User login
Reassuring data on long-term outcomes among kids with MIS-C
Most children who develop multisystemic inflammatory syndrome (MIS-C) after infection with SARS-CoV-2 recover relatively quickly and without significant sequelae, according to a research letter published online in JAMA Pediatrics.
“The results of this research letter offer some reassurance as has been the case with other longitudinal reports, that children with MIS-C largely recover from the illness with minimal sequelae,” said Kanwal M. Farooqi, MD, a pediatric cardiologist from Columbia University Irving Medical Center, New York.
“This is despite the severity of the initial clinical presentation, which can be quite significant with signs of systemic inflammation, hypotension, and need for ICU-level care,” continued Dr. Farooqi, who was not involved in the study.
Given that little is known about the medium- and long-term effects of MIS-C following infection with COVID-19, Patrick Davies, MRCPCH, Nottingham (England) University Hospitals NHS Trust, and colleagues reviewed data from one of the earliest multicenter national cohorts of children in the United Kingdom. The cohort included children admitted to the hospital prior to May 10, 2020, and the analysis was based on data from 68 of 76 (89%) patients of the initial surviving cohort. Information regarding critical care readmissions and outpatient follow-up visits up to April 1, 2021 (1-year post admission), was included in the analysis.
Overall laboratory results appeared normal for most children at 50 days post admission, including neutrophils, platelets, ferritin, creatinine, and alanine transaminase. Just 3% (2/65 test results) of children showed elevated levels of C-reactive protein, 3% (2/59 test results) for D-dimer, and 2% (1/60 test results) for troponin.
Based on echocardiographic data, 14 of the 19 patients who presented with aneurysms had resolution. Nine of 10 patients who presented with “bright” coronary arteries had resolution and only one progressed to having unresolved coronary artery aneurysms with the latest follow-up at 86 days post admission. All of the 38 patients who presented with impaired function without aneurysm had recovered by day 74.
Of the six patients with ongoing echocardiographic abnormalities, all had aneurysmal changes noted on echocardiograms performed between 86 and 336 days post admission. The authors were surprised to find that troponin levels in this group were lower when compared with others in the cohort (0.06 ng/mL [interquartile range, 0.02-0.418 ng/mL] vs. 0.157 ng/mL [0.033-0.81 ng/mL]; P = .02).
These six patients ranged in age from 0 to 13 years (median age, 8.75 years); five were Afro Caribbean boys and one was a White girl.
The researchers acknowledged that, despite coming from a nationwide data set, the interpretation of this data is limited given the small size of the cohort and the lack of standardized follow-up protocol available at the time.
When asked how this data might inform follow-up guidance for children post COVID infection, Dr. Farooqi said, “although it appears from the data that we have seen in the last few months that the patients recover relatively quickly from MIS-C, I believe it is reasonable to evaluate them at 6-month intervals for the second year until we have more information regarding longer-term outcomes.”
The study authors and Dr. Farooqi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most children who develop multisystemic inflammatory syndrome (MIS-C) after infection with SARS-CoV-2 recover relatively quickly and without significant sequelae, according to a research letter published online in JAMA Pediatrics.
“The results of this research letter offer some reassurance as has been the case with other longitudinal reports, that children with MIS-C largely recover from the illness with minimal sequelae,” said Kanwal M. Farooqi, MD, a pediatric cardiologist from Columbia University Irving Medical Center, New York.
“This is despite the severity of the initial clinical presentation, which can be quite significant with signs of systemic inflammation, hypotension, and need for ICU-level care,” continued Dr. Farooqi, who was not involved in the study.
Given that little is known about the medium- and long-term effects of MIS-C following infection with COVID-19, Patrick Davies, MRCPCH, Nottingham (England) University Hospitals NHS Trust, and colleagues reviewed data from one of the earliest multicenter national cohorts of children in the United Kingdom. The cohort included children admitted to the hospital prior to May 10, 2020, and the analysis was based on data from 68 of 76 (89%) patients of the initial surviving cohort. Information regarding critical care readmissions and outpatient follow-up visits up to April 1, 2021 (1-year post admission), was included in the analysis.
Overall laboratory results appeared normal for most children at 50 days post admission, including neutrophils, platelets, ferritin, creatinine, and alanine transaminase. Just 3% (2/65 test results) of children showed elevated levels of C-reactive protein, 3% (2/59 test results) for D-dimer, and 2% (1/60 test results) for troponin.
Based on echocardiographic data, 14 of the 19 patients who presented with aneurysms had resolution. Nine of 10 patients who presented with “bright” coronary arteries had resolution and only one progressed to having unresolved coronary artery aneurysms with the latest follow-up at 86 days post admission. All of the 38 patients who presented with impaired function without aneurysm had recovered by day 74.
Of the six patients with ongoing echocardiographic abnormalities, all had aneurysmal changes noted on echocardiograms performed between 86 and 336 days post admission. The authors were surprised to find that troponin levels in this group were lower when compared with others in the cohort (0.06 ng/mL [interquartile range, 0.02-0.418 ng/mL] vs. 0.157 ng/mL [0.033-0.81 ng/mL]; P = .02).
These six patients ranged in age from 0 to 13 years (median age, 8.75 years); five were Afro Caribbean boys and one was a White girl.
The researchers acknowledged that, despite coming from a nationwide data set, the interpretation of this data is limited given the small size of the cohort and the lack of standardized follow-up protocol available at the time.
When asked how this data might inform follow-up guidance for children post COVID infection, Dr. Farooqi said, “although it appears from the data that we have seen in the last few months that the patients recover relatively quickly from MIS-C, I believe it is reasonable to evaluate them at 6-month intervals for the second year until we have more information regarding longer-term outcomes.”
The study authors and Dr. Farooqi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Most children who develop multisystemic inflammatory syndrome (MIS-C) after infection with SARS-CoV-2 recover relatively quickly and without significant sequelae, according to a research letter published online in JAMA Pediatrics.
“The results of this research letter offer some reassurance as has been the case with other longitudinal reports, that children with MIS-C largely recover from the illness with minimal sequelae,” said Kanwal M. Farooqi, MD, a pediatric cardiologist from Columbia University Irving Medical Center, New York.
“This is despite the severity of the initial clinical presentation, which can be quite significant with signs of systemic inflammation, hypotension, and need for ICU-level care,” continued Dr. Farooqi, who was not involved in the study.
Given that little is known about the medium- and long-term effects of MIS-C following infection with COVID-19, Patrick Davies, MRCPCH, Nottingham (England) University Hospitals NHS Trust, and colleagues reviewed data from one of the earliest multicenter national cohorts of children in the United Kingdom. The cohort included children admitted to the hospital prior to May 10, 2020, and the analysis was based on data from 68 of 76 (89%) patients of the initial surviving cohort. Information regarding critical care readmissions and outpatient follow-up visits up to April 1, 2021 (1-year post admission), was included in the analysis.
Overall laboratory results appeared normal for most children at 50 days post admission, including neutrophils, platelets, ferritin, creatinine, and alanine transaminase. Just 3% (2/65 test results) of children showed elevated levels of C-reactive protein, 3% (2/59 test results) for D-dimer, and 2% (1/60 test results) for troponin.
Based on echocardiographic data, 14 of the 19 patients who presented with aneurysms had resolution. Nine of 10 patients who presented with “bright” coronary arteries had resolution and only one progressed to having unresolved coronary artery aneurysms with the latest follow-up at 86 days post admission. All of the 38 patients who presented with impaired function without aneurysm had recovered by day 74.
Of the six patients with ongoing echocardiographic abnormalities, all had aneurysmal changes noted on echocardiograms performed between 86 and 336 days post admission. The authors were surprised to find that troponin levels in this group were lower when compared with others in the cohort (0.06 ng/mL [interquartile range, 0.02-0.418 ng/mL] vs. 0.157 ng/mL [0.033-0.81 ng/mL]; P = .02).
These six patients ranged in age from 0 to 13 years (median age, 8.75 years); five were Afro Caribbean boys and one was a White girl.
The researchers acknowledged that, despite coming from a nationwide data set, the interpretation of this data is limited given the small size of the cohort and the lack of standardized follow-up protocol available at the time.
When asked how this data might inform follow-up guidance for children post COVID infection, Dr. Farooqi said, “although it appears from the data that we have seen in the last few months that the patients recover relatively quickly from MIS-C, I believe it is reasonable to evaluate them at 6-month intervals for the second year until we have more information regarding longer-term outcomes.”
The study authors and Dr. Farooqi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long COVID symptoms can persist for more than 1 year, study shows
Nearly half of people who are hospitalized with COVID-19 suffer at least one lingering symptom 1 year after discharge, according to the largest study yet to assess the dynamic recovery of a group of COVID-19 survivors 12 months after the illness.
The most common lingering symptoms are fatigue and muscle weakness. One-third continue to have shortness of breath.
Overall, at 12 months, COVID-19 survivors had more problems with mobility, pain or discomfort, and anxiety or depression, and had lower self-assessment scores of quality of life than matched COVID-free peers, the investigators report.
The study was published online Aug. 28 in The Lancet.
“While most had made a good recovery, health problems persisted in some patients, especially those who had been critically ill during their hospital stay,” Bin Cao, MD, from the National Center for Respiratory Medicine at the China-Japan Friendship Hospital and Capital Medical University, both in Beijing, said in a Lancet news release.
“Our findings suggest that recovery for some patients will take longer than 1 year, and this should be taken into account when planning delivery of health care services post pandemic,” Dr. Cao said.
“As the COVID-19 pandemic continues, the need to understand and respond to long COVID is increasingly pressing,” says a Lancet editorial.
“Symptoms such as persistent fatigue, breathlessness, brain fog, and depression could debilitate many millions of people globally. Long COVID is a modern medical challenge of the first order,” it reads.
Study details
Dr. Cao and colleagues studied 1,276 COVID-19 patients (median age 59; 53% men) discharged from a hospital in Wuhan, China, between Jan. 7 and May 29, 2020. The patients were assessed at 6 and 12 months from the date they first experienced COVID-19 symptoms.
Many symptoms resolved over time, regardless of the severity of illness. Yet 49% of patients still had at least one symptom 12 months after their acute illness, down from 68% at the 6-month mark, the authors report.
Fatigue and muscle weakness were the most commonly reported symptoms seen in 52% of patients at 6 months and 20% at 12 months. Compared with men, women were 1.4 times more likely to report fatigue or muscle weakness.
Patients treated with corticosteroids during the acute phase of COVID-19 were 1.5 times as likely to experience fatigue or muscle weakness after 12 months, compared with those who had not received corticosteroids.
Thirty percent of patients reported dyspnea at 12 months, slightly more than at 6 months (26%). Dyspnea was more common in the most severely ill patients needing a ventilator during their hospital stay (39%), compared with those who did not need oxygen treatment (25%).
At the 6-month check, 349 study participants underwent pulmonary function tests and 244 of those patients completed the same test at 12 months.
Spirometric and lung volume parameters of most of these patients were within normal limits at 12 months. But lung diffusion impairment was observed in about 20%-30% of patients who had been moderately ill with COVID-19 and as high as 54% in critically ill patients.
Compared with men, women were almost three times as likely to have lung diffusion impairment after 12 months.
Of 186 patients with abnormal lung CT scan at 6 months, 118 patients had a repeat CT scan at 12 months. The lung imaging abnormality gradually recovered during follow-up, yet 76% of the most critically ill patients still had ground glass opacity at 12 months.
Mental health hit
Among those patients who had been employed full- or part-time before catching COVID, the majority had returned to their original job (88%) and most had returned to their pre-COVID-19 level of work (76%) within 12 months.
Among those who did not return to their original work, 32% cited decreased physical function, 25% were unwilling to do their previous job, and 18% were unemployed.
As shown in multiple other studies, COVID-19 can take a toll on mental health. In this cohort, slightly more patients reported anxiety or depression at 12 months than at 6 months (23% vs. 26%), and the proportion was much greater than in matched community-dwelling adults without COVID-19 (5%).
Compared with men, women were twice as likely to report anxiety or depression.
“We do not yet fully understand why psychiatric symptoms are slightly more common at 1 year than at 6 months in COVID-19 survivors,” study author Xiaoying Gu, PhD, from the Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, said in the news release.
“These could be caused by a biological process linked to the virus infection itself, or the body’s immune response to it. Or they could be linked to reduced social contact, loneliness, incomplete recovery of physical health, or loss of employment associated with illness. Large, long-term studies of COVID-19 survivors are needed so that we can better understand the long-term physical and mental health consequences of COVID-19,” Dr. Gu said.
The authors caution that the findings represent a group of patients from a single hospital in China and the cohort included only a small number of patients who had been admitted to intensive care (94 of 1,276; 7.4%).
The Lancet editorial urges the scientific and medical community to “collaborate to explore the mechanism and pathogenesis of long COVID, estimate the global and regional disease burdens, better delineate who is most at risk, understand how vaccines might affect the condition, and find effective treatments via randomized controlled trials.”
“At the same time, health care providers must acknowledge and validate the toll of the persistent symptoms of long COVID on patients, and health systems need to be prepared to meet individualized, patient-oriented goals, with an appropriately trained workforce involving physical, cognitive, social, and occupational elements,” the editorial states.
“Answering these research questions while providing compassionate and multidisciplinary care will require the full breadth of scientific and medical ingenuity. It is a challenge to which the whole health community must rise,” the editorialists conclude.
The study was funded by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, the National Key Research and Development Program of China, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, the China Evergrande Group, the Jack Ma Foundation, Sino Biopharmaceutical, the Ping An Insurance (Group), and the New Sunshine Charity Foundation. The full list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Nearly half of people who are hospitalized with COVID-19 suffer at least one lingering symptom 1 year after discharge, according to the largest study yet to assess the dynamic recovery of a group of COVID-19 survivors 12 months after the illness.
The most common lingering symptoms are fatigue and muscle weakness. One-third continue to have shortness of breath.
Overall, at 12 months, COVID-19 survivors had more problems with mobility, pain or discomfort, and anxiety or depression, and had lower self-assessment scores of quality of life than matched COVID-free peers, the investigators report.
The study was published online Aug. 28 in The Lancet.
“While most had made a good recovery, health problems persisted in some patients, especially those who had been critically ill during their hospital stay,” Bin Cao, MD, from the National Center for Respiratory Medicine at the China-Japan Friendship Hospital and Capital Medical University, both in Beijing, said in a Lancet news release.
“Our findings suggest that recovery for some patients will take longer than 1 year, and this should be taken into account when planning delivery of health care services post pandemic,” Dr. Cao said.
“As the COVID-19 pandemic continues, the need to understand and respond to long COVID is increasingly pressing,” says a Lancet editorial.
“Symptoms such as persistent fatigue, breathlessness, brain fog, and depression could debilitate many millions of people globally. Long COVID is a modern medical challenge of the first order,” it reads.
Study details
Dr. Cao and colleagues studied 1,276 COVID-19 patients (median age 59; 53% men) discharged from a hospital in Wuhan, China, between Jan. 7 and May 29, 2020. The patients were assessed at 6 and 12 months from the date they first experienced COVID-19 symptoms.
Many symptoms resolved over time, regardless of the severity of illness. Yet 49% of patients still had at least one symptom 12 months after their acute illness, down from 68% at the 6-month mark, the authors report.
Fatigue and muscle weakness were the most commonly reported symptoms seen in 52% of patients at 6 months and 20% at 12 months. Compared with men, women were 1.4 times more likely to report fatigue or muscle weakness.
Patients treated with corticosteroids during the acute phase of COVID-19 were 1.5 times as likely to experience fatigue or muscle weakness after 12 months, compared with those who had not received corticosteroids.
Thirty percent of patients reported dyspnea at 12 months, slightly more than at 6 months (26%). Dyspnea was more common in the most severely ill patients needing a ventilator during their hospital stay (39%), compared with those who did not need oxygen treatment (25%).
At the 6-month check, 349 study participants underwent pulmonary function tests and 244 of those patients completed the same test at 12 months.
Spirometric and lung volume parameters of most of these patients were within normal limits at 12 months. But lung diffusion impairment was observed in about 20%-30% of patients who had been moderately ill with COVID-19 and as high as 54% in critically ill patients.
Compared with men, women were almost three times as likely to have lung diffusion impairment after 12 months.
Of 186 patients with abnormal lung CT scan at 6 months, 118 patients had a repeat CT scan at 12 months. The lung imaging abnormality gradually recovered during follow-up, yet 76% of the most critically ill patients still had ground glass opacity at 12 months.
Mental health hit
Among those patients who had been employed full- or part-time before catching COVID, the majority had returned to their original job (88%) and most had returned to their pre-COVID-19 level of work (76%) within 12 months.
Among those who did not return to their original work, 32% cited decreased physical function, 25% were unwilling to do their previous job, and 18% were unemployed.
As shown in multiple other studies, COVID-19 can take a toll on mental health. In this cohort, slightly more patients reported anxiety or depression at 12 months than at 6 months (23% vs. 26%), and the proportion was much greater than in matched community-dwelling adults without COVID-19 (5%).
Compared with men, women were twice as likely to report anxiety or depression.
“We do not yet fully understand why psychiatric symptoms are slightly more common at 1 year than at 6 months in COVID-19 survivors,” study author Xiaoying Gu, PhD, from the Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, said in the news release.
“These could be caused by a biological process linked to the virus infection itself, or the body’s immune response to it. Or they could be linked to reduced social contact, loneliness, incomplete recovery of physical health, or loss of employment associated with illness. Large, long-term studies of COVID-19 survivors are needed so that we can better understand the long-term physical and mental health consequences of COVID-19,” Dr. Gu said.
The authors caution that the findings represent a group of patients from a single hospital in China and the cohort included only a small number of patients who had been admitted to intensive care (94 of 1,276; 7.4%).
The Lancet editorial urges the scientific and medical community to “collaborate to explore the mechanism and pathogenesis of long COVID, estimate the global and regional disease burdens, better delineate who is most at risk, understand how vaccines might affect the condition, and find effective treatments via randomized controlled trials.”
“At the same time, health care providers must acknowledge and validate the toll of the persistent symptoms of long COVID on patients, and health systems need to be prepared to meet individualized, patient-oriented goals, with an appropriately trained workforce involving physical, cognitive, social, and occupational elements,” the editorial states.
“Answering these research questions while providing compassionate and multidisciplinary care will require the full breadth of scientific and medical ingenuity. It is a challenge to which the whole health community must rise,” the editorialists conclude.
The study was funded by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, the National Key Research and Development Program of China, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, the China Evergrande Group, the Jack Ma Foundation, Sino Biopharmaceutical, the Ping An Insurance (Group), and the New Sunshine Charity Foundation. The full list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
Nearly half of people who are hospitalized with COVID-19 suffer at least one lingering symptom 1 year after discharge, according to the largest study yet to assess the dynamic recovery of a group of COVID-19 survivors 12 months after the illness.
The most common lingering symptoms are fatigue and muscle weakness. One-third continue to have shortness of breath.
Overall, at 12 months, COVID-19 survivors had more problems with mobility, pain or discomfort, and anxiety or depression, and had lower self-assessment scores of quality of life than matched COVID-free peers, the investigators report.
The study was published online Aug. 28 in The Lancet.
“While most had made a good recovery, health problems persisted in some patients, especially those who had been critically ill during their hospital stay,” Bin Cao, MD, from the National Center for Respiratory Medicine at the China-Japan Friendship Hospital and Capital Medical University, both in Beijing, said in a Lancet news release.
“Our findings suggest that recovery for some patients will take longer than 1 year, and this should be taken into account when planning delivery of health care services post pandemic,” Dr. Cao said.
“As the COVID-19 pandemic continues, the need to understand and respond to long COVID is increasingly pressing,” says a Lancet editorial.
“Symptoms such as persistent fatigue, breathlessness, brain fog, and depression could debilitate many millions of people globally. Long COVID is a modern medical challenge of the first order,” it reads.
Study details
Dr. Cao and colleagues studied 1,276 COVID-19 patients (median age 59; 53% men) discharged from a hospital in Wuhan, China, between Jan. 7 and May 29, 2020. The patients were assessed at 6 and 12 months from the date they first experienced COVID-19 symptoms.
Many symptoms resolved over time, regardless of the severity of illness. Yet 49% of patients still had at least one symptom 12 months after their acute illness, down from 68% at the 6-month mark, the authors report.
Fatigue and muscle weakness were the most commonly reported symptoms seen in 52% of patients at 6 months and 20% at 12 months. Compared with men, women were 1.4 times more likely to report fatigue or muscle weakness.
Patients treated with corticosteroids during the acute phase of COVID-19 were 1.5 times as likely to experience fatigue or muscle weakness after 12 months, compared with those who had not received corticosteroids.
Thirty percent of patients reported dyspnea at 12 months, slightly more than at 6 months (26%). Dyspnea was more common in the most severely ill patients needing a ventilator during their hospital stay (39%), compared with those who did not need oxygen treatment (25%).
At the 6-month check, 349 study participants underwent pulmonary function tests and 244 of those patients completed the same test at 12 months.
Spirometric and lung volume parameters of most of these patients were within normal limits at 12 months. But lung diffusion impairment was observed in about 20%-30% of patients who had been moderately ill with COVID-19 and as high as 54% in critically ill patients.
Compared with men, women were almost three times as likely to have lung diffusion impairment after 12 months.
Of 186 patients with abnormal lung CT scan at 6 months, 118 patients had a repeat CT scan at 12 months. The lung imaging abnormality gradually recovered during follow-up, yet 76% of the most critically ill patients still had ground glass opacity at 12 months.
Mental health hit
Among those patients who had been employed full- or part-time before catching COVID, the majority had returned to their original job (88%) and most had returned to their pre-COVID-19 level of work (76%) within 12 months.
Among those who did not return to their original work, 32% cited decreased physical function, 25% were unwilling to do their previous job, and 18% were unemployed.
As shown in multiple other studies, COVID-19 can take a toll on mental health. In this cohort, slightly more patients reported anxiety or depression at 12 months than at 6 months (23% vs. 26%), and the proportion was much greater than in matched community-dwelling adults without COVID-19 (5%).
Compared with men, women were twice as likely to report anxiety or depression.
“We do not yet fully understand why psychiatric symptoms are slightly more common at 1 year than at 6 months in COVID-19 survivors,” study author Xiaoying Gu, PhD, from the Institute of Clinical Medical Sciences, China-Japan Friendship Hospital, said in the news release.
“These could be caused by a biological process linked to the virus infection itself, or the body’s immune response to it. Or they could be linked to reduced social contact, loneliness, incomplete recovery of physical health, or loss of employment associated with illness. Large, long-term studies of COVID-19 survivors are needed so that we can better understand the long-term physical and mental health consequences of COVID-19,” Dr. Gu said.
The authors caution that the findings represent a group of patients from a single hospital in China and the cohort included only a small number of patients who had been admitted to intensive care (94 of 1,276; 7.4%).
The Lancet editorial urges the scientific and medical community to “collaborate to explore the mechanism and pathogenesis of long COVID, estimate the global and regional disease burdens, better delineate who is most at risk, understand how vaccines might affect the condition, and find effective treatments via randomized controlled trials.”
“At the same time, health care providers must acknowledge and validate the toll of the persistent symptoms of long COVID on patients, and health systems need to be prepared to meet individualized, patient-oriented goals, with an appropriately trained workforce involving physical, cognitive, social, and occupational elements,” the editorial states.
“Answering these research questions while providing compassionate and multidisciplinary care will require the full breadth of scientific and medical ingenuity. It is a challenge to which the whole health community must rise,” the editorialists conclude.
The study was funded by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences, the National Natural Science Foundation of China, the National Key Research and Development Program of China, Major Projects of National Science and Technology on New Drug Creation and Development of Pulmonary Tuberculosis, the China Evergrande Group, the Jack Ma Foundation, Sino Biopharmaceutical, the Ping An Insurance (Group), and the New Sunshine Charity Foundation. The full list of author disclosures is available with the original article.
A version of this article first appeared on Medscape.com.
EMPEROR-Preserved spouts torrent of reports on empagliflozin treatment of HFpEF
The featured report from the 6,000-patient EMPEROR-Preserved trial at the virtual annual congress of the European Society of Cardiology drew lots of attention for its headline finding: the first unequivocal demonstration that a medication, empagliflozin, can significantly reduce the rate of cardiovascular death and hospitalization for heart failure in patients with heart failure with preserved ejection fraction (HFpEF, a left ventricular ejection fraction of more than 40%), with the details simultaneously published online.
But at the same time, the EMPEROR-Preserved investigators released four additional reports with a lot more outcome analyses that also deserve some attention.
The puzzling neutral effect on renal events
Perhaps the most surprising and complicated set of findings among the main EMPEROR-Preserved outcomes involved renal outcomes.
The trial’s primary outcome was the combined rate of cardiovascular death or hospitalization for heart failure (HHF), and the results showed that treatment with empagliflozin (Jardiance) for a median of 26 months on top of standard treatment for patients with HFpEF led to a significant 21% relative risk reduction, compared with placebo-treated patients.
The trial had two prespecified secondary outcomes. One was the total number of HHF, which dropped by a significant 27%, compared with placebo. The second was the mean change in slope of estimated glomerular filtration rate (eGFR) on an annualized basis, and the empagliflozin regimen reduced the cumulative annual deficit, compared with placebo by an average of 1.36 mL/min per 1.73 m2, a significant difference.
This preservation of renal function was consistent with results from many prior studies of empagliflozin and all of the other U.S.-approved agents from the sodium-glucose cotransporter 2 inhibitor class. Preservation of renal function and a reduction in renal events has become a hallmark property of all agents in the SGLT2 inhibitor class both in patients with type 2 diabetes, as well as in those without diabetes but with heart failure with reduced ejection fraction (HFrEF) or with chronic kidney disease.
EMPEROR-Preserved threw a wrench into what had been an unbroken history of renal protection by SGLT2 inhibitors. That happened when a prespecified endpoint of the study – a composite renal outcome defined as time to first occurrence of chronic dialysis, renal transplantation, a sustained reduction of at least 40% in eGFR, or a sustained drop in eGFR of more than 10 or 15 mL/min per 1.73 m2 from baseline – yielded an unexpected neutral finding.
For this composite renal outcome, EMPEROR-Preserved showed a nonsignificant 5% reduction, compared with placebo, a result that both differed from what had been seen in essentially all the other SGLT2 inhibitor trials that had looked at this, but which also seemed at odds with the observed significant preservation of renal function that seemed substantial enough to produce a clinically meaningful benefit.
Renal effects blunted in HFpEF
The immediate upshot was a letter published by several EMPEROR-Preserved investigators that spelled out this discrepancy and came to the jolting conclusion that “eGFR slope analysis has limitations as a surrogate for predicting the effect of drugs on renal outcomes in patients with heart failure.”
The same authors, along with some additional associates, also published a second letter that noted a further unexpected twist with the renal outcome: “In prior large-scale clinical trials, the effect of SGLT2 inhibitors on heart failure and renal outcomes had consistently tracked together,” they noted, but in this case it didn’t, a discordance they said was “extraordinarily puzzling”.
This led the study’s leaders to reanalyze the renal outcomes using a different definition, one that Milton Packer, MD, who helped design the trial and oversaw several of its analyses, called “a more conventional definition of renal events,” during his presentation of these findings at the congress. The researchers swapped out a 40% drop from baseline eGFR as an event and replaced it with a 50% decline, a change designed to screen out less severe, and often transient, reductions in kidney function that have less lasting impact on health. They also added an additional component to the composite endpoint, renal death. A revised analysis using this new renal composite outcome appeared in the European Journal of Heart Failure letter.
This change cut the total number of renal events tallied in the trial nearly in half, down to 112, and showed a more robust decline in renal events with empagliflozin treatment compared with the initial analysis, although the drop remained nonsignificant. The revised analysis also showed that the overall, nonsignificant 22% relative reduction in renal events in patients on empagliflozin, compared with placebo, dwindled down to completely nonexistent in the tertile of patients with a left ventricular ejection fraction of 60% or greater. In this tertile the hazard ratio actually showed a nonsignificant point estimate of a 24% increased rate of renal events on empagliflozin, with the caveat that this subgroup now included a total of just 40 total events between the two treatment arms. (Each of the two other tertiles also had roughly the same number of total events.)
The biggest effect on renal-event reduction was in the tertile of patients with an ejection fraction of 41%-49%, in which empagliflozin treatment was linked with a significant 59% cut in renal events, compared with placebo. The analysis also showed significant heterogeneity in thus outcome between this subgroup and the other two tertiles that had higher ejection fractions and showed reduced rates of protection by empagliflozin against renal events.
This apparent blunting of a renal effect despite preservation of renal function seemed to mimic the blunting of the primary cardiovascular outcome effect that also appeared in patients with ejection fractions in the 60%-65% range or above.
“If we knew what blunted the effect of empagliflozin on heart failure outcomes at higher ejection fraction levels, we think the same explanation may also apply to the blunting of effect on renal outcomes, but right now we do not know the answer to either question,” Dr. Packer said in an interview. He’s suggested that one possibility is that many of the enrolled patients identified as having HFpEF, but with these high ejection fractions may have not actually had HFpEF, and their signs and symptoms may have instead resulted from atrial fibrillation.
“Many patients with an ejection fraction of 60%-65% and above had atrial fibrillation,” he noted, with a prevalence at enrollment in this subgroup of about 50%. Atrial fibrillation can cause dyspnea, a hallmark symptom leading to diagnosis of heart failure, and it also increases levels of N-terminal of the prohormone brain natriuretic peptide, a metric that served as a gatekeeper for entry into the trial. “Essentially, we are saying that many of the criteria that we specified to ensure that patients had heart failure probably did not work very well in patients with an ejection fraction of 65% or greater,” said Dr. Packer, a cardiologist at Baylor University Medical Center in Dallas. “We need to figure out who these patients are.”
Some experts not involved with the study voiced skepticism that the renal findings reflected a real issue.
“I’m quite optimistic that in the long-term the effect on eGFR will translate into renal protection,” said Rudolf A. de Boer, MD, PhD, a professor of translational cardiology at University Medical Center Groningen (the Netherlands), and designated discussant at the congress for the presentation by Dr. Packer.
John J.V. McMurray, MD, a professor of cardiology and a heart failure specialist at Glasgow University, speculated that the unexpected renal outcomes data may relate to the initial decline in renal function produced by treatment with SGLT2 inhibitors despite their longer-term enhancement of renal protection.
“If you use a treatment that protects the kidneys in the long-term but causes an initial dip in eGFR, more patients receiving that treatment will have an early ‘event,’ ” he noted in an interview. He also cautioned about the dangers of subgroup analyses that dice the study population into small cohorts.
“Trials are powered to look at the effect of treatment in the overall population. Everything else is exploratory, underpowered, and subject to the play of chance,” Dr. McMurray stressed.
Counting additional cardiovascular disease events allows more analyses
A third auxiliary report from the EMPEROR-Preserved investigators performed several prespecified analyses that depended on adding additional cardiovascular disease endpoints to the core tallies of cardiovascular death or HHF – such as emergent, urgent, and outpatient events that reflected worsening heart failure – and also included information on diuretic and vasopressor use because of worsening heart failure. The increased event numbers allowed the researchers to perform 30 additional analyses included in this report, according to the count kept by Dr. Packer who was the lead author.
He highlighted several of the additional results in this paper that documented benefits from empagliflozin treatment, compared with placebo:
- A significant 29% reduction in the need for admission to a cardiac care unit or intensive care unit during an HHF.
- A nonsignificant 33% reduction in the need for intravenous vasopressors or positive inotropic drugs during HHF.
- A significantly increased rate of patients achieving a higher New York Heart Association functional class. For example, after the first year of treatment patients who received empagliflozin had a 37% higher rate of functional class improvement, compared with patients who received placebo.
Dr. McMurray had his own list of key takeaways from this paper, including:
- Among patients who needed hospitalization, “those treated with empagliflozin were less sick than those in the placebo group.”
- In addition to reducing HHF empagliflozin treatment also reduced episodes of outpatient worsening as reflected by their receipt of intensified diuretic treatment, which occurred a significant 27% less often, compared with patients on placebo.
- Treatment with empagliflozin also linked with a significant 39% relative reduction in emergency or urgent-care visits that required intravenous therapy.
Empagliflozin’s performance relative to sacubitril/valsartan
The fourth additional report focused on a post hoc, cross-trial comparison of the results from EMPEROR-Preserved and from another recent trial that, like EMPEROR-Preserved, assessed in patients with HFpEF a drug previously proven to work quite well in patients with HFrEF. The comparator drug was sacubitril/valsartan (Entresto), which underwent testing in patients with HFpEF in the PARAGON-HF trial.
The primary outcome of PARAGON-HF, which randomized 4,822 patients, was reduction in cardiovascular death and in total HHF. This dropped by a relative 13%, compared with placebo, during a median of 35 months, a between-group difference that came close to but did not achieve significance (P = .06). Despite this limitation, the Food and Drug Administration in February 2021 loosened the indication for using sacubitril/valsartan in patients with heart failure and a “below normal” ejection fraction, a category that can include many patients considered to have HFpEF.
Although the researchers who ran this analysis, including Dr. Packer, who was the first author, admitted that “comparison of effect sizes across trials is fraught with difficulties,” they nonetheless concluded from their analysis that “for all outcomes that included HHF the effect size was larger for empagliflozin than for sacubitril/valsartan.”
Dr. McMurray, a lead instigator for PARAGON-HF, said there was little to take away from this analysis.
“The patient populations were different, and sacubitril/valsartan was compared against an active therapy, valsartan,” while in EMPEROR-Preserved empagliflozin compared against placebo. “Most of us believe that sacubitril/valsartan and SGLT2 inhibitors work in different but complementary ways, and their benefits are additive. You would want patients with HFpEF or HFrEF to take both,” he said in an interview.
Dr. Packer agreed with that approach and added that he would probably also prescribe a third agent, spironolactone, to many patients with HFpEF.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and Eli Lilly, which jointly market empagliflozin (Jardiance). PARAGON-HF was sponsored by Novartis, which markets sacubitril/valsartan (Entresto). Dr. Packer has received consulting fees from Boehringer Ingelheim and from numerous other companies. Dr. de Boer has research contracts with Boehringer Ingelheim as well as from Abbott, AstraZeneca, Cardior, Ionis, Novo Nordisk, and Roche, and he has been a consultant to Novartis as well as to Abbott, AstraZeneca, Gayer, and Roche. Dr. McMurray led trials of sacubitril/valsartan sponsored by Novartis, and his institution has received compensation for his participation in studies sponsored by Abbvie, AstraZeneca, Cardurion, DalCor, GlaxoSmithKline, Pfizer, and Theracos.
The featured report from the 6,000-patient EMPEROR-Preserved trial at the virtual annual congress of the European Society of Cardiology drew lots of attention for its headline finding: the first unequivocal demonstration that a medication, empagliflozin, can significantly reduce the rate of cardiovascular death and hospitalization for heart failure in patients with heart failure with preserved ejection fraction (HFpEF, a left ventricular ejection fraction of more than 40%), with the details simultaneously published online.
But at the same time, the EMPEROR-Preserved investigators released four additional reports with a lot more outcome analyses that also deserve some attention.
The puzzling neutral effect on renal events
Perhaps the most surprising and complicated set of findings among the main EMPEROR-Preserved outcomes involved renal outcomes.
The trial’s primary outcome was the combined rate of cardiovascular death or hospitalization for heart failure (HHF), and the results showed that treatment with empagliflozin (Jardiance) for a median of 26 months on top of standard treatment for patients with HFpEF led to a significant 21% relative risk reduction, compared with placebo-treated patients.
The trial had two prespecified secondary outcomes. One was the total number of HHF, which dropped by a significant 27%, compared with placebo. The second was the mean change in slope of estimated glomerular filtration rate (eGFR) on an annualized basis, and the empagliflozin regimen reduced the cumulative annual deficit, compared with placebo by an average of 1.36 mL/min per 1.73 m2, a significant difference.
This preservation of renal function was consistent with results from many prior studies of empagliflozin and all of the other U.S.-approved agents from the sodium-glucose cotransporter 2 inhibitor class. Preservation of renal function and a reduction in renal events has become a hallmark property of all agents in the SGLT2 inhibitor class both in patients with type 2 diabetes, as well as in those without diabetes but with heart failure with reduced ejection fraction (HFrEF) or with chronic kidney disease.
EMPEROR-Preserved threw a wrench into what had been an unbroken history of renal protection by SGLT2 inhibitors. That happened when a prespecified endpoint of the study – a composite renal outcome defined as time to first occurrence of chronic dialysis, renal transplantation, a sustained reduction of at least 40% in eGFR, or a sustained drop in eGFR of more than 10 or 15 mL/min per 1.73 m2 from baseline – yielded an unexpected neutral finding.
For this composite renal outcome, EMPEROR-Preserved showed a nonsignificant 5% reduction, compared with placebo, a result that both differed from what had been seen in essentially all the other SGLT2 inhibitor trials that had looked at this, but which also seemed at odds with the observed significant preservation of renal function that seemed substantial enough to produce a clinically meaningful benefit.
Renal effects blunted in HFpEF
The immediate upshot was a letter published by several EMPEROR-Preserved investigators that spelled out this discrepancy and came to the jolting conclusion that “eGFR slope analysis has limitations as a surrogate for predicting the effect of drugs on renal outcomes in patients with heart failure.”
The same authors, along with some additional associates, also published a second letter that noted a further unexpected twist with the renal outcome: “In prior large-scale clinical trials, the effect of SGLT2 inhibitors on heart failure and renal outcomes had consistently tracked together,” they noted, but in this case it didn’t, a discordance they said was “extraordinarily puzzling”.
This led the study’s leaders to reanalyze the renal outcomes using a different definition, one that Milton Packer, MD, who helped design the trial and oversaw several of its analyses, called “a more conventional definition of renal events,” during his presentation of these findings at the congress. The researchers swapped out a 40% drop from baseline eGFR as an event and replaced it with a 50% decline, a change designed to screen out less severe, and often transient, reductions in kidney function that have less lasting impact on health. They also added an additional component to the composite endpoint, renal death. A revised analysis using this new renal composite outcome appeared in the European Journal of Heart Failure letter.
This change cut the total number of renal events tallied in the trial nearly in half, down to 112, and showed a more robust decline in renal events with empagliflozin treatment compared with the initial analysis, although the drop remained nonsignificant. The revised analysis also showed that the overall, nonsignificant 22% relative reduction in renal events in patients on empagliflozin, compared with placebo, dwindled down to completely nonexistent in the tertile of patients with a left ventricular ejection fraction of 60% or greater. In this tertile the hazard ratio actually showed a nonsignificant point estimate of a 24% increased rate of renal events on empagliflozin, with the caveat that this subgroup now included a total of just 40 total events between the two treatment arms. (Each of the two other tertiles also had roughly the same number of total events.)
The biggest effect on renal-event reduction was in the tertile of patients with an ejection fraction of 41%-49%, in which empagliflozin treatment was linked with a significant 59% cut in renal events, compared with placebo. The analysis also showed significant heterogeneity in thus outcome between this subgroup and the other two tertiles that had higher ejection fractions and showed reduced rates of protection by empagliflozin against renal events.
This apparent blunting of a renal effect despite preservation of renal function seemed to mimic the blunting of the primary cardiovascular outcome effect that also appeared in patients with ejection fractions in the 60%-65% range or above.
“If we knew what blunted the effect of empagliflozin on heart failure outcomes at higher ejection fraction levels, we think the same explanation may also apply to the blunting of effect on renal outcomes, but right now we do not know the answer to either question,” Dr. Packer said in an interview. He’s suggested that one possibility is that many of the enrolled patients identified as having HFpEF, but with these high ejection fractions may have not actually had HFpEF, and their signs and symptoms may have instead resulted from atrial fibrillation.
“Many patients with an ejection fraction of 60%-65% and above had atrial fibrillation,” he noted, with a prevalence at enrollment in this subgroup of about 50%. Atrial fibrillation can cause dyspnea, a hallmark symptom leading to diagnosis of heart failure, and it also increases levels of N-terminal of the prohormone brain natriuretic peptide, a metric that served as a gatekeeper for entry into the trial. “Essentially, we are saying that many of the criteria that we specified to ensure that patients had heart failure probably did not work very well in patients with an ejection fraction of 65% or greater,” said Dr. Packer, a cardiologist at Baylor University Medical Center in Dallas. “We need to figure out who these patients are.”
Some experts not involved with the study voiced skepticism that the renal findings reflected a real issue.
“I’m quite optimistic that in the long-term the effect on eGFR will translate into renal protection,” said Rudolf A. de Boer, MD, PhD, a professor of translational cardiology at University Medical Center Groningen (the Netherlands), and designated discussant at the congress for the presentation by Dr. Packer.
John J.V. McMurray, MD, a professor of cardiology and a heart failure specialist at Glasgow University, speculated that the unexpected renal outcomes data may relate to the initial decline in renal function produced by treatment with SGLT2 inhibitors despite their longer-term enhancement of renal protection.
“If you use a treatment that protects the kidneys in the long-term but causes an initial dip in eGFR, more patients receiving that treatment will have an early ‘event,’ ” he noted in an interview. He also cautioned about the dangers of subgroup analyses that dice the study population into small cohorts.
“Trials are powered to look at the effect of treatment in the overall population. Everything else is exploratory, underpowered, and subject to the play of chance,” Dr. McMurray stressed.
Counting additional cardiovascular disease events allows more analyses
A third auxiliary report from the EMPEROR-Preserved investigators performed several prespecified analyses that depended on adding additional cardiovascular disease endpoints to the core tallies of cardiovascular death or HHF – such as emergent, urgent, and outpatient events that reflected worsening heart failure – and also included information on diuretic and vasopressor use because of worsening heart failure. The increased event numbers allowed the researchers to perform 30 additional analyses included in this report, according to the count kept by Dr. Packer who was the lead author.
He highlighted several of the additional results in this paper that documented benefits from empagliflozin treatment, compared with placebo:
- A significant 29% reduction in the need for admission to a cardiac care unit or intensive care unit during an HHF.
- A nonsignificant 33% reduction in the need for intravenous vasopressors or positive inotropic drugs during HHF.
- A significantly increased rate of patients achieving a higher New York Heart Association functional class. For example, after the first year of treatment patients who received empagliflozin had a 37% higher rate of functional class improvement, compared with patients who received placebo.
Dr. McMurray had his own list of key takeaways from this paper, including:
- Among patients who needed hospitalization, “those treated with empagliflozin were less sick than those in the placebo group.”
- In addition to reducing HHF empagliflozin treatment also reduced episodes of outpatient worsening as reflected by their receipt of intensified diuretic treatment, which occurred a significant 27% less often, compared with patients on placebo.
- Treatment with empagliflozin also linked with a significant 39% relative reduction in emergency or urgent-care visits that required intravenous therapy.
Empagliflozin’s performance relative to sacubitril/valsartan
The fourth additional report focused on a post hoc, cross-trial comparison of the results from EMPEROR-Preserved and from another recent trial that, like EMPEROR-Preserved, assessed in patients with HFpEF a drug previously proven to work quite well in patients with HFrEF. The comparator drug was sacubitril/valsartan (Entresto), which underwent testing in patients with HFpEF in the PARAGON-HF trial.
The primary outcome of PARAGON-HF, which randomized 4,822 patients, was reduction in cardiovascular death and in total HHF. This dropped by a relative 13%, compared with placebo, during a median of 35 months, a between-group difference that came close to but did not achieve significance (P = .06). Despite this limitation, the Food and Drug Administration in February 2021 loosened the indication for using sacubitril/valsartan in patients with heart failure and a “below normal” ejection fraction, a category that can include many patients considered to have HFpEF.
Although the researchers who ran this analysis, including Dr. Packer, who was the first author, admitted that “comparison of effect sizes across trials is fraught with difficulties,” they nonetheless concluded from their analysis that “for all outcomes that included HHF the effect size was larger for empagliflozin than for sacubitril/valsartan.”
Dr. McMurray, a lead instigator for PARAGON-HF, said there was little to take away from this analysis.
“The patient populations were different, and sacubitril/valsartan was compared against an active therapy, valsartan,” while in EMPEROR-Preserved empagliflozin compared against placebo. “Most of us believe that sacubitril/valsartan and SGLT2 inhibitors work in different but complementary ways, and their benefits are additive. You would want patients with HFpEF or HFrEF to take both,” he said in an interview.
Dr. Packer agreed with that approach and added that he would probably also prescribe a third agent, spironolactone, to many patients with HFpEF.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and Eli Lilly, which jointly market empagliflozin (Jardiance). PARAGON-HF was sponsored by Novartis, which markets sacubitril/valsartan (Entresto). Dr. Packer has received consulting fees from Boehringer Ingelheim and from numerous other companies. Dr. de Boer has research contracts with Boehringer Ingelheim as well as from Abbott, AstraZeneca, Cardior, Ionis, Novo Nordisk, and Roche, and he has been a consultant to Novartis as well as to Abbott, AstraZeneca, Gayer, and Roche. Dr. McMurray led trials of sacubitril/valsartan sponsored by Novartis, and his institution has received compensation for his participation in studies sponsored by Abbvie, AstraZeneca, Cardurion, DalCor, GlaxoSmithKline, Pfizer, and Theracos.
The featured report from the 6,000-patient EMPEROR-Preserved trial at the virtual annual congress of the European Society of Cardiology drew lots of attention for its headline finding: the first unequivocal demonstration that a medication, empagliflozin, can significantly reduce the rate of cardiovascular death and hospitalization for heart failure in patients with heart failure with preserved ejection fraction (HFpEF, a left ventricular ejection fraction of more than 40%), with the details simultaneously published online.
But at the same time, the EMPEROR-Preserved investigators released four additional reports with a lot more outcome analyses that also deserve some attention.
The puzzling neutral effect on renal events
Perhaps the most surprising and complicated set of findings among the main EMPEROR-Preserved outcomes involved renal outcomes.
The trial’s primary outcome was the combined rate of cardiovascular death or hospitalization for heart failure (HHF), and the results showed that treatment with empagliflozin (Jardiance) for a median of 26 months on top of standard treatment for patients with HFpEF led to a significant 21% relative risk reduction, compared with placebo-treated patients.
The trial had two prespecified secondary outcomes. One was the total number of HHF, which dropped by a significant 27%, compared with placebo. The second was the mean change in slope of estimated glomerular filtration rate (eGFR) on an annualized basis, and the empagliflozin regimen reduced the cumulative annual deficit, compared with placebo by an average of 1.36 mL/min per 1.73 m2, a significant difference.
This preservation of renal function was consistent with results from many prior studies of empagliflozin and all of the other U.S.-approved agents from the sodium-glucose cotransporter 2 inhibitor class. Preservation of renal function and a reduction in renal events has become a hallmark property of all agents in the SGLT2 inhibitor class both in patients with type 2 diabetes, as well as in those without diabetes but with heart failure with reduced ejection fraction (HFrEF) or with chronic kidney disease.
EMPEROR-Preserved threw a wrench into what had been an unbroken history of renal protection by SGLT2 inhibitors. That happened when a prespecified endpoint of the study – a composite renal outcome defined as time to first occurrence of chronic dialysis, renal transplantation, a sustained reduction of at least 40% in eGFR, or a sustained drop in eGFR of more than 10 or 15 mL/min per 1.73 m2 from baseline – yielded an unexpected neutral finding.
For this composite renal outcome, EMPEROR-Preserved showed a nonsignificant 5% reduction, compared with placebo, a result that both differed from what had been seen in essentially all the other SGLT2 inhibitor trials that had looked at this, but which also seemed at odds with the observed significant preservation of renal function that seemed substantial enough to produce a clinically meaningful benefit.
Renal effects blunted in HFpEF
The immediate upshot was a letter published by several EMPEROR-Preserved investigators that spelled out this discrepancy and came to the jolting conclusion that “eGFR slope analysis has limitations as a surrogate for predicting the effect of drugs on renal outcomes in patients with heart failure.”
The same authors, along with some additional associates, also published a second letter that noted a further unexpected twist with the renal outcome: “In prior large-scale clinical trials, the effect of SGLT2 inhibitors on heart failure and renal outcomes had consistently tracked together,” they noted, but in this case it didn’t, a discordance they said was “extraordinarily puzzling”.
This led the study’s leaders to reanalyze the renal outcomes using a different definition, one that Milton Packer, MD, who helped design the trial and oversaw several of its analyses, called “a more conventional definition of renal events,” during his presentation of these findings at the congress. The researchers swapped out a 40% drop from baseline eGFR as an event and replaced it with a 50% decline, a change designed to screen out less severe, and often transient, reductions in kidney function that have less lasting impact on health. They also added an additional component to the composite endpoint, renal death. A revised analysis using this new renal composite outcome appeared in the European Journal of Heart Failure letter.
This change cut the total number of renal events tallied in the trial nearly in half, down to 112, and showed a more robust decline in renal events with empagliflozin treatment compared with the initial analysis, although the drop remained nonsignificant. The revised analysis also showed that the overall, nonsignificant 22% relative reduction in renal events in patients on empagliflozin, compared with placebo, dwindled down to completely nonexistent in the tertile of patients with a left ventricular ejection fraction of 60% or greater. In this tertile the hazard ratio actually showed a nonsignificant point estimate of a 24% increased rate of renal events on empagliflozin, with the caveat that this subgroup now included a total of just 40 total events between the two treatment arms. (Each of the two other tertiles also had roughly the same number of total events.)
The biggest effect on renal-event reduction was in the tertile of patients with an ejection fraction of 41%-49%, in which empagliflozin treatment was linked with a significant 59% cut in renal events, compared with placebo. The analysis also showed significant heterogeneity in thus outcome between this subgroup and the other two tertiles that had higher ejection fractions and showed reduced rates of protection by empagliflozin against renal events.
This apparent blunting of a renal effect despite preservation of renal function seemed to mimic the blunting of the primary cardiovascular outcome effect that also appeared in patients with ejection fractions in the 60%-65% range or above.
“If we knew what blunted the effect of empagliflozin on heart failure outcomes at higher ejection fraction levels, we think the same explanation may also apply to the blunting of effect on renal outcomes, but right now we do not know the answer to either question,” Dr. Packer said in an interview. He’s suggested that one possibility is that many of the enrolled patients identified as having HFpEF, but with these high ejection fractions may have not actually had HFpEF, and their signs and symptoms may have instead resulted from atrial fibrillation.
“Many patients with an ejection fraction of 60%-65% and above had atrial fibrillation,” he noted, with a prevalence at enrollment in this subgroup of about 50%. Atrial fibrillation can cause dyspnea, a hallmark symptom leading to diagnosis of heart failure, and it also increases levels of N-terminal of the prohormone brain natriuretic peptide, a metric that served as a gatekeeper for entry into the trial. “Essentially, we are saying that many of the criteria that we specified to ensure that patients had heart failure probably did not work very well in patients with an ejection fraction of 65% or greater,” said Dr. Packer, a cardiologist at Baylor University Medical Center in Dallas. “We need to figure out who these patients are.”
Some experts not involved with the study voiced skepticism that the renal findings reflected a real issue.
“I’m quite optimistic that in the long-term the effect on eGFR will translate into renal protection,” said Rudolf A. de Boer, MD, PhD, a professor of translational cardiology at University Medical Center Groningen (the Netherlands), and designated discussant at the congress for the presentation by Dr. Packer.
John J.V. McMurray, MD, a professor of cardiology and a heart failure specialist at Glasgow University, speculated that the unexpected renal outcomes data may relate to the initial decline in renal function produced by treatment with SGLT2 inhibitors despite their longer-term enhancement of renal protection.
“If you use a treatment that protects the kidneys in the long-term but causes an initial dip in eGFR, more patients receiving that treatment will have an early ‘event,’ ” he noted in an interview. He also cautioned about the dangers of subgroup analyses that dice the study population into small cohorts.
“Trials are powered to look at the effect of treatment in the overall population. Everything else is exploratory, underpowered, and subject to the play of chance,” Dr. McMurray stressed.
Counting additional cardiovascular disease events allows more analyses
A third auxiliary report from the EMPEROR-Preserved investigators performed several prespecified analyses that depended on adding additional cardiovascular disease endpoints to the core tallies of cardiovascular death or HHF – such as emergent, urgent, and outpatient events that reflected worsening heart failure – and also included information on diuretic and vasopressor use because of worsening heart failure. The increased event numbers allowed the researchers to perform 30 additional analyses included in this report, according to the count kept by Dr. Packer who was the lead author.
He highlighted several of the additional results in this paper that documented benefits from empagliflozin treatment, compared with placebo:
- A significant 29% reduction in the need for admission to a cardiac care unit or intensive care unit during an HHF.
- A nonsignificant 33% reduction in the need for intravenous vasopressors or positive inotropic drugs during HHF.
- A significantly increased rate of patients achieving a higher New York Heart Association functional class. For example, after the first year of treatment patients who received empagliflozin had a 37% higher rate of functional class improvement, compared with patients who received placebo.
Dr. McMurray had his own list of key takeaways from this paper, including:
- Among patients who needed hospitalization, “those treated with empagliflozin were less sick than those in the placebo group.”
- In addition to reducing HHF empagliflozin treatment also reduced episodes of outpatient worsening as reflected by their receipt of intensified diuretic treatment, which occurred a significant 27% less often, compared with patients on placebo.
- Treatment with empagliflozin also linked with a significant 39% relative reduction in emergency or urgent-care visits that required intravenous therapy.
Empagliflozin’s performance relative to sacubitril/valsartan
The fourth additional report focused on a post hoc, cross-trial comparison of the results from EMPEROR-Preserved and from another recent trial that, like EMPEROR-Preserved, assessed in patients with HFpEF a drug previously proven to work quite well in patients with HFrEF. The comparator drug was sacubitril/valsartan (Entresto), which underwent testing in patients with HFpEF in the PARAGON-HF trial.
The primary outcome of PARAGON-HF, which randomized 4,822 patients, was reduction in cardiovascular death and in total HHF. This dropped by a relative 13%, compared with placebo, during a median of 35 months, a between-group difference that came close to but did not achieve significance (P = .06). Despite this limitation, the Food and Drug Administration in February 2021 loosened the indication for using sacubitril/valsartan in patients with heart failure and a “below normal” ejection fraction, a category that can include many patients considered to have HFpEF.
Although the researchers who ran this analysis, including Dr. Packer, who was the first author, admitted that “comparison of effect sizes across trials is fraught with difficulties,” they nonetheless concluded from their analysis that “for all outcomes that included HHF the effect size was larger for empagliflozin than for sacubitril/valsartan.”
Dr. McMurray, a lead instigator for PARAGON-HF, said there was little to take away from this analysis.
“The patient populations were different, and sacubitril/valsartan was compared against an active therapy, valsartan,” while in EMPEROR-Preserved empagliflozin compared against placebo. “Most of us believe that sacubitril/valsartan and SGLT2 inhibitors work in different but complementary ways, and their benefits are additive. You would want patients with HFpEF or HFrEF to take both,” he said in an interview.
Dr. Packer agreed with that approach and added that he would probably also prescribe a third agent, spironolactone, to many patients with HFpEF.
EMPEROR-Preserved was sponsored by Boehringer Ingelheim and Eli Lilly, which jointly market empagliflozin (Jardiance). PARAGON-HF was sponsored by Novartis, which markets sacubitril/valsartan (Entresto). Dr. Packer has received consulting fees from Boehringer Ingelheim and from numerous other companies. Dr. de Boer has research contracts with Boehringer Ingelheim as well as from Abbott, AstraZeneca, Cardior, Ionis, Novo Nordisk, and Roche, and he has been a consultant to Novartis as well as to Abbott, AstraZeneca, Gayer, and Roche. Dr. McMurray led trials of sacubitril/valsartan sponsored by Novartis, and his institution has received compensation for his participation in studies sponsored by Abbvie, AstraZeneca, Cardurion, DalCor, GlaxoSmithKline, Pfizer, and Theracos.
FROM ESC 2021
Early end for trial of experimental oxygenation strategies in ARDS
Background: Both observational studies and clinical trials have found that a liberal oxygenation strategy in multiple inpatient settings may be harmful. Furthermore, a conservative strategy is what has been recommended in guidelines. Conversely, the relevance of this recent concept has been challenged in a large trial of a critically ill population (ICU-ROX).
Study design: Randomized clinical trial, unblinded.
Setting: Thirteen sites in France.
Synopsis: In a multicenter randomized clinical trial, investigators enrolled patients with ARDS to either a liberal oxygenation group (PaO2 target 90-105 mm Hg or SpO2 of 96% or greater) or a conservative oxygenation group (PaO2 target 55-70 mm Hg or SpO2 88%-92%). The trial was planned for inclusion of 850 patients, but the data and safety monitoring board decided to stop the trial after inclusion of 205 patients. Although the primary outcome (28-day all-cause mortality) was not significantly different between groups (34.3% vs 26.5%; absolute difference, 7.8%; 95% confidence interval, –4.8 to 20.6), the direction was signaling possible harm and there were five episodes of mesenteric ischemia in the conservative oxygenation group (none in the liberal oxygenation group).
Bottom line: A conservative oxygenation strategy cannot be currently recommended to patients with ARDS in the ICU. A minimum SpO2 of 90% was suggested in an accompanying editorial.
Editorial commentary: Interestingly, the supplemental results of the article show that prone positioning was used much less frequently in the conservative oxygenation group (34.3 vs 51.0%). If the impressive results of Guerin (2013) would be repeated in this population, this difference could help explain the higher observed mortality in the conservative oxygenation group. It is possible that, by aiming to be less aggressive in improving the PaO2, clinicians inadvertently withheld effective treatments for ARDS. The results of this trial bring up several interesting questions, but provide the bedside clinician with few answers. The complex interplay of treatment factors needs to be dissected in future trials.
Citation: Barrot L et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Eng J Med. 2020;382:999-1008.
Dr. Saraiva is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Both observational studies and clinical trials have found that a liberal oxygenation strategy in multiple inpatient settings may be harmful. Furthermore, a conservative strategy is what has been recommended in guidelines. Conversely, the relevance of this recent concept has been challenged in a large trial of a critically ill population (ICU-ROX).
Study design: Randomized clinical trial, unblinded.
Setting: Thirteen sites in France.
Synopsis: In a multicenter randomized clinical trial, investigators enrolled patients with ARDS to either a liberal oxygenation group (PaO2 target 90-105 mm Hg or SpO2 of 96% or greater) or a conservative oxygenation group (PaO2 target 55-70 mm Hg or SpO2 88%-92%). The trial was planned for inclusion of 850 patients, but the data and safety monitoring board decided to stop the trial after inclusion of 205 patients. Although the primary outcome (28-day all-cause mortality) was not significantly different between groups (34.3% vs 26.5%; absolute difference, 7.8%; 95% confidence interval, –4.8 to 20.6), the direction was signaling possible harm and there were five episodes of mesenteric ischemia in the conservative oxygenation group (none in the liberal oxygenation group).
Bottom line: A conservative oxygenation strategy cannot be currently recommended to patients with ARDS in the ICU. A minimum SpO2 of 90% was suggested in an accompanying editorial.
Editorial commentary: Interestingly, the supplemental results of the article show that prone positioning was used much less frequently in the conservative oxygenation group (34.3 vs 51.0%). If the impressive results of Guerin (2013) would be repeated in this population, this difference could help explain the higher observed mortality in the conservative oxygenation group. It is possible that, by aiming to be less aggressive in improving the PaO2, clinicians inadvertently withheld effective treatments for ARDS. The results of this trial bring up several interesting questions, but provide the bedside clinician with few answers. The complex interplay of treatment factors needs to be dissected in future trials.
Citation: Barrot L et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Eng J Med. 2020;382:999-1008.
Dr. Saraiva is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Both observational studies and clinical trials have found that a liberal oxygenation strategy in multiple inpatient settings may be harmful. Furthermore, a conservative strategy is what has been recommended in guidelines. Conversely, the relevance of this recent concept has been challenged in a large trial of a critically ill population (ICU-ROX).
Study design: Randomized clinical trial, unblinded.
Setting: Thirteen sites in France.
Synopsis: In a multicenter randomized clinical trial, investigators enrolled patients with ARDS to either a liberal oxygenation group (PaO2 target 90-105 mm Hg or SpO2 of 96% or greater) or a conservative oxygenation group (PaO2 target 55-70 mm Hg or SpO2 88%-92%). The trial was planned for inclusion of 850 patients, but the data and safety monitoring board decided to stop the trial after inclusion of 205 patients. Although the primary outcome (28-day all-cause mortality) was not significantly different between groups (34.3% vs 26.5%; absolute difference, 7.8%; 95% confidence interval, –4.8 to 20.6), the direction was signaling possible harm and there were five episodes of mesenteric ischemia in the conservative oxygenation group (none in the liberal oxygenation group).
Bottom line: A conservative oxygenation strategy cannot be currently recommended to patients with ARDS in the ICU. A minimum SpO2 of 90% was suggested in an accompanying editorial.
Editorial commentary: Interestingly, the supplemental results of the article show that prone positioning was used much less frequently in the conservative oxygenation group (34.3 vs 51.0%). If the impressive results of Guerin (2013) would be repeated in this population, this difference could help explain the higher observed mortality in the conservative oxygenation group. It is possible that, by aiming to be less aggressive in improving the PaO2, clinicians inadvertently withheld effective treatments for ARDS. The results of this trial bring up several interesting questions, but provide the bedside clinician with few answers. The complex interplay of treatment factors needs to be dissected in future trials.
Citation: Barrot L et al. Liberal or conservative oxygen therapy for acute respiratory distress syndrome. N Eng J Med. 2020;382:999-1008.
Dr. Saraiva is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
CDC panel unanimously backs Pfizer vax, fortifying FDA approval
An independent expert panel within the Centers for Disease Control and Prevention (CDC) has studied the potential benefits and risks of the Pfizer-BioNTech COVID-19 vaccine and voted unanimously to recommend the shots for all Americans ages 16 and older.
The vaccine was fully approved by the U.S. Food and Drug Administration (FDA) last week.
The inoculation is still available to teens ages 12 to 15 under an emergency use authorization from the FDA.
ACIP now sends its recommendation to the CDC Director Rochelle Walensky, MD, for her sign off.
After reviewing the evidence behind the vaccine, panel member Sarah Long, MD, a professor of pediatrics at Drexel University College of Medicine, Philadelphia, said she couldn’t recall another instance where panelists had so much data on which to base their recommendation.
“This vaccine is worthy of the trust of the American people,” she said.
Doctors across the country use vaccines in line with the recommendations made by the ACIP. Their approval typically means that private and government insurers will cover the cost of the shots. In the case of the COVID-19 vaccines, the government is already picking up the tab.
Few surprises
The panel’s independent review of the vaccine’s effectiveness from nine studies held few surprises.
They found the Pfizer vaccine prevented a COVID infection with symptoms about 90%–92% of the time, at least for the first 4 months after the second shot. Protection against hospitalization and death was even higher.
The vaccine was about 89% effective at preventing a COVID infection without symptoms, according to a pooled estimate of five studies.
The data included in the review was updated only through March 13 of this year, however, and does not reflect the impact of further waning of immunity or the impact of the Delta variant.
In making their recommendation, the panel got an update on the safety of the vaccines, which have now been used in the United States for about 9 months.
The rate of anaphylaxis has settled at around five cases for every million shots given, according to the ACIP’s review of the evidence.
Cases of myocarditis and pericarditis were more common after getting a Pfizer-BioNTech vaccine than would be expected to happen naturally in the general population, but the risk was still very rare, and elevated primarily for men younger than age 30.
Out of 17 million second doses of Pfizer-BioNTech vaccines in the United States, there have been 327 confirmed cases of myocarditis reported to the Vaccine Adverse Event Reporting System in people who are younger than age 30. The average hospital stay for a myocarditis cases is 1 to 2 days.
So far, no one in the United States diagnosed with myocarditis after vaccination has died.
What’s more, the risk of myocarditis after vaccination was dwarfed by the risk of myocarditis after a COVID infection. The risk of myocarditis after a COVID infection was 6 to 34 times higher than the risk after receiving an mRNA vaccine.
About 11% of people who get the vaccine experience a serious reaction to the shot, compared with about 3% in the placebo group. Serious reactions were defined as pain; swelling or redness at the injection site that interferes with activity; needing to visit the hospital or ER for pain; tissue necrosis, or having skin slough off; high fever; vomiting that requires hydration; persistent diarrhea; severe headache; or muscle pain/severe joint pain.
“Safe and effective”
After hearing a presentation on the state of the pandemic in the US, some panel members were struck and shaken that 38% of Americans who are eligible are still not fully vaccinated.
Pablo Sanchez, MD, a pediatrician at Nationwide Children’s Hospital in Columbus, Ohio, said, “We’re doing an abysmal job vaccinating the American people. The message has to go out that the vaccines are safe and effective.”
A version of this story first appeared on Medscape.com.
An independent expert panel within the Centers for Disease Control and Prevention (CDC) has studied the potential benefits and risks of the Pfizer-BioNTech COVID-19 vaccine and voted unanimously to recommend the shots for all Americans ages 16 and older.
The vaccine was fully approved by the U.S. Food and Drug Administration (FDA) last week.
The inoculation is still available to teens ages 12 to 15 under an emergency use authorization from the FDA.
ACIP now sends its recommendation to the CDC Director Rochelle Walensky, MD, for her sign off.
After reviewing the evidence behind the vaccine, panel member Sarah Long, MD, a professor of pediatrics at Drexel University College of Medicine, Philadelphia, said she couldn’t recall another instance where panelists had so much data on which to base their recommendation.
“This vaccine is worthy of the trust of the American people,” she said.
Doctors across the country use vaccines in line with the recommendations made by the ACIP. Their approval typically means that private and government insurers will cover the cost of the shots. In the case of the COVID-19 vaccines, the government is already picking up the tab.
Few surprises
The panel’s independent review of the vaccine’s effectiveness from nine studies held few surprises.
They found the Pfizer vaccine prevented a COVID infection with symptoms about 90%–92% of the time, at least for the first 4 months after the second shot. Protection against hospitalization and death was even higher.
The vaccine was about 89% effective at preventing a COVID infection without symptoms, according to a pooled estimate of five studies.
The data included in the review was updated only through March 13 of this year, however, and does not reflect the impact of further waning of immunity or the impact of the Delta variant.
In making their recommendation, the panel got an update on the safety of the vaccines, which have now been used in the United States for about 9 months.
The rate of anaphylaxis has settled at around five cases for every million shots given, according to the ACIP’s review of the evidence.
Cases of myocarditis and pericarditis were more common after getting a Pfizer-BioNTech vaccine than would be expected to happen naturally in the general population, but the risk was still very rare, and elevated primarily for men younger than age 30.
Out of 17 million second doses of Pfizer-BioNTech vaccines in the United States, there have been 327 confirmed cases of myocarditis reported to the Vaccine Adverse Event Reporting System in people who are younger than age 30. The average hospital stay for a myocarditis cases is 1 to 2 days.
So far, no one in the United States diagnosed with myocarditis after vaccination has died.
What’s more, the risk of myocarditis after vaccination was dwarfed by the risk of myocarditis after a COVID infection. The risk of myocarditis after a COVID infection was 6 to 34 times higher than the risk after receiving an mRNA vaccine.
About 11% of people who get the vaccine experience a serious reaction to the shot, compared with about 3% in the placebo group. Serious reactions were defined as pain; swelling or redness at the injection site that interferes with activity; needing to visit the hospital or ER for pain; tissue necrosis, or having skin slough off; high fever; vomiting that requires hydration; persistent diarrhea; severe headache; or muscle pain/severe joint pain.
“Safe and effective”
After hearing a presentation on the state of the pandemic in the US, some panel members were struck and shaken that 38% of Americans who are eligible are still not fully vaccinated.
Pablo Sanchez, MD, a pediatrician at Nationwide Children’s Hospital in Columbus, Ohio, said, “We’re doing an abysmal job vaccinating the American people. The message has to go out that the vaccines are safe and effective.”
A version of this story first appeared on Medscape.com.
An independent expert panel within the Centers for Disease Control and Prevention (CDC) has studied the potential benefits and risks of the Pfizer-BioNTech COVID-19 vaccine and voted unanimously to recommend the shots for all Americans ages 16 and older.
The vaccine was fully approved by the U.S. Food and Drug Administration (FDA) last week.
The inoculation is still available to teens ages 12 to 15 under an emergency use authorization from the FDA.
ACIP now sends its recommendation to the CDC Director Rochelle Walensky, MD, for her sign off.
After reviewing the evidence behind the vaccine, panel member Sarah Long, MD, a professor of pediatrics at Drexel University College of Medicine, Philadelphia, said she couldn’t recall another instance where panelists had so much data on which to base their recommendation.
“This vaccine is worthy of the trust of the American people,” she said.
Doctors across the country use vaccines in line with the recommendations made by the ACIP. Their approval typically means that private and government insurers will cover the cost of the shots. In the case of the COVID-19 vaccines, the government is already picking up the tab.
Few surprises
The panel’s independent review of the vaccine’s effectiveness from nine studies held few surprises.
They found the Pfizer vaccine prevented a COVID infection with symptoms about 90%–92% of the time, at least for the first 4 months after the second shot. Protection against hospitalization and death was even higher.
The vaccine was about 89% effective at preventing a COVID infection without symptoms, according to a pooled estimate of five studies.
The data included in the review was updated only through March 13 of this year, however, and does not reflect the impact of further waning of immunity or the impact of the Delta variant.
In making their recommendation, the panel got an update on the safety of the vaccines, which have now been used in the United States for about 9 months.
The rate of anaphylaxis has settled at around five cases for every million shots given, according to the ACIP’s review of the evidence.
Cases of myocarditis and pericarditis were more common after getting a Pfizer-BioNTech vaccine than would be expected to happen naturally in the general population, but the risk was still very rare, and elevated primarily for men younger than age 30.
Out of 17 million second doses of Pfizer-BioNTech vaccines in the United States, there have been 327 confirmed cases of myocarditis reported to the Vaccine Adverse Event Reporting System in people who are younger than age 30. The average hospital stay for a myocarditis cases is 1 to 2 days.
So far, no one in the United States diagnosed with myocarditis after vaccination has died.
What’s more, the risk of myocarditis after vaccination was dwarfed by the risk of myocarditis after a COVID infection. The risk of myocarditis after a COVID infection was 6 to 34 times higher than the risk after receiving an mRNA vaccine.
About 11% of people who get the vaccine experience a serious reaction to the shot, compared with about 3% in the placebo group. Serious reactions were defined as pain; swelling or redness at the injection site that interferes with activity; needing to visit the hospital or ER for pain; tissue necrosis, or having skin slough off; high fever; vomiting that requires hydration; persistent diarrhea; severe headache; or muscle pain/severe joint pain.
“Safe and effective”
After hearing a presentation on the state of the pandemic in the US, some panel members were struck and shaken that 38% of Americans who are eligible are still not fully vaccinated.
Pablo Sanchez, MD, a pediatrician at Nationwide Children’s Hospital in Columbus, Ohio, said, “We’re doing an abysmal job vaccinating the American people. The message has to go out that the vaccines are safe and effective.”
A version of this story first appeared on Medscape.com.
Children’s upper airways primed to combat SARS-CoV-2 infection
Epithelial and immune cells of the upper airways of children are preactivated and primed to detect SARS-CoV-2 infection, which may contribute to stronger early immune responses to SARS-CoV-2 infection than adults, new research suggests.
The findings may help to explain why children have a lower risk of developing severe COVID-19 illness or becoming infected with SARS-CoV-2 in the first place, the researchers say.
The study was published online Aug. 18 in Nature Biotechnology.
Primed for action
Children appear to be better able than adults to control SARS-CoV-2 infection, but, until now, the exact molecular mechanisms have been unclear.
A team of investigators from Germany did an in-depth analysis of nasal swab samples obtained from 24 children and 21 adults who tested positive for SARS-CoV-2, as well as a control group of 18 children and 23 adults who tested negative for SARS-CoV-2.
“We wanted to understand why viral defense appears to work so much better in children than in adults,” Irina Lehmann, PhD, head of the molecular epidemiology unit at the Berlin Institute of Health Charité – Universitätsmedizin Berlin, explained in a news release.
Single-cell sequencing showed that children had higher baseline levels of certain RNA-sensing receptors that are relevant to SARS-CoV-2 detection, such as MDA5 and RIG-I, in the epithelial and immune cells of their noses.
This differential expression led to stronger early immune responses to SARS-CoV-2 infection in children than in adults.
Children were also more likely than adults to have distinct immune cell subpopulations, including KLRC1+ cytotoxic T cells, involved in fighting infection, and memory CD8+ T cells, associated with the development of long-lasting immunity.
‘Clear evidence’
The study provides “clear evidence” that upper-airway immune cells of children are “primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults,” the investigators say.
Primed virus sensing and a preactivated innate immune response in children leads to efficient early production of interferons (IFNs) in the infected airways, likely mediating substantial antiviral effects, they note.
Ultimately, this may lead to lower viral replication and faster clearance in children. In fact, several studies have already shown that children eliminate the virus more quickly than adults, consistent with the concept that they shut down viral replication earlier, the study team says.
Weighing in on the findings for this news organization, John Wherry, PhD, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, said this “interesting study highlights potential differences in innate immunity and possibly geographic immunity in the upper respiratory tract in children versus adults.”
“We know there are differences in innate immunity over a lifespan, but exactly how these differences might relate to viral infection remains unclear,” said Dr. Wherry, who was not involved in the study.
“Children, of course, often have more respiratory infections than adults [but] whether this is due to exposure [i.e., daycare, schools, etc.] or susceptibility [lack of accumulated adaptive immunity over a greater number of years of exposure] is unclear,” Dr. Wherry noted.
“These data may help reveal what kinds of innate immune responses in the upper respiratory tract might help restrain SARS-CoV-2 and [perhaps partially] explain why children typically have milder COVID-19 disease,” he added.
The study was supported by the Berlin Institute of Health COVID-19 research program and fightCOVID@DKFZ initiative, European Commission, German Federal Ministry for Education and Research (BMBF), and German Research Foundation. Dr. Lehmann and Dr. Wherry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Epithelial and immune cells of the upper airways of children are preactivated and primed to detect SARS-CoV-2 infection, which may contribute to stronger early immune responses to SARS-CoV-2 infection than adults, new research suggests.
The findings may help to explain why children have a lower risk of developing severe COVID-19 illness or becoming infected with SARS-CoV-2 in the first place, the researchers say.
The study was published online Aug. 18 in Nature Biotechnology.
Primed for action
Children appear to be better able than adults to control SARS-CoV-2 infection, but, until now, the exact molecular mechanisms have been unclear.
A team of investigators from Germany did an in-depth analysis of nasal swab samples obtained from 24 children and 21 adults who tested positive for SARS-CoV-2, as well as a control group of 18 children and 23 adults who tested negative for SARS-CoV-2.
“We wanted to understand why viral defense appears to work so much better in children than in adults,” Irina Lehmann, PhD, head of the molecular epidemiology unit at the Berlin Institute of Health Charité – Universitätsmedizin Berlin, explained in a news release.
Single-cell sequencing showed that children had higher baseline levels of certain RNA-sensing receptors that are relevant to SARS-CoV-2 detection, such as MDA5 and RIG-I, in the epithelial and immune cells of their noses.
This differential expression led to stronger early immune responses to SARS-CoV-2 infection in children than in adults.
Children were also more likely than adults to have distinct immune cell subpopulations, including KLRC1+ cytotoxic T cells, involved in fighting infection, and memory CD8+ T cells, associated with the development of long-lasting immunity.
‘Clear evidence’
The study provides “clear evidence” that upper-airway immune cells of children are “primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults,” the investigators say.
Primed virus sensing and a preactivated innate immune response in children leads to efficient early production of interferons (IFNs) in the infected airways, likely mediating substantial antiviral effects, they note.
Ultimately, this may lead to lower viral replication and faster clearance in children. In fact, several studies have already shown that children eliminate the virus more quickly than adults, consistent with the concept that they shut down viral replication earlier, the study team says.
Weighing in on the findings for this news organization, John Wherry, PhD, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, said this “interesting study highlights potential differences in innate immunity and possibly geographic immunity in the upper respiratory tract in children versus adults.”
“We know there are differences in innate immunity over a lifespan, but exactly how these differences might relate to viral infection remains unclear,” said Dr. Wherry, who was not involved in the study.
“Children, of course, often have more respiratory infections than adults [but] whether this is due to exposure [i.e., daycare, schools, etc.] or susceptibility [lack of accumulated adaptive immunity over a greater number of years of exposure] is unclear,” Dr. Wherry noted.
“These data may help reveal what kinds of innate immune responses in the upper respiratory tract might help restrain SARS-CoV-2 and [perhaps partially] explain why children typically have milder COVID-19 disease,” he added.
The study was supported by the Berlin Institute of Health COVID-19 research program and fightCOVID@DKFZ initiative, European Commission, German Federal Ministry for Education and Research (BMBF), and German Research Foundation. Dr. Lehmann and Dr. Wherry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Epithelial and immune cells of the upper airways of children are preactivated and primed to detect SARS-CoV-2 infection, which may contribute to stronger early immune responses to SARS-CoV-2 infection than adults, new research suggests.
The findings may help to explain why children have a lower risk of developing severe COVID-19 illness or becoming infected with SARS-CoV-2 in the first place, the researchers say.
The study was published online Aug. 18 in Nature Biotechnology.
Primed for action
Children appear to be better able than adults to control SARS-CoV-2 infection, but, until now, the exact molecular mechanisms have been unclear.
A team of investigators from Germany did an in-depth analysis of nasal swab samples obtained from 24 children and 21 adults who tested positive for SARS-CoV-2, as well as a control group of 18 children and 23 adults who tested negative for SARS-CoV-2.
“We wanted to understand why viral defense appears to work so much better in children than in adults,” Irina Lehmann, PhD, head of the molecular epidemiology unit at the Berlin Institute of Health Charité – Universitätsmedizin Berlin, explained in a news release.
Single-cell sequencing showed that children had higher baseline levels of certain RNA-sensing receptors that are relevant to SARS-CoV-2 detection, such as MDA5 and RIG-I, in the epithelial and immune cells of their noses.
This differential expression led to stronger early immune responses to SARS-CoV-2 infection in children than in adults.
Children were also more likely than adults to have distinct immune cell subpopulations, including KLRC1+ cytotoxic T cells, involved in fighting infection, and memory CD8+ T cells, associated with the development of long-lasting immunity.
‘Clear evidence’
The study provides “clear evidence” that upper-airway immune cells of children are “primed for virus sensing, resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults,” the investigators say.
Primed virus sensing and a preactivated innate immune response in children leads to efficient early production of interferons (IFNs) in the infected airways, likely mediating substantial antiviral effects, they note.
Ultimately, this may lead to lower viral replication and faster clearance in children. In fact, several studies have already shown that children eliminate the virus more quickly than adults, consistent with the concept that they shut down viral replication earlier, the study team says.
Weighing in on the findings for this news organization, John Wherry, PhD, director of the Institute for Immunology at the University of Pennsylvania, Philadelphia, said this “interesting study highlights potential differences in innate immunity and possibly geographic immunity in the upper respiratory tract in children versus adults.”
“We know there are differences in innate immunity over a lifespan, but exactly how these differences might relate to viral infection remains unclear,” said Dr. Wherry, who was not involved in the study.
“Children, of course, often have more respiratory infections than adults [but] whether this is due to exposure [i.e., daycare, schools, etc.] or susceptibility [lack of accumulated adaptive immunity over a greater number of years of exposure] is unclear,” Dr. Wherry noted.
“These data may help reveal what kinds of innate immune responses in the upper respiratory tract might help restrain SARS-CoV-2 and [perhaps partially] explain why children typically have milder COVID-19 disease,” he added.
The study was supported by the Berlin Institute of Health COVID-19 research program and fightCOVID@DKFZ initiative, European Commission, German Federal Ministry for Education and Research (BMBF), and German Research Foundation. Dr. Lehmann and Dr. Wherry have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
ICU infections and all-cause hospital mortality rate
Background: Many articles have been published on sepsis and mortality in ICUs, but there are not many analyzing outcomes in patients with infections, nor types of infections. More information on the infection rate, types of infection, and possible impact on mortality should heighten awareness of infection effects, as well as guide resource allocation and help direct policy development for diagnosis and treatment.
Study design: 24-hour point-prevalence study with longitudinal follow-up.
Setting: ICUs in 1,150 centers in 88 countries.
Synopsis: The study included 15,202 patients who were aged 18 or older (mean, 61.6) within a 24-hour time period on Sept. 13, 2017, who were admitted to the ICU in participating centers and had documented, confirmed, or suspected infection. The investigators looked at prevalence of infection and antibiotic exposure on the study day and the main outcome measure was all cause in-hospital mortality, which was compiled 60 days later. The prevalence of suspected or proven infection in ICUs was 54% (8,135) and that of ICU-acquired infection was 22%. Of confirmed or suspected infection, 65% (5,259) had at least one positive microbiology culture. Of those cultures, 67% were gram-negative and 37% gram-positive bacteria, and 16% were fungal. 70% of ICU patients received at least one antibiotic. The in-hospital mortality rate with proven or suspected infection was 30% (2,404 of 7,936). Multilevel analysis disclosed two independent risk factors for mortality, which were ICU-acquired infections and antibiotic-resistant organisms, specifically, vancomycin-resistant Enterococcus, Klebsiella resistant to beta-lactam antibiotics, and carbapenem-resistant Acinetobacter.
Despite limitations related to being an observational study, 24-hour point evaluation, a centrally controlled database, and different geographic locations, this study elucidated the world-wide prevalence of ICU infection and high hospital-in mortality in those patients.
Bottom line: There is a high prevalence of infection in ICUs: 43%-60% depending on location. This is associated with 30% in-hospital mortality.
Citation: Vincent J-L et al. Prevalance and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020 Mar 24;323(15):1478-87.
Dr. Rogozinska is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Many articles have been published on sepsis and mortality in ICUs, but there are not many analyzing outcomes in patients with infections, nor types of infections. More information on the infection rate, types of infection, and possible impact on mortality should heighten awareness of infection effects, as well as guide resource allocation and help direct policy development for diagnosis and treatment.
Study design: 24-hour point-prevalence study with longitudinal follow-up.
Setting: ICUs in 1,150 centers in 88 countries.
Synopsis: The study included 15,202 patients who were aged 18 or older (mean, 61.6) within a 24-hour time period on Sept. 13, 2017, who were admitted to the ICU in participating centers and had documented, confirmed, or suspected infection. The investigators looked at prevalence of infection and antibiotic exposure on the study day and the main outcome measure was all cause in-hospital mortality, which was compiled 60 days later. The prevalence of suspected or proven infection in ICUs was 54% (8,135) and that of ICU-acquired infection was 22%. Of confirmed or suspected infection, 65% (5,259) had at least one positive microbiology culture. Of those cultures, 67% were gram-negative and 37% gram-positive bacteria, and 16% were fungal. 70% of ICU patients received at least one antibiotic. The in-hospital mortality rate with proven or suspected infection was 30% (2,404 of 7,936). Multilevel analysis disclosed two independent risk factors for mortality, which were ICU-acquired infections and antibiotic-resistant organisms, specifically, vancomycin-resistant Enterococcus, Klebsiella resistant to beta-lactam antibiotics, and carbapenem-resistant Acinetobacter.
Despite limitations related to being an observational study, 24-hour point evaluation, a centrally controlled database, and different geographic locations, this study elucidated the world-wide prevalence of ICU infection and high hospital-in mortality in those patients.
Bottom line: There is a high prevalence of infection in ICUs: 43%-60% depending on location. This is associated with 30% in-hospital mortality.
Citation: Vincent J-L et al. Prevalance and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020 Mar 24;323(15):1478-87.
Dr. Rogozinska is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Many articles have been published on sepsis and mortality in ICUs, but there are not many analyzing outcomes in patients with infections, nor types of infections. More information on the infection rate, types of infection, and possible impact on mortality should heighten awareness of infection effects, as well as guide resource allocation and help direct policy development for diagnosis and treatment.
Study design: 24-hour point-prevalence study with longitudinal follow-up.
Setting: ICUs in 1,150 centers in 88 countries.
Synopsis: The study included 15,202 patients who were aged 18 or older (mean, 61.6) within a 24-hour time period on Sept. 13, 2017, who were admitted to the ICU in participating centers and had documented, confirmed, or suspected infection. The investigators looked at prevalence of infection and antibiotic exposure on the study day and the main outcome measure was all cause in-hospital mortality, which was compiled 60 days later. The prevalence of suspected or proven infection in ICUs was 54% (8,135) and that of ICU-acquired infection was 22%. Of confirmed or suspected infection, 65% (5,259) had at least one positive microbiology culture. Of those cultures, 67% were gram-negative and 37% gram-positive bacteria, and 16% were fungal. 70% of ICU patients received at least one antibiotic. The in-hospital mortality rate with proven or suspected infection was 30% (2,404 of 7,936). Multilevel analysis disclosed two independent risk factors for mortality, which were ICU-acquired infections and antibiotic-resistant organisms, specifically, vancomycin-resistant Enterococcus, Klebsiella resistant to beta-lactam antibiotics, and carbapenem-resistant Acinetobacter.
Despite limitations related to being an observational study, 24-hour point evaluation, a centrally controlled database, and different geographic locations, this study elucidated the world-wide prevalence of ICU infection and high hospital-in mortality in those patients.
Bottom line: There is a high prevalence of infection in ICUs: 43%-60% depending on location. This is associated with 30% in-hospital mortality.
Citation: Vincent J-L et al. Prevalance and outcomes of infection among patients in intensive care units in 2017. JAMA. 2020 Mar 24;323(15):1478-87.
Dr. Rogozinska is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Dapagliflozin in HFrEF may cut arrhythmias, sudden death: DAPA-HF
Dapagliflozin might reduce the risk for ventricular arrhythmias and sudden death in patients with heart failure and reduced ejection fraction (HFrEF), a post hoc analysis of the DAPA-HF trial suggests.
The addition of dapagliflozin to standard therapy reduced the relative risk for the primary composite endpoint of any serious ventricular arrhythmia, resuscitated cardiac arrest, or sudden death by 21%, compared with placebo (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99). The absolute risk reduction was 1.5% (5.9% vs. 7.4%).
The benefit was consistent in a competing-risks analysis that included all-cause mortality (HR, 0.80; P = .043) and across the individual components of the composite outcome, James Curtain, MD, Cardiovascular Research Centre of Glasgow, said at the annual congress of the European Society of Cardiology.
As previously reported from the main trial, treatment with the sodium-glucose cotransporter 2 (SGLT2) inhibitor cut the primary endpoint of cardiovascular death or worsening heart failure by 26% among 4,744 patients with HFrEF and in New York Heart Association functional class 2-4.
Cochair of the late-breaking science session, Lars Lund, MD, Karolinska Institute, Stockholm, pointed out that dapagliflozin reduced sudden cardiac deaths and related events to an extent similar to that observed for cardiovascular deaths, total mortality, and the main trial’s primary endpoint.
“So does that mean it has any particular effect on arrhythmic events or does it mean, such as a beta-blocker, for example, [it] reduces calcium transience and improves handling of calcium, or does it have an effect simply by improving heart failure?” he asked.
Dr. Curtain replied they are still trying to understand the effects of this new class of drug but that studies have shown dapagliflozin and other SGLT2 inhibitors have favorable effects on adverse cardiac remodeling, which contributes to sudden death and ventricular arrhythmia. They’ve also been shown to reduce cardiac chamber size, left ventricular hypertrophy, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels over time, consistent with a reduction in myocardial wall stress. “So it could indeed be one of several mechanisms by which they may exert a beneficial cardiac effect.”
Speaking with this news organization, Dr. Curtain pointed out that the Kaplan-Meier curves for the composite outcome began to separate early on, but that the clearest separation was after 9 months, suggestive of a positive action on adverse cardiac remodeling over time.
“This would improve the patients’ heart failure situation, but also thick ventricles are a key risk factor for the occurrence of sudden death and ventricular arrhythmias,” he said. “The effects on adverse cardiac remodeling, given its plausibility in terms of our Kaplan-Meier curves, are one [mechanism] that I’d look to in the first instance, but I’m sure there are more than one actions at play.”
According to the new analysis, the primary outcome occurred in 315 (6.6%) patients; there were 203 adjudicated sudden deaths (64%), 104 investigator-reported ventricular arrhythmias (33%), and 8 resuscitated cardiac arrests (3%). Independent predictors of the primary outcome were higher NT-proBNP levels (odds ratio, 1.54), previous ventricular arrhythmia (OR, 1.93), previous myocardial infarction (OR, 1.42), male sex (OR, 1.53), and higher body mass index (OR, 1.03).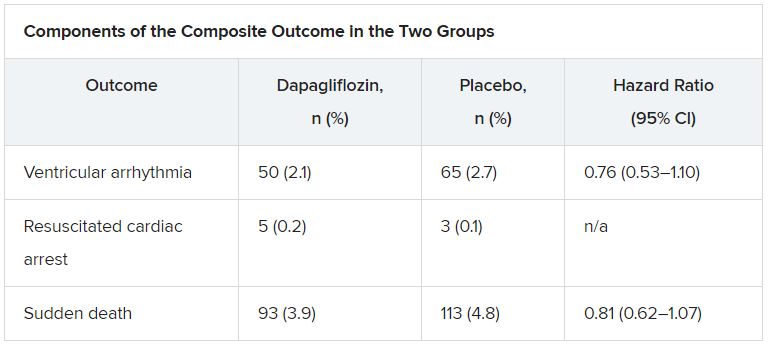
The effect of dapagliflozin on the primary outcome was consistent in several sensitivity analyses and “generally consistent” across key subgroups, Dr. Curtain said.
During a discussion of the results, session cochair Mitja Lainscak, MD, General Hospital Murska Sobota, Slovenia, called out two exceptions. “With regard to patients with implanted ICDs, the effect was neutral, and in the patients without diabetes, the benefit was less than in diabetic patients. Any explanations for that?”
Dr. Curtain responded that “it’s important to note that in the subgroup analyses the point estimates were all on the side favoring dapagliflozin and the interaction test was not significant in that subgroup. The numbers of patients who were in the defibrillator group were modest, and there was a relatively smaller number of events, so it may be harder to show benefit in that group.”
In the dapagliflozin and placebo groups, the event rates per 100 person-years were 3.9 and 5.8, respectively, in patients with diabetes, and 4.1 and 4.7, respectively, in those without diabetes (P for interaction = .273).
Event rates per 100 person-years were 5.8 and 5.9, respectively, in patients with a defibrillator at baseline, and 3.5 and 4.9, respectively, in those without a defibrillator (P for interaction = .174).
Asked to comment on the study, which was simultaneously published in the European Heart Journal, Milton Packer, MD, Baylor University Medical Center, Dallas, said he had “very little confidence” in the findings.
“This was entirely post hoc and the investigators combined events – with markedly different levels of clinical importance – in order to achieve a P value less than 0.05,” he told this news organization. “If one takes asymptomatic ventricular arrhythmias out of the analysis, the effect is no longer statistically significant. Furthermore, half of sudden deaths in patients with heart failure are not related to a ventricular arrhythmia.”
The authors note in their report that the analysis was not prespecified and the findings should be regarded as “hypothesis generating and require confirmation,” but also point out that a recent meta-analysis showed that SGLT2 inhibitor use was associated with a lower risk for ventricular tachycardia. Other limitations to the post hoc analysis are that adverse-event reporting likely underestimated the true prevalence of ventricular arrhythmias, and that these events were not adjudicated.
DAPA-HF was funded by AstraZeneca. Dr. Curtain reports no relevant financial relationships. Disclosures for the coauthors are listed in the paper.
A version of this article first appeared on Medscape.com.
Dapagliflozin might reduce the risk for ventricular arrhythmias and sudden death in patients with heart failure and reduced ejection fraction (HFrEF), a post hoc analysis of the DAPA-HF trial suggests.
The addition of dapagliflozin to standard therapy reduced the relative risk for the primary composite endpoint of any serious ventricular arrhythmia, resuscitated cardiac arrest, or sudden death by 21%, compared with placebo (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99). The absolute risk reduction was 1.5% (5.9% vs. 7.4%).
The benefit was consistent in a competing-risks analysis that included all-cause mortality (HR, 0.80; P = .043) and across the individual components of the composite outcome, James Curtain, MD, Cardiovascular Research Centre of Glasgow, said at the annual congress of the European Society of Cardiology.
As previously reported from the main trial, treatment with the sodium-glucose cotransporter 2 (SGLT2) inhibitor cut the primary endpoint of cardiovascular death or worsening heart failure by 26% among 4,744 patients with HFrEF and in New York Heart Association functional class 2-4.
Cochair of the late-breaking science session, Lars Lund, MD, Karolinska Institute, Stockholm, pointed out that dapagliflozin reduced sudden cardiac deaths and related events to an extent similar to that observed for cardiovascular deaths, total mortality, and the main trial’s primary endpoint.
“So does that mean it has any particular effect on arrhythmic events or does it mean, such as a beta-blocker, for example, [it] reduces calcium transience and improves handling of calcium, or does it have an effect simply by improving heart failure?” he asked.
Dr. Curtain replied they are still trying to understand the effects of this new class of drug but that studies have shown dapagliflozin and other SGLT2 inhibitors have favorable effects on adverse cardiac remodeling, which contributes to sudden death and ventricular arrhythmia. They’ve also been shown to reduce cardiac chamber size, left ventricular hypertrophy, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels over time, consistent with a reduction in myocardial wall stress. “So it could indeed be one of several mechanisms by which they may exert a beneficial cardiac effect.”
Speaking with this news organization, Dr. Curtain pointed out that the Kaplan-Meier curves for the composite outcome began to separate early on, but that the clearest separation was after 9 months, suggestive of a positive action on adverse cardiac remodeling over time.
“This would improve the patients’ heart failure situation, but also thick ventricles are a key risk factor for the occurrence of sudden death and ventricular arrhythmias,” he said. “The effects on adverse cardiac remodeling, given its plausibility in terms of our Kaplan-Meier curves, are one [mechanism] that I’d look to in the first instance, but I’m sure there are more than one actions at play.”
According to the new analysis, the primary outcome occurred in 315 (6.6%) patients; there were 203 adjudicated sudden deaths (64%), 104 investigator-reported ventricular arrhythmias (33%), and 8 resuscitated cardiac arrests (3%). Independent predictors of the primary outcome were higher NT-proBNP levels (odds ratio, 1.54), previous ventricular arrhythmia (OR, 1.93), previous myocardial infarction (OR, 1.42), male sex (OR, 1.53), and higher body mass index (OR, 1.03).
The effect of dapagliflozin on the primary outcome was consistent in several sensitivity analyses and “generally consistent” across key subgroups, Dr. Curtain said.
During a discussion of the results, session cochair Mitja Lainscak, MD, General Hospital Murska Sobota, Slovenia, called out two exceptions. “With regard to patients with implanted ICDs, the effect was neutral, and in the patients without diabetes, the benefit was less than in diabetic patients. Any explanations for that?”
Dr. Curtain responded that “it’s important to note that in the subgroup analyses the point estimates were all on the side favoring dapagliflozin and the interaction test was not significant in that subgroup. The numbers of patients who were in the defibrillator group were modest, and there was a relatively smaller number of events, so it may be harder to show benefit in that group.”
In the dapagliflozin and placebo groups, the event rates per 100 person-years were 3.9 and 5.8, respectively, in patients with diabetes, and 4.1 and 4.7, respectively, in those without diabetes (P for interaction = .273).
Event rates per 100 person-years were 5.8 and 5.9, respectively, in patients with a defibrillator at baseline, and 3.5 and 4.9, respectively, in those without a defibrillator (P for interaction = .174).
Asked to comment on the study, which was simultaneously published in the European Heart Journal, Milton Packer, MD, Baylor University Medical Center, Dallas, said he had “very little confidence” in the findings.
“This was entirely post hoc and the investigators combined events – with markedly different levels of clinical importance – in order to achieve a P value less than 0.05,” he told this news organization. “If one takes asymptomatic ventricular arrhythmias out of the analysis, the effect is no longer statistically significant. Furthermore, half of sudden deaths in patients with heart failure are not related to a ventricular arrhythmia.”
The authors note in their report that the analysis was not prespecified and the findings should be regarded as “hypothesis generating and require confirmation,” but also point out that a recent meta-analysis showed that SGLT2 inhibitor use was associated with a lower risk for ventricular tachycardia. Other limitations to the post hoc analysis are that adverse-event reporting likely underestimated the true prevalence of ventricular arrhythmias, and that these events were not adjudicated.
DAPA-HF was funded by AstraZeneca. Dr. Curtain reports no relevant financial relationships. Disclosures for the coauthors are listed in the paper.
A version of this article first appeared on Medscape.com.
Dapagliflozin might reduce the risk for ventricular arrhythmias and sudden death in patients with heart failure and reduced ejection fraction (HFrEF), a post hoc analysis of the DAPA-HF trial suggests.
The addition of dapagliflozin to standard therapy reduced the relative risk for the primary composite endpoint of any serious ventricular arrhythmia, resuscitated cardiac arrest, or sudden death by 21%, compared with placebo (hazard ratio, 0.79; 95% confidence interval, 0.63-0.99). The absolute risk reduction was 1.5% (5.9% vs. 7.4%).
The benefit was consistent in a competing-risks analysis that included all-cause mortality (HR, 0.80; P = .043) and across the individual components of the composite outcome, James Curtain, MD, Cardiovascular Research Centre of Glasgow, said at the annual congress of the European Society of Cardiology.
As previously reported from the main trial, treatment with the sodium-glucose cotransporter 2 (SGLT2) inhibitor cut the primary endpoint of cardiovascular death or worsening heart failure by 26% among 4,744 patients with HFrEF and in New York Heart Association functional class 2-4.
Cochair of the late-breaking science session, Lars Lund, MD, Karolinska Institute, Stockholm, pointed out that dapagliflozin reduced sudden cardiac deaths and related events to an extent similar to that observed for cardiovascular deaths, total mortality, and the main trial’s primary endpoint.
“So does that mean it has any particular effect on arrhythmic events or does it mean, such as a beta-blocker, for example, [it] reduces calcium transience and improves handling of calcium, or does it have an effect simply by improving heart failure?” he asked.
Dr. Curtain replied they are still trying to understand the effects of this new class of drug but that studies have shown dapagliflozin and other SGLT2 inhibitors have favorable effects on adverse cardiac remodeling, which contributes to sudden death and ventricular arrhythmia. They’ve also been shown to reduce cardiac chamber size, left ventricular hypertrophy, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels over time, consistent with a reduction in myocardial wall stress. “So it could indeed be one of several mechanisms by which they may exert a beneficial cardiac effect.”
Speaking with this news organization, Dr. Curtain pointed out that the Kaplan-Meier curves for the composite outcome began to separate early on, but that the clearest separation was after 9 months, suggestive of a positive action on adverse cardiac remodeling over time.
“This would improve the patients’ heart failure situation, but also thick ventricles are a key risk factor for the occurrence of sudden death and ventricular arrhythmias,” he said. “The effects on adverse cardiac remodeling, given its plausibility in terms of our Kaplan-Meier curves, are one [mechanism] that I’d look to in the first instance, but I’m sure there are more than one actions at play.”
According to the new analysis, the primary outcome occurred in 315 (6.6%) patients; there were 203 adjudicated sudden deaths (64%), 104 investigator-reported ventricular arrhythmias (33%), and 8 resuscitated cardiac arrests (3%). Independent predictors of the primary outcome were higher NT-proBNP levels (odds ratio, 1.54), previous ventricular arrhythmia (OR, 1.93), previous myocardial infarction (OR, 1.42), male sex (OR, 1.53), and higher body mass index (OR, 1.03).
The effect of dapagliflozin on the primary outcome was consistent in several sensitivity analyses and “generally consistent” across key subgroups, Dr. Curtain said.
During a discussion of the results, session cochair Mitja Lainscak, MD, General Hospital Murska Sobota, Slovenia, called out two exceptions. “With regard to patients with implanted ICDs, the effect was neutral, and in the patients without diabetes, the benefit was less than in diabetic patients. Any explanations for that?”
Dr. Curtain responded that “it’s important to note that in the subgroup analyses the point estimates were all on the side favoring dapagliflozin and the interaction test was not significant in that subgroup. The numbers of patients who were in the defibrillator group were modest, and there was a relatively smaller number of events, so it may be harder to show benefit in that group.”
In the dapagliflozin and placebo groups, the event rates per 100 person-years were 3.9 and 5.8, respectively, in patients with diabetes, and 4.1 and 4.7, respectively, in those without diabetes (P for interaction = .273).
Event rates per 100 person-years were 5.8 and 5.9, respectively, in patients with a defibrillator at baseline, and 3.5 and 4.9, respectively, in those without a defibrillator (P for interaction = .174).
Asked to comment on the study, which was simultaneously published in the European Heart Journal, Milton Packer, MD, Baylor University Medical Center, Dallas, said he had “very little confidence” in the findings.
“This was entirely post hoc and the investigators combined events – with markedly different levels of clinical importance – in order to achieve a P value less than 0.05,” he told this news organization. “If one takes asymptomatic ventricular arrhythmias out of the analysis, the effect is no longer statistically significant. Furthermore, half of sudden deaths in patients with heart failure are not related to a ventricular arrhythmia.”
The authors note in their report that the analysis was not prespecified and the findings should be regarded as “hypothesis generating and require confirmation,” but also point out that a recent meta-analysis showed that SGLT2 inhibitor use was associated with a lower risk for ventricular tachycardia. Other limitations to the post hoc analysis are that adverse-event reporting likely underestimated the true prevalence of ventricular arrhythmias, and that these events were not adjudicated.
DAPA-HF was funded by AstraZeneca. Dr. Curtain reports no relevant financial relationships. Disclosures for the coauthors are listed in the paper.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2021
ICMs detect serious arrhythmias in high-risk post-MI patients: SMART-MI
Prevention strategies may be next
After a myocardial infarction, implantable cardiac monitors (ICMs) are sensitive for detecting serious arrhythmias in patients with cardiac autonomic dysfunction but only moderately reduced left ventricular ejection fraction (LVEF), according to results of the randomized SMART-MI trial.
When remote monitoring with the ICM was compared with conventional follow-up in this group of patients, serious arrhythmic events were detected at a nearly sixfold greater rate, reported Axel Bauer, MD, at the annual congress of the European Society of Cardiology.
The study further showed that these events were closely associated with subsequent major adverse cardiac and cerebrovascular events (MACCE).
“SMART-MI is the first study to test an implantable device in high-risk MI patients with a LVEF greater than 35%,” reported Dr. Bauer, a cardiologist and director of the internal medicine clinic, University of Innsbruck (Austria). It showed that the types and frequency of arrhythmias were “comparable to those of post-MI patients with reduced LVEF.”
The ability to assess risk is potentially significant because “the majority of cardiovascular complications, including sudden death, occur in patients with only moderately reduced LVEF,” explained Dr. Bauer.
Despite the greater risk, “there are no preventive strategies so far” currently available for this group, he said.
The SMART-MI study confirms the need for treatments, confirms a method for monitoring risk, and might provide the basis for trials designed to test treatments to modify this risk, he added.
ECG used to define autonomic dysfunction
In the SMART MI protocol, 1,305 survivors of MI with LVEF of 36%-50% at 33 participating centers in Austria and Germany were evaluated with a 20-minute high resolution electrocardiogram. They were enrolled and randomized if they demonstrated cardiac autonomic dysfunction on at least two validated ECG biomarkers.
The 400 participants were randomized to implantation of a ICM, which transmitted daily reports to a ICM core laboratory, or to conventional follow-up.
After a median follow-up of 21 months, serious events were detected in 60 of the 201 patients in the ICM group and 12 of the 199 patients in the control group (29% vs. 6%). Serious adverse events were defined as those that would typically warrant therapy, such as prolonged atrial fibrillation (at least 6 minutes) high-degree atrioventricular block, and sustained ventricular tachycardia.
The difference in the detection rate, which was the primary endpoint, was highly significant (P < .0001), but the study was also able to confirm that these events predicted MACCE, a secondary study endpoint. In those with a serious arrhythmia, the hazard ratio for subsequent MACCE was approximately sevenfold greater relative to those without a serious arrhythmia. This was true of those in the ICM group (HR, 6.8; P < .001) and controls (HR 7.3; P < .001).
Arrhythmias warn of impending complications
“The data show that the prognostic impact of detecting a serious arrhythmia does not depend on the mode of detection,” Dr. Bauer reported. The data also confirm that “subclinical serious arrhythmia events are a warning signal for an impending complication.”
Although more interventions – including pacemakers, catheter ablations, and oral anticoagulants – were offered to patients in the experimental arm, “the study was not powered to show differences in outcomes,” and, in fact, no significant differences were observed, according to Dr. Bauer. However, the evidence that ICM is effective for detecting arrhythmias does provide a structure on which to build clinical trials.
“We now need the trials to see if ICM can change practice and improve outcomes,” said Carlos Aguiar, MD, a staff cardiologist at the Hospital Santa Cruz, Lisbon. He acknowledged that this study proves that ICM can detect serious arrhythmias in patients with moderate left ventricular dysfunction, but “we need to develop and test treatment paths.”
Dr. Aguiar considers SMART-MI an important study that “goes to the heart” of a common clinical dilemma.
“In clinical practice, we see patients with LVEF that is not that suppressed and so do not have a class I indication for ICM, but there are often features that might have you concerned and make you think it would be great if the LVEF was 35% or lower [to justify intervention],” Dr. Aguiar said.
Data provide insight on unaddressed risk group
SMART-MI confirms earlier evidence that post-MI patients with cardiac autonomic dysfunction are at high risk. Currently, this relative increase in risk goes “unaddressed,” according to Dr. Bauer. Although he contended that the risk itself “could be an indication for ICM in a high-risk patient group without classically defined left ventricular dysfunction,” he agreed that the ultimate value of this trial might be that it “opens a window” for a rationale to test preventive strategies.
An invited ESC discussant, Gerhard Hindricks, MD, PhD, praised the study for drawing attention to the risk of events in a subset of post-MI patients with LVEF of 35% or greater. However, he suggested that criteria other than those based on ECG might be more sensitive for selecting patients who might benefit from intervention.
“We do not know whether additional methods of establishing risk, such as imaging, might be valuable,” said Dr. Hindricks, chief of the department of arrhythmology in the Heart Institute of the University of Leipzig (Germany). He believes work in this area is needed to ensure appropriate entry criteria for interventional trials designed to modify risk in post-MI patients who do not meet the traditional definition of reduced ejection fraction.
Dr. Bauer reports financial relationships with Medtronic, which sponsored this study, as well as Bayer, Boehringer Ingelheim, Edwards, and Novartis. Dr. Aguiar reports no relevant financial conflicts.
Prevention strategies may be next
Prevention strategies may be next
After a myocardial infarction, implantable cardiac monitors (ICMs) are sensitive for detecting serious arrhythmias in patients with cardiac autonomic dysfunction but only moderately reduced left ventricular ejection fraction (LVEF), according to results of the randomized SMART-MI trial.
When remote monitoring with the ICM was compared with conventional follow-up in this group of patients, serious arrhythmic events were detected at a nearly sixfold greater rate, reported Axel Bauer, MD, at the annual congress of the European Society of Cardiology.
The study further showed that these events were closely associated with subsequent major adverse cardiac and cerebrovascular events (MACCE).
“SMART-MI is the first study to test an implantable device in high-risk MI patients with a LVEF greater than 35%,” reported Dr. Bauer, a cardiologist and director of the internal medicine clinic, University of Innsbruck (Austria). It showed that the types and frequency of arrhythmias were “comparable to those of post-MI patients with reduced LVEF.”
The ability to assess risk is potentially significant because “the majority of cardiovascular complications, including sudden death, occur in patients with only moderately reduced LVEF,” explained Dr. Bauer.
Despite the greater risk, “there are no preventive strategies so far” currently available for this group, he said.
The SMART-MI study confirms the need for treatments, confirms a method for monitoring risk, and might provide the basis for trials designed to test treatments to modify this risk, he added.
ECG used to define autonomic dysfunction
In the SMART MI protocol, 1,305 survivors of MI with LVEF of 36%-50% at 33 participating centers in Austria and Germany were evaluated with a 20-minute high resolution electrocardiogram. They were enrolled and randomized if they demonstrated cardiac autonomic dysfunction on at least two validated ECG biomarkers.
The 400 participants were randomized to implantation of a ICM, which transmitted daily reports to a ICM core laboratory, or to conventional follow-up.
After a median follow-up of 21 months, serious events were detected in 60 of the 201 patients in the ICM group and 12 of the 199 patients in the control group (29% vs. 6%). Serious adverse events were defined as those that would typically warrant therapy, such as prolonged atrial fibrillation (at least 6 minutes) high-degree atrioventricular block, and sustained ventricular tachycardia.
The difference in the detection rate, which was the primary endpoint, was highly significant (P < .0001), but the study was also able to confirm that these events predicted MACCE, a secondary study endpoint. In those with a serious arrhythmia, the hazard ratio for subsequent MACCE was approximately sevenfold greater relative to those without a serious arrhythmia. This was true of those in the ICM group (HR, 6.8; P < .001) and controls (HR 7.3; P < .001).
Arrhythmias warn of impending complications
“The data show that the prognostic impact of detecting a serious arrhythmia does not depend on the mode of detection,” Dr. Bauer reported. The data also confirm that “subclinical serious arrhythmia events are a warning signal for an impending complication.”
Although more interventions – including pacemakers, catheter ablations, and oral anticoagulants – were offered to patients in the experimental arm, “the study was not powered to show differences in outcomes,” and, in fact, no significant differences were observed, according to Dr. Bauer. However, the evidence that ICM is effective for detecting arrhythmias does provide a structure on which to build clinical trials.
“We now need the trials to see if ICM can change practice and improve outcomes,” said Carlos Aguiar, MD, a staff cardiologist at the Hospital Santa Cruz, Lisbon. He acknowledged that this study proves that ICM can detect serious arrhythmias in patients with moderate left ventricular dysfunction, but “we need to develop and test treatment paths.”
Dr. Aguiar considers SMART-MI an important study that “goes to the heart” of a common clinical dilemma.
“In clinical practice, we see patients with LVEF that is not that suppressed and so do not have a class I indication for ICM, but there are often features that might have you concerned and make you think it would be great if the LVEF was 35% or lower [to justify intervention],” Dr. Aguiar said.
Data provide insight on unaddressed risk group
SMART-MI confirms earlier evidence that post-MI patients with cardiac autonomic dysfunction are at high risk. Currently, this relative increase in risk goes “unaddressed,” according to Dr. Bauer. Although he contended that the risk itself “could be an indication for ICM in a high-risk patient group without classically defined left ventricular dysfunction,” he agreed that the ultimate value of this trial might be that it “opens a window” for a rationale to test preventive strategies.
An invited ESC discussant, Gerhard Hindricks, MD, PhD, praised the study for drawing attention to the risk of events in a subset of post-MI patients with LVEF of 35% or greater. However, he suggested that criteria other than those based on ECG might be more sensitive for selecting patients who might benefit from intervention.
“We do not know whether additional methods of establishing risk, such as imaging, might be valuable,” said Dr. Hindricks, chief of the department of arrhythmology in the Heart Institute of the University of Leipzig (Germany). He believes work in this area is needed to ensure appropriate entry criteria for interventional trials designed to modify risk in post-MI patients who do not meet the traditional definition of reduced ejection fraction.
Dr. Bauer reports financial relationships with Medtronic, which sponsored this study, as well as Bayer, Boehringer Ingelheim, Edwards, and Novartis. Dr. Aguiar reports no relevant financial conflicts.
After a myocardial infarction, implantable cardiac monitors (ICMs) are sensitive for detecting serious arrhythmias in patients with cardiac autonomic dysfunction but only moderately reduced left ventricular ejection fraction (LVEF), according to results of the randomized SMART-MI trial.
When remote monitoring with the ICM was compared with conventional follow-up in this group of patients, serious arrhythmic events were detected at a nearly sixfold greater rate, reported Axel Bauer, MD, at the annual congress of the European Society of Cardiology.
The study further showed that these events were closely associated with subsequent major adverse cardiac and cerebrovascular events (MACCE).
“SMART-MI is the first study to test an implantable device in high-risk MI patients with a LVEF greater than 35%,” reported Dr. Bauer, a cardiologist and director of the internal medicine clinic, University of Innsbruck (Austria). It showed that the types and frequency of arrhythmias were “comparable to those of post-MI patients with reduced LVEF.”
The ability to assess risk is potentially significant because “the majority of cardiovascular complications, including sudden death, occur in patients with only moderately reduced LVEF,” explained Dr. Bauer.
Despite the greater risk, “there are no preventive strategies so far” currently available for this group, he said.
The SMART-MI study confirms the need for treatments, confirms a method for monitoring risk, and might provide the basis for trials designed to test treatments to modify this risk, he added.
ECG used to define autonomic dysfunction
In the SMART MI protocol, 1,305 survivors of MI with LVEF of 36%-50% at 33 participating centers in Austria and Germany were evaluated with a 20-minute high resolution electrocardiogram. They were enrolled and randomized if they demonstrated cardiac autonomic dysfunction on at least two validated ECG biomarkers.
The 400 participants were randomized to implantation of a ICM, which transmitted daily reports to a ICM core laboratory, or to conventional follow-up.
After a median follow-up of 21 months, serious events were detected in 60 of the 201 patients in the ICM group and 12 of the 199 patients in the control group (29% vs. 6%). Serious adverse events were defined as those that would typically warrant therapy, such as prolonged atrial fibrillation (at least 6 minutes) high-degree atrioventricular block, and sustained ventricular tachycardia.
The difference in the detection rate, which was the primary endpoint, was highly significant (P < .0001), but the study was also able to confirm that these events predicted MACCE, a secondary study endpoint. In those with a serious arrhythmia, the hazard ratio for subsequent MACCE was approximately sevenfold greater relative to those without a serious arrhythmia. This was true of those in the ICM group (HR, 6.8; P < .001) and controls (HR 7.3; P < .001).
Arrhythmias warn of impending complications
“The data show that the prognostic impact of detecting a serious arrhythmia does not depend on the mode of detection,” Dr. Bauer reported. The data also confirm that “subclinical serious arrhythmia events are a warning signal for an impending complication.”
Although more interventions – including pacemakers, catheter ablations, and oral anticoagulants – were offered to patients in the experimental arm, “the study was not powered to show differences in outcomes,” and, in fact, no significant differences were observed, according to Dr. Bauer. However, the evidence that ICM is effective for detecting arrhythmias does provide a structure on which to build clinical trials.
“We now need the trials to see if ICM can change practice and improve outcomes,” said Carlos Aguiar, MD, a staff cardiologist at the Hospital Santa Cruz, Lisbon. He acknowledged that this study proves that ICM can detect serious arrhythmias in patients with moderate left ventricular dysfunction, but “we need to develop and test treatment paths.”
Dr. Aguiar considers SMART-MI an important study that “goes to the heart” of a common clinical dilemma.
“In clinical practice, we see patients with LVEF that is not that suppressed and so do not have a class I indication for ICM, but there are often features that might have you concerned and make you think it would be great if the LVEF was 35% or lower [to justify intervention],” Dr. Aguiar said.
Data provide insight on unaddressed risk group
SMART-MI confirms earlier evidence that post-MI patients with cardiac autonomic dysfunction are at high risk. Currently, this relative increase in risk goes “unaddressed,” according to Dr. Bauer. Although he contended that the risk itself “could be an indication for ICM in a high-risk patient group without classically defined left ventricular dysfunction,” he agreed that the ultimate value of this trial might be that it “opens a window” for a rationale to test preventive strategies.
An invited ESC discussant, Gerhard Hindricks, MD, PhD, praised the study for drawing attention to the risk of events in a subset of post-MI patients with LVEF of 35% or greater. However, he suggested that criteria other than those based on ECG might be more sensitive for selecting patients who might benefit from intervention.
“We do not know whether additional methods of establishing risk, such as imaging, might be valuable,” said Dr. Hindricks, chief of the department of arrhythmology in the Heart Institute of the University of Leipzig (Germany). He believes work in this area is needed to ensure appropriate entry criteria for interventional trials designed to modify risk in post-MI patients who do not meet the traditional definition of reduced ejection fraction.
Dr. Bauer reports financial relationships with Medtronic, which sponsored this study, as well as Bayer, Boehringer Ingelheim, Edwards, and Novartis. Dr. Aguiar reports no relevant financial conflicts.
FROM ESC CONGRESS 2021
Optimizing screening for asymptomatic Afib
Background: Afib is often asymptomatic until a patient presents with an acute stroke. Current screening strategies for Afib fail to detect a large portion of patients, especially since most Afib is paroxysmal. Better screening strategies that increase diagnostic yield are needed.
Study design: Randomized controlled trial (part of the LOOP trial).
Setting: Four centers in Denmark.
Synopsis: Patients over the age of 70 years, with at least one stroke risk factor, were monitored over the course of 3 years using an implantable loop recorder to obtain complete heart rhythm histories and to monitor for the development of Afib. Researchers then applied different sampling strategies to simulate different Afib screening scenarios on this set of rhythm data. A single 10-second EKG yielded a sensitivity of 1.5% for Afib detection and a negative predictive value (NPV) of 66%, increasing to 2.3% and 71% for annual EKGs during 3 years. Twice-daily 30-second EKGs during 14 consecutive days yielded a sensitivity of 8.3%, while a single 24-h monitoring yielded a sensitivity of 11%, increasing to 13%, 15%, and 21% for a 48-hour, 72-hour, and 7-day monitoring, respectively. The highest performance was achieved with annual 30-day monitoring which had a sensitivity of 34%-55% and a NPV of 74%-84% over 1-3 years.
The authors acknowledged many limitations including: The algorithm used had a sensitivity of 95%, there is no valid cutoffs for time-in-Afib, and the simulations assumed 100% patient compliance.
Bottom line: Screening for atrial fibrillation improves by increasing the duration of, spacing between, and number of screenings.
Citation: Diederichsen SZ et al. Comprehensive evaluation of rhythm monitoring strategies in screening for atrial fibrillation: Insights from patients at risk long-term monitored with implantable loop recorder. Circulation. 2020 May 12;141(19):1510-22.
Dr. Mastbergen is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Afib is often asymptomatic until a patient presents with an acute stroke. Current screening strategies for Afib fail to detect a large portion of patients, especially since most Afib is paroxysmal. Better screening strategies that increase diagnostic yield are needed.
Study design: Randomized controlled trial (part of the LOOP trial).
Setting: Four centers in Denmark.
Synopsis: Patients over the age of 70 years, with at least one stroke risk factor, were monitored over the course of 3 years using an implantable loop recorder to obtain complete heart rhythm histories and to monitor for the development of Afib. Researchers then applied different sampling strategies to simulate different Afib screening scenarios on this set of rhythm data. A single 10-second EKG yielded a sensitivity of 1.5% for Afib detection and a negative predictive value (NPV) of 66%, increasing to 2.3% and 71% for annual EKGs during 3 years. Twice-daily 30-second EKGs during 14 consecutive days yielded a sensitivity of 8.3%, while a single 24-h monitoring yielded a sensitivity of 11%, increasing to 13%, 15%, and 21% for a 48-hour, 72-hour, and 7-day monitoring, respectively. The highest performance was achieved with annual 30-day monitoring which had a sensitivity of 34%-55% and a NPV of 74%-84% over 1-3 years.
The authors acknowledged many limitations including: The algorithm used had a sensitivity of 95%, there is no valid cutoffs for time-in-Afib, and the simulations assumed 100% patient compliance.
Bottom line: Screening for atrial fibrillation improves by increasing the duration of, spacing between, and number of screenings.
Citation: Diederichsen SZ et al. Comprehensive evaluation of rhythm monitoring strategies in screening for atrial fibrillation: Insights from patients at risk long-term monitored with implantable loop recorder. Circulation. 2020 May 12;141(19):1510-22.
Dr. Mastbergen is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.
Background: Afib is often asymptomatic until a patient presents with an acute stroke. Current screening strategies for Afib fail to detect a large portion of patients, especially since most Afib is paroxysmal. Better screening strategies that increase diagnostic yield are needed.
Study design: Randomized controlled trial (part of the LOOP trial).
Setting: Four centers in Denmark.
Synopsis: Patients over the age of 70 years, with at least one stroke risk factor, were monitored over the course of 3 years using an implantable loop recorder to obtain complete heart rhythm histories and to monitor for the development of Afib. Researchers then applied different sampling strategies to simulate different Afib screening scenarios on this set of rhythm data. A single 10-second EKG yielded a sensitivity of 1.5% for Afib detection and a negative predictive value (NPV) of 66%, increasing to 2.3% and 71% for annual EKGs during 3 years. Twice-daily 30-second EKGs during 14 consecutive days yielded a sensitivity of 8.3%, while a single 24-h monitoring yielded a sensitivity of 11%, increasing to 13%, 15%, and 21% for a 48-hour, 72-hour, and 7-day monitoring, respectively. The highest performance was achieved with annual 30-day monitoring which had a sensitivity of 34%-55% and a NPV of 74%-84% over 1-3 years.
The authors acknowledged many limitations including: The algorithm used had a sensitivity of 95%, there is no valid cutoffs for time-in-Afib, and the simulations assumed 100% patient compliance.
Bottom line: Screening for atrial fibrillation improves by increasing the duration of, spacing between, and number of screenings.
Citation: Diederichsen SZ et al. Comprehensive evaluation of rhythm monitoring strategies in screening for atrial fibrillation: Insights from patients at risk long-term monitored with implantable loop recorder. Circulation. 2020 May 12;141(19):1510-22.
Dr. Mastbergen is a hospitalist and assistant professor of medicine at UK HealthCare, Lexington, Ky.