User login
A review of 2015 drug approvals: Safety in pregnancy and lactation
The Food and Drug Administration approved 45 new drugs in 2015. Currently, lesinurad (Zurampic) to treat high blood uric levels associated with gout, is not yet available. Another agent, aripiprazole (Aristada) for the treatment of schizophrenia, was initially approved in 2004 and is included in “Drugs in Pregnancy and Lactation,” 10th ed. (Philadelphia: Walters Kluwer: 2014, pp. 83-5).
There are three new multidrug combinations. Ceftazidime-avibactam (Avycaz) is indicated for complicated intra-abdominal and urinary tract infections, including pyelonephritis, when there are limited or no alternative treatment options. Lumacaftor-ivacaftor (Orkambi) is used to treat cystic fibrosis. There are no human pregnancy data for these two combination products but, based on animal data, they can be classified as low risk (no developmental toxicity in two or more animal species). There also is a four-drug combination that is a complete regimen for the treatment of HIV-1 infections: elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide (Genvoya). There is one reference citing the use of this combination drug in human pregnancy. It resulted in a healthy full-term infant. Because of its indication, this drug product should not be withheld because of pregnancy.
Asfotase (Strensiq) is a tissue nonspecific alkaline phosphatase. It is indicated for the treatment of perinatal/infantile and juvenile-onset hypophosphatasia. The animal data suggest low risk but it is doubtful that this drug will be used in pregnancy. In studies conducted by the manufacturer, the oldest patient age was 12.6 years.
Dinutuximab (Unituxin) is indicated for the treatment of neuroblastoma, an extracranial solid cancer that occurs in children. There are no studies in pregnant animals or humans.
The remaining 38 agents can be classified into the following categories: anticoagulant (2), antidiarrheal (1), antidiabetic (1), antidote (3), antiemetic (1), antifungal (1), antilipemic (2), antineoplastic (13), antipsychotic (2), antiviral (1), bile acid (2), cardiovascular (2), female sexual dysfunction (1), immunomodulator (1), lysosomal acid lipase deficiency (1), parathyroid hormone (1), pyrimidine analog (1), and respiratory (2).
Only two (cholic acid and ivabradine) of these 38 drugs have any human pregnancy data. Thus, the embryo-fetal risk has to be estimated using only animal data. However, an analysis of 1,154 drugs found that animal data raised the possibility of human developmental toxicity in 311 drugs, which was eventually confirmed in 75 drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). Nevertheless, in some cases the maternal benefit will outweigh the risk based on animal data.
Anticoagulants
The two anticoagulants are cangrelor (Kengreal), indicated as an adjunct to percutaneous coronary intervention to reduce the risk of myocardial infarction, and edoxaban (Savaysa), to reduce the risk of stroke and embolism in patients with atrial fibrillation and for the treatment of deep vein thrombosis and pulmonary embolism. Although the animal data for cangrelor suggest moderate risk (developmental toxicity in one animal species), the potential benefit to the mother outweighs the risk. The animal data for edoxaban suggest low risk.
Antidiarrheal
Eluxadoline (Viberzi) is indicated for the treatment of irritable bowel syndrome with diarrhea. The animal data suggest low risk.
Antidiabetic
The animal data also suggest low risk for insulin degludec (Tresiba), a long-acting human insulin analog.
Antidote
Sugammadex (Bridion), an antidote, is indicated for the reversal of neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. The animal data suggest risk (developmental toxicity in two or more animal species). Another antidote, idarucizumab (Praxbind) is a humanized monoclonal antibody fragment. It is indicated to reverse the anticoagulant effects of dabigatran. Animal reproduction studies have not been conducted. If indicated, the drug should not be withheld because of pregnancy. The third antidote is patiromer (Veltassa). It is a potassium binder used for the treatment of hyperkalemia. It should not be used for life-threatening hyperkalemia because of its delayed onset of action. It is not absorbed systemically. Because it can bind other drugs in the gut, other oral medications should be given at least 6 hours before or after patiromer.
Antiemetic
The antiemetic drug rolapitant (Varubi) is used, in combination with other antiemetics, for patients receiving antineoplastics. Animal data suggest moderate risk.
Antifungal
The triazole antifungal, isavuconazonium (Cresemba) is used for the treatment of invasive aspergillosis or invasive mucormycosis. Low doses of the drug caused perinatal death and skeletal defects in rats, similar to other azole antifungal agents.
Antilipemic agents
There are two antilipemic agents; alirocumab (Praluent) and evolocumab (Repatha). Alirocumab, a monoclonal antibody, is combined with statin therapy. Statins are contraindicated in pregnancy because interruption of cholesterol-lowering therapy during pregnancy should have no effect on the long-term treatment of hyperlipidemia and there is potential for embryo-fetal risk. Thus, alirocumab is also contraindicated during pregnancy. The same reasoning applies to evolocumab, an immunoglobulin, that is also given with statins.
Antineoplastic
There are 13 new antineoplastic agents that are often combined with other agents. They all can be classified as contraindicated in pregnancy, but the maternal benefit has to be weighed against the potential for embryo-fetal harm. Moreover, they are usually indicated when older drugs and treatments have not been effective. If the drug must be used in pregnancy, avoiding the first trimester should be a priority.
Sonidegib (Odomzo) is used for the treatment of basal cell carcinoma. The new agent for breast cancer is palbociclib (Ibrance). It is combined with letrozole, an antineoplastic that has caused spontaneous abortions and birth defects. A combination drug indicated for metastatic colorectal cancer is trifluridine/tipiracil (Lonsurf).
There are three new drugs that are indicated for non–small-cell lung cancer: alectinib (Alecensa), necitumumab (Portrazza), and osimertinib (Tagrisso). Alectinib and osimertinib are kinase inhibitors, whereas necitumumab is a epidermal growth factor receptor antagonist. The latter agent is a first-line drug that is combined with gemcitabine and cisplatin, both of which can cause birth defects.
The drug used for metastatic liposarcoma or leiomyosarcoma is trabectedin (Yondelis), an alkylating drug. There are no animal data, but the drug’s mechanism of action suggests that it can cause fetal harm. Cobimetinib (Cotellic), a kinase inhibitor, is indicated for the treatment of metastatic melanoma in combination with vemurafenib, another kinase inhibitor. The animal data and mechanism of action suggest that the drug could cause fetal harm.
There are four new agents for the treatment of multiple myeloma: panobinostat (Farydak), daratumumab (Darzalex), elotuzumab (Empliciti), and ixazomib (Ninlaro). Panobinostat, a histone deacetylase inhibitor, is combined with bortezomib (a proteasome inhibitor) and dexamethasone. The animal data suggest risk. The manufacturer recommends effective contraception while taking the drug and for at least 1 month after the last dose. Daratumumab is a monoclonal antibody. There are no animal data, but based on its mechanism of action, the drug may cause fetal myeloid or lymphoid-cell depletion and decreased bone density. The immunostimulatory antibody elotuzumab is given in combination with lenalidomide, an analog of thalidomide, and dexamethasone. There are no pregnancy data in animals or humans with lenalidomide, but this agent is contraindicated in pregnancy because of its relationship to thalidomide. Ixazomib, a proteasome inhibitor, is also given in combination with lenalidomide and dexamethasone, so the concerns are the same.
A new drug for thyroid cancer is lenvatinib (Lenvima), a tyrosine kinase inhibitor. The animal data suggest risk.
Antipsychotics
The two atypical antipsychotics are brexpiprazole (Rexulti) and cariprazine (Vraylar). Both drugs are included in National Pregnancy Registry for Atypical Antipsychotics. Information concerning the registry can be obtained by calling 1-866-961-2388 or at http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Brexpiprazole is indicated for use as an adjunctive therapy to antidepressants for the treatment of major depressive disorder and for the treatment of schizophrenia. The animal data suggest low risk. Cariprazine is indicated for the treatment of schizophrenia and for the treatment of manic or mixed episodes associated with bipolar I disorder. The animal data suggest moderate risk.
Antiviral
Daclatasvir (Daklinza) is an antiviral agent indicated for use with sofosbuvir, with or without ribavirin, for the treatment of chronic hepatitis C virus genotype 1 or 3 infection. The animal data suggest low risk but, if ribavirin is used, the combination is contraindicated.
Bile acid
There are two new bile acid products. Cholic acid (Cholbam), is indicated for the treatment of bile acid synthesis disorders due to single enzyme defects and for peroxisomal disorders, including Zellweger spectrum disorders. Although studies in pregnant women or animals have not been conducted, the manufacturer is aware of some case reports that resulted in healthy infants.
Deoxycholic acid (Kybella) is a cytolytic agent that is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. The drug caused no fetal harm in rats but did in rabbits, which may have been due to maternal toxicity.
Cardiovascular agents
There are two new cardiovascular agents. Ivabradine (Corlanor) is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that is indicated to reduce the risk of hospitalization for worsening heart failure. The animal data suggest risk. A 2001 case report described the use of the drug in a pregnant woman. She suffered a cardiopulmonary arrest in her 10th week of pregnancy and was successfully treated with several drugs, including ivabradine. Ivabradine was stopped 1 month later. She gave birth at term to a normal healthy male infant.
Entresto is a combination of sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin II receptor blocker. The combination is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure and reduced ejection fraction. There is ample evidence that exposure to valsartan in the second half of pregnancy can cause severe fetal/neonatal toxicity including death.
Sexual dysfunction
Flibanserin (Addyi) is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder. The animal data suggest moderate risk. The simultaneous use of alcohol increases the risk of severe hypotension and syncope. The drug is available only through a restricted program called the Addyi REMS Program.
Immunomodulator
The new immunomodulator is secukinumab (Cosentyx). It is a human interleukin-17A antagonist indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy of phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis. The animal data suggest low risk.
Lysosomal acid lipase deficiency treatment
Sebelipase (Kanuma) is a lysosomal cholesteryl ester and triacylglycerol-specific enzyme that is indicated for the treatment of lysosomal acid lipase deficiency. The animal data suggest low risk.
Parathyroid hormone
Parathyroid hormone (Natpara) is used as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathryoidism. The animal data in one species suggest moderate risk. Moreover, because of the risk of osteosarcoma, the drug should be used only in patients who cannot be well controlled on calcium and active forms of vitamin D alone.
Pyrimidine analog
Uridine (Xuriden) is a pyrimidine analog for uridine replacement. It is indicated for the treatment of hereditary orotic aciduria. The animal data suggest low risk.
Respiratory agents
The two respiratory agents are mepolizumab (Nucala) and selexipag (Uptravi). Mepolizumab is an interleukin-5 monoclonal antibody. It is indicated for the add-on maintenance treatment of patients with severe asthma aged 12 years and older and with an eosinophilic phenotype. The animal data suggest low risk. Selexipag is a prostacyclin receptor agonist that is used for the treatment of pulmonary arterial hypertension to delay disease progression and reduce the risk of hospitalization. The animal data suggest low risk.
Lactation
Use of the above drugs during human lactation has not been reported. It is likely that many of these agents will be excreted into breast milk. If a new agent is being taken by a woman who is or will be breastfeeding, the drug’s product information should be checked for the most common adverse reactions noted in nonpregnant patients and the nursing infant should be monitored for these effects.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
The Food and Drug Administration approved 45 new drugs in 2015. Currently, lesinurad (Zurampic) to treat high blood uric levels associated with gout, is not yet available. Another agent, aripiprazole (Aristada) for the treatment of schizophrenia, was initially approved in 2004 and is included in “Drugs in Pregnancy and Lactation,” 10th ed. (Philadelphia: Walters Kluwer: 2014, pp. 83-5).
There are three new multidrug combinations. Ceftazidime-avibactam (Avycaz) is indicated for complicated intra-abdominal and urinary tract infections, including pyelonephritis, when there are limited or no alternative treatment options. Lumacaftor-ivacaftor (Orkambi) is used to treat cystic fibrosis. There are no human pregnancy data for these two combination products but, based on animal data, they can be classified as low risk (no developmental toxicity in two or more animal species). There also is a four-drug combination that is a complete regimen for the treatment of HIV-1 infections: elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide (Genvoya). There is one reference citing the use of this combination drug in human pregnancy. It resulted in a healthy full-term infant. Because of its indication, this drug product should not be withheld because of pregnancy.
Asfotase (Strensiq) is a tissue nonspecific alkaline phosphatase. It is indicated for the treatment of perinatal/infantile and juvenile-onset hypophosphatasia. The animal data suggest low risk but it is doubtful that this drug will be used in pregnancy. In studies conducted by the manufacturer, the oldest patient age was 12.6 years.
Dinutuximab (Unituxin) is indicated for the treatment of neuroblastoma, an extracranial solid cancer that occurs in children. There are no studies in pregnant animals or humans.
The remaining 38 agents can be classified into the following categories: anticoagulant (2), antidiarrheal (1), antidiabetic (1), antidote (3), antiemetic (1), antifungal (1), antilipemic (2), antineoplastic (13), antipsychotic (2), antiviral (1), bile acid (2), cardiovascular (2), female sexual dysfunction (1), immunomodulator (1), lysosomal acid lipase deficiency (1), parathyroid hormone (1), pyrimidine analog (1), and respiratory (2).
Only two (cholic acid and ivabradine) of these 38 drugs have any human pregnancy data. Thus, the embryo-fetal risk has to be estimated using only animal data. However, an analysis of 1,154 drugs found that animal data raised the possibility of human developmental toxicity in 311 drugs, which was eventually confirmed in 75 drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). Nevertheless, in some cases the maternal benefit will outweigh the risk based on animal data.
Anticoagulants
The two anticoagulants are cangrelor (Kengreal), indicated as an adjunct to percutaneous coronary intervention to reduce the risk of myocardial infarction, and edoxaban (Savaysa), to reduce the risk of stroke and embolism in patients with atrial fibrillation and for the treatment of deep vein thrombosis and pulmonary embolism. Although the animal data for cangrelor suggest moderate risk (developmental toxicity in one animal species), the potential benefit to the mother outweighs the risk. The animal data for edoxaban suggest low risk.
Antidiarrheal
Eluxadoline (Viberzi) is indicated for the treatment of irritable bowel syndrome with diarrhea. The animal data suggest low risk.
Antidiabetic
The animal data also suggest low risk for insulin degludec (Tresiba), a long-acting human insulin analog.
Antidote
Sugammadex (Bridion), an antidote, is indicated for the reversal of neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. The animal data suggest risk (developmental toxicity in two or more animal species). Another antidote, idarucizumab (Praxbind) is a humanized monoclonal antibody fragment. It is indicated to reverse the anticoagulant effects of dabigatran. Animal reproduction studies have not been conducted. If indicated, the drug should not be withheld because of pregnancy. The third antidote is patiromer (Veltassa). It is a potassium binder used for the treatment of hyperkalemia. It should not be used for life-threatening hyperkalemia because of its delayed onset of action. It is not absorbed systemically. Because it can bind other drugs in the gut, other oral medications should be given at least 6 hours before or after patiromer.
Antiemetic
The antiemetic drug rolapitant (Varubi) is used, in combination with other antiemetics, for patients receiving antineoplastics. Animal data suggest moderate risk.
Antifungal
The triazole antifungal, isavuconazonium (Cresemba) is used for the treatment of invasive aspergillosis or invasive mucormycosis. Low doses of the drug caused perinatal death and skeletal defects in rats, similar to other azole antifungal agents.
Antilipemic agents
There are two antilipemic agents; alirocumab (Praluent) and evolocumab (Repatha). Alirocumab, a monoclonal antibody, is combined with statin therapy. Statins are contraindicated in pregnancy because interruption of cholesterol-lowering therapy during pregnancy should have no effect on the long-term treatment of hyperlipidemia and there is potential for embryo-fetal risk. Thus, alirocumab is also contraindicated during pregnancy. The same reasoning applies to evolocumab, an immunoglobulin, that is also given with statins.
Antineoplastic
There are 13 new antineoplastic agents that are often combined with other agents. They all can be classified as contraindicated in pregnancy, but the maternal benefit has to be weighed against the potential for embryo-fetal harm. Moreover, they are usually indicated when older drugs and treatments have not been effective. If the drug must be used in pregnancy, avoiding the first trimester should be a priority.
Sonidegib (Odomzo) is used for the treatment of basal cell carcinoma. The new agent for breast cancer is palbociclib (Ibrance). It is combined with letrozole, an antineoplastic that has caused spontaneous abortions and birth defects. A combination drug indicated for metastatic colorectal cancer is trifluridine/tipiracil (Lonsurf).
There are three new drugs that are indicated for non–small-cell lung cancer: alectinib (Alecensa), necitumumab (Portrazza), and osimertinib (Tagrisso). Alectinib and osimertinib are kinase inhibitors, whereas necitumumab is a epidermal growth factor receptor antagonist. The latter agent is a first-line drug that is combined with gemcitabine and cisplatin, both of which can cause birth defects.
The drug used for metastatic liposarcoma or leiomyosarcoma is trabectedin (Yondelis), an alkylating drug. There are no animal data, but the drug’s mechanism of action suggests that it can cause fetal harm. Cobimetinib (Cotellic), a kinase inhibitor, is indicated for the treatment of metastatic melanoma in combination with vemurafenib, another kinase inhibitor. The animal data and mechanism of action suggest that the drug could cause fetal harm.
There are four new agents for the treatment of multiple myeloma: panobinostat (Farydak), daratumumab (Darzalex), elotuzumab (Empliciti), and ixazomib (Ninlaro). Panobinostat, a histone deacetylase inhibitor, is combined with bortezomib (a proteasome inhibitor) and dexamethasone. The animal data suggest risk. The manufacturer recommends effective contraception while taking the drug and for at least 1 month after the last dose. Daratumumab is a monoclonal antibody. There are no animal data, but based on its mechanism of action, the drug may cause fetal myeloid or lymphoid-cell depletion and decreased bone density. The immunostimulatory antibody elotuzumab is given in combination with lenalidomide, an analog of thalidomide, and dexamethasone. There are no pregnancy data in animals or humans with lenalidomide, but this agent is contraindicated in pregnancy because of its relationship to thalidomide. Ixazomib, a proteasome inhibitor, is also given in combination with lenalidomide and dexamethasone, so the concerns are the same.
A new drug for thyroid cancer is lenvatinib (Lenvima), a tyrosine kinase inhibitor. The animal data suggest risk.
Antipsychotics
The two atypical antipsychotics are brexpiprazole (Rexulti) and cariprazine (Vraylar). Both drugs are included in National Pregnancy Registry for Atypical Antipsychotics. Information concerning the registry can be obtained by calling 1-866-961-2388 or at http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Brexpiprazole is indicated for use as an adjunctive therapy to antidepressants for the treatment of major depressive disorder and for the treatment of schizophrenia. The animal data suggest low risk. Cariprazine is indicated for the treatment of schizophrenia and for the treatment of manic or mixed episodes associated with bipolar I disorder. The animal data suggest moderate risk.
Antiviral
Daclatasvir (Daklinza) is an antiviral agent indicated for use with sofosbuvir, with or without ribavirin, for the treatment of chronic hepatitis C virus genotype 1 or 3 infection. The animal data suggest low risk but, if ribavirin is used, the combination is contraindicated.
Bile acid
There are two new bile acid products. Cholic acid (Cholbam), is indicated for the treatment of bile acid synthesis disorders due to single enzyme defects and for peroxisomal disorders, including Zellweger spectrum disorders. Although studies in pregnant women or animals have not been conducted, the manufacturer is aware of some case reports that resulted in healthy infants.
Deoxycholic acid (Kybella) is a cytolytic agent that is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. The drug caused no fetal harm in rats but did in rabbits, which may have been due to maternal toxicity.
Cardiovascular agents
There are two new cardiovascular agents. Ivabradine (Corlanor) is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that is indicated to reduce the risk of hospitalization for worsening heart failure. The animal data suggest risk. A 2001 case report described the use of the drug in a pregnant woman. She suffered a cardiopulmonary arrest in her 10th week of pregnancy and was successfully treated with several drugs, including ivabradine. Ivabradine was stopped 1 month later. She gave birth at term to a normal healthy male infant.
Entresto is a combination of sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin II receptor blocker. The combination is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure and reduced ejection fraction. There is ample evidence that exposure to valsartan in the second half of pregnancy can cause severe fetal/neonatal toxicity including death.
Sexual dysfunction
Flibanserin (Addyi) is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder. The animal data suggest moderate risk. The simultaneous use of alcohol increases the risk of severe hypotension and syncope. The drug is available only through a restricted program called the Addyi REMS Program.
Immunomodulator
The new immunomodulator is secukinumab (Cosentyx). It is a human interleukin-17A antagonist indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy of phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis. The animal data suggest low risk.
Lysosomal acid lipase deficiency treatment
Sebelipase (Kanuma) is a lysosomal cholesteryl ester and triacylglycerol-specific enzyme that is indicated for the treatment of lysosomal acid lipase deficiency. The animal data suggest low risk.
Parathyroid hormone
Parathyroid hormone (Natpara) is used as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathryoidism. The animal data in one species suggest moderate risk. Moreover, because of the risk of osteosarcoma, the drug should be used only in patients who cannot be well controlled on calcium and active forms of vitamin D alone.
Pyrimidine analog
Uridine (Xuriden) is a pyrimidine analog for uridine replacement. It is indicated for the treatment of hereditary orotic aciduria. The animal data suggest low risk.
Respiratory agents
The two respiratory agents are mepolizumab (Nucala) and selexipag (Uptravi). Mepolizumab is an interleukin-5 monoclonal antibody. It is indicated for the add-on maintenance treatment of patients with severe asthma aged 12 years and older and with an eosinophilic phenotype. The animal data suggest low risk. Selexipag is a prostacyclin receptor agonist that is used for the treatment of pulmonary arterial hypertension to delay disease progression and reduce the risk of hospitalization. The animal data suggest low risk.
Lactation
Use of the above drugs during human lactation has not been reported. It is likely that many of these agents will be excreted into breast milk. If a new agent is being taken by a woman who is or will be breastfeeding, the drug’s product information should be checked for the most common adverse reactions noted in nonpregnant patients and the nursing infant should be monitored for these effects.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
The Food and Drug Administration approved 45 new drugs in 2015. Currently, lesinurad (Zurampic) to treat high blood uric levels associated with gout, is not yet available. Another agent, aripiprazole (Aristada) for the treatment of schizophrenia, was initially approved in 2004 and is included in “Drugs in Pregnancy and Lactation,” 10th ed. (Philadelphia: Walters Kluwer: 2014, pp. 83-5).
There are three new multidrug combinations. Ceftazidime-avibactam (Avycaz) is indicated for complicated intra-abdominal and urinary tract infections, including pyelonephritis, when there are limited or no alternative treatment options. Lumacaftor-ivacaftor (Orkambi) is used to treat cystic fibrosis. There are no human pregnancy data for these two combination products but, based on animal data, they can be classified as low risk (no developmental toxicity in two or more animal species). There also is a four-drug combination that is a complete regimen for the treatment of HIV-1 infections: elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide (Genvoya). There is one reference citing the use of this combination drug in human pregnancy. It resulted in a healthy full-term infant. Because of its indication, this drug product should not be withheld because of pregnancy.
Asfotase (Strensiq) is a tissue nonspecific alkaline phosphatase. It is indicated for the treatment of perinatal/infantile and juvenile-onset hypophosphatasia. The animal data suggest low risk but it is doubtful that this drug will be used in pregnancy. In studies conducted by the manufacturer, the oldest patient age was 12.6 years.
Dinutuximab (Unituxin) is indicated for the treatment of neuroblastoma, an extracranial solid cancer that occurs in children. There are no studies in pregnant animals or humans.
The remaining 38 agents can be classified into the following categories: anticoagulant (2), antidiarrheal (1), antidiabetic (1), antidote (3), antiemetic (1), antifungal (1), antilipemic (2), antineoplastic (13), antipsychotic (2), antiviral (1), bile acid (2), cardiovascular (2), female sexual dysfunction (1), immunomodulator (1), lysosomal acid lipase deficiency (1), parathyroid hormone (1), pyrimidine analog (1), and respiratory (2).
Only two (cholic acid and ivabradine) of these 38 drugs have any human pregnancy data. Thus, the embryo-fetal risk has to be estimated using only animal data. However, an analysis of 1,154 drugs found that animal data raised the possibility of human developmental toxicity in 311 drugs, which was eventually confirmed in 75 drugs (Am J Obstet Gynecol. 2015 Dec;213[6]:810-5). Nevertheless, in some cases the maternal benefit will outweigh the risk based on animal data.
Anticoagulants
The two anticoagulants are cangrelor (Kengreal), indicated as an adjunct to percutaneous coronary intervention to reduce the risk of myocardial infarction, and edoxaban (Savaysa), to reduce the risk of stroke and embolism in patients with atrial fibrillation and for the treatment of deep vein thrombosis and pulmonary embolism. Although the animal data for cangrelor suggest moderate risk (developmental toxicity in one animal species), the potential benefit to the mother outweighs the risk. The animal data for edoxaban suggest low risk.
Antidiarrheal
Eluxadoline (Viberzi) is indicated for the treatment of irritable bowel syndrome with diarrhea. The animal data suggest low risk.
Antidiabetic
The animal data also suggest low risk for insulin degludec (Tresiba), a long-acting human insulin analog.
Antidote
Sugammadex (Bridion), an antidote, is indicated for the reversal of neuromuscular blockade induced by rocuronium and vecuronium in adults undergoing surgery. The animal data suggest risk (developmental toxicity in two or more animal species). Another antidote, idarucizumab (Praxbind) is a humanized monoclonal antibody fragment. It is indicated to reverse the anticoagulant effects of dabigatran. Animal reproduction studies have not been conducted. If indicated, the drug should not be withheld because of pregnancy. The third antidote is patiromer (Veltassa). It is a potassium binder used for the treatment of hyperkalemia. It should not be used for life-threatening hyperkalemia because of its delayed onset of action. It is not absorbed systemically. Because it can bind other drugs in the gut, other oral medications should be given at least 6 hours before or after patiromer.
Antiemetic
The antiemetic drug rolapitant (Varubi) is used, in combination with other antiemetics, for patients receiving antineoplastics. Animal data suggest moderate risk.
Antifungal
The triazole antifungal, isavuconazonium (Cresemba) is used for the treatment of invasive aspergillosis or invasive mucormycosis. Low doses of the drug caused perinatal death and skeletal defects in rats, similar to other azole antifungal agents.
Antilipemic agents
There are two antilipemic agents; alirocumab (Praluent) and evolocumab (Repatha). Alirocumab, a monoclonal antibody, is combined with statin therapy. Statins are contraindicated in pregnancy because interruption of cholesterol-lowering therapy during pregnancy should have no effect on the long-term treatment of hyperlipidemia and there is potential for embryo-fetal risk. Thus, alirocumab is also contraindicated during pregnancy. The same reasoning applies to evolocumab, an immunoglobulin, that is also given with statins.
Antineoplastic
There are 13 new antineoplastic agents that are often combined with other agents. They all can be classified as contraindicated in pregnancy, but the maternal benefit has to be weighed against the potential for embryo-fetal harm. Moreover, they are usually indicated when older drugs and treatments have not been effective. If the drug must be used in pregnancy, avoiding the first trimester should be a priority.
Sonidegib (Odomzo) is used for the treatment of basal cell carcinoma. The new agent for breast cancer is palbociclib (Ibrance). It is combined with letrozole, an antineoplastic that has caused spontaneous abortions and birth defects. A combination drug indicated for metastatic colorectal cancer is trifluridine/tipiracil (Lonsurf).
There are three new drugs that are indicated for non–small-cell lung cancer: alectinib (Alecensa), necitumumab (Portrazza), and osimertinib (Tagrisso). Alectinib and osimertinib are kinase inhibitors, whereas necitumumab is a epidermal growth factor receptor antagonist. The latter agent is a first-line drug that is combined with gemcitabine and cisplatin, both of which can cause birth defects.
The drug used for metastatic liposarcoma or leiomyosarcoma is trabectedin (Yondelis), an alkylating drug. There are no animal data, but the drug’s mechanism of action suggests that it can cause fetal harm. Cobimetinib (Cotellic), a kinase inhibitor, is indicated for the treatment of metastatic melanoma in combination with vemurafenib, another kinase inhibitor. The animal data and mechanism of action suggest that the drug could cause fetal harm.
There are four new agents for the treatment of multiple myeloma: panobinostat (Farydak), daratumumab (Darzalex), elotuzumab (Empliciti), and ixazomib (Ninlaro). Panobinostat, a histone deacetylase inhibitor, is combined with bortezomib (a proteasome inhibitor) and dexamethasone. The animal data suggest risk. The manufacturer recommends effective contraception while taking the drug and for at least 1 month after the last dose. Daratumumab is a monoclonal antibody. There are no animal data, but based on its mechanism of action, the drug may cause fetal myeloid or lymphoid-cell depletion and decreased bone density. The immunostimulatory antibody elotuzumab is given in combination with lenalidomide, an analog of thalidomide, and dexamethasone. There are no pregnancy data in animals or humans with lenalidomide, but this agent is contraindicated in pregnancy because of its relationship to thalidomide. Ixazomib, a proteasome inhibitor, is also given in combination with lenalidomide and dexamethasone, so the concerns are the same.
A new drug for thyroid cancer is lenvatinib (Lenvima), a tyrosine kinase inhibitor. The animal data suggest risk.
Antipsychotics
The two atypical antipsychotics are brexpiprazole (Rexulti) and cariprazine (Vraylar). Both drugs are included in National Pregnancy Registry for Atypical Antipsychotics. Information concerning the registry can be obtained by calling 1-866-961-2388 or at http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Brexpiprazole is indicated for use as an adjunctive therapy to antidepressants for the treatment of major depressive disorder and for the treatment of schizophrenia. The animal data suggest low risk. Cariprazine is indicated for the treatment of schizophrenia and for the treatment of manic or mixed episodes associated with bipolar I disorder. The animal data suggest moderate risk.
Antiviral
Daclatasvir (Daklinza) is an antiviral agent indicated for use with sofosbuvir, with or without ribavirin, for the treatment of chronic hepatitis C virus genotype 1 or 3 infection. The animal data suggest low risk but, if ribavirin is used, the combination is contraindicated.
Bile acid
There are two new bile acid products. Cholic acid (Cholbam), is indicated for the treatment of bile acid synthesis disorders due to single enzyme defects and for peroxisomal disorders, including Zellweger spectrum disorders. Although studies in pregnant women or animals have not been conducted, the manufacturer is aware of some case reports that resulted in healthy infants.
Deoxycholic acid (Kybella) is a cytolytic agent that is indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. The drug caused no fetal harm in rats but did in rabbits, which may have been due to maternal toxicity.
Cardiovascular agents
There are two new cardiovascular agents. Ivabradine (Corlanor) is a hyperpolarization-activated cyclic nucleotide-gated channel blocker that is indicated to reduce the risk of hospitalization for worsening heart failure. The animal data suggest risk. A 2001 case report described the use of the drug in a pregnant woman. She suffered a cardiopulmonary arrest in her 10th week of pregnancy and was successfully treated with several drugs, including ivabradine. Ivabradine was stopped 1 month later. She gave birth at term to a normal healthy male infant.
Entresto is a combination of sacubitril, a neprilysin inhibitor, and valsartan, an angiotensin II receptor blocker. The combination is indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure and reduced ejection fraction. There is ample evidence that exposure to valsartan in the second half of pregnancy can cause severe fetal/neonatal toxicity including death.
Sexual dysfunction
Flibanserin (Addyi) is indicated for the treatment of premenopausal women with acquired, generalized hypoactive sexual desire disorder. The animal data suggest moderate risk. The simultaneous use of alcohol increases the risk of severe hypotension and syncope. The drug is available only through a restricted program called the Addyi REMS Program.
Immunomodulator
The new immunomodulator is secukinumab (Cosentyx). It is a human interleukin-17A antagonist indicated for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy of phototherapy, adults with psoriatic arthritis, and adults with active ankylosing spondylitis. The animal data suggest low risk.
Lysosomal acid lipase deficiency treatment
Sebelipase (Kanuma) is a lysosomal cholesteryl ester and triacylglycerol-specific enzyme that is indicated for the treatment of lysosomal acid lipase deficiency. The animal data suggest low risk.
Parathyroid hormone
Parathyroid hormone (Natpara) is used as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathryoidism. The animal data in one species suggest moderate risk. Moreover, because of the risk of osteosarcoma, the drug should be used only in patients who cannot be well controlled on calcium and active forms of vitamin D alone.
Pyrimidine analog
Uridine (Xuriden) is a pyrimidine analog for uridine replacement. It is indicated for the treatment of hereditary orotic aciduria. The animal data suggest low risk.
Respiratory agents
The two respiratory agents are mepolizumab (Nucala) and selexipag (Uptravi). Mepolizumab is an interleukin-5 monoclonal antibody. It is indicated for the add-on maintenance treatment of patients with severe asthma aged 12 years and older and with an eosinophilic phenotype. The animal data suggest low risk. Selexipag is a prostacyclin receptor agonist that is used for the treatment of pulmonary arterial hypertension to delay disease progression and reduce the risk of hospitalization. The animal data suggest low risk.
Lactation
Use of the above drugs during human lactation has not been reported. It is likely that many of these agents will be excreted into breast milk. If a new agent is being taken by a woman who is or will be breastfeeding, the drug’s product information should be checked for the most common adverse reactions noted in nonpregnant patients and the nursing infant should be monitored for these effects.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco, and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
Ophthalmic drugs in pregnancy and lactation
A number of drugs are available for ophthalmic use. This review focuses on single drug products and, although combination drug products are not discussed, these formulations typically include the drugs reviewed here.
Since it appears that ophthalmic medications are commonly used for a wide range of conditions and ages, one would expect to see numerous reports of their use in the eyes of pregnant or breastfeeding patients. Unfortunately, the opposite is true. The majority of the drugs have no human pregnancy or lactation data. When there are human data, it invariably involves the systemic use of the drug for other indications, rather than its ophthalmic use. Moreover, the animal reproduction data are usually not relevant because they involve systemic drugs (e.g., IV or oral). Consequently, determining the level of risk an ophthalmic drug presents to an embryo and/or fetus is primarily based on time and dose, the two cardinal principles of teratology.
Avoiding exposure during organogenesis – the period when a drug can cause developmental toxicity (altered growth, structural anomalies, functional and/or behavioral deficits, or death) – is usually best, but may not be possible in some cases, including glaucoma, eye infections, and eye surgery. Fortunately, the systemic concentrations of drugs applied topically to the eye are typically thought to be low, even though the levels of most drugs have not been studied. Thus, the risk to the embryo and/or fetus in most cases can be considered low and the drug classified as compatible in pregnancy and breastfeeding.
If a topical eye drug must be used during pregnancy or lactation, teach the patient how to decrease the amount of drug reaching the systemic circulation. This involves placing pressure over the tear duct in the corner of the eye for 1 minute or more, then removing any excess solution with absorbent tissue.
In the sections below, drugs are shown by indication or by pharmacologic class. The term “human eye data” refers to the use of the drug in pregnancy and/or lactation.
Glaucoma
If you are caring for a pregnant patient who is being treated for glaucoma, two recent reviews may be helpful: Surv Ophthalmol. 2011 Jul-Aug;56(4):324-35 and Curr Opin Ophthalmol. 2014 Mar;25(2):93-7.
Sympathomimetics (alpha-adrenergic agonists) include apraclonidine (Iopidine), which has no human eye data, and brimonidine (Alphagan P), which has one case report in pregnancy and breastfeeding showing no fetal or nursing infant harm.
Four of the five beta-adrenergic blockers have no human eye data: betaxolol (Betoptic), carteolol (Ocupress), levobunolol (Betagan), and metipranolol, but there are two case reports for timolol (Betimol, Istalol, Timoptic, Timoptic-XE) showing no fetal harm in one newborn and growth restriction in the other.
Among the miotics, there are limited human eye data for pilocarpine (Isopto Carpine) and no fetal harm was observed. For echothiophate iodide (Phospholine Iodide), there is one case report of a normal full-term infant whose mother was treated with the agent up to 32 weeks’ gestation and then with pilocarpine for 8 weeks (Arch Ophthalmol. 1968 Mar;79[3]:283-5).
Carbonic anhydrase inhibitors include brinzolamide (Azopt), which has no human eye data, and dorzolamide (Trusopt), which has growth restriction in one case treated with fixed combination of dorzolamide and timolol. There are no human eye data for unoprostone isopropyl (Rescula), a synthetic docosanoid.
Of the four prostaglandin analogs, three have no human eye data, bimatoprost (Lumigan), tafluprost (Zioptan), and travoprost (Travatan Z). There were 11 pregnancies exposed to latanoprost (Xalatan). The outcomes of these cases were one lost to follow-up, one miscarriage, and nine infants without congenital anomalies (Am J Ophthalmol. 2004 Aug;138[2]:305-6).
Mitomycin (Mitosol) is an antimetabolite that is given topically to the surgical site of glaucoma filtration surgery. No reports of its use in pregnant humans have been located. According to the manufacturer, clinically significant systemic concentrations are not expected.
Antiseptics
Povidone-iodine (Betadine) is indicated for prepping of the periocular region. There does not appear to be any risk to the embryo-fetus or nursing infant from this indication.
Antihistamines
The four ophthalmic agents in this class are alcaftadine (Lastacaft), azelastine (Optivar), emedastine (Emadine), and epinastine (Elestat). There are no human eye data for these agents but, like antihistamines given systemically, they are probably compatible in pregnancy and lactation.
Antihistamine-mast cell stabilizers
There are no human eye data for bepotastine (Bepreve), ketotifen (Alaway), and olopatadine (Pataday, Patanol). Peak plasma concentrations of bepotastine were 5.1-7.3 ng/mL for 1-2 hours after instillation and were less than 2 ng/mL at 24 hours. It appears that these drugs can also be classified as compatible in pregnancy and lactation.
Anti-infectives
Ten anti-infectives are available for topical treatment of eye infections (systemic concentrations if known): besifloxacin (Besivance) (0.43 ng/mL), ciprofloxacin (less than 2.5 ng/mL), gentamicin, gatifloxacin (Zymaxid) (less than 5 ng/mL), levofloxacin (Iquix) (10.9 ng/mL), moxifloxacin (Vigamox) (2.7 ng/mL), ofloxacin (1.9 ng/mL), sulfacetamide (Isopto Cetamide), and tobramycin. The tenth agent, natamycin (Natacyn), is an antifungal. According to the manufacturer, systemic absorption is not expected.
None of these agents have human eye data, but they are usually considered compatible in pregnancy and breastfeeding when systemic formulations are used, so they should be compatible when used in the eye.
Antivirals
Ganciclovir (Zirgan) has no human eye data but, according to the manufacturer, the daily ophthalmic dose is about 0.04% and 0.1% of the oral and IV doses, respectively. Thus, minimal systemic exposure is expected. Trifluridine (Viroptic) also has no eye human data. As reported in the product information, detectable blood concentrations of the drug were not found in healthy normal subjects indicating that systemic absorption was negligible.
Corticosteroids
Among the nine corticosteroid products, six are suspensions or ointments, one is an injection, and two are implants. In most nonpregnant patients receiving the dexamethasone (Ozurdex) intravitreal implant, plasma dexamethasone concentrations were undetectable (less than 50 pg/mL) but, in some, ranged from 52 pg/mL to 102 pg/mL. There is only one case report describing the use of topical dexamethasone suspension (Maxidex) in pregnancy. In that case, dexamethasone and clindamycin were given in the first and second trimesters and the woman eventually gave birth to a normal full term infant (Int Ophthalmol. 1998-1999;22[2]:85-8).
For the fluocinolone (Retisert) ocular implant, systemic absorption of detectable amounts of the drug have not been observed. In a second report, a patient who had type 1 diabetes and was 6 months pregnant received a 2-mg intravitreal injection of triamcinolone (Triesence) in both eyes. No adverse effects in the mother or fetus were noted (Clin Ophthalmol. 2011;5:439-41).
There are no human eye data for five topical corticosteroids: difluprednate (Durezol), fluorometholone (Flarex, Fluor-OP, FML), loteprednol (Alrex, Lotemax), prednisolone (Econopred), and rimexolone (Vexol). Only two of the five drugs had information about systemic absorption. For difluprednate, blood levels were below the quantification limit (50 ng/mL). Extremely low levels were detected after the use of rimexolone with a mean serum concentration of 130 pg/mL (range less than 80-470 pg/mL).
Cycloplegics-mydriatics
This class includes four anticholinergic agents: atropine, cyclopentolate (Cyclogyl), homatropine, and tropicamide (Mydriacyl). Systemic absorption has not been studied for these drugs and there are no reports of their use in pregnancy or lactation.
Cystine-depleting agents
There are no reports describing the use of cysteamine (Cystaran) in human pregnancy or lactation. Since the drug is given as one drop in each eye every waking hour, transfer to the systemic circulation should be expected, but the amount, if it occurs, has not been reported.
Immunologics
There are no reports on the use of cyclosporine (Restasis) eye drops in human pregnancy or during breastfeeding. However, data for the systemic use of the drug during these conditions has shown it to be low risk. After long term ophthalmic use, blood concentrations of the drug were below the quantitation limit of 0.1 ng/mL.
Local anesthetics
The three drugs in this class are lidocaine, proparacaine (Alcaine), and tetracaine (Altacaine, Tetravisc). There is no information regarding the use of these agents in pregnancy or lactation. Since they are used for brief periods, the risk to an embryo or nursing infant appears to be nil.
Mast cell stabilizers
There are three drugs in this class that can be used topically in the eye: cromolyn, lodoxamide (Alomide), and nedocromil (Alocril). There are no human eye data for these agents. Systemic concentrations of lodoxamide were below the detection limit of 2.5 ng/mL.
Miotics
The two miotics are acetylcholine (Miochol E) and carbachol (Carbastat, Miostat). Both are used immediately before eye surgery. The amount, if any, in the systemic circulation is unknown. No reports of human eye use in pregnancy or lactation have been found.
NSAIDs
The five drugs in this class are bromfenac (Xibrom), diclofenac (Voltaren), flurbiprofen (Ocufen), ketorolac, and nepafenac (Nevanac). Although it has not been studied, the estimated plasma level for bromfenac is less than 50 ng/mL. For diclofenac, the plasma concentration was below the limit of quantification (10 ng/mL). In a study conducted with ketorolac, only 5 of 26 subjects had detectable plasma concentrations (10.7 to 22.5 ng/mL). These levels were about 2% of the peak plasma levels after oral dosing. For nepafenac, the peak plasma concentrations of the parent drug and active metabolite were 0.3 and 0.4 ng/mL, respectively. These data suggest that the risk to the embryo-fetus or a nursing infant are nil.
Photodynamic therapy
Verteporfin (Visudyne) is given intravenously. Three reports have described its use in three pregnancies. In all cases, the infants were healthy at birth and had normal growth (Acta Ophthalmol Scand. 2004 Oct;82[5]:623-4, Eye [Lond]. 2009 Jun;23[6]:1479, and Aust N Z J Obstet Gynaecol. 2009 Apr;49[2]:236-7).
Proteolytic enzymes
No human eye data were found for ocriplasmin (Jetrea), an agent given as an intravitreal injection. Although it was not studied, detectable levels of the drug in the systemic circulation are not expected, according to the manufacturer.
Selective vascular endothelial growth factor antagonists
There are three agents in this class: aflibercept (Eylea), pegaptanib (Macugen), and ranibizumab (Lucentis). There are no human eye data for these drugs. All are given as intravitreal injection. The systemic concentrations of the three drugs were a mean 0.02 mcg/mL; 80 ng/mL (after a dose of 10 times the recommended dose); and an estimated minimum 0.22 ng/mL, respectively. These data suggest that the risk to the embryo-fetus or a nursing infant is nil.
Sympathomimetics (decongestants)
There are four ophthalmic agents in this class and none have human eye data. Naphazoline (Naphcon, Vasocon) is the only one of the four that requires a prescription. No reports describing the systemic absorption, if any, have been found. The other three agents are available over the counter. They are oxymetazoline (Visine L.R.), phenylephrine, and tetrahydrozoline (Opti-Clear). Although the amount reaching the systemic circulation was not provided by the manufacturers for these three agents, a caution was placed on phenylephrine stating that systemic absorption, although rare, might cause alpha-adrenergic effects, such as a rise in blood pressure and reflex atropine-sensitive bradycardia.
The risk to the embryo-fetus or nursing infant from ophthalmic drugs appears to be low, with the possible exception of phenylephrine. Nevertheless, if any of these agents are used in pregnancy or during breastfeeding, careful assessment of the embryo-fetus and nursing infant should be conducted. Moreover, research on the potential effects of ophthalmic drugs on the embryo-fetus and nursing infant are desperately needed.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
A number of drugs are available for ophthalmic use. This review focuses on single drug products and, although combination drug products are not discussed, these formulations typically include the drugs reviewed here.
Since it appears that ophthalmic medications are commonly used for a wide range of conditions and ages, one would expect to see numerous reports of their use in the eyes of pregnant or breastfeeding patients. Unfortunately, the opposite is true. The majority of the drugs have no human pregnancy or lactation data. When there are human data, it invariably involves the systemic use of the drug for other indications, rather than its ophthalmic use. Moreover, the animal reproduction data are usually not relevant because they involve systemic drugs (e.g., IV or oral). Consequently, determining the level of risk an ophthalmic drug presents to an embryo and/or fetus is primarily based on time and dose, the two cardinal principles of teratology.
Avoiding exposure during organogenesis – the period when a drug can cause developmental toxicity (altered growth, structural anomalies, functional and/or behavioral deficits, or death) – is usually best, but may not be possible in some cases, including glaucoma, eye infections, and eye surgery. Fortunately, the systemic concentrations of drugs applied topically to the eye are typically thought to be low, even though the levels of most drugs have not been studied. Thus, the risk to the embryo and/or fetus in most cases can be considered low and the drug classified as compatible in pregnancy and breastfeeding.
If a topical eye drug must be used during pregnancy or lactation, teach the patient how to decrease the amount of drug reaching the systemic circulation. This involves placing pressure over the tear duct in the corner of the eye for 1 minute or more, then removing any excess solution with absorbent tissue.
In the sections below, drugs are shown by indication or by pharmacologic class. The term “human eye data” refers to the use of the drug in pregnancy and/or lactation.
Glaucoma
If you are caring for a pregnant patient who is being treated for glaucoma, two recent reviews may be helpful: Surv Ophthalmol. 2011 Jul-Aug;56(4):324-35 and Curr Opin Ophthalmol. 2014 Mar;25(2):93-7.
Sympathomimetics (alpha-adrenergic agonists) include apraclonidine (Iopidine), which has no human eye data, and brimonidine (Alphagan P), which has one case report in pregnancy and breastfeeding showing no fetal or nursing infant harm.
Four of the five beta-adrenergic blockers have no human eye data: betaxolol (Betoptic), carteolol (Ocupress), levobunolol (Betagan), and metipranolol, but there are two case reports for timolol (Betimol, Istalol, Timoptic, Timoptic-XE) showing no fetal harm in one newborn and growth restriction in the other.
Among the miotics, there are limited human eye data for pilocarpine (Isopto Carpine) and no fetal harm was observed. For echothiophate iodide (Phospholine Iodide), there is one case report of a normal full-term infant whose mother was treated with the agent up to 32 weeks’ gestation and then with pilocarpine for 8 weeks (Arch Ophthalmol. 1968 Mar;79[3]:283-5).
Carbonic anhydrase inhibitors include brinzolamide (Azopt), which has no human eye data, and dorzolamide (Trusopt), which has growth restriction in one case treated with fixed combination of dorzolamide and timolol. There are no human eye data for unoprostone isopropyl (Rescula), a synthetic docosanoid.
Of the four prostaglandin analogs, three have no human eye data, bimatoprost (Lumigan), tafluprost (Zioptan), and travoprost (Travatan Z). There were 11 pregnancies exposed to latanoprost (Xalatan). The outcomes of these cases were one lost to follow-up, one miscarriage, and nine infants without congenital anomalies (Am J Ophthalmol. 2004 Aug;138[2]:305-6).
Mitomycin (Mitosol) is an antimetabolite that is given topically to the surgical site of glaucoma filtration surgery. No reports of its use in pregnant humans have been located. According to the manufacturer, clinically significant systemic concentrations are not expected.
Antiseptics
Povidone-iodine (Betadine) is indicated for prepping of the periocular region. There does not appear to be any risk to the embryo-fetus or nursing infant from this indication.
Antihistamines
The four ophthalmic agents in this class are alcaftadine (Lastacaft), azelastine (Optivar), emedastine (Emadine), and epinastine (Elestat). There are no human eye data for these agents but, like antihistamines given systemically, they are probably compatible in pregnancy and lactation.
Antihistamine-mast cell stabilizers
There are no human eye data for bepotastine (Bepreve), ketotifen (Alaway), and olopatadine (Pataday, Patanol). Peak plasma concentrations of bepotastine were 5.1-7.3 ng/mL for 1-2 hours after instillation and were less than 2 ng/mL at 24 hours. It appears that these drugs can also be classified as compatible in pregnancy and lactation.
Anti-infectives
Ten anti-infectives are available for topical treatment of eye infections (systemic concentrations if known): besifloxacin (Besivance) (0.43 ng/mL), ciprofloxacin (less than 2.5 ng/mL), gentamicin, gatifloxacin (Zymaxid) (less than 5 ng/mL), levofloxacin (Iquix) (10.9 ng/mL), moxifloxacin (Vigamox) (2.7 ng/mL), ofloxacin (1.9 ng/mL), sulfacetamide (Isopto Cetamide), and tobramycin. The tenth agent, natamycin (Natacyn), is an antifungal. According to the manufacturer, systemic absorption is not expected.
None of these agents have human eye data, but they are usually considered compatible in pregnancy and breastfeeding when systemic formulations are used, so they should be compatible when used in the eye.
Antivirals
Ganciclovir (Zirgan) has no human eye data but, according to the manufacturer, the daily ophthalmic dose is about 0.04% and 0.1% of the oral and IV doses, respectively. Thus, minimal systemic exposure is expected. Trifluridine (Viroptic) also has no eye human data. As reported in the product information, detectable blood concentrations of the drug were not found in healthy normal subjects indicating that systemic absorption was negligible.
Corticosteroids
Among the nine corticosteroid products, six are suspensions or ointments, one is an injection, and two are implants. In most nonpregnant patients receiving the dexamethasone (Ozurdex) intravitreal implant, plasma dexamethasone concentrations were undetectable (less than 50 pg/mL) but, in some, ranged from 52 pg/mL to 102 pg/mL. There is only one case report describing the use of topical dexamethasone suspension (Maxidex) in pregnancy. In that case, dexamethasone and clindamycin were given in the first and second trimesters and the woman eventually gave birth to a normal full term infant (Int Ophthalmol. 1998-1999;22[2]:85-8).
For the fluocinolone (Retisert) ocular implant, systemic absorption of detectable amounts of the drug have not been observed. In a second report, a patient who had type 1 diabetes and was 6 months pregnant received a 2-mg intravitreal injection of triamcinolone (Triesence) in both eyes. No adverse effects in the mother or fetus were noted (Clin Ophthalmol. 2011;5:439-41).
There are no human eye data for five topical corticosteroids: difluprednate (Durezol), fluorometholone (Flarex, Fluor-OP, FML), loteprednol (Alrex, Lotemax), prednisolone (Econopred), and rimexolone (Vexol). Only two of the five drugs had information about systemic absorption. For difluprednate, blood levels were below the quantification limit (50 ng/mL). Extremely low levels were detected after the use of rimexolone with a mean serum concentration of 130 pg/mL (range less than 80-470 pg/mL).
Cycloplegics-mydriatics
This class includes four anticholinergic agents: atropine, cyclopentolate (Cyclogyl), homatropine, and tropicamide (Mydriacyl). Systemic absorption has not been studied for these drugs and there are no reports of their use in pregnancy or lactation.
Cystine-depleting agents
There are no reports describing the use of cysteamine (Cystaran) in human pregnancy or lactation. Since the drug is given as one drop in each eye every waking hour, transfer to the systemic circulation should be expected, but the amount, if it occurs, has not been reported.
Immunologics
There are no reports on the use of cyclosporine (Restasis) eye drops in human pregnancy or during breastfeeding. However, data for the systemic use of the drug during these conditions has shown it to be low risk. After long term ophthalmic use, blood concentrations of the drug were below the quantitation limit of 0.1 ng/mL.
Local anesthetics
The three drugs in this class are lidocaine, proparacaine (Alcaine), and tetracaine (Altacaine, Tetravisc). There is no information regarding the use of these agents in pregnancy or lactation. Since they are used for brief periods, the risk to an embryo or nursing infant appears to be nil.
Mast cell stabilizers
There are three drugs in this class that can be used topically in the eye: cromolyn, lodoxamide (Alomide), and nedocromil (Alocril). There are no human eye data for these agents. Systemic concentrations of lodoxamide were below the detection limit of 2.5 ng/mL.
Miotics
The two miotics are acetylcholine (Miochol E) and carbachol (Carbastat, Miostat). Both are used immediately before eye surgery. The amount, if any, in the systemic circulation is unknown. No reports of human eye use in pregnancy or lactation have been found.
NSAIDs
The five drugs in this class are bromfenac (Xibrom), diclofenac (Voltaren), flurbiprofen (Ocufen), ketorolac, and nepafenac (Nevanac). Although it has not been studied, the estimated plasma level for bromfenac is less than 50 ng/mL. For diclofenac, the plasma concentration was below the limit of quantification (10 ng/mL). In a study conducted with ketorolac, only 5 of 26 subjects had detectable plasma concentrations (10.7 to 22.5 ng/mL). These levels were about 2% of the peak plasma levels after oral dosing. For nepafenac, the peak plasma concentrations of the parent drug and active metabolite were 0.3 and 0.4 ng/mL, respectively. These data suggest that the risk to the embryo-fetus or a nursing infant are nil.
Photodynamic therapy
Verteporfin (Visudyne) is given intravenously. Three reports have described its use in three pregnancies. In all cases, the infants were healthy at birth and had normal growth (Acta Ophthalmol Scand. 2004 Oct;82[5]:623-4, Eye [Lond]. 2009 Jun;23[6]:1479, and Aust N Z J Obstet Gynaecol. 2009 Apr;49[2]:236-7).
Proteolytic enzymes
No human eye data were found for ocriplasmin (Jetrea), an agent given as an intravitreal injection. Although it was not studied, detectable levels of the drug in the systemic circulation are not expected, according to the manufacturer.
Selective vascular endothelial growth factor antagonists
There are three agents in this class: aflibercept (Eylea), pegaptanib (Macugen), and ranibizumab (Lucentis). There are no human eye data for these drugs. All are given as intravitreal injection. The systemic concentrations of the three drugs were a mean 0.02 mcg/mL; 80 ng/mL (after a dose of 10 times the recommended dose); and an estimated minimum 0.22 ng/mL, respectively. These data suggest that the risk to the embryo-fetus or a nursing infant is nil.
Sympathomimetics (decongestants)
There are four ophthalmic agents in this class and none have human eye data. Naphazoline (Naphcon, Vasocon) is the only one of the four that requires a prescription. No reports describing the systemic absorption, if any, have been found. The other three agents are available over the counter. They are oxymetazoline (Visine L.R.), phenylephrine, and tetrahydrozoline (Opti-Clear). Although the amount reaching the systemic circulation was not provided by the manufacturers for these three agents, a caution was placed on phenylephrine stating that systemic absorption, although rare, might cause alpha-adrenergic effects, such as a rise in blood pressure and reflex atropine-sensitive bradycardia.
The risk to the embryo-fetus or nursing infant from ophthalmic drugs appears to be low, with the possible exception of phenylephrine. Nevertheless, if any of these agents are used in pregnancy or during breastfeeding, careful assessment of the embryo-fetus and nursing infant should be conducted. Moreover, research on the potential effects of ophthalmic drugs on the embryo-fetus and nursing infant are desperately needed.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
A number of drugs are available for ophthalmic use. This review focuses on single drug products and, although combination drug products are not discussed, these formulations typically include the drugs reviewed here.
Since it appears that ophthalmic medications are commonly used for a wide range of conditions and ages, one would expect to see numerous reports of their use in the eyes of pregnant or breastfeeding patients. Unfortunately, the opposite is true. The majority of the drugs have no human pregnancy or lactation data. When there are human data, it invariably involves the systemic use of the drug for other indications, rather than its ophthalmic use. Moreover, the animal reproduction data are usually not relevant because they involve systemic drugs (e.g., IV or oral). Consequently, determining the level of risk an ophthalmic drug presents to an embryo and/or fetus is primarily based on time and dose, the two cardinal principles of teratology.
Avoiding exposure during organogenesis – the period when a drug can cause developmental toxicity (altered growth, structural anomalies, functional and/or behavioral deficits, or death) – is usually best, but may not be possible in some cases, including glaucoma, eye infections, and eye surgery. Fortunately, the systemic concentrations of drugs applied topically to the eye are typically thought to be low, even though the levels of most drugs have not been studied. Thus, the risk to the embryo and/or fetus in most cases can be considered low and the drug classified as compatible in pregnancy and breastfeeding.
If a topical eye drug must be used during pregnancy or lactation, teach the patient how to decrease the amount of drug reaching the systemic circulation. This involves placing pressure over the tear duct in the corner of the eye for 1 minute or more, then removing any excess solution with absorbent tissue.
In the sections below, drugs are shown by indication or by pharmacologic class. The term “human eye data” refers to the use of the drug in pregnancy and/or lactation.
Glaucoma
If you are caring for a pregnant patient who is being treated for glaucoma, two recent reviews may be helpful: Surv Ophthalmol. 2011 Jul-Aug;56(4):324-35 and Curr Opin Ophthalmol. 2014 Mar;25(2):93-7.
Sympathomimetics (alpha-adrenergic agonists) include apraclonidine (Iopidine), which has no human eye data, and brimonidine (Alphagan P), which has one case report in pregnancy and breastfeeding showing no fetal or nursing infant harm.
Four of the five beta-adrenergic blockers have no human eye data: betaxolol (Betoptic), carteolol (Ocupress), levobunolol (Betagan), and metipranolol, but there are two case reports for timolol (Betimol, Istalol, Timoptic, Timoptic-XE) showing no fetal harm in one newborn and growth restriction in the other.
Among the miotics, there are limited human eye data for pilocarpine (Isopto Carpine) and no fetal harm was observed. For echothiophate iodide (Phospholine Iodide), there is one case report of a normal full-term infant whose mother was treated with the agent up to 32 weeks’ gestation and then with pilocarpine for 8 weeks (Arch Ophthalmol. 1968 Mar;79[3]:283-5).
Carbonic anhydrase inhibitors include brinzolamide (Azopt), which has no human eye data, and dorzolamide (Trusopt), which has growth restriction in one case treated with fixed combination of dorzolamide and timolol. There are no human eye data for unoprostone isopropyl (Rescula), a synthetic docosanoid.
Of the four prostaglandin analogs, three have no human eye data, bimatoprost (Lumigan), tafluprost (Zioptan), and travoprost (Travatan Z). There were 11 pregnancies exposed to latanoprost (Xalatan). The outcomes of these cases were one lost to follow-up, one miscarriage, and nine infants without congenital anomalies (Am J Ophthalmol. 2004 Aug;138[2]:305-6).
Mitomycin (Mitosol) is an antimetabolite that is given topically to the surgical site of glaucoma filtration surgery. No reports of its use in pregnant humans have been located. According to the manufacturer, clinically significant systemic concentrations are not expected.
Antiseptics
Povidone-iodine (Betadine) is indicated for prepping of the periocular region. There does not appear to be any risk to the embryo-fetus or nursing infant from this indication.
Antihistamines
The four ophthalmic agents in this class are alcaftadine (Lastacaft), azelastine (Optivar), emedastine (Emadine), and epinastine (Elestat). There are no human eye data for these agents but, like antihistamines given systemically, they are probably compatible in pregnancy and lactation.
Antihistamine-mast cell stabilizers
There are no human eye data for bepotastine (Bepreve), ketotifen (Alaway), and olopatadine (Pataday, Patanol). Peak plasma concentrations of bepotastine were 5.1-7.3 ng/mL for 1-2 hours after instillation and were less than 2 ng/mL at 24 hours. It appears that these drugs can also be classified as compatible in pregnancy and lactation.
Anti-infectives
Ten anti-infectives are available for topical treatment of eye infections (systemic concentrations if known): besifloxacin (Besivance) (0.43 ng/mL), ciprofloxacin (less than 2.5 ng/mL), gentamicin, gatifloxacin (Zymaxid) (less than 5 ng/mL), levofloxacin (Iquix) (10.9 ng/mL), moxifloxacin (Vigamox) (2.7 ng/mL), ofloxacin (1.9 ng/mL), sulfacetamide (Isopto Cetamide), and tobramycin. The tenth agent, natamycin (Natacyn), is an antifungal. According to the manufacturer, systemic absorption is not expected.
None of these agents have human eye data, but they are usually considered compatible in pregnancy and breastfeeding when systemic formulations are used, so they should be compatible when used in the eye.
Antivirals
Ganciclovir (Zirgan) has no human eye data but, according to the manufacturer, the daily ophthalmic dose is about 0.04% and 0.1% of the oral and IV doses, respectively. Thus, minimal systemic exposure is expected. Trifluridine (Viroptic) also has no eye human data. As reported in the product information, detectable blood concentrations of the drug were not found in healthy normal subjects indicating that systemic absorption was negligible.
Corticosteroids
Among the nine corticosteroid products, six are suspensions or ointments, one is an injection, and two are implants. In most nonpregnant patients receiving the dexamethasone (Ozurdex) intravitreal implant, plasma dexamethasone concentrations were undetectable (less than 50 pg/mL) but, in some, ranged from 52 pg/mL to 102 pg/mL. There is only one case report describing the use of topical dexamethasone suspension (Maxidex) in pregnancy. In that case, dexamethasone and clindamycin were given in the first and second trimesters and the woman eventually gave birth to a normal full term infant (Int Ophthalmol. 1998-1999;22[2]:85-8).
For the fluocinolone (Retisert) ocular implant, systemic absorption of detectable amounts of the drug have not been observed. In a second report, a patient who had type 1 diabetes and was 6 months pregnant received a 2-mg intravitreal injection of triamcinolone (Triesence) in both eyes. No adverse effects in the mother or fetus were noted (Clin Ophthalmol. 2011;5:439-41).
There are no human eye data for five topical corticosteroids: difluprednate (Durezol), fluorometholone (Flarex, Fluor-OP, FML), loteprednol (Alrex, Lotemax), prednisolone (Econopred), and rimexolone (Vexol). Only two of the five drugs had information about systemic absorption. For difluprednate, blood levels were below the quantification limit (50 ng/mL). Extremely low levels were detected after the use of rimexolone with a mean serum concentration of 130 pg/mL (range less than 80-470 pg/mL).
Cycloplegics-mydriatics
This class includes four anticholinergic agents: atropine, cyclopentolate (Cyclogyl), homatropine, and tropicamide (Mydriacyl). Systemic absorption has not been studied for these drugs and there are no reports of their use in pregnancy or lactation.
Cystine-depleting agents
There are no reports describing the use of cysteamine (Cystaran) in human pregnancy or lactation. Since the drug is given as one drop in each eye every waking hour, transfer to the systemic circulation should be expected, but the amount, if it occurs, has not been reported.
Immunologics
There are no reports on the use of cyclosporine (Restasis) eye drops in human pregnancy or during breastfeeding. However, data for the systemic use of the drug during these conditions has shown it to be low risk. After long term ophthalmic use, blood concentrations of the drug were below the quantitation limit of 0.1 ng/mL.
Local anesthetics
The three drugs in this class are lidocaine, proparacaine (Alcaine), and tetracaine (Altacaine, Tetravisc). There is no information regarding the use of these agents in pregnancy or lactation. Since they are used for brief periods, the risk to an embryo or nursing infant appears to be nil.
Mast cell stabilizers
There are three drugs in this class that can be used topically in the eye: cromolyn, lodoxamide (Alomide), and nedocromil (Alocril). There are no human eye data for these agents. Systemic concentrations of lodoxamide were below the detection limit of 2.5 ng/mL.
Miotics
The two miotics are acetylcholine (Miochol E) and carbachol (Carbastat, Miostat). Both are used immediately before eye surgery. The amount, if any, in the systemic circulation is unknown. No reports of human eye use in pregnancy or lactation have been found.
NSAIDs
The five drugs in this class are bromfenac (Xibrom), diclofenac (Voltaren), flurbiprofen (Ocufen), ketorolac, and nepafenac (Nevanac). Although it has not been studied, the estimated plasma level for bromfenac is less than 50 ng/mL. For diclofenac, the plasma concentration was below the limit of quantification (10 ng/mL). In a study conducted with ketorolac, only 5 of 26 subjects had detectable plasma concentrations (10.7 to 22.5 ng/mL). These levels were about 2% of the peak plasma levels after oral dosing. For nepafenac, the peak plasma concentrations of the parent drug and active metabolite were 0.3 and 0.4 ng/mL, respectively. These data suggest that the risk to the embryo-fetus or a nursing infant are nil.
Photodynamic therapy
Verteporfin (Visudyne) is given intravenously. Three reports have described its use in three pregnancies. In all cases, the infants were healthy at birth and had normal growth (Acta Ophthalmol Scand. 2004 Oct;82[5]:623-4, Eye [Lond]. 2009 Jun;23[6]:1479, and Aust N Z J Obstet Gynaecol. 2009 Apr;49[2]:236-7).
Proteolytic enzymes
No human eye data were found for ocriplasmin (Jetrea), an agent given as an intravitreal injection. Although it was not studied, detectable levels of the drug in the systemic circulation are not expected, according to the manufacturer.
Selective vascular endothelial growth factor antagonists
There are three agents in this class: aflibercept (Eylea), pegaptanib (Macugen), and ranibizumab (Lucentis). There are no human eye data for these drugs. All are given as intravitreal injection. The systemic concentrations of the three drugs were a mean 0.02 mcg/mL; 80 ng/mL (after a dose of 10 times the recommended dose); and an estimated minimum 0.22 ng/mL, respectively. These data suggest that the risk to the embryo-fetus or a nursing infant is nil.
Sympathomimetics (decongestants)
There are four ophthalmic agents in this class and none have human eye data. Naphazoline (Naphcon, Vasocon) is the only one of the four that requires a prescription. No reports describing the systemic absorption, if any, have been found. The other three agents are available over the counter. They are oxymetazoline (Visine L.R.), phenylephrine, and tetrahydrozoline (Opti-Clear). Although the amount reaching the systemic circulation was not provided by the manufacturers for these three agents, a caution was placed on phenylephrine stating that systemic absorption, although rare, might cause alpha-adrenergic effects, such as a rise in blood pressure and reflex atropine-sensitive bradycardia.
The risk to the embryo-fetus or nursing infant from ophthalmic drugs appears to be low, with the possible exception of phenylephrine. Nevertheless, if any of these agents are used in pregnancy or during breastfeeding, careful assessment of the embryo-fetus and nursing infant should be conducted. Moreover, research on the potential effects of ophthalmic drugs on the embryo-fetus and nursing infant are desperately needed.
Mr. Briggs is clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He has no relevant financial disclosures.
Nipple Raynaud’s can freeze out breastfeeding desire
NEW YORK – The “perfect storm” of pregnancy and lactation can throw breastfeeding moms into a painful deep freeze.
Almost 25% of women with lactation pain may actually be experiencing symptoms of Raynaud’s, Dr. Honor Fullerton Stone said at the American Academy of Dermatology summer meeting.
“Breastfeeding mothers are in a perfect storm,” of physical developments that predispose them to this vasoconstrictive phenomenon, said Dr. Fullerton Stone, who practices dermatology in Menlo Park, Ca. “Estrogen increases the alpha-adrenergic receptors on smooth muscle. Nerve irritation from constant breastfeeding upregulates those receptors. And emotional stress – crying baby, anyone? – increases epinephrine, which contributes further to vasoconstriction.”
She conducted a review of 88 of her own patients with nursing-related breast pain. Of these, about a quarter ended up with a diagnosis of nipple vasoconstriction. None of the women had a history of Raynaud’s disease.
Pain during a nursing session is the presenting complaint, but breastfeeding pain is incredibly nonspecific as a symptom. It’s the quality of the pain, Dr Stone said, that should ring a bell.
“There’s let-down pain, which occurs at latch and then comes on again later, with refill. There’s pain from candidiasis, which is dramatic at latch, like a radiating heat, but goes away rapidly after a few days of antifungals,” she noted. But with Raynaud’s, “the pain is persistent. It’s throbbing, which makes sense since it’s vascular. And it’s constant,” lasting through every nursing session, which she said is the kind of experience that makes mothers stop breastfeeding.
Since pain is such a pervasive symptom in breastfeeding complaints, women with vasoconstriction of the nipple are often misdiagnosed. They can go for months trying to improve latch technique or receiving antifungal therapy with no improvement, she said.
Dr. Stone considers a diagnosis of Raynaud’s if two of the following criteria are met:
• Color change of nipple, cold sensitivity, or color change of acral surfaces with cold exposure.
• Chronic deep breast pain for 4 or more weeks.
• Failure of oral antifungals and or antibiotics.
Treatment is both supportive and systemic and avoiding cold is key. She recalled a young mother who arrived in her office bundled up in a down parka on a warm California spring day. “I don’t know why, but this really seems to help,” she said.
“I suggest taking two hot showers a day and all the better if they can be right before nursing. Hot pads and compresses are not going to help. You need to warm up the entire body to calm this vascular reactivity.”
Women should also avoid consuming anything that can cause vasoconstriction, including caffeine and tobacco, she advised.
Nifedipine is a very effective medication, and is safe for nursing infants, Dr. Stone said. The sustained-released 30 mg/day dose is typically recommended, but she has changed her thinking on this a bit.
Her internal study showed that, although 83% responded very well to the drug, about a third of them also had a vasodilation-related side effect.
“For this reason, I usually now start with the 10 mg nonsustained form, and warn about things like headache, dizziness, postural hypotension, and fainting,” she said. Typically, patients adjust well to the drug’s effects and then the dose can be individually titrated.
Dr. Fullerton Stone had no relevant financial disclosures.
On Twitter @Alz_Gal
NEW YORK – The “perfect storm” of pregnancy and lactation can throw breastfeeding moms into a painful deep freeze.
Almost 25% of women with lactation pain may actually be experiencing symptoms of Raynaud’s, Dr. Honor Fullerton Stone said at the American Academy of Dermatology summer meeting.
“Breastfeeding mothers are in a perfect storm,” of physical developments that predispose them to this vasoconstrictive phenomenon, said Dr. Fullerton Stone, who practices dermatology in Menlo Park, Ca. “Estrogen increases the alpha-adrenergic receptors on smooth muscle. Nerve irritation from constant breastfeeding upregulates those receptors. And emotional stress – crying baby, anyone? – increases epinephrine, which contributes further to vasoconstriction.”
She conducted a review of 88 of her own patients with nursing-related breast pain. Of these, about a quarter ended up with a diagnosis of nipple vasoconstriction. None of the women had a history of Raynaud’s disease.
Pain during a nursing session is the presenting complaint, but breastfeeding pain is incredibly nonspecific as a symptom. It’s the quality of the pain, Dr Stone said, that should ring a bell.
“There’s let-down pain, which occurs at latch and then comes on again later, with refill. There’s pain from candidiasis, which is dramatic at latch, like a radiating heat, but goes away rapidly after a few days of antifungals,” she noted. But with Raynaud’s, “the pain is persistent. It’s throbbing, which makes sense since it’s vascular. And it’s constant,” lasting through every nursing session, which she said is the kind of experience that makes mothers stop breastfeeding.
Since pain is such a pervasive symptom in breastfeeding complaints, women with vasoconstriction of the nipple are often misdiagnosed. They can go for months trying to improve latch technique or receiving antifungal therapy with no improvement, she said.
Dr. Stone considers a diagnosis of Raynaud’s if two of the following criteria are met:
• Color change of nipple, cold sensitivity, or color change of acral surfaces with cold exposure.
• Chronic deep breast pain for 4 or more weeks.
• Failure of oral antifungals and or antibiotics.
Treatment is both supportive and systemic and avoiding cold is key. She recalled a young mother who arrived in her office bundled up in a down parka on a warm California spring day. “I don’t know why, but this really seems to help,” she said.
“I suggest taking two hot showers a day and all the better if they can be right before nursing. Hot pads and compresses are not going to help. You need to warm up the entire body to calm this vascular reactivity.”
Women should also avoid consuming anything that can cause vasoconstriction, including caffeine and tobacco, she advised.
Nifedipine is a very effective medication, and is safe for nursing infants, Dr. Stone said. The sustained-released 30 mg/day dose is typically recommended, but she has changed her thinking on this a bit.
Her internal study showed that, although 83% responded very well to the drug, about a third of them also had a vasodilation-related side effect.
“For this reason, I usually now start with the 10 mg nonsustained form, and warn about things like headache, dizziness, postural hypotension, and fainting,” she said. Typically, patients adjust well to the drug’s effects and then the dose can be individually titrated.
Dr. Fullerton Stone had no relevant financial disclosures.
On Twitter @Alz_Gal
NEW YORK – The “perfect storm” of pregnancy and lactation can throw breastfeeding moms into a painful deep freeze.
Almost 25% of women with lactation pain may actually be experiencing symptoms of Raynaud’s, Dr. Honor Fullerton Stone said at the American Academy of Dermatology summer meeting.
“Breastfeeding mothers are in a perfect storm,” of physical developments that predispose them to this vasoconstrictive phenomenon, said Dr. Fullerton Stone, who practices dermatology in Menlo Park, Ca. “Estrogen increases the alpha-adrenergic receptors on smooth muscle. Nerve irritation from constant breastfeeding upregulates those receptors. And emotional stress – crying baby, anyone? – increases epinephrine, which contributes further to vasoconstriction.”
She conducted a review of 88 of her own patients with nursing-related breast pain. Of these, about a quarter ended up with a diagnosis of nipple vasoconstriction. None of the women had a history of Raynaud’s disease.
Pain during a nursing session is the presenting complaint, but breastfeeding pain is incredibly nonspecific as a symptom. It’s the quality of the pain, Dr Stone said, that should ring a bell.
“There’s let-down pain, which occurs at latch and then comes on again later, with refill. There’s pain from candidiasis, which is dramatic at latch, like a radiating heat, but goes away rapidly after a few days of antifungals,” she noted. But with Raynaud’s, “the pain is persistent. It’s throbbing, which makes sense since it’s vascular. And it’s constant,” lasting through every nursing session, which she said is the kind of experience that makes mothers stop breastfeeding.
Since pain is such a pervasive symptom in breastfeeding complaints, women with vasoconstriction of the nipple are often misdiagnosed. They can go for months trying to improve latch technique or receiving antifungal therapy with no improvement, she said.
Dr. Stone considers a diagnosis of Raynaud’s if two of the following criteria are met:
• Color change of nipple, cold sensitivity, or color change of acral surfaces with cold exposure.
• Chronic deep breast pain for 4 or more weeks.
• Failure of oral antifungals and or antibiotics.
Treatment is both supportive and systemic and avoiding cold is key. She recalled a young mother who arrived in her office bundled up in a down parka on a warm California spring day. “I don’t know why, but this really seems to help,” she said.
“I suggest taking two hot showers a day and all the better if they can be right before nursing. Hot pads and compresses are not going to help. You need to warm up the entire body to calm this vascular reactivity.”
Women should also avoid consuming anything that can cause vasoconstriction, including caffeine and tobacco, she advised.
Nifedipine is a very effective medication, and is safe for nursing infants, Dr. Stone said. The sustained-released 30 mg/day dose is typically recommended, but she has changed her thinking on this a bit.
Her internal study showed that, although 83% responded very well to the drug, about a third of them also had a vasodilation-related side effect.
“For this reason, I usually now start with the 10 mg nonsustained form, and warn about things like headache, dizziness, postural hypotension, and fainting,” she said. Typically, patients adjust well to the drug’s effects and then the dose can be individually titrated.
Dr. Fullerton Stone had no relevant financial disclosures.
On Twitter @Alz_Gal
AT THE AAD SUMMER ACADEMY 2015
Pregnancy registries add to the clinical picture
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of human pregnancy experience. The new Food and Drug Administration prescription drug information format may increase the amount of information available, at least from registries conducted by drug manufacturers, because they will be required to list all substantive changes made within the year. Although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of pregnancy registries are their prospective nature and enrollment across a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions).
Registries can identify early signals of teratogenicity, but they have several limitations, including voluntary reporting that results in selection bias, pregnancies lost to follow-up that may have had different outcomes than those with documented outcomes, and a lack of control groups (with some exceptions).
Other limitations are that registry cases are not representative of target populations, and pregnancies lost to follow-up lack details on elective terminations and fetal deaths without birth defects, as well as all spontaneous abortions. Additionally, publication of results may be delayed and does not appear in a peer-reviewed journal.
Since the total number of exposed pregnancies is unknown, registry data cannot be used to calculate prevalence, but can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports, which are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. However, they may be helpful in detecting unusual patterns of birth defects.
MothertoBaby Registry
The large Organization of Teratology Information Specialists (OTIS) MothertoBaby registry (877-311-8972) involves four different categories: autoimmune diseases(rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma; antiviral agents; and vaccines.
The drugs for autoimmune diseases are tocilizumab (Actemra), leflunomide (Arava), teriflunomide (Aubagio), certolizumab pegol (Cimzia), etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), apremilast (Otezla), ustekinumab (Stelara), tofacitinib (Xeljanz), infliximab (Remicade), and methotrexate.
The asthma drugs are formoterol (Foradil, Perforomist, Symbicort, Dulera), albuterol, levalbuterol, metaproterenol, pirbuterol (Maxair), and terbutaline.
The antiviral agents are oseltamivir (Tamiflu) and zanamivir (Relenza).
The vaccines include pertussis (Tdap), seasonal influenza, and meningococcal disease (Menveo). In addition to a control group in each category, a unique aspect of this registry is that many exposed infants will undergo dysmorphology examinations.
Vaccine registries
A second vaccine for meningococcal disease (Menactra) is being studied in the Menactra vaccine Pregnancy Registry (800-822-2463 / www.sanofipasteurpregnancy.com).
Two other registries also include influenza vaccines: Flu vaccine Pregnancy Registry, PPD (Flucelvax) (877-413-4759 / email: [email protected]) and the GSK Seasonal Influenza Vaccine Pregnancy Registry, GlaxoSmithKline (888-825-5249 / pregnancyregistry.gsk.com/seasonalinfluenzavaccines.html), which includes the Fluarix and FluLaval vaccines.
Asthma
Women with asthma who are being treated with omalizumab (Xolair) are the target population for the EXPECT Pregnancy Registry (866-496-5247 Option 3 / www.xolairpregnancyregistry.com).
GSK registries
GlaxoSmithKline is also conducting three other registries: the Belimumab (Benlysta) Pregnancy Registry for patients with systemic lupus erythematosus treated with belimumab (877-681-6296 / [email protected]); and the Twinrix Pregnancy Registry for women who have received the Twinrix hepatitis A and B vaccine (888-825-5249).
Atypical antipsychotics
TheNational Pregnancy Registry for Atypical Antipsychotics (866-961-2388 / registry@womens mentalhealth.org) is studying 10 drugs: aripiprazole (Abilify), clozapine (Clozaril), iloperidone (Fanapt), paliperidone (Invega), lurasidone (Latuda), risperidone (Risperdal), asenapine (Saphris), quetiapine (Seroquel), ziprasidone (Geodon), and olanzapine (Zyprexa).
TAPP registry
Acitretin is under study by the Take Action to Prevent Pregnancy (T.A.P.P.) registry (855-850-2138 / www.tevagenerics.com/acitretin).
MPS I registry
The MPS I (Mucopolysaccharidosis I) Registry, Genzyme Corp (617-591-5500 / [email protected]), is studying the use of laronidase (Aldurazyme) for Hurler syndrome, Scheie syndrome, and Hurler-Scheie syndrome.
MPS VI registry
The use of galsulfase (Naglazyme) for Maroteaux-Lamy syndrome during pregnancy is under study by the Mucopolysaccharidosis VI (MPS VI) Clinical Surveillance Program(415-506-6849 or 415-506-6703)
Epilepsy drugs
The Antiepileptic Drug Pregnancy Registry (888-233-2334 / www.aedpregnancyregistry.org) is studying antiepileptic drugs.
Type 2 diabetes
The Exenatide Pregnancy Registry, conducted by INC Research for AstraZeneca (800-633-9081 / www.exenatidepregnancyregistry.com), examines the use of exenatide (Byetta, Bydureon) for the treatment of type 2 diabetes.
Renal transplant
Renal transplant patients exposed to mycophenolate (Cellcept) can be enrolled in the Mycophenolate Pregnancy Registry (800-617-8191) or the National Transplantation Pregnancy Registry (877-955-6877). The NTPR is enrolling renal transplant patients exposed to belatacept (Nulojix).
Cymbalta
The antidepressant duloxetine (Cymbalta), when used for major depressive or generalized anxiety disorders, diabetic peripheral neuropathic pain, or fibromyalgia is being studied by the Cymbalta Pregnancy Registry, INC Research ([email protected] / www.cymbaltapregancyregistry.com).
Fabry disease
The Fabry Registry, Genzyme Corp (617-581-5500 / [email protected]) is studying the use in pregnancy of agalsidase beta (Fabrazyme) for Fabry disease.
MS registry
Novartis Pharmaceuticals is conducting the Gilenya (fingolimod) Pregnancy Registry (877-598-7237 / [email protected]) for patients with multiple sclerosis who are taking fingolimod (Gilenya).
Cancer
The MotHER Pregnancy Registry, INC Research (800-690-6720 / [email protected] / themotherpregnancyregistry.com) is enrolling breast cancer patients who have been treated during pregnancy with ado-trastuzumab (Kadcyla), trastuzumab (Herceptin), or pertuzumab (Perjeta).
Merck registries
Merck Pregnancy Registries (800-986-8999) include a registry for type 2 diabetes – sitagliptin/metformin (Janumet) or sitagliptin (Januvia) (www.merckpregancyregistries.com/januvia.html); a registry for rizatriptan (Maxalt) (www.merckpregancyregistries.com/maxalt.html), for migraine headaches; and a registry for the 9-valent human papillomavirus (HPV) (Gardasil 9) vaccine (www.merckpregnancyregistries.com/gardasil9.html).
The registry for the asthma drug montelukast (Singulair) and the registry for Gardasil, the quadrivalent HPV vaccine, are now closed to new enrollment, but new cases of pregnancy exposures can be reported to the company at: 877-888-4231.
Osteoporosis
Amgen’s Pregnancy Surveillance Program (800-772-6436) is enrolling pregnant subjects with osteoporosis who are being treated with denosumab (Prolia).
Hepatitis C
The Ribavirin Pregnancy Registry, INC Research (800-593-2214/ [email protected] / ribavirinpregnancyregistry.com) is looking for subjects with hepatitis C who have been treated with ribavirin (Copegus).
Fibromyalgia
The Savella Pregnancy Registry (877-643-3010/ [email protected] / savellapregnancyregistry.com) is looking for patients with fibromyalgia who are being treated with milnacipran (Savella).
Skin infections
An antibacterial, telavancin (Vibativ), indicated for skin infections is being studied by the Vibativ Pregnancy Registry (888-658-4228 / www.clinicaltrial.gov).
Pompe disease
Pregnant women treated with alglucosidase alfa (Myozyme) for Pompe disease can enroll in the Pompe Registry (800-745-4447 x 15500 / www.pompe.com/en/healthcare-professionals/pompe-registry.aspx).
Sleep disorders
Armodafinil (Nuvigil) used to treat excessive sleepiness associated with narcolepsy and other sleep disorders is being studied in the Nuvigil Pregnancy Registry (866-404-4106 / www.nuvigilpregnancyregistry.com). A second drug with the same indication and telephone number, modafinil (Provigil), is in the Provigil Pregnancy Registry (provigilpregnancyregistry.com).
Additional details, including fax numbers and addresses of the registries reviewed above, can be obtained from the FDA website, List of Pregnancy Exposure Registries. Since the strength of a registry is based on numbers, health care professionals are encouraged to enroll potential subjects or have their patients call to enroll themselves.
Before retirement, Mr. Briggs was a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; he remains a clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He had no relevant financial disclosures. Contact him at [email protected].
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of human pregnancy experience. The new Food and Drug Administration prescription drug information format may increase the amount of information available, at least from registries conducted by drug manufacturers, because they will be required to list all substantive changes made within the year. Although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of pregnancy registries are their prospective nature and enrollment across a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions).
Registries can identify early signals of teratogenicity, but they have several limitations, including voluntary reporting that results in selection bias, pregnancies lost to follow-up that may have had different outcomes than those with documented outcomes, and a lack of control groups (with some exceptions).
Other limitations are that registry cases are not representative of target populations, and pregnancies lost to follow-up lack details on elective terminations and fetal deaths without birth defects, as well as all spontaneous abortions. Additionally, publication of results may be delayed and does not appear in a peer-reviewed journal.
Since the total number of exposed pregnancies is unknown, registry data cannot be used to calculate prevalence, but can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports, which are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. However, they may be helpful in detecting unusual patterns of birth defects.
MothertoBaby Registry
The large Organization of Teratology Information Specialists (OTIS) MothertoBaby registry (877-311-8972) involves four different categories: autoimmune diseases(rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma; antiviral agents; and vaccines.
The drugs for autoimmune diseases are tocilizumab (Actemra), leflunomide (Arava), teriflunomide (Aubagio), certolizumab pegol (Cimzia), etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), apremilast (Otezla), ustekinumab (Stelara), tofacitinib (Xeljanz), infliximab (Remicade), and methotrexate.
The asthma drugs are formoterol (Foradil, Perforomist, Symbicort, Dulera), albuterol, levalbuterol, metaproterenol, pirbuterol (Maxair), and terbutaline.
The antiviral agents are oseltamivir (Tamiflu) and zanamivir (Relenza).
The vaccines include pertussis (Tdap), seasonal influenza, and meningococcal disease (Menveo). In addition to a control group in each category, a unique aspect of this registry is that many exposed infants will undergo dysmorphology examinations.
Vaccine registries
A second vaccine for meningococcal disease (Menactra) is being studied in the Menactra vaccine Pregnancy Registry (800-822-2463 / www.sanofipasteurpregnancy.com).
Two other registries also include influenza vaccines: Flu vaccine Pregnancy Registry, PPD (Flucelvax) (877-413-4759 / email: [email protected]) and the GSK Seasonal Influenza Vaccine Pregnancy Registry, GlaxoSmithKline (888-825-5249 / pregnancyregistry.gsk.com/seasonalinfluenzavaccines.html), which includes the Fluarix and FluLaval vaccines.
Asthma
Women with asthma who are being treated with omalizumab (Xolair) are the target population for the EXPECT Pregnancy Registry (866-496-5247 Option 3 / www.xolairpregnancyregistry.com).
GSK registries
GlaxoSmithKline is also conducting three other registries: the Belimumab (Benlysta) Pregnancy Registry for patients with systemic lupus erythematosus treated with belimumab (877-681-6296 / [email protected]); and the Twinrix Pregnancy Registry for women who have received the Twinrix hepatitis A and B vaccine (888-825-5249).
Atypical antipsychotics
TheNational Pregnancy Registry for Atypical Antipsychotics (866-961-2388 / registry@womens mentalhealth.org) is studying 10 drugs: aripiprazole (Abilify), clozapine (Clozaril), iloperidone (Fanapt), paliperidone (Invega), lurasidone (Latuda), risperidone (Risperdal), asenapine (Saphris), quetiapine (Seroquel), ziprasidone (Geodon), and olanzapine (Zyprexa).
TAPP registry
Acitretin is under study by the Take Action to Prevent Pregnancy (T.A.P.P.) registry (855-850-2138 / www.tevagenerics.com/acitretin).
MPS I registry
The MPS I (Mucopolysaccharidosis I) Registry, Genzyme Corp (617-591-5500 / [email protected]), is studying the use of laronidase (Aldurazyme) for Hurler syndrome, Scheie syndrome, and Hurler-Scheie syndrome.
MPS VI registry
The use of galsulfase (Naglazyme) for Maroteaux-Lamy syndrome during pregnancy is under study by the Mucopolysaccharidosis VI (MPS VI) Clinical Surveillance Program(415-506-6849 or 415-506-6703)
Epilepsy drugs
The Antiepileptic Drug Pregnancy Registry (888-233-2334 / www.aedpregnancyregistry.org) is studying antiepileptic drugs.
Type 2 diabetes
The Exenatide Pregnancy Registry, conducted by INC Research for AstraZeneca (800-633-9081 / www.exenatidepregnancyregistry.com), examines the use of exenatide (Byetta, Bydureon) for the treatment of type 2 diabetes.
Renal transplant
Renal transplant patients exposed to mycophenolate (Cellcept) can be enrolled in the Mycophenolate Pregnancy Registry (800-617-8191) or the National Transplantation Pregnancy Registry (877-955-6877). The NTPR is enrolling renal transplant patients exposed to belatacept (Nulojix).
Cymbalta
The antidepressant duloxetine (Cymbalta), when used for major depressive or generalized anxiety disorders, diabetic peripheral neuropathic pain, or fibromyalgia is being studied by the Cymbalta Pregnancy Registry, INC Research ([email protected] / www.cymbaltapregancyregistry.com).
Fabry disease
The Fabry Registry, Genzyme Corp (617-581-5500 / [email protected]) is studying the use in pregnancy of agalsidase beta (Fabrazyme) for Fabry disease.
MS registry
Novartis Pharmaceuticals is conducting the Gilenya (fingolimod) Pregnancy Registry (877-598-7237 / [email protected]) for patients with multiple sclerosis who are taking fingolimod (Gilenya).
Cancer
The MotHER Pregnancy Registry, INC Research (800-690-6720 / [email protected] / themotherpregnancyregistry.com) is enrolling breast cancer patients who have been treated during pregnancy with ado-trastuzumab (Kadcyla), trastuzumab (Herceptin), or pertuzumab (Perjeta).
Merck registries
Merck Pregnancy Registries (800-986-8999) include a registry for type 2 diabetes – sitagliptin/metformin (Janumet) or sitagliptin (Januvia) (www.merckpregancyregistries.com/januvia.html); a registry for rizatriptan (Maxalt) (www.merckpregancyregistries.com/maxalt.html), for migraine headaches; and a registry for the 9-valent human papillomavirus (HPV) (Gardasil 9) vaccine (www.merckpregnancyregistries.com/gardasil9.html).
The registry for the asthma drug montelukast (Singulair) and the registry for Gardasil, the quadrivalent HPV vaccine, are now closed to new enrollment, but new cases of pregnancy exposures can be reported to the company at: 877-888-4231.
Osteoporosis
Amgen’s Pregnancy Surveillance Program (800-772-6436) is enrolling pregnant subjects with osteoporosis who are being treated with denosumab (Prolia).
Hepatitis C
The Ribavirin Pregnancy Registry, INC Research (800-593-2214/ [email protected] / ribavirinpregnancyregistry.com) is looking for subjects with hepatitis C who have been treated with ribavirin (Copegus).
Fibromyalgia
The Savella Pregnancy Registry (877-643-3010/ [email protected] / savellapregnancyregistry.com) is looking for patients with fibromyalgia who are being treated with milnacipran (Savella).
Skin infections
An antibacterial, telavancin (Vibativ), indicated for skin infections is being studied by the Vibativ Pregnancy Registry (888-658-4228 / www.clinicaltrial.gov).
Pompe disease
Pregnant women treated with alglucosidase alfa (Myozyme) for Pompe disease can enroll in the Pompe Registry (800-745-4447 x 15500 / www.pompe.com/en/healthcare-professionals/pompe-registry.aspx).
Sleep disorders
Armodafinil (Nuvigil) used to treat excessive sleepiness associated with narcolepsy and other sleep disorders is being studied in the Nuvigil Pregnancy Registry (866-404-4106 / www.nuvigilpregnancyregistry.com). A second drug with the same indication and telephone number, modafinil (Provigil), is in the Provigil Pregnancy Registry (provigilpregnancyregistry.com).
Additional details, including fax numbers and addresses of the registries reviewed above, can be obtained from the FDA website, List of Pregnancy Exposure Registries. Since the strength of a registry is based on numbers, health care professionals are encouraged to enroll potential subjects or have their patients call to enroll themselves.
Before retirement, Mr. Briggs was a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; he remains a clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He had no relevant financial disclosures. Contact him at [email protected].
Pregnancy registries are valuable sources of information. For many drugs, they are the primary source of human pregnancy experience. The new Food and Drug Administration prescription drug information format may increase the amount of information available, at least from registries conducted by drug manufacturers, because they will be required to list all substantive changes made within the year. Although most of the registries use the word “pregnancy,” it is important to note that many also enroll women who took the target drug shortly before conception.
The strengths of pregnancy registries are their prospective nature and enrollment across a wide geographical area. Typically, two types of pregnancy outcomes are obtained: those with birth defects and those without known birth defects (classified as live births, fetal deaths, and spontaneous abortions).
Registries can identify early signals of teratogenicity, but they have several limitations, including voluntary reporting that results in selection bias, pregnancies lost to follow-up that may have had different outcomes than those with documented outcomes, and a lack of control groups (with some exceptions).
Other limitations are that registry cases are not representative of target populations, and pregnancies lost to follow-up lack details on elective terminations and fetal deaths without birth defects, as well as all spontaneous abortions. Additionally, publication of results may be delayed and does not appear in a peer-reviewed journal.
Since the total number of exposed pregnancies is unknown, registry data cannot be used to calculate prevalence, but can be used to estimate the proportion of birth defects. Some registries also collect data on retrospective reports, which are less representative of the target population because they can be biased toward the reporting of more unusual and severe outcomes. However, they may be helpful in detecting unusual patterns of birth defects.
MothertoBaby Registry
The large Organization of Teratology Information Specialists (OTIS) MothertoBaby registry (877-311-8972) involves four different categories: autoimmune diseases(rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease, and multiple sclerosis); asthma; antiviral agents; and vaccines.
The drugs for autoimmune diseases are tocilizumab (Actemra), leflunomide (Arava), teriflunomide (Aubagio), certolizumab pegol (Cimzia), etanercept (Enbrel), adalimumab (Humira), abatacept (Orencia), apremilast (Otezla), ustekinumab (Stelara), tofacitinib (Xeljanz), infliximab (Remicade), and methotrexate.
The asthma drugs are formoterol (Foradil, Perforomist, Symbicort, Dulera), albuterol, levalbuterol, metaproterenol, pirbuterol (Maxair), and terbutaline.
The antiviral agents are oseltamivir (Tamiflu) and zanamivir (Relenza).
The vaccines include pertussis (Tdap), seasonal influenza, and meningococcal disease (Menveo). In addition to a control group in each category, a unique aspect of this registry is that many exposed infants will undergo dysmorphology examinations.
Vaccine registries
A second vaccine for meningococcal disease (Menactra) is being studied in the Menactra vaccine Pregnancy Registry (800-822-2463 / www.sanofipasteurpregnancy.com).
Two other registries also include influenza vaccines: Flu vaccine Pregnancy Registry, PPD (Flucelvax) (877-413-4759 / email: [email protected]) and the GSK Seasonal Influenza Vaccine Pregnancy Registry, GlaxoSmithKline (888-825-5249 / pregnancyregistry.gsk.com/seasonalinfluenzavaccines.html), which includes the Fluarix and FluLaval vaccines.
Asthma
Women with asthma who are being treated with omalizumab (Xolair) are the target population for the EXPECT Pregnancy Registry (866-496-5247 Option 3 / www.xolairpregnancyregistry.com).
GSK registries
GlaxoSmithKline is also conducting three other registries: the Belimumab (Benlysta) Pregnancy Registry for patients with systemic lupus erythematosus treated with belimumab (877-681-6296 / [email protected]); and the Twinrix Pregnancy Registry for women who have received the Twinrix hepatitis A and B vaccine (888-825-5249).
Atypical antipsychotics
TheNational Pregnancy Registry for Atypical Antipsychotics (866-961-2388 / registry@womens mentalhealth.org) is studying 10 drugs: aripiprazole (Abilify), clozapine (Clozaril), iloperidone (Fanapt), paliperidone (Invega), lurasidone (Latuda), risperidone (Risperdal), asenapine (Saphris), quetiapine (Seroquel), ziprasidone (Geodon), and olanzapine (Zyprexa).
TAPP registry
Acitretin is under study by the Take Action to Prevent Pregnancy (T.A.P.P.) registry (855-850-2138 / www.tevagenerics.com/acitretin).
MPS I registry
The MPS I (Mucopolysaccharidosis I) Registry, Genzyme Corp (617-591-5500 / [email protected]), is studying the use of laronidase (Aldurazyme) for Hurler syndrome, Scheie syndrome, and Hurler-Scheie syndrome.
MPS VI registry
The use of galsulfase (Naglazyme) for Maroteaux-Lamy syndrome during pregnancy is under study by the Mucopolysaccharidosis VI (MPS VI) Clinical Surveillance Program(415-506-6849 or 415-506-6703)
Epilepsy drugs
The Antiepileptic Drug Pregnancy Registry (888-233-2334 / www.aedpregnancyregistry.org) is studying antiepileptic drugs.
Type 2 diabetes
The Exenatide Pregnancy Registry, conducted by INC Research for AstraZeneca (800-633-9081 / www.exenatidepregnancyregistry.com), examines the use of exenatide (Byetta, Bydureon) for the treatment of type 2 diabetes.
Renal transplant
Renal transplant patients exposed to mycophenolate (Cellcept) can be enrolled in the Mycophenolate Pregnancy Registry (800-617-8191) or the National Transplantation Pregnancy Registry (877-955-6877). The NTPR is enrolling renal transplant patients exposed to belatacept (Nulojix).
Cymbalta
The antidepressant duloxetine (Cymbalta), when used for major depressive or generalized anxiety disorders, diabetic peripheral neuropathic pain, or fibromyalgia is being studied by the Cymbalta Pregnancy Registry, INC Research ([email protected] / www.cymbaltapregancyregistry.com).
Fabry disease
The Fabry Registry, Genzyme Corp (617-581-5500 / [email protected]) is studying the use in pregnancy of agalsidase beta (Fabrazyme) for Fabry disease.
MS registry
Novartis Pharmaceuticals is conducting the Gilenya (fingolimod) Pregnancy Registry (877-598-7237 / [email protected]) for patients with multiple sclerosis who are taking fingolimod (Gilenya).
Cancer
The MotHER Pregnancy Registry, INC Research (800-690-6720 / [email protected] / themotherpregnancyregistry.com) is enrolling breast cancer patients who have been treated during pregnancy with ado-trastuzumab (Kadcyla), trastuzumab (Herceptin), or pertuzumab (Perjeta).
Merck registries
Merck Pregnancy Registries (800-986-8999) include a registry for type 2 diabetes – sitagliptin/metformin (Janumet) or sitagliptin (Januvia) (www.merckpregancyregistries.com/januvia.html); a registry for rizatriptan (Maxalt) (www.merckpregancyregistries.com/maxalt.html), for migraine headaches; and a registry for the 9-valent human papillomavirus (HPV) (Gardasil 9) vaccine (www.merckpregnancyregistries.com/gardasil9.html).
The registry for the asthma drug montelukast (Singulair) and the registry for Gardasil, the quadrivalent HPV vaccine, are now closed to new enrollment, but new cases of pregnancy exposures can be reported to the company at: 877-888-4231.
Osteoporosis
Amgen’s Pregnancy Surveillance Program (800-772-6436) is enrolling pregnant subjects with osteoporosis who are being treated with denosumab (Prolia).
Hepatitis C
The Ribavirin Pregnancy Registry, INC Research (800-593-2214/ [email protected] / ribavirinpregnancyregistry.com) is looking for subjects with hepatitis C who have been treated with ribavirin (Copegus).
Fibromyalgia
The Savella Pregnancy Registry (877-643-3010/ [email protected] / savellapregnancyregistry.com) is looking for patients with fibromyalgia who are being treated with milnacipran (Savella).
Skin infections
An antibacterial, telavancin (Vibativ), indicated for skin infections is being studied by the Vibativ Pregnancy Registry (888-658-4228 / www.clinicaltrial.gov).
Pompe disease
Pregnant women treated with alglucosidase alfa (Myozyme) for Pompe disease can enroll in the Pompe Registry (800-745-4447 x 15500 / www.pompe.com/en/healthcare-professionals/pompe-registry.aspx).
Sleep disorders
Armodafinil (Nuvigil) used to treat excessive sleepiness associated with narcolepsy and other sleep disorders is being studied in the Nuvigil Pregnancy Registry (866-404-4106 / www.nuvigilpregnancyregistry.com). A second drug with the same indication and telephone number, modafinil (Provigil), is in the Provigil Pregnancy Registry (provigilpregnancyregistry.com).
Additional details, including fax numbers and addresses of the registries reviewed above, can be obtained from the FDA website, List of Pregnancy Exposure Registries. Since the strength of a registry is based on numbers, health care professionals are encouraged to enroll potential subjects or have their patients call to enroll themselves.
Before retirement, Mr. Briggs was a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; he remains a clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He had no relevant financial disclosures. Contact him at [email protected].
Q&A with the FDA: Implementing the Pregnancy and Lactation Labeling Rule
On June 30, pharmaceutical and biological manufacturers will begin implementing the Food and Drug Administration’s revised rules for pregnancy and lactation drug labeling. The Pregnancy and Lactation Labeling Rule (PLLR) amends the Physician Labeling Rule (PLR, issued in 2006) and new labels aimed at providing clearer and more accurate information on drug risks will gradually roll out over the next 5 years.
The new rule – several years in the making – replaces pregnancy categories A, B, C, D, and X with short, integrated summaries that provide physicians with more information for discussing with patients the risks and benefits of a medication. The summaries will be brief and easy to read, and will contain evidence-based information that specifically addresses drug risks for women during pregnancy and lactation, as well as for men and women of reproductive potential. Under the PLLR, the pregnancy subsection of the risk summary will include for the first time information for any drug that has a pregnancy exposure registry.
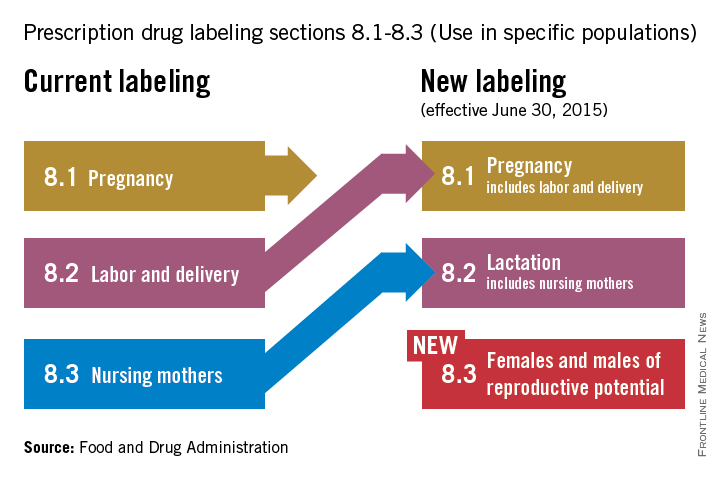
Dr. John Whyte, director of Professional Affairs and Stakeholder Engagment (PASE) at the FDA Center for Drug Evaluation and Research, answers questions about the new rule from Ob.Gyn. News journalists and our Drugs, Pregnancy & Lactation columnists.
Question: Instead of the traditional letter categories, prescribers will now have to read the pregnancy and lactation subsections of the labeling. What can the agency do to ensure prescribers are reading all of the new contextual information in the drug label?
Answer: The agency has initiated outreach efforts to help prescribers become familiar with the new information in labeling. The most important information is now under a new heading called Risk Summary. This narrative summary replaces the pregnancy letter categories with a summary of information that is known about a product. The new labeling rule explains, based on available information, the potential benefits and risks for the mother, the fetus, and the breastfeeding child. The decision to replace the pregnancy letter categories with the summary paragraph was reached after extensive consultation with experts and stakeholders who were concerned that the traditional letter categories were overly simplistic.
Q: PLLR praises the value of pregnancy exposure registries and mandates that if a registry exists for an approved product, then the label must include the registry’s website address. There is no requirement, however, that industry actually fund these registries. Why did the agency stop short of requiring companies to provide ongoing support for pregnancy exposure registries?
A: FDA has the authority to require the establishment of pregnancy registries when there is a safety concern that would benefit from the collection of data in a postapproval study, or when a particular product will be used by a large number of females of reproductive age. However, FDA does not have the authority to require companies to fund existing pregnancy exposure registries.
Q: How does the FDA plan to review all of the summary statements of available data regarding reproductive safety considering the breadth of drugs in various therapeutic areas that will need to be reviewed?
A: All labeling changes, including the changes required by the PLLR, must be submitted to the FDA for review and approval. Changes to the Pregnancy and Lactation subsections of labeling have been integrated into standard labeling review processes. In addition, the Division of Pediatric and Maternal Health, in the Office of New Drugs, works with the primary review divisions to help coordinate PLLR review processes.
Q: Are the manufacturers of generic drugs expected to “piggyback” the label for a branded molecule with respect to the pregnancy label?
A: Generic drug products (Abbreviated New Drug Applications) are required by regulation to have the “same” labeling as the reference drug listed. When the labeling is revised for the referenced drug, generic drug manufacturers also are required to update their labeling.
Q: In the new system, how will labeling stay up to date when important new data are published on a drug sometimes two to three times a year?
A: When new information becomes available that causes the labeling to be inaccurate, false, or misleading, drug manufacturers are required by regulation to update labeling.
Q: The rule is phased in over time. Drugs already on the market are given more time to switch to the new system. How long will physicians need to deal simultaneously with the old and the new formats?
A: There have been two labeling formats in use for some time as products approved prior to 2001 are not required to conform to either PLLR or PLR. The agency encourages manufacturers to voluntarily convert labeling of older products into PLR format. However, products approved prior to 2001 will be required to remove the pregnancy letter category by June 30, 2018, (3 years after the implementation of the PLLR).
Q: Does the FDA have any plans to address labeling for over-the-counter products in terms of their impact on pregnancy, lactation, and reproductive potential?
A: The PLLR does not apply to over-the-counter products. However, the agency is continually reviewing the safety of products used over the counter, including impacts on pregnancy, lactation, and reproductive potential.
Q: How does the FDA plan to assess over time the usefulness of the new labeling for prescribers and patients and make revisions?
A: The draft guidance was issued concurrently with PLLR. Based on the comments received from the public on the draft, as well as learning from the initial revisions of labeling, the guidance will be revised as needed. Guidance statements issued by FDA are regularly reviewed and revised as needed.
Dr. Whyte, a board-certified internist, is the director of Professional Affairs and Stakeholder Engagement at the FDA. Do you have other questions about the PLLR? Send them to [email protected].
On June 30, pharmaceutical and biological manufacturers will begin implementing the Food and Drug Administration’s revised rules for pregnancy and lactation drug labeling. The Pregnancy and Lactation Labeling Rule (PLLR) amends the Physician Labeling Rule (PLR, issued in 2006) and new labels aimed at providing clearer and more accurate information on drug risks will gradually roll out over the next 5 years.
The new rule – several years in the making – replaces pregnancy categories A, B, C, D, and X with short, integrated summaries that provide physicians with more information for discussing with patients the risks and benefits of a medication. The summaries will be brief and easy to read, and will contain evidence-based information that specifically addresses drug risks for women during pregnancy and lactation, as well as for men and women of reproductive potential. Under the PLLR, the pregnancy subsection of the risk summary will include for the first time information for any drug that has a pregnancy exposure registry.

Dr. John Whyte, director of Professional Affairs and Stakeholder Engagment (PASE) at the FDA Center for Drug Evaluation and Research, answers questions about the new rule from Ob.Gyn. News journalists and our Drugs, Pregnancy & Lactation columnists.
Question: Instead of the traditional letter categories, prescribers will now have to read the pregnancy and lactation subsections of the labeling. What can the agency do to ensure prescribers are reading all of the new contextual information in the drug label?
Answer: The agency has initiated outreach efforts to help prescribers become familiar with the new information in labeling. The most important information is now under a new heading called Risk Summary. This narrative summary replaces the pregnancy letter categories with a summary of information that is known about a product. The new labeling rule explains, based on available information, the potential benefits and risks for the mother, the fetus, and the breastfeeding child. The decision to replace the pregnancy letter categories with the summary paragraph was reached after extensive consultation with experts and stakeholders who were concerned that the traditional letter categories were overly simplistic.
Q: PLLR praises the value of pregnancy exposure registries and mandates that if a registry exists for an approved product, then the label must include the registry’s website address. There is no requirement, however, that industry actually fund these registries. Why did the agency stop short of requiring companies to provide ongoing support for pregnancy exposure registries?
A: FDA has the authority to require the establishment of pregnancy registries when there is a safety concern that would benefit from the collection of data in a postapproval study, or when a particular product will be used by a large number of females of reproductive age. However, FDA does not have the authority to require companies to fund existing pregnancy exposure registries.
Q: How does the FDA plan to review all of the summary statements of available data regarding reproductive safety considering the breadth of drugs in various therapeutic areas that will need to be reviewed?
A: All labeling changes, including the changes required by the PLLR, must be submitted to the FDA for review and approval. Changes to the Pregnancy and Lactation subsections of labeling have been integrated into standard labeling review processes. In addition, the Division of Pediatric and Maternal Health, in the Office of New Drugs, works with the primary review divisions to help coordinate PLLR review processes.
Q: Are the manufacturers of generic drugs expected to “piggyback” the label for a branded molecule with respect to the pregnancy label?
A: Generic drug products (Abbreviated New Drug Applications) are required by regulation to have the “same” labeling as the reference drug listed. When the labeling is revised for the referenced drug, generic drug manufacturers also are required to update their labeling.
Q: In the new system, how will labeling stay up to date when important new data are published on a drug sometimes two to three times a year?
A: When new information becomes available that causes the labeling to be inaccurate, false, or misleading, drug manufacturers are required by regulation to update labeling.
Q: The rule is phased in over time. Drugs already on the market are given more time to switch to the new system. How long will physicians need to deal simultaneously with the old and the new formats?
A: There have been two labeling formats in use for some time as products approved prior to 2001 are not required to conform to either PLLR or PLR. The agency encourages manufacturers to voluntarily convert labeling of older products into PLR format. However, products approved prior to 2001 will be required to remove the pregnancy letter category by June 30, 2018, (3 years after the implementation of the PLLR).
Q: Does the FDA have any plans to address labeling for over-the-counter products in terms of their impact on pregnancy, lactation, and reproductive potential?
A: The PLLR does not apply to over-the-counter products. However, the agency is continually reviewing the safety of products used over the counter, including impacts on pregnancy, lactation, and reproductive potential.
Q: How does the FDA plan to assess over time the usefulness of the new labeling for prescribers and patients and make revisions?
A: The draft guidance was issued concurrently with PLLR. Based on the comments received from the public on the draft, as well as learning from the initial revisions of labeling, the guidance will be revised as needed. Guidance statements issued by FDA are regularly reviewed and revised as needed.
Dr. Whyte, a board-certified internist, is the director of Professional Affairs and Stakeholder Engagement at the FDA. Do you have other questions about the PLLR? Send them to [email protected].
On June 30, pharmaceutical and biological manufacturers will begin implementing the Food and Drug Administration’s revised rules for pregnancy and lactation drug labeling. The Pregnancy and Lactation Labeling Rule (PLLR) amends the Physician Labeling Rule (PLR, issued in 2006) and new labels aimed at providing clearer and more accurate information on drug risks will gradually roll out over the next 5 years.
The new rule – several years in the making – replaces pregnancy categories A, B, C, D, and X with short, integrated summaries that provide physicians with more information for discussing with patients the risks and benefits of a medication. The summaries will be brief and easy to read, and will contain evidence-based information that specifically addresses drug risks for women during pregnancy and lactation, as well as for men and women of reproductive potential. Under the PLLR, the pregnancy subsection of the risk summary will include for the first time information for any drug that has a pregnancy exposure registry.

Dr. John Whyte, director of Professional Affairs and Stakeholder Engagment (PASE) at the FDA Center for Drug Evaluation and Research, answers questions about the new rule from Ob.Gyn. News journalists and our Drugs, Pregnancy & Lactation columnists.
Question: Instead of the traditional letter categories, prescribers will now have to read the pregnancy and lactation subsections of the labeling. What can the agency do to ensure prescribers are reading all of the new contextual information in the drug label?
Answer: The agency has initiated outreach efforts to help prescribers become familiar with the new information in labeling. The most important information is now under a new heading called Risk Summary. This narrative summary replaces the pregnancy letter categories with a summary of information that is known about a product. The new labeling rule explains, based on available information, the potential benefits and risks for the mother, the fetus, and the breastfeeding child. The decision to replace the pregnancy letter categories with the summary paragraph was reached after extensive consultation with experts and stakeholders who were concerned that the traditional letter categories were overly simplistic.
Q: PLLR praises the value of pregnancy exposure registries and mandates that if a registry exists for an approved product, then the label must include the registry’s website address. There is no requirement, however, that industry actually fund these registries. Why did the agency stop short of requiring companies to provide ongoing support for pregnancy exposure registries?
A: FDA has the authority to require the establishment of pregnancy registries when there is a safety concern that would benefit from the collection of data in a postapproval study, or when a particular product will be used by a large number of females of reproductive age. However, FDA does not have the authority to require companies to fund existing pregnancy exposure registries.
Q: How does the FDA plan to review all of the summary statements of available data regarding reproductive safety considering the breadth of drugs in various therapeutic areas that will need to be reviewed?
A: All labeling changes, including the changes required by the PLLR, must be submitted to the FDA for review and approval. Changes to the Pregnancy and Lactation subsections of labeling have been integrated into standard labeling review processes. In addition, the Division of Pediatric and Maternal Health, in the Office of New Drugs, works with the primary review divisions to help coordinate PLLR review processes.
Q: Are the manufacturers of generic drugs expected to “piggyback” the label for a branded molecule with respect to the pregnancy label?
A: Generic drug products (Abbreviated New Drug Applications) are required by regulation to have the “same” labeling as the reference drug listed. When the labeling is revised for the referenced drug, generic drug manufacturers also are required to update their labeling.
Q: In the new system, how will labeling stay up to date when important new data are published on a drug sometimes two to three times a year?
A: When new information becomes available that causes the labeling to be inaccurate, false, or misleading, drug manufacturers are required by regulation to update labeling.
Q: The rule is phased in over time. Drugs already on the market are given more time to switch to the new system. How long will physicians need to deal simultaneously with the old and the new formats?
A: There have been two labeling formats in use for some time as products approved prior to 2001 are not required to conform to either PLLR or PLR. The agency encourages manufacturers to voluntarily convert labeling of older products into PLR format. However, products approved prior to 2001 will be required to remove the pregnancy letter category by June 30, 2018, (3 years after the implementation of the PLLR).
Q: Does the FDA have any plans to address labeling for over-the-counter products in terms of their impact on pregnancy, lactation, and reproductive potential?
A: The PLLR does not apply to over-the-counter products. However, the agency is continually reviewing the safety of products used over the counter, including impacts on pregnancy, lactation, and reproductive potential.
Q: How does the FDA plan to assess over time the usefulness of the new labeling for prescribers and patients and make revisions?
A: The draft guidance was issued concurrently with PLLR. Based on the comments received from the public on the draft, as well as learning from the initial revisions of labeling, the guidance will be revised as needed. Guidance statements issued by FDA are regularly reviewed and revised as needed.
Dr. Whyte, a board-certified internist, is the director of Professional Affairs and Stakeholder Engagement at the FDA. Do you have other questions about the PLLR? Send them to [email protected].
Expert: Don’t discourage pregnancy in MS patients; manage it
INDIANAPOLIS – If you feel overwhelmed by the notion of managing multiple sclerosis patients who seek your guidance in navigating their pregnancy, you’re not alone.
Comprehensive management programs for pregnant MS patients currently do not exist, even within most dedicated MS centers and clinics, Dr. Maria K. Houtchens said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“Individual providers have an interest in this area, but there are no guidelines for most physicians or nurse practitioners to follow yet,” she said. “The level of care these patients receive varies dramatically, from one part of the country to another and from one provider to the next.”
An estimated 50% of all pregnancies in the United States and about 40% of all pregnancies worldwide are unplanned.
“I believe that women with MS should receive support and counseling from their MS specialist on issues about possible pregnancy,” said Dr. Houtchens, a neurologist who directs the Women’s Health Program at the Partners MS Center at Brigham and Women’s Hospital, Boston. “I believe that we should all feel comfortable discussing with our patients the effects of pregnancy on their MS course and the reciprocal effect on pregnancy outcomes of their disease, the genetic risk of disease in their offspring, and optimal conception timing. We need to be able to talk to them freely about disease control before, during, and after pregnancy.”
Dr. Houtchens, one of the authors of a recent multinational systematic review on the topic noted that increasing numbers of MS patients with stable disease are choosing not to become pregnant (Obstet. Gynecol. 2014;124:1157-68). Others “want to get pregnant and feel cheated out of their life goal,” she said.
A large survey of female patients with MS from Canada found that more than three-quarters had not become pregnant since being diagnosed with the disease. The most common contributing factor across both MS-related and non–MS-related categories was completion of families prior to an MS diagnosis (53%). The top MS-related reasons that contributed to not having children were symptoms interfering with parenting, burdening the partner, and finances (Mult. Scler. 2013;19:351-8).
“It’s only in the last 2-3 decades that this issue has come to the forefront in caring for MS patients,” she said. “Previous to that, a lot of colleagues would discourage patients from becoming pregnant. Some of those attitudes persist to this day. There’s a perception of disapproval from health care providers on the part of the patient, and from their family and peers, and historic misconceptions. Our goal as physicians is to help women live lives to their fullest potential with a disease that can’t be cured. We should not discourage our patients from becoming pregnant.”
To optimize chances of conception, she recommended that oral contraceptives be stopped 2-3 months prior to conception attempts, and patients should be advised to transition to mechanical birth control. The optimal “fertility window” is a 6-day period, ending with the ovulation day. This window can be estimated based on duration of menstrual cycle, cervical mucus, and basal body temperature, as well as commercially available ovulation kits. Intercourse is most likely going to result in a pregnancy if attempted within a 3-day period, ending with the ovulation day. Moderate alcohol consumption, smoking, drug use, and vaginal lubricant use decrease the chance of contraception, she said.
Assisted reproductive technologies, if associated with failed pregnancy attempts, can lead to an increased relapse rate.
“If somebody really wants to have a child and are not able to conceive on their own, they may certainly consider ART; it’s all about education,” Dr. Houtchens said. “You just need to tell them what to expect and that they might have a higher risk of relapses if their attempt is unsuccessful.”
According to the medical literature, an MS patient’s prepregnancy annualized relapse rate predicts her risk for relapse during the postpartum period. “From that perspective, if you have the luxury of time, you ideally try to make sure that her disease is stabilized for the year before she becomes pregnant,” she said. “That means if she has an active MRI or has had a couple of attacks on whatever drug she’s taking, you talk to her about that, and you change the medication and repeat the MRI after the medication is changed to try to make sure that her disease is stable before she becomes pregnant. Hopefully that will help prevent postpartum attacks.”
Dr. Houtchens recommends standard preconception care including prenatal vitamins with 0.4 mg-1 mg of daily folate, smoking and alcohol cessation, improved sleep hygiene, and vitamin D3 supplementation. Low levels of vitamin D3 are associated with adverse pregnancy outcomes, and a poorer clinical and radiologic MS course. Low levels may also be associated with increased MS risk in offspring.
“A pregnancy test should be administered prior to every treatment with a chemotherapeutic or cytotoxic agent in a woman of child-bearing age, even if she thinks her periods have ceased as a result of chemotherapy treatment,” she said. “They may have ceased due to pregnancy, and we don’t want to treat our pregnant patients with chemotherapy.”
The approximate risk of MS is 1 in 500 in the general population, 1 in 100 in people with an affected second-degree relative, 1 in 50 with an affected first-degree relative, and 1 in 4 in monozygotic twins born to an affected mother, according to Dr. Houtchens.
Babies born to MS moms face a risk of being slightly smaller for gestational age by weight (odds ratio, 1.45) but their Apgar scores are the same as their healthy mom counterpart babies. MS moms face a slight risk for operative deliveries but no increased risk for birth defects or other adverse fetal outcomes specifically related to MS. The method of labor and delivery does not impact the postpartum course of the disease, she said.
“Epidural anesthesia is perfectly safe and does not impact the postpartum course of MS,” Dr. Houtchens said. “There’s still an ongoing misconception [about this], especially among obstetric providers and anesthesiologists. Multiple studies have been published stating that there is no increased risk of relapse in these patients after they get an epidural. If they don’t want an epidural, that’s okay, but they shouldn’t be denied it because they have MS.”
Secondary MS symptoms may be affected by pregnancy, including fatigue, bladder symptoms, and mobility difficulty due to increased weight. IV corticosteroids are used widely to treat MS relapses during pregnancy, as well as in obstetrics to speed fetal lung maturity.
Steroids “cross the placental barrier and may increase the risk of cleft palate when used in the first trimester or may cause lower birth weight in the baby and earlier than expected delivery. They could also in theory delay healing for the mother after giving birth,” she said.
However, prednisone, prednisolone, and methylprednisolone can be administered with low levels of fetal exposure. “These agents are metabolized to inactive forms by 11 beta-hydroxysteroid dehydrogenase in the placenta, allowing less than 10% of the maternal dose to reach the fetus,” she said. Betamethasone and dexamethasone cross the placenta with minimal metabolism, leading to direct full-dose effects on the fetus.
Dr. Houtchens cautioned that none of the available MS drugs should be used in pregnant patients.
“It appears that pregnancy itself is protective enough that we don’t need to use a drug to keep them healthier,” she said.
For a nonlactating patient, it’s safe to resume MS therapy within 1 week after birth. For a lactating patient with previously active disease, it may be safe to administer monthly steroids or monthly IVIG, instructing them to discontinue breastfeeding for 24 hours after treatment. In breast milk, beta-interferon agents are found at 0.006% of the maternal dose. Oral small molecules such as fingolimod and dimethyl fumarate “are freely passed in breast milk at a lower level than in sera but are more likely to directly affect the infant’s immune/neurological systems,” Dr. Houtchens said. “Hepatic clearance is slower in infants. We don’t have anyone at our MS center taking any MS medications and breastfeeding. Is this the right thing to do? I don’t know. But if you have someone who really wants to breastfeed, you could theoretically put them on an injectable medication.”
MRI should be repeated within 6 months postpartum to assess radiographic disease activity, she recommended.
Dr. Houtchers pointed out that postpartum depression is common in mothers with MS, “and it’s probably under-studied in general.” In fact, the lifetime prevalence of major depressive disorder in people with MS is estimated to be approximately 50%, while the rate of suicides among people with MS is 7.5 times greater than that of the general population.
Helping MS patients navigate conception, pregnancy, and the postpartum period is just the beginning.
“You’re still going to be her doctor,” Dr. Houtchens said. “She’s still going to have that child for the rest of her life. How is she going to deal with raising the child with all of the symptoms of her disease over time? How is she going to relate to her child? You’re going to walk this road with your patients, as one of their most important health care providers.”
Dr. Houtchens disclosed that she has received research grants from Genzyme Sanofi, Biogen Idec, and Novartis. She has also served as a consultant for Teva Pharmaceuticals, Genzyme Sanofi, Questcor, Biogen Idec, and Novartis.
On Twitter @dougbrunk
INDIANAPOLIS – If you feel overwhelmed by the notion of managing multiple sclerosis patients who seek your guidance in navigating their pregnancy, you’re not alone.
Comprehensive management programs for pregnant MS patients currently do not exist, even within most dedicated MS centers and clinics, Dr. Maria K. Houtchens said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“Individual providers have an interest in this area, but there are no guidelines for most physicians or nurse practitioners to follow yet,” she said. “The level of care these patients receive varies dramatically, from one part of the country to another and from one provider to the next.”
An estimated 50% of all pregnancies in the United States and about 40% of all pregnancies worldwide are unplanned.
“I believe that women with MS should receive support and counseling from their MS specialist on issues about possible pregnancy,” said Dr. Houtchens, a neurologist who directs the Women’s Health Program at the Partners MS Center at Brigham and Women’s Hospital, Boston. “I believe that we should all feel comfortable discussing with our patients the effects of pregnancy on their MS course and the reciprocal effect on pregnancy outcomes of their disease, the genetic risk of disease in their offspring, and optimal conception timing. We need to be able to talk to them freely about disease control before, during, and after pregnancy.”
Dr. Houtchens, one of the authors of a recent multinational systematic review on the topic noted that increasing numbers of MS patients with stable disease are choosing not to become pregnant (Obstet. Gynecol. 2014;124:1157-68). Others “want to get pregnant and feel cheated out of their life goal,” she said.
A large survey of female patients with MS from Canada found that more than three-quarters had not become pregnant since being diagnosed with the disease. The most common contributing factor across both MS-related and non–MS-related categories was completion of families prior to an MS diagnosis (53%). The top MS-related reasons that contributed to not having children were symptoms interfering with parenting, burdening the partner, and finances (Mult. Scler. 2013;19:351-8).
“It’s only in the last 2-3 decades that this issue has come to the forefront in caring for MS patients,” she said. “Previous to that, a lot of colleagues would discourage patients from becoming pregnant. Some of those attitudes persist to this day. There’s a perception of disapproval from health care providers on the part of the patient, and from their family and peers, and historic misconceptions. Our goal as physicians is to help women live lives to their fullest potential with a disease that can’t be cured. We should not discourage our patients from becoming pregnant.”
To optimize chances of conception, she recommended that oral contraceptives be stopped 2-3 months prior to conception attempts, and patients should be advised to transition to mechanical birth control. The optimal “fertility window” is a 6-day period, ending with the ovulation day. This window can be estimated based on duration of menstrual cycle, cervical mucus, and basal body temperature, as well as commercially available ovulation kits. Intercourse is most likely going to result in a pregnancy if attempted within a 3-day period, ending with the ovulation day. Moderate alcohol consumption, smoking, drug use, and vaginal lubricant use decrease the chance of contraception, she said.
Assisted reproductive technologies, if associated with failed pregnancy attempts, can lead to an increased relapse rate.
“If somebody really wants to have a child and are not able to conceive on their own, they may certainly consider ART; it’s all about education,” Dr. Houtchens said. “You just need to tell them what to expect and that they might have a higher risk of relapses if their attempt is unsuccessful.”
According to the medical literature, an MS patient’s prepregnancy annualized relapse rate predicts her risk for relapse during the postpartum period. “From that perspective, if you have the luxury of time, you ideally try to make sure that her disease is stabilized for the year before she becomes pregnant,” she said. “That means if she has an active MRI or has had a couple of attacks on whatever drug she’s taking, you talk to her about that, and you change the medication and repeat the MRI after the medication is changed to try to make sure that her disease is stable before she becomes pregnant. Hopefully that will help prevent postpartum attacks.”
Dr. Houtchens recommends standard preconception care including prenatal vitamins with 0.4 mg-1 mg of daily folate, smoking and alcohol cessation, improved sleep hygiene, and vitamin D3 supplementation. Low levels of vitamin D3 are associated with adverse pregnancy outcomes, and a poorer clinical and radiologic MS course. Low levels may also be associated with increased MS risk in offspring.
“A pregnancy test should be administered prior to every treatment with a chemotherapeutic or cytotoxic agent in a woman of child-bearing age, even if she thinks her periods have ceased as a result of chemotherapy treatment,” she said. “They may have ceased due to pregnancy, and we don’t want to treat our pregnant patients with chemotherapy.”
The approximate risk of MS is 1 in 500 in the general population, 1 in 100 in people with an affected second-degree relative, 1 in 50 with an affected first-degree relative, and 1 in 4 in monozygotic twins born to an affected mother, according to Dr. Houtchens.
Babies born to MS moms face a risk of being slightly smaller for gestational age by weight (odds ratio, 1.45) but their Apgar scores are the same as their healthy mom counterpart babies. MS moms face a slight risk for operative deliveries but no increased risk for birth defects or other adverse fetal outcomes specifically related to MS. The method of labor and delivery does not impact the postpartum course of the disease, she said.
“Epidural anesthesia is perfectly safe and does not impact the postpartum course of MS,” Dr. Houtchens said. “There’s still an ongoing misconception [about this], especially among obstetric providers and anesthesiologists. Multiple studies have been published stating that there is no increased risk of relapse in these patients after they get an epidural. If they don’t want an epidural, that’s okay, but they shouldn’t be denied it because they have MS.”
Secondary MS symptoms may be affected by pregnancy, including fatigue, bladder symptoms, and mobility difficulty due to increased weight. IV corticosteroids are used widely to treat MS relapses during pregnancy, as well as in obstetrics to speed fetal lung maturity.
Steroids “cross the placental barrier and may increase the risk of cleft palate when used in the first trimester or may cause lower birth weight in the baby and earlier than expected delivery. They could also in theory delay healing for the mother after giving birth,” she said.
However, prednisone, prednisolone, and methylprednisolone can be administered with low levels of fetal exposure. “These agents are metabolized to inactive forms by 11 beta-hydroxysteroid dehydrogenase in the placenta, allowing less than 10% of the maternal dose to reach the fetus,” she said. Betamethasone and dexamethasone cross the placenta with minimal metabolism, leading to direct full-dose effects on the fetus.
Dr. Houtchens cautioned that none of the available MS drugs should be used in pregnant patients.
“It appears that pregnancy itself is protective enough that we don’t need to use a drug to keep them healthier,” she said.
For a nonlactating patient, it’s safe to resume MS therapy within 1 week after birth. For a lactating patient with previously active disease, it may be safe to administer monthly steroids or monthly IVIG, instructing them to discontinue breastfeeding for 24 hours after treatment. In breast milk, beta-interferon agents are found at 0.006% of the maternal dose. Oral small molecules such as fingolimod and dimethyl fumarate “are freely passed in breast milk at a lower level than in sera but are more likely to directly affect the infant’s immune/neurological systems,” Dr. Houtchens said. “Hepatic clearance is slower in infants. We don’t have anyone at our MS center taking any MS medications and breastfeeding. Is this the right thing to do? I don’t know. But if you have someone who really wants to breastfeed, you could theoretically put them on an injectable medication.”
MRI should be repeated within 6 months postpartum to assess radiographic disease activity, she recommended.
Dr. Houtchers pointed out that postpartum depression is common in mothers with MS, “and it’s probably under-studied in general.” In fact, the lifetime prevalence of major depressive disorder in people with MS is estimated to be approximately 50%, while the rate of suicides among people with MS is 7.5 times greater than that of the general population.
Helping MS patients navigate conception, pregnancy, and the postpartum period is just the beginning.
“You’re still going to be her doctor,” Dr. Houtchens said. “She’s still going to have that child for the rest of her life. How is she going to deal with raising the child with all of the symptoms of her disease over time? How is she going to relate to her child? You’re going to walk this road with your patients, as one of their most important health care providers.”
Dr. Houtchens disclosed that she has received research grants from Genzyme Sanofi, Biogen Idec, and Novartis. She has also served as a consultant for Teva Pharmaceuticals, Genzyme Sanofi, Questcor, Biogen Idec, and Novartis.
On Twitter @dougbrunk
INDIANAPOLIS – If you feel overwhelmed by the notion of managing multiple sclerosis patients who seek your guidance in navigating their pregnancy, you’re not alone.
Comprehensive management programs for pregnant MS patients currently do not exist, even within most dedicated MS centers and clinics, Dr. Maria K. Houtchens said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“Individual providers have an interest in this area, but there are no guidelines for most physicians or nurse practitioners to follow yet,” she said. “The level of care these patients receive varies dramatically, from one part of the country to another and from one provider to the next.”
An estimated 50% of all pregnancies in the United States and about 40% of all pregnancies worldwide are unplanned.
“I believe that women with MS should receive support and counseling from their MS specialist on issues about possible pregnancy,” said Dr. Houtchens, a neurologist who directs the Women’s Health Program at the Partners MS Center at Brigham and Women’s Hospital, Boston. “I believe that we should all feel comfortable discussing with our patients the effects of pregnancy on their MS course and the reciprocal effect on pregnancy outcomes of their disease, the genetic risk of disease in their offspring, and optimal conception timing. We need to be able to talk to them freely about disease control before, during, and after pregnancy.”
Dr. Houtchens, one of the authors of a recent multinational systematic review on the topic noted that increasing numbers of MS patients with stable disease are choosing not to become pregnant (Obstet. Gynecol. 2014;124:1157-68). Others “want to get pregnant and feel cheated out of their life goal,” she said.
A large survey of female patients with MS from Canada found that more than three-quarters had not become pregnant since being diagnosed with the disease. The most common contributing factor across both MS-related and non–MS-related categories was completion of families prior to an MS diagnosis (53%). The top MS-related reasons that contributed to not having children were symptoms interfering with parenting, burdening the partner, and finances (Mult. Scler. 2013;19:351-8).
“It’s only in the last 2-3 decades that this issue has come to the forefront in caring for MS patients,” she said. “Previous to that, a lot of colleagues would discourage patients from becoming pregnant. Some of those attitudes persist to this day. There’s a perception of disapproval from health care providers on the part of the patient, and from their family and peers, and historic misconceptions. Our goal as physicians is to help women live lives to their fullest potential with a disease that can’t be cured. We should not discourage our patients from becoming pregnant.”
To optimize chances of conception, she recommended that oral contraceptives be stopped 2-3 months prior to conception attempts, and patients should be advised to transition to mechanical birth control. The optimal “fertility window” is a 6-day period, ending with the ovulation day. This window can be estimated based on duration of menstrual cycle, cervical mucus, and basal body temperature, as well as commercially available ovulation kits. Intercourse is most likely going to result in a pregnancy if attempted within a 3-day period, ending with the ovulation day. Moderate alcohol consumption, smoking, drug use, and vaginal lubricant use decrease the chance of contraception, she said.
Assisted reproductive technologies, if associated with failed pregnancy attempts, can lead to an increased relapse rate.
“If somebody really wants to have a child and are not able to conceive on their own, they may certainly consider ART; it’s all about education,” Dr. Houtchens said. “You just need to tell them what to expect and that they might have a higher risk of relapses if their attempt is unsuccessful.”
According to the medical literature, an MS patient’s prepregnancy annualized relapse rate predicts her risk for relapse during the postpartum period. “From that perspective, if you have the luxury of time, you ideally try to make sure that her disease is stabilized for the year before she becomes pregnant,” she said. “That means if she has an active MRI or has had a couple of attacks on whatever drug she’s taking, you talk to her about that, and you change the medication and repeat the MRI after the medication is changed to try to make sure that her disease is stable before she becomes pregnant. Hopefully that will help prevent postpartum attacks.”
Dr. Houtchens recommends standard preconception care including prenatal vitamins with 0.4 mg-1 mg of daily folate, smoking and alcohol cessation, improved sleep hygiene, and vitamin D3 supplementation. Low levels of vitamin D3 are associated with adverse pregnancy outcomes, and a poorer clinical and radiologic MS course. Low levels may also be associated with increased MS risk in offspring.
“A pregnancy test should be administered prior to every treatment with a chemotherapeutic or cytotoxic agent in a woman of child-bearing age, even if she thinks her periods have ceased as a result of chemotherapy treatment,” she said. “They may have ceased due to pregnancy, and we don’t want to treat our pregnant patients with chemotherapy.”
The approximate risk of MS is 1 in 500 in the general population, 1 in 100 in people with an affected second-degree relative, 1 in 50 with an affected first-degree relative, and 1 in 4 in monozygotic twins born to an affected mother, according to Dr. Houtchens.
Babies born to MS moms face a risk of being slightly smaller for gestational age by weight (odds ratio, 1.45) but their Apgar scores are the same as their healthy mom counterpart babies. MS moms face a slight risk for operative deliveries but no increased risk for birth defects or other adverse fetal outcomes specifically related to MS. The method of labor and delivery does not impact the postpartum course of the disease, she said.
“Epidural anesthesia is perfectly safe and does not impact the postpartum course of MS,” Dr. Houtchens said. “There’s still an ongoing misconception [about this], especially among obstetric providers and anesthesiologists. Multiple studies have been published stating that there is no increased risk of relapse in these patients after they get an epidural. If they don’t want an epidural, that’s okay, but they shouldn’t be denied it because they have MS.”
Secondary MS symptoms may be affected by pregnancy, including fatigue, bladder symptoms, and mobility difficulty due to increased weight. IV corticosteroids are used widely to treat MS relapses during pregnancy, as well as in obstetrics to speed fetal lung maturity.
Steroids “cross the placental barrier and may increase the risk of cleft palate when used in the first trimester or may cause lower birth weight in the baby and earlier than expected delivery. They could also in theory delay healing for the mother after giving birth,” she said.
However, prednisone, prednisolone, and methylprednisolone can be administered with low levels of fetal exposure. “These agents are metabolized to inactive forms by 11 beta-hydroxysteroid dehydrogenase in the placenta, allowing less than 10% of the maternal dose to reach the fetus,” she said. Betamethasone and dexamethasone cross the placenta with minimal metabolism, leading to direct full-dose effects on the fetus.
Dr. Houtchens cautioned that none of the available MS drugs should be used in pregnant patients.
“It appears that pregnancy itself is protective enough that we don’t need to use a drug to keep them healthier,” she said.
For a nonlactating patient, it’s safe to resume MS therapy within 1 week after birth. For a lactating patient with previously active disease, it may be safe to administer monthly steroids or monthly IVIG, instructing them to discontinue breastfeeding for 24 hours after treatment. In breast milk, beta-interferon agents are found at 0.006% of the maternal dose. Oral small molecules such as fingolimod and dimethyl fumarate “are freely passed in breast milk at a lower level than in sera but are more likely to directly affect the infant’s immune/neurological systems,” Dr. Houtchens said. “Hepatic clearance is slower in infants. We don’t have anyone at our MS center taking any MS medications and breastfeeding. Is this the right thing to do? I don’t know. But if you have someone who really wants to breastfeed, you could theoretically put them on an injectable medication.”
MRI should be repeated within 6 months postpartum to assess radiographic disease activity, she recommended.
Dr. Houtchers pointed out that postpartum depression is common in mothers with MS, “and it’s probably under-studied in general.” In fact, the lifetime prevalence of major depressive disorder in people with MS is estimated to be approximately 50%, while the rate of suicides among people with MS is 7.5 times greater than that of the general population.
Helping MS patients navigate conception, pregnancy, and the postpartum period is just the beginning.
“You’re still going to be her doctor,” Dr. Houtchens said. “She’s still going to have that child for the rest of her life. How is she going to deal with raising the child with all of the symptoms of her disease over time? How is she going to relate to her child? You’re going to walk this road with your patients, as one of their most important health care providers.”
Dr. Houtchens disclosed that she has received research grants from Genzyme Sanofi, Biogen Idec, and Novartis. She has also served as a consultant for Teva Pharmaceuticals, Genzyme Sanofi, Questcor, Biogen Idec, and Novartis.
On Twitter @dougbrunk
EXPERT ANALYSIS AT THE CMSC ANNUAL MEETING
FDA’s new labeling rule: clinical implications
As reviewed in a previous column, in December 2014, the Food and Drug Administration released the Pregnancy and Lactation Labeling Rule (PLLR), which will go into effect on June 30, 2015. This replaces and addresses the limitations of the system that has been in place for more than 30 years, which ascribed a pregnancy risk category of A,B,C,D, or X to drugs, with the purpose of informing the clinician and patient about the reproductive safety of medications during pregnancy. Though well intentioned, criticisms of this system have been abundant.
The system certainly simplified the interaction between physicians and patients, who presumably would be reassured that the risk of a certain medicine had been quantified by a regulatory body and therefore could be used as a basis for making a decision about whether or not to take a medicine during pregnancy. While the purpose of the labeling system was to provide some overarching guidance about available reproductive safety information of a medicine, it was ultimately used by clinicians and patients either to somehow garner reassurance about a medicine, or to heighten concern about a medicine.
From the outset, the system could not take into account the accruing reproductive safety information regarding compounds across therapeutic categories, and as a result, the risk category could be inadvertently reassuring or even misleading to patients with respect to medicines they might decide to stop or to continue.
With the older labeling system, some medicines are in the same category, despite very different amounts of reproductive safety information available on the drugs. In the 1990s, there were more reproductive safety data available on certain selective serotonin reuptake inhibitors (SSRIs), compared with others, but now the amount of such data available across SSRIs is fairly consistent. Yet SSRI labels have not been updated with the abundance of new reproductive safety information that has become available.
Almost 10 years ago, paroxetine (Paxil) was switched from a category C to D, when first-trimester exposure was linked to an increased risk of birth defects, particularly heart defects. But it was not switched back to category C when data became available that did not support that level of concern. Because of some of its side effects, paroxetine may not be considered by many to be a first-line treatment for major depression, but it certainly would not be absolutely contraindicated during pregnancy as might be presumed by the assignment of a category D label.
Lithium and sodium valproate provide another example of the limitations of the old system, which will be addressed in the new system. While the teratogenicity of both agents has been well described, the absolute risk of malformations with fetal exposure to lithium is approximately 0.05%- 0.1%, but the risk of neural tube defects with sodium valproate is estimated at 8%. Complicating the issue further, in 2013, the FDA announced that sodium valproate had been changed from a category D to X for migraine prevention, but retained the category D classification for other indications.
Placing lithium in category D suggests a relative contraindication and yet discontinuing that medication during pregnancy can put the mother and her baby at risk, given the data supporting the rapid onset of relapse in women who stop mood stabilizers during pregnancy.
For women maintained on lithium for recurrent or brittle bipolar disorder, the drug would certainly not be contraindicated and may afford critical emotional well-being and protection from relapse during pregnancy; the clinical scenario of discontinuation of lithium proximate to or during pregnancy and subsequent relapse of underlying illness is a serious clinical matter frequently demanding urgent intervention.
Still another example of the incomplete informative value of the older system is found in the assignment of atypical antipsychotics into different risk categories. Lurasidone (Latuda), approved in 2010, is in category B, but other atypical antipsychotics are in category C. One might assume that this implies that there are more reproductive safety data available on lurasidone supporting safety, but in fact, reproductive safety data for this molecule are extremely limited, and the absence of adverse event information resulted in a category B. This is a great example of the clinical maxim that incomplete or sparse data is just that; it does not imply safety, it implies that we do not know a lot about the safety of a medication.
If the old system of pregnancy labeling was arbitrary, the PLLR will be more descriptive. Safety information during pregnancy and lactation in the drug label will appear in a section on pregnancy, reformatted to include a risk summary, clinical considerations, and data subsections, as well as a section on lactation, and a section on females and males of reproductive potential.
Ongoing revision of the label as information becomes outdated is a requirement, and manufacturers will be obligated to include information on whether there is a pregnancy registry for the given drug. The goal of the PLLR is thus to provide the patient and clinician with information which addresses both sides of the risk-benefit decision for a given medicine – risks of fetal drug exposure and the risk of untreated illness for the woman and baby, a factor that is not addressed at all with the current system.
Certainly, the new label system will be a charge to industry to establish, support, and encourage enrollment in well-designed pregnancy registries across therapeutic areas to provide ample amounts of good quality data that can then be used by patients along with their physicians to make the most appropriate clinical decisions.
Much of the currently available reproductive safety information on drugs is derived from spontaneous reports, where there has been inconsistent information and variable levels of scrutiny with respect to outcomes assessment, and from small, underpowered cohort studies or large administrative databases. Postmarketing surveillance efforts have been rather modest and have not been a priority for manufacturers in most cases. Hopefully, this will change as pregnancy registries become part of routine postmarketing surveillance.
The new system will not be a panacea, and I expect there will be growing pains, considering the huge challenge of reducing the available data of varying quality into distinct paragraphs. It may also be difficult to synthesize the volume of data and the nuanced differences between certain studies into a paragraph on risk assessment. The task will be simpler for some agents and more challenging for others where the data are less consistent. Questions also remain as to how data will be revised over time.
But despite these challenges, the new system represents a monumental change, and in my mind, will bring a focus to the importance of the issue of quantifying reproductive safety of medications used by women either planning to get pregnant or who are pregnant or breastfeeding, across therapeutic areas. Of particular importance, the new system will hopefully lead to more discussion between physician and patient about what is and is not known about the reproductive safety of a medication, versus a cursory reference to some previously assigned category label.
Our group has shown that when it comes to making decisions about using medication during pregnancy, even when given the same information, women will make different decisions. This is critical since people make personal decisions about the use of these medications in collaboration with their doctors on a case-by-case basis, based on personal preference, available information, and clinical conditions across a spectrum of severity.
As the FDA requirements shift from the arbitrary category label assignment to a more descriptive explanation of risk, based on available data, an important question will be what mechanism will be used by regulators collaborating with industry to update labels with the growing amounts of information on reproductive safety, particularly if there is a commitment from industry to enhance postmarketing surveillance with more pregnancy registries.
Better data can catalyze thoughtful discussions between doctor and patient regarding decisions to use or defer treatment with a given medicine. One might wonder if the new system will open a Pandora’s box. But I believe in this case, opening Pandora’s box would be welcome because it will hopefully lead to a more careful examination of the available information regarding reproductive safety and more informed decisions on the part of patients.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information about reproductive mental health. He has been a consultant to manufacturers of antidepressant medications and is the principal investigator of the National Pregnancy Registry for Atypical Antipsychotics, which receives support from the manufacturers of those drugs. To comment, e-mail him at [email protected]. Scan this QR code or go to obgynnews.com to view similar columns.
As reviewed in a previous column, in December 2014, the Food and Drug Administration released the Pregnancy and Lactation Labeling Rule (PLLR), which will go into effect on June 30, 2015. This replaces and addresses the limitations of the system that has been in place for more than 30 years, which ascribed a pregnancy risk category of A,B,C,D, or X to drugs, with the purpose of informing the clinician and patient about the reproductive safety of medications during pregnancy. Though well intentioned, criticisms of this system have been abundant.
The system certainly simplified the interaction between physicians and patients, who presumably would be reassured that the risk of a certain medicine had been quantified by a regulatory body and therefore could be used as a basis for making a decision about whether or not to take a medicine during pregnancy. While the purpose of the labeling system was to provide some overarching guidance about available reproductive safety information of a medicine, it was ultimately used by clinicians and patients either to somehow garner reassurance about a medicine, or to heighten concern about a medicine.
From the outset, the system could not take into account the accruing reproductive safety information regarding compounds across therapeutic categories, and as a result, the risk category could be inadvertently reassuring or even misleading to patients with respect to medicines they might decide to stop or to continue.
With the older labeling system, some medicines are in the same category, despite very different amounts of reproductive safety information available on the drugs. In the 1990s, there were more reproductive safety data available on certain selective serotonin reuptake inhibitors (SSRIs), compared with others, but now the amount of such data available across SSRIs is fairly consistent. Yet SSRI labels have not been updated with the abundance of new reproductive safety information that has become available.
Almost 10 years ago, paroxetine (Paxil) was switched from a category C to D, when first-trimester exposure was linked to an increased risk of birth defects, particularly heart defects. But it was not switched back to category C when data became available that did not support that level of concern. Because of some of its side effects, paroxetine may not be considered by many to be a first-line treatment for major depression, but it certainly would not be absolutely contraindicated during pregnancy as might be presumed by the assignment of a category D label.
Lithium and sodium valproate provide another example of the limitations of the old system, which will be addressed in the new system. While the teratogenicity of both agents has been well described, the absolute risk of malformations with fetal exposure to lithium is approximately 0.05%- 0.1%, but the risk of neural tube defects with sodium valproate is estimated at 8%. Complicating the issue further, in 2013, the FDA announced that sodium valproate had been changed from a category D to X for migraine prevention, but retained the category D classification for other indications.
Placing lithium in category D suggests a relative contraindication and yet discontinuing that medication during pregnancy can put the mother and her baby at risk, given the data supporting the rapid onset of relapse in women who stop mood stabilizers during pregnancy.
For women maintained on lithium for recurrent or brittle bipolar disorder, the drug would certainly not be contraindicated and may afford critical emotional well-being and protection from relapse during pregnancy; the clinical scenario of discontinuation of lithium proximate to or during pregnancy and subsequent relapse of underlying illness is a serious clinical matter frequently demanding urgent intervention.
Still another example of the incomplete informative value of the older system is found in the assignment of atypical antipsychotics into different risk categories. Lurasidone (Latuda), approved in 2010, is in category B, but other atypical antipsychotics are in category C. One might assume that this implies that there are more reproductive safety data available on lurasidone supporting safety, but in fact, reproductive safety data for this molecule are extremely limited, and the absence of adverse event information resulted in a category B. This is a great example of the clinical maxim that incomplete or sparse data is just that; it does not imply safety, it implies that we do not know a lot about the safety of a medication.
If the old system of pregnancy labeling was arbitrary, the PLLR will be more descriptive. Safety information during pregnancy and lactation in the drug label will appear in a section on pregnancy, reformatted to include a risk summary, clinical considerations, and data subsections, as well as a section on lactation, and a section on females and males of reproductive potential.
Ongoing revision of the label as information becomes outdated is a requirement, and manufacturers will be obligated to include information on whether there is a pregnancy registry for the given drug. The goal of the PLLR is thus to provide the patient and clinician with information which addresses both sides of the risk-benefit decision for a given medicine – risks of fetal drug exposure and the risk of untreated illness for the woman and baby, a factor that is not addressed at all with the current system.
Certainly, the new label system will be a charge to industry to establish, support, and encourage enrollment in well-designed pregnancy registries across therapeutic areas to provide ample amounts of good quality data that can then be used by patients along with their physicians to make the most appropriate clinical decisions.
Much of the currently available reproductive safety information on drugs is derived from spontaneous reports, where there has been inconsistent information and variable levels of scrutiny with respect to outcomes assessment, and from small, underpowered cohort studies or large administrative databases. Postmarketing surveillance efforts have been rather modest and have not been a priority for manufacturers in most cases. Hopefully, this will change as pregnancy registries become part of routine postmarketing surveillance.
The new system will not be a panacea, and I expect there will be growing pains, considering the huge challenge of reducing the available data of varying quality into distinct paragraphs. It may also be difficult to synthesize the volume of data and the nuanced differences between certain studies into a paragraph on risk assessment. The task will be simpler for some agents and more challenging for others where the data are less consistent. Questions also remain as to how data will be revised over time.
But despite these challenges, the new system represents a monumental change, and in my mind, will bring a focus to the importance of the issue of quantifying reproductive safety of medications used by women either planning to get pregnant or who are pregnant or breastfeeding, across therapeutic areas. Of particular importance, the new system will hopefully lead to more discussion between physician and patient about what is and is not known about the reproductive safety of a medication, versus a cursory reference to some previously assigned category label.
Our group has shown that when it comes to making decisions about using medication during pregnancy, even when given the same information, women will make different decisions. This is critical since people make personal decisions about the use of these medications in collaboration with their doctors on a case-by-case basis, based on personal preference, available information, and clinical conditions across a spectrum of severity.
As the FDA requirements shift from the arbitrary category label assignment to a more descriptive explanation of risk, based on available data, an important question will be what mechanism will be used by regulators collaborating with industry to update labels with the growing amounts of information on reproductive safety, particularly if there is a commitment from industry to enhance postmarketing surveillance with more pregnancy registries.
Better data can catalyze thoughtful discussions between doctor and patient regarding decisions to use or defer treatment with a given medicine. One might wonder if the new system will open a Pandora’s box. But I believe in this case, opening Pandora’s box would be welcome because it will hopefully lead to a more careful examination of the available information regarding reproductive safety and more informed decisions on the part of patients.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information about reproductive mental health. He has been a consultant to manufacturers of antidepressant medications and is the principal investigator of the National Pregnancy Registry for Atypical Antipsychotics, which receives support from the manufacturers of those drugs. To comment, e-mail him at [email protected]. Scan this QR code or go to obgynnews.com to view similar columns.
As reviewed in a previous column, in December 2014, the Food and Drug Administration released the Pregnancy and Lactation Labeling Rule (PLLR), which will go into effect on June 30, 2015. This replaces and addresses the limitations of the system that has been in place for more than 30 years, which ascribed a pregnancy risk category of A,B,C,D, or X to drugs, with the purpose of informing the clinician and patient about the reproductive safety of medications during pregnancy. Though well intentioned, criticisms of this system have been abundant.
The system certainly simplified the interaction between physicians and patients, who presumably would be reassured that the risk of a certain medicine had been quantified by a regulatory body and therefore could be used as a basis for making a decision about whether or not to take a medicine during pregnancy. While the purpose of the labeling system was to provide some overarching guidance about available reproductive safety information of a medicine, it was ultimately used by clinicians and patients either to somehow garner reassurance about a medicine, or to heighten concern about a medicine.
From the outset, the system could not take into account the accruing reproductive safety information regarding compounds across therapeutic categories, and as a result, the risk category could be inadvertently reassuring or even misleading to patients with respect to medicines they might decide to stop or to continue.
With the older labeling system, some medicines are in the same category, despite very different amounts of reproductive safety information available on the drugs. In the 1990s, there were more reproductive safety data available on certain selective serotonin reuptake inhibitors (SSRIs), compared with others, but now the amount of such data available across SSRIs is fairly consistent. Yet SSRI labels have not been updated with the abundance of new reproductive safety information that has become available.
Almost 10 years ago, paroxetine (Paxil) was switched from a category C to D, when first-trimester exposure was linked to an increased risk of birth defects, particularly heart defects. But it was not switched back to category C when data became available that did not support that level of concern. Because of some of its side effects, paroxetine may not be considered by many to be a first-line treatment for major depression, but it certainly would not be absolutely contraindicated during pregnancy as might be presumed by the assignment of a category D label.
Lithium and sodium valproate provide another example of the limitations of the old system, which will be addressed in the new system. While the teratogenicity of both agents has been well described, the absolute risk of malformations with fetal exposure to lithium is approximately 0.05%- 0.1%, but the risk of neural tube defects with sodium valproate is estimated at 8%. Complicating the issue further, in 2013, the FDA announced that sodium valproate had been changed from a category D to X for migraine prevention, but retained the category D classification for other indications.
Placing lithium in category D suggests a relative contraindication and yet discontinuing that medication during pregnancy can put the mother and her baby at risk, given the data supporting the rapid onset of relapse in women who stop mood stabilizers during pregnancy.
For women maintained on lithium for recurrent or brittle bipolar disorder, the drug would certainly not be contraindicated and may afford critical emotional well-being and protection from relapse during pregnancy; the clinical scenario of discontinuation of lithium proximate to or during pregnancy and subsequent relapse of underlying illness is a serious clinical matter frequently demanding urgent intervention.
Still another example of the incomplete informative value of the older system is found in the assignment of atypical antipsychotics into different risk categories. Lurasidone (Latuda), approved in 2010, is in category B, but other atypical antipsychotics are in category C. One might assume that this implies that there are more reproductive safety data available on lurasidone supporting safety, but in fact, reproductive safety data for this molecule are extremely limited, and the absence of adverse event information resulted in a category B. This is a great example of the clinical maxim that incomplete or sparse data is just that; it does not imply safety, it implies that we do not know a lot about the safety of a medication.
If the old system of pregnancy labeling was arbitrary, the PLLR will be more descriptive. Safety information during pregnancy and lactation in the drug label will appear in a section on pregnancy, reformatted to include a risk summary, clinical considerations, and data subsections, as well as a section on lactation, and a section on females and males of reproductive potential.
Ongoing revision of the label as information becomes outdated is a requirement, and manufacturers will be obligated to include information on whether there is a pregnancy registry for the given drug. The goal of the PLLR is thus to provide the patient and clinician with information which addresses both sides of the risk-benefit decision for a given medicine – risks of fetal drug exposure and the risk of untreated illness for the woman and baby, a factor that is not addressed at all with the current system.
Certainly, the new label system will be a charge to industry to establish, support, and encourage enrollment in well-designed pregnancy registries across therapeutic areas to provide ample amounts of good quality data that can then be used by patients along with their physicians to make the most appropriate clinical decisions.
Much of the currently available reproductive safety information on drugs is derived from spontaneous reports, where there has been inconsistent information and variable levels of scrutiny with respect to outcomes assessment, and from small, underpowered cohort studies or large administrative databases. Postmarketing surveillance efforts have been rather modest and have not been a priority for manufacturers in most cases. Hopefully, this will change as pregnancy registries become part of routine postmarketing surveillance.
The new system will not be a panacea, and I expect there will be growing pains, considering the huge challenge of reducing the available data of varying quality into distinct paragraphs. It may also be difficult to synthesize the volume of data and the nuanced differences between certain studies into a paragraph on risk assessment. The task will be simpler for some agents and more challenging for others where the data are less consistent. Questions also remain as to how data will be revised over time.
But despite these challenges, the new system represents a monumental change, and in my mind, will bring a focus to the importance of the issue of quantifying reproductive safety of medications used by women either planning to get pregnant or who are pregnant or breastfeeding, across therapeutic areas. Of particular importance, the new system will hopefully lead to more discussion between physician and patient about what is and is not known about the reproductive safety of a medication, versus a cursory reference to some previously assigned category label.
Our group has shown that when it comes to making decisions about using medication during pregnancy, even when given the same information, women will make different decisions. This is critical since people make personal decisions about the use of these medications in collaboration with their doctors on a case-by-case basis, based on personal preference, available information, and clinical conditions across a spectrum of severity.
As the FDA requirements shift from the arbitrary category label assignment to a more descriptive explanation of risk, based on available data, an important question will be what mechanism will be used by regulators collaborating with industry to update labels with the growing amounts of information on reproductive safety, particularly if there is a commitment from industry to enhance postmarketing surveillance with more pregnancy registries.
Better data can catalyze thoughtful discussions between doctor and patient regarding decisions to use or defer treatment with a given medicine. One might wonder if the new system will open a Pandora’s box. But I believe in this case, opening Pandora’s box would be welcome because it will hopefully lead to a more careful examination of the available information regarding reproductive safety and more informed decisions on the part of patients.
Dr. Cohen is the director of the Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information about reproductive mental health. He has been a consultant to manufacturers of antidepressant medications and is the principal investigator of the National Pregnancy Registry for Atypical Antipsychotics, which receives support from the manufacturers of those drugs. To comment, e-mail him at [email protected]. Scan this QR code or go to obgynnews.com to view similar columns.
Evaluating the impact of FDA’s pregnancy and lactation labeling rule
Since 1979, obstetric and other health care providers who treat pregnant or potentially pregnant and breastfeeding women have relied heavily on the Food and Drug Administration’s pregnancy labeling categories for pharmaceuticals – the familiar A, B, C, D, X. However, as early as 1997, a public hearing was held that challenged the value of these labels as typically used in clinical practice by both providers and patients.
Now, 17 years later, in December 2014, the FDA has released the “Pregnancy and Lactation Labeling Rule” (also known as PLLR or final rule). In brief, the revised label will require that:
• Contact information be prominently listed for a pregnancy exposure registry for the drug, when one is available.
• Narrative sections be presented that include a risk summary, clinical considerations, and the supporting data.
• A lactation subsection be included that provides information about using the drug while breastfeeding, such as the amount of drug in breast milk and potential effects on the breastfed infant.
• A subsection on females and males of reproductive potential be included, when necessary, with information about the need for pregnancy testing, contraception recommendations, and information about infertility as it relates to the drug.
The labeling changes go into effect on June 30, 2015. Prescription drugs and biologic products submitted for FDA review after that date will use the new format immediately, while labeling for prescription drugs approved on or after June 30, 2001, will be phased in gradually.
Why are these changes needed, and what impact will they likely have for clinical practice?
First a bit of history – the pregnancy label A, B, C, D, X categories were initially introduced in the 1970s, following the recognition that thalidomide was a human teratogen. The intention was to help the clinician deal in a more standardized way with the increasing amount of experimental animal data and human reports generated for drugs that might be used by women of reproductive potential.
However, the letter categories, and their accompanying standard text statements, were frequently misinterpreted in oversimplistic and inaccurate ways (Birth Defects Res. Part A 2007,79:627-30). Clinicians and patients often believe that risk increases as you move across the letter categories; for example, that a category C drug is worse than a category B drug for a given patient.
Clinicians and patients also commonly think that drugs in the same category have the same level of risk, or that there is a similar quantity and quality of information to support that risk category. Frequently cited examples of misinterpretation include those drugs assigned a category X, for example, label text indicating that the drug is “contraindicated in women who are or may become pregnant.” In reality, in some cases, the X category has been applied to drugs with known human teratogenic potential (such as isotretinoin or thalidomide). However, in other cases, the X has been assigned to drugs for which there were no or very limited human data indicating risk (such as ribavirin or leflunomide) or for which the treatment for the underlying condition would not be necessary or advisable in pregnancy (such as statins or some weight loss drugs).
There is no differentiation made in the category X label for varying risks specifically related to dose and timing of exposure in gestation. In each of these situations where there are no clear-cut human data, inadvertent exposure to the drug in an unplanned pregnancy can easily lead to the misunderstanding that the drug is known to cause birth defects in humans.
The immediate impact of the PLLR label revision will be to require narrative sections that describe the actual data, provide a summary of risks, and also present clinical considerations that may include the risk of undertreatment or no treatment with the drug. The new format is intended to provide the clinician (and the patient) with more comprehensive information on which to base decisions.
The downside of the label revision is that clinicians will have to learn to interpret more comprehensive information and deal with the unknowns, which are many.
The other major impact of the label revision will be to highlight the clear lack of sufficient human data for most drugs currently marketed in the United States. A recent review of drugs (both prescription and over the counter) approved by the FDA between 2000 and 2010 found that 73.3% had no human data available that was relevant to pregnancy safety (Am. J. Med. Genet. C. Semin. Med. Genet. 157C:175-82).
In the short term, the learning curve for label writers and the end users of such labels will be steep. However, clinicians and patients can contribute to the compilation of data for most drugs by more proactively engaging in pregnancy and lactation safety studies. Information about the existence of any pregnancy registries in drug labeling has been recommended in the past, but will now be required. Acting on that information by enrolling patients in these studies can ensure that labels can more quickly be populated with evidence-based data that can better inform patient care.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures. To comment, e-mail her at [email protected].
Since 1979, obstetric and other health care providers who treat pregnant or potentially pregnant and breastfeeding women have relied heavily on the Food and Drug Administration’s pregnancy labeling categories for pharmaceuticals – the familiar A, B, C, D, X. However, as early as 1997, a public hearing was held that challenged the value of these labels as typically used in clinical practice by both providers and patients.
Now, 17 years later, in December 2014, the FDA has released the “Pregnancy and Lactation Labeling Rule” (also known as PLLR or final rule). In brief, the revised label will require that:
• Contact information be prominently listed for a pregnancy exposure registry for the drug, when one is available.
• Narrative sections be presented that include a risk summary, clinical considerations, and the supporting data.
• A lactation subsection be included that provides information about using the drug while breastfeeding, such as the amount of drug in breast milk and potential effects on the breastfed infant.
• A subsection on females and males of reproductive potential be included, when necessary, with information about the need for pregnancy testing, contraception recommendations, and information about infertility as it relates to the drug.
The labeling changes go into effect on June 30, 2015. Prescription drugs and biologic products submitted for FDA review after that date will use the new format immediately, while labeling for prescription drugs approved on or after June 30, 2001, will be phased in gradually.
Why are these changes needed, and what impact will they likely have for clinical practice?
First a bit of history – the pregnancy label A, B, C, D, X categories were initially introduced in the 1970s, following the recognition that thalidomide was a human teratogen. The intention was to help the clinician deal in a more standardized way with the increasing amount of experimental animal data and human reports generated for drugs that might be used by women of reproductive potential.
However, the letter categories, and their accompanying standard text statements, were frequently misinterpreted in oversimplistic and inaccurate ways (Birth Defects Res. Part A 2007,79:627-30). Clinicians and patients often believe that risk increases as you move across the letter categories; for example, that a category C drug is worse than a category B drug for a given patient.
Clinicians and patients also commonly think that drugs in the same category have the same level of risk, or that there is a similar quantity and quality of information to support that risk category. Frequently cited examples of misinterpretation include those drugs assigned a category X, for example, label text indicating that the drug is “contraindicated in women who are or may become pregnant.” In reality, in some cases, the X category has been applied to drugs with known human teratogenic potential (such as isotretinoin or thalidomide). However, in other cases, the X has been assigned to drugs for which there were no or very limited human data indicating risk (such as ribavirin or leflunomide) or for which the treatment for the underlying condition would not be necessary or advisable in pregnancy (such as statins or some weight loss drugs).
There is no differentiation made in the category X label for varying risks specifically related to dose and timing of exposure in gestation. In each of these situations where there are no clear-cut human data, inadvertent exposure to the drug in an unplanned pregnancy can easily lead to the misunderstanding that the drug is known to cause birth defects in humans.
The immediate impact of the PLLR label revision will be to require narrative sections that describe the actual data, provide a summary of risks, and also present clinical considerations that may include the risk of undertreatment or no treatment with the drug. The new format is intended to provide the clinician (and the patient) with more comprehensive information on which to base decisions.
The downside of the label revision is that clinicians will have to learn to interpret more comprehensive information and deal with the unknowns, which are many.
The other major impact of the label revision will be to highlight the clear lack of sufficient human data for most drugs currently marketed in the United States. A recent review of drugs (both prescription and over the counter) approved by the FDA between 2000 and 2010 found that 73.3% had no human data available that was relevant to pregnancy safety (Am. J. Med. Genet. C. Semin. Med. Genet. 157C:175-82).
In the short term, the learning curve for label writers and the end users of such labels will be steep. However, clinicians and patients can contribute to the compilation of data for most drugs by more proactively engaging in pregnancy and lactation safety studies. Information about the existence of any pregnancy registries in drug labeling has been recommended in the past, but will now be required. Acting on that information by enrolling patients in these studies can ensure that labels can more quickly be populated with evidence-based data that can better inform patient care.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures. To comment, e-mail her at [email protected].
Since 1979, obstetric and other health care providers who treat pregnant or potentially pregnant and breastfeeding women have relied heavily on the Food and Drug Administration’s pregnancy labeling categories for pharmaceuticals – the familiar A, B, C, D, X. However, as early as 1997, a public hearing was held that challenged the value of these labels as typically used in clinical practice by both providers and patients.
Now, 17 years later, in December 2014, the FDA has released the “Pregnancy and Lactation Labeling Rule” (also known as PLLR or final rule). In brief, the revised label will require that:
• Contact information be prominently listed for a pregnancy exposure registry for the drug, when one is available.
• Narrative sections be presented that include a risk summary, clinical considerations, and the supporting data.
• A lactation subsection be included that provides information about using the drug while breastfeeding, such as the amount of drug in breast milk and potential effects on the breastfed infant.
• A subsection on females and males of reproductive potential be included, when necessary, with information about the need for pregnancy testing, contraception recommendations, and information about infertility as it relates to the drug.
The labeling changes go into effect on June 30, 2015. Prescription drugs and biologic products submitted for FDA review after that date will use the new format immediately, while labeling for prescription drugs approved on or after June 30, 2001, will be phased in gradually.
Why are these changes needed, and what impact will they likely have for clinical practice?
First a bit of history – the pregnancy label A, B, C, D, X categories were initially introduced in the 1970s, following the recognition that thalidomide was a human teratogen. The intention was to help the clinician deal in a more standardized way with the increasing amount of experimental animal data and human reports generated for drugs that might be used by women of reproductive potential.
However, the letter categories, and their accompanying standard text statements, were frequently misinterpreted in oversimplistic and inaccurate ways (Birth Defects Res. Part A 2007,79:627-30). Clinicians and patients often believe that risk increases as you move across the letter categories; for example, that a category C drug is worse than a category B drug for a given patient.
Clinicians and patients also commonly think that drugs in the same category have the same level of risk, or that there is a similar quantity and quality of information to support that risk category. Frequently cited examples of misinterpretation include those drugs assigned a category X, for example, label text indicating that the drug is “contraindicated in women who are or may become pregnant.” In reality, in some cases, the X category has been applied to drugs with known human teratogenic potential (such as isotretinoin or thalidomide). However, in other cases, the X has been assigned to drugs for which there were no or very limited human data indicating risk (such as ribavirin or leflunomide) or for which the treatment for the underlying condition would not be necessary or advisable in pregnancy (such as statins or some weight loss drugs).
There is no differentiation made in the category X label for varying risks specifically related to dose and timing of exposure in gestation. In each of these situations where there are no clear-cut human data, inadvertent exposure to the drug in an unplanned pregnancy can easily lead to the misunderstanding that the drug is known to cause birth defects in humans.
The immediate impact of the PLLR label revision will be to require narrative sections that describe the actual data, provide a summary of risks, and also present clinical considerations that may include the risk of undertreatment or no treatment with the drug. The new format is intended to provide the clinician (and the patient) with more comprehensive information on which to base decisions.
The downside of the label revision is that clinicians will have to learn to interpret more comprehensive information and deal with the unknowns, which are many.
The other major impact of the label revision will be to highlight the clear lack of sufficient human data for most drugs currently marketed in the United States. A recent review of drugs (both prescription and over the counter) approved by the FDA between 2000 and 2010 found that 73.3% had no human data available that was relevant to pregnancy safety (Am. J. Med. Genet. C. Semin. Med. Genet. 157C:175-82).
In the short term, the learning curve for label writers and the end users of such labels will be steep. However, clinicians and patients can contribute to the compilation of data for most drugs by more proactively engaging in pregnancy and lactation safety studies. Information about the existence of any pregnancy registries in drug labeling has been recommended in the past, but will now be required. Acting on that information by enrolling patients in these studies can ensure that labels can more quickly be populated with evidence-based data that can better inform patient care.
Dr. Chambers is a professor of pediatrics and director of clinical research at Rady Children’s Hospital, and associate director of the Clinical and Translational Research Institute at the University of California, San Diego. She is director of MotherToBaby California, a past president of the Organization of Teratology Information Specialists, and past president of the Teratology Society. She has no relevant financial disclosures. To comment, e-mail her at [email protected].
FDA issues new pregnancy/lactation drug label standards
The U.S. Food and Drug Administration has issued a final rule requiring content and format changes to pregnancy and lactation labeling information for prescription drugs and biologic products.
The long-awaited “Content and Format of Labeling for Human Prescription Drug and Biological Products; Requirements for Pregnancy and Lactation Labeling, or the Pregnancy and Lactation Labeling Rule”(PLLR) is part of broad effort by the FDA to improve the content and format of prescription drug labeling. The PLLR, which finalizes many of the provisions in a proposed rule issued in May 2008 after input from numerous stakeholders, calls for replacement of the current A, B, C, D, and X drug classification system with more detailed information about the risks and benefits of use during pregnancy and breastfeeding.
The rule will take effect June 30, 2015.
Under the PLLR, labels will be required to include three detailed subsections entitled Pregnancy, Lactation, and Females and Males of Reproductive Potential. Each will include a risk summary, a discussion of the supporting data, and relevant information to help providers make prescribing and counseling decisions, according to the FDA. If no data are available to guide decision making, this must be stated.
The Pregnancy subsection combines the existing Pregnancy and Labor and Delivery subsections, and will address use of the drug during pregnancy as well as provide information about relevant registries that collect and maintain data on the use of the product in pregnant women. The Lactation subsection replaces the existing Nursing Mothers subsection, and will include information about use of the product during breastfeeding, including the amount of drug in breast milk and potential effects on the breastfed child. The new Females and Males of Reproductive Potential subsection will address pregnancy testing, contraception, and fertility issues as they relate to use of the product.
The existing A, B, C, D, and X categories were frequently misinterpreted as a grading system, giving an over simplified view of product risk, according to Dr. Sandra Kweder, deputy director of the Office of New Drugs in the FDA’s Center for Drug Evaluation and Research.
The new, more detailed approach to labeling will better address the complex risk-benefit considerations inherent in prescribing decisions during pregnancy and lactation, she said during a press briefing.
“I’m excited because clinicians will, going forward, be able to rely on FDA-approved drug labeling for comprehensive, chronically relevant, and user-friendly information in this part of labeling – something that has been missing for many years,” she said, noting that the changes are particularly important, given that the more than 6 million women who become pregnant each year in the United States take an average of 3-5 different prescription products during the course of their pregnancy and while breastfeeding.
“It is our hope that this new system will help their health care professionals and these women as they discuss treatment options,” she said.
Importantly, the PLLR ensures that more robust and informative data about drugs will be provided than ever before – and in a manner that speaks directly to the concerns that are common among providers, she said.
In addition to the elimination of the letter categories and the addition of the three new subsections, the use of standardized risk statement also was eliminated, as these had the same limitations as the letter categories. A section on inadvertent exposure also was eliminated due to redundancy, as the risk would be the same as with intentional exposure.
The rule also requires that labels be updated as they become outdated.
Much of the information that will be included on the new labels, which will be phased in for existing drugs and required immediately for drugs approved after June 30, 2015, was already included, but was scattered and difficult to find. The new formatting requirements provides for consistency across labels by pulling this information together in one place, Dr. Kweder said.
In an official statement, the American College of Obstetricians and Gynecologists applauded the rule for “taking needed steps to increase understanding about the effect of prescription medicine on women during pregnancy and lactation.”
“The FDA’s updated method of presenting information about both risk and benefit will improve the ability of all physicians to treat their pregnant and breastfeeding patients, as well as women who may become pregnant. It will also help more women to understand and take part in their health care decision making,” according to the ACOG statement, which also noted that the organization hopes the new content on prescription drug and biological product labels will “provide added incentives for clinical research as well as participation in patient registries.”
Christina Chambers, Ph.D., professor of pediatrics and director of clinical research for the department of pediatrics at the University of California, San Diego, also praised the new labeling rule, noting in an interview that “the final rule has been long awaited by many who work in the field of counseling pregnant and breastfeeding women about risks and safety of prescription medications, such as counselors with organizations like MotherToBaby, a service of the Organization of Teratology Information Specialists, which provides information about medication and other exposures during pregnancy and breastfeeding, and which was involved in development of the final rule.
“The MotherToBaby counselors located throughout the United States who answer questions about medication exposures for hundreds of women every day, have struggled for years with trying to explain the not-so-useful A, B, C, D, X pregnancy categories to patients and providers alike who commonly misinterpret their meaning. The new label format is much more content rich and evidence-based, and encompasses the larger picture of the safety data in the context of treatment (or lack of treatment) of the maternal condition. This is a huge step forward – and will make even more clear how critical the need is for more human pregnancy data for all medications likely to be used by women of reproductive age,” she said.
Dr. Chambers is the program director for MotherToBaby California, and director of the MotherToBaby research center at the University of California, San Diego. She reported having no relevant disclosures.
The U.S. Food and Drug Administration has issued a final rule requiring content and format changes to pregnancy and lactation labeling information for prescription drugs and biologic products.
The long-awaited “Content and Format of Labeling for Human Prescription Drug and Biological Products; Requirements for Pregnancy and Lactation Labeling, or the Pregnancy and Lactation Labeling Rule”(PLLR) is part of broad effort by the FDA to improve the content and format of prescription drug labeling. The PLLR, which finalizes many of the provisions in a proposed rule issued in May 2008 after input from numerous stakeholders, calls for replacement of the current A, B, C, D, and X drug classification system with more detailed information about the risks and benefits of use during pregnancy and breastfeeding.
The rule will take effect June 30, 2015.
Under the PLLR, labels will be required to include three detailed subsections entitled Pregnancy, Lactation, and Females and Males of Reproductive Potential. Each will include a risk summary, a discussion of the supporting data, and relevant information to help providers make prescribing and counseling decisions, according to the FDA. If no data are available to guide decision making, this must be stated.
The Pregnancy subsection combines the existing Pregnancy and Labor and Delivery subsections, and will address use of the drug during pregnancy as well as provide information about relevant registries that collect and maintain data on the use of the product in pregnant women. The Lactation subsection replaces the existing Nursing Mothers subsection, and will include information about use of the product during breastfeeding, including the amount of drug in breast milk and potential effects on the breastfed child. The new Females and Males of Reproductive Potential subsection will address pregnancy testing, contraception, and fertility issues as they relate to use of the product.
The existing A, B, C, D, and X categories were frequently misinterpreted as a grading system, giving an over simplified view of product risk, according to Dr. Sandra Kweder, deputy director of the Office of New Drugs in the FDA’s Center for Drug Evaluation and Research.
The new, more detailed approach to labeling will better address the complex risk-benefit considerations inherent in prescribing decisions during pregnancy and lactation, she said during a press briefing.
“I’m excited because clinicians will, going forward, be able to rely on FDA-approved drug labeling for comprehensive, chronically relevant, and user-friendly information in this part of labeling – something that has been missing for many years,” she said, noting that the changes are particularly important, given that the more than 6 million women who become pregnant each year in the United States take an average of 3-5 different prescription products during the course of their pregnancy and while breastfeeding.
“It is our hope that this new system will help their health care professionals and these women as they discuss treatment options,” she said.
Importantly, the PLLR ensures that more robust and informative data about drugs will be provided than ever before – and in a manner that speaks directly to the concerns that are common among providers, she said.
In addition to the elimination of the letter categories and the addition of the three new subsections, the use of standardized risk statement also was eliminated, as these had the same limitations as the letter categories. A section on inadvertent exposure also was eliminated due to redundancy, as the risk would be the same as with intentional exposure.
The rule also requires that labels be updated as they become outdated.
Much of the information that will be included on the new labels, which will be phased in for existing drugs and required immediately for drugs approved after June 30, 2015, was already included, but was scattered and difficult to find. The new formatting requirements provides for consistency across labels by pulling this information together in one place, Dr. Kweder said.
In an official statement, the American College of Obstetricians and Gynecologists applauded the rule for “taking needed steps to increase understanding about the effect of prescription medicine on women during pregnancy and lactation.”
“The FDA’s updated method of presenting information about both risk and benefit will improve the ability of all physicians to treat their pregnant and breastfeeding patients, as well as women who may become pregnant. It will also help more women to understand and take part in their health care decision making,” according to the ACOG statement, which also noted that the organization hopes the new content on prescription drug and biological product labels will “provide added incentives for clinical research as well as participation in patient registries.”
Christina Chambers, Ph.D., professor of pediatrics and director of clinical research for the department of pediatrics at the University of California, San Diego, also praised the new labeling rule, noting in an interview that “the final rule has been long awaited by many who work in the field of counseling pregnant and breastfeeding women about risks and safety of prescription medications, such as counselors with organizations like MotherToBaby, a service of the Organization of Teratology Information Specialists, which provides information about medication and other exposures during pregnancy and breastfeeding, and which was involved in development of the final rule.
“The MotherToBaby counselors located throughout the United States who answer questions about medication exposures for hundreds of women every day, have struggled for years with trying to explain the not-so-useful A, B, C, D, X pregnancy categories to patients and providers alike who commonly misinterpret their meaning. The new label format is much more content rich and evidence-based, and encompasses the larger picture of the safety data in the context of treatment (or lack of treatment) of the maternal condition. This is a huge step forward – and will make even more clear how critical the need is for more human pregnancy data for all medications likely to be used by women of reproductive age,” she said.
Dr. Chambers is the program director for MotherToBaby California, and director of the MotherToBaby research center at the University of California, San Diego. She reported having no relevant disclosures.
The U.S. Food and Drug Administration has issued a final rule requiring content and format changes to pregnancy and lactation labeling information for prescription drugs and biologic products.
The long-awaited “Content and Format of Labeling for Human Prescription Drug and Biological Products; Requirements for Pregnancy and Lactation Labeling, or the Pregnancy and Lactation Labeling Rule”(PLLR) is part of broad effort by the FDA to improve the content and format of prescription drug labeling. The PLLR, which finalizes many of the provisions in a proposed rule issued in May 2008 after input from numerous stakeholders, calls for replacement of the current A, B, C, D, and X drug classification system with more detailed information about the risks and benefits of use during pregnancy and breastfeeding.
The rule will take effect June 30, 2015.
Under the PLLR, labels will be required to include three detailed subsections entitled Pregnancy, Lactation, and Females and Males of Reproductive Potential. Each will include a risk summary, a discussion of the supporting data, and relevant information to help providers make prescribing and counseling decisions, according to the FDA. If no data are available to guide decision making, this must be stated.
The Pregnancy subsection combines the existing Pregnancy and Labor and Delivery subsections, and will address use of the drug during pregnancy as well as provide information about relevant registries that collect and maintain data on the use of the product in pregnant women. The Lactation subsection replaces the existing Nursing Mothers subsection, and will include information about use of the product during breastfeeding, including the amount of drug in breast milk and potential effects on the breastfed child. The new Females and Males of Reproductive Potential subsection will address pregnancy testing, contraception, and fertility issues as they relate to use of the product.
The existing A, B, C, D, and X categories were frequently misinterpreted as a grading system, giving an over simplified view of product risk, according to Dr. Sandra Kweder, deputy director of the Office of New Drugs in the FDA’s Center for Drug Evaluation and Research.
The new, more detailed approach to labeling will better address the complex risk-benefit considerations inherent in prescribing decisions during pregnancy and lactation, she said during a press briefing.
“I’m excited because clinicians will, going forward, be able to rely on FDA-approved drug labeling for comprehensive, chronically relevant, and user-friendly information in this part of labeling – something that has been missing for many years,” she said, noting that the changes are particularly important, given that the more than 6 million women who become pregnant each year in the United States take an average of 3-5 different prescription products during the course of their pregnancy and while breastfeeding.
“It is our hope that this new system will help their health care professionals and these women as they discuss treatment options,” she said.
Importantly, the PLLR ensures that more robust and informative data about drugs will be provided than ever before – and in a manner that speaks directly to the concerns that are common among providers, she said.
In addition to the elimination of the letter categories and the addition of the three new subsections, the use of standardized risk statement also was eliminated, as these had the same limitations as the letter categories. A section on inadvertent exposure also was eliminated due to redundancy, as the risk would be the same as with intentional exposure.
The rule also requires that labels be updated as they become outdated.
Much of the information that will be included on the new labels, which will be phased in for existing drugs and required immediately for drugs approved after June 30, 2015, was already included, but was scattered and difficult to find. The new formatting requirements provides for consistency across labels by pulling this information together in one place, Dr. Kweder said.
In an official statement, the American College of Obstetricians and Gynecologists applauded the rule for “taking needed steps to increase understanding about the effect of prescription medicine on women during pregnancy and lactation.”
“The FDA’s updated method of presenting information about both risk and benefit will improve the ability of all physicians to treat their pregnant and breastfeeding patients, as well as women who may become pregnant. It will also help more women to understand and take part in their health care decision making,” according to the ACOG statement, which also noted that the organization hopes the new content on prescription drug and biological product labels will “provide added incentives for clinical research as well as participation in patient registries.”
Christina Chambers, Ph.D., professor of pediatrics and director of clinical research for the department of pediatrics at the University of California, San Diego, also praised the new labeling rule, noting in an interview that “the final rule has been long awaited by many who work in the field of counseling pregnant and breastfeeding women about risks and safety of prescription medications, such as counselors with organizations like MotherToBaby, a service of the Organization of Teratology Information Specialists, which provides information about medication and other exposures during pregnancy and breastfeeding, and which was involved in development of the final rule.
“The MotherToBaby counselors located throughout the United States who answer questions about medication exposures for hundreds of women every day, have struggled for years with trying to explain the not-so-useful A, B, C, D, X pregnancy categories to patients and providers alike who commonly misinterpret their meaning. The new label format is much more content rich and evidence-based, and encompasses the larger picture of the safety data in the context of treatment (or lack of treatment) of the maternal condition. This is a huge step forward – and will make even more clear how critical the need is for more human pregnancy data for all medications likely to be used by women of reproductive age,” she said.
Dr. Chambers is the program director for MotherToBaby California, and director of the MotherToBaby research center at the University of California, San Diego. She reported having no relevant disclosures.
Drugs, Pregnancy, and Lactation: Herbs
Herbs are commonly consumed by pregnant and breast-feeding women, possibly because they believe that “natural products” are safer than drugs. However, even though some have been available for hundreds or thousands of years, little is known about their effects on the embryo, fetus, newborn, or nursing infant. Moreover, as unregulated products, the concentration, contents, and presence of contaminants cannot be easily determined. Detailed reviews of the 22 most commonly used herbs discussed here can be found in “Drugs in Pregnancy and Lactation,” Briggs GG, Freeman RK, 10th ed., Philadelphia: Wolters Kluwer Health, 2014).
In the following discussions, dose, one of the two key factors that determine the risk of developmental toxicity (abnormal growth, structural anomalies, functional and/or neurobehavioral deficits, or death), is rarely reported. In addition, all herbs contain multiple chemical compounds, few of which have been studied during pregnancy or lactation. Thus, with few exceptions, a woman who takes an herb in pregnancy should be informed that the risk to her developing baby is unknown.
Six herbs are considered contraindicated in pregnancy: arnica, black seed /kalanji, blue cohosh, feverfew, salvia divinorum, and valerian.
• Arnica. The dried flowers, and sometimes the roots and rhizomes, are the parts of this perennial plant that are used topically for their anti-inflammatory and analgesic effects. There is no clinical evidence to support this use. Occasional topical use probably represents a low risk, but absorption may occur when it is applied to broken skin. The Food and Drug Administration has classified arnica as an unsafe herb and, when used orally, it is considered a poison. It is a uterine stimulant and an abortifacient. Nevertheless, in homeopathic formulations, it has been promoted for use before and during labor for internal and external bruising of the mother and newborn. In Italy, it is one of the top 10 herbs taken by women (Pharmacoepidemiol. Drug Saf. 2006;15:354-9).
• Black seed/kalanji. This herb has been used for thousands of year as a medicine, food, or spice. Because of this, it is unlikely that it causes teratogenesis. Nevertheless, its use to stimulate menstruation and its potential contraceptive properties suggest that it is contraindicated in pregnancy.
• Blue cohosh. Some of the components of this herb have been shown to be teratogenic and toxic in various animal species, so it should be avoided in the first trimester. The herb has uterine stimulant properties that are used by nurse-midwives to stimulate labor. Blue cohosh was the most frequently used herbal preparation for this purpose. However, some sources believe that the potential fetal and newborn toxicity may outweigh any medical benefit (“PDR for Herbal Medicine,” 2nd ed., Montvale, N.J.: Medical Economics, 2000:109-10; “The Review of Natural Products,” St. Louis, MO: Facts and Comparisons, 2000).
• Feverfew. This herb has been used for labor, menstrual disorders, potential miscarriage, and morning sickness; as an abortifacient; and for several other indications. Because of its antipyretic properties, it has been known as “medieval aspirin.” The doses used for these indications have not been quantified. Because of its emmenagogic (capable of provoking menstruation) activity, the herb should not be used in pregnancy.
• Salvia divinorum. This herb has hallucinogenic effects and is used in certain regions of Mexico for healing and divinatory rituals. It is also thought to have antidiarrheal properties. The herb is either smoked or chewed, or its juices are ingested. When taken orally, systemic effects are dependent upon absorption across the oral mucosa as the active ingredient is destroyed in the GI tract. Persistent psychosis has been observed in people who smoked the herb, so it is contraindicated in pregnancy.
• Valerian. A large number of preparations containing valerian are available. It has been used as a sedative and hypnotic for anxiety, restlessness, and sleep disturbances, as well as several other pharmacologic claims. Because of the risk of cytotoxicity in the fetus and hepatotoxicity in the mother, the herb should be avoided during gestation.
For the remaining 16 herbs, small, infrequent doses probably cause no harm to the mother, embryo, fetus, or newborn. Nevertheless, as noted below, some of these herbs are best avoided during pregnancy.
• Chamomile. Excessive use of this herb should be avoided because it is thought to have uterine stimulant, emmenagogic, and abortifacient properties. Although controversial, some nurse-midwives prescribe chamomile teas for the treatment of morning sickness. Because the plant sources of the herb contain coumarin compounds, ingesting chamomile by pregnant women with coagulation disorders is a concern. However, the herb has been used for thousands of years, so the risk of harm, at least from occasional use, must be very rare.
• Echinacea. This herb is used topically to enhance wound healing and systemically as an immunostimulant. An IV formulation is used in Germany but is not available in the United States. It also has been recommended to assist in the prevention or treatment of viral upper respiratory tract infections. Its use in pregnancy is limited to one small study.
• Evening primrose oil. The oil contains two essential fatty acids: cis-linoleic and gamma-linolenic acid. In a national survey of nurse-midwives, it was the most frequently used herbal preparation for the induction of labor. No adverse effects have been reported in the fetus or newborn from this use. The doses used varied widely and included both oral and vaginal routes of administration. In addition, the oil has been used for rheumatoid arthritis and diabetic neuropathy, but there are no reports of these uses in pregnancy.
• Garlic. Garlic has been used for food flavoring since ancient times and appears to be safe during pregnancy. Some components cross the placenta, as shown by garlic odor in the amniotic fluid and on the newborn’s breath. Very high doses have the potential to induce menstruation or uterine contractions, but apparently these effects have not been reported.
• Ginger. No reports of ginger-induced developmental toxicity have been located. Ginger has been used as antiemetic for nausea and vomiting of pregnancy.
• Ginseng. The root is the most important part of this plant that is found throughout the world and has been used in medicine for more than 2,000 years. The herb has been promoted for multiple pharmacologic effects, including adaptogenic, CNS, cardiovascular, endocrine, ergogenic, antineoplastic, and immunomodulatory effects.
Hypertension and hypoglycemia have been reported in nonpregnant patients, but not in the limited human pregnancy data. A brief 1991 study compared 88 women who took the herb during pregnancy with 88 controls. No differences between the groups were found with regard to the mode of delivery, birth weight, low birth weight (< 2,500 ), preterm delivery (< 37 weeks), low Apgar score (< 7), stillbirths, neonatal deaths, or maternal complications (Asia Oceania J. Obstet. Gynaecol. 1991;17:379-80).
• Ginkgo biloba. The limited animal reproduction data suggest low risk, but there is no reported human pregnancy experience. Nevertheless, it is an ancient herbal preparation that is commonly used for organic brain syndrome, circulatory disorders, asthma, vertigo, and tinnitus. Because of its widespread use, it is doubtful that a major teratogenic effect would have escaped notice, but more subtle or low-incidence toxic effects may not have been detected.
• Kudzu. No human or animal data regarding pregnancy have been located. The herb has been used for more than 2,500 years for the treatment of alcohol hangover, drunkenness, alcoholism, muscle pain, and measles. Many of its chemical constituents can be found in foods. Nevertheless, high, frequent doses should be avoided.
• Nutmeg. This is a commonly used spice but, as with any herb, high doses can produce toxicity. The toxicity is caused by a chemical in the seeds, myristicin, which has anticholinergic properties. A woman at 30 weeks’ gestation misread a recipe and used a whole grated nutmeg rather than 1/8 teaspoon when making cookies. When she ate a cookie, she experienced sinus tachycardia, hypertension, and a sensation of impending doom. The fetus had tachycardia, and atropine-like poisoning was diagnosed. After about 12 hours, both mother and fetus made an uneventful recovery and a healthy infant was born at term.
• Passion flower. The name of this herb may refer to about 400 species of the genus Passiflora. It is available in both oral and topical forms and is used for nervousness, neuralgia, insomnia, pain, asthma, seizures, burns, hemorrhoids, and menopausal complaints. As with many herbs, it contains a large number of chemicals, none of which have undergone reproductive testing. No reports describing the use of this herb in human pregnancy have been located. However, because it has uterine stimulant properties, the oral formulation is best avoided in pregnancy.
• Peppermint. This popular flavoring appears to be harmless for the mother and developing baby when low, recommended doses are ingested. Peppermint oil is available in numerous topical and oral formulations. High oral doses, however, can cause significant toxicity, including death. During pregnancy, ingestion of more than the recommended doses is unsafe because of possible emmenagogic and abortifacient properties.
• Pumpkin seed. This herb, when used as a food, appears to be harmless for the mother and embryo-fetus, but no reports describing its use in pregnancy have been located. High doses, such as those used in traditional medicine or in eating disorders, should be avoided because of the potential for toxic effects from the many chemicals these seeds contain.
• Raspberry leaf. Raspberry leaf tea is commonly used by pregnant women. Nurse-midwives often prescribe the tea to treat nausea and vomiting and as a uterine tonic to shorten labor. A double-blind, randomized, placebo-controlled study evaluated the effect of raspberry leaf tablets (2 tablets/day) on pregnancy outcomes. Compared with controls, no differences were found for length of labor or stages of labor, mode of delivery, admission to the neonatal intensive care unit, Apgar score, and birth weight (J. Midwifery Womens Health 2001;46:51-9).
• Safflower. Safflower oil is commonly used in cooking and has been given for its laxative action. There are no reports describing the use of the herb in pregnancy. It is doubtful if such use would have any adverse effect on a pregnancy. Although abortifacient and emmenagogic effects have been suggested, there is no evidence supporting these effects when used as a food.
• St. John’s wort. No toxicity in pregnant humans has been reported. The use of the herb is widespread and dates back thousands of years. Thus, it is doubtful that the herb is a major teratogen or causes other elements of developmental toxicity. The herb has been used for the management of anxiety, depression, insomnia, inflammation, and gastritis. It is also used as a diuretic and, topically, for the treatment of hemorrhoids and enhanced wound healing.
• Yohimbine. The use of this herb in human pregnancies has not been reported. It has been used as an aphrodisiac and for weight loss, sexual dysfunction, and the treatment of orthostatic hypotension. Although it has no Food and Drug Administration–sanctioned indications, it is also available by prescription for male erectile dysfunction. Due to the lack of data regarding pregnancy, the herb is best avoided during pregnancy.
There are few data regarding the effects of the above herbs on a breast-feeding infant. Depending upon the herb, nursing infants will be exposed to many chemical compounds. For those herbs used as food, nursing is probably safe. The safety of the other herbs during lactation is unknown. However, toxicity has been reported in a 9-day-old term infant whose mother was taking arnica (Clin. Toxicol. 2009;47:726, abstract 120). The infant presented with lethargy, decreased milk intake, anemia, and jaundice but recovered with treatment. After the mother stopped the herb and resumed nursing, no further problems were noted in the infant.
Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He had no relevant financial disclosures. Contact him at [email protected].
Herbs are commonly consumed by pregnant and breast-feeding women, possibly because they believe that “natural products” are safer than drugs. However, even though some have been available for hundreds or thousands of years, little is known about their effects on the embryo, fetus, newborn, or nursing infant. Moreover, as unregulated products, the concentration, contents, and presence of contaminants cannot be easily determined. Detailed reviews of the 22 most commonly used herbs discussed here can be found in “Drugs in Pregnancy and Lactation,” Briggs GG, Freeman RK, 10th ed., Philadelphia: Wolters Kluwer Health, 2014).
In the following discussions, dose, one of the two key factors that determine the risk of developmental toxicity (abnormal growth, structural anomalies, functional and/or neurobehavioral deficits, or death), is rarely reported. In addition, all herbs contain multiple chemical compounds, few of which have been studied during pregnancy or lactation. Thus, with few exceptions, a woman who takes an herb in pregnancy should be informed that the risk to her developing baby is unknown.
Six herbs are considered contraindicated in pregnancy: arnica, black seed /kalanji, blue cohosh, feverfew, salvia divinorum, and valerian.
• Arnica. The dried flowers, and sometimes the roots and rhizomes, are the parts of this perennial plant that are used topically for their anti-inflammatory and analgesic effects. There is no clinical evidence to support this use. Occasional topical use probably represents a low risk, but absorption may occur when it is applied to broken skin. The Food and Drug Administration has classified arnica as an unsafe herb and, when used orally, it is considered a poison. It is a uterine stimulant and an abortifacient. Nevertheless, in homeopathic formulations, it has been promoted for use before and during labor for internal and external bruising of the mother and newborn. In Italy, it is one of the top 10 herbs taken by women (Pharmacoepidemiol. Drug Saf. 2006;15:354-9).
• Black seed/kalanji. This herb has been used for thousands of year as a medicine, food, or spice. Because of this, it is unlikely that it causes teratogenesis. Nevertheless, its use to stimulate menstruation and its potential contraceptive properties suggest that it is contraindicated in pregnancy.
• Blue cohosh. Some of the components of this herb have been shown to be teratogenic and toxic in various animal species, so it should be avoided in the first trimester. The herb has uterine stimulant properties that are used by nurse-midwives to stimulate labor. Blue cohosh was the most frequently used herbal preparation for this purpose. However, some sources believe that the potential fetal and newborn toxicity may outweigh any medical benefit (“PDR for Herbal Medicine,” 2nd ed., Montvale, N.J.: Medical Economics, 2000:109-10; “The Review of Natural Products,” St. Louis, MO: Facts and Comparisons, 2000).
• Feverfew. This herb has been used for labor, menstrual disorders, potential miscarriage, and morning sickness; as an abortifacient; and for several other indications. Because of its antipyretic properties, it has been known as “medieval aspirin.” The doses used for these indications have not been quantified. Because of its emmenagogic (capable of provoking menstruation) activity, the herb should not be used in pregnancy.
• Salvia divinorum. This herb has hallucinogenic effects and is used in certain regions of Mexico for healing and divinatory rituals. It is also thought to have antidiarrheal properties. The herb is either smoked or chewed, or its juices are ingested. When taken orally, systemic effects are dependent upon absorption across the oral mucosa as the active ingredient is destroyed in the GI tract. Persistent psychosis has been observed in people who smoked the herb, so it is contraindicated in pregnancy.
• Valerian. A large number of preparations containing valerian are available. It has been used as a sedative and hypnotic for anxiety, restlessness, and sleep disturbances, as well as several other pharmacologic claims. Because of the risk of cytotoxicity in the fetus and hepatotoxicity in the mother, the herb should be avoided during gestation.
For the remaining 16 herbs, small, infrequent doses probably cause no harm to the mother, embryo, fetus, or newborn. Nevertheless, as noted below, some of these herbs are best avoided during pregnancy.
• Chamomile. Excessive use of this herb should be avoided because it is thought to have uterine stimulant, emmenagogic, and abortifacient properties. Although controversial, some nurse-midwives prescribe chamomile teas for the treatment of morning sickness. Because the plant sources of the herb contain coumarin compounds, ingesting chamomile by pregnant women with coagulation disorders is a concern. However, the herb has been used for thousands of years, so the risk of harm, at least from occasional use, must be very rare.
• Echinacea. This herb is used topically to enhance wound healing and systemically as an immunostimulant. An IV formulation is used in Germany but is not available in the United States. It also has been recommended to assist in the prevention or treatment of viral upper respiratory tract infections. Its use in pregnancy is limited to one small study.
• Evening primrose oil. The oil contains two essential fatty acids: cis-linoleic and gamma-linolenic acid. In a national survey of nurse-midwives, it was the most frequently used herbal preparation for the induction of labor. No adverse effects have been reported in the fetus or newborn from this use. The doses used varied widely and included both oral and vaginal routes of administration. In addition, the oil has been used for rheumatoid arthritis and diabetic neuropathy, but there are no reports of these uses in pregnancy.
• Garlic. Garlic has been used for food flavoring since ancient times and appears to be safe during pregnancy. Some components cross the placenta, as shown by garlic odor in the amniotic fluid and on the newborn’s breath. Very high doses have the potential to induce menstruation or uterine contractions, but apparently these effects have not been reported.
• Ginger. No reports of ginger-induced developmental toxicity have been located. Ginger has been used as antiemetic for nausea and vomiting of pregnancy.
• Ginseng. The root is the most important part of this plant that is found throughout the world and has been used in medicine for more than 2,000 years. The herb has been promoted for multiple pharmacologic effects, including adaptogenic, CNS, cardiovascular, endocrine, ergogenic, antineoplastic, and immunomodulatory effects.
Hypertension and hypoglycemia have been reported in nonpregnant patients, but not in the limited human pregnancy data. A brief 1991 study compared 88 women who took the herb during pregnancy with 88 controls. No differences between the groups were found with regard to the mode of delivery, birth weight, low birth weight (< 2,500 ), preterm delivery (< 37 weeks), low Apgar score (< 7), stillbirths, neonatal deaths, or maternal complications (Asia Oceania J. Obstet. Gynaecol. 1991;17:379-80).
• Ginkgo biloba. The limited animal reproduction data suggest low risk, but there is no reported human pregnancy experience. Nevertheless, it is an ancient herbal preparation that is commonly used for organic brain syndrome, circulatory disorders, asthma, vertigo, and tinnitus. Because of its widespread use, it is doubtful that a major teratogenic effect would have escaped notice, but more subtle or low-incidence toxic effects may not have been detected.
• Kudzu. No human or animal data regarding pregnancy have been located. The herb has been used for more than 2,500 years for the treatment of alcohol hangover, drunkenness, alcoholism, muscle pain, and measles. Many of its chemical constituents can be found in foods. Nevertheless, high, frequent doses should be avoided.
• Nutmeg. This is a commonly used spice but, as with any herb, high doses can produce toxicity. The toxicity is caused by a chemical in the seeds, myristicin, which has anticholinergic properties. A woman at 30 weeks’ gestation misread a recipe and used a whole grated nutmeg rather than 1/8 teaspoon when making cookies. When she ate a cookie, she experienced sinus tachycardia, hypertension, and a sensation of impending doom. The fetus had tachycardia, and atropine-like poisoning was diagnosed. After about 12 hours, both mother and fetus made an uneventful recovery and a healthy infant was born at term.
• Passion flower. The name of this herb may refer to about 400 species of the genus Passiflora. It is available in both oral and topical forms and is used for nervousness, neuralgia, insomnia, pain, asthma, seizures, burns, hemorrhoids, and menopausal complaints. As with many herbs, it contains a large number of chemicals, none of which have undergone reproductive testing. No reports describing the use of this herb in human pregnancy have been located. However, because it has uterine stimulant properties, the oral formulation is best avoided in pregnancy.
• Peppermint. This popular flavoring appears to be harmless for the mother and developing baby when low, recommended doses are ingested. Peppermint oil is available in numerous topical and oral formulations. High oral doses, however, can cause significant toxicity, including death. During pregnancy, ingestion of more than the recommended doses is unsafe because of possible emmenagogic and abortifacient properties.
• Pumpkin seed. This herb, when used as a food, appears to be harmless for the mother and embryo-fetus, but no reports describing its use in pregnancy have been located. High doses, such as those used in traditional medicine or in eating disorders, should be avoided because of the potential for toxic effects from the many chemicals these seeds contain.
• Raspberry leaf. Raspberry leaf tea is commonly used by pregnant women. Nurse-midwives often prescribe the tea to treat nausea and vomiting and as a uterine tonic to shorten labor. A double-blind, randomized, placebo-controlled study evaluated the effect of raspberry leaf tablets (2 tablets/day) on pregnancy outcomes. Compared with controls, no differences were found for length of labor or stages of labor, mode of delivery, admission to the neonatal intensive care unit, Apgar score, and birth weight (J. Midwifery Womens Health 2001;46:51-9).
• Safflower. Safflower oil is commonly used in cooking and has been given for its laxative action. There are no reports describing the use of the herb in pregnancy. It is doubtful if such use would have any adverse effect on a pregnancy. Although abortifacient and emmenagogic effects have been suggested, there is no evidence supporting these effects when used as a food.
• St. John’s wort. No toxicity in pregnant humans has been reported. The use of the herb is widespread and dates back thousands of years. Thus, it is doubtful that the herb is a major teratogen or causes other elements of developmental toxicity. The herb has been used for the management of anxiety, depression, insomnia, inflammation, and gastritis. It is also used as a diuretic and, topically, for the treatment of hemorrhoids and enhanced wound healing.
• Yohimbine. The use of this herb in human pregnancies has not been reported. It has been used as an aphrodisiac and for weight loss, sexual dysfunction, and the treatment of orthostatic hypotension. Although it has no Food and Drug Administration–sanctioned indications, it is also available by prescription for male erectile dysfunction. Due to the lack of data regarding pregnancy, the herb is best avoided during pregnancy.
There are few data regarding the effects of the above herbs on a breast-feeding infant. Depending upon the herb, nursing infants will be exposed to many chemical compounds. For those herbs used as food, nursing is probably safe. The safety of the other herbs during lactation is unknown. However, toxicity has been reported in a 9-day-old term infant whose mother was taking arnica (Clin. Toxicol. 2009;47:726, abstract 120). The infant presented with lethargy, decreased milk intake, anemia, and jaundice but recovered with treatment. After the mother stopped the herb and resumed nursing, no further problems were noted in the infant.
Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He had no relevant financial disclosures. Contact him at [email protected].
Herbs are commonly consumed by pregnant and breast-feeding women, possibly because they believe that “natural products” are safer than drugs. However, even though some have been available for hundreds or thousands of years, little is known about their effects on the embryo, fetus, newborn, or nursing infant. Moreover, as unregulated products, the concentration, contents, and presence of contaminants cannot be easily determined. Detailed reviews of the 22 most commonly used herbs discussed here can be found in “Drugs in Pregnancy and Lactation,” Briggs GG, Freeman RK, 10th ed., Philadelphia: Wolters Kluwer Health, 2014).
In the following discussions, dose, one of the two key factors that determine the risk of developmental toxicity (abnormal growth, structural anomalies, functional and/or neurobehavioral deficits, or death), is rarely reported. In addition, all herbs contain multiple chemical compounds, few of which have been studied during pregnancy or lactation. Thus, with few exceptions, a woman who takes an herb in pregnancy should be informed that the risk to her developing baby is unknown.
Six herbs are considered contraindicated in pregnancy: arnica, black seed /kalanji, blue cohosh, feverfew, salvia divinorum, and valerian.
• Arnica. The dried flowers, and sometimes the roots and rhizomes, are the parts of this perennial plant that are used topically for their anti-inflammatory and analgesic effects. There is no clinical evidence to support this use. Occasional topical use probably represents a low risk, but absorption may occur when it is applied to broken skin. The Food and Drug Administration has classified arnica as an unsafe herb and, when used orally, it is considered a poison. It is a uterine stimulant and an abortifacient. Nevertheless, in homeopathic formulations, it has been promoted for use before and during labor for internal and external bruising of the mother and newborn. In Italy, it is one of the top 10 herbs taken by women (Pharmacoepidemiol. Drug Saf. 2006;15:354-9).
• Black seed/kalanji. This herb has been used for thousands of year as a medicine, food, or spice. Because of this, it is unlikely that it causes teratogenesis. Nevertheless, its use to stimulate menstruation and its potential contraceptive properties suggest that it is contraindicated in pregnancy.
• Blue cohosh. Some of the components of this herb have been shown to be teratogenic and toxic in various animal species, so it should be avoided in the first trimester. The herb has uterine stimulant properties that are used by nurse-midwives to stimulate labor. Blue cohosh was the most frequently used herbal preparation for this purpose. However, some sources believe that the potential fetal and newborn toxicity may outweigh any medical benefit (“PDR for Herbal Medicine,” 2nd ed., Montvale, N.J.: Medical Economics, 2000:109-10; “The Review of Natural Products,” St. Louis, MO: Facts and Comparisons, 2000).
• Feverfew. This herb has been used for labor, menstrual disorders, potential miscarriage, and morning sickness; as an abortifacient; and for several other indications. Because of its antipyretic properties, it has been known as “medieval aspirin.” The doses used for these indications have not been quantified. Because of its emmenagogic (capable of provoking menstruation) activity, the herb should not be used in pregnancy.
• Salvia divinorum. This herb has hallucinogenic effects and is used in certain regions of Mexico for healing and divinatory rituals. It is also thought to have antidiarrheal properties. The herb is either smoked or chewed, or its juices are ingested. When taken orally, systemic effects are dependent upon absorption across the oral mucosa as the active ingredient is destroyed in the GI tract. Persistent psychosis has been observed in people who smoked the herb, so it is contraindicated in pregnancy.
• Valerian. A large number of preparations containing valerian are available. It has been used as a sedative and hypnotic for anxiety, restlessness, and sleep disturbances, as well as several other pharmacologic claims. Because of the risk of cytotoxicity in the fetus and hepatotoxicity in the mother, the herb should be avoided during gestation.
For the remaining 16 herbs, small, infrequent doses probably cause no harm to the mother, embryo, fetus, or newborn. Nevertheless, as noted below, some of these herbs are best avoided during pregnancy.
• Chamomile. Excessive use of this herb should be avoided because it is thought to have uterine stimulant, emmenagogic, and abortifacient properties. Although controversial, some nurse-midwives prescribe chamomile teas for the treatment of morning sickness. Because the plant sources of the herb contain coumarin compounds, ingesting chamomile by pregnant women with coagulation disorders is a concern. However, the herb has been used for thousands of years, so the risk of harm, at least from occasional use, must be very rare.
• Echinacea. This herb is used topically to enhance wound healing and systemically as an immunostimulant. An IV formulation is used in Germany but is not available in the United States. It also has been recommended to assist in the prevention or treatment of viral upper respiratory tract infections. Its use in pregnancy is limited to one small study.
• Evening primrose oil. The oil contains two essential fatty acids: cis-linoleic and gamma-linolenic acid. In a national survey of nurse-midwives, it was the most frequently used herbal preparation for the induction of labor. No adverse effects have been reported in the fetus or newborn from this use. The doses used varied widely and included both oral and vaginal routes of administration. In addition, the oil has been used for rheumatoid arthritis and diabetic neuropathy, but there are no reports of these uses in pregnancy.
• Garlic. Garlic has been used for food flavoring since ancient times and appears to be safe during pregnancy. Some components cross the placenta, as shown by garlic odor in the amniotic fluid and on the newborn’s breath. Very high doses have the potential to induce menstruation or uterine contractions, but apparently these effects have not been reported.
• Ginger. No reports of ginger-induced developmental toxicity have been located. Ginger has been used as antiemetic for nausea and vomiting of pregnancy.
• Ginseng. The root is the most important part of this plant that is found throughout the world and has been used in medicine for more than 2,000 years. The herb has been promoted for multiple pharmacologic effects, including adaptogenic, CNS, cardiovascular, endocrine, ergogenic, antineoplastic, and immunomodulatory effects.
Hypertension and hypoglycemia have been reported in nonpregnant patients, but not in the limited human pregnancy data. A brief 1991 study compared 88 women who took the herb during pregnancy with 88 controls. No differences between the groups were found with regard to the mode of delivery, birth weight, low birth weight (< 2,500 ), preterm delivery (< 37 weeks), low Apgar score (< 7), stillbirths, neonatal deaths, or maternal complications (Asia Oceania J. Obstet. Gynaecol. 1991;17:379-80).
• Ginkgo biloba. The limited animal reproduction data suggest low risk, but there is no reported human pregnancy experience. Nevertheless, it is an ancient herbal preparation that is commonly used for organic brain syndrome, circulatory disorders, asthma, vertigo, and tinnitus. Because of its widespread use, it is doubtful that a major teratogenic effect would have escaped notice, but more subtle or low-incidence toxic effects may not have been detected.
• Kudzu. No human or animal data regarding pregnancy have been located. The herb has been used for more than 2,500 years for the treatment of alcohol hangover, drunkenness, alcoholism, muscle pain, and measles. Many of its chemical constituents can be found in foods. Nevertheless, high, frequent doses should be avoided.
• Nutmeg. This is a commonly used spice but, as with any herb, high doses can produce toxicity. The toxicity is caused by a chemical in the seeds, myristicin, which has anticholinergic properties. A woman at 30 weeks’ gestation misread a recipe and used a whole grated nutmeg rather than 1/8 teaspoon when making cookies. When she ate a cookie, she experienced sinus tachycardia, hypertension, and a sensation of impending doom. The fetus had tachycardia, and atropine-like poisoning was diagnosed. After about 12 hours, both mother and fetus made an uneventful recovery and a healthy infant was born at term.
• Passion flower. The name of this herb may refer to about 400 species of the genus Passiflora. It is available in both oral and topical forms and is used for nervousness, neuralgia, insomnia, pain, asthma, seizures, burns, hemorrhoids, and menopausal complaints. As with many herbs, it contains a large number of chemicals, none of which have undergone reproductive testing. No reports describing the use of this herb in human pregnancy have been located. However, because it has uterine stimulant properties, the oral formulation is best avoided in pregnancy.
• Peppermint. This popular flavoring appears to be harmless for the mother and developing baby when low, recommended doses are ingested. Peppermint oil is available in numerous topical and oral formulations. High oral doses, however, can cause significant toxicity, including death. During pregnancy, ingestion of more than the recommended doses is unsafe because of possible emmenagogic and abortifacient properties.
• Pumpkin seed. This herb, when used as a food, appears to be harmless for the mother and embryo-fetus, but no reports describing its use in pregnancy have been located. High doses, such as those used in traditional medicine or in eating disorders, should be avoided because of the potential for toxic effects from the many chemicals these seeds contain.
• Raspberry leaf. Raspberry leaf tea is commonly used by pregnant women. Nurse-midwives often prescribe the tea to treat nausea and vomiting and as a uterine tonic to shorten labor. A double-blind, randomized, placebo-controlled study evaluated the effect of raspberry leaf tablets (2 tablets/day) on pregnancy outcomes. Compared with controls, no differences were found for length of labor or stages of labor, mode of delivery, admission to the neonatal intensive care unit, Apgar score, and birth weight (J. Midwifery Womens Health 2001;46:51-9).
• Safflower. Safflower oil is commonly used in cooking and has been given for its laxative action. There are no reports describing the use of the herb in pregnancy. It is doubtful if such use would have any adverse effect on a pregnancy. Although abortifacient and emmenagogic effects have been suggested, there is no evidence supporting these effects when used as a food.
• St. John’s wort. No toxicity in pregnant humans has been reported. The use of the herb is widespread and dates back thousands of years. Thus, it is doubtful that the herb is a major teratogen or causes other elements of developmental toxicity. The herb has been used for the management of anxiety, depression, insomnia, inflammation, and gastritis. It is also used as a diuretic and, topically, for the treatment of hemorrhoids and enhanced wound healing.
• Yohimbine. The use of this herb in human pregnancies has not been reported. It has been used as an aphrodisiac and for weight loss, sexual dysfunction, and the treatment of orthostatic hypotension. Although it has no Food and Drug Administration–sanctioned indications, it is also available by prescription for male erectile dysfunction. Due to the lack of data regarding pregnancy, the herb is best avoided during pregnancy.
There are few data regarding the effects of the above herbs on a breast-feeding infant. Depending upon the herb, nursing infants will be exposed to many chemical compounds. For those herbs used as food, nursing is probably safe. The safety of the other herbs during lactation is unknown. However, toxicity has been reported in a 9-day-old term infant whose mother was taking arnica (Clin. Toxicol. 2009;47:726, abstract 120). The infant presented with lethargy, decreased milk intake, anemia, and jaundice but recovered with treatment. After the mother stopped the herb and resumed nursing, no further problems were noted in the infant.
Mr. Briggs is a pharmacist clinical specialist at the outpatient clinics of Memorial Care Center for Women at Miller Children’s Hospital in Long Beach, Calif.; clinical professor of pharmacy at the University of California, San Francisco; and adjunct professor of pharmacy at the University of Southern California, Los Angeles, and Washington State University, Spokane. He also is coauthor of “Drugs in Pregnancy and Lactation,” and coeditor of “Diseases, Complications, and Drug Therapy in Obstetrics.” He had no relevant financial disclosures. Contact him at [email protected].