User login
Reactivation of a BCG Vaccination Scar Following the First Dose of the Moderna COVID-19 Vaccine
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
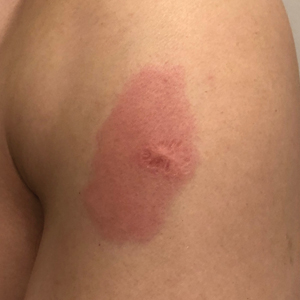
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).
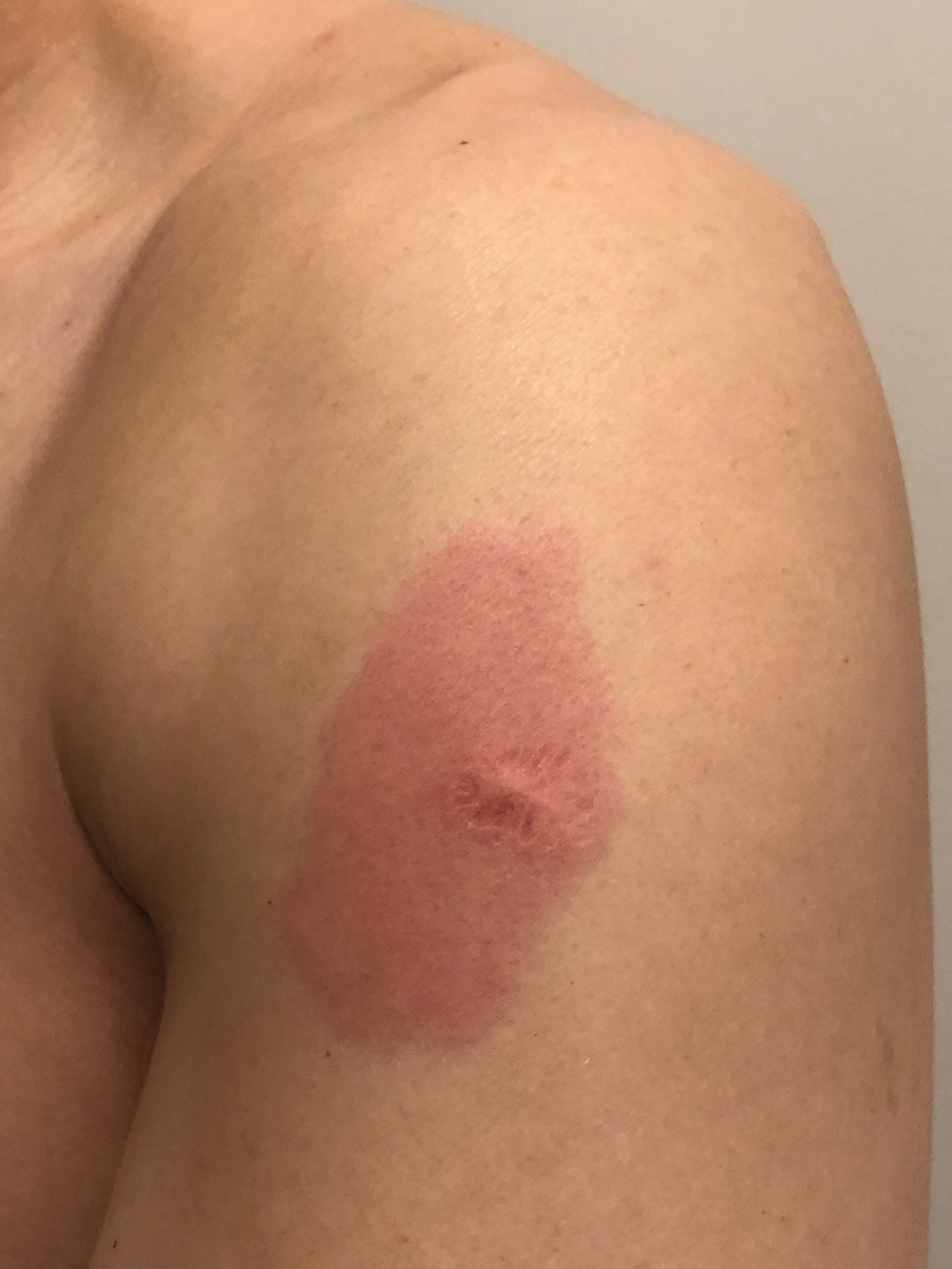
The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).

The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
The COVID-19 pandemic has resulted in notable morbidity and mortality worldwide. In December 2020, the US Food and Drug Administration issued an Emergency Use Authorization for 2 messenger RNA (mRNA) vaccines—produced by Pfizer-BioNTech and Moderna—for the prevention of COVID-19. Phase 3 trials of the vaccine developed by Moderna showed 94.1% efficacy at preventing COVID-19 after 2 doses.1
Common cutaneous adverse effects of the Moderna COVID-19 Vaccine include injection-site reactions, such as pain, induration, and erythema. Less frequently reported dermatologic adverse effects include diffuse bullous rash and hypersensitivity reactions.1 We report a case of reactivation of a BCG vaccination scar after the first dose of the Moderna COVID-19 Vaccine.
Case Report
A 48-year-old Asian man who was otherwise healthy presented with erythema, induration, and mild pruritus on the deltoid muscle of the left arm, near the scar from an earlier BCG vaccine, which he received at approximately 5 years of age when living in Taiwan. The patient received the first dose of the Moderna COVID-19 Vaccine approximately 5 to 7 cm distant from the BCG vaccination scar. One to 2 days after inoculation, the patient endorsed tenderness at the site of COVID-19 vaccination but denied systemic symptoms. He had never been given a diagnosis of COVID-19. His SARS-CoV-2 antibody status was unknown.
Eight days later, the patient noticed a well-defined, erythematous, indurated plaque with mild itchiness overlying and around the BCG vaccination scar that did not involve the COVID-19 vaccination site. The following day, the redness and induration became worse (Figure).

The patient was otherwise well. Vital signs were normal; there was no lymphadenopathy. The rash resolved without treatment over the next 4 days.
Comment
The BCG vaccine is an intradermal live attenuated virus vaccine used to prevent certain forms of tuberculosis and potentially other Mycobacterium infections. Although the vaccine is not routinely administered in the United States, it is part of the vaccination schedule in most countries, administered most often to newborns and infants. Administration of the BCG vaccine commonly results in mild localized erythema, swelling, and pain at the injection site. Most inoculated patients also develop an ulcer that heals with the characteristic BCG vaccination scar.2,3
There is evidence that the BCG vaccine can enhance the innate immune system response and might decrease the rate of infection by unrelated pathogens, including viruses.4 Several epidemiologic studies have suggested that the BCG vaccine might offer some protection against COVID-19, possibly due to a resemblance of the amino acid sequences of BCG and SARS-CoV-2, which might provoke cross-reactive T cells.5,6 Further studies are underway to determine whether the BCG vaccine is truly protective against COVID-19.
BCG vaccination scar reactivation presents as redness, swelling, or ulceration at the BCG injection site months to years after inoculation. Although erythema and induration of the BCG scar are not included in the diagnostic criteria of Kawasaki disease, likely due to variable vaccine requirements in different countries, these findings are largely recognized as specific for Kawasaki disease and present in approximately half of affected patients who received the BCG vaccine.2
Heat Shock Proteins—Heat shock proteins (HSPs) are produced by cells in response to stressors. The proposed mechanism of BCG vaccination scar reactivation is a cross-reaction between human homologue HSP 63 and Mycobacterium HSP 65, leading to hyperactivity of the immune system against BCG.7 There also are reports of reactivation of a BCG vaccination scar from measles infection and influenza vaccination.2,8,9 Most prior reports of BCG vaccination scar reactivation have been in pediatric patients; our patient is an adult who received the BCG vaccine more than 40 years ago.
Mechanism of Reactivation—The mechanism of BCG vaccination scar reactivation in our patient, who received the Moderna COVID-19 Vaccine, is unclear. Possible mechanisms include (1) release of HSP mediated by the COVID-19 vaccine, leading to an immune response at the BCG vaccine scar, or (2) another immune-mediated cross-reaction between BCG and the Moderna COVID-19 Vaccine mRNA nanoparticle or encoded spike protein antigen. It has been hypothesized that the BCG vaccine might offer some protection against COVID-19; this remains uncertain and is under further investigation.10 A recent retrospective cohort study showed that a BCG vaccination booster may decrease COVID-19 infection rates in higher-risk populations.11
Conclusion
We present a case of BCG vaccine scar reactivation occurring after a dose of the Moderna COVID-19 Vaccine, a likely underreported, self-limiting, cutaneous adverse effect of this mRNA vaccine.
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
- Baden LR, El Sahly HM, Essink B, et al; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403-416. doi:10.1056/NEJMoa2035389
- Muthuvelu S, Lim KS, Huang L-Y, et al. Measles infection causing bacillus Calmette-Guérin reactivation: a case report. BMC Pediatr. 2019;19:251. doi:10.1186/s12887-019-1635-z
- Fatima S, Kumari A, Das G, et al. Tuberculosis vaccine: a journey from BCG to present. Life Sci. 2020;252:117594. doi:10.1016/j.lfs.2020.117594
- O’Neill LAJ, Netea MG. BCG-induced trained immunity: can it offer protection against COVID-19? Nat Rev Immunol. 2020;20:335-337. doi:10.1038/s41577-020-0337-y
- Brooks NA, Puri A, Garg S, et al. The association of coronavirus disease-19 mortality and prior bacille Calmette-Guérin vaccination: a robust ecological analysis using unsupervised machine learning. Sci Rep. 2021;11:774. doi:10.1038/s41598-020-80787-z
- Tomita Y, Sato R, Ikeda T, et al. BCG vaccine may generate cross-reactive T-cells against SARS-CoV-2: in silico analyses and a hypothesis. Vaccine. 2020;38:6352-6356. doi:10.1016/j.vaccine.2020.08.045
- Lim KYY, Chua MC, Tan NWH, et al. Reactivation of BCG inoculation site in a child with febrile exanthema of 3 days duration: an early indicator of incomplete Kawasaki disease. BMJ Case Rep. 2020;13:E239648. doi:10.1136/bcr-2020-239648
- Kondo M, Goto H, Yamamoto S. First case of redness and erosion at bacillus Calmette-Guérin inoculation site after vaccination against influenza. J Dermatol. 2016;43:1229-1231. doi:10.1111/1346-8138.13365
- Chavarri-Guerra Y, Soto-Pérez-de-Celis E. Erythema at the bacillus Calmette-Guerin scar after influenza vaccination. Rev Soc Bras Med Trop. 2019;53:E20190390. doi:10.1590/0037-8682-0390-2019
- Fu W, Ho P-C, Liu C-L, et al. Reconcile the debate over protective effects of BCG vaccine against COVID-19. Sci Rep. 2021;11:8356. doi:10.1038/s41598-021-87731-9
- Amirlak L, Haddad R, Hardy JD, et al. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum Vaccin Immunother. 2021;17:3913-3915. doi:10.1080/21645515.2021.1956228
Practice Points
- BCG vaccination scar reactivation is a potential benign, self-limited reaction in patients who receive the Moderna COVID-19 Vaccine.
- Symptoms of BCG vaccination scar reactivation, which is seen more commonly in children with Kawasaki disease, include redness, swelling, and ulceration.
NY radiation oncologist loses license, poses ‘potential danger’
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
The state Board for Professional Medical Conduct has revoked the medical license of Won Sam Yi, MD, following a lengthy review of the care he provided to seven cancer patients; six of them died.
“He is a danger to potential new patients should he be reinstated as a radiation oncologist,” board members wrote, according to a news report in the Buffalo News.
Dr. Yi’s lawyer said that he is appealing the decision.
Dr. Yi was the former CEO of the now-defunct private cancer practice CCS Oncology, located in western New York.
In 2018, the state health department brought numerous charges of professional misconduct against Dr. Yi, including charges that he had failed to “account for prior doses of radiotherapy” as well as exceeding “appropriate tissue tolerances” during the treatment.
Now, the state’s Board for Professional Medical Conduct has upheld nearly all of the departmental charges that had been levied against him, and also found that Dr. Yi failed to take responsibility or show contrition for his treatment decisions.
However, whistleblower claims from a former CSS Oncology employee were dismissed.
Troubled history
CCS Oncology was once one of the largest private cancer practices in Erie and Niagara counties, both in the Buffalo metropolitan area.
Dr. Yi purchased CCS Oncology in 2008 and was its sole shareholder, and in 2012 he also acquired CCS Medical. As of 2016, the practices provided care to about 30% of cancer patients in the region. CCS also began acquiring other practices as it expanded into noncancer specialties, including primary care.
However, CCS began to struggle financially in late 2016, when health insurance provider Independent Health announced it was removing CCS Oncology from its network, and several vendors and lenders subsequently sued CCS and Dr. Yi for nonpayment.
The announcement from Independent Health was “financially devastating to CCS,” and also was “the direct cause” of the practice defaulting on its Bank of America loan and of the practice’s inability to pay not only its vendors but state and federal tax agencies, the Buffalo News reported. As a result, several vendors and lenders had sued CCS and Dr. Yi for nonpayment.
The FBI raided numerous CCS locations in March 2018, seizing financial and other data as part of an investigation into possible Medicare billing fraud. The following month, CCS filed for Chapter 11 bankruptcy, citing it owed millions of dollars to Bank of America and other creditors. Shortly afterward, the practice closed.
Medical misconduct
The state’s charges of professional misconduct accused Dr. Yi of “gross negligence,” “gross incompetence,” and several other cases of misconduct in treating seven patients between 2009 and 2013 at various CCS locations. The patients ranged in age from 27 to 72. Six of the seven patients died.
In one case, Dr. Yi was accused of providing whole-brain radiation therapy to a 43-year-old woman for about 6 weeks in 2012, but the treatment was “contrary to medical indications” and did not take into account prior doses of such treatment. The patient died in December of that year, and the board concluded that Dr. Yi had improperly treated her with a high dose of radiation that was intended to cure her cancer even though she was at a stage where her disease was incurable.
The state board eventually concluded that for all but one of the patients in question, Dr. Yi was guilty of misconduct in his treatment decisions. They wrote that Dr. Yi had frequently administered radiation doses without taking into account how much radiation therapy the patients had received previously and without considering the risk of serious complications for them.
Dr. Yi plans to appeal the board’s decision in state court, according to his attorney, Anthony Scher.
“Dr Yi has treated over 10,000 patients in his career,” Mr. Scher told the Buffalo News. “These handful of cases don’t represent the thousands of success stories that he’s had.”
A version of this article first appeared on Medscape.com.
Why pregnant people were left behind while vaccines moved at ‘warp speed’ to help the masses
Kia Slade was 7 months pregnant, unvaccinated, and fighting for breath, her oxygen levels plummeting, when her son came into the world last May.
A severe case of COVID-19 pneumonia had left Ms. Slade delirious. When the intensive care team tried to place an oxygen mask on her face, she snatched it away, she recalled. Her baby’s heart rate began to drop.
Ms. Slade’s doctor performed an emergency cesarean section at her bedside in the intensive care unit, delivering baby Tristan 10 weeks early. He weighed just 2 pounds, 14 ounces, about half the size of small full-term baby.
But Ms. Slade wouldn’t meet him until July. She was on a ventilator in a medically-induced coma for 8 weeks, and she developed a serious infection and blood clot while unconscious. It was only after a perilous 2½ months in the hospital, during which her heart stopped twice, that Ms. Slade was vaccinated against COVID-19.
“I wish I had gotten the vaccine earlier,” said Ms. Slade, 42, who remains too sick to return to work as a special education teacher in Baltimore. Doctors “kept pushing me to get vaccinated, but there just wasn’t enough information out there for me to do it.”
A year ago, there was little to no vaccine safety data for pregnant people like Ms. Slade, because they had been excluded from clinical trials run by Pfizer, Moderna, and other vaccine makers.
Lacking data, health experts were unsure and divided about how to advise expectant parents. Although U.S. health officials permitted pregnant people to be vaccinated, the World Health Organization in January 2021 actually discouraged them from doing so; it later reversed that recommendation.
The uncertainty led many women to delay vaccination, and only about two-thirds of the pregnant people who have been tracked by the Centers for Disease Control and Prevention were fully vaccinated as of Feb. 5, 2022, leaving many expectant moms at a high risk of infection and life-threatening complications.
More than 29,000 pregnant people have been hospitalized with COVID-19 and 274 have died, according to the CDC.
“There were surely women who were hospitalized because there wasn’t information available to them,” said Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Vaccine developers say that pregnant people – who have special health needs and risks – were excluded from clinical trials to protect them from potential side effects of novel technologies, including the Pfizer and Moderna mRNA vaccines and formulations made with cold viruses, such as the Johnson & Johnson vaccine.
But a KHN analysis also shows that pregnant people were left behind because including them in vaccine studies would have complicated and potentially delayed the delivery of COVID-19 vaccines to the broader population.
A growing number of women’s health researchers and advocates say that excluding pregnant people – and the months-long delay in recommending that they be immunized – helped fuel widespread vaccine hesitancy in this vulnerable group.
“Women and their unborn fetuses are dying of COVID infection,” said Jane Van Dis, MD, an ob.gyn. at the University of Rochester (N.Y.) Medical Center who has treated many patients like Ms. Slade. “Our failure as a society to vaccinate women in pregnancy will be remembered by the children and families who lost their mothers to this disease.”
New technology, uncertain risks
At the time COVID-19 vaccines were being developed, scientists had very little experience using mRNA vaccines in pregnant women, said Jacqueline Miller, MD, a senior vice president involved in vaccine research at Moderna.
“When you study anything in pregnant women, you have two patients, the mom and the unborn child,” Dr. Miller said. “Until we had more safety data on the platform, it wasn’t something we wanted to undertake.”
But Dr. Offit noted that vaccines have a strong record of safety in pregnancy and he sees no reason to have excluded pregnant people. None of the vaccines currently in use – including the chickenpox and rubella vaccines, which contain live viruses – have been shown to harm fetuses, he said. Doctors routinely recommend that pregnant people receive pertussis and flu vaccinations.
Dr. Offit, the coinventor of a rotavirus vaccine, said that some concerns about vaccines stem from commercial, not medical, interests. Drug makers don’t want to risk that their product will be blamed for any problems occurring in pregnant people, even if coincidental, he said.
“These companies don’t want bad news,” Dr. Offit said.
In the United States, health officials typically would have told expectant mothers not to take a vaccine that was untested during pregnancy, said Dr. Offit, a member of a committee that advises the Food and Drug Administration on vaccines.
Due to the urgency of the pandemic, health agencies instead permitted pregnant people to make up their own minds about vaccines without recommending them.
Women’s medical associations were also hampered by the lack of data. Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine actively encouraged pregnant people to be vaccinated until July 30, 2021, after the first real-world vaccine studies had been published. The CDC followed suit in August of 2021.
“If we had had this data in the beginning, we would have been able to vaccinate more women,” said Kelli Burroughs, MD, the department chair of obstetrics and gynecology at Memorial Hermann Sugar Land Hospital near Houston.
Yet anti-vaccine groups wasted no time in scaring pregnant people, flooding social media with misinformation about impaired fertility and harm to the fetus.
In the first few months after the COVID-19 vaccines were approved, some doctors were ambivalent about recommending them, and some still advise pregnant patients against vaccination.
An estimated 67% of pregnant people today are fully vaccinated, compared with about 89% of people 65 and older, another high-risk group, and 65% of Americans overall. Vaccination rates are lower among minorities, with 65% of expectant Hispanic mothers and 53% of pregnant African Americans fully vaccinated, according to the CDC.
Vaccination is especially important during pregnancy, because of increased risks of hospitalization, ICU admission, and mechanical ventilation, Dr. Burroughs said. A study released in February from the National Institutes of Health found that pregnant people with a moderate to severe COVID-19 infection also were more likely to have a C-section, deliver preterm, or develop a postpartum hemorrhage.
Black moms such as Ms. Slade were already at higher risk of maternal and infant mortality before the pandemic, because of higher underlying risks, unequal access to health care, and other factors. COVID-19 has magnified those risks, said Dr. Burroughs, who has persuaded reluctant patients by revealing that she had a healthy pregnancy and child after being vaccinated.
Ms. Slade said she has never opposed vaccines and had no hesitation about receiving other vaccines while pregnant. But she said she “just wasn’t comfortable” with COVID-19 shots.
“If there had been data out there saying the COVID shot was safe, and that nothing would happen to my baby and there was no risk of birth defects, I would have taken it,” said Ms. Slade, who has had type 2 diabetes for 12 years.
Working at warp speed
Government scientists at the NIH were concerned about the risk of COVID-19 to pregnant people from the very beginning and knew that expectant moms needed vaccines as much or more than anyone else, said Larry Corey, MD, a leader of the COVID-19 Prevention Network, which coordinated COVID-19 vaccine trials for the federal government.
But including pregnant volunteers in the larger vaccine trials could have led to interruptions and delays, Dr. Corey said. Researchers would have had to enroll thousands of pregnant volunteers to achieve statistically robust results that weren’t due to chance, he said.
Pregnancy can bring on a wide range of complications: gestational diabetes, hypertension, anemia, bleeding, blood clots, or problems with the placenta, for example. Up to 20% of people who know they’re pregnant miscarry. Because researchers would have been obliged to investigate any medical problem to make sure it wasn’t caused by one of the COVID-19 vaccines, including pregnant people might have meant having to hit pause on those trials, Dr. Corey said.
With death tolls from the pandemic mounting, “we had a mission to do this as quickly and as thoroughly as possible,” Dr. Corey said. Making COVID-19 vaccines available within a year “saved hundreds of thousands of lives.”
The first data on COVID-19 vaccine safety in pregnancy was published in April of 2021 when the CDC released an analysis of nearly 36,000 vaccinated pregnant people who had enrolled in a registry called V-safe, which allows users to log the dates of their vaccinations and any subsequent symptoms.
Later research showed that COVID-19 vaccines weren’t associated with increased risk of miscarriage or premature delivery.
Brenna Hughes, MD, a maternal-fetal medicine specialist and member of the American College of Obstetricians and Gynecologists’ COVID-19 expert group, agrees that adding pregnant people to large-scale COVID-19 vaccine and drug trials may have been impractical. But researchers could have launched parallel trials of pregnant women, once early studies showed the vaccines were safe in humans, she said.
“Would it have been hard? Everything with COVID is hard,” Dr. Hughes said. “But it would have been feasible.”
The FDA requires that researchers perform additional animal studies – called developmental and reproductive toxicity studies – before testing vaccines in pregnant people. Although these studies are essential, they take 5-6 months, and weren’t completed until late 2020, around the time the first COVID-19 vaccines were authorized for adults, said Emily Erbelding, MD, director of microbiology and infectious diseases at the National Institute of Allergy and Infectious Diseases, part of the NIH.
Pregnancy studies “were an afterthought,” said Irina Burd, MD, director of Johns Hopkins’ Integrated Research Center for Fetal Medicine and a professor of gynecology and obstetrics. “They should have been done sooner.”
The NIH is conducting a study of pregnant and postpartum people who decided on their own to be vaccinated, Dr. Erbelding said. The study is due to be completed by July 2023.
Janssen and Moderna are also conducting studies in pregnant people, both due to be completed in 2024.
Pfizer scientists encountered problems when they initiated a clinical trial, which would have randomly assigned pregnant people to receive either a vaccine or placebo. Once vaccines were widely available, many patients weren’t willing to take a chance on being unvaccinated until after delivery.
Pfizer has stopped recruiting patients and has not said whether it will publicly report any data from the trial.
Dr. Hughes said vaccine developers need to include pregnant people from the very beginning.
“There is this notion of protecting pregnant people from research,” Dr. Hughes said. “But we should be protecting patients through research, not from research.”
Recovering physically and emotionally
Ms. Slade still regrets being deprived of time with her children while she fought the disease.
Being on a ventilator kept her from spending those early weeks with her newborn, or from seeing her 9-year-old daughter, Zoe.
Even when Ms. Slade was finally able to see her son, she wasn’t able to tell him she loved him or sing a lullaby, or even talk at all, because of a breathing tube in her throat.
Today, Ms. Slade is a strong advocate of COVID-19 vaccinations, urging her friends and family to get their shots to avoid suffering the way she has.
Ms. Slade had to relearn to walk after being bedridden for weeks. Her many weeks on a ventilator may have contributed to her stomach paralysis, which often causes intense pain, nausea, and even vomiting when she eats or drinks. Ms. Slade weighs 50 pounds less today than before she became pregnant and has resorted to going to the emergency department when the pain is unbearable. “Most days, I’m just miserable,” she said.
Her family suffered as well. Like many babies born prematurely, Tristan, now nearly 9 months old and crawling, receives physical therapy to strengthen his muscles. At 15 pounds, Tristan is largely healthy, although his doctor said he has symptoms of asthma.
Ms. Slade said she would like to attend family counseling with Zoe, who rarely complains and tends to keep her feelings to herself. Ms. Slade said she knows her illness must have been terrifying for her little girl.
“The other day she was talking to me,” Ms. Slade said, “and she said, ‘You know, I almost had to bury you.’ ”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Kia Slade was 7 months pregnant, unvaccinated, and fighting for breath, her oxygen levels plummeting, when her son came into the world last May.
A severe case of COVID-19 pneumonia had left Ms. Slade delirious. When the intensive care team tried to place an oxygen mask on her face, she snatched it away, she recalled. Her baby’s heart rate began to drop.
Ms. Slade’s doctor performed an emergency cesarean section at her bedside in the intensive care unit, delivering baby Tristan 10 weeks early. He weighed just 2 pounds, 14 ounces, about half the size of small full-term baby.
But Ms. Slade wouldn’t meet him until July. She was on a ventilator in a medically-induced coma for 8 weeks, and she developed a serious infection and blood clot while unconscious. It was only after a perilous 2½ months in the hospital, during which her heart stopped twice, that Ms. Slade was vaccinated against COVID-19.
“I wish I had gotten the vaccine earlier,” said Ms. Slade, 42, who remains too sick to return to work as a special education teacher in Baltimore. Doctors “kept pushing me to get vaccinated, but there just wasn’t enough information out there for me to do it.”
A year ago, there was little to no vaccine safety data for pregnant people like Ms. Slade, because they had been excluded from clinical trials run by Pfizer, Moderna, and other vaccine makers.
Lacking data, health experts were unsure and divided about how to advise expectant parents. Although U.S. health officials permitted pregnant people to be vaccinated, the World Health Organization in January 2021 actually discouraged them from doing so; it later reversed that recommendation.
The uncertainty led many women to delay vaccination, and only about two-thirds of the pregnant people who have been tracked by the Centers for Disease Control and Prevention were fully vaccinated as of Feb. 5, 2022, leaving many expectant moms at a high risk of infection and life-threatening complications.
More than 29,000 pregnant people have been hospitalized with COVID-19 and 274 have died, according to the CDC.
“There were surely women who were hospitalized because there wasn’t information available to them,” said Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Vaccine developers say that pregnant people – who have special health needs and risks – were excluded from clinical trials to protect them from potential side effects of novel technologies, including the Pfizer and Moderna mRNA vaccines and formulations made with cold viruses, such as the Johnson & Johnson vaccine.
But a KHN analysis also shows that pregnant people were left behind because including them in vaccine studies would have complicated and potentially delayed the delivery of COVID-19 vaccines to the broader population.
A growing number of women’s health researchers and advocates say that excluding pregnant people – and the months-long delay in recommending that they be immunized – helped fuel widespread vaccine hesitancy in this vulnerable group.
“Women and their unborn fetuses are dying of COVID infection,” said Jane Van Dis, MD, an ob.gyn. at the University of Rochester (N.Y.) Medical Center who has treated many patients like Ms. Slade. “Our failure as a society to vaccinate women in pregnancy will be remembered by the children and families who lost their mothers to this disease.”
New technology, uncertain risks
At the time COVID-19 vaccines were being developed, scientists had very little experience using mRNA vaccines in pregnant women, said Jacqueline Miller, MD, a senior vice president involved in vaccine research at Moderna.
“When you study anything in pregnant women, you have two patients, the mom and the unborn child,” Dr. Miller said. “Until we had more safety data on the platform, it wasn’t something we wanted to undertake.”
But Dr. Offit noted that vaccines have a strong record of safety in pregnancy and he sees no reason to have excluded pregnant people. None of the vaccines currently in use – including the chickenpox and rubella vaccines, which contain live viruses – have been shown to harm fetuses, he said. Doctors routinely recommend that pregnant people receive pertussis and flu vaccinations.
Dr. Offit, the coinventor of a rotavirus vaccine, said that some concerns about vaccines stem from commercial, not medical, interests. Drug makers don’t want to risk that their product will be blamed for any problems occurring in pregnant people, even if coincidental, he said.
“These companies don’t want bad news,” Dr. Offit said.
In the United States, health officials typically would have told expectant mothers not to take a vaccine that was untested during pregnancy, said Dr. Offit, a member of a committee that advises the Food and Drug Administration on vaccines.
Due to the urgency of the pandemic, health agencies instead permitted pregnant people to make up their own minds about vaccines without recommending them.
Women’s medical associations were also hampered by the lack of data. Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine actively encouraged pregnant people to be vaccinated until July 30, 2021, after the first real-world vaccine studies had been published. The CDC followed suit in August of 2021.
“If we had had this data in the beginning, we would have been able to vaccinate more women,” said Kelli Burroughs, MD, the department chair of obstetrics and gynecology at Memorial Hermann Sugar Land Hospital near Houston.
Yet anti-vaccine groups wasted no time in scaring pregnant people, flooding social media with misinformation about impaired fertility and harm to the fetus.
In the first few months after the COVID-19 vaccines were approved, some doctors were ambivalent about recommending them, and some still advise pregnant patients against vaccination.
An estimated 67% of pregnant people today are fully vaccinated, compared with about 89% of people 65 and older, another high-risk group, and 65% of Americans overall. Vaccination rates are lower among minorities, with 65% of expectant Hispanic mothers and 53% of pregnant African Americans fully vaccinated, according to the CDC.
Vaccination is especially important during pregnancy, because of increased risks of hospitalization, ICU admission, and mechanical ventilation, Dr. Burroughs said. A study released in February from the National Institutes of Health found that pregnant people with a moderate to severe COVID-19 infection also were more likely to have a C-section, deliver preterm, or develop a postpartum hemorrhage.
Black moms such as Ms. Slade were already at higher risk of maternal and infant mortality before the pandemic, because of higher underlying risks, unequal access to health care, and other factors. COVID-19 has magnified those risks, said Dr. Burroughs, who has persuaded reluctant patients by revealing that she had a healthy pregnancy and child after being vaccinated.
Ms. Slade said she has never opposed vaccines and had no hesitation about receiving other vaccines while pregnant. But she said she “just wasn’t comfortable” with COVID-19 shots.
“If there had been data out there saying the COVID shot was safe, and that nothing would happen to my baby and there was no risk of birth defects, I would have taken it,” said Ms. Slade, who has had type 2 diabetes for 12 years.
Working at warp speed
Government scientists at the NIH were concerned about the risk of COVID-19 to pregnant people from the very beginning and knew that expectant moms needed vaccines as much or more than anyone else, said Larry Corey, MD, a leader of the COVID-19 Prevention Network, which coordinated COVID-19 vaccine trials for the federal government.
But including pregnant volunteers in the larger vaccine trials could have led to interruptions and delays, Dr. Corey said. Researchers would have had to enroll thousands of pregnant volunteers to achieve statistically robust results that weren’t due to chance, he said.
Pregnancy can bring on a wide range of complications: gestational diabetes, hypertension, anemia, bleeding, blood clots, or problems with the placenta, for example. Up to 20% of people who know they’re pregnant miscarry. Because researchers would have been obliged to investigate any medical problem to make sure it wasn’t caused by one of the COVID-19 vaccines, including pregnant people might have meant having to hit pause on those trials, Dr. Corey said.
With death tolls from the pandemic mounting, “we had a mission to do this as quickly and as thoroughly as possible,” Dr. Corey said. Making COVID-19 vaccines available within a year “saved hundreds of thousands of lives.”
The first data on COVID-19 vaccine safety in pregnancy was published in April of 2021 when the CDC released an analysis of nearly 36,000 vaccinated pregnant people who had enrolled in a registry called V-safe, which allows users to log the dates of their vaccinations and any subsequent symptoms.
Later research showed that COVID-19 vaccines weren’t associated with increased risk of miscarriage or premature delivery.
Brenna Hughes, MD, a maternal-fetal medicine specialist and member of the American College of Obstetricians and Gynecologists’ COVID-19 expert group, agrees that adding pregnant people to large-scale COVID-19 vaccine and drug trials may have been impractical. But researchers could have launched parallel trials of pregnant women, once early studies showed the vaccines were safe in humans, she said.
“Would it have been hard? Everything with COVID is hard,” Dr. Hughes said. “But it would have been feasible.”
The FDA requires that researchers perform additional animal studies – called developmental and reproductive toxicity studies – before testing vaccines in pregnant people. Although these studies are essential, they take 5-6 months, and weren’t completed until late 2020, around the time the first COVID-19 vaccines were authorized for adults, said Emily Erbelding, MD, director of microbiology and infectious diseases at the National Institute of Allergy and Infectious Diseases, part of the NIH.
Pregnancy studies “were an afterthought,” said Irina Burd, MD, director of Johns Hopkins’ Integrated Research Center for Fetal Medicine and a professor of gynecology and obstetrics. “They should have been done sooner.”
The NIH is conducting a study of pregnant and postpartum people who decided on their own to be vaccinated, Dr. Erbelding said. The study is due to be completed by July 2023.
Janssen and Moderna are also conducting studies in pregnant people, both due to be completed in 2024.
Pfizer scientists encountered problems when they initiated a clinical trial, which would have randomly assigned pregnant people to receive either a vaccine or placebo. Once vaccines were widely available, many patients weren’t willing to take a chance on being unvaccinated until after delivery.
Pfizer has stopped recruiting patients and has not said whether it will publicly report any data from the trial.
Dr. Hughes said vaccine developers need to include pregnant people from the very beginning.
“There is this notion of protecting pregnant people from research,” Dr. Hughes said. “But we should be protecting patients through research, not from research.”
Recovering physically and emotionally
Ms. Slade still regrets being deprived of time with her children while she fought the disease.
Being on a ventilator kept her from spending those early weeks with her newborn, or from seeing her 9-year-old daughter, Zoe.
Even when Ms. Slade was finally able to see her son, she wasn’t able to tell him she loved him or sing a lullaby, or even talk at all, because of a breathing tube in her throat.
Today, Ms. Slade is a strong advocate of COVID-19 vaccinations, urging her friends and family to get their shots to avoid suffering the way she has.
Ms. Slade had to relearn to walk after being bedridden for weeks. Her many weeks on a ventilator may have contributed to her stomach paralysis, which often causes intense pain, nausea, and even vomiting when she eats or drinks. Ms. Slade weighs 50 pounds less today than before she became pregnant and has resorted to going to the emergency department when the pain is unbearable. “Most days, I’m just miserable,” she said.
Her family suffered as well. Like many babies born prematurely, Tristan, now nearly 9 months old and crawling, receives physical therapy to strengthen his muscles. At 15 pounds, Tristan is largely healthy, although his doctor said he has symptoms of asthma.
Ms. Slade said she would like to attend family counseling with Zoe, who rarely complains and tends to keep her feelings to herself. Ms. Slade said she knows her illness must have been terrifying for her little girl.
“The other day she was talking to me,” Ms. Slade said, “and she said, ‘You know, I almost had to bury you.’ ”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Kia Slade was 7 months pregnant, unvaccinated, and fighting for breath, her oxygen levels plummeting, when her son came into the world last May.
A severe case of COVID-19 pneumonia had left Ms. Slade delirious. When the intensive care team tried to place an oxygen mask on her face, she snatched it away, she recalled. Her baby’s heart rate began to drop.
Ms. Slade’s doctor performed an emergency cesarean section at her bedside in the intensive care unit, delivering baby Tristan 10 weeks early. He weighed just 2 pounds, 14 ounces, about half the size of small full-term baby.
But Ms. Slade wouldn’t meet him until July. She was on a ventilator in a medically-induced coma for 8 weeks, and she developed a serious infection and blood clot while unconscious. It was only after a perilous 2½ months in the hospital, during which her heart stopped twice, that Ms. Slade was vaccinated against COVID-19.
“I wish I had gotten the vaccine earlier,” said Ms. Slade, 42, who remains too sick to return to work as a special education teacher in Baltimore. Doctors “kept pushing me to get vaccinated, but there just wasn’t enough information out there for me to do it.”
A year ago, there was little to no vaccine safety data for pregnant people like Ms. Slade, because they had been excluded from clinical trials run by Pfizer, Moderna, and other vaccine makers.
Lacking data, health experts were unsure and divided about how to advise expectant parents. Although U.S. health officials permitted pregnant people to be vaccinated, the World Health Organization in January 2021 actually discouraged them from doing so; it later reversed that recommendation.
The uncertainty led many women to delay vaccination, and only about two-thirds of the pregnant people who have been tracked by the Centers for Disease Control and Prevention were fully vaccinated as of Feb. 5, 2022, leaving many expectant moms at a high risk of infection and life-threatening complications.
More than 29,000 pregnant people have been hospitalized with COVID-19 and 274 have died, according to the CDC.
“There were surely women who were hospitalized because there wasn’t information available to them,” said Paul A. Offit, MD, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Vaccine developers say that pregnant people – who have special health needs and risks – were excluded from clinical trials to protect them from potential side effects of novel technologies, including the Pfizer and Moderna mRNA vaccines and formulations made with cold viruses, such as the Johnson & Johnson vaccine.
But a KHN analysis also shows that pregnant people were left behind because including them in vaccine studies would have complicated and potentially delayed the delivery of COVID-19 vaccines to the broader population.
A growing number of women’s health researchers and advocates say that excluding pregnant people – and the months-long delay in recommending that they be immunized – helped fuel widespread vaccine hesitancy in this vulnerable group.
“Women and their unborn fetuses are dying of COVID infection,” said Jane Van Dis, MD, an ob.gyn. at the University of Rochester (N.Y.) Medical Center who has treated many patients like Ms. Slade. “Our failure as a society to vaccinate women in pregnancy will be remembered by the children and families who lost their mothers to this disease.”
New technology, uncertain risks
At the time COVID-19 vaccines were being developed, scientists had very little experience using mRNA vaccines in pregnant women, said Jacqueline Miller, MD, a senior vice president involved in vaccine research at Moderna.
“When you study anything in pregnant women, you have two patients, the mom and the unborn child,” Dr. Miller said. “Until we had more safety data on the platform, it wasn’t something we wanted to undertake.”
But Dr. Offit noted that vaccines have a strong record of safety in pregnancy and he sees no reason to have excluded pregnant people. None of the vaccines currently in use – including the chickenpox and rubella vaccines, which contain live viruses – have been shown to harm fetuses, he said. Doctors routinely recommend that pregnant people receive pertussis and flu vaccinations.
Dr. Offit, the coinventor of a rotavirus vaccine, said that some concerns about vaccines stem from commercial, not medical, interests. Drug makers don’t want to risk that their product will be blamed for any problems occurring in pregnant people, even if coincidental, he said.
“These companies don’t want bad news,” Dr. Offit said.
In the United States, health officials typically would have told expectant mothers not to take a vaccine that was untested during pregnancy, said Dr. Offit, a member of a committee that advises the Food and Drug Administration on vaccines.
Due to the urgency of the pandemic, health agencies instead permitted pregnant people to make up their own minds about vaccines without recommending them.
Women’s medical associations were also hampered by the lack of data. Neither the American College of Obstetricians and Gynecologists nor the Society for Maternal-Fetal Medicine actively encouraged pregnant people to be vaccinated until July 30, 2021, after the first real-world vaccine studies had been published. The CDC followed suit in August of 2021.
“If we had had this data in the beginning, we would have been able to vaccinate more women,” said Kelli Burroughs, MD, the department chair of obstetrics and gynecology at Memorial Hermann Sugar Land Hospital near Houston.
Yet anti-vaccine groups wasted no time in scaring pregnant people, flooding social media with misinformation about impaired fertility and harm to the fetus.
In the first few months after the COVID-19 vaccines were approved, some doctors were ambivalent about recommending them, and some still advise pregnant patients against vaccination.
An estimated 67% of pregnant people today are fully vaccinated, compared with about 89% of people 65 and older, another high-risk group, and 65% of Americans overall. Vaccination rates are lower among minorities, with 65% of expectant Hispanic mothers and 53% of pregnant African Americans fully vaccinated, according to the CDC.
Vaccination is especially important during pregnancy, because of increased risks of hospitalization, ICU admission, and mechanical ventilation, Dr. Burroughs said. A study released in February from the National Institutes of Health found that pregnant people with a moderate to severe COVID-19 infection also were more likely to have a C-section, deliver preterm, or develop a postpartum hemorrhage.
Black moms such as Ms. Slade were already at higher risk of maternal and infant mortality before the pandemic, because of higher underlying risks, unequal access to health care, and other factors. COVID-19 has magnified those risks, said Dr. Burroughs, who has persuaded reluctant patients by revealing that she had a healthy pregnancy and child after being vaccinated.
Ms. Slade said she has never opposed vaccines and had no hesitation about receiving other vaccines while pregnant. But she said she “just wasn’t comfortable” with COVID-19 shots.
“If there had been data out there saying the COVID shot was safe, and that nothing would happen to my baby and there was no risk of birth defects, I would have taken it,” said Ms. Slade, who has had type 2 diabetes for 12 years.
Working at warp speed
Government scientists at the NIH were concerned about the risk of COVID-19 to pregnant people from the very beginning and knew that expectant moms needed vaccines as much or more than anyone else, said Larry Corey, MD, a leader of the COVID-19 Prevention Network, which coordinated COVID-19 vaccine trials for the federal government.
But including pregnant volunteers in the larger vaccine trials could have led to interruptions and delays, Dr. Corey said. Researchers would have had to enroll thousands of pregnant volunteers to achieve statistically robust results that weren’t due to chance, he said.
Pregnancy can bring on a wide range of complications: gestational diabetes, hypertension, anemia, bleeding, blood clots, or problems with the placenta, for example. Up to 20% of people who know they’re pregnant miscarry. Because researchers would have been obliged to investigate any medical problem to make sure it wasn’t caused by one of the COVID-19 vaccines, including pregnant people might have meant having to hit pause on those trials, Dr. Corey said.
With death tolls from the pandemic mounting, “we had a mission to do this as quickly and as thoroughly as possible,” Dr. Corey said. Making COVID-19 vaccines available within a year “saved hundreds of thousands of lives.”
The first data on COVID-19 vaccine safety in pregnancy was published in April of 2021 when the CDC released an analysis of nearly 36,000 vaccinated pregnant people who had enrolled in a registry called V-safe, which allows users to log the dates of their vaccinations and any subsequent symptoms.
Later research showed that COVID-19 vaccines weren’t associated with increased risk of miscarriage or premature delivery.
Brenna Hughes, MD, a maternal-fetal medicine specialist and member of the American College of Obstetricians and Gynecologists’ COVID-19 expert group, agrees that adding pregnant people to large-scale COVID-19 vaccine and drug trials may have been impractical. But researchers could have launched parallel trials of pregnant women, once early studies showed the vaccines were safe in humans, she said.
“Would it have been hard? Everything with COVID is hard,” Dr. Hughes said. “But it would have been feasible.”
The FDA requires that researchers perform additional animal studies – called developmental and reproductive toxicity studies – before testing vaccines in pregnant people. Although these studies are essential, they take 5-6 months, and weren’t completed until late 2020, around the time the first COVID-19 vaccines were authorized for adults, said Emily Erbelding, MD, director of microbiology and infectious diseases at the National Institute of Allergy and Infectious Diseases, part of the NIH.
Pregnancy studies “were an afterthought,” said Irina Burd, MD, director of Johns Hopkins’ Integrated Research Center for Fetal Medicine and a professor of gynecology and obstetrics. “They should have been done sooner.”
The NIH is conducting a study of pregnant and postpartum people who decided on their own to be vaccinated, Dr. Erbelding said. The study is due to be completed by July 2023.
Janssen and Moderna are also conducting studies in pregnant people, both due to be completed in 2024.
Pfizer scientists encountered problems when they initiated a clinical trial, which would have randomly assigned pregnant people to receive either a vaccine or placebo. Once vaccines were widely available, many patients weren’t willing to take a chance on being unvaccinated until after delivery.
Pfizer has stopped recruiting patients and has not said whether it will publicly report any data from the trial.
Dr. Hughes said vaccine developers need to include pregnant people from the very beginning.
“There is this notion of protecting pregnant people from research,” Dr. Hughes said. “But we should be protecting patients through research, not from research.”
Recovering physically and emotionally
Ms. Slade still regrets being deprived of time with her children while she fought the disease.
Being on a ventilator kept her from spending those early weeks with her newborn, or from seeing her 9-year-old daughter, Zoe.
Even when Ms. Slade was finally able to see her son, she wasn’t able to tell him she loved him or sing a lullaby, or even talk at all, because of a breathing tube in her throat.
Today, Ms. Slade is a strong advocate of COVID-19 vaccinations, urging her friends and family to get their shots to avoid suffering the way she has.
Ms. Slade had to relearn to walk after being bedridden for weeks. Her many weeks on a ventilator may have contributed to her stomach paralysis, which often causes intense pain, nausea, and even vomiting when she eats or drinks. Ms. Slade weighs 50 pounds less today than before she became pregnant and has resorted to going to the emergency department when the pain is unbearable. “Most days, I’m just miserable,” she said.
Her family suffered as well. Like many babies born prematurely, Tristan, now nearly 9 months old and crawling, receives physical therapy to strengthen his muscles. At 15 pounds, Tristan is largely healthy, although his doctor said he has symptoms of asthma.
Ms. Slade said she would like to attend family counseling with Zoe, who rarely complains and tends to keep her feelings to herself. Ms. Slade said she knows her illness must have been terrifying for her little girl.
“The other day she was talking to me,” Ms. Slade said, “and she said, ‘You know, I almost had to bury you.’ ”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Most Americans unaware alcohol can cause cancer
The majority of Americans are not aware that alcohol consumption causes a variety of cancers and especially do not consider wine and beer to have a link with cancer, suggest the results from a national survey.
write lead author Andrew Seidenberg, PhD, MPH, National Cancer Institute, Rockville, Md., and colleagues.
“Increasing awareness of the alcohol-cancer link, such as through multimedia campaigns and patient-provider communication, may be an important new strategy for health advocates working to implement preventive alcohol policies,” they add.
The findings were published in the February issue of the American Journal of Preventive Medicine.
“This is the first study to examine the relationship between alcohol control policy support and awareness of the alcohol-cancer link among a national U.S. sample,” the authors write.
The results show that there is some public support for the idea of adding written warnings about the alcohol-cancer risk to alcoholic beverages, which is something that a number of cancer organizations have been petitioning for.
A petition filed by the American Society of Clinical Oncology, the American Institute for Cancer Research, and Breast Cancer Prevention Partners, all in collaboration with several public health organizations, proposes labeling that would read: “WARNING: According to the Surgeon General, consumption of alcoholic beverages can cause cancer, including breast and colon cancers.”
Such labeling has “the potential to save lives by ensuring that consumers have a more accurate understanding of the link between alcohol and cancer, which will empower them to better protect their health,” the groups said in the petition.
Public support
The findings come from an analysis of the 2020 Health Information National Trends Survey 5 Cycle 4. A total of 3,865 adults participated in the survey, approximately half of whom were nondrinkers.
As well as investigating how aware people were of the alcohol-cancer link, the investigators looked at how prevalent public support might be for the following three communication-focused alcohol policies:
- Banning outdoor alcohol-related advertising
- Requiring health warnings on alcohol beverage containers
- Requiring recommended drinking guidelines on alcoholic beverage containers
“Awareness of the alcohol-cancer link was measured separately for wine, beer, and liquor by asking: In your opinion, how much does drinking the following types of alcohol affect the risk of getting cancer?” the authors explain.
“Awareness of the alcohol-cancer link was low,” the investigators comment; only about one-third (31.8%) of participants were aware that alcohol increases the risk of cancer. The figures were even lower for individual beverage type, at 20.3% for wine, 24.9% for beer, and 31.2% for liquor. Furthermore, approximately half of participants responded with “don’t know” to the three awareness items, investigators noted.
On the other hand, more than half of the Americans surveyed supported adding both health warning labels (65.1%) and information on recommended drinking guidelines (63.9%) to alcoholic beverage containers. Support was lower (34.4% of respondents) for banning outdoor alcohol advertising.
Among Americans who were aware that alcohol increased cancer risk, support was also higher for all three policies.
For example, about 75% of respondents who were aware that alcohol increases cancer risk supported adding health warnings and drinking guidelines to beverage containers, compared with about half of Americans who felt that alcohol consumption had either no effect on or decreased cancer risk.
Even among those who were aware of the alcohol-cancer link, public support for outdoor advertising was not high (37.8%), but it was even lower (23.6%) among respondents who felt alcohol had no effect on or decreased the risk of cancer.
“Policy support was highest among nondrinkers, followed by drinkers, and was lowest among heavier drinkers,” the authors report.
For example, almost 43% of nondrinkers supported restrictions on outdoor alcohol advertising, compared with only about 28.6% of drinkers and 22% of heavier drinkers. More respondents supported adding health warning labels on alcoholic beverages – 70% of nondrinkers, 65% of drinkers, and 57% of heavier drinkers, investigators observe.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The majority of Americans are not aware that alcohol consumption causes a variety of cancers and especially do not consider wine and beer to have a link with cancer, suggest the results from a national survey.
write lead author Andrew Seidenberg, PhD, MPH, National Cancer Institute, Rockville, Md., and colleagues.
“Increasing awareness of the alcohol-cancer link, such as through multimedia campaigns and patient-provider communication, may be an important new strategy for health advocates working to implement preventive alcohol policies,” they add.
The findings were published in the February issue of the American Journal of Preventive Medicine.
“This is the first study to examine the relationship between alcohol control policy support and awareness of the alcohol-cancer link among a national U.S. sample,” the authors write.
The results show that there is some public support for the idea of adding written warnings about the alcohol-cancer risk to alcoholic beverages, which is something that a number of cancer organizations have been petitioning for.
A petition filed by the American Society of Clinical Oncology, the American Institute for Cancer Research, and Breast Cancer Prevention Partners, all in collaboration with several public health organizations, proposes labeling that would read: “WARNING: According to the Surgeon General, consumption of alcoholic beverages can cause cancer, including breast and colon cancers.”
Such labeling has “the potential to save lives by ensuring that consumers have a more accurate understanding of the link between alcohol and cancer, which will empower them to better protect their health,” the groups said in the petition.
Public support
The findings come from an analysis of the 2020 Health Information National Trends Survey 5 Cycle 4. A total of 3,865 adults participated in the survey, approximately half of whom were nondrinkers.
As well as investigating how aware people were of the alcohol-cancer link, the investigators looked at how prevalent public support might be for the following three communication-focused alcohol policies:
- Banning outdoor alcohol-related advertising
- Requiring health warnings on alcohol beverage containers
- Requiring recommended drinking guidelines on alcoholic beverage containers
“Awareness of the alcohol-cancer link was measured separately for wine, beer, and liquor by asking: In your opinion, how much does drinking the following types of alcohol affect the risk of getting cancer?” the authors explain.
“Awareness of the alcohol-cancer link was low,” the investigators comment; only about one-third (31.8%) of participants were aware that alcohol increases the risk of cancer. The figures were even lower for individual beverage type, at 20.3% for wine, 24.9% for beer, and 31.2% for liquor. Furthermore, approximately half of participants responded with “don’t know” to the three awareness items, investigators noted.
On the other hand, more than half of the Americans surveyed supported adding both health warning labels (65.1%) and information on recommended drinking guidelines (63.9%) to alcoholic beverage containers. Support was lower (34.4% of respondents) for banning outdoor alcohol advertising.
Among Americans who were aware that alcohol increased cancer risk, support was also higher for all three policies.
For example, about 75% of respondents who were aware that alcohol increases cancer risk supported adding health warnings and drinking guidelines to beverage containers, compared with about half of Americans who felt that alcohol consumption had either no effect on or decreased cancer risk.
Even among those who were aware of the alcohol-cancer link, public support for outdoor advertising was not high (37.8%), but it was even lower (23.6%) among respondents who felt alcohol had no effect on or decreased the risk of cancer.
“Policy support was highest among nondrinkers, followed by drinkers, and was lowest among heavier drinkers,” the authors report.
For example, almost 43% of nondrinkers supported restrictions on outdoor alcohol advertising, compared with only about 28.6% of drinkers and 22% of heavier drinkers. More respondents supported adding health warning labels on alcoholic beverages – 70% of nondrinkers, 65% of drinkers, and 57% of heavier drinkers, investigators observe.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The majority of Americans are not aware that alcohol consumption causes a variety of cancers and especially do not consider wine and beer to have a link with cancer, suggest the results from a national survey.
write lead author Andrew Seidenberg, PhD, MPH, National Cancer Institute, Rockville, Md., and colleagues.
“Increasing awareness of the alcohol-cancer link, such as through multimedia campaigns and patient-provider communication, may be an important new strategy for health advocates working to implement preventive alcohol policies,” they add.
The findings were published in the February issue of the American Journal of Preventive Medicine.
“This is the first study to examine the relationship between alcohol control policy support and awareness of the alcohol-cancer link among a national U.S. sample,” the authors write.
The results show that there is some public support for the idea of adding written warnings about the alcohol-cancer risk to alcoholic beverages, which is something that a number of cancer organizations have been petitioning for.
A petition filed by the American Society of Clinical Oncology, the American Institute for Cancer Research, and Breast Cancer Prevention Partners, all in collaboration with several public health organizations, proposes labeling that would read: “WARNING: According to the Surgeon General, consumption of alcoholic beverages can cause cancer, including breast and colon cancers.”
Such labeling has “the potential to save lives by ensuring that consumers have a more accurate understanding of the link between alcohol and cancer, which will empower them to better protect their health,” the groups said in the petition.
Public support
The findings come from an analysis of the 2020 Health Information National Trends Survey 5 Cycle 4. A total of 3,865 adults participated in the survey, approximately half of whom were nondrinkers.
As well as investigating how aware people were of the alcohol-cancer link, the investigators looked at how prevalent public support might be for the following three communication-focused alcohol policies:
- Banning outdoor alcohol-related advertising
- Requiring health warnings on alcohol beverage containers
- Requiring recommended drinking guidelines on alcoholic beverage containers
“Awareness of the alcohol-cancer link was measured separately for wine, beer, and liquor by asking: In your opinion, how much does drinking the following types of alcohol affect the risk of getting cancer?” the authors explain.
“Awareness of the alcohol-cancer link was low,” the investigators comment; only about one-third (31.8%) of participants were aware that alcohol increases the risk of cancer. The figures were even lower for individual beverage type, at 20.3% for wine, 24.9% for beer, and 31.2% for liquor. Furthermore, approximately half of participants responded with “don’t know” to the three awareness items, investigators noted.
On the other hand, more than half of the Americans surveyed supported adding both health warning labels (65.1%) and information on recommended drinking guidelines (63.9%) to alcoholic beverage containers. Support was lower (34.4% of respondents) for banning outdoor alcohol advertising.
Among Americans who were aware that alcohol increased cancer risk, support was also higher for all three policies.
For example, about 75% of respondents who were aware that alcohol increases cancer risk supported adding health warnings and drinking guidelines to beverage containers, compared with about half of Americans who felt that alcohol consumption had either no effect on or decreased cancer risk.
Even among those who were aware of the alcohol-cancer link, public support for outdoor advertising was not high (37.8%), but it was even lower (23.6%) among respondents who felt alcohol had no effect on or decreased the risk of cancer.
“Policy support was highest among nondrinkers, followed by drinkers, and was lowest among heavier drinkers,” the authors report.
For example, almost 43% of nondrinkers supported restrictions on outdoor alcohol advertising, compared with only about 28.6% of drinkers and 22% of heavier drinkers. More respondents supported adding health warning labels on alcoholic beverages – 70% of nondrinkers, 65% of drinkers, and 57% of heavier drinkers, investigators observe.
The study had no specific funding. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Perinatal deaths from COVID show ‘extensive’ placental damage
Recent evidence has shown that women who contract COVID-19 during pregnancy are at increased risk for pregnancy loss and neonatal death. Now, an analysis of pathology data from dozens of perinatal deaths shows how.
Unlike numerous pathogens that kill the fetus by infecting it directly, SARS-CoV-2 causes “widespread and severe” destruction of the placenta that deprives the fetus of oxygen, a team of 44 researchers in 12 countries concluded after examining 64 stillbirths and four neonatal deaths in which the placentas were infected with the virus. They noted that such damage occurs in a small percentage of pregnant women with COVID and that all the women in the study had not been vaccinated against the disease.
The findings were published online Feb. 10 in the Archives of Pathology & Laboratory Medicine.
Nearly all placentas had each of three features that pathologists have dubbed SARS-CoV-2 placentitis: large deposits of fibrin, a clotting protein that obstructs the flow of blood, death of cells in the trophoblast, and an unusual form of inflammation called chronic histiocytic intervillositis. Some had other abnormalities that could have exacerbated the condition.
The researchers called the extent of damage “striking,” affecting 77.7% of the placenta on average. The virus did not appear to harm fetal tissue, but placental damage “was extensive and highly destructive,” they write. Notably, none of the women in the analysis were known to have severe COVID.
Virus seen ‘chewing up the placenta’
David Schwartz, MD, a pathologist in Atlanta, and the lead author of the study, said COVID appears to be unique in destroying the placenta.
“I don’t know of any infection that does that to this degree or with this uniformity,” Dr. Schwartz told this news organization. “The simple message is that this infection is chewing up the placenta and destroying its capability to oxygenate the fetus.”
In November, the Centers for Disease Control and Prevention reported that maternal COVID increases the risk of losing a pregnancy. From March 2020 to September 2021, 8,154 stillbirths were reported, affecting 0.65% of births by women without COVID and 1.26% of births by women with COVID, for a relative risk of 1.90 (95% confidence interval, 1.69-2.15).
Delta, the variant that dominated in mid-2021, appears to have been particularly harmful. The CDC reported that the relative risk for stillbirth for mothers with COVID-19 during that period increased to 4.04 (95% CI, 3.28-4.97). Many cases in the new analysis coincided with Delta.
Dr. Schwartz and his colleagues said immunization, along with antiviral therapy, might reduce the chance of SARS-CoV-2 infecting the placenta. None of the mothers in the analysis was vaccinated, and Dr. Schwartz said he is not aware of a single case in a vaccinated woman.
The analysis comes on the heels of a study from the National Institutes of Health linking severe to moderate COVID infection to greater risk of other pregnancy complications: cesarean and preterm delivery, death during childbirth, postpartum hemorrhaging, and non-COVID infections.
Diana Bianchi, MD, director of NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development, said those findings underscore the need for pregnant women to be vaccinated. (The shots have been shown to be safe for pregnant women.)
Denise Jamieson, MD, MPH, chair of the department of gynecology and obstetrics at Emory University, Atlanta, who was not involved in the new analysis, said the findings may have important clinical implications. In addition to ensuring that pregnant patients are fully vaccinated, she said “there may be opportunities to more closely monitor the placenta during pregnancy using imaging modalities such as ultrasound.”
Even in the presence of severe abnormalities, a fetus that has reached a viable gestational age could potentially be delivered prior to stillbirth, Dr. Jamieson said. The 64 stillbirths in the analysis ranged from 15 to 39.2 weeks of gestation, with an average of 30 weeks. Eight were delivered at full term.
However, additional studies are needed to support monitoring of placental changes, she said: “It is not ready for prime time now.”
Christopher Zahn, MD, vice president of practice activities the American College of Obstetricians and Gynecologists, cautioned that data on COVID and pregnancy complications remain limited.
The findings in this analysis “do not prove the association between COVID-19 infection and neonatal outcomes,” Dr. Zahn said. “While stillbirth could potentially be another adverse outcome for pregnant people who contract COVID-19, currently we don’t have enough data to confirm that a COVID-19 infection at any point in pregnancy indicates increased risk of stillbirth.”
He added that ACOG continues to strongly recommend vaccination against COVID for women who are pregnant, recently pregnant, or planning to be pregnant.
Dr. Schwartz and Dr. Jamieson have disclosed no relevant financial relationships. One author reported receiving financial support from the Slovak Research and Development Agency. Another reported funding from the Belgian Fund for Scientific Research and the Fetus for Life charity.
A version of this article first appeared on Medscape.com.
Recent evidence has shown that women who contract COVID-19 during pregnancy are at increased risk for pregnancy loss and neonatal death. Now, an analysis of pathology data from dozens of perinatal deaths shows how.
Unlike numerous pathogens that kill the fetus by infecting it directly, SARS-CoV-2 causes “widespread and severe” destruction of the placenta that deprives the fetus of oxygen, a team of 44 researchers in 12 countries concluded after examining 64 stillbirths and four neonatal deaths in which the placentas were infected with the virus. They noted that such damage occurs in a small percentage of pregnant women with COVID and that all the women in the study had not been vaccinated against the disease.
The findings were published online Feb. 10 in the Archives of Pathology & Laboratory Medicine.
Nearly all placentas had each of three features that pathologists have dubbed SARS-CoV-2 placentitis: large deposits of fibrin, a clotting protein that obstructs the flow of blood, death of cells in the trophoblast, and an unusual form of inflammation called chronic histiocytic intervillositis. Some had other abnormalities that could have exacerbated the condition.
The researchers called the extent of damage “striking,” affecting 77.7% of the placenta on average. The virus did not appear to harm fetal tissue, but placental damage “was extensive and highly destructive,” they write. Notably, none of the women in the analysis were known to have severe COVID.
Virus seen ‘chewing up the placenta’
David Schwartz, MD, a pathologist in Atlanta, and the lead author of the study, said COVID appears to be unique in destroying the placenta.
“I don’t know of any infection that does that to this degree or with this uniformity,” Dr. Schwartz told this news organization. “The simple message is that this infection is chewing up the placenta and destroying its capability to oxygenate the fetus.”
In November, the Centers for Disease Control and Prevention reported that maternal COVID increases the risk of losing a pregnancy. From March 2020 to September 2021, 8,154 stillbirths were reported, affecting 0.65% of births by women without COVID and 1.26% of births by women with COVID, for a relative risk of 1.90 (95% confidence interval, 1.69-2.15).
Delta, the variant that dominated in mid-2021, appears to have been particularly harmful. The CDC reported that the relative risk for stillbirth for mothers with COVID-19 during that period increased to 4.04 (95% CI, 3.28-4.97). Many cases in the new analysis coincided with Delta.
Dr. Schwartz and his colleagues said immunization, along with antiviral therapy, might reduce the chance of SARS-CoV-2 infecting the placenta. None of the mothers in the analysis was vaccinated, and Dr. Schwartz said he is not aware of a single case in a vaccinated woman.
The analysis comes on the heels of a study from the National Institutes of Health linking severe to moderate COVID infection to greater risk of other pregnancy complications: cesarean and preterm delivery, death during childbirth, postpartum hemorrhaging, and non-COVID infections.
Diana Bianchi, MD, director of NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development, said those findings underscore the need for pregnant women to be vaccinated. (The shots have been shown to be safe for pregnant women.)
Denise Jamieson, MD, MPH, chair of the department of gynecology and obstetrics at Emory University, Atlanta, who was not involved in the new analysis, said the findings may have important clinical implications. In addition to ensuring that pregnant patients are fully vaccinated, she said “there may be opportunities to more closely monitor the placenta during pregnancy using imaging modalities such as ultrasound.”
Even in the presence of severe abnormalities, a fetus that has reached a viable gestational age could potentially be delivered prior to stillbirth, Dr. Jamieson said. The 64 stillbirths in the analysis ranged from 15 to 39.2 weeks of gestation, with an average of 30 weeks. Eight were delivered at full term.
However, additional studies are needed to support monitoring of placental changes, she said: “It is not ready for prime time now.”
Christopher Zahn, MD, vice president of practice activities the American College of Obstetricians and Gynecologists, cautioned that data on COVID and pregnancy complications remain limited.
The findings in this analysis “do not prove the association between COVID-19 infection and neonatal outcomes,” Dr. Zahn said. “While stillbirth could potentially be another adverse outcome for pregnant people who contract COVID-19, currently we don’t have enough data to confirm that a COVID-19 infection at any point in pregnancy indicates increased risk of stillbirth.”
He added that ACOG continues to strongly recommend vaccination against COVID for women who are pregnant, recently pregnant, or planning to be pregnant.
Dr. Schwartz and Dr. Jamieson have disclosed no relevant financial relationships. One author reported receiving financial support from the Slovak Research and Development Agency. Another reported funding from the Belgian Fund for Scientific Research and the Fetus for Life charity.
A version of this article first appeared on Medscape.com.
Recent evidence has shown that women who contract COVID-19 during pregnancy are at increased risk for pregnancy loss and neonatal death. Now, an analysis of pathology data from dozens of perinatal deaths shows how.
Unlike numerous pathogens that kill the fetus by infecting it directly, SARS-CoV-2 causes “widespread and severe” destruction of the placenta that deprives the fetus of oxygen, a team of 44 researchers in 12 countries concluded after examining 64 stillbirths and four neonatal deaths in which the placentas were infected with the virus. They noted that such damage occurs in a small percentage of pregnant women with COVID and that all the women in the study had not been vaccinated against the disease.
The findings were published online Feb. 10 in the Archives of Pathology & Laboratory Medicine.
Nearly all placentas had each of three features that pathologists have dubbed SARS-CoV-2 placentitis: large deposits of fibrin, a clotting protein that obstructs the flow of blood, death of cells in the trophoblast, and an unusual form of inflammation called chronic histiocytic intervillositis. Some had other abnormalities that could have exacerbated the condition.
The researchers called the extent of damage “striking,” affecting 77.7% of the placenta on average. The virus did not appear to harm fetal tissue, but placental damage “was extensive and highly destructive,” they write. Notably, none of the women in the analysis were known to have severe COVID.
Virus seen ‘chewing up the placenta’
David Schwartz, MD, a pathologist in Atlanta, and the lead author of the study, said COVID appears to be unique in destroying the placenta.
“I don’t know of any infection that does that to this degree or with this uniformity,” Dr. Schwartz told this news organization. “The simple message is that this infection is chewing up the placenta and destroying its capability to oxygenate the fetus.”
In November, the Centers for Disease Control and Prevention reported that maternal COVID increases the risk of losing a pregnancy. From March 2020 to September 2021, 8,154 stillbirths were reported, affecting 0.65% of births by women without COVID and 1.26% of births by women with COVID, for a relative risk of 1.90 (95% confidence interval, 1.69-2.15).
Delta, the variant that dominated in mid-2021, appears to have been particularly harmful. The CDC reported that the relative risk for stillbirth for mothers with COVID-19 during that period increased to 4.04 (95% CI, 3.28-4.97). Many cases in the new analysis coincided with Delta.
Dr. Schwartz and his colleagues said immunization, along with antiviral therapy, might reduce the chance of SARS-CoV-2 infecting the placenta. None of the mothers in the analysis was vaccinated, and Dr. Schwartz said he is not aware of a single case in a vaccinated woman.
The analysis comes on the heels of a study from the National Institutes of Health linking severe to moderate COVID infection to greater risk of other pregnancy complications: cesarean and preterm delivery, death during childbirth, postpartum hemorrhaging, and non-COVID infections.
Diana Bianchi, MD, director of NIH’s Eunice Kennedy Shriver National Institute of Child Health and Human Development, said those findings underscore the need for pregnant women to be vaccinated. (The shots have been shown to be safe for pregnant women.)
Denise Jamieson, MD, MPH, chair of the department of gynecology and obstetrics at Emory University, Atlanta, who was not involved in the new analysis, said the findings may have important clinical implications. In addition to ensuring that pregnant patients are fully vaccinated, she said “there may be opportunities to more closely monitor the placenta during pregnancy using imaging modalities such as ultrasound.”
Even in the presence of severe abnormalities, a fetus that has reached a viable gestational age could potentially be delivered prior to stillbirth, Dr. Jamieson said. The 64 stillbirths in the analysis ranged from 15 to 39.2 weeks of gestation, with an average of 30 weeks. Eight were delivered at full term.
However, additional studies are needed to support monitoring of placental changes, she said: “It is not ready for prime time now.”
Christopher Zahn, MD, vice president of practice activities the American College of Obstetricians and Gynecologists, cautioned that data on COVID and pregnancy complications remain limited.
The findings in this analysis “do not prove the association between COVID-19 infection and neonatal outcomes,” Dr. Zahn said. “While stillbirth could potentially be another adverse outcome for pregnant people who contract COVID-19, currently we don’t have enough data to confirm that a COVID-19 infection at any point in pregnancy indicates increased risk of stillbirth.”
He added that ACOG continues to strongly recommend vaccination against COVID for women who are pregnant, recently pregnant, or planning to be pregnant.
Dr. Schwartz and Dr. Jamieson have disclosed no relevant financial relationships. One author reported receiving financial support from the Slovak Research and Development Agency. Another reported funding from the Belgian Fund for Scientific Research and the Fetus for Life charity.
A version of this article first appeared on Medscape.com.
Agreement reached for research definition of ‘long COVID’ in children and young people
Long COVID can affect adults, young people, and children, and now for the first time, in a landmark study accepted for publication in the Archives of Disease in Childhood, formal agreement has been made on a research definition for post–acute COVID-19, or “long COVID” as it is commonly known, in children and young people.
The researchers charged themselves with a single objective – to derive a research definition for long COVID (post–acute COVID-19) in children and young people to allow comparisons between research studies. Specifically, so studies on prevalence, course, and outcome of long COVID in this age group can be reliably compared, because to date there has been no consensus. In fact, the authors pointed out how the “slew of definitions” currently used all differ in number, type, and duration of symptoms, which hampers research efforts. In addition, the lack of definition consensus has contributed to very wide reported variations in the estimated prevalence of long COVID in children of 1%-51%, with the authors saying that a “consistently applied definition of long COVID will help reduce the variability of prevalence estimates.”
Statements sequentially whittled down
“Using robust consensus methodology,” the authors said, “we derived a research definition for long COVID in children and young people.”
To achieve the definition consensus, a three-phase online Delphi process was used, followed by a virtual consensus meeting. The 123 participants registered to take part in the study included 23 people (19%) in a lived experience panel, 50 (42%) in the researcher or researcher/service delivery combined panel and 47 (39%) in the service delivery panel. Of 120 registered participants, 105 (88%) completed phase 1, 86 eligible participants (82% of those completing phase 1) completed phase 2 and 77 eligible participants (90% of those completing phase 2) completed phase 3. Seventeen participants attended and voted at the consensus meeting – 4 (23%) from the service delivery panel, 11 (65%) from the researcher panel, and 2 (12%) from the lived experience panel.
Presented with 49 statements in each phase, participants scored these from 1-9 based on how important they were perceived to be with regards inclusion in the research definition of long COVID in children and young people. Having been sequentially whittled down in three phases, 10 statements were discussed at the consensus meeting, and a panel of eight 11- to 17-year-olds affected by long COVID also reviewed the statements to reach a final agreement.
Five of the statements were agreed to be included in the definition, which stated that long COVID in children and young people is a condition in which a child or young person has symptoms (at least one of which is a physical symptom) that have continued or developed after a diagnosis of COVID-19 (confirmed with one or more positive COVID tests); impact their physical, mental, or social well-being; are interfering with some aspect of daily living (for example, school, work, home, or relationships); and persist for a minimum duration of 12 weeks after initial testing for COVID-19 (even if symptoms have waxed and waned over that period).
David Strain, MBChB, MD, chair of the BMA board of science and clinical senior lecturer and honorary consultant, University of Exeter (England), told the Science Media Centre: “A Delphi study builds a consensus from the world’s experts by presenting a series of statements and continuing to refine them until there is agreement as to what the definition of pediatric long COVID should be.” He added: “This is vitally important in order to align the global research effort into long COVID.”
Reassuringly similar
From the agreed five statements, a further research definition was proposed to align with the World Health Organization definition for adults: “Post–COVID-19 condition occurs in young people with a history of confirmed SARS CoV-2 infection, with at least one persisting physical symptom for a minimum duration of 12 weeks after initial testing that cannot be explained by an alternative diagnosis. The symptoms have an impact on everyday functioning, may continue or develop after COVID-19 infection, and may fluctuate or relapse over time.”
The authors concluded: “This is the first research definition of long COVID (post–COVID-19 condition) in children and young people and complements the clinical case definition in adults proposed by WHO,” adding that the two definitions are “reassuringly similar.”
They reiterated how widespread adoption of this definition would allow comparisons between studies such that a core outcome set can be developed and the prevalence, course and outcome of long COVID in children and young people can be reliably evaluated, which “will substantially help strengthen the evidence base on this debilitating condition.”
In addition, the authors said that a consistently applied definition of long COVID will help to provide a “more accurate picture on the true impact of the condition.”
The researchers emphasized the need to differentiate between a clinical case definition and a research definition of long COVID and explained: “It is understandable that the patient groups representing people with long COVID are concerned about a definition that could restrict access to services that are needed.”
They went on to say that in their view the decision whether a child or young person can see a health care professional, access any support needed, or be referred, investigated, or treated for long COVID should be a “shared decision involving the young person, their carers, and clinicians.”
Dr. Strain reinforced that it was important that the definition was a research one and not a clinical one, pointing out that the 12-week period in the research definition “does not necessarily mean that a child or young person should need to wait 3 months before being offered help or assistance from their health care team, indeed a 3-month delay in offering support to a child or young person, at this vitally important period of their educational development, could have lasting long-term impacts.”
A version of this article first appeared on Medscape.co.uk.
Long COVID can affect adults, young people, and children, and now for the first time, in a landmark study accepted for publication in the Archives of Disease in Childhood, formal agreement has been made on a research definition for post–acute COVID-19, or “long COVID” as it is commonly known, in children and young people.
The researchers charged themselves with a single objective – to derive a research definition for long COVID (post–acute COVID-19) in children and young people to allow comparisons between research studies. Specifically, so studies on prevalence, course, and outcome of long COVID in this age group can be reliably compared, because to date there has been no consensus. In fact, the authors pointed out how the “slew of definitions” currently used all differ in number, type, and duration of symptoms, which hampers research efforts. In addition, the lack of definition consensus has contributed to very wide reported variations in the estimated prevalence of long COVID in children of 1%-51%, with the authors saying that a “consistently applied definition of long COVID will help reduce the variability of prevalence estimates.”
Statements sequentially whittled down
“Using robust consensus methodology,” the authors said, “we derived a research definition for long COVID in children and young people.”
To achieve the definition consensus, a three-phase online Delphi process was used, followed by a virtual consensus meeting. The 123 participants registered to take part in the study included 23 people (19%) in a lived experience panel, 50 (42%) in the researcher or researcher/service delivery combined panel and 47 (39%) in the service delivery panel. Of 120 registered participants, 105 (88%) completed phase 1, 86 eligible participants (82% of those completing phase 1) completed phase 2 and 77 eligible participants (90% of those completing phase 2) completed phase 3. Seventeen participants attended and voted at the consensus meeting – 4 (23%) from the service delivery panel, 11 (65%) from the researcher panel, and 2 (12%) from the lived experience panel.
Presented with 49 statements in each phase, participants scored these from 1-9 based on how important they were perceived to be with regards inclusion in the research definition of long COVID in children and young people. Having been sequentially whittled down in three phases, 10 statements were discussed at the consensus meeting, and a panel of eight 11- to 17-year-olds affected by long COVID also reviewed the statements to reach a final agreement.
Five of the statements were agreed to be included in the definition, which stated that long COVID in children and young people is a condition in which a child or young person has symptoms (at least one of which is a physical symptom) that have continued or developed after a diagnosis of COVID-19 (confirmed with one or more positive COVID tests); impact their physical, mental, or social well-being; are interfering with some aspect of daily living (for example, school, work, home, or relationships); and persist for a minimum duration of 12 weeks after initial testing for COVID-19 (even if symptoms have waxed and waned over that period).
David Strain, MBChB, MD, chair of the BMA board of science and clinical senior lecturer and honorary consultant, University of Exeter (England), told the Science Media Centre: “A Delphi study builds a consensus from the world’s experts by presenting a series of statements and continuing to refine them until there is agreement as to what the definition of pediatric long COVID should be.” He added: “This is vitally important in order to align the global research effort into long COVID.”
Reassuringly similar
From the agreed five statements, a further research definition was proposed to align with the World Health Organization definition for adults: “Post–COVID-19 condition occurs in young people with a history of confirmed SARS CoV-2 infection, with at least one persisting physical symptom for a minimum duration of 12 weeks after initial testing that cannot be explained by an alternative diagnosis. The symptoms have an impact on everyday functioning, may continue or develop after COVID-19 infection, and may fluctuate or relapse over time.”
The authors concluded: “This is the first research definition of long COVID (post–COVID-19 condition) in children and young people and complements the clinical case definition in adults proposed by WHO,” adding that the two definitions are “reassuringly similar.”
They reiterated how widespread adoption of this definition would allow comparisons between studies such that a core outcome set can be developed and the prevalence, course and outcome of long COVID in children and young people can be reliably evaluated, which “will substantially help strengthen the evidence base on this debilitating condition.”
In addition, the authors said that a consistently applied definition of long COVID will help to provide a “more accurate picture on the true impact of the condition.”
The researchers emphasized the need to differentiate between a clinical case definition and a research definition of long COVID and explained: “It is understandable that the patient groups representing people with long COVID are concerned about a definition that could restrict access to services that are needed.”
They went on to say that in their view the decision whether a child or young person can see a health care professional, access any support needed, or be referred, investigated, or treated for long COVID should be a “shared decision involving the young person, their carers, and clinicians.”
Dr. Strain reinforced that it was important that the definition was a research one and not a clinical one, pointing out that the 12-week period in the research definition “does not necessarily mean that a child or young person should need to wait 3 months before being offered help or assistance from their health care team, indeed a 3-month delay in offering support to a child or young person, at this vitally important period of their educational development, could have lasting long-term impacts.”
A version of this article first appeared on Medscape.co.uk.
Long COVID can affect adults, young people, and children, and now for the first time, in a landmark study accepted for publication in the Archives of Disease in Childhood, formal agreement has been made on a research definition for post–acute COVID-19, or “long COVID” as it is commonly known, in children and young people.
The researchers charged themselves with a single objective – to derive a research definition for long COVID (post–acute COVID-19) in children and young people to allow comparisons between research studies. Specifically, so studies on prevalence, course, and outcome of long COVID in this age group can be reliably compared, because to date there has been no consensus. In fact, the authors pointed out how the “slew of definitions” currently used all differ in number, type, and duration of symptoms, which hampers research efforts. In addition, the lack of definition consensus has contributed to very wide reported variations in the estimated prevalence of long COVID in children of 1%-51%, with the authors saying that a “consistently applied definition of long COVID will help reduce the variability of prevalence estimates.”
Statements sequentially whittled down
“Using robust consensus methodology,” the authors said, “we derived a research definition for long COVID in children and young people.”
To achieve the definition consensus, a three-phase online Delphi process was used, followed by a virtual consensus meeting. The 123 participants registered to take part in the study included 23 people (19%) in a lived experience panel, 50 (42%) in the researcher or researcher/service delivery combined panel and 47 (39%) in the service delivery panel. Of 120 registered participants, 105 (88%) completed phase 1, 86 eligible participants (82% of those completing phase 1) completed phase 2 and 77 eligible participants (90% of those completing phase 2) completed phase 3. Seventeen participants attended and voted at the consensus meeting – 4 (23%) from the service delivery panel, 11 (65%) from the researcher panel, and 2 (12%) from the lived experience panel.
Presented with 49 statements in each phase, participants scored these from 1-9 based on how important they were perceived to be with regards inclusion in the research definition of long COVID in children and young people. Having been sequentially whittled down in three phases, 10 statements were discussed at the consensus meeting, and a panel of eight 11- to 17-year-olds affected by long COVID also reviewed the statements to reach a final agreement.
Five of the statements were agreed to be included in the definition, which stated that long COVID in children and young people is a condition in which a child or young person has symptoms (at least one of which is a physical symptom) that have continued or developed after a diagnosis of COVID-19 (confirmed with one or more positive COVID tests); impact their physical, mental, or social well-being; are interfering with some aspect of daily living (for example, school, work, home, or relationships); and persist for a minimum duration of 12 weeks after initial testing for COVID-19 (even if symptoms have waxed and waned over that period).
David Strain, MBChB, MD, chair of the BMA board of science and clinical senior lecturer and honorary consultant, University of Exeter (England), told the Science Media Centre: “A Delphi study builds a consensus from the world’s experts by presenting a series of statements and continuing to refine them until there is agreement as to what the definition of pediatric long COVID should be.” He added: “This is vitally important in order to align the global research effort into long COVID.”
Reassuringly similar
From the agreed five statements, a further research definition was proposed to align with the World Health Organization definition for adults: “Post–COVID-19 condition occurs in young people with a history of confirmed SARS CoV-2 infection, with at least one persisting physical symptom for a minimum duration of 12 weeks after initial testing that cannot be explained by an alternative diagnosis. The symptoms have an impact on everyday functioning, may continue or develop after COVID-19 infection, and may fluctuate or relapse over time.”
The authors concluded: “This is the first research definition of long COVID (post–COVID-19 condition) in children and young people and complements the clinical case definition in adults proposed by WHO,” adding that the two definitions are “reassuringly similar.”
They reiterated how widespread adoption of this definition would allow comparisons between studies such that a core outcome set can be developed and the prevalence, course and outcome of long COVID in children and young people can be reliably evaluated, which “will substantially help strengthen the evidence base on this debilitating condition.”
In addition, the authors said that a consistently applied definition of long COVID will help to provide a “more accurate picture on the true impact of the condition.”
The researchers emphasized the need to differentiate between a clinical case definition and a research definition of long COVID and explained: “It is understandable that the patient groups representing people with long COVID are concerned about a definition that could restrict access to services that are needed.”
They went on to say that in their view the decision whether a child or young person can see a health care professional, access any support needed, or be referred, investigated, or treated for long COVID should be a “shared decision involving the young person, their carers, and clinicians.”
Dr. Strain reinforced that it was important that the definition was a research one and not a clinical one, pointing out that the 12-week period in the research definition “does not necessarily mean that a child or young person should need to wait 3 months before being offered help or assistance from their health care team, indeed a 3-month delay in offering support to a child or young person, at this vitally important period of their educational development, could have lasting long-term impacts.”
A version of this article first appeared on Medscape.co.uk.
FROM THE ARCHIVES OF DISEASE IN CHILDHOOD
Improved follow-up needed to find late-stage pancreatic cancers
A relatively large number of late-stage pancreatic ductal adenocarcinomas (PDACs) are detected during follow-up surveillance, yet no single patient- or protocol-specific factor appears to be significantly associated with detecting late-stage disease during this period, according to a new systematic literature review and meta-analysis.
The researchers, led by Ankit Chhoda, MD, of Yale University, New Haven, Conn., wrote in Gastroenterology that interval progression in high-risk individuals “highlights the need for improved follow-up methodology with higher accuracy to detect prognostically significant and treatable lesions.”
Individuals at high risk for PDAC are encouraged to undergo routine surveillance for the disease because early detection and resection of T1N0M0 PDAC and high-grade precursors may improve survival outcomes. According to Dr. Chhoda and colleagues, challenges of interval progression of cancers during the surveillance period for gastrointestinal malignancies have been well described in the general and at-risk patient populations. Previous studies, the authors explained, have not scrutinized the issues associated with late-stage PDACs detected during follow-up surveillance.
“Late-stage PDACs necessitate critical appraisal of current follow-up strategies to detect successful targets and perform timely resections,” the authors wrote. The researchers added that the diagnosis of late-stage PDACs during follow-up emphasizes the need for implementing “quality measures to avoid preventable causes, including surveillance adherence and diagnostic errors.”
To understand the incidence rates of late-stage PDACs during follow-up in high-risk individuals, Dr. Chhoda and researchers performed a systematic literature review and meta-analysis of data that included follow-up strategies for early PDAC detection among a high-risk population.
Outcomes of interest for the analysis included the overall diagnosis of advanced neoplasia as well as surveillance-detected/interval late-stage PDACs (T2–4N0M0/metastatic stage PDAC) during follow-up. The investigators defined surveillance-detected and interval late-stage PDACs as late-stage PDACs that were detected during surveillance and as those presenting symptomatically between visits, respectively.
The researchers also performed metaregression of the incidence rates of late-stage PDACs to examine the relationship with clinicoradiologic features in high-risk individuals.
A total of 13 studies on surveillance in 2,169 high-risk individuals were included in the systematic review, while 12 studies were included in the meta-analysis. Across studies, high-risk individuals were followed for over 7,302.72 patient-years for the purposes of detecting incident lesions or progression of preexisting pancreatic abnormalities.
In all high-risk individuals who underwent follow-up, the investigators identified a total yield of advanced neoplasia of 53. This total yield consisted of 7 high-grade pancreatic intraepithelial neoplasms, 7 high-grade intraductal papillary mucinous neoplasms, and 39 PDACs. According to the meta-analysis, the cumulative incidence of advanced neoplasia was 3.3 (95% confidence interval, 0.6-7.4; P < .001) per 1,000 patient-years. During follow-up, the cumulative incidence of surveillance-detected/interval late-stage PDACs was 1.7 per 1,000 patient-years (95% CI, 0.2-4.0; P = .03).
In a separate analysis, the investigators sought to identify the relationship between the modality of follow-up imaging and late-stage PDAC incidence. Imaging modalities used during follow-up were mostly cross-sectional imaging, such as computed tomography or magnetic resonance imaging with cholangiopancreatography (n = 4) or endoscopic ultrasound and cross-sectional modalities (n = 8).
The investigators found no significant associations between late-stage PDACs and surveillance imaging, baseline pancreatic morphology, study location, genetic background, gender, or age. Incidence of late-stage PDACs in studies with mostly cross-sectional imaging was 0.7 per 1,000 patient-years (95% CI, 0.0-8.0). This incidence rate was lower than that reported with EUS and cross-sectional modalities (2.5 per 1,000 patient-years; 95% CI, 0.6-5.4), but this difference was not statistically significant (P = .2).
No significant difference was found during follow-up in the incidence of late-stage PDACs between high-risk individuals with baseline pancreatic abnormalities (0.0 no significant difference; 95% CI, 0.0-0.3) vs. high-risk individuals with normal baseline (0.9 per 1,000 patient-years; 95% CI, 0.0-2.8) (P = .9).
Most studies included in the analysis did not report on diagnostic errors and surveillance adherence, the researchers wrote. Nonadherence to surveillance as well as delays in surveillance accounted for four late-stage PDACs, and surveillance cessation and/or delays were reported in 4 out of 19 high-risk individuals. There was limited information on symptoms, presentation timing, site of lesion, and surveillance adherence, which the investigators indicated prevented a formal meta-analysis.
In their summary, the study authors noted that in clinical practice there is a need for improved quality measures and adherence to surveillance programs to reduce the risk of diagnostic errors. The authors stated that evidence on the impact of these quality measures “on surveillance outcomes will not only improve quality of surveillance practices, but also enrich our communication with patients who undergo surveillance.”
The researchers reported no conflicts of interest with the pharmaceutical industry, and the study did not receive any funding.
A relatively large number of late-stage pancreatic ductal adenocarcinomas (PDACs) are detected during follow-up surveillance, yet no single patient- or protocol-specific factor appears to be significantly associated with detecting late-stage disease during this period, according to a new systematic literature review and meta-analysis.
The researchers, led by Ankit Chhoda, MD, of Yale University, New Haven, Conn., wrote in Gastroenterology that interval progression in high-risk individuals “highlights the need for improved follow-up methodology with higher accuracy to detect prognostically significant and treatable lesions.”
Individuals at high risk for PDAC are encouraged to undergo routine surveillance for the disease because early detection and resection of T1N0M0 PDAC and high-grade precursors may improve survival outcomes. According to Dr. Chhoda and colleagues, challenges of interval progression of cancers during the surveillance period for gastrointestinal malignancies have been well described in the general and at-risk patient populations. Previous studies, the authors explained, have not scrutinized the issues associated with late-stage PDACs detected during follow-up surveillance.
“Late-stage PDACs necessitate critical appraisal of current follow-up strategies to detect successful targets and perform timely resections,” the authors wrote. The researchers added that the diagnosis of late-stage PDACs during follow-up emphasizes the need for implementing “quality measures to avoid preventable causes, including surveillance adherence and diagnostic errors.”
To understand the incidence rates of late-stage PDACs during follow-up in high-risk individuals, Dr. Chhoda and researchers performed a systematic literature review and meta-analysis of data that included follow-up strategies for early PDAC detection among a high-risk population.
Outcomes of interest for the analysis included the overall diagnosis of advanced neoplasia as well as surveillance-detected/interval late-stage PDACs (T2–4N0M0/metastatic stage PDAC) during follow-up. The investigators defined surveillance-detected and interval late-stage PDACs as late-stage PDACs that were detected during surveillance and as those presenting symptomatically between visits, respectively.
The researchers also performed metaregression of the incidence rates of late-stage PDACs to examine the relationship with clinicoradiologic features in high-risk individuals.
A total of 13 studies on surveillance in 2,169 high-risk individuals were included in the systematic review, while 12 studies were included in the meta-analysis. Across studies, high-risk individuals were followed for over 7,302.72 patient-years for the purposes of detecting incident lesions or progression of preexisting pancreatic abnormalities.
In all high-risk individuals who underwent follow-up, the investigators identified a total yield of advanced neoplasia of 53. This total yield consisted of 7 high-grade pancreatic intraepithelial neoplasms, 7 high-grade intraductal papillary mucinous neoplasms, and 39 PDACs. According to the meta-analysis, the cumulative incidence of advanced neoplasia was 3.3 (95% confidence interval, 0.6-7.4; P < .001) per 1,000 patient-years. During follow-up, the cumulative incidence of surveillance-detected/interval late-stage PDACs was 1.7 per 1,000 patient-years (95% CI, 0.2-4.0; P = .03).
In a separate analysis, the investigators sought to identify the relationship between the modality of follow-up imaging and late-stage PDAC incidence. Imaging modalities used during follow-up were mostly cross-sectional imaging, such as computed tomography or magnetic resonance imaging with cholangiopancreatography (n = 4) or endoscopic ultrasound and cross-sectional modalities (n = 8).
The investigators found no significant associations between late-stage PDACs and surveillance imaging, baseline pancreatic morphology, study location, genetic background, gender, or age. Incidence of late-stage PDACs in studies with mostly cross-sectional imaging was 0.7 per 1,000 patient-years (95% CI, 0.0-8.0). This incidence rate was lower than that reported with EUS and cross-sectional modalities (2.5 per 1,000 patient-years; 95% CI, 0.6-5.4), but this difference was not statistically significant (P = .2).
No significant difference was found during follow-up in the incidence of late-stage PDACs between high-risk individuals with baseline pancreatic abnormalities (0.0 no significant difference; 95% CI, 0.0-0.3) vs. high-risk individuals with normal baseline (0.9 per 1,000 patient-years; 95% CI, 0.0-2.8) (P = .9).
Most studies included in the analysis did not report on diagnostic errors and surveillance adherence, the researchers wrote. Nonadherence to surveillance as well as delays in surveillance accounted for four late-stage PDACs, and surveillance cessation and/or delays were reported in 4 out of 19 high-risk individuals. There was limited information on symptoms, presentation timing, site of lesion, and surveillance adherence, which the investigators indicated prevented a formal meta-analysis.
In their summary, the study authors noted that in clinical practice there is a need for improved quality measures and adherence to surveillance programs to reduce the risk of diagnostic errors. The authors stated that evidence on the impact of these quality measures “on surveillance outcomes will not only improve quality of surveillance practices, but also enrich our communication with patients who undergo surveillance.”
The researchers reported no conflicts of interest with the pharmaceutical industry, and the study did not receive any funding.
A relatively large number of late-stage pancreatic ductal adenocarcinomas (PDACs) are detected during follow-up surveillance, yet no single patient- or protocol-specific factor appears to be significantly associated with detecting late-stage disease during this period, according to a new systematic literature review and meta-analysis.
The researchers, led by Ankit Chhoda, MD, of Yale University, New Haven, Conn., wrote in Gastroenterology that interval progression in high-risk individuals “highlights the need for improved follow-up methodology with higher accuracy to detect prognostically significant and treatable lesions.”
Individuals at high risk for PDAC are encouraged to undergo routine surveillance for the disease because early detection and resection of T1N0M0 PDAC and high-grade precursors may improve survival outcomes. According to Dr. Chhoda and colleagues, challenges of interval progression of cancers during the surveillance period for gastrointestinal malignancies have been well described in the general and at-risk patient populations. Previous studies, the authors explained, have not scrutinized the issues associated with late-stage PDACs detected during follow-up surveillance.
“Late-stage PDACs necessitate critical appraisal of current follow-up strategies to detect successful targets and perform timely resections,” the authors wrote. The researchers added that the diagnosis of late-stage PDACs during follow-up emphasizes the need for implementing “quality measures to avoid preventable causes, including surveillance adherence and diagnostic errors.”
To understand the incidence rates of late-stage PDACs during follow-up in high-risk individuals, Dr. Chhoda and researchers performed a systematic literature review and meta-analysis of data that included follow-up strategies for early PDAC detection among a high-risk population.
Outcomes of interest for the analysis included the overall diagnosis of advanced neoplasia as well as surveillance-detected/interval late-stage PDACs (T2–4N0M0/metastatic stage PDAC) during follow-up. The investigators defined surveillance-detected and interval late-stage PDACs as late-stage PDACs that were detected during surveillance and as those presenting symptomatically between visits, respectively.
The researchers also performed metaregression of the incidence rates of late-stage PDACs to examine the relationship with clinicoradiologic features in high-risk individuals.
A total of 13 studies on surveillance in 2,169 high-risk individuals were included in the systematic review, while 12 studies were included in the meta-analysis. Across studies, high-risk individuals were followed for over 7,302.72 patient-years for the purposes of detecting incident lesions or progression of preexisting pancreatic abnormalities.
In all high-risk individuals who underwent follow-up, the investigators identified a total yield of advanced neoplasia of 53. This total yield consisted of 7 high-grade pancreatic intraepithelial neoplasms, 7 high-grade intraductal papillary mucinous neoplasms, and 39 PDACs. According to the meta-analysis, the cumulative incidence of advanced neoplasia was 3.3 (95% confidence interval, 0.6-7.4; P < .001) per 1,000 patient-years. During follow-up, the cumulative incidence of surveillance-detected/interval late-stage PDACs was 1.7 per 1,000 patient-years (95% CI, 0.2-4.0; P = .03).
In a separate analysis, the investigators sought to identify the relationship between the modality of follow-up imaging and late-stage PDAC incidence. Imaging modalities used during follow-up were mostly cross-sectional imaging, such as computed tomography or magnetic resonance imaging with cholangiopancreatography (n = 4) or endoscopic ultrasound and cross-sectional modalities (n = 8).
The investigators found no significant associations between late-stage PDACs and surveillance imaging, baseline pancreatic morphology, study location, genetic background, gender, or age. Incidence of late-stage PDACs in studies with mostly cross-sectional imaging was 0.7 per 1,000 patient-years (95% CI, 0.0-8.0). This incidence rate was lower than that reported with EUS and cross-sectional modalities (2.5 per 1,000 patient-years; 95% CI, 0.6-5.4), but this difference was not statistically significant (P = .2).
No significant difference was found during follow-up in the incidence of late-stage PDACs between high-risk individuals with baseline pancreatic abnormalities (0.0 no significant difference; 95% CI, 0.0-0.3) vs. high-risk individuals with normal baseline (0.9 per 1,000 patient-years; 95% CI, 0.0-2.8) (P = .9).
Most studies included in the analysis did not report on diagnostic errors and surveillance adherence, the researchers wrote. Nonadherence to surveillance as well as delays in surveillance accounted for four late-stage PDACs, and surveillance cessation and/or delays were reported in 4 out of 19 high-risk individuals. There was limited information on symptoms, presentation timing, site of lesion, and surveillance adherence, which the investigators indicated prevented a formal meta-analysis.
In their summary, the study authors noted that in clinical practice there is a need for improved quality measures and adherence to surveillance programs to reduce the risk of diagnostic errors. The authors stated that evidence on the impact of these quality measures “on surveillance outcomes will not only improve quality of surveillance practices, but also enrich our communication with patients who undergo surveillance.”
The researchers reported no conflicts of interest with the pharmaceutical industry, and the study did not receive any funding.
FROM GASTROENTEROLOGY
Oncologists in malpractice suits: Less than other specialties
, notes the latest Medscape Malpractice Report.
Over a third (39%) of oncologists who were surveyed said that they had been named in at least one malpractice suit, according to the Medscape Oncologist Malpractice Report 2021.
This number is considerably lower than that reported by physicians across all specialties (51%), and it is also much lower than that reported by oncologists in past years. In the 2019 report, 54% of oncologists surveyed said they had been named in a malpractice suit, while in the 2017 report, the figure was 53%.
The dramatic decline in malpractice suits may have much to do with the COVID-19 pandemic, when oncology care was in a state of flux.
“Fewer people were seeking cancer care during the COVID pandemic, which might have impacted the number of lawsuits brought against oncologists,” says Paul Walker, a New York–based malpractice attorney at Walker Medical Law, who represents physicians and other healthcare professionals.
“Additionally, a fair number of people who died of COVID were also older,” he pointed out, and it is often older people who get cancer, so there were fewer older people who consulted an oncologist or were treated by one, he added.
However, the pandemic may be storing up trouble for future years. “Patient fears of contracting COVID-19 have led many to avoid seeking or resuming care, so delays in diagnosing new cancer cases could mean that more patients are diagnosed at a later stage of their disease, leading to potential adverse events and malpractice claims,” commented David L. Feldman, MD, MBA, chief medical officer of The Doctors Company Group.
This latest 2021 Medscape Malpractice Report was compiled from an online survey that included more than 4,300 physicians from 29 specialties. It included 106 oncologists. More than half of respondents (56%) had been in practice for more than 25 years, and 54% were aged 60 years or older. The survey was available from May 21 to August 28, 2021.
Similar to findings in previous years, complications from treatment/surgery were the most common reason for the lawsuits (31%). Failure to make a correct diagnosis or a delay in diagnosis was the second most common reason (23%), while 20% of patients sued because of a poor outcome or disease progression.
Surprise at being sued
Among the oncologists who reported involvement in a lawsuit in 2021, the majority (86%) said they were “very surprised” or “somewhat surprised” by the malpractice suit, which is similar to that of other physicians surveyed. However, fewer were surprised this year as compared to 2019 and 2017 (90% and 94%).
One reason for the surprise over the litigation was that it concerned a patient who had been treated a long time ago. One oncologist wrote that “the patient had not seen me for over 7 years and during that time, he did not call me with his new symptomatology. I was only named in the suit because I had previously been involved.”
Another common scenario reported by oncologists was being named in a lawsuit which was brought by another clinician’s patient. “I was the chairperson of the department, and one of the doctors in the practice was involved in the suit,” wrote one respondent. “I was named as an accomplice.”
More than half of surveyed oncologists said that they were able to identify the patient who bought the suit, and these figures are again comparable to those of other physicians. One oncologist commented that in the case he was involved with, the family did not understand or accept the nature of cancer and the different ways that a patient could die of complications. This patient had died of sepsis and pneumonia related to decubitus ulcers that were completely unrelated to her radiation therapy.
As in the case above, sometimes it is the family who filed the lawsuit, not the patient.
“The patient may even recognize that you did your best and be grateful for your skill and efforts, but the family can’t accept that grandma died of cancer and brings a lawsuit,” said Dennis Hursh, an attorney with Physicians Agreement Health Law in Pennsylvania.
When looking at outcomes of the lawsuit, 40% of oncologists were dismissed from the suit within the first few months, or the case was settled before going to trial. This trend is also consistent with the results from the 2019 and 2017 surveys. When the case did go to trial, 10% received a favorable verdict, which was the same in 2019.
“It seems that most of my clients end up being released from lawsuits, and many lawsuits are dismissed prior to proceeding to trial,” Mr. Hursh commented.
Murdering psychopath
Some oncologists weighed in on what they felt was the worst experience of being sued.
“Mental anguish, knowing that I did nothing wrong,” said one physician. Another reported that it was a feeling of being “inadequate and totally alone.”
Another oncologist commented that the “depositions from lawyers implied that I was worse than a murdering psychopath. My reputation was permanently damaged.”
However, the vast majority of oncologists (88%) did not believe that the lawsuit negatively affected their career, which was similar to physicians in general. That said, many did complain about the ongoing requirement to report the lawsuit to the credentialing committee, even if it was dismissed, and then having to pay increased malpractice premiums. “I still need to document this episode every single time I apply for any medical position, even more than 29 years after I was dismissed from the case,” said one respondent.
When asked if they would do anything differently, many oncologists (42%) said no, they would not have done anything differently. This is similar to the responses from physicians in general and with 2019 responses from oncologists. However, 15% of the respondents said that in retrospect, they would not have taken on that patient to begin with.
Some oncologists noted that they would have been more conscientious in relaying the information to the referring physician. Evan Lyman, an associate attorney at Voute, Lohrfink, McAndrew, Meisner & Roberts, LLP, in White Plains, N.Y., pointed out that a common reason for lawsuits is a slip-up of communication between the specialist and the referring physician.
Oncologists who had been sued have some insights to offer to colleagues, should they find themselves in a similar situation.
“Only answer with short and precise statements,” wrote one oncologist. “Attend all the depositions as much as you can; they are more likely to fabricate or exaggerate if you are not sitting in the room.”
Another physician said to base “everything on the medical record and do not answer hypothetical questions.”
“Document all interactions with patients as if a jury will be reading them, word by word,” said one respondent.
As for the public or patients, oncologists had this message: “malpractice suits should be rarely launched and only when gross errors can be absolutely proven.”
Another oncologist pointed out that communication is key. “Speak to the physicians against whom you have distrust. Lots of things could be cleared by good communication.”
A version of this article first appeared on Medscape.com.
, notes the latest Medscape Malpractice Report.
Over a third (39%) of oncologists who were surveyed said that they had been named in at least one malpractice suit, according to the Medscape Oncologist Malpractice Report 2021.
This number is considerably lower than that reported by physicians across all specialties (51%), and it is also much lower than that reported by oncologists in past years. In the 2019 report, 54% of oncologists surveyed said they had been named in a malpractice suit, while in the 2017 report, the figure was 53%.
The dramatic decline in malpractice suits may have much to do with the COVID-19 pandemic, when oncology care was in a state of flux.
“Fewer people were seeking cancer care during the COVID pandemic, which might have impacted the number of lawsuits brought against oncologists,” says Paul Walker, a New York–based malpractice attorney at Walker Medical Law, who represents physicians and other healthcare professionals.
“Additionally, a fair number of people who died of COVID were also older,” he pointed out, and it is often older people who get cancer, so there were fewer older people who consulted an oncologist or were treated by one, he added.
However, the pandemic may be storing up trouble for future years. “Patient fears of contracting COVID-19 have led many to avoid seeking or resuming care, so delays in diagnosing new cancer cases could mean that more patients are diagnosed at a later stage of their disease, leading to potential adverse events and malpractice claims,” commented David L. Feldman, MD, MBA, chief medical officer of The Doctors Company Group.
This latest 2021 Medscape Malpractice Report was compiled from an online survey that included more than 4,300 physicians from 29 specialties. It included 106 oncologists. More than half of respondents (56%) had been in practice for more than 25 years, and 54% were aged 60 years or older. The survey was available from May 21 to August 28, 2021.
Similar to findings in previous years, complications from treatment/surgery were the most common reason for the lawsuits (31%). Failure to make a correct diagnosis or a delay in diagnosis was the second most common reason (23%), while 20% of patients sued because of a poor outcome or disease progression.
Surprise at being sued
Among the oncologists who reported involvement in a lawsuit in 2021, the majority (86%) said they were “very surprised” or “somewhat surprised” by the malpractice suit, which is similar to that of other physicians surveyed. However, fewer were surprised this year as compared to 2019 and 2017 (90% and 94%).
One reason for the surprise over the litigation was that it concerned a patient who had been treated a long time ago. One oncologist wrote that “the patient had not seen me for over 7 years and during that time, he did not call me with his new symptomatology. I was only named in the suit because I had previously been involved.”
Another common scenario reported by oncologists was being named in a lawsuit which was brought by another clinician’s patient. “I was the chairperson of the department, and one of the doctors in the practice was involved in the suit,” wrote one respondent. “I was named as an accomplice.”
More than half of surveyed oncologists said that they were able to identify the patient who bought the suit, and these figures are again comparable to those of other physicians. One oncologist commented that in the case he was involved with, the family did not understand or accept the nature of cancer and the different ways that a patient could die of complications. This patient had died of sepsis and pneumonia related to decubitus ulcers that were completely unrelated to her radiation therapy.
As in the case above, sometimes it is the family who filed the lawsuit, not the patient.
“The patient may even recognize that you did your best and be grateful for your skill and efforts, but the family can’t accept that grandma died of cancer and brings a lawsuit,” said Dennis Hursh, an attorney with Physicians Agreement Health Law in Pennsylvania.
When looking at outcomes of the lawsuit, 40% of oncologists were dismissed from the suit within the first few months, or the case was settled before going to trial. This trend is also consistent with the results from the 2019 and 2017 surveys. When the case did go to trial, 10% received a favorable verdict, which was the same in 2019.
“It seems that most of my clients end up being released from lawsuits, and many lawsuits are dismissed prior to proceeding to trial,” Mr. Hursh commented.
Murdering psychopath
Some oncologists weighed in on what they felt was the worst experience of being sued.
“Mental anguish, knowing that I did nothing wrong,” said one physician. Another reported that it was a feeling of being “inadequate and totally alone.”
Another oncologist commented that the “depositions from lawyers implied that I was worse than a murdering psychopath. My reputation was permanently damaged.”
However, the vast majority of oncologists (88%) did not believe that the lawsuit negatively affected their career, which was similar to physicians in general. That said, many did complain about the ongoing requirement to report the lawsuit to the credentialing committee, even if it was dismissed, and then having to pay increased malpractice premiums. “I still need to document this episode every single time I apply for any medical position, even more than 29 years after I was dismissed from the case,” said one respondent.
When asked if they would do anything differently, many oncologists (42%) said no, they would not have done anything differently. This is similar to the responses from physicians in general and with 2019 responses from oncologists. However, 15% of the respondents said that in retrospect, they would not have taken on that patient to begin with.
Some oncologists noted that they would have been more conscientious in relaying the information to the referring physician. Evan Lyman, an associate attorney at Voute, Lohrfink, McAndrew, Meisner & Roberts, LLP, in White Plains, N.Y., pointed out that a common reason for lawsuits is a slip-up of communication between the specialist and the referring physician.
Oncologists who had been sued have some insights to offer to colleagues, should they find themselves in a similar situation.
“Only answer with short and precise statements,” wrote one oncologist. “Attend all the depositions as much as you can; they are more likely to fabricate or exaggerate if you are not sitting in the room.”
Another physician said to base “everything on the medical record and do not answer hypothetical questions.”
“Document all interactions with patients as if a jury will be reading them, word by word,” said one respondent.
As for the public or patients, oncologists had this message: “malpractice suits should be rarely launched and only when gross errors can be absolutely proven.”
Another oncologist pointed out that communication is key. “Speak to the physicians against whom you have distrust. Lots of things could be cleared by good communication.”
A version of this article first appeared on Medscape.com.
, notes the latest Medscape Malpractice Report.
Over a third (39%) of oncologists who were surveyed said that they had been named in at least one malpractice suit, according to the Medscape Oncologist Malpractice Report 2021.
This number is considerably lower than that reported by physicians across all specialties (51%), and it is also much lower than that reported by oncologists in past years. In the 2019 report, 54% of oncologists surveyed said they had been named in a malpractice suit, while in the 2017 report, the figure was 53%.
The dramatic decline in malpractice suits may have much to do with the COVID-19 pandemic, when oncology care was in a state of flux.
“Fewer people were seeking cancer care during the COVID pandemic, which might have impacted the number of lawsuits brought against oncologists,” says Paul Walker, a New York–based malpractice attorney at Walker Medical Law, who represents physicians and other healthcare professionals.
“Additionally, a fair number of people who died of COVID were also older,” he pointed out, and it is often older people who get cancer, so there were fewer older people who consulted an oncologist or were treated by one, he added.
However, the pandemic may be storing up trouble for future years. “Patient fears of contracting COVID-19 have led many to avoid seeking or resuming care, so delays in diagnosing new cancer cases could mean that more patients are diagnosed at a later stage of their disease, leading to potential adverse events and malpractice claims,” commented David L. Feldman, MD, MBA, chief medical officer of The Doctors Company Group.
This latest 2021 Medscape Malpractice Report was compiled from an online survey that included more than 4,300 physicians from 29 specialties. It included 106 oncologists. More than half of respondents (56%) had been in practice for more than 25 years, and 54% were aged 60 years or older. The survey was available from May 21 to August 28, 2021.
Similar to findings in previous years, complications from treatment/surgery were the most common reason for the lawsuits (31%). Failure to make a correct diagnosis or a delay in diagnosis was the second most common reason (23%), while 20% of patients sued because of a poor outcome or disease progression.
Surprise at being sued
Among the oncologists who reported involvement in a lawsuit in 2021, the majority (86%) said they were “very surprised” or “somewhat surprised” by the malpractice suit, which is similar to that of other physicians surveyed. However, fewer were surprised this year as compared to 2019 and 2017 (90% and 94%).
One reason for the surprise over the litigation was that it concerned a patient who had been treated a long time ago. One oncologist wrote that “the patient had not seen me for over 7 years and during that time, he did not call me with his new symptomatology. I was only named in the suit because I had previously been involved.”
Another common scenario reported by oncologists was being named in a lawsuit which was brought by another clinician’s patient. “I was the chairperson of the department, and one of the doctors in the practice was involved in the suit,” wrote one respondent. “I was named as an accomplice.”
More than half of surveyed oncologists said that they were able to identify the patient who bought the suit, and these figures are again comparable to those of other physicians. One oncologist commented that in the case he was involved with, the family did not understand or accept the nature of cancer and the different ways that a patient could die of complications. This patient had died of sepsis and pneumonia related to decubitus ulcers that were completely unrelated to her radiation therapy.
As in the case above, sometimes it is the family who filed the lawsuit, not the patient.
“The patient may even recognize that you did your best and be grateful for your skill and efforts, but the family can’t accept that grandma died of cancer and brings a lawsuit,” said Dennis Hursh, an attorney with Physicians Agreement Health Law in Pennsylvania.
When looking at outcomes of the lawsuit, 40% of oncologists were dismissed from the suit within the first few months, or the case was settled before going to trial. This trend is also consistent with the results from the 2019 and 2017 surveys. When the case did go to trial, 10% received a favorable verdict, which was the same in 2019.
“It seems that most of my clients end up being released from lawsuits, and many lawsuits are dismissed prior to proceeding to trial,” Mr. Hursh commented.
Murdering psychopath
Some oncologists weighed in on what they felt was the worst experience of being sued.
“Mental anguish, knowing that I did nothing wrong,” said one physician. Another reported that it was a feeling of being “inadequate and totally alone.”
Another oncologist commented that the “depositions from lawyers implied that I was worse than a murdering psychopath. My reputation was permanently damaged.”
However, the vast majority of oncologists (88%) did not believe that the lawsuit negatively affected their career, which was similar to physicians in general. That said, many did complain about the ongoing requirement to report the lawsuit to the credentialing committee, even if it was dismissed, and then having to pay increased malpractice premiums. “I still need to document this episode every single time I apply for any medical position, even more than 29 years after I was dismissed from the case,” said one respondent.
When asked if they would do anything differently, many oncologists (42%) said no, they would not have done anything differently. This is similar to the responses from physicians in general and with 2019 responses from oncologists. However, 15% of the respondents said that in retrospect, they would not have taken on that patient to begin with.
Some oncologists noted that they would have been more conscientious in relaying the information to the referring physician. Evan Lyman, an associate attorney at Voute, Lohrfink, McAndrew, Meisner & Roberts, LLP, in White Plains, N.Y., pointed out that a common reason for lawsuits is a slip-up of communication between the specialist and the referring physician.
Oncologists who had been sued have some insights to offer to colleagues, should they find themselves in a similar situation.
“Only answer with short and precise statements,” wrote one oncologist. “Attend all the depositions as much as you can; they are more likely to fabricate or exaggerate if you are not sitting in the room.”
Another physician said to base “everything on the medical record and do not answer hypothetical questions.”
“Document all interactions with patients as if a jury will be reading them, word by word,” said one respondent.
As for the public or patients, oncologists had this message: “malpractice suits should be rarely launched and only when gross errors can be absolutely proven.”
Another oncologist pointed out that communication is key. “Speak to the physicians against whom you have distrust. Lots of things could be cleared by good communication.”
A version of this article first appeared on Medscape.com.
Guttate Psoriasis Following COVID-19 Infection
Psoriasis is an inflammatory skin condition affecting 1% to 5% of the world population. 1 Guttate psoriasis is a subgroup of psoriasis that most commonly presents as raindroplike, erythematous, silvery, scaly papules. There have been limited reports of guttate psoriasis caused by rhinovirus and COVID-19 infection, but a PubMed search of articles indexed for MEDLINE using the term COVID-19 guttate psoriasis yielded only 3 documented cases of a psoriatic flare secondary to SARS-CoV-2 infection. 1-4 Herein, we detail a case in which a patient with mild SARS-CoV-2 infection who did not have a personal or family history of psoriasis experienced a moderate psoriatic flare 3 weeks after diagnosis of COVID-19.
Case Report
A 55-year-old woman was diagnosed with COVID-19 after SARS-CoV-2 RNA was detected from a nasopharyngeal swab. She reported moderate fatigue but no other symptoms. At the time of infection, she was not taking medications and reported neither a personal nor family history of psoriasis.
Three weeks after the COVID-19 diagnosis, she reported erythematous scaly papules only on the trunk and backs of the legs. Two months after the COVID-19 diagnosis, she was evaluated in our practice and diagnosed with guttate psoriasis. The patient refused biopsy. Physical examination revealed that the affected body surface area had increased to 5%; erythematous, silvery, scaly papules were found on the trunk, anterior and posterior legs, and lateral thighs (Figure). At the time of evaluation, she did not report joint pain or nail changes.
The patient was treated with triamcinolone acetonide cream 0.1% twice daily for 2 to 4 weeks. The guttate psoriasis resolved.
Comment
A sudden psoriatic flare can be linked to dysregulation of the innate immune response. Guttate psoriasis and generalized plaque-type psoriasis are postulated to have similar pathogenetic mechanisms, but guttate psoriasis is the only type of psoriasis that originates from viral infection. Initially, viral RNA will stimulate the toll-like receptor 3 protein, leading to increased production of the pathogenic cytokine IL-36γ and pathogenic chemokine CXCL8 (also known as IL-8), both of which are biomarkers for psoriasis.1 Specifically, IL-36γ and CXCL8 are known to further stimulate the proinflammatory cascade during the innate immune response displayed in guttate psoriasis.5,6
Our patient had a mild case of COVID-19, and she first reported the erythematous and scaly papules 3 weeks after infection. Dysregulation of proinflammatory cytokines must have started in the initial stages—within 7 days—of the viral infection. Guttate psoriasis arises within 3 weeks of infection with other viral and bacterial triggers, most commonly with streptococcal infections.1
Rodríguez et al7 described a phenomenon in which both SARS-CoV-2 and Middle East respiratory syndrome, both caused by a coronavirus, can lead to a reduction of type I interferon, which in turn leads to failure of control of viral replication during initial stages of a viral infection. This triggers an increase in proinflammatory cytokines and chemokines, including IL‐36γ and CXCL8. This pathologic mechanism might apply to SARS-CoV-2, as demonstrated in our patient’s sudden psoriatic flare 3 weeks after the COVID-19 diagnosis. However, further investigation and quantification of the putatively involved cytokines is necessary for confirmation.
Conclusion
Psoriasis, a chronic inflammatory skin condition, has been linked predominantly to genetic and environmental factors. Guttate psoriasis as a secondary reaction after streptococcal tonsillar and respiratory infections has been reported.1
Our case is the fourth documented case of guttate psoriasis secondary to COVID-19 infection.2-4 However, it is the second documented case of a patient with a diagnosis of guttate psoriasis secondary to COVID-19 infection who had neither a personal nor family history of psoriasis.
Because SARS-CoV-2 is a novel virus, the long-term effects of COVID-19 remain unclear. We report this case and its findings to introduce a novel clinical manifestation of SARS-CoV-2 infection.
- Sbidian E, Madrange M, Viguier M, et al. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br J Dermatol. 2019;181:1304-1306. doi:10.1111/bjd.18203
- Gananandan K, Sacks B, Ewing I. Guttate psoriasis secondary to COVID-19. BMJ Case Rep. 2020;13:e237367. doi:10.1136/bcr-2020-237367
- Rouai M, Rabhi F, Mansouri N, et al. New-onset guttate psoriasis secondary to COVID-19. Clin Case Rep. 2021;9:e04542. doi:10.1002/ccr3.4542
- Agarwal A, Tripathy T, Kar BR. Guttate flare in a patient with chronic plaque psoriasis following COVID-19 infection: a case report. J Cosmet Dermatol. 2021;20:3064-3065. doi:10.1111/jocd.14396
- Madonna S, Girolomoni G, Dinarello CA, et al. The significance of IL-36 hyperactivation and IL-36R targeting in psoriasis. Int J Mol Sci. 2019;20:3318. doi:10.3390/ijms20133318
- Nedoszytko B, Sokołowska-Wojdyło M, Ruckemann-Dziurdzin´ska K, et al. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatol Alergol. 2014;31:84-91. doi:10.5114/pdia.2014.40920
- Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020;114:102506. doi:10.1016/j.jaut.2020.102506
Psoriasis is an inflammatory skin condition affecting 1% to 5% of the world population. 1 Guttate psoriasis is a subgroup of psoriasis that most commonly presents as raindroplike, erythematous, silvery, scaly papules. There have been limited reports of guttate psoriasis caused by rhinovirus and COVID-19 infection, but a PubMed search of articles indexed for MEDLINE using the term COVID-19 guttate psoriasis yielded only 3 documented cases of a psoriatic flare secondary to SARS-CoV-2 infection. 1-4 Herein, we detail a case in which a patient with mild SARS-CoV-2 infection who did not have a personal or family history of psoriasis experienced a moderate psoriatic flare 3 weeks after diagnosis of COVID-19.
Case Report
A 55-year-old woman was diagnosed with COVID-19 after SARS-CoV-2 RNA was detected from a nasopharyngeal swab. She reported moderate fatigue but no other symptoms. At the time of infection, she was not taking medications and reported neither a personal nor family history of psoriasis.
Three weeks after the COVID-19 diagnosis, she reported erythematous scaly papules only on the trunk and backs of the legs. Two months after the COVID-19 diagnosis, she was evaluated in our practice and diagnosed with guttate psoriasis. The patient refused biopsy. Physical examination revealed that the affected body surface area had increased to 5%; erythematous, silvery, scaly papules were found on the trunk, anterior and posterior legs, and lateral thighs (Figure). At the time of evaluation, she did not report joint pain or nail changes.

The patient was treated with triamcinolone acetonide cream 0.1% twice daily for 2 to 4 weeks. The guttate psoriasis resolved.
Comment
A sudden psoriatic flare can be linked to dysregulation of the innate immune response. Guttate psoriasis and generalized plaque-type psoriasis are postulated to have similar pathogenetic mechanisms, but guttate psoriasis is the only type of psoriasis that originates from viral infection. Initially, viral RNA will stimulate the toll-like receptor 3 protein, leading to increased production of the pathogenic cytokine IL-36γ and pathogenic chemokine CXCL8 (also known as IL-8), both of which are biomarkers for psoriasis.1 Specifically, IL-36γ and CXCL8 are known to further stimulate the proinflammatory cascade during the innate immune response displayed in guttate psoriasis.5,6
Our patient had a mild case of COVID-19, and she first reported the erythematous and scaly papules 3 weeks after infection. Dysregulation of proinflammatory cytokines must have started in the initial stages—within 7 days—of the viral infection. Guttate psoriasis arises within 3 weeks of infection with other viral and bacterial triggers, most commonly with streptococcal infections.1
Rodríguez et al7 described a phenomenon in which both SARS-CoV-2 and Middle East respiratory syndrome, both caused by a coronavirus, can lead to a reduction of type I interferon, which in turn leads to failure of control of viral replication during initial stages of a viral infection. This triggers an increase in proinflammatory cytokines and chemokines, including IL‐36γ and CXCL8. This pathologic mechanism might apply to SARS-CoV-2, as demonstrated in our patient’s sudden psoriatic flare 3 weeks after the COVID-19 diagnosis. However, further investigation and quantification of the putatively involved cytokines is necessary for confirmation.
Conclusion
Psoriasis, a chronic inflammatory skin condition, has been linked predominantly to genetic and environmental factors. Guttate psoriasis as a secondary reaction after streptococcal tonsillar and respiratory infections has been reported.1
Our case is the fourth documented case of guttate psoriasis secondary to COVID-19 infection.2-4 However, it is the second documented case of a patient with a diagnosis of guttate psoriasis secondary to COVID-19 infection who had neither a personal nor family history of psoriasis.
Because SARS-CoV-2 is a novel virus, the long-term effects of COVID-19 remain unclear. We report this case and its findings to introduce a novel clinical manifestation of SARS-CoV-2 infection.
Psoriasis is an inflammatory skin condition affecting 1% to 5% of the world population. 1 Guttate psoriasis is a subgroup of psoriasis that most commonly presents as raindroplike, erythematous, silvery, scaly papules. There have been limited reports of guttate psoriasis caused by rhinovirus and COVID-19 infection, but a PubMed search of articles indexed for MEDLINE using the term COVID-19 guttate psoriasis yielded only 3 documented cases of a psoriatic flare secondary to SARS-CoV-2 infection. 1-4 Herein, we detail a case in which a patient with mild SARS-CoV-2 infection who did not have a personal or family history of psoriasis experienced a moderate psoriatic flare 3 weeks after diagnosis of COVID-19.
Case Report
A 55-year-old woman was diagnosed with COVID-19 after SARS-CoV-2 RNA was detected from a nasopharyngeal swab. She reported moderate fatigue but no other symptoms. At the time of infection, she was not taking medications and reported neither a personal nor family history of psoriasis.
Three weeks after the COVID-19 diagnosis, she reported erythematous scaly papules only on the trunk and backs of the legs. Two months after the COVID-19 diagnosis, she was evaluated in our practice and diagnosed with guttate psoriasis. The patient refused biopsy. Physical examination revealed that the affected body surface area had increased to 5%; erythematous, silvery, scaly papules were found on the trunk, anterior and posterior legs, and lateral thighs (Figure). At the time of evaluation, she did not report joint pain or nail changes.

The patient was treated with triamcinolone acetonide cream 0.1% twice daily for 2 to 4 weeks. The guttate psoriasis resolved.
Comment
A sudden psoriatic flare can be linked to dysregulation of the innate immune response. Guttate psoriasis and generalized plaque-type psoriasis are postulated to have similar pathogenetic mechanisms, but guttate psoriasis is the only type of psoriasis that originates from viral infection. Initially, viral RNA will stimulate the toll-like receptor 3 protein, leading to increased production of the pathogenic cytokine IL-36γ and pathogenic chemokine CXCL8 (also known as IL-8), both of which are biomarkers for psoriasis.1 Specifically, IL-36γ and CXCL8 are known to further stimulate the proinflammatory cascade during the innate immune response displayed in guttate psoriasis.5,6
Our patient had a mild case of COVID-19, and she first reported the erythematous and scaly papules 3 weeks after infection. Dysregulation of proinflammatory cytokines must have started in the initial stages—within 7 days—of the viral infection. Guttate psoriasis arises within 3 weeks of infection with other viral and bacterial triggers, most commonly with streptococcal infections.1
Rodríguez et al7 described a phenomenon in which both SARS-CoV-2 and Middle East respiratory syndrome, both caused by a coronavirus, can lead to a reduction of type I interferon, which in turn leads to failure of control of viral replication during initial stages of a viral infection. This triggers an increase in proinflammatory cytokines and chemokines, including IL‐36γ and CXCL8. This pathologic mechanism might apply to SARS-CoV-2, as demonstrated in our patient’s sudden psoriatic flare 3 weeks after the COVID-19 diagnosis. However, further investigation and quantification of the putatively involved cytokines is necessary for confirmation.
Conclusion
Psoriasis, a chronic inflammatory skin condition, has been linked predominantly to genetic and environmental factors. Guttate psoriasis as a secondary reaction after streptococcal tonsillar and respiratory infections has been reported.1
Our case is the fourth documented case of guttate psoriasis secondary to COVID-19 infection.2-4 However, it is the second documented case of a patient with a diagnosis of guttate psoriasis secondary to COVID-19 infection who had neither a personal nor family history of psoriasis.
Because SARS-CoV-2 is a novel virus, the long-term effects of COVID-19 remain unclear. We report this case and its findings to introduce a novel clinical manifestation of SARS-CoV-2 infection.
- Sbidian E, Madrange M, Viguier M, et al. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br J Dermatol. 2019;181:1304-1306. doi:10.1111/bjd.18203
- Gananandan K, Sacks B, Ewing I. Guttate psoriasis secondary to COVID-19. BMJ Case Rep. 2020;13:e237367. doi:10.1136/bcr-2020-237367
- Rouai M, Rabhi F, Mansouri N, et al. New-onset guttate psoriasis secondary to COVID-19. Clin Case Rep. 2021;9:e04542. doi:10.1002/ccr3.4542
- Agarwal A, Tripathy T, Kar BR. Guttate flare in a patient with chronic plaque psoriasis following COVID-19 infection: a case report. J Cosmet Dermatol. 2021;20:3064-3065. doi:10.1111/jocd.14396
- Madonna S, Girolomoni G, Dinarello CA, et al. The significance of IL-36 hyperactivation and IL-36R targeting in psoriasis. Int J Mol Sci. 2019;20:3318. doi:10.3390/ijms20133318
- Nedoszytko B, Sokołowska-Wojdyło M, Ruckemann-Dziurdzin´ska K, et al. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatol Alergol. 2014;31:84-91. doi:10.5114/pdia.2014.40920
- Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020;114:102506. doi:10.1016/j.jaut.2020.102506
- Sbidian E, Madrange M, Viguier M, et al. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br J Dermatol. 2019;181:1304-1306. doi:10.1111/bjd.18203
- Gananandan K, Sacks B, Ewing I. Guttate psoriasis secondary to COVID-19. BMJ Case Rep. 2020;13:e237367. doi:10.1136/bcr-2020-237367
- Rouai M, Rabhi F, Mansouri N, et al. New-onset guttate psoriasis secondary to COVID-19. Clin Case Rep. 2021;9:e04542. doi:10.1002/ccr3.4542
- Agarwal A, Tripathy T, Kar BR. Guttate flare in a patient with chronic plaque psoriasis following COVID-19 infection: a case report. J Cosmet Dermatol. 2021;20:3064-3065. doi:10.1111/jocd.14396
- Madonna S, Girolomoni G, Dinarello CA, et al. The significance of IL-36 hyperactivation and IL-36R targeting in psoriasis. Int J Mol Sci. 2019;20:3318. doi:10.3390/ijms20133318
- Nedoszytko B, Sokołowska-Wojdyło M, Ruckemann-Dziurdzin´ska K, et al. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatol Alergol. 2014;31:84-91. doi:10.5114/pdia.2014.40920
- Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020;114:102506. doi:10.1016/j.jaut.2020.102506
Practice Points
- Guttate psoriasis is the only type of psoriasis that originates from viral infection.
- Dysregulation of proinflammatory cytokines during COVID-19 infection in our patient led to development of guttate psoriasis 3 weeks later.
EMA gives green light to new CAR T-cell therapy
At its late January meeting, the Committee for Medicinal Products for Human Use of the European Medicines Agency recommended for approval lisocabtagene maraleucel (Breyanzi, Bristol-Myers Squibb). This chimeric antigen receptor T-cell therapy is indicated for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma (PMBCL), and follicular lymphoma grade 3B (FL3B). The indication is for use in patients who have received at least two lines of treatment.
The benefits of lisocabtagene maraleucel, noted the CHMP, are its ability to provide high and durable responses in patients with relapsed or refractory DLBCL, PMBCL, and FL3B. The most common side effects reported are neutropenia, anemia, cytokine release syndrome, fatigue, and thrombocytopenia.
The product is already approved in the United States for the same indication. The Food and Drug Administration’s approval came with a Risk Evaluation and Mitigation Strategy because of the risk for serious adverse events, including cytokine release syndrome.
During development, it was designated as an orphan medicine. The EMA will now review the information available to date to determine if the orphan designation can be maintained.
Biosimilar pegfilgrastim
At the same meeting, the committee recommended approval of a biosimilar product for pegfilgrastim (Stimufend, Fresenius Kabi Deutschland), which is used to reduce the duration of neutropenia and the incidence of febrile neutropenia after cytotoxic chemotherapy.
The committee noted that this product has been shown to be highly similar to the reference product Neulasta (pegfilgrastim), which has been available in the EU for 2 decades (authorized in 2002). Data have demonstrated that Stimufend has comparable quality, safety, and efficacy to Neulasta.
Its full indication is to reduce the duration of neutropenia and incidence of febrile neutropenia in adult patients treated with cytotoxic chemotherapy for malignancies, with the exception of chronic myeloid leukemia (CML) and myelodysplastic syndromes.
Generic versions of dasatinib
Also recommended for approval were for two generic formulations of dasatinib (Dasatinib Accord and Dasatinib Accordpharma, both from Accord Healthcare) for the treatment of various leukemias.
These are generic versions of dasatinib (Sprycel), which has been available in the European Union since 2006.
The CHMP noted that studies have demonstrated the satisfactory quality of Dasatinib Accord, as well as its bioequivalence to the reference product. This generic is indicated for the treatment of adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia with resistance or intolerance to prior therapy and pediatric patients with newly diagnosed Ph+ ALL in combination with chemotherapy.
Dasatinib Accordpharma has a wider set of indications, which include the treatment of adult patients with newly diagnosed Ph+ CML in the chronic phase; chronic, accelerated, or blast phase CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL and lymphoid blast CML with resistance or intolerance to prior therapy. In addition, this generic is indicated for the treatment of pediatric patients with newly diagnosed Ph+ CML in the chronic phase or Ph+ CML-CP resistant or intolerant to prior therapy including imatinib and newly diagnosed Ph+ ALL in combination with chemotherapy.
A version of this article first appeared on Medscape.com.
At its late January meeting, the Committee for Medicinal Products for Human Use of the European Medicines Agency recommended for approval lisocabtagene maraleucel (Breyanzi, Bristol-Myers Squibb). This chimeric antigen receptor T-cell therapy is indicated for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma (PMBCL), and follicular lymphoma grade 3B (FL3B). The indication is for use in patients who have received at least two lines of treatment.
The benefits of lisocabtagene maraleucel, noted the CHMP, are its ability to provide high and durable responses in patients with relapsed or refractory DLBCL, PMBCL, and FL3B. The most common side effects reported are neutropenia, anemia, cytokine release syndrome, fatigue, and thrombocytopenia.
The product is already approved in the United States for the same indication. The Food and Drug Administration’s approval came with a Risk Evaluation and Mitigation Strategy because of the risk for serious adverse events, including cytokine release syndrome.
During development, it was designated as an orphan medicine. The EMA will now review the information available to date to determine if the orphan designation can be maintained.
Biosimilar pegfilgrastim
At the same meeting, the committee recommended approval of a biosimilar product for pegfilgrastim (Stimufend, Fresenius Kabi Deutschland), which is used to reduce the duration of neutropenia and the incidence of febrile neutropenia after cytotoxic chemotherapy.
The committee noted that this product has been shown to be highly similar to the reference product Neulasta (pegfilgrastim), which has been available in the EU for 2 decades (authorized in 2002). Data have demonstrated that Stimufend has comparable quality, safety, and efficacy to Neulasta.
Its full indication is to reduce the duration of neutropenia and incidence of febrile neutropenia in adult patients treated with cytotoxic chemotherapy for malignancies, with the exception of chronic myeloid leukemia (CML) and myelodysplastic syndromes.
Generic versions of dasatinib
Also recommended for approval were for two generic formulations of dasatinib (Dasatinib Accord and Dasatinib Accordpharma, both from Accord Healthcare) for the treatment of various leukemias.
These are generic versions of dasatinib (Sprycel), which has been available in the European Union since 2006.
The CHMP noted that studies have demonstrated the satisfactory quality of Dasatinib Accord, as well as its bioequivalence to the reference product. This generic is indicated for the treatment of adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia with resistance or intolerance to prior therapy and pediatric patients with newly diagnosed Ph+ ALL in combination with chemotherapy.
Dasatinib Accordpharma has a wider set of indications, which include the treatment of adult patients with newly diagnosed Ph+ CML in the chronic phase; chronic, accelerated, or blast phase CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL and lymphoid blast CML with resistance or intolerance to prior therapy. In addition, this generic is indicated for the treatment of pediatric patients with newly diagnosed Ph+ CML in the chronic phase or Ph+ CML-CP resistant or intolerant to prior therapy including imatinib and newly diagnosed Ph+ ALL in combination with chemotherapy.
A version of this article first appeared on Medscape.com.
At its late January meeting, the Committee for Medicinal Products for Human Use of the European Medicines Agency recommended for approval lisocabtagene maraleucel (Breyanzi, Bristol-Myers Squibb). This chimeric antigen receptor T-cell therapy is indicated for the treatment of relapsed or refractory diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma (PMBCL), and follicular lymphoma grade 3B (FL3B). The indication is for use in patients who have received at least two lines of treatment.
The benefits of lisocabtagene maraleucel, noted the CHMP, are its ability to provide high and durable responses in patients with relapsed or refractory DLBCL, PMBCL, and FL3B. The most common side effects reported are neutropenia, anemia, cytokine release syndrome, fatigue, and thrombocytopenia.
The product is already approved in the United States for the same indication. The Food and Drug Administration’s approval came with a Risk Evaluation and Mitigation Strategy because of the risk for serious adverse events, including cytokine release syndrome.
During development, it was designated as an orphan medicine. The EMA will now review the information available to date to determine if the orphan designation can be maintained.
Biosimilar pegfilgrastim
At the same meeting, the committee recommended approval of a biosimilar product for pegfilgrastim (Stimufend, Fresenius Kabi Deutschland), which is used to reduce the duration of neutropenia and the incidence of febrile neutropenia after cytotoxic chemotherapy.
The committee noted that this product has been shown to be highly similar to the reference product Neulasta (pegfilgrastim), which has been available in the EU for 2 decades (authorized in 2002). Data have demonstrated that Stimufend has comparable quality, safety, and efficacy to Neulasta.
Its full indication is to reduce the duration of neutropenia and incidence of febrile neutropenia in adult patients treated with cytotoxic chemotherapy for malignancies, with the exception of chronic myeloid leukemia (CML) and myelodysplastic syndromes.
Generic versions of dasatinib
Also recommended for approval were for two generic formulations of dasatinib (Dasatinib Accord and Dasatinib Accordpharma, both from Accord Healthcare) for the treatment of various leukemias.
These are generic versions of dasatinib (Sprycel), which has been available in the European Union since 2006.
The CHMP noted that studies have demonstrated the satisfactory quality of Dasatinib Accord, as well as its bioequivalence to the reference product. This generic is indicated for the treatment of adult patients with Philadelphia chromosome–positive acute lymphoblastic leukemia with resistance or intolerance to prior therapy and pediatric patients with newly diagnosed Ph+ ALL in combination with chemotherapy.
Dasatinib Accordpharma has a wider set of indications, which include the treatment of adult patients with newly diagnosed Ph+ CML in the chronic phase; chronic, accelerated, or blast phase CML with resistance or intolerance to prior therapy including imatinib; and Ph+ ALL and lymphoid blast CML with resistance or intolerance to prior therapy. In addition, this generic is indicated for the treatment of pediatric patients with newly diagnosed Ph+ CML in the chronic phase or Ph+ CML-CP resistant or intolerant to prior therapy including imatinib and newly diagnosed Ph+ ALL in combination with chemotherapy.
A version of this article first appeared on Medscape.com.