User login
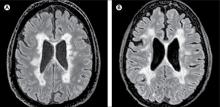
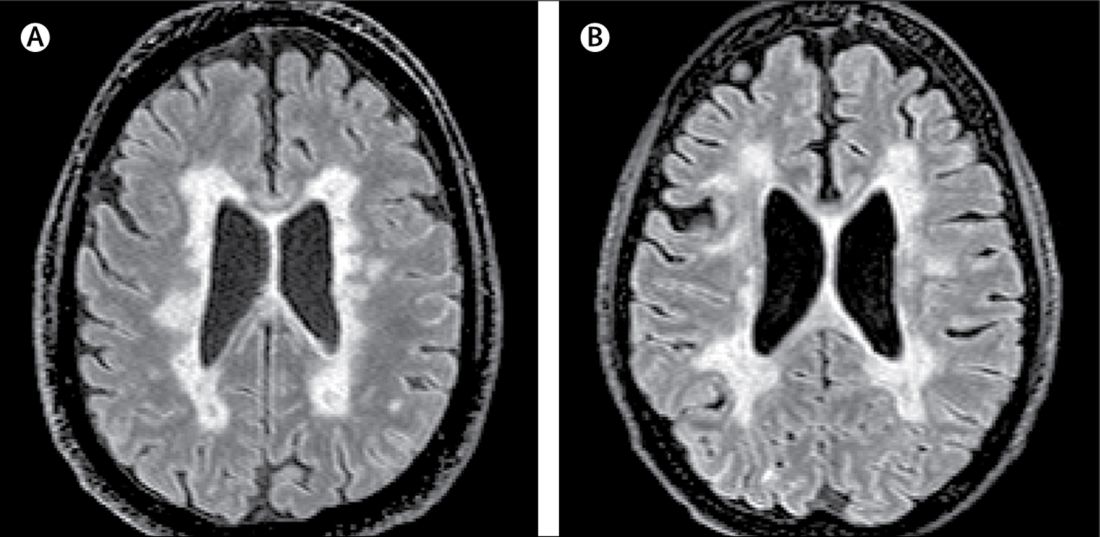
How Many Patients Have Benign MS?
Patients and physicians interpret the term differently, thus making its use in the clinical setting problematic.
An estimated 3% of patients with multiple sclerosis (MS) have a benign course of disease, according to findings from a population-based UK study published online ahead of print September 3 in Journal of Neurology, Neurosurgery & Psychiatry. The term “benign MS” remains problematic, however.
“The study of the individuals with extremely favorable outcomes may uncover insights about disease pathogenesis or repair. However, the insensitivity of Expanded Disability Status Scale [EDSS]–based definitions of benign MS and the discrepancy between patient and clinician perception of benign MS undermine use of the term ‘benign’ in the clinical setting,” said Emma Clare Tallantyre, BMBS, PhD, Clinical Senior Lecturer in Neurosciences at Cardiff University in the UK, and her colleagues.
The investigators found that of 1,049 patients with a disease duration of longer than 15 years, 200 had a recent EDSS score of less than 4.0. Of those patients, 60 were clinically assessed, and nine (15%) had benign MS, which was defined as an EDSS score less than 3.0 and lack of significant fatigue, mood disturbance, cognitive impairment, and disruption to employment in the absence of disease-modifying therapy at at least 15 years after symptom onset.
Extrapolating these data, the investigators estimated that 30 patients in the study population of 1,049 had benign MS, yielding a prevalence of 2.9%. Of the 60 patients who were clinically assessed, 39 thought they had benign MS, based on the following definition: “When referring to illness, ‘benign’ usually means a condition which has little or no harmful effects on a person. There are no complications, and there is a good outcome or prognosis.”
Patients who self-reported benign MS had significantly lower EDSS scores, fewer depressive symptoms, lower fatigue severity, and lower reported MS impact than did patients who did not report benign MS. “Self-reported benign MS status showed poor agreement with our composite definition of benign MS status and only fair agreement with EDSS-based definitions of benign MS status,” said the investigators.
—Jeff Evans
Suggested Reading
Tallantyre EC, Major PC, Atherton MJ, et al. How common is truly benign MS in a UK population? J Neurol Neurosurg Psychiatry. 2018 Sep 3 [Epub ahead of print].
Patients and physicians interpret the term differently, thus making its use in the clinical setting problematic.
Patients and physicians interpret the term differently, thus making its use in the clinical setting problematic.
An estimated 3% of patients with multiple sclerosis (MS) have a benign course of disease, according to findings from a population-based UK study published online ahead of print September 3 in Journal of Neurology, Neurosurgery & Psychiatry. The term “benign MS” remains problematic, however.
“The study of the individuals with extremely favorable outcomes may uncover insights about disease pathogenesis or repair. However, the insensitivity of Expanded Disability Status Scale [EDSS]–based definitions of benign MS and the discrepancy between patient and clinician perception of benign MS undermine use of the term ‘benign’ in the clinical setting,” said Emma Clare Tallantyre, BMBS, PhD, Clinical Senior Lecturer in Neurosciences at Cardiff University in the UK, and her colleagues.
The investigators found that of 1,049 patients with a disease duration of longer than 15 years, 200 had a recent EDSS score of less than 4.0. Of those patients, 60 were clinically assessed, and nine (15%) had benign MS, which was defined as an EDSS score less than 3.0 and lack of significant fatigue, mood disturbance, cognitive impairment, and disruption to employment in the absence of disease-modifying therapy at at least 15 years after symptom onset.
Extrapolating these data, the investigators estimated that 30 patients in the study population of 1,049 had benign MS, yielding a prevalence of 2.9%. Of the 60 patients who were clinically assessed, 39 thought they had benign MS, based on the following definition: “When referring to illness, ‘benign’ usually means a condition which has little or no harmful effects on a person. There are no complications, and there is a good outcome or prognosis.”
Patients who self-reported benign MS had significantly lower EDSS scores, fewer depressive symptoms, lower fatigue severity, and lower reported MS impact than did patients who did not report benign MS. “Self-reported benign MS status showed poor agreement with our composite definition of benign MS status and only fair agreement with EDSS-based definitions of benign MS status,” said the investigators.
—Jeff Evans
Suggested Reading
Tallantyre EC, Major PC, Atherton MJ, et al. How common is truly benign MS in a UK population? J Neurol Neurosurg Psychiatry. 2018 Sep 3 [Epub ahead of print].
An estimated 3% of patients with multiple sclerosis (MS) have a benign course of disease, according to findings from a population-based UK study published online ahead of print September 3 in Journal of Neurology, Neurosurgery & Psychiatry. The term “benign MS” remains problematic, however.
“The study of the individuals with extremely favorable outcomes may uncover insights about disease pathogenesis or repair. However, the insensitivity of Expanded Disability Status Scale [EDSS]–based definitions of benign MS and the discrepancy between patient and clinician perception of benign MS undermine use of the term ‘benign’ in the clinical setting,” said Emma Clare Tallantyre, BMBS, PhD, Clinical Senior Lecturer in Neurosciences at Cardiff University in the UK, and her colleagues.
The investigators found that of 1,049 patients with a disease duration of longer than 15 years, 200 had a recent EDSS score of less than 4.0. Of those patients, 60 were clinically assessed, and nine (15%) had benign MS, which was defined as an EDSS score less than 3.0 and lack of significant fatigue, mood disturbance, cognitive impairment, and disruption to employment in the absence of disease-modifying therapy at at least 15 years after symptom onset.
Extrapolating these data, the investigators estimated that 30 patients in the study population of 1,049 had benign MS, yielding a prevalence of 2.9%. Of the 60 patients who were clinically assessed, 39 thought they had benign MS, based on the following definition: “When referring to illness, ‘benign’ usually means a condition which has little or no harmful effects on a person. There are no complications, and there is a good outcome or prognosis.”
Patients who self-reported benign MS had significantly lower EDSS scores, fewer depressive symptoms, lower fatigue severity, and lower reported MS impact than did patients who did not report benign MS. “Self-reported benign MS status showed poor agreement with our composite definition of benign MS status and only fair agreement with EDSS-based definitions of benign MS status,” said the investigators.
—Jeff Evans
Suggested Reading
Tallantyre EC, Major PC, Atherton MJ, et al. How common is truly benign MS in a UK population? J Neurol Neurosurg Psychiatry. 2018 Sep 3 [Epub ahead of print].
Teva Announces FDA Approval of Ajovy (fremanezumab-vfrm)
Teva Pharmaceutical Industries Ltd. announced that the FDA approved Ajovy (fremanezumab-vfrm) injection for the preventive treatment of migraine in adults. Ajovy, a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor, is the first and only anti-CGRP treatment for the prevention of migraine with quarterly (675 mg) and monthly (225 mg) dosing options.
“Migraine is a disabling neurological disease that affects more than 36 million people in the United States,” said Stephen Silberstein
Ajovy was evaluated in two Phase III, placebo-controlled clinical trials that enrolled patients with disabling migraine and was studied as both a stand-alone preventive treatment and in combination with oral preventive treatments. In these trials, patients experienced a reduction in monthly migraine days during a 12-week period. The most common adverse reactions (≥ 5% and greater than placebo) were injection site reactions.
Teva Pharmaceutical Industries Ltd. announced that the FDA approved Ajovy (fremanezumab-vfrm) injection for the preventive treatment of migraine in adults. Ajovy, a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor, is the first and only anti-CGRP treatment for the prevention of migraine with quarterly (675 mg) and monthly (225 mg) dosing options.
“Migraine is a disabling neurological disease that affects more than 36 million people in the United States,” said Stephen Silberstein
Ajovy was evaluated in two Phase III, placebo-controlled clinical trials that enrolled patients with disabling migraine and was studied as both a stand-alone preventive treatment and in combination with oral preventive treatments. In these trials, patients experienced a reduction in monthly migraine days during a 12-week period. The most common adverse reactions (≥ 5% and greater than placebo) were injection site reactions.
Teva Pharmaceutical Industries Ltd. announced that the FDA approved Ajovy (fremanezumab-vfrm) injection for the preventive treatment of migraine in adults. Ajovy, a humanized monoclonal antibody that binds to the calcitonin gene-related peptide (CGRP) ligand and blocks its binding to the receptor, is the first and only anti-CGRP treatment for the prevention of migraine with quarterly (675 mg) and monthly (225 mg) dosing options.
“Migraine is a disabling neurological disease that affects more than 36 million people in the United States,” said Stephen Silberstein
Ajovy was evaluated in two Phase III, placebo-controlled clinical trials that enrolled patients with disabling migraine and was studied as both a stand-alone preventive treatment and in combination with oral preventive treatments. In these trials, patients experienced a reduction in monthly migraine days during a 12-week period. The most common adverse reactions (≥ 5% and greater than placebo) were injection site reactions.
Benign MS is real in small minority of patients
Nearly 3% of patients with multiple sclerosis (MS) are estimated to have a truly benign course of disease over at least 15 years without the use of disease-modifying therapy, based on findings from a U.K. population-based study that also showed how poorly benign disease tracks with disability measures and lacks agreement between patients and physicians.
“The study of the individuals with extremely favorable outcomes may uncover insights about disease pathogenesis or repair. However, the insensitivity of EDSS [Expanded Disability Status Scale]–based definitions of benign MS and the discrepancy between patient and clinician perception of benign MS undermine use of the term ‘benign’ in the clinical setting,” Emma Clare Tallantyre, MD, of Cardiff (Wales) University, and her colleagues wrote in the Journal of Neurology, Neurosurgery & Psychiatry.
Dr. Tallantyre and her colleagues found that, of 1,049 patients with disease duration longer than 15 years, 200 had a recent EDSS score of less than 4.0. Of those 200, 60 were clinically assessed and 9 (15%) were found to have truly benign MS, defined as having an EDSS less than 3.0 and having no significant fatigue, mood disturbance, cognitive impairment, or disruption to employment in the absence of disease-modifying therapy at least 15 years after symptom onset.
The investigators extrapolated these data to estimate that 30 patients in the study population of 1,049 had truly benign MS, for a prevalence of 2.9%. However, of the 60 patients who were clinically assessed, 39 thought they had benign MS based on the lay definition provided: “When referring to illness, ‘benign’ usually means a condition which has little or no harmful effects on a person. There are no complications and there is a good outcome or prognosis.”
Patients who self-reported benign MS had significantly lower EDSS scores, fewer depressive symptoms, lower fatigue severity, and lower reported MS impact than did patients who did not report benign MS. “Self-reported benign MS status showed poor agreement with our composite definition of benign MS status and only fair agreement with EDSS-based definitions of benign MS status,” the investigators wrote.
SOURCE: Tallantyre EC et al. J Neurol Neurosurg Psychiatry. 2018 Sep 3. doi: 10.1136/jnnp-2018-318802.
Nearly 3% of patients with multiple sclerosis (MS) are estimated to have a truly benign course of disease over at least 15 years without the use of disease-modifying therapy, based on findings from a U.K. population-based study that also showed how poorly benign disease tracks with disability measures and lacks agreement between patients and physicians.
“The study of the individuals with extremely favorable outcomes may uncover insights about disease pathogenesis or repair. However, the insensitivity of EDSS [Expanded Disability Status Scale]–based definitions of benign MS and the discrepancy between patient and clinician perception of benign MS undermine use of the term ‘benign’ in the clinical setting,” Emma Clare Tallantyre, MD, of Cardiff (Wales) University, and her colleagues wrote in the Journal of Neurology, Neurosurgery & Psychiatry.
Dr. Tallantyre and her colleagues found that, of 1,049 patients with disease duration longer than 15 years, 200 had a recent EDSS score of less than 4.0. Of those 200, 60 were clinically assessed and 9 (15%) were found to have truly benign MS, defined as having an EDSS less than 3.0 and having no significant fatigue, mood disturbance, cognitive impairment, or disruption to employment in the absence of disease-modifying therapy at least 15 years after symptom onset.
The investigators extrapolated these data to estimate that 30 patients in the study population of 1,049 had truly benign MS, for a prevalence of 2.9%. However, of the 60 patients who were clinically assessed, 39 thought they had benign MS based on the lay definition provided: “When referring to illness, ‘benign’ usually means a condition which has little or no harmful effects on a person. There are no complications and there is a good outcome or prognosis.”
Patients who self-reported benign MS had significantly lower EDSS scores, fewer depressive symptoms, lower fatigue severity, and lower reported MS impact than did patients who did not report benign MS. “Self-reported benign MS status showed poor agreement with our composite definition of benign MS status and only fair agreement with EDSS-based definitions of benign MS status,” the investigators wrote.
SOURCE: Tallantyre EC et al. J Neurol Neurosurg Psychiatry. 2018 Sep 3. doi: 10.1136/jnnp-2018-318802.
Nearly 3% of patients with multiple sclerosis (MS) are estimated to have a truly benign course of disease over at least 15 years without the use of disease-modifying therapy, based on findings from a U.K. population-based study that also showed how poorly benign disease tracks with disability measures and lacks agreement between patients and physicians.
“The study of the individuals with extremely favorable outcomes may uncover insights about disease pathogenesis or repair. However, the insensitivity of EDSS [Expanded Disability Status Scale]–based definitions of benign MS and the discrepancy between patient and clinician perception of benign MS undermine use of the term ‘benign’ in the clinical setting,” Emma Clare Tallantyre, MD, of Cardiff (Wales) University, and her colleagues wrote in the Journal of Neurology, Neurosurgery & Psychiatry.
Dr. Tallantyre and her colleagues found that, of 1,049 patients with disease duration longer than 15 years, 200 had a recent EDSS score of less than 4.0. Of those 200, 60 were clinically assessed and 9 (15%) were found to have truly benign MS, defined as having an EDSS less than 3.0 and having no significant fatigue, mood disturbance, cognitive impairment, or disruption to employment in the absence of disease-modifying therapy at least 15 years after symptom onset.
The investigators extrapolated these data to estimate that 30 patients in the study population of 1,049 had truly benign MS, for a prevalence of 2.9%. However, of the 60 patients who were clinically assessed, 39 thought they had benign MS based on the lay definition provided: “When referring to illness, ‘benign’ usually means a condition which has little or no harmful effects on a person. There are no complications and there is a good outcome or prognosis.”
Patients who self-reported benign MS had significantly lower EDSS scores, fewer depressive symptoms, lower fatigue severity, and lower reported MS impact than did patients who did not report benign MS. “Self-reported benign MS status showed poor agreement with our composite definition of benign MS status and only fair agreement with EDSS-based definitions of benign MS status,” the investigators wrote.
SOURCE: Tallantyre EC et al. J Neurol Neurosurg Psychiatry. 2018 Sep 3. doi: 10.1136/jnnp-2018-318802.
FROM THE JOURNAL OF NEUROLOGY, NEUROSURGERY & PSYCHIATRY
Serum Nf-L shows promise as biomarker for BMT response in MS
LOS ANGELES – Serum neurofilament light chain shows promise as a biomarker for disease severity and response to treatment with autologous bone marrow transplant in patients with multiple sclerosis, according to findings from an analysis of paired samples.
Serum neurofilament light chain (Nf-L) levels were found to be significantly elevated in both the serum and cerebrospinal fluid (CSF) of 23 patients with aggressive multiple sclerosis (MS), compared with samples from 5 controls with noninflammatory neurologic conditions such as chronic fatigue syndrome or migraine. The levels in the patients with MS were significantly reduced following autologous bone marrow transplant (BMT), Simon Thebault, MD, reported in a poster at the annual meeting of the American Academy of Neurology.
Nf-L has been shown to be a biomarker for neuronal damage and to have potential for denoting disease severity and treatment response in MS; for this analysis, samples from patients were collected at baseline and annually for 3 years after transplant. Samples from controls were obtained for comparison.
“Our objective, really, was [to determine if we could] detect a treatment response in neurofilament light chain, and secondly, did the degree of neurofilament light chain being high at baseline predict anything about subsequent disease outcome?” Dr. Thebault, a third-year resident at the University of Ottawa, reported in the poster.
Indeed, a treatment response was detected; baseline levels were high, and levels in both serum and CSF were down significantly (P = .05) at both 1 and 3 years following bone marrow transplant, and they stayed down. “In fact, they were so low, they were not significantly different from [the levels] in noninflammatory controls,” he said, noting that serum and CSF NfL levels were highly correlated (r = .833; P less than .001).
Study subjects were patients with aggressive MS, characterized by early disease onset, rapid progression, frequent relapses, and poor treatment responses. Such patients who have received autologous BMT represent an interesting group for examining treatment responses, he said.
At baseline, these patients presumably have a high burden of tissue injury, which would be representative of high levels of Nf-L; good, prospective data show these patients have no evidence of ongoing inflammatory disease following an intensive regimen that includes chemoablation and reinfusion of autologous stem cells, which is representative of a significant reduction of neurofilament levels, he explained.
Importantly, serum and CSF levels were correlated in this study, he said, noting that most prior work has involved CSF, but “the real clinical utility in neurofilament light chain is probably in the serum, because patients don’t like having lumbar punctures too often.”
With respect to the second question pertaining to disease outcomes, the study did show that patients with baseline Nf-L levels above the median for the group had worse T1 lesion volumes at baseline and after transplant (P = .0021 at 36 months), and also had worsening brain atrophy even after transplant (P = .0389 at 60 months).
The curves appeared to be diverging, suggesting that, in the absence of inflammatory disease activity, patients with high Nf-L levels at baseline continue to have ongoing atrophy at a differential rate, compared with those with low baseline levels, Dr. Thebault said.
Other findings included better Expanded Disability Status Scale outcomes after transplant in patients with lower baseline Nf-L, and a trend toward worsening outcomes among those with higher baseline Nf-L levels, although the study may have been underpowered for this. Additionally, lower baseline Nf-L predicted significantly lower T1 and T2 lesion volume, number of gadolinium-enhancing lesions, and a reduction in brain atrophy, whereas higher baseline Nf-L predicted the opposite, he noted, adding that N-acetylaspartate-to-creatinine ratios also tended to vary inversely with Nf-L levels.
The most important findings of the study are “the sustained reduction in Nf-L levels after BMT, and that higher baseline levels predicted worse MRI outcomes,” Dr. Thebault noted. “Together these data add to a growing body of evidence that suggests that serum Nf-L has a role in monitoring treatment responses and even a predictive value in determining disease severity.”
This study was supported by Quanterix, which provided Nf-L assay kits. Dr. Thebault reported having no disclosures.
SOURCE: Thebault S et al. Neurology. 2018 Apr;90(15 Suppl.):P5.036.
LOS ANGELES – Serum neurofilament light chain shows promise as a biomarker for disease severity and response to treatment with autologous bone marrow transplant in patients with multiple sclerosis, according to findings from an analysis of paired samples.
Serum neurofilament light chain (Nf-L) levels were found to be significantly elevated in both the serum and cerebrospinal fluid (CSF) of 23 patients with aggressive multiple sclerosis (MS), compared with samples from 5 controls with noninflammatory neurologic conditions such as chronic fatigue syndrome or migraine. The levels in the patients with MS were significantly reduced following autologous bone marrow transplant (BMT), Simon Thebault, MD, reported in a poster at the annual meeting of the American Academy of Neurology.
Nf-L has been shown to be a biomarker for neuronal damage and to have potential for denoting disease severity and treatment response in MS; for this analysis, samples from patients were collected at baseline and annually for 3 years after transplant. Samples from controls were obtained for comparison.
“Our objective, really, was [to determine if we could] detect a treatment response in neurofilament light chain, and secondly, did the degree of neurofilament light chain being high at baseline predict anything about subsequent disease outcome?” Dr. Thebault, a third-year resident at the University of Ottawa, reported in the poster.
Indeed, a treatment response was detected; baseline levels were high, and levels in both serum and CSF were down significantly (P = .05) at both 1 and 3 years following bone marrow transplant, and they stayed down. “In fact, they were so low, they were not significantly different from [the levels] in noninflammatory controls,” he said, noting that serum and CSF NfL levels were highly correlated (r = .833; P less than .001).
Study subjects were patients with aggressive MS, characterized by early disease onset, rapid progression, frequent relapses, and poor treatment responses. Such patients who have received autologous BMT represent an interesting group for examining treatment responses, he said.
At baseline, these patients presumably have a high burden of tissue injury, which would be representative of high levels of Nf-L; good, prospective data show these patients have no evidence of ongoing inflammatory disease following an intensive regimen that includes chemoablation and reinfusion of autologous stem cells, which is representative of a significant reduction of neurofilament levels, he explained.
Importantly, serum and CSF levels were correlated in this study, he said, noting that most prior work has involved CSF, but “the real clinical utility in neurofilament light chain is probably in the serum, because patients don’t like having lumbar punctures too often.”
With respect to the second question pertaining to disease outcomes, the study did show that patients with baseline Nf-L levels above the median for the group had worse T1 lesion volumes at baseline and after transplant (P = .0021 at 36 months), and also had worsening brain atrophy even after transplant (P = .0389 at 60 months).
The curves appeared to be diverging, suggesting that, in the absence of inflammatory disease activity, patients with high Nf-L levels at baseline continue to have ongoing atrophy at a differential rate, compared with those with low baseline levels, Dr. Thebault said.
Other findings included better Expanded Disability Status Scale outcomes after transplant in patients with lower baseline Nf-L, and a trend toward worsening outcomes among those with higher baseline Nf-L levels, although the study may have been underpowered for this. Additionally, lower baseline Nf-L predicted significantly lower T1 and T2 lesion volume, number of gadolinium-enhancing lesions, and a reduction in brain atrophy, whereas higher baseline Nf-L predicted the opposite, he noted, adding that N-acetylaspartate-to-creatinine ratios also tended to vary inversely with Nf-L levels.
The most important findings of the study are “the sustained reduction in Nf-L levels after BMT, and that higher baseline levels predicted worse MRI outcomes,” Dr. Thebault noted. “Together these data add to a growing body of evidence that suggests that serum Nf-L has a role in monitoring treatment responses and even a predictive value in determining disease severity.”
This study was supported by Quanterix, which provided Nf-L assay kits. Dr. Thebault reported having no disclosures.
SOURCE: Thebault S et al. Neurology. 2018 Apr;90(15 Suppl.):P5.036.
LOS ANGELES – Serum neurofilament light chain shows promise as a biomarker for disease severity and response to treatment with autologous bone marrow transplant in patients with multiple sclerosis, according to findings from an analysis of paired samples.
Serum neurofilament light chain (Nf-L) levels were found to be significantly elevated in both the serum and cerebrospinal fluid (CSF) of 23 patients with aggressive multiple sclerosis (MS), compared with samples from 5 controls with noninflammatory neurologic conditions such as chronic fatigue syndrome or migraine. The levels in the patients with MS were significantly reduced following autologous bone marrow transplant (BMT), Simon Thebault, MD, reported in a poster at the annual meeting of the American Academy of Neurology.
Nf-L has been shown to be a biomarker for neuronal damage and to have potential for denoting disease severity and treatment response in MS; for this analysis, samples from patients were collected at baseline and annually for 3 years after transplant. Samples from controls were obtained for comparison.
“Our objective, really, was [to determine if we could] detect a treatment response in neurofilament light chain, and secondly, did the degree of neurofilament light chain being high at baseline predict anything about subsequent disease outcome?” Dr. Thebault, a third-year resident at the University of Ottawa, reported in the poster.
Indeed, a treatment response was detected; baseline levels were high, and levels in both serum and CSF were down significantly (P = .05) at both 1 and 3 years following bone marrow transplant, and they stayed down. “In fact, they were so low, they were not significantly different from [the levels] in noninflammatory controls,” he said, noting that serum and CSF NfL levels were highly correlated (r = .833; P less than .001).
Study subjects were patients with aggressive MS, characterized by early disease onset, rapid progression, frequent relapses, and poor treatment responses. Such patients who have received autologous BMT represent an interesting group for examining treatment responses, he said.
At baseline, these patients presumably have a high burden of tissue injury, which would be representative of high levels of Nf-L; good, prospective data show these patients have no evidence of ongoing inflammatory disease following an intensive regimen that includes chemoablation and reinfusion of autologous stem cells, which is representative of a significant reduction of neurofilament levels, he explained.
Importantly, serum and CSF levels were correlated in this study, he said, noting that most prior work has involved CSF, but “the real clinical utility in neurofilament light chain is probably in the serum, because patients don’t like having lumbar punctures too often.”
With respect to the second question pertaining to disease outcomes, the study did show that patients with baseline Nf-L levels above the median for the group had worse T1 lesion volumes at baseline and after transplant (P = .0021 at 36 months), and also had worsening brain atrophy even after transplant (P = .0389 at 60 months).
The curves appeared to be diverging, suggesting that, in the absence of inflammatory disease activity, patients with high Nf-L levels at baseline continue to have ongoing atrophy at a differential rate, compared with those with low baseline levels, Dr. Thebault said.
Other findings included better Expanded Disability Status Scale outcomes after transplant in patients with lower baseline Nf-L, and a trend toward worsening outcomes among those with higher baseline Nf-L levels, although the study may have been underpowered for this. Additionally, lower baseline Nf-L predicted significantly lower T1 and T2 lesion volume, number of gadolinium-enhancing lesions, and a reduction in brain atrophy, whereas higher baseline Nf-L predicted the opposite, he noted, adding that N-acetylaspartate-to-creatinine ratios also tended to vary inversely with Nf-L levels.
The most important findings of the study are “the sustained reduction in Nf-L levels after BMT, and that higher baseline levels predicted worse MRI outcomes,” Dr. Thebault noted. “Together these data add to a growing body of evidence that suggests that serum Nf-L has a role in monitoring treatment responses and even a predictive value in determining disease severity.”
This study was supported by Quanterix, which provided Nf-L assay kits. Dr. Thebault reported having no disclosures.
SOURCE: Thebault S et al. Neurology. 2018 Apr;90(15 Suppl.):P5.036.
REPORTING FROM AAN 2018
Key clinical point:
Major finding: Serum and cerebrospinal fluid Nf-L levels declines significantly after bone marrow transplant (P less than .05) and did not differ from the levels in controls.
Study details: An analysis of paired samples from 23 patients with multiple sclerosis and 5 controls.
Disclosures: This study was supported by Quanterix, which provided Nf-L assay kits. Dr. Thebault reported having no disclosures.
Source: Thebault S et al. Neurology. 2018 Apr;90(15 Suppl.):P5.036.
New MS subtype shows absence of cerebral white matter demyelination
A new subtype of multiple sclerosis called myelocortical multiple sclerosis is characterized by demyelination only in the spinal cord and cerebral cortex and not in the cerebral white matter.
A paper published online Aug. 21 in Lancet Neurology presents the results of a study of the brains and spinal cords of 100 patients who died of multiple sclerosis.
Bruce D. Trapp, PhD, of the Lerner Research Institute at the Cleveland Clinic in Ohio, and his coauthors wrote that while the demyelination of cerebral white matter is a pathologic hallmark of multiple sclerosis, previous research has found only around half of cerebral T2-weighted hyperintense white matter lesions are demyelinated, and these lesions account for less than a third of variance in the rate of brain atrophy.
“In the absence of specific MRI metrics for demyelination, the relationship between cerebral white-matter demyelination and neurodegeneration remains speculative,” they wrote.
In this study, researchers scanned the brains with MRI before autopsy, then took centimeter-thick hemispheric slices to study the white-matter lesions. They identified 12 individuals as having what they describe as ‘myelocortical multiple sclerosis,’ characterized by the absence of areas of cerebral white-matter discoloration indicative of demyelinated lesions.
The authors then compared these individuals to 12 individuals with typical multiple sclerosis matched by age, sex, MRI protocol, multiple sclerosis disease subtype, disease duration, and Expanded Disability Status Scale.
They found that while individuals with myelocortical multiple sclerosis did not have demyelinated lesions in the cerebral white matter, they had similar areas of demyelinated lesions in the cerebral cortex to individuals with typical multiple sclerosis (median 4.45% vs. 9.74% respectively, P = .5512).
However, the individuals with myelocortical multiple sclerosis had a significantly smaller area of spinal cord demyelination (median 3.81% vs. 13.81%, P = .0083).
Individuals with myelocortical multiple sclerosis also had significantly lower mean cortical neuronal densities, compared with healthy control brains in layer III, layer V, and layer VI. But individuals with typical multiple sclerosis only had a lower cortical neuronal density in layer V when compared with controls.
Researchers also saw that in typical multiple sclerosis, neuronal density decreased as the area of brain white-matter demyelination increased. However, this negative linear correlation was not seen in myelocortical multiple sclerosis.
On MRI, researchers were still able to see abnormalities in the cerebral white matter in individuals with myelocortical multiple sclerosis, in T2-weighted, T1-weighted and magnetization transfer ratios (MTR) images.
They also found similar total T2-weighted and T1-weighted lesion volumes in individuals with myelocortical and with typical multiple sclerosis, although individuals with typical multiple sclerosis had significantly greater MTR lesion volumes.
“We propose that myelocortical multiple sclerosis is characterized by spinal cord demyelination, subpial cortical demyelination, and an absence of cerebral white-matter demyelination,” the authors wrote. “Our findings indicate that abnormal cerebral white-matter T2-T1-MTR regions of interest are not always demyelinated, and this pathological evidence suggests that cerebral white-matter demyelination and cortical neuronal degeneration can be independent events in myelocortical multiple sclerosis.”
The authors noted that their study may have been affected by selection bias, as all the patients in the study had died from complications of advanced multiple sclerosis. They suggested that it was therefore not appropriate to conclude that the prevalence of myelocortical multiple sclerosis seen in their sample would be similar across the multiple sclerosis population, nor were the findings likely to apply to people with earlier stage disease.
The study was funded by the U.S. National Institutes of Health and National Multiple Sclerosis Society. One author was an employee of Renovo Neural, and three authors were employees of Biogen. One author declared a pending patent related to automated lesion segmentation from MRI images, and four authors declared funding, fees, and non-financial support from pharmaceutical companies.
SOURCE: Trapp B et al. Lancet Neurol. 2018 Aug 21. doi: 10.1016/ S1474-4422(18)30245-X.
A new subtype of multiple sclerosis called myelocortical multiple sclerosis is characterized by demyelination only in the spinal cord and cerebral cortex and not in the cerebral white matter.
A paper published online Aug. 21 in Lancet Neurology presents the results of a study of the brains and spinal cords of 100 patients who died of multiple sclerosis.
Bruce D. Trapp, PhD, of the Lerner Research Institute at the Cleveland Clinic in Ohio, and his coauthors wrote that while the demyelination of cerebral white matter is a pathologic hallmark of multiple sclerosis, previous research has found only around half of cerebral T2-weighted hyperintense white matter lesions are demyelinated, and these lesions account for less than a third of variance in the rate of brain atrophy.
“In the absence of specific MRI metrics for demyelination, the relationship between cerebral white-matter demyelination and neurodegeneration remains speculative,” they wrote.
In this study, researchers scanned the brains with MRI before autopsy, then took centimeter-thick hemispheric slices to study the white-matter lesions. They identified 12 individuals as having what they describe as ‘myelocortical multiple sclerosis,’ characterized by the absence of areas of cerebral white-matter discoloration indicative of demyelinated lesions.
The authors then compared these individuals to 12 individuals with typical multiple sclerosis matched by age, sex, MRI protocol, multiple sclerosis disease subtype, disease duration, and Expanded Disability Status Scale.
They found that while individuals with myelocortical multiple sclerosis did not have demyelinated lesions in the cerebral white matter, they had similar areas of demyelinated lesions in the cerebral cortex to individuals with typical multiple sclerosis (median 4.45% vs. 9.74% respectively, P = .5512).
However, the individuals with myelocortical multiple sclerosis had a significantly smaller area of spinal cord demyelination (median 3.81% vs. 13.81%, P = .0083).
Individuals with myelocortical multiple sclerosis also had significantly lower mean cortical neuronal densities, compared with healthy control brains in layer III, layer V, and layer VI. But individuals with typical multiple sclerosis only had a lower cortical neuronal density in layer V when compared with controls.
Researchers also saw that in typical multiple sclerosis, neuronal density decreased as the area of brain white-matter demyelination increased. However, this negative linear correlation was not seen in myelocortical multiple sclerosis.
On MRI, researchers were still able to see abnormalities in the cerebral white matter in individuals with myelocortical multiple sclerosis, in T2-weighted, T1-weighted and magnetization transfer ratios (MTR) images.
They also found similar total T2-weighted and T1-weighted lesion volumes in individuals with myelocortical and with typical multiple sclerosis, although individuals with typical multiple sclerosis had significantly greater MTR lesion volumes.
“We propose that myelocortical multiple sclerosis is characterized by spinal cord demyelination, subpial cortical demyelination, and an absence of cerebral white-matter demyelination,” the authors wrote. “Our findings indicate that abnormal cerebral white-matter T2-T1-MTR regions of interest are not always demyelinated, and this pathological evidence suggests that cerebral white-matter demyelination and cortical neuronal degeneration can be independent events in myelocortical multiple sclerosis.”
The authors noted that their study may have been affected by selection bias, as all the patients in the study had died from complications of advanced multiple sclerosis. They suggested that it was therefore not appropriate to conclude that the prevalence of myelocortical multiple sclerosis seen in their sample would be similar across the multiple sclerosis population, nor were the findings likely to apply to people with earlier stage disease.
The study was funded by the U.S. National Institutes of Health and National Multiple Sclerosis Society. One author was an employee of Renovo Neural, and three authors were employees of Biogen. One author declared a pending patent related to automated lesion segmentation from MRI images, and four authors declared funding, fees, and non-financial support from pharmaceutical companies.
SOURCE: Trapp B et al. Lancet Neurol. 2018 Aug 21. doi: 10.1016/ S1474-4422(18)30245-X.
A new subtype of multiple sclerosis called myelocortical multiple sclerosis is characterized by demyelination only in the spinal cord and cerebral cortex and not in the cerebral white matter.
A paper published online Aug. 21 in Lancet Neurology presents the results of a study of the brains and spinal cords of 100 patients who died of multiple sclerosis.
Bruce D. Trapp, PhD, of the Lerner Research Institute at the Cleveland Clinic in Ohio, and his coauthors wrote that while the demyelination of cerebral white matter is a pathologic hallmark of multiple sclerosis, previous research has found only around half of cerebral T2-weighted hyperintense white matter lesions are demyelinated, and these lesions account for less than a third of variance in the rate of brain atrophy.
“In the absence of specific MRI metrics for demyelination, the relationship between cerebral white-matter demyelination and neurodegeneration remains speculative,” they wrote.
In this study, researchers scanned the brains with MRI before autopsy, then took centimeter-thick hemispheric slices to study the white-matter lesions. They identified 12 individuals as having what they describe as ‘myelocortical multiple sclerosis,’ characterized by the absence of areas of cerebral white-matter discoloration indicative of demyelinated lesions.
The authors then compared these individuals to 12 individuals with typical multiple sclerosis matched by age, sex, MRI protocol, multiple sclerosis disease subtype, disease duration, and Expanded Disability Status Scale.
They found that while individuals with myelocortical multiple sclerosis did not have demyelinated lesions in the cerebral white matter, they had similar areas of demyelinated lesions in the cerebral cortex to individuals with typical multiple sclerosis (median 4.45% vs. 9.74% respectively, P = .5512).
However, the individuals with myelocortical multiple sclerosis had a significantly smaller area of spinal cord demyelination (median 3.81% vs. 13.81%, P = .0083).
Individuals with myelocortical multiple sclerosis also had significantly lower mean cortical neuronal densities, compared with healthy control brains in layer III, layer V, and layer VI. But individuals with typical multiple sclerosis only had a lower cortical neuronal density in layer V when compared with controls.
Researchers also saw that in typical multiple sclerosis, neuronal density decreased as the area of brain white-matter demyelination increased. However, this negative linear correlation was not seen in myelocortical multiple sclerosis.
On MRI, researchers were still able to see abnormalities in the cerebral white matter in individuals with myelocortical multiple sclerosis, in T2-weighted, T1-weighted and magnetization transfer ratios (MTR) images.
They also found similar total T2-weighted and T1-weighted lesion volumes in individuals with myelocortical and with typical multiple sclerosis, although individuals with typical multiple sclerosis had significantly greater MTR lesion volumes.
“We propose that myelocortical multiple sclerosis is characterized by spinal cord demyelination, subpial cortical demyelination, and an absence of cerebral white-matter demyelination,” the authors wrote. “Our findings indicate that abnormal cerebral white-matter T2-T1-MTR regions of interest are not always demyelinated, and this pathological evidence suggests that cerebral white-matter demyelination and cortical neuronal degeneration can be independent events in myelocortical multiple sclerosis.”
The authors noted that their study may have been affected by selection bias, as all the patients in the study had died from complications of advanced multiple sclerosis. They suggested that it was therefore not appropriate to conclude that the prevalence of myelocortical multiple sclerosis seen in their sample would be similar across the multiple sclerosis population, nor were the findings likely to apply to people with earlier stage disease.
The study was funded by the U.S. National Institutes of Health and National Multiple Sclerosis Society. One author was an employee of Renovo Neural, and three authors were employees of Biogen. One author declared a pending patent related to automated lesion segmentation from MRI images, and four authors declared funding, fees, and non-financial support from pharmaceutical companies.
SOURCE: Trapp B et al. Lancet Neurol. 2018 Aug 21. doi: 10.1016/ S1474-4422(18)30245-X.
FROM LANCET NEUROLOGY
Key clinical point: Researchers have identified a new subtype of multiple sclerosis.
Major finding: Individuals with myelocortical multiple sclerosis show demyelination in the spinal cord and cortex only.
Study details: Post-mortem study of brains and spinal cords of 100 individuals with multiple sclerosis.
Disclosures: The study was funded by the U.S. National Institutes of Health and National Multiple Sclerosis Society. One author was an employee of Renovo Neural, three authors were employees of Biogen. One author declared a pending patent related to automated lesion segmentation from MRI images, and four authors declared funding, fees and non-financial support from pharmaceutical companies.
Source: Trapp B et al. Lancet Neurol. 2018 Aug 21. doi: 10.1016/ S1474-4422(18)30245-X.
Latex Allergy From Biologic Injectable Devices



Innovations Lead to More Targeted Prostate Cancer Treatments (FULL)
The main treatment for prostate cancer—the third leading cause of cancer death in American men—often is “watchful waiting.” But what happens before, during, and after that waiting period has changed tremendously in recent years. Innovative and improved methods and drugs allow for a more precise diagnosis, better risk stratification, targeted treatment options, and longer survival.
Innovations in diagnosis include a revised histologic grading system, which was incorporated into the 2016 World Health Organization classification of tumors. The new grading system ranks prostate cancer on a 1-to-5 scale, making it more discriminating, as validated in a study of more than 25,000 men.
The use of new prognostic biomarkers has advanced risk stratification. According to a recent review, biopsy guided by ultrasound misses between 21% and 28% of prostate cancers and undergrades between 14% and 17%.1 But new serum-, tissue-, and image-based biomarkers may help identify potential false negatives. The prostate cancer antigen 3 test, for example, has an 88% negative predictive value for subsequent biopsy. Molecular biomarkers also can predict clinical progression, risk of adverse pathology, and metastatic risk.
Fortunately, biopsy guided by ultrasound is getting more precise. Advances in magnetic resonance imaging (MRI) now allow for “targeted biopsies.” The enhanced MRI has 89% sensitivity and 73% specificity for identifying prostate cancer. According to one study of 1,003 men, targeted prostate biopsy using MRI-ultrasound fusion identified 30% more cases of Gleason score ≥ 4 + 3 than did systematic prostate biopsy.1 Updates in positron emission tomography are garnering interest for improved staging because this technology allows for better detection of local recurrence, regional lymph node metastases, and distant metastases.
Once a prostate cancer diagnosis has been confirmed, the decision of what to do next may be watchful waiting (treating symptoms palliatively), but recent research suggests that active surveillance that includes regular prostate-specific antigen testing, physical examinations, and prostate biopsies may be a better choice, particularly for men with less aggressive cancer. One study of 1,298 men with mostly very low-risk disease followed for up to 60 months found metastasis in only 5; only 2 died. The Prostate Testing for Cancer and Treatment (ProtecT) trial found that the number of deaths in the active monitoring group did not differ significantly from those in the surgery or radiation groups.
What should be the contemporary standard of care? Androgen deprivation therapy (ADT) is still the go-to treatment for men with metastatic prostate cancer. Although ADT has been associated with toxicity, a meta-analysis found continuous ADT was better than intermittent in terms of disease progression and survival.1
Other research has focused on which types of prostate cancer respond best to specific therapies. Molecular subtyping (already available in bladder and breast cancer) is gaining popularity. Prostate cancer was thought to derive from glandular luminal cells, but recent evidence supports the idea that basal cells play a role as well. Researchers who analyzed nearly 4,000 samples suggest that luminal B tumors respond better to postoperative ADT than do nonluminal B cancers. These findings suggest that “personalized” ADT treatment may be possible.2
Several drugs have been shown to improve survival: Among them, docetaxel, abiraterone acetate, enzalutamide, and cabazitaxel. In the STAMPEDE trial, men with locally advanced or metastatic prostate cancer who received ADT plus abiraterone and prednisolone had significantly higher rates of overall and failure-free survival.3
Docetaxel, which can extend survival by 10 to 13 months compared with standard ADT, is taking on a bigger role for its ability to delay progression and recurrence while being well tolerated. Options for men whose cancer does not respond to ADT include abiraterone and enzalutamide. Both act on the androgen axis to slow progression and improve survival.
More than 30% of patients treated with radical prostatectomy will have recurrent cancer as will 50% of those treated with salvage radiation therapy. Bicalutamide has shown extremely promising action against recurrent cancer. In one study, the cumulative incidence of metastatic prostate cancer at 12 years was 14.5% in the bicalutamide group, compared with 23.0% in the placebo group.4
But while that study was going on, it was superseded by injectable gonadotropin-releasing hormone agonists as first-choice hormonal therapy with radiation. However, the researchers say that does not
Multimodal therapy and precision medicine are becoming bywords in prostate cancer treatment. Drugs on the horizon likely will be tailored to tumor molecular biology, with genetic information used to specifically guide diagnosis and treatment. Prostate cancer may still be a slow killer, but immunotherapies (like sipuleucel-T, the first FDA-approved cancer vaccine), hormonal therapies, and bone-targeting agents enable men with prostate cancer to not only live longer but also with a better quality of life.
Click here to read the digital edition.
1. Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317(24):2532-2542.
2. Zhao SG, Chang SL, Erho N, et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol. 2017. [Epub ahead of print.]
3. James ND, de Bono JS, Spears MR, et al; for the STAMPEDE Investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017. [Epub ahead of print.]
4. Shipley WU, Seiferheld W, Lukka HR, et al; NRG Oncology RTOG. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376(5):417-428.
The main treatment for prostate cancer—the third leading cause of cancer death in American men—often is “watchful waiting.” But what happens before, during, and after that waiting period has changed tremendously in recent years. Innovative and improved methods and drugs allow for a more precise diagnosis, better risk stratification, targeted treatment options, and longer survival.
Innovations in diagnosis include a revised histologic grading system, which was incorporated into the 2016 World Health Organization classification of tumors. The new grading system ranks prostate cancer on a 1-to-5 scale, making it more discriminating, as validated in a study of more than 25,000 men.
The use of new prognostic biomarkers has advanced risk stratification. According to a recent review, biopsy guided by ultrasound misses between 21% and 28% of prostate cancers and undergrades between 14% and 17%.1 But new serum-, tissue-, and image-based biomarkers may help identify potential false negatives. The prostate cancer antigen 3 test, for example, has an 88% negative predictive value for subsequent biopsy. Molecular biomarkers also can predict clinical progression, risk of adverse pathology, and metastatic risk.
Fortunately, biopsy guided by ultrasound is getting more precise. Advances in magnetic resonance imaging (MRI) now allow for “targeted biopsies.” The enhanced MRI has 89% sensitivity and 73% specificity for identifying prostate cancer. According to one study of 1,003 men, targeted prostate biopsy using MRI-ultrasound fusion identified 30% more cases of Gleason score ≥ 4 + 3 than did systematic prostate biopsy.1 Updates in positron emission tomography are garnering interest for improved staging because this technology allows for better detection of local recurrence, regional lymph node metastases, and distant metastases.
Once a prostate cancer diagnosis has been confirmed, the decision of what to do next may be watchful waiting (treating symptoms palliatively), but recent research suggests that active surveillance that includes regular prostate-specific antigen testing, physical examinations, and prostate biopsies may be a better choice, particularly for men with less aggressive cancer. One study of 1,298 men with mostly very low-risk disease followed for up to 60 months found metastasis in only 5; only 2 died. The Prostate Testing for Cancer and Treatment (ProtecT) trial found that the number of deaths in the active monitoring group did not differ significantly from those in the surgery or radiation groups.
What should be the contemporary standard of care? Androgen deprivation therapy (ADT) is still the go-to treatment for men with metastatic prostate cancer. Although ADT has been associated with toxicity, a meta-analysis found continuous ADT was better than intermittent in terms of disease progression and survival.1
Other research has focused on which types of prostate cancer respond best to specific therapies. Molecular subtyping (already available in bladder and breast cancer) is gaining popularity. Prostate cancer was thought to derive from glandular luminal cells, but recent evidence supports the idea that basal cells play a role as well. Researchers who analyzed nearly 4,000 samples suggest that luminal B tumors respond better to postoperative ADT than do nonluminal B cancers. These findings suggest that “personalized” ADT treatment may be possible.2
Several drugs have been shown to improve survival: Among them, docetaxel, abiraterone acetate, enzalutamide, and cabazitaxel. In the STAMPEDE trial, men with locally advanced or metastatic prostate cancer who received ADT plus abiraterone and prednisolone had significantly higher rates of overall and failure-free survival.3
Docetaxel, which can extend survival by 10 to 13 months compared with standard ADT, is taking on a bigger role for its ability to delay progression and recurrence while being well tolerated. Options for men whose cancer does not respond to ADT include abiraterone and enzalutamide. Both act on the androgen axis to slow progression and improve survival.
More than 30% of patients treated with radical prostatectomy will have recurrent cancer as will 50% of those treated with salvage radiation therapy. Bicalutamide has shown extremely promising action against recurrent cancer. In one study, the cumulative incidence of metastatic prostate cancer at 12 years was 14.5% in the bicalutamide group, compared with 23.0% in the placebo group.4
But while that study was going on, it was superseded by injectable gonadotropin-releasing hormone agonists as first-choice hormonal therapy with radiation. However, the researchers say that does not
Multimodal therapy and precision medicine are becoming bywords in prostate cancer treatment. Drugs on the horizon likely will be tailored to tumor molecular biology, with genetic information used to specifically guide diagnosis and treatment. Prostate cancer may still be a slow killer, but immunotherapies (like sipuleucel-T, the first FDA-approved cancer vaccine), hormonal therapies, and bone-targeting agents enable men with prostate cancer to not only live longer but also with a better quality of life.
Click here to read the digital edition.
The main treatment for prostate cancer—the third leading cause of cancer death in American men—often is “watchful waiting.” But what happens before, during, and after that waiting period has changed tremendously in recent years. Innovative and improved methods and drugs allow for a more precise diagnosis, better risk stratification, targeted treatment options, and longer survival.
Innovations in diagnosis include a revised histologic grading system, which was incorporated into the 2016 World Health Organization classification of tumors. The new grading system ranks prostate cancer on a 1-to-5 scale, making it more discriminating, as validated in a study of more than 25,000 men.
The use of new prognostic biomarkers has advanced risk stratification. According to a recent review, biopsy guided by ultrasound misses between 21% and 28% of prostate cancers and undergrades between 14% and 17%.1 But new serum-, tissue-, and image-based biomarkers may help identify potential false negatives. The prostate cancer antigen 3 test, for example, has an 88% negative predictive value for subsequent biopsy. Molecular biomarkers also can predict clinical progression, risk of adverse pathology, and metastatic risk.
Fortunately, biopsy guided by ultrasound is getting more precise. Advances in magnetic resonance imaging (MRI) now allow for “targeted biopsies.” The enhanced MRI has 89% sensitivity and 73% specificity for identifying prostate cancer. According to one study of 1,003 men, targeted prostate biopsy using MRI-ultrasound fusion identified 30% more cases of Gleason score ≥ 4 + 3 than did systematic prostate biopsy.1 Updates in positron emission tomography are garnering interest for improved staging because this technology allows for better detection of local recurrence, regional lymph node metastases, and distant metastases.
Once a prostate cancer diagnosis has been confirmed, the decision of what to do next may be watchful waiting (treating symptoms palliatively), but recent research suggests that active surveillance that includes regular prostate-specific antigen testing, physical examinations, and prostate biopsies may be a better choice, particularly for men with less aggressive cancer. One study of 1,298 men with mostly very low-risk disease followed for up to 60 months found metastasis in only 5; only 2 died. The Prostate Testing for Cancer and Treatment (ProtecT) trial found that the number of deaths in the active monitoring group did not differ significantly from those in the surgery or radiation groups.
What should be the contemporary standard of care? Androgen deprivation therapy (ADT) is still the go-to treatment for men with metastatic prostate cancer. Although ADT has been associated with toxicity, a meta-analysis found continuous ADT was better than intermittent in terms of disease progression and survival.1
Other research has focused on which types of prostate cancer respond best to specific therapies. Molecular subtyping (already available in bladder and breast cancer) is gaining popularity. Prostate cancer was thought to derive from glandular luminal cells, but recent evidence supports the idea that basal cells play a role as well. Researchers who analyzed nearly 4,000 samples suggest that luminal B tumors respond better to postoperative ADT than do nonluminal B cancers. These findings suggest that “personalized” ADT treatment may be possible.2
Several drugs have been shown to improve survival: Among them, docetaxel, abiraterone acetate, enzalutamide, and cabazitaxel. In the STAMPEDE trial, men with locally advanced or metastatic prostate cancer who received ADT plus abiraterone and prednisolone had significantly higher rates of overall and failure-free survival.3
Docetaxel, which can extend survival by 10 to 13 months compared with standard ADT, is taking on a bigger role for its ability to delay progression and recurrence while being well tolerated. Options for men whose cancer does not respond to ADT include abiraterone and enzalutamide. Both act on the androgen axis to slow progression and improve survival.
More than 30% of patients treated with radical prostatectomy will have recurrent cancer as will 50% of those treated with salvage radiation therapy. Bicalutamide has shown extremely promising action against recurrent cancer. In one study, the cumulative incidence of metastatic prostate cancer at 12 years was 14.5% in the bicalutamide group, compared with 23.0% in the placebo group.4
But while that study was going on, it was superseded by injectable gonadotropin-releasing hormone agonists as first-choice hormonal therapy with radiation. However, the researchers say that does not
Multimodal therapy and precision medicine are becoming bywords in prostate cancer treatment. Drugs on the horizon likely will be tailored to tumor molecular biology, with genetic information used to specifically guide diagnosis and treatment. Prostate cancer may still be a slow killer, but immunotherapies (like sipuleucel-T, the first FDA-approved cancer vaccine), hormonal therapies, and bone-targeting agents enable men with prostate cancer to not only live longer but also with a better quality of life.
Click here to read the digital edition.
1. Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317(24):2532-2542.
2. Zhao SG, Chang SL, Erho N, et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol. 2017. [Epub ahead of print.]
3. James ND, de Bono JS, Spears MR, et al; for the STAMPEDE Investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017. [Epub ahead of print.]
4. Shipley WU, Seiferheld W, Lukka HR, et al; NRG Oncology RTOG. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376(5):417-428.
1. Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317(24):2532-2542.
2. Zhao SG, Chang SL, Erho N, et al. Associations of luminal and basal subtyping of prostate cancer with prognosis and response to androgen deprivation therapy. JAMA Oncol. 2017. [Epub ahead of print.]
3. James ND, de Bono JS, Spears MR, et al; for the STAMPEDE Investigators. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017. [Epub ahead of print.]
4. Shipley WU, Seiferheld W, Lukka HR, et al; NRG Oncology RTOG. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;376(5):417-428.
Acute Leukemia of Ambiguous Lineage in Elderly Patients: A SEER-Medicare Database Analysis (FULL)
About Research in Context
In this article, the authors of recent scholarship have been asked to discuss the implications of their research on federal health care providers and specifically the veteran and active-duty service member patient populations. Because the article does not include new research and cannot be blinded, it has undergone an abbreviated peer review process. The original article can be found at Guru Murthy GS, Dhakal I, Lee JY, Mehta P. Acute leukemia of ambiguous lineage in elderly patients - analysis of survival using surveillance epidemiology and end results-Medicare database. Clin Lymphoma Myeloma Leuk. 2017;17(2):100-107.
Acute leukemia of ambiguous lineage (ALAL) is a rare disorder in adults, constituting about 3% to 5% of acute leukemia cases. Unlike acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), ALAL cannot be clearly differentiated into a single subtype based on immunophenotyping. The diagnostic criteria for accurately identifying ALAL has evolved over time. There is paucity of information regarding the outcomes and management of this rare leukemia especially in elderly patients, and it is unclear whether treatment improves survival in these patients.
We performed a retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database to describe the outcomes of ALAL in the elderly population in U.S.1 Patients included in the analysis were aged > 65 years, with a pathologically confirmed diagnosis of ALAL, diagnosed between 1992-2010, and on active follow-up. Information on patient demographics, treatment, chemotherapeutic agents used in treatment, and survival was obtained and analyzed using appropriate statistical methods. A total of 705 patients with a median age of 80 years were included. There was a higher proportion of males than females and a higher proportion of white patients compared with African Americans and other races. We found that the overall survival (OS) declined significantly with increasing age, and treatment with chemotherapy improved the survival. However, factors such as gender, race, or type of chemotherapy received (ALL based, AML based, or other regimens) did not significantly influence the survival.
Even in the current era, the optimal therapy for ALAL is not well established. Although options such as AML-based or ALL-based chemotherapy are available, the best chemotherapy regimen and its sequence is unknown as prior studies have demonstrated varying results.2-5 Among elderly patients, numerous factors such as performance status, comorbidities, and ability to tolerate therapy influence the treatment decision. In light of the poor prognosis in elderly patients, a question often arises in the clinician’s mind about whether chemotherapy would provide any benefit for the patient.
Our study results showed that chemotherapy likely improves survival in these patients. However, due to the smaller number of patients, caution is needed in interpreting the result that there was no significant difference between AML-directed or ALL-directed chemotherapy. Another factor highlighted in the study was that only about 21.5% of patients had been treated with chemotherapy. Due to the inherent nature of the database, we could not identify the factors that may have influenced treatment decisions in these patients. Additionally, patients with stem cell transplantation-related claims could not be included in the analysis due to noncontinuous Medicare coverage during the study period. Hence, the role of stem cell transplantation in these patients could not be determined.
Implications Among Veterans
Actual incidence of ALAL among veterans is not known. Whether the incidence of ALAL relates to exposures to chemicals or toxins during military training and service also is unknown. However, ALAL is likely to be at least as prevalent as it is in the nonveteran population and perhaps more so because of exposures and stresses during military training and service.
It is unclear whether veterans attending VA hospitals receive less or different treatment given the higher comorbidities. Finally, it also is not known whether the outcomes for veterans would be different with or without treatment.
Our findings suggest that treatment should be seriously considered in all patients (veterans or not) who are healthy enough to receive chemotherapy regardless of their age. More research is needed to determine the disease incidence and prevalence among veterans and to evaluate whether there are specific etiologic correlations between ALAL and military exposures, whether the natural history is similar to other populations, and to delineate responsiveness to treatment.
Conclusion
This study suggests a poor survival for elderly patients with ALAL in the U.S. Although treatment is associated with an improvement in survival, only 21.5% of patients have received therapy. The optimal choice of chemotherapy for this disease is still not known and warrants prospective studies.
Click here to read the digital edition.
1. Guru Murthy GS, Dhakal I, Lee JY, Mehta P. Acute leukemia of ambiguous lineage in elderly patients—analysis of survival using surveillance epidemiology and end results—Medicare database. Clin Lymphoma Myeloma Leuk. 2017;17(2):100-107.
2. Rubnitz JE, Onciu M, Pounds S, et al. Acute mixed lineage leukemia in children: the experience of St Jude Children’s Research Hospital. Blood. 2009;113(21):5083-5089.
3. Matutes E, Pickl WF, Van’t Veer M, et al. Mixed phenotype acute leukemia: clinical and laboratory features and out-come in 100 patients defined according to the WHO classification. Blood. 2011;117(11):3163-3171.
4. Wolach O, Stone RM. How I treat mixed-phenotype acute leukemia. Blood. 2015;125(16):2477-2485.
5. Lee JH, Min YH, Chung CW, et al; Korean Society of Hematology AML/MDS Working Party. Prognostic implications of the immunophenotype in biphenotypic acute leukemia. Leuk Lymphoma. 2008;49(4):700-709.
About Research in Context
In this article, the authors of recent scholarship have been asked to discuss the implications of their research on federal health care providers and specifically the veteran and active-duty service member patient populations. Because the article does not include new research and cannot be blinded, it has undergone an abbreviated peer review process. The original article can be found at Guru Murthy GS, Dhakal I, Lee JY, Mehta P. Acute leukemia of ambiguous lineage in elderly patients - analysis of survival using surveillance epidemiology and end results-Medicare database. Clin Lymphoma Myeloma Leuk. 2017;17(2):100-107.
Acute leukemia of ambiguous lineage (ALAL) is a rare disorder in adults, constituting about 3% to 5% of acute leukemia cases. Unlike acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), ALAL cannot be clearly differentiated into a single subtype based on immunophenotyping. The diagnostic criteria for accurately identifying ALAL has evolved over time. There is paucity of information regarding the outcomes and management of this rare leukemia especially in elderly patients, and it is unclear whether treatment improves survival in these patients.
We performed a retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database to describe the outcomes of ALAL in the elderly population in U.S.1 Patients included in the analysis were aged > 65 years, with a pathologically confirmed diagnosis of ALAL, diagnosed between 1992-2010, and on active follow-up. Information on patient demographics, treatment, chemotherapeutic agents used in treatment, and survival was obtained and analyzed using appropriate statistical methods. A total of 705 patients with a median age of 80 years were included. There was a higher proportion of males than females and a higher proportion of white patients compared with African Americans and other races. We found that the overall survival (OS) declined significantly with increasing age, and treatment with chemotherapy improved the survival. However, factors such as gender, race, or type of chemotherapy received (ALL based, AML based, or other regimens) did not significantly influence the survival.
Even in the current era, the optimal therapy for ALAL is not well established. Although options such as AML-based or ALL-based chemotherapy are available, the best chemotherapy regimen and its sequence is unknown as prior studies have demonstrated varying results.2-5 Among elderly patients, numerous factors such as performance status, comorbidities, and ability to tolerate therapy influence the treatment decision. In light of the poor prognosis in elderly patients, a question often arises in the clinician’s mind about whether chemotherapy would provide any benefit for the patient.
Our study results showed that chemotherapy likely improves survival in these patients. However, due to the smaller number of patients, caution is needed in interpreting the result that there was no significant difference between AML-directed or ALL-directed chemotherapy. Another factor highlighted in the study was that only about 21.5% of patients had been treated with chemotherapy. Due to the inherent nature of the database, we could not identify the factors that may have influenced treatment decisions in these patients. Additionally, patients with stem cell transplantation-related claims could not be included in the analysis due to noncontinuous Medicare coverage during the study period. Hence, the role of stem cell transplantation in these patients could not be determined.
Implications Among Veterans
Actual incidence of ALAL among veterans is not known. Whether the incidence of ALAL relates to exposures to chemicals or toxins during military training and service also is unknown. However, ALAL is likely to be at least as prevalent as it is in the nonveteran population and perhaps more so because of exposures and stresses during military training and service.
It is unclear whether veterans attending VA hospitals receive less or different treatment given the higher comorbidities. Finally, it also is not known whether the outcomes for veterans would be different with or without treatment.
Our findings suggest that treatment should be seriously considered in all patients (veterans or not) who are healthy enough to receive chemotherapy regardless of their age. More research is needed to determine the disease incidence and prevalence among veterans and to evaluate whether there are specific etiologic correlations between ALAL and military exposures, whether the natural history is similar to other populations, and to delineate responsiveness to treatment.
Conclusion
This study suggests a poor survival for elderly patients with ALAL in the U.S. Although treatment is associated with an improvement in survival, only 21.5% of patients have received therapy. The optimal choice of chemotherapy for this disease is still not known and warrants prospective studies.
Click here to read the digital edition.
About Research in Context
In this article, the authors of recent scholarship have been asked to discuss the implications of their research on federal health care providers and specifically the veteran and active-duty service member patient populations. Because the article does not include new research and cannot be blinded, it has undergone an abbreviated peer review process. The original article can be found at Guru Murthy GS, Dhakal I, Lee JY, Mehta P. Acute leukemia of ambiguous lineage in elderly patients - analysis of survival using surveillance epidemiology and end results-Medicare database. Clin Lymphoma Myeloma Leuk. 2017;17(2):100-107.
Acute leukemia of ambiguous lineage (ALAL) is a rare disorder in adults, constituting about 3% to 5% of acute leukemia cases. Unlike acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), ALAL cannot be clearly differentiated into a single subtype based on immunophenotyping. The diagnostic criteria for accurately identifying ALAL has evolved over time. There is paucity of information regarding the outcomes and management of this rare leukemia especially in elderly patients, and it is unclear whether treatment improves survival in these patients.
We performed a retrospective analysis of the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database to describe the outcomes of ALAL in the elderly population in U.S.1 Patients included in the analysis were aged > 65 years, with a pathologically confirmed diagnosis of ALAL, diagnosed between 1992-2010, and on active follow-up. Information on patient demographics, treatment, chemotherapeutic agents used in treatment, and survival was obtained and analyzed using appropriate statistical methods. A total of 705 patients with a median age of 80 years were included. There was a higher proportion of males than females and a higher proportion of white patients compared with African Americans and other races. We found that the overall survival (OS) declined significantly with increasing age, and treatment with chemotherapy improved the survival. However, factors such as gender, race, or type of chemotherapy received (ALL based, AML based, or other regimens) did not significantly influence the survival.
Even in the current era, the optimal therapy for ALAL is not well established. Although options such as AML-based or ALL-based chemotherapy are available, the best chemotherapy regimen and its sequence is unknown as prior studies have demonstrated varying results.2-5 Among elderly patients, numerous factors such as performance status, comorbidities, and ability to tolerate therapy influence the treatment decision. In light of the poor prognosis in elderly patients, a question often arises in the clinician’s mind about whether chemotherapy would provide any benefit for the patient.
Our study results showed that chemotherapy likely improves survival in these patients. However, due to the smaller number of patients, caution is needed in interpreting the result that there was no significant difference between AML-directed or ALL-directed chemotherapy. Another factor highlighted in the study was that only about 21.5% of patients had been treated with chemotherapy. Due to the inherent nature of the database, we could not identify the factors that may have influenced treatment decisions in these patients. Additionally, patients with stem cell transplantation-related claims could not be included in the analysis due to noncontinuous Medicare coverage during the study period. Hence, the role of stem cell transplantation in these patients could not be determined.
Implications Among Veterans
Actual incidence of ALAL among veterans is not known. Whether the incidence of ALAL relates to exposures to chemicals or toxins during military training and service also is unknown. However, ALAL is likely to be at least as prevalent as it is in the nonveteran population and perhaps more so because of exposures and stresses during military training and service.
It is unclear whether veterans attending VA hospitals receive less or different treatment given the higher comorbidities. Finally, it also is not known whether the outcomes for veterans would be different with or without treatment.
Our findings suggest that treatment should be seriously considered in all patients (veterans or not) who are healthy enough to receive chemotherapy regardless of their age. More research is needed to determine the disease incidence and prevalence among veterans and to evaluate whether there are specific etiologic correlations between ALAL and military exposures, whether the natural history is similar to other populations, and to delineate responsiveness to treatment.
Conclusion
This study suggests a poor survival for elderly patients with ALAL in the U.S. Although treatment is associated with an improvement in survival, only 21.5% of patients have received therapy. The optimal choice of chemotherapy for this disease is still not known and warrants prospective studies.
Click here to read the digital edition.
1. Guru Murthy GS, Dhakal I, Lee JY, Mehta P. Acute leukemia of ambiguous lineage in elderly patients—analysis of survival using surveillance epidemiology and end results—Medicare database. Clin Lymphoma Myeloma Leuk. 2017;17(2):100-107.
2. Rubnitz JE, Onciu M, Pounds S, et al. Acute mixed lineage leukemia in children: the experience of St Jude Children’s Research Hospital. Blood. 2009;113(21):5083-5089.
3. Matutes E, Pickl WF, Van’t Veer M, et al. Mixed phenotype acute leukemia: clinical and laboratory features and out-come in 100 patients defined according to the WHO classification. Blood. 2011;117(11):3163-3171.
4. Wolach O, Stone RM. How I treat mixed-phenotype acute leukemia. Blood. 2015;125(16):2477-2485.
5. Lee JH, Min YH, Chung CW, et al; Korean Society of Hematology AML/MDS Working Party. Prognostic implications of the immunophenotype in biphenotypic acute leukemia. Leuk Lymphoma. 2008;49(4):700-709.
1. Guru Murthy GS, Dhakal I, Lee JY, Mehta P. Acute leukemia of ambiguous lineage in elderly patients—analysis of survival using surveillance epidemiology and end results—Medicare database. Clin Lymphoma Myeloma Leuk. 2017;17(2):100-107.
2. Rubnitz JE, Onciu M, Pounds S, et al. Acute mixed lineage leukemia in children: the experience of St Jude Children’s Research Hospital. Blood. 2009;113(21):5083-5089.
3. Matutes E, Pickl WF, Van’t Veer M, et al. Mixed phenotype acute leukemia: clinical and laboratory features and out-come in 100 patients defined according to the WHO classification. Blood. 2011;117(11):3163-3171.
4. Wolach O, Stone RM. How I treat mixed-phenotype acute leukemia. Blood. 2015;125(16):2477-2485.
5. Lee JH, Min YH, Chung CW, et al; Korean Society of Hematology AML/MDS Working Party. Prognostic implications of the immunophenotype in biphenotypic acute leukemia. Leuk Lymphoma. 2008;49(4):700-709.
Open Clinical Trials for Patients With Renal Cell Carcinoma (FULL)
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting from those populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors more than 430 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of July 24, 2017; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for kidney cancer/renal cell carninoma. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
NCI-MATCH: Targeted Therapy Directed by Genetic Testing in Treating Patients With Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma
This phase II trial studies how well treatment that is directed by genetic testing works in patients with solid tumors or lymphomas that have progressed following at least 1 line of standard treatment or for which no agreed upon treatment approach exists. Genetic tests look at the unique genetic material (genes) of patients’ tumor cells. Patients with genetic abnormalities (such as mutations, amplifications, or translocations) may benefit more from treatment that targets their tumor’s particular genetic abnormality. Identifying these genetic abnormalities first may help doctors plan better treatment for patients with solid tumors, lymphomas, or multiple myeloma.
ID: NCT02465060
Sponsor: National Cancer Institute
Locations (contact): Naval Medical Center-San Diego, California (Preston S. Gable); VA Connecticut Healthcare System-West Haven Campus (Herta H. Chao); Durham VAMC, North Carolina (Michael J. Kelley); Walter Reed National Military Medical Center, Bethesda, Maryland (Jeremy G. Perkins)
Bevacizumab, Sorafenib Tosylate, and Temsirolimus in Treating Patients With Metastatic Kidney Cancer
This randomized phase II trial studies different combinations of bevacizumab, temsirolimus, and sorafenib tosylate to see how well they work compared with bevacizumab alone in treating patients with kidney cancer that has spread to other places in the body. Monoclonal antibodies, such as bevacizumab, may interfere with the ability of tumor cells to grow and spread. Bevacizumab and sorafenib tosylate may stop the growth of tumor cells by blocking blood flow to the tumor. Temsirolimus and sorafenib tosylate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving different combinations of bevacizumab, sorafenib tosylate, and temsirolimus may be more effective than bevacizumab alone in treating metastatic kidney cancer.
ID: NCT00378703
Sponsor: National Cancer Institute
Locations: VA San Diego Medical Center, California; Jesse Brown VAMC, Chicago, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; VA New Jersey Health Care System East Orange; Dayton VAMC, Ohio; Michael E. DeBakey VAMC, Houston, Texas
Everolimus in Treating Patients With Kidney Cancer Who Have Undergone Surgery (S0931)
Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth or by blocking blood flow to the tumor. This phase III trial is studying everolimus to see how well it works in treating patients with kidney cancer who have undergone surgery.
ID: NCT01120249
Sponsor: Southwest Oncology Group
Locations: Central Arkansas Veterans Healthcare System, Little Rock; Denver VAMC, Colorado; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC Indianapolis, Indiana; VAMC Baltimore, Maryland; Minneapolis VeteransMedical Center, Minnesota; VA New Jersey Health Care System, East Orange; VA New York Harbor Healthcare System-Brooklyn Campus; Wright-Patterson, Medical Center, Ohio; Michael E.DeBakey VAMC, Houston, Texas; Audie L. Murphy VA Hospital, San Antonio, Texas
Cabozantinib-s-malate or Sunitinib Malate in Treating Patients With Previously Untreated Locally Advanced or Metastatic Kidney Cancer
This randomized phase II trial studies how well cabozantinib-s-malate works compared to sunitinib malate in treating patients with previously untreated kidney cancer that has spread from where it started to nearby tissue or lymph nodes or to other places in the body. Cabozantinib-s-malate and sunitinib malate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. It is not yet known whether cabozantinib-s-malate is more effective than sunitinib malate in treating patients with kidney cancer.
ID: NCT01835158
Sponsor: National Cancer Institute
Locations: VA San Diego Medical Center, California; Minneapolis Veterans Medical Center, Minnesota; VAMC Columbia, Missouri; VA Western New York Health Care System, Buffalo
Everolimus With or Without Bevacizumab in Treating Patients With Advanced Kidney Cancer That Progressed After First-Line Therapy
This randomized phase III trial studies giving everolimus together with bevacizumab to see how well it works compared to everolimus alone in treating patients with advanced kidney cancer that progressed after first-line therapy. Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Monoclonal antibodies, such as bevacizumab, can interfere with tumor growth by blocking the ability of tumor cells to grow and spread. Everolimus and bevacizumab may also stop the growth of kidney cancer by blocking blood flow to the tumor. It is not yet known whether giving everolimus together with bevacizumab is better than everolimus alone in treating patients with advanced kidney cancer that has progressed after first-line therapy.
Sponsor: National Cancer Institute
ID: NCT01198158
Locations: Jesse Brown VAMC, Chicago, Illinois; Walter Reed National Military Medical Center, Bethesda, Maryland; VA Western New York Health Care System, Buffalo
Tivantinib With or Without Erlotinib Hydrochloride in Treating Patients With Metastatic or Locally Advanced Kidney Cancer That Cannot Be Removed by Surgery
This randomized phase II trial studies how well tivantinib with or without erlotinib hydrochloride works in treating patients with metastatic or locally advanced kidney cancer that cannot be removed by surgery. Tivantinib and erlotinib hydrochloride may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth.
Sponsor: National Cancer Institute (NCI)
ID: NCT01688973
Locations: Hines VA Hospital, Illinois; VA New Jersey Health Care System, East Orange; Audie L. Murphy VA Hospital, San Antonio, Texas
Bioequivalence & Food Effect Study in Patients With Solid Tumor or Hematologic Malignancies
This study will enroll approximately 60 subjects in stage I and 60 subjects in stage II with hematologic or solid tumor malignancies, excluding gastrointestinal tumors and tumors that have originated or metastasized to the liver for which no standard treatment exists or have progressed or recurred following prior therapy. Subjects must not be eligible for therapy of higher curative potential where an alternative treatment has been shown to prolong survival in an analogous population. Approximately 23 sites in the U.S. and 2 in Canada will participate in this study.
Sponsor: Celgene
ID: NCT02223052
Location: VAMC Kansas City, Missouri
Gemcitabine Hydrochloride and Cisplatin With or Without Bevacizumab in Treating Patients With Advanced Urinary Tract Cancer
This randomized phase III trial studies gemcitabine hydrochloride, cisplatin, and bevacizumab to see how well they work compared with gemcitabine hydrochloride and cisplatin in treating patients with urinary tract cancer that has spread to other places in the body. Drugs used in chemotherapy, such as gemcitabine hydrochloride and cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Monoclonal antibodies, such as bevacizumab, may interfere with the ability of tumor cells to grow and spread. It is not yet known whether gemcitabine hydrochloride and cisplatin are more effective when given with or without bevacizumab in treating patients with urinary tract cancer.
Sponsor: National Cancer Institute
ID: NCT00942331
Locations: Central Arkansas Veterans Healthcare System, Little Rock; Denver VAMC, Colorado; Baltimore VAMC, Maryland; Columbia VA, Missouri; VA New Jersey Health Care System, East Orange; VA New York Harbor Healthcare System-Brooklyn Campus; VA Western New York Health Care System, Buffalo; Dayton VAMC, Ohio
Eribulin Mesylate in Treating Patients With Locally Advanced or Metastatic Cancer of the Urothelium and Kidney Dysfunction
This phase I/II trial studies the side effects and best dose of eribulin mesylate and to see how well it works in treating patients with cancer of the urothelium that has spread to nearby tissue or to other places in the body and kidney dysfunction. Drugs used in chemotherapy, such as eribulin mesylate, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Chemotherapy drugs may have different effects in patients who have changes in their kidney function.
Sponsor: National Cancer Institute
ID: NCT00365157
Location: VA Hospital-Martinez, California
Click here to read the digital edition.
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting from those populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors more than 430 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of July 24, 2017; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for kidney cancer/renal cell carninoma. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
NCI-MATCH: Targeted Therapy Directed by Genetic Testing in Treating Patients With Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma
This phase II trial studies how well treatment that is directed by genetic testing works in patients with solid tumors or lymphomas that have progressed following at least 1 line of standard treatment or for which no agreed upon treatment approach exists. Genetic tests look at the unique genetic material (genes) of patients’ tumor cells. Patients with genetic abnormalities (such as mutations, amplifications, or translocations) may benefit more from treatment that targets their tumor’s particular genetic abnormality. Identifying these genetic abnormalities first may help doctors plan better treatment for patients with solid tumors, lymphomas, or multiple myeloma.
ID: NCT02465060
Sponsor: National Cancer Institute
Locations (contact): Naval Medical Center-San Diego, California (Preston S. Gable); VA Connecticut Healthcare System-West Haven Campus (Herta H. Chao); Durham VAMC, North Carolina (Michael J. Kelley); Walter Reed National Military Medical Center, Bethesda, Maryland (Jeremy G. Perkins)
Bevacizumab, Sorafenib Tosylate, and Temsirolimus in Treating Patients With Metastatic Kidney Cancer
This randomized phase II trial studies different combinations of bevacizumab, temsirolimus, and sorafenib tosylate to see how well they work compared with bevacizumab alone in treating patients with kidney cancer that has spread to other places in the body. Monoclonal antibodies, such as bevacizumab, may interfere with the ability of tumor cells to grow and spread. Bevacizumab and sorafenib tosylate may stop the growth of tumor cells by blocking blood flow to the tumor. Temsirolimus and sorafenib tosylate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving different combinations of bevacizumab, sorafenib tosylate, and temsirolimus may be more effective than bevacizumab alone in treating metastatic kidney cancer.
ID: NCT00378703
Sponsor: National Cancer Institute
Locations: VA San Diego Medical Center, California; Jesse Brown VAMC, Chicago, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; VA New Jersey Health Care System East Orange; Dayton VAMC, Ohio; Michael E. DeBakey VAMC, Houston, Texas
Everolimus in Treating Patients With Kidney Cancer Who Have Undergone Surgery (S0931)
Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth or by blocking blood flow to the tumor. This phase III trial is studying everolimus to see how well it works in treating patients with kidney cancer who have undergone surgery.
ID: NCT01120249
Sponsor: Southwest Oncology Group
Locations: Central Arkansas Veterans Healthcare System, Little Rock; Denver VAMC, Colorado; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC Indianapolis, Indiana; VAMC Baltimore, Maryland; Minneapolis VeteransMedical Center, Minnesota; VA New Jersey Health Care System, East Orange; VA New York Harbor Healthcare System-Brooklyn Campus; Wright-Patterson, Medical Center, Ohio; Michael E.DeBakey VAMC, Houston, Texas; Audie L. Murphy VA Hospital, San Antonio, Texas
Cabozantinib-s-malate or Sunitinib Malate in Treating Patients With Previously Untreated Locally Advanced or Metastatic Kidney Cancer
This randomized phase II trial studies how well cabozantinib-s-malate works compared to sunitinib malate in treating patients with previously untreated kidney cancer that has spread from where it started to nearby tissue or lymph nodes or to other places in the body. Cabozantinib-s-malate and sunitinib malate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. It is not yet known whether cabozantinib-s-malate is more effective than sunitinib malate in treating patients with kidney cancer.
ID: NCT01835158
Sponsor: National Cancer Institute
Locations: VA San Diego Medical Center, California; Minneapolis Veterans Medical Center, Minnesota; VAMC Columbia, Missouri; VA Western New York Health Care System, Buffalo
Everolimus With or Without Bevacizumab in Treating Patients With Advanced Kidney Cancer That Progressed After First-Line Therapy
This randomized phase III trial studies giving everolimus together with bevacizumab to see how well it works compared to everolimus alone in treating patients with advanced kidney cancer that progressed after first-line therapy. Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Monoclonal antibodies, such as bevacizumab, can interfere with tumor growth by blocking the ability of tumor cells to grow and spread. Everolimus and bevacizumab may also stop the growth of kidney cancer by blocking blood flow to the tumor. It is not yet known whether giving everolimus together with bevacizumab is better than everolimus alone in treating patients with advanced kidney cancer that has progressed after first-line therapy.
Sponsor: National Cancer Institute
ID: NCT01198158
Locations: Jesse Brown VAMC, Chicago, Illinois; Walter Reed National Military Medical Center, Bethesda, Maryland; VA Western New York Health Care System, Buffalo
Tivantinib With or Without Erlotinib Hydrochloride in Treating Patients With Metastatic or Locally Advanced Kidney Cancer That Cannot Be Removed by Surgery
This randomized phase II trial studies how well tivantinib with or without erlotinib hydrochloride works in treating patients with metastatic or locally advanced kidney cancer that cannot be removed by surgery. Tivantinib and erlotinib hydrochloride may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth.
Sponsor: National Cancer Institute (NCI)
ID: NCT01688973
Locations: Hines VA Hospital, Illinois; VA New Jersey Health Care System, East Orange; Audie L. Murphy VA Hospital, San Antonio, Texas
Bioequivalence & Food Effect Study in Patients With Solid Tumor or Hematologic Malignancies
This study will enroll approximately 60 subjects in stage I and 60 subjects in stage II with hematologic or solid tumor malignancies, excluding gastrointestinal tumors and tumors that have originated or metastasized to the liver for which no standard treatment exists or have progressed or recurred following prior therapy. Subjects must not be eligible for therapy of higher curative potential where an alternative treatment has been shown to prolong survival in an analogous population. Approximately 23 sites in the U.S. and 2 in Canada will participate in this study.
Sponsor: Celgene
ID: NCT02223052
Location: VAMC Kansas City, Missouri
Gemcitabine Hydrochloride and Cisplatin With or Without Bevacizumab in Treating Patients With Advanced Urinary Tract Cancer
This randomized phase III trial studies gemcitabine hydrochloride, cisplatin, and bevacizumab to see how well they work compared with gemcitabine hydrochloride and cisplatin in treating patients with urinary tract cancer that has spread to other places in the body. Drugs used in chemotherapy, such as gemcitabine hydrochloride and cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Monoclonal antibodies, such as bevacizumab, may interfere with the ability of tumor cells to grow and spread. It is not yet known whether gemcitabine hydrochloride and cisplatin are more effective when given with or without bevacizumab in treating patients with urinary tract cancer.
Sponsor: National Cancer Institute
ID: NCT00942331
Locations: Central Arkansas Veterans Healthcare System, Little Rock; Denver VAMC, Colorado; Baltimore VAMC, Maryland; Columbia VA, Missouri; VA New Jersey Health Care System, East Orange; VA New York Harbor Healthcare System-Brooklyn Campus; VA Western New York Health Care System, Buffalo; Dayton VAMC, Ohio
Eribulin Mesylate in Treating Patients With Locally Advanced or Metastatic Cancer of the Urothelium and Kidney Dysfunction
This phase I/II trial studies the side effects and best dose of eribulin mesylate and to see how well it works in treating patients with cancer of the urothelium that has spread to nearby tissue or to other places in the body and kidney dysfunction. Drugs used in chemotherapy, such as eribulin mesylate, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Chemotherapy drugs may have different effects in patients who have changes in their kidney function.
Sponsor: National Cancer Institute
ID: NCT00365157
Location: VA Hospital-Martinez, California
Click here to read the digital edition.
Providing access to clinical trials for veteran and active-duty military patients can be a challenge, but a significant number of trials are now recruiting from those populations. Many trials explicitly recruit patients from the VA, the military, and IHS. The VA Office of Research and Development alone sponsors more than 430 research initiatives, and many more are sponsored by Walter Reed National Medical Center and other major defense and VA facilities. The clinical trials listed below are all open as of July 24, 2017; have at least 1 VA, DoD, or IHS location recruiting patients; and are focused on treatment for kidney cancer/renal cell carninoma. For additional information and full inclusion/exclusion criteria, please consult clinicaltrials.gov.
NCI-MATCH: Targeted Therapy Directed by Genetic Testing in Treating Patients With Advanced Refractory Solid Tumors, Lymphomas, or Multiple Myeloma
This phase II trial studies how well treatment that is directed by genetic testing works in patients with solid tumors or lymphomas that have progressed following at least 1 line of standard treatment or for which no agreed upon treatment approach exists. Genetic tests look at the unique genetic material (genes) of patients’ tumor cells. Patients with genetic abnormalities (such as mutations, amplifications, or translocations) may benefit more from treatment that targets their tumor’s particular genetic abnormality. Identifying these genetic abnormalities first may help doctors plan better treatment for patients with solid tumors, lymphomas, or multiple myeloma.
ID: NCT02465060
Sponsor: National Cancer Institute
Locations (contact): Naval Medical Center-San Diego, California (Preston S. Gable); VA Connecticut Healthcare System-West Haven Campus (Herta H. Chao); Durham VAMC, North Carolina (Michael J. Kelley); Walter Reed National Military Medical Center, Bethesda, Maryland (Jeremy G. Perkins)
Bevacizumab, Sorafenib Tosylate, and Temsirolimus in Treating Patients With Metastatic Kidney Cancer
This randomized phase II trial studies different combinations of bevacizumab, temsirolimus, and sorafenib tosylate to see how well they work compared with bevacizumab alone in treating patients with kidney cancer that has spread to other places in the body. Monoclonal antibodies, such as bevacizumab, may interfere with the ability of tumor cells to grow and spread. Bevacizumab and sorafenib tosylate may stop the growth of tumor cells by blocking blood flow to the tumor. Temsirolimus and sorafenib tosylate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Giving different combinations of bevacizumab, sorafenib tosylate, and temsirolimus may be more effective than bevacizumab alone in treating metastatic kidney cancer.
ID: NCT00378703
Sponsor: National Cancer Institute
Locations: VA San Diego Medical Center, California; Jesse Brown VAMC, Chicago, Illinois; Richard L. Roudebush VAMC, Indianapolis, Indiana; VA New Jersey Health Care System East Orange; Dayton VAMC, Ohio; Michael E. DeBakey VAMC, Houston, Texas
Everolimus in Treating Patients With Kidney Cancer Who Have Undergone Surgery (S0931)
Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth or by blocking blood flow to the tumor. This phase III trial is studying everolimus to see how well it works in treating patients with kidney cancer who have undergone surgery.
ID: NCT01120249
Sponsor: Southwest Oncology Group
Locations: Central Arkansas Veterans Healthcare System, Little Rock; Denver VAMC, Colorado; Hines VA Hospital, Illinois; Richard L. Roudebush VAMC Indianapolis, Indiana; VAMC Baltimore, Maryland; Minneapolis VeteransMedical Center, Minnesota; VA New Jersey Health Care System, East Orange; VA New York Harbor Healthcare System-Brooklyn Campus; Wright-Patterson, Medical Center, Ohio; Michael E.DeBakey VAMC, Houston, Texas; Audie L. Murphy VA Hospital, San Antonio, Texas
Cabozantinib-s-malate or Sunitinib Malate in Treating Patients With Previously Untreated Locally Advanced or Metastatic Kidney Cancer
This randomized phase II trial studies how well cabozantinib-s-malate works compared to sunitinib malate in treating patients with previously untreated kidney cancer that has spread from where it started to nearby tissue or lymph nodes or to other places in the body. Cabozantinib-s-malate and sunitinib malate may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. It is not yet known whether cabozantinib-s-malate is more effective than sunitinib malate in treating patients with kidney cancer.
ID: NCT01835158
Sponsor: National Cancer Institute
Locations: VA San Diego Medical Center, California; Minneapolis Veterans Medical Center, Minnesota; VAMC Columbia, Missouri; VA Western New York Health Care System, Buffalo
Everolimus With or Without Bevacizumab in Treating Patients With Advanced Kidney Cancer That Progressed After First-Line Therapy
This randomized phase III trial studies giving everolimus together with bevacizumab to see how well it works compared to everolimus alone in treating patients with advanced kidney cancer that progressed after first-line therapy. Everolimus may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth. Monoclonal antibodies, such as bevacizumab, can interfere with tumor growth by blocking the ability of tumor cells to grow and spread. Everolimus and bevacizumab may also stop the growth of kidney cancer by blocking blood flow to the tumor. It is not yet known whether giving everolimus together with bevacizumab is better than everolimus alone in treating patients with advanced kidney cancer that has progressed after first-line therapy.
Sponsor: National Cancer Institute
ID: NCT01198158
Locations: Jesse Brown VAMC, Chicago, Illinois; Walter Reed National Military Medical Center, Bethesda, Maryland; VA Western New York Health Care System, Buffalo
Tivantinib With or Without Erlotinib Hydrochloride in Treating Patients With Metastatic or Locally Advanced Kidney Cancer That Cannot Be Removed by Surgery
This randomized phase II trial studies how well tivantinib with or without erlotinib hydrochloride works in treating patients with metastatic or locally advanced kidney cancer that cannot be removed by surgery. Tivantinib and erlotinib hydrochloride may stop the growth of tumor cells by blocking some of the enzymes needed for cell growth.
Sponsor: National Cancer Institute (NCI)
ID: NCT01688973
Locations: Hines VA Hospital, Illinois; VA New Jersey Health Care System, East Orange; Audie L. Murphy VA Hospital, San Antonio, Texas
Bioequivalence & Food Effect Study in Patients With Solid Tumor or Hematologic Malignancies
This study will enroll approximately 60 subjects in stage I and 60 subjects in stage II with hematologic or solid tumor malignancies, excluding gastrointestinal tumors and tumors that have originated or metastasized to the liver for which no standard treatment exists or have progressed or recurred following prior therapy. Subjects must not be eligible for therapy of higher curative potential where an alternative treatment has been shown to prolong survival in an analogous population. Approximately 23 sites in the U.S. and 2 in Canada will participate in this study.
Sponsor: Celgene
ID: NCT02223052
Location: VAMC Kansas City, Missouri
Gemcitabine Hydrochloride and Cisplatin With or Without Bevacizumab in Treating Patients With Advanced Urinary Tract Cancer
This randomized phase III trial studies gemcitabine hydrochloride, cisplatin, and bevacizumab to see how well they work compared with gemcitabine hydrochloride and cisplatin in treating patients with urinary tract cancer that has spread to other places in the body. Drugs used in chemotherapy, such as gemcitabine hydrochloride and cisplatin, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Monoclonal antibodies, such as bevacizumab, may interfere with the ability of tumor cells to grow and spread. It is not yet known whether gemcitabine hydrochloride and cisplatin are more effective when given with or without bevacizumab in treating patients with urinary tract cancer.
Sponsor: National Cancer Institute
ID: NCT00942331
Locations: Central Arkansas Veterans Healthcare System, Little Rock; Denver VAMC, Colorado; Baltimore VAMC, Maryland; Columbia VA, Missouri; VA New Jersey Health Care System, East Orange; VA New York Harbor Healthcare System-Brooklyn Campus; VA Western New York Health Care System, Buffalo; Dayton VAMC, Ohio
Eribulin Mesylate in Treating Patients With Locally Advanced or Metastatic Cancer of the Urothelium and Kidney Dysfunction
This phase I/II trial studies the side effects and best dose of eribulin mesylate and to see how well it works in treating patients with cancer of the urothelium that has spread to nearby tissue or to other places in the body and kidney dysfunction. Drugs used in chemotherapy, such as eribulin mesylate, work in different ways to stop the growth of tumor cells, either by killing the cells, by stopping them from dividing, or by stopping them from spreading. Chemotherapy drugs may have different effects in patients who have changes in their kidney function.
Sponsor: National Cancer Institute
ID: NCT00365157
Location: VA Hospital-Martinez, California
Click here to read the digital edition.
Depression Screening and Treatment: A Missed Opportunity in Lung Cancer Care (FULL)
About Research in Context
In this article, the authors of recent scholarship have been asked to discuss the implications of their research on federal health care providers and specifically the veteran and active-duty service member patient populations. Because the article does not include new research and cannot be blinded, it has undergone an abbreviated peer review process. The original article can be found at Sullivan DR, Forsberg CW, Ganzini L, et al. Longitudinal changes in depression symptoms and survival among patients with lung cancer: a national cohort assessment. J Clin Oncol. 2016;34(33):3984-3991.
Although depression is common among patients with cancer, patients with lung cancer are at particularly high risk. The prevalence of major depressive disorder (MDD) among patients with cancer can be as high as 13%, whereas up to 44% of patients with lung cancer experience depression symptoms at some point following their cancer diagnosis.1-3 These estimates are consistently higher than those of other types of cancer, possibly related to the stigma of the disease and the associated morbidity and mortality that are its hallmarks.4-8 This potentially life-threatening cancer diagnosis often evokes psychological distress; however, additional stressors contribute to the development of depression, including the effects of chemotherapeutic agents, surgical procedures, radiotherapy, and the consequences of physical symptoms and paraneoplastic syndromes.
In addition to the crippling effects of comorbid depression on patients’ quality of life (QOL), severe and persistent depression among patients with cancer is associated with prolonged hospital stays, worse treatment adherence, physical distress and pain, and increased desire for hastened death.9-11 During treatment, depression can amplify physical symptoms and interfere with effective coping.12,13
Depression also is likely a significant factor for the risk of suicide, which is 4 times higher in patients with lung cancer than that of the general population.14 Most important, as our recent study demonstrated, depression that develops at cancer diagnosis or during cancer treatment may contribute to worse survival. This effect was strongest among patients with early stage disease, in other words, the patients who are most likely to achieve cure.3 This association with early stage disease also has been observed in a strictly veteran population from the northwest U.S.15
Another key finding of our study was the similar survival among patients who experienced a remission of their depression and those who were never depressed. This finding reinforces the importance of effective depression treatment, which has the potential to reduce depression-related mortality; however, depression treatment was not fully captured and could not be directly compared in our study. Unfortunately, comorbid depression often goes undiagnosed and untreated in cancer patients as they report unmet emotional needs and a desire for psychological support during and after completion of cancer treatment.16,17
Given the general lack of depression treatment that occurs in patients with cancer, the negative consequences of depression can be sustained well into survivorship—defined clinically as someone who is free of any sign of cancer for 5 years. Cancer survivors frequently report fatigue, mood disturbance, sleep disruption, pain, and cognitive limitations that significantly impact QOL and are associated with disability and increased health care use.18 These symptoms likely are intertwined with and contribute to the development and persistence of depression. The ramifications of untreated depression on long-term cancer survivor outcomes are not completely understood, as few high-quality studies of depression in cancer survivors exist. However, in a mixed group of patients with cancer, there was a 2-fold risk of mortality in survivors with depression symptoms when these patients were assessed from 1 to 10 years into survivorship.19 The impact of depression on cancer survivorship is an important aspect of cancer care that deserves significantly more attention from both a research and clinical perspective.
Special Considerations for Veterans
There is a higher prevalence of mental health diagnoses in veterans than that in the general population, and depressive disorders are the most common.20-22 According to the VA National Registry for Depression, 11% of veterans aged ≥ 65 years have a diagnosis of MDD, a rate more than twice that in the general population of a similar age.23 However, the actual rate of depression among veterans may be even higher, as studies suggest depression is underdiagnosed in the veteran population.24 In addition to depression, veterans experience other disabling psychological illnesses, such as posttraumatic stress disorder (PTSD) related to deployment and combat duty or combat-related injuries, such as traumatic brain injuries. The negative consequences of PTSD on cancer outcomes are largely unexplored, but PTSD can contribute to increased health care utilization and costs.25,26 A similar psychological construct, cancer-related posttraumatic stress (PTS), which develops as a result of a cancer diagnosis or treatment, is associated with missed medical appointments and procedures, which could impact survival.27
Depression Screening and Treatment
Given the negative consequences of comorbid mental illness, professional oncology societies have started developing guidelines regarding the assessments and care of patients with cancer who are experiencing symptoms of depression and/or anxiety.11,28,29 Among these, the American Society of Clinical Oncology (ASCO) has adapted the Pan-Canadian Practice Guideline on Screening, Assessment, and Care of Psychosocial Distress (Depression, Anxiety) in Adults With Cancer.28 Per ASCO, the target audience for these guidelines is health care providers (eg, medical, surgical, and radiation oncologists; psychiatrists; psychologists; primary care providers; nurses; and others involved in the delivery of care for adults with cancer) as well as patients with cancer and their family members and caregivers.28 These guidelines address the optimum screening, assessment, and psychosocial-supportive care interventions for adults with cancer who are identified as experiencing symptoms of depression. Among the most imperative recommendations are periodic assessments across the trajectory of cancer care, including after cure, as well as employing institutional and community resources for depression treatment.
In clinical practice in a VA setting, implementing these guidelines might involve various interventions. First, it is vital for providers to conduct depression screening during periodic health care encounters. Given the high prevalence of depression in patients with lung cancer, we suggest using the 9-item Patient Health Questionnaire (PHQ-9) as an initial screening tool.30 Unlike the abridged 2-item PHQ-2 commonly used in the VA, the PHQ-9 provides an assessment of the full range of depressive symptoms. An elevated PHQ-9 score (≥ 10) is consistent with a major depressive episode and should trigger next steps.30
Once clinically significant depression is identified, initiation of treatment should occur next. The VA is well suited to assist and support non-mental health clinicians—particularly primary care—in treatment initiation and monitoring. This model of partnership is frequently called collaborative care, or integrated care, and it is well positioned to help patients with lung cancer with concomitant depression. In the VA, this model of care is called primary care-mental healthintegration (PC-MHI). One PC-MHI resource is called TIDES (Translating Initiatives for Depression into Effective Solutions), and when a patient is referred, a mental health nurse care manager helps to track the patients’ antidepressant adherence and treatment response while reporting results to primary care clinicians, who are generally responsible for initiating and continuing the antidepressant prescription. For patients preferring nonpharmacologic approaches or for whom an antidepressant may be contraindicated, PC-MHI can provide other assistance. For example, psychologists working in PC-MHI are equipped to provide a brief course of cognitive behavioral therapy sessions, another first-line, evidence-based treatment for clinical depression.
Clinician follow-up to ensure patient adherence, response, and satisfaction, and to adjust treatment as needed is essential. Besides ongoing coordination with PC-MHI services, including mental health clinicians as part of multidisciplinary cancer clinics could offer substantial added value to patients’ comprehensive cancer care. Indeed, the initiation of multicomponent depression care has been shown to improve QOL and role functioning in patients with cancer.31 Besides the established benefits on QOL, patients with lung cancer who achieve depression symptom remission also may enjoy a significant survival benefit over patients whose depression symptoms remain untreated during lung cancer treatment as our study suggests.3
Conclusion
Depression is a common comorbid disease among patients with lung cancer with important negative implications for QOL and survival. When it occurs after a cancer diagnosis, depression is expected to impact all phases of a patient’s life through treatment and survivorshi —ultimately affecting long-term survival. Veterans may be at particularly high risk given the increased prevalence of mental illness, including depression and PTSD in this group compared with that of the general population. Early detection and prompt treatment can promote depression remission, prevent relapse, and reduce the eventual emotional and financial burden of the disease. This approach may ultimately diminish the prevalence and persistence of depression symptoms and decrease the associated negative effects of this disease on patients with lung cancer.
The importance of integrated systems of depression treatment for patients with cancer as part of comprehensive cancer care cannot be overstated. Development and implementation of these systems should be a priority of lung cancer clinicians and treatment centers. The integrated system within the VA is well positioned to be a leader in this area, and VA clinicians who care for patients with lung cancer are encouraged to take advantage of available mental health resources. Additional research is urgently needed to explore optimal implementation of depression screening and subsequent treatment delivery to improve cancer patient outcomes in VA and non-VA health care settings.
Overall, there is minimal evidence that depression treatment can improve lung cancer survival; however, the lack of high-quality studies is a considerable limitation. Given the significant impact of depression on survival among patients with lung cancer, additional funding and resources are urgently needed to combat this debilitating comorbid disease.
Acknowledgments
This project was supported in part by the National Cancer Institute of the National Institutes of Health under award K07CA190706 to Dr. Sullivan, a Career Development Award from the Veterans Health Administration Health Service Research and Development (CDA 14-428) to Dr. Teo and the HSR&D Center to Improve Veteran Involvement in Care (CIVIC) (CIN 13-404) at the VA Portland Health Care System.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The VA had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249(6):751-757.
2. Walker J, Holm Hansen C, Martin P, et al. Prevalence of depression in adults with cancer: a systematic review. Ann Oncol. 2013;24(4):895-900.
3. Sullivan DR, Forsberg CW, Ganzini L, et al. Longitudinal changes in depression symptoms and survival among patients with lung cancer: a national cohort assessment. J Clin Oncol. 2016;34(33):3984-3991.
4. Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141(2-3):343-351.
5. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71.
6. Brown Johnson CG, Brodsky JL, Cataldo JK. Lung cancer stigma, anxiety, depression, and quality of life. J Psychosoc Oncol. 2014;32(1):59-73.
7. Cataldo JK, Jahan TM, Pongquan VL. Lung cancer stigma, depression, and quality of life among ever and never smokers. Eur J Oncol Nurs. 2012;16(3):264-269.
8. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2010. https://seer.cancer.gov/archive/csr/1975_2010/. Revised February 21, 2014. Accessed July 12, 2017.
9. Li M, Boquiren V, Lo C, et al. Depression and anxiety in supportive oncology. In: Davis M, Feyer P, Ortner P, Zimmermann C, eds. Supportive Oncology. 1st ed. Philadelphia, PA: Elsevier; 2011:528-540.
10. Brown LF, Kroenke K, Theobald DE, Wu J, Tu W. The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psychooncology. 2010;19(7):734-741.
11. Lazenby M, Ercolano E, Grant M, Holland JC, Jacobsen PB, McCorkle R. Supporting Commission on Cancer-mandated psychosocial distress screening with implementation strategies. J Oncol Pract. 2015;11(3):e413-e420.
12. Mystakidou K, Tsilika E, Parpa E, Katsouda E, Galanos A, Vlahos L. Psychological distress of patients with advanced cancer: influence and contribution of pain severity and pain interference. Cancer Nurs. 2006;29(5):400-405.
13. Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobald DE, Edgerton S. Oncologists’ recognition of depression in their patients with cancer. J Clin Oncol. 1998;16(4):1594-1600.
14. Rahuma M, Kamel M, Nasar A, et al. Lung cancer patients have the highest malignancy-associated suicide rate in USA: a population based analysis. Am J Respir Crit Care Med. 2017;195:A6730.
15. Sullivan DR, Ganzini L, Duckart JP, et al. Treatment receipt and outcomes among lung cancer patients with depression. Clin Oncol (R Coll Radiol). 2014;26(1):25-31.
16. Merckaert I, Libert Y, Messin S, Milani M, Slachmuylder JL, Razavi D. Cancer patients’ desire for psychological support: prevalence and implications for screening patients psychological needs. Psychooncology. 2010;19(2):141-149.
17. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17(8):1117-1128.
18. Wu HS, Harden JK. Symptom burden and quality of life in survivorship: a review of the literature. Cancer Nurs. 2015;38(1):E29-E54.
19. Mols F, Husson O, Roukema JA, van de Poll-Franse LV. Depressive symptoms are a risk factor for all-cause mortality: results from a prospective population-based study among 3,080 cancer survivors from the PROFILES registry. J Cancer Surviv. 2013;7(3):484-492.
20. Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13-22.
21. Fortney JC, Curran GM, Hunt JB, et al. Prevalence of probable mental disorders and help-seeking behaviors among veteran and non-veteran community college students. Gen Hosp Psychiatry. 2016;38:99-104.
22. Pickett T, Rothman D, Crawford EF, Brancu M, Fairbank JA, Kudler HS. Mental health among military personnel and veterans. N C Med J. 2015;76(5):299-306.
23. U.S. Department of Veterans Affairs, Veterans Health Administration. One in ten older vets is depressed. https://www.va.gov/health/NewsFeatures/20110624a.asp. Updated April 17, 2015. Accessed July 12, 2017.
24. Fontana A, Rosenheck R. Treatment-seeking veterans of Iraq and Afghanistan: comparison with veterans of previous wars. J Nerv Ment Dis. 2008;196(7):513-521.
25. Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(suppl 5):4-12; discussion, 13-14.
26. Kartha A, Brower V, Saitz R, Samet JH, Keane TM, Liebschutz J. The impact of trauma exposure and post-traumatic stress disorder on healthcare utilization among primary care patients. Med Care. 2008;46(4):388-393.
27. National Cancer Institute. Cancer-related post-traumatic stress (PDQ®)–Patient version. https://www.cancer.gov/about-cancer/coping/survivorship/new-normal/ptsd-pdq. Updated July 7, 2015. Accessed July 12, 2017.
28. Andersen BL, DeRubeis RJ, Berman BS, et al; American Society of Clinical Oncology. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619.
29. Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol. 2013;20(3):e233-e246.
30. Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12(9):964-973.
31. Walker J, Hansen CH, Martin P, et al; SMaRT (Symptom Management Research Trials) Oncology-3 Team. Integrated collaborative care for major depression comorbid with a poor prognosis cancer (SMaRT oncology-3): a multicentre randomised controlled trial in patients with lung cancer. Lancet Oncol. 2014;15(10):1168-1176.
About Research in Context
In this article, the authors of recent scholarship have been asked to discuss the implications of their research on federal health care providers and specifically the veteran and active-duty service member patient populations. Because the article does not include new research and cannot be blinded, it has undergone an abbreviated peer review process. The original article can be found at Sullivan DR, Forsberg CW, Ganzini L, et al. Longitudinal changes in depression symptoms and survival among patients with lung cancer: a national cohort assessment. J Clin Oncol. 2016;34(33):3984-3991.
Although depression is common among patients with cancer, patients with lung cancer are at particularly high risk. The prevalence of major depressive disorder (MDD) among patients with cancer can be as high as 13%, whereas up to 44% of patients with lung cancer experience depression symptoms at some point following their cancer diagnosis.1-3 These estimates are consistently higher than those of other types of cancer, possibly related to the stigma of the disease and the associated morbidity and mortality that are its hallmarks.4-8 This potentially life-threatening cancer diagnosis often evokes psychological distress; however, additional stressors contribute to the development of depression, including the effects of chemotherapeutic agents, surgical procedures, radiotherapy, and the consequences of physical symptoms and paraneoplastic syndromes.
In addition to the crippling effects of comorbid depression on patients’ quality of life (QOL), severe and persistent depression among patients with cancer is associated with prolonged hospital stays, worse treatment adherence, physical distress and pain, and increased desire for hastened death.9-11 During treatment, depression can amplify physical symptoms and interfere with effective coping.12,13
Depression also is likely a significant factor for the risk of suicide, which is 4 times higher in patients with lung cancer than that of the general population.14 Most important, as our recent study demonstrated, depression that develops at cancer diagnosis or during cancer treatment may contribute to worse survival. This effect was strongest among patients with early stage disease, in other words, the patients who are most likely to achieve cure.3 This association with early stage disease also has been observed in a strictly veteran population from the northwest U.S.15
Another key finding of our study was the similar survival among patients who experienced a remission of their depression and those who were never depressed. This finding reinforces the importance of effective depression treatment, which has the potential to reduce depression-related mortality; however, depression treatment was not fully captured and could not be directly compared in our study. Unfortunately, comorbid depression often goes undiagnosed and untreated in cancer patients as they report unmet emotional needs and a desire for psychological support during and after completion of cancer treatment.16,17
Given the general lack of depression treatment that occurs in patients with cancer, the negative consequences of depression can be sustained well into survivorship—defined clinically as someone who is free of any sign of cancer for 5 years. Cancer survivors frequently report fatigue, mood disturbance, sleep disruption, pain, and cognitive limitations that significantly impact QOL and are associated with disability and increased health care use.18 These symptoms likely are intertwined with and contribute to the development and persistence of depression. The ramifications of untreated depression on long-term cancer survivor outcomes are not completely understood, as few high-quality studies of depression in cancer survivors exist. However, in a mixed group of patients with cancer, there was a 2-fold risk of mortality in survivors with depression symptoms when these patients were assessed from 1 to 10 years into survivorship.19 The impact of depression on cancer survivorship is an important aspect of cancer care that deserves significantly more attention from both a research and clinical perspective.
Special Considerations for Veterans
There is a higher prevalence of mental health diagnoses in veterans than that in the general population, and depressive disorders are the most common.20-22 According to the VA National Registry for Depression, 11% of veterans aged ≥ 65 years have a diagnosis of MDD, a rate more than twice that in the general population of a similar age.23 However, the actual rate of depression among veterans may be even higher, as studies suggest depression is underdiagnosed in the veteran population.24 In addition to depression, veterans experience other disabling psychological illnesses, such as posttraumatic stress disorder (PTSD) related to deployment and combat duty or combat-related injuries, such as traumatic brain injuries. The negative consequences of PTSD on cancer outcomes are largely unexplored, but PTSD can contribute to increased health care utilization and costs.25,26 A similar psychological construct, cancer-related posttraumatic stress (PTS), which develops as a result of a cancer diagnosis or treatment, is associated with missed medical appointments and procedures, which could impact survival.27
Depression Screening and Treatment
Given the negative consequences of comorbid mental illness, professional oncology societies have started developing guidelines regarding the assessments and care of patients with cancer who are experiencing symptoms of depression and/or anxiety.11,28,29 Among these, the American Society of Clinical Oncology (ASCO) has adapted the Pan-Canadian Practice Guideline on Screening, Assessment, and Care of Psychosocial Distress (Depression, Anxiety) in Adults With Cancer.28 Per ASCO, the target audience for these guidelines is health care providers (eg, medical, surgical, and radiation oncologists; psychiatrists; psychologists; primary care providers; nurses; and others involved in the delivery of care for adults with cancer) as well as patients with cancer and their family members and caregivers.28 These guidelines address the optimum screening, assessment, and psychosocial-supportive care interventions for adults with cancer who are identified as experiencing symptoms of depression. Among the most imperative recommendations are periodic assessments across the trajectory of cancer care, including after cure, as well as employing institutional and community resources for depression treatment.
In clinical practice in a VA setting, implementing these guidelines might involve various interventions. First, it is vital for providers to conduct depression screening during periodic health care encounters. Given the high prevalence of depression in patients with lung cancer, we suggest using the 9-item Patient Health Questionnaire (PHQ-9) as an initial screening tool.30 Unlike the abridged 2-item PHQ-2 commonly used in the VA, the PHQ-9 provides an assessment of the full range of depressive symptoms. An elevated PHQ-9 score (≥ 10) is consistent with a major depressive episode and should trigger next steps.30
Once clinically significant depression is identified, initiation of treatment should occur next. The VA is well suited to assist and support non-mental health clinicians—particularly primary care—in treatment initiation and monitoring. This model of partnership is frequently called collaborative care, or integrated care, and it is well positioned to help patients with lung cancer with concomitant depression. In the VA, this model of care is called primary care-mental healthintegration (PC-MHI). One PC-MHI resource is called TIDES (Translating Initiatives for Depression into Effective Solutions), and when a patient is referred, a mental health nurse care manager helps to track the patients’ antidepressant adherence and treatment response while reporting results to primary care clinicians, who are generally responsible for initiating and continuing the antidepressant prescription. For patients preferring nonpharmacologic approaches or for whom an antidepressant may be contraindicated, PC-MHI can provide other assistance. For example, psychologists working in PC-MHI are equipped to provide a brief course of cognitive behavioral therapy sessions, another first-line, evidence-based treatment for clinical depression.
Clinician follow-up to ensure patient adherence, response, and satisfaction, and to adjust treatment as needed is essential. Besides ongoing coordination with PC-MHI services, including mental health clinicians as part of multidisciplinary cancer clinics could offer substantial added value to patients’ comprehensive cancer care. Indeed, the initiation of multicomponent depression care has been shown to improve QOL and role functioning in patients with cancer.31 Besides the established benefits on QOL, patients with lung cancer who achieve depression symptom remission also may enjoy a significant survival benefit over patients whose depression symptoms remain untreated during lung cancer treatment as our study suggests.3
Conclusion
Depression is a common comorbid disease among patients with lung cancer with important negative implications for QOL and survival. When it occurs after a cancer diagnosis, depression is expected to impact all phases of a patient’s life through treatment and survivorshi —ultimately affecting long-term survival. Veterans may be at particularly high risk given the increased prevalence of mental illness, including depression and PTSD in this group compared with that of the general population. Early detection and prompt treatment can promote depression remission, prevent relapse, and reduce the eventual emotional and financial burden of the disease. This approach may ultimately diminish the prevalence and persistence of depression symptoms and decrease the associated negative effects of this disease on patients with lung cancer.
The importance of integrated systems of depression treatment for patients with cancer as part of comprehensive cancer care cannot be overstated. Development and implementation of these systems should be a priority of lung cancer clinicians and treatment centers. The integrated system within the VA is well positioned to be a leader in this area, and VA clinicians who care for patients with lung cancer are encouraged to take advantage of available mental health resources. Additional research is urgently needed to explore optimal implementation of depression screening and subsequent treatment delivery to improve cancer patient outcomes in VA and non-VA health care settings.
Overall, there is minimal evidence that depression treatment can improve lung cancer survival; however, the lack of high-quality studies is a considerable limitation. Given the significant impact of depression on survival among patients with lung cancer, additional funding and resources are urgently needed to combat this debilitating comorbid disease.
Acknowledgments
This project was supported in part by the National Cancer Institute of the National Institutes of Health under award K07CA190706 to Dr. Sullivan, a Career Development Award from the Veterans Health Administration Health Service Research and Development (CDA 14-428) to Dr. Teo and the HSR&D Center to Improve Veteran Involvement in Care (CIVIC) (CIN 13-404) at the VA Portland Health Care System.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The VA had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
About Research in Context
In this article, the authors of recent scholarship have been asked to discuss the implications of their research on federal health care providers and specifically the veteran and active-duty service member patient populations. Because the article does not include new research and cannot be blinded, it has undergone an abbreviated peer review process. The original article can be found at Sullivan DR, Forsberg CW, Ganzini L, et al. Longitudinal changes in depression symptoms and survival among patients with lung cancer: a national cohort assessment. J Clin Oncol. 2016;34(33):3984-3991.
Although depression is common among patients with cancer, patients with lung cancer are at particularly high risk. The prevalence of major depressive disorder (MDD) among patients with cancer can be as high as 13%, whereas up to 44% of patients with lung cancer experience depression symptoms at some point following their cancer diagnosis.1-3 These estimates are consistently higher than those of other types of cancer, possibly related to the stigma of the disease and the associated morbidity and mortality that are its hallmarks.4-8 This potentially life-threatening cancer diagnosis often evokes psychological distress; however, additional stressors contribute to the development of depression, including the effects of chemotherapeutic agents, surgical procedures, radiotherapy, and the consequences of physical symptoms and paraneoplastic syndromes.
In addition to the crippling effects of comorbid depression on patients’ quality of life (QOL), severe and persistent depression among patients with cancer is associated with prolonged hospital stays, worse treatment adherence, physical distress and pain, and increased desire for hastened death.9-11 During treatment, depression can amplify physical symptoms and interfere with effective coping.12,13
Depression also is likely a significant factor for the risk of suicide, which is 4 times higher in patients with lung cancer than that of the general population.14 Most important, as our recent study demonstrated, depression that develops at cancer diagnosis or during cancer treatment may contribute to worse survival. This effect was strongest among patients with early stage disease, in other words, the patients who are most likely to achieve cure.3 This association with early stage disease also has been observed in a strictly veteran population from the northwest U.S.15
Another key finding of our study was the similar survival among patients who experienced a remission of their depression and those who were never depressed. This finding reinforces the importance of effective depression treatment, which has the potential to reduce depression-related mortality; however, depression treatment was not fully captured and could not be directly compared in our study. Unfortunately, comorbid depression often goes undiagnosed and untreated in cancer patients as they report unmet emotional needs and a desire for psychological support during and after completion of cancer treatment.16,17
Given the general lack of depression treatment that occurs in patients with cancer, the negative consequences of depression can be sustained well into survivorship—defined clinically as someone who is free of any sign of cancer for 5 years. Cancer survivors frequently report fatigue, mood disturbance, sleep disruption, pain, and cognitive limitations that significantly impact QOL and are associated with disability and increased health care use.18 These symptoms likely are intertwined with and contribute to the development and persistence of depression. The ramifications of untreated depression on long-term cancer survivor outcomes are not completely understood, as few high-quality studies of depression in cancer survivors exist. However, in a mixed group of patients with cancer, there was a 2-fold risk of mortality in survivors with depression symptoms when these patients were assessed from 1 to 10 years into survivorship.19 The impact of depression on cancer survivorship is an important aspect of cancer care that deserves significantly more attention from both a research and clinical perspective.
Special Considerations for Veterans
There is a higher prevalence of mental health diagnoses in veterans than that in the general population, and depressive disorders are the most common.20-22 According to the VA National Registry for Depression, 11% of veterans aged ≥ 65 years have a diagnosis of MDD, a rate more than twice that in the general population of a similar age.23 However, the actual rate of depression among veterans may be even higher, as studies suggest depression is underdiagnosed in the veteran population.24 In addition to depression, veterans experience other disabling psychological illnesses, such as posttraumatic stress disorder (PTSD) related to deployment and combat duty or combat-related injuries, such as traumatic brain injuries. The negative consequences of PTSD on cancer outcomes are largely unexplored, but PTSD can contribute to increased health care utilization and costs.25,26 A similar psychological construct, cancer-related posttraumatic stress (PTS), which develops as a result of a cancer diagnosis or treatment, is associated with missed medical appointments and procedures, which could impact survival.27
Depression Screening and Treatment
Given the negative consequences of comorbid mental illness, professional oncology societies have started developing guidelines regarding the assessments and care of patients with cancer who are experiencing symptoms of depression and/or anxiety.11,28,29 Among these, the American Society of Clinical Oncology (ASCO) has adapted the Pan-Canadian Practice Guideline on Screening, Assessment, and Care of Psychosocial Distress (Depression, Anxiety) in Adults With Cancer.28 Per ASCO, the target audience for these guidelines is health care providers (eg, medical, surgical, and radiation oncologists; psychiatrists; psychologists; primary care providers; nurses; and others involved in the delivery of care for adults with cancer) as well as patients with cancer and their family members and caregivers.28 These guidelines address the optimum screening, assessment, and psychosocial-supportive care interventions for adults with cancer who are identified as experiencing symptoms of depression. Among the most imperative recommendations are periodic assessments across the trajectory of cancer care, including after cure, as well as employing institutional and community resources for depression treatment.
In clinical practice in a VA setting, implementing these guidelines might involve various interventions. First, it is vital for providers to conduct depression screening during periodic health care encounters. Given the high prevalence of depression in patients with lung cancer, we suggest using the 9-item Patient Health Questionnaire (PHQ-9) as an initial screening tool.30 Unlike the abridged 2-item PHQ-2 commonly used in the VA, the PHQ-9 provides an assessment of the full range of depressive symptoms. An elevated PHQ-9 score (≥ 10) is consistent with a major depressive episode and should trigger next steps.30
Once clinically significant depression is identified, initiation of treatment should occur next. The VA is well suited to assist and support non-mental health clinicians—particularly primary care—in treatment initiation and monitoring. This model of partnership is frequently called collaborative care, or integrated care, and it is well positioned to help patients with lung cancer with concomitant depression. In the VA, this model of care is called primary care-mental healthintegration (PC-MHI). One PC-MHI resource is called TIDES (Translating Initiatives for Depression into Effective Solutions), and when a patient is referred, a mental health nurse care manager helps to track the patients’ antidepressant adherence and treatment response while reporting results to primary care clinicians, who are generally responsible for initiating and continuing the antidepressant prescription. For patients preferring nonpharmacologic approaches or for whom an antidepressant may be contraindicated, PC-MHI can provide other assistance. For example, psychologists working in PC-MHI are equipped to provide a brief course of cognitive behavioral therapy sessions, another first-line, evidence-based treatment for clinical depression.
Clinician follow-up to ensure patient adherence, response, and satisfaction, and to adjust treatment as needed is essential. Besides ongoing coordination with PC-MHI services, including mental health clinicians as part of multidisciplinary cancer clinics could offer substantial added value to patients’ comprehensive cancer care. Indeed, the initiation of multicomponent depression care has been shown to improve QOL and role functioning in patients with cancer.31 Besides the established benefits on QOL, patients with lung cancer who achieve depression symptom remission also may enjoy a significant survival benefit over patients whose depression symptoms remain untreated during lung cancer treatment as our study suggests.3
Conclusion
Depression is a common comorbid disease among patients with lung cancer with important negative implications for QOL and survival. When it occurs after a cancer diagnosis, depression is expected to impact all phases of a patient’s life through treatment and survivorshi —ultimately affecting long-term survival. Veterans may be at particularly high risk given the increased prevalence of mental illness, including depression and PTSD in this group compared with that of the general population. Early detection and prompt treatment can promote depression remission, prevent relapse, and reduce the eventual emotional and financial burden of the disease. This approach may ultimately diminish the prevalence and persistence of depression symptoms and decrease the associated negative effects of this disease on patients with lung cancer.
The importance of integrated systems of depression treatment for patients with cancer as part of comprehensive cancer care cannot be overstated. Development and implementation of these systems should be a priority of lung cancer clinicians and treatment centers. The integrated system within the VA is well positioned to be a leader in this area, and VA clinicians who care for patients with lung cancer are encouraged to take advantage of available mental health resources. Additional research is urgently needed to explore optimal implementation of depression screening and subsequent treatment delivery to improve cancer patient outcomes in VA and non-VA health care settings.
Overall, there is minimal evidence that depression treatment can improve lung cancer survival; however, the lack of high-quality studies is a considerable limitation. Given the significant impact of depression on survival among patients with lung cancer, additional funding and resources are urgently needed to combat this debilitating comorbid disease.
Acknowledgments
This project was supported in part by the National Cancer Institute of the National Institutes of Health under award K07CA190706 to Dr. Sullivan, a Career Development Award from the Veterans Health Administration Health Service Research and Development (CDA 14-428) to Dr. Teo and the HSR&D Center to Improve Veteran Involvement in Care (CIVIC) (CIN 13-404) at the VA Portland Health Care System.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The VA had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies.
Click here to read the digital edition.
1. Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249(6):751-757.
2. Walker J, Holm Hansen C, Martin P, et al. Prevalence of depression in adults with cancer: a systematic review. Ann Oncol. 2013;24(4):895-900.
3. Sullivan DR, Forsberg CW, Ganzini L, et al. Longitudinal changes in depression symptoms and survival among patients with lung cancer: a national cohort assessment. J Clin Oncol. 2016;34(33):3984-3991.
4. Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141(2-3):343-351.
5. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71.
6. Brown Johnson CG, Brodsky JL, Cataldo JK. Lung cancer stigma, anxiety, depression, and quality of life. J Psychosoc Oncol. 2014;32(1):59-73.
7. Cataldo JK, Jahan TM, Pongquan VL. Lung cancer stigma, depression, and quality of life among ever and never smokers. Eur J Oncol Nurs. 2012;16(3):264-269.
8. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2010. https://seer.cancer.gov/archive/csr/1975_2010/. Revised February 21, 2014. Accessed July 12, 2017.
9. Li M, Boquiren V, Lo C, et al. Depression and anxiety in supportive oncology. In: Davis M, Feyer P, Ortner P, Zimmermann C, eds. Supportive Oncology. 1st ed. Philadelphia, PA: Elsevier; 2011:528-540.
10. Brown LF, Kroenke K, Theobald DE, Wu J, Tu W. The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psychooncology. 2010;19(7):734-741.
11. Lazenby M, Ercolano E, Grant M, Holland JC, Jacobsen PB, McCorkle R. Supporting Commission on Cancer-mandated psychosocial distress screening with implementation strategies. J Oncol Pract. 2015;11(3):e413-e420.
12. Mystakidou K, Tsilika E, Parpa E, Katsouda E, Galanos A, Vlahos L. Psychological distress of patients with advanced cancer: influence and contribution of pain severity and pain interference. Cancer Nurs. 2006;29(5):400-405.
13. Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobald DE, Edgerton S. Oncologists’ recognition of depression in their patients with cancer. J Clin Oncol. 1998;16(4):1594-1600.
14. Rahuma M, Kamel M, Nasar A, et al. Lung cancer patients have the highest malignancy-associated suicide rate in USA: a population based analysis. Am J Respir Crit Care Med. 2017;195:A6730.
15. Sullivan DR, Ganzini L, Duckart JP, et al. Treatment receipt and outcomes among lung cancer patients with depression. Clin Oncol (R Coll Radiol). 2014;26(1):25-31.
16. Merckaert I, Libert Y, Messin S, Milani M, Slachmuylder JL, Razavi D. Cancer patients’ desire for psychological support: prevalence and implications for screening patients psychological needs. Psychooncology. 2010;19(2):141-149.
17. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17(8):1117-1128.
18. Wu HS, Harden JK. Symptom burden and quality of life in survivorship: a review of the literature. Cancer Nurs. 2015;38(1):E29-E54.
19. Mols F, Husson O, Roukema JA, van de Poll-Franse LV. Depressive symptoms are a risk factor for all-cause mortality: results from a prospective population-based study among 3,080 cancer survivors from the PROFILES registry. J Cancer Surviv. 2013;7(3):484-492.
20. Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13-22.
21. Fortney JC, Curran GM, Hunt JB, et al. Prevalence of probable mental disorders and help-seeking behaviors among veteran and non-veteran community college students. Gen Hosp Psychiatry. 2016;38:99-104.
22. Pickett T, Rothman D, Crawford EF, Brancu M, Fairbank JA, Kudler HS. Mental health among military personnel and veterans. N C Med J. 2015;76(5):299-306.
23. U.S. Department of Veterans Affairs, Veterans Health Administration. One in ten older vets is depressed. https://www.va.gov/health/NewsFeatures/20110624a.asp. Updated April 17, 2015. Accessed July 12, 2017.
24. Fontana A, Rosenheck R. Treatment-seeking veterans of Iraq and Afghanistan: comparison with veterans of previous wars. J Nerv Ment Dis. 2008;196(7):513-521.
25. Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(suppl 5):4-12; discussion, 13-14.
26. Kartha A, Brower V, Saitz R, Samet JH, Keane TM, Liebschutz J. The impact of trauma exposure and post-traumatic stress disorder on healthcare utilization among primary care patients. Med Care. 2008;46(4):388-393.
27. National Cancer Institute. Cancer-related post-traumatic stress (PDQ®)–Patient version. https://www.cancer.gov/about-cancer/coping/survivorship/new-normal/ptsd-pdq. Updated July 7, 2015. Accessed July 12, 2017.
28. Andersen BL, DeRubeis RJ, Berman BS, et al; American Society of Clinical Oncology. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619.
29. Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol. 2013;20(3):e233-e246.
30. Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12(9):964-973.
31. Walker J, Hansen CH, Martin P, et al; SMaRT (Symptom Management Research Trials) Oncology-3 Team. Integrated collaborative care for major depression comorbid with a poor prognosis cancer (SMaRT oncology-3): a multicentre randomised controlled trial in patients with lung cancer. Lancet Oncol. 2014;15(10):1168-1176.
1. Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249(6):751-757.
2. Walker J, Holm Hansen C, Martin P, et al. Prevalence of depression in adults with cancer: a systematic review. Ann Oncol. 2013;24(4):895-900.
3. Sullivan DR, Forsberg CW, Ganzini L, et al. Longitudinal changes in depression symptoms and survival among patients with lung cancer: a national cohort assessment. J Clin Oncol. 2016;34(33):3984-3991.
4. Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141(2-3):343-351.
5. Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;(32):57-71.
6. Brown Johnson CG, Brodsky JL, Cataldo JK. Lung cancer stigma, anxiety, depression, and quality of life. J Psychosoc Oncol. 2014;32(1):59-73.
7. Cataldo JK, Jahan TM, Pongquan VL. Lung cancer stigma, depression, and quality of life among ever and never smokers. Eur J Oncol Nurs. 2012;16(3):264-269.
8. Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975-2010. https://seer.cancer.gov/archive/csr/1975_2010/. Revised February 21, 2014. Accessed July 12, 2017.
9. Li M, Boquiren V, Lo C, et al. Depression and anxiety in supportive oncology. In: Davis M, Feyer P, Ortner P, Zimmermann C, eds. Supportive Oncology. 1st ed. Philadelphia, PA: Elsevier; 2011:528-540.
10. Brown LF, Kroenke K, Theobald DE, Wu J, Tu W. The association of depression and anxiety with health-related quality of life in cancer patients with depression and/or pain. Psychooncology. 2010;19(7):734-741.
11. Lazenby M, Ercolano E, Grant M, Holland JC, Jacobsen PB, McCorkle R. Supporting Commission on Cancer-mandated psychosocial distress screening with implementation strategies. J Oncol Pract. 2015;11(3):e413-e420.
12. Mystakidou K, Tsilika E, Parpa E, Katsouda E, Galanos A, Vlahos L. Psychological distress of patients with advanced cancer: influence and contribution of pain severity and pain interference. Cancer Nurs. 2006;29(5):400-405.
13. Passik SD, Dugan W, McDonald MV, Rosenfeld B, Theobald DE, Edgerton S. Oncologists’ recognition of depression in their patients with cancer. J Clin Oncol. 1998;16(4):1594-1600.
14. Rahuma M, Kamel M, Nasar A, et al. Lung cancer patients have the highest malignancy-associated suicide rate in USA: a population based analysis. Am J Respir Crit Care Med. 2017;195:A6730.
15. Sullivan DR, Ganzini L, Duckart JP, et al. Treatment receipt and outcomes among lung cancer patients with depression. Clin Oncol (R Coll Radiol). 2014;26(1):25-31.
16. Merckaert I, Libert Y, Messin S, Milani M, Slachmuylder JL, Razavi D. Cancer patients’ desire for psychological support: prevalence and implications for screening patients psychological needs. Psychooncology. 2010;19(2):141-149.
17. Harrison JD, Young JM, Price MA, Butow PN, Solomon MJ. What are the unmet supportive care needs of people with cancer? A systematic review. Support Care Cancer. 2009;17(8):1117-1128.
18. Wu HS, Harden JK. Symptom burden and quality of life in survivorship: a review of the literature. Cancer Nurs. 2015;38(1):E29-E54.
19. Mols F, Husson O, Roukema JA, van de Poll-Franse LV. Depressive symptoms are a risk factor for all-cause mortality: results from a prospective population-based study among 3,080 cancer survivors from the PROFILES registry. J Cancer Surviv. 2013;7(3):484-492.
20. Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13-22.
21. Fortney JC, Curran GM, Hunt JB, et al. Prevalence of probable mental disorders and help-seeking behaviors among veteran and non-veteran community college students. Gen Hosp Psychiatry. 2016;38:99-104.
22. Pickett T, Rothman D, Crawford EF, Brancu M, Fairbank JA, Kudler HS. Mental health among military personnel and veterans. N C Med J. 2015;76(5):299-306.
23. U.S. Department of Veterans Affairs, Veterans Health Administration. One in ten older vets is depressed. https://www.va.gov/health/NewsFeatures/20110624a.asp. Updated April 17, 2015. Accessed July 12, 2017.
24. Fontana A, Rosenheck R. Treatment-seeking veterans of Iraq and Afghanistan: comparison with veterans of previous wars. J Nerv Ment Dis. 2008;196(7):513-521.
25. Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(suppl 5):4-12; discussion, 13-14.
26. Kartha A, Brower V, Saitz R, Samet JH, Keane TM, Liebschutz J. The impact of trauma exposure and post-traumatic stress disorder on healthcare utilization among primary care patients. Med Care. 2008;46(4):388-393.
27. National Cancer Institute. Cancer-related post-traumatic stress (PDQ®)–Patient version. https://www.cancer.gov/about-cancer/coping/survivorship/new-normal/ptsd-pdq. Updated July 7, 2015. Accessed July 12, 2017.
28. Andersen BL, DeRubeis RJ, Berman BS, et al; American Society of Clinical Oncology. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: an American Society of Clinical Oncology guideline adaptation. J Clin Oncol. 2014;32(15):1605-1619.
29. Howell D, Keller-Olaman S, Oliver TK, et al. A pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigue. Curr Oncol. 2013;20(3):e233-e246.
30. Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain. 2011;12(9):964-973.
31. Walker J, Hansen CH, Martin P, et al; SMaRT (Symptom Management Research Trials) Oncology-3 Team. Integrated collaborative care for major depression comorbid with a poor prognosis cancer (SMaRT oncology-3): a multicentre randomised controlled trial in patients with lung cancer. Lancet Oncol. 2014;15(10):1168-1176.