJ, et al. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood 1997;89:2067–78.
80. Zukerberg LR, Yang WI, Arnold A, Harris NL. Cyclin D1 expression in non-Hodgkin’s lymphomas. Detection by immunohistochemistry. Am J Clin Pathol 1995;103:756–60.
81. Wiestner A, Tehrani M, Chiorazzi M, et al. Point mutations and genomic deletions in CCND1 create stable truncated cyclin D1 mRNAs that are associated with increased proliferation rate and shorter survival. Blood 2007;109:4599–606.
82. Fu K, Weisenburger DD, Greiner TC, et al. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood 2005;106:4315–21.
83. Mozos A, Royo C, Hartmann E, et al. SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1-negative subtype. Haematologica 2009;94:1555–62.
84. Norton AJ, Matthews J, Pappa V, et al. Mantle cell lymphoma: Natural history defined in a serially biopsied population over a 20-year period. Ann Oncol 1995;6:249–56.
85. Chihara D, Cheah CY, Westin JR, et al. Rituximab plus hyper-CVAD alternating with MTX/Ara-C in patients with newly diagnosed mantle cell lymphoma: 15-year follow-up of a phase II study from the MD Anderson Cancer Center. Br J Haematol 2016;172:80–8.
86. Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood 2013;121:48–53.
87. Eskelund CW, Kolstad A, Jerkeman M, et al. 15-year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol 2016;175:410–8.
88. Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008;111:558–65.
89. Hoster E, Klapper W, Hermine O, et al. Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. J Clin Oncol 2014;32:1338–46.
90. Hoster E, Rosenwald A, Berger F, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: Results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol 2016;34:1386–94.
91. Bernard M, Gressin R, Lefrère F, et al. Blastic variant of mantle cell lymphoma: a rare but highly aggressive subtype. Leukemia 2001;15:1785–91.
92. Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol 2009;27:1209–13.
93. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017 Sep 28;377(13):1250–60.
94. Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet 2016;388:565–75.
95. Fenske TS, Zhang M-J, Carreras J, et al. Autologous or reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chemotherapy-sensitive mantle-cell lymphoma: analysis of transplantation timing and modality. J Clin Oncol 2014;32:273–81.
96. Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med 2012;367:520–31.
97. Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014;123:2944–52.
98. Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203–10.
99. Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol 2005;23:1984–92.
100. Belch A, Kouroukis CT, Crump M, et al. A phase II study of bortezomib in mantle cell lymphoma: the National Cancer Institute of Canada Clinical Trials Group trial IND.150. Ann Oncol Off J Eur Soc Med Oncol 2007;18:116–21.
101. Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol 2006;24:4867–74.
102. Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet 2016;387:770–8.
103. Wang ML, Rule S, Martin P, Goy A, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507–16.
104. Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet 2018;391:659–67.
105. Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol 2013;31:3688–95.
106. Khouri IF, Lee M-S, Saliba RM, et al. Nonablative allogeneic stem-cell transplantation for advanced/recurrent mantle-cell lymphoma. J Clin Oncol 2003;21:4407–12.
107. Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood 2004;104:3009–20.
108. Roschewski M, Dunleavy K, Abramson JS, et al. Risk-adapted therapy in adults with Burkitt lymphoma: results of NCI 9177, a multicenter prospective phase II study of DA-EPOCH-R. Blood American Society of Hematology;2017;130(Suppl 1):188.
109. Maramattom L V, Hari PN, Burns LJ, et al. Autologous and allogeneic transplantation for burkitt lymphoma outcomes and changes in utilization: a report from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2013;19:173–9.
110. Zinzani PL, Bendandi M, Visani G, et al. Adult lymphoblastic lymphoma: clinical features and prognostic factors in 53 patients. Leuk Lymphoma 1996;23:577–82.
111. Thomas DA, O’Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood 2004;104:1624–30.
112. Aljurf M, Zaidi SZA. Chemotherapy and hematopoietic stem cell transplantation for adult T-cell lymphoblastic lymphoma: current status and controversies. Biol Blood Marrow Transplant 2005;11:739–54.
113. Sweetenham JW, Santini G, Qian W, et al. High-dose therapy and autologous stem-cell transplantation versus conventional-dose consolidation/maintenance therapy as postremission therapy for adult patients with lymphoblastic lymphoma: results of a randomized trial of the European Group for Blood and Marrow Transplantation and the United Kingdom Lymphoma Group. J Clin Oncol 2001;19:2927–36.
114. Zwaan CM, Kowalczyk J, Schmitt C, et al. Safety and efficacy of nelarabine in children and young adults with relapsed or refractory T-lineage acute lymphoblastic leukaemia or T-lineage lymphoblastic lymphoma: results of a phase 4 study. Br J Haematol 2017;179:284–93.
1. Swerdlow, SH, Campo E, Harris NL, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th edition. Lyon, France: World Health Organization; 2017.
2. Surveillance, Epidemiology, and End Results (SEER) Program. www.seer.cancer.gov. Research Data 2017.
3. Boffetta P, de Vocht F. Occupation and the risk of non-Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev 2007;16:369–72.
4. Bower M. Acquired immunodeficiency syndrome-related systemic non-Hodgkin’s lymphoma. Br J Haematol 2001;112:863–73.
5. Ekstrom Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood 2008;111:4029–38.
6. Clarke CA, Morton LM, Lynch C, et al. Risk of lymphoma subtypes after solid organ transplantation in the United States. Br J Cancer 2013;109:280–8.
7. Wang SS, Slager SL, Brennan P, et al. Family history of hematopoietic malignancies and risk of non-Hodgkin lymphoma (NHL): a pooled analysis of 10 211 cases and 11 905 controls from the International Lymphoma Epidemiology Consortium (InterLymph). Blood 2007;109:3479–88.
8. Dong C, Hemminki K. Second primary neoplasms among 53 159 haematolymphoproliferative malignancy patients in Sweden, 1958–1996: a search for common mechanisms. Br J Cancer 2001;85:997–1005.
9. Hummel M, Anagnostopoulos I, Korbjuhn P, Stein H. Epstein-Barr virus in B-cell non-Hodgkin’s lymphomas: unexpected infection patterns and different infection incidence in low- and high-grade types. J Pathol 1995;175:263–71.
10. Cesarman E, Chang Y, Moore PS, et al. Kaposi’s sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med 1995;332:1186–91.
11. Viswanatha DS, Dogan A. Hepatitis C virus and lymphoma. J Clin Pathol 2007;60:1378–83.
12. Engels EA, Cho ER, Jee SH. Hepatitis B virus infection and risk of non-Hodgkin lymphoma in South Korea: a cohort study. Lancet Oncol 2010;11:827–34.
13. Marcucci F, Mele A. Hepatitis viruses and non-Hodgkin lymphoma: epidemiology, mechanisms of tumorigenesis, and therapeutic opportunities. Blood 2011;117:1792–8.
14. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014;32:3059–68.
15. Rosenwald A, Wright G, Chan WC, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002;346:1937–47.
16. Teras LR, DeSantis CE, Cerhan JR, et al. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin 2016;66:443–59.
17. Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood 2006;107:265–76.
18. Møller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation--a population-based study of 1575 cases. Br J Haematol 2004;124:151–9.
19. Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol 1998;16:2780–95.
20. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin’s lymphoma. The Non-Hodgkin’s Lymphoma Classification Project. Blood 1997;89:3909–18.
21. Sehn LH, Scott DW, Chhanabhai M, et al. Impact of concordant and discordant bone marrow involvement on outcome in diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 2011;29:1452–7.
22. Fisher DE, Jacobson JO, Ault KA, Harris NL. Diffuse large cell lymphoma with discordant bone marrow histology. Clinical features and biological implications. Cancer 1989;64:1879–87.
23. Yao Z, Deng L, Xu-Monette ZY, et al. Concordant bone marrow involvement of diffuse large B-cell lymphoma represents a distinct clinical and biological entity in the era of immunotherapy. Leukemia 2018;32:353–63.
24. Gascoyne RD, Adomat SA, Krajewski S, et al. Prognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin’s lymphoma. Blood 1997;90:244–51.
25. Skinnider BF, Horsman DE, Dupuis B, Gascoyne RD. Bcl-6 and Bcl-2 protein expression in diffuse large B-cell lymphoma and follicular lymphoma: correlation with 3q27 and 18q21 chromosomal abnormalities. Hum Pathol 1999;30:803–8.
26. Chisholm KM, Bangs CD, Bacchi CE, et al. Expression profiles of MYC protein and MYC gene rearrangement in lymphomas. Am J Surg Pathol 2015;39:294–303.
27. Zhou Z, Sehn LH, Rademaker AW, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 2014;123:837–42.
28. Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004;103:275–82.
29. Horn H, Ziepert M, Becher C, et al. MYC status in concert with BCL2 and BCL6 expression predicts outcome in diffuse large B-cell lymphoma. Blood 2013;121:2253–63.
30. Barrans S, Crouch S, Smith A, et al. Rearrangement of MYC is associated with poor prognosis in patients with diffuse large B-cell lymphoma treated in the era of rituximab. J Clin Oncol 2010;28:3360–5.
31. Hu S, Xu-Monette ZY, Tzankov A, et al. MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood 2013;121:4021–31.
32. Green TM, Young KH, Visco C, et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012;30:3460–7.
33. Fisher RI, Gaynor ER, Dahlberg S, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin’s lymphoma. N Engl J Med 1993;328:1002–6.
34. Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol 2011;12:1013–22.
35. Coiffier B, Lepage E, Brière J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med 2002;346:235–42.
36. Persky DO, Unger JM, Spier CM, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol 2008;26:2258–63.
37. Lamy T, Damaj G, Soubeyran P, et al. R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood 2018;131:174–81.
38. Peyrade F, Jardin F, Thieblemont C, et al. Attenuated immunochemotherapy regimen (R-miniCHOP) in elderly patients older than 80 years with diffuse large B-cell lymphoma: a multicentre, single-arm, phase 2 trial. Lancet Oncol 2011;12:460–8.
39. Wilson WH, sin-Ho J, Pitcher BN, et al. Phase III randomized study of R-CHOP versus DA-EPOCH-R and molecular analysis of untreated diffuse large B-cell lymphoma: CALGB/Alliance 50303. Blood 2016;128:469 LP-469. 38.
40. Vitolo U, Trne˘ný M, Belada D, et al. Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol 2017;35:3529–37.
41. Leonard JP, Kolibaba KS, Reeves JA, et al. Randomized phase II study of R-CHOP with or without bortezomib in previously untreated patients with non-germinal center B-cell-like diffuse large B-cell lymphoma. J Clin Oncol 2017;35:3538–46.
42. Nowakowski GS, LaPlant B, Macon WR, et al. Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-Cell lymphoma: a phase II study. J Clin Oncol 2015;33:251–7.
43. Younes A, Thieblemont C, Morschhauser F, et al. Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol 2014;15:1019–26.
44. Younes A, Zinzani PL, Sehn LH, et al. A randomized, double-blind, placebo-controlled phase 3 study of ibrutinib in combination with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in subjects with newly diagnosed nongerminal center B-cell subtype of diffuse large B-cell lymphoma (DLBCL). J Clin Oncol 2014;32(15_suppl):TPS8615.
45. Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol 2013;14:525–33.
46. Leppä S, Fayad LE, Lee J-J, et al. A phase III study of enzastaurin in patients with high-risk diffuse large B cell lymphoma following response to primary treatment: the Prelude trial. Blood 2013;122:371 LP-371.
47. Witzig TE, Tobinai K, Rigacci L, et al. Adjuvant everolimus in high-risk diffuse large B-cell lymphoma: final results from the PILLAR-2 randomized phase III trial. Ann Oncol 2018;29:707–14.
48. Strehl J, Mey U, Glasmacher A, et al. High-dose chemotherapy followed by autologous stem cell transplantation as first-line therapy in aggressive non-Hodgkin’s lymphoma: a meta-analysis. Haematologica 2003;88:1304–15.
49. Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med 2013;369:1681–90.
50. Oki Y, Noorani M, Lin P, et al. Double hit lymphoma: the MD Anderson Cancer Center clinical experience. Br J Haematol 2014;166:891–901.
51. Petrich AM, Gandhi M, Jovanovic B, et al. Impact of induction regimen and stem cell transplantation on outcomes in double-hit lymphoma: a multicenter retrospective analysis. Blood 2014;124:2354–61.
52. Howlett C, Snedecor SJ, Landsburg DJ, et al. Front-line, dose-escalated immunochemotherapy is associated with a significant progression-free survival advantage in patients with double-hit lymphomas: a systematic review and meta-analysis. Br J Haematol 2015;170:504–14.
53. Landsburg DJ, Falkiewicz MK, Maly J, et al. Outcomes of patients with double-hit lymphoma who achieve first complete remission. J Clin Oncol 2017;35:2260–7.
54. Schmitz N, Zeynalova S, Nickelsen M, et al. CNS International Prognostic Index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J Clin Oncol 2016;34:3150–6.
55. Abramson JS, Hellmann M, Barnes JA, et al. Intravenous methotrexate as central nervous system (CNS) prophylaxis is associated with a low risk of CNS recurrence in high-risk patients with diffuse large B-cell lymphoma. Cancer 2010;116:4283–90.
56. Dunleavy K, Roschewski M, Abramson JS, et al. Risk-adapted therapy in adults with Burkitt lymphoma: updated results of a multicenter prospective phase II study of DA-EPOCH-R. Hematol Oncol 2017;35(S2):133–4.
57. Damaj G, Ivanoff S, Coso D, et al. Concomitant systemic and central nervous system non-Hodgkin lymphoma: the role of consolidation in terms of high dose therapy and autologous stem cell transplantation. A 60-case retrospective study from LYSA and the LOC network. Haematologica 2015;100:1199–206.
58. Thieblemont C, Briere J, Mounier N, et al. The germinal center/activated B-cell subclassification has a prognostic impact for response to salvage therapy in relapsed/refractory diffuse large B-cell lymphoma: a bio-CORAL study. J Clin Oncol 2011;29:4079–87.
59. Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with dalvage vhemotherapy in relapses of chemotherapy-densitive non-Hodgkin’s lymphoma. N Engl J Med 1995;333:1540–5.
60. Hamadani M, Hari PN, Zhang Y, et al. Early failure of frontline rituximab-containing chemo-immunotherapy in diffuse large B cell lymphoma does not predict futility of autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant 2014;20:1729–36.
61. Costa LJ, Maddocks K, Epperla N, et al. Diffuse large B-cell lymphoma with primary treatment failure: Ultra-high risk features and benchmarking for experimental therapies. Am J Hematol 2017;92:e24615.
62. Fenske TS, Ahn KW, Graff TM, et al. Allogeneic transplantation provides durable remission in a subset of DLBCL patients relapsing after autologous transplantation. Br J Haematol 2016;174:235–48.
63. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;377:2531–44.
64. van Besien K, Kelta M, Bahaguna P. Primary mediastinal B-cell lymphoma: a review of pathology and management. J Clin Oncol 2001;19:1855–64.
65. Savage KJ, Al-Rajhi N, Voss N, et al. Favorable outcome of primary mediastinal large B-cell lymphoma in a single institution: the British Columbia experience. Ann Oncol Off J Eur Soc Med Oncol 2006;17:123–30.
66. Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med 2003;198:851–62.
67. Lavabre-Bertrand T, Donadio D, Fegueux N, et al. A study of 15 cases of primary mediastinal lymphoma of B-cell type. Cancer 1992;69:2561–6.
68. Lazzarino M, Orlandi E, Paulli M, et al. Treatment outcome and prognostic factors for primary mediastinal (thymic) B-cell lymphoma: a multicenter study of 106 patients. J Clin Oncol 1997;15:1646–53.
69. Zinzani PL, Martelli M, Magagnoli M, et al. Treatment and clinical management of primary mediastinal large B-cell lymphoma with sclerosis: MACOP-B regimen and mediastinal radiotherapy monitored by (67)Gallium scan in 50 patients. Blood 1999;94:3289–93.
70. Todeschini G, Secchi S, Morra E, et al. Primary mediastinal large B-cell lymphoma (PMLBCL): long-term results from a retrospective multicentre Italian experience in 138 patients treated with CHOP or MACOP-B/VACOP-B. Br J Cancer 2004;90:372–6.
71. Rieger M, Osterborg A, Pettengell R, et al. Primary mediastinal B-cell lymphoma treated with CHOP-like chemotherapy with or without rituximab: results of the Mabthera International Trial Group study. Ann Oncol Off J Eur Soc Med Oncol 2011;22:664–70.
72. Shah NN, Szabo A, Huntington SF, et al. R-CHOP versus dose-adjusted R-EPOCH in frontline management of primary mediastinal B-cell lymphoma: a multi-centre analysis. Br J Haematol 2018;180:534–44.
73. Dunleavy K, Pittaluga S, Maeda LS, et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N Engl J Med 2013;368:1408–16.
74. Aoki T, Shimada K, Suzuki R, et al. High-dose chemotherapy followed by autologous stem cell transplantation for relapsed/refractory primary mediastinal large B-cell lymphoma. Blood Cancer J 2015;5:e372–e372.
75. Sehn LH, Antin JH, Shulman LN, et al. Primary diffuse large B-cell lymphoma of the mediastinum: outcome following high-dose chemotherapy and autologous hematopoietic cell transplantation. Blood 1998;91:717–23.
76. Kuruvilla J, Pintilie M, Tsang R, et al. Salvage chemotherapy and autologous stem cell transplantation are inferior for relapsed or refractory primary mediastinal large B-cell lymphoma compared with diffuse large B-cell lymphoma. Leuk Lymphoma 2008;49:1329–36.
77. Zinzani PL, Ribrag V, Moskowitz CH, et al. Safety and tolerability of pembrolizumab in patients with relapsed/refractory primary mediastinal large B-cell lymphoma. Blood 2017;130:267–70.
78. Smith A, Howell D, Patmore R, et al. Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network. Br J Cancer 2011;105:1684–92.
79. Argatoff LH, Connors JM, Klasa RJ, et al. Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood 1997;89:2067–78.
80. Zukerberg LR, Yang WI, Arnold A, Harris NL. Cyclin D1 expression in non-Hodgkin’s lymphomas. Detection by immunohistochemistry. Am J Clin Pathol 1995;103:756–60.
81. Wiestner A, Tehrani M, Chiorazzi M, et al. Point mutations and genomic deletions in CCND1 create stable truncated cyclin D1 mRNAs that are associated with increased proliferation rate and shorter survival. Blood 2007;109:4599–606.
82. Fu K, Weisenburger DD, Greiner TC, et al. Cyclin D1-negative mantle cell lymphoma: a clinicopathologic study based on gene expression profiling. Blood 2005;106:4315–21.
83. Mozos A, Royo C, Hartmann E, et al. SOX11 expression is highly specific for mantle cell lymphoma and identifies the cyclin D1-negative subtype. Haematologica 2009;94:1555–62.
84. Norton AJ, Matthews J, Pappa V, et al. Mantle cell lymphoma: Natural history defined in a serially biopsied population over a 20-year period. Ann Oncol 1995;6:249–56.
85. Chihara D, Cheah CY, Westin JR, et al. Rituximab plus hyper-CVAD alternating with MTX/Ara-C in patients with newly diagnosed mantle cell lymphoma: 15-year follow-up of a phase II study from the MD Anderson Cancer Center. Br J Haematol 2016;172:80–8.
86. Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood 2013;121:48–53.
87. Eskelund CW, Kolstad A, Jerkeman M, et al. 15-year follow-up of the Second Nordic Mantle Cell Lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol 2016;175:410–8.
88. Hoster E, Dreyling M, Klapper W, et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008;111:558–65.
89. Hoster E, Klapper W, Hermine O, et al. Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. J Clin Oncol 2014;32:1338–46.
90. Hoster E, Rosenwald A, Berger F, et al. Prognostic value of Ki-67 index, cytology, and growth pattern in mantle-cell lymphoma: Results from randomized trials of the European Mantle Cell Lymphoma Network. J Clin Oncol 2016;34:1386–94.
91. Bernard M, Gressin R, Lefrère F, et al. Blastic variant of mantle cell lymphoma: a rare but highly aggressive subtype. Leukemia 2001;15:1785–91.
92. Martin P, Chadburn A, Christos P, et al. Outcome of deferred initial therapy in mantle-cell lymphoma. J Clin Oncol 2009;27:1209–13.
93. Le Gouill S, Thieblemont C, Oberic L, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017 Sep 28;377(13):1250–60.
94. Hermine O, Hoster E, Walewski J, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet 2016;388:565–75.
95. Fenske TS, Zhang M-J, Carreras J, et al. Autologous or reduced-intensity conditioning allogeneic hematopoietic cell transplantation for chemotherapy-sensitive mantle-cell lymphoma: analysis of transplantation timing and modality. J Clin Oncol 2014;32:273–81.
96. Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. N Engl J Med 2012;367:520–31.
97. Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014;123:2944–52.
98. Rummel MJ, Niederle N, Maschmeyer G, et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: an open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013;381:1203–10.
99. Lenz G, Dreyling M, Hoster E, et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J Clin Oncol 2005;23:1984–92.
100. Belch A, Kouroukis CT, Crump M, et al. A phase II study of bortezomib in mantle cell lymphoma: the National Cancer Institute of Canada Clinical Trials Group trial IND.150. Ann Oncol Off J Eur Soc Med Oncol 2007;18:116–21.
101. Fisher RI, Bernstein SH, Kahl BS, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol 2006;24:4867–74.
102. Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet 2016;387:770–8.
103. Wang ML, Rule S, Martin P, Goy A, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507–16.
104. Wang M, Rule S, Zinzani PL, et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): a single-arm, multicentre, phase 2 trial. Lancet 2018;391:659–67.
105. Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol 2013;31:3688–95.
106. Khouri IF, Lee M-S, Saliba RM, et al. Nonablative allogeneic stem-cell transplantation for advanced/recurrent mantle-cell lymphoma. J Clin Oncol 2003;21:4407–12.
107. Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood 2004;104:3009–20.
108. Roschewski M, Dunleavy K, Abramson JS, et al. Risk-adapted therapy in adults with Burkitt lymphoma: results of NCI 9177, a multicenter prospective phase II study of DA-EPOCH-R. Blood American Society of Hematology;2017;130(Suppl 1):188.
109. Maramattom L V, Hari PN, Burns LJ, et al. Autologous and allogeneic transplantation for burkitt lymphoma outcomes and changes in utilization: a report from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2013;19:173–9.
110. Zinzani PL, Bendandi M, Visani G, et al. Adult lymphoblastic lymphoma: clinical features and prognostic factors in 53 patients. Leuk Lymphoma 1996;23:577–82.
111. Thomas DA, O’Brien S, Cortes J, et al. Outcome with the hyper-CVAD regimens in lymphoblastic lymphoma. Blood 2004;104:1624–30.
112. Aljurf M, Zaidi SZA. Chemotherapy and hematopoietic stem cell transplantation for adult T-cell lymphoblastic lymphoma: current status and controversies. Biol Blood Marrow Transplant 2005;11:739–54.
113. Sweetenham JW, Santini G, Qian W, et al. High-dose therapy and autologous stem-cell transplantation versus conventional-dose consolidation/maintenance therapy as postremission therapy for adult patients with lymphoblastic lymphoma: results of a randomized trial of the European Group for Blood and Marrow Transplantation and the United Kingdom Lymphoma Group. J Clin Oncol 2001;19:2927–36.
114. Zwaan CM, Kowalczyk J, Schmitt C, et al. Safety and efficacy of nelarabine in children and young adults with relapsed or refractory T-lineage acute lymphoblastic leukaemia or T-lineage lymphoblastic lymphoma: results of a phase 4 study. Br J Haematol 2017;179:284–93.
Management of Dyslipidemia in the Elderly
From the Harrison School of Pharmacy, Auburn University, Mobile, AL.
Abstract
- Objective: To summarize the literature relevant to managing dyslipidemia in the elderly and review recommendations for initiating lipid-lowering therapy.
- Methods: Review of the literature.
- Results: Statins are the most commonly utilized medication class for lipid-lowering in the general population, and they are recommended for primary prevention in patients between the ages of 40 to 75 with at least 1 risk factor for cardiovascular disease as well as for any patient needing secondary prevention. In the elderly, statins may be appropriate for both primary and secondary prevention if the benefits outweigh the risks. Based on the available evidence, it is safe to recommend statin therapy to elderly patients who require secondary prevention given the known benefits in reducing cardiovascular morbidity and mortality for patients up to the age of 80. For primary prevention, statin therapy may be beneficial, but one must carefully evaluate for comorbid conditions, life expectancy, concomitant medications, overall health status, frailty, and patient or family preference. Several other classes of lipid-lowering agents exist; however, there is not enough evidence for us to recommend use in the elderly population for cardiovascular risk reduction in either primary or secondary scenarios.
- Conclusion: Although clinical research in the elderly population is limited, evidence supports the use of statins in elderly patients for secondary prevention and in patients up to age 75 for primary prevention; however clinicians must use clinical judgement and take into consideration the patient’s situation regarding comorbidities, polypharmacy, and possible adverse effects. More high-quality evidence is necessary.
Key words: hyperlipidemia; geriatrics; elderly; patient-centered care; statin; cardiovascular disease.
The number of Americans age 65 years and older is projected to more than double, from 46 million today to over 98 million by 2060, and the 65-and-older age group’s share of the total population will rise to nearly 24% [1]. Life expectancy is now predicted to be > 20 years for women at age 65 and > 17 years for men at age 65 in many high-income countries, including the United States [2]. This demographic shift toward an older population will result in a higher burden of coronary heart disease and stroke, with atherosclerotic cardiovascular disease (ASCVD) prevalence and costs projected to increase substantially [3].
Among adults seeking medical care in the United States, roughly 95 million have a total cholesterol (TC) level of ≥ 200 mg/dL or more, and approximately 29 million have a TC > 240 mg/dL [4]. Cholesterol screening is important since most patients suffering from dyslipidemia are asymptomatic. Dyslipidemia is a major risk factor for the development of atherosclerotic disease. Because of the complications associated with dyslipidemia, it is vital that patients are provided with primary and/or secondary prevention strategies to reduce the risk of cardiovascular disease (CVD) and protect high-risk patients from recurring events. A clinical controversy exists surrounding the elderly population, concerning whether or not clinicians should be providing lipid-lowering treatment to this group of individuals for dyslipidemia. The evidence is limited for patients over age 65, and even more so for the very elderly (> 80 years); therefore, it is necessary to review the available evidence to make an appropriate decision when it comes to managing dyslipidemia in the elderly population
Currently, HMG-CoA reductase inhibitors (statins) are the only known class of medications for the treatment of dyslipidemia that will prevent both primary and secondary cardiovascular (CV) events, including death. Statin intensity (Table 1)
Guideline Recommendations
Current guidelines differ in their recommendations for treating dyslipidemia in the elderly population. In 2016, the Task Force for the Management of Dyslipidemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) released updated guidelines for managing dyslipidemia. These guidelines recommend that older patients with established CVD be treated in the same way as younger patients because of the many benefits statin therapy demonstrated in clinical trials. They also suggest that statin therapy be started at a lower doses to achieve goals for primary prevention in the older population. In addition, CVD risk factors (hypertension, diabetes, dyslipidemia, smoking) should be addressed in this population to reduce CVD risk. They also acknowledged that primary prevention may not prolong life in the older adult, but treatment does reduce cardiovascular mortality and statin therapy is recommended to reduce the overall risk of CV morbidity in this population [11]. In contrast, The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines changed the management and treatment of dyslipidemia by highlighting “statin benefit groups” rather than recommending a treat-to-target goal as guidelines had done for many years. ACC/AHA recommends a moderate-intensity statin for patients > 75 years of age for secondary prevention versus the use of a high-intensity statin for patients who are between the ages of 40 and 75 based on the pooled cohort risk equation. In patients over age 75 with no history of CVD, no specific recommendation is available for the use of lipid-lowering therapy at this time [12]. ACC/AHA is expected to publish a new set of guidelines sometime in 2018 and they are projected to utilize lipid-lowering goals in combination with the pooled cohort equation to assess overall risk in patients with dyslipidemia.
The 2015 National Lipid Association (NLA) released “Part 1” guidelines for the management of dyslipidemia and then provided “Part 2” about a year later, which focuses on management for special populations. To summarize, the NLA guidelines recommend that elderly patients between the ages of 65 and 80 receive a high-intensity statin for secondary prevention after special consideration of the potential risks and benefits. In patients over the age of 80, NLA recommends a moderate-intensity statin for secondary prevention. For primary prevention, NLA recommends utilizing the pooled cohort risk equation to analyze patient characteristics, keeping in mind that age is a driving factor for increased risk of CVD and that the actual risk for developing a CV event may be “overestimated” if the patient has no other risk factors other than their age. When evaluating patients between the ages of 65 and 79 for primary prevention, NLA suggests following Part 1 of the guidelines. In Part 1, NLA recommends evaluating the patient’s characteristics and suggests a moderate- or high-intensity statin if the patient is considered “very high risk” or “high risk” and a moderate-intensity statin for patients who are considered “moderate risk”. For patients over the age of 80, they recommend utilizing a moderate- or a low-intensity statin depending on frailty status or if significant comorbidities or polypharmacy exist [13,14].
In 2017, the American Association of Clinical Endocrinologist (AACE) released guidelines for the management of dyslipidemia and CVD prevention. AACE recommends that patients over age 65 be screened for dyslipidemia, and those who have multiple risk factors, other than age, should be considered for treatment with lipid-lowering therapy. AACE focuses on specific target LDL-C levels as treatment goals [15].
In addition to statins, other lipid-lowering therapies are used to treat dyslipidemia. The 2016 American College of Cardiology (ACC) Task Force reported on the use of non-statin therapies for the management of dyslipidemia and prevention of clinical ASCVD [16]. The committee concluded that ezetimibe added to statin therapy, bile acid sequestrants as monotherapy, and niacin as monotherapy all have some benefit for the prevention of clinical ASCVD. These guidelines also discuss the use of PCSK-9 inhibitors and their potential to decrease the risk of clinical ASCVD, but trials are currently ongoing to determine actual benefit. These guidelines address special populations but they do not consider the elderly in their recommendations. Currently, the only special populations included are patients with heart failure, those on hemodialysis, women who are of childbearing age or pregnant, and those with autoimmune diseases [16]. The literature available for each individual medication is discussed in further detail below.
Evidence for Secondary Prevention
The benefits of statin therapy for secondary prevention in the elderly is more established than it is for primary prevention (Table 2).
The ASCOT–LLA (Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm), published in 2003, evaluated the effect of atorvastatin 10 mg on reducing ASCVD events in moderate-risk patients between 40–79 years of age who had hypertension and normal or slightly elevated LDL-C levels, with at least 2 other risk factors for CVD (age > 55 years was considered a risk factor). The primary outcome was non-fatal MI including silent MI and fatal CHD. A significant reduction was seen in the primary endpoint. Over half of the study population was > 60 years of age, with a mean age of 63 years. In a post-hoc analysis, stroke prevention was found to be similar in patients who were > 70 years of age and those < 70 years of age [19].
One of the first trials to specifically analyze the impact of age on lipid-lowering therapy in secondary ASCVD prevention was the Scandinavian Simvastatin Survival Study (4S), published in 1994. They evaluated the effect of simvastatin 20 mg on CV-related mortality and morbidity in patients 35–70 years of age with hyperlipidemia and a history of angina or acute MI occurring > 6 months of the study starting. The primary outcome was all-cause mortality. The secondary endpoint was time to first major CV event, which included coronary death, non-fatal acute MI, resuscitated cardiac arrest, and silent MI. Simvastatin significantly reduced the primary outcome and CHD-related deaths. A subgroup analysis of the study population > 60 years of age showed that age made no significant impact on primary or secondary outcomes; however, investigators noted that these subgroup analyses had less statistical power than the population as a whole [20].
Published in 1998, the LIPID (Long-Term Intervention with Pravastatin in Ischemic Disease) study evaluated the effects of pravastatin 40 mg daily on CHD-related mortality and overall mortality in patients with hyperlipidemia and clinical ASCVD (previous MI or unstable angina). The primary outcome observed was fatal CHD. Pravastatin significantly reduced the primary outcome, overall mortality, and pre-specified CV events. In a subgroup analysis, age group ( 65, and > 70 years) had no significant impact on the combined outcome of death from CHD and nonfatal MI; however, patients 65 to 70 years of age made up less than half of the study population [21].
The Cholesterol and Recurrent Events (CARE) trial, published in 1996, looked at the effect of pravastatin 40mg therapy for secondary ASCVD prevention following an MI in patients who had average cholesterol levels (defined as TC < 240 mg/dL and LDL-C 115–174 mg/dL). The primary endpoint assessed was time to fatal CHD or nonfatal MI. To meet statistical power they looked at subgroups for a broader outcome of a major coronary event (including fatal CHD, nonfatal MI, bypass surgery, and angioplasty). Pravastatin significantly reduced the primary outcome. The significant reduction in coronary events produced by pravastatin was noted to be significantly greater in women and in patients with higher pretreatment levels of LDL-C, but was not significantly impacted by age group (24–59 vs. 60–75 years) [22].
The Heart Protection Study (HPS), published in 2002, looked at the long-term effects of lowering LDL-C with simvastatin 40 mg in patients 40 to 80 years of age at high risk for mortality due to either vascular or nonvascular causes. The primary outcome assessed was all-cause mortality, with fatal or nonfatal vascular events as co-primary outcomes for subcategory analyses. Simvastatin significantly reduced both primary and co-primary outcomes, but there was no significant difference when they looked at nonvascular mortality between groups. Neither age nor baseline LDL levels were reported to have had a significant impact on outcomes. Over half the population was > 65 years of age, and about one-third of the population was > 70 years of age [23].
The PROVE-IT/TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, published in 2004, compared pravastatin 40 mg (moderate-intensity) to atorvastatin 80 mg (high-intensity) for secondary ASCVD prevention in patients with recent acute coronary syndrome (ACS) 65 years of age and the mean age was 58 years [24].
The TNT (Treating to New Targets) trial, published in 2005, looked at secondary ASCVD prevention in regards to targeting LDL-C levels to < 100 mg/dL or < 70 mg/dL with atorvastatin 10 mg and atorvastatin 80 mg. Patients had stable coronary artery disease (CAD) and baseline LDL-C levels < 130 mg/dL. The primary endpoint was the occurrence of a CV event (CAD mortality, nonfatal MI not related to procedure, resuscitation after cardiac arrest, or fatal or nonfatal stroke). High-intensity atorvastatin (80 mg) significantly reduced the primary outcome. The mean age of the study population was approximately 61 years. The study reported no statistical interaction for age or sex in the primary outcome measure [25].
The Study Assessing Goals in the Elderly (SAGE), published in 2007, evaluated the effects of pravastatin 40 mg (moderate-intensity) vs atorvastatin 80 mg (high-intensity) on secondary ASCVD prevention in patients 65 to 85 years (mean age 72) with stable CHD, LDL-C 100–250 mg/dL, with at least 1 episode of myocardial ischemia with total ischemia duration > 3 minutes. The primary efficacy outcome observed was absolute change in total duration of myocardial ischemia on 48-hour ambulatory electrocardiographic monitoring from baseline to month 12. No significant difference was observed in efficacy between the two groups for the primary endpoint, but the intensive statin therapy group showed greater benefit respective to several secondary outcomes, including major acute CV events and death [26].
In summary, while these trials provide evidence that statin therapy is beneficial in a wide range of patients with clinical ASCVD and dyslipidemia, the trial data does not provide definitive guidance for treating elderly patients at this time. Given the small percentage of elderly patients that were included, some of the trial results reporting statistical significance in this age group hold less clinical significance. It appears that high-intensity statin therapy was more likely to effectively prevent clinical ASCVD and death than moderate-intensity statin therapy, but more evidence is needed regarding secondary prevention in patients over age 75.
Evidence for Primary Prevention
The PROSPER (PROspective Study of Pravastatin in the Elderly at Risk) was published in 2002 to assess the efficacy of pravastatin in patients between the ages of 70 and 82 (mean age 75 years) with pre-existing vascular disease (coronary, cerebral, or peripheral) or at an elevated risk (smoking, hypertension, or diabetes). Patients were randomized to receive either placebo or pravastatin 40 mg (a moderate-intensity statin). They found that pravastatin therapy reduced the risk of the composite outcome of CHD-related death, nonfatal MI, and fatal or nonfatal stroke in this elderly population. A post-hoc analysis comparing primary versus secondary prevention groups found no significant differences between these subgroups [7].
Han et al recently conducted a post hoc secondary analysis of older participants (65 years and older) in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial–Lipid-Lowering Trial (ALLHAT-LLT). The intervention for ALLHAT-LLT was 40 mg pravastatin. They found no significant differences in all-cause mortality or cardiovascular outcomes between the pravastatin and usual care groups [27]
JUPITER (Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin), published in 2008, examined the efficacy of rosuvastatin vs. placebo in low- to moderate-risk men 50 years and older and women 60 years and older using a composite outcome of MI, unstable angina, stroke, arterial revascularization, or CVD death. Rosuvastatin did significantly decrease the primary endpoint, however it did not reduce the risk of overall death [28]. A subgroup analysis was performed on the elderly (65–75 years) study participants in JUPITER demonstrating a significant risk reduction for the combined CV endpoint and a nonsignificant reduction of all-cause mortality [29].
CARDS (Collaborative Atorvastatin Diabetes Study), published in 2004, looked at statin use for primary prevention in high-risk patients with type 2 diabetes without high LDL-C, but they had to have at least 1 additional risk factor for CVD. The primary outcome was first acute CHD event (myocardial infarction including silent infarction, unstable angina, acute coronary heart disease death, resuscitated cardiac arrest), coronary revascularization procedures, or stroke. Atorvastatin 10 mg, a moderate-intensity statin, significantly decreased occurrence of the primary outcome [30]. A subgroup analysis was performed to evaluate patients specifically between the ages of 65 and 75 and found a similar outcome in the elderly with a significant reduction in first major CV event and stroke [31].
A recent study evaluating primary prevention in patients with an intermediate risk for CVD was the HOPE-3 (Heart Outcomes Prevention Evaluation), published in 2016. Two co-primary outcomes were evaluated: the composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, while the second primary outcome also included revascularization, heart failure, and resuscitated cardiac arrest. Rosuvastatin significantly decreased occurrence of both co-primary endpoints. About half of the study populations was over the age of 65 with a median age of 71 [32].
In addition to these trials of primary prevention, summarized in Table 3, a meta-analysis was published in 2013 to assess whether statins reduce all-cause mortality and CV events in elderly people without established CV disease.
As demonstrated by the above studies, it is evident that statins do help reduce the risk of CV events, regardless of statin intensity, but they do not consistently prevent death. However, the trials that did not demonstrate a significant outcome related to death utilized a moderate-intensity statin; if a high-intensity statin was used in those trials, there may have been a benefit [7,27]. More study is needed to evaluate the use of high-intensity statins in the elderly for the prevention of all-cause mortality and CV-related death.
Fortunately, the ongoing STAREE (STAtin Therapy for Reducing Events in the Elderly) study is looking specifically at the impact of statin therapy in adults 70 and older. Patients with a history of CVD or dementia are excluded. Results are set to be released in 2020 [34].
Risks of Using Statins in Older Adults
Statin use has been linked to a number of unwanted adverse effects.
Myalgia
Myalgia is variable but may occur in up to 25% of patients using statin therapy, and elderly patients typically experience more statin-associated myalgia than younger patients [35,36]. Elderly patients are more prone to decreased muscle mass and therefore may be at a higher risk of developing myalgia pain. Elderly patients are also utilizing more medications, leading to the potential for increased drug-drug interactions that could lead to myalgia. Elderly patients may also lose the function of drug metabolizing enzymes responsible for breaking down statin therapy, which may also increase the risk for statin-associated myalgia. One study demonstrated that elderly patients were more likely to discontinue statin therapy due to muscle pain and elderly patients reported more muscle side effects than their younger cohorts [37]. It is important to monitor for muscle pain and weakness in every patient. If they experience any myalgia, it is recommended to either lower the dose or discontinue the statin once it is determined to be statin-related. After myalgia resolves, therapy can be reinitiated at a lower dose or with a different statin if the patient is deemed high-risk. If creatine phosphokinase levels are greater than 10 times above the upper limit of normal, then discontinue the statin and wait for levels to return to normal. Re-initiation may be appropriate, but the the risks and benefits must be weighed. Simvastatin and atorvastatin are associated with higher rates of myalgia while pravastatin and rosuvastatin have the least myalgia pain associated with use [38,39].
Statin Intolerance
Statin intolerance, while not very common, is typically seen more often in special populations such as women, Asian patients, and the elderly. For a patient to be considered intolerant to statins, they need to have documented muscle symptoms or an elevated creatine phosphokinase level. Although not well defined, many clinicians consider improvement of symptoms with statin withdrawal as a diagnosis for statin intolerance. Typically patients are then rechallenged with 1 to 2 other statins and if still unable to tolerate, then different lipid-lowering therapies may be utilized [40]. In the elderly, it is important to rule out other causes for myalgia and monitor for significant drug interactions that may lead to muscle pain, particularly if the patient is requiring secondary prevention with statin therapy, before discontinuation.
Dementia
In 2012, the FDA issued a warning about the potential risk of cognitive impairment with the use of statins, which was based on case reports, not clinical trial data [41]. The NLA guidelines do not recommend baseline cognitive assessments prior to starting therapy and recommend that if patients do report cognitive impairment, other contributing factors and the risk associated with stopping statin therapy must be considered. Statin therapy may be discontinued to assess reversibility of symptoms, and if symptoms resolve, then it may be more beneficial to keep the patient off statin therapy. Clinicians may also consider lowering the dose or switching to another statin if they feel it is necessary for the patient to continue with a statin, particularly if the patient requires secondary prevention. Evidence suggests that statins are not associated with adverse effects on cognition and should not be withheld due to the potential for causing cognitive impairment alone [42]. The prevalence of cognitive impairment increases with age, so it is important for a clinician to rule out age-related processes or other disease states, such as Alzheimer’s, before discontinuation of previously tolerated statin therapy.
Renal Impairment
Kidney function must be evaluated prior to initiation of a statin in an elderly person as well as during the time the patient is taking a statin. Because statins are eliminated via the kidney, and because most elderly patients have decreased kidney function, the potential for drug build-up in the body is higher than in a younger patient and may lead to more adverse effects. Atorvastatin is the only option that does not require dose adjustment. All other statins should be adjusted based upon the level of renal impairment. The results from the SHARP study, published in 2011, showed that the combination of ezetimibe and simvastatin versus placebo significantly reduced ASCVD events in patients with moderate to severe chronic kidney disease, including those receiving dialysis. Specifically, this trial showed a significant reduction of ischemic events and occurrence of arterial revascularization procedures. Although the trial did not show a significant difference in incidence of MI or CHD-related mortality, the trial was not adequately powered to show differences in results among the individual ASCVD events and it is not clear whether the results can guide the use of statin therapy in all patients with chronic kidney disease [43]. Statins may be beneficial in renal insufficiency to lower LDL-C, but more studies are needed to assess CVD outcomes related to statin use in patients with a history of kidney disease [44].
Hepatic Function
Statins have been known to increase liver enzymes and in rare cases lead to liver injury, which typically has led to underutilization of therapy in clinical practice. Risk factors associated with this include preexisting hepatitis, advanced age, chronic alcohol use, and use of concomitant medications that may also cause hepatotoxicity, such as acetaminophen. When a statin-induced hepatic effect is suspected, it is important to first rule out other causes or disease states that may be undiagnosed. If no other cause can be found, clinicians may choose to reduce the statin dose, switch the statin, or discontinue the statin altogether if the risk outweighs the benefit. Additionally, statins do not have to be held in patients who have preexisting hepatic dysfunction if use is clearly indicated because the cardiovascular benefits typically outweigh the risks of causing liver injury. Clinical judgement is still warranted and patients with preexisting liver conditions should be monitored regularly [45].
Cost Considerations
Several studies have demonstrated that statin therapy, in the general population, is economical for both primary and secondary prevention of CVD [46,47]. The 4S study found simvastatin therapy to be cost-effective; for example, the cost per life year gained for a 70-year-old man with high chlesterol was $3800 [48]. In contrast, primary prevention in middle-aged men, based on the West of Scotland trial, averages about $35,000 per year of life gained [46]. In a 2015 study that utilized an established Markov simulation model, researchers studied adults 75 to 94 years and examined the cost-effectiveness of generic statins for primary prevention in this population. The authors estimated treating this population with statins over the next decade would be cost-effective. However, the researchers cautioned that the CV benefits and cost-effectiveness would be offset with even a modest increased risk of cognitive impairments or functional limitations. Statin use was not cost-effective in diabetes patients who did not have elevated LDL-C levels [49].
Non-Statin Therapies
Several other classes of medications are available for the management of hyperlipidemia; however, none of these lipid-lowering therapies have been found to reduce CVD events or mortality in the elderly population.
Ezetimibe
Ezetimibe blocks the absorption of intestinal cholesterol and is typically combined with statin therapy to lower LDL-C. Up until the IMPROVE-IT trial was published in 2015, ezetimibe did not have much use in clinical practice. This landmark trial was a large double-blind study that looked at secondary prevention in patients with ACS, comparing ezetimibe 10 mg and simvastatin 40 mg versus simvastatin 40 mg alone. The authors included patients over the age of 50 (mean age 64) with clinical ASCVD. They found that the addition of ezetimibe to simvastatin did reduce the primary composite outcome (CV mortality, major CV events, or nonfatal stroke) when compared to simvastatin alone [50]. This trial demonstrates clinical benefit with the addition of ezetimibe to statin therapy and adds additional evidence to support a target LDL-C of less than 70 mg/dL; however, the elderly population was not adequately represented in the study to allow extrapolation of these results to older patients.
PCSK-9 Inhibitors
The proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors are a newer class of monoclonal antibodies that were first approved by the US Food and Drug Administration in 2015. Alirocumab and evolocumab, both approved PCSK-9 inhibitors, bind to LDL receptors on the surface of hepatocytes and assist in the internalization of LDL receptors for lysosomal degradation. By inhibiting the binding of PCSK-9 to the LDL receptors, there is an overall increase in LDL receptors available on the cell surface to bind to LDL particles, thereby lowering LDL-C levels. Treatment with these agents are currently considered (in addition to diet and maximally tolerated statin therapy) in adult patients with heterozygous familial hypercholesterolemia or clinical ASCVD requiring further reduction in LDL-C. Two studies were published focusing on the use of PCSK-9 inhibitors: Open-label Study of Long-term Evaluation against LDL Cholesterol (OSLER) and the Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy (ODYSSEY LONG TERM). Overall, these studies demonstrated a 60% reduction of LDL-C among patients with high CVD risk on maximum-tolerated statin therapy. Furthermore, the ODYSSEY LONG TERM trial did find that the rate of major CVD adverse events was significantly lower with alirocumab added to maximum-tolerated statin therapy, with a hazard ratio of 0.52 [51].
One recent study of evolocumab, named the Further Cardiovascular OUtcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER), enrolled patients between the ages of 40 and 85 with 1 major CV risk factor or 2 minor CV risk factors. The primary endpoint was a composite of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization. Evolocumab lowered major CV events by roughly 15% when added to statin therapy in patients who were at high risk for clinical ASCVD. The mean age of the patients in the trial was 63; however, it is unclear how many of the study participants were elderly [52].
Unfortunately, the studies discussed above do not represent the elderly population well and the agents have not been studied long-term to determine the effects of continued use beyond 2 years. Long-term outcome studies are currently underway; however, it is unknown at this time whether elderly patients are being considered in these studies. It is known that genetic variation of the PCSK-9 locus does lower LDL-C in the elderly but does not significantly lower their risk of vascular disease [51]. At this time, until further evidence is available, we do not recommend the use of PCSK-9 inhibitors in elderly patients.
Nicotinic Acid
Nicotinic acid (Niacin, Niacin ER), also known as vitamin B3, has been utilized for decades as a vitamin supplement, an anti-wrinkle agent, and is known to have neuroprotective effects. It has also been utilized for dyslipidemia and has had some benefits when used alone to decrease cardiovascular disease [53]. Unfortunately the Coronary Drug Project was completed in the 1980s and did not incorporate patients over the age of 64, therefore making the results difficult to apply to elderly patients today [54]. Other literature has been published in recent years to refute that study, claiming there is no additional benefit to using niacin for cardiovascular protection and these studies have included elderly patients. In the AIM-HIGH trial, published in 2011, approximately 46% of the patients were 65 or older. Patients who were previously taking statin therapy that had known cardiovascular disease were enrolled. Niacin added to simvastatin 40–80 mg lowered LDL-C, triglycerides, and increased HDL-C, but the addition of niacin was not proven to help lower the risk of cardiovascular events [55]. The HPS2-THRIVE study enrolled patients with known cardiovascular disease between the ages of 50 and 80 years and found no benefit in preventing CVD when adding niacin to statin therapy [56]. With its side effect profile, risk for increased glucose intolerance, and lack of evidence to demonstrate benefit for prevention of CV events, we do not recommend niacin for use in the elderly at this time.
Bile Acid Sequestrants
The ATP III guidelines [57] noted that when statins are not sufficient to lower high cholesterol, bile acid sequestrants also known as resins could be added. More recently, the 2016 ACC expert consensus on non-statin therapies for LDL-C lowering [16] stated resins may be considered in select circumstances as a second-line agent for adults with ezetimibe intolerance and with triglycerides
Fibrates
While fibrates (gemfibrozil, fenofibrate, clofibrate) have not been studied to demonstrate a reduction in CVD or CVD mortality in the elderly population, this medication class is beneficial in patients with hypertriglyceridemia to lower triglyceride levels and prevent pancreatitis. Fibrates are recommended for patients with triglyceride levels approaching 500 mg/dL. Fibrates can also increase high-density lipoproteins, which tend to be lower in the elderly population and considered a risk factor for CVD. Gemfibrozil is not recommended in combination with statin therapy due to an increased risk of myalgia. Fenofibrate is the drug of choice, particularly for diabetic patients with very uncontrolled triglyceride levels because it will not affect glucose levels [57]. At this time, we do not recommend the use of fibrates in the elderly population unless they are at risk for developing pancreatitis and have elevated triglyceride levels.
Patient-Centered Care
Evidence-based medicine can aid in making sound clinical decisions for proper patient care; however, treatment plans should consider the individual patient’s perspectives and needs, beliefs, expectations, and goals. In the elderly population, we must also consider factors such as finances, pill-burden, drug-drug interactions, physiological needs, comorbid disease states, and overall life expectancy. In addition, the elderly population is physiologically heterogeneous group and recommendations for therapy need to be individualized. Chronological age does not necessarily correspond to vascular age and risk factors for cardiovascular disease do not predict outcomes as well in the elderly as they do in younger patients. While older patients may view having to take 1 less medication as more important than preventing a heart attack or stroke at the age of 80, it is advisable to discuss all potential outcomes related to morbidity associated with the occurrence of an MI or stroke due to the lack of statin therapy. Additionally, pharmacists can play a vital role in evaluating elderly patients and their medication regimens. Elderly patients should undergo a medication reconciliation at each visit to evaluate drug-drug interactions, side effects, and potentially harmful medication combinations that may lead to increased adverse drug outcomes.
Conclusion
CHD increases with age, and most patients who have a CV event are more likely to die with advancing age. Based on the the limited available evidence, statin therapy is beneficial in the elderly population in reducing overall CV morbidity. We recommend beginning with with a moderate-intensity statin and adjusting accordingly. High-intensity statin therapy appears to be effective for elderly patients for secondary prevention, but clinicians should use clinical judgment and monitor for adverse events, particularly myalgia pain. At this time, we are unable to determine if non-statin therapies for the elderly would be beneficial and do not recommend their use unless the patient is at risk for pancreatitis, in which case a fenofibrate is recommended.
Corresponding author: Nicole A. Slater, PharmD, BCACP, Auburn University, Harrison School of Pharmacy, 650 Clinic Dr., Mobile, AL 36688.
Financial disclosures: None.
1. Fact sheet: Aging in the United States. Accessed at www.prb.org/aging-unitedstates-fact-sheet/.
2. Kontis JE, Bennett CD, Mathers G, et al. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet 2017;389:1323–35.
3. Odden MD, Coxson PG, Moran A, et al. The impact of the aging population on coronary heart disease in the United States. Am J Med 2011;124:827–33.
4. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017;135:e1–e458.
5. Mills EJ, Rachlis B, Wu P, et al. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Amer Col Cardiol 2008;52:1769–81.
6. Stone NJ. Statins in secondary prevention: intensity matters. J Am Coll Cardiol 2017;69: 2707–9.
7. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–30.
8. Heart Protection Study Collaborative Group Writers. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.
9. Lefevre F, Nishida L. Special report: the efficacy and safety of statins in the elderly. TEC Assessment Program 2007;21
10. Pravastatin benefits elderly patients: results of PROSPER study. Cardiovasc J S Afr 2003;14:48.
11. Catapano AL, Graham I, Backer GD, et al. ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058.
12. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934.
13. Jacobson TA, Ito MK, Maki K, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1-full report. J Clin Lipidol 2015;9:129-169
14. Jacobson TA, Maki KC, Orringer CE, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol 2015;9:S1–22.e1.
15. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologist and american college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 2017;23:1–87.
16. Lloyd-Jones DM, Morris PB, Minissian MB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 2016;68:92–125.
17. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–59.
18. Chaturvedi S, Zivin J, Breazna A, et al. Effect of atorvastatin in elderly patients with a recent stroke or transient ischemic attack. Neurology 2009;72:688–94.
19. Sever PS, Dahior B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian cardiac outcomes trial—lipid lowering arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149–58.
20. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383–9.
21. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349–57.
22. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin of coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001–09.
23. Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.
24. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–04.
25. LaRosa JC, Grundy SM, Waters DD, et al; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–35.
26. Deedwania P, Stone PH, Bairey CN, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the study assessing goals in the elderly (SAGE). Circulation 2007;115:700–7.
27. Han BH, Sutin D, Williamson JD, et al; ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: the ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017;177:955–65.
28. Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C Reactive protein. N Engl J Med 2008; 359:2195–207.
29. Glynn RJ, Koenig W, Nordestgaard BG, et al. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: Exploratory analysis of a randomized trial. Ann Intern Med 2010;152:488–96.
30. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
31. Neil HA, DeMicco DA, Luo D, et al. Analysis of efficacy and safety in patients aged 65-75 years at randomization: collaborative atorvastatin diabetes study (CARDS). Diabetes Care 2006;29:2378–84.
32. Yusuf S, Bosch J, Dagenais G, et al; HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–31.
33. Savarese G, Gotta AM Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol 2013;62:2090–99.
34. National Institute of Health. A clinical trial of STAtin therapy for reducing events in the elderly (STAREE). Clinical Trials. https://clinicaltrials.gov/ct2/show/NCT02099123. Accessed June 5, 2018.
35. Gaist D, Rodríquez, LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001;12:565–9.
36. Pasternak RC., Smith SC Jr, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002;40:567–72.
37. Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding statin use in America and gaps in patient education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012;6:208–15.
38. Harper CR, Jacobson TA. Evidence-based management of statin myopathy. Curr Atheroscler Rep 2010;12:322–30.
39. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005;19:403–14.
40. Ahmad Z. Statin intolerance. Am J Cardiol 2014;113:1765–71.
41. Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Published February 28, 2012. Accessed June 5, 2018.
42. Gauthier JM, Massicotte A. Statins and their effect on cognition: let’s clear up the confusion. Can Pharm J (Ott) 2015;148:150–55.
43. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92.
44. Vaziri ND, Anzalone DA, Catini J. Statins in chronic didney disease: when and when not to use them. J Fam Pract 2016;65:8 Suppl. www.mdedge.com/jfp/custom/statins-chronic-kidney-disease-when-and-when-not-use-them-1
45. Jose J. Statins and its hepatic effects: newer data, implications, and changing recommendations. J Pharm Bioallied Sci 2016;8:23–8.
46. Caro J, Klittich W, McGuire A, et al. The West of Scotland coronary prevention study: Economic benefit analysis of primary prevention with pravastatin. BMJ 1997;315:1577–82.
47. Schectman G, Wolff N, Byrd JC, et al. Physician extenders for cost-effective management of hypercholesterolemia. J Gen Intern Med 1996;11:277–86.
48. Johannesson M, Jonsson B, Kjekshus J, et al. Cost effectiveness of simvastatin treatment to lower cholesterol levels in patients with coronary heart disease. Scandinavian simvastatin survival study group. N Engl J Med 1997;336:332–6.
49. Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015;162:533–41.
50. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97.
51. Polisecki E, Peter I, Robertson M, et al. Genetic variation at the PCSK9 locus moderately lowers low-density lipoprotein cholesterol levels, but does not significantly lower vascular disease risk in an elderly population. Atherosclerosis 2008;200:95–101.
52. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22.
53. Sinthupoom N, Prachayasittikul V, Prachayasittikul S, et al. Nicotinic acid and derivatives as multifunctional pharmacophores for medical applications. Eur Food Res Technol 2015;240: 1–17.
54. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245–55.
55. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67.
56. HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–12.
57. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002;106:3143–421.
58. The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351–64.
From the Harrison School of Pharmacy, Auburn University, Mobile, AL.
Abstract
- Objective: To summarize the literature relevant to managing dyslipidemia in the elderly and review recommendations for initiating lipid-lowering therapy.
- Methods: Review of the literature.
- Results: Statins are the most commonly utilized medication class for lipid-lowering in the general population, and they are recommended for primary prevention in patients between the ages of 40 to 75 with at least 1 risk factor for cardiovascular disease as well as for any patient needing secondary prevention. In the elderly, statins may be appropriate for both primary and secondary prevention if the benefits outweigh the risks. Based on the available evidence, it is safe to recommend statin therapy to elderly patients who require secondary prevention given the known benefits in reducing cardiovascular morbidity and mortality for patients up to the age of 80. For primary prevention, statin therapy may be beneficial, but one must carefully evaluate for comorbid conditions, life expectancy, concomitant medications, overall health status, frailty, and patient or family preference. Several other classes of lipid-lowering agents exist; however, there is not enough evidence for us to recommend use in the elderly population for cardiovascular risk reduction in either primary or secondary scenarios.
- Conclusion: Although clinical research in the elderly population is limited, evidence supports the use of statins in elderly patients for secondary prevention and in patients up to age 75 for primary prevention; however clinicians must use clinical judgement and take into consideration the patient’s situation regarding comorbidities, polypharmacy, and possible adverse effects. More high-quality evidence is necessary.
Key words: hyperlipidemia; geriatrics; elderly; patient-centered care; statin; cardiovascular disease.
The number of Americans age 65 years and older is projected to more than double, from 46 million today to over 98 million by 2060, and the 65-and-older age group’s share of the total population will rise to nearly 24% [1]. Life expectancy is now predicted to be > 20 years for women at age 65 and > 17 years for men at age 65 in many high-income countries, including the United States [2]. This demographic shift toward an older population will result in a higher burden of coronary heart disease and stroke, with atherosclerotic cardiovascular disease (ASCVD) prevalence and costs projected to increase substantially [3].
Among adults seeking medical care in the United States, roughly 95 million have a total cholesterol (TC) level of ≥ 200 mg/dL or more, and approximately 29 million have a TC > 240 mg/dL [4]. Cholesterol screening is important since most patients suffering from dyslipidemia are asymptomatic. Dyslipidemia is a major risk factor for the development of atherosclerotic disease. Because of the complications associated with dyslipidemia, it is vital that patients are provided with primary and/or secondary prevention strategies to reduce the risk of cardiovascular disease (CVD) and protect high-risk patients from recurring events. A clinical controversy exists surrounding the elderly population, concerning whether or not clinicians should be providing lipid-lowering treatment to this group of individuals for dyslipidemia. The evidence is limited for patients over age 65, and even more so for the very elderly (> 80 years); therefore, it is necessary to review the available evidence to make an appropriate decision when it comes to managing dyslipidemia in the elderly population
Currently, HMG-CoA reductase inhibitors (statins) are the only known class of medications for the treatment of dyslipidemia that will prevent both primary and secondary cardiovascular (CV) events, including death. Statin intensity (Table 1)
Guideline Recommendations
Current guidelines differ in their recommendations for treating dyslipidemia in the elderly population. In 2016, the Task Force for the Management of Dyslipidemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) released updated guidelines for managing dyslipidemia. These guidelines recommend that older patients with established CVD be treated in the same way as younger patients because of the many benefits statin therapy demonstrated in clinical trials. They also suggest that statin therapy be started at a lower doses to achieve goals for primary prevention in the older population. In addition, CVD risk factors (hypertension, diabetes, dyslipidemia, smoking) should be addressed in this population to reduce CVD risk. They also acknowledged that primary prevention may not prolong life in the older adult, but treatment does reduce cardiovascular mortality and statin therapy is recommended to reduce the overall risk of CV morbidity in this population [11]. In contrast, The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines changed the management and treatment of dyslipidemia by highlighting “statin benefit groups” rather than recommending a treat-to-target goal as guidelines had done for many years. ACC/AHA recommends a moderate-intensity statin for patients > 75 years of age for secondary prevention versus the use of a high-intensity statin for patients who are between the ages of 40 and 75 based on the pooled cohort risk equation. In patients over age 75 with no history of CVD, no specific recommendation is available for the use of lipid-lowering therapy at this time [12]. ACC/AHA is expected to publish a new set of guidelines sometime in 2018 and they are projected to utilize lipid-lowering goals in combination with the pooled cohort equation to assess overall risk in patients with dyslipidemia.
The 2015 National Lipid Association (NLA) released “Part 1” guidelines for the management of dyslipidemia and then provided “Part 2” about a year later, which focuses on management for special populations. To summarize, the NLA guidelines recommend that elderly patients between the ages of 65 and 80 receive a high-intensity statin for secondary prevention after special consideration of the potential risks and benefits. In patients over the age of 80, NLA recommends a moderate-intensity statin for secondary prevention. For primary prevention, NLA recommends utilizing the pooled cohort risk equation to analyze patient characteristics, keeping in mind that age is a driving factor for increased risk of CVD and that the actual risk for developing a CV event may be “overestimated” if the patient has no other risk factors other than their age. When evaluating patients between the ages of 65 and 79 for primary prevention, NLA suggests following Part 1 of the guidelines. In Part 1, NLA recommends evaluating the patient’s characteristics and suggests a moderate- or high-intensity statin if the patient is considered “very high risk” or “high risk” and a moderate-intensity statin for patients who are considered “moderate risk”. For patients over the age of 80, they recommend utilizing a moderate- or a low-intensity statin depending on frailty status or if significant comorbidities or polypharmacy exist [13,14].
In 2017, the American Association of Clinical Endocrinologist (AACE) released guidelines for the management of dyslipidemia and CVD prevention. AACE recommends that patients over age 65 be screened for dyslipidemia, and those who have multiple risk factors, other than age, should be considered for treatment with lipid-lowering therapy. AACE focuses on specific target LDL-C levels as treatment goals [15].
In addition to statins, other lipid-lowering therapies are used to treat dyslipidemia. The 2016 American College of Cardiology (ACC) Task Force reported on the use of non-statin therapies for the management of dyslipidemia and prevention of clinical ASCVD [16]. The committee concluded that ezetimibe added to statin therapy, bile acid sequestrants as monotherapy, and niacin as monotherapy all have some benefit for the prevention of clinical ASCVD. These guidelines also discuss the use of PCSK-9 inhibitors and their potential to decrease the risk of clinical ASCVD, but trials are currently ongoing to determine actual benefit. These guidelines address special populations but they do not consider the elderly in their recommendations. Currently, the only special populations included are patients with heart failure, those on hemodialysis, women who are of childbearing age or pregnant, and those with autoimmune diseases [16]. The literature available for each individual medication is discussed in further detail below.
Evidence for Secondary Prevention
The benefits of statin therapy for secondary prevention in the elderly is more established than it is for primary prevention (Table 2).
The ASCOT–LLA (Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm), published in 2003, evaluated the effect of atorvastatin 10 mg on reducing ASCVD events in moderate-risk patients between 40–79 years of age who had hypertension and normal or slightly elevated LDL-C levels, with at least 2 other risk factors for CVD (age > 55 years was considered a risk factor). The primary outcome was non-fatal MI including silent MI and fatal CHD. A significant reduction was seen in the primary endpoint. Over half of the study population was > 60 years of age, with a mean age of 63 years. In a post-hoc analysis, stroke prevention was found to be similar in patients who were > 70 years of age and those < 70 years of age [19].
One of the first trials to specifically analyze the impact of age on lipid-lowering therapy in secondary ASCVD prevention was the Scandinavian Simvastatin Survival Study (4S), published in 1994. They evaluated the effect of simvastatin 20 mg on CV-related mortality and morbidity in patients 35–70 years of age with hyperlipidemia and a history of angina or acute MI occurring > 6 months of the study starting. The primary outcome was all-cause mortality. The secondary endpoint was time to first major CV event, which included coronary death, non-fatal acute MI, resuscitated cardiac arrest, and silent MI. Simvastatin significantly reduced the primary outcome and CHD-related deaths. A subgroup analysis of the study population > 60 years of age showed that age made no significant impact on primary or secondary outcomes; however, investigators noted that these subgroup analyses had less statistical power than the population as a whole [20].
Published in 1998, the LIPID (Long-Term Intervention with Pravastatin in Ischemic Disease) study evaluated the effects of pravastatin 40 mg daily on CHD-related mortality and overall mortality in patients with hyperlipidemia and clinical ASCVD (previous MI or unstable angina). The primary outcome observed was fatal CHD. Pravastatin significantly reduced the primary outcome, overall mortality, and pre-specified CV events. In a subgroup analysis, age group ( 65, and > 70 years) had no significant impact on the combined outcome of death from CHD and nonfatal MI; however, patients 65 to 70 years of age made up less than half of the study population [21].
The Cholesterol and Recurrent Events (CARE) trial, published in 1996, looked at the effect of pravastatin 40mg therapy for secondary ASCVD prevention following an MI in patients who had average cholesterol levels (defined as TC < 240 mg/dL and LDL-C 115–174 mg/dL). The primary endpoint assessed was time to fatal CHD or nonfatal MI. To meet statistical power they looked at subgroups for a broader outcome of a major coronary event (including fatal CHD, nonfatal MI, bypass surgery, and angioplasty). Pravastatin significantly reduced the primary outcome. The significant reduction in coronary events produced by pravastatin was noted to be significantly greater in women and in patients with higher pretreatment levels of LDL-C, but was not significantly impacted by age group (24–59 vs. 60–75 years) [22].
The Heart Protection Study (HPS), published in 2002, looked at the long-term effects of lowering LDL-C with simvastatin 40 mg in patients 40 to 80 years of age at high risk for mortality due to either vascular or nonvascular causes. The primary outcome assessed was all-cause mortality, with fatal or nonfatal vascular events as co-primary outcomes for subcategory analyses. Simvastatin significantly reduced both primary and co-primary outcomes, but there was no significant difference when they looked at nonvascular mortality between groups. Neither age nor baseline LDL levels were reported to have had a significant impact on outcomes. Over half the population was > 65 years of age, and about one-third of the population was > 70 years of age [23].
The PROVE-IT/TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, published in 2004, compared pravastatin 40 mg (moderate-intensity) to atorvastatin 80 mg (high-intensity) for secondary ASCVD prevention in patients with recent acute coronary syndrome (ACS) 65 years of age and the mean age was 58 years [24].
The TNT (Treating to New Targets) trial, published in 2005, looked at secondary ASCVD prevention in regards to targeting LDL-C levels to < 100 mg/dL or < 70 mg/dL with atorvastatin 10 mg and atorvastatin 80 mg. Patients had stable coronary artery disease (CAD) and baseline LDL-C levels < 130 mg/dL. The primary endpoint was the occurrence of a CV event (CAD mortality, nonfatal MI not related to procedure, resuscitation after cardiac arrest, or fatal or nonfatal stroke). High-intensity atorvastatin (80 mg) significantly reduced the primary outcome. The mean age of the study population was approximately 61 years. The study reported no statistical interaction for age or sex in the primary outcome measure [25].
The Study Assessing Goals in the Elderly (SAGE), published in 2007, evaluated the effects of pravastatin 40 mg (moderate-intensity) vs atorvastatin 80 mg (high-intensity) on secondary ASCVD prevention in patients 65 to 85 years (mean age 72) with stable CHD, LDL-C 100–250 mg/dL, with at least 1 episode of myocardial ischemia with total ischemia duration > 3 minutes. The primary efficacy outcome observed was absolute change in total duration of myocardial ischemia on 48-hour ambulatory electrocardiographic monitoring from baseline to month 12. No significant difference was observed in efficacy between the two groups for the primary endpoint, but the intensive statin therapy group showed greater benefit respective to several secondary outcomes, including major acute CV events and death [26].
In summary, while these trials provide evidence that statin therapy is beneficial in a wide range of patients with clinical ASCVD and dyslipidemia, the trial data does not provide definitive guidance for treating elderly patients at this time. Given the small percentage of elderly patients that were included, some of the trial results reporting statistical significance in this age group hold less clinical significance. It appears that high-intensity statin therapy was more likely to effectively prevent clinical ASCVD and death than moderate-intensity statin therapy, but more evidence is needed regarding secondary prevention in patients over age 75.
Evidence for Primary Prevention
The PROSPER (PROspective Study of Pravastatin in the Elderly at Risk) was published in 2002 to assess the efficacy of pravastatin in patients between the ages of 70 and 82 (mean age 75 years) with pre-existing vascular disease (coronary, cerebral, or peripheral) or at an elevated risk (smoking, hypertension, or diabetes). Patients were randomized to receive either placebo or pravastatin 40 mg (a moderate-intensity statin). They found that pravastatin therapy reduced the risk of the composite outcome of CHD-related death, nonfatal MI, and fatal or nonfatal stroke in this elderly population. A post-hoc analysis comparing primary versus secondary prevention groups found no significant differences between these subgroups [7].
Han et al recently conducted a post hoc secondary analysis of older participants (65 years and older) in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial–Lipid-Lowering Trial (ALLHAT-LLT). The intervention for ALLHAT-LLT was 40 mg pravastatin. They found no significant differences in all-cause mortality or cardiovascular outcomes between the pravastatin and usual care groups [27]
JUPITER (Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin), published in 2008, examined the efficacy of rosuvastatin vs. placebo in low- to moderate-risk men 50 years and older and women 60 years and older using a composite outcome of MI, unstable angina, stroke, arterial revascularization, or CVD death. Rosuvastatin did significantly decrease the primary endpoint, however it did not reduce the risk of overall death [28]. A subgroup analysis was performed on the elderly (65–75 years) study participants in JUPITER demonstrating a significant risk reduction for the combined CV endpoint and a nonsignificant reduction of all-cause mortality [29].
CARDS (Collaborative Atorvastatin Diabetes Study), published in 2004, looked at statin use for primary prevention in high-risk patients with type 2 diabetes without high LDL-C, but they had to have at least 1 additional risk factor for CVD. The primary outcome was first acute CHD event (myocardial infarction including silent infarction, unstable angina, acute coronary heart disease death, resuscitated cardiac arrest), coronary revascularization procedures, or stroke. Atorvastatin 10 mg, a moderate-intensity statin, significantly decreased occurrence of the primary outcome [30]. A subgroup analysis was performed to evaluate patients specifically between the ages of 65 and 75 and found a similar outcome in the elderly with a significant reduction in first major CV event and stroke [31].
A recent study evaluating primary prevention in patients with an intermediate risk for CVD was the HOPE-3 (Heart Outcomes Prevention Evaluation), published in 2016. Two co-primary outcomes were evaluated: the composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, while the second primary outcome also included revascularization, heart failure, and resuscitated cardiac arrest. Rosuvastatin significantly decreased occurrence of both co-primary endpoints. About half of the study populations was over the age of 65 with a median age of 71 [32].
In addition to these trials of primary prevention, summarized in Table 3, a meta-analysis was published in 2013 to assess whether statins reduce all-cause mortality and CV events in elderly people without established CV disease.
As demonstrated by the above studies, it is evident that statins do help reduce the risk of CV events, regardless of statin intensity, but they do not consistently prevent death. However, the trials that did not demonstrate a significant outcome related to death utilized a moderate-intensity statin; if a high-intensity statin was used in those trials, there may have been a benefit [7,27]. More study is needed to evaluate the use of high-intensity statins in the elderly for the prevention of all-cause mortality and CV-related death.
Fortunately, the ongoing STAREE (STAtin Therapy for Reducing Events in the Elderly) study is looking specifically at the impact of statin therapy in adults 70 and older. Patients with a history of CVD or dementia are excluded. Results are set to be released in 2020 [34].
Risks of Using Statins in Older Adults
Statin use has been linked to a number of unwanted adverse effects.
Myalgia
Myalgia is variable but may occur in up to 25% of patients using statin therapy, and elderly patients typically experience more statin-associated myalgia than younger patients [35,36]. Elderly patients are more prone to decreased muscle mass and therefore may be at a higher risk of developing myalgia pain. Elderly patients are also utilizing more medications, leading to the potential for increased drug-drug interactions that could lead to myalgia. Elderly patients may also lose the function of drug metabolizing enzymes responsible for breaking down statin therapy, which may also increase the risk for statin-associated myalgia. One study demonstrated that elderly patients were more likely to discontinue statin therapy due to muscle pain and elderly patients reported more muscle side effects than their younger cohorts [37]. It is important to monitor for muscle pain and weakness in every patient. If they experience any myalgia, it is recommended to either lower the dose or discontinue the statin once it is determined to be statin-related. After myalgia resolves, therapy can be reinitiated at a lower dose or with a different statin if the patient is deemed high-risk. If creatine phosphokinase levels are greater than 10 times above the upper limit of normal, then discontinue the statin and wait for levels to return to normal. Re-initiation may be appropriate, but the the risks and benefits must be weighed. Simvastatin and atorvastatin are associated with higher rates of myalgia while pravastatin and rosuvastatin have the least myalgia pain associated with use [38,39].
Statin Intolerance
Statin intolerance, while not very common, is typically seen more often in special populations such as women, Asian patients, and the elderly. For a patient to be considered intolerant to statins, they need to have documented muscle symptoms or an elevated creatine phosphokinase level. Although not well defined, many clinicians consider improvement of symptoms with statin withdrawal as a diagnosis for statin intolerance. Typically patients are then rechallenged with 1 to 2 other statins and if still unable to tolerate, then different lipid-lowering therapies may be utilized [40]. In the elderly, it is important to rule out other causes for myalgia and monitor for significant drug interactions that may lead to muscle pain, particularly if the patient is requiring secondary prevention with statin therapy, before discontinuation.
Dementia
In 2012, the FDA issued a warning about the potential risk of cognitive impairment with the use of statins, which was based on case reports, not clinical trial data [41]. The NLA guidelines do not recommend baseline cognitive assessments prior to starting therapy and recommend that if patients do report cognitive impairment, other contributing factors and the risk associated with stopping statin therapy must be considered. Statin therapy may be discontinued to assess reversibility of symptoms, and if symptoms resolve, then it may be more beneficial to keep the patient off statin therapy. Clinicians may also consider lowering the dose or switching to another statin if they feel it is necessary for the patient to continue with a statin, particularly if the patient requires secondary prevention. Evidence suggests that statins are not associated with adverse effects on cognition and should not be withheld due to the potential for causing cognitive impairment alone [42]. The prevalence of cognitive impairment increases with age, so it is important for a clinician to rule out age-related processes or other disease states, such as Alzheimer’s, before discontinuation of previously tolerated statin therapy.
Renal Impairment
Kidney function must be evaluated prior to initiation of a statin in an elderly person as well as during the time the patient is taking a statin. Because statins are eliminated via the kidney, and because most elderly patients have decreased kidney function, the potential for drug build-up in the body is higher than in a younger patient and may lead to more adverse effects. Atorvastatin is the only option that does not require dose adjustment. All other statins should be adjusted based upon the level of renal impairment. The results from the SHARP study, published in 2011, showed that the combination of ezetimibe and simvastatin versus placebo significantly reduced ASCVD events in patients with moderate to severe chronic kidney disease, including those receiving dialysis. Specifically, this trial showed a significant reduction of ischemic events and occurrence of arterial revascularization procedures. Although the trial did not show a significant difference in incidence of MI or CHD-related mortality, the trial was not adequately powered to show differences in results among the individual ASCVD events and it is not clear whether the results can guide the use of statin therapy in all patients with chronic kidney disease [43]. Statins may be beneficial in renal insufficiency to lower LDL-C, but more studies are needed to assess CVD outcomes related to statin use in patients with a history of kidney disease [44].
Hepatic Function
Statins have been known to increase liver enzymes and in rare cases lead to liver injury, which typically has led to underutilization of therapy in clinical practice. Risk factors associated with this include preexisting hepatitis, advanced age, chronic alcohol use, and use of concomitant medications that may also cause hepatotoxicity, such as acetaminophen. When a statin-induced hepatic effect is suspected, it is important to first rule out other causes or disease states that may be undiagnosed. If no other cause can be found, clinicians may choose to reduce the statin dose, switch the statin, or discontinue the statin altogether if the risk outweighs the benefit. Additionally, statins do not have to be held in patients who have preexisting hepatic dysfunction if use is clearly indicated because the cardiovascular benefits typically outweigh the risks of causing liver injury. Clinical judgement is still warranted and patients with preexisting liver conditions should be monitored regularly [45].
Cost Considerations
Several studies have demonstrated that statin therapy, in the general population, is economical for both primary and secondary prevention of CVD [46,47]. The 4S study found simvastatin therapy to be cost-effective; for example, the cost per life year gained for a 70-year-old man with high chlesterol was $3800 [48]. In contrast, primary prevention in middle-aged men, based on the West of Scotland trial, averages about $35,000 per year of life gained [46]. In a 2015 study that utilized an established Markov simulation model, researchers studied adults 75 to 94 years and examined the cost-effectiveness of generic statins for primary prevention in this population. The authors estimated treating this population with statins over the next decade would be cost-effective. However, the researchers cautioned that the CV benefits and cost-effectiveness would be offset with even a modest increased risk of cognitive impairments or functional limitations. Statin use was not cost-effective in diabetes patients who did not have elevated LDL-C levels [49].
Non-Statin Therapies
Several other classes of medications are available for the management of hyperlipidemia; however, none of these lipid-lowering therapies have been found to reduce CVD events or mortality in the elderly population.
Ezetimibe
Ezetimibe blocks the absorption of intestinal cholesterol and is typically combined with statin therapy to lower LDL-C. Up until the IMPROVE-IT trial was published in 2015, ezetimibe did not have much use in clinical practice. This landmark trial was a large double-blind study that looked at secondary prevention in patients with ACS, comparing ezetimibe 10 mg and simvastatin 40 mg versus simvastatin 40 mg alone. The authors included patients over the age of 50 (mean age 64) with clinical ASCVD. They found that the addition of ezetimibe to simvastatin did reduce the primary composite outcome (CV mortality, major CV events, or nonfatal stroke) when compared to simvastatin alone [50]. This trial demonstrates clinical benefit with the addition of ezetimibe to statin therapy and adds additional evidence to support a target LDL-C of less than 70 mg/dL; however, the elderly population was not adequately represented in the study to allow extrapolation of these results to older patients.
PCSK-9 Inhibitors
The proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors are a newer class of monoclonal antibodies that were first approved by the US Food and Drug Administration in 2015. Alirocumab and evolocumab, both approved PCSK-9 inhibitors, bind to LDL receptors on the surface of hepatocytes and assist in the internalization of LDL receptors for lysosomal degradation. By inhibiting the binding of PCSK-9 to the LDL receptors, there is an overall increase in LDL receptors available on the cell surface to bind to LDL particles, thereby lowering LDL-C levels. Treatment with these agents are currently considered (in addition to diet and maximally tolerated statin therapy) in adult patients with heterozygous familial hypercholesterolemia or clinical ASCVD requiring further reduction in LDL-C. Two studies were published focusing on the use of PCSK-9 inhibitors: Open-label Study of Long-term Evaluation against LDL Cholesterol (OSLER) and the Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy (ODYSSEY LONG TERM). Overall, these studies demonstrated a 60% reduction of LDL-C among patients with high CVD risk on maximum-tolerated statin therapy. Furthermore, the ODYSSEY LONG TERM trial did find that the rate of major CVD adverse events was significantly lower with alirocumab added to maximum-tolerated statin therapy, with a hazard ratio of 0.52 [51].
One recent study of evolocumab, named the Further Cardiovascular OUtcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER), enrolled patients between the ages of 40 and 85 with 1 major CV risk factor or 2 minor CV risk factors. The primary endpoint was a composite of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization. Evolocumab lowered major CV events by roughly 15% when added to statin therapy in patients who were at high risk for clinical ASCVD. The mean age of the patients in the trial was 63; however, it is unclear how many of the study participants were elderly [52].
Unfortunately, the studies discussed above do not represent the elderly population well and the agents have not been studied long-term to determine the effects of continued use beyond 2 years. Long-term outcome studies are currently underway; however, it is unknown at this time whether elderly patients are being considered in these studies. It is known that genetic variation of the PCSK-9 locus does lower LDL-C in the elderly but does not significantly lower their risk of vascular disease [51]. At this time, until further evidence is available, we do not recommend the use of PCSK-9 inhibitors in elderly patients.
Nicotinic Acid
Nicotinic acid (Niacin, Niacin ER), also known as vitamin B3, has been utilized for decades as a vitamin supplement, an anti-wrinkle agent, and is known to have neuroprotective effects. It has also been utilized for dyslipidemia and has had some benefits when used alone to decrease cardiovascular disease [53]. Unfortunately the Coronary Drug Project was completed in the 1980s and did not incorporate patients over the age of 64, therefore making the results difficult to apply to elderly patients today [54]. Other literature has been published in recent years to refute that study, claiming there is no additional benefit to using niacin for cardiovascular protection and these studies have included elderly patients. In the AIM-HIGH trial, published in 2011, approximately 46% of the patients were 65 or older. Patients who were previously taking statin therapy that had known cardiovascular disease were enrolled. Niacin added to simvastatin 40–80 mg lowered LDL-C, triglycerides, and increased HDL-C, but the addition of niacin was not proven to help lower the risk of cardiovascular events [55]. The HPS2-THRIVE study enrolled patients with known cardiovascular disease between the ages of 50 and 80 years and found no benefit in preventing CVD when adding niacin to statin therapy [56]. With its side effect profile, risk for increased glucose intolerance, and lack of evidence to demonstrate benefit for prevention of CV events, we do not recommend niacin for use in the elderly at this time.
Bile Acid Sequestrants
The ATP III guidelines [57] noted that when statins are not sufficient to lower high cholesterol, bile acid sequestrants also known as resins could be added. More recently, the 2016 ACC expert consensus on non-statin therapies for LDL-C lowering [16] stated resins may be considered in select circumstances as a second-line agent for adults with ezetimibe intolerance and with triglycerides
Fibrates
While fibrates (gemfibrozil, fenofibrate, clofibrate) have not been studied to demonstrate a reduction in CVD or CVD mortality in the elderly population, this medication class is beneficial in patients with hypertriglyceridemia to lower triglyceride levels and prevent pancreatitis. Fibrates are recommended for patients with triglyceride levels approaching 500 mg/dL. Fibrates can also increase high-density lipoproteins, which tend to be lower in the elderly population and considered a risk factor for CVD. Gemfibrozil is not recommended in combination with statin therapy due to an increased risk of myalgia. Fenofibrate is the drug of choice, particularly for diabetic patients with very uncontrolled triglyceride levels because it will not affect glucose levels [57]. At this time, we do not recommend the use of fibrates in the elderly population unless they are at risk for developing pancreatitis and have elevated triglyceride levels.
Patient-Centered Care
Evidence-based medicine can aid in making sound clinical decisions for proper patient care; however, treatment plans should consider the individual patient’s perspectives and needs, beliefs, expectations, and goals. In the elderly population, we must also consider factors such as finances, pill-burden, drug-drug interactions, physiological needs, comorbid disease states, and overall life expectancy. In addition, the elderly population is physiologically heterogeneous group and recommendations for therapy need to be individualized. Chronological age does not necessarily correspond to vascular age and risk factors for cardiovascular disease do not predict outcomes as well in the elderly as they do in younger patients. While older patients may view having to take 1 less medication as more important than preventing a heart attack or stroke at the age of 80, it is advisable to discuss all potential outcomes related to morbidity associated with the occurrence of an MI or stroke due to the lack of statin therapy. Additionally, pharmacists can play a vital role in evaluating elderly patients and their medication regimens. Elderly patients should undergo a medication reconciliation at each visit to evaluate drug-drug interactions, side effects, and potentially harmful medication combinations that may lead to increased adverse drug outcomes.
Conclusion
CHD increases with age, and most patients who have a CV event are more likely to die with advancing age. Based on the the limited available evidence, statin therapy is beneficial in the elderly population in reducing overall CV morbidity. We recommend beginning with with a moderate-intensity statin and adjusting accordingly. High-intensity statin therapy appears to be effective for elderly patients for secondary prevention, but clinicians should use clinical judgment and monitor for adverse events, particularly myalgia pain. At this time, we are unable to determine if non-statin therapies for the elderly would be beneficial and do not recommend their use unless the patient is at risk for pancreatitis, in which case a fenofibrate is recommended.
Corresponding author: Nicole A. Slater, PharmD, BCACP, Auburn University, Harrison School of Pharmacy, 650 Clinic Dr., Mobile, AL 36688.
Financial disclosures: None.
From the Harrison School of Pharmacy, Auburn University, Mobile, AL.
Abstract
- Objective: To summarize the literature relevant to managing dyslipidemia in the elderly and review recommendations for initiating lipid-lowering therapy.
- Methods: Review of the literature.
- Results: Statins are the most commonly utilized medication class for lipid-lowering in the general population, and they are recommended for primary prevention in patients between the ages of 40 to 75 with at least 1 risk factor for cardiovascular disease as well as for any patient needing secondary prevention. In the elderly, statins may be appropriate for both primary and secondary prevention if the benefits outweigh the risks. Based on the available evidence, it is safe to recommend statin therapy to elderly patients who require secondary prevention given the known benefits in reducing cardiovascular morbidity and mortality for patients up to the age of 80. For primary prevention, statin therapy may be beneficial, but one must carefully evaluate for comorbid conditions, life expectancy, concomitant medications, overall health status, frailty, and patient or family preference. Several other classes of lipid-lowering agents exist; however, there is not enough evidence for us to recommend use in the elderly population for cardiovascular risk reduction in either primary or secondary scenarios.
- Conclusion: Although clinical research in the elderly population is limited, evidence supports the use of statins in elderly patients for secondary prevention and in patients up to age 75 for primary prevention; however clinicians must use clinical judgement and take into consideration the patient’s situation regarding comorbidities, polypharmacy, and possible adverse effects. More high-quality evidence is necessary.
Key words: hyperlipidemia; geriatrics; elderly; patient-centered care; statin; cardiovascular disease.
The number of Americans age 65 years and older is projected to more than double, from 46 million today to over 98 million by 2060, and the 65-and-older age group’s share of the total population will rise to nearly 24% [1]. Life expectancy is now predicted to be > 20 years for women at age 65 and > 17 years for men at age 65 in many high-income countries, including the United States [2]. This demographic shift toward an older population will result in a higher burden of coronary heart disease and stroke, with atherosclerotic cardiovascular disease (ASCVD) prevalence and costs projected to increase substantially [3].
Among adults seeking medical care in the United States, roughly 95 million have a total cholesterol (TC) level of ≥ 200 mg/dL or more, and approximately 29 million have a TC > 240 mg/dL [4]. Cholesterol screening is important since most patients suffering from dyslipidemia are asymptomatic. Dyslipidemia is a major risk factor for the development of atherosclerotic disease. Because of the complications associated with dyslipidemia, it is vital that patients are provided with primary and/or secondary prevention strategies to reduce the risk of cardiovascular disease (CVD) and protect high-risk patients from recurring events. A clinical controversy exists surrounding the elderly population, concerning whether or not clinicians should be providing lipid-lowering treatment to this group of individuals for dyslipidemia. The evidence is limited for patients over age 65, and even more so for the very elderly (> 80 years); therefore, it is necessary to review the available evidence to make an appropriate decision when it comes to managing dyslipidemia in the elderly population
Currently, HMG-CoA reductase inhibitors (statins) are the only known class of medications for the treatment of dyslipidemia that will prevent both primary and secondary cardiovascular (CV) events, including death. Statin intensity (Table 1)
Guideline Recommendations
Current guidelines differ in their recommendations for treating dyslipidemia in the elderly population. In 2016, the Task Force for the Management of Dyslipidemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) released updated guidelines for managing dyslipidemia. These guidelines recommend that older patients with established CVD be treated in the same way as younger patients because of the many benefits statin therapy demonstrated in clinical trials. They also suggest that statin therapy be started at a lower doses to achieve goals for primary prevention in the older population. In addition, CVD risk factors (hypertension, diabetes, dyslipidemia, smoking) should be addressed in this population to reduce CVD risk. They also acknowledged that primary prevention may not prolong life in the older adult, but treatment does reduce cardiovascular mortality and statin therapy is recommended to reduce the overall risk of CV morbidity in this population [11]. In contrast, The 2013 American College of Cardiology/American Heart Association (ACC/AHA) guidelines changed the management and treatment of dyslipidemia by highlighting “statin benefit groups” rather than recommending a treat-to-target goal as guidelines had done for many years. ACC/AHA recommends a moderate-intensity statin for patients > 75 years of age for secondary prevention versus the use of a high-intensity statin for patients who are between the ages of 40 and 75 based on the pooled cohort risk equation. In patients over age 75 with no history of CVD, no specific recommendation is available for the use of lipid-lowering therapy at this time [12]. ACC/AHA is expected to publish a new set of guidelines sometime in 2018 and they are projected to utilize lipid-lowering goals in combination with the pooled cohort equation to assess overall risk in patients with dyslipidemia.
The 2015 National Lipid Association (NLA) released “Part 1” guidelines for the management of dyslipidemia and then provided “Part 2” about a year later, which focuses on management for special populations. To summarize, the NLA guidelines recommend that elderly patients between the ages of 65 and 80 receive a high-intensity statin for secondary prevention after special consideration of the potential risks and benefits. In patients over the age of 80, NLA recommends a moderate-intensity statin for secondary prevention. For primary prevention, NLA recommends utilizing the pooled cohort risk equation to analyze patient characteristics, keeping in mind that age is a driving factor for increased risk of CVD and that the actual risk for developing a CV event may be “overestimated” if the patient has no other risk factors other than their age. When evaluating patients between the ages of 65 and 79 for primary prevention, NLA suggests following Part 1 of the guidelines. In Part 1, NLA recommends evaluating the patient’s characteristics and suggests a moderate- or high-intensity statin if the patient is considered “very high risk” or “high risk” and a moderate-intensity statin for patients who are considered “moderate risk”. For patients over the age of 80, they recommend utilizing a moderate- or a low-intensity statin depending on frailty status or if significant comorbidities or polypharmacy exist [13,14].
In 2017, the American Association of Clinical Endocrinologist (AACE) released guidelines for the management of dyslipidemia and CVD prevention. AACE recommends that patients over age 65 be screened for dyslipidemia, and those who have multiple risk factors, other than age, should be considered for treatment with lipid-lowering therapy. AACE focuses on specific target LDL-C levels as treatment goals [15].
In addition to statins, other lipid-lowering therapies are used to treat dyslipidemia. The 2016 American College of Cardiology (ACC) Task Force reported on the use of non-statin therapies for the management of dyslipidemia and prevention of clinical ASCVD [16]. The committee concluded that ezetimibe added to statin therapy, bile acid sequestrants as monotherapy, and niacin as monotherapy all have some benefit for the prevention of clinical ASCVD. These guidelines also discuss the use of PCSK-9 inhibitors and their potential to decrease the risk of clinical ASCVD, but trials are currently ongoing to determine actual benefit. These guidelines address special populations but they do not consider the elderly in their recommendations. Currently, the only special populations included are patients with heart failure, those on hemodialysis, women who are of childbearing age or pregnant, and those with autoimmune diseases [16]. The literature available for each individual medication is discussed in further detail below.
Evidence for Secondary Prevention
The benefits of statin therapy for secondary prevention in the elderly is more established than it is for primary prevention (Table 2).
The ASCOT–LLA (Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm), published in 2003, evaluated the effect of atorvastatin 10 mg on reducing ASCVD events in moderate-risk patients between 40–79 years of age who had hypertension and normal or slightly elevated LDL-C levels, with at least 2 other risk factors for CVD (age > 55 years was considered a risk factor). The primary outcome was non-fatal MI including silent MI and fatal CHD. A significant reduction was seen in the primary endpoint. Over half of the study population was > 60 years of age, with a mean age of 63 years. In a post-hoc analysis, stroke prevention was found to be similar in patients who were > 70 years of age and those < 70 years of age [19].
One of the first trials to specifically analyze the impact of age on lipid-lowering therapy in secondary ASCVD prevention was the Scandinavian Simvastatin Survival Study (4S), published in 1994. They evaluated the effect of simvastatin 20 mg on CV-related mortality and morbidity in patients 35–70 years of age with hyperlipidemia and a history of angina or acute MI occurring > 6 months of the study starting. The primary outcome was all-cause mortality. The secondary endpoint was time to first major CV event, which included coronary death, non-fatal acute MI, resuscitated cardiac arrest, and silent MI. Simvastatin significantly reduced the primary outcome and CHD-related deaths. A subgroup analysis of the study population > 60 years of age showed that age made no significant impact on primary or secondary outcomes; however, investigators noted that these subgroup analyses had less statistical power than the population as a whole [20].
Published in 1998, the LIPID (Long-Term Intervention with Pravastatin in Ischemic Disease) study evaluated the effects of pravastatin 40 mg daily on CHD-related mortality and overall mortality in patients with hyperlipidemia and clinical ASCVD (previous MI or unstable angina). The primary outcome observed was fatal CHD. Pravastatin significantly reduced the primary outcome, overall mortality, and pre-specified CV events. In a subgroup analysis, age group ( 65, and > 70 years) had no significant impact on the combined outcome of death from CHD and nonfatal MI; however, patients 65 to 70 years of age made up less than half of the study population [21].
The Cholesterol and Recurrent Events (CARE) trial, published in 1996, looked at the effect of pravastatin 40mg therapy for secondary ASCVD prevention following an MI in patients who had average cholesterol levels (defined as TC < 240 mg/dL and LDL-C 115–174 mg/dL). The primary endpoint assessed was time to fatal CHD or nonfatal MI. To meet statistical power they looked at subgroups for a broader outcome of a major coronary event (including fatal CHD, nonfatal MI, bypass surgery, and angioplasty). Pravastatin significantly reduced the primary outcome. The significant reduction in coronary events produced by pravastatin was noted to be significantly greater in women and in patients with higher pretreatment levels of LDL-C, but was not significantly impacted by age group (24–59 vs. 60–75 years) [22].
The Heart Protection Study (HPS), published in 2002, looked at the long-term effects of lowering LDL-C with simvastatin 40 mg in patients 40 to 80 years of age at high risk for mortality due to either vascular or nonvascular causes. The primary outcome assessed was all-cause mortality, with fatal or nonfatal vascular events as co-primary outcomes for subcategory analyses. Simvastatin significantly reduced both primary and co-primary outcomes, but there was no significant difference when they looked at nonvascular mortality between groups. Neither age nor baseline LDL levels were reported to have had a significant impact on outcomes. Over half the population was > 65 years of age, and about one-third of the population was > 70 years of age [23].
The PROVE-IT/TIMI 22 (Pravastatin or Atorvastatin Evaluation and Infection Therapy–Thrombolysis in Myocardial Infarction 22) trial, published in 2004, compared pravastatin 40 mg (moderate-intensity) to atorvastatin 80 mg (high-intensity) for secondary ASCVD prevention in patients with recent acute coronary syndrome (ACS) 65 years of age and the mean age was 58 years [24].
The TNT (Treating to New Targets) trial, published in 2005, looked at secondary ASCVD prevention in regards to targeting LDL-C levels to < 100 mg/dL or < 70 mg/dL with atorvastatin 10 mg and atorvastatin 80 mg. Patients had stable coronary artery disease (CAD) and baseline LDL-C levels < 130 mg/dL. The primary endpoint was the occurrence of a CV event (CAD mortality, nonfatal MI not related to procedure, resuscitation after cardiac arrest, or fatal or nonfatal stroke). High-intensity atorvastatin (80 mg) significantly reduced the primary outcome. The mean age of the study population was approximately 61 years. The study reported no statistical interaction for age or sex in the primary outcome measure [25].
The Study Assessing Goals in the Elderly (SAGE), published in 2007, evaluated the effects of pravastatin 40 mg (moderate-intensity) vs atorvastatin 80 mg (high-intensity) on secondary ASCVD prevention in patients 65 to 85 years (mean age 72) with stable CHD, LDL-C 100–250 mg/dL, with at least 1 episode of myocardial ischemia with total ischemia duration > 3 minutes. The primary efficacy outcome observed was absolute change in total duration of myocardial ischemia on 48-hour ambulatory electrocardiographic monitoring from baseline to month 12. No significant difference was observed in efficacy between the two groups for the primary endpoint, but the intensive statin therapy group showed greater benefit respective to several secondary outcomes, including major acute CV events and death [26].
In summary, while these trials provide evidence that statin therapy is beneficial in a wide range of patients with clinical ASCVD and dyslipidemia, the trial data does not provide definitive guidance for treating elderly patients at this time. Given the small percentage of elderly patients that were included, some of the trial results reporting statistical significance in this age group hold less clinical significance. It appears that high-intensity statin therapy was more likely to effectively prevent clinical ASCVD and death than moderate-intensity statin therapy, but more evidence is needed regarding secondary prevention in patients over age 75.
Evidence for Primary Prevention
The PROSPER (PROspective Study of Pravastatin in the Elderly at Risk) was published in 2002 to assess the efficacy of pravastatin in patients between the ages of 70 and 82 (mean age 75 years) with pre-existing vascular disease (coronary, cerebral, or peripheral) or at an elevated risk (smoking, hypertension, or diabetes). Patients were randomized to receive either placebo or pravastatin 40 mg (a moderate-intensity statin). They found that pravastatin therapy reduced the risk of the composite outcome of CHD-related death, nonfatal MI, and fatal or nonfatal stroke in this elderly population. A post-hoc analysis comparing primary versus secondary prevention groups found no significant differences between these subgroups [7].
Han et al recently conducted a post hoc secondary analysis of older participants (65 years and older) in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial–Lipid-Lowering Trial (ALLHAT-LLT). The intervention for ALLHAT-LLT was 40 mg pravastatin. They found no significant differences in all-cause mortality or cardiovascular outcomes between the pravastatin and usual care groups [27]
JUPITER (Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin), published in 2008, examined the efficacy of rosuvastatin vs. placebo in low- to moderate-risk men 50 years and older and women 60 years and older using a composite outcome of MI, unstable angina, stroke, arterial revascularization, or CVD death. Rosuvastatin did significantly decrease the primary endpoint, however it did not reduce the risk of overall death [28]. A subgroup analysis was performed on the elderly (65–75 years) study participants in JUPITER demonstrating a significant risk reduction for the combined CV endpoint and a nonsignificant reduction of all-cause mortality [29].
CARDS (Collaborative Atorvastatin Diabetes Study), published in 2004, looked at statin use for primary prevention in high-risk patients with type 2 diabetes without high LDL-C, but they had to have at least 1 additional risk factor for CVD. The primary outcome was first acute CHD event (myocardial infarction including silent infarction, unstable angina, acute coronary heart disease death, resuscitated cardiac arrest), coronary revascularization procedures, or stroke. Atorvastatin 10 mg, a moderate-intensity statin, significantly decreased occurrence of the primary outcome [30]. A subgroup analysis was performed to evaluate patients specifically between the ages of 65 and 75 and found a similar outcome in the elderly with a significant reduction in first major CV event and stroke [31].
A recent study evaluating primary prevention in patients with an intermediate risk for CVD was the HOPE-3 (Heart Outcomes Prevention Evaluation), published in 2016. Two co-primary outcomes were evaluated: the composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, while the second primary outcome also included revascularization, heart failure, and resuscitated cardiac arrest. Rosuvastatin significantly decreased occurrence of both co-primary endpoints. About half of the study populations was over the age of 65 with a median age of 71 [32].
In addition to these trials of primary prevention, summarized in Table 3, a meta-analysis was published in 2013 to assess whether statins reduce all-cause mortality and CV events in elderly people without established CV disease.
As demonstrated by the above studies, it is evident that statins do help reduce the risk of CV events, regardless of statin intensity, but they do not consistently prevent death. However, the trials that did not demonstrate a significant outcome related to death utilized a moderate-intensity statin; if a high-intensity statin was used in those trials, there may have been a benefit [7,27]. More study is needed to evaluate the use of high-intensity statins in the elderly for the prevention of all-cause mortality and CV-related death.
Fortunately, the ongoing STAREE (STAtin Therapy for Reducing Events in the Elderly) study is looking specifically at the impact of statin therapy in adults 70 and older. Patients with a history of CVD or dementia are excluded. Results are set to be released in 2020 [34].
Risks of Using Statins in Older Adults
Statin use has been linked to a number of unwanted adverse effects.
Myalgia
Myalgia is variable but may occur in up to 25% of patients using statin therapy, and elderly patients typically experience more statin-associated myalgia than younger patients [35,36]. Elderly patients are more prone to decreased muscle mass and therefore may be at a higher risk of developing myalgia pain. Elderly patients are also utilizing more medications, leading to the potential for increased drug-drug interactions that could lead to myalgia. Elderly patients may also lose the function of drug metabolizing enzymes responsible for breaking down statin therapy, which may also increase the risk for statin-associated myalgia. One study demonstrated that elderly patients were more likely to discontinue statin therapy due to muscle pain and elderly patients reported more muscle side effects than their younger cohorts [37]. It is important to monitor for muscle pain and weakness in every patient. If they experience any myalgia, it is recommended to either lower the dose or discontinue the statin once it is determined to be statin-related. After myalgia resolves, therapy can be reinitiated at a lower dose or with a different statin if the patient is deemed high-risk. If creatine phosphokinase levels are greater than 10 times above the upper limit of normal, then discontinue the statin and wait for levels to return to normal. Re-initiation may be appropriate, but the the risks and benefits must be weighed. Simvastatin and atorvastatin are associated with higher rates of myalgia while pravastatin and rosuvastatin have the least myalgia pain associated with use [38,39].
Statin Intolerance
Statin intolerance, while not very common, is typically seen more often in special populations such as women, Asian patients, and the elderly. For a patient to be considered intolerant to statins, they need to have documented muscle symptoms or an elevated creatine phosphokinase level. Although not well defined, many clinicians consider improvement of symptoms with statin withdrawal as a diagnosis for statin intolerance. Typically patients are then rechallenged with 1 to 2 other statins and if still unable to tolerate, then different lipid-lowering therapies may be utilized [40]. In the elderly, it is important to rule out other causes for myalgia and monitor for significant drug interactions that may lead to muscle pain, particularly if the patient is requiring secondary prevention with statin therapy, before discontinuation.
Dementia
In 2012, the FDA issued a warning about the potential risk of cognitive impairment with the use of statins, which was based on case reports, not clinical trial data [41]. The NLA guidelines do not recommend baseline cognitive assessments prior to starting therapy and recommend that if patients do report cognitive impairment, other contributing factors and the risk associated with stopping statin therapy must be considered. Statin therapy may be discontinued to assess reversibility of symptoms, and if symptoms resolve, then it may be more beneficial to keep the patient off statin therapy. Clinicians may also consider lowering the dose or switching to another statin if they feel it is necessary for the patient to continue with a statin, particularly if the patient requires secondary prevention. Evidence suggests that statins are not associated with adverse effects on cognition and should not be withheld due to the potential for causing cognitive impairment alone [42]. The prevalence of cognitive impairment increases with age, so it is important for a clinician to rule out age-related processes or other disease states, such as Alzheimer’s, before discontinuation of previously tolerated statin therapy.
Renal Impairment
Kidney function must be evaluated prior to initiation of a statin in an elderly person as well as during the time the patient is taking a statin. Because statins are eliminated via the kidney, and because most elderly patients have decreased kidney function, the potential for drug build-up in the body is higher than in a younger patient and may lead to more adverse effects. Atorvastatin is the only option that does not require dose adjustment. All other statins should be adjusted based upon the level of renal impairment. The results from the SHARP study, published in 2011, showed that the combination of ezetimibe and simvastatin versus placebo significantly reduced ASCVD events in patients with moderate to severe chronic kidney disease, including those receiving dialysis. Specifically, this trial showed a significant reduction of ischemic events and occurrence of arterial revascularization procedures. Although the trial did not show a significant difference in incidence of MI or CHD-related mortality, the trial was not adequately powered to show differences in results among the individual ASCVD events and it is not clear whether the results can guide the use of statin therapy in all patients with chronic kidney disease [43]. Statins may be beneficial in renal insufficiency to lower LDL-C, but more studies are needed to assess CVD outcomes related to statin use in patients with a history of kidney disease [44].
Hepatic Function
Statins have been known to increase liver enzymes and in rare cases lead to liver injury, which typically has led to underutilization of therapy in clinical practice. Risk factors associated with this include preexisting hepatitis, advanced age, chronic alcohol use, and use of concomitant medications that may also cause hepatotoxicity, such as acetaminophen. When a statin-induced hepatic effect is suspected, it is important to first rule out other causes or disease states that may be undiagnosed. If no other cause can be found, clinicians may choose to reduce the statin dose, switch the statin, or discontinue the statin altogether if the risk outweighs the benefit. Additionally, statins do not have to be held in patients who have preexisting hepatic dysfunction if use is clearly indicated because the cardiovascular benefits typically outweigh the risks of causing liver injury. Clinical judgement is still warranted and patients with preexisting liver conditions should be monitored regularly [45].
Cost Considerations
Several studies have demonstrated that statin therapy, in the general population, is economical for both primary and secondary prevention of CVD [46,47]. The 4S study found simvastatin therapy to be cost-effective; for example, the cost per life year gained for a 70-year-old man with high chlesterol was $3800 [48]. In contrast, primary prevention in middle-aged men, based on the West of Scotland trial, averages about $35,000 per year of life gained [46]. In a 2015 study that utilized an established Markov simulation model, researchers studied adults 75 to 94 years and examined the cost-effectiveness of generic statins for primary prevention in this population. The authors estimated treating this population with statins over the next decade would be cost-effective. However, the researchers cautioned that the CV benefits and cost-effectiveness would be offset with even a modest increased risk of cognitive impairments or functional limitations. Statin use was not cost-effective in diabetes patients who did not have elevated LDL-C levels [49].
Non-Statin Therapies
Several other classes of medications are available for the management of hyperlipidemia; however, none of these lipid-lowering therapies have been found to reduce CVD events or mortality in the elderly population.
Ezetimibe
Ezetimibe blocks the absorption of intestinal cholesterol and is typically combined with statin therapy to lower LDL-C. Up until the IMPROVE-IT trial was published in 2015, ezetimibe did not have much use in clinical practice. This landmark trial was a large double-blind study that looked at secondary prevention in patients with ACS, comparing ezetimibe 10 mg and simvastatin 40 mg versus simvastatin 40 mg alone. The authors included patients over the age of 50 (mean age 64) with clinical ASCVD. They found that the addition of ezetimibe to simvastatin did reduce the primary composite outcome (CV mortality, major CV events, or nonfatal stroke) when compared to simvastatin alone [50]. This trial demonstrates clinical benefit with the addition of ezetimibe to statin therapy and adds additional evidence to support a target LDL-C of less than 70 mg/dL; however, the elderly population was not adequately represented in the study to allow extrapolation of these results to older patients.
PCSK-9 Inhibitors
The proprotein convertase subtilisin/kexin type 9 (PCSK-9) inhibitors are a newer class of monoclonal antibodies that were first approved by the US Food and Drug Administration in 2015. Alirocumab and evolocumab, both approved PCSK-9 inhibitors, bind to LDL receptors on the surface of hepatocytes and assist in the internalization of LDL receptors for lysosomal degradation. By inhibiting the binding of PCSK-9 to the LDL receptors, there is an overall increase in LDL receptors available on the cell surface to bind to LDL particles, thereby lowering LDL-C levels. Treatment with these agents are currently considered (in addition to diet and maximally tolerated statin therapy) in adult patients with heterozygous familial hypercholesterolemia or clinical ASCVD requiring further reduction in LDL-C. Two studies were published focusing on the use of PCSK-9 inhibitors: Open-label Study of Long-term Evaluation against LDL Cholesterol (OSLER) and the Tolerability of Alirocumab in High Cardiovascular Risk Patients with Hypercholesterolemia Not Adequately Controlled with Their Lipid Modifying Therapy (ODYSSEY LONG TERM). Overall, these studies demonstrated a 60% reduction of LDL-C among patients with high CVD risk on maximum-tolerated statin therapy. Furthermore, the ODYSSEY LONG TERM trial did find that the rate of major CVD adverse events was significantly lower with alirocumab added to maximum-tolerated statin therapy, with a hazard ratio of 0.52 [51].
One recent study of evolocumab, named the Further Cardiovascular OUtcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk (FOURIER), enrolled patients between the ages of 40 and 85 with 1 major CV risk factor or 2 minor CV risk factors. The primary endpoint was a composite of cardiovascular death, MI, stroke, hospitalization for unstable angina, or coronary revascularization. Evolocumab lowered major CV events by roughly 15% when added to statin therapy in patients who were at high risk for clinical ASCVD. The mean age of the patients in the trial was 63; however, it is unclear how many of the study participants were elderly [52].
Unfortunately, the studies discussed above do not represent the elderly population well and the agents have not been studied long-term to determine the effects of continued use beyond 2 years. Long-term outcome studies are currently underway; however, it is unknown at this time whether elderly patients are being considered in these studies. It is known that genetic variation of the PCSK-9 locus does lower LDL-C in the elderly but does not significantly lower their risk of vascular disease [51]. At this time, until further evidence is available, we do not recommend the use of PCSK-9 inhibitors in elderly patients.
Nicotinic Acid
Nicotinic acid (Niacin, Niacin ER), also known as vitamin B3, has been utilized for decades as a vitamin supplement, an anti-wrinkle agent, and is known to have neuroprotective effects. It has also been utilized for dyslipidemia and has had some benefits when used alone to decrease cardiovascular disease [53]. Unfortunately the Coronary Drug Project was completed in the 1980s and did not incorporate patients over the age of 64, therefore making the results difficult to apply to elderly patients today [54]. Other literature has been published in recent years to refute that study, claiming there is no additional benefit to using niacin for cardiovascular protection and these studies have included elderly patients. In the AIM-HIGH trial, published in 2011, approximately 46% of the patients were 65 or older. Patients who were previously taking statin therapy that had known cardiovascular disease were enrolled. Niacin added to simvastatin 40–80 mg lowered LDL-C, triglycerides, and increased HDL-C, but the addition of niacin was not proven to help lower the risk of cardiovascular events [55]. The HPS2-THRIVE study enrolled patients with known cardiovascular disease between the ages of 50 and 80 years and found no benefit in preventing CVD when adding niacin to statin therapy [56]. With its side effect profile, risk for increased glucose intolerance, and lack of evidence to demonstrate benefit for prevention of CV events, we do not recommend niacin for use in the elderly at this time.
Bile Acid Sequestrants
The ATP III guidelines [57] noted that when statins are not sufficient to lower high cholesterol, bile acid sequestrants also known as resins could be added. More recently, the 2016 ACC expert consensus on non-statin therapies for LDL-C lowering [16] stated resins may be considered in select circumstances as a second-line agent for adults with ezetimibe intolerance and with triglycerides
Fibrates
While fibrates (gemfibrozil, fenofibrate, clofibrate) have not been studied to demonstrate a reduction in CVD or CVD mortality in the elderly population, this medication class is beneficial in patients with hypertriglyceridemia to lower triglyceride levels and prevent pancreatitis. Fibrates are recommended for patients with triglyceride levels approaching 500 mg/dL. Fibrates can also increase high-density lipoproteins, which tend to be lower in the elderly population and considered a risk factor for CVD. Gemfibrozil is not recommended in combination with statin therapy due to an increased risk of myalgia. Fenofibrate is the drug of choice, particularly for diabetic patients with very uncontrolled triglyceride levels because it will not affect glucose levels [57]. At this time, we do not recommend the use of fibrates in the elderly population unless they are at risk for developing pancreatitis and have elevated triglyceride levels.
Patient-Centered Care
Evidence-based medicine can aid in making sound clinical decisions for proper patient care; however, treatment plans should consider the individual patient’s perspectives and needs, beliefs, expectations, and goals. In the elderly population, we must also consider factors such as finances, pill-burden, drug-drug interactions, physiological needs, comorbid disease states, and overall life expectancy. In addition, the elderly population is physiologically heterogeneous group and recommendations for therapy need to be individualized. Chronological age does not necessarily correspond to vascular age and risk factors for cardiovascular disease do not predict outcomes as well in the elderly as they do in younger patients. While older patients may view having to take 1 less medication as more important than preventing a heart attack or stroke at the age of 80, it is advisable to discuss all potential outcomes related to morbidity associated with the occurrence of an MI or stroke due to the lack of statin therapy. Additionally, pharmacists can play a vital role in evaluating elderly patients and their medication regimens. Elderly patients should undergo a medication reconciliation at each visit to evaluate drug-drug interactions, side effects, and potentially harmful medication combinations that may lead to increased adverse drug outcomes.
Conclusion
CHD increases with age, and most patients who have a CV event are more likely to die with advancing age. Based on the the limited available evidence, statin therapy is beneficial in the elderly population in reducing overall CV morbidity. We recommend beginning with with a moderate-intensity statin and adjusting accordingly. High-intensity statin therapy appears to be effective for elderly patients for secondary prevention, but clinicians should use clinical judgment and monitor for adverse events, particularly myalgia pain. At this time, we are unable to determine if non-statin therapies for the elderly would be beneficial and do not recommend their use unless the patient is at risk for pancreatitis, in which case a fenofibrate is recommended.
Corresponding author: Nicole A. Slater, PharmD, BCACP, Auburn University, Harrison School of Pharmacy, 650 Clinic Dr., Mobile, AL 36688.
Financial disclosures: None.
1. Fact sheet: Aging in the United States. Accessed at www.prb.org/aging-unitedstates-fact-sheet/.
2. Kontis JE, Bennett CD, Mathers G, et al. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet 2017;389:1323–35.
3. Odden MD, Coxson PG, Moran A, et al. The impact of the aging population on coronary heart disease in the United States. Am J Med 2011;124:827–33.
4. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017;135:e1–e458.
5. Mills EJ, Rachlis B, Wu P, et al. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Amer Col Cardiol 2008;52:1769–81.
6. Stone NJ. Statins in secondary prevention: intensity matters. J Am Coll Cardiol 2017;69: 2707–9.
7. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–30.
8. Heart Protection Study Collaborative Group Writers. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.
9. Lefevre F, Nishida L. Special report: the efficacy and safety of statins in the elderly. TEC Assessment Program 2007;21
10. Pravastatin benefits elderly patients: results of PROSPER study. Cardiovasc J S Afr 2003;14:48.
11. Catapano AL, Graham I, Backer GD, et al. ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058.
12. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934.
13. Jacobson TA, Ito MK, Maki K, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1-full report. J Clin Lipidol 2015;9:129-169
14. Jacobson TA, Maki KC, Orringer CE, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol 2015;9:S1–22.e1.
15. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologist and american college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 2017;23:1–87.
16. Lloyd-Jones DM, Morris PB, Minissian MB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 2016;68:92–125.
17. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–59.
18. Chaturvedi S, Zivin J, Breazna A, et al. Effect of atorvastatin in elderly patients with a recent stroke or transient ischemic attack. Neurology 2009;72:688–94.
19. Sever PS, Dahior B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian cardiac outcomes trial—lipid lowering arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149–58.
20. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383–9.
21. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349–57.
22. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin of coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001–09.
23. Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.
24. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–04.
25. LaRosa JC, Grundy SM, Waters DD, et al; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–35.
26. Deedwania P, Stone PH, Bairey CN, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the study assessing goals in the elderly (SAGE). Circulation 2007;115:700–7.
27. Han BH, Sutin D, Williamson JD, et al; ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: the ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017;177:955–65.
28. Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C Reactive protein. N Engl J Med 2008; 359:2195–207.
29. Glynn RJ, Koenig W, Nordestgaard BG, et al. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: Exploratory analysis of a randomized trial. Ann Intern Med 2010;152:488–96.
30. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
31. Neil HA, DeMicco DA, Luo D, et al. Analysis of efficacy and safety in patients aged 65-75 years at randomization: collaborative atorvastatin diabetes study (CARDS). Diabetes Care 2006;29:2378–84.
32. Yusuf S, Bosch J, Dagenais G, et al; HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–31.
33. Savarese G, Gotta AM Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol 2013;62:2090–99.
34. National Institute of Health. A clinical trial of STAtin therapy for reducing events in the elderly (STAREE). Clinical Trials. https://clinicaltrials.gov/ct2/show/NCT02099123. Accessed June 5, 2018.
35. Gaist D, Rodríquez, LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001;12:565–9.
36. Pasternak RC., Smith SC Jr, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002;40:567–72.
37. Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding statin use in America and gaps in patient education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012;6:208–15.
38. Harper CR, Jacobson TA. Evidence-based management of statin myopathy. Curr Atheroscler Rep 2010;12:322–30.
39. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005;19:403–14.
40. Ahmad Z. Statin intolerance. Am J Cardiol 2014;113:1765–71.
41. Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Published February 28, 2012. Accessed June 5, 2018.
42. Gauthier JM, Massicotte A. Statins and their effect on cognition: let’s clear up the confusion. Can Pharm J (Ott) 2015;148:150–55.
43. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92.
44. Vaziri ND, Anzalone DA, Catini J. Statins in chronic didney disease: when and when not to use them. J Fam Pract 2016;65:8 Suppl. www.mdedge.com/jfp/custom/statins-chronic-kidney-disease-when-and-when-not-use-them-1
45. Jose J. Statins and its hepatic effects: newer data, implications, and changing recommendations. J Pharm Bioallied Sci 2016;8:23–8.
46. Caro J, Klittich W, McGuire A, et al. The West of Scotland coronary prevention study: Economic benefit analysis of primary prevention with pravastatin. BMJ 1997;315:1577–82.
47. Schectman G, Wolff N, Byrd JC, et al. Physician extenders for cost-effective management of hypercholesterolemia. J Gen Intern Med 1996;11:277–86.
48. Johannesson M, Jonsson B, Kjekshus J, et al. Cost effectiveness of simvastatin treatment to lower cholesterol levels in patients with coronary heart disease. Scandinavian simvastatin survival study group. N Engl J Med 1997;336:332–6.
49. Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015;162:533–41.
50. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97.
51. Polisecki E, Peter I, Robertson M, et al. Genetic variation at the PCSK9 locus moderately lowers low-density lipoprotein cholesterol levels, but does not significantly lower vascular disease risk in an elderly population. Atherosclerosis 2008;200:95–101.
52. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22.
53. Sinthupoom N, Prachayasittikul V, Prachayasittikul S, et al. Nicotinic acid and derivatives as multifunctional pharmacophores for medical applications. Eur Food Res Technol 2015;240: 1–17.
54. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245–55.
55. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67.
56. HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–12.
57. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002;106:3143–421.
58. The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351–64.
1. Fact sheet: Aging in the United States. Accessed at www.prb.org/aging-unitedstates-fact-sheet/.
2. Kontis JE, Bennett CD, Mathers G, et al. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet 2017;389:1323–35.
3. Odden MD, Coxson PG, Moran A, et al. The impact of the aging population on coronary heart disease in the United States. Am J Med 2011;124:827–33.
4. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation 2017;135:e1–e458.
5. Mills EJ, Rachlis B, Wu P, et al. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Amer Col Cardiol 2008;52:1769–81.
6. Stone NJ. Statins in secondary prevention: intensity matters. J Am Coll Cardiol 2017;69: 2707–9.
7. Shepherd J, Blauw GJ, Murphy MB, et al; PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–30.
8. Heart Protection Study Collaborative Group Writers. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.
9. Lefevre F, Nishida L. Special report: the efficacy and safety of statins in the elderly. TEC Assessment Program 2007;21
10. Pravastatin benefits elderly patients: results of PROSPER study. Cardiovasc J S Afr 2003;14:48.
11. Catapano AL, Graham I, Backer GD, et al. ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058.
12. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2889–934.
13. Jacobson TA, Ito MK, Maki K, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1-full report. J Clin Lipidol 2015;9:129-169
14. Jacobson TA, Maki KC, Orringer CE, et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol 2015;9:S1–22.e1.
15. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American association of clinical endocrinologist and american college of endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract 2017;23:1–87.
16. Lloyd-Jones DM, Morris PB, Minissian MB, et al. 2016 ACC expert consensus decision pathway on the role of non-statin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American college of cardiology task force on clinical expert consensus documents. J Am Coll Cardiol 2016;68:92–125.
17. Amarenco P, Bogousslavsky J, Callahan A 3rd, et al; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006;355:549–59.
18. Chaturvedi S, Zivin J, Breazna A, et al. Effect of atorvastatin in elderly patients with a recent stroke or transient ischemic attack. Neurology 2009;72:688–94.
19. Sever PS, Dahior B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian cardiac outcomes trial—lipid lowering arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149–58.
20. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4S). Lancet 1994;344:1383–9.
21. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349–57.
22. Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin of coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001–09.
23. Heart Protection Study Collaborative Group. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–22.
24. Cannon CP, Braunwald E, McCabe CH, et al; Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 Investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–04.
25. LaRosa JC, Grundy SM, Waters DD, et al; Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005;352:1425–35.
26. Deedwania P, Stone PH, Bairey CN, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the study assessing goals in the elderly (SAGE). Circulation 2007;115:700–7.
27. Han BH, Sutin D, Williamson JD, et al; ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: the ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017;177:955–65.
28. Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C Reactive protein. N Engl J Med 2008; 359:2195–207.
29. Glynn RJ, Koenig W, Nordestgaard BG, et al. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: Exploratory analysis of a randomized trial. Ann Intern Med 2010;152:488–96.
30. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96.
31. Neil HA, DeMicco DA, Luo D, et al. Analysis of efficacy and safety in patients aged 65-75 years at randomization: collaborative atorvastatin diabetes study (CARDS). Diabetes Care 2006;29:2378–84.
32. Yusuf S, Bosch J, Dagenais G, et al; HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–31.
33. Savarese G, Gotta AM Jr, Paolillo S, et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol 2013;62:2090–99.
34. National Institute of Health. A clinical trial of STAtin therapy for reducing events in the elderly (STAREE). Clinical Trials. https://clinicaltrials.gov/ct2/show/NCT02099123. Accessed June 5, 2018.
35. Gaist D, Rodríquez, LA, Huerta C, et al. Lipid-lowering drugs and risk of myopathy: a population-based follow-up study. Epidemiology 2001;12:565–9.
36. Pasternak RC., Smith SC Jr, Bairey-Merz CN, et al. ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol 2002;40:567–72.
37. Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding statin use in America and gaps in patient education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol 2012;6:208–15.
38. Harper CR, Jacobson TA. Evidence-based management of statin myopathy. Curr Atheroscler Rep 2010;12:322–30.
39. Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005;19:403–14.
40. Ahmad Z. Statin intolerance. Am J Cardiol 2014;113:1765–71.
41. Food and Drug Administration. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Published February 28, 2012. Accessed June 5, 2018.
42. Gauthier JM, Massicotte A. Statins and their effect on cognition: let’s clear up the confusion. Can Pharm J (Ott) 2015;148:150–55.
43. Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (study of heart and renal protection): a randomised placebo-controlled trial. Lancet 2011;377:2181–92.
44. Vaziri ND, Anzalone DA, Catini J. Statins in chronic didney disease: when and when not to use them. J Fam Pract 2016;65:8 Suppl. www.mdedge.com/jfp/custom/statins-chronic-kidney-disease-when-and-when-not-use-them-1
45. Jose J. Statins and its hepatic effects: newer data, implications, and changing recommendations. J Pharm Bioallied Sci 2016;8:23–8.
46. Caro J, Klittich W, McGuire A, et al. The West of Scotland coronary prevention study: Economic benefit analysis of primary prevention with pravastatin. BMJ 1997;315:1577–82.
47. Schectman G, Wolff N, Byrd JC, et al. Physician extenders for cost-effective management of hypercholesterolemia. J Gen Intern Med 1996;11:277–86.
48. Johannesson M, Jonsson B, Kjekshus J, et al. Cost effectiveness of simvastatin treatment to lower cholesterol levels in patients with coronary heart disease. Scandinavian simvastatin survival study group. N Engl J Med 1997;336:332–6.
49. Odden MC, Pletcher MJ, Coxson PG, et al. Cost-effectiveness and population impact of statins for primary prevention in adults aged 75 years or older in the United States. Ann Intern Med 2015;162:533–41.
50. Cannon CP, Blazing MA, Giugliano RP, et al; IMPROVE-IT Investigators. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–97.
51. Polisecki E, Peter I, Robertson M, et al. Genetic variation at the PCSK9 locus moderately lowers low-density lipoprotein cholesterol levels, but does not significantly lower vascular disease risk in an elderly population. Atherosclerosis 2008;200:95–101.
52. Sabatine MS, Giugliano RP, Keech AC, et al; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22.
53. Sinthupoom N, Prachayasittikul V, Prachayasittikul S, et al. Nicotinic acid and derivatives as multifunctional pharmacophores for medical applications. Eur Food Res Technol 2015;240: 1–17.
54. Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J Am Coll Cardiol 1986;8:1245–55.
55. AIM-HIGH Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–67.
56. HPS2-THRIVE Collaborative Group, Landray MJ, Haynes R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–12.
57. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002;106:3143–421.
58. The lipid research clinics coronary primary prevention trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984;251:351–64.
Supporting Suicidal Patients After Discharge from the Emergency Department
From the Department of Psychiatry, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Abstract
- Objective: To provide a review of emergency department (ED)-based psychosocial interventions that support adult patients with an identified suicide risk towards a goal of reducing subsequent suicidal behavior through the period after discharge, which is known to be a time of high risk for suicidal behavior.
- Methods: Non-systematic review of the literature.
- Results: Multiple methods of engaging patients after discharge from the ED have been shown to reduce subsequent suicidal behaviors. These methods include sending caring letters in the mail, facilitating supportive phone conversations, case management, and protocols that combine different services. Overall, the existing literature is insufficient to recommend widespread adoption of any individual strategy or protocol. However, providing psychosocial and emotional support to patients with an identified suicide risk after they are discharged from the ED is feasible and may reduce subsequent suicidal behaviors. Templates for providing supportive outreach using different modalities now exist, and these may help guide the ongoing development and widespread adoption of more effective and cost-effective solutions.
- Conclusion: Many ED–based interventions that provide enhanced support to patients with suicide risk after they are discharged have demonstrated a potential to reduce the risk of future suicidal behavior.
Key words: suicide; emergency department.
Despite the fact that emergency department (ED) providers often feel unprepared to manage suicide risk, patients with significant suicide risk frequently receive care in EDs, whether or not they have sustained physical injuries resulting from suicidal behavior [1,2]. Patients make greater than 400,000 visits to EDs in the United States each year for suicidal and self-injurious behaviors (suicide attempts and self-injurious behaviors are typically coded in ways that make them indistinguishable from each other in retrospective analyses) [3], and it is estimated that 6% to 10% of all patients in EDs endorse suicidal ideation when asked, regardless of their original chief complaints [4]. Meanwhile, suicide has become the 10th leading cause of death in the United States [5], and the Joint Commission has charged all accredited health care organizations with providing comprehensive treatment to suicidal patients, which may range from immediately containing an acute risk to ensuring continuity of care in follow-up [5].
When an acute suicide risk is identified in the ED, the provider’s immediate next steps should be to place the patient in a safe area under constant observation and to provide an emergency assessment [5,6]. Although psychiatric consultation and/or psychiatric admission may follow this assessment, suicide risk does not require admission in all cases; and some patients with suicide risk may be discharged to an outpatient setting even without receiving a psychiatric consultation [1]. Regardless of whether an outpatient disposition from the ED is appropriate, however, the period that immediately follows discharge is a time of high risk for repeated suicidal behavior and suicide death [7–9], and only 30% to 50% of patients who are discharged from EDs after a self-harm incident actually keep a follow-up mental health appointment [9,10]. Therefore, any support given to patients through this transition out of the emergency care setting could be especially high-yield.
The Joint Commission recommends that all patients with suicidal ideation receive, at minimum, a referral to treatment, telephone numbers for local and national crisis support resources (including the National Suicide Prevention Lifeline 1-800-273-TALK), collaborative safety planning, and counseling to restrict access to lethal means upon discharge [5]. However, some programs have demonstrated the capacity to provide enhanced support to patients beyond discharge from the ED, with some success in reducing the rates of subsequent suicidal behaviors. This non-systematic review describes interventions that can be initiated in the context of an ED encounter with the purpose of reducing future suicidal behavior among adult patients. They are primarily psychosocial rather than clinical. Clinical interventions that apply psychotherapy [11–13] psychopharmacology [14], and specialized inpatient treatments [15] have been studied as well but are beyond the scope of this review.
[polldaddy:10107269]
Interventions to Support Patients At Risk of Suicide After Discharge from the ED
Brief Contact Interventions
The idea that maintaining written correspondence with patients who have a known suicide risk after discharge can reduce subsequent suicide rates originated with a study of psychiatric inpatients conducted by Motto and Bostrom, in which patients who had been admitted for depression but had declined outpatient treatment were randomly assigned to periodically receive letters containing supportive messages from staff members over a period of 5 years [16]. This study remarkably found that these so-called brief contact interventions (BCIs), which were personalized to each recipient but did not contain psychotherapy per se, were associated with a reduced rate of suicide throughout the duration of the program compared with no written contacts [16].
BCIs have since been adapted to other communication formats and have been studied in patients who were discharged directly from the ED after an evaluation of suicide risk or suicidal behavior. Typically, BCIs consist of short, supportive messages that are delivered at regular intervals (often once every 1–2 months) over a period of 1 to 5 years [17,18]. They notably do not contain psychotherapy content, although they may reinforce coping strategies or remind recipients of how to access help if needed [17,19]. They may arrive as postcards [20,21], letters [22], telephone outreach [23–25], or a combination of modalities [26].
Protocols that rely on BCIs alone vary in their structure and have yielded mixed results [18]. A meta-analysis of 12 BCI protocols conducted by Milner et al found that, overall, BCIs administered after a presentation to the ED for self-harm have been associated with a significant reduction in repeat suicide attempts per recipient but not in total suicide deaths [27]. Milner’s group did not recommend large-scale promotion of BCIs based on the inadequacy of data so far, but suggested that this strategy may yet show promise upon further study [27]. A key advantage of BCIs is that they are inexpensive to implement, particularly if they do not include a telephone outreach component [28]. Thus, even if the potential benefit to patients is small, administering BCIs can be cost-effective [28].
It should not come as a surprise, therefore, that the potential for incorporation of BCIs into mobile smartphone technology is currently under investigation. Individuals who own mobile phones typically keep them on their persons and turned on continuously, and thus this is a reliable platform for maintaining contact with a wide range of patients in real-time [17,29]. Developers of at least 2 BCI smartphone programs that rely on mobile text messaging have published their protocols [17,30]. However, whether these programs will succeed in meaningfully reducing suicide rates remains to be determined by future research.
Green Cards
Morgan et al conducted a study in the United Kingdom in which individuals who presented to EDs after a self-harm event received a “green card,” which contained encouraging messages about seeking help and provided contact information for emergency services with 24-hour availability [31]. The green card also facilitated access to a crisis admission if necessary. The green card was distributed first in the ED and a second time by mail 3 weeks later. No suicides occurred in either the intervention or control group, which received usual care, and no statistically significant differences in suicide reattempt rate were found between groups after 1 year [31].
Evans et al studied an updated version of the green card intervention in which the green card facilitated access to an on-call psychiatrist with 24-hour availability by telephone [32]. The updated card included encouraging messages about seeking help similar to the original green card described by Morgan; however, the psychiatry consultation via telephone replaced the offer of hospital admission [32]. This second trial of green cards also failed to show a reduction in the rate of suicide reattempts among green card recipients at 6 months and 1 year [32,33].
Brief Intervention and Contact
The World Health Organization’s Brief Intervention and Contact (BIC) protocol is a standardized, multi-step suicide prevention program that has been studied primarily in patients who present to EDs after a suicide attempt in middle-income countries [34]. BIC includes a 1-hour information session that is administered shortly prior to discharge, and subsequently provides 9 follow-up contact interventions at specified intervals over an 18-month period. Unlike in a typical BCI, the contacts in BIC are conducted by a clinician either face-to-face or over the phone and include standardized assessments of the patient’s condition, although they still do not include psychotherapy. BIC has been shown to reduce suicide attempts, suicide deaths, or both in India [34–36], Iran [34,36,37], China [34,36], Brazil [34,36], and Sri Lanka [34,36] but was not found to directly improve clinical outcomes in a study conducted in French Polynesia [38]. A meta-analysis conducted by Riblet et al concluded that BIC is effective in reducing suicide risk overall [39].
ED-SAFE
The Emergency Department Safety Assessment and Follow-up Evaluation (ED-SAFE) protocol was validated in 8 EDs in 7 states in the US that did not already provide psychiatric services internally [40]. Under this model, all patients in the ED receive a screening for suicide risk, and those with an initial positive screen receive a secondary screen administered by the ED physician, a self-administered safety plan, and a series of up to 11 phone contacts over the following year that are administered by trained mental health clinicians in a central location. The ED-SAFE phone contacts follow the Coping Long Term with Active Suicide Program (CLASP) protocol [41] and provide support around safety planning and treatment engagement. They have the capacity to engage the patients’ significant others directly if a significant other is available and the patient chooses to involve that person.
In a single multicenter study, ED-SAFE reduced the absolute risk of suicide attempt by 5%, and the relative risk by 20% compared to usual treatment [40]. An intermediate phase of the study compared the universal suicide screening alone (ie, without the safety plan or follow-up contacts) with usual care and did not find this to improve outcomes [40].
Case Management
Kawanishi et al conducted a randomized controlled trial of assertive case management, the ACTION-J study, for patients with psychiatric diagnoses who presented with self-harm to 17 participating EDs in Japan [42]. In the ACTION-J study, case managers were mental health clinicians who provided clinical evaluations, treatment planning, encouragement, and care coordination over the course of 7 scheduled face-to-face or phone contacts in the first 18 months, and additional contacts at 6-month intervals until the completion of the trial (up to a total of 5 years) [43]. The comparison intervention, enhanced usual care, consisted of psychoeducation provided at the time of the encounter in the ED without case management services. The assertive case management intervention was associated with a decrease in suicidal behavior in the first 6 months but not for the duration of the study, except in women, for whom the benefit lasted the full 18 months [42]. A subsequent analysis also found a decrease in the total number of self-harm episodes per person-year compared to enhanced usual care, although there was not a difference in the number of participants who experienced a repeat self-harm episode [43]. The benefit was most strongly pronounced among patients who had presented with an index suicide attempt [43].
Morthorst et al applied an alternative case management model for the assertive intervention for deliberate self harm (AID) trial, which took place in Denmark [44]. Participants were aged 12 and older and could have been recruited from medical or pediatric inpatient units as well as the ED after a self-harm event. AID employed psychiatric nurses to provide crisis intervention, crisis planning, problem solving, motivational support, family mediation, and assistance with keeping appointments over a period of 6 months following discharge. Outreach took place over the phone, by text message, in participants’ homes, in cafes, and at health and social services appointments. The intervention required at least 4 contacts, although additional contacts could be made if appropriate. In comparison with a control group, in which participants received only usual care (which included ready access to short-term psychotherapy), the AID intervention was not associated with statistically significant differences in recurrent suicidal behaviors [44]. Subgroup analyses examining adult participants aged 20–39 and 40 and older also did not find differences in recurrent suicidal behavior between groups [44].
The Baerum Model and OPAC
A municipal suicide prevention team that provides comprehensive social services to suicide attempters has operated in Baerum, Norway, since 1983 [45]. Under the Baerum model, patients who attempt suicide, can be discharged from the general hospital without psychiatric admission, and are determined to have a high level of need for support are connected by a hospital-based suicide prevention team to a community-based team consisting of nurses and a consulting psychologist, who subsequently engage patients in own their homes and through follow-up phone calls. The services they provide include care coordination, encouragement, activation of social networks, psychological first-aid, and counseling focused on problem-solving. The ostensible goal of the suicide prevention team is to provide a bridge between inpatient medical care and outpatient mental health treatment; however, the intervention lasts approximately 1 year regardless of whether the patient connects with a treatment program [45].
A retrospective comparison of outcomes between recipients of the original Baerum program and non-recipients failed to find a difference in suicide attempts or suicide deaths between groups [45]. However, this was not a controlled study, and suicide attempters were preferentially referred to the program based on whether they had a higher level of need at baseline. Hvid and Wang adapted this model to patients who presented to EDs and general hospitals in Amager, Denmark [46] and have since conducted a series of randomized controlled trials comparing their adaptation to usual care. The Danish version of the Baerum model, renamed OPAC (for “outreach, problem solving, adherence, continuity”), provides similar case management and counseling services but for a maximum of 6 months. In their studies, OPAC significantly reduced the number of patients with a repeat suicide attempt and the total number of repeat suicide attempts at a 1-year interval, and this effect on total number of suicide attempts was sustained at 5 years [47,48]. Although the OPAC protocol begins with a patient’s presentation to the ED, the intervention is initiated after admission to the general hospital. Therefore, while this may inspire a model that provides similar services directly from the ED to patients who do not require general hospital admission, the existing model is not entirely based in the ED.
Discussion
The needs of suicidal patients are often multidimensional, and in some cases their risks are driven by psychosocial problems in addition to, or instead of, medically modifiable psychiatric conditions [49]. However, developing an ED-based program to support patients who are at risk of suicide after they are discharged from the ED is possible. Many such programs that provide or facilitate caring contacts, family support, case management, and/or treatment engagement with discharged patients have demonstrated that similar strategies may have the potential to impact future suicidal behavior. Nonetheless, it would be a stretch to say that all hospital systems should immediately begin doing so.
A new post-discharge support program is an investment of financial resources, personnel, and sometimes technology. Successful delivery of support or messages in any format requires that the intended recipient be able to receive it via reliable access to a working address, telephone number, or electronic device. Nonetheless, programs that rely on BCIs alone (excluding those conducted via telephone) cost relatively little to implement and thus would require a smaller investment than programs that require synchronous telephone or face-to-face contacts with staff in addition to or instead of BCIs. Costs for synchronous programs will also vary depending on the frequency and duration of contacts and the licensure and training required of the staff who provide them.
A trend toward better outcomes associating with more resource-intensive programs is easy to imagine but has not been definitively demonstrated. The wide variation between protocols in all types of programs makes comparisons between those that do and do not include synchronous contacts, and between types of synchronous contacts, difficult. Meanwhile, the low cost of BCIs alone could increase their attractiveness as an investment regardless of the magnitude of outcome improvement.
Denchev et al constructed a cost/benefit comparison model that included the postcard BCI study conducted by Carter et al [20], the telephone outreach study conducted by Vaiva et al [23], and a study of cognitive behavioral therapy (CBT) [11], all of which showed a clinical benefit. This model relied upon some numeric estimations and did not account for variation in outcomes between individual studies of each intervention strategy. However, it concluded that both telephone outreach and CBT were likely to be cost-prohibitive compared to asynchronous BCIs, which were associated with a reduction in costs overall [28].
Conclusion
There remains much to learn regarding how best to reduce suicide risk among adult patients in the period after discharge from the ED, during which patients with an identified suicide risk are known to be vulnerable. However, providing psychosocial and emotional support to patients with an identified suicide risk after they are discharged from the ED is feasible and may reduce subsequent suicidal behaviors. Templates for providing supportive outreach using different modalities now exist, and these may help guide the ongoing development and widespread adoption of more effective and cost-effective solutions.
Corresponding author: David S. Kroll, MD, [email protected].
Financial disclosure: Dr. Kroll has received research funding from Brigham and Women’s Hospital to study and develop technological solutions for supporting suicidal patients after discharge from the emergency department. He has additionally received research funding and a speaking honorarium from Avasure.
1. Betz ME, Boudreaux ED. Managing suicidal patients in the emergency department. Ann Emerg Med 2016;67:276–82.
2. McManus MC, Cramer RJ, Boshier M, et al. Mental health and drivers of need in emergent and non-emergent emergency department (ED) use: do living location and non-emergent care sources matter? Int J Environ Res Public Health 2018;15:129.
3. Ting SA, Sullivan AF, Boudreaux ED, et al. Trends in US emergency department visits for attempted suicide and self-inflicted injury, 1993-2008. Gen Hosp Psychiatry 2012;34:557–65.
4. Betz ME, Wintersteen M, Boudreaux ED, Brown G, Capoccia L, Currier G, et al. reducing suicide risk: challenges and opportunities in the emergency department. Ann Emerg Med 2016;68:758–65.
5. The Joint Commission. Sentinel event alert 56: detecting and treating suicide ideation in all settings. www.jointcommission.org/sea_issue_56/. Published February 24, 2016. Accessed June 4, 2018.
6. Mills PD, Watts BV, Hemphill RR. Suicide attempts and completions on medical-surgical and intensive care units. J Hosp Med 2014;9:182–5.
7. Crane EH. Patients with drug-related emergency department visits involving suicide attempts who left against medical advice. The CBHSQ Report. http://www.ncbi.nlm.nih.gov/books/NBK396153/ . Published September 13, 2016. Accessed June 4, 2018.
8. Fedyszyn IE, Erlangsen A, Hjorthøj C, et al. Repeated suicide attempts and suicide among individuals with a first emergency department contact for attempted suicide: a prospective, nationwide, Danish register-based study. J Clin Psychiatry 2016;77:832–40.
9. Hunter J, Maunder R, Kurdyak P, et al. Mental health follow-up after deliberate self-harm and risk for repeat self-harm and death. Psychiatry Res 2018;259:333–9.
10. Costemale-Lacoste JF, Balaguer E, Boniface B, et al. Outpatient treatment engagement after suicidal attempt: a multisite prospective study. Psychiatry Res 2017;258:21–3.
11. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA 2005;294:563–70.
12. Gysin-Maillart A, Schwab S, Soravia L, Megert M, Michel K. A novel brief therapy for patients who attempt suicide: a 24-months follow-up randomized controlled study of the attempted suicide short intervention program (ASSIP). PLoS Med 2016;13:e1001968.
13. Hawton K, Witt KG, Salisbury TLT, et al. Psychosocial interventions following self-harm in adults: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3:740–50.
14. Battaglia J, Wolff TK, Wagner-Johnson DS, et al. Structured diagnostic assessment and depot fluphenazine treatment of multiple suicide attempters in the emergency department. Int Clin Psychopharmacol 1999;14:361–72.
15. van der Sande R, van Rooijen L, Buskens E, et al. Intensive in-patient and community intervention versus routine care after attempted suicide. A randomised controlled intervention study. Br J Psychiatry 1997;171:35–41.
16. Motto JA, Bostrom AG. A randomized controlled trial of postcrisis suicide prevention. Psychiatr Serv 2001;52:828–33.
17. Berrouiguet S, Larsen ME, Mesmeur C, Gravey M, Billot R, Walter M, et al. Toward mHealth brief contact interventions in suicide prevention: case series from the suicide intervention assisted by messages (SIAM) randomized controlled trial. JMIR MHealth UHealth 2018;6:e8.
18. Falcone G, Nardella A, Lamis DA, et al. Taking care of suicidal patients with new technologies and reaching-out means in the post-discharge period. World J Psychiatry 2017;7:163–76.
19. Milner A, Spittal MJ, Kapur N, et al. Mechanisms of brief contact interventions in clinical populations: a systematic review. BMC Psychiatry 2016;16:194.
20. Carter GL, Clover K, Whyte IM, et al. Postcards from the EDge: 5-year outcomes of a randomised controlled trial for hospital-treated self-poisoning. Br J Psychiatry 2013;202:372–80.
21. Hassanian-Moghaddam H, Sarjami S, Kolahi AA, Carter GL. Postcards in Persia: randomised controlled trial to reduce suicidal behaviours 12 months after hospital-treated self-poisoning. Br J Psychiatry 2011;198:309–16.
22. Luxton DD, Thomas EK, Chipps J, et al. Caring letters for suicide prevention: implementation of a multi-site randomized clinical trial in the U.S. military and Veteran Affairs healthcare systems. Contemp Clin Trials 2014;37(2):252–60.
23. Vaiva G, Vaiva G, Ducrocq F, et al. Effect of telephone contact on further suicide attempts in patients discharged from an emergency department: randomised controlled study. BMJ 2006;332:1241–5.
24. Cebrià AI, Parra I, Pàmias M, et al. Effectiveness of a telephone management programme for patients discharged from an emergency department after a suicide attempt: controlled study in a Spanish population. J Affect Disord 2013;147:269–76.
25. Cedereke M, Monti K, Ojehagen A. Telephone contact with patients in the year after a suicide attempt: does it affect treatment attendance and outcome? A randomised controlled study. Eur Psychiatry. 2002;17:82–91.
26. Vaiva G, Walter M, Al Arab AS, et al. ALGOS: the development of a randomized controlled trial testing a case management algorithm designed to reduce suicide risk among suicide attempters. BMC Psychiatry 2011;11:1.
27. Milner AJ, Carter G, Pirkis J, et al. Letters, green cards, telephone calls and postcards: systematic and meta-analytic review of brief contact interventions for reducing self-harm, suicide attempts and suicide. Br J Psychiatry. 2015;206:184–90.
28. Denchev P, Pearson JL, Allen MH, Claassen CA, Currier GW, Zatzick DF, et al. Modeling the cost-effectiveness of interventions to reduce suicide risk among hospital emergency department patients. Psychiatr Serv 2018;69:23–31.
29. Berrouiguet S, Courtet P, Larsen ME, et al. Suicide prevention: towards integrative, innovative and individualized brief contact interventions. Eur Psychiatry 2018;47:25–6.
30. Larsen ME, Shand F, Morley K, Batterham PJ, Petrie K, Reda B, et al. A mobile text message intervention to reduce repeat suicidal episodes: design and development of reconnecting after a suicide attempt (RAFT). JMIR Ment Health 2017;4:e56.
31. Morgan HG, Jones EM, Owen JH. Secondary prevention of non-fatal deliberate self-harm. The green card study. Br J Psychiatry 1993;163:111–2.
32. Evans MO, Morgan HG, Hayward A, Gunnell DJ. Crisis telephone consultation for deliberate self-harm patients: effects on repetition. Br J Psychiatry 1999;175:23–7.
33. Evans J, Evans M, Morgan HG, et al. Crisis card following self-harm: 12-month follow-up of a randomised controlled trial. Br J Psychiatry J 2005;187:186–7.
34. Fleischmann A, Bertolote JM, Wasserman D, et al. Effectiveness of brief intervention and contact for suicide attempters: a randomized controlled trial in five countries. Bull World Health Organ 2008;86:703–9.
35. Vijayakumar L, Umamaheswari C, Shujaath Ali ZS, et al. Intervention for suicide attempters: a randomized controlled study. Indian J Psychiatry 2011;53:244–8.
36. Bertolote JM, Fleischmann A, De Leo D, et al. Repetition of suicide attempts: data from emergency care settings in five culturally different low- and middle-income countries participating in the WHO SUPRE-MISS Study. Crisis 2010;31:194–201.
37. Mousavi SG, Zohreh R, Maracy MR, et al. The efficacy of telephonic follow up in prevention of suicidal reattempt in patients with suicide attempt history. Adv Biomed Res 2014;3:198.
38. Amadéo S, Rereao M, Malogne A, et al. Testing brief intervention and phone contact among subjects with suicidal behavior: a randomized controlled trial in French Polynesia in the frames of the World Health Organization/suicide trends in at-risk territories study. Ment Illn 2015;7:5818.
39. Riblet NBV, Shiner B, Young-Xu Y, Watts BV. Strategies to prevent death by suicide: meta-analysis of randomised controlled trials. Br J Psychiatry 2017;210:396–402.
40. Miller IW, Camargo CA Jr, Arias SA, et al. Suicide prevention in an emergency department population: the ED-SAFE study. JAMA Psychiatry 2017;74:563–70.
41. Miller IW, Gaudiano BA, Weinstock LM. The coping long term with active suicide program: description and pilot data. Suicide Life Threat Behav 2016;46:752–61.
42. Kawanishi C, Aruga T, Ishizuka N, et al. Assertive case management versus enhanced usual care for people with mental health problems who had attempted suicide and were admitted to hospital emergency departments in Japan (ACTION-J): a multicentre, randomised controlled trial. Lancet Psychiatry 2014;1:193–201.
43. Furuno T, Nakagawa M, Hino K, et al. Effectiveness of assertive case management on repeat self-harm in patients admitted for suicide attempt: findings from ACTION-J study. J Affect Disord 2018;225:460–5.
44. Morthorst B, Krogh J, Erlangsen A, et al. Effect of assertive outreach after suicide attempt in the AID (assertive intervention for deliberate self harm) trial: randomised controlled trial. BMJ 2012;345:e4972.
45. Johannessen HA, Dieserud G, De Leo D, Claussen B, et al. Chain of care for patients who have attempted suicide: a follow-up study from Bærum, Norway. BMC Public Health 2011;11:81.
46. Hvid M, Wang AG. Preventing repetition of attempted suicide—I. Feasibility (acceptability, adherence, and effectiveness) of a Baerum-model like aftercare. Nord J Psychiatry 2009;63:148–53.
47. Hvid M, Vangborg K, Sørensen HJ, et al. Preventing repetition of attempted suicide-II. The Amager project, a randomized controlled trial. Nord J Psychiatry 2011;65:292–8.
48. Lahoz T, Hvid M, Wang AG. Preventing repetition of attempted suicide-III. The Amager project, 5-year follow-up of a randomized controlled trial. Nord J Psychiatry 2016;70:547–53.
49. Kroll DS, Karno J, Mullen B, et al. Clinical severity alone does not determine disposition decisions for patients in the emergency department with suicide risk. Psychosomatics 2017; pii: S0033-3182(17)30247–5.
From the Department of Psychiatry, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Abstract
- Objective: To provide a review of emergency department (ED)-based psychosocial interventions that support adult patients with an identified suicide risk towards a goal of reducing subsequent suicidal behavior through the period after discharge, which is known to be a time of high risk for suicidal behavior.
- Methods: Non-systematic review of the literature.
- Results: Multiple methods of engaging patients after discharge from the ED have been shown to reduce subsequent suicidal behaviors. These methods include sending caring letters in the mail, facilitating supportive phone conversations, case management, and protocols that combine different services. Overall, the existing literature is insufficient to recommend widespread adoption of any individual strategy or protocol. However, providing psychosocial and emotional support to patients with an identified suicide risk after they are discharged from the ED is feasible and may reduce subsequent suicidal behaviors. Templates for providing supportive outreach using different modalities now exist, and these may help guide the ongoing development and widespread adoption of more effective and cost-effective solutions.
- Conclusion: Many ED–based interventions that provide enhanced support to patients with suicide risk after they are discharged have demonstrated a potential to reduce the risk of future suicidal behavior.
Key words: suicide; emergency department.
Despite the fact that emergency department (ED) providers often feel unprepared to manage suicide risk, patients with significant suicide risk frequently receive care in EDs, whether or not they have sustained physical injuries resulting from suicidal behavior [1,2]. Patients make greater than 400,000 visits to EDs in the United States each year for suicidal and self-injurious behaviors (suicide attempts and self-injurious behaviors are typically coded in ways that make them indistinguishable from each other in retrospective analyses) [3], and it is estimated that 6% to 10% of all patients in EDs endorse suicidal ideation when asked, regardless of their original chief complaints [4]. Meanwhile, suicide has become the 10th leading cause of death in the United States [5], and the Joint Commission has charged all accredited health care organizations with providing comprehensive treatment to suicidal patients, which may range from immediately containing an acute risk to ensuring continuity of care in follow-up [5].
When an acute suicide risk is identified in the ED, the provider’s immediate next steps should be to place the patient in a safe area under constant observation and to provide an emergency assessment [5,6]. Although psychiatric consultation and/or psychiatric admission may follow this assessment, suicide risk does not require admission in all cases; and some patients with suicide risk may be discharged to an outpatient setting even without receiving a psychiatric consultation [1]. Regardless of whether an outpatient disposition from the ED is appropriate, however, the period that immediately follows discharge is a time of high risk for repeated suicidal behavior and suicide death [7–9], and only 30% to 50% of patients who are discharged from EDs after a self-harm incident actually keep a follow-up mental health appointment [9,10]. Therefore, any support given to patients through this transition out of the emergency care setting could be especially high-yield.
The Joint Commission recommends that all patients with suicidal ideation receive, at minimum, a referral to treatment, telephone numbers for local and national crisis support resources (including the National Suicide Prevention Lifeline 1-800-273-TALK), collaborative safety planning, and counseling to restrict access to lethal means upon discharge [5]. However, some programs have demonstrated the capacity to provide enhanced support to patients beyond discharge from the ED, with some success in reducing the rates of subsequent suicidal behaviors. This non-systematic review describes interventions that can be initiated in the context of an ED encounter with the purpose of reducing future suicidal behavior among adult patients. They are primarily psychosocial rather than clinical. Clinical interventions that apply psychotherapy [11–13] psychopharmacology [14], and specialized inpatient treatments [15] have been studied as well but are beyond the scope of this review.
[polldaddy:10107269]
Interventions to Support Patients At Risk of Suicide After Discharge from the ED
Brief Contact Interventions
The idea that maintaining written correspondence with patients who have a known suicide risk after discharge can reduce subsequent suicide rates originated with a study of psychiatric inpatients conducted by Motto and Bostrom, in which patients who had been admitted for depression but had declined outpatient treatment were randomly assigned to periodically receive letters containing supportive messages from staff members over a period of 5 years [16]. This study remarkably found that these so-called brief contact interventions (BCIs), which were personalized to each recipient but did not contain psychotherapy per se, were associated with a reduced rate of suicide throughout the duration of the program compared with no written contacts [16].
BCIs have since been adapted to other communication formats and have been studied in patients who were discharged directly from the ED after an evaluation of suicide risk or suicidal behavior. Typically, BCIs consist of short, supportive messages that are delivered at regular intervals (often once every 1–2 months) over a period of 1 to 5 years [17,18]. They notably do not contain psychotherapy content, although they may reinforce coping strategies or remind recipients of how to access help if needed [17,19]. They may arrive as postcards [20,21], letters [22], telephone outreach [23–25], or a combination of modalities [26].
Protocols that rely on BCIs alone vary in their structure and have yielded mixed results [18]. A meta-analysis of 12 BCI protocols conducted by Milner et al found that, overall, BCIs administered after a presentation to the ED for self-harm have been associated with a significant reduction in repeat suicide attempts per recipient but not in total suicide deaths [27]. Milner’s group did not recommend large-scale promotion of BCIs based on the inadequacy of data so far, but suggested that this strategy may yet show promise upon further study [27]. A key advantage of BCIs is that they are inexpensive to implement, particularly if they do not include a telephone outreach component [28]. Thus, even if the potential benefit to patients is small, administering BCIs can be cost-effective [28].
It should not come as a surprise, therefore, that the potential for incorporation of BCIs into mobile smartphone technology is currently under investigation. Individuals who own mobile phones typically keep them on their persons and turned on continuously, and thus this is a reliable platform for maintaining contact with a wide range of patients in real-time [17,29]. Developers of at least 2 BCI smartphone programs that rely on mobile text messaging have published their protocols [17,30]. However, whether these programs will succeed in meaningfully reducing suicide rates remains to be determined by future research.
Green Cards
Morgan et al conducted a study in the United Kingdom in which individuals who presented to EDs after a self-harm event received a “green card,” which contained encouraging messages about seeking help and provided contact information for emergency services with 24-hour availability [31]. The green card also facilitated access to a crisis admission if necessary. The green card was distributed first in the ED and a second time by mail 3 weeks later. No suicides occurred in either the intervention or control group, which received usual care, and no statistically significant differences in suicide reattempt rate were found between groups after 1 year [31].
Evans et al studied an updated version of the green card intervention in which the green card facilitated access to an on-call psychiatrist with 24-hour availability by telephone [32]. The updated card included encouraging messages about seeking help similar to the original green card described by Morgan; however, the psychiatry consultation via telephone replaced the offer of hospital admission [32]. This second trial of green cards also failed to show a reduction in the rate of suicide reattempts among green card recipients at 6 months and 1 year [32,33].
Brief Intervention and Contact
The World Health Organization’s Brief Intervention and Contact (BIC) protocol is a standardized, multi-step suicide prevention program that has been studied primarily in patients who present to EDs after a suicide attempt in middle-income countries [34]. BIC includes a 1-hour information session that is administered shortly prior to discharge, and subsequently provides 9 follow-up contact interventions at specified intervals over an 18-month period. Unlike in a typical BCI, the contacts in BIC are conducted by a clinician either face-to-face or over the phone and include standardized assessments of the patient’s condition, although they still do not include psychotherapy. BIC has been shown to reduce suicide attempts, suicide deaths, or both in India [34–36], Iran [34,36,37], China [34,36], Brazil [34,36], and Sri Lanka [34,36] but was not found to directly improve clinical outcomes in a study conducted in French Polynesia [38]. A meta-analysis conducted by Riblet et al concluded that BIC is effective in reducing suicide risk overall [39].
ED-SAFE
The Emergency Department Safety Assessment and Follow-up Evaluation (ED-SAFE) protocol was validated in 8 EDs in 7 states in the US that did not already provide psychiatric services internally [40]. Under this model, all patients in the ED receive a screening for suicide risk, and those with an initial positive screen receive a secondary screen administered by the ED physician, a self-administered safety plan, and a series of up to 11 phone contacts over the following year that are administered by trained mental health clinicians in a central location. The ED-SAFE phone contacts follow the Coping Long Term with Active Suicide Program (CLASP) protocol [41] and provide support around safety planning and treatment engagement. They have the capacity to engage the patients’ significant others directly if a significant other is available and the patient chooses to involve that person.
In a single multicenter study, ED-SAFE reduced the absolute risk of suicide attempt by 5%, and the relative risk by 20% compared to usual treatment [40]. An intermediate phase of the study compared the universal suicide screening alone (ie, without the safety plan or follow-up contacts) with usual care and did not find this to improve outcomes [40].
Case Management
Kawanishi et al conducted a randomized controlled trial of assertive case management, the ACTION-J study, for patients with psychiatric diagnoses who presented with self-harm to 17 participating EDs in Japan [42]. In the ACTION-J study, case managers were mental health clinicians who provided clinical evaluations, treatment planning, encouragement, and care coordination over the course of 7 scheduled face-to-face or phone contacts in the first 18 months, and additional contacts at 6-month intervals until the completion of the trial (up to a total of 5 years) [43]. The comparison intervention, enhanced usual care, consisted of psychoeducation provided at the time of the encounter in the ED without case management services. The assertive case management intervention was associated with a decrease in suicidal behavior in the first 6 months but not for the duration of the study, except in women, for whom the benefit lasted the full 18 months [42]. A subsequent analysis also found a decrease in the total number of self-harm episodes per person-year compared to enhanced usual care, although there was not a difference in the number of participants who experienced a repeat self-harm episode [43]. The benefit was most strongly pronounced among patients who had presented with an index suicide attempt [43].
Morthorst et al applied an alternative case management model for the assertive intervention for deliberate self harm (AID) trial, which took place in Denmark [44]. Participants were aged 12 and older and could have been recruited from medical or pediatric inpatient units as well as the ED after a self-harm event. AID employed psychiatric nurses to provide crisis intervention, crisis planning, problem solving, motivational support, family mediation, and assistance with keeping appointments over a period of 6 months following discharge. Outreach took place over the phone, by text message, in participants’ homes, in cafes, and at health and social services appointments. The intervention required at least 4 contacts, although additional contacts could be made if appropriate. In comparison with a control group, in which participants received only usual care (which included ready access to short-term psychotherapy), the AID intervention was not associated with statistically significant differences in recurrent suicidal behaviors [44]. Subgroup analyses examining adult participants aged 20–39 and 40 and older also did not find differences in recurrent suicidal behavior between groups [44].
The Baerum Model and OPAC
A municipal suicide prevention team that provides comprehensive social services to suicide attempters has operated in Baerum, Norway, since 1983 [45]. Under the Baerum model, patients who attempt suicide, can be discharged from the general hospital without psychiatric admission, and are determined to have a high level of need for support are connected by a hospital-based suicide prevention team to a community-based team consisting of nurses and a consulting psychologist, who subsequently engage patients in own their homes and through follow-up phone calls. The services they provide include care coordination, encouragement, activation of social networks, psychological first-aid, and counseling focused on problem-solving. The ostensible goal of the suicide prevention team is to provide a bridge between inpatient medical care and outpatient mental health treatment; however, the intervention lasts approximately 1 year regardless of whether the patient connects with a treatment program [45].
A retrospective comparison of outcomes between recipients of the original Baerum program and non-recipients failed to find a difference in suicide attempts or suicide deaths between groups [45]. However, this was not a controlled study, and suicide attempters were preferentially referred to the program based on whether they had a higher level of need at baseline. Hvid and Wang adapted this model to patients who presented to EDs and general hospitals in Amager, Denmark [46] and have since conducted a series of randomized controlled trials comparing their adaptation to usual care. The Danish version of the Baerum model, renamed OPAC (for “outreach, problem solving, adherence, continuity”), provides similar case management and counseling services but for a maximum of 6 months. In their studies, OPAC significantly reduced the number of patients with a repeat suicide attempt and the total number of repeat suicide attempts at a 1-year interval, and this effect on total number of suicide attempts was sustained at 5 years [47,48]. Although the OPAC protocol begins with a patient’s presentation to the ED, the intervention is initiated after admission to the general hospital. Therefore, while this may inspire a model that provides similar services directly from the ED to patients who do not require general hospital admission, the existing model is not entirely based in the ED.
Discussion
The needs of suicidal patients are often multidimensional, and in some cases their risks are driven by psychosocial problems in addition to, or instead of, medically modifiable psychiatric conditions [49]. However, developing an ED-based program to support patients who are at risk of suicide after they are discharged from the ED is possible. Many such programs that provide or facilitate caring contacts, family support, case management, and/or treatment engagement with discharged patients have demonstrated that similar strategies may have the potential to impact future suicidal behavior. Nonetheless, it would be a stretch to say that all hospital systems should immediately begin doing so.
A new post-discharge support program is an investment of financial resources, personnel, and sometimes technology. Successful delivery of support or messages in any format requires that the intended recipient be able to receive it via reliable access to a working address, telephone number, or electronic device. Nonetheless, programs that rely on BCIs alone (excluding those conducted via telephone) cost relatively little to implement and thus would require a smaller investment than programs that require synchronous telephone or face-to-face contacts with staff in addition to or instead of BCIs. Costs for synchronous programs will also vary depending on the frequency and duration of contacts and the licensure and training required of the staff who provide them.
A trend toward better outcomes associating with more resource-intensive programs is easy to imagine but has not been definitively demonstrated. The wide variation between protocols in all types of programs makes comparisons between those that do and do not include synchronous contacts, and between types of synchronous contacts, difficult. Meanwhile, the low cost of BCIs alone could increase their attractiveness as an investment regardless of the magnitude of outcome improvement.
Denchev et al constructed a cost/benefit comparison model that included the postcard BCI study conducted by Carter et al [20], the telephone outreach study conducted by Vaiva et al [23], and a study of cognitive behavioral therapy (CBT) [11], all of which showed a clinical benefit. This model relied upon some numeric estimations and did not account for variation in outcomes between individual studies of each intervention strategy. However, it concluded that both telephone outreach and CBT were likely to be cost-prohibitive compared to asynchronous BCIs, which were associated with a reduction in costs overall [28].
Conclusion
There remains much to learn regarding how best to reduce suicide risk among adult patients in the period after discharge from the ED, during which patients with an identified suicide risk are known to be vulnerable. However, providing psychosocial and emotional support to patients with an identified suicide risk after they are discharged from the ED is feasible and may reduce subsequent suicidal behaviors. Templates for providing supportive outreach using different modalities now exist, and these may help guide the ongoing development and widespread adoption of more effective and cost-effective solutions.
Corresponding author: David S. Kroll, MD, [email protected].
Financial disclosure: Dr. Kroll has received research funding from Brigham and Women’s Hospital to study and develop technological solutions for supporting suicidal patients after discharge from the emergency department. He has additionally received research funding and a speaking honorarium from Avasure.
From the Department of Psychiatry, Brigham and Women’s Hospital, and Harvard Medical School, Boston, MA.
Abstract
- Objective: To provide a review of emergency department (ED)-based psychosocial interventions that support adult patients with an identified suicide risk towards a goal of reducing subsequent suicidal behavior through the period after discharge, which is known to be a time of high risk for suicidal behavior.
- Methods: Non-systematic review of the literature.
- Results: Multiple methods of engaging patients after discharge from the ED have been shown to reduce subsequent suicidal behaviors. These methods include sending caring letters in the mail, facilitating supportive phone conversations, case management, and protocols that combine different services. Overall, the existing literature is insufficient to recommend widespread adoption of any individual strategy or protocol. However, providing psychosocial and emotional support to patients with an identified suicide risk after they are discharged from the ED is feasible and may reduce subsequent suicidal behaviors. Templates for providing supportive outreach using different modalities now exist, and these may help guide the ongoing development and widespread adoption of more effective and cost-effective solutions.
- Conclusion: Many ED–based interventions that provide enhanced support to patients with suicide risk after they are discharged have demonstrated a potential to reduce the risk of future suicidal behavior.
Key words: suicide; emergency department.
Despite the fact that emergency department (ED) providers often feel unprepared to manage suicide risk, patients with significant suicide risk frequently receive care in EDs, whether or not they have sustained physical injuries resulting from suicidal behavior [1,2]. Patients make greater than 400,000 visits to EDs in the United States each year for suicidal and self-injurious behaviors (suicide attempts and self-injurious behaviors are typically coded in ways that make them indistinguishable from each other in retrospective analyses) [3], and it is estimated that 6% to 10% of all patients in EDs endorse suicidal ideation when asked, regardless of their original chief complaints [4]. Meanwhile, suicide has become the 10th leading cause of death in the United States [5], and the Joint Commission has charged all accredited health care organizations with providing comprehensive treatment to suicidal patients, which may range from immediately containing an acute risk to ensuring continuity of care in follow-up [5].
When an acute suicide risk is identified in the ED, the provider’s immediate next steps should be to place the patient in a safe area under constant observation and to provide an emergency assessment [5,6]. Although psychiatric consultation and/or psychiatric admission may follow this assessment, suicide risk does not require admission in all cases; and some patients with suicide risk may be discharged to an outpatient setting even without receiving a psychiatric consultation [1]. Regardless of whether an outpatient disposition from the ED is appropriate, however, the period that immediately follows discharge is a time of high risk for repeated suicidal behavior and suicide death [7–9], and only 30% to 50% of patients who are discharged from EDs after a self-harm incident actually keep a follow-up mental health appointment [9,10]. Therefore, any support given to patients through this transition out of the emergency care setting could be especially high-yield.
The Joint Commission recommends that all patients with suicidal ideation receive, at minimum, a referral to treatment, telephone numbers for local and national crisis support resources (including the National Suicide Prevention Lifeline 1-800-273-TALK), collaborative safety planning, and counseling to restrict access to lethal means upon discharge [5]. However, some programs have demonstrated the capacity to provide enhanced support to patients beyond discharge from the ED, with some success in reducing the rates of subsequent suicidal behaviors. This non-systematic review describes interventions that can be initiated in the context of an ED encounter with the purpose of reducing future suicidal behavior among adult patients. They are primarily psychosocial rather than clinical. Clinical interventions that apply psychotherapy [11–13] psychopharmacology [14], and specialized inpatient treatments [15] have been studied as well but are beyond the scope of this review.
[polldaddy:10107269]
Interventions to Support Patients At Risk of Suicide After Discharge from the ED
Brief Contact Interventions
The idea that maintaining written correspondence with patients who have a known suicide risk after discharge can reduce subsequent suicide rates originated with a study of psychiatric inpatients conducted by Motto and Bostrom, in which patients who had been admitted for depression but had declined outpatient treatment were randomly assigned to periodically receive letters containing supportive messages from staff members over a period of 5 years [16]. This study remarkably found that these so-called brief contact interventions (BCIs), which were personalized to each recipient but did not contain psychotherapy per se, were associated with a reduced rate of suicide throughout the duration of the program compared with no written contacts [16].
BCIs have since been adapted to other communication formats and have been studied in patients who were discharged directly from the ED after an evaluation of suicide risk or suicidal behavior. Typically, BCIs consist of short, supportive messages that are delivered at regular intervals (often once every 1–2 months) over a period of 1 to 5 years [17,18]. They notably do not contain psychotherapy content, although they may reinforce coping strategies or remind recipients of how to access help if needed [17,19]. They may arrive as postcards [20,21], letters [22], telephone outreach [23–25], or a combination of modalities [26].
Protocols that rely on BCIs alone vary in their structure and have yielded mixed results [18]. A meta-analysis of 12 BCI protocols conducted by Milner et al found that, overall, BCIs administered after a presentation to the ED for self-harm have been associated with a significant reduction in repeat suicide attempts per recipient but not in total suicide deaths [27]. Milner’s group did not recommend large-scale promotion of BCIs based on the inadequacy of data so far, but suggested that this strategy may yet show promise upon further study [27]. A key advantage of BCIs is that they are inexpensive to implement, particularly if they do not include a telephone outreach component [28]. Thus, even if the potential benefit to patients is small, administering BCIs can be cost-effective [28].
It should not come as a surprise, therefore, that the potential for incorporation of BCIs into mobile smartphone technology is currently under investigation. Individuals who own mobile phones typically keep them on their persons and turned on continuously, and thus this is a reliable platform for maintaining contact with a wide range of patients in real-time [17,29]. Developers of at least 2 BCI smartphone programs that rely on mobile text messaging have published their protocols [17,30]. However, whether these programs will succeed in meaningfully reducing suicide rates remains to be determined by future research.
Green Cards
Morgan et al conducted a study in the United Kingdom in which individuals who presented to EDs after a self-harm event received a “green card,” which contained encouraging messages about seeking help and provided contact information for emergency services with 24-hour availability [31]. The green card also facilitated access to a crisis admission if necessary. The green card was distributed first in the ED and a second time by mail 3 weeks later. No suicides occurred in either the intervention or control group, which received usual care, and no statistically significant differences in suicide reattempt rate were found between groups after 1 year [31].
Evans et al studied an updated version of the green card intervention in which the green card facilitated access to an on-call psychiatrist with 24-hour availability by telephone [32]. The updated card included encouraging messages about seeking help similar to the original green card described by Morgan; however, the psychiatry consultation via telephone replaced the offer of hospital admission [32]. This second trial of green cards also failed to show a reduction in the rate of suicide reattempts among green card recipients at 6 months and 1 year [32,33].
Brief Intervention and Contact
The World Health Organization’s Brief Intervention and Contact (BIC) protocol is a standardized, multi-step suicide prevention program that has been studied primarily in patients who present to EDs after a suicide attempt in middle-income countries [34]. BIC includes a 1-hour information session that is administered shortly prior to discharge, and subsequently provides 9 follow-up contact interventions at specified intervals over an 18-month period. Unlike in a typical BCI, the contacts in BIC are conducted by a clinician either face-to-face or over the phone and include standardized assessments of the patient’s condition, although they still do not include psychotherapy. BIC has been shown to reduce suicide attempts, suicide deaths, or both in India [34–36], Iran [34,36,37], China [34,36], Brazil [34,36], and Sri Lanka [34,36] but was not found to directly improve clinical outcomes in a study conducted in French Polynesia [38]. A meta-analysis conducted by Riblet et al concluded that BIC is effective in reducing suicide risk overall [39].
ED-SAFE
The Emergency Department Safety Assessment and Follow-up Evaluation (ED-SAFE) protocol was validated in 8 EDs in 7 states in the US that did not already provide psychiatric services internally [40]. Under this model, all patients in the ED receive a screening for suicide risk, and those with an initial positive screen receive a secondary screen administered by the ED physician, a self-administered safety plan, and a series of up to 11 phone contacts over the following year that are administered by trained mental health clinicians in a central location. The ED-SAFE phone contacts follow the Coping Long Term with Active Suicide Program (CLASP) protocol [41] and provide support around safety planning and treatment engagement. They have the capacity to engage the patients’ significant others directly if a significant other is available and the patient chooses to involve that person.
In a single multicenter study, ED-SAFE reduced the absolute risk of suicide attempt by 5%, and the relative risk by 20% compared to usual treatment [40]. An intermediate phase of the study compared the universal suicide screening alone (ie, without the safety plan or follow-up contacts) with usual care and did not find this to improve outcomes [40].
Case Management
Kawanishi et al conducted a randomized controlled trial of assertive case management, the ACTION-J study, for patients with psychiatric diagnoses who presented with self-harm to 17 participating EDs in Japan [42]. In the ACTION-J study, case managers were mental health clinicians who provided clinical evaluations, treatment planning, encouragement, and care coordination over the course of 7 scheduled face-to-face or phone contacts in the first 18 months, and additional contacts at 6-month intervals until the completion of the trial (up to a total of 5 years) [43]. The comparison intervention, enhanced usual care, consisted of psychoeducation provided at the time of the encounter in the ED without case management services. The assertive case management intervention was associated with a decrease in suicidal behavior in the first 6 months but not for the duration of the study, except in women, for whom the benefit lasted the full 18 months [42]. A subsequent analysis also found a decrease in the total number of self-harm episodes per person-year compared to enhanced usual care, although there was not a difference in the number of participants who experienced a repeat self-harm episode [43]. The benefit was most strongly pronounced among patients who had presented with an index suicide attempt [43].
Morthorst et al applied an alternative case management model for the assertive intervention for deliberate self harm (AID) trial, which took place in Denmark [44]. Participants were aged 12 and older and could have been recruited from medical or pediatric inpatient units as well as the ED after a self-harm event. AID employed psychiatric nurses to provide crisis intervention, crisis planning, problem solving, motivational support, family mediation, and assistance with keeping appointments over a period of 6 months following discharge. Outreach took place over the phone, by text message, in participants’ homes, in cafes, and at health and social services appointments. The intervention required at least 4 contacts, although additional contacts could be made if appropriate. In comparison with a control group, in which participants received only usual care (which included ready access to short-term psychotherapy), the AID intervention was not associated with statistically significant differences in recurrent suicidal behaviors [44]. Subgroup analyses examining adult participants aged 20–39 and 40 and older also did not find differences in recurrent suicidal behavior between groups [44].
The Baerum Model and OPAC
A municipal suicide prevention team that provides comprehensive social services to suicide attempters has operated in Baerum, Norway, since 1983 [45]. Under the Baerum model, patients who attempt suicide, can be discharged from the general hospital without psychiatric admission, and are determined to have a high level of need for support are connected by a hospital-based suicide prevention team to a community-based team consisting of nurses and a consulting psychologist, who subsequently engage patients in own their homes and through follow-up phone calls. The services they provide include care coordination, encouragement, activation of social networks, psychological first-aid, and counseling focused on problem-solving. The ostensible goal of the suicide prevention team is to provide a bridge between inpatient medical care and outpatient mental health treatment; however, the intervention lasts approximately 1 year regardless of whether the patient connects with a treatment program [45].
A retrospective comparison of outcomes between recipients of the original Baerum program and non-recipients failed to find a difference in suicide attempts or suicide deaths between groups [45]. However, this was not a controlled study, and suicide attempters were preferentially referred to the program based on whether they had a higher level of need at baseline. Hvid and Wang adapted this model to patients who presented to EDs and general hospitals in Amager, Denmark [46] and have since conducted a series of randomized controlled trials comparing their adaptation to usual care. The Danish version of the Baerum model, renamed OPAC (for “outreach, problem solving, adherence, continuity”), provides similar case management and counseling services but for a maximum of 6 months. In their studies, OPAC significantly reduced the number of patients with a repeat suicide attempt and the total number of repeat suicide attempts at a 1-year interval, and this effect on total number of suicide attempts was sustained at 5 years [47,48]. Although the OPAC protocol begins with a patient’s presentation to the ED, the intervention is initiated after admission to the general hospital. Therefore, while this may inspire a model that provides similar services directly from the ED to patients who do not require general hospital admission, the existing model is not entirely based in the ED.
Discussion
The needs of suicidal patients are often multidimensional, and in some cases their risks are driven by psychosocial problems in addition to, or instead of, medically modifiable psychiatric conditions [49]. However, developing an ED-based program to support patients who are at risk of suicide after they are discharged from the ED is possible. Many such programs that provide or facilitate caring contacts, family support, case management, and/or treatment engagement with discharged patients have demonstrated that similar strategies may have the potential to impact future suicidal behavior. Nonetheless, it would be a stretch to say that all hospital systems should immediately begin doing so.
A new post-discharge support program is an investment of financial resources, personnel, and sometimes technology. Successful delivery of support or messages in any format requires that the intended recipient be able to receive it via reliable access to a working address, telephone number, or electronic device. Nonetheless, programs that rely on BCIs alone (excluding those conducted via telephone) cost relatively little to implement and thus would require a smaller investment than programs that require synchronous telephone or face-to-face contacts with staff in addition to or instead of BCIs. Costs for synchronous programs will also vary depending on the frequency and duration of contacts and the licensure and training required of the staff who provide them.
A trend toward better outcomes associating with more resource-intensive programs is easy to imagine but has not been definitively demonstrated. The wide variation between protocols in all types of programs makes comparisons between those that do and do not include synchronous contacts, and between types of synchronous contacts, difficult. Meanwhile, the low cost of BCIs alone could increase their attractiveness as an investment regardless of the magnitude of outcome improvement.
Denchev et al constructed a cost/benefit comparison model that included the postcard BCI study conducted by Carter et al [20], the telephone outreach study conducted by Vaiva et al [23], and a study of cognitive behavioral therapy (CBT) [11], all of which showed a clinical benefit. This model relied upon some numeric estimations and did not account for variation in outcomes between individual studies of each intervention strategy. However, it concluded that both telephone outreach and CBT were likely to be cost-prohibitive compared to asynchronous BCIs, which were associated with a reduction in costs overall [28].
Conclusion
There remains much to learn regarding how best to reduce suicide risk among adult patients in the period after discharge from the ED, during which patients with an identified suicide risk are known to be vulnerable. However, providing psychosocial and emotional support to patients with an identified suicide risk after they are discharged from the ED is feasible and may reduce subsequent suicidal behaviors. Templates for providing supportive outreach using different modalities now exist, and these may help guide the ongoing development and widespread adoption of more effective and cost-effective solutions.
Corresponding author: David S. Kroll, MD, [email protected].
Financial disclosure: Dr. Kroll has received research funding from Brigham and Women’s Hospital to study and develop technological solutions for supporting suicidal patients after discharge from the emergency department. He has additionally received research funding and a speaking honorarium from Avasure.
1. Betz ME, Boudreaux ED. Managing suicidal patients in the emergency department. Ann Emerg Med 2016;67:276–82.
2. McManus MC, Cramer RJ, Boshier M, et al. Mental health and drivers of need in emergent and non-emergent emergency department (ED) use: do living location and non-emergent care sources matter? Int J Environ Res Public Health 2018;15:129.
3. Ting SA, Sullivan AF, Boudreaux ED, et al. Trends in US emergency department visits for attempted suicide and self-inflicted injury, 1993-2008. Gen Hosp Psychiatry 2012;34:557–65.
4. Betz ME, Wintersteen M, Boudreaux ED, Brown G, Capoccia L, Currier G, et al. reducing suicide risk: challenges and opportunities in the emergency department. Ann Emerg Med 2016;68:758–65.
5. The Joint Commission. Sentinel event alert 56: detecting and treating suicide ideation in all settings. www.jointcommission.org/sea_issue_56/. Published February 24, 2016. Accessed June 4, 2018.
6. Mills PD, Watts BV, Hemphill RR. Suicide attempts and completions on medical-surgical and intensive care units. J Hosp Med 2014;9:182–5.
7. Crane EH. Patients with drug-related emergency department visits involving suicide attempts who left against medical advice. The CBHSQ Report. http://www.ncbi.nlm.nih.gov/books/NBK396153/ . Published September 13, 2016. Accessed June 4, 2018.
8. Fedyszyn IE, Erlangsen A, Hjorthøj C, et al. Repeated suicide attempts and suicide among individuals with a first emergency department contact for attempted suicide: a prospective, nationwide, Danish register-based study. J Clin Psychiatry 2016;77:832–40.
9. Hunter J, Maunder R, Kurdyak P, et al. Mental health follow-up after deliberate self-harm and risk for repeat self-harm and death. Psychiatry Res 2018;259:333–9.
10. Costemale-Lacoste JF, Balaguer E, Boniface B, et al. Outpatient treatment engagement after suicidal attempt: a multisite prospective study. Psychiatry Res 2017;258:21–3.
11. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA 2005;294:563–70.
12. Gysin-Maillart A, Schwab S, Soravia L, Megert M, Michel K. A novel brief therapy for patients who attempt suicide: a 24-months follow-up randomized controlled study of the attempted suicide short intervention program (ASSIP). PLoS Med 2016;13:e1001968.
13. Hawton K, Witt KG, Salisbury TLT, et al. Psychosocial interventions following self-harm in adults: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3:740–50.
14. Battaglia J, Wolff TK, Wagner-Johnson DS, et al. Structured diagnostic assessment and depot fluphenazine treatment of multiple suicide attempters in the emergency department. Int Clin Psychopharmacol 1999;14:361–72.
15. van der Sande R, van Rooijen L, Buskens E, et al. Intensive in-patient and community intervention versus routine care after attempted suicide. A randomised controlled intervention study. Br J Psychiatry 1997;171:35–41.
16. Motto JA, Bostrom AG. A randomized controlled trial of postcrisis suicide prevention. Psychiatr Serv 2001;52:828–33.
17. Berrouiguet S, Larsen ME, Mesmeur C, Gravey M, Billot R, Walter M, et al. Toward mHealth brief contact interventions in suicide prevention: case series from the suicide intervention assisted by messages (SIAM) randomized controlled trial. JMIR MHealth UHealth 2018;6:e8.
18. Falcone G, Nardella A, Lamis DA, et al. Taking care of suicidal patients with new technologies and reaching-out means in the post-discharge period. World J Psychiatry 2017;7:163–76.
19. Milner A, Spittal MJ, Kapur N, et al. Mechanisms of brief contact interventions in clinical populations: a systematic review. BMC Psychiatry 2016;16:194.
20. Carter GL, Clover K, Whyte IM, et al. Postcards from the EDge: 5-year outcomes of a randomised controlled trial for hospital-treated self-poisoning. Br J Psychiatry 2013;202:372–80.
21. Hassanian-Moghaddam H, Sarjami S, Kolahi AA, Carter GL. Postcards in Persia: randomised controlled trial to reduce suicidal behaviours 12 months after hospital-treated self-poisoning. Br J Psychiatry 2011;198:309–16.
22. Luxton DD, Thomas EK, Chipps J, et al. Caring letters for suicide prevention: implementation of a multi-site randomized clinical trial in the U.S. military and Veteran Affairs healthcare systems. Contemp Clin Trials 2014;37(2):252–60.
23. Vaiva G, Vaiva G, Ducrocq F, et al. Effect of telephone contact on further suicide attempts in patients discharged from an emergency department: randomised controlled study. BMJ 2006;332:1241–5.
24. Cebrià AI, Parra I, Pàmias M, et al. Effectiveness of a telephone management programme for patients discharged from an emergency department after a suicide attempt: controlled study in a Spanish population. J Affect Disord 2013;147:269–76.
25. Cedereke M, Monti K, Ojehagen A. Telephone contact with patients in the year after a suicide attempt: does it affect treatment attendance and outcome? A randomised controlled study. Eur Psychiatry. 2002;17:82–91.
26. Vaiva G, Walter M, Al Arab AS, et al. ALGOS: the development of a randomized controlled trial testing a case management algorithm designed to reduce suicide risk among suicide attempters. BMC Psychiatry 2011;11:1.
27. Milner AJ, Carter G, Pirkis J, et al. Letters, green cards, telephone calls and postcards: systematic and meta-analytic review of brief contact interventions for reducing self-harm, suicide attempts and suicide. Br J Psychiatry. 2015;206:184–90.
28. Denchev P, Pearson JL, Allen MH, Claassen CA, Currier GW, Zatzick DF, et al. Modeling the cost-effectiveness of interventions to reduce suicide risk among hospital emergency department patients. Psychiatr Serv 2018;69:23–31.
29. Berrouiguet S, Courtet P, Larsen ME, et al. Suicide prevention: towards integrative, innovative and individualized brief contact interventions. Eur Psychiatry 2018;47:25–6.
30. Larsen ME, Shand F, Morley K, Batterham PJ, Petrie K, Reda B, et al. A mobile text message intervention to reduce repeat suicidal episodes: design and development of reconnecting after a suicide attempt (RAFT). JMIR Ment Health 2017;4:e56.
31. Morgan HG, Jones EM, Owen JH. Secondary prevention of non-fatal deliberate self-harm. The green card study. Br J Psychiatry 1993;163:111–2.
32. Evans MO, Morgan HG, Hayward A, Gunnell DJ. Crisis telephone consultation for deliberate self-harm patients: effects on repetition. Br J Psychiatry 1999;175:23–7.
33. Evans J, Evans M, Morgan HG, et al. Crisis card following self-harm: 12-month follow-up of a randomised controlled trial. Br J Psychiatry J 2005;187:186–7.
34. Fleischmann A, Bertolote JM, Wasserman D, et al. Effectiveness of brief intervention and contact for suicide attempters: a randomized controlled trial in five countries. Bull World Health Organ 2008;86:703–9.
35. Vijayakumar L, Umamaheswari C, Shujaath Ali ZS, et al. Intervention for suicide attempters: a randomized controlled study. Indian J Psychiatry 2011;53:244–8.
36. Bertolote JM, Fleischmann A, De Leo D, et al. Repetition of suicide attempts: data from emergency care settings in five culturally different low- and middle-income countries participating in the WHO SUPRE-MISS Study. Crisis 2010;31:194–201.
37. Mousavi SG, Zohreh R, Maracy MR, et al. The efficacy of telephonic follow up in prevention of suicidal reattempt in patients with suicide attempt history. Adv Biomed Res 2014;3:198.
38. Amadéo S, Rereao M, Malogne A, et al. Testing brief intervention and phone contact among subjects with suicidal behavior: a randomized controlled trial in French Polynesia in the frames of the World Health Organization/suicide trends in at-risk territories study. Ment Illn 2015;7:5818.
39. Riblet NBV, Shiner B, Young-Xu Y, Watts BV. Strategies to prevent death by suicide: meta-analysis of randomised controlled trials. Br J Psychiatry 2017;210:396–402.
40. Miller IW, Camargo CA Jr, Arias SA, et al. Suicide prevention in an emergency department population: the ED-SAFE study. JAMA Psychiatry 2017;74:563–70.
41. Miller IW, Gaudiano BA, Weinstock LM. The coping long term with active suicide program: description and pilot data. Suicide Life Threat Behav 2016;46:752–61.
42. Kawanishi C, Aruga T, Ishizuka N, et al. Assertive case management versus enhanced usual care for people with mental health problems who had attempted suicide and were admitted to hospital emergency departments in Japan (ACTION-J): a multicentre, randomised controlled trial. Lancet Psychiatry 2014;1:193–201.
43. Furuno T, Nakagawa M, Hino K, et al. Effectiveness of assertive case management on repeat self-harm in patients admitted for suicide attempt: findings from ACTION-J study. J Affect Disord 2018;225:460–5.
44. Morthorst B, Krogh J, Erlangsen A, et al. Effect of assertive outreach after suicide attempt in the AID (assertive intervention for deliberate self harm) trial: randomised controlled trial. BMJ 2012;345:e4972.
45. Johannessen HA, Dieserud G, De Leo D, Claussen B, et al. Chain of care for patients who have attempted suicide: a follow-up study from Bærum, Norway. BMC Public Health 2011;11:81.
46. Hvid M, Wang AG. Preventing repetition of attempted suicide—I. Feasibility (acceptability, adherence, and effectiveness) of a Baerum-model like aftercare. Nord J Psychiatry 2009;63:148–53.
47. Hvid M, Vangborg K, Sørensen HJ, et al. Preventing repetition of attempted suicide-II. The Amager project, a randomized controlled trial. Nord J Psychiatry 2011;65:292–8.
48. Lahoz T, Hvid M, Wang AG. Preventing repetition of attempted suicide-III. The Amager project, 5-year follow-up of a randomized controlled trial. Nord J Psychiatry 2016;70:547–53.
49. Kroll DS, Karno J, Mullen B, et al. Clinical severity alone does not determine disposition decisions for patients in the emergency department with suicide risk. Psychosomatics 2017; pii: S0033-3182(17)30247–5.
1. Betz ME, Boudreaux ED. Managing suicidal patients in the emergency department. Ann Emerg Med 2016;67:276–82.
2. McManus MC, Cramer RJ, Boshier M, et al. Mental health and drivers of need in emergent and non-emergent emergency department (ED) use: do living location and non-emergent care sources matter? Int J Environ Res Public Health 2018;15:129.
3. Ting SA, Sullivan AF, Boudreaux ED, et al. Trends in US emergency department visits for attempted suicide and self-inflicted injury, 1993-2008. Gen Hosp Psychiatry 2012;34:557–65.
4. Betz ME, Wintersteen M, Boudreaux ED, Brown G, Capoccia L, Currier G, et al. reducing suicide risk: challenges and opportunities in the emergency department. Ann Emerg Med 2016;68:758–65.
5. The Joint Commission. Sentinel event alert 56: detecting and treating suicide ideation in all settings. www.jointcommission.org/sea_issue_56/. Published February 24, 2016. Accessed June 4, 2018.
6. Mills PD, Watts BV, Hemphill RR. Suicide attempts and completions on medical-surgical and intensive care units. J Hosp Med 2014;9:182–5.
7. Crane EH. Patients with drug-related emergency department visits involving suicide attempts who left against medical advice. The CBHSQ Report. http://www.ncbi.nlm.nih.gov/books/NBK396153/ . Published September 13, 2016. Accessed June 4, 2018.
8. Fedyszyn IE, Erlangsen A, Hjorthøj C, et al. Repeated suicide attempts and suicide among individuals with a first emergency department contact for attempted suicide: a prospective, nationwide, Danish register-based study. J Clin Psychiatry 2016;77:832–40.
9. Hunter J, Maunder R, Kurdyak P, et al. Mental health follow-up after deliberate self-harm and risk for repeat self-harm and death. Psychiatry Res 2018;259:333–9.
10. Costemale-Lacoste JF, Balaguer E, Boniface B, et al. Outpatient treatment engagement after suicidal attempt: a multisite prospective study. Psychiatry Res 2017;258:21–3.
11. Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA 2005;294:563–70.
12. Gysin-Maillart A, Schwab S, Soravia L, Megert M, Michel K. A novel brief therapy for patients who attempt suicide: a 24-months follow-up randomized controlled study of the attempted suicide short intervention program (ASSIP). PLoS Med 2016;13:e1001968.
13. Hawton K, Witt KG, Salisbury TLT, et al. Psychosocial interventions following self-harm in adults: a systematic review and meta-analysis. Lancet Psychiatry. 2016;3:740–50.
14. Battaglia J, Wolff TK, Wagner-Johnson DS, et al. Structured diagnostic assessment and depot fluphenazine treatment of multiple suicide attempters in the emergency department. Int Clin Psychopharmacol 1999;14:361–72.
15. van der Sande R, van Rooijen L, Buskens E, et al. Intensive in-patient and community intervention versus routine care after attempted suicide. A randomised controlled intervention study. Br J Psychiatry 1997;171:35–41.
16. Motto JA, Bostrom AG. A randomized controlled trial of postcrisis suicide prevention. Psychiatr Serv 2001;52:828–33.
17. Berrouiguet S, Larsen ME, Mesmeur C, Gravey M, Billot R, Walter M, et al. Toward mHealth brief contact interventions in suicide prevention: case series from the suicide intervention assisted by messages (SIAM) randomized controlled trial. JMIR MHealth UHealth 2018;6:e8.
18. Falcone G, Nardella A, Lamis DA, et al. Taking care of suicidal patients with new technologies and reaching-out means in the post-discharge period. World J Psychiatry 2017;7:163–76.
19. Milner A, Spittal MJ, Kapur N, et al. Mechanisms of brief contact interventions in clinical populations: a systematic review. BMC Psychiatry 2016;16:194.
20. Carter GL, Clover K, Whyte IM, et al. Postcards from the EDge: 5-year outcomes of a randomised controlled trial for hospital-treated self-poisoning. Br J Psychiatry 2013;202:372–80.
21. Hassanian-Moghaddam H, Sarjami S, Kolahi AA, Carter GL. Postcards in Persia: randomised controlled trial to reduce suicidal behaviours 12 months after hospital-treated self-poisoning. Br J Psychiatry 2011;198:309–16.
22. Luxton DD, Thomas EK, Chipps J, et al. Caring letters for suicide prevention: implementation of a multi-site randomized clinical trial in the U.S. military and Veteran Affairs healthcare systems. Contemp Clin Trials 2014;37(2):252–60.
23. Vaiva G, Vaiva G, Ducrocq F, et al. Effect of telephone contact on further suicide attempts in patients discharged from an emergency department: randomised controlled study. BMJ 2006;332:1241–5.
24. Cebrià AI, Parra I, Pàmias M, et al. Effectiveness of a telephone management programme for patients discharged from an emergency department after a suicide attempt: controlled study in a Spanish population. J Affect Disord 2013;147:269–76.
25. Cedereke M, Monti K, Ojehagen A. Telephone contact with patients in the year after a suicide attempt: does it affect treatment attendance and outcome? A randomised controlled study. Eur Psychiatry. 2002;17:82–91.
26. Vaiva G, Walter M, Al Arab AS, et al. ALGOS: the development of a randomized controlled trial testing a case management algorithm designed to reduce suicide risk among suicide attempters. BMC Psychiatry 2011;11:1.
27. Milner AJ, Carter G, Pirkis J, et al. Letters, green cards, telephone calls and postcards: systematic and meta-analytic review of brief contact interventions for reducing self-harm, suicide attempts and suicide. Br J Psychiatry. 2015;206:184–90.
28. Denchev P, Pearson JL, Allen MH, Claassen CA, Currier GW, Zatzick DF, et al. Modeling the cost-effectiveness of interventions to reduce suicide risk among hospital emergency department patients. Psychiatr Serv 2018;69:23–31.
29. Berrouiguet S, Courtet P, Larsen ME, et al. Suicide prevention: towards integrative, innovative and individualized brief contact interventions. Eur Psychiatry 2018;47:25–6.
30. Larsen ME, Shand F, Morley K, Batterham PJ, Petrie K, Reda B, et al. A mobile text message intervention to reduce repeat suicidal episodes: design and development of reconnecting after a suicide attempt (RAFT). JMIR Ment Health 2017;4:e56.
31. Morgan HG, Jones EM, Owen JH. Secondary prevention of non-fatal deliberate self-harm. The green card study. Br J Psychiatry 1993;163:111–2.
32. Evans MO, Morgan HG, Hayward A, Gunnell DJ. Crisis telephone consultation for deliberate self-harm patients: effects on repetition. Br J Psychiatry 1999;175:23–7.
33. Evans J, Evans M, Morgan HG, et al. Crisis card following self-harm: 12-month follow-up of a randomised controlled trial. Br J Psychiatry J 2005;187:186–7.
34. Fleischmann A, Bertolote JM, Wasserman D, et al. Effectiveness of brief intervention and contact for suicide attempters: a randomized controlled trial in five countries. Bull World Health Organ 2008;86:703–9.
35. Vijayakumar L, Umamaheswari C, Shujaath Ali ZS, et al. Intervention for suicide attempters: a randomized controlled study. Indian J Psychiatry 2011;53:244–8.
36. Bertolote JM, Fleischmann A, De Leo D, et al. Repetition of suicide attempts: data from emergency care settings in five culturally different low- and middle-income countries participating in the WHO SUPRE-MISS Study. Crisis 2010;31:194–201.
37. Mousavi SG, Zohreh R, Maracy MR, et al. The efficacy of telephonic follow up in prevention of suicidal reattempt in patients with suicide attempt history. Adv Biomed Res 2014;3:198.
38. Amadéo S, Rereao M, Malogne A, et al. Testing brief intervention and phone contact among subjects with suicidal behavior: a randomized controlled trial in French Polynesia in the frames of the World Health Organization/suicide trends in at-risk territories study. Ment Illn 2015;7:5818.
39. Riblet NBV, Shiner B, Young-Xu Y, Watts BV. Strategies to prevent death by suicide: meta-analysis of randomised controlled trials. Br J Psychiatry 2017;210:396–402.
40. Miller IW, Camargo CA Jr, Arias SA, et al. Suicide prevention in an emergency department population: the ED-SAFE study. JAMA Psychiatry 2017;74:563–70.
41. Miller IW, Gaudiano BA, Weinstock LM. The coping long term with active suicide program: description and pilot data. Suicide Life Threat Behav 2016;46:752–61.
42. Kawanishi C, Aruga T, Ishizuka N, et al. Assertive case management versus enhanced usual care for people with mental health problems who had attempted suicide and were admitted to hospital emergency departments in Japan (ACTION-J): a multicentre, randomised controlled trial. Lancet Psychiatry 2014;1:193–201.
43. Furuno T, Nakagawa M, Hino K, et al. Effectiveness of assertive case management on repeat self-harm in patients admitted for suicide attempt: findings from ACTION-J study. J Affect Disord 2018;225:460–5.
44. Morthorst B, Krogh J, Erlangsen A, et al. Effect of assertive outreach after suicide attempt in the AID (assertive intervention for deliberate self harm) trial: randomised controlled trial. BMJ 2012;345:e4972.
45. Johannessen HA, Dieserud G, De Leo D, Claussen B, et al. Chain of care for patients who have attempted suicide: a follow-up study from Bærum, Norway. BMC Public Health 2011;11:81.
46. Hvid M, Wang AG. Preventing repetition of attempted suicide—I. Feasibility (acceptability, adherence, and effectiveness) of a Baerum-model like aftercare. Nord J Psychiatry 2009;63:148–53.
47. Hvid M, Vangborg K, Sørensen HJ, et al. Preventing repetition of attempted suicide-II. The Amager project, a randomized controlled trial. Nord J Psychiatry 2011;65:292–8.
48. Lahoz T, Hvid M, Wang AG. Preventing repetition of attempted suicide-III. The Amager project, 5-year follow-up of a randomized controlled trial. Nord J Psychiatry 2016;70:547–53.
49. Kroll DS, Karno J, Mullen B, et al. Clinical severity alone does not determine disposition decisions for patients in the emergency department with suicide risk. Psychosomatics 2017; pii: S0033-3182(17)30247–5.
Complementary treatments for anxiety: Beyond pharmacotherapy and psychotherapy
Anxiety disorders are the most common psychiatric illnesses in the United States, with a prevalence of nearly 29%.1 These disorders typically are treated with pharmacotherapy, psychotherapy, or a combination of both. Pharmacotherapy for anxiety has evolved considerably during the last 30 years, but medications are not efficacious for or tolerated by all patients. For example, selective serotonin reuptake inhibitors, which are frequently used for treating anxiety, can cause sexual dysfunction,2 weight gain,2 drug interactions,2 coagulopathies,3 and gastrointestinal disturbances.4 Psychotherapeutic techniques, such as cognitive behavioral therapy (CBT) and interpersonal therapy (IPT), are efficacious for mild to moderate anxiety.5-7
In addition to standard pharmacotherapy and psychotherapy, some evidence suggests that complementary therapies, such as yoga, massage, and relaxation techniques, may be beneficial as adjunctive treatments for anxiety. In placebo-controlled trials, several of these complementary therapies have been shown to decrease serum levels of the inflammatory biomarker cortisol. Anxiety is associated with inflammation,8 so therapies that reduce inflammation may help reduce symptoms of anxiety. Here, we describe the results of select positive randomized controlled trials (RCTs) of several complementary interventions for anxiety that might be useful as adjunctive treatments to psychotherapy or pharmacotherapy.
A look at RCTs that measured both anxiety and cortisol
We searched PubMed, Google Scholar, and Scopus to identify RCTs of complementary nonpharmacologic and nonpsychotherapeutic therapies for anxiety published from January 2010 to May 2017. We included only studies that:
- blindly assessed anxiety levels through a validated instrument (the State-Trait Anxiety Inventory [STAI])9
- measured cortisol concentrations before and after treatment.
Evaluating both STAI scores and cortisol levels is useful because doing so gives insight into both the clinical and biological efficacy of the therapies. Studies were excluded if they employed a pharmacologic agent in addition to the approach being evaluated.
We identified 26 studies, of which 14 met the inclusion/exclusion criteria. These studies found beneficial effects for yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Yoga
Yoga has become increasingly popular in the Western world during the last 2 decades.10 There are a variety of yoga practices; common forms include hatha yoga, power yoga, kripalu yoga, and forrest yoga.11
A study of 92 depressed pregnant women monito
Hatha yoga consists of a combination of postural exercises, breathing techniques, relaxation, and meditation. In a 12-week study of 88 postmenopausal women, those who practiced hatha yoga for 75 minutes a day had significantly lower STAI scores compared with women who exercised for 75 minutes a day and those who performed no physical activity.13
Continue to: Massage therapy
Massage therapy
Receiving as little as 15 minutes of back massage has proven to be beneficial for individuals with anxiety. In an RCT conducted in Turkey, 44 caregivers of patients with cancer were assigned to receive a back massage or to rest quietly in a room for 15 minutes once each day for 1 week.14 By the end of the week, compared with those who quietly rested, those who received the back massage had a statistically significant reduction in serum cortisol levels and STAI scores.14
Aromatherapy massage
Aromatherapy is the use of essential oils from plants through distillation.15 The scent of the oils is purported to provide medical benefits. More than 60 essential oils are used therapeutically, including rose, lavender, lemon, and orange.16 These essential oils are frequently used in combination with a massage.
In South Korea, researchers investigated the effects of aromatherapy massage on 25 women who had children diagnosed with attention-deficit/hyperactivity disorder.17 Women assigned to the treatment group received a 40-minute aromatherapy massage using mixed essential oils that contained lavender and geranium twice a week for 4 weeks. Women in the control group received no treatment. Compared with those in the control group, women who received the aromatherapy massages had a statistically significant decrease in STAI scores and salivary cortisol levels. Plasma cortisol was not significantly different between groups.17
Pet therapy
The psychological benefits of animal-assisted therapy were not evident until World War II, when dogs were used to cheer up injured soldiers.18 Today, pet therapy has been used on many inpatient units.19
In a U.S. study, 48 healthy undergraduate students were assigned to a room with a dog, a room with a friend, or a room by themselves.20 All participants were given the Trier Social Stress Test (TSST), a protocol that measures stress by having participants give a speech and perform mental arithmetic in front of an audience.The TSST is known to induce increases in cortisol levels. Although no differences in STAI scores were found among groups, students in the room with the dog had a lower spike in salivary cortisol after the TSST compared with participants who were in a room with a friend or in a room alone.20
Continue to: Qigong
Qigong
In Chinese medicine, Qi is known as a vital life force that flows through the body. The disruption of Qi is hypothesized to contribute to disease.21
Qigong is a medical therapy that focuses on uniting the body, breath, and mind to improve health.21 It consists of rhythmic, choreographed movements used to position the body into postures believed to help direct Qi to specific areas in the body. Qigong also uses sound exercises, in which an individual creates certain syllables while breathing. Six syllables are used, each of which is believed to affect a certain organ.21
Korean researchers randomly assigned 32 healthy men to a Qigong training group or a sham Qigong control group.22 Individuals in the training group performed 25 minutes of sound exercises, 20 minutes of meditation, and 15 minutes of movements. The control group learned the same movements as the experimental group, but without the conscious effort of moving Qi. After 3 sessions, those in the Qigong training group had significantly decreased STAI scores and serum cortisol levels compared with those in the sham group.22
In a different Korean study, researchers randomly assigned 50 participants with elevated distress levels to a Qigong training group or a waitlist control group in which participants called a trainer to describe stressful events.23 After 4 weeks, participants in the Qigong group had significant decreases in STAI scores compared with the control group. However, there were no changes in salivary cortisol levels.23
Auricular acupressure
Auricular acupressure involves applying pressure on certain portions of the auricle (outer ear) to alleviate pain and disease.24 Similar to Qigong, auricular acupressure focuses on reestablishing Qi in the body. Researchers randomly assigned 80 post-caesarean section women in Taiwan to 5 days of auricular acupressure or usual care.25 The women who received auricular acupressure had significantly lower STAI scores and serum cortisol levels compared with women who received routine care.25
Continue to: Reiki touch therapy
Reiki touch therapy
Reiki touch therapy originated in Japan. In this therapy, healers apply a light touch or hover their hands above an individual’s body to help direct energy.26
The effects of reiki touch therapy were recently evaluated in a U.S. study.27 Researchers randomly assigned 37 patients with human immunodeficiency virus to an experimental group that received 30 minutes of reiki touch therapy plus music therapy 6 times a week for 10 weeks, or to a music therapy–only control group. Patients who received reiki touch therapy had a significant decrease in STAI scores. Patients in this group also had a statistically significant drop in salivary cortisol levels after the first week.27
Acupuncture
Acupuncture is the application of needles to specific areas on the body. Acupuncture has been proposed to activate pain receptors, thereby producing an analgesic response.28
Researchers in Brazil randomly assigned 57 lactating women with preterm infants to an experimental group that received acupuncture or to a control group that received sham acupuncture.29 Treatment was administered at 5 points on the ear unilaterally for 5 minutes once a week for 16 months. Custom-made needles that did not actually puncture the skin were used in the sham group; a toothpick was used to create the sensation of needle perforations. STAI scores were reduced in both groups, although there was no statistically significant difference in scores between the acupuncture and sham groups.29
Music therapy
Music has been long believed to have beneficial psychological effects. In Turkey, researchers evaluated the effects of music therapy in 100 oncology patients who received port catheters.30 Patients were randomly assigned to an experimental group that received music therapy throughout the procedure or to a control group that received normal care. Patients who listened to music during port catheter placement had significantly reduced STAI scores and serum cortisol levels compared with those in the control group.30
Continue to: Relaxation techniques
Relaxation techniques
A wide range of relaxation techniques are used for therapeutic purposes. In Switzerland, researchers evaluated the anxiolytic effects of 10 minutes of progressive muscle relaxation and guided imagery in 39 pregnant women.31 Women randomly assigned to progressive muscle relaxation were instructed to systematically tense and then release muscle groups throughout their body in sequential order. Women assigned to the guided imagery intervention were told to imagine a safe place and to think of someone who could confer security and reassurance. The remainder of the women were assigned to a control group, where they sat quietly without any formal instructions. Researchers found that each group had a decrease in STAI scores and salivary cortisol levels immediately after the intervention.31
The relaxation response was first described in 1975 by Herbert Benson, MD, as a deep meditative state characterized by a decrease in tension, heart rate, and breathing rate. Several techniques can induce this state, including hypnosis, progressive muscle relaxation, yoga, and transcendental meditation.32 In a study of 15 healthy older adults (age 65 to 80), researchers randomly assigned participants to a relaxation response training group or to a control group.33 The relaxation response training included meditation, imagery, and relaxation techniques. After 5 weeks, participants who received the relaxation response training had marginally significant decreases in STAI scores compared with those in the control group.33
Consider these therapies as adjuncts
Our review of select positive RCTs (Table12-14,17,20,22,23,25,27,29-31,33) suggests that some nonpharmacologic/nonpsychotherapeutic adjunctive interventions may have beneficial effects for patients who have anxiety. Several of the controlled studies we reviewed demonstrated that these interventions are superior to placebo. The reductions in both anxiety severity as measured by the STAI and cortisol levels suggests that some of these complementary therapies deserve a second look as useful adjuncts to established anxiety treatments.
Bottom Line
A review of select randomized controlled trials suggests that some complementary therapies may be helpful as adjunctive therapy in patients with anxiety. These include yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Related Resources
- Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214.
- National Institute of Mental Health. Anxiety disorders. https://www.nimh.nih.gov/health/topics/anxiety-disorders/index.shtml.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Masand PS, Gupta S. Long-term side effects of newer-generation antidepressants: SSRIs, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry. 2002;14(3):175-182.
3. Siddiqui R, Gawande S, Shende T, et al. SSRI-induced coagulopathy: is it reality? Therapeutic Advances in Psychopharmacology. 2011;1(6):169-174.
4. Brambilla P, Cipriani A, Hotopf M, et al. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38(2):69-77.
5. Slomski A. Blended CBT controls anxiety in cancer survivors. JAMA. 2017;318(4):323.
6. Forsell E, Bendix M, Holländare F, et al. Internet delivered cognitive behavior therapy for antenatal depression: a randomised controlled trial. J Affect Disord. 2017;221:56-64.
7. Lilliengren P, Johansson R, Town JM, et al. Intensive Short-Term Dynamic Psychotherapy for generalized anxiety disorder: A pilot effectiveness and process-outcome study. Clin Psychol Psychother. 2017;24(6):1313-1321.
8. Furtado M, Katzman MA. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015;229(1-2):37-48.
9. Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
10. Saper RB, Eisenberg DM, Davis RB, et al. Prevalence and patterns of adult yoga use in the United States: results of a national survey. Altern Ther Health Med. 2004;10(2):44-49.
11. Farmer J. Americanasana. Reviews in American history. 2012;40(1):145-158.
12. Field T, Diego M, Delgado J, et al. Yoga and social support reduce prenatal depression, anxiety and cortisol. J Bodyw Mov Ther: 2013;17(4):397-403.
13. Jorge MP, Santaella DF, Pontes IM, et al. Hatha Yoga practice decreases menopause symptoms and improves quality of life: a randomized controlled trial. Complement Ther Med. 2016;26:128-135.
14. Pinar R, Afsar F. Back massage to decrease state anxiety, cortisol level, blood pressure, heart rate and increase sleep quality in family caregivers of patients with cancer: a randomised controlled trial. Asian Pac J Cancer Prev. 2015;16(18):8127-8133.
15. Kuriyama H, Watanabe S, Nakaya, et al. Immunological and psychological benefits of aromatherapy massage. Evid Based Complement Alternat Med. 2005;2(2):179-184.
16. Setzer WN. Essential oils and anxiolytic aromatherapy. Nat Prod Commun. 2009;4(9):1305-1316.
17. Wu JJ, Cui Y, Yang YS, et al. Modulatory effects of aromatherapy massage intervention on electroencephalogram, psychological assessments, salivary cortisol and plasma brain-derived neurotrophic factor. Complement Ther Med. 2014;22(3):456-462.
18. Fine A. Forward. In: Fine A, ed. Handbook on animal-assisted therapy-theoretical foundations and guidelines for practice. 3rd ed. Academic Press; 2010:xvii-xviii.
19. Snipelisky D, Burton MC. Canine-assisted therapy in the inpatient setting. South Med J. 2014;107(4):265-273.
20. Polheber JP, Matchock RL. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. J Behav Med. 2014;37(5):860-867.
21. Liu T, Qiang X, eds. Chinese medical Qigong. Philadelphia, PA: Singing Dragon; 2013:1-100,192,238,511.
22. Lee MS, Kang CW, Lim HJ, et al. Effects of Qi-training on anxiety and plasma concentrations of cortisol, ACTH, and aldosterone: a randomized placebo-controlled pilot study. Stress Health. 2004;20(5):243-248.
23. Hwang EY, Chung SY, Cho JH, et al. Effects of a brief Qigong-based stress reduction program (BQSRP) in a distressed Korean population: a randomized trial. BMC Complement Altern Med. 2013;13:113.
24. Oleson, T. Overview and history of auriculotherapy. In: Auriculotherapy manual: Chinese and Western systems of ear acupuncture. 4th ed. London: Churchill Livingstone; 2014:1.
25. Kuo SY, Tsai SH, Chen SL, et al. Auricular acupressure relieves anxiety and fatigue, and reduces cortisol levels in post-caesarean section women: a single-blind, randomised controlled study. Int J Nurs Stud. 2016;53:17-26.
26. Horan P. Introduction. In: Horan P. Empowerment through reiki: the path to personal and global transformation. 8th ed. Twin Lakes, WI: Lotus Press; 1998:13-15.
27. Bremner MN, Blake BJ, Wagner VD, et al. Effects of reiki with music compared to music only among people living with HIV. J Assoc Nurses AIDS Care. 2016;27(5):635-647.
28. Helmes JM. The basic, clinical, and speculative science of acupuncture. In: Acupuncture energetics: a clinical approach for physicians. Volume 1. Berkeley, CA: Medical Acupuncture Publishers; 1995:19-32.
29. Haddad-Rodrigues M, Spanó Nakano A, Stefanello J, et al. Acupuncture for anxiety in lactating mothers with preterm infants: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:169184. doi: 10.1155/2013/169184.
30. Zengin S, Kabul S, Al B, et al. Effects of music therapy on pain and anxiety in patients undergoing port catheter placement procedure. Complement Ther Med. 2013;21(6):689-696.
31. Urech C, Fink NS, Hoesli I, et al. Effects of relaxation on psychobiological wellbeing during pregnancy: a randomized controlled trial. Psychoneuroendocrinology. 2010;35(9):1348-1355.
32. Goleman D. The relaxation response. In: Mind body medicine: how to use your mind for better health. Yonkers, NY: Consumer Reports; 1993:125-149.
33. Galvin JA, Benson H, Deckro GR, et al. The relaxation response: reducing stress and improving cognition in healthy aging adults. Complement Ther Clin Pract. 2006;12(3):186-191.
Anxiety disorders are the most common psychiatric illnesses in the United States, with a prevalence of nearly 29%.1 These disorders typically are treated with pharmacotherapy, psychotherapy, or a combination of both. Pharmacotherapy for anxiety has evolved considerably during the last 30 years, but medications are not efficacious for or tolerated by all patients. For example, selective serotonin reuptake inhibitors, which are frequently used for treating anxiety, can cause sexual dysfunction,2 weight gain,2 drug interactions,2 coagulopathies,3 and gastrointestinal disturbances.4 Psychotherapeutic techniques, such as cognitive behavioral therapy (CBT) and interpersonal therapy (IPT), are efficacious for mild to moderate anxiety.5-7
In addition to standard pharmacotherapy and psychotherapy, some evidence suggests that complementary therapies, such as yoga, massage, and relaxation techniques, may be beneficial as adjunctive treatments for anxiety. In placebo-controlled trials, several of these complementary therapies have been shown to decrease serum levels of the inflammatory biomarker cortisol. Anxiety is associated with inflammation,8 so therapies that reduce inflammation may help reduce symptoms of anxiety. Here, we describe the results of select positive randomized controlled trials (RCTs) of several complementary interventions for anxiety that might be useful as adjunctive treatments to psychotherapy or pharmacotherapy.
A look at RCTs that measured both anxiety and cortisol
We searched PubMed, Google Scholar, and Scopus to identify RCTs of complementary nonpharmacologic and nonpsychotherapeutic therapies for anxiety published from January 2010 to May 2017. We included only studies that:
- blindly assessed anxiety levels through a validated instrument (the State-Trait Anxiety Inventory [STAI])9
- measured cortisol concentrations before and after treatment.
Evaluating both STAI scores and cortisol levels is useful because doing so gives insight into both the clinical and biological efficacy of the therapies. Studies were excluded if they employed a pharmacologic agent in addition to the approach being evaluated.
We identified 26 studies, of which 14 met the inclusion/exclusion criteria. These studies found beneficial effects for yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Yoga
Yoga has become increasingly popular in the Western world during the last 2 decades.10 There are a variety of yoga practices; common forms include hatha yoga, power yoga, kripalu yoga, and forrest yoga.11
A study of 92 depressed pregnant women monito
Hatha yoga consists of a combination of postural exercises, breathing techniques, relaxation, and meditation. In a 12-week study of 88 postmenopausal women, those who practiced hatha yoga for 75 minutes a day had significantly lower STAI scores compared with women who exercised for 75 minutes a day and those who performed no physical activity.13
Continue to: Massage therapy
Massage therapy
Receiving as little as 15 minutes of back massage has proven to be beneficial for individuals with anxiety. In an RCT conducted in Turkey, 44 caregivers of patients with cancer were assigned to receive a back massage or to rest quietly in a room for 15 minutes once each day for 1 week.14 By the end of the week, compared with those who quietly rested, those who received the back massage had a statistically significant reduction in serum cortisol levels and STAI scores.14
Aromatherapy massage
Aromatherapy is the use of essential oils from plants through distillation.15 The scent of the oils is purported to provide medical benefits. More than 60 essential oils are used therapeutically, including rose, lavender, lemon, and orange.16 These essential oils are frequently used in combination with a massage.
In South Korea, researchers investigated the effects of aromatherapy massage on 25 women who had children diagnosed with attention-deficit/hyperactivity disorder.17 Women assigned to the treatment group received a 40-minute aromatherapy massage using mixed essential oils that contained lavender and geranium twice a week for 4 weeks. Women in the control group received no treatment. Compared with those in the control group, women who received the aromatherapy massages had a statistically significant decrease in STAI scores and salivary cortisol levels. Plasma cortisol was not significantly different between groups.17
Pet therapy
The psychological benefits of animal-assisted therapy were not evident until World War II, when dogs were used to cheer up injured soldiers.18 Today, pet therapy has been used on many inpatient units.19
In a U.S. study, 48 healthy undergraduate students were assigned to a room with a dog, a room with a friend, or a room by themselves.20 All participants were given the Trier Social Stress Test (TSST), a protocol that measures stress by having participants give a speech and perform mental arithmetic in front of an audience.The TSST is known to induce increases in cortisol levels. Although no differences in STAI scores were found among groups, students in the room with the dog had a lower spike in salivary cortisol after the TSST compared with participants who were in a room with a friend or in a room alone.20
Continue to: Qigong
Qigong
In Chinese medicine, Qi is known as a vital life force that flows through the body. The disruption of Qi is hypothesized to contribute to disease.21
Qigong is a medical therapy that focuses on uniting the body, breath, and mind to improve health.21 It consists of rhythmic, choreographed movements used to position the body into postures believed to help direct Qi to specific areas in the body. Qigong also uses sound exercises, in which an individual creates certain syllables while breathing. Six syllables are used, each of which is believed to affect a certain organ.21
Korean researchers randomly assigned 32 healthy men to a Qigong training group or a sham Qigong control group.22 Individuals in the training group performed 25 minutes of sound exercises, 20 minutes of meditation, and 15 minutes of movements. The control group learned the same movements as the experimental group, but without the conscious effort of moving Qi. After 3 sessions, those in the Qigong training group had significantly decreased STAI scores and serum cortisol levels compared with those in the sham group.22
In a different Korean study, researchers randomly assigned 50 participants with elevated distress levels to a Qigong training group or a waitlist control group in which participants called a trainer to describe stressful events.23 After 4 weeks, participants in the Qigong group had significant decreases in STAI scores compared with the control group. However, there were no changes in salivary cortisol levels.23
Auricular acupressure
Auricular acupressure involves applying pressure on certain portions of the auricle (outer ear) to alleviate pain and disease.24 Similar to Qigong, auricular acupressure focuses on reestablishing Qi in the body. Researchers randomly assigned 80 post-caesarean section women in Taiwan to 5 days of auricular acupressure or usual care.25 The women who received auricular acupressure had significantly lower STAI scores and serum cortisol levels compared with women who received routine care.25
Continue to: Reiki touch therapy
Reiki touch therapy
Reiki touch therapy originated in Japan. In this therapy, healers apply a light touch or hover their hands above an individual’s body to help direct energy.26
The effects of reiki touch therapy were recently evaluated in a U.S. study.27 Researchers randomly assigned 37 patients with human immunodeficiency virus to an experimental group that received 30 minutes of reiki touch therapy plus music therapy 6 times a week for 10 weeks, or to a music therapy–only control group. Patients who received reiki touch therapy had a significant decrease in STAI scores. Patients in this group also had a statistically significant drop in salivary cortisol levels after the first week.27
Acupuncture
Acupuncture is the application of needles to specific areas on the body. Acupuncture has been proposed to activate pain receptors, thereby producing an analgesic response.28
Researchers in Brazil randomly assigned 57 lactating women with preterm infants to an experimental group that received acupuncture or to a control group that received sham acupuncture.29 Treatment was administered at 5 points on the ear unilaterally for 5 minutes once a week for 16 months. Custom-made needles that did not actually puncture the skin were used in the sham group; a toothpick was used to create the sensation of needle perforations. STAI scores were reduced in both groups, although there was no statistically significant difference in scores between the acupuncture and sham groups.29
Music therapy
Music has been long believed to have beneficial psychological effects. In Turkey, researchers evaluated the effects of music therapy in 100 oncology patients who received port catheters.30 Patients were randomly assigned to an experimental group that received music therapy throughout the procedure or to a control group that received normal care. Patients who listened to music during port catheter placement had significantly reduced STAI scores and serum cortisol levels compared with those in the control group.30
Continue to: Relaxation techniques
Relaxation techniques
A wide range of relaxation techniques are used for therapeutic purposes. In Switzerland, researchers evaluated the anxiolytic effects of 10 minutes of progressive muscle relaxation and guided imagery in 39 pregnant women.31 Women randomly assigned to progressive muscle relaxation were instructed to systematically tense and then release muscle groups throughout their body in sequential order. Women assigned to the guided imagery intervention were told to imagine a safe place and to think of someone who could confer security and reassurance. The remainder of the women were assigned to a control group, where they sat quietly without any formal instructions. Researchers found that each group had a decrease in STAI scores and salivary cortisol levels immediately after the intervention.31
The relaxation response was first described in 1975 by Herbert Benson, MD, as a deep meditative state characterized by a decrease in tension, heart rate, and breathing rate. Several techniques can induce this state, including hypnosis, progressive muscle relaxation, yoga, and transcendental meditation.32 In a study of 15 healthy older adults (age 65 to 80), researchers randomly assigned participants to a relaxation response training group or to a control group.33 The relaxation response training included meditation, imagery, and relaxation techniques. After 5 weeks, participants who received the relaxation response training had marginally significant decreases in STAI scores compared with those in the control group.33
Consider these therapies as adjuncts
Our review of select positive RCTs (Table12-14,17,20,22,23,25,27,29-31,33) suggests that some nonpharmacologic/nonpsychotherapeutic adjunctive interventions may have beneficial effects for patients who have anxiety. Several of the controlled studies we reviewed demonstrated that these interventions are superior to placebo. The reductions in both anxiety severity as measured by the STAI and cortisol levels suggests that some of these complementary therapies deserve a second look as useful adjuncts to established anxiety treatments.
Bottom Line
A review of select randomized controlled trials suggests that some complementary therapies may be helpful as adjunctive therapy in patients with anxiety. These include yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Related Resources
- Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214.
- National Institute of Mental Health. Anxiety disorders. https://www.nimh.nih.gov/health/topics/anxiety-disorders/index.shtml.
Anxiety disorders are the most common psychiatric illnesses in the United States, with a prevalence of nearly 29%.1 These disorders typically are treated with pharmacotherapy, psychotherapy, or a combination of both. Pharmacotherapy for anxiety has evolved considerably during the last 30 years, but medications are not efficacious for or tolerated by all patients. For example, selective serotonin reuptake inhibitors, which are frequently used for treating anxiety, can cause sexual dysfunction,2 weight gain,2 drug interactions,2 coagulopathies,3 and gastrointestinal disturbances.4 Psychotherapeutic techniques, such as cognitive behavioral therapy (CBT) and interpersonal therapy (IPT), are efficacious for mild to moderate anxiety.5-7
In addition to standard pharmacotherapy and psychotherapy, some evidence suggests that complementary therapies, such as yoga, massage, and relaxation techniques, may be beneficial as adjunctive treatments for anxiety. In placebo-controlled trials, several of these complementary therapies have been shown to decrease serum levels of the inflammatory biomarker cortisol. Anxiety is associated with inflammation,8 so therapies that reduce inflammation may help reduce symptoms of anxiety. Here, we describe the results of select positive randomized controlled trials (RCTs) of several complementary interventions for anxiety that might be useful as adjunctive treatments to psychotherapy or pharmacotherapy.
A look at RCTs that measured both anxiety and cortisol
We searched PubMed, Google Scholar, and Scopus to identify RCTs of complementary nonpharmacologic and nonpsychotherapeutic therapies for anxiety published from January 2010 to May 2017. We included only studies that:
- blindly assessed anxiety levels through a validated instrument (the State-Trait Anxiety Inventory [STAI])9
- measured cortisol concentrations before and after treatment.
Evaluating both STAI scores and cortisol levels is useful because doing so gives insight into both the clinical and biological efficacy of the therapies. Studies were excluded if they employed a pharmacologic agent in addition to the approach being evaluated.
We identified 26 studies, of which 14 met the inclusion/exclusion criteria. These studies found beneficial effects for yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Yoga
Yoga has become increasingly popular in the Western world during the last 2 decades.10 There are a variety of yoga practices; common forms include hatha yoga, power yoga, kripalu yoga, and forrest yoga.11
A study of 92 depressed pregnant women monito
Hatha yoga consists of a combination of postural exercises, breathing techniques, relaxation, and meditation. In a 12-week study of 88 postmenopausal women, those who practiced hatha yoga for 75 minutes a day had significantly lower STAI scores compared with women who exercised for 75 minutes a day and those who performed no physical activity.13
Continue to: Massage therapy
Massage therapy
Receiving as little as 15 minutes of back massage has proven to be beneficial for individuals with anxiety. In an RCT conducted in Turkey, 44 caregivers of patients with cancer were assigned to receive a back massage or to rest quietly in a room for 15 minutes once each day for 1 week.14 By the end of the week, compared with those who quietly rested, those who received the back massage had a statistically significant reduction in serum cortisol levels and STAI scores.14
Aromatherapy massage
Aromatherapy is the use of essential oils from plants through distillation.15 The scent of the oils is purported to provide medical benefits. More than 60 essential oils are used therapeutically, including rose, lavender, lemon, and orange.16 These essential oils are frequently used in combination with a massage.
In South Korea, researchers investigated the effects of aromatherapy massage on 25 women who had children diagnosed with attention-deficit/hyperactivity disorder.17 Women assigned to the treatment group received a 40-minute aromatherapy massage using mixed essential oils that contained lavender and geranium twice a week for 4 weeks. Women in the control group received no treatment. Compared with those in the control group, women who received the aromatherapy massages had a statistically significant decrease in STAI scores and salivary cortisol levels. Plasma cortisol was not significantly different between groups.17
Pet therapy
The psychological benefits of animal-assisted therapy were not evident until World War II, when dogs were used to cheer up injured soldiers.18 Today, pet therapy has been used on many inpatient units.19
In a U.S. study, 48 healthy undergraduate students were assigned to a room with a dog, a room with a friend, or a room by themselves.20 All participants were given the Trier Social Stress Test (TSST), a protocol that measures stress by having participants give a speech and perform mental arithmetic in front of an audience.The TSST is known to induce increases in cortisol levels. Although no differences in STAI scores were found among groups, students in the room with the dog had a lower spike in salivary cortisol after the TSST compared with participants who were in a room with a friend or in a room alone.20
Continue to: Qigong
Qigong
In Chinese medicine, Qi is known as a vital life force that flows through the body. The disruption of Qi is hypothesized to contribute to disease.21
Qigong is a medical therapy that focuses on uniting the body, breath, and mind to improve health.21 It consists of rhythmic, choreographed movements used to position the body into postures believed to help direct Qi to specific areas in the body. Qigong also uses sound exercises, in which an individual creates certain syllables while breathing. Six syllables are used, each of which is believed to affect a certain organ.21
Korean researchers randomly assigned 32 healthy men to a Qigong training group or a sham Qigong control group.22 Individuals in the training group performed 25 minutes of sound exercises, 20 minutes of meditation, and 15 minutes of movements. The control group learned the same movements as the experimental group, but without the conscious effort of moving Qi. After 3 sessions, those in the Qigong training group had significantly decreased STAI scores and serum cortisol levels compared with those in the sham group.22
In a different Korean study, researchers randomly assigned 50 participants with elevated distress levels to a Qigong training group or a waitlist control group in which participants called a trainer to describe stressful events.23 After 4 weeks, participants in the Qigong group had significant decreases in STAI scores compared with the control group. However, there were no changes in salivary cortisol levels.23
Auricular acupressure
Auricular acupressure involves applying pressure on certain portions of the auricle (outer ear) to alleviate pain and disease.24 Similar to Qigong, auricular acupressure focuses on reestablishing Qi in the body. Researchers randomly assigned 80 post-caesarean section women in Taiwan to 5 days of auricular acupressure or usual care.25 The women who received auricular acupressure had significantly lower STAI scores and serum cortisol levels compared with women who received routine care.25
Continue to: Reiki touch therapy
Reiki touch therapy
Reiki touch therapy originated in Japan. In this therapy, healers apply a light touch or hover their hands above an individual’s body to help direct energy.26
The effects of reiki touch therapy were recently evaluated in a U.S. study.27 Researchers randomly assigned 37 patients with human immunodeficiency virus to an experimental group that received 30 minutes of reiki touch therapy plus music therapy 6 times a week for 10 weeks, or to a music therapy–only control group. Patients who received reiki touch therapy had a significant decrease in STAI scores. Patients in this group also had a statistically significant drop in salivary cortisol levels after the first week.27
Acupuncture
Acupuncture is the application of needles to specific areas on the body. Acupuncture has been proposed to activate pain receptors, thereby producing an analgesic response.28
Researchers in Brazil randomly assigned 57 lactating women with preterm infants to an experimental group that received acupuncture or to a control group that received sham acupuncture.29 Treatment was administered at 5 points on the ear unilaterally for 5 minutes once a week for 16 months. Custom-made needles that did not actually puncture the skin were used in the sham group; a toothpick was used to create the sensation of needle perforations. STAI scores were reduced in both groups, although there was no statistically significant difference in scores between the acupuncture and sham groups.29
Music therapy
Music has been long believed to have beneficial psychological effects. In Turkey, researchers evaluated the effects of music therapy in 100 oncology patients who received port catheters.30 Patients were randomly assigned to an experimental group that received music therapy throughout the procedure or to a control group that received normal care. Patients who listened to music during port catheter placement had significantly reduced STAI scores and serum cortisol levels compared with those in the control group.30
Continue to: Relaxation techniques
Relaxation techniques
A wide range of relaxation techniques are used for therapeutic purposes. In Switzerland, researchers evaluated the anxiolytic effects of 10 minutes of progressive muscle relaxation and guided imagery in 39 pregnant women.31 Women randomly assigned to progressive muscle relaxation were instructed to systematically tense and then release muscle groups throughout their body in sequential order. Women assigned to the guided imagery intervention were told to imagine a safe place and to think of someone who could confer security and reassurance. The remainder of the women were assigned to a control group, where they sat quietly without any formal instructions. Researchers found that each group had a decrease in STAI scores and salivary cortisol levels immediately after the intervention.31
The relaxation response was first described in 1975 by Herbert Benson, MD, as a deep meditative state characterized by a decrease in tension, heart rate, and breathing rate. Several techniques can induce this state, including hypnosis, progressive muscle relaxation, yoga, and transcendental meditation.32 In a study of 15 healthy older adults (age 65 to 80), researchers randomly assigned participants to a relaxation response training group or to a control group.33 The relaxation response training included meditation, imagery, and relaxation techniques. After 5 weeks, participants who received the relaxation response training had marginally significant decreases in STAI scores compared with those in the control group.33
Consider these therapies as adjuncts
Our review of select positive RCTs (Table12-14,17,20,22,23,25,27,29-31,33) suggests that some nonpharmacologic/nonpsychotherapeutic adjunctive interventions may have beneficial effects for patients who have anxiety. Several of the controlled studies we reviewed demonstrated that these interventions are superior to placebo. The reductions in both anxiety severity as measured by the STAI and cortisol levels suggests that some of these complementary therapies deserve a second look as useful adjuncts to established anxiety treatments.
Bottom Line
A review of select randomized controlled trials suggests that some complementary therapies may be helpful as adjunctive therapy in patients with anxiety. These include yoga, massage therapy, aromatherapy massage, pet therapy, Qigong, auricular acupressure, reiki touch therapy, acupuncture, music therapy, and relaxation techniques.
Related Resources
- Bandelow B, Baldwin D, Abelli M, et al. Biological markers for anxiety disorders, OCD and PTSD: a consensus statement. Part II: neurochemistry, neurophysiology and neurocognition. World J Biol Psychiatry. 2017;18(3):162-214.
- National Institute of Mental Health. Anxiety disorders. https://www.nimh.nih.gov/health/topics/anxiety-disorders/index.shtml.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Masand PS, Gupta S. Long-term side effects of newer-generation antidepressants: SSRIs, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry. 2002;14(3):175-182.
3. Siddiqui R, Gawande S, Shende T, et al. SSRI-induced coagulopathy: is it reality? Therapeutic Advances in Psychopharmacology. 2011;1(6):169-174.
4. Brambilla P, Cipriani A, Hotopf M, et al. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38(2):69-77.
5. Slomski A. Blended CBT controls anxiety in cancer survivors. JAMA. 2017;318(4):323.
6. Forsell E, Bendix M, Holländare F, et al. Internet delivered cognitive behavior therapy for antenatal depression: a randomised controlled trial. J Affect Disord. 2017;221:56-64.
7. Lilliengren P, Johansson R, Town JM, et al. Intensive Short-Term Dynamic Psychotherapy for generalized anxiety disorder: A pilot effectiveness and process-outcome study. Clin Psychol Psychother. 2017;24(6):1313-1321.
8. Furtado M, Katzman MA. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015;229(1-2):37-48.
9. Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
10. Saper RB, Eisenberg DM, Davis RB, et al. Prevalence and patterns of adult yoga use in the United States: results of a national survey. Altern Ther Health Med. 2004;10(2):44-49.
11. Farmer J. Americanasana. Reviews in American history. 2012;40(1):145-158.
12. Field T, Diego M, Delgado J, et al. Yoga and social support reduce prenatal depression, anxiety and cortisol. J Bodyw Mov Ther: 2013;17(4):397-403.
13. Jorge MP, Santaella DF, Pontes IM, et al. Hatha Yoga practice decreases menopause symptoms and improves quality of life: a randomized controlled trial. Complement Ther Med. 2016;26:128-135.
14. Pinar R, Afsar F. Back massage to decrease state anxiety, cortisol level, blood pressure, heart rate and increase sleep quality in family caregivers of patients with cancer: a randomised controlled trial. Asian Pac J Cancer Prev. 2015;16(18):8127-8133.
15. Kuriyama H, Watanabe S, Nakaya, et al. Immunological and psychological benefits of aromatherapy massage. Evid Based Complement Alternat Med. 2005;2(2):179-184.
16. Setzer WN. Essential oils and anxiolytic aromatherapy. Nat Prod Commun. 2009;4(9):1305-1316.
17. Wu JJ, Cui Y, Yang YS, et al. Modulatory effects of aromatherapy massage intervention on electroencephalogram, psychological assessments, salivary cortisol and plasma brain-derived neurotrophic factor. Complement Ther Med. 2014;22(3):456-462.
18. Fine A. Forward. In: Fine A, ed. Handbook on animal-assisted therapy-theoretical foundations and guidelines for practice. 3rd ed. Academic Press; 2010:xvii-xviii.
19. Snipelisky D, Burton MC. Canine-assisted therapy in the inpatient setting. South Med J. 2014;107(4):265-273.
20. Polheber JP, Matchock RL. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. J Behav Med. 2014;37(5):860-867.
21. Liu T, Qiang X, eds. Chinese medical Qigong. Philadelphia, PA: Singing Dragon; 2013:1-100,192,238,511.
22. Lee MS, Kang CW, Lim HJ, et al. Effects of Qi-training on anxiety and plasma concentrations of cortisol, ACTH, and aldosterone: a randomized placebo-controlled pilot study. Stress Health. 2004;20(5):243-248.
23. Hwang EY, Chung SY, Cho JH, et al. Effects of a brief Qigong-based stress reduction program (BQSRP) in a distressed Korean population: a randomized trial. BMC Complement Altern Med. 2013;13:113.
24. Oleson, T. Overview and history of auriculotherapy. In: Auriculotherapy manual: Chinese and Western systems of ear acupuncture. 4th ed. London: Churchill Livingstone; 2014:1.
25. Kuo SY, Tsai SH, Chen SL, et al. Auricular acupressure relieves anxiety and fatigue, and reduces cortisol levels in post-caesarean section women: a single-blind, randomised controlled study. Int J Nurs Stud. 2016;53:17-26.
26. Horan P. Introduction. In: Horan P. Empowerment through reiki: the path to personal and global transformation. 8th ed. Twin Lakes, WI: Lotus Press; 1998:13-15.
27. Bremner MN, Blake BJ, Wagner VD, et al. Effects of reiki with music compared to music only among people living with HIV. J Assoc Nurses AIDS Care. 2016;27(5):635-647.
28. Helmes JM. The basic, clinical, and speculative science of acupuncture. In: Acupuncture energetics: a clinical approach for physicians. Volume 1. Berkeley, CA: Medical Acupuncture Publishers; 1995:19-32.
29. Haddad-Rodrigues M, Spanó Nakano A, Stefanello J, et al. Acupuncture for anxiety in lactating mothers with preterm infants: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:169184. doi: 10.1155/2013/169184.
30. Zengin S, Kabul S, Al B, et al. Effects of music therapy on pain and anxiety in patients undergoing port catheter placement procedure. Complement Ther Med. 2013;21(6):689-696.
31. Urech C, Fink NS, Hoesli I, et al. Effects of relaxation on psychobiological wellbeing during pregnancy: a randomized controlled trial. Psychoneuroendocrinology. 2010;35(9):1348-1355.
32. Goleman D. The relaxation response. In: Mind body medicine: how to use your mind for better health. Yonkers, NY: Consumer Reports; 1993:125-149.
33. Galvin JA, Benson H, Deckro GR, et al. The relaxation response: reducing stress and improving cognition in healthy aging adults. Complement Ther Clin Pract. 2006;12(3):186-191.
1. Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Masand PS, Gupta S. Long-term side effects of newer-generation antidepressants: SSRIs, venlafaxine, nefazodone, bupropion, and mirtazapine. Ann Clin Psychiatry. 2002;14(3):175-182.
3. Siddiqui R, Gawande S, Shende T, et al. SSRI-induced coagulopathy: is it reality? Therapeutic Advances in Psychopharmacology. 2011;1(6):169-174.
4. Brambilla P, Cipriani A, Hotopf M, et al. Side-effect profile of fluoxetine in comparison with other SSRIs, tricyclic and newer antidepressants: a meta-analysis of clinical trial data. Pharmacopsychiatry. 2005;38(2):69-77.
5. Slomski A. Blended CBT controls anxiety in cancer survivors. JAMA. 2017;318(4):323.
6. Forsell E, Bendix M, Holländare F, et al. Internet delivered cognitive behavior therapy for antenatal depression: a randomised controlled trial. J Affect Disord. 2017;221:56-64.
7. Lilliengren P, Johansson R, Town JM, et al. Intensive Short-Term Dynamic Psychotherapy for generalized anxiety disorder: A pilot effectiveness and process-outcome study. Clin Psychol Psychother. 2017;24(6):1313-1321.
8. Furtado M, Katzman MA. Neuroinflammatory pathways in anxiety, posttraumatic stress, and obsessive compulsive disorders. Psychiatry Res. 2015;229(1-2):37-48.
9. Spielberger CD, Gorsuch RL, Lushene R, et al. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983.
10. Saper RB, Eisenberg DM, Davis RB, et al. Prevalence and patterns of adult yoga use in the United States: results of a national survey. Altern Ther Health Med. 2004;10(2):44-49.
11. Farmer J. Americanasana. Reviews in American history. 2012;40(1):145-158.
12. Field T, Diego M, Delgado J, et al. Yoga and social support reduce prenatal depression, anxiety and cortisol. J Bodyw Mov Ther: 2013;17(4):397-403.
13. Jorge MP, Santaella DF, Pontes IM, et al. Hatha Yoga practice decreases menopause symptoms and improves quality of life: a randomized controlled trial. Complement Ther Med. 2016;26:128-135.
14. Pinar R, Afsar F. Back massage to decrease state anxiety, cortisol level, blood pressure, heart rate and increase sleep quality in family caregivers of patients with cancer: a randomised controlled trial. Asian Pac J Cancer Prev. 2015;16(18):8127-8133.
15. Kuriyama H, Watanabe S, Nakaya, et al. Immunological and psychological benefits of aromatherapy massage. Evid Based Complement Alternat Med. 2005;2(2):179-184.
16. Setzer WN. Essential oils and anxiolytic aromatherapy. Nat Prod Commun. 2009;4(9):1305-1316.
17. Wu JJ, Cui Y, Yang YS, et al. Modulatory effects of aromatherapy massage intervention on electroencephalogram, psychological assessments, salivary cortisol and plasma brain-derived neurotrophic factor. Complement Ther Med. 2014;22(3):456-462.
18. Fine A. Forward. In: Fine A, ed. Handbook on animal-assisted therapy-theoretical foundations and guidelines for practice. 3rd ed. Academic Press; 2010:xvii-xviii.
19. Snipelisky D, Burton MC. Canine-assisted therapy in the inpatient setting. South Med J. 2014;107(4):265-273.
20. Polheber JP, Matchock RL. The presence of a dog attenuates cortisol and heart rate in the Trier Social Stress Test compared to human friends. J Behav Med. 2014;37(5):860-867.
21. Liu T, Qiang X, eds. Chinese medical Qigong. Philadelphia, PA: Singing Dragon; 2013:1-100,192,238,511.
22. Lee MS, Kang CW, Lim HJ, et al. Effects of Qi-training on anxiety and plasma concentrations of cortisol, ACTH, and aldosterone: a randomized placebo-controlled pilot study. Stress Health. 2004;20(5):243-248.
23. Hwang EY, Chung SY, Cho JH, et al. Effects of a brief Qigong-based stress reduction program (BQSRP) in a distressed Korean population: a randomized trial. BMC Complement Altern Med. 2013;13:113.
24. Oleson, T. Overview and history of auriculotherapy. In: Auriculotherapy manual: Chinese and Western systems of ear acupuncture. 4th ed. London: Churchill Livingstone; 2014:1.
25. Kuo SY, Tsai SH, Chen SL, et al. Auricular acupressure relieves anxiety and fatigue, and reduces cortisol levels in post-caesarean section women: a single-blind, randomised controlled study. Int J Nurs Stud. 2016;53:17-26.
26. Horan P. Introduction. In: Horan P. Empowerment through reiki: the path to personal and global transformation. 8th ed. Twin Lakes, WI: Lotus Press; 1998:13-15.
27. Bremner MN, Blake BJ, Wagner VD, et al. Effects of reiki with music compared to music only among people living with HIV. J Assoc Nurses AIDS Care. 2016;27(5):635-647.
28. Helmes JM. The basic, clinical, and speculative science of acupuncture. In: Acupuncture energetics: a clinical approach for physicians. Volume 1. Berkeley, CA: Medical Acupuncture Publishers; 1995:19-32.
29. Haddad-Rodrigues M, Spanó Nakano A, Stefanello J, et al. Acupuncture for anxiety in lactating mothers with preterm infants: a randomized controlled trial. Evid Based Complement Alternat Med. 2013;2013:169184. doi: 10.1155/2013/169184.
30. Zengin S, Kabul S, Al B, et al. Effects of music therapy on pain and anxiety in patients undergoing port catheter placement procedure. Complement Ther Med. 2013;21(6):689-696.
31. Urech C, Fink NS, Hoesli I, et al. Effects of relaxation on psychobiological wellbeing during pregnancy: a randomized controlled trial. Psychoneuroendocrinology. 2010;35(9):1348-1355.
32. Goleman D. The relaxation response. In: Mind body medicine: how to use your mind for better health. Yonkers, NY: Consumer Reports; 1993:125-149.
33. Galvin JA, Benson H, Deckro GR, et al. The relaxation response: reducing stress and improving cognition in healthy aging adults. Complement Ther Clin Pract. 2006;12(3):186-191.
Can CBT effectively treat adult insomnia disorder?
EVIDENCE SUMMARY
Three meta-analyses that included only randomized controlled trials (RCTs) compared various CBT delivery methods with controls (wait-listed for treatment or general sleep hygiene education) to assess sleep outcomes for adults with insomnia.1-3 TABLE 11-3 summarizes the results.

CBT is comparable to pharmacotherapy
A 2002 comparative meta-analysis of 21 RCTs with a total of 470 patients examined the effectiveness of CBT (stimulus control and/or sleep restriction) compared with pharmacotherapy (benzodiazepines or benzodiazepine agonists) for treating primary insomnia of longer than one month’s duration in adults with no comorbid medical or psychiatric diagnoses.4 The CBT group received intervention over an average of 5 weeks, and the pharmacotherapy group received intervention over an average of 2 weeks.
CBT produced greater reductions in sleep onset latency than pharmacotherapy based on mean weighted effect size (1.05 vs 0.45 weighted effect size; 95% confidence interval, 0.17-1.04; P=.01). Although both CBT and pharmacotherapy improved sleep outcomes, no statistical differences were found in wake after sleep onset time, total sleep time, number of awakenings, or sleep quality ratings (TABLE 24).

Continue to: CBT has significant benefit for comorbid insomnia
CBT has significant benefit for comorbid insomnia
A 2015 meta-analysis of 23 studies enrolling a total of 1379 adults with a number of illnesses (chronic pain, alcohol dependence, breast cancer, psychiatric disorders, chronic obstructive pulmonary disease, fibromyalgia) and comorbid insomnia investigated the qualitative effectiveness of individual or group CBT therapy.5 Subjects received at least 4 face-to-face sessions and at least 2 components of CBT.
The primary outcome showed that sleep quality improved, as measured by a 6.36-point reduction in the Insomnia Severity Index (ISI; a 7-question scale on which 0=no insomnia and 28=severe insomnia) and a 3.3-point reduction in the Pittsburgh Sleep Quality Index (PSQI; a 7-category assessment tool on which 0=perfect quality and 21=poor quality). The effect size was large for both ISI and PSQI, as indicated by standard mean differences greater than 0.8 (1.22 and 0.88, respectively) and was sustained for as long as 18 months.
RECOMMENDATIONS
The American College of Physicians strongly recommends that all adult patients receive CBT as initial treatment for chronic insomnia disorder. It can be performed in multiple settings, including the primary care setting. Compared with hypnotics, CBT is unlikely to have any adverse effects.6
1. Trauer J, Qian M, Doyle J, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:191-204.
2. Koffel E, Koffel J, Gehrman P. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6-16.
3. Ye Y, Chen N, Chen J, et al. Internet-based cognitive-behavioral therapy for insomnia (ICBT-i): a meta-analysis of randomized controlled trials. BMJ Open. 2016;6:e010707.
4. Smith M, Perlis M, Park S, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5-11.
5. Geiger-Brown J, Rogers V, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67.
6. Qaseem A, Kansagara D, Forciea M, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133.
EVIDENCE SUMMARY
Three meta-analyses that included only randomized controlled trials (RCTs) compared various CBT delivery methods with controls (wait-listed for treatment or general sleep hygiene education) to assess sleep outcomes for adults with insomnia.1-3 TABLE 11-3 summarizes the results.

CBT is comparable to pharmacotherapy
A 2002 comparative meta-analysis of 21 RCTs with a total of 470 patients examined the effectiveness of CBT (stimulus control and/or sleep restriction) compared with pharmacotherapy (benzodiazepines or benzodiazepine agonists) for treating primary insomnia of longer than one month’s duration in adults with no comorbid medical or psychiatric diagnoses.4 The CBT group received intervention over an average of 5 weeks, and the pharmacotherapy group received intervention over an average of 2 weeks.
CBT produced greater reductions in sleep onset latency than pharmacotherapy based on mean weighted effect size (1.05 vs 0.45 weighted effect size; 95% confidence interval, 0.17-1.04; P=.01). Although both CBT and pharmacotherapy improved sleep outcomes, no statistical differences were found in wake after sleep onset time, total sleep time, number of awakenings, or sleep quality ratings (TABLE 24).

Continue to: CBT has significant benefit for comorbid insomnia
CBT has significant benefit for comorbid insomnia
A 2015 meta-analysis of 23 studies enrolling a total of 1379 adults with a number of illnesses (chronic pain, alcohol dependence, breast cancer, psychiatric disorders, chronic obstructive pulmonary disease, fibromyalgia) and comorbid insomnia investigated the qualitative effectiveness of individual or group CBT therapy.5 Subjects received at least 4 face-to-face sessions and at least 2 components of CBT.
The primary outcome showed that sleep quality improved, as measured by a 6.36-point reduction in the Insomnia Severity Index (ISI; a 7-question scale on which 0=no insomnia and 28=severe insomnia) and a 3.3-point reduction in the Pittsburgh Sleep Quality Index (PSQI; a 7-category assessment tool on which 0=perfect quality and 21=poor quality). The effect size was large for both ISI and PSQI, as indicated by standard mean differences greater than 0.8 (1.22 and 0.88, respectively) and was sustained for as long as 18 months.
RECOMMENDATIONS
The American College of Physicians strongly recommends that all adult patients receive CBT as initial treatment for chronic insomnia disorder. It can be performed in multiple settings, including the primary care setting. Compared with hypnotics, CBT is unlikely to have any adverse effects.6
EVIDENCE SUMMARY
Three meta-analyses that included only randomized controlled trials (RCTs) compared various CBT delivery methods with controls (wait-listed for treatment or general sleep hygiene education) to assess sleep outcomes for adults with insomnia.1-3 TABLE 11-3 summarizes the results.

CBT is comparable to pharmacotherapy
A 2002 comparative meta-analysis of 21 RCTs with a total of 470 patients examined the effectiveness of CBT (stimulus control and/or sleep restriction) compared with pharmacotherapy (benzodiazepines or benzodiazepine agonists) for treating primary insomnia of longer than one month’s duration in adults with no comorbid medical or psychiatric diagnoses.4 The CBT group received intervention over an average of 5 weeks, and the pharmacotherapy group received intervention over an average of 2 weeks.
CBT produced greater reductions in sleep onset latency than pharmacotherapy based on mean weighted effect size (1.05 vs 0.45 weighted effect size; 95% confidence interval, 0.17-1.04; P=.01). Although both CBT and pharmacotherapy improved sleep outcomes, no statistical differences were found in wake after sleep onset time, total sleep time, number of awakenings, or sleep quality ratings (TABLE 24).

Continue to: CBT has significant benefit for comorbid insomnia
CBT has significant benefit for comorbid insomnia
A 2015 meta-analysis of 23 studies enrolling a total of 1379 adults with a number of illnesses (chronic pain, alcohol dependence, breast cancer, psychiatric disorders, chronic obstructive pulmonary disease, fibromyalgia) and comorbid insomnia investigated the qualitative effectiveness of individual or group CBT therapy.5 Subjects received at least 4 face-to-face sessions and at least 2 components of CBT.
The primary outcome showed that sleep quality improved, as measured by a 6.36-point reduction in the Insomnia Severity Index (ISI; a 7-question scale on which 0=no insomnia and 28=severe insomnia) and a 3.3-point reduction in the Pittsburgh Sleep Quality Index (PSQI; a 7-category assessment tool on which 0=perfect quality and 21=poor quality). The effect size was large for both ISI and PSQI, as indicated by standard mean differences greater than 0.8 (1.22 and 0.88, respectively) and was sustained for as long as 18 months.
RECOMMENDATIONS
The American College of Physicians strongly recommends that all adult patients receive CBT as initial treatment for chronic insomnia disorder. It can be performed in multiple settings, including the primary care setting. Compared with hypnotics, CBT is unlikely to have any adverse effects.6
1. Trauer J, Qian M, Doyle J, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:191-204.
2. Koffel E, Koffel J, Gehrman P. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6-16.
3. Ye Y, Chen N, Chen J, et al. Internet-based cognitive-behavioral therapy for insomnia (ICBT-i): a meta-analysis of randomized controlled trials. BMJ Open. 2016;6:e010707.
4. Smith M, Perlis M, Park S, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5-11.
5. Geiger-Brown J, Rogers V, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67.
6. Qaseem A, Kansagara D, Forciea M, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133.
1. Trauer J, Qian M, Doyle J, et al. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:191-204.
2. Koffel E, Koffel J, Gehrman P. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6-16.
3. Ye Y, Chen N, Chen J, et al. Internet-based cognitive-behavioral therapy for insomnia (ICBT-i): a meta-analysis of randomized controlled trials. BMJ Open. 2016;6:e010707.
4. Smith M, Perlis M, Park S, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry. 2002;159:5-11.
5. Geiger-Brown J, Rogers V, Liu W, et al. Cognitive behavioral therapy in persons with comorbid insomnia: a meta-analysis. Sleep Med Rev. 2015;23:54-67.
6. Qaseem A, Kansagara D, Forciea M, et al. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165:125-133.
EVIDENCE-BASED ANSWER:
Yes. Cognitive behavioral therapy (CBT) administered individually, in a group setting, or on the internet is effective for treating insomnia in adults compared with control (strength of recommendation [SOR]: A, meta-analyses).
CBT is comparable to pharmacotherapy for improving measures of sleep (SOR: A, comparative meta-analysis).
CBT produces sustainable improvements in subjective sleep quality for adults with comorbid insomnia (SOR: A, meta-analysis).
How to differentiate maternal from fetal heart rate patterns on electronic fetal monitoring
Continuous electronic fetal heart rate monitoring (EFM) is used in the vast majority of all labors in the United States. With the use of EFM categories and definitions from the American College of Obstetricians and Gynecologists, the National Institutes of Health, and the Society for Maternal-Fetal Medicine, clinicians can now better define and communicate tracing assessments. Except for reducing neonatal seizure activity, however, EFM use during labor has not been demonstrated to significantly improve fetal and neonatal outcomes, yet EFM is associated with an increase in cesarean deliveries and instrument-assisted vaginal births.1
The negative predictive value of EFM for fetal hypoxia/acidosis is high, but its positive predictive value is only 30%, and the false-positive rate is as high as 60%.2 Although a false-positive assessment may result in a potentially unnecessary operative vaginal or cesarean delivery, a falsely reassuring strip may produce devastating consequences in the newborn and, not infrequently, medical malpractice liability. One etiology associated with falsely reassuring assessments is that of EFM monitoring of the maternal heart rate and the failure to recognize the tracing as maternal.
In this article, I discuss the mechanisms and periods of labor that often are associated with the maternal heart rate masquerading as the fetal heart rate. I review common EFM patterns associated with the maternal heart rate so as to aid in recognizing the maternal heart rate. In addition, I provide 3 case scenarios that illustrate the simple yet critical steps that clinicians can take to remedy the situation. Being aware of the potential for a maternal heart rate recording, investigating the EFM signals, and correcting the monitoring can help prevent significant morbidity.
CASE 1 EFM shows seesaw decelerations and returns to baseline rate
A 29-year-old woman (G3P2) at 39 weeks’ gestation was admitted to the hospital with spontaneous labor. Continuous EFM external monitoring was initiated. After membranes spontaneously ruptured at 4 cm dilation, an epidural was placed. Throughout the active phase of labor, the fetus demonstrated intermittent mild variable decelerations, and the fetal heart rate baseline increased to 180 beats per minute (BPM). With complete dilation, the patient initiated pushing. During the first several pushes, the EFM demonstrated an initial heart rate deceleration, and a loss of signal, but the heart rate returned to a baseline rate of 150 BPM. With the patient’s continued pushing efforts, the EFM baseline increased to 180 BPM, with evidence of variable decelerations to a nadir of 120 BPM, although with some signal gaps (FIGURE 1, red arrow). The tracing then appeared to have a baseline of 120 BPM with variability or accelerations (FIGURE 1, green arrow) before shifting again to 170 to 180 BPM.
What was happening?
Why does the EFM record the maternal heart rate?
Most commonly, EFM recording of the maternal heart rate occurs during the second stage of labor. Early in labor, the normal fetal heart rate (110–160 BPM) typically exceeds the basal maternal heart rate. However, in the presence of chorioamnionitis and maternal fever or with the stress of maternal pushing, the maternal heart rate frequently approaches or exceeds that of the fetal heart rate. The maximum maternal heart rate can be estimated as 220 BPM minus the maternal age. Thus, the heart rate in a 20-year-old gravida may reach rates of 160 to 180 BPM, equivalent to 80% to 90% of her maximum heart rate during second-stage pushing.
The external Doppler fetal monitor, having a somewhat narrow acoustic window, may lose the focus on the fetal heart as a result of descent of the baby, the abdominal shape-altering effect of uterine contractions, and the patient’s pushing. During the second stage, the EFM may record the maternal heart rate from the uterine arteries. Although some clinicians claim to differentiate the maternal from the fetal heart rate by the “whooshing” maternal uterine artery signal as compared with the “thumping” fetal heart rate signal, this auditory assessment is unproven and likely unreliable.
CASE 1 Problem recognized and addressed
In this case, the obstetrician recognized that “slipping” from the fetal to the maternal heart rate recording occurred with the onset of maternal pushing. After the pushing ceased, the maternal heart rate slipped back to the fetal heart rate. With the next several contractions, only the maternal heart rate was recorded. A fetal scalp electrode was then placed, and fetal variable decelerations were recognized. In view of the category II EFM recording, a vacuum procedure was performed from +3 station and a female infant was delivered. She had Apgar scores of 6 and 8 at 1 and 5 minutes, respectively, and she did well in the nursery.
Read what happened in Case 2 when the EFM demonstrated breaks in the tracing
CASE 2 EFM tracings belie the clinical situation
A 20-year-old woman (G1P0) presented for induction of labor at 41 weeks’ gestation. Continuous EFM recording was initiated, and the patient was given dinoprostone and, subsequently, oxytocin. Rupture of membranes at 3 cm demonstrated a small amount of fluid with thick meconium. The patient progressed to complete dilation and developed a temperature of 38.5°C; the EFM baseline increased to 180 BPM. Throughout the first hour of the second stage of labor, the EFM demonstrated breaks in the tracing and a heart rate of 130 to 150 BPM with each pushing effort (FIGURE 2A). The Doppler monitor was subsequently adjusted to focus on the fetal heart and repetitive late decelerations were observed (FIGURE 2B). An emergent cesarean delivery was performed. A depressed newborn male was delivered, with Apgar scores of 2 and 4 at 1 and 5 minutes, respectively, and significant metabolic acidosis.
What happened?
Fetal versus maternal responses to pushing
The fetal variable deceleration pattern is well recognized by clinicians. As a result of umbilical cord occlusion (due to compression, stretching, or twisting of the cord), fetal variable decelerations have a typical pattern. An initial acceleration shoulder resulting from umbilical vein occlusion (due to reduced venous return) is followed by an umbilical artery occlusion–induced sharp deceleration. The relief of the occlusion allows the sharp return toward baseline with the secondary shoulder overshoot.
In some cases, partial umbilical cord occlusion that affects only the fetal umbilical vein may result in an acceleration, although these usually resolve or evolve into variable decelerations within 30 minutes. By contrast, the maternal heart rate typically increases with contractions and with maternal pushing efforts. Thus, a repetitive pattern of heart rate accelerations with each contraction should warn of a possible maternal heart rate recording.
How maternal heart rate responds to pushing. Maternal pushing is a Valsalva maneuver. Although there are 4 classic cardiovascular phases of Valsalva responses, the typical maternal pushing effort results in an increase in the maternal heart rate. With the common sequence of three 10-second pushes during each contraction, the maternal heart rate often exhibits 3 acceleration and deceleration responses. The maternal heart rate tracing looks similar to the shape of the Three Sisters mountain peaks in Oregon (FIGURE 3). Due to Valsalva physiology, the 3 peaks of the Sisters mirror the 3 uterine wave form peaks, although with a 5- to 10-second delay in the heart rate responses (mountain peaks) from the pushing efforts.
Pre- and postcontraction changes offer clues. Several classic findings aid in differentiating the maternal from the fetal heart rate. If the tracing is maternal, typically the heart rate gradually decreases following the end of the contraction/pushing and continues to decrease until the start of the next contraction/pushing, at which time it increases. During the push, the Three Sisters wave form, with the 5- to 10-second offset, should alert the clinician to possible maternal heart rate recordings. By contrast, the fetal heart rate variable deceleration typically increases following the end of the maternal contraction/pushing and is either stable or increases further (variable with slow recovery) prior to the next uterine contraction/pushing effort. These differences in the patterns of precontraction and postcontraction changes can be very valuable in differentiating periods of maternal versus fetal heart rate recordings.
With “slipping” between fetal and maternal recording, it is not uncommon to record fetal heart rate between contractions, slip to the maternal heart rate during the pushing effort, and return again to the fetal heart rate with the end of the contraction. When confounded with the potential for other EFM artifacts, including doubling of a low maternal or fetal heart rate, or halving of a tachycardic signal, it is not surprising that it is challenging to recognize an EFM maternal heart rate recording.
CASE 2 Check the monitor for accurate focus
A retrospective analysis of this case revealed that the maternal heart rate was recorded with each contraction throughout the second stage. The actual fetal heart rate pattern of decelerations was revealed with the refocusing of the Doppler monitor.
Read how subtle slipping manifested in the EFM tracing of Case 3
CASE 3 Low fetal heart rate and variability during contractions
A 22-year-old woman (G2P1) in spontaneous labor at term progressed to complete dilation. Fetal heart rate accelerations occurred for approximately 30 minutes. With the advent of pushing, the fetal heart rate showed a rate of 130 to 140 BPM and mild decelerations with each contraction (FIGURE 4A). As the second stage progressed, the tracing demonstrated an undulating baseline heart rate between 100 and 130 BPM with possible variability during contractions (FIGURE 4B). This pattern continued for an additional 60 minutes. At vaginal delivery, the ObGyn was surprised to deliver a depressed newborn with Apgar scores of 1 and 3 at 1 and 5 minutes, respectively.
Slipping from the fetal to the maternal heart rate may be imperceptible
In contrast to the breaks in the tracings seen in Case 1 and Case 2, the EFM tracing in Case 3 appears continuous. Yet, slipping from the fetal to the maternal recording was occurring.
As seen in FIGURE 4C, the maternal heart rate with variability was recorded during pushing efforts, and the fetal heart rate was seen rising back toward a baseline between contractions. Note that the fetal heart rate did not reach a level baseline, but rather decelerated with the next contraction. The slipping to the maternal heart rate occurred without a perceptible break in the recording, making this tracing extremely difficult to interpret.
CASE 3 Be ever vigilant
The lack of recognition that the EFM recording had slipped to the maternal heart rate resulted in fetal and newborn hypoxia and acidosis, accounting for the infant’s low Apgar scores.
Read how using 3 steps can help you distinguish fetal from maternal heart rate patterns
Follow 3 steps to discern fetal vs maternal heart rate
These cases illustrate the difficulties in recognizing maternal heart rate patterns on the fetal monitor tracing. The 3 simple steps described below can aid in differentiating maternal from fetal heart rate patterns.
1 Be aware and alert
Recognize that EFM monitoring of the maternal heart rate may occur during periods of monitoring, particularly in second-stage labor. Often, the recorded tracing is a mix of fetal and maternal patterns. Remember that the maternal heart rate may increase markedly during the second stage and rise even higher during pushing efforts. When presented with a tracing that ostensibly represents the fetus, it may be challenging for the clinician to question that assumption. Thus, be aware that tracings may not represent what they seem to be.
Often, clinicians view only the 10-minute portion of the tracing displayed on the monitor screen. I recommend, however, that clinicians review the tracing over the past 30 to 60 minutes, or since their last EFM assessment, for an understanding of the recent fetal baseline heart rate and decelerations.
2 Investigate
Although it is sometimes challenging to recognize EFM maternal heart rate recordings, this is relatively easy to investigate. Even without a pulse oximeter in place, carefully examine the EFM recording for maternal signs to determine if the maternal heart rate is within the range of the recording. You can confirm that the recording is maternal through 1 of 3 easy measures:
- First, check the maternal radial pulse and correlate it with the heart rate baseline.
- Second, place a maternal electrocardiographic (EKG) heart rate monitor.
- Last, and often the simplest approach for continuous tracings, place a finger pulse oximeter to provide a continuous maternal pulse reading. Should the maternal heart rate superimpose on the EFM recording, maternal patterns are likely being detected. However, since the pulse oximeter and EFM Doppler devices use different technologies, they will provide similar—but not precisely identical—heart rate numerical readings if both are assessing the maternal heart rate. In that case, take steps to assure that the EFM truly is recording the fetal heart rate.
3 Treat and correct
If the EFM is recording a maternal signal or if a significant question remains, place a fetal scalp electrode (unless contraindicated), as this may likely occur during the second stage. Alternatively, place a maternal surface fetal EKG monitor, or use ultrasonography to visually assess the fetal heart rate in real time.
Key point summary
The use of a maternal finger pulse oximeter, combined with a careful assessment of the EFM tracing, and/or a fetal scalp electrode are appropriate measures for confirming a fetal heart rate recording.
The 3 steps described (be aware and alert, investigate, treat and correct) can help you effectively monitor the fetal heart rate and avoid the potentially dangerous outcomes that might occur when the maternal heart rate masquerades as the fetal heart rate.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Alfirevic Z, Devane D, Gyte GM, Cuthbert A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2017; doi:10.1002/14651858.CD006066.pub3.
- Pinas A, Chandraharan E. Continuous cardiotocography during labour: analysis, classification and management. Best Pract Res Clin Obstet Gynaecol. 2016;30:33–47.
Continuous electronic fetal heart rate monitoring (EFM) is used in the vast majority of all labors in the United States. With the use of EFM categories and definitions from the American College of Obstetricians and Gynecologists, the National Institutes of Health, and the Society for Maternal-Fetal Medicine, clinicians can now better define and communicate tracing assessments. Except for reducing neonatal seizure activity, however, EFM use during labor has not been demonstrated to significantly improve fetal and neonatal outcomes, yet EFM is associated with an increase in cesarean deliveries and instrument-assisted vaginal births.1
The negative predictive value of EFM for fetal hypoxia/acidosis is high, but its positive predictive value is only 30%, and the false-positive rate is as high as 60%.2 Although a false-positive assessment may result in a potentially unnecessary operative vaginal or cesarean delivery, a falsely reassuring strip may produce devastating consequences in the newborn and, not infrequently, medical malpractice liability. One etiology associated with falsely reassuring assessments is that of EFM monitoring of the maternal heart rate and the failure to recognize the tracing as maternal.
In this article, I discuss the mechanisms and periods of labor that often are associated with the maternal heart rate masquerading as the fetal heart rate. I review common EFM patterns associated with the maternal heart rate so as to aid in recognizing the maternal heart rate. In addition, I provide 3 case scenarios that illustrate the simple yet critical steps that clinicians can take to remedy the situation. Being aware of the potential for a maternal heart rate recording, investigating the EFM signals, and correcting the monitoring can help prevent significant morbidity.
CASE 1 EFM shows seesaw decelerations and returns to baseline rate
A 29-year-old woman (G3P2) at 39 weeks’ gestation was admitted to the hospital with spontaneous labor. Continuous EFM external monitoring was initiated. After membranes spontaneously ruptured at 4 cm dilation, an epidural was placed. Throughout the active phase of labor, the fetus demonstrated intermittent mild variable decelerations, and the fetal heart rate baseline increased to 180 beats per minute (BPM). With complete dilation, the patient initiated pushing. During the first several pushes, the EFM demonstrated an initial heart rate deceleration, and a loss of signal, but the heart rate returned to a baseline rate of 150 BPM. With the patient’s continued pushing efforts, the EFM baseline increased to 180 BPM, with evidence of variable decelerations to a nadir of 120 BPM, although with some signal gaps (FIGURE 1, red arrow). The tracing then appeared to have a baseline of 120 BPM with variability or accelerations (FIGURE 1, green arrow) before shifting again to 170 to 180 BPM.
What was happening?
Why does the EFM record the maternal heart rate?
Most commonly, EFM recording of the maternal heart rate occurs during the second stage of labor. Early in labor, the normal fetal heart rate (110–160 BPM) typically exceeds the basal maternal heart rate. However, in the presence of chorioamnionitis and maternal fever or with the stress of maternal pushing, the maternal heart rate frequently approaches or exceeds that of the fetal heart rate. The maximum maternal heart rate can be estimated as 220 BPM minus the maternal age. Thus, the heart rate in a 20-year-old gravida may reach rates of 160 to 180 BPM, equivalent to 80% to 90% of her maximum heart rate during second-stage pushing.
The external Doppler fetal monitor, having a somewhat narrow acoustic window, may lose the focus on the fetal heart as a result of descent of the baby, the abdominal shape-altering effect of uterine contractions, and the patient’s pushing. During the second stage, the EFM may record the maternal heart rate from the uterine arteries. Although some clinicians claim to differentiate the maternal from the fetal heart rate by the “whooshing” maternal uterine artery signal as compared with the “thumping” fetal heart rate signal, this auditory assessment is unproven and likely unreliable.
CASE 1 Problem recognized and addressed
In this case, the obstetrician recognized that “slipping” from the fetal to the maternal heart rate recording occurred with the onset of maternal pushing. After the pushing ceased, the maternal heart rate slipped back to the fetal heart rate. With the next several contractions, only the maternal heart rate was recorded. A fetal scalp electrode was then placed, and fetal variable decelerations were recognized. In view of the category II EFM recording, a vacuum procedure was performed from +3 station and a female infant was delivered. She had Apgar scores of 6 and 8 at 1 and 5 minutes, respectively, and she did well in the nursery.
Read what happened in Case 2 when the EFM demonstrated breaks in the tracing
CASE 2 EFM tracings belie the clinical situation
A 20-year-old woman (G1P0) presented for induction of labor at 41 weeks’ gestation. Continuous EFM recording was initiated, and the patient was given dinoprostone and, subsequently, oxytocin. Rupture of membranes at 3 cm demonstrated a small amount of fluid with thick meconium. The patient progressed to complete dilation and developed a temperature of 38.5°C; the EFM baseline increased to 180 BPM. Throughout the first hour of the second stage of labor, the EFM demonstrated breaks in the tracing and a heart rate of 130 to 150 BPM with each pushing effort (FIGURE 2A). The Doppler monitor was subsequently adjusted to focus on the fetal heart and repetitive late decelerations were observed (FIGURE 2B). An emergent cesarean delivery was performed. A depressed newborn male was delivered, with Apgar scores of 2 and 4 at 1 and 5 minutes, respectively, and significant metabolic acidosis.
What happened?
Fetal versus maternal responses to pushing
The fetal variable deceleration pattern is well recognized by clinicians. As a result of umbilical cord occlusion (due to compression, stretching, or twisting of the cord), fetal variable decelerations have a typical pattern. An initial acceleration shoulder resulting from umbilical vein occlusion (due to reduced venous return) is followed by an umbilical artery occlusion–induced sharp deceleration. The relief of the occlusion allows the sharp return toward baseline with the secondary shoulder overshoot.
In some cases, partial umbilical cord occlusion that affects only the fetal umbilical vein may result in an acceleration, although these usually resolve or evolve into variable decelerations within 30 minutes. By contrast, the maternal heart rate typically increases with contractions and with maternal pushing efforts. Thus, a repetitive pattern of heart rate accelerations with each contraction should warn of a possible maternal heart rate recording.
How maternal heart rate responds to pushing. Maternal pushing is a Valsalva maneuver. Although there are 4 classic cardiovascular phases of Valsalva responses, the typical maternal pushing effort results in an increase in the maternal heart rate. With the common sequence of three 10-second pushes during each contraction, the maternal heart rate often exhibits 3 acceleration and deceleration responses. The maternal heart rate tracing looks similar to the shape of the Three Sisters mountain peaks in Oregon (FIGURE 3). Due to Valsalva physiology, the 3 peaks of the Sisters mirror the 3 uterine wave form peaks, although with a 5- to 10-second delay in the heart rate responses (mountain peaks) from the pushing efforts.
Pre- and postcontraction changes offer clues. Several classic findings aid in differentiating the maternal from the fetal heart rate. If the tracing is maternal, typically the heart rate gradually decreases following the end of the contraction/pushing and continues to decrease until the start of the next contraction/pushing, at which time it increases. During the push, the Three Sisters wave form, with the 5- to 10-second offset, should alert the clinician to possible maternal heart rate recordings. By contrast, the fetal heart rate variable deceleration typically increases following the end of the maternal contraction/pushing and is either stable or increases further (variable with slow recovery) prior to the next uterine contraction/pushing effort. These differences in the patterns of precontraction and postcontraction changes can be very valuable in differentiating periods of maternal versus fetal heart rate recordings.
With “slipping” between fetal and maternal recording, it is not uncommon to record fetal heart rate between contractions, slip to the maternal heart rate during the pushing effort, and return again to the fetal heart rate with the end of the contraction. When confounded with the potential for other EFM artifacts, including doubling of a low maternal or fetal heart rate, or halving of a tachycardic signal, it is not surprising that it is challenging to recognize an EFM maternal heart rate recording.
CASE 2 Check the monitor for accurate focus
A retrospective analysis of this case revealed that the maternal heart rate was recorded with each contraction throughout the second stage. The actual fetal heart rate pattern of decelerations was revealed with the refocusing of the Doppler monitor.
Read how subtle slipping manifested in the EFM tracing of Case 3
CASE 3 Low fetal heart rate and variability during contractions
A 22-year-old woman (G2P1) in spontaneous labor at term progressed to complete dilation. Fetal heart rate accelerations occurred for approximately 30 minutes. With the advent of pushing, the fetal heart rate showed a rate of 130 to 140 BPM and mild decelerations with each contraction (FIGURE 4A). As the second stage progressed, the tracing demonstrated an undulating baseline heart rate between 100 and 130 BPM with possible variability during contractions (FIGURE 4B). This pattern continued for an additional 60 minutes. At vaginal delivery, the ObGyn was surprised to deliver a depressed newborn with Apgar scores of 1 and 3 at 1 and 5 minutes, respectively.
Slipping from the fetal to the maternal heart rate may be imperceptible
In contrast to the breaks in the tracings seen in Case 1 and Case 2, the EFM tracing in Case 3 appears continuous. Yet, slipping from the fetal to the maternal recording was occurring.
As seen in FIGURE 4C, the maternal heart rate with variability was recorded during pushing efforts, and the fetal heart rate was seen rising back toward a baseline between contractions. Note that the fetal heart rate did not reach a level baseline, but rather decelerated with the next contraction. The slipping to the maternal heart rate occurred without a perceptible break in the recording, making this tracing extremely difficult to interpret.
CASE 3 Be ever vigilant
The lack of recognition that the EFM recording had slipped to the maternal heart rate resulted in fetal and newborn hypoxia and acidosis, accounting for the infant’s low Apgar scores.
Read how using 3 steps can help you distinguish fetal from maternal heart rate patterns
Follow 3 steps to discern fetal vs maternal heart rate
These cases illustrate the difficulties in recognizing maternal heart rate patterns on the fetal monitor tracing. The 3 simple steps described below can aid in differentiating maternal from fetal heart rate patterns.
1 Be aware and alert
Recognize that EFM monitoring of the maternal heart rate may occur during periods of monitoring, particularly in second-stage labor. Often, the recorded tracing is a mix of fetal and maternal patterns. Remember that the maternal heart rate may increase markedly during the second stage and rise even higher during pushing efforts. When presented with a tracing that ostensibly represents the fetus, it may be challenging for the clinician to question that assumption. Thus, be aware that tracings may not represent what they seem to be.
Often, clinicians view only the 10-minute portion of the tracing displayed on the monitor screen. I recommend, however, that clinicians review the tracing over the past 30 to 60 minutes, or since their last EFM assessment, for an understanding of the recent fetal baseline heart rate and decelerations.
2 Investigate
Although it is sometimes challenging to recognize EFM maternal heart rate recordings, this is relatively easy to investigate. Even without a pulse oximeter in place, carefully examine the EFM recording for maternal signs to determine if the maternal heart rate is within the range of the recording. You can confirm that the recording is maternal through 1 of 3 easy measures:
- First, check the maternal radial pulse and correlate it with the heart rate baseline.
- Second, place a maternal electrocardiographic (EKG) heart rate monitor.
- Last, and often the simplest approach for continuous tracings, place a finger pulse oximeter to provide a continuous maternal pulse reading. Should the maternal heart rate superimpose on the EFM recording, maternal patterns are likely being detected. However, since the pulse oximeter and EFM Doppler devices use different technologies, they will provide similar—but not precisely identical—heart rate numerical readings if both are assessing the maternal heart rate. In that case, take steps to assure that the EFM truly is recording the fetal heart rate.
3 Treat and correct
If the EFM is recording a maternal signal or if a significant question remains, place a fetal scalp electrode (unless contraindicated), as this may likely occur during the second stage. Alternatively, place a maternal surface fetal EKG monitor, or use ultrasonography to visually assess the fetal heart rate in real time.
Key point summary
The use of a maternal finger pulse oximeter, combined with a careful assessment of the EFM tracing, and/or a fetal scalp electrode are appropriate measures for confirming a fetal heart rate recording.
The 3 steps described (be aware and alert, investigate, treat and correct) can help you effectively monitor the fetal heart rate and avoid the potentially dangerous outcomes that might occur when the maternal heart rate masquerades as the fetal heart rate.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Continuous electronic fetal heart rate monitoring (EFM) is used in the vast majority of all labors in the United States. With the use of EFM categories and definitions from the American College of Obstetricians and Gynecologists, the National Institutes of Health, and the Society for Maternal-Fetal Medicine, clinicians can now better define and communicate tracing assessments. Except for reducing neonatal seizure activity, however, EFM use during labor has not been demonstrated to significantly improve fetal and neonatal outcomes, yet EFM is associated with an increase in cesarean deliveries and instrument-assisted vaginal births.1
The negative predictive value of EFM for fetal hypoxia/acidosis is high, but its positive predictive value is only 30%, and the false-positive rate is as high as 60%.2 Although a false-positive assessment may result in a potentially unnecessary operative vaginal or cesarean delivery, a falsely reassuring strip may produce devastating consequences in the newborn and, not infrequently, medical malpractice liability. One etiology associated with falsely reassuring assessments is that of EFM monitoring of the maternal heart rate and the failure to recognize the tracing as maternal.
In this article, I discuss the mechanisms and periods of labor that often are associated with the maternal heart rate masquerading as the fetal heart rate. I review common EFM patterns associated with the maternal heart rate so as to aid in recognizing the maternal heart rate. In addition, I provide 3 case scenarios that illustrate the simple yet critical steps that clinicians can take to remedy the situation. Being aware of the potential for a maternal heart rate recording, investigating the EFM signals, and correcting the monitoring can help prevent significant morbidity.
CASE 1 EFM shows seesaw decelerations and returns to baseline rate
A 29-year-old woman (G3P2) at 39 weeks’ gestation was admitted to the hospital with spontaneous labor. Continuous EFM external monitoring was initiated. After membranes spontaneously ruptured at 4 cm dilation, an epidural was placed. Throughout the active phase of labor, the fetus demonstrated intermittent mild variable decelerations, and the fetal heart rate baseline increased to 180 beats per minute (BPM). With complete dilation, the patient initiated pushing. During the first several pushes, the EFM demonstrated an initial heart rate deceleration, and a loss of signal, but the heart rate returned to a baseline rate of 150 BPM. With the patient’s continued pushing efforts, the EFM baseline increased to 180 BPM, with evidence of variable decelerations to a nadir of 120 BPM, although with some signal gaps (FIGURE 1, red arrow). The tracing then appeared to have a baseline of 120 BPM with variability or accelerations (FIGURE 1, green arrow) before shifting again to 170 to 180 BPM.
What was happening?
Why does the EFM record the maternal heart rate?
Most commonly, EFM recording of the maternal heart rate occurs during the second stage of labor. Early in labor, the normal fetal heart rate (110–160 BPM) typically exceeds the basal maternal heart rate. However, in the presence of chorioamnionitis and maternal fever or with the stress of maternal pushing, the maternal heart rate frequently approaches or exceeds that of the fetal heart rate. The maximum maternal heart rate can be estimated as 220 BPM minus the maternal age. Thus, the heart rate in a 20-year-old gravida may reach rates of 160 to 180 BPM, equivalent to 80% to 90% of her maximum heart rate during second-stage pushing.
The external Doppler fetal monitor, having a somewhat narrow acoustic window, may lose the focus on the fetal heart as a result of descent of the baby, the abdominal shape-altering effect of uterine contractions, and the patient’s pushing. During the second stage, the EFM may record the maternal heart rate from the uterine arteries. Although some clinicians claim to differentiate the maternal from the fetal heart rate by the “whooshing” maternal uterine artery signal as compared with the “thumping” fetal heart rate signal, this auditory assessment is unproven and likely unreliable.
CASE 1 Problem recognized and addressed
In this case, the obstetrician recognized that “slipping” from the fetal to the maternal heart rate recording occurred with the onset of maternal pushing. After the pushing ceased, the maternal heart rate slipped back to the fetal heart rate. With the next several contractions, only the maternal heart rate was recorded. A fetal scalp electrode was then placed, and fetal variable decelerations were recognized. In view of the category II EFM recording, a vacuum procedure was performed from +3 station and a female infant was delivered. She had Apgar scores of 6 and 8 at 1 and 5 minutes, respectively, and she did well in the nursery.
Read what happened in Case 2 when the EFM demonstrated breaks in the tracing
CASE 2 EFM tracings belie the clinical situation
A 20-year-old woman (G1P0) presented for induction of labor at 41 weeks’ gestation. Continuous EFM recording was initiated, and the patient was given dinoprostone and, subsequently, oxytocin. Rupture of membranes at 3 cm demonstrated a small amount of fluid with thick meconium. The patient progressed to complete dilation and developed a temperature of 38.5°C; the EFM baseline increased to 180 BPM. Throughout the first hour of the second stage of labor, the EFM demonstrated breaks in the tracing and a heart rate of 130 to 150 BPM with each pushing effort (FIGURE 2A). The Doppler monitor was subsequently adjusted to focus on the fetal heart and repetitive late decelerations were observed (FIGURE 2B). An emergent cesarean delivery was performed. A depressed newborn male was delivered, with Apgar scores of 2 and 4 at 1 and 5 minutes, respectively, and significant metabolic acidosis.
What happened?
Fetal versus maternal responses to pushing
The fetal variable deceleration pattern is well recognized by clinicians. As a result of umbilical cord occlusion (due to compression, stretching, or twisting of the cord), fetal variable decelerations have a typical pattern. An initial acceleration shoulder resulting from umbilical vein occlusion (due to reduced venous return) is followed by an umbilical artery occlusion–induced sharp deceleration. The relief of the occlusion allows the sharp return toward baseline with the secondary shoulder overshoot.
In some cases, partial umbilical cord occlusion that affects only the fetal umbilical vein may result in an acceleration, although these usually resolve or evolve into variable decelerations within 30 minutes. By contrast, the maternal heart rate typically increases with contractions and with maternal pushing efforts. Thus, a repetitive pattern of heart rate accelerations with each contraction should warn of a possible maternal heart rate recording.
How maternal heart rate responds to pushing. Maternal pushing is a Valsalva maneuver. Although there are 4 classic cardiovascular phases of Valsalva responses, the typical maternal pushing effort results in an increase in the maternal heart rate. With the common sequence of three 10-second pushes during each contraction, the maternal heart rate often exhibits 3 acceleration and deceleration responses. The maternal heart rate tracing looks similar to the shape of the Three Sisters mountain peaks in Oregon (FIGURE 3). Due to Valsalva physiology, the 3 peaks of the Sisters mirror the 3 uterine wave form peaks, although with a 5- to 10-second delay in the heart rate responses (mountain peaks) from the pushing efforts.
Pre- and postcontraction changes offer clues. Several classic findings aid in differentiating the maternal from the fetal heart rate. If the tracing is maternal, typically the heart rate gradually decreases following the end of the contraction/pushing and continues to decrease until the start of the next contraction/pushing, at which time it increases. During the push, the Three Sisters wave form, with the 5- to 10-second offset, should alert the clinician to possible maternal heart rate recordings. By contrast, the fetal heart rate variable deceleration typically increases following the end of the maternal contraction/pushing and is either stable or increases further (variable with slow recovery) prior to the next uterine contraction/pushing effort. These differences in the patterns of precontraction and postcontraction changes can be very valuable in differentiating periods of maternal versus fetal heart rate recordings.
With “slipping” between fetal and maternal recording, it is not uncommon to record fetal heart rate between contractions, slip to the maternal heart rate during the pushing effort, and return again to the fetal heart rate with the end of the contraction. When confounded with the potential for other EFM artifacts, including doubling of a low maternal or fetal heart rate, or halving of a tachycardic signal, it is not surprising that it is challenging to recognize an EFM maternal heart rate recording.
CASE 2 Check the monitor for accurate focus
A retrospective analysis of this case revealed that the maternal heart rate was recorded with each contraction throughout the second stage. The actual fetal heart rate pattern of decelerations was revealed with the refocusing of the Doppler monitor.
Read how subtle slipping manifested in the EFM tracing of Case 3
CASE 3 Low fetal heart rate and variability during contractions
A 22-year-old woman (G2P1) in spontaneous labor at term progressed to complete dilation. Fetal heart rate accelerations occurred for approximately 30 minutes. With the advent of pushing, the fetal heart rate showed a rate of 130 to 140 BPM and mild decelerations with each contraction (FIGURE 4A). As the second stage progressed, the tracing demonstrated an undulating baseline heart rate between 100 and 130 BPM with possible variability during contractions (FIGURE 4B). This pattern continued for an additional 60 minutes. At vaginal delivery, the ObGyn was surprised to deliver a depressed newborn with Apgar scores of 1 and 3 at 1 and 5 minutes, respectively.
Slipping from the fetal to the maternal heart rate may be imperceptible
In contrast to the breaks in the tracings seen in Case 1 and Case 2, the EFM tracing in Case 3 appears continuous. Yet, slipping from the fetal to the maternal recording was occurring.
As seen in FIGURE 4C, the maternal heart rate with variability was recorded during pushing efforts, and the fetal heart rate was seen rising back toward a baseline between contractions. Note that the fetal heart rate did not reach a level baseline, but rather decelerated with the next contraction. The slipping to the maternal heart rate occurred without a perceptible break in the recording, making this tracing extremely difficult to interpret.
CASE 3 Be ever vigilant
The lack of recognition that the EFM recording had slipped to the maternal heart rate resulted in fetal and newborn hypoxia and acidosis, accounting for the infant’s low Apgar scores.
Read how using 3 steps can help you distinguish fetal from maternal heart rate patterns
Follow 3 steps to discern fetal vs maternal heart rate
These cases illustrate the difficulties in recognizing maternal heart rate patterns on the fetal monitor tracing. The 3 simple steps described below can aid in differentiating maternal from fetal heart rate patterns.
1 Be aware and alert
Recognize that EFM monitoring of the maternal heart rate may occur during periods of monitoring, particularly in second-stage labor. Often, the recorded tracing is a mix of fetal and maternal patterns. Remember that the maternal heart rate may increase markedly during the second stage and rise even higher during pushing efforts. When presented with a tracing that ostensibly represents the fetus, it may be challenging for the clinician to question that assumption. Thus, be aware that tracings may not represent what they seem to be.
Often, clinicians view only the 10-minute portion of the tracing displayed on the monitor screen. I recommend, however, that clinicians review the tracing over the past 30 to 60 minutes, or since their last EFM assessment, for an understanding of the recent fetal baseline heart rate and decelerations.
2 Investigate
Although it is sometimes challenging to recognize EFM maternal heart rate recordings, this is relatively easy to investigate. Even without a pulse oximeter in place, carefully examine the EFM recording for maternal signs to determine if the maternal heart rate is within the range of the recording. You can confirm that the recording is maternal through 1 of 3 easy measures:
- First, check the maternal radial pulse and correlate it with the heart rate baseline.
- Second, place a maternal electrocardiographic (EKG) heart rate monitor.
- Last, and often the simplest approach for continuous tracings, place a finger pulse oximeter to provide a continuous maternal pulse reading. Should the maternal heart rate superimpose on the EFM recording, maternal patterns are likely being detected. However, since the pulse oximeter and EFM Doppler devices use different technologies, they will provide similar—but not precisely identical—heart rate numerical readings if both are assessing the maternal heart rate. In that case, take steps to assure that the EFM truly is recording the fetal heart rate.
3 Treat and correct
If the EFM is recording a maternal signal or if a significant question remains, place a fetal scalp electrode (unless contraindicated), as this may likely occur during the second stage. Alternatively, place a maternal surface fetal EKG monitor, or use ultrasonography to visually assess the fetal heart rate in real time.
Key point summary
The use of a maternal finger pulse oximeter, combined with a careful assessment of the EFM tracing, and/or a fetal scalp electrode are appropriate measures for confirming a fetal heart rate recording.
The 3 steps described (be aware and alert, investigate, treat and correct) can help you effectively monitor the fetal heart rate and avoid the potentially dangerous outcomes that might occur when the maternal heart rate masquerades as the fetal heart rate.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Alfirevic Z, Devane D, Gyte GM, Cuthbert A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2017; doi:10.1002/14651858.CD006066.pub3.
- Pinas A, Chandraharan E. Continuous cardiotocography during labour: analysis, classification and management. Best Pract Res Clin Obstet Gynaecol. 2016;30:33–47.
- Alfirevic Z, Devane D, Gyte GM, Cuthbert A. Continuous cardiotocography (CTG) as a form of electronic fetal monitoring (EFM) for fetal assessment during labour. Cochrane Database Syst Rev. 2017; doi:10.1002/14651858.CD006066.pub3.
- Pinas A, Chandraharan E. Continuous cardiotocography during labour: analysis, classification and management. Best Pract Res Clin Obstet Gynaecol. 2016;30:33–47.
2018 Update on infectious disease
In this Update I highlight 5 interesting investigations on infectious diseases. The first addresses the value of applying prophylactically a negative-pressure wound dressing to prevent surgical site infection (SSI) in obese women having cesarean delivery (CD). The second report assesses the effectiveness of a preoperative vaginal wash in reducing the frequency of postcesarean endometritis. The third investigation examines the role of systemic antibiotics, combined with surgical drainage, for patients who have subcutaneous abscesses ranging in size up to 5 cm. The fourth study presents new information about the major risk factors for Clostridium difficile infections in obstetric patients. The final study presents valuable sobering new data about the risks of congenital Zika virus infection.
Negative-pressure wound therapy after CD shows some benefit in preventing SSI
Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200-210.e1.
Yu and colleagues sought to determine if the prophylactic use of negative-pressure devices, compared with standard wound dressing, was effective in reducing the frequency of SSI after CD.
The authors searched multiple databases and initially identified 161 randomized controlled trials and cohort studies for further assessment. After applying rigorous exclusion criteria, they ultimately selected 9 studies for systematic review and meta-analysis. Six studies were randomized controlled trials (RCTs), 2 were retrospective cohort studies, and 1 was a prospective cohort study. Five studies were considered high quality; 4 were of low quality.
Details of the study
Several types of negative-pressure devices were used, but the 2 most common were the Prevena incision management system (KCI, San Antonio, Texas) and PICO negative- pressure wound therapy (Smith & Nephew, St. Petersburg, Florida). The majority of patients in all groups were at high risk for wound complications because of obesity.
The primary outcome of interest was the frequency of SSI. Secondary outcomes included dehiscence, seroma, endometritis, a composite measure for all wound complications, and hospital readmission.
The absolute risk of SSI in the intervention group was 5% (95% confidence interval [CI], 2.0%-7.0%) compared with 11% (95% CI, 7.0%-16.0%) in the standard dressing group. The pooled risk ratio was 0.45 (95% CI, 0.31-0.66). The absolute risk reduction was 6% (95% CI, -10.0% to -3.0%), and the number needed to treat was 17.
There were no significant differences in the rate of any of the secondary outcomes other than the composite of all wound complications. This difference was largely accounted for by the difference in the rate of SSI.
How negative-pressure devices aid wound healing
Yu and colleagues explained that negative-pressure devices exert their beneficial effects in various ways, including:
- shrinking the wound
- inducing cellular stretch
- removing extracellular fluids
- creating a favorable environment for healing
- promoting angiogenesis and neurogenesis.
Multiple studies in nonobstetric patients have shown that prophylactic use of negative-pressure devices is beneficial in reducing the rate of SSI.1 Yu and colleagues' systematic review and meta-analysis confirms those findings in a high-risk population of women having CD.
Study limitations
Before routinely adopting the use of negative-pressure devices for all women having CD, however, obstetricians should consider the following caveats:
- The investigations included in the study by Yu and colleagues did not consistently distinguish between scheduled versus unscheduled CDs.
- The reports did not systematically consider other major risk factors for wound complications besides obesity, and they did not control for these confounders in the statistical analyses.
- The studies included in the meta-analysis did not provide full descriptions of other measures that might influence the rate of SSIs, such as timing and selection of prophylactic antibiotics, selection of suture material, preoperative skin preparation, and closure techniques for the deep subcutaneous tissue and skin.
- None of the included studies systematically considered the cost-effectiveness of the negative-pressure devices. This is an important consideration given that the acquisition cost of these devices ranges from $200 to $500.
Results of the systematic review and meta-analysis by Yu and colleagues suggest that prophylactic negative-pressure wound therapy in high-risk mostly obese women after CD reduces SSI and overall wound complications. The study's limitations, however, must be kept in mind, and more data are needed. It would be most helpful if a large, well-designed RCT was conducted and included 2 groups with comparable multiple major risk factors for wound complications, and in which all women received the following important interventions2-4:
- removal of hair in the surgical site with a clipper, not a razor
- cleansing of the skin with a chlorhexidine rather than an iodophor solution
- closure of the deep subcutaneous tissue if the total subcutaneous layer exceeds 2 cm in depth
- closure of the skin with suture rather than staples
- administration of antibiotic prophylaxis, ideally with a combination of cefazolin plus azithromycin, prior to the surgical incision.
Read about vaginal cleansing’s effect on post-CD endometritis
Vaginal cleansing before CD lowers risk of postop endometritis
Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(3):527-538.
Caissutti and colleagues aimed to determine if cleansing the vagina with an antiseptic solution prior to surgery reduced the frequency of postcesarean endometritis. They included 16 RCTs (4,837 patients) in their systematic review and meta-analysis. The primary outcome was the frequency of postoperative endometritis.
Details of the study
The studies were conducted in several countries and included patients of various socioeconomic classes. Six trials included only patients having a scheduled CD; 9 included both scheduled and unscheduled cesareans; and 1 included only unscheduled cesareans. In 11 studies, povidone-iodine was the antiseptic solution used. Two trials used chlorhexidine diacetate 0.2%, and 1 used chlorhexidine diacetate 0.4%. One trial used metronidazole 0.5% gel, and another used the antiseptic cetrimide, which is a mixture of different quaternary ammonium salts, including cetrimonium bromide.
In all trials, patients received prophylactic antibiotics. The antibiotics were administered prior to the surgical incision in 6 trials; they were given after the umbilical cord was clamped in 6 trials. In 2 trials, the antibiotics were given at varying times, and in the final 2 trials, the timing of antibiotic administration was not reported. Of note, no trials described the method of placenta removal, a factor of considerable significance in influencing the rate of postoperative endometritis.5,6
Endometritis frequency reduced with vaginal cleansing; benefit greater in certain groups. Overall, in the 15 trials in which vaginal cleansing was compared with placebo or with no treatment, women in the treatment group had a significantly lower rate of endometritis (4.5% compared with 8.8%; relative risk [RR], 0.52; 95% CI, 0.37-0.72). When only women in labor were considered, the frequency of endometritis was 8.1% in the intervention group compared with 13.8% in the control group (RR, 0.52; 95% CI, 0.28-0.97). In the women who were not in labor, the difference in the incidence of endometritis was not statistically significant (3.5% vs 6.6%; RR, 0.62; 95% CI, 0.34-1.15).
In the subgroup analysis of women with ruptured membranes at the time of surgery, the incidence of endometritis was 4.3% in the treatment group compared with 20.1% in the control group (RR, 0.23; 95% CI, 0.10-0.52). In women with intact membranes at the time of surgery, the incidence of endometritis was not significantly reduced in the treatment group.
Interestingly, in the subgroup analysis of the 10 trials that used povidone-iodine, the reduction in the frequency of postcesarean endometritis was statistically significant (2.8% vs 6.3%; RR, 0.42; 95% CI, 0.25-0.71). However, this same protective effect was not observed in the women treated with chlorhexidine. In the 1 trial that directly compared povidone-iodine with chlorhexidine, there was no statistically significant difference in outcome.
Simple intervention, solid benefit
Endometritis is the most common complication following CD. The infection is polymicrobial, with mixed aerobic and anaerobic organisms. The principal risk factors for postcesarean endometritis are low socioeconomic status, extended duration of labor and ruptured membranes, multiple vaginal examinations, internal fetal monitoring, and pre-existing vaginal infections (principally, bacterial vaginosis and group B streptococcal colonization).
Two interventions are clearly of value in reducing the incidence of endometritis: administration of prophylactic antibiotics prior to the surgical incision and removal of the placenta by traction on the cord as opposed to manual extraction.5,6
The assessment by Caissutti and colleagues confirms that a third measure — preoperative vaginal cleansing — also helps reduce the incidence of postcesarean endometritis. The principal benefit is seen in women who have been in labor with ruptured membranes, although certainly it is not harmful in lower-risk patients. The intervention is simple and straightforward: a 30-second vaginal wash with a povidone-iodine solution just prior to surgery.
From my perspective, the interesting unanswered question is why a chlorhexidine solution with low alcohol content was not more effective than povidone-iodine, given that a chlorhexidine abdominal wash is superior to povidone-iodine in preventing wound infection after cesarean delivery.7 Until additional studies confirm the effectiveness of vaginal cleansing with chlorhexidine, I recommend the routine use of the povidone-iodine solution in all women having CD.
Read about management approaches for skin abscesses
Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
Daum RS, Miller LG, Immergluck L, et al; for the DMID 07-0051 Team. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376(26):2545-2555.
For treatment of subcutaneous abscesses that were 5 cm or smaller in diameter, investigators sought to determine if surgical drainage alone was equivalent to surgical drainage plus systemic antibiotics. After their abscess was drained, patients were randomly assigned to receive either clindamycin (300 mg 3 times daily) or trimethoprim-sulfamethoxazole (80 mg/400 mg twice daily) or placebo for 10 days. The primary outcome was clinical cure 7 to 10 days after treatment.
Details of the study
Daum and colleagues enrolled 786 participants (505 adults, 281 children) in the prospective double-blind study. Staphylococcus aureus was isolated from 527 patients (67.0%); methicillin-resistant S aureus (MRSA) was isolated from 388 (49.4%). The cure rate was similar in patients in the clindamycin group (83.1%) and the trimethoprim-sulfamethoxazole group (81.7%), and the cure rate in each antibiotic group was significantly higher than that in the placebo group (68.9%; P<.001 for both comparisons). The difference in treatment effect was specifically limited to patients who had S aureus isolated from their lesions.
Findings at follow-up. At 1 month of follow-up, new infections were less common in the clindamycin group (6.8%) than in the trimethoprim-sulfamethoxazole group (13.5%; P = .03) or the placebo group (12.4%; P = .06). However, the highest frequency of adverse effects occurred in the patients who received clindamycin (21.9% vs 11.1% vs 12.5%). No adverse effects were judged to be serious, and all resolved without sequela.
Controversy remains on antibiotic use after drainage
This study is important for 2 major reasons. First, soft tissue infections are quite commonand can evolve into serious problems, especially when the offending pathogen is MRSA. Second, controversy exists about whether systemic antibiotics are indicated if the subcutaneous abscess is relatively small and is adequately drained. For example, Talan and colleagues demonstrated that, in settings with a high prevalence of MRSA, surgical drainage combined with trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily) was superior to drainage plus placebo.8 However, Daum and Gold recently debated the issue of drainage plus antibiotics in a case vignette and reached opposite conclusions.9
In my opinion, this investigation by Daum and colleagues supports a role for consistent use of systemic antibiotics following surgical drainage of clinically significant subcutaneous abscesses that have a 5 cm or smaller diameter. Several oral antibiotics are effective against S aureus, including MRSA.10 These drugs include trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily), clindamycin (300-450 mg 3 times daily), doxycycline (100 mg twice daily), and minocycline (200 mg initially, then 100 mg every 12 hours).
Of these drugs, I prefer trimethoprim-sulfamethoxazole, provided that the patient does not have an allergy to sulfonamides. Trimethoprim-sulfamethoxazole is significantly less expensive than the other 3 drugs and usually is better tolerated. In particular, compared with clindamycin, trimethoprim-sulfamethoxazole is less likely to cause antibiotic-associated diarrhea, including Clostridium difficile infection. Trimethoprim-sulfamethoxazole should not be used in the first trimester of pregnancy because of concerns about fetal teratogenicity.
Read how to avoid C difficile infections in pregnant patients
Antibiotic use, common in the obstetric population, raises risk for C difficile infection
Ruiter-Ligeti J, Vincent S, Czuzoj-Shulman N, Abenhaim HA. Risk factors, incidence, and morbidity associated with obstetric Clostridium difficile infection. Obstet Gynecol. 2018;131(2):387-391.
The objective of this investigation was to identify risk factors for Clostridium difficile infection (previously termed pseudomembranous enterocolitis) in obstetric patients. The authors performed a retrospective cohort study using information from a large database maintained by the Agency for Healthcare Research and Quality. This database provides information about inpatient hospital stays in the United States, and it is the largest repository of its kind. It includes data from a sample of 1,000 US hospitals.
Details of the study
Ruiter-Ligeti and colleagues reviewed 13,881,592 births during 1999-2013 and identified 2,757 (0.02%) admissions for delivery complicated by C difficile infection, a rate of 20 admissions per 100,000 deliveries per year (95% CI, 19.13-20.62). The rate of admissions with this diagnosis doubled from 1999 (15 per 100,000) to 2013 (30 per 100,000, P<.001).
Among these obstetric patients, the principal risk factors for C difficile infection were older age, multiple gestation, long-term antibiotic use (not precisely defined), and concurrent diagnosis of inflammatory bowel disease. In addition, patients with pyelonephritis, perineal or cesarean wound infections, or pneumonia also were at increased risk, presumably because those patients required longer courses of broad-spectrum antibiotics.
Of additional note, when compared with women who did not have C difficile infection, patients with infection were more likely to develop a thromboembolic event (38.4 per 1,000), paralytic ileus (58.0 per 1,000), sepsis (46.4 per 1,000), and death (8.0 per 1,000).
Be on guard for C difficile infection in antibiotic-treated obstetric patients
C difficile infection is an uncommon but potentially very serious complication of antibiotic therapy. Given that approximately half of all women admitted for delivery are exposed to antibiotics because of prophylaxis for group B streptococcus infection, prophylaxis for CD, and treatment of chorioamnionitis and puerperal endometritis, clinicians constantly need to be vigilant for this complication.11
Affected patients typically present with frequent loose, watery stools and lower abdominal cramping. In severe cases, blood may be present in the stool, and signs of intestinal distention and even acute peritonitis may be evident. The diagnosis can be established by documenting a positive culture or polymerase chain reaction (PCR) assay for C difficile and a positive cytotoxin assay for toxins A and/or B. In addition, if endoscopy is performed, the characteristic gray membranous plaques can be visualized on the rectal and colonic mucosa.11
Discontinue antibiotic therapy. The first step in managing affected patients is to stop all antibiotics, if possible, or at least the one most likely to be the causative agent of C difficile infection. Patients with relatively mild clinical findings should be treated with oral metronidazole, 500 mg every 8 hours for 10 to 14 days. Patients with severe findings should be treated with oral vancomycin, 500 mg every 6 hours, plus IV metronidazole, 500 mg every 8 hours. The more seriously ill patient must be observed carefully for signs of bowel obstruction, intestinal perforation, peritonitis, and sepsis.
Clearly, clinicians should make every effort to prevent C difficile infection in the first place. The following preventive measures are essential:
- Avoid the use of extremely broad-spectrum antibiotics for prophylaxis for CD.
- When using therapeutic antibiotics, keep the spectrum as narrow as possible, consistent with adequately treating the pathogens causing the infection.
- Administer antibiotics for the shortest time possible, consistent with achieving a clinical cure or providing appropriate prophylaxis for surgical procedures (usually, a maximum of 3 doses).
- If a patient receiving antibiotics experiences more than 3 loose stools in 24 hours, either discontinue all antibiotics or substitute another drug for the most likely offending agent, depending on the clinical situation.
- If, after stopping or changing antibiotics, the clinical findings do not resolve promptly, perform a culture or PCR assay for C difficile and assays for the C difficile toxin. Treat as outlined above if these tests are positive.
Read about pregnancy outcomes and trimester of maternal Zika infection
Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994.
To estimate the risk of congenital neurologic defects associated with Zika virus infection, Hoen and colleagues conducted a prospective cohort study of pregnant women with symptomatic Zika virus infection who were enrolled during March through November 2016 in French Guiana, Guadeloupe, and Martinique. All women had Zika virus infection confirmed by PCR assay.
Details of the study
The investigators reviewed 546 pregnancies, which resulted in the birth of 555 fetuses and infants. Thirty-nine fetuses and neonates (7%; 95% CI, 5.0-9.5) had neurologic and ocular findings known to be associated with Zika virus infection. Of these, 10 pregnancies were terminated, 1 fetus was stillborn, and 28 were live-born.
Microcephaly (defined as head circumference more than 2 SD below the mean) was present in 32 fetuses and infants (5.8%); 9 had severe microcephaly, defined as head circumference more than 3 SD below the mean. Neurologic and ocular abnormalities were more common when maternal infection occurred during the first trimester (24 of 189 fetuses and infants, 12.7%) compared with infection during the second trimester (9 of 252, 3.6%) or third trimester (6 of 114, 5.3%) (P = .001).
Studies report similar rates of fetal injury
Zika virus infection primarily is caused by a bite from the Aedes aegypti mosquito. The infection also can be transmitted by sexual contact, laboratory accident, and blood transfusion. Eighty percent of infected persons are asymptomatic. In symptomatic patients, the most common clinical manifestations are low-grade fever, a disseminated maculopapular rash, arthralgias, swelling of the hands and feet, and nonpurulent conjunctivitis.
The most ominous manifestation of congenital Zika virus infection is microcephaly. Other important manifestations include lissencephaly, pachygyria, cortical atrophy, ventriculomegaly, subcortical calcifications, ocular abnormalities, and arthrogryposis. Although most of these abnormalities are immediately visible in the neonate, some may not appear until the child is older.
The present study is an excellent complement to 2 recent reports that defined the risk of Zika virus-related fetal injury in patients in the United States and its territories. Based on an analysis of data from the US Zika Pregnancy Registry, Honein and colleagues reported an overall rate of congenital infection of 6%.12 The rate of fetal injury was 11% when the mother was infected in the first trimester and 0% when the infection occurred in the second or third trimester. The overall rate of infection and the first trimester rate of infection were similar to those reported by Hoen and colleagues.
Conversely, Shapiro-Mendoza and colleagues evaluated rates of infection in US territories (American Samoa, Puerto Rico, and the US Virgin Islands) and observed cases of fetal injury associated with second- and third-trimester maternal infection.13 These authors reported an overall rate of infection of 5% and an 8% rate of infection with first-trimester maternal infection. When maternal infection occurred in the second and third trimesters, the rates of fetal injury were 5% and 4%, respectively, figures almost identical to those reported by Hoen and colleagues. Of note, the investigations by Honein and Shapiro-Mendoza included women with both symptomatic and asymptomatic infection.
Taken together, the studies discussed provide 2 clear take-home messages:
- Both symptomatic and asymptomatic maternal infection pose a significant risk of injury to the fetus and neonate.
- Although the risk of fetal injury is greatest when maternal infection occurs in the first trimester, exposure in the second and third trimesters is still dangerous. The Zika virus is quite pathogenic and can cause debilitating injury to the developing fetus at any stage of gestation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477–486.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41–47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidence-based measures to consider. OBG Manag. 2016;28(12):18–22.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374(9):823–832.
- Wilbur MB, Daum RS, Gold HS. Skin abscess. N Engl J Med. 2016;374(9): 882–884.
- Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370(11):1039–1047.
- Unger JA, Whimbey E, Gravett MG, Eschenbach DA. The emergence of Clostridium difficile infection among peripartum women: a case-control study of a C difficile outbreak on an obstetrical service. Infect Dis Obstet Gynecol. 2011;267249. doi:10.1155/2011/267249.
- Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59–68.
- Shapiro-Mendoza CK, Rice ME, Galang RR, et al; Zika Pregnancy and Infant Registries Working Group. Pregnancy outcomes after maternal Zika virus infection during pregnancy — US territories. January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621.
In this Update I highlight 5 interesting investigations on infectious diseases. The first addresses the value of applying prophylactically a negative-pressure wound dressing to prevent surgical site infection (SSI) in obese women having cesarean delivery (CD). The second report assesses the effectiveness of a preoperative vaginal wash in reducing the frequency of postcesarean endometritis. The third investigation examines the role of systemic antibiotics, combined with surgical drainage, for patients who have subcutaneous abscesses ranging in size up to 5 cm. The fourth study presents new information about the major risk factors for Clostridium difficile infections in obstetric patients. The final study presents valuable sobering new data about the risks of congenital Zika virus infection.
Negative-pressure wound therapy after CD shows some benefit in preventing SSI
Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200-210.e1.
Yu and colleagues sought to determine if the prophylactic use of negative-pressure devices, compared with standard wound dressing, was effective in reducing the frequency of SSI after CD.
The authors searched multiple databases and initially identified 161 randomized controlled trials and cohort studies for further assessment. After applying rigorous exclusion criteria, they ultimately selected 9 studies for systematic review and meta-analysis. Six studies were randomized controlled trials (RCTs), 2 were retrospective cohort studies, and 1 was a prospective cohort study. Five studies were considered high quality; 4 were of low quality.
Details of the study
Several types of negative-pressure devices were used, but the 2 most common were the Prevena incision management system (KCI, San Antonio, Texas) and PICO negative- pressure wound therapy (Smith & Nephew, St. Petersburg, Florida). The majority of patients in all groups were at high risk for wound complications because of obesity.
The primary outcome of interest was the frequency of SSI. Secondary outcomes included dehiscence, seroma, endometritis, a composite measure for all wound complications, and hospital readmission.
The absolute risk of SSI in the intervention group was 5% (95% confidence interval [CI], 2.0%-7.0%) compared with 11% (95% CI, 7.0%-16.0%) in the standard dressing group. The pooled risk ratio was 0.45 (95% CI, 0.31-0.66). The absolute risk reduction was 6% (95% CI, -10.0% to -3.0%), and the number needed to treat was 17.
There were no significant differences in the rate of any of the secondary outcomes other than the composite of all wound complications. This difference was largely accounted for by the difference in the rate of SSI.

How negative-pressure devices aid wound healing
Yu and colleagues explained that negative-pressure devices exert their beneficial effects in various ways, including:
- shrinking the wound
- inducing cellular stretch
- removing extracellular fluids
- creating a favorable environment for healing
- promoting angiogenesis and neurogenesis.
Multiple studies in nonobstetric patients have shown that prophylactic use of negative-pressure devices is beneficial in reducing the rate of SSI.1 Yu and colleagues' systematic review and meta-analysis confirms those findings in a high-risk population of women having CD.
Study limitations
Before routinely adopting the use of negative-pressure devices for all women having CD, however, obstetricians should consider the following caveats:
- The investigations included in the study by Yu and colleagues did not consistently distinguish between scheduled versus unscheduled CDs.
- The reports did not systematically consider other major risk factors for wound complications besides obesity, and they did not control for these confounders in the statistical analyses.
- The studies included in the meta-analysis did not provide full descriptions of other measures that might influence the rate of SSIs, such as timing and selection of prophylactic antibiotics, selection of suture material, preoperative skin preparation, and closure techniques for the deep subcutaneous tissue and skin.
- None of the included studies systematically considered the cost-effectiveness of the negative-pressure devices. This is an important consideration given that the acquisition cost of these devices ranges from $200 to $500.
Results of the systematic review and meta-analysis by Yu and colleagues suggest that prophylactic negative-pressure wound therapy in high-risk mostly obese women after CD reduces SSI and overall wound complications. The study's limitations, however, must be kept in mind, and more data are needed. It would be most helpful if a large, well-designed RCT was conducted and included 2 groups with comparable multiple major risk factors for wound complications, and in which all women received the following important interventions2-4:
- removal of hair in the surgical site with a clipper, not a razor
- cleansing of the skin with a chlorhexidine rather than an iodophor solution
- closure of the deep subcutaneous tissue if the total subcutaneous layer exceeds 2 cm in depth
- closure of the skin with suture rather than staples
- administration of antibiotic prophylaxis, ideally with a combination of cefazolin plus azithromycin, prior to the surgical incision.
Read about vaginal cleansing’s effect on post-CD endometritis
Vaginal cleansing before CD lowers risk of postop endometritis
Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(3):527-538.
Caissutti and colleagues aimed to determine if cleansing the vagina with an antiseptic solution prior to surgery reduced the frequency of postcesarean endometritis. They included 16 RCTs (4,837 patients) in their systematic review and meta-analysis. The primary outcome was the frequency of postoperative endometritis.
Details of the study
The studies were conducted in several countries and included patients of various socioeconomic classes. Six trials included only patients having a scheduled CD; 9 included both scheduled and unscheduled cesareans; and 1 included only unscheduled cesareans. In 11 studies, povidone-iodine was the antiseptic solution used. Two trials used chlorhexidine diacetate 0.2%, and 1 used chlorhexidine diacetate 0.4%. One trial used metronidazole 0.5% gel, and another used the antiseptic cetrimide, which is a mixture of different quaternary ammonium salts, including cetrimonium bromide.
In all trials, patients received prophylactic antibiotics. The antibiotics were administered prior to the surgical incision in 6 trials; they were given after the umbilical cord was clamped in 6 trials. In 2 trials, the antibiotics were given at varying times, and in the final 2 trials, the timing of antibiotic administration was not reported. Of note, no trials described the method of placenta removal, a factor of considerable significance in influencing the rate of postoperative endometritis.5,6
Endometritis frequency reduced with vaginal cleansing; benefit greater in certain groups. Overall, in the 15 trials in which vaginal cleansing was compared with placebo or with no treatment, women in the treatment group had a significantly lower rate of endometritis (4.5% compared with 8.8%; relative risk [RR], 0.52; 95% CI, 0.37-0.72). When only women in labor were considered, the frequency of endometritis was 8.1% in the intervention group compared with 13.8% in the control group (RR, 0.52; 95% CI, 0.28-0.97). In the women who were not in labor, the difference in the incidence of endometritis was not statistically significant (3.5% vs 6.6%; RR, 0.62; 95% CI, 0.34-1.15).
In the subgroup analysis of women with ruptured membranes at the time of surgery, the incidence of endometritis was 4.3% in the treatment group compared with 20.1% in the control group (RR, 0.23; 95% CI, 0.10-0.52). In women with intact membranes at the time of surgery, the incidence of endometritis was not significantly reduced in the treatment group.
Interestingly, in the subgroup analysis of the 10 trials that used povidone-iodine, the reduction in the frequency of postcesarean endometritis was statistically significant (2.8% vs 6.3%; RR, 0.42; 95% CI, 0.25-0.71). However, this same protective effect was not observed in the women treated with chlorhexidine. In the 1 trial that directly compared povidone-iodine with chlorhexidine, there was no statistically significant difference in outcome.
Simple intervention, solid benefit
Endometritis is the most common complication following CD. The infection is polymicrobial, with mixed aerobic and anaerobic organisms. The principal risk factors for postcesarean endometritis are low socioeconomic status, extended duration of labor and ruptured membranes, multiple vaginal examinations, internal fetal monitoring, and pre-existing vaginal infections (principally, bacterial vaginosis and group B streptococcal colonization).
Two interventions are clearly of value in reducing the incidence of endometritis: administration of prophylactic antibiotics prior to the surgical incision and removal of the placenta by traction on the cord as opposed to manual extraction.5,6
The assessment by Caissutti and colleagues confirms that a third measure — preoperative vaginal cleansing — also helps reduce the incidence of postcesarean endometritis. The principal benefit is seen in women who have been in labor with ruptured membranes, although certainly it is not harmful in lower-risk patients. The intervention is simple and straightforward: a 30-second vaginal wash with a povidone-iodine solution just prior to surgery.
From my perspective, the interesting unanswered question is why a chlorhexidine solution with low alcohol content was not more effective than povidone-iodine, given that a chlorhexidine abdominal wash is superior to povidone-iodine in preventing wound infection after cesarean delivery.7 Until additional studies confirm the effectiveness of vaginal cleansing with chlorhexidine, I recommend the routine use of the povidone-iodine solution in all women having CD.
Read about management approaches for skin abscesses
Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
Daum RS, Miller LG, Immergluck L, et al; for the DMID 07-0051 Team. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376(26):2545-2555.
For treatment of subcutaneous abscesses that were 5 cm or smaller in diameter, investigators sought to determine if surgical drainage alone was equivalent to surgical drainage plus systemic antibiotics. After their abscess was drained, patients were randomly assigned to receive either clindamycin (300 mg 3 times daily) or trimethoprim-sulfamethoxazole (80 mg/400 mg twice daily) or placebo for 10 days. The primary outcome was clinical cure 7 to 10 days after treatment.
Details of the study
Daum and colleagues enrolled 786 participants (505 adults, 281 children) in the prospective double-blind study. Staphylococcus aureus was isolated from 527 patients (67.0%); methicillin-resistant S aureus (MRSA) was isolated from 388 (49.4%). The cure rate was similar in patients in the clindamycin group (83.1%) and the trimethoprim-sulfamethoxazole group (81.7%), and the cure rate in each antibiotic group was significantly higher than that in the placebo group (68.9%; P<.001 for both comparisons). The difference in treatment effect was specifically limited to patients who had S aureus isolated from their lesions.
Findings at follow-up. At 1 month of follow-up, new infections were less common in the clindamycin group (6.8%) than in the trimethoprim-sulfamethoxazole group (13.5%; P = .03) or the placebo group (12.4%; P = .06). However, the highest frequency of adverse effects occurred in the patients who received clindamycin (21.9% vs 11.1% vs 12.5%). No adverse effects were judged to be serious, and all resolved without sequela.
Controversy remains on antibiotic use after drainage
This study is important for 2 major reasons. First, soft tissue infections are quite commonand can evolve into serious problems, especially when the offending pathogen is MRSA. Second, controversy exists about whether systemic antibiotics are indicated if the subcutaneous abscess is relatively small and is adequately drained. For example, Talan and colleagues demonstrated that, in settings with a high prevalence of MRSA, surgical drainage combined with trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily) was superior to drainage plus placebo.8 However, Daum and Gold recently debated the issue of drainage plus antibiotics in a case vignette and reached opposite conclusions.9
In my opinion, this investigation by Daum and colleagues supports a role for consistent use of systemic antibiotics following surgical drainage of clinically significant subcutaneous abscesses that have a 5 cm or smaller diameter. Several oral antibiotics are effective against S aureus, including MRSA.10 These drugs include trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily), clindamycin (300-450 mg 3 times daily), doxycycline (100 mg twice daily), and minocycline (200 mg initially, then 100 mg every 12 hours).
Of these drugs, I prefer trimethoprim-sulfamethoxazole, provided that the patient does not have an allergy to sulfonamides. Trimethoprim-sulfamethoxazole is significantly less expensive than the other 3 drugs and usually is better tolerated. In particular, compared with clindamycin, trimethoprim-sulfamethoxazole is less likely to cause antibiotic-associated diarrhea, including Clostridium difficile infection. Trimethoprim-sulfamethoxazole should not be used in the first trimester of pregnancy because of concerns about fetal teratogenicity.
Read how to avoid C difficile infections in pregnant patients
Antibiotic use, common in the obstetric population, raises risk for C difficile infection
Ruiter-Ligeti J, Vincent S, Czuzoj-Shulman N, Abenhaim HA. Risk factors, incidence, and morbidity associated with obstetric Clostridium difficile infection. Obstet Gynecol. 2018;131(2):387-391.
The objective of this investigation was to identify risk factors for Clostridium difficile infection (previously termed pseudomembranous enterocolitis) in obstetric patients. The authors performed a retrospective cohort study using information from a large database maintained by the Agency for Healthcare Research and Quality. This database provides information about inpatient hospital stays in the United States, and it is the largest repository of its kind. It includes data from a sample of 1,000 US hospitals.
Details of the study
Ruiter-Ligeti and colleagues reviewed 13,881,592 births during 1999-2013 and identified 2,757 (0.02%) admissions for delivery complicated by C difficile infection, a rate of 20 admissions per 100,000 deliveries per year (95% CI, 19.13-20.62). The rate of admissions with this diagnosis doubled from 1999 (15 per 100,000) to 2013 (30 per 100,000, P<.001).
Among these obstetric patients, the principal risk factors for C difficile infection were older age, multiple gestation, long-term antibiotic use (not precisely defined), and concurrent diagnosis of inflammatory bowel disease. In addition, patients with pyelonephritis, perineal or cesarean wound infections, or pneumonia also were at increased risk, presumably because those patients required longer courses of broad-spectrum antibiotics.
Of additional note, when compared with women who did not have C difficile infection, patients with infection were more likely to develop a thromboembolic event (38.4 per 1,000), paralytic ileus (58.0 per 1,000), sepsis (46.4 per 1,000), and death (8.0 per 1,000).
Be on guard for C difficile infection in antibiotic-treated obstetric patients
C difficile infection is an uncommon but potentially very serious complication of antibiotic therapy. Given that approximately half of all women admitted for delivery are exposed to antibiotics because of prophylaxis for group B streptococcus infection, prophylaxis for CD, and treatment of chorioamnionitis and puerperal endometritis, clinicians constantly need to be vigilant for this complication.11
Affected patients typically present with frequent loose, watery stools and lower abdominal cramping. In severe cases, blood may be present in the stool, and signs of intestinal distention and even acute peritonitis may be evident. The diagnosis can be established by documenting a positive culture or polymerase chain reaction (PCR) assay for C difficile and a positive cytotoxin assay for toxins A and/or B. In addition, if endoscopy is performed, the characteristic gray membranous plaques can be visualized on the rectal and colonic mucosa.11
Discontinue antibiotic therapy. The first step in managing affected patients is to stop all antibiotics, if possible, or at least the one most likely to be the causative agent of C difficile infection. Patients with relatively mild clinical findings should be treated with oral metronidazole, 500 mg every 8 hours for 10 to 14 days. Patients with severe findings should be treated with oral vancomycin, 500 mg every 6 hours, plus IV metronidazole, 500 mg every 8 hours. The more seriously ill patient must be observed carefully for signs of bowel obstruction, intestinal perforation, peritonitis, and sepsis.
Clearly, clinicians should make every effort to prevent C difficile infection in the first place. The following preventive measures are essential:
- Avoid the use of extremely broad-spectrum antibiotics for prophylaxis for CD.
- When using therapeutic antibiotics, keep the spectrum as narrow as possible, consistent with adequately treating the pathogens causing the infection.
- Administer antibiotics for the shortest time possible, consistent with achieving a clinical cure or providing appropriate prophylaxis for surgical procedures (usually, a maximum of 3 doses).
- If a patient receiving antibiotics experiences more than 3 loose stools in 24 hours, either discontinue all antibiotics or substitute another drug for the most likely offending agent, depending on the clinical situation.
- If, after stopping or changing antibiotics, the clinical findings do not resolve promptly, perform a culture or PCR assay for C difficile and assays for the C difficile toxin. Treat as outlined above if these tests are positive.
Read about pregnancy outcomes and trimester of maternal Zika infection
Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994.
To estimate the risk of congenital neurologic defects associated with Zika virus infection, Hoen and colleagues conducted a prospective cohort study of pregnant women with symptomatic Zika virus infection who were enrolled during March through November 2016 in French Guiana, Guadeloupe, and Martinique. All women had Zika virus infection confirmed by PCR assay.
Details of the study
The investigators reviewed 546 pregnancies, which resulted in the birth of 555 fetuses and infants. Thirty-nine fetuses and neonates (7%; 95% CI, 5.0-9.5) had neurologic and ocular findings known to be associated with Zika virus infection. Of these, 10 pregnancies were terminated, 1 fetus was stillborn, and 28 were live-born.
Microcephaly (defined as head circumference more than 2 SD below the mean) was present in 32 fetuses and infants (5.8%); 9 had severe microcephaly, defined as head circumference more than 3 SD below the mean. Neurologic and ocular abnormalities were more common when maternal infection occurred during the first trimester (24 of 189 fetuses and infants, 12.7%) compared with infection during the second trimester (9 of 252, 3.6%) or third trimester (6 of 114, 5.3%) (P = .001).
Studies report similar rates of fetal injury
Zika virus infection primarily is caused by a bite from the Aedes aegypti mosquito. The infection also can be transmitted by sexual contact, laboratory accident, and blood transfusion. Eighty percent of infected persons are asymptomatic. In symptomatic patients, the most common clinical manifestations are low-grade fever, a disseminated maculopapular rash, arthralgias, swelling of the hands and feet, and nonpurulent conjunctivitis.
The most ominous manifestation of congenital Zika virus infection is microcephaly. Other important manifestations include lissencephaly, pachygyria, cortical atrophy, ventriculomegaly, subcortical calcifications, ocular abnormalities, and arthrogryposis. Although most of these abnormalities are immediately visible in the neonate, some may not appear until the child is older.
The present study is an excellent complement to 2 recent reports that defined the risk of Zika virus-related fetal injury in patients in the United States and its territories. Based on an analysis of data from the US Zika Pregnancy Registry, Honein and colleagues reported an overall rate of congenital infection of 6%.12 The rate of fetal injury was 11% when the mother was infected in the first trimester and 0% when the infection occurred in the second or third trimester. The overall rate of infection and the first trimester rate of infection were similar to those reported by Hoen and colleagues.
Conversely, Shapiro-Mendoza and colleagues evaluated rates of infection in US territories (American Samoa, Puerto Rico, and the US Virgin Islands) and observed cases of fetal injury associated with second- and third-trimester maternal infection.13 These authors reported an overall rate of infection of 5% and an 8% rate of infection with first-trimester maternal infection. When maternal infection occurred in the second and third trimesters, the rates of fetal injury were 5% and 4%, respectively, figures almost identical to those reported by Hoen and colleagues. Of note, the investigations by Honein and Shapiro-Mendoza included women with both symptomatic and asymptomatic infection.
Taken together, the studies discussed provide 2 clear take-home messages:
- Both symptomatic and asymptomatic maternal infection pose a significant risk of injury to the fetus and neonate.
- Although the risk of fetal injury is greatest when maternal infection occurs in the first trimester, exposure in the second and third trimesters is still dangerous. The Zika virus is quite pathogenic and can cause debilitating injury to the developing fetus at any stage of gestation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Update I highlight 5 interesting investigations on infectious diseases. The first addresses the value of applying prophylactically a negative-pressure wound dressing to prevent surgical site infection (SSI) in obese women having cesarean delivery (CD). The second report assesses the effectiveness of a preoperative vaginal wash in reducing the frequency of postcesarean endometritis. The third investigation examines the role of systemic antibiotics, combined with surgical drainage, for patients who have subcutaneous abscesses ranging in size up to 5 cm. The fourth study presents new information about the major risk factors for Clostridium difficile infections in obstetric patients. The final study presents valuable sobering new data about the risks of congenital Zika virus infection.
Negative-pressure wound therapy after CD shows some benefit in preventing SSI
Yu L, Kronen RJ, Simon LE, Stoll CR, Colditz GA, Tuuli MG. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218(2):200-210.e1.
Yu and colleagues sought to determine if the prophylactic use of negative-pressure devices, compared with standard wound dressing, was effective in reducing the frequency of SSI after CD.
The authors searched multiple databases and initially identified 161 randomized controlled trials and cohort studies for further assessment. After applying rigorous exclusion criteria, they ultimately selected 9 studies for systematic review and meta-analysis. Six studies were randomized controlled trials (RCTs), 2 were retrospective cohort studies, and 1 was a prospective cohort study. Five studies were considered high quality; 4 were of low quality.
Details of the study
Several types of negative-pressure devices were used, but the 2 most common were the Prevena incision management system (KCI, San Antonio, Texas) and PICO negative- pressure wound therapy (Smith & Nephew, St. Petersburg, Florida). The majority of patients in all groups were at high risk for wound complications because of obesity.
The primary outcome of interest was the frequency of SSI. Secondary outcomes included dehiscence, seroma, endometritis, a composite measure for all wound complications, and hospital readmission.
The absolute risk of SSI in the intervention group was 5% (95% confidence interval [CI], 2.0%-7.0%) compared with 11% (95% CI, 7.0%-16.0%) in the standard dressing group. The pooled risk ratio was 0.45 (95% CI, 0.31-0.66). The absolute risk reduction was 6% (95% CI, -10.0% to -3.0%), and the number needed to treat was 17.
There were no significant differences in the rate of any of the secondary outcomes other than the composite of all wound complications. This difference was largely accounted for by the difference in the rate of SSI.

How negative-pressure devices aid wound healing
Yu and colleagues explained that negative-pressure devices exert their beneficial effects in various ways, including:
- shrinking the wound
- inducing cellular stretch
- removing extracellular fluids
- creating a favorable environment for healing
- promoting angiogenesis and neurogenesis.
Multiple studies in nonobstetric patients have shown that prophylactic use of negative-pressure devices is beneficial in reducing the rate of SSI.1 Yu and colleagues' systematic review and meta-analysis confirms those findings in a high-risk population of women having CD.
Study limitations
Before routinely adopting the use of negative-pressure devices for all women having CD, however, obstetricians should consider the following caveats:
- The investigations included in the study by Yu and colleagues did not consistently distinguish between scheduled versus unscheduled CDs.
- The reports did not systematically consider other major risk factors for wound complications besides obesity, and they did not control for these confounders in the statistical analyses.
- The studies included in the meta-analysis did not provide full descriptions of other measures that might influence the rate of SSIs, such as timing and selection of prophylactic antibiotics, selection of suture material, preoperative skin preparation, and closure techniques for the deep subcutaneous tissue and skin.
- None of the included studies systematically considered the cost-effectiveness of the negative-pressure devices. This is an important consideration given that the acquisition cost of these devices ranges from $200 to $500.
Results of the systematic review and meta-analysis by Yu and colleagues suggest that prophylactic negative-pressure wound therapy in high-risk mostly obese women after CD reduces SSI and overall wound complications. The study's limitations, however, must be kept in mind, and more data are needed. It would be most helpful if a large, well-designed RCT was conducted and included 2 groups with comparable multiple major risk factors for wound complications, and in which all women received the following important interventions2-4:
- removal of hair in the surgical site with a clipper, not a razor
- cleansing of the skin with a chlorhexidine rather than an iodophor solution
- closure of the deep subcutaneous tissue if the total subcutaneous layer exceeds 2 cm in depth
- closure of the skin with suture rather than staples
- administration of antibiotic prophylaxis, ideally with a combination of cefazolin plus azithromycin, prior to the surgical incision.
Read about vaginal cleansing’s effect on post-CD endometritis
Vaginal cleansing before CD lowers risk of postop endometritis
Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery: a systematic review and meta-analysis. Obstet Gynecol. 2017;130(3):527-538.
Caissutti and colleagues aimed to determine if cleansing the vagina with an antiseptic solution prior to surgery reduced the frequency of postcesarean endometritis. They included 16 RCTs (4,837 patients) in their systematic review and meta-analysis. The primary outcome was the frequency of postoperative endometritis.
Details of the study
The studies were conducted in several countries and included patients of various socioeconomic classes. Six trials included only patients having a scheduled CD; 9 included both scheduled and unscheduled cesareans; and 1 included only unscheduled cesareans. In 11 studies, povidone-iodine was the antiseptic solution used. Two trials used chlorhexidine diacetate 0.2%, and 1 used chlorhexidine diacetate 0.4%. One trial used metronidazole 0.5% gel, and another used the antiseptic cetrimide, which is a mixture of different quaternary ammonium salts, including cetrimonium bromide.
In all trials, patients received prophylactic antibiotics. The antibiotics were administered prior to the surgical incision in 6 trials; they were given after the umbilical cord was clamped in 6 trials. In 2 trials, the antibiotics were given at varying times, and in the final 2 trials, the timing of antibiotic administration was not reported. Of note, no trials described the method of placenta removal, a factor of considerable significance in influencing the rate of postoperative endometritis.5,6
Endometritis frequency reduced with vaginal cleansing; benefit greater in certain groups. Overall, in the 15 trials in which vaginal cleansing was compared with placebo or with no treatment, women in the treatment group had a significantly lower rate of endometritis (4.5% compared with 8.8%; relative risk [RR], 0.52; 95% CI, 0.37-0.72). When only women in labor were considered, the frequency of endometritis was 8.1% in the intervention group compared with 13.8% in the control group (RR, 0.52; 95% CI, 0.28-0.97). In the women who were not in labor, the difference in the incidence of endometritis was not statistically significant (3.5% vs 6.6%; RR, 0.62; 95% CI, 0.34-1.15).
In the subgroup analysis of women with ruptured membranes at the time of surgery, the incidence of endometritis was 4.3% in the treatment group compared with 20.1% in the control group (RR, 0.23; 95% CI, 0.10-0.52). In women with intact membranes at the time of surgery, the incidence of endometritis was not significantly reduced in the treatment group.
Interestingly, in the subgroup analysis of the 10 trials that used povidone-iodine, the reduction in the frequency of postcesarean endometritis was statistically significant (2.8% vs 6.3%; RR, 0.42; 95% CI, 0.25-0.71). However, this same protective effect was not observed in the women treated with chlorhexidine. In the 1 trial that directly compared povidone-iodine with chlorhexidine, there was no statistically significant difference in outcome.
Simple intervention, solid benefit
Endometritis is the most common complication following CD. The infection is polymicrobial, with mixed aerobic and anaerobic organisms. The principal risk factors for postcesarean endometritis are low socioeconomic status, extended duration of labor and ruptured membranes, multiple vaginal examinations, internal fetal monitoring, and pre-existing vaginal infections (principally, bacterial vaginosis and group B streptococcal colonization).
Two interventions are clearly of value in reducing the incidence of endometritis: administration of prophylactic antibiotics prior to the surgical incision and removal of the placenta by traction on the cord as opposed to manual extraction.5,6
The assessment by Caissutti and colleagues confirms that a third measure — preoperative vaginal cleansing — also helps reduce the incidence of postcesarean endometritis. The principal benefit is seen in women who have been in labor with ruptured membranes, although certainly it is not harmful in lower-risk patients. The intervention is simple and straightforward: a 30-second vaginal wash with a povidone-iodine solution just prior to surgery.
From my perspective, the interesting unanswered question is why a chlorhexidine solution with low alcohol content was not more effective than povidone-iodine, given that a chlorhexidine abdominal wash is superior to povidone-iodine in preventing wound infection after cesarean delivery.7 Until additional studies confirm the effectiveness of vaginal cleansing with chlorhexidine, I recommend the routine use of the povidone-iodine solution in all women having CD.
Read about management approaches for skin abscesses
Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
Daum RS, Miller LG, Immergluck L, et al; for the DMID 07-0051 Team. A placebo-controlled trial of antibiotics for smaller skin abscesses. N Engl J Med. 2017;376(26):2545-2555.
For treatment of subcutaneous abscesses that were 5 cm or smaller in diameter, investigators sought to determine if surgical drainage alone was equivalent to surgical drainage plus systemic antibiotics. After their abscess was drained, patients were randomly assigned to receive either clindamycin (300 mg 3 times daily) or trimethoprim-sulfamethoxazole (80 mg/400 mg twice daily) or placebo for 10 days. The primary outcome was clinical cure 7 to 10 days after treatment.
Details of the study
Daum and colleagues enrolled 786 participants (505 adults, 281 children) in the prospective double-blind study. Staphylococcus aureus was isolated from 527 patients (67.0%); methicillin-resistant S aureus (MRSA) was isolated from 388 (49.4%). The cure rate was similar in patients in the clindamycin group (83.1%) and the trimethoprim-sulfamethoxazole group (81.7%), and the cure rate in each antibiotic group was significantly higher than that in the placebo group (68.9%; P<.001 for both comparisons). The difference in treatment effect was specifically limited to patients who had S aureus isolated from their lesions.
Findings at follow-up. At 1 month of follow-up, new infections were less common in the clindamycin group (6.8%) than in the trimethoprim-sulfamethoxazole group (13.5%; P = .03) or the placebo group (12.4%; P = .06). However, the highest frequency of adverse effects occurred in the patients who received clindamycin (21.9% vs 11.1% vs 12.5%). No adverse effects were judged to be serious, and all resolved without sequela.
Controversy remains on antibiotic use after drainage
This study is important for 2 major reasons. First, soft tissue infections are quite commonand can evolve into serious problems, especially when the offending pathogen is MRSA. Second, controversy exists about whether systemic antibiotics are indicated if the subcutaneous abscess is relatively small and is adequately drained. For example, Talan and colleagues demonstrated that, in settings with a high prevalence of MRSA, surgical drainage combined with trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily) was superior to drainage plus placebo.8 However, Daum and Gold recently debated the issue of drainage plus antibiotics in a case vignette and reached opposite conclusions.9
In my opinion, this investigation by Daum and colleagues supports a role for consistent use of systemic antibiotics following surgical drainage of clinically significant subcutaneous abscesses that have a 5 cm or smaller diameter. Several oral antibiotics are effective against S aureus, including MRSA.10 These drugs include trimethoprim-sulfamethoxazole (1 double-strength tablet orally twice daily), clindamycin (300-450 mg 3 times daily), doxycycline (100 mg twice daily), and minocycline (200 mg initially, then 100 mg every 12 hours).
Of these drugs, I prefer trimethoprim-sulfamethoxazole, provided that the patient does not have an allergy to sulfonamides. Trimethoprim-sulfamethoxazole is significantly less expensive than the other 3 drugs and usually is better tolerated. In particular, compared with clindamycin, trimethoprim-sulfamethoxazole is less likely to cause antibiotic-associated diarrhea, including Clostridium difficile infection. Trimethoprim-sulfamethoxazole should not be used in the first trimester of pregnancy because of concerns about fetal teratogenicity.
Read how to avoid C difficile infections in pregnant patients
Antibiotic use, common in the obstetric population, raises risk for C difficile infection
Ruiter-Ligeti J, Vincent S, Czuzoj-Shulman N, Abenhaim HA. Risk factors, incidence, and morbidity associated with obstetric Clostridium difficile infection. Obstet Gynecol. 2018;131(2):387-391.
The objective of this investigation was to identify risk factors for Clostridium difficile infection (previously termed pseudomembranous enterocolitis) in obstetric patients. The authors performed a retrospective cohort study using information from a large database maintained by the Agency for Healthcare Research and Quality. This database provides information about inpatient hospital stays in the United States, and it is the largest repository of its kind. It includes data from a sample of 1,000 US hospitals.
Details of the study
Ruiter-Ligeti and colleagues reviewed 13,881,592 births during 1999-2013 and identified 2,757 (0.02%) admissions for delivery complicated by C difficile infection, a rate of 20 admissions per 100,000 deliveries per year (95% CI, 19.13-20.62). The rate of admissions with this diagnosis doubled from 1999 (15 per 100,000) to 2013 (30 per 100,000, P<.001).
Among these obstetric patients, the principal risk factors for C difficile infection were older age, multiple gestation, long-term antibiotic use (not precisely defined), and concurrent diagnosis of inflammatory bowel disease. In addition, patients with pyelonephritis, perineal or cesarean wound infections, or pneumonia also were at increased risk, presumably because those patients required longer courses of broad-spectrum antibiotics.
Of additional note, when compared with women who did not have C difficile infection, patients with infection were more likely to develop a thromboembolic event (38.4 per 1,000), paralytic ileus (58.0 per 1,000), sepsis (46.4 per 1,000), and death (8.0 per 1,000).
Be on guard for C difficile infection in antibiotic-treated obstetric patients
C difficile infection is an uncommon but potentially very serious complication of antibiotic therapy. Given that approximately half of all women admitted for delivery are exposed to antibiotics because of prophylaxis for group B streptococcus infection, prophylaxis for CD, and treatment of chorioamnionitis and puerperal endometritis, clinicians constantly need to be vigilant for this complication.11
Affected patients typically present with frequent loose, watery stools and lower abdominal cramping. In severe cases, blood may be present in the stool, and signs of intestinal distention and even acute peritonitis may be evident. The diagnosis can be established by documenting a positive culture or polymerase chain reaction (PCR) assay for C difficile and a positive cytotoxin assay for toxins A and/or B. In addition, if endoscopy is performed, the characteristic gray membranous plaques can be visualized on the rectal and colonic mucosa.11
Discontinue antibiotic therapy. The first step in managing affected patients is to stop all antibiotics, if possible, or at least the one most likely to be the causative agent of C difficile infection. Patients with relatively mild clinical findings should be treated with oral metronidazole, 500 mg every 8 hours for 10 to 14 days. Patients with severe findings should be treated with oral vancomycin, 500 mg every 6 hours, plus IV metronidazole, 500 mg every 8 hours. The more seriously ill patient must be observed carefully for signs of bowel obstruction, intestinal perforation, peritonitis, and sepsis.
Clearly, clinicians should make every effort to prevent C difficile infection in the first place. The following preventive measures are essential:
- Avoid the use of extremely broad-spectrum antibiotics for prophylaxis for CD.
- When using therapeutic antibiotics, keep the spectrum as narrow as possible, consistent with adequately treating the pathogens causing the infection.
- Administer antibiotics for the shortest time possible, consistent with achieving a clinical cure or providing appropriate prophylaxis for surgical procedures (usually, a maximum of 3 doses).
- If a patient receiving antibiotics experiences more than 3 loose stools in 24 hours, either discontinue all antibiotics or substitute another drug for the most likely offending agent, depending on the clinical situation.
- If, after stopping or changing antibiotics, the clinical findings do not resolve promptly, perform a culture or PCR assay for C difficile and assays for the C difficile toxin. Treat as outlined above if these tests are positive.
Read about pregnancy outcomes and trimester of maternal Zika infection
Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Hoen B, Schaub B, Funk AL, et al. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378(11):985-994.
To estimate the risk of congenital neurologic defects associated with Zika virus infection, Hoen and colleagues conducted a prospective cohort study of pregnant women with symptomatic Zika virus infection who were enrolled during March through November 2016 in French Guiana, Guadeloupe, and Martinique. All women had Zika virus infection confirmed by PCR assay.
Details of the study
The investigators reviewed 546 pregnancies, which resulted in the birth of 555 fetuses and infants. Thirty-nine fetuses and neonates (7%; 95% CI, 5.0-9.5) had neurologic and ocular findings known to be associated with Zika virus infection. Of these, 10 pregnancies were terminated, 1 fetus was stillborn, and 28 were live-born.
Microcephaly (defined as head circumference more than 2 SD below the mean) was present in 32 fetuses and infants (5.8%); 9 had severe microcephaly, defined as head circumference more than 3 SD below the mean. Neurologic and ocular abnormalities were more common when maternal infection occurred during the first trimester (24 of 189 fetuses and infants, 12.7%) compared with infection during the second trimester (9 of 252, 3.6%) or third trimester (6 of 114, 5.3%) (P = .001).
Studies report similar rates of fetal injury
Zika virus infection primarily is caused by a bite from the Aedes aegypti mosquito. The infection also can be transmitted by sexual contact, laboratory accident, and blood transfusion. Eighty percent of infected persons are asymptomatic. In symptomatic patients, the most common clinical manifestations are low-grade fever, a disseminated maculopapular rash, arthralgias, swelling of the hands and feet, and nonpurulent conjunctivitis.
The most ominous manifestation of congenital Zika virus infection is microcephaly. Other important manifestations include lissencephaly, pachygyria, cortical atrophy, ventriculomegaly, subcortical calcifications, ocular abnormalities, and arthrogryposis. Although most of these abnormalities are immediately visible in the neonate, some may not appear until the child is older.
The present study is an excellent complement to 2 recent reports that defined the risk of Zika virus-related fetal injury in patients in the United States and its territories. Based on an analysis of data from the US Zika Pregnancy Registry, Honein and colleagues reported an overall rate of congenital infection of 6%.12 The rate of fetal injury was 11% when the mother was infected in the first trimester and 0% when the infection occurred in the second or third trimester. The overall rate of infection and the first trimester rate of infection were similar to those reported by Hoen and colleagues.
Conversely, Shapiro-Mendoza and colleagues evaluated rates of infection in US territories (American Samoa, Puerto Rico, and the US Virgin Islands) and observed cases of fetal injury associated with second- and third-trimester maternal infection.13 These authors reported an overall rate of infection of 5% and an 8% rate of infection with first-trimester maternal infection. When maternal infection occurred in the second and third trimesters, the rates of fetal injury were 5% and 4%, respectively, figures almost identical to those reported by Hoen and colleagues. Of note, the investigations by Honein and Shapiro-Mendoza included women with both symptomatic and asymptomatic infection.
Taken together, the studies discussed provide 2 clear take-home messages:
- Both symptomatic and asymptomatic maternal infection pose a significant risk of injury to the fetus and neonate.
- Although the risk of fetal injury is greatest when maternal infection occurs in the first trimester, exposure in the second and third trimesters is still dangerous. The Zika virus is quite pathogenic and can cause debilitating injury to the developing fetus at any stage of gestation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477–486.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41–47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidence-based measures to consider. OBG Manag. 2016;28(12):18–22.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374(9):823–832.
- Wilbur MB, Daum RS, Gold HS. Skin abscess. N Engl J Med. 2016;374(9): 882–884.
- Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370(11):1039–1047.
- Unger JA, Whimbey E, Gravett MG, Eschenbach DA. The emergence of Clostridium difficile infection among peripartum women: a case-control study of a C difficile outbreak on an obstetrical service. Infect Dis Obstet Gynecol. 2011;267249. doi:10.1155/2011/267249.
- Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59–68.
- Shapiro-Mendoza CK, Rice ME, Galang RR, et al; Zika Pregnancy and Infant Registries Working Group. Pregnancy outcomes after maternal Zika virus infection during pregnancy — US territories. January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621.
- Hyldig N, Birke-Sorensen H, Kruse M, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016;103(5):477–486.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: evidence-based guidance. OBG Manag. 2016;28(11):41–47.
- Patrick KE, Deatsman SL, Duff P. Preventing infection after cesarean delivery: 5 more evidence-based measures to consider. OBG Manag. 2016;28(12):18–22.
- Lasley DS, Eblen A, Yancey MK, Duff P. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176(6):1250–1254.
- Duff P. A simple checklist for preventing major complications associated with cesarean delivery. Obstet Gynecol. 2010;116(6):1393–1396.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374(7):647–655.
- Talan DA, Mower WR, Krishnadasan A, et al. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med. 2016;374(9):823–832.
- Wilbur MB, Daum RS, Gold HS. Skin abscess. N Engl J Med. 2016;374(9): 882–884.
- Singer AJ, Talan DA. Management of skin abscesses in the era of methicillin-resistant Staphylococcus aureus. N Engl J Med. 2014;370(11):1039–1047.
- Unger JA, Whimbey E, Gravett MG, Eschenbach DA. The emergence of Clostridium difficile infection among peripartum women: a case-control study of a C difficile outbreak on an obstetrical service. Infect Dis Obstet Gynecol. 2011;267249. doi:10.1155/2011/267249.
- Honein MA, Dawson AL, Petersen EE, et al; US Zika Pregnancy Registry Collaboration. Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA. 2017;317(1):59–68.
- Shapiro-Mendoza CK, Rice ME, Galang RR, et al; Zika Pregnancy and Infant Registries Working Group. Pregnancy outcomes after maternal Zika virus infection during pregnancy — US territories. January 1, 2016-April 25, 2017. MMWR Morb Mortal Wkly Rep. 2017;66(23):615–621.
IN THIS ARTICLE
- Vaginal cleansing before CD lowers risk of postop endometritis
- Treat smaller skin abscesses with antibiotics after surgical drainage? Yes.
- Antibiotic use, common in the obstetric population, raises risk for C difficile infection
- Danger for birth defects with maternal Zika infection present in all trimesters, but greatest in first
Human trafficking: How ObGyns can—and should—be helping survivors

Despite increasing media coverage of human trafficking and the gravity of its many ramifications, I am struck by how often trainees and other clinicians present to me patients for which trafficking is a real potential concern—yet who give me a blank expression when I ask if anyone has screened these patients for being victims of trafficking. I suspect that few of us anticipated, during medical training, that we would be providing care to women who are enslaved.
How large is the problem?
It is impossible to comprehend the true scope of human trafficking. Estimates are that 20.9 million men, women, and children globally are forced into work that they are not free to leave.1
Although human trafficking is recognized as a global phenomenon, its prevalence in the United States is significant enough that it should prompt the health care community to engage in helping identify and assist victims/survivors: From January until June of 2017, the National Human Trafficking Hotline received 13,807 telephone calls, resulting in reporting of 4,460 cases.2 Indeed, from 2015 to 2016 there was a 35.7% increase in the number of hotline cases reported, for a total of 7,572 (6,340—more than 80%—of which regarded females). California had the most cases reported (1,323), followed by Texas (670) and Florida (550); those 3 states also reported an increase in trafficking crime. Vermont (5), Rhode Island (9), and Alaska (10) reported the fewest calls.3
How is trafficking defined?
The United Nations Office on Drugs and Crime defines “trafficking in persons” as:
… recruitment, transportation, transfer, harbouring or receipt of persons, by means of the threat or use of force or other forms of coercion, of abduction, of fraud, of deception, of the abuse of power or of a position of vulnerability or of the giving or receiving of payments or benefits to achieve the consent of a person having control over another person, for the purpose of exploitation. Exploitation shall include, at a minimum, the exploitation of the prostitution of others or other forms of sexual exploitation, forced labour or services, slavery or practices similar to slavery, servitude or the removal of organs.4
Traffickers prey on potentially vulnerable people. Girls and young women who have experienced poverty, homelessness, childhood sexual abuse, substance abuse, gender nonconformity, mental illness, or developmental delay are at particular risk.5 Children who have had interactions with Child Protective Services, come from a dysfunctional family, or have lived in a community with high crime, political or social unrest, corruption, or gender bias and discrimination are also at increased risk.6
Read about clues that raise clinical suspicion
Clues that raise clinical suspicion
A number of potential signs should make providers suspicious about potential human trafficking. Some of those signs are similar to the red flags we see in intimate partner violence, such as:
- having a difficult time talking to the patient alone
- having the accompanying person answer the patient’s questions
- body language that suggests fear, anxiety, or distrust (eg, shifting positions, looking away, appearing withdrawn)
- physical examination inconsistent with the history
- physical injury (especially multiple injuries or injuries in various stages of healing)
- refusal of interpreter services.
Trafficked girls or women may appear overly familiar with sex, have unexpected material possessions, or appear to be giving scripted or memorized answers to queries.7 Traffickers often confiscate their victims’ personal identification. They try to prevent victims from knowing their geographic locales: Patients might not have any documentation or awareness of exact surroundings (eg, their home address). Patients may be wearing clothes considered inappropriate for the weather or venue. They may have tattoos that are marks of branding.8
Medical consequences of being trafficked are obvious, numerous, and serious
Many medical sequelae that result from trafficking are obvious, given the nature of work that victims are forced to do. For example, overcrowding can lead to infectious disease, such as tuberculosis.9 Inadequate access to preventive or basic medical services can result in weight loss, poor dentition, and untreated chronic medical conditions.
If victims are experiencing physical or sexual abuse, they can present with evidence of blunt trauma, ligature marks, skin burns, wounds inflicted by weapons, and vaginal lacerations.10 A study found that 63% of survivors reported at least 10 somatic symptoms, including headache, fatigue, dizziness, back pain, abdominal or pelvic pain, memory loss, and symptoms of genital infectious disease.11
Girls and women being trafficked for sex may experience many of the sequelae of unprotected intercourse: irregular bleeding, unintended pregnancy, unwanted or unsafe pregnancy termination, vaginal trauma, and sexually transmitted infection (STI).12 In a study of trafficking survivors, 38% were HIV-positive.13
Trafficking survivors can suffer myriad mental health conditions, with high rates of depression, anxiety, posttraumatic stress, and suicidal ideation.14 A study of 387 survivors found that 12% had attempted to harm themselves or commit suicide the month before they were interviewed.15
Substance abuse is also a common problem among trafficking victims.16 One survivor interviewed in a recent study said:
It was much more difficult to work sober because I was dealing with emotions or the pain that I was feeling during intercourse, because when you have sex with people 8, 9, 10 times a day, even more than that, it starts to hurt a lot. And being high made it easier to deal with that and also it made it easier for me to get away from my body while it was happening, place my brain somewhere else.17
Because of the substantial risk of mental health problems, including substance abuse, among trafficking survivors, the physical exam of a patient should include careful assessment of demeanor and mental health status. Of course, comprehensive inspection for signs of physical or blunt trauma is paramount.
Read about Patient and staff safety during the visit
Patient and staff safety during the visit
Providers should be aware of potential safety concerns, both for the patient and for the staff. Creative strategies should be utilized to screen the patient in private. The use of interpreter services—either in person or over the telephone—should be presented and facilitated as being a routine part of practice. Any person who accompanies the patient should be asked to leave the examining room, either as a statement of practice routine or under the guise of having him (or her) step out to obtain paperwork or provide documentation.
Care of victims
Trauma-informed care should be a guiding principle for trafficking survivors. This involves empowering the patient, who may feel victimized again if asked to undress and undergo multiple physical examinations. Macias-Konstantopoulos noted: “A trauma-informed approach to care acknowledges the pervasiveness and effect of trauma across the life span of the individual, recognizes the vulnerabilities and emotional triggers of trauma survivors, minimizes repeated traumatization and fosters physical, psychological, and emotional safety, recovery, health and well-being.”18
The patient should be counseled that she has control over her body and can guide different aspects of the examination. For example the provider should discuss: 1) the amount of clothing deemed optimal for an examination, 2) the availability of a support person during the exam (for instance, a nurse or a social worker) if the patient requests one, and 3) utilization of whatever strategies the patient deems optimal for her to be most comfortable during the exam (such as leaving the door slightly ajar or having a mutually agreed-on signal to interrupt the exam).
Routine health care maintenance should be offered, including an assessment of overall physical and dental health and screening for STI and mental health. Screening for substances of abuse should be considered. If indicated, emergency contraception, postexposure HIV prophylaxis, immunizations, and empiric antibiotics for STI should be offered.19
Screening when indicated by evidence, suspicion, or concern
Unlike the case with intimate partner violence, experts do not recommend universal screening for human trafficking. Clinicians should be comfortable, however, trying to elicit that history when a concern arises, either because of identified risk factors, red flags, or concerns that arise from the findings of the history or physical. Ideally, clinicians should consider becoming comfortable choosing a few screening questions to regularly incorporate into their assessment. The US Department of Health & Human Services (HHS) offers a list of questions that can be utilized (TABLE).20
In January 2018, the Office on Trafficking in Persons, a unit of the HHS Administration for Children and Families, released an “Adult Human Trafficking Screening Tool and Guide.” The document includes 2 excellent tools21 that clinicians can utilize to identify patients who should be screened and how to identify and assist survivors (FIGURE 1 and FIGURE 2).
Clinicians, in their encounters with patients, are particularly well-positioned to intersect with, and identify, survivors. Regrettably, such opportunities are often missed—and victims thus remain unidentified and trapped in their circumstances. A study revealed that one-half of survivors who were interviewed reported seeing a physician while they were being trafficked.22 Even more alarming, another study showed that 87.8% of survivors had received health care during their captivity.23 It is dismaying to know that these patients left those health care settings without receiving the assistance they truly need and with their true circumstances remaining unidentified.
Read about Finding assistance and support
Finding assistance and support
Centers in the United States now provide trauma-informed care for trafficking survivors in a confidential setting (see “Specialized care is increasingly available”).24 A physician who works at a center in New York City noted: “Our survivors told us that more than fear or pain, the feelings that sat with them most often were worthlessness and invisibility. We can do better as physicians and as educators to expose this epidemic and care for its victims.”24
Here is a sampling of the growing number of centers in the United States that provide trauma-centered care for survivors of human trafficking:
- Survivor Clinic at New York Presbyterian Hospital-Weill Cornell Medical College, New York, New York
- EMPOWER Clinic for Survivors of Sex Trafficking and Sexual Violence at NYU Langone Health, New York, New York
- Freedom Clinic at Massachusetts General Hospital, Boston
- The Hope Through Health Clinic, Austin, Texas
- Pacific Survivor Center, Honolulu, Hawaii
Most clinicians practice in settings that do not have easy access to such subspecialized centers, however. For them, the National Human Trafficking Hotline can be an invaluable resource (see “Hotline is a valuable resource”).25 Law enforcement and social services colleagues also can be useful allies.
Uncertain how you can help a patient who is a victim of human trafficking? For assistance and support, contact the National Human Trafficking Hotline--24 hours a day, 7 days a week, and in 200 languages--in any of 3 ways:
- By telephone: (888) 373-7888
- By text: 233733
- On the web: https://humantraffickinghotline.orga
aIncludes a search field that clinicians can use to look up the nearest resources for additional assistance.
Let’s turn our concern and awareness into results
We, as providers of women’s health care, are uniquely positioned to help these most vulnerable of people, many of whom have been stripped of personal documents and denied access to financial resources and community support. As a medical community, we should strive to combat this tragic epidemic, 1 patient at a time.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- International Labour Organization. New ILO Global Estimate of Forced Labour: 20.9 million victims. http://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_182109/lang--en/index.htm. Published June 2012. Accessed May 30, 2018.
- National Human Trafficking Hotline. Hotline statistics. https://humantraffickinghotline.org/states. Accessed May 30, 2018.
- Cone A. Report: Human trafficking in U.S. rose 35.7 percent in one year. United Press International (UPI). https://www.upi.com/Report-Human-trafficking-in-US-rose-357-percent-in-one-year/5571486328579. Published February 5, 2017. Accessed May 30, 2018.
- United Nations Office on Drugs and Crime. Human trafficking. http://www.unodc.org/unodc/en/human-trafficking/what-is-human-trafficking.html. Accessed May 30, 2018.
- Risk factors for and consequences of commercial sexual exploitation and sex trafficking of minors. In Clayton E, Krugman R, Simon P, eds; Committee on the Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States; Board on Children, Youth, and Families; Committee on Law and Justice; Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
- Greenbaum J, Crawford-Jakubiak JE. Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015:135(3);566–574.
- Alpert E, Ahn R, Albright E, Purcell G, Burke T, Macias-Konstantanopoulos W. Human Trafficking: Guidebook on Identification, Assessment, and Response in the Health Care Setting. Waltham, MA: Massachusetts General Hospital and Massachusetts Medical Society; 2014. http://www.massmed.org/Patient-Care/Health-Topics/Violence-Prevention-and-Intervention/Human-Trafficking-(pdf). Accessed May 30, 2018.
- National Human Trafficking Training and Technical Assistance Center. Adult human trafficking screening tool and guide. http://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Published January 2018. Accessed May 30, 2018.
- Steele S. Human trafficking, labor brokering, and mining in southern Africa: responding to a decentralized and hidden public health disaster. Int J Health Serv. 2013;43(4):665–680.
- Becker HJ, Bechtel K. Recognizing victims of human trafficking in the pediatric emergency department. Pediatr Emerg Care. 2015;31(2):144–147.
- Zimmerman C, Hossain M, Yun K, et al. The health of trafficked women: a survey of women entering postrafficking services in Europe. Am J Public Health. 2008;98(1):55–59.
- Tracy EE, Macias-Konstantopoulos W. Identifying and assisting sexually exploited and trafficked patients seeking women’s health care services. Obstet Gynecol. 2017;130(2):443–453.
- Silverman JG, Decker MR, Gupta J, Maheshwari A, Willis BM, Raj A. HIV prevalence and predictors of infection in sex-trafficked Nepalese girls and women. JAMA. 2007;298(5):536–542.
- Rafferty Y. Child trafficking and commercial sexual exploitation: a review of promising prevention policies and programs. Am J Orthopsychiatry. 2013;83(4):559–575.
- Kiss L, Yun K, Pocock N, Zimmerman C. Exploitation, violence, and suicide risk among child and adolescent survivors of human trafficking in the Greater Mekong Subregion. JAMA Pediatr. 2015;169(9):e152278.
- Stoklosa H, MacGibbon M, Stoklosa J. Human trafficking, mental illness, and addiction: avoiding diagnostic overshadowing. AMA J Ethics. 2017;19(1):23–34.
- Ravi A, Pfeiffer MR, Rosner Z, Shea JA. Trafficking and trauma: insight and advice for the healthcare system from sex-trafficked women incarcerated on Rikers Island. Med Care. 2017;55(12):1017–1022.
- Macias-Konstantopoulos W. Human trafficking: the role of medicine in interrupting the cycle of abuse and violence. Ann Intern Med. 2016:165(8):582–588.
- Chung RJ, English A. Commercial sexual exploitation and sex trafficking of adolescents. Curr Opin Pediatr. 2015;27(4):427–433.
- Resources: Screening tool for victims of human trafficking. Washington, DC: US Department of Health and Human Services. https://www.justice.gov/sites/default/files/usao-ndia/legacy/2011/10/14/health_screen_questions.pdf. Accessed May 30, 2018.
- US Department of Health and Human Services. Adult human trafficking screening tool and guide. January 2018. https://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Accessed May 30, 2018.
- Baldwin SB, Eisenman DP, Sayles JN, Ryan G, Chuang KS. Identification of human trafficking victims in health care settings. Health Hum Rights. 2011;13(1):e36–e49.
- Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in health-care facilities. Ann Health Law. 2014;23:61–91.
- Geynisman-Tan JM, Taylor JS, Edersheim T, Taubel D. All the darkness we don’t see. Am J Obstet Gynecol. 2017;216(2):135.e1–e5.
- National Human Trafficking Hotline. https://humantraffickinghotline.org. Accessed May 30, 2018.

Despite increasing media coverage of human trafficking and the gravity of its many ramifications, I am struck by how often trainees and other clinicians present to me patients for which trafficking is a real potential concern—yet who give me a blank expression when I ask if anyone has screened these patients for being victims of trafficking. I suspect that few of us anticipated, during medical training, that we would be providing care to women who are enslaved.
How large is the problem?
It is impossible to comprehend the true scope of human trafficking. Estimates are that 20.9 million men, women, and children globally are forced into work that they are not free to leave.1
Although human trafficking is recognized as a global phenomenon, its prevalence in the United States is significant enough that it should prompt the health care community to engage in helping identify and assist victims/survivors: From January until June of 2017, the National Human Trafficking Hotline received 13,807 telephone calls, resulting in reporting of 4,460 cases.2 Indeed, from 2015 to 2016 there was a 35.7% increase in the number of hotline cases reported, for a total of 7,572 (6,340—more than 80%—of which regarded females). California had the most cases reported (1,323), followed by Texas (670) and Florida (550); those 3 states also reported an increase in trafficking crime. Vermont (5), Rhode Island (9), and Alaska (10) reported the fewest calls.3
How is trafficking defined?
The United Nations Office on Drugs and Crime defines “trafficking in persons” as:
… recruitment, transportation, transfer, harbouring or receipt of persons, by means of the threat or use of force or other forms of coercion, of abduction, of fraud, of deception, of the abuse of power or of a position of vulnerability or of the giving or receiving of payments or benefits to achieve the consent of a person having control over another person, for the purpose of exploitation. Exploitation shall include, at a minimum, the exploitation of the prostitution of others or other forms of sexual exploitation, forced labour or services, slavery or practices similar to slavery, servitude or the removal of organs.4
Traffickers prey on potentially vulnerable people. Girls and young women who have experienced poverty, homelessness, childhood sexual abuse, substance abuse, gender nonconformity, mental illness, or developmental delay are at particular risk.5 Children who have had interactions with Child Protective Services, come from a dysfunctional family, or have lived in a community with high crime, political or social unrest, corruption, or gender bias and discrimination are also at increased risk.6
Read about clues that raise clinical suspicion
Clues that raise clinical suspicion
A number of potential signs should make providers suspicious about potential human trafficking. Some of those signs are similar to the red flags we see in intimate partner violence, such as:
- having a difficult time talking to the patient alone
- having the accompanying person answer the patient’s questions
- body language that suggests fear, anxiety, or distrust (eg, shifting positions, looking away, appearing withdrawn)
- physical examination inconsistent with the history
- physical injury (especially multiple injuries or injuries in various stages of healing)
- refusal of interpreter services.
Trafficked girls or women may appear overly familiar with sex, have unexpected material possessions, or appear to be giving scripted or memorized answers to queries.7 Traffickers often confiscate their victims’ personal identification. They try to prevent victims from knowing their geographic locales: Patients might not have any documentation or awareness of exact surroundings (eg, their home address). Patients may be wearing clothes considered inappropriate for the weather or venue. They may have tattoos that are marks of branding.8
Medical consequences of being trafficked are obvious, numerous, and serious
Many medical sequelae that result from trafficking are obvious, given the nature of work that victims are forced to do. For example, overcrowding can lead to infectious disease, such as tuberculosis.9 Inadequate access to preventive or basic medical services can result in weight loss, poor dentition, and untreated chronic medical conditions.
If victims are experiencing physical or sexual abuse, they can present with evidence of blunt trauma, ligature marks, skin burns, wounds inflicted by weapons, and vaginal lacerations.10 A study found that 63% of survivors reported at least 10 somatic symptoms, including headache, fatigue, dizziness, back pain, abdominal or pelvic pain, memory loss, and symptoms of genital infectious disease.11
Girls and women being trafficked for sex may experience many of the sequelae of unprotected intercourse: irregular bleeding, unintended pregnancy, unwanted or unsafe pregnancy termination, vaginal trauma, and sexually transmitted infection (STI).12 In a study of trafficking survivors, 38% were HIV-positive.13
Trafficking survivors can suffer myriad mental health conditions, with high rates of depression, anxiety, posttraumatic stress, and suicidal ideation.14 A study of 387 survivors found that 12% had attempted to harm themselves or commit suicide the month before they were interviewed.15
Substance abuse is also a common problem among trafficking victims.16 One survivor interviewed in a recent study said:
It was much more difficult to work sober because I was dealing with emotions or the pain that I was feeling during intercourse, because when you have sex with people 8, 9, 10 times a day, even more than that, it starts to hurt a lot. And being high made it easier to deal with that and also it made it easier for me to get away from my body while it was happening, place my brain somewhere else.17
Because of the substantial risk of mental health problems, including substance abuse, among trafficking survivors, the physical exam of a patient should include careful assessment of demeanor and mental health status. Of course, comprehensive inspection for signs of physical or blunt trauma is paramount.
Read about Patient and staff safety during the visit
Patient and staff safety during the visit
Providers should be aware of potential safety concerns, both for the patient and for the staff. Creative strategies should be utilized to screen the patient in private. The use of interpreter services—either in person or over the telephone—should be presented and facilitated as being a routine part of practice. Any person who accompanies the patient should be asked to leave the examining room, either as a statement of practice routine or under the guise of having him (or her) step out to obtain paperwork or provide documentation.
Care of victims
Trauma-informed care should be a guiding principle for trafficking survivors. This involves empowering the patient, who may feel victimized again if asked to undress and undergo multiple physical examinations. Macias-Konstantopoulos noted: “A trauma-informed approach to care acknowledges the pervasiveness and effect of trauma across the life span of the individual, recognizes the vulnerabilities and emotional triggers of trauma survivors, minimizes repeated traumatization and fosters physical, psychological, and emotional safety, recovery, health and well-being.”18
The patient should be counseled that she has control over her body and can guide different aspects of the examination. For example the provider should discuss: 1) the amount of clothing deemed optimal for an examination, 2) the availability of a support person during the exam (for instance, a nurse or a social worker) if the patient requests one, and 3) utilization of whatever strategies the patient deems optimal for her to be most comfortable during the exam (such as leaving the door slightly ajar or having a mutually agreed-on signal to interrupt the exam).
Routine health care maintenance should be offered, including an assessment of overall physical and dental health and screening for STI and mental health. Screening for substances of abuse should be considered. If indicated, emergency contraception, postexposure HIV prophylaxis, immunizations, and empiric antibiotics for STI should be offered.19
Screening when indicated by evidence, suspicion, or concern
Unlike the case with intimate partner violence, experts do not recommend universal screening for human trafficking. Clinicians should be comfortable, however, trying to elicit that history when a concern arises, either because of identified risk factors, red flags, or concerns that arise from the findings of the history or physical. Ideally, clinicians should consider becoming comfortable choosing a few screening questions to regularly incorporate into their assessment. The US Department of Health & Human Services (HHS) offers a list of questions that can be utilized (TABLE).20
In January 2018, the Office on Trafficking in Persons, a unit of the HHS Administration for Children and Families, released an “Adult Human Trafficking Screening Tool and Guide.” The document includes 2 excellent tools21 that clinicians can utilize to identify patients who should be screened and how to identify and assist survivors (FIGURE 1 and FIGURE 2).
Clinicians, in their encounters with patients, are particularly well-positioned to intersect with, and identify, survivors. Regrettably, such opportunities are often missed—and victims thus remain unidentified and trapped in their circumstances. A study revealed that one-half of survivors who were interviewed reported seeing a physician while they were being trafficked.22 Even more alarming, another study showed that 87.8% of survivors had received health care during their captivity.23 It is dismaying to know that these patients left those health care settings without receiving the assistance they truly need and with their true circumstances remaining unidentified.
Read about Finding assistance and support
Finding assistance and support
Centers in the United States now provide trauma-informed care for trafficking survivors in a confidential setting (see “Specialized care is increasingly available”).24 A physician who works at a center in New York City noted: “Our survivors told us that more than fear or pain, the feelings that sat with them most often were worthlessness and invisibility. We can do better as physicians and as educators to expose this epidemic and care for its victims.”24
Here is a sampling of the growing number of centers in the United States that provide trauma-centered care for survivors of human trafficking:
- Survivor Clinic at New York Presbyterian Hospital-Weill Cornell Medical College, New York, New York
- EMPOWER Clinic for Survivors of Sex Trafficking and Sexual Violence at NYU Langone Health, New York, New York
- Freedom Clinic at Massachusetts General Hospital, Boston
- The Hope Through Health Clinic, Austin, Texas
- Pacific Survivor Center, Honolulu, Hawaii
Most clinicians practice in settings that do not have easy access to such subspecialized centers, however. For them, the National Human Trafficking Hotline can be an invaluable resource (see “Hotline is a valuable resource”).25 Law enforcement and social services colleagues also can be useful allies.
Uncertain how you can help a patient who is a victim of human trafficking? For assistance and support, contact the National Human Trafficking Hotline--24 hours a day, 7 days a week, and in 200 languages--in any of 3 ways:
- By telephone: (888) 373-7888
- By text: 233733
- On the web: https://humantraffickinghotline.orga
aIncludes a search field that clinicians can use to look up the nearest resources for additional assistance.
Let’s turn our concern and awareness into results
We, as providers of women’s health care, are uniquely positioned to help these most vulnerable of people, many of whom have been stripped of personal documents and denied access to financial resources and community support. As a medical community, we should strive to combat this tragic epidemic, 1 patient at a time.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Despite increasing media coverage of human trafficking and the gravity of its many ramifications, I am struck by how often trainees and other clinicians present to me patients for which trafficking is a real potential concern—yet who give me a blank expression when I ask if anyone has screened these patients for being victims of trafficking. I suspect that few of us anticipated, during medical training, that we would be providing care to women who are enslaved.
How large is the problem?
It is impossible to comprehend the true scope of human trafficking. Estimates are that 20.9 million men, women, and children globally are forced into work that they are not free to leave.1
Although human trafficking is recognized as a global phenomenon, its prevalence in the United States is significant enough that it should prompt the health care community to engage in helping identify and assist victims/survivors: From January until June of 2017, the National Human Trafficking Hotline received 13,807 telephone calls, resulting in reporting of 4,460 cases.2 Indeed, from 2015 to 2016 there was a 35.7% increase in the number of hotline cases reported, for a total of 7,572 (6,340—more than 80%—of which regarded females). California had the most cases reported (1,323), followed by Texas (670) and Florida (550); those 3 states also reported an increase in trafficking crime. Vermont (5), Rhode Island (9), and Alaska (10) reported the fewest calls.3
How is trafficking defined?
The United Nations Office on Drugs and Crime defines “trafficking in persons” as:
… recruitment, transportation, transfer, harbouring or receipt of persons, by means of the threat or use of force or other forms of coercion, of abduction, of fraud, of deception, of the abuse of power or of a position of vulnerability or of the giving or receiving of payments or benefits to achieve the consent of a person having control over another person, for the purpose of exploitation. Exploitation shall include, at a minimum, the exploitation of the prostitution of others or other forms of sexual exploitation, forced labour or services, slavery or practices similar to slavery, servitude or the removal of organs.4
Traffickers prey on potentially vulnerable people. Girls and young women who have experienced poverty, homelessness, childhood sexual abuse, substance abuse, gender nonconformity, mental illness, or developmental delay are at particular risk.5 Children who have had interactions with Child Protective Services, come from a dysfunctional family, or have lived in a community with high crime, political or social unrest, corruption, or gender bias and discrimination are also at increased risk.6
Read about clues that raise clinical suspicion
Clues that raise clinical suspicion
A number of potential signs should make providers suspicious about potential human trafficking. Some of those signs are similar to the red flags we see in intimate partner violence, such as:
- having a difficult time talking to the patient alone
- having the accompanying person answer the patient’s questions
- body language that suggests fear, anxiety, or distrust (eg, shifting positions, looking away, appearing withdrawn)
- physical examination inconsistent with the history
- physical injury (especially multiple injuries or injuries in various stages of healing)
- refusal of interpreter services.
Trafficked girls or women may appear overly familiar with sex, have unexpected material possessions, or appear to be giving scripted or memorized answers to queries.7 Traffickers often confiscate their victims’ personal identification. They try to prevent victims from knowing their geographic locales: Patients might not have any documentation or awareness of exact surroundings (eg, their home address). Patients may be wearing clothes considered inappropriate for the weather or venue. They may have tattoos that are marks of branding.8
Medical consequences of being trafficked are obvious, numerous, and serious
Many medical sequelae that result from trafficking are obvious, given the nature of work that victims are forced to do. For example, overcrowding can lead to infectious disease, such as tuberculosis.9 Inadequate access to preventive or basic medical services can result in weight loss, poor dentition, and untreated chronic medical conditions.
If victims are experiencing physical or sexual abuse, they can present with evidence of blunt trauma, ligature marks, skin burns, wounds inflicted by weapons, and vaginal lacerations.10 A study found that 63% of survivors reported at least 10 somatic symptoms, including headache, fatigue, dizziness, back pain, abdominal or pelvic pain, memory loss, and symptoms of genital infectious disease.11
Girls and women being trafficked for sex may experience many of the sequelae of unprotected intercourse: irregular bleeding, unintended pregnancy, unwanted or unsafe pregnancy termination, vaginal trauma, and sexually transmitted infection (STI).12 In a study of trafficking survivors, 38% were HIV-positive.13
Trafficking survivors can suffer myriad mental health conditions, with high rates of depression, anxiety, posttraumatic stress, and suicidal ideation.14 A study of 387 survivors found that 12% had attempted to harm themselves or commit suicide the month before they were interviewed.15
Substance abuse is also a common problem among trafficking victims.16 One survivor interviewed in a recent study said:
It was much more difficult to work sober because I was dealing with emotions or the pain that I was feeling during intercourse, because when you have sex with people 8, 9, 10 times a day, even more than that, it starts to hurt a lot. And being high made it easier to deal with that and also it made it easier for me to get away from my body while it was happening, place my brain somewhere else.17
Because of the substantial risk of mental health problems, including substance abuse, among trafficking survivors, the physical exam of a patient should include careful assessment of demeanor and mental health status. Of course, comprehensive inspection for signs of physical or blunt trauma is paramount.
Read about Patient and staff safety during the visit
Patient and staff safety during the visit
Providers should be aware of potential safety concerns, both for the patient and for the staff. Creative strategies should be utilized to screen the patient in private. The use of interpreter services—either in person or over the telephone—should be presented and facilitated as being a routine part of practice. Any person who accompanies the patient should be asked to leave the examining room, either as a statement of practice routine or under the guise of having him (or her) step out to obtain paperwork or provide documentation.
Care of victims
Trauma-informed care should be a guiding principle for trafficking survivors. This involves empowering the patient, who may feel victimized again if asked to undress and undergo multiple physical examinations. Macias-Konstantopoulos noted: “A trauma-informed approach to care acknowledges the pervasiveness and effect of trauma across the life span of the individual, recognizes the vulnerabilities and emotional triggers of trauma survivors, minimizes repeated traumatization and fosters physical, psychological, and emotional safety, recovery, health and well-being.”18
The patient should be counseled that she has control over her body and can guide different aspects of the examination. For example the provider should discuss: 1) the amount of clothing deemed optimal for an examination, 2) the availability of a support person during the exam (for instance, a nurse or a social worker) if the patient requests one, and 3) utilization of whatever strategies the patient deems optimal for her to be most comfortable during the exam (such as leaving the door slightly ajar or having a mutually agreed-on signal to interrupt the exam).
Routine health care maintenance should be offered, including an assessment of overall physical and dental health and screening for STI and mental health. Screening for substances of abuse should be considered. If indicated, emergency contraception, postexposure HIV prophylaxis, immunizations, and empiric antibiotics for STI should be offered.19
Screening when indicated by evidence, suspicion, or concern
Unlike the case with intimate partner violence, experts do not recommend universal screening for human trafficking. Clinicians should be comfortable, however, trying to elicit that history when a concern arises, either because of identified risk factors, red flags, or concerns that arise from the findings of the history or physical. Ideally, clinicians should consider becoming comfortable choosing a few screening questions to regularly incorporate into their assessment. The US Department of Health & Human Services (HHS) offers a list of questions that can be utilized (TABLE).20
In January 2018, the Office on Trafficking in Persons, a unit of the HHS Administration for Children and Families, released an “Adult Human Trafficking Screening Tool and Guide.” The document includes 2 excellent tools21 that clinicians can utilize to identify patients who should be screened and how to identify and assist survivors (FIGURE 1 and FIGURE 2).
Clinicians, in their encounters with patients, are particularly well-positioned to intersect with, and identify, survivors. Regrettably, such opportunities are often missed—and victims thus remain unidentified and trapped in their circumstances. A study revealed that one-half of survivors who were interviewed reported seeing a physician while they were being trafficked.22 Even more alarming, another study showed that 87.8% of survivors had received health care during their captivity.23 It is dismaying to know that these patients left those health care settings without receiving the assistance they truly need and with their true circumstances remaining unidentified.
Read about Finding assistance and support
Finding assistance and support
Centers in the United States now provide trauma-informed care for trafficking survivors in a confidential setting (see “Specialized care is increasingly available”).24 A physician who works at a center in New York City noted: “Our survivors told us that more than fear or pain, the feelings that sat with them most often were worthlessness and invisibility. We can do better as physicians and as educators to expose this epidemic and care for its victims.”24
Here is a sampling of the growing number of centers in the United States that provide trauma-centered care for survivors of human trafficking:
- Survivor Clinic at New York Presbyterian Hospital-Weill Cornell Medical College, New York, New York
- EMPOWER Clinic for Survivors of Sex Trafficking and Sexual Violence at NYU Langone Health, New York, New York
- Freedom Clinic at Massachusetts General Hospital, Boston
- The Hope Through Health Clinic, Austin, Texas
- Pacific Survivor Center, Honolulu, Hawaii
Most clinicians practice in settings that do not have easy access to such subspecialized centers, however. For them, the National Human Trafficking Hotline can be an invaluable resource (see “Hotline is a valuable resource”).25 Law enforcement and social services colleagues also can be useful allies.
Uncertain how you can help a patient who is a victim of human trafficking? For assistance and support, contact the National Human Trafficking Hotline--24 hours a day, 7 days a week, and in 200 languages--in any of 3 ways:
- By telephone: (888) 373-7888
- By text: 233733
- On the web: https://humantraffickinghotline.orga
aIncludes a search field that clinicians can use to look up the nearest resources for additional assistance.
Let’s turn our concern and awareness into results
We, as providers of women’s health care, are uniquely positioned to help these most vulnerable of people, many of whom have been stripped of personal documents and denied access to financial resources and community support. As a medical community, we should strive to combat this tragic epidemic, 1 patient at a time.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- International Labour Organization. New ILO Global Estimate of Forced Labour: 20.9 million victims. http://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_182109/lang--en/index.htm. Published June 2012. Accessed May 30, 2018.
- National Human Trafficking Hotline. Hotline statistics. https://humantraffickinghotline.org/states. Accessed May 30, 2018.
- Cone A. Report: Human trafficking in U.S. rose 35.7 percent in one year. United Press International (UPI). https://www.upi.com/Report-Human-trafficking-in-US-rose-357-percent-in-one-year/5571486328579. Published February 5, 2017. Accessed May 30, 2018.
- United Nations Office on Drugs and Crime. Human trafficking. http://www.unodc.org/unodc/en/human-trafficking/what-is-human-trafficking.html. Accessed May 30, 2018.
- Risk factors for and consequences of commercial sexual exploitation and sex trafficking of minors. In Clayton E, Krugman R, Simon P, eds; Committee on the Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States; Board on Children, Youth, and Families; Committee on Law and Justice; Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
- Greenbaum J, Crawford-Jakubiak JE. Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015:135(3);566–574.
- Alpert E, Ahn R, Albright E, Purcell G, Burke T, Macias-Konstantanopoulos W. Human Trafficking: Guidebook on Identification, Assessment, and Response in the Health Care Setting. Waltham, MA: Massachusetts General Hospital and Massachusetts Medical Society; 2014. http://www.massmed.org/Patient-Care/Health-Topics/Violence-Prevention-and-Intervention/Human-Trafficking-(pdf). Accessed May 30, 2018.
- National Human Trafficking Training and Technical Assistance Center. Adult human trafficking screening tool and guide. http://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Published January 2018. Accessed May 30, 2018.
- Steele S. Human trafficking, labor brokering, and mining in southern Africa: responding to a decentralized and hidden public health disaster. Int J Health Serv. 2013;43(4):665–680.
- Becker HJ, Bechtel K. Recognizing victims of human trafficking in the pediatric emergency department. Pediatr Emerg Care. 2015;31(2):144–147.
- Zimmerman C, Hossain M, Yun K, et al. The health of trafficked women: a survey of women entering postrafficking services in Europe. Am J Public Health. 2008;98(1):55–59.
- Tracy EE, Macias-Konstantopoulos W. Identifying and assisting sexually exploited and trafficked patients seeking women’s health care services. Obstet Gynecol. 2017;130(2):443–453.
- Silverman JG, Decker MR, Gupta J, Maheshwari A, Willis BM, Raj A. HIV prevalence and predictors of infection in sex-trafficked Nepalese girls and women. JAMA. 2007;298(5):536–542.
- Rafferty Y. Child trafficking and commercial sexual exploitation: a review of promising prevention policies and programs. Am J Orthopsychiatry. 2013;83(4):559–575.
- Kiss L, Yun K, Pocock N, Zimmerman C. Exploitation, violence, and suicide risk among child and adolescent survivors of human trafficking in the Greater Mekong Subregion. JAMA Pediatr. 2015;169(9):e152278.
- Stoklosa H, MacGibbon M, Stoklosa J. Human trafficking, mental illness, and addiction: avoiding diagnostic overshadowing. AMA J Ethics. 2017;19(1):23–34.
- Ravi A, Pfeiffer MR, Rosner Z, Shea JA. Trafficking and trauma: insight and advice for the healthcare system from sex-trafficked women incarcerated on Rikers Island. Med Care. 2017;55(12):1017–1022.
- Macias-Konstantopoulos W. Human trafficking: the role of medicine in interrupting the cycle of abuse and violence. Ann Intern Med. 2016:165(8):582–588.
- Chung RJ, English A. Commercial sexual exploitation and sex trafficking of adolescents. Curr Opin Pediatr. 2015;27(4):427–433.
- Resources: Screening tool for victims of human trafficking. Washington, DC: US Department of Health and Human Services. https://www.justice.gov/sites/default/files/usao-ndia/legacy/2011/10/14/health_screen_questions.pdf. Accessed May 30, 2018.
- US Department of Health and Human Services. Adult human trafficking screening tool and guide. January 2018. https://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Accessed May 30, 2018.
- Baldwin SB, Eisenman DP, Sayles JN, Ryan G, Chuang KS. Identification of human trafficking victims in health care settings. Health Hum Rights. 2011;13(1):e36–e49.
- Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in health-care facilities. Ann Health Law. 2014;23:61–91.
- Geynisman-Tan JM, Taylor JS, Edersheim T, Taubel D. All the darkness we don’t see. Am J Obstet Gynecol. 2017;216(2):135.e1–e5.
- National Human Trafficking Hotline. https://humantraffickinghotline.org. Accessed May 30, 2018.
- International Labour Organization. New ILO Global Estimate of Forced Labour: 20.9 million victims. http://www.ilo.org/global/about-the-ilo/newsroom/news/WCMS_182109/lang--en/index.htm. Published June 2012. Accessed May 30, 2018.
- National Human Trafficking Hotline. Hotline statistics. https://humantraffickinghotline.org/states. Accessed May 30, 2018.
- Cone A. Report: Human trafficking in U.S. rose 35.7 percent in one year. United Press International (UPI). https://www.upi.com/Report-Human-trafficking-in-US-rose-357-percent-in-one-year/5571486328579. Published February 5, 2017. Accessed May 30, 2018.
- United Nations Office on Drugs and Crime. Human trafficking. http://www.unodc.org/unodc/en/human-trafficking/what-is-human-trafficking.html. Accessed May 30, 2018.
- Risk factors for and consequences of commercial sexual exploitation and sex trafficking of minors. In Clayton E, Krugman R, Simon P, eds; Committee on the Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States; Board on Children, Youth, and Families; Committee on Law and Justice; Institute of Medicine; National Research Council. Confronting Commercial Sexual Exploitation and Sex Trafficking of Minors in the United States. Washington, DC: The National Academies Press; 2013.
- Greenbaum J, Crawford-Jakubiak JE. Committee on Child Abuse and Neglect. Child sex trafficking and commercial sexual exploitation: health care needs of victims. Pediatrics. 2015:135(3);566–574.
- Alpert E, Ahn R, Albright E, Purcell G, Burke T, Macias-Konstantanopoulos W. Human Trafficking: Guidebook on Identification, Assessment, and Response in the Health Care Setting. Waltham, MA: Massachusetts General Hospital and Massachusetts Medical Society; 2014. http://www.massmed.org/Patient-Care/Health-Topics/Violence-Prevention-and-Intervention/Human-Trafficking-(pdf). Accessed May 30, 2018.
- National Human Trafficking Training and Technical Assistance Center. Adult human trafficking screening tool and guide. http://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Published January 2018. Accessed May 30, 2018.
- Steele S. Human trafficking, labor brokering, and mining in southern Africa: responding to a decentralized and hidden public health disaster. Int J Health Serv. 2013;43(4):665–680.
- Becker HJ, Bechtel K. Recognizing victims of human trafficking in the pediatric emergency department. Pediatr Emerg Care. 2015;31(2):144–147.
- Zimmerman C, Hossain M, Yun K, et al. The health of trafficked women: a survey of women entering postrafficking services in Europe. Am J Public Health. 2008;98(1):55–59.
- Tracy EE, Macias-Konstantopoulos W. Identifying and assisting sexually exploited and trafficked patients seeking women’s health care services. Obstet Gynecol. 2017;130(2):443–453.
- Silverman JG, Decker MR, Gupta J, Maheshwari A, Willis BM, Raj A. HIV prevalence and predictors of infection in sex-trafficked Nepalese girls and women. JAMA. 2007;298(5):536–542.
- Rafferty Y. Child trafficking and commercial sexual exploitation: a review of promising prevention policies and programs. Am J Orthopsychiatry. 2013;83(4):559–575.
- Kiss L, Yun K, Pocock N, Zimmerman C. Exploitation, violence, and suicide risk among child and adolescent survivors of human trafficking in the Greater Mekong Subregion. JAMA Pediatr. 2015;169(9):e152278.
- Stoklosa H, MacGibbon M, Stoklosa J. Human trafficking, mental illness, and addiction: avoiding diagnostic overshadowing. AMA J Ethics. 2017;19(1):23–34.
- Ravi A, Pfeiffer MR, Rosner Z, Shea JA. Trafficking and trauma: insight and advice for the healthcare system from sex-trafficked women incarcerated on Rikers Island. Med Care. 2017;55(12):1017–1022.
- Macias-Konstantopoulos W. Human trafficking: the role of medicine in interrupting the cycle of abuse and violence. Ann Intern Med. 2016:165(8):582–588.
- Chung RJ, English A. Commercial sexual exploitation and sex trafficking of adolescents. Curr Opin Pediatr. 2015;27(4):427–433.
- Resources: Screening tool for victims of human trafficking. Washington, DC: US Department of Health and Human Services. https://www.justice.gov/sites/default/files/usao-ndia/legacy/2011/10/14/health_screen_questions.pdf. Accessed May 30, 2018.
- US Department of Health and Human Services. Adult human trafficking screening tool and guide. January 2018. https://www.acf.hhs.gov/sites/default/files/otip/adult_human_trafficking_screening_tool_and_guide.pdf. Accessed May 30, 2018.
- Baldwin SB, Eisenman DP, Sayles JN, Ryan G, Chuang KS. Identification of human trafficking victims in health care settings. Health Hum Rights. 2011;13(1):e36–e49.
- Lederer LJ, Wetzel CA. The health consequences of sex trafficking and their implications for identifying victims in health-care facilities. Ann Health Law. 2014;23:61–91.
- Geynisman-Tan JM, Taylor JS, Edersheim T, Taubel D. All the darkness we don’t see. Am J Obstet Gynecol. 2017;216(2):135.e1–e5.
- National Human Trafficking Hotline. https://humantraffickinghotline.org. Accessed May 30, 2018.
IN THIS ARTICLE
- Clues to raise suspicion
- Medical consequences of trafficking
- Screening algorithm