User login
It Is What It Is…. For Now.
This issue of the Journal of Hospital Medicine addresses an emerging trend in internal medicine graduate medical education: the hospitalist rotation.
In the article, Training Residents in Hospital Medicine: The Hospitalist Elective National Survey (HENS). by Ludwin et al., the authors present a descriptive overview of the composition of hospital medicine rotations, as described by program directors from some of the largest training programs. 1 It can be said for sure that hospital medicine rotations exist: half of the 82 programs that replied to the survey noted that a hospital medicine rotation was already in place. That is where the certainty ends. Although there are common themes across these rotations, there is no one clear definition of such a rotation. Like all good contributions to the medical literature, this study inspires more questions than it answers.
The Mark Twain-inspired cynic would be quick to make an interpretation of the hospital medicine rotation: Is this not just a clever way to coax residents into using their elective time to cover the service needs left over from Accreditation Council for Graduate Medical Education (ACGME)-mandated shift limits and admission caps? Seventy-one percent of these rotations were involved in “admitting new patients.” And since forty-six percent were tasked with taking hold-over admissions, it is reasonable to surmise that these rotations are playing a role in covering patient care duties left over from traditional ward services.
But is there anything wrong with that? Within the confines of reasonable intensity, caring for more patients usually benefits a resident’s education. And if the resident is learning knowledge, skills and attitudes that are unique from those that are acquired on a traditional ward service, painting the fence for free might not be that bad. The question is: “Does the hospitalist rotation help in the acquisition of those unique knowledge, skills and attitudes?” Although this study alludes to such unique components via its qualitative analysis (ie, more autonomy, co-management of non-medicine services, etc.), it does not fully answer that question. It does, however, inspire the next study: How do residents perceive the unique and additional value (if any) of the hospital medicine rotation?
For the sake of argument, let’s say that residents’ perception of the hospital medicine rotation is one of meaning and value. Does that matter? It is great if they do, but equally important is the question of whether or not hospital medicine rotations are effective in preparing resident graduates for a career in hospital medicine. This study suggests that those who have designed these rotations have tried to anticipate and address this need. Components such as quality, patient safety, co-management, and billing and compliance are all clearly a part of a hospitalist’s practice, and all are elements that have not been traditionally emphasized in residency training. The question is: ”Are these elements the knowledge, skills and attitudes that are most lacking in the residency graduate as he/she enters the practice of hospital medicine?” The unfortunate answer is that we do not know for sure, and this uncertainty has been the Achilles heel of our current residency-training infrastructure. Not unique to hospital medicine, there is simply not a well-defined feedback loop between practice requirements and residency training requirements. A structured and regular gap analysis comparing the residents’ areas of competence at the end of training to what they need in practice, would go a long way in answering questions such as this one, and would most certainly inform the components of a hospital medicine elective going forward.
Even if the components of a hospital medicine rotation are valuable, and even if they do align with what the practice needs, there is still the question of whether a month-long hospital medicine rotation can even come close to closing the gap of what is needed versus what is delivered. One can surmise that the answer to that question is what has extended the “hospital medicine rotation” to the “hospital medicine track,” comprised of a multiple of such rotations. Like all discussions on time-constrained medical education curricula, what will be discarded to make room for these rotations? In thirty-six months of training, there is opportunity cost: every month spent on a hospital medicine elective is a month that could have been spent on something else (rheumatology, nephrology, etc.). Again, this is not unique to hospital medicine; the same could be said of the resident who does too many cardiology electives at the exclusion of learning about endocrinology. It would be overly dramatic to say that devoting a month to a hospital medicine rotation, or any elective for that matter, meaningfully compromises the resident’s overall competence as an internist. It is, instead, a question of degree: an excessive number of these electives would likely compromise the resident’s overall competence. The likelihood of this happening is proportional to the size of the gap between what is required to effectively enter hospital medicine practice and what can be delivered in a month-long hospital medicine rotation. We return, then, to the question: How much hospital medicine training in residency would be required to fully prepare a resident for the current practice of a hospitalist?
Whatever the answer might be, that question takes us to a difficult dilemma that has lurked in the background of residency training for some time now; one that is not at all unique to hospital medicine. Should residency training be “voc-tech” or “liberal arts”? A purist would argue that an understanding and appreciation of all things not hospital medicine is what truly makes for the great hospitalist. An understanding of primary care, for example, would seem to optimize a hospitalist’s performance with respect to transitions of care. Adding to the gravity of such an argument is that residency might be the last time to acquire such “non-hospital-medicine” experiences.
Noting that the practice of hospital medicine being so dynamic and heterogeneous, the realist might pile on by saying that it is simply impossible to fully prepare a resident for the actual practice of hospital medicine. Further, many of these skills might be impossible to fully master outside of being fully immersed in the practice of hospital medicine (i.e., billing and coding). The best that can be done is to set a solid foundation that would enable them to learn further as they practice; there will be opportunities to learn the specific components of the field later on.
On the other hand, it is hard to justify residency training if the graduate is unprepared to practice, and without the fundamental knowledge, skills and attitudes specific to their career as they practice. For example, it is reasonable to suspect that a new hospitalist who has had no prior training in quality improvement will, because of the inertia that comes with engaging in any new and foreign skill, find it much harder to engage in quality improvement as a part of her career. It is also worth considering the role that mastery, autonomy and purpose have upon the overall residency experience. Engaging in electives that have a palpable purpose for the resident’s eventual career, and engender an opportunity to begin developing a sense of mastery in that field, could be an effective antidote in mitigating the burn-out that is far too common in residency training today.
For residents engaged in a future practice of hospital medicine, the hospital medicine rotation seems like a promising way out of this dilemma. An effectively designed elective approach could enable maintaining a core foundational education, while getting an early start on the specific components necessary for a promising career in hospital medicine. The operative words, of course, are “effectively designed.” What exactly does that entail? That is why this study is so important; even if we do not fully know what it should look like, we now have our first glimpse of what it is.
Disclosures
The author has nothing to disclose.
1. Ludwin S, Harrison J, Ranji S, et al. Training Residents in Hospital Medicine: The Hospitalist Elective National Survey (HENS). J Hosp Med. 2018;13(9):623-625. doi: 10.12788/jhm.2952. PubMed
This issue of the Journal of Hospital Medicine addresses an emerging trend in internal medicine graduate medical education: the hospitalist rotation.
In the article, Training Residents in Hospital Medicine: The Hospitalist Elective National Survey (HENS). by Ludwin et al., the authors present a descriptive overview of the composition of hospital medicine rotations, as described by program directors from some of the largest training programs. 1 It can be said for sure that hospital medicine rotations exist: half of the 82 programs that replied to the survey noted that a hospital medicine rotation was already in place. That is where the certainty ends. Although there are common themes across these rotations, there is no one clear definition of such a rotation. Like all good contributions to the medical literature, this study inspires more questions than it answers.
The Mark Twain-inspired cynic would be quick to make an interpretation of the hospital medicine rotation: Is this not just a clever way to coax residents into using their elective time to cover the service needs left over from Accreditation Council for Graduate Medical Education (ACGME)-mandated shift limits and admission caps? Seventy-one percent of these rotations were involved in “admitting new patients.” And since forty-six percent were tasked with taking hold-over admissions, it is reasonable to surmise that these rotations are playing a role in covering patient care duties left over from traditional ward services.
But is there anything wrong with that? Within the confines of reasonable intensity, caring for more patients usually benefits a resident’s education. And if the resident is learning knowledge, skills and attitudes that are unique from those that are acquired on a traditional ward service, painting the fence for free might not be that bad. The question is: “Does the hospitalist rotation help in the acquisition of those unique knowledge, skills and attitudes?” Although this study alludes to such unique components via its qualitative analysis (ie, more autonomy, co-management of non-medicine services, etc.), it does not fully answer that question. It does, however, inspire the next study: How do residents perceive the unique and additional value (if any) of the hospital medicine rotation?
For the sake of argument, let’s say that residents’ perception of the hospital medicine rotation is one of meaning and value. Does that matter? It is great if they do, but equally important is the question of whether or not hospital medicine rotations are effective in preparing resident graduates for a career in hospital medicine. This study suggests that those who have designed these rotations have tried to anticipate and address this need. Components such as quality, patient safety, co-management, and billing and compliance are all clearly a part of a hospitalist’s practice, and all are elements that have not been traditionally emphasized in residency training. The question is: ”Are these elements the knowledge, skills and attitudes that are most lacking in the residency graduate as he/she enters the practice of hospital medicine?” The unfortunate answer is that we do not know for sure, and this uncertainty has been the Achilles heel of our current residency-training infrastructure. Not unique to hospital medicine, there is simply not a well-defined feedback loop between practice requirements and residency training requirements. A structured and regular gap analysis comparing the residents’ areas of competence at the end of training to what they need in practice, would go a long way in answering questions such as this one, and would most certainly inform the components of a hospital medicine elective going forward.
Even if the components of a hospital medicine rotation are valuable, and even if they do align with what the practice needs, there is still the question of whether a month-long hospital medicine rotation can even come close to closing the gap of what is needed versus what is delivered. One can surmise that the answer to that question is what has extended the “hospital medicine rotation” to the “hospital medicine track,” comprised of a multiple of such rotations. Like all discussions on time-constrained medical education curricula, what will be discarded to make room for these rotations? In thirty-six months of training, there is opportunity cost: every month spent on a hospital medicine elective is a month that could have been spent on something else (rheumatology, nephrology, etc.). Again, this is not unique to hospital medicine; the same could be said of the resident who does too many cardiology electives at the exclusion of learning about endocrinology. It would be overly dramatic to say that devoting a month to a hospital medicine rotation, or any elective for that matter, meaningfully compromises the resident’s overall competence as an internist. It is, instead, a question of degree: an excessive number of these electives would likely compromise the resident’s overall competence. The likelihood of this happening is proportional to the size of the gap between what is required to effectively enter hospital medicine practice and what can be delivered in a month-long hospital medicine rotation. We return, then, to the question: How much hospital medicine training in residency would be required to fully prepare a resident for the current practice of a hospitalist?
Whatever the answer might be, that question takes us to a difficult dilemma that has lurked in the background of residency training for some time now; one that is not at all unique to hospital medicine. Should residency training be “voc-tech” or “liberal arts”? A purist would argue that an understanding and appreciation of all things not hospital medicine is what truly makes for the great hospitalist. An understanding of primary care, for example, would seem to optimize a hospitalist’s performance with respect to transitions of care. Adding to the gravity of such an argument is that residency might be the last time to acquire such “non-hospital-medicine” experiences.
Noting that the practice of hospital medicine being so dynamic and heterogeneous, the realist might pile on by saying that it is simply impossible to fully prepare a resident for the actual practice of hospital medicine. Further, many of these skills might be impossible to fully master outside of being fully immersed in the practice of hospital medicine (i.e., billing and coding). The best that can be done is to set a solid foundation that would enable them to learn further as they practice; there will be opportunities to learn the specific components of the field later on.
On the other hand, it is hard to justify residency training if the graduate is unprepared to practice, and without the fundamental knowledge, skills and attitudes specific to their career as they practice. For example, it is reasonable to suspect that a new hospitalist who has had no prior training in quality improvement will, because of the inertia that comes with engaging in any new and foreign skill, find it much harder to engage in quality improvement as a part of her career. It is also worth considering the role that mastery, autonomy and purpose have upon the overall residency experience. Engaging in electives that have a palpable purpose for the resident’s eventual career, and engender an opportunity to begin developing a sense of mastery in that field, could be an effective antidote in mitigating the burn-out that is far too common in residency training today.
For residents engaged in a future practice of hospital medicine, the hospital medicine rotation seems like a promising way out of this dilemma. An effectively designed elective approach could enable maintaining a core foundational education, while getting an early start on the specific components necessary for a promising career in hospital medicine. The operative words, of course, are “effectively designed.” What exactly does that entail? That is why this study is so important; even if we do not fully know what it should look like, we now have our first glimpse of what it is.
Disclosures
The author has nothing to disclose.
This issue of the Journal of Hospital Medicine addresses an emerging trend in internal medicine graduate medical education: the hospitalist rotation.
In the article, Training Residents in Hospital Medicine: The Hospitalist Elective National Survey (HENS). by Ludwin et al., the authors present a descriptive overview of the composition of hospital medicine rotations, as described by program directors from some of the largest training programs. 1 It can be said for sure that hospital medicine rotations exist: half of the 82 programs that replied to the survey noted that a hospital medicine rotation was already in place. That is where the certainty ends. Although there are common themes across these rotations, there is no one clear definition of such a rotation. Like all good contributions to the medical literature, this study inspires more questions than it answers.
The Mark Twain-inspired cynic would be quick to make an interpretation of the hospital medicine rotation: Is this not just a clever way to coax residents into using their elective time to cover the service needs left over from Accreditation Council for Graduate Medical Education (ACGME)-mandated shift limits and admission caps? Seventy-one percent of these rotations were involved in “admitting new patients.” And since forty-six percent were tasked with taking hold-over admissions, it is reasonable to surmise that these rotations are playing a role in covering patient care duties left over from traditional ward services.
But is there anything wrong with that? Within the confines of reasonable intensity, caring for more patients usually benefits a resident’s education. And if the resident is learning knowledge, skills and attitudes that are unique from those that are acquired on a traditional ward service, painting the fence for free might not be that bad. The question is: “Does the hospitalist rotation help in the acquisition of those unique knowledge, skills and attitudes?” Although this study alludes to such unique components via its qualitative analysis (ie, more autonomy, co-management of non-medicine services, etc.), it does not fully answer that question. It does, however, inspire the next study: How do residents perceive the unique and additional value (if any) of the hospital medicine rotation?
For the sake of argument, let’s say that residents’ perception of the hospital medicine rotation is one of meaning and value. Does that matter? It is great if they do, but equally important is the question of whether or not hospital medicine rotations are effective in preparing resident graduates for a career in hospital medicine. This study suggests that those who have designed these rotations have tried to anticipate and address this need. Components such as quality, patient safety, co-management, and billing and compliance are all clearly a part of a hospitalist’s practice, and all are elements that have not been traditionally emphasized in residency training. The question is: ”Are these elements the knowledge, skills and attitudes that are most lacking in the residency graduate as he/she enters the practice of hospital medicine?” The unfortunate answer is that we do not know for sure, and this uncertainty has been the Achilles heel of our current residency-training infrastructure. Not unique to hospital medicine, there is simply not a well-defined feedback loop between practice requirements and residency training requirements. A structured and regular gap analysis comparing the residents’ areas of competence at the end of training to what they need in practice, would go a long way in answering questions such as this one, and would most certainly inform the components of a hospital medicine elective going forward.
Even if the components of a hospital medicine rotation are valuable, and even if they do align with what the practice needs, there is still the question of whether a month-long hospital medicine rotation can even come close to closing the gap of what is needed versus what is delivered. One can surmise that the answer to that question is what has extended the “hospital medicine rotation” to the “hospital medicine track,” comprised of a multiple of such rotations. Like all discussions on time-constrained medical education curricula, what will be discarded to make room for these rotations? In thirty-six months of training, there is opportunity cost: every month spent on a hospital medicine elective is a month that could have been spent on something else (rheumatology, nephrology, etc.). Again, this is not unique to hospital medicine; the same could be said of the resident who does too many cardiology electives at the exclusion of learning about endocrinology. It would be overly dramatic to say that devoting a month to a hospital medicine rotation, or any elective for that matter, meaningfully compromises the resident’s overall competence as an internist. It is, instead, a question of degree: an excessive number of these electives would likely compromise the resident’s overall competence. The likelihood of this happening is proportional to the size of the gap between what is required to effectively enter hospital medicine practice and what can be delivered in a month-long hospital medicine rotation. We return, then, to the question: How much hospital medicine training in residency would be required to fully prepare a resident for the current practice of a hospitalist?
Whatever the answer might be, that question takes us to a difficult dilemma that has lurked in the background of residency training for some time now; one that is not at all unique to hospital medicine. Should residency training be “voc-tech” or “liberal arts”? A purist would argue that an understanding and appreciation of all things not hospital medicine is what truly makes for the great hospitalist. An understanding of primary care, for example, would seem to optimize a hospitalist’s performance with respect to transitions of care. Adding to the gravity of such an argument is that residency might be the last time to acquire such “non-hospital-medicine” experiences.
Noting that the practice of hospital medicine being so dynamic and heterogeneous, the realist might pile on by saying that it is simply impossible to fully prepare a resident for the actual practice of hospital medicine. Further, many of these skills might be impossible to fully master outside of being fully immersed in the practice of hospital medicine (i.e., billing and coding). The best that can be done is to set a solid foundation that would enable them to learn further as they practice; there will be opportunities to learn the specific components of the field later on.
On the other hand, it is hard to justify residency training if the graduate is unprepared to practice, and without the fundamental knowledge, skills and attitudes specific to their career as they practice. For example, it is reasonable to suspect that a new hospitalist who has had no prior training in quality improvement will, because of the inertia that comes with engaging in any new and foreign skill, find it much harder to engage in quality improvement as a part of her career. It is also worth considering the role that mastery, autonomy and purpose have upon the overall residency experience. Engaging in electives that have a palpable purpose for the resident’s eventual career, and engender an opportunity to begin developing a sense of mastery in that field, could be an effective antidote in mitigating the burn-out that is far too common in residency training today.
For residents engaged in a future practice of hospital medicine, the hospital medicine rotation seems like a promising way out of this dilemma. An effectively designed elective approach could enable maintaining a core foundational education, while getting an early start on the specific components necessary for a promising career in hospital medicine. The operative words, of course, are “effectively designed.” What exactly does that entail? That is why this study is so important; even if we do not fully know what it should look like, we now have our first glimpse of what it is.
Disclosures
The author has nothing to disclose.
1. Ludwin S, Harrison J, Ranji S, et al. Training Residents in Hospital Medicine: The Hospitalist Elective National Survey (HENS). J Hosp Med. 2018;13(9):623-625. doi: 10.12788/jhm.2952. PubMed
1. Ludwin S, Harrison J, Ranji S, et al. Training Residents in Hospital Medicine: The Hospitalist Elective National Survey (HENS). J Hosp Med. 2018;13(9):623-625. doi: 10.12788/jhm.2952. PubMed
© 2018 Society of Hospital Medicine
Healthy Skepticism and Due Process
For more than 75 years, pediatrics has sought sound guidelines for prescribing maintenance intravenous fluid (mIVF) for children. In 1957, Holliday and Segar (H&S)1 introduced a breakthrough method for estimating mIVF needs. Their guidelines for calculating free-water and electrolyte needs for mIVF gained wide-spread acceptance and became the standard of care for decades.
Over the last two decades, awareness has grown around the occurrence of rare, life-threatening hyponatremic conditions, especially hyponatremic encephalopathy, in hospitalized children. Concomitantly, an increasing awareness shows that serum levels of antidiuretic hormone (ADH) are often elevated in sick children and triggered by nonosmotic conditions (pain, vomiting, perioperative state, meningitis, and pulmonary disease). This situation led to heightened concern of clinicians and investigators who assumed that hospitalized patients would exhibit reduced tolerance for hypotonic mIVF the mainstay of the H&S method. The possibility that the H&S method could be a significant contributing factor to the development of hyponatremic encephalopathy in hospitalized children became a research topic. This research speculated that even mildly reduced serum sodium levels might be a marker for the much rarer condition of hyponatremic encephalopathy. A number of hospitalists also switched from quarter-normal to half-normal saline in mIVF.
The substitution of hypotonic fluids with isotonic fluids (eg, 0.9% normal saline or lactated Ringer’s) is the current front-runner alternative to increase sodium delivery. The hypothesis is that the delivery of additional sodium, while maintaining the same H&S method volume/rate of fluid delivery, will protect against life-threatening hyponatremic events.
The challenge we face is whether we are moving from mIVF therapy, which features a long track record of success and an excellent safety profile, to a safer or more effective therapeutic approach. We should consider the burden of proof which should be satisfied to support creating new guidelines which center on changing from hypotonic mIVF to isotonic mIVF.
Is there sufficient scientific proof that isotonic mIVF is safer and/or more effective than hypotonic mIVF in preventing life-threatening hyponatremic events?
Is there compelling biologic plausibility for this change for patients with risk factors that are associated with elevated serum ADH levels?
What is the magnitude of the benefit?
What is the magnitude of unintended harms?
We offer our perspective on each of these questions.
The primary difficulty with addressing the adverse events of catastrophic hyponatremia (encephalopathy, seizures, cerebral edema, and death) is their rarity. The events stand out when they occur, prompting mortality and morbidity (M&M) conferences to blunder into action. But that action is not evidence-based, even if a rationale mentions a meta-analysis, because the rationales lack estimates of the number needed to treat (NNT) to prevent one catastrophic event. Estimates of the NNT to prevent mild hypernatremia are not useful. Furthermore, estimates of the number needed to harm (NNH) via unintended consequences of infusing extra sodium chloride are unavailable. True evidence-based medicine (EBM) is rigorous in requiring NNT and NNH. Anything less is considered M&M-based medicine masquerading as EBM.
No technical jargon distinguishes the profound and catastrophic events from the common, mild hyponatremia frequently observed in ill toddlers upon admission. As an analogy, in dealing with fever, astute pediatricians recognize that a moderate fever of 103.4 °F is not halfway to a heatstroke of 108 °F. Fever is not a near miss for heatstroke. Physicians do not recommend acetaminophen to prevent heatstroke, although many parents act that way.
No published randomized controlled trials (RCTs) showed the incidence of these catastrophic hyponatremic events. In the meta-analysis of 10 disparate and uncoordinated trials in 2014,2 no serious adverse events were noted among the 1,000 patients involved. Since then, newer RCTs have added another 1,000 patients to the meta-analysis pool, but still no serious adverse event has been observed.
The H&S method features 60 years of proven safety and remains the appropriate estimate when composing long-term parenteral nutrition. No recommendation is perfect for all situations. Many hospitalized children will exhibit an increased level of ADH. A very small fraction of those children will present a sufficiently elevated ADH level long enough to risk creating profound hyponatremia. An approximation is in the order of magnitude of 1 per 100,000 pediatric medical admissions and 1 per 10,000 postoperative patients. With 3 million pediatric admissions yearly in the United States, such numbers mean that large children’s hospitals might see one or two catastrophic adverse events each decade due to mIVF in previously healthy children. The risk in chronically ill children and in the ICU will be higher. The potential for causing unintended greater harm amongst the other millions of patients is high, requiring application of the precautionary principle.
Thus, EBM and RCTs are poor methodologies for quality improvement of this issue. Assigning surrogate measures, such as moderate hyponatremia or even mild hyponatremia, to increase sensitivity and incidence for research purposes lacks a validated scientific link to the much rarer profound hyponatremic events. The resulting nonvalid extrapolation is precisely what true EBM seeks to avoid. A serum sodium of 132 mEq/L is not a near miss. The NNT to prevent the catastrophic events is unknown. Indeed, no paper advocating adoption of isotonic mIVF has even ventured an approximation.
The RCTs are also, therefore, underpowered to identify harms from using normal saline as a maintenance fluid. A few studies mention hypernatremia, but serum sodium is not a statistical variable. Renal physiology predicts that kidneys can easily handle excess infused sodium and can protect against hypernatremia. However, the extra chloride load risks creating hyperchloremic acidosis, particularly when a patient with respiratory insufficiency cannot compensate by lowering pCO2 through increased minute ventilation. Edema is another risk. Both respiratory insufficiency and edema already occur more frequently (by orders of magnitude) in hospitalized patients on any mIVF than the profound hyponatremia events in hospitalized patients on hypotonic mIVF. For instance, about 1% of hospitalized infants with bronchiolitis are ventilated for respiratory failure. If hyperchloremic acidosis unintentionally caused by isotonic mIVF slightly increases the frequency of intubation, then such result far outweighs any benefit from reducing catastrophic hyponatremic events. Difficulty will also arise in detecting this unintended increase in the rate of intubation compared with the current background frequency. Detecting these unintended harms becomes impossible if the RCT is underpowered by 100-fold due to utilizing a surrogate measure, such as serum sodium <135 mEq/L, as the dependent variable instead of measuring serious hyponatremic adverse events.
All claims that “no evidence of harm” was found from using normal saline as mIVF are type II statistical errors. There is little chance of detecting any harm with a grossly underpowered study or a meta-analysis of 10 such studies. Simply put, EBM is impossible to use for events that occur less than 1 per 10,000 patients using RCTs with 1,000 patients. No usable safety data are available for normal saline as mIVF in any published RCT. As the RCTs are underpowered, one should rely on science to guide therapy, rather than on invalid statistics.
Using the precautionary principle, hypothetically, adding extra sodium chloride to maintenance fluids should be considered in the same manner as adding any other drug. Based on the current evidence, would the Food and Drug Administration approve the drug intravenous sodium chloride for the prevention of hyponatremia induced by maintenance fluids? An increasing evidence of a minimal beneficial effect is observed, but no evidence of safety nor physiology is available. A new drug application for using normal saline as a default maintenance fluid would be soundly rejected by an FDA panel, just as it has been rejected by the majority of pediatric hospitalists throughout the past 15 years since the idea was proposed in 2003.
With the lack of compelling statistical evidence to guide practice, clinicians often rely on biologic plausibility. Relatively recent studies have revealed that many sick children develop elevated blood levels of ADH due to nonosmotic and nonhemodynamic triggers. Fortunately, we also possess a strong body of knowledge around management of children with syndrome of inappropriate secretion of antidiuretic hormone (SIADH). We understand that elevated levels of ADH in the blood causes an increase in the resorption of free water from the renal collecting tubules. No increase in loss of renal sodium nor chloride is associated with this hormonal influence. The resultant hyponatremia is due to excess free-water retention and not the excess loss of sodium or chloride. To manage this condition, patients are not given a salt shaker and then allowed to drink ad libitum. The standard and well-accepted management of patients with SIADH is the restriction of free-water intake because this step addresses the dysfunctional renal process. Administering sodium chloride to a child with SIADH might possibly slow down the progression of hyponatremia but would also expand the total fluid volumes of the patient and would indirectly deal with a problem that could be addressed directly.
Understandably, in an intensive care setting, when hemodynamics is dicey, and when fluid-restriction could risk hypovolemia, employing a volume-expanding solution for mIVF therapy might be reasonable. However, in an ICU setting, SIADH is routinely treated with free-water restriction, and careful calculations of an individual patient’s fluid and electrolyte losses and needs are made.
In conclusion, we recognize the motivation for questioning the H&S method for mIVF as our field surveilles more than a half-century of accumulated experience with this method and the advances in our understanding of physiology and pathophysiology. However, we believe that the current body of evidence fails to substantiate the proposed recommendations.3 The avoidance of laboratory-detectable decreases in serum sodium levels is an unproven marker for the development of life-threatening hyponatremic events. Concerns for untoward effects (eg, excessive volume expansion and effects of hyperchloremia toward acidosis) and the exploration of alternative approaches (eg, modifications in volumes/rates of fluid delivery) have been inadequately explored. The proposed changes in practice may provide no mitigation in the rare events we hope to avoid, may fail to serve all subpopulations within the proposed scope of patients, and will likely
Disclosures
Dr. Powell and Dr. Zaoutis have nothing to disclose.
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics 1957;19(5):823-832. PubMed
2. Wang J, Xu E, Xiao Y. Isotonic versus hypotonic maintenance IV fluids in hospitalized children: a meta-analysis. Pediatrics 2014;133(1):105-113. doi: 10.1542/peds.2013-2041. PubMed
3. Hall AM, Ayus JC, Moritz ML. The default use of hypotonic maintenance intravenous fluids in pediatrics. J Hosp Med. 2018;13(9)637-640. doi: 10.12788/jhm.3040. PubMed
For more than 75 years, pediatrics has sought sound guidelines for prescribing maintenance intravenous fluid (mIVF) for children. In 1957, Holliday and Segar (H&S)1 introduced a breakthrough method for estimating mIVF needs. Their guidelines for calculating free-water and electrolyte needs for mIVF gained wide-spread acceptance and became the standard of care for decades.
Over the last two decades, awareness has grown around the occurrence of rare, life-threatening hyponatremic conditions, especially hyponatremic encephalopathy, in hospitalized children. Concomitantly, an increasing awareness shows that serum levels of antidiuretic hormone (ADH) are often elevated in sick children and triggered by nonosmotic conditions (pain, vomiting, perioperative state, meningitis, and pulmonary disease). This situation led to heightened concern of clinicians and investigators who assumed that hospitalized patients would exhibit reduced tolerance for hypotonic mIVF the mainstay of the H&S method. The possibility that the H&S method could be a significant contributing factor to the development of hyponatremic encephalopathy in hospitalized children became a research topic. This research speculated that even mildly reduced serum sodium levels might be a marker for the much rarer condition of hyponatremic encephalopathy. A number of hospitalists also switched from quarter-normal to half-normal saline in mIVF.
The substitution of hypotonic fluids with isotonic fluids (eg, 0.9% normal saline or lactated Ringer’s) is the current front-runner alternative to increase sodium delivery. The hypothesis is that the delivery of additional sodium, while maintaining the same H&S method volume/rate of fluid delivery, will protect against life-threatening hyponatremic events.
The challenge we face is whether we are moving from mIVF therapy, which features a long track record of success and an excellent safety profile, to a safer or more effective therapeutic approach. We should consider the burden of proof which should be satisfied to support creating new guidelines which center on changing from hypotonic mIVF to isotonic mIVF.
Is there sufficient scientific proof that isotonic mIVF is safer and/or more effective than hypotonic mIVF in preventing life-threatening hyponatremic events?
Is there compelling biologic plausibility for this change for patients with risk factors that are associated with elevated serum ADH levels?
What is the magnitude of the benefit?
What is the magnitude of unintended harms?
We offer our perspective on each of these questions.
The primary difficulty with addressing the adverse events of catastrophic hyponatremia (encephalopathy, seizures, cerebral edema, and death) is their rarity. The events stand out when they occur, prompting mortality and morbidity (M&M) conferences to blunder into action. But that action is not evidence-based, even if a rationale mentions a meta-analysis, because the rationales lack estimates of the number needed to treat (NNT) to prevent one catastrophic event. Estimates of the NNT to prevent mild hypernatremia are not useful. Furthermore, estimates of the number needed to harm (NNH) via unintended consequences of infusing extra sodium chloride are unavailable. True evidence-based medicine (EBM) is rigorous in requiring NNT and NNH. Anything less is considered M&M-based medicine masquerading as EBM.
No technical jargon distinguishes the profound and catastrophic events from the common, mild hyponatremia frequently observed in ill toddlers upon admission. As an analogy, in dealing with fever, astute pediatricians recognize that a moderate fever of 103.4 °F is not halfway to a heatstroke of 108 °F. Fever is not a near miss for heatstroke. Physicians do not recommend acetaminophen to prevent heatstroke, although many parents act that way.
No published randomized controlled trials (RCTs) showed the incidence of these catastrophic hyponatremic events. In the meta-analysis of 10 disparate and uncoordinated trials in 2014,2 no serious adverse events were noted among the 1,000 patients involved. Since then, newer RCTs have added another 1,000 patients to the meta-analysis pool, but still no serious adverse event has been observed.
The H&S method features 60 years of proven safety and remains the appropriate estimate when composing long-term parenteral nutrition. No recommendation is perfect for all situations. Many hospitalized children will exhibit an increased level of ADH. A very small fraction of those children will present a sufficiently elevated ADH level long enough to risk creating profound hyponatremia. An approximation is in the order of magnitude of 1 per 100,000 pediatric medical admissions and 1 per 10,000 postoperative patients. With 3 million pediatric admissions yearly in the United States, such numbers mean that large children’s hospitals might see one or two catastrophic adverse events each decade due to mIVF in previously healthy children. The risk in chronically ill children and in the ICU will be higher. The potential for causing unintended greater harm amongst the other millions of patients is high, requiring application of the precautionary principle.
Thus, EBM and RCTs are poor methodologies for quality improvement of this issue. Assigning surrogate measures, such as moderate hyponatremia or even mild hyponatremia, to increase sensitivity and incidence for research purposes lacks a validated scientific link to the much rarer profound hyponatremic events. The resulting nonvalid extrapolation is precisely what true EBM seeks to avoid. A serum sodium of 132 mEq/L is not a near miss. The NNT to prevent the catastrophic events is unknown. Indeed, no paper advocating adoption of isotonic mIVF has even ventured an approximation.
The RCTs are also, therefore, underpowered to identify harms from using normal saline as a maintenance fluid. A few studies mention hypernatremia, but serum sodium is not a statistical variable. Renal physiology predicts that kidneys can easily handle excess infused sodium and can protect against hypernatremia. However, the extra chloride load risks creating hyperchloremic acidosis, particularly when a patient with respiratory insufficiency cannot compensate by lowering pCO2 through increased minute ventilation. Edema is another risk. Both respiratory insufficiency and edema already occur more frequently (by orders of magnitude) in hospitalized patients on any mIVF than the profound hyponatremia events in hospitalized patients on hypotonic mIVF. For instance, about 1% of hospitalized infants with bronchiolitis are ventilated for respiratory failure. If hyperchloremic acidosis unintentionally caused by isotonic mIVF slightly increases the frequency of intubation, then such result far outweighs any benefit from reducing catastrophic hyponatremic events. Difficulty will also arise in detecting this unintended increase in the rate of intubation compared with the current background frequency. Detecting these unintended harms becomes impossible if the RCT is underpowered by 100-fold due to utilizing a surrogate measure, such as serum sodium <135 mEq/L, as the dependent variable instead of measuring serious hyponatremic adverse events.
All claims that “no evidence of harm” was found from using normal saline as mIVF are type II statistical errors. There is little chance of detecting any harm with a grossly underpowered study or a meta-analysis of 10 such studies. Simply put, EBM is impossible to use for events that occur less than 1 per 10,000 patients using RCTs with 1,000 patients. No usable safety data are available for normal saline as mIVF in any published RCT. As the RCTs are underpowered, one should rely on science to guide therapy, rather than on invalid statistics.
Using the precautionary principle, hypothetically, adding extra sodium chloride to maintenance fluids should be considered in the same manner as adding any other drug. Based on the current evidence, would the Food and Drug Administration approve the drug intravenous sodium chloride for the prevention of hyponatremia induced by maintenance fluids? An increasing evidence of a minimal beneficial effect is observed, but no evidence of safety nor physiology is available. A new drug application for using normal saline as a default maintenance fluid would be soundly rejected by an FDA panel, just as it has been rejected by the majority of pediatric hospitalists throughout the past 15 years since the idea was proposed in 2003.
With the lack of compelling statistical evidence to guide practice, clinicians often rely on biologic plausibility. Relatively recent studies have revealed that many sick children develop elevated blood levels of ADH due to nonosmotic and nonhemodynamic triggers. Fortunately, we also possess a strong body of knowledge around management of children with syndrome of inappropriate secretion of antidiuretic hormone (SIADH). We understand that elevated levels of ADH in the blood causes an increase in the resorption of free water from the renal collecting tubules. No increase in loss of renal sodium nor chloride is associated with this hormonal influence. The resultant hyponatremia is due to excess free-water retention and not the excess loss of sodium or chloride. To manage this condition, patients are not given a salt shaker and then allowed to drink ad libitum. The standard and well-accepted management of patients with SIADH is the restriction of free-water intake because this step addresses the dysfunctional renal process. Administering sodium chloride to a child with SIADH might possibly slow down the progression of hyponatremia but would also expand the total fluid volumes of the patient and would indirectly deal with a problem that could be addressed directly.
Understandably, in an intensive care setting, when hemodynamics is dicey, and when fluid-restriction could risk hypovolemia, employing a volume-expanding solution for mIVF therapy might be reasonable. However, in an ICU setting, SIADH is routinely treated with free-water restriction, and careful calculations of an individual patient’s fluid and electrolyte losses and needs are made.
In conclusion, we recognize the motivation for questioning the H&S method for mIVF as our field surveilles more than a half-century of accumulated experience with this method and the advances in our understanding of physiology and pathophysiology. However, we believe that the current body of evidence fails to substantiate the proposed recommendations.3 The avoidance of laboratory-detectable decreases in serum sodium levels is an unproven marker for the development of life-threatening hyponatremic events. Concerns for untoward effects (eg, excessive volume expansion and effects of hyperchloremia toward acidosis) and the exploration of alternative approaches (eg, modifications in volumes/rates of fluid delivery) have been inadequately explored. The proposed changes in practice may provide no mitigation in the rare events we hope to avoid, may fail to serve all subpopulations within the proposed scope of patients, and will likely
Disclosures
Dr. Powell and Dr. Zaoutis have nothing to disclose.
For more than 75 years, pediatrics has sought sound guidelines for prescribing maintenance intravenous fluid (mIVF) for children. In 1957, Holliday and Segar (H&S)1 introduced a breakthrough method for estimating mIVF needs. Their guidelines for calculating free-water and electrolyte needs for mIVF gained wide-spread acceptance and became the standard of care for decades.
Over the last two decades, awareness has grown around the occurrence of rare, life-threatening hyponatremic conditions, especially hyponatremic encephalopathy, in hospitalized children. Concomitantly, an increasing awareness shows that serum levels of antidiuretic hormone (ADH) are often elevated in sick children and triggered by nonosmotic conditions (pain, vomiting, perioperative state, meningitis, and pulmonary disease). This situation led to heightened concern of clinicians and investigators who assumed that hospitalized patients would exhibit reduced tolerance for hypotonic mIVF the mainstay of the H&S method. The possibility that the H&S method could be a significant contributing factor to the development of hyponatremic encephalopathy in hospitalized children became a research topic. This research speculated that even mildly reduced serum sodium levels might be a marker for the much rarer condition of hyponatremic encephalopathy. A number of hospitalists also switched from quarter-normal to half-normal saline in mIVF.
The substitution of hypotonic fluids with isotonic fluids (eg, 0.9% normal saline or lactated Ringer’s) is the current front-runner alternative to increase sodium delivery. The hypothesis is that the delivery of additional sodium, while maintaining the same H&S method volume/rate of fluid delivery, will protect against life-threatening hyponatremic events.
The challenge we face is whether we are moving from mIVF therapy, which features a long track record of success and an excellent safety profile, to a safer or more effective therapeutic approach. We should consider the burden of proof which should be satisfied to support creating new guidelines which center on changing from hypotonic mIVF to isotonic mIVF.
Is there sufficient scientific proof that isotonic mIVF is safer and/or more effective than hypotonic mIVF in preventing life-threatening hyponatremic events?
Is there compelling biologic plausibility for this change for patients with risk factors that are associated with elevated serum ADH levels?
What is the magnitude of the benefit?
What is the magnitude of unintended harms?
We offer our perspective on each of these questions.
The primary difficulty with addressing the adverse events of catastrophic hyponatremia (encephalopathy, seizures, cerebral edema, and death) is their rarity. The events stand out when they occur, prompting mortality and morbidity (M&M) conferences to blunder into action. But that action is not evidence-based, even if a rationale mentions a meta-analysis, because the rationales lack estimates of the number needed to treat (NNT) to prevent one catastrophic event. Estimates of the NNT to prevent mild hypernatremia are not useful. Furthermore, estimates of the number needed to harm (NNH) via unintended consequences of infusing extra sodium chloride are unavailable. True evidence-based medicine (EBM) is rigorous in requiring NNT and NNH. Anything less is considered M&M-based medicine masquerading as EBM.
No technical jargon distinguishes the profound and catastrophic events from the common, mild hyponatremia frequently observed in ill toddlers upon admission. As an analogy, in dealing with fever, astute pediatricians recognize that a moderate fever of 103.4 °F is not halfway to a heatstroke of 108 °F. Fever is not a near miss for heatstroke. Physicians do not recommend acetaminophen to prevent heatstroke, although many parents act that way.
No published randomized controlled trials (RCTs) showed the incidence of these catastrophic hyponatremic events. In the meta-analysis of 10 disparate and uncoordinated trials in 2014,2 no serious adverse events were noted among the 1,000 patients involved. Since then, newer RCTs have added another 1,000 patients to the meta-analysis pool, but still no serious adverse event has been observed.
The H&S method features 60 years of proven safety and remains the appropriate estimate when composing long-term parenteral nutrition. No recommendation is perfect for all situations. Many hospitalized children will exhibit an increased level of ADH. A very small fraction of those children will present a sufficiently elevated ADH level long enough to risk creating profound hyponatremia. An approximation is in the order of magnitude of 1 per 100,000 pediatric medical admissions and 1 per 10,000 postoperative patients. With 3 million pediatric admissions yearly in the United States, such numbers mean that large children’s hospitals might see one or two catastrophic adverse events each decade due to mIVF in previously healthy children. The risk in chronically ill children and in the ICU will be higher. The potential for causing unintended greater harm amongst the other millions of patients is high, requiring application of the precautionary principle.
Thus, EBM and RCTs are poor methodologies for quality improvement of this issue. Assigning surrogate measures, such as moderate hyponatremia or even mild hyponatremia, to increase sensitivity and incidence for research purposes lacks a validated scientific link to the much rarer profound hyponatremic events. The resulting nonvalid extrapolation is precisely what true EBM seeks to avoid. A serum sodium of 132 mEq/L is not a near miss. The NNT to prevent the catastrophic events is unknown. Indeed, no paper advocating adoption of isotonic mIVF has even ventured an approximation.
The RCTs are also, therefore, underpowered to identify harms from using normal saline as a maintenance fluid. A few studies mention hypernatremia, but serum sodium is not a statistical variable. Renal physiology predicts that kidneys can easily handle excess infused sodium and can protect against hypernatremia. However, the extra chloride load risks creating hyperchloremic acidosis, particularly when a patient with respiratory insufficiency cannot compensate by lowering pCO2 through increased minute ventilation. Edema is another risk. Both respiratory insufficiency and edema already occur more frequently (by orders of magnitude) in hospitalized patients on any mIVF than the profound hyponatremia events in hospitalized patients on hypotonic mIVF. For instance, about 1% of hospitalized infants with bronchiolitis are ventilated for respiratory failure. If hyperchloremic acidosis unintentionally caused by isotonic mIVF slightly increases the frequency of intubation, then such result far outweighs any benefit from reducing catastrophic hyponatremic events. Difficulty will also arise in detecting this unintended increase in the rate of intubation compared with the current background frequency. Detecting these unintended harms becomes impossible if the RCT is underpowered by 100-fold due to utilizing a surrogate measure, such as serum sodium <135 mEq/L, as the dependent variable instead of measuring serious hyponatremic adverse events.
All claims that “no evidence of harm” was found from using normal saline as mIVF are type II statistical errors. There is little chance of detecting any harm with a grossly underpowered study or a meta-analysis of 10 such studies. Simply put, EBM is impossible to use for events that occur less than 1 per 10,000 patients using RCTs with 1,000 patients. No usable safety data are available for normal saline as mIVF in any published RCT. As the RCTs are underpowered, one should rely on science to guide therapy, rather than on invalid statistics.
Using the precautionary principle, hypothetically, adding extra sodium chloride to maintenance fluids should be considered in the same manner as adding any other drug. Based on the current evidence, would the Food and Drug Administration approve the drug intravenous sodium chloride for the prevention of hyponatremia induced by maintenance fluids? An increasing evidence of a minimal beneficial effect is observed, but no evidence of safety nor physiology is available. A new drug application for using normal saline as a default maintenance fluid would be soundly rejected by an FDA panel, just as it has been rejected by the majority of pediatric hospitalists throughout the past 15 years since the idea was proposed in 2003.
With the lack of compelling statistical evidence to guide practice, clinicians often rely on biologic plausibility. Relatively recent studies have revealed that many sick children develop elevated blood levels of ADH due to nonosmotic and nonhemodynamic triggers. Fortunately, we also possess a strong body of knowledge around management of children with syndrome of inappropriate secretion of antidiuretic hormone (SIADH). We understand that elevated levels of ADH in the blood causes an increase in the resorption of free water from the renal collecting tubules. No increase in loss of renal sodium nor chloride is associated with this hormonal influence. The resultant hyponatremia is due to excess free-water retention and not the excess loss of sodium or chloride. To manage this condition, patients are not given a salt shaker and then allowed to drink ad libitum. The standard and well-accepted management of patients with SIADH is the restriction of free-water intake because this step addresses the dysfunctional renal process. Administering sodium chloride to a child with SIADH might possibly slow down the progression of hyponatremia but would also expand the total fluid volumes of the patient and would indirectly deal with a problem that could be addressed directly.
Understandably, in an intensive care setting, when hemodynamics is dicey, and when fluid-restriction could risk hypovolemia, employing a volume-expanding solution for mIVF therapy might be reasonable. However, in an ICU setting, SIADH is routinely treated with free-water restriction, and careful calculations of an individual patient’s fluid and electrolyte losses and needs are made.
In conclusion, we recognize the motivation for questioning the H&S method for mIVF as our field surveilles more than a half-century of accumulated experience with this method and the advances in our understanding of physiology and pathophysiology. However, we believe that the current body of evidence fails to substantiate the proposed recommendations.3 The avoidance of laboratory-detectable decreases in serum sodium levels is an unproven marker for the development of life-threatening hyponatremic events. Concerns for untoward effects (eg, excessive volume expansion and effects of hyperchloremia toward acidosis) and the exploration of alternative approaches (eg, modifications in volumes/rates of fluid delivery) have been inadequately explored. The proposed changes in practice may provide no mitigation in the rare events we hope to avoid, may fail to serve all subpopulations within the proposed scope of patients, and will likely
Disclosures
Dr. Powell and Dr. Zaoutis have nothing to disclose.
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics 1957;19(5):823-832. PubMed
2. Wang J, Xu E, Xiao Y. Isotonic versus hypotonic maintenance IV fluids in hospitalized children: a meta-analysis. Pediatrics 2014;133(1):105-113. doi: 10.1542/peds.2013-2041. PubMed
3. Hall AM, Ayus JC, Moritz ML. The default use of hypotonic maintenance intravenous fluids in pediatrics. J Hosp Med. 2018;13(9)637-640. doi: 10.12788/jhm.3040. PubMed
1. Holliday MA, Segar WE. The maintenance need for water in parenteral fluid therapy. Pediatrics 1957;19(5):823-832. PubMed
2. Wang J, Xu E, Xiao Y. Isotonic versus hypotonic maintenance IV fluids in hospitalized children: a meta-analysis. Pediatrics 2014;133(1):105-113. doi: 10.1542/peds.2013-2041. PubMed
3. Hall AM, Ayus JC, Moritz ML. The default use of hypotonic maintenance intravenous fluids in pediatrics. J Hosp Med. 2018;13(9)637-640. doi: 10.12788/jhm.3040. PubMed
© 2018 Society of Hospital Medicine
Phosphorus in kidney disease: Culprit or bystander?
Phosphorus is essential for life. However, both low and high levels of phosphorus in the body have consequences, and its concentration in the blood is tightly regulated through dietary absorption, bone flux, and renal excretion and is influenced by calcitriol (1,25 hydroxyvitamin D3), parathyroid hormone, and fibroblast growth factor 23 (FGF23).
See related articles by M. Shetty and A. Sekar
Sekar et al,1 in this issue of the Journal, provide an extensive review of the pathophysiology of phosphorus metabolism and strategies to control phosphorus levels in patients with hyperphosphatemia and end-stage kidney disease.
PHOSPHORUS OR PHOSPHATE?
What's in a name? That which we call a rose
By any other word would smell as sweet.
—Shakespeare, Romeo and Juliet
The terms phosphate and phosphorus are often used interchangeably, though most writers still prefer phosphate over phosphorus.
The serum concentrations of phosphate and phosphorus are the same when expressed in millimoles per liter, as every mole of phosphate contains 1 mole of phosphorus, but not the same when expressed in milligrams per deciliter.2 The molecular weight of phosphorus is 30.97, whereas the molecular weight of the phosphate ion (PO43–) is 94.97—more than 3 times higher. Therefore, using these terms interchangeably in this context can lead to numerical error.3
Phosphorus, being highly reactive, does not exist by itself in nature and is typically present as phosphates in biologic systems. When describing phosphorus metabolism, the term phosphates should ideally be used because phosphates are the actual participants in the bodily processes. But in the clinical laboratory, all methods that measure serum phosphorus in fact measure inorganic phosphate and are expressed in terms of milligrams of phosphorus per deciliter rather than milligrams of phosphate per deciliter, and using these 2 terms interchangeably in clinical practice should not be of concern.4
THE PROBLEM
US adults typically ingest 1,200 mg of phosphorus each day, and about 60% to 70% of the ingested phosphorus is absorbed both by passive paracellular diffusion via tight junctions and by active transcellular transport via sodium-phosphate cotransport. The kidneys must excrete the same amount daily to maintain a steady state. As kidney function declines, phosphorus accumulates in the blood, leading to hyperphosphatemia.
Hyperphosphatemia is often asymptomatic, but it can cause generalized itching, red eyes, and adverse effects on the bone and parathyroid glands. Higher serum phosphorus levels have been shown to be associated with vascular calcification,5 cardiovascular events, and higher all-cause mortality rates in the general population,6 in patients with diabetes,7 and in those with chronic kidney disease.8 This association between higher serum phosphorus levels and the all-cause mortality rate led to the assumption that lowering serum phosphorus levels in these patients could reduce the rates of cardiovascular events and death, and to efforts to correct hyperphosphatemia.
Research into FGF23 continues, especially its role in cardiovascular complications of chronic kidney disease, as both phosphorus and FGF23 levels are elevated in chronic kidney disease and are implicated in poor clinical outcomes in these patients. However, both FGF23 and parathyroid hormone levels rise early in the course of kidney disease, long before overt hyperphosphatemia develops. Further, FGF23 rises earlier than parathyroid hormone and has been found to be an independent risk factor for cardiovascular events and death from any cause in end-stage kidney disease.9
Whether hyperphosphatemia is the culprit or merely an epiphenomenon of metabolic complications of chronic kidney disease is still unclear, as more molecules are being identified in the complex process of cardiovascular calcification.10
However, one thing is clear: vascular calcification is not just a simple precipitation of calcium and phosphorus. Instead, it is an active process that involves many regulators of mineral metabolism.10 The complex nature of this process is likely one of the reasons that evidence is conflicting11 about the benefits of phosphorus binders in terms of cardiovascular events or all-cause mortality in these patients.
STRATEGIES TO CONTROL HYPERPHOSPHATEMIA
Reducing intake
Dietary phosphorus restriction is the first step in controlling serum phosphorus. But reducing phosphorus intake while otherwise trying to optimize the nutritional status can be challenging.
The recommended daily protein intake is 1.0 to 1.2 g/kg. But phosphorus is typically found in foods rich in proteins, and restricting protein severely can compromise nutritional status and may be as bad as elevated phosphate levels in terms of outcomes.
Although plant-based foods contain more phosphate per gram of protein (ie, they have a higher ratio of phosphorus to protein) than animal-based foods, the bioavailability of phosphorus from plant foods is lower. Phosphorus in plant-based foods is mainly in the form of phytate. Humans cannot hydrolyze phytate because we lack the phytase enzyme; hence, the phosphorus in plant-based foods is not well absorbed. Therefore, a vegetarian diet may be preferable and beneficial in patients with chronic kidney disease. A small study in humans showed that a vegetarian diet resulted in lower serum phosphorus and FGF23 levels, but the study was limited by its small sample size.12
Patients should be advised to avoid foods that have a high phosphate content, such as processed foods, fast foods, and cola beverages, which often have phosphate-based food additives.
Further, one should be cautious about using supplements with healthy-sounding names. A case in point is “vitamin water”: 12 oz of this fruit punch-flavored beverage contains 392 mg of phosphorus,13 and this alone would require 12 to 15 phosphate binder tablets to bind its phosphorus content.
In addition, many prescription drugs have significant amounts of phosphorus, and this is often unrecognized.
Sherman et al14 reviewed 200 of the most commonly prescribed drugs in dialysis patients and found that 23 (11.5%) of the drug labels listed phosphorus-containing ingredients, but the actual amount of phosphorus was not listed. The phosphorus content ranged from 1.4 mg (clonidine 0.2 mg, Blue Point Laboratories, Dublin, Ireland) to 111.5 mg (paroxetine 40 mg, GlaxoSmith Kline, Philadelphia, PA). The phosphorus content was inconsistent and varied with the dose of the agent, type of formulation (tablet or syrup), branded or generic formulation, and manufacturer.
Branded lisinopril (Merck, Kenilworth, NJ) had 21.4 mg of phosphorus per 10-mg dose, while a generic product (Blue Point Laboratories, Dublin, Ireland) had 32.6 mg. Different brands of generic amlodipine 10 mg varied in their phosphorus content from 8.6 mg (Lupin Pharmaceuticals, Mumbai, India) to 27.8 mg (Greenstone LLC, Peapack, NJ) to 40.1 mg (Qualitest Pharmaceuticals, Huntsville, AL. Rena-Vite (Cypress Pharmaceuticals, Madison, MS), a multivitamin marketed to patients with kidney disease, had 37.7 mg of phosphorus per tablet. Thus, just to bind the phosphorus content of these 3 tablets (lisinopril, amlodipine, and Rena-Vite), a patient could need at least 3 to 4 extra doses of phosphate binder.
The phosphate content of medications should be considered when prescribing. For example, Reno Caps (Nnodum Pharmaceuticals, Cincinnati, OH), another vitamin supplement, has only 1.7 mg of phosphorus per tablet and should be considered, especially in patients with poorly controlled serum phosphorus levels. However, the challenge is that medication labels do not provide the phosphorus content.
Reducing phosphorus absorption
Although these agents reduce serum phosphorus and help reduce symptoms, an important quality-of-life measure, it is uncertain whether they improve clinical outcomes.11 To date, no specific phosphorus binder offers a survival benefit over placebo.11
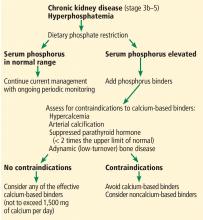
Based on the limited and conflicting evidence, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, recently updated, suggest that oral phosphorus binders should be used in patients with hyperphosphatemia to lower serum phosphorus levels toward the normal range.15 They further recommend not exceeding 1,500 mg of elemental calcium per day if a calcium-based binder is used, and they recommend avoiding calcium-based binders in patients with hypercalcemia, adynamic bone disease, or vascular calcification.
Phosphorus binders may account for up to 50% of the daily pill burden and may contribute to poor medication adherence.16 Dialysis patients need to take a lot of these drugs: by weight, 5 to 6 pounds per year.
These drugs can bind and interfere with the absorption of other vital medications and so should be taken with meals and separately from other medications.
Removing phosphorus
Removal of phosphorus by adequate dialysis or kidney transplant is the final strategy.
New agents under study
To improve phosphorus control, other agents that inhibit absorption of phosphate are being investigated.
Nicotinamide reduces expression of the sodium-phosphorus cotransporter NTP2b. Its use in combination with a low-phosphorus diet and phosphorus binders may maximize reductions in phosphorus absorption and is being studied in the CKD Optimal Management With Binders and Nicotinamide (COMBINE) study.
Tenapanor, an inhibitor of the sodium-hydrogen transporter NHE3, has been shown in animal studies to increase fecal phosphate excretion and decrease urinary phosphate excretion17 but requires further evaluation.
- Sekar A, Kaur T, Nally JV Jr, Rincon-Choles H, Jolly S, Nakhoul G. Phosphorus binders: the new and the old, and how to choose. Cleve Clin J Med 2018; 85(8):629–638. doi:10.3949/ccjm.85a.17054
- Young DS. "Phosphorus" or "phosphate." Ann Intern Med 1980; 93(4):631. pmid:7436198
- Bartter FC. Reporting of phosphate and phosphorus plasma values. Am J Med 1981; 71(5):848. pmid:7304659.
- Iheagwara OS, Ing TS, Kjellstrand CM, Lew SQ. Phosphorus, phosphorous, and phosphate. Hemodial Int 2013; 17(4):479–482. doi:10.1111/hdi.12010
- Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 2009; 20(2):381–387. doi:10.1681/ASN.2008040349
- Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007; 167(9):879–885. doi:10.1001/archinte.167.9.879
- Chonchol M, Dale R, Schrier RW, Estacio R. Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med 2009; 122(4):380–386. doi:10.1016/j.amjmed.2008.09.039
- Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 2009; 24(5):1506–1523. doi:10.1093/ndt/gfn613
- Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359(6):584–592. doi:10.1056/NEJMoa0706130
- Lullo LD, Barbera V, Bellasi A, et al. Vascular and valvular calcifications in chronic kidney disease: an update. EMJ Nephrol 2016; 4(1):84–91. https://pdfs.semanticscholar.org/150f/c7b5dfe671c9b61e4c76d54b7d713b60ba6a.pdf. Accesssed June 5, 2018.
- Palmer SC, Gardner S, Tonelli M, et al. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis 2016; 68(5):691–702. doi:10.1053/j.ajkd.2016.05.015
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Moser M, White K, Henry B, et al. Phosphorus content of popular beverages. Am J Kidney Dis 2015; 65(6):969–971. doi:10.1053/j.ajkd.2015.02.330
- Sherman RA, Ravella S, Kapoian T. A dearth of data: the problem of phosphorus in prescription medications. Kidney Int 2015; 87(6):1097–1099. doi:10.1038/ki.2015.67
- KDIGO 2017 clinical practice guideline update for diagnosis, evaluation, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Supplements 2017; 7(1 suppl): 1–59. www.kisupplements.org/article/S2157-1716(17)30001-1/pdf. Accessed June 5, 2018.
- Fissell RB, Karaboyas A, Bieber BA, et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int 2016; 20(1):38–49. doi:10.1111/hdi.12315
- Labonté ED, Carreras CW, Leadbetter MR, et al. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 2015; 26(5):1138–1149. doi:10.1681/ASN.2014030317
Phosphorus is essential for life. However, both low and high levels of phosphorus in the body have consequences, and its concentration in the blood is tightly regulated through dietary absorption, bone flux, and renal excretion and is influenced by calcitriol (1,25 hydroxyvitamin D3), parathyroid hormone, and fibroblast growth factor 23 (FGF23).
See related articles by M. Shetty and A. Sekar
Sekar et al,1 in this issue of the Journal, provide an extensive review of the pathophysiology of phosphorus metabolism and strategies to control phosphorus levels in patients with hyperphosphatemia and end-stage kidney disease.
PHOSPHORUS OR PHOSPHATE?
What's in a name? That which we call a rose
By any other word would smell as sweet.
—Shakespeare, Romeo and Juliet
The terms phosphate and phosphorus are often used interchangeably, though most writers still prefer phosphate over phosphorus.
The serum concentrations of phosphate and phosphorus are the same when expressed in millimoles per liter, as every mole of phosphate contains 1 mole of phosphorus, but not the same when expressed in milligrams per deciliter.2 The molecular weight of phosphorus is 30.97, whereas the molecular weight of the phosphate ion (PO43–) is 94.97—more than 3 times higher. Therefore, using these terms interchangeably in this context can lead to numerical error.3
Phosphorus, being highly reactive, does not exist by itself in nature and is typically present as phosphates in biologic systems. When describing phosphorus metabolism, the term phosphates should ideally be used because phosphates are the actual participants in the bodily processes. But in the clinical laboratory, all methods that measure serum phosphorus in fact measure inorganic phosphate and are expressed in terms of milligrams of phosphorus per deciliter rather than milligrams of phosphate per deciliter, and using these 2 terms interchangeably in clinical practice should not be of concern.4
THE PROBLEM
US adults typically ingest 1,200 mg of phosphorus each day, and about 60% to 70% of the ingested phosphorus is absorbed both by passive paracellular diffusion via tight junctions and by active transcellular transport via sodium-phosphate cotransport. The kidneys must excrete the same amount daily to maintain a steady state. As kidney function declines, phosphorus accumulates in the blood, leading to hyperphosphatemia.
Hyperphosphatemia is often asymptomatic, but it can cause generalized itching, red eyes, and adverse effects on the bone and parathyroid glands. Higher serum phosphorus levels have been shown to be associated with vascular calcification,5 cardiovascular events, and higher all-cause mortality rates in the general population,6 in patients with diabetes,7 and in those with chronic kidney disease.8 This association between higher serum phosphorus levels and the all-cause mortality rate led to the assumption that lowering serum phosphorus levels in these patients could reduce the rates of cardiovascular events and death, and to efforts to correct hyperphosphatemia.
Research into FGF23 continues, especially its role in cardiovascular complications of chronic kidney disease, as both phosphorus and FGF23 levels are elevated in chronic kidney disease and are implicated in poor clinical outcomes in these patients. However, both FGF23 and parathyroid hormone levels rise early in the course of kidney disease, long before overt hyperphosphatemia develops. Further, FGF23 rises earlier than parathyroid hormone and has been found to be an independent risk factor for cardiovascular events and death from any cause in end-stage kidney disease.9
Whether hyperphosphatemia is the culprit or merely an epiphenomenon of metabolic complications of chronic kidney disease is still unclear, as more molecules are being identified in the complex process of cardiovascular calcification.10
However, one thing is clear: vascular calcification is not just a simple precipitation of calcium and phosphorus. Instead, it is an active process that involves many regulators of mineral metabolism.10 The complex nature of this process is likely one of the reasons that evidence is conflicting11 about the benefits of phosphorus binders in terms of cardiovascular events or all-cause mortality in these patients.
STRATEGIES TO CONTROL HYPERPHOSPHATEMIA
Reducing intake
Dietary phosphorus restriction is the first step in controlling serum phosphorus. But reducing phosphorus intake while otherwise trying to optimize the nutritional status can be challenging.
The recommended daily protein intake is 1.0 to 1.2 g/kg. But phosphorus is typically found in foods rich in proteins, and restricting protein severely can compromise nutritional status and may be as bad as elevated phosphate levels in terms of outcomes.
Although plant-based foods contain more phosphate per gram of protein (ie, they have a higher ratio of phosphorus to protein) than animal-based foods, the bioavailability of phosphorus from plant foods is lower. Phosphorus in plant-based foods is mainly in the form of phytate. Humans cannot hydrolyze phytate because we lack the phytase enzyme; hence, the phosphorus in plant-based foods is not well absorbed. Therefore, a vegetarian diet may be preferable and beneficial in patients with chronic kidney disease. A small study in humans showed that a vegetarian diet resulted in lower serum phosphorus and FGF23 levels, but the study was limited by its small sample size.12
Patients should be advised to avoid foods that have a high phosphate content, such as processed foods, fast foods, and cola beverages, which often have phosphate-based food additives.
Further, one should be cautious about using supplements with healthy-sounding names. A case in point is “vitamin water”: 12 oz of this fruit punch-flavored beverage contains 392 mg of phosphorus,13 and this alone would require 12 to 15 phosphate binder tablets to bind its phosphorus content.
In addition, many prescription drugs have significant amounts of phosphorus, and this is often unrecognized.
Sherman et al14 reviewed 200 of the most commonly prescribed drugs in dialysis patients and found that 23 (11.5%) of the drug labels listed phosphorus-containing ingredients, but the actual amount of phosphorus was not listed. The phosphorus content ranged from 1.4 mg (clonidine 0.2 mg, Blue Point Laboratories, Dublin, Ireland) to 111.5 mg (paroxetine 40 mg, GlaxoSmith Kline, Philadelphia, PA). The phosphorus content was inconsistent and varied with the dose of the agent, type of formulation (tablet or syrup), branded or generic formulation, and manufacturer.
Branded lisinopril (Merck, Kenilworth, NJ) had 21.4 mg of phosphorus per 10-mg dose, while a generic product (Blue Point Laboratories, Dublin, Ireland) had 32.6 mg. Different brands of generic amlodipine 10 mg varied in their phosphorus content from 8.6 mg (Lupin Pharmaceuticals, Mumbai, India) to 27.8 mg (Greenstone LLC, Peapack, NJ) to 40.1 mg (Qualitest Pharmaceuticals, Huntsville, AL. Rena-Vite (Cypress Pharmaceuticals, Madison, MS), a multivitamin marketed to patients with kidney disease, had 37.7 mg of phosphorus per tablet. Thus, just to bind the phosphorus content of these 3 tablets (lisinopril, amlodipine, and Rena-Vite), a patient could need at least 3 to 4 extra doses of phosphate binder.
The phosphate content of medications should be considered when prescribing. For example, Reno Caps (Nnodum Pharmaceuticals, Cincinnati, OH), another vitamin supplement, has only 1.7 mg of phosphorus per tablet and should be considered, especially in patients with poorly controlled serum phosphorus levels. However, the challenge is that medication labels do not provide the phosphorus content.
Reducing phosphorus absorption
Although these agents reduce serum phosphorus and help reduce symptoms, an important quality-of-life measure, it is uncertain whether they improve clinical outcomes.11 To date, no specific phosphorus binder offers a survival benefit over placebo.11
Based on the limited and conflicting evidence, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, recently updated, suggest that oral phosphorus binders should be used in patients with hyperphosphatemia to lower serum phosphorus levels toward the normal range.15 They further recommend not exceeding 1,500 mg of elemental calcium per day if a calcium-based binder is used, and they recommend avoiding calcium-based binders in patients with hypercalcemia, adynamic bone disease, or vascular calcification.
Phosphorus binders may account for up to 50% of the daily pill burden and may contribute to poor medication adherence.16 Dialysis patients need to take a lot of these drugs: by weight, 5 to 6 pounds per year.
These drugs can bind and interfere with the absorption of other vital medications and so should be taken with meals and separately from other medications.
Removing phosphorus
Removal of phosphorus by adequate dialysis or kidney transplant is the final strategy.
New agents under study
To improve phosphorus control, other agents that inhibit absorption of phosphate are being investigated.
Nicotinamide reduces expression of the sodium-phosphorus cotransporter NTP2b. Its use in combination with a low-phosphorus diet and phosphorus binders may maximize reductions in phosphorus absorption and is being studied in the CKD Optimal Management With Binders and Nicotinamide (COMBINE) study.
Tenapanor, an inhibitor of the sodium-hydrogen transporter NHE3, has been shown in animal studies to increase fecal phosphate excretion and decrease urinary phosphate excretion17 but requires further evaluation.
Phosphorus is essential for life. However, both low and high levels of phosphorus in the body have consequences, and its concentration in the blood is tightly regulated through dietary absorption, bone flux, and renal excretion and is influenced by calcitriol (1,25 hydroxyvitamin D3), parathyroid hormone, and fibroblast growth factor 23 (FGF23).
See related articles by M. Shetty and A. Sekar
Sekar et al,1 in this issue of the Journal, provide an extensive review of the pathophysiology of phosphorus metabolism and strategies to control phosphorus levels in patients with hyperphosphatemia and end-stage kidney disease.
PHOSPHORUS OR PHOSPHATE?
What's in a name? That which we call a rose
By any other word would smell as sweet.
—Shakespeare, Romeo and Juliet
The terms phosphate and phosphorus are often used interchangeably, though most writers still prefer phosphate over phosphorus.
The serum concentrations of phosphate and phosphorus are the same when expressed in millimoles per liter, as every mole of phosphate contains 1 mole of phosphorus, but not the same when expressed in milligrams per deciliter.2 The molecular weight of phosphorus is 30.97, whereas the molecular weight of the phosphate ion (PO43–) is 94.97—more than 3 times higher. Therefore, using these terms interchangeably in this context can lead to numerical error.3
Phosphorus, being highly reactive, does not exist by itself in nature and is typically present as phosphates in biologic systems. When describing phosphorus metabolism, the term phosphates should ideally be used because phosphates are the actual participants in the bodily processes. But in the clinical laboratory, all methods that measure serum phosphorus in fact measure inorganic phosphate and are expressed in terms of milligrams of phosphorus per deciliter rather than milligrams of phosphate per deciliter, and using these 2 terms interchangeably in clinical practice should not be of concern.4
THE PROBLEM
US adults typically ingest 1,200 mg of phosphorus each day, and about 60% to 70% of the ingested phosphorus is absorbed both by passive paracellular diffusion via tight junctions and by active transcellular transport via sodium-phosphate cotransport. The kidneys must excrete the same amount daily to maintain a steady state. As kidney function declines, phosphorus accumulates in the blood, leading to hyperphosphatemia.
Hyperphosphatemia is often asymptomatic, but it can cause generalized itching, red eyes, and adverse effects on the bone and parathyroid glands. Higher serum phosphorus levels have been shown to be associated with vascular calcification,5 cardiovascular events, and higher all-cause mortality rates in the general population,6 in patients with diabetes,7 and in those with chronic kidney disease.8 This association between higher serum phosphorus levels and the all-cause mortality rate led to the assumption that lowering serum phosphorus levels in these patients could reduce the rates of cardiovascular events and death, and to efforts to correct hyperphosphatemia.
Research into FGF23 continues, especially its role in cardiovascular complications of chronic kidney disease, as both phosphorus and FGF23 levels are elevated in chronic kidney disease and are implicated in poor clinical outcomes in these patients. However, both FGF23 and parathyroid hormone levels rise early in the course of kidney disease, long before overt hyperphosphatemia develops. Further, FGF23 rises earlier than parathyroid hormone and has been found to be an independent risk factor for cardiovascular events and death from any cause in end-stage kidney disease.9
Whether hyperphosphatemia is the culprit or merely an epiphenomenon of metabolic complications of chronic kidney disease is still unclear, as more molecules are being identified in the complex process of cardiovascular calcification.10
However, one thing is clear: vascular calcification is not just a simple precipitation of calcium and phosphorus. Instead, it is an active process that involves many regulators of mineral metabolism.10 The complex nature of this process is likely one of the reasons that evidence is conflicting11 about the benefits of phosphorus binders in terms of cardiovascular events or all-cause mortality in these patients.
STRATEGIES TO CONTROL HYPERPHOSPHATEMIA
Reducing intake
Dietary phosphorus restriction is the first step in controlling serum phosphorus. But reducing phosphorus intake while otherwise trying to optimize the nutritional status can be challenging.
The recommended daily protein intake is 1.0 to 1.2 g/kg. But phosphorus is typically found in foods rich in proteins, and restricting protein severely can compromise nutritional status and may be as bad as elevated phosphate levels in terms of outcomes.
Although plant-based foods contain more phosphate per gram of protein (ie, they have a higher ratio of phosphorus to protein) than animal-based foods, the bioavailability of phosphorus from plant foods is lower. Phosphorus in plant-based foods is mainly in the form of phytate. Humans cannot hydrolyze phytate because we lack the phytase enzyme; hence, the phosphorus in plant-based foods is not well absorbed. Therefore, a vegetarian diet may be preferable and beneficial in patients with chronic kidney disease. A small study in humans showed that a vegetarian diet resulted in lower serum phosphorus and FGF23 levels, but the study was limited by its small sample size.12
Patients should be advised to avoid foods that have a high phosphate content, such as processed foods, fast foods, and cola beverages, which often have phosphate-based food additives.
Further, one should be cautious about using supplements with healthy-sounding names. A case in point is “vitamin water”: 12 oz of this fruit punch-flavored beverage contains 392 mg of phosphorus,13 and this alone would require 12 to 15 phosphate binder tablets to bind its phosphorus content.
In addition, many prescription drugs have significant amounts of phosphorus, and this is often unrecognized.
Sherman et al14 reviewed 200 of the most commonly prescribed drugs in dialysis patients and found that 23 (11.5%) of the drug labels listed phosphorus-containing ingredients, but the actual amount of phosphorus was not listed. The phosphorus content ranged from 1.4 mg (clonidine 0.2 mg, Blue Point Laboratories, Dublin, Ireland) to 111.5 mg (paroxetine 40 mg, GlaxoSmith Kline, Philadelphia, PA). The phosphorus content was inconsistent and varied with the dose of the agent, type of formulation (tablet or syrup), branded or generic formulation, and manufacturer.
Branded lisinopril (Merck, Kenilworth, NJ) had 21.4 mg of phosphorus per 10-mg dose, while a generic product (Blue Point Laboratories, Dublin, Ireland) had 32.6 mg. Different brands of generic amlodipine 10 mg varied in their phosphorus content from 8.6 mg (Lupin Pharmaceuticals, Mumbai, India) to 27.8 mg (Greenstone LLC, Peapack, NJ) to 40.1 mg (Qualitest Pharmaceuticals, Huntsville, AL. Rena-Vite (Cypress Pharmaceuticals, Madison, MS), a multivitamin marketed to patients with kidney disease, had 37.7 mg of phosphorus per tablet. Thus, just to bind the phosphorus content of these 3 tablets (lisinopril, amlodipine, and Rena-Vite), a patient could need at least 3 to 4 extra doses of phosphate binder.
The phosphate content of medications should be considered when prescribing. For example, Reno Caps (Nnodum Pharmaceuticals, Cincinnati, OH), another vitamin supplement, has only 1.7 mg of phosphorus per tablet and should be considered, especially in patients with poorly controlled serum phosphorus levels. However, the challenge is that medication labels do not provide the phosphorus content.
Reducing phosphorus absorption
Although these agents reduce serum phosphorus and help reduce symptoms, an important quality-of-life measure, it is uncertain whether they improve clinical outcomes.11 To date, no specific phosphorus binder offers a survival benefit over placebo.11
Based on the limited and conflicting evidence, the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines, recently updated, suggest that oral phosphorus binders should be used in patients with hyperphosphatemia to lower serum phosphorus levels toward the normal range.15 They further recommend not exceeding 1,500 mg of elemental calcium per day if a calcium-based binder is used, and they recommend avoiding calcium-based binders in patients with hypercalcemia, adynamic bone disease, or vascular calcification.
Phosphorus binders may account for up to 50% of the daily pill burden and may contribute to poor medication adherence.16 Dialysis patients need to take a lot of these drugs: by weight, 5 to 6 pounds per year.
These drugs can bind and interfere with the absorption of other vital medications and so should be taken with meals and separately from other medications.
Removing phosphorus
Removal of phosphorus by adequate dialysis or kidney transplant is the final strategy.
New agents under study
To improve phosphorus control, other agents that inhibit absorption of phosphate are being investigated.
Nicotinamide reduces expression of the sodium-phosphorus cotransporter NTP2b. Its use in combination with a low-phosphorus diet and phosphorus binders may maximize reductions in phosphorus absorption and is being studied in the CKD Optimal Management With Binders and Nicotinamide (COMBINE) study.
Tenapanor, an inhibitor of the sodium-hydrogen transporter NHE3, has been shown in animal studies to increase fecal phosphate excretion and decrease urinary phosphate excretion17 but requires further evaluation.
- Sekar A, Kaur T, Nally JV Jr, Rincon-Choles H, Jolly S, Nakhoul G. Phosphorus binders: the new and the old, and how to choose. Cleve Clin J Med 2018; 85(8):629–638. doi:10.3949/ccjm.85a.17054
- Young DS. "Phosphorus" or "phosphate." Ann Intern Med 1980; 93(4):631. pmid:7436198
- Bartter FC. Reporting of phosphate and phosphorus plasma values. Am J Med 1981; 71(5):848. pmid:7304659.
- Iheagwara OS, Ing TS, Kjellstrand CM, Lew SQ. Phosphorus, phosphorous, and phosphate. Hemodial Int 2013; 17(4):479–482. doi:10.1111/hdi.12010
- Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 2009; 20(2):381–387. doi:10.1681/ASN.2008040349
- Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007; 167(9):879–885. doi:10.1001/archinte.167.9.879
- Chonchol M, Dale R, Schrier RW, Estacio R. Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med 2009; 122(4):380–386. doi:10.1016/j.amjmed.2008.09.039
- Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 2009; 24(5):1506–1523. doi:10.1093/ndt/gfn613
- Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359(6):584–592. doi:10.1056/NEJMoa0706130
- Lullo LD, Barbera V, Bellasi A, et al. Vascular and valvular calcifications in chronic kidney disease: an update. EMJ Nephrol 2016; 4(1):84–91. https://pdfs.semanticscholar.org/150f/c7b5dfe671c9b61e4c76d54b7d713b60ba6a.pdf. Accesssed June 5, 2018.
- Palmer SC, Gardner S, Tonelli M, et al. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis 2016; 68(5):691–702. doi:10.1053/j.ajkd.2016.05.015
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Moser M, White K, Henry B, et al. Phosphorus content of popular beverages. Am J Kidney Dis 2015; 65(6):969–971. doi:10.1053/j.ajkd.2015.02.330
- Sherman RA, Ravella S, Kapoian T. A dearth of data: the problem of phosphorus in prescription medications. Kidney Int 2015; 87(6):1097–1099. doi:10.1038/ki.2015.67
- KDIGO 2017 clinical practice guideline update for diagnosis, evaluation, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Supplements 2017; 7(1 suppl): 1–59. www.kisupplements.org/article/S2157-1716(17)30001-1/pdf. Accessed June 5, 2018.
- Fissell RB, Karaboyas A, Bieber BA, et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int 2016; 20(1):38–49. doi:10.1111/hdi.12315
- Labonté ED, Carreras CW, Leadbetter MR, et al. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 2015; 26(5):1138–1149. doi:10.1681/ASN.2014030317
- Sekar A, Kaur T, Nally JV Jr, Rincon-Choles H, Jolly S, Nakhoul G. Phosphorus binders: the new and the old, and how to choose. Cleve Clin J Med 2018; 85(8):629–638. doi:10.3949/ccjm.85a.17054
- Young DS. "Phosphorus" or "phosphate." Ann Intern Med 1980; 93(4):631. pmid:7436198
- Bartter FC. Reporting of phosphate and phosphorus plasma values. Am J Med 1981; 71(5):848. pmid:7304659.
- Iheagwara OS, Ing TS, Kjellstrand CM, Lew SQ. Phosphorus, phosphorous, and phosphate. Hemodial Int 2013; 17(4):479–482. doi:10.1111/hdi.12010
- Adeney KL, Siscovick DS, Ix JH, et al. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol 2009; 20(2):381–387. doi:10.1681/ASN.2008040349
- Dhingra R, Sullivan LM, Fox CS, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 2007; 167(9):879–885. doi:10.1001/archinte.167.9.879
- Chonchol M, Dale R, Schrier RW, Estacio R. Serum phosphorus and cardiovascular mortality in type 2 diabetes. Am J Med 2009; 122(4):380–386. doi:10.1016/j.amjmed.2008.09.039
- Covic A, Kothawala P, Bernal M, Robbins S, Chalian A, Goldsmith D. Systematic review of the evidence underlying the association between mineral metabolism disturbances and risk of all-cause mortality, cardiovascular mortality and cardiovascular events in chronic kidney disease. Nephrol Dial Transplant 2009; 24(5):1506–1523. doi:10.1093/ndt/gfn613
- Gutiérrez OM, Mannstadt M, Isakova T, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359(6):584–592. doi:10.1056/NEJMoa0706130
- Lullo LD, Barbera V, Bellasi A, et al. Vascular and valvular calcifications in chronic kidney disease: an update. EMJ Nephrol 2016; 4(1):84–91. https://pdfs.semanticscholar.org/150f/c7b5dfe671c9b61e4c76d54b7d713b60ba6a.pdf. Accesssed June 5, 2018.
- Palmer SC, Gardner S, Tonelli M, et al. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis 2016; 68(5):691–702. doi:10.1053/j.ajkd.2016.05.015
- Moe SM, Zidehsarai MP, Chambers MA, et al. Vegetarian compared with meat dietary protein source and phosphorus homeostasis in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6(2):257–264. doi:10.2215/CJN.05040610
- Moser M, White K, Henry B, et al. Phosphorus content of popular beverages. Am J Kidney Dis 2015; 65(6):969–971. doi:10.1053/j.ajkd.2015.02.330
- Sherman RA, Ravella S, Kapoian T. A dearth of data: the problem of phosphorus in prescription medications. Kidney Int 2015; 87(6):1097–1099. doi:10.1038/ki.2015.67
- KDIGO 2017 clinical practice guideline update for diagnosis, evaluation, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Supplements 2017; 7(1 suppl): 1–59. www.kisupplements.org/article/S2157-1716(17)30001-1/pdf. Accessed June 5, 2018.
- Fissell RB, Karaboyas A, Bieber BA, et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int 2016; 20(1):38–49. doi:10.1111/hdi.12315
- Labonté ED, Carreras CW, Leadbetter MR, et al. Gastrointestinal inhibition of sodium-hydrogen exchanger 3 reduces phosphorus absorption and protects against vascular calcification in CKD. J Am Soc Nephrol 2015; 26(5):1138–1149. doi:10.1681/ASN.2014030317
S aureus bacteremia: TEE and infectious disease consultation
Morbidity and mortality rates in patients with Staphylococcus aureus bacteremia remain high even though diagnostic tests have improved and antibiotic therapy is effective. Diagnosis and management are made more complex by difficulties in finding the source of bacteremia and sites of metastatic infection.
S aureus bacteremia is a finding that demands further investigation, since up to 25% of people who have it may have endocarditis, a condition with even worse consequences.1 The ability of S aureus to infect normal valves2,3 adds to the challenge. In the mid-20th century, Wilson and Hamburger4 demonstrated that 64% of patients with S aureus bacteremia had evidence of valvular infection at autopsy. In a more recent case series of patients with S aureus endocarditis, the diagnosis was established at autopsy in 32%.5
Specific clinical findings in patients with complicated S aureus bacteremia—those who have a site of infection remote from or extended beyond the primary focus—may be useful in determining the need for additional diagnostic and therapeutic measures.
In a prospective cohort study, Fowler et al6 identified several factors that predicted complicated S aureus bacteremia (including but not limited to endocarditis):
- Prolonged bacteremia (> 48–72 hours after initiation of therapy)
- Community onset
- Fever persisting more than 72 hours
- Skin findings suggesting systemic infection.
THE ROLE OF ECHOCARDIOGRAPHY
Infective endocarditis may be difficult to detect in patients with S aureus bacteremia; experts recommend routine use of echocardiography in this process.7,8 Transesophageal echocardiography (TEE) detects more cases of endocarditis than transthoracic echocardiography (TTE),9,10 but access, cost, and risks lead to questions about its utility.
Guidance for the use of echocardiography in S aureus bacteremia1,10–14 continues to evolve. Consensus seems to be emerging that the risk of endocarditis is lower in patients with S aureus bacteremia who:
- Do not have a prosthetic valve or other permanent intracardiac device
- Have sterile blood cultures within 96 hours after the initial set
- Are not hemodialysis-dependent
- Developed the bacteremia in a healthcare setting
- Have no secondary focus of infection
- Have no clinical signs of infective endocarditis.
Heriot et al14 point out that studies of risk-stratification approaches to echocardiography in patients with S aureus bacteremia are difficult to interpret, as there are questions regarding the validity of the studies and the balance of the risks and benefits.1 The question of timing of TEE remains largely unexplored, both in initial screening and in follow-up of previously undiagnosed cases of S aureus endocarditis.
In this issue of the Journal, Mirrakhimov et al15 weigh in on use of a risk-stratification model to guide use of TEE in patients with S aureus bacteremia. Their comments about avoiding TEE in patients who have an alternative explanation for S aureus bacteremia and a low pretest probability for infectious endocarditis and in patients with a disease focus that requires extended treatment are derived from a survey of infectious disease physicians.16
ROLE OF INFECTIOUS DISEASE CONSULTATION
Infectious disease consultation reduces mortality rates and healthcare costs for a variety of infections, with endocarditis as a prime example.17 For S aureus bacteremia, a large and growing body of literature demonstrates the impact of infectious disease consultation, including improved adherence to guidelines and quality measures,18–20 lower in-hospital mortality rates18–21 and earlier hospital discharge.18 In the era of “curbside consults” and “e-consultation,” it is interesting to note the enduring value of bedside, in-person consultation in the management of S aureus bacteremia.20
Many people with S aureus bacteremia should undergo TEE. Until the evidence becomes more robust, the decision to forgo TEE must be made with caution. The expertise of infectious disease physicians in the diagnosis and management of endocarditis can assist clinicians working with the often-complex patients who develop S aureus bacteremia. If the goal is to improve outcomes, infectious disease consultation may be at least as important as appropriate selection of patients for TEE.
- Rasmussen RV, Høst U, Arpi M, et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr 2011; 12(6):414–420. doi:10.1093/ejechocard/jer023
- Vogler, WR, Dorney ER. Bacterial endocarditis in normal heart. Bull Emory Univ Clin 1961; 1:21–31.
- Thayer WS. Bacterial or infective endocarditis. Edinburgh Med J 1931; 38:237–265, 307–334.
- Wilson R, Hamburger M. Fifteen years’ experience with staphylococcus septicemia in large city hospital: analysis of fifty-five cases in Cincinnati General Hospital 1940 to 1954. Am J Med 1957; 22(3):437–457. pmid:13402795
- Røder BL, Wandall DA, Frimodt-Møllar N, Espersen F, Skinhøj P, Rosdahl VT. Clinical features of Staphylococcus aureus endocarditis: a 10-year experience in Denmark. Arch Intern Med 1999; 159(5):462–469. pmid:10074954
- Fowler VG Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163(17):2066–2072. doi:10.1001/archinte.163.17.2066
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52(3):285–292. doi:10.1093/cid/cir034
- Reynolds HR, Jagen MA, Tunick PA, Kronzon I. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J Am Soc Echocardiogr 2003; 16(1):67–70. doi:10.1067/mje.2003.43
- Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312(13):1330–1341. doi:10.1001/jama.2014.9743
- Kaasch AJ, Folwler VG Jr, Rieg S, et al. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis 2011; 53(1):1–9. doi:10.1093/cid/cir320
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Bai AD, Agarawal A, Steinberg M, et al. Clinical predictors and clinical prediction rules to estimate initial patient risk for infective endocarditis in Staphylococcus aureus bacteremia: a systematic review and meta-analysis. Clin Microbiol Infect 2017; 23(12):900-906. doi:10.1016/j.cmi.2017.04.025
- Heriot GS, Cronin K, Tong SYC, Cheng AC, Liew D. Criteria for identifying patients with Staphylococcus aureus bacteremia who are at low risk of endocarditis: a systematic review. Open Forum Infect Dis 2017; 4(4):ofx261. doi:10.1093/ofid/ofx261
- Mirrakhimov AE, Jesinger ME, Ayach T, Gray A. When does S aureus bacteremia require transesophageal echocardiography? Cleve Clin J Med 2018; 85(7):517–520. doi:10.3949/ccjm.85a.16095
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Schmitt S, McQuillen DP, Nahass R, et al. Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin Infect Dis 2014; 58(1):22–28. doi:10.1093/cid/cit610
- Bai AD, Showler A, Burry L, et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis. 2015; 60(10):1451–1461. doi:10.1093/cid/civ120
- Buehrle K, Pisano J, Han Z, Pettit NN. Guideline compliance and clinical outcomes among patients with Staphylococcus aureus bacteremia with infectious diseases consultation in addition to antimicrobial stewardship-directed review. Am J Infect Control 2017; 45(7):713–716. doi:10.1016/j.ajic.2017.02.030
- Saunderson RB, Gouliouris T, Nickerson EK, et al. Impact of routine bedside infectious disease consultation on clinical management and outcome of Staphylococcus aureus bacteremia in adults. Clin Microbiol Infect 2015; 21(8):779–785. doi:10.1016/j.cmi.2015.05.026
- Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009; 88(5):263–267. doi:10.1097/MD.0b013e3181b8fccb
Morbidity and mortality rates in patients with Staphylococcus aureus bacteremia remain high even though diagnostic tests have improved and antibiotic therapy is effective. Diagnosis and management are made more complex by difficulties in finding the source of bacteremia and sites of metastatic infection.
S aureus bacteremia is a finding that demands further investigation, since up to 25% of people who have it may have endocarditis, a condition with even worse consequences.1 The ability of S aureus to infect normal valves2,3 adds to the challenge. In the mid-20th century, Wilson and Hamburger4 demonstrated that 64% of patients with S aureus bacteremia had evidence of valvular infection at autopsy. In a more recent case series of patients with S aureus endocarditis, the diagnosis was established at autopsy in 32%.5
Specific clinical findings in patients with complicated S aureus bacteremia—those who have a site of infection remote from or extended beyond the primary focus—may be useful in determining the need for additional diagnostic and therapeutic measures.
In a prospective cohort study, Fowler et al6 identified several factors that predicted complicated S aureus bacteremia (including but not limited to endocarditis):
- Prolonged bacteremia (> 48–72 hours after initiation of therapy)
- Community onset
- Fever persisting more than 72 hours
- Skin findings suggesting systemic infection.
THE ROLE OF ECHOCARDIOGRAPHY
Infective endocarditis may be difficult to detect in patients with S aureus bacteremia; experts recommend routine use of echocardiography in this process.7,8 Transesophageal echocardiography (TEE) detects more cases of endocarditis than transthoracic echocardiography (TTE),9,10 but access, cost, and risks lead to questions about its utility.
Guidance for the use of echocardiography in S aureus bacteremia1,10–14 continues to evolve. Consensus seems to be emerging that the risk of endocarditis is lower in patients with S aureus bacteremia who:
- Do not have a prosthetic valve or other permanent intracardiac device
- Have sterile blood cultures within 96 hours after the initial set
- Are not hemodialysis-dependent
- Developed the bacteremia in a healthcare setting
- Have no secondary focus of infection
- Have no clinical signs of infective endocarditis.
Heriot et al14 point out that studies of risk-stratification approaches to echocardiography in patients with S aureus bacteremia are difficult to interpret, as there are questions regarding the validity of the studies and the balance of the risks and benefits.1 The question of timing of TEE remains largely unexplored, both in initial screening and in follow-up of previously undiagnosed cases of S aureus endocarditis.
In this issue of the Journal, Mirrakhimov et al15 weigh in on use of a risk-stratification model to guide use of TEE in patients with S aureus bacteremia. Their comments about avoiding TEE in patients who have an alternative explanation for S aureus bacteremia and a low pretest probability for infectious endocarditis and in patients with a disease focus that requires extended treatment are derived from a survey of infectious disease physicians.16
ROLE OF INFECTIOUS DISEASE CONSULTATION
Infectious disease consultation reduces mortality rates and healthcare costs for a variety of infections, with endocarditis as a prime example.17 For S aureus bacteremia, a large and growing body of literature demonstrates the impact of infectious disease consultation, including improved adherence to guidelines and quality measures,18–20 lower in-hospital mortality rates18–21 and earlier hospital discharge.18 In the era of “curbside consults” and “e-consultation,” it is interesting to note the enduring value of bedside, in-person consultation in the management of S aureus bacteremia.20
Many people with S aureus bacteremia should undergo TEE. Until the evidence becomes more robust, the decision to forgo TEE must be made with caution. The expertise of infectious disease physicians in the diagnosis and management of endocarditis can assist clinicians working with the often-complex patients who develop S aureus bacteremia. If the goal is to improve outcomes, infectious disease consultation may be at least as important as appropriate selection of patients for TEE.
Morbidity and mortality rates in patients with Staphylococcus aureus bacteremia remain high even though diagnostic tests have improved and antibiotic therapy is effective. Diagnosis and management are made more complex by difficulties in finding the source of bacteremia and sites of metastatic infection.
S aureus bacteremia is a finding that demands further investigation, since up to 25% of people who have it may have endocarditis, a condition with even worse consequences.1 The ability of S aureus to infect normal valves2,3 adds to the challenge. In the mid-20th century, Wilson and Hamburger4 demonstrated that 64% of patients with S aureus bacteremia had evidence of valvular infection at autopsy. In a more recent case series of patients with S aureus endocarditis, the diagnosis was established at autopsy in 32%.5
Specific clinical findings in patients with complicated S aureus bacteremia—those who have a site of infection remote from or extended beyond the primary focus—may be useful in determining the need for additional diagnostic and therapeutic measures.
In a prospective cohort study, Fowler et al6 identified several factors that predicted complicated S aureus bacteremia (including but not limited to endocarditis):
- Prolonged bacteremia (> 48–72 hours after initiation of therapy)
- Community onset
- Fever persisting more than 72 hours
- Skin findings suggesting systemic infection.
THE ROLE OF ECHOCARDIOGRAPHY
Infective endocarditis may be difficult to detect in patients with S aureus bacteremia; experts recommend routine use of echocardiography in this process.7,8 Transesophageal echocardiography (TEE) detects more cases of endocarditis than transthoracic echocardiography (TTE),9,10 but access, cost, and risks lead to questions about its utility.
Guidance for the use of echocardiography in S aureus bacteremia1,10–14 continues to evolve. Consensus seems to be emerging that the risk of endocarditis is lower in patients with S aureus bacteremia who:
- Do not have a prosthetic valve or other permanent intracardiac device
- Have sterile blood cultures within 96 hours after the initial set
- Are not hemodialysis-dependent
- Developed the bacteremia in a healthcare setting
- Have no secondary focus of infection
- Have no clinical signs of infective endocarditis.
Heriot et al14 point out that studies of risk-stratification approaches to echocardiography in patients with S aureus bacteremia are difficult to interpret, as there are questions regarding the validity of the studies and the balance of the risks and benefits.1 The question of timing of TEE remains largely unexplored, both in initial screening and in follow-up of previously undiagnosed cases of S aureus endocarditis.
In this issue of the Journal, Mirrakhimov et al15 weigh in on use of a risk-stratification model to guide use of TEE in patients with S aureus bacteremia. Their comments about avoiding TEE in patients who have an alternative explanation for S aureus bacteremia and a low pretest probability for infectious endocarditis and in patients with a disease focus that requires extended treatment are derived from a survey of infectious disease physicians.16
ROLE OF INFECTIOUS DISEASE CONSULTATION
Infectious disease consultation reduces mortality rates and healthcare costs for a variety of infections, with endocarditis as a prime example.17 For S aureus bacteremia, a large and growing body of literature demonstrates the impact of infectious disease consultation, including improved adherence to guidelines and quality measures,18–20 lower in-hospital mortality rates18–21 and earlier hospital discharge.18 In the era of “curbside consults” and “e-consultation,” it is interesting to note the enduring value of bedside, in-person consultation in the management of S aureus bacteremia.20
Many people with S aureus bacteremia should undergo TEE. Until the evidence becomes more robust, the decision to forgo TEE must be made with caution. The expertise of infectious disease physicians in the diagnosis and management of endocarditis can assist clinicians working with the often-complex patients who develop S aureus bacteremia. If the goal is to improve outcomes, infectious disease consultation may be at least as important as appropriate selection of patients for TEE.
- Rasmussen RV, Høst U, Arpi M, et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr 2011; 12(6):414–420. doi:10.1093/ejechocard/jer023
- Vogler, WR, Dorney ER. Bacterial endocarditis in normal heart. Bull Emory Univ Clin 1961; 1:21–31.
- Thayer WS. Bacterial or infective endocarditis. Edinburgh Med J 1931; 38:237–265, 307–334.
- Wilson R, Hamburger M. Fifteen years’ experience with staphylococcus septicemia in large city hospital: analysis of fifty-five cases in Cincinnati General Hospital 1940 to 1954. Am J Med 1957; 22(3):437–457. pmid:13402795
- Røder BL, Wandall DA, Frimodt-Møllar N, Espersen F, Skinhøj P, Rosdahl VT. Clinical features of Staphylococcus aureus endocarditis: a 10-year experience in Denmark. Arch Intern Med 1999; 159(5):462–469. pmid:10074954
- Fowler VG Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163(17):2066–2072. doi:10.1001/archinte.163.17.2066
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52(3):285–292. doi:10.1093/cid/cir034
- Reynolds HR, Jagen MA, Tunick PA, Kronzon I. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J Am Soc Echocardiogr 2003; 16(1):67–70. doi:10.1067/mje.2003.43
- Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312(13):1330–1341. doi:10.1001/jama.2014.9743
- Kaasch AJ, Folwler VG Jr, Rieg S, et al. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis 2011; 53(1):1–9. doi:10.1093/cid/cir320
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Bai AD, Agarawal A, Steinberg M, et al. Clinical predictors and clinical prediction rules to estimate initial patient risk for infective endocarditis in Staphylococcus aureus bacteremia: a systematic review and meta-analysis. Clin Microbiol Infect 2017; 23(12):900-906. doi:10.1016/j.cmi.2017.04.025
- Heriot GS, Cronin K, Tong SYC, Cheng AC, Liew D. Criteria for identifying patients with Staphylococcus aureus bacteremia who are at low risk of endocarditis: a systematic review. Open Forum Infect Dis 2017; 4(4):ofx261. doi:10.1093/ofid/ofx261
- Mirrakhimov AE, Jesinger ME, Ayach T, Gray A. When does S aureus bacteremia require transesophageal echocardiography? Cleve Clin J Med 2018; 85(7):517–520. doi:10.3949/ccjm.85a.16095
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Schmitt S, McQuillen DP, Nahass R, et al. Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin Infect Dis 2014; 58(1):22–28. doi:10.1093/cid/cit610
- Bai AD, Showler A, Burry L, et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis. 2015; 60(10):1451–1461. doi:10.1093/cid/civ120
- Buehrle K, Pisano J, Han Z, Pettit NN. Guideline compliance and clinical outcomes among patients with Staphylococcus aureus bacteremia with infectious diseases consultation in addition to antimicrobial stewardship-directed review. Am J Infect Control 2017; 45(7):713–716. doi:10.1016/j.ajic.2017.02.030
- Saunderson RB, Gouliouris T, Nickerson EK, et al. Impact of routine bedside infectious disease consultation on clinical management and outcome of Staphylococcus aureus bacteremia in adults. Clin Microbiol Infect 2015; 21(8):779–785. doi:10.1016/j.cmi.2015.05.026
- Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009; 88(5):263–267. doi:10.1097/MD.0b013e3181b8fccb
- Rasmussen RV, Høst U, Arpi M, et al. Prevalence of infective endocarditis in patients with Staphylococcus aureus bacteraemia: the value of screening with echocardiography. Eur J Echocardiogr 2011; 12(6):414–420. doi:10.1093/ejechocard/jer023
- Vogler, WR, Dorney ER. Bacterial endocarditis in normal heart. Bull Emory Univ Clin 1961; 1:21–31.
- Thayer WS. Bacterial or infective endocarditis. Edinburgh Med J 1931; 38:237–265, 307–334.
- Wilson R, Hamburger M. Fifteen years’ experience with staphylococcus septicemia in large city hospital: analysis of fifty-five cases in Cincinnati General Hospital 1940 to 1954. Am J Med 1957; 22(3):437–457. pmid:13402795
- Røder BL, Wandall DA, Frimodt-Møllar N, Espersen F, Skinhøj P, Rosdahl VT. Clinical features of Staphylococcus aureus endocarditis: a 10-year experience in Denmark. Arch Intern Med 1999; 159(5):462–469. pmid:10074954
- Fowler VG Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163(17):2066–2072. doi:10.1001/archinte.163.17.2066
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132(15):1435–1486. doi:10.1161/CIR.0000000000000296
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis 2011; 52(3):285–292. doi:10.1093/cid/cir034
- Reynolds HR, Jagen MA, Tunick PA, Kronzon I. Sensitivity of transthoracic versus transesophageal echocardiography for the detection of native valve vegetations in the modern era. J Am Soc Echocardiogr 2003; 16(1):67–70. doi:10.1067/mje.2003.43
- Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312(13):1330–1341. doi:10.1001/jama.2014.9743
- Kaasch AJ, Folwler VG Jr, Rieg S, et al. Use of a simple criteria set for guiding echocardiography in nosocomial Staphylococcus aureus bacteremia. Clin Infect Dis 2011; 53(1):1–9. doi:10.1093/cid/cir320
- Palraj BR, Baddour LM, Hess EP, et al. Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 2015; 61(1):18–28. doi:10.1093/cid/civ235
- Bai AD, Agarawal A, Steinberg M, et al. Clinical predictors and clinical prediction rules to estimate initial patient risk for infective endocarditis in Staphylococcus aureus bacteremia: a systematic review and meta-analysis. Clin Microbiol Infect 2017; 23(12):900-906. doi:10.1016/j.cmi.2017.04.025
- Heriot GS, Cronin K, Tong SYC, Cheng AC, Liew D. Criteria for identifying patients with Staphylococcus aureus bacteremia who are at low risk of endocarditis: a systematic review. Open Forum Infect Dis 2017; 4(4):ofx261. doi:10.1093/ofid/ofx261
- Mirrakhimov AE, Jesinger ME, Ayach T, Gray A. When does S aureus bacteremia require transesophageal echocardiography? Cleve Clin J Med 2018; 85(7):517–520. doi:10.3949/ccjm.85a.16095
- Young H, Knepper BC, Price CS, Heard S, Jenkins TC. Clinical reasoning of infectious diseases physicians behind the use or nonuse of transesophageal echocardiography in Staphylococcus aureus bacteremia. Open Forum Infect Dis 2016; 3(4):ofw204. doi:10.1093/ofid/ofw204
- Schmitt S, McQuillen DP, Nahass R, et al. Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin Infect Dis 2014; 58(1):22–28. doi:10.1093/cid/cit610
- Bai AD, Showler A, Burry L, et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis. 2015; 60(10):1451–1461. doi:10.1093/cid/civ120
- Buehrle K, Pisano J, Han Z, Pettit NN. Guideline compliance and clinical outcomes among patients with Staphylococcus aureus bacteremia with infectious diseases consultation in addition to antimicrobial stewardship-directed review. Am J Infect Control 2017; 45(7):713–716. doi:10.1016/j.ajic.2017.02.030
- Saunderson RB, Gouliouris T, Nickerson EK, et al. Impact of routine bedside infectious disease consultation on clinical management and outcome of Staphylococcus aureus bacteremia in adults. Clin Microbiol Infect 2015; 21(8):779–785. doi:10.1016/j.cmi.2015.05.026
- Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009; 88(5):263–267. doi:10.1097/MD.0b013e3181b8fccb
Critical care medicine: An ongoing journey
My introduction to critical care medicine came about during the summer between my third and fourth years of medical school. During that brief break, I, like most of my classmates, was drawn to the classic medical satire The House of God by Samuel Shem,1 which had become a cult classic in the medical field for its ghoulish medical wisdom and dark humor. In “the house,” the intensive care unit (ICU) is “that mausoleum down the hall,” its patients “perched precariously on the edge of that slick bobsled ride down to death.”1 This sentiment persisted even as I began my critical care medicine fellowship in the mid-1990s.
The science and practice of critical care medicine have changed, evolved, and advanced over the past several decades reflecting newer technology, but also an aging population with higher acuity.2 Critical care medicine has established itself as a specialty in its own right, and the importance of the physician intensivist-led multidisciplinary care teams in optimizing outcome has been demonstrated.3,4 These teams have been associated with improved quality of care, reduced length of stay, improved resource utilization, and reduced rates of complications, morbidity, and death.
While there have been few medical miracles and limited advances in therapeutics over the last 30 years, advances in patient management, adherence to processes of care, better use of technology, and more timely diagnosis and treatment have facilitated improved outcomes.5 Collaboration with nurses, respiratory therapists, pharmacists, and other healthcare personnel is invaluable, as these providers are responsible for executing management protocols such as weaning sedation and mechanical ventilation, nutrition, glucose control, vasopressor and electrolyte titration, positioning, and early ambulation.
Unfortunately, as an increasing number of patients are being discharged from the ICU, evidence is accumulating that ICU survivors may develop persistent organ dysfunction requiring prolonged stays in the ICU and resulting in chronic critical illness. A 2015 study estimated 380,000 cases of chronic critical illness annually, particularly among the elderly population, with attendant hospital costs of up to $26 billion.6 While 70% of these patients may survive their hospitalization, the Society of Critical Care Medicine (SCCM) estimates that the 1-year post-discharge mortality rate may exceed 50%.7
We can take pride and comfort in knowing that the past several decades have seen growth in critical care training, more engaged practice, and heightened communication resulting in lower mortality rates.8 However, a majority of survivors suffer significant morbidities that may be severe and persist for a prolonged period after hospital discharge. These worsening impairments after discharge are termed postintensive care syndrome (PICS), which manifests as a new or worsening mental, cognitive, and physical condition and may affect up to 50% of ICU survivors.6
The impact on daily functioning and quality of life can be devastating, and primary care physicians will be increasingly called on to diagnose and participate in ongoing post-discharge management. Additionally, the impact of critical illness on relatives and informal caregivers can be long-lasting and profound, increasing their own risk of depression, posttraumatic stress disorder, and financial hardship.
In this issue of the Journal, Golovyan and colleagues identify several potential complications and sequelae of critical illness after discharge from the ICU.9 Primary care providers will see these patients in outpatient settings and need to be prepared to triage and treat the new-onset and chronic conditions for which these patients are at high risk.
In addition, as the authors point out, family members and informal caregivers need to be counseled about the proper care of these patients as well as themselves.
The current healthcare system does not appropriately address these survivors and their families. In 2015, the Society of Critical Care Medicine announced the THRIVE initiative, designed to improve support for the patient and family after critical illness. Given the many survivors and caregivers touched by critical illness, the Society has invested in THRIVE with the intent of helping those affected to work together with clinicians to advance recovery. Through peer support groups, post-ICU clinics, and continuing research into quality improvement, THRIVE may help to reduce readmissions and improve quality of life for critical care survivors and their loved ones.
Things have changed since the days of The House of God. Critical care medicine has become a vibrant medical specialty and an integral part of our healthcare system. Dedicated critical care physicians and the multidisciplinary teams they lead have improved outcomes and resource utilization.2–5
The demand for ICU care will continue to increase as our population ages and the need for medical and surgical services increases commensurately. The ratio of ICU beds to hospital beds continues to escalate, and it is feared that the demand for critical care professionals may outstrip the supply.
While we no longer see that mournful shaking of the head when a patient is admitted to the ICU, we need to have the proper vision and use the most up-to-date scientific knowledge and research in treating underlying illness to ensure that once these patients are discharged, communication continues between critical care and primary care providers. This ongoing support will ensure these patients the best possible quality of life.
- Shem S. The House of God: A Novel. New York: R. Marek Publishers, 1978; chapter 18.
- Lilly CM, Swami S, Liu X, Riker RR, Badawi O. Five year trends of critical care practice and outcomes. Chest 2017; 152(4):723–735. doi:10.1016/j.chest.2017.06.050
- Yoo EJ, Edwards JD, Dean ML, Dudley RA. Multidisciplinary critical care and intensivist staffing: results of a statewide survey and association with mortality. J Intensive Care Med 2016; 31(5):325–332. doi:10.1177/0885066614534605
- Levy MM, Rapoport J, Lemeshow S, Chalfin DB, Phillips G, Danis M. Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med 2008; 148(11):801–809. pmid:18519926
- Vincent JL, Singer M, Marini JJ, et al. Thirty years of critical care medicine. Crit Care 2010; 14(3):311. doi:10.1186/cc8979
- Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 2012; 60(6):1070–1077. doi:10.1111/j.1532-5415.2012.03989.x
- Kahn JM, Le T, Angus DC, et al; ProVent Study Group Investigators. The epidemiology of chronic critical illness in the United States. Crit Care Med 2015; 43(2):282–287. doi:10.1097/CCM.0000000000000710
- Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long term acute care hospital utilization after critical illness. JAMA 2010; 303(22):2253–2259. doi:10.1001/jama.2010.761
- Golovyan DM, Khan SH, Wang S, Khan BA. What should I address at follow-up of patients who survive critical illness? Cleve Clin J Med 2018; 85(7):523–526. doi:10.3949/ccjm.85a.17104
My introduction to critical care medicine came about during the summer between my third and fourth years of medical school. During that brief break, I, like most of my classmates, was drawn to the classic medical satire The House of God by Samuel Shem,1 which had become a cult classic in the medical field for its ghoulish medical wisdom and dark humor. In “the house,” the intensive care unit (ICU) is “that mausoleum down the hall,” its patients “perched precariously on the edge of that slick bobsled ride down to death.”1 This sentiment persisted even as I began my critical care medicine fellowship in the mid-1990s.
The science and practice of critical care medicine have changed, evolved, and advanced over the past several decades reflecting newer technology, but also an aging population with higher acuity.2 Critical care medicine has established itself as a specialty in its own right, and the importance of the physician intensivist-led multidisciplinary care teams in optimizing outcome has been demonstrated.3,4 These teams have been associated with improved quality of care, reduced length of stay, improved resource utilization, and reduced rates of complications, morbidity, and death.
While there have been few medical miracles and limited advances in therapeutics over the last 30 years, advances in patient management, adherence to processes of care, better use of technology, and more timely diagnosis and treatment have facilitated improved outcomes.5 Collaboration with nurses, respiratory therapists, pharmacists, and other healthcare personnel is invaluable, as these providers are responsible for executing management protocols such as weaning sedation and mechanical ventilation, nutrition, glucose control, vasopressor and electrolyte titration, positioning, and early ambulation.
Unfortunately, as an increasing number of patients are being discharged from the ICU, evidence is accumulating that ICU survivors may develop persistent organ dysfunction requiring prolonged stays in the ICU and resulting in chronic critical illness. A 2015 study estimated 380,000 cases of chronic critical illness annually, particularly among the elderly population, with attendant hospital costs of up to $26 billion.6 While 70% of these patients may survive their hospitalization, the Society of Critical Care Medicine (SCCM) estimates that the 1-year post-discharge mortality rate may exceed 50%.7
We can take pride and comfort in knowing that the past several decades have seen growth in critical care training, more engaged practice, and heightened communication resulting in lower mortality rates.8 However, a majority of survivors suffer significant morbidities that may be severe and persist for a prolonged period after hospital discharge. These worsening impairments after discharge are termed postintensive care syndrome (PICS), which manifests as a new or worsening mental, cognitive, and physical condition and may affect up to 50% of ICU survivors.6
The impact on daily functioning and quality of life can be devastating, and primary care physicians will be increasingly called on to diagnose and participate in ongoing post-discharge management. Additionally, the impact of critical illness on relatives and informal caregivers can be long-lasting and profound, increasing their own risk of depression, posttraumatic stress disorder, and financial hardship.
In this issue of the Journal, Golovyan and colleagues identify several potential complications and sequelae of critical illness after discharge from the ICU.9 Primary care providers will see these patients in outpatient settings and need to be prepared to triage and treat the new-onset and chronic conditions for which these patients are at high risk.
In addition, as the authors point out, family members and informal caregivers need to be counseled about the proper care of these patients as well as themselves.
The current healthcare system does not appropriately address these survivors and their families. In 2015, the Society of Critical Care Medicine announced the THRIVE initiative, designed to improve support for the patient and family after critical illness. Given the many survivors and caregivers touched by critical illness, the Society has invested in THRIVE with the intent of helping those affected to work together with clinicians to advance recovery. Through peer support groups, post-ICU clinics, and continuing research into quality improvement, THRIVE may help to reduce readmissions and improve quality of life for critical care survivors and their loved ones.
Things have changed since the days of The House of God. Critical care medicine has become a vibrant medical specialty and an integral part of our healthcare system. Dedicated critical care physicians and the multidisciplinary teams they lead have improved outcomes and resource utilization.2–5
The demand for ICU care will continue to increase as our population ages and the need for medical and surgical services increases commensurately. The ratio of ICU beds to hospital beds continues to escalate, and it is feared that the demand for critical care professionals may outstrip the supply.
While we no longer see that mournful shaking of the head when a patient is admitted to the ICU, we need to have the proper vision and use the most up-to-date scientific knowledge and research in treating underlying illness to ensure that once these patients are discharged, communication continues between critical care and primary care providers. This ongoing support will ensure these patients the best possible quality of life.
My introduction to critical care medicine came about during the summer between my third and fourth years of medical school. During that brief break, I, like most of my classmates, was drawn to the classic medical satire The House of God by Samuel Shem,1 which had become a cult classic in the medical field for its ghoulish medical wisdom and dark humor. In “the house,” the intensive care unit (ICU) is “that mausoleum down the hall,” its patients “perched precariously on the edge of that slick bobsled ride down to death.”1 This sentiment persisted even as I began my critical care medicine fellowship in the mid-1990s.
The science and practice of critical care medicine have changed, evolved, and advanced over the past several decades reflecting newer technology, but also an aging population with higher acuity.2 Critical care medicine has established itself as a specialty in its own right, and the importance of the physician intensivist-led multidisciplinary care teams in optimizing outcome has been demonstrated.3,4 These teams have been associated with improved quality of care, reduced length of stay, improved resource utilization, and reduced rates of complications, morbidity, and death.
While there have been few medical miracles and limited advances in therapeutics over the last 30 years, advances in patient management, adherence to processes of care, better use of technology, and more timely diagnosis and treatment have facilitated improved outcomes.5 Collaboration with nurses, respiratory therapists, pharmacists, and other healthcare personnel is invaluable, as these providers are responsible for executing management protocols such as weaning sedation and mechanical ventilation, nutrition, glucose control, vasopressor and electrolyte titration, positioning, and early ambulation.
Unfortunately, as an increasing number of patients are being discharged from the ICU, evidence is accumulating that ICU survivors may develop persistent organ dysfunction requiring prolonged stays in the ICU and resulting in chronic critical illness. A 2015 study estimated 380,000 cases of chronic critical illness annually, particularly among the elderly population, with attendant hospital costs of up to $26 billion.6 While 70% of these patients may survive their hospitalization, the Society of Critical Care Medicine (SCCM) estimates that the 1-year post-discharge mortality rate may exceed 50%.7
We can take pride and comfort in knowing that the past several decades have seen growth in critical care training, more engaged practice, and heightened communication resulting in lower mortality rates.8 However, a majority of survivors suffer significant morbidities that may be severe and persist for a prolonged period after hospital discharge. These worsening impairments after discharge are termed postintensive care syndrome (PICS), which manifests as a new or worsening mental, cognitive, and physical condition and may affect up to 50% of ICU survivors.6
The impact on daily functioning and quality of life can be devastating, and primary care physicians will be increasingly called on to diagnose and participate in ongoing post-discharge management. Additionally, the impact of critical illness on relatives and informal caregivers can be long-lasting and profound, increasing their own risk of depression, posttraumatic stress disorder, and financial hardship.
In this issue of the Journal, Golovyan and colleagues identify several potential complications and sequelae of critical illness after discharge from the ICU.9 Primary care providers will see these patients in outpatient settings and need to be prepared to triage and treat the new-onset and chronic conditions for which these patients are at high risk.
In addition, as the authors point out, family members and informal caregivers need to be counseled about the proper care of these patients as well as themselves.
The current healthcare system does not appropriately address these survivors and their families. In 2015, the Society of Critical Care Medicine announced the THRIVE initiative, designed to improve support for the patient and family after critical illness. Given the many survivors and caregivers touched by critical illness, the Society has invested in THRIVE with the intent of helping those affected to work together with clinicians to advance recovery. Through peer support groups, post-ICU clinics, and continuing research into quality improvement, THRIVE may help to reduce readmissions and improve quality of life for critical care survivors and their loved ones.
Things have changed since the days of The House of God. Critical care medicine has become a vibrant medical specialty and an integral part of our healthcare system. Dedicated critical care physicians and the multidisciplinary teams they lead have improved outcomes and resource utilization.2–5
The demand for ICU care will continue to increase as our population ages and the need for medical and surgical services increases commensurately. The ratio of ICU beds to hospital beds continues to escalate, and it is feared that the demand for critical care professionals may outstrip the supply.
While we no longer see that mournful shaking of the head when a patient is admitted to the ICU, we need to have the proper vision and use the most up-to-date scientific knowledge and research in treating underlying illness to ensure that once these patients are discharged, communication continues between critical care and primary care providers. This ongoing support will ensure these patients the best possible quality of life.
- Shem S. The House of God: A Novel. New York: R. Marek Publishers, 1978; chapter 18.
- Lilly CM, Swami S, Liu X, Riker RR, Badawi O. Five year trends of critical care practice and outcomes. Chest 2017; 152(4):723–735. doi:10.1016/j.chest.2017.06.050
- Yoo EJ, Edwards JD, Dean ML, Dudley RA. Multidisciplinary critical care and intensivist staffing: results of a statewide survey and association with mortality. J Intensive Care Med 2016; 31(5):325–332. doi:10.1177/0885066614534605
- Levy MM, Rapoport J, Lemeshow S, Chalfin DB, Phillips G, Danis M. Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med 2008; 148(11):801–809. pmid:18519926
- Vincent JL, Singer M, Marini JJ, et al. Thirty years of critical care medicine. Crit Care 2010; 14(3):311. doi:10.1186/cc8979
- Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 2012; 60(6):1070–1077. doi:10.1111/j.1532-5415.2012.03989.x
- Kahn JM, Le T, Angus DC, et al; ProVent Study Group Investigators. The epidemiology of chronic critical illness in the United States. Crit Care Med 2015; 43(2):282–287. doi:10.1097/CCM.0000000000000710
- Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long term acute care hospital utilization after critical illness. JAMA 2010; 303(22):2253–2259. doi:10.1001/jama.2010.761
- Golovyan DM, Khan SH, Wang S, Khan BA. What should I address at follow-up of patients who survive critical illness? Cleve Clin J Med 2018; 85(7):523–526. doi:10.3949/ccjm.85a.17104
- Shem S. The House of God: A Novel. New York: R. Marek Publishers, 1978; chapter 18.
- Lilly CM, Swami S, Liu X, Riker RR, Badawi O. Five year trends of critical care practice and outcomes. Chest 2017; 152(4):723–735. doi:10.1016/j.chest.2017.06.050
- Yoo EJ, Edwards JD, Dean ML, Dudley RA. Multidisciplinary critical care and intensivist staffing: results of a statewide survey and association with mortality. J Intensive Care Med 2016; 31(5):325–332. doi:10.1177/0885066614534605
- Levy MM, Rapoport J, Lemeshow S, Chalfin DB, Phillips G, Danis M. Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med 2008; 148(11):801–809. pmid:18519926
- Vincent JL, Singer M, Marini JJ, et al. Thirty years of critical care medicine. Crit Care 2010; 14(3):311. doi:10.1186/cc8979
- Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long term survivorship after severe sepsis in older Americans. J Am Geriatr Soc 2012; 60(6):1070–1077. doi:10.1111/j.1532-5415.2012.03989.x
- Kahn JM, Le T, Angus DC, et al; ProVent Study Group Investigators. The epidemiology of chronic critical illness in the United States. Crit Care Med 2015; 43(2):282–287. doi:10.1097/CCM.0000000000000710
- Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long term acute care hospital utilization after critical illness. JAMA 2010; 303(22):2253–2259. doi:10.1001/jama.2010.761
- Golovyan DM, Khan SH, Wang S, Khan BA. What should I address at follow-up of patients who survive critical illness? Cleve Clin J Med 2018; 85(7):523–526. doi:10.3949/ccjm.85a.17104
The Inpatient Blindside: Comorbid Mental Health Conditions and Readmissions among Hospitalized Children
To ensure hospital quality, the Centers for Medicaid & Medicare Services have tied payments to performance measures, including readmissions.1 One readmission metric, the Potentially Preventable Readmission measure (3M, PPR), was initially developed for Medicare and defined as readmissions related to an index admission, excluding those for treatment of cancer, related to trauma or burns, or following neonatal hospitalization. The PPR includes readmissions for both primary mental health conditions (MHCs) and for other hospitalizations with comorbid MHCs.2 Although controversies surround equating a hospital’s quality with its rate of readmissions, the PPR has been expanded to include numerous states. Since the PPR is also used for the Medicaid population in these states, it also measures pediatric readmissions. Hospitals in states adopting PPR calculations, including children’s hospitals, must either meet these new quality metrics or risk financial penalties. In light of evidence of high readmission rates among adult patients with MHCs, several states have modified the PPR to exclude MHCs and claims for mental health services.3–9
In their study, “Mental Health Conditions and Unplanned Hospital Readmissions in Children,” Doupnik et al. provided compelling evidence that MHCs in children (similar to adults) are closely associated with readmissions.10 MHCs are possibly underappreciated risk factors for readmission penalties and therefore represent a necessary point for increased awareness. Doupnik et al. calculated 30-day unplanned hospital readmissions of children with versus without comorbid MHCs using another standard measure, the Pediatric All-Condition Readmission (PACR) measure. The PACR measure excludes index admissions with a MHC as primary diagnosis but includes children with comorbid MHCs.
Doupnik et al. used a nationally representative cohort of all index hospitalizations of children aged 3–21 years from the 2013 Nationwide Readmission Database that allowed for estimates of MHC prevalence in the study population.11 A comorbid MHC was identified in almost 1 in 5 medical admissions and 1 in 7 procedural admissions. Comorbid substance abuse was identified in 5.4% of medical admissions and 4.7% of procedure admissions, making this diagnosis the most frequently coded stand-alone MHC. The authors’ findings are particularly noteworthy given that diagnosis of MHCs is highly dependent upon coding and is therefore almost certainly underreported. In pediatric inpatient populations, the true prevalence of comorbid MHCs is probably higher.
Doupnik et al. observed that comorbid MHCs are a significant risk factor for readmission. After adjustment for demographic, clinical, and hospital characteristics, children with MHCs presented a nearly 25% higher chance of readmission for both medical and procedural hospitalizations. Children admitted with medical conditions and multiple MHCs yielded odds of readmission 50% higher than that of children without MHCs. Overall, the presence of MHCs was associated with more than 2,500 medical and 200 procedure readmissions.
Previous studies in adult populations have also found that comorbid MHCs are an important risk factor for readmissions.12,13 Other research describes that children with MHCs have increased hospital resource use, including longer lengths of stay and higher hospitalization costs.14-17 Further, children with MHCs as a primary diagnosis are more prone to readmission, with readmission rates approaching those observed in children with medical complexity in some cases.18,19 MHCs are common among hospitalized children and have become an increasingly present comorbidity in primary medical or surgical admissions.17
One particular strength of this study lies in its description of the relationship between comorbid (not primary) MHCs and readmission following medical or surgical procedures in hospitalized children. This relationship has been examined in adult inpatient populations but less so in pediatric inpatient populations.12,13 This study provides insights into the relationships between specific MHCs and unplanned readmissions for certain primary medical or surgical diagnoses, including those for attention deficit disorder and autism that are not well-recognized in adult populations.
High-quality inpatient pediatric practice depends not only upon recognition of concurrent MHCs during hospitalizations but also assurance of follow-up outside of such institutions. During the inpatient care of children, pediatric hospitalists often perform myopic inpatient care which fails to routinely address underlying MHCs.20 For example, among children who are admitted with primary medical or procedure diagnoses, it is possible, or perhaps likely, that providers give little attention to an underlying MHC outside of continuation of a current medication. Comorbid MHCs are not accounted for within readmission calculations that directly affect hospital reimbursement. This study suggests that comorbid MHCs in hospitalized children may worsen readmission penalty status. In this manner, comorbid MHCs may represent a hospital’s blindside.
We agree with Doupnik et al. that an integrated approach with medical and mental health professionals may improve the care of children with MHCs in hospitalized settings. This improvement in care may eventually affect hospital-level national quality metrics, such as readmissions. The findings of Doupnik et al. also provide a strong argument that pediatric inpatient providers should consider mental health consultations for patients with frequent admissions associated with chronic conditions, as comorbid MHCs are associated with worsened disease states and account for a disproportionate share of admissions for children with chronic conditions.21,22 Recognition of comorbid MHCs may improve baseline chronic disease states for hospitalized children.
We assert that the current silos in inpatient pediatrics of medical and mental healthcare are outdated. Pediatric hospitalists need to assess for and access effective MHC treatment options in the inpatient setting. In addition to the provision of mental health care within hospital settings, providers should also ensure that appropriate follow-up is arranged at the time of discharge. From a health policy standpoint, providers should clarify how both primary and comorbid MHCs are included within readmission measures while considering the close association of these conditions with readmission. Although the care of children with MHCs requires a long-term and coordinated approach, identification and treatment during hospitalization offer unique opportunities to modify outcomes of MHCs and coexistent medical and surgical diagnoses.
Disclosures
The authors declare no conflict of interest.
1. Centers for Medicare & Medicaid Services. Hospital Readmission Reduction Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HRRP/Hospital-Readmission-Reduction-Program.html. Published September 28, 2015. Accessed February 9, 2018.
2. 3M. Potentially Preventable Readmissions Classification System. http://multimedia.3m.com/mws/media/1042610O/resources-and-references-his-2015.pdf. Accessed February 9, 2018.
3. Illinois Department of Family and Healthcare Services. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed February 9, 2018.
4. New York State Department of Health. Potentially preventable hospital readmissions among medicaid recipients with mental health and/or substance abuse health conditions compared with all others: New York State, 2007. https://www.health.ny.gov/health_care/managed_care/reports/statistics_data/3hospital_readmissions_mentahealth.pdf. Accessed February 9, 2018.
5. Texas Health and Human Services Commission. Potentially preventable readmissions in Texas Medicaid and CHIP Programs, Fiscal Year 2013. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year-2013. Accessed February 9, 2018.
6. Oklahoma Healthcare Association. Provider reimbursement notice. https://www.okhca.org/providers.aspx?id=2538. Accessed February 9, 2018.
7. Washington State Hospital Association. Potentially preventable readmission (PPR) adjustments. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed February 9, 2018.
8. State of Colorado. HQIP 30-day All cause readmission. https://www.colorado.gov/pacific/sites/default/files/2016%20March%20HQIP%2030-day%20all-cause%20readmission%20measure.pdf. Accessed February 9, 2018.
9. Maryland Health Services Cost Review Commission. Readmission reduction incentive program. http://www.hscrc.state.md.us/Pages/init-readm-rip.aspx. Accessed February 9, 2018.
10. Doupnik SK, Lawlor J, Zima BT, et al. Mental health conditions and unplanned hospital readmissions in children. J Hosp Med. 2018(13):445-452. PubMed
11. NRD Overview. https://www.hcup-us.ahrq.gov/nrdoverview.jsp. Accessed February 9, 2018.
12. Singh G, Zhang W, Kuo Y-F, Sharma G. Association of psychological disorders with 30-day readmission rates in patients with COPD. Chest. 2016;149(4):905-915. doi:10.1378/chest.15-0449 PubMed
13. McIntyre LK, Arbabi S, Robinson EF, Maier RV. Analysis of risk factors for patient readmission 30 days following discharge from general surgery. JAMA Surg. 2016;151(9):855-861. doi:10.1001/jamasurg.2016.1258 PubMed
14. Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics. 2014;133(4):602-609. doi:10.1542/peds.2013-3165 PubMed
15. Doupnik SK, Lawlor J, Zima BT, et al. Mental health conditions and medical and surgical hospital utilization. Pediatrics. 2016;138(6): e20162416. doi:10.1542/peds.2016-2416 PubMed
16. Doupnik SK, Mitra N, Feudtner C, Marcus SC. The influence of comorbid mood and anxiety disorders on outcomes of pediatric patients hospitalized for pneumonia. Hosp Pediatr. 2016;6(3):135-142. doi:10.1542/hpeds.2015-0177 PubMed
17. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric disorders and trends in resource use in pediatric hospitals. Pediatrics. 2016;138(5): e20160909. doi:10.1542/peds.2016-0909 PubMed
18. Feng JY, Toomey SL, Zaslavsky AM, Nakamura MM, Schuster MA. Readmission after pediatric mental health admissions. Pediatrics. 2017;140(6):e20171571. doi:10.1542/peds.2017-1571 PubMed
19. Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6):e1463-e1470. doi:10.1542/peds.2012-0175 PubMed
20. Doupnik SK, Walter JK. Collaboration is key to improving hospital care for patients with medical and psychiatric comorbidity. Hosp Pediatr. 2016;6(12):760-762. doi:10.1542/hpeds.2016-0165 PubMed
21. Richardson LP, Russo JE, Lozano P, McCauley E, Katon W. The effect of comorbid anxiety and depressive disorders on health care utilization and costs among adolescents with asthma. Gen Hosp Psychiatry. 2008;30(5):398-406. doi:10.1016/j.genhosppsych.2008.06.004 PubMed
22. Malik FS, Hall M, Mangione-Smith R, et al. Patient characteristics associated with differences in admission frequency for diabetic ketoacidosis in United States children’s hospitals. J Pediatr. 2016;171:104-110. doi:10.1016/j.jpeds.2015.12.015 PubMed
To ensure hospital quality, the Centers for Medicaid & Medicare Services have tied payments to performance measures, including readmissions.1 One readmission metric, the Potentially Preventable Readmission measure (3M, PPR), was initially developed for Medicare and defined as readmissions related to an index admission, excluding those for treatment of cancer, related to trauma or burns, or following neonatal hospitalization. The PPR includes readmissions for both primary mental health conditions (MHCs) and for other hospitalizations with comorbid MHCs.2 Although controversies surround equating a hospital’s quality with its rate of readmissions, the PPR has been expanded to include numerous states. Since the PPR is also used for the Medicaid population in these states, it also measures pediatric readmissions. Hospitals in states adopting PPR calculations, including children’s hospitals, must either meet these new quality metrics or risk financial penalties. In light of evidence of high readmission rates among adult patients with MHCs, several states have modified the PPR to exclude MHCs and claims for mental health services.3–9
In their study, “Mental Health Conditions and Unplanned Hospital Readmissions in Children,” Doupnik et al. provided compelling evidence that MHCs in children (similar to adults) are closely associated with readmissions.10 MHCs are possibly underappreciated risk factors for readmission penalties and therefore represent a necessary point for increased awareness. Doupnik et al. calculated 30-day unplanned hospital readmissions of children with versus without comorbid MHCs using another standard measure, the Pediatric All-Condition Readmission (PACR) measure. The PACR measure excludes index admissions with a MHC as primary diagnosis but includes children with comorbid MHCs.
Doupnik et al. used a nationally representative cohort of all index hospitalizations of children aged 3–21 years from the 2013 Nationwide Readmission Database that allowed for estimates of MHC prevalence in the study population.11 A comorbid MHC was identified in almost 1 in 5 medical admissions and 1 in 7 procedural admissions. Comorbid substance abuse was identified in 5.4% of medical admissions and 4.7% of procedure admissions, making this diagnosis the most frequently coded stand-alone MHC. The authors’ findings are particularly noteworthy given that diagnosis of MHCs is highly dependent upon coding and is therefore almost certainly underreported. In pediatric inpatient populations, the true prevalence of comorbid MHCs is probably higher.
Doupnik et al. observed that comorbid MHCs are a significant risk factor for readmission. After adjustment for demographic, clinical, and hospital characteristics, children with MHCs presented a nearly 25% higher chance of readmission for both medical and procedural hospitalizations. Children admitted with medical conditions and multiple MHCs yielded odds of readmission 50% higher than that of children without MHCs. Overall, the presence of MHCs was associated with more than 2,500 medical and 200 procedure readmissions.
Previous studies in adult populations have also found that comorbid MHCs are an important risk factor for readmissions.12,13 Other research describes that children with MHCs have increased hospital resource use, including longer lengths of stay and higher hospitalization costs.14-17 Further, children with MHCs as a primary diagnosis are more prone to readmission, with readmission rates approaching those observed in children with medical complexity in some cases.18,19 MHCs are common among hospitalized children and have become an increasingly present comorbidity in primary medical or surgical admissions.17
One particular strength of this study lies in its description of the relationship between comorbid (not primary) MHCs and readmission following medical or surgical procedures in hospitalized children. This relationship has been examined in adult inpatient populations but less so in pediatric inpatient populations.12,13 This study provides insights into the relationships between specific MHCs and unplanned readmissions for certain primary medical or surgical diagnoses, including those for attention deficit disorder and autism that are not well-recognized in adult populations.
High-quality inpatient pediatric practice depends not only upon recognition of concurrent MHCs during hospitalizations but also assurance of follow-up outside of such institutions. During the inpatient care of children, pediatric hospitalists often perform myopic inpatient care which fails to routinely address underlying MHCs.20 For example, among children who are admitted with primary medical or procedure diagnoses, it is possible, or perhaps likely, that providers give little attention to an underlying MHC outside of continuation of a current medication. Comorbid MHCs are not accounted for within readmission calculations that directly affect hospital reimbursement. This study suggests that comorbid MHCs in hospitalized children may worsen readmission penalty status. In this manner, comorbid MHCs may represent a hospital’s blindside.
We agree with Doupnik et al. that an integrated approach with medical and mental health professionals may improve the care of children with MHCs in hospitalized settings. This improvement in care may eventually affect hospital-level national quality metrics, such as readmissions. The findings of Doupnik et al. also provide a strong argument that pediatric inpatient providers should consider mental health consultations for patients with frequent admissions associated with chronic conditions, as comorbid MHCs are associated with worsened disease states and account for a disproportionate share of admissions for children with chronic conditions.21,22 Recognition of comorbid MHCs may improve baseline chronic disease states for hospitalized children.
We assert that the current silos in inpatient pediatrics of medical and mental healthcare are outdated. Pediatric hospitalists need to assess for and access effective MHC treatment options in the inpatient setting. In addition to the provision of mental health care within hospital settings, providers should also ensure that appropriate follow-up is arranged at the time of discharge. From a health policy standpoint, providers should clarify how both primary and comorbid MHCs are included within readmission measures while considering the close association of these conditions with readmission. Although the care of children with MHCs requires a long-term and coordinated approach, identification and treatment during hospitalization offer unique opportunities to modify outcomes of MHCs and coexistent medical and surgical diagnoses.
Disclosures
The authors declare no conflict of interest.
To ensure hospital quality, the Centers for Medicaid & Medicare Services have tied payments to performance measures, including readmissions.1 One readmission metric, the Potentially Preventable Readmission measure (3M, PPR), was initially developed for Medicare and defined as readmissions related to an index admission, excluding those for treatment of cancer, related to trauma or burns, or following neonatal hospitalization. The PPR includes readmissions for both primary mental health conditions (MHCs) and for other hospitalizations with comorbid MHCs.2 Although controversies surround equating a hospital’s quality with its rate of readmissions, the PPR has been expanded to include numerous states. Since the PPR is also used for the Medicaid population in these states, it also measures pediatric readmissions. Hospitals in states adopting PPR calculations, including children’s hospitals, must either meet these new quality metrics or risk financial penalties. In light of evidence of high readmission rates among adult patients with MHCs, several states have modified the PPR to exclude MHCs and claims for mental health services.3–9
In their study, “Mental Health Conditions and Unplanned Hospital Readmissions in Children,” Doupnik et al. provided compelling evidence that MHCs in children (similar to adults) are closely associated with readmissions.10 MHCs are possibly underappreciated risk factors for readmission penalties and therefore represent a necessary point for increased awareness. Doupnik et al. calculated 30-day unplanned hospital readmissions of children with versus without comorbid MHCs using another standard measure, the Pediatric All-Condition Readmission (PACR) measure. The PACR measure excludes index admissions with a MHC as primary diagnosis but includes children with comorbid MHCs.
Doupnik et al. used a nationally representative cohort of all index hospitalizations of children aged 3–21 years from the 2013 Nationwide Readmission Database that allowed for estimates of MHC prevalence in the study population.11 A comorbid MHC was identified in almost 1 in 5 medical admissions and 1 in 7 procedural admissions. Comorbid substance abuse was identified in 5.4% of medical admissions and 4.7% of procedure admissions, making this diagnosis the most frequently coded stand-alone MHC. The authors’ findings are particularly noteworthy given that diagnosis of MHCs is highly dependent upon coding and is therefore almost certainly underreported. In pediatric inpatient populations, the true prevalence of comorbid MHCs is probably higher.
Doupnik et al. observed that comorbid MHCs are a significant risk factor for readmission. After adjustment for demographic, clinical, and hospital characteristics, children with MHCs presented a nearly 25% higher chance of readmission for both medical and procedural hospitalizations. Children admitted with medical conditions and multiple MHCs yielded odds of readmission 50% higher than that of children without MHCs. Overall, the presence of MHCs was associated with more than 2,500 medical and 200 procedure readmissions.
Previous studies in adult populations have also found that comorbid MHCs are an important risk factor for readmissions.12,13 Other research describes that children with MHCs have increased hospital resource use, including longer lengths of stay and higher hospitalization costs.14-17 Further, children with MHCs as a primary diagnosis are more prone to readmission, with readmission rates approaching those observed in children with medical complexity in some cases.18,19 MHCs are common among hospitalized children and have become an increasingly present comorbidity in primary medical or surgical admissions.17
One particular strength of this study lies in its description of the relationship between comorbid (not primary) MHCs and readmission following medical or surgical procedures in hospitalized children. This relationship has been examined in adult inpatient populations but less so in pediatric inpatient populations.12,13 This study provides insights into the relationships between specific MHCs and unplanned readmissions for certain primary medical or surgical diagnoses, including those for attention deficit disorder and autism that are not well-recognized in adult populations.
High-quality inpatient pediatric practice depends not only upon recognition of concurrent MHCs during hospitalizations but also assurance of follow-up outside of such institutions. During the inpatient care of children, pediatric hospitalists often perform myopic inpatient care which fails to routinely address underlying MHCs.20 For example, among children who are admitted with primary medical or procedure diagnoses, it is possible, or perhaps likely, that providers give little attention to an underlying MHC outside of continuation of a current medication. Comorbid MHCs are not accounted for within readmission calculations that directly affect hospital reimbursement. This study suggests that comorbid MHCs in hospitalized children may worsen readmission penalty status. In this manner, comorbid MHCs may represent a hospital’s blindside.
We agree with Doupnik et al. that an integrated approach with medical and mental health professionals may improve the care of children with MHCs in hospitalized settings. This improvement in care may eventually affect hospital-level national quality metrics, such as readmissions. The findings of Doupnik et al. also provide a strong argument that pediatric inpatient providers should consider mental health consultations for patients with frequent admissions associated with chronic conditions, as comorbid MHCs are associated with worsened disease states and account for a disproportionate share of admissions for children with chronic conditions.21,22 Recognition of comorbid MHCs may improve baseline chronic disease states for hospitalized children.
We assert that the current silos in inpatient pediatrics of medical and mental healthcare are outdated. Pediatric hospitalists need to assess for and access effective MHC treatment options in the inpatient setting. In addition to the provision of mental health care within hospital settings, providers should also ensure that appropriate follow-up is arranged at the time of discharge. From a health policy standpoint, providers should clarify how both primary and comorbid MHCs are included within readmission measures while considering the close association of these conditions with readmission. Although the care of children with MHCs requires a long-term and coordinated approach, identification and treatment during hospitalization offer unique opportunities to modify outcomes of MHCs and coexistent medical and surgical diagnoses.
Disclosures
The authors declare no conflict of interest.
1. Centers for Medicare & Medicaid Services. Hospital Readmission Reduction Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HRRP/Hospital-Readmission-Reduction-Program.html. Published September 28, 2015. Accessed February 9, 2018.
2. 3M. Potentially Preventable Readmissions Classification System. http://multimedia.3m.com/mws/media/1042610O/resources-and-references-his-2015.pdf. Accessed February 9, 2018.
3. Illinois Department of Family and Healthcare Services. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed February 9, 2018.
4. New York State Department of Health. Potentially preventable hospital readmissions among medicaid recipients with mental health and/or substance abuse health conditions compared with all others: New York State, 2007. https://www.health.ny.gov/health_care/managed_care/reports/statistics_data/3hospital_readmissions_mentahealth.pdf. Accessed February 9, 2018.
5. Texas Health and Human Services Commission. Potentially preventable readmissions in Texas Medicaid and CHIP Programs, Fiscal Year 2013. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year-2013. Accessed February 9, 2018.
6. Oklahoma Healthcare Association. Provider reimbursement notice. https://www.okhca.org/providers.aspx?id=2538. Accessed February 9, 2018.
7. Washington State Hospital Association. Potentially preventable readmission (PPR) adjustments. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed February 9, 2018.
8. State of Colorado. HQIP 30-day All cause readmission. https://www.colorado.gov/pacific/sites/default/files/2016%20March%20HQIP%2030-day%20all-cause%20readmission%20measure.pdf. Accessed February 9, 2018.
9. Maryland Health Services Cost Review Commission. Readmission reduction incentive program. http://www.hscrc.state.md.us/Pages/init-readm-rip.aspx. Accessed February 9, 2018.
10. Doupnik SK, Lawlor J, Zima BT, et al. Mental health conditions and unplanned hospital readmissions in children. J Hosp Med. 2018(13):445-452. PubMed
11. NRD Overview. https://www.hcup-us.ahrq.gov/nrdoverview.jsp. Accessed February 9, 2018.
12. Singh G, Zhang W, Kuo Y-F, Sharma G. Association of psychological disorders with 30-day readmission rates in patients with COPD. Chest. 2016;149(4):905-915. doi:10.1378/chest.15-0449 PubMed
13. McIntyre LK, Arbabi S, Robinson EF, Maier RV. Analysis of risk factors for patient readmission 30 days following discharge from general surgery. JAMA Surg. 2016;151(9):855-861. doi:10.1001/jamasurg.2016.1258 PubMed
14. Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics. 2014;133(4):602-609. doi:10.1542/peds.2013-3165 PubMed
15. Doupnik SK, Lawlor J, Zima BT, et al. Mental health conditions and medical and surgical hospital utilization. Pediatrics. 2016;138(6): e20162416. doi:10.1542/peds.2016-2416 PubMed
16. Doupnik SK, Mitra N, Feudtner C, Marcus SC. The influence of comorbid mood and anxiety disorders on outcomes of pediatric patients hospitalized for pneumonia. Hosp Pediatr. 2016;6(3):135-142. doi:10.1542/hpeds.2015-0177 PubMed
17. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric disorders and trends in resource use in pediatric hospitals. Pediatrics. 2016;138(5): e20160909. doi:10.1542/peds.2016-0909 PubMed
18. Feng JY, Toomey SL, Zaslavsky AM, Nakamura MM, Schuster MA. Readmission after pediatric mental health admissions. Pediatrics. 2017;140(6):e20171571. doi:10.1542/peds.2017-1571 PubMed
19. Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6):e1463-e1470. doi:10.1542/peds.2012-0175 PubMed
20. Doupnik SK, Walter JK. Collaboration is key to improving hospital care for patients with medical and psychiatric comorbidity. Hosp Pediatr. 2016;6(12):760-762. doi:10.1542/hpeds.2016-0165 PubMed
21. Richardson LP, Russo JE, Lozano P, McCauley E, Katon W. The effect of comorbid anxiety and depressive disorders on health care utilization and costs among adolescents with asthma. Gen Hosp Psychiatry. 2008;30(5):398-406. doi:10.1016/j.genhosppsych.2008.06.004 PubMed
22. Malik FS, Hall M, Mangione-Smith R, et al. Patient characteristics associated with differences in admission frequency for diabetic ketoacidosis in United States children’s hospitals. J Pediatr. 2016;171:104-110. doi:10.1016/j.jpeds.2015.12.015 PubMed
1. Centers for Medicare & Medicaid Services. Hospital Readmission Reduction Program. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HRRP/Hospital-Readmission-Reduction-Program.html. Published September 28, 2015. Accessed February 9, 2018.
2. 3M. Potentially Preventable Readmissions Classification System. http://multimedia.3m.com/mws/media/1042610O/resources-and-references-his-2015.pdf. Accessed February 9, 2018.
3. Illinois Department of Family and Healthcare Services. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed February 9, 2018.
4. New York State Department of Health. Potentially preventable hospital readmissions among medicaid recipients with mental health and/or substance abuse health conditions compared with all others: New York State, 2007. https://www.health.ny.gov/health_care/managed_care/reports/statistics_data/3hospital_readmissions_mentahealth.pdf. Accessed February 9, 2018.
5. Texas Health and Human Services Commission. Potentially preventable readmissions in Texas Medicaid and CHIP Programs, Fiscal Year 2013. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year-2013. Accessed February 9, 2018.
6. Oklahoma Healthcare Association. Provider reimbursement notice. https://www.okhca.org/providers.aspx?id=2538. Accessed February 9, 2018.
7. Washington State Hospital Association. Potentially preventable readmission (PPR) adjustments. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed February 9, 2018.
8. State of Colorado. HQIP 30-day All cause readmission. https://www.colorado.gov/pacific/sites/default/files/2016%20March%20HQIP%2030-day%20all-cause%20readmission%20measure.pdf. Accessed February 9, 2018.
9. Maryland Health Services Cost Review Commission. Readmission reduction incentive program. http://www.hscrc.state.md.us/Pages/init-readm-rip.aspx. Accessed February 9, 2018.
10. Doupnik SK, Lawlor J, Zima BT, et al. Mental health conditions and unplanned hospital readmissions in children. J Hosp Med. 2018(13):445-452. PubMed
11. NRD Overview. https://www.hcup-us.ahrq.gov/nrdoverview.jsp. Accessed February 9, 2018.
12. Singh G, Zhang W, Kuo Y-F, Sharma G. Association of psychological disorders with 30-day readmission rates in patients with COPD. Chest. 2016;149(4):905-915. doi:10.1378/chest.15-0449 PubMed
13. McIntyre LK, Arbabi S, Robinson EF, Maier RV. Analysis of risk factors for patient readmission 30 days following discharge from general surgery. JAMA Surg. 2016;151(9):855-861. doi:10.1001/jamasurg.2016.1258 PubMed
14. Bardach NS, Coker TR, Zima BT, et al. Common and costly hospitalizations for pediatric mental health disorders. Pediatrics. 2014;133(4):602-609. doi:10.1542/peds.2013-3165 PubMed
15. Doupnik SK, Lawlor J, Zima BT, et al. Mental health conditions and medical and surgical hospital utilization. Pediatrics. 2016;138(6): e20162416. doi:10.1542/peds.2016-2416 PubMed
16. Doupnik SK, Mitra N, Feudtner C, Marcus SC. The influence of comorbid mood and anxiety disorders on outcomes of pediatric patients hospitalized for pneumonia. Hosp Pediatr. 2016;6(3):135-142. doi:10.1542/hpeds.2015-0177 PubMed
17. Zima BT, Rodean J, Hall M, Bardach NS, Coker TR, Berry JG. Psychiatric disorders and trends in resource use in pediatric hospitals. Pediatrics. 2016;138(5): e20160909. doi:10.1542/peds.2016-0909 PubMed
18. Feng JY, Toomey SL, Zaslavsky AM, Nakamura MM, Schuster MA. Readmission after pediatric mental health admissions. Pediatrics. 2017;140(6):e20171571. doi:10.1542/peds.2017-1571 PubMed
19. Cohen E, Berry JG, Camacho X, Anderson G, Wodchis W, Guttmann A. Patterns and costs of health care use of children with medical complexity. Pediatrics. 2012;130(6):e1463-e1470. doi:10.1542/peds.2012-0175 PubMed
20. Doupnik SK, Walter JK. Collaboration is key to improving hospital care for patients with medical and psychiatric comorbidity. Hosp Pediatr. 2016;6(12):760-762. doi:10.1542/hpeds.2016-0165 PubMed
21. Richardson LP, Russo JE, Lozano P, McCauley E, Katon W. The effect of comorbid anxiety and depressive disorders on health care utilization and costs among adolescents with asthma. Gen Hosp Psychiatry. 2008;30(5):398-406. doi:10.1016/j.genhosppsych.2008.06.004 PubMed
22. Malik FS, Hall M, Mangione-Smith R, et al. Patient characteristics associated with differences in admission frequency for diabetic ketoacidosis in United States children’s hospitals. J Pediatr. 2016;171:104-110. doi:10.1016/j.jpeds.2015.12.015 PubMed
© 2018 Society of Hospital Medicine
Is a detailed neurologic physical examination always necessary?
The article in this issue by Shikino et al1 on a mimic of Bell palsy gives us an opportunity to discuss the question posed by the title of this editorial. The obvious short answer is “no.”
Any experienced clinician will acknowledge that the extent of the physical examination and the extent of information obtained during the history should be determined by the problem being evaluated at the time and by the setting in which it takes place. The difficulty, of course, is that this relies on the judgment of the clinician, and this may or may not pass the test of hindsight.
Verghese et al2 have eloquently emphasized the hazards of an incomplete or inadequate physical examination. Their study was not designed to determine the prevalence of deficient physical examination, either in its extent or its accuracy. Their purpose was to promote the necessity of proper teaching and performance of examination technique.
The neurologic examination is one of the last bastions of physical assessment.3 Despite remarkable advances in imaging and physiologic techniques, the neurologic physical assessment remains critical for diagnosis and management of the neurologic patient. One of my mentors in neurology used to urge residents to examine patients and record the results of the examination as if every patient would subsequently be the subject of a clinicopathologic conference. Anyone who has reviewed a case for a conference or a case report can identify with that sentiment, wishing that some missing piece of information were available. Yet everyone also recognizes the difficulties, if not the impossibility, of achieving that ideal result.
But recording information obtained during the history or physical examination is important even in the course of a daily routine evaluation. I find myself wishing that a previous examiner had commented on whether the muscle stretch reflexes were somewhat hypoactive (eg, “1+”) or on the brisk side (“3+”) rather than “physiologic.” Was the right leg actually globally weak (“4/5”), or was there a discrepancy between proximal and distal muscles or between the physiologic flexors and the extensors?
This can make a big difference in following a patient’s neurologic progress, even over a short time span. It might tell us whether we are dealing with weakness from a peripheral neuromuscular disorder (eg, Guillain-Barré syndrome) or from a myelopathy due to impending spinal cord compression.
It should be mentioned that although Guillain-Barré syndrome is characterized as an ascending paralysis, ie, beginning distally and spreading rostrally, it is one of the few peripheral neuropathies that can present with predominant proximal weakness. It is, in fact, a radiculoneuropathy. But spinal cord (upper motor neuron) disorders preferentially weaken the physiologic flexors of the lower limbs (hamstrings and ankle dorsiflexors), leading to the characteristic extensor posture of the spastic leg. Other findings that can help differential peripheral vs spinal cord disorders include distal sensory loss and hypoactive or absent muscle stretch reflexes in a peripheral neuropathy, compared with dissociated sensory loss (eg, impaired pain and temperature sensation in one leg with reduced vibration perception and proprioception in the other) along with hyperreflexia with cord lesions.
Therefore, a careful neurologic examination may tell us whether magnetic resonance imaging of the spine or an electrodiagnostic study should be the next step.
Shikino et al describe a patient who presented with what looked like idiopathic facial palsy (Bell palsy) but turned out to be the result of a primary central nervous system (CNS) cause. Would a more detailed neurologic examination have identified this as a CNS disorder? Would more specific information about the degree and distribution of facial paresis have facilitated earlier recognition of a progressive process, making idiopathic facial palsy less likely? How much elevation of the eyebrow occurred with voluntary activation, how many millimeters of sclera were visible with gentle eyelid closure? How much space remained between the lips on attempted lip closure?
Upper facial muscle weakness is typically not seen in CNS disorders, although facial nerve or nucleus involvement at the pontine level can impair eyelid and frontalis function. Such lesions would usually be accompanied by “neighborhood” signs such as subtle ipsilateral lateral rectus or abducens palsy, involvement of the vestibular nuclei with vertigo, or facial sensory impairment from disruption of the descending trigeminal nucleus and tract. These would be “pertinent negatives” for excluding a brainstem lesion, and ipsilateral motor, sensory, or “higher cortical” functions would obviously signal a supratentorial CNS disorder.
In the case described by Shikino et al, observation and recording of the amount of facial motor function at the initial visit, 3 days after onset, could facilitate recognition of an aberrant course even a few days later and prompt further investigation at an early follow-up visit (idiopathic palsy is almost invariably maximal by 72 hours). I would assume that no additional clinical information was available to the subsequent examiner in this case, 2 months later, rather than suggesting that such information was omitted for the sake of parsimony.
Would any of this have made a difference? Probably not, but we need all the help we can get in medicine. Remember that every bit of information you obtain from your history or physical examination that you do not record disappears with you and is irretrievably lost.
- Shikino K, Suzuki S, Uehara T, Ikusaka M. Primary central nervous system lymphoma mimicking Bell palsy. Cleve Clin J Med 2018: 85(6)442–443. doi:10.3949/ccjm.85a.17061
- Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JP. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med 2015; 128(12):1322–1324.e3. doi:10.1016/j.amjmed.2015.06.004
- Berger JR. Neurologists: the last bedside physician-scientists. JAMA Neurol 2013; 70(8):965–966. doi:10.1001/jamaneurol.2013.2977
The article in this issue by Shikino et al1 on a mimic of Bell palsy gives us an opportunity to discuss the question posed by the title of this editorial. The obvious short answer is “no.”
Any experienced clinician will acknowledge that the extent of the physical examination and the extent of information obtained during the history should be determined by the problem being evaluated at the time and by the setting in which it takes place. The difficulty, of course, is that this relies on the judgment of the clinician, and this may or may not pass the test of hindsight.
Verghese et al2 have eloquently emphasized the hazards of an incomplete or inadequate physical examination. Their study was not designed to determine the prevalence of deficient physical examination, either in its extent or its accuracy. Their purpose was to promote the necessity of proper teaching and performance of examination technique.
The neurologic examination is one of the last bastions of physical assessment.3 Despite remarkable advances in imaging and physiologic techniques, the neurologic physical assessment remains critical for diagnosis and management of the neurologic patient. One of my mentors in neurology used to urge residents to examine patients and record the results of the examination as if every patient would subsequently be the subject of a clinicopathologic conference. Anyone who has reviewed a case for a conference or a case report can identify with that sentiment, wishing that some missing piece of information were available. Yet everyone also recognizes the difficulties, if not the impossibility, of achieving that ideal result.
But recording information obtained during the history or physical examination is important even in the course of a daily routine evaluation. I find myself wishing that a previous examiner had commented on whether the muscle stretch reflexes were somewhat hypoactive (eg, “1+”) or on the brisk side (“3+”) rather than “physiologic.” Was the right leg actually globally weak (“4/5”), or was there a discrepancy between proximal and distal muscles or between the physiologic flexors and the extensors?
This can make a big difference in following a patient’s neurologic progress, even over a short time span. It might tell us whether we are dealing with weakness from a peripheral neuromuscular disorder (eg, Guillain-Barré syndrome) or from a myelopathy due to impending spinal cord compression.
It should be mentioned that although Guillain-Barré syndrome is characterized as an ascending paralysis, ie, beginning distally and spreading rostrally, it is one of the few peripheral neuropathies that can present with predominant proximal weakness. It is, in fact, a radiculoneuropathy. But spinal cord (upper motor neuron) disorders preferentially weaken the physiologic flexors of the lower limbs (hamstrings and ankle dorsiflexors), leading to the characteristic extensor posture of the spastic leg. Other findings that can help differential peripheral vs spinal cord disorders include distal sensory loss and hypoactive or absent muscle stretch reflexes in a peripheral neuropathy, compared with dissociated sensory loss (eg, impaired pain and temperature sensation in one leg with reduced vibration perception and proprioception in the other) along with hyperreflexia with cord lesions.
Therefore, a careful neurologic examination may tell us whether magnetic resonance imaging of the spine or an electrodiagnostic study should be the next step.
Shikino et al describe a patient who presented with what looked like idiopathic facial palsy (Bell palsy) but turned out to be the result of a primary central nervous system (CNS) cause. Would a more detailed neurologic examination have identified this as a CNS disorder? Would more specific information about the degree and distribution of facial paresis have facilitated earlier recognition of a progressive process, making idiopathic facial palsy less likely? How much elevation of the eyebrow occurred with voluntary activation, how many millimeters of sclera were visible with gentle eyelid closure? How much space remained between the lips on attempted lip closure?
Upper facial muscle weakness is typically not seen in CNS disorders, although facial nerve or nucleus involvement at the pontine level can impair eyelid and frontalis function. Such lesions would usually be accompanied by “neighborhood” signs such as subtle ipsilateral lateral rectus or abducens palsy, involvement of the vestibular nuclei with vertigo, or facial sensory impairment from disruption of the descending trigeminal nucleus and tract. These would be “pertinent negatives” for excluding a brainstem lesion, and ipsilateral motor, sensory, or “higher cortical” functions would obviously signal a supratentorial CNS disorder.
In the case described by Shikino et al, observation and recording of the amount of facial motor function at the initial visit, 3 days after onset, could facilitate recognition of an aberrant course even a few days later and prompt further investigation at an early follow-up visit (idiopathic palsy is almost invariably maximal by 72 hours). I would assume that no additional clinical information was available to the subsequent examiner in this case, 2 months later, rather than suggesting that such information was omitted for the sake of parsimony.
Would any of this have made a difference? Probably not, but we need all the help we can get in medicine. Remember that every bit of information you obtain from your history or physical examination that you do not record disappears with you and is irretrievably lost.
The article in this issue by Shikino et al1 on a mimic of Bell palsy gives us an opportunity to discuss the question posed by the title of this editorial. The obvious short answer is “no.”
Any experienced clinician will acknowledge that the extent of the physical examination and the extent of information obtained during the history should be determined by the problem being evaluated at the time and by the setting in which it takes place. The difficulty, of course, is that this relies on the judgment of the clinician, and this may or may not pass the test of hindsight.
Verghese et al2 have eloquently emphasized the hazards of an incomplete or inadequate physical examination. Their study was not designed to determine the prevalence of deficient physical examination, either in its extent or its accuracy. Their purpose was to promote the necessity of proper teaching and performance of examination technique.
The neurologic examination is one of the last bastions of physical assessment.3 Despite remarkable advances in imaging and physiologic techniques, the neurologic physical assessment remains critical for diagnosis and management of the neurologic patient. One of my mentors in neurology used to urge residents to examine patients and record the results of the examination as if every patient would subsequently be the subject of a clinicopathologic conference. Anyone who has reviewed a case for a conference or a case report can identify with that sentiment, wishing that some missing piece of information were available. Yet everyone also recognizes the difficulties, if not the impossibility, of achieving that ideal result.
But recording information obtained during the history or physical examination is important even in the course of a daily routine evaluation. I find myself wishing that a previous examiner had commented on whether the muscle stretch reflexes were somewhat hypoactive (eg, “1+”) or on the brisk side (“3+”) rather than “physiologic.” Was the right leg actually globally weak (“4/5”), or was there a discrepancy between proximal and distal muscles or between the physiologic flexors and the extensors?
This can make a big difference in following a patient’s neurologic progress, even over a short time span. It might tell us whether we are dealing with weakness from a peripheral neuromuscular disorder (eg, Guillain-Barré syndrome) or from a myelopathy due to impending spinal cord compression.
It should be mentioned that although Guillain-Barré syndrome is characterized as an ascending paralysis, ie, beginning distally and spreading rostrally, it is one of the few peripheral neuropathies that can present with predominant proximal weakness. It is, in fact, a radiculoneuropathy. But spinal cord (upper motor neuron) disorders preferentially weaken the physiologic flexors of the lower limbs (hamstrings and ankle dorsiflexors), leading to the characteristic extensor posture of the spastic leg. Other findings that can help differential peripheral vs spinal cord disorders include distal sensory loss and hypoactive or absent muscle stretch reflexes in a peripheral neuropathy, compared with dissociated sensory loss (eg, impaired pain and temperature sensation in one leg with reduced vibration perception and proprioception in the other) along with hyperreflexia with cord lesions.
Therefore, a careful neurologic examination may tell us whether magnetic resonance imaging of the spine or an electrodiagnostic study should be the next step.
Shikino et al describe a patient who presented with what looked like idiopathic facial palsy (Bell palsy) but turned out to be the result of a primary central nervous system (CNS) cause. Would a more detailed neurologic examination have identified this as a CNS disorder? Would more specific information about the degree and distribution of facial paresis have facilitated earlier recognition of a progressive process, making idiopathic facial palsy less likely? How much elevation of the eyebrow occurred with voluntary activation, how many millimeters of sclera were visible with gentle eyelid closure? How much space remained between the lips on attempted lip closure?
Upper facial muscle weakness is typically not seen in CNS disorders, although facial nerve or nucleus involvement at the pontine level can impair eyelid and frontalis function. Such lesions would usually be accompanied by “neighborhood” signs such as subtle ipsilateral lateral rectus or abducens palsy, involvement of the vestibular nuclei with vertigo, or facial sensory impairment from disruption of the descending trigeminal nucleus and tract. These would be “pertinent negatives” for excluding a brainstem lesion, and ipsilateral motor, sensory, or “higher cortical” functions would obviously signal a supratentorial CNS disorder.
In the case described by Shikino et al, observation and recording of the amount of facial motor function at the initial visit, 3 days after onset, could facilitate recognition of an aberrant course even a few days later and prompt further investigation at an early follow-up visit (idiopathic palsy is almost invariably maximal by 72 hours). I would assume that no additional clinical information was available to the subsequent examiner in this case, 2 months later, rather than suggesting that such information was omitted for the sake of parsimony.
Would any of this have made a difference? Probably not, but we need all the help we can get in medicine. Remember that every bit of information you obtain from your history or physical examination that you do not record disappears with you and is irretrievably lost.
- Shikino K, Suzuki S, Uehara T, Ikusaka M. Primary central nervous system lymphoma mimicking Bell palsy. Cleve Clin J Med 2018: 85(6)442–443. doi:10.3949/ccjm.85a.17061
- Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JP. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med 2015; 128(12):1322–1324.e3. doi:10.1016/j.amjmed.2015.06.004
- Berger JR. Neurologists: the last bedside physician-scientists. JAMA Neurol 2013; 70(8):965–966. doi:10.1001/jamaneurol.2013.2977
- Shikino K, Suzuki S, Uehara T, Ikusaka M. Primary central nervous system lymphoma mimicking Bell palsy. Cleve Clin J Med 2018: 85(6)442–443. doi:10.3949/ccjm.85a.17061
- Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JP. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med 2015; 128(12):1322–1324.e3. doi:10.1016/j.amjmed.2015.06.004
- Berger JR. Neurologists: the last bedside physician-scientists. JAMA Neurol 2013; 70(8):965–966. doi:10.1001/jamaneurol.2013.2977