User login
Circumscribed Nodule in a Renal Transplant Patient
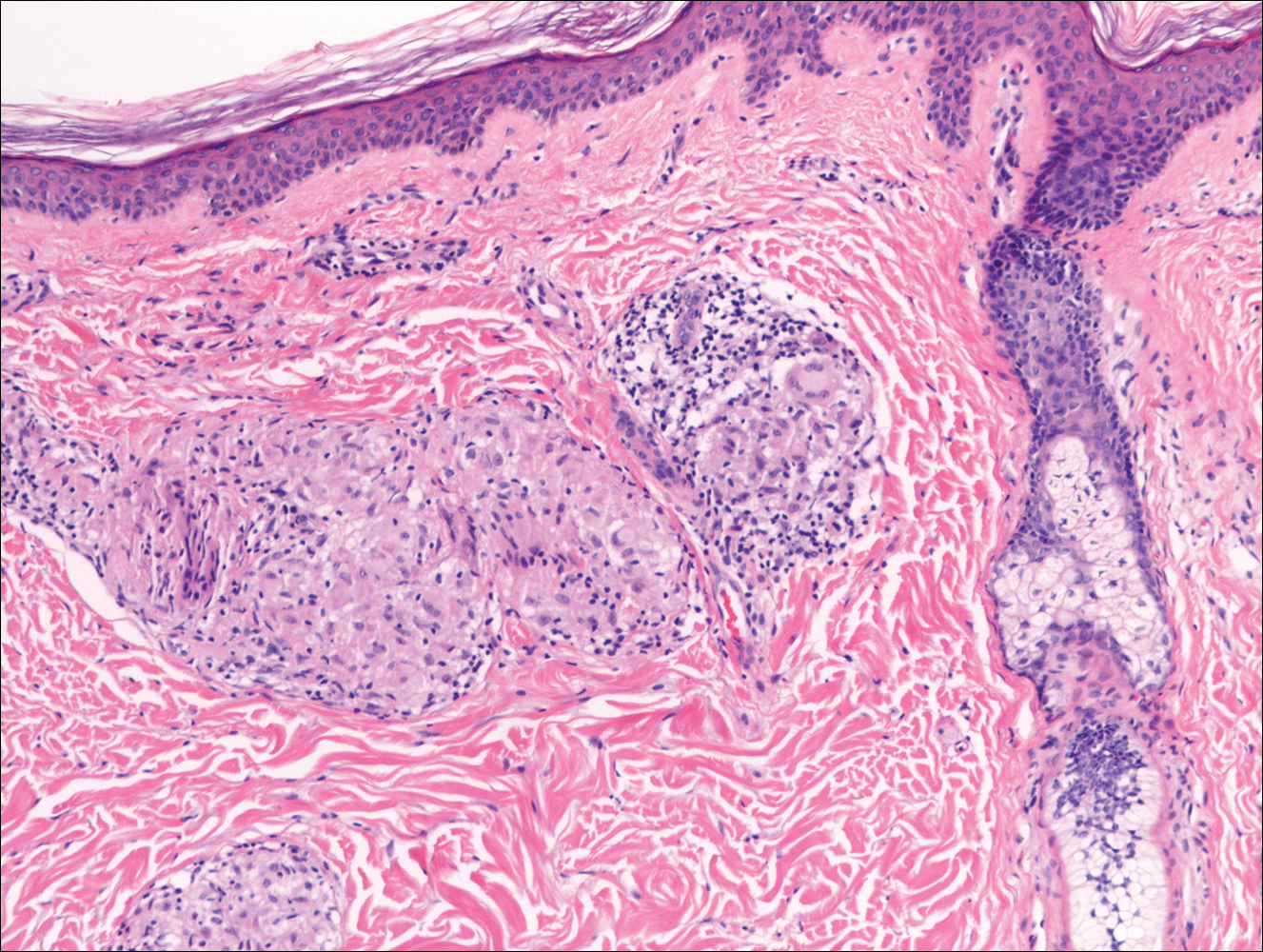
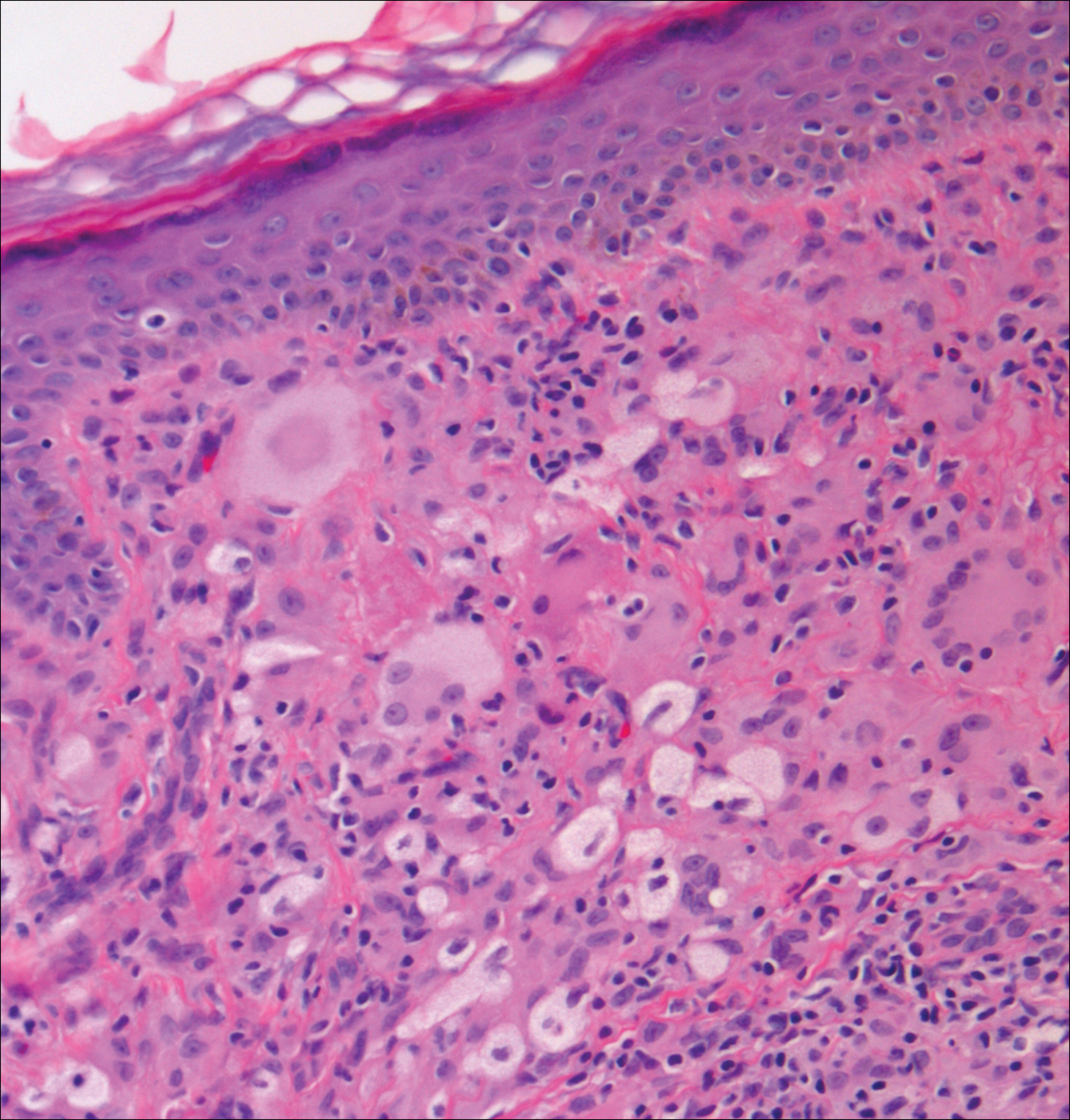
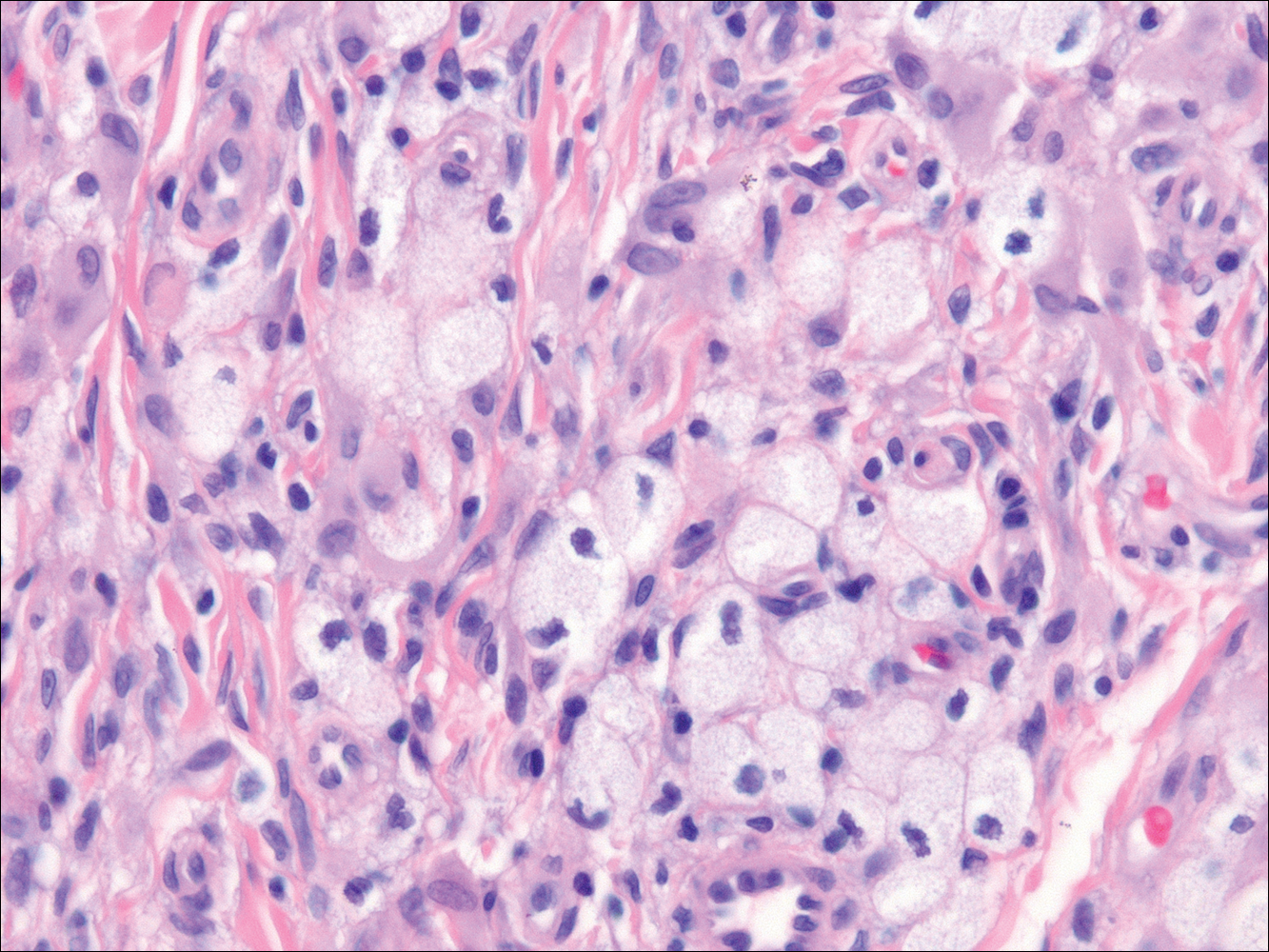
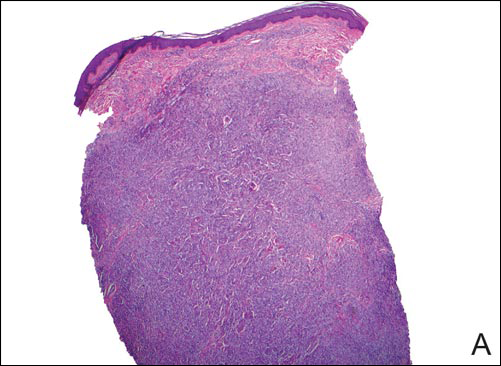
The Diagnosis: Subcutaneous Phaeohyphomycosis
Subcutaneous phaeohyphomycosis (SP), also called mycotic cyst, is characterized by a painless, nodular lesion that develops in response to traumatic implantation of dematiaceous, pigment-forming fungi.1 Similar to other fungal infections, SP can arise opportunistically in immunocompromised patients.2,3 More than 60 genera (and more than 100 species) are known etiologic agents of phaeohyphomycosis; the 2 main causes of infection are Bipolaris spicifera and Exophiala jeanselmei.4,5 Given this variety, phaeohyphomycosis can present superficially as black piedra or tinea nigra, cutaneously as scytalidiosis, subcutaneously as SP, or disseminated as sinusitis or systemic phaeohyphomycosis.
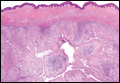
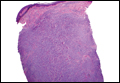
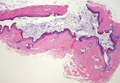
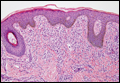
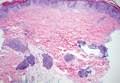
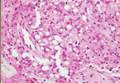
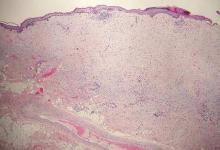
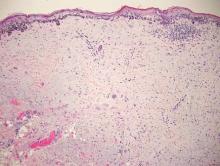
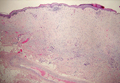
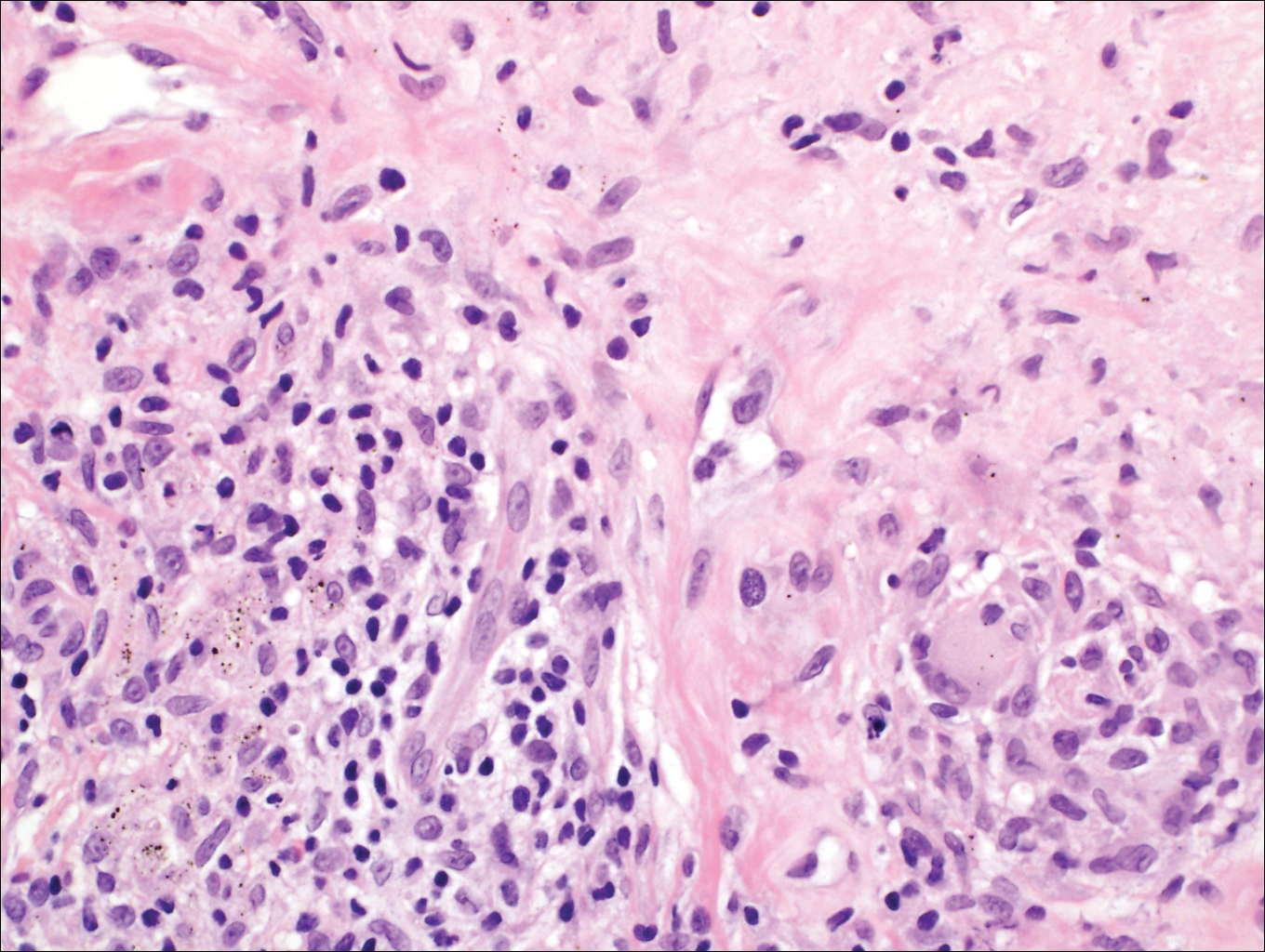
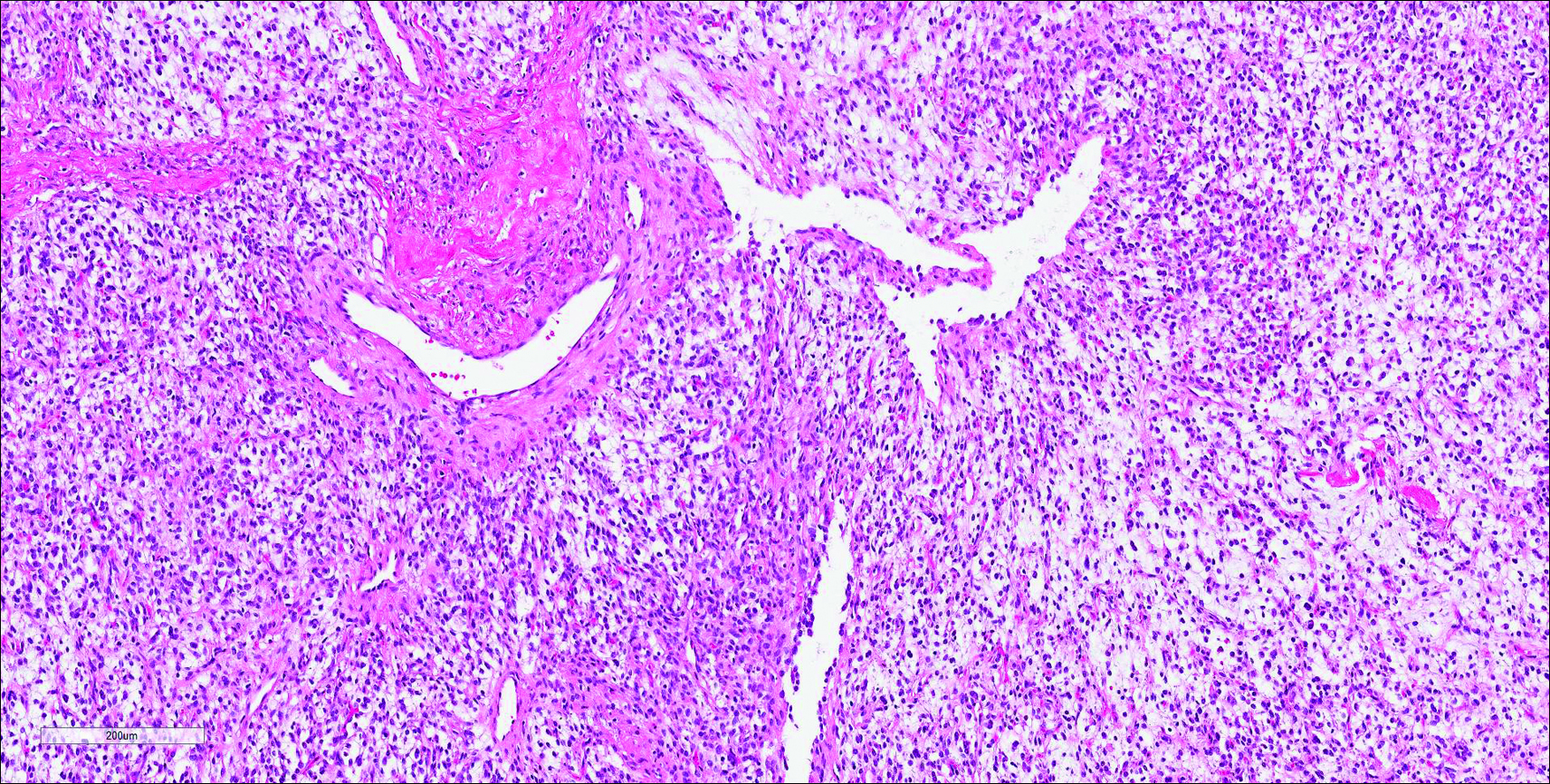
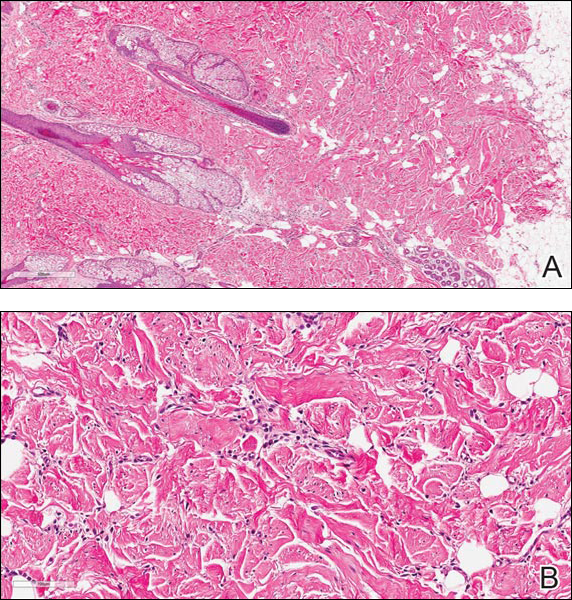
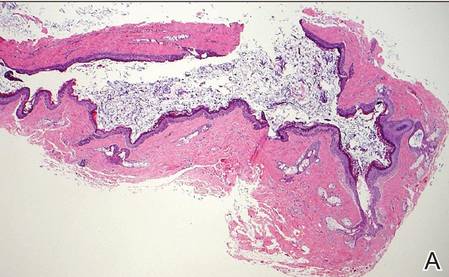
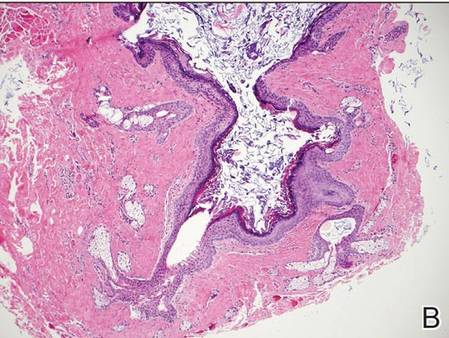
Coined in 1974 by Ajello et al,6 the term phaeohyphomycosis translates to “condition of dark hyphal fungus,” a term used to designate mycoses caused by fungi with melanized hyphae. Histologically, SP demonstrates a circumscribed chronic cyst or abscess with a dense fibrous wall (quiz image A). At high power, the wall is composed of chronic granulomatous inflammation with foamy macrophages, and the cystic cavity contains necrotic debris admixed with neutrophils. Pigmented filamentous hyphae and yeastlike entities can be seen in the cyst wall, in multinucleated giant cells, in the necrotic debris, or directly attached to the implanted foreign material (quiz image B).7 The first-line treatment of SP is wide local excision and oral itraconazole. It often requires adjustments to dosage or change to antifungal due to recurrence and etiologic variation.8 Furthermore, if SP is not definitively treated, immunocompromised patients are at an increased risk for developing potentially fatal systemic phaeohyphomycosis.3
Chromoblastomycosis (CBM), also caused by dematiaceous fungi, is characterized by an initially indolent clinical presentation. Typically found on the legs and lower thighs of agricultural workers, the lesion begins as a slow-growing, nodular papule with subsequent transformation into an edematous verrucous plaque with peripheral erythema.9 Lesions can be annular with central clearing, and lymphedema with elephantiasis may be present.10 Histologically, CBM shows pseudoepitheliomatous hyperplasia and intraepidermal pustules as the host rids the infection via transepithelial elimination. Dematiaceous fungi often are seen in the dermis, either freestanding or attached to foreign plant material. Medlar bodies, also called copper penny spores or sclerotic bodies, are the most defining histologic finding and are characterized by groups of brown, thick-walled cells found in giant cells or neutrophil abscesses (Figure 1). Hyphae are not typically found in this type of infection.11

Granulomatous foreign body reactions occur in response to the inoculation of nonhuman material and are characterized by dermal or subcutaneous nodules. Tissue macrophages phagocytize material not removed shortly after implantation, which initiates an inflammatory response that attempts to isolate the material from the uninvolved surrounding tissue. Vegetative foreign bodies will cause the most severe inflammatory reactions.12 Histologically, foreign body granulomas are noncaseating with epithelioid histiocytes surrounding a central foreign body (Figure 2). Occasionally, foreign bodies may be difficult to detect; some are birefringent to polarized light.13 Additionally, inoculation injuries can predispose patients to SP, CBM, and other fungal infections.

Tattoos are characterized by exogenous pigment deposition into the dermis.14 Histologically, tattoos display exogenous pigment deposited throughout the reticular dermis, attached to collagen bundles, within macrophages, or adjacent to adnexal structures (eg, pilosebaceous units or eccrine glands). Although all tattoo pigments can cause adverse reactions, hypersensitivity reactions occur most commonly in response to red pigment, resulting in discrete areas of spongiosis and granulomatous or lichenoid inflammation. Occasionally, hypersensitivity reactions can induce necrobiotic granulomatous reactions characterized by collagen alteration surrounded by palisaded histiocytes and lymphocytes (Figure 3).15,16 There also may be focally dense areas of superficial and deep perivascular lymphohistiocytic infiltrate. Clinical context is important, as brown tattoo pigment (Figure 3) can be easily confused with the pigmented hyphae of phaeohyphomycosis, melanin, or hemosiderin.
Subcutaneous hyalohyphomycosis is a nondemat-iaceous (nonpigmented) infection that is caused by hyaline septate hyphal cells.17 Hyalohyphomycosis skin lesions can present as painful erythematous nodules that evolve into excoriated pustules.18 Hyalohyphomycosis most often arises in immunocompromised patients. Causative organisms are ubiquitous soil saprophytes and plant pathogens, most often Aspergillus and Fusarium species, with a predilection for affecting severely immunocompromised hosts, particularly children.19 These species tend to be vasculotropic, which can result in tissue necrosis and systemic dissemination. Histologically, fungi are dispersed within tissue. They have a bright, bubbly, mildly basophilic cytoplasm and are nonpigmented, branching, and septate (Figure 4).11

- Isa-Isa R, García C, Isa M, et al. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin Dermatol. 2012;30:425-431.
- Rubin RH. Infectious disease complications of renal transplantation. Kidney Int. 1993;44:221-236.
- Ogawa MM, Galante NZ, Godoy P, et al. Treatment of subcutaneous phaeohyphomycosis and prospective follow-up of 17 kidney transplant recipients. J Am Acad Dermatol. 2009;61:977-985.
- Matsumoto T, Ajello L, Matsuda T, et al. Developments in hyalohyphomycosis and phaeohyphomycosis. J Med Vet Mycol. 1994;32(suppl 1):329-349.
- Rinaldi MG. Phaeohyphomycosis. Dermatol Clin. 1996;14:147-153.
- Ajello L, Georg LK, Steigbigel RT, et al. A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia. 1974;66:490-498.
- Patterson J. Weedon’s Skin Pathology. 4th ed. London, England: Churchill Livingstone Elsevier; 2014.
- Patel U, Chu J, Patel R, et al. Subcutaneous dematiaceous fungal infection. Dermatol Online J. 2011;17:19.
- Bonifaz A, Carrasco-Gerard E, Saúl A. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses. 2001;44:1-7.
- Ameen M. Chromoblastomycosis: clinical presentation and management. Clin Exp Dermatol. 2009;34:849-854.
- Elston D, Ferringer T, Peckham S, et al, eds. Dermatopathology. 2nd ed. St. Louis, MO: Elsevier Saunders; 2014.
- Lammers RL. Soft tissue foreign bodies. In: Tintinalli J, Stapczynski S, Ma O, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw Hill Professional; 2011.
- Murphy GF, Saavedra AP, Mihm MC. Nodular/interstitial dermatitis. In: Murphy GF, Saavedra AP, Mihm MC, eds. Atlas of Nontumor Pathology: Inflammatory Disorders of the Skin. Vol 10. Washington, DC: American Registry of Pathology; 2012:337-395.
- Laumann A. Body art. In: Goldsmith L, Katz S, Gilchrest B, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012. http://access medicine.mhmedical.com.proxy.lib.uiowa.edu/content.aspx?bookid=392&Sectionid=41138811. Accessed July 17,2016.
- Wood A, Hamilton SA, Wallace WA, et al. Necrobiotic granulomatous tattoo reaction: report of an unusual case showing features of both necrobiosis lipoidica and granuloma annulare patterns. Am J Dermatopathol. 2014;36:e152-e155.
- Mortimer N, Chave T, Johnston G. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Ajello L. Hyalohyphomycosis and phaeohyphomycosis: two global disease entities of public health importance. Eur J Epidemiol. 1986;2:243-251.
- Safdar A. Progressive cutaneous hyalohyphomycosis due to Paecilomyces lilacinus: rapid response to treatment with caspofungin and itraconazole. Clin Infect Dis. 2002;34:1415-1417.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
The Diagnosis: Subcutaneous Phaeohyphomycosis
Subcutaneous phaeohyphomycosis (SP), also called mycotic cyst, is characterized by a painless, nodular lesion that develops in response to traumatic implantation of dematiaceous, pigment-forming fungi.1 Similar to other fungal infections, SP can arise opportunistically in immunocompromised patients.2,3 More than 60 genera (and more than 100 species) are known etiologic agents of phaeohyphomycosis; the 2 main causes of infection are Bipolaris spicifera and Exophiala jeanselmei.4,5 Given this variety, phaeohyphomycosis can present superficially as black piedra or tinea nigra, cutaneously as scytalidiosis, subcutaneously as SP, or disseminated as sinusitis or systemic phaeohyphomycosis.
Coined in 1974 by Ajello et al,6 the term phaeohyphomycosis translates to “condition of dark hyphal fungus,” a term used to designate mycoses caused by fungi with melanized hyphae. Histologically, SP demonstrates a circumscribed chronic cyst or abscess with a dense fibrous wall (quiz image A). At high power, the wall is composed of chronic granulomatous inflammation with foamy macrophages, and the cystic cavity contains necrotic debris admixed with neutrophils. Pigmented filamentous hyphae and yeastlike entities can be seen in the cyst wall, in multinucleated giant cells, in the necrotic debris, or directly attached to the implanted foreign material (quiz image B).7 The first-line treatment of SP is wide local excision and oral itraconazole. It often requires adjustments to dosage or change to antifungal due to recurrence and etiologic variation.8 Furthermore, if SP is not definitively treated, immunocompromised patients are at an increased risk for developing potentially fatal systemic phaeohyphomycosis.3
Chromoblastomycosis (CBM), also caused by dematiaceous fungi, is characterized by an initially indolent clinical presentation. Typically found on the legs and lower thighs of agricultural workers, the lesion begins as a slow-growing, nodular papule with subsequent transformation into an edematous verrucous plaque with peripheral erythema.9 Lesions can be annular with central clearing, and lymphedema with elephantiasis may be present.10 Histologically, CBM shows pseudoepitheliomatous hyperplasia and intraepidermal pustules as the host rids the infection via transepithelial elimination. Dematiaceous fungi often are seen in the dermis, either freestanding or attached to foreign plant material. Medlar bodies, also called copper penny spores or sclerotic bodies, are the most defining histologic finding and are characterized by groups of brown, thick-walled cells found in giant cells or neutrophil abscesses (Figure 1). Hyphae are not typically found in this type of infection.11

Granulomatous foreign body reactions occur in response to the inoculation of nonhuman material and are characterized by dermal or subcutaneous nodules. Tissue macrophages phagocytize material not removed shortly after implantation, which initiates an inflammatory response that attempts to isolate the material from the uninvolved surrounding tissue. Vegetative foreign bodies will cause the most severe inflammatory reactions.12 Histologically, foreign body granulomas are noncaseating with epithelioid histiocytes surrounding a central foreign body (Figure 2). Occasionally, foreign bodies may be difficult to detect; some are birefringent to polarized light.13 Additionally, inoculation injuries can predispose patients to SP, CBM, and other fungal infections.

Tattoos are characterized by exogenous pigment deposition into the dermis.14 Histologically, tattoos display exogenous pigment deposited throughout the reticular dermis, attached to collagen bundles, within macrophages, or adjacent to adnexal structures (eg, pilosebaceous units or eccrine glands). Although all tattoo pigments can cause adverse reactions, hypersensitivity reactions occur most commonly in response to red pigment, resulting in discrete areas of spongiosis and granulomatous or lichenoid inflammation. Occasionally, hypersensitivity reactions can induce necrobiotic granulomatous reactions characterized by collagen alteration surrounded by palisaded histiocytes and lymphocytes (Figure 3).15,16 There also may be focally dense areas of superficial and deep perivascular lymphohistiocytic infiltrate. Clinical context is important, as brown tattoo pigment (Figure 3) can be easily confused with the pigmented hyphae of phaeohyphomycosis, melanin, or hemosiderin.

Subcutaneous hyalohyphomycosis is a nondemat-iaceous (nonpigmented) infection that is caused by hyaline septate hyphal cells.17 Hyalohyphomycosis skin lesions can present as painful erythematous nodules that evolve into excoriated pustules.18 Hyalohyphomycosis most often arises in immunocompromised patients. Causative organisms are ubiquitous soil saprophytes and plant pathogens, most often Aspergillus and Fusarium species, with a predilection for affecting severely immunocompromised hosts, particularly children.19 These species tend to be vasculotropic, which can result in tissue necrosis and systemic dissemination. Histologically, fungi are dispersed within tissue. They have a bright, bubbly, mildly basophilic cytoplasm and are nonpigmented, branching, and septate (Figure 4).11

The Diagnosis: Subcutaneous Phaeohyphomycosis
Subcutaneous phaeohyphomycosis (SP), also called mycotic cyst, is characterized by a painless, nodular lesion that develops in response to traumatic implantation of dematiaceous, pigment-forming fungi.1 Similar to other fungal infections, SP can arise opportunistically in immunocompromised patients.2,3 More than 60 genera (and more than 100 species) are known etiologic agents of phaeohyphomycosis; the 2 main causes of infection are Bipolaris spicifera and Exophiala jeanselmei.4,5 Given this variety, phaeohyphomycosis can present superficially as black piedra or tinea nigra, cutaneously as scytalidiosis, subcutaneously as SP, or disseminated as sinusitis or systemic phaeohyphomycosis.
Coined in 1974 by Ajello et al,6 the term phaeohyphomycosis translates to “condition of dark hyphal fungus,” a term used to designate mycoses caused by fungi with melanized hyphae. Histologically, SP demonstrates a circumscribed chronic cyst or abscess with a dense fibrous wall (quiz image A). At high power, the wall is composed of chronic granulomatous inflammation with foamy macrophages, and the cystic cavity contains necrotic debris admixed with neutrophils. Pigmented filamentous hyphae and yeastlike entities can be seen in the cyst wall, in multinucleated giant cells, in the necrotic debris, or directly attached to the implanted foreign material (quiz image B).7 The first-line treatment of SP is wide local excision and oral itraconazole. It often requires adjustments to dosage or change to antifungal due to recurrence and etiologic variation.8 Furthermore, if SP is not definitively treated, immunocompromised patients are at an increased risk for developing potentially fatal systemic phaeohyphomycosis.3
Chromoblastomycosis (CBM), also caused by dematiaceous fungi, is characterized by an initially indolent clinical presentation. Typically found on the legs and lower thighs of agricultural workers, the lesion begins as a slow-growing, nodular papule with subsequent transformation into an edematous verrucous plaque with peripheral erythema.9 Lesions can be annular with central clearing, and lymphedema with elephantiasis may be present.10 Histologically, CBM shows pseudoepitheliomatous hyperplasia and intraepidermal pustules as the host rids the infection via transepithelial elimination. Dematiaceous fungi often are seen in the dermis, either freestanding or attached to foreign plant material. Medlar bodies, also called copper penny spores or sclerotic bodies, are the most defining histologic finding and are characterized by groups of brown, thick-walled cells found in giant cells or neutrophil abscesses (Figure 1). Hyphae are not typically found in this type of infection.11

Granulomatous foreign body reactions occur in response to the inoculation of nonhuman material and are characterized by dermal or subcutaneous nodules. Tissue macrophages phagocytize material not removed shortly after implantation, which initiates an inflammatory response that attempts to isolate the material from the uninvolved surrounding tissue. Vegetative foreign bodies will cause the most severe inflammatory reactions.12 Histologically, foreign body granulomas are noncaseating with epithelioid histiocytes surrounding a central foreign body (Figure 2). Occasionally, foreign bodies may be difficult to detect; some are birefringent to polarized light.13 Additionally, inoculation injuries can predispose patients to SP, CBM, and other fungal infections.

Tattoos are characterized by exogenous pigment deposition into the dermis.14 Histologically, tattoos display exogenous pigment deposited throughout the reticular dermis, attached to collagen bundles, within macrophages, or adjacent to adnexal structures (eg, pilosebaceous units or eccrine glands). Although all tattoo pigments can cause adverse reactions, hypersensitivity reactions occur most commonly in response to red pigment, resulting in discrete areas of spongiosis and granulomatous or lichenoid inflammation. Occasionally, hypersensitivity reactions can induce necrobiotic granulomatous reactions characterized by collagen alteration surrounded by palisaded histiocytes and lymphocytes (Figure 3).15,16 There also may be focally dense areas of superficial and deep perivascular lymphohistiocytic infiltrate. Clinical context is important, as brown tattoo pigment (Figure 3) can be easily confused with the pigmented hyphae of phaeohyphomycosis, melanin, or hemosiderin.

Subcutaneous hyalohyphomycosis is a nondemat-iaceous (nonpigmented) infection that is caused by hyaline septate hyphal cells.17 Hyalohyphomycosis skin lesions can present as painful erythematous nodules that evolve into excoriated pustules.18 Hyalohyphomycosis most often arises in immunocompromised patients. Causative organisms are ubiquitous soil saprophytes and plant pathogens, most often Aspergillus and Fusarium species, with a predilection for affecting severely immunocompromised hosts, particularly children.19 These species tend to be vasculotropic, which can result in tissue necrosis and systemic dissemination. Histologically, fungi are dispersed within tissue. They have a bright, bubbly, mildly basophilic cytoplasm and are nonpigmented, branching, and septate (Figure 4).11

- Isa-Isa R, García C, Isa M, et al. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin Dermatol. 2012;30:425-431.
- Rubin RH. Infectious disease complications of renal transplantation. Kidney Int. 1993;44:221-236.
- Ogawa MM, Galante NZ, Godoy P, et al. Treatment of subcutaneous phaeohyphomycosis and prospective follow-up of 17 kidney transplant recipients. J Am Acad Dermatol. 2009;61:977-985.
- Matsumoto T, Ajello L, Matsuda T, et al. Developments in hyalohyphomycosis and phaeohyphomycosis. J Med Vet Mycol. 1994;32(suppl 1):329-349.
- Rinaldi MG. Phaeohyphomycosis. Dermatol Clin. 1996;14:147-153.
- Ajello L, Georg LK, Steigbigel RT, et al. A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia. 1974;66:490-498.
- Patterson J. Weedon’s Skin Pathology. 4th ed. London, England: Churchill Livingstone Elsevier; 2014.
- Patel U, Chu J, Patel R, et al. Subcutaneous dematiaceous fungal infection. Dermatol Online J. 2011;17:19.
- Bonifaz A, Carrasco-Gerard E, Saúl A. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses. 2001;44:1-7.
- Ameen M. Chromoblastomycosis: clinical presentation and management. Clin Exp Dermatol. 2009;34:849-854.
- Elston D, Ferringer T, Peckham S, et al, eds. Dermatopathology. 2nd ed. St. Louis, MO: Elsevier Saunders; 2014.
- Lammers RL. Soft tissue foreign bodies. In: Tintinalli J, Stapczynski S, Ma O, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw Hill Professional; 2011.
- Murphy GF, Saavedra AP, Mihm MC. Nodular/interstitial dermatitis. In: Murphy GF, Saavedra AP, Mihm MC, eds. Atlas of Nontumor Pathology: Inflammatory Disorders of the Skin. Vol 10. Washington, DC: American Registry of Pathology; 2012:337-395.
- Laumann A. Body art. In: Goldsmith L, Katz S, Gilchrest B, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012. http://access medicine.mhmedical.com.proxy.lib.uiowa.edu/content.aspx?bookid=392&Sectionid=41138811. Accessed July 17,2016.
- Wood A, Hamilton SA, Wallace WA, et al. Necrobiotic granulomatous tattoo reaction: report of an unusual case showing features of both necrobiosis lipoidica and granuloma annulare patterns. Am J Dermatopathol. 2014;36:e152-e155.
- Mortimer N, Chave T, Johnston G. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Ajello L. Hyalohyphomycosis and phaeohyphomycosis: two global disease entities of public health importance. Eur J Epidemiol. 1986;2:243-251.
- Safdar A. Progressive cutaneous hyalohyphomycosis due to Paecilomyces lilacinus: rapid response to treatment with caspofungin and itraconazole. Clin Infect Dis. 2002;34:1415-1417.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
- Isa-Isa R, García C, Isa M, et al. Subcutaneous phaeohyphomycosis (mycotic cyst). Clin Dermatol. 2012;30:425-431.
- Rubin RH. Infectious disease complications of renal transplantation. Kidney Int. 1993;44:221-236.
- Ogawa MM, Galante NZ, Godoy P, et al. Treatment of subcutaneous phaeohyphomycosis and prospective follow-up of 17 kidney transplant recipients. J Am Acad Dermatol. 2009;61:977-985.
- Matsumoto T, Ajello L, Matsuda T, et al. Developments in hyalohyphomycosis and phaeohyphomycosis. J Med Vet Mycol. 1994;32(suppl 1):329-349.
- Rinaldi MG. Phaeohyphomycosis. Dermatol Clin. 1996;14:147-153.
- Ajello L, Georg LK, Steigbigel RT, et al. A case of phaeohyphomycosis caused by a new species of Phialophora. Mycologia. 1974;66:490-498.
- Patterson J. Weedon’s Skin Pathology. 4th ed. London, England: Churchill Livingstone Elsevier; 2014.
- Patel U, Chu J, Patel R, et al. Subcutaneous dematiaceous fungal infection. Dermatol Online J. 2011;17:19.
- Bonifaz A, Carrasco-Gerard E, Saúl A. Chromoblastomycosis: clinical and mycologic experience of 51 cases. Mycoses. 2001;44:1-7.
- Ameen M. Chromoblastomycosis: clinical presentation and management. Clin Exp Dermatol. 2009;34:849-854.
- Elston D, Ferringer T, Peckham S, et al, eds. Dermatopathology. 2nd ed. St. Louis, MO: Elsevier Saunders; 2014.
- Lammers RL. Soft tissue foreign bodies. In: Tintinalli J, Stapczynski S, Ma O, et al, eds. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. 7th ed. New York, NY: McGraw Hill Professional; 2011.
- Murphy GF, Saavedra AP, Mihm MC. Nodular/interstitial dermatitis. In: Murphy GF, Saavedra AP, Mihm MC, eds. Atlas of Nontumor Pathology: Inflammatory Disorders of the Skin. Vol 10. Washington, DC: American Registry of Pathology; 2012:337-395.
- Laumann A. Body art. In: Goldsmith L, Katz S, Gilchrest B, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012. http://access medicine.mhmedical.com.proxy.lib.uiowa.edu/content.aspx?bookid=392&Sectionid=41138811. Accessed July 17,2016.
- Wood A, Hamilton SA, Wallace WA, et al. Necrobiotic granulomatous tattoo reaction: report of an unusual case showing features of both necrobiosis lipoidica and granuloma annulare patterns. Am J Dermatopathol. 2014;36:e152-e155.
- Mortimer N, Chave T, Johnston G. Red tattoo reactions. Clin Exp Dermatol. 2003;28:508-510.
- Ajello L. Hyalohyphomycosis and phaeohyphomycosis: two global disease entities of public health importance. Eur J Epidemiol. 1986;2:243-251.
- Safdar A. Progressive cutaneous hyalohyphomycosis due to Paecilomyces lilacinus: rapid response to treatment with caspofungin and itraconazole. Clin Infect Dis. 2002;34:1415-1417.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
A 63-year-old man on immunosuppressive therapy following renal transplantation 5 years prior presented with a nontender circumscribed nodule above the left knee of 6 months’ duration. The patient denied any trauma or injury to the site.
Erythema and Induration on the Right Ear and Maxilla
Lepromatous Leprosy
Lepromatous leprosy (LL) is a chronic, cutaneous, granulomatous infection caused by Mycobacterium leprae or the newly discovered Mycobacterium lepromatosis, both acid-fast, intracellular, bacillus bacterium.1 Although decreasing in prevalence due to effective treatment with antimicrobials, LL continues to be endemic in warm tropical or subtropical areas in Southeast Asia, sub-Saharan Africa, the Indian subcontinent, and South America.1 The mode of transmission of infection is not well established.
The cutaneous manifestation of leprosy was previously classified based on the cell-mediated immune response of the patient, as described by Ridley and Jopling,2 ranging from tuberculoid leprosy (TT) to LL. In this spectrum of leprosy are the borderline lesions including borderline tuberculoid, borderline, and borderline lepromatous.2,3 Although this classification is popular, in 2012 the World Health Organization implemented a new 2-category classification system to standardize treatment regimens: paucibacillary (2–5 lesions or 1 nerve involvement) and multibacillary (>5 lesions or multiple nerve involvement).4
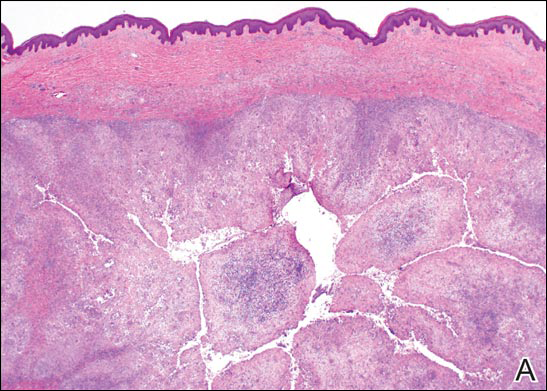
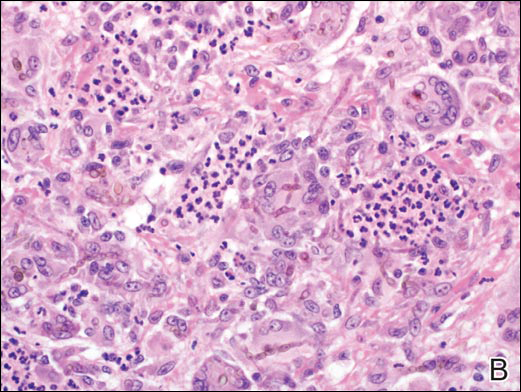
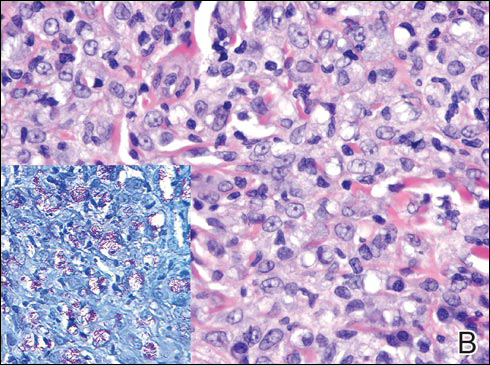
In LL, a cell-mediated immune response is not mounted against the infection in the patient. Clinically, the disease can manifest as macular and nodular erythematous cutaneous lesions with poorly defined borders that are preferentially located on the face, earlobes, and nasal mucosa. Chronic infections are associated with sensory loss. Histologically, the dermis is densely infiltrated by foamy macrophages (Virchow cells or lepra cells), which do not form granulomas (quiz image A). The infiltrate may have varying accompanying lymphocytes and plasma cells, which can extend deep into the subcutaneous adipose tissue. Between the dermal infiltrate and epidermis is an uninvolved band of superficial dermis called the Grenz zone. The epidermis is flattened and atrophic. Nerves often are surrounded by macrophages with degrees of hyalinization but rarely are swollen. On acid-fast staining (Wade-Fite or Ziehl-Neelsen), numerous acid-fast bacilli are present within dermal cells in densely packed, intracellular collections called globi (quiz image B).2,3,5
In TT, the robust immune response causes epithelioid granuloma formation, similar to cutaneous sarcoidosis, and few, if any, organisms can be found on special stains. The remaining borderline lesions have varying numbers of bacilli and varying amounts of granuloma formation.3,6,7 Many cases of TT resolve without specific treatment. For most leprous diseases, the World Health Organization currently recommends a regimen of dapsone, rifampin, and clofazimine combination treatments for 6 to 12 months depending on the type of leprosy.8
Cutaneous leishmaniasis should be included in the differential diagnosis for patients from LL endemic areas. Early lesions can have a histiocytic infiltration with associated mixed inflammation and prominent epidermal hyperplasia. These early lesions usually have parasitic organisms located within the periphery of the cytoplasm of macrophages (“marquee sign”) to help differentiate it from leprous diseases (Figure 1).9
In nonendemic areas, leprous diseases often are mistaken for sarcoidosis, xanthomas, granular cell tumors, paraffinomas, or other histiocytic-rich lesions.10 Cutaneous sarcoidosis may be difficult to distinguish from TT, as both have noncaseating granulomas (Figure 2). Rare acid-fast bacilli may aid in the diagnosis, and sarcoid granulomas are not typically associated with cutaneous nerve involvement. New diagnostic tools such as polymerase chain reaction or genome sequencing can pick up rare organisms.
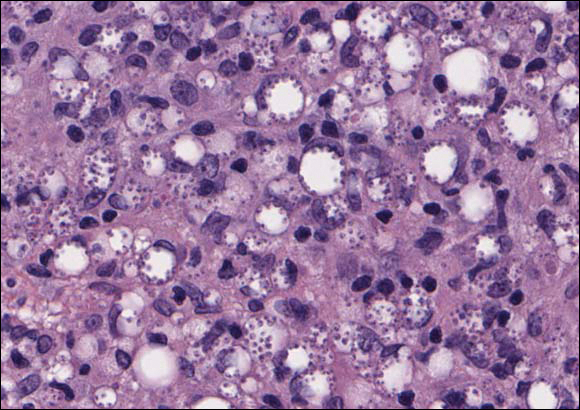
Xanthogranuolomas and xanthomas may histologically resemble LL with a dense dermal infiltrate of foamy histiocytes. No organisms are found in the infiltrate. Histologically, xanthogranulomas (juvenile or adult) will be a mixed infiltrate with foamy histiocytes; giant cell formation, especially Touton giant cells; lymphocytes; and granulocytes (Figure 3). Touton giant cells have a wreathlike formation of nuclei and an outer vacuolated cytoplasm. Xanthomas have sheets of large histiocytes with a foamy, lipid-filled interior and mild lymphocytic infiltrate (Figure 4).
- Han XY, Seo YH, Sizer KC, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130:856-864.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. a five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255-273.
- Ridley DS. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51:451-465.
- World Health Organization. WHO Expert Committee on Leprosy. World Health Organ Tech Rep Ser. 2012;968:1-61.
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- Crowson AN, Magro C, Mihm M Jr. Treponemal diseases. In: Elder DE, Elenitsas R, Johnson Jr BL, et al. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:540-579.
- Dacso MM, Jacobson RR, Scollard DM, et al. Evaluation of multi-drug therapy for leprosy in the United States using daily rifampin. South Med J. 2011;104:689-694.
- Handler MZ, Patel PA, Kapila R, et al. Cutaneous and mucocutaneous leishmaniasis: differential diagnosis, diagnosis, histopathology, and management. J Am Acad Dermatol. 2015;73:911-926.
- Massone C, Nunzi E, Cerroni L. Histopathologic diagnosis of leprosy in a nonendemic area. Am J Dermatopathol. 2010;32:417-419.
Lepromatous Leprosy
Lepromatous leprosy (LL) is a chronic, cutaneous, granulomatous infection caused by Mycobacterium leprae or the newly discovered Mycobacterium lepromatosis, both acid-fast, intracellular, bacillus bacterium.1 Although decreasing in prevalence due to effective treatment with antimicrobials, LL continues to be endemic in warm tropical or subtropical areas in Southeast Asia, sub-Saharan Africa, the Indian subcontinent, and South America.1 The mode of transmission of infection is not well established.
The cutaneous manifestation of leprosy was previously classified based on the cell-mediated immune response of the patient, as described by Ridley and Jopling,2 ranging from tuberculoid leprosy (TT) to LL. In this spectrum of leprosy are the borderline lesions including borderline tuberculoid, borderline, and borderline lepromatous.2,3 Although this classification is popular, in 2012 the World Health Organization implemented a new 2-category classification system to standardize treatment regimens: paucibacillary (2–5 lesions or 1 nerve involvement) and multibacillary (>5 lesions or multiple nerve involvement).4
In LL, a cell-mediated immune response is not mounted against the infection in the patient. Clinically, the disease can manifest as macular and nodular erythematous cutaneous lesions with poorly defined borders that are preferentially located on the face, earlobes, and nasal mucosa. Chronic infections are associated with sensory loss. Histologically, the dermis is densely infiltrated by foamy macrophages (Virchow cells or lepra cells), which do not form granulomas (quiz image A). The infiltrate may have varying accompanying lymphocytes and plasma cells, which can extend deep into the subcutaneous adipose tissue. Between the dermal infiltrate and epidermis is an uninvolved band of superficial dermis called the Grenz zone. The epidermis is flattened and atrophic. Nerves often are surrounded by macrophages with degrees of hyalinization but rarely are swollen. On acid-fast staining (Wade-Fite or Ziehl-Neelsen), numerous acid-fast bacilli are present within dermal cells in densely packed, intracellular collections called globi (quiz image B).2,3,5
In TT, the robust immune response causes epithelioid granuloma formation, similar to cutaneous sarcoidosis, and few, if any, organisms can be found on special stains. The remaining borderline lesions have varying numbers of bacilli and varying amounts of granuloma formation.3,6,7 Many cases of TT resolve without specific treatment. For most leprous diseases, the World Health Organization currently recommends a regimen of dapsone, rifampin, and clofazimine combination treatments for 6 to 12 months depending on the type of leprosy.8
Cutaneous leishmaniasis should be included in the differential diagnosis for patients from LL endemic areas. Early lesions can have a histiocytic infiltration with associated mixed inflammation and prominent epidermal hyperplasia. These early lesions usually have parasitic organisms located within the periphery of the cytoplasm of macrophages (“marquee sign”) to help differentiate it from leprous diseases (Figure 1).9

In nonendemic areas, leprous diseases often are mistaken for sarcoidosis, xanthomas, granular cell tumors, paraffinomas, or other histiocytic-rich lesions.10 Cutaneous sarcoidosis may be difficult to distinguish from TT, as both have noncaseating granulomas (Figure 2). Rare acid-fast bacilli may aid in the diagnosis, and sarcoid granulomas are not typically associated with cutaneous nerve involvement. New diagnostic tools such as polymerase chain reaction or genome sequencing can pick up rare organisms.

Xanthogranuolomas and xanthomas may histologically resemble LL with a dense dermal infiltrate of foamy histiocytes. No organisms are found in the infiltrate. Histologically, xanthogranulomas (juvenile or adult) will be a mixed infiltrate with foamy histiocytes; giant cell formation, especially Touton giant cells; lymphocytes; and granulocytes (Figure 3). Touton giant cells have a wreathlike formation of nuclei and an outer vacuolated cytoplasm. Xanthomas have sheets of large histiocytes with a foamy, lipid-filled interior and mild lymphocytic infiltrate (Figure 4).


Lepromatous Leprosy
Lepromatous leprosy (LL) is a chronic, cutaneous, granulomatous infection caused by Mycobacterium leprae or the newly discovered Mycobacterium lepromatosis, both acid-fast, intracellular, bacillus bacterium.1 Although decreasing in prevalence due to effective treatment with antimicrobials, LL continues to be endemic in warm tropical or subtropical areas in Southeast Asia, sub-Saharan Africa, the Indian subcontinent, and South America.1 The mode of transmission of infection is not well established.
The cutaneous manifestation of leprosy was previously classified based on the cell-mediated immune response of the patient, as described by Ridley and Jopling,2 ranging from tuberculoid leprosy (TT) to LL. In this spectrum of leprosy are the borderline lesions including borderline tuberculoid, borderline, and borderline lepromatous.2,3 Although this classification is popular, in 2012 the World Health Organization implemented a new 2-category classification system to standardize treatment regimens: paucibacillary (2–5 lesions or 1 nerve involvement) and multibacillary (>5 lesions or multiple nerve involvement).4
In LL, a cell-mediated immune response is not mounted against the infection in the patient. Clinically, the disease can manifest as macular and nodular erythematous cutaneous lesions with poorly defined borders that are preferentially located on the face, earlobes, and nasal mucosa. Chronic infections are associated with sensory loss. Histologically, the dermis is densely infiltrated by foamy macrophages (Virchow cells or lepra cells), which do not form granulomas (quiz image A). The infiltrate may have varying accompanying lymphocytes and plasma cells, which can extend deep into the subcutaneous adipose tissue. Between the dermal infiltrate and epidermis is an uninvolved band of superficial dermis called the Grenz zone. The epidermis is flattened and atrophic. Nerves often are surrounded by macrophages with degrees of hyalinization but rarely are swollen. On acid-fast staining (Wade-Fite or Ziehl-Neelsen), numerous acid-fast bacilli are present within dermal cells in densely packed, intracellular collections called globi (quiz image B).2,3,5
In TT, the robust immune response causes epithelioid granuloma formation, similar to cutaneous sarcoidosis, and few, if any, organisms can be found on special stains. The remaining borderline lesions have varying numbers of bacilli and varying amounts of granuloma formation.3,6,7 Many cases of TT resolve without specific treatment. For most leprous diseases, the World Health Organization currently recommends a regimen of dapsone, rifampin, and clofazimine combination treatments for 6 to 12 months depending on the type of leprosy.8
Cutaneous leishmaniasis should be included in the differential diagnosis for patients from LL endemic areas. Early lesions can have a histiocytic infiltration with associated mixed inflammation and prominent epidermal hyperplasia. These early lesions usually have parasitic organisms located within the periphery of the cytoplasm of macrophages (“marquee sign”) to help differentiate it from leprous diseases (Figure 1).9

In nonendemic areas, leprous diseases often are mistaken for sarcoidosis, xanthomas, granular cell tumors, paraffinomas, or other histiocytic-rich lesions.10 Cutaneous sarcoidosis may be difficult to distinguish from TT, as both have noncaseating granulomas (Figure 2). Rare acid-fast bacilli may aid in the diagnosis, and sarcoid granulomas are not typically associated with cutaneous nerve involvement. New diagnostic tools such as polymerase chain reaction or genome sequencing can pick up rare organisms.

Xanthogranuolomas and xanthomas may histologically resemble LL with a dense dermal infiltrate of foamy histiocytes. No organisms are found in the infiltrate. Histologically, xanthogranulomas (juvenile or adult) will be a mixed infiltrate with foamy histiocytes; giant cell formation, especially Touton giant cells; lymphocytes; and granulocytes (Figure 3). Touton giant cells have a wreathlike formation of nuclei and an outer vacuolated cytoplasm. Xanthomas have sheets of large histiocytes with a foamy, lipid-filled interior and mild lymphocytic infiltrate (Figure 4).


- Han XY, Seo YH, Sizer KC, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130:856-864.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. a five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255-273.
- Ridley DS. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51:451-465.
- World Health Organization. WHO Expert Committee on Leprosy. World Health Organ Tech Rep Ser. 2012;968:1-61.
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- Crowson AN, Magro C, Mihm M Jr. Treponemal diseases. In: Elder DE, Elenitsas R, Johnson Jr BL, et al. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:540-579.
- Dacso MM, Jacobson RR, Scollard DM, et al. Evaluation of multi-drug therapy for leprosy in the United States using daily rifampin. South Med J. 2011;104:689-694.
- Handler MZ, Patel PA, Kapila R, et al. Cutaneous and mucocutaneous leishmaniasis: differential diagnosis, diagnosis, histopathology, and management. J Am Acad Dermatol. 2015;73:911-926.
- Massone C, Nunzi E, Cerroni L. Histopathologic diagnosis of leprosy in a nonendemic area. Am J Dermatopathol. 2010;32:417-419.
- Han XY, Seo YH, Sizer KC, et al. A new Mycobacterium species causing diffuse lepromatous leprosy. Am J Clin Pathol. 2008;130:856-864.
- Ridley DS, Jopling WH. Classification of leprosy according to immunity. a five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255-273.
- Ridley DS. Histological classification and the immunological spectrum of leprosy. Bull World Health Organ. 1974;51:451-465.
- World Health Organization. WHO Expert Committee on Leprosy. World Health Organ Tech Rep Ser. 2012;968:1-61.
- Massone C, Belachew WA, Schettini A. Histopathology of the lepromatous skin biopsy. Clin Dermatol. 2015;33:38-45.
- Britton WJ, Lockwood DN. Leprosy. Lancet. 2004;363:1209-1219.
- Crowson AN, Magro C, Mihm M Jr. Treponemal diseases. In: Elder DE, Elenitsas R, Johnson Jr BL, et al. Lever’s Histopathology of the Skin. 10th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:540-579.
- Dacso MM, Jacobson RR, Scollard DM, et al. Evaluation of multi-drug therapy for leprosy in the United States using daily rifampin. South Med J. 2011;104:689-694.
- Handler MZ, Patel PA, Kapila R, et al. Cutaneous and mucocutaneous leishmaniasis: differential diagnosis, diagnosis, histopathology, and management. J Am Acad Dermatol. 2015;73:911-926.
- Massone C, Nunzi E, Cerroni L. Histopathologic diagnosis of leprosy in a nonendemic area. Am J Dermatopathol. 2010;32:417-419.
A 43-year-old man from Ghana presented with erythema and induration on the skin of the right maxillary region and right ear of several weeks’ duration.
The best diagnosis is:
a. cutaneous leishmaniasis
b. lepromatous leprosy
c. sarcoidosis
d. xanthogranuloma
e. xanthoma
Growing Papule on the Right Shoulder of an Elderly Man
Granular Cell Basal Cell Carcinoma
Basal cell carcinoma (BCC) is the most common human epithelial malignancy. There are several histologic variants, the rarest being granular cell BCC (GBCC).1 Granular cell BCC is reported most commonly in men with a mean age of 63 years. Sixty-four percent of cases develop on the face, with the remainder arising on the chest or trunk. Granular cell BCC has distinct histologic features but has no specific epidemiologic or clinical features that differentiate it from more common forms of BCC. Treatment of GBCC is identical to BCC and demonstrates similar outcomes. The presence of granular cells can make GBCC difficult to differentiate from other benign and malignant lesions that display similar granular histologic changes.1,2 Rarely, tumors that are histologically similar to human GBCC have been reported in animals.1
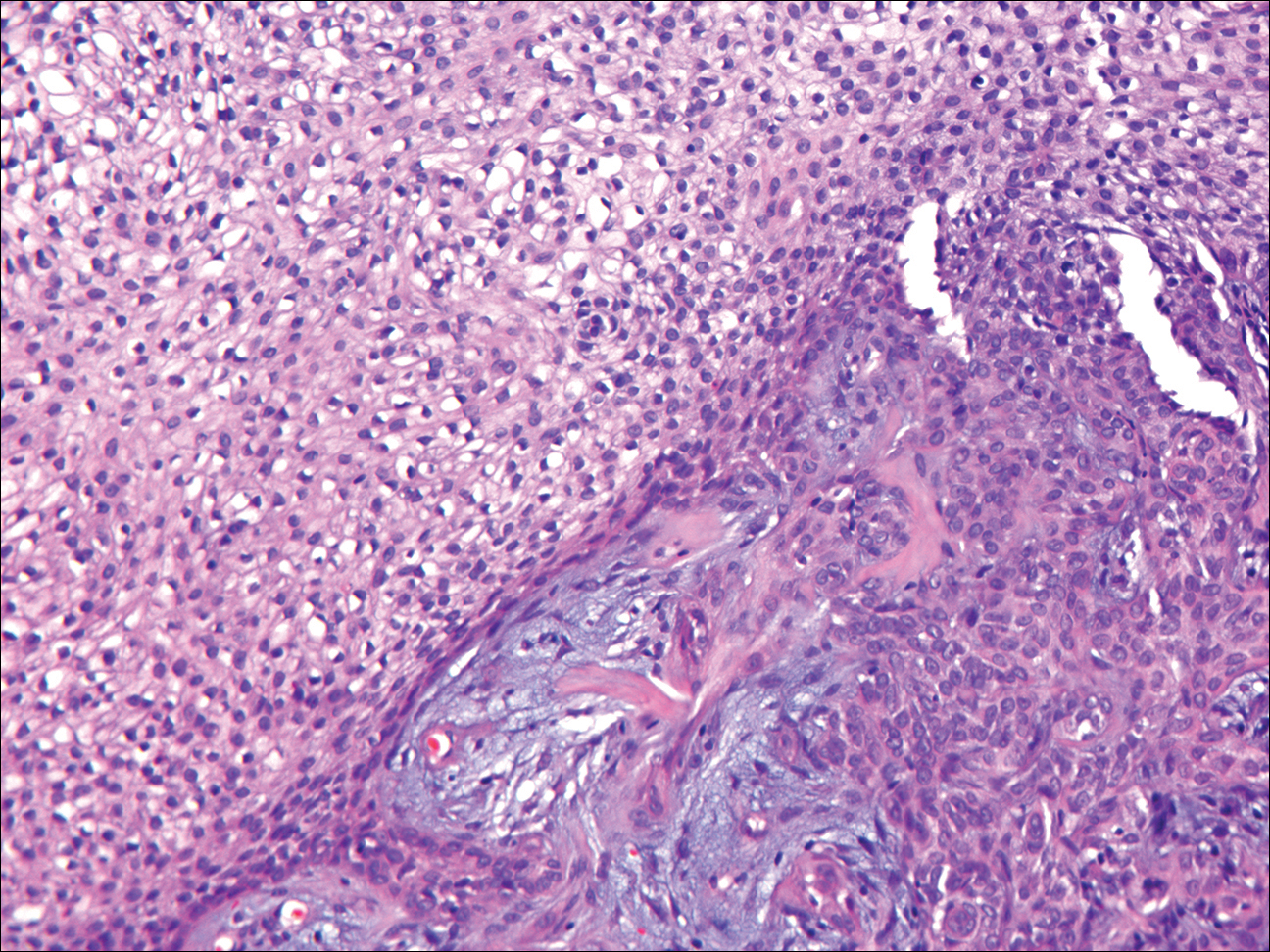
Histologically, GBCC commonly demonstrates the architecture of a nodular BCC or may extend from an existing nodular BCC (quiz images A and B). Granular cell BCC is comprised of large islands of basaloid cells extending from the epidermis with rare mitotic activity. Certain variants showing no epidermal attachments have been described,1,3 as in the current case. Classically, BCC and GBCC both demonstrate a peripheral palisade of blue basal cells; however, GBCC may lack this palisading feature in some cases. Therefore, GBCC may be comprised of granular cells only, which may be more easily confused with other tumors with granular cell differentiation. Even when GBCC retains the traditional peripheral palisade of blue basal cells, the central cells are filled with eosinophilic granules.1,2
Electron microscopy of GBCC usually reveals bundles of cytoplasmic tonofilaments and desmosomes in both granular cells and the peripherally palisaded cells. Electron microscopy imaging also demonstrates 0.1- to 0.5-µm membrane-bound lysosomelike structures. In certain reports, these structures show focal positivity for lysozymes.1,2 The etiology of the granules is unclear; however, they are thought to represent degenerative changes related to metabolic alteration and accumulation of lysosomelike structures. These lysosomelike structures have been highlighted with CD68 staining, which was negative in our case.1,2 The lesional cells in GBCC stain positively for cytokeratins, p63, and Ber-EP4, and negatively for S-100 protein, epithelial membrane antigen, and carcinoembryonic antigen. The granules in GBCC generally are positive on periodic acid–Schiff staining.1-4
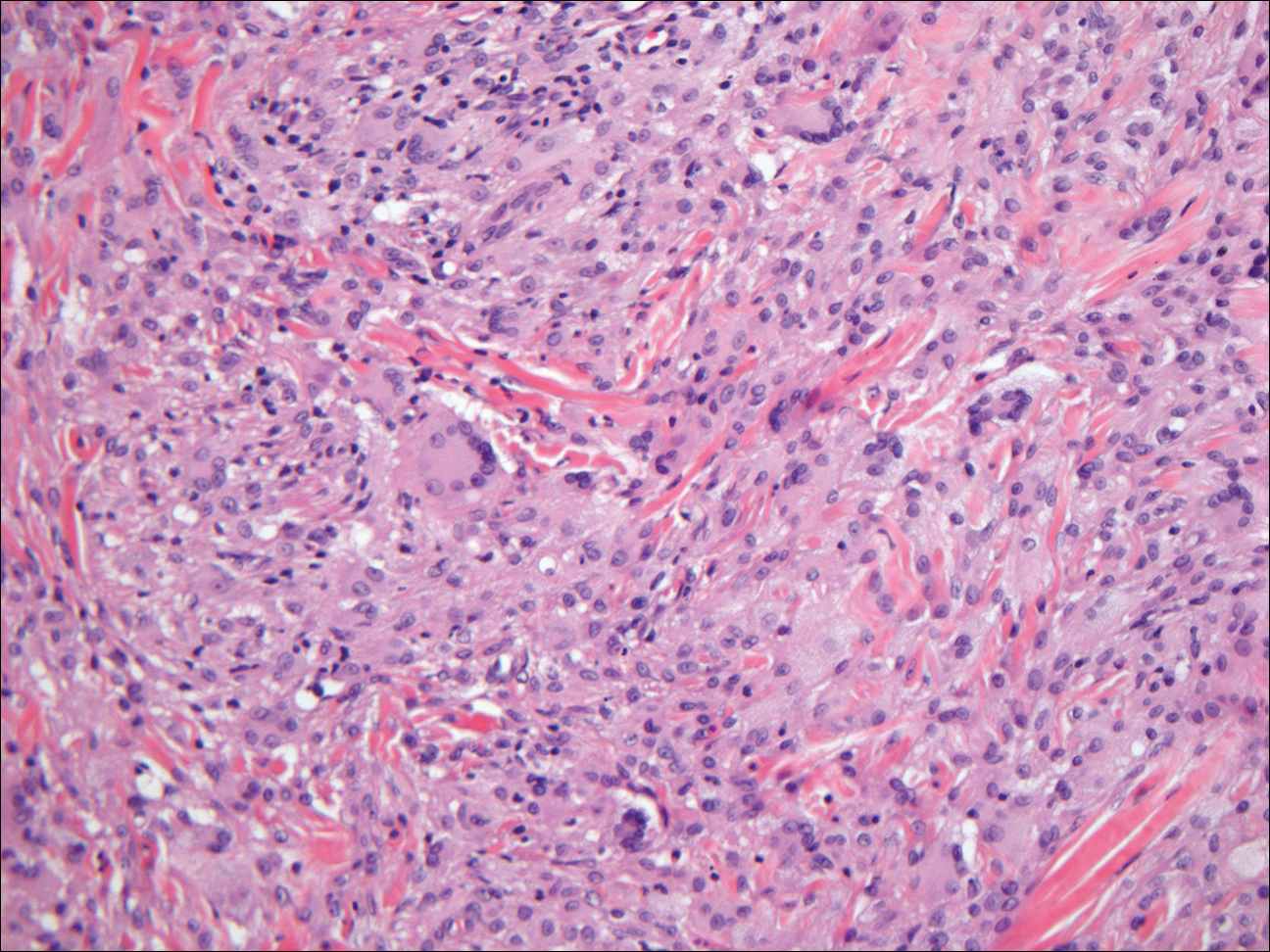
The histologic differential diagnosis for GBCC includes granular cell tumor as well as other tumors that can present with granular cell changes such as ameloblastoma, leiomyoma, leiomyosarcoma, angiosarcoma, malignant peripheral nerve sheath tumor, and granular cell trichoblastoma. Granular cell ameloblastomas have histologic features and staining patterns that are identical to GBCC; however, ameloblastomas are distinguished by their location within the oral cavity. Granular cell tumors and malignant peripheral nerve sheath tumors stain positive for S-100 protein, and angiosarcomas stain positive for D2-40 and CD31. Leiomyomas and leiomyosarcomas can be differentiated by staining with smooth muscle actin or desmin.1 Granular cell trichoblastomas can be differentiated by the follicular stem cell marker protein PHLDA1 positivity.5
Desmoplastic trichilemmoma is difficult to distinguish from BCC. These tumors are comprised of superficial lobules of basaloid cells with a perilobular hyaline mantel surrounding a central desmoplastic stroma (Figure 1). The basaloid cells in desmoplastic trichoepithelioma demonstrate clear cell change; however, granular features are not seen. The cells within the desmoplastic areas are arranged haphazardly in cords and nests and can mimic an invasive carcinoma; however, nuclear atypia and mitotic activity generally are absent in desmoplastic trichilemmoma.6
Granular cell tumors generally are poorly circumscribed dermal nodules comprised of large polygonal cells with an eosinophilic granular cytoplasm (Figure 2). The nuclei are generally small and round, and cytological atypia, necrosis, and mitotic activity are uncommon. The cells are positive for S-100 protein and neuron-specific enolase but negative for CD68. The granules are positive for periodic acid–Schiff stain and are diastase resistant. Rarely, these tumors can be malignant.7

Sebaceous adenoma is a well-circumscribed tumor comprised of lobules of characteristic mature sebocytes with bubbly or multivacuolated cytoplasm and crenated nuclei (Figure 3). There is an expansion and increased prominence of the peripherally located basaloid cells; however, in contrast to sebaceous epithelioma, less than 50% of the tumor usually is comprised of these basaloid cells.8
Xanthogranuloma demonstrates a dense collection of histiocytes in the dermis, commonly with Touton giant cell formation (Figure 4). The cells often have a foamy cytoplasm and cytoplasmic vacuoles are observed. The histiocytes are positive for factor XIIIa and CD68, and generally negative for S-100 protein and CD1a, which allows for differentiation from Langerhans cells.9
- Kanitakis J, Chouvet B. Granular-cell basal cell carcinoma of the skin. Eur J Dermatol. 2005;15:301-303.
- Dundr P, Stork J, Povysil C, et al. Granular cell basal cell carcinoma. Australas J Dermatol. 2004;45:70-72.
- Hayden AA, Shamma HN. Ber-EP4 and MNF-116 in a previously undescribed morphologic pattern of granular basal cell carcinoma. Am J Dermatopathol. 2001;23:530-532.
- Ansai S, Takayama R, Kimura T, et al. Ber-EP4 is a useful marker for follicular germinative cell differentiation of cutaneous epithelial neoplasms. J Dermatol. 2012;39:688-692.
- Battistella M, Peltre B, Cribier B. PHLDA1, a follicular stem cell marker, differentiates clear-cell/granular-cell trichoblastoma and clear-cell/granular cell basal cell carcinoma: a case-control study, with first description of granular-cell trichoblastoma. Am J Dermatopathol. 2014;36:643-650.
- Tellechea O, Reis JP, Baptista AP. Desmoplastic trichilemmoma. Am J Dermatopathol. 1992;14:107-114.
- Battistella M, Cribier B, Feugeas JP, et al. Vascular invasion and other invasive features in granular cell tumours of the skin: a multicentre study of 119 cases. J Clin Pathol. 2014;67:19-25.
- Shalin SC, Lyle S, Calonje E, et al. Sebaceous neoplasia and the Muir-Torre syndrome: important connections with clinical implications. Histopathology. 2010;56:133-147.
- Janssen D, Harms D. Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the kiel pediatric tumor registry. Am J Surg Pathol. 2005;29:21-28.
Granular Cell Basal Cell Carcinoma
Basal cell carcinoma (BCC) is the most common human epithelial malignancy. There are several histologic variants, the rarest being granular cell BCC (GBCC).1 Granular cell BCC is reported most commonly in men with a mean age of 63 years. Sixty-four percent of cases develop on the face, with the remainder arising on the chest or trunk. Granular cell BCC has distinct histologic features but has no specific epidemiologic or clinical features that differentiate it from more common forms of BCC. Treatment of GBCC is identical to BCC and demonstrates similar outcomes. The presence of granular cells can make GBCC difficult to differentiate from other benign and malignant lesions that display similar granular histologic changes.1,2 Rarely, tumors that are histologically similar to human GBCC have been reported in animals.1
Histologically, GBCC commonly demonstrates the architecture of a nodular BCC or may extend from an existing nodular BCC (quiz images A and B). Granular cell BCC is comprised of large islands of basaloid cells extending from the epidermis with rare mitotic activity. Certain variants showing no epidermal attachments have been described,1,3 as in the current case. Classically, BCC and GBCC both demonstrate a peripheral palisade of blue basal cells; however, GBCC may lack this palisading feature in some cases. Therefore, GBCC may be comprised of granular cells only, which may be more easily confused with other tumors with granular cell differentiation. Even when GBCC retains the traditional peripheral palisade of blue basal cells, the central cells are filled with eosinophilic granules.1,2
Electron microscopy of GBCC usually reveals bundles of cytoplasmic tonofilaments and desmosomes in both granular cells and the peripherally palisaded cells. Electron microscopy imaging also demonstrates 0.1- to 0.5-µm membrane-bound lysosomelike structures. In certain reports, these structures show focal positivity for lysozymes.1,2 The etiology of the granules is unclear; however, they are thought to represent degenerative changes related to metabolic alteration and accumulation of lysosomelike structures. These lysosomelike structures have been highlighted with CD68 staining, which was negative in our case.1,2 The lesional cells in GBCC stain positively for cytokeratins, p63, and Ber-EP4, and negatively for S-100 protein, epithelial membrane antigen, and carcinoembryonic antigen. The granules in GBCC generally are positive on periodic acid–Schiff staining.1-4
The histologic differential diagnosis for GBCC includes granular cell tumor as well as other tumors that can present with granular cell changes such as ameloblastoma, leiomyoma, leiomyosarcoma, angiosarcoma, malignant peripheral nerve sheath tumor, and granular cell trichoblastoma. Granular cell ameloblastomas have histologic features and staining patterns that are identical to GBCC; however, ameloblastomas are distinguished by their location within the oral cavity. Granular cell tumors and malignant peripheral nerve sheath tumors stain positive for S-100 protein, and angiosarcomas stain positive for D2-40 and CD31. Leiomyomas and leiomyosarcomas can be differentiated by staining with smooth muscle actin or desmin.1 Granular cell trichoblastomas can be differentiated by the follicular stem cell marker protein PHLDA1 positivity.5
Desmoplastic trichilemmoma is difficult to distinguish from BCC. These tumors are comprised of superficial lobules of basaloid cells with a perilobular hyaline mantel surrounding a central desmoplastic stroma (Figure 1). The basaloid cells in desmoplastic trichoepithelioma demonstrate clear cell change; however, granular features are not seen. The cells within the desmoplastic areas are arranged haphazardly in cords and nests and can mimic an invasive carcinoma; however, nuclear atypia and mitotic activity generally are absent in desmoplastic trichilemmoma.6

Granular cell tumors generally are poorly circumscribed dermal nodules comprised of large polygonal cells with an eosinophilic granular cytoplasm (Figure 2). The nuclei are generally small and round, and cytological atypia, necrosis, and mitotic activity are uncommon. The cells are positive for S-100 protein and neuron-specific enolase but negative for CD68. The granules are positive for periodic acid–Schiff stain and are diastase resistant. Rarely, these tumors can be malignant.7

Sebaceous adenoma is a well-circumscribed tumor comprised of lobules of characteristic mature sebocytes with bubbly or multivacuolated cytoplasm and crenated nuclei (Figure 3). There is an expansion and increased prominence of the peripherally located basaloid cells; however, in contrast to sebaceous epithelioma, less than 50% of the tumor usually is comprised of these basaloid cells.8

Xanthogranuloma demonstrates a dense collection of histiocytes in the dermis, commonly with Touton giant cell formation (Figure 4). The cells often have a foamy cytoplasm and cytoplasmic vacuoles are observed. The histiocytes are positive for factor XIIIa and CD68, and generally negative for S-100 protein and CD1a, which allows for differentiation from Langerhans cells.9

Granular Cell Basal Cell Carcinoma
Basal cell carcinoma (BCC) is the most common human epithelial malignancy. There are several histologic variants, the rarest being granular cell BCC (GBCC).1 Granular cell BCC is reported most commonly in men with a mean age of 63 years. Sixty-four percent of cases develop on the face, with the remainder arising on the chest or trunk. Granular cell BCC has distinct histologic features but has no specific epidemiologic or clinical features that differentiate it from more common forms of BCC. Treatment of GBCC is identical to BCC and demonstrates similar outcomes. The presence of granular cells can make GBCC difficult to differentiate from other benign and malignant lesions that display similar granular histologic changes.1,2 Rarely, tumors that are histologically similar to human GBCC have been reported in animals.1
Histologically, GBCC commonly demonstrates the architecture of a nodular BCC or may extend from an existing nodular BCC (quiz images A and B). Granular cell BCC is comprised of large islands of basaloid cells extending from the epidermis with rare mitotic activity. Certain variants showing no epidermal attachments have been described,1,3 as in the current case. Classically, BCC and GBCC both demonstrate a peripheral palisade of blue basal cells; however, GBCC may lack this palisading feature in some cases. Therefore, GBCC may be comprised of granular cells only, which may be more easily confused with other tumors with granular cell differentiation. Even when GBCC retains the traditional peripheral palisade of blue basal cells, the central cells are filled with eosinophilic granules.1,2
Electron microscopy of GBCC usually reveals bundles of cytoplasmic tonofilaments and desmosomes in both granular cells and the peripherally palisaded cells. Electron microscopy imaging also demonstrates 0.1- to 0.5-µm membrane-bound lysosomelike structures. In certain reports, these structures show focal positivity for lysozymes.1,2 The etiology of the granules is unclear; however, they are thought to represent degenerative changes related to metabolic alteration and accumulation of lysosomelike structures. These lysosomelike structures have been highlighted with CD68 staining, which was negative in our case.1,2 The lesional cells in GBCC stain positively for cytokeratins, p63, and Ber-EP4, and negatively for S-100 protein, epithelial membrane antigen, and carcinoembryonic antigen. The granules in GBCC generally are positive on periodic acid–Schiff staining.1-4
The histologic differential diagnosis for GBCC includes granular cell tumor as well as other tumors that can present with granular cell changes such as ameloblastoma, leiomyoma, leiomyosarcoma, angiosarcoma, malignant peripheral nerve sheath tumor, and granular cell trichoblastoma. Granular cell ameloblastomas have histologic features and staining patterns that are identical to GBCC; however, ameloblastomas are distinguished by their location within the oral cavity. Granular cell tumors and malignant peripheral nerve sheath tumors stain positive for S-100 protein, and angiosarcomas stain positive for D2-40 and CD31. Leiomyomas and leiomyosarcomas can be differentiated by staining with smooth muscle actin or desmin.1 Granular cell trichoblastomas can be differentiated by the follicular stem cell marker protein PHLDA1 positivity.5
Desmoplastic trichilemmoma is difficult to distinguish from BCC. These tumors are comprised of superficial lobules of basaloid cells with a perilobular hyaline mantel surrounding a central desmoplastic stroma (Figure 1). The basaloid cells in desmoplastic trichoepithelioma demonstrate clear cell change; however, granular features are not seen. The cells within the desmoplastic areas are arranged haphazardly in cords and nests and can mimic an invasive carcinoma; however, nuclear atypia and mitotic activity generally are absent in desmoplastic trichilemmoma.6

Granular cell tumors generally are poorly circumscribed dermal nodules comprised of large polygonal cells with an eosinophilic granular cytoplasm (Figure 2). The nuclei are generally small and round, and cytological atypia, necrosis, and mitotic activity are uncommon. The cells are positive for S-100 protein and neuron-specific enolase but negative for CD68. The granules are positive for periodic acid–Schiff stain and are diastase resistant. Rarely, these tumors can be malignant.7

Sebaceous adenoma is a well-circumscribed tumor comprised of lobules of characteristic mature sebocytes with bubbly or multivacuolated cytoplasm and crenated nuclei (Figure 3). There is an expansion and increased prominence of the peripherally located basaloid cells; however, in contrast to sebaceous epithelioma, less than 50% of the tumor usually is comprised of these basaloid cells.8

Xanthogranuloma demonstrates a dense collection of histiocytes in the dermis, commonly with Touton giant cell formation (Figure 4). The cells often have a foamy cytoplasm and cytoplasmic vacuoles are observed. The histiocytes are positive for factor XIIIa and CD68, and generally negative for S-100 protein and CD1a, which allows for differentiation from Langerhans cells.9

- Kanitakis J, Chouvet B. Granular-cell basal cell carcinoma of the skin. Eur J Dermatol. 2005;15:301-303.
- Dundr P, Stork J, Povysil C, et al. Granular cell basal cell carcinoma. Australas J Dermatol. 2004;45:70-72.
- Hayden AA, Shamma HN. Ber-EP4 and MNF-116 in a previously undescribed morphologic pattern of granular basal cell carcinoma. Am J Dermatopathol. 2001;23:530-532.
- Ansai S, Takayama R, Kimura T, et al. Ber-EP4 is a useful marker for follicular germinative cell differentiation of cutaneous epithelial neoplasms. J Dermatol. 2012;39:688-692.
- Battistella M, Peltre B, Cribier B. PHLDA1, a follicular stem cell marker, differentiates clear-cell/granular-cell trichoblastoma and clear-cell/granular cell basal cell carcinoma: a case-control study, with first description of granular-cell trichoblastoma. Am J Dermatopathol. 2014;36:643-650.
- Tellechea O, Reis JP, Baptista AP. Desmoplastic trichilemmoma. Am J Dermatopathol. 1992;14:107-114.
- Battistella M, Cribier B, Feugeas JP, et al. Vascular invasion and other invasive features in granular cell tumours of the skin: a multicentre study of 119 cases. J Clin Pathol. 2014;67:19-25.
- Shalin SC, Lyle S, Calonje E, et al. Sebaceous neoplasia and the Muir-Torre syndrome: important connections with clinical implications. Histopathology. 2010;56:133-147.
- Janssen D, Harms D. Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the kiel pediatric tumor registry. Am J Surg Pathol. 2005;29:21-28.
- Kanitakis J, Chouvet B. Granular-cell basal cell carcinoma of the skin. Eur J Dermatol. 2005;15:301-303.
- Dundr P, Stork J, Povysil C, et al. Granular cell basal cell carcinoma. Australas J Dermatol. 2004;45:70-72.
- Hayden AA, Shamma HN. Ber-EP4 and MNF-116 in a previously undescribed morphologic pattern of granular basal cell carcinoma. Am J Dermatopathol. 2001;23:530-532.
- Ansai S, Takayama R, Kimura T, et al. Ber-EP4 is a useful marker for follicular germinative cell differentiation of cutaneous epithelial neoplasms. J Dermatol. 2012;39:688-692.
- Battistella M, Peltre B, Cribier B. PHLDA1, a follicular stem cell marker, differentiates clear-cell/granular-cell trichoblastoma and clear-cell/granular cell basal cell carcinoma: a case-control study, with first description of granular-cell trichoblastoma. Am J Dermatopathol. 2014;36:643-650.
- Tellechea O, Reis JP, Baptista AP. Desmoplastic trichilemmoma. Am J Dermatopathol. 1992;14:107-114.
- Battistella M, Cribier B, Feugeas JP, et al. Vascular invasion and other invasive features in granular cell tumours of the skin: a multicentre study of 119 cases. J Clin Pathol. 2014;67:19-25.
- Shalin SC, Lyle S, Calonje E, et al. Sebaceous neoplasia and the Muir-Torre syndrome: important connections with clinical implications. Histopathology. 2010;56:133-147.
- Janssen D, Harms D. Juvenile xanthogranuloma in childhood and adolescence: a clinicopathologic study of 129 patients from the kiel pediatric tumor registry. Am J Surg Pathol. 2005;29:21-28.

The best diagnosis is:
a. desmoplastic trichilemmoma
b. granular cell basal cell carcinoma
c. granular cell tumor
d. sebaceous adenoma
e. xanthogranuloma
Continue to the next page for the diagnosis >>
Benign Lesion on the Posterior Aspect of the Neck
Nuchal-Type Fibroma
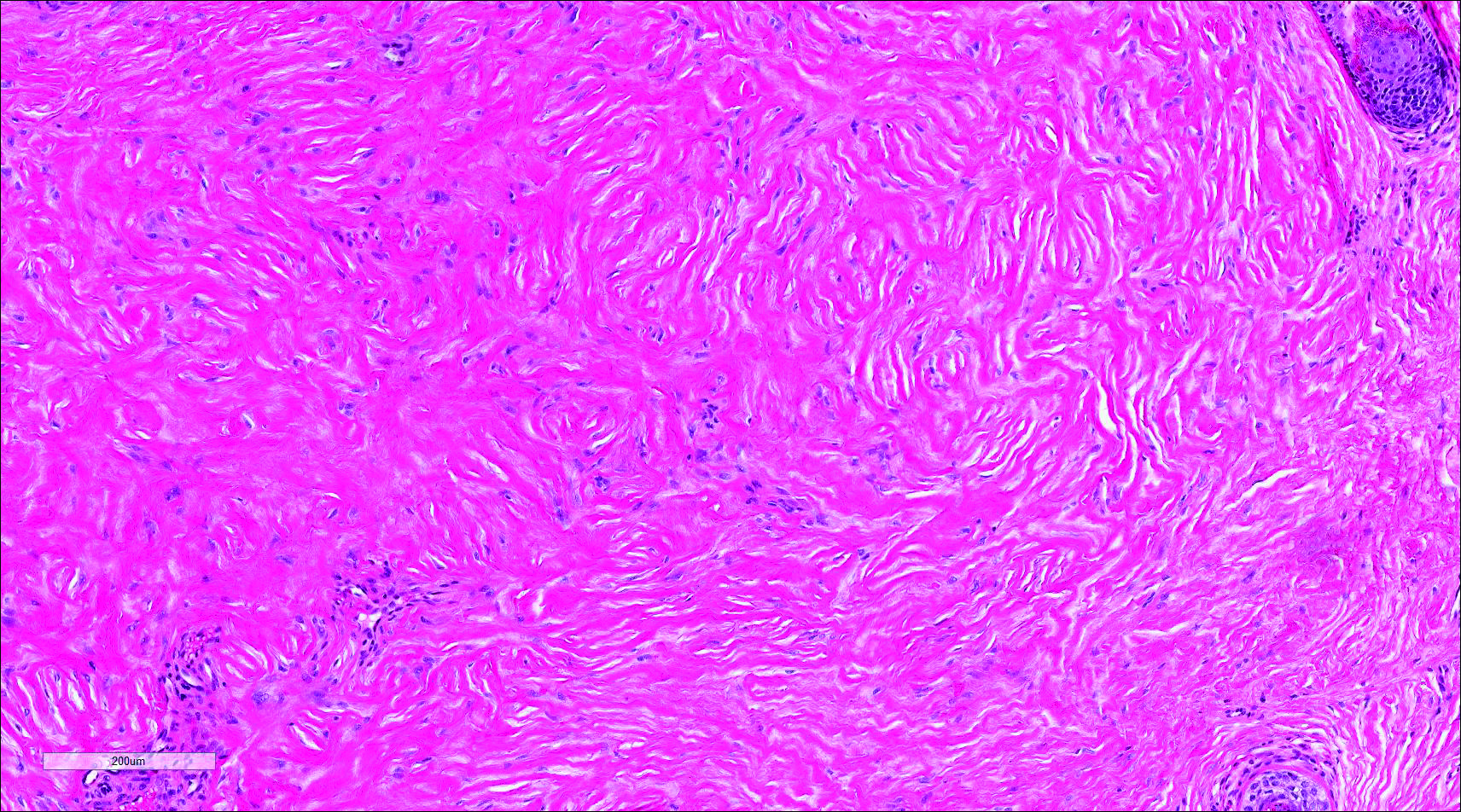
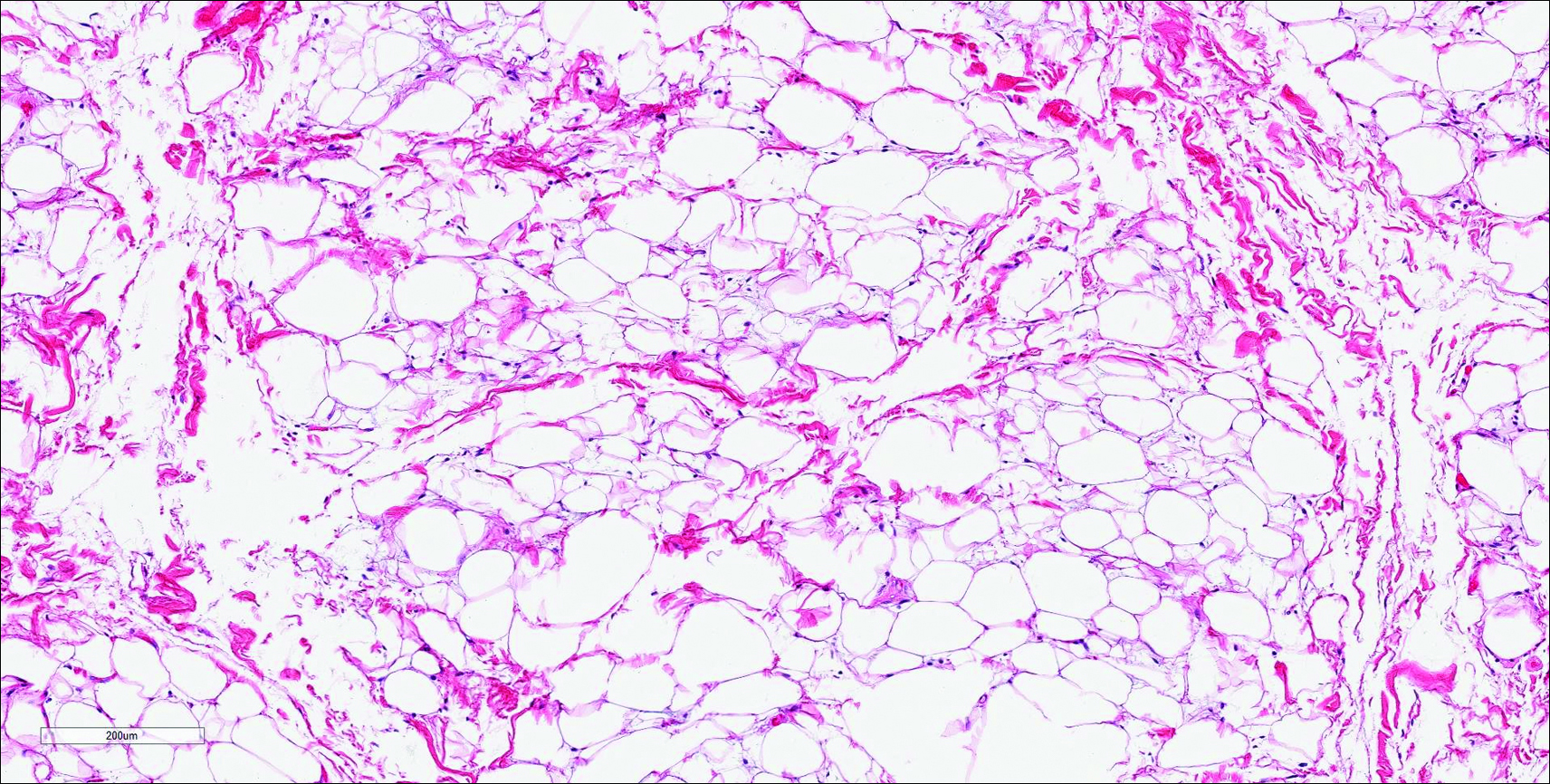
Nuchal-type fibroma (NTF) is a rare benign proliferation of the dermis and subcutis associated with diabetes mellitus and Gardner syndrome.1,2 Forty-four percent of patients with NTF have diabetes mellitus.2 The posterior aspect of the neck is the most frequently affected site, but lesions also may present on the upper back, lumbosacral area, buttocks, and face. Physical examination generally reveals an indurated, asymptomatic, ill-defined, 3-cm or smaller nodule that is hard and white, unencapsulated, and poorly circumscribed.
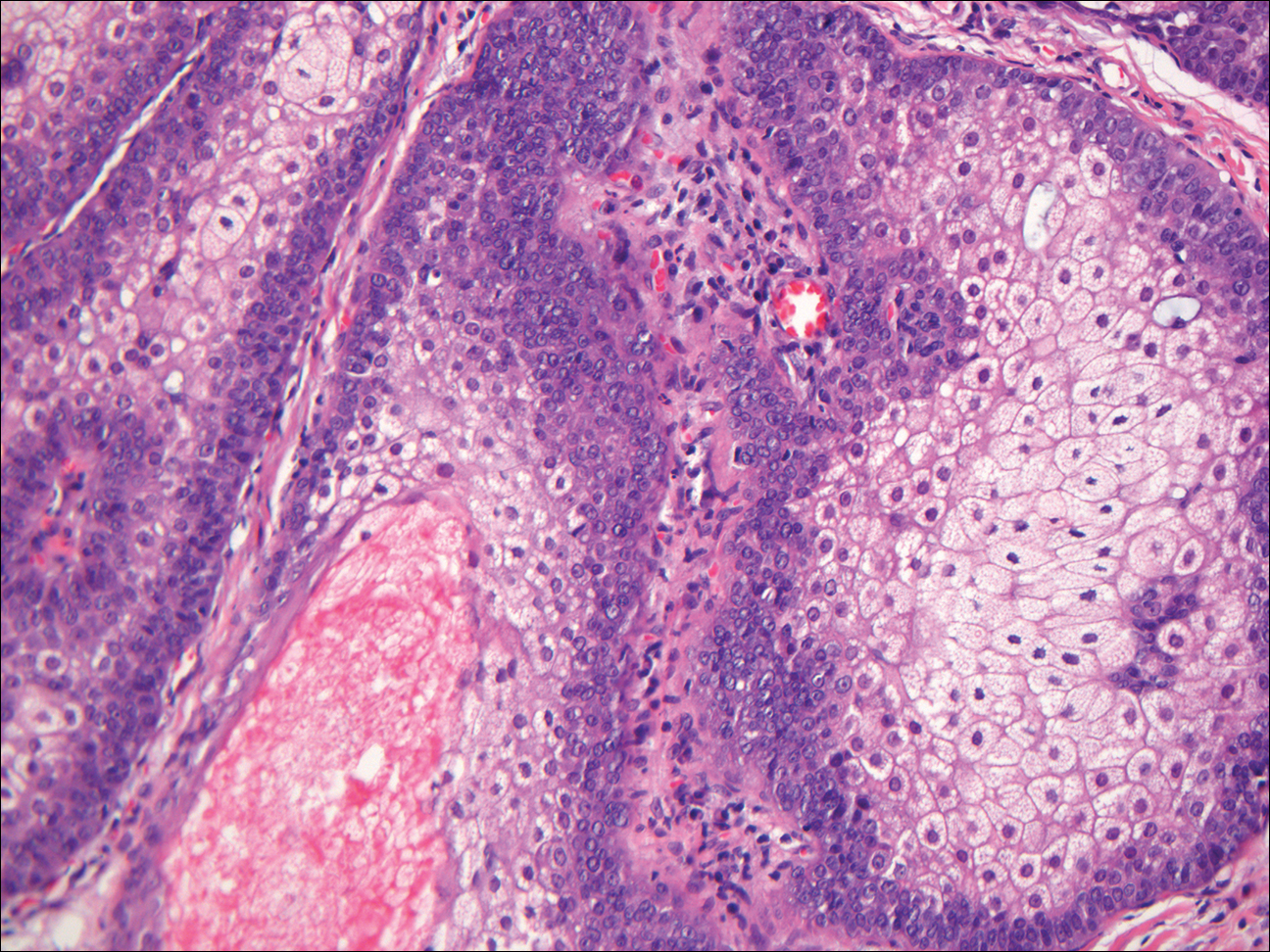
Histopathologic examination of NTF typically reveals a nodular paucicellular proliferation of thick collagen bundles with inconspicuous fibroblasts, radiation of collagenous septa into the subcutaneous fat, and entrapment of mature adipose tissue and small nerves (quiz image A). Collagen bundles are thickened with entrapment of adipose tissue without increased cellularity (quiz image B). S-100 staining can show the entrapped nerves.
Similar to NTF, sclerotic fibroma is a firm dermal nodule with histologic examination usually demonstrating a paucicellular collagenous tumor. In sclerotic fibromas, the collagen pattern resembles Vincent van Gogh’s painting “The Starry Night” and may be a marker for Cowden disease (Figure 1).3 Solitary fibrous tumors are distinguished by more hypercellular areas, patternless pattern, and staghorn-shaped blood vessels (Figure 2).4 Spindle cell lipoma classically demonstrates a mixture of mature adipocytes and bland spindle cells in a mucinous or fibrous background with thick collagen bundles with no storiform pattern (Figure 3). Some variants of spindle cell lipoma have minimal or no fat.5 All of these conditions have positive immunohistochemical staining for CD34.
However, dermatofibroma is CD34‒. Dermatofibroma is characterized by an interstitial spindle cell proliferation with a loose storiform pattern, collagen trapping at the outer edges of the tumor, overlying platelike acanthosis, and sometimes follicular induction (Figure 4).

Nuchal-type fibroma also can resemble scleredema. Both lesions can show increased and thickened collagen bundles without notable fibroblast proliferation; the difference is the occurrence of mucin in scleredema. However, incases of late-stage scleredema, mucin is not always demonstrated. Therefore, one can conclude that histologically NTF is closely associated with late-stage scleredema.6
- Dawes LC, La Hei ER, Tobias V, et al. Nuchal fibroma should be recognized as a new extracolonic manifestation of Gardner-variant familial adenomatous polyposis. Aust N Z J Surg. 2000;70:824-826.
- Michal M, Fetsch JF, Hes O, et al. Nuchal-type fibroma: a clinicopathologic study of 52 cases. Cancer. 1999;85:156-163.
- Pernet C, Durand L, Bessis D, et al. Solitary sclerotic fibroma of the skin: a possible clue for Cowden syndrome. Eur J Dermatol. 2012;22:278-279.
- Omori Y, Saeki H, Ito K, et al. Solitary fibrous tumour of the scalp. Clin Exp Dermatol. 2014;39:539-541.
- Billings SD, Folpe AL. Diagnostically challenging spindle cell lipomas: a report of 34 “low-fat” and “fat-free” variants. Am J Dermatopathol. 2007;29:437-442.
- Banney LA, Weedon D, Muir JB. Nuchal fibroma associated with scleredema, diabetes mellitus and organic solvent exposure. Australas J Dermatol. 2000;41:39-41.
Nuchal-Type Fibroma
Nuchal-type fibroma (NTF) is a rare benign proliferation of the dermis and subcutis associated with diabetes mellitus and Gardner syndrome.1,2 Forty-four percent of patients with NTF have diabetes mellitus.2 The posterior aspect of the neck is the most frequently affected site, but lesions also may present on the upper back, lumbosacral area, buttocks, and face. Physical examination generally reveals an indurated, asymptomatic, ill-defined, 3-cm or smaller nodule that is hard and white, unencapsulated, and poorly circumscribed.
Histopathologic examination of NTF typically reveals a nodular paucicellular proliferation of thick collagen bundles with inconspicuous fibroblasts, radiation of collagenous septa into the subcutaneous fat, and entrapment of mature adipose tissue and small nerves (quiz image A). Collagen bundles are thickened with entrapment of adipose tissue without increased cellularity (quiz image B). S-100 staining can show the entrapped nerves.
Similar to NTF, sclerotic fibroma is a firm dermal nodule with histologic examination usually demonstrating a paucicellular collagenous tumor. In sclerotic fibromas, the collagen pattern resembles Vincent van Gogh’s painting “The Starry Night” and may be a marker for Cowden disease (Figure 1).3 Solitary fibrous tumors are distinguished by more hypercellular areas, patternless pattern, and staghorn-shaped blood vessels (Figure 2).4 Spindle cell lipoma classically demonstrates a mixture of mature adipocytes and bland spindle cells in a mucinous or fibrous background with thick collagen bundles with no storiform pattern (Figure 3). Some variants of spindle cell lipoma have minimal or no fat.5 All of these conditions have positive immunohistochemical staining for CD34.



However, dermatofibroma is CD34‒. Dermatofibroma is characterized by an interstitial spindle cell proliferation with a loose storiform pattern, collagen trapping at the outer edges of the tumor, overlying platelike acanthosis, and sometimes follicular induction (Figure 4).

Nuchal-type fibroma also can resemble scleredema. Both lesions can show increased and thickened collagen bundles without notable fibroblast proliferation; the difference is the occurrence of mucin in scleredema. However, incases of late-stage scleredema, mucin is not always demonstrated. Therefore, one can conclude that histologically NTF is closely associated with late-stage scleredema.6
Nuchal-Type Fibroma
Nuchal-type fibroma (NTF) is a rare benign proliferation of the dermis and subcutis associated with diabetes mellitus and Gardner syndrome.1,2 Forty-four percent of patients with NTF have diabetes mellitus.2 The posterior aspect of the neck is the most frequently affected site, but lesions also may present on the upper back, lumbosacral area, buttocks, and face. Physical examination generally reveals an indurated, asymptomatic, ill-defined, 3-cm or smaller nodule that is hard and white, unencapsulated, and poorly circumscribed.
Histopathologic examination of NTF typically reveals a nodular paucicellular proliferation of thick collagen bundles with inconspicuous fibroblasts, radiation of collagenous septa into the subcutaneous fat, and entrapment of mature adipose tissue and small nerves (quiz image A). Collagen bundles are thickened with entrapment of adipose tissue without increased cellularity (quiz image B). S-100 staining can show the entrapped nerves.
Similar to NTF, sclerotic fibroma is a firm dermal nodule with histologic examination usually demonstrating a paucicellular collagenous tumor. In sclerotic fibromas, the collagen pattern resembles Vincent van Gogh’s painting “The Starry Night” and may be a marker for Cowden disease (Figure 1).3 Solitary fibrous tumors are distinguished by more hypercellular areas, patternless pattern, and staghorn-shaped blood vessels (Figure 2).4 Spindle cell lipoma classically demonstrates a mixture of mature adipocytes and bland spindle cells in a mucinous or fibrous background with thick collagen bundles with no storiform pattern (Figure 3). Some variants of spindle cell lipoma have minimal or no fat.5 All of these conditions have positive immunohistochemical staining for CD34.



However, dermatofibroma is CD34‒. Dermatofibroma is characterized by an interstitial spindle cell proliferation with a loose storiform pattern, collagen trapping at the outer edges of the tumor, overlying platelike acanthosis, and sometimes follicular induction (Figure 4).

Nuchal-type fibroma also can resemble scleredema. Both lesions can show increased and thickened collagen bundles without notable fibroblast proliferation; the difference is the occurrence of mucin in scleredema. However, incases of late-stage scleredema, mucin is not always demonstrated. Therefore, one can conclude that histologically NTF is closely associated with late-stage scleredema.6
- Dawes LC, La Hei ER, Tobias V, et al. Nuchal fibroma should be recognized as a new extracolonic manifestation of Gardner-variant familial adenomatous polyposis. Aust N Z J Surg. 2000;70:824-826.
- Michal M, Fetsch JF, Hes O, et al. Nuchal-type fibroma: a clinicopathologic study of 52 cases. Cancer. 1999;85:156-163.
- Pernet C, Durand L, Bessis D, et al. Solitary sclerotic fibroma of the skin: a possible clue for Cowden syndrome. Eur J Dermatol. 2012;22:278-279.
- Omori Y, Saeki H, Ito K, et al. Solitary fibrous tumour of the scalp. Clin Exp Dermatol. 2014;39:539-541.
- Billings SD, Folpe AL. Diagnostically challenging spindle cell lipomas: a report of 34 “low-fat” and “fat-free” variants. Am J Dermatopathol. 2007;29:437-442.
- Banney LA, Weedon D, Muir JB. Nuchal fibroma associated with scleredema, diabetes mellitus and organic solvent exposure. Australas J Dermatol. 2000;41:39-41.
- Dawes LC, La Hei ER, Tobias V, et al. Nuchal fibroma should be recognized as a new extracolonic manifestation of Gardner-variant familial adenomatous polyposis. Aust N Z J Surg. 2000;70:824-826.
- Michal M, Fetsch JF, Hes O, et al. Nuchal-type fibroma: a clinicopathologic study of 52 cases. Cancer. 1999;85:156-163.
- Pernet C, Durand L, Bessis D, et al. Solitary sclerotic fibroma of the skin: a possible clue for Cowden syndrome. Eur J Dermatol. 2012;22:278-279.
- Omori Y, Saeki H, Ito K, et al. Solitary fibrous tumour of the scalp. Clin Exp Dermatol. 2014;39:539-541.
- Billings SD, Folpe AL. Diagnostically challenging spindle cell lipomas: a report of 34 “low-fat” and “fat-free” variants. Am J Dermatopathol. 2007;29:437-442.
- Banney LA, Weedon D, Muir JB. Nuchal fibroma associated with scleredema, diabetes mellitus and organic solvent exposure. Australas J Dermatol. 2000;41:39-41.
The best diagnosis is:
a. dermatofibroma
b. nuchal-type fibroma
c. sclerotic fibroma
d. solitary fibrous tumor
e. spindle cell lipoma
Continue to the next page for the diagnosis >>
Cyst on the Eyebrow
The best diagnosis is:
a. bronchogenic cyst
b. dermoid cyst
c. epidermal inclusion cyst
d. hidrocystoma
e. steatocystoma
|
|
| H&E, original magnification ×40. |
|
| H&E, original magnification ×100. |
Continue to the next page for the diagnosis >>
Dermoid Cyst
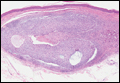
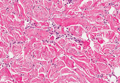
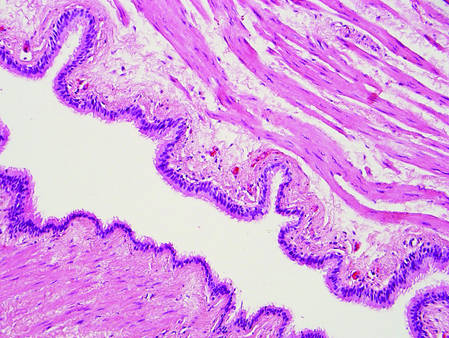
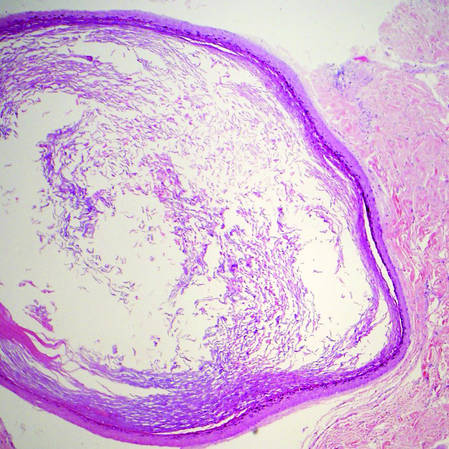
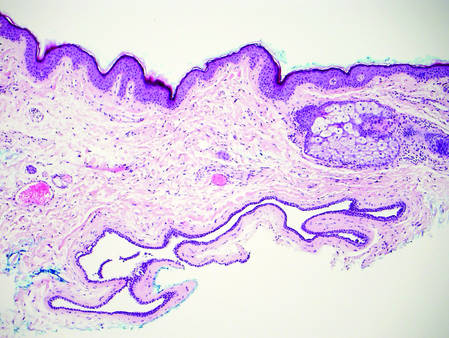
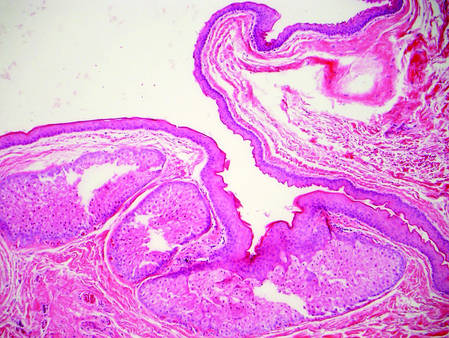
Dermoid cysts often present clinically as firm subcutaneous nodules on the head or neck in young children. They tend to arise along the lateral aspect of the eyebrow but also can occur on the nose, forehead, neck, chest, or scalp.1 Dermoid cysts are thought to arise from the sequestration of ectodermal tissues along the embryonic fusion planes during development.2 As such, they represent congenital defects and often are identified at birth; however, some are not noticed until much later when they enlarge or become inflamed or infected. Midline dermoid cysts may be associated with underlying dysraphism or intracranial extension.3,4 Thus, any midline lesion warrants evaluation that incorporates imaging with computed tomography or magnetic resonance imaging.4,5 Histologically, dermoid cysts are lined by a keratinizing stratified squamous epithelium (quiz image A), but the lining may be brightly eosinophilic and wavy resembling shark teeth.1,3 The wall of a dermoid cyst commonly contains mature adnexal structures such as terminal hair follicles, sebaceous glands, apocrine glands, and/or eccrine glands (quiz image B).1 Smooth muscle also may be seen within the lining; however, bone and cartilage are not commonly reported in dermoid cysts.2 Lamellar keratin is typical of the cyst contents, and terminal hair shafts also are sometimes noted within the cystic space (quiz image B).1,2 Treatment options include excision at the time of diagnosis or close clinical monitoring with subsequent excision if the lesion grows or becomes symptomatic.4,5 Many practitioners opt to excise these cysts at diagnosis, as untreated lesions are at risk for infection and/or inflammation or may be cosmetically deforming.6,7 Surgical resection, including removal of the wall of the cyst, is curative and reoccurrence is rare.5
| |
Figure 1. Bronchogenic cyst demonstrating a ciliated pseudostratified epithelial lining encircled by smooth muscle (H&E, original magnification ×200). | |
| |
| Figure 2. Epidermal inclusion cyst containing loose lamellar keratin and a lining that closely resembles the surface epidermis (H&E, original magnification ×40). |
|
Bronchogenic cysts demonstrate an epithelial lining that often is pseudostratified cuboidal or columnar as well as ciliated (Figure 1). Goblet cells are present in the lining in approximately 50% of cases. Smooth muscle may be seen circumferentially surrounding the cyst lining, and rare cases also contain cartilage.1 In contrast to dermoid cysts, other types of adnexal structures are not found within the lining. Bronchogenic cysts that arise in the skin are extremely rare.2 These cysts are thought to arise from respiratory epithelium that has been sequestered during embryologic formation of the tracheobronchial tree. They often are seen overlying the suprasternal notch and occasionally are found on the anterior aspect of the neck or chin. These cysts also are present at birth, similar to dermoid cysts.3
Epidermal inclusion cysts have a lining that histologically bears close resemblance to the surface epidermis. These cysts contain loose lamellar keratin, similar to a dermoid cyst. In contrast, the lining of an epidermal inclusion cyst will lack adnexal structures (Figure 2).1 Clinically, epidermal inclusion cysts often present as smooth, dome-shaped papules and nodules with a central punctum. They are classically found on the face, neck, and trunk. These cysts are thought to arise after a traumatic insult to the pilosebaceous unit.2
Hidrocystomas can be apocrine or eccrine.3 Eccrine hidrocystomas are unilocular cysts that are lined by 2 layers of flattened to cuboidal epithelial cells (Figure 3). The cysts are filled with clear fluid and often are found adjacent to normal eccrine glands.1 Apocrine hidrocystomas are unilocular or multilocular cysts that are lined by 1 to several layers of epithelial cells. The lining of an apocrine hidrocystoma will often exhibit luminal decapitation secretion.3 Apocrine and eccrine hidrocystomas are clinically identical and appear as blue translucent papules on the cheeks or eyelids of adults.1-3 They usually occur periorbitally but also can be seen on the trunk, popliteal fossa, external ears, or vulva. Eccrine hidrocystomas can wax and wane in accordance with the amount of sweat produced; thus, they often expand in size during the summer months.2
Steatocystomas, or simple sebaceous duct cysts, histologically demonstrate a characteristically wavy and eosinophilic cuticle resembling shark teeth (Figure 4) similar to the lining of the sebaceous duct where it enters the follicle.1 Sebaceous glands are an almost invariable feature, either present within the lining of the cyst (Figure 4) or in the adjacent tissue.2 In comparison, dermoid cysts may have a red wavy cuticle but also will usually have terminal hair follicles or eccrine or apocrine glands within the wall of the cyst. Steatocystomas typically are collapsed and empty or only contain sebaceous debris (Figure 4) rather than the lamellar keratin seen in dermoid and epidermoid inclusion cysts. Steatocystomas can occur as solitary (steatocystoma simplex) or multiple (steatocystoma multiplex) lesions.1,3 They are clinically comprised of small dome-shaped papules that often are translucent and yellow. These cysts are commonly found on the sternum of males and the axillae or groin of females.2
| 
| |
Figure 3. Eccrine hidrocystoma with clear contents and lined by 2 layers of cuboidal epithelial cells (H&E, original magnification ×100). | Figure 4. Steatocystoma with a red wavy cuticle, sparse sebaceous contents, and sebaceous glands within the lining (H&E, original magnification ×100). |
|
1. Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
2. Calonje JE, Brenn T, Lazar AJ, et al. McKee’s Pathology of the Skin. 4th ed. St Louis, MO: Elsevier/Saunders; 2012.
3. Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
4. Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
5. Sorenson EP, Powel JE, Rozzelle CJ, et al. Scalp dermoids: a review of their anatomy, diagnosis, and treatment. Childs Nerv Syst. 2013;29:375-380.
6. Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolarynol Head Neck Surg. 2005;132:938-942.
7. Abou-Rayyah Y, Rose GE, Konrad H, et al. Clinical, radiological and pathological examination of periocular dermoid cysts: evidence of inflammation from an early age. Eye (Lond). 2002;16:507-512.
The best diagnosis is:
a. bronchogenic cyst
b. dermoid cyst
c. epidermal inclusion cyst
d. hidrocystoma
e. steatocystoma
|

|
| H&E, original magnification ×40. |

|
| H&E, original magnification ×100. |
Continue to the next page for the diagnosis >>
Dermoid Cyst
Dermoid cysts often present clinically as firm subcutaneous nodules on the head or neck in young children. They tend to arise along the lateral aspect of the eyebrow but also can occur on the nose, forehead, neck, chest, or scalp.1 Dermoid cysts are thought to arise from the sequestration of ectodermal tissues along the embryonic fusion planes during development.2 As such, they represent congenital defects and often are identified at birth; however, some are not noticed until much later when they enlarge or become inflamed or infected. Midline dermoid cysts may be associated with underlying dysraphism or intracranial extension.3,4 Thus, any midline lesion warrants evaluation that incorporates imaging with computed tomography or magnetic resonance imaging.4,5 Histologically, dermoid cysts are lined by a keratinizing stratified squamous epithelium (quiz image A), but the lining may be brightly eosinophilic and wavy resembling shark teeth.1,3 The wall of a dermoid cyst commonly contains mature adnexal structures such as terminal hair follicles, sebaceous glands, apocrine glands, and/or eccrine glands (quiz image B).1 Smooth muscle also may be seen within the lining; however, bone and cartilage are not commonly reported in dermoid cysts.2 Lamellar keratin is typical of the cyst contents, and terminal hair shafts also are sometimes noted within the cystic space (quiz image B).1,2 Treatment options include excision at the time of diagnosis or close clinical monitoring with subsequent excision if the lesion grows or becomes symptomatic.4,5 Many practitioners opt to excise these cysts at diagnosis, as untreated lesions are at risk for infection and/or inflammation or may be cosmetically deforming.6,7 Surgical resection, including removal of the wall of the cyst, is curative and reoccurrence is rare.5

| |
Figure 1. Bronchogenic cyst demonstrating a ciliated pseudostratified epithelial lining encircled by smooth muscle (H&E, original magnification ×200). | |

| |
| Figure 2. Epidermal inclusion cyst containing loose lamellar keratin and a lining that closely resembles the surface epidermis (H&E, original magnification ×40). |
|
Bronchogenic cysts demonstrate an epithelial lining that often is pseudostratified cuboidal or columnar as well as ciliated (Figure 1). Goblet cells are present in the lining in approximately 50% of cases. Smooth muscle may be seen circumferentially surrounding the cyst lining, and rare cases also contain cartilage.1 In contrast to dermoid cysts, other types of adnexal structures are not found within the lining. Bronchogenic cysts that arise in the skin are extremely rare.2 These cysts are thought to arise from respiratory epithelium that has been sequestered during embryologic formation of the tracheobronchial tree. They often are seen overlying the suprasternal notch and occasionally are found on the anterior aspect of the neck or chin. These cysts also are present at birth, similar to dermoid cysts.3
Epidermal inclusion cysts have a lining that histologically bears close resemblance to the surface epidermis. These cysts contain loose lamellar keratin, similar to a dermoid cyst. In contrast, the lining of an epidermal inclusion cyst will lack adnexal structures (Figure 2).1 Clinically, epidermal inclusion cysts often present as smooth, dome-shaped papules and nodules with a central punctum. They are classically found on the face, neck, and trunk. These cysts are thought to arise after a traumatic insult to the pilosebaceous unit.2
Hidrocystomas can be apocrine or eccrine.3 Eccrine hidrocystomas are unilocular cysts that are lined by 2 layers of flattened to cuboidal epithelial cells (Figure 3). The cysts are filled with clear fluid and often are found adjacent to normal eccrine glands.1 Apocrine hidrocystomas are unilocular or multilocular cysts that are lined by 1 to several layers of epithelial cells. The lining of an apocrine hidrocystoma will often exhibit luminal decapitation secretion.3 Apocrine and eccrine hidrocystomas are clinically identical and appear as blue translucent papules on the cheeks or eyelids of adults.1-3 They usually occur periorbitally but also can be seen on the trunk, popliteal fossa, external ears, or vulva. Eccrine hidrocystomas can wax and wane in accordance with the amount of sweat produced; thus, they often expand in size during the summer months.2
Steatocystomas, or simple sebaceous duct cysts, histologically demonstrate a characteristically wavy and eosinophilic cuticle resembling shark teeth (Figure 4) similar to the lining of the sebaceous duct where it enters the follicle.1 Sebaceous glands are an almost invariable feature, either present within the lining of the cyst (Figure 4) or in the adjacent tissue.2 In comparison, dermoid cysts may have a red wavy cuticle but also will usually have terminal hair follicles or eccrine or apocrine glands within the wall of the cyst. Steatocystomas typically are collapsed and empty or only contain sebaceous debris (Figure 4) rather than the lamellar keratin seen in dermoid and epidermoid inclusion cysts. Steatocystomas can occur as solitary (steatocystoma simplex) or multiple (steatocystoma multiplex) lesions.1,3 They are clinically comprised of small dome-shaped papules that often are translucent and yellow. These cysts are commonly found on the sternum of males and the axillae or groin of females.2

| 
| |
Figure 3. Eccrine hidrocystoma with clear contents and lined by 2 layers of cuboidal epithelial cells (H&E, original magnification ×100). | Figure 4. Steatocystoma with a red wavy cuticle, sparse sebaceous contents, and sebaceous glands within the lining (H&E, original magnification ×100). |
|
The best diagnosis is:
a. bronchogenic cyst
b. dermoid cyst
c. epidermal inclusion cyst
d. hidrocystoma
e. steatocystoma
|

|
| H&E, original magnification ×40. |

|
| H&E, original magnification ×100. |
Continue to the next page for the diagnosis >>
Dermoid Cyst
Dermoid cysts often present clinically as firm subcutaneous nodules on the head or neck in young children. They tend to arise along the lateral aspect of the eyebrow but also can occur on the nose, forehead, neck, chest, or scalp.1 Dermoid cysts are thought to arise from the sequestration of ectodermal tissues along the embryonic fusion planes during development.2 As such, they represent congenital defects and often are identified at birth; however, some are not noticed until much later when they enlarge or become inflamed or infected. Midline dermoid cysts may be associated with underlying dysraphism or intracranial extension.3,4 Thus, any midline lesion warrants evaluation that incorporates imaging with computed tomography or magnetic resonance imaging.4,5 Histologically, dermoid cysts are lined by a keratinizing stratified squamous epithelium (quiz image A), but the lining may be brightly eosinophilic and wavy resembling shark teeth.1,3 The wall of a dermoid cyst commonly contains mature adnexal structures such as terminal hair follicles, sebaceous glands, apocrine glands, and/or eccrine glands (quiz image B).1 Smooth muscle also may be seen within the lining; however, bone and cartilage are not commonly reported in dermoid cysts.2 Lamellar keratin is typical of the cyst contents, and terminal hair shafts also are sometimes noted within the cystic space (quiz image B).1,2 Treatment options include excision at the time of diagnosis or close clinical monitoring with subsequent excision if the lesion grows or becomes symptomatic.4,5 Many practitioners opt to excise these cysts at diagnosis, as untreated lesions are at risk for infection and/or inflammation or may be cosmetically deforming.6,7 Surgical resection, including removal of the wall of the cyst, is curative and reoccurrence is rare.5

| |
Figure 1. Bronchogenic cyst demonstrating a ciliated pseudostratified epithelial lining encircled by smooth muscle (H&E, original magnification ×200). | |

| |
| Figure 2. Epidermal inclusion cyst containing loose lamellar keratin and a lining that closely resembles the surface epidermis (H&E, original magnification ×40). |
|
Bronchogenic cysts demonstrate an epithelial lining that often is pseudostratified cuboidal or columnar as well as ciliated (Figure 1). Goblet cells are present in the lining in approximately 50% of cases. Smooth muscle may be seen circumferentially surrounding the cyst lining, and rare cases also contain cartilage.1 In contrast to dermoid cysts, other types of adnexal structures are not found within the lining. Bronchogenic cysts that arise in the skin are extremely rare.2 These cysts are thought to arise from respiratory epithelium that has been sequestered during embryologic formation of the tracheobronchial tree. They often are seen overlying the suprasternal notch and occasionally are found on the anterior aspect of the neck or chin. These cysts also are present at birth, similar to dermoid cysts.3
Epidermal inclusion cysts have a lining that histologically bears close resemblance to the surface epidermis. These cysts contain loose lamellar keratin, similar to a dermoid cyst. In contrast, the lining of an epidermal inclusion cyst will lack adnexal structures (Figure 2).1 Clinically, epidermal inclusion cysts often present as smooth, dome-shaped papules and nodules with a central punctum. They are classically found on the face, neck, and trunk. These cysts are thought to arise after a traumatic insult to the pilosebaceous unit.2
Hidrocystomas can be apocrine or eccrine.3 Eccrine hidrocystomas are unilocular cysts that are lined by 2 layers of flattened to cuboidal epithelial cells (Figure 3). The cysts are filled with clear fluid and often are found adjacent to normal eccrine glands.1 Apocrine hidrocystomas are unilocular or multilocular cysts that are lined by 1 to several layers of epithelial cells. The lining of an apocrine hidrocystoma will often exhibit luminal decapitation secretion.3 Apocrine and eccrine hidrocystomas are clinically identical and appear as blue translucent papules on the cheeks or eyelids of adults.1-3 They usually occur periorbitally but also can be seen on the trunk, popliteal fossa, external ears, or vulva. Eccrine hidrocystomas can wax and wane in accordance with the amount of sweat produced; thus, they often expand in size during the summer months.2
Steatocystomas, or simple sebaceous duct cysts, histologically demonstrate a characteristically wavy and eosinophilic cuticle resembling shark teeth (Figure 4) similar to the lining of the sebaceous duct where it enters the follicle.1 Sebaceous glands are an almost invariable feature, either present within the lining of the cyst (Figure 4) or in the adjacent tissue.2 In comparison, dermoid cysts may have a red wavy cuticle but also will usually have terminal hair follicles or eccrine or apocrine glands within the wall of the cyst. Steatocystomas typically are collapsed and empty or only contain sebaceous debris (Figure 4) rather than the lamellar keratin seen in dermoid and epidermoid inclusion cysts. Steatocystomas can occur as solitary (steatocystoma simplex) or multiple (steatocystoma multiplex) lesions.1,3 They are clinically comprised of small dome-shaped papules that often are translucent and yellow. These cysts are commonly found on the sternum of males and the axillae or groin of females.2

| 
| |
Figure 3. Eccrine hidrocystoma with clear contents and lined by 2 layers of cuboidal epithelial cells (H&E, original magnification ×100). | Figure 4. Steatocystoma with a red wavy cuticle, sparse sebaceous contents, and sebaceous glands within the lining (H&E, original magnification ×100). |
|
1. Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
2. Calonje JE, Brenn T, Lazar AJ, et al. McKee’s Pathology of the Skin. 4th ed. St Louis, MO: Elsevier/Saunders; 2012.
3. Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
4. Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
5. Sorenson EP, Powel JE, Rozzelle CJ, et al. Scalp dermoids: a review of their anatomy, diagnosis, and treatment. Childs Nerv Syst. 2013;29:375-380.
6. Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolarynol Head Neck Surg. 2005;132:938-942.
7. Abou-Rayyah Y, Rose GE, Konrad H, et al. Clinical, radiological and pathological examination of periocular dermoid cysts: evidence of inflammation from an early age. Eye (Lond). 2002;16:507-512.
1. Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
2. Calonje JE, Brenn T, Lazar AJ, et al. McKee’s Pathology of the Skin. 4th ed. St Louis, MO: Elsevier/Saunders; 2012.
3. Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012.
4. Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, et al. Dermoid cysts: a report of 75 pediatric patients. Pediatr Dermatol. 2013;30:706-711.
5. Sorenson EP, Powel JE, Rozzelle CJ, et al. Scalp dermoids: a review of their anatomy, diagnosis, and treatment. Childs Nerv Syst. 2013;29:375-380.
6. Pryor SG, Lewis JE, Weaver AL, et al. Pediatric dermoid cysts of the head and neck. Otolarynol Head Neck Surg. 2005;132:938-942.
7. Abou-Rayyah Y, Rose GE, Konrad H, et al. Clinical, radiological and pathological examination of periocular dermoid cysts: evidence of inflammation from an early age. Eye (Lond). 2002;16:507-512.
Brown Macule on the Waist
The best diagnosis is:
a. granular cell tumor
b. intradermal nevus
c. Langerhans cell disease
d. mastocytosis
e. multicentric reticulohistiocytosis
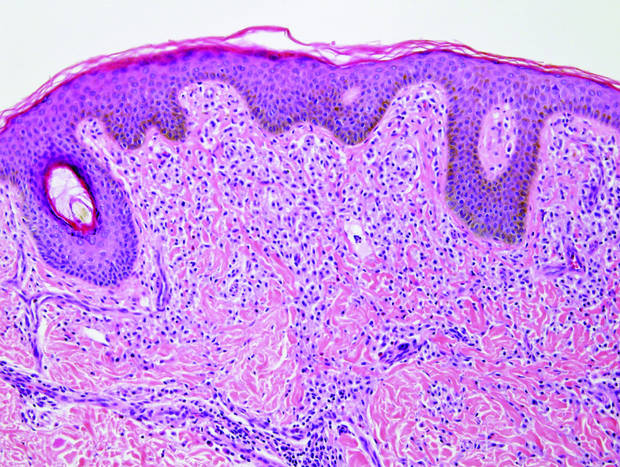
|
| Monomorphic cell infiltrate in the upper dermis (H&E, original magnification ×100). |
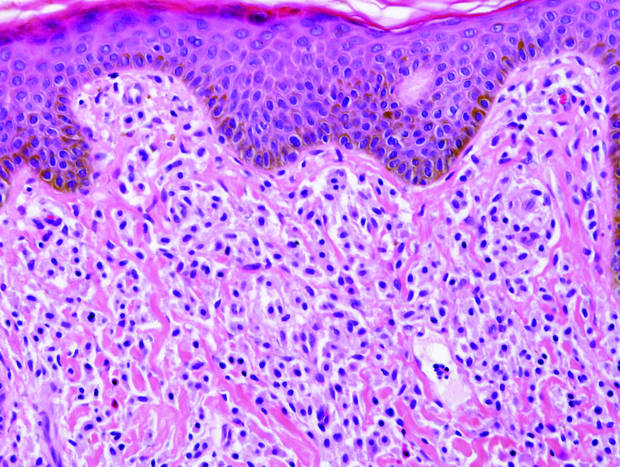
|
| A closer view reveals cuboidal or spindle cells with basal hyperpigmentation (H&E, original magnification ×200). |
Continue to the next page for the diagnosis >>
Mastocytosis
Mastocytosis is a clonal proliferation of mast cells in the skin and various systems of the body including the bone marrow, liver, lymph nodes, and gastrointestinal tract.1,2 Mast cell proliferation is closely associated with germline and acquired activating KIT mutations.3-5 Adult-onset mastocytosis is likely to involve several organs, whereas pediatric mastocytosis usually affects only the skin and is self-limiting. Patients with profound mast cell infiltration in the skin or other organs are likely to have attacks of flushing, palpitation, or diarrhea resulting from the degranulation of mast cells and release of histamine.6,7 In a majority of patients with advanced systemic mastocytosis, mast cells are positive for the Ki-1 antigen (CD30), whereas in most patients with indolent systemic mastocytosis, only a few mast cells are positive for CD30.8 Recently, CD30 was reported as a new drug target in patients with CD30+ advanced systemic mastocytosis.9 Because the skin frequently is involved and easily accessible in comparison with other organs, skin biopsy often is performed to establish a diagnosis of mastocytosis. Cutaneous mastocytosis comprises urticaria pigmentosa, solitary mastocytoma, diffuse cutaneous mastocytosis, and telangiectasia macularis eruptiva perstans; approximately 80% of all cases have urticaria pigmentosa.10-12 In cutaneous mastocytosis, skin biopsy typically shows monomorphous mast cell infiltrate mostly in the upper third of the dermis. The density of mast cells varies according to the clinical variant. For example, a lesion of telangiectasia macularis eruptiva perstans has only a perivascular mast cell infiltrate, whereas a solitary mastocytoma has sheets of mast cells in the dermis, sometimes extending into the subcutis. A skin biopsy of the brown macule on the waist showed a number of cuboidal or spindle mast cells in the upper dermis with occasional eosinophils. These mast cells are monomorphous, and no mitotic figures, necrotic cells, or atypical cells are seen. Mast cells have metachromatic granules in the cytoplasm, which can be seen with toluidine blue or Giemsa stain. CD117 (c-kit) also is positive. Mast cells in urticaria pigmentosa easily may be mistaken for nevus cells. Hyperpigmentation of the basal layer, a characteristic feature seen in urticaria pigmentosa, also may erroneously suggest a diagnosis of a melanocytic nevus.
Granular cell tumors predominantly affect the oral cavity, but the skin also can be involved. It comprises a fascicular infiltrate of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (Figure 1).13 Cell membranes are not always distinct. Although the nuclei usually are small and centrally located, irregular and plump nuclei with distinct nucleoli also may be seen. The overlying epidermis tends to be hyperplastic. Granular cell tumor is considered a group of lesions of varying histogenesis. Cases in which tumors originated from a neural crest–derived peripheral nerve–related cell as well as a Schwann cell have been reported.14,15 The origin of granular cell tumors is controversial.
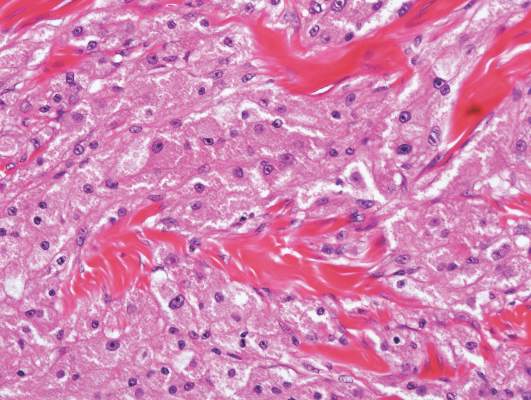
|
| Figure 1. Granular cell tumor showing fascicles of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (H&E, original magnification ×200). |

|
| Figure 2. Intradermal nevus showing nests with melanin in the uppermost area of the lesion and neurotized nevus cells in the lower part (H&E, original magnification ×100). Pseudovascular spaces are seen on the right side. |
Intradermal nevus usually has nests and cords of nevus cells in the upper dermis. The uppermost melanocytes often contain a moderate amount of melanin, whereas nevus cells in the mid and lower dermis usually do not contain melanin (Figure 2). Shrinkage during tissue processing maycause clefts between nevus cells, resulting in pseudovascular spaces.16 The deeper dermis may have a neuroid appearance with spindle-shaped cells and Meissner corpuscle–like structures.17
Although Langerhans cell disease was formerly known as Langerhans cell histiocytosis and subdivided into several clinical subtypes, including Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma, these clinical subtypes commonly overlapped. Langerhans cell disease is now used as a terminology that encompasses all subtypes.18,19 Langerhans cell disease is characterized by a proliferation of Langerhans cells with a variable mixture of other inflammatory cells. The constituent cells are large and ovoid with a distinct folded or lobulated, often kidney-shaped nucleus.20 Langerhans cells usually infiltrate the upper dermis and occasionally the epidermis (Figure 3). CD1a, HLA-DR, S-100 protein, and langerin are positive in Langerhans cells.21

|
| Figure 3. Langerhans cell disease showing an infiltrate of large and ovoid Langerhans cells with a distinct folded or lobulated, often kidney-shaped nucleus in the upper dermis and epidermis (H&E, original magnification ×200). |

|
| Figure 4. Multicentric reticulohistiocytosis showing a mixture of mononuclear and multinucleate histiocytes with abundant eosinophilic and finely granular cytoplasm (H&E, original magnification ×200). |
Multicentric reticulohistiocytosis is characterized by a combination of papulonodular cutaneous lesions and severe arthropathy.22 An irregular mixture of mononuclear and multinucleate histiocytes showing abundant eosinophilic and finely granular cytoplasm, often with a ground-glass appearance, is seen along with lymphocytic infiltration (Figure 4).23 A few giant cells may be seen in early lesions; older lesions more commonly have giant cells and fibrosis.
1. Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497-516.
2. Pardanani A. Systemic mastocytosis in adults: 2013 update on diagnosis, risk stratification, and management. Am J Hematol. 2013;88:612-624.
3. Orfao A, Garcia-Montero AC, Sanchez L, et al. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12-30.
4. Yanagihori H, Oyama N, Nakamura K, et al. c-KIT mutations in patients with childhood-onset mastocytosis and genotype-phenotype correlation. J Mol Diagn. 2005;7:252-257.
5. Bodemer C, Hermine O, Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804-815.
6. Kettelhut BV, Metcalfe DD. Pediatric mastocytosis. Ann Allergy. 1994;73:197-202; quiz 202-207.
7. Longley J, Duffy TP, Kohn S. The mast cell and mast cell disease. J Am Acad Dermatol. 1995;32:545-561; quiz 562-564.
8. Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24:585-595.
9. Blatt K, Cerny-Reiterer S, Schwaab J, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis [published online October 20, 2015]. Blood. 2015;126:2832-2841.
10. Kiszewski AE, Duran-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
11. Akoglu G, Erkin G, Cakir B, et al. Cutaneous mastocytosis: demographic aspects and clinical features of 55 patients. J Eur Acad Dermatol Venereol. 2006;20:969-973.
12. Sarkany RP, Monk BE, Handfield-Jones SE. Telangiectasia macularis eruptiva perstans: a case report and review of the literature. Clin Exp Dermatol. 1998;23:38-39.
13. Lack EE, Worsham GF, Callihan MD, et al. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301-316.
14. Buley ID, Gatter KC, Kelly PM, et al. Granular cell tumours revisited. an immunohistological and ultrastructural study. Histopathology. 1988;12:263-274.
15. Penneys NS, Adachi K, Ziegels-Weissman J, et al. Granular cell tumors of the skin contain myelin basic protein. Arch Pathol Lab Med. 1983;107:302-303.
16. Modlin RL, Gottlieb B, Taylor C, et al. Identification of cells lining pseudovascular spaces of benign pigmented nevi. Am J Dermatopathol. 1984;(suppl 6):25-29.
17. Fullen DR, Reed JA, Finnerty B, et al. S100A6 preferentially labels type C nevus cells and nevic corpuscles: additional support for Schwannian differentiation of intradermal nevi. J Cutan Pathol. 2001;28:393-399.
18. Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
19. Weedon D. Cutaneous infiltrates—non-lymphoid. In: Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Amsterdam, Netherlands: Elsevier; 2010:937-970.
20. Harrist TJ, Bhan AK, Murphy GF, et al. Histiocytosis-X: in situ characterization of cutaneous infiltrates with monoclonal antibodies. Am J Clin Pathol. 1983;79:294-300.
21. Lau SK, Chu PG, Weiss LM. Immunohistochemical expression of langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol. 2008;32:615-619.
22. Lesher JL Jr, Allen BS. Multicentric reticulohistiocytosis. J Am Acad Dermatol. 1984;11:713-723.
23. Heathcote JG, Guenther LC, Wallace AC. Multicentric reticulohistiocytosis: a report of a case and a review of the pathology. Pathology. 1985;17:601-608.
The best diagnosis is:
a. granular cell tumor
b. intradermal nevus
c. Langerhans cell disease
d. mastocytosis
e. multicentric reticulohistiocytosis

|
| Monomorphic cell infiltrate in the upper dermis (H&E, original magnification ×100). |

|
| A closer view reveals cuboidal or spindle cells with basal hyperpigmentation (H&E, original magnification ×200). |
Continue to the next page for the diagnosis >>
Mastocytosis
Mastocytosis is a clonal proliferation of mast cells in the skin and various systems of the body including the bone marrow, liver, lymph nodes, and gastrointestinal tract.1,2 Mast cell proliferation is closely associated with germline and acquired activating KIT mutations.3-5 Adult-onset mastocytosis is likely to involve several organs, whereas pediatric mastocytosis usually affects only the skin and is self-limiting. Patients with profound mast cell infiltration in the skin or other organs are likely to have attacks of flushing, palpitation, or diarrhea resulting from the degranulation of mast cells and release of histamine.6,7 In a majority of patients with advanced systemic mastocytosis, mast cells are positive for the Ki-1 antigen (CD30), whereas in most patients with indolent systemic mastocytosis, only a few mast cells are positive for CD30.8 Recently, CD30 was reported as a new drug target in patients with CD30+ advanced systemic mastocytosis.9 Because the skin frequently is involved and easily accessible in comparison with other organs, skin biopsy often is performed to establish a diagnosis of mastocytosis. Cutaneous mastocytosis comprises urticaria pigmentosa, solitary mastocytoma, diffuse cutaneous mastocytosis, and telangiectasia macularis eruptiva perstans; approximately 80% of all cases have urticaria pigmentosa.10-12 In cutaneous mastocytosis, skin biopsy typically shows monomorphous mast cell infiltrate mostly in the upper third of the dermis. The density of mast cells varies according to the clinical variant. For example, a lesion of telangiectasia macularis eruptiva perstans has only a perivascular mast cell infiltrate, whereas a solitary mastocytoma has sheets of mast cells in the dermis, sometimes extending into the subcutis. A skin biopsy of the brown macule on the waist showed a number of cuboidal or spindle mast cells in the upper dermis with occasional eosinophils. These mast cells are monomorphous, and no mitotic figures, necrotic cells, or atypical cells are seen. Mast cells have metachromatic granules in the cytoplasm, which can be seen with toluidine blue or Giemsa stain. CD117 (c-kit) also is positive. Mast cells in urticaria pigmentosa easily may be mistaken for nevus cells. Hyperpigmentation of the basal layer, a characteristic feature seen in urticaria pigmentosa, also may erroneously suggest a diagnosis of a melanocytic nevus.
Granular cell tumors predominantly affect the oral cavity, but the skin also can be involved. It comprises a fascicular infiltrate of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (Figure 1).13 Cell membranes are not always distinct. Although the nuclei usually are small and centrally located, irregular and plump nuclei with distinct nucleoli also may be seen. The overlying epidermis tends to be hyperplastic. Granular cell tumor is considered a group of lesions of varying histogenesis. Cases in which tumors originated from a neural crest–derived peripheral nerve–related cell as well as a Schwann cell have been reported.14,15 The origin of granular cell tumors is controversial.

|
| Figure 1. Granular cell tumor showing fascicles of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (H&E, original magnification ×200). |

|
| Figure 2. Intradermal nevus showing nests with melanin in the uppermost area of the lesion and neurotized nevus cells in the lower part (H&E, original magnification ×100). Pseudovascular spaces are seen on the right side. |
Intradermal nevus usually has nests and cords of nevus cells in the upper dermis. The uppermost melanocytes often contain a moderate amount of melanin, whereas nevus cells in the mid and lower dermis usually do not contain melanin (Figure 2). Shrinkage during tissue processing maycause clefts between nevus cells, resulting in pseudovascular spaces.16 The deeper dermis may have a neuroid appearance with spindle-shaped cells and Meissner corpuscle–like structures.17
Although Langerhans cell disease was formerly known as Langerhans cell histiocytosis and subdivided into several clinical subtypes, including Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma, these clinical subtypes commonly overlapped. Langerhans cell disease is now used as a terminology that encompasses all subtypes.18,19 Langerhans cell disease is characterized by a proliferation of Langerhans cells with a variable mixture of other inflammatory cells. The constituent cells are large and ovoid with a distinct folded or lobulated, often kidney-shaped nucleus.20 Langerhans cells usually infiltrate the upper dermis and occasionally the epidermis (Figure 3). CD1a, HLA-DR, S-100 protein, and langerin are positive in Langerhans cells.21

|
| Figure 3. Langerhans cell disease showing an infiltrate of large and ovoid Langerhans cells with a distinct folded or lobulated, often kidney-shaped nucleus in the upper dermis and epidermis (H&E, original magnification ×200). |

|
| Figure 4. Multicentric reticulohistiocytosis showing a mixture of mononuclear and multinucleate histiocytes with abundant eosinophilic and finely granular cytoplasm (H&E, original magnification ×200). |
Multicentric reticulohistiocytosis is characterized by a combination of papulonodular cutaneous lesions and severe arthropathy.22 An irregular mixture of mononuclear and multinucleate histiocytes showing abundant eosinophilic and finely granular cytoplasm, often with a ground-glass appearance, is seen along with lymphocytic infiltration (Figure 4).23 A few giant cells may be seen in early lesions; older lesions more commonly have giant cells and fibrosis.
The best diagnosis is:
a. granular cell tumor
b. intradermal nevus
c. Langerhans cell disease
d. mastocytosis
e. multicentric reticulohistiocytosis

|
| Monomorphic cell infiltrate in the upper dermis (H&E, original magnification ×100). |

|
| A closer view reveals cuboidal or spindle cells with basal hyperpigmentation (H&E, original magnification ×200). |
Continue to the next page for the diagnosis >>
Mastocytosis
Mastocytosis is a clonal proliferation of mast cells in the skin and various systems of the body including the bone marrow, liver, lymph nodes, and gastrointestinal tract.1,2 Mast cell proliferation is closely associated with germline and acquired activating KIT mutations.3-5 Adult-onset mastocytosis is likely to involve several organs, whereas pediatric mastocytosis usually affects only the skin and is self-limiting. Patients with profound mast cell infiltration in the skin or other organs are likely to have attacks of flushing, palpitation, or diarrhea resulting from the degranulation of mast cells and release of histamine.6,7 In a majority of patients with advanced systemic mastocytosis, mast cells are positive for the Ki-1 antigen (CD30), whereas in most patients with indolent systemic mastocytosis, only a few mast cells are positive for CD30.8 Recently, CD30 was reported as a new drug target in patients with CD30+ advanced systemic mastocytosis.9 Because the skin frequently is involved and easily accessible in comparison with other organs, skin biopsy often is performed to establish a diagnosis of mastocytosis. Cutaneous mastocytosis comprises urticaria pigmentosa, solitary mastocytoma, diffuse cutaneous mastocytosis, and telangiectasia macularis eruptiva perstans; approximately 80% of all cases have urticaria pigmentosa.10-12 In cutaneous mastocytosis, skin biopsy typically shows monomorphous mast cell infiltrate mostly in the upper third of the dermis. The density of mast cells varies according to the clinical variant. For example, a lesion of telangiectasia macularis eruptiva perstans has only a perivascular mast cell infiltrate, whereas a solitary mastocytoma has sheets of mast cells in the dermis, sometimes extending into the subcutis. A skin biopsy of the brown macule on the waist showed a number of cuboidal or spindle mast cells in the upper dermis with occasional eosinophils. These mast cells are monomorphous, and no mitotic figures, necrotic cells, or atypical cells are seen. Mast cells have metachromatic granules in the cytoplasm, which can be seen with toluidine blue or Giemsa stain. CD117 (c-kit) also is positive. Mast cells in urticaria pigmentosa easily may be mistaken for nevus cells. Hyperpigmentation of the basal layer, a characteristic feature seen in urticaria pigmentosa, also may erroneously suggest a diagnosis of a melanocytic nevus.
Granular cell tumors predominantly affect the oral cavity, but the skin also can be involved. It comprises a fascicular infiltrate of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (Figure 1).13 Cell membranes are not always distinct. Although the nuclei usually are small and centrally located, irregular and plump nuclei with distinct nucleoli also may be seen. The overlying epidermis tends to be hyperplastic. Granular cell tumor is considered a group of lesions of varying histogenesis. Cases in which tumors originated from a neural crest–derived peripheral nerve–related cell as well as a Schwann cell have been reported.14,15 The origin of granular cell tumors is controversial.

|
| Figure 1. Granular cell tumor showing fascicles of large and polygonal cells with characteristic eosinophilic granular cytoplasm in the dermis (H&E, original magnification ×200). |

|
| Figure 2. Intradermal nevus showing nests with melanin in the uppermost area of the lesion and neurotized nevus cells in the lower part (H&E, original magnification ×100). Pseudovascular spaces are seen on the right side. |
Intradermal nevus usually has nests and cords of nevus cells in the upper dermis. The uppermost melanocytes often contain a moderate amount of melanin, whereas nevus cells in the mid and lower dermis usually do not contain melanin (Figure 2). Shrinkage during tissue processing maycause clefts between nevus cells, resulting in pseudovascular spaces.16 The deeper dermis may have a neuroid appearance with spindle-shaped cells and Meissner corpuscle–like structures.17
Although Langerhans cell disease was formerly known as Langerhans cell histiocytosis and subdivided into several clinical subtypes, including Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma, these clinical subtypes commonly overlapped. Langerhans cell disease is now used as a terminology that encompasses all subtypes.18,19 Langerhans cell disease is characterized by a proliferation of Langerhans cells with a variable mixture of other inflammatory cells. The constituent cells are large and ovoid with a distinct folded or lobulated, often kidney-shaped nucleus.20 Langerhans cells usually infiltrate the upper dermis and occasionally the epidermis (Figure 3). CD1a, HLA-DR, S-100 protein, and langerin are positive in Langerhans cells.21

|
| Figure 3. Langerhans cell disease showing an infiltrate of large and ovoid Langerhans cells with a distinct folded or lobulated, often kidney-shaped nucleus in the upper dermis and epidermis (H&E, original magnification ×200). |

|
| Figure 4. Multicentric reticulohistiocytosis showing a mixture of mononuclear and multinucleate histiocytes with abundant eosinophilic and finely granular cytoplasm (H&E, original magnification ×200). |
Multicentric reticulohistiocytosis is characterized by a combination of papulonodular cutaneous lesions and severe arthropathy.22 An irregular mixture of mononuclear and multinucleate histiocytes showing abundant eosinophilic and finely granular cytoplasm, often with a ground-glass appearance, is seen along with lymphocytic infiltration (Figure 4).23 A few giant cells may be seen in early lesions; older lesions more commonly have giant cells and fibrosis.
1. Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497-516.
2. Pardanani A. Systemic mastocytosis in adults: 2013 update on diagnosis, risk stratification, and management. Am J Hematol. 2013;88:612-624.
3. Orfao A, Garcia-Montero AC, Sanchez L, et al. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12-30.
4. Yanagihori H, Oyama N, Nakamura K, et al. c-KIT mutations in patients with childhood-onset mastocytosis and genotype-phenotype correlation. J Mol Diagn. 2005;7:252-257.
5. Bodemer C, Hermine O, Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804-815.
6. Kettelhut BV, Metcalfe DD. Pediatric mastocytosis. Ann Allergy. 1994;73:197-202; quiz 202-207.
7. Longley J, Duffy TP, Kohn S. The mast cell and mast cell disease. J Am Acad Dermatol. 1995;32:545-561; quiz 562-564.
8. Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24:585-595.
9. Blatt K, Cerny-Reiterer S, Schwaab J, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis [published online October 20, 2015]. Blood. 2015;126:2832-2841.
10. Kiszewski AE, Duran-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
11. Akoglu G, Erkin G, Cakir B, et al. Cutaneous mastocytosis: demographic aspects and clinical features of 55 patients. J Eur Acad Dermatol Venereol. 2006;20:969-973.
12. Sarkany RP, Monk BE, Handfield-Jones SE. Telangiectasia macularis eruptiva perstans: a case report and review of the literature. Clin Exp Dermatol. 1998;23:38-39.
13. Lack EE, Worsham GF, Callihan MD, et al. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301-316.
14. Buley ID, Gatter KC, Kelly PM, et al. Granular cell tumours revisited. an immunohistological and ultrastructural study. Histopathology. 1988;12:263-274.
15. Penneys NS, Adachi K, Ziegels-Weissman J, et al. Granular cell tumors of the skin contain myelin basic protein. Arch Pathol Lab Med. 1983;107:302-303.
16. Modlin RL, Gottlieb B, Taylor C, et al. Identification of cells lining pseudovascular spaces of benign pigmented nevi. Am J Dermatopathol. 1984;(suppl 6):25-29.
17. Fullen DR, Reed JA, Finnerty B, et al. S100A6 preferentially labels type C nevus cells and nevic corpuscles: additional support for Schwannian differentiation of intradermal nevi. J Cutan Pathol. 2001;28:393-399.
18. Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
19. Weedon D. Cutaneous infiltrates—non-lymphoid. In: Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Amsterdam, Netherlands: Elsevier; 2010:937-970.
20. Harrist TJ, Bhan AK, Murphy GF, et al. Histiocytosis-X: in situ characterization of cutaneous infiltrates with monoclonal antibodies. Am J Clin Pathol. 1983;79:294-300.
21. Lau SK, Chu PG, Weiss LM. Immunohistochemical expression of langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol. 2008;32:615-619.
22. Lesher JL Jr, Allen BS. Multicentric reticulohistiocytosis. J Am Acad Dermatol. 1984;11:713-723.
23. Heathcote JG, Guenther LC, Wallace AC. Multicentric reticulohistiocytosis: a report of a case and a review of the pathology. Pathology. 1985;17:601-608.
1. Arock M, Valent P. Pathogenesis, classification and treatment of mastocytosis: state of the art in 2010 and future perspectives. Expert Rev Hematol. 2010;3:497-516.
2. Pardanani A. Systemic mastocytosis in adults: 2013 update on diagnosis, risk stratification, and management. Am J Hematol. 2013;88:612-624.
3. Orfao A, Garcia-Montero AC, Sanchez L, et al. Recent advances in the understanding of mastocytosis: the role of KIT mutations. Br J Haematol. 2007;138:12-30.
4. Yanagihori H, Oyama N, Nakamura K, et al. c-KIT mutations in patients with childhood-onset mastocytosis and genotype-phenotype correlation. J Mol Diagn. 2005;7:252-257.
5. Bodemer C, Hermine O, Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol. 2010;130:804-815.
6. Kettelhut BV, Metcalfe DD. Pediatric mastocytosis. Ann Allergy. 1994;73:197-202; quiz 202-207.
7. Longley J, Duffy TP, Kohn S. The mast cell and mast cell disease. J Am Acad Dermatol. 1995;32:545-561; quiz 562-564.
8. Sotlar K, Cerny-Reiterer S, Petat-Dutter K, et al. Aberrant expression of CD30 in neoplastic mast cells in high-grade mastocytosis. Mod Pathol. 2011;24:585-595.
9. Blatt K, Cerny-Reiterer S, Schwaab J, et al. Identification of the Ki-1 antigen (CD30) as a novel therapeutic target in systemic mastocytosis [published online October 20, 2015]. Blood. 2015;126:2832-2841.
10. Kiszewski AE, Duran-Mckinster C, Orozco-Covarrubias L, et al. Cutaneous mastocytosis in children: a clinical analysis of 71 cases. J Eur Acad Dermatol Venereol. 2004;18:285-290.
11. Akoglu G, Erkin G, Cakir B, et al. Cutaneous mastocytosis: demographic aspects and clinical features of 55 patients. J Eur Acad Dermatol Venereol. 2006;20:969-973.
12. Sarkany RP, Monk BE, Handfield-Jones SE. Telangiectasia macularis eruptiva perstans: a case report and review of the literature. Clin Exp Dermatol. 1998;23:38-39.
13. Lack EE, Worsham GF, Callihan MD, et al. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301-316.
14. Buley ID, Gatter KC, Kelly PM, et al. Granular cell tumours revisited. an immunohistological and ultrastructural study. Histopathology. 1988;12:263-274.
15. Penneys NS, Adachi K, Ziegels-Weissman J, et al. Granular cell tumors of the skin contain myelin basic protein. Arch Pathol Lab Med. 1983;107:302-303.
16. Modlin RL, Gottlieb B, Taylor C, et al. Identification of cells lining pseudovascular spaces of benign pigmented nevi. Am J Dermatopathol. 1984;(suppl 6):25-29.
17. Fullen DR, Reed JA, Finnerty B, et al. S100A6 preferentially labels type C nevus cells and nevic corpuscles: additional support for Schwannian differentiation of intradermal nevi. J Cutan Pathol. 2001;28:393-399.
18. Newman B, Hu W, Nigro K, et al. Aggressive histiocytic disorders that can involve the skin. J Am Acad Dermatol. 2007;56:302-316.
19. Weedon D. Cutaneous infiltrates—non-lymphoid. In: Weedon D, ed. Weedon’s Skin Pathology. 3rd ed. Amsterdam, Netherlands: Elsevier; 2010:937-970.
20. Harrist TJ, Bhan AK, Murphy GF, et al. Histiocytosis-X: in situ characterization of cutaneous infiltrates with monoclonal antibodies. Am J Clin Pathol. 1983;79:294-300.
21. Lau SK, Chu PG, Weiss LM. Immunohistochemical expression of langerin in Langerhans cell histiocytosis and non-Langerhans cell histiocytic disorders. Am J Surg Pathol. 2008;32:615-619.
22. Lesher JL Jr, Allen BS. Multicentric reticulohistiocytosis. J Am Acad Dermatol. 1984;11:713-723.
23. Heathcote JG, Guenther LC, Wallace AC. Multicentric reticulohistiocytosis: a report of a case and a review of the pathology. Pathology. 1985;17:601-608.
Lichen Striatus
Lichen striatus (LS) is a benign, uncommon, self-limited, linear inflammatory skin disorder that primarily affects children up to 15 years of age, most commonly around 2 to 3 years of age, and is seen more frequently in girls.1 It presents with a sudden eruption of asymptomatic small, flat-topped, lichenoid, scaly papules in a linear array on a single extremity. The lesions may be erythematous, flesh colored, or hypopigmented.1,2 Multiple lesions appear over days to weeks and coalesce into linear plaques in a continuous or interrupted pattern along the lines of Blaschko, indicating possible somatic mosaicism.1 Although typically asymptomatic, it may be pruritic. Most cases spontaneously resolve within 1 year.3 Recurrences are unusual. Digital involvement may result in onycholysis, longitudinal ridging, splitting, and nail loss.1 The underlying cause of LS may be an abnormal immunologic reaction or genetic predisposition that is precipitated by some trigger such as a viral infection, trauma, hypersensitivity reaction, vaccine, seasonal variation, medication, or pregnancy.1,2 An association with atopy has been described. Treatment is not necessary but options include topical steroids, topical retinoids, and topical calcineurin inhibitors.2
Histologically, findings in LS are somewhat variable but typically show a combination of spongiotic and lichenoid interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate (Figure 1). Epidermal changes include intercellular and intracellular edema, focal spongiosis, lymphocytic exocytosis, parakeratosis, patchy hyperkeratosis, and keratinocyte necrosis (Figure 2A).1,3 The epidermis is normal or slightly acanthotic, and dyskeratotic keratinocytes can be found in the granular and horny layers or at the dermoepidermal junction.2 The lymphohistiocytic infiltrate in the superficial and deep dermis surrounds vascular plexuses and cutaneous adnexa such as eccrine glands and hair follicles.1 Perivascular lymphoid aggregates and eccrine coil involvement are particularly distinctive of LS (Figure 2B).4 Pigment incontinence also may be seen.
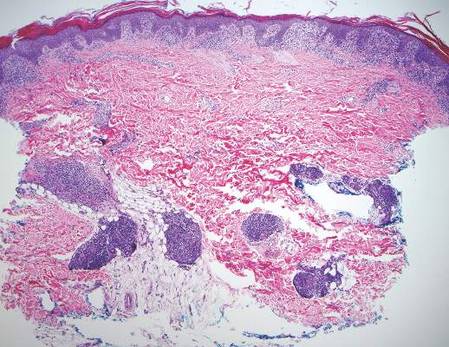
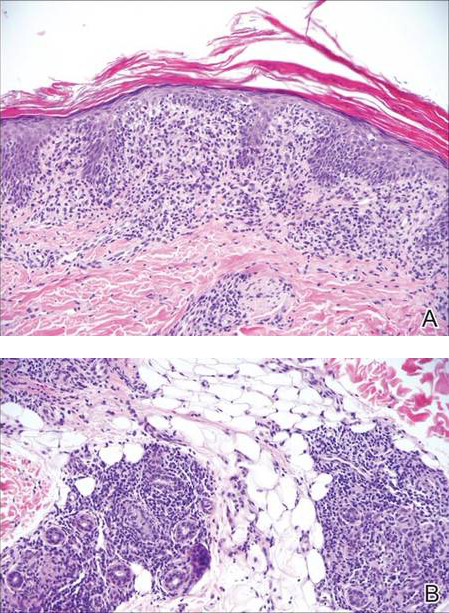
Another condition that distributes linearly along the lines of Blaschko is linear epidermolytic hyperkeratosis (EHK). Similar to LS, histology shows hyperkeratosis, focal parakeratosis, and acanthosis of the epidermis.5 However, EHK shows epidermolysis, acantholysis, and perinuclear vacuolization in spinous and granular layers (Figure 3).5 The lack of perivascular and periadnexal inflammation also can help differentiate EHK from LS.
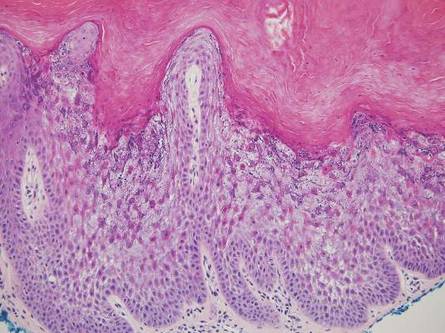
Linear lichen planus (LLP), similar to LS, histologically shows a lichenoid lymphocytic bandlike infiltrate obscuring the dermoepidermal junction, vacuolization of the basal cell layer, and pigment incontinence.1,2 Although LS and LLP can have histologic overlap, the absence of adnexal or perieccrine lymphocytic inflammation can help distinguish the two.3 The histopathologic changes of intercellular edema or mild spongiosis, exocytosis, and parakeratosis present in LS also are typically absent in LLP. Linear lichen planus characteristically consists of wedge-shaped hypergranulosis and irregular acanthosis with saw-toothed rete ridges (Figure 4).2 In addition, lobular eosinophilic deposits known as cytoid or Civatte bodies representing degenerated keratinocytes can be visualized at the dermoepidermal junction in LLP.2 Immunofluorescence will highlight Civatte bodies with IgM, IgG, and C3, also helping to differentiate these 2 conditions.1
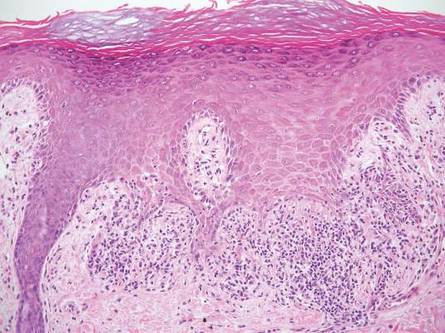
Linear porokeratosis can be mistaken for the linear lesion of LS. Both entities may reveal perivascular lymphocytes in the dermis, and porokeratosis can be lichenoid in the central portion of the lesion.6 However, porokeratosis is unique in that it contains a cornoid lamella, characterized by a thin column of tightly packed parakeratotic cells extending from an invagination of the epidermis through the adjacent stratum corneum (Figure 5).6 Beneath the cornoid lamella, the granular layer is either absent or markedly attenuated, and pyknotic keratinocytes with perinuclear edema are present in the spinous layer.6 The epidermis in the central portion of the porokeratotic lesion may be normal, hyperplastic, or atrophic with effacement of rete ridges.
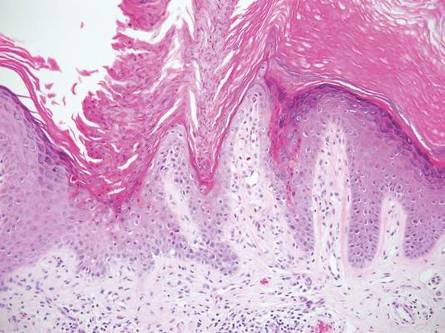
Similar to LS, linear psoriasis follows lines of Blaschko clinically. However, it is distinguished by its characteristic psoriatic epidermal changes as well as its lack of lichenoid or perieccrine inflammation.3 Typical findings in linear psoriasis include hyperkeratosis, confluent parakeratosis with entrapped neutrophilic microabscesses, acanthosis with regular elongation of rete ridges, intraepidermal neutrophils, thinned suprapapillary plates, dilated capillaries in the tips of the dermal papillae, and a chronic dermal inflammatory infiltrate (Figure 6).4
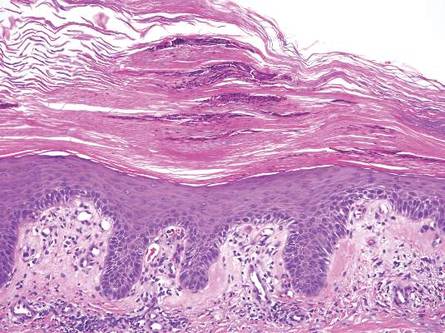
- Wang WL, Lazar A. Lichenoid and interface dermatitis. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin. 4th ed. London, England: Elsevier/Saunders; 2011:219-258.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:183-202.
- Zhang Y, McNutt NS. Lichen striatus. histological, immunohistochemical, and ultrastructural study of 37 cases. J Cutan Pathol. 2001;28:65-71.
- Johnson M, Walker D, Galloway W, et al. Interface dermatitis along Blaschko’s lines. J Cutan Pathol. 2014;41:950-954.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Requena L, Requena C, Cockerell C. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:1795-1815.
Lichen striatus (LS) is a benign, uncommon, self-limited, linear inflammatory skin disorder that primarily affects children up to 15 years of age, most commonly around 2 to 3 years of age, and is seen more frequently in girls.1 It presents with a sudden eruption of asymptomatic small, flat-topped, lichenoid, scaly papules in a linear array on a single extremity. The lesions may be erythematous, flesh colored, or hypopigmented.1,2 Multiple lesions appear over days to weeks and coalesce into linear plaques in a continuous or interrupted pattern along the lines of Blaschko, indicating possible somatic mosaicism.1 Although typically asymptomatic, it may be pruritic. Most cases spontaneously resolve within 1 year.3 Recurrences are unusual. Digital involvement may result in onycholysis, longitudinal ridging, splitting, and nail loss.1 The underlying cause of LS may be an abnormal immunologic reaction or genetic predisposition that is precipitated by some trigger such as a viral infection, trauma, hypersensitivity reaction, vaccine, seasonal variation, medication, or pregnancy.1,2 An association with atopy has been described. Treatment is not necessary but options include topical steroids, topical retinoids, and topical calcineurin inhibitors.2
Histologically, findings in LS are somewhat variable but typically show a combination of spongiotic and lichenoid interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate (Figure 1). Epidermal changes include intercellular and intracellular edema, focal spongiosis, lymphocytic exocytosis, parakeratosis, patchy hyperkeratosis, and keratinocyte necrosis (Figure 2A).1,3 The epidermis is normal or slightly acanthotic, and dyskeratotic keratinocytes can be found in the granular and horny layers or at the dermoepidermal junction.2 The lymphohistiocytic infiltrate in the superficial and deep dermis surrounds vascular plexuses and cutaneous adnexa such as eccrine glands and hair follicles.1 Perivascular lymphoid aggregates and eccrine coil involvement are particularly distinctive of LS (Figure 2B).4 Pigment incontinence also may be seen.


Another condition that distributes linearly along the lines of Blaschko is linear epidermolytic hyperkeratosis (EHK). Similar to LS, histology shows hyperkeratosis, focal parakeratosis, and acanthosis of the epidermis.5 However, EHK shows epidermolysis, acantholysis, and perinuclear vacuolization in spinous and granular layers (Figure 3).5 The lack of perivascular and periadnexal inflammation also can help differentiate EHK from LS.

Linear lichen planus (LLP), similar to LS, histologically shows a lichenoid lymphocytic bandlike infiltrate obscuring the dermoepidermal junction, vacuolization of the basal cell layer, and pigment incontinence.1,2 Although LS and LLP can have histologic overlap, the absence of adnexal or perieccrine lymphocytic inflammation can help distinguish the two.3 The histopathologic changes of intercellular edema or mild spongiosis, exocytosis, and parakeratosis present in LS also are typically absent in LLP. Linear lichen planus characteristically consists of wedge-shaped hypergranulosis and irregular acanthosis with saw-toothed rete ridges (Figure 4).2 In addition, lobular eosinophilic deposits known as cytoid or Civatte bodies representing degenerated keratinocytes can be visualized at the dermoepidermal junction in LLP.2 Immunofluorescence will highlight Civatte bodies with IgM, IgG, and C3, also helping to differentiate these 2 conditions.1

Linear porokeratosis can be mistaken for the linear lesion of LS. Both entities may reveal perivascular lymphocytes in the dermis, and porokeratosis can be lichenoid in the central portion of the lesion.6 However, porokeratosis is unique in that it contains a cornoid lamella, characterized by a thin column of tightly packed parakeratotic cells extending from an invagination of the epidermis through the adjacent stratum corneum (Figure 5).6 Beneath the cornoid lamella, the granular layer is either absent or markedly attenuated, and pyknotic keratinocytes with perinuclear edema are present in the spinous layer.6 The epidermis in the central portion of the porokeratotic lesion may be normal, hyperplastic, or atrophic with effacement of rete ridges.

Similar to LS, linear psoriasis follows lines of Blaschko clinically. However, it is distinguished by its characteristic psoriatic epidermal changes as well as its lack of lichenoid or perieccrine inflammation.3 Typical findings in linear psoriasis include hyperkeratosis, confluent parakeratosis with entrapped neutrophilic microabscesses, acanthosis with regular elongation of rete ridges, intraepidermal neutrophils, thinned suprapapillary plates, dilated capillaries in the tips of the dermal papillae, and a chronic dermal inflammatory infiltrate (Figure 6).4

Lichen striatus (LS) is a benign, uncommon, self-limited, linear inflammatory skin disorder that primarily affects children up to 15 years of age, most commonly around 2 to 3 years of age, and is seen more frequently in girls.1 It presents with a sudden eruption of asymptomatic small, flat-topped, lichenoid, scaly papules in a linear array on a single extremity. The lesions may be erythematous, flesh colored, or hypopigmented.1,2 Multiple lesions appear over days to weeks and coalesce into linear plaques in a continuous or interrupted pattern along the lines of Blaschko, indicating possible somatic mosaicism.1 Although typically asymptomatic, it may be pruritic. Most cases spontaneously resolve within 1 year.3 Recurrences are unusual. Digital involvement may result in onycholysis, longitudinal ridging, splitting, and nail loss.1 The underlying cause of LS may be an abnormal immunologic reaction or genetic predisposition that is precipitated by some trigger such as a viral infection, trauma, hypersensitivity reaction, vaccine, seasonal variation, medication, or pregnancy.1,2 An association with atopy has been described. Treatment is not necessary but options include topical steroids, topical retinoids, and topical calcineurin inhibitors.2
Histologically, findings in LS are somewhat variable but typically show a combination of spongiotic and lichenoid interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate (Figure 1). Epidermal changes include intercellular and intracellular edema, focal spongiosis, lymphocytic exocytosis, parakeratosis, patchy hyperkeratosis, and keratinocyte necrosis (Figure 2A).1,3 The epidermis is normal or slightly acanthotic, and dyskeratotic keratinocytes can be found in the granular and horny layers or at the dermoepidermal junction.2 The lymphohistiocytic infiltrate in the superficial and deep dermis surrounds vascular plexuses and cutaneous adnexa such as eccrine glands and hair follicles.1 Perivascular lymphoid aggregates and eccrine coil involvement are particularly distinctive of LS (Figure 2B).4 Pigment incontinence also may be seen.


Another condition that distributes linearly along the lines of Blaschko is linear epidermolytic hyperkeratosis (EHK). Similar to LS, histology shows hyperkeratosis, focal parakeratosis, and acanthosis of the epidermis.5 However, EHK shows epidermolysis, acantholysis, and perinuclear vacuolization in spinous and granular layers (Figure 3).5 The lack of perivascular and periadnexal inflammation also can help differentiate EHK from LS.

Linear lichen planus (LLP), similar to LS, histologically shows a lichenoid lymphocytic bandlike infiltrate obscuring the dermoepidermal junction, vacuolization of the basal cell layer, and pigment incontinence.1,2 Although LS and LLP can have histologic overlap, the absence of adnexal or perieccrine lymphocytic inflammation can help distinguish the two.3 The histopathologic changes of intercellular edema or mild spongiosis, exocytosis, and parakeratosis present in LS also are typically absent in LLP. Linear lichen planus characteristically consists of wedge-shaped hypergranulosis and irregular acanthosis with saw-toothed rete ridges (Figure 4).2 In addition, lobular eosinophilic deposits known as cytoid or Civatte bodies representing degenerated keratinocytes can be visualized at the dermoepidermal junction in LLP.2 Immunofluorescence will highlight Civatte bodies with IgM, IgG, and C3, also helping to differentiate these 2 conditions.1

Linear porokeratosis can be mistaken for the linear lesion of LS. Both entities may reveal perivascular lymphocytes in the dermis, and porokeratosis can be lichenoid in the central portion of the lesion.6 However, porokeratosis is unique in that it contains a cornoid lamella, characterized by a thin column of tightly packed parakeratotic cells extending from an invagination of the epidermis through the adjacent stratum corneum (Figure 5).6 Beneath the cornoid lamella, the granular layer is either absent or markedly attenuated, and pyknotic keratinocytes with perinuclear edema are present in the spinous layer.6 The epidermis in the central portion of the porokeratotic lesion may be normal, hyperplastic, or atrophic with effacement of rete ridges.

Similar to LS, linear psoriasis follows lines of Blaschko clinically. However, it is distinguished by its characteristic psoriatic epidermal changes as well as its lack of lichenoid or perieccrine inflammation.3 Typical findings in linear psoriasis include hyperkeratosis, confluent parakeratosis with entrapped neutrophilic microabscesses, acanthosis with regular elongation of rete ridges, intraepidermal neutrophils, thinned suprapapillary plates, dilated capillaries in the tips of the dermal papillae, and a chronic dermal inflammatory infiltrate (Figure 6).4

- Wang WL, Lazar A. Lichenoid and interface dermatitis. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin. 4th ed. London, England: Elsevier/Saunders; 2011:219-258.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:183-202.
- Zhang Y, McNutt NS. Lichen striatus. histological, immunohistochemical, and ultrastructural study of 37 cases. J Cutan Pathol. 2001;28:65-71.
- Johnson M, Walker D, Galloway W, et al. Interface dermatitis along Blaschko’s lines. J Cutan Pathol. 2014;41:950-954.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Requena L, Requena C, Cockerell C. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:1795-1815.
- Wang WL, Lazar A. Lichenoid and interface dermatitis. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin. 4th ed. London, England: Elsevier/Saunders; 2011:219-258.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:183-202.
- Zhang Y, McNutt NS. Lichen striatus. histological, immunohistochemical, and ultrastructural study of 37 cases. J Cutan Pathol. 2001;28:65-71.
- Johnson M, Walker D, Galloway W, et al. Interface dermatitis along Blaschko’s lines. J Cutan Pathol. 2014;41:950-954.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Requena L, Requena C, Cockerell C. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:1795-1815.
Secondary Syphilis
Syphilis often is referred to as the “great imitator” due to the protean presentations of secondary-stage disease, the most common of which are skin manifestations.1 Secondary syphilis typically begins 3 to 10 weeks after initial exposure due to systemic dissemination of Treponema pallidum, and although presentations can vary widely, the classic presentation includes nonspecific generalized symptoms (eg, fever, malaise, lymphadenopathy), variable skin findings (eg, nonpruritic papulosquamous eruption), and mucosal ulcerations or plaques.1 Early and accurate diagnosis of syphilis is critical to avoid the morbidity associated with advanced disease.
The classic histopathologic appearance of secondary syphilis is characterized by psoriasiform epidermal changes; a dermal inflammatory infiltrate of lymphocytes, histiocytes, and plasma cells in a lichenoid and/or superficial and deep perivascular distribution (Figure 1); and endothelial swelling of dermal blood vessels.1 The presence of plasma cells in the infiltrate (Figure 2) is particularly useful for differentiating secondary syphilis from other clinicopathological mimickers, but this finding is not always present. Silver-based histochemical stains (eg, Warthin-Starry silver stain) can be used to high-light T pallidum organisms; however, histochemical staining is plagued by low diagnostic sensitivity for identifying the causative organism, making immunohistochemical and/or serologic testing the preferred method for confirming the diagnosis.1

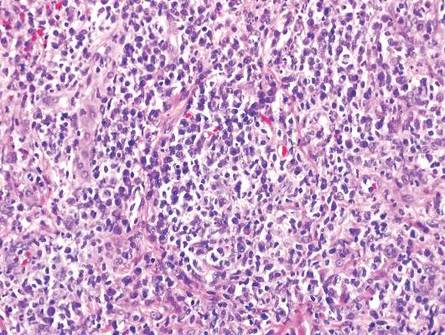
Arthropod assault is characterized by a superficial and deep perivascular lymphocytic inflammatory infiltrate with a variable number of polymorphonuclear cells.2 Overlying spongiosis or focal epidermal necrosis and increased eosinophils are typical of arthropod assault (Figure 3).2 The infiltrate seen following insect bites is classically described as wedge-shaped, although recent literature has disputed the sensitivity of this finding, identifying adnexal structure involvement as an alternative sensitive marker for identifying insect bites.2
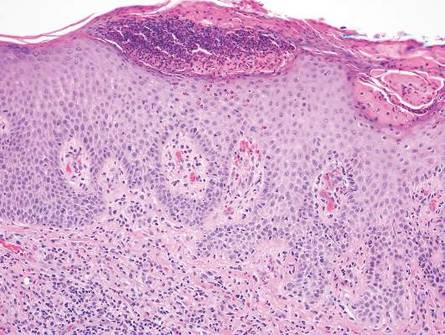
Chronic cutaneous lupus erythematosus demonstrates a spectrum of histopathologic changes depending on the age of the lesion biopsied; however, characteristic histopathologic features typically include variable epidermal atrophy or acanthosis with basal layer vacuolar degeneration, basement membrane thickening, follicular plugging, superficial and deep perivascular and periappendageal lymphocytic inflammation, and dermal mucin deposition (Figure 4).4
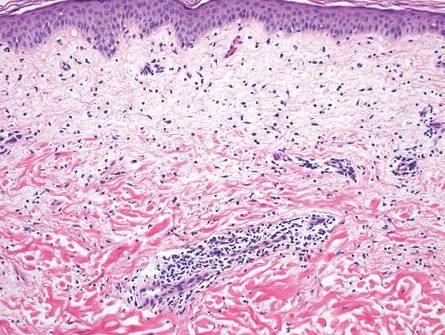
Fixed drug eruption histopathologically presents as an interface tissue reaction–associated single-cell necrosis to broader areas of epidermal necrosis, as well as superficial to mid-dermal lymphocytic infiltrate. Unlike secondary syphilis, a fixed drug eruption is characterized by prominent melanin pigment incontinence and eosinophils (Figure 5).5

Similar to secondary syphilis, pityriasis lichenoides et varioliformis acuta (PLEVA) demonstrates variable psoriasiform epidermal hyperplasia with a lichenoid and perivascular lymphocytic infiltrate. Other findings in PLEVA include parakeratosis, variable epidermal necrosis, and prominent exocytosis of lymphocytes. Unlike typical secondary syphilis, PLEVA often is associated with lymphocytic vasculitis, consisting of the invasion of vessel walls by lymphocytes with extravasation of erythrocytes and an absence of conspicuous plasma cells (Figure 6).6
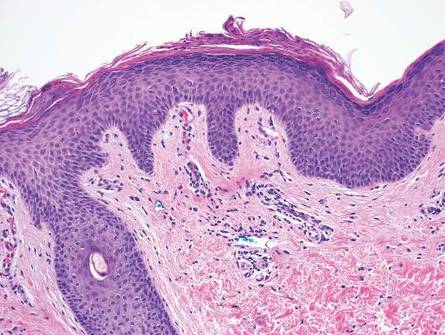
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;3:595-599.
- Miteva M, Elsner P, Ziemer M. A histopathologic study of arthropod bite reactions in 20 patients highlights relevant adnexal involvement. J Cutan Pathol. 2009;36:26-33.
- Winkelmann RK, Reizner GT. Diffuse dermal neutrophilia in urticarial. Human Pathol. 1988;19:389-393.
- Sepehr A, Wenson S, Tahan SR. Histopathologic manifestations of systemic diseases: the example of cutaneous lupus erythematosus. J Cutan Pathol. 2010;37 (suppl 1):112-124.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727.
- Fernandes NF, Rozdeba PJ, Schwartz RA, et al. Pityriasis lichenoides et varioliformis acuta: a disease spectrum. Int J Dermatol. 2010;49:257-261.
Syphilis often is referred to as the “great imitator” due to the protean presentations of secondary-stage disease, the most common of which are skin manifestations.1 Secondary syphilis typically begins 3 to 10 weeks after initial exposure due to systemic dissemination of Treponema pallidum, and although presentations can vary widely, the classic presentation includes nonspecific generalized symptoms (eg, fever, malaise, lymphadenopathy), variable skin findings (eg, nonpruritic papulosquamous eruption), and mucosal ulcerations or plaques.1 Early and accurate diagnosis of syphilis is critical to avoid the morbidity associated with advanced disease.
The classic histopathologic appearance of secondary syphilis is characterized by psoriasiform epidermal changes; a dermal inflammatory infiltrate of lymphocytes, histiocytes, and plasma cells in a lichenoid and/or superficial and deep perivascular distribution (Figure 1); and endothelial swelling of dermal blood vessels.1 The presence of plasma cells in the infiltrate (Figure 2) is particularly useful for differentiating secondary syphilis from other clinicopathological mimickers, but this finding is not always present. Silver-based histochemical stains (eg, Warthin-Starry silver stain) can be used to high-light T pallidum organisms; however, histochemical staining is plagued by low diagnostic sensitivity for identifying the causative organism, making immunohistochemical and/or serologic testing the preferred method for confirming the diagnosis.1


Arthropod assault is characterized by a superficial and deep perivascular lymphocytic inflammatory infiltrate with a variable number of polymorphonuclear cells.2 Overlying spongiosis or focal epidermal necrosis and increased eosinophils are typical of arthropod assault (Figure 3).2 The infiltrate seen following insect bites is classically described as wedge-shaped, although recent literature has disputed the sensitivity of this finding, identifying adnexal structure involvement as an alternative sensitive marker for identifying insect bites.2

Chronic cutaneous lupus erythematosus demonstrates a spectrum of histopathologic changes depending on the age of the lesion biopsied; however, characteristic histopathologic features typically include variable epidermal atrophy or acanthosis with basal layer vacuolar degeneration, basement membrane thickening, follicular plugging, superficial and deep perivascular and periappendageal lymphocytic inflammation, and dermal mucin deposition (Figure 4).4

Fixed drug eruption histopathologically presents as an interface tissue reaction–associated single-cell necrosis to broader areas of epidermal necrosis, as well as superficial to mid-dermal lymphocytic infiltrate. Unlike secondary syphilis, a fixed drug eruption is characterized by prominent melanin pigment incontinence and eosinophils (Figure 5).5

Similar to secondary syphilis, pityriasis lichenoides et varioliformis acuta (PLEVA) demonstrates variable psoriasiform epidermal hyperplasia with a lichenoid and perivascular lymphocytic infiltrate. Other findings in PLEVA include parakeratosis, variable epidermal necrosis, and prominent exocytosis of lymphocytes. Unlike typical secondary syphilis, PLEVA often is associated with lymphocytic vasculitis, consisting of the invasion of vessel walls by lymphocytes with extravasation of erythrocytes and an absence of conspicuous plasma cells (Figure 6).6

Syphilis often is referred to as the “great imitator” due to the protean presentations of secondary-stage disease, the most common of which are skin manifestations.1 Secondary syphilis typically begins 3 to 10 weeks after initial exposure due to systemic dissemination of Treponema pallidum, and although presentations can vary widely, the classic presentation includes nonspecific generalized symptoms (eg, fever, malaise, lymphadenopathy), variable skin findings (eg, nonpruritic papulosquamous eruption), and mucosal ulcerations or plaques.1 Early and accurate diagnosis of syphilis is critical to avoid the morbidity associated with advanced disease.
The classic histopathologic appearance of secondary syphilis is characterized by psoriasiform epidermal changes; a dermal inflammatory infiltrate of lymphocytes, histiocytes, and plasma cells in a lichenoid and/or superficial and deep perivascular distribution (Figure 1); and endothelial swelling of dermal blood vessels.1 The presence of plasma cells in the infiltrate (Figure 2) is particularly useful for differentiating secondary syphilis from other clinicopathological mimickers, but this finding is not always present. Silver-based histochemical stains (eg, Warthin-Starry silver stain) can be used to high-light T pallidum organisms; however, histochemical staining is plagued by low diagnostic sensitivity for identifying the causative organism, making immunohistochemical and/or serologic testing the preferred method for confirming the diagnosis.1


Arthropod assault is characterized by a superficial and deep perivascular lymphocytic inflammatory infiltrate with a variable number of polymorphonuclear cells.2 Overlying spongiosis or focal epidermal necrosis and increased eosinophils are typical of arthropod assault (Figure 3).2 The infiltrate seen following insect bites is classically described as wedge-shaped, although recent literature has disputed the sensitivity of this finding, identifying adnexal structure involvement as an alternative sensitive marker for identifying insect bites.2

Chronic cutaneous lupus erythematosus demonstrates a spectrum of histopathologic changes depending on the age of the lesion biopsied; however, characteristic histopathologic features typically include variable epidermal atrophy or acanthosis with basal layer vacuolar degeneration, basement membrane thickening, follicular plugging, superficial and deep perivascular and periappendageal lymphocytic inflammation, and dermal mucin deposition (Figure 4).4

Fixed drug eruption histopathologically presents as an interface tissue reaction–associated single-cell necrosis to broader areas of epidermal necrosis, as well as superficial to mid-dermal lymphocytic infiltrate. Unlike secondary syphilis, a fixed drug eruption is characterized by prominent melanin pigment incontinence and eosinophils (Figure 5).5

Similar to secondary syphilis, pityriasis lichenoides et varioliformis acuta (PLEVA) demonstrates variable psoriasiform epidermal hyperplasia with a lichenoid and perivascular lymphocytic infiltrate. Other findings in PLEVA include parakeratosis, variable epidermal necrosis, and prominent exocytosis of lymphocytes. Unlike typical secondary syphilis, PLEVA often is associated with lymphocytic vasculitis, consisting of the invasion of vessel walls by lymphocytes with extravasation of erythrocytes and an absence of conspicuous plasma cells (Figure 6).6

- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;3:595-599.
- Miteva M, Elsner P, Ziemer M. A histopathologic study of arthropod bite reactions in 20 patients highlights relevant adnexal involvement. J Cutan Pathol. 2009;36:26-33.
- Winkelmann RK, Reizner GT. Diffuse dermal neutrophilia in urticarial. Human Pathol. 1988;19:389-393.
- Sepehr A, Wenson S, Tahan SR. Histopathologic manifestations of systemic diseases: the example of cutaneous lupus erythematosus. J Cutan Pathol. 2010;37 (suppl 1):112-124.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727.
- Fernandes NF, Rozdeba PJ, Schwartz RA, et al. Pityriasis lichenoides et varioliformis acuta: a disease spectrum. Int J Dermatol. 2010;49:257-261.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;3:595-599.
- Miteva M, Elsner P, Ziemer M. A histopathologic study of arthropod bite reactions in 20 patients highlights relevant adnexal involvement. J Cutan Pathol. 2009;36:26-33.
- Winkelmann RK, Reizner GT. Diffuse dermal neutrophilia in urticarial. Human Pathol. 1988;19:389-393.
- Sepehr A, Wenson S, Tahan SR. Histopathologic manifestations of systemic diseases: the example of cutaneous lupus erythematosus. J Cutan Pathol. 2010;37 (suppl 1):112-124.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727.
- Fernandes NF, Rozdeba PJ, Schwartz RA, et al. Pityriasis lichenoides et varioliformis acuta: a disease spectrum. Int J Dermatol. 2010;49:257-261.
Cutaneous Leishmaniasis
Cutaneous leishmaniasis is a parasitic infection caused by intracellular organisms found in tropical climates. Old World leishmaniasis is endemic to Asia, Africa, and parts of Europe, while New World leishmaniasis is native to Central and South Americas.1 Depending upon a host’s immune status and the specific Leishmania species, clinical presentations vary in appearance and severity, ranging from self-limited, localized cutaneous disease to potentially fatal visceral and mucocutaneous involvement. Most cutaneous manifestations of leishmaniasis begin as distinct, painless papules that may progress to nodules or become ulcerated over time.1 Histologically, leishmaniasis is diagnosed by the identification of intracellular organisms that characteristically align along the peripheral rim inside the vacuole of a histiocyte.2 This unique finding is called the “marquee sign” due to its resemblance to light bulbs arranged around a dressing room mirror (Figure 1).2Leishmania amastigotes (also known as Leishman-Donovan bodies) have kinetoplasts that are helpful in diagnosis but also may be difficult to detect.2 Along with the Leishmania parasites, there typically is a mixed inflammatory infiltrate of plasma cells, lymphocytes, histiocytes, and neutrophils (Figure 2).1,2 There also may be varying degrees of pseudoepitheliomatous hyperplasia and overlying epidermal ulceration.1
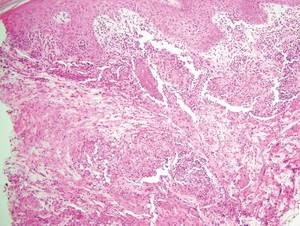

Cutaneous botryomycosis can present clinically as a number of various primary lesions, including papules, nodules, or ulcers that may resemble leishmaniasis.3 Botryomycosis represents a specific histologic collection of bacterial granules, most commonly caused by Staphylococcus aureus.3 The dermal granulomatous infiltrate seen in botryomycosis often is similar to that seen in chronic leishmaniasis; however, one histologic feature unique to botryomycosis is the presence of characteristic basophilic staphylococcal grains that are arranged in clusters resembling bunches of grapes (the term botryo means “bunch of grapes” in Greek).3 A thin, eosinophilic rim consisting of antibodies, bacterial debris, and complement proteins and glycoproteins may encircle the basophilic grains but does not need to be present for diagnosis (Figure 3).3
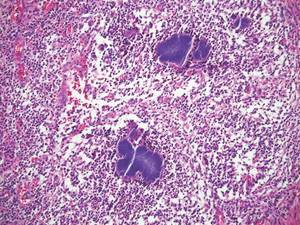
Lepromatous leprosy presents as a symmetric, widespread eruption of macules, patches, plaques, or papules that are most prominent in acral areas.4 Perivascular infiltration of lymphocytes and histiocytes is characteristic of lepromatous leprosy.2 Mycobacteria bacilli also are seen within histiocytic vacuoles, similarly to leishmaniasis; however, collections of these bacilli congregate within the center of a foamy histiocyte to form a distinctive histologic finding known as a globus. These individual histiocytes containing central globi are called Virchow cells (Figure 4).2 However, lepromatous leprosy can be distinguished from leishmaniasis histologically by carefully observing the intracellular location of the infectious organism. Mycobacteria bacilli are located in the center of a histiocyte vacuole whereas Leishmania parasites demonstrate a peripheral alignment along a histiocyte vacuole. If any uncertainty remains between a diagnosis of leishmaniasis and lepromatous leprosy, positive Fite staining for mycobacteria easily differentiates between the 2 conditions.2,4
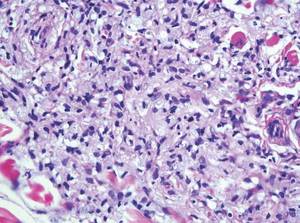
Cutaneous lobomycosis, a rare fungal infection transmitted by dolphins, manifests clinically as an asymptomatic nodule that is similar in appearance to a keloid. Histologic similarities to leishmaniasis include pseudoepitheliomatous hyperplasia and dermal granulomatous inflammation.4 The most distinguishing characteristic of lobomycosis is the presence of round, thick-walled, white organisms connected in a “string of beads” or chainlike configuration (Figure 5).2 Unlike leishmaniasis, lobomycosis fungal organisms would stain positive on periodic acid–Schiff staining.4

Cutaneous protothecosis is a rare clinical entity that presents as an isolated nodule or plaque or bursitis.4 It occurs following minor trauma and inoculation with Prototheca organisms, a genus of algae found in contaminated water.2,4 In its morula form, Prototheca adopts a characteristic arrangement within histiocytes that strikingly resembles a soccer ball (Figure 6).2 Conversely, nonmorulating forms of protothecosis can also be seen; these exhibit a central basophilic, dotlike structure within the histiocytes surrounded by a white halo.2 Definitive diagnosis of protothecosis can only be made upon successful culture of the algae.5
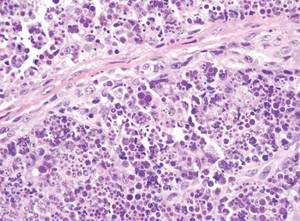
- Kevric I, Cappel MA, Keeling JH. New World and Old World leishmania infections: a practical review. Dermatol Clin. 2015;33:579-593.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 2nd ed. London, England: Elsevier Saunders; 2013.
- De Vries HJ, Van Noesel CJ, Hoekzema R, et al. Botryomycosis in an HIV-positive subject. J Eur Acad Dermatol Venereol. 2003;17:87-90.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Health Sciences UK; 2012.
- Hillesheim PB, Bahrami S. Cutaneous protothecosis. Arch Pathol Lab Med. 2011;135:941-944.
Cutaneous leishmaniasis is a parasitic infection caused by intracellular organisms found in tropical climates. Old World leishmaniasis is endemic to Asia, Africa, and parts of Europe, while New World leishmaniasis is native to Central and South Americas.1 Depending upon a host’s immune status and the specific Leishmania species, clinical presentations vary in appearance and severity, ranging from self-limited, localized cutaneous disease to potentially fatal visceral and mucocutaneous involvement. Most cutaneous manifestations of leishmaniasis begin as distinct, painless papules that may progress to nodules or become ulcerated over time.1 Histologically, leishmaniasis is diagnosed by the identification of intracellular organisms that characteristically align along the peripheral rim inside the vacuole of a histiocyte.2 This unique finding is called the “marquee sign” due to its resemblance to light bulbs arranged around a dressing room mirror (Figure 1).2Leishmania amastigotes (also known as Leishman-Donovan bodies) have kinetoplasts that are helpful in diagnosis but also may be difficult to detect.2 Along with the Leishmania parasites, there typically is a mixed inflammatory infiltrate of plasma cells, lymphocytes, histiocytes, and neutrophils (Figure 2).1,2 There also may be varying degrees of pseudoepitheliomatous hyperplasia and overlying epidermal ulceration.1


Cutaneous botryomycosis can present clinically as a number of various primary lesions, including papules, nodules, or ulcers that may resemble leishmaniasis.3 Botryomycosis represents a specific histologic collection of bacterial granules, most commonly caused by Staphylococcus aureus.3 The dermal granulomatous infiltrate seen in botryomycosis often is similar to that seen in chronic leishmaniasis; however, one histologic feature unique to botryomycosis is the presence of characteristic basophilic staphylococcal grains that are arranged in clusters resembling bunches of grapes (the term botryo means “bunch of grapes” in Greek).3 A thin, eosinophilic rim consisting of antibodies, bacterial debris, and complement proteins and glycoproteins may encircle the basophilic grains but does not need to be present for diagnosis (Figure 3).3

Lepromatous leprosy presents as a symmetric, widespread eruption of macules, patches, plaques, or papules that are most prominent in acral areas.4 Perivascular infiltration of lymphocytes and histiocytes is characteristic of lepromatous leprosy.2 Mycobacteria bacilli also are seen within histiocytic vacuoles, similarly to leishmaniasis; however, collections of these bacilli congregate within the center of a foamy histiocyte to form a distinctive histologic finding known as a globus. These individual histiocytes containing central globi are called Virchow cells (Figure 4).2 However, lepromatous leprosy can be distinguished from leishmaniasis histologically by carefully observing the intracellular location of the infectious organism. Mycobacteria bacilli are located in the center of a histiocyte vacuole whereas Leishmania parasites demonstrate a peripheral alignment along a histiocyte vacuole. If any uncertainty remains between a diagnosis of leishmaniasis and lepromatous leprosy, positive Fite staining for mycobacteria easily differentiates between the 2 conditions.2,4

Cutaneous lobomycosis, a rare fungal infection transmitted by dolphins, manifests clinically as an asymptomatic nodule that is similar in appearance to a keloid. Histologic similarities to leishmaniasis include pseudoepitheliomatous hyperplasia and dermal granulomatous inflammation.4 The most distinguishing characteristic of lobomycosis is the presence of round, thick-walled, white organisms connected in a “string of beads” or chainlike configuration (Figure 5).2 Unlike leishmaniasis, lobomycosis fungal organisms would stain positive on periodic acid–Schiff staining.4

Cutaneous protothecosis is a rare clinical entity that presents as an isolated nodule or plaque or bursitis.4 It occurs following minor trauma and inoculation with Prototheca organisms, a genus of algae found in contaminated water.2,4 In its morula form, Prototheca adopts a characteristic arrangement within histiocytes that strikingly resembles a soccer ball (Figure 6).2 Conversely, nonmorulating forms of protothecosis can also be seen; these exhibit a central basophilic, dotlike structure within the histiocytes surrounded by a white halo.2 Definitive diagnosis of protothecosis can only be made upon successful culture of the algae.5

Cutaneous leishmaniasis is a parasitic infection caused by intracellular organisms found in tropical climates. Old World leishmaniasis is endemic to Asia, Africa, and parts of Europe, while New World leishmaniasis is native to Central and South Americas.1 Depending upon a host’s immune status and the specific Leishmania species, clinical presentations vary in appearance and severity, ranging from self-limited, localized cutaneous disease to potentially fatal visceral and mucocutaneous involvement. Most cutaneous manifestations of leishmaniasis begin as distinct, painless papules that may progress to nodules or become ulcerated over time.1 Histologically, leishmaniasis is diagnosed by the identification of intracellular organisms that characteristically align along the peripheral rim inside the vacuole of a histiocyte.2 This unique finding is called the “marquee sign” due to its resemblance to light bulbs arranged around a dressing room mirror (Figure 1).2Leishmania amastigotes (also known as Leishman-Donovan bodies) have kinetoplasts that are helpful in diagnosis but also may be difficult to detect.2 Along with the Leishmania parasites, there typically is a mixed inflammatory infiltrate of plasma cells, lymphocytes, histiocytes, and neutrophils (Figure 2).1,2 There also may be varying degrees of pseudoepitheliomatous hyperplasia and overlying epidermal ulceration.1


Cutaneous botryomycosis can present clinically as a number of various primary lesions, including papules, nodules, or ulcers that may resemble leishmaniasis.3 Botryomycosis represents a specific histologic collection of bacterial granules, most commonly caused by Staphylococcus aureus.3 The dermal granulomatous infiltrate seen in botryomycosis often is similar to that seen in chronic leishmaniasis; however, one histologic feature unique to botryomycosis is the presence of characteristic basophilic staphylococcal grains that are arranged in clusters resembling bunches of grapes (the term botryo means “bunch of grapes” in Greek).3 A thin, eosinophilic rim consisting of antibodies, bacterial debris, and complement proteins and glycoproteins may encircle the basophilic grains but does not need to be present for diagnosis (Figure 3).3

Lepromatous leprosy presents as a symmetric, widespread eruption of macules, patches, plaques, or papules that are most prominent in acral areas.4 Perivascular infiltration of lymphocytes and histiocytes is characteristic of lepromatous leprosy.2 Mycobacteria bacilli also are seen within histiocytic vacuoles, similarly to leishmaniasis; however, collections of these bacilli congregate within the center of a foamy histiocyte to form a distinctive histologic finding known as a globus. These individual histiocytes containing central globi are called Virchow cells (Figure 4).2 However, lepromatous leprosy can be distinguished from leishmaniasis histologically by carefully observing the intracellular location of the infectious organism. Mycobacteria bacilli are located in the center of a histiocyte vacuole whereas Leishmania parasites demonstrate a peripheral alignment along a histiocyte vacuole. If any uncertainty remains between a diagnosis of leishmaniasis and lepromatous leprosy, positive Fite staining for mycobacteria easily differentiates between the 2 conditions.2,4

Cutaneous lobomycosis, a rare fungal infection transmitted by dolphins, manifests clinically as an asymptomatic nodule that is similar in appearance to a keloid. Histologic similarities to leishmaniasis include pseudoepitheliomatous hyperplasia and dermal granulomatous inflammation.4 The most distinguishing characteristic of lobomycosis is the presence of round, thick-walled, white organisms connected in a “string of beads” or chainlike configuration (Figure 5).2 Unlike leishmaniasis, lobomycosis fungal organisms would stain positive on periodic acid–Schiff staining.4

Cutaneous protothecosis is a rare clinical entity that presents as an isolated nodule or plaque or bursitis.4 It occurs following minor trauma and inoculation with Prototheca organisms, a genus of algae found in contaminated water.2,4 In its morula form, Prototheca adopts a characteristic arrangement within histiocytes that strikingly resembles a soccer ball (Figure 6).2 Conversely, nonmorulating forms of protothecosis can also be seen; these exhibit a central basophilic, dotlike structure within the histiocytes surrounded by a white halo.2 Definitive diagnosis of protothecosis can only be made upon successful culture of the algae.5

- Kevric I, Cappel MA, Keeling JH. New World and Old World leishmania infections: a practical review. Dermatol Clin. 2015;33:579-593.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 2nd ed. London, England: Elsevier Saunders; 2013.
- De Vries HJ, Van Noesel CJ, Hoekzema R, et al. Botryomycosis in an HIV-positive subject. J Eur Acad Dermatol Venereol. 2003;17:87-90.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Health Sciences UK; 2012.
- Hillesheim PB, Bahrami S. Cutaneous protothecosis. Arch Pathol Lab Med. 2011;135:941-944.
- Kevric I, Cappel MA, Keeling JH. New World and Old World leishmania infections: a practical review. Dermatol Clin. 2015;33:579-593.
- Elston DM, Ferringer T, Ko CJ, et al. Dermatopathology. 2nd ed. London, England: Elsevier Saunders; 2013.
- De Vries HJ, Van Noesel CJ, Hoekzema R, et al. Botryomycosis in an HIV-positive subject. J Eur Acad Dermatol Venereol. 2003;17:87-90.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier Health Sciences UK; 2012.
- Hillesheim PB, Bahrami S. Cutaneous protothecosis. Arch Pathol Lab Med. 2011;135:941-944.
Desmoplastic Melanoma
Desmoplastic melanoma, an uncommon variant of melanoma, poses a diagnostic challenge to the clinician because the tumors frequently appear as nonspecific flesh-colored or amelanotic plaques or nodules. They are more common in men than in women and are frequently found on the head and neck.1,2 Their innocuous appearance may lead to a delay in diagnosis and may explain why desmoplastic melanomas often are deeply infiltrative at the time of biopsy. Desmoplastic melanoma arises de novo in approximately one-third of cases.1 In the remainder of cases, it is seen in conjunction with overlying melanoma in situ, most commonly lentigo maligna melanoma.1 Histologically, desmoplastic melanomas are characterized by malignant spindle cells within a densely fibrotic stroma (Figure 1). Adjacent lymphoid aggregates and perineural involvement are common features,2 while pigment and atypical mitoses can be infrequent. Desmoplastic melanoma can be classified as mixed or pure based on the degree of desmoplasia and cellularity. Within mixed desmoplastic melanomas, there are areas that have histologic features of conventional melanomas while others demonstrate more typical desmoplastic characteristics. Pure desmoplastic melanoma has a higher degree of desmoplasia and fewer tumor cells than the mixed type.1 The pure subtype tends to be less aggressive and is less likely to metastasize to the lymph nodes.1 In the absence of an in situ component (Figure 2), desmoplastic melanoma may be indistinguishable from other spindle cell tumors on routine hematoxylin and eosin staining; thus, immunohistochemical staining generally is required. The most reliable stains in confirming a diagnosis of desmoplastic melanoma are S100 and SOX10 (SRY-related HMG-box 10)(Figure 3)(eTable).3
Atypical fibroxathoma typically presents as a nodule in the head and neck region or other sun-exposed areas in elderly individuals and is more commonly seen in men than in women.4 Histologically, atypical fibroxanthomas are composed of pleomorphic spindle, epithelioid, and multinucleated giant cells with numerous and atypical mitoses (Figure 4).5 Atypical fibroxanthoma is considered a diagnosis of exclusion; therefore, other dermal spindle cell tumors need to be ruled out before diagnosis can be made. Atypical fibroxanthomas generally stain negative for cytokeratin, S100, SOX10, and desmin, but in some cases there is positive focal staining for smooth muscle actin.4 Multiple immunohistochemical markers, including CD10, have shown reactivity in atypical fibroxanthomas,4 but none of these markers has a high specificity for this tumor; thus, it remains a diagnosis of exclusion.
Cutaneous angiosarcomas are aggressive tumors associated with a high mortality rate despite appropriate treatment with surgical resection and postoperative radiation treatment. They typically present as ecchymotic macules or nodules on the face or scalp of elderly patients.6,7 Ionizing radiation and chronic lymphedema are risk factors for cutaneous angiosarcoma.6 Histologically, well-differentiated cutaneous angiosarcomas are composed of irregular, anastomosing vascular channels that dissect through the dermis (Figure 5).6,7 Less well-differentiated tumors may contain spindle cells and lack obvious vascular structures; thus immunohistochemistry is essential for making the correct diagnosis in these cases. Cutaneous angiosarcomas typically stain positive for ERG (ETS-related gene) protein, CD31, CD34, and factor VIII.6,8 Unfortunately these tumors may also occasionally stain with cytokeratin, which may lead to the erroneous diagnosis of a carcinoma.6
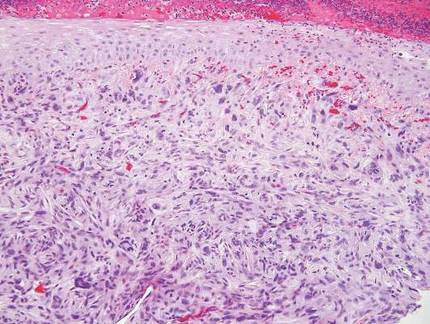
| 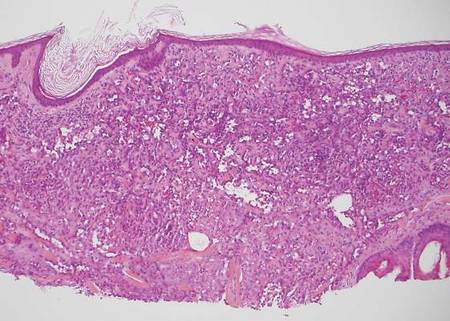
| |
| Figure 4. Pleomorphic spindle, epithelioid, and multinucleate giant cells with atypical mitoses filling the dermis in atypical fibroxanthoma (H&E, original magnification ×200). | Figure 5. Anastamosing vascular channels dissecting through collagen bundles and consuming the epidermis in cutaneous angiosarcoma (H&E, original magnification ×100). |
Cutaneous leiomyosarcoma is a smooth muscle neoplasm that arises from arrector pili muscles, genital smooth muscles, or vascular smooth muscles. It typically presents as a single plaque or nodule on the arms and legs of individuals older than 50 years of age.9 Cutaneous leiomyosarcomas can be classified as either dermal, in which at least 90% of the tumor is confined to the dermis, or subcutaneous; this distinction is important because the latter type has a higher rate of metastasis and a poorer prognosis.9 Because of this tumor’s smooth muscle derivation, well-differentiated tumors may retain features of typical smooth muscle cells, including cigar-shaped nuclei with adjacent glycogen vacuoles (Figure 6). If fascicle formation is observed, this may be an additional clue to the diagnosis. In poorly differentiated tumors, immunohistochemistry is invaluable. Leiomyosarcoma often stains positive for smooth muscle actin, muscle specific actin, h-caldesmon, desmin, and calponin.9-11
Spindle cell squamous cell carcinomas often present as ulcerated nodules on sun-exposed skin or on sites of prior ionizing radiation.2,12 Like desmoplastic melanoma, spindle cell squamous cell carcinomas are characterized by spindle cells in the dermis. Helpful diagnostic clues may include evidence of squamous differentiation, including keratin pearls or overlying actinic keratosis (Figure 7). However, actinic keratosis is common on sun-damaged skin and cannot be used to definitively confirm this diagnosis. There also may be areas of the tumor with more typical epithelioid cells that are easily identified as squamous cell carcinoma.2 Spindle cell squamous cell carcinoma stains positive for high–molecular weight cytokeratin antibodies and p63,2 which can help to differentiate it from the other spindle cell tumors in the differential.
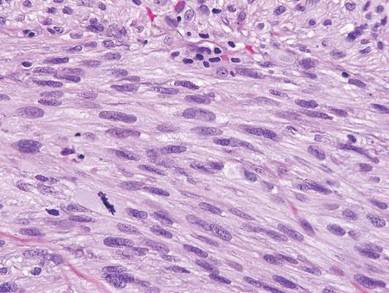
| 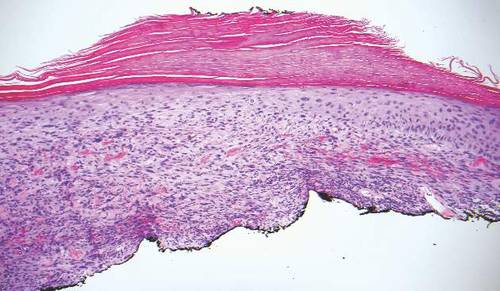
| |
| Figure 6. Spindle cells of leiomyosarcoma with cigar-shaped nuclei and adjacent glycogen vacuoles (H&E, original magnification ×600). | Figure 7. Spindle cell squamous cell carcinoma with overlying epidermal atypia that blends with the underlying dermal spindle cells (H&E, original magnification ×100). |
1. Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
2. Calonje JE, Brenn T, Lazar AJ, et al. McKee’s Pathology of the Skin. 4th ed. St Louis, MO: Elsevier Saunders; 2012.
3. Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
4. Luzar B, Calonje E. Morphological and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37:301-309.
5. Iorizzo LJ III, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
6. Luca DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
7. Mendenhall WM, Mendenhall CM, Werning JW, et al. Cutaneous angiosarcoma. Am J Oncol. 2006;29:524-528.
8. Thum C, Husain EA, Mulholland K, et al. Atypical fibroxanthoma with pseudoangiomatous features: a histological and immunohistochemical mimic of cutaneous angiosarcoma. Ann Diagn Pathol. 2013;17:502-507.
9. Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
10. Hall BJ, Grossmann AH, Webber NP, et al. Atypical intradermal smooth muscle neoplasms (formerly cutaneous leiomyosarcomas): case series, immunohistochemical profile and review of the literature. Appl Immunohistochem Mol Morphol. 2013;21:132-138.
11. Perez-Montiel MD, Plaza JA, Dominguez-Malagon H, et al. Differential expression of smooth muscle myosin, smooth muscle actin, h-caldesmon, and calponin in the diagnosis of myofibroblastic and smooth muscle lesions of skin and soft tissue. Am J Dermatopathol. 2006;28:105-111.
12. Cassarino DS, DeRienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. part one. J Cutan Pathol. 2006;33:191-205.
Desmoplastic melanoma, an uncommon variant of melanoma, poses a diagnostic challenge to the clinician because the tumors frequently appear as nonspecific flesh-colored or amelanotic plaques or nodules. They are more common in men than in women and are frequently found on the head and neck.1,2 Their innocuous appearance may lead to a delay in diagnosis and may explain why desmoplastic melanomas often are deeply infiltrative at the time of biopsy. Desmoplastic melanoma arises de novo in approximately one-third of cases.1 In the remainder of cases, it is seen in conjunction with overlying melanoma in situ, most commonly lentigo maligna melanoma.1 Histologically, desmoplastic melanomas are characterized by malignant spindle cells within a densely fibrotic stroma (Figure 1). Adjacent lymphoid aggregates and perineural involvement are common features,2 while pigment and atypical mitoses can be infrequent. Desmoplastic melanoma can be classified as mixed or pure based on the degree of desmoplasia and cellularity. Within mixed desmoplastic melanomas, there are areas that have histologic features of conventional melanomas while others demonstrate more typical desmoplastic characteristics. Pure desmoplastic melanoma has a higher degree of desmoplasia and fewer tumor cells than the mixed type.1 The pure subtype tends to be less aggressive and is less likely to metastasize to the lymph nodes.1 In the absence of an in situ component (Figure 2), desmoplastic melanoma may be indistinguishable from other spindle cell tumors on routine hematoxylin and eosin staining; thus, immunohistochemical staining generally is required. The most reliable stains in confirming a diagnosis of desmoplastic melanoma are S100 and SOX10 (SRY-related HMG-box 10)(Figure 3)(eTable).3
Atypical fibroxathoma typically presents as a nodule in the head and neck region or other sun-exposed areas in elderly individuals and is more commonly seen in men than in women.4 Histologically, atypical fibroxanthomas are composed of pleomorphic spindle, epithelioid, and multinucleated giant cells with numerous and atypical mitoses (Figure 4).5 Atypical fibroxanthoma is considered a diagnosis of exclusion; therefore, other dermal spindle cell tumors need to be ruled out before diagnosis can be made. Atypical fibroxanthomas generally stain negative for cytokeratin, S100, SOX10, and desmin, but in some cases there is positive focal staining for smooth muscle actin.4 Multiple immunohistochemical markers, including CD10, have shown reactivity in atypical fibroxanthomas,4 but none of these markers has a high specificity for this tumor; thus, it remains a diagnosis of exclusion.
Cutaneous angiosarcomas are aggressive tumors associated with a high mortality rate despite appropriate treatment with surgical resection and postoperative radiation treatment. They typically present as ecchymotic macules or nodules on the face or scalp of elderly patients.6,7 Ionizing radiation and chronic lymphedema are risk factors for cutaneous angiosarcoma.6 Histologically, well-differentiated cutaneous angiosarcomas are composed of irregular, anastomosing vascular channels that dissect through the dermis (Figure 5).6,7 Less well-differentiated tumors may contain spindle cells and lack obvious vascular structures; thus immunohistochemistry is essential for making the correct diagnosis in these cases. Cutaneous angiosarcomas typically stain positive for ERG (ETS-related gene) protein, CD31, CD34, and factor VIII.6,8 Unfortunately these tumors may also occasionally stain with cytokeratin, which may lead to the erroneous diagnosis of a carcinoma.6

| 
| |
| Figure 4. Pleomorphic spindle, epithelioid, and multinucleate giant cells with atypical mitoses filling the dermis in atypical fibroxanthoma (H&E, original magnification ×200). | Figure 5. Anastamosing vascular channels dissecting through collagen bundles and consuming the epidermis in cutaneous angiosarcoma (H&E, original magnification ×100). |
Cutaneous leiomyosarcoma is a smooth muscle neoplasm that arises from arrector pili muscles, genital smooth muscles, or vascular smooth muscles. It typically presents as a single plaque or nodule on the arms and legs of individuals older than 50 years of age.9 Cutaneous leiomyosarcomas can be classified as either dermal, in which at least 90% of the tumor is confined to the dermis, or subcutaneous; this distinction is important because the latter type has a higher rate of metastasis and a poorer prognosis.9 Because of this tumor’s smooth muscle derivation, well-differentiated tumors may retain features of typical smooth muscle cells, including cigar-shaped nuclei with adjacent glycogen vacuoles (Figure 6). If fascicle formation is observed, this may be an additional clue to the diagnosis. In poorly differentiated tumors, immunohistochemistry is invaluable. Leiomyosarcoma often stains positive for smooth muscle actin, muscle specific actin, h-caldesmon, desmin, and calponin.9-11
Spindle cell squamous cell carcinomas often present as ulcerated nodules on sun-exposed skin or on sites of prior ionizing radiation.2,12 Like desmoplastic melanoma, spindle cell squamous cell carcinomas are characterized by spindle cells in the dermis. Helpful diagnostic clues may include evidence of squamous differentiation, including keratin pearls or overlying actinic keratosis (Figure 7). However, actinic keratosis is common on sun-damaged skin and cannot be used to definitively confirm this diagnosis. There also may be areas of the tumor with more typical epithelioid cells that are easily identified as squamous cell carcinoma.2 Spindle cell squamous cell carcinoma stains positive for high–molecular weight cytokeratin antibodies and p63,2 which can help to differentiate it from the other spindle cell tumors in the differential.

| 
| |
| Figure 6. Spindle cells of leiomyosarcoma with cigar-shaped nuclei and adjacent glycogen vacuoles (H&E, original magnification ×600). | Figure 7. Spindle cell squamous cell carcinoma with overlying epidermal atypia that blends with the underlying dermal spindle cells (H&E, original magnification ×100). |
Desmoplastic melanoma, an uncommon variant of melanoma, poses a diagnostic challenge to the clinician because the tumors frequently appear as nonspecific flesh-colored or amelanotic plaques or nodules. They are more common in men than in women and are frequently found on the head and neck.1,2 Their innocuous appearance may lead to a delay in diagnosis and may explain why desmoplastic melanomas often are deeply infiltrative at the time of biopsy. Desmoplastic melanoma arises de novo in approximately one-third of cases.1 In the remainder of cases, it is seen in conjunction with overlying melanoma in situ, most commonly lentigo maligna melanoma.1 Histologically, desmoplastic melanomas are characterized by malignant spindle cells within a densely fibrotic stroma (Figure 1). Adjacent lymphoid aggregates and perineural involvement are common features,2 while pigment and atypical mitoses can be infrequent. Desmoplastic melanoma can be classified as mixed or pure based on the degree of desmoplasia and cellularity. Within mixed desmoplastic melanomas, there are areas that have histologic features of conventional melanomas while others demonstrate more typical desmoplastic characteristics. Pure desmoplastic melanoma has a higher degree of desmoplasia and fewer tumor cells than the mixed type.1 The pure subtype tends to be less aggressive and is less likely to metastasize to the lymph nodes.1 In the absence of an in situ component (Figure 2), desmoplastic melanoma may be indistinguishable from other spindle cell tumors on routine hematoxylin and eosin staining; thus, immunohistochemical staining generally is required. The most reliable stains in confirming a diagnosis of desmoplastic melanoma are S100 and SOX10 (SRY-related HMG-box 10)(Figure 3)(eTable).3
Atypical fibroxathoma typically presents as a nodule in the head and neck region or other sun-exposed areas in elderly individuals and is more commonly seen in men than in women.4 Histologically, atypical fibroxanthomas are composed of pleomorphic spindle, epithelioid, and multinucleated giant cells with numerous and atypical mitoses (Figure 4).5 Atypical fibroxanthoma is considered a diagnosis of exclusion; therefore, other dermal spindle cell tumors need to be ruled out before diagnosis can be made. Atypical fibroxanthomas generally stain negative for cytokeratin, S100, SOX10, and desmin, but in some cases there is positive focal staining for smooth muscle actin.4 Multiple immunohistochemical markers, including CD10, have shown reactivity in atypical fibroxanthomas,4 but none of these markers has a high specificity for this tumor; thus, it remains a diagnosis of exclusion.
Cutaneous angiosarcomas are aggressive tumors associated with a high mortality rate despite appropriate treatment with surgical resection and postoperative radiation treatment. They typically present as ecchymotic macules or nodules on the face or scalp of elderly patients.6,7 Ionizing radiation and chronic lymphedema are risk factors for cutaneous angiosarcoma.6 Histologically, well-differentiated cutaneous angiosarcomas are composed of irregular, anastomosing vascular channels that dissect through the dermis (Figure 5).6,7 Less well-differentiated tumors may contain spindle cells and lack obvious vascular structures; thus immunohistochemistry is essential for making the correct diagnosis in these cases. Cutaneous angiosarcomas typically stain positive for ERG (ETS-related gene) protein, CD31, CD34, and factor VIII.6,8 Unfortunately these tumors may also occasionally stain with cytokeratin, which may lead to the erroneous diagnosis of a carcinoma.6

| 
| |
| Figure 4. Pleomorphic spindle, epithelioid, and multinucleate giant cells with atypical mitoses filling the dermis in atypical fibroxanthoma (H&E, original magnification ×200). | Figure 5. Anastamosing vascular channels dissecting through collagen bundles and consuming the epidermis in cutaneous angiosarcoma (H&E, original magnification ×100). |
Cutaneous leiomyosarcoma is a smooth muscle neoplasm that arises from arrector pili muscles, genital smooth muscles, or vascular smooth muscles. It typically presents as a single plaque or nodule on the arms and legs of individuals older than 50 years of age.9 Cutaneous leiomyosarcomas can be classified as either dermal, in which at least 90% of the tumor is confined to the dermis, or subcutaneous; this distinction is important because the latter type has a higher rate of metastasis and a poorer prognosis.9 Because of this tumor’s smooth muscle derivation, well-differentiated tumors may retain features of typical smooth muscle cells, including cigar-shaped nuclei with adjacent glycogen vacuoles (Figure 6). If fascicle formation is observed, this may be an additional clue to the diagnosis. In poorly differentiated tumors, immunohistochemistry is invaluable. Leiomyosarcoma often stains positive for smooth muscle actin, muscle specific actin, h-caldesmon, desmin, and calponin.9-11
Spindle cell squamous cell carcinomas often present as ulcerated nodules on sun-exposed skin or on sites of prior ionizing radiation.2,12 Like desmoplastic melanoma, spindle cell squamous cell carcinomas are characterized by spindle cells in the dermis. Helpful diagnostic clues may include evidence of squamous differentiation, including keratin pearls or overlying actinic keratosis (Figure 7). However, actinic keratosis is common on sun-damaged skin and cannot be used to definitively confirm this diagnosis. There also may be areas of the tumor with more typical epithelioid cells that are easily identified as squamous cell carcinoma.2 Spindle cell squamous cell carcinoma stains positive for high–molecular weight cytokeratin antibodies and p63,2 which can help to differentiate it from the other spindle cell tumors in the differential.

| 
| |
| Figure 6. Spindle cells of leiomyosarcoma with cigar-shaped nuclei and adjacent glycogen vacuoles (H&E, original magnification ×600). | Figure 7. Spindle cell squamous cell carcinoma with overlying epidermal atypia that blends with the underlying dermal spindle cells (H&E, original magnification ×100). |
1. Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
2. Calonje JE, Brenn T, Lazar AJ, et al. McKee’s Pathology of the Skin. 4th ed. St Louis, MO: Elsevier Saunders; 2012.
3. Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
4. Luzar B, Calonje E. Morphological and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37:301-309.
5. Iorizzo LJ III, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
6. Luca DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
7. Mendenhall WM, Mendenhall CM, Werning JW, et al. Cutaneous angiosarcoma. Am J Oncol. 2006;29:524-528.
8. Thum C, Husain EA, Mulholland K, et al. Atypical fibroxanthoma with pseudoangiomatous features: a histological and immunohistochemical mimic of cutaneous angiosarcoma. Ann Diagn Pathol. 2013;17:502-507.
9. Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
10. Hall BJ, Grossmann AH, Webber NP, et al. Atypical intradermal smooth muscle neoplasms (formerly cutaneous leiomyosarcomas): case series, immunohistochemical profile and review of the literature. Appl Immunohistochem Mol Morphol. 2013;21:132-138.
11. Perez-Montiel MD, Plaza JA, Dominguez-Malagon H, et al. Differential expression of smooth muscle myosin, smooth muscle actin, h-caldesmon, and calponin in the diagnosis of myofibroblastic and smooth muscle lesions of skin and soft tissue. Am J Dermatopathol. 2006;28:105-111.
12. Cassarino DS, DeRienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. part one. J Cutan Pathol. 2006;33:191-205.
1. Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
2. Calonje JE, Brenn T, Lazar AJ, et al. McKee’s Pathology of the Skin. 4th ed. St Louis, MO: Elsevier Saunders; 2012.
3. Elston DM, Ferringer TC, Ko C, et al. Dermatopathology: Requisites in Dermatology. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2014.
4. Luzar B, Calonje E. Morphological and immunohistochemical characteristics of atypical fibroxanthoma with a special emphasis on potential diagnostic pitfalls: a review. J Cutan Pathol. 2010;37:301-309.
5. Iorizzo LJ III, Brown MD. Atypical fibroxanthoma: a review of the literature. Dermatol Surg. 2011;37:146-157.
6. Luca DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133:1804-1809.
7. Mendenhall WM, Mendenhall CM, Werning JW, et al. Cutaneous angiosarcoma. Am J Oncol. 2006;29:524-528.
8. Thum C, Husain EA, Mulholland K, et al. Atypical fibroxanthoma with pseudoangiomatous features: a histological and immunohistochemical mimic of cutaneous angiosarcoma. Ann Diagn Pathol. 2013;17:502-507.
9. Bolognia JL, Jorizzo JL, Shaffer JV. Dermatology. 3rd ed. Philadelphia, PA: Elsevier; 2012.
10. Hall BJ, Grossmann AH, Webber NP, et al. Atypical intradermal smooth muscle neoplasms (formerly cutaneous leiomyosarcomas): case series, immunohistochemical profile and review of the literature. Appl Immunohistochem Mol Morphol. 2013;21:132-138.
11. Perez-Montiel MD, Plaza JA, Dominguez-Malagon H, et al. Differential expression of smooth muscle myosin, smooth muscle actin, h-caldesmon, and calponin in the diagnosis of myofibroblastic and smooth muscle lesions of skin and soft tissue. Am J Dermatopathol. 2006;28:105-111.
12. Cassarino DS, DeRienzo DP, Barr RJ. Cutaneous squamous cell carcinoma: a comprehensive clinicopathologic classification. part one. J Cutan Pathol. 2006;33:191-205.