User login
The Virtual Hospitalist: The Future is Now
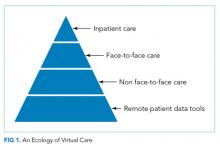
Compared with other industries, medicine has been slow to embrace the digital age. Electronic health records have only recently become ubiquitous, and that was only realized after governmental prodding through Meaningful Use legislation. Other digital tools, such as video or remote sensor technologies, have been available for decades but had not been introduced into routine medical care until recently for various reasons, ranging from costs to security to reimbursement rules. However, we are currently in the midst of a paradigm shift in medicine toward virtual care, as exemplified by the Kaiser Permanente CEO’s proclamation in 2017 that this capitated care system had moved over half of its 100 million annual patient encounters to the virtual environment.1
Regulation – both at the state and federal levels – has been the largest barrier to the adoption of virtual care. State licensure regulations for practicing medicine hamper virtual visits, which can otherwise be easily achieved without regard to geography. Although the Centers for Medicare & Medicaid Services (CMS) has had provisions for telehealth billing, these have been largely limited to rural areas. However, regulations are constantly evolving as the Interstate Medical Licensure Compact list is not CMS. The Interstate Medical Licensure Compact (www.imlcc.org) is an agreement involving 24 states that permits licensed physicians to practice medicine across state lines. CMS has recently proposed to add payments for virtual check-in visits, which will not be subject to the prior limitations on Medicare telehealth services.2 These and future changes in regulation will likely spur the rapid adoption and evolution of virtual services.
In this context, the article by Kuperman et al.3 provides a welcoming view of the future of hospital medicine. The authors demonstrated the feasibility of using a “virtual hospitalist” to manage patients admitted to a small rural hospital that lacked the patient volumes and resources to justify on-site hospitalist staffing. The patients benefited from the clinical expertise of an experienced inpatient provider while staying near their homes. This article adds to the growing literature on the use of these technologies in the hospital settings, which range from the management of patients in the intensive care unit4 to stroke patients in the ED5 and to inpatient psychiatric consultation.6
What are the implications for hospitalists? We need to prepare the current and future generations of hospitalists for practice in an evolving digital environment. “Choosing Wisely®: Things We Do For No Reason” is one of the most popular segments of JHM for a good reason: there are many things in the field of medicine because “that’s the way we always did it.” The capabilities unleashed by digital technologies will require hospitalists to rethink how we manage patients in acute and subacute settings and after discharge. Although these tools show a substantial promise to help us achieve the Triple Aim, we will need considerably more research to understand the costs and effectiveness of these new digital technologies and approaches.7,8 We also need new payment models that recognize their value. Finally, we also need to be aware that doctoring elements, such as human touch, physical presence, and emotional connection, can be encumbered and not enhanced by digital technologies.9
Disclosures
Dr. Ong and Dr. Brotman have nothing to disclose.
1. Why Digital Transformations Are Hard. Wall Street Journal. March 7, 2017, 2017.
2. Medicare Program: Revisions to Payment Policies under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; etc. In: Centers for Medicare & Medicaid Services, ed: Federal Register; 2018:1472.
3. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018. In Press. PubMed
4. Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305(21):2175-2183. doi: 10.1001/jama.2011.697. PubMed
5. Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7(9):787-795. doi: 10.1016/S1474-4422(08)70171-6. PubMed
6. Arevian AC, Jeffrey J, Young AS, Ong MK. Opportunities for flexible, on-demand care delivery through telemedicine. Psychiatr Serv. 2018;69(1):5-8. doi: 10.1176/appi.ps.201600589. PubMed
7. Ashwood JS, Mehrotra A, Cowling D, Uscher-Pines L. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Aff (Millwood). 2017;36(3):485-491. doi: 10.1377/hlthaff.2016.1130. PubMed
8. Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016;176(3):310-318. doi: 10.1001/jamainternmed.2015.7712. PubMed
9. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. doi: 10.1056/NEJMp0807461. PubMed
Compared with other industries, medicine has been slow to embrace the digital age. Electronic health records have only recently become ubiquitous, and that was only realized after governmental prodding through Meaningful Use legislation. Other digital tools, such as video or remote sensor technologies, have been available for decades but had not been introduced into routine medical care until recently for various reasons, ranging from costs to security to reimbursement rules. However, we are currently in the midst of a paradigm shift in medicine toward virtual care, as exemplified by the Kaiser Permanente CEO’s proclamation in 2017 that this capitated care system had moved over half of its 100 million annual patient encounters to the virtual environment.1
Regulation – both at the state and federal levels – has been the largest barrier to the adoption of virtual care. State licensure regulations for practicing medicine hamper virtual visits, which can otherwise be easily achieved without regard to geography. Although the Centers for Medicare & Medicaid Services (CMS) has had provisions for telehealth billing, these have been largely limited to rural areas. However, regulations are constantly evolving as the Interstate Medical Licensure Compact list is not CMS. The Interstate Medical Licensure Compact (www.imlcc.org) is an agreement involving 24 states that permits licensed physicians to practice medicine across state lines. CMS has recently proposed to add payments for virtual check-in visits, which will not be subject to the prior limitations on Medicare telehealth services.2 These and future changes in regulation will likely spur the rapid adoption and evolution of virtual services.
In this context, the article by Kuperman et al.3 provides a welcoming view of the future of hospital medicine. The authors demonstrated the feasibility of using a “virtual hospitalist” to manage patients admitted to a small rural hospital that lacked the patient volumes and resources to justify on-site hospitalist staffing. The patients benefited from the clinical expertise of an experienced inpatient provider while staying near their homes. This article adds to the growing literature on the use of these technologies in the hospital settings, which range from the management of patients in the intensive care unit4 to stroke patients in the ED5 and to inpatient psychiatric consultation.6
What are the implications for hospitalists? We need to prepare the current and future generations of hospitalists for practice in an evolving digital environment. “Choosing Wisely®: Things We Do For No Reason” is one of the most popular segments of JHM for a good reason: there are many things in the field of medicine because “that’s the way we always did it.” The capabilities unleashed by digital technologies will require hospitalists to rethink how we manage patients in acute and subacute settings and after discharge. Although these tools show a substantial promise to help us achieve the Triple Aim, we will need considerably more research to understand the costs and effectiveness of these new digital technologies and approaches.7,8 We also need new payment models that recognize their value. Finally, we also need to be aware that doctoring elements, such as human touch, physical presence, and emotional connection, can be encumbered and not enhanced by digital technologies.9
Disclosures
Dr. Ong and Dr. Brotman have nothing to disclose.
Compared with other industries, medicine has been slow to embrace the digital age. Electronic health records have only recently become ubiquitous, and that was only realized after governmental prodding through Meaningful Use legislation. Other digital tools, such as video or remote sensor technologies, have been available for decades but had not been introduced into routine medical care until recently for various reasons, ranging from costs to security to reimbursement rules. However, we are currently in the midst of a paradigm shift in medicine toward virtual care, as exemplified by the Kaiser Permanente CEO’s proclamation in 2017 that this capitated care system had moved over half of its 100 million annual patient encounters to the virtual environment.1
Regulation – both at the state and federal levels – has been the largest barrier to the adoption of virtual care. State licensure regulations for practicing medicine hamper virtual visits, which can otherwise be easily achieved without regard to geography. Although the Centers for Medicare & Medicaid Services (CMS) has had provisions for telehealth billing, these have been largely limited to rural areas. However, regulations are constantly evolving as the Interstate Medical Licensure Compact list is not CMS. The Interstate Medical Licensure Compact (www.imlcc.org) is an agreement involving 24 states that permits licensed physicians to practice medicine across state lines. CMS has recently proposed to add payments for virtual check-in visits, which will not be subject to the prior limitations on Medicare telehealth services.2 These and future changes in regulation will likely spur the rapid adoption and evolution of virtual services.
In this context, the article by Kuperman et al.3 provides a welcoming view of the future of hospital medicine. The authors demonstrated the feasibility of using a “virtual hospitalist” to manage patients admitted to a small rural hospital that lacked the patient volumes and resources to justify on-site hospitalist staffing. The patients benefited from the clinical expertise of an experienced inpatient provider while staying near their homes. This article adds to the growing literature on the use of these technologies in the hospital settings, which range from the management of patients in the intensive care unit4 to stroke patients in the ED5 and to inpatient psychiatric consultation.6
What are the implications for hospitalists? We need to prepare the current and future generations of hospitalists for practice in an evolving digital environment. “Choosing Wisely®: Things We Do For No Reason” is one of the most popular segments of JHM for a good reason: there are many things in the field of medicine because “that’s the way we always did it.” The capabilities unleashed by digital technologies will require hospitalists to rethink how we manage patients in acute and subacute settings and after discharge. Although these tools show a substantial promise to help us achieve the Triple Aim, we will need considerably more research to understand the costs and effectiveness of these new digital technologies and approaches.7,8 We also need new payment models that recognize their value. Finally, we also need to be aware that doctoring elements, such as human touch, physical presence, and emotional connection, can be encumbered and not enhanced by digital technologies.9
Disclosures
Dr. Ong and Dr. Brotman have nothing to disclose.
1. Why Digital Transformations Are Hard. Wall Street Journal. March 7, 2017, 2017.
2. Medicare Program: Revisions to Payment Policies under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; etc. In: Centers for Medicare & Medicaid Services, ed: Federal Register; 2018:1472.
3. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018. In Press. PubMed
4. Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305(21):2175-2183. doi: 10.1001/jama.2011.697. PubMed
5. Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7(9):787-795. doi: 10.1016/S1474-4422(08)70171-6. PubMed
6. Arevian AC, Jeffrey J, Young AS, Ong MK. Opportunities for flexible, on-demand care delivery through telemedicine. Psychiatr Serv. 2018;69(1):5-8. doi: 10.1176/appi.ps.201600589. PubMed
7. Ashwood JS, Mehrotra A, Cowling D, Uscher-Pines L. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Aff (Millwood). 2017;36(3):485-491. doi: 10.1377/hlthaff.2016.1130. PubMed
8. Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016;176(3):310-318. doi: 10.1001/jamainternmed.2015.7712. PubMed
9. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. doi: 10.1056/NEJMp0807461. PubMed
1. Why Digital Transformations Are Hard. Wall Street Journal. March 7, 2017, 2017.
2. Medicare Program: Revisions to Payment Policies under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; etc. In: Centers for Medicare & Medicaid Services, ed: Federal Register; 2018:1472.
3. Kuperman EF, Linson EL, Klefstad K, Perry E, Glenn K. The virtual hospitalist: a single-site implementation bringing hospitalist coverage to critical access hospitals. J Hosp Med. 2018. In Press. PubMed
4. Lilly CM, Cody S, Zhao H, et al. Hospital mortality, length of stay, and preventable complications among critically ill patients before and after tele-ICU reengineering of critical care processes. JAMA. 2011;305(21):2175-2183. doi: 10.1001/jama.2011.697. PubMed
5. Meyer BC, Raman R, Hemmen T, et al. Efficacy of site-independent telemedicine in the STRokE DOC trial: a randomised, blinded, prospective study. Lancet Neurol. 2008;7(9):787-795. doi: 10.1016/S1474-4422(08)70171-6. PubMed
6. Arevian AC, Jeffrey J, Young AS, Ong MK. Opportunities for flexible, on-demand care delivery through telemedicine. Psychiatr Serv. 2018;69(1):5-8. doi: 10.1176/appi.ps.201600589. PubMed
7. Ashwood JS, Mehrotra A, Cowling D, Uscher-Pines L. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Aff (Millwood). 2017;36(3):485-491. doi: 10.1377/hlthaff.2016.1130. PubMed
8. Ong MK, Romano PS, Edgington S, et al. Effectiveness of remote patient monitoring after discharge of hospitalized patients with heart failure: the better effectiveness after transition -- Heart Failure (BEAT-HF) Randomized Clinical Trial. JAMA Intern Med. 2016;176(3):310-318. doi: 10.1001/jamainternmed.2015.7712. PubMed
9. Verghese A. Culture shock--patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751. doi: 10.1056/NEJMp0807461. PubMed
© 2018 Society of Hospital Medicine
The Continued Quest for Pediatric Readmission Risk Prediction
While the use of pediatric readmission rates as a quality metric remains controversial, pediatric hospital-to-home transitions need improvement.1 As many as a third of pediatric readmissions are preventable,2 but the multifactorial and complex nature of factors that contribute to pediatric readmissions presents a challenge in tackling readmission. Several factors are associated with increased risk of readmission; these factors include both clinical and sociodemographic characteristics;3 however, we still have much to learn. Further, the only large trial of an intervention to prevent pediatric readmissions across all comers (nontargeted) was unsuccessful in decreasing reutilization.4 By contrast, various studies have succeeded in reducing readmission and/or emergency department revisit rates associated with inpatient interventions in select populations.5 Currently, however, no standardized or validated pediatric risk prediction tool can reliably identify the high-risk patients who may benefit from interventions. In the Journal of Hospital Medicine, Brittan and colleagues add to the literature base exploring the factors associated with an increased 30-day readmission risk by trialing an electronic health record (EHR)-based tool composed of three components: presence of home health, polypharmacy in the form of ≥6 medications, and presence of a caregiver who prefers a language other than English.6
This brief report contributes significantly to the literature. First, the presence of a tool embedded within the pediatric EHR and readily accessible at the point of clinical care is novel. Study authors purposefully chose components easily extractable from the EHR which update automatically. This infrastructure generates an automated score that is easily accessible to clinicians in real-time. Second, the transparency of the tool is notable given its display via the EHR’s “Discharge Readiness Report,” where a clinician can view not only the total composite score (1 point for each component) but also the specific components for which a point was allocated. Although a composite score in and of itself is potentially helpful, understanding specific factors that contribute to a patient’s increased risk of readmission allows for better targeting of interventions. For example, in Brittan’s simple, three-component model, the presence of polypharmacy might trigger a pharmacist to meet with the family prior to discharge to discuss indications for and how to properly administer medications. Finally, a multidisciplinary team composed of clinicians, nurse-family educators, case managers, social workers, and informatics experts developed and implemented this tool. Although the roll-out and longitudinal use of this tool is not described, the engagement of these multiple provider-types is likely to increase successful roll-out and utilization of the tool.
Unfortunately, the utility of this tool in predicting readmission is limited as evidenced by its low c-statistic. This limitation may be due to several reasons. The tool was not originally built as a tool to predict readmissions but rather an instrument to identify complex discharge care as part of a quality improvement initiative to improve discharge processes. Given the questions about readmission risk prediction, the authors explored the potential for the tool to predict readmission risk. The authors acknowledge that the tool excluded many known readmission risk factors based upon inconsistent documentation within the EHR and the desire to emphasize only modifiable factors. Thus, variables, including prior hospitalization which is a well-documented risk factor for readmissions (but not modifiable) and social determinants of health (which are not consistently documented), were excluded. Additionally, the included variable of “language preference” may have been a considerably broad characteristic. Limited English proficiency has been increasingly recognized as a construct placing patients at higher risk for adverse outcomes. However, caregivers with high English proficiency also exhibit varying degrees of health literacy. The inclusion of health literacy may be additive to a readmission risk prediction tool. Finally, the outcome is not well-described with regard to identification of “unplanned” events. Thus, their outcome measure may have included planned admissions for which the readmission risk prediction tool would be irrelevant.
In summary, Brittan and colleagues engaged a multidisciplinary group of providers to address discharge planning processes and leveraged the EHR to support their efforts in the form of a brief screening tool. Although this tool was not predictive of hospital readmissions, it highlights the opportunity to better utilize the EHR to gather meaningful, real-time data and subsequently use this information to positively impact our clinical care and allocation of resources. The tool should serve as a stepping stone to building a more extensive tool with inclusion of other known and potential readmission risk factors, thus resulting in a clinically relevant readmission risk prediction tool.
The authors have nothing to disclose.
1. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics 2015;136(6):e1539-1549. doi: 10.1542/peds.2015-2098. PubMed
2. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-Day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2). doi: 10.1542/peds.2015-4182. PubMed
3. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi: 10.1001/jama.2011.122. PubMed
4. Auger KA, Simmons JM, Tubbs-Cooley H, et al. Hospital to home outcomes (H2O) randomized trial of a post-discharge nurse home visit. Pediatrics. In press. PubMed
5. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. doi: 10.1002/jhm.2134. PubMed
6. Brittan MS, Martin SL, Anderson, Moss A,Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions [published online ahead of print August 29, 2018]. J Hosp Med. doi: 10.12788/jhm.3043. PubMed
While the use of pediatric readmission rates as a quality metric remains controversial, pediatric hospital-to-home transitions need improvement.1 As many as a third of pediatric readmissions are preventable,2 but the multifactorial and complex nature of factors that contribute to pediatric readmissions presents a challenge in tackling readmission. Several factors are associated with increased risk of readmission; these factors include both clinical and sociodemographic characteristics;3 however, we still have much to learn. Further, the only large trial of an intervention to prevent pediatric readmissions across all comers (nontargeted) was unsuccessful in decreasing reutilization.4 By contrast, various studies have succeeded in reducing readmission and/or emergency department revisit rates associated with inpatient interventions in select populations.5 Currently, however, no standardized or validated pediatric risk prediction tool can reliably identify the high-risk patients who may benefit from interventions. In the Journal of Hospital Medicine, Brittan and colleagues add to the literature base exploring the factors associated with an increased 30-day readmission risk by trialing an electronic health record (EHR)-based tool composed of three components: presence of home health, polypharmacy in the form of ≥6 medications, and presence of a caregiver who prefers a language other than English.6
This brief report contributes significantly to the literature. First, the presence of a tool embedded within the pediatric EHR and readily accessible at the point of clinical care is novel. Study authors purposefully chose components easily extractable from the EHR which update automatically. This infrastructure generates an automated score that is easily accessible to clinicians in real-time. Second, the transparency of the tool is notable given its display via the EHR’s “Discharge Readiness Report,” where a clinician can view not only the total composite score (1 point for each component) but also the specific components for which a point was allocated. Although a composite score in and of itself is potentially helpful, understanding specific factors that contribute to a patient’s increased risk of readmission allows for better targeting of interventions. For example, in Brittan’s simple, three-component model, the presence of polypharmacy might trigger a pharmacist to meet with the family prior to discharge to discuss indications for and how to properly administer medications. Finally, a multidisciplinary team composed of clinicians, nurse-family educators, case managers, social workers, and informatics experts developed and implemented this tool. Although the roll-out and longitudinal use of this tool is not described, the engagement of these multiple provider-types is likely to increase successful roll-out and utilization of the tool.
Unfortunately, the utility of this tool in predicting readmission is limited as evidenced by its low c-statistic. This limitation may be due to several reasons. The tool was not originally built as a tool to predict readmissions but rather an instrument to identify complex discharge care as part of a quality improvement initiative to improve discharge processes. Given the questions about readmission risk prediction, the authors explored the potential for the tool to predict readmission risk. The authors acknowledge that the tool excluded many known readmission risk factors based upon inconsistent documentation within the EHR and the desire to emphasize only modifiable factors. Thus, variables, including prior hospitalization which is a well-documented risk factor for readmissions (but not modifiable) and social determinants of health (which are not consistently documented), were excluded. Additionally, the included variable of “language preference” may have been a considerably broad characteristic. Limited English proficiency has been increasingly recognized as a construct placing patients at higher risk for adverse outcomes. However, caregivers with high English proficiency also exhibit varying degrees of health literacy. The inclusion of health literacy may be additive to a readmission risk prediction tool. Finally, the outcome is not well-described with regard to identification of “unplanned” events. Thus, their outcome measure may have included planned admissions for which the readmission risk prediction tool would be irrelevant.
In summary, Brittan and colleagues engaged a multidisciplinary group of providers to address discharge planning processes and leveraged the EHR to support their efforts in the form of a brief screening tool. Although this tool was not predictive of hospital readmissions, it highlights the opportunity to better utilize the EHR to gather meaningful, real-time data and subsequently use this information to positively impact our clinical care and allocation of resources. The tool should serve as a stepping stone to building a more extensive tool with inclusion of other known and potential readmission risk factors, thus resulting in a clinically relevant readmission risk prediction tool.
The authors have nothing to disclose.
While the use of pediatric readmission rates as a quality metric remains controversial, pediatric hospital-to-home transitions need improvement.1 As many as a third of pediatric readmissions are preventable,2 but the multifactorial and complex nature of factors that contribute to pediatric readmissions presents a challenge in tackling readmission. Several factors are associated with increased risk of readmission; these factors include both clinical and sociodemographic characteristics;3 however, we still have much to learn. Further, the only large trial of an intervention to prevent pediatric readmissions across all comers (nontargeted) was unsuccessful in decreasing reutilization.4 By contrast, various studies have succeeded in reducing readmission and/or emergency department revisit rates associated with inpatient interventions in select populations.5 Currently, however, no standardized or validated pediatric risk prediction tool can reliably identify the high-risk patients who may benefit from interventions. In the Journal of Hospital Medicine, Brittan and colleagues add to the literature base exploring the factors associated with an increased 30-day readmission risk by trialing an electronic health record (EHR)-based tool composed of three components: presence of home health, polypharmacy in the form of ≥6 medications, and presence of a caregiver who prefers a language other than English.6
This brief report contributes significantly to the literature. First, the presence of a tool embedded within the pediatric EHR and readily accessible at the point of clinical care is novel. Study authors purposefully chose components easily extractable from the EHR which update automatically. This infrastructure generates an automated score that is easily accessible to clinicians in real-time. Second, the transparency of the tool is notable given its display via the EHR’s “Discharge Readiness Report,” where a clinician can view not only the total composite score (1 point for each component) but also the specific components for which a point was allocated. Although a composite score in and of itself is potentially helpful, understanding specific factors that contribute to a patient’s increased risk of readmission allows for better targeting of interventions. For example, in Brittan’s simple, three-component model, the presence of polypharmacy might trigger a pharmacist to meet with the family prior to discharge to discuss indications for and how to properly administer medications. Finally, a multidisciplinary team composed of clinicians, nurse-family educators, case managers, social workers, and informatics experts developed and implemented this tool. Although the roll-out and longitudinal use of this tool is not described, the engagement of these multiple provider-types is likely to increase successful roll-out and utilization of the tool.
Unfortunately, the utility of this tool in predicting readmission is limited as evidenced by its low c-statistic. This limitation may be due to several reasons. The tool was not originally built as a tool to predict readmissions but rather an instrument to identify complex discharge care as part of a quality improvement initiative to improve discharge processes. Given the questions about readmission risk prediction, the authors explored the potential for the tool to predict readmission risk. The authors acknowledge that the tool excluded many known readmission risk factors based upon inconsistent documentation within the EHR and the desire to emphasize only modifiable factors. Thus, variables, including prior hospitalization which is a well-documented risk factor for readmissions (but not modifiable) and social determinants of health (which are not consistently documented), were excluded. Additionally, the included variable of “language preference” may have been a considerably broad characteristic. Limited English proficiency has been increasingly recognized as a construct placing patients at higher risk for adverse outcomes. However, caregivers with high English proficiency also exhibit varying degrees of health literacy. The inclusion of health literacy may be additive to a readmission risk prediction tool. Finally, the outcome is not well-described with regard to identification of “unplanned” events. Thus, their outcome measure may have included planned admissions for which the readmission risk prediction tool would be irrelevant.
In summary, Brittan and colleagues engaged a multidisciplinary group of providers to address discharge planning processes and leveraged the EHR to support their efforts in the form of a brief screening tool. Although this tool was not predictive of hospital readmissions, it highlights the opportunity to better utilize the EHR to gather meaningful, real-time data and subsequently use this information to positively impact our clinical care and allocation of resources. The tool should serve as a stepping stone to building a more extensive tool with inclusion of other known and potential readmission risk factors, thus resulting in a clinically relevant readmission risk prediction tool.
The authors have nothing to disclose.
1. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics 2015;136(6):e1539-1549. doi: 10.1542/peds.2015-2098. PubMed
2. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-Day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2). doi: 10.1542/peds.2015-4182. PubMed
3. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi: 10.1001/jama.2011.122. PubMed
4. Auger KA, Simmons JM, Tubbs-Cooley H, et al. Hospital to home outcomes (H2O) randomized trial of a post-discharge nurse home visit. Pediatrics. In press. PubMed
5. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. doi: 10.1002/jhm.2134. PubMed
6. Brittan MS, Martin SL, Anderson, Moss A,Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions [published online ahead of print August 29, 2018]. J Hosp Med. doi: 10.12788/jhm.3043. PubMed
1. Solan LG, Beck AF, Brunswick SA, et al. The family perspective on hospital to home transitions: a qualitative study. Pediatrics 2015;136(6):e1539-1549. doi: 10.1542/peds.2015-2098. PubMed
2. Toomey SL, Peltz A, Loren S, et al. Potentially preventable 30-Day hospital readmissions at a children’s hospital. Pediatrics. 2016;138(2). doi: 10.1542/peds.2015-4182. PubMed
3. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi: 10.1001/jama.2011.122. PubMed
4. Auger KA, Simmons JM, Tubbs-Cooley H, et al. Hospital to home outcomes (H2O) randomized trial of a post-discharge nurse home visit. Pediatrics. In press. PubMed
5. Auger KA, Kenyon CC, Feudtner C, Davis MM. Pediatric hospital discharge interventions to reduce subsequent utilization: a systematic review. J Hosp Med. 2014;9(4):251-260. doi: 10.1002/jhm.2134. PubMed
6. Brittan MS, Martin SL, Anderson, Moss A,Torok MR. An electronic health record tool designed to improve pediatric hospital discharge has low predictive utility for readmissions [published online ahead of print August 29, 2018]. J Hosp Med. doi: 10.12788/jhm.3043. PubMed
© 2018 Society of Hospital Medicine
Transcatheter aortic valve replacement for bicuspid aortic valve stenosis
Bicuspid aortic valve is the most common congenital cardiac abnormality in humans and is a significant risk factor for premature aortic valve dysfunction due to accelerated leaflet deterioration and calcification from altered hemodynamics. From 20% to 50% of patients with bicuspid aortic valve need aortic valve replacement during their lifetime, mostly for aortic stenosis.1,2
While 0.5% to 2% of the general population are born with a bicuspid aortic valve, more than 40% of patients (mainly younger patients) who undergo surgical or transcatheter intervention for aortic valve disease in some cohorts have this abnormality, suggesting that its true prevalence may be underreported.3
In the past decade, transcatheter aortic valve replacement (TAVR) has cemented its place as an option for patients with severe tricuspid aortic stenosis who cannot undergo surgery because their surgical risk is intermediate or high.4 However, most of the studies of balloon-expandable and self-expanding TAVR devices have excluded patients with bicuspid aortic valve.
BICUSPID AORTIC VALVE POSES CHALLENGES FOR TAVR
As TAVR is explored in younger and lower-risk populations, in which the prevalence of bicuspid aortic valve is presumably higher, the discussion of feasibility, safety, and efficacy of TAVR in patients with bicuspid aortic valve is both important and timely.
Bicuspid aortic valve is commonly categorized according to the Sievers classification,5 which describes 3 main morphologic types (designated types 0, 1, and 2) according to the number of raphes connecting the leaflets. Unique anatomic features of bicuspid aortic valve render the TAVR procedure challenging in these patients and merit consideration. These include, but are not limited to:
- Asymmetric calcification of the valve leaflets and calcified raphes. This results in asymmetric and incomplete expansion of the prosthesis, leading to incomplete sealing and paravalvular leak.
- A larger and more elliptically shaped aortic annulus, leading to challenges with proper sizing and apposition of the prosthesis
- Concomitant aortopathy, posing a higher risk of aortic rupture, dissection, paravalvular leak, and other complications during implantation.
Thus, compared with patients with tricuspid degenerative aortic stenosis, patients undergoing TAVR who have bicuspid aortic valve have a higher short-term risk of death and a higher risk of residual aortic regurgitation, and are more likely to need implantation of a second valve.
PARAVALVULAR LEAK
Paravalvular leak, arguably an independent marker of higher morbidity and mortality risk after TAVR, is more common in patients with bicuspid aortic valve undergoing TAVR than in those with tricuspid aortic valve. Earlier studies reported rates of moderate or severe paravalvular leak between 16% and 32%.6,7
The newer-generation balloon-expandable Sapien 3 valve (Edwards Lifesciences, Irvine, CA) is associated with a lower incidence of moderate or severe paravalvular leak than earlier devices, mainly attributable to its outer skirt, which allows more complete sealing.8 There are also reports of successful treatment of bicuspid aortic valve stenosis using the Lotus device (Boston Scientific, Marlborough, MA).9 This device features adaptive sealing along with retrievability and repositioning ability, which may facilitate optimal positioning and prevent paravalvular leak.
SIZING OF THE PROSTHESIS
Sizing of the prosthesis in patients with bicuspid aortic valve stenosis remains a challenge: some experts advocate the usual practice of measuring the perimeter and area at the level of the annulus, while others advocate measuring at the level of the commissures, 4 to 8 mm above the annulus. Balloon valvuloplasty may be a useful sizing tool, though it carries the hazards of severe aortic regurgitation and periprocedural stroke.
Angiography of the ascending aorta during balloon valvuloplasty can help verify whether an adequate seal is achievable and aid in selecting an appropriately sized prosthesis. Liu et al10 performed sequential balloon aortic valvuloplasty before TAVR with a self-expanding valve in 12 patients. Of these, 11 (91.7%) received a smaller device than they would have with multidetector computed tomography-guided annulus measurement.
Given that a larger valve may increase the risk of annular rupture, implantation of a smaller valve is always reasonable in bicuspid aortic valve as long as it achieves appropriate sealing with no paravalvular leak.
THE NEED FOR A PACEMAKER
After undergoing TAVR, more patients who have a bicuspid aortic valve need a permanent pacemaker than those who have a tricuspid aortic valve. This group appears to be more vulnerable to conduction abnormalities after TAVR, and rates of new pacemaker implantation as high as 25% have been reported with the newer-generation devices. Perlman et al8 observed that even when the Sapien 3 valve was implanted high in the left ventricular outflow tract, nearly 10% of patients needed a new pacemaker.
This is an important issue, as most patients with bicuspid aortic valve with severe aortic stenosis are relatively young and may endure deleterious effects from long-term pacing.
LONG-TERM OUTCOMES
The data on long-term outcomes of patients with bicuspid aortic valve who undergo TAVR are limited, and the available studies were small, with relatively short-term follow-up. However, Yoon et al compared TAVR outcomes between bicuspid and tricuspid aortic stenosis patients using propensity-score matching and demonstrated comparable all-cause mortality rates at 2 years (17.2% vs 19.4%, P = .28).6
Given the relatively long life expectancy of patients with bicuspid aortic valve undergoing TAVR, who tend to be younger than those with tricuspid aortic valve stenosis, longer-term data are needed to draw meaningful conclusions about the durability of transcatheter valves in this population. The bicuspid aortic valve is asymmetric, so that during TAVR the stent may not expand completely, and this in theory may result in more strain on the prosthesis and accelerate its structural deterioration.
In a recent meta-analysis, Reddy et al11 analyzed 13 observational studies in 758 patients with severe bicuspid aortic valve stenosis undergoing TAVR with older and newer devices. The mean Society of Thoracic Surgeons Predicted Risk of Mortality score, which predicts the risk of death within 30 days, was 5.0%, but the actual rate was 3.7% (95% confidence interval [CI] 2.1%–5.6%). A high procedural success rate of 95% (95% CI 90.2%–98.5%] was also noted, but the rates of new permanent pacemaker implantation (17.9%, 95% CI 14.2%–22%) and severe perivalvular leak (12.2%, 95% CI 3.1%–24.8%) were somewhat elevated.11
NOT FOR ALL, BUT AN EMERGING, VIABLE OPTION
As implanted prostheses and TAVR techniques undergo continuous improvement and as the experience of operators and institutions advances, procedural outcomes will likely improve.
The available data suggest that TAVR with the newer devices, when performed by experienced hands, is a viable option across most of the risk spectrum in patients with severe tricuspid aortic stenosis, including low-risk patients,12 and selectively in patients with bicuspid aortic valve stenosis. However, for patients with bicuspid aortic valve with severe aortic stenosis and associated aortopathy, surgery remains the standard of care.
More study is needed to identify patients with bicuspid aortic valve who can be safely and effectively treated with TAVR.
- Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000; 83(1):81–85. pmid:10618341
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Jilaihawi H, Wu Y, Yang Y, et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv 2015; 85(suppl 1):752–761. doi:10.1002/ccd.25863
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice. J Am Coll Cardiol 2017; 70(2):252–289. doi:10.1016/j.jacc.2017.03.011
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133(5):1226–1233. doi:10.1016/j.jtcvs.2007.01.039
- Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol 2017; 69(21):2579–2589. doi:10.1016/j.jacc.2017.03.017
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Perlman GY, Blanke P, Dvir D, et al. Bicuspid aortic valve stenosis: favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study. JACC Cardiovasc Interv 2016; 9(8):817–824. doi:10.1016/j.jcin.2016.01.002
- Chan AW, Wong D, Charania J. Transcatheter aortic valve replacement in bicuspid aortic stenosis using Lotus valve system. Catheter Cardiovasc Interv 2017; 90(1):157–163. doi:10.1002/ccd.26506
- Liu X, He Y, Zhu Q, et al. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter Cardiovasc Interv 2018; 91(5):986–994. doi:10.1002/ccd.27467
- Reddy G, Wang Z, Nishimura RA, et al. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: systematic review and meta analyses of observational studies. Catheter Cardiovasc Interv 2018; 91(5):975–983. doi:10.1002/ccd.27340
- Waksman R, Rogers T, Torguson R, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol 2018; pii:S0735-1097(18)36852–36859. Epub ahead of print. doi:10.1016/j.jacc.2018.08.1033
Bicuspid aortic valve is the most common congenital cardiac abnormality in humans and is a significant risk factor for premature aortic valve dysfunction due to accelerated leaflet deterioration and calcification from altered hemodynamics. From 20% to 50% of patients with bicuspid aortic valve need aortic valve replacement during their lifetime, mostly for aortic stenosis.1,2
While 0.5% to 2% of the general population are born with a bicuspid aortic valve, more than 40% of patients (mainly younger patients) who undergo surgical or transcatheter intervention for aortic valve disease in some cohorts have this abnormality, suggesting that its true prevalence may be underreported.3
In the past decade, transcatheter aortic valve replacement (TAVR) has cemented its place as an option for patients with severe tricuspid aortic stenosis who cannot undergo surgery because their surgical risk is intermediate or high.4 However, most of the studies of balloon-expandable and self-expanding TAVR devices have excluded patients with bicuspid aortic valve.
BICUSPID AORTIC VALVE POSES CHALLENGES FOR TAVR
As TAVR is explored in younger and lower-risk populations, in which the prevalence of bicuspid aortic valve is presumably higher, the discussion of feasibility, safety, and efficacy of TAVR in patients with bicuspid aortic valve is both important and timely.
Bicuspid aortic valve is commonly categorized according to the Sievers classification,5 which describes 3 main morphologic types (designated types 0, 1, and 2) according to the number of raphes connecting the leaflets. Unique anatomic features of bicuspid aortic valve render the TAVR procedure challenging in these patients and merit consideration. These include, but are not limited to:
- Asymmetric calcification of the valve leaflets and calcified raphes. This results in asymmetric and incomplete expansion of the prosthesis, leading to incomplete sealing and paravalvular leak.
- A larger and more elliptically shaped aortic annulus, leading to challenges with proper sizing and apposition of the prosthesis
- Concomitant aortopathy, posing a higher risk of aortic rupture, dissection, paravalvular leak, and other complications during implantation.
Thus, compared with patients with tricuspid degenerative aortic stenosis, patients undergoing TAVR who have bicuspid aortic valve have a higher short-term risk of death and a higher risk of residual aortic regurgitation, and are more likely to need implantation of a second valve.
PARAVALVULAR LEAK
Paravalvular leak, arguably an independent marker of higher morbidity and mortality risk after TAVR, is more common in patients with bicuspid aortic valve undergoing TAVR than in those with tricuspid aortic valve. Earlier studies reported rates of moderate or severe paravalvular leak between 16% and 32%.6,7
The newer-generation balloon-expandable Sapien 3 valve (Edwards Lifesciences, Irvine, CA) is associated with a lower incidence of moderate or severe paravalvular leak than earlier devices, mainly attributable to its outer skirt, which allows more complete sealing.8 There are also reports of successful treatment of bicuspid aortic valve stenosis using the Lotus device (Boston Scientific, Marlborough, MA).9 This device features adaptive sealing along with retrievability and repositioning ability, which may facilitate optimal positioning and prevent paravalvular leak.
SIZING OF THE PROSTHESIS
Sizing of the prosthesis in patients with bicuspid aortic valve stenosis remains a challenge: some experts advocate the usual practice of measuring the perimeter and area at the level of the annulus, while others advocate measuring at the level of the commissures, 4 to 8 mm above the annulus. Balloon valvuloplasty may be a useful sizing tool, though it carries the hazards of severe aortic regurgitation and periprocedural stroke.
Angiography of the ascending aorta during balloon valvuloplasty can help verify whether an adequate seal is achievable and aid in selecting an appropriately sized prosthesis. Liu et al10 performed sequential balloon aortic valvuloplasty before TAVR with a self-expanding valve in 12 patients. Of these, 11 (91.7%) received a smaller device than they would have with multidetector computed tomography-guided annulus measurement.
Given that a larger valve may increase the risk of annular rupture, implantation of a smaller valve is always reasonable in bicuspid aortic valve as long as it achieves appropriate sealing with no paravalvular leak.
THE NEED FOR A PACEMAKER
After undergoing TAVR, more patients who have a bicuspid aortic valve need a permanent pacemaker than those who have a tricuspid aortic valve. This group appears to be more vulnerable to conduction abnormalities after TAVR, and rates of new pacemaker implantation as high as 25% have been reported with the newer-generation devices. Perlman et al8 observed that even when the Sapien 3 valve was implanted high in the left ventricular outflow tract, nearly 10% of patients needed a new pacemaker.
This is an important issue, as most patients with bicuspid aortic valve with severe aortic stenosis are relatively young and may endure deleterious effects from long-term pacing.
LONG-TERM OUTCOMES
The data on long-term outcomes of patients with bicuspid aortic valve who undergo TAVR are limited, and the available studies were small, with relatively short-term follow-up. However, Yoon et al compared TAVR outcomes between bicuspid and tricuspid aortic stenosis patients using propensity-score matching and demonstrated comparable all-cause mortality rates at 2 years (17.2% vs 19.4%, P = .28).6
Given the relatively long life expectancy of patients with bicuspid aortic valve undergoing TAVR, who tend to be younger than those with tricuspid aortic valve stenosis, longer-term data are needed to draw meaningful conclusions about the durability of transcatheter valves in this population. The bicuspid aortic valve is asymmetric, so that during TAVR the stent may not expand completely, and this in theory may result in more strain on the prosthesis and accelerate its structural deterioration.
In a recent meta-analysis, Reddy et al11 analyzed 13 observational studies in 758 patients with severe bicuspid aortic valve stenosis undergoing TAVR with older and newer devices. The mean Society of Thoracic Surgeons Predicted Risk of Mortality score, which predicts the risk of death within 30 days, was 5.0%, but the actual rate was 3.7% (95% confidence interval [CI] 2.1%–5.6%). A high procedural success rate of 95% (95% CI 90.2%–98.5%] was also noted, but the rates of new permanent pacemaker implantation (17.9%, 95% CI 14.2%–22%) and severe perivalvular leak (12.2%, 95% CI 3.1%–24.8%) were somewhat elevated.11
NOT FOR ALL, BUT AN EMERGING, VIABLE OPTION
As implanted prostheses and TAVR techniques undergo continuous improvement and as the experience of operators and institutions advances, procedural outcomes will likely improve.
The available data suggest that TAVR with the newer devices, when performed by experienced hands, is a viable option across most of the risk spectrum in patients with severe tricuspid aortic stenosis, including low-risk patients,12 and selectively in patients with bicuspid aortic valve stenosis. However, for patients with bicuspid aortic valve with severe aortic stenosis and associated aortopathy, surgery remains the standard of care.
More study is needed to identify patients with bicuspid aortic valve who can be safely and effectively treated with TAVR.
Bicuspid aortic valve is the most common congenital cardiac abnormality in humans and is a significant risk factor for premature aortic valve dysfunction due to accelerated leaflet deterioration and calcification from altered hemodynamics. From 20% to 50% of patients with bicuspid aortic valve need aortic valve replacement during their lifetime, mostly for aortic stenosis.1,2
While 0.5% to 2% of the general population are born with a bicuspid aortic valve, more than 40% of patients (mainly younger patients) who undergo surgical or transcatheter intervention for aortic valve disease in some cohorts have this abnormality, suggesting that its true prevalence may be underreported.3
In the past decade, transcatheter aortic valve replacement (TAVR) has cemented its place as an option for patients with severe tricuspid aortic stenosis who cannot undergo surgery because their surgical risk is intermediate or high.4 However, most of the studies of balloon-expandable and self-expanding TAVR devices have excluded patients with bicuspid aortic valve.
BICUSPID AORTIC VALVE POSES CHALLENGES FOR TAVR
As TAVR is explored in younger and lower-risk populations, in which the prevalence of bicuspid aortic valve is presumably higher, the discussion of feasibility, safety, and efficacy of TAVR in patients with bicuspid aortic valve is both important and timely.
Bicuspid aortic valve is commonly categorized according to the Sievers classification,5 which describes 3 main morphologic types (designated types 0, 1, and 2) according to the number of raphes connecting the leaflets. Unique anatomic features of bicuspid aortic valve render the TAVR procedure challenging in these patients and merit consideration. These include, but are not limited to:
- Asymmetric calcification of the valve leaflets and calcified raphes. This results in asymmetric and incomplete expansion of the prosthesis, leading to incomplete sealing and paravalvular leak.
- A larger and more elliptically shaped aortic annulus, leading to challenges with proper sizing and apposition of the prosthesis
- Concomitant aortopathy, posing a higher risk of aortic rupture, dissection, paravalvular leak, and other complications during implantation.
Thus, compared with patients with tricuspid degenerative aortic stenosis, patients undergoing TAVR who have bicuspid aortic valve have a higher short-term risk of death and a higher risk of residual aortic regurgitation, and are more likely to need implantation of a second valve.
PARAVALVULAR LEAK
Paravalvular leak, arguably an independent marker of higher morbidity and mortality risk after TAVR, is more common in patients with bicuspid aortic valve undergoing TAVR than in those with tricuspid aortic valve. Earlier studies reported rates of moderate or severe paravalvular leak between 16% and 32%.6,7
The newer-generation balloon-expandable Sapien 3 valve (Edwards Lifesciences, Irvine, CA) is associated with a lower incidence of moderate or severe paravalvular leak than earlier devices, mainly attributable to its outer skirt, which allows more complete sealing.8 There are also reports of successful treatment of bicuspid aortic valve stenosis using the Lotus device (Boston Scientific, Marlborough, MA).9 This device features adaptive sealing along with retrievability and repositioning ability, which may facilitate optimal positioning and prevent paravalvular leak.
SIZING OF THE PROSTHESIS
Sizing of the prosthesis in patients with bicuspid aortic valve stenosis remains a challenge: some experts advocate the usual practice of measuring the perimeter and area at the level of the annulus, while others advocate measuring at the level of the commissures, 4 to 8 mm above the annulus. Balloon valvuloplasty may be a useful sizing tool, though it carries the hazards of severe aortic regurgitation and periprocedural stroke.
Angiography of the ascending aorta during balloon valvuloplasty can help verify whether an adequate seal is achievable and aid in selecting an appropriately sized prosthesis. Liu et al10 performed sequential balloon aortic valvuloplasty before TAVR with a self-expanding valve in 12 patients. Of these, 11 (91.7%) received a smaller device than they would have with multidetector computed tomography-guided annulus measurement.
Given that a larger valve may increase the risk of annular rupture, implantation of a smaller valve is always reasonable in bicuspid aortic valve as long as it achieves appropriate sealing with no paravalvular leak.
THE NEED FOR A PACEMAKER
After undergoing TAVR, more patients who have a bicuspid aortic valve need a permanent pacemaker than those who have a tricuspid aortic valve. This group appears to be more vulnerable to conduction abnormalities after TAVR, and rates of new pacemaker implantation as high as 25% have been reported with the newer-generation devices. Perlman et al8 observed that even when the Sapien 3 valve was implanted high in the left ventricular outflow tract, nearly 10% of patients needed a new pacemaker.
This is an important issue, as most patients with bicuspid aortic valve with severe aortic stenosis are relatively young and may endure deleterious effects from long-term pacing.
LONG-TERM OUTCOMES
The data on long-term outcomes of patients with bicuspid aortic valve who undergo TAVR are limited, and the available studies were small, with relatively short-term follow-up. However, Yoon et al compared TAVR outcomes between bicuspid and tricuspid aortic stenosis patients using propensity-score matching and demonstrated comparable all-cause mortality rates at 2 years (17.2% vs 19.4%, P = .28).6
Given the relatively long life expectancy of patients with bicuspid aortic valve undergoing TAVR, who tend to be younger than those with tricuspid aortic valve stenosis, longer-term data are needed to draw meaningful conclusions about the durability of transcatheter valves in this population. The bicuspid aortic valve is asymmetric, so that during TAVR the stent may not expand completely, and this in theory may result in more strain on the prosthesis and accelerate its structural deterioration.
In a recent meta-analysis, Reddy et al11 analyzed 13 observational studies in 758 patients with severe bicuspid aortic valve stenosis undergoing TAVR with older and newer devices. The mean Society of Thoracic Surgeons Predicted Risk of Mortality score, which predicts the risk of death within 30 days, was 5.0%, but the actual rate was 3.7% (95% confidence interval [CI] 2.1%–5.6%). A high procedural success rate of 95% (95% CI 90.2%–98.5%] was also noted, but the rates of new permanent pacemaker implantation (17.9%, 95% CI 14.2%–22%) and severe perivalvular leak (12.2%, 95% CI 3.1%–24.8%) were somewhat elevated.11
NOT FOR ALL, BUT AN EMERGING, VIABLE OPTION
As implanted prostheses and TAVR techniques undergo continuous improvement and as the experience of operators and institutions advances, procedural outcomes will likely improve.
The available data suggest that TAVR with the newer devices, when performed by experienced hands, is a viable option across most of the risk spectrum in patients with severe tricuspid aortic stenosis, including low-risk patients,12 and selectively in patients with bicuspid aortic valve stenosis. However, for patients with bicuspid aortic valve with severe aortic stenosis and associated aortopathy, surgery remains the standard of care.
More study is needed to identify patients with bicuspid aortic valve who can be safely and effectively treated with TAVR.
- Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000; 83(1):81–85. pmid:10618341
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Jilaihawi H, Wu Y, Yang Y, et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv 2015; 85(suppl 1):752–761. doi:10.1002/ccd.25863
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice. J Am Coll Cardiol 2017; 70(2):252–289. doi:10.1016/j.jacc.2017.03.011
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133(5):1226–1233. doi:10.1016/j.jtcvs.2007.01.039
- Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol 2017; 69(21):2579–2589. doi:10.1016/j.jacc.2017.03.017
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Perlman GY, Blanke P, Dvir D, et al. Bicuspid aortic valve stenosis: favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study. JACC Cardiovasc Interv 2016; 9(8):817–824. doi:10.1016/j.jcin.2016.01.002
- Chan AW, Wong D, Charania J. Transcatheter aortic valve replacement in bicuspid aortic stenosis using Lotus valve system. Catheter Cardiovasc Interv 2017; 90(1):157–163. doi:10.1002/ccd.26506
- Liu X, He Y, Zhu Q, et al. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter Cardiovasc Interv 2018; 91(5):986–994. doi:10.1002/ccd.27467
- Reddy G, Wang Z, Nishimura RA, et al. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: systematic review and meta analyses of observational studies. Catheter Cardiovasc Interv 2018; 91(5):975–983. doi:10.1002/ccd.27340
- Waksman R, Rogers T, Torguson R, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol 2018; pii:S0735-1097(18)36852–36859. Epub ahead of print. doi:10.1016/j.jacc.2018.08.1033
- Ward C. Clinical significance of the bicuspid aortic valve. Heart 2000; 83(1):81–85. pmid:10618341
- Michelena HI, Desjardins VA, Avierinos JF, et al. Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008; 117(21):2776–2784. doi:10.1161/CIRCULATIONAHA.107.740878
- Jilaihawi H, Wu Y, Yang Y, et al. Morphological characteristics of severe aortic stenosis in China: imaging corelab observations from the first Chinese transcatheter aortic valve trial. Catheter Cardiovasc Interv 2015; 85(suppl 1):752–761. doi:10.1002/ccd.25863
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice. J Am Coll Cardiol 2017; 70(2):252–289. doi:10.1016/j.jacc.2017.03.011
- Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg 2007; 133(5):1226–1233. doi:10.1016/j.jtcvs.2007.01.039
- Yoon SH, Bleiziffer S, De Backer O, et al. Outcomes in transcatheter aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Am Coll Cardiol 2017; 69(21):2579–2589. doi:10.1016/j.jacc.2017.03.017
- Mylotte D, Lefevre T, Sondergaard L, et al. Transcatheter aortic valve replacement in bicuspid aortic valve disease. J Am Coll Cardiol 2014; 64(22):2330–2339. doi:10.1016/j.jacc.2014.09.039
- Perlman GY, Blanke P, Dvir D, et al. Bicuspid aortic valve stenosis: favorable early outcomes with a next-generation transcatheter heart valve in a multicenter study. JACC Cardiovasc Interv 2016; 9(8):817–824. doi:10.1016/j.jcin.2016.01.002
- Chan AW, Wong D, Charania J. Transcatheter aortic valve replacement in bicuspid aortic stenosis using Lotus valve system. Catheter Cardiovasc Interv 2017; 90(1):157–163. doi:10.1002/ccd.26506
- Liu X, He Y, Zhu Q, et al. Supra-annular structure assessment for self-expanding transcatheter heart valve size selection in patients with bicuspid aortic valve. Catheter Cardiovasc Interv 2018; 91(5):986–994. doi:10.1002/ccd.27467
- Reddy G, Wang Z, Nishimura RA, et al. Transcatheter aortic valve replacement for stenotic bicuspid aortic valves: systematic review and meta analyses of observational studies. Catheter Cardiovasc Interv 2018; 91(5):975–983. doi:10.1002/ccd.27340
- Waksman R, Rogers T, Torguson R, et al. Transcatheter aortic valve replacement in low-risk patients with symptomatic severe aortic stenosis. J Am Coll Cardiol 2018; pii:S0735-1097(18)36852–36859. Epub ahead of print. doi:10.1016/j.jacc.2018.08.1033
Perioperative Management of ACE Inhibitor Therapy: Challenges of Clinical Decision-Making Based on Surrogate Endpoints
Renin-angiotensin inhibitors, which include angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), have demonstrated benefits in the treatment of several common cardiovascular and renal conditions. For example, they are prescribed to individuals with hypertension, heart failure with reduced ejection fraction (HFrEF), prior myocardial infarction, and chronic kidney disease with proteinuria. Perhaps unsurprisingly, many individuals presenting for surgery are already on long-term ACE inhibitor or ARB therapy. For example, such individuals comprised approximately one-third of the sample in the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) multicenter prospective cohort study of major inpatient noncardiac surgery.1
There is considerable controversy regarding how best to manage these cardiovascular medications during the perioperative period. The critical question pertains to whether renin-angiotensin inhibitors should be temporarily withdrawn 24 hours before surgery or continued uninterrupted up to the day of surgery. The main argument for withdrawing these medications is concern that they cause perioperative hypotension. For example, a recent systematic review of randomized controlled trials (RCTs) and cohort studies found that preoperative continuation of renin-angiotensin inhibitor therapy led to a significantly increased risk of intraoperative hypotension, albeit without associated effects on rates of death, major adverse cardiac events, or postoperative hypotension.2 Notably, randomized trial evidence in this meta-analysis was limited to only five trials with a total of 774 participants. Conversely, preoperative interruption of renin-angiotensin inhibitor therapy also has risks. For example, there is a potential for unintended permanent discontinuation of medications with long-term benefits.3 Furthermore, some prior cohort studies have demonstrated that the failure to resume renin-angiotensin inhibitor therapy promptly after surgery is associated with an elevated risk of postoperative mortality.4,5 While these studies have methodological limitations related to survivorship bias and unmeasured confounders, they still raise concerns that the abrupt withdrawal of long-term cardiovascular therapy before major surgery can have adverse effects. While ACE inhibitor withdrawal has not shown adverse physiological effects in the perioperative setting, it has led to rebound myocardial ischemia in patients with prior myocardial infarction.6
Given this controversy, there is variation across hospitals1 and practice guidelines with respect to perioperative management of renin-angiotensin inhibitors. For example, the 2017 Canadian Cardiovascular Society guidelines recommend that renin-angiotensin inhibitors be stopped temporarily 24 hours before major inpatient surgery,7 and the 2014 European guidelines recommend continuing therapy in patients with HFrEF but temporarily interrupting therapy in patients with hypertension.8 The 2014 American Heart Association and American College of Cardiology guidelines suggest that either continuation or interruption are reasonable options, but any interrupted therapy should be restarted postoperatively as soon as clinically feasible.9
In this issue of the Journal of Hospital Medicine, Shiffermiller and colleagues present a single-center RCT that provides additional high-quality data to improve our understanding of this important clinical issue.10 In a sample of 275 patients undergoing nonvascular inpatient noncardiac surgery, omission of the final dose of preoperative ACE inhibitor therapy reduced the risk of intraoperative hypotension across multiple definitions, including any episode of systolic blood pressure less than 80 mmHg (number needed to treat: 8), any episode of a systolic blood pressure less than 80 mmHg necessitating vasopressor therapy (number needed to treat: 6), and total cumulative duration of intraoperative systolic blood pressure less than 80 mmHg. In addition, the investigators found that preoperative interruption of ACE inhibitor therapy reduced the risk of postoperative hypotension (number needed to treat: 9), increased the risk of severe postoperative hypertension (number needed to harm: 9), and had no effect on clinical outcomes (eg, acute kidney injury, major adverse cardiac events). In conjunction with a recent systematic review,2 these new data demonstrate that temporary preoperative discontinuation of renin-angiotensin inhibitors leads to reduced risks of intraoperative and postoperative hypotension, with the only major identified risk being episodes of postoperative hypertension.
This current evidence base suggests that, in most cases, perioperative physicians should temporarily interrupt renin-angiotensin inhibitor therapy before inpatient noncardiac surgery, provided that protocols are in place to resume treatment postoperatively as soon as clinically feasible. Nonetheless, clinicians must also be cognizant of the key limitations to current data, namely that hypotension, be it intraoperative or postoperative, remains essentially a surrogate endpoint.11,12 Stated otherwise, the clinical importance of perioperative hypotension is largely predicated on its close association with clinically important or patient-relevant outcomes such as cardiovascular complications, acute kidney injury, and death.13–16 There is an implicit assumption that a reduction in the risk of hypotension will necessarily lead to reduced rates of clinical adverse events. This assumption is unlikely to be true, especially since many different underlying mechanisms lead to hypotension in the dynamic perioperative environment, including decreased cardiac contractility, decreased heart rate, decreased intravascular volume status, and vasodilation. Consistent with this possibility, different perioperative interventions with similar effects on hypotension have shown quite different effects on clinical outcomes. For example, epidural analgesia invariably reduces perioperative blood pressure, yet it does not appear to increase the risk of postoperative complications.17 Similarly, both beta-blockers and clonidine increase the risk of significant perioperative hypotension and bradycardia, yet only beta-blockers appear to lead to increased rates of mortality after noncardiac surgery.18,19 Thus, the relationship between perioperative hypotension and outcomes is clearly complex. Unless a RCT demonstrates that a hypotension-reduction strategy leads to an improvement in clinical outcomes,20 perioperative physicians should not assume that prevention of hypotension will always lead to improvements in patient-relevant clinical outcomes. Similar assumptions about other surrogate endpoints in cardiovascular medicine have sometimes been spectacularly incorrect.12,21 To more definitively address this important clinical issue, RCTs must be specifically designed to compare the effects of renin-angiotensin inhibitor therapy withdrawal versus continuation on patient-relevant and clinically important outcomes, such as death, myocardial infarction, and stroke. Fortunately, some ongoing trials will address this question, either directly (ClinicalTrials.gov NCT03374449) or as a component of a hypotension-avoidance strategy (ClinicalTrials.gov NCT03505723).
Overall, perioperative physicians should now adopt the standard approach of temporarily withdrawing renin-angiotensin inhibitor therapy 24 hours before major inpatient noncardiac surgery. Nonetheless, they should do so cautiously, recognizing that the data underpinning this strategy remain weak. As with many aspects of perioperative medicine, more research remains needed.
Disclosures
The authors have nothing to report.Funding: DNW is supported in part by a New Investigator Award from the Canadian Institutes of Health Research, and a Merit Award from the Department of Anesthesia at the University of Toronto.
1. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: An Analysis of the Vascular events In noncardiac Surgery patients cohort evaluation prospective cohort. Anesthesiology. 2017;126(1):16-27. doi: 10.1097/ALN.0000000000001404. PubMed
2. Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery [published online ahead of print January 29, 2018]. Anesth Analg. doi: 10.1213/ANE.0000000000002837. PubMed
3. Bell CM, Bajcar J, Bierman AS, Li P, Mamdani MM, Urbach DR. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Intern Med. 2006;166(22):2525-2531. doi: 10.1001/archinte.166.22.2525. PubMed
4. Mudumbai SC, Takemoto S, Cason BA, Au S, Upadhyay A, Wallace AW. Thirty-day mortality risk associated with the postoperative nonresumption of angiotensin-converting enzyme inhibitors: a retrospective study of the Veterans Affairs Healthcare System. J Hosp Med. 2014;9(5):289-296. doi: 10.1002/jhm.2182. PubMed
5. Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology. 2015;123(2):288-306. doi: 10.1097/ALN.0000000000000739. PubMed
6. van den Heuvel AF, van Gilst WH, van Veldhuisen DJ, de Vries RJ, Dunselman PH, Kingma JH. Long-term anti-ischemic effects of angiotensin-converting enzyme inhibition in patients after myocardial infarction. J Am Coll Cardiol. 1997;30(2):400-405. doi: 10.1016/S0735-1097(97)00183-6 PubMed
7. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. doi: 10.1016/j.cjca.2016.09.008. PubMed
8. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management./ The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. doi: 10.1093/eurheartj/ehu282. PubMed
9. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):e278-e333. doi: 10.1161/CIR.0000000000000105. PubMed
10. Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin converting enzyme inhibition (PREOP-ACEI) [published online ahead of print July 25, 2018]. J Hosp Med. doi: 10.12788/jhm.3036. PubMed
11. Psaty BM, Weiss NS, Furberg CD, et al. Surrogate end points, health outcomes, and the drug-approval process for the treatment of risk factors for cardiovascular disease. JAMA. 1999;282(8):786-790. doi: 10.1001/jama.282.8.786. PubMed
12. Vanderweele TJ. Surrogate measures and consistent surrogates. Biometrics. 2013;69(3):561-569. doi: 10.1111/biom.12071. PubMed
13. Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515-523. doi: 10.1097/ALN.0000000000000765. PubMed
14. van Waes JA, van Klei WA, Wijeysundera DN, van Wolfswinkel L, Lindsay TF, Beattie WS. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124(1):35-44. doi: 10.1097/ALN.0000000000000922. PubMed
15. Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47-65. doi: 10.1097/ALN.0000000000001432. PubMed
16. Monk TG, Bronsert MR, Henderson WG, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123(2):307-319.doi: 10.1097/ALN.0000000000000756. PubMed
17. Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359(9314):1276-1282. doi: 10.1016/S0140-6736(02)08266-1. PubMed
18. POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839-1847. doi: 10.1016/S0140-6736(08)60601-7. PubMed
19. Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1504-1513. doi: 10.1056/NEJMoa1401106. PubMed
20. Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346-1357. doi: 10.1001/jama.2017.14172. PubMed
21. Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321(6):406-412. doi:10.1056/NEJM198908103210629 PubMed
Renin-angiotensin inhibitors, which include angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), have demonstrated benefits in the treatment of several common cardiovascular and renal conditions. For example, they are prescribed to individuals with hypertension, heart failure with reduced ejection fraction (HFrEF), prior myocardial infarction, and chronic kidney disease with proteinuria. Perhaps unsurprisingly, many individuals presenting for surgery are already on long-term ACE inhibitor or ARB therapy. For example, such individuals comprised approximately one-third of the sample in the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) multicenter prospective cohort study of major inpatient noncardiac surgery.1
There is considerable controversy regarding how best to manage these cardiovascular medications during the perioperative period. The critical question pertains to whether renin-angiotensin inhibitors should be temporarily withdrawn 24 hours before surgery or continued uninterrupted up to the day of surgery. The main argument for withdrawing these medications is concern that they cause perioperative hypotension. For example, a recent systematic review of randomized controlled trials (RCTs) and cohort studies found that preoperative continuation of renin-angiotensin inhibitor therapy led to a significantly increased risk of intraoperative hypotension, albeit without associated effects on rates of death, major adverse cardiac events, or postoperative hypotension.2 Notably, randomized trial evidence in this meta-analysis was limited to only five trials with a total of 774 participants. Conversely, preoperative interruption of renin-angiotensin inhibitor therapy also has risks. For example, there is a potential for unintended permanent discontinuation of medications with long-term benefits.3 Furthermore, some prior cohort studies have demonstrated that the failure to resume renin-angiotensin inhibitor therapy promptly after surgery is associated with an elevated risk of postoperative mortality.4,5 While these studies have methodological limitations related to survivorship bias and unmeasured confounders, they still raise concerns that the abrupt withdrawal of long-term cardiovascular therapy before major surgery can have adverse effects. While ACE inhibitor withdrawal has not shown adverse physiological effects in the perioperative setting, it has led to rebound myocardial ischemia in patients with prior myocardial infarction.6
Given this controversy, there is variation across hospitals1 and practice guidelines with respect to perioperative management of renin-angiotensin inhibitors. For example, the 2017 Canadian Cardiovascular Society guidelines recommend that renin-angiotensin inhibitors be stopped temporarily 24 hours before major inpatient surgery,7 and the 2014 European guidelines recommend continuing therapy in patients with HFrEF but temporarily interrupting therapy in patients with hypertension.8 The 2014 American Heart Association and American College of Cardiology guidelines suggest that either continuation or interruption are reasonable options, but any interrupted therapy should be restarted postoperatively as soon as clinically feasible.9
In this issue of the Journal of Hospital Medicine, Shiffermiller and colleagues present a single-center RCT that provides additional high-quality data to improve our understanding of this important clinical issue.10 In a sample of 275 patients undergoing nonvascular inpatient noncardiac surgery, omission of the final dose of preoperative ACE inhibitor therapy reduced the risk of intraoperative hypotension across multiple definitions, including any episode of systolic blood pressure less than 80 mmHg (number needed to treat: 8), any episode of a systolic blood pressure less than 80 mmHg necessitating vasopressor therapy (number needed to treat: 6), and total cumulative duration of intraoperative systolic blood pressure less than 80 mmHg. In addition, the investigators found that preoperative interruption of ACE inhibitor therapy reduced the risk of postoperative hypotension (number needed to treat: 9), increased the risk of severe postoperative hypertension (number needed to harm: 9), and had no effect on clinical outcomes (eg, acute kidney injury, major adverse cardiac events). In conjunction with a recent systematic review,2 these new data demonstrate that temporary preoperative discontinuation of renin-angiotensin inhibitors leads to reduced risks of intraoperative and postoperative hypotension, with the only major identified risk being episodes of postoperative hypertension.
This current evidence base suggests that, in most cases, perioperative physicians should temporarily interrupt renin-angiotensin inhibitor therapy before inpatient noncardiac surgery, provided that protocols are in place to resume treatment postoperatively as soon as clinically feasible. Nonetheless, clinicians must also be cognizant of the key limitations to current data, namely that hypotension, be it intraoperative or postoperative, remains essentially a surrogate endpoint.11,12 Stated otherwise, the clinical importance of perioperative hypotension is largely predicated on its close association with clinically important or patient-relevant outcomes such as cardiovascular complications, acute kidney injury, and death.13–16 There is an implicit assumption that a reduction in the risk of hypotension will necessarily lead to reduced rates of clinical adverse events. This assumption is unlikely to be true, especially since many different underlying mechanisms lead to hypotension in the dynamic perioperative environment, including decreased cardiac contractility, decreased heart rate, decreased intravascular volume status, and vasodilation. Consistent with this possibility, different perioperative interventions with similar effects on hypotension have shown quite different effects on clinical outcomes. For example, epidural analgesia invariably reduces perioperative blood pressure, yet it does not appear to increase the risk of postoperative complications.17 Similarly, both beta-blockers and clonidine increase the risk of significant perioperative hypotension and bradycardia, yet only beta-blockers appear to lead to increased rates of mortality after noncardiac surgery.18,19 Thus, the relationship between perioperative hypotension and outcomes is clearly complex. Unless a RCT demonstrates that a hypotension-reduction strategy leads to an improvement in clinical outcomes,20 perioperative physicians should not assume that prevention of hypotension will always lead to improvements in patient-relevant clinical outcomes. Similar assumptions about other surrogate endpoints in cardiovascular medicine have sometimes been spectacularly incorrect.12,21 To more definitively address this important clinical issue, RCTs must be specifically designed to compare the effects of renin-angiotensin inhibitor therapy withdrawal versus continuation on patient-relevant and clinically important outcomes, such as death, myocardial infarction, and stroke. Fortunately, some ongoing trials will address this question, either directly (ClinicalTrials.gov NCT03374449) or as a component of a hypotension-avoidance strategy (ClinicalTrials.gov NCT03505723).
Overall, perioperative physicians should now adopt the standard approach of temporarily withdrawing renin-angiotensin inhibitor therapy 24 hours before major inpatient noncardiac surgery. Nonetheless, they should do so cautiously, recognizing that the data underpinning this strategy remain weak. As with many aspects of perioperative medicine, more research remains needed.
Disclosures
The authors have nothing to report.Funding: DNW is supported in part by a New Investigator Award from the Canadian Institutes of Health Research, and a Merit Award from the Department of Anesthesia at the University of Toronto.
Renin-angiotensin inhibitors, which include angiotensin-converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), have demonstrated benefits in the treatment of several common cardiovascular and renal conditions. For example, they are prescribed to individuals with hypertension, heart failure with reduced ejection fraction (HFrEF), prior myocardial infarction, and chronic kidney disease with proteinuria. Perhaps unsurprisingly, many individuals presenting for surgery are already on long-term ACE inhibitor or ARB therapy. For example, such individuals comprised approximately one-third of the sample in the Vascular Events In Noncardiac Surgery Patients Cohort Evaluation (VISION) multicenter prospective cohort study of major inpatient noncardiac surgery.1
There is considerable controversy regarding how best to manage these cardiovascular medications during the perioperative period. The critical question pertains to whether renin-angiotensin inhibitors should be temporarily withdrawn 24 hours before surgery or continued uninterrupted up to the day of surgery. The main argument for withdrawing these medications is concern that they cause perioperative hypotension. For example, a recent systematic review of randomized controlled trials (RCTs) and cohort studies found that preoperative continuation of renin-angiotensin inhibitor therapy led to a significantly increased risk of intraoperative hypotension, albeit without associated effects on rates of death, major adverse cardiac events, or postoperative hypotension.2 Notably, randomized trial evidence in this meta-analysis was limited to only five trials with a total of 774 participants. Conversely, preoperative interruption of renin-angiotensin inhibitor therapy also has risks. For example, there is a potential for unintended permanent discontinuation of medications with long-term benefits.3 Furthermore, some prior cohort studies have demonstrated that the failure to resume renin-angiotensin inhibitor therapy promptly after surgery is associated with an elevated risk of postoperative mortality.4,5 While these studies have methodological limitations related to survivorship bias and unmeasured confounders, they still raise concerns that the abrupt withdrawal of long-term cardiovascular therapy before major surgery can have adverse effects. While ACE inhibitor withdrawal has not shown adverse physiological effects in the perioperative setting, it has led to rebound myocardial ischemia in patients with prior myocardial infarction.6
Given this controversy, there is variation across hospitals1 and practice guidelines with respect to perioperative management of renin-angiotensin inhibitors. For example, the 2017 Canadian Cardiovascular Society guidelines recommend that renin-angiotensin inhibitors be stopped temporarily 24 hours before major inpatient surgery,7 and the 2014 European guidelines recommend continuing therapy in patients with HFrEF but temporarily interrupting therapy in patients with hypertension.8 The 2014 American Heart Association and American College of Cardiology guidelines suggest that either continuation or interruption are reasonable options, but any interrupted therapy should be restarted postoperatively as soon as clinically feasible.9
In this issue of the Journal of Hospital Medicine, Shiffermiller and colleagues present a single-center RCT that provides additional high-quality data to improve our understanding of this important clinical issue.10 In a sample of 275 patients undergoing nonvascular inpatient noncardiac surgery, omission of the final dose of preoperative ACE inhibitor therapy reduced the risk of intraoperative hypotension across multiple definitions, including any episode of systolic blood pressure less than 80 mmHg (number needed to treat: 8), any episode of a systolic blood pressure less than 80 mmHg necessitating vasopressor therapy (number needed to treat: 6), and total cumulative duration of intraoperative systolic blood pressure less than 80 mmHg. In addition, the investigators found that preoperative interruption of ACE inhibitor therapy reduced the risk of postoperative hypotension (number needed to treat: 9), increased the risk of severe postoperative hypertension (number needed to harm: 9), and had no effect on clinical outcomes (eg, acute kidney injury, major adverse cardiac events). In conjunction with a recent systematic review,2 these new data demonstrate that temporary preoperative discontinuation of renin-angiotensin inhibitors leads to reduced risks of intraoperative and postoperative hypotension, with the only major identified risk being episodes of postoperative hypertension.
This current evidence base suggests that, in most cases, perioperative physicians should temporarily interrupt renin-angiotensin inhibitor therapy before inpatient noncardiac surgery, provided that protocols are in place to resume treatment postoperatively as soon as clinically feasible. Nonetheless, clinicians must also be cognizant of the key limitations to current data, namely that hypotension, be it intraoperative or postoperative, remains essentially a surrogate endpoint.11,12 Stated otherwise, the clinical importance of perioperative hypotension is largely predicated on its close association with clinically important or patient-relevant outcomes such as cardiovascular complications, acute kidney injury, and death.13–16 There is an implicit assumption that a reduction in the risk of hypotension will necessarily lead to reduced rates of clinical adverse events. This assumption is unlikely to be true, especially since many different underlying mechanisms lead to hypotension in the dynamic perioperative environment, including decreased cardiac contractility, decreased heart rate, decreased intravascular volume status, and vasodilation. Consistent with this possibility, different perioperative interventions with similar effects on hypotension have shown quite different effects on clinical outcomes. For example, epidural analgesia invariably reduces perioperative blood pressure, yet it does not appear to increase the risk of postoperative complications.17 Similarly, both beta-blockers and clonidine increase the risk of significant perioperative hypotension and bradycardia, yet only beta-blockers appear to lead to increased rates of mortality after noncardiac surgery.18,19 Thus, the relationship between perioperative hypotension and outcomes is clearly complex. Unless a RCT demonstrates that a hypotension-reduction strategy leads to an improvement in clinical outcomes,20 perioperative physicians should not assume that prevention of hypotension will always lead to improvements in patient-relevant clinical outcomes. Similar assumptions about other surrogate endpoints in cardiovascular medicine have sometimes been spectacularly incorrect.12,21 To more definitively address this important clinical issue, RCTs must be specifically designed to compare the effects of renin-angiotensin inhibitor therapy withdrawal versus continuation on patient-relevant and clinically important outcomes, such as death, myocardial infarction, and stroke. Fortunately, some ongoing trials will address this question, either directly (ClinicalTrials.gov NCT03374449) or as a component of a hypotension-avoidance strategy (ClinicalTrials.gov NCT03505723).
Overall, perioperative physicians should now adopt the standard approach of temporarily withdrawing renin-angiotensin inhibitor therapy 24 hours before major inpatient noncardiac surgery. Nonetheless, they should do so cautiously, recognizing that the data underpinning this strategy remain weak. As with many aspects of perioperative medicine, more research remains needed.
Disclosures
The authors have nothing to report.Funding: DNW is supported in part by a New Investigator Award from the Canadian Institutes of Health Research, and a Merit Award from the Department of Anesthesia at the University of Toronto.
1. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: An Analysis of the Vascular events In noncardiac Surgery patients cohort evaluation prospective cohort. Anesthesiology. 2017;126(1):16-27. doi: 10.1097/ALN.0000000000001404. PubMed
2. Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery [published online ahead of print January 29, 2018]. Anesth Analg. doi: 10.1213/ANE.0000000000002837. PubMed
3. Bell CM, Bajcar J, Bierman AS, Li P, Mamdani MM, Urbach DR. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Intern Med. 2006;166(22):2525-2531. doi: 10.1001/archinte.166.22.2525. PubMed
4. Mudumbai SC, Takemoto S, Cason BA, Au S, Upadhyay A, Wallace AW. Thirty-day mortality risk associated with the postoperative nonresumption of angiotensin-converting enzyme inhibitors: a retrospective study of the Veterans Affairs Healthcare System. J Hosp Med. 2014;9(5):289-296. doi: 10.1002/jhm.2182. PubMed
5. Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology. 2015;123(2):288-306. doi: 10.1097/ALN.0000000000000739. PubMed
6. van den Heuvel AF, van Gilst WH, van Veldhuisen DJ, de Vries RJ, Dunselman PH, Kingma JH. Long-term anti-ischemic effects of angiotensin-converting enzyme inhibition in patients after myocardial infarction. J Am Coll Cardiol. 1997;30(2):400-405. doi: 10.1016/S0735-1097(97)00183-6 PubMed
7. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. doi: 10.1016/j.cjca.2016.09.008. PubMed
8. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management./ The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. doi: 10.1093/eurheartj/ehu282. PubMed
9. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):e278-e333. doi: 10.1161/CIR.0000000000000105. PubMed
10. Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin converting enzyme inhibition (PREOP-ACEI) [published online ahead of print July 25, 2018]. J Hosp Med. doi: 10.12788/jhm.3036. PubMed
11. Psaty BM, Weiss NS, Furberg CD, et al. Surrogate end points, health outcomes, and the drug-approval process for the treatment of risk factors for cardiovascular disease. JAMA. 1999;282(8):786-790. doi: 10.1001/jama.282.8.786. PubMed
12. Vanderweele TJ. Surrogate measures and consistent surrogates. Biometrics. 2013;69(3):561-569. doi: 10.1111/biom.12071. PubMed
13. Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515-523. doi: 10.1097/ALN.0000000000000765. PubMed
14. van Waes JA, van Klei WA, Wijeysundera DN, van Wolfswinkel L, Lindsay TF, Beattie WS. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124(1):35-44. doi: 10.1097/ALN.0000000000000922. PubMed
15. Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47-65. doi: 10.1097/ALN.0000000000001432. PubMed
16. Monk TG, Bronsert MR, Henderson WG, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123(2):307-319.doi: 10.1097/ALN.0000000000000756. PubMed
17. Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359(9314):1276-1282. doi: 10.1016/S0140-6736(02)08266-1. PubMed
18. POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839-1847. doi: 10.1016/S0140-6736(08)60601-7. PubMed
19. Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1504-1513. doi: 10.1056/NEJMoa1401106. PubMed
20. Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346-1357. doi: 10.1001/jama.2017.14172. PubMed
21. Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321(6):406-412. doi:10.1056/NEJM198908103210629 PubMed
1. Roshanov PS, Rochwerg B, Patel A, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: An Analysis of the Vascular events In noncardiac Surgery patients cohort evaluation prospective cohort. Anesthesiology. 2017;126(1):16-27. doi: 10.1097/ALN.0000000000001404. PubMed
2. Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery [published online ahead of print January 29, 2018]. Anesth Analg. doi: 10.1213/ANE.0000000000002837. PubMed
3. Bell CM, Bajcar J, Bierman AS, Li P, Mamdani MM, Urbach DR. Potentially unintended discontinuation of long-term medication use after elective surgical procedures. Arch Intern Med. 2006;166(22):2525-2531. doi: 10.1001/archinte.166.22.2525. PubMed
4. Mudumbai SC, Takemoto S, Cason BA, Au S, Upadhyay A, Wallace AW. Thirty-day mortality risk associated with the postoperative nonresumption of angiotensin-converting enzyme inhibitors: a retrospective study of the Veterans Affairs Healthcare System. J Hosp Med. 2014;9(5):289-296. doi: 10.1002/jhm.2182. PubMed
5. Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the Veterans Affairs Healthcare System. Anesthesiology. 2015;123(2):288-306. doi: 10.1097/ALN.0000000000000739. PubMed
6. van den Heuvel AF, van Gilst WH, van Veldhuisen DJ, de Vries RJ, Dunselman PH, Kingma JH. Long-term anti-ischemic effects of angiotensin-converting enzyme inhibition in patients after myocardial infarction. J Am Coll Cardiol. 1997;30(2):400-405. doi: 10.1016/S0735-1097(97)00183-6 PubMed
7. Duceppe E, Parlow J, MacDonald P, et al. Canadian Cardiovascular Society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33(1):17-32. doi: 10.1016/j.cjca.2016.09.008. PubMed
8. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management./ The Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur Heart J. 2014;35(35):2383-2431. doi: 10.1093/eurheartj/ehu282. PubMed
9. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(24):e278-e333. doi: 10.1161/CIR.0000000000000105. PubMed
10. Shiffermiller JF, Monson BJ, Vokoun CW, et al. Prospective randomized evaluation of preoperative angiotensin converting enzyme inhibition (PREOP-ACEI) [published online ahead of print July 25, 2018]. J Hosp Med. doi: 10.12788/jhm.3036. PubMed
11. Psaty BM, Weiss NS, Furberg CD, et al. Surrogate end points, health outcomes, and the drug-approval process for the treatment of risk factors for cardiovascular disease. JAMA. 1999;282(8):786-790. doi: 10.1001/jama.282.8.786. PubMed
12. Vanderweele TJ. Surrogate measures and consistent surrogates. Biometrics. 2013;69(3):561-569. doi: 10.1111/biom.12071. PubMed
13. Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515-523. doi: 10.1097/ALN.0000000000000765. PubMed
14. van Waes JA, van Klei WA, Wijeysundera DN, van Wolfswinkel L, Lindsay TF, Beattie WS. Association between intraoperative hypotension and myocardial injury after vascular surgery. Anesthesiology. 2016;124(1):35-44. doi: 10.1097/ALN.0000000000000922. PubMed
15. Salmasi V, Maheshwari K, Yang D, et al. Relationship between intraoperative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology. 2017;126(1):47-65. doi: 10.1097/ALN.0000000000001432. PubMed
16. Monk TG, Bronsert MR, Henderson WG, et al. Association between intraoperative hypotension and hypertension and 30-day postoperative mortality in noncardiac surgery. Anesthesiology. 2015;123(2):307-319.doi: 10.1097/ALN.0000000000000756. PubMed
17. Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet. 2002;359(9314):1276-1282. doi: 10.1016/S0140-6736(02)08266-1. PubMed
18. POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet. 2008;371(9627):1839-1847. doi: 10.1016/S0140-6736(08)60601-7. PubMed
19. Devereaux PJ, Sessler DI, Leslie K, et al. Clonidine in patients undergoing noncardiac surgery. N Engl J Med. 2014;370(16):1504-1513. doi: 10.1056/NEJMoa1401106. PubMed
20. Futier E, Lefrant JY, Guinot PG, et al. Effect of individualized vs standard blood pressure management strategies on postoperative organ dysfunction among high-risk patients undergoing major surgery: a randomized clinical trial. JAMA. 2017;318(14):1346-1357. doi: 10.1001/jama.2017.14172. PubMed
21. Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321(6):406-412. doi:10.1056/NEJM198908103210629 PubMed
© 2018 Society of Hospital Medicine
Coronary artery calcium scoring: A valuable tool in primary care
In 1984, Jim Fixx, who wrote The Complete Book of Running,1 went out for his daily run and died of a massive heart attack. He was 48. Unbeknownst to him, he had 3-vessel coronary artery disease.
His case illustrates the difficulty of diagnosing coronary artery disease in patients who have no symptoms of it. For many, the initial presentation is myocardial infarction or death. Until recently, there was no reliable way to diagnose subclinical coronary artery disease other than angiography, and there is still no way to rule it out. As a result, physicians have concentrated less on diagnosing subclinical disease and more on assessing the risk of myocardial infarction.
ASSESSING RISK
The risk factors for coronary artery disease (age, male sex, smoking, hypertension, and cholesterol) have been well known for half a century. By combining risk factors with the appropriate weighting, it is possible to predict an individual’s risk of a myocardial infarction.
In 2013, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines applied this risk-based approach to prescribing statins for primary prevention.2 Instead of focusing on low-density lipoprotein cholesterol concentration, which by itself is a poor predictor of myocardial infarction, they recommended using the Pooled Cohort Equation3 to determine the risk of a cardiovascular event within 10 years. For patients at high risk (> 7.5%), the benefits of a statin generally outweigh the harms. For those at low risk (< 5%), the opposite is true. For patients in between, there is room for shared decision-making.
Debate has focused on the predictive accuracy of the equation, the threshold for treatment, and the fact that almost all men over 60 qualify for treatment.4 These objections stem from the focus on risk rather than on diagnosis of the underlying disease.
Because one-third of “high-risk” patients never develop cardiovascular disease,5 the risk-based approach necessitates overtreatment. Those without disease cannot benefit from treatment but nonetheless suffer its side effects, cost, and inconvenience. Raising treatment thresholds (eg, treating only patients whose 10-year risk exceeds 10%) improves the ratio of patients with disease to those without but also misses diseased patients who have few risk factors. “Low risk” is not “no risk.”
TESTING FOR DISEASE IN THOSE AT INTERMEDIATE RISK
Diagnostic testing is preferred if such testing is safe and inexpensive.
In this issue of Cleveland Clinic Journal of Medicine, Parikh and colleagues6 review coronary artery calcium scoring, a diagnostic test for coronary artery disease. They conclude that calcium scoring is strongly predictive but should be reserved for patients at intermediate risk to help them decide about treatment. This is clearly the right approach, but the authors leave the term “intermediate” undefined, and their clinical examples offer little guidance as to where the borders lie.
The ACC/AHA guidelines specify a narrow intermediate range (5.0%–7.4%). For these patients, calcium scoring could reclassify most as being at high or low risk, helping to clarify whether statins are indicated.
However, only 12% of patients fall into this category.7 What about patients at higher risk? Could they be reclassified as being at low risk if their calcium score was 0?8 Conversely, could some low-risk patients discover that they are at high risk and perhaps take action?
The ACC/AHA guidelines recommend against calcium scoring in these circumstances. One concern was that calcium scoring had not been tested with the Pooled Cohort Equation. Another concern related to cost and radiation exposure, but as Parikh et al point out, the cost has now fallen to less than $100, and radiation exposure is similar to that with mammography.
SHOULD WE TEST PATIENTS AT HIGH OR LOW RISK?
Who, then, should we test? For patients at high or low risk according to the Pooled Cohort Equation, 2 questions determine whether calcium scoring is warranted: how much would an extremely high or low score (ie, 0 or > 400) change the risk of an event, and how likely is an extreme score?
The first question relates to the usefulness of the test, the second to its cost-effectiveness. If even an extreme score cannot move a patient’s risk into or out of the treatment range, then testing is unwarranted. At the same time, if few patients have an extreme score, then cost per test that changes practice will be high.
Because calcium scoring is a direct test for disease, it is extremely predictive. When added to risk-factor models, it substantially improves discrimination9 and exhibits excellent calibration.10 This is true whether the outcome is a major cardiovascular event or death from any cause.
But the calcium score is not strong enough to override all other risk factors. A patient with a predicted 10-year risk of 18% according to the Pooled Cohort Equation and a calcium score of 0 could be reclassified as being at low risk, but a patient with a 10-year predicted risk of 35% could not. The same is true for patients at low risk. A patient with a 4% risk and a calcium score higher than 400 would be reclassified as being at high risk, but not a patient with a 1% risk.
Extreme calcium scores are common, especially in patients at high risk. In the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, 45% of patients with a 10-year predicted risk of 7.5% to 20% had a calcium score of 0, reclassifying them into the low-risk category.11 Even if the predicted risk was greater than 20%, 1 in 4 patients had a score of 0. In contrast, if the 10-year predicted risk was below 5%, one-fifth of patients had a calcium score greater than 0, but only 4% had a score greater than 100.
Nevertheless, patients in the low-risk category whose baseline risk is close to 5% may wish to undergo calcium scoring, because a positive test opens the door to a potentially lifesaving treatment. In general, the closer patients are to the treatment threshold, the more likely they are to be reclassified by calcium scoring.
The Society for Cardiovascular Computed Tomography currently recommends coronary artery calcium scoring for patients whose 10-year risk is between 5% and 20%.12 These numbers are easy to remember and a reasonable approximation of the number of patients likely to benefit from testing.
COMBINING CALCIUM SCORING WITH TRADITIONAL RISK FACTORS
Primary care physicians interested in more exact personalized medicine can use a risk calculator derived from the MESA cohort.13 Based on 10-year outcomes for 6,814 participants, Blaha et al8 derived and validated this risk-prediction tool incorporating all the elements of the Pooled Cohort Equation in addition to family history, race, and calcium score.
The tool offered good discrimination and calibration when validated against 2 external cohorts (the Heinz Nixdorf Recall Study and the Dallas Heart Study).10 The C statistics were 0.78 and 0.82, with 10-year risk predicted by the tool within half a percent of the observed event rate in each cohort.
The online calculator displays the 10-year risk based on risk factors alone or including a calcium score, allowing the clinician to gauge the value of testing. For example, a 70-year-old nonsmoking white man with a total cholesterol level of 240 mg/dL, high-density lipoprotein cholesterol 40 mg/dL, and systolic blood pressure 130 mm Hg on amlodipine has a 15.2% 10-year risk (well above the 7.5% threshold for statin therapy). However, if his calcium score is 0, his risk falls to 4.3% (well below the threshold). Sharing such information with patients could help them to decide whether to undergo coronary artery calcium scoring.
Ultimately, the decision to take a statin for primary prevention of coronary artery disease is a personal one. It involves weighing risks, benefits, and preferences. Physicians can facilitate the process by providing information and guidance. Patients are best served by having the most accurate information. In many cases, that information should include calcium scoring.
- Fixx JF. The Complete Book of Running. New York: Random House, 1977.
- Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(25 suppl 2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
- American Heart Association, American College of Cardiology. 2013 Prevention guidelines tools. CV risk calculator. ASCVD risk calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_ASCVD-Risk-Calculator.jsp. Accessed August 17, 2018.
- Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014; 370(15):1422–1431. doi:10.1056/NEJMoa1315665
- Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012; 308(17):1795–1801. doi:10.1001/jama.2012.14312
- Parikh P, Shah N, Ahmed H, Schoenhagen P, Fares M. Coronary artery calcium scoring: its practicality and clinical utility in primary care. Cleve Clin J Med 2018; 85(9):707–716. doi:10.3949/ccjm.85a.17097
- Blaha MJ, Dardari ZA, Blumenthal RS, Martin SS, Nasir K, Al-Mallah MH. The new “intermediate risk” group: a comparative analysis of the new 2013 ACC/AHA risk assessment guidelines versus prior guidelines in men. Atherosclerosis 2014; 237(1):1–4. doi:10.1016/j.atherosclerosis.2014.08.024
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016; 133(9):849–858. doi:10.1161/CIRCULATIONAHA.115.018524
- Peters SAE, den Ruijter HM, Bots ML, Moons KGM. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart 2012; 98(3):177–184. doi:10.1136/heartjnl-2011-300747
- McClelland RL, Jorgensen NW, Budoff M, et al. Ten-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the Multi-Ethnic Study of Atherosclerosis with validation in the Heinz Nixdorf Recall Study and the Dallas Heart Study. J Am Coll Cardiol 2015; 66(15):1643–1653. doi:10.1016/j.jacc.2015.08.035
- Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015; 66(15):1657–1668. doi:10.1016/j.jacc.2015.07.066
- Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017; 11(2):157–168. doi:10.1016/j.jcct.2017.02.010
- MESA. The Multi-Ethnic Study of Atherosclerosis. MESA 10-year CHD risk with coronary artery calcification. www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx. Accessed August 17, 2018.
In 1984, Jim Fixx, who wrote The Complete Book of Running,1 went out for his daily run and died of a massive heart attack. He was 48. Unbeknownst to him, he had 3-vessel coronary artery disease.
His case illustrates the difficulty of diagnosing coronary artery disease in patients who have no symptoms of it. For many, the initial presentation is myocardial infarction or death. Until recently, there was no reliable way to diagnose subclinical coronary artery disease other than angiography, and there is still no way to rule it out. As a result, physicians have concentrated less on diagnosing subclinical disease and more on assessing the risk of myocardial infarction.
ASSESSING RISK
The risk factors for coronary artery disease (age, male sex, smoking, hypertension, and cholesterol) have been well known for half a century. By combining risk factors with the appropriate weighting, it is possible to predict an individual’s risk of a myocardial infarction.
In 2013, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines applied this risk-based approach to prescribing statins for primary prevention.2 Instead of focusing on low-density lipoprotein cholesterol concentration, which by itself is a poor predictor of myocardial infarction, they recommended using the Pooled Cohort Equation3 to determine the risk of a cardiovascular event within 10 years. For patients at high risk (> 7.5%), the benefits of a statin generally outweigh the harms. For those at low risk (< 5%), the opposite is true. For patients in between, there is room for shared decision-making.
Debate has focused on the predictive accuracy of the equation, the threshold for treatment, and the fact that almost all men over 60 qualify for treatment.4 These objections stem from the focus on risk rather than on diagnosis of the underlying disease.
Because one-third of “high-risk” patients never develop cardiovascular disease,5 the risk-based approach necessitates overtreatment. Those without disease cannot benefit from treatment but nonetheless suffer its side effects, cost, and inconvenience. Raising treatment thresholds (eg, treating only patients whose 10-year risk exceeds 10%) improves the ratio of patients with disease to those without but also misses diseased patients who have few risk factors. “Low risk” is not “no risk.”
TESTING FOR DISEASE IN THOSE AT INTERMEDIATE RISK
Diagnostic testing is preferred if such testing is safe and inexpensive.
In this issue of Cleveland Clinic Journal of Medicine, Parikh and colleagues6 review coronary artery calcium scoring, a diagnostic test for coronary artery disease. They conclude that calcium scoring is strongly predictive but should be reserved for patients at intermediate risk to help them decide about treatment. This is clearly the right approach, but the authors leave the term “intermediate” undefined, and their clinical examples offer little guidance as to where the borders lie.
The ACC/AHA guidelines specify a narrow intermediate range (5.0%–7.4%). For these patients, calcium scoring could reclassify most as being at high or low risk, helping to clarify whether statins are indicated.
However, only 12% of patients fall into this category.7 What about patients at higher risk? Could they be reclassified as being at low risk if their calcium score was 0?8 Conversely, could some low-risk patients discover that they are at high risk and perhaps take action?
The ACC/AHA guidelines recommend against calcium scoring in these circumstances. One concern was that calcium scoring had not been tested with the Pooled Cohort Equation. Another concern related to cost and radiation exposure, but as Parikh et al point out, the cost has now fallen to less than $100, and radiation exposure is similar to that with mammography.
SHOULD WE TEST PATIENTS AT HIGH OR LOW RISK?
Who, then, should we test? For patients at high or low risk according to the Pooled Cohort Equation, 2 questions determine whether calcium scoring is warranted: how much would an extremely high or low score (ie, 0 or > 400) change the risk of an event, and how likely is an extreme score?
The first question relates to the usefulness of the test, the second to its cost-effectiveness. If even an extreme score cannot move a patient’s risk into or out of the treatment range, then testing is unwarranted. At the same time, if few patients have an extreme score, then cost per test that changes practice will be high.
Because calcium scoring is a direct test for disease, it is extremely predictive. When added to risk-factor models, it substantially improves discrimination9 and exhibits excellent calibration.10 This is true whether the outcome is a major cardiovascular event or death from any cause.
But the calcium score is not strong enough to override all other risk factors. A patient with a predicted 10-year risk of 18% according to the Pooled Cohort Equation and a calcium score of 0 could be reclassified as being at low risk, but a patient with a 10-year predicted risk of 35% could not. The same is true for patients at low risk. A patient with a 4% risk and a calcium score higher than 400 would be reclassified as being at high risk, but not a patient with a 1% risk.
Extreme calcium scores are common, especially in patients at high risk. In the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, 45% of patients with a 10-year predicted risk of 7.5% to 20% had a calcium score of 0, reclassifying them into the low-risk category.11 Even if the predicted risk was greater than 20%, 1 in 4 patients had a score of 0. In contrast, if the 10-year predicted risk was below 5%, one-fifth of patients had a calcium score greater than 0, but only 4% had a score greater than 100.
Nevertheless, patients in the low-risk category whose baseline risk is close to 5% may wish to undergo calcium scoring, because a positive test opens the door to a potentially lifesaving treatment. In general, the closer patients are to the treatment threshold, the more likely they are to be reclassified by calcium scoring.
The Society for Cardiovascular Computed Tomography currently recommends coronary artery calcium scoring for patients whose 10-year risk is between 5% and 20%.12 These numbers are easy to remember and a reasonable approximation of the number of patients likely to benefit from testing.
COMBINING CALCIUM SCORING WITH TRADITIONAL RISK FACTORS
Primary care physicians interested in more exact personalized medicine can use a risk calculator derived from the MESA cohort.13 Based on 10-year outcomes for 6,814 participants, Blaha et al8 derived and validated this risk-prediction tool incorporating all the elements of the Pooled Cohort Equation in addition to family history, race, and calcium score.
The tool offered good discrimination and calibration when validated against 2 external cohorts (the Heinz Nixdorf Recall Study and the Dallas Heart Study).10 The C statistics were 0.78 and 0.82, with 10-year risk predicted by the tool within half a percent of the observed event rate in each cohort.
The online calculator displays the 10-year risk based on risk factors alone or including a calcium score, allowing the clinician to gauge the value of testing. For example, a 70-year-old nonsmoking white man with a total cholesterol level of 240 mg/dL, high-density lipoprotein cholesterol 40 mg/dL, and systolic blood pressure 130 mm Hg on amlodipine has a 15.2% 10-year risk (well above the 7.5% threshold for statin therapy). However, if his calcium score is 0, his risk falls to 4.3% (well below the threshold). Sharing such information with patients could help them to decide whether to undergo coronary artery calcium scoring.
Ultimately, the decision to take a statin for primary prevention of coronary artery disease is a personal one. It involves weighing risks, benefits, and preferences. Physicians can facilitate the process by providing information and guidance. Patients are best served by having the most accurate information. In many cases, that information should include calcium scoring.
In 1984, Jim Fixx, who wrote The Complete Book of Running,1 went out for his daily run and died of a massive heart attack. He was 48. Unbeknownst to him, he had 3-vessel coronary artery disease.
His case illustrates the difficulty of diagnosing coronary artery disease in patients who have no symptoms of it. For many, the initial presentation is myocardial infarction or death. Until recently, there was no reliable way to diagnose subclinical coronary artery disease other than angiography, and there is still no way to rule it out. As a result, physicians have concentrated less on diagnosing subclinical disease and more on assessing the risk of myocardial infarction.
ASSESSING RISK
The risk factors for coronary artery disease (age, male sex, smoking, hypertension, and cholesterol) have been well known for half a century. By combining risk factors with the appropriate weighting, it is possible to predict an individual’s risk of a myocardial infarction.
In 2013, the American College of Cardiology/American Heart Association (ACC/AHA) guidelines applied this risk-based approach to prescribing statins for primary prevention.2 Instead of focusing on low-density lipoprotein cholesterol concentration, which by itself is a poor predictor of myocardial infarction, they recommended using the Pooled Cohort Equation3 to determine the risk of a cardiovascular event within 10 years. For patients at high risk (> 7.5%), the benefits of a statin generally outweigh the harms. For those at low risk (< 5%), the opposite is true. For patients in between, there is room for shared decision-making.
Debate has focused on the predictive accuracy of the equation, the threshold for treatment, and the fact that almost all men over 60 qualify for treatment.4 These objections stem from the focus on risk rather than on diagnosis of the underlying disease.
Because one-third of “high-risk” patients never develop cardiovascular disease,5 the risk-based approach necessitates overtreatment. Those without disease cannot benefit from treatment but nonetheless suffer its side effects, cost, and inconvenience. Raising treatment thresholds (eg, treating only patients whose 10-year risk exceeds 10%) improves the ratio of patients with disease to those without but also misses diseased patients who have few risk factors. “Low risk” is not “no risk.”
TESTING FOR DISEASE IN THOSE AT INTERMEDIATE RISK
Diagnostic testing is preferred if such testing is safe and inexpensive.
In this issue of Cleveland Clinic Journal of Medicine, Parikh and colleagues6 review coronary artery calcium scoring, a diagnostic test for coronary artery disease. They conclude that calcium scoring is strongly predictive but should be reserved for patients at intermediate risk to help them decide about treatment. This is clearly the right approach, but the authors leave the term “intermediate” undefined, and their clinical examples offer little guidance as to where the borders lie.
The ACC/AHA guidelines specify a narrow intermediate range (5.0%–7.4%). For these patients, calcium scoring could reclassify most as being at high or low risk, helping to clarify whether statins are indicated.
However, only 12% of patients fall into this category.7 What about patients at higher risk? Could they be reclassified as being at low risk if their calcium score was 0?8 Conversely, could some low-risk patients discover that they are at high risk and perhaps take action?
The ACC/AHA guidelines recommend against calcium scoring in these circumstances. One concern was that calcium scoring had not been tested with the Pooled Cohort Equation. Another concern related to cost and radiation exposure, but as Parikh et al point out, the cost has now fallen to less than $100, and radiation exposure is similar to that with mammography.
SHOULD WE TEST PATIENTS AT HIGH OR LOW RISK?
Who, then, should we test? For patients at high or low risk according to the Pooled Cohort Equation, 2 questions determine whether calcium scoring is warranted: how much would an extremely high or low score (ie, 0 or > 400) change the risk of an event, and how likely is an extreme score?
The first question relates to the usefulness of the test, the second to its cost-effectiveness. If even an extreme score cannot move a patient’s risk into or out of the treatment range, then testing is unwarranted. At the same time, if few patients have an extreme score, then cost per test that changes practice will be high.
Because calcium scoring is a direct test for disease, it is extremely predictive. When added to risk-factor models, it substantially improves discrimination9 and exhibits excellent calibration.10 This is true whether the outcome is a major cardiovascular event or death from any cause.
But the calcium score is not strong enough to override all other risk factors. A patient with a predicted 10-year risk of 18% according to the Pooled Cohort Equation and a calcium score of 0 could be reclassified as being at low risk, but a patient with a 10-year predicted risk of 35% could not. The same is true for patients at low risk. A patient with a 4% risk and a calcium score higher than 400 would be reclassified as being at high risk, but not a patient with a 1% risk.
Extreme calcium scores are common, especially in patients at high risk. In the Multi-Ethnic Study of Atherosclerosis (MESA) cohort, 45% of patients with a 10-year predicted risk of 7.5% to 20% had a calcium score of 0, reclassifying them into the low-risk category.11 Even if the predicted risk was greater than 20%, 1 in 4 patients had a score of 0. In contrast, if the 10-year predicted risk was below 5%, one-fifth of patients had a calcium score greater than 0, but only 4% had a score greater than 100.
Nevertheless, patients in the low-risk category whose baseline risk is close to 5% may wish to undergo calcium scoring, because a positive test opens the door to a potentially lifesaving treatment. In general, the closer patients are to the treatment threshold, the more likely they are to be reclassified by calcium scoring.
The Society for Cardiovascular Computed Tomography currently recommends coronary artery calcium scoring for patients whose 10-year risk is between 5% and 20%.12 These numbers are easy to remember and a reasonable approximation of the number of patients likely to benefit from testing.
COMBINING CALCIUM SCORING WITH TRADITIONAL RISK FACTORS
Primary care physicians interested in more exact personalized medicine can use a risk calculator derived from the MESA cohort.13 Based on 10-year outcomes for 6,814 participants, Blaha et al8 derived and validated this risk-prediction tool incorporating all the elements of the Pooled Cohort Equation in addition to family history, race, and calcium score.
The tool offered good discrimination and calibration when validated against 2 external cohorts (the Heinz Nixdorf Recall Study and the Dallas Heart Study).10 The C statistics were 0.78 and 0.82, with 10-year risk predicted by the tool within half a percent of the observed event rate in each cohort.
The online calculator displays the 10-year risk based on risk factors alone or including a calcium score, allowing the clinician to gauge the value of testing. For example, a 70-year-old nonsmoking white man with a total cholesterol level of 240 mg/dL, high-density lipoprotein cholesterol 40 mg/dL, and systolic blood pressure 130 mm Hg on amlodipine has a 15.2% 10-year risk (well above the 7.5% threshold for statin therapy). However, if his calcium score is 0, his risk falls to 4.3% (well below the threshold). Sharing such information with patients could help them to decide whether to undergo coronary artery calcium scoring.
Ultimately, the decision to take a statin for primary prevention of coronary artery disease is a personal one. It involves weighing risks, benefits, and preferences. Physicians can facilitate the process by providing information and guidance. Patients are best served by having the most accurate information. In many cases, that information should include calcium scoring.
- Fixx JF. The Complete Book of Running. New York: Random House, 1977.
- Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(25 suppl 2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
- American Heart Association, American College of Cardiology. 2013 Prevention guidelines tools. CV risk calculator. ASCVD risk calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_ASCVD-Risk-Calculator.jsp. Accessed August 17, 2018.
- Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014; 370(15):1422–1431. doi:10.1056/NEJMoa1315665
- Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012; 308(17):1795–1801. doi:10.1001/jama.2012.14312
- Parikh P, Shah N, Ahmed H, Schoenhagen P, Fares M. Coronary artery calcium scoring: its practicality and clinical utility in primary care. Cleve Clin J Med 2018; 85(9):707–716. doi:10.3949/ccjm.85a.17097
- Blaha MJ, Dardari ZA, Blumenthal RS, Martin SS, Nasir K, Al-Mallah MH. The new “intermediate risk” group: a comparative analysis of the new 2013 ACC/AHA risk assessment guidelines versus prior guidelines in men. Atherosclerosis 2014; 237(1):1–4. doi:10.1016/j.atherosclerosis.2014.08.024
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016; 133(9):849–858. doi:10.1161/CIRCULATIONAHA.115.018524
- Peters SAE, den Ruijter HM, Bots ML, Moons KGM. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart 2012; 98(3):177–184. doi:10.1136/heartjnl-2011-300747
- McClelland RL, Jorgensen NW, Budoff M, et al. Ten-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the Multi-Ethnic Study of Atherosclerosis with validation in the Heinz Nixdorf Recall Study and the Dallas Heart Study. J Am Coll Cardiol 2015; 66(15):1643–1653. doi:10.1016/j.jacc.2015.08.035
- Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015; 66(15):1657–1668. doi:10.1016/j.jacc.2015.07.066
- Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017; 11(2):157–168. doi:10.1016/j.jcct.2017.02.010
- MESA. The Multi-Ethnic Study of Atherosclerosis. MESA 10-year CHD risk with coronary artery calcification. www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx. Accessed August 17, 2018.
- Fixx JF. The Complete Book of Running. New York: Random House, 1977.
- Goff DC, Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129(25 suppl 2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
- American Heart Association, American College of Cardiology. 2013 Prevention guidelines tools. CV risk calculator. ASCVD risk calculator. https://professional.heart.org/professional/GuidelinesStatements/PreventionGuidelines/UCM_457698_ASCVD-Risk-Calculator.jsp. Accessed August 17, 2018.
- Pencina MJ, Navar-Boggan AM, D’Agostino RB, Sr, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014; 370(15):1422–1431. doi:10.1056/NEJMoa1315665
- Wilkins JT, Ning H, Berry J, Zhao L, Dyer AR, Lloyd-Jones DM. Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012; 308(17):1795–1801. doi:10.1001/jama.2012.14312
- Parikh P, Shah N, Ahmed H, Schoenhagen P, Fares M. Coronary artery calcium scoring: its practicality and clinical utility in primary care. Cleve Clin J Med 2018; 85(9):707–716. doi:10.3949/ccjm.85a.17097
- Blaha MJ, Dardari ZA, Blumenthal RS, Martin SS, Nasir K, Al-Mallah MH. The new “intermediate risk” group: a comparative analysis of the new 2013 ACC/AHA risk assessment guidelines versus prior guidelines in men. Atherosclerosis 2014; 237(1):1–4. doi:10.1016/j.atherosclerosis.2014.08.024
- Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2016; 133(9):849–858. doi:10.1161/CIRCULATIONAHA.115.018524
- Peters SAE, den Ruijter HM, Bots ML, Moons KGM. Improvements in risk stratification for the occurrence of cardiovascular disease by imaging subclinical atherosclerosis: a systematic review. Heart 2012; 98(3):177–184. doi:10.1136/heartjnl-2011-300747
- McClelland RL, Jorgensen NW, Budoff M, et al. Ten-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the Multi-Ethnic Study of Atherosclerosis with validation in the Heinz Nixdorf Recall Study and the Dallas Heart Study. J Am Coll Cardiol 2015; 66(15):1643–1653. doi:10.1016/j.jacc.2015.08.035
- Nasir K, Bittencourt MS, Blaha MJ, et al. Implications of coronary artery calcium testing among statin candidates according to American College of Cardiology/American Heart Association cholesterol management guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol 2015; 66(15):1657–1668. doi:10.1016/j.jacc.2015.07.066
- Hecht H, Blaha MJ, Berman DS, et al. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr 2017; 11(2):157–168. doi:10.1016/j.jcct.2017.02.010
- MESA. The Multi-Ethnic Study of Atherosclerosis. MESA 10-year CHD risk with coronary artery calcification. www.mesa-nhlbi.org/MESACHDRisk/MesaRiskScore/RiskScore.aspx. Accessed August 17, 2018.
Postdischarge Emergency Department Visits: Good, Bad, or Ugly?
Once upon a time, discharges were easy to categorize: good, bad, or ugly. Good discharges allowed the patient to leave before noon, while bad discharges allowed the patient to leave without follow-up appointments. The worst discharges were defined by the two ugly cousins of acute care re-escalation: return emergency department (ED) visits and readmissions. Recently, however, much of this conventional wisdom has been turned on its head. For example, pre-noon discharges and provider-scheduled follow-up appointments may lead to unintended negative consequences and futility.1,2 In contrast, weekend discharges, which were often viewed to be unsafe, may reduce lengths of stay without compromising care even in high-risk patients.3
Having obfuscated the line between good and bad, we can now turn our attention to the ugly. Comparing return ED visits with readmissions, hospitalists may be forgiven for judging the latter cousin as uglier – and not just for reimbursement reasons. Readmitted patients are sicker, more vulnerable, and have poorer outcomes. In our healthcare system’s resultant quest to eliminate readmissions, return ED visits that do not end in readmission are generally either ignored or grouped with readmissions. Ignoring these treat-and-discharge ED visits is problematic because of their incidence, which rivals that of ED visits ending in readmission.4 On the other hand, grouping these visits with readmissions only makes sense if the two are considered to be equally ugly outcomes. Is this a valid assumption to make?
In this issue of the Journal of Hospital Medicine, Venkatesh et al5 tackle that question by studying Medicare beneficiaries hospitalized for acute myocardial infarction, heart failure, or pneumonia over a 1-year period. The authors differentiate 30-day treat-and-discharge ED visit rates from 30-day readmission rates before risk-standardizing these rates based on visit codes and hospital characteristics. Similar to the results of prior studies, the authors observe an 8%–9% overall incidence of treat-and-discharge ED visits within 30 days of hospital discharge.6 Mapping treat-and-discharge ED visit rates versus readmission rates for each hospital, the authors detect modest but noticeable inverse correlations between the two. Among hospitals discharging heart failure patients, for example, every 10% increase in postdischarge ED visit rates corresponds to a roughly 2% decrease in readmission rates.
The authors are correct to tread cautiously with their interpretation of this correlation. Dispositions for ED patients exist on a continuum, so hospitals with higher propensities to discharge patients from EDs (whether directly or from observation units) will inherently have lower admission rates. The authors hint at a causal relationship nonetheless, suggesting that ED providers may be able to intervene on high-risk patients earlier before Readmission Road becomes a one-way street. Proving this hypothesis will require careful research that controls for patient, disease, and ED factors as well as their complex interactions in the post-discharge timeline. That being said, most analyses of outpatient follow-up visits (except for heart failure patients) have failed to find any anti-readmission correlation analogous to that identified by Venkatesh et al. What powers do ED providers have that outpatient providers lack? Many, admittedly: stat phlebotomy services, on-demand consultations, and observation units. Additionally, while ED visits invariably require a patient’s presence in person, 25% of provider-scheduled posthospitalization outpatient visits end in no-shows.2 Whether patient-triggered follow-up through rapid access clinics or even urgent care centers can replicate ED functionality in recently discharged patients is unknown and warrants further study.
Venkatesh et al5 also find that reasons for postdischarge ED visits bear only a slight resemblance to reasons for index hospitalizations. For example, of all ED visits by patients recovering from hospitalizations for pneumonia, only 20% involve respiratory or pulmonary complaints. What explains the other 80%? Some variability may be attributable to the study’s use of visit codes instead of chart reviews or stakeholder interviews; in surveys of patients and ED physicians during these postdischarge visits, the two groups may have very different perceptions of why the encounter is occurring and whether it is preventable.7 Regardless of who is “right,” the heterogeneity of reasons that prompt care re-escalation lends further credence to the existence of a distinct posthospitalization syndrome:8 in the immediate postdischarge interval, patients experience many transient but real physiological risks for which they may identify the ED as their best recourse.
Whether the ED actually provides secondary prophylaxis against the posthospitalization syndrome is highly debatable, and Venkatesh et al wisely refrain from assigning a positive or negative valence to treat-and-discharge ED visits. Ultimately, postdischarge ED visits are neither inherently good nor bad (nor ugly, for that matter). Their unique nature is attracting newfound appreciation, and their potential ability to prevent readmission merits further research. If hospitals with high postdischarge ED visit rates can deliver high-quality care while truly arresting or reversing readmission-bound trajectories, then the strategies employed by these hospitals should inspire emulation, innovation, and dissemination.
Disclosures
The authors have nothing to disclose.
1. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. 10.1002/jhm.2529. PubMed
2. Banerjee R, Suarez A, Kier M, et al. If you book it, will they come? Attendance at postdischarge follow-up visits scheduled by inpatient providers. J Hosp Med. 2017;12(8):618-625. 10.12788/jhm.2777. PubMed
3. McAlister FA, Youngson E, Padwal RS, Majumdar SR. Similar outcomes among general medicine patients discharged on weekends. J Hosp Med. 2015;10(2):69-74. 10.1002/jhm.2310. PubMed
4. Rising KL, White LF, Fernandez WG, Boutwell AE. Emergency department visits after hospital discharge: a missing part of the equation. Ann Emerg Med. 2013;62(2):145-150. 10.1016/j.annemergmed.2013.01.024. PubMed
5. Venkatesh A, Wang C, Wang Y, Altaf F, Bernheim S, Horwitz L. Association between post-discharge emergency department visitation and readmission rates J Hosp Med. 2018;13(9):589-594. doi: 10.12788/jhm.2937.
6. Vashi AA, Fox JP, Carr BG, et al. Use of hospital-based acute care among patients recently discharged from the hospital. JAMA. 2013;309(4):364-371. 10.1001/jama.2012.216219. PubMed
7. Suffoletto B, Hu J, Guyette M, Callaway C. Factors contributing to emergency department care within 30 days of hospital discharge and potential ways to prevent it: differences in perspectives of patients, caregivers, and emergency physicians. J Hosp Med. 2014;9(5):315-319. 10.1002/jhm.2167. PubMed
8. Krumholz HM. Post-hospital syndrome: an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. 10.1056/NEJMp1212324. PubMed
Once upon a time, discharges were easy to categorize: good, bad, or ugly. Good discharges allowed the patient to leave before noon, while bad discharges allowed the patient to leave without follow-up appointments. The worst discharges were defined by the two ugly cousins of acute care re-escalation: return emergency department (ED) visits and readmissions. Recently, however, much of this conventional wisdom has been turned on its head. For example, pre-noon discharges and provider-scheduled follow-up appointments may lead to unintended negative consequences and futility.1,2 In contrast, weekend discharges, which were often viewed to be unsafe, may reduce lengths of stay without compromising care even in high-risk patients.3
Having obfuscated the line between good and bad, we can now turn our attention to the ugly. Comparing return ED visits with readmissions, hospitalists may be forgiven for judging the latter cousin as uglier – and not just for reimbursement reasons. Readmitted patients are sicker, more vulnerable, and have poorer outcomes. In our healthcare system’s resultant quest to eliminate readmissions, return ED visits that do not end in readmission are generally either ignored or grouped with readmissions. Ignoring these treat-and-discharge ED visits is problematic because of their incidence, which rivals that of ED visits ending in readmission.4 On the other hand, grouping these visits with readmissions only makes sense if the two are considered to be equally ugly outcomes. Is this a valid assumption to make?
In this issue of the Journal of Hospital Medicine, Venkatesh et al5 tackle that question by studying Medicare beneficiaries hospitalized for acute myocardial infarction, heart failure, or pneumonia over a 1-year period. The authors differentiate 30-day treat-and-discharge ED visit rates from 30-day readmission rates before risk-standardizing these rates based on visit codes and hospital characteristics. Similar to the results of prior studies, the authors observe an 8%–9% overall incidence of treat-and-discharge ED visits within 30 days of hospital discharge.6 Mapping treat-and-discharge ED visit rates versus readmission rates for each hospital, the authors detect modest but noticeable inverse correlations between the two. Among hospitals discharging heart failure patients, for example, every 10% increase in postdischarge ED visit rates corresponds to a roughly 2% decrease in readmission rates.
The authors are correct to tread cautiously with their interpretation of this correlation. Dispositions for ED patients exist on a continuum, so hospitals with higher propensities to discharge patients from EDs (whether directly or from observation units) will inherently have lower admission rates. The authors hint at a causal relationship nonetheless, suggesting that ED providers may be able to intervene on high-risk patients earlier before Readmission Road becomes a one-way street. Proving this hypothesis will require careful research that controls for patient, disease, and ED factors as well as their complex interactions in the post-discharge timeline. That being said, most analyses of outpatient follow-up visits (except for heart failure patients) have failed to find any anti-readmission correlation analogous to that identified by Venkatesh et al. What powers do ED providers have that outpatient providers lack? Many, admittedly: stat phlebotomy services, on-demand consultations, and observation units. Additionally, while ED visits invariably require a patient’s presence in person, 25% of provider-scheduled posthospitalization outpatient visits end in no-shows.2 Whether patient-triggered follow-up through rapid access clinics or even urgent care centers can replicate ED functionality in recently discharged patients is unknown and warrants further study.
Venkatesh et al5 also find that reasons for postdischarge ED visits bear only a slight resemblance to reasons for index hospitalizations. For example, of all ED visits by patients recovering from hospitalizations for pneumonia, only 20% involve respiratory or pulmonary complaints. What explains the other 80%? Some variability may be attributable to the study’s use of visit codes instead of chart reviews or stakeholder interviews; in surveys of patients and ED physicians during these postdischarge visits, the two groups may have very different perceptions of why the encounter is occurring and whether it is preventable.7 Regardless of who is “right,” the heterogeneity of reasons that prompt care re-escalation lends further credence to the existence of a distinct posthospitalization syndrome:8 in the immediate postdischarge interval, patients experience many transient but real physiological risks for which they may identify the ED as their best recourse.
Whether the ED actually provides secondary prophylaxis against the posthospitalization syndrome is highly debatable, and Venkatesh et al wisely refrain from assigning a positive or negative valence to treat-and-discharge ED visits. Ultimately, postdischarge ED visits are neither inherently good nor bad (nor ugly, for that matter). Their unique nature is attracting newfound appreciation, and their potential ability to prevent readmission merits further research. If hospitals with high postdischarge ED visit rates can deliver high-quality care while truly arresting or reversing readmission-bound trajectories, then the strategies employed by these hospitals should inspire emulation, innovation, and dissemination.
Disclosures
The authors have nothing to disclose.
Once upon a time, discharges were easy to categorize: good, bad, or ugly. Good discharges allowed the patient to leave before noon, while bad discharges allowed the patient to leave without follow-up appointments. The worst discharges were defined by the two ugly cousins of acute care re-escalation: return emergency department (ED) visits and readmissions. Recently, however, much of this conventional wisdom has been turned on its head. For example, pre-noon discharges and provider-scheduled follow-up appointments may lead to unintended negative consequences and futility.1,2 In contrast, weekend discharges, which were often viewed to be unsafe, may reduce lengths of stay without compromising care even in high-risk patients.3
Having obfuscated the line between good and bad, we can now turn our attention to the ugly. Comparing return ED visits with readmissions, hospitalists may be forgiven for judging the latter cousin as uglier – and not just for reimbursement reasons. Readmitted patients are sicker, more vulnerable, and have poorer outcomes. In our healthcare system’s resultant quest to eliminate readmissions, return ED visits that do not end in readmission are generally either ignored or grouped with readmissions. Ignoring these treat-and-discharge ED visits is problematic because of their incidence, which rivals that of ED visits ending in readmission.4 On the other hand, grouping these visits with readmissions only makes sense if the two are considered to be equally ugly outcomes. Is this a valid assumption to make?
In this issue of the Journal of Hospital Medicine, Venkatesh et al5 tackle that question by studying Medicare beneficiaries hospitalized for acute myocardial infarction, heart failure, or pneumonia over a 1-year period. The authors differentiate 30-day treat-and-discharge ED visit rates from 30-day readmission rates before risk-standardizing these rates based on visit codes and hospital characteristics. Similar to the results of prior studies, the authors observe an 8%–9% overall incidence of treat-and-discharge ED visits within 30 days of hospital discharge.6 Mapping treat-and-discharge ED visit rates versus readmission rates for each hospital, the authors detect modest but noticeable inverse correlations between the two. Among hospitals discharging heart failure patients, for example, every 10% increase in postdischarge ED visit rates corresponds to a roughly 2% decrease in readmission rates.
The authors are correct to tread cautiously with their interpretation of this correlation. Dispositions for ED patients exist on a continuum, so hospitals with higher propensities to discharge patients from EDs (whether directly or from observation units) will inherently have lower admission rates. The authors hint at a causal relationship nonetheless, suggesting that ED providers may be able to intervene on high-risk patients earlier before Readmission Road becomes a one-way street. Proving this hypothesis will require careful research that controls for patient, disease, and ED factors as well as their complex interactions in the post-discharge timeline. That being said, most analyses of outpatient follow-up visits (except for heart failure patients) have failed to find any anti-readmission correlation analogous to that identified by Venkatesh et al. What powers do ED providers have that outpatient providers lack? Many, admittedly: stat phlebotomy services, on-demand consultations, and observation units. Additionally, while ED visits invariably require a patient’s presence in person, 25% of provider-scheduled posthospitalization outpatient visits end in no-shows.2 Whether patient-triggered follow-up through rapid access clinics or even urgent care centers can replicate ED functionality in recently discharged patients is unknown and warrants further study.
Venkatesh et al5 also find that reasons for postdischarge ED visits bear only a slight resemblance to reasons for index hospitalizations. For example, of all ED visits by patients recovering from hospitalizations for pneumonia, only 20% involve respiratory or pulmonary complaints. What explains the other 80%? Some variability may be attributable to the study’s use of visit codes instead of chart reviews or stakeholder interviews; in surveys of patients and ED physicians during these postdischarge visits, the two groups may have very different perceptions of why the encounter is occurring and whether it is preventable.7 Regardless of who is “right,” the heterogeneity of reasons that prompt care re-escalation lends further credence to the existence of a distinct posthospitalization syndrome:8 in the immediate postdischarge interval, patients experience many transient but real physiological risks for which they may identify the ED as their best recourse.
Whether the ED actually provides secondary prophylaxis against the posthospitalization syndrome is highly debatable, and Venkatesh et al wisely refrain from assigning a positive or negative valence to treat-and-discharge ED visits. Ultimately, postdischarge ED visits are neither inherently good nor bad (nor ugly, for that matter). Their unique nature is attracting newfound appreciation, and their potential ability to prevent readmission merits further research. If hospitals with high postdischarge ED visit rates can deliver high-quality care while truly arresting or reversing readmission-bound trajectories, then the strategies employed by these hospitals should inspire emulation, innovation, and dissemination.
Disclosures
The authors have nothing to disclose.
1. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. 10.1002/jhm.2529. PubMed
2. Banerjee R, Suarez A, Kier M, et al. If you book it, will they come? Attendance at postdischarge follow-up visits scheduled by inpatient providers. J Hosp Med. 2017;12(8):618-625. 10.12788/jhm.2777. PubMed
3. McAlister FA, Youngson E, Padwal RS, Majumdar SR. Similar outcomes among general medicine patients discharged on weekends. J Hosp Med. 2015;10(2):69-74. 10.1002/jhm.2310. PubMed
4. Rising KL, White LF, Fernandez WG, Boutwell AE. Emergency department visits after hospital discharge: a missing part of the equation. Ann Emerg Med. 2013;62(2):145-150. 10.1016/j.annemergmed.2013.01.024. PubMed
5. Venkatesh A, Wang C, Wang Y, Altaf F, Bernheim S, Horwitz L. Association between post-discharge emergency department visitation and readmission rates J Hosp Med. 2018;13(9):589-594. doi: 10.12788/jhm.2937.
6. Vashi AA, Fox JP, Carr BG, et al. Use of hospital-based acute care among patients recently discharged from the hospital. JAMA. 2013;309(4):364-371. 10.1001/jama.2012.216219. PubMed
7. Suffoletto B, Hu J, Guyette M, Callaway C. Factors contributing to emergency department care within 30 days of hospital discharge and potential ways to prevent it: differences in perspectives of patients, caregivers, and emergency physicians. J Hosp Med. 2014;9(5):315-319. 10.1002/jhm.2167. PubMed
8. Krumholz HM. Post-hospital syndrome: an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. 10.1056/NEJMp1212324. PubMed
1. Rajkomar A, Valencia V, Novelero M, Mourad M, Auerbach A. The association between discharge before noon and length of stay in medical and surgical patients. J Hosp Med. 2016;11(12):859-861. 10.1002/jhm.2529. PubMed
2. Banerjee R, Suarez A, Kier M, et al. If you book it, will they come? Attendance at postdischarge follow-up visits scheduled by inpatient providers. J Hosp Med. 2017;12(8):618-625. 10.12788/jhm.2777. PubMed
3. McAlister FA, Youngson E, Padwal RS, Majumdar SR. Similar outcomes among general medicine patients discharged on weekends. J Hosp Med. 2015;10(2):69-74. 10.1002/jhm.2310. PubMed
4. Rising KL, White LF, Fernandez WG, Boutwell AE. Emergency department visits after hospital discharge: a missing part of the equation. Ann Emerg Med. 2013;62(2):145-150. 10.1016/j.annemergmed.2013.01.024. PubMed
5. Venkatesh A, Wang C, Wang Y, Altaf F, Bernheim S, Horwitz L. Association between post-discharge emergency department visitation and readmission rates J Hosp Med. 2018;13(9):589-594. doi: 10.12788/jhm.2937.
6. Vashi AA, Fox JP, Carr BG, et al. Use of hospital-based acute care among patients recently discharged from the hospital. JAMA. 2013;309(4):364-371. 10.1001/jama.2012.216219. PubMed
7. Suffoletto B, Hu J, Guyette M, Callaway C. Factors contributing to emergency department care within 30 days of hospital discharge and potential ways to prevent it: differences in perspectives of patients, caregivers, and emergency physicians. J Hosp Med. 2014;9(5):315-319. 10.1002/jhm.2167. PubMed
8. Krumholz HM. Post-hospital syndrome: an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-102. 10.1056/NEJMp1212324. PubMed
© 2018 Society of Hospital Medicine
Relative Weights for Pediatric Inpatients: Children Now Have a Scale of Their Own
For the last 35 years, Medicare’s prospective payment system has transformed reimbursement for hospital-based care of patients. This “revolutionary” system shifted payment from being retrospective—the government paid hospitals for what they did—to prospective—the government paid hospitals against a predetermined fee schedule based on a patient’s condition and other factors.1 When the system started in 1983, the then-new payment system classified patients into 467 Diagnosis-Related Groups (DRGs). In those early days, Medicare paid hospitals “an average price for an average patient within the DRG.”2 Not surprisingly, early critics were concerned that this average payment would disadvantage hospitals that cared for more complex patients, such as teaching hospitals; studies then demonstrated that theoretical concern.3 The Severity of Illness (SOI) index, which was developed in the 1980s, attempted to correct this problem by using SOI-stratified DRGs as a payment mechanism. By adding SOI to DRGs, the homogeneity of resource consumption in each group increased, resulting in more accurate comparisons about complexity, outcomes, resource utilization, and ultimately payment. Eventually, along with the risk of mortality, the SOI made its way into the All Patients Refined (APR) DRG system, which is more representative of non-Medicare populations and thus could be applied to children.
The ongoing challenge with SOI classification is that its 4-level categories (1-mild, 2-moderate, 3-severe, 4-extreme) is not comparable across DRGs; that is, a “moderate” patient in one DRG may be sicker and use more resources than an “extreme” patient in another DRG. For this reason, more than a decade ago, Medicare replaced the DRG/SOI approach with the Medicare Severity (MS)-DRG for Medicare payments to hospitals. The distinguishing feature of MS-DRGs is that they represent a complete relative scale; the relative weights are not categorical but can be lined up and payments assigned relative to the average Medicare patient. For example, a look at the 2015 tables shows that heart transplant has the highest relative weight and is the most expensive one, whereas false labor has the lowest relative weight and is the least expensive.4 Due to its exclusive intent for use on Medicare patients, the system could not be used for pediatrics. Interestingly, New York State developed a Service Intensity Weight (SIW) in 2009 by using 3 years of Medicaid and commercial payer data to create a relative scale for payment within the state.5
Thanks to Richardson, et al, in this issue of Journal of Hospital Medicine, pediatrics has its first relative weight system for hospitalized children across the United States.6 Similar to the MS-DRG system, those with the interest or need can line up the APR-DRGs into a relative scale and see that a normal newborn has a relative weight on their H-RISK scale of 0.18, while a heart transplant patient has a weight of 91.66. This is a welcome and much-needed addition to the world of pediatric health services and health service research. Stakeholders can use this system for comparative analyses, risk adjustment, resource utilization comparison, and payment. For those inclined, one can explore the comparisons of relative weights on different scales; for example, the ratio between simple pneumonia and heart transplant is 21 on the MS-DRG, 60 on the NY State SIW scale,7 and 187 on H-RISK. A generation of health service researchers and economists may find great satisfaction in elucidating why this relativity in relative scales exists!
There are limitations to all weighting and relative weighting systems. The H-RISK is based on DRG and SOI, which rely on accurate coding. In addition, as the authors note, iatrogenic complications are not differentiated from naturally occurring ones. Thus, a hospital may obtain a higher relative weight applied to a patient who did not enter the hospital as sick as the final score suggests. Researchers noted this problem from the start of the DRG/SOI journey, and all systems that rely on post hoc scoring based on coded diagnoses and activities, without differentiation of presence on admission, have this limitation.8 Furthermore, children’s hospitals have far more variable use of observation status than in Medicare, and many DRG analyses exclude observation-status patients.
Despite these limitations, this is an important first step for children’s hospitals to be better able to do comparative analyses and benchmarking with a true relative weight scale that is appropriate for use among hospitalized children.
Disclosure
The author declares no conflicts of interest.
1. Mayes R. The origins, development, and passage of Medicare’s revolutionary prospective payment system. J Hist Med Allied Sci. 2007;62(1):21-55. DOI: 10.1093/jhmas/jrj038. PubMed
2. Iglehart JK. Medicare begins prospective payment of hospitals. N Engl J Med. 1983;303(23):1428-1432. DOI: 10.1056/NEJM198306093082331. PubMed
3. Horn SD, Sharkey PD, Chambers AF, Horn RA. Severity of illness within DRGs: impact on prospective payment. Am J Public Health. 1985;75(10):1195-1199. PMCID: PMC1646367 PubMed
4. Inpatient Charge Data FY2015, https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Inpatient2015.html. Accessed February 20, 2018.
5. Service Intensity Weights (SIW) and average length-of-stay (LOS). https://regs.health.ny.gov/content/section-86-118-service-intensity-weights-siw-and-average-length-stay-los. Accessed February 22, 2018.
6. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of hospitalization resource intensity scores for kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9); 602-608. doi: 10.12788/jhm.2948 PubMed
7. APR-DRG Service Intensity Weights and Average Length of Stay, July 1, 2014. Department of Health, New York State. https://www.health.ny.gov/facilities/hospital/reimbursement/apr-drg/weights/siw_alos_2014.htm. Accessed February 20, 2018.
8. Horn SD, Horn RA, Sharkey PD. The severity of illness index as a severity adjustment to diagnosis-related groups. Health Care Financ Rev. 1984;(Suppl):33-45. PubMed
For the last 35 years, Medicare’s prospective payment system has transformed reimbursement for hospital-based care of patients. This “revolutionary” system shifted payment from being retrospective—the government paid hospitals for what they did—to prospective—the government paid hospitals against a predetermined fee schedule based on a patient’s condition and other factors.1 When the system started in 1983, the then-new payment system classified patients into 467 Diagnosis-Related Groups (DRGs). In those early days, Medicare paid hospitals “an average price for an average patient within the DRG.”2 Not surprisingly, early critics were concerned that this average payment would disadvantage hospitals that cared for more complex patients, such as teaching hospitals; studies then demonstrated that theoretical concern.3 The Severity of Illness (SOI) index, which was developed in the 1980s, attempted to correct this problem by using SOI-stratified DRGs as a payment mechanism. By adding SOI to DRGs, the homogeneity of resource consumption in each group increased, resulting in more accurate comparisons about complexity, outcomes, resource utilization, and ultimately payment. Eventually, along with the risk of mortality, the SOI made its way into the All Patients Refined (APR) DRG system, which is more representative of non-Medicare populations and thus could be applied to children.
The ongoing challenge with SOI classification is that its 4-level categories (1-mild, 2-moderate, 3-severe, 4-extreme) is not comparable across DRGs; that is, a “moderate” patient in one DRG may be sicker and use more resources than an “extreme” patient in another DRG. For this reason, more than a decade ago, Medicare replaced the DRG/SOI approach with the Medicare Severity (MS)-DRG for Medicare payments to hospitals. The distinguishing feature of MS-DRGs is that they represent a complete relative scale; the relative weights are not categorical but can be lined up and payments assigned relative to the average Medicare patient. For example, a look at the 2015 tables shows that heart transplant has the highest relative weight and is the most expensive one, whereas false labor has the lowest relative weight and is the least expensive.4 Due to its exclusive intent for use on Medicare patients, the system could not be used for pediatrics. Interestingly, New York State developed a Service Intensity Weight (SIW) in 2009 by using 3 years of Medicaid and commercial payer data to create a relative scale for payment within the state.5
Thanks to Richardson, et al, in this issue of Journal of Hospital Medicine, pediatrics has its first relative weight system for hospitalized children across the United States.6 Similar to the MS-DRG system, those with the interest or need can line up the APR-DRGs into a relative scale and see that a normal newborn has a relative weight on their H-RISK scale of 0.18, while a heart transplant patient has a weight of 91.66. This is a welcome and much-needed addition to the world of pediatric health services and health service research. Stakeholders can use this system for comparative analyses, risk adjustment, resource utilization comparison, and payment. For those inclined, one can explore the comparisons of relative weights on different scales; for example, the ratio between simple pneumonia and heart transplant is 21 on the MS-DRG, 60 on the NY State SIW scale,7 and 187 on H-RISK. A generation of health service researchers and economists may find great satisfaction in elucidating why this relativity in relative scales exists!
There are limitations to all weighting and relative weighting systems. The H-RISK is based on DRG and SOI, which rely on accurate coding. In addition, as the authors note, iatrogenic complications are not differentiated from naturally occurring ones. Thus, a hospital may obtain a higher relative weight applied to a patient who did not enter the hospital as sick as the final score suggests. Researchers noted this problem from the start of the DRG/SOI journey, and all systems that rely on post hoc scoring based on coded diagnoses and activities, without differentiation of presence on admission, have this limitation.8 Furthermore, children’s hospitals have far more variable use of observation status than in Medicare, and many DRG analyses exclude observation-status patients.
Despite these limitations, this is an important first step for children’s hospitals to be better able to do comparative analyses and benchmarking with a true relative weight scale that is appropriate for use among hospitalized children.
Disclosure
The author declares no conflicts of interest.
For the last 35 years, Medicare’s prospective payment system has transformed reimbursement for hospital-based care of patients. This “revolutionary” system shifted payment from being retrospective—the government paid hospitals for what they did—to prospective—the government paid hospitals against a predetermined fee schedule based on a patient’s condition and other factors.1 When the system started in 1983, the then-new payment system classified patients into 467 Diagnosis-Related Groups (DRGs). In those early days, Medicare paid hospitals “an average price for an average patient within the DRG.”2 Not surprisingly, early critics were concerned that this average payment would disadvantage hospitals that cared for more complex patients, such as teaching hospitals; studies then demonstrated that theoretical concern.3 The Severity of Illness (SOI) index, which was developed in the 1980s, attempted to correct this problem by using SOI-stratified DRGs as a payment mechanism. By adding SOI to DRGs, the homogeneity of resource consumption in each group increased, resulting in more accurate comparisons about complexity, outcomes, resource utilization, and ultimately payment. Eventually, along with the risk of mortality, the SOI made its way into the All Patients Refined (APR) DRG system, which is more representative of non-Medicare populations and thus could be applied to children.
The ongoing challenge with SOI classification is that its 4-level categories (1-mild, 2-moderate, 3-severe, 4-extreme) is not comparable across DRGs; that is, a “moderate” patient in one DRG may be sicker and use more resources than an “extreme” patient in another DRG. For this reason, more than a decade ago, Medicare replaced the DRG/SOI approach with the Medicare Severity (MS)-DRG for Medicare payments to hospitals. The distinguishing feature of MS-DRGs is that they represent a complete relative scale; the relative weights are not categorical but can be lined up and payments assigned relative to the average Medicare patient. For example, a look at the 2015 tables shows that heart transplant has the highest relative weight and is the most expensive one, whereas false labor has the lowest relative weight and is the least expensive.4 Due to its exclusive intent for use on Medicare patients, the system could not be used for pediatrics. Interestingly, New York State developed a Service Intensity Weight (SIW) in 2009 by using 3 years of Medicaid and commercial payer data to create a relative scale for payment within the state.5
Thanks to Richardson, et al, in this issue of Journal of Hospital Medicine, pediatrics has its first relative weight system for hospitalized children across the United States.6 Similar to the MS-DRG system, those with the interest or need can line up the APR-DRGs into a relative scale and see that a normal newborn has a relative weight on their H-RISK scale of 0.18, while a heart transplant patient has a weight of 91.66. This is a welcome and much-needed addition to the world of pediatric health services and health service research. Stakeholders can use this system for comparative analyses, risk adjustment, resource utilization comparison, and payment. For those inclined, one can explore the comparisons of relative weights on different scales; for example, the ratio between simple pneumonia and heart transplant is 21 on the MS-DRG, 60 on the NY State SIW scale,7 and 187 on H-RISK. A generation of health service researchers and economists may find great satisfaction in elucidating why this relativity in relative scales exists!
There are limitations to all weighting and relative weighting systems. The H-RISK is based on DRG and SOI, which rely on accurate coding. In addition, as the authors note, iatrogenic complications are not differentiated from naturally occurring ones. Thus, a hospital may obtain a higher relative weight applied to a patient who did not enter the hospital as sick as the final score suggests. Researchers noted this problem from the start of the DRG/SOI journey, and all systems that rely on post hoc scoring based on coded diagnoses and activities, without differentiation of presence on admission, have this limitation.8 Furthermore, children’s hospitals have far more variable use of observation status than in Medicare, and many DRG analyses exclude observation-status patients.
Despite these limitations, this is an important first step for children’s hospitals to be better able to do comparative analyses and benchmarking with a true relative weight scale that is appropriate for use among hospitalized children.
Disclosure
The author declares no conflicts of interest.
1. Mayes R. The origins, development, and passage of Medicare’s revolutionary prospective payment system. J Hist Med Allied Sci. 2007;62(1):21-55. DOI: 10.1093/jhmas/jrj038. PubMed
2. Iglehart JK. Medicare begins prospective payment of hospitals. N Engl J Med. 1983;303(23):1428-1432. DOI: 10.1056/NEJM198306093082331. PubMed
3. Horn SD, Sharkey PD, Chambers AF, Horn RA. Severity of illness within DRGs: impact on prospective payment. Am J Public Health. 1985;75(10):1195-1199. PMCID: PMC1646367 PubMed
4. Inpatient Charge Data FY2015, https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Inpatient2015.html. Accessed February 20, 2018.
5. Service Intensity Weights (SIW) and average length-of-stay (LOS). https://regs.health.ny.gov/content/section-86-118-service-intensity-weights-siw-and-average-length-stay-los. Accessed February 22, 2018.
6. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of hospitalization resource intensity scores for kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9); 602-608. doi: 10.12788/jhm.2948 PubMed
7. APR-DRG Service Intensity Weights and Average Length of Stay, July 1, 2014. Department of Health, New York State. https://www.health.ny.gov/facilities/hospital/reimbursement/apr-drg/weights/siw_alos_2014.htm. Accessed February 20, 2018.
8. Horn SD, Horn RA, Sharkey PD. The severity of illness index as a severity adjustment to diagnosis-related groups. Health Care Financ Rev. 1984;(Suppl):33-45. PubMed
1. Mayes R. The origins, development, and passage of Medicare’s revolutionary prospective payment system. J Hist Med Allied Sci. 2007;62(1):21-55. DOI: 10.1093/jhmas/jrj038. PubMed
2. Iglehart JK. Medicare begins prospective payment of hospitals. N Engl J Med. 1983;303(23):1428-1432. DOI: 10.1056/NEJM198306093082331. PubMed
3. Horn SD, Sharkey PD, Chambers AF, Horn RA. Severity of illness within DRGs: impact on prospective payment. Am J Public Health. 1985;75(10):1195-1199. PMCID: PMC1646367 PubMed
4. Inpatient Charge Data FY2015, https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/Medicare-Provider-Charge-Data/Inpatient2015.html. Accessed February 20, 2018.
5. Service Intensity Weights (SIW) and average length-of-stay (LOS). https://regs.health.ny.gov/content/section-86-118-service-intensity-weights-siw-and-average-length-stay-los. Accessed February 22, 2018.
6. Richardson T, Rodean J, Harris M, Berry J, Gay JC, Hall M. Development of hospitalization resource intensity scores for kids (H-RISK) and comparison across pediatric populations. J Hosp Med. 2018;13(9); 602-608. doi: 10.12788/jhm.2948 PubMed
7. APR-DRG Service Intensity Weights and Average Length of Stay, July 1, 2014. Department of Health, New York State. https://www.health.ny.gov/facilities/hospital/reimbursement/apr-drg/weights/siw_alos_2014.htm. Accessed February 20, 2018.
8. Horn SD, Horn RA, Sharkey PD. The severity of illness index as a severity adjustment to diagnosis-related groups. Health Care Financ Rev. 1984;(Suppl):33-45. PubMed
© 2018 Society of Hospital Medicine
FYI: This Message Will Interrupt You – Texting Impact on Clinical Learning Environment
Fifteen years ago, beepers with 5-digit call-back numbers were the norm. Pushing a call light button outside the patient’s room to flag the desk clerk that a new order had been hand-written was all part of the lived experience of residency. Using that as our baseline, we have clearly come a long way in the way that we communicate with other clinicians in hospitals. Communication among the patient care team in the digital age predominantly involves bidirectional messaging using mobile devices. The approach is both immediate and convenient. Mobile devices can improve work efficiency, patient safety, and quality of care, but their main advantage may be real-time bedside decision support.1,2 However, the widespread use of mobile devices for communication in healthcare is not without its concerns. First and foremost, there has been abundant literature around short message service (SMS) use in the healthcare setting, and there are concerns surrounding both threats to privacy and the prevalence and impact of interruptions in clinical care.
The first SMS was sent in 1992.3 Text messaging since then has become ubiquitous, even in healthcare, raising concerns around the protection of patient health information under the Health Insurance Portability and Accountability Act (HIPAA). Interestingly, the United States Department of Health and Human Services Office for Civil Rights, enforcer of HIPAA, is tech neutral on the subject.3 Multiple studies have assessed physician stances on SMS communication in the healthcare setting using routine, non-HIPAA-compliant mobile phones. Overall, 60%-80% of respondents admitted to using SMS in patient care, while in another study, 72% and 80% of Internal Medicine residents surveyed found SMS to be the most efficient form of communication and overall preferred method of communication, respectively.3,4 Interestingly, 82.5% of those same residents preferred the hospital-based alphanumeric paging system for security purposes, even though Freundlich et al. make a compelling argument that unidirectional alphanumeric paging systems are most certainly less HIPAA compliant, lacking encryption and password protection.5 Newer platforms that enable HIPAA-compliant messaging are promising, although they may not be fully adopted by clinical teams without full-scale implementation in hospitals.6In addition to privacy concerns with SMS applications on mobile phones, interruptions in healthcare – be it from phone calls, emails, text messages, or in-person conversations – are common. In fact, famed communication researcher Enrico Coeira has notoriously described healthcare communication as ”interrupt-driven.”7 Prior work has shown that frequent interruptions in the healthcare setting can lead to medication prescription errors, errors in computerized physician order entry, and even surgical procedural errors.8-10
While studies have focused on interruptions in clinical care in the healthcare setting, little is known about how education may be compromised by interruptions due to mobile devices. Text messaging during dedicated conference time can lead to inadequate learning and a sense of frustration among residents. In this issue of the Journal of Hospital Medicine, Mendel et al. performed a quality improvement study involving eight academic inpatient clinical training units with the aim of reducing nonurgent text messages during education rounds.11 Their unique interventions included learning sessions, posters, adding alerts to the digital communication platform, and alternative messaging options. Of four sequential interventions, a message alerting the sender that they will be interrupting educational rounds and suggesting a “delayed send” or “send as an FYI” showed the greatest impact, reducing the number of text interruptions per team per educational hour from 0.81 to 0.59 (95% CI 0.51-0.67). When comparing a four-week pre-intervention sample with a four-week end-intervention sample, the percentage of nonurgent messages decreased from 82% to 68% (P < .01).
While these results are promising, challenges to large-scale implementation of such a program exist. Buy-in from the ancillary healthcare team is critical for such interventions to succeed and be sustained. It also places a burden of “point triage” on the healthcare team members, who must assess the patient situation and determine the level of urgency and whether to immediately interrupt, delay interrupt or send an FYI message. For example, in the study by Mendel et al.,11 it is noteworthy that urgent patient care issues were mislabeled as “FYI” in 2% of patients. While this is a seemingly low rate, even one of these mislabeled messages could result in significant adverse patient outcomes and should be considered a “never event.” Finally, the study used a messaging platform with programming flexibility and IT personnel to assist. This could be cost prohibitive for some programs, especially if rolled out to an entire institution.
Communication is critical for effective patient care and unfortunately, the timing of such communication is often not orderly but rather, chaotic. Text message communication can introduce interruptions into all aspects of patient care and education, not only dedicated learning conferences. If the goal is for all residents to attend all conferences, it seems impossible (and likely dangerous) to eliminate all messaging interruptions during conference hours. Nevertheless, it is worth noting that Mandel et al. have moved us creatively toward that goal with a multifaceted approach.11 Future work should address more downstream outcomes, such as objective resident learning retention and adverse patient events relative to the number of interruptions per educational hour. If such studies showed improved learning outcomes and fewer adverse patient events, the next step would be to further strengthen and refine their protocol with real-time and scheduled feedback sessions between providers and other patient care team members in addition to the continued search for additional innovative approaches. In addition, combining artificial intelligence or predictive modeling may help us delineate when an interruption is warranted, for example, when a patient is at high clinical risk without intervention. Likewise, human factors research may help us understand the best way to time and execute an interruption to minimize the risk to clinical care or education. After all, the ideal system would not eliminate interruptions entirely but allow clinicians to know when someone should be interrupted and when they do not need to be interrupted.
Disclosures
The authors have no financial relationships relevant to this article to disclose.
1. Berner ES, Houston TK, Ray MN, et al. Improving ambulatory prescribing safety with a handheld decision support system: a ran domized controlled trial. J Am Med Inform Assoc. 2006;13(2):171-179. doi: 10.1197/jamia.M1961. PubMed
2. Sintchenko V, Iredell JR, Gilbert GL, et al. Handheld computer-based decision support reduces patient length of stay and antibiotic prescribing in critical care. J Am Med Inform Assoc. 2005;12(4):398-402. doi: 10.1197/jamia.M1798. PubMed
3. Drolet BC. Text messaging and protected health information: what is permitted? JAMA. 2017;317(23):2369-2370. doi: 10.1001/jama.2017.5646. PubMed
4. Prochaska MT, Bird AN, Chadaga A, Arora VM. Resident use of text messaging for patient care: ease of use or breach of privacy? JMIR Med Inform. 2015;3(4):e37. doi: 10.2196/medinform.4797. PubMed
5. Samora JB, Blazar PE, Lifchez SD, et al. Mobile messaging communication in health care rules, regulations, penalties, and safety of provider use. JBJS Rev. 2018;6(3):e4. doi: 10.2106/JBJS.RVW.17.00070 PubMed
6. Freundlich RE, Freundlich KL, Drolet BC. Pagers, smartphones, and HIPAA: finding the best solution for electronic communication of protected health information. J Med Syst. 2017;42(1):9. doi: 10.1007/s10916-017-0870-9. PubMed
7. Coiera E. Clinical communication—a new informatics paradigm. In Proceedings of the American. Medical Informatics Association Autumn Symposium. 1996;17-21.
8. Feuerbacher RL, Funk KH, Spight DH, et al. Realistic distractions and interruptions that impair simulated surgical performance by novice surgeons. Arch Surg. 2012;147(11):1026-1030. doi: 10.1001/archsurg.2012.1480. PubMed
9. Agency for Healthcare Research and Quality–Patient Safety Network (AHRQ-PSNet). https://psnet.ahrq.gov/webmm/case/257/order-interrupted-by-text-multitasking-mishapCases & Commentaries. Order Interrupted by Text: Multitasking Mishap. December 2011. Commentary by John Halamka, MD, MS.
10. Westbrook JI, Raban MZ, Walter SR, et al. Task errors by emergency physicians are associated with interruptions, multitasking, fatigue and working memory capacity: a prospective, direct observation study [published online ahead of print January 9, 2018]. BMJ Qual Saf. doi: 10.1136/bmjqs-2017-007333. [Epub ahead of print]. PubMed
11. Mendel A, Lott A, Lo L, et al. A matter of urgency: reducing clinical text message interruptions during educational sessions. J Hosp Med. 2018;13(9):616-622. doi: 10.12788/jhm.2959. PubMed
Fifteen years ago, beepers with 5-digit call-back numbers were the norm. Pushing a call light button outside the patient’s room to flag the desk clerk that a new order had been hand-written was all part of the lived experience of residency. Using that as our baseline, we have clearly come a long way in the way that we communicate with other clinicians in hospitals. Communication among the patient care team in the digital age predominantly involves bidirectional messaging using mobile devices. The approach is both immediate and convenient. Mobile devices can improve work efficiency, patient safety, and quality of care, but their main advantage may be real-time bedside decision support.1,2 However, the widespread use of mobile devices for communication in healthcare is not without its concerns. First and foremost, there has been abundant literature around short message service (SMS) use in the healthcare setting, and there are concerns surrounding both threats to privacy and the prevalence and impact of interruptions in clinical care.
The first SMS was sent in 1992.3 Text messaging since then has become ubiquitous, even in healthcare, raising concerns around the protection of patient health information under the Health Insurance Portability and Accountability Act (HIPAA). Interestingly, the United States Department of Health and Human Services Office for Civil Rights, enforcer of HIPAA, is tech neutral on the subject.3 Multiple studies have assessed physician stances on SMS communication in the healthcare setting using routine, non-HIPAA-compliant mobile phones. Overall, 60%-80% of respondents admitted to using SMS in patient care, while in another study, 72% and 80% of Internal Medicine residents surveyed found SMS to be the most efficient form of communication and overall preferred method of communication, respectively.3,4 Interestingly, 82.5% of those same residents preferred the hospital-based alphanumeric paging system for security purposes, even though Freundlich et al. make a compelling argument that unidirectional alphanumeric paging systems are most certainly less HIPAA compliant, lacking encryption and password protection.5 Newer platforms that enable HIPAA-compliant messaging are promising, although they may not be fully adopted by clinical teams without full-scale implementation in hospitals.6In addition to privacy concerns with SMS applications on mobile phones, interruptions in healthcare – be it from phone calls, emails, text messages, or in-person conversations – are common. In fact, famed communication researcher Enrico Coeira has notoriously described healthcare communication as ”interrupt-driven.”7 Prior work has shown that frequent interruptions in the healthcare setting can lead to medication prescription errors, errors in computerized physician order entry, and even surgical procedural errors.8-10
While studies have focused on interruptions in clinical care in the healthcare setting, little is known about how education may be compromised by interruptions due to mobile devices. Text messaging during dedicated conference time can lead to inadequate learning and a sense of frustration among residents. In this issue of the Journal of Hospital Medicine, Mendel et al. performed a quality improvement study involving eight academic inpatient clinical training units with the aim of reducing nonurgent text messages during education rounds.11 Their unique interventions included learning sessions, posters, adding alerts to the digital communication platform, and alternative messaging options. Of four sequential interventions, a message alerting the sender that they will be interrupting educational rounds and suggesting a “delayed send” or “send as an FYI” showed the greatest impact, reducing the number of text interruptions per team per educational hour from 0.81 to 0.59 (95% CI 0.51-0.67). When comparing a four-week pre-intervention sample with a four-week end-intervention sample, the percentage of nonurgent messages decreased from 82% to 68% (P < .01).
While these results are promising, challenges to large-scale implementation of such a program exist. Buy-in from the ancillary healthcare team is critical for such interventions to succeed and be sustained. It also places a burden of “point triage” on the healthcare team members, who must assess the patient situation and determine the level of urgency and whether to immediately interrupt, delay interrupt or send an FYI message. For example, in the study by Mendel et al.,11 it is noteworthy that urgent patient care issues were mislabeled as “FYI” in 2% of patients. While this is a seemingly low rate, even one of these mislabeled messages could result in significant adverse patient outcomes and should be considered a “never event.” Finally, the study used a messaging platform with programming flexibility and IT personnel to assist. This could be cost prohibitive for some programs, especially if rolled out to an entire institution.
Communication is critical for effective patient care and unfortunately, the timing of such communication is often not orderly but rather, chaotic. Text message communication can introduce interruptions into all aspects of patient care and education, not only dedicated learning conferences. If the goal is for all residents to attend all conferences, it seems impossible (and likely dangerous) to eliminate all messaging interruptions during conference hours. Nevertheless, it is worth noting that Mandel et al. have moved us creatively toward that goal with a multifaceted approach.11 Future work should address more downstream outcomes, such as objective resident learning retention and adverse patient events relative to the number of interruptions per educational hour. If such studies showed improved learning outcomes and fewer adverse patient events, the next step would be to further strengthen and refine their protocol with real-time and scheduled feedback sessions between providers and other patient care team members in addition to the continued search for additional innovative approaches. In addition, combining artificial intelligence or predictive modeling may help us delineate when an interruption is warranted, for example, when a patient is at high clinical risk without intervention. Likewise, human factors research may help us understand the best way to time and execute an interruption to minimize the risk to clinical care or education. After all, the ideal system would not eliminate interruptions entirely but allow clinicians to know when someone should be interrupted and when they do not need to be interrupted.
Disclosures
The authors have no financial relationships relevant to this article to disclose.
Fifteen years ago, beepers with 5-digit call-back numbers were the norm. Pushing a call light button outside the patient’s room to flag the desk clerk that a new order had been hand-written was all part of the lived experience of residency. Using that as our baseline, we have clearly come a long way in the way that we communicate with other clinicians in hospitals. Communication among the patient care team in the digital age predominantly involves bidirectional messaging using mobile devices. The approach is both immediate and convenient. Mobile devices can improve work efficiency, patient safety, and quality of care, but their main advantage may be real-time bedside decision support.1,2 However, the widespread use of mobile devices for communication in healthcare is not without its concerns. First and foremost, there has been abundant literature around short message service (SMS) use in the healthcare setting, and there are concerns surrounding both threats to privacy and the prevalence and impact of interruptions in clinical care.
The first SMS was sent in 1992.3 Text messaging since then has become ubiquitous, even in healthcare, raising concerns around the protection of patient health information under the Health Insurance Portability and Accountability Act (HIPAA). Interestingly, the United States Department of Health and Human Services Office for Civil Rights, enforcer of HIPAA, is tech neutral on the subject.3 Multiple studies have assessed physician stances on SMS communication in the healthcare setting using routine, non-HIPAA-compliant mobile phones. Overall, 60%-80% of respondents admitted to using SMS in patient care, while in another study, 72% and 80% of Internal Medicine residents surveyed found SMS to be the most efficient form of communication and overall preferred method of communication, respectively.3,4 Interestingly, 82.5% of those same residents preferred the hospital-based alphanumeric paging system for security purposes, even though Freundlich et al. make a compelling argument that unidirectional alphanumeric paging systems are most certainly less HIPAA compliant, lacking encryption and password protection.5 Newer platforms that enable HIPAA-compliant messaging are promising, although they may not be fully adopted by clinical teams without full-scale implementation in hospitals.6In addition to privacy concerns with SMS applications on mobile phones, interruptions in healthcare – be it from phone calls, emails, text messages, or in-person conversations – are common. In fact, famed communication researcher Enrico Coeira has notoriously described healthcare communication as ”interrupt-driven.”7 Prior work has shown that frequent interruptions in the healthcare setting can lead to medication prescription errors, errors in computerized physician order entry, and even surgical procedural errors.8-10
While studies have focused on interruptions in clinical care in the healthcare setting, little is known about how education may be compromised by interruptions due to mobile devices. Text messaging during dedicated conference time can lead to inadequate learning and a sense of frustration among residents. In this issue of the Journal of Hospital Medicine, Mendel et al. performed a quality improvement study involving eight academic inpatient clinical training units with the aim of reducing nonurgent text messages during education rounds.11 Their unique interventions included learning sessions, posters, adding alerts to the digital communication platform, and alternative messaging options. Of four sequential interventions, a message alerting the sender that they will be interrupting educational rounds and suggesting a “delayed send” or “send as an FYI” showed the greatest impact, reducing the number of text interruptions per team per educational hour from 0.81 to 0.59 (95% CI 0.51-0.67). When comparing a four-week pre-intervention sample with a four-week end-intervention sample, the percentage of nonurgent messages decreased from 82% to 68% (P < .01).
While these results are promising, challenges to large-scale implementation of such a program exist. Buy-in from the ancillary healthcare team is critical for such interventions to succeed and be sustained. It also places a burden of “point triage” on the healthcare team members, who must assess the patient situation and determine the level of urgency and whether to immediately interrupt, delay interrupt or send an FYI message. For example, in the study by Mendel et al.,11 it is noteworthy that urgent patient care issues were mislabeled as “FYI” in 2% of patients. While this is a seemingly low rate, even one of these mislabeled messages could result in significant adverse patient outcomes and should be considered a “never event.” Finally, the study used a messaging platform with programming flexibility and IT personnel to assist. This could be cost prohibitive for some programs, especially if rolled out to an entire institution.
Communication is critical for effective patient care and unfortunately, the timing of such communication is often not orderly but rather, chaotic. Text message communication can introduce interruptions into all aspects of patient care and education, not only dedicated learning conferences. If the goal is for all residents to attend all conferences, it seems impossible (and likely dangerous) to eliminate all messaging interruptions during conference hours. Nevertheless, it is worth noting that Mandel et al. have moved us creatively toward that goal with a multifaceted approach.11 Future work should address more downstream outcomes, such as objective resident learning retention and adverse patient events relative to the number of interruptions per educational hour. If such studies showed improved learning outcomes and fewer adverse patient events, the next step would be to further strengthen and refine their protocol with real-time and scheduled feedback sessions between providers and other patient care team members in addition to the continued search for additional innovative approaches. In addition, combining artificial intelligence or predictive modeling may help us delineate when an interruption is warranted, for example, when a patient is at high clinical risk without intervention. Likewise, human factors research may help us understand the best way to time and execute an interruption to minimize the risk to clinical care or education. After all, the ideal system would not eliminate interruptions entirely but allow clinicians to know when someone should be interrupted and when they do not need to be interrupted.
Disclosures
The authors have no financial relationships relevant to this article to disclose.
1. Berner ES, Houston TK, Ray MN, et al. Improving ambulatory prescribing safety with a handheld decision support system: a ran domized controlled trial. J Am Med Inform Assoc. 2006;13(2):171-179. doi: 10.1197/jamia.M1961. PubMed
2. Sintchenko V, Iredell JR, Gilbert GL, et al. Handheld computer-based decision support reduces patient length of stay and antibiotic prescribing in critical care. J Am Med Inform Assoc. 2005;12(4):398-402. doi: 10.1197/jamia.M1798. PubMed
3. Drolet BC. Text messaging and protected health information: what is permitted? JAMA. 2017;317(23):2369-2370. doi: 10.1001/jama.2017.5646. PubMed
4. Prochaska MT, Bird AN, Chadaga A, Arora VM. Resident use of text messaging for patient care: ease of use or breach of privacy? JMIR Med Inform. 2015;3(4):e37. doi: 10.2196/medinform.4797. PubMed
5. Samora JB, Blazar PE, Lifchez SD, et al. Mobile messaging communication in health care rules, regulations, penalties, and safety of provider use. JBJS Rev. 2018;6(3):e4. doi: 10.2106/JBJS.RVW.17.00070 PubMed
6. Freundlich RE, Freundlich KL, Drolet BC. Pagers, smartphones, and HIPAA: finding the best solution for electronic communication of protected health information. J Med Syst. 2017;42(1):9. doi: 10.1007/s10916-017-0870-9. PubMed
7. Coiera E. Clinical communication—a new informatics paradigm. In Proceedings of the American. Medical Informatics Association Autumn Symposium. 1996;17-21.
8. Feuerbacher RL, Funk KH, Spight DH, et al. Realistic distractions and interruptions that impair simulated surgical performance by novice surgeons. Arch Surg. 2012;147(11):1026-1030. doi: 10.1001/archsurg.2012.1480. PubMed
9. Agency for Healthcare Research and Quality–Patient Safety Network (AHRQ-PSNet). https://psnet.ahrq.gov/webmm/case/257/order-interrupted-by-text-multitasking-mishapCases & Commentaries. Order Interrupted by Text: Multitasking Mishap. December 2011. Commentary by John Halamka, MD, MS.
10. Westbrook JI, Raban MZ, Walter SR, et al. Task errors by emergency physicians are associated with interruptions, multitasking, fatigue and working memory capacity: a prospective, direct observation study [published online ahead of print January 9, 2018]. BMJ Qual Saf. doi: 10.1136/bmjqs-2017-007333. [Epub ahead of print]. PubMed
11. Mendel A, Lott A, Lo L, et al. A matter of urgency: reducing clinical text message interruptions during educational sessions. J Hosp Med. 2018;13(9):616-622. doi: 10.12788/jhm.2959. PubMed
1. Berner ES, Houston TK, Ray MN, et al. Improving ambulatory prescribing safety with a handheld decision support system: a ran domized controlled trial. J Am Med Inform Assoc. 2006;13(2):171-179. doi: 10.1197/jamia.M1961. PubMed
2. Sintchenko V, Iredell JR, Gilbert GL, et al. Handheld computer-based decision support reduces patient length of stay and antibiotic prescribing in critical care. J Am Med Inform Assoc. 2005;12(4):398-402. doi: 10.1197/jamia.M1798. PubMed
3. Drolet BC. Text messaging and protected health information: what is permitted? JAMA. 2017;317(23):2369-2370. doi: 10.1001/jama.2017.5646. PubMed
4. Prochaska MT, Bird AN, Chadaga A, Arora VM. Resident use of text messaging for patient care: ease of use or breach of privacy? JMIR Med Inform. 2015;3(4):e37. doi: 10.2196/medinform.4797. PubMed
5. Samora JB, Blazar PE, Lifchez SD, et al. Mobile messaging communication in health care rules, regulations, penalties, and safety of provider use. JBJS Rev. 2018;6(3):e4. doi: 10.2106/JBJS.RVW.17.00070 PubMed
6. Freundlich RE, Freundlich KL, Drolet BC. Pagers, smartphones, and HIPAA: finding the best solution for electronic communication of protected health information. J Med Syst. 2017;42(1):9. doi: 10.1007/s10916-017-0870-9. PubMed
7. Coiera E. Clinical communication—a new informatics paradigm. In Proceedings of the American. Medical Informatics Association Autumn Symposium. 1996;17-21.
8. Feuerbacher RL, Funk KH, Spight DH, et al. Realistic distractions and interruptions that impair simulated surgical performance by novice surgeons. Arch Surg. 2012;147(11):1026-1030. doi: 10.1001/archsurg.2012.1480. PubMed
9. Agency for Healthcare Research and Quality–Patient Safety Network (AHRQ-PSNet). https://psnet.ahrq.gov/webmm/case/257/order-interrupted-by-text-multitasking-mishapCases & Commentaries. Order Interrupted by Text: Multitasking Mishap. December 2011. Commentary by John Halamka, MD, MS.
10. Westbrook JI, Raban MZ, Walter SR, et al. Task errors by emergency physicians are associated with interruptions, multitasking, fatigue and working memory capacity: a prospective, direct observation study [published online ahead of print January 9, 2018]. BMJ Qual Saf. doi: 10.1136/bmjqs-2017-007333. [Epub ahead of print]. PubMed
11. Mendel A, Lott A, Lo L, et al. A matter of urgency: reducing clinical text message interruptions during educational sessions. J Hosp Med. 2018;13(9):616-622. doi: 10.12788/jhm.2959. PubMed
© 2018 Society of Hospital Medicine