User login
Cesarean myomectomy: Safe operation or surgical folly?

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
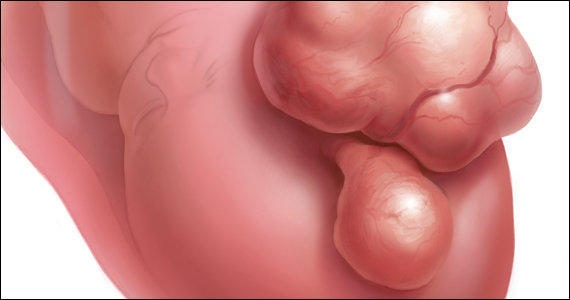
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●

Uterine leiomyomata (fibroids) are the most common pelvic tumor of women. When women are planning to conceive, and their fibroid(s) are clinically significant, causing abnormal uterine bleeding or bulk symptoms, it is often optimal to remove the uterine tumor(s) before conception. Advances in minimally invasive surgery offer women the option of laparoscopic or robot-assisted myomectomy with a low rate of operative complications, including excessive blood loss and hysterectomy, and a low rate of postoperative complications, including major pelvic adhesions and uterine rupture during subsequent pregnancy.1-3 However, many women become pregnant when they have clinically significant fibroids, and at least one-third of these women will have a cesarean birth.
Important clinical issues are the relative benefits and risks of performing a myomectomy at the time of the cesarean birth, so called cesarean myomectomy. Cesarean myomectomy offers carefully selected women the opportunity to have a cesarean birth and myomectomy in one operation, thereby avoiding a second major operation. Over the past 6 decades, most experts in the United States and the United Kingdom have strongly recommended against myomectomy at the time of cesarean delivery because of the risk of excessive blood loss and hysterectomy. Recently, expert opinion has shifted, especially in continental Europe and Asia, and cesarean myomectomy is now viewed as an acceptable surgical option in a limited number of clinical situations, including removal of pedunculated fibroids, excision of large solitary subserosal fibroids, and to achieve optimal management of the hysterotomy incision.
Decades of expert guidance: Avoid cesarean myomectomy at all costs
Dr. K.S.J. Olah succinctly captured the standard teaching that cesarean myomectomy should be avoided in this personal vignette:
Many years ago as a trainee I removed a subserosal fibroid during a cesarean section that was hanging by a thin stalk on the back of the uterus. The berating I received was severe and disproportionate to the crime. The rule was that myomectomy performed at cesarean section was not just frowned upon but expressly forbidden. It has always been considered foolish to consider removing fibroids at cesarean section, mostly because of the associated morbidity and the risk of haemorrhage requiring hysterectomy.4
Dr. Olah quoted guidance from Shaw’s Textbook of Operative Gynaecology,5 “It should be stressed that myomectomy in pregnancy should be avoided at all costs, including at caesarean section.” However, large case series published over the past 10 years report that, in limited clinical situations, cesarean myomectomy is a viable surgical option, where benefit may outweigh risk.6-14 The current literature has many weaknesses, including failure to specifically identify the indication for the cesarean myomectomy and lack of controlled prospective clinical trials. In almost all cases, cesarean myomectomy is performed after delivery of the fetus and placenta.
Continue to: The pedunculated, FIGO type 7 fibroid...
The pedunculated, FIGO type 7 fibroid
The International Federation of Gynecology and Obstetrics (FIGO) leiomyoma classification system identifies subserosal pedunculated fibroids as type 7 (FIGURE).15 Pedunculated fibroids are attached to the uterus by a stalk that is ≤10% of the mean of the 3 diameters of the fibroid. When a clinically significant pedunculated fibroid, causing bulk symptoms, is encountered at cesarean birth, I recommend that it be removed. This will save many patients a second major operation to perform a myomectomy. The surgical risk of removing a pedunculated is low.

The solitary FIGO type 6 fibroid
Type 6 fibroids are subserosal fibroids with less than 50% of their mass being subserosal. The type 6 fibroid is relatively easy to enucleate from the uterus. Following removal of a type 6 fibroid, closure of the serosal defect is relatively straightforward. In carefully selected cases, if the type 6 fibroid is causing bulk symptoms, cesarean myomectomy may be indicated with a low risk of operative complications.
The FIGO type 2-5 fibroid
The type 2-5 fibroid is a transmural fibroid with significant mass abutting both the endometrial cavity and serosal surface. Excision of a type 2-5 fibroid is likely to result in a large transmyometrial defect that will be more difficult to close and could be associated with greater blood loss. Although data are limited, I would recommend against cesarean myomectomy for type 2-5 fibroids in most clinical situations.
Myomectomy to achieve optimal management of the cesarean hysterotomy incision
Many surgeons performing a cesarean birth for a woman with clinically significant fibroids will plan the hysterotomy incision to avoid the fibroids. However, following delivery and contraction of the uterus, proper closure of the hysterotomy incision may be very difficult without removing a fibroid that is abutting the hysterotomy incision. Surgeons have reported performing myomectomy on lower uterine segment fibroids before making the hysterotomy incision in order to facilitate the hysterotomy incision and closure.16 Myomectomy prior to delivery of the newborn must be associated with additional risks to the fetus. I would prefer to identify an optimal site to perform a hysterotomy, deliver the newborn and placenta, and then consider myomectomy.
Complications associated with cesarean myomectomy
The evidence concerning the complications of cesarean birth plus myomectomy compared with cesarean birth alone in women with fibroids is limited to case series. There are no reported controlled clinical trials to guide practice. The largest single case series reported on 1,242 women with fibroids who had a cesarean birth plus myomectomy compared with 3 control groups, including 200 women without fibroids who had a cesarean birth, 145 women with fibroids who had a cesarean birth and no myomectomy, and 51 women with fibroids who had a cesarean hysterectomy. The investigators reported no significant differences in preoperative to postoperative hemoglobin change, incidence of postoperative fever, or length of hospital stay among the 4 groups.8 The authors concluded that myomectomy during cesarean birth was a safe and effective procedure.
Continue to: A systematic review and meta-analysis reported...
A systematic review and meta-analysis reported on the results of 17 studies which included 4,702 women who had a cesarean myomectomy and 1,843 women with cesarean birth without myomectomy.17 The authors of the meta-analysis noted that most reported case series had excluded women with a high risk of bleeding, including women with placenta previa, placenta accreta, coagulation disorders, and a history of multiple myomectomy operations. The investigators reported that, compared with the control women, the women undergoing cesarean myomectomy had a statistically significant but clinically insignificant decrease in mean hemoglobin concentration (-0.27 g/dL), a significant increase in mean operative time (+15 minutes) and a significant increase in the length of hospital stay (+0.36 days). There was an increase in the need for blood transfusion (risk ratio, 1.45; 95% confidence interval, 1.05–1.99), but only 3% of women undergoing cesarean myomectomy received a blood transfusion. There was no significant difference between the two groups in the incidence of postoperative fever. The authors concluded that cesarean myomectomy is a safe procedure when performed by experienced surgeons with appropriate hemostatic techniques.
Techniques to reduce blood loss at the time of cesarean myomectomy
A detailed review of all the available techniques to reduce blood loss at the time of cesarean myomectomy is beyond the scope of this editorial. All gynecologists know that control of uterine blood flow through the uterine artery, infundibulopelvic vessels and internal iliac artery can help to reduce bleeding at the time of myomectomy. Tourniquets, vascular clamps, and artery ligation all have been reported to be useful at the time of cesarean myomectomy. In addition, intravenous infusion of oxytocin and tranexamic acid is often used at the time of cesarean myomectomy. Direct injection of uterotonics, including carbetocin, oxytocin, and vasopressin, into the uterus also has been reported. Cell saver blood salvage technology has been utilized in a limited number of cases of cesarean myomectomy.8,18,19
Medicine is not a static field
Discoveries and new data help guide advances in medical practice. After 6 decades of strict adherence to the advice that myomectomy in pregnancy should be avoided at all costs, including at caesarean delivery, new data indicate that in carefully selected cases cesarean myomectomy is an acceptable operation. ●
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
- Pitter MC, Gargiulo AR, Bonaventura LM, et al. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28:99-108.
- Pitter MC, Srouji SS, Gargiulo AR, et al. Fertility and symptom relief following robot-assisted laparoscopic myomectomy. Obstet Gynecol Int. 2015;2015:967568.
- Huberlant S, Lenot J, Neron M, et al. Fertility and obstetric outcomes after robot-assisted laparoscopic myomectomy. Int J Med Robot. 2020;16:e2059.
- Olah KSJ. Caesarean myomectomy: TE or not TE? BJOG. 2018;125:501.
- Shaw, et al. Textbook of Operative Gynaecology. Edinburgh: Churchill Livingston; 1977.
- Burton CA, Grimes DA, March CM. Surgical management of leiomyomata during pregnancy. Obstet Gynecol. 1989;74:707-709.
- Ortac F, Gungor M, Sonmezer M. Myomectomy during cesarean section. Int J Gynaecol Obstet. 1999;67:189-193.
- Li H, Du J, Jin L, et al. Myomectomy during cesarean section. Acta Obstetricia et Gynecologica. 2009;88:183-186.
- Kwon DH, Song JE, Yoon KR, et al. Obstet Gynecol Sci. 2014;57:367-372.
- Senturk MB, Polat M, Dogan O, et al. Outcome of cesarean myomectomy: is it a safe procedure? Geburtshilfe Frauenheilkd. 2017;77:1200-1206.
- Chauhan AR. Cesarean myomectomy: necessity or opportunity? J Obstet Gynecol India. 2018;68:432-436.
- Sparic R, Kadija S, Stefanovic A, et al. Cesarean myomectomy in modern obstetrics: more light and fewer shadows. J Obstet Gynaecol Res. 2017;43:798-804.
- Ramya T, Sabnis SS, Chitra TV, et al. Cesarean myomectomy: an experience from a tertiary care teaching hospital. J Obstet Gynaecol India. 2019;69:426-430.
- Zhao R, Wang X, Zou L, et al. Outcomes of myomectomy at the time of cesarean section among pregnant women with uterine fibroids: a retrospective cohort study. Biomed Res Int. 2019;7576934.
- Munro MG, Critchley HOD, Fraser IS; FIGO Menstrual Disorders Committee. The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions. In J Gynaecol Obstet. 2018;143:393.
- Omar SZ, Sivanesaratnam V, Damodaran P. Large lower segment myoma—myomectomy at lower segment caesarean section—a report of two cases. Singapore Med J. 1999;40:109-110.
- Goyal M, Dawood AS, Elbohoty SB, et al. Cesarean myomectomy in the last ten years; A true shift from contraindication to indication: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2021;256:145-157.
- Lin JY, Lee WL, Wang PH, et al. Uterine artery occlusion and myomectomy for treatment of pregnant women with uterine leiomyomas who are undergoing caesarean section. J Obstet Gynecol Res. 2010;36:284-290.
- Alfred E, Joy G, Uduak O, et al. Cesarean myomectomy outcome in a Nigerian hospital district hospital. J Basic Clin Reprod Sci. 2013;2:115-118.
Plagues that will haunt us long after the COVID-19 pandemic is gone
As we struggle to gradually emerge from the horrid coronavirus disease 2019 (COVID-19) pandemic that has disrupted our lives and killed hundreds of thousands of people in the United States, we harbor the hope that life will return to “normal.” But while it will certainly be a great relief to put this deadly virus behind us, many other epidemics will continue to plague our society and taint our culture.
Scientific ingenuity has led to the development of several vaccines in record time (aka “warp speed”) that will help defeat the deadly scourge of COVID-19. The pandemic is likely to peter out 2 years after its onset. We will all be grateful for such a rapid resolution of the worst health crisis the world has faced in a century, which will enable medical, economic, and social recovery. But as we eventually resume our lives and rejoice in resuming the pursuit of happiness, we will quickly realize that all is not well in our society just because the viral pandemic is gone.
Perhaps the ordeal of the COVID-19 pandemic, and the agony that was universally shared, will open our collective eyes to a jarring reality: many other epidemics will continue to permeate society and cause endless grief and suffering to many of our fellow humans. And thanks to our training as psychiatric physicians, we have developed extra “receptors” to the darker side of the human condition. As we help many of our psychiatric patients rendered sicker under the unbearable stress of the pandemic, we must not overlook the plight of so many others who do not show up in our clinics for health care, yet suffer enormously but imperceptibly. And no vaccine can come to the rescue of those who continue to live in quiet desperation.
Long-standing epidemics
It is truly unfortunate that many of the epidemics I am referring to have persisted for so long that they have become “fixtures” of contemporary societies. They have become “endemic epidemics” with no urgency to squelch them, as with the COVID-19 pandemic. The benign neglect that perpetuates these serious epidemics has had a malignant effect of “grudging resignation” that nothing can be done to reverse them. Unlike the viral epidemic that engulfed everyone around the world and triggered a massive and unified push to defeat the virus, these long-standing epidemics continue to afflict subgroups who are left to fend for themselves. These individuals deserve our empathy and warrant our determination to lift them from their miserable existence.
Consider some of the widespread epidemics that preceded the pandemic and will, in all likelihood, persist after the pandemic’s burden is lifted:
- millions of people living in poverty and hunger
- widespread racism
- smoldering social injustice
- appalling human trafficking, especially targeting children and women
- child abuse and neglect that leads to psychosis, depression, and suicide in adulthood
- gun violence, which kills many innocent people
- domestic violence that inflicts both physical and mental harm on families
- suicide, both attempts and completions, which continues to increase annually
- the festering stigma of mental illness that adds insult to injury for psychiatric patients
- alcohol and drug addictions, which destroy lives and corrode the fabric of society
- lack of access to mental health care for millions of people who need it
- lack of parity for psychiatric disorders, which is so unjust for our patients
- venomous political hatred and hyperpartisanship, which permeates our culture and can lead to violence, as we recently witnessed
- physician burnout, due to many causes, even before the stresses of COVID-19
- the ongoing agony of wars and terrorism, including dangerous cyberattacks
- the deleterious effect of social media on everyone, especially children.
Most of these epidemics claim thousands of lives each year, and yet no concerted public health effort is being mounted to counteract them, as we are seeing with the COVID-19 pandemic. Much is being written about each of them, but there has been little tangible action, so they persist. They have become a perpetual underbelly of our society that is essentially ignored or simply given the usual lip service.
It will take a herculean effort by policymakers, the judicial system, the medical establishment, and faith organizations to put an end to these life-threatening epidemics. It may appear too daunting to mount a war on so many fronts, but that should not deter us all from launching a strategic plan to create meaningful tactics and solutions. And just as was done with the COVID-19 pandemic, both mitigation measures as well as effective interventions must be employed in this campaign against the epidemic “hydra.”
Continue to: It is tragic...
It is tragic that so many fellow humans are allowed to suffer or die while the rest of us watch, or worse, turn a blind eye and never get involved. A civilized society must never neglect so many of its suffering citizens. As psychiatrists, we are aware of those human travesties around us, but we are often so overwhelmed with our work and personal responsibilities that few of us are passionately advocating or setting aside some time for those victimized by one or more of these endemic pandemics. And unless we all decide to be actively, meaningfully involved, many lives will continue to be lost every day, but without the daily “casualty count” displayed on television screens, as is the case with COVID-19 causalities.
Regrettably, maybe that old saw is true: out of sight, out of mind.
As we struggle to gradually emerge from the horrid coronavirus disease 2019 (COVID-19) pandemic that has disrupted our lives and killed hundreds of thousands of people in the United States, we harbor the hope that life will return to “normal.” But while it will certainly be a great relief to put this deadly virus behind us, many other epidemics will continue to plague our society and taint our culture.
Scientific ingenuity has led to the development of several vaccines in record time (aka “warp speed”) that will help defeat the deadly scourge of COVID-19. The pandemic is likely to peter out 2 years after its onset. We will all be grateful for such a rapid resolution of the worst health crisis the world has faced in a century, which will enable medical, economic, and social recovery. But as we eventually resume our lives and rejoice in resuming the pursuit of happiness, we will quickly realize that all is not well in our society just because the viral pandemic is gone.
Perhaps the ordeal of the COVID-19 pandemic, and the agony that was universally shared, will open our collective eyes to a jarring reality: many other epidemics will continue to permeate society and cause endless grief and suffering to many of our fellow humans. And thanks to our training as psychiatric physicians, we have developed extra “receptors” to the darker side of the human condition. As we help many of our psychiatric patients rendered sicker under the unbearable stress of the pandemic, we must not overlook the plight of so many others who do not show up in our clinics for health care, yet suffer enormously but imperceptibly. And no vaccine can come to the rescue of those who continue to live in quiet desperation.
Long-standing epidemics
It is truly unfortunate that many of the epidemics I am referring to have persisted for so long that they have become “fixtures” of contemporary societies. They have become “endemic epidemics” with no urgency to squelch them, as with the COVID-19 pandemic. The benign neglect that perpetuates these serious epidemics has had a malignant effect of “grudging resignation” that nothing can be done to reverse them. Unlike the viral epidemic that engulfed everyone around the world and triggered a massive and unified push to defeat the virus, these long-standing epidemics continue to afflict subgroups who are left to fend for themselves. These individuals deserve our empathy and warrant our determination to lift them from their miserable existence.
Consider some of the widespread epidemics that preceded the pandemic and will, in all likelihood, persist after the pandemic’s burden is lifted:
- millions of people living in poverty and hunger
- widespread racism
- smoldering social injustice
- appalling human trafficking, especially targeting children and women
- child abuse and neglect that leads to psychosis, depression, and suicide in adulthood
- gun violence, which kills many innocent people
- domestic violence that inflicts both physical and mental harm on families
- suicide, both attempts and completions, which continues to increase annually
- the festering stigma of mental illness that adds insult to injury for psychiatric patients
- alcohol and drug addictions, which destroy lives and corrode the fabric of society
- lack of access to mental health care for millions of people who need it
- lack of parity for psychiatric disorders, which is so unjust for our patients
- venomous political hatred and hyperpartisanship, which permeates our culture and can lead to violence, as we recently witnessed
- physician burnout, due to many causes, even before the stresses of COVID-19
- the ongoing agony of wars and terrorism, including dangerous cyberattacks
- the deleterious effect of social media on everyone, especially children.
Most of these epidemics claim thousands of lives each year, and yet no concerted public health effort is being mounted to counteract them, as we are seeing with the COVID-19 pandemic. Much is being written about each of them, but there has been little tangible action, so they persist. They have become a perpetual underbelly of our society that is essentially ignored or simply given the usual lip service.
It will take a herculean effort by policymakers, the judicial system, the medical establishment, and faith organizations to put an end to these life-threatening epidemics. It may appear too daunting to mount a war on so many fronts, but that should not deter us all from launching a strategic plan to create meaningful tactics and solutions. And just as was done with the COVID-19 pandemic, both mitigation measures as well as effective interventions must be employed in this campaign against the epidemic “hydra.”
Continue to: It is tragic...
It is tragic that so many fellow humans are allowed to suffer or die while the rest of us watch, or worse, turn a blind eye and never get involved. A civilized society must never neglect so many of its suffering citizens. As psychiatrists, we are aware of those human travesties around us, but we are often so overwhelmed with our work and personal responsibilities that few of us are passionately advocating or setting aside some time for those victimized by one or more of these endemic pandemics. And unless we all decide to be actively, meaningfully involved, many lives will continue to be lost every day, but without the daily “casualty count” displayed on television screens, as is the case with COVID-19 causalities.
Regrettably, maybe that old saw is true: out of sight, out of mind.
As we struggle to gradually emerge from the horrid coronavirus disease 2019 (COVID-19) pandemic that has disrupted our lives and killed hundreds of thousands of people in the United States, we harbor the hope that life will return to “normal.” But while it will certainly be a great relief to put this deadly virus behind us, many other epidemics will continue to plague our society and taint our culture.
Scientific ingenuity has led to the development of several vaccines in record time (aka “warp speed”) that will help defeat the deadly scourge of COVID-19. The pandemic is likely to peter out 2 years after its onset. We will all be grateful for such a rapid resolution of the worst health crisis the world has faced in a century, which will enable medical, economic, and social recovery. But as we eventually resume our lives and rejoice in resuming the pursuit of happiness, we will quickly realize that all is not well in our society just because the viral pandemic is gone.
Perhaps the ordeal of the COVID-19 pandemic, and the agony that was universally shared, will open our collective eyes to a jarring reality: many other epidemics will continue to permeate society and cause endless grief and suffering to many of our fellow humans. And thanks to our training as psychiatric physicians, we have developed extra “receptors” to the darker side of the human condition. As we help many of our psychiatric patients rendered sicker under the unbearable stress of the pandemic, we must not overlook the plight of so many others who do not show up in our clinics for health care, yet suffer enormously but imperceptibly. And no vaccine can come to the rescue of those who continue to live in quiet desperation.
Long-standing epidemics
It is truly unfortunate that many of the epidemics I am referring to have persisted for so long that they have become “fixtures” of contemporary societies. They have become “endemic epidemics” with no urgency to squelch them, as with the COVID-19 pandemic. The benign neglect that perpetuates these serious epidemics has had a malignant effect of “grudging resignation” that nothing can be done to reverse them. Unlike the viral epidemic that engulfed everyone around the world and triggered a massive and unified push to defeat the virus, these long-standing epidemics continue to afflict subgroups who are left to fend for themselves. These individuals deserve our empathy and warrant our determination to lift them from their miserable existence.
Consider some of the widespread epidemics that preceded the pandemic and will, in all likelihood, persist after the pandemic’s burden is lifted:
- millions of people living in poverty and hunger
- widespread racism
- smoldering social injustice
- appalling human trafficking, especially targeting children and women
- child abuse and neglect that leads to psychosis, depression, and suicide in adulthood
- gun violence, which kills many innocent people
- domestic violence that inflicts both physical and mental harm on families
- suicide, both attempts and completions, which continues to increase annually
- the festering stigma of mental illness that adds insult to injury for psychiatric patients
- alcohol and drug addictions, which destroy lives and corrode the fabric of society
- lack of access to mental health care for millions of people who need it
- lack of parity for psychiatric disorders, which is so unjust for our patients
- venomous political hatred and hyperpartisanship, which permeates our culture and can lead to violence, as we recently witnessed
- physician burnout, due to many causes, even before the stresses of COVID-19
- the ongoing agony of wars and terrorism, including dangerous cyberattacks
- the deleterious effect of social media on everyone, especially children.
Most of these epidemics claim thousands of lives each year, and yet no concerted public health effort is being mounted to counteract them, as we are seeing with the COVID-19 pandemic. Much is being written about each of them, but there has been little tangible action, so they persist. They have become a perpetual underbelly of our society that is essentially ignored or simply given the usual lip service.
It will take a herculean effort by policymakers, the judicial system, the medical establishment, and faith organizations to put an end to these life-threatening epidemics. It may appear too daunting to mount a war on so many fronts, but that should not deter us all from launching a strategic plan to create meaningful tactics and solutions. And just as was done with the COVID-19 pandemic, both mitigation measures as well as effective interventions must be employed in this campaign against the epidemic “hydra.”
Continue to: It is tragic...
It is tragic that so many fellow humans are allowed to suffer or die while the rest of us watch, or worse, turn a blind eye and never get involved. A civilized society must never neglect so many of its suffering citizens. As psychiatrists, we are aware of those human travesties around us, but we are often so overwhelmed with our work and personal responsibilities that few of us are passionately advocating or setting aside some time for those victimized by one or more of these endemic pandemics. And unless we all decide to be actively, meaningfully involved, many lives will continue to be lost every day, but without the daily “casualty count” displayed on television screens, as is the case with COVID-19 causalities.
Regrettably, maybe that old saw is true: out of sight, out of mind.
Optimizing the use of oxytocin on labor and delivery

Oxytocin is the hormone most commonly administered to women on labor and delivery. It is used for induction of labor, augmentation of labor, and to reduce the risk of postpartum hemorrhage. Licensed independent prescribers, including physicians and nurse midwives, order oxytocin, and licensed professional nurses execute the order by administering the hormone. Optimal management of oxytocin infusion requires effective interprofessional communication and collaboration. During labor it is common for disagreements to arise between the professionals ordering and the professionals administering oxytocin. The disagreements are usually caused by differing perspectives on the appropriate oxytocin dose. Standardized protocols and checklists reduce practice variation and improve patient safety.
Oxytocin hormone
Oxytocin is a cyclic nonapeptide synthesized in the hypothalamus and secreted into the circulation from axonal terminals in the posterior pituitary. In the myometrium, oxytocin activates a membrane G protein-coupled receptor, increasing phospholipase C and intracellular calcium. Following several intracellular chemical cascades, oxytocin stimulation results in myosin and actin filaments sliding over each other initiating shortening of the smooth muscle cell. Myometrial smooth muscle cells are connected by gap junctions, facilitating the coordinated contraction of the uterus.1
Oxytocin pulse frequency and uterine oxytocin receptor concentration both increase during pregnancy and labor, facilitating the birth process. Oxytocin pulse frequency increases from 2.4 pulses per hour before labor to 13.4 pulses per hour in the second stage.2 In addition, uterine oxytocin receptor concentration increases 12-fold from the early second trimester of pregnancy to term.3
Oxytocin has a half-life of approximately 10 to 15 minutes. Many pharmacologists believe that for a given dose of a drug, it takes 4 to 5 half-lives for a stabilized circulating concentration to be achieved. Therefore, during an oxytocin infusion, when the dose is increased it may take 40 to 50 minutes to achieve a new higher, stabile circulating concentration.4
Low-dose vs high-dose oxytocin protocols
Oxytocin is often used in a premixed solution of 30 units of oxytocin in 500 mL of lactated Ringer’s solution. With this mixture, an infusion of 1 mL/hour results in the administration of 1 mU of oxytocin per minute (1 mU/min). There is no national consensus on an optimal oxytocin infusion regimen for induction or augmentation of labor. A commonly used low-dose regimen is an initial dose of 1 to 2 mU/min, with a dose increase of 1 to 2 mU/min every 30 to 40 minutes until regular uterine contractions occur every 2 to 3 minutes.5 An example of a high-dose oxytocin regimen is an initial dose of 6 mU/min with an increase of 3 to 6 mU/min every 30 to 40 minutes (induction of labor).6
A randomized trial reported that, compared with a low-dose oxytocin regimen, a high-dose regimen increased the risk of tachysystole without a significant change in cesarean birth rate.7 A Cochrane review concluded that, compared with low-dose regimens, high-dose oxytocin regimens were more likely to be associated with tachysystole.8 Based on these reports, I would suggest avoiding the use of a high-dose oxytocin regimen. Experts have reported that an oxytocin dose of approximately 6 mU/min achieves a circulating oxytocin concentration similar to that observed in normal spontaneous labor.9
Continue to: Maximum dose of oxytocin infusion...
Maximum dose of oxytocin infusion
There is no national consensus on the maximum safe dose of oxytocin for induction or augmentation of labor. Many labor and delivery units have a protocol where the maximum dose of oxytocin is 20 mU/min for women in the following clinical situations: previous vaginal delivery, prior cesarean delivery, multiple gestation, and nulliparous women in the second stage of labor. A maximum oxytocin dose of 30 mU/min may be appropriate for nulliparous women in the first stage of labor. Some units permit an oxytocin dose of 40 mU/min. Many labor nurses are concerned that an oxytocin dose that high may be associated with an increased frequency of adverse effects.
Management of the oxytocin dose when tachysystole is diagnosed
Tachysystole is defined as more than 5 uterine contractions in 10 minutes averaged over 30 minutes.5,6 Because uterine contractions cause a reduction in oxygen delivery to the fetus, tachysystole, prolonged uterine contractions, and sustained elevated intrauterine pressure can result in fetal hypoxia and an abnormal fetal heart rate (FHR) pattern. If tachysystole is detected and the FHR pattern is Category 1, the oxytocin dose should be reduced. If tachysystole is detected and the FHR pattern is a concerning Category 2 or Category 3 pattern, the oxytocin infusion should be discontinued until the concerning FHR pattern resolves. If tachysystole is diagnosed, changing the maternal position (ensuring a lateral maternal position) and administering an intravenous bolus of 500 mL of lactated Ringer’s solution may help resolve an abnormal FHR. Terbutaline 0.25 mg, administered by subcutaneous injection, may be given to reduce myometrial contractility. Following resolution of an episode of tachysystole with a concerning FHR tracing, the oxytocin infusion can be restarted at a dose less than the dose that was associated with the tachysystole.
Inadvertent excess oxytocin administration
Oxytocin only should be administered using a computerized medication infusion pump with the oxytocin line piggybacked into a main infusion line.5 Occasionally, an excessively large bolus of oxytocin is administered inadvertently because the oxytocin line was mistakenly thought to be the main line or because of an infusion pump failure. These situations usually result in a tetanic contraction that will need to be treated by the immediate discontinuation of the oxytocin infusion, a fluid bolus, and one or more doses of terbutaline.
Reduction in oxytocin dose as labor progresses
Many investigators have reported that once rapid cervical dilation is occurring, or in the second stage of labor, the dose of exogenous oxytocin often can be reduced without stalling the progress of labor. Dilation of the vagina and pelvic floor, which occurs late in the process of labor, is a powerful stimulus for the release of oxytocin from the posterior pituitary.10,11 The marked increase in endogenous secretion of oxytocin during the second stage of labor may be the reason that the exogenous oxytocin infusion can be reduced or discontinued.
In a systematic review and meta-analysis, discontinuation of oxytocin after 5 cm of cervical dilation was associated with a reduced rate of uterine tachysystole and no increase in cesarean delivery.12 A Cochrane evidence-based review also concluded that once rapid cervical dilation is occurring, the dose of oxytocin can be reduced with a decrease in the rate of tachysystole with an abnormal FHR and without an increase in the rate of cesarean delivery.13
Continue to: Management of the oxytocin dose is a common cause of clinical disagreement...
Management of the oxytocin dose is a common cause of clinical disagreement
As noted in two recent research studies, experienced independent professional labor nurses often feel pressured by obstetricians to increase the dose of oxytocin. One nurse reported that physicians “like the pit pushed and you’d better push it and go, go, go, otherwise they’ll be…really mad if it is not going.” Many obstetricians favor working with a labor nurse who will actively manage labor by aggressively increasing the oxytocin dose. One obstetrician reported, “When I hear I’ve got a nurse who will go up on the pit, I know it’s going to be a good day.”14
Obstetricians and labor nurses with a good relationship can openly discuss differing perspectives and find a compromise solution. However, if the relationship is not good, the conflict may not be resolved, and the labor nurse may use a passive-aggressive approach to the situation. As one nurse reported, “It actually depends on the doctor and his personality. I know that there were times when I had a doc who would throw a fit if I didn’t up the pitocin, so I would pacify him by agreeing to, but never would.”15
An oxytocin checklist may help to reduce conflict over the optimal management of oxytocin infusion and improve patient safety.16 Practice variation among nurses, obstetricians, and nurse midwives may contribute to difficulty in achieving a consensus on how to manage oxytocin. One approach to reducing practice variation is to use checklists to improve collaboration and uniformity on a clinical team. Clark and colleagues describe the beneficial effect of both a pre-oxytocin checklist and an oxytocin in-use checklist.16 Their in-use checklist, which is completed every 30 minutes by the labor nurse, recommended decreasing the dose of oxytocin unless the FHR is reassuring and no tachysystole has occurred. In one retrospective study, when compared against outcomes prior to the use of a checklist, the use of the checklist resulted in a lower maximum dose of oxytocin (11.4 vs 13.8 mU/min; P = .003), a greater 1-minute Apgar score at birth (7.9 vs 7.6; P = .048), and no increase in time to delivery (8.2 vs 8.5 hours) or cesarean delivery rate (13% vs 15%).16 When nurses and obstetricians collaborate using an oxytocin in-use checklist, both clinical variation and probability of conflict are reduced.
Consider use of a checklist to reduce conflict
Oxytocin infusion for induction or augmentation of labor is one of the most common and most important interventions on labor and delivery units. Oxytocin infusion practices vary widely among labor and delivery units. In addition to the lack of a consensus national standard, within any one labor unit the perspectives of obstetricians and labor nurses regarding the management of oxytocin infusions often differ, leading to conflict. The use of an oxytocin in-use checklist may help to reduce variability and improve patient outcomes.17 ●
- Blanks AM, Shmygol A, Thornton S. Regulation of oxytocin receptors and oxytocin receptor signaling. Semin Reprod Med. 2007;25:52-59.
- Fuchs AM, Romero R, Keefe D, et al. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol. 1991;165:1515-1523.
- Fuchs AR, Fuchs F, Husslein P, et al. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734-741.
- Seitchik J, Amico J, Robinson AG, et al. Oxytocin augmentation of dysfunctional labor. IV. Oxytocin pharmacokinetics Am J Obstet Gynecol. 1984;150:225-228.
- Simpson KR. Cervical ripening, labor induction and labor augmentation, 5th edition. Nurs Womens Health. 2020;24:S1-S43.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 107: induction of labor. Obstet Gynecol. 2009;114:386-397.
- Selin L, Wennerholm UB, Jonsson M, et al. High-dose versus low-dose of oxytocin for labor augmentation: a randomized controlled trial. Women Birth. 2019;32:356-363.
- Budden A, Chen LJ, Henry A. High-dose versus low-dose oxytocin infusion regimens for induction of labor at term. Cochrane Database Syst Rev. 2014;CD00970.
- Cuppett CD, Caritis SN. Uterine contraction agents and tocolytics. In: Mattison DR (Ed.) Clinical Pharmacology During Pregnancy. London, United Kingdom: Elsevier;2013:307-330.
- Ferguson JK. A study of the motility of the intact uterus at term. Surg Gynecol Obstet. 1941;73:359-366.
- Fisher DA. Maternal-fetal neurohypophyseal system. Clin Perinatol. 1983;10:695-707.
- Saccone G, Ciadulli A, Baxter JK, et al. Discontinuing oxytocin in the active phase of labor: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:1090-1096.
- Boie S, Glavind J, Velu AV, et al. Discontinuation of oxytocin in the active phase of induced labour. Cochrane Database Syst Rev. 2018;CD012274.
- Simpson KR, James DC, Knox GE. Nurse-physician communication during labor and birth: implications for patient safety. J Obstet Gynecol Neonatal Nursing. 2006;35:547-566.
- Simpson KR, Lyndon A. Clinical disagreements during labor and birth: how does real life compare to best practice? MCN Am J Matern Child Nurs. 2009;34:31-39.
- Clark S, Belfort M, Saade G, et al. Implementation of a conservative checklist-based protocol for oxytocin administration: maternal and newborn outcomes. Am J Obstet Gynecol. 2007;197:480.e1-e5.

Oxytocin is the hormone most commonly administered to women on labor and delivery. It is used for induction of labor, augmentation of labor, and to reduce the risk of postpartum hemorrhage. Licensed independent prescribers, including physicians and nurse midwives, order oxytocin, and licensed professional nurses execute the order by administering the hormone. Optimal management of oxytocin infusion requires effective interprofessional communication and collaboration. During labor it is common for disagreements to arise between the professionals ordering and the professionals administering oxytocin. The disagreements are usually caused by differing perspectives on the appropriate oxytocin dose. Standardized protocols and checklists reduce practice variation and improve patient safety.
Oxytocin hormone
Oxytocin is a cyclic nonapeptide synthesized in the hypothalamus and secreted into the circulation from axonal terminals in the posterior pituitary. In the myometrium, oxytocin activates a membrane G protein-coupled receptor, increasing phospholipase C and intracellular calcium. Following several intracellular chemical cascades, oxytocin stimulation results in myosin and actin filaments sliding over each other initiating shortening of the smooth muscle cell. Myometrial smooth muscle cells are connected by gap junctions, facilitating the coordinated contraction of the uterus.1
Oxytocin pulse frequency and uterine oxytocin receptor concentration both increase during pregnancy and labor, facilitating the birth process. Oxytocin pulse frequency increases from 2.4 pulses per hour before labor to 13.4 pulses per hour in the second stage.2 In addition, uterine oxytocin receptor concentration increases 12-fold from the early second trimester of pregnancy to term.3
Oxytocin has a half-life of approximately 10 to 15 minutes. Many pharmacologists believe that for a given dose of a drug, it takes 4 to 5 half-lives for a stabilized circulating concentration to be achieved. Therefore, during an oxytocin infusion, when the dose is increased it may take 40 to 50 minutes to achieve a new higher, stabile circulating concentration.4
Low-dose vs high-dose oxytocin protocols
Oxytocin is often used in a premixed solution of 30 units of oxytocin in 500 mL of lactated Ringer’s solution. With this mixture, an infusion of 1 mL/hour results in the administration of 1 mU of oxytocin per minute (1 mU/min). There is no national consensus on an optimal oxytocin infusion regimen for induction or augmentation of labor. A commonly used low-dose regimen is an initial dose of 1 to 2 mU/min, with a dose increase of 1 to 2 mU/min every 30 to 40 minutes until regular uterine contractions occur every 2 to 3 minutes.5 An example of a high-dose oxytocin regimen is an initial dose of 6 mU/min with an increase of 3 to 6 mU/min every 30 to 40 minutes (induction of labor).6
A randomized trial reported that, compared with a low-dose oxytocin regimen, a high-dose regimen increased the risk of tachysystole without a significant change in cesarean birth rate.7 A Cochrane review concluded that, compared with low-dose regimens, high-dose oxytocin regimens were more likely to be associated with tachysystole.8 Based on these reports, I would suggest avoiding the use of a high-dose oxytocin regimen. Experts have reported that an oxytocin dose of approximately 6 mU/min achieves a circulating oxytocin concentration similar to that observed in normal spontaneous labor.9
Continue to: Maximum dose of oxytocin infusion...
Maximum dose of oxytocin infusion
There is no national consensus on the maximum safe dose of oxytocin for induction or augmentation of labor. Many labor and delivery units have a protocol where the maximum dose of oxytocin is 20 mU/min for women in the following clinical situations: previous vaginal delivery, prior cesarean delivery, multiple gestation, and nulliparous women in the second stage of labor. A maximum oxytocin dose of 30 mU/min may be appropriate for nulliparous women in the first stage of labor. Some units permit an oxytocin dose of 40 mU/min. Many labor nurses are concerned that an oxytocin dose that high may be associated with an increased frequency of adverse effects.
Management of the oxytocin dose when tachysystole is diagnosed
Tachysystole is defined as more than 5 uterine contractions in 10 minutes averaged over 30 minutes.5,6 Because uterine contractions cause a reduction in oxygen delivery to the fetus, tachysystole, prolonged uterine contractions, and sustained elevated intrauterine pressure can result in fetal hypoxia and an abnormal fetal heart rate (FHR) pattern. If tachysystole is detected and the FHR pattern is Category 1, the oxytocin dose should be reduced. If tachysystole is detected and the FHR pattern is a concerning Category 2 or Category 3 pattern, the oxytocin infusion should be discontinued until the concerning FHR pattern resolves. If tachysystole is diagnosed, changing the maternal position (ensuring a lateral maternal position) and administering an intravenous bolus of 500 mL of lactated Ringer’s solution may help resolve an abnormal FHR. Terbutaline 0.25 mg, administered by subcutaneous injection, may be given to reduce myometrial contractility. Following resolution of an episode of tachysystole with a concerning FHR tracing, the oxytocin infusion can be restarted at a dose less than the dose that was associated with the tachysystole.
Inadvertent excess oxytocin administration
Oxytocin only should be administered using a computerized medication infusion pump with the oxytocin line piggybacked into a main infusion line.5 Occasionally, an excessively large bolus of oxytocin is administered inadvertently because the oxytocin line was mistakenly thought to be the main line or because of an infusion pump failure. These situations usually result in a tetanic contraction that will need to be treated by the immediate discontinuation of the oxytocin infusion, a fluid bolus, and one or more doses of terbutaline.
Reduction in oxytocin dose as labor progresses
Many investigators have reported that once rapid cervical dilation is occurring, or in the second stage of labor, the dose of exogenous oxytocin often can be reduced without stalling the progress of labor. Dilation of the vagina and pelvic floor, which occurs late in the process of labor, is a powerful stimulus for the release of oxytocin from the posterior pituitary.10,11 The marked increase in endogenous secretion of oxytocin during the second stage of labor may be the reason that the exogenous oxytocin infusion can be reduced or discontinued.
In a systematic review and meta-analysis, discontinuation of oxytocin after 5 cm of cervical dilation was associated with a reduced rate of uterine tachysystole and no increase in cesarean delivery.12 A Cochrane evidence-based review also concluded that once rapid cervical dilation is occurring, the dose of oxytocin can be reduced with a decrease in the rate of tachysystole with an abnormal FHR and without an increase in the rate of cesarean delivery.13
Continue to: Management of the oxytocin dose is a common cause of clinical disagreement...
Management of the oxytocin dose is a common cause of clinical disagreement
As noted in two recent research studies, experienced independent professional labor nurses often feel pressured by obstetricians to increase the dose of oxytocin. One nurse reported that physicians “like the pit pushed and you’d better push it and go, go, go, otherwise they’ll be…really mad if it is not going.” Many obstetricians favor working with a labor nurse who will actively manage labor by aggressively increasing the oxytocin dose. One obstetrician reported, “When I hear I’ve got a nurse who will go up on the pit, I know it’s going to be a good day.”14
Obstetricians and labor nurses with a good relationship can openly discuss differing perspectives and find a compromise solution. However, if the relationship is not good, the conflict may not be resolved, and the labor nurse may use a passive-aggressive approach to the situation. As one nurse reported, “It actually depends on the doctor and his personality. I know that there were times when I had a doc who would throw a fit if I didn’t up the pitocin, so I would pacify him by agreeing to, but never would.”15
An oxytocin checklist may help to reduce conflict over the optimal management of oxytocin infusion and improve patient safety.16 Practice variation among nurses, obstetricians, and nurse midwives may contribute to difficulty in achieving a consensus on how to manage oxytocin. One approach to reducing practice variation is to use checklists to improve collaboration and uniformity on a clinical team. Clark and colleagues describe the beneficial effect of both a pre-oxytocin checklist and an oxytocin in-use checklist.16 Their in-use checklist, which is completed every 30 minutes by the labor nurse, recommended decreasing the dose of oxytocin unless the FHR is reassuring and no tachysystole has occurred. In one retrospective study, when compared against outcomes prior to the use of a checklist, the use of the checklist resulted in a lower maximum dose of oxytocin (11.4 vs 13.8 mU/min; P = .003), a greater 1-minute Apgar score at birth (7.9 vs 7.6; P = .048), and no increase in time to delivery (8.2 vs 8.5 hours) or cesarean delivery rate (13% vs 15%).16 When nurses and obstetricians collaborate using an oxytocin in-use checklist, both clinical variation and probability of conflict are reduced.
Consider use of a checklist to reduce conflict
Oxytocin infusion for induction or augmentation of labor is one of the most common and most important interventions on labor and delivery units. Oxytocin infusion practices vary widely among labor and delivery units. In addition to the lack of a consensus national standard, within any one labor unit the perspectives of obstetricians and labor nurses regarding the management of oxytocin infusions often differ, leading to conflict. The use of an oxytocin in-use checklist may help to reduce variability and improve patient outcomes.17 ●

Oxytocin is the hormone most commonly administered to women on labor and delivery. It is used for induction of labor, augmentation of labor, and to reduce the risk of postpartum hemorrhage. Licensed independent prescribers, including physicians and nurse midwives, order oxytocin, and licensed professional nurses execute the order by administering the hormone. Optimal management of oxytocin infusion requires effective interprofessional communication and collaboration. During labor it is common for disagreements to arise between the professionals ordering and the professionals administering oxytocin. The disagreements are usually caused by differing perspectives on the appropriate oxytocin dose. Standardized protocols and checklists reduce practice variation and improve patient safety.
Oxytocin hormone
Oxytocin is a cyclic nonapeptide synthesized in the hypothalamus and secreted into the circulation from axonal terminals in the posterior pituitary. In the myometrium, oxytocin activates a membrane G protein-coupled receptor, increasing phospholipase C and intracellular calcium. Following several intracellular chemical cascades, oxytocin stimulation results in myosin and actin filaments sliding over each other initiating shortening of the smooth muscle cell. Myometrial smooth muscle cells are connected by gap junctions, facilitating the coordinated contraction of the uterus.1
Oxytocin pulse frequency and uterine oxytocin receptor concentration both increase during pregnancy and labor, facilitating the birth process. Oxytocin pulse frequency increases from 2.4 pulses per hour before labor to 13.4 pulses per hour in the second stage.2 In addition, uterine oxytocin receptor concentration increases 12-fold from the early second trimester of pregnancy to term.3
Oxytocin has a half-life of approximately 10 to 15 minutes. Many pharmacologists believe that for a given dose of a drug, it takes 4 to 5 half-lives for a stabilized circulating concentration to be achieved. Therefore, during an oxytocin infusion, when the dose is increased it may take 40 to 50 minutes to achieve a new higher, stabile circulating concentration.4
Low-dose vs high-dose oxytocin protocols
Oxytocin is often used in a premixed solution of 30 units of oxytocin in 500 mL of lactated Ringer’s solution. With this mixture, an infusion of 1 mL/hour results in the administration of 1 mU of oxytocin per minute (1 mU/min). There is no national consensus on an optimal oxytocin infusion regimen for induction or augmentation of labor. A commonly used low-dose regimen is an initial dose of 1 to 2 mU/min, with a dose increase of 1 to 2 mU/min every 30 to 40 minutes until regular uterine contractions occur every 2 to 3 minutes.5 An example of a high-dose oxytocin regimen is an initial dose of 6 mU/min with an increase of 3 to 6 mU/min every 30 to 40 minutes (induction of labor).6
A randomized trial reported that, compared with a low-dose oxytocin regimen, a high-dose regimen increased the risk of tachysystole without a significant change in cesarean birth rate.7 A Cochrane review concluded that, compared with low-dose regimens, high-dose oxytocin regimens were more likely to be associated with tachysystole.8 Based on these reports, I would suggest avoiding the use of a high-dose oxytocin regimen. Experts have reported that an oxytocin dose of approximately 6 mU/min achieves a circulating oxytocin concentration similar to that observed in normal spontaneous labor.9
Continue to: Maximum dose of oxytocin infusion...
Maximum dose of oxytocin infusion
There is no national consensus on the maximum safe dose of oxytocin for induction or augmentation of labor. Many labor and delivery units have a protocol where the maximum dose of oxytocin is 20 mU/min for women in the following clinical situations: previous vaginal delivery, prior cesarean delivery, multiple gestation, and nulliparous women in the second stage of labor. A maximum oxytocin dose of 30 mU/min may be appropriate for nulliparous women in the first stage of labor. Some units permit an oxytocin dose of 40 mU/min. Many labor nurses are concerned that an oxytocin dose that high may be associated with an increased frequency of adverse effects.
Management of the oxytocin dose when tachysystole is diagnosed
Tachysystole is defined as more than 5 uterine contractions in 10 minutes averaged over 30 minutes.5,6 Because uterine contractions cause a reduction in oxygen delivery to the fetus, tachysystole, prolonged uterine contractions, and sustained elevated intrauterine pressure can result in fetal hypoxia and an abnormal fetal heart rate (FHR) pattern. If tachysystole is detected and the FHR pattern is Category 1, the oxytocin dose should be reduced. If tachysystole is detected and the FHR pattern is a concerning Category 2 or Category 3 pattern, the oxytocin infusion should be discontinued until the concerning FHR pattern resolves. If tachysystole is diagnosed, changing the maternal position (ensuring a lateral maternal position) and administering an intravenous bolus of 500 mL of lactated Ringer’s solution may help resolve an abnormal FHR. Terbutaline 0.25 mg, administered by subcutaneous injection, may be given to reduce myometrial contractility. Following resolution of an episode of tachysystole with a concerning FHR tracing, the oxytocin infusion can be restarted at a dose less than the dose that was associated with the tachysystole.
Inadvertent excess oxytocin administration
Oxytocin only should be administered using a computerized medication infusion pump with the oxytocin line piggybacked into a main infusion line.5 Occasionally, an excessively large bolus of oxytocin is administered inadvertently because the oxytocin line was mistakenly thought to be the main line or because of an infusion pump failure. These situations usually result in a tetanic contraction that will need to be treated by the immediate discontinuation of the oxytocin infusion, a fluid bolus, and one or more doses of terbutaline.
Reduction in oxytocin dose as labor progresses
Many investigators have reported that once rapid cervical dilation is occurring, or in the second stage of labor, the dose of exogenous oxytocin often can be reduced without stalling the progress of labor. Dilation of the vagina and pelvic floor, which occurs late in the process of labor, is a powerful stimulus for the release of oxytocin from the posterior pituitary.10,11 The marked increase in endogenous secretion of oxytocin during the second stage of labor may be the reason that the exogenous oxytocin infusion can be reduced or discontinued.
In a systematic review and meta-analysis, discontinuation of oxytocin after 5 cm of cervical dilation was associated with a reduced rate of uterine tachysystole and no increase in cesarean delivery.12 A Cochrane evidence-based review also concluded that once rapid cervical dilation is occurring, the dose of oxytocin can be reduced with a decrease in the rate of tachysystole with an abnormal FHR and without an increase in the rate of cesarean delivery.13
Continue to: Management of the oxytocin dose is a common cause of clinical disagreement...
Management of the oxytocin dose is a common cause of clinical disagreement
As noted in two recent research studies, experienced independent professional labor nurses often feel pressured by obstetricians to increase the dose of oxytocin. One nurse reported that physicians “like the pit pushed and you’d better push it and go, go, go, otherwise they’ll be…really mad if it is not going.” Many obstetricians favor working with a labor nurse who will actively manage labor by aggressively increasing the oxytocin dose. One obstetrician reported, “When I hear I’ve got a nurse who will go up on the pit, I know it’s going to be a good day.”14
Obstetricians and labor nurses with a good relationship can openly discuss differing perspectives and find a compromise solution. However, if the relationship is not good, the conflict may not be resolved, and the labor nurse may use a passive-aggressive approach to the situation. As one nurse reported, “It actually depends on the doctor and his personality. I know that there were times when I had a doc who would throw a fit if I didn’t up the pitocin, so I would pacify him by agreeing to, but never would.”15
An oxytocin checklist may help to reduce conflict over the optimal management of oxytocin infusion and improve patient safety.16 Practice variation among nurses, obstetricians, and nurse midwives may contribute to difficulty in achieving a consensus on how to manage oxytocin. One approach to reducing practice variation is to use checklists to improve collaboration and uniformity on a clinical team. Clark and colleagues describe the beneficial effect of both a pre-oxytocin checklist and an oxytocin in-use checklist.16 Their in-use checklist, which is completed every 30 minutes by the labor nurse, recommended decreasing the dose of oxytocin unless the FHR is reassuring and no tachysystole has occurred. In one retrospective study, when compared against outcomes prior to the use of a checklist, the use of the checklist resulted in a lower maximum dose of oxytocin (11.4 vs 13.8 mU/min; P = .003), a greater 1-minute Apgar score at birth (7.9 vs 7.6; P = .048), and no increase in time to delivery (8.2 vs 8.5 hours) or cesarean delivery rate (13% vs 15%).16 When nurses and obstetricians collaborate using an oxytocin in-use checklist, both clinical variation and probability of conflict are reduced.
Consider use of a checklist to reduce conflict
Oxytocin infusion for induction or augmentation of labor is one of the most common and most important interventions on labor and delivery units. Oxytocin infusion practices vary widely among labor and delivery units. In addition to the lack of a consensus national standard, within any one labor unit the perspectives of obstetricians and labor nurses regarding the management of oxytocin infusions often differ, leading to conflict. The use of an oxytocin in-use checklist may help to reduce variability and improve patient outcomes.17 ●
- Blanks AM, Shmygol A, Thornton S. Regulation of oxytocin receptors and oxytocin receptor signaling. Semin Reprod Med. 2007;25:52-59.
- Fuchs AM, Romero R, Keefe D, et al. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol. 1991;165:1515-1523.
- Fuchs AR, Fuchs F, Husslein P, et al. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734-741.
- Seitchik J, Amico J, Robinson AG, et al. Oxytocin augmentation of dysfunctional labor. IV. Oxytocin pharmacokinetics Am J Obstet Gynecol. 1984;150:225-228.
- Simpson KR. Cervical ripening, labor induction and labor augmentation, 5th edition. Nurs Womens Health. 2020;24:S1-S43.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 107: induction of labor. Obstet Gynecol. 2009;114:386-397.
- Selin L, Wennerholm UB, Jonsson M, et al. High-dose versus low-dose of oxytocin for labor augmentation: a randomized controlled trial. Women Birth. 2019;32:356-363.
- Budden A, Chen LJ, Henry A. High-dose versus low-dose oxytocin infusion regimens for induction of labor at term. Cochrane Database Syst Rev. 2014;CD00970.
- Cuppett CD, Caritis SN. Uterine contraction agents and tocolytics. In: Mattison DR (Ed.) Clinical Pharmacology During Pregnancy. London, United Kingdom: Elsevier;2013:307-330.
- Ferguson JK. A study of the motility of the intact uterus at term. Surg Gynecol Obstet. 1941;73:359-366.
- Fisher DA. Maternal-fetal neurohypophyseal system. Clin Perinatol. 1983;10:695-707.
- Saccone G, Ciadulli A, Baxter JK, et al. Discontinuing oxytocin in the active phase of labor: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:1090-1096.
- Boie S, Glavind J, Velu AV, et al. Discontinuation of oxytocin in the active phase of induced labour. Cochrane Database Syst Rev. 2018;CD012274.
- Simpson KR, James DC, Knox GE. Nurse-physician communication during labor and birth: implications for patient safety. J Obstet Gynecol Neonatal Nursing. 2006;35:547-566.
- Simpson KR, Lyndon A. Clinical disagreements during labor and birth: how does real life compare to best practice? MCN Am J Matern Child Nurs. 2009;34:31-39.
- Clark S, Belfort M, Saade G, et al. Implementation of a conservative checklist-based protocol for oxytocin administration: maternal and newborn outcomes. Am J Obstet Gynecol. 2007;197:480.e1-e5.
- Blanks AM, Shmygol A, Thornton S. Regulation of oxytocin receptors and oxytocin receptor signaling. Semin Reprod Med. 2007;25:52-59.
- Fuchs AM, Romero R, Keefe D, et al. Oxytocin secretion and human parturition: pulse frequency and duration increase during spontaneous labor in women. Am J Obstet Gynecol. 1991;165:1515-1523.
- Fuchs AR, Fuchs F, Husslein P, et al. Oxytocin receptors in the human uterus during pregnancy and parturition. Am J Obstet Gynecol. 1984;150:734-741.
- Seitchik J, Amico J, Robinson AG, et al. Oxytocin augmentation of dysfunctional labor. IV. Oxytocin pharmacokinetics Am J Obstet Gynecol. 1984;150:225-228.
- Simpson KR. Cervical ripening, labor induction and labor augmentation, 5th edition. Nurs Womens Health. 2020;24:S1-S43.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 107: induction of labor. Obstet Gynecol. 2009;114:386-397.
- Selin L, Wennerholm UB, Jonsson M, et al. High-dose versus low-dose of oxytocin for labor augmentation: a randomized controlled trial. Women Birth. 2019;32:356-363.
- Budden A, Chen LJ, Henry A. High-dose versus low-dose oxytocin infusion regimens for induction of labor at term. Cochrane Database Syst Rev. 2014;CD00970.
- Cuppett CD, Caritis SN. Uterine contraction agents and tocolytics. In: Mattison DR (Ed.) Clinical Pharmacology During Pregnancy. London, United Kingdom: Elsevier;2013:307-330.
- Ferguson JK. A study of the motility of the intact uterus at term. Surg Gynecol Obstet. 1941;73:359-366.
- Fisher DA. Maternal-fetal neurohypophyseal system. Clin Perinatol. 1983;10:695-707.
- Saccone G, Ciadulli A, Baxter JK, et al. Discontinuing oxytocin in the active phase of labor: a systematic review and meta-analysis. Obstet Gynecol. 2017;130:1090-1096.
- Boie S, Glavind J, Velu AV, et al. Discontinuation of oxytocin in the active phase of induced labour. Cochrane Database Syst Rev. 2018;CD012274.
- Simpson KR, James DC, Knox GE. Nurse-physician communication during labor and birth: implications for patient safety. J Obstet Gynecol Neonatal Nursing. 2006;35:547-566.
- Simpson KR, Lyndon A. Clinical disagreements during labor and birth: how does real life compare to best practice? MCN Am J Matern Child Nurs. 2009;34:31-39.
- Clark S, Belfort M, Saade G, et al. Implementation of a conservative checklist-based protocol for oxytocin administration: maternal and newborn outcomes. Am J Obstet Gynecol. 2007;197:480.e1-e5.
Building (or rebuilding) trust amid vaccine hesitancy
Nearly 10 months since the onset of the COVID-19 pandemic in the United States, ICUs across the country are reaching maximum capacity and hospitalizations are outnumbering the available providers and staff to care for them. Clinicians everywhere are becoming exhausted and frustrated, and the world is all asking when an end to this pandemic will be in sight? The Food and Drug Administration issued emergency authorization for two multidose COVID-19 vaccines that are now being deployed across the country.
At this writing, 2.8 million Americans have received their first COVID-19 vaccine dose, a number far short of the projected 100 million. The limited production capacity and tiered distribution are the main determinants of who gets the vaccine and when, but a third and extremely important factor in whether people will choose to get vaccinated is their level of awareness of and trust in the scientific and medical processes behind wide-scale vaccination.
As medical professionals, many of us wouldn’t hesitate to get vaccinated against a pandemic virus. Concerns about safety and the integrity of the COVID-19 vaccine development process in light of the “warp speed” of its production has many Americans concerned about getting vaccinated. We may not be able to relate to some patients’ reluctance to receive a vaccine that has been confirmed by phase 3 clinical trials with collectively over 66,000 participants (nearly 10% African American in each study) to have an effectiveness of over 90%. We are so intimately familiar with the vaccine development process, the medical terminology used to describe these results and the effectiveness of vaccines overall in eliminating infectious diseases like polio and smallpox. To many of us, receiving the COVID-19 vaccine may be considered a no-brainer. However, and especially for BIPOC (Black, Indigenous, and people of color) patients with sickle cell disease or other hematologic disorders, the history of medical racism and a pattern of negative health care experiences have sown a distrust of the medical research community that spurs vaccine hesitancy despite the far-reaching impact of this pandemic.
I asked an African American friend of mine who is a pediatrician if she would get the vaccine, to which she replied: “People of color are already aware of the experiments and trials performed on our communities without the knowledge and informed consent of those being tested – many of whom were children, impoverished or disenfranchised – so while I personally will get vaccinated, I understand why some wouldn’t be as trusting.”
In December 2020, a poll by the Kaiser Family Foundation found that a primary factor behind COVID-19 vaccine hesitancy among Black respondents was the fear of catching the virus from the vaccine.1 While this is an understandable concern for the general public, there needs to be a wide-reaching patient education effort to teach about the vaccine and how it was designed to work, (especially around the use of messenger RNA technology) so as to put such fears to rest and empower patients to go into this process as knowledgeable advocates for their own health.
With so many sources of information about the pandemic, there are just as many sources of misinformation being spouted by biased outlets on all sides of the political spectrum.
Physicians are most likely to influence their patients’ willingness to take the flu vaccine, accept treatment recommendations as well as potentially accept the COVID-19 vaccine. Our responsibility as care providers is to help our patients filter through the information and provide them with the tools, they need to understand what is fact and what is fiction. We are to answer their questions and concerns, correct any misunderstandings, and address their individual reasons for hesitancy. We must also pay particular attention to our BIPOC patient populations who may have unique reasons for declining the vaccine, compared with the general population. Our conversations should not only reassure patients that the vaccines currently available won’t give them COVID-19, but also address concerns about the efficacy and safety of the vaccine and reiterate that no corners were cut in the development and approval process.
As a hematologist I have had to become very comfortable with having uncomfortable conversations with my patients about the history of maltreatment and discrimination toward minorities in health care, while reassuring them of the current attempts to right those wrongs and the major wins we have had in research when it comes to adapting therapeutics to diverse populations for optimal outcomes.
The conversation about vaccine hesitancy should be held with patience and humility, acknowledging the past and validating patient concerns that will influence their decisions. We need to be more humane and relatable, and use real-world language to clearly share the facts without buzzwords and jargon that may confuse or even reinforce perceptions of lack of transparency.
I received my COVID-19 vaccine on Dec. 29, 2020, and my experience was similar to that of anyone else’s. I had the same concerns most of my patients and colleagues have expressed, but when I saw my sister share her “postvax selfie” on WhatsApp and discussed her experience with her, I felt more comfortable. I then spoke with my allergist, my primary care provider, my husband, and other people in my personal circles before I scheduled my appointment. After my first dose, I called my sister-in-law, a nurse in Canada, who expressed the same concerns that I had and was about to cancel her appointment for that afternoon. I shared my selfie, I shared my experience, and that afternoon she got her vaccine.
The best way to restore a fundamental trust in science and medicine in our patients is to relate to them as humans. Our patients need to know we have the same concerns and fears that they do and that sometimes we have just as many questions too. Communicating openly and authentically, not only with our patients but in all our spheres of influence, can help rebuild the relationship between the public and the health care system. By giving them a glimpse of our humanity, we can support each other as we hopefully eventually see an end to this pandemic.
Ifeyinwa (Ify) Osunkwo, MD, MPH, is a professor of medicine and the director of the Sickle Cell Disease Enterprise at the Levine Cancer Institute, Atrium Health, Charlotte, N.C. She is the editor in chief of Hematology News.
References
1. www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
Nearly 10 months since the onset of the COVID-19 pandemic in the United States, ICUs across the country are reaching maximum capacity and hospitalizations are outnumbering the available providers and staff to care for them. Clinicians everywhere are becoming exhausted and frustrated, and the world is all asking when an end to this pandemic will be in sight? The Food and Drug Administration issued emergency authorization for two multidose COVID-19 vaccines that are now being deployed across the country.
At this writing, 2.8 million Americans have received their first COVID-19 vaccine dose, a number far short of the projected 100 million. The limited production capacity and tiered distribution are the main determinants of who gets the vaccine and when, but a third and extremely important factor in whether people will choose to get vaccinated is their level of awareness of and trust in the scientific and medical processes behind wide-scale vaccination.
As medical professionals, many of us wouldn’t hesitate to get vaccinated against a pandemic virus. Concerns about safety and the integrity of the COVID-19 vaccine development process in light of the “warp speed” of its production has many Americans concerned about getting vaccinated. We may not be able to relate to some patients’ reluctance to receive a vaccine that has been confirmed by phase 3 clinical trials with collectively over 66,000 participants (nearly 10% African American in each study) to have an effectiveness of over 90%. We are so intimately familiar with the vaccine development process, the medical terminology used to describe these results and the effectiveness of vaccines overall in eliminating infectious diseases like polio and smallpox. To many of us, receiving the COVID-19 vaccine may be considered a no-brainer. However, and especially for BIPOC (Black, Indigenous, and people of color) patients with sickle cell disease or other hematologic disorders, the history of medical racism and a pattern of negative health care experiences have sown a distrust of the medical research community that spurs vaccine hesitancy despite the far-reaching impact of this pandemic.
I asked an African American friend of mine who is a pediatrician if she would get the vaccine, to which she replied: “People of color are already aware of the experiments and trials performed on our communities without the knowledge and informed consent of those being tested – many of whom were children, impoverished or disenfranchised – so while I personally will get vaccinated, I understand why some wouldn’t be as trusting.”
In December 2020, a poll by the Kaiser Family Foundation found that a primary factor behind COVID-19 vaccine hesitancy among Black respondents was the fear of catching the virus from the vaccine.1 While this is an understandable concern for the general public, there needs to be a wide-reaching patient education effort to teach about the vaccine and how it was designed to work, (especially around the use of messenger RNA technology) so as to put such fears to rest and empower patients to go into this process as knowledgeable advocates for their own health.
With so many sources of information about the pandemic, there are just as many sources of misinformation being spouted by biased outlets on all sides of the political spectrum.
Physicians are most likely to influence their patients’ willingness to take the flu vaccine, accept treatment recommendations as well as potentially accept the COVID-19 vaccine. Our responsibility as care providers is to help our patients filter through the information and provide them with the tools, they need to understand what is fact and what is fiction. We are to answer their questions and concerns, correct any misunderstandings, and address their individual reasons for hesitancy. We must also pay particular attention to our BIPOC patient populations who may have unique reasons for declining the vaccine, compared with the general population. Our conversations should not only reassure patients that the vaccines currently available won’t give them COVID-19, but also address concerns about the efficacy and safety of the vaccine and reiterate that no corners were cut in the development and approval process.
As a hematologist I have had to become very comfortable with having uncomfortable conversations with my patients about the history of maltreatment and discrimination toward minorities in health care, while reassuring them of the current attempts to right those wrongs and the major wins we have had in research when it comes to adapting therapeutics to diverse populations for optimal outcomes.
The conversation about vaccine hesitancy should be held with patience and humility, acknowledging the past and validating patient concerns that will influence their decisions. We need to be more humane and relatable, and use real-world language to clearly share the facts without buzzwords and jargon that may confuse or even reinforce perceptions of lack of transparency.
I received my COVID-19 vaccine on Dec. 29, 2020, and my experience was similar to that of anyone else’s. I had the same concerns most of my patients and colleagues have expressed, but when I saw my sister share her “postvax selfie” on WhatsApp and discussed her experience with her, I felt more comfortable. I then spoke with my allergist, my primary care provider, my husband, and other people in my personal circles before I scheduled my appointment. After my first dose, I called my sister-in-law, a nurse in Canada, who expressed the same concerns that I had and was about to cancel her appointment for that afternoon. I shared my selfie, I shared my experience, and that afternoon she got her vaccine.
The best way to restore a fundamental trust in science and medicine in our patients is to relate to them as humans. Our patients need to know we have the same concerns and fears that they do and that sometimes we have just as many questions too. Communicating openly and authentically, not only with our patients but in all our spheres of influence, can help rebuild the relationship between the public and the health care system. By giving them a glimpse of our humanity, we can support each other as we hopefully eventually see an end to this pandemic.
Ifeyinwa (Ify) Osunkwo, MD, MPH, is a professor of medicine and the director of the Sickle Cell Disease Enterprise at the Levine Cancer Institute, Atrium Health, Charlotte, N.C. She is the editor in chief of Hematology News.
References
1. www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
Nearly 10 months since the onset of the COVID-19 pandemic in the United States, ICUs across the country are reaching maximum capacity and hospitalizations are outnumbering the available providers and staff to care for them. Clinicians everywhere are becoming exhausted and frustrated, and the world is all asking when an end to this pandemic will be in sight? The Food and Drug Administration issued emergency authorization for two multidose COVID-19 vaccines that are now being deployed across the country.
At this writing, 2.8 million Americans have received their first COVID-19 vaccine dose, a number far short of the projected 100 million. The limited production capacity and tiered distribution are the main determinants of who gets the vaccine and when, but a third and extremely important factor in whether people will choose to get vaccinated is their level of awareness of and trust in the scientific and medical processes behind wide-scale vaccination.
As medical professionals, many of us wouldn’t hesitate to get vaccinated against a pandemic virus. Concerns about safety and the integrity of the COVID-19 vaccine development process in light of the “warp speed” of its production has many Americans concerned about getting vaccinated. We may not be able to relate to some patients’ reluctance to receive a vaccine that has been confirmed by phase 3 clinical trials with collectively over 66,000 participants (nearly 10% African American in each study) to have an effectiveness of over 90%. We are so intimately familiar with the vaccine development process, the medical terminology used to describe these results and the effectiveness of vaccines overall in eliminating infectious diseases like polio and smallpox. To many of us, receiving the COVID-19 vaccine may be considered a no-brainer. However, and especially for BIPOC (Black, Indigenous, and people of color) patients with sickle cell disease or other hematologic disorders, the history of medical racism and a pattern of negative health care experiences have sown a distrust of the medical research community that spurs vaccine hesitancy despite the far-reaching impact of this pandemic.
I asked an African American friend of mine who is a pediatrician if she would get the vaccine, to which she replied: “People of color are already aware of the experiments and trials performed on our communities without the knowledge and informed consent of those being tested – many of whom were children, impoverished or disenfranchised – so while I personally will get vaccinated, I understand why some wouldn’t be as trusting.”
In December 2020, a poll by the Kaiser Family Foundation found that a primary factor behind COVID-19 vaccine hesitancy among Black respondents was the fear of catching the virus from the vaccine.1 While this is an understandable concern for the general public, there needs to be a wide-reaching patient education effort to teach about the vaccine and how it was designed to work, (especially around the use of messenger RNA technology) so as to put such fears to rest and empower patients to go into this process as knowledgeable advocates for their own health.
With so many sources of information about the pandemic, there are just as many sources of misinformation being spouted by biased outlets on all sides of the political spectrum.
Physicians are most likely to influence their patients’ willingness to take the flu vaccine, accept treatment recommendations as well as potentially accept the COVID-19 vaccine. Our responsibility as care providers is to help our patients filter through the information and provide them with the tools, they need to understand what is fact and what is fiction. We are to answer their questions and concerns, correct any misunderstandings, and address their individual reasons for hesitancy. We must also pay particular attention to our BIPOC patient populations who may have unique reasons for declining the vaccine, compared with the general population. Our conversations should not only reassure patients that the vaccines currently available won’t give them COVID-19, but also address concerns about the efficacy and safety of the vaccine and reiterate that no corners were cut in the development and approval process.
As a hematologist I have had to become very comfortable with having uncomfortable conversations with my patients about the history of maltreatment and discrimination toward minorities in health care, while reassuring them of the current attempts to right those wrongs and the major wins we have had in research when it comes to adapting therapeutics to diverse populations for optimal outcomes.
The conversation about vaccine hesitancy should be held with patience and humility, acknowledging the past and validating patient concerns that will influence their decisions. We need to be more humane and relatable, and use real-world language to clearly share the facts without buzzwords and jargon that may confuse or even reinforce perceptions of lack of transparency.
I received my COVID-19 vaccine on Dec. 29, 2020, and my experience was similar to that of anyone else’s. I had the same concerns most of my patients and colleagues have expressed, but when I saw my sister share her “postvax selfie” on WhatsApp and discussed her experience with her, I felt more comfortable. I then spoke with my allergist, my primary care provider, my husband, and other people in my personal circles before I scheduled my appointment. After my first dose, I called my sister-in-law, a nurse in Canada, who expressed the same concerns that I had and was about to cancel her appointment for that afternoon. I shared my selfie, I shared my experience, and that afternoon she got her vaccine.
The best way to restore a fundamental trust in science and medicine in our patients is to relate to them as humans. Our patients need to know we have the same concerns and fears that they do and that sometimes we have just as many questions too. Communicating openly and authentically, not only with our patients but in all our spheres of influence, can help rebuild the relationship between the public and the health care system. By giving them a glimpse of our humanity, we can support each other as we hopefully eventually see an end to this pandemic.
Ifeyinwa (Ify) Osunkwo, MD, MPH, is a professor of medicine and the director of the Sickle Cell Disease Enterprise at the Levine Cancer Institute, Atrium Health, Charlotte, N.C. She is the editor in chief of Hematology News.
References
1. www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
Let’s ‘cancel’ these obsolete terms in DSM
Psychiatry has made significant scientific advances over the past century. However, it is still saddled with archaic terms, with pejorative connotations, disguised as official medical diagnoses. It is time to “cancel” those terms and replace them with ones that are neutral and have not accumulated baggage.
This process of “creative destruction” of psychiatric terminology is long overdue. It is frankly disturbing that the psychiatric jargon used around the time that the American Psychiatric Association was established 175 years ago (1844) is now considered insults and epithets. We no longer work in “lunatic asylums for the insane,” and our patients with intellectual disabilities are no longer classified as “morons,” “idiots,” or “imbeciles.” Such “diagnoses” have certainly contributed to the stigma of psychiatric brain disorders. Even the noble word “asylum” has acquired a negative valence because in the past it referred to hospitals that housed persons with serious mental illness.
Thankfully, some of the outrageous terms fabricated during the condemnable and dark era of slavery 2 centuries ago were never adopted by organized psychiatry. The absurd diagnosis of “negritude,” whose tenet was that black skin is a disease curable by whitening the skin, was “invented” by none other than Benjamin Rush, the Father of Psychiatry, whose conflicted soul was depicted by concomitantly owning a slave and positioning himself as an ardent abolitionist!
Terms that need to be replaced
Fast-forward to the modern era and consider the following:
Borderline personality disorder. It is truly tragic how this confusing and non-scientific term is used as an official diagnosis for a set of seriously ill persons. It is loaded with obloquy, indignity, and derision that completely ignore the tumult, self-harm, and disability with which patients who carry this label are burdened throughout their lives, despite being intelligent. This is a serious brain disorder that has been shown to be highly genetic and is characterized by many well-established structural brain abnormalities that have been documented in neuroimaging studies.1,2 Borderline personality should not be classified as a personality disorder but as an illness with multiple signs and symptoms, including mood lability, anger, impulsivity, self-cutting, suicidal urges, feelings of abandonment, and micro-psychotic episodes. A more clinically accurate term should be coined very soon to replace borderline personality, which should be discarded to the trash heap of obsolete psychiatric terms, and no longer inflicted on patients.
Neurosis. What is the justification for continuing to use the term “neurotic” for a person who has an anxiety disorder? Is it used because Jung and Freud propagated the term “neurosis” (after it was coined by William Cullen in 1769)? Neurosis has degenerated from a psychiatric diagnosis to a scornful snub that must never be used for any patient.
Schizophrenia. This diagnosis, coined by Eugen Bleuler to replace the narrow and pessimistic “dementia praecox” proposed by Emil Kraepelin in the 1920s, initially seemed to be a neutral description of a thought disorder (split associations, not split personality). Bleuler was perceptive enough to call his book Dementia Praecox or the Group of Schizophrenias, which is consistent with the modern scientific research that confirms schizophrenia is a very heterogeneous syndrome with hundreds of genetic and environmental biotypes with a similar phenotype but a wide range of severity, treatment response, and functional outcomes. However, in subsequent decades, schizophrenia became one of the most demeaning labels in psychiatry, casting a shadow of hopelessness and disability on the people who have this serious neurologic condition with many psychiatric symptoms. The term that should replace schizophrenia should be no more degrading than stroke, multiple sclerosis, or myocardial infarction.
Continue to: Over the past 15 years...
Over the past 15 years, an expanding group of schizophrenia experts have agreed that this term must be changed to one that reflects the core features of this syndrome, and have proposed terms such as “salience syndrome,” “psychosis-spectrum,” and “reality distortion and cognitive impairment disorder.”3 In fact, several countries have already adopted a new official diagnosis for schizophrenia.4 Japan now uses the term “integration disorder,” which has significantly reduced the stigma of this brain disorder.5 South Korea changed the name to “attunement disorder.” Hong Kong and Taiwan now use “dysfunction of thought and perception.” Some researchers recommend calling schizophrenia “Bleuler’s syndrome,” a neutral eponymous designation.
One of the most irritating things about the term schizophrenia is the widespread misconception that it means “split personality.” This prompts some sports announcers to call a football team “schizophrenic” if they play well in the first half and badly in the second. The stock market is labeled “schizophrenic” if it goes up one day and way down on the next. No other medical term is misused by the media as often as the term schizophrenia.
Narcissistic personality disorder. The origin of this diagnostic category is the concept of “malignant narcissism” coined by Erich Fromm in 1964, which he designated as “the quintessence of evil.” I strongly object to implying that evil is part of any psychiatric diagnosis. Numerous studies have found structural brain abnormalities (in both gray and white matter) in patients diagnosed with psychopathic traits.6 Later, malignant narcissism was reframed as narcissistic personality disorder in 1971 by Herbert Rosenfeld. Although malignant narcissism was never accepted by either the DSM or the International Classification of Diseases, narcissistic personality disorder has been included in the DSM for the past few decades. This diagnosis reeks of disparagement and negativity. Persons with narcissistic personality disorder have been shown to have pathological brain changes in resting-state functional connectivity,7 weakened frontostriatal white matter connectivity,8,9 and a reduced frontal thickness and cortical volume.10 A distorted sense of self and others is a socially disabling disorder that should generate empathy, not disdain. Narcissistic personality disorder should be replaced by a term that accurately describes its behavioral pathology, and should not incorporate Greek mythology.
Mania. This is another unfortunate diagnosis that immediately evokes a negative image of patients who suffer from a potentially lethal brain disorder. It was fortunate that Robert Kendall coined the term “bipolar disorder” to replace “manic-depressive illness,” but mania is still being used within bipolar disorder as a prominent clinical phase. While depression accurately describes the mood in the other phase of this disorder, the term mania evokes wild, irrational behavior. Because the actual mood symptom cluster in mania is either elation/grandiosity or irritability/anger, why not replace mania with “elation/irritability phase of bipolar disorder”? It is more descriptive of the patient’s mood and is less pejorative.
Nomenclature is vital, and words do matter, especially when used as a diagnostic medical term. Psychiatry must “cancel” its archaic names, which are infused with negative connotations. Reinventing the psychiatric lexicon is a necessary act of renewal in a specialty where a poorly worded diagnostic label can morph into the equivalent of a “scarlet letter.” Think of other contemptuous terms, such as refrigerator mother, male hysteria, moral insanity, toxic parents, inadequate personality disorder, neurasthenia, or catastrophic schizophrenia.
General medicine regularly discards many of its obsolete terms.11 These include terms such as ablepsy, ague, camp fever, bloody flux, chlorosis, catarrh, consumption, dropsy, French pox, phthisis, milk sickness, and scrumpox.
Think also of how society abandoned the antediluvian names of boys and girls. Few parents these days would name their son Ackley, Allard, Arundel, Awarnach, Beldon, Durward, Grower, Kenlm, or Legolan, or name their daughter Afton, Agrona, Arantxa, Corliss, Demelza, Eartha, Maida, Obsession, Radella, or Sacrifice.In summary, a necessary part of psychiatry’s progress is shedding obsolete terminology, even if it means slaughtering some widely used “traditional” vocabulary. It is a necessary act of renewal, and the image of psychiatry will be burnished by it.
1. Nasrallah HA. Borderline personality disorder is a heritable brain disease. Current Psychiatry. 2014;13(4):19-20,32.
2. Sagarwala R, Nasrallah HA. White matter pathology in patients with borderline personality disorder: a review of controlled DTI studies. Ann Clin Psychiatry. 2020;32(4):281-286.
3. Keshavan MS, Tandon R, Nasrallah HA. Renaming schizophrenia: keeping up with the facts. Schizophr Res. 2013;148(1-3):1-2.
4. Lasalvia A, Penta E, Sartorius N, et al. Should the label “schizophrenia” be abandoned? Schizophr Res. 2015;162(1-3):276-284.
5. Takahashi H, Ideno T, Okubo S, et al. Impact of changing the Japanese term for “schizophrenia” for reasons of stereotypical beliefs of schizophrenia in Japanese youth. Schizophr Res. 2009;112(1-3):149-152.
6. Johanson M, Vaurio D, Tiihunen J, et al. A systematic literature review of neuroimaging of psychopathic traits. Front Psychiatry. 2020;10:1027.
7. Yang, W, Cun L, Du X, et al. Gender differences in brain structure and resting-state functional connectivity related to narcissistic personality. Sci Rep. 2015;5:10924.
8. Chester DS, Cynam DR, Powell DK, et al. Narcissismis associated with weakened frontostriatal connectivity: a DTI study. Soc Cogn Affect Neurosci. 2016;11(7):1036-1040.
9. Nenadic I, Gullmar D, Dietzek M, et al. Brain structure in narcissistic personality disorder: a VBM and DTI pilot study. Psychiatry Res. 2015;231(2):184-186.
10. Mao Y, Sang N, Wang Y, et al. Reduced frontal cortex thickness and cortical volume associated with pathological narcissism. Neuroscience. 2016;378:51-57.
11. Nasrallah HA. The transient truths of medical ‘progress.’ Current Psychiatry. 2014;13(6):23-24.
Psychiatry has made significant scientific advances over the past century. However, it is still saddled with archaic terms, with pejorative connotations, disguised as official medical diagnoses. It is time to “cancel” those terms and replace them with ones that are neutral and have not accumulated baggage.
This process of “creative destruction” of psychiatric terminology is long overdue. It is frankly disturbing that the psychiatric jargon used around the time that the American Psychiatric Association was established 175 years ago (1844) is now considered insults and epithets. We no longer work in “lunatic asylums for the insane,” and our patients with intellectual disabilities are no longer classified as “morons,” “idiots,” or “imbeciles.” Such “diagnoses” have certainly contributed to the stigma of psychiatric brain disorders. Even the noble word “asylum” has acquired a negative valence because in the past it referred to hospitals that housed persons with serious mental illness.
Thankfully, some of the outrageous terms fabricated during the condemnable and dark era of slavery 2 centuries ago were never adopted by organized psychiatry. The absurd diagnosis of “negritude,” whose tenet was that black skin is a disease curable by whitening the skin, was “invented” by none other than Benjamin Rush, the Father of Psychiatry, whose conflicted soul was depicted by concomitantly owning a slave and positioning himself as an ardent abolitionist!
Terms that need to be replaced
Fast-forward to the modern era and consider the following:
Borderline personality disorder. It is truly tragic how this confusing and non-scientific term is used as an official diagnosis for a set of seriously ill persons. It is loaded with obloquy, indignity, and derision that completely ignore the tumult, self-harm, and disability with which patients who carry this label are burdened throughout their lives, despite being intelligent. This is a serious brain disorder that has been shown to be highly genetic and is characterized by many well-established structural brain abnormalities that have been documented in neuroimaging studies.1,2 Borderline personality should not be classified as a personality disorder but as an illness with multiple signs and symptoms, including mood lability, anger, impulsivity, self-cutting, suicidal urges, feelings of abandonment, and micro-psychotic episodes. A more clinically accurate term should be coined very soon to replace borderline personality, which should be discarded to the trash heap of obsolete psychiatric terms, and no longer inflicted on patients.
Neurosis. What is the justification for continuing to use the term “neurotic” for a person who has an anxiety disorder? Is it used because Jung and Freud propagated the term “neurosis” (after it was coined by William Cullen in 1769)? Neurosis has degenerated from a psychiatric diagnosis to a scornful snub that must never be used for any patient.
Schizophrenia. This diagnosis, coined by Eugen Bleuler to replace the narrow and pessimistic “dementia praecox” proposed by Emil Kraepelin in the 1920s, initially seemed to be a neutral description of a thought disorder (split associations, not split personality). Bleuler was perceptive enough to call his book Dementia Praecox or the Group of Schizophrenias, which is consistent with the modern scientific research that confirms schizophrenia is a very heterogeneous syndrome with hundreds of genetic and environmental biotypes with a similar phenotype but a wide range of severity, treatment response, and functional outcomes. However, in subsequent decades, schizophrenia became one of the most demeaning labels in psychiatry, casting a shadow of hopelessness and disability on the people who have this serious neurologic condition with many psychiatric symptoms. The term that should replace schizophrenia should be no more degrading than stroke, multiple sclerosis, or myocardial infarction.
Continue to: Over the past 15 years...
Over the past 15 years, an expanding group of schizophrenia experts have agreed that this term must be changed to one that reflects the core features of this syndrome, and have proposed terms such as “salience syndrome,” “psychosis-spectrum,” and “reality distortion and cognitive impairment disorder.”3 In fact, several countries have already adopted a new official diagnosis for schizophrenia.4 Japan now uses the term “integration disorder,” which has significantly reduced the stigma of this brain disorder.5 South Korea changed the name to “attunement disorder.” Hong Kong and Taiwan now use “dysfunction of thought and perception.” Some researchers recommend calling schizophrenia “Bleuler’s syndrome,” a neutral eponymous designation.
One of the most irritating things about the term schizophrenia is the widespread misconception that it means “split personality.” This prompts some sports announcers to call a football team “schizophrenic” if they play well in the first half and badly in the second. The stock market is labeled “schizophrenic” if it goes up one day and way down on the next. No other medical term is misused by the media as often as the term schizophrenia.
Narcissistic personality disorder. The origin of this diagnostic category is the concept of “malignant narcissism” coined by Erich Fromm in 1964, which he designated as “the quintessence of evil.” I strongly object to implying that evil is part of any psychiatric diagnosis. Numerous studies have found structural brain abnormalities (in both gray and white matter) in patients diagnosed with psychopathic traits.6 Later, malignant narcissism was reframed as narcissistic personality disorder in 1971 by Herbert Rosenfeld. Although malignant narcissism was never accepted by either the DSM or the International Classification of Diseases, narcissistic personality disorder has been included in the DSM for the past few decades. This diagnosis reeks of disparagement and negativity. Persons with narcissistic personality disorder have been shown to have pathological brain changes in resting-state functional connectivity,7 weakened frontostriatal white matter connectivity,8,9 and a reduced frontal thickness and cortical volume.10 A distorted sense of self and others is a socially disabling disorder that should generate empathy, not disdain. Narcissistic personality disorder should be replaced by a term that accurately describes its behavioral pathology, and should not incorporate Greek mythology.
Mania. This is another unfortunate diagnosis that immediately evokes a negative image of patients who suffer from a potentially lethal brain disorder. It was fortunate that Robert Kendall coined the term “bipolar disorder” to replace “manic-depressive illness,” but mania is still being used within bipolar disorder as a prominent clinical phase. While depression accurately describes the mood in the other phase of this disorder, the term mania evokes wild, irrational behavior. Because the actual mood symptom cluster in mania is either elation/grandiosity or irritability/anger, why not replace mania with “elation/irritability phase of bipolar disorder”? It is more descriptive of the patient’s mood and is less pejorative.
Nomenclature is vital, and words do matter, especially when used as a diagnostic medical term. Psychiatry must “cancel” its archaic names, which are infused with negative connotations. Reinventing the psychiatric lexicon is a necessary act of renewal in a specialty where a poorly worded diagnostic label can morph into the equivalent of a “scarlet letter.” Think of other contemptuous terms, such as refrigerator mother, male hysteria, moral insanity, toxic parents, inadequate personality disorder, neurasthenia, or catastrophic schizophrenia.
General medicine regularly discards many of its obsolete terms.11 These include terms such as ablepsy, ague, camp fever, bloody flux, chlorosis, catarrh, consumption, dropsy, French pox, phthisis, milk sickness, and scrumpox.
Think also of how society abandoned the antediluvian names of boys and girls. Few parents these days would name their son Ackley, Allard, Arundel, Awarnach, Beldon, Durward, Grower, Kenlm, or Legolan, or name their daughter Afton, Agrona, Arantxa, Corliss, Demelza, Eartha, Maida, Obsession, Radella, or Sacrifice.In summary, a necessary part of psychiatry’s progress is shedding obsolete terminology, even if it means slaughtering some widely used “traditional” vocabulary. It is a necessary act of renewal, and the image of psychiatry will be burnished by it.
Psychiatry has made significant scientific advances over the past century. However, it is still saddled with archaic terms, with pejorative connotations, disguised as official medical diagnoses. It is time to “cancel” those terms and replace them with ones that are neutral and have not accumulated baggage.
This process of “creative destruction” of psychiatric terminology is long overdue. It is frankly disturbing that the psychiatric jargon used around the time that the American Psychiatric Association was established 175 years ago (1844) is now considered insults and epithets. We no longer work in “lunatic asylums for the insane,” and our patients with intellectual disabilities are no longer classified as “morons,” “idiots,” or “imbeciles.” Such “diagnoses” have certainly contributed to the stigma of psychiatric brain disorders. Even the noble word “asylum” has acquired a negative valence because in the past it referred to hospitals that housed persons with serious mental illness.
Thankfully, some of the outrageous terms fabricated during the condemnable and dark era of slavery 2 centuries ago were never adopted by organized psychiatry. The absurd diagnosis of “negritude,” whose tenet was that black skin is a disease curable by whitening the skin, was “invented” by none other than Benjamin Rush, the Father of Psychiatry, whose conflicted soul was depicted by concomitantly owning a slave and positioning himself as an ardent abolitionist!
Terms that need to be replaced
Fast-forward to the modern era and consider the following:
Borderline personality disorder. It is truly tragic how this confusing and non-scientific term is used as an official diagnosis for a set of seriously ill persons. It is loaded with obloquy, indignity, and derision that completely ignore the tumult, self-harm, and disability with which patients who carry this label are burdened throughout their lives, despite being intelligent. This is a serious brain disorder that has been shown to be highly genetic and is characterized by many well-established structural brain abnormalities that have been documented in neuroimaging studies.1,2 Borderline personality should not be classified as a personality disorder but as an illness with multiple signs and symptoms, including mood lability, anger, impulsivity, self-cutting, suicidal urges, feelings of abandonment, and micro-psychotic episodes. A more clinically accurate term should be coined very soon to replace borderline personality, which should be discarded to the trash heap of obsolete psychiatric terms, and no longer inflicted on patients.
Neurosis. What is the justification for continuing to use the term “neurotic” for a person who has an anxiety disorder? Is it used because Jung and Freud propagated the term “neurosis” (after it was coined by William Cullen in 1769)? Neurosis has degenerated from a psychiatric diagnosis to a scornful snub that must never be used for any patient.
Schizophrenia. This diagnosis, coined by Eugen Bleuler to replace the narrow and pessimistic “dementia praecox” proposed by Emil Kraepelin in the 1920s, initially seemed to be a neutral description of a thought disorder (split associations, not split personality). Bleuler was perceptive enough to call his book Dementia Praecox or the Group of Schizophrenias, which is consistent with the modern scientific research that confirms schizophrenia is a very heterogeneous syndrome with hundreds of genetic and environmental biotypes with a similar phenotype but a wide range of severity, treatment response, and functional outcomes. However, in subsequent decades, schizophrenia became one of the most demeaning labels in psychiatry, casting a shadow of hopelessness and disability on the people who have this serious neurologic condition with many psychiatric symptoms. The term that should replace schizophrenia should be no more degrading than stroke, multiple sclerosis, or myocardial infarction.
Continue to: Over the past 15 years...
Over the past 15 years, an expanding group of schizophrenia experts have agreed that this term must be changed to one that reflects the core features of this syndrome, and have proposed terms such as “salience syndrome,” “psychosis-spectrum,” and “reality distortion and cognitive impairment disorder.”3 In fact, several countries have already adopted a new official diagnosis for schizophrenia.4 Japan now uses the term “integration disorder,” which has significantly reduced the stigma of this brain disorder.5 South Korea changed the name to “attunement disorder.” Hong Kong and Taiwan now use “dysfunction of thought and perception.” Some researchers recommend calling schizophrenia “Bleuler’s syndrome,” a neutral eponymous designation.
One of the most irritating things about the term schizophrenia is the widespread misconception that it means “split personality.” This prompts some sports announcers to call a football team “schizophrenic” if they play well in the first half and badly in the second. The stock market is labeled “schizophrenic” if it goes up one day and way down on the next. No other medical term is misused by the media as often as the term schizophrenia.
Narcissistic personality disorder. The origin of this diagnostic category is the concept of “malignant narcissism” coined by Erich Fromm in 1964, which he designated as “the quintessence of evil.” I strongly object to implying that evil is part of any psychiatric diagnosis. Numerous studies have found structural brain abnormalities (in both gray and white matter) in patients diagnosed with psychopathic traits.6 Later, malignant narcissism was reframed as narcissistic personality disorder in 1971 by Herbert Rosenfeld. Although malignant narcissism was never accepted by either the DSM or the International Classification of Diseases, narcissistic personality disorder has been included in the DSM for the past few decades. This diagnosis reeks of disparagement and negativity. Persons with narcissistic personality disorder have been shown to have pathological brain changes in resting-state functional connectivity,7 weakened frontostriatal white matter connectivity,8,9 and a reduced frontal thickness and cortical volume.10 A distorted sense of self and others is a socially disabling disorder that should generate empathy, not disdain. Narcissistic personality disorder should be replaced by a term that accurately describes its behavioral pathology, and should not incorporate Greek mythology.
Mania. This is another unfortunate diagnosis that immediately evokes a negative image of patients who suffer from a potentially lethal brain disorder. It was fortunate that Robert Kendall coined the term “bipolar disorder” to replace “manic-depressive illness,” but mania is still being used within bipolar disorder as a prominent clinical phase. While depression accurately describes the mood in the other phase of this disorder, the term mania evokes wild, irrational behavior. Because the actual mood symptom cluster in mania is either elation/grandiosity or irritability/anger, why not replace mania with “elation/irritability phase of bipolar disorder”? It is more descriptive of the patient’s mood and is less pejorative.
Nomenclature is vital, and words do matter, especially when used as a diagnostic medical term. Psychiatry must “cancel” its archaic names, which are infused with negative connotations. Reinventing the psychiatric lexicon is a necessary act of renewal in a specialty where a poorly worded diagnostic label can morph into the equivalent of a “scarlet letter.” Think of other contemptuous terms, such as refrigerator mother, male hysteria, moral insanity, toxic parents, inadequate personality disorder, neurasthenia, or catastrophic schizophrenia.
General medicine regularly discards many of its obsolete terms.11 These include terms such as ablepsy, ague, camp fever, bloody flux, chlorosis, catarrh, consumption, dropsy, French pox, phthisis, milk sickness, and scrumpox.
Think also of how society abandoned the antediluvian names of boys and girls. Few parents these days would name their son Ackley, Allard, Arundel, Awarnach, Beldon, Durward, Grower, Kenlm, or Legolan, or name their daughter Afton, Agrona, Arantxa, Corliss, Demelza, Eartha, Maida, Obsession, Radella, or Sacrifice.In summary, a necessary part of psychiatry’s progress is shedding obsolete terminology, even if it means slaughtering some widely used “traditional” vocabulary. It is a necessary act of renewal, and the image of psychiatry will be burnished by it.
1. Nasrallah HA. Borderline personality disorder is a heritable brain disease. Current Psychiatry. 2014;13(4):19-20,32.
2. Sagarwala R, Nasrallah HA. White matter pathology in patients with borderline personality disorder: a review of controlled DTI studies. Ann Clin Psychiatry. 2020;32(4):281-286.
3. Keshavan MS, Tandon R, Nasrallah HA. Renaming schizophrenia: keeping up with the facts. Schizophr Res. 2013;148(1-3):1-2.
4. Lasalvia A, Penta E, Sartorius N, et al. Should the label “schizophrenia” be abandoned? Schizophr Res. 2015;162(1-3):276-284.
5. Takahashi H, Ideno T, Okubo S, et al. Impact of changing the Japanese term for “schizophrenia” for reasons of stereotypical beliefs of schizophrenia in Japanese youth. Schizophr Res. 2009;112(1-3):149-152.
6. Johanson M, Vaurio D, Tiihunen J, et al. A systematic literature review of neuroimaging of psychopathic traits. Front Psychiatry. 2020;10:1027.
7. Yang, W, Cun L, Du X, et al. Gender differences in brain structure and resting-state functional connectivity related to narcissistic personality. Sci Rep. 2015;5:10924.
8. Chester DS, Cynam DR, Powell DK, et al. Narcissismis associated with weakened frontostriatal connectivity: a DTI study. Soc Cogn Affect Neurosci. 2016;11(7):1036-1040.
9. Nenadic I, Gullmar D, Dietzek M, et al. Brain structure in narcissistic personality disorder: a VBM and DTI pilot study. Psychiatry Res. 2015;231(2):184-186.
10. Mao Y, Sang N, Wang Y, et al. Reduced frontal cortex thickness and cortical volume associated with pathological narcissism. Neuroscience. 2016;378:51-57.
11. Nasrallah HA. The transient truths of medical ‘progress.’ Current Psychiatry. 2014;13(6):23-24.
1. Nasrallah HA. Borderline personality disorder is a heritable brain disease. Current Psychiatry. 2014;13(4):19-20,32.
2. Sagarwala R, Nasrallah HA. White matter pathology in patients with borderline personality disorder: a review of controlled DTI studies. Ann Clin Psychiatry. 2020;32(4):281-286.
3. Keshavan MS, Tandon R, Nasrallah HA. Renaming schizophrenia: keeping up with the facts. Schizophr Res. 2013;148(1-3):1-2.
4. Lasalvia A, Penta E, Sartorius N, et al. Should the label “schizophrenia” be abandoned? Schizophr Res. 2015;162(1-3):276-284.
5. Takahashi H, Ideno T, Okubo S, et al. Impact of changing the Japanese term for “schizophrenia” for reasons of stereotypical beliefs of schizophrenia in Japanese youth. Schizophr Res. 2009;112(1-3):149-152.
6. Johanson M, Vaurio D, Tiihunen J, et al. A systematic literature review of neuroimaging of psychopathic traits. Front Psychiatry. 2020;10:1027.
7. Yang, W, Cun L, Du X, et al. Gender differences in brain structure and resting-state functional connectivity related to narcissistic personality. Sci Rep. 2015;5:10924.
8. Chester DS, Cynam DR, Powell DK, et al. Narcissismis associated with weakened frontostriatal connectivity: a DTI study. Soc Cogn Affect Neurosci. 2016;11(7):1036-1040.
9. Nenadic I, Gullmar D, Dietzek M, et al. Brain structure in narcissistic personality disorder: a VBM and DTI pilot study. Psychiatry Res. 2015;231(2):184-186.
10. Mao Y, Sang N, Wang Y, et al. Reduced frontal cortex thickness and cortical volume associated with pathological narcissism. Neuroscience. 2016;378:51-57.
11. Nasrallah HA. The transient truths of medical ‘progress.’ Current Psychiatry. 2014;13(6):23-24.
For obese postmenopausal women, what options may decrease endometrial cancer risk?
Endometrial cancer is the most common gynecologic malignancy, with approximately 59,000 cases diagnosed annually,1 and a lifetime risk of approximately 3.1% in the United States.2 Type I endometrial cancer includes tumors with endometrioid histology that are grade 1 or 2. Type II endometrial cancer includes tumors that have grade 3 endometrioid or nonendometrioid histology, including serous, clear cell, mucinous, squamous transitional cell, mesonephric, and undifferentiated tumors.3 Type I endometrial cancer is hormone sensitive, generally stimulated by estrogen and suppressed by progestins.
Endometrial cancer is diagnosed at a mean age of 63 years,4 and only 15% of cases occur before age 50.5 Women with an elevated body mass index (BMI) have a markedly increased risk of both Types I and II endometrial cancer (TABLE).6 Hence, endometrial cancer is highly prevalent in obese postmenopausal women. For these women health interventions that may reduce the risk of developing endometrial cancer include dieting, physical activity, bariatric surgery, and progestin therapy.

Educating patients is a priority
Many women do not know that postmenopausal bleeding is a sign of endometrial cancer. All postmenopausal women should be advised that if they develop vaginal bleeding they need to be evaluated by a clinician.7 Women who are knowledgeable about the link between postmenopausal vaginal bleeding and endometrial cancer can be encouraged to share this information with their postmenopausal friends in order to reach more people with this important information. All obese postmenopausal women should be advised that weight loss and increased physical activity can reduce the risk of developing endometrial cancer.
How weight loss and physical activity affect risk
Intentional weight loss has been reported to reduce the risk of endometrial cancer in postmenopausal women. As part of the Women’s Health Initiative observational study, 36,794 postmenopausal women aged 50 to 79 years with a uterus had their body weight and height measured at entry into the study and after 3 years of follow-up.8 During the 11 years following study entry, there were 566 incident cases of endometrial cancer. Compared with women who had a stable weight, intentional weight loss of ≥5% was associated with a 40% reduction in the risk of endometrial cancer (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.42–0.86). Compared with women who had a stable weight, women who had weight gain ≥10% had an increased risk of endometrial cancer (HR, 1.26; 95% CI, 1.00–1.57).
High levels of physical activity may be associated with a decreased risk of endometrial cancer. In one study, compared with a sedentary lifestyle, higher levels of physical activity were reported to be associated with a decreased risk of endometrial cancer.9
Continue to: How bariatric surgery affects risk...
How bariatric surgery affects risk
Many cancers are associated with obesity, including endometrial, breast, colon, pancreas, gallbladder, and renal. Obesity is associated with increased conversion of androgens to estrogens in fat tissue, stimulating excessive endometrial proliferation and increasing the risk of endometrial hyperplasia and cancer. Bariatric surgery reliably causes sustained weight reduction. Multiple studies have reported that bariatric surgery reduces the risk of endometrial cancer.
Schauer and colleagues used data from the Kaiser Permanente health system to identify 22,198 obese people who had undergone bariatric surgery and 66,427 matched controls who were obese but did not have surgery.10 The study population was 81% female, with a mean age of 45 years and a mean BMI of 45 kg/m2. After an average 3.5 years of follow-up there were 2,542 incident cases of cancer, including 322 cases of endometrial cancer. Compared with conventional weight loss treatment, bariatric surgery reduced the risk of endometrial cancer by 50% (HR, 0.50; 95% CI, 0.37–0.67; P<.001).10 In addition, bariatric surgery reduced the risk of colon and pancreatic cancer by 41% and 54%, respectively.10
In the Swedish Obese Subjects (SOS) study, 1,420 women who underwent bariatric surgery and 1,447 matched controls who received conventional obesity treatment were followed for 18 years.11 At study entry, the mean age of the women was approximately 48 years, and the mean BMI was approximately 42 kg/m2. In follow-up there were 76 incident cases of endometrial cancer. Compared with women receiving conventional obesity treatment, women who had bariatric surgery had a non–statistically significant 49% decrease in the risk of developing endometrial cancer (HR, 0.51; 95% CI, 0.24–1.10)
In a systematic review of 5 additional studies (not including publications 10 or 11) of the impact of bariatric surgery on the risk of developing endometrial cancer, the surgery was associated with a 68% risk reduction (odds ratio [OR], 0.32; 95% CI, 0.16–0.63) compared with matched obese women that did not have surgery.12
Although there are no randomized prospective studies showing that bariatric surgery reduces the risk of endometrial cancer, the weight of the observation evidence is strong. In addition, bariatric surgery was reported to reduce all-cause mortality in the SOS study.13 Hence, for obese postmenopausal women, if lifestyle changes do not result in sustained weight loss, bariatric surgery may be an optimal approach to improving health outcomes.
Continue to: Progestin treatment and endometrial cancer risk...
Progestin treatment and endometrial cancer risk
Estrogen stimulates endometrial cell proliferation. Hence, unopposed chronic exposure to estrogen is a major risk factor for developing endometrial hyperplasia and cancer. Progestins block the proliferative effect of estrogen and cause cell differentiation, resulting in stromal decidualization. Progestins also reduce the concentration of estrogen and progesterone receptors and increase the activity of enzymes that convert estradiol to estrone, blocking estrogen-induced endometrial proliferation.14
In women with endometrial hyperplasia, progestins have been shown to be effective in resolving the hyperplasia in approximately 80% of cases. Both oral progestins and the 52-mg levonorgestrel-containing intrauterine device (LNG-IUD) have been reported to be effective in the treatment of endometrial hyperplasia. In a Cochrane systematic review and meta-analysis, the 52-mg LNG-IUD was reported to be somewhat more effective in resolving endometrial hyperplasia than cyclic oral progestins (89% vs 72%, respectively).15
Other studies have also reported that the 52 mg LNG-IUD was more effective than oral progestin therapy for women with complex atypical endometrial hyperplasia.16 There are no large randomized clinical trials of progestin therapy on prevention for future development of endometrial cancer in obese postmenopausal women who have a normal endometrial histology. However, for an obese perimenopausal woman, insertion of a 52-mg LNG-IUD may help to minimize excessive uterine bleeding during the menopause transition and reduce the risk of developing endometrial hyperplasia during the early postmenopause.
We can help our patients reduce their risk of endometrial cancer
Obese postmenopausal women are at increased risk for developing endometrial cancer. Gynecologists play an important role in the prevention and early detection of endometrial cancer. We can make a difference and improve the health of our obese peri- and postmenopausal women by recommending interventions that reduce the risk of endometrial cancer, thereby improving the health of our patients. ●
- American Society of Clinical Oncology. Uterine cancer statistics. https://www.cancer.net/cancer-types/uterine-cancer/statistics#:~:text=This%20year%2C%20an%20
estimated%2065%2C620,cancers%20occur%20in%20the%20endometrium. Accessed November 23, 2020. - Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2017. National Cancer Institute: Bethesda, MD. April 15, 2020. https://seer.cancer.gov/csr/1975_2017/. Accessed November 23, 2020.
- Noer MC, Antonsen SL, Ottesen B, et al. Type I versus Type II endometrial cancer: differential impact of comorbidity. Int J Gynecol Cancer. 2018;28:586-593.
- Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-437.
- Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417-420.
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors. J Clin Oncol. 2013;31:2607-2618.
- Saccardi C, Vitagliano A, Marchetti M, et al. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in postmenopausal women. Diagnostics (Basel). 2020;10:257.e1-e11.
- Luo J, Chlebowski RT, Hendryx M, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncology. 2017;35:1189-1193.
- Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biological mechanisms. Mol Oncol. August 2, 2020. doi: 10.1001/1878-0261.12772.
- Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95-101.
- Anvenden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224-229.
- Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. 2018;28:1433-1440.
- Carlsson LM, Sjoholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535-1543.
- Lessey BA, Young SL. In: Strauss JF, Barbieri RL (eds.) Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 8th ed. Elsevier Saunders: Philadelphia, PA; 2018:208-212.
- Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev. 2020;CD012658.
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-e13.
Endometrial cancer is the most common gynecologic malignancy, with approximately 59,000 cases diagnosed annually,1 and a lifetime risk of approximately 3.1% in the United States.2 Type I endometrial cancer includes tumors with endometrioid histology that are grade 1 or 2. Type II endometrial cancer includes tumors that have grade 3 endometrioid or nonendometrioid histology, including serous, clear cell, mucinous, squamous transitional cell, mesonephric, and undifferentiated tumors.3 Type I endometrial cancer is hormone sensitive, generally stimulated by estrogen and suppressed by progestins.
Endometrial cancer is diagnosed at a mean age of 63 years,4 and only 15% of cases occur before age 50.5 Women with an elevated body mass index (BMI) have a markedly increased risk of both Types I and II endometrial cancer (TABLE).6 Hence, endometrial cancer is highly prevalent in obese postmenopausal women. For these women health interventions that may reduce the risk of developing endometrial cancer include dieting, physical activity, bariatric surgery, and progestin therapy.

Educating patients is a priority
Many women do not know that postmenopausal bleeding is a sign of endometrial cancer. All postmenopausal women should be advised that if they develop vaginal bleeding they need to be evaluated by a clinician.7 Women who are knowledgeable about the link between postmenopausal vaginal bleeding and endometrial cancer can be encouraged to share this information with their postmenopausal friends in order to reach more people with this important information. All obese postmenopausal women should be advised that weight loss and increased physical activity can reduce the risk of developing endometrial cancer.
How weight loss and physical activity affect risk
Intentional weight loss has been reported to reduce the risk of endometrial cancer in postmenopausal women. As part of the Women’s Health Initiative observational study, 36,794 postmenopausal women aged 50 to 79 years with a uterus had their body weight and height measured at entry into the study and after 3 years of follow-up.8 During the 11 years following study entry, there were 566 incident cases of endometrial cancer. Compared with women who had a stable weight, intentional weight loss of ≥5% was associated with a 40% reduction in the risk of endometrial cancer (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.42–0.86). Compared with women who had a stable weight, women who had weight gain ≥10% had an increased risk of endometrial cancer (HR, 1.26; 95% CI, 1.00–1.57).
High levels of physical activity may be associated with a decreased risk of endometrial cancer. In one study, compared with a sedentary lifestyle, higher levels of physical activity were reported to be associated with a decreased risk of endometrial cancer.9
Continue to: How bariatric surgery affects risk...
How bariatric surgery affects risk
Many cancers are associated with obesity, including endometrial, breast, colon, pancreas, gallbladder, and renal. Obesity is associated with increased conversion of androgens to estrogens in fat tissue, stimulating excessive endometrial proliferation and increasing the risk of endometrial hyperplasia and cancer. Bariatric surgery reliably causes sustained weight reduction. Multiple studies have reported that bariatric surgery reduces the risk of endometrial cancer.
Schauer and colleagues used data from the Kaiser Permanente health system to identify 22,198 obese people who had undergone bariatric surgery and 66,427 matched controls who were obese but did not have surgery.10 The study population was 81% female, with a mean age of 45 years and a mean BMI of 45 kg/m2. After an average 3.5 years of follow-up there were 2,542 incident cases of cancer, including 322 cases of endometrial cancer. Compared with conventional weight loss treatment, bariatric surgery reduced the risk of endometrial cancer by 50% (HR, 0.50; 95% CI, 0.37–0.67; P<.001).10 In addition, bariatric surgery reduced the risk of colon and pancreatic cancer by 41% and 54%, respectively.10
In the Swedish Obese Subjects (SOS) study, 1,420 women who underwent bariatric surgery and 1,447 matched controls who received conventional obesity treatment were followed for 18 years.11 At study entry, the mean age of the women was approximately 48 years, and the mean BMI was approximately 42 kg/m2. In follow-up there were 76 incident cases of endometrial cancer. Compared with women receiving conventional obesity treatment, women who had bariatric surgery had a non–statistically significant 49% decrease in the risk of developing endometrial cancer (HR, 0.51; 95% CI, 0.24–1.10)
In a systematic review of 5 additional studies (not including publications 10 or 11) of the impact of bariatric surgery on the risk of developing endometrial cancer, the surgery was associated with a 68% risk reduction (odds ratio [OR], 0.32; 95% CI, 0.16–0.63) compared with matched obese women that did not have surgery.12
Although there are no randomized prospective studies showing that bariatric surgery reduces the risk of endometrial cancer, the weight of the observation evidence is strong. In addition, bariatric surgery was reported to reduce all-cause mortality in the SOS study.13 Hence, for obese postmenopausal women, if lifestyle changes do not result in sustained weight loss, bariatric surgery may be an optimal approach to improving health outcomes.
Continue to: Progestin treatment and endometrial cancer risk...
Progestin treatment and endometrial cancer risk
Estrogen stimulates endometrial cell proliferation. Hence, unopposed chronic exposure to estrogen is a major risk factor for developing endometrial hyperplasia and cancer. Progestins block the proliferative effect of estrogen and cause cell differentiation, resulting in stromal decidualization. Progestins also reduce the concentration of estrogen and progesterone receptors and increase the activity of enzymes that convert estradiol to estrone, blocking estrogen-induced endometrial proliferation.14
In women with endometrial hyperplasia, progestins have been shown to be effective in resolving the hyperplasia in approximately 80% of cases. Both oral progestins and the 52-mg levonorgestrel-containing intrauterine device (LNG-IUD) have been reported to be effective in the treatment of endometrial hyperplasia. In a Cochrane systematic review and meta-analysis, the 52-mg LNG-IUD was reported to be somewhat more effective in resolving endometrial hyperplasia than cyclic oral progestins (89% vs 72%, respectively).15
Other studies have also reported that the 52 mg LNG-IUD was more effective than oral progestin therapy for women with complex atypical endometrial hyperplasia.16 There are no large randomized clinical trials of progestin therapy on prevention for future development of endometrial cancer in obese postmenopausal women who have a normal endometrial histology. However, for an obese perimenopausal woman, insertion of a 52-mg LNG-IUD may help to minimize excessive uterine bleeding during the menopause transition and reduce the risk of developing endometrial hyperplasia during the early postmenopause.
We can help our patients reduce their risk of endometrial cancer
Obese postmenopausal women are at increased risk for developing endometrial cancer. Gynecologists play an important role in the prevention and early detection of endometrial cancer. We can make a difference and improve the health of our obese peri- and postmenopausal women by recommending interventions that reduce the risk of endometrial cancer, thereby improving the health of our patients. ●
Endometrial cancer is the most common gynecologic malignancy, with approximately 59,000 cases diagnosed annually,1 and a lifetime risk of approximately 3.1% in the United States.2 Type I endometrial cancer includes tumors with endometrioid histology that are grade 1 or 2. Type II endometrial cancer includes tumors that have grade 3 endometrioid or nonendometrioid histology, including serous, clear cell, mucinous, squamous transitional cell, mesonephric, and undifferentiated tumors.3 Type I endometrial cancer is hormone sensitive, generally stimulated by estrogen and suppressed by progestins.
Endometrial cancer is diagnosed at a mean age of 63 years,4 and only 15% of cases occur before age 50.5 Women with an elevated body mass index (BMI) have a markedly increased risk of both Types I and II endometrial cancer (TABLE).6 Hence, endometrial cancer is highly prevalent in obese postmenopausal women. For these women health interventions that may reduce the risk of developing endometrial cancer include dieting, physical activity, bariatric surgery, and progestin therapy.

Educating patients is a priority
Many women do not know that postmenopausal bleeding is a sign of endometrial cancer. All postmenopausal women should be advised that if they develop vaginal bleeding they need to be evaluated by a clinician.7 Women who are knowledgeable about the link between postmenopausal vaginal bleeding and endometrial cancer can be encouraged to share this information with their postmenopausal friends in order to reach more people with this important information. All obese postmenopausal women should be advised that weight loss and increased physical activity can reduce the risk of developing endometrial cancer.
How weight loss and physical activity affect risk
Intentional weight loss has been reported to reduce the risk of endometrial cancer in postmenopausal women. As part of the Women’s Health Initiative observational study, 36,794 postmenopausal women aged 50 to 79 years with a uterus had their body weight and height measured at entry into the study and after 3 years of follow-up.8 During the 11 years following study entry, there were 566 incident cases of endometrial cancer. Compared with women who had a stable weight, intentional weight loss of ≥5% was associated with a 40% reduction in the risk of endometrial cancer (hazard ratio [HR], 0.60; 95% confidence interval [CI], 0.42–0.86). Compared with women who had a stable weight, women who had weight gain ≥10% had an increased risk of endometrial cancer (HR, 1.26; 95% CI, 1.00–1.57).
High levels of physical activity may be associated with a decreased risk of endometrial cancer. In one study, compared with a sedentary lifestyle, higher levels of physical activity were reported to be associated with a decreased risk of endometrial cancer.9
Continue to: How bariatric surgery affects risk...
How bariatric surgery affects risk
Many cancers are associated with obesity, including endometrial, breast, colon, pancreas, gallbladder, and renal. Obesity is associated with increased conversion of androgens to estrogens in fat tissue, stimulating excessive endometrial proliferation and increasing the risk of endometrial hyperplasia and cancer. Bariatric surgery reliably causes sustained weight reduction. Multiple studies have reported that bariatric surgery reduces the risk of endometrial cancer.
Schauer and colleagues used data from the Kaiser Permanente health system to identify 22,198 obese people who had undergone bariatric surgery and 66,427 matched controls who were obese but did not have surgery.10 The study population was 81% female, with a mean age of 45 years and a mean BMI of 45 kg/m2. After an average 3.5 years of follow-up there were 2,542 incident cases of cancer, including 322 cases of endometrial cancer. Compared with conventional weight loss treatment, bariatric surgery reduced the risk of endometrial cancer by 50% (HR, 0.50; 95% CI, 0.37–0.67; P<.001).10 In addition, bariatric surgery reduced the risk of colon and pancreatic cancer by 41% and 54%, respectively.10
In the Swedish Obese Subjects (SOS) study, 1,420 women who underwent bariatric surgery and 1,447 matched controls who received conventional obesity treatment were followed for 18 years.11 At study entry, the mean age of the women was approximately 48 years, and the mean BMI was approximately 42 kg/m2. In follow-up there were 76 incident cases of endometrial cancer. Compared with women receiving conventional obesity treatment, women who had bariatric surgery had a non–statistically significant 49% decrease in the risk of developing endometrial cancer (HR, 0.51; 95% CI, 0.24–1.10)
In a systematic review of 5 additional studies (not including publications 10 or 11) of the impact of bariatric surgery on the risk of developing endometrial cancer, the surgery was associated with a 68% risk reduction (odds ratio [OR], 0.32; 95% CI, 0.16–0.63) compared with matched obese women that did not have surgery.12
Although there are no randomized prospective studies showing that bariatric surgery reduces the risk of endometrial cancer, the weight of the observation evidence is strong. In addition, bariatric surgery was reported to reduce all-cause mortality in the SOS study.13 Hence, for obese postmenopausal women, if lifestyle changes do not result in sustained weight loss, bariatric surgery may be an optimal approach to improving health outcomes.
Continue to: Progestin treatment and endometrial cancer risk...
Progestin treatment and endometrial cancer risk
Estrogen stimulates endometrial cell proliferation. Hence, unopposed chronic exposure to estrogen is a major risk factor for developing endometrial hyperplasia and cancer. Progestins block the proliferative effect of estrogen and cause cell differentiation, resulting in stromal decidualization. Progestins also reduce the concentration of estrogen and progesterone receptors and increase the activity of enzymes that convert estradiol to estrone, blocking estrogen-induced endometrial proliferation.14
In women with endometrial hyperplasia, progestins have been shown to be effective in resolving the hyperplasia in approximately 80% of cases. Both oral progestins and the 52-mg levonorgestrel-containing intrauterine device (LNG-IUD) have been reported to be effective in the treatment of endometrial hyperplasia. In a Cochrane systematic review and meta-analysis, the 52-mg LNG-IUD was reported to be somewhat more effective in resolving endometrial hyperplasia than cyclic oral progestins (89% vs 72%, respectively).15
Other studies have also reported that the 52 mg LNG-IUD was more effective than oral progestin therapy for women with complex atypical endometrial hyperplasia.16 There are no large randomized clinical trials of progestin therapy on prevention for future development of endometrial cancer in obese postmenopausal women who have a normal endometrial histology. However, for an obese perimenopausal woman, insertion of a 52-mg LNG-IUD may help to minimize excessive uterine bleeding during the menopause transition and reduce the risk of developing endometrial hyperplasia during the early postmenopause.
We can help our patients reduce their risk of endometrial cancer
Obese postmenopausal women are at increased risk for developing endometrial cancer. Gynecologists play an important role in the prevention and early detection of endometrial cancer. We can make a difference and improve the health of our obese peri- and postmenopausal women by recommending interventions that reduce the risk of endometrial cancer, thereby improving the health of our patients. ●
- American Society of Clinical Oncology. Uterine cancer statistics. https://www.cancer.net/cancer-types/uterine-cancer/statistics#:~:text=This%20year%2C%20an%20
estimated%2065%2C620,cancers%20occur%20in%20the%20endometrium. Accessed November 23, 2020. - Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2017. National Cancer Institute: Bethesda, MD. April 15, 2020. https://seer.cancer.gov/csr/1975_2017/. Accessed November 23, 2020.
- Noer MC, Antonsen SL, Ottesen B, et al. Type I versus Type II endometrial cancer: differential impact of comorbidity. Int J Gynecol Cancer. 2018;28:586-593.
- Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-437.
- Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417-420.
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors. J Clin Oncol. 2013;31:2607-2618.
- Saccardi C, Vitagliano A, Marchetti M, et al. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in postmenopausal women. Diagnostics (Basel). 2020;10:257.e1-e11.
- Luo J, Chlebowski RT, Hendryx M, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncology. 2017;35:1189-1193.
- Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biological mechanisms. Mol Oncol. August 2, 2020. doi: 10.1001/1878-0261.12772.
- Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95-101.
- Anvenden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224-229.
- Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. 2018;28:1433-1440.
- Carlsson LM, Sjoholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535-1543.
- Lessey BA, Young SL. In: Strauss JF, Barbieri RL (eds.) Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 8th ed. Elsevier Saunders: Philadelphia, PA; 2018:208-212.
- Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev. 2020;CD012658.
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-e13.
- American Society of Clinical Oncology. Uterine cancer statistics. https://www.cancer.net/cancer-types/uterine-cancer/statistics#:~:text=This%20year%2C%20an%20
estimated%2065%2C620,cancers%20occur%20in%20the%20endometrium. Accessed November 23, 2020. - Howlader N, Noone AM, Krapcho M, et al (eds). SEER Cancer Statistics Review, 1975-2017. National Cancer Institute: Bethesda, MD. April 15, 2020. https://seer.cancer.gov/csr/1975_2017/. Accessed November 23, 2020.
- Noer MC, Antonsen SL, Ottesen B, et al. Type I versus Type II endometrial cancer: differential impact of comorbidity. Int J Gynecol Cancer. 2018;28:586-593.
- Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436-437.
- Gallup DG, Stock RJ. Adenocarcinoma of the endometrium in women 40 years of age or younger. Obstet Gynecol. 1984;64:417-420.
- Setiawan VW, Yang HP, Pike MC, et al. Type I and II endometrial cancers: have they different risk factors. J Clin Oncol. 2013;31:2607-2618.
- Saccardi C, Vitagliano A, Marchetti M, et al. Endometrial cancer risk prediction according to indication of diagnostic hysteroscopy in postmenopausal women. Diagnostics (Basel). 2020;10:257.e1-e11.
- Luo J, Chlebowski RT, Hendryx M, et al. Intentional weight loss and endometrial cancer risk. J Clin Oncology. 2017;35:1189-1193.
- Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biological mechanisms. Mol Oncol. August 2, 2020. doi: 10.1001/1878-0261.12772.
- Schauer DP, Feigelson HS, Koebnick C, et al. Bariatric surgery and the risk of cancer in a large multisite cohort. Ann Surg. 2019;269:95-101.
- Anvenden A, Taube M, Peltonen M, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the Swedish Obese Subjects Study. Gynecol Oncol. 2017;145:224-229.
- Winder AA, Kularatna M, MacCormick AD. Does bariatric surgery affect the incidence of endometrial cancer development? A systematic review. Obes Surg. 2018;28:1433-1440.
- Carlsson LM, Sjoholm K, Jacobson P, et al. Life expectancy after bariatric surgery in the Swedish Obese Subjects Study. N Engl J Med. 2020;383:1535-1543.
- Lessey BA, Young SL. In: Strauss JF, Barbieri RL (eds.) Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management. 8th ed. Elsevier Saunders: Philadelphia, PA; 2018:208-212.
- Mittermeier T, Farrant C, Wise MR. Levonorgestrel-releasing intrauterine system for endometrial hyperplasia. Cochrane Database Syst Rev. 2020;CD012658.
- Mandelbaum RS, Ciccone MA, Nusbaum DJ, et al. Progestin therapy for obese women with complex atypical hyperplasia: levonorgestrel-releasing intrauterine device vs systemic therapy. Am J Obstet Gynecol. 2020;223:103.e1-e13.