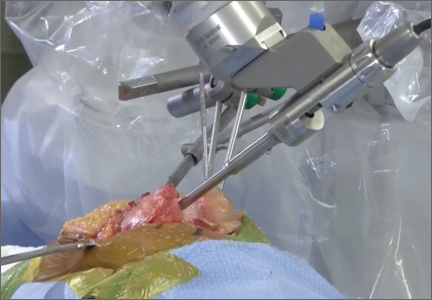
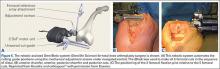
User login
Medical Mimics of Psychiatric Conditions, Part 1
The chaos of a busy ED can test the cognitive reserve of even the most focused practitioner. To streamline the challenge of serial diagnosis and treatment, clinicians employ heuristics while honing the skills of pattern recognition. However, by definition, heuristics employs shortcuts, leaving out information for the sake of efficiency—sometimes at the expense of accuracy. Whether a patient presents with chest pain, abdominal pain, headache, or (the dreaded) dizziness, emergency physicians (EPs) employ algorithms based on a combination of education and prior experience.
Most of the time, these models lead the EP along the correct path, but not always. For example, when a clinician evaluating a patient presenting with psychotic behavior assumes the patient has schizophrenia, he or she will be correct eight or nine times out of 10. However, in some cases, a patient’s bizarre behavior may not be due to a true psychiatric disorder but, for example, from ingestion of an illicit substance.
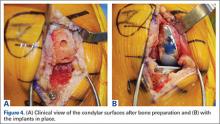
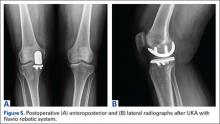
In addition, in such patients, psychiatric symptoms may be masking a serious acute, organic condition—one requiring prompt intervention and therapy to avoid morbidity or death. To help prevent diagnostic errors, this 2-part series reviews several of the most common medical mimics of psychiatric conditions. Part 1 of this series reviews the psychiatric presentations associated with medical conditions of an infectious, pharmacological withdrawal, metabolic, autoimmune, traumatic, or central nervous system etiology (Table 1). This article also discusses clinical signs and symptoms that suggest an increased likelihood that a patient’s psychiatric symptoms are from an underlying medical condition (Table 2).
Case Scenarios
Case 1
A 58-year-old woman with a history of smoking 40 packs of cigarettes per year presented to the ED 1 hour after onset of intermittent chest pain. Upon arrival at the ED, the patient stated that she had trouble catching her breath on and off throughout the day. The patient’s vital signs, electrocardiogram (ECG), and chest X-ray were all normal. The physical examination was unremarkable except for mild diaphoresis. The patient denied experiencing palpitations, recent travel, or previous episodes; she further stated that she was currently not on any medications. There was no previous history of visits to this hospital. The patient’s husband, who accompanied her to the ED, noted that his wife’s behavior had been atypical for approximately 1 week.
After receiving aspirin, the patient appeared symptom-free. Pending the results of another chest radiograph and laboratory evaluation, the EP anticipated moving her to the chest-pain observation unit.
Case 2
A 36-year-old woman presented with altered mental status to the ED via emergency medical services (EMS). Her vital signs, including temperature, were normal. Despite intermittently appearing to be asleep, the patient was alternatingly cooperative and combative. She repetitively whispered, “Who am I?” and randomly shouted at staff members as they walked by her room.
Her neurological examination was nonfocal. The hospital’s electronic medical record (EMR) for this patient showed nearly monthly ED visits for behavioral symptoms. Precipitating events noted in the EMR included job loss and separation from her husband. While waiting for the results of the basic laboratory work-up and toxicology screening to medically clear the patient for psychiatric evaluation, the EP contemplated a computed tomography (CT) study. Realizing the patient would not be able to remain still for the scan, the EP ordered 10 mg of intramuscular ziprasidone for sedation. When the patient’s husband arrived, the EP placed the CT scan on hold until she could obtain additional history from him.
Infections
Herpes Simplex Encephalitis
Herpes simplex encephalitis (HSE) is a serious but treatable disease—one that requires early detection and treatment to avoid severe morbidity. While the classic symptoms are fever and altered mental status, recent literature has noted that afebrile patients with HSE may present with behavioral changes, cognitive decline, aggression, and disinhibition. Therefore, diagnosis of a functional psychiatric complaint, if made initially, could delay appropriate treatment with acyclovir.1
Human Immunodeficiency Virus
Progression of human immunodeficiency virus (HIV) is a well-known cause of various neurocognitive disorders, including early-onset dementia. Since the availability of highly active antiretroviral therapy, the incidence of HIV dementia has decreased, but HIV remains the most common preventable cause of dementia in persons younger than age 50 years. Recent literature has described HIV dementia presenting as an early-onset, rapidly progressing dementia in a young person. Thus, the EP should consider early HIV testing in any young patient who presents with dementia, especially one with a history of fever of unknown origin.2
Progressive Multifocal Encephalopathy
Caused by reactivation of the John Cunningham virus, progressive multifocal encephalopathy has been classically described as a potentially lethal complication of a severely immunocompromised state, often presenting with clumsiness, weakness, visual changes, speech difficulty, and behavioral changes. Though typically described as occurring in the context of acquired immunodeficiency disease syndrome, hematological malignancy, or organ transplant, the condition can occur in the setting of minimal or occult immunosuppression—especially in patients with a history of cirrhosis. If the condition is detected early, immunotherapy can result in significant clinical improvement.3
Syphilis
Late stages of syphilis can present with a wide variety of psychiatric symptoms, including personality disorder, psychosis, delirium, and dementia. As with HIV, there has been a resurgence of syphilis cases, and screening is now often a routine part of a neuropsychiatric work-up. The EP should consider syphilis in the differential for any new-onset psychiatric complaint.4,5
Typhoid Fever
Although this severe febrile illness is uncommon in the United States, it is endemic to many tropical countries within Africa, Southeast Asia, and Central and South America. Typhoid is characterized by a stepwise fever that can progress to abdominal distension, toxemia, and potentially bowel perforation. It is also known to present with psychiatric symptoms such as acute confusion, psychosis, generalized anxiety disorder, and, though rare, depressive disorder. Physicians traveling to rural endemic areas should be aware of these neuropsychiatric presentations to avoid misdiagnosis and delay of treatment.6 Other infectious endemic diseases with reports of neuropsychiatric components are neurocystercercosis, Lyme disease, and African trypanosomiasis.
Pharmacological Withdrawal Syndromes
Alcohol
Alcohol withdrawal is a common presentation in the ED, and up to 24% of US adults brought to the ED by EMS suffer from alcoholism. Typically characterized by tachycardia, hypertension, and tremors, alcohol withdrawal syndrome can also feature psychiatric components such as agitation, hallucinations, persecutory delusions, and even self-mutilation.7 Evidence-based protocols indicate loading doses of benzodiazepines as a mainstay of treatment, with supplemental barbiturates or propofol in cases of treatment failure.8
Benzodiazepines
Withdrawal from therapeutic doses of benzodiazepines can potentially cause psychiatric symptoms, including sleep disturbances, irritability, anxiety, panic attacks, tremor, and perceptual changes. Withdrawal from higher doses of benzodiazepines can lead to more serious presentations, such as seizures and acute psychosis.9 Withdrawal symptoms can develop from discontinuation of the drug and with non-tapered switching between benzodiazepines.10
Opiates
Opiate withdrawal is an unpleasant experience characterized by generalized pain, nausea and vomiting, sweating, and tachycardia. Neuropsychiatric complaints such as anxiety, agitation, and irritability can also be present. More severe agitation has been described in naltrexone-accelerated detoxification.11
Cannabis
Recent literature on cannabis use indicates a high prevalence and clinical significance of associated withdrawal symptoms in frequent users. There appear to be two subsets of cannabis withdrawal—one characterized by weakness and hypersomnia, and the other by anxiety, depression, restlessness, and insomnia.12
Estrogen
Withdrawal from endogenous estrogen has been hypothesized as a possible cause of puerperal psychosis.13 Estrogen withdrawal outside of this setting, however, can and does occur, and recent literature has shown episodes of reversible psychosis associated with the discontinuation of both oral contraceptive regimens and hormonal therapy for menopausal symptoms.
Acute Metabolic Conditions
Hypoglycemia
Hypoglycemia, most often encountered as a side effect of insulin or oral hypoglycemic therapies, is a potentially lethal cause of confusion, anxiety, nervousness, and seizures. Nocturnal hypoglycemia can manifest as nightmares, crying out, and confusion upon awakening. A fingerstick blood-glucose test is an absolutely vital part of the initial work-up of any patient with an altered mental status or overt psychiatric complaint.14
Central Pontine Myelinolysis
A potentially devastating neurological condition associated with malnourishment and alcohol dependence, central pontine myelinolysis (CPM) is classically exacerbated by rapid overcorrection of hyponatremia. While the disease can manifest primarily with quadriplegia or pseudobulbar palsy and eventual progress to the dreaded “locked-in” syndrome, early presentations can include psychiatric symptoms such as behavioral changes, psychosis, and cognitive disturbances. Patients with early signs and symptoms of CPM have been misdiagnosed as having schizophrenia with catatonia, leading to delayed treatment and poor outcomes. The EP should remain vigilant when evaluating for this condition and consider a magnetic resonance imaging study in patients with psychiatric symptoms in the setting of fluctuating hyponatremia.15
Autoimmune Disorders
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is one of the most common autoimmune disorders, and has a higher incidence in young women. The disease affects multiple organ systems. Though the classic initial presentation of SLE is fever, joint pain, and rash, the associated neuropsychiatric syndromes of this disease are diverse and surprisingly common, and can be the initial manifestation of the disease. Common psychiatric manifestations of SLE include cognitive dysfunction, anxiety, mood disorders such as depression, acute confusion, psychosis, paranoia, and auditory or visual hallucinations.16
Anti-N-methyl-D-Aspartate Receptor Encephalitis
Initially described as a paraneoplastic effect of ovarian teratomas, anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis is actually an autoimmune disorder that can occur even in the absence of a primary tumor. As with SLE, the condition primarily occurs in young women. Antibodies in the cerebrospinal fluid cause prominent psychiatric symptoms such as acute psychosis, delusional thinking, hallucinations, agitation, and confusion. Although the disease can progress to seizures, movement disorders, autonomic dysregulation, and ultimately death, early recognition and treatment can lead to positive outcomes in up to 80% of cases.17 While the prevalence of anti-NMDAR antibodies in new-onset psychosis remains unclear, recent literature has suggested widespread screening for the disease in all first presentations of psychotic episodes.18,19
Multiple Sclerosis
Multiple sclerosis (MS) is another autoimmune disorder that has a higher prevalence in young women. The disease is characterized by central nervous system involvement that occurs over a period of months to years, with symptoms corresponding to different anatomic locations. Though the classic presenting symptom of MS is optic neuritis, neuropsychiatric syndromes are a common co-occurrence and can be the initial presenting symptom. The most commonly associated psychiatric complaints are anxiety, depression, and bipolar disorder, though case reports of SLE have described acute psychosis, psychotic depression, and adult-onset tic disorder.20
Trauma
Subarachnoid Hemorrhage
Long-term psychiatric sequelae from subarachnoid hemorrhage, either traumatic or aneurysmal, manifest most commonly as personality changes, intellectual impairment, depression, and anxiety. This condition is also known to cause a host of more bizarre psychiatric presentations, such as new-onset kleptomania, akinetic mutism, confabulatory amnesia, acute psychosis, and Capgras syndrome (the delusion that familiar individuals have been replaced by imposters). These symptoms can occur at initial presentation, and may show variable improvement with shunt surgery.21
Subdural Hematoma
Acute or chronic subdural hematoma can result from major head trauma, or even quite minor head trauma in an elderly or coagulopathic patient. Some common psychiatric manifestations of subdural hematoma include cognitive impairment, withdrawn behavior, blunted affect otherwise mimicking schizophrenic psychosis, and catatonia. The EP should consider early imaging studies in patients with new-onset psychotic symptoms—especially when they are refractory to typical antipsychotics.22
Central Nervous Symptom Diseases
Huntington Disease
Huntington disease (HD) is an autosomal dominant inherited, progressive neurodegenerative disorder characterized by mental decline, mood disorder, and muscle coordination problems that eventually become the classic involuntary writhing termed chorea. Due to its progressive nature, precise onset of the disease is difficult to describe; however, HD can manifest initially as schizophrenia-like psychotic episodes with only minimal apparent motor difficulty. Family history, including movement disorders and suicide, is important to obtain when available.23
Parkinson Disease
A progressive and disabling neurodegenerative disorder, Parkinson disease (PD) is classically characterized by fine resting tremor, cogwheeling rigidity, akinesia and mask-like facies, and postural instability. Comorbidity of psychiatric disorders is high, both as a result of the underlying disease process and as a side effect of dopaminergic treatment regimes. Common presentations of psychiatric disorders in PD include schizophrenia-like psychosis with visual hallucinations and mood disorders with prominent apathy and executive dysfunction. Recognition of the comorbidity is important because psychiatric disorders in PD respond differently to treatment than classic psychiatric disorders.24
Temporal Lobe Epilepsy
Epilepsy is a complex group of related neurological disorders involving unregulated nerve cell firing with a large variability in clinical presentation. Characteristically there is recurrent seizure activity. Temporal lobe epilepsy (TLE) is a subset of epilepsy known to present as a number of behavioral and neuropsychiatric complaints. Most presentations of TLE involve auras of emotional phenomena such as depression, fear, or anxiety, which can occur alone or with subsequent progression to complex partial or secondary generalized seizures.25 Many other bizarre presentations of TLE have been reported, including recurrent, potentially debilitating déjà vu, vivid recollection of past traumatic events mimicking posttraumatic stress disorder, paranoid delusions following olfactory triggers; and unprovoked attacks of depersonalization, derealization, anxiety, and dyspnea originally misdiagnosed as panic attack.
Stroke
The term “stroke chameleon” refers to presentations suggestive of other diseases that actually represent underlying strokes. Altered mental status is by far the largest block of these chameleons, with up to 30% of misdiagnosed strokes being misdiagnosed as altered mental status. The positive predictive value of altered mental status alone (ie, the chance that the diagnosis of altered mental status actually represents an undiagnosed acute stroke) is 7%.26
Case Scenarios Continued
Case 1
[The 58-year-old woman with intermittent chest pain.]
The patient’s D-dimer and troponin I levels were normal. Before the EP had an opportunity to discuss the results and next steps with the patient, the nurse asked him to see the patient immediately. Upon entering her room, the EP noted that the patient appeared anxious. The patient said the shortness of breath had returned, and also that she felt as if she were “floating” off the gurney, outside of her body. A check of her vital signs revealed a heart rate of 106 beats/minute and blood pressure of 160/100 mm Hg. A repeat ECG was significant only for sinus tachycardia. In an effort to calm the patient, the EP reassured her that the ECG, chest X-ray, and screening laboratory studies were normal, and that there was no evidence of a heart attack. Relieved, the patient asked for an Ativan to calm her nerves. Upon further questioning, the patient sheepishly reported that she had been taking 3 to 6 mg lorazepam for about 10 years, as prescribed by her family physician (FP) for anxiety. She further admitted that she abruptly discontinued taking the drug about one week before this ED visit after she’d heard on a daytime TV show that the medication was addictive.
After receiving lorazepam, the patient showed marked improvement. The EP’s final impressions were atypical chest pain and acute panic attack precipitated by abrupt benzodiazepine withdrawal. After discussing the case with the patient’s FP, the EP discharged the patient home with instructions to complete the cardiac evaluation as an outpatient. The EP also recommended that the patient resume taking lorazepam and follow-up with her FP within one week to discuss a benzodiazepine taper and alternative therapy for anxiety.
Case 2
[The 36-year-old woman with altered mental status.]
When the EP entered the patient’s room, he witnessed the patient staring at her husband and striking him repetitively with her right arm. When the EP asked the patient to stop hitting, her husband told the EP that everything was alright and that the patient’s neurologist had previously told them this behavior was caused by a seizure. While in the next examination room, one of the EP’s colleagues had overheard some of the patient’s history and recognized the name of the patient’s neurologist as a specialist in partial complex seizures—one who had retired from the local medical school about 10 years ago.
After records from the local university hospital confirmed the patient’s diagnosis of partial complex seizures, she was given intravenous lorazepam 2 mg; she became alert, conversational, and stopped flailing her right arm. She was then admitted to the hospital for medical stabilization of her frequent seizures.
Editor’s Note: Part 2 of this article will appear in the June 2016 issue of Emergency Medicine and will cover psychiatric presentations related to dementia, cancer, cardiac disease, nutritional deficiencies, endocrine disorders, and toxins.
1. Boyapati R, Papadopoulos G, Olver J, Geluk M, Johnson PD. An unusual presentation of herpes simplex virus encephalitis. Case Rep Med. 2012;241710.
2. Verma R, Anand KS. HIV presenting as young-onset dementia. J Int Assoc Provid AIDS Care. 2014;13(2):110-112.
3. Gheuens S, Pierone G, Peeters P, Koralnik IJ. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J Neurol Neurosurg Psychiatry. 2010;81(3):247-254.
4. Sobhan T, Rowe HM, Ryan WG, Munoz C. Unusual case report: three cases of psychiatric manifestations of neurosyphilis. Psychiatr Serv. 2004;55(7):830-832.
5. Noblett J, Roberts E. The importance of not jumping to conclusions: syphilis as an organic cause of neurological, psychiatric and endocrine presentations. BMJ Case Rep. 2015;25:2015.
6. Ukwaja KN. Typhoid fever presenting as a depressive disorder—a case report. Rural Remote Health. 2010;10(2):1276.
7. Patra BN, Sharma A, Mehra A, Singh S. Complicated alcohol withdrawal presenting as self mutilation. J Forensic Leg Med. 2014;21:46-47.
8. Stehman CR, Mycyk MB. A rational approach to the treatment of alcohol withdrawal in the ED. Am J Emerg Med. 2013;31(4):734-742.
9. Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455-1459.
10. Bosshart H. Withdrawal-induced delirium associated with a benzodiazepine switch: a case report. J Med Case Rep. 2011;5:207207.
11. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567. doi:10.1177/0960327112450901
12. Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF. Cannabis withdrawal in the United States: results from NESARC. J Clin Psychiatry. 69(9):1354-1363.
13. Okazaki Y. The epidemiology and pathogenesis of postpartum depression. Nihon Rinsho. 2001;59(8):1555-1559.
14. Sinert R, Su M, Secko M, Zehtabchi S. The utility of routine laboratory testing in hypoglycaemic emergency department patients. Emerg Med J. 2009;26(1):28-31.
15. Schneider P, Nejtek VA, Hurd CL. A case of mistaken identity: alcohol withdrawal, schizophrenia, or central pontine myelinolysis? Neuropsychiatr Dis Treat. 2012;8:49-54.
16. Stojanovich L, Zandman-Goddard G, Pavlovich S, Sikanich N. Psychiatric manifestations in systemic lupus erythematosus. Autoimmun Rev. 2007;6(6):421-426.
17. Kayser MS, J Dalmau. Anti-NMDA receptor encephalitis, autoimmunity, and psychosis. Schizophr Res. 2014;pi:S0920-9964(14)00546-5.
18. Tidswell J, Kleinig T, Ash D, Thompson P, Galletly C. Early recognition of anti-N-methyl D-aspartate (NMDA) receptor encephalitis presenting as acute psychosis. Australas Psychiatry. 2013;21(6):596-599.
19. Masopust J, Andrýs C, Bažant J, Vyšata O, Kuca K, Vališ M. Anti-NMDA receptor antibodies in patients with a first episode of schizophrenia. Neuropsychiatr Dis Treat. 2015;11:619-623.
20. de Cerqueira AC, Semionato de Andrade P, Godoy Barreiros JM, Teixeira AL, Nardi AE. Psychiatric disorders in patients with multiple sclerosis. Compr Psychiatry. 2015;63:10-14.
21. Mobbs RJ, Chandran KN, Newcombe RL. Psychiatric presentation of aneurysmal subarachnoid haemorrhage. ANZ J Surg. 2001;71(1):69-70.
22. Kar SK, Kumar D, Singh P, Upadhyay PK. Psychiatric manifestation of chronic subdural hematoma: the unfolding of mystery in a homeless patient. Indian J Psychol Med. 2015;37(2):239-242.
23. Nagel M, Rumpf HJ, Kasten M. Acute psychosis in a verified Huntington disease gene carrier with subtle motor signs: psychiatric criteria should be considered for the diagnosis. Gen Hosp Psychiatry. 2014;36(3):361.e3-e4.
24. Buoli M, Caldiroli A, Altamura AC. Psychiatric conditions in Parkinson disease: a comparison with classical psychiatric disorders. J Geriatr Psychiatry Neurol. 2016;29(2):72-91.
25. Bortz JJ. Neuropsychiatric and memory issues in epilepsy.” Mayo Clin Proc. 2003;78(6):781-787.
26. Dupre CM, Libman R, Dupre SI, Katz JM, Rybinnik I, Kwiatkowski T. Stroke chameleons. J Stroke Cerebrovasc Dis. 2014;23(2):374-378.
The chaos of a busy ED can test the cognitive reserve of even the most focused practitioner. To streamline the challenge of serial diagnosis and treatment, clinicians employ heuristics while honing the skills of pattern recognition. However, by definition, heuristics employs shortcuts, leaving out information for the sake of efficiency—sometimes at the expense of accuracy. Whether a patient presents with chest pain, abdominal pain, headache, or (the dreaded) dizziness, emergency physicians (EPs) employ algorithms based on a combination of education and prior experience.
Most of the time, these models lead the EP along the correct path, but not always. For example, when a clinician evaluating a patient presenting with psychotic behavior assumes the patient has schizophrenia, he or she will be correct eight or nine times out of 10. However, in some cases, a patient’s bizarre behavior may not be due to a true psychiatric disorder but, for example, from ingestion of an illicit substance.
In addition, in such patients, psychiatric symptoms may be masking a serious acute, organic condition—one requiring prompt intervention and therapy to avoid morbidity or death. To help prevent diagnostic errors, this 2-part series reviews several of the most common medical mimics of psychiatric conditions. Part 1 of this series reviews the psychiatric presentations associated with medical conditions of an infectious, pharmacological withdrawal, metabolic, autoimmune, traumatic, or central nervous system etiology (Table 1). This article also discusses clinical signs and symptoms that suggest an increased likelihood that a patient’s psychiatric symptoms are from an underlying medical condition (Table 2).
Case Scenarios
Case 1
A 58-year-old woman with a history of smoking 40 packs of cigarettes per year presented to the ED 1 hour after onset of intermittent chest pain. Upon arrival at the ED, the patient stated that she had trouble catching her breath on and off throughout the day. The patient’s vital signs, electrocardiogram (ECG), and chest X-ray were all normal. The physical examination was unremarkable except for mild diaphoresis. The patient denied experiencing palpitations, recent travel, or previous episodes; she further stated that she was currently not on any medications. There was no previous history of visits to this hospital. The patient’s husband, who accompanied her to the ED, noted that his wife’s behavior had been atypical for approximately 1 week.
After receiving aspirin, the patient appeared symptom-free. Pending the results of another chest radiograph and laboratory evaluation, the EP anticipated moving her to the chest-pain observation unit.
Case 2
A 36-year-old woman presented with altered mental status to the ED via emergency medical services (EMS). Her vital signs, including temperature, were normal. Despite intermittently appearing to be asleep, the patient was alternatingly cooperative and combative. She repetitively whispered, “Who am I?” and randomly shouted at staff members as they walked by her room.
Her neurological examination was nonfocal. The hospital’s electronic medical record (EMR) for this patient showed nearly monthly ED visits for behavioral symptoms. Precipitating events noted in the EMR included job loss and separation from her husband. While waiting for the results of the basic laboratory work-up and toxicology screening to medically clear the patient for psychiatric evaluation, the EP contemplated a computed tomography (CT) study. Realizing the patient would not be able to remain still for the scan, the EP ordered 10 mg of intramuscular ziprasidone for sedation. When the patient’s husband arrived, the EP placed the CT scan on hold until she could obtain additional history from him.
Infections
Herpes Simplex Encephalitis
Herpes simplex encephalitis (HSE) is a serious but treatable disease—one that requires early detection and treatment to avoid severe morbidity. While the classic symptoms are fever and altered mental status, recent literature has noted that afebrile patients with HSE may present with behavioral changes, cognitive decline, aggression, and disinhibition. Therefore, diagnosis of a functional psychiatric complaint, if made initially, could delay appropriate treatment with acyclovir.1
Human Immunodeficiency Virus
Progression of human immunodeficiency virus (HIV) is a well-known cause of various neurocognitive disorders, including early-onset dementia. Since the availability of highly active antiretroviral therapy, the incidence of HIV dementia has decreased, but HIV remains the most common preventable cause of dementia in persons younger than age 50 years. Recent literature has described HIV dementia presenting as an early-onset, rapidly progressing dementia in a young person. Thus, the EP should consider early HIV testing in any young patient who presents with dementia, especially one with a history of fever of unknown origin.2
Progressive Multifocal Encephalopathy
Caused by reactivation of the John Cunningham virus, progressive multifocal encephalopathy has been classically described as a potentially lethal complication of a severely immunocompromised state, often presenting with clumsiness, weakness, visual changes, speech difficulty, and behavioral changes. Though typically described as occurring in the context of acquired immunodeficiency disease syndrome, hematological malignancy, or organ transplant, the condition can occur in the setting of minimal or occult immunosuppression—especially in patients with a history of cirrhosis. If the condition is detected early, immunotherapy can result in significant clinical improvement.3
Syphilis
Late stages of syphilis can present with a wide variety of psychiatric symptoms, including personality disorder, psychosis, delirium, and dementia. As with HIV, there has been a resurgence of syphilis cases, and screening is now often a routine part of a neuropsychiatric work-up. The EP should consider syphilis in the differential for any new-onset psychiatric complaint.4,5
Typhoid Fever
Although this severe febrile illness is uncommon in the United States, it is endemic to many tropical countries within Africa, Southeast Asia, and Central and South America. Typhoid is characterized by a stepwise fever that can progress to abdominal distension, toxemia, and potentially bowel perforation. It is also known to present with psychiatric symptoms such as acute confusion, psychosis, generalized anxiety disorder, and, though rare, depressive disorder. Physicians traveling to rural endemic areas should be aware of these neuropsychiatric presentations to avoid misdiagnosis and delay of treatment.6 Other infectious endemic diseases with reports of neuropsychiatric components are neurocystercercosis, Lyme disease, and African trypanosomiasis.
Pharmacological Withdrawal Syndromes
Alcohol
Alcohol withdrawal is a common presentation in the ED, and up to 24% of US adults brought to the ED by EMS suffer from alcoholism. Typically characterized by tachycardia, hypertension, and tremors, alcohol withdrawal syndrome can also feature psychiatric components such as agitation, hallucinations, persecutory delusions, and even self-mutilation.7 Evidence-based protocols indicate loading doses of benzodiazepines as a mainstay of treatment, with supplemental barbiturates or propofol in cases of treatment failure.8
Benzodiazepines
Withdrawal from therapeutic doses of benzodiazepines can potentially cause psychiatric symptoms, including sleep disturbances, irritability, anxiety, panic attacks, tremor, and perceptual changes. Withdrawal from higher doses of benzodiazepines can lead to more serious presentations, such as seizures and acute psychosis.9 Withdrawal symptoms can develop from discontinuation of the drug and with non-tapered switching between benzodiazepines.10
Opiates
Opiate withdrawal is an unpleasant experience characterized by generalized pain, nausea and vomiting, sweating, and tachycardia. Neuropsychiatric complaints such as anxiety, agitation, and irritability can also be present. More severe agitation has been described in naltrexone-accelerated detoxification.11
Cannabis
Recent literature on cannabis use indicates a high prevalence and clinical significance of associated withdrawal symptoms in frequent users. There appear to be two subsets of cannabis withdrawal—one characterized by weakness and hypersomnia, and the other by anxiety, depression, restlessness, and insomnia.12
Estrogen
Withdrawal from endogenous estrogen has been hypothesized as a possible cause of puerperal psychosis.13 Estrogen withdrawal outside of this setting, however, can and does occur, and recent literature has shown episodes of reversible psychosis associated with the discontinuation of both oral contraceptive regimens and hormonal therapy for menopausal symptoms.
Acute Metabolic Conditions
Hypoglycemia
Hypoglycemia, most often encountered as a side effect of insulin or oral hypoglycemic therapies, is a potentially lethal cause of confusion, anxiety, nervousness, and seizures. Nocturnal hypoglycemia can manifest as nightmares, crying out, and confusion upon awakening. A fingerstick blood-glucose test is an absolutely vital part of the initial work-up of any patient with an altered mental status or overt psychiatric complaint.14
Central Pontine Myelinolysis
A potentially devastating neurological condition associated with malnourishment and alcohol dependence, central pontine myelinolysis (CPM) is classically exacerbated by rapid overcorrection of hyponatremia. While the disease can manifest primarily with quadriplegia or pseudobulbar palsy and eventual progress to the dreaded “locked-in” syndrome, early presentations can include psychiatric symptoms such as behavioral changes, psychosis, and cognitive disturbances. Patients with early signs and symptoms of CPM have been misdiagnosed as having schizophrenia with catatonia, leading to delayed treatment and poor outcomes. The EP should remain vigilant when evaluating for this condition and consider a magnetic resonance imaging study in patients with psychiatric symptoms in the setting of fluctuating hyponatremia.15
Autoimmune Disorders
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is one of the most common autoimmune disorders, and has a higher incidence in young women. The disease affects multiple organ systems. Though the classic initial presentation of SLE is fever, joint pain, and rash, the associated neuropsychiatric syndromes of this disease are diverse and surprisingly common, and can be the initial manifestation of the disease. Common psychiatric manifestations of SLE include cognitive dysfunction, anxiety, mood disorders such as depression, acute confusion, psychosis, paranoia, and auditory or visual hallucinations.16
Anti-N-methyl-D-Aspartate Receptor Encephalitis
Initially described as a paraneoplastic effect of ovarian teratomas, anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis is actually an autoimmune disorder that can occur even in the absence of a primary tumor. As with SLE, the condition primarily occurs in young women. Antibodies in the cerebrospinal fluid cause prominent psychiatric symptoms such as acute psychosis, delusional thinking, hallucinations, agitation, and confusion. Although the disease can progress to seizures, movement disorders, autonomic dysregulation, and ultimately death, early recognition and treatment can lead to positive outcomes in up to 80% of cases.17 While the prevalence of anti-NMDAR antibodies in new-onset psychosis remains unclear, recent literature has suggested widespread screening for the disease in all first presentations of psychotic episodes.18,19
Multiple Sclerosis
Multiple sclerosis (MS) is another autoimmune disorder that has a higher prevalence in young women. The disease is characterized by central nervous system involvement that occurs over a period of months to years, with symptoms corresponding to different anatomic locations. Though the classic presenting symptom of MS is optic neuritis, neuropsychiatric syndromes are a common co-occurrence and can be the initial presenting symptom. The most commonly associated psychiatric complaints are anxiety, depression, and bipolar disorder, though case reports of SLE have described acute psychosis, psychotic depression, and adult-onset tic disorder.20
Trauma
Subarachnoid Hemorrhage
Long-term psychiatric sequelae from subarachnoid hemorrhage, either traumatic or aneurysmal, manifest most commonly as personality changes, intellectual impairment, depression, and anxiety. This condition is also known to cause a host of more bizarre psychiatric presentations, such as new-onset kleptomania, akinetic mutism, confabulatory amnesia, acute psychosis, and Capgras syndrome (the delusion that familiar individuals have been replaced by imposters). These symptoms can occur at initial presentation, and may show variable improvement with shunt surgery.21
Subdural Hematoma
Acute or chronic subdural hematoma can result from major head trauma, or even quite minor head trauma in an elderly or coagulopathic patient. Some common psychiatric manifestations of subdural hematoma include cognitive impairment, withdrawn behavior, blunted affect otherwise mimicking schizophrenic psychosis, and catatonia. The EP should consider early imaging studies in patients with new-onset psychotic symptoms—especially when they are refractory to typical antipsychotics.22
Central Nervous Symptom Diseases
Huntington Disease
Huntington disease (HD) is an autosomal dominant inherited, progressive neurodegenerative disorder characterized by mental decline, mood disorder, and muscle coordination problems that eventually become the classic involuntary writhing termed chorea. Due to its progressive nature, precise onset of the disease is difficult to describe; however, HD can manifest initially as schizophrenia-like psychotic episodes with only minimal apparent motor difficulty. Family history, including movement disorders and suicide, is important to obtain when available.23
Parkinson Disease
A progressive and disabling neurodegenerative disorder, Parkinson disease (PD) is classically characterized by fine resting tremor, cogwheeling rigidity, akinesia and mask-like facies, and postural instability. Comorbidity of psychiatric disorders is high, both as a result of the underlying disease process and as a side effect of dopaminergic treatment regimes. Common presentations of psychiatric disorders in PD include schizophrenia-like psychosis with visual hallucinations and mood disorders with prominent apathy and executive dysfunction. Recognition of the comorbidity is important because psychiatric disorders in PD respond differently to treatment than classic psychiatric disorders.24
Temporal Lobe Epilepsy
Epilepsy is a complex group of related neurological disorders involving unregulated nerve cell firing with a large variability in clinical presentation. Characteristically there is recurrent seizure activity. Temporal lobe epilepsy (TLE) is a subset of epilepsy known to present as a number of behavioral and neuropsychiatric complaints. Most presentations of TLE involve auras of emotional phenomena such as depression, fear, or anxiety, which can occur alone or with subsequent progression to complex partial or secondary generalized seizures.25 Many other bizarre presentations of TLE have been reported, including recurrent, potentially debilitating déjà vu, vivid recollection of past traumatic events mimicking posttraumatic stress disorder, paranoid delusions following olfactory triggers; and unprovoked attacks of depersonalization, derealization, anxiety, and dyspnea originally misdiagnosed as panic attack.
Stroke
The term “stroke chameleon” refers to presentations suggestive of other diseases that actually represent underlying strokes. Altered mental status is by far the largest block of these chameleons, with up to 30% of misdiagnosed strokes being misdiagnosed as altered mental status. The positive predictive value of altered mental status alone (ie, the chance that the diagnosis of altered mental status actually represents an undiagnosed acute stroke) is 7%.26
Case Scenarios Continued
Case 1
[The 58-year-old woman with intermittent chest pain.]
The patient’s D-dimer and troponin I levels were normal. Before the EP had an opportunity to discuss the results and next steps with the patient, the nurse asked him to see the patient immediately. Upon entering her room, the EP noted that the patient appeared anxious. The patient said the shortness of breath had returned, and also that she felt as if she were “floating” off the gurney, outside of her body. A check of her vital signs revealed a heart rate of 106 beats/minute and blood pressure of 160/100 mm Hg. A repeat ECG was significant only for sinus tachycardia. In an effort to calm the patient, the EP reassured her that the ECG, chest X-ray, and screening laboratory studies were normal, and that there was no evidence of a heart attack. Relieved, the patient asked for an Ativan to calm her nerves. Upon further questioning, the patient sheepishly reported that she had been taking 3 to 6 mg lorazepam for about 10 years, as prescribed by her family physician (FP) for anxiety. She further admitted that she abruptly discontinued taking the drug about one week before this ED visit after she’d heard on a daytime TV show that the medication was addictive.
After receiving lorazepam, the patient showed marked improvement. The EP’s final impressions were atypical chest pain and acute panic attack precipitated by abrupt benzodiazepine withdrawal. After discussing the case with the patient’s FP, the EP discharged the patient home with instructions to complete the cardiac evaluation as an outpatient. The EP also recommended that the patient resume taking lorazepam and follow-up with her FP within one week to discuss a benzodiazepine taper and alternative therapy for anxiety.
Case 2
[The 36-year-old woman with altered mental status.]
When the EP entered the patient’s room, he witnessed the patient staring at her husband and striking him repetitively with her right arm. When the EP asked the patient to stop hitting, her husband told the EP that everything was alright and that the patient’s neurologist had previously told them this behavior was caused by a seizure. While in the next examination room, one of the EP’s colleagues had overheard some of the patient’s history and recognized the name of the patient’s neurologist as a specialist in partial complex seizures—one who had retired from the local medical school about 10 years ago.
After records from the local university hospital confirmed the patient’s diagnosis of partial complex seizures, she was given intravenous lorazepam 2 mg; she became alert, conversational, and stopped flailing her right arm. She was then admitted to the hospital for medical stabilization of her frequent seizures.
Editor’s Note: Part 2 of this article will appear in the June 2016 issue of Emergency Medicine and will cover psychiatric presentations related to dementia, cancer, cardiac disease, nutritional deficiencies, endocrine disorders, and toxins.
The chaos of a busy ED can test the cognitive reserve of even the most focused practitioner. To streamline the challenge of serial diagnosis and treatment, clinicians employ heuristics while honing the skills of pattern recognition. However, by definition, heuristics employs shortcuts, leaving out information for the sake of efficiency—sometimes at the expense of accuracy. Whether a patient presents with chest pain, abdominal pain, headache, or (the dreaded) dizziness, emergency physicians (EPs) employ algorithms based on a combination of education and prior experience.
Most of the time, these models lead the EP along the correct path, but not always. For example, when a clinician evaluating a patient presenting with psychotic behavior assumes the patient has schizophrenia, he or she will be correct eight or nine times out of 10. However, in some cases, a patient’s bizarre behavior may not be due to a true psychiatric disorder but, for example, from ingestion of an illicit substance.
In addition, in such patients, psychiatric symptoms may be masking a serious acute, organic condition—one requiring prompt intervention and therapy to avoid morbidity or death. To help prevent diagnostic errors, this 2-part series reviews several of the most common medical mimics of psychiatric conditions. Part 1 of this series reviews the psychiatric presentations associated with medical conditions of an infectious, pharmacological withdrawal, metabolic, autoimmune, traumatic, or central nervous system etiology (Table 1). This article also discusses clinical signs and symptoms that suggest an increased likelihood that a patient’s psychiatric symptoms are from an underlying medical condition (Table 2).
Case Scenarios
Case 1
A 58-year-old woman with a history of smoking 40 packs of cigarettes per year presented to the ED 1 hour after onset of intermittent chest pain. Upon arrival at the ED, the patient stated that she had trouble catching her breath on and off throughout the day. The patient’s vital signs, electrocardiogram (ECG), and chest X-ray were all normal. The physical examination was unremarkable except for mild diaphoresis. The patient denied experiencing palpitations, recent travel, or previous episodes; she further stated that she was currently not on any medications. There was no previous history of visits to this hospital. The patient’s husband, who accompanied her to the ED, noted that his wife’s behavior had been atypical for approximately 1 week.
After receiving aspirin, the patient appeared symptom-free. Pending the results of another chest radiograph and laboratory evaluation, the EP anticipated moving her to the chest-pain observation unit.
Case 2
A 36-year-old woman presented with altered mental status to the ED via emergency medical services (EMS). Her vital signs, including temperature, were normal. Despite intermittently appearing to be asleep, the patient was alternatingly cooperative and combative. She repetitively whispered, “Who am I?” and randomly shouted at staff members as they walked by her room.
Her neurological examination was nonfocal. The hospital’s electronic medical record (EMR) for this patient showed nearly monthly ED visits for behavioral symptoms. Precipitating events noted in the EMR included job loss and separation from her husband. While waiting for the results of the basic laboratory work-up and toxicology screening to medically clear the patient for psychiatric evaluation, the EP contemplated a computed tomography (CT) study. Realizing the patient would not be able to remain still for the scan, the EP ordered 10 mg of intramuscular ziprasidone for sedation. When the patient’s husband arrived, the EP placed the CT scan on hold until she could obtain additional history from him.
Infections
Herpes Simplex Encephalitis
Herpes simplex encephalitis (HSE) is a serious but treatable disease—one that requires early detection and treatment to avoid severe morbidity. While the classic symptoms are fever and altered mental status, recent literature has noted that afebrile patients with HSE may present with behavioral changes, cognitive decline, aggression, and disinhibition. Therefore, diagnosis of a functional psychiatric complaint, if made initially, could delay appropriate treatment with acyclovir.1
Human Immunodeficiency Virus
Progression of human immunodeficiency virus (HIV) is a well-known cause of various neurocognitive disorders, including early-onset dementia. Since the availability of highly active antiretroviral therapy, the incidence of HIV dementia has decreased, but HIV remains the most common preventable cause of dementia in persons younger than age 50 years. Recent literature has described HIV dementia presenting as an early-onset, rapidly progressing dementia in a young person. Thus, the EP should consider early HIV testing in any young patient who presents with dementia, especially one with a history of fever of unknown origin.2
Progressive Multifocal Encephalopathy
Caused by reactivation of the John Cunningham virus, progressive multifocal encephalopathy has been classically described as a potentially lethal complication of a severely immunocompromised state, often presenting with clumsiness, weakness, visual changes, speech difficulty, and behavioral changes. Though typically described as occurring in the context of acquired immunodeficiency disease syndrome, hematological malignancy, or organ transplant, the condition can occur in the setting of minimal or occult immunosuppression—especially in patients with a history of cirrhosis. If the condition is detected early, immunotherapy can result in significant clinical improvement.3
Syphilis
Late stages of syphilis can present with a wide variety of psychiatric symptoms, including personality disorder, psychosis, delirium, and dementia. As with HIV, there has been a resurgence of syphilis cases, and screening is now often a routine part of a neuropsychiatric work-up. The EP should consider syphilis in the differential for any new-onset psychiatric complaint.4,5
Typhoid Fever
Although this severe febrile illness is uncommon in the United States, it is endemic to many tropical countries within Africa, Southeast Asia, and Central and South America. Typhoid is characterized by a stepwise fever that can progress to abdominal distension, toxemia, and potentially bowel perforation. It is also known to present with psychiatric symptoms such as acute confusion, psychosis, generalized anxiety disorder, and, though rare, depressive disorder. Physicians traveling to rural endemic areas should be aware of these neuropsychiatric presentations to avoid misdiagnosis and delay of treatment.6 Other infectious endemic diseases with reports of neuropsychiatric components are neurocystercercosis, Lyme disease, and African trypanosomiasis.
Pharmacological Withdrawal Syndromes
Alcohol
Alcohol withdrawal is a common presentation in the ED, and up to 24% of US adults brought to the ED by EMS suffer from alcoholism. Typically characterized by tachycardia, hypertension, and tremors, alcohol withdrawal syndrome can also feature psychiatric components such as agitation, hallucinations, persecutory delusions, and even self-mutilation.7 Evidence-based protocols indicate loading doses of benzodiazepines as a mainstay of treatment, with supplemental barbiturates or propofol in cases of treatment failure.8
Benzodiazepines
Withdrawal from therapeutic doses of benzodiazepines can potentially cause psychiatric symptoms, including sleep disturbances, irritability, anxiety, panic attacks, tremor, and perceptual changes. Withdrawal from higher doses of benzodiazepines can lead to more serious presentations, such as seizures and acute psychosis.9 Withdrawal symptoms can develop from discontinuation of the drug and with non-tapered switching between benzodiazepines.10
Opiates
Opiate withdrawal is an unpleasant experience characterized by generalized pain, nausea and vomiting, sweating, and tachycardia. Neuropsychiatric complaints such as anxiety, agitation, and irritability can also be present. More severe agitation has been described in naltrexone-accelerated detoxification.11
Cannabis
Recent literature on cannabis use indicates a high prevalence and clinical significance of associated withdrawal symptoms in frequent users. There appear to be two subsets of cannabis withdrawal—one characterized by weakness and hypersomnia, and the other by anxiety, depression, restlessness, and insomnia.12
Estrogen
Withdrawal from endogenous estrogen has been hypothesized as a possible cause of puerperal psychosis.13 Estrogen withdrawal outside of this setting, however, can and does occur, and recent literature has shown episodes of reversible psychosis associated with the discontinuation of both oral contraceptive regimens and hormonal therapy for menopausal symptoms.
Acute Metabolic Conditions
Hypoglycemia
Hypoglycemia, most often encountered as a side effect of insulin or oral hypoglycemic therapies, is a potentially lethal cause of confusion, anxiety, nervousness, and seizures. Nocturnal hypoglycemia can manifest as nightmares, crying out, and confusion upon awakening. A fingerstick blood-glucose test is an absolutely vital part of the initial work-up of any patient with an altered mental status or overt psychiatric complaint.14
Central Pontine Myelinolysis
A potentially devastating neurological condition associated with malnourishment and alcohol dependence, central pontine myelinolysis (CPM) is classically exacerbated by rapid overcorrection of hyponatremia. While the disease can manifest primarily with quadriplegia or pseudobulbar palsy and eventual progress to the dreaded “locked-in” syndrome, early presentations can include psychiatric symptoms such as behavioral changes, psychosis, and cognitive disturbances. Patients with early signs and symptoms of CPM have been misdiagnosed as having schizophrenia with catatonia, leading to delayed treatment and poor outcomes. The EP should remain vigilant when evaluating for this condition and consider a magnetic resonance imaging study in patients with psychiatric symptoms in the setting of fluctuating hyponatremia.15
Autoimmune Disorders
Systemic Lupus Erythematosus
Systemic lupus erythematosus (SLE) is one of the most common autoimmune disorders, and has a higher incidence in young women. The disease affects multiple organ systems. Though the classic initial presentation of SLE is fever, joint pain, and rash, the associated neuropsychiatric syndromes of this disease are diverse and surprisingly common, and can be the initial manifestation of the disease. Common psychiatric manifestations of SLE include cognitive dysfunction, anxiety, mood disorders such as depression, acute confusion, psychosis, paranoia, and auditory or visual hallucinations.16
Anti-N-methyl-D-Aspartate Receptor Encephalitis
Initially described as a paraneoplastic effect of ovarian teratomas, anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis is actually an autoimmune disorder that can occur even in the absence of a primary tumor. As with SLE, the condition primarily occurs in young women. Antibodies in the cerebrospinal fluid cause prominent psychiatric symptoms such as acute psychosis, delusional thinking, hallucinations, agitation, and confusion. Although the disease can progress to seizures, movement disorders, autonomic dysregulation, and ultimately death, early recognition and treatment can lead to positive outcomes in up to 80% of cases.17 While the prevalence of anti-NMDAR antibodies in new-onset psychosis remains unclear, recent literature has suggested widespread screening for the disease in all first presentations of psychotic episodes.18,19
Multiple Sclerosis
Multiple sclerosis (MS) is another autoimmune disorder that has a higher prevalence in young women. The disease is characterized by central nervous system involvement that occurs over a period of months to years, with symptoms corresponding to different anatomic locations. Though the classic presenting symptom of MS is optic neuritis, neuropsychiatric syndromes are a common co-occurrence and can be the initial presenting symptom. The most commonly associated psychiatric complaints are anxiety, depression, and bipolar disorder, though case reports of SLE have described acute psychosis, psychotic depression, and adult-onset tic disorder.20
Trauma
Subarachnoid Hemorrhage
Long-term psychiatric sequelae from subarachnoid hemorrhage, either traumatic or aneurysmal, manifest most commonly as personality changes, intellectual impairment, depression, and anxiety. This condition is also known to cause a host of more bizarre psychiatric presentations, such as new-onset kleptomania, akinetic mutism, confabulatory amnesia, acute psychosis, and Capgras syndrome (the delusion that familiar individuals have been replaced by imposters). These symptoms can occur at initial presentation, and may show variable improvement with shunt surgery.21
Subdural Hematoma
Acute or chronic subdural hematoma can result from major head trauma, or even quite minor head trauma in an elderly or coagulopathic patient. Some common psychiatric manifestations of subdural hematoma include cognitive impairment, withdrawn behavior, blunted affect otherwise mimicking schizophrenic psychosis, and catatonia. The EP should consider early imaging studies in patients with new-onset psychotic symptoms—especially when they are refractory to typical antipsychotics.22
Central Nervous Symptom Diseases
Huntington Disease
Huntington disease (HD) is an autosomal dominant inherited, progressive neurodegenerative disorder characterized by mental decline, mood disorder, and muscle coordination problems that eventually become the classic involuntary writhing termed chorea. Due to its progressive nature, precise onset of the disease is difficult to describe; however, HD can manifest initially as schizophrenia-like psychotic episodes with only minimal apparent motor difficulty. Family history, including movement disorders and suicide, is important to obtain when available.23
Parkinson Disease
A progressive and disabling neurodegenerative disorder, Parkinson disease (PD) is classically characterized by fine resting tremor, cogwheeling rigidity, akinesia and mask-like facies, and postural instability. Comorbidity of psychiatric disorders is high, both as a result of the underlying disease process and as a side effect of dopaminergic treatment regimes. Common presentations of psychiatric disorders in PD include schizophrenia-like psychosis with visual hallucinations and mood disorders with prominent apathy and executive dysfunction. Recognition of the comorbidity is important because psychiatric disorders in PD respond differently to treatment than classic psychiatric disorders.24
Temporal Lobe Epilepsy
Epilepsy is a complex group of related neurological disorders involving unregulated nerve cell firing with a large variability in clinical presentation. Characteristically there is recurrent seizure activity. Temporal lobe epilepsy (TLE) is a subset of epilepsy known to present as a number of behavioral and neuropsychiatric complaints. Most presentations of TLE involve auras of emotional phenomena such as depression, fear, or anxiety, which can occur alone or with subsequent progression to complex partial or secondary generalized seizures.25 Many other bizarre presentations of TLE have been reported, including recurrent, potentially debilitating déjà vu, vivid recollection of past traumatic events mimicking posttraumatic stress disorder, paranoid delusions following olfactory triggers; and unprovoked attacks of depersonalization, derealization, anxiety, and dyspnea originally misdiagnosed as panic attack.
Stroke
The term “stroke chameleon” refers to presentations suggestive of other diseases that actually represent underlying strokes. Altered mental status is by far the largest block of these chameleons, with up to 30% of misdiagnosed strokes being misdiagnosed as altered mental status. The positive predictive value of altered mental status alone (ie, the chance that the diagnosis of altered mental status actually represents an undiagnosed acute stroke) is 7%.26
Case Scenarios Continued
Case 1
[The 58-year-old woman with intermittent chest pain.]
The patient’s D-dimer and troponin I levels were normal. Before the EP had an opportunity to discuss the results and next steps with the patient, the nurse asked him to see the patient immediately. Upon entering her room, the EP noted that the patient appeared anxious. The patient said the shortness of breath had returned, and also that she felt as if she were “floating” off the gurney, outside of her body. A check of her vital signs revealed a heart rate of 106 beats/minute and blood pressure of 160/100 mm Hg. A repeat ECG was significant only for sinus tachycardia. In an effort to calm the patient, the EP reassured her that the ECG, chest X-ray, and screening laboratory studies were normal, and that there was no evidence of a heart attack. Relieved, the patient asked for an Ativan to calm her nerves. Upon further questioning, the patient sheepishly reported that she had been taking 3 to 6 mg lorazepam for about 10 years, as prescribed by her family physician (FP) for anxiety. She further admitted that she abruptly discontinued taking the drug about one week before this ED visit after she’d heard on a daytime TV show that the medication was addictive.
After receiving lorazepam, the patient showed marked improvement. The EP’s final impressions were atypical chest pain and acute panic attack precipitated by abrupt benzodiazepine withdrawal. After discussing the case with the patient’s FP, the EP discharged the patient home with instructions to complete the cardiac evaluation as an outpatient. The EP also recommended that the patient resume taking lorazepam and follow-up with her FP within one week to discuss a benzodiazepine taper and alternative therapy for anxiety.
Case 2
[The 36-year-old woman with altered mental status.]
When the EP entered the patient’s room, he witnessed the patient staring at her husband and striking him repetitively with her right arm. When the EP asked the patient to stop hitting, her husband told the EP that everything was alright and that the patient’s neurologist had previously told them this behavior was caused by a seizure. While in the next examination room, one of the EP’s colleagues had overheard some of the patient’s history and recognized the name of the patient’s neurologist as a specialist in partial complex seizures—one who had retired from the local medical school about 10 years ago.
After records from the local university hospital confirmed the patient’s diagnosis of partial complex seizures, she was given intravenous lorazepam 2 mg; she became alert, conversational, and stopped flailing her right arm. She was then admitted to the hospital for medical stabilization of her frequent seizures.
Editor’s Note: Part 2 of this article will appear in the June 2016 issue of Emergency Medicine and will cover psychiatric presentations related to dementia, cancer, cardiac disease, nutritional deficiencies, endocrine disorders, and toxins.
1. Boyapati R, Papadopoulos G, Olver J, Geluk M, Johnson PD. An unusual presentation of herpes simplex virus encephalitis. Case Rep Med. 2012;241710.
2. Verma R, Anand KS. HIV presenting as young-onset dementia. J Int Assoc Provid AIDS Care. 2014;13(2):110-112.
3. Gheuens S, Pierone G, Peeters P, Koralnik IJ. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J Neurol Neurosurg Psychiatry. 2010;81(3):247-254.
4. Sobhan T, Rowe HM, Ryan WG, Munoz C. Unusual case report: three cases of psychiatric manifestations of neurosyphilis. Psychiatr Serv. 2004;55(7):830-832.
5. Noblett J, Roberts E. The importance of not jumping to conclusions: syphilis as an organic cause of neurological, psychiatric and endocrine presentations. BMJ Case Rep. 2015;25:2015.
6. Ukwaja KN. Typhoid fever presenting as a depressive disorder—a case report. Rural Remote Health. 2010;10(2):1276.
7. Patra BN, Sharma A, Mehra A, Singh S. Complicated alcohol withdrawal presenting as self mutilation. J Forensic Leg Med. 2014;21:46-47.
8. Stehman CR, Mycyk MB. A rational approach to the treatment of alcohol withdrawal in the ED. Am J Emerg Med. 2013;31(4):734-742.
9. Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455-1459.
10. Bosshart H. Withdrawal-induced delirium associated with a benzodiazepine switch: a case report. J Med Case Rep. 2011;5:207207.
11. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567. doi:10.1177/0960327112450901
12. Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF. Cannabis withdrawal in the United States: results from NESARC. J Clin Psychiatry. 69(9):1354-1363.
13. Okazaki Y. The epidemiology and pathogenesis of postpartum depression. Nihon Rinsho. 2001;59(8):1555-1559.
14. Sinert R, Su M, Secko M, Zehtabchi S. The utility of routine laboratory testing in hypoglycaemic emergency department patients. Emerg Med J. 2009;26(1):28-31.
15. Schneider P, Nejtek VA, Hurd CL. A case of mistaken identity: alcohol withdrawal, schizophrenia, or central pontine myelinolysis? Neuropsychiatr Dis Treat. 2012;8:49-54.
16. Stojanovich L, Zandman-Goddard G, Pavlovich S, Sikanich N. Psychiatric manifestations in systemic lupus erythematosus. Autoimmun Rev. 2007;6(6):421-426.
17. Kayser MS, J Dalmau. Anti-NMDA receptor encephalitis, autoimmunity, and psychosis. Schizophr Res. 2014;pi:S0920-9964(14)00546-5.
18. Tidswell J, Kleinig T, Ash D, Thompson P, Galletly C. Early recognition of anti-N-methyl D-aspartate (NMDA) receptor encephalitis presenting as acute psychosis. Australas Psychiatry. 2013;21(6):596-599.
19. Masopust J, Andrýs C, Bažant J, Vyšata O, Kuca K, Vališ M. Anti-NMDA receptor antibodies in patients with a first episode of schizophrenia. Neuropsychiatr Dis Treat. 2015;11:619-623.
20. de Cerqueira AC, Semionato de Andrade P, Godoy Barreiros JM, Teixeira AL, Nardi AE. Psychiatric disorders in patients with multiple sclerosis. Compr Psychiatry. 2015;63:10-14.
21. Mobbs RJ, Chandran KN, Newcombe RL. Psychiatric presentation of aneurysmal subarachnoid haemorrhage. ANZ J Surg. 2001;71(1):69-70.
22. Kar SK, Kumar D, Singh P, Upadhyay PK. Psychiatric manifestation of chronic subdural hematoma: the unfolding of mystery in a homeless patient. Indian J Psychol Med. 2015;37(2):239-242.
23. Nagel M, Rumpf HJ, Kasten M. Acute psychosis in a verified Huntington disease gene carrier with subtle motor signs: psychiatric criteria should be considered for the diagnosis. Gen Hosp Psychiatry. 2014;36(3):361.e3-e4.
24. Buoli M, Caldiroli A, Altamura AC. Psychiatric conditions in Parkinson disease: a comparison with classical psychiatric disorders. J Geriatr Psychiatry Neurol. 2016;29(2):72-91.
25. Bortz JJ. Neuropsychiatric and memory issues in epilepsy.” Mayo Clin Proc. 2003;78(6):781-787.
26. Dupre CM, Libman R, Dupre SI, Katz JM, Rybinnik I, Kwiatkowski T. Stroke chameleons. J Stroke Cerebrovasc Dis. 2014;23(2):374-378.
1. Boyapati R, Papadopoulos G, Olver J, Geluk M, Johnson PD. An unusual presentation of herpes simplex virus encephalitis. Case Rep Med. 2012;241710.
2. Verma R, Anand KS. HIV presenting as young-onset dementia. J Int Assoc Provid AIDS Care. 2014;13(2):110-112.
3. Gheuens S, Pierone G, Peeters P, Koralnik IJ. Progressive multifocal leukoencephalopathy in individuals with minimal or occult immunosuppression. J Neurol Neurosurg Psychiatry. 2010;81(3):247-254.
4. Sobhan T, Rowe HM, Ryan WG, Munoz C. Unusual case report: three cases of psychiatric manifestations of neurosyphilis. Psychiatr Serv. 2004;55(7):830-832.
5. Noblett J, Roberts E. The importance of not jumping to conclusions: syphilis as an organic cause of neurological, psychiatric and endocrine presentations. BMJ Case Rep. 2015;25:2015.
6. Ukwaja KN. Typhoid fever presenting as a depressive disorder—a case report. Rural Remote Health. 2010;10(2):1276.
7. Patra BN, Sharma A, Mehra A, Singh S. Complicated alcohol withdrawal presenting as self mutilation. J Forensic Leg Med. 2014;21:46-47.
8. Stehman CR, Mycyk MB. A rational approach to the treatment of alcohol withdrawal in the ED. Am J Emerg Med. 2013;31(4):734-742.
9. Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89(11):1455-1459.
10. Bosshart H. Withdrawal-induced delirium associated with a benzodiazepine switch: a case report. J Med Case Rep. 2011;5:207207.
11. Hassanian-Moghaddam H, Afzali S, Pooya A. Withdrawal syndrome caused by naltrexone in opioid abusers. Hum Exp Toxicol. 2014;33(6):561-567. doi:10.1177/0960327112450901
12. Hasin DS, Keyes KM, Alderson D, Wang S, Aharonovich E, Grant BF. Cannabis withdrawal in the United States: results from NESARC. J Clin Psychiatry. 69(9):1354-1363.
13. Okazaki Y. The epidemiology and pathogenesis of postpartum depression. Nihon Rinsho. 2001;59(8):1555-1559.
14. Sinert R, Su M, Secko M, Zehtabchi S. The utility of routine laboratory testing in hypoglycaemic emergency department patients. Emerg Med J. 2009;26(1):28-31.
15. Schneider P, Nejtek VA, Hurd CL. A case of mistaken identity: alcohol withdrawal, schizophrenia, or central pontine myelinolysis? Neuropsychiatr Dis Treat. 2012;8:49-54.
16. Stojanovich L, Zandman-Goddard G, Pavlovich S, Sikanich N. Psychiatric manifestations in systemic lupus erythematosus. Autoimmun Rev. 2007;6(6):421-426.
17. Kayser MS, J Dalmau. Anti-NMDA receptor encephalitis, autoimmunity, and psychosis. Schizophr Res. 2014;pi:S0920-9964(14)00546-5.
18. Tidswell J, Kleinig T, Ash D, Thompson P, Galletly C. Early recognition of anti-N-methyl D-aspartate (NMDA) receptor encephalitis presenting as acute psychosis. Australas Psychiatry. 2013;21(6):596-599.
19. Masopust J, Andrýs C, Bažant J, Vyšata O, Kuca K, Vališ M. Anti-NMDA receptor antibodies in patients with a first episode of schizophrenia. Neuropsychiatr Dis Treat. 2015;11:619-623.
20. de Cerqueira AC, Semionato de Andrade P, Godoy Barreiros JM, Teixeira AL, Nardi AE. Psychiatric disorders in patients with multiple sclerosis. Compr Psychiatry. 2015;63:10-14.
21. Mobbs RJ, Chandran KN, Newcombe RL. Psychiatric presentation of aneurysmal subarachnoid haemorrhage. ANZ J Surg. 2001;71(1):69-70.
22. Kar SK, Kumar D, Singh P, Upadhyay PK. Psychiatric manifestation of chronic subdural hematoma: the unfolding of mystery in a homeless patient. Indian J Psychol Med. 2015;37(2):239-242.
23. Nagel M, Rumpf HJ, Kasten M. Acute psychosis in a verified Huntington disease gene carrier with subtle motor signs: psychiatric criteria should be considered for the diagnosis. Gen Hosp Psychiatry. 2014;36(3):361.e3-e4.
24. Buoli M, Caldiroli A, Altamura AC. Psychiatric conditions in Parkinson disease: a comparison with classical psychiatric disorders. J Geriatr Psychiatry Neurol. 2016;29(2):72-91.
25. Bortz JJ. Neuropsychiatric and memory issues in epilepsy.” Mayo Clin Proc. 2003;78(6):781-787.
26. Dupre CM, Libman R, Dupre SI, Katz JM, Rybinnik I, Kwiatkowski T. Stroke chameleons. J Stroke Cerebrovasc Dis. 2014;23(2):374-378.
10 tips for overcoming common challenges of intrapartum fetal monitoring
Interpreting continuous fetal heart rate (FHR) monitoring is one of the most common tasks obstetricians perform during the course of intrapartum care. Notably, many providers do not seek ongoing training to optimize their ability to reliably and accurately interpret the FHR. Yet FHR interpretation is one of the most frequent causes of litigation in the modern obstetric practice. Failure to interpret continuous FHR monitoring appropriately is estimated to account for 75% of obstetric-related litigation.1
Continuous FHR monitoring during labor was introduced to identify infants at risk for developing hypoxic-ischemic encephalopathy (HIE). The rate of HIE has not declined, however, despite almost universal adoption of continuous FHR monitoring.2 Numerous reasons account for this failure, including ad hoc interpretation of terminology, lack of standardized protocols for management and intervention, and the oftentimes challenging patterns that must be interpreted.3 The confusion about and dissatisfaction with the current state of FHR monitoring has led to attempts to enhance our ability to identify infants at risk with additional approaches (such as fetal pulse oximetry and fetal ST-segment evaluation), and some have called for a complete overhaul of our approach to interpreting the FHR. Clark and colleagues stated recently, "It is time to start over and establish some common language, standard interpretation, and reasonable management principles and guidelines."3
We must recognize that, as a stand-alone tool, continuous FHR monitoring is ineffective for avoiding preventable adverse outcomes. It is most likely to be effective when used in accordance with published standard guidelines by professionals skilled in interpretation and when timely, appropriate interventions are performed based on that interpretation. Optimal FHR monitoring requires a collaborative perinatal team that performs the monitoring correctly, interprets it appropriately, and communicates the findings effectively, and in a timely fashion, to all members of the care team when a high-risk pattern is detected.
In this article we review some common challenges that clinicians encounter during intrapartum FHR monitoring and we offer 10 simple tips to help overcome these challenges. The clinical scenarios described are derived from published reports in the medical literature, published malpractice claims, and from our personal experience working in a major health care system as part of a team charged with overseeing ongoing certification and training of labor and delivery nurses.
Challenge: Signal ambiguity
CASE 1 Young woman in labor with first pregnancy
A 19-year-old woman presents in spontaneous labor with her first pregnancy, which has been uncomplicated. During the course of her care, it is noted that the FHR changes to a lower baseline than previously recorded. Evaluation reveals that the external monitor is tracking the maternal heart rate and not the FHR (FIGURE 1). After the monitor is adjusted, both the fetal and maternal rates are documented for a short period. Ultimately, continuous monitoring of the maternal heart rate is discontinued. After delivery of the infant several hours later, it is noted that the FHR continues to register on the monitor, and it is determined that for the last few hours the maternal heart rate has been traced.
| FIGURE 1 FHR tracing indicates signal ambiguity | ||
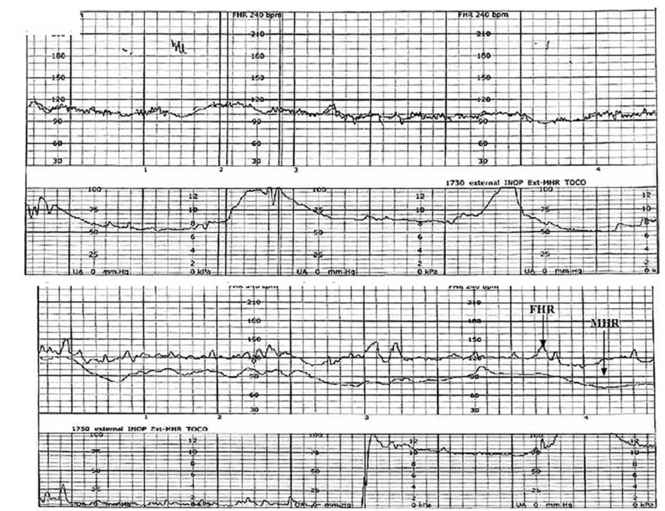 | ||
As described in Case 1, the upper panel of this tracing demonstrates the maternal heart rate confused as the fetal heart rate, while the segment in the lower panel shows a clear distinction between the maternal and fetal heart rates. | ||
TIP #10: Ensure the FHR monitor is tracking the fetal, not the maternal, heart rate
Confusing the maternal and the fetal heart rate with external cardiotocography is common. When the mix-up is noted and corrected expeditiously, it is unlikely to result in an adverse outcome. Signal ambiguity may arise from faulty Doppler equipment or the inability of the cardiotocograph to differentiate between maternal and fetal heart rates. It commonly occurs after repositioning the patient, after fetal movement, or during pushing in the second stage when the maternal heart rate may increase to a baseline that is similar to that of the fetus.
Signal ambiguity should be suspected when the FHR runs in the low-normal range or when FHR accelerations are noted with greater than 50% of contractions (especially when pushing).4 Signal ambiguity also should be ruled out when there is an apparent FHR deceleration to the maternal range that does not recover.
Evaluating for suspected signal ambiguity involves 2 key steps: (1) documentation and verification of the maternal heart rate and (2) definitive documentation of the true FHR. To document the maternal heart rate, manually count the radial pulse for 1 minute or use a pulse oximeter for continuous monitoring. Using a pulse oximeter is a less labor-intensive approach and has the advantage of allowing continuous assessment of the maternal heart rate for comparison. Recording the maternal pulse continuously on the same screen as the FHR enables ongoing differentiation of the mother and fetus in difficult cases, particularly if internal fetal monitoring is not an option (because of maternal infectious disease, low suspicion for an abnormal FHR pattern, or strong maternal preference against internal monitoring, for example).
When clinically appropriate, use of a fetal scalp electrode (FSE) can document the FHR. If intrauterine fetal death has occurred, however, the FSE may transmit the maternal heart rate.5 Using ultrasonography to confirm the FHR prior to placing the FSE is a reliable method of definitive differentiation. If a newly placed FSE shows a clear differentiation of 5 to 10 beats per minute from a continuously assessed maternal pulse rate, then this is also a reliable way to assure that the FHR monitoring represents the fetus, particularly if ultrasonography is not immediately available.
Ultimately, before intervening based on an abnormal FHR tracing, it is paramount to confirm that the data are adequate for interpretation and represent the actual FHR. If signal ambiguity is identified or suspected, correct it by using ultrasonography to locate the FHR and replace the external monitor until a rate that is at least 5 to 10 beats per minute different from the maternal rate is obtained. Alternatively, this is an indication for internal fetal monitoring with an FSE.
Challenge: Inadequate FHR tracing, poor communication, lack of clinical context
CASE 2 Woman with uncomplicated postdates pregnancy presents for induction
A 28-year-old woman (G3P2) at 41 weeks 0 days of gestation presents to labor and delivery for induction of labor for the indication of postdates. There have been no complications with the current pregnancy. The initial cervical exam reveals 1+ cm dilation, 90% effacement, and −3 station, and the patient is started on oxytocin per the hospital protocol. What is your interpretation of the continuous FHR tracing shown in FIGURE 2?
| FIGURE 2 Inadequate, uninterpretable FHR tracing | ||
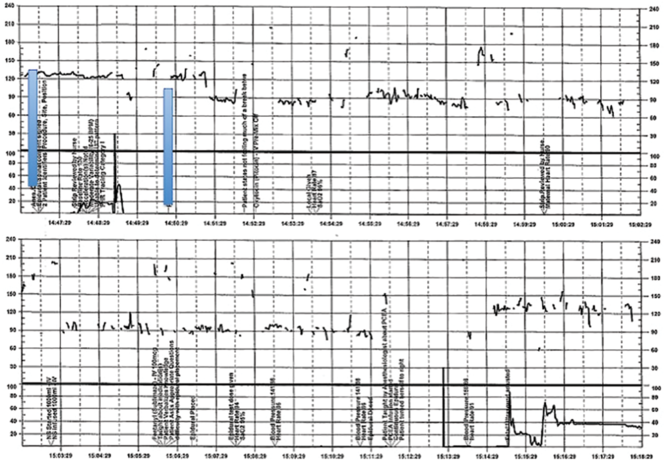 | ||
This FHR tracing, from the patient described in Case 2, is unusable because of the absence of data. | ||
TIP #9: Check that the monitors are providing useful data
The ability to accurately interpret a continuous FHR tracing depends on the quality of data recorded. Unfortunately, the absence of data makes interpretation impossible. This includes both FHR and tocometry data, since both pieces of information are required for appropriate interpretation of a continuous FHR tracing.
Prolonged periods of uninterpretable FHR and uterine activity tracings imply that no one was attending the mother and fetus.6 If it is difficult to obtain an interpretable FHR tracing, document in the medical record that you made ongoing efforts to maintain an adequate tracing, including the amount of time spent holding the external monitor, use of ultrasonography to document the FHR, and plans for potential internal monitoring.
CASE 2 Continued
After several hours, the patient requests an epidural for pain management and one is placed without difficulty. She reports adequate pain relief and is comfortable for the next 1 to 2 hours. Subsequently, the patient reports a sudden onset of increasing pain that does not respond to additional patient-administered doses of anesthesia over a 30-minute period. The labor and delivery nurse becomes concerned about the patient's pain level and contacts the attending physician to discuss her concerns. The physician, who is currently attending to patients in clinic, listens to the nurse and asks her to contact the anesthesia department with her concerns (FIGURE 3).
| FIGURE 3 FHR tracing reveals recurrent variables in a patient with evolving clinical concerns | ||
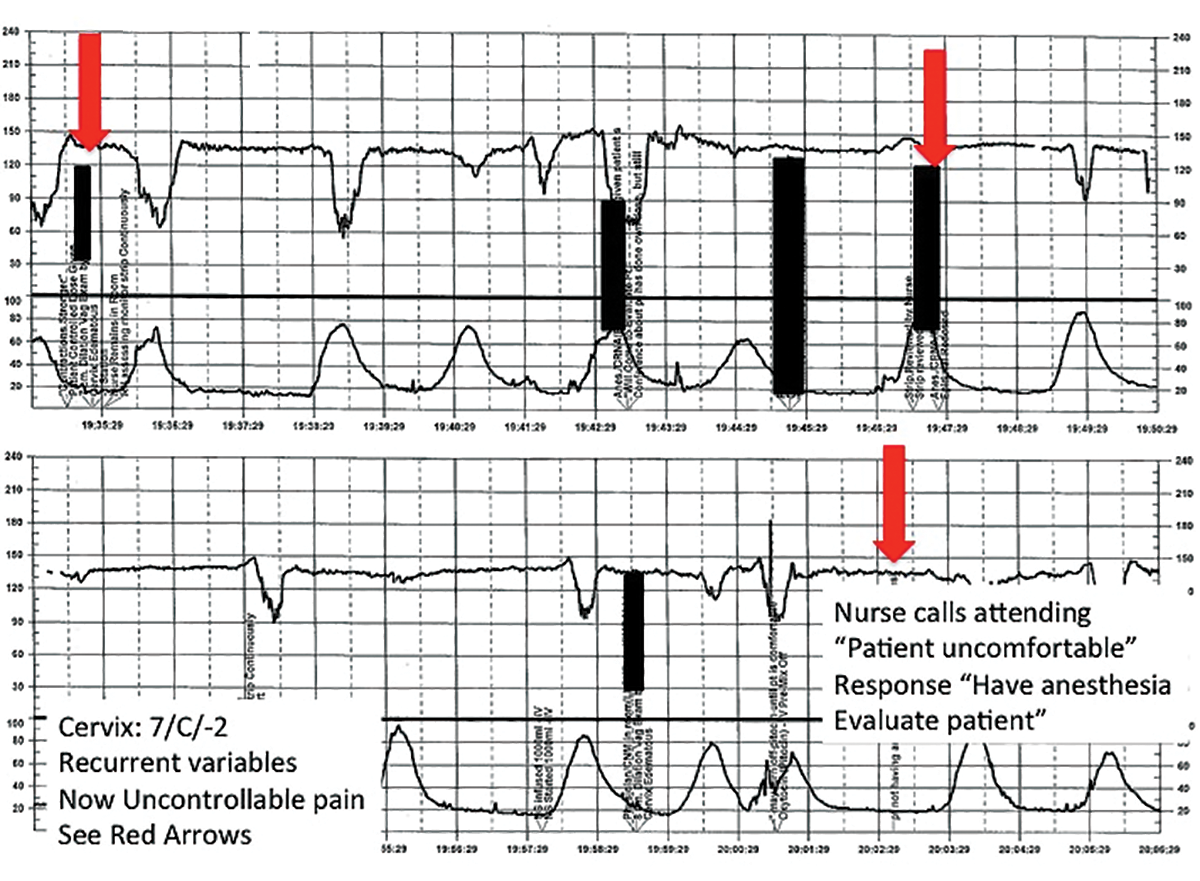 | ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #8: Clearly communicate an urgent situation to the care team
Poor communication underlies many preventable adverse outcomes in medicine.7 Effective communication requires an adequate description of the clinical scenario or problem. A root cause analysis of a series of intrapartum adverse events involving fetal death or injury showed that poor communication about a concerning FHR tracing played a role in 72% of cases.1
In this clinical scenario, the nurse believed that the patient's pain level was unusual or more than anticipated. The person who is communicating his or her concern (the sender) must be sure that the person receiving the message (the responder) clearly understands the sender's level of concern. In this case, it would have been appropriate for the sender to state clearly that she felt the patient's pain was outside of normal expectations and to request that the attending physician come to evaluate the patient.
Clear and effective communication includes (1) an appropriate description of the urgency of the situation and (2) an indication by the sender as to the desired response to this information ("please come evaluate the patient").8 In all cases, both steps are necessary to elicit an appropriate response.
CASE 2 Continued
Over the next 2 hours, recurrent variable decelerations develop, and then sudden, prolonged fetal bradycardia leads to urgent cesarean delivery. At delivery, a uterine rupture is diagnosed and a fetal hand is observed protruding through a lower-uterine segment defect into the maternal abdomen.
TIP #7: Always consider the entire clinical scenario
In this case, the team caring for the patient was not aware that her previous pregnancy had ended with a low transverse cesarean delivery. How does this information change your interpretation of the clinical scenario? The importance of understanding the entire clinical context when interpreting individual characteristics of cardiotocography cannot be overstated. For example, the sudden onset of recurrent, significant variable decelerations is more concerning in the context of a prior cesarean delivery, and late decelerations are more concerning in a patient with placental abruption, fetal growth restriction, or poorly controlled maternal diabetes.
An estimated 70% of fetuses will have an indeterminate FHR pattern (category II) at some time during labor.9 To appropriately interpret the FHR tracing, it is crucial to know the a priori risk for fetal hypoxia and metabolic acidosis (the precursor of fetal injury) due to such identified clinical risk factors as placental insufficiency, medical comorbidities (hypertension, diabetes), or postdates gestational age.
It is well established that cardiotocography has a good negative predictive value for the absence of fetal metabolic acidosis when there is moderate variability and spontaneous or induced accelerations. When attempting to risk stratify the fetus with a category II (indeterminate) FHR tracing, consider these 3 important questions:
- What are the risk factors for this particular patient and her fetus?
- What is the state of the fetus right now, and when was the last time metabolic acidosis could be excluded reasonably (by the presence of moderate variability and accelerations)?
- What is the risk that the fetus will develop acidemia prior to delivery?
The presence of decelerations indicates interruption of oxygen delivery to the fetus, and recurrent decelerations may indicate an evolving process of accumulated oxygen deprivation, hypoxia, and eventually, metabolic acidosis. Most authorities agree that, for the fetus with a previously normal FHR tracing, the onset of significant, recurrent decelerations with slowly cumulative oxygen deficit can lead to fetal acidemia over the course of approximately 1 hour.10 Of course, acidosis also can occur much more quickly with acute events, such as placental abruption or uterine rupture. In deciding whether or not to intervene based on an FHR tracing, the clinician must take into account the clinical context to determine if delivery is likely to occur before significant acidemia develops.
Challenge: Lack of situational awareness, failure to address nursing concerns, reluctance to initiate the chain of command
CASE 3 Spontaneous labor in a second pregnancy
A 28-year-old woman (G2P1) at 40 weeks' gestation presents in spontaneous labor. She has a history of a previous uncomplicated vaginal delivery. After 6 hours she reaches complete dilation with the fetus at −1 station and begins pushing. After 60 minutes, the patient has only progressed to +1 station. She is contracting every 1 to 2 minutes with recurrent variable decelerations (FIGURE 4).
| FIGURE 4 FHR tracing shows time points for initiation and continuation of pushing | ||
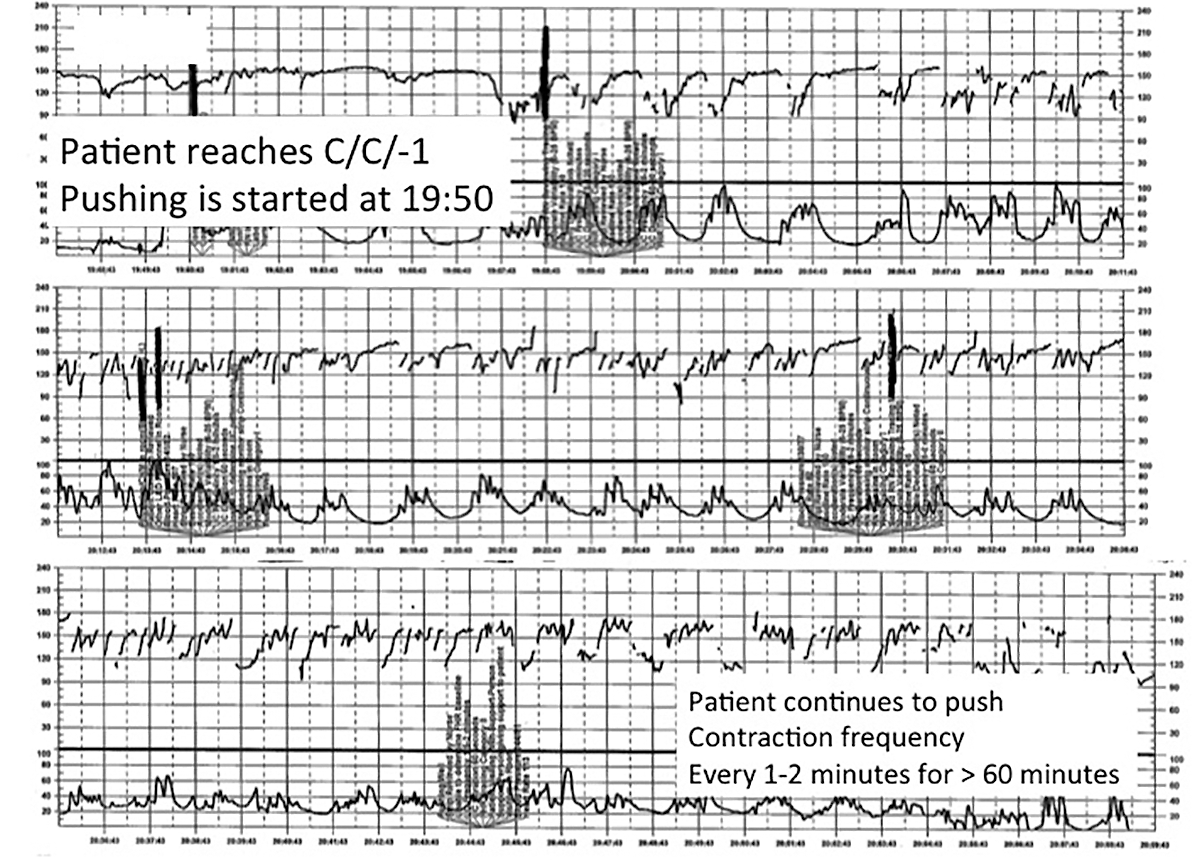 | ||
This tracing, from the patient described in Case 3, documents contraction frequency every 1-2 minutes for more than 60 minutes while the patient continues to push. The fetal heart rate demonstrates repetitive moderate variable decelerations with every push. | ||
TIP #6: Maintain situational awareness
A state of situational awareness exists when caregivers have a clear understanding of all of the factors at play in a clinical situation.11 This can be lost when caregivers focus too intensely on one aspect of care. It often happens when the patient is pushing in the second stage and the provider, focused on the progress of fetal descent, loses track of the amount of time that has passed without reassuring features (such as variability and induced or spontaneous accelerations) in the FHR tracing. The nurse, seeing the physician at the bedside, presumes he or she is aware of the tracing and is thus reluctant to point out the concerning features for fear of appearing insubordinate.
Situational awareness also may be lost at the time of patient hand off between providers wherein critical information, such as a history of previous cesarean delivery, is not communicated to the next care team. When receiving an intrapartum patient hand off, providers must have heightened vigilance to ensure they quickly reach situational awareness and are cognizant of the entire clinical context. Maintaining an environment in which all members of the care team, regardless of their training level, are encouraged to voice their concerns is another way to promote ongoing situational awareness.
CASE 3 Continued
The patient continues pushing for another 20 minutes without delivery, and the nurse raises a concern about the FHR tracing to the physician, who remains in the room but does not respond (FIGURE 5).
| FIGURE 5 FHR tracing reveals ongoing repetitive variable decelerations | ||
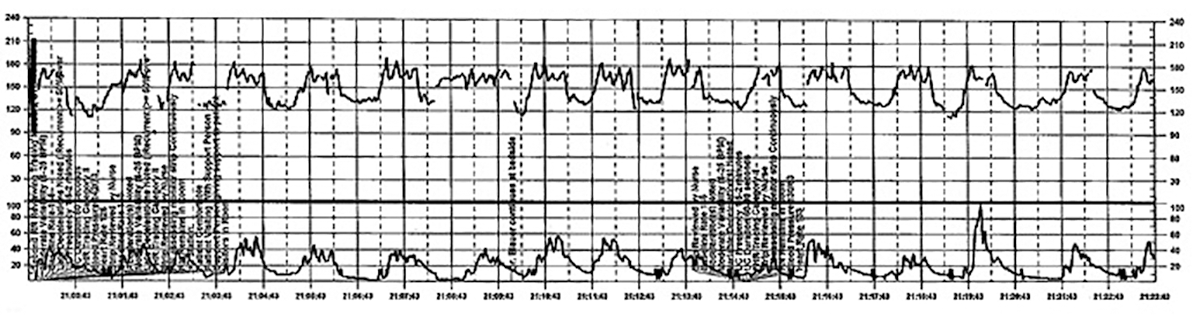 | ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #5: Acknowledge and respond to other caregivers' concerns
A team approach to patient care is essential in all areas of medicine, perhaps none more so than in obstetrics. Each member of the team is engaged in trying to provide optimal patient care and the concerns of every team member--regardless of title or level of training--must be acknowledged and addressed. Good communication requires creating a safe environment wherein each member of the team feels comfortable raising concerns without fear of reprisal. Rather than becoming angry or frustrated when questioned, providers should remain cognizant that these are ongoing efforts to maintain situational awareness and ensure the best possible outcome for mother and baby.
CASE 3 Continued
Pushing continues for another 30 minutes despite the nurse's repeated effort to express concern to the physician about the FHR tracing. After more than 2 hours of pushing, the infant is delivered; Apgar scores are 1, 5, and 7. No cord gas is obtained.
TIP #4: Initiate the chain of command when necessary
Any caregiver, regardless of job title, has a duty to initiate the institution's chain-of-command policy and procedure if he or she has a concern about patient well-being that is not being addressed adequately. It can be uncomfortable for a nurse, midwife, or resident physician to question an attending physician, particularly if that person responds in a dismissive, condescending, or angry manner. If a caregiver has made several attempts to engage the attending physician and feels the concerns are being inadequately addressed, then he or she must respectfully initiate the chain of command to seek additional objective review of the clinical situation.
Failure to follow oxytocin protocols, inadequate surveillance, poor documentation
CASE 4 Induction of an uncomplicated pregnancy due to postdates
A 20-year-old woman (G1P0) at 42 weeks' gestation with an otherwise uncomplicated first pregnancy presents for postdates induction with oxytocin. After 6 hours, she develops uterine tachysystole with recurrent variable decelerations but the oxytocin infusion is continued at the same rate (FIGURE 6).
| FIGURE 6 FHR tracing indicates uterine tachysystole | ||
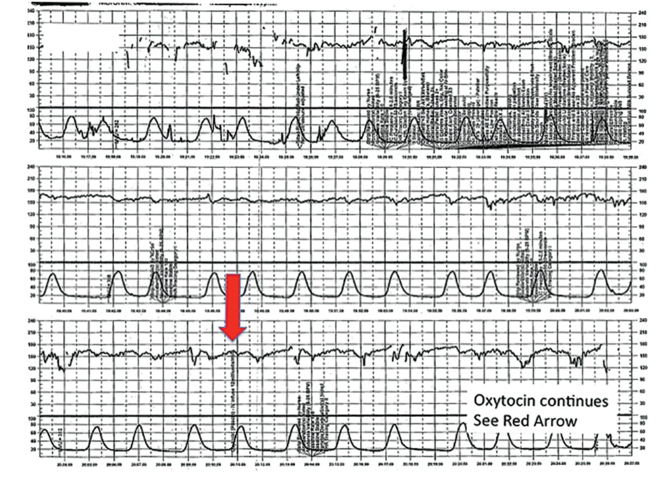 | ||
The patient in Case 4 received oxytocin for induction of postdates pregnancy. The red arrow shown on the FHR tracing points out that oxytocin augmentation continues despite the presence of uterine contractions that are too frequent and initial changes, including subtle late decelerations in the FHR, that suggest early fetal compromise. | ||
TIP #3: Manage oxytocin infusion according to protocol
Inappropriate use of oxytocin is common, including the improper management of oxytocin infusion in the setting of uterine tachysystole (defined as the presence of >5 contractions over a 10-minute period averaged over 30 minutes) and/or an abnormal FHR tracing. The mismanagement of uterine tachysystole is cited in more than two-thirds of obstetric malpractice cases.12
Uterine contractions alter blood flow through the spiral arteries and transiently reduce placental perfusion. Prolonged uterine tachysystole can lead to fetal oxygen debt and early signs of hypoxia, including the loss of spontaneous accelerations, tachycardia, and reduced variability. Continuing or increasing the oxytocin in the setting of such changes is hard to justify. One study found that the use of oxytocin in the setting of tachysystole was significantly associated with signs of fetal asphyxia (odds ratio [OR], 5.6).13 When the FHR pattern suggests significant interruption of fetal oxygen delivery and possible hypoxia, continuing or increasing an oxytocin infusion suggests a lack of understanding of the physiology that is the basis for FHR interpretation.
Appropriate management of tachysystole depends on the accompanying FHR.14 In the setting of a category I (normal) FHR tracing, tachysystole can be treated first with maternal repositioning (left or right lateral) and administration of a 500-cm3 maternal IV fluid bolus. If uterine activity does not return to normal after 10 to 15 minutes, decrease the oxytocin rate by at least half. If it does not return to normal after another 10 to 15 minutes, discontinue oxytocin until the tachysystole has resolved.
In the setting of a concerning category IIFHR tracing, discontinuation of oxytocin should be the first step along with maternal repositioning and administration of a fluid bolus. If these measures do not improve the FHR tracing and tachysystole persists, administration of an acute uterine relaxant, such as terbutaline, should be considered to slow contraction frequency.
If interventions result in normalization of the FHR tracing and resolution of tachysystole for 20 to 30 minutes, then oxytocin may be restarted if necessary for labor progress at no more than half the rate that produced tachysystole.
TIP #2: Recognize an abnormal FHR tracing--and what it means
Misinterpretation of the FHR tracing occurs when there is a failure to recognize characteristics that should raise concern about fetal well-being. Failure to recognize an abnormal FHR tracing occurred in 77% of sentinel cases involving intrapartum birth injury or death.1,12,13 To limit misinterpretation of the FHR tracing, it is critical for nurses and physicians to use standardized terminology for clear, effective communication.
In 2008, the Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) published guidelines standardizing the terminology used to describe cardiotocography and to create consensus around its interpretation.15 Any description of an intrapartum FHR tracing should include a designation of category (I, II, or III). Fetal well-being is reasonably established with a category I FHR tracing. A category III tracing indicates the high likelihood of fetal acidemia and the need for immediate intervention. A category II FHR tracing is considered indeterminate, and further characterization is required to reasonably exclude fetal metabolic acidosis and a risk of fetal injury.
The presence of moderate variability and fetal response to scalp stimulation are considered reassuring findings that reasonably exclude significant metabolic acidosis. In assessing variability, one pitfall is mistaking the appearance of "variability" within a deceleration (including during return to baseline) for baseline FHR variability. In the event of a persistent category II FHR tracing (>30 minutes), nursing staff should request direct physician review of the FHR tracing. In any case in which fetal well-being is uncertain, nursing staff should request direct physician evaluation of the mother in person and also the FHR tracing, with clear documentation of the findings, interpretation, and plan of care.16
TIP #1: Document, document, document
Nursing and physician documentation about the FHR tracing within the patient-specific clinical context is crucial for effective caregiver communication and patient safety. Thoughtful documentation also reduces liability exposure for providers by demonstrating maternal-fetal surveillance, early identification and treatment of an abnormal or indeterminate FHR tracing, and timely intervention on fetal behalf when necessary.
When the medical record aligns with the electronic FHR tracing and includes appropriate descriptions, interpretations, and interventions in line with national guidelines and institutional policy, the record demonstrates that the providers have a thorough understanding of the physiology behind cardiotocography and, more importantly, that they are able to apply that knowledge in clinical practice.6
Minimizing missteps
Several straightforward interventions can help clinicians overcome the most common pitfalls during FHR monitoring. These include accurate and high-quality cardiotocography, a collaborative team-based approach to patient care, and sustained situational awareness among providers. The consistent use of common language for the description and interpretation of FHR monitoring, adherence to hospital oxytocin protocols, and well-defined expectations for fetal surveillance and provider communication are critical to overcoming these challenges. Regularly scheduled nursing and physician education sessions and interdisciplinary case review can promote the adoption and sustained incorporation of these simple techniques into daily practice.3
Some have advocated for an "electronic fetal monitoring bundle," which would serve as a checklist of clinical evaluation steps that should occur every time a given process occurs.17 This approach would ensure that all providers on labor and delivery are qualified to read, accurately interpret, and respond to FHR tracings. It would require a credentialing process to confirm the competency of team members and reinforce the presence of a common language. It would also include an explicit escalation policy for rapid initiation of the chain of command in cases wherein there is a disagreement among team members about the FHR interpretation. Finally, each patient would be required to have, at all times, an identified responsible provider capable of a rapid response.
Although continuous FHR monitoring may not effectively reduce intrapartum fetal asphyxia, it is clearly here to stay. Recognizing--and addressing--the most common challenges encountered during intrapartum FHR monitoring may reduce unnecessary morbidity and potential liability for caregivers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Sentinel event alert issue 30--July 21, 2004. Preventing infant death and injury during delivery. Adv Neonatal Care. 2004;4(4):180–181.
- Shy KK, Luthy DA, Bennett FC, et al. Effects of electronic fetal-heart-rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. N Engl J Med. 1990;322(9):588–593.
- Clark SL, Nageotte MP, Garite TJ, et al. Intrapartum management of category II fetal heart rate tracings: towards standardization of care. Am J Obstet Gynecol. 2013;209(2):89–97.
- Neilson DR Jr, Freeman RK, Mangan S. Signal ambiguity resulting in unexpected outcome with external fetal heart rate monitoring. Am J Obstet Gynecol. 2008;198(6):717–724.
- McWhinney NA, Knowles S, Green HL, Gordon H. Transmission of the maternal electrocardiograph via a fetal scalp electrode in the presence of intrauterine death. Case report. Br J Obstet Gynaecol. 1984;91(10):1046–1048.
- Simpson KR, Knox GE. Risk management and electronic fetal monitoring: decreasing risk of adverse outcomes and liability exposure. J Perinat Neonatal Nurs. 2000;14(3):40–52.
- Gluck PA. Patient safety in women's health care: a framework for progress. Best Pract Res Clin Obstet Gynaecol. 2007;21(4):525–536.
- Lyndon A, Zlatnik MG, Wachter RM. Effective physician-nurse communication: a patient safety essential for labor and delivery. Am J Obstet Gynecol. 2011;205(2):91–96.
- Jackson M, Holmgren CM, Esplin MS, Henry E, Varner MW. Frequency of fetal heart rate categories and short-term neonatal outcome. Obstet Gynecol. 2011;118(4):803–808.
- Parer JT, Ikeda T. A framework for standardized management of intrapartum fetal heart rate patterns. Am J Obstet Gynecol. 2007;197(1):26.e1-e6.
- MacEachin SR, Lopez CM, Powell KJ, Corbett NL. The fetal heart rate collaborative practice project: situational awareness in electronic fetal monitoring--a Kaiser Permanente Perinatal Patient Safety Program Initiative. J Perinat Neonatal Nurs. 2009;23(4):314–323; quiz 24–25.
- Jonsson M, Norden SL, Hanson U. Analysis of malpractice claims with a focus on oxytocin use in labour. Acta Obstet Gynecol Scand. 2007;86(3):315–319.
- Berglund S, Pettersson H, Cnattingius S, Grunewald C. How often is a low Apgar score the result of substandard care during labour? BJOG. 2010;117(8):968–978.
- Doyle J, Kenny TH, Burkett AM, von Gruenigen VE. A performance improvement process to tackle tachysystole. J Obstet Gynecol Neonatal Nurs. 2011;40(5):512–519.
- Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661–666.
- Knox GE, Simpson KR, Garite TJ. High reliability perinatal units: an approach to the prevention of patient injury and medical malpractice claims. J Healthc Risk Manag. 1999;19(2):24–32.
- Minkoff H, Berkowitz R; Greater New York Hospital Association's Perinatal Safety C. Fetal monitoring bundle. Obstet Gynecol. 2009;114(6):1332–1335.
Interpreting continuous fetal heart rate (FHR) monitoring is one of the most common tasks obstetricians perform during the course of intrapartum care. Notably, many providers do not seek ongoing training to optimize their ability to reliably and accurately interpret the FHR. Yet FHR interpretation is one of the most frequent causes of litigation in the modern obstetric practice. Failure to interpret continuous FHR monitoring appropriately is estimated to account for 75% of obstetric-related litigation.1
Continuous FHR monitoring during labor was introduced to identify infants at risk for developing hypoxic-ischemic encephalopathy (HIE). The rate of HIE has not declined, however, despite almost universal adoption of continuous FHR monitoring.2 Numerous reasons account for this failure, including ad hoc interpretation of terminology, lack of standardized protocols for management and intervention, and the oftentimes challenging patterns that must be interpreted.3 The confusion about and dissatisfaction with the current state of FHR monitoring has led to attempts to enhance our ability to identify infants at risk with additional approaches (such as fetal pulse oximetry and fetal ST-segment evaluation), and some have called for a complete overhaul of our approach to interpreting the FHR. Clark and colleagues stated recently, "It is time to start over and establish some common language, standard interpretation, and reasonable management principles and guidelines."3
We must recognize that, as a stand-alone tool, continuous FHR monitoring is ineffective for avoiding preventable adverse outcomes. It is most likely to be effective when used in accordance with published standard guidelines by professionals skilled in interpretation and when timely, appropriate interventions are performed based on that interpretation. Optimal FHR monitoring requires a collaborative perinatal team that performs the monitoring correctly, interprets it appropriately, and communicates the findings effectively, and in a timely fashion, to all members of the care team when a high-risk pattern is detected.
In this article we review some common challenges that clinicians encounter during intrapartum FHR monitoring and we offer 10 simple tips to help overcome these challenges. The clinical scenarios described are derived from published reports in the medical literature, published malpractice claims, and from our personal experience working in a major health care system as part of a team charged with overseeing ongoing certification and training of labor and delivery nurses.
Challenge: Signal ambiguity
CASE 1 Young woman in labor with first pregnancy
A 19-year-old woman presents in spontaneous labor with her first pregnancy, which has been uncomplicated. During the course of her care, it is noted that the FHR changes to a lower baseline than previously recorded. Evaluation reveals that the external monitor is tracking the maternal heart rate and not the FHR (FIGURE 1). After the monitor is adjusted, both the fetal and maternal rates are documented for a short period. Ultimately, continuous monitoring of the maternal heart rate is discontinued. After delivery of the infant several hours later, it is noted that the FHR continues to register on the monitor, and it is determined that for the last few hours the maternal heart rate has been traced.
| FIGURE 1 FHR tracing indicates signal ambiguity | ||
 | ||
As described in Case 1, the upper panel of this tracing demonstrates the maternal heart rate confused as the fetal heart rate, while the segment in the lower panel shows a clear distinction between the maternal and fetal heart rates. | ||
TIP #10: Ensure the FHR monitor is tracking the fetal, not the maternal, heart rate
Confusing the maternal and the fetal heart rate with external cardiotocography is common. When the mix-up is noted and corrected expeditiously, it is unlikely to result in an adverse outcome. Signal ambiguity may arise from faulty Doppler equipment or the inability of the cardiotocograph to differentiate between maternal and fetal heart rates. It commonly occurs after repositioning the patient, after fetal movement, or during pushing in the second stage when the maternal heart rate may increase to a baseline that is similar to that of the fetus.
Signal ambiguity should be suspected when the FHR runs in the low-normal range or when FHR accelerations are noted with greater than 50% of contractions (especially when pushing).4 Signal ambiguity also should be ruled out when there is an apparent FHR deceleration to the maternal range that does not recover.
Evaluating for suspected signal ambiguity involves 2 key steps: (1) documentation and verification of the maternal heart rate and (2) definitive documentation of the true FHR. To document the maternal heart rate, manually count the radial pulse for 1 minute or use a pulse oximeter for continuous monitoring. Using a pulse oximeter is a less labor-intensive approach and has the advantage of allowing continuous assessment of the maternal heart rate for comparison. Recording the maternal pulse continuously on the same screen as the FHR enables ongoing differentiation of the mother and fetus in difficult cases, particularly if internal fetal monitoring is not an option (because of maternal infectious disease, low suspicion for an abnormal FHR pattern, or strong maternal preference against internal monitoring, for example).
When clinically appropriate, use of a fetal scalp electrode (FSE) can document the FHR. If intrauterine fetal death has occurred, however, the FSE may transmit the maternal heart rate.5 Using ultrasonography to confirm the FHR prior to placing the FSE is a reliable method of definitive differentiation. If a newly placed FSE shows a clear differentiation of 5 to 10 beats per minute from a continuously assessed maternal pulse rate, then this is also a reliable way to assure that the FHR monitoring represents the fetus, particularly if ultrasonography is not immediately available.
Ultimately, before intervening based on an abnormal FHR tracing, it is paramount to confirm that the data are adequate for interpretation and represent the actual FHR. If signal ambiguity is identified or suspected, correct it by using ultrasonography to locate the FHR and replace the external monitor until a rate that is at least 5 to 10 beats per minute different from the maternal rate is obtained. Alternatively, this is an indication for internal fetal monitoring with an FSE.
Challenge: Inadequate FHR tracing, poor communication, lack of clinical context
CASE 2 Woman with uncomplicated postdates pregnancy presents for induction
A 28-year-old woman (G3P2) at 41 weeks 0 days of gestation presents to labor and delivery for induction of labor for the indication of postdates. There have been no complications with the current pregnancy. The initial cervical exam reveals 1+ cm dilation, 90% effacement, and −3 station, and the patient is started on oxytocin per the hospital protocol. What is your interpretation of the continuous FHR tracing shown in FIGURE 2?
| FIGURE 2 Inadequate, uninterpretable FHR tracing | ||
 | ||
This FHR tracing, from the patient described in Case 2, is unusable because of the absence of data. | ||
TIP #9: Check that the monitors are providing useful data
The ability to accurately interpret a continuous FHR tracing depends on the quality of data recorded. Unfortunately, the absence of data makes interpretation impossible. This includes both FHR and tocometry data, since both pieces of information are required for appropriate interpretation of a continuous FHR tracing.
Prolonged periods of uninterpretable FHR and uterine activity tracings imply that no one was attending the mother and fetus.6 If it is difficult to obtain an interpretable FHR tracing, document in the medical record that you made ongoing efforts to maintain an adequate tracing, including the amount of time spent holding the external monitor, use of ultrasonography to document the FHR, and plans for potential internal monitoring.
CASE 2 Continued
After several hours, the patient requests an epidural for pain management and one is placed without difficulty. She reports adequate pain relief and is comfortable for the next 1 to 2 hours. Subsequently, the patient reports a sudden onset of increasing pain that does not respond to additional patient-administered doses of anesthesia over a 30-minute period. The labor and delivery nurse becomes concerned about the patient's pain level and contacts the attending physician to discuss her concerns. The physician, who is currently attending to patients in clinic, listens to the nurse and asks her to contact the anesthesia department with her concerns (FIGURE 3).
| FIGURE 3 FHR tracing reveals recurrent variables in a patient with evolving clinical concerns | ||
 | ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #8: Clearly communicate an urgent situation to the care team
Poor communication underlies many preventable adverse outcomes in medicine.7 Effective communication requires an adequate description of the clinical scenario or problem. A root cause analysis of a series of intrapartum adverse events involving fetal death or injury showed that poor communication about a concerning FHR tracing played a role in 72% of cases.1
In this clinical scenario, the nurse believed that the patient's pain level was unusual or more than anticipated. The person who is communicating his or her concern (the sender) must be sure that the person receiving the message (the responder) clearly understands the sender's level of concern. In this case, it would have been appropriate for the sender to state clearly that she felt the patient's pain was outside of normal expectations and to request that the attending physician come to evaluate the patient.
Clear and effective communication includes (1) an appropriate description of the urgency of the situation and (2) an indication by the sender as to the desired response to this information ("please come evaluate the patient").8 In all cases, both steps are necessary to elicit an appropriate response.
CASE 2 Continued
Over the next 2 hours, recurrent variable decelerations develop, and then sudden, prolonged fetal bradycardia leads to urgent cesarean delivery. At delivery, a uterine rupture is diagnosed and a fetal hand is observed protruding through a lower-uterine segment defect into the maternal abdomen.
TIP #7: Always consider the entire clinical scenario
In this case, the team caring for the patient was not aware that her previous pregnancy had ended with a low transverse cesarean delivery. How does this information change your interpretation of the clinical scenario? The importance of understanding the entire clinical context when interpreting individual characteristics of cardiotocography cannot be overstated. For example, the sudden onset of recurrent, significant variable decelerations is more concerning in the context of a prior cesarean delivery, and late decelerations are more concerning in a patient with placental abruption, fetal growth restriction, or poorly controlled maternal diabetes.
An estimated 70% of fetuses will have an indeterminate FHR pattern (category II) at some time during labor.9 To appropriately interpret the FHR tracing, it is crucial to know the a priori risk for fetal hypoxia and metabolic acidosis (the precursor of fetal injury) due to such identified clinical risk factors as placental insufficiency, medical comorbidities (hypertension, diabetes), or postdates gestational age.
It is well established that cardiotocography has a good negative predictive value for the absence of fetal metabolic acidosis when there is moderate variability and spontaneous or induced accelerations. When attempting to risk stratify the fetus with a category II (indeterminate) FHR tracing, consider these 3 important questions:
- What are the risk factors for this particular patient and her fetus?
- What is the state of the fetus right now, and when was the last time metabolic acidosis could be excluded reasonably (by the presence of moderate variability and accelerations)?
- What is the risk that the fetus will develop acidemia prior to delivery?
The presence of decelerations indicates interruption of oxygen delivery to the fetus, and recurrent decelerations may indicate an evolving process of accumulated oxygen deprivation, hypoxia, and eventually, metabolic acidosis. Most authorities agree that, for the fetus with a previously normal FHR tracing, the onset of significant, recurrent decelerations with slowly cumulative oxygen deficit can lead to fetal acidemia over the course of approximately 1 hour.10 Of course, acidosis also can occur much more quickly with acute events, such as placental abruption or uterine rupture. In deciding whether or not to intervene based on an FHR tracing, the clinician must take into account the clinical context to determine if delivery is likely to occur before significant acidemia develops.
Challenge: Lack of situational awareness, failure to address nursing concerns, reluctance to initiate the chain of command
CASE 3 Spontaneous labor in a second pregnancy
A 28-year-old woman (G2P1) at 40 weeks' gestation presents in spontaneous labor. She has a history of a previous uncomplicated vaginal delivery. After 6 hours she reaches complete dilation with the fetus at −1 station and begins pushing. After 60 minutes, the patient has only progressed to +1 station. She is contracting every 1 to 2 minutes with recurrent variable decelerations (FIGURE 4).
| FIGURE 4 FHR tracing shows time points for initiation and continuation of pushing | ||
 | ||
This tracing, from the patient described in Case 3, documents contraction frequency every 1-2 minutes for more than 60 minutes while the patient continues to push. The fetal heart rate demonstrates repetitive moderate variable decelerations with every push. | ||
TIP #6: Maintain situational awareness
A state of situational awareness exists when caregivers have a clear understanding of all of the factors at play in a clinical situation.11 This can be lost when caregivers focus too intensely on one aspect of care. It often happens when the patient is pushing in the second stage and the provider, focused on the progress of fetal descent, loses track of the amount of time that has passed without reassuring features (such as variability and induced or spontaneous accelerations) in the FHR tracing. The nurse, seeing the physician at the bedside, presumes he or she is aware of the tracing and is thus reluctant to point out the concerning features for fear of appearing insubordinate.
Situational awareness also may be lost at the time of patient hand off between providers wherein critical information, such as a history of previous cesarean delivery, is not communicated to the next care team. When receiving an intrapartum patient hand off, providers must have heightened vigilance to ensure they quickly reach situational awareness and are cognizant of the entire clinical context. Maintaining an environment in which all members of the care team, regardless of their training level, are encouraged to voice their concerns is another way to promote ongoing situational awareness.
CASE 3 Continued
The patient continues pushing for another 20 minutes without delivery, and the nurse raises a concern about the FHR tracing to the physician, who remains in the room but does not respond (FIGURE 5).
| FIGURE 5 FHR tracing reveals ongoing repetitive variable decelerations | ||
 | ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #5: Acknowledge and respond to other caregivers' concerns
A team approach to patient care is essential in all areas of medicine, perhaps none more so than in obstetrics. Each member of the team is engaged in trying to provide optimal patient care and the concerns of every team member--regardless of title or level of training--must be acknowledged and addressed. Good communication requires creating a safe environment wherein each member of the team feels comfortable raising concerns without fear of reprisal. Rather than becoming angry or frustrated when questioned, providers should remain cognizant that these are ongoing efforts to maintain situational awareness and ensure the best possible outcome for mother and baby.
CASE 3 Continued
Pushing continues for another 30 minutes despite the nurse's repeated effort to express concern to the physician about the FHR tracing. After more than 2 hours of pushing, the infant is delivered; Apgar scores are 1, 5, and 7. No cord gas is obtained.
TIP #4: Initiate the chain of command when necessary
Any caregiver, regardless of job title, has a duty to initiate the institution's chain-of-command policy and procedure if he or she has a concern about patient well-being that is not being addressed adequately. It can be uncomfortable for a nurse, midwife, or resident physician to question an attending physician, particularly if that person responds in a dismissive, condescending, or angry manner. If a caregiver has made several attempts to engage the attending physician and feels the concerns are being inadequately addressed, then he or she must respectfully initiate the chain of command to seek additional objective review of the clinical situation.
Failure to follow oxytocin protocols, inadequate surveillance, poor documentation
CASE 4 Induction of an uncomplicated pregnancy due to postdates
A 20-year-old woman (G1P0) at 42 weeks' gestation with an otherwise uncomplicated first pregnancy presents for postdates induction with oxytocin. After 6 hours, she develops uterine tachysystole with recurrent variable decelerations but the oxytocin infusion is continued at the same rate (FIGURE 6).
| FIGURE 6 FHR tracing indicates uterine tachysystole | ||
 | ||
The patient in Case 4 received oxytocin for induction of postdates pregnancy. The red arrow shown on the FHR tracing points out that oxytocin augmentation continues despite the presence of uterine contractions that are too frequent and initial changes, including subtle late decelerations in the FHR, that suggest early fetal compromise. | ||
TIP #3: Manage oxytocin infusion according to protocol
Inappropriate use of oxytocin is common, including the improper management of oxytocin infusion in the setting of uterine tachysystole (defined as the presence of >5 contractions over a 10-minute period averaged over 30 minutes) and/or an abnormal FHR tracing. The mismanagement of uterine tachysystole is cited in more than two-thirds of obstetric malpractice cases.12
Uterine contractions alter blood flow through the spiral arteries and transiently reduce placental perfusion. Prolonged uterine tachysystole can lead to fetal oxygen debt and early signs of hypoxia, including the loss of spontaneous accelerations, tachycardia, and reduced variability. Continuing or increasing the oxytocin in the setting of such changes is hard to justify. One study found that the use of oxytocin in the setting of tachysystole was significantly associated with signs of fetal asphyxia (odds ratio [OR], 5.6).13 When the FHR pattern suggests significant interruption of fetal oxygen delivery and possible hypoxia, continuing or increasing an oxytocin infusion suggests a lack of understanding of the physiology that is the basis for FHR interpretation.
Appropriate management of tachysystole depends on the accompanying FHR.14 In the setting of a category I (normal) FHR tracing, tachysystole can be treated first with maternal repositioning (left or right lateral) and administration of a 500-cm3 maternal IV fluid bolus. If uterine activity does not return to normal after 10 to 15 minutes, decrease the oxytocin rate by at least half. If it does not return to normal after another 10 to 15 minutes, discontinue oxytocin until the tachysystole has resolved.
In the setting of a concerning category IIFHR tracing, discontinuation of oxytocin should be the first step along with maternal repositioning and administration of a fluid bolus. If these measures do not improve the FHR tracing and tachysystole persists, administration of an acute uterine relaxant, such as terbutaline, should be considered to slow contraction frequency.
If interventions result in normalization of the FHR tracing and resolution of tachysystole for 20 to 30 minutes, then oxytocin may be restarted if necessary for labor progress at no more than half the rate that produced tachysystole.
TIP #2: Recognize an abnormal FHR tracing--and what it means
Misinterpretation of the FHR tracing occurs when there is a failure to recognize characteristics that should raise concern about fetal well-being. Failure to recognize an abnormal FHR tracing occurred in 77% of sentinel cases involving intrapartum birth injury or death.1,12,13 To limit misinterpretation of the FHR tracing, it is critical for nurses and physicians to use standardized terminology for clear, effective communication.
In 2008, the Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) published guidelines standardizing the terminology used to describe cardiotocography and to create consensus around its interpretation.15 Any description of an intrapartum FHR tracing should include a designation of category (I, II, or III). Fetal well-being is reasonably established with a category I FHR tracing. A category III tracing indicates the high likelihood of fetal acidemia and the need for immediate intervention. A category II FHR tracing is considered indeterminate, and further characterization is required to reasonably exclude fetal metabolic acidosis and a risk of fetal injury.
The presence of moderate variability and fetal response to scalp stimulation are considered reassuring findings that reasonably exclude significant metabolic acidosis. In assessing variability, one pitfall is mistaking the appearance of "variability" within a deceleration (including during return to baseline) for baseline FHR variability. In the event of a persistent category II FHR tracing (>30 minutes), nursing staff should request direct physician review of the FHR tracing. In any case in which fetal well-being is uncertain, nursing staff should request direct physician evaluation of the mother in person and also the FHR tracing, with clear documentation of the findings, interpretation, and plan of care.16
TIP #1: Document, document, document
Nursing and physician documentation about the FHR tracing within the patient-specific clinical context is crucial for effective caregiver communication and patient safety. Thoughtful documentation also reduces liability exposure for providers by demonstrating maternal-fetal surveillance, early identification and treatment of an abnormal or indeterminate FHR tracing, and timely intervention on fetal behalf when necessary.
When the medical record aligns with the electronic FHR tracing and includes appropriate descriptions, interpretations, and interventions in line with national guidelines and institutional policy, the record demonstrates that the providers have a thorough understanding of the physiology behind cardiotocography and, more importantly, that they are able to apply that knowledge in clinical practice.6
Minimizing missteps
Several straightforward interventions can help clinicians overcome the most common pitfalls during FHR monitoring. These include accurate and high-quality cardiotocography, a collaborative team-based approach to patient care, and sustained situational awareness among providers. The consistent use of common language for the description and interpretation of FHR monitoring, adherence to hospital oxytocin protocols, and well-defined expectations for fetal surveillance and provider communication are critical to overcoming these challenges. Regularly scheduled nursing and physician education sessions and interdisciplinary case review can promote the adoption and sustained incorporation of these simple techniques into daily practice.3
Some have advocated for an "electronic fetal monitoring bundle," which would serve as a checklist of clinical evaluation steps that should occur every time a given process occurs.17 This approach would ensure that all providers on labor and delivery are qualified to read, accurately interpret, and respond to FHR tracings. It would require a credentialing process to confirm the competency of team members and reinforce the presence of a common language. It would also include an explicit escalation policy for rapid initiation of the chain of command in cases wherein there is a disagreement among team members about the FHR interpretation. Finally, each patient would be required to have, at all times, an identified responsible provider capable of a rapid response.
Although continuous FHR monitoring may not effectively reduce intrapartum fetal asphyxia, it is clearly here to stay. Recognizing--and addressing--the most common challenges encountered during intrapartum FHR monitoring may reduce unnecessary morbidity and potential liability for caregivers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Interpreting continuous fetal heart rate (FHR) monitoring is one of the most common tasks obstetricians perform during the course of intrapartum care. Notably, many providers do not seek ongoing training to optimize their ability to reliably and accurately interpret the FHR. Yet FHR interpretation is one of the most frequent causes of litigation in the modern obstetric practice. Failure to interpret continuous FHR monitoring appropriately is estimated to account for 75% of obstetric-related litigation.1
Continuous FHR monitoring during labor was introduced to identify infants at risk for developing hypoxic-ischemic encephalopathy (HIE). The rate of HIE has not declined, however, despite almost universal adoption of continuous FHR monitoring.2 Numerous reasons account for this failure, including ad hoc interpretation of terminology, lack of standardized protocols for management and intervention, and the oftentimes challenging patterns that must be interpreted.3 The confusion about and dissatisfaction with the current state of FHR monitoring has led to attempts to enhance our ability to identify infants at risk with additional approaches (such as fetal pulse oximetry and fetal ST-segment evaluation), and some have called for a complete overhaul of our approach to interpreting the FHR. Clark and colleagues stated recently, "It is time to start over and establish some common language, standard interpretation, and reasonable management principles and guidelines."3
We must recognize that, as a stand-alone tool, continuous FHR monitoring is ineffective for avoiding preventable adverse outcomes. It is most likely to be effective when used in accordance with published standard guidelines by professionals skilled in interpretation and when timely, appropriate interventions are performed based on that interpretation. Optimal FHR monitoring requires a collaborative perinatal team that performs the monitoring correctly, interprets it appropriately, and communicates the findings effectively, and in a timely fashion, to all members of the care team when a high-risk pattern is detected.
In this article we review some common challenges that clinicians encounter during intrapartum FHR monitoring and we offer 10 simple tips to help overcome these challenges. The clinical scenarios described are derived from published reports in the medical literature, published malpractice claims, and from our personal experience working in a major health care system as part of a team charged with overseeing ongoing certification and training of labor and delivery nurses.
Challenge: Signal ambiguity
CASE 1 Young woman in labor with first pregnancy
A 19-year-old woman presents in spontaneous labor with her first pregnancy, which has been uncomplicated. During the course of her care, it is noted that the FHR changes to a lower baseline than previously recorded. Evaluation reveals that the external monitor is tracking the maternal heart rate and not the FHR (FIGURE 1). After the monitor is adjusted, both the fetal and maternal rates are documented for a short period. Ultimately, continuous monitoring of the maternal heart rate is discontinued. After delivery of the infant several hours later, it is noted that the FHR continues to register on the monitor, and it is determined that for the last few hours the maternal heart rate has been traced.
| FIGURE 1 FHR tracing indicates signal ambiguity | ||
 | ||
As described in Case 1, the upper panel of this tracing demonstrates the maternal heart rate confused as the fetal heart rate, while the segment in the lower panel shows a clear distinction between the maternal and fetal heart rates. | ||
TIP #10: Ensure the FHR monitor is tracking the fetal, not the maternal, heart rate
Confusing the maternal and the fetal heart rate with external cardiotocography is common. When the mix-up is noted and corrected expeditiously, it is unlikely to result in an adverse outcome. Signal ambiguity may arise from faulty Doppler equipment or the inability of the cardiotocograph to differentiate between maternal and fetal heart rates. It commonly occurs after repositioning the patient, after fetal movement, or during pushing in the second stage when the maternal heart rate may increase to a baseline that is similar to that of the fetus.
Signal ambiguity should be suspected when the FHR runs in the low-normal range or when FHR accelerations are noted with greater than 50% of contractions (especially when pushing).4 Signal ambiguity also should be ruled out when there is an apparent FHR deceleration to the maternal range that does not recover.
Evaluating for suspected signal ambiguity involves 2 key steps: (1) documentation and verification of the maternal heart rate and (2) definitive documentation of the true FHR. To document the maternal heart rate, manually count the radial pulse for 1 minute or use a pulse oximeter for continuous monitoring. Using a pulse oximeter is a less labor-intensive approach and has the advantage of allowing continuous assessment of the maternal heart rate for comparison. Recording the maternal pulse continuously on the same screen as the FHR enables ongoing differentiation of the mother and fetus in difficult cases, particularly if internal fetal monitoring is not an option (because of maternal infectious disease, low suspicion for an abnormal FHR pattern, or strong maternal preference against internal monitoring, for example).
When clinically appropriate, use of a fetal scalp electrode (FSE) can document the FHR. If intrauterine fetal death has occurred, however, the FSE may transmit the maternal heart rate.5 Using ultrasonography to confirm the FHR prior to placing the FSE is a reliable method of definitive differentiation. If a newly placed FSE shows a clear differentiation of 5 to 10 beats per minute from a continuously assessed maternal pulse rate, then this is also a reliable way to assure that the FHR monitoring represents the fetus, particularly if ultrasonography is not immediately available.
Ultimately, before intervening based on an abnormal FHR tracing, it is paramount to confirm that the data are adequate for interpretation and represent the actual FHR. If signal ambiguity is identified or suspected, correct it by using ultrasonography to locate the FHR and replace the external monitor until a rate that is at least 5 to 10 beats per minute different from the maternal rate is obtained. Alternatively, this is an indication for internal fetal monitoring with an FSE.
Challenge: Inadequate FHR tracing, poor communication, lack of clinical context
CASE 2 Woman with uncomplicated postdates pregnancy presents for induction
A 28-year-old woman (G3P2) at 41 weeks 0 days of gestation presents to labor and delivery for induction of labor for the indication of postdates. There have been no complications with the current pregnancy. The initial cervical exam reveals 1+ cm dilation, 90% effacement, and −3 station, and the patient is started on oxytocin per the hospital protocol. What is your interpretation of the continuous FHR tracing shown in FIGURE 2?
| FIGURE 2 Inadequate, uninterpretable FHR tracing | ||
 | ||
This FHR tracing, from the patient described in Case 2, is unusable because of the absence of data. | ||
TIP #9: Check that the monitors are providing useful data
The ability to accurately interpret a continuous FHR tracing depends on the quality of data recorded. Unfortunately, the absence of data makes interpretation impossible. This includes both FHR and tocometry data, since both pieces of information are required for appropriate interpretation of a continuous FHR tracing.
Prolonged periods of uninterpretable FHR and uterine activity tracings imply that no one was attending the mother and fetus.6 If it is difficult to obtain an interpretable FHR tracing, document in the medical record that you made ongoing efforts to maintain an adequate tracing, including the amount of time spent holding the external monitor, use of ultrasonography to document the FHR, and plans for potential internal monitoring.
CASE 2 Continued
After several hours, the patient requests an epidural for pain management and one is placed without difficulty. She reports adequate pain relief and is comfortable for the next 1 to 2 hours. Subsequently, the patient reports a sudden onset of increasing pain that does not respond to additional patient-administered doses of anesthesia over a 30-minute period. The labor and delivery nurse becomes concerned about the patient's pain level and contacts the attending physician to discuss her concerns. The physician, who is currently attending to patients in clinic, listens to the nurse and asks her to contact the anesthesia department with her concerns (FIGURE 3).
| FIGURE 3 FHR tracing reveals recurrent variables in a patient with evolving clinical concerns | ||
 | ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #8: Clearly communicate an urgent situation to the care team
Poor communication underlies many preventable adverse outcomes in medicine.7 Effective communication requires an adequate description of the clinical scenario or problem. A root cause analysis of a series of intrapartum adverse events involving fetal death or injury showed that poor communication about a concerning FHR tracing played a role in 72% of cases.1
In this clinical scenario, the nurse believed that the patient's pain level was unusual or more than anticipated. The person who is communicating his or her concern (the sender) must be sure that the person receiving the message (the responder) clearly understands the sender's level of concern. In this case, it would have been appropriate for the sender to state clearly that she felt the patient's pain was outside of normal expectations and to request that the attending physician come to evaluate the patient.
Clear and effective communication includes (1) an appropriate description of the urgency of the situation and (2) an indication by the sender as to the desired response to this information ("please come evaluate the patient").8 In all cases, both steps are necessary to elicit an appropriate response.
CASE 2 Continued
Over the next 2 hours, recurrent variable decelerations develop, and then sudden, prolonged fetal bradycardia leads to urgent cesarean delivery. At delivery, a uterine rupture is diagnosed and a fetal hand is observed protruding through a lower-uterine segment defect into the maternal abdomen.
TIP #7: Always consider the entire clinical scenario
In this case, the team caring for the patient was not aware that her previous pregnancy had ended with a low transverse cesarean delivery. How does this information change your interpretation of the clinical scenario? The importance of understanding the entire clinical context when interpreting individual characteristics of cardiotocography cannot be overstated. For example, the sudden onset of recurrent, significant variable decelerations is more concerning in the context of a prior cesarean delivery, and late decelerations are more concerning in a patient with placental abruption, fetal growth restriction, or poorly controlled maternal diabetes.
An estimated 70% of fetuses will have an indeterminate FHR pattern (category II) at some time during labor.9 To appropriately interpret the FHR tracing, it is crucial to know the a priori risk for fetal hypoxia and metabolic acidosis (the precursor of fetal injury) due to such identified clinical risk factors as placental insufficiency, medical comorbidities (hypertension, diabetes), or postdates gestational age.
It is well established that cardiotocography has a good negative predictive value for the absence of fetal metabolic acidosis when there is moderate variability and spontaneous or induced accelerations. When attempting to risk stratify the fetus with a category II (indeterminate) FHR tracing, consider these 3 important questions:
- What are the risk factors for this particular patient and her fetus?
- What is the state of the fetus right now, and when was the last time metabolic acidosis could be excluded reasonably (by the presence of moderate variability and accelerations)?
- What is the risk that the fetus will develop acidemia prior to delivery?
The presence of decelerations indicates interruption of oxygen delivery to the fetus, and recurrent decelerations may indicate an evolving process of accumulated oxygen deprivation, hypoxia, and eventually, metabolic acidosis. Most authorities agree that, for the fetus with a previously normal FHR tracing, the onset of significant, recurrent decelerations with slowly cumulative oxygen deficit can lead to fetal acidemia over the course of approximately 1 hour.10 Of course, acidosis also can occur much more quickly with acute events, such as placental abruption or uterine rupture. In deciding whether or not to intervene based on an FHR tracing, the clinician must take into account the clinical context to determine if delivery is likely to occur before significant acidemia develops.
Challenge: Lack of situational awareness, failure to address nursing concerns, reluctance to initiate the chain of command
CASE 3 Spontaneous labor in a second pregnancy
A 28-year-old woman (G2P1) at 40 weeks' gestation presents in spontaneous labor. She has a history of a previous uncomplicated vaginal delivery. After 6 hours she reaches complete dilation with the fetus at −1 station and begins pushing. After 60 minutes, the patient has only progressed to +1 station. She is contracting every 1 to 2 minutes with recurrent variable decelerations (FIGURE 4).
| FIGURE 4 FHR tracing shows time points for initiation and continuation of pushing | ||
 | ||
This tracing, from the patient described in Case 3, documents contraction frequency every 1-2 minutes for more than 60 minutes while the patient continues to push. The fetal heart rate demonstrates repetitive moderate variable decelerations with every push. | ||
TIP #6: Maintain situational awareness
A state of situational awareness exists when caregivers have a clear understanding of all of the factors at play in a clinical situation.11 This can be lost when caregivers focus too intensely on one aspect of care. It often happens when the patient is pushing in the second stage and the provider, focused on the progress of fetal descent, loses track of the amount of time that has passed without reassuring features (such as variability and induced or spontaneous accelerations) in the FHR tracing. The nurse, seeing the physician at the bedside, presumes he or she is aware of the tracing and is thus reluctant to point out the concerning features for fear of appearing insubordinate.
Situational awareness also may be lost at the time of patient hand off between providers wherein critical information, such as a history of previous cesarean delivery, is not communicated to the next care team. When receiving an intrapartum patient hand off, providers must have heightened vigilance to ensure they quickly reach situational awareness and are cognizant of the entire clinical context. Maintaining an environment in which all members of the care team, regardless of their training level, are encouraged to voice their concerns is another way to promote ongoing situational awareness.
CASE 3 Continued
The patient continues pushing for another 20 minutes without delivery, and the nurse raises a concern about the FHR tracing to the physician, who remains in the room but does not respond (FIGURE 5).
| FIGURE 5 FHR tracing reveals ongoing repetitive variable decelerations | ||
 | ||
This tracing, from the patient described in Case 2, shows variables in the FHR while the patient experiences increasing discomfort. Each of the red arrows indicates documentation by the nurse of increasing pain reported by the patient. The black bars are used to cover names of caregivers. | ||
TIP #5: Acknowledge and respond to other caregivers' concerns
A team approach to patient care is essential in all areas of medicine, perhaps none more so than in obstetrics. Each member of the team is engaged in trying to provide optimal patient care and the concerns of every team member--regardless of title or level of training--must be acknowledged and addressed. Good communication requires creating a safe environment wherein each member of the team feels comfortable raising concerns without fear of reprisal. Rather than becoming angry or frustrated when questioned, providers should remain cognizant that these are ongoing efforts to maintain situational awareness and ensure the best possible outcome for mother and baby.
CASE 3 Continued
Pushing continues for another 30 minutes despite the nurse's repeated effort to express concern to the physician about the FHR tracing. After more than 2 hours of pushing, the infant is delivered; Apgar scores are 1, 5, and 7. No cord gas is obtained.
TIP #4: Initiate the chain of command when necessary
Any caregiver, regardless of job title, has a duty to initiate the institution's chain-of-command policy and procedure if he or she has a concern about patient well-being that is not being addressed adequately. It can be uncomfortable for a nurse, midwife, or resident physician to question an attending physician, particularly if that person responds in a dismissive, condescending, or angry manner. If a caregiver has made several attempts to engage the attending physician and feels the concerns are being inadequately addressed, then he or she must respectfully initiate the chain of command to seek additional objective review of the clinical situation.
Failure to follow oxytocin protocols, inadequate surveillance, poor documentation
CASE 4 Induction of an uncomplicated pregnancy due to postdates
A 20-year-old woman (G1P0) at 42 weeks' gestation with an otherwise uncomplicated first pregnancy presents for postdates induction with oxytocin. After 6 hours, she develops uterine tachysystole with recurrent variable decelerations but the oxytocin infusion is continued at the same rate (FIGURE 6).
| FIGURE 6 FHR tracing indicates uterine tachysystole | ||
 | ||
The patient in Case 4 received oxytocin for induction of postdates pregnancy. The red arrow shown on the FHR tracing points out that oxytocin augmentation continues despite the presence of uterine contractions that are too frequent and initial changes, including subtle late decelerations in the FHR, that suggest early fetal compromise. | ||
TIP #3: Manage oxytocin infusion according to protocol
Inappropriate use of oxytocin is common, including the improper management of oxytocin infusion in the setting of uterine tachysystole (defined as the presence of >5 contractions over a 10-minute period averaged over 30 minutes) and/or an abnormal FHR tracing. The mismanagement of uterine tachysystole is cited in more than two-thirds of obstetric malpractice cases.12
Uterine contractions alter blood flow through the spiral arteries and transiently reduce placental perfusion. Prolonged uterine tachysystole can lead to fetal oxygen debt and early signs of hypoxia, including the loss of spontaneous accelerations, tachycardia, and reduced variability. Continuing or increasing the oxytocin in the setting of such changes is hard to justify. One study found that the use of oxytocin in the setting of tachysystole was significantly associated with signs of fetal asphyxia (odds ratio [OR], 5.6).13 When the FHR pattern suggests significant interruption of fetal oxygen delivery and possible hypoxia, continuing or increasing an oxytocin infusion suggests a lack of understanding of the physiology that is the basis for FHR interpretation.
Appropriate management of tachysystole depends on the accompanying FHR.14 In the setting of a category I (normal) FHR tracing, tachysystole can be treated first with maternal repositioning (left or right lateral) and administration of a 500-cm3 maternal IV fluid bolus. If uterine activity does not return to normal after 10 to 15 minutes, decrease the oxytocin rate by at least half. If it does not return to normal after another 10 to 15 minutes, discontinue oxytocin until the tachysystole has resolved.
In the setting of a concerning category IIFHR tracing, discontinuation of oxytocin should be the first step along with maternal repositioning and administration of a fluid bolus. If these measures do not improve the FHR tracing and tachysystole persists, administration of an acute uterine relaxant, such as terbutaline, should be considered to slow contraction frequency.
If interventions result in normalization of the FHR tracing and resolution of tachysystole for 20 to 30 minutes, then oxytocin may be restarted if necessary for labor progress at no more than half the rate that produced tachysystole.
TIP #2: Recognize an abnormal FHR tracing--and what it means
Misinterpretation of the FHR tracing occurs when there is a failure to recognize characteristics that should raise concern about fetal well-being. Failure to recognize an abnormal FHR tracing occurred in 77% of sentinel cases involving intrapartum birth injury or death.1,12,13 To limit misinterpretation of the FHR tracing, it is critical for nurses and physicians to use standardized terminology for clear, effective communication.
In 2008, the Eunice Kennedy Schriver National Institute of Child Health and Human Development (NICHD) published guidelines standardizing the terminology used to describe cardiotocography and to create consensus around its interpretation.15 Any description of an intrapartum FHR tracing should include a designation of category (I, II, or III). Fetal well-being is reasonably established with a category I FHR tracing. A category III tracing indicates the high likelihood of fetal acidemia and the need for immediate intervention. A category II FHR tracing is considered indeterminate, and further characterization is required to reasonably exclude fetal metabolic acidosis and a risk of fetal injury.
The presence of moderate variability and fetal response to scalp stimulation are considered reassuring findings that reasonably exclude significant metabolic acidosis. In assessing variability, one pitfall is mistaking the appearance of "variability" within a deceleration (including during return to baseline) for baseline FHR variability. In the event of a persistent category II FHR tracing (>30 minutes), nursing staff should request direct physician review of the FHR tracing. In any case in which fetal well-being is uncertain, nursing staff should request direct physician evaluation of the mother in person and also the FHR tracing, with clear documentation of the findings, interpretation, and plan of care.16
TIP #1: Document, document, document
Nursing and physician documentation about the FHR tracing within the patient-specific clinical context is crucial for effective caregiver communication and patient safety. Thoughtful documentation also reduces liability exposure for providers by demonstrating maternal-fetal surveillance, early identification and treatment of an abnormal or indeterminate FHR tracing, and timely intervention on fetal behalf when necessary.
When the medical record aligns with the electronic FHR tracing and includes appropriate descriptions, interpretations, and interventions in line with national guidelines and institutional policy, the record demonstrates that the providers have a thorough understanding of the physiology behind cardiotocography and, more importantly, that they are able to apply that knowledge in clinical practice.6
Minimizing missteps
Several straightforward interventions can help clinicians overcome the most common pitfalls during FHR monitoring. These include accurate and high-quality cardiotocography, a collaborative team-based approach to patient care, and sustained situational awareness among providers. The consistent use of common language for the description and interpretation of FHR monitoring, adherence to hospital oxytocin protocols, and well-defined expectations for fetal surveillance and provider communication are critical to overcoming these challenges. Regularly scheduled nursing and physician education sessions and interdisciplinary case review can promote the adoption and sustained incorporation of these simple techniques into daily practice.3
Some have advocated for an "electronic fetal monitoring bundle," which would serve as a checklist of clinical evaluation steps that should occur every time a given process occurs.17 This approach would ensure that all providers on labor and delivery are qualified to read, accurately interpret, and respond to FHR tracings. It would require a credentialing process to confirm the competency of team members and reinforce the presence of a common language. It would also include an explicit escalation policy for rapid initiation of the chain of command in cases wherein there is a disagreement among team members about the FHR interpretation. Finally, each patient would be required to have, at all times, an identified responsible provider capable of a rapid response.
Although continuous FHR monitoring may not effectively reduce intrapartum fetal asphyxia, it is clearly here to stay. Recognizing--and addressing--the most common challenges encountered during intrapartum FHR monitoring may reduce unnecessary morbidity and potential liability for caregivers.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Sentinel event alert issue 30--July 21, 2004. Preventing infant death and injury during delivery. Adv Neonatal Care. 2004;4(4):180–181.
- Shy KK, Luthy DA, Bennett FC, et al. Effects of electronic fetal-heart-rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. N Engl J Med. 1990;322(9):588–593.
- Clark SL, Nageotte MP, Garite TJ, et al. Intrapartum management of category II fetal heart rate tracings: towards standardization of care. Am J Obstet Gynecol. 2013;209(2):89–97.
- Neilson DR Jr, Freeman RK, Mangan S. Signal ambiguity resulting in unexpected outcome with external fetal heart rate monitoring. Am J Obstet Gynecol. 2008;198(6):717–724.
- McWhinney NA, Knowles S, Green HL, Gordon H. Transmission of the maternal electrocardiograph via a fetal scalp electrode in the presence of intrauterine death. Case report. Br J Obstet Gynaecol. 1984;91(10):1046–1048.
- Simpson KR, Knox GE. Risk management and electronic fetal monitoring: decreasing risk of adverse outcomes and liability exposure. J Perinat Neonatal Nurs. 2000;14(3):40–52.
- Gluck PA. Patient safety in women's health care: a framework for progress. Best Pract Res Clin Obstet Gynaecol. 2007;21(4):525–536.
- Lyndon A, Zlatnik MG, Wachter RM. Effective physician-nurse communication: a patient safety essential for labor and delivery. Am J Obstet Gynecol. 2011;205(2):91–96.
- Jackson M, Holmgren CM, Esplin MS, Henry E, Varner MW. Frequency of fetal heart rate categories and short-term neonatal outcome. Obstet Gynecol. 2011;118(4):803–808.
- Parer JT, Ikeda T. A framework for standardized management of intrapartum fetal heart rate patterns. Am J Obstet Gynecol. 2007;197(1):26.e1-e6.
- MacEachin SR, Lopez CM, Powell KJ, Corbett NL. The fetal heart rate collaborative practice project: situational awareness in electronic fetal monitoring--a Kaiser Permanente Perinatal Patient Safety Program Initiative. J Perinat Neonatal Nurs. 2009;23(4):314–323; quiz 24–25.
- Jonsson M, Norden SL, Hanson U. Analysis of malpractice claims with a focus on oxytocin use in labour. Acta Obstet Gynecol Scand. 2007;86(3):315–319.
- Berglund S, Pettersson H, Cnattingius S, Grunewald C. How often is a low Apgar score the result of substandard care during labour? BJOG. 2010;117(8):968–978.
- Doyle J, Kenny TH, Burkett AM, von Gruenigen VE. A performance improvement process to tackle tachysystole. J Obstet Gynecol Neonatal Nurs. 2011;40(5):512–519.
- Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661–666.
- Knox GE, Simpson KR, Garite TJ. High reliability perinatal units: an approach to the prevention of patient injury and medical malpractice claims. J Healthc Risk Manag. 1999;19(2):24–32.
- Minkoff H, Berkowitz R; Greater New York Hospital Association's Perinatal Safety C. Fetal monitoring bundle. Obstet Gynecol. 2009;114(6):1332–1335.
- Sentinel event alert issue 30--July 21, 2004. Preventing infant death and injury during delivery. Adv Neonatal Care. 2004;4(4):180–181.
- Shy KK, Luthy DA, Bennett FC, et al. Effects of electronic fetal-heart-rate monitoring, as compared with periodic auscultation, on the neurologic development of premature infants. N Engl J Med. 1990;322(9):588–593.
- Clark SL, Nageotte MP, Garite TJ, et al. Intrapartum management of category II fetal heart rate tracings: towards standardization of care. Am J Obstet Gynecol. 2013;209(2):89–97.
- Neilson DR Jr, Freeman RK, Mangan S. Signal ambiguity resulting in unexpected outcome with external fetal heart rate monitoring. Am J Obstet Gynecol. 2008;198(6):717–724.
- McWhinney NA, Knowles S, Green HL, Gordon H. Transmission of the maternal electrocardiograph via a fetal scalp electrode in the presence of intrauterine death. Case report. Br J Obstet Gynaecol. 1984;91(10):1046–1048.
- Simpson KR, Knox GE. Risk management and electronic fetal monitoring: decreasing risk of adverse outcomes and liability exposure. J Perinat Neonatal Nurs. 2000;14(3):40–52.
- Gluck PA. Patient safety in women's health care: a framework for progress. Best Pract Res Clin Obstet Gynaecol. 2007;21(4):525–536.
- Lyndon A, Zlatnik MG, Wachter RM. Effective physician-nurse communication: a patient safety essential for labor and delivery. Am J Obstet Gynecol. 2011;205(2):91–96.
- Jackson M, Holmgren CM, Esplin MS, Henry E, Varner MW. Frequency of fetal heart rate categories and short-term neonatal outcome. Obstet Gynecol. 2011;118(4):803–808.
- Parer JT, Ikeda T. A framework for standardized management of intrapartum fetal heart rate patterns. Am J Obstet Gynecol. 2007;197(1):26.e1-e6.
- MacEachin SR, Lopez CM, Powell KJ, Corbett NL. The fetal heart rate collaborative practice project: situational awareness in electronic fetal monitoring--a Kaiser Permanente Perinatal Patient Safety Program Initiative. J Perinat Neonatal Nurs. 2009;23(4):314–323; quiz 24–25.
- Jonsson M, Norden SL, Hanson U. Analysis of malpractice claims with a focus on oxytocin use in labour. Acta Obstet Gynecol Scand. 2007;86(3):315–319.
- Berglund S, Pettersson H, Cnattingius S, Grunewald C. How often is a low Apgar score the result of substandard care during labour? BJOG. 2010;117(8):968–978.
- Doyle J, Kenny TH, Burkett AM, von Gruenigen VE. A performance improvement process to tackle tachysystole. J Obstet Gynecol Neonatal Nurs. 2011;40(5):512–519.
- Macones GA, Hankins GD, Spong CY, Hauth J, Moore T. The 2008 National Institute of Child Health and Human Development workshop report on electronic fetal monitoring: update on definitions, interpretation, and research guidelines. Obstet Gynecol. 2008;112(3):661–666.
- Knox GE, Simpson KR, Garite TJ. High reliability perinatal units: an approach to the prevention of patient injury and medical malpractice claims. J Healthc Risk Manag. 1999;19(2):24–32.
- Minkoff H, Berkowitz R; Greater New York Hospital Association's Perinatal Safety C. Fetal monitoring bundle. Obstet Gynecol. 2009;114(6):1332–1335.
In this article
• Communicate urgency
• Situational awareness
Acute Bacterial Sinusitis in Children: Evaluation and Treatment
IN THIS ARTICLE
- Complications of acute bacterial sinusitis
- What are the medical options?
- AAP 2013 recommendations for initial antimicrobial treatment
Acute bacterial sinusitis (ABS) is a common diagnosis in pediatric patients. Of children who are evaluated for respiratory complaints, 6% to 7% meet clinical criteria for ABS.1 In addition to being a frequent complication of upper respiratory infection (URI), ABS in children has a significant financial impact. Direct health care expenditures attributed to sinusitis in children ages 12 or younger is $1.8 billion annually.2
Differentiating between viral URI and ABS is a diagnostic challenge for health care providers. In recent years, the American Academy of Pediatrics (AAP) and the Infectious Diseases Society of America (IDSA) have released clinical practice guidelines on the clinical diagnosis and management of ABS in children.3,4
URI AND ABS MANIFESTATIONS
URIs manifest with a predictable pattern of symptoms. Children may experience one to two days of fever, accompanied by constitutional symptoms, such as fatigue, headache, decreased appetite, and myalgia. Nasal discharge typically begins clear and becomes mucopurulent over the next few days; subsequently, it either resolves or becomes serous again at the end of the URI. Cough, hoarseness, malodorous breath, and pharyngitis may be present.3,5 Symptoms typically resolve over five to 10 days, with respiratory symptoms peaking at days 3 to 5. Importantly, in viral URIs, nasal congestion and cough improve toward the end of the illness.1,3
Persistent illness. Patients with ABS may experience persistent illness with nasal discharge (of any quality) and/or daytime cough that persists more than 10 days without improvement.3,4 These patients are differentiated from those with a viral URI by a lack of improvement in congestion and/or cough after 10 days of symptoms.3 While some children with viral illnesses may have persistant upper respiratory symptoms, these should be gradually resolving. Clinicians must take a thorough history to identify children who may have multiple consecutive URIs rather than one persistent illness that is not resolving.
Other diagnoses, such as allergic rhinitis, nasal foreign body, pertussis, influenza, and bacterial pharyngitis, must also be excluded. Children who present with persistent nasal congestion, with or without cough, after 10 days of illness without signs of improvement meet the criteria for ABS.3
Severe symptom onset. Children with ABS may experience severe onset of symptoms.3,4 These children have purulent nasal discharge for at least three consecutive days at onset of illness and concurrent fever (temperature, ≥ 102.2°F). In contrast, a viral URI typically presents with fever for less than 48 hours and clear nasal discharge that becomes purulent after the first few days of symptoms.
“Double sickening.” Finally, children with ABS may have a worsening course of symptoms or a “double sickening.”3,4 These patients experience typical URI symptoms that initially begin to improve, then worsen on day 6 or 7 of illness with increasing or new onset of considerable nasal drainage, daytime cough, or fever.
Continue for physical findings >>
PHYSICAL FINDINGS
Physical exam findings are not sufficient to distinguish between viral URI and ABS.1,3,5 Typical findings on examination may include nasal congestion, postnasal drip, erythematous turbinates, and/or injected posterior oropharynx. Malodorous breath may be present but is not diagnostic.3,5 The tympanic membranes should be examined for signs of concomitant acute otitis media (AOM) or otitis media with effusion. Swelling of the eyelids may be present. Facial pain may also be noted on physical exam.3
Clinicians should be mindful of the complications of sinusitis when performing the physical examination (see Table 1). The most common complications of ABS are orbital and may manifest with eyelid swelling, proptosis, or decreased extraocular movements. Patients with intracranial complications may present with headache, photophobia, seizure, meningeal signs, or focal neurologic signs.3
Continue to making the diagnosis >>
MAKING THE DIAGNOSIS
The diagnosis of ABS is a clinical one. Clinical guidelines from the AAP and IDSA for the diagnosis of ABS in children are very similar; both describe clinical presentations of persistent, severe, or worsening symptoms.3,4 This display of expert consensus allows clinicians to confidently distinguish between viral URIs and ABS by adhering to the strict diagnostic criteria already discussed.
Imaging
Radiographs and CT scans are not necessary to confirm the diagnosis of ABS. Imaging studies may reveal current or recent upper respiratory symptoms, including mucosal inflammation, opacities, and air-fluid levels6,7; however, no imaging study is available that can distinguish among mucosal inflammation and viral or bacterial infections. CT scans or MRI may be useful if clinicians suspect a complication of sinusitis.3,7
Microbiology
Historically, the microbiology of ABS has been determined by maxillary sinus aspiration. The most recent studies of this method, published in the 1980s, identified Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis as the most common bacterial pathogens.3,6,9 More recently, the microbiology of ABS has been linked with causative pathogens in AOM. The pathogenesis of ABS and AOM are similar, therefore allowing data from tympanocentesis in children with AOM to be used to determine the microbiology of children with ABS.10 Although S pneumoniae, H influenzae, and M catarrhalis remain common causative pathogens in ABS, the introduction of the 7- and 13-valent pneumococcal vaccines has altered the microbiology of AOM, and presumably ABS.11
Importantly, numbers of cases of AOM attributed to S pneumoniae have decreased while those attributed to H influenzae have increased.3,11 In addition, antimicrobial susceptibility of S pneumoniae and the prevalence of β-lactamase–positive H influenzae are important considerations when choosing appropriate antibiotics and can vary significantly by geographic region. Therefore, it is important that clinicians be aware of susceptibility patterns in the communities in which they practice.
Continue for the medical options >>
WHAT ARE THE MEDICAL OPTIONS?
Initial management of ABS in children is contingent on the symptom profile at presentation (persistent, worsening, or severe) and consideration of causative pathogens. While the consensus among experts is that children presenting with severe or worsening symptoms be treated initially with antibiotics,3,4 children who present with mild symptoms consistent with persistent illness may initially be treated with antibiotics or observed for an additional three days. This decision should be made thoughtfully and in collaboration with the patient’s parents or guardians. Any child with ABS who presents with persistent symptoms of illness and is managed initially by observation alone should be reassessed or treated with an antibiotic if symptoms worsen, if new symptoms appear, or if the child fails to improve within 72 hours.
Amoxicillin with or without clavulanate is the antibiotic of choice when ABS has been diagnosed and antibiotic treatment is indicated.1,3 AAP recommendations for antimicrobial treatment of ABS in children are stratified on the basis of age, severity of illness, day care attendance, and history of treatment with amoxicillin in the previous 30 days (see Table 2). Clinicians should rely on their knowledge of drug resistance in their community and judgment regarding severity of illness when choosing between amoxicillin and amoxicillin/clavulanate as initial treatment for ABS.3
Recommendations for duration of antimicrobial treatment for ABS vary considerably. A reasonable suggestion is to continue to treat patients until they are symptom-free for seven days. For many patients, this will mean a 10-day treatment course, with flexibility to increase the duration as needed.3
Children with ABS should be reassessed if there are worsening signs or symptoms or lack of clinical improvement within 72 hours of initial management. Clinicians should evaluate whether the patient has been appropriately diagnosed and assessed for complications of ABS. If the diagnosis is confirmed and ABS complications are not suspected, the clinician may change antibiotics or initiate antibiotic therapy if the child was previously managed by observation only.3,4
A child who is vomiting or unable to tolerate PO medications may benefit from a single 50-gm/kg dose of ceftriaxone IV or IM with follow-up in 24 hours.3 Oral antibiotics may be started at the follow-up visit if the patient demonstrates clinical improvement.
When considering a change in antibiotic, the clinician should consider the possibility of drug resistance. If the child was initially treated with amoxicillin, high-dose amoxicillin/clavulanate may be prescribed. If high-dose amoxicillin/clavulanate was initially prescribed and the patient has not improved or is experiencing worsening symptoms, clindamycin with cefixime, linezolid with cefixime, or levofloxacin may be considered.3,4
Penicillin allergy
Patients with a history of a non–type 1 hypersensitivity reaction to amoxicillin may be treated with cefdinir, cefuroxime, cefpodoxime, or combination therapy with clindamycin plus a third-generation oral cephalosporin. Allergist referral for penicillin or cephalosporin skin testing may be considered before initiation of therapy.3,4,13
Adjuvant therapy
Decongestants, antihistamines, and nasal irrigation are frequently recommended in the management of ABS in children; however, the authors of a 2012 Cochrane review found no properly designed studies to evaluate the effectiveness of these treatments.13 Furthermore, there is insufficient evidence to clearly recommend the use of intranasal steroids as an adjuvant therapy in the treatment of ABS in children (although several randomized controlled studies demonstrate their effectiveness in adolescents and adults).3
Continue for the conclusion >>
CONCLUSION
ABS in children is diagnosed clinically by following a strict set of clinical criteria. Patients commonly present with one of three types of symptoms: persistent URI; severe purulent nasal discharge and fever for at least three consecutive days; or a double sickening. Physical examination findings vary and will not differentiate viral URI symptoms from a diagnosis of ABS. Imaging is not recommended for diagnosis but may be helpful if an orbital or intracranial complication of ABS is suspected.
S pneumoniae, H influenzae, and M catarrhalis continue to be the most common pathogens associated with ABS. However, since the introduction of the 7-valent pneumococcal vaccine, prevalence of H influenzae and β-lactamase–positive H influenzae has increased.
Treatment recommendations vary, based on suspected causative pathogens and presenting symptoms. Amoxicillin or amoxicillin-clavulanate is recommended as firstline antimicrobial treatment for ABS, with alternate antibiotic choices for patients with worsening symptoms or lack of improvement within 72 hours. An awareness of the community’s susceptibility patterns is essential for the clinician who cares for children at risk for ABS.
REFERENCES
1. Wald ER, Nash D, Eickhoff J. Effectiveness of amoxicillin/clavulanate potassium in the treatment of acute bacterial sinusitis in children. Pediatrics. 2009;124(1):9-15.
2. Ray NF, Baraniuk JN, Thamer M, et al. Healthcare expenditures for sinusitis in 1996: contributions of asthma, rhinitis, and other airway disorders. J Allergy Clin Immunol. 1999;103(3 pt 1):408-414.
3. Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132(1):e262-e280.
4. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):1041-1045.
5. Shaikh N, Hoberman A, Kearney DH, et al. Signs and symptoms that differentiate acute sinusitis from viral upper respiratory tract infection. Pediatr Infect Dis J. 2013;32(10):1061-1065.
6. Kovatch AL, Wald ER, Ledesma-Medina J, et al. Maxillary sinus radiographs in children with nonrespiratory complaints. Pediatrics. 1984; 73(3):306-308.
7. Triulzi F, Zirpoli S. Imaging techniques in the diagnosis and management of rhinosinusitis in children. Pediatr Allergy Immunol. 2007;18(suppl 18):46-49.
8. Wald ER, Milmoe GJ, Bowen A, et al. Acute maxillary sinusitis in children. N Engl J Med. 1981;304(13):749-754.
9. Wald ER, Reilly JS, Casselbrant M, et al. Treatment of acute maxillary sinusitis in childhood: a comparative study of amoxicillin and cefaclor. J Pediatr. 1984;104(2):297-302.
10. Wald ER. Acute otitis media and acute bacterial sinusitis. Clin Infect Dis. 2011;52(suppl 4):S277-S283.
11. Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304-309.
12. Pichichero ME. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics. 2004;115(4):1048-1057.
13. Shaikh N, Wald ER, Pi M. Decongestants, antihistamines and nasal irrigation for acute sinusitis in children. Cochrane Database Syst Rev. 2012;9:CD007909.
IN THIS ARTICLE
- Complications of acute bacterial sinusitis
- What are the medical options?
- AAP 2013 recommendations for initial antimicrobial treatment
Acute bacterial sinusitis (ABS) is a common diagnosis in pediatric patients. Of children who are evaluated for respiratory complaints, 6% to 7% meet clinical criteria for ABS.1 In addition to being a frequent complication of upper respiratory infection (URI), ABS in children has a significant financial impact. Direct health care expenditures attributed to sinusitis in children ages 12 or younger is $1.8 billion annually.2
Differentiating between viral URI and ABS is a diagnostic challenge for health care providers. In recent years, the American Academy of Pediatrics (AAP) and the Infectious Diseases Society of America (IDSA) have released clinical practice guidelines on the clinical diagnosis and management of ABS in children.3,4
URI AND ABS MANIFESTATIONS
URIs manifest with a predictable pattern of symptoms. Children may experience one to two days of fever, accompanied by constitutional symptoms, such as fatigue, headache, decreased appetite, and myalgia. Nasal discharge typically begins clear and becomes mucopurulent over the next few days; subsequently, it either resolves or becomes serous again at the end of the URI. Cough, hoarseness, malodorous breath, and pharyngitis may be present.3,5 Symptoms typically resolve over five to 10 days, with respiratory symptoms peaking at days 3 to 5. Importantly, in viral URIs, nasal congestion and cough improve toward the end of the illness.1,3
Persistent illness. Patients with ABS may experience persistent illness with nasal discharge (of any quality) and/or daytime cough that persists more than 10 days without improvement.3,4 These patients are differentiated from those with a viral URI by a lack of improvement in congestion and/or cough after 10 days of symptoms.3 While some children with viral illnesses may have persistant upper respiratory symptoms, these should be gradually resolving. Clinicians must take a thorough history to identify children who may have multiple consecutive URIs rather than one persistent illness that is not resolving.
Other diagnoses, such as allergic rhinitis, nasal foreign body, pertussis, influenza, and bacterial pharyngitis, must also be excluded. Children who present with persistent nasal congestion, with or without cough, after 10 days of illness without signs of improvement meet the criteria for ABS.3
Severe symptom onset. Children with ABS may experience severe onset of symptoms.3,4 These children have purulent nasal discharge for at least three consecutive days at onset of illness and concurrent fever (temperature, ≥ 102.2°F). In contrast, a viral URI typically presents with fever for less than 48 hours and clear nasal discharge that becomes purulent after the first few days of symptoms.
“Double sickening.” Finally, children with ABS may have a worsening course of symptoms or a “double sickening.”3,4 These patients experience typical URI symptoms that initially begin to improve, then worsen on day 6 or 7 of illness with increasing or new onset of considerable nasal drainage, daytime cough, or fever.
Continue for physical findings >>
PHYSICAL FINDINGS
Physical exam findings are not sufficient to distinguish between viral URI and ABS.1,3,5 Typical findings on examination may include nasal congestion, postnasal drip, erythematous turbinates, and/or injected posterior oropharynx. Malodorous breath may be present but is not diagnostic.3,5 The tympanic membranes should be examined for signs of concomitant acute otitis media (AOM) or otitis media with effusion. Swelling of the eyelids may be present. Facial pain may also be noted on physical exam.3
Clinicians should be mindful of the complications of sinusitis when performing the physical examination (see Table 1). The most common complications of ABS are orbital and may manifest with eyelid swelling, proptosis, or decreased extraocular movements. Patients with intracranial complications may present with headache, photophobia, seizure, meningeal signs, or focal neurologic signs.3
Continue to making the diagnosis >>
MAKING THE DIAGNOSIS
The diagnosis of ABS is a clinical one. Clinical guidelines from the AAP and IDSA for the diagnosis of ABS in children are very similar; both describe clinical presentations of persistent, severe, or worsening symptoms.3,4 This display of expert consensus allows clinicians to confidently distinguish between viral URIs and ABS by adhering to the strict diagnostic criteria already discussed.
Imaging
Radiographs and CT scans are not necessary to confirm the diagnosis of ABS. Imaging studies may reveal current or recent upper respiratory symptoms, including mucosal inflammation, opacities, and air-fluid levels6,7; however, no imaging study is available that can distinguish among mucosal inflammation and viral or bacterial infections. CT scans or MRI may be useful if clinicians suspect a complication of sinusitis.3,7
Microbiology
Historically, the microbiology of ABS has been determined by maxillary sinus aspiration. The most recent studies of this method, published in the 1980s, identified Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis as the most common bacterial pathogens.3,6,9 More recently, the microbiology of ABS has been linked with causative pathogens in AOM. The pathogenesis of ABS and AOM are similar, therefore allowing data from tympanocentesis in children with AOM to be used to determine the microbiology of children with ABS.10 Although S pneumoniae, H influenzae, and M catarrhalis remain common causative pathogens in ABS, the introduction of the 7- and 13-valent pneumococcal vaccines has altered the microbiology of AOM, and presumably ABS.11
Importantly, numbers of cases of AOM attributed to S pneumoniae have decreased while those attributed to H influenzae have increased.3,11 In addition, antimicrobial susceptibility of S pneumoniae and the prevalence of β-lactamase–positive H influenzae are important considerations when choosing appropriate antibiotics and can vary significantly by geographic region. Therefore, it is important that clinicians be aware of susceptibility patterns in the communities in which they practice.
Continue for the medical options >>
WHAT ARE THE MEDICAL OPTIONS?
Initial management of ABS in children is contingent on the symptom profile at presentation (persistent, worsening, or severe) and consideration of causative pathogens. While the consensus among experts is that children presenting with severe or worsening symptoms be treated initially with antibiotics,3,4 children who present with mild symptoms consistent with persistent illness may initially be treated with antibiotics or observed for an additional three days. This decision should be made thoughtfully and in collaboration with the patient’s parents or guardians. Any child with ABS who presents with persistent symptoms of illness and is managed initially by observation alone should be reassessed or treated with an antibiotic if symptoms worsen, if new symptoms appear, or if the child fails to improve within 72 hours.
Amoxicillin with or without clavulanate is the antibiotic of choice when ABS has been diagnosed and antibiotic treatment is indicated.1,3 AAP recommendations for antimicrobial treatment of ABS in children are stratified on the basis of age, severity of illness, day care attendance, and history of treatment with amoxicillin in the previous 30 days (see Table 2). Clinicians should rely on their knowledge of drug resistance in their community and judgment regarding severity of illness when choosing between amoxicillin and amoxicillin/clavulanate as initial treatment for ABS.3
Recommendations for duration of antimicrobial treatment for ABS vary considerably. A reasonable suggestion is to continue to treat patients until they are symptom-free for seven days. For many patients, this will mean a 10-day treatment course, with flexibility to increase the duration as needed.3
Children with ABS should be reassessed if there are worsening signs or symptoms or lack of clinical improvement within 72 hours of initial management. Clinicians should evaluate whether the patient has been appropriately diagnosed and assessed for complications of ABS. If the diagnosis is confirmed and ABS complications are not suspected, the clinician may change antibiotics or initiate antibiotic therapy if the child was previously managed by observation only.3,4
A child who is vomiting or unable to tolerate PO medications may benefit from a single 50-gm/kg dose of ceftriaxone IV or IM with follow-up in 24 hours.3 Oral antibiotics may be started at the follow-up visit if the patient demonstrates clinical improvement.
When considering a change in antibiotic, the clinician should consider the possibility of drug resistance. If the child was initially treated with amoxicillin, high-dose amoxicillin/clavulanate may be prescribed. If high-dose amoxicillin/clavulanate was initially prescribed and the patient has not improved or is experiencing worsening symptoms, clindamycin with cefixime, linezolid with cefixime, or levofloxacin may be considered.3,4
Penicillin allergy
Patients with a history of a non–type 1 hypersensitivity reaction to amoxicillin may be treated with cefdinir, cefuroxime, cefpodoxime, or combination therapy with clindamycin plus a third-generation oral cephalosporin. Allergist referral for penicillin or cephalosporin skin testing may be considered before initiation of therapy.3,4,13
Adjuvant therapy
Decongestants, antihistamines, and nasal irrigation are frequently recommended in the management of ABS in children; however, the authors of a 2012 Cochrane review found no properly designed studies to evaluate the effectiveness of these treatments.13 Furthermore, there is insufficient evidence to clearly recommend the use of intranasal steroids as an adjuvant therapy in the treatment of ABS in children (although several randomized controlled studies demonstrate their effectiveness in adolescents and adults).3
Continue for the conclusion >>
CONCLUSION
ABS in children is diagnosed clinically by following a strict set of clinical criteria. Patients commonly present with one of three types of symptoms: persistent URI; severe purulent nasal discharge and fever for at least three consecutive days; or a double sickening. Physical examination findings vary and will not differentiate viral URI symptoms from a diagnosis of ABS. Imaging is not recommended for diagnosis but may be helpful if an orbital or intracranial complication of ABS is suspected.
S pneumoniae, H influenzae, and M catarrhalis continue to be the most common pathogens associated with ABS. However, since the introduction of the 7-valent pneumococcal vaccine, prevalence of H influenzae and β-lactamase–positive H influenzae has increased.
Treatment recommendations vary, based on suspected causative pathogens and presenting symptoms. Amoxicillin or amoxicillin-clavulanate is recommended as firstline antimicrobial treatment for ABS, with alternate antibiotic choices for patients with worsening symptoms or lack of improvement within 72 hours. An awareness of the community’s susceptibility patterns is essential for the clinician who cares for children at risk for ABS.
REFERENCES
1. Wald ER, Nash D, Eickhoff J. Effectiveness of amoxicillin/clavulanate potassium in the treatment of acute bacterial sinusitis in children. Pediatrics. 2009;124(1):9-15.
2. Ray NF, Baraniuk JN, Thamer M, et al. Healthcare expenditures for sinusitis in 1996: contributions of asthma, rhinitis, and other airway disorders. J Allergy Clin Immunol. 1999;103(3 pt 1):408-414.
3. Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132(1):e262-e280.
4. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):1041-1045.
5. Shaikh N, Hoberman A, Kearney DH, et al. Signs and symptoms that differentiate acute sinusitis from viral upper respiratory tract infection. Pediatr Infect Dis J. 2013;32(10):1061-1065.
6. Kovatch AL, Wald ER, Ledesma-Medina J, et al. Maxillary sinus radiographs in children with nonrespiratory complaints. Pediatrics. 1984; 73(3):306-308.
7. Triulzi F, Zirpoli S. Imaging techniques in the diagnosis and management of rhinosinusitis in children. Pediatr Allergy Immunol. 2007;18(suppl 18):46-49.
8. Wald ER, Milmoe GJ, Bowen A, et al. Acute maxillary sinusitis in children. N Engl J Med. 1981;304(13):749-754.
9. Wald ER, Reilly JS, Casselbrant M, et al. Treatment of acute maxillary sinusitis in childhood: a comparative study of amoxicillin and cefaclor. J Pediatr. 1984;104(2):297-302.
10. Wald ER. Acute otitis media and acute bacterial sinusitis. Clin Infect Dis. 2011;52(suppl 4):S277-S283.
11. Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304-309.
12. Pichichero ME. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics. 2004;115(4):1048-1057.
13. Shaikh N, Wald ER, Pi M. Decongestants, antihistamines and nasal irrigation for acute sinusitis in children. Cochrane Database Syst Rev. 2012;9:CD007909.
IN THIS ARTICLE
- Complications of acute bacterial sinusitis
- What are the medical options?
- AAP 2013 recommendations for initial antimicrobial treatment
Acute bacterial sinusitis (ABS) is a common diagnosis in pediatric patients. Of children who are evaluated for respiratory complaints, 6% to 7% meet clinical criteria for ABS.1 In addition to being a frequent complication of upper respiratory infection (URI), ABS in children has a significant financial impact. Direct health care expenditures attributed to sinusitis in children ages 12 or younger is $1.8 billion annually.2
Differentiating between viral URI and ABS is a diagnostic challenge for health care providers. In recent years, the American Academy of Pediatrics (AAP) and the Infectious Diseases Society of America (IDSA) have released clinical practice guidelines on the clinical diagnosis and management of ABS in children.3,4
URI AND ABS MANIFESTATIONS
URIs manifest with a predictable pattern of symptoms. Children may experience one to two days of fever, accompanied by constitutional symptoms, such as fatigue, headache, decreased appetite, and myalgia. Nasal discharge typically begins clear and becomes mucopurulent over the next few days; subsequently, it either resolves or becomes serous again at the end of the URI. Cough, hoarseness, malodorous breath, and pharyngitis may be present.3,5 Symptoms typically resolve over five to 10 days, with respiratory symptoms peaking at days 3 to 5. Importantly, in viral URIs, nasal congestion and cough improve toward the end of the illness.1,3
Persistent illness. Patients with ABS may experience persistent illness with nasal discharge (of any quality) and/or daytime cough that persists more than 10 days without improvement.3,4 These patients are differentiated from those with a viral URI by a lack of improvement in congestion and/or cough after 10 days of symptoms.3 While some children with viral illnesses may have persistant upper respiratory symptoms, these should be gradually resolving. Clinicians must take a thorough history to identify children who may have multiple consecutive URIs rather than one persistent illness that is not resolving.
Other diagnoses, such as allergic rhinitis, nasal foreign body, pertussis, influenza, and bacterial pharyngitis, must also be excluded. Children who present with persistent nasal congestion, with or without cough, after 10 days of illness without signs of improvement meet the criteria for ABS.3
Severe symptom onset. Children with ABS may experience severe onset of symptoms.3,4 These children have purulent nasal discharge for at least three consecutive days at onset of illness and concurrent fever (temperature, ≥ 102.2°F). In contrast, a viral URI typically presents with fever for less than 48 hours and clear nasal discharge that becomes purulent after the first few days of symptoms.
“Double sickening.” Finally, children with ABS may have a worsening course of symptoms or a “double sickening.”3,4 These patients experience typical URI symptoms that initially begin to improve, then worsen on day 6 or 7 of illness with increasing or new onset of considerable nasal drainage, daytime cough, or fever.
Continue for physical findings >>
PHYSICAL FINDINGS
Physical exam findings are not sufficient to distinguish between viral URI and ABS.1,3,5 Typical findings on examination may include nasal congestion, postnasal drip, erythematous turbinates, and/or injected posterior oropharynx. Malodorous breath may be present but is not diagnostic.3,5 The tympanic membranes should be examined for signs of concomitant acute otitis media (AOM) or otitis media with effusion. Swelling of the eyelids may be present. Facial pain may also be noted on physical exam.3
Clinicians should be mindful of the complications of sinusitis when performing the physical examination (see Table 1). The most common complications of ABS are orbital and may manifest with eyelid swelling, proptosis, or decreased extraocular movements. Patients with intracranial complications may present with headache, photophobia, seizure, meningeal signs, or focal neurologic signs.3
Continue to making the diagnosis >>
MAKING THE DIAGNOSIS
The diagnosis of ABS is a clinical one. Clinical guidelines from the AAP and IDSA for the diagnosis of ABS in children are very similar; both describe clinical presentations of persistent, severe, or worsening symptoms.3,4 This display of expert consensus allows clinicians to confidently distinguish between viral URIs and ABS by adhering to the strict diagnostic criteria already discussed.
Imaging
Radiographs and CT scans are not necessary to confirm the diagnosis of ABS. Imaging studies may reveal current or recent upper respiratory symptoms, including mucosal inflammation, opacities, and air-fluid levels6,7; however, no imaging study is available that can distinguish among mucosal inflammation and viral or bacterial infections. CT scans or MRI may be useful if clinicians suspect a complication of sinusitis.3,7
Microbiology
Historically, the microbiology of ABS has been determined by maxillary sinus aspiration. The most recent studies of this method, published in the 1980s, identified Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis as the most common bacterial pathogens.3,6,9 More recently, the microbiology of ABS has been linked with causative pathogens in AOM. The pathogenesis of ABS and AOM are similar, therefore allowing data from tympanocentesis in children with AOM to be used to determine the microbiology of children with ABS.10 Although S pneumoniae, H influenzae, and M catarrhalis remain common causative pathogens in ABS, the introduction of the 7- and 13-valent pneumococcal vaccines has altered the microbiology of AOM, and presumably ABS.11
Importantly, numbers of cases of AOM attributed to S pneumoniae have decreased while those attributed to H influenzae have increased.3,11 In addition, antimicrobial susceptibility of S pneumoniae and the prevalence of β-lactamase–positive H influenzae are important considerations when choosing appropriate antibiotics and can vary significantly by geographic region. Therefore, it is important that clinicians be aware of susceptibility patterns in the communities in which they practice.
Continue for the medical options >>
WHAT ARE THE MEDICAL OPTIONS?
Initial management of ABS in children is contingent on the symptom profile at presentation (persistent, worsening, or severe) and consideration of causative pathogens. While the consensus among experts is that children presenting with severe or worsening symptoms be treated initially with antibiotics,3,4 children who present with mild symptoms consistent with persistent illness may initially be treated with antibiotics or observed for an additional three days. This decision should be made thoughtfully and in collaboration with the patient’s parents or guardians. Any child with ABS who presents with persistent symptoms of illness and is managed initially by observation alone should be reassessed or treated with an antibiotic if symptoms worsen, if new symptoms appear, or if the child fails to improve within 72 hours.
Amoxicillin with or without clavulanate is the antibiotic of choice when ABS has been diagnosed and antibiotic treatment is indicated.1,3 AAP recommendations for antimicrobial treatment of ABS in children are stratified on the basis of age, severity of illness, day care attendance, and history of treatment with amoxicillin in the previous 30 days (see Table 2). Clinicians should rely on their knowledge of drug resistance in their community and judgment regarding severity of illness when choosing between amoxicillin and amoxicillin/clavulanate as initial treatment for ABS.3
Recommendations for duration of antimicrobial treatment for ABS vary considerably. A reasonable suggestion is to continue to treat patients until they are symptom-free for seven days. For many patients, this will mean a 10-day treatment course, with flexibility to increase the duration as needed.3
Children with ABS should be reassessed if there are worsening signs or symptoms or lack of clinical improvement within 72 hours of initial management. Clinicians should evaluate whether the patient has been appropriately diagnosed and assessed for complications of ABS. If the diagnosis is confirmed and ABS complications are not suspected, the clinician may change antibiotics or initiate antibiotic therapy if the child was previously managed by observation only.3,4
A child who is vomiting or unable to tolerate PO medications may benefit from a single 50-gm/kg dose of ceftriaxone IV or IM with follow-up in 24 hours.3 Oral antibiotics may be started at the follow-up visit if the patient demonstrates clinical improvement.
When considering a change in antibiotic, the clinician should consider the possibility of drug resistance. If the child was initially treated with amoxicillin, high-dose amoxicillin/clavulanate may be prescribed. If high-dose amoxicillin/clavulanate was initially prescribed and the patient has not improved or is experiencing worsening symptoms, clindamycin with cefixime, linezolid with cefixime, or levofloxacin may be considered.3,4
Penicillin allergy
Patients with a history of a non–type 1 hypersensitivity reaction to amoxicillin may be treated with cefdinir, cefuroxime, cefpodoxime, or combination therapy with clindamycin plus a third-generation oral cephalosporin. Allergist referral for penicillin or cephalosporin skin testing may be considered before initiation of therapy.3,4,13
Adjuvant therapy
Decongestants, antihistamines, and nasal irrigation are frequently recommended in the management of ABS in children; however, the authors of a 2012 Cochrane review found no properly designed studies to evaluate the effectiveness of these treatments.13 Furthermore, there is insufficient evidence to clearly recommend the use of intranasal steroids as an adjuvant therapy in the treatment of ABS in children (although several randomized controlled studies demonstrate their effectiveness in adolescents and adults).3
Continue for the conclusion >>
CONCLUSION
ABS in children is diagnosed clinically by following a strict set of clinical criteria. Patients commonly present with one of three types of symptoms: persistent URI; severe purulent nasal discharge and fever for at least three consecutive days; or a double sickening. Physical examination findings vary and will not differentiate viral URI symptoms from a diagnosis of ABS. Imaging is not recommended for diagnosis but may be helpful if an orbital or intracranial complication of ABS is suspected.
S pneumoniae, H influenzae, and M catarrhalis continue to be the most common pathogens associated with ABS. However, since the introduction of the 7-valent pneumococcal vaccine, prevalence of H influenzae and β-lactamase–positive H influenzae has increased.
Treatment recommendations vary, based on suspected causative pathogens and presenting symptoms. Amoxicillin or amoxicillin-clavulanate is recommended as firstline antimicrobial treatment for ABS, with alternate antibiotic choices for patients with worsening symptoms or lack of improvement within 72 hours. An awareness of the community’s susceptibility patterns is essential for the clinician who cares for children at risk for ABS.
REFERENCES
1. Wald ER, Nash D, Eickhoff J. Effectiveness of amoxicillin/clavulanate potassium in the treatment of acute bacterial sinusitis in children. Pediatrics. 2009;124(1):9-15.
2. Ray NF, Baraniuk JN, Thamer M, et al. Healthcare expenditures for sinusitis in 1996: contributions of asthma, rhinitis, and other airway disorders. J Allergy Clin Immunol. 1999;103(3 pt 1):408-414.
3. Wald ER, Applegate KE, Bordley C, et al. Clinical practice guideline for the diagnosis and management of acute bacterial sinusitis in children aged 1 to 18 years. Pediatrics. 2013;132(1):e262-e280.
4. Chow AW, Benninger MS, Brook I, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):1041-1045.
5. Shaikh N, Hoberman A, Kearney DH, et al. Signs and symptoms that differentiate acute sinusitis from viral upper respiratory tract infection. Pediatr Infect Dis J. 2013;32(10):1061-1065.
6. Kovatch AL, Wald ER, Ledesma-Medina J, et al. Maxillary sinus radiographs in children with nonrespiratory complaints. Pediatrics. 1984; 73(3):306-308.
7. Triulzi F, Zirpoli S. Imaging techniques in the diagnosis and management of rhinosinusitis in children. Pediatr Allergy Immunol. 2007;18(suppl 18):46-49.
8. Wald ER, Milmoe GJ, Bowen A, et al. Acute maxillary sinusitis in children. N Engl J Med. 1981;304(13):749-754.
9. Wald ER, Reilly JS, Casselbrant M, et al. Treatment of acute maxillary sinusitis in childhood: a comparative study of amoxicillin and cefaclor. J Pediatr. 1984;104(2):297-302.
10. Wald ER. Acute otitis media and acute bacterial sinusitis. Clin Infect Dis. 2011;52(suppl 4):S277-S283.
11. Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29(4):304-309.
12. Pichichero ME. A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients. Pediatrics. 2004;115(4):1048-1057.
13. Shaikh N, Wald ER, Pi M. Decongestants, antihistamines and nasal irrigation for acute sinusitis in children. Cochrane Database Syst Rev. 2012;9:CD007909.
Shoulder dystocia: Taking the fear out of management
Proximal Periprosthetic Femur Fractures: Strategies for Internal Fixation
The rate of total hip arthroplasty (THA) is rising and demand is expected to increase by 174% to 572,000 by 2030.1 The rate of periprosthetic fracture around primary THA is frequently reported at around 1%,2-4 though a recent study of over 32,000 THAs quotes the 20-year probability of periprosthetic fracture at 3.5%.5 Revision THA is also increasing in frequency and associated rates of periprosthetic fracture range from 1.5% to 7.8% following revision THA,3,4,6 with the probability of fracture at 20 years of 11%.7 Projection models predict that the number of periprosthetic fractures will rise by 4.6% per decade over the next 30 years.8
Broadly, treatment options include open reduction internal fixation (ORIF), revision THA, and combined approaches. The Vancouver classification, based on fracture location, stem stability, and bone loss, is often used to guide fracture treatment, with stable implants treated with ORIF and unstable implants requiring revision arthroplasty.
Fixation strategies for treatment of periprosthetic fracture around a well-fixed arthroplasty stem have evolved over time, and there continue to be a variety of available internal fixation options with no clear consensus on the optimal strategy.9 Rates of reoperation following ORIF of periprosthetic femur fracture are reported from 13% to 23%,8,10-12 confirming that there remains room for improvement in management of these injuries.
Locking Plate Fixation
Early fixation strategies included allograft and cables alone as well as nonlocked plate and cerclage constructs. In response to the complication and reoperation rate for nonlocked plate constructs, reported at 33%,13 locking plates were introduced as a treatment option, allowing for both improved osseous vascularity and added screw options.14 When compared to the traditional nonlocked Ogden construct, locking plate constructs are more resistant to axial and torsional load.15 Clinically, the relative risk of nonunion after nonlocking plate fixation is reported at 11.9 times that of fixation with locking plate technology.16
Successful use of lateral locking plate fixation for treatment of this injury has been reported on in several clinical series.17-20 Froberg and colleagues12 evaluated 60 Vancouver B1 and C fractures treated by locking plate osteosynthesis and reported no nonunions, an improvement from previous constructs. However, 8 out of 60 patients with 2-year follow-up required reoperation—4 for infection, 3 for refracture, and 1 for stem loosening—making it clear that the locking plate alone was not a panacea.
With locking plate fixation a mainstay of modern treatment of periprosthetic femur fractures, many questions still remain.
Proximal Fixation
Even with the introduction of locked plates, treatment success after ORIF of Vancouver B1 fractures relies on adequate proximal fixation. Options for proximal fixation around the stem include cerclage wires or cables, unicortical locked screws, obliquely directed bicortical screws, and use of the locking attachment plate to insert bicortical locked screws. These strategies can be used in the presence of cemented or uncemented stems, with biomechanical evidence that screw fixation through the cement mantle does not cause failure.21
Several biomechanical studies address the stiffness and strength of varying proximal fixation strategies. While early fixation relied heavily on cables, the use of cables alone as proximal fixation has been linked to significantly higher rates of failure when compared to other constructs in a large clinical series.11 Multiple biomechanical studies have shown that newer methods of proximal fixation provide more rigid constructs.22,23
Unicortical locked screws appear to outperform cables biomechanically. The use of unicortical screws in lieu of or in addition to cables provides added resistance to lateral bending as well as torsion when compared to cables alone.24 A second group found that unicortical locked screws alone were superior to combined fixation with cerclage wires and unicortical locked screws.25
Added stability can be demonstrated by bicortical fixation strategies, which offer increased rigidity when compared to cables or unicortical screws.22 In vitro work has shown enhanced fixation stability with bicortical screw fixation using the locking attachment plate when compared to cerclage wires alone.23,26 Clinically, some authors have demonstrated success with the use of reversed distal femoral locking plates in order to enhance proximal locking options and allow for bicortical fixation around the stem.19 As noted above, the data favor the opinion that clinical failure rates with cerclage wires alone are high, and biomechanically, bicortical fixation around the femoral stem appears to be superior to unicortical locked screw fixation or cerclage wires. If rigid proximal fixation is desired, an effort should be made to obtain bicortical fixation around the femoral stem.
Allograft
Allograft strut, either alone or in addition to plate osteosynthesis, has long been used in treatment of periprosthetic fractures. Proponents of this technique cite improved biomechanical stability17 and allograft incorporation resulting in restoration of bone stock.
Early treatment of periprosthetic femur fractures consisted solely of allograft and cable fixation, but data on the technique is limited. A small series reported reasonable success, with only 2 out of 19 patients developing nonunion.27 More recently Haddad and colleagues28 reported malunions in 3 out of 19 patients treated with allograft and cables alone. Allograft alone has been largely abandoned in favor of plate fixation, and biomechanical evidence shows that plate and screw or cerclage constructs are more resistant to torsion and lateral bending than allograft with cables alone.29
However, the role of allograft in treatment of periprosthetic femur fractures is not clearly defined. Some authors advocate routinely supplementing plate fixation with allograft28,30 and others go as far as to suggest superior union rates of strut allograft augmented plate fixation when compared to plate fixation alone for periprosthetic fractures around a stable femoral stem.31 However, in that series, the failure rate of 5/11 patients treated with plate alone is higher than current series,12 and others have demonstrated good success without allograft, even with nonlocked plates.32
As recently as 2016, a lateral locking plate supplemented with allograft has been described as a successful technique, with no nonunions reported in a small series.30 However, without a comparison group, it is unclear what role the allograft plays in success in that construct.
Despite some proposed benefits, the additional soft tissue stripping required to place allograft has raised the question of delayed healing and increased infection rate as a result of this technique. A systematic review by Moore and colleagues33 looking at the use of allograft strut in Vancouver B1 fractures found increased time to union (4.4 vs 6.6 months) and deep infection rate (3.8% vs 8.3%) with the use of allograft strut, leading them to recommend cautious use of allograft when treating Vancouver B1 fractures.
With improved fixation strategies available, the role of allograft may be best reserved for patients with inadequate bone stock.
Dual Plate Fixation
Dual plate fixation has been proposed as one mechanism to increase construct strength. A periprosthetic fracture model has shown that, biomechanically, orthogonal plates have higher bending stiffness, torsional stiffness, cycles to failure, and load to failure when compared to a single lateral plate with use of a locking attachment plate proximally.34 Choi and colleagues35 compared lateral locking plates alone, lateral locking plates with allograft, and lateral locking plates with an orthogonal anterior plate and found the addition of an anterior plate resulted in the strongest construct.
Clinically, Müller and colleagues36 reported on a series of 10 patients treated with orthogonal (anterior and lateral) plating for periprosthetic femur fractures, including 3 nonunions. In their series, there was 1 plate failure and they conclude that dual plating is not associated with an increased risk of complications, and can also be used as a salvage procedure.
While the evidence for dual plating is limited, it may provide needed additional stability in certain cases without the added cost and exposure required for allograft.
Minimally Invasive Plate Osteosynthesis
Contrary to the extensive exposure required to place allograft, minimally invasive plate osteosynthesis (MIPO) of periprosthetic femur fractures is advocated by some authors.18,20 Ricci and colleagues18 reported no nonunions in 50 patients treated with indirect reduction techniques and laterally based plating alone without use of allograft. A combination of cables, locking, and nonlocking screws were used. Critical to their technique was preservation of the soft tissue envelope at the level of the fracture.
In further support of MIPO techniques, a systematic review of 1571 periprosthetic hip fractures reported significantly increased risk of nonunion with open approaches when compared to minimally invasive osteosynthesis,16 emphasizing the role of preservation of vascularity in treating these fractures.
Length of Fixation
For some time it was recommended that fixation of Vancouver B1 fractures end 2 cortical diameters below the level of the fracture.37,38 More recently there has been interest in the potential benefits of increased length of fixation.
A biomechanical study comparing long (20-hole) and short (12-hole) plates for periprosthetic fracture with regard to failure found no difference in failure rates between groups.39 While plate length did not appear to affect construct stiffness, the issue of subsequent fracture distal to the construct remains.
Moloney and colleagues40 proposed fixation of Vancouver B1 fractures using plates that span the length of the femur to the level of the femoral condyles to minimize peri-implant failures in osteoporotic patients. In 36 patients treated with standard-length plates, there were 2 fractures distal to the previous fixation compared to no subsequent fractures in 21 patients treated with spanning fixation.
Similarly, in Vancouver C fractures there is some evidence that fixation should span the femoral stem, regardless of available bone for fixation proximal to the fracture. Kubiak and colleagues41 found increasing load to failure and decreased cortical strain in a biomechanical model comparing plates that stop short of the femoral stem with those that span the stem.
Clinically, this concept is supported by Froberg and colleagues.12 In their series of 60 Vancouver B1 and C fractures treated with laterally based locked plating, 3 patients went on to refracture. All of these fractures occurred in patients with Vancouver C fractures treated with plates overlapping the preexisting stem by <50%. The fractures all occurred at the high stress area between the tip of the stem and the end of the plate.
Further support of extended plate length comes from Drew and colleagues,8 who demonstrated a significantly decreased risk of reoperation following ORIF of periprosthetic femur fracture when >75% of the length of the femur was spanned compared to <50%. Although in some settings short fixation may produce satisfactory results, consideration should be given to extending the length of fixation, especially in the osteoporotic population.
Interprosthetic Fractures
With a rising number of patients with ipsilateral hip and knee arthroplasty, the rate of interprosthetic fractures is rising. These fractures present additional challenges given preexisting implants above and below the level of the fracture. The use of a single precontoured laterally based locked plate has been reported with good union rates approaching 90%.42,43 In one series, all nonunions occurred in Vancouver B1 fractures,43 again bringing to light the challenging nature of the B1 fracture.
Nonunion
Success in treating periprosthetic femur fractures has improved with improved fixation methods and understanding of technique. However, current rates of nonunion are still reported up to 27% for B1 and C fractures.44
There is limited evidence on the treatment of periprosthetic femur fracture nonunion. However, treatment is difficult and complication rates are high. Crockarell and colleagues45 reported a 52% overall complication rate in their series of 23 periprosthetic femur fracture nonunions.
Nonunions of the femur near a prosthesis can be treated by revision of the fracture fixation using compression and grafting to achieve bone healing vs revision of the joint prosthesis to span the area of the nonunited bone. Case-by-case decision-making is based on the remaining bone stock and the type of revision prosthesis necessary to span the problem area. Given the challenges associated with their treatment, a focus on prevention of nonunion is of paramount importance.
Authors’ Preferred Treatment
Our treatment of periprosthetic femur fractures with a well-fixed hip arthroplasty stem adheres to the principles supported in the literature (Figures 1A-1D and Figures 2A, 2B).
- Soft tissue friendly dissection with limited exposure at the fracture site is preferred as the fracture allows, particularly in cases with comminution where a direct assessment of the reduction is not available.
- Plate fixation strategy is dictated by the characteristics of the fracture. Fracture patterns amenable to anatomic reduction receive interfragmentary compression and absolute stability constructs. Highly comminuted fractures receive relatively stable bridging constructs to encourage callous.
- Locking screws are used rarely in diaphyseal fracture patterns, and when employed, are applied to only one side of the fracture to limit “over stiffening” the construct.
- Liberal use of dual plating, both as a method of maintaining fracture reduction while a structural plate is applied and increasing construct rigidity.
- Proximal fixation relies heavily on bicortical screws placed through the holes of the lateral plate. Cerclage wires and unicortical screws are rarely used in our practice. In the case of larger stems, a bicortical 3.5-mm screw can be placed through a 4.5-mm plate using a reduction washer.
Summary
Techniques for treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty stem are constantly evolving. Several principles have emerged to decrease rates of treatment failure and subsequent reoperation. While there are several methods to do so, it is critical to achieve stable proximal fixation. Long spanning fixation constructs are linked to lower failure and reoperation rates in both B1 and C type fractures. Additionally, the importance of soft tissue management and maintenance of local vascularity should not be underestimated.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Lewallen DG, Berry DJ. Periprosthetic fracture of the femur after total hip arthroplasty: treatment and results to date. Instr Course Lect. 1998;47:243-249.
3. Kavanagh BF. Femoral fractures associated with total hip arthroplasty. Orthop Clin North Am. 1992;23(2):249-257.
4. Meek RM, Norwood T, Smith R, Brenkel IJ, Howie CR. The risk of peri-prosthetic fracture after primary and revision total hip and knee replacement. J Bone Joint Surg Br. 2011;93(1):96-101.
5. Abdel MP, Watts CD, Houdek MT, Lewallen DG, Berry DJ. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J. 2016;98-B(4):461-467.
6. Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am. 1999;30(2):183-190.
7. Abdel MP, Houdek MT, Watts CD, Lewallen DG, Berry DJ. Epidemiology of periprosthetic femoral fractures in 5417 revision total hip arthrolasties: a 40-year experience. Bone Joint J. 2016;98-B(4):468-474.
8. Drew JM, Griffin WL, Odum SM, Van Doren B, Weston BT, Stryker LS. Survivorship after periprosthetic femur fracture: factors affecting outcome. J Arthroplasty. 2015. [Epub ahead of print]
9. Dehghan N, McKee MD, Nauth A, Ristevski B, Schemitsch EH. Surgical fixation of Vancouver type B1 periprosthetic femur fractures: a systematic review. J Orthop Trauma. 2014;28(12):721-727.
10. Mukundan C, Rayan F, Kheir E, Macdonald D. Management of late periprosthetic femur fractures: a retrospective cohort of 72 patients. Int Orthop. 2010;34(4):485-489.
11. Lindahl H, Malchau H, Odén A, Garellick G. Risk factors for failure after treatment of a periprosthetic fracture of the femur. J Bone Joint Surg Br. 2006;88(1):26-30.
12. Froberg L, Troelsen A, Brix M. Periprosthetic Vancouver type B1 and C fractures treated by locking-plate osteosynthesis: fracture union and reoperations in 60 consecutive fractures. Acta Orthop. 2012;83(6):648-652.
13. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996(327):238-246.
14. Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84(8):1093-1110.
15. Fulkerson E, Koval K, Preston CF, Iesaka K, Kummer FJ, Egol KA. Fixation of periprosthetic femoral shaft fractures associated with cemented femoral stems: a biomechanical comparison of locked plating and conventional cable plates. J Orthop Trauma. 2006;20(2):89-93.
16. Stoffel K, Sommer C, Kalampoki V, Blumenthal A, Joeris A. The influence of the operation technique and implant used in the treatment of periprosthetic hip and interprosthetic femur fractures: a systematic literature review of 1571 cases. Arch Orthop Trauma Surg. 2016;136(4):553-561.
17. Fulkerson E, Tejwani N, Stuchin S, Egol K. Management of periprosthetic femur fractures with a first generation locking plate. Injury. 2007;38(8):965-972.
18. Ricci WM, Bolhofner BR, Loftus T, Cox C, Mitchell S, Borrelli J Jr. Indirect reduction and plate fixation, without grafting, for periprosthetic femoral shaft fractures about a stable intramedullary implant. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 2:275-282.
19. Ebraheim NA, Gomez C, Ramineni SK, Liu J. Fixation of periprosthetic femoral shaft fractures adjacent to a well-fixed femoral stem with reversed distal femoral locking plate. J Trauma. 2009;66(4):1152-1157.
20. Bryant GK, Morshed S, Agel J, et al. Isolated locked compression plating for Vancouver Type B1 periprosthetic femoral fractures. Injury. 2009;40(11):1180-1186.
21. Giesinger K, Ebneter L, Day RE, Stoffel KK, Yates PJ, Kuster MS. Can plate osteosynthesis of periprosthethic femoral fractures cause cement mantle failure around a stable hip stem? A biomechanical analysis. J Arthroplasty. 2014;29(6):1308-1312.
22. Lewis GS, Caroom CT, Wee H, et al. Tangential bicortical locked fixation improves stability in vancouver B1 periprosthetic femur fractures: a biomechanical study. J Orthop Trauma. 2015;29(10):e364-e370.
23. Lenz M, Perren SM, Gueorguiev B, et al. A biomechanical study on proximal plate fixation techniques in periprosthetic femur fractures. Injury. 2014;45 Suppl 1:S71-S75.
24. Dennis MG, Simon JA, Kummer FJ, Koval KJ, DiCesare PE. Fixation of periprosthetic femoral shaft fractures occurring at the tip of the stem: a biomechanical study of 5 techniques. J Arthroplasty. 2000;15(4):523-528.
25. Graham SM, Mak JH, Moazen M, et al. Periprosthetic femoral fracture fixation: a biomechanical comparison between proximal locking screws and cables. J Orthop Sci. 2015;20(5):875-880.
26. Griffiths JT, Taheri A, Day RE, Yates PJ. Better axial stiffness of a bicortical screw construct compared to a cable construct for comminuted Vancouver B1 proximal femoral fractures. J Arthroplasty. 2015;30(12):2333-2337.
27. Chandler HP, King D, Limbird R, et al. The use of cortical allograft struts for fixation of fractures associated with well-fixed total joint prostheses. Semin Arthroplasty. 1993;4(2):99-107.
28. Haddad FS, Duncan CP, Berry DJ, Lewallen DG, Gross AE, Chandler HP. Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am. 2002;84-A(6):945-950.
29. Dennis MG, Simon JA, Kummer FJ, Koval KJ, Di Cesare PE. Fixation of periprosthetic femoral shaft fractures: a biomechanical comparison of two techniques. J Orthop Trauma. 2001;15(3):177-180.
30. Yeo I, Rhyu KH, Kim SM, Park YS, Lim SJ. High union rates of locking compression plating with cortical strut allograft for type B1 periprosthetic femoral fractures. Int Orthop. 2016. [Epub ahead of print]
31. Khashan M, Amar E, Drexler M, Chechik Ok, Cohen Z, Steinberg EL. Superior outcome of strut allograft-augmented plate fixation for the treatment of periprosthetic fractures around a stable femoral stem. Injury. 2013;44(11):1556-1560.
32. Old AB, McGrory BJ, White RR, Babikian GM. Fixation of Vancouver B1 peri-prosthetic fractures by broad metal plates without the application of strut allografts. J Bone Joint Surg Br. 2006;88(11):1425-1429.
33. Moore RE, Baldwin K, Austin MS, Mehta S. A systematic review of open reduction and internal fixation of periprosthetic femur fractures with or without allograft strut, cerclage, and locked plates. J Arthroplasty. 2014;29(5):872-876.
34. Lenz M, Stoffel K, Gueorguiev B, Klos K, Kielstein H, Hofmann GO. Enhancing fixation strength in periprosthetic femur fractures by orthogonal plating-a biomechanical study. J Orthop Res. 2016;34(4):591-596.
35. Choi JK, Gardner TR, Yoon E, Morrison TA, Macaulay WB, Geller JA. The effect of fixation technique on the stiffness of comminuted Vancouver B1 periprosthetic femur fractures. J Arthroplasty. 2010;25(6 Suppl):124-128.
36. Müller FJ, Galler M Füchtmeier B. Clinical and radiological results of patients treated with orthogonal double plating for periprosthetic femoral fractures. Int Orthop. 2014;38(12):2469-2472.
37. Pike J, Davidson D, Garbuz D, Duncan CP, O’Brien PJ, Masri BA. Principles of treatment for periprosthetic femoral shaft fractures around well-fixed total hip arthroplasty. J Am Acad Orthop Surg. 2009;17(11):677-688.
38. Serocki JH, Chandler RW, Dorr LD. Treatment of fractures about hip prostheses with compression plating. J Arthroplasty. 1992;7(2):129-135.
39. Pletka JD, Marsland D, Belkoff SM, Mears SC, Kates SL. Biomechanical comparison of 2 different locking plate fixation methods in vancouver b1 periprosthetic femur fractures. Geriatr Orthop Surg Rehabil. 2011;2(2):51-55.
40. Moloney GB, Westrick ER, Siska PA, Tarkin IS. Treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty implant: span the whole bone. Arch Orthop Trauma Surg. 2014;134(1):9-14.
41. Kubiak EN, Haller JM, Kemper DD, Presson AP, Higgins TF, Horowitz DS. Does the lateral plate need to overlap the stem to mitigate stress concentration when treating Vancouver C periprosthetic supracondylar femur fracture? J Arthroplasty. 2015;30(1):104-108.
42. Sah AP, Marshall A, Virkus WV, Estok DM 2nd, Della Valle CJ. Interprosthetic fractures of the femur: treatment with a single-locked plate. J Arthroplasty. 2010;25(2):280-286.
43. Hoffmann MF, Lotzien S, Schildhauer TA. Clinical outcome of interprosthetic femoral fractures treated with polyaxial locking plates. Injury. 2016. [Epub ahead of print]
44. Holder N, Papp S, Gofton W, Beaulé PE. Outcomes following surgical treatment of periprosthetic femur fractures: a single centre series. Can J Surg. 2014;57(3):209-213.
45. Crockarell JR Jr, Berry DJ, Lewallen DG. Nonunion after periprosthetic femoral fracture associated with total hip arthroplasty. J Bone Joint Surg Am. 1999;81(8):1073-1079.
The rate of total hip arthroplasty (THA) is rising and demand is expected to increase by 174% to 572,000 by 2030.1 The rate of periprosthetic fracture around primary THA is frequently reported at around 1%,2-4 though a recent study of over 32,000 THAs quotes the 20-year probability of periprosthetic fracture at 3.5%.5 Revision THA is also increasing in frequency and associated rates of periprosthetic fracture range from 1.5% to 7.8% following revision THA,3,4,6 with the probability of fracture at 20 years of 11%.7 Projection models predict that the number of periprosthetic fractures will rise by 4.6% per decade over the next 30 years.8
Broadly, treatment options include open reduction internal fixation (ORIF), revision THA, and combined approaches. The Vancouver classification, based on fracture location, stem stability, and bone loss, is often used to guide fracture treatment, with stable implants treated with ORIF and unstable implants requiring revision arthroplasty.
Fixation strategies for treatment of periprosthetic fracture around a well-fixed arthroplasty stem have evolved over time, and there continue to be a variety of available internal fixation options with no clear consensus on the optimal strategy.9 Rates of reoperation following ORIF of periprosthetic femur fracture are reported from 13% to 23%,8,10-12 confirming that there remains room for improvement in management of these injuries.
Locking Plate Fixation
Early fixation strategies included allograft and cables alone as well as nonlocked plate and cerclage constructs. In response to the complication and reoperation rate for nonlocked plate constructs, reported at 33%,13 locking plates were introduced as a treatment option, allowing for both improved osseous vascularity and added screw options.14 When compared to the traditional nonlocked Ogden construct, locking plate constructs are more resistant to axial and torsional load.15 Clinically, the relative risk of nonunion after nonlocking plate fixation is reported at 11.9 times that of fixation with locking plate technology.16
Successful use of lateral locking plate fixation for treatment of this injury has been reported on in several clinical series.17-20 Froberg and colleagues12 evaluated 60 Vancouver B1 and C fractures treated by locking plate osteosynthesis and reported no nonunions, an improvement from previous constructs. However, 8 out of 60 patients with 2-year follow-up required reoperation—4 for infection, 3 for refracture, and 1 for stem loosening—making it clear that the locking plate alone was not a panacea.
With locking plate fixation a mainstay of modern treatment of periprosthetic femur fractures, many questions still remain.
Proximal Fixation
Even with the introduction of locked plates, treatment success after ORIF of Vancouver B1 fractures relies on adequate proximal fixation. Options for proximal fixation around the stem include cerclage wires or cables, unicortical locked screws, obliquely directed bicortical screws, and use of the locking attachment plate to insert bicortical locked screws. These strategies can be used in the presence of cemented or uncemented stems, with biomechanical evidence that screw fixation through the cement mantle does not cause failure.21
Several biomechanical studies address the stiffness and strength of varying proximal fixation strategies. While early fixation relied heavily on cables, the use of cables alone as proximal fixation has been linked to significantly higher rates of failure when compared to other constructs in a large clinical series.11 Multiple biomechanical studies have shown that newer methods of proximal fixation provide more rigid constructs.22,23
Unicortical locked screws appear to outperform cables biomechanically. The use of unicortical screws in lieu of or in addition to cables provides added resistance to lateral bending as well as torsion when compared to cables alone.24 A second group found that unicortical locked screws alone were superior to combined fixation with cerclage wires and unicortical locked screws.25
Added stability can be demonstrated by bicortical fixation strategies, which offer increased rigidity when compared to cables or unicortical screws.22 In vitro work has shown enhanced fixation stability with bicortical screw fixation using the locking attachment plate when compared to cerclage wires alone.23,26 Clinically, some authors have demonstrated success with the use of reversed distal femoral locking plates in order to enhance proximal locking options and allow for bicortical fixation around the stem.19 As noted above, the data favor the opinion that clinical failure rates with cerclage wires alone are high, and biomechanically, bicortical fixation around the femoral stem appears to be superior to unicortical locked screw fixation or cerclage wires. If rigid proximal fixation is desired, an effort should be made to obtain bicortical fixation around the femoral stem.
Allograft
Allograft strut, either alone or in addition to plate osteosynthesis, has long been used in treatment of periprosthetic fractures. Proponents of this technique cite improved biomechanical stability17 and allograft incorporation resulting in restoration of bone stock.
Early treatment of periprosthetic femur fractures consisted solely of allograft and cable fixation, but data on the technique is limited. A small series reported reasonable success, with only 2 out of 19 patients developing nonunion.27 More recently Haddad and colleagues28 reported malunions in 3 out of 19 patients treated with allograft and cables alone. Allograft alone has been largely abandoned in favor of plate fixation, and biomechanical evidence shows that plate and screw or cerclage constructs are more resistant to torsion and lateral bending than allograft with cables alone.29
However, the role of allograft in treatment of periprosthetic femur fractures is not clearly defined. Some authors advocate routinely supplementing plate fixation with allograft28,30 and others go as far as to suggest superior union rates of strut allograft augmented plate fixation when compared to plate fixation alone for periprosthetic fractures around a stable femoral stem.31 However, in that series, the failure rate of 5/11 patients treated with plate alone is higher than current series,12 and others have demonstrated good success without allograft, even with nonlocked plates.32
As recently as 2016, a lateral locking plate supplemented with allograft has been described as a successful technique, with no nonunions reported in a small series.30 However, without a comparison group, it is unclear what role the allograft plays in success in that construct.
Despite some proposed benefits, the additional soft tissue stripping required to place allograft has raised the question of delayed healing and increased infection rate as a result of this technique. A systematic review by Moore and colleagues33 looking at the use of allograft strut in Vancouver B1 fractures found increased time to union (4.4 vs 6.6 months) and deep infection rate (3.8% vs 8.3%) with the use of allograft strut, leading them to recommend cautious use of allograft when treating Vancouver B1 fractures.
With improved fixation strategies available, the role of allograft may be best reserved for patients with inadequate bone stock.
Dual Plate Fixation
Dual plate fixation has been proposed as one mechanism to increase construct strength. A periprosthetic fracture model has shown that, biomechanically, orthogonal plates have higher bending stiffness, torsional stiffness, cycles to failure, and load to failure when compared to a single lateral plate with use of a locking attachment plate proximally.34 Choi and colleagues35 compared lateral locking plates alone, lateral locking plates with allograft, and lateral locking plates with an orthogonal anterior plate and found the addition of an anterior plate resulted in the strongest construct.
Clinically, Müller and colleagues36 reported on a series of 10 patients treated with orthogonal (anterior and lateral) plating for periprosthetic femur fractures, including 3 nonunions. In their series, there was 1 plate failure and they conclude that dual plating is not associated with an increased risk of complications, and can also be used as a salvage procedure.
While the evidence for dual plating is limited, it may provide needed additional stability in certain cases without the added cost and exposure required for allograft.
Minimally Invasive Plate Osteosynthesis
Contrary to the extensive exposure required to place allograft, minimally invasive plate osteosynthesis (MIPO) of periprosthetic femur fractures is advocated by some authors.18,20 Ricci and colleagues18 reported no nonunions in 50 patients treated with indirect reduction techniques and laterally based plating alone without use of allograft. A combination of cables, locking, and nonlocking screws were used. Critical to their technique was preservation of the soft tissue envelope at the level of the fracture.
In further support of MIPO techniques, a systematic review of 1571 periprosthetic hip fractures reported significantly increased risk of nonunion with open approaches when compared to minimally invasive osteosynthesis,16 emphasizing the role of preservation of vascularity in treating these fractures.
Length of Fixation
For some time it was recommended that fixation of Vancouver B1 fractures end 2 cortical diameters below the level of the fracture.37,38 More recently there has been interest in the potential benefits of increased length of fixation.
A biomechanical study comparing long (20-hole) and short (12-hole) plates for periprosthetic fracture with regard to failure found no difference in failure rates between groups.39 While plate length did not appear to affect construct stiffness, the issue of subsequent fracture distal to the construct remains.
Moloney and colleagues40 proposed fixation of Vancouver B1 fractures using plates that span the length of the femur to the level of the femoral condyles to minimize peri-implant failures in osteoporotic patients. In 36 patients treated with standard-length plates, there were 2 fractures distal to the previous fixation compared to no subsequent fractures in 21 patients treated with spanning fixation.
Similarly, in Vancouver C fractures there is some evidence that fixation should span the femoral stem, regardless of available bone for fixation proximal to the fracture. Kubiak and colleagues41 found increasing load to failure and decreased cortical strain in a biomechanical model comparing plates that stop short of the femoral stem with those that span the stem.
Clinically, this concept is supported by Froberg and colleagues.12 In their series of 60 Vancouver B1 and C fractures treated with laterally based locked plating, 3 patients went on to refracture. All of these fractures occurred in patients with Vancouver C fractures treated with plates overlapping the preexisting stem by <50%. The fractures all occurred at the high stress area between the tip of the stem and the end of the plate.
Further support of extended plate length comes from Drew and colleagues,8 who demonstrated a significantly decreased risk of reoperation following ORIF of periprosthetic femur fracture when >75% of the length of the femur was spanned compared to <50%. Although in some settings short fixation may produce satisfactory results, consideration should be given to extending the length of fixation, especially in the osteoporotic population.
Interprosthetic Fractures
With a rising number of patients with ipsilateral hip and knee arthroplasty, the rate of interprosthetic fractures is rising. These fractures present additional challenges given preexisting implants above and below the level of the fracture. The use of a single precontoured laterally based locked plate has been reported with good union rates approaching 90%.42,43 In one series, all nonunions occurred in Vancouver B1 fractures,43 again bringing to light the challenging nature of the B1 fracture.
Nonunion
Success in treating periprosthetic femur fractures has improved with improved fixation methods and understanding of technique. However, current rates of nonunion are still reported up to 27% for B1 and C fractures.44
There is limited evidence on the treatment of periprosthetic femur fracture nonunion. However, treatment is difficult and complication rates are high. Crockarell and colleagues45 reported a 52% overall complication rate in their series of 23 periprosthetic femur fracture nonunions.
Nonunions of the femur near a prosthesis can be treated by revision of the fracture fixation using compression and grafting to achieve bone healing vs revision of the joint prosthesis to span the area of the nonunited bone. Case-by-case decision-making is based on the remaining bone stock and the type of revision prosthesis necessary to span the problem area. Given the challenges associated with their treatment, a focus on prevention of nonunion is of paramount importance.
Authors’ Preferred Treatment
Our treatment of periprosthetic femur fractures with a well-fixed hip arthroplasty stem adheres to the principles supported in the literature (Figures 1A-1D and Figures 2A, 2B).
- Soft tissue friendly dissection with limited exposure at the fracture site is preferred as the fracture allows, particularly in cases with comminution where a direct assessment of the reduction is not available.
- Plate fixation strategy is dictated by the characteristics of the fracture. Fracture patterns amenable to anatomic reduction receive interfragmentary compression and absolute stability constructs. Highly comminuted fractures receive relatively stable bridging constructs to encourage callous.
- Locking screws are used rarely in diaphyseal fracture patterns, and when employed, are applied to only one side of the fracture to limit “over stiffening” the construct.
- Liberal use of dual plating, both as a method of maintaining fracture reduction while a structural plate is applied and increasing construct rigidity.
- Proximal fixation relies heavily on bicortical screws placed through the holes of the lateral plate. Cerclage wires and unicortical screws are rarely used in our practice. In the case of larger stems, a bicortical 3.5-mm screw can be placed through a 4.5-mm plate using a reduction washer.
Summary
Techniques for treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty stem are constantly evolving. Several principles have emerged to decrease rates of treatment failure and subsequent reoperation. While there are several methods to do so, it is critical to achieve stable proximal fixation. Long spanning fixation constructs are linked to lower failure and reoperation rates in both B1 and C type fractures. Additionally, the importance of soft tissue management and maintenance of local vascularity should not be underestimated.
The rate of total hip arthroplasty (THA) is rising and demand is expected to increase by 174% to 572,000 by 2030.1 The rate of periprosthetic fracture around primary THA is frequently reported at around 1%,2-4 though a recent study of over 32,000 THAs quotes the 20-year probability of periprosthetic fracture at 3.5%.5 Revision THA is also increasing in frequency and associated rates of periprosthetic fracture range from 1.5% to 7.8% following revision THA,3,4,6 with the probability of fracture at 20 years of 11%.7 Projection models predict that the number of periprosthetic fractures will rise by 4.6% per decade over the next 30 years.8
Broadly, treatment options include open reduction internal fixation (ORIF), revision THA, and combined approaches. The Vancouver classification, based on fracture location, stem stability, and bone loss, is often used to guide fracture treatment, with stable implants treated with ORIF and unstable implants requiring revision arthroplasty.
Fixation strategies for treatment of periprosthetic fracture around a well-fixed arthroplasty stem have evolved over time, and there continue to be a variety of available internal fixation options with no clear consensus on the optimal strategy.9 Rates of reoperation following ORIF of periprosthetic femur fracture are reported from 13% to 23%,8,10-12 confirming that there remains room for improvement in management of these injuries.
Locking Plate Fixation
Early fixation strategies included allograft and cables alone as well as nonlocked plate and cerclage constructs. In response to the complication and reoperation rate for nonlocked plate constructs, reported at 33%,13 locking plates were introduced as a treatment option, allowing for both improved osseous vascularity and added screw options.14 When compared to the traditional nonlocked Ogden construct, locking plate constructs are more resistant to axial and torsional load.15 Clinically, the relative risk of nonunion after nonlocking plate fixation is reported at 11.9 times that of fixation with locking plate technology.16
Successful use of lateral locking plate fixation for treatment of this injury has been reported on in several clinical series.17-20 Froberg and colleagues12 evaluated 60 Vancouver B1 and C fractures treated by locking plate osteosynthesis and reported no nonunions, an improvement from previous constructs. However, 8 out of 60 patients with 2-year follow-up required reoperation—4 for infection, 3 for refracture, and 1 for stem loosening—making it clear that the locking plate alone was not a panacea.
With locking plate fixation a mainstay of modern treatment of periprosthetic femur fractures, many questions still remain.
Proximal Fixation
Even with the introduction of locked plates, treatment success after ORIF of Vancouver B1 fractures relies on adequate proximal fixation. Options for proximal fixation around the stem include cerclage wires or cables, unicortical locked screws, obliquely directed bicortical screws, and use of the locking attachment plate to insert bicortical locked screws. These strategies can be used in the presence of cemented or uncemented stems, with biomechanical evidence that screw fixation through the cement mantle does not cause failure.21
Several biomechanical studies address the stiffness and strength of varying proximal fixation strategies. While early fixation relied heavily on cables, the use of cables alone as proximal fixation has been linked to significantly higher rates of failure when compared to other constructs in a large clinical series.11 Multiple biomechanical studies have shown that newer methods of proximal fixation provide more rigid constructs.22,23
Unicortical locked screws appear to outperform cables biomechanically. The use of unicortical screws in lieu of or in addition to cables provides added resistance to lateral bending as well as torsion when compared to cables alone.24 A second group found that unicortical locked screws alone were superior to combined fixation with cerclage wires and unicortical locked screws.25
Added stability can be demonstrated by bicortical fixation strategies, which offer increased rigidity when compared to cables or unicortical screws.22 In vitro work has shown enhanced fixation stability with bicortical screw fixation using the locking attachment plate when compared to cerclage wires alone.23,26 Clinically, some authors have demonstrated success with the use of reversed distal femoral locking plates in order to enhance proximal locking options and allow for bicortical fixation around the stem.19 As noted above, the data favor the opinion that clinical failure rates with cerclage wires alone are high, and biomechanically, bicortical fixation around the femoral stem appears to be superior to unicortical locked screw fixation or cerclage wires. If rigid proximal fixation is desired, an effort should be made to obtain bicortical fixation around the femoral stem.
Allograft
Allograft strut, either alone or in addition to plate osteosynthesis, has long been used in treatment of periprosthetic fractures. Proponents of this technique cite improved biomechanical stability17 and allograft incorporation resulting in restoration of bone stock.
Early treatment of periprosthetic femur fractures consisted solely of allograft and cable fixation, but data on the technique is limited. A small series reported reasonable success, with only 2 out of 19 patients developing nonunion.27 More recently Haddad and colleagues28 reported malunions in 3 out of 19 patients treated with allograft and cables alone. Allograft alone has been largely abandoned in favor of plate fixation, and biomechanical evidence shows that plate and screw or cerclage constructs are more resistant to torsion and lateral bending than allograft with cables alone.29
However, the role of allograft in treatment of periprosthetic femur fractures is not clearly defined. Some authors advocate routinely supplementing plate fixation with allograft28,30 and others go as far as to suggest superior union rates of strut allograft augmented plate fixation when compared to plate fixation alone for periprosthetic fractures around a stable femoral stem.31 However, in that series, the failure rate of 5/11 patients treated with plate alone is higher than current series,12 and others have demonstrated good success without allograft, even with nonlocked plates.32
As recently as 2016, a lateral locking plate supplemented with allograft has been described as a successful technique, with no nonunions reported in a small series.30 However, without a comparison group, it is unclear what role the allograft plays in success in that construct.
Despite some proposed benefits, the additional soft tissue stripping required to place allograft has raised the question of delayed healing and increased infection rate as a result of this technique. A systematic review by Moore and colleagues33 looking at the use of allograft strut in Vancouver B1 fractures found increased time to union (4.4 vs 6.6 months) and deep infection rate (3.8% vs 8.3%) with the use of allograft strut, leading them to recommend cautious use of allograft when treating Vancouver B1 fractures.
With improved fixation strategies available, the role of allograft may be best reserved for patients with inadequate bone stock.
Dual Plate Fixation
Dual plate fixation has been proposed as one mechanism to increase construct strength. A periprosthetic fracture model has shown that, biomechanically, orthogonal plates have higher bending stiffness, torsional stiffness, cycles to failure, and load to failure when compared to a single lateral plate with use of a locking attachment plate proximally.34 Choi and colleagues35 compared lateral locking plates alone, lateral locking plates with allograft, and lateral locking plates with an orthogonal anterior plate and found the addition of an anterior plate resulted in the strongest construct.
Clinically, Müller and colleagues36 reported on a series of 10 patients treated with orthogonal (anterior and lateral) plating for periprosthetic femur fractures, including 3 nonunions. In their series, there was 1 plate failure and they conclude that dual plating is not associated with an increased risk of complications, and can also be used as a salvage procedure.
While the evidence for dual plating is limited, it may provide needed additional stability in certain cases without the added cost and exposure required for allograft.
Minimally Invasive Plate Osteosynthesis
Contrary to the extensive exposure required to place allograft, minimally invasive plate osteosynthesis (MIPO) of periprosthetic femur fractures is advocated by some authors.18,20 Ricci and colleagues18 reported no nonunions in 50 patients treated with indirect reduction techniques and laterally based plating alone without use of allograft. A combination of cables, locking, and nonlocking screws were used. Critical to their technique was preservation of the soft tissue envelope at the level of the fracture.
In further support of MIPO techniques, a systematic review of 1571 periprosthetic hip fractures reported significantly increased risk of nonunion with open approaches when compared to minimally invasive osteosynthesis,16 emphasizing the role of preservation of vascularity in treating these fractures.
Length of Fixation
For some time it was recommended that fixation of Vancouver B1 fractures end 2 cortical diameters below the level of the fracture.37,38 More recently there has been interest in the potential benefits of increased length of fixation.
A biomechanical study comparing long (20-hole) and short (12-hole) plates for periprosthetic fracture with regard to failure found no difference in failure rates between groups.39 While plate length did not appear to affect construct stiffness, the issue of subsequent fracture distal to the construct remains.
Moloney and colleagues40 proposed fixation of Vancouver B1 fractures using plates that span the length of the femur to the level of the femoral condyles to minimize peri-implant failures in osteoporotic patients. In 36 patients treated with standard-length plates, there were 2 fractures distal to the previous fixation compared to no subsequent fractures in 21 patients treated with spanning fixation.
Similarly, in Vancouver C fractures there is some evidence that fixation should span the femoral stem, regardless of available bone for fixation proximal to the fracture. Kubiak and colleagues41 found increasing load to failure and decreased cortical strain in a biomechanical model comparing plates that stop short of the femoral stem with those that span the stem.
Clinically, this concept is supported by Froberg and colleagues.12 In their series of 60 Vancouver B1 and C fractures treated with laterally based locked plating, 3 patients went on to refracture. All of these fractures occurred in patients with Vancouver C fractures treated with plates overlapping the preexisting stem by <50%. The fractures all occurred at the high stress area between the tip of the stem and the end of the plate.
Further support of extended plate length comes from Drew and colleagues,8 who demonstrated a significantly decreased risk of reoperation following ORIF of periprosthetic femur fracture when >75% of the length of the femur was spanned compared to <50%. Although in some settings short fixation may produce satisfactory results, consideration should be given to extending the length of fixation, especially in the osteoporotic population.
Interprosthetic Fractures
With a rising number of patients with ipsilateral hip and knee arthroplasty, the rate of interprosthetic fractures is rising. These fractures present additional challenges given preexisting implants above and below the level of the fracture. The use of a single precontoured laterally based locked plate has been reported with good union rates approaching 90%.42,43 In one series, all nonunions occurred in Vancouver B1 fractures,43 again bringing to light the challenging nature of the B1 fracture.
Nonunion
Success in treating periprosthetic femur fractures has improved with improved fixation methods and understanding of technique. However, current rates of nonunion are still reported up to 27% for B1 and C fractures.44
There is limited evidence on the treatment of periprosthetic femur fracture nonunion. However, treatment is difficult and complication rates are high. Crockarell and colleagues45 reported a 52% overall complication rate in their series of 23 periprosthetic femur fracture nonunions.
Nonunions of the femur near a prosthesis can be treated by revision of the fracture fixation using compression and grafting to achieve bone healing vs revision of the joint prosthesis to span the area of the nonunited bone. Case-by-case decision-making is based on the remaining bone stock and the type of revision prosthesis necessary to span the problem area. Given the challenges associated with their treatment, a focus on prevention of nonunion is of paramount importance.
Authors’ Preferred Treatment
Our treatment of periprosthetic femur fractures with a well-fixed hip arthroplasty stem adheres to the principles supported in the literature (Figures 1A-1D and Figures 2A, 2B).
- Soft tissue friendly dissection with limited exposure at the fracture site is preferred as the fracture allows, particularly in cases with comminution where a direct assessment of the reduction is not available.
- Plate fixation strategy is dictated by the characteristics of the fracture. Fracture patterns amenable to anatomic reduction receive interfragmentary compression and absolute stability constructs. Highly comminuted fractures receive relatively stable bridging constructs to encourage callous.
- Locking screws are used rarely in diaphyseal fracture patterns, and when employed, are applied to only one side of the fracture to limit “over stiffening” the construct.
- Liberal use of dual plating, both as a method of maintaining fracture reduction while a structural plate is applied and increasing construct rigidity.
- Proximal fixation relies heavily on bicortical screws placed through the holes of the lateral plate. Cerclage wires and unicortical screws are rarely used in our practice. In the case of larger stems, a bicortical 3.5-mm screw can be placed through a 4.5-mm plate using a reduction washer.
Summary
Techniques for treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty stem are constantly evolving. Several principles have emerged to decrease rates of treatment failure and subsequent reoperation. While there are several methods to do so, it is critical to achieve stable proximal fixation. Long spanning fixation constructs are linked to lower failure and reoperation rates in both B1 and C type fractures. Additionally, the importance of soft tissue management and maintenance of local vascularity should not be underestimated.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Lewallen DG, Berry DJ. Periprosthetic fracture of the femur after total hip arthroplasty: treatment and results to date. Instr Course Lect. 1998;47:243-249.
3. Kavanagh BF. Femoral fractures associated with total hip arthroplasty. Orthop Clin North Am. 1992;23(2):249-257.
4. Meek RM, Norwood T, Smith R, Brenkel IJ, Howie CR. The risk of peri-prosthetic fracture after primary and revision total hip and knee replacement. J Bone Joint Surg Br. 2011;93(1):96-101.
5. Abdel MP, Watts CD, Houdek MT, Lewallen DG, Berry DJ. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J. 2016;98-B(4):461-467.
6. Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am. 1999;30(2):183-190.
7. Abdel MP, Houdek MT, Watts CD, Lewallen DG, Berry DJ. Epidemiology of periprosthetic femoral fractures in 5417 revision total hip arthrolasties: a 40-year experience. Bone Joint J. 2016;98-B(4):468-474.
8. Drew JM, Griffin WL, Odum SM, Van Doren B, Weston BT, Stryker LS. Survivorship after periprosthetic femur fracture: factors affecting outcome. J Arthroplasty. 2015. [Epub ahead of print]
9. Dehghan N, McKee MD, Nauth A, Ristevski B, Schemitsch EH. Surgical fixation of Vancouver type B1 periprosthetic femur fractures: a systematic review. J Orthop Trauma. 2014;28(12):721-727.
10. Mukundan C, Rayan F, Kheir E, Macdonald D. Management of late periprosthetic femur fractures: a retrospective cohort of 72 patients. Int Orthop. 2010;34(4):485-489.
11. Lindahl H, Malchau H, Odén A, Garellick G. Risk factors for failure after treatment of a periprosthetic fracture of the femur. J Bone Joint Surg Br. 2006;88(1):26-30.
12. Froberg L, Troelsen A, Brix M. Periprosthetic Vancouver type B1 and C fractures treated by locking-plate osteosynthesis: fracture union and reoperations in 60 consecutive fractures. Acta Orthop. 2012;83(6):648-652.
13. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996(327):238-246.
14. Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84(8):1093-1110.
15. Fulkerson E, Koval K, Preston CF, Iesaka K, Kummer FJ, Egol KA. Fixation of periprosthetic femoral shaft fractures associated with cemented femoral stems: a biomechanical comparison of locked plating and conventional cable plates. J Orthop Trauma. 2006;20(2):89-93.
16. Stoffel K, Sommer C, Kalampoki V, Blumenthal A, Joeris A. The influence of the operation technique and implant used in the treatment of periprosthetic hip and interprosthetic femur fractures: a systematic literature review of 1571 cases. Arch Orthop Trauma Surg. 2016;136(4):553-561.
17. Fulkerson E, Tejwani N, Stuchin S, Egol K. Management of periprosthetic femur fractures with a first generation locking plate. Injury. 2007;38(8):965-972.
18. Ricci WM, Bolhofner BR, Loftus T, Cox C, Mitchell S, Borrelli J Jr. Indirect reduction and plate fixation, without grafting, for periprosthetic femoral shaft fractures about a stable intramedullary implant. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 2:275-282.
19. Ebraheim NA, Gomez C, Ramineni SK, Liu J. Fixation of periprosthetic femoral shaft fractures adjacent to a well-fixed femoral stem with reversed distal femoral locking plate. J Trauma. 2009;66(4):1152-1157.
20. Bryant GK, Morshed S, Agel J, et al. Isolated locked compression plating for Vancouver Type B1 periprosthetic femoral fractures. Injury. 2009;40(11):1180-1186.
21. Giesinger K, Ebneter L, Day RE, Stoffel KK, Yates PJ, Kuster MS. Can plate osteosynthesis of periprosthethic femoral fractures cause cement mantle failure around a stable hip stem? A biomechanical analysis. J Arthroplasty. 2014;29(6):1308-1312.
22. Lewis GS, Caroom CT, Wee H, et al. Tangential bicortical locked fixation improves stability in vancouver B1 periprosthetic femur fractures: a biomechanical study. J Orthop Trauma. 2015;29(10):e364-e370.
23. Lenz M, Perren SM, Gueorguiev B, et al. A biomechanical study on proximal plate fixation techniques in periprosthetic femur fractures. Injury. 2014;45 Suppl 1:S71-S75.
24. Dennis MG, Simon JA, Kummer FJ, Koval KJ, DiCesare PE. Fixation of periprosthetic femoral shaft fractures occurring at the tip of the stem: a biomechanical study of 5 techniques. J Arthroplasty. 2000;15(4):523-528.
25. Graham SM, Mak JH, Moazen M, et al. Periprosthetic femoral fracture fixation: a biomechanical comparison between proximal locking screws and cables. J Orthop Sci. 2015;20(5):875-880.
26. Griffiths JT, Taheri A, Day RE, Yates PJ. Better axial stiffness of a bicortical screw construct compared to a cable construct for comminuted Vancouver B1 proximal femoral fractures. J Arthroplasty. 2015;30(12):2333-2337.
27. Chandler HP, King D, Limbird R, et al. The use of cortical allograft struts for fixation of fractures associated with well-fixed total joint prostheses. Semin Arthroplasty. 1993;4(2):99-107.
28. Haddad FS, Duncan CP, Berry DJ, Lewallen DG, Gross AE, Chandler HP. Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am. 2002;84-A(6):945-950.
29. Dennis MG, Simon JA, Kummer FJ, Koval KJ, Di Cesare PE. Fixation of periprosthetic femoral shaft fractures: a biomechanical comparison of two techniques. J Orthop Trauma. 2001;15(3):177-180.
30. Yeo I, Rhyu KH, Kim SM, Park YS, Lim SJ. High union rates of locking compression plating with cortical strut allograft for type B1 periprosthetic femoral fractures. Int Orthop. 2016. [Epub ahead of print]
31. Khashan M, Amar E, Drexler M, Chechik Ok, Cohen Z, Steinberg EL. Superior outcome of strut allograft-augmented plate fixation for the treatment of periprosthetic fractures around a stable femoral stem. Injury. 2013;44(11):1556-1560.
32. Old AB, McGrory BJ, White RR, Babikian GM. Fixation of Vancouver B1 peri-prosthetic fractures by broad metal plates without the application of strut allografts. J Bone Joint Surg Br. 2006;88(11):1425-1429.
33. Moore RE, Baldwin K, Austin MS, Mehta S. A systematic review of open reduction and internal fixation of periprosthetic femur fractures with or without allograft strut, cerclage, and locked plates. J Arthroplasty. 2014;29(5):872-876.
34. Lenz M, Stoffel K, Gueorguiev B, Klos K, Kielstein H, Hofmann GO. Enhancing fixation strength in periprosthetic femur fractures by orthogonal plating-a biomechanical study. J Orthop Res. 2016;34(4):591-596.
35. Choi JK, Gardner TR, Yoon E, Morrison TA, Macaulay WB, Geller JA. The effect of fixation technique on the stiffness of comminuted Vancouver B1 periprosthetic femur fractures. J Arthroplasty. 2010;25(6 Suppl):124-128.
36. Müller FJ, Galler M Füchtmeier B. Clinical and radiological results of patients treated with orthogonal double plating for periprosthetic femoral fractures. Int Orthop. 2014;38(12):2469-2472.
37. Pike J, Davidson D, Garbuz D, Duncan CP, O’Brien PJ, Masri BA. Principles of treatment for periprosthetic femoral shaft fractures around well-fixed total hip arthroplasty. J Am Acad Orthop Surg. 2009;17(11):677-688.
38. Serocki JH, Chandler RW, Dorr LD. Treatment of fractures about hip prostheses with compression plating. J Arthroplasty. 1992;7(2):129-135.
39. Pletka JD, Marsland D, Belkoff SM, Mears SC, Kates SL. Biomechanical comparison of 2 different locking plate fixation methods in vancouver b1 periprosthetic femur fractures. Geriatr Orthop Surg Rehabil. 2011;2(2):51-55.
40. Moloney GB, Westrick ER, Siska PA, Tarkin IS. Treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty implant: span the whole bone. Arch Orthop Trauma Surg. 2014;134(1):9-14.
41. Kubiak EN, Haller JM, Kemper DD, Presson AP, Higgins TF, Horowitz DS. Does the lateral plate need to overlap the stem to mitigate stress concentration when treating Vancouver C periprosthetic supracondylar femur fracture? J Arthroplasty. 2015;30(1):104-108.
42. Sah AP, Marshall A, Virkus WV, Estok DM 2nd, Della Valle CJ. Interprosthetic fractures of the femur: treatment with a single-locked plate. J Arthroplasty. 2010;25(2):280-286.
43. Hoffmann MF, Lotzien S, Schildhauer TA. Clinical outcome of interprosthetic femoral fractures treated with polyaxial locking plates. Injury. 2016. [Epub ahead of print]
44. Holder N, Papp S, Gofton W, Beaulé PE. Outcomes following surgical treatment of periprosthetic femur fractures: a single centre series. Can J Surg. 2014;57(3):209-213.
45. Crockarell JR Jr, Berry DJ, Lewallen DG. Nonunion after periprosthetic femoral fracture associated with total hip arthroplasty. J Bone Joint Surg Am. 1999;81(8):1073-1079.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Lewallen DG, Berry DJ. Periprosthetic fracture of the femur after total hip arthroplasty: treatment and results to date. Instr Course Lect. 1998;47:243-249.
3. Kavanagh BF. Femoral fractures associated with total hip arthroplasty. Orthop Clin North Am. 1992;23(2):249-257.
4. Meek RM, Norwood T, Smith R, Brenkel IJ, Howie CR. The risk of peri-prosthetic fracture after primary and revision total hip and knee replacement. J Bone Joint Surg Br. 2011;93(1):96-101.
5. Abdel MP, Watts CD, Houdek MT, Lewallen DG, Berry DJ. Epidemiology of periprosthetic fracture of the femur in 32 644 primary total hip arthroplasties: a 40-year experience. Bone Joint J. 2016;98-B(4):461-467.
6. Berry DJ. Epidemiology: hip and knee. Orthop Clin North Am. 1999;30(2):183-190.
7. Abdel MP, Houdek MT, Watts CD, Lewallen DG, Berry DJ. Epidemiology of periprosthetic femoral fractures in 5417 revision total hip arthrolasties: a 40-year experience. Bone Joint J. 2016;98-B(4):468-474.
8. Drew JM, Griffin WL, Odum SM, Van Doren B, Weston BT, Stryker LS. Survivorship after periprosthetic femur fracture: factors affecting outcome. J Arthroplasty. 2015. [Epub ahead of print]
9. Dehghan N, McKee MD, Nauth A, Ristevski B, Schemitsch EH. Surgical fixation of Vancouver type B1 periprosthetic femur fractures: a systematic review. J Orthop Trauma. 2014;28(12):721-727.
10. Mukundan C, Rayan F, Kheir E, Macdonald D. Management of late periprosthetic femur fractures: a retrospective cohort of 72 patients. Int Orthop. 2010;34(4):485-489.
11. Lindahl H, Malchau H, Odén A, Garellick G. Risk factors for failure after treatment of a periprosthetic fracture of the femur. J Bone Joint Surg Br. 2006;88(1):26-30.
12. Froberg L, Troelsen A, Brix M. Periprosthetic Vancouver type B1 and C fractures treated by locking-plate osteosynthesis: fracture union and reoperations in 60 consecutive fractures. Acta Orthop. 2012;83(6):648-652.
13. Beals RK, Tower SS. Periprosthetic fractures of the femur. An analysis of 93 fractures. Clin Orthop Relat Res. 1996(327):238-246.
14. Perren SM. Evolution of the internal fixation of long bone fractures. The scientific basis of biological internal fixation: choosing a new balance between stability and biology. J Bone Joint Surg Br. 2002;84(8):1093-1110.
15. Fulkerson E, Koval K, Preston CF, Iesaka K, Kummer FJ, Egol KA. Fixation of periprosthetic femoral shaft fractures associated with cemented femoral stems: a biomechanical comparison of locked plating and conventional cable plates. J Orthop Trauma. 2006;20(2):89-93.
16. Stoffel K, Sommer C, Kalampoki V, Blumenthal A, Joeris A. The influence of the operation technique and implant used in the treatment of periprosthetic hip and interprosthetic femur fractures: a systematic literature review of 1571 cases. Arch Orthop Trauma Surg. 2016;136(4):553-561.
17. Fulkerson E, Tejwani N, Stuchin S, Egol K. Management of periprosthetic femur fractures with a first generation locking plate. Injury. 2007;38(8):965-972.
18. Ricci WM, Bolhofner BR, Loftus T, Cox C, Mitchell S, Borrelli J Jr. Indirect reduction and plate fixation, without grafting, for periprosthetic femoral shaft fractures about a stable intramedullary implant. Surgical technique. J Bone Joint Surg Am. 2006;88 Suppl 1 Pt 2:275-282.
19. Ebraheim NA, Gomez C, Ramineni SK, Liu J. Fixation of periprosthetic femoral shaft fractures adjacent to a well-fixed femoral stem with reversed distal femoral locking plate. J Trauma. 2009;66(4):1152-1157.
20. Bryant GK, Morshed S, Agel J, et al. Isolated locked compression plating for Vancouver Type B1 periprosthetic femoral fractures. Injury. 2009;40(11):1180-1186.
21. Giesinger K, Ebneter L, Day RE, Stoffel KK, Yates PJ, Kuster MS. Can plate osteosynthesis of periprosthethic femoral fractures cause cement mantle failure around a stable hip stem? A biomechanical analysis. J Arthroplasty. 2014;29(6):1308-1312.
22. Lewis GS, Caroom CT, Wee H, et al. Tangential bicortical locked fixation improves stability in vancouver B1 periprosthetic femur fractures: a biomechanical study. J Orthop Trauma. 2015;29(10):e364-e370.
23. Lenz M, Perren SM, Gueorguiev B, et al. A biomechanical study on proximal plate fixation techniques in periprosthetic femur fractures. Injury. 2014;45 Suppl 1:S71-S75.
24. Dennis MG, Simon JA, Kummer FJ, Koval KJ, DiCesare PE. Fixation of periprosthetic femoral shaft fractures occurring at the tip of the stem: a biomechanical study of 5 techniques. J Arthroplasty. 2000;15(4):523-528.
25. Graham SM, Mak JH, Moazen M, et al. Periprosthetic femoral fracture fixation: a biomechanical comparison between proximal locking screws and cables. J Orthop Sci. 2015;20(5):875-880.
26. Griffiths JT, Taheri A, Day RE, Yates PJ. Better axial stiffness of a bicortical screw construct compared to a cable construct for comminuted Vancouver B1 proximal femoral fractures. J Arthroplasty. 2015;30(12):2333-2337.
27. Chandler HP, King D, Limbird R, et al. The use of cortical allograft struts for fixation of fractures associated with well-fixed total joint prostheses. Semin Arthroplasty. 1993;4(2):99-107.
28. Haddad FS, Duncan CP, Berry DJ, Lewallen DG, Gross AE, Chandler HP. Periprosthetic femoral fractures around well-fixed implants: use of cortical onlay allografts with or without a plate. J Bone Joint Surg Am. 2002;84-A(6):945-950.
29. Dennis MG, Simon JA, Kummer FJ, Koval KJ, Di Cesare PE. Fixation of periprosthetic femoral shaft fractures: a biomechanical comparison of two techniques. J Orthop Trauma. 2001;15(3):177-180.
30. Yeo I, Rhyu KH, Kim SM, Park YS, Lim SJ. High union rates of locking compression plating with cortical strut allograft for type B1 periprosthetic femoral fractures. Int Orthop. 2016. [Epub ahead of print]
31. Khashan M, Amar E, Drexler M, Chechik Ok, Cohen Z, Steinberg EL. Superior outcome of strut allograft-augmented plate fixation for the treatment of periprosthetic fractures around a stable femoral stem. Injury. 2013;44(11):1556-1560.
32. Old AB, McGrory BJ, White RR, Babikian GM. Fixation of Vancouver B1 peri-prosthetic fractures by broad metal plates without the application of strut allografts. J Bone Joint Surg Br. 2006;88(11):1425-1429.
33. Moore RE, Baldwin K, Austin MS, Mehta S. A systematic review of open reduction and internal fixation of periprosthetic femur fractures with or without allograft strut, cerclage, and locked plates. J Arthroplasty. 2014;29(5):872-876.
34. Lenz M, Stoffel K, Gueorguiev B, Klos K, Kielstein H, Hofmann GO. Enhancing fixation strength in periprosthetic femur fractures by orthogonal plating-a biomechanical study. J Orthop Res. 2016;34(4):591-596.
35. Choi JK, Gardner TR, Yoon E, Morrison TA, Macaulay WB, Geller JA. The effect of fixation technique on the stiffness of comminuted Vancouver B1 periprosthetic femur fractures. J Arthroplasty. 2010;25(6 Suppl):124-128.
36. Müller FJ, Galler M Füchtmeier B. Clinical and radiological results of patients treated with orthogonal double plating for periprosthetic femoral fractures. Int Orthop. 2014;38(12):2469-2472.
37. Pike J, Davidson D, Garbuz D, Duncan CP, O’Brien PJ, Masri BA. Principles of treatment for periprosthetic femoral shaft fractures around well-fixed total hip arthroplasty. J Am Acad Orthop Surg. 2009;17(11):677-688.
38. Serocki JH, Chandler RW, Dorr LD. Treatment of fractures about hip prostheses with compression plating. J Arthroplasty. 1992;7(2):129-135.
39. Pletka JD, Marsland D, Belkoff SM, Mears SC, Kates SL. Biomechanical comparison of 2 different locking plate fixation methods in vancouver b1 periprosthetic femur fractures. Geriatr Orthop Surg Rehabil. 2011;2(2):51-55.
40. Moloney GB, Westrick ER, Siska PA, Tarkin IS. Treatment of periprosthetic femur fractures around a well-fixed hip arthroplasty implant: span the whole bone. Arch Orthop Trauma Surg. 2014;134(1):9-14.
41. Kubiak EN, Haller JM, Kemper DD, Presson AP, Higgins TF, Horowitz DS. Does the lateral plate need to overlap the stem to mitigate stress concentration when treating Vancouver C periprosthetic supracondylar femur fracture? J Arthroplasty. 2015;30(1):104-108.
42. Sah AP, Marshall A, Virkus WV, Estok DM 2nd, Della Valle CJ. Interprosthetic fractures of the femur: treatment with a single-locked plate. J Arthroplasty. 2010;25(2):280-286.
43. Hoffmann MF, Lotzien S, Schildhauer TA. Clinical outcome of interprosthetic femoral fractures treated with polyaxial locking plates. Injury. 2016. [Epub ahead of print]
44. Holder N, Papp S, Gofton W, Beaulé PE. Outcomes following surgical treatment of periprosthetic femur fractures: a single centre series. Can J Surg. 2014;57(3):209-213.
45. Crockarell JR Jr, Berry DJ, Lewallen DG. Nonunion after periprosthetic femoral fracture associated with total hip arthroplasty. J Bone Joint Surg Am. 1999;81(8):1073-1079.
Active Robotics for Total Hip Arthroplasty
Total hip arthroplasty (THA) is a successful surgery with positive clinical outcomes and over 95% survivorship at 10-year follow-up and 80% survivorship at 25-year follow-up.1,2 A hip replacement requires strong osteointegration3,4 to prevent femoral osteolysis, and correct implant alignment has been shown to correlate with prolonged implant survivorship and reduced dislocation.5,6 Robotics and computer-assisted navigation have been developed to increase the accuracy of implant placement and reduce outliers with the overall goal of improving long-term results. These technologies have shown significant improvements in implant positioning when compared to conventional techniques.7
The first active robotic system for use in orthopedic procedures, Robodoc (Think Surgical, Inc.), was based on a traditional computer-aided design/computer-aided manufacturing system. Currently, only 3 robotic systems for THA have clearance in the US: The Mako System (Stryker), Robodoc, and TSolution One (Think Surgical, Inc.). The TSolution One system is based on the legacy technology developed as Robodoc and currently provides assistance during preparation of the femoral canal as well as guidance and positioning assistance during acetabular cup reaming and implanting. The following is a summary of the author’s (DSD) preferred technique for robotic-assisted THA using TSolution One.
How It Works
The process begins with preoperative planning (Figure 1). A computed tomography (CT) scan is used to create a detailed 3-dimensional (3D) reconstruction of the patient’s pathologic hip anatomy. The CT scan images are uploaded to TPLAN, a preoperative planning station.
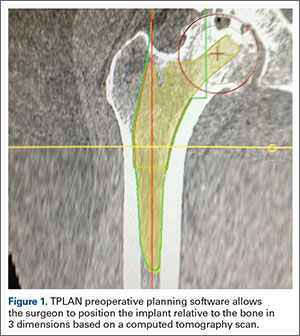
In TPLAN, the user creates a 3D template of the surgical plan for both the femoral and acetabular portions of the procedure. The system has an open platform, meaning that the user is not limited to a single implant design or manufacturer. The surgeon can control every aspect of implant positioning: rotation, anteversion, fit and fill on the femoral side and anteversion, inclination/lateral opening, and depth on the acetabular side. Additional features available to the surgeon include accurately defining bony deficits, identifying outlier implant sizes, and checking for excess native version. The system allows the surgeon to determine the native center of hip rotation, which can then be used during templating to give the patient a hip that feels natural because the native muscle tension is restored. Once the desired plan has been achieved, it is uploaded to the robot.The TCAT robot is an active system similar to those used in manufacturing assembly plants (eg, automobiles) in that it follows a predetermined path and can do so in an efficient manner. More specifically, once the user has defined the patient’s anatomy within its workspace, it will proceed with actively milling the femur as planned with sub-millimeter accuracy without the use of navigation. This is in contrast to a haptic system, where the user manually guides the robotic arm within a predefined boundary.
The acetabular portion of the procedure currently uses a standard reamer system and power tools, but the TCAT guides the surgeon to the planned cup orientation, which is maintained during reaming and impaction.
In the Operating Suite
Once in the operating suite, the plan is uploaded into TCAT. Confirmation of the plan and the patient are incorporated into the surgical “time out.” Currently, the system supports patient positioning in standard lateral decubitus using a posterior approach with a standard operating room table. A posterior approach is undertaken to expose and dislocate the hip, with retractors placed to protect the soft tissues and allow the robot its working space.
One procedural difference from the standard THA technique is that the femoral head is initially retained to fixate the femur relative to the robot. A 5-mm Schanz pin is placed in the femoral head and then rigidly attached to the base of the robot. During a process called registration, a series of points on the surface of the exposed bone are collected by the surgeon via a digitizer probe attached to the robot. The TCAT monitor will guide the surgeon through point collection using a map showing the patient’s 3D bone model generated from the CT scan. The software then “finds” the patient’s femur in space and matches it to the 3D CT plan. Milling begins with a burr spinning at 80,000 rpm and saline to irrigate and remove bone debris (Figure 2). The actual milling process takes 5 to 15 minutes, depending on the choice and size of the implant.
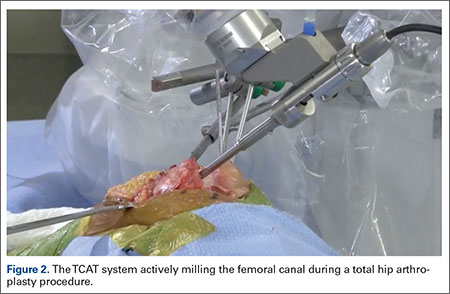
A bone motion monitor (BMM) is also attached to the femur, along with recovery markers (RM). The BMM immediately pauses the robot during any active bone milling if it senses femoral motion from the original position. The surgeon then touches the RM with the digitizer to re-register the bone’s position and resume the milling process.
Attention is then turned to the acetabular portion of the procedure. Again, the robot must be rigidly fixed to the patient’s pelvis, along with the RM. Once the surgeon has registered the acetabular position using the digitizer, the robotic arm moves into the preoperatively planned orientation. A universal quick-release allows the surgeon to attach a standard reamer to the robot arm and ream while the robot holds the reamer in place. Once the acetabular preparation is complete, the cup impactor is placed onto the robotic arm and the implant is impacted into the patient. Thereafter, the digitizer can be used to collect points on the surface of the cup and confirm the exact cup placement (Figure 3).
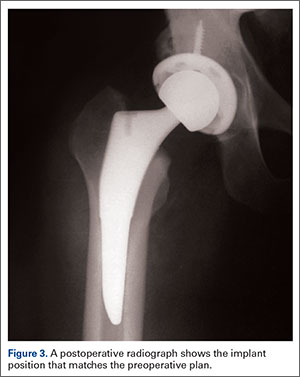
Outcomes
The legacy system, Robodoc, has been used in thousands of clinical cases for both THA and total knee arthroplasty. The Table represents a summary of the THA clinical studies during a time frame in which only the femoral portion of the procedure was available to surgeons.
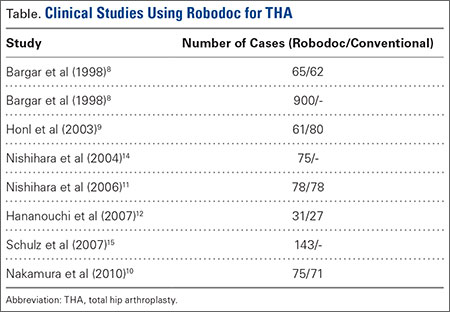
Bargar and colleagues8 describe the first Robodoc clinical trial in the US, along with the first 900 THA procedures performed in Germany. In the US, researchers conducted a prospective, randomized control study with 65 robotic cases and 62 conventional control cases. In terms of functional outcomes, there were no differences between the 2 groups. The robot group had improved radiographic fit and component positioning but significantly increased surgical time and blood loss. There were no femoral fractures in the robot group but 3 cases in the control group. In Germany, they reported on 870 primary THAs and 30 revision THA cases. For the primary cases, Harris hip scores rose from 43.7 preoperatively to 91.5 postoperatively. Complication rates were similar to conventional techniques, except the robot cases had no intraoperative femoral fractures.
Several prospective randomized clinical studies compared use of the Robodoc system with a conventional technique. The group studied by Honl and colleagues9 included 61 robotic cases and 80 conventional cases. The robot group had significant improvements in limb-length equality and varus-valgus orientation of the stem. When the revision cases were excluded, the authors found the Harris hip scores, prosthetic alignment, and limb length differentials were better for the robotic group at both 6 and 12 months.
Nakamura and colleagues10 looked at 75 robotic cases and 71 conventional cases. The results showed that at 2 and 3 years postoperatively, the robotic group had better Japanese Orthopaedic Association (JOA) scores, but by 5 years postoperatively, the differences were no longer significant. The robotic group had a smaller range for leg length inequality (0-12 mm) compared to the conventional group (0-29 mm). The results also showed that at both 2 and 5 years postoperatively, there was more significant stress shielding of the proximal femur, suggesting greater bone loss in the conventional group.
Nishihara and colleagues11 had 78 subjects in each of the robotic and conventional groups and found significantly better Merle d’Aubigné hip scores at 2 years postoperatively in the robotic group. The conventional group suffered 5 intraoperative fractures compared with none in the robotic group, along with greater estimated blood loss, an increased use of undersized stems, higher-than-expected vertical seating, and unexpected femoral anteversion. The robotic cases did, however, take 19 minutes longer than the conventional cases.
Hananouchi and colleagues12 looked at periprosthetic bone remodeling in 31 robotic hips and 27 conventional hips to determine whether load was effectively transferred from implant to bone after using the Robodoc system to prepare the femoral canal. Using dual energy X-ray absorptiometry (DEXA) to measure bone density, they found significantly less bone loss in the proximal periprosthetic areas in the robotic group compared to the conventional group; however, there were no differences in the Merle d’Aubigné hip scores.
Lim and colleagues13 looked specifically at alignment accuracy and clinical outcomes specifically for short femoral stem implants. In a group of 24 robotic cases and 25 conventional cases, they found significantly improved stem alignment and leg length inequality and no differences in Harris Hip score, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, or complications at 24 months.
In 2004, Nishihara and colleagues14 evaluated the accuracy of femoral canal preparation using postoperative CT images for 75 cases of THA performed with the original pin-based version of Robodoc. The results showed that the differences between the preoperative plan and the postoperative CT were <5% in terms of canal fill, <1 mm in gap, and <1° in mediolateral and anteroposterior alignment with no reported fractures or complications. They concluded that the Robodoc system resulted in a high degree of accuracy.
Schulz and colleagues15 reported on 97 of 143 consecutive cases performed from 1997 to 2002. Technical complications were described in 9 cases. Five of the reported complications included the BMM pausing cutting as designed for patient safety, which led to re-registration, and slightly prolonged surgery. The remaining 4 complications included 2 femoral shaft fissures requiring wire cerclage, 1 case of damage to the acetabular rim from the milling device, and 1 defect of the greater trochanter that was milled. In terms of clinical results, they found that the complications, functional outcomes, and radiographic outcomes were comparable to conventional techniques. The rate of femoral shaft fissures, which had been zero in all other studies with Robodoc, was comparable to conventional technique.
Conclusion
The most significant change in hip arthroplasty until now has been the transition from a cemented technique to a press-fit or ingrowth prosthesis.16 The first robotic surgery was performed in the US in 1992 using the legacy system upon which the current TSolution One was based. Since then, the use of surgical robots has seen a 30% increase annually over the last decade in a variety of surgical fields.17 In orthopedics, specifically THA, the results have shown that robotics clearly offers benefits in terms of accuracy, precision, and reproducibility. These benefits will likely translate into improved long-term outcomes and increased survivorship in future studies.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. National Joint Registry. National Joint Registry for England and Wales. 7th annual report. Available at: http://www.njrcentre.org.uk/njrcentre/portals/0/njr%207th%20annual%20report%202010.pdf. Accessed April 12, 2016.
3. Paul HA, Bargar WL, Mittlestadt B, et al. Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res. 1992;285:57-66.
4. Bobyn JD, Engh CA. Human histology of bone-porous metal implant interface. Orthopedics. 1984;7(9):1410.
5. Barrack RL. Dislocation after total hip arthroplasty: Implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
6. Miki H, Sugano N, Yonenobu K, Tsuda K, Hattori M, Suzuki N. Detecting cause of dislocation after total hip arthroplasty by patient-specific four-dimensional motion analysis. Clin Biomech. 2013;28(2):182-186.
7. Sugano N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg. 2013;5(1):1-9.
8. Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Rel Res. 1998;354:82-91.
9. Honl M, Dierk O, Gauck C, et al. Comparison of robotic-assisted and manual implantation of primary total hip replacement: a prospective study. J Bone Joint Surg Am. 2003;85-A(8):1470-1478.
10. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res. 2010;468(4):1072-1081.
11. Nishihara S, Sugano N, Nishii T, Miki H, Nakamura N, Yoshikawa H. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplasty. 2006;21(7):957-966.
12. Hananouchi T, Sugano N, Nishii T, et al. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res. 2007;25(8):1062-1069.
13. Lim SJ, Ko KR, Park CW, Moon YW, Park YS. Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: a prospective randomized short-term outcome study. Comput Aided Surg. 2015;20(1):41-46.
14. Nishihara S, Sugano N, Nishii T, et al. Clinical accuracy evaluation of femoral canal preparation using the ROBODOC system. J Orthop Sci. 2004;9(5):452-461.
15. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
16. Wyatt M, Hooper G, Framptom C, Rothwell A. Survival outcomes of cemented compared to uncemented stems in primary total hip replacement. World J Orthop. 2014;5(5):591-596.
17. Howard B. Is robotic surgery right for you? AARP The Magazine. December 2013/January 2014. Available at: http://www.aarp.org/health/conditions-treatments/info-12-2013/robotic-surgery-risks-benefits.html. Accessed April 12, 2016.
Total hip arthroplasty (THA) is a successful surgery with positive clinical outcomes and over 95% survivorship at 10-year follow-up and 80% survivorship at 25-year follow-up.1,2 A hip replacement requires strong osteointegration3,4 to prevent femoral osteolysis, and correct implant alignment has been shown to correlate with prolonged implant survivorship and reduced dislocation.5,6 Robotics and computer-assisted navigation have been developed to increase the accuracy of implant placement and reduce outliers with the overall goal of improving long-term results. These technologies have shown significant improvements in implant positioning when compared to conventional techniques.7
The first active robotic system for use in orthopedic procedures, Robodoc (Think Surgical, Inc.), was based on a traditional computer-aided design/computer-aided manufacturing system. Currently, only 3 robotic systems for THA have clearance in the US: The Mako System (Stryker), Robodoc, and TSolution One (Think Surgical, Inc.). The TSolution One system is based on the legacy technology developed as Robodoc and currently provides assistance during preparation of the femoral canal as well as guidance and positioning assistance during acetabular cup reaming and implanting. The following is a summary of the author’s (DSD) preferred technique for robotic-assisted THA using TSolution One.
How It Works
The process begins with preoperative planning (Figure 1). A computed tomography (CT) scan is used to create a detailed 3-dimensional (3D) reconstruction of the patient’s pathologic hip anatomy. The CT scan images are uploaded to TPLAN, a preoperative planning station.

In TPLAN, the user creates a 3D template of the surgical plan for both the femoral and acetabular portions of the procedure. The system has an open platform, meaning that the user is not limited to a single implant design or manufacturer. The surgeon can control every aspect of implant positioning: rotation, anteversion, fit and fill on the femoral side and anteversion, inclination/lateral opening, and depth on the acetabular side. Additional features available to the surgeon include accurately defining bony deficits, identifying outlier implant sizes, and checking for excess native version. The system allows the surgeon to determine the native center of hip rotation, which can then be used during templating to give the patient a hip that feels natural because the native muscle tension is restored. Once the desired plan has been achieved, it is uploaded to the robot.The TCAT robot is an active system similar to those used in manufacturing assembly plants (eg, automobiles) in that it follows a predetermined path and can do so in an efficient manner. More specifically, once the user has defined the patient’s anatomy within its workspace, it will proceed with actively milling the femur as planned with sub-millimeter accuracy without the use of navigation. This is in contrast to a haptic system, where the user manually guides the robotic arm within a predefined boundary.
The acetabular portion of the procedure currently uses a standard reamer system and power tools, but the TCAT guides the surgeon to the planned cup orientation, which is maintained during reaming and impaction.
In the Operating Suite
Once in the operating suite, the plan is uploaded into TCAT. Confirmation of the plan and the patient are incorporated into the surgical “time out.” Currently, the system supports patient positioning in standard lateral decubitus using a posterior approach with a standard operating room table. A posterior approach is undertaken to expose and dislocate the hip, with retractors placed to protect the soft tissues and allow the robot its working space.
One procedural difference from the standard THA technique is that the femoral head is initially retained to fixate the femur relative to the robot. A 5-mm Schanz pin is placed in the femoral head and then rigidly attached to the base of the robot. During a process called registration, a series of points on the surface of the exposed bone are collected by the surgeon via a digitizer probe attached to the robot. The TCAT monitor will guide the surgeon through point collection using a map showing the patient’s 3D bone model generated from the CT scan. The software then “finds” the patient’s femur in space and matches it to the 3D CT plan. Milling begins with a burr spinning at 80,000 rpm and saline to irrigate and remove bone debris (Figure 2). The actual milling process takes 5 to 15 minutes, depending on the choice and size of the implant.

A bone motion monitor (BMM) is also attached to the femur, along with recovery markers (RM). The BMM immediately pauses the robot during any active bone milling if it senses femoral motion from the original position. The surgeon then touches the RM with the digitizer to re-register the bone’s position and resume the milling process.
Attention is then turned to the acetabular portion of the procedure. Again, the robot must be rigidly fixed to the patient’s pelvis, along with the RM. Once the surgeon has registered the acetabular position using the digitizer, the robotic arm moves into the preoperatively planned orientation. A universal quick-release allows the surgeon to attach a standard reamer to the robot arm and ream while the robot holds the reamer in place. Once the acetabular preparation is complete, the cup impactor is placed onto the robotic arm and the implant is impacted into the patient. Thereafter, the digitizer can be used to collect points on the surface of the cup and confirm the exact cup placement (Figure 3).

Outcomes
The legacy system, Robodoc, has been used in thousands of clinical cases for both THA and total knee arthroplasty. The Table represents a summary of the THA clinical studies during a time frame in which only the femoral portion of the procedure was available to surgeons.

Bargar and colleagues8 describe the first Robodoc clinical trial in the US, along with the first 900 THA procedures performed in Germany. In the US, researchers conducted a prospective, randomized control study with 65 robotic cases and 62 conventional control cases. In terms of functional outcomes, there were no differences between the 2 groups. The robot group had improved radiographic fit and component positioning but significantly increased surgical time and blood loss. There were no femoral fractures in the robot group but 3 cases in the control group. In Germany, they reported on 870 primary THAs and 30 revision THA cases. For the primary cases, Harris hip scores rose from 43.7 preoperatively to 91.5 postoperatively. Complication rates were similar to conventional techniques, except the robot cases had no intraoperative femoral fractures.
Several prospective randomized clinical studies compared use of the Robodoc system with a conventional technique. The group studied by Honl and colleagues9 included 61 robotic cases and 80 conventional cases. The robot group had significant improvements in limb-length equality and varus-valgus orientation of the stem. When the revision cases were excluded, the authors found the Harris hip scores, prosthetic alignment, and limb length differentials were better for the robotic group at both 6 and 12 months.
Nakamura and colleagues10 looked at 75 robotic cases and 71 conventional cases. The results showed that at 2 and 3 years postoperatively, the robotic group had better Japanese Orthopaedic Association (JOA) scores, but by 5 years postoperatively, the differences were no longer significant. The robotic group had a smaller range for leg length inequality (0-12 mm) compared to the conventional group (0-29 mm). The results also showed that at both 2 and 5 years postoperatively, there was more significant stress shielding of the proximal femur, suggesting greater bone loss in the conventional group.
Nishihara and colleagues11 had 78 subjects in each of the robotic and conventional groups and found significantly better Merle d’Aubigné hip scores at 2 years postoperatively in the robotic group. The conventional group suffered 5 intraoperative fractures compared with none in the robotic group, along with greater estimated blood loss, an increased use of undersized stems, higher-than-expected vertical seating, and unexpected femoral anteversion. The robotic cases did, however, take 19 minutes longer than the conventional cases.
Hananouchi and colleagues12 looked at periprosthetic bone remodeling in 31 robotic hips and 27 conventional hips to determine whether load was effectively transferred from implant to bone after using the Robodoc system to prepare the femoral canal. Using dual energy X-ray absorptiometry (DEXA) to measure bone density, they found significantly less bone loss in the proximal periprosthetic areas in the robotic group compared to the conventional group; however, there were no differences in the Merle d’Aubigné hip scores.
Lim and colleagues13 looked specifically at alignment accuracy and clinical outcomes specifically for short femoral stem implants. In a group of 24 robotic cases and 25 conventional cases, they found significantly improved stem alignment and leg length inequality and no differences in Harris Hip score, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, or complications at 24 months.
In 2004, Nishihara and colleagues14 evaluated the accuracy of femoral canal preparation using postoperative CT images for 75 cases of THA performed with the original pin-based version of Robodoc. The results showed that the differences between the preoperative plan and the postoperative CT were <5% in terms of canal fill, <1 mm in gap, and <1° in mediolateral and anteroposterior alignment with no reported fractures or complications. They concluded that the Robodoc system resulted in a high degree of accuracy.
Schulz and colleagues15 reported on 97 of 143 consecutive cases performed from 1997 to 2002. Technical complications were described in 9 cases. Five of the reported complications included the BMM pausing cutting as designed for patient safety, which led to re-registration, and slightly prolonged surgery. The remaining 4 complications included 2 femoral shaft fissures requiring wire cerclage, 1 case of damage to the acetabular rim from the milling device, and 1 defect of the greater trochanter that was milled. In terms of clinical results, they found that the complications, functional outcomes, and radiographic outcomes were comparable to conventional techniques. The rate of femoral shaft fissures, which had been zero in all other studies with Robodoc, was comparable to conventional technique.
Conclusion
The most significant change in hip arthroplasty until now has been the transition from a cemented technique to a press-fit or ingrowth prosthesis.16 The first robotic surgery was performed in the US in 1992 using the legacy system upon which the current TSolution One was based. Since then, the use of surgical robots has seen a 30% increase annually over the last decade in a variety of surgical fields.17 In orthopedics, specifically THA, the results have shown that robotics clearly offers benefits in terms of accuracy, precision, and reproducibility. These benefits will likely translate into improved long-term outcomes and increased survivorship in future studies.
Total hip arthroplasty (THA) is a successful surgery with positive clinical outcomes and over 95% survivorship at 10-year follow-up and 80% survivorship at 25-year follow-up.1,2 A hip replacement requires strong osteointegration3,4 to prevent femoral osteolysis, and correct implant alignment has been shown to correlate with prolonged implant survivorship and reduced dislocation.5,6 Robotics and computer-assisted navigation have been developed to increase the accuracy of implant placement and reduce outliers with the overall goal of improving long-term results. These technologies have shown significant improvements in implant positioning when compared to conventional techniques.7
The first active robotic system for use in orthopedic procedures, Robodoc (Think Surgical, Inc.), was based on a traditional computer-aided design/computer-aided manufacturing system. Currently, only 3 robotic systems for THA have clearance in the US: The Mako System (Stryker), Robodoc, and TSolution One (Think Surgical, Inc.). The TSolution One system is based on the legacy technology developed as Robodoc and currently provides assistance during preparation of the femoral canal as well as guidance and positioning assistance during acetabular cup reaming and implanting. The following is a summary of the author’s (DSD) preferred technique for robotic-assisted THA using TSolution One.
How It Works
The process begins with preoperative planning (Figure 1). A computed tomography (CT) scan is used to create a detailed 3-dimensional (3D) reconstruction of the patient’s pathologic hip anatomy. The CT scan images are uploaded to TPLAN, a preoperative planning station.

In TPLAN, the user creates a 3D template of the surgical plan for both the femoral and acetabular portions of the procedure. The system has an open platform, meaning that the user is not limited to a single implant design or manufacturer. The surgeon can control every aspect of implant positioning: rotation, anteversion, fit and fill on the femoral side and anteversion, inclination/lateral opening, and depth on the acetabular side. Additional features available to the surgeon include accurately defining bony deficits, identifying outlier implant sizes, and checking for excess native version. The system allows the surgeon to determine the native center of hip rotation, which can then be used during templating to give the patient a hip that feels natural because the native muscle tension is restored. Once the desired plan has been achieved, it is uploaded to the robot.The TCAT robot is an active system similar to those used in manufacturing assembly plants (eg, automobiles) in that it follows a predetermined path and can do so in an efficient manner. More specifically, once the user has defined the patient’s anatomy within its workspace, it will proceed with actively milling the femur as planned with sub-millimeter accuracy without the use of navigation. This is in contrast to a haptic system, where the user manually guides the robotic arm within a predefined boundary.
The acetabular portion of the procedure currently uses a standard reamer system and power tools, but the TCAT guides the surgeon to the planned cup orientation, which is maintained during reaming and impaction.
In the Operating Suite
Once in the operating suite, the plan is uploaded into TCAT. Confirmation of the plan and the patient are incorporated into the surgical “time out.” Currently, the system supports patient positioning in standard lateral decubitus using a posterior approach with a standard operating room table. A posterior approach is undertaken to expose and dislocate the hip, with retractors placed to protect the soft tissues and allow the robot its working space.
One procedural difference from the standard THA technique is that the femoral head is initially retained to fixate the femur relative to the robot. A 5-mm Schanz pin is placed in the femoral head and then rigidly attached to the base of the robot. During a process called registration, a series of points on the surface of the exposed bone are collected by the surgeon via a digitizer probe attached to the robot. The TCAT monitor will guide the surgeon through point collection using a map showing the patient’s 3D bone model generated from the CT scan. The software then “finds” the patient’s femur in space and matches it to the 3D CT plan. Milling begins with a burr spinning at 80,000 rpm and saline to irrigate and remove bone debris (Figure 2). The actual milling process takes 5 to 15 minutes, depending on the choice and size of the implant.

A bone motion monitor (BMM) is also attached to the femur, along with recovery markers (RM). The BMM immediately pauses the robot during any active bone milling if it senses femoral motion from the original position. The surgeon then touches the RM with the digitizer to re-register the bone’s position and resume the milling process.
Attention is then turned to the acetabular portion of the procedure. Again, the robot must be rigidly fixed to the patient’s pelvis, along with the RM. Once the surgeon has registered the acetabular position using the digitizer, the robotic arm moves into the preoperatively planned orientation. A universal quick-release allows the surgeon to attach a standard reamer to the robot arm and ream while the robot holds the reamer in place. Once the acetabular preparation is complete, the cup impactor is placed onto the robotic arm and the implant is impacted into the patient. Thereafter, the digitizer can be used to collect points on the surface of the cup and confirm the exact cup placement (Figure 3).

Outcomes
The legacy system, Robodoc, has been used in thousands of clinical cases for both THA and total knee arthroplasty. The Table represents a summary of the THA clinical studies during a time frame in which only the femoral portion of the procedure was available to surgeons.

Bargar and colleagues8 describe the first Robodoc clinical trial in the US, along with the first 900 THA procedures performed in Germany. In the US, researchers conducted a prospective, randomized control study with 65 robotic cases and 62 conventional control cases. In terms of functional outcomes, there were no differences between the 2 groups. The robot group had improved radiographic fit and component positioning but significantly increased surgical time and blood loss. There were no femoral fractures in the robot group but 3 cases in the control group. In Germany, they reported on 870 primary THAs and 30 revision THA cases. For the primary cases, Harris hip scores rose from 43.7 preoperatively to 91.5 postoperatively. Complication rates were similar to conventional techniques, except the robot cases had no intraoperative femoral fractures.
Several prospective randomized clinical studies compared use of the Robodoc system with a conventional technique. The group studied by Honl and colleagues9 included 61 robotic cases and 80 conventional cases. The robot group had significant improvements in limb-length equality and varus-valgus orientation of the stem. When the revision cases were excluded, the authors found the Harris hip scores, prosthetic alignment, and limb length differentials were better for the robotic group at both 6 and 12 months.
Nakamura and colleagues10 looked at 75 robotic cases and 71 conventional cases. The results showed that at 2 and 3 years postoperatively, the robotic group had better Japanese Orthopaedic Association (JOA) scores, but by 5 years postoperatively, the differences were no longer significant. The robotic group had a smaller range for leg length inequality (0-12 mm) compared to the conventional group (0-29 mm). The results also showed that at both 2 and 5 years postoperatively, there was more significant stress shielding of the proximal femur, suggesting greater bone loss in the conventional group.
Nishihara and colleagues11 had 78 subjects in each of the robotic and conventional groups and found significantly better Merle d’Aubigné hip scores at 2 years postoperatively in the robotic group. The conventional group suffered 5 intraoperative fractures compared with none in the robotic group, along with greater estimated blood loss, an increased use of undersized stems, higher-than-expected vertical seating, and unexpected femoral anteversion. The robotic cases did, however, take 19 minutes longer than the conventional cases.
Hananouchi and colleagues12 looked at periprosthetic bone remodeling in 31 robotic hips and 27 conventional hips to determine whether load was effectively transferred from implant to bone after using the Robodoc system to prepare the femoral canal. Using dual energy X-ray absorptiometry (DEXA) to measure bone density, they found significantly less bone loss in the proximal periprosthetic areas in the robotic group compared to the conventional group; however, there were no differences in the Merle d’Aubigné hip scores.
Lim and colleagues13 looked specifically at alignment accuracy and clinical outcomes specifically for short femoral stem implants. In a group of 24 robotic cases and 25 conventional cases, they found significantly improved stem alignment and leg length inequality and no differences in Harris Hip score, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, or complications at 24 months.
In 2004, Nishihara and colleagues14 evaluated the accuracy of femoral canal preparation using postoperative CT images for 75 cases of THA performed with the original pin-based version of Robodoc. The results showed that the differences between the preoperative plan and the postoperative CT were <5% in terms of canal fill, <1 mm in gap, and <1° in mediolateral and anteroposterior alignment with no reported fractures or complications. They concluded that the Robodoc system resulted in a high degree of accuracy.
Schulz and colleagues15 reported on 97 of 143 consecutive cases performed from 1997 to 2002. Technical complications were described in 9 cases. Five of the reported complications included the BMM pausing cutting as designed for patient safety, which led to re-registration, and slightly prolonged surgery. The remaining 4 complications included 2 femoral shaft fissures requiring wire cerclage, 1 case of damage to the acetabular rim from the milling device, and 1 defect of the greater trochanter that was milled. In terms of clinical results, they found that the complications, functional outcomes, and radiographic outcomes were comparable to conventional techniques. The rate of femoral shaft fissures, which had been zero in all other studies with Robodoc, was comparable to conventional technique.
Conclusion
The most significant change in hip arthroplasty until now has been the transition from a cemented technique to a press-fit or ingrowth prosthesis.16 The first robotic surgery was performed in the US in 1992 using the legacy system upon which the current TSolution One was based. Since then, the use of surgical robots has seen a 30% increase annually over the last decade in a variety of surgical fields.17 In orthopedics, specifically THA, the results have shown that robotics clearly offers benefits in terms of accuracy, precision, and reproducibility. These benefits will likely translate into improved long-term outcomes and increased survivorship in future studies.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. National Joint Registry. National Joint Registry for England and Wales. 7th annual report. Available at: http://www.njrcentre.org.uk/njrcentre/portals/0/njr%207th%20annual%20report%202010.pdf. Accessed April 12, 2016.
3. Paul HA, Bargar WL, Mittlestadt B, et al. Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res. 1992;285:57-66.
4. Bobyn JD, Engh CA. Human histology of bone-porous metal implant interface. Orthopedics. 1984;7(9):1410.
5. Barrack RL. Dislocation after total hip arthroplasty: Implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
6. Miki H, Sugano N, Yonenobu K, Tsuda K, Hattori M, Suzuki N. Detecting cause of dislocation after total hip arthroplasty by patient-specific four-dimensional motion analysis. Clin Biomech. 2013;28(2):182-186.
7. Sugano N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg. 2013;5(1):1-9.
8. Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Rel Res. 1998;354:82-91.
9. Honl M, Dierk O, Gauck C, et al. Comparison of robotic-assisted and manual implantation of primary total hip replacement: a prospective study. J Bone Joint Surg Am. 2003;85-A(8):1470-1478.
10. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res. 2010;468(4):1072-1081.
11. Nishihara S, Sugano N, Nishii T, Miki H, Nakamura N, Yoshikawa H. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplasty. 2006;21(7):957-966.
12. Hananouchi T, Sugano N, Nishii T, et al. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res. 2007;25(8):1062-1069.
13. Lim SJ, Ko KR, Park CW, Moon YW, Park YS. Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: a prospective randomized short-term outcome study. Comput Aided Surg. 2015;20(1):41-46.
14. Nishihara S, Sugano N, Nishii T, et al. Clinical accuracy evaluation of femoral canal preparation using the ROBODOC system. J Orthop Sci. 2004;9(5):452-461.
15. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
16. Wyatt M, Hooper G, Framptom C, Rothwell A. Survival outcomes of cemented compared to uncemented stems in primary total hip replacement. World J Orthop. 2014;5(5):591-596.
17. Howard B. Is robotic surgery right for you? AARP The Magazine. December 2013/January 2014. Available at: http://www.aarp.org/health/conditions-treatments/info-12-2013/robotic-surgery-risks-benefits.html. Accessed April 12, 2016.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. National Joint Registry. National Joint Registry for England and Wales. 7th annual report. Available at: http://www.njrcentre.org.uk/njrcentre/portals/0/njr%207th%20annual%20report%202010.pdf. Accessed April 12, 2016.
3. Paul HA, Bargar WL, Mittlestadt B, et al. Development of a surgical robot for cementless total hip arthroplasty. Clin Orthop Relat Res. 1992;285:57-66.
4. Bobyn JD, Engh CA. Human histology of bone-porous metal implant interface. Orthopedics. 1984;7(9):1410.
5. Barrack RL. Dislocation after total hip arthroplasty: Implant design and orientation. J Am Acad Orthop Surg. 2003;11(2):89-99.
6. Miki H, Sugano N, Yonenobu K, Tsuda K, Hattori M, Suzuki N. Detecting cause of dislocation after total hip arthroplasty by patient-specific four-dimensional motion analysis. Clin Biomech. 2013;28(2):182-186.
7. Sugano N. Computer-assisted orthopaedic surgery and robotic surgery in total hip arthroplasty. Clin Orthop Surg. 2013;5(1):1-9.
8. Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Rel Res. 1998;354:82-91.
9. Honl M, Dierk O, Gauck C, et al. Comparison of robotic-assisted and manual implantation of primary total hip replacement: a prospective study. J Bone Joint Surg Am. 2003;85-A(8):1470-1478.
10. Nakamura N, Sugano N, Nishii T, Kakimoto A, Miki H. A comparison between robotic-assisted and manual implantation of cementless total hip arthroplasty. Clin Orthop Relat Res. 2010;468(4):1072-1081.
11. Nishihara S, Sugano N, Nishii T, Miki H, Nakamura N, Yoshikawa H. Comparison between hand rasping and robotic milling for stem implantation in cementless total hip arthroplasty. J Arthroplasty. 2006;21(7):957-966.
12. Hananouchi T, Sugano N, Nishii T, et al. Effect of robotic milling on periprosthetic bone remodeling. J Orthop Res. 2007;25(8):1062-1069.
13. Lim SJ, Ko KR, Park CW, Moon YW, Park YS. Robot-assisted primary cementless total hip arthroplasty with a short femoral stem: a prospective randomized short-term outcome study. Comput Aided Surg. 2015;20(1):41-46.
14. Nishihara S, Sugano N, Nishii T, et al. Clinical accuracy evaluation of femoral canal preparation using the ROBODOC system. J Orthop Sci. 2004;9(5):452-461.
15. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
16. Wyatt M, Hooper G, Framptom C, Rothwell A. Survival outcomes of cemented compared to uncemented stems in primary total hip replacement. World J Orthop. 2014;5(5):591-596.
17. Howard B. Is robotic surgery right for you? AARP The Magazine. December 2013/January 2014. Available at: http://www.aarp.org/health/conditions-treatments/info-12-2013/robotic-surgery-risks-benefits.html. Accessed April 12, 2016.
The Evolution of Image-Free Robotic Assistance in Unicompartmental Knee Arthroplasty
The concept of robotics is relatively new in medical practice. The term “robot” itself is less than 100 years old, having been first introduced to popular culture in 1917 by Joseph Capek in the science fiction story Opilec.1,2 Robots eventually transitioned from this initial fictional literary setting to reality in 1958, when General Motors began adding automated machines to its assembly lines.1 However, it was not until the 1980s that robotics and their exacting efficiencies would be introduced in the medical field, and it would take another decade before they would enter the specialty of orthopedics.1-4
The first robotic-assisted orthopedic surgery was reportedly performed in 1992, when the Robodoc autonomous system was utilized for total hip arthroplasty.2-4 A robotic system for total knee arthroplasty (TKA) was first described in 1993, but it would take several more years until a system for unicompartmental knee arthroplasty (UKA) would be commercialized and used clinically.5,6 The rationale for advancement of robotic technology for isolated medial or lateral knee arthritis stems from the recognition that while UKA is effective and durable when components and limb are well aligned and soft tissues appropriately balanced, they are less forgiving of even slight component malalignment of as little as 2° to 3° and prone to premature loosening or wear in those circumstances.7-13,14 In the mid 2000s, Cobb and colleagues6 reported using a semiautonomous robot for UKA. Since then, emergence of other semiautonomous robotic systems has led to greater market penetration and technology utilization.15
Currently, an estimated 15% to 20% of UKA surgeries are being performed with robotic assistance.16 Further, patent activity and peer-reviewed publications related to robotic technology in UKA (which can be considered surrogate measures of interest and evolving development and experience with robotic technologies) have increased dramatically over the past few years.2,6,14,17,18-34 To date, while the most dramatic growth of robotic utilization and case volumes has occurred in the subspecialty of UKA, semiautonomous robotic systems have been used with increasing frequency for patellofemoral and bicompartmental knee arthroplasty.35,36 Robotics have been used sparingly for TKA, and limited to autonomous systems;37,38 however, it is anticipated that emergence of semiautonomous platforms for TKA will further expand the role of robotics over the next decade, particularly as our focus shifts beyond component and limb alignment in TKA and more towards the role of robotics in soft tissue balancing, reduction in instrumentation and inventory and its attendant cost savings, and surgical efficiencies. One semiautonomous robotic technology first used in 2006 (Mako, Stryker) reported a 130% increase in robotic volume from 2011 to 2012; another, first used in 2013, reported growth of 480% between 2013 and 2014, due to its improved cost structure, ease of use, smaller footprint, image-free platform and applicability in ambulatory surgery centers (Navio, Smith & Nephew; data supplied by manufacturer), demonstrating the growing popularity of robotic technology.17,39 Further, a recent analysis of potential market penetration over the next decade published by Medical Device and Diagnostic Industry (http://www.mddionline.com) projected that nearly 37% of UKAs and 23% of TKAs will be performed with robotics in 10 years.
Distinction Between Robotic-Assisted Technologies
Autonomous systems involve pre-programming the system with parameters that define the amount and orientation of bone to be removed, after which the system prepares the surfaces independent of surgeon control, other than having access to a “shutdown” switch. There are currently no autonomous robotic tools approved by the US Food and Drug Administration (FDA) for knee arthroplasty.
Semiautonomous systems involve the mapping of condylar landmarks and determination of alignment indices, which also defines the volume and orientation of bone to be removed. While the systems remove bone and cartilage within the pre-established parameters, the robotic tools are controlled and manipulated by the surgeon (Figure 1). The predetermined safe zones modulate and safeguard the surgical actions. These systems also provide real-time quantification of soft tissue balancing, which may contribute to the reported successful clinical and functional outcomes with semiautonomous systems (Figure 2).2,4,19,22 There are several semiautonomous robotic systems that are approved for use by the FDA.
Each robotic-assisted surgery (RAS) system utilizes some sort of 3-dimensional digital map of the surgical surfaces after a process of surface mapping and landmark registration.2 In the case of Mako, this planning process also requires a preoperative computed tomography (CT) scan. Over the past few years, the requirement of a CT scan has proven problematic and costly, as increasingly third-party payers and insurers are denying coverage for additional studies used for preoperative planning, leaving the burden of cost on the patients and/or hospitals. Additionally, in an era in which bundled payment arrangements are commonplace or in which providers are held accountable for costly care, the use of costly preoperative imaging is untenable. Furthermore, there is a growing concern regarding the risk of radiation exposure from CT scans that makes image-free technologies, such as Navio, an alternative for stakeholders.40
At this time, the 2 semiautonomous systems in use for UKA employ different methods to safeguard against inadvertent bone preparation: one by providing haptic constraint beyond which movement of the bur is limited (Mako); the other by modulating the exposure or speed of the handheld robotic bur (Navio) (Figure 3).
Outcomes of RAS in UKA
Compared to conventional UKA, robotic assistance has consistently demonstrated improved surgical accuracy, even through minimally invasive incisions (Figures 4, 5).6,20-28 Several studies have found substantial reduction in variability and error of component positioning with use of semiautonomous robotic tools.6,21,25 In fact, precision appears to be comparable regardless of whether an image-free system or one requiring a preoperative CT scan is used (Table). Further, in addition to improving component and limb alignment, Plate and colleagues22 demonstrated that RAS-based UKA systems can help the surgeon precisely reproduce plans for soft-tissue balancing. The authors reported ligament balancing to be accurate up to .53 mm compared to the preoperative plan, with approximately 83% of cases balanced within 1 mm of the plan through a full range of flexion.22
When evaluating advanced and novel technologies, there is undoubtedly concern that there will be increased operative time and a substantial learning curve with those technologies. Karia and colleagues30 found that when inexperienced surgeons performed UKA on synthetic bone models using robotics, the mean compound rotational and translational errors were lower than when conventional techniques were used. Among those using conventional techniques, although surgical times improved during the learning period, positional inaccuracies persisted. On the other hand, robotic assistance enabled surgeons to achieve precision and accuracy when positioning UKA components irrespective of their learning experience.30 Another study, by Coon,31 similarly suggested a shorter learning curve and greater accuracy with RAS using the Mako system compared to conventional techniques. A prospective, multicenter, observational study evaluated the operative times of 11 surgeons during their initial clinical cases using Navio robotic technology for medial UKA after a period of training using cadaver knees and sawbones.41 The learning curve for total surgical time (tracker placement to implant trial phase) indicates that it takes 8 cases to achieve 95% of total learning and maintain a steady state surgical time.
Potential Disadvantages of RAS in UKA
RAS for UKA has several potential disadvantages that must be weighed against their potential benefits. One major barrier to broader use of RAS is the increased cost associated with the technologies.17,19,27,32 Capital and maintenance costs for these systems can be high, and those that require additional advanced imaging, such as CT scans, further challenge the return on investment.17,19,32 In a Markov analysis of one robotic system (Mako), Moschetti and colleagues17 found that if one assumes a system cost of $1.362 million, value can be attained due to slightly better outcomes despite being more expensive than traditional methods. Nonetheless, their analysis of the Mako system estimated that each robot-assisted UKA case cost $19,219, compared to $16,476 with traditional UKA, and was associated with an incremental cost of $47,180 per quality-adjusted life-year. Their analysis further demonstrated that the cost-effectiveness was very sensitive to case volume, with lower costs realized once volumes surpassed 94 cases per year. On the other hand, costs (and thus value) will also obviously vary depending on the capital costs, annual service charges, and avoidance of unnecessary preoperative scans.19 For instance, assuming a cost of $500,000 for the image-free Navio robotic system, return on investment is achievable within 25 cases annually, roughly one-quarter of the cases necessary with the image-based system.
Another disadvantage of RAS systems in UKA is the unique complications associated with their use. Both RAS and conventional UKA can be complicated by similar problems such as component loosening, polyethylene wear, progressive arthritis, infection, stiffness, instability, and thromboembolism. RAS systems, however, carry the additional risk of specific robot-related issues.19,27 Perhaps most notably, the pin tracts for the required optical tracking arrays can create a stress riser in the cortical bone,19,27,33,42 highlighting the importance of inserting these pins in metaphyseal, and not diaphyseal, bone. Soft tissue complications have been reported during bone preparation with autonomous systems in total knee and hip arthroplasty;37,38 however, the senior author (JHL) has not observed that in 1000 consecutive cases with either semiautonomous surgeon-driven robotic tool.19
Finally, systems that require a preoperative CT scan pose an increased radiation risk.40 Ponzio and Lonner40 recently reported that each preoperative CT scan for robotic-assisted knee arthroplasty (using a Mako protocol) is associated with a mean effective dose of radiation of 4.8 mSv, which is approximately equivalent to 48 chest radiographs.34 Further, in that study, at least 25% of patients had been subjected to multiple scans, with some being exposed to cumulative effective doses of up to 103 mSv. This risk should not be considered completely negligible given that 10 mSv may be associated with an increase in the possibility of fatal cancer, and an estimated 29,000 excess cancer cases in the United States annually are reportedly caused by CT scans.40,43,44 However, this increased radiation risk is not inherent to all RAS systems. Image-free systems, such as Navio, do not require CT scans and are thus not associated with this potential disadvantage.
Conclusion
Robotics has come a long way from its humble conceptual beginnings nearly a century ago. Rapid advances in medical technology over the past 10 years have led to the development and growing popularity of RAS in orthopedic surgery, particularly during UKA. Component placement, quantified soft tissue balance, and radiographic alignment appear to be improved and the incidence of outliers reduced with the use of RAS during UKA. Further assessment is needed to determine whether the improved alignment and balance will impact clinical function and/or durability. Early results are very promising, though, creating optimism that the full benefits of RAS in UKA will be further confirmed with additional time and research.
1. Hockstein NG, Gourin CG, Faust RA, Terris DJ. A history of robots: from science fiction to surgical robotics. J Robot Surg. 2007;1(2):113-118.
2. Tamam C, Poehling GG. Robotic-assisted unicompartmental knee arthroplasty. Sports Med Arthrosc. 2014;22(4):219-222.
3. Beasley RA. Medical robots: current systems and research directions. Journal of Robotics. 2012. doi:10.1155/2012/401613.
4. Bargar WL. Robots in orthopaedic surgery: past, present, and future. Clin Orthop Relat Res. 2007;463:31-36.
5. Matsen FA 3rd, Garbini JL, Sidles JA, Pratt B, Baumgarten D, Kaiura R. Robotic assistance in orthopaedic surgery. A proof of principle using distal femoral arthroplasty. Clin Orthop Relat Res. 1993;(296):178-186.
6. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
7. Borus T, Thornhill T. Unicompartmental knee arthroplasty.
J Am Acad Orthop Surg. 2008;16(1):9-18.
8. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
9. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
10. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
11. Hamilton WG, Collier MB, Tarabee E, McAuley JP, Engh CA Jr, Engh GA. Incidence and reasons for reoperation after minimally invasive unicompartmental knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):98-107.
12. Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;(423):161-165.
13. Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(3):506-511.
14. Lonner JH. Indications for unicompartmental knee arthroplasty and rationale for robotic arm-assisted technology. Am J Orthop. 2009;38(2 Suppl):3-6.
15. Lonner JH. Robotically-assisted unicompartmental knee arthroplasty with a hand-held image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
16. Orthopedic Network News. 2013 Hip and Knee Implant Review. http://www.OrthopedicNetworkNews.com. Published July 2013. Accessed March 7, 2016.
17. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A Markov decision analysis. J Arthroplasty. 2016;31(4):759-765.
18. Roche M. Robotic-assisted unicompartmental knee arthroplasty: the MAKO experience. Orthop Clin North Am. 2015;46(1):125-131.
19. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Oper Tech Orthop. 2015;25:104-113.
20. Mofidi A, Plate JF, Lu B, et al. Assessment of accuracy of robotically assisted unicompartmental arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1918-1925.
21. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
22. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
23. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
24. Smith JR, Picard F, Lonner J, et al. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. J Bone Joint Surg Br. 2014;96-B(Suppl 16):12.
25. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
26. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
27. Sinha RK. Outcomes of robotic arm-assisted unicompartmental knee arthroplasty. Am J Orthop. 2009;38(2 Suppl):20-22.
28. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
29. Mozes A, Chang TC, Arata L, Zhao W. Three-dimensional A-mode ultrasound calibration and registration for robotic orthopaedic knee surgery. Int J Med Robot. 2010;6(1):91-101.
30. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
31. Coon TM. Integrating robotic technology into the operating room. Am J Orthop. 2009;38(2 Suppl):7-9.
32. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
33. Roche M, Augustin D, Conditt M. One year outcomes of robotically guided UKA. In: Proceedings of the 21st Annual Congress of the International Society of Technology in Arthroplasty. Sacramento, CA: International Society for Technology in Arthroplasty; 2008:175.
34. Dalton DM, Burke TP, Kelly EG, Curtin PD. Quantitative analysis of technological innovation in knee arthroplasty: using patent and publication metrics to identify developments and trends. J Arthroplasty. 2015. [Epub ahead of print]
35. Lonner JH. Modular bicompartmental knee arthroplasty with robotic arm assistance. Am J Orthop. 2009;38(2 Suppl):28-31.
36. Kamath AF, Levack A, John T, Thomas BS, Lonner JH. Minimum two-year outcomes of modular bicompartmental knee arthroplasty. J Arthroplasty. 2014;29(1):75-79.
37. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
38. Chun YS, Kim KI, Cho YJ, Kim YH, Yoo MC, Rhyu KH. Causes and patterns of aborting a robot-assisted arthroplasty. J Arthroplasty. 2011;26(4):621-625.
39. MAKO Surgical Corp. Fact Sheet. http://www.makosurgical.com/assets/files/Company/newsroom/Corporate_Fact_Sheet_208578r00.pdf. Published 2013. Accessed March 7, 2016.
40. Ponzio DY, Lonner JH. Preoperative mapping in unicompartmental knee arthroplasty using computed tomography scans is associated with radiation exposure and carries high cost. J Arthroplasty. 2015;30(6):964-967.
41. Wallace D, Gregori A, Picard F, et al. The learning curve of a novel handheld robotic system for unicondylar knee arthroplasty. Paper presented at: 14th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. June 18-21, 2014; Milan, Italy.
42. Wysocki RW, Sheinkop MB, Virkus WW, Della Valle CJ. Femoral fracture through a previous pin site after computer-assisted total knee arthroplasty. J Arthroplasty. 2008;23(3):462-465.
43. Costello JE, Cecava ND, Tucker JE, Bau JL. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol. 2013;201(6):1283-1290.
44. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
The concept of robotics is relatively new in medical practice. The term “robot” itself is less than 100 years old, having been first introduced to popular culture in 1917 by Joseph Capek in the science fiction story Opilec.1,2 Robots eventually transitioned from this initial fictional literary setting to reality in 1958, when General Motors began adding automated machines to its assembly lines.1 However, it was not until the 1980s that robotics and their exacting efficiencies would be introduced in the medical field, and it would take another decade before they would enter the specialty of orthopedics.1-4
The first robotic-assisted orthopedic surgery was reportedly performed in 1992, when the Robodoc autonomous system was utilized for total hip arthroplasty.2-4 A robotic system for total knee arthroplasty (TKA) was first described in 1993, but it would take several more years until a system for unicompartmental knee arthroplasty (UKA) would be commercialized and used clinically.5,6 The rationale for advancement of robotic technology for isolated medial or lateral knee arthritis stems from the recognition that while UKA is effective and durable when components and limb are well aligned and soft tissues appropriately balanced, they are less forgiving of even slight component malalignment of as little as 2° to 3° and prone to premature loosening or wear in those circumstances.7-13,14 In the mid 2000s, Cobb and colleagues6 reported using a semiautonomous robot for UKA. Since then, emergence of other semiautonomous robotic systems has led to greater market penetration and technology utilization.15
Currently, an estimated 15% to 20% of UKA surgeries are being performed with robotic assistance.16 Further, patent activity and peer-reviewed publications related to robotic technology in UKA (which can be considered surrogate measures of interest and evolving development and experience with robotic technologies) have increased dramatically over the past few years.2,6,14,17,18-34 To date, while the most dramatic growth of robotic utilization and case volumes has occurred in the subspecialty of UKA, semiautonomous robotic systems have been used with increasing frequency for patellofemoral and bicompartmental knee arthroplasty.35,36 Robotics have been used sparingly for TKA, and limited to autonomous systems;37,38 however, it is anticipated that emergence of semiautonomous platforms for TKA will further expand the role of robotics over the next decade, particularly as our focus shifts beyond component and limb alignment in TKA and more towards the role of robotics in soft tissue balancing, reduction in instrumentation and inventory and its attendant cost savings, and surgical efficiencies. One semiautonomous robotic technology first used in 2006 (Mako, Stryker) reported a 130% increase in robotic volume from 2011 to 2012; another, first used in 2013, reported growth of 480% between 2013 and 2014, due to its improved cost structure, ease of use, smaller footprint, image-free platform and applicability in ambulatory surgery centers (Navio, Smith & Nephew; data supplied by manufacturer), demonstrating the growing popularity of robotic technology.17,39 Further, a recent analysis of potential market penetration over the next decade published by Medical Device and Diagnostic Industry (http://www.mddionline.com) projected that nearly 37% of UKAs and 23% of TKAs will be performed with robotics in 10 years.
Distinction Between Robotic-Assisted Technologies
Autonomous systems involve pre-programming the system with parameters that define the amount and orientation of bone to be removed, after which the system prepares the surfaces independent of surgeon control, other than having access to a “shutdown” switch. There are currently no autonomous robotic tools approved by the US Food and Drug Administration (FDA) for knee arthroplasty.
Semiautonomous systems involve the mapping of condylar landmarks and determination of alignment indices, which also defines the volume and orientation of bone to be removed. While the systems remove bone and cartilage within the pre-established parameters, the robotic tools are controlled and manipulated by the surgeon (Figure 1). The predetermined safe zones modulate and safeguard the surgical actions. These systems also provide real-time quantification of soft tissue balancing, which may contribute to the reported successful clinical and functional outcomes with semiautonomous systems (Figure 2).2,4,19,22 There are several semiautonomous robotic systems that are approved for use by the FDA.
Each robotic-assisted surgery (RAS) system utilizes some sort of 3-dimensional digital map of the surgical surfaces after a process of surface mapping and landmark registration.2 In the case of Mako, this planning process also requires a preoperative computed tomography (CT) scan. Over the past few years, the requirement of a CT scan has proven problematic and costly, as increasingly third-party payers and insurers are denying coverage for additional studies used for preoperative planning, leaving the burden of cost on the patients and/or hospitals. Additionally, in an era in which bundled payment arrangements are commonplace or in which providers are held accountable for costly care, the use of costly preoperative imaging is untenable. Furthermore, there is a growing concern regarding the risk of radiation exposure from CT scans that makes image-free technologies, such as Navio, an alternative for stakeholders.40
At this time, the 2 semiautonomous systems in use for UKA employ different methods to safeguard against inadvertent bone preparation: one by providing haptic constraint beyond which movement of the bur is limited (Mako); the other by modulating the exposure or speed of the handheld robotic bur (Navio) (Figure 3).
Outcomes of RAS in UKA
Compared to conventional UKA, robotic assistance has consistently demonstrated improved surgical accuracy, even through minimally invasive incisions (Figures 4, 5).6,20-28 Several studies have found substantial reduction in variability and error of component positioning with use of semiautonomous robotic tools.6,21,25 In fact, precision appears to be comparable regardless of whether an image-free system or one requiring a preoperative CT scan is used (Table). Further, in addition to improving component and limb alignment, Plate and colleagues22 demonstrated that RAS-based UKA systems can help the surgeon precisely reproduce plans for soft-tissue balancing. The authors reported ligament balancing to be accurate up to .53 mm compared to the preoperative plan, with approximately 83% of cases balanced within 1 mm of the plan through a full range of flexion.22
When evaluating advanced and novel technologies, there is undoubtedly concern that there will be increased operative time and a substantial learning curve with those technologies. Karia and colleagues30 found that when inexperienced surgeons performed UKA on synthetic bone models using robotics, the mean compound rotational and translational errors were lower than when conventional techniques were used. Among those using conventional techniques, although surgical times improved during the learning period, positional inaccuracies persisted. On the other hand, robotic assistance enabled surgeons to achieve precision and accuracy when positioning UKA components irrespective of their learning experience.30 Another study, by Coon,31 similarly suggested a shorter learning curve and greater accuracy with RAS using the Mako system compared to conventional techniques. A prospective, multicenter, observational study evaluated the operative times of 11 surgeons during their initial clinical cases using Navio robotic technology for medial UKA after a period of training using cadaver knees and sawbones.41 The learning curve for total surgical time (tracker placement to implant trial phase) indicates that it takes 8 cases to achieve 95% of total learning and maintain a steady state surgical time.
Potential Disadvantages of RAS in UKA
RAS for UKA has several potential disadvantages that must be weighed against their potential benefits. One major barrier to broader use of RAS is the increased cost associated with the technologies.17,19,27,32 Capital and maintenance costs for these systems can be high, and those that require additional advanced imaging, such as CT scans, further challenge the return on investment.17,19,32 In a Markov analysis of one robotic system (Mako), Moschetti and colleagues17 found that if one assumes a system cost of $1.362 million, value can be attained due to slightly better outcomes despite being more expensive than traditional methods. Nonetheless, their analysis of the Mako system estimated that each robot-assisted UKA case cost $19,219, compared to $16,476 with traditional UKA, and was associated with an incremental cost of $47,180 per quality-adjusted life-year. Their analysis further demonstrated that the cost-effectiveness was very sensitive to case volume, with lower costs realized once volumes surpassed 94 cases per year. On the other hand, costs (and thus value) will also obviously vary depending on the capital costs, annual service charges, and avoidance of unnecessary preoperative scans.19 For instance, assuming a cost of $500,000 for the image-free Navio robotic system, return on investment is achievable within 25 cases annually, roughly one-quarter of the cases necessary with the image-based system.
Another disadvantage of RAS systems in UKA is the unique complications associated with their use. Both RAS and conventional UKA can be complicated by similar problems such as component loosening, polyethylene wear, progressive arthritis, infection, stiffness, instability, and thromboembolism. RAS systems, however, carry the additional risk of specific robot-related issues.19,27 Perhaps most notably, the pin tracts for the required optical tracking arrays can create a stress riser in the cortical bone,19,27,33,42 highlighting the importance of inserting these pins in metaphyseal, and not diaphyseal, bone. Soft tissue complications have been reported during bone preparation with autonomous systems in total knee and hip arthroplasty;37,38 however, the senior author (JHL) has not observed that in 1000 consecutive cases with either semiautonomous surgeon-driven robotic tool.19
Finally, systems that require a preoperative CT scan pose an increased radiation risk.40 Ponzio and Lonner40 recently reported that each preoperative CT scan for robotic-assisted knee arthroplasty (using a Mako protocol) is associated with a mean effective dose of radiation of 4.8 mSv, which is approximately equivalent to 48 chest radiographs.34 Further, in that study, at least 25% of patients had been subjected to multiple scans, with some being exposed to cumulative effective doses of up to 103 mSv. This risk should not be considered completely negligible given that 10 mSv may be associated with an increase in the possibility of fatal cancer, and an estimated 29,000 excess cancer cases in the United States annually are reportedly caused by CT scans.40,43,44 However, this increased radiation risk is not inherent to all RAS systems. Image-free systems, such as Navio, do not require CT scans and are thus not associated with this potential disadvantage.
Conclusion
Robotics has come a long way from its humble conceptual beginnings nearly a century ago. Rapid advances in medical technology over the past 10 years have led to the development and growing popularity of RAS in orthopedic surgery, particularly during UKA. Component placement, quantified soft tissue balance, and radiographic alignment appear to be improved and the incidence of outliers reduced with the use of RAS during UKA. Further assessment is needed to determine whether the improved alignment and balance will impact clinical function and/or durability. Early results are very promising, though, creating optimism that the full benefits of RAS in UKA will be further confirmed with additional time and research.
The concept of robotics is relatively new in medical practice. The term “robot” itself is less than 100 years old, having been first introduced to popular culture in 1917 by Joseph Capek in the science fiction story Opilec.1,2 Robots eventually transitioned from this initial fictional literary setting to reality in 1958, when General Motors began adding automated machines to its assembly lines.1 However, it was not until the 1980s that robotics and their exacting efficiencies would be introduced in the medical field, and it would take another decade before they would enter the specialty of orthopedics.1-4
The first robotic-assisted orthopedic surgery was reportedly performed in 1992, when the Robodoc autonomous system was utilized for total hip arthroplasty.2-4 A robotic system for total knee arthroplasty (TKA) was first described in 1993, but it would take several more years until a system for unicompartmental knee arthroplasty (UKA) would be commercialized and used clinically.5,6 The rationale for advancement of robotic technology for isolated medial or lateral knee arthritis stems from the recognition that while UKA is effective and durable when components and limb are well aligned and soft tissues appropriately balanced, they are less forgiving of even slight component malalignment of as little as 2° to 3° and prone to premature loosening or wear in those circumstances.7-13,14 In the mid 2000s, Cobb and colleagues6 reported using a semiautonomous robot for UKA. Since then, emergence of other semiautonomous robotic systems has led to greater market penetration and technology utilization.15
Currently, an estimated 15% to 20% of UKA surgeries are being performed with robotic assistance.16 Further, patent activity and peer-reviewed publications related to robotic technology in UKA (which can be considered surrogate measures of interest and evolving development and experience with robotic technologies) have increased dramatically over the past few years.2,6,14,17,18-34 To date, while the most dramatic growth of robotic utilization and case volumes has occurred in the subspecialty of UKA, semiautonomous robotic systems have been used with increasing frequency for patellofemoral and bicompartmental knee arthroplasty.35,36 Robotics have been used sparingly for TKA, and limited to autonomous systems;37,38 however, it is anticipated that emergence of semiautonomous platforms for TKA will further expand the role of robotics over the next decade, particularly as our focus shifts beyond component and limb alignment in TKA and more towards the role of robotics in soft tissue balancing, reduction in instrumentation and inventory and its attendant cost savings, and surgical efficiencies. One semiautonomous robotic technology first used in 2006 (Mako, Stryker) reported a 130% increase in robotic volume from 2011 to 2012; another, first used in 2013, reported growth of 480% between 2013 and 2014, due to its improved cost structure, ease of use, smaller footprint, image-free platform and applicability in ambulatory surgery centers (Navio, Smith & Nephew; data supplied by manufacturer), demonstrating the growing popularity of robotic technology.17,39 Further, a recent analysis of potential market penetration over the next decade published by Medical Device and Diagnostic Industry (http://www.mddionline.com) projected that nearly 37% of UKAs and 23% of TKAs will be performed with robotics in 10 years.
Distinction Between Robotic-Assisted Technologies
Autonomous systems involve pre-programming the system with parameters that define the amount and orientation of bone to be removed, after which the system prepares the surfaces independent of surgeon control, other than having access to a “shutdown” switch. There are currently no autonomous robotic tools approved by the US Food and Drug Administration (FDA) for knee arthroplasty.
Semiautonomous systems involve the mapping of condylar landmarks and determination of alignment indices, which also defines the volume and orientation of bone to be removed. While the systems remove bone and cartilage within the pre-established parameters, the robotic tools are controlled and manipulated by the surgeon (Figure 1). The predetermined safe zones modulate and safeguard the surgical actions. These systems also provide real-time quantification of soft tissue balancing, which may contribute to the reported successful clinical and functional outcomes with semiautonomous systems (Figure 2).2,4,19,22 There are several semiautonomous robotic systems that are approved for use by the FDA.
Each robotic-assisted surgery (RAS) system utilizes some sort of 3-dimensional digital map of the surgical surfaces after a process of surface mapping and landmark registration.2 In the case of Mako, this planning process also requires a preoperative computed tomography (CT) scan. Over the past few years, the requirement of a CT scan has proven problematic and costly, as increasingly third-party payers and insurers are denying coverage for additional studies used for preoperative planning, leaving the burden of cost on the patients and/or hospitals. Additionally, in an era in which bundled payment arrangements are commonplace or in which providers are held accountable for costly care, the use of costly preoperative imaging is untenable. Furthermore, there is a growing concern regarding the risk of radiation exposure from CT scans that makes image-free technologies, such as Navio, an alternative for stakeholders.40
At this time, the 2 semiautonomous systems in use for UKA employ different methods to safeguard against inadvertent bone preparation: one by providing haptic constraint beyond which movement of the bur is limited (Mako); the other by modulating the exposure or speed of the handheld robotic bur (Navio) (Figure 3).
Outcomes of RAS in UKA
Compared to conventional UKA, robotic assistance has consistently demonstrated improved surgical accuracy, even through minimally invasive incisions (Figures 4, 5).6,20-28 Several studies have found substantial reduction in variability and error of component positioning with use of semiautonomous robotic tools.6,21,25 In fact, precision appears to be comparable regardless of whether an image-free system or one requiring a preoperative CT scan is used (Table). Further, in addition to improving component and limb alignment, Plate and colleagues22 demonstrated that RAS-based UKA systems can help the surgeon precisely reproduce plans for soft-tissue balancing. The authors reported ligament balancing to be accurate up to .53 mm compared to the preoperative plan, with approximately 83% of cases balanced within 1 mm of the plan through a full range of flexion.22
When evaluating advanced and novel technologies, there is undoubtedly concern that there will be increased operative time and a substantial learning curve with those technologies. Karia and colleagues30 found that when inexperienced surgeons performed UKA on synthetic bone models using robotics, the mean compound rotational and translational errors were lower than when conventional techniques were used. Among those using conventional techniques, although surgical times improved during the learning period, positional inaccuracies persisted. On the other hand, robotic assistance enabled surgeons to achieve precision and accuracy when positioning UKA components irrespective of their learning experience.30 Another study, by Coon,31 similarly suggested a shorter learning curve and greater accuracy with RAS using the Mako system compared to conventional techniques. A prospective, multicenter, observational study evaluated the operative times of 11 surgeons during their initial clinical cases using Navio robotic technology for medial UKA after a period of training using cadaver knees and sawbones.41 The learning curve for total surgical time (tracker placement to implant trial phase) indicates that it takes 8 cases to achieve 95% of total learning and maintain a steady state surgical time.
Potential Disadvantages of RAS in UKA
RAS for UKA has several potential disadvantages that must be weighed against their potential benefits. One major barrier to broader use of RAS is the increased cost associated with the technologies.17,19,27,32 Capital and maintenance costs for these systems can be high, and those that require additional advanced imaging, such as CT scans, further challenge the return on investment.17,19,32 In a Markov analysis of one robotic system (Mako), Moschetti and colleagues17 found that if one assumes a system cost of $1.362 million, value can be attained due to slightly better outcomes despite being more expensive than traditional methods. Nonetheless, their analysis of the Mako system estimated that each robot-assisted UKA case cost $19,219, compared to $16,476 with traditional UKA, and was associated with an incremental cost of $47,180 per quality-adjusted life-year. Their analysis further demonstrated that the cost-effectiveness was very sensitive to case volume, with lower costs realized once volumes surpassed 94 cases per year. On the other hand, costs (and thus value) will also obviously vary depending on the capital costs, annual service charges, and avoidance of unnecessary preoperative scans.19 For instance, assuming a cost of $500,000 for the image-free Navio robotic system, return on investment is achievable within 25 cases annually, roughly one-quarter of the cases necessary with the image-based system.
Another disadvantage of RAS systems in UKA is the unique complications associated with their use. Both RAS and conventional UKA can be complicated by similar problems such as component loosening, polyethylene wear, progressive arthritis, infection, stiffness, instability, and thromboembolism. RAS systems, however, carry the additional risk of specific robot-related issues.19,27 Perhaps most notably, the pin tracts for the required optical tracking arrays can create a stress riser in the cortical bone,19,27,33,42 highlighting the importance of inserting these pins in metaphyseal, and not diaphyseal, bone. Soft tissue complications have been reported during bone preparation with autonomous systems in total knee and hip arthroplasty;37,38 however, the senior author (JHL) has not observed that in 1000 consecutive cases with either semiautonomous surgeon-driven robotic tool.19
Finally, systems that require a preoperative CT scan pose an increased radiation risk.40 Ponzio and Lonner40 recently reported that each preoperative CT scan for robotic-assisted knee arthroplasty (using a Mako protocol) is associated with a mean effective dose of radiation of 4.8 mSv, which is approximately equivalent to 48 chest radiographs.34 Further, in that study, at least 25% of patients had been subjected to multiple scans, with some being exposed to cumulative effective doses of up to 103 mSv. This risk should not be considered completely negligible given that 10 mSv may be associated with an increase in the possibility of fatal cancer, and an estimated 29,000 excess cancer cases in the United States annually are reportedly caused by CT scans.40,43,44 However, this increased radiation risk is not inherent to all RAS systems. Image-free systems, such as Navio, do not require CT scans and are thus not associated with this potential disadvantage.
Conclusion
Robotics has come a long way from its humble conceptual beginnings nearly a century ago. Rapid advances in medical technology over the past 10 years have led to the development and growing popularity of RAS in orthopedic surgery, particularly during UKA. Component placement, quantified soft tissue balance, and radiographic alignment appear to be improved and the incidence of outliers reduced with the use of RAS during UKA. Further assessment is needed to determine whether the improved alignment and balance will impact clinical function and/or durability. Early results are very promising, though, creating optimism that the full benefits of RAS in UKA will be further confirmed with additional time and research.
1. Hockstein NG, Gourin CG, Faust RA, Terris DJ. A history of robots: from science fiction to surgical robotics. J Robot Surg. 2007;1(2):113-118.
2. Tamam C, Poehling GG. Robotic-assisted unicompartmental knee arthroplasty. Sports Med Arthrosc. 2014;22(4):219-222.
3. Beasley RA. Medical robots: current systems and research directions. Journal of Robotics. 2012. doi:10.1155/2012/401613.
4. Bargar WL. Robots in orthopaedic surgery: past, present, and future. Clin Orthop Relat Res. 2007;463:31-36.
5. Matsen FA 3rd, Garbini JL, Sidles JA, Pratt B, Baumgarten D, Kaiura R. Robotic assistance in orthopaedic surgery. A proof of principle using distal femoral arthroplasty. Clin Orthop Relat Res. 1993;(296):178-186.
6. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
7. Borus T, Thornhill T. Unicompartmental knee arthroplasty.
J Am Acad Orthop Surg. 2008;16(1):9-18.
8. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
9. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
10. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
11. Hamilton WG, Collier MB, Tarabee E, McAuley JP, Engh CA Jr, Engh GA. Incidence and reasons for reoperation after minimally invasive unicompartmental knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):98-107.
12. Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;(423):161-165.
13. Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(3):506-511.
14. Lonner JH. Indications for unicompartmental knee arthroplasty and rationale for robotic arm-assisted technology. Am J Orthop. 2009;38(2 Suppl):3-6.
15. Lonner JH. Robotically-assisted unicompartmental knee arthroplasty with a hand-held image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
16. Orthopedic Network News. 2013 Hip and Knee Implant Review. http://www.OrthopedicNetworkNews.com. Published July 2013. Accessed March 7, 2016.
17. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A Markov decision analysis. J Arthroplasty. 2016;31(4):759-765.
18. Roche M. Robotic-assisted unicompartmental knee arthroplasty: the MAKO experience. Orthop Clin North Am. 2015;46(1):125-131.
19. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Oper Tech Orthop. 2015;25:104-113.
20. Mofidi A, Plate JF, Lu B, et al. Assessment of accuracy of robotically assisted unicompartmental arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1918-1925.
21. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
22. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
23. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
24. Smith JR, Picard F, Lonner J, et al. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. J Bone Joint Surg Br. 2014;96-B(Suppl 16):12.
25. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
26. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
27. Sinha RK. Outcomes of robotic arm-assisted unicompartmental knee arthroplasty. Am J Orthop. 2009;38(2 Suppl):20-22.
28. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
29. Mozes A, Chang TC, Arata L, Zhao W. Three-dimensional A-mode ultrasound calibration and registration for robotic orthopaedic knee surgery. Int J Med Robot. 2010;6(1):91-101.
30. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
31. Coon TM. Integrating robotic technology into the operating room. Am J Orthop. 2009;38(2 Suppl):7-9.
32. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
33. Roche M, Augustin D, Conditt M. One year outcomes of robotically guided UKA. In: Proceedings of the 21st Annual Congress of the International Society of Technology in Arthroplasty. Sacramento, CA: International Society for Technology in Arthroplasty; 2008:175.
34. Dalton DM, Burke TP, Kelly EG, Curtin PD. Quantitative analysis of technological innovation in knee arthroplasty: using patent and publication metrics to identify developments and trends. J Arthroplasty. 2015. [Epub ahead of print]
35. Lonner JH. Modular bicompartmental knee arthroplasty with robotic arm assistance. Am J Orthop. 2009;38(2 Suppl):28-31.
36. Kamath AF, Levack A, John T, Thomas BS, Lonner JH. Minimum two-year outcomes of modular bicompartmental knee arthroplasty. J Arthroplasty. 2014;29(1):75-79.
37. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
38. Chun YS, Kim KI, Cho YJ, Kim YH, Yoo MC, Rhyu KH. Causes and patterns of aborting a robot-assisted arthroplasty. J Arthroplasty. 2011;26(4):621-625.
39. MAKO Surgical Corp. Fact Sheet. http://www.makosurgical.com/assets/files/Company/newsroom/Corporate_Fact_Sheet_208578r00.pdf. Published 2013. Accessed March 7, 2016.
40. Ponzio DY, Lonner JH. Preoperative mapping in unicompartmental knee arthroplasty using computed tomography scans is associated with radiation exposure and carries high cost. J Arthroplasty. 2015;30(6):964-967.
41. Wallace D, Gregori A, Picard F, et al. The learning curve of a novel handheld robotic system for unicondylar knee arthroplasty. Paper presented at: 14th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. June 18-21, 2014; Milan, Italy.
42. Wysocki RW, Sheinkop MB, Virkus WW, Della Valle CJ. Femoral fracture through a previous pin site after computer-assisted total knee arthroplasty. J Arthroplasty. 2008;23(3):462-465.
43. Costello JE, Cecava ND, Tucker JE, Bau JL. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol. 2013;201(6):1283-1290.
44. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
1. Hockstein NG, Gourin CG, Faust RA, Terris DJ. A history of robots: from science fiction to surgical robotics. J Robot Surg. 2007;1(2):113-118.
2. Tamam C, Poehling GG. Robotic-assisted unicompartmental knee arthroplasty. Sports Med Arthrosc. 2014;22(4):219-222.
3. Beasley RA. Medical robots: current systems and research directions. Journal of Robotics. 2012. doi:10.1155/2012/401613.
4. Bargar WL. Robots in orthopaedic surgery: past, present, and future. Clin Orthop Relat Res. 2007;463:31-36.
5. Matsen FA 3rd, Garbini JL, Sidles JA, Pratt B, Baumgarten D, Kaiura R. Robotic assistance in orthopaedic surgery. A proof of principle using distal femoral arthroplasty. Clin Orthop Relat Res. 1993;(296):178-186.
6. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
7. Borus T, Thornhill T. Unicompartmental knee arthroplasty.
J Am Acad Orthop Surg. 2008;16(1):9-18.
8. Berger RA, Meneghini RM, Jacobs JJ, et al. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87(5):999-1006.
9. Price AJ, Waite JC, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;(435):171-180.
10. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
11. Hamilton WG, Collier MB, Tarabee E, McAuley JP, Engh CA Jr, Engh GA. Incidence and reasons for reoperation after minimally invasive unicompartmental knee arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):98-107.
12. Hernigou P, Deschamps G. Alignment influences wear in the knee after medial unicompartmental arthroplasty. Clin Orthop Relat Res. 2004;(423):161-165.
13. Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2004;86-A(3):506-511.
14. Lonner JH. Indications for unicompartmental knee arthroplasty and rationale for robotic arm-assisted technology. Am J Orthop. 2009;38(2 Suppl):3-6.
15. Lonner JH. Robotically-assisted unicompartmental knee arthroplasty with a hand-held image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
16. Orthopedic Network News. 2013 Hip and Knee Implant Review. http://www.OrthopedicNetworkNews.com. Published July 2013. Accessed March 7, 2016.
17. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A Markov decision analysis. J Arthroplasty. 2016;31(4):759-765.
18. Roche M. Robotic-assisted unicompartmental knee arthroplasty: the MAKO experience. Orthop Clin North Am. 2015;46(1):125-131.
19. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Oper Tech Orthop. 2015;25:104-113.
20. Mofidi A, Plate JF, Lu B, et al. Assessment of accuracy of robotically assisted unicompartmental arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2014;22(8):1918-1925.
21. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
22. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
23. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
24. Smith JR, Picard F, Lonner J, et al. The accuracy of a robotically-controlled freehand sculpting tool for unicondylar knee arthroplasty. J Bone Joint Surg Br. 2014;96-B(Suppl 16):12.
25. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
26. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
27. Sinha RK. Outcomes of robotic arm-assisted unicompartmental knee arthroplasty. Am J Orthop. 2009;38(2 Suppl):20-22.
28. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
29. Mozes A, Chang TC, Arata L, Zhao W. Three-dimensional A-mode ultrasound calibration and registration for robotic orthopaedic knee surgery. Int J Med Robot. 2010;6(1):91-101.
30. Karia M, Masjedi M, Andrews B, Jaffry Z, Cobb J. Robotic assistance enables inexperienced surgeons to perform unicompartmental knee arthroplasties on dry bone models with accuracy superior to conventional methods. Adv Orthop. 2013;2013:481039.
31. Coon TM. Integrating robotic technology into the operating room. Am J Orthop. 2009;38(2 Suppl):7-9.
32. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
33. Roche M, Augustin D, Conditt M. One year outcomes of robotically guided UKA. In: Proceedings of the 21st Annual Congress of the International Society of Technology in Arthroplasty. Sacramento, CA: International Society for Technology in Arthroplasty; 2008:175.
34. Dalton DM, Burke TP, Kelly EG, Curtin PD. Quantitative analysis of technological innovation in knee arthroplasty: using patent and publication metrics to identify developments and trends. J Arthroplasty. 2015. [Epub ahead of print]
35. Lonner JH. Modular bicompartmental knee arthroplasty with robotic arm assistance. Am J Orthop. 2009;38(2 Suppl):28-31.
36. Kamath AF, Levack A, John T, Thomas BS, Lonner JH. Minimum two-year outcomes of modular bicompartmental knee arthroplasty. J Arthroplasty. 2014;29(1):75-79.
37. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
38. Chun YS, Kim KI, Cho YJ, Kim YH, Yoo MC, Rhyu KH. Causes and patterns of aborting a robot-assisted arthroplasty. J Arthroplasty. 2011;26(4):621-625.
39. MAKO Surgical Corp. Fact Sheet. http://www.makosurgical.com/assets/files/Company/newsroom/Corporate_Fact_Sheet_208578r00.pdf. Published 2013. Accessed March 7, 2016.
40. Ponzio DY, Lonner JH. Preoperative mapping in unicompartmental knee arthroplasty using computed tomography scans is associated with radiation exposure and carries high cost. J Arthroplasty. 2015;30(6):964-967.
41. Wallace D, Gregori A, Picard F, et al. The learning curve of a novel handheld robotic system for unicondylar knee arthroplasty. Paper presented at: 14th Annual Meeting of the International Society for Computer Assisted Orthopaedic Surgery. June 18-21, 2014; Milan, Italy.
42. Wysocki RW, Sheinkop MB, Virkus WW, Della Valle CJ. Femoral fracture through a previous pin site after computer-assisted total knee arthroplasty. J Arthroplasty. 2008;23(3):462-465.
43. Costello JE, Cecava ND, Tucker JE, Bau JL. CT radiation dose: current controversies and dose reduction strategies. AJR Am J Roentgenol. 2013;201(6):1283-1290.
44. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077.
Disposable Navigation for Total Knee Arthroplasty
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
Total knee arthroplasty (TKA) continues to be a widely utilized treatment option for end-stage knee osteoarthritis, and the number of patients undergoing TKA is projected to continually increase over the next decade.1 Although TKA is highly successful for many patients, studies continue to report that approximately 20% of patients are dissatisfied after undergoing TKA, and nearly 25% of knee revisions are performed for instability or malalignment.2,3 Technological advances have been developed to help improve clinical outcomes and implant survivorship. Over the past decade, computer navigation and intraoperative guides have been introduced to help control surgical variables and overcome the limitations and inaccuracies of traditional mechanical instrumentation. Currently, there are a variety of technologies available to assist surgeons with component alignment, including extramedullary devices, computer-assisted navigation systems (CAS), and patient-specific instrumentation (PSI) that help achieve desired alignment goals.4,5
Computer-assisted navigation tools were introduced in an effort to improve implant alignment and clinical outcomes compared to traditional mechanical guides. Some argue that the use of computer-assisted surgery has a steep learning curve and successful use is dependent on the user’s experience; however, studies have suggested computer-assisted surgery may allow less experienced surgeons to reliably achieve anticipated intraoperative alignment goals with a low complication rate.6,7 Various studies have looked at computer-assisted TKA at short to mid-term follow-up, but few studies have reported long-term outcomes.6-9 de Steiger and colleagues10 recently found that computer-assisted TKA reduced the overall revision rate for aseptic loosening following TKA in patients younger than age 65 years, which suggests benefit of CAS for younger patients. Short-term follow-up has also shown the benefit of CAS TKA in patients with severe extra-articular deformity, where traditional instrumentation cannot be utilized.11 Currently, there is no consensus that computer-assisted TKA leads to improved postoperative patient reported outcomes, because many studies are limited by study design or small cohorts; however, current literature does show an improvement in component alignment as compared to mechanical instrumentation.9,12,13 As future implant and position targets are defined to improve implant survivorship and clinical outcomes in total joint arthroplasty, computer-assisted devices will be useful to help achieve more precise and accurate component positioning.
In addition to CAS devices, some companies have sought to improve TKA surgery by introducing PSI. PSI was introduced to improve component alignment in TKA, with the purported advantages of a shorter surgical time, decrease in the number of instruments needed, and improved clinical outcomes. PSI accuracy remains variable, which may be attributed to the various systems and implant designs in each study.14-17 In addition, advanced preoperative imaging is necessary, which further adds to the overall cost.17 While the recent advancement in technology may provide decreased costs at the time of surgery, the increased cost and time incurred by the patient preoperatively has not resulted in significantly better clinical outcomes.18,19 Additionally, recent work has not shown PSI to have superior accuracy as compared to currently available CAS devices.14 These findings suggest that the additional cost and time incurred by patients may limit the widespread use of PSI.
Although computer navigation has been shown to be more accurate than conventional instrumentation and PSI, the lack of improvement in long-term clinical outcome data has limited its use. In a meta-analysis, Bauwens and colleagues20 suggested that while navigated TKAs have improved component alignment outcomes as compared to conventional surgery, the clinical benefit remains unclear. Less than 5% of surgeons are currently using navigation systems due to the perceived learning curve, cost, additional surgical time, and imaging required to utilize these systems. Certain navigation systems can be seemingly cumbersome, with large consoles, increased number of instruments required, and optical instruments with line-of-sight issues. Recent technological advances have worked to decrease this challenge by using accelerometer- and gyroscope-based electronic components, which combine the accuracy of computer-assisted technology with the ease of use of mechanical guides.
Accelerometer and gyroscope technology systems, such as the iAssist system, are portable devices that do not require a large computer console or navigation arrays. This technology relies on accelerator-based navigation without additional preoperative imaging. A recent study demonstrated the iAssist had reproducible accuracy in component alignment that could be easily incorporated into the operating room without optical trackers.21 The use of portable computer-assisted devices provides a more compact and easily accessible technology that can be used to achieve accurate component alignment without additional large equipment in the operating room.22 These new handheld intraoperative tools have been introduced to place implants according to a preoperative plan in order to minimize failure due to preoperative extra-articular deformity or intraoperative technical miscues.23 Nam and colleagues24 used an accelerometer-based surgical navigation system to perform tibial resections in cadaveric models, and found that the accelerometer-based guide was accurate for tibial resection in both the coronal and sagittal planes. In a prospective randomized controlled trial evaluating 100 patients undergoing a TKA using either an accelerometer-based guide or conventional alignment methods, the authors showed that the accelerometer-based guide decreased outliers in tibial component alignment compared to conventional guides.25 In the accelerometer-based guide cohort, 95.7% of tibial components were within 2° of perpendicular to the tibial mechanical axis, compared to 68.1% in the conventional group (P < .001). These results suggested that portable accelerometer-based navigation allows surgeons to achieve satisfactory tibial component alignment with a decrease in the number of potential outliers.24,25 Similarly, Bugbee and colleagues26 found that accelerometer-based handheld navigation was accurate for tibial coronal and sagittal alignment and no additional surgical time was required compared to conventional techniques.
The relationship between knee alignment and clinical outcomes for TKA remains controversial. Regardless of the surgeon’s alignment preference, it is important to reliably and accurately execute the preoperative plan in a reproducible fashion. Advances in technology that assist with intraoperative component alignment can be useful, and may help decrease the incidence of implant malalignment in clinical practice.
Preoperative Planning and Intraoperative Technique
Preoperative planning is carried out in a manner identical to the use of conventional mechanical guides. Long leg films are taken for evaluation of overall limb alignment, and calibrated lateral images are taken for templating implant sizes. Lines are drawn on the images to determine the difference between the mechanical and anatomic axis of the femur, and a line drawn perpendicular to the mechanical axis is placed to show the expected bone cut. In similar fashion a perpendicular line to the tibial mechanical axis is also drawn to show the expected tibial resection. This preoperative plan allows the surgeon to have an additional intraoperative guide to ensure accuracy of the computer-assisted device.
After standard exposure, the distal femoral or proximal tibial cut can be made based on surgeon preference. The system being demonstrated in the accompanying photos is the KneeAlign 2 system (OrthAlign).
Distal Femoral Cut
The KneeAlign 2 femoral cutting guide is attached to the distal femur with a central pin that is placed in the middle of the distal femur measured from medial to lateral, and 1 cm anterior to the intercondylar notch. It is important to note that this spot is often more medial than traditionally used for insertion of an intramedullary rod. This central point sets the distal point of the femoral mechanical axis. The device is then held in place with 2 oblique pins, and is solidly fixed to the bone. Using a rotating motion, the femur is rotated around the hip joint. The accelerometer and gyroscope in the unit are able to determine the center of the hip joint from this motion, creating the proximal point of the mechanical axis of the femur. Once the mechanical axis of the femur is determined, varus/valgus and flexion/extension can be adjusted on the guide. One adjustment screw is available for varus/valgus, and a second is available for flexion/extension. Numbers on the device screen show real-time alignment, and are easily adjusted to set the desired alignment (Figure 1). Once alignment is obtained, a mechanical stylus is used to determine depth of resection, and the distal femoral cutting block is pinned. After pinning the block, the 3 pins in the device are removed, and the device is removed from the bone. This leaves only the distal femoral cutting block in place. In experienced hands, this part of the procedure takes less than 3 minutes.
Proximal Tibial Cut
The KneeAlign 2 proximal tibial guide is similar in appearance to a standard mechanical tibial cutting guide. It is attached to the proximal tibia with a spring around the calf and 2 pins that hold the device aligned with the medial third of the tibial tubercle. A stylus is then centered on the anterior cruciate ligament (ACL) footprint, which sets the proximal mechanical axis point of the tibia (Figure 2). An offset number is read off the stylus on the ACL footprint, and this number is matched on the ankle offset portion of the guide. The device has 2 sensors. One sensor is on the chassis of the device, and the other is on a mobile arm. Movements between the 2 are monitored by the accelerometers, allowing for accurate maintenance of alignment position even with motion in the leg. A point is taken from the lateral malleolus and then a second point is taken from the medial malleolus. These points are used to determine the center of the ankle joint, which sets the distal mechanical axis point. Once mechanical axis of the tibia is determined, the tibial cutting guide is pinned in place, and can be adjusted with real-time guidance of the varus/valgus and posterior slope values seen on the device (Figure 3). Cut depth is once again determined with a mechanical stylus.
Limitations
Although these devices have proven to be very accurate, surgeons must continue to recognize that all tools can have errors. With computerized guides of any sort, these errors are usually user errors that cannot be detected by the device. Surgeons need to be able to recognize this and always double-check bone cuts for accuracy. Templating the bone cuts prior to surgery is an effective double-check. In addition, many handheld accelerometer devices do not currently assist with the rotational alignment of the femoral component. This is still performed using the surgeon’s preferred technique, and is a limitation of these systems.
Discussion
Currently, TKA provides satisfactory 10-year results with modern implant designs and survival rates as high as 90% to 95%.27,28 Even with good survival rates, a percentage of patients fail within the first 5 years.3 At a single institution, 50% of revision TKAs were related to instability, malalignment, or failure of fixation that occurred less than 2 years after the index procedure.29 In general, TKA with mechanical instrumentation provides satisfactory pain relief and postoperative knee function; however, studies have consistently shown that the use of advanced technology decreases the risk of implant malalignment, which may decrease early implant failure rates as compared to mechanical and some PSI.13,14,22 While there is a paucity of literature that has shown better clinical outcomes with the use of advanced technology, there are studies supporting the notion that proper limb alignment and component positioning improves implant survivorship.23,30
CAS may have additional advantages if the surgeon chooses to place the TKA in an alignment other than a neutral mechanical axis. Kinematic alignment for TKA has gained increasing popularity, where the target of a neutral mechanical axis alignment is not always the goal.31,32 The reported benefit is a more natural ligament tension with the hope of improving patient satisfaction. One concern with this technique is that it is a departure from the long-held teaching that a TKA aligned to a neutral mechanical axis is necessary for long-term implant survivorship.33,34 In addition, if the goal of surgery is to cut the tibia and femur at a specific varus/valgus cut, standard instrumentation may not allow for this level of accuracy. This in turn increases the risk of having a tibial or femoral cut that is outside the commonly accepted standards of alignment, which may lead to early implant failure. If further research suggests alignment is a variable that differs from patient to patient, the use of precise tools to ensure accuracy of executing the preoperatively templated alignment becomes even more important.
As the number of TKAs continues to rise each year, even a small percentage of malaligned knees that go on to revision surgery will create a large burden on the healthcare system.1,3 Although the short-term clinical benefits of CAS have not shown substantial differences as compared to conventional TKA, the number of knees aligned outside of a desired neutral mechanical axis alignment has been shown in multiple studies to be decreased with the use of advanced technology.7,12,34 Although CAS is an additional cost to a primary TKA, if the orthopedic community can decrease the number of TKA revisions due to malalignment from 6.6% to nearly zero, this may decrease the revision burden and overall cost to the healthcare system.1,3
TKA technology continues to evolve, and we must continue to assess each new advance not only to understand how it works, but also to ensure it addresses a specific clinical problem, and to be aware of the costs associated before incorporating it into routine practice. Some argue that the use of advanced technology requires increased surgical time, which in turn ultimately increases costs; however, one study has documented no increase in surgical time with handheld navigation while maintaining the accuracy of the device.34 In addition, no significant radiographic or clinical differences have been found between handheld navigation and larger console CAS systems, but large console systems have been associated with increased surgical times.22 The use of handheld accelerometer- and gyroscope-based guides has proven to provide reliable coronal and sagittal implant alignment that can easily be incorporated into the operating room. More widespread use of such technology will help decrease alignment outliers for TKA, and future long-term clinical outcome studies will be necessary to assess functional outcomes.
Conclusion
Advanced computer based technology offers an additional tool to the surgeon for reliably improving component positioning during TKA. The use of handheld accelerometer- and gyroscope-based guides increases the accuracy of component placement while decreasing the incidence of outliers compared to standard mechanical guides, without the need for a large computer console. Long-term radiographic and patient-reported outcomes are necessary to further validate these devices.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
1. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
2. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KD. Patient satisfaction after total knee arthroplasty: who is satisfied and who is not? Clin Orthop Relat Res. 2010;468(1):57-63.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28( 8 Suppl):116-119.
4. Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473(1):151-158.
5. Anderson KC, Buehler KC, Markel DC. Computer assisted navigation in total knee arthroplasty: comparison with conventional methods. J Arthroplasty. 2005;20(7 Suppl 3):132-138.
6. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
7. Khakha RS, Chowdhry M, Sivaprakasam M, Kheiran A, Chauhan SK. Radiological and functional outcomes in computer assisted total knee arthroplasty between consultants and trainees - a prospective randomized controlled trial. J Arthroplasty. 2015;30(8):1344-1347.
8. Zhu M, Ang CL, Yeo SJ, Lo NN, Chia SL, Chong HC. Minimally invasive computer-assisted total knee arthroplasty compared with conventional total knee arthroplasty: a prospective 9-year follow-up. J Arthroplasty. 2015. [Epub ahead of print]
9. Roberts TD, Clatworthy MG, Frampton CM, Young SW. Does computer assisted navigation improve functional outcomes and implant survivability after total knee arthroplasty? J Arthroplasty. 2015;30(9 Suppl):59-63.
10. de Steiger RN, Liu YL, Graves SE. Computer navigation for total knee arthroplasty reduces revision rate for patients less than sixty-five years of age. J Bone Joint Surg Am. 2015;97(8):635-642.
11. Fehring TK, Mason JB, Moskal J, Pollock DC, Mann J, Williams VJ. When computer-assisted knee replacement is the best alternative. Clin Orthop Relat Res. 2006;452:132-136.
12. Iorio R, Mazza D, Drogo P, et al. Clinical and radiographic outcomes of an accelerometer-based system for the tibial resection in total knee arthroplasty. Int Orthop. 2015;39(3):461-466.
13. Haaker RG, Stockheim M, Kamp M, Proff G, Breitenfelder J, Ottersbach A. Computer-assisted navigation increases precision of component placement in total knee arthroplasty. Clin Orthop Relat Res. 2005;433:152-159.
14. Ollivier M, Tribot-Laspiere Q, Amzallag J, Boisrenoult P, Pujol N, Beaufils P. Abnormal rate of intraoperative and postoperative implant positioning outliers using “MRI-based patient-specific” compared to “computer assisted” instrumentation in total knee replacement. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
15. Nunley RM, Ellison BS, Zhu J, Ruh EL, Howell SM, Barrack RL. Do patient-specific guides improve coronal alignment in total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):895-902.
16. Nunley RM, Ellison BS, Ruh EL, et al. Are patient-specific cutting blocks cost-effective for total knee arthroplasty? Clin Orthop Relat Res. 2012;470(3):889-894.
17. Barrack RL, Ruh EL, Williams BM, Ford AD, Foreman K, Nunley RM. Patient specific cutting blocks are currently of no proven value. J Bone Joint Surg Br. 2012;94(11 Suppl A):95-99.
18. Chen JY, Chin PL, Tay DK, Chia SL, Lo NN, Yeo SJ. Functional outcome and quality of life after patient-specific instrumentation in total knee arthroplasty. J Arthroplasty. 2015;30(10):1724-1728.
19. Goyal N, Patel AR, Yaffe MA, Luo MY, Stulberg SD. Does implant design influence the accuracy of patient specific instrumentation in total knee arthroplasty? J Arthroplasty. 2015;30(9):1526-1530.
20. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
21. Scuderi GR, Fallaha M, Masse V, Lavigne P, Amiot LP, Berthiaume MJ. Total knee arthroplasty with a novel navigation system within the surgical field. Orthop Clin North Am. 2014;45(2):167-173.
22. Goh GS, Liow MH, Lim WS, Tay DK, Yeo SJ, Tan MH. Accelerometer-based navigation is as accurate as optical computer navigation in restoring the joint line and mechanical axis after total knee arthroplasty: a prospective matched study. J Arthroplasty. 2016;31(1):92-97.
23. Berend KR, Lombardi AV Jr. Liberal indications for minimally invasive oxford unicondylar arthroplasty provide rapid functional recovery and pain relief. Surg Technol Int. 2007;16:193-197.
24. Nam D, Jerabek SA, Cross MB, Mayman DJ. Cadaveric analysis of an accelerometer-based portable navigation device for distal femoral cutting block alignment in total knee arthroplasty. Comput Aided Surg. 2012;17(4):205-210.
25. Nam D, Cody EA, Nguyen JT, Figgie MP, Mayman DJ. Extramedullary guides versus portable, accelerometer-based navigation for tibial alignment in total knee arthroplasty: a randomized, controlled trial: winner of the 2013 HAP PAUL award. J Arthroplasty. 2014;29(2):288-294.
26. Bugbee WD, Kermanshahi AY, Munro MM, McCauley JC, Copp SN. Accuracy of a hand-held surgical navigation system for tibial resection in total knee arthroplasty. Knee. 2014;21(6):1225-1228.
27. Schai PA, Thornhill TS, Scott RD. Total knee arthroplasty with the PFC system. Results at a minimum of ten years and survivorship analysis. J Bone Joint Surg Br. 1998;80(5):850-858.
28. Pradhan NR, Gambhir A, Porter ML. Survivorship analysis of 3234 primary knee arthroplasties implanted over a 26-year period: a study of eight different implant designs. Knee. 2006;13(1):7-11.
29. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
30. Fang DM, Ritter MA, Davis KE. Coronal alignment in total knee arthroplasty: just how important is it? J Arthroplasty. 2009;24(6 Suppl):39-43.
31. Cherian JJ, Kapadia BH, Banerjee S, Jauregui JJ, Issa K, Mont MA. Mechanical, anatomical, and kinematic axis in TKA: concepts and practical applications. Curr Rev Musculoskelet Med. 2014;7(2):89-95.
32. Howell SM, Papadopoulos S, Kuznik K, Ghaly LR, Hull ML. Does varus alignment adversely affect implant survival and function six years after kinematically aligned total knee arthroplasty? Int Orthop. 2015;39(11):2117-2124.
33. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
34. Huang EH, Copp SN, Bugbee WD. Accuracy of a handheld accelerometer-based navigation system for femoral and tibial resection in total knee arthroplasty. J Arthroplasty. 2015;30(11):1906-1910.
Robotic-Assisted Knee Arthroplasty: An Overview
Unicompartmental knee arthroplasty (UKA) and total knee arthroplasty (TKA) are 2 reliable treatment options for patients with primary osteoarthritis. Recently published systematic reviews of cohort studies have shown that 10-year survivorship of medial and lateral UKA is 92% and 91%, respectively,1 while 10-year survivorship of TKA in cohort studies is 95%.2 National and annual registries show a similar trend, although the reported survivorship is lower.1,3-7
In order to improve these survivorship rates, the surgical variables that can intraoperatively be controlled by the orthopedic surgeon have been evaluated. These variables include lower leg alignment, soft tissue balance, joint line maintenance, and alignment, size, and fixation of the tibial and femoral component. Several studies have shown that tight control of lower leg alignment,8-14 balancing of the soft tissues,15-19 joint line maintenance,20-23 component alignment,24-28 component size,29-34 and component fixation35-40 can improve the outcomes of UKA and TKA. As a result, over the past 2 decades, several computer-assisted surgery systems have been developed with the goal of more accurate and reliable control of these factors, and thus improved outcomes of knee arthroplasty.
These systems differ with regard to the number and type of variables they control. Computer navigation systems aim to control one or more of these surgical variables, and several meta-analyses have shown that these systems, when compared to conventional surgery, improve mechanical axis accuracy, decrease the risk for mechanical axis outliers, and improve component positioning in TKA41-49 and UKA surgery.50,51 Interestingly, however, meta-analyses have failed to show the expected superiority in clinical outcomes following computer navigation compared to conventional knee arthroplasty.48,52-55 Furthermore, authors have shown that, despite the fact that computer-navigated surgery increases the accuracy of mechanical alignment and surgical cutting, there is still room for improvement.56 As a consequence, robotic-assisted systems have been developed.
Similar to computer navigation, these robotic-assisted systems aim to control the surgical variables; in addition, they aim to improve the surgical precision of the procedure. Interestingly, 2 recent studies have shown that robotic-assisted systems are superior to computer navigation systems with regard to less cutting time and less resection deviations in coronal and sagittal plane in a cadaveric study,57 and shorter total surgery time, more accurate mechanical axis, and shorter hospital stay in a clinical study.58 Although these results are promising, the exact role of robotic surgery in knee arthroplasty remains unclear. In this review, we aim to report the current state of robotic-assisted knee arthroplasty by discussing (1) the different robotic-assisted knee arthroplasty systems that are available for UKA and TKA surgery, (2) studies that assessed the role of robotic-assisted knee arthroplasty in controlling the aforementioned surgical variables, (3) cadaveric and clinical comparative studies that compared how accurate robotic-assisted and conventional knee surgery control these surgical variables, and (4) studies that assessed the cost-effectiveness of robotic-assisted knee arthroplasty surgery.
Robotic-Assisted Knee Arthroplasty Systems
Several systems have been developed over the years for knee arthroplasty, and these are usually defined as active, semi-active, or passive.59 Active systems are capable of performing tasks or processes autonomously under the watchful eye of the surgeon, while passive systems do not perform actions independently but provide the surgeon with information. In semi-active systems, the surgical action is physically constrained in order to follow a predefined strategy.
In the United States, 3 robotic systems are FDA-approved for knee arthroplasty. The Stryker/Mako haptic guided robot (Mako Surgical Corp.) was introduced in 2005 and has been used for over 50,000 UKA procedures (Figure 1). There are nearly 300 robotic systems used nationally, as it has 20% of the market share for UKA in the United States. The Mako system is a semi-active tactile robotic system that requires preoperative imaging, after which a preoperative planning is performed. Intraoperatively, the robotic arm is under direct surgeon control and gives real-time tactile feedback during the procedure (Figure 2).
Furthermore, the surgeon can intraoperatively virtually adjust component positioning and alignment and move the knee through the range of motion, after which the system can provide information on alignment, component position, and balance of the soft tissue (eg, if the knee is overtight or lax through the flexion-arch).60 This system has a burr that resects the bone and when the surgeon directs the burr outside the preplanned area, the burr stops and prevents unnecessary and unwanted resections (Figure 3).
The Navio Precision Free-Hand Sculptor (PFS) system (Blue Belt Technologies) has been used for 1500 UKA procedures, with 50 robots in use in the United States (Figure 4). This system is an image-free semi-active robotic system and has the same characteristics as the aforementioned Mako system.61 Finally, the OmniBotic robotic system (Omnilife Science) has been released for TKA and has been used for over 7300 procedures (Figure 5). This system has an automated cutting-guide technique in which the surgeon designs a virtual plan on the computer system. After this, the cutting-guides are placed by the robotic system at the planned location for all 5 femoral cuts (ie, distal, anterior chamfer, anterior, posterior chamfer, and posterior) and the surgeon then makes the final cuts.57,62
Three robotic systems for knee arthroplasty surgery have been used in Europe. The Caspar system (URS Ortho) is an active robotic system in which a computed tomography (CT) scan is performed preoperatively, after which a virtual implantation is performed on the screen. The surgeon can then obtain information on lower leg alignment, gap balancing, and component positioning, and after an operative plan is made, the surgical resections are performed by the robot. Reflective markers are attached to the leg and all robotic movements are monitored using an infrared camera system. Any undesired motion will be detected by this camera system and will stop all movements.63 A second and more frequently reported system in the literature is the active Robodoc surgical system (Curexo Technology Corporation). This system is designed for TKA and total hip arthroplasty (THA) surgery. Although initial studies reported a high incidence of system-related complications in THA,64 the use of this system for TKA has frequently been reported in the literature.56,63,65-69 A third robotic system that has been used in Europe is the Acrobot surgical system (Acrobot Company Ltd), which is an image-based semi-active robotic system70 used for both UKA and TKA surgery.70,71
Accuracy of Controlling Surgical Variables in Robotic-Assisted Knee Arthroplasty
Several studies have assessed the accuracy of robotic-assisted surgery in UKA surgery with regard to control of the aforementioned surgical variables. Pearle and colleagues72 assessed the mechanical axis accuracy of the Mako system in 10 patients undergoing medial UKA robotic-assisted surgery. They reported that the intraoperative registration lasted 7.5 minutes and the duration of time needed for robotic-assisted burring was 34.8 minutes. They compared the actual postoperative alignment at 6 weeks follow-up with the planned lower leg alignment and found that all measurements were within 1.6° of the planned lower leg alignment. Dunbar and colleagues73 assessed the accuracy of component positioning of the Mako system in 20 patients undergoing medial UKA surgery by comparing preoperative and postoperative 3-dimensional CT scans. They found that the femoral component was within 0.8 mm and 0.9° in all directions and that the tibial component was within 0.9 mm and 1.7° in all directions. They concluded that the accuracy of component positioning with the Mako system was excellent. Finally, Plate and colleagues17 assessed the accuracy of soft tissue balancing in the Mako system in 52 patients undergoing medial UKA surgery. They compared the balance plan with the soft tissue balance after implantation and the Mako system quantified soft tissue balance as the amount of mm of the knee being tight or loose at 0°, 30°, 60°, 90°, and 110° of flexion. They found that at all flexion angles the ligament balancing was accurate up to 0.53 mm of the original plan. Furthermore, they noted that in 83% of cases the accuracy was within 1 mm at all flexion angles.
For the Navio system, Smith and colleagues74 assessed the accuracy of component positioning using 20 synthetic femurs and tibia. They reported a maximum rotational error of 3.2°, an angular error of 1.46° in all orientations, and a maximum translational error of 1.18 mm for both the tibial and femoral implants. Lonner and colleagues75 assessed the accuracy of component positioning in 25 cadaveric specimens. They found similar results as were found in the study of Smith and colleagues74 and concluded that these results were similar to other semi-active robotic systems designed for UKA.
For TKA surgery, Ponder and colleagues76 assessed the accuracy of the OmniBotic system and found that the average error in the anterior-posterior dimension between the targeted and measured cuts was -0.14 mm, and that the standard deviation in guide positioning for the distal, anterior chamfer, and posterior chamfer resections was 0.03° and 0.17 mm. Koenig and colleagues62 assessed the accuracy of the OmniBotic system in the first 100 cases and found that 98% of the cases were within 3° of the neutral mechanical axis. Furthermore, they found a learning curve with regard to tourniquet time between the first and second 10 patients in which they performed robotic-assisted TKA surgery. Siebert and colleagues63 assessed the accuracy of mechanical alignment in the Caspar system in 70 patients treated with the robotic system. They found that the difference between preoperatively planned and postoperatively achieved mechanical alignment was 0.8°. Similarly, Bellemans and colleagues77 assessed mechanical alignment and the positioning and rotation of the tibial and femoral components in a clinical study of 25 cases using the Caspar system. They noted that none of the patients had mechanical alignment, tibial or femoral component positioning, or rotation beyond 1° of the neutral axis. Liow and colleagues56 assessed the accuracy of mechanical axis alignment and component sizing accuracy using the Robodoc system in 25 patients. They reported that the mean postoperative alignment was 0.4° valgus and that all cases were within 3° of the neutral mechanical axis. Furthermore, they reported a mean surgical time of 96 minutes and reported that preoperative planning yielded femoral and tibial component size accuracy of 100%.
These studies have shown that robotic systems for UKA and TKA are accurate in the surgical variables they aim to control. These studies validated tight control of mechanical axis alignment, decrease for outliers, and component positioning and rotation, and also found that the balancing of soft tissues was improved using robotic-assisted surgery.
Robotic-Assisted vs Conventional Knee Arthroplasty
Despite the fact that these systems are accurate in the variables they aim to control, these systems have to be compared to the gold standard of conventional knee arthroplasty. For UKA, Cobb and colleagues70 performed a randomized clinical trial for patients treated undergoing UKA with robotic-assistance of the Acrobot systems compared to conventional UKA and assessed differences in mechanical accuracy. A total of 27 patients were randomly assigned to one of both treatments. They found that in the group of robotic-assisted surgery, 100% of the patients had a mechanical axis within 2° of neutral, while this was only 40% in the conventional UKA groups (difference P < .001). They also assessed the increase in functional outcomes and noted a trend towards improvement in performance with increasing accuracy at 6 weeks and 3 months postoperatively. Lonner and colleagues78 also compared the tibial component positioning between robotic-assisted UKA surgery using the Mako system and conventional UKA surgery. The authors found that the variance in tibial slope, in coronal plane of the tibial component, and varus/valgus alignment were all larger with conventional UKA when compared to robotic-assisted UKA. Citak and colleagues79 compared the accuracy of tibial and femoral implant positioning between robotic-assisted surgery using the Mako system and conventional UKA in a cadaveric study. They reported that the root mean square (RMS) error of femoral component was 1.9 mm and 3.7° in robotic-assisted surgery and 5.4 mm and 10.2° for conventional UKA, while the RMS error for tibial component were 1.4 mm and 5.0° for robotic-assisted surgery and 5.7 mm and 19.2° for conventional UKA surgery. MacCallum and colleagues80 compared the tibial base plate position in a prospective clinical study of 177 patients treated with conventional UKA and 87 patients treated with robotic-assisted surgery using the Mako system. They found that surgery with robotic-assistance was more precise in the coronal and sagittal plane and was more accurate in coronal alignment when compared to conventional UKA. Finally, the first results of robotic-assisted UKA surgery have been presented. Coon and colleagues81 reported the preliminary results of a multicenter study of 854 patients and found a survivorship of 98.9% and satisfaction rate of 92% at minimum 2-year follow-up. Comparing these results to other large conventional UKA cohorts82,83 suggests that robotic-assisted surgery may improve survivorship at short-term follow-up. However, comparative studies and studies with longer follow-up are necessary to assess the additional value of robotic-assisted UKA surgery. Due to the relatively new concept of robotic-assisted surgery, these studies have not been performed or published yet.
For TKA, several studies also have compared how these robotic-systems control the surgical variables compared to conventional TKA surgery. Siebert and colleagues63 assessed mechanical axis accuracy and mechanical outliers following robotic-assisted TKA surgery using the Caspar system and conventional TKA surgery. They reported the difference between preoperative planned and postoperative achieved alignment was 0.8° for robotic-assisted surgery and 2.6° for conventional TKA surgery. Furthermore, they showed that 1 patient in the robotic-assisted group (1.4%) and 18 patients in the conventional TKA group (35%) had mechanical alignment greater than 3° from the neutral mechanical axis. Liow and colleagues56 found similar differences in their prospective randomized study in which they reported that 0% outliers greater than 3° from the neutral mechanical axis were found in the robotic-assisted group while 19.4% of the patients in the conventional TKA group had mechanical axis outliers. They also assessed the joint-line outliers in both procedures and found that 3.2% had joint-line outliers greater than 5 mm in the robotic-assisted group compared to 20.6% in the conventional TKA group. Kim and colleagues65 assessed implant accuracy in robotic-assisted surgery using the ROBODOC system and in conventional surgery and reported higher implant accuracy and fewer outliers using robotic-assisted surgery. Moon and colleagues66 compared robotic-assisted TKA surgery using the Robodoc system with conventional TKA surgery in 10 cadavers. They found that robotic-assisted surgery had excellent precision in all planes and had better accuracy in femoral rotation alignment compared to conventional TKA surgery. Park and Lee67 compared Robodoc robotic-assisted TKA surgery with conventional TKA surgery in a randomized clinical trial of 72 patients. They found that robotic-assisted surgery had definitive advantages in preoperative planning, accuracy of the procedure, and postoperative follow-up regarding femoral and tibial component flexion angles. Finally, Song and colleagues68,69 performed 2 randomized clinical trials in which they compared mechanical axis alignment, component positioning, soft tissue balancing, and patient preference between conventional TKA surgery and robotic-assisted surgery using the Robodoc system. In the first study,68 they simultaneously performed robotic-assisted surgery in one leg and conventional TKA surgery in the other leg. They found that robotic-assisted surgery resulted in less outlier in mechanical axis and component positioning. Furthermore, they found at latest follow-up of 2 years that 12 patients preferred the leg treated with robotic-assisted surgery while 6 preferred the conventional leg. Despite this finding, no significant differences in functional outcome scores were detected between both treatment options. Furthermore, they found that flexion-extension balance was achieved in 92% of patients treated with robotic-assisted TKA surgery and in 77% of patients treated with conventional TKA surgery. In the other study,69 the authors found that more patients treated with robotic-assisted surgery had <2 mm flexion-extension gap and more satisfactory posterior cruciate ligament tension when compared to conventional surgery.
These studies have shown that robotic-assisted surgery is accurate in controlling surgical variables, such as mechanical lower leg alignment, maintaining joint-line, implant positioning, and soft tissue balancing. Furthermore, these studies have shown that controlling these variables is better than the current gold standard of manual knee arthroplasty. Until now, not many studies have assessed survivorship of robotic-assisted surgery. Furthermore, no studies have, to our knowledge, compared survivorship of robotic-assisted with conventional knee replacement surgery. Finally, studies comparing functional outcomes following robotic-assisted surgery and conventional knee arthroplasty surgery are frequently underpowered due to their small sample sizes.68,70 Since many studies have shown that the surgical variables are more tightly controlled using robotic-assisted surgery when compared to conventional surgery, large comparative studies are necessary to assess the role of robotic-assisted surgery in functional outcomes and survivorship of UKA and TKA.
Cost-Effectiveness of Robotic-Assisted Surgery
High initial capital costs of robotic-assisted surgery is one of the factors that constitute a barrier to the widespread implementation of this technique. Multiple authors have suggested that improved implant survivorship afforded by robotic-assisted surgery may justify the expenditure from both societal and provider perspective.84-86 Two studies have performed a cost-effectiveness analysis for UKA surgery. Swank and colleagues84 reviewed the hospital expenditures and profits associated with robot-assisted knee arthroplasty, citing upfront costs of approximately $800,000. The authors estimated a mean per-case contribution profit of $5790 for robotic-assisted UKA, assuming an inpatient-to-outpatient ratio of 1 to 3. Based on this data, Swank and colleagues84 proposed that the capital costs of robotic-assisted UKA may be recovered in as little as 2 years when in the first 3 consecutive years 50, 70, and 90 cases were performed using robotic-assisted UKA. Moschetti and colleagues85 recently published the first formal cost-effectiveness analysis of robotic-assisted compared to manual UKA. The authors used an annual revision risk of 0.55% for the first 2 years following robot-assisted UKA, based on the aforementioned presented data by Coon and colleagues.81 They based their data on the Mako system and assumed an initial capital expenditure of $934,728 with annual servicing costs of 10% (discounted annually) for 4 years thereafter, resulting in a total cost of the robotic system of $1.362 million. These costs were divided by the number of patients estimated to undergo robotic-assisted UKA per year, which was varied to estimate the effect of case volume on cost-effectiveness. The authors reported that robotic-assisted UKA was associated with higher lifetime costs and net utilities compared to manual UKA, at an incremental cost-effectiveness ratio of $47,180 per quality-adjusted life year (QALY) in a high-volume center. This falls well within the societal willingness-to-pay threshold of $100,000/QALY. Sensitivity analysis showed that robotic-assisted UKA is cost-effective under the following conditions: (1) centers performing at least 94 cases annually, (2) in patients younger than age 67 years, and (3) 2-year revision rate does not exceed 1.2%. While the results of this initial analysis are promising, follow-up cost-effectiveness analysis studies will be required as long-term survivorship data become available.
Conclusion
Tighter control of intraoperative surgical variables, such as lower leg alignment, soft tissue balance, joint-line maintenance, and component alignment and positioning, have been associated with improved survivorship and functional outcomes. Upon reviewing the available literature on robotic-assisted surgery, it becomes clear that this technique can improve the accuracy of these surgical variables and is superior to conventional manual UKA and TKA. Although larger and comparative survivorship studies are necessary to compare robotic-assisted knee arthroplasty to conventional techniques, the early results and cost-effectiveness analysis seem promising.
1. van der List JP, McDonald LS, Pearle AD. Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee. 2015;22(6):454-460.
2. Mont MA, Pivec R, Issa K, Kapadia BH, Maheshwari A, Harwin SF. Long-term implant survivorship of cementless total knee arthroplasty: a systematic review of the literature and meta-analysis. J Knee Surg. 2014;27(5):369-376.
3. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2014 Australian Hip and Knee Arthroplasty Register. https://aoanjrr.sahmri.com/documents/10180/172286/Annual%20Report%202014. Accessed April 6, 2016.
4. The Swedish Knee Arthroplasty Register. Annual Report 2015 Swedish Knee Arthroplasty Register. http://www.myknee.se/pdf/SVK_2015_Eng_1.0.pdf. Published December 1, 2015. Accessed April 6, 2016.
5. Centre of excellence of joint replacements. The Norwegian Arthroplasty Register. http://nrlweb.ihelse.net/eng/Report_2010.pdf. Published June 2010. Accessed June 3, 2015.
6. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 12th Annual Report 2015. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/12th%20annual%20report/NJR%20Online%20Annual%20Report%202015.pdf. Accessed April 6, 2016.
7. The New Zealand Joint Registry. Fourteen Year Report January 1999 to December 2012. http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed April 6, 2016.
8. Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73(5):709-714.
9. Rand JA, Coventry MB. Ten-year evaluation of geometric total knee arthroplasty. Clin Orthop Relat Res. 1988;232:168-173.
10. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
11. Ryd L, Lindstrand A, Stenström A, Selvik G. Porous coated anatomic tricompartmental tibial components. The relationship between prosthetic position and micromotion. Clin Orthop Relat Res. 1990;251:189-197.
12. van der List JP, Chawla H, Villa JC, Zuiderbaan HA, Pearle AD. Early functional outcome after lateral UKA is sensitive to postoperative lower limb alignment. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
13. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty. 2015. [Epub ahead of print]
14. Vasso M, Del Regno C, D’Amelio A, Viggiano D, Corona K, Schiavone Panni A. Minor varus alignment provides better results than neutral alignment in medial UKA. Knee. 2015;22(2):117-121.
15. Attfield SF, Wilton TJ, Pratt DJ, Sambatakakis A. Soft-tissue balance and recovery of proprioception after total knee replacement. J Bone Joint Surg Br. 1996;78(4):540-545.
16. Pagnano MW, Hanssen AD, Lewallen DG, Stuart MJ. Flexion instability after primary posterior cruciate retaining total knee arthroplasty. Clin Orthop Relat Res. 1998;356:39-46.
17. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
18. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
19. Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31-43.
20. Ji HM, Han J, Jin DS, Seo H, Won YY. Kinematically aligned TKA can align knee joint line to horizontal. Knee Surg Sports Traumatol Arthrosc. 2016. [Epub ahead of print]
21. Khamaisy S, Zuiderbaan HA, van der List JP, Nam D, Pearle AD. Medial unicompartmental knee arthroplasty improves congruence and restores joint space width of the lateral compartment. Knee. 2016. [Epub ahead of print]
22. Niinimaki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
23. Zuiderbaan HA, Khamaisy S, Thein R, Nawabi DH, Pearle AD. Congruence and joint space width alterations of the medial compartment following lateral unicompartmental knee arthroplasty. Bone Joint J. 2015;97-B(1):50-55.
24. Barbadoro P, Ensini A, Leardini A, et al. Tibial component alignment and risk of loosening in unicompartmental knee arthroplasty: a radiographic and radiostereometric study. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3157-3162.
25. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
26. Nedopil AJ, Howell SM, Hull ML. Does malrotation of the tibial and femoral components compromise function in kinematically aligned total knee arthroplasty? Orthop Clin North Am. 2016;47(1):41-50.
27. Rosskopf J, Singh PK, Wolf P, Strauch M, Graichen H. Influence of intentional femoral component flexion in navigated TKA on gap balance and sagittal anatomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(3):687-693.
28. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech (Bristol, Avon). 2005;20(7):661-668.
29. Bonnin MP, Saffarini M, Shepherd D, Bossard N, Dantony E. Oversizing the tibial component in TKAs: incidence, consequences and risk factors. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
30. Bonnin MP, Schmidt A, Basiglini L, Bossard N, Dantony E. Mediolateral oversizing influences pain, function, and flexion after TKA. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2314-2324.
31. Chau R, Gulati A, Pandit H, et al. Tibial component overhang following unicompartmental knee replacement--does it matter? Knee. 2009;16(5):310-313.
32. Mueller JK, Wentorf FA, Moore RE. Femoral and tibial insert downsizing increases the laxity envelope in TKA. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3003-3011.
33. Sriphirom P, Raungthong N, Chutchawan P, Thiranon C, Sukandhavesa N. Influence of a secondary downsizing of the femoral component on the extension gap: a cadaveric study. Orthopedics. 2012;35(10 Suppl):56-59.
34. Young SW, Clarke HD, Graves SE, Liu YL, de Steiger RN. Higher rate of revision in PFC sigma primary total knee arthroplasty with mismatch of femoro-tibial component sizes. J Arthroplasty. 2015;30(5):813-817.
35. Barink M, Verdonschot N, de Waal Malefijt M. A different fixation of the femoral component in total knee arthroplasty may lead to preservation of femoral bone stock. Proc Inst Mech Eng H. 2003;217(5):325-332.
36. Eagar P, Hull ML, Howell SM. How the fixation method stiffness and initial tension affect anterior load-displacement of the knee and tension in anterior cruciate ligament grafts: a study in cadaveric knees using a double-loop hamstrings graft. J Orthop Res. 2004;22(3):613-624.
37. Fricka KB, Sritulanondha S, McAsey CJ. To cement or not? Two-year results of a prospective, randomized study comparing cemented vs. cementless total knee arthroplasty (TKA). J Arthroplasty. 2015;30(9 Suppl):55-58.
38. Kendrick BJ, Kaptein BL, Valstar ER, et al. Cemented versus cementless Oxford unicompartmental knee arthroplasty using radiostereometric analysis: a randomised controlled trial. Bone Joint J. 2015;97-B(2):185-191.
39. Kim TK, Chang CB, Kang YG, Chung BJ, Cho HJ, Seong SC. Execution accuracy of bone resection and implant fixation in computer assisted minimally invasive total knee arthroplasty. Knee. 2010;17(1):23-28.
40. Whiteside LA. Making your next unicompartmental knee arthroplasty last: three keys to success. J Arthroplasty. 2005;20(4 Suppl 2):2-3.
41. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
42. Brin YS, Nikolaou VS, Joseph L, Zukor DJ, Antoniou J. Imageless computer assisted versus conventional total knee replacement. A Bayesian meta-analysis of 23 comparative studies. Int Orthop. 2011;35(3):331-339.
43. Cheng T, Zhang G, Zhang X. Imageless navigation system does not improve component rotational alignment in total knee arthroplasty. J Surg Res. 2011;171(2):590-600.
44. Conteduca F, Iorio R, Mazza D, Ferretti A. Patient-specific instruments in total knee arthroplasty. Int Orthop. 2014;38(2):259-265.
45. Fu Y, Wang M, Liu Y, Fu Q. Alignment outcomes in navigated total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1075-1082.
46. Hetaimish BM, Khan MM, Simunovic N, Al-Harbi HH, Bhandari M, Zalzal PK. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27(6):1177-1182.
47. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
48. Moskal JT, Capps SG, Mann JW, Scanelli JA. Navigated versus conventional total knee arthroplasty. J Knee Surg. 2014;27(3):235-248.
49. Shi J, Wei Y, Wang S, et al. Computer navigation and total knee arthroplasty. Orthopedics. 2014;37(1):e39-e43.
50. Nair R, Tripathy G, Deysine GR. Computer navigation systems in unicompartmental knee arthroplasty: a systematic review. Am J Orthop. 2014;43(6):256-261.
51. Weber P, Crispin A, Schmidutz F, et al. Improved accuracy in computer-assisted unicondylar knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2453-2461.
52. Alcelik IA, Blomfield MI, Diana G, Gibbon AJ, Carrington N, Burr S. A comparison of short-term outcomes of minimally invasive computer-assisted vs minimally invasive conventional instrumentation for primary total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2016;31(2):410-418.
53. Cheng T, Pan XY, Mao X, Zhang GY, Zhang XL. Little clinical advantage of computer-assisted navigation over conventional instrumentation in primary total knee arthroplasty at early follow-up. Knee. 2012;19(4):237-245.
54. Rebal BA, Babatunde OM, Lee JH, Geller JA, Patrick DA Jr, Macaulay W. Imageless computer navigation in total knee arthroplasty provides superior short term functional outcomes: a meta-analysis. J Arthroplasty. 2014;29(5):938-944.
55. Zamora LA, Humphreys KJ, Watt AM, Forel D, Cameron AL. Systematic review of computer-navigated total knee arthroplasty. ANZ J Surg. 2013;83(1-2):22-30.
56. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplasty. 2014;29(12):2373-2377.
57. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
58. Clark TC, Schmidt FH. Robot-assisted navigation versus computer-assisted navigation in primary total knee arthroplasty: efficiency and accuracy. ISRN Orthop. 2013;2013:794827.
59. DiGioia AM 3rd, Jaramaz B, Colgan BD. Computer assisted orthopaedic surgery. Image guided and robotic assistive technologies. Clin Orthop Relat Res. 1998(354):8-16.
60. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
61. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
62. Koenig JA, Suero EM, Plaskos C. Surgical accuracy and efficiency of computer-navigated TKA with a robotic cutting guide–report on the first 100 cases. J Bone Joint Surg Br. 2012;94-B(SUPP XLIV):103. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/94-B/SUPP_XLIV/103. Accessed April 6, 2016.
63. Siebert W, Mai S, Kober R, Heeckt PF. Technique and first clinical results of robot-assisted total knee replacement. Knee. 2002;9(3):173-180.
64. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
65. Kim SM, Park YS, Ha CW, Lim SJ, Moon YW. Robot-assisted implantation improves the precision of component position in minimally invasive TKA. Orthopedics. 2012;35(9):e1334-e1339.
66. Moon YW, Ha CW, Do KH, et al. Comparison of robot-assisted and conventional total knee arthroplasty: a controlled cadaver study using multiparameter quantitative three-dimensional CT assessment of alignment. Comput Aided Surg. 2012;17(2):86-95.
67. Park SE, Lee CT. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty. 2007;22(7):1054-1059.
68. Song EK, Seon JK, Park SJ, Jung WB, Park HW, Lee GW. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1069-1076.
69. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
70. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
71. Jakopec M, Harris SJ, Rodriguez y Baena F, Gomes P, Cobb J, Davies BL. The first clinical application of a “hands-on” robotic knee surgery system. Comput Aided Surg. 2001;6(6):329-339.
72. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
73. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
74. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
75. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
76. Ponder C, Plaskos C, Cheal E. Press-fit total knee arthroplasty with a robotic-cutting guide: proof of concept and initial clinical experience. Bone & Joint Journal Orthopaedic Proceedings Supplement. 2013;95(SUPP 28):61. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/95-B/SUPP_28/61.abstract. Accessed April 6, 2016.
77. Bellemans J, Vandenneucker H, Vanlauwe J. Robot-assisted total knee arthroplasty. Clin Orthop Relat Res. 2007;464:111-116.
78. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
79. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
80. MacCallum KP, Danoff JR, Geller JA. Tibial baseplate positioning in robotic-assisted and conventional unicompartmental knee arthroplasty. Eur J Orthop Surg Traumatol. 2016;26(1):93-98.
81. Coon T, Roche M, Pearle AD, Dounchis J, Borus T, Buechel F Jr. Two year survivorship of robotically guided unicompartmental knee arthroplasty. Paper presented at: International Society for Technology in Arthroplasty 26th Annual Congress; October 16-19, 2013; Palm Beach, FL.
82. Pandit H, Jenkins C, Gill HS, Barker K, Dodd CA, Murray DW. Minimally invasive Oxford phase 3 unicompartmental knee replacement: results of 1000 cases. J Bone Joint Surg Br. 2011;93(2):198-204.
83. Yoshida K, Tada M, Yoshida H, Takei S, Fukuoka S, Nakamura H. Oxford phase 3 unicompartmental knee arthroplasty in Japan--clinical results in greater than one thousand cases over ten years. J Arthroplasty. 2013;28(9 Suppl):168-171.
84. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
85. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A markovdecision analysis. J Arthroplasty. 2015. [Epub ahead of print]
86. Thienpont E. Improving Accuracy in Knee Arthroplasty. 1st ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2012.
Unicompartmental knee arthroplasty (UKA) and total knee arthroplasty (TKA) are 2 reliable treatment options for patients with primary osteoarthritis. Recently published systematic reviews of cohort studies have shown that 10-year survivorship of medial and lateral UKA is 92% and 91%, respectively,1 while 10-year survivorship of TKA in cohort studies is 95%.2 National and annual registries show a similar trend, although the reported survivorship is lower.1,3-7
In order to improve these survivorship rates, the surgical variables that can intraoperatively be controlled by the orthopedic surgeon have been evaluated. These variables include lower leg alignment, soft tissue balance, joint line maintenance, and alignment, size, and fixation of the tibial and femoral component. Several studies have shown that tight control of lower leg alignment,8-14 balancing of the soft tissues,15-19 joint line maintenance,20-23 component alignment,24-28 component size,29-34 and component fixation35-40 can improve the outcomes of UKA and TKA. As a result, over the past 2 decades, several computer-assisted surgery systems have been developed with the goal of more accurate and reliable control of these factors, and thus improved outcomes of knee arthroplasty.
These systems differ with regard to the number and type of variables they control. Computer navigation systems aim to control one or more of these surgical variables, and several meta-analyses have shown that these systems, when compared to conventional surgery, improve mechanical axis accuracy, decrease the risk for mechanical axis outliers, and improve component positioning in TKA41-49 and UKA surgery.50,51 Interestingly, however, meta-analyses have failed to show the expected superiority in clinical outcomes following computer navigation compared to conventional knee arthroplasty.48,52-55 Furthermore, authors have shown that, despite the fact that computer-navigated surgery increases the accuracy of mechanical alignment and surgical cutting, there is still room for improvement.56 As a consequence, robotic-assisted systems have been developed.
Similar to computer navigation, these robotic-assisted systems aim to control the surgical variables; in addition, they aim to improve the surgical precision of the procedure. Interestingly, 2 recent studies have shown that robotic-assisted systems are superior to computer navigation systems with regard to less cutting time and less resection deviations in coronal and sagittal plane in a cadaveric study,57 and shorter total surgery time, more accurate mechanical axis, and shorter hospital stay in a clinical study.58 Although these results are promising, the exact role of robotic surgery in knee arthroplasty remains unclear. In this review, we aim to report the current state of robotic-assisted knee arthroplasty by discussing (1) the different robotic-assisted knee arthroplasty systems that are available for UKA and TKA surgery, (2) studies that assessed the role of robotic-assisted knee arthroplasty in controlling the aforementioned surgical variables, (3) cadaveric and clinical comparative studies that compared how accurate robotic-assisted and conventional knee surgery control these surgical variables, and (4) studies that assessed the cost-effectiveness of robotic-assisted knee arthroplasty surgery.
Robotic-Assisted Knee Arthroplasty Systems
Several systems have been developed over the years for knee arthroplasty, and these are usually defined as active, semi-active, or passive.59 Active systems are capable of performing tasks or processes autonomously under the watchful eye of the surgeon, while passive systems do not perform actions independently but provide the surgeon with information. In semi-active systems, the surgical action is physically constrained in order to follow a predefined strategy.
In the United States, 3 robotic systems are FDA-approved for knee arthroplasty. The Stryker/Mako haptic guided robot (Mako Surgical Corp.) was introduced in 2005 and has been used for over 50,000 UKA procedures (Figure 1). There are nearly 300 robotic systems used nationally, as it has 20% of the market share for UKA in the United States. The Mako system is a semi-active tactile robotic system that requires preoperative imaging, after which a preoperative planning is performed. Intraoperatively, the robotic arm is under direct surgeon control and gives real-time tactile feedback during the procedure (Figure 2).
Furthermore, the surgeon can intraoperatively virtually adjust component positioning and alignment and move the knee through the range of motion, after which the system can provide information on alignment, component position, and balance of the soft tissue (eg, if the knee is overtight or lax through the flexion-arch).60 This system has a burr that resects the bone and when the surgeon directs the burr outside the preplanned area, the burr stops and prevents unnecessary and unwanted resections (Figure 3).
The Navio Precision Free-Hand Sculptor (PFS) system (Blue Belt Technologies) has been used for 1500 UKA procedures, with 50 robots in use in the United States (Figure 4). This system is an image-free semi-active robotic system and has the same characteristics as the aforementioned Mako system.61 Finally, the OmniBotic robotic system (Omnilife Science) has been released for TKA and has been used for over 7300 procedures (Figure 5). This system has an automated cutting-guide technique in which the surgeon designs a virtual plan on the computer system. After this, the cutting-guides are placed by the robotic system at the planned location for all 5 femoral cuts (ie, distal, anterior chamfer, anterior, posterior chamfer, and posterior) and the surgeon then makes the final cuts.57,62
Three robotic systems for knee arthroplasty surgery have been used in Europe. The Caspar system (URS Ortho) is an active robotic system in which a computed tomography (CT) scan is performed preoperatively, after which a virtual implantation is performed on the screen. The surgeon can then obtain information on lower leg alignment, gap balancing, and component positioning, and after an operative plan is made, the surgical resections are performed by the robot. Reflective markers are attached to the leg and all robotic movements are monitored using an infrared camera system. Any undesired motion will be detected by this camera system and will stop all movements.63 A second and more frequently reported system in the literature is the active Robodoc surgical system (Curexo Technology Corporation). This system is designed for TKA and total hip arthroplasty (THA) surgery. Although initial studies reported a high incidence of system-related complications in THA,64 the use of this system for TKA has frequently been reported in the literature.56,63,65-69 A third robotic system that has been used in Europe is the Acrobot surgical system (Acrobot Company Ltd), which is an image-based semi-active robotic system70 used for both UKA and TKA surgery.70,71
Accuracy of Controlling Surgical Variables in Robotic-Assisted Knee Arthroplasty
Several studies have assessed the accuracy of robotic-assisted surgery in UKA surgery with regard to control of the aforementioned surgical variables. Pearle and colleagues72 assessed the mechanical axis accuracy of the Mako system in 10 patients undergoing medial UKA robotic-assisted surgery. They reported that the intraoperative registration lasted 7.5 minutes and the duration of time needed for robotic-assisted burring was 34.8 minutes. They compared the actual postoperative alignment at 6 weeks follow-up with the planned lower leg alignment and found that all measurements were within 1.6° of the planned lower leg alignment. Dunbar and colleagues73 assessed the accuracy of component positioning of the Mako system in 20 patients undergoing medial UKA surgery by comparing preoperative and postoperative 3-dimensional CT scans. They found that the femoral component was within 0.8 mm and 0.9° in all directions and that the tibial component was within 0.9 mm and 1.7° in all directions. They concluded that the accuracy of component positioning with the Mako system was excellent. Finally, Plate and colleagues17 assessed the accuracy of soft tissue balancing in the Mako system in 52 patients undergoing medial UKA surgery. They compared the balance plan with the soft tissue balance after implantation and the Mako system quantified soft tissue balance as the amount of mm of the knee being tight or loose at 0°, 30°, 60°, 90°, and 110° of flexion. They found that at all flexion angles the ligament balancing was accurate up to 0.53 mm of the original plan. Furthermore, they noted that in 83% of cases the accuracy was within 1 mm at all flexion angles.
For the Navio system, Smith and colleagues74 assessed the accuracy of component positioning using 20 synthetic femurs and tibia. They reported a maximum rotational error of 3.2°, an angular error of 1.46° in all orientations, and a maximum translational error of 1.18 mm for both the tibial and femoral implants. Lonner and colleagues75 assessed the accuracy of component positioning in 25 cadaveric specimens. They found similar results as were found in the study of Smith and colleagues74 and concluded that these results were similar to other semi-active robotic systems designed for UKA.
For TKA surgery, Ponder and colleagues76 assessed the accuracy of the OmniBotic system and found that the average error in the anterior-posterior dimension between the targeted and measured cuts was -0.14 mm, and that the standard deviation in guide positioning for the distal, anterior chamfer, and posterior chamfer resections was 0.03° and 0.17 mm. Koenig and colleagues62 assessed the accuracy of the OmniBotic system in the first 100 cases and found that 98% of the cases were within 3° of the neutral mechanical axis. Furthermore, they found a learning curve with regard to tourniquet time between the first and second 10 patients in which they performed robotic-assisted TKA surgery. Siebert and colleagues63 assessed the accuracy of mechanical alignment in the Caspar system in 70 patients treated with the robotic system. They found that the difference between preoperatively planned and postoperatively achieved mechanical alignment was 0.8°. Similarly, Bellemans and colleagues77 assessed mechanical alignment and the positioning and rotation of the tibial and femoral components in a clinical study of 25 cases using the Caspar system. They noted that none of the patients had mechanical alignment, tibial or femoral component positioning, or rotation beyond 1° of the neutral axis. Liow and colleagues56 assessed the accuracy of mechanical axis alignment and component sizing accuracy using the Robodoc system in 25 patients. They reported that the mean postoperative alignment was 0.4° valgus and that all cases were within 3° of the neutral mechanical axis. Furthermore, they reported a mean surgical time of 96 minutes and reported that preoperative planning yielded femoral and tibial component size accuracy of 100%.
These studies have shown that robotic systems for UKA and TKA are accurate in the surgical variables they aim to control. These studies validated tight control of mechanical axis alignment, decrease for outliers, and component positioning and rotation, and also found that the balancing of soft tissues was improved using robotic-assisted surgery.
Robotic-Assisted vs Conventional Knee Arthroplasty
Despite the fact that these systems are accurate in the variables they aim to control, these systems have to be compared to the gold standard of conventional knee arthroplasty. For UKA, Cobb and colleagues70 performed a randomized clinical trial for patients treated undergoing UKA with robotic-assistance of the Acrobot systems compared to conventional UKA and assessed differences in mechanical accuracy. A total of 27 patients were randomly assigned to one of both treatments. They found that in the group of robotic-assisted surgery, 100% of the patients had a mechanical axis within 2° of neutral, while this was only 40% in the conventional UKA groups (difference P < .001). They also assessed the increase in functional outcomes and noted a trend towards improvement in performance with increasing accuracy at 6 weeks and 3 months postoperatively. Lonner and colleagues78 also compared the tibial component positioning between robotic-assisted UKA surgery using the Mako system and conventional UKA surgery. The authors found that the variance in tibial slope, in coronal plane of the tibial component, and varus/valgus alignment were all larger with conventional UKA when compared to robotic-assisted UKA. Citak and colleagues79 compared the accuracy of tibial and femoral implant positioning between robotic-assisted surgery using the Mako system and conventional UKA in a cadaveric study. They reported that the root mean square (RMS) error of femoral component was 1.9 mm and 3.7° in robotic-assisted surgery and 5.4 mm and 10.2° for conventional UKA, while the RMS error for tibial component were 1.4 mm and 5.0° for robotic-assisted surgery and 5.7 mm and 19.2° for conventional UKA surgery. MacCallum and colleagues80 compared the tibial base plate position in a prospective clinical study of 177 patients treated with conventional UKA and 87 patients treated with robotic-assisted surgery using the Mako system. They found that surgery with robotic-assistance was more precise in the coronal and sagittal plane and was more accurate in coronal alignment when compared to conventional UKA. Finally, the first results of robotic-assisted UKA surgery have been presented. Coon and colleagues81 reported the preliminary results of a multicenter study of 854 patients and found a survivorship of 98.9% and satisfaction rate of 92% at minimum 2-year follow-up. Comparing these results to other large conventional UKA cohorts82,83 suggests that robotic-assisted surgery may improve survivorship at short-term follow-up. However, comparative studies and studies with longer follow-up are necessary to assess the additional value of robotic-assisted UKA surgery. Due to the relatively new concept of robotic-assisted surgery, these studies have not been performed or published yet.
For TKA, several studies also have compared how these robotic-systems control the surgical variables compared to conventional TKA surgery. Siebert and colleagues63 assessed mechanical axis accuracy and mechanical outliers following robotic-assisted TKA surgery using the Caspar system and conventional TKA surgery. They reported the difference between preoperative planned and postoperative achieved alignment was 0.8° for robotic-assisted surgery and 2.6° for conventional TKA surgery. Furthermore, they showed that 1 patient in the robotic-assisted group (1.4%) and 18 patients in the conventional TKA group (35%) had mechanical alignment greater than 3° from the neutral mechanical axis. Liow and colleagues56 found similar differences in their prospective randomized study in which they reported that 0% outliers greater than 3° from the neutral mechanical axis were found in the robotic-assisted group while 19.4% of the patients in the conventional TKA group had mechanical axis outliers. They also assessed the joint-line outliers in both procedures and found that 3.2% had joint-line outliers greater than 5 mm in the robotic-assisted group compared to 20.6% in the conventional TKA group. Kim and colleagues65 assessed implant accuracy in robotic-assisted surgery using the ROBODOC system and in conventional surgery and reported higher implant accuracy and fewer outliers using robotic-assisted surgery. Moon and colleagues66 compared robotic-assisted TKA surgery using the Robodoc system with conventional TKA surgery in 10 cadavers. They found that robotic-assisted surgery had excellent precision in all planes and had better accuracy in femoral rotation alignment compared to conventional TKA surgery. Park and Lee67 compared Robodoc robotic-assisted TKA surgery with conventional TKA surgery in a randomized clinical trial of 72 patients. They found that robotic-assisted surgery had definitive advantages in preoperative planning, accuracy of the procedure, and postoperative follow-up regarding femoral and tibial component flexion angles. Finally, Song and colleagues68,69 performed 2 randomized clinical trials in which they compared mechanical axis alignment, component positioning, soft tissue balancing, and patient preference between conventional TKA surgery and robotic-assisted surgery using the Robodoc system. In the first study,68 they simultaneously performed robotic-assisted surgery in one leg and conventional TKA surgery in the other leg. They found that robotic-assisted surgery resulted in less outlier in mechanical axis and component positioning. Furthermore, they found at latest follow-up of 2 years that 12 patients preferred the leg treated with robotic-assisted surgery while 6 preferred the conventional leg. Despite this finding, no significant differences in functional outcome scores were detected between both treatment options. Furthermore, they found that flexion-extension balance was achieved in 92% of patients treated with robotic-assisted TKA surgery and in 77% of patients treated with conventional TKA surgery. In the other study,69 the authors found that more patients treated with robotic-assisted surgery had <2 mm flexion-extension gap and more satisfactory posterior cruciate ligament tension when compared to conventional surgery.
These studies have shown that robotic-assisted surgery is accurate in controlling surgical variables, such as mechanical lower leg alignment, maintaining joint-line, implant positioning, and soft tissue balancing. Furthermore, these studies have shown that controlling these variables is better than the current gold standard of manual knee arthroplasty. Until now, not many studies have assessed survivorship of robotic-assisted surgery. Furthermore, no studies have, to our knowledge, compared survivorship of robotic-assisted with conventional knee replacement surgery. Finally, studies comparing functional outcomes following robotic-assisted surgery and conventional knee arthroplasty surgery are frequently underpowered due to their small sample sizes.68,70 Since many studies have shown that the surgical variables are more tightly controlled using robotic-assisted surgery when compared to conventional surgery, large comparative studies are necessary to assess the role of robotic-assisted surgery in functional outcomes and survivorship of UKA and TKA.
Cost-Effectiveness of Robotic-Assisted Surgery
High initial capital costs of robotic-assisted surgery is one of the factors that constitute a barrier to the widespread implementation of this technique. Multiple authors have suggested that improved implant survivorship afforded by robotic-assisted surgery may justify the expenditure from both societal and provider perspective.84-86 Two studies have performed a cost-effectiveness analysis for UKA surgery. Swank and colleagues84 reviewed the hospital expenditures and profits associated with robot-assisted knee arthroplasty, citing upfront costs of approximately $800,000. The authors estimated a mean per-case contribution profit of $5790 for robotic-assisted UKA, assuming an inpatient-to-outpatient ratio of 1 to 3. Based on this data, Swank and colleagues84 proposed that the capital costs of robotic-assisted UKA may be recovered in as little as 2 years when in the first 3 consecutive years 50, 70, and 90 cases were performed using robotic-assisted UKA. Moschetti and colleagues85 recently published the first formal cost-effectiveness analysis of robotic-assisted compared to manual UKA. The authors used an annual revision risk of 0.55% for the first 2 years following robot-assisted UKA, based on the aforementioned presented data by Coon and colleagues.81 They based their data on the Mako system and assumed an initial capital expenditure of $934,728 with annual servicing costs of 10% (discounted annually) for 4 years thereafter, resulting in a total cost of the robotic system of $1.362 million. These costs were divided by the number of patients estimated to undergo robotic-assisted UKA per year, which was varied to estimate the effect of case volume on cost-effectiveness. The authors reported that robotic-assisted UKA was associated with higher lifetime costs and net utilities compared to manual UKA, at an incremental cost-effectiveness ratio of $47,180 per quality-adjusted life year (QALY) in a high-volume center. This falls well within the societal willingness-to-pay threshold of $100,000/QALY. Sensitivity analysis showed that robotic-assisted UKA is cost-effective under the following conditions: (1) centers performing at least 94 cases annually, (2) in patients younger than age 67 years, and (3) 2-year revision rate does not exceed 1.2%. While the results of this initial analysis are promising, follow-up cost-effectiveness analysis studies will be required as long-term survivorship data become available.
Conclusion
Tighter control of intraoperative surgical variables, such as lower leg alignment, soft tissue balance, joint-line maintenance, and component alignment and positioning, have been associated with improved survivorship and functional outcomes. Upon reviewing the available literature on robotic-assisted surgery, it becomes clear that this technique can improve the accuracy of these surgical variables and is superior to conventional manual UKA and TKA. Although larger and comparative survivorship studies are necessary to compare robotic-assisted knee arthroplasty to conventional techniques, the early results and cost-effectiveness analysis seem promising.
Unicompartmental knee arthroplasty (UKA) and total knee arthroplasty (TKA) are 2 reliable treatment options for patients with primary osteoarthritis. Recently published systematic reviews of cohort studies have shown that 10-year survivorship of medial and lateral UKA is 92% and 91%, respectively,1 while 10-year survivorship of TKA in cohort studies is 95%.2 National and annual registries show a similar trend, although the reported survivorship is lower.1,3-7
In order to improve these survivorship rates, the surgical variables that can intraoperatively be controlled by the orthopedic surgeon have been evaluated. These variables include lower leg alignment, soft tissue balance, joint line maintenance, and alignment, size, and fixation of the tibial and femoral component. Several studies have shown that tight control of lower leg alignment,8-14 balancing of the soft tissues,15-19 joint line maintenance,20-23 component alignment,24-28 component size,29-34 and component fixation35-40 can improve the outcomes of UKA and TKA. As a result, over the past 2 decades, several computer-assisted surgery systems have been developed with the goal of more accurate and reliable control of these factors, and thus improved outcomes of knee arthroplasty.
These systems differ with regard to the number and type of variables they control. Computer navigation systems aim to control one or more of these surgical variables, and several meta-analyses have shown that these systems, when compared to conventional surgery, improve mechanical axis accuracy, decrease the risk for mechanical axis outliers, and improve component positioning in TKA41-49 and UKA surgery.50,51 Interestingly, however, meta-analyses have failed to show the expected superiority in clinical outcomes following computer navigation compared to conventional knee arthroplasty.48,52-55 Furthermore, authors have shown that, despite the fact that computer-navigated surgery increases the accuracy of mechanical alignment and surgical cutting, there is still room for improvement.56 As a consequence, robotic-assisted systems have been developed.
Similar to computer navigation, these robotic-assisted systems aim to control the surgical variables; in addition, they aim to improve the surgical precision of the procedure. Interestingly, 2 recent studies have shown that robotic-assisted systems are superior to computer navigation systems with regard to less cutting time and less resection deviations in coronal and sagittal plane in a cadaveric study,57 and shorter total surgery time, more accurate mechanical axis, and shorter hospital stay in a clinical study.58 Although these results are promising, the exact role of robotic surgery in knee arthroplasty remains unclear. In this review, we aim to report the current state of robotic-assisted knee arthroplasty by discussing (1) the different robotic-assisted knee arthroplasty systems that are available for UKA and TKA surgery, (2) studies that assessed the role of robotic-assisted knee arthroplasty in controlling the aforementioned surgical variables, (3) cadaveric and clinical comparative studies that compared how accurate robotic-assisted and conventional knee surgery control these surgical variables, and (4) studies that assessed the cost-effectiveness of robotic-assisted knee arthroplasty surgery.
Robotic-Assisted Knee Arthroplasty Systems
Several systems have been developed over the years for knee arthroplasty, and these are usually defined as active, semi-active, or passive.59 Active systems are capable of performing tasks or processes autonomously under the watchful eye of the surgeon, while passive systems do not perform actions independently but provide the surgeon with information. In semi-active systems, the surgical action is physically constrained in order to follow a predefined strategy.
In the United States, 3 robotic systems are FDA-approved for knee arthroplasty. The Stryker/Mako haptic guided robot (Mako Surgical Corp.) was introduced in 2005 and has been used for over 50,000 UKA procedures (Figure 1). There are nearly 300 robotic systems used nationally, as it has 20% of the market share for UKA in the United States. The Mako system is a semi-active tactile robotic system that requires preoperative imaging, after which a preoperative planning is performed. Intraoperatively, the robotic arm is under direct surgeon control and gives real-time tactile feedback during the procedure (Figure 2).
Furthermore, the surgeon can intraoperatively virtually adjust component positioning and alignment and move the knee through the range of motion, after which the system can provide information on alignment, component position, and balance of the soft tissue (eg, if the knee is overtight or lax through the flexion-arch).60 This system has a burr that resects the bone and when the surgeon directs the burr outside the preplanned area, the burr stops and prevents unnecessary and unwanted resections (Figure 3).
The Navio Precision Free-Hand Sculptor (PFS) system (Blue Belt Technologies) has been used for 1500 UKA procedures, with 50 robots in use in the United States (Figure 4). This system is an image-free semi-active robotic system and has the same characteristics as the aforementioned Mako system.61 Finally, the OmniBotic robotic system (Omnilife Science) has been released for TKA and has been used for over 7300 procedures (Figure 5). This system has an automated cutting-guide technique in which the surgeon designs a virtual plan on the computer system. After this, the cutting-guides are placed by the robotic system at the planned location for all 5 femoral cuts (ie, distal, anterior chamfer, anterior, posterior chamfer, and posterior) and the surgeon then makes the final cuts.57,62
Three robotic systems for knee arthroplasty surgery have been used in Europe. The Caspar system (URS Ortho) is an active robotic system in which a computed tomography (CT) scan is performed preoperatively, after which a virtual implantation is performed on the screen. The surgeon can then obtain information on lower leg alignment, gap balancing, and component positioning, and after an operative plan is made, the surgical resections are performed by the robot. Reflective markers are attached to the leg and all robotic movements are monitored using an infrared camera system. Any undesired motion will be detected by this camera system and will stop all movements.63 A second and more frequently reported system in the literature is the active Robodoc surgical system (Curexo Technology Corporation). This system is designed for TKA and total hip arthroplasty (THA) surgery. Although initial studies reported a high incidence of system-related complications in THA,64 the use of this system for TKA has frequently been reported in the literature.56,63,65-69 A third robotic system that has been used in Europe is the Acrobot surgical system (Acrobot Company Ltd), which is an image-based semi-active robotic system70 used for both UKA and TKA surgery.70,71
Accuracy of Controlling Surgical Variables in Robotic-Assisted Knee Arthroplasty
Several studies have assessed the accuracy of robotic-assisted surgery in UKA surgery with regard to control of the aforementioned surgical variables. Pearle and colleagues72 assessed the mechanical axis accuracy of the Mako system in 10 patients undergoing medial UKA robotic-assisted surgery. They reported that the intraoperative registration lasted 7.5 minutes and the duration of time needed for robotic-assisted burring was 34.8 minutes. They compared the actual postoperative alignment at 6 weeks follow-up with the planned lower leg alignment and found that all measurements were within 1.6° of the planned lower leg alignment. Dunbar and colleagues73 assessed the accuracy of component positioning of the Mako system in 20 patients undergoing medial UKA surgery by comparing preoperative and postoperative 3-dimensional CT scans. They found that the femoral component was within 0.8 mm and 0.9° in all directions and that the tibial component was within 0.9 mm and 1.7° in all directions. They concluded that the accuracy of component positioning with the Mako system was excellent. Finally, Plate and colleagues17 assessed the accuracy of soft tissue balancing in the Mako system in 52 patients undergoing medial UKA surgery. They compared the balance plan with the soft tissue balance after implantation and the Mako system quantified soft tissue balance as the amount of mm of the knee being tight or loose at 0°, 30°, 60°, 90°, and 110° of flexion. They found that at all flexion angles the ligament balancing was accurate up to 0.53 mm of the original plan. Furthermore, they noted that in 83% of cases the accuracy was within 1 mm at all flexion angles.
For the Navio system, Smith and colleagues74 assessed the accuracy of component positioning using 20 synthetic femurs and tibia. They reported a maximum rotational error of 3.2°, an angular error of 1.46° in all orientations, and a maximum translational error of 1.18 mm for both the tibial and femoral implants. Lonner and colleagues75 assessed the accuracy of component positioning in 25 cadaveric specimens. They found similar results as were found in the study of Smith and colleagues74 and concluded that these results were similar to other semi-active robotic systems designed for UKA.
For TKA surgery, Ponder and colleagues76 assessed the accuracy of the OmniBotic system and found that the average error in the anterior-posterior dimension between the targeted and measured cuts was -0.14 mm, and that the standard deviation in guide positioning for the distal, anterior chamfer, and posterior chamfer resections was 0.03° and 0.17 mm. Koenig and colleagues62 assessed the accuracy of the OmniBotic system in the first 100 cases and found that 98% of the cases were within 3° of the neutral mechanical axis. Furthermore, they found a learning curve with regard to tourniquet time between the first and second 10 patients in which they performed robotic-assisted TKA surgery. Siebert and colleagues63 assessed the accuracy of mechanical alignment in the Caspar system in 70 patients treated with the robotic system. They found that the difference between preoperatively planned and postoperatively achieved mechanical alignment was 0.8°. Similarly, Bellemans and colleagues77 assessed mechanical alignment and the positioning and rotation of the tibial and femoral components in a clinical study of 25 cases using the Caspar system. They noted that none of the patients had mechanical alignment, tibial or femoral component positioning, or rotation beyond 1° of the neutral axis. Liow and colleagues56 assessed the accuracy of mechanical axis alignment and component sizing accuracy using the Robodoc system in 25 patients. They reported that the mean postoperative alignment was 0.4° valgus and that all cases were within 3° of the neutral mechanical axis. Furthermore, they reported a mean surgical time of 96 minutes and reported that preoperative planning yielded femoral and tibial component size accuracy of 100%.
These studies have shown that robotic systems for UKA and TKA are accurate in the surgical variables they aim to control. These studies validated tight control of mechanical axis alignment, decrease for outliers, and component positioning and rotation, and also found that the balancing of soft tissues was improved using robotic-assisted surgery.
Robotic-Assisted vs Conventional Knee Arthroplasty
Despite the fact that these systems are accurate in the variables they aim to control, these systems have to be compared to the gold standard of conventional knee arthroplasty. For UKA, Cobb and colleagues70 performed a randomized clinical trial for patients treated undergoing UKA with robotic-assistance of the Acrobot systems compared to conventional UKA and assessed differences in mechanical accuracy. A total of 27 patients were randomly assigned to one of both treatments. They found that in the group of robotic-assisted surgery, 100% of the patients had a mechanical axis within 2° of neutral, while this was only 40% in the conventional UKA groups (difference P < .001). They also assessed the increase in functional outcomes and noted a trend towards improvement in performance with increasing accuracy at 6 weeks and 3 months postoperatively. Lonner and colleagues78 also compared the tibial component positioning between robotic-assisted UKA surgery using the Mako system and conventional UKA surgery. The authors found that the variance in tibial slope, in coronal plane of the tibial component, and varus/valgus alignment were all larger with conventional UKA when compared to robotic-assisted UKA. Citak and colleagues79 compared the accuracy of tibial and femoral implant positioning between robotic-assisted surgery using the Mako system and conventional UKA in a cadaveric study. They reported that the root mean square (RMS) error of femoral component was 1.9 mm and 3.7° in robotic-assisted surgery and 5.4 mm and 10.2° for conventional UKA, while the RMS error for tibial component were 1.4 mm and 5.0° for robotic-assisted surgery and 5.7 mm and 19.2° for conventional UKA surgery. MacCallum and colleagues80 compared the tibial base plate position in a prospective clinical study of 177 patients treated with conventional UKA and 87 patients treated with robotic-assisted surgery using the Mako system. They found that surgery with robotic-assistance was more precise in the coronal and sagittal plane and was more accurate in coronal alignment when compared to conventional UKA. Finally, the first results of robotic-assisted UKA surgery have been presented. Coon and colleagues81 reported the preliminary results of a multicenter study of 854 patients and found a survivorship of 98.9% and satisfaction rate of 92% at minimum 2-year follow-up. Comparing these results to other large conventional UKA cohorts82,83 suggests that robotic-assisted surgery may improve survivorship at short-term follow-up. However, comparative studies and studies with longer follow-up are necessary to assess the additional value of robotic-assisted UKA surgery. Due to the relatively new concept of robotic-assisted surgery, these studies have not been performed or published yet.
For TKA, several studies also have compared how these robotic-systems control the surgical variables compared to conventional TKA surgery. Siebert and colleagues63 assessed mechanical axis accuracy and mechanical outliers following robotic-assisted TKA surgery using the Caspar system and conventional TKA surgery. They reported the difference between preoperative planned and postoperative achieved alignment was 0.8° for robotic-assisted surgery and 2.6° for conventional TKA surgery. Furthermore, they showed that 1 patient in the robotic-assisted group (1.4%) and 18 patients in the conventional TKA group (35%) had mechanical alignment greater than 3° from the neutral mechanical axis. Liow and colleagues56 found similar differences in their prospective randomized study in which they reported that 0% outliers greater than 3° from the neutral mechanical axis were found in the robotic-assisted group while 19.4% of the patients in the conventional TKA group had mechanical axis outliers. They also assessed the joint-line outliers in both procedures and found that 3.2% had joint-line outliers greater than 5 mm in the robotic-assisted group compared to 20.6% in the conventional TKA group. Kim and colleagues65 assessed implant accuracy in robotic-assisted surgery using the ROBODOC system and in conventional surgery and reported higher implant accuracy and fewer outliers using robotic-assisted surgery. Moon and colleagues66 compared robotic-assisted TKA surgery using the Robodoc system with conventional TKA surgery in 10 cadavers. They found that robotic-assisted surgery had excellent precision in all planes and had better accuracy in femoral rotation alignment compared to conventional TKA surgery. Park and Lee67 compared Robodoc robotic-assisted TKA surgery with conventional TKA surgery in a randomized clinical trial of 72 patients. They found that robotic-assisted surgery had definitive advantages in preoperative planning, accuracy of the procedure, and postoperative follow-up regarding femoral and tibial component flexion angles. Finally, Song and colleagues68,69 performed 2 randomized clinical trials in which they compared mechanical axis alignment, component positioning, soft tissue balancing, and patient preference between conventional TKA surgery and robotic-assisted surgery using the Robodoc system. In the first study,68 they simultaneously performed robotic-assisted surgery in one leg and conventional TKA surgery in the other leg. They found that robotic-assisted surgery resulted in less outlier in mechanical axis and component positioning. Furthermore, they found at latest follow-up of 2 years that 12 patients preferred the leg treated with robotic-assisted surgery while 6 preferred the conventional leg. Despite this finding, no significant differences in functional outcome scores were detected between both treatment options. Furthermore, they found that flexion-extension balance was achieved in 92% of patients treated with robotic-assisted TKA surgery and in 77% of patients treated with conventional TKA surgery. In the other study,69 the authors found that more patients treated with robotic-assisted surgery had <2 mm flexion-extension gap and more satisfactory posterior cruciate ligament tension when compared to conventional surgery.
These studies have shown that robotic-assisted surgery is accurate in controlling surgical variables, such as mechanical lower leg alignment, maintaining joint-line, implant positioning, and soft tissue balancing. Furthermore, these studies have shown that controlling these variables is better than the current gold standard of manual knee arthroplasty. Until now, not many studies have assessed survivorship of robotic-assisted surgery. Furthermore, no studies have, to our knowledge, compared survivorship of robotic-assisted with conventional knee replacement surgery. Finally, studies comparing functional outcomes following robotic-assisted surgery and conventional knee arthroplasty surgery are frequently underpowered due to their small sample sizes.68,70 Since many studies have shown that the surgical variables are more tightly controlled using robotic-assisted surgery when compared to conventional surgery, large comparative studies are necessary to assess the role of robotic-assisted surgery in functional outcomes and survivorship of UKA and TKA.
Cost-Effectiveness of Robotic-Assisted Surgery
High initial capital costs of robotic-assisted surgery is one of the factors that constitute a barrier to the widespread implementation of this technique. Multiple authors have suggested that improved implant survivorship afforded by robotic-assisted surgery may justify the expenditure from both societal and provider perspective.84-86 Two studies have performed a cost-effectiveness analysis for UKA surgery. Swank and colleagues84 reviewed the hospital expenditures and profits associated with robot-assisted knee arthroplasty, citing upfront costs of approximately $800,000. The authors estimated a mean per-case contribution profit of $5790 for robotic-assisted UKA, assuming an inpatient-to-outpatient ratio of 1 to 3. Based on this data, Swank and colleagues84 proposed that the capital costs of robotic-assisted UKA may be recovered in as little as 2 years when in the first 3 consecutive years 50, 70, and 90 cases were performed using robotic-assisted UKA. Moschetti and colleagues85 recently published the first formal cost-effectiveness analysis of robotic-assisted compared to manual UKA. The authors used an annual revision risk of 0.55% for the first 2 years following robot-assisted UKA, based on the aforementioned presented data by Coon and colleagues.81 They based their data on the Mako system and assumed an initial capital expenditure of $934,728 with annual servicing costs of 10% (discounted annually) for 4 years thereafter, resulting in a total cost of the robotic system of $1.362 million. These costs were divided by the number of patients estimated to undergo robotic-assisted UKA per year, which was varied to estimate the effect of case volume on cost-effectiveness. The authors reported that robotic-assisted UKA was associated with higher lifetime costs and net utilities compared to manual UKA, at an incremental cost-effectiveness ratio of $47,180 per quality-adjusted life year (QALY) in a high-volume center. This falls well within the societal willingness-to-pay threshold of $100,000/QALY. Sensitivity analysis showed that robotic-assisted UKA is cost-effective under the following conditions: (1) centers performing at least 94 cases annually, (2) in patients younger than age 67 years, and (3) 2-year revision rate does not exceed 1.2%. While the results of this initial analysis are promising, follow-up cost-effectiveness analysis studies will be required as long-term survivorship data become available.
Conclusion
Tighter control of intraoperative surgical variables, such as lower leg alignment, soft tissue balance, joint-line maintenance, and component alignment and positioning, have been associated with improved survivorship and functional outcomes. Upon reviewing the available literature on robotic-assisted surgery, it becomes clear that this technique can improve the accuracy of these surgical variables and is superior to conventional manual UKA and TKA. Although larger and comparative survivorship studies are necessary to compare robotic-assisted knee arthroplasty to conventional techniques, the early results and cost-effectiveness analysis seem promising.
1. van der List JP, McDonald LS, Pearle AD. Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee. 2015;22(6):454-460.
2. Mont MA, Pivec R, Issa K, Kapadia BH, Maheshwari A, Harwin SF. Long-term implant survivorship of cementless total knee arthroplasty: a systematic review of the literature and meta-analysis. J Knee Surg. 2014;27(5):369-376.
3. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2014 Australian Hip and Knee Arthroplasty Register. https://aoanjrr.sahmri.com/documents/10180/172286/Annual%20Report%202014. Accessed April 6, 2016.
4. The Swedish Knee Arthroplasty Register. Annual Report 2015 Swedish Knee Arthroplasty Register. http://www.myknee.se/pdf/SVK_2015_Eng_1.0.pdf. Published December 1, 2015. Accessed April 6, 2016.
5. Centre of excellence of joint replacements. The Norwegian Arthroplasty Register. http://nrlweb.ihelse.net/eng/Report_2010.pdf. Published June 2010. Accessed June 3, 2015.
6. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 12th Annual Report 2015. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/12th%20annual%20report/NJR%20Online%20Annual%20Report%202015.pdf. Accessed April 6, 2016.
7. The New Zealand Joint Registry. Fourteen Year Report January 1999 to December 2012. http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed April 6, 2016.
8. Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73(5):709-714.
9. Rand JA, Coventry MB. Ten-year evaluation of geometric total knee arthroplasty. Clin Orthop Relat Res. 1988;232:168-173.
10. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
11. Ryd L, Lindstrand A, Stenström A, Selvik G. Porous coated anatomic tricompartmental tibial components. The relationship between prosthetic position and micromotion. Clin Orthop Relat Res. 1990;251:189-197.
12. van der List JP, Chawla H, Villa JC, Zuiderbaan HA, Pearle AD. Early functional outcome after lateral UKA is sensitive to postoperative lower limb alignment. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
13. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty. 2015. [Epub ahead of print]
14. Vasso M, Del Regno C, D’Amelio A, Viggiano D, Corona K, Schiavone Panni A. Minor varus alignment provides better results than neutral alignment in medial UKA. Knee. 2015;22(2):117-121.
15. Attfield SF, Wilton TJ, Pratt DJ, Sambatakakis A. Soft-tissue balance and recovery of proprioception after total knee replacement. J Bone Joint Surg Br. 1996;78(4):540-545.
16. Pagnano MW, Hanssen AD, Lewallen DG, Stuart MJ. Flexion instability after primary posterior cruciate retaining total knee arthroplasty. Clin Orthop Relat Res. 1998;356:39-46.
17. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
18. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
19. Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31-43.
20. Ji HM, Han J, Jin DS, Seo H, Won YY. Kinematically aligned TKA can align knee joint line to horizontal. Knee Surg Sports Traumatol Arthrosc. 2016. [Epub ahead of print]
21. Khamaisy S, Zuiderbaan HA, van der List JP, Nam D, Pearle AD. Medial unicompartmental knee arthroplasty improves congruence and restores joint space width of the lateral compartment. Knee. 2016. [Epub ahead of print]
22. Niinimaki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
23. Zuiderbaan HA, Khamaisy S, Thein R, Nawabi DH, Pearle AD. Congruence and joint space width alterations of the medial compartment following lateral unicompartmental knee arthroplasty. Bone Joint J. 2015;97-B(1):50-55.
24. Barbadoro P, Ensini A, Leardini A, et al. Tibial component alignment and risk of loosening in unicompartmental knee arthroplasty: a radiographic and radiostereometric study. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3157-3162.
25. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
26. Nedopil AJ, Howell SM, Hull ML. Does malrotation of the tibial and femoral components compromise function in kinematically aligned total knee arthroplasty? Orthop Clin North Am. 2016;47(1):41-50.
27. Rosskopf J, Singh PK, Wolf P, Strauch M, Graichen H. Influence of intentional femoral component flexion in navigated TKA on gap balance and sagittal anatomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(3):687-693.
28. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech (Bristol, Avon). 2005;20(7):661-668.
29. Bonnin MP, Saffarini M, Shepherd D, Bossard N, Dantony E. Oversizing the tibial component in TKAs: incidence, consequences and risk factors. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
30. Bonnin MP, Schmidt A, Basiglini L, Bossard N, Dantony E. Mediolateral oversizing influences pain, function, and flexion after TKA. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2314-2324.
31. Chau R, Gulati A, Pandit H, et al. Tibial component overhang following unicompartmental knee replacement--does it matter? Knee. 2009;16(5):310-313.
32. Mueller JK, Wentorf FA, Moore RE. Femoral and tibial insert downsizing increases the laxity envelope in TKA. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3003-3011.
33. Sriphirom P, Raungthong N, Chutchawan P, Thiranon C, Sukandhavesa N. Influence of a secondary downsizing of the femoral component on the extension gap: a cadaveric study. Orthopedics. 2012;35(10 Suppl):56-59.
34. Young SW, Clarke HD, Graves SE, Liu YL, de Steiger RN. Higher rate of revision in PFC sigma primary total knee arthroplasty with mismatch of femoro-tibial component sizes. J Arthroplasty. 2015;30(5):813-817.
35. Barink M, Verdonschot N, de Waal Malefijt M. A different fixation of the femoral component in total knee arthroplasty may lead to preservation of femoral bone stock. Proc Inst Mech Eng H. 2003;217(5):325-332.
36. Eagar P, Hull ML, Howell SM. How the fixation method stiffness and initial tension affect anterior load-displacement of the knee and tension in anterior cruciate ligament grafts: a study in cadaveric knees using a double-loop hamstrings graft. J Orthop Res. 2004;22(3):613-624.
37. Fricka KB, Sritulanondha S, McAsey CJ. To cement or not? Two-year results of a prospective, randomized study comparing cemented vs. cementless total knee arthroplasty (TKA). J Arthroplasty. 2015;30(9 Suppl):55-58.
38. Kendrick BJ, Kaptein BL, Valstar ER, et al. Cemented versus cementless Oxford unicompartmental knee arthroplasty using radiostereometric analysis: a randomised controlled trial. Bone Joint J. 2015;97-B(2):185-191.
39. Kim TK, Chang CB, Kang YG, Chung BJ, Cho HJ, Seong SC. Execution accuracy of bone resection and implant fixation in computer assisted minimally invasive total knee arthroplasty. Knee. 2010;17(1):23-28.
40. Whiteside LA. Making your next unicompartmental knee arthroplasty last: three keys to success. J Arthroplasty. 2005;20(4 Suppl 2):2-3.
41. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
42. Brin YS, Nikolaou VS, Joseph L, Zukor DJ, Antoniou J. Imageless computer assisted versus conventional total knee replacement. A Bayesian meta-analysis of 23 comparative studies. Int Orthop. 2011;35(3):331-339.
43. Cheng T, Zhang G, Zhang X. Imageless navigation system does not improve component rotational alignment in total knee arthroplasty. J Surg Res. 2011;171(2):590-600.
44. Conteduca F, Iorio R, Mazza D, Ferretti A. Patient-specific instruments in total knee arthroplasty. Int Orthop. 2014;38(2):259-265.
45. Fu Y, Wang M, Liu Y, Fu Q. Alignment outcomes in navigated total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1075-1082.
46. Hetaimish BM, Khan MM, Simunovic N, Al-Harbi HH, Bhandari M, Zalzal PK. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27(6):1177-1182.
47. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
48. Moskal JT, Capps SG, Mann JW, Scanelli JA. Navigated versus conventional total knee arthroplasty. J Knee Surg. 2014;27(3):235-248.
49. Shi J, Wei Y, Wang S, et al. Computer navigation and total knee arthroplasty. Orthopedics. 2014;37(1):e39-e43.
50. Nair R, Tripathy G, Deysine GR. Computer navigation systems in unicompartmental knee arthroplasty: a systematic review. Am J Orthop. 2014;43(6):256-261.
51. Weber P, Crispin A, Schmidutz F, et al. Improved accuracy in computer-assisted unicondylar knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2453-2461.
52. Alcelik IA, Blomfield MI, Diana G, Gibbon AJ, Carrington N, Burr S. A comparison of short-term outcomes of minimally invasive computer-assisted vs minimally invasive conventional instrumentation for primary total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2016;31(2):410-418.
53. Cheng T, Pan XY, Mao X, Zhang GY, Zhang XL. Little clinical advantage of computer-assisted navigation over conventional instrumentation in primary total knee arthroplasty at early follow-up. Knee. 2012;19(4):237-245.
54. Rebal BA, Babatunde OM, Lee JH, Geller JA, Patrick DA Jr, Macaulay W. Imageless computer navigation in total knee arthroplasty provides superior short term functional outcomes: a meta-analysis. J Arthroplasty. 2014;29(5):938-944.
55. Zamora LA, Humphreys KJ, Watt AM, Forel D, Cameron AL. Systematic review of computer-navigated total knee arthroplasty. ANZ J Surg. 2013;83(1-2):22-30.
56. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplasty. 2014;29(12):2373-2377.
57. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
58. Clark TC, Schmidt FH. Robot-assisted navigation versus computer-assisted navigation in primary total knee arthroplasty: efficiency and accuracy. ISRN Orthop. 2013;2013:794827.
59. DiGioia AM 3rd, Jaramaz B, Colgan BD. Computer assisted orthopaedic surgery. Image guided and robotic assistive technologies. Clin Orthop Relat Res. 1998(354):8-16.
60. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
61. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
62. Koenig JA, Suero EM, Plaskos C. Surgical accuracy and efficiency of computer-navigated TKA with a robotic cutting guide–report on the first 100 cases. J Bone Joint Surg Br. 2012;94-B(SUPP XLIV):103. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/94-B/SUPP_XLIV/103. Accessed April 6, 2016.
63. Siebert W, Mai S, Kober R, Heeckt PF. Technique and first clinical results of robot-assisted total knee replacement. Knee. 2002;9(3):173-180.
64. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
65. Kim SM, Park YS, Ha CW, Lim SJ, Moon YW. Robot-assisted implantation improves the precision of component position in minimally invasive TKA. Orthopedics. 2012;35(9):e1334-e1339.
66. Moon YW, Ha CW, Do KH, et al. Comparison of robot-assisted and conventional total knee arthroplasty: a controlled cadaver study using multiparameter quantitative three-dimensional CT assessment of alignment. Comput Aided Surg. 2012;17(2):86-95.
67. Park SE, Lee CT. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty. 2007;22(7):1054-1059.
68. Song EK, Seon JK, Park SJ, Jung WB, Park HW, Lee GW. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1069-1076.
69. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
70. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
71. Jakopec M, Harris SJ, Rodriguez y Baena F, Gomes P, Cobb J, Davies BL. The first clinical application of a “hands-on” robotic knee surgery system. Comput Aided Surg. 2001;6(6):329-339.
72. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
73. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
74. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
75. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
76. Ponder C, Plaskos C, Cheal E. Press-fit total knee arthroplasty with a robotic-cutting guide: proof of concept and initial clinical experience. Bone & Joint Journal Orthopaedic Proceedings Supplement. 2013;95(SUPP 28):61. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/95-B/SUPP_28/61.abstract. Accessed April 6, 2016.
77. Bellemans J, Vandenneucker H, Vanlauwe J. Robot-assisted total knee arthroplasty. Clin Orthop Relat Res. 2007;464:111-116.
78. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
79. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
80. MacCallum KP, Danoff JR, Geller JA. Tibial baseplate positioning in robotic-assisted and conventional unicompartmental knee arthroplasty. Eur J Orthop Surg Traumatol. 2016;26(1):93-98.
81. Coon T, Roche M, Pearle AD, Dounchis J, Borus T, Buechel F Jr. Two year survivorship of robotically guided unicompartmental knee arthroplasty. Paper presented at: International Society for Technology in Arthroplasty 26th Annual Congress; October 16-19, 2013; Palm Beach, FL.
82. Pandit H, Jenkins C, Gill HS, Barker K, Dodd CA, Murray DW. Minimally invasive Oxford phase 3 unicompartmental knee replacement: results of 1000 cases. J Bone Joint Surg Br. 2011;93(2):198-204.
83. Yoshida K, Tada M, Yoshida H, Takei S, Fukuoka S, Nakamura H. Oxford phase 3 unicompartmental knee arthroplasty in Japan--clinical results in greater than one thousand cases over ten years. J Arthroplasty. 2013;28(9 Suppl):168-171.
84. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
85. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A markovdecision analysis. J Arthroplasty. 2015. [Epub ahead of print]
86. Thienpont E. Improving Accuracy in Knee Arthroplasty. 1st ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2012.
1. van der List JP, McDonald LS, Pearle AD. Systematic review of medial versus lateral survivorship in unicompartmental knee arthroplasty. Knee. 2015;22(6):454-460.
2. Mont MA, Pivec R, Issa K, Kapadia BH, Maheshwari A, Harwin SF. Long-term implant survivorship of cementless total knee arthroplasty: a systematic review of the literature and meta-analysis. J Knee Surg. 2014;27(5):369-376.
3. Australian Orthopaedic Association National Joint Replacement Registry. Annual Report 2014 Australian Hip and Knee Arthroplasty Register. https://aoanjrr.sahmri.com/documents/10180/172286/Annual%20Report%202014. Accessed April 6, 2016.
4. The Swedish Knee Arthroplasty Register. Annual Report 2015 Swedish Knee Arthroplasty Register. http://www.myknee.se/pdf/SVK_2015_Eng_1.0.pdf. Published December 1, 2015. Accessed April 6, 2016.
5. Centre of excellence of joint replacements. The Norwegian Arthroplasty Register. http://nrlweb.ihelse.net/eng/Report_2010.pdf. Published June 2010. Accessed June 3, 2015.
6. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. 12th Annual Report 2015. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Reports/12th%20annual%20report/NJR%20Online%20Annual%20Report%202015.pdf. Accessed April 6, 2016.
7. The New Zealand Joint Registry. Fourteen Year Report January 1999 to December 2012. http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed April 6, 2016.
8. Jeffery RS, Morris RW, Denham RA. Coronal alignment after total knee replacement. J Bone Joint Surg Br. 1991;73(5):709-714.
9. Rand JA, Coventry MB. Ten-year evaluation of geometric total knee arthroplasty. Clin Orthop Relat Res. 1988;232:168-173.
10. Ritter MA, Faris PM, Keating EM, Meding JB. Postoperative alignment of total knee replacement. Its effect on survival. Clin Orthop Relat Res. 1994;299:153-156.
11. Ryd L, Lindstrand A, Stenström A, Selvik G. Porous coated anatomic tricompartmental tibial components. The relationship between prosthetic position and micromotion. Clin Orthop Relat Res. 1990;251:189-197.
12. van der List JP, Chawla H, Villa JC, Zuiderbaan HA, Pearle AD. Early functional outcome after lateral UKA is sensitive to postoperative lower limb alignment. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
13. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty. 2015. [Epub ahead of print]
14. Vasso M, Del Regno C, D’Amelio A, Viggiano D, Corona K, Schiavone Panni A. Minor varus alignment provides better results than neutral alignment in medial UKA. Knee. 2015;22(2):117-121.
15. Attfield SF, Wilton TJ, Pratt DJ, Sambatakakis A. Soft-tissue balance and recovery of proprioception after total knee replacement. J Bone Joint Surg Br. 1996;78(4):540-545.
16. Pagnano MW, Hanssen AD, Lewallen DG, Stuart MJ. Flexion instability after primary posterior cruciate retaining total knee arthroplasty. Clin Orthop Relat Res. 1998;356:39-46.
17. Plate JF, Mofidi A, Mannava S, et al. Achieving accurate ligament balancing using robotic-assisted unicompartmental knee arthroplasty. Adv Orthop. 2013;2013:837167.
18. Roche M, Elson L, Anderson C. Dynamic soft tissue balancing in total knee arthroplasty. Orthop Clin North Am. 2014;45(2):157-165.
19. Wasielewski RC, Galante JO, Leighty RM, Natarajan RN, Rosenberg AG. Wear patterns on retrieved polyethylene tibial inserts and their relationship to technical considerations during total knee arthroplasty. Clin Orthop Relat Res. 1994;299:31-43.
20. Ji HM, Han J, Jin DS, Seo H, Won YY. Kinematically aligned TKA can align knee joint line to horizontal. Knee Surg Sports Traumatol Arthrosc. 2016. [Epub ahead of print]
21. Khamaisy S, Zuiderbaan HA, van der List JP, Nam D, Pearle AD. Medial unicompartmental knee arthroplasty improves congruence and restores joint space width of the lateral compartment. Knee. 2016. [Epub ahead of print]
22. Niinimaki TT, Murray DW, Partanen J, Pajala A, Leppilahti JI. Unicompartmental knee arthroplasties implanted for osteoarthritis with partial loss of joint space have high re-operation rates. Knee. 2011;18(6):432-435.
23. Zuiderbaan HA, Khamaisy S, Thein R, Nawabi DH, Pearle AD. Congruence and joint space width alterations of the medial compartment following lateral unicompartmental knee arthroplasty. Bone Joint J. 2015;97-B(1):50-55.
24. Barbadoro P, Ensini A, Leardini A, et al. Tibial component alignment and risk of loosening in unicompartmental knee arthroplasty: a radiographic and radiostereometric study. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3157-3162.
25. Collier MB, Eickmann TH, Sukezaki F, McAuley JP, Engh GA. Patient, implant, and alignment factors associated with revision of medial compartment unicondylar arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):108-115.
26. Nedopil AJ, Howell SM, Hull ML. Does malrotation of the tibial and femoral components compromise function in kinematically aligned total knee arthroplasty? Orthop Clin North Am. 2016;47(1):41-50.
27. Rosskopf J, Singh PK, Wolf P, Strauch M, Graichen H. Influence of intentional femoral component flexion in navigated TKA on gap balance and sagittal anatomy. Knee Surg Sports Traumatol Arthrosc. 2014;22(3):687-693.
28. Zihlmann MS, Stacoff A, Romero J, Quervain IK, Stüssi E. Biomechanical background and clinical observations of rotational malalignment in TKA: literature review and consequences. Clin Biomech (Bristol, Avon). 2005;20(7):661-668.
29. Bonnin MP, Saffarini M, Shepherd D, Bossard N, Dantony E. Oversizing the tibial component in TKAs: incidence, consequences and risk factors. Knee Surg Sports Traumatol Arthrosc. 2015. [Epub ahead of print]
30. Bonnin MP, Schmidt A, Basiglini L, Bossard N, Dantony E. Mediolateral oversizing influences pain, function, and flexion after TKA. Knee Surg Sports Traumatol Arthrosc. 2013;21(10):2314-2324.
31. Chau R, Gulati A, Pandit H, et al. Tibial component overhang following unicompartmental knee replacement--does it matter? Knee. 2009;16(5):310-313.
32. Mueller JK, Wentorf FA, Moore RE. Femoral and tibial insert downsizing increases the laxity envelope in TKA. Knee Surg Sports Traumatol Arthrosc. 2014;22(12):3003-3011.
33. Sriphirom P, Raungthong N, Chutchawan P, Thiranon C, Sukandhavesa N. Influence of a secondary downsizing of the femoral component on the extension gap: a cadaveric study. Orthopedics. 2012;35(10 Suppl):56-59.
34. Young SW, Clarke HD, Graves SE, Liu YL, de Steiger RN. Higher rate of revision in PFC sigma primary total knee arthroplasty with mismatch of femoro-tibial component sizes. J Arthroplasty. 2015;30(5):813-817.
35. Barink M, Verdonschot N, de Waal Malefijt M. A different fixation of the femoral component in total knee arthroplasty may lead to preservation of femoral bone stock. Proc Inst Mech Eng H. 2003;217(5):325-332.
36. Eagar P, Hull ML, Howell SM. How the fixation method stiffness and initial tension affect anterior load-displacement of the knee and tension in anterior cruciate ligament grafts: a study in cadaveric knees using a double-loop hamstrings graft. J Orthop Res. 2004;22(3):613-624.
37. Fricka KB, Sritulanondha S, McAsey CJ. To cement or not? Two-year results of a prospective, randomized study comparing cemented vs. cementless total knee arthroplasty (TKA). J Arthroplasty. 2015;30(9 Suppl):55-58.
38. Kendrick BJ, Kaptein BL, Valstar ER, et al. Cemented versus cementless Oxford unicompartmental knee arthroplasty using radiostereometric analysis: a randomised controlled trial. Bone Joint J. 2015;97-B(2):185-191.
39. Kim TK, Chang CB, Kang YG, Chung BJ, Cho HJ, Seong SC. Execution accuracy of bone resection and implant fixation in computer assisted minimally invasive total knee arthroplasty. Knee. 2010;17(1):23-28.
40. Whiteside LA. Making your next unicompartmental knee arthroplasty last: three keys to success. J Arthroplasty. 2005;20(4 Suppl 2):2-3.
41. Bauwens K, Matthes G, Wich M, et al. Navigated total knee replacement. A meta-analysis. J Bone Joint Surg Am. 2007;89(2):261-269.
42. Brin YS, Nikolaou VS, Joseph L, Zukor DJ, Antoniou J. Imageless computer assisted versus conventional total knee replacement. A Bayesian meta-analysis of 23 comparative studies. Int Orthop. 2011;35(3):331-339.
43. Cheng T, Zhang G, Zhang X. Imageless navigation system does not improve component rotational alignment in total knee arthroplasty. J Surg Res. 2011;171(2):590-600.
44. Conteduca F, Iorio R, Mazza D, Ferretti A. Patient-specific instruments in total knee arthroplasty. Int Orthop. 2014;38(2):259-265.
45. Fu Y, Wang M, Liu Y, Fu Q. Alignment outcomes in navigated total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20(6):1075-1082.
46. Hetaimish BM, Khan MM, Simunovic N, Al-Harbi HH, Bhandari M, Zalzal PK. Meta-analysis of navigation vs conventional total knee arthroplasty. J Arthroplasty. 2012;27(6):1177-1182.
47. Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;22(8):1097-1106.
48. Moskal JT, Capps SG, Mann JW, Scanelli JA. Navigated versus conventional total knee arthroplasty. J Knee Surg. 2014;27(3):235-248.
49. Shi J, Wei Y, Wang S, et al. Computer navigation and total knee arthroplasty. Orthopedics. 2014;37(1):e39-e43.
50. Nair R, Tripathy G, Deysine GR. Computer navigation systems in unicompartmental knee arthroplasty: a systematic review. Am J Orthop. 2014;43(6):256-261.
51. Weber P, Crispin A, Schmidutz F, et al. Improved accuracy in computer-assisted unicondylar knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2453-2461.
52. Alcelik IA, Blomfield MI, Diana G, Gibbon AJ, Carrington N, Burr S. A comparison of short-term outcomes of minimally invasive computer-assisted vs minimally invasive conventional instrumentation for primary total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2016;31(2):410-418.
53. Cheng T, Pan XY, Mao X, Zhang GY, Zhang XL. Little clinical advantage of computer-assisted navigation over conventional instrumentation in primary total knee arthroplasty at early follow-up. Knee. 2012;19(4):237-245.
54. Rebal BA, Babatunde OM, Lee JH, Geller JA, Patrick DA Jr, Macaulay W. Imageless computer navigation in total knee arthroplasty provides superior short term functional outcomes: a meta-analysis. J Arthroplasty. 2014;29(5):938-944.
55. Zamora LA, Humphreys KJ, Watt AM, Forel D, Cameron AL. Systematic review of computer-navigated total knee arthroplasty. ANZ J Surg. 2013;83(1-2):22-30.
56. Liow MH, Xia Z, Wong MK, Tay KJ, Yeo SJ, Chin PL. Robot-assisted total knee arthroplasty accurately restores the joint line and mechanical axis. A prospective randomised study. J Arthroplasty. 2014;29(12):2373-2377.
57. Koulalis D, O’Loughlin PF, Plaskos C, Kendoff D, Cross MB, Pearle AD. Sequential versus automated cutting guides in computer-assisted total knee arthroplasty. Knee. 2011;18(6):436-442.
58. Clark TC, Schmidt FH. Robot-assisted navigation versus computer-assisted navigation in primary total knee arthroplasty: efficiency and accuracy. ISRN Orthop. 2013;2013:794827.
59. DiGioia AM 3rd, Jaramaz B, Colgan BD. Computer assisted orthopaedic surgery. Image guided and robotic assistive technologies. Clin Orthop Relat Res. 1998(354):8-16.
60. Conditt MA, Roche MW. Minimally invasive robotic-arm-guided unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2009;91 Suppl 1:63-68.
61. Lonner JH. Robotically assisted unicompartmental knee arthroplasty with a handheld image-free sculpting tool. Orthop Clin North Am. 2016;47(1):29-40.
62. Koenig JA, Suero EM, Plaskos C. Surgical accuracy and efficiency of computer-navigated TKA with a robotic cutting guide–report on the first 100 cases. J Bone Joint Surg Br. 2012;94-B(SUPP XLIV):103. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/94-B/SUPP_XLIV/103. Accessed April 6, 2016.
63. Siebert W, Mai S, Kober R, Heeckt PF. Technique and first clinical results of robot-assisted total knee replacement. Knee. 2002;9(3):173-180.
64. Schulz AP, Seide K, Queitsch C, et al. Results of total hip replacement using the Robodoc surgical assistant system: clinical outcome and evaluation of complications for 97 procedures. Int J Med Robot. 2007;3(4):301-306.
65. Kim SM, Park YS, Ha CW, Lim SJ, Moon YW. Robot-assisted implantation improves the precision of component position in minimally invasive TKA. Orthopedics. 2012;35(9):e1334-e1339.
66. Moon YW, Ha CW, Do KH, et al. Comparison of robot-assisted and conventional total knee arthroplasty: a controlled cadaver study using multiparameter quantitative three-dimensional CT assessment of alignment. Comput Aided Surg. 2012;17(2):86-95.
67. Park SE, Lee CT. Comparison of robotic-assisted and conventional manual implantation of a primary total knee arthroplasty. J Arthroplasty. 2007;22(7):1054-1059.
68. Song EK, Seon JK, Park SJ, Jung WB, Park HW, Lee GW. Simultaneous bilateral total knee arthroplasty with robotic and conventional techniques: a prospective, randomized study. Knee Surg Sports Traumatol Arthrosc. 2011;19(7):1069-1076.
69. Song EK, Seon JK, Yim JH, Netravali NA, Bargar WL. Robotic-assisted TKA reduces postoperative alignment outliers and improves gap balance compared to conventional TKA. Clin Orthop Relat Res. 2013;471(1):118-126.
70. Cobb J, Henckel J, Gomes P, et al. Hands-on robotic unicompartmental knee replacement: a prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88(2):188-197.
71. Jakopec M, Harris SJ, Rodriguez y Baena F, Gomes P, Cobb J, Davies BL. The first clinical application of a “hands-on” robotic knee surgery system. Comput Aided Surg. 2001;6(6):329-339.
72. Pearle AD, O’Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty. 2010;25(2):230-237.
73. Dunbar NJ, Roche MW, Park BH, Branch SH, Conditt MA, Banks SA. Accuracy of dynamic tactile-guided unicompartmental knee arthroplasty. J Arthroplasty. 2012;27(5):803-808.e1.
74. Smith JR, Riches PE, Rowe PJ. Accuracy of a freehand sculpting tool for unicondylar knee replacement. Int J Med Robot. 2014;10(2):162-169.
75. Lonner JH, Smith JR, Picard F, Hamlin B, Rowe PJ, Riches PE. High degree of accuracy of a novel image-free handheld robot for unicondylar knee arthroplasty in a cadaveric study. Clin Orthop Relat Res. 2015;473(1):206-212.
76. Ponder C, Plaskos C, Cheal E. Press-fit total knee arthroplasty with a robotic-cutting guide: proof of concept and initial clinical experience. Bone & Joint Journal Orthopaedic Proceedings Supplement. 2013;95(SUPP 28):61. Available at: http://www.bjjprocs.boneandjoint.org.uk/content/95-B/SUPP_28/61.abstract. Accessed April 6, 2016.
77. Bellemans J, Vandenneucker H, Vanlauwe J. Robot-assisted total knee arthroplasty. Clin Orthop Relat Res. 2007;464:111-116.
78. Lonner JH, John TK, Conditt MA. Robotic arm-assisted UKA improves tibial component alignment: a pilot study. Clin Orthop Relat Res. 2010;468(1):141-146.
79. Citak M, Suero EM, Citak M, et al. Unicompartmental knee arthroplasty: is robotic technology more accurate than conventional technique? Knee. 2013;20(4):268-271.
80. MacCallum KP, Danoff JR, Geller JA. Tibial baseplate positioning in robotic-assisted and conventional unicompartmental knee arthroplasty. Eur J Orthop Surg Traumatol. 2016;26(1):93-98.
81. Coon T, Roche M, Pearle AD, Dounchis J, Borus T, Buechel F Jr. Two year survivorship of robotically guided unicompartmental knee arthroplasty. Paper presented at: International Society for Technology in Arthroplasty 26th Annual Congress; October 16-19, 2013; Palm Beach, FL.
82. Pandit H, Jenkins C, Gill HS, Barker K, Dodd CA, Murray DW. Minimally invasive Oxford phase 3 unicompartmental knee replacement: results of 1000 cases. J Bone Joint Surg Br. 2011;93(2):198-204.
83. Yoshida K, Tada M, Yoshida H, Takei S, Fukuoka S, Nakamura H. Oxford phase 3 unicompartmental knee arthroplasty in Japan--clinical results in greater than one thousand cases over ten years. J Arthroplasty. 2013;28(9 Suppl):168-171.
84. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop. 2009;38(2 Suppl):32-36.
85. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A markovdecision analysis. J Arthroplasty. 2015. [Epub ahead of print]
86. Thienpont E. Improving Accuracy in Knee Arthroplasty. 1st ed. New Delhi, India: Jaypee Brothers Medical Publishers; 2012.
Changing Treatment Landscape of Hepatitis C Virus Infection Among Penitentiary Inmates
The incidence of hepatitis C virus (HCV) infection increased markedly in the 1970s and 1980s. These increases were mainly attributable to blood transfusions and injection drug use.1,2 The blood supply was not screened for HCV before 1992 (now, HCV infection by blood transfusion is rare).2,3 Surveillance of the period 1992-2003 showed a substantial decrease in the incidence of acute hepatitis C cases, and the rate remained steady through 2010.2,3 Over the past 5 years, HCV has returned to national attention with a rising infection rate (2.5-fold increase during 2010-2013) and a rapid succession of FDA approvals of direct-acting antiviral agents (DAAs).4 Currently, the most prevalent mode of infection is injection drug use, accounting for > 50% of all cases of HCV infection and 84% of acute HCV infections.5
Baby boomers (people born between 1945 and 1965) account for three-fourths of the population currently living with chronic HCV infection resulting from past infection.6 Historically, rates of acute and chronic infection have been far higher for blacks than for whites and Hispanics.2,4,7,8 In 2004, that trend started to reverse, with the incidence in whites surpassing that in blacks.4 Recent reports have identified a new cohort of HCV-infected injection drug users (IDUs) who are young (aged ≤ 24 years) and white nonurban dwellers.5
HCV Infection Among High Risk Individuals
In the U.S., unlike in other parts of the world, HCV infection is more prevalent than hepatitis B virus (HBV) infection.4,9,10 According to the National Health and Nutrition Examination Survey (NHANES), about 2.7 million Americans have chronic HCV infection. However, NHANES surveys do not sample certain populations, including the incarcerated and the homeless, in whom infection rates are thought to be higher.11 The incarcerated, the largest institutionalized group, have the highest incidence: One in 3 is infected with HCV.12 This rate is attributable to high rates of injection drug use and other high-risk behaviors. Drug-related offenses account for 50% of federal prison incarceration.13 For IDUs, the HCV infection rate is as high as 70% to 90%. Despite widespread implementation of needle-exchange programs after the discovery of HIV in the 1980s, recent surveys have indicated that about one-third of 18- to 30-year-old active IDUs are infected with HCV.14
Penitentiary Inmates Infected With HCV
A 2015 search of the Federal Bureau of Prisons (BOP) electronic medical records at the U.S. Penitentiary Canaan (USP Canaan) found that out of a population of about 1,600 inmates, 182 (11%) had tested positive for HCV antibodies (anti-HCV). This percentage likely is an underestimation, because HCV testing is not mandatory, and many (45%-85%) of the infected are unaware of their HCV infection status.2 Most of the infected remain chronically infected and are not being treated.
Prevalence of HCV infection commonly refers to chronic HCV infection. The diagnosis of chronic HCV infection is established by presence of HCV RNA on polymerase chain reaction assays. Of the 182 inmates who tested positive for anti-HCV, 45 had their cases resolved (undetectable HCV RNA), 34 spontaneously, and the other 11 after treatment, primarily with peginterferon and ribavirin (pegINF/RBV) dual therapies. The remaining 137 who tested positive remained chronically infected. This chronically infected group represented 9% of the population of 1,600 inmates. Although the infection rate is significantly higher than that in the general population (1% incidence), the inmates’ true rate of infection in all probability is much higher.11
Earlier NHANES data showed HCV more prevalent in minorities, particularly blacks, compared with whites.2,7,8 At USP Canaan, however, the incidence of chronic HCV infection was 21% in whites (mean age, 42 years), 4% in blacks (mean age, 51 years), and 7% in Hispanics (mean age, 39 years). The lower rates in blacks and Hispanics could have resulted from a lack of awareness about getting tested or from having fewer opportunities to obtain medical care in the community before incarceration (the infection can remain asymptomatic for several decades).
HCV genotype 1 is the most common HCV genotype in the U.S.5,15 At USP Canaan, genotype 1 accounted for 56% of the cases of chronic HCV infection in whites, 90% in blacks, and 79% in Hispanics. The majority genotype was subtype 1a.
Of the 137 inmates with HCV co-infections, 8 (6%) had HIV/HCV co-infection, and 4 (3%) had HBV/HCV co-infection. Also, 7 (5%) were diabetic. According to the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD/IDSA) guidelines, patients with comorbidities are a high priority for treatment, as there is a high risk for complications, with liver fibrosis progressing more rapidly.16
Changing Landscape of HCV Treatment
Treatments for chronic HCV infection have never been more promising. There is the prospect of a cure with the new DAAs. These new medications are active against HCV and interfere with viral enzymes and other proteins essential for viral replication. Until recently, the mainstay of treatment for chronic HCV infection was pegINF/RBV. However, INF-based dual therapies were associated with high rates of adverse effects (AEs) and treatment discontinuation. In 2011, release of the protease inhibitors (PIs) boceprevir and telaprevir improved sustained virologic response (SVR) rates for treatment-naïve patients with genotype 1 from about 50% (pegINF/RBV dual therapies) to 70% to 75% (PI in combination with pegINF/RBV triple therapies). However, first-generation DAAs were INF-based, and their dosing was cumbersome.15,17-19
Starting with the 2013 approval of simeprevir (second-wave PI) and sofosbuvir (polymerase inhibitor), most patients’ SVR rates improved to 75% to 90%.20,21 Sustained virologic response rates were boosted to > 95% after the 2014 approval of Harvoni, coformulated ledispasvir (replication complex inhibitor) and sofosbuvir, and Viekira Pak, a combination of ombitasvir (replication complex inhibitor), paritaprevir (PI), and ritonavir (inhibitor of CYP3A4 enzyme) co-packaged with dasabuvir (polymerase inhibitor).22-24 In 2015, daclatasvir (replication complex inhibitor) was approved, followed in 2016 by Zepatier, coformulated elbasvir (replication complex inhibitor) and grazoprevir (PI). Harvoni has simplified the treatment regimen to 1 pill a day and shortened the duration of treatment to as few as 8 weeks for some
patients.25
The option of an all-oral, INF-free treatment regimen and the prospect of freedom from the HCV could not come at a more opportune time, given the aging of baby boomers with chronic HCV infection and the high rates of HCV and HIV infections contracted in the 1970s and 1980s. An estimated one-third of those infected is expected to develop cirrhosis within 20 years.26
Cost of HCV Treatment
The U.S. has the highest health care costs in the world—consuming 17% of the nation’s gross domestic product.27,28 Health care costs also have been steadily increasing in U.S. prisons because of the expanding and aging incarcerated population. The Eighth Amendment provides inmates with the constitutional right to health care. The BOP’s overall expense of pharmaceuticals for HCV treatment has soared in recent years. It was kept below $2 million in fiscal years 2010 and 2011 but more than doubled the next 2 years, to more than $4 million in 2012 and 2013, and increased in 2014 to about $6 million. Hepititis C treatment accounted for 3% of the BOP pharmaceutical budget in 2011 and 7% in 2014.29 Increased HCV pharmaceutical expenses were attributable to the introduction of DAAs. Even so, the majority of inmates with chronic HCV infection remained untreated.
Compared with DAA PIs, sofosbuvir is a game changer. Its all-oral, INF-free regimen makes treatment of chronic HCV infection more effective and safer. However, its cost is prohibitive, even in rich countries: A 12-week course costs $84,000, and Harvoni (ledispasvir/ sofosbuvir) costs $94,000.30,31 A generic version of sofosbuvir is not expected until 2025.32 Many studies have been conducted on the cost-effectiveness of these newer DAAs, but the picture is unclear, as the results were sensitive to drug price, drug efficacy (SVR rates vary with genotype and patient profile), quality of life after successful treatment, and the willingness-to-pay threshold.30 Ironically, treatment cost could be the primary barrier to HCV eradication.
At USP Canaan, 137 inmates with chronic HCV infection potentially could have benefited from treatment. A majority (91 inmates) were infected with HCV genotype 1; of the other 46 inmates, 12 had genotype 2, 18 had genotype 3, 2 had genotype 4, and 14 lacked genotyping.
The all-oral, INF-free regimen obviates the need for weekly injection, and treatment duration is shorter. The AASLD/IDSA treatment guidelines recommend all-oral, INF-free treatment regimens for all patients with genotype 1. Typically, treatment lasts 12 or 24 weeks, depending on presence or absence of liver cirrhosis, among other considerations.16
Because of the high cost of treating all inmates with chronic HCV infection, the large number of inmates who are asymptomatic, and the potential decrease in medication costs after the introduction of generic versions, inmates are being prioritized for treatment based primarily on staging (presence or absence of liver disease). The rationale for using staging for prioritization is that patients with chronic HCV infection and no or minimal fibrosis at presentation seem to progress slowly, and treatment possibly can be delayed or withheld; whereas patients with significant fibrosis (septal or bridging fibrosis) progress almost invariably to cirrhosis over a 10- to 20-year period, so antiviral treatment becomes urgent.33
APRI: Biomarker for Liver Fibrosis
A liver biopsy is the gold standard for the diagnosis of liver fibrosis. Although generally safe, it is costly. It is also subject to sampling error and examiner discrepancy, which lead to incorrect staging of fibrosis in 20% of patients.5,33 Alternatively, various biologic markers can be used to diagnose liver disease. The aspartate aminotransferase (AST) platelet ratio index (APRI) is a simple and useful index based on readily available laboratory results: AST level and platelet count. APRI correlated significantly with fibrosis stage in patients with chronic HCV infection.33
At USP Canaan, 16 (12%) of the 137 inmates with chronic HCV infection had an APRI higher than 1, and 5 of the 16 had an APRI higher than 2.
Conclusion
In coming years, treatment of chronic HCV infection will consume a more significant portion of the health care budget. As treatment becomes more efficacious and safer, the paradigm may shift from a stage-based strategy to a treat-all strategy. Possibly, more inmates will ask for treatment as the treatment burden lessens due to fewer treatment-associated AEs. However, despite the efficacy of HCV treatment, there is no reduction in the overall lifetime medical costs, because the offset in downstream direct medical costs (from successful treatment) is less than the cost of a cure.30
In the BOP, many challenges remain: HCV infection rates are expected to be high, treatment costs astronomical, resources limited, and treated patients may become reinfected if high-risk behavior continues. Patient education is, therefore, an important component of effective prevention and treatment strategies. The U.S. Preventive Services Task Force recommends HCV screening for all high-risk persons and a onetime screening for all baby boomers.34 Federal prisons offer HCV testing to all inmates with risk factors, when clinically indicated, or on
request.
All inmates with chronic HCV infection were being monitored for treatment prioritization, as some were at higher risk for complications or disease progression and required more urgent treatment.35 Ideally, all inmates should be treated, as incarcerated persons are at elevated risk for HCV transmission, and successful treatment would benefit public health by reducing infection rates in the community.16 However, resource constraints are a reality in health care, particularly among underserved populations, and this situation provides the rationale for screening, monitoring, and treatment prioritization. This step-by-step approach, which rests on sound clinical judgment, helps control the budget.
Click here for the digital edition.
1. Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39.
2. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691-698.
3. Daniels D, Grytdal S, Wasley A; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis - United States, 2007. MMWR Surveill Summ. 2009;58(3):1-27.
4. Centers for Disease Control and Prevention, Division of Viral Hepatitis and National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Viral Hepatitis Surveillance—United States, 2013. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/statistics/2013surveillance/pdfs/2013hepsurveillancerpt.pdf. Updated April 24, 2015. Accessed May 20, 2015.
5. Hepatitis C Online. Hepatitis C Online Website. http://www.hepatitisc.uw.edu. Accessed March 3, 2016.
6. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32.
7. Alter KJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
8. Liu G, Holmberg SD, Kamili S, Xu F. Racial disparities in the proportion of current, unresolved hepatitis C virus infections in the United States, 2003-2010. Dig Dis Sci. 2014;59(8):1950-1957.
9. World Health Organization. Hepatitis B [fact sheet 204]. World Health Organization Website. http://www.who.int/mediacentre/factsheets/fs204/en. Updated July 2015. Accessed March 3, 2016.
10. World Health Organization. Hepatitis C [fact sheet 164]. World Health Organization Website. http://www.who.int/mediacentre/factsheets/fs164/en. Updated July 2015. Accessed March 3, 2016.
11. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
12. Centers for Disease Control and Prevention. Hepatitis C and Incarceration. Publication No. 21-1306. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/HCV/PDFs/HepCIncarcerationFactSheet.pdf. October 2013. Accessed March 3, 2016.
13. Federal Bureau of Prisons. Inmate statistics: offenses. Federal Bureau of Prisons Website. http://www.bop.gov/about/statistics/statistics_inmate_offenses.jsp. Updated January 30, 2016. Accessed March 3, 2016.
14. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm. Updated January 8, 2016. Accessed March 4, 2016.
15. Saab S, Gordon SC, Park H, Sulkowski M, Ahmed A, Younossi Z. Cost-effectiveness analysis of sofosbuvir plus peginterferon/ribavirin in the treatment of chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. 2014;40(6):657-675.
16. American Association for the Study of Liver Diseases, Infectious Diseases Society of America. Recommendations for Testing, Managing, and Treating Hepatitis C. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America Website. http://hcvguidelines.org. Updated February 2016. Accessed March 4, 2016.
17. Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405-2416.
18. Kwo PY. Boceprevir: a novel nonstructural 3 (NS3) protease inhibitor for the treatment of chronic hepatitis C infection. Therap Adv Gastroenterol. 2012;5(3):179-188.
19. Stahmeyer JT, Rossol S, Krauth C. Outcomes, costs and cost-effectiveness of treating
hepatitis C with direct acting antivirals. J Comp Eff Res. 2015;4(3):267-277.
20. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
21. Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58(6):1918-1929.
22. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
23. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594-1603.
24. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898.
25. Kowdley KV, Gordon SC, Reddy KR, et al; ION-3 Investigators. Ledipasvir and
sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
26. Younossi ZM, Singer ME, Mir HM, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol. 2014;60(3):530-537.
27. The Economist Don’t kill Obamacare. The Economist Website. http://www.economist.com/news/leaders/21645730-supreme-court-considers-whether-gut-obamacare-evidence-mounting-law. Published May 7, 2015. Accessed March 4, 2016.
28. The World Bank. Health expenditure, total (% of GDP). The World Bank Website. http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Published 2015. Accessed March 4, 2016.
29. Federal Bureau of Prisons, Health Services Division. 2015 BOP National P&T Minutes. Federal Bureau of Prisons intranet website. http://sallyport.bop.gov/co/hsd/pharmacy/docs/BOP_National_P&T_Minutes/2015%20BOP%20National%20P&T%20Minutes_Final.pdf. Published August 13, 2014. Accessed November 9, 2015.
30. Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397-406.
31. Sachs J. The drug that is bankrupting America. Huffington Post Website. http://www.huffingtonpost.com/jeffrey-sachs/the-drug-that-is-bankrupt_b_6692340
.html. Published February 16, 2015. Updated April 18, 2015. Accessed March 4, 2016.
32. Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58(7):928-936.
33. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518-526.
34. U.S. Preventive Services Task Force. Hepatitis C: screening. U.S. Preventive Services Task Force Website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening?ds=1&s=hepatitis c. Updated July 2015. Accessed March 4, 2016.
35. Federal Bureau of Prisons. Evaluation and Management of Chronic Hepatitis C Virus (HCV) Infection [clinical practice guidelines]. Federal Bureau of Prisons Website. http://www.bop.gov/resources/pdfs/hepatitis_c.pdf. Published July 2015. Accessed March 4, 2016.
The incidence of hepatitis C virus (HCV) infection increased markedly in the 1970s and 1980s. These increases were mainly attributable to blood transfusions and injection drug use.1,2 The blood supply was not screened for HCV before 1992 (now, HCV infection by blood transfusion is rare).2,3 Surveillance of the period 1992-2003 showed a substantial decrease in the incidence of acute hepatitis C cases, and the rate remained steady through 2010.2,3 Over the past 5 years, HCV has returned to national attention with a rising infection rate (2.5-fold increase during 2010-2013) and a rapid succession of FDA approvals of direct-acting antiviral agents (DAAs).4 Currently, the most prevalent mode of infection is injection drug use, accounting for > 50% of all cases of HCV infection and 84% of acute HCV infections.5
Baby boomers (people born between 1945 and 1965) account for three-fourths of the population currently living with chronic HCV infection resulting from past infection.6 Historically, rates of acute and chronic infection have been far higher for blacks than for whites and Hispanics.2,4,7,8 In 2004, that trend started to reverse, with the incidence in whites surpassing that in blacks.4 Recent reports have identified a new cohort of HCV-infected injection drug users (IDUs) who are young (aged ≤ 24 years) and white nonurban dwellers.5
HCV Infection Among High Risk Individuals
In the U.S., unlike in other parts of the world, HCV infection is more prevalent than hepatitis B virus (HBV) infection.4,9,10 According to the National Health and Nutrition Examination Survey (NHANES), about 2.7 million Americans have chronic HCV infection. However, NHANES surveys do not sample certain populations, including the incarcerated and the homeless, in whom infection rates are thought to be higher.11 The incarcerated, the largest institutionalized group, have the highest incidence: One in 3 is infected with HCV.12 This rate is attributable to high rates of injection drug use and other high-risk behaviors. Drug-related offenses account for 50% of federal prison incarceration.13 For IDUs, the HCV infection rate is as high as 70% to 90%. Despite widespread implementation of needle-exchange programs after the discovery of HIV in the 1980s, recent surveys have indicated that about one-third of 18- to 30-year-old active IDUs are infected with HCV.14
Penitentiary Inmates Infected With HCV
A 2015 search of the Federal Bureau of Prisons (BOP) electronic medical records at the U.S. Penitentiary Canaan (USP Canaan) found that out of a population of about 1,600 inmates, 182 (11%) had tested positive for HCV antibodies (anti-HCV). This percentage likely is an underestimation, because HCV testing is not mandatory, and many (45%-85%) of the infected are unaware of their HCV infection status.2 Most of the infected remain chronically infected and are not being treated.
Prevalence of HCV infection commonly refers to chronic HCV infection. The diagnosis of chronic HCV infection is established by presence of HCV RNA on polymerase chain reaction assays. Of the 182 inmates who tested positive for anti-HCV, 45 had their cases resolved (undetectable HCV RNA), 34 spontaneously, and the other 11 after treatment, primarily with peginterferon and ribavirin (pegINF/RBV) dual therapies. The remaining 137 who tested positive remained chronically infected. This chronically infected group represented 9% of the population of 1,600 inmates. Although the infection rate is significantly higher than that in the general population (1% incidence), the inmates’ true rate of infection in all probability is much higher.11
Earlier NHANES data showed HCV more prevalent in minorities, particularly blacks, compared with whites.2,7,8 At USP Canaan, however, the incidence of chronic HCV infection was 21% in whites (mean age, 42 years), 4% in blacks (mean age, 51 years), and 7% in Hispanics (mean age, 39 years). The lower rates in blacks and Hispanics could have resulted from a lack of awareness about getting tested or from having fewer opportunities to obtain medical care in the community before incarceration (the infection can remain asymptomatic for several decades).
HCV genotype 1 is the most common HCV genotype in the U.S.5,15 At USP Canaan, genotype 1 accounted for 56% of the cases of chronic HCV infection in whites, 90% in blacks, and 79% in Hispanics. The majority genotype was subtype 1a.
Of the 137 inmates with HCV co-infections, 8 (6%) had HIV/HCV co-infection, and 4 (3%) had HBV/HCV co-infection. Also, 7 (5%) were diabetic. According to the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD/IDSA) guidelines, patients with comorbidities are a high priority for treatment, as there is a high risk for complications, with liver fibrosis progressing more rapidly.16
Changing Landscape of HCV Treatment
Treatments for chronic HCV infection have never been more promising. There is the prospect of a cure with the new DAAs. These new medications are active against HCV and interfere with viral enzymes and other proteins essential for viral replication. Until recently, the mainstay of treatment for chronic HCV infection was pegINF/RBV. However, INF-based dual therapies were associated with high rates of adverse effects (AEs) and treatment discontinuation. In 2011, release of the protease inhibitors (PIs) boceprevir and telaprevir improved sustained virologic response (SVR) rates for treatment-naïve patients with genotype 1 from about 50% (pegINF/RBV dual therapies) to 70% to 75% (PI in combination with pegINF/RBV triple therapies). However, first-generation DAAs were INF-based, and their dosing was cumbersome.15,17-19
Starting with the 2013 approval of simeprevir (second-wave PI) and sofosbuvir (polymerase inhibitor), most patients’ SVR rates improved to 75% to 90%.20,21 Sustained virologic response rates were boosted to > 95% after the 2014 approval of Harvoni, coformulated ledispasvir (replication complex inhibitor) and sofosbuvir, and Viekira Pak, a combination of ombitasvir (replication complex inhibitor), paritaprevir (PI), and ritonavir (inhibitor of CYP3A4 enzyme) co-packaged with dasabuvir (polymerase inhibitor).22-24 In 2015, daclatasvir (replication complex inhibitor) was approved, followed in 2016 by Zepatier, coformulated elbasvir (replication complex inhibitor) and grazoprevir (PI). Harvoni has simplified the treatment regimen to 1 pill a day and shortened the duration of treatment to as few as 8 weeks for some
patients.25
The option of an all-oral, INF-free treatment regimen and the prospect of freedom from the HCV could not come at a more opportune time, given the aging of baby boomers with chronic HCV infection and the high rates of HCV and HIV infections contracted in the 1970s and 1980s. An estimated one-third of those infected is expected to develop cirrhosis within 20 years.26
Cost of HCV Treatment
The U.S. has the highest health care costs in the world—consuming 17% of the nation’s gross domestic product.27,28 Health care costs also have been steadily increasing in U.S. prisons because of the expanding and aging incarcerated population. The Eighth Amendment provides inmates with the constitutional right to health care. The BOP’s overall expense of pharmaceuticals for HCV treatment has soared in recent years. It was kept below $2 million in fiscal years 2010 and 2011 but more than doubled the next 2 years, to more than $4 million in 2012 and 2013, and increased in 2014 to about $6 million. Hepititis C treatment accounted for 3% of the BOP pharmaceutical budget in 2011 and 7% in 2014.29 Increased HCV pharmaceutical expenses were attributable to the introduction of DAAs. Even so, the majority of inmates with chronic HCV infection remained untreated.
Compared with DAA PIs, sofosbuvir is a game changer. Its all-oral, INF-free regimen makes treatment of chronic HCV infection more effective and safer. However, its cost is prohibitive, even in rich countries: A 12-week course costs $84,000, and Harvoni (ledispasvir/ sofosbuvir) costs $94,000.30,31 A generic version of sofosbuvir is not expected until 2025.32 Many studies have been conducted on the cost-effectiveness of these newer DAAs, but the picture is unclear, as the results were sensitive to drug price, drug efficacy (SVR rates vary with genotype and patient profile), quality of life after successful treatment, and the willingness-to-pay threshold.30 Ironically, treatment cost could be the primary barrier to HCV eradication.
At USP Canaan, 137 inmates with chronic HCV infection potentially could have benefited from treatment. A majority (91 inmates) were infected with HCV genotype 1; of the other 46 inmates, 12 had genotype 2, 18 had genotype 3, 2 had genotype 4, and 14 lacked genotyping.
The all-oral, INF-free regimen obviates the need for weekly injection, and treatment duration is shorter. The AASLD/IDSA treatment guidelines recommend all-oral, INF-free treatment regimens for all patients with genotype 1. Typically, treatment lasts 12 or 24 weeks, depending on presence or absence of liver cirrhosis, among other considerations.16
Because of the high cost of treating all inmates with chronic HCV infection, the large number of inmates who are asymptomatic, and the potential decrease in medication costs after the introduction of generic versions, inmates are being prioritized for treatment based primarily on staging (presence or absence of liver disease). The rationale for using staging for prioritization is that patients with chronic HCV infection and no or minimal fibrosis at presentation seem to progress slowly, and treatment possibly can be delayed or withheld; whereas patients with significant fibrosis (septal or bridging fibrosis) progress almost invariably to cirrhosis over a 10- to 20-year period, so antiviral treatment becomes urgent.33
APRI: Biomarker for Liver Fibrosis
A liver biopsy is the gold standard for the diagnosis of liver fibrosis. Although generally safe, it is costly. It is also subject to sampling error and examiner discrepancy, which lead to incorrect staging of fibrosis in 20% of patients.5,33 Alternatively, various biologic markers can be used to diagnose liver disease. The aspartate aminotransferase (AST) platelet ratio index (APRI) is a simple and useful index based on readily available laboratory results: AST level and platelet count. APRI correlated significantly with fibrosis stage in patients with chronic HCV infection.33
At USP Canaan, 16 (12%) of the 137 inmates with chronic HCV infection had an APRI higher than 1, and 5 of the 16 had an APRI higher than 2.
Conclusion
In coming years, treatment of chronic HCV infection will consume a more significant portion of the health care budget. As treatment becomes more efficacious and safer, the paradigm may shift from a stage-based strategy to a treat-all strategy. Possibly, more inmates will ask for treatment as the treatment burden lessens due to fewer treatment-associated AEs. However, despite the efficacy of HCV treatment, there is no reduction in the overall lifetime medical costs, because the offset in downstream direct medical costs (from successful treatment) is less than the cost of a cure.30
In the BOP, many challenges remain: HCV infection rates are expected to be high, treatment costs astronomical, resources limited, and treated patients may become reinfected if high-risk behavior continues. Patient education is, therefore, an important component of effective prevention and treatment strategies. The U.S. Preventive Services Task Force recommends HCV screening for all high-risk persons and a onetime screening for all baby boomers.34 Federal prisons offer HCV testing to all inmates with risk factors, when clinically indicated, or on
request.
All inmates with chronic HCV infection were being monitored for treatment prioritization, as some were at higher risk for complications or disease progression and required more urgent treatment.35 Ideally, all inmates should be treated, as incarcerated persons are at elevated risk for HCV transmission, and successful treatment would benefit public health by reducing infection rates in the community.16 However, resource constraints are a reality in health care, particularly among underserved populations, and this situation provides the rationale for screening, monitoring, and treatment prioritization. This step-by-step approach, which rests on sound clinical judgment, helps control the budget.
Click here for the digital edition.
The incidence of hepatitis C virus (HCV) infection increased markedly in the 1970s and 1980s. These increases were mainly attributable to blood transfusions and injection drug use.1,2 The blood supply was not screened for HCV before 1992 (now, HCV infection by blood transfusion is rare).2,3 Surveillance of the period 1992-2003 showed a substantial decrease in the incidence of acute hepatitis C cases, and the rate remained steady through 2010.2,3 Over the past 5 years, HCV has returned to national attention with a rising infection rate (2.5-fold increase during 2010-2013) and a rapid succession of FDA approvals of direct-acting antiviral agents (DAAs).4 Currently, the most prevalent mode of infection is injection drug use, accounting for > 50% of all cases of HCV infection and 84% of acute HCV infections.5
Baby boomers (people born between 1945 and 1965) account for three-fourths of the population currently living with chronic HCV infection resulting from past infection.6 Historically, rates of acute and chronic infection have been far higher for blacks than for whites and Hispanics.2,4,7,8 In 2004, that trend started to reverse, with the incidence in whites surpassing that in blacks.4 Recent reports have identified a new cohort of HCV-infected injection drug users (IDUs) who are young (aged ≤ 24 years) and white nonurban dwellers.5
HCV Infection Among High Risk Individuals
In the U.S., unlike in other parts of the world, HCV infection is more prevalent than hepatitis B virus (HBV) infection.4,9,10 According to the National Health and Nutrition Examination Survey (NHANES), about 2.7 million Americans have chronic HCV infection. However, NHANES surveys do not sample certain populations, including the incarcerated and the homeless, in whom infection rates are thought to be higher.11 The incarcerated, the largest institutionalized group, have the highest incidence: One in 3 is infected with HCV.12 This rate is attributable to high rates of injection drug use and other high-risk behaviors. Drug-related offenses account for 50% of federal prison incarceration.13 For IDUs, the HCV infection rate is as high as 70% to 90%. Despite widespread implementation of needle-exchange programs after the discovery of HIV in the 1980s, recent surveys have indicated that about one-third of 18- to 30-year-old active IDUs are infected with HCV.14
Penitentiary Inmates Infected With HCV
A 2015 search of the Federal Bureau of Prisons (BOP) electronic medical records at the U.S. Penitentiary Canaan (USP Canaan) found that out of a population of about 1,600 inmates, 182 (11%) had tested positive for HCV antibodies (anti-HCV). This percentage likely is an underestimation, because HCV testing is not mandatory, and many (45%-85%) of the infected are unaware of their HCV infection status.2 Most of the infected remain chronically infected and are not being treated.
Prevalence of HCV infection commonly refers to chronic HCV infection. The diagnosis of chronic HCV infection is established by presence of HCV RNA on polymerase chain reaction assays. Of the 182 inmates who tested positive for anti-HCV, 45 had their cases resolved (undetectable HCV RNA), 34 spontaneously, and the other 11 after treatment, primarily with peginterferon and ribavirin (pegINF/RBV) dual therapies. The remaining 137 who tested positive remained chronically infected. This chronically infected group represented 9% of the population of 1,600 inmates. Although the infection rate is significantly higher than that in the general population (1% incidence), the inmates’ true rate of infection in all probability is much higher.11
Earlier NHANES data showed HCV more prevalent in minorities, particularly blacks, compared with whites.2,7,8 At USP Canaan, however, the incidence of chronic HCV infection was 21% in whites (mean age, 42 years), 4% in blacks (mean age, 51 years), and 7% in Hispanics (mean age, 39 years). The lower rates in blacks and Hispanics could have resulted from a lack of awareness about getting tested or from having fewer opportunities to obtain medical care in the community before incarceration (the infection can remain asymptomatic for several decades).
HCV genotype 1 is the most common HCV genotype in the U.S.5,15 At USP Canaan, genotype 1 accounted for 56% of the cases of chronic HCV infection in whites, 90% in blacks, and 79% in Hispanics. The majority genotype was subtype 1a.
Of the 137 inmates with HCV co-infections, 8 (6%) had HIV/HCV co-infection, and 4 (3%) had HBV/HCV co-infection. Also, 7 (5%) were diabetic. According to the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD/IDSA) guidelines, patients with comorbidities are a high priority for treatment, as there is a high risk for complications, with liver fibrosis progressing more rapidly.16
Changing Landscape of HCV Treatment
Treatments for chronic HCV infection have never been more promising. There is the prospect of a cure with the new DAAs. These new medications are active against HCV and interfere with viral enzymes and other proteins essential for viral replication. Until recently, the mainstay of treatment for chronic HCV infection was pegINF/RBV. However, INF-based dual therapies were associated with high rates of adverse effects (AEs) and treatment discontinuation. In 2011, release of the protease inhibitors (PIs) boceprevir and telaprevir improved sustained virologic response (SVR) rates for treatment-naïve patients with genotype 1 from about 50% (pegINF/RBV dual therapies) to 70% to 75% (PI in combination with pegINF/RBV triple therapies). However, first-generation DAAs were INF-based, and their dosing was cumbersome.15,17-19
Starting with the 2013 approval of simeprevir (second-wave PI) and sofosbuvir (polymerase inhibitor), most patients’ SVR rates improved to 75% to 90%.20,21 Sustained virologic response rates were boosted to > 95% after the 2014 approval of Harvoni, coformulated ledispasvir (replication complex inhibitor) and sofosbuvir, and Viekira Pak, a combination of ombitasvir (replication complex inhibitor), paritaprevir (PI), and ritonavir (inhibitor of CYP3A4 enzyme) co-packaged with dasabuvir (polymerase inhibitor).22-24 In 2015, daclatasvir (replication complex inhibitor) was approved, followed in 2016 by Zepatier, coformulated elbasvir (replication complex inhibitor) and grazoprevir (PI). Harvoni has simplified the treatment regimen to 1 pill a day and shortened the duration of treatment to as few as 8 weeks for some
patients.25
The option of an all-oral, INF-free treatment regimen and the prospect of freedom from the HCV could not come at a more opportune time, given the aging of baby boomers with chronic HCV infection and the high rates of HCV and HIV infections contracted in the 1970s and 1980s. An estimated one-third of those infected is expected to develop cirrhosis within 20 years.26
Cost of HCV Treatment
The U.S. has the highest health care costs in the world—consuming 17% of the nation’s gross domestic product.27,28 Health care costs also have been steadily increasing in U.S. prisons because of the expanding and aging incarcerated population. The Eighth Amendment provides inmates with the constitutional right to health care. The BOP’s overall expense of pharmaceuticals for HCV treatment has soared in recent years. It was kept below $2 million in fiscal years 2010 and 2011 but more than doubled the next 2 years, to more than $4 million in 2012 and 2013, and increased in 2014 to about $6 million. Hepititis C treatment accounted for 3% of the BOP pharmaceutical budget in 2011 and 7% in 2014.29 Increased HCV pharmaceutical expenses were attributable to the introduction of DAAs. Even so, the majority of inmates with chronic HCV infection remained untreated.
Compared with DAA PIs, sofosbuvir is a game changer. Its all-oral, INF-free regimen makes treatment of chronic HCV infection more effective and safer. However, its cost is prohibitive, even in rich countries: A 12-week course costs $84,000, and Harvoni (ledispasvir/ sofosbuvir) costs $94,000.30,31 A generic version of sofosbuvir is not expected until 2025.32 Many studies have been conducted on the cost-effectiveness of these newer DAAs, but the picture is unclear, as the results were sensitive to drug price, drug efficacy (SVR rates vary with genotype and patient profile), quality of life after successful treatment, and the willingness-to-pay threshold.30 Ironically, treatment cost could be the primary barrier to HCV eradication.
At USP Canaan, 137 inmates with chronic HCV infection potentially could have benefited from treatment. A majority (91 inmates) were infected with HCV genotype 1; of the other 46 inmates, 12 had genotype 2, 18 had genotype 3, 2 had genotype 4, and 14 lacked genotyping.
The all-oral, INF-free regimen obviates the need for weekly injection, and treatment duration is shorter. The AASLD/IDSA treatment guidelines recommend all-oral, INF-free treatment regimens for all patients with genotype 1. Typically, treatment lasts 12 or 24 weeks, depending on presence or absence of liver cirrhosis, among other considerations.16
Because of the high cost of treating all inmates with chronic HCV infection, the large number of inmates who are asymptomatic, and the potential decrease in medication costs after the introduction of generic versions, inmates are being prioritized for treatment based primarily on staging (presence or absence of liver disease). The rationale for using staging for prioritization is that patients with chronic HCV infection and no or minimal fibrosis at presentation seem to progress slowly, and treatment possibly can be delayed or withheld; whereas patients with significant fibrosis (septal or bridging fibrosis) progress almost invariably to cirrhosis over a 10- to 20-year period, so antiviral treatment becomes urgent.33
APRI: Biomarker for Liver Fibrosis
A liver biopsy is the gold standard for the diagnosis of liver fibrosis. Although generally safe, it is costly. It is also subject to sampling error and examiner discrepancy, which lead to incorrect staging of fibrosis in 20% of patients.5,33 Alternatively, various biologic markers can be used to diagnose liver disease. The aspartate aminotransferase (AST) platelet ratio index (APRI) is a simple and useful index based on readily available laboratory results: AST level and platelet count. APRI correlated significantly with fibrosis stage in patients with chronic HCV infection.33
At USP Canaan, 16 (12%) of the 137 inmates with chronic HCV infection had an APRI higher than 1, and 5 of the 16 had an APRI higher than 2.
Conclusion
In coming years, treatment of chronic HCV infection will consume a more significant portion of the health care budget. As treatment becomes more efficacious and safer, the paradigm may shift from a stage-based strategy to a treat-all strategy. Possibly, more inmates will ask for treatment as the treatment burden lessens due to fewer treatment-associated AEs. However, despite the efficacy of HCV treatment, there is no reduction in the overall lifetime medical costs, because the offset in downstream direct medical costs (from successful treatment) is less than the cost of a cure.30
In the BOP, many challenges remain: HCV infection rates are expected to be high, treatment costs astronomical, resources limited, and treated patients may become reinfected if high-risk behavior continues. Patient education is, therefore, an important component of effective prevention and treatment strategies. The U.S. Preventive Services Task Force recommends HCV screening for all high-risk persons and a onetime screening for all baby boomers.34 Federal prisons offer HCV testing to all inmates with risk factors, when clinically indicated, or on
request.
All inmates with chronic HCV infection were being monitored for treatment prioritization, as some were at higher risk for complications or disease progression and required more urgent treatment.35 Ideally, all inmates should be treated, as incarcerated persons are at elevated risk for HCV transmission, and successful treatment would benefit public health by reducing infection rates in the community.16 However, resource constraints are a reality in health care, particularly among underserved populations, and this situation provides the rationale for screening, monitoring, and treatment prioritization. This step-by-step approach, which rests on sound clinical judgment, helps control the budget.
Click here for the digital edition.
1. Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39.
2. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691-698.
3. Daniels D, Grytdal S, Wasley A; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis - United States, 2007. MMWR Surveill Summ. 2009;58(3):1-27.
4. Centers for Disease Control and Prevention, Division of Viral Hepatitis and National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Viral Hepatitis Surveillance—United States, 2013. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/statistics/2013surveillance/pdfs/2013hepsurveillancerpt.pdf. Updated April 24, 2015. Accessed May 20, 2015.
5. Hepatitis C Online. Hepatitis C Online Website. http://www.hepatitisc.uw.edu. Accessed March 3, 2016.
6. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32.
7. Alter KJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
8. Liu G, Holmberg SD, Kamili S, Xu F. Racial disparities in the proportion of current, unresolved hepatitis C virus infections in the United States, 2003-2010. Dig Dis Sci. 2014;59(8):1950-1957.
9. World Health Organization. Hepatitis B [fact sheet 204]. World Health Organization Website. http://www.who.int/mediacentre/factsheets/fs204/en. Updated July 2015. Accessed March 3, 2016.
10. World Health Organization. Hepatitis C [fact sheet 164]. World Health Organization Website. http://www.who.int/mediacentre/factsheets/fs164/en. Updated July 2015. Accessed March 3, 2016.
11. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
12. Centers for Disease Control and Prevention. Hepatitis C and Incarceration. Publication No. 21-1306. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/HCV/PDFs/HepCIncarcerationFactSheet.pdf. October 2013. Accessed March 3, 2016.
13. Federal Bureau of Prisons. Inmate statistics: offenses. Federal Bureau of Prisons Website. http://www.bop.gov/about/statistics/statistics_inmate_offenses.jsp. Updated January 30, 2016. Accessed March 3, 2016.
14. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm. Updated January 8, 2016. Accessed March 4, 2016.
15. Saab S, Gordon SC, Park H, Sulkowski M, Ahmed A, Younossi Z. Cost-effectiveness analysis of sofosbuvir plus peginterferon/ribavirin in the treatment of chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. 2014;40(6):657-675.
16. American Association for the Study of Liver Diseases, Infectious Diseases Society of America. Recommendations for Testing, Managing, and Treating Hepatitis C. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America Website. http://hcvguidelines.org. Updated February 2016. Accessed March 4, 2016.
17. Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405-2416.
18. Kwo PY. Boceprevir: a novel nonstructural 3 (NS3) protease inhibitor for the treatment of chronic hepatitis C infection. Therap Adv Gastroenterol. 2012;5(3):179-188.
19. Stahmeyer JT, Rossol S, Krauth C. Outcomes, costs and cost-effectiveness of treating
hepatitis C with direct acting antivirals. J Comp Eff Res. 2015;4(3):267-277.
20. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
21. Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58(6):1918-1929.
22. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
23. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594-1603.
24. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898.
25. Kowdley KV, Gordon SC, Reddy KR, et al; ION-3 Investigators. Ledipasvir and
sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
26. Younossi ZM, Singer ME, Mir HM, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol. 2014;60(3):530-537.
27. The Economist Don’t kill Obamacare. The Economist Website. http://www.economist.com/news/leaders/21645730-supreme-court-considers-whether-gut-obamacare-evidence-mounting-law. Published May 7, 2015. Accessed March 4, 2016.
28. The World Bank. Health expenditure, total (% of GDP). The World Bank Website. http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Published 2015. Accessed March 4, 2016.
29. Federal Bureau of Prisons, Health Services Division. 2015 BOP National P&T Minutes. Federal Bureau of Prisons intranet website. http://sallyport.bop.gov/co/hsd/pharmacy/docs/BOP_National_P&T_Minutes/2015%20BOP%20National%20P&T%20Minutes_Final.pdf. Published August 13, 2014. Accessed November 9, 2015.
30. Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397-406.
31. Sachs J. The drug that is bankrupting America. Huffington Post Website. http://www.huffingtonpost.com/jeffrey-sachs/the-drug-that-is-bankrupt_b_6692340
.html. Published February 16, 2015. Updated April 18, 2015. Accessed March 4, 2016.
32. Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58(7):928-936.
33. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518-526.
34. U.S. Preventive Services Task Force. Hepatitis C: screening. U.S. Preventive Services Task Force Website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening?ds=1&s=hepatitis c. Updated July 2015. Accessed March 4, 2016.
35. Federal Bureau of Prisons. Evaluation and Management of Chronic Hepatitis C Virus (HCV) Infection [clinical practice guidelines]. Federal Bureau of Prisons Website. http://www.bop.gov/resources/pdfs/hepatitis_c.pdf. Published July 2015. Accessed March 4, 2016.
1. Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39.
2. Ditah I, Ditah F, Devaki P, et al. The changing epidemiology of hepatitis C virus infection in the United States: National Health and Nutrition Examination Survey 2001 through 2010. J Hepatol. 2014;60(4):691-698.
3. Daniels D, Grytdal S, Wasley A; Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis - United States, 2007. MMWR Surveill Summ. 2009;58(3):1-27.
4. Centers for Disease Control and Prevention, Division of Viral Hepatitis and National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. Viral Hepatitis Surveillance—United States, 2013. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/statistics/2013surveillance/pdfs/2013hepsurveillancerpt.pdf. Updated April 24, 2015. Accessed May 20, 2015.
5. Hepatitis C Online. Hepatitis C Online Website. http://www.hepatitisc.uw.edu. Accessed March 3, 2016.
6. Smith BD, Morgan RL, Beckett GA, et al; Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32.
7. Alter KJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
8. Liu G, Holmberg SD, Kamili S, Xu F. Racial disparities in the proportion of current, unresolved hepatitis C virus infections in the United States, 2003-2010. Dig Dis Sci. 2014;59(8):1950-1957.
9. World Health Organization. Hepatitis B [fact sheet 204]. World Health Organization Website. http://www.who.int/mediacentre/factsheets/fs204/en. Updated July 2015. Accessed March 3, 2016.
10. World Health Organization. Hepatitis C [fact sheet 164]. World Health Organization Website. http://www.who.int/mediacentre/factsheets/fs164/en. Updated July 2015. Accessed March 3, 2016.
11. Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann Intern Med. 2014;160(5):293-300.
12. Centers for Disease Control and Prevention. Hepatitis C and Incarceration. Publication No. 21-1306. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/HCV/PDFs/HepCIncarcerationFactSheet.pdf. October 2013. Accessed March 3, 2016.
13. Federal Bureau of Prisons. Inmate statistics: offenses. Federal Bureau of Prisons Website. http://www.bop.gov/about/statistics/statistics_inmate_offenses.jsp. Updated January 30, 2016. Accessed March 3, 2016.
14. Centers for Disease Control and Prevention. Hepatitis C FAQs for health professionals. Centers for Disease Control and Prevention Website. http://www.cdc.gov/hepatitis/HCV/HCVfaq.htm. Updated January 8, 2016. Accessed March 4, 2016.
15. Saab S, Gordon SC, Park H, Sulkowski M, Ahmed A, Younossi Z. Cost-effectiveness analysis of sofosbuvir plus peginterferon/ribavirin in the treatment of chronic hepatitis C virus genotype 1 infection. Aliment Pharmacol Ther. 2014;40(6):657-675.
16. American Association for the Study of Liver Diseases, Infectious Diseases Society of America. Recommendations for Testing, Managing, and Treating Hepatitis C. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America Website. http://hcvguidelines.org. Updated February 2016. Accessed March 4, 2016.
17. Jacobson IM, McHutchison JG, Dusheiko G, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011;364(25):2405-2416.
18. Kwo PY. Boceprevir: a novel nonstructural 3 (NS3) protease inhibitor for the treatment of chronic hepatitis C infection. Therap Adv Gastroenterol. 2012;5(3):179-188.
19. Stahmeyer JT, Rossol S, Krauth C. Outcomes, costs and cost-effectiveness of treating
hepatitis C with direct acting antivirals. J Comp Eff Res. 2015;4(3):267-277.
20. Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368(20):1878-1887.
21. Fried MW, Buti M, Dore GJ, et al. Once-daily simeprevir (TMC435) with pegylated interferon and ribavirin in treatment-naïve genotype 1 hepatitis C: the randomized PILLAR study. Hepatology. 2013;58(6):1918-1929.
22. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
23. Feld JJ, Kowdley KV, Coakley E, et al. Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 2014;370(17):1594-1603.
24. Afdhal N, Zeuzem S, Kwo P, et al; ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898.
25. Kowdley KV, Gordon SC, Reddy KR, et al; ION-3 Investigators. Ledipasvir and
sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014;370(20):1879-1888.
26. Younossi ZM, Singer ME, Mir HM, Henry L, Hunt S. Impact of interferon free regimens on clinical and cost outcomes for chronic hepatitis C genotype 1 patients. J Hepatol. 2014;60(3):530-537.
27. The Economist Don’t kill Obamacare. The Economist Website. http://www.economist.com/news/leaders/21645730-supreme-court-considers-whether-gut-obamacare-evidence-mounting-law. Published May 7, 2015. Accessed March 4, 2016.
28. The World Bank. Health expenditure, total (% of GDP). The World Bank Website. http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Published 2015. Accessed March 4, 2016.
29. Federal Bureau of Prisons, Health Services Division. 2015 BOP National P&T Minutes. Federal Bureau of Prisons intranet website. http://sallyport.bop.gov/co/hsd/pharmacy/docs/BOP_National_P&T_Minutes/2015%20BOP%20National%20P&T%20Minutes_Final.pdf. Published August 13, 2014. Accessed November 9, 2015.
30. Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162(6):397-406.
31. Sachs J. The drug that is bankrupting America. Huffington Post Website. http://www.huffingtonpost.com/jeffrey-sachs/the-drug-that-is-bankrupt_b_6692340
.html. Published February 16, 2015. Updated April 18, 2015. Accessed March 4, 2016.
32. Hill A, Khoo S, Fortunak J, Simmons B, Ford N. Minimum costs for producing hepatitis C direct-acting antivirals for use in large-scale treatment access programs in developing countries. Clin Infect Dis. 2014;58(7):928-936.
33. Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518-526.
34. U.S. Preventive Services Task Force. Hepatitis C: screening. U.S. Preventive Services Task Force Website. http://www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/hepatitis-c-screening?ds=1&s=hepatitis c. Updated July 2015. Accessed March 4, 2016.
35. Federal Bureau of Prisons. Evaluation and Management of Chronic Hepatitis C Virus (HCV) Infection [clinical practice guidelines]. Federal Bureau of Prisons Website. http://www.bop.gov/resources/pdfs/hepatitis_c.pdf. Published July 2015. Accessed March 4, 2016.