User login
Gestational diabetes and the Barker Hypothesis
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).
While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
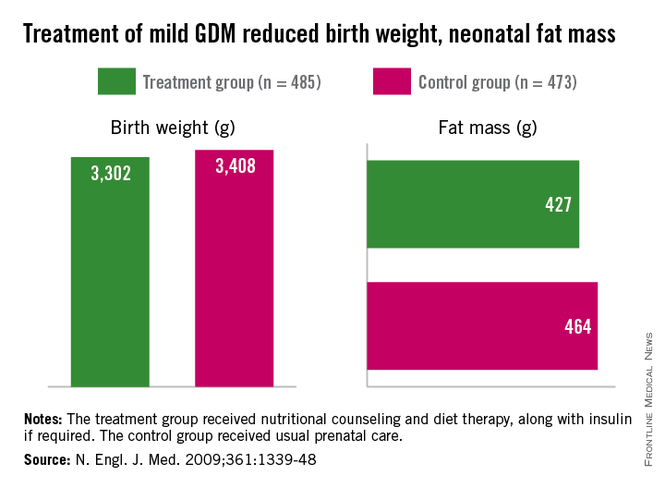
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.
Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
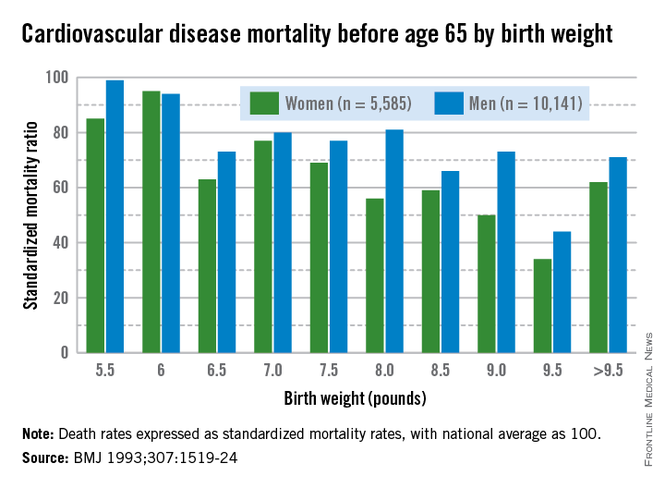
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.
Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
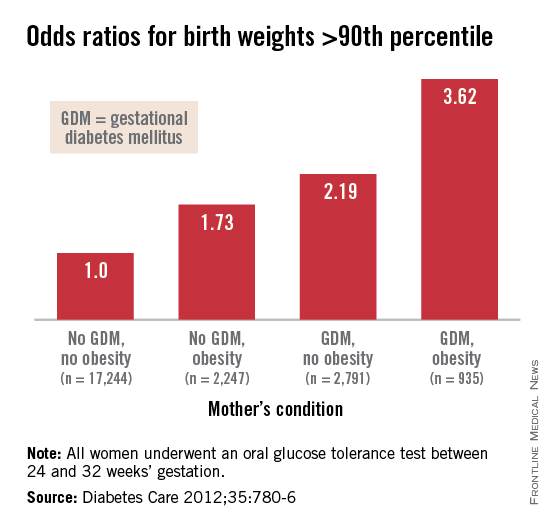
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
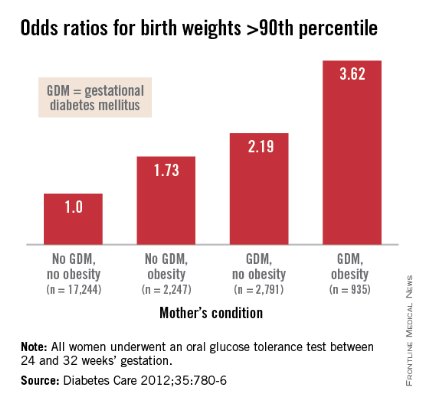
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).

While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.

Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.

Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
Although there are some glimmers of hope that U.S. birthweights may be declining, the average infant birthweight has remained significantly tilted toward obesity. Moreover, and alarming number of infants, children, and adolescents are obese.
In 2007-2008, 9.5% of infants and toddlers were at or above the 95th percentile of the weight-for-recumbent-length growth charts. Among children and adolescents aged 2-19 years, 11.9% were at or above the 97th percentile of the body-mass-index-for-age growth charts; 16.9% were at or above the 95th percentile; and 31.7% were at or above the 85th percentile of BMI for age (JAMA 2010;303:242-9).

While more recent reports of obesity in children indicate a modest decline in obesity among 2- to 5-year-olds (JAMA 2014;311:806-14), an alarming number of infants and children have excess adiposity (roughly twice what is expected). In addition, cardiovascular mortality later in life continues to rise.
The question arises, have childhood and adult obesity rates remained high because mothers are feeding their children the wrong foods or because these children were born obese? One also wonders, with respect to cardiovascular mortality in adulthood, is the in utero environment playing a role?
Old lessons, growing relevance
More than 3 decades ago, the late British physician Dr. David Barker got us thinking about how a challenging life in the womb can set us up for downstream ill health. He studied births from 1910 to 1945 and found that the cardiovascular mortality of individuals born during that time was inversely related to birthweight. Smaller babies, he found, could have cardiovascular mortality risks that were double or even quadruple the risks of larger babies.

Dr. Barker theorized that, when faced with undernutrition, the fetus adapts by sending more blood to the brain and sacrificing blood flow to less essential tissues. His theory about how growth and nutrition before birth may affect the heart became known as the "Barker Hypothesis." It was initially controversial, but it led to an explosion of research – especially since 2000 – on various downstream effects of the intrauterine environment.
Investigators have learned that it is not only cardiovascular mortality that is affected by low birthweight, but also the risk of developing diabetes and being overweight. This is because the fetus makes less essential systems insulin resistant. Insulin resistance persists in the womb and after birth as well, predisposing individuals to insulin resistance and obesity, both of which are closely linked to the risk of metabolic syndrome – a group of risk factors that raises the likelihood of developing heart disease, stroke, and diabetes.
In fact, further research on cohorts of Barker children – individuals who had low birthweights – has shown that not only have they had higher rates of cardiovascular disease, but they have had higher blood sugars and higher rates of insulin resistance as well.
Today, we appreciate a fuller picture of the Barker data, one that shows a reversal of this trend when birthweights reach 4,000-4,500 grams. At this point, what was a progressively downward slope of cardiovascular mortality rates with increasing birthweight suddenly shoots upward again when birthweight exceeds 4,000 g.
It is this end of the curve that is most relevant – and most concerning – for ob.gyns. today. Our problem in the United States is not so much one of starving or growth-restricted newborns, as these babies account for 5% or less of all births. It is one of overweight and obese newborns who now represent as many as 1 in 7 births. Just like the Barker babies who were growth restricted, these newborns have high insulin levels and increased risk of cardiovascular disease as adults.
Changing the trajectory
Both maternal obesity and gestational diabetes get at the heart of the Barker Hypothesis, albeit a twist, in that excessive maternal adiposity and associated insulin resistance results in high maternal blood glucose, transferring excessive nutrients to the fetus. This causes accumulation of fat in the fetus and programs the fetus for an increased and persistent risk of adiposity after birth, early-onset metabolic syndrome, and downstream cardiovascular disease in adulthood.

Dr. Dana Dabelea’s sibling study of almost 15 years ago demonstrated the long-term impact of the adverse intrauterine environment associated with maternal diabetes. Matched siblings who were born after their mothers had developed diabetes had almost double the rate of obesity as adolescents, compared with the siblings born before their mothers were diagnosed with diabetes. In childhood, these siblings ate at the same table and came from the same gene pools (with the same fathers), but they experienced dramatically different health outcomes (Diabetes 2000:49:2208-11).
This landmark study has been reproduced by other investigators who have compared children of mothers who had gestational diabetes and/or were overweight, with children whose mothers did not have gestational diabetes mellitus (GDM) or were of normal weight. Such studies have consistently shown that, faced with either or both maternal obesity and diabetes in utero, offspring were significantly more likely to become overweight children and adults with insulin resistance and other components of the metabolic syndrome.
Importantly, we have evidence from randomized trials that interventions to treat GDM can effectively reduce rates of newborn obesity. While differences in birthweight between treatment and no-treatment arms have been modest, reductions in neonatal body fat, as measured by skin-fold thickness, the ponderal index, and birthweight percentile, have been highly significant.
The offspring of mothers who were treated in these trials, the Australian Carbohydrate Intolerance Study in Pregnant Women (N. Engl. J. Med. 2005;352:2477-86), and a study by Dr. Mark B. Landon and his colleagues (N. Engl. J. Med. 2009;361:1339-48), had approximately half of the newborn adiposity than did offspring of mothers who were not treated. In the latter study, maternal dietary measures alone were successful in reducing neonatal adiposity in over 80% of infants.
While published follow-up data of the offspring in these cohorts have covered only 5-8 years (showing persistently less adiposity in the treated groups), the offspring in the Australian cohort are still being monitored. Based on the cohort and case-control studies summarized above, it seems fair to expect that the children of mothers who were treated for GDM will have significantly better health profiles into and through adulthood.
We know from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study that what were formerly considered mild and inconsequential maternal blood glucose levels are instead potentially quite harmful. The study showed a clear linear relationship between maternal fasting blood glucose levels, fetal cord blood insulin concentrations (a reflection of fetal glucose levels), and newborn body fat percentage (N. Engl. J. Med. 2008;358:1991-2002).
Interestingly, Dr. Patrick Catalano’s analysis of data from the HAPO study (Diabetes Care 2012;35:780-6) shows us more: Maternal obesity is almost as strong a driver of newborn obesity as is GDM. Compared with GDM (which increased the percentage of infant birthweights to greater than the 90th percentile by a factor of 2.19), maternal obesity alone increased the frequency of LGA by a factor of 1.73, and maternal obesity and GDM together increased LGA newborns by 3.62-fold.
In light of these recent findings, it is critical that we not only treat our patients who have GDM, but that we attempt to interrupt the chain of obesity that passes from mother to fetus, and from obese newborns onto their subsequent offspring.
A growing proportion of women across all race and ethnicity groups gain more than 40 pounds during pregnancy for singleton births, and many of them do not lose the weight between pregnancies. Increasingly, we have patients whose first child may not have been exposed to obesity in utero, but whose second child is exposed to overweight or obesity and higher levels of insulin resistance and glycemia.
The Institute of Medicine documented these issues in its 2009 report, "Weight Gain During Pregnancy: Reexamining the Guidelines." Data on maternal postpartum weights are not widely available, but data that have been collected suggest that gaining above recommended ranges is associated with excess maternal weight retention post partum, regardless of prepregnancy BMI. Women who gained above the range recommended by the IOM in 1990 had postpartum weight retention of 15-20 pounds. Among women who gained excessive amounts of weight, moreover, more than 40% retained more than 20 pounds, according to the report.
We must break the intergenerational transfer of obesity and insulin resistance by liberally treating GDM and optimizing glucose control during pregnancy. More importantly, we must emphasize to women the importance of having healthy weights at the time of conception. Recent research affirms that moderately simple interventions, such as dietary improvements and exercise can go a long way to achieving these goals. If we don’t – in keeping with the knowledge spurred on by Dr. Barker – we will be programming more newborns for life with insulin resistance, obesity, and disease.
Dr. Moore is a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego. He said he had no relevant financial disclosures.
The fetal origins hypothesis
On Aug. 27, 2013, the field of obstetrics and gynecology suffered a great loss: the passing of Dr. David J.P. Barker. Dr. Barker was a visionary and leader whose hypothesis about the links between a mother’s health and the long-term health of her children was controversial when he first posed it in the late 1980s. However, after subsequent decades of maternal-fetal practice and research, his idea that preventing chronic disease starts with a healthy mother and baby, is embraced by the medical and science communities today.
I was just starting my career when Dr. Barker’s hypothesis, known then as the "fetal origins hypothesis" but subsequently referred to as the "Barker Hypothesis," was published. During my fellowship in maternal-fetal medicine at Yale University, I had the privilege of attending one of Dr. Barker’s lectures and meeting him. While my colleagues and I thought him an eloquent and impassioned speaker, Dr. Barker’s theory – that there was a relationship between a person’s birth weight and his or her lifetime risk for chronic disease – was highly contentious. He had based his hypothesis on large epidemiological studies conducted in Finland, India, the Netherlands, the United Kingdom, and the United States – all of which revealed that the lower a person’s birth weight, the higher his or her risk for developing coronary heart disease. In addition, he concluded that the lower a person’s birth weight, but the faster the weight gain after age 2 years, the higher a person’s risk for hypertension, stroke, and type 2 diabetes.
At that time, we did not fully realize the significance, gravity, and enormity of Dr. Barker’s contribution to the ob.gyn. field. I could not imagine the impact that his work would have on my professional path. Dr. Barker’s early papers often concluded with a section looking to the future, and in one of them he stated that "we now need to progress beyond epidemiologic associations [between in utero conditions and health later in life] to greater understanding of the cellular and molecular processes that underlie them"(Am. J. Clin. Nutr. 2000;71:1344s-52s). From my work as a physician with diabetic pregnant women to my scientific research devoted to understanding how, at the molecular level, maternal diabetes affects the developing fetus, I have a great appreciation and respect for Dr. Barker’s work.
Therefore, I am very pleased that this month’s Master Class is devoted to a discussion of how the Barker Hypothesis applies today. We have invited Dr. Thomas R. Moore, a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego, to give his reflection on how Dr. Barker’s once radical ideas revolutionized our field.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of the Master Class column. Contact him at [email protected].
On Aug. 27, 2013, the field of obstetrics and gynecology suffered a great loss: the passing of Dr. David J.P. Barker. Dr. Barker was a visionary and leader whose hypothesis about the links between a mother’s health and the long-term health of her children was controversial when he first posed it in the late 1980s. However, after subsequent decades of maternal-fetal practice and research, his idea that preventing chronic disease starts with a healthy mother and baby, is embraced by the medical and science communities today.
I was just starting my career when Dr. Barker’s hypothesis, known then as the "fetal origins hypothesis" but subsequently referred to as the "Barker Hypothesis," was published. During my fellowship in maternal-fetal medicine at Yale University, I had the privilege of attending one of Dr. Barker’s lectures and meeting him. While my colleagues and I thought him an eloquent and impassioned speaker, Dr. Barker’s theory – that there was a relationship between a person’s birth weight and his or her lifetime risk for chronic disease – was highly contentious. He had based his hypothesis on large epidemiological studies conducted in Finland, India, the Netherlands, the United Kingdom, and the United States – all of which revealed that the lower a person’s birth weight, the higher his or her risk for developing coronary heart disease. In addition, he concluded that the lower a person’s birth weight, but the faster the weight gain after age 2 years, the higher a person’s risk for hypertension, stroke, and type 2 diabetes.
At that time, we did not fully realize the significance, gravity, and enormity of Dr. Barker’s contribution to the ob.gyn. field. I could not imagine the impact that his work would have on my professional path. Dr. Barker’s early papers often concluded with a section looking to the future, and in one of them he stated that "we now need to progress beyond epidemiologic associations [between in utero conditions and health later in life] to greater understanding of the cellular and molecular processes that underlie them"(Am. J. Clin. Nutr. 2000;71:1344s-52s). From my work as a physician with diabetic pregnant women to my scientific research devoted to understanding how, at the molecular level, maternal diabetes affects the developing fetus, I have a great appreciation and respect for Dr. Barker’s work.
Therefore, I am very pleased that this month’s Master Class is devoted to a discussion of how the Barker Hypothesis applies today. We have invited Dr. Thomas R. Moore, a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego, to give his reflection on how Dr. Barker’s once radical ideas revolutionized our field.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of the Master Class column. Contact him at [email protected].
On Aug. 27, 2013, the field of obstetrics and gynecology suffered a great loss: the passing of Dr. David J.P. Barker. Dr. Barker was a visionary and leader whose hypothesis about the links between a mother’s health and the long-term health of her children was controversial when he first posed it in the late 1980s. However, after subsequent decades of maternal-fetal practice and research, his idea that preventing chronic disease starts with a healthy mother and baby, is embraced by the medical and science communities today.
I was just starting my career when Dr. Barker’s hypothesis, known then as the "fetal origins hypothesis" but subsequently referred to as the "Barker Hypothesis," was published. During my fellowship in maternal-fetal medicine at Yale University, I had the privilege of attending one of Dr. Barker’s lectures and meeting him. While my colleagues and I thought him an eloquent and impassioned speaker, Dr. Barker’s theory – that there was a relationship between a person’s birth weight and his or her lifetime risk for chronic disease – was highly contentious. He had based his hypothesis on large epidemiological studies conducted in Finland, India, the Netherlands, the United Kingdom, and the United States – all of which revealed that the lower a person’s birth weight, the higher his or her risk for developing coronary heart disease. In addition, he concluded that the lower a person’s birth weight, but the faster the weight gain after age 2 years, the higher a person’s risk for hypertension, stroke, and type 2 diabetes.
At that time, we did not fully realize the significance, gravity, and enormity of Dr. Barker’s contribution to the ob.gyn. field. I could not imagine the impact that his work would have on my professional path. Dr. Barker’s early papers often concluded with a section looking to the future, and in one of them he stated that "we now need to progress beyond epidemiologic associations [between in utero conditions and health later in life] to greater understanding of the cellular and molecular processes that underlie them"(Am. J. Clin. Nutr. 2000;71:1344s-52s). From my work as a physician with diabetic pregnant women to my scientific research devoted to understanding how, at the molecular level, maternal diabetes affects the developing fetus, I have a great appreciation and respect for Dr. Barker’s work.
Therefore, I am very pleased that this month’s Master Class is devoted to a discussion of how the Barker Hypothesis applies today. We have invited Dr. Thomas R. Moore, a perinatologist who is chair of the department of reproductive medicine at the University of California, San Diego, to give his reflection on how Dr. Barker’s once radical ideas revolutionized our field.
Dr. Reece, who specializes in maternal-fetal medicine, is vice president for medical affairs at the University of Maryland, Baltimore, as well as the John Z. and Akiko K. Bowers Distinguished Professor and dean of the school of medicine. Dr. Reece said he had no relevant financial disclosures. He is the medical editor of the Master Class column. Contact him at [email protected].
Need for more data
The need for more and better quality data on medication safety in human pregnancy has been in the public and regulatory spotlight for decades. The issue is becoming even more urgent with the impending revisions to the product label format that will eliminate the traditional A, B, C, D, X pregnancy categories, and substitute more data-driven narratives.
In response to this need, over the last several years there has been a steady increase in the number of regulatory requests/requirements for postmarketing surveillance studies for new medications likely to be used by women of reproductive age or by pregnant women. These requests most often have been fulfilled by the initiation of pregnancy registries. However, concern has been raised that pregnancy registries by and large have been challenged to recruit sufficient numbers of study subjects, and the time to completion of these studies is typically longer than desirable. Other questions have been raised about the adequacy of pregnancy registries alone (especially those with small sample sizes and no internal comparison groups) to test hypotheses related to birth outcomes.
In May 2014, the Food and Drug Administration hosted a public meeting to highlight some of these issues and to seek advice and solutions, titled "Study Approaches and Methods to Evaluate the Safety of Drugs and Biological Products During Pregnancy in the Post-Approval Setting." Panelists included representation from federal agencies including the Centers for Disease Control and Prevention, Department of Defense Naval Health Research Center, the Agency for Healthcare Research and Quality, and the Food and Drug Administration. Other panelists represented academic and HMO-based research networks, contract research organizations, the pharmaceutical industry, obstetric providers, and patients. Comments also were provided by audience members who represented a variety of entities including academic societies, professional associations, and advocacy groups.
Specific strategies for obtaining better data in a more timely fashion were presented by the panelists. These included the value and efficiency of utilizing large administrative claims databases or systemwide electronic capture of pregnancy exposure and outcome data. Using this approach, representative samples of patients can be accrued while not requiring individual active informed consent. Limitations of such sources of data also were mentioned, including inability to validate that the pregnant woman actually took a medication of interest, when, and at what dose, and the usual absence of information in claims data on some important confounders such as alcohol use and folic acid supplementation.
Panelists representing various pregnancy registry study designs described approaches to addressing some of the limitations of these studies. For example, some panelists emphasized that "disease-based" registries that involve examination of birth outcomes for several medications used for the treatment of one or more similar maternal conditions have several distinct advantages. These include a more streamlined referral process, whereby clinicians can more easily identify and refer all pregnant patients who have the same underlying condition, irrespective of treatment with any specific medication under study. Examples of successful disease-based approaches that were highlighted by panelists included the Antiretroviral Drugs in Pregnancy Registry, the North American Antiepileptic Drug Pregnancy Registry, and the OTIS/MotherToBaby Autoimmune Diseases in Pregnancy Project. Each of these studies allows for comparison of outcomes across various treatments, while accounting for the possible contribution of the underlying maternal condition to adverse pregnancy outcomes.
Panelists also emphasized that more effective methods are needed for raising awareness of the existence and value of pregnancy registries for providers and consumers alike, including more efficient and extensive use of social media. Panelists, and in particular obstetric providers, indicated a need for better methods of identifying women who are eligible for participation in a pregnancy study, and facilitating the referral process. The obstetric provider panelist suggested that existing electronic medical records systems could be adapted to generate automated alerts to providers regarding patients who qualify for referral to a registry.
The patient representative panelist emphasized the need to engage the pregnant woman in the research process. This was confirmed by research groups whose primary interaction in pregnancy studies is with the pregnant woman herself, resulting in minimal loss-to-follow-up and high participant satisfaction.
Last, there was extensive discussion about the use of other alternative study designs, such as case-control studies, that could help address the limitations of pregnancy registries, especially in terms of statistical power for evaluating rare outcomes such as specific birth defects. The Vaccines and Medications in Pregnancy Surveillance System (VAMPSS) was described as one such alternative, combining the benefits of a cohort (registry-type) study with a concurrent case-control study to address the same exposures in pregnancy.
There was general consensus among panel members that no single approach to postmarketing safety studies would likely be sufficient to evaluate new or existing products, and that complementary approaches are needed.
A complete set of the panelists’ slide presentations from the public meeting as well as webcasts of the 2-day proceedings in their entirety are available for viewing here.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She said that she had no relevant financial disclosures. To comment, e-mail her at [email protected].
The need for more and better quality data on medication safety in human pregnancy has been in the public and regulatory spotlight for decades. The issue is becoming even more urgent with the impending revisions to the product label format that will eliminate the traditional A, B, C, D, X pregnancy categories, and substitute more data-driven narratives.
In response to this need, over the last several years there has been a steady increase in the number of regulatory requests/requirements for postmarketing surveillance studies for new medications likely to be used by women of reproductive age or by pregnant women. These requests most often have been fulfilled by the initiation of pregnancy registries. However, concern has been raised that pregnancy registries by and large have been challenged to recruit sufficient numbers of study subjects, and the time to completion of these studies is typically longer than desirable. Other questions have been raised about the adequacy of pregnancy registries alone (especially those with small sample sizes and no internal comparison groups) to test hypotheses related to birth outcomes.
In May 2014, the Food and Drug Administration hosted a public meeting to highlight some of these issues and to seek advice and solutions, titled "Study Approaches and Methods to Evaluate the Safety of Drugs and Biological Products During Pregnancy in the Post-Approval Setting." Panelists included representation from federal agencies including the Centers for Disease Control and Prevention, Department of Defense Naval Health Research Center, the Agency for Healthcare Research and Quality, and the Food and Drug Administration. Other panelists represented academic and HMO-based research networks, contract research organizations, the pharmaceutical industry, obstetric providers, and patients. Comments also were provided by audience members who represented a variety of entities including academic societies, professional associations, and advocacy groups.
Specific strategies for obtaining better data in a more timely fashion were presented by the panelists. These included the value and efficiency of utilizing large administrative claims databases or systemwide electronic capture of pregnancy exposure and outcome data. Using this approach, representative samples of patients can be accrued while not requiring individual active informed consent. Limitations of such sources of data also were mentioned, including inability to validate that the pregnant woman actually took a medication of interest, when, and at what dose, and the usual absence of information in claims data on some important confounders such as alcohol use and folic acid supplementation.
Panelists representing various pregnancy registry study designs described approaches to addressing some of the limitations of these studies. For example, some panelists emphasized that "disease-based" registries that involve examination of birth outcomes for several medications used for the treatment of one or more similar maternal conditions have several distinct advantages. These include a more streamlined referral process, whereby clinicians can more easily identify and refer all pregnant patients who have the same underlying condition, irrespective of treatment with any specific medication under study. Examples of successful disease-based approaches that were highlighted by panelists included the Antiretroviral Drugs in Pregnancy Registry, the North American Antiepileptic Drug Pregnancy Registry, and the OTIS/MotherToBaby Autoimmune Diseases in Pregnancy Project. Each of these studies allows for comparison of outcomes across various treatments, while accounting for the possible contribution of the underlying maternal condition to adverse pregnancy outcomes.
Panelists also emphasized that more effective methods are needed for raising awareness of the existence and value of pregnancy registries for providers and consumers alike, including more efficient and extensive use of social media. Panelists, and in particular obstetric providers, indicated a need for better methods of identifying women who are eligible for participation in a pregnancy study, and facilitating the referral process. The obstetric provider panelist suggested that existing electronic medical records systems could be adapted to generate automated alerts to providers regarding patients who qualify for referral to a registry.
The patient representative panelist emphasized the need to engage the pregnant woman in the research process. This was confirmed by research groups whose primary interaction in pregnancy studies is with the pregnant woman herself, resulting in minimal loss-to-follow-up and high participant satisfaction.
Last, there was extensive discussion about the use of other alternative study designs, such as case-control studies, that could help address the limitations of pregnancy registries, especially in terms of statistical power for evaluating rare outcomes such as specific birth defects. The Vaccines and Medications in Pregnancy Surveillance System (VAMPSS) was described as one such alternative, combining the benefits of a cohort (registry-type) study with a concurrent case-control study to address the same exposures in pregnancy.
There was general consensus among panel members that no single approach to postmarketing safety studies would likely be sufficient to evaluate new or existing products, and that complementary approaches are needed.
A complete set of the panelists’ slide presentations from the public meeting as well as webcasts of the 2-day proceedings in their entirety are available for viewing here.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She said that she had no relevant financial disclosures. To comment, e-mail her at [email protected].
The need for more and better quality data on medication safety in human pregnancy has been in the public and regulatory spotlight for decades. The issue is becoming even more urgent with the impending revisions to the product label format that will eliminate the traditional A, B, C, D, X pregnancy categories, and substitute more data-driven narratives.
In response to this need, over the last several years there has been a steady increase in the number of regulatory requests/requirements for postmarketing surveillance studies for new medications likely to be used by women of reproductive age or by pregnant women. These requests most often have been fulfilled by the initiation of pregnancy registries. However, concern has been raised that pregnancy registries by and large have been challenged to recruit sufficient numbers of study subjects, and the time to completion of these studies is typically longer than desirable. Other questions have been raised about the adequacy of pregnancy registries alone (especially those with small sample sizes and no internal comparison groups) to test hypotheses related to birth outcomes.
In May 2014, the Food and Drug Administration hosted a public meeting to highlight some of these issues and to seek advice and solutions, titled "Study Approaches and Methods to Evaluate the Safety of Drugs and Biological Products During Pregnancy in the Post-Approval Setting." Panelists included representation from federal agencies including the Centers for Disease Control and Prevention, Department of Defense Naval Health Research Center, the Agency for Healthcare Research and Quality, and the Food and Drug Administration. Other panelists represented academic and HMO-based research networks, contract research organizations, the pharmaceutical industry, obstetric providers, and patients. Comments also were provided by audience members who represented a variety of entities including academic societies, professional associations, and advocacy groups.
Specific strategies for obtaining better data in a more timely fashion were presented by the panelists. These included the value and efficiency of utilizing large administrative claims databases or systemwide electronic capture of pregnancy exposure and outcome data. Using this approach, representative samples of patients can be accrued while not requiring individual active informed consent. Limitations of such sources of data also were mentioned, including inability to validate that the pregnant woman actually took a medication of interest, when, and at what dose, and the usual absence of information in claims data on some important confounders such as alcohol use and folic acid supplementation.
Panelists representing various pregnancy registry study designs described approaches to addressing some of the limitations of these studies. For example, some panelists emphasized that "disease-based" registries that involve examination of birth outcomes for several medications used for the treatment of one or more similar maternal conditions have several distinct advantages. These include a more streamlined referral process, whereby clinicians can more easily identify and refer all pregnant patients who have the same underlying condition, irrespective of treatment with any specific medication under study. Examples of successful disease-based approaches that were highlighted by panelists included the Antiretroviral Drugs in Pregnancy Registry, the North American Antiepileptic Drug Pregnancy Registry, and the OTIS/MotherToBaby Autoimmune Diseases in Pregnancy Project. Each of these studies allows for comparison of outcomes across various treatments, while accounting for the possible contribution of the underlying maternal condition to adverse pregnancy outcomes.
Panelists also emphasized that more effective methods are needed for raising awareness of the existence and value of pregnancy registries for providers and consumers alike, including more efficient and extensive use of social media. Panelists, and in particular obstetric providers, indicated a need for better methods of identifying women who are eligible for participation in a pregnancy study, and facilitating the referral process. The obstetric provider panelist suggested that existing electronic medical records systems could be adapted to generate automated alerts to providers regarding patients who qualify for referral to a registry.
The patient representative panelist emphasized the need to engage the pregnant woman in the research process. This was confirmed by research groups whose primary interaction in pregnancy studies is with the pregnant woman herself, resulting in minimal loss-to-follow-up and high participant satisfaction.
Last, there was extensive discussion about the use of other alternative study designs, such as case-control studies, that could help address the limitations of pregnancy registries, especially in terms of statistical power for evaluating rare outcomes such as specific birth defects. The Vaccines and Medications in Pregnancy Surveillance System (VAMPSS) was described as one such alternative, combining the benefits of a cohort (registry-type) study with a concurrent case-control study to address the same exposures in pregnancy.
There was general consensus among panel members that no single approach to postmarketing safety studies would likely be sufficient to evaluate new or existing products, and that complementary approaches are needed.
A complete set of the panelists’ slide presentations from the public meeting as well as webcasts of the 2-day proceedings in their entirety are available for viewing here.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She said that she had no relevant financial disclosures. To comment, e-mail her at [email protected].
The Medical Roundtable: Heart Disease in Women
We're going to discuss an issue that has been in the headlines for more than a decade: heart disease in women. We’ll discuss what we know about it, where we've been in terms of problems that have been elucidated, and how we've addressed those problems. We all know that heart disease is the chief cause of mortality in women over their lifetime. Heart disease is actually the second highest cause of mortality in women until the age of 75; it is second to cancer until that age. But it is so great after the age of 75 that when averaged over the lifetime, heart disease becomes the chief cause of mortality in women. I think that may provide some perspective. Heart disease, particularly coronary heart disease, which is the major cause of mortality in women among the cardiovascular diseases, rises sharply after menopause. Coronary heart disease is unusual in women prior to menopause, unless there are outstanding risk factors such as familial hypercholesterolemia or diabetes. Finally, the risk of heart disease in women is always less than men throughout the lifetime.
Kristin, you authored the chapter with Pamela Douglas on heart disease in women in the latest edition of the Braunwald text on heart disease.1 Can you tell us about the situation as you see it now in terms of what we recognize as previous underdiagnosis and undertreatment of heart disease in women with a focus on coronary heart disease.
Dr. Newby: Thank you. I think that's an important question. We can look at a number of sources and I think most of them suggest that we have underappreciated the risk of heart disease in women. In part that's because women develop coronary artery disease later in life or the consequences of coronary artery disease are manifest later in life. I think every signal tells us that we are doing better, including physicians’ perception of risk and treatment. There was a recent study by Lori Mosca published maybe a couple of years ago that shows that women are increasingly aware of heart disease as a major risk factor.2 That said, however, only about half of women think that heart disease is their leading lifetime risk; many still believe it is cancer. So it's about 50/50 now, where it was about 30/70 maybe 5 to 10 years ago. We're making progress, and I think the Red Dress3 campaign and other awareness campaigns have been effective.
We still see gaps in treatment; use of evidence-based therapy in women is lower than it is in men, although it has improved. I think importantly on the side of clinical trials and testing new therapies we're seeing a better balance. But there is still underrepresentation of women in clinical trials that establish evidence for treatment.
Dr. Amsterdam: Your last point is key, and leads me to note that an age cutoff of 75 for enrollment in many clinical trials may be partly responsible for female under-representation, because it’s after 75 that cardiac disease really proliferates in women.
Jeff, what are your views on how much progress we’ve made?
Dr. Borer: Of course, I agree with everything Kristin said. I would emphasize something that we don't talk about so often, and that is that when women do manifest evidence of coronary disease, and particularly when they have a myocardial infarction (MI), they’re about twice as likely as men to die within a year of that first infarction. If only we were more attentive we would do something to modulate that bad outcome. Women are much less likely to get the needed drugs such as beta-blockers, angiotensin-converting enzyme inhibitors, aspirin, and other therapies known to improve survival after MI than are men. If we were more aggressive about that, maybe the outcome would be better. However, the problem is that men who have prognostically important coronary disease, which may be heralded by a first infarction, actually are best treated, or I should say their outcome is best improved, if they undergo coronary artery bypass grafting. One might think, therefore, that this strategy should then be applied to women. However, women are much more likely to die during a bypass grafting procedure than men.
Therefore, the appropriate therapy for women to reduce this extraordinarily higher risk after MI is not clear. I don't think we’re as good at giving medicines as we ought to be, but going the next step and using invasive therapy, that’s something else again. It’s not clear whether women benefit sufficiently to warrant that approach, and that's something we have to think about. We need a great deal more data on that subject.
Dr. Amsterdam: I would add a couple of short points. Kristin indicated that things have gotten better. In fact, in the past 10 years there's been a sharp decrease in the rate of death from cardiovascular disease in women. If you look at the curve of the decrease in mortality from heart disease for the past 50 years in this country, there is a steady downtrend. But when we break up the data, the curve for women was pretty flat, indicating that they did not share in this benefit until recently. It was mostly men until the past decade, when we saw a sharp drop in mortality in women. The death rate in women has fallen almost to that of men. This is attributable to programs such as the Red Dress campaign, as Kristin mentioned, and the “Get with the Guidelines” campaign. When women and men are risk-adjusted after an MI, the outlook is similar—women are older and have more comorbidities when they have their MI. So the question is, is there something intrinsic that puts women at higher risk, or are they just older, sicker, and with more comorbidities, which leads to a different outcome? Jeff?
Dr. Borer: I think it's a good point and I don't really know the answer, but the fact is that the disease is different in women than in men, whether that accounts for some of the difference in outcomes that we see or not. Typically, atherosclerosis in the coronary artery is much more diffuse in women than it is in men, so that angiographic findings often suggest narrow normal arteries when in fact they’re loaded with plaque. In contrast, men’s obstructions tend to be relatively discrete. That difference in the manifestations of atherosclerosis in the coronary arteries in women and in men may well have an important impact on outcome.So while it may be that risk adjustment makes things look more similar among men and women, the fact is the disease is different.
Dr. Amsterdam: Important point. Kristin, would you like to add something on that topic?
Dr. Newby: I think the points that you both have made are good in that looking at risk-adjusted outcomes is important and it gives us an idea of one group relative to another. Unfortunately, sometimes that masks the issue, which is that women are dying at a pretty high rate. Whether it's because they're older or not, it suggests that we've got a high-risk population in which we need to figure out what is different in the way they respond to therapy or whether there are other interventions that could be applied that might improve outcome.
Risk adjustment is important, but I don't think it is the whole answer and it doesn't do away with the challenge of the high event rates in women.
Dr. Borer: I agree with that and if you just look at patients younger than 50, just under age 50, a MI in a woman is twice as likely to be fatal as it is in a man.4
Dr. Amsterdam: On the other hand, of women under 35 who come to the emergency department (ED) with chest pain, only about 1 in 1000 turn out to have an MI. So there's a high frequency of chest pain in women and they generally tend to use the ED and medical system more than men for symptoms. This may be because there’s less denial in women, but there are lots of negative tests in women for symptoms like chest pain or chest discomfort. Would you agree with that, Kristin?
Dr. Newby: It is true. I think there are a number of other features, unfortunately, that may lead, perhaps not in young women but in older women, to missed or delayed diagnosis that may also contribute to their worse prognosis. That may explain some of the difference as well. I would just throw that out for discussion.
Dr. Amsterdam: A lot of the data that we’re talking about in underdiagnosis and undertreatment come from papers, many of which are 10 years and 20 years old. In your own institutions, are you seeing a persistence of these issues, because I'm not aware of any recent studies indicating that there's been an improvement in these trends, other than that we know that cardiovascular disease leading to mortality is decreasing in women.
In your own institutions, do you see a persistence of the woman who sits in the ED with chest pain and doesn't get an electrocardiogram (ECG) within 10 minutes, or a good workup, or a referral from a clinic or office for chest pain? Jeff, what’s your experience?
Dr. Borer: The issue, I suppose, is, what does the cardiologist do? I think over the past decade or more, maybe 2 decades, there's been a heightened sensitivity among cardiologists of the fact that women do in fact have atherosclerosis, they do have coronary disease, they do have MIs, and they do die from it. But I think the problem is a little bit different.
It's not so much now, I think, allowing a woman to sit in the ED with chest pain for too long. I don't think that happens terribly often, at least not from what I've seen. It's that the symptom pattern is different in women than in men, and while a lot of women have chest discomfort and often the chest discomfort isn't associated with coronary disease, a lot of women have coronary disease with symptoms that don't include chest discomfort. The difficulty in the sensitivity to that possibility is something that we still have to improve.
Dr. Amsterdam: Kristin, Jeff raised an important point about the difference of symptoms in women and men when they present with coronary disease. We know that symptoms in the elderly are different than those who are younger when presenting with ischemic heart disease. So is it that the symptoms are different in women per se, or is it that they comprise a large part of the elderly population with coronary artery disease, and if you compare elderly men with elderly women, would there be a difference in symptom presentation?
Dr. Newby: That's a good question. You know one of the better studies that's been done on this actually revealed that the majority of patients who were having an acute MI presented with some type of chest-related symptoms, such as tightness and pressure.1
My personal experience, and again I don't think this has been well studied specifically in the elderly, is that the issue with atypical symptoms is in part a phenomenon of age, where the elderly tend to present more frequently with just dyspnea or fatigue. One really has to be paying attention to that. That disproportionately affects women who present later in life with acute ischemic syndromes.
I want to move to the risk factors for coronary disease in men and women. Jeff, any differences or similarities—I will tell you my view right off the bat that the risk factors for coronary artery disease other than pregnancy and menopause, which act through the traditional risk factors, are the same in men and women. There are some differences, and in her chapter in Braunwald, Kristin pointed them out nicely with regard to diabetes from the INTERHEART study.5 What's your view on the risk factors in women? Do we need preventive cardiology clinics for women and for women with heart disease; or can women come to a good preventive cardiology clinic that men also attend?
Dr. Borer: I think the risk factors are pretty much the same. You know there may be some difference in outcomes associated with them, depending on age, with diabetes being the major issue. I think it has more of an impact on the risk of second MI in women than in men, so you have to be more cognizant of that particular risk factor in women.
But I think the risk factors are the same and the outcomes are qualitatively the same and so managing patients by trying to modulate risk factors could be done in a preventive cardiology unit. I don't think you need a separate one for women because I don't think we have sufficient data to suggest a different strategy for men and for women.
Dr. Amsterdam: Kristin?
Dr. Newby: I fully agree. I think that the risk factors, with the possible exception that diabetes may be a bit more potent in women, are still the same risk factors and we know the same treatments work in both groups. What I think is probably more interesting that came out of INTERHEART is the differential strength (stronger in women) of protective factors in women, such as exercise, eating a diet high in fruits and vegetables, and stress management. I found that to be interesting and potentially more informative than focusing on the proven risk factors.
Dr. Amsterdam: I think INTERHEART was a very good confirmation of what the Framingham Heart Study showed of the traditional risk factors.6
Would you agree, Kristin, that there is more stress in general, more psychosocial stress, in women than in men? Women work and take care of the kids—men do better now, but we don't do an equal share, and that seems to be having an impact on women and disease.
Dr. Newby: Yes, I think that's an interesting topic, and I think women's response to stress and the stress that they're dealing with may be different from that of men. I think there’s an interesting analysis that’s been done several times about support systems and how men do better when they have a spouse who’s taking care of them. Women on the other hand who have an MI who have a spouse actually do worse. So I'm not sure what that tells us, but I do think there's either some disproportionate amount of reaction to or ability to cope with stress among women. That is important for us to understand and work through.
Dr. Amsterdam: It's too bad we can't measure that the way we measure other data. Now let’s consider a stable patient who comes to the physician’s office with chest pain; it may be typical or atypical, but it's suspicious enough for the patient to require a careful evaluation.
Jeff, which tests do you use to detect objective evidence of myocardial ischemia and, by that factor, the likelihood of coronary disease? Exercise treadmill test has really been criticized for its value in women because of its repute for false-positive results. Some experts have advised going straight to a stress imaging study rather than a plain treadmill test.
Dr. Borer: Yes, well that's what I do, actually. The fact is that the specificity of every noninvasive test for coronary disease is lower in women than in men. The positive predictive value is less in women than in men, whether it be a radionuclide test, an echocardiography-based test, it's not just the exercise ECG. On the other hand, it seems to me that radionuclide based studies do provide greater accuracy in women than the exercise ECG, and that's what I use.
Dr. Amsterdam: This is good because thus far we’ve had little divergence of opinion and it’s good to have some differences. Thus, I am a strong proponent of the standard treadmill exercise test without imaging if the patient has a normal baseline ECG and the history indicates adequate exercise capacity, which is what the guidelines have advocated since 1998. So in these circumstances, my first test is always a plain treadmill study, and we train our fellows to follow this approach. Kristin, your method?
Dr. Newby: Yes, I think I fall a little bit more to Jeff's side of things. So there are two things I think about: one is if I see someone in the clinic who gives me a very good story for symptoms and they can't exercise and can’t do their usual activities. I either move to treat them with medicines and cardiac rehabilitation or with catheterization if I have a high index of suspicion that I'm dealing with coronary disease. So I think that's the first thing. Then the question is, how do we think about the individuals who aren't clear-cut? We want to rule out the group that has the highest likelihood to have a false positive—the ones with very atypical symptoms, which I know we all see in our clinics—and try to work with them without doing any kind of further workup if at all possible. But if I do a stress test in the clinic and I'm on the fence, I, like Jeff, add imaging.7 We tend to use more stress echocardiography than stress nuclear testing, but I don't think there's any evidence that one or the other, if you're adding an imaging study, is of a major advantage given the certain limitations of each study in terms of acquiring the images.
Dr. Amsterdam: I agree. They're very similar. Perhaps nuclear is a little more sensitive but less specific than echocardiography.
So I will say quickly, I think we all know the guidelines of the American Heart Association/American College of Cardiology (AHA/ACC)8—that I just referred to. They’re very clear in stating that the first test, even in a stable female patient with a normal ECG who is able to exercise should be a plain old treadmill test. I'll make a couple of points; one, we have in press a paper on how to improve the positive predictive value of the exercise ECG in women. The more risk factors the patient has, the deeper the ST depression, the longer the persistence of ST depression post-exercise—these factors can increase the positive predictive value up to about 80% in women. These ECG factors and non-ECG factors can help. We have also shown that if the patient, man or woman, has a positive treadmill ECG and can exercise to 10 metabolic equivalents (METs) (Stage III of the Bruce test),9,10 and then is referred for a stress imaging test, about a 94% likelihood that the stress imaging test will be negative.
So the key point for me is that assessment of the exercise test by ECG criteria alone is really obsolete. The poor positive predictive value and the high rate of false-positives are based solely on the ST segment when we know that the best prognostic indicator is functional capacity. So that's why we always do the treadmill test as the initial assessment unless there's a baseline abnormality or the patient can't exercise.
Do you have any comments on any of that, Jeff?
Dr. Borer: I would like to pick up on two things that Kristin said. She talked about symptoms, and I would say that if a person, and it doesn't matter whether it's a man or a woman, has typical symptoms, when you take a history and you put that person on a treadmill and you reproduce the symptoms and you do an ECG alone, the concordance of the ECG criteria and the symptoms has a very high predictive value. But that presumes that the person is going to get symptoms on the treadmill, and that may not happen.
The other point that Kristin made was that in certain patients with a compelling clinical presentation, she might go right to a catheterization. I would have to say that I don't do that. I guess it depends on how you define “compelling.” If somebody has a history that is consistent with crescendo angina, yes, I would send him or her to catheterization. But absent that, I would not because I believe that the noninvasive testing provides prognostic information even in the presence of true coronary disease.
The problem that I fear in sending someone to catheterization is that, in the absence of prognostically important disease, they'll come away with an angioplasty with no evidence at all that anything will be done to their natural history, only that their symptoms will be relieved and that could probably have been done with medication. So I'm a little more wary about sending people directly to catheterization. There really has to be what I would call a “compelling” history, and it may be the same that Kristin would call “compelling” or it may not.
Dr. Amsterdam: We’re into the art of medicine, and it’s legitimate to differ if we have good reasons to go down different paths. Kristin, your comments on this?
Dr. Newby: When I'm thinking of somebody whose symptoms are compelling enough that I would send them to catheterization, it's the woman who was active, doing her gardening, mowing her yard, whatever last summer, and has over the past several months progressively deteriorated in terms of activity levels. So you already know their functional capacity has declined by what they're telling you through their history. In those individuals, I don't think you gain a lot more information by documenting that on a treadmill.
You're right, maybe you take that person and you try medication or maybe you commit that they have coronary disease, you're going to take care of their angina, get them immediately back to functional capacity. I think those are style points, as you said, the art of medicine. To some extent it's driven by patient preferences and I think that's the thing that sometimes we also forget.
There are patients who, once they know what you're thinking, don't want to deal with multiple steps to get to an answer—they want to know. Maybe that's right, maybe that's wrong, maybe that's part of what's wrong with our healthcare system. We could debate those points for another hour probably, but I think those factors all play into how we manage patients.
Then you think about taking somebody when you're pretty sure you know what the diagnosis is, or maybe you're pretty sure you don't think it's the diagnosis, but you put them on a treadmill or a treadmill with imaging and it's positive, and now you've got them worried. They want to know what's going on, and they may or not may not tolerate medicines.
These are some practical issues in medicine, as well, that we have to think about, and again, as you said, that's the art of medicine.
Dr. Amsterdam: Kristin, can you tell us a little about the Duke treadmill score? Because it's a very good prognostic indicator.
Dr. Newby: Absolutely, and that's very much like the study that you described of Dr. Beller’s; if they could do 10 METs they did well. We look at our treadmill information, often we get it in conjunction with an echocardiogram, but we don't ignore the key physiologic information and functional information from the treadmill for that very reason; there's a high correlation between either METs done or various scores. The Duke treadmill score being one type of score that one can use to estimate risk of dying in the next 5 to 10 years. So it is critically important that we don't ignore the wealth of information that can come from a treadmill test.
Dr. Amsterdam: Jeff, you mentioned chest pain and it reminded me of something important. One of the values of stress testing is whether we can reproduce symptoms that the patient complains of. I notice in our chest pain unit that patients are admitted because of chest pain and they have a normal resting ECG and negative troponins. Our first test in these stable patients is, again, a treadmill test, symptom-limited. They do more activity on that stress test than they do in life but it's very rare that we reproduce chest pain. Even the patients with coronary disease who go to catheterization because of a very positive stress test infrequently have chest pain during treadmill testing. Does your experience differ?
Dr. Borer: No, my experience doesn't differ. When people come out of our chest pain unit they have an imaging study, they don't have a straight treadmill exercise ECG, but that's irrelevant. One thing that is perhaps not irrelevant is that in New York State, in the absence of unstable angina, you now cannot send a patient to catheterization unless you have demonstrated a positive exercise test.
If they have unstable symptoms certainly you can catheterize them, but in a patient who has relatively stable symptomatology, you can't catheterize (and expect to be paid for the procedure) unless you have evidence of ischemia by a noninvasive test. Those are called “appropriate use” criteria that have been set up here in New York, and if you don't follow them, the insurance companies don't pay. So there is sort of a brake here that may not be true in other states.
Dr. Amsterdam: Kristin, do you have such a proscription in North Carolina? What about your experience of chest pain on the treadmill?
Dr. Newby: First of all, we don't have such a proscription here in North Carolina, although I think we’re going to see more and more of that. New York State has historically been a leader in that kind of thinking through and monitoring and implementing criteria like these, and I don't think it's all a bad thing.
In our chest pain unit or in referrals from clinic, I agree that most of the time we don't reproduce symptoms on the treadmill and I find it very reassuring if we do reproduce symptoms and we don't see anything. Again we’re doing imaging, but imaging in conjunction with the ECG, I think, is actually helpful information to have, but in the majority of cases we don't reproduce the symptoms.
Dr. Amsterdam: It’s a really interesting phenomenon, and as Jeff has said, the symptoms on the treadmill are very important.
Let's make a couple of comments on heart failure. There are data that women present more typically with heart failure as an indication of underlying coronary disease and also have more diastolic dysfunction than men in terms of the underlying cause of heart failure. Your experience with this, Jeff?
Dr. Borer: I think that's true but, in fact, the truth also is that most heart failure trials include relatively few women. It was the same point you made earlier. I'm one of the principal investigators of the “Systolic Heart failure treatment with the If inhibitor ivabradine Trial” (SHIFT), the largest heart failure trial that was ever done, which used a heart rate–slowing strategy for therapy. We had a marked paucity of women compared with men, so it's hard to draw conclusions.11 I don't know how many women were excluded because they had diastolic dysfunction. It certainly is my perception and my bias that among those with heart failure, women commonly do have diastolic dysfunction, probably more commonly than men, but I think we lack a lot of information about heart failure.
Let me talk about the area on which I focus most closely, which is valve disease. The manifestations of valve disease are different in women than in men. That was demonstrated most clearly in the Placement of Aortic Transcatheter Valve Trial (PARTNER),12 which was the first randomized trial of any mechanical therapy for valve disease in patients with aortic stenosis. In fact, approximately 50% of the population in PARTNER comprised women. The average age was the mid-80s, and by that age there are a lot more women around than men. So perhaps that had something to do with the fact that many of those who entered the trial were women.
The extraordinary finding was that women did better with percutaneous valve therapy than men. Not only did they do better than men, the relationship of percutaneous therapy to conventional surgery was better in women than in men. We could speculate on why that is (smaller arteries, harder to do, etc.), and maybe we’d be correct. However, the finding does suggest that there is a difference, at least for that valve disease, in women compared to men.
Another piece of evidence is the affects of treadmill testing in patients with aortic stenosis. Asymptomatic patients with aortic stenosis are marked out as being at relatively high risk for the imminent development of symptoms or worse based on treadmill studies. One of the criteria that seems to separate those at high risk is ST-segment depression, but it’s 1 mm in men and 2 mm in women. We see the valve dysfunction more commonly in women than in men and the manifestations of the disease differ.
Dr. Amsterdam: These are fascinating data. We're doing an investigation in aortic stenosis and the overwhelming number of patients are men, but, in general, I agree with your point that because we’re seeing patients with critical aortic stenosis who are now in their 80s, women comprise a very large component of that group.
Kristin, can you comment on heart failure and whether there is more diastolic dysfunction, more hypertension in women, and so relative to their body size they have more left ventricular hypertrophy?
Dr. Newby: Yes I think that diastolic dysfunction is more common and more severe with aging. There are also the effects of hypertension. Both of those obviously are issues with older women. I do share Jeff's concern not just related to diastolic dysfunction, but related to any heart failure, of the underrepresentation of women in our studies that are designed to help us understand how to manage heart failure from any cause.
Dr. Amsterdam: Jeff; do you remember the age cutoff in the SHIFT trial?
Dr. Borer: There was no upper age limit. However, the average age was 60 years and 11% were at least 75 years old.
Dr. Newby: And if diastolic dysfunction is more prevalent in the older population than systolic dysfunction, the age of the patients will be a big factor in the etiology of the heart failure.
Dr. Amsterdam: In the past decade there has been a proliferation of publications on women with normal epicardial coronary arteries in whom myocardial ischemia and its complications are caused by (or associated with) coronary microvascular dysfunction (which is also presumed to be the underlying cause of the elusive Syndrome X). What are your comments on this issue?
Dr. Borer: Regarding microvascular dysfunction and Syndrome X, it has been my impression that some people, more often women than men, present with atypical chest pain and even typical angina pectoris in the absence of large-vessel coronary artery disease or any other definable cause, but that the problem of demonstrable microvascular dysfunction in these settings is relatively uncommon. Patients who present in this way may benefit from certain types of vasodilating drugs with relief of symptoms. However, I believe the outcomes for these people are substantially more benign than for those with large coronary artery obstruction. In other words, frank MI or death are relatively infrequently associated with this syndrome. Moreover, although it is plausible to associate such a clinical picture with endothelial dysfunction and small-vessel (arteriolar) hyperreactivity, I think the pathophysiology of the syndrome is not clear. Thus, while Syndrome X may contribute to debility and activity limitation particularly in women, I do not think the syndrome is very clearly understood and do not think it is an important cause of premature death or major morbidity.
Dr. Amsterdam: We're going to have concluding comments from each of you. So briefly, Jeff, are there any comments you'd like to make?
Dr. Borer: I would summarize that the situation with regard to sensitivity about heart disease in women has improved considerably over the past decade or two, but we still have a long way to go to understand the differences between women and men in terms of the manifestations of heart disease and how to manage them.
Dr. Amsterdam: And Kristin.
Dr. Newby: I completely agree with Jeff's summary. The only thing I would add is that of the therapies that we have available, they are all equally effective in men and women, and I think we just need to focus right now as we're trying to understand the underlying differences in pathophysiology and symptoms, among other things, on applying these therapies as appropriately as we can across both sexes.
Dr. Amsterdam: Thank you.
FoxP2 Media LLC is the publisher of The Medical Roundtable.
We're going to discuss an issue that has been in the headlines for more than a decade: heart disease in women. We’ll discuss what we know about it, where we've been in terms of problems that have been elucidated, and how we've addressed those problems. We all know that heart disease is the chief cause of mortality in women over their lifetime. Heart disease is actually the second highest cause of mortality in women until the age of 75; it is second to cancer until that age. But it is so great after the age of 75 that when averaged over the lifetime, heart disease becomes the chief cause of mortality in women. I think that may provide some perspective. Heart disease, particularly coronary heart disease, which is the major cause of mortality in women among the cardiovascular diseases, rises sharply after menopause. Coronary heart disease is unusual in women prior to menopause, unless there are outstanding risk factors such as familial hypercholesterolemia or diabetes. Finally, the risk of heart disease in women is always less than men throughout the lifetime.
Kristin, you authored the chapter with Pamela Douglas on heart disease in women in the latest edition of the Braunwald text on heart disease.1 Can you tell us about the situation as you see it now in terms of what we recognize as previous underdiagnosis and undertreatment of heart disease in women with a focus on coronary heart disease.
Dr. Newby: Thank you. I think that's an important question. We can look at a number of sources and I think most of them suggest that we have underappreciated the risk of heart disease in women. In part that's because women develop coronary artery disease later in life or the consequences of coronary artery disease are manifest later in life. I think every signal tells us that we are doing better, including physicians’ perception of risk and treatment. There was a recent study by Lori Mosca published maybe a couple of years ago that shows that women are increasingly aware of heart disease as a major risk factor.2 That said, however, only about half of women think that heart disease is their leading lifetime risk; many still believe it is cancer. So it's about 50/50 now, where it was about 30/70 maybe 5 to 10 years ago. We're making progress, and I think the Red Dress3 campaign and other awareness campaigns have been effective.
We still see gaps in treatment; use of evidence-based therapy in women is lower than it is in men, although it has improved. I think importantly on the side of clinical trials and testing new therapies we're seeing a better balance. But there is still underrepresentation of women in clinical trials that establish evidence for treatment.
Dr. Amsterdam: Your last point is key, and leads me to note that an age cutoff of 75 for enrollment in many clinical trials may be partly responsible for female under-representation, because it’s after 75 that cardiac disease really proliferates in women.
Jeff, what are your views on how much progress we’ve made?
Dr. Borer: Of course, I agree with everything Kristin said. I would emphasize something that we don't talk about so often, and that is that when women do manifest evidence of coronary disease, and particularly when they have a myocardial infarction (MI), they’re about twice as likely as men to die within a year of that first infarction. If only we were more attentive we would do something to modulate that bad outcome. Women are much less likely to get the needed drugs such as beta-blockers, angiotensin-converting enzyme inhibitors, aspirin, and other therapies known to improve survival after MI than are men. If we were more aggressive about that, maybe the outcome would be better. However, the problem is that men who have prognostically important coronary disease, which may be heralded by a first infarction, actually are best treated, or I should say their outcome is best improved, if they undergo coronary artery bypass grafting. One might think, therefore, that this strategy should then be applied to women. However, women are much more likely to die during a bypass grafting procedure than men.
Therefore, the appropriate therapy for women to reduce this extraordinarily higher risk after MI is not clear. I don't think we’re as good at giving medicines as we ought to be, but going the next step and using invasive therapy, that’s something else again. It’s not clear whether women benefit sufficiently to warrant that approach, and that's something we have to think about. We need a great deal more data on that subject.
Dr. Amsterdam: I would add a couple of short points. Kristin indicated that things have gotten better. In fact, in the past 10 years there's been a sharp decrease in the rate of death from cardiovascular disease in women. If you look at the curve of the decrease in mortality from heart disease for the past 50 years in this country, there is a steady downtrend. But when we break up the data, the curve for women was pretty flat, indicating that they did not share in this benefit until recently. It was mostly men until the past decade, when we saw a sharp drop in mortality in women. The death rate in women has fallen almost to that of men. This is attributable to programs such as the Red Dress campaign, as Kristin mentioned, and the “Get with the Guidelines” campaign. When women and men are risk-adjusted after an MI, the outlook is similar—women are older and have more comorbidities when they have their MI. So the question is, is there something intrinsic that puts women at higher risk, or are they just older, sicker, and with more comorbidities, which leads to a different outcome? Jeff?
Dr. Borer: I think it's a good point and I don't really know the answer, but the fact is that the disease is different in women than in men, whether that accounts for some of the difference in outcomes that we see or not. Typically, atherosclerosis in the coronary artery is much more diffuse in women than it is in men, so that angiographic findings often suggest narrow normal arteries when in fact they’re loaded with plaque. In contrast, men’s obstructions tend to be relatively discrete. That difference in the manifestations of atherosclerosis in the coronary arteries in women and in men may well have an important impact on outcome.So while it may be that risk adjustment makes things look more similar among men and women, the fact is the disease is different.
Dr. Amsterdam: Important point. Kristin, would you like to add something on that topic?
Dr. Newby: I think the points that you both have made are good in that looking at risk-adjusted outcomes is important and it gives us an idea of one group relative to another. Unfortunately, sometimes that masks the issue, which is that women are dying at a pretty high rate. Whether it's because they're older or not, it suggests that we've got a high-risk population in which we need to figure out what is different in the way they respond to therapy or whether there are other interventions that could be applied that might improve outcome.
Risk adjustment is important, but I don't think it is the whole answer and it doesn't do away with the challenge of the high event rates in women.
Dr. Borer: I agree with that and if you just look at patients younger than 50, just under age 50, a MI in a woman is twice as likely to be fatal as it is in a man.4
Dr. Amsterdam: On the other hand, of women under 35 who come to the emergency department (ED) with chest pain, only about 1 in 1000 turn out to have an MI. So there's a high frequency of chest pain in women and they generally tend to use the ED and medical system more than men for symptoms. This may be because there’s less denial in women, but there are lots of negative tests in women for symptoms like chest pain or chest discomfort. Would you agree with that, Kristin?
Dr. Newby: It is true. I think there are a number of other features, unfortunately, that may lead, perhaps not in young women but in older women, to missed or delayed diagnosis that may also contribute to their worse prognosis. That may explain some of the difference as well. I would just throw that out for discussion.
Dr. Amsterdam: A lot of the data that we’re talking about in underdiagnosis and undertreatment come from papers, many of which are 10 years and 20 years old. In your own institutions, are you seeing a persistence of these issues, because I'm not aware of any recent studies indicating that there's been an improvement in these trends, other than that we know that cardiovascular disease leading to mortality is decreasing in women.
In your own institutions, do you see a persistence of the woman who sits in the ED with chest pain and doesn't get an electrocardiogram (ECG) within 10 minutes, or a good workup, or a referral from a clinic or office for chest pain? Jeff, what’s your experience?
Dr. Borer: The issue, I suppose, is, what does the cardiologist do? I think over the past decade or more, maybe 2 decades, there's been a heightened sensitivity among cardiologists of the fact that women do in fact have atherosclerosis, they do have coronary disease, they do have MIs, and they do die from it. But I think the problem is a little bit different.
It's not so much now, I think, allowing a woman to sit in the ED with chest pain for too long. I don't think that happens terribly often, at least not from what I've seen. It's that the symptom pattern is different in women than in men, and while a lot of women have chest discomfort and often the chest discomfort isn't associated with coronary disease, a lot of women have coronary disease with symptoms that don't include chest discomfort. The difficulty in the sensitivity to that possibility is something that we still have to improve.
Dr. Amsterdam: Kristin, Jeff raised an important point about the difference of symptoms in women and men when they present with coronary disease. We know that symptoms in the elderly are different than those who are younger when presenting with ischemic heart disease. So is it that the symptoms are different in women per se, or is it that they comprise a large part of the elderly population with coronary artery disease, and if you compare elderly men with elderly women, would there be a difference in symptom presentation?
Dr. Newby: That's a good question. You know one of the better studies that's been done on this actually revealed that the majority of patients who were having an acute MI presented with some type of chest-related symptoms, such as tightness and pressure.1
My personal experience, and again I don't think this has been well studied specifically in the elderly, is that the issue with atypical symptoms is in part a phenomenon of age, where the elderly tend to present more frequently with just dyspnea or fatigue. One really has to be paying attention to that. That disproportionately affects women who present later in life with acute ischemic syndromes.
I want to move to the risk factors for coronary disease in men and women. Jeff, any differences or similarities—I will tell you my view right off the bat that the risk factors for coronary artery disease other than pregnancy and menopause, which act through the traditional risk factors, are the same in men and women. There are some differences, and in her chapter in Braunwald, Kristin pointed them out nicely with regard to diabetes from the INTERHEART study.5 What's your view on the risk factors in women? Do we need preventive cardiology clinics for women and for women with heart disease; or can women come to a good preventive cardiology clinic that men also attend?
Dr. Borer: I think the risk factors are pretty much the same. You know there may be some difference in outcomes associated with them, depending on age, with diabetes being the major issue. I think it has more of an impact on the risk of second MI in women than in men, so you have to be more cognizant of that particular risk factor in women.
But I think the risk factors are the same and the outcomes are qualitatively the same and so managing patients by trying to modulate risk factors could be done in a preventive cardiology unit. I don't think you need a separate one for women because I don't think we have sufficient data to suggest a different strategy for men and for women.
Dr. Amsterdam: Kristin?
Dr. Newby: I fully agree. I think that the risk factors, with the possible exception that diabetes may be a bit more potent in women, are still the same risk factors and we know the same treatments work in both groups. What I think is probably more interesting that came out of INTERHEART is the differential strength (stronger in women) of protective factors in women, such as exercise, eating a diet high in fruits and vegetables, and stress management. I found that to be interesting and potentially more informative than focusing on the proven risk factors.
Dr. Amsterdam: I think INTERHEART was a very good confirmation of what the Framingham Heart Study showed of the traditional risk factors.6
Would you agree, Kristin, that there is more stress in general, more psychosocial stress, in women than in men? Women work and take care of the kids—men do better now, but we don't do an equal share, and that seems to be having an impact on women and disease.
Dr. Newby: Yes, I think that's an interesting topic, and I think women's response to stress and the stress that they're dealing with may be different from that of men. I think there’s an interesting analysis that’s been done several times about support systems and how men do better when they have a spouse who’s taking care of them. Women on the other hand who have an MI who have a spouse actually do worse. So I'm not sure what that tells us, but I do think there's either some disproportionate amount of reaction to or ability to cope with stress among women. That is important for us to understand and work through.
Dr. Amsterdam: It's too bad we can't measure that the way we measure other data. Now let’s consider a stable patient who comes to the physician’s office with chest pain; it may be typical or atypical, but it's suspicious enough for the patient to require a careful evaluation.
Jeff, which tests do you use to detect objective evidence of myocardial ischemia and, by that factor, the likelihood of coronary disease? Exercise treadmill test has really been criticized for its value in women because of its repute for false-positive results. Some experts have advised going straight to a stress imaging study rather than a plain treadmill test.
Dr. Borer: Yes, well that's what I do, actually. The fact is that the specificity of every noninvasive test for coronary disease is lower in women than in men. The positive predictive value is less in women than in men, whether it be a radionuclide test, an echocardiography-based test, it's not just the exercise ECG. On the other hand, it seems to me that radionuclide based studies do provide greater accuracy in women than the exercise ECG, and that's what I use.
Dr. Amsterdam: This is good because thus far we’ve had little divergence of opinion and it’s good to have some differences. Thus, I am a strong proponent of the standard treadmill exercise test without imaging if the patient has a normal baseline ECG and the history indicates adequate exercise capacity, which is what the guidelines have advocated since 1998. So in these circumstances, my first test is always a plain treadmill study, and we train our fellows to follow this approach. Kristin, your method?
Dr. Newby: Yes, I think I fall a little bit more to Jeff's side of things. So there are two things I think about: one is if I see someone in the clinic who gives me a very good story for symptoms and they can't exercise and can’t do their usual activities. I either move to treat them with medicines and cardiac rehabilitation or with catheterization if I have a high index of suspicion that I'm dealing with coronary disease. So I think that's the first thing. Then the question is, how do we think about the individuals who aren't clear-cut? We want to rule out the group that has the highest likelihood to have a false positive—the ones with very atypical symptoms, which I know we all see in our clinics—and try to work with them without doing any kind of further workup if at all possible. But if I do a stress test in the clinic and I'm on the fence, I, like Jeff, add imaging.7 We tend to use more stress echocardiography than stress nuclear testing, but I don't think there's any evidence that one or the other, if you're adding an imaging study, is of a major advantage given the certain limitations of each study in terms of acquiring the images.
Dr. Amsterdam: I agree. They're very similar. Perhaps nuclear is a little more sensitive but less specific than echocardiography.
So I will say quickly, I think we all know the guidelines of the American Heart Association/American College of Cardiology (AHA/ACC)8—that I just referred to. They’re very clear in stating that the first test, even in a stable female patient with a normal ECG who is able to exercise should be a plain old treadmill test. I'll make a couple of points; one, we have in press a paper on how to improve the positive predictive value of the exercise ECG in women. The more risk factors the patient has, the deeper the ST depression, the longer the persistence of ST depression post-exercise—these factors can increase the positive predictive value up to about 80% in women. These ECG factors and non-ECG factors can help. We have also shown that if the patient, man or woman, has a positive treadmill ECG and can exercise to 10 metabolic equivalents (METs) (Stage III of the Bruce test),9,10 and then is referred for a stress imaging test, about a 94% likelihood that the stress imaging test will be negative.
So the key point for me is that assessment of the exercise test by ECG criteria alone is really obsolete. The poor positive predictive value and the high rate of false-positives are based solely on the ST segment when we know that the best prognostic indicator is functional capacity. So that's why we always do the treadmill test as the initial assessment unless there's a baseline abnormality or the patient can't exercise.
Do you have any comments on any of that, Jeff?
Dr. Borer: I would like to pick up on two things that Kristin said. She talked about symptoms, and I would say that if a person, and it doesn't matter whether it's a man or a woman, has typical symptoms, when you take a history and you put that person on a treadmill and you reproduce the symptoms and you do an ECG alone, the concordance of the ECG criteria and the symptoms has a very high predictive value. But that presumes that the person is going to get symptoms on the treadmill, and that may not happen.
The other point that Kristin made was that in certain patients with a compelling clinical presentation, she might go right to a catheterization. I would have to say that I don't do that. I guess it depends on how you define “compelling.” If somebody has a history that is consistent with crescendo angina, yes, I would send him or her to catheterization. But absent that, I would not because I believe that the noninvasive testing provides prognostic information even in the presence of true coronary disease.
The problem that I fear in sending someone to catheterization is that, in the absence of prognostically important disease, they'll come away with an angioplasty with no evidence at all that anything will be done to their natural history, only that their symptoms will be relieved and that could probably have been done with medication. So I'm a little more wary about sending people directly to catheterization. There really has to be what I would call a “compelling” history, and it may be the same that Kristin would call “compelling” or it may not.
Dr. Amsterdam: We’re into the art of medicine, and it’s legitimate to differ if we have good reasons to go down different paths. Kristin, your comments on this?
Dr. Newby: When I'm thinking of somebody whose symptoms are compelling enough that I would send them to catheterization, it's the woman who was active, doing her gardening, mowing her yard, whatever last summer, and has over the past several months progressively deteriorated in terms of activity levels. So you already know their functional capacity has declined by what they're telling you through their history. In those individuals, I don't think you gain a lot more information by documenting that on a treadmill.
You're right, maybe you take that person and you try medication or maybe you commit that they have coronary disease, you're going to take care of their angina, get them immediately back to functional capacity. I think those are style points, as you said, the art of medicine. To some extent it's driven by patient preferences and I think that's the thing that sometimes we also forget.
There are patients who, once they know what you're thinking, don't want to deal with multiple steps to get to an answer—they want to know. Maybe that's right, maybe that's wrong, maybe that's part of what's wrong with our healthcare system. We could debate those points for another hour probably, but I think those factors all play into how we manage patients.
Then you think about taking somebody when you're pretty sure you know what the diagnosis is, or maybe you're pretty sure you don't think it's the diagnosis, but you put them on a treadmill or a treadmill with imaging and it's positive, and now you've got them worried. They want to know what's going on, and they may or not may not tolerate medicines.
These are some practical issues in medicine, as well, that we have to think about, and again, as you said, that's the art of medicine.
Dr. Amsterdam: Kristin, can you tell us a little about the Duke treadmill score? Because it's a very good prognostic indicator.
Dr. Newby: Absolutely, and that's very much like the study that you described of Dr. Beller’s; if they could do 10 METs they did well. We look at our treadmill information, often we get it in conjunction with an echocardiogram, but we don't ignore the key physiologic information and functional information from the treadmill for that very reason; there's a high correlation between either METs done or various scores. The Duke treadmill score being one type of score that one can use to estimate risk of dying in the next 5 to 10 years. So it is critically important that we don't ignore the wealth of information that can come from a treadmill test.
Dr. Amsterdam: Jeff, you mentioned chest pain and it reminded me of something important. One of the values of stress testing is whether we can reproduce symptoms that the patient complains of. I notice in our chest pain unit that patients are admitted because of chest pain and they have a normal resting ECG and negative troponins. Our first test in these stable patients is, again, a treadmill test, symptom-limited. They do more activity on that stress test than they do in life but it's very rare that we reproduce chest pain. Even the patients with coronary disease who go to catheterization because of a very positive stress test infrequently have chest pain during treadmill testing. Does your experience differ?
Dr. Borer: No, my experience doesn't differ. When people come out of our chest pain unit they have an imaging study, they don't have a straight treadmill exercise ECG, but that's irrelevant. One thing that is perhaps not irrelevant is that in New York State, in the absence of unstable angina, you now cannot send a patient to catheterization unless you have demonstrated a positive exercise test.
If they have unstable symptoms certainly you can catheterize them, but in a patient who has relatively stable symptomatology, you can't catheterize (and expect to be paid for the procedure) unless you have evidence of ischemia by a noninvasive test. Those are called “appropriate use” criteria that have been set up here in New York, and if you don't follow them, the insurance companies don't pay. So there is sort of a brake here that may not be true in other states.
Dr. Amsterdam: Kristin, do you have such a proscription in North Carolina? What about your experience of chest pain on the treadmill?
Dr. Newby: First of all, we don't have such a proscription here in North Carolina, although I think we’re going to see more and more of that. New York State has historically been a leader in that kind of thinking through and monitoring and implementing criteria like these, and I don't think it's all a bad thing.
In our chest pain unit or in referrals from clinic, I agree that most of the time we don't reproduce symptoms on the treadmill and I find it very reassuring if we do reproduce symptoms and we don't see anything. Again we’re doing imaging, but imaging in conjunction with the ECG, I think, is actually helpful information to have, but in the majority of cases we don't reproduce the symptoms.
Dr. Amsterdam: It’s a really interesting phenomenon, and as Jeff has said, the symptoms on the treadmill are very important.
Let's make a couple of comments on heart failure. There are data that women present more typically with heart failure as an indication of underlying coronary disease and also have more diastolic dysfunction than men in terms of the underlying cause of heart failure. Your experience with this, Jeff?
Dr. Borer: I think that's true but, in fact, the truth also is that most heart failure trials include relatively few women. It was the same point you made earlier. I'm one of the principal investigators of the “Systolic Heart failure treatment with the If inhibitor ivabradine Trial” (SHIFT), the largest heart failure trial that was ever done, which used a heart rate–slowing strategy for therapy. We had a marked paucity of women compared with men, so it's hard to draw conclusions.11 I don't know how many women were excluded because they had diastolic dysfunction. It certainly is my perception and my bias that among those with heart failure, women commonly do have diastolic dysfunction, probably more commonly than men, but I think we lack a lot of information about heart failure.
Let me talk about the area on which I focus most closely, which is valve disease. The manifestations of valve disease are different in women than in men. That was demonstrated most clearly in the Placement of Aortic Transcatheter Valve Trial (PARTNER),12 which was the first randomized trial of any mechanical therapy for valve disease in patients with aortic stenosis. In fact, approximately 50% of the population in PARTNER comprised women. The average age was the mid-80s, and by that age there are a lot more women around than men. So perhaps that had something to do with the fact that many of those who entered the trial were women.
The extraordinary finding was that women did better with percutaneous valve therapy than men. Not only did they do better than men, the relationship of percutaneous therapy to conventional surgery was better in women than in men. We could speculate on why that is (smaller arteries, harder to do, etc.), and maybe we’d be correct. However, the finding does suggest that there is a difference, at least for that valve disease, in women compared to men.
Another piece of evidence is the affects of treadmill testing in patients with aortic stenosis. Asymptomatic patients with aortic stenosis are marked out as being at relatively high risk for the imminent development of symptoms or worse based on treadmill studies. One of the criteria that seems to separate those at high risk is ST-segment depression, but it’s 1 mm in men and 2 mm in women. We see the valve dysfunction more commonly in women than in men and the manifestations of the disease differ.
Dr. Amsterdam: These are fascinating data. We're doing an investigation in aortic stenosis and the overwhelming number of patients are men, but, in general, I agree with your point that because we’re seeing patients with critical aortic stenosis who are now in their 80s, women comprise a very large component of that group.
Kristin, can you comment on heart failure and whether there is more diastolic dysfunction, more hypertension in women, and so relative to their body size they have more left ventricular hypertrophy?
Dr. Newby: Yes I think that diastolic dysfunction is more common and more severe with aging. There are also the effects of hypertension. Both of those obviously are issues with older women. I do share Jeff's concern not just related to diastolic dysfunction, but related to any heart failure, of the underrepresentation of women in our studies that are designed to help us understand how to manage heart failure from any cause.
Dr. Amsterdam: Jeff; do you remember the age cutoff in the SHIFT trial?
Dr. Borer: There was no upper age limit. However, the average age was 60 years and 11% were at least 75 years old.
Dr. Newby: And if diastolic dysfunction is more prevalent in the older population than systolic dysfunction, the age of the patients will be a big factor in the etiology of the heart failure.
Dr. Amsterdam: In the past decade there has been a proliferation of publications on women with normal epicardial coronary arteries in whom myocardial ischemia and its complications are caused by (or associated with) coronary microvascular dysfunction (which is also presumed to be the underlying cause of the elusive Syndrome X). What are your comments on this issue?
Dr. Borer: Regarding microvascular dysfunction and Syndrome X, it has been my impression that some people, more often women than men, present with atypical chest pain and even typical angina pectoris in the absence of large-vessel coronary artery disease or any other definable cause, but that the problem of demonstrable microvascular dysfunction in these settings is relatively uncommon. Patients who present in this way may benefit from certain types of vasodilating drugs with relief of symptoms. However, I believe the outcomes for these people are substantially more benign than for those with large coronary artery obstruction. In other words, frank MI or death are relatively infrequently associated with this syndrome. Moreover, although it is plausible to associate such a clinical picture with endothelial dysfunction and small-vessel (arteriolar) hyperreactivity, I think the pathophysiology of the syndrome is not clear. Thus, while Syndrome X may contribute to debility and activity limitation particularly in women, I do not think the syndrome is very clearly understood and do not think it is an important cause of premature death or major morbidity.
Dr. Amsterdam: We're going to have concluding comments from each of you. So briefly, Jeff, are there any comments you'd like to make?
Dr. Borer: I would summarize that the situation with regard to sensitivity about heart disease in women has improved considerably over the past decade or two, but we still have a long way to go to understand the differences between women and men in terms of the manifestations of heart disease and how to manage them.
Dr. Amsterdam: And Kristin.
Dr. Newby: I completely agree with Jeff's summary. The only thing I would add is that of the therapies that we have available, they are all equally effective in men and women, and I think we just need to focus right now as we're trying to understand the underlying differences in pathophysiology and symptoms, among other things, on applying these therapies as appropriately as we can across both sexes.
Dr. Amsterdam: Thank you.
FoxP2 Media LLC is the publisher of The Medical Roundtable.
We're going to discuss an issue that has been in the headlines for more than a decade: heart disease in women. We’ll discuss what we know about it, where we've been in terms of problems that have been elucidated, and how we've addressed those problems. We all know that heart disease is the chief cause of mortality in women over their lifetime. Heart disease is actually the second highest cause of mortality in women until the age of 75; it is second to cancer until that age. But it is so great after the age of 75 that when averaged over the lifetime, heart disease becomes the chief cause of mortality in women. I think that may provide some perspective. Heart disease, particularly coronary heart disease, which is the major cause of mortality in women among the cardiovascular diseases, rises sharply after menopause. Coronary heart disease is unusual in women prior to menopause, unless there are outstanding risk factors such as familial hypercholesterolemia or diabetes. Finally, the risk of heart disease in women is always less than men throughout the lifetime.
Kristin, you authored the chapter with Pamela Douglas on heart disease in women in the latest edition of the Braunwald text on heart disease.1 Can you tell us about the situation as you see it now in terms of what we recognize as previous underdiagnosis and undertreatment of heart disease in women with a focus on coronary heart disease.
Dr. Newby: Thank you. I think that's an important question. We can look at a number of sources and I think most of them suggest that we have underappreciated the risk of heart disease in women. In part that's because women develop coronary artery disease later in life or the consequences of coronary artery disease are manifest later in life. I think every signal tells us that we are doing better, including physicians’ perception of risk and treatment. There was a recent study by Lori Mosca published maybe a couple of years ago that shows that women are increasingly aware of heart disease as a major risk factor.2 That said, however, only about half of women think that heart disease is their leading lifetime risk; many still believe it is cancer. So it's about 50/50 now, where it was about 30/70 maybe 5 to 10 years ago. We're making progress, and I think the Red Dress3 campaign and other awareness campaigns have been effective.
We still see gaps in treatment; use of evidence-based therapy in women is lower than it is in men, although it has improved. I think importantly on the side of clinical trials and testing new therapies we're seeing a better balance. But there is still underrepresentation of women in clinical trials that establish evidence for treatment.
Dr. Amsterdam: Your last point is key, and leads me to note that an age cutoff of 75 for enrollment in many clinical trials may be partly responsible for female under-representation, because it’s after 75 that cardiac disease really proliferates in women.
Jeff, what are your views on how much progress we’ve made?
Dr. Borer: Of course, I agree with everything Kristin said. I would emphasize something that we don't talk about so often, and that is that when women do manifest evidence of coronary disease, and particularly when they have a myocardial infarction (MI), they’re about twice as likely as men to die within a year of that first infarction. If only we were more attentive we would do something to modulate that bad outcome. Women are much less likely to get the needed drugs such as beta-blockers, angiotensin-converting enzyme inhibitors, aspirin, and other therapies known to improve survival after MI than are men. If we were more aggressive about that, maybe the outcome would be better. However, the problem is that men who have prognostically important coronary disease, which may be heralded by a first infarction, actually are best treated, or I should say their outcome is best improved, if they undergo coronary artery bypass grafting. One might think, therefore, that this strategy should then be applied to women. However, women are much more likely to die during a bypass grafting procedure than men.
Therefore, the appropriate therapy for women to reduce this extraordinarily higher risk after MI is not clear. I don't think we’re as good at giving medicines as we ought to be, but going the next step and using invasive therapy, that’s something else again. It’s not clear whether women benefit sufficiently to warrant that approach, and that's something we have to think about. We need a great deal more data on that subject.
Dr. Amsterdam: I would add a couple of short points. Kristin indicated that things have gotten better. In fact, in the past 10 years there's been a sharp decrease in the rate of death from cardiovascular disease in women. If you look at the curve of the decrease in mortality from heart disease for the past 50 years in this country, there is a steady downtrend. But when we break up the data, the curve for women was pretty flat, indicating that they did not share in this benefit until recently. It was mostly men until the past decade, when we saw a sharp drop in mortality in women. The death rate in women has fallen almost to that of men. This is attributable to programs such as the Red Dress campaign, as Kristin mentioned, and the “Get with the Guidelines” campaign. When women and men are risk-adjusted after an MI, the outlook is similar—women are older and have more comorbidities when they have their MI. So the question is, is there something intrinsic that puts women at higher risk, or are they just older, sicker, and with more comorbidities, which leads to a different outcome? Jeff?
Dr. Borer: I think it's a good point and I don't really know the answer, but the fact is that the disease is different in women than in men, whether that accounts for some of the difference in outcomes that we see or not. Typically, atherosclerosis in the coronary artery is much more diffuse in women than it is in men, so that angiographic findings often suggest narrow normal arteries when in fact they’re loaded with plaque. In contrast, men’s obstructions tend to be relatively discrete. That difference in the manifestations of atherosclerosis in the coronary arteries in women and in men may well have an important impact on outcome.So while it may be that risk adjustment makes things look more similar among men and women, the fact is the disease is different.
Dr. Amsterdam: Important point. Kristin, would you like to add something on that topic?
Dr. Newby: I think the points that you both have made are good in that looking at risk-adjusted outcomes is important and it gives us an idea of one group relative to another. Unfortunately, sometimes that masks the issue, which is that women are dying at a pretty high rate. Whether it's because they're older or not, it suggests that we've got a high-risk population in which we need to figure out what is different in the way they respond to therapy or whether there are other interventions that could be applied that might improve outcome.
Risk adjustment is important, but I don't think it is the whole answer and it doesn't do away with the challenge of the high event rates in women.
Dr. Borer: I agree with that and if you just look at patients younger than 50, just under age 50, a MI in a woman is twice as likely to be fatal as it is in a man.4
Dr. Amsterdam: On the other hand, of women under 35 who come to the emergency department (ED) with chest pain, only about 1 in 1000 turn out to have an MI. So there's a high frequency of chest pain in women and they generally tend to use the ED and medical system more than men for symptoms. This may be because there’s less denial in women, but there are lots of negative tests in women for symptoms like chest pain or chest discomfort. Would you agree with that, Kristin?
Dr. Newby: It is true. I think there are a number of other features, unfortunately, that may lead, perhaps not in young women but in older women, to missed or delayed diagnosis that may also contribute to their worse prognosis. That may explain some of the difference as well. I would just throw that out for discussion.
Dr. Amsterdam: A lot of the data that we’re talking about in underdiagnosis and undertreatment come from papers, many of which are 10 years and 20 years old. In your own institutions, are you seeing a persistence of these issues, because I'm not aware of any recent studies indicating that there's been an improvement in these trends, other than that we know that cardiovascular disease leading to mortality is decreasing in women.
In your own institutions, do you see a persistence of the woman who sits in the ED with chest pain and doesn't get an electrocardiogram (ECG) within 10 minutes, or a good workup, or a referral from a clinic or office for chest pain? Jeff, what’s your experience?
Dr. Borer: The issue, I suppose, is, what does the cardiologist do? I think over the past decade or more, maybe 2 decades, there's been a heightened sensitivity among cardiologists of the fact that women do in fact have atherosclerosis, they do have coronary disease, they do have MIs, and they do die from it. But I think the problem is a little bit different.
It's not so much now, I think, allowing a woman to sit in the ED with chest pain for too long. I don't think that happens terribly often, at least not from what I've seen. It's that the symptom pattern is different in women than in men, and while a lot of women have chest discomfort and often the chest discomfort isn't associated with coronary disease, a lot of women have coronary disease with symptoms that don't include chest discomfort. The difficulty in the sensitivity to that possibility is something that we still have to improve.
Dr. Amsterdam: Kristin, Jeff raised an important point about the difference of symptoms in women and men when they present with coronary disease. We know that symptoms in the elderly are different than those who are younger when presenting with ischemic heart disease. So is it that the symptoms are different in women per se, or is it that they comprise a large part of the elderly population with coronary artery disease, and if you compare elderly men with elderly women, would there be a difference in symptom presentation?
Dr. Newby: That's a good question. You know one of the better studies that's been done on this actually revealed that the majority of patients who were having an acute MI presented with some type of chest-related symptoms, such as tightness and pressure.1
My personal experience, and again I don't think this has been well studied specifically in the elderly, is that the issue with atypical symptoms is in part a phenomenon of age, where the elderly tend to present more frequently with just dyspnea or fatigue. One really has to be paying attention to that. That disproportionately affects women who present later in life with acute ischemic syndromes.
I want to move to the risk factors for coronary disease in men and women. Jeff, any differences or similarities—I will tell you my view right off the bat that the risk factors for coronary artery disease other than pregnancy and menopause, which act through the traditional risk factors, are the same in men and women. There are some differences, and in her chapter in Braunwald, Kristin pointed them out nicely with regard to diabetes from the INTERHEART study.5 What's your view on the risk factors in women? Do we need preventive cardiology clinics for women and for women with heart disease; or can women come to a good preventive cardiology clinic that men also attend?
Dr. Borer: I think the risk factors are pretty much the same. You know there may be some difference in outcomes associated with them, depending on age, with diabetes being the major issue. I think it has more of an impact on the risk of second MI in women than in men, so you have to be more cognizant of that particular risk factor in women.
But I think the risk factors are the same and the outcomes are qualitatively the same and so managing patients by trying to modulate risk factors could be done in a preventive cardiology unit. I don't think you need a separate one for women because I don't think we have sufficient data to suggest a different strategy for men and for women.
Dr. Amsterdam: Kristin?
Dr. Newby: I fully agree. I think that the risk factors, with the possible exception that diabetes may be a bit more potent in women, are still the same risk factors and we know the same treatments work in both groups. What I think is probably more interesting that came out of INTERHEART is the differential strength (stronger in women) of protective factors in women, such as exercise, eating a diet high in fruits and vegetables, and stress management. I found that to be interesting and potentially more informative than focusing on the proven risk factors.
Dr. Amsterdam: I think INTERHEART was a very good confirmation of what the Framingham Heart Study showed of the traditional risk factors.6
Would you agree, Kristin, that there is more stress in general, more psychosocial stress, in women than in men? Women work and take care of the kids—men do better now, but we don't do an equal share, and that seems to be having an impact on women and disease.
Dr. Newby: Yes, I think that's an interesting topic, and I think women's response to stress and the stress that they're dealing with may be different from that of men. I think there’s an interesting analysis that’s been done several times about support systems and how men do better when they have a spouse who’s taking care of them. Women on the other hand who have an MI who have a spouse actually do worse. So I'm not sure what that tells us, but I do think there's either some disproportionate amount of reaction to or ability to cope with stress among women. That is important for us to understand and work through.
Dr. Amsterdam: It's too bad we can't measure that the way we measure other data. Now let’s consider a stable patient who comes to the physician’s office with chest pain; it may be typical or atypical, but it's suspicious enough for the patient to require a careful evaluation.
Jeff, which tests do you use to detect objective evidence of myocardial ischemia and, by that factor, the likelihood of coronary disease? Exercise treadmill test has really been criticized for its value in women because of its repute for false-positive results. Some experts have advised going straight to a stress imaging study rather than a plain treadmill test.
Dr. Borer: Yes, well that's what I do, actually. The fact is that the specificity of every noninvasive test for coronary disease is lower in women than in men. The positive predictive value is less in women than in men, whether it be a radionuclide test, an echocardiography-based test, it's not just the exercise ECG. On the other hand, it seems to me that radionuclide based studies do provide greater accuracy in women than the exercise ECG, and that's what I use.
Dr. Amsterdam: This is good because thus far we’ve had little divergence of opinion and it’s good to have some differences. Thus, I am a strong proponent of the standard treadmill exercise test without imaging if the patient has a normal baseline ECG and the history indicates adequate exercise capacity, which is what the guidelines have advocated since 1998. So in these circumstances, my first test is always a plain treadmill study, and we train our fellows to follow this approach. Kristin, your method?
Dr. Newby: Yes, I think I fall a little bit more to Jeff's side of things. So there are two things I think about: one is if I see someone in the clinic who gives me a very good story for symptoms and they can't exercise and can’t do their usual activities. I either move to treat them with medicines and cardiac rehabilitation or with catheterization if I have a high index of suspicion that I'm dealing with coronary disease. So I think that's the first thing. Then the question is, how do we think about the individuals who aren't clear-cut? We want to rule out the group that has the highest likelihood to have a false positive—the ones with very atypical symptoms, which I know we all see in our clinics—and try to work with them without doing any kind of further workup if at all possible. But if I do a stress test in the clinic and I'm on the fence, I, like Jeff, add imaging.7 We tend to use more stress echocardiography than stress nuclear testing, but I don't think there's any evidence that one or the other, if you're adding an imaging study, is of a major advantage given the certain limitations of each study in terms of acquiring the images.
Dr. Amsterdam: I agree. They're very similar. Perhaps nuclear is a little more sensitive but less specific than echocardiography.
So I will say quickly, I think we all know the guidelines of the American Heart Association/American College of Cardiology (AHA/ACC)8—that I just referred to. They’re very clear in stating that the first test, even in a stable female patient with a normal ECG who is able to exercise should be a plain old treadmill test. I'll make a couple of points; one, we have in press a paper on how to improve the positive predictive value of the exercise ECG in women. The more risk factors the patient has, the deeper the ST depression, the longer the persistence of ST depression post-exercise—these factors can increase the positive predictive value up to about 80% in women. These ECG factors and non-ECG factors can help. We have also shown that if the patient, man or woman, has a positive treadmill ECG and can exercise to 10 metabolic equivalents (METs) (Stage III of the Bruce test),9,10 and then is referred for a stress imaging test, about a 94% likelihood that the stress imaging test will be negative.
So the key point for me is that assessment of the exercise test by ECG criteria alone is really obsolete. The poor positive predictive value and the high rate of false-positives are based solely on the ST segment when we know that the best prognostic indicator is functional capacity. So that's why we always do the treadmill test as the initial assessment unless there's a baseline abnormality or the patient can't exercise.
Do you have any comments on any of that, Jeff?
Dr. Borer: I would like to pick up on two things that Kristin said. She talked about symptoms, and I would say that if a person, and it doesn't matter whether it's a man or a woman, has typical symptoms, when you take a history and you put that person on a treadmill and you reproduce the symptoms and you do an ECG alone, the concordance of the ECG criteria and the symptoms has a very high predictive value. But that presumes that the person is going to get symptoms on the treadmill, and that may not happen.
The other point that Kristin made was that in certain patients with a compelling clinical presentation, she might go right to a catheterization. I would have to say that I don't do that. I guess it depends on how you define “compelling.” If somebody has a history that is consistent with crescendo angina, yes, I would send him or her to catheterization. But absent that, I would not because I believe that the noninvasive testing provides prognostic information even in the presence of true coronary disease.
The problem that I fear in sending someone to catheterization is that, in the absence of prognostically important disease, they'll come away with an angioplasty with no evidence at all that anything will be done to their natural history, only that their symptoms will be relieved and that could probably have been done with medication. So I'm a little more wary about sending people directly to catheterization. There really has to be what I would call a “compelling” history, and it may be the same that Kristin would call “compelling” or it may not.
Dr. Amsterdam: We’re into the art of medicine, and it’s legitimate to differ if we have good reasons to go down different paths. Kristin, your comments on this?
Dr. Newby: When I'm thinking of somebody whose symptoms are compelling enough that I would send them to catheterization, it's the woman who was active, doing her gardening, mowing her yard, whatever last summer, and has over the past several months progressively deteriorated in terms of activity levels. So you already know their functional capacity has declined by what they're telling you through their history. In those individuals, I don't think you gain a lot more information by documenting that on a treadmill.
You're right, maybe you take that person and you try medication or maybe you commit that they have coronary disease, you're going to take care of their angina, get them immediately back to functional capacity. I think those are style points, as you said, the art of medicine. To some extent it's driven by patient preferences and I think that's the thing that sometimes we also forget.
There are patients who, once they know what you're thinking, don't want to deal with multiple steps to get to an answer—they want to know. Maybe that's right, maybe that's wrong, maybe that's part of what's wrong with our healthcare system. We could debate those points for another hour probably, but I think those factors all play into how we manage patients.
Then you think about taking somebody when you're pretty sure you know what the diagnosis is, or maybe you're pretty sure you don't think it's the diagnosis, but you put them on a treadmill or a treadmill with imaging and it's positive, and now you've got them worried. They want to know what's going on, and they may or not may not tolerate medicines.
These are some practical issues in medicine, as well, that we have to think about, and again, as you said, that's the art of medicine.
Dr. Amsterdam: Kristin, can you tell us a little about the Duke treadmill score? Because it's a very good prognostic indicator.
Dr. Newby: Absolutely, and that's very much like the study that you described of Dr. Beller’s; if they could do 10 METs they did well. We look at our treadmill information, often we get it in conjunction with an echocardiogram, but we don't ignore the key physiologic information and functional information from the treadmill for that very reason; there's a high correlation between either METs done or various scores. The Duke treadmill score being one type of score that one can use to estimate risk of dying in the next 5 to 10 years. So it is critically important that we don't ignore the wealth of information that can come from a treadmill test.
Dr. Amsterdam: Jeff, you mentioned chest pain and it reminded me of something important. One of the values of stress testing is whether we can reproduce symptoms that the patient complains of. I notice in our chest pain unit that patients are admitted because of chest pain and they have a normal resting ECG and negative troponins. Our first test in these stable patients is, again, a treadmill test, symptom-limited. They do more activity on that stress test than they do in life but it's very rare that we reproduce chest pain. Even the patients with coronary disease who go to catheterization because of a very positive stress test infrequently have chest pain during treadmill testing. Does your experience differ?
Dr. Borer: No, my experience doesn't differ. When people come out of our chest pain unit they have an imaging study, they don't have a straight treadmill exercise ECG, but that's irrelevant. One thing that is perhaps not irrelevant is that in New York State, in the absence of unstable angina, you now cannot send a patient to catheterization unless you have demonstrated a positive exercise test.
If they have unstable symptoms certainly you can catheterize them, but in a patient who has relatively stable symptomatology, you can't catheterize (and expect to be paid for the procedure) unless you have evidence of ischemia by a noninvasive test. Those are called “appropriate use” criteria that have been set up here in New York, and if you don't follow them, the insurance companies don't pay. So there is sort of a brake here that may not be true in other states.
Dr. Amsterdam: Kristin, do you have such a proscription in North Carolina? What about your experience of chest pain on the treadmill?
Dr. Newby: First of all, we don't have such a proscription here in North Carolina, although I think we’re going to see more and more of that. New York State has historically been a leader in that kind of thinking through and monitoring and implementing criteria like these, and I don't think it's all a bad thing.
In our chest pain unit or in referrals from clinic, I agree that most of the time we don't reproduce symptoms on the treadmill and I find it very reassuring if we do reproduce symptoms and we don't see anything. Again we’re doing imaging, but imaging in conjunction with the ECG, I think, is actually helpful information to have, but in the majority of cases we don't reproduce the symptoms.
Dr. Amsterdam: It’s a really interesting phenomenon, and as Jeff has said, the symptoms on the treadmill are very important.
Let's make a couple of comments on heart failure. There are data that women present more typically with heart failure as an indication of underlying coronary disease and also have more diastolic dysfunction than men in terms of the underlying cause of heart failure. Your experience with this, Jeff?
Dr. Borer: I think that's true but, in fact, the truth also is that most heart failure trials include relatively few women. It was the same point you made earlier. I'm one of the principal investigators of the “Systolic Heart failure treatment with the If inhibitor ivabradine Trial” (SHIFT), the largest heart failure trial that was ever done, which used a heart rate–slowing strategy for therapy. We had a marked paucity of women compared with men, so it's hard to draw conclusions.11 I don't know how many women were excluded because they had diastolic dysfunction. It certainly is my perception and my bias that among those with heart failure, women commonly do have diastolic dysfunction, probably more commonly than men, but I think we lack a lot of information about heart failure.
Let me talk about the area on which I focus most closely, which is valve disease. The manifestations of valve disease are different in women than in men. That was demonstrated most clearly in the Placement of Aortic Transcatheter Valve Trial (PARTNER),12 which was the first randomized trial of any mechanical therapy for valve disease in patients with aortic stenosis. In fact, approximately 50% of the population in PARTNER comprised women. The average age was the mid-80s, and by that age there are a lot more women around than men. So perhaps that had something to do with the fact that many of those who entered the trial were women.
The extraordinary finding was that women did better with percutaneous valve therapy than men. Not only did they do better than men, the relationship of percutaneous therapy to conventional surgery was better in women than in men. We could speculate on why that is (smaller arteries, harder to do, etc.), and maybe we’d be correct. However, the finding does suggest that there is a difference, at least for that valve disease, in women compared to men.
Another piece of evidence is the affects of treadmill testing in patients with aortic stenosis. Asymptomatic patients with aortic stenosis are marked out as being at relatively high risk for the imminent development of symptoms or worse based on treadmill studies. One of the criteria that seems to separate those at high risk is ST-segment depression, but it’s 1 mm in men and 2 mm in women. We see the valve dysfunction more commonly in women than in men and the manifestations of the disease differ.
Dr. Amsterdam: These are fascinating data. We're doing an investigation in aortic stenosis and the overwhelming number of patients are men, but, in general, I agree with your point that because we’re seeing patients with critical aortic stenosis who are now in their 80s, women comprise a very large component of that group.
Kristin, can you comment on heart failure and whether there is more diastolic dysfunction, more hypertension in women, and so relative to their body size they have more left ventricular hypertrophy?
Dr. Newby: Yes I think that diastolic dysfunction is more common and more severe with aging. There are also the effects of hypertension. Both of those obviously are issues with older women. I do share Jeff's concern not just related to diastolic dysfunction, but related to any heart failure, of the underrepresentation of women in our studies that are designed to help us understand how to manage heart failure from any cause.
Dr. Amsterdam: Jeff; do you remember the age cutoff in the SHIFT trial?
Dr. Borer: There was no upper age limit. However, the average age was 60 years and 11% were at least 75 years old.
Dr. Newby: And if diastolic dysfunction is more prevalent in the older population than systolic dysfunction, the age of the patients will be a big factor in the etiology of the heart failure.
Dr. Amsterdam: In the past decade there has been a proliferation of publications on women with normal epicardial coronary arteries in whom myocardial ischemia and its complications are caused by (or associated with) coronary microvascular dysfunction (which is also presumed to be the underlying cause of the elusive Syndrome X). What are your comments on this issue?
Dr. Borer: Regarding microvascular dysfunction and Syndrome X, it has been my impression that some people, more often women than men, present with atypical chest pain and even typical angina pectoris in the absence of large-vessel coronary artery disease or any other definable cause, but that the problem of demonstrable microvascular dysfunction in these settings is relatively uncommon. Patients who present in this way may benefit from certain types of vasodilating drugs with relief of symptoms. However, I believe the outcomes for these people are substantially more benign than for those with large coronary artery obstruction. In other words, frank MI or death are relatively infrequently associated with this syndrome. Moreover, although it is plausible to associate such a clinical picture with endothelial dysfunction and small-vessel (arteriolar) hyperreactivity, I think the pathophysiology of the syndrome is not clear. Thus, while Syndrome X may contribute to debility and activity limitation particularly in women, I do not think the syndrome is very clearly understood and do not think it is an important cause of premature death or major morbidity.
Dr. Amsterdam: We're going to have concluding comments from each of you. So briefly, Jeff, are there any comments you'd like to make?
Dr. Borer: I would summarize that the situation with regard to sensitivity about heart disease in women has improved considerably over the past decade or two, but we still have a long way to go to understand the differences between women and men in terms of the manifestations of heart disease and how to manage them.
Dr. Amsterdam: And Kristin.
Dr. Newby: I completely agree with Jeff's summary. The only thing I would add is that of the therapies that we have available, they are all equally effective in men and women, and I think we just need to focus right now as we're trying to understand the underlying differences in pathophysiology and symptoms, among other things, on applying these therapies as appropriately as we can across both sexes.
Dr. Amsterdam: Thank you.
FoxP2 Media LLC is the publisher of The Medical Roundtable.
FoxP2 Media LLC
Disoriented
It’s late August, and finally I feel ready for summer. You don’t need to remind me that I’m late: Our five children have no fewer than eight school orientations this week. The extras are because new high schoolers need two sessions, one for their parents and another just for students, where they tell them all the really important stuff, which the kids then forget until second semester when it’s too late. Can I go to the beach now?
Of course the orientations are mainly just a chance for teachers to pass out supply lists, which, with severe budget cuts in our state, now include items like, “one bottle of hand sanitizer, five dry-erase markers in various colors, one qualified teaching assistant.” Also, I know why my 4th-grader needs to start the year with 24 #2 pencils: He chews them. But do all the kids have an oral fixation? Or is it that when my son has polished off his small forest of Ticonderogas, his classmates are expected to lend him theirs? I guess I’ll ask at orientation.
Hand-me-downs
Here’s my question: If we saved all the money parents are about to spend on classroom hand sanitizer, could we actually afford a teaching assistant? Or at least a few more dry-erase markers? Because a study from New Zealand suggests that as long as soap and water are available, hand sanitizer does nothing but to give schoolchildren another excuse to get up out of their seats.
Patricia Priest and her colleagues from the University of Otego in Dunedin (I, too think it sounds made up) studied school absences among 2,443 students aged 5 to 11 in 68 schools. They performed the experiment during the winter (read “summer”), placing alcohol-based hand sanitizers in half the schools and relying on soap, water, and Kiwi common sense in the other half. Then they sat back and counted the absences, going as far as to call parents and force them to invent diseases for children who were actually playing hooky to quest after the One Ring to Rule Them All.
When the investigators tallied everything up, hand sanitizer appeared to make no difference in the number of absences from all illnesses or from any specific illness (respiratory or GI). Also unaffected were the length of illness, the length of the absence, or the likelihood of another family member contracting the illness. (That last endpoint confuses me, unless the kids were taking hand sanitizer home every night.) What this study proved was that whether or not children in New Zealand have access to hand sanitizer, they will still all be mistaken for Australians.
I think we’re alone now
If you can just spend a little one-on-one time with teenagers, they’ll reveal the most amazing things about their lives. That’s why I have five children: to make sure that kind of thing never happens. But in the pediatrician’s office, it’s a good thing. We need to know what’s going on in adolescents’ lives in order to help them avoid the dangers of substance abuse, high-risk sexual behavior, and whatever boneheaded dare has been going around YouTube this week. A new study out of Indianapolis confirms what common sense already tells us: If you don’t talk with teens’ parents out of the exam room for a few minutes, you miss the good stuff, (read “the bad stuff”).
To prove it, the researchers surveyed around 500 adolescents (ages 13-17 years) and their parents regarding conversations they had with providers during their wellness exams. The best single statistic from their study was this: 89% of parents believed adolescents should be able to talk with their doctors privately, while 61% of parents preferred to stay in the exam room for the entire visit. That means that 50% of parents misunderstand one of the following terms: adolescent, private, or exam room.
Kids who did get to talk to their doctors alone discussed almost twice as many sensitive topics as did kids whose parents policed their conversations. On the up side, when parents refused to leave, kids were more likely to say “ma’am” and “sir,” to sit up straight for once please, and to brush that hair out of their eyes so the doctor can tell you’re looking at them, honey, thank you.
Call me maybe
Speaking of counseling teens and parents, do you sometimes think we’re talking with the wrong person? The National Institutes of Health just completed a study of distracted driving, a cause of 11% of fatal crashes among adolescents. Since 21% of those crashes involve mobile phone use, researchers asked 400 15- to 18-year-old drivers who in the heck they were talking to on the phone that made it worth risking their lives. Of the 86% of teens who reported talking on the phone while driving, 100% of them said the people they were talking to were their parents.
Fellow parents of teens, this does not make us look good. I like chatting with my kids, too, but it’s easier to converse with them when they’re alive, or at least not in a coma (if you can’t tell whether or not your adolescent is in a coma, have him brush that hair out of his eyes). We’ve got to do better, y’all; no one really wants to miss out on next year’s school orientation.
David L. Hill, M.D., FAAP, is the author of Dad to Dad: Parenting Like a Pro (AAP Publishing, 2012). He is also vice president of Cape Fear Pediatrics in Wilmington, N.C., and adjunct assistant professor of pediatrics at the University of North Carolina at Chapel Hill. He serves as Program Director for the AAP Council on Communications and Media and as an executive committee member of the North Carolina Pediatric Society. He has recorded commentaries for NPR's All Things Considered and provided content for various print, television, and Internet outlets.
It’s late August, and finally I feel ready for summer. You don’t need to remind me that I’m late: Our five children have no fewer than eight school orientations this week. The extras are because new high schoolers need two sessions, one for their parents and another just for students, where they tell them all the really important stuff, which the kids then forget until second semester when it’s too late. Can I go to the beach now?
Of course the orientations are mainly just a chance for teachers to pass out supply lists, which, with severe budget cuts in our state, now include items like, “one bottle of hand sanitizer, five dry-erase markers in various colors, one qualified teaching assistant.” Also, I know why my 4th-grader needs to start the year with 24 #2 pencils: He chews them. But do all the kids have an oral fixation? Or is it that when my son has polished off his small forest of Ticonderogas, his classmates are expected to lend him theirs? I guess I’ll ask at orientation.
Hand-me-downs
Here’s my question: If we saved all the money parents are about to spend on classroom hand sanitizer, could we actually afford a teaching assistant? Or at least a few more dry-erase markers? Because a study from New Zealand suggests that as long as soap and water are available, hand sanitizer does nothing but to give schoolchildren another excuse to get up out of their seats.
Patricia Priest and her colleagues from the University of Otego in Dunedin (I, too think it sounds made up) studied school absences among 2,443 students aged 5 to 11 in 68 schools. They performed the experiment during the winter (read “summer”), placing alcohol-based hand sanitizers in half the schools and relying on soap, water, and Kiwi common sense in the other half. Then they sat back and counted the absences, going as far as to call parents and force them to invent diseases for children who were actually playing hooky to quest after the One Ring to Rule Them All.
When the investigators tallied everything up, hand sanitizer appeared to make no difference in the number of absences from all illnesses or from any specific illness (respiratory or GI). Also unaffected were the length of illness, the length of the absence, or the likelihood of another family member contracting the illness. (That last endpoint confuses me, unless the kids were taking hand sanitizer home every night.) What this study proved was that whether or not children in New Zealand have access to hand sanitizer, they will still all be mistaken for Australians.
I think we’re alone now
If you can just spend a little one-on-one time with teenagers, they’ll reveal the most amazing things about their lives. That’s why I have five children: to make sure that kind of thing never happens. But in the pediatrician’s office, it’s a good thing. We need to know what’s going on in adolescents’ lives in order to help them avoid the dangers of substance abuse, high-risk sexual behavior, and whatever boneheaded dare has been going around YouTube this week. A new study out of Indianapolis confirms what common sense already tells us: If you don’t talk with teens’ parents out of the exam room for a few minutes, you miss the good stuff, (read “the bad stuff”).
To prove it, the researchers surveyed around 500 adolescents (ages 13-17 years) and their parents regarding conversations they had with providers during their wellness exams. The best single statistic from their study was this: 89% of parents believed adolescents should be able to talk with their doctors privately, while 61% of parents preferred to stay in the exam room for the entire visit. That means that 50% of parents misunderstand one of the following terms: adolescent, private, or exam room.
Kids who did get to talk to their doctors alone discussed almost twice as many sensitive topics as did kids whose parents policed their conversations. On the up side, when parents refused to leave, kids were more likely to say “ma’am” and “sir,” to sit up straight for once please, and to brush that hair out of their eyes so the doctor can tell you’re looking at them, honey, thank you.
Call me maybe
Speaking of counseling teens and parents, do you sometimes think we’re talking with the wrong person? The National Institutes of Health just completed a study of distracted driving, a cause of 11% of fatal crashes among adolescents. Since 21% of those crashes involve mobile phone use, researchers asked 400 15- to 18-year-old drivers who in the heck they were talking to on the phone that made it worth risking their lives. Of the 86% of teens who reported talking on the phone while driving, 100% of them said the people they were talking to were their parents.
Fellow parents of teens, this does not make us look good. I like chatting with my kids, too, but it’s easier to converse with them when they’re alive, or at least not in a coma (if you can’t tell whether or not your adolescent is in a coma, have him brush that hair out of his eyes). We’ve got to do better, y’all; no one really wants to miss out on next year’s school orientation.
David L. Hill, M.D., FAAP, is the author of Dad to Dad: Parenting Like a Pro (AAP Publishing, 2012). He is also vice president of Cape Fear Pediatrics in Wilmington, N.C., and adjunct assistant professor of pediatrics at the University of North Carolina at Chapel Hill. He serves as Program Director for the AAP Council on Communications and Media and as an executive committee member of the North Carolina Pediatric Society. He has recorded commentaries for NPR's All Things Considered and provided content for various print, television, and Internet outlets.
It’s late August, and finally I feel ready for summer. You don’t need to remind me that I’m late: Our five children have no fewer than eight school orientations this week. The extras are because new high schoolers need two sessions, one for their parents and another just for students, where they tell them all the really important stuff, which the kids then forget until second semester when it’s too late. Can I go to the beach now?
Of course the orientations are mainly just a chance for teachers to pass out supply lists, which, with severe budget cuts in our state, now include items like, “one bottle of hand sanitizer, five dry-erase markers in various colors, one qualified teaching assistant.” Also, I know why my 4th-grader needs to start the year with 24 #2 pencils: He chews them. But do all the kids have an oral fixation? Or is it that when my son has polished off his small forest of Ticonderogas, his classmates are expected to lend him theirs? I guess I’ll ask at orientation.
Hand-me-downs
Here’s my question: If we saved all the money parents are about to spend on classroom hand sanitizer, could we actually afford a teaching assistant? Or at least a few more dry-erase markers? Because a study from New Zealand suggests that as long as soap and water are available, hand sanitizer does nothing but to give schoolchildren another excuse to get up out of their seats.
Patricia Priest and her colleagues from the University of Otego in Dunedin (I, too think it sounds made up) studied school absences among 2,443 students aged 5 to 11 in 68 schools. They performed the experiment during the winter (read “summer”), placing alcohol-based hand sanitizers in half the schools and relying on soap, water, and Kiwi common sense in the other half. Then they sat back and counted the absences, going as far as to call parents and force them to invent diseases for children who were actually playing hooky to quest after the One Ring to Rule Them All.
When the investigators tallied everything up, hand sanitizer appeared to make no difference in the number of absences from all illnesses or from any specific illness (respiratory or GI). Also unaffected were the length of illness, the length of the absence, or the likelihood of another family member contracting the illness. (That last endpoint confuses me, unless the kids were taking hand sanitizer home every night.) What this study proved was that whether or not children in New Zealand have access to hand sanitizer, they will still all be mistaken for Australians.
I think we’re alone now
If you can just spend a little one-on-one time with teenagers, they’ll reveal the most amazing things about their lives. That’s why I have five children: to make sure that kind of thing never happens. But in the pediatrician’s office, it’s a good thing. We need to know what’s going on in adolescents’ lives in order to help them avoid the dangers of substance abuse, high-risk sexual behavior, and whatever boneheaded dare has been going around YouTube this week. A new study out of Indianapolis confirms what common sense already tells us: If you don’t talk with teens’ parents out of the exam room for a few minutes, you miss the good stuff, (read “the bad stuff”).
To prove it, the researchers surveyed around 500 adolescents (ages 13-17 years) and their parents regarding conversations they had with providers during their wellness exams. The best single statistic from their study was this: 89% of parents believed adolescents should be able to talk with their doctors privately, while 61% of parents preferred to stay in the exam room for the entire visit. That means that 50% of parents misunderstand one of the following terms: adolescent, private, or exam room.
Kids who did get to talk to their doctors alone discussed almost twice as many sensitive topics as did kids whose parents policed their conversations. On the up side, when parents refused to leave, kids were more likely to say “ma’am” and “sir,” to sit up straight for once please, and to brush that hair out of their eyes so the doctor can tell you’re looking at them, honey, thank you.
Call me maybe
Speaking of counseling teens and parents, do you sometimes think we’re talking with the wrong person? The National Institutes of Health just completed a study of distracted driving, a cause of 11% of fatal crashes among adolescents. Since 21% of those crashes involve mobile phone use, researchers asked 400 15- to 18-year-old drivers who in the heck they were talking to on the phone that made it worth risking their lives. Of the 86% of teens who reported talking on the phone while driving, 100% of them said the people they were talking to were their parents.
Fellow parents of teens, this does not make us look good. I like chatting with my kids, too, but it’s easier to converse with them when they’re alive, or at least not in a coma (if you can’t tell whether or not your adolescent is in a coma, have him brush that hair out of his eyes). We’ve got to do better, y’all; no one really wants to miss out on next year’s school orientation.
David L. Hill, M.D., FAAP, is the author of Dad to Dad: Parenting Like a Pro (AAP Publishing, 2012). He is also vice president of Cape Fear Pediatrics in Wilmington, N.C., and adjunct assistant professor of pediatrics at the University of North Carolina at Chapel Hill. He serves as Program Director for the AAP Council on Communications and Media and as an executive committee member of the North Carolina Pediatric Society. He has recorded commentaries for NPR's All Things Considered and provided content for various print, television, and Internet outlets.
Estrogen does not cause breast cancer
“OOPHORECTOMY OR SALPINGECTOMY—WHICH MAKES MORE SENSE?”
William H. Parker, MD (March 2014)
Estrogen does not cause breast cancer
Excellent article by Dr. Parker, with one exception—he continues to promulgate the erroneous misconception that estrogen is what causes breast cancer. He repeats the inaccurate early conclusion of the Women’s Health Initiative (WHI) in 2002 that reported an increased risk of breast cancer. Yet review of that early data suggested that progestin was responsible for the increase, because patients taking estrogen alone had a lower breast cancer risk. Indeed, final and comprehensive review of the WHI studies in 2011 indicates that the estrogen-only arm of the study demonstrated lower breast cancer risks than the placebo group; that distinction continued in the post-intervention period as well.
We need to stop perpetuating the misconception that estrogen causes breast cancer, as the idea continues to be a major deterrent for patients to take estrogen during menopause, to the detriment of their health.
Rafael Haciski, MD
Naples, Florida
Dr. Parker responds:
Dr. Haciski is absolutely right about the fact that the estrogen-only arm of the WHI found a lower risk of breast cancer. The point I was trying to make, perhaps unsuccessfully, was that, in response to the 2002 WHI publication findings of an increased risk of breast cancer after estrogen and progesterone administration, the rate of oophorectomy declined. One interpretation of this trend toward ovarian conservation is that it reflected women deciding to keep their own ovarian hormones, rather than take exogenous hormones associated with health-related risks. While the 2004 WHI estrogen-only publication found a trend toward lower breast cancer risk, this association was not fully confirmed until 2012.1 Even now, many women find the WHI estrogen-progestin and the estrogen-only publications confusing. It was not my intention to add to that confusion.
Reference
1. Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486.
“UPDATE ON MINIMALLY INVASIVE GYNECOLOGY”
Amy Garcia, MD (April 2014)
Terminology is important: A cesarean scar pregnancy is not an ectopic pregnancy
I read with great interest the excellent “Update on minimally invasive gynecology,” by Amy Garcia, MD, co-member of OBG Management’s Board of Editors. In it she gives an excellent definition and discussion of an increase in the epidemic of cesarean scar defects (CSD). Her update focuses on intermenstrual bleeding when the menstrual blood presumably collects in the defect and comes out externally, and unpredictably, as old dark blood. I strongly agree with how she managed her clinical case, as I too have had success in such cases using the lowest dosed birth control pills.
My concern, however, is for a potentially fatal outcome because of our use of the term “cesarean scar ectopic pregnancy,” which she mentions as being an additional clinical outcome as described in the review article by Tower and colleagues.1 The strictest definition of ectopic pregnancy is “a pregnancy that occurs outside the uterus.” Many clinicians, however, refer to any pregnancy outside the normal endometrial cavity as being “ectopic.” Regardless of the definition used, I am aware of cases when this nomenclature has been responsible (at least in part) for maternal mortality.
Here is a scenario: A clinician gets an imaging report that there is a cesarean scar ectopic pregnancy. The patient is then treated with a methotrexate protocol2 for the ectopic pregnancy, even though the sac size is small and there is no embryo or cardiac activity.
This patient did not actually have a cesarean scar ectopic pregnancy. Ninety-eight percent of ectopic pregnancies are tubal and these are the ones in which methotrexate has been studied adequately and used. Cesarean scar pregnancies (and, in my opinion, cervical pregnancies as well as cornual pregnancies) are very different from “garden variety” tubal ectopic pregnancies, and should not automatically be plugged into existing methotrexate protocols. In my experience, the existing methotrexate protocols do not work for cesarean scar pregnancies, and place these women at risk for potential harm, such as severe hemorrhage.
We should be meticulous in referring to these as cesarean scar pregnancies—not cesarean scar ectopic pregnancies—to help keep well-meaning clinicians from being misled.
Steven R. Goldstein, MD
Professor, Department of Obstetrics and Gynecology
New York University School of Medicine, New York, New York
References
1. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications.
J Minim Invasive Gynecol. 2013;20(5):562–572.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479–1485.
Dr. Garcia responds:
While I can appreciate the comments from my co-editor, Dr. Goldstein, I believe he is defocusing the real issue regarding pregnancies occurring in scar defects caused by previous cesarean section. Rather than creating an issue with the semantics of “ectopic,” I would have preferred to read that Dr. Goldstein was emphasizing the potentially significant, if not fatal, complications and management of cesarean scar pregnancies as he did with co-authors Dr. Timor-Trisch and Dr. Monteagudo in a recent OBG Management article.1
To clarify the terminology, I used “cesarean scar ectopic pregnancy” as it was quoted from the work of Tower and colleagues.2 This terminology adequately describes the location of the pregnancy, as many cesarean scar defects are not just located in the isthmus of the uterus but in the cervix itself.3 And by all accounts a pregnancy at this location would be considered to be a cervical pregnancy, which ACOG defines as ectopic. The following definition of ectopic pregnancy is taken from Dr. Goldstein’s reference to ACOG Practice Bulletin #94: “Nearly all ectopic pregnancies (97%) are implanted within the fallopian tube, although implantation can occur within the abdomen, cervix, ovary, or uterine cornua.”4 Indeed if ACOG uses “ectopic” to describe a pregnancy within the cervix, then “cesarean scar ectopic pregnancy” should be adequate to describe the location of a potentially dangerous pregnancy.
Dr. Goldstein is concerned that our colleagues may become confused by the terminology “cesarean scar ectopic pregnancy” because the word “ectopic” might denote a less clinically significant scenario. Yet I say that anyone confused by the location of the pregnancy has not understood the part of the descriptive term that is “cesarean scar” regardless of the use of “ectopic.” Any clinician treating a patient with a cesarean scar pregnancy would benefit from Dr. Goldstein and his colleagues’ article for diagnosis and management of this critical and potentially life-threatening scenario.
References
1. Timor-Trisch IE, Monteagudo A, Goldstein SR. How to identify and manage cesarean scar pregnancy. OBG Manag. 2014;26(6):19–27.
2. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20(5):562–572.
3. Garcia A. Update on minimally invasive gynecology. OBG Manag. 2014;26(4):18–32.
4. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479–1485.
Totally painless birth: A new concept in obstetrical anesthesia and labor management
A recent review on epidural anesthesia for pain management during labor, which was published in the New England Journal of Medicine,1 starts with the following case presentation: “A 30-year-old nulliparous woman at 39 weeks gestation is undergoing induction of labor for premature rupture of membranes. She is currently receiving an oxytocin infusion, and her cervical dilatation is 1 cm. Her obstetrician has ordered intermittent intravenous administration of Fentanyl for pain relief, but she feels nauseated, has been unable to rest, and describes her pain as 9 on scale of 10.”
The case we present in this letter sounds totally different: A 26-year-old multiparous woman at 39 weeks’ gestation is scheduled for induction of labor because of a decreased amount of amniotic fluid detected on prenatal ultrasonography. On admission, her cervix is 1-cm dilated and 60% effaced. She has no uterine contractions, and she describes her pain as 0 on the scale of 10. Anticipating a long induction and unwilling to experience any pain, the patient requests and is offered epidural anesthesia prior to oxytocin administration. Labor takes longer than expected but ultimately results in the birth of a healthy child. At no time during the course of labor and delivery does the mother experience any pain or discomfort, and she reports an extremely satisfying experience.
Traditionally, epidural anesthesia is administered upon maternal request when labor pain becomes difficult or intolerable. Many obstetricians discourage the use of epidural anesthesia prior to the onset of the active stage of labor, where the cervix is 4- to 6-cm dilated and the patient is in a great deal of pain.
This concept of administering an analgesic after the onset of pain is very different from the one where anesthesia is provided to patients prior to surgical procedures. No physician would begin a scheduled surgical procedure before confirming administration of adequate anesthesia. Why should labor be any different?
The pain of labor caused by uterine contractions and cervical dilatation is transmitted through visceral afferent nerves entering the spinal cord from T10 through L1. Perineal stretching transmits pain through the pudendal and sacral nerves. Epidural analgesia for labor and delivery involves injection of a local anesthetic agent and an opioid analgesic into the lumbar epidural space. The injected agents gradually diffuse across the dura into the subarachnoid cavity. In spinal analgesia, which is often combined with epidural analgesia, the medication is injected directly into the subarachnoid cavity, resulting in a more rapid effect.2
The American Society of Anesthesiologists and the American College of Obstetrics and Gynecology jointly state, “Labor causes severe pain for many women. There is no other circumstance where it is considered acceptable for an individual to experience severe pain, untreated. In the absence of medical contraindications, maternal request is a sufficient medical indication for pain relief during labor.”3
We decided to stretch this concept by allowing epidural anesthesia to be administered upon maternal consent in anticipation of pain, prior to onset. Our extensive literature search failed to find any comprehensive studies on the use of epidural anesthesia in anticipation of labor pain.
In theory, neuraxial local anesthetic in clinically relevant doses affect only skeletal muscles, not smooth muscles; therefore, this agent should not significantly decrease the amplitude or frequency of contractions in the myometrium.4 Randomized controlled trials of the effects of analgesia during labor do not exist since it is impossible to randomly assign women to a placebo group (no pain relief). Most trials have compared the use of epidural analgesia with that of systemic narcotics. In one large trial, 992 multiparous women were randomly assigned to either epidural analgesia or midwifery support supplemented by intramuscular narcotic injections. When pain was rated on a scale of 0–100 (100 is high), the median score before the study interventions was 80 in the group of patients who did not receive an epidural analgesia. With the administration of epidural analgesia, the median score was 27.5
Clinicians and patients also have been concerned about whether the use of epidural analgesia increases the risk of cesarean delivery. The results of three randomized controlled trials demonstrated that early administration of epidural analgesia does not increase the rate of cesarean delivery among women with spontaneous or induced labor as compared with early initiation of opioids.6–8 The fact that early epidural placement does not appear to increase the incidence of cesarean delivery is encouraging for our ongoing study but doesn’t necessarily mean that epidural analgesia prior to the onset of pain (preventive epidural) will have the same outcome. More research is needed.
Boris M. Petrikovsky, MD, PhD; Elya Kozlov, MD; Matthew Ackert, MD; Ralph Ruggiero, MD; and Crystal Behofsits, DO
Wyckoff Heights Medical Center, Brooklyn NY
References
1. Haukins GL. Epidural analgesia for labor and delivery. N Engl J Med. 2010;362(16):1502–1510.
2. Catterall WA, Mackie K. Local anesthetics. In: Brunton LL, Lazo JS, Parker KL, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, New York: McGraw-Hill; 2005;369–386.
3. American College of Obstetricians and Gynecologists. ACOG Committee Opinion #295: pain relief during labor. Obstet Gynecol. 2004;104(1):213.
4. Fanning RA, Campion DP, Collins CB, et al. A comparison of the inhibitory effects of bupivacaine and levobupivacaine on isolated human pregnant myometrium contractility. Anesth Analg. 2008;107(4):1303–1307.
5. Dickinson JE, Paech MJ, McDonald SJ, Evans SF. Maternal satisfaction with childbirth and intrapartum analgesia in nulliparous labor. Aust N Z J Obstet Gynecol. 2003;43(6):463–468.
6. Wong CA, Scavone BM, Peaceman AM, et al. The risk of cesarean delivery with neuraxial analgesia in labor. N Engl J Med. 2005;352(7):655–665.
7. Ohel G, Gonen R, Vaida S, Barak S, Gaitini L. Early versus late initiation of epidural analgesia in labor: does it increase the risk of cesarean section? A randomized trial. Am J Obstet Gynecol. 2006:194(3):600–605.
8. Wong CA, McCarthy RJ, Sullivan JT, Scavone BM, Gerber SE, Yaghmour EA. Early compared with late neuraxial analgesia in nulliparous labor induction: a randomized controlled trial. Obstet Gynecol. 2009;113(5):1066–1074.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Boris M. Petrikovsky,Elya Kozlov,Matthew Ackert,Ralph Ruggiero,Crystal Behofsits,totally painless birth,obstetrical anesthesia,labor management,labor and delivery,New England Journal of Medicine,epidural anesthesia,pain management during labor,nulliparous,premature rupture of membranes,multiparous,prenatal ultrasonography,uterine contractions,cervical dilation,visceral afferent nerves,American Society of Anesthesiologists,American College of Obstetricians and Gynecologists,
“OOPHORECTOMY OR SALPINGECTOMY—WHICH MAKES MORE SENSE?”
William H. Parker, MD (March 2014)
Estrogen does not cause breast cancer
Excellent article by Dr. Parker, with one exception—he continues to promulgate the erroneous misconception that estrogen is what causes breast cancer. He repeats the inaccurate early conclusion of the Women’s Health Initiative (WHI) in 2002 that reported an increased risk of breast cancer. Yet review of that early data suggested that progestin was responsible for the increase, because patients taking estrogen alone had a lower breast cancer risk. Indeed, final and comprehensive review of the WHI studies in 2011 indicates that the estrogen-only arm of the study demonstrated lower breast cancer risks than the placebo group; that distinction continued in the post-intervention period as well.
We need to stop perpetuating the misconception that estrogen causes breast cancer, as the idea continues to be a major deterrent for patients to take estrogen during menopause, to the detriment of their health.
Rafael Haciski, MD
Naples, Florida
Dr. Parker responds:
Dr. Haciski is absolutely right about the fact that the estrogen-only arm of the WHI found a lower risk of breast cancer. The point I was trying to make, perhaps unsuccessfully, was that, in response to the 2002 WHI publication findings of an increased risk of breast cancer after estrogen and progesterone administration, the rate of oophorectomy declined. One interpretation of this trend toward ovarian conservation is that it reflected women deciding to keep their own ovarian hormones, rather than take exogenous hormones associated with health-related risks. While the 2004 WHI estrogen-only publication found a trend toward lower breast cancer risk, this association was not fully confirmed until 2012.1 Even now, many women find the WHI estrogen-progestin and the estrogen-only publications confusing. It was not my intention to add to that confusion.
Reference
1. Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486.
“UPDATE ON MINIMALLY INVASIVE GYNECOLOGY”
Amy Garcia, MD (April 2014)
Terminology is important: A cesarean scar pregnancy is not an ectopic pregnancy
I read with great interest the excellent “Update on minimally invasive gynecology,” by Amy Garcia, MD, co-member of OBG Management’s Board of Editors. In it she gives an excellent definition and discussion of an increase in the epidemic of cesarean scar defects (CSD). Her update focuses on intermenstrual bleeding when the menstrual blood presumably collects in the defect and comes out externally, and unpredictably, as old dark blood. I strongly agree with how she managed her clinical case, as I too have had success in such cases using the lowest dosed birth control pills.
My concern, however, is for a potentially fatal outcome because of our use of the term “cesarean scar ectopic pregnancy,” which she mentions as being an additional clinical outcome as described in the review article by Tower and colleagues.1 The strictest definition of ectopic pregnancy is “a pregnancy that occurs outside the uterus.” Many clinicians, however, refer to any pregnancy outside the normal endometrial cavity as being “ectopic.” Regardless of the definition used, I am aware of cases when this nomenclature has been responsible (at least in part) for maternal mortality.
Here is a scenario: A clinician gets an imaging report that there is a cesarean scar ectopic pregnancy. The patient is then treated with a methotrexate protocol2 for the ectopic pregnancy, even though the sac size is small and there is no embryo or cardiac activity.
This patient did not actually have a cesarean scar ectopic pregnancy. Ninety-eight percent of ectopic pregnancies are tubal and these are the ones in which methotrexate has been studied adequately and used. Cesarean scar pregnancies (and, in my opinion, cervical pregnancies as well as cornual pregnancies) are very different from “garden variety” tubal ectopic pregnancies, and should not automatically be plugged into existing methotrexate protocols. In my experience, the existing methotrexate protocols do not work for cesarean scar pregnancies, and place these women at risk for potential harm, such as severe hemorrhage.
We should be meticulous in referring to these as cesarean scar pregnancies—not cesarean scar ectopic pregnancies—to help keep well-meaning clinicians from being misled.
Steven R. Goldstein, MD
Professor, Department of Obstetrics and Gynecology
New York University School of Medicine, New York, New York
References
1. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications.
J Minim Invasive Gynecol. 2013;20(5):562–572.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479–1485.
Dr. Garcia responds:
While I can appreciate the comments from my co-editor, Dr. Goldstein, I believe he is defocusing the real issue regarding pregnancies occurring in scar defects caused by previous cesarean section. Rather than creating an issue with the semantics of “ectopic,” I would have preferred to read that Dr. Goldstein was emphasizing the potentially significant, if not fatal, complications and management of cesarean scar pregnancies as he did with co-authors Dr. Timor-Trisch and Dr. Monteagudo in a recent OBG Management article.1
To clarify the terminology, I used “cesarean scar ectopic pregnancy” as it was quoted from the work of Tower and colleagues.2 This terminology adequately describes the location of the pregnancy, as many cesarean scar defects are not just located in the isthmus of the uterus but in the cervix itself.3 And by all accounts a pregnancy at this location would be considered to be a cervical pregnancy, which ACOG defines as ectopic. The following definition of ectopic pregnancy is taken from Dr. Goldstein’s reference to ACOG Practice Bulletin #94: “Nearly all ectopic pregnancies (97%) are implanted within the fallopian tube, although implantation can occur within the abdomen, cervix, ovary, or uterine cornua.”4 Indeed if ACOG uses “ectopic” to describe a pregnancy within the cervix, then “cesarean scar ectopic pregnancy” should be adequate to describe the location of a potentially dangerous pregnancy.
Dr. Goldstein is concerned that our colleagues may become confused by the terminology “cesarean scar ectopic pregnancy” because the word “ectopic” might denote a less clinically significant scenario. Yet I say that anyone confused by the location of the pregnancy has not understood the part of the descriptive term that is “cesarean scar” regardless of the use of “ectopic.” Any clinician treating a patient with a cesarean scar pregnancy would benefit from Dr. Goldstein and his colleagues’ article for diagnosis and management of this critical and potentially life-threatening scenario.
References
1. Timor-Trisch IE, Monteagudo A, Goldstein SR. How to identify and manage cesarean scar pregnancy. OBG Manag. 2014;26(6):19–27.
2. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20(5):562–572.
3. Garcia A. Update on minimally invasive gynecology. OBG Manag. 2014;26(4):18–32.
4. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479–1485.
Totally painless birth: A new concept in obstetrical anesthesia and labor management
A recent review on epidural anesthesia for pain management during labor, which was published in the New England Journal of Medicine,1 starts with the following case presentation: “A 30-year-old nulliparous woman at 39 weeks gestation is undergoing induction of labor for premature rupture of membranes. She is currently receiving an oxytocin infusion, and her cervical dilatation is 1 cm. Her obstetrician has ordered intermittent intravenous administration of Fentanyl for pain relief, but she feels nauseated, has been unable to rest, and describes her pain as 9 on scale of 10.”
The case we present in this letter sounds totally different: A 26-year-old multiparous woman at 39 weeks’ gestation is scheduled for induction of labor because of a decreased amount of amniotic fluid detected on prenatal ultrasonography. On admission, her cervix is 1-cm dilated and 60% effaced. She has no uterine contractions, and she describes her pain as 0 on the scale of 10. Anticipating a long induction and unwilling to experience any pain, the patient requests and is offered epidural anesthesia prior to oxytocin administration. Labor takes longer than expected but ultimately results in the birth of a healthy child. At no time during the course of labor and delivery does the mother experience any pain or discomfort, and she reports an extremely satisfying experience.
Traditionally, epidural anesthesia is administered upon maternal request when labor pain becomes difficult or intolerable. Many obstetricians discourage the use of epidural anesthesia prior to the onset of the active stage of labor, where the cervix is 4- to 6-cm dilated and the patient is in a great deal of pain.
This concept of administering an analgesic after the onset of pain is very different from the one where anesthesia is provided to patients prior to surgical procedures. No physician would begin a scheduled surgical procedure before confirming administration of adequate anesthesia. Why should labor be any different?
The pain of labor caused by uterine contractions and cervical dilatation is transmitted through visceral afferent nerves entering the spinal cord from T10 through L1. Perineal stretching transmits pain through the pudendal and sacral nerves. Epidural analgesia for labor and delivery involves injection of a local anesthetic agent and an opioid analgesic into the lumbar epidural space. The injected agents gradually diffuse across the dura into the subarachnoid cavity. In spinal analgesia, which is often combined with epidural analgesia, the medication is injected directly into the subarachnoid cavity, resulting in a more rapid effect.2
The American Society of Anesthesiologists and the American College of Obstetrics and Gynecology jointly state, “Labor causes severe pain for many women. There is no other circumstance where it is considered acceptable for an individual to experience severe pain, untreated. In the absence of medical contraindications, maternal request is a sufficient medical indication for pain relief during labor.”3
We decided to stretch this concept by allowing epidural anesthesia to be administered upon maternal consent in anticipation of pain, prior to onset. Our extensive literature search failed to find any comprehensive studies on the use of epidural anesthesia in anticipation of labor pain.
In theory, neuraxial local anesthetic in clinically relevant doses affect only skeletal muscles, not smooth muscles; therefore, this agent should not significantly decrease the amplitude or frequency of contractions in the myometrium.4 Randomized controlled trials of the effects of analgesia during labor do not exist since it is impossible to randomly assign women to a placebo group (no pain relief). Most trials have compared the use of epidural analgesia with that of systemic narcotics. In one large trial, 992 multiparous women were randomly assigned to either epidural analgesia or midwifery support supplemented by intramuscular narcotic injections. When pain was rated on a scale of 0–100 (100 is high), the median score before the study interventions was 80 in the group of patients who did not receive an epidural analgesia. With the administration of epidural analgesia, the median score was 27.5
Clinicians and patients also have been concerned about whether the use of epidural analgesia increases the risk of cesarean delivery. The results of three randomized controlled trials demonstrated that early administration of epidural analgesia does not increase the rate of cesarean delivery among women with spontaneous or induced labor as compared with early initiation of opioids.6–8 The fact that early epidural placement does not appear to increase the incidence of cesarean delivery is encouraging for our ongoing study but doesn’t necessarily mean that epidural analgesia prior to the onset of pain (preventive epidural) will have the same outcome. More research is needed.
Boris M. Petrikovsky, MD, PhD; Elya Kozlov, MD; Matthew Ackert, MD; Ralph Ruggiero, MD; and Crystal Behofsits, DO
Wyckoff Heights Medical Center, Brooklyn NY
References
1. Haukins GL. Epidural analgesia for labor and delivery. N Engl J Med. 2010;362(16):1502–1510.
2. Catterall WA, Mackie K. Local anesthetics. In: Brunton LL, Lazo JS, Parker KL, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, New York: McGraw-Hill; 2005;369–386.
3. American College of Obstetricians and Gynecologists. ACOG Committee Opinion #295: pain relief during labor. Obstet Gynecol. 2004;104(1):213.
4. Fanning RA, Campion DP, Collins CB, et al. A comparison of the inhibitory effects of bupivacaine and levobupivacaine on isolated human pregnant myometrium contractility. Anesth Analg. 2008;107(4):1303–1307.
5. Dickinson JE, Paech MJ, McDonald SJ, Evans SF. Maternal satisfaction with childbirth and intrapartum analgesia in nulliparous labor. Aust N Z J Obstet Gynecol. 2003;43(6):463–468.
6. Wong CA, Scavone BM, Peaceman AM, et al. The risk of cesarean delivery with neuraxial analgesia in labor. N Engl J Med. 2005;352(7):655–665.
7. Ohel G, Gonen R, Vaida S, Barak S, Gaitini L. Early versus late initiation of epidural analgesia in labor: does it increase the risk of cesarean section? A randomized trial. Am J Obstet Gynecol. 2006:194(3):600–605.
8. Wong CA, McCarthy RJ, Sullivan JT, Scavone BM, Gerber SE, Yaghmour EA. Early compared with late neuraxial analgesia in nulliparous labor induction: a randomized controlled trial. Obstet Gynecol. 2009;113(5):1066–1074.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
“OOPHORECTOMY OR SALPINGECTOMY—WHICH MAKES MORE SENSE?”
William H. Parker, MD (March 2014)
Estrogen does not cause breast cancer
Excellent article by Dr. Parker, with one exception—he continues to promulgate the erroneous misconception that estrogen is what causes breast cancer. He repeats the inaccurate early conclusion of the Women’s Health Initiative (WHI) in 2002 that reported an increased risk of breast cancer. Yet review of that early data suggested that progestin was responsible for the increase, because patients taking estrogen alone had a lower breast cancer risk. Indeed, final and comprehensive review of the WHI studies in 2011 indicates that the estrogen-only arm of the study demonstrated lower breast cancer risks than the placebo group; that distinction continued in the post-intervention period as well.
We need to stop perpetuating the misconception that estrogen causes breast cancer, as the idea continues to be a major deterrent for patients to take estrogen during menopause, to the detriment of their health.
Rafael Haciski, MD
Naples, Florida
Dr. Parker responds:
Dr. Haciski is absolutely right about the fact that the estrogen-only arm of the WHI found a lower risk of breast cancer. The point I was trying to make, perhaps unsuccessfully, was that, in response to the 2002 WHI publication findings of an increased risk of breast cancer after estrogen and progesterone administration, the rate of oophorectomy declined. One interpretation of this trend toward ovarian conservation is that it reflected women deciding to keep their own ovarian hormones, rather than take exogenous hormones associated with health-related risks. While the 2004 WHI estrogen-only publication found a trend toward lower breast cancer risk, this association was not fully confirmed until 2012.1 Even now, many women find the WHI estrogen-progestin and the estrogen-only publications confusing. It was not my intention to add to that confusion.
Reference
1. Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomised placebo-controlled trial. Lancet Oncol. 2012;13(5):476–486.
“UPDATE ON MINIMALLY INVASIVE GYNECOLOGY”
Amy Garcia, MD (April 2014)
Terminology is important: A cesarean scar pregnancy is not an ectopic pregnancy
I read with great interest the excellent “Update on minimally invasive gynecology,” by Amy Garcia, MD, co-member of OBG Management’s Board of Editors. In it she gives an excellent definition and discussion of an increase in the epidemic of cesarean scar defects (CSD). Her update focuses on intermenstrual bleeding when the menstrual blood presumably collects in the defect and comes out externally, and unpredictably, as old dark blood. I strongly agree with how she managed her clinical case, as I too have had success in such cases using the lowest dosed birth control pills.
My concern, however, is for a potentially fatal outcome because of our use of the term “cesarean scar ectopic pregnancy,” which she mentions as being an additional clinical outcome as described in the review article by Tower and colleagues.1 The strictest definition of ectopic pregnancy is “a pregnancy that occurs outside the uterus.” Many clinicians, however, refer to any pregnancy outside the normal endometrial cavity as being “ectopic.” Regardless of the definition used, I am aware of cases when this nomenclature has been responsible (at least in part) for maternal mortality.
Here is a scenario: A clinician gets an imaging report that there is a cesarean scar ectopic pregnancy. The patient is then treated with a methotrexate protocol2 for the ectopic pregnancy, even though the sac size is small and there is no embryo or cardiac activity.
This patient did not actually have a cesarean scar ectopic pregnancy. Ninety-eight percent of ectopic pregnancies are tubal and these are the ones in which methotrexate has been studied adequately and used. Cesarean scar pregnancies (and, in my opinion, cervical pregnancies as well as cornual pregnancies) are very different from “garden variety” tubal ectopic pregnancies, and should not automatically be plugged into existing methotrexate protocols. In my experience, the existing methotrexate protocols do not work for cesarean scar pregnancies, and place these women at risk for potential harm, such as severe hemorrhage.
We should be meticulous in referring to these as cesarean scar pregnancies—not cesarean scar ectopic pregnancies—to help keep well-meaning clinicians from being misled.
Steven R. Goldstein, MD
Professor, Department of Obstetrics and Gynecology
New York University School of Medicine, New York, New York
References
1. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications.
J Minim Invasive Gynecol. 2013;20(5):562–572.
2. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479–1485.
Dr. Garcia responds:
While I can appreciate the comments from my co-editor, Dr. Goldstein, I believe he is defocusing the real issue regarding pregnancies occurring in scar defects caused by previous cesarean section. Rather than creating an issue with the semantics of “ectopic,” I would have preferred to read that Dr. Goldstein was emphasizing the potentially significant, if not fatal, complications and management of cesarean scar pregnancies as he did with co-authors Dr. Timor-Trisch and Dr. Monteagudo in a recent OBG Management article.1
To clarify the terminology, I used “cesarean scar ectopic pregnancy” as it was quoted from the work of Tower and colleagues.2 This terminology adequately describes the location of the pregnancy, as many cesarean scar defects are not just located in the isthmus of the uterus but in the cervix itself.3 And by all accounts a pregnancy at this location would be considered to be a cervical pregnancy, which ACOG defines as ectopic. The following definition of ectopic pregnancy is taken from Dr. Goldstein’s reference to ACOG Practice Bulletin #94: “Nearly all ectopic pregnancies (97%) are implanted within the fallopian tube, although implantation can occur within the abdomen, cervix, ovary, or uterine cornua.”4 Indeed if ACOG uses “ectopic” to describe a pregnancy within the cervix, then “cesarean scar ectopic pregnancy” should be adequate to describe the location of a potentially dangerous pregnancy.
Dr. Goldstein is concerned that our colleagues may become confused by the terminology “cesarean scar ectopic pregnancy” because the word “ectopic” might denote a less clinically significant scenario. Yet I say that anyone confused by the location of the pregnancy has not understood the part of the descriptive term that is “cesarean scar” regardless of the use of “ectopic.” Any clinician treating a patient with a cesarean scar pregnancy would benefit from Dr. Goldstein and his colleagues’ article for diagnosis and management of this critical and potentially life-threatening scenario.
References
1. Timor-Trisch IE, Monteagudo A, Goldstein SR. How to identify and manage cesarean scar pregnancy. OBG Manag. 2014;26(6):19–27.
2. Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20(5):562–572.
3. Garcia A. Update on minimally invasive gynecology. OBG Manag. 2014;26(4):18–32.
4. American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 94: Medical management of ectopic pregnancy. Obstet Gynecol. 2008;111(6):1479–1485.
Totally painless birth: A new concept in obstetrical anesthesia and labor management
A recent review on epidural anesthesia for pain management during labor, which was published in the New England Journal of Medicine,1 starts with the following case presentation: “A 30-year-old nulliparous woman at 39 weeks gestation is undergoing induction of labor for premature rupture of membranes. She is currently receiving an oxytocin infusion, and her cervical dilatation is 1 cm. Her obstetrician has ordered intermittent intravenous administration of Fentanyl for pain relief, but she feels nauseated, has been unable to rest, and describes her pain as 9 on scale of 10.”
The case we present in this letter sounds totally different: A 26-year-old multiparous woman at 39 weeks’ gestation is scheduled for induction of labor because of a decreased amount of amniotic fluid detected on prenatal ultrasonography. On admission, her cervix is 1-cm dilated and 60% effaced. She has no uterine contractions, and she describes her pain as 0 on the scale of 10. Anticipating a long induction and unwilling to experience any pain, the patient requests and is offered epidural anesthesia prior to oxytocin administration. Labor takes longer than expected but ultimately results in the birth of a healthy child. At no time during the course of labor and delivery does the mother experience any pain or discomfort, and she reports an extremely satisfying experience.
Traditionally, epidural anesthesia is administered upon maternal request when labor pain becomes difficult or intolerable. Many obstetricians discourage the use of epidural anesthesia prior to the onset of the active stage of labor, where the cervix is 4- to 6-cm dilated and the patient is in a great deal of pain.
This concept of administering an analgesic after the onset of pain is very different from the one where anesthesia is provided to patients prior to surgical procedures. No physician would begin a scheduled surgical procedure before confirming administration of adequate anesthesia. Why should labor be any different?
The pain of labor caused by uterine contractions and cervical dilatation is transmitted through visceral afferent nerves entering the spinal cord from T10 through L1. Perineal stretching transmits pain through the pudendal and sacral nerves. Epidural analgesia for labor and delivery involves injection of a local anesthetic agent and an opioid analgesic into the lumbar epidural space. The injected agents gradually diffuse across the dura into the subarachnoid cavity. In spinal analgesia, which is often combined with epidural analgesia, the medication is injected directly into the subarachnoid cavity, resulting in a more rapid effect.2
The American Society of Anesthesiologists and the American College of Obstetrics and Gynecology jointly state, “Labor causes severe pain for many women. There is no other circumstance where it is considered acceptable for an individual to experience severe pain, untreated. In the absence of medical contraindications, maternal request is a sufficient medical indication for pain relief during labor.”3
We decided to stretch this concept by allowing epidural anesthesia to be administered upon maternal consent in anticipation of pain, prior to onset. Our extensive literature search failed to find any comprehensive studies on the use of epidural anesthesia in anticipation of labor pain.
In theory, neuraxial local anesthetic in clinically relevant doses affect only skeletal muscles, not smooth muscles; therefore, this agent should not significantly decrease the amplitude or frequency of contractions in the myometrium.4 Randomized controlled trials of the effects of analgesia during labor do not exist since it is impossible to randomly assign women to a placebo group (no pain relief). Most trials have compared the use of epidural analgesia with that of systemic narcotics. In one large trial, 992 multiparous women were randomly assigned to either epidural analgesia or midwifery support supplemented by intramuscular narcotic injections. When pain was rated on a scale of 0–100 (100 is high), the median score before the study interventions was 80 in the group of patients who did not receive an epidural analgesia. With the administration of epidural analgesia, the median score was 27.5
Clinicians and patients also have been concerned about whether the use of epidural analgesia increases the risk of cesarean delivery. The results of three randomized controlled trials demonstrated that early administration of epidural analgesia does not increase the rate of cesarean delivery among women with spontaneous or induced labor as compared with early initiation of opioids.6–8 The fact that early epidural placement does not appear to increase the incidence of cesarean delivery is encouraging for our ongoing study but doesn’t necessarily mean that epidural analgesia prior to the onset of pain (preventive epidural) will have the same outcome. More research is needed.
Boris M. Petrikovsky, MD, PhD; Elya Kozlov, MD; Matthew Ackert, MD; Ralph Ruggiero, MD; and Crystal Behofsits, DO
Wyckoff Heights Medical Center, Brooklyn NY
References
1. Haukins GL. Epidural analgesia for labor and delivery. N Engl J Med. 2010;362(16):1502–1510.
2. Catterall WA, Mackie K. Local anesthetics. In: Brunton LL, Lazo JS, Parker KL, eds. Goodman & Gilman’s The Pharmacological Basis of Therapeutics. 11th ed. New York, New York: McGraw-Hill; 2005;369–386.
3. American College of Obstetricians and Gynecologists. ACOG Committee Opinion #295: pain relief during labor. Obstet Gynecol. 2004;104(1):213.
4. Fanning RA, Campion DP, Collins CB, et al. A comparison of the inhibitory effects of bupivacaine and levobupivacaine on isolated human pregnant myometrium contractility. Anesth Analg. 2008;107(4):1303–1307.
5. Dickinson JE, Paech MJ, McDonald SJ, Evans SF. Maternal satisfaction with childbirth and intrapartum analgesia in nulliparous labor. Aust N Z J Obstet Gynecol. 2003;43(6):463–468.
6. Wong CA, Scavone BM, Peaceman AM, et al. The risk of cesarean delivery with neuraxial analgesia in labor. N Engl J Med. 2005;352(7):655–665.
7. Ohel G, Gonen R, Vaida S, Barak S, Gaitini L. Early versus late initiation of epidural analgesia in labor: does it increase the risk of cesarean section? A randomized trial. Am J Obstet Gynecol. 2006:194(3):600–605.
8. Wong CA, McCarthy RJ, Sullivan JT, Scavone BM, Gerber SE, Yaghmour EA. Early compared with late neuraxial analgesia in nulliparous labor induction: a randomized controlled trial. Obstet Gynecol. 2009;113(5):1066–1074.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Boris M. Petrikovsky,Elya Kozlov,Matthew Ackert,Ralph Ruggiero,Crystal Behofsits,totally painless birth,obstetrical anesthesia,labor management,labor and delivery,New England Journal of Medicine,epidural anesthesia,pain management during labor,nulliparous,premature rupture of membranes,multiparous,prenatal ultrasonography,uterine contractions,cervical dilation,visceral afferent nerves,American Society of Anesthesiologists,American College of Obstetricians and Gynecologists,
Boris M. Petrikovsky,Elya Kozlov,Matthew Ackert,Ralph Ruggiero,Crystal Behofsits,totally painless birth,obstetrical anesthesia,labor management,labor and delivery,New England Journal of Medicine,epidural anesthesia,pain management during labor,nulliparous,premature rupture of membranes,multiparous,prenatal ultrasonography,uterine contractions,cervical dilation,visceral afferent nerves,American Society of Anesthesiologists,American College of Obstetricians and Gynecologists,
Gatekeeper, provider, HCP: The slow degradation of the doctor title
What’s in a name? A lot, if you’re a doctor.
"Doctor" is our formal title, although technically it means anyone with a higher educational degree. We’re also called physicians, or healers. In other times, we may have been medicine men, witch doctors, or shamans. Or we may prefer a more specific name based on our chosen field: neurologist, internist, or surgeon, for example.
Recently, though, we’ve had specific (and less flattering) names hung on us – health care providers, primary care physicians, gatekeepers – not to mention the alphabet soup that longer names bring (HCP, PCP).
It kind of puts us in a semantic identity crisis. Especially with all the HCPs out there who aren’t MDs or DOs.
But no matter what title they hang on me, I know what I do. I care for people who need me. I provide treatment for those I can help and support to those I can’t. I hold hands. I write prescriptions. I discuss test results. I talk, and I listen. I fill out forms. I argue with insurance companies. I go home each night and in the morning come back and do it all over again.
Somehow saying I’m an HCP doesn’t seem to do the job description justice.
I remember a residency meeting I attended in the mid-90s. I was in training, and the meeting was held to introduce all the residents to the hospital’s new health plan. The insurance lady running it told us not to use the word "patients" but instead call them "lives." Doctors, in her doublespeak, were "providers" unless you were in internal medicine or family practice, in which case you had the even less flattering name of "gatekeeper."
The meeting got increasingly acrimonious, and the insurance lady looked more and more uncomfortable. Finally, a family practice resident named Barb stood up and said, "You can change words all you want, but here’s the truth. We are not providers, or PCPs, or gatekeepers. We are doctors. And we do our best to care for people, even when your company won’t."
Barb stood up, turned on her heel, and walked out with her long skirt swirling. The insurance company lady was obviously angry and left through the exit behind her.
It’s now 20 years later, Barb, and I don’t think anyone could have said it better, then or now. It still holds true.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
What’s in a name? A lot, if you’re a doctor.
"Doctor" is our formal title, although technically it means anyone with a higher educational degree. We’re also called physicians, or healers. In other times, we may have been medicine men, witch doctors, or shamans. Or we may prefer a more specific name based on our chosen field: neurologist, internist, or surgeon, for example.
Recently, though, we’ve had specific (and less flattering) names hung on us – health care providers, primary care physicians, gatekeepers – not to mention the alphabet soup that longer names bring (HCP, PCP).
It kind of puts us in a semantic identity crisis. Especially with all the HCPs out there who aren’t MDs or DOs.
But no matter what title they hang on me, I know what I do. I care for people who need me. I provide treatment for those I can help and support to those I can’t. I hold hands. I write prescriptions. I discuss test results. I talk, and I listen. I fill out forms. I argue with insurance companies. I go home each night and in the morning come back and do it all over again.
Somehow saying I’m an HCP doesn’t seem to do the job description justice.
I remember a residency meeting I attended in the mid-90s. I was in training, and the meeting was held to introduce all the residents to the hospital’s new health plan. The insurance lady running it told us not to use the word "patients" but instead call them "lives." Doctors, in her doublespeak, were "providers" unless you were in internal medicine or family practice, in which case you had the even less flattering name of "gatekeeper."
The meeting got increasingly acrimonious, and the insurance lady looked more and more uncomfortable. Finally, a family practice resident named Barb stood up and said, "You can change words all you want, but here’s the truth. We are not providers, or PCPs, or gatekeepers. We are doctors. And we do our best to care for people, even when your company won’t."
Barb stood up, turned on her heel, and walked out with her long skirt swirling. The insurance company lady was obviously angry and left through the exit behind her.
It’s now 20 years later, Barb, and I don’t think anyone could have said it better, then or now. It still holds true.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
What’s in a name? A lot, if you’re a doctor.
"Doctor" is our formal title, although technically it means anyone with a higher educational degree. We’re also called physicians, or healers. In other times, we may have been medicine men, witch doctors, or shamans. Or we may prefer a more specific name based on our chosen field: neurologist, internist, or surgeon, for example.
Recently, though, we’ve had specific (and less flattering) names hung on us – health care providers, primary care physicians, gatekeepers – not to mention the alphabet soup that longer names bring (HCP, PCP).
It kind of puts us in a semantic identity crisis. Especially with all the HCPs out there who aren’t MDs or DOs.
But no matter what title they hang on me, I know what I do. I care for people who need me. I provide treatment for those I can help and support to those I can’t. I hold hands. I write prescriptions. I discuss test results. I talk, and I listen. I fill out forms. I argue with insurance companies. I go home each night and in the morning come back and do it all over again.
Somehow saying I’m an HCP doesn’t seem to do the job description justice.
I remember a residency meeting I attended in the mid-90s. I was in training, and the meeting was held to introduce all the residents to the hospital’s new health plan. The insurance lady running it told us not to use the word "patients" but instead call them "lives." Doctors, in her doublespeak, were "providers" unless you were in internal medicine or family practice, in which case you had the even less flattering name of "gatekeeper."
The meeting got increasingly acrimonious, and the insurance lady looked more and more uncomfortable. Finally, a family practice resident named Barb stood up and said, "You can change words all you want, but here’s the truth. We are not providers, or PCPs, or gatekeepers. We are doctors. And we do our best to care for people, even when your company won’t."
Barb stood up, turned on her heel, and walked out with her long skirt swirling. The insurance company lady was obviously angry and left through the exit behind her.
It’s now 20 years later, Barb, and I don’t think anyone could have said it better, then or now. It still holds true.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The Medical Roundtable: Current and Future Strategies for Overcoming Diuretic Resistance in Acute Heart Failure
It is well recognized that diuretic therapy remains the main approach to volume management in heart failure patients and carries a first-line recommendation according to the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.1 However, in more advanced patients and those who have been on chronic diuretic therapy, many fail to respond to escalating doses of intermittent loop diuretics, a common clinical scenario referred to as “diuretic resistance.”
In patients exhibiting diuretic resistance, several approaches have been utilized. This includes increasing the frequency of administration or initiating a continuous infusion of the loop diuretic, changing to a more potent (on a milligram per milligram basis) loop, or combining a loop diuretic with a thiazide agent. Other approaches to enhancing fluid removal such as ultrafiltration and the addition of other intravenous (IV) vasoactive agents have also been considered. Only a few studies have evaluated the individual efficacy and safety of these different approaches.
There is a paucity of head-to-head studies to guide our approach, with most of the comparative data being limited to the comparison of intermittent bolus to continuous infusion loop diuretic administration. In those studies the results have been somewhat conflicting. In addition, all these data have inherent limitations and are open to interpretation. Thus there continues to be a debate as to the best method for volume management in acute heart failure. In the future we may have new pharmacologic therapies and the role of ultrafiltration and other nonpharmacologic approaches may be clarified.
I thought we could start with the issue of continuous infusion and what is the role of continuous infusion compared to intermittent bolus loop diuretic administration. Perhaps Dr. Mehra could start us off by giving us his impressions on this issue.
DR. MEHRA: Let me first begin by amplifying one important item on the clinical definition of diuretic resistance. We tend to think of a patient with advanced heart failure or heart failure with decompensation as having evidence of diuretic resistance if a certain threshold of exposure to incremental IV diuretic bolus therapy does not evoke an adequate diuretic response. Clinicians consider unresponsiveness to 160 mg or 200 mg of IV furosemide administered as a bolus evokes an anemic urine output response in the subsequent two to three hours as demonstrating clinical diuretic resistance.
It is important to note also that many of these trials did not in fact include just diuretic resistance or diuretic refractory patients. Most of the recent clinical trials, such as Diuretic Optimization Strategies Evaluation (DOSE) looked at patients with congestion in the setting of decompensated heart failure who were being treated with a diuretic regimen with the idea of testing two very specific strategies of diuretic use. First, a low dose compared to a high dose strategy, and the second a continuous infusion strategy compated to a bolus dosing strategy.2 These are clinically useful strategies, which until now have not been subjected to randomized control trials. In that sense the DOSE trial has helped us move forward in a clinical direction.
To summarize the DOSE trial,2 this study suggested that neither of these specific clinical strategies were superior to the other ones, including a low dose/ high dose or bolus dose/continuous dosing with a diuretic. The study found some signals that suggested that the higher dose strategy was associated with less dyspnea but at a risk of a greater incidence of worsening of renal function, at least in the short-term. Thus, the appropriate interpretation of the DOSE trial is that it has shown us evidence of the reasonable safety and effectiveness of all of these strategies when used early in the course of decompensated heart failure. Would you agree with that, Tien and Jo Ellen?
DR. RODGERS: I definitely agree that the DOSE trial demonstrated, in a broad population of patients presenting with acute decompensated heart failure and requiring diuretic therapy ,that there is no difference between IV bolus and continuous diuretic administration, but that there is some benefit with high dose, specifically 2.5 times the oral dose, compared to a low dose. In addition to a reduced area under the curve for dyspnea at 72 hours, patients receiving high dose therapy also experienced a significant reduction in weight and net fluid loss at 72 hours. As Dr. Mehra pointed out, this benefit may be at the expense of temporary worsening of renal function. And thus, in practice, we need to be mindful of patient selection and which patients need more aggressive diuretic response compared to those patients in whom you are not willing to tolerate worsening renal function to gain the additional diuretic response. The former may be the patient who needs more aggressive relief of dyspnea—perhaps the patient in the emergency room with poor oxygen saturation. This patient should be managed with the high dose diuretic strategy. The latter may be the patient with acute or chronic renal failure in whom the risk of worsening renal function does not outweigh the benefit of more aggressive diuresis.
The issue that is critical to emphasize is that the DOSE trial assessed diuretic effectiveness of various diuretic strategies in patients who were requiring diuretic therapy, but may or may not be truly diuretic resistant. And thus, the patients enrolled were simply in decompensated heart failure secondary to fluid overload. Looking at the demographic characteristics of the trial population including baseline systolic blood pressure, serum urea nitrogen and serum creatinine as well as serum sodium, it would be anticipated that these patients would have fairly low in-hospital mortality rates compared to patients with systolic blood pressures less than 115 mm Hg, serum urea nitrogen greater than 43, and serum creatinine >3.75 mg/dL, as demonstrated by prior Acute Decompensated Heart Failure National Registry (ADHERE) data. Patients who are developing diuretic resistance are more apt to be in this latter group. Such patients may be more prone to renal dysfunction and other adverse outcomes with aggressive diuretic therapy. Diuretic response in this patient population has not been assessed.
DR. NG: Yes, I have to agree with both of you. Overall, the trial does give us some sense of the safety and the efficacy of the high versus the low dose. However, one important caveat when it comes to the interpretation of the continuous infusion compared to the intermittent infusion results was that the dosing was fixed in these two arms for the first 48 hours of the study, but then from 48 to 72 hours it became open label. There definitely was a suggestion that the continuous infusion arm had a lower dose requirement overall and required less dose escalation.
In terms of the interpretation of the DOSE trial for the continuous infusion versus intermittent infusion comparison, the lack of difference in overall urine output is in agreement with the recently published study by Dr. O’Connor’s group showing absolutely no difference between continuous infusion and intermittent bolus infusions using the same daily dose.3 On the surface it seems in contrast to the Thompson study,4 which found a trend toward an increase in urine output with continuous infusion. However, it is consistent if one considers that in the latter study the actual dose received over 24 hours was not the same. I still have some issue with the amount of diuretic that was received in the DOSE study for the intermittent versus continuous infusion comparison when the take home message of the study was that there was no difference between the two regimens.
DR. MEHRA: The fundamental issue relates to the application of these findings to clinical practice. Does it not affect clinical practice at all, or do we take comfort in the finding from the DOSE trial that, hey, the continuous high-dose diuretic strategy is reasonably safe and one should not run away from it if you deem as a clinician that that is the appropriate strategy to use by a particular patient? For example, someone with chronic long-standing heart failure with exposure to chronic high dose diuretic therapy, and some renal dysfunction at the time of inception of continuous IV diuretic therapy may be more suitable for chronic infusion therapy. At that point in time I think that my clinician gestalt would be to use a higher dose continuous infusion strategy, not simply because I am worried about the amount of urine output, but perhaps also because of the toxicity associated with the higher dose diuretic bolus regimens that one may encounter. The DOSE trial did not look at toxicity, particularly oto-toxicity; it mainly focused its safety endpoints on renal function.
DR. NG: I think the issue of other safety signals not addressed in the DOSE trial have come up in some of the other studies. A Cochrane Review,5 which was completed prior to the three latest trials, looked at all the smaller studies and found continuous infusion was associated with a lower incidence of ototoxicity compared to intermittent bolus therapy.
DR. MEHRA: That’s correct. In fact, much of that information comes from the chronic renal failure literature as well, where continuous infusions with drugs like bumetanide have been shown to result in a lower incidence of cochlear toxicity.
DR. NG: Okay, so Dr. Rodgers, I think we’re all in agreement that the DOSE trial allows us to feel better about using higher doses of loop diuretics in select acute heart failure patients. But based on the data from the most recent trials by Thompson, Allen, and the DOSE trial, do you believe there is still a strong rationale for using continuous infusion as opposed to intermittent bolus.
Any time a clinician is starting to push to higher diuretic doses with no response or is starting to consider additional therapies, one should be cognizant of the risk of additional electrolyte wasting and worsening renal function and assured that these safety parameters are closely monitored. This is when patient selection for more aggressive diuretic regimens must be carefully considered. In addition, there remain many unanswered questions about appropriate alternative therapies and the role of such in further increasing or perhaps mitigating these risks. Alternative therapies studied to date include low dose dopamine, low dose nesiritide, and other vasoactive agents.
With regard specifically to ototoxicity, this is an adverse effect that is probably very underappreciated and perhaps it’s because clinicians rarely monitor it. Most clinicians are very aware that if you administer a large, single, IV bolus dose of 180 mg of furosemide too rapidly (for example, greater than 4 mg per minute), the risk of ototoxicity is increased, but I’m less certain at what precise dose of a furosemide continuous infusion a clinician should avoid or more assertively monitor for ototoxicity. Perhaps the focus of future research should include assessing the role of these additional or alternative therapies in minimizing toxicities including ototoxicity. Do you have any suggestions, Dr. Mehra?
DR. MEHRA: I think that the data suggest that cochlear toxicity or ototoxicity is related to the peak effects and not the trough effect, so the higher the bolus dosing, the more the chance that one could have cochlear toxicity, particularly if there are other ototoxic agents being administered concomitantly. For example, if someone is receiving a drug like a gentamycin-type analog, for instance, along with a diuretic infusion or a diuretic bolus, I think there’s magnification of that ototoxicity. Frankly that isn’t the overriding concern of most clinicians, and in fact we do not systematically study it, as you’ve pointed out. I often am more concerned in the diuretic resistant patients with the best dosing strategy. I know that, Tien, you have done some work in that regard and I wonder if we could turn the conversation toward discussing what kind of combination diuretic strategies really work in reducing fluid overload.
DR. NG: Clearly another option which is commonly used in practice is the combination of diuretic agents, specifically a loop diuretic added to a thiazide agent, a thiazide which is still effective even in the setting of poor renal perfusion. There are a lot of observational and small studies that have evaluated whether or not the addition of a thiazide, such as metolazone, to a loop diuretic, such as furosemide or bumetanide, augments sodium excretion and urine output. A paper that was published in the Journal of the American College of Cardiology summarized some of these data found a signal for increased urine output.6 Our group recently evaluated the addition of metolazone to furosemide, compared to just a continuous infusion of furosemide, and found a statistically significant greater increase in mean hourly urine output with the combination compared to furosemide used by itself.
What was interesting about our data, which are consistent with some of the existing data, is there was actually no signal for worsening renal function in our patients who received the combination, although there was a signal for an increased risk of electrolyte disturbances. So the conclusion of our study, since it was an observational study, was because of potential differences in clinical efficacy and safety, there is a great need for further study in prospective trials of these alternative strategies, such as combining agents, to truly understand whether these therapies are more effective while remaining relatively safe.
DR. RODGERS: These studies have made important contributions to the literature, but as Tien explains, are not optimally designed trials. Thus, there is need for prospective evaluations to address these unanswered questions. When conducting these future prospective studies, it will be important to include endpoints such as 30-day rehospitalization. If these alternative regimens more effectively diurese, then length of hospital stay may even be reduced by a day or two. With that said, I would be concerned that patients might subsequently suffer other adverse consequences such as continued unwanted diuresis and related adverse effects post-discharge. This is an important question to address with agents such as metolazone, given the long half-life and considerable intra- and inter-patient variability in onset of action with this agent. Future prospective trials need to address outcomes, such as length of stay and rehospitalization at 30 days, for these very reasons.
There are many reasons these trials have not been prospectively conducted in the past, and one major rate-limiting factor is the uniqueness of the population of patients with refractory fluid overload. When clinicians are faced with these patients, they often start to inquire about the appropriateness of a right heart catheter and inotropic therapy simply because diuretic refractoriness may be an indicator of low cardiac output. Patient characteristics such as concomitant inotropic therapy was most often an exclusion criteria for the previously discussed trials.
DR. MEHRA: So let me respond to that, Jo Ellen. First of all, I was taught to use a lot of metolazone, and frankly, as I matured clinically, I became less enamored by that drug as I realized how tough a drug it was on the kidneys and on electrolyte balance. There is this notion of what is the appropriate clinical endpoint, and I think you alluded to it. The endpoint needs to be clinically relevant. The endpoint should focus on the patient and not be one where the clinician feels better. Essentially we have been a bit swayed by how much urine output can be evoked and how much fluid can be removed. We have a large number of papers and data emerging that tell us that there may be a significant disconnect between the amount of fluid removal and clinical outcomes. As an example, studies like the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST)7 with a vasopressin receptor antagonist showed effectiveness of the drug tolvaptan in improving hyponatremia, and in reducing weight in patients significantly, compared to placebo, but was no better than placebo in influencing 24 month outcomes from a cardiovascular endpoint. So we have to fundamentally ask the question, do any of these strategies actually make a real difference in patients?
The other issue is that we need to do a better job of discriminating among the distinct patient groups that present with diuretic resistance syndrome. So, for example, it would be clinically less useful to start using incremental diuretic strategies and multiple drug regimens in a patient who has a clear low output state causing diuretic resistance. In such a patient, perhaps, a strategy that improves cardiac output with or without the need for mechanical circulatory support might be the appropriate one to overcome diuretic resistance.
In another situation where you have acute pulmonary edema, for instance, in the setting of severe hypertension or acute ischemic syndromes, I think the treatment ought to be very different. In such situations, step number one from a clinical standpoint is to segregate the patients into classifications of why the diuretic resistance has occurred and what is the relationship of that diuretic resistance to pump function. As an example, most of the multiple drug use strategies that work do so in the setting where true renal causes of diuretic resistance have occurred. This is manifested pathologically by the development of tubular hypertrophy, which determines classical diuretic resistance. In those situations, even considering a “renal rest” such as with ultrafiltration for a few days in some series has shown to be of benefit. It is in those situations where using multiple agents may be beneficial from a fluid removal standpoint. You’re right, Jo Ellen, we have to ask ourselves to what end we are actually making patients better. And our definitions of how we make patients better are equally critical.
Tien, do you have some thoughts about that?
DR. NG: Yes, I have to agree. I think an issue has always been, what is the appropriate endpoint for our acute heart failure patients? This has been debated for years and years now, and when you look at the safety signals or the safety endpoints that are currently being used and the efficacy endpoints, we really don’t know whether these are the optimal safety and efficacy endpoints. In one sense we’re using urine output, and as you’ve pointed out there are clearly data showing that urine output isn’t necessarily the greatest indicator of an improved prognosis in these patients. At the same time, looking at serum creatinine changes as a measure of renal function obviously has its limitations as well. With serum creatinine one wouldn’t expect large changes in these relatively short-term trials, as it is a delayed marker of renal injury. So in the future, to look at the renal safety of these agents it will be important to incorporate some novel, more accurate and early markers of acute kidney injury. I think that will help us understand whether or not we are actually doing something beneficial for these patients or whether we are just treating ourselves.
DR. RODGERS: I completely agree, Dr. Ng. I think that many of our future trials are focusing on either the right population or the right endpoint. Some good examples, Dr. Mehra just mentioned ultrafiltration and the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS) trial,8 which is currently recruiting patients, and this study is specifically enrolling patients who are congested and fluid overloaded, but who have experienced renal insufficiency. The primary endpoint is a combination of weight and renal function changes. I am optimistic that any follow-up studies to CARRESS will assess endpoints such as mortality and rehospitalization. Other trials such as the Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure (RELAX) trial9 with the agent relaxin, also are enrolling patients with mild to moderate renal insufficiency. This trial is designed to assess clinically meaningful endpoints such as worsening heart failure associated with longer length of stay, as well as 60-day outcomes. It is great that these trials are addressing how to manage the patients that clinicians most struggle with.
DR. NG: Would either of you like to comment on some of the novel pharmacologic approaches which are currently being evaluated? This includes low dose nesiritide compared to low dose dopamine, the Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE) trial,10 designer natriuretic peptides such as CDNP or CUNP. Thus far agents such as vasopressin antagonists and adenosine receptor antagonists haven’t been associated with positive results from a cardiorenal standpoint; however, I was wondering if you believe these agents may still have a potential role?
DR. RODGERS: The ROSE trial, again alludes to what we previously discussed regarding the addition of other agents in an attempt to minimize toxicity of diuretic therapy. This trial is assessing our ability to effectively manage the fluid overload without causing renal injury. Similar to RELAX,9 the ROSE trial is enrolling patients with impaired renal function as defined by a creatinine clearance between 15 and 60 mL/min. As Dr. Ng had suggested, they are utilizing a new marker for renal dysfunction in this trial. Their primary endpoint is change in cystatin C. Cystatin C is an excellent marker of kidney function because it is devoid of some of the limitations or inaccuracies of measuring serum creatinine, specifically it may more readily detect milder forms of renal impairment. Also, it is more reliable in patients with varying muscle mass or protein intake, which is commonly a problem in our heart failure patients who have cachexia due to low cardiac output or fluid overload.
DR. MEHRA: I think that the future is clearly in many of these trials that are being undertaken. I’m particularly optimistic about the combined use of diuretic infusions with low dose dopamine, for instance. That has been a strategy that’s been used in the past, but it’s very controversial. I understand that one of the components of the ROSE trial that is testing these various strategies includes low dose dopamine. It should be an interesting facet to investigate to see what is the role of an old drug that’s easily available and won’t cost us incremental dollars in a cost-restrained system.
I actually do think that agents need to continue to be investigated, but I have been very disappointed with the way the current field of agents looks. As you look at the vasopressin receptor antagonists, they’ve all come out fairly short in enhancing clinical outcomes. My suspicion is that there will be sub-populations that benefit and sub-populations that are harmed by these therapies. That is the uniform outcome in almost all situations where you have high morbidity and mortality, such as in acute decompensated heart failure. My personal feeling is that we need to do a better job of finding which populations are most likely to benefit and our attention ought to be focused there. I still think that there will be selective roles for these agents in the appropriately defined groups of patients.
The problem with any such study that looks either at surrogate biomarkers like cystatin C or looks at just patient derived global assessment scales or renal function, is the very small study size, often with a small number of patients in each group. It is therefore very difficult to pull out the groups of patients in whom harm occurred and distinguish them very clearly from the groups of patients in whom clear benefit was observed. I suspect that one of the studies from which we ought to get more information in this regard is the large Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND), with more than 7000 patients.11 I think the value of that particular trial is probably confined to developing a very clear interface of investigations that will guide us to pick out those particular subgroups of patients in whom we should use targeted approaches for incremental diuretic or fluid removing strategies. What do you guys think?
DR. NG: I fully agree with both of your previous sentiments. Clearly the general sense is loop diuretics, regardless of how they’re administered or dosed, represents a suboptimal method to affect fluid removal from our volume overloaded patients. There remains hope that in the future new therapies that are more sparing from a pathophysiology standpoint will be developed so that we can effectively manage volume status in our patients while also having a positive effect on their outcomes.
So, yes, I hold hope that different combinations of pharmacologic agents as well as device therapies will be developed, and we’ll gain a greater appreciation of how to optimally select between these therapeutic options for individual patients.
Before concluding, I want to get your thoughts on one last issue. In the past we’ve approached diuresis as being a consequence of hypoperfusion. More recently, data focusing on venous congestion and how that influences diuretic responsiveness has emerged. My feeling is that as we understand the contribution of venous congestion to renal function in acute heart failure, new physiologic targets may be identified.
DR. MEHRA: Let me make a few comments about that. If you just think about the person who is most likely to develop diuretic resistance, it would be a person who has low blood pressure, chronic renal insufficiency, and very high right sided pressures. I think we’re learning now that the frequency of cardiorenal syndrome in patients with chronic heart failure is singularly linked to the severity of right heart function. And right heart failure is probably the closest correlate. Small observational studies have addressed the issue of intra-abdominal pressure as a surrogate marker for worsening renal function and improvements following release of intra-abdominal pressure, by removal of ascitic fluid or by decongesting these patients to reduce right atrial pressure, these have all shown some signals towards reduction in serum creatinine and recovery from cardiorenal syndrome.
So I think we are beginning to move in the direction that congestion is bad, that right-sided heart failure is probably the focus of our therapy, and that one surrogate for right heart failure other than the jugular venous pressure is intra-abdominal pressure, which can be very easily measured by placing a Foley in the bladders of these patients and attaching that to a pressure manometer.
DR. NG: Jo Ellen, did you have anything to add there?
DR. RODGERS: No.
DR. NG: Okay, in that case I would like to thank you all for your participation. To conclude I’ll provide a brief summary of what we’ve discussed. We began with a discussion of the role of continuous infusion and high-dose compared to low-dose loop diuretics as a method for overcoming clinical diuretic resistance. We all agree that, although the data are not airtight, they do suggest that there doesn’t seem to be large differences in terms of efficacy and in safety using high versus low or intermittent versus continuous infusion. The actual selection of the strategy should take into account the clinical status and characteristics of the individual patient.
We then moved to the issue of different diuretic strategies. We are hopeful that the addition of other agents which help augment overall diuresis will allow for a loop diuretic sparing effect, and may represent a better approach. Some of the current trials will help clarify that issue. We ended with just a very brief discussion on the need in future studies to identify optimal patients and optimal endpoints, as well as looking at the overall model of congestion, right-sided failure, and its contribution to the cardiorenal syndrome that we manage daily in our acute heart failure patients.
FoxP2 Media LLC is the publisher of The Medical Roundtable.
It is well recognized that diuretic therapy remains the main approach to volume management in heart failure patients and carries a first-line recommendation according to the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.1 However, in more advanced patients and those who have been on chronic diuretic therapy, many fail to respond to escalating doses of intermittent loop diuretics, a common clinical scenario referred to as “diuretic resistance.”
In patients exhibiting diuretic resistance, several approaches have been utilized. This includes increasing the frequency of administration or initiating a continuous infusion of the loop diuretic, changing to a more potent (on a milligram per milligram basis) loop, or combining a loop diuretic with a thiazide agent. Other approaches to enhancing fluid removal such as ultrafiltration and the addition of other intravenous (IV) vasoactive agents have also been considered. Only a few studies have evaluated the individual efficacy and safety of these different approaches.
There is a paucity of head-to-head studies to guide our approach, with most of the comparative data being limited to the comparison of intermittent bolus to continuous infusion loop diuretic administration. In those studies the results have been somewhat conflicting. In addition, all these data have inherent limitations and are open to interpretation. Thus there continues to be a debate as to the best method for volume management in acute heart failure. In the future we may have new pharmacologic therapies and the role of ultrafiltration and other nonpharmacologic approaches may be clarified.
I thought we could start with the issue of continuous infusion and what is the role of continuous infusion compared to intermittent bolus loop diuretic administration. Perhaps Dr. Mehra could start us off by giving us his impressions on this issue.
DR. MEHRA: Let me first begin by amplifying one important item on the clinical definition of diuretic resistance. We tend to think of a patient with advanced heart failure or heart failure with decompensation as having evidence of diuretic resistance if a certain threshold of exposure to incremental IV diuretic bolus therapy does not evoke an adequate diuretic response. Clinicians consider unresponsiveness to 160 mg or 200 mg of IV furosemide administered as a bolus evokes an anemic urine output response in the subsequent two to three hours as demonstrating clinical diuretic resistance.
It is important to note also that many of these trials did not in fact include just diuretic resistance or diuretic refractory patients. Most of the recent clinical trials, such as Diuretic Optimization Strategies Evaluation (DOSE) looked at patients with congestion in the setting of decompensated heart failure who were being treated with a diuretic regimen with the idea of testing two very specific strategies of diuretic use. First, a low dose compared to a high dose strategy, and the second a continuous infusion strategy compated to a bolus dosing strategy.2 These are clinically useful strategies, which until now have not been subjected to randomized control trials. In that sense the DOSE trial has helped us move forward in a clinical direction.
To summarize the DOSE trial,2 this study suggested that neither of these specific clinical strategies were superior to the other ones, including a low dose/ high dose or bolus dose/continuous dosing with a diuretic. The study found some signals that suggested that the higher dose strategy was associated with less dyspnea but at a risk of a greater incidence of worsening of renal function, at least in the short-term. Thus, the appropriate interpretation of the DOSE trial is that it has shown us evidence of the reasonable safety and effectiveness of all of these strategies when used early in the course of decompensated heart failure. Would you agree with that, Tien and Jo Ellen?
DR. RODGERS: I definitely agree that the DOSE trial demonstrated, in a broad population of patients presenting with acute decompensated heart failure and requiring diuretic therapy ,that there is no difference between IV bolus and continuous diuretic administration, but that there is some benefit with high dose, specifically 2.5 times the oral dose, compared to a low dose. In addition to a reduced area under the curve for dyspnea at 72 hours, patients receiving high dose therapy also experienced a significant reduction in weight and net fluid loss at 72 hours. As Dr. Mehra pointed out, this benefit may be at the expense of temporary worsening of renal function. And thus, in practice, we need to be mindful of patient selection and which patients need more aggressive diuretic response compared to those patients in whom you are not willing to tolerate worsening renal function to gain the additional diuretic response. The former may be the patient who needs more aggressive relief of dyspnea—perhaps the patient in the emergency room with poor oxygen saturation. This patient should be managed with the high dose diuretic strategy. The latter may be the patient with acute or chronic renal failure in whom the risk of worsening renal function does not outweigh the benefit of more aggressive diuresis.
The issue that is critical to emphasize is that the DOSE trial assessed diuretic effectiveness of various diuretic strategies in patients who were requiring diuretic therapy, but may or may not be truly diuretic resistant. And thus, the patients enrolled were simply in decompensated heart failure secondary to fluid overload. Looking at the demographic characteristics of the trial population including baseline systolic blood pressure, serum urea nitrogen and serum creatinine as well as serum sodium, it would be anticipated that these patients would have fairly low in-hospital mortality rates compared to patients with systolic blood pressures less than 115 mm Hg, serum urea nitrogen greater than 43, and serum creatinine >3.75 mg/dL, as demonstrated by prior Acute Decompensated Heart Failure National Registry (ADHERE) data. Patients who are developing diuretic resistance are more apt to be in this latter group. Such patients may be more prone to renal dysfunction and other adverse outcomes with aggressive diuretic therapy. Diuretic response in this patient population has not been assessed.
DR. NG: Yes, I have to agree with both of you. Overall, the trial does give us some sense of the safety and the efficacy of the high versus the low dose. However, one important caveat when it comes to the interpretation of the continuous infusion compared to the intermittent infusion results was that the dosing was fixed in these two arms for the first 48 hours of the study, but then from 48 to 72 hours it became open label. There definitely was a suggestion that the continuous infusion arm had a lower dose requirement overall and required less dose escalation.
In terms of the interpretation of the DOSE trial for the continuous infusion versus intermittent infusion comparison, the lack of difference in overall urine output is in agreement with the recently published study by Dr. O’Connor’s group showing absolutely no difference between continuous infusion and intermittent bolus infusions using the same daily dose.3 On the surface it seems in contrast to the Thompson study,4 which found a trend toward an increase in urine output with continuous infusion. However, it is consistent if one considers that in the latter study the actual dose received over 24 hours was not the same. I still have some issue with the amount of diuretic that was received in the DOSE study for the intermittent versus continuous infusion comparison when the take home message of the study was that there was no difference between the two regimens.
DR. MEHRA: The fundamental issue relates to the application of these findings to clinical practice. Does it not affect clinical practice at all, or do we take comfort in the finding from the DOSE trial that, hey, the continuous high-dose diuretic strategy is reasonably safe and one should not run away from it if you deem as a clinician that that is the appropriate strategy to use by a particular patient? For example, someone with chronic long-standing heart failure with exposure to chronic high dose diuretic therapy, and some renal dysfunction at the time of inception of continuous IV diuretic therapy may be more suitable for chronic infusion therapy. At that point in time I think that my clinician gestalt would be to use a higher dose continuous infusion strategy, not simply because I am worried about the amount of urine output, but perhaps also because of the toxicity associated with the higher dose diuretic bolus regimens that one may encounter. The DOSE trial did not look at toxicity, particularly oto-toxicity; it mainly focused its safety endpoints on renal function.
DR. NG: I think the issue of other safety signals not addressed in the DOSE trial have come up in some of the other studies. A Cochrane Review,5 which was completed prior to the three latest trials, looked at all the smaller studies and found continuous infusion was associated with a lower incidence of ototoxicity compared to intermittent bolus therapy.
DR. MEHRA: That’s correct. In fact, much of that information comes from the chronic renal failure literature as well, where continuous infusions with drugs like bumetanide have been shown to result in a lower incidence of cochlear toxicity.
DR. NG: Okay, so Dr. Rodgers, I think we’re all in agreement that the DOSE trial allows us to feel better about using higher doses of loop diuretics in select acute heart failure patients. But based on the data from the most recent trials by Thompson, Allen, and the DOSE trial, do you believe there is still a strong rationale for using continuous infusion as opposed to intermittent bolus.
Any time a clinician is starting to push to higher diuretic doses with no response or is starting to consider additional therapies, one should be cognizant of the risk of additional electrolyte wasting and worsening renal function and assured that these safety parameters are closely monitored. This is when patient selection for more aggressive diuretic regimens must be carefully considered. In addition, there remain many unanswered questions about appropriate alternative therapies and the role of such in further increasing or perhaps mitigating these risks. Alternative therapies studied to date include low dose dopamine, low dose nesiritide, and other vasoactive agents.
With regard specifically to ototoxicity, this is an adverse effect that is probably very underappreciated and perhaps it’s because clinicians rarely monitor it. Most clinicians are very aware that if you administer a large, single, IV bolus dose of 180 mg of furosemide too rapidly (for example, greater than 4 mg per minute), the risk of ototoxicity is increased, but I’m less certain at what precise dose of a furosemide continuous infusion a clinician should avoid or more assertively monitor for ototoxicity. Perhaps the focus of future research should include assessing the role of these additional or alternative therapies in minimizing toxicities including ototoxicity. Do you have any suggestions, Dr. Mehra?
DR. MEHRA: I think that the data suggest that cochlear toxicity or ototoxicity is related to the peak effects and not the trough effect, so the higher the bolus dosing, the more the chance that one could have cochlear toxicity, particularly if there are other ototoxic agents being administered concomitantly. For example, if someone is receiving a drug like a gentamycin-type analog, for instance, along with a diuretic infusion or a diuretic bolus, I think there’s magnification of that ototoxicity. Frankly that isn’t the overriding concern of most clinicians, and in fact we do not systematically study it, as you’ve pointed out. I often am more concerned in the diuretic resistant patients with the best dosing strategy. I know that, Tien, you have done some work in that regard and I wonder if we could turn the conversation toward discussing what kind of combination diuretic strategies really work in reducing fluid overload.
DR. NG: Clearly another option which is commonly used in practice is the combination of diuretic agents, specifically a loop diuretic added to a thiazide agent, a thiazide which is still effective even in the setting of poor renal perfusion. There are a lot of observational and small studies that have evaluated whether or not the addition of a thiazide, such as metolazone, to a loop diuretic, such as furosemide or bumetanide, augments sodium excretion and urine output. A paper that was published in the Journal of the American College of Cardiology summarized some of these data found a signal for increased urine output.6 Our group recently evaluated the addition of metolazone to furosemide, compared to just a continuous infusion of furosemide, and found a statistically significant greater increase in mean hourly urine output with the combination compared to furosemide used by itself.
What was interesting about our data, which are consistent with some of the existing data, is there was actually no signal for worsening renal function in our patients who received the combination, although there was a signal for an increased risk of electrolyte disturbances. So the conclusion of our study, since it was an observational study, was because of potential differences in clinical efficacy and safety, there is a great need for further study in prospective trials of these alternative strategies, such as combining agents, to truly understand whether these therapies are more effective while remaining relatively safe.
DR. RODGERS: These studies have made important contributions to the literature, but as Tien explains, are not optimally designed trials. Thus, there is need for prospective evaluations to address these unanswered questions. When conducting these future prospective studies, it will be important to include endpoints such as 30-day rehospitalization. If these alternative regimens more effectively diurese, then length of hospital stay may even be reduced by a day or two. With that said, I would be concerned that patients might subsequently suffer other adverse consequences such as continued unwanted diuresis and related adverse effects post-discharge. This is an important question to address with agents such as metolazone, given the long half-life and considerable intra- and inter-patient variability in onset of action with this agent. Future prospective trials need to address outcomes, such as length of stay and rehospitalization at 30 days, for these very reasons.
There are many reasons these trials have not been prospectively conducted in the past, and one major rate-limiting factor is the uniqueness of the population of patients with refractory fluid overload. When clinicians are faced with these patients, they often start to inquire about the appropriateness of a right heart catheter and inotropic therapy simply because diuretic refractoriness may be an indicator of low cardiac output. Patient characteristics such as concomitant inotropic therapy was most often an exclusion criteria for the previously discussed trials.
DR. MEHRA: So let me respond to that, Jo Ellen. First of all, I was taught to use a lot of metolazone, and frankly, as I matured clinically, I became less enamored by that drug as I realized how tough a drug it was on the kidneys and on electrolyte balance. There is this notion of what is the appropriate clinical endpoint, and I think you alluded to it. The endpoint needs to be clinically relevant. The endpoint should focus on the patient and not be one where the clinician feels better. Essentially we have been a bit swayed by how much urine output can be evoked and how much fluid can be removed. We have a large number of papers and data emerging that tell us that there may be a significant disconnect between the amount of fluid removal and clinical outcomes. As an example, studies like the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST)7 with a vasopressin receptor antagonist showed effectiveness of the drug tolvaptan in improving hyponatremia, and in reducing weight in patients significantly, compared to placebo, but was no better than placebo in influencing 24 month outcomes from a cardiovascular endpoint. So we have to fundamentally ask the question, do any of these strategies actually make a real difference in patients?
The other issue is that we need to do a better job of discriminating among the distinct patient groups that present with diuretic resistance syndrome. So, for example, it would be clinically less useful to start using incremental diuretic strategies and multiple drug regimens in a patient who has a clear low output state causing diuretic resistance. In such a patient, perhaps, a strategy that improves cardiac output with or without the need for mechanical circulatory support might be the appropriate one to overcome diuretic resistance.
In another situation where you have acute pulmonary edema, for instance, in the setting of severe hypertension or acute ischemic syndromes, I think the treatment ought to be very different. In such situations, step number one from a clinical standpoint is to segregate the patients into classifications of why the diuretic resistance has occurred and what is the relationship of that diuretic resistance to pump function. As an example, most of the multiple drug use strategies that work do so in the setting where true renal causes of diuretic resistance have occurred. This is manifested pathologically by the development of tubular hypertrophy, which determines classical diuretic resistance. In those situations, even considering a “renal rest” such as with ultrafiltration for a few days in some series has shown to be of benefit. It is in those situations where using multiple agents may be beneficial from a fluid removal standpoint. You’re right, Jo Ellen, we have to ask ourselves to what end we are actually making patients better. And our definitions of how we make patients better are equally critical.
Tien, do you have some thoughts about that?
DR. NG: Yes, I have to agree. I think an issue has always been, what is the appropriate endpoint for our acute heart failure patients? This has been debated for years and years now, and when you look at the safety signals or the safety endpoints that are currently being used and the efficacy endpoints, we really don’t know whether these are the optimal safety and efficacy endpoints. In one sense we’re using urine output, and as you’ve pointed out there are clearly data showing that urine output isn’t necessarily the greatest indicator of an improved prognosis in these patients. At the same time, looking at serum creatinine changes as a measure of renal function obviously has its limitations as well. With serum creatinine one wouldn’t expect large changes in these relatively short-term trials, as it is a delayed marker of renal injury. So in the future, to look at the renal safety of these agents it will be important to incorporate some novel, more accurate and early markers of acute kidney injury. I think that will help us understand whether or not we are actually doing something beneficial for these patients or whether we are just treating ourselves.
DR. RODGERS: I completely agree, Dr. Ng. I think that many of our future trials are focusing on either the right population or the right endpoint. Some good examples, Dr. Mehra just mentioned ultrafiltration and the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS) trial,8 which is currently recruiting patients, and this study is specifically enrolling patients who are congested and fluid overloaded, but who have experienced renal insufficiency. The primary endpoint is a combination of weight and renal function changes. I am optimistic that any follow-up studies to CARRESS will assess endpoints such as mortality and rehospitalization. Other trials such as the Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure (RELAX) trial9 with the agent relaxin, also are enrolling patients with mild to moderate renal insufficiency. This trial is designed to assess clinically meaningful endpoints such as worsening heart failure associated with longer length of stay, as well as 60-day outcomes. It is great that these trials are addressing how to manage the patients that clinicians most struggle with.
DR. NG: Would either of you like to comment on some of the novel pharmacologic approaches which are currently being evaluated? This includes low dose nesiritide compared to low dose dopamine, the Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE) trial,10 designer natriuretic peptides such as CDNP or CUNP. Thus far agents such as vasopressin antagonists and adenosine receptor antagonists haven’t been associated with positive results from a cardiorenal standpoint; however, I was wondering if you believe these agents may still have a potential role?
DR. RODGERS: The ROSE trial, again alludes to what we previously discussed regarding the addition of other agents in an attempt to minimize toxicity of diuretic therapy. This trial is assessing our ability to effectively manage the fluid overload without causing renal injury. Similar to RELAX,9 the ROSE trial is enrolling patients with impaired renal function as defined by a creatinine clearance between 15 and 60 mL/min. As Dr. Ng had suggested, they are utilizing a new marker for renal dysfunction in this trial. Their primary endpoint is change in cystatin C. Cystatin C is an excellent marker of kidney function because it is devoid of some of the limitations or inaccuracies of measuring serum creatinine, specifically it may more readily detect milder forms of renal impairment. Also, it is more reliable in patients with varying muscle mass or protein intake, which is commonly a problem in our heart failure patients who have cachexia due to low cardiac output or fluid overload.
DR. MEHRA: I think that the future is clearly in many of these trials that are being undertaken. I’m particularly optimistic about the combined use of diuretic infusions with low dose dopamine, for instance. That has been a strategy that’s been used in the past, but it’s very controversial. I understand that one of the components of the ROSE trial that is testing these various strategies includes low dose dopamine. It should be an interesting facet to investigate to see what is the role of an old drug that’s easily available and won’t cost us incremental dollars in a cost-restrained system.
I actually do think that agents need to continue to be investigated, but I have been very disappointed with the way the current field of agents looks. As you look at the vasopressin receptor antagonists, they’ve all come out fairly short in enhancing clinical outcomes. My suspicion is that there will be sub-populations that benefit and sub-populations that are harmed by these therapies. That is the uniform outcome in almost all situations where you have high morbidity and mortality, such as in acute decompensated heart failure. My personal feeling is that we need to do a better job of finding which populations are most likely to benefit and our attention ought to be focused there. I still think that there will be selective roles for these agents in the appropriately defined groups of patients.
The problem with any such study that looks either at surrogate biomarkers like cystatin C or looks at just patient derived global assessment scales or renal function, is the very small study size, often with a small number of patients in each group. It is therefore very difficult to pull out the groups of patients in whom harm occurred and distinguish them very clearly from the groups of patients in whom clear benefit was observed. I suspect that one of the studies from which we ought to get more information in this regard is the large Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND), with more than 7000 patients.11 I think the value of that particular trial is probably confined to developing a very clear interface of investigations that will guide us to pick out those particular subgroups of patients in whom we should use targeted approaches for incremental diuretic or fluid removing strategies. What do you guys think?
DR. NG: I fully agree with both of your previous sentiments. Clearly the general sense is loop diuretics, regardless of how they’re administered or dosed, represents a suboptimal method to affect fluid removal from our volume overloaded patients. There remains hope that in the future new therapies that are more sparing from a pathophysiology standpoint will be developed so that we can effectively manage volume status in our patients while also having a positive effect on their outcomes.
So, yes, I hold hope that different combinations of pharmacologic agents as well as device therapies will be developed, and we’ll gain a greater appreciation of how to optimally select between these therapeutic options for individual patients.
Before concluding, I want to get your thoughts on one last issue. In the past we’ve approached diuresis as being a consequence of hypoperfusion. More recently, data focusing on venous congestion and how that influences diuretic responsiveness has emerged. My feeling is that as we understand the contribution of venous congestion to renal function in acute heart failure, new physiologic targets may be identified.
DR. MEHRA: Let me make a few comments about that. If you just think about the person who is most likely to develop diuretic resistance, it would be a person who has low blood pressure, chronic renal insufficiency, and very high right sided pressures. I think we’re learning now that the frequency of cardiorenal syndrome in patients with chronic heart failure is singularly linked to the severity of right heart function. And right heart failure is probably the closest correlate. Small observational studies have addressed the issue of intra-abdominal pressure as a surrogate marker for worsening renal function and improvements following release of intra-abdominal pressure, by removal of ascitic fluid or by decongesting these patients to reduce right atrial pressure, these have all shown some signals towards reduction in serum creatinine and recovery from cardiorenal syndrome.
So I think we are beginning to move in the direction that congestion is bad, that right-sided heart failure is probably the focus of our therapy, and that one surrogate for right heart failure other than the jugular venous pressure is intra-abdominal pressure, which can be very easily measured by placing a Foley in the bladders of these patients and attaching that to a pressure manometer.
DR. NG: Jo Ellen, did you have anything to add there?
DR. RODGERS: No.
DR. NG: Okay, in that case I would like to thank you all for your participation. To conclude I’ll provide a brief summary of what we’ve discussed. We began with a discussion of the role of continuous infusion and high-dose compared to low-dose loop diuretics as a method for overcoming clinical diuretic resistance. We all agree that, although the data are not airtight, they do suggest that there doesn’t seem to be large differences in terms of efficacy and in safety using high versus low or intermittent versus continuous infusion. The actual selection of the strategy should take into account the clinical status and characteristics of the individual patient.
We then moved to the issue of different diuretic strategies. We are hopeful that the addition of other agents which help augment overall diuresis will allow for a loop diuretic sparing effect, and may represent a better approach. Some of the current trials will help clarify that issue. We ended with just a very brief discussion on the need in future studies to identify optimal patients and optimal endpoints, as well as looking at the overall model of congestion, right-sided failure, and its contribution to the cardiorenal syndrome that we manage daily in our acute heart failure patients.
FoxP2 Media LLC is the publisher of The Medical Roundtable.
It is well recognized that diuretic therapy remains the main approach to volume management in heart failure patients and carries a first-line recommendation according to the American College of Cardiology/American Heart Association (ACC/AHA) guidelines.1 However, in more advanced patients and those who have been on chronic diuretic therapy, many fail to respond to escalating doses of intermittent loop diuretics, a common clinical scenario referred to as “diuretic resistance.”
In patients exhibiting diuretic resistance, several approaches have been utilized. This includes increasing the frequency of administration or initiating a continuous infusion of the loop diuretic, changing to a more potent (on a milligram per milligram basis) loop, or combining a loop diuretic with a thiazide agent. Other approaches to enhancing fluid removal such as ultrafiltration and the addition of other intravenous (IV) vasoactive agents have also been considered. Only a few studies have evaluated the individual efficacy and safety of these different approaches.
There is a paucity of head-to-head studies to guide our approach, with most of the comparative data being limited to the comparison of intermittent bolus to continuous infusion loop diuretic administration. In those studies the results have been somewhat conflicting. In addition, all these data have inherent limitations and are open to interpretation. Thus there continues to be a debate as to the best method for volume management in acute heart failure. In the future we may have new pharmacologic therapies and the role of ultrafiltration and other nonpharmacologic approaches may be clarified.
I thought we could start with the issue of continuous infusion and what is the role of continuous infusion compared to intermittent bolus loop diuretic administration. Perhaps Dr. Mehra could start us off by giving us his impressions on this issue.
DR. MEHRA: Let me first begin by amplifying one important item on the clinical definition of diuretic resistance. We tend to think of a patient with advanced heart failure or heart failure with decompensation as having evidence of diuretic resistance if a certain threshold of exposure to incremental IV diuretic bolus therapy does not evoke an adequate diuretic response. Clinicians consider unresponsiveness to 160 mg or 200 mg of IV furosemide administered as a bolus evokes an anemic urine output response in the subsequent two to three hours as demonstrating clinical diuretic resistance.
It is important to note also that many of these trials did not in fact include just diuretic resistance or diuretic refractory patients. Most of the recent clinical trials, such as Diuretic Optimization Strategies Evaluation (DOSE) looked at patients with congestion in the setting of decompensated heart failure who were being treated with a diuretic regimen with the idea of testing two very specific strategies of diuretic use. First, a low dose compared to a high dose strategy, and the second a continuous infusion strategy compated to a bolus dosing strategy.2 These are clinically useful strategies, which until now have not been subjected to randomized control trials. In that sense the DOSE trial has helped us move forward in a clinical direction.
To summarize the DOSE trial,2 this study suggested that neither of these specific clinical strategies were superior to the other ones, including a low dose/ high dose or bolus dose/continuous dosing with a diuretic. The study found some signals that suggested that the higher dose strategy was associated with less dyspnea but at a risk of a greater incidence of worsening of renal function, at least in the short-term. Thus, the appropriate interpretation of the DOSE trial is that it has shown us evidence of the reasonable safety and effectiveness of all of these strategies when used early in the course of decompensated heart failure. Would you agree with that, Tien and Jo Ellen?
DR. RODGERS: I definitely agree that the DOSE trial demonstrated, in a broad population of patients presenting with acute decompensated heart failure and requiring diuretic therapy ,that there is no difference between IV bolus and continuous diuretic administration, but that there is some benefit with high dose, specifically 2.5 times the oral dose, compared to a low dose. In addition to a reduced area under the curve for dyspnea at 72 hours, patients receiving high dose therapy also experienced a significant reduction in weight and net fluid loss at 72 hours. As Dr. Mehra pointed out, this benefit may be at the expense of temporary worsening of renal function. And thus, in practice, we need to be mindful of patient selection and which patients need more aggressive diuretic response compared to those patients in whom you are not willing to tolerate worsening renal function to gain the additional diuretic response. The former may be the patient who needs more aggressive relief of dyspnea—perhaps the patient in the emergency room with poor oxygen saturation. This patient should be managed with the high dose diuretic strategy. The latter may be the patient with acute or chronic renal failure in whom the risk of worsening renal function does not outweigh the benefit of more aggressive diuresis.
The issue that is critical to emphasize is that the DOSE trial assessed diuretic effectiveness of various diuretic strategies in patients who were requiring diuretic therapy, but may or may not be truly diuretic resistant. And thus, the patients enrolled were simply in decompensated heart failure secondary to fluid overload. Looking at the demographic characteristics of the trial population including baseline systolic blood pressure, serum urea nitrogen and serum creatinine as well as serum sodium, it would be anticipated that these patients would have fairly low in-hospital mortality rates compared to patients with systolic blood pressures less than 115 mm Hg, serum urea nitrogen greater than 43, and serum creatinine >3.75 mg/dL, as demonstrated by prior Acute Decompensated Heart Failure National Registry (ADHERE) data. Patients who are developing diuretic resistance are more apt to be in this latter group. Such patients may be more prone to renal dysfunction and other adverse outcomes with aggressive diuretic therapy. Diuretic response in this patient population has not been assessed.
DR. NG: Yes, I have to agree with both of you. Overall, the trial does give us some sense of the safety and the efficacy of the high versus the low dose. However, one important caveat when it comes to the interpretation of the continuous infusion compared to the intermittent infusion results was that the dosing was fixed in these two arms for the first 48 hours of the study, but then from 48 to 72 hours it became open label. There definitely was a suggestion that the continuous infusion arm had a lower dose requirement overall and required less dose escalation.
In terms of the interpretation of the DOSE trial for the continuous infusion versus intermittent infusion comparison, the lack of difference in overall urine output is in agreement with the recently published study by Dr. O’Connor’s group showing absolutely no difference between continuous infusion and intermittent bolus infusions using the same daily dose.3 On the surface it seems in contrast to the Thompson study,4 which found a trend toward an increase in urine output with continuous infusion. However, it is consistent if one considers that in the latter study the actual dose received over 24 hours was not the same. I still have some issue with the amount of diuretic that was received in the DOSE study for the intermittent versus continuous infusion comparison when the take home message of the study was that there was no difference between the two regimens.
DR. MEHRA: The fundamental issue relates to the application of these findings to clinical practice. Does it not affect clinical practice at all, or do we take comfort in the finding from the DOSE trial that, hey, the continuous high-dose diuretic strategy is reasonably safe and one should not run away from it if you deem as a clinician that that is the appropriate strategy to use by a particular patient? For example, someone with chronic long-standing heart failure with exposure to chronic high dose diuretic therapy, and some renal dysfunction at the time of inception of continuous IV diuretic therapy may be more suitable for chronic infusion therapy. At that point in time I think that my clinician gestalt would be to use a higher dose continuous infusion strategy, not simply because I am worried about the amount of urine output, but perhaps also because of the toxicity associated with the higher dose diuretic bolus regimens that one may encounter. The DOSE trial did not look at toxicity, particularly oto-toxicity; it mainly focused its safety endpoints on renal function.
DR. NG: I think the issue of other safety signals not addressed in the DOSE trial have come up in some of the other studies. A Cochrane Review,5 which was completed prior to the three latest trials, looked at all the smaller studies and found continuous infusion was associated with a lower incidence of ototoxicity compared to intermittent bolus therapy.
DR. MEHRA: That’s correct. In fact, much of that information comes from the chronic renal failure literature as well, where continuous infusions with drugs like bumetanide have been shown to result in a lower incidence of cochlear toxicity.
DR. NG: Okay, so Dr. Rodgers, I think we’re all in agreement that the DOSE trial allows us to feel better about using higher doses of loop diuretics in select acute heart failure patients. But based on the data from the most recent trials by Thompson, Allen, and the DOSE trial, do you believe there is still a strong rationale for using continuous infusion as opposed to intermittent bolus.
Any time a clinician is starting to push to higher diuretic doses with no response or is starting to consider additional therapies, one should be cognizant of the risk of additional electrolyte wasting and worsening renal function and assured that these safety parameters are closely monitored. This is when patient selection for more aggressive diuretic regimens must be carefully considered. In addition, there remain many unanswered questions about appropriate alternative therapies and the role of such in further increasing or perhaps mitigating these risks. Alternative therapies studied to date include low dose dopamine, low dose nesiritide, and other vasoactive agents.
With regard specifically to ototoxicity, this is an adverse effect that is probably very underappreciated and perhaps it’s because clinicians rarely monitor it. Most clinicians are very aware that if you administer a large, single, IV bolus dose of 180 mg of furosemide too rapidly (for example, greater than 4 mg per minute), the risk of ototoxicity is increased, but I’m less certain at what precise dose of a furosemide continuous infusion a clinician should avoid or more assertively monitor for ototoxicity. Perhaps the focus of future research should include assessing the role of these additional or alternative therapies in minimizing toxicities including ototoxicity. Do you have any suggestions, Dr. Mehra?
DR. MEHRA: I think that the data suggest that cochlear toxicity or ototoxicity is related to the peak effects and not the trough effect, so the higher the bolus dosing, the more the chance that one could have cochlear toxicity, particularly if there are other ototoxic agents being administered concomitantly. For example, if someone is receiving a drug like a gentamycin-type analog, for instance, along with a diuretic infusion or a diuretic bolus, I think there’s magnification of that ototoxicity. Frankly that isn’t the overriding concern of most clinicians, and in fact we do not systematically study it, as you’ve pointed out. I often am more concerned in the diuretic resistant patients with the best dosing strategy. I know that, Tien, you have done some work in that regard and I wonder if we could turn the conversation toward discussing what kind of combination diuretic strategies really work in reducing fluid overload.
DR. NG: Clearly another option which is commonly used in practice is the combination of diuretic agents, specifically a loop diuretic added to a thiazide agent, a thiazide which is still effective even in the setting of poor renal perfusion. There are a lot of observational and small studies that have evaluated whether or not the addition of a thiazide, such as metolazone, to a loop diuretic, such as furosemide or bumetanide, augments sodium excretion and urine output. A paper that was published in the Journal of the American College of Cardiology summarized some of these data found a signal for increased urine output.6 Our group recently evaluated the addition of metolazone to furosemide, compared to just a continuous infusion of furosemide, and found a statistically significant greater increase in mean hourly urine output with the combination compared to furosemide used by itself.
What was interesting about our data, which are consistent with some of the existing data, is there was actually no signal for worsening renal function in our patients who received the combination, although there was a signal for an increased risk of electrolyte disturbances. So the conclusion of our study, since it was an observational study, was because of potential differences in clinical efficacy and safety, there is a great need for further study in prospective trials of these alternative strategies, such as combining agents, to truly understand whether these therapies are more effective while remaining relatively safe.
DR. RODGERS: These studies have made important contributions to the literature, but as Tien explains, are not optimally designed trials. Thus, there is need for prospective evaluations to address these unanswered questions. When conducting these future prospective studies, it will be important to include endpoints such as 30-day rehospitalization. If these alternative regimens more effectively diurese, then length of hospital stay may even be reduced by a day or two. With that said, I would be concerned that patients might subsequently suffer other adverse consequences such as continued unwanted diuresis and related adverse effects post-discharge. This is an important question to address with agents such as metolazone, given the long half-life and considerable intra- and inter-patient variability in onset of action with this agent. Future prospective trials need to address outcomes, such as length of stay and rehospitalization at 30 days, for these very reasons.
There are many reasons these trials have not been prospectively conducted in the past, and one major rate-limiting factor is the uniqueness of the population of patients with refractory fluid overload. When clinicians are faced with these patients, they often start to inquire about the appropriateness of a right heart catheter and inotropic therapy simply because diuretic refractoriness may be an indicator of low cardiac output. Patient characteristics such as concomitant inotropic therapy was most often an exclusion criteria for the previously discussed trials.
DR. MEHRA: So let me respond to that, Jo Ellen. First of all, I was taught to use a lot of metolazone, and frankly, as I matured clinically, I became less enamored by that drug as I realized how tough a drug it was on the kidneys and on electrolyte balance. There is this notion of what is the appropriate clinical endpoint, and I think you alluded to it. The endpoint needs to be clinically relevant. The endpoint should focus on the patient and not be one where the clinician feels better. Essentially we have been a bit swayed by how much urine output can be evoked and how much fluid can be removed. We have a large number of papers and data emerging that tell us that there may be a significant disconnect between the amount of fluid removal and clinical outcomes. As an example, studies like the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST)7 with a vasopressin receptor antagonist showed effectiveness of the drug tolvaptan in improving hyponatremia, and in reducing weight in patients significantly, compared to placebo, but was no better than placebo in influencing 24 month outcomes from a cardiovascular endpoint. So we have to fundamentally ask the question, do any of these strategies actually make a real difference in patients?
The other issue is that we need to do a better job of discriminating among the distinct patient groups that present with diuretic resistance syndrome. So, for example, it would be clinically less useful to start using incremental diuretic strategies and multiple drug regimens in a patient who has a clear low output state causing diuretic resistance. In such a patient, perhaps, a strategy that improves cardiac output with or without the need for mechanical circulatory support might be the appropriate one to overcome diuretic resistance.
In another situation where you have acute pulmonary edema, for instance, in the setting of severe hypertension or acute ischemic syndromes, I think the treatment ought to be very different. In such situations, step number one from a clinical standpoint is to segregate the patients into classifications of why the diuretic resistance has occurred and what is the relationship of that diuretic resistance to pump function. As an example, most of the multiple drug use strategies that work do so in the setting where true renal causes of diuretic resistance have occurred. This is manifested pathologically by the development of tubular hypertrophy, which determines classical diuretic resistance. In those situations, even considering a “renal rest” such as with ultrafiltration for a few days in some series has shown to be of benefit. It is in those situations where using multiple agents may be beneficial from a fluid removal standpoint. You’re right, Jo Ellen, we have to ask ourselves to what end we are actually making patients better. And our definitions of how we make patients better are equally critical.
Tien, do you have some thoughts about that?
DR. NG: Yes, I have to agree. I think an issue has always been, what is the appropriate endpoint for our acute heart failure patients? This has been debated for years and years now, and when you look at the safety signals or the safety endpoints that are currently being used and the efficacy endpoints, we really don’t know whether these are the optimal safety and efficacy endpoints. In one sense we’re using urine output, and as you’ve pointed out there are clearly data showing that urine output isn’t necessarily the greatest indicator of an improved prognosis in these patients. At the same time, looking at serum creatinine changes as a measure of renal function obviously has its limitations as well. With serum creatinine one wouldn’t expect large changes in these relatively short-term trials, as it is a delayed marker of renal injury. So in the future, to look at the renal safety of these agents it will be important to incorporate some novel, more accurate and early markers of acute kidney injury. I think that will help us understand whether or not we are actually doing something beneficial for these patients or whether we are just treating ourselves.
DR. RODGERS: I completely agree, Dr. Ng. I think that many of our future trials are focusing on either the right population or the right endpoint. Some good examples, Dr. Mehra just mentioned ultrafiltration and the Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS) trial,8 which is currently recruiting patients, and this study is specifically enrolling patients who are congested and fluid overloaded, but who have experienced renal insufficiency. The primary endpoint is a combination of weight and renal function changes. I am optimistic that any follow-up studies to CARRESS will assess endpoints such as mortality and rehospitalization. Other trials such as the Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure (RELAX) trial9 with the agent relaxin, also are enrolling patients with mild to moderate renal insufficiency. This trial is designed to assess clinically meaningful endpoints such as worsening heart failure associated with longer length of stay, as well as 60-day outcomes. It is great that these trials are addressing how to manage the patients that clinicians most struggle with.
DR. NG: Would either of you like to comment on some of the novel pharmacologic approaches which are currently being evaluated? This includes low dose nesiritide compared to low dose dopamine, the Renal Optimization Strategies Evaluation in Acute Heart Failure (ROSE) trial,10 designer natriuretic peptides such as CDNP or CUNP. Thus far agents such as vasopressin antagonists and adenosine receptor antagonists haven’t been associated with positive results from a cardiorenal standpoint; however, I was wondering if you believe these agents may still have a potential role?
DR. RODGERS: The ROSE trial, again alludes to what we previously discussed regarding the addition of other agents in an attempt to minimize toxicity of diuretic therapy. This trial is assessing our ability to effectively manage the fluid overload without causing renal injury. Similar to RELAX,9 the ROSE trial is enrolling patients with impaired renal function as defined by a creatinine clearance between 15 and 60 mL/min. As Dr. Ng had suggested, they are utilizing a new marker for renal dysfunction in this trial. Their primary endpoint is change in cystatin C. Cystatin C is an excellent marker of kidney function because it is devoid of some of the limitations or inaccuracies of measuring serum creatinine, specifically it may more readily detect milder forms of renal impairment. Also, it is more reliable in patients with varying muscle mass or protein intake, which is commonly a problem in our heart failure patients who have cachexia due to low cardiac output or fluid overload.
DR. MEHRA: I think that the future is clearly in many of these trials that are being undertaken. I’m particularly optimistic about the combined use of diuretic infusions with low dose dopamine, for instance. That has been a strategy that’s been used in the past, but it’s very controversial. I understand that one of the components of the ROSE trial that is testing these various strategies includes low dose dopamine. It should be an interesting facet to investigate to see what is the role of an old drug that’s easily available and won’t cost us incremental dollars in a cost-restrained system.
I actually do think that agents need to continue to be investigated, but I have been very disappointed with the way the current field of agents looks. As you look at the vasopressin receptor antagonists, they’ve all come out fairly short in enhancing clinical outcomes. My suspicion is that there will be sub-populations that benefit and sub-populations that are harmed by these therapies. That is the uniform outcome in almost all situations where you have high morbidity and mortality, such as in acute decompensated heart failure. My personal feeling is that we need to do a better job of finding which populations are most likely to benefit and our attention ought to be focused there. I still think that there will be selective roles for these agents in the appropriately defined groups of patients.
The problem with any such study that looks either at surrogate biomarkers like cystatin C or looks at just patient derived global assessment scales or renal function, is the very small study size, often with a small number of patients in each group. It is therefore very difficult to pull out the groups of patients in whom harm occurred and distinguish them very clearly from the groups of patients in whom clear benefit was observed. I suspect that one of the studies from which we ought to get more information in this regard is the large Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND), with more than 7000 patients.11 I think the value of that particular trial is probably confined to developing a very clear interface of investigations that will guide us to pick out those particular subgroups of patients in whom we should use targeted approaches for incremental diuretic or fluid removing strategies. What do you guys think?
DR. NG: I fully agree with both of your previous sentiments. Clearly the general sense is loop diuretics, regardless of how they’re administered or dosed, represents a suboptimal method to affect fluid removal from our volume overloaded patients. There remains hope that in the future new therapies that are more sparing from a pathophysiology standpoint will be developed so that we can effectively manage volume status in our patients while also having a positive effect on their outcomes.
So, yes, I hold hope that different combinations of pharmacologic agents as well as device therapies will be developed, and we’ll gain a greater appreciation of how to optimally select between these therapeutic options for individual patients.
Before concluding, I want to get your thoughts on one last issue. In the past we’ve approached diuresis as being a consequence of hypoperfusion. More recently, data focusing on venous congestion and how that influences diuretic responsiveness has emerged. My feeling is that as we understand the contribution of venous congestion to renal function in acute heart failure, new physiologic targets may be identified.
DR. MEHRA: Let me make a few comments about that. If you just think about the person who is most likely to develop diuretic resistance, it would be a person who has low blood pressure, chronic renal insufficiency, and very high right sided pressures. I think we’re learning now that the frequency of cardiorenal syndrome in patients with chronic heart failure is singularly linked to the severity of right heart function. And right heart failure is probably the closest correlate. Small observational studies have addressed the issue of intra-abdominal pressure as a surrogate marker for worsening renal function and improvements following release of intra-abdominal pressure, by removal of ascitic fluid or by decongesting these patients to reduce right atrial pressure, these have all shown some signals towards reduction in serum creatinine and recovery from cardiorenal syndrome.
So I think we are beginning to move in the direction that congestion is bad, that right-sided heart failure is probably the focus of our therapy, and that one surrogate for right heart failure other than the jugular venous pressure is intra-abdominal pressure, which can be very easily measured by placing a Foley in the bladders of these patients and attaching that to a pressure manometer.
DR. NG: Jo Ellen, did you have anything to add there?
DR. RODGERS: No.
DR. NG: Okay, in that case I would like to thank you all for your participation. To conclude I’ll provide a brief summary of what we’ve discussed. We began with a discussion of the role of continuous infusion and high-dose compared to low-dose loop diuretics as a method for overcoming clinical diuretic resistance. We all agree that, although the data are not airtight, they do suggest that there doesn’t seem to be large differences in terms of efficacy and in safety using high versus low or intermittent versus continuous infusion. The actual selection of the strategy should take into account the clinical status and characteristics of the individual patient.
We then moved to the issue of different diuretic strategies. We are hopeful that the addition of other agents which help augment overall diuresis will allow for a loop diuretic sparing effect, and may represent a better approach. Some of the current trials will help clarify that issue. We ended with just a very brief discussion on the need in future studies to identify optimal patients and optimal endpoints, as well as looking at the overall model of congestion, right-sided failure, and its contribution to the cardiorenal syndrome that we manage daily in our acute heart failure patients.
FoxP2 Media LLC is the publisher of The Medical Roundtable.
FoxP2 Media LLC
BVS and renal denervation fuel a continental divide
It’s no secret that the Food and Drug Administration and the European Commission take very different approaches to how they allow medical devices onto the market. Their different tacks have produced a profound disconnect in how cardiologists on the two continents use two of the newest technologies in their specialty: bioabsorbable vascular scaffolds for coronary disease and renal denervation for controlling treatment-resistant hypertension.
The European Commission granted a CE mark to the first bioabsorbable vascular stent (BVS), Abbott’s Absorb, in 2011, and Abbott announced the launch of international marketing in September 2012. By last May, an Absorb BVS had been placed in more than 50,000 patients worldwide, according to a company spokesperson.
In contrast, the U.S. total of patients who received a BVS is much lower, and is limited to those randomized to receive a BVS in one of the two ongoing U.S. pivotal trials comparing its performance with a second-generation, drug-eluting metallic stent. One of those trials, with 2,000 total patients, completed enrollment in April 2014, but a second slated to have 3,000 total continues to enroll, so the soonest a BVS might come onto the U.S. market is at least 3 years off.
Not only does Europe have tens of thousands of BVS-treated patients, but several established interventional programs there, such as the Thoraxcenter in Rotterdam (the Netherlands), and others in Italy, Spain, Poland, and the Czech Republic, have adopted the BVS as their default approach for treating patients with percutaneous coronary intervention (PCI). For now, a BVS-first approach does not translate into universal use because only about a fifth of all PCI patients have anatomy suitable for BVS placement. But these BVS-first centers are using them in any PCI patient with anatomy that’s appropriate regardless of the severity of their coronary disease or the complexity of their coronary lesion. This means that BVSs have been placed in growing numbers of European patients with myocardial infarctions, acute coronary syndrome, and bifurcations, as well as in other types of clinically advanced PCI patients.
For renal denervation the numbers are not as disparate, but the prevailing attitudes of many thought leaders are. In Europe, Asia, and a few other parts of the world, interventionalists have used the Symplicity catheter, the first renal denervation device on the world market starting in 2010, in more than 5,500 patients as of this August, according to a spokesperson for Medtronic, the company that sells this device.
The consensus among many European interventional cardiologists and hypertension specialists at the annual EuroPCR meeting was that renal denervation is an effective and valuable option for managing otherwise uncontrolled hypertension, when performed carefully and thoroughly in well-selected patients with true drug-resistant hypertension. They stuck with that opinion despite the report in March that renal denervation failed to produce a significant blood pressure reduction compared with a sham procedure in the pivotal U.S. trial, SYMPLICITY HTN 3 (New Engl. J. Med. 2014;370:1393-1401).
A statement from three leading European cardiologists issued under the auspices of the EuroPCR meeting last May spelled out that despite the negative trial findings, "renal denervation is an option in patients with difficult-to-control hypertension, in whom other treatments have failed."
In the United States, the failed pivotal trial seems to have temporarily put U.S. testing of renal denervation on hold as companies and their consultants scramble to figure out how to avoid the problems that doomed SYMPLICITY HTN 3.
U.S. and European cardiologists show agreement in several other new areas of practice, but for BVS and renal denervation the transatlantic divide is stark.
On Twitter @mitchelzoler
It’s no secret that the Food and Drug Administration and the European Commission take very different approaches to how they allow medical devices onto the market. Their different tacks have produced a profound disconnect in how cardiologists on the two continents use two of the newest technologies in their specialty: bioabsorbable vascular scaffolds for coronary disease and renal denervation for controlling treatment-resistant hypertension.
The European Commission granted a CE mark to the first bioabsorbable vascular stent (BVS), Abbott’s Absorb, in 2011, and Abbott announced the launch of international marketing in September 2012. By last May, an Absorb BVS had been placed in more than 50,000 patients worldwide, according to a company spokesperson.
In contrast, the U.S. total of patients who received a BVS is much lower, and is limited to those randomized to receive a BVS in one of the two ongoing U.S. pivotal trials comparing its performance with a second-generation, drug-eluting metallic stent. One of those trials, with 2,000 total patients, completed enrollment in April 2014, but a second slated to have 3,000 total continues to enroll, so the soonest a BVS might come onto the U.S. market is at least 3 years off.
Not only does Europe have tens of thousands of BVS-treated patients, but several established interventional programs there, such as the Thoraxcenter in Rotterdam (the Netherlands), and others in Italy, Spain, Poland, and the Czech Republic, have adopted the BVS as their default approach for treating patients with percutaneous coronary intervention (PCI). For now, a BVS-first approach does not translate into universal use because only about a fifth of all PCI patients have anatomy suitable for BVS placement. But these BVS-first centers are using them in any PCI patient with anatomy that’s appropriate regardless of the severity of their coronary disease or the complexity of their coronary lesion. This means that BVSs have been placed in growing numbers of European patients with myocardial infarctions, acute coronary syndrome, and bifurcations, as well as in other types of clinically advanced PCI patients.
For renal denervation the numbers are not as disparate, but the prevailing attitudes of many thought leaders are. In Europe, Asia, and a few other parts of the world, interventionalists have used the Symplicity catheter, the first renal denervation device on the world market starting in 2010, in more than 5,500 patients as of this August, according to a spokesperson for Medtronic, the company that sells this device.
The consensus among many European interventional cardiologists and hypertension specialists at the annual EuroPCR meeting was that renal denervation is an effective and valuable option for managing otherwise uncontrolled hypertension, when performed carefully and thoroughly in well-selected patients with true drug-resistant hypertension. They stuck with that opinion despite the report in March that renal denervation failed to produce a significant blood pressure reduction compared with a sham procedure in the pivotal U.S. trial, SYMPLICITY HTN 3 (New Engl. J. Med. 2014;370:1393-1401).
A statement from three leading European cardiologists issued under the auspices of the EuroPCR meeting last May spelled out that despite the negative trial findings, "renal denervation is an option in patients with difficult-to-control hypertension, in whom other treatments have failed."
In the United States, the failed pivotal trial seems to have temporarily put U.S. testing of renal denervation on hold as companies and their consultants scramble to figure out how to avoid the problems that doomed SYMPLICITY HTN 3.
U.S. and European cardiologists show agreement in several other new areas of practice, but for BVS and renal denervation the transatlantic divide is stark.
On Twitter @mitchelzoler
It’s no secret that the Food and Drug Administration and the European Commission take very different approaches to how they allow medical devices onto the market. Their different tacks have produced a profound disconnect in how cardiologists on the two continents use two of the newest technologies in their specialty: bioabsorbable vascular scaffolds for coronary disease and renal denervation for controlling treatment-resistant hypertension.
The European Commission granted a CE mark to the first bioabsorbable vascular stent (BVS), Abbott’s Absorb, in 2011, and Abbott announced the launch of international marketing in September 2012. By last May, an Absorb BVS had been placed in more than 50,000 patients worldwide, according to a company spokesperson.
In contrast, the U.S. total of patients who received a BVS is much lower, and is limited to those randomized to receive a BVS in one of the two ongoing U.S. pivotal trials comparing its performance with a second-generation, drug-eluting metallic stent. One of those trials, with 2,000 total patients, completed enrollment in April 2014, but a second slated to have 3,000 total continues to enroll, so the soonest a BVS might come onto the U.S. market is at least 3 years off.
Not only does Europe have tens of thousands of BVS-treated patients, but several established interventional programs there, such as the Thoraxcenter in Rotterdam (the Netherlands), and others in Italy, Spain, Poland, and the Czech Republic, have adopted the BVS as their default approach for treating patients with percutaneous coronary intervention (PCI). For now, a BVS-first approach does not translate into universal use because only about a fifth of all PCI patients have anatomy suitable for BVS placement. But these BVS-first centers are using them in any PCI patient with anatomy that’s appropriate regardless of the severity of their coronary disease or the complexity of their coronary lesion. This means that BVSs have been placed in growing numbers of European patients with myocardial infarctions, acute coronary syndrome, and bifurcations, as well as in other types of clinically advanced PCI patients.
For renal denervation the numbers are not as disparate, but the prevailing attitudes of many thought leaders are. In Europe, Asia, and a few other parts of the world, interventionalists have used the Symplicity catheter, the first renal denervation device on the world market starting in 2010, in more than 5,500 patients as of this August, according to a spokesperson for Medtronic, the company that sells this device.
The consensus among many European interventional cardiologists and hypertension specialists at the annual EuroPCR meeting was that renal denervation is an effective and valuable option for managing otherwise uncontrolled hypertension, when performed carefully and thoroughly in well-selected patients with true drug-resistant hypertension. They stuck with that opinion despite the report in March that renal denervation failed to produce a significant blood pressure reduction compared with a sham procedure in the pivotal U.S. trial, SYMPLICITY HTN 3 (New Engl. J. Med. 2014;370:1393-1401).
A statement from three leading European cardiologists issued under the auspices of the EuroPCR meeting last May spelled out that despite the negative trial findings, "renal denervation is an option in patients with difficult-to-control hypertension, in whom other treatments have failed."
In the United States, the failed pivotal trial seems to have temporarily put U.S. testing of renal denervation on hold as companies and their consultants scramble to figure out how to avoid the problems that doomed SYMPLICITY HTN 3.
U.S. and European cardiologists show agreement in several other new areas of practice, but for BVS and renal denervation the transatlantic divide is stark.
On Twitter @mitchelzoler
Responding to the heroin epidemic one patient at a time
Once regarded as a curse of the urban poor, heroin now has a stronghold on many suburban, middle class communities.I was stunned to learn that heroin use is so prevalent in the quiet Baltimore suburb where I work that our local police officers carry Narcan kits. Use has become so ubiquitous in our country that U.S. Attorney General Eric Holder has said law enforcement officials should consider carrying heroin’s antidote. From Smalltown U.S.A. to booming metropolises, this inexpensive narcotic is wreaking havoc on individuals and their families.
Sure, I admit an occasional patient with "a history of heroin abuse" who takes methadone. Rarely, I may see physical evidence of heroin use. But in most cases, patients who use heroin present as overdose cases in the ED, where they are discharged home when they are stable or admitted directly to the intensive care unit. Sadly, I recently received from the ICU a transfer of a heroin overdose patient – a handsome young man in his 20s, his entire life ahead of him, loving parents and siblings at his bedside. He was completely oblivious to everything around him – comatose, on hospice, dying before he had a real chance to live.
There was nothing I could do for him or his family other than to provide comfort. But perhaps I can do more for future potential overdose victims. You know, the heroin users admitted for a skin abscess after missing a vein or for DKA because they were too high to remember to take their insulin. Yes, we are busy; but we need to take 5-10 minutes to address the issue, to listen to the user’s story and encourage them, uplift them. Substance abuse counselors are invaluable, but by taking the time to care about our patients as individuals, hospitalists might just be the straw to break the camel’s back of heroin use for someone. Considering that most heroin addicts these days are young adults, a 10 minute investment of our time now may help buy those patients back another 40 or 50 years of their lives.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
Once regarded as a curse of the urban poor, heroin now has a stronghold on many suburban, middle class communities.I was stunned to learn that heroin use is so prevalent in the quiet Baltimore suburb where I work that our local police officers carry Narcan kits. Use has become so ubiquitous in our country that U.S. Attorney General Eric Holder has said law enforcement officials should consider carrying heroin’s antidote. From Smalltown U.S.A. to booming metropolises, this inexpensive narcotic is wreaking havoc on individuals and their families.
Sure, I admit an occasional patient with "a history of heroin abuse" who takes methadone. Rarely, I may see physical evidence of heroin use. But in most cases, patients who use heroin present as overdose cases in the ED, where they are discharged home when they are stable or admitted directly to the intensive care unit. Sadly, I recently received from the ICU a transfer of a heroin overdose patient – a handsome young man in his 20s, his entire life ahead of him, loving parents and siblings at his bedside. He was completely oblivious to everything around him – comatose, on hospice, dying before he had a real chance to live.
There was nothing I could do for him or his family other than to provide comfort. But perhaps I can do more for future potential overdose victims. You know, the heroin users admitted for a skin abscess after missing a vein or for DKA because they were too high to remember to take their insulin. Yes, we are busy; but we need to take 5-10 minutes to address the issue, to listen to the user’s story and encourage them, uplift them. Substance abuse counselors are invaluable, but by taking the time to care about our patients as individuals, hospitalists might just be the straw to break the camel’s back of heroin use for someone. Considering that most heroin addicts these days are young adults, a 10 minute investment of our time now may help buy those patients back another 40 or 50 years of their lives.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].
Once regarded as a curse of the urban poor, heroin now has a stronghold on many suburban, middle class communities.I was stunned to learn that heroin use is so prevalent in the quiet Baltimore suburb where I work that our local police officers carry Narcan kits. Use has become so ubiquitous in our country that U.S. Attorney General Eric Holder has said law enforcement officials should consider carrying heroin’s antidote. From Smalltown U.S.A. to booming metropolises, this inexpensive narcotic is wreaking havoc on individuals and their families.
Sure, I admit an occasional patient with "a history of heroin abuse" who takes methadone. Rarely, I may see physical evidence of heroin use. But in most cases, patients who use heroin present as overdose cases in the ED, where they are discharged home when they are stable or admitted directly to the intensive care unit. Sadly, I recently received from the ICU a transfer of a heroin overdose patient – a handsome young man in his 20s, his entire life ahead of him, loving parents and siblings at his bedside. He was completely oblivious to everything around him – comatose, on hospice, dying before he had a real chance to live.
There was nothing I could do for him or his family other than to provide comfort. But perhaps I can do more for future potential overdose victims. You know, the heroin users admitted for a skin abscess after missing a vein or for DKA because they were too high to remember to take their insulin. Yes, we are busy; but we need to take 5-10 minutes to address the issue, to listen to the user’s story and encourage them, uplift them. Substance abuse counselors are invaluable, but by taking the time to care about our patients as individuals, hospitalists might just be the straw to break the camel’s back of heroin use for someone. Considering that most heroin addicts these days are young adults, a 10 minute investment of our time now may help buy those patients back another 40 or 50 years of their lives.
Dr. Hester is a hospitalist at Baltimore-Washington Medical Center in Glen Burnie, Md. She is the creator of the Patient Whiz, a patient-engagement app for iOS. Reach her at [email protected].