User login
VA Choice Bill Defeated in the House
A U.S. House of Representatives appropriation to fund the Veterans Choice Program surprisingly went down to defeat on Monday. The VA Choice Program is set to run out of money in September, and VA officials have been calling for Congress to provide additional funding for the program. Republican leaders, hoping to expedite the bill’s passage and thinking that it was not controversial, submitted the bill in a process that required the votes of two-thirds of the representatives. The 219-186 vote fell well short of the necessary two-thirds, and voting fell largely along party lines.
Many veterans service organizations (VSOs) were critical of the bill and called on the House to make substantial changes to it. Seven VSOs signed a joint statement calling for the bill’s defeat. “As organizations who represent and support the interests of America’s 21 million veterans, and in fulfillment of our mandate to ensure that the men and women who served are able to receive the health care and benefits they need and deserve, we are calling on Members of Congress to defeat the House vote on unacceptable choice funding legislation (S. 114, with amendments),” the statement read.
AMVETS, Disabled American Veterans , Military Officers Association of America, Military Order of the Purple Heart, Veterans of Foreign Wars, Vietnam Veterans of America, and Wounded Warrior Project all signed on to the statement. The chief complaint was that the legislation “includes funding only for the ‘choice’ program which provides additional community care options, but makes no investment in VA and uses ‘savings’ from other veterans benefits or services to ‘pay’ for the ‘choice’ program.”
The bill would have allocated $2 billion for the Veterans Choice Program, taken funding for veteran housing loan fees, and would reduce the pensions for some veterans living in nursing facilities that also could be paid for under the Medicaid program.
The fate of the bill and funding for the Veterans Choice Program remains unclear. Senate and House veterans committees seem to be far apart on how to fund the program and for efforts to make more substantive changes to the program. Although House Republicans eventually may be able to pass a bill without Democrats, in the Senate, they will need the support of at least a handful of Democrats to move the bill to the President’s desk.
A U.S. House of Representatives appropriation to fund the Veterans Choice Program surprisingly went down to defeat on Monday. The VA Choice Program is set to run out of money in September, and VA officials have been calling for Congress to provide additional funding for the program. Republican leaders, hoping to expedite the bill’s passage and thinking that it was not controversial, submitted the bill in a process that required the votes of two-thirds of the representatives. The 219-186 vote fell well short of the necessary two-thirds, and voting fell largely along party lines.
Many veterans service organizations (VSOs) were critical of the bill and called on the House to make substantial changes to it. Seven VSOs signed a joint statement calling for the bill’s defeat. “As organizations who represent and support the interests of America’s 21 million veterans, and in fulfillment of our mandate to ensure that the men and women who served are able to receive the health care and benefits they need and deserve, we are calling on Members of Congress to defeat the House vote on unacceptable choice funding legislation (S. 114, with amendments),” the statement read.
AMVETS, Disabled American Veterans , Military Officers Association of America, Military Order of the Purple Heart, Veterans of Foreign Wars, Vietnam Veterans of America, and Wounded Warrior Project all signed on to the statement. The chief complaint was that the legislation “includes funding only for the ‘choice’ program which provides additional community care options, but makes no investment in VA and uses ‘savings’ from other veterans benefits or services to ‘pay’ for the ‘choice’ program.”
The bill would have allocated $2 billion for the Veterans Choice Program, taken funding for veteran housing loan fees, and would reduce the pensions for some veterans living in nursing facilities that also could be paid for under the Medicaid program.
The fate of the bill and funding for the Veterans Choice Program remains unclear. Senate and House veterans committees seem to be far apart on how to fund the program and for efforts to make more substantive changes to the program. Although House Republicans eventually may be able to pass a bill without Democrats, in the Senate, they will need the support of at least a handful of Democrats to move the bill to the President’s desk.
A U.S. House of Representatives appropriation to fund the Veterans Choice Program surprisingly went down to defeat on Monday. The VA Choice Program is set to run out of money in September, and VA officials have been calling for Congress to provide additional funding for the program. Republican leaders, hoping to expedite the bill’s passage and thinking that it was not controversial, submitted the bill in a process that required the votes of two-thirds of the representatives. The 219-186 vote fell well short of the necessary two-thirds, and voting fell largely along party lines.
Many veterans service organizations (VSOs) were critical of the bill and called on the House to make substantial changes to it. Seven VSOs signed a joint statement calling for the bill’s defeat. “As organizations who represent and support the interests of America’s 21 million veterans, and in fulfillment of our mandate to ensure that the men and women who served are able to receive the health care and benefits they need and deserve, we are calling on Members of Congress to defeat the House vote on unacceptable choice funding legislation (S. 114, with amendments),” the statement read.
AMVETS, Disabled American Veterans , Military Officers Association of America, Military Order of the Purple Heart, Veterans of Foreign Wars, Vietnam Veterans of America, and Wounded Warrior Project all signed on to the statement. The chief complaint was that the legislation “includes funding only for the ‘choice’ program which provides additional community care options, but makes no investment in VA and uses ‘savings’ from other veterans benefits or services to ‘pay’ for the ‘choice’ program.”
The bill would have allocated $2 billion for the Veterans Choice Program, taken funding for veteran housing loan fees, and would reduce the pensions for some veterans living in nursing facilities that also could be paid for under the Medicaid program.
The fate of the bill and funding for the Veterans Choice Program remains unclear. Senate and House veterans committees seem to be far apart on how to fund the program and for efforts to make more substantive changes to the program. Although House Republicans eventually may be able to pass a bill without Democrats, in the Senate, they will need the support of at least a handful of Democrats to move the bill to the President’s desk.
How is VA Doing? Report Card Grades Are In
The US Department of Veterans Affairs (VA) is earning high marks for the quality of care provided to veterans, according to multiple sources. For instance, systematic reviews published in 2023 found that VA health care is consistently as good as, or surpasses, non-VA health care. In the latest Centers for Medicare & Medicaid Services (CMS) annual Overall Hospital Quality Star Ratings, 67% of VA hospitals received either 4 or 5 stars, compared with only 41% of non-VA hospitals.
Veterans themselves are awarding high marks. According to the Medicare nationwide survey of patients, VA hospitals outperformed non-VA hospitals on all 10 core patient satisfaction metrics, including overall hospital rating, communication with doctors, communication about medications, and willingness to recommend the hospital. Furthermore, trust in VA outpatient care has reached an all-time record high of 92%, according to a survey of more than 440,000 veterans.
This year, in fact, the VA has broken a number of its own records. The VA cites other high points:
- More than 127.5 million health care appointments, a 6% increase over last year;
- Shorter wait times: new patients saw an 11% reduction in average wait times for VA primary care and a 7% reduction for mental health care compared to last year;
- $187 billion in benefits to 6.7 million veterans and survivors this year—an all-time record;
- 2,517,519 disability benefit claims processed, a 27% increase over 2023;
- No-cost emergency health care is provided to more than 50,000 veterans in acute suicidal crises; the Veterans Crisis Line supported 1,123,591 million calls, texts, and chats, up 12% from 2023;
- 47,925 veterans experiencing homelessness were housed in fiscal year 2024 and 96% remain housed long-term;
- 519,453 spouses and dependents received survivor benefits, a 4.5% increase from 2023;
- Services, resources, and assistance provided to a record 88,095 veteran family caregivers, an 18.6% increase over the 2023 record;
- A record 741,259 women veterans received compensation payments, 8.2% more than 2023;
- VA dental clinics provided > 6 million procedures to > 630,000 veterans; through community care, the VA delivered a record additional 3.4 million procedures to > 330,000 veterans.
Other actions this year include: expanding eligibility for VA healthcare to all toxin-exposed veterans years earlier than called for by the PACT Act; expanding access to care across the nation through VA Access Sprints, adding night and weekend clinics, and increasing the number of veterans scheduled into daily clinic schedules; removing copays for the first 3 outpatient mental health care and substance use disorder visits of each calendar year through 2027; expanding access to VA cancer care through establishing new cancer presumptive conditions, expanding access to genetic, lung, and colorectal cancer screening, and expanding the Close to Me cancer care program; expanding access to in vitro fertilization for eligible unmarried veterans and eligible veterans in same-sex marriages; expanding access to VA care and benefits for some former service members discharged under other than honorable conditions; and launching tele-emergency care for veterans nationwide.
The VA will continue to “aggressively reach out to and engage veterans to encourage them to come to VA for the care and benefits they have earned.”
“Veterans deserve the very best from VA and our nation, and we will never settle for anything less,” said VA Secretary Denis McDonough. “We’re honored that more veterans are getting their earned health care and benefits from VA than ever before, but make no mistake: there is still work to do. We will continue to work each and every day to earn the trust of those we serve — and ensure that all Veterans, their families, and their survivors get the care and benefits they so rightly deserve.”
The US Department of Veterans Affairs (VA) is earning high marks for the quality of care provided to veterans, according to multiple sources. For instance, systematic reviews published in 2023 found that VA health care is consistently as good as, or surpasses, non-VA health care. In the latest Centers for Medicare & Medicaid Services (CMS) annual Overall Hospital Quality Star Ratings, 67% of VA hospitals received either 4 or 5 stars, compared with only 41% of non-VA hospitals.
Veterans themselves are awarding high marks. According to the Medicare nationwide survey of patients, VA hospitals outperformed non-VA hospitals on all 10 core patient satisfaction metrics, including overall hospital rating, communication with doctors, communication about medications, and willingness to recommend the hospital. Furthermore, trust in VA outpatient care has reached an all-time record high of 92%, according to a survey of more than 440,000 veterans.
This year, in fact, the VA has broken a number of its own records. The VA cites other high points:
- More than 127.5 million health care appointments, a 6% increase over last year;
- Shorter wait times: new patients saw an 11% reduction in average wait times for VA primary care and a 7% reduction for mental health care compared to last year;
- $187 billion in benefits to 6.7 million veterans and survivors this year—an all-time record;
- 2,517,519 disability benefit claims processed, a 27% increase over 2023;
- No-cost emergency health care is provided to more than 50,000 veterans in acute suicidal crises; the Veterans Crisis Line supported 1,123,591 million calls, texts, and chats, up 12% from 2023;
- 47,925 veterans experiencing homelessness were housed in fiscal year 2024 and 96% remain housed long-term;
- 519,453 spouses and dependents received survivor benefits, a 4.5% increase from 2023;
- Services, resources, and assistance provided to a record 88,095 veteran family caregivers, an 18.6% increase over the 2023 record;
- A record 741,259 women veterans received compensation payments, 8.2% more than 2023;
- VA dental clinics provided > 6 million procedures to > 630,000 veterans; through community care, the VA delivered a record additional 3.4 million procedures to > 330,000 veterans.
Other actions this year include: expanding eligibility for VA healthcare to all toxin-exposed veterans years earlier than called for by the PACT Act; expanding access to care across the nation through VA Access Sprints, adding night and weekend clinics, and increasing the number of veterans scheduled into daily clinic schedules; removing copays for the first 3 outpatient mental health care and substance use disorder visits of each calendar year through 2027; expanding access to VA cancer care through establishing new cancer presumptive conditions, expanding access to genetic, lung, and colorectal cancer screening, and expanding the Close to Me cancer care program; expanding access to in vitro fertilization for eligible unmarried veterans and eligible veterans in same-sex marriages; expanding access to VA care and benefits for some former service members discharged under other than honorable conditions; and launching tele-emergency care for veterans nationwide.
The VA will continue to “aggressively reach out to and engage veterans to encourage them to come to VA for the care and benefits they have earned.”
“Veterans deserve the very best from VA and our nation, and we will never settle for anything less,” said VA Secretary Denis McDonough. “We’re honored that more veterans are getting their earned health care and benefits from VA than ever before, but make no mistake: there is still work to do. We will continue to work each and every day to earn the trust of those we serve — and ensure that all Veterans, their families, and their survivors get the care and benefits they so rightly deserve.”
The US Department of Veterans Affairs (VA) is earning high marks for the quality of care provided to veterans, according to multiple sources. For instance, systematic reviews published in 2023 found that VA health care is consistently as good as, or surpasses, non-VA health care. In the latest Centers for Medicare & Medicaid Services (CMS) annual Overall Hospital Quality Star Ratings, 67% of VA hospitals received either 4 or 5 stars, compared with only 41% of non-VA hospitals.
Veterans themselves are awarding high marks. According to the Medicare nationwide survey of patients, VA hospitals outperformed non-VA hospitals on all 10 core patient satisfaction metrics, including overall hospital rating, communication with doctors, communication about medications, and willingness to recommend the hospital. Furthermore, trust in VA outpatient care has reached an all-time record high of 92%, according to a survey of more than 440,000 veterans.
This year, in fact, the VA has broken a number of its own records. The VA cites other high points:
- More than 127.5 million health care appointments, a 6% increase over last year;
- Shorter wait times: new patients saw an 11% reduction in average wait times for VA primary care and a 7% reduction for mental health care compared to last year;
- $187 billion in benefits to 6.7 million veterans and survivors this year—an all-time record;
- 2,517,519 disability benefit claims processed, a 27% increase over 2023;
- No-cost emergency health care is provided to more than 50,000 veterans in acute suicidal crises; the Veterans Crisis Line supported 1,123,591 million calls, texts, and chats, up 12% from 2023;
- 47,925 veterans experiencing homelessness were housed in fiscal year 2024 and 96% remain housed long-term;
- 519,453 spouses and dependents received survivor benefits, a 4.5% increase from 2023;
- Services, resources, and assistance provided to a record 88,095 veteran family caregivers, an 18.6% increase over the 2023 record;
- A record 741,259 women veterans received compensation payments, 8.2% more than 2023;
- VA dental clinics provided > 6 million procedures to > 630,000 veterans; through community care, the VA delivered a record additional 3.4 million procedures to > 330,000 veterans.
Other actions this year include: expanding eligibility for VA healthcare to all toxin-exposed veterans years earlier than called for by the PACT Act; expanding access to care across the nation through VA Access Sprints, adding night and weekend clinics, and increasing the number of veterans scheduled into daily clinic schedules; removing copays for the first 3 outpatient mental health care and substance use disorder visits of each calendar year through 2027; expanding access to VA cancer care through establishing new cancer presumptive conditions, expanding access to genetic, lung, and colorectal cancer screening, and expanding the Close to Me cancer care program; expanding access to in vitro fertilization for eligible unmarried veterans and eligible veterans in same-sex marriages; expanding access to VA care and benefits for some former service members discharged under other than honorable conditions; and launching tele-emergency care for veterans nationwide.
The VA will continue to “aggressively reach out to and engage veterans to encourage them to come to VA for the care and benefits they have earned.”
“Veterans deserve the very best from VA and our nation, and we will never settle for anything less,” said VA Secretary Denis McDonough. “We’re honored that more veterans are getting their earned health care and benefits from VA than ever before, but make no mistake: there is still work to do. We will continue to work each and every day to earn the trust of those we serve — and ensure that all Veterans, their families, and their survivors get the care and benefits they so rightly deserve.”
VA Awards Grants to Support Adaptive Sports
The US Department of Veterans Affairs (VA) is awarding $15.9 million in grants to fund adaptive sports, recreational activities, and equine therapy for > 15,000 veterans and service members living with disabilities.
Marine Corps veteran Jataya Taylor — who competed in wheelchair fencing at the 2024 Paralympics — experienced mental health symptoms until she began participating in adaptive sports through an organization supported by the VA Adaptive Sports Grant Program.
“Getting involved in adaptive sports was a saving grace for me,” Taylor said. “Participating in these programs got me on the bike to start with, then got me climbing, and eventually it became an important part of my mental health to participate. I found my people. I found my new network of friends.”
Adaptive sports, which are customized to fit the needs of veterans with disabilities, include paralympic sports, archery, cycling, skiing, hunting, rock climbing, and sky diving. Mike Gooler, another Marine Corps veteran, praised the Adaptive Sports Center’s facilities in Crested Butte, Colorado, calling it “nothing short of amazing.”
“[S]ki therapy has been instrumental in helping me navigate through my experiences and injuries,” Gooler said. “Skiing provides me with sense of freedom and empowerment … and having my family by my side, witnessing my progress and sharing the joy of skiing, was truly special.”
The grant program is facilitated and managed by the National Veterans Sports Programs and Special Events Office and will provide grants to 91 national, regional, and community-based programs for fiscal year 2024 across all 50 states, the District of Columbia, Guam, and Puerto Rico.
“These grants give veterans life-changing opportunities,” Secretary of VA Denis McDonough said. “We know adaptive sports and recreational activities can be transformational for veterans living with disabilities, improving their overall physical and mental health, and also giving them important community with fellow heroes who served.”
Information about the awardees and details of the program are available at www.va.gov/adaptivesports and on Facebook at Sports4Vets.
The US Department of Veterans Affairs (VA) is awarding $15.9 million in grants to fund adaptive sports, recreational activities, and equine therapy for > 15,000 veterans and service members living with disabilities.
Marine Corps veteran Jataya Taylor — who competed in wheelchair fencing at the 2024 Paralympics — experienced mental health symptoms until she began participating in adaptive sports through an organization supported by the VA Adaptive Sports Grant Program.
“Getting involved in adaptive sports was a saving grace for me,” Taylor said. “Participating in these programs got me on the bike to start with, then got me climbing, and eventually it became an important part of my mental health to participate. I found my people. I found my new network of friends.”
Adaptive sports, which are customized to fit the needs of veterans with disabilities, include paralympic sports, archery, cycling, skiing, hunting, rock climbing, and sky diving. Mike Gooler, another Marine Corps veteran, praised the Adaptive Sports Center’s facilities in Crested Butte, Colorado, calling it “nothing short of amazing.”
“[S]ki therapy has been instrumental in helping me navigate through my experiences and injuries,” Gooler said. “Skiing provides me with sense of freedom and empowerment … and having my family by my side, witnessing my progress and sharing the joy of skiing, was truly special.”
The grant program is facilitated and managed by the National Veterans Sports Programs and Special Events Office and will provide grants to 91 national, regional, and community-based programs for fiscal year 2024 across all 50 states, the District of Columbia, Guam, and Puerto Rico.
“These grants give veterans life-changing opportunities,” Secretary of VA Denis McDonough said. “We know adaptive sports and recreational activities can be transformational for veterans living with disabilities, improving their overall physical and mental health, and also giving them important community with fellow heroes who served.”
Information about the awardees and details of the program are available at www.va.gov/adaptivesports and on Facebook at Sports4Vets.
The US Department of Veterans Affairs (VA) is awarding $15.9 million in grants to fund adaptive sports, recreational activities, and equine therapy for > 15,000 veterans and service members living with disabilities.
Marine Corps veteran Jataya Taylor — who competed in wheelchair fencing at the 2024 Paralympics — experienced mental health symptoms until she began participating in adaptive sports through an organization supported by the VA Adaptive Sports Grant Program.
“Getting involved in adaptive sports was a saving grace for me,” Taylor said. “Participating in these programs got me on the bike to start with, then got me climbing, and eventually it became an important part of my mental health to participate. I found my people. I found my new network of friends.”
Adaptive sports, which are customized to fit the needs of veterans with disabilities, include paralympic sports, archery, cycling, skiing, hunting, rock climbing, and sky diving. Mike Gooler, another Marine Corps veteran, praised the Adaptive Sports Center’s facilities in Crested Butte, Colorado, calling it “nothing short of amazing.”
“[S]ki therapy has been instrumental in helping me navigate through my experiences and injuries,” Gooler said. “Skiing provides me with sense of freedom and empowerment … and having my family by my side, witnessing my progress and sharing the joy of skiing, was truly special.”
The grant program is facilitated and managed by the National Veterans Sports Programs and Special Events Office and will provide grants to 91 national, regional, and community-based programs for fiscal year 2024 across all 50 states, the District of Columbia, Guam, and Puerto Rico.
“These grants give veterans life-changing opportunities,” Secretary of VA Denis McDonough said. “We know adaptive sports and recreational activities can be transformational for veterans living with disabilities, improving their overall physical and mental health, and also giving them important community with fellow heroes who served.”
Information about the awardees and details of the program are available at www.va.gov/adaptivesports and on Facebook at Sports4Vets.
Bipartisan Bill to Provide Free Gun Lockboxes to Veterans
About 7 of every 10 veterans who die by suicide involve the use of a firearm. A reason for this high rate is access, as half of veterans report owning ≥ 1 personal firearms. Of those individuals, more than half report storing firearms loaded and/or unsecured and one-third of veterans who store their firearms loaded and unlocked do not own a lockbox or safe.
Suicide death prevention has improved as firearms have become more difficult to obtain. That’s why Navy veteran Rep. Chris Deluzio (D-PA), former FBI Special Agent and federal prosecutor Rep. Brian Fitzpatrick (R-PA), and Rep. Greg Landsman (D-OH) have teamed up to introduce the Saving Our Veterans Lives Act of 2024. Under the proposed act, any veteran would be able to get a free lockbox from the US Department of Veterans Affairs (VA).
Suicidal crises can be brief. According to the VA, if a person experiencing a suicidal crisis can’t access the method they planned to use, they generally do not seek out other lethal means. Lockboxes are a way of “putting space between thought and trigger,” the VA said.
The VA Suicide Prevention Program distributes free firearm cable locks to any veteran who requests one. However, many veterans favor lockboxes and safes to secure their guns. A VA pilot program offers free lockboxes to veterans enrolled in the Veterans Health Administration who are at an elevated risk for suicide. The program is set to launch in late 2024 and is a collaboration between the Rocky Mountain Mental Illness Research, Education, and Clinical Center for Suicide Prevention, VA National Prosthetics Service, and VA Office of Suicide Prevention.
The proposed bill would make the lockboxes (which typically cost between $25 and $350) free to any veteran, regardless of VA enrollment status or diagnosis. It ensures “sufficient funding for many tens of thousands of lockboxes to be distributed.” The bill would also direct the VA to create a public education campaign on the availability of lockboxes and the importance of secure firearm storage in suicide prevention.
“The alarming and tragic reality is that our veterans face a suicide rate 57% higher than that of civilians,” Rep. Fitzpatrick said. “This commonsense, bipartisan initiative is more than a solution—it's a lifeline.”
The representatives report that the bill has been endorsed by an “unprecedented” number of organizations, including the National Shooting Sports Foundation, Disabled American Veterans, The American Legion, GIFFORDS, Everytown for Gun Safety, Brady, American Psychological Association, American Foundation for Suicide Prevention, and Association of VA Psychologist Leaders.
“Did you know that in some cases only 10 minutes elapse between an individual having suicidal ideation and acting?” American Legion National Commander James LaCoursiere said in the representatives’ press release. “The Saving Our Veterans Lives Act is an important part of preventing suicide as it will provide veterans with the information and means to securely store their firearms to prevent suicide, while still protecting their Second Amendment rights. The Legion commends Rep. Deluzio and his team for bringing this bill forward and for their continued dedication to the welfare of our nation’s veterans.”
"I hear colleagues all the time talk about veteran suicide," Rep. Deluzio said in an interview with Military.com. "It is a problem in my community. It's a problem across the country. Let's take action. This is a chance where we can do it that I think can cut through the politics that normally divide us on these [gun] issues. And I think the coalition supporting the bill tells you, we've got a path to pass it."
About 7 of every 10 veterans who die by suicide involve the use of a firearm. A reason for this high rate is access, as half of veterans report owning ≥ 1 personal firearms. Of those individuals, more than half report storing firearms loaded and/or unsecured and one-third of veterans who store their firearms loaded and unlocked do not own a lockbox or safe.
Suicide death prevention has improved as firearms have become more difficult to obtain. That’s why Navy veteran Rep. Chris Deluzio (D-PA), former FBI Special Agent and federal prosecutor Rep. Brian Fitzpatrick (R-PA), and Rep. Greg Landsman (D-OH) have teamed up to introduce the Saving Our Veterans Lives Act of 2024. Under the proposed act, any veteran would be able to get a free lockbox from the US Department of Veterans Affairs (VA).
Suicidal crises can be brief. According to the VA, if a person experiencing a suicidal crisis can’t access the method they planned to use, they generally do not seek out other lethal means. Lockboxes are a way of “putting space between thought and trigger,” the VA said.
The VA Suicide Prevention Program distributes free firearm cable locks to any veteran who requests one. However, many veterans favor lockboxes and safes to secure their guns. A VA pilot program offers free lockboxes to veterans enrolled in the Veterans Health Administration who are at an elevated risk for suicide. The program is set to launch in late 2024 and is a collaboration between the Rocky Mountain Mental Illness Research, Education, and Clinical Center for Suicide Prevention, VA National Prosthetics Service, and VA Office of Suicide Prevention.
The proposed bill would make the lockboxes (which typically cost between $25 and $350) free to any veteran, regardless of VA enrollment status or diagnosis. It ensures “sufficient funding for many tens of thousands of lockboxes to be distributed.” The bill would also direct the VA to create a public education campaign on the availability of lockboxes and the importance of secure firearm storage in suicide prevention.
“The alarming and tragic reality is that our veterans face a suicide rate 57% higher than that of civilians,” Rep. Fitzpatrick said. “This commonsense, bipartisan initiative is more than a solution—it's a lifeline.”
The representatives report that the bill has been endorsed by an “unprecedented” number of organizations, including the National Shooting Sports Foundation, Disabled American Veterans, The American Legion, GIFFORDS, Everytown for Gun Safety, Brady, American Psychological Association, American Foundation for Suicide Prevention, and Association of VA Psychologist Leaders.
“Did you know that in some cases only 10 minutes elapse between an individual having suicidal ideation and acting?” American Legion National Commander James LaCoursiere said in the representatives’ press release. “The Saving Our Veterans Lives Act is an important part of preventing suicide as it will provide veterans with the information and means to securely store their firearms to prevent suicide, while still protecting their Second Amendment rights. The Legion commends Rep. Deluzio and his team for bringing this bill forward and for their continued dedication to the welfare of our nation’s veterans.”
"I hear colleagues all the time talk about veteran suicide," Rep. Deluzio said in an interview with Military.com. "It is a problem in my community. It's a problem across the country. Let's take action. This is a chance where we can do it that I think can cut through the politics that normally divide us on these [gun] issues. And I think the coalition supporting the bill tells you, we've got a path to pass it."
About 7 of every 10 veterans who die by suicide involve the use of a firearm. A reason for this high rate is access, as half of veterans report owning ≥ 1 personal firearms. Of those individuals, more than half report storing firearms loaded and/or unsecured and one-third of veterans who store their firearms loaded and unlocked do not own a lockbox or safe.
Suicide death prevention has improved as firearms have become more difficult to obtain. That’s why Navy veteran Rep. Chris Deluzio (D-PA), former FBI Special Agent and federal prosecutor Rep. Brian Fitzpatrick (R-PA), and Rep. Greg Landsman (D-OH) have teamed up to introduce the Saving Our Veterans Lives Act of 2024. Under the proposed act, any veteran would be able to get a free lockbox from the US Department of Veterans Affairs (VA).
Suicidal crises can be brief. According to the VA, if a person experiencing a suicidal crisis can’t access the method they planned to use, they generally do not seek out other lethal means. Lockboxes are a way of “putting space between thought and trigger,” the VA said.
The VA Suicide Prevention Program distributes free firearm cable locks to any veteran who requests one. However, many veterans favor lockboxes and safes to secure their guns. A VA pilot program offers free lockboxes to veterans enrolled in the Veterans Health Administration who are at an elevated risk for suicide. The program is set to launch in late 2024 and is a collaboration between the Rocky Mountain Mental Illness Research, Education, and Clinical Center for Suicide Prevention, VA National Prosthetics Service, and VA Office of Suicide Prevention.
The proposed bill would make the lockboxes (which typically cost between $25 and $350) free to any veteran, regardless of VA enrollment status or diagnosis. It ensures “sufficient funding for many tens of thousands of lockboxes to be distributed.” The bill would also direct the VA to create a public education campaign on the availability of lockboxes and the importance of secure firearm storage in suicide prevention.
“The alarming and tragic reality is that our veterans face a suicide rate 57% higher than that of civilians,” Rep. Fitzpatrick said. “This commonsense, bipartisan initiative is more than a solution—it's a lifeline.”
The representatives report that the bill has been endorsed by an “unprecedented” number of organizations, including the National Shooting Sports Foundation, Disabled American Veterans, The American Legion, GIFFORDS, Everytown for Gun Safety, Brady, American Psychological Association, American Foundation for Suicide Prevention, and Association of VA Psychologist Leaders.
“Did you know that in some cases only 10 minutes elapse between an individual having suicidal ideation and acting?” American Legion National Commander James LaCoursiere said in the representatives’ press release. “The Saving Our Veterans Lives Act is an important part of preventing suicide as it will provide veterans with the information and means to securely store their firearms to prevent suicide, while still protecting their Second Amendment rights. The Legion commends Rep. Deluzio and his team for bringing this bill forward and for their continued dedication to the welfare of our nation’s veterans.”
"I hear colleagues all the time talk about veteran suicide," Rep. Deluzio said in an interview with Military.com. "It is a problem in my community. It's a problem across the country. Let's take action. This is a chance where we can do it that I think can cut through the politics that normally divide us on these [gun] issues. And I think the coalition supporting the bill tells you, we've got a path to pass it."
Has the VA Fulfilled its Commitment to Trust and Healing?
Trust is built step by step, commitment by commitment, on every level.
Robert C. Solomon1
The US Department of Veterans Affairs (VA) was created in response to criticism of its predecessors. Since its establishment in 1930, the VA has never been short of critics who denounced its corruption, called for its dismantling in favor of privatization, and derided its incompetence.2 Despite multiple scandals that have handed more ammunition to those who object to its continued existence, the VA has not only survived, but thrived. This editorial is written in the form of a debate between exemplar opponents and defenders of the VA on whether it is currently fulfilling its commitment to veterans.
In May 2024, the Veterans Signals survey found that 80.4% of respondents reported trust in the VA, the highest level ever recorded.3 At its 2016 launch, the survey found that only 55% of veterans expressed trust in the VA. The survey was conducted 2 years after the scandal over access to care for veterans in Phoenix. Scores would surely have been even lower than 55% during that period when the critique of the VA—even from those who believe in its mission—was most trenchant.4 Administered quarterly, the survey samples > 38,000 of the 9 million enrolled veterans. Veterans surveyed were using services from all 3 branches of the VA: Veterans Health Administration, Veterans Benefits Administration, and National Cemetery Administration. Participants are asked whether they trust the VA to fulfill the country’s commitment to veterans and specifically how they rate the VA in 3 specific criteria: effectiveness, emotional resonance, and overall ease. In the latest survey, 80.5% of veterans rated the VA positively for effectiveness, 78.4% for emotional resonance, and 75.9% for overall ease. Even more impressive is the 91.8% of participants who reported they trust the VA for outpatient health care, capping a 7-year upward trend.3
The paradigmatic VA antagonist will rightly point out the well-known methodological limitations of this type of survey, including self-selection, sampling bias, and especially low response rates. However, VA researchers will counter that the 18% response rate for the latest Veterans Signals survey is higher than the industry average.5
VA critics might say that it would not matter if the response rate were 4 times higher; what matters is not what veterans say on a survey but what decisions they make about their care. The VA defender would be constrained to concede that even the most statistically sophisticated survey remains an indirect measure of veteran trust. They could, though, marshal far stronger evidence. Two direct demonstrations published in the literature suggest that veterans do as they say and are acting on their trust in the agency. First, the VA delivered more services, health care, and benefits to veterans during the 2023 fiscal year than ever before. Importantly for Federal Practitioner readers, the 16 million documented health care visits were 3 million more than previous records.6 Second, and in some ways even more encouraging for the future of the VA as a health care system, is that due in large part to the passage of the PACT Act, there has been a surge in VA enrollment by veterans. The VA recently announced that in the last year, > 400,000 veterans signed up for its health care and services. Enrollments are 30% more than the previous year and represented the highest figure in the past 5 years, a remarkable 50% increase over 2020 pandemic levels.7
VA critics could legitimately rebut this data by asking, “So more veterans are signing up for VA, and you are delivering more care, but what about the quality of that care? Has it improved?” The VA proponent’s rejoinder from multiple converging empirical studies would be a resounding yes. We have space to cite only a few examples of that rigorous recent research. What stands out ethically about these studies is that the VA has a broad program of research into the quality of the care it delivers and then transparently publishes those findings. The VA quality improvement research mission is truly unique and provides a shared open set of data for both critics and defenders to objectively examine VA successes and failures.
Among the most persuasive analysis was a systematic review of 37 studies contrasting VA with non-VA care from 2015 to 2023. The authors examined clinical quality, safety, patient access, experience, cost-efficiency, and equity of outcome. “VA care is consistently as good as or better than non-VA care in terms of clinical quality and safety,” the systematic review authors stated while qualifying that “Access, cost/efficiency, and patient experience between the 2 systems are not well studied.”8
A second systematic review looked specifically at similar key areas of quality, safety, access, patient experience, and comparative cost-efficiency for surgical treatment delivered in the VA and the community from 2015 to 2021. Only 18 studies met the inclusion criteria, but as the authors argued:
Based on limited data, these findings suggest that expanding eligibility for veterans to get care in the community may not provide benefits in terms of increasing access to surgical procedures, will not result in better quality, and may result in worse quality of care, but may reduce inpatient length of stay and perhaps cost less.9
At this juncture, the faultfinder may become frustrated and resort to a new tactic, challenging the very assumption that is the subject of the debate and demanding proof that there is any connection between veterans’ trust in the VA and their health and well-being. “Fair enough,” the VA side would reply, “here is some research that bolsters that connection.” Kopacz and colleagues examined the relationship between trust and healing at 6 sites and included 427 veterans and active-duty service members with combat posttraumatic stress disorder (PTSD) symptoms. The researchers found that trust and lack thereof are related to several significant mental, social, and physical health outcomes. The authors indicate the need for more research to better understand the importance and impact of trust and healing, but they show it is significant.10 Finally, veterans recognize the crucial link between trust in the unique expertise of VA practitioners in the treatment of PTSD. In a 2019 study, a majority expressed a preference to receive their PTSD treatment at the VA compared to a smaller group choosing care in the community.11
You be the judge of who won the debate, but knowing the dedication of my fellow federal practitioners, many of you will endorse my sentiment that we all need to stop talking and get back to doing our best to enhance veteran trust and healing; doing our essential part to keep fulfilling our commitment.
1. Solomon RC, Fernando F. Building Trust: In Business, Politics, Relationships, and Life. Oxford University Press; 2003:49.
2. Seiken J. 1921: Veterans Bureau is born - precursor to Department of Veterans Affairs. November 12, 2021. Updated September 4, 2023. Accessed July 22, 2024. https://department.va.gov/history/featured-stories/veterans-bureau/
3. US Department of Veterans Affairs. Serving America’s veterans, January 1 - March 31, 2024. Accessed July 22, 2024. https://department.va.gov/veterans-experience/wp-content/uploads/sites/2/2024/05/veteran-trust-report-fiscal-year-2024-quarter-2.pdf
4. Kizer KW, Jha AK. Restoring trust in VA health care. N Engl J Med. 2014;371(4):295-297. doi:10.1056/NEJMp1406852
5. Veteran trust in VA has increased 25% since 2016, reached an all-time high. News release. US Department of Veterans Affairs. May 28, 2024. Accessed July 22, 2024. https://news.va.gov/press-room/veteran-trust-va-increased-25-since-2016-high
6. VA sets all-time records for care and benefits delivered to Veterans in fiscal year 2023. News release. US Department of Veterans Affairs. November 6, 2023. Accessed July 23, 2024. https://news.va.gov/press-room/va-all-time-record-care-benefits-veterans-fy-2023/
7. 400,000+ Veterans enrolled in VA health care over the past 365 days, a 30% increase over last year. News release. US Department of Veterans Affairs. March 29, 2024. Accessed July 23, 2024. https://news.va.gov/press-room/va-enrolled-401006-veterans-healthcare-365/
8. Apaydin EA, Paige NM, Begashaw MM, Larkin J, Miake-Lye IM, Shekelle PG. Veterans Health Administration (VA) vs. non-VA healthcare quality: a systematic review. J Gen Intern Med. 2023;38(9):2179-2188. doi:10.1007/s11606-023-08207-2
9. Blegen M, Ko J, Salzman G, et al. Comparing quality of surgical care between the US Department of Veterans Affairs and non-veterans affairs settings: a systematic review. J Am Coll Surg. 2023;237(2):352-361. doi:10.1097/XCS.0000000000000720
10. Kopacz MS, Ames D, Koenig HG. Association between trust and mental, social, and physical health outcomes in veterans and active duty service members with combat-related PTSD symptomatology. Front Psychiatry. 2018;9:408. doi:10.3389/fpsyt.2018.00408
11. Haro E, Mader M, Noël PH, et al. The impact of trust, satisfaction, and perceived quality on preference for setting of future care among veterans with PTSD. Mil Med. 2019;184(11-12):e708-e714. doi:10.1093/milmed/usz078
Trust is built step by step, commitment by commitment, on every level.
Robert C. Solomon1
The US Department of Veterans Affairs (VA) was created in response to criticism of its predecessors. Since its establishment in 1930, the VA has never been short of critics who denounced its corruption, called for its dismantling in favor of privatization, and derided its incompetence.2 Despite multiple scandals that have handed more ammunition to those who object to its continued existence, the VA has not only survived, but thrived. This editorial is written in the form of a debate between exemplar opponents and defenders of the VA on whether it is currently fulfilling its commitment to veterans.
In May 2024, the Veterans Signals survey found that 80.4% of respondents reported trust in the VA, the highest level ever recorded.3 At its 2016 launch, the survey found that only 55% of veterans expressed trust in the VA. The survey was conducted 2 years after the scandal over access to care for veterans in Phoenix. Scores would surely have been even lower than 55% during that period when the critique of the VA—even from those who believe in its mission—was most trenchant.4 Administered quarterly, the survey samples > 38,000 of the 9 million enrolled veterans. Veterans surveyed were using services from all 3 branches of the VA: Veterans Health Administration, Veterans Benefits Administration, and National Cemetery Administration. Participants are asked whether they trust the VA to fulfill the country’s commitment to veterans and specifically how they rate the VA in 3 specific criteria: effectiveness, emotional resonance, and overall ease. In the latest survey, 80.5% of veterans rated the VA positively for effectiveness, 78.4% for emotional resonance, and 75.9% for overall ease. Even more impressive is the 91.8% of participants who reported they trust the VA for outpatient health care, capping a 7-year upward trend.3
The paradigmatic VA antagonist will rightly point out the well-known methodological limitations of this type of survey, including self-selection, sampling bias, and especially low response rates. However, VA researchers will counter that the 18% response rate for the latest Veterans Signals survey is higher than the industry average.5
VA critics might say that it would not matter if the response rate were 4 times higher; what matters is not what veterans say on a survey but what decisions they make about their care. The VA defender would be constrained to concede that even the most statistically sophisticated survey remains an indirect measure of veteran trust. They could, though, marshal far stronger evidence. Two direct demonstrations published in the literature suggest that veterans do as they say and are acting on their trust in the agency. First, the VA delivered more services, health care, and benefits to veterans during the 2023 fiscal year than ever before. Importantly for Federal Practitioner readers, the 16 million documented health care visits were 3 million more than previous records.6 Second, and in some ways even more encouraging for the future of the VA as a health care system, is that due in large part to the passage of the PACT Act, there has been a surge in VA enrollment by veterans. The VA recently announced that in the last year, > 400,000 veterans signed up for its health care and services. Enrollments are 30% more than the previous year and represented the highest figure in the past 5 years, a remarkable 50% increase over 2020 pandemic levels.7
VA critics could legitimately rebut this data by asking, “So more veterans are signing up for VA, and you are delivering more care, but what about the quality of that care? Has it improved?” The VA proponent’s rejoinder from multiple converging empirical studies would be a resounding yes. We have space to cite only a few examples of that rigorous recent research. What stands out ethically about these studies is that the VA has a broad program of research into the quality of the care it delivers and then transparently publishes those findings. The VA quality improvement research mission is truly unique and provides a shared open set of data for both critics and defenders to objectively examine VA successes and failures.
Among the most persuasive analysis was a systematic review of 37 studies contrasting VA with non-VA care from 2015 to 2023. The authors examined clinical quality, safety, patient access, experience, cost-efficiency, and equity of outcome. “VA care is consistently as good as or better than non-VA care in terms of clinical quality and safety,” the systematic review authors stated while qualifying that “Access, cost/efficiency, and patient experience between the 2 systems are not well studied.”8
A second systematic review looked specifically at similar key areas of quality, safety, access, patient experience, and comparative cost-efficiency for surgical treatment delivered in the VA and the community from 2015 to 2021. Only 18 studies met the inclusion criteria, but as the authors argued:
Based on limited data, these findings suggest that expanding eligibility for veterans to get care in the community may not provide benefits in terms of increasing access to surgical procedures, will not result in better quality, and may result in worse quality of care, but may reduce inpatient length of stay and perhaps cost less.9
At this juncture, the faultfinder may become frustrated and resort to a new tactic, challenging the very assumption that is the subject of the debate and demanding proof that there is any connection between veterans’ trust in the VA and their health and well-being. “Fair enough,” the VA side would reply, “here is some research that bolsters that connection.” Kopacz and colleagues examined the relationship between trust and healing at 6 sites and included 427 veterans and active-duty service members with combat posttraumatic stress disorder (PTSD) symptoms. The researchers found that trust and lack thereof are related to several significant mental, social, and physical health outcomes. The authors indicate the need for more research to better understand the importance and impact of trust and healing, but they show it is significant.10 Finally, veterans recognize the crucial link between trust in the unique expertise of VA practitioners in the treatment of PTSD. In a 2019 study, a majority expressed a preference to receive their PTSD treatment at the VA compared to a smaller group choosing care in the community.11
You be the judge of who won the debate, but knowing the dedication of my fellow federal practitioners, many of you will endorse my sentiment that we all need to stop talking and get back to doing our best to enhance veteran trust and healing; doing our essential part to keep fulfilling our commitment.
Trust is built step by step, commitment by commitment, on every level.
Robert C. Solomon1
The US Department of Veterans Affairs (VA) was created in response to criticism of its predecessors. Since its establishment in 1930, the VA has never been short of critics who denounced its corruption, called for its dismantling in favor of privatization, and derided its incompetence.2 Despite multiple scandals that have handed more ammunition to those who object to its continued existence, the VA has not only survived, but thrived. This editorial is written in the form of a debate between exemplar opponents and defenders of the VA on whether it is currently fulfilling its commitment to veterans.
In May 2024, the Veterans Signals survey found that 80.4% of respondents reported trust in the VA, the highest level ever recorded.3 At its 2016 launch, the survey found that only 55% of veterans expressed trust in the VA. The survey was conducted 2 years after the scandal over access to care for veterans in Phoenix. Scores would surely have been even lower than 55% during that period when the critique of the VA—even from those who believe in its mission—was most trenchant.4 Administered quarterly, the survey samples > 38,000 of the 9 million enrolled veterans. Veterans surveyed were using services from all 3 branches of the VA: Veterans Health Administration, Veterans Benefits Administration, and National Cemetery Administration. Participants are asked whether they trust the VA to fulfill the country’s commitment to veterans and specifically how they rate the VA in 3 specific criteria: effectiveness, emotional resonance, and overall ease. In the latest survey, 80.5% of veterans rated the VA positively for effectiveness, 78.4% for emotional resonance, and 75.9% for overall ease. Even more impressive is the 91.8% of participants who reported they trust the VA for outpatient health care, capping a 7-year upward trend.3
The paradigmatic VA antagonist will rightly point out the well-known methodological limitations of this type of survey, including self-selection, sampling bias, and especially low response rates. However, VA researchers will counter that the 18% response rate for the latest Veterans Signals survey is higher than the industry average.5
VA critics might say that it would not matter if the response rate were 4 times higher; what matters is not what veterans say on a survey but what decisions they make about their care. The VA defender would be constrained to concede that even the most statistically sophisticated survey remains an indirect measure of veteran trust. They could, though, marshal far stronger evidence. Two direct demonstrations published in the literature suggest that veterans do as they say and are acting on their trust in the agency. First, the VA delivered more services, health care, and benefits to veterans during the 2023 fiscal year than ever before. Importantly for Federal Practitioner readers, the 16 million documented health care visits were 3 million more than previous records.6 Second, and in some ways even more encouraging for the future of the VA as a health care system, is that due in large part to the passage of the PACT Act, there has been a surge in VA enrollment by veterans. The VA recently announced that in the last year, > 400,000 veterans signed up for its health care and services. Enrollments are 30% more than the previous year and represented the highest figure in the past 5 years, a remarkable 50% increase over 2020 pandemic levels.7
VA critics could legitimately rebut this data by asking, “So more veterans are signing up for VA, and you are delivering more care, but what about the quality of that care? Has it improved?” The VA proponent’s rejoinder from multiple converging empirical studies would be a resounding yes. We have space to cite only a few examples of that rigorous recent research. What stands out ethically about these studies is that the VA has a broad program of research into the quality of the care it delivers and then transparently publishes those findings. The VA quality improvement research mission is truly unique and provides a shared open set of data for both critics and defenders to objectively examine VA successes and failures.
Among the most persuasive analysis was a systematic review of 37 studies contrasting VA with non-VA care from 2015 to 2023. The authors examined clinical quality, safety, patient access, experience, cost-efficiency, and equity of outcome. “VA care is consistently as good as or better than non-VA care in terms of clinical quality and safety,” the systematic review authors stated while qualifying that “Access, cost/efficiency, and patient experience between the 2 systems are not well studied.”8
A second systematic review looked specifically at similar key areas of quality, safety, access, patient experience, and comparative cost-efficiency for surgical treatment delivered in the VA and the community from 2015 to 2021. Only 18 studies met the inclusion criteria, but as the authors argued:
Based on limited data, these findings suggest that expanding eligibility for veterans to get care in the community may not provide benefits in terms of increasing access to surgical procedures, will not result in better quality, and may result in worse quality of care, but may reduce inpatient length of stay and perhaps cost less.9
At this juncture, the faultfinder may become frustrated and resort to a new tactic, challenging the very assumption that is the subject of the debate and demanding proof that there is any connection between veterans’ trust in the VA and their health and well-being. “Fair enough,” the VA side would reply, “here is some research that bolsters that connection.” Kopacz and colleagues examined the relationship between trust and healing at 6 sites and included 427 veterans and active-duty service members with combat posttraumatic stress disorder (PTSD) symptoms. The researchers found that trust and lack thereof are related to several significant mental, social, and physical health outcomes. The authors indicate the need for more research to better understand the importance and impact of trust and healing, but they show it is significant.10 Finally, veterans recognize the crucial link between trust in the unique expertise of VA practitioners in the treatment of PTSD. In a 2019 study, a majority expressed a preference to receive their PTSD treatment at the VA compared to a smaller group choosing care in the community.11
You be the judge of who won the debate, but knowing the dedication of my fellow federal practitioners, many of you will endorse my sentiment that we all need to stop talking and get back to doing our best to enhance veteran trust and healing; doing our essential part to keep fulfilling our commitment.
1. Solomon RC, Fernando F. Building Trust: In Business, Politics, Relationships, and Life. Oxford University Press; 2003:49.
2. Seiken J. 1921: Veterans Bureau is born - precursor to Department of Veterans Affairs. November 12, 2021. Updated September 4, 2023. Accessed July 22, 2024. https://department.va.gov/history/featured-stories/veterans-bureau/
3. US Department of Veterans Affairs. Serving America’s veterans, January 1 - March 31, 2024. Accessed July 22, 2024. https://department.va.gov/veterans-experience/wp-content/uploads/sites/2/2024/05/veteran-trust-report-fiscal-year-2024-quarter-2.pdf
4. Kizer KW, Jha AK. Restoring trust in VA health care. N Engl J Med. 2014;371(4):295-297. doi:10.1056/NEJMp1406852
5. Veteran trust in VA has increased 25% since 2016, reached an all-time high. News release. US Department of Veterans Affairs. May 28, 2024. Accessed July 22, 2024. https://news.va.gov/press-room/veteran-trust-va-increased-25-since-2016-high
6. VA sets all-time records for care and benefits delivered to Veterans in fiscal year 2023. News release. US Department of Veterans Affairs. November 6, 2023. Accessed July 23, 2024. https://news.va.gov/press-room/va-all-time-record-care-benefits-veterans-fy-2023/
7. 400,000+ Veterans enrolled in VA health care over the past 365 days, a 30% increase over last year. News release. US Department of Veterans Affairs. March 29, 2024. Accessed July 23, 2024. https://news.va.gov/press-room/va-enrolled-401006-veterans-healthcare-365/
8. Apaydin EA, Paige NM, Begashaw MM, Larkin J, Miake-Lye IM, Shekelle PG. Veterans Health Administration (VA) vs. non-VA healthcare quality: a systematic review. J Gen Intern Med. 2023;38(9):2179-2188. doi:10.1007/s11606-023-08207-2
9. Blegen M, Ko J, Salzman G, et al. Comparing quality of surgical care between the US Department of Veterans Affairs and non-veterans affairs settings: a systematic review. J Am Coll Surg. 2023;237(2):352-361. doi:10.1097/XCS.0000000000000720
10. Kopacz MS, Ames D, Koenig HG. Association between trust and mental, social, and physical health outcomes in veterans and active duty service members with combat-related PTSD symptomatology. Front Psychiatry. 2018;9:408. doi:10.3389/fpsyt.2018.00408
11. Haro E, Mader M, Noël PH, et al. The impact of trust, satisfaction, and perceived quality on preference for setting of future care among veterans with PTSD. Mil Med. 2019;184(11-12):e708-e714. doi:10.1093/milmed/usz078
1. Solomon RC, Fernando F. Building Trust: In Business, Politics, Relationships, and Life. Oxford University Press; 2003:49.
2. Seiken J. 1921: Veterans Bureau is born - precursor to Department of Veterans Affairs. November 12, 2021. Updated September 4, 2023. Accessed July 22, 2024. https://department.va.gov/history/featured-stories/veterans-bureau/
3. US Department of Veterans Affairs. Serving America’s veterans, January 1 - March 31, 2024. Accessed July 22, 2024. https://department.va.gov/veterans-experience/wp-content/uploads/sites/2/2024/05/veteran-trust-report-fiscal-year-2024-quarter-2.pdf
4. Kizer KW, Jha AK. Restoring trust in VA health care. N Engl J Med. 2014;371(4):295-297. doi:10.1056/NEJMp1406852
5. Veteran trust in VA has increased 25% since 2016, reached an all-time high. News release. US Department of Veterans Affairs. May 28, 2024. Accessed July 22, 2024. https://news.va.gov/press-room/veteran-trust-va-increased-25-since-2016-high
6. VA sets all-time records for care and benefits delivered to Veterans in fiscal year 2023. News release. US Department of Veterans Affairs. November 6, 2023. Accessed July 23, 2024. https://news.va.gov/press-room/va-all-time-record-care-benefits-veterans-fy-2023/
7. 400,000+ Veterans enrolled in VA health care over the past 365 days, a 30% increase over last year. News release. US Department of Veterans Affairs. March 29, 2024. Accessed July 23, 2024. https://news.va.gov/press-room/va-enrolled-401006-veterans-healthcare-365/
8. Apaydin EA, Paige NM, Begashaw MM, Larkin J, Miake-Lye IM, Shekelle PG. Veterans Health Administration (VA) vs. non-VA healthcare quality: a systematic review. J Gen Intern Med. 2023;38(9):2179-2188. doi:10.1007/s11606-023-08207-2
9. Blegen M, Ko J, Salzman G, et al. Comparing quality of surgical care between the US Department of Veterans Affairs and non-veterans affairs settings: a systematic review. J Am Coll Surg. 2023;237(2):352-361. doi:10.1097/XCS.0000000000000720
10. Kopacz MS, Ames D, Koenig HG. Association between trust and mental, social, and physical health outcomes in veterans and active duty service members with combat-related PTSD symptomatology. Front Psychiatry. 2018;9:408. doi:10.3389/fpsyt.2018.00408
11. Haro E, Mader M, Noël PH, et al. The impact of trust, satisfaction, and perceived quality on preference for setting of future care among veterans with PTSD. Mil Med. 2019;184(11-12):e708-e714. doi:10.1093/milmed/usz078
Graduate Medical Education Financing in the US Department of Veterans Affairs
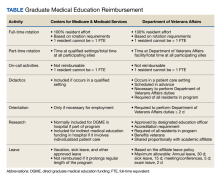
The US Department of Veterans Affairs (VA) has partnered with academic medical centers and programs since 1946 to provide clinical training for physician residents. Ranking second in federal graduate medical education (GME) funding to the Centers for Medicare and Medicaid Services (CMS), the $850 million VA GME budget annually reimburses > 250 GME-sponsoring institutions (affiliates) of 8000 GME programs for the clinical training of 49,000 individual residents rotating through > 11,000 full-time equivalent (FTE) positions.1 The VA also distributes $1.6 billion to VA facilities to offset the costs of conducting health professions education (HPE) (eg, facility infrastructure, salary support for VA instructors and preceptors, education office administration, and instructional equipment).2 The VA financial and educational contributions account for payment of 11% of resident positions nationally and allow academic medical centers to be less reliant on CMS GME funding.3,4 The VA contributions also provide opportunities for GME expansion,1,5,6 educational innovations,5,7 interprofessional and team-based care,8,9 and quality and safety training.10,11 The Table provides a comparison of CMS and VA GME reimbursability based on activity.
GME financing is complex, particularly the formulaic approach used by CMS, the details of which are often obscured in federal regulations. Due to this complexity and the $16 billion CMS GME budget, academic publications have focused on CMS GME financing while not fully explaining the VA GME policies and processes.4,12-14 By comparison, the VA GME financing model is relatively straightforward and governed by different statues and VA regulations, yet sharing some of the same principles as CMS regulations. Given the challenges in CMS reimbursement to fully support the cost of resident education, as well as the educational opportunities at the VA, the VA designs its reimbursement model to assure that affiliates receive appropriate payments.4,12,15 To ensure the continued success of VA GME partnerships, knowledge of VA GME financing has become increasingly important for designated institutional officers (DIOs) and residency program directors, particularly in light of recent investigations into oversight of the VA’s reimbursement to academic affiliates.
VA AUTHORITY
While the VA’s primary mission is “to provide a complete hospital medical service for the medical care and treatment of veterans,” early VA leaders recognized the importance of affiliating with the nation’s academic institutions.19 In 1946, the VA Policy Memorandum Number 2 established a partnership between the VA and the academic medical community.20 Additional legislation authorized specific agreements with academic affiliates for the central administration of salary and benefits for residents rotating at VA facilities. This process, known as disbursement, is an alternative payroll mechanism whereby the VA reimburses the academic affiliate for resident salary and benefits and the affiliate acts as the disbursing agent, issuing paychecks to residents.21,22
Resident FUNDING
By policy, with rare exceptions, the VA does not sponsor residency programs due to the challenges of providing an appropriate patient mix of age, sex, and medical conditions to meet accreditation standards.4 Nearly all VA reimbursements are for residents in affiliate-sponsored programs, while just 1% pays for residents in legacy, VA-sponsored residency programs at 2 VA facilities. The VA budget for resident (including fellows) salary and benefits is managed by the VA Office of Academic Affiliations (OAA), the national VA office responsible for oversight, policy, and funding of VA HPE programs.
Resident Salaries and Benefits
VA funding of resident salary and benefits are analogous with CMS direct GME (DGME), which is designed to cover resident salary and benefits costs.4,14,23 CMS DGME payments depend on a hospital’s volume of CMS inpatients and are based on a statutory formula, which uses the hospital’s resident FTE positions, the per-resident amount, and Medicare’s share of inpatient beds (Medicare patient load) to determine payments.12 The per-resident amount is set by statute, varies geographically, and is calculated by dividing the hospital’s allowable costs of GME (percentage of CMS inpatient days) divided by the number of residents.12,24
By comparison, the VA GME payment reimburses for each FTE based on the salary and benefits rate set by the academic affiliate. Reimbursement is calculated based on resident time spent at the VA multiplied by a daily salary rate. The daily salary rate is determined by dividing the resident’s total compensation (salary and benefits) by the number of calendar days in an academic year. Resident time spent at the VA facility is determined by obtaining rotation schedules provided by the academic affiliate and verifying resident clinical and educational activity during scheduled rotations.
Indirect Medical Education Funding
In addition to resident salary and benefits, funds to offset the cost of conducting HPE are provided to VA facilities. These funds are intended to improve and maintain necessary infrastructure for all HPE programs not just GME, including education office administration needs, teaching costs (ie, a portion of VA preceptors salary), and instructional equipment.
The Veterans Equitable Resource Allocation (VERA) is a national budgeting process for VA medical facilities that funds facility operational needs such as staff salary and benefits, infrastructure, and equipment.2 The education portion of the VERA, the VERA Education Support Component (VESC), is not managed by the OAA, but rather is distributed through the VERA model to the general budget of VA facilities hosting HPE (Figure). VESC funding in the VA budget is based on labor mapping of physician time spent in education; other labor mapping categories include clinical care, research, and administration. VA facility VESC funding is calculated based on the number of paid health profession trainees (HPTs) from all professions, apportioned according to the number of FTEs for physician residents and VA-paid HPTs in other disciplines. In fiscal year 2024, VA facilities received $115,812 for each physician resident FTE position and $84,906 for each VA-paid, non-GME FTE position.
The VESC is like CMS's indirect GME funding, termed Indirect Medical Education (IME), an additional payment for each Medicare patient discharged reflecting teaching hospitals’ higher patient care costs relative to nonteaching hospitals. Described elsewhere, IME is calculated using a resident-to-bed ratio and a multiplier, which is set by statute.4,25 While IME can be used for reimbursement for some resident clinical and educational activities(eg, research), VA VESC funds cannot be used for such activities and are part of the general facility budget and appropriated per the discretion of the medical facility director.
ESTABLISHING GME PARTNERSHIPS
An affiliation agreement establishes the administrative and legal requirements for educational relationships with academic affiliates and includes standards for conducting HPE, responsibilities for accreditation standards, program leadership, faculty, resources, supervision, academic policies, and procedures. The VA uses standardized affiliation agreement templates that have been vetted with accrediting bodies and the VA Office of General Counsel.
A disbursement agreement authorizes the VA to reimburse affiliates for resident salary and benefits for VA clinical and educational activities. The disbursement agreement details the fiscal arrangements (eg, payment in advance vs arrears, salary, and benefit rates, leave) for the reimbursement payments. Veterans Health Administration (VHA) Directive 1400.05 provides the policy and procedures for calculating reimbursement for HPT educational activities.26
The VA facility designated education officer (DEO) oversees all HPE programs and coordinates the affiliation and disbursement agreement processes.27 The DEO, affiliate DIO, residency program director, and VA residency site director determine the physician resident FTE positions assigned to a VA facility based on educational objectives and availability of educational resources at the VA facility, such as patient care opportunities, faculty supervisors, space, and equipment. The VA facility requests for resident FTE positions are submitted to the OAA by the facility DEO.
Once GME FTE positions are approved by the OAA, VA facilities work with their academic affiliate to submit the physician resident salary and benefit rate. Affiliate DIOs attest to the accuracy of the salary rate schedule and the local DEO submits the budget request to the OAA. Upon approval, the funds are transferred to the VA facility each fiscal year, which begins October 1. DEOs report quarterly to the OAA both budget needs and excesses based on variations in the approved FTEs due to additional VA rotations, physician resident attrition, or reassignment.
Resident Position Allocation
VA GME financing provides flexibility through periodic needs assessments and expansion initiatives. In August and December, DEOs collaborate with an academic affiliate to submit reports to the OAA confirming their projected GME needs for the next academic year. Additional positions requests are reviewed by the OAA; funding depends on budget and the educational justification. The OAA periodically issues GME expansion requests for proposal, which typically arise from legislation to address specific VA workforce needs. The VA facility DEO and affiliate GME leaders collaborate to apply for additional positions. For example, a VA GME expansion under the Veterans Access, Choice, and Accountability Act of 2014 added 1500 GME positions in 8 years for critically needed specialties and in rural and underserved areas.5 The Maintaining Internal Systems and Strengthening Outside Networks (MISSION) Act of 2018 authorized a pilot program for VA to fund residents at non-VA facilities with priority for Indian Health Services, Tribes and Tribal Organizations, Federally Qualified Health Centers, and US Department of Defense facilities to provide access to veterans in underserved areas.6
The VA GME financing system has flexibility to meet local needs for additional resident positions and to address broader VA workforce gaps through targeted expansion. Generally, CMS does not fund positions to address workforce needs, place residents in specific geographic areas, or require the training of certain types of residents.4 However, the Consolidated Appropriations Act of 2021 has provided the opportunity to address rural workforce needs.28
Reimbursement
The VA provides reimbursement for clinical and educational activities performed in VA facilities for the benefit of veterans as well as research, didactics, meetings and conferences, annual and sick leave, and orientation. The VA also may provide reimbursement for educational activities that occur off VA grounds (eg, the VA proportional share of a residency program’s didactic sessions). The VA does not reimburse for affiliate clinical duties or administrative costs, although a national policy allows VA facilities to reimburse affiliates for some GME overhead costs.29
CMS similarly reimburses for residency training time spent in patient care activities as well as orientation activities, didactics, leave, and, in some cases, research.4,30,31 CMS makes payments to hospitals, which may include sponsoring institutions and Medicare-eligible participating training sites.4,30,31 For both the VA and CMS, residents may not be counted twice for reimbursement by 2 federal agencies; in other words, a resident may not count for > 1 FTE.4,30-32
GME Oversight
VA GME funding came under significant scrutiny. At a 2016 House Veterans Affairs Committee hearing, Representative Phil Roe, MD (R-Tennessee), noted that no process existed at many VA facilities for “determining trainee presence” and that many VA medical centers had “difficulty tracking resident rotations”16 A VA Office of the Inspector General investigation recommended that the VA implement policies and procedures to improve oversight to “ensure residents are fully participating in educational activities” and that the VA is “paying the correct amount” to the affiliate.17 A 2020 General Accountability Office report outlined unclear policy guidance, incomplete tracking of resident activities, and improper fiscal processes for reimbursement and reconciliation of affiliate invoices.18
In response, the OAA created an oversight and compliance unit, revised VHA Directive 1400.05 (the policy for disbursement), and improved resident tracking procedures.26 The standard operating procedure that accompanied VHA Directive 1400.05 provides detailed information for the DEO and VA facility staff for tracking resident clinical and educational activities. FTE counts are essential to both VA and CMS for accurate reimbursement. The eAppendix and the Table provide a guide to reimbursable activities in the VA for the calculation of reimbursement, with a comparison to CMS.33,34 The OAA in cooperation with other VA staff and officers periodically conducts audits to assess compliance with disbursement policy and affiliate reimbursement accuracy.
In the VA, resident activities are captured on the VA Educational Activity Record, a standardized spreadsheet to track activities and calculate reimbursement. Each VA facility hosting resident physicians manually records resident activity by the half-day. This process is labor intensive, involving both VA and affiliate staff to accurately reconcile payments. To address the workload demands, the OAA is developing an online tool that will automate aspects of the tracking process. Also, to ensure adequate staffing, the OAA is in the process of implementing an office optimization project, providing standardized position descriptions, an organizational chart, and staffing levels for DEO offices in VA facilities.
Conclusions
This report describes the key policies and principles of VA GME financing, highlighting the essential similarities and differences between VA and CMS. Neither the VA nor CMS regulations allow for reimbursement for > 1 FTE position per resident, a principle that underpins the assignment of resident rotations and federal funding for GME and are similar with respect to reimbursement for patient care activities, didactics, research, orientation, and scholarly activity. While reimbursable activities in the VA require physical presence and care of veteran patients, CMS also limits reimbursement to resident activities in the hospital and approved other settings if the hospital is paying for resident salary and benefits in these settings. The VA provides some flexibility for offsite activities including didactics and, in specific circumstances, remote care of veteran patients (eg, teleradiology).
The VA and CMS use different GME financing models. For example, the CMS calculations for resident FTEs are complex, whereas VA calculations reimburse the salary and benefits as set by the academic affiliate. The VA process accounts for local variation in salary rates, whereas the per-resident amount set by CMS varies regionally and does not fully account for differences in the cost of living.24 Because all patients in VA facilities are veterans, VA calculations for reimbursement do not involve ratios of beds like the CMS calculations to determine a proportional share of reimbursement. The VA GME expansion tends to be more directed to VA health workforce needs than CMS, specifying the types of programs and geographic locations to address these needs.
The VA regularly reevaluates how affiliates are reimbursed for VA resident activity, balancing compliance with VA policies and the workload for VA and its affiliates. The VA obtains input from key stakeholders including DEOs, DIOs, and professional organizations such as the Association of American Medical Colleges and the Accreditation Council for Graduate Medical Education.35,36
Looking ahead, the VA is developing an online tool to improve the accuracy of affiliate reimbursement. The VA will also implement a standardized staffing model, organizational structure, and position descriptions for DEO offices. These initiatives will help reduce the burden of tracking and verifying resident activity and continue to support the 77-year partnership between VA and its affiliated institutions.
1. Klink KA, Albanese AP, Bope ET, Sanders KM. Veterans Affairs graduate medical education expansion addresses US physician workforce needs. Acad Med. 2022;97(8):1144-1150. doi:10.1097/ACM.0000000000004545
2. Andrus CH, Johnson K, Pierce E, Romito PJ, Hartel P, Berrios‐Guccione S, Best W. Finance modeling in the delivery of medical care in tertiary‐care hospitals in the Department of Veterans Affairs. J Surg Res. 2001;96(2):152-157. doi:10.1006/jsre.1999.5728
3. Petrakis IL, Kozal M. Academic medical centers and the U.S. Department of Veterans Affairs: a 75-year partnership influences medical education, scientific discovery, and clinical care. Acad Med. 2022;97(8):1110-1113. doi:10.1097/ACM.0000000000004734
4. Heisler EJ, Mendez BH, Mitchell A, Panangala SV, Villagrana MA. Federal support for graduate medical education: an overview (R44376). Congressional Research Service report R44376; version 11. Updated December 27, 2018. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/R/R44376/11
5. Chang BK, Brannen JL. The Veterans Access, Choice, and Accountability Act of 2014: examining graduate medical education enhancement in the Department of Veterans Affairs. Acad Med. 2015;90(9):1196-1198. doi:10.1097/ACM.0000000000000795
6. Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
7. Lypson ML, Roberts LW. Valuing the partnership between the Veterans Health Administration and academic medicine. Acad Med. 2022;97(8):1091-1093. doi:10.1097/ACM.0000000000004748
8. Harada ND, Traylor L, Rugen KW, et al. Interprofessional transformation of clinical education: the first six years of the Veterans Affairs Centers of Excellence in Primary Care Education. J Interprof Care. 2023;37(suppl 1):S86-S94. doi:10.1080/13561820.2018.1433642

9. Harada ND, Rajashekara S, Sansgiry S, et al. Developing interprofessional primary care teams: alumni evaluation of the Department of Veterans Affairs Centers of Excellence in Primary Care Education Program. J Med Educ Curric Dev. 2019;6:2382120519875455. doi:10.1177/2382120519875455
10. Splaine ME, Ogrinc G, Gilman SC, et al. The Department of Veterans Affairs National Quality Scholars Fellowship Program: experience from 10 years of training quality scholars. Acad Med. 2009;84(12):1741-1748. doi:10.1097/ACM.0b013e3181bfdcef
11. Watts BV, Paull DE, Williams LC, Neily J, Hemphill RR, Brannen JL. Department of Veterans Affairs chief resident in quality and patient safety program: a model to spread change. Am J Med Qual. 2016;31(6):598-600. doi:10.1177/1062860616643403
12. He K, Whang E, Kristo G. Graduate medical education funding mechanisms, challenges, and solutions: a narrative review. Am J Surg. 2021;221(1):65-71. doi:10.1016/j.amjsurg.2020.06.007
13. Villagrana M. Medicare graduate medical education payments: an overview. Congressional Research Service report IF10960. Updated September 29, 2022. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/IF/IF10960
14. Committee on the Governance and Financing of Graduate Medical Education; Board on Health Care Services; Institute of Medicine. Graduate Medical Education That Meets the Nation’s Health Needs. Eden J, Berwick DM, Wilensky GR, eds. Washington, DC: National Academies Press; 2014. doi:10.17226/18754
15. Physician workforce: caps on Medicare-funded graduate medical education at teaching hospitals. Report to congressional requesters. GAO-21-391. May 21, 2021. Accessed March 1, 2024. https://www.gao.gov/assets/gao-21-391.pdf
16. VA and Academic Affiliates: Who Benefits? Hearing Before the Subcommittee on Oversight and Investigations of the Committee on Veterans’ Affairs, 114th Cong, 2nd Sess (2016). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CHRG-115hhrg29685/html/CHRG-115hhrg29685.htm
17. US Department of Veterans Affairs, Office of Inspector General (OIG). Veterans Health Administration. Review of resident and part-time physician time and attendance at the Oklahoma City VA Health Care System. OIG report 17-00253-93. March 28, 2018. Accessed March 1, 2024. https://www.oversight.gov/sites/default/files/oig-reports/VAOIG-17-00253-93.pdf
18. VA health care: actions needed to improve oversight of graduate medical education reimbursement. Report to the ranking member, Committee on Veterans’ Affairs, House of Representatives. GAO-20-553. July 2020. Accessed March 1, 2024. https://www.gao.gov/assets/710/708275.pdf
19. Functions of Veterans Health Administration: in general, 38 USC §7301 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap73-subchapI-sec7301.pdf
20. US Department of Veterans Affairs. Policy memorandum no. 2, policy in association of veterans’ hospitals with medical schools. January 30, 1946.
21. Veterans Health Care Expansion Act of 1973, Public Law 93-82. August 2, 1973. Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/STATUTE-87/pdf/STATUTE-87-Pg179.pdf
22. Residencies and internships, 38 USC § 7406 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap74-subchapI-sec7406.pdf
23. Direct graduate medical education (DGME). Centers for Medicaid and Medicare Services. Updated December 5, 2023. Accessed March 1, 2024. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/DGME
24. Drezdzon MK, Cowley NJ, Sweeney DP, et al. Going for broke: the impact of cost of living on surgery resident stipend value. Ann Surg. 2023;278(6):1053-1059. doi:10.1097/SLA.0000000000005923
25. Special treatment: hospitals that incur indirect costs for graduate medical education programs, 42 CFR § 412.105 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec412-105.pdf
26. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.05, Disbursement agreements for health professions trainees appointed under 38 U.S.C. § 7406. June 2, 2021. Accessed March 1, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9293
27. Harada ND, Sanders KM, Bowman MA. Health systems education leadership: learning from the VA designated education officer role. Fed Pract. 2022;39(6):266-273. doi:10.12788/fp.0278
28. Schleiter Hitchell K, Johnson L. CMS finalizes rules for distribution of 1000 new Medicare-funded residency positions and changes to rural training track programs. J Grad Med Educ. 2022;14(2):245-249. doi:10.4300/JGME-D-22-00193.1

29. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.10, Educational cost contracts for health professions education. September 25, 2023. Accessed March 1, 2024. https://www.va.gov/VHAPUBLICATIONS/ViewPublication.asp?pub_ID=11480
30. Direct GME payments: general requirements, 42 CFR § 413.75 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-75.pdf
31. Direct GME payments: determination of the total number of FTE residents, 42 CFR § 413.78 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-78.pdf
32. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Medicare financial management manual, chapter 8. Contractor procedures for provider audits. Accessed March 1, 2024. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/fin106c08.pdf
33. US Department of Health and Human Services, Office of Inspector General. CMS did not always ensure hospitals complied with Medicare reimbursement requirements for graduate medical education. OIG report A-02-17-01017. November 2018. Accessed March 1, 2024. https://oig.hhs.gov/oas/reports/region2/21701017.pdf
34. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Interns and Residents Information System (IRIS) XML format. Publication 100-20. Transmittal 11418. Change request 12724. May 19, 2022. Accessed March 1, 2024. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/R11418OTN.pdf
35. Birnbaum AD, Byrne J, on behalf of the VA Office of Academic Affiliations. VHA Updates: Disbursement Policy and Education Cost Contracts. Presented at: American Association of Medical Colleges Webinar; June 2021. Accessed March 1, 2024. https://vimeo.com/644415670
36. Byrne JM, on behalf of the VA Office of Academic Affiliations. Disbursement procedures update for AY 23-24. Accessed March 1, 2024. https://www.va.gov/oaa/Videos/AffiliatePresentationDisbursementandEARsAY23-24.pptx
The US Department of Veterans Affairs (VA) has partnered with academic medical centers and programs since 1946 to provide clinical training for physician residents. Ranking second in federal graduate medical education (GME) funding to the Centers for Medicare and Medicaid Services (CMS), the $850 million VA GME budget annually reimburses > 250 GME-sponsoring institutions (affiliates) of 8000 GME programs for the clinical training of 49,000 individual residents rotating through > 11,000 full-time equivalent (FTE) positions.1 The VA also distributes $1.6 billion to VA facilities to offset the costs of conducting health professions education (HPE) (eg, facility infrastructure, salary support for VA instructors and preceptors, education office administration, and instructional equipment).2 The VA financial and educational contributions account for payment of 11% of resident positions nationally and allow academic medical centers to be less reliant on CMS GME funding.3,4 The VA contributions also provide opportunities for GME expansion,1,5,6 educational innovations,5,7 interprofessional and team-based care,8,9 and quality and safety training.10,11 The Table provides a comparison of CMS and VA GME reimbursability based on activity.
GME financing is complex, particularly the formulaic approach used by CMS, the details of which are often obscured in federal regulations. Due to this complexity and the $16 billion CMS GME budget, academic publications have focused on CMS GME financing while not fully explaining the VA GME policies and processes.4,12-14 By comparison, the VA GME financing model is relatively straightforward and governed by different statues and VA regulations, yet sharing some of the same principles as CMS regulations. Given the challenges in CMS reimbursement to fully support the cost of resident education, as well as the educational opportunities at the VA, the VA designs its reimbursement model to assure that affiliates receive appropriate payments.4,12,15 To ensure the continued success of VA GME partnerships, knowledge of VA GME financing has become increasingly important for designated institutional officers (DIOs) and residency program directors, particularly in light of recent investigations into oversight of the VA’s reimbursement to academic affiliates.
VA AUTHORITY
While the VA’s primary mission is “to provide a complete hospital medical service for the medical care and treatment of veterans,” early VA leaders recognized the importance of affiliating with the nation’s academic institutions.19 In 1946, the VA Policy Memorandum Number 2 established a partnership between the VA and the academic medical community.20 Additional legislation authorized specific agreements with academic affiliates for the central administration of salary and benefits for residents rotating at VA facilities. This process, known as disbursement, is an alternative payroll mechanism whereby the VA reimburses the academic affiliate for resident salary and benefits and the affiliate acts as the disbursing agent, issuing paychecks to residents.21,22
Resident FUNDING
By policy, with rare exceptions, the VA does not sponsor residency programs due to the challenges of providing an appropriate patient mix of age, sex, and medical conditions to meet accreditation standards.4 Nearly all VA reimbursements are for residents in affiliate-sponsored programs, while just 1% pays for residents in legacy, VA-sponsored residency programs at 2 VA facilities. The VA budget for resident (including fellows) salary and benefits is managed by the VA Office of Academic Affiliations (OAA), the national VA office responsible for oversight, policy, and funding of VA HPE programs.
Resident Salaries and Benefits
VA funding of resident salary and benefits are analogous with CMS direct GME (DGME), which is designed to cover resident salary and benefits costs.4,14,23 CMS DGME payments depend on a hospital’s volume of CMS inpatients and are based on a statutory formula, which uses the hospital’s resident FTE positions, the per-resident amount, and Medicare’s share of inpatient beds (Medicare patient load) to determine payments.12 The per-resident amount is set by statute, varies geographically, and is calculated by dividing the hospital’s allowable costs of GME (percentage of CMS inpatient days) divided by the number of residents.12,24
By comparison, the VA GME payment reimburses for each FTE based on the salary and benefits rate set by the academic affiliate. Reimbursement is calculated based on resident time spent at the VA multiplied by a daily salary rate. The daily salary rate is determined by dividing the resident’s total compensation (salary and benefits) by the number of calendar days in an academic year. Resident time spent at the VA facility is determined by obtaining rotation schedules provided by the academic affiliate and verifying resident clinical and educational activity during scheduled rotations.
Indirect Medical Education Funding
In addition to resident salary and benefits, funds to offset the cost of conducting HPE are provided to VA facilities. These funds are intended to improve and maintain necessary infrastructure for all HPE programs not just GME, including education office administration needs, teaching costs (ie, a portion of VA preceptors salary), and instructional equipment.
The Veterans Equitable Resource Allocation (VERA) is a national budgeting process for VA medical facilities that funds facility operational needs such as staff salary and benefits, infrastructure, and equipment.2 The education portion of the VERA, the VERA Education Support Component (VESC), is not managed by the OAA, but rather is distributed through the VERA model to the general budget of VA facilities hosting HPE (Figure). VESC funding in the VA budget is based on labor mapping of physician time spent in education; other labor mapping categories include clinical care, research, and administration. VA facility VESC funding is calculated based on the number of paid health profession trainees (HPTs) from all professions, apportioned according to the number of FTEs for physician residents and VA-paid HPTs in other disciplines. In fiscal year 2024, VA facilities received $115,812 for each physician resident FTE position and $84,906 for each VA-paid, non-GME FTE position.
The VESC is like CMS's indirect GME funding, termed Indirect Medical Education (IME), an additional payment for each Medicare patient discharged reflecting teaching hospitals’ higher patient care costs relative to nonteaching hospitals. Described elsewhere, IME is calculated using a resident-to-bed ratio and a multiplier, which is set by statute.4,25 While IME can be used for reimbursement for some resident clinical and educational activities(eg, research), VA VESC funds cannot be used for such activities and are part of the general facility budget and appropriated per the discretion of the medical facility director.
ESTABLISHING GME PARTNERSHIPS
An affiliation agreement establishes the administrative and legal requirements for educational relationships with academic affiliates and includes standards for conducting HPE, responsibilities for accreditation standards, program leadership, faculty, resources, supervision, academic policies, and procedures. The VA uses standardized affiliation agreement templates that have been vetted with accrediting bodies and the VA Office of General Counsel.
A disbursement agreement authorizes the VA to reimburse affiliates for resident salary and benefits for VA clinical and educational activities. The disbursement agreement details the fiscal arrangements (eg, payment in advance vs arrears, salary, and benefit rates, leave) for the reimbursement payments. Veterans Health Administration (VHA) Directive 1400.05 provides the policy and procedures for calculating reimbursement for HPT educational activities.26
The VA facility designated education officer (DEO) oversees all HPE programs and coordinates the affiliation and disbursement agreement processes.27 The DEO, affiliate DIO, residency program director, and VA residency site director determine the physician resident FTE positions assigned to a VA facility based on educational objectives and availability of educational resources at the VA facility, such as patient care opportunities, faculty supervisors, space, and equipment. The VA facility requests for resident FTE positions are submitted to the OAA by the facility DEO.
Once GME FTE positions are approved by the OAA, VA facilities work with their academic affiliate to submit the physician resident salary and benefit rate. Affiliate DIOs attest to the accuracy of the salary rate schedule and the local DEO submits the budget request to the OAA. Upon approval, the funds are transferred to the VA facility each fiscal year, which begins October 1. DEOs report quarterly to the OAA both budget needs and excesses based on variations in the approved FTEs due to additional VA rotations, physician resident attrition, or reassignment.
Resident Position Allocation
VA GME financing provides flexibility through periodic needs assessments and expansion initiatives. In August and December, DEOs collaborate with an academic affiliate to submit reports to the OAA confirming their projected GME needs for the next academic year. Additional positions requests are reviewed by the OAA; funding depends on budget and the educational justification. The OAA periodically issues GME expansion requests for proposal, which typically arise from legislation to address specific VA workforce needs. The VA facility DEO and affiliate GME leaders collaborate to apply for additional positions. For example, a VA GME expansion under the Veterans Access, Choice, and Accountability Act of 2014 added 1500 GME positions in 8 years for critically needed specialties and in rural and underserved areas.5 The Maintaining Internal Systems and Strengthening Outside Networks (MISSION) Act of 2018 authorized a pilot program for VA to fund residents at non-VA facilities with priority for Indian Health Services, Tribes and Tribal Organizations, Federally Qualified Health Centers, and US Department of Defense facilities to provide access to veterans in underserved areas.6
The VA GME financing system has flexibility to meet local needs for additional resident positions and to address broader VA workforce gaps through targeted expansion. Generally, CMS does not fund positions to address workforce needs, place residents in specific geographic areas, or require the training of certain types of residents.4 However, the Consolidated Appropriations Act of 2021 has provided the opportunity to address rural workforce needs.28
Reimbursement
The VA provides reimbursement for clinical and educational activities performed in VA facilities for the benefit of veterans as well as research, didactics, meetings and conferences, annual and sick leave, and orientation. The VA also may provide reimbursement for educational activities that occur off VA grounds (eg, the VA proportional share of a residency program’s didactic sessions). The VA does not reimburse for affiliate clinical duties or administrative costs, although a national policy allows VA facilities to reimburse affiliates for some GME overhead costs.29
CMS similarly reimburses for residency training time spent in patient care activities as well as orientation activities, didactics, leave, and, in some cases, research.4,30,31 CMS makes payments to hospitals, which may include sponsoring institutions and Medicare-eligible participating training sites.4,30,31 For both the VA and CMS, residents may not be counted twice for reimbursement by 2 federal agencies; in other words, a resident may not count for > 1 FTE.4,30-32
GME Oversight
VA GME funding came under significant scrutiny. At a 2016 House Veterans Affairs Committee hearing, Representative Phil Roe, MD (R-Tennessee), noted that no process existed at many VA facilities for “determining trainee presence” and that many VA medical centers had “difficulty tracking resident rotations”16 A VA Office of the Inspector General investigation recommended that the VA implement policies and procedures to improve oversight to “ensure residents are fully participating in educational activities” and that the VA is “paying the correct amount” to the affiliate.17 A 2020 General Accountability Office report outlined unclear policy guidance, incomplete tracking of resident activities, and improper fiscal processes for reimbursement and reconciliation of affiliate invoices.18
In response, the OAA created an oversight and compliance unit, revised VHA Directive 1400.05 (the policy for disbursement), and improved resident tracking procedures.26 The standard operating procedure that accompanied VHA Directive 1400.05 provides detailed information for the DEO and VA facility staff for tracking resident clinical and educational activities. FTE counts are essential to both VA and CMS for accurate reimbursement. The eAppendix and the Table provide a guide to reimbursable activities in the VA for the calculation of reimbursement, with a comparison to CMS.33,34 The OAA in cooperation with other VA staff and officers periodically conducts audits to assess compliance with disbursement policy and affiliate reimbursement accuracy.
In the VA, resident activities are captured on the VA Educational Activity Record, a standardized spreadsheet to track activities and calculate reimbursement. Each VA facility hosting resident physicians manually records resident activity by the half-day. This process is labor intensive, involving both VA and affiliate staff to accurately reconcile payments. To address the workload demands, the OAA is developing an online tool that will automate aspects of the tracking process. Also, to ensure adequate staffing, the OAA is in the process of implementing an office optimization project, providing standardized position descriptions, an organizational chart, and staffing levels for DEO offices in VA facilities.
Conclusions
This report describes the key policies and principles of VA GME financing, highlighting the essential similarities and differences between VA and CMS. Neither the VA nor CMS regulations allow for reimbursement for > 1 FTE position per resident, a principle that underpins the assignment of resident rotations and federal funding for GME and are similar with respect to reimbursement for patient care activities, didactics, research, orientation, and scholarly activity. While reimbursable activities in the VA require physical presence and care of veteran patients, CMS also limits reimbursement to resident activities in the hospital and approved other settings if the hospital is paying for resident salary and benefits in these settings. The VA provides some flexibility for offsite activities including didactics and, in specific circumstances, remote care of veteran patients (eg, teleradiology).
The VA and CMS use different GME financing models. For example, the CMS calculations for resident FTEs are complex, whereas VA calculations reimburse the salary and benefits as set by the academic affiliate. The VA process accounts for local variation in salary rates, whereas the per-resident amount set by CMS varies regionally and does not fully account for differences in the cost of living.24 Because all patients in VA facilities are veterans, VA calculations for reimbursement do not involve ratios of beds like the CMS calculations to determine a proportional share of reimbursement. The VA GME expansion tends to be more directed to VA health workforce needs than CMS, specifying the types of programs and geographic locations to address these needs.
The VA regularly reevaluates how affiliates are reimbursed for VA resident activity, balancing compliance with VA policies and the workload for VA and its affiliates. The VA obtains input from key stakeholders including DEOs, DIOs, and professional organizations such as the Association of American Medical Colleges and the Accreditation Council for Graduate Medical Education.35,36
Looking ahead, the VA is developing an online tool to improve the accuracy of affiliate reimbursement. The VA will also implement a standardized staffing model, organizational structure, and position descriptions for DEO offices. These initiatives will help reduce the burden of tracking and verifying resident activity and continue to support the 77-year partnership between VA and its affiliated institutions.
The US Department of Veterans Affairs (VA) has partnered with academic medical centers and programs since 1946 to provide clinical training for physician residents. Ranking second in federal graduate medical education (GME) funding to the Centers for Medicare and Medicaid Services (CMS), the $850 million VA GME budget annually reimburses > 250 GME-sponsoring institutions (affiliates) of 8000 GME programs for the clinical training of 49,000 individual residents rotating through > 11,000 full-time equivalent (FTE) positions.1 The VA also distributes $1.6 billion to VA facilities to offset the costs of conducting health professions education (HPE) (eg, facility infrastructure, salary support for VA instructors and preceptors, education office administration, and instructional equipment).2 The VA financial and educational contributions account for payment of 11% of resident positions nationally and allow academic medical centers to be less reliant on CMS GME funding.3,4 The VA contributions also provide opportunities for GME expansion,1,5,6 educational innovations,5,7 interprofessional and team-based care,8,9 and quality and safety training.10,11 The Table provides a comparison of CMS and VA GME reimbursability based on activity.
GME financing is complex, particularly the formulaic approach used by CMS, the details of which are often obscured in federal regulations. Due to this complexity and the $16 billion CMS GME budget, academic publications have focused on CMS GME financing while not fully explaining the VA GME policies and processes.4,12-14 By comparison, the VA GME financing model is relatively straightforward and governed by different statues and VA regulations, yet sharing some of the same principles as CMS regulations. Given the challenges in CMS reimbursement to fully support the cost of resident education, as well as the educational opportunities at the VA, the VA designs its reimbursement model to assure that affiliates receive appropriate payments.4,12,15 To ensure the continued success of VA GME partnerships, knowledge of VA GME financing has become increasingly important for designated institutional officers (DIOs) and residency program directors, particularly in light of recent investigations into oversight of the VA’s reimbursement to academic affiliates.
VA AUTHORITY
While the VA’s primary mission is “to provide a complete hospital medical service for the medical care and treatment of veterans,” early VA leaders recognized the importance of affiliating with the nation’s academic institutions.19 In 1946, the VA Policy Memorandum Number 2 established a partnership between the VA and the academic medical community.20 Additional legislation authorized specific agreements with academic affiliates for the central administration of salary and benefits for residents rotating at VA facilities. This process, known as disbursement, is an alternative payroll mechanism whereby the VA reimburses the academic affiliate for resident salary and benefits and the affiliate acts as the disbursing agent, issuing paychecks to residents.21,22
Resident FUNDING
By policy, with rare exceptions, the VA does not sponsor residency programs due to the challenges of providing an appropriate patient mix of age, sex, and medical conditions to meet accreditation standards.4 Nearly all VA reimbursements are for residents in affiliate-sponsored programs, while just 1% pays for residents in legacy, VA-sponsored residency programs at 2 VA facilities. The VA budget for resident (including fellows) salary and benefits is managed by the VA Office of Academic Affiliations (OAA), the national VA office responsible for oversight, policy, and funding of VA HPE programs.
Resident Salaries and Benefits
VA funding of resident salary and benefits are analogous with CMS direct GME (DGME), which is designed to cover resident salary and benefits costs.4,14,23 CMS DGME payments depend on a hospital’s volume of CMS inpatients and are based on a statutory formula, which uses the hospital’s resident FTE positions, the per-resident amount, and Medicare’s share of inpatient beds (Medicare patient load) to determine payments.12 The per-resident amount is set by statute, varies geographically, and is calculated by dividing the hospital’s allowable costs of GME (percentage of CMS inpatient days) divided by the number of residents.12,24
By comparison, the VA GME payment reimburses for each FTE based on the salary and benefits rate set by the academic affiliate. Reimbursement is calculated based on resident time spent at the VA multiplied by a daily salary rate. The daily salary rate is determined by dividing the resident’s total compensation (salary and benefits) by the number of calendar days in an academic year. Resident time spent at the VA facility is determined by obtaining rotation schedules provided by the academic affiliate and verifying resident clinical and educational activity during scheduled rotations.
Indirect Medical Education Funding
In addition to resident salary and benefits, funds to offset the cost of conducting HPE are provided to VA facilities. These funds are intended to improve and maintain necessary infrastructure for all HPE programs not just GME, including education office administration needs, teaching costs (ie, a portion of VA preceptors salary), and instructional equipment.
The Veterans Equitable Resource Allocation (VERA) is a national budgeting process for VA medical facilities that funds facility operational needs such as staff salary and benefits, infrastructure, and equipment.2 The education portion of the VERA, the VERA Education Support Component (VESC), is not managed by the OAA, but rather is distributed through the VERA model to the general budget of VA facilities hosting HPE (Figure). VESC funding in the VA budget is based on labor mapping of physician time spent in education; other labor mapping categories include clinical care, research, and administration. VA facility VESC funding is calculated based on the number of paid health profession trainees (HPTs) from all professions, apportioned according to the number of FTEs for physician residents and VA-paid HPTs in other disciplines. In fiscal year 2024, VA facilities received $115,812 for each physician resident FTE position and $84,906 for each VA-paid, non-GME FTE position.
The VESC is like CMS's indirect GME funding, termed Indirect Medical Education (IME), an additional payment for each Medicare patient discharged reflecting teaching hospitals’ higher patient care costs relative to nonteaching hospitals. Described elsewhere, IME is calculated using a resident-to-bed ratio and a multiplier, which is set by statute.4,25 While IME can be used for reimbursement for some resident clinical and educational activities(eg, research), VA VESC funds cannot be used for such activities and are part of the general facility budget and appropriated per the discretion of the medical facility director.
ESTABLISHING GME PARTNERSHIPS
An affiliation agreement establishes the administrative and legal requirements for educational relationships with academic affiliates and includes standards for conducting HPE, responsibilities for accreditation standards, program leadership, faculty, resources, supervision, academic policies, and procedures. The VA uses standardized affiliation agreement templates that have been vetted with accrediting bodies and the VA Office of General Counsel.
A disbursement agreement authorizes the VA to reimburse affiliates for resident salary and benefits for VA clinical and educational activities. The disbursement agreement details the fiscal arrangements (eg, payment in advance vs arrears, salary, and benefit rates, leave) for the reimbursement payments. Veterans Health Administration (VHA) Directive 1400.05 provides the policy and procedures for calculating reimbursement for HPT educational activities.26
The VA facility designated education officer (DEO) oversees all HPE programs and coordinates the affiliation and disbursement agreement processes.27 The DEO, affiliate DIO, residency program director, and VA residency site director determine the physician resident FTE positions assigned to a VA facility based on educational objectives and availability of educational resources at the VA facility, such as patient care opportunities, faculty supervisors, space, and equipment. The VA facility requests for resident FTE positions are submitted to the OAA by the facility DEO.
Once GME FTE positions are approved by the OAA, VA facilities work with their academic affiliate to submit the physician resident salary and benefit rate. Affiliate DIOs attest to the accuracy of the salary rate schedule and the local DEO submits the budget request to the OAA. Upon approval, the funds are transferred to the VA facility each fiscal year, which begins October 1. DEOs report quarterly to the OAA both budget needs and excesses based on variations in the approved FTEs due to additional VA rotations, physician resident attrition, or reassignment.
Resident Position Allocation
VA GME financing provides flexibility through periodic needs assessments and expansion initiatives. In August and December, DEOs collaborate with an academic affiliate to submit reports to the OAA confirming their projected GME needs for the next academic year. Additional positions requests are reviewed by the OAA; funding depends on budget and the educational justification. The OAA periodically issues GME expansion requests for proposal, which typically arise from legislation to address specific VA workforce needs. The VA facility DEO and affiliate GME leaders collaborate to apply for additional positions. For example, a VA GME expansion under the Veterans Access, Choice, and Accountability Act of 2014 added 1500 GME positions in 8 years for critically needed specialties and in rural and underserved areas.5 The Maintaining Internal Systems and Strengthening Outside Networks (MISSION) Act of 2018 authorized a pilot program for VA to fund residents at non-VA facilities with priority for Indian Health Services, Tribes and Tribal Organizations, Federally Qualified Health Centers, and US Department of Defense facilities to provide access to veterans in underserved areas.6
The VA GME financing system has flexibility to meet local needs for additional resident positions and to address broader VA workforce gaps through targeted expansion. Generally, CMS does not fund positions to address workforce needs, place residents in specific geographic areas, or require the training of certain types of residents.4 However, the Consolidated Appropriations Act of 2021 has provided the opportunity to address rural workforce needs.28
Reimbursement
The VA provides reimbursement for clinical and educational activities performed in VA facilities for the benefit of veterans as well as research, didactics, meetings and conferences, annual and sick leave, and orientation. The VA also may provide reimbursement for educational activities that occur off VA grounds (eg, the VA proportional share of a residency program’s didactic sessions). The VA does not reimburse for affiliate clinical duties or administrative costs, although a national policy allows VA facilities to reimburse affiliates for some GME overhead costs.29
CMS similarly reimburses for residency training time spent in patient care activities as well as orientation activities, didactics, leave, and, in some cases, research.4,30,31 CMS makes payments to hospitals, which may include sponsoring institutions and Medicare-eligible participating training sites.4,30,31 For both the VA and CMS, residents may not be counted twice for reimbursement by 2 federal agencies; in other words, a resident may not count for > 1 FTE.4,30-32
GME Oversight
VA GME funding came under significant scrutiny. At a 2016 House Veterans Affairs Committee hearing, Representative Phil Roe, MD (R-Tennessee), noted that no process existed at many VA facilities for “determining trainee presence” and that many VA medical centers had “difficulty tracking resident rotations”16 A VA Office of the Inspector General investigation recommended that the VA implement policies and procedures to improve oversight to “ensure residents are fully participating in educational activities” and that the VA is “paying the correct amount” to the affiliate.17 A 2020 General Accountability Office report outlined unclear policy guidance, incomplete tracking of resident activities, and improper fiscal processes for reimbursement and reconciliation of affiliate invoices.18
In response, the OAA created an oversight and compliance unit, revised VHA Directive 1400.05 (the policy for disbursement), and improved resident tracking procedures.26 The standard operating procedure that accompanied VHA Directive 1400.05 provides detailed information for the DEO and VA facility staff for tracking resident clinical and educational activities. FTE counts are essential to both VA and CMS for accurate reimbursement. The eAppendix and the Table provide a guide to reimbursable activities in the VA for the calculation of reimbursement, with a comparison to CMS.33,34 The OAA in cooperation with other VA staff and officers periodically conducts audits to assess compliance with disbursement policy and affiliate reimbursement accuracy.
In the VA, resident activities are captured on the VA Educational Activity Record, a standardized spreadsheet to track activities and calculate reimbursement. Each VA facility hosting resident physicians manually records resident activity by the half-day. This process is labor intensive, involving both VA and affiliate staff to accurately reconcile payments. To address the workload demands, the OAA is developing an online tool that will automate aspects of the tracking process. Also, to ensure adequate staffing, the OAA is in the process of implementing an office optimization project, providing standardized position descriptions, an organizational chart, and staffing levels for DEO offices in VA facilities.
Conclusions
This report describes the key policies and principles of VA GME financing, highlighting the essential similarities and differences between VA and CMS. Neither the VA nor CMS regulations allow for reimbursement for > 1 FTE position per resident, a principle that underpins the assignment of resident rotations and federal funding for GME and are similar with respect to reimbursement for patient care activities, didactics, research, orientation, and scholarly activity. While reimbursable activities in the VA require physical presence and care of veteran patients, CMS also limits reimbursement to resident activities in the hospital and approved other settings if the hospital is paying for resident salary and benefits in these settings. The VA provides some flexibility for offsite activities including didactics and, in specific circumstances, remote care of veteran patients (eg, teleradiology).
The VA and CMS use different GME financing models. For example, the CMS calculations for resident FTEs are complex, whereas VA calculations reimburse the salary and benefits as set by the academic affiliate. The VA process accounts for local variation in salary rates, whereas the per-resident amount set by CMS varies regionally and does not fully account for differences in the cost of living.24 Because all patients in VA facilities are veterans, VA calculations for reimbursement do not involve ratios of beds like the CMS calculations to determine a proportional share of reimbursement. The VA GME expansion tends to be more directed to VA health workforce needs than CMS, specifying the types of programs and geographic locations to address these needs.
The VA regularly reevaluates how affiliates are reimbursed for VA resident activity, balancing compliance with VA policies and the workload for VA and its affiliates. The VA obtains input from key stakeholders including DEOs, DIOs, and professional organizations such as the Association of American Medical Colleges and the Accreditation Council for Graduate Medical Education.35,36
Looking ahead, the VA is developing an online tool to improve the accuracy of affiliate reimbursement. The VA will also implement a standardized staffing model, organizational structure, and position descriptions for DEO offices. These initiatives will help reduce the burden of tracking and verifying resident activity and continue to support the 77-year partnership between VA and its affiliated institutions.
1. Klink KA, Albanese AP, Bope ET, Sanders KM. Veterans Affairs graduate medical education expansion addresses US physician workforce needs. Acad Med. 2022;97(8):1144-1150. doi:10.1097/ACM.0000000000004545
2. Andrus CH, Johnson K, Pierce E, Romito PJ, Hartel P, Berrios‐Guccione S, Best W. Finance modeling in the delivery of medical care in tertiary‐care hospitals in the Department of Veterans Affairs. J Surg Res. 2001;96(2):152-157. doi:10.1006/jsre.1999.5728
3. Petrakis IL, Kozal M. Academic medical centers and the U.S. Department of Veterans Affairs: a 75-year partnership influences medical education, scientific discovery, and clinical care. Acad Med. 2022;97(8):1110-1113. doi:10.1097/ACM.0000000000004734
4. Heisler EJ, Mendez BH, Mitchell A, Panangala SV, Villagrana MA. Federal support for graduate medical education: an overview (R44376). Congressional Research Service report R44376; version 11. Updated December 27, 2018. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/R/R44376/11
5. Chang BK, Brannen JL. The Veterans Access, Choice, and Accountability Act of 2014: examining graduate medical education enhancement in the Department of Veterans Affairs. Acad Med. 2015;90(9):1196-1198. doi:10.1097/ACM.0000000000000795
6. Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
7. Lypson ML, Roberts LW. Valuing the partnership between the Veterans Health Administration and academic medicine. Acad Med. 2022;97(8):1091-1093. doi:10.1097/ACM.0000000000004748
8. Harada ND, Traylor L, Rugen KW, et al. Interprofessional transformation of clinical education: the first six years of the Veterans Affairs Centers of Excellence in Primary Care Education. J Interprof Care. 2023;37(suppl 1):S86-S94. doi:10.1080/13561820.2018.1433642

9. Harada ND, Rajashekara S, Sansgiry S, et al. Developing interprofessional primary care teams: alumni evaluation of the Department of Veterans Affairs Centers of Excellence in Primary Care Education Program. J Med Educ Curric Dev. 2019;6:2382120519875455. doi:10.1177/2382120519875455
10. Splaine ME, Ogrinc G, Gilman SC, et al. The Department of Veterans Affairs National Quality Scholars Fellowship Program: experience from 10 years of training quality scholars. Acad Med. 2009;84(12):1741-1748. doi:10.1097/ACM.0b013e3181bfdcef
11. Watts BV, Paull DE, Williams LC, Neily J, Hemphill RR, Brannen JL. Department of Veterans Affairs chief resident in quality and patient safety program: a model to spread change. Am J Med Qual. 2016;31(6):598-600. doi:10.1177/1062860616643403
12. He K, Whang E, Kristo G. Graduate medical education funding mechanisms, challenges, and solutions: a narrative review. Am J Surg. 2021;221(1):65-71. doi:10.1016/j.amjsurg.2020.06.007
13. Villagrana M. Medicare graduate medical education payments: an overview. Congressional Research Service report IF10960. Updated September 29, 2022. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/IF/IF10960
14. Committee on the Governance and Financing of Graduate Medical Education; Board on Health Care Services; Institute of Medicine. Graduate Medical Education That Meets the Nation’s Health Needs. Eden J, Berwick DM, Wilensky GR, eds. Washington, DC: National Academies Press; 2014. doi:10.17226/18754
15. Physician workforce: caps on Medicare-funded graduate medical education at teaching hospitals. Report to congressional requesters. GAO-21-391. May 21, 2021. Accessed March 1, 2024. https://www.gao.gov/assets/gao-21-391.pdf
16. VA and Academic Affiliates: Who Benefits? Hearing Before the Subcommittee on Oversight and Investigations of the Committee on Veterans’ Affairs, 114th Cong, 2nd Sess (2016). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CHRG-115hhrg29685/html/CHRG-115hhrg29685.htm
17. US Department of Veterans Affairs, Office of Inspector General (OIG). Veterans Health Administration. Review of resident and part-time physician time and attendance at the Oklahoma City VA Health Care System. OIG report 17-00253-93. March 28, 2018. Accessed March 1, 2024. https://www.oversight.gov/sites/default/files/oig-reports/VAOIG-17-00253-93.pdf
18. VA health care: actions needed to improve oversight of graduate medical education reimbursement. Report to the ranking member, Committee on Veterans’ Affairs, House of Representatives. GAO-20-553. July 2020. Accessed March 1, 2024. https://www.gao.gov/assets/710/708275.pdf
19. Functions of Veterans Health Administration: in general, 38 USC §7301 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap73-subchapI-sec7301.pdf
20. US Department of Veterans Affairs. Policy memorandum no. 2, policy in association of veterans’ hospitals with medical schools. January 30, 1946.
21. Veterans Health Care Expansion Act of 1973, Public Law 93-82. August 2, 1973. Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/STATUTE-87/pdf/STATUTE-87-Pg179.pdf
22. Residencies and internships, 38 USC § 7406 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap74-subchapI-sec7406.pdf
23. Direct graduate medical education (DGME). Centers for Medicaid and Medicare Services. Updated December 5, 2023. Accessed March 1, 2024. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/DGME
24. Drezdzon MK, Cowley NJ, Sweeney DP, et al. Going for broke: the impact of cost of living on surgery resident stipend value. Ann Surg. 2023;278(6):1053-1059. doi:10.1097/SLA.0000000000005923
25. Special treatment: hospitals that incur indirect costs for graduate medical education programs, 42 CFR § 412.105 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec412-105.pdf
26. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.05, Disbursement agreements for health professions trainees appointed under 38 U.S.C. § 7406. June 2, 2021. Accessed March 1, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9293
27. Harada ND, Sanders KM, Bowman MA. Health systems education leadership: learning from the VA designated education officer role. Fed Pract. 2022;39(6):266-273. doi:10.12788/fp.0278
28. Schleiter Hitchell K, Johnson L. CMS finalizes rules for distribution of 1000 new Medicare-funded residency positions and changes to rural training track programs. J Grad Med Educ. 2022;14(2):245-249. doi:10.4300/JGME-D-22-00193.1

29. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.10, Educational cost contracts for health professions education. September 25, 2023. Accessed March 1, 2024. https://www.va.gov/VHAPUBLICATIONS/ViewPublication.asp?pub_ID=11480
30. Direct GME payments: general requirements, 42 CFR § 413.75 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-75.pdf
31. Direct GME payments: determination of the total number of FTE residents, 42 CFR § 413.78 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-78.pdf
32. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Medicare financial management manual, chapter 8. Contractor procedures for provider audits. Accessed March 1, 2024. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/fin106c08.pdf
33. US Department of Health and Human Services, Office of Inspector General. CMS did not always ensure hospitals complied with Medicare reimbursement requirements for graduate medical education. OIG report A-02-17-01017. November 2018. Accessed March 1, 2024. https://oig.hhs.gov/oas/reports/region2/21701017.pdf
34. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Interns and Residents Information System (IRIS) XML format. Publication 100-20. Transmittal 11418. Change request 12724. May 19, 2022. Accessed March 1, 2024. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/R11418OTN.pdf
35. Birnbaum AD, Byrne J, on behalf of the VA Office of Academic Affiliations. VHA Updates: Disbursement Policy and Education Cost Contracts. Presented at: American Association of Medical Colleges Webinar; June 2021. Accessed March 1, 2024. https://vimeo.com/644415670
36. Byrne JM, on behalf of the VA Office of Academic Affiliations. Disbursement procedures update for AY 23-24. Accessed March 1, 2024. https://www.va.gov/oaa/Videos/AffiliatePresentationDisbursementandEARsAY23-24.pptx
1. Klink KA, Albanese AP, Bope ET, Sanders KM. Veterans Affairs graduate medical education expansion addresses US physician workforce needs. Acad Med. 2022;97(8):1144-1150. doi:10.1097/ACM.0000000000004545
2. Andrus CH, Johnson K, Pierce E, Romito PJ, Hartel P, Berrios‐Guccione S, Best W. Finance modeling in the delivery of medical care in tertiary‐care hospitals in the Department of Veterans Affairs. J Surg Res. 2001;96(2):152-157. doi:10.1006/jsre.1999.5728
3. Petrakis IL, Kozal M. Academic medical centers and the U.S. Department of Veterans Affairs: a 75-year partnership influences medical education, scientific discovery, and clinical care. Acad Med. 2022;97(8):1110-1113. doi:10.1097/ACM.0000000000004734
4. Heisler EJ, Mendez BH, Mitchell A, Panangala SV, Villagrana MA. Federal support for graduate medical education: an overview (R44376). Congressional Research Service report R44376; version 11. Updated December 27, 2018. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/R/R44376/11
5. Chang BK, Brannen JL. The Veterans Access, Choice, and Accountability Act of 2014: examining graduate medical education enhancement in the Department of Veterans Affairs. Acad Med. 2015;90(9):1196-1198. doi:10.1097/ACM.0000000000000795
6. Albanese AP, Bope ET, Sanders KM, Bowman M. The VA MISSION Act of 2018: a potential game changer for rural GME expansion and veteran health care. J Rural Health. 2020;36(1):133-136. doi:10.1111/jrh.12360
7. Lypson ML, Roberts LW. Valuing the partnership between the Veterans Health Administration and academic medicine. Acad Med. 2022;97(8):1091-1093. doi:10.1097/ACM.0000000000004748
8. Harada ND, Traylor L, Rugen KW, et al. Interprofessional transformation of clinical education: the first six years of the Veterans Affairs Centers of Excellence in Primary Care Education. J Interprof Care. 2023;37(suppl 1):S86-S94. doi:10.1080/13561820.2018.1433642

9. Harada ND, Rajashekara S, Sansgiry S, et al. Developing interprofessional primary care teams: alumni evaluation of the Department of Veterans Affairs Centers of Excellence in Primary Care Education Program. J Med Educ Curric Dev. 2019;6:2382120519875455. doi:10.1177/2382120519875455
10. Splaine ME, Ogrinc G, Gilman SC, et al. The Department of Veterans Affairs National Quality Scholars Fellowship Program: experience from 10 years of training quality scholars. Acad Med. 2009;84(12):1741-1748. doi:10.1097/ACM.0b013e3181bfdcef
11. Watts BV, Paull DE, Williams LC, Neily J, Hemphill RR, Brannen JL. Department of Veterans Affairs chief resident in quality and patient safety program: a model to spread change. Am J Med Qual. 2016;31(6):598-600. doi:10.1177/1062860616643403
12. He K, Whang E, Kristo G. Graduate medical education funding mechanisms, challenges, and solutions: a narrative review. Am J Surg. 2021;221(1):65-71. doi:10.1016/j.amjsurg.2020.06.007
13. Villagrana M. Medicare graduate medical education payments: an overview. Congressional Research Service report IF10960. Updated September 29, 2022. Accessed March 2, 2024. https://crsreports.congress.gov/product/pdf/IF/IF10960
14. Committee on the Governance and Financing of Graduate Medical Education; Board on Health Care Services; Institute of Medicine. Graduate Medical Education That Meets the Nation’s Health Needs. Eden J, Berwick DM, Wilensky GR, eds. Washington, DC: National Academies Press; 2014. doi:10.17226/18754
15. Physician workforce: caps on Medicare-funded graduate medical education at teaching hospitals. Report to congressional requesters. GAO-21-391. May 21, 2021. Accessed March 1, 2024. https://www.gao.gov/assets/gao-21-391.pdf
16. VA and Academic Affiliates: Who Benefits? Hearing Before the Subcommittee on Oversight and Investigations of the Committee on Veterans’ Affairs, 114th Cong, 2nd Sess (2016). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CHRG-115hhrg29685/html/CHRG-115hhrg29685.htm
17. US Department of Veterans Affairs, Office of Inspector General (OIG). Veterans Health Administration. Review of resident and part-time physician time and attendance at the Oklahoma City VA Health Care System. OIG report 17-00253-93. March 28, 2018. Accessed March 1, 2024. https://www.oversight.gov/sites/default/files/oig-reports/VAOIG-17-00253-93.pdf
18. VA health care: actions needed to improve oversight of graduate medical education reimbursement. Report to the ranking member, Committee on Veterans’ Affairs, House of Representatives. GAO-20-553. July 2020. Accessed March 1, 2024. https://www.gao.gov/assets/710/708275.pdf
19. Functions of Veterans Health Administration: in general, 38 USC §7301 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap73-subchapI-sec7301.pdf
20. US Department of Veterans Affairs. Policy memorandum no. 2, policy in association of veterans’ hospitals with medical schools. January 30, 1946.
21. Veterans Health Care Expansion Act of 1973, Public Law 93-82. August 2, 1973. Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/STATUTE-87/pdf/STATUTE-87-Pg179.pdf
22. Residencies and internships, 38 USC § 7406 (2022). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/USCODE-2022-title38/pdf/USCODE-2022-title38-partV-chap74-subchapI-sec7406.pdf
23. Direct graduate medical education (DGME). Centers for Medicaid and Medicare Services. Updated December 5, 2023. Accessed March 1, 2024. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/DGME
24. Drezdzon MK, Cowley NJ, Sweeney DP, et al. Going for broke: the impact of cost of living on surgery resident stipend value. Ann Surg. 2023;278(6):1053-1059. doi:10.1097/SLA.0000000000005923
25. Special treatment: hospitals that incur indirect costs for graduate medical education programs, 42 CFR § 412.105 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec412-105.pdf
26. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.05, Disbursement agreements for health professions trainees appointed under 38 U.S.C. § 7406. June 2, 2021. Accessed March 1, 2024. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9293
27. Harada ND, Sanders KM, Bowman MA. Health systems education leadership: learning from the VA designated education officer role. Fed Pract. 2022;39(6):266-273. doi:10.12788/fp.0278
28. Schleiter Hitchell K, Johnson L. CMS finalizes rules for distribution of 1000 new Medicare-funded residency positions and changes to rural training track programs. J Grad Med Educ. 2022;14(2):245-249. doi:10.4300/JGME-D-22-00193.1

29. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1400.10, Educational cost contracts for health professions education. September 25, 2023. Accessed March 1, 2024. https://www.va.gov/VHAPUBLICATIONS/ViewPublication.asp?pub_ID=11480
30. Direct GME payments: general requirements, 42 CFR § 413.75 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-75.pdf
31. Direct GME payments: determination of the total number of FTE residents, 42 CFR § 413.78 (2023). Accessed March 1, 2024. https://www.govinfo.gov/content/pkg/CFR-2023-title42-vol2/pdf/CFR-2023-title42-vol2-sec413-78.pdf
32. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Medicare financial management manual, chapter 8. Contractor procedures for provider audits. Accessed March 1, 2024. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/fin106c08.pdf
33. US Department of Health and Human Services, Office of Inspector General. CMS did not always ensure hospitals complied with Medicare reimbursement requirements for graduate medical education. OIG report A-02-17-01017. November 2018. Accessed March 1, 2024. https://oig.hhs.gov/oas/reports/region2/21701017.pdf
34. US Department of Health and Human Services, Centers for Medicare and Medicaid Services. Interns and Residents Information System (IRIS) XML format. Publication 100-20. Transmittal 11418. Change request 12724. May 19, 2022. Accessed March 1, 2024. https://www.hhs.gov/guidance/sites/default/files/hhs-guidance-documents/R11418OTN.pdf
35. Birnbaum AD, Byrne J, on behalf of the VA Office of Academic Affiliations. VHA Updates: Disbursement Policy and Education Cost Contracts. Presented at: American Association of Medical Colleges Webinar; June 2021. Accessed March 1, 2024. https://vimeo.com/644415670
36. Byrne JM, on behalf of the VA Office of Academic Affiliations. Disbursement procedures update for AY 23-24. Accessed March 1, 2024. https://www.va.gov/oaa/Videos/AffiliatePresentationDisbursementandEARsAY23-24.pptx
Where Have All the Future Veterans Gone?
Word to the Nation: Guard zealously your right to serve in the Armed Forces, for without them, there will be no other rights to guard.
John F. Kennedy 1
The title of this Veterans Day editorial is a paraphrase of the legendary folk artist Pete Seeger’s protest song popularized during the Vietnam War. On January 27, 1973, in the wake of the widespread antiwar movement, Secretary of Defense Melvin Laird announced an end to the dreaded draft.2
For nearly 50 years, the all-volunteer military was celebrated as an outstanding achievement that professionalized the armed services and arguably made the US military among the most highly trained and effective fighting forces in the world. That was until an ongoing recruitment crisis threatened to write a different and far more disturbing conclusion to what the government had heralded as a “success story.”3
The recruiting crisis is a complicated problem with many facets that have received increasing attention from journalists, the media, experts, think tanks, and the government. Given this complexity, this will be a 2-part editorial: This column examines the scope of the crisis and the putative causes of the problem with recruiting Americans to serve in uniform. The next column will examine the potential impact of the shortage of service members on federal health care practice.
The Recruiting Crisis
Over the past several years, nearly every branch of the armed forces has struggled with recruitment, especially the Army. In April of this year, the US Department of Defense (DoD) reported that the Army, Navy, and Air Force would all fail to meet recruitment goals; only the Marines and Space Forces were expected to reach their targets.4 At the end of its fiscal year (October 1), the Army acknowledged that its 55,000 recruits were 10,000 fewer soldiers than it had aimed to enlist.5 But this was still more people joining the ranks than in 2022 when the Army was 15,000 recruits below the mark.6
Challenging Trends
There are many putative causes and proposed solutions for the recruitment crisis. Among the most serious is a marked drop in the American public’s confidence in the military. A June 2023 Gallup poll found that only 60% of citizens expressed “a great deal” or “quite a lot” of confidence in the military. This was the nadir of a 5-year decline that this year reached the lowest point since 1997/1998.7 For many Americans in and out of uniform, the ignoble end to the long war in Afghanistan leaving behind friends and allies contrary to the military ethos is cited as a significant contributor to both the loss of confidence in the military and the recruiting crisis.8
These cultural developments reinforce each other. Now, many veterans do not want their relatives and friends to follow them into the armed services. A 2021 survey by the Military Family Advisory Network found that slightly more than 60% of veterans and active-duty service members would recommend a military career to a potential recruit. This was down from 75% in 2019.9 Veterans cite a variety of reasons for discouraging their fellow citizens from serving, including low pay compared with civilian employment, especially in a labor-hungry job market; and the military failure to fulfill health care promises, housing, and other social services, especially for the growing number experiencing mental health disorders related to their service.10
Two facts about recruitment heighten the negative impact of some veterans’ change of attitude toward joining the services. First, since the end of the draft, military life in the US has become a family tradition. Published in 2011, a Pew Research Center study found that even then, a decreasing number of Americans had a family connection to the military. More respondents aged ≥ 50 years had a parent, child, spouse, or sibling who had served compared with those aged 30 to 49 years and those aged 18 to 29 (77%, 57%, and 33%, respectively).11 Second, since the end of the draft, far fewer Americans have had military experience. Only 1% of the nation is currently in military service, and the veteran population is steadily declining. In 1980, 18% of adult Americans were veterans; 20 years later, that number is only 7%.12 This makes it less likely that a high school or college student will have a personal or even a passing relationship with a teacher, coach, or other mentoring adult who is or has been a military member. This demographic discrepancy has generated what sociologists call the military-civilian gap.10 That division has been manipulated in the increasingly vehement culture wars and generational struggles that are splitting the country.12
This relatively recent sociological trend is reflected in a growing lack of interest among many young Americans in armed forces service. A DoD survey of participants aged 16 to 24 years regarding their intention to serve in the military found that 89% were probably not going to pursue a career in uniform. More than 65% of respondents indicated that the possibility of physical injury, death, or psychological trauma was the primary deterrent for considering enlisting.13 The latter barrier is directly related to our work as practitioners caring for service members and veterans, and through our compassion and competence, we may help bridge the widening divide between the military and civilian spheres. These numbers speak to the unwilling; there is also a significant group of Americans who want to serve yet are unable to due to their history, diagnoses, or condition.14 Their motivation to be military members in the face of the recruitment challenges highlighted here present federal practitioners with ethical questions that will be the subject of the next column.
Armed Forces and Veterans Day
This column’s epigraph is from President John F. Kennedy, a decorated World War II Navy combat veteran who decreed Armed Forces Day an official holiday a decade before conscription ended.1 The commemoration was to thank and honor all individuals currently serving in the military for their patriotism and sacrifice. President Kennedy’s Word to the Nation could not be timelier on Veterans Day 2023. The data reviewed here raise profound questions as to where tomorrow’s service members and the veterans of the future will come from, and how we will persuade them that though there are real risks to military service, the rewards are both tangible and transcendent.
1. US Department of Defense. Armed Forces Day. Accessed October 17, 2023. https://afd.defense.gov/History
2. Zipkin A. The military draft ended 50 years ago, dividing a generation. The Washington Post. January 27, 2023. Accessed October 17, 2023. https://www.washingtonpost.com/history/2023/01/27/draft-end-conscription-1973
3. Lopez TC. All-volunteer force proves successful for U.S. military. March 2, 2023. Accessed October 17, 2023. https://www.defense.gov/News/News-Stories/Article/Article/3316678/all-volunteer-force-proves-successful-for-us-military
4. Garamone J. Vice-chiefs talk recruiting shortfalls, readiness issues. April 20, 2023. Accessed October 17, 2023. https://www.defense.gov/News/News-Stories/Article/Article/3369472/vice-chiefs-talk-recruiting-shortfalls-readiness-issues
5. Winkie D. Army recruiters at two-thirds of contract goals as the fiscal year closes. Military Times. September 7, 2023. Accessed October 17, 2023. https://www.armytimes.com/news/recruiting/2023/09/07/army-recruiters-at-two-thirds-of-contract-goals-as-fiscal-year-closes
6. Baldor LC. Army misses recruiting goal by 15,000 soldiers. Accessed October 17, 2023. https://www.armytimes.com/news/your-army/2022/10/02/army-misses-recruiting-goal-by-15000-soldiers
7. Younis M. Confidence in U.S. military lowest in over two decades. Accessed October 17, 2023. https://news.gallup.com/poll/509189/confidence-military-lowest-two-decades.aspx
8. Rogin A, Corkery A. Why recruiting and confidence in America’s armed forces is so low right now? Accessed October 17, 2023. https://www.pbs.org/newshour/show/why-recruiting-and-confidence-in-americas-armed-forces-is-so-low-right-now
9. Military Family Advisory Network. 2021 military family support programming survey. Accessed October 17, 2023. https://www.mfan.org/wp-content/uploads/2022/07/Executive-Summary-MFAN-Programming-Survey-Results-2021.pdf
10. Kesling B. The military recruiting crisis: even veterans don’t want their family to join. Wall Street Journal. 30 June 2023. Accessed October 17, 2023. https://www.wsj.com/articles/military-recruiting-crisis-veterans-dont-want-their-children-to-join-510e1a25
11. Pew Research Center. The military-civilian gap: fewer family connections. Accessed October 17, 2023. https://www.pewresearch.org/social-trends/2011/11/23/the-military-civilian-gap-fewer-family-connections
12. Myers M. Is the military too ‘woke’ to recruit? Accessed October 17, 2023. https://www.militarytimes.com/news/your-military/2022/10/13/is-the-military-too-woke-to-recruit
13. Schaeffer K. The changing face of America’s veteran population. Accessed October 17, 2023. https://www.pewresearch.org/short-reads/2021/04/05/the-changing-face-of-americas-veteran-population
14. Phillips D. With few able and fewer willing, U.S. military can’t find recruits. New York Times. July 14, 2023. Accessed October 17, 2023. https://www.nytimes.com/2022/07/14/us/us-military-recruiting-enlistment.html
Word to the Nation: Guard zealously your right to serve in the Armed Forces, for without them, there will be no other rights to guard.
John F. Kennedy 1
The title of this Veterans Day editorial is a paraphrase of the legendary folk artist Pete Seeger’s protest song popularized during the Vietnam War. On January 27, 1973, in the wake of the widespread antiwar movement, Secretary of Defense Melvin Laird announced an end to the dreaded draft.2
For nearly 50 years, the all-volunteer military was celebrated as an outstanding achievement that professionalized the armed services and arguably made the US military among the most highly trained and effective fighting forces in the world. That was until an ongoing recruitment crisis threatened to write a different and far more disturbing conclusion to what the government had heralded as a “success story.”3
The recruiting crisis is a complicated problem with many facets that have received increasing attention from journalists, the media, experts, think tanks, and the government. Given this complexity, this will be a 2-part editorial: This column examines the scope of the crisis and the putative causes of the problem with recruiting Americans to serve in uniform. The next column will examine the potential impact of the shortage of service members on federal health care practice.
The Recruiting Crisis
Over the past several years, nearly every branch of the armed forces has struggled with recruitment, especially the Army. In April of this year, the US Department of Defense (DoD) reported that the Army, Navy, and Air Force would all fail to meet recruitment goals; only the Marines and Space Forces were expected to reach their targets.4 At the end of its fiscal year (October 1), the Army acknowledged that its 55,000 recruits were 10,000 fewer soldiers than it had aimed to enlist.5 But this was still more people joining the ranks than in 2022 when the Army was 15,000 recruits below the mark.6
Challenging Trends
There are many putative causes and proposed solutions for the recruitment crisis. Among the most serious is a marked drop in the American public’s confidence in the military. A June 2023 Gallup poll found that only 60% of citizens expressed “a great deal” or “quite a lot” of confidence in the military. This was the nadir of a 5-year decline that this year reached the lowest point since 1997/1998.7 For many Americans in and out of uniform, the ignoble end to the long war in Afghanistan leaving behind friends and allies contrary to the military ethos is cited as a significant contributor to both the loss of confidence in the military and the recruiting crisis.8
These cultural developments reinforce each other. Now, many veterans do not want their relatives and friends to follow them into the armed services. A 2021 survey by the Military Family Advisory Network found that slightly more than 60% of veterans and active-duty service members would recommend a military career to a potential recruit. This was down from 75% in 2019.9 Veterans cite a variety of reasons for discouraging their fellow citizens from serving, including low pay compared with civilian employment, especially in a labor-hungry job market; and the military failure to fulfill health care promises, housing, and other social services, especially for the growing number experiencing mental health disorders related to their service.10
Two facts about recruitment heighten the negative impact of some veterans’ change of attitude toward joining the services. First, since the end of the draft, military life in the US has become a family tradition. Published in 2011, a Pew Research Center study found that even then, a decreasing number of Americans had a family connection to the military. More respondents aged ≥ 50 years had a parent, child, spouse, or sibling who had served compared with those aged 30 to 49 years and those aged 18 to 29 (77%, 57%, and 33%, respectively).11 Second, since the end of the draft, far fewer Americans have had military experience. Only 1% of the nation is currently in military service, and the veteran population is steadily declining. In 1980, 18% of adult Americans were veterans; 20 years later, that number is only 7%.12 This makes it less likely that a high school or college student will have a personal or even a passing relationship with a teacher, coach, or other mentoring adult who is or has been a military member. This demographic discrepancy has generated what sociologists call the military-civilian gap.10 That division has been manipulated in the increasingly vehement culture wars and generational struggles that are splitting the country.12
This relatively recent sociological trend is reflected in a growing lack of interest among many young Americans in armed forces service. A DoD survey of participants aged 16 to 24 years regarding their intention to serve in the military found that 89% were probably not going to pursue a career in uniform. More than 65% of respondents indicated that the possibility of physical injury, death, or psychological trauma was the primary deterrent for considering enlisting.13 The latter barrier is directly related to our work as practitioners caring for service members and veterans, and through our compassion and competence, we may help bridge the widening divide between the military and civilian spheres. These numbers speak to the unwilling; there is also a significant group of Americans who want to serve yet are unable to due to their history, diagnoses, or condition.14 Their motivation to be military members in the face of the recruitment challenges highlighted here present federal practitioners with ethical questions that will be the subject of the next column.
Armed Forces and Veterans Day
This column’s epigraph is from President John F. Kennedy, a decorated World War II Navy combat veteran who decreed Armed Forces Day an official holiday a decade before conscription ended.1 The commemoration was to thank and honor all individuals currently serving in the military for their patriotism and sacrifice. President Kennedy’s Word to the Nation could not be timelier on Veterans Day 2023. The data reviewed here raise profound questions as to where tomorrow’s service members and the veterans of the future will come from, and how we will persuade them that though there are real risks to military service, the rewards are both tangible and transcendent.
Word to the Nation: Guard zealously your right to serve in the Armed Forces, for without them, there will be no other rights to guard.
John F. Kennedy 1
The title of this Veterans Day editorial is a paraphrase of the legendary folk artist Pete Seeger’s protest song popularized during the Vietnam War. On January 27, 1973, in the wake of the widespread antiwar movement, Secretary of Defense Melvin Laird announced an end to the dreaded draft.2
For nearly 50 years, the all-volunteer military was celebrated as an outstanding achievement that professionalized the armed services and arguably made the US military among the most highly trained and effective fighting forces in the world. That was until an ongoing recruitment crisis threatened to write a different and far more disturbing conclusion to what the government had heralded as a “success story.”3
The recruiting crisis is a complicated problem with many facets that have received increasing attention from journalists, the media, experts, think tanks, and the government. Given this complexity, this will be a 2-part editorial: This column examines the scope of the crisis and the putative causes of the problem with recruiting Americans to serve in uniform. The next column will examine the potential impact of the shortage of service members on federal health care practice.
The Recruiting Crisis
Over the past several years, nearly every branch of the armed forces has struggled with recruitment, especially the Army. In April of this year, the US Department of Defense (DoD) reported that the Army, Navy, and Air Force would all fail to meet recruitment goals; only the Marines and Space Forces were expected to reach their targets.4 At the end of its fiscal year (October 1), the Army acknowledged that its 55,000 recruits were 10,000 fewer soldiers than it had aimed to enlist.5 But this was still more people joining the ranks than in 2022 when the Army was 15,000 recruits below the mark.6
Challenging Trends
There are many putative causes and proposed solutions for the recruitment crisis. Among the most serious is a marked drop in the American public’s confidence in the military. A June 2023 Gallup poll found that only 60% of citizens expressed “a great deal” or “quite a lot” of confidence in the military. This was the nadir of a 5-year decline that this year reached the lowest point since 1997/1998.7 For many Americans in and out of uniform, the ignoble end to the long war in Afghanistan leaving behind friends and allies contrary to the military ethos is cited as a significant contributor to both the loss of confidence in the military and the recruiting crisis.8
These cultural developments reinforce each other. Now, many veterans do not want their relatives and friends to follow them into the armed services. A 2021 survey by the Military Family Advisory Network found that slightly more than 60% of veterans and active-duty service members would recommend a military career to a potential recruit. This was down from 75% in 2019.9 Veterans cite a variety of reasons for discouraging their fellow citizens from serving, including low pay compared with civilian employment, especially in a labor-hungry job market; and the military failure to fulfill health care promises, housing, and other social services, especially for the growing number experiencing mental health disorders related to their service.10
Two facts about recruitment heighten the negative impact of some veterans’ change of attitude toward joining the services. First, since the end of the draft, military life in the US has become a family tradition. Published in 2011, a Pew Research Center study found that even then, a decreasing number of Americans had a family connection to the military. More respondents aged ≥ 50 years had a parent, child, spouse, or sibling who had served compared with those aged 30 to 49 years and those aged 18 to 29 (77%, 57%, and 33%, respectively).11 Second, since the end of the draft, far fewer Americans have had military experience. Only 1% of the nation is currently in military service, and the veteran population is steadily declining. In 1980, 18% of adult Americans were veterans; 20 years later, that number is only 7%.12 This makes it less likely that a high school or college student will have a personal or even a passing relationship with a teacher, coach, or other mentoring adult who is or has been a military member. This demographic discrepancy has generated what sociologists call the military-civilian gap.10 That division has been manipulated in the increasingly vehement culture wars and generational struggles that are splitting the country.12
This relatively recent sociological trend is reflected in a growing lack of interest among many young Americans in armed forces service. A DoD survey of participants aged 16 to 24 years regarding their intention to serve in the military found that 89% were probably not going to pursue a career in uniform. More than 65% of respondents indicated that the possibility of physical injury, death, or psychological trauma was the primary deterrent for considering enlisting.13 The latter barrier is directly related to our work as practitioners caring for service members and veterans, and through our compassion and competence, we may help bridge the widening divide between the military and civilian spheres. These numbers speak to the unwilling; there is also a significant group of Americans who want to serve yet are unable to due to their history, diagnoses, or condition.14 Their motivation to be military members in the face of the recruitment challenges highlighted here present federal practitioners with ethical questions that will be the subject of the next column.
Armed Forces and Veterans Day
This column’s epigraph is from President John F. Kennedy, a decorated World War II Navy combat veteran who decreed Armed Forces Day an official holiday a decade before conscription ended.1 The commemoration was to thank and honor all individuals currently serving in the military for their patriotism and sacrifice. President Kennedy’s Word to the Nation could not be timelier on Veterans Day 2023. The data reviewed here raise profound questions as to where tomorrow’s service members and the veterans of the future will come from, and how we will persuade them that though there are real risks to military service, the rewards are both tangible and transcendent.
1. US Department of Defense. Armed Forces Day. Accessed October 17, 2023. https://afd.defense.gov/History
2. Zipkin A. The military draft ended 50 years ago, dividing a generation. The Washington Post. January 27, 2023. Accessed October 17, 2023. https://www.washingtonpost.com/history/2023/01/27/draft-end-conscription-1973
3. Lopez TC. All-volunteer force proves successful for U.S. military. March 2, 2023. Accessed October 17, 2023. https://www.defense.gov/News/News-Stories/Article/Article/3316678/all-volunteer-force-proves-successful-for-us-military
4. Garamone J. Vice-chiefs talk recruiting shortfalls, readiness issues. April 20, 2023. Accessed October 17, 2023. https://www.defense.gov/News/News-Stories/Article/Article/3369472/vice-chiefs-talk-recruiting-shortfalls-readiness-issues
5. Winkie D. Army recruiters at two-thirds of contract goals as the fiscal year closes. Military Times. September 7, 2023. Accessed October 17, 2023. https://www.armytimes.com/news/recruiting/2023/09/07/army-recruiters-at-two-thirds-of-contract-goals-as-fiscal-year-closes
6. Baldor LC. Army misses recruiting goal by 15,000 soldiers. Accessed October 17, 2023. https://www.armytimes.com/news/your-army/2022/10/02/army-misses-recruiting-goal-by-15000-soldiers
7. Younis M. Confidence in U.S. military lowest in over two decades. Accessed October 17, 2023. https://news.gallup.com/poll/509189/confidence-military-lowest-two-decades.aspx
8. Rogin A, Corkery A. Why recruiting and confidence in America’s armed forces is so low right now? Accessed October 17, 2023. https://www.pbs.org/newshour/show/why-recruiting-and-confidence-in-americas-armed-forces-is-so-low-right-now
9. Military Family Advisory Network. 2021 military family support programming survey. Accessed October 17, 2023. https://www.mfan.org/wp-content/uploads/2022/07/Executive-Summary-MFAN-Programming-Survey-Results-2021.pdf
10. Kesling B. The military recruiting crisis: even veterans don’t want their family to join. Wall Street Journal. 30 June 2023. Accessed October 17, 2023. https://www.wsj.com/articles/military-recruiting-crisis-veterans-dont-want-their-children-to-join-510e1a25
11. Pew Research Center. The military-civilian gap: fewer family connections. Accessed October 17, 2023. https://www.pewresearch.org/social-trends/2011/11/23/the-military-civilian-gap-fewer-family-connections
12. Myers M. Is the military too ‘woke’ to recruit? Accessed October 17, 2023. https://www.militarytimes.com/news/your-military/2022/10/13/is-the-military-too-woke-to-recruit
13. Schaeffer K. The changing face of America’s veteran population. Accessed October 17, 2023. https://www.pewresearch.org/short-reads/2021/04/05/the-changing-face-of-americas-veteran-population
14. Phillips D. With few able and fewer willing, U.S. military can’t find recruits. New York Times. July 14, 2023. Accessed October 17, 2023. https://www.nytimes.com/2022/07/14/us/us-military-recruiting-enlistment.html
1. US Department of Defense. Armed Forces Day. Accessed October 17, 2023. https://afd.defense.gov/History
2. Zipkin A. The military draft ended 50 years ago, dividing a generation. The Washington Post. January 27, 2023. Accessed October 17, 2023. https://www.washingtonpost.com/history/2023/01/27/draft-end-conscription-1973
3. Lopez TC. All-volunteer force proves successful for U.S. military. March 2, 2023. Accessed October 17, 2023. https://www.defense.gov/News/News-Stories/Article/Article/3316678/all-volunteer-force-proves-successful-for-us-military
4. Garamone J. Vice-chiefs talk recruiting shortfalls, readiness issues. April 20, 2023. Accessed October 17, 2023. https://www.defense.gov/News/News-Stories/Article/Article/3369472/vice-chiefs-talk-recruiting-shortfalls-readiness-issues
5. Winkie D. Army recruiters at two-thirds of contract goals as the fiscal year closes. Military Times. September 7, 2023. Accessed October 17, 2023. https://www.armytimes.com/news/recruiting/2023/09/07/army-recruiters-at-two-thirds-of-contract-goals-as-fiscal-year-closes
6. Baldor LC. Army misses recruiting goal by 15,000 soldiers. Accessed October 17, 2023. https://www.armytimes.com/news/your-army/2022/10/02/army-misses-recruiting-goal-by-15000-soldiers
7. Younis M. Confidence in U.S. military lowest in over two decades. Accessed October 17, 2023. https://news.gallup.com/poll/509189/confidence-military-lowest-two-decades.aspx
8. Rogin A, Corkery A. Why recruiting and confidence in America’s armed forces is so low right now? Accessed October 17, 2023. https://www.pbs.org/newshour/show/why-recruiting-and-confidence-in-americas-armed-forces-is-so-low-right-now
9. Military Family Advisory Network. 2021 military family support programming survey. Accessed October 17, 2023. https://www.mfan.org/wp-content/uploads/2022/07/Executive-Summary-MFAN-Programming-Survey-Results-2021.pdf
10. Kesling B. The military recruiting crisis: even veterans don’t want their family to join. Wall Street Journal. 30 June 2023. Accessed October 17, 2023. https://www.wsj.com/articles/military-recruiting-crisis-veterans-dont-want-their-children-to-join-510e1a25
11. Pew Research Center. The military-civilian gap: fewer family connections. Accessed October 17, 2023. https://www.pewresearch.org/social-trends/2011/11/23/the-military-civilian-gap-fewer-family-connections
12. Myers M. Is the military too ‘woke’ to recruit? Accessed October 17, 2023. https://www.militarytimes.com/news/your-military/2022/10/13/is-the-military-too-woke-to-recruit
13. Schaeffer K. The changing face of America’s veteran population. Accessed October 17, 2023. https://www.pewresearch.org/short-reads/2021/04/05/the-changing-face-of-americas-veteran-population
14. Phillips D. With few able and fewer willing, U.S. military can’t find recruits. New York Times. July 14, 2023. Accessed October 17, 2023. https://www.nytimes.com/2022/07/14/us/us-military-recruiting-enlistment.html
How VA Innovative Partnerships and Health Care Systems Can Respond to National Needs: NOSE Trial Example
Traditional manufacturing concentrates capacity into a few discrete locations while applying lean and just-in-time philosophies to maximize profit during times of somewhat predictable supply and demand. This approach exposed nationwide vulnerabilities even during local crises, such as the United States saline shortages following closure of a single plant in Puerto Rico following Hurricane Maria in 2017.1 Interruptions to the supply chain due to pandemic plant closure, weather, politics, or surge demand can cause immediate and lasting shortages. Nasal swabs were a clear example.
At the onset of COVID-19, 2 companies—Puritan in Guilford, Maine, and Copan in Italy—manufactured nearly all of the highly specialized nasopharyngeal (NP) swabs singled out by the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration (FDA) to test patients for COVID-19. Demand for swabs skyrocketed as the virus spread, and they became unattainable. The lack of swabs meant patients went undiagnosed. Without knowing who was positive, people with symptoms and known contacts were presumed positive and quarantined, impacting isolated patients, the health care professionals treating them, and the entire US economy.
3-Dimensional Printing Solutions
Manufacturing NP swabs is not trivial. Their simple shape conceals complexity and requires highly specialized equipment. The lead time for one non-US machine manufacturer was > 6 months at the start of the pandemic.
Digital manufacturing/3-dimensional (3D) printing represented a potential solution to the supply chain crisis.2 Designers created digital blueprints for 3D-printed goods, face masks, face shields, and ventilator splitters were rapidly created and shared.3,4 Scrambling to fill the critical need for NP swabs, many hospitals, businesses, and academic centers began 3D printing swabs. This effort was spearheaded by University of South Florida (USF) and Northwell Health researchers and clinicians, who designed and tested a 3D-printed NP swab from photocurable resin that was printable on 2 models of Formlabs printers.5 Several other 3D-printed NP swab designs soon followed. This innovation and problem-solving renaissance faced several challenges well known to traditional manufacturers of regulated products but novel to newcomers.
The first challlenge was that these NP swabs predate FDA oversight of medical device development and manufacturing and no testing standards existed. Designers began casting prototypes out without guidance about the critical features and clinical functions required. Many of these designs did not have a clinical evaluation pathway to test safety and efficacy.
The second challlenge was that these swabs were being produced by facilities not registered with the FDA. This raised concerns about the quality of unlisted medical products developed and manufactured at novel facilities.
The third challenge was that small-scale novel approaches may offset local shortages but could not address national needs. The self-organized infrastructure for this crisis was ad hoc, local, and lacked coordinated federal support. This led to rolling shortages of these materials for years.
Two studies were performed early in the pandemic. The first study evaluated 4 prototypes of different manufacturer designs, finding excellent concordance among them and their control swab.6 A second study demonstrated the USF swab to be noninferior to the standard of care.7 Both studies acknowledged and addressed the first challenge for their designs.
COLLABORATIONS
Interagency
Before the pandemic, the US Department of Veterans Affairs (VA) had been coordinating with the FDA, the National Institutes of Health (NIH), and the nonprofit America Makes to bring medical product development and manufacturing closer to the point of care.
At the outset of the COVID-19 pandemic, the collaboration was formalized to address new challenges.8 The objectives of this collaboration were the following: (1) host a digital repository for 3D-printed digital designs for personal protectice equipment and other medical supplies in or at risk of shortage; (2) provide scientifically based ratings for designs according to clinical and field testing; and (3) offer education to health care workers and the public about the digital manufacturing of medical goods and devices.4,9
A key output of this collaboration was the COVID 3D Trusted Repository For Users And Suppliers Through Testing (COVID 3D TRUST), a curated archive of designs. In most cases, existing FDA standards and guidance formed the basis of testing strategies with deviations due to limited access to traditional testing facilities and reagents.
To address novel NP swabs, working with its COVID 3D TRUST partners, the VA gathered a combined list of clinical- and engineering-informed customer requirements and performed a hazard analysis. The result was a list of design inputs for NP swabs and 8 standard test protocols to evaluate key functions (Table).10 These protocols are meant to benchmark novel 3D-printed swabs against the key functions of established, traditionally manufactured swabs, which have a long record of safety and efficacy. The protocols, developed by the VA and undergoing validation by the US Army, empower and inform consumers and provide performance metrics to swab designers and manufacturers. The testing protocols and preliminary test results developed by the VA are publicly available at the NIH.11
Intra-agency
The use of the inputs and verification tests noted in the Table may reduce the risk of poor design but were inadequate to evaluate the clinical safety and efficacy of novel swabs. Recognizing this, the VA Office of Healthcare Innovation and Learning (OHIL) and the Office of Research and Development (ORD) launched the Nasal Swab Objective and Statistical Evaluation (NOSE) study to formally evaluate the safety and efficacy of 3D-printed swabs in the field. This multisite clinical study was a close collaboration between the OHIL and ORD. The OHIL provided the quality system and manufacturing oversight and delivery of the swabs, and the ORD provided scientific review, research infrastructure, human subjects oversight, administrative support, and funding and fiscal oversight. The OHIL/ORD collaboration resulted in the successful completion of the NOSE study.
This study (manuscript under preparation) yielded two 3D-printing production processes and swab designs that had comparable performance to the standard of care, were manufacturable compliant with FDA guidelines, and could be produced at scale in a distributed manner. This approach directly addressed the 3 challenges described earlier.
LESSONS LEARNED
Swabs were an example of supply challenges in the pandemic, but advanced manufacturing (notably, digital designs leading to 3D-printed solutions) also served as a temporary solution to device and product shortages during the COVID-19 pandemic. Digital designs and 3D printing as manufacturing techniques have the following key advantages: (1) they are distributed in nature, both in the breadth of locations that have access to these manufacturing platforms and in the depth of material choice that can be used to fabricate products, which alleviates the threat of a disaster impacting manufacturing capacity or a material stream; (2) they do not require retooling of machinery so new products can deploy rapidly and on demand; and (3) the speed of digital iteration, printing, and revision allows for rapid product development and production.
There also are notable disadvantages to these techniques. First, because 3D printing is a newer technology, there is less general depth of knowledge regarding design and material choice for additive manufacturing. Second, the flexibility of 3D printing means that operators must increase awareness of the factors that might cause the fabrication of a part to fail in either printing or postprocessing. Third, there are significant gaps in understanding how materials and manufacturing processes will perform in high-stakes settings such as health care, where performance and biocompatibility may be critical to support life-sustaining functions. Fourth, digital files are vulnerable to intentional or unintentional alteration. These alterations might weaken design integrity and be imperceptible to the manufacturer or end user. This is a prevalent challenge in all open-source designs.
The pandemic materialized quickly and created vast supply chain challenges. To address this crisis, it was clear that the average 17-year interval between research and translation in the US was unacceptable. The VA was able to accelerate swiftly many existing processes to meet this need, build new capabilities, and establish new practices for the rapid evaluation and deployment of health care products and guidance. This agile and innovative cooperation was critical in the success of the VA’s national support for pandemic solutions.
Finally, although COVID 3D TRUST was able to provide testing of submitted designs, this collaboration was not a substitute for the “peacetime” process of manufacturing site registration with the FDA and product listing. COVID 3D TRUST could evaluate designs only, not the production process, safety, and efficacy.
CALLS TO ACTION
The pandemic's impact on medical supply chain security persists, as does the need for greater foresight and crisis preparation. We must act now to avoid experiencing again the magnitude of fatalities (civilian and veteran) and the devastation to the US economy and livelihoods that occurred during this single biological event. To this end, creating a digital stockpile of federally curated, crisis-ready designs for as-needed distribution across our US industrial base would offer a second line of defense against life-threatening supply chain interruptions. The realization of such a digital stockpile requires calls to action among multiple contributors.
Collaborations
The VA’s Fourth Mission is to improve the nation’s preparedness for response to war, terrorism, national emergencies, and natural disasters. The VA does this by developing plans and taking actions to ensure continued service to veterans, as well as to support national, state, and local emergency management, public health, safety, and homeland security efforts.
The VA partnership with the FDA and NIH during the pandemic enabled successful coordination among federal agencies. Numerous other agencies, including the US Department of Defense (DoD), the Biomedical Advanced Research and Development Authority (BARDA), and the Defense Advanced Research Projects Agency (DARPA), also developed and executed successful initiatives.12-14 The joint awareness and management of these efforts, however, could be strengthened through more formal agreements and processes in peacetime. The VA/FDA/NIH Memorandum of Understanding is a prototype example of each agency lending its subject matter expertise to address a host of pandemic challenges collectively, cooperatively, and efficiently.8
Public-private partnerships (eg, VA/FDA/NIH and America Makes) led to coordinated responses for crisis readiness. The Advanced Manufacturing Crisis Product Response Program, a multipartner collaboration that included VA, addressed 7 crisis scenarios, 3 of which were specifically related to COVID-19.15 In addition, both BARDA and DARPA had successful public-private collaborations, and the DoD supported national logistics and other efforts.12-14 Clearly, industry and government both recognize complementary synergies: (1) the depth of resources of US industry; and (2) the national resources, coordination, and clinical insight available through federal agencies that can address the challenges of future crises quickly and efficiently.
When traditional supply chains and manufacturing processes failed during the pandemic, new techniques were exploited to fill the unmet material needs. Novel techniques and product pathways, however, are untested or undeveloped. The collaboration between the ORD and OHIL in support of NP swab testing and production is an example of bringing research insight, regulated product development, and manufacturing together to support a complete product life cycle.
Joint Awareness and Management
The VA continues to refine the joint awareness and management (JAM) process of products from ideation to translation, to shorten the time from research to product delivery. JAM is a VA collaborative committee of partners from ORD research offices and technology transfer program, and the OHIL Office of Advanced Manufacturing, which seeks additional support and guidance from VHA clinical service lines, VA Office of General Council, and VA Office of Acquisitions, Logistics, and Construction.
This team enables the rapid identification of unmet veteran health care product needs. In addition, JAM leverages the resources of each group to support products from problem identification to solution ideation, regulated development, production, and delivery into clinical service lines. While the concept of JAM arose to meet the crisis needs of the pandemic, it persists in delivering advanced health care solutions to veterans.
A Proposed Plan
The next national crisis is likely to involve and threaten national health care security. We propose that federal agencies be brought together to form a federally supported digital stockpile. This digital stockpile must encompass, at minimum, the following features: (1) preservation of novel, scalable medical supplies and products generated during the COVID-19 pandemic, to avoid the loss of this work; (2) clinical maturation of those existing supplies and products to refine their features and functions under the guidance of clinical, regulatory, and manufacturing experts—and validate those outputs with clinical evidence; (3) manufacturing maturation of those existing supplies and products, such that complete design and production processes are developed with the intent to distribute to multiple public manufacturers during the next crisis; (4) a call for new designs/intake portal for new designs to be matured and curated as vulnerabilities are identified; (5) supply chain crisis drills executed to test public-private preparedness to ensure design transfer is turnkey and can be engaged quickly during the next crisis; and (6) public-private engagement to develop strategy, scenarios, and policy to ensure that when supply chains next fail, additional surge capacity can be quickly added to protect American lives and health care, and that when supply chains resume, surge capacity can be redirected or stood down to protect the competitive markets.
This digital stockpile can complement and be part of the Strategic National Stockpile. Whereas the Strategic National Stockpile is a reserve of physical products that may offset product shortages, the digital stockpile is a reserve of turnkey, transferable designs that may offset supply chain disruptions and production-capacity shortages.
CONCLUSIONS
The success of 3D-printed NP swabs is a specific example of the importance of collaborations across industry, government, innovators, and researchers. More important than a sole product, however, these collaborations demonstrated the potential for game-changing approaches to how public-private partnerships support the continuity of health care operations nationally and prevent the potential for unnecessary loss of life due to capacity and supply chain disruptions.
As the largest health care system in the US, the VA has a unique capability to lead in the assessment of other novel 3D-printed medical devices in partnership with the FDA. The VA has a unique patient-centered perspective on medical device efficacy, and as a government institution, it is a trusted independent source for medical device evaluation. The VA’s role in the evaluation of 3D-printed medical devices will benefit veterans and their families, clinicians, hospitals, and the broader public by providing a gold-standard evaluation for the growing medical 3D-printing industry to follow. By creating new pathways and expectations for how federal agencies maintain crisis preparedness—such as establishing a digital stockpile—we can be equipped to serve the US health care system and minimize the effects of supply chain disruptions.
1. Sacks CA, Kesselheim AS, Fralick M. The shortage of normal saline in the wake of Hurricane Maria. JAMA Intern Med. 2018;178(7):885–886. doi:10.1001/jamainternmed.2018.1936
2. Bauchner H, Fontanarosa PB, Livingston EH. Conserving supply of personal protective equipment–a call for ideas. JAMA. 2020;323(19):1911. doi:10.1001/jama.2020.4770
3. Sinha MS, Bourgeois FT, Sorger PK. Personal protective equipment for COVID-19: distributed fabrication and additive manufacturing. Am J Public Health. 2020;110(8):1162-1164. doi:10.2105/AJPH.2020.305753
4. McCarthy MC, Di Prima M, Cruz P, et al. Trust in the time of Covid-19: 3D printing and additive manufacturing (3DP/AM) as a solution to supply chain gaps. NEJM Catalyst. 2021;2(6). doi:10.1056/CAT.21.0321
5. Ford J, Goldstein T, Trahan S, Neuwirth A, Tatoris K, Decker S. A 3D-printed nasopharyngeal swab for COVID-19 diagnostic testing. 3D Print Med. 2020;6(1):21. Published 2020 Aug 15. doi:10.1186/s41205-020-00076-3
6. Callahan CJ, Lee R, Zulauf K, et al. Open development and clinical validation of multiple 3D-printed sample-collection swabs: rapid resolution of a critical COVID-19 testing bottleneck. Preprint. medRxiv. 2020;2020.04.14.20065094. Published 2020 Apr 17. doi:10.1101/2020.04.14.20065094
7. Decker SJ, Goldstein TA, Ford JM, et al. 3-dimensional printed alternative to the standard synthetic flocked nasopharyngeal swabs used for coronavirus disease 2019 testing. Clin Infect Dis. 2021;73(9):e3027-e3032. doi:10.1093/cid/ciaa1366
8. US Food and Drug Administration. Memorandum of understanding: rapid response to Covid-19 using 3d printing between National Institutes of Health within U.S. Department of Health and Human Services and Food and Drug Administration, U.S. Department of Health and Human Services and Veterans Health Administration within the U.S. Department of Veterans Affairs. March 26, 2020. Accessed August 31, 2023. https://www.fda.gov/about-fda/domestic-mous/mou-225-20-008
9. National Institutes of Health, NIH 3D Print Exchange. Covid 3D trust: trusted repository for users and suppliers through testing. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=search
10. National Institutes of Health, NIH 3D Print Exchange. 3D printed nasal swabs - assessment criteria. August 17, 2020. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=swabassessment
11. National Institutes of Health, NIH 3D Print Exchange. 3D printed nasal swabs - general information. August 17, 2020. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=swabinfo
12. US Department of Defense. Coronavirus: DOD response. December 20, 2022. Accessed August 31, 2023. https://www.defense.gov/Spotlights/Coronavirus-DoD-Response
13. US Department of Health and Human Services, Biomedical Advanced Research and Development Authority. BARDA COVID-19 response. Updated May 25, 2023. Accessed August 31, 2023. https://www.medicalcountermeasures.gov/barda/barda-covid-19-response
14. Green S. Pandemic prevention platform (P3). Accessed August 31, 2023. https://www.darpa.mil/program/pandemic-prevention-platform
15. America Makes. America makes completes successful scenario testing for crisis response program [press release]. May 25, 2021. Accessed August 31, 2023. https://www.americamakes.us/america-makes-completes-successful-scenario-testing-for-crisis-response-program
Traditional manufacturing concentrates capacity into a few discrete locations while applying lean and just-in-time philosophies to maximize profit during times of somewhat predictable supply and demand. This approach exposed nationwide vulnerabilities even during local crises, such as the United States saline shortages following closure of a single plant in Puerto Rico following Hurricane Maria in 2017.1 Interruptions to the supply chain due to pandemic plant closure, weather, politics, or surge demand can cause immediate and lasting shortages. Nasal swabs were a clear example.
At the onset of COVID-19, 2 companies—Puritan in Guilford, Maine, and Copan in Italy—manufactured nearly all of the highly specialized nasopharyngeal (NP) swabs singled out by the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration (FDA) to test patients for COVID-19. Demand for swabs skyrocketed as the virus spread, and they became unattainable. The lack of swabs meant patients went undiagnosed. Without knowing who was positive, people with symptoms and known contacts were presumed positive and quarantined, impacting isolated patients, the health care professionals treating them, and the entire US economy.
3-Dimensional Printing Solutions
Manufacturing NP swabs is not trivial. Their simple shape conceals complexity and requires highly specialized equipment. The lead time for one non-US machine manufacturer was > 6 months at the start of the pandemic.
Digital manufacturing/3-dimensional (3D) printing represented a potential solution to the supply chain crisis.2 Designers created digital blueprints for 3D-printed goods, face masks, face shields, and ventilator splitters were rapidly created and shared.3,4 Scrambling to fill the critical need for NP swabs, many hospitals, businesses, and academic centers began 3D printing swabs. This effort was spearheaded by University of South Florida (USF) and Northwell Health researchers and clinicians, who designed and tested a 3D-printed NP swab from photocurable resin that was printable on 2 models of Formlabs printers.5 Several other 3D-printed NP swab designs soon followed. This innovation and problem-solving renaissance faced several challenges well known to traditional manufacturers of regulated products but novel to newcomers.
The first challlenge was that these NP swabs predate FDA oversight of medical device development and manufacturing and no testing standards existed. Designers began casting prototypes out without guidance about the critical features and clinical functions required. Many of these designs did not have a clinical evaluation pathway to test safety and efficacy.
The second challlenge was that these swabs were being produced by facilities not registered with the FDA. This raised concerns about the quality of unlisted medical products developed and manufactured at novel facilities.
The third challenge was that small-scale novel approaches may offset local shortages but could not address national needs. The self-organized infrastructure for this crisis was ad hoc, local, and lacked coordinated federal support. This led to rolling shortages of these materials for years.
Two studies were performed early in the pandemic. The first study evaluated 4 prototypes of different manufacturer designs, finding excellent concordance among them and their control swab.6 A second study demonstrated the USF swab to be noninferior to the standard of care.7 Both studies acknowledged and addressed the first challenge for their designs.
COLLABORATIONS
Interagency
Before the pandemic, the US Department of Veterans Affairs (VA) had been coordinating with the FDA, the National Institutes of Health (NIH), and the nonprofit America Makes to bring medical product development and manufacturing closer to the point of care.
At the outset of the COVID-19 pandemic, the collaboration was formalized to address new challenges.8 The objectives of this collaboration were the following: (1) host a digital repository for 3D-printed digital designs for personal protectice equipment and other medical supplies in or at risk of shortage; (2) provide scientifically based ratings for designs according to clinical and field testing; and (3) offer education to health care workers and the public about the digital manufacturing of medical goods and devices.4,9
A key output of this collaboration was the COVID 3D Trusted Repository For Users And Suppliers Through Testing (COVID 3D TRUST), a curated archive of designs. In most cases, existing FDA standards and guidance formed the basis of testing strategies with deviations due to limited access to traditional testing facilities and reagents.
To address novel NP swabs, working with its COVID 3D TRUST partners, the VA gathered a combined list of clinical- and engineering-informed customer requirements and performed a hazard analysis. The result was a list of design inputs for NP swabs and 8 standard test protocols to evaluate key functions (Table).10 These protocols are meant to benchmark novel 3D-printed swabs against the key functions of established, traditionally manufactured swabs, which have a long record of safety and efficacy. The protocols, developed by the VA and undergoing validation by the US Army, empower and inform consumers and provide performance metrics to swab designers and manufacturers. The testing protocols and preliminary test results developed by the VA are publicly available at the NIH.11
Intra-agency
The use of the inputs and verification tests noted in the Table may reduce the risk of poor design but were inadequate to evaluate the clinical safety and efficacy of novel swabs. Recognizing this, the VA Office of Healthcare Innovation and Learning (OHIL) and the Office of Research and Development (ORD) launched the Nasal Swab Objective and Statistical Evaluation (NOSE) study to formally evaluate the safety and efficacy of 3D-printed swabs in the field. This multisite clinical study was a close collaboration between the OHIL and ORD. The OHIL provided the quality system and manufacturing oversight and delivery of the swabs, and the ORD provided scientific review, research infrastructure, human subjects oversight, administrative support, and funding and fiscal oversight. The OHIL/ORD collaboration resulted in the successful completion of the NOSE study.
This study (manuscript under preparation) yielded two 3D-printing production processes and swab designs that had comparable performance to the standard of care, were manufacturable compliant with FDA guidelines, and could be produced at scale in a distributed manner. This approach directly addressed the 3 challenges described earlier.
LESSONS LEARNED
Swabs were an example of supply challenges in the pandemic, but advanced manufacturing (notably, digital designs leading to 3D-printed solutions) also served as a temporary solution to device and product shortages during the COVID-19 pandemic. Digital designs and 3D printing as manufacturing techniques have the following key advantages: (1) they are distributed in nature, both in the breadth of locations that have access to these manufacturing platforms and in the depth of material choice that can be used to fabricate products, which alleviates the threat of a disaster impacting manufacturing capacity or a material stream; (2) they do not require retooling of machinery so new products can deploy rapidly and on demand; and (3) the speed of digital iteration, printing, and revision allows for rapid product development and production.
There also are notable disadvantages to these techniques. First, because 3D printing is a newer technology, there is less general depth of knowledge regarding design and material choice for additive manufacturing. Second, the flexibility of 3D printing means that operators must increase awareness of the factors that might cause the fabrication of a part to fail in either printing or postprocessing. Third, there are significant gaps in understanding how materials and manufacturing processes will perform in high-stakes settings such as health care, where performance and biocompatibility may be critical to support life-sustaining functions. Fourth, digital files are vulnerable to intentional or unintentional alteration. These alterations might weaken design integrity and be imperceptible to the manufacturer or end user. This is a prevalent challenge in all open-source designs.
The pandemic materialized quickly and created vast supply chain challenges. To address this crisis, it was clear that the average 17-year interval between research and translation in the US was unacceptable. The VA was able to accelerate swiftly many existing processes to meet this need, build new capabilities, and establish new practices for the rapid evaluation and deployment of health care products and guidance. This agile and innovative cooperation was critical in the success of the VA’s national support for pandemic solutions.
Finally, although COVID 3D TRUST was able to provide testing of submitted designs, this collaboration was not a substitute for the “peacetime” process of manufacturing site registration with the FDA and product listing. COVID 3D TRUST could evaluate designs only, not the production process, safety, and efficacy.
CALLS TO ACTION
The pandemic's impact on medical supply chain security persists, as does the need for greater foresight and crisis preparation. We must act now to avoid experiencing again the magnitude of fatalities (civilian and veteran) and the devastation to the US economy and livelihoods that occurred during this single biological event. To this end, creating a digital stockpile of federally curated, crisis-ready designs for as-needed distribution across our US industrial base would offer a second line of defense against life-threatening supply chain interruptions. The realization of such a digital stockpile requires calls to action among multiple contributors.
Collaborations
The VA’s Fourth Mission is to improve the nation’s preparedness for response to war, terrorism, national emergencies, and natural disasters. The VA does this by developing plans and taking actions to ensure continued service to veterans, as well as to support national, state, and local emergency management, public health, safety, and homeland security efforts.
The VA partnership with the FDA and NIH during the pandemic enabled successful coordination among federal agencies. Numerous other agencies, including the US Department of Defense (DoD), the Biomedical Advanced Research and Development Authority (BARDA), and the Defense Advanced Research Projects Agency (DARPA), also developed and executed successful initiatives.12-14 The joint awareness and management of these efforts, however, could be strengthened through more formal agreements and processes in peacetime. The VA/FDA/NIH Memorandum of Understanding is a prototype example of each agency lending its subject matter expertise to address a host of pandemic challenges collectively, cooperatively, and efficiently.8
Public-private partnerships (eg, VA/FDA/NIH and America Makes) led to coordinated responses for crisis readiness. The Advanced Manufacturing Crisis Product Response Program, a multipartner collaboration that included VA, addressed 7 crisis scenarios, 3 of which were specifically related to COVID-19.15 In addition, both BARDA and DARPA had successful public-private collaborations, and the DoD supported national logistics and other efforts.12-14 Clearly, industry and government both recognize complementary synergies: (1) the depth of resources of US industry; and (2) the national resources, coordination, and clinical insight available through federal agencies that can address the challenges of future crises quickly and efficiently.
When traditional supply chains and manufacturing processes failed during the pandemic, new techniques were exploited to fill the unmet material needs. Novel techniques and product pathways, however, are untested or undeveloped. The collaboration between the ORD and OHIL in support of NP swab testing and production is an example of bringing research insight, regulated product development, and manufacturing together to support a complete product life cycle.
Joint Awareness and Management
The VA continues to refine the joint awareness and management (JAM) process of products from ideation to translation, to shorten the time from research to product delivery. JAM is a VA collaborative committee of partners from ORD research offices and technology transfer program, and the OHIL Office of Advanced Manufacturing, which seeks additional support and guidance from VHA clinical service lines, VA Office of General Council, and VA Office of Acquisitions, Logistics, and Construction.
This team enables the rapid identification of unmet veteran health care product needs. In addition, JAM leverages the resources of each group to support products from problem identification to solution ideation, regulated development, production, and delivery into clinical service lines. While the concept of JAM arose to meet the crisis needs of the pandemic, it persists in delivering advanced health care solutions to veterans.
A Proposed Plan
The next national crisis is likely to involve and threaten national health care security. We propose that federal agencies be brought together to form a federally supported digital stockpile. This digital stockpile must encompass, at minimum, the following features: (1) preservation of novel, scalable medical supplies and products generated during the COVID-19 pandemic, to avoid the loss of this work; (2) clinical maturation of those existing supplies and products to refine their features and functions under the guidance of clinical, regulatory, and manufacturing experts—and validate those outputs with clinical evidence; (3) manufacturing maturation of those existing supplies and products, such that complete design and production processes are developed with the intent to distribute to multiple public manufacturers during the next crisis; (4) a call for new designs/intake portal for new designs to be matured and curated as vulnerabilities are identified; (5) supply chain crisis drills executed to test public-private preparedness to ensure design transfer is turnkey and can be engaged quickly during the next crisis; and (6) public-private engagement to develop strategy, scenarios, and policy to ensure that when supply chains next fail, additional surge capacity can be quickly added to protect American lives and health care, and that when supply chains resume, surge capacity can be redirected or stood down to protect the competitive markets.
This digital stockpile can complement and be part of the Strategic National Stockpile. Whereas the Strategic National Stockpile is a reserve of physical products that may offset product shortages, the digital stockpile is a reserve of turnkey, transferable designs that may offset supply chain disruptions and production-capacity shortages.
CONCLUSIONS
The success of 3D-printed NP swabs is a specific example of the importance of collaborations across industry, government, innovators, and researchers. More important than a sole product, however, these collaborations demonstrated the potential for game-changing approaches to how public-private partnerships support the continuity of health care operations nationally and prevent the potential for unnecessary loss of life due to capacity and supply chain disruptions.
As the largest health care system in the US, the VA has a unique capability to lead in the assessment of other novel 3D-printed medical devices in partnership with the FDA. The VA has a unique patient-centered perspective on medical device efficacy, and as a government institution, it is a trusted independent source for medical device evaluation. The VA’s role in the evaluation of 3D-printed medical devices will benefit veterans and their families, clinicians, hospitals, and the broader public by providing a gold-standard evaluation for the growing medical 3D-printing industry to follow. By creating new pathways and expectations for how federal agencies maintain crisis preparedness—such as establishing a digital stockpile—we can be equipped to serve the US health care system and minimize the effects of supply chain disruptions.
Traditional manufacturing concentrates capacity into a few discrete locations while applying lean and just-in-time philosophies to maximize profit during times of somewhat predictable supply and demand. This approach exposed nationwide vulnerabilities even during local crises, such as the United States saline shortages following closure of a single plant in Puerto Rico following Hurricane Maria in 2017.1 Interruptions to the supply chain due to pandemic plant closure, weather, politics, or surge demand can cause immediate and lasting shortages. Nasal swabs were a clear example.
At the onset of COVID-19, 2 companies—Puritan in Guilford, Maine, and Copan in Italy—manufactured nearly all of the highly specialized nasopharyngeal (NP) swabs singled out by the Centers for Disease Control and Prevention (CDC) and the US Food and Drug Administration (FDA) to test patients for COVID-19. Demand for swabs skyrocketed as the virus spread, and they became unattainable. The lack of swabs meant patients went undiagnosed. Without knowing who was positive, people with symptoms and known contacts were presumed positive and quarantined, impacting isolated patients, the health care professionals treating them, and the entire US economy.
3-Dimensional Printing Solutions
Manufacturing NP swabs is not trivial. Their simple shape conceals complexity and requires highly specialized equipment. The lead time for one non-US machine manufacturer was > 6 months at the start of the pandemic.
Digital manufacturing/3-dimensional (3D) printing represented a potential solution to the supply chain crisis.2 Designers created digital blueprints for 3D-printed goods, face masks, face shields, and ventilator splitters were rapidly created and shared.3,4 Scrambling to fill the critical need for NP swabs, many hospitals, businesses, and academic centers began 3D printing swabs. This effort was spearheaded by University of South Florida (USF) and Northwell Health researchers and clinicians, who designed and tested a 3D-printed NP swab from photocurable resin that was printable on 2 models of Formlabs printers.5 Several other 3D-printed NP swab designs soon followed. This innovation and problem-solving renaissance faced several challenges well known to traditional manufacturers of regulated products but novel to newcomers.
The first challlenge was that these NP swabs predate FDA oversight of medical device development and manufacturing and no testing standards existed. Designers began casting prototypes out without guidance about the critical features and clinical functions required. Many of these designs did not have a clinical evaluation pathway to test safety and efficacy.
The second challlenge was that these swabs were being produced by facilities not registered with the FDA. This raised concerns about the quality of unlisted medical products developed and manufactured at novel facilities.
The third challenge was that small-scale novel approaches may offset local shortages but could not address national needs. The self-organized infrastructure for this crisis was ad hoc, local, and lacked coordinated federal support. This led to rolling shortages of these materials for years.
Two studies were performed early in the pandemic. The first study evaluated 4 prototypes of different manufacturer designs, finding excellent concordance among them and their control swab.6 A second study demonstrated the USF swab to be noninferior to the standard of care.7 Both studies acknowledged and addressed the first challenge for their designs.
COLLABORATIONS
Interagency
Before the pandemic, the US Department of Veterans Affairs (VA) had been coordinating with the FDA, the National Institutes of Health (NIH), and the nonprofit America Makes to bring medical product development and manufacturing closer to the point of care.
At the outset of the COVID-19 pandemic, the collaboration was formalized to address new challenges.8 The objectives of this collaboration were the following: (1) host a digital repository for 3D-printed digital designs for personal protectice equipment and other medical supplies in or at risk of shortage; (2) provide scientifically based ratings for designs according to clinical and field testing; and (3) offer education to health care workers and the public about the digital manufacturing of medical goods and devices.4,9
A key output of this collaboration was the COVID 3D Trusted Repository For Users And Suppliers Through Testing (COVID 3D TRUST), a curated archive of designs. In most cases, existing FDA standards and guidance formed the basis of testing strategies with deviations due to limited access to traditional testing facilities and reagents.
To address novel NP swabs, working with its COVID 3D TRUST partners, the VA gathered a combined list of clinical- and engineering-informed customer requirements and performed a hazard analysis. The result was a list of design inputs for NP swabs and 8 standard test protocols to evaluate key functions (Table).10 These protocols are meant to benchmark novel 3D-printed swabs against the key functions of established, traditionally manufactured swabs, which have a long record of safety and efficacy. The protocols, developed by the VA and undergoing validation by the US Army, empower and inform consumers and provide performance metrics to swab designers and manufacturers. The testing protocols and preliminary test results developed by the VA are publicly available at the NIH.11
Intra-agency
The use of the inputs and verification tests noted in the Table may reduce the risk of poor design but were inadequate to evaluate the clinical safety and efficacy of novel swabs. Recognizing this, the VA Office of Healthcare Innovation and Learning (OHIL) and the Office of Research and Development (ORD) launched the Nasal Swab Objective and Statistical Evaluation (NOSE) study to formally evaluate the safety and efficacy of 3D-printed swabs in the field. This multisite clinical study was a close collaboration between the OHIL and ORD. The OHIL provided the quality system and manufacturing oversight and delivery of the swabs, and the ORD provided scientific review, research infrastructure, human subjects oversight, administrative support, and funding and fiscal oversight. The OHIL/ORD collaboration resulted in the successful completion of the NOSE study.
This study (manuscript under preparation) yielded two 3D-printing production processes and swab designs that had comparable performance to the standard of care, were manufacturable compliant with FDA guidelines, and could be produced at scale in a distributed manner. This approach directly addressed the 3 challenges described earlier.
LESSONS LEARNED
Swabs were an example of supply challenges in the pandemic, but advanced manufacturing (notably, digital designs leading to 3D-printed solutions) also served as a temporary solution to device and product shortages during the COVID-19 pandemic. Digital designs and 3D printing as manufacturing techniques have the following key advantages: (1) they are distributed in nature, both in the breadth of locations that have access to these manufacturing platforms and in the depth of material choice that can be used to fabricate products, which alleviates the threat of a disaster impacting manufacturing capacity or a material stream; (2) they do not require retooling of machinery so new products can deploy rapidly and on demand; and (3) the speed of digital iteration, printing, and revision allows for rapid product development and production.
There also are notable disadvantages to these techniques. First, because 3D printing is a newer technology, there is less general depth of knowledge regarding design and material choice for additive manufacturing. Second, the flexibility of 3D printing means that operators must increase awareness of the factors that might cause the fabrication of a part to fail in either printing or postprocessing. Third, there are significant gaps in understanding how materials and manufacturing processes will perform in high-stakes settings such as health care, where performance and biocompatibility may be critical to support life-sustaining functions. Fourth, digital files are vulnerable to intentional or unintentional alteration. These alterations might weaken design integrity and be imperceptible to the manufacturer or end user. This is a prevalent challenge in all open-source designs.
The pandemic materialized quickly and created vast supply chain challenges. To address this crisis, it was clear that the average 17-year interval between research and translation in the US was unacceptable. The VA was able to accelerate swiftly many existing processes to meet this need, build new capabilities, and establish new practices for the rapid evaluation and deployment of health care products and guidance. This agile and innovative cooperation was critical in the success of the VA’s national support for pandemic solutions.
Finally, although COVID 3D TRUST was able to provide testing of submitted designs, this collaboration was not a substitute for the “peacetime” process of manufacturing site registration with the FDA and product listing. COVID 3D TRUST could evaluate designs only, not the production process, safety, and efficacy.
CALLS TO ACTION
The pandemic's impact on medical supply chain security persists, as does the need for greater foresight and crisis preparation. We must act now to avoid experiencing again the magnitude of fatalities (civilian and veteran) and the devastation to the US economy and livelihoods that occurred during this single biological event. To this end, creating a digital stockpile of federally curated, crisis-ready designs for as-needed distribution across our US industrial base would offer a second line of defense against life-threatening supply chain interruptions. The realization of such a digital stockpile requires calls to action among multiple contributors.
Collaborations
The VA’s Fourth Mission is to improve the nation’s preparedness for response to war, terrorism, national emergencies, and natural disasters. The VA does this by developing plans and taking actions to ensure continued service to veterans, as well as to support national, state, and local emergency management, public health, safety, and homeland security efforts.
The VA partnership with the FDA and NIH during the pandemic enabled successful coordination among federal agencies. Numerous other agencies, including the US Department of Defense (DoD), the Biomedical Advanced Research and Development Authority (BARDA), and the Defense Advanced Research Projects Agency (DARPA), also developed and executed successful initiatives.12-14 The joint awareness and management of these efforts, however, could be strengthened through more formal agreements and processes in peacetime. The VA/FDA/NIH Memorandum of Understanding is a prototype example of each agency lending its subject matter expertise to address a host of pandemic challenges collectively, cooperatively, and efficiently.8
Public-private partnerships (eg, VA/FDA/NIH and America Makes) led to coordinated responses for crisis readiness. The Advanced Manufacturing Crisis Product Response Program, a multipartner collaboration that included VA, addressed 7 crisis scenarios, 3 of which were specifically related to COVID-19.15 In addition, both BARDA and DARPA had successful public-private collaborations, and the DoD supported national logistics and other efforts.12-14 Clearly, industry and government both recognize complementary synergies: (1) the depth of resources of US industry; and (2) the national resources, coordination, and clinical insight available through federal agencies that can address the challenges of future crises quickly and efficiently.
When traditional supply chains and manufacturing processes failed during the pandemic, new techniques were exploited to fill the unmet material needs. Novel techniques and product pathways, however, are untested or undeveloped. The collaboration between the ORD and OHIL in support of NP swab testing and production is an example of bringing research insight, regulated product development, and manufacturing together to support a complete product life cycle.
Joint Awareness and Management
The VA continues to refine the joint awareness and management (JAM) process of products from ideation to translation, to shorten the time from research to product delivery. JAM is a VA collaborative committee of partners from ORD research offices and technology transfer program, and the OHIL Office of Advanced Manufacturing, which seeks additional support and guidance from VHA clinical service lines, VA Office of General Council, and VA Office of Acquisitions, Logistics, and Construction.
This team enables the rapid identification of unmet veteran health care product needs. In addition, JAM leverages the resources of each group to support products from problem identification to solution ideation, regulated development, production, and delivery into clinical service lines. While the concept of JAM arose to meet the crisis needs of the pandemic, it persists in delivering advanced health care solutions to veterans.
A Proposed Plan
The next national crisis is likely to involve and threaten national health care security. We propose that federal agencies be brought together to form a federally supported digital stockpile. This digital stockpile must encompass, at minimum, the following features: (1) preservation of novel, scalable medical supplies and products generated during the COVID-19 pandemic, to avoid the loss of this work; (2) clinical maturation of those existing supplies and products to refine their features and functions under the guidance of clinical, regulatory, and manufacturing experts—and validate those outputs with clinical evidence; (3) manufacturing maturation of those existing supplies and products, such that complete design and production processes are developed with the intent to distribute to multiple public manufacturers during the next crisis; (4) a call for new designs/intake portal for new designs to be matured and curated as vulnerabilities are identified; (5) supply chain crisis drills executed to test public-private preparedness to ensure design transfer is turnkey and can be engaged quickly during the next crisis; and (6) public-private engagement to develop strategy, scenarios, and policy to ensure that when supply chains next fail, additional surge capacity can be quickly added to protect American lives and health care, and that when supply chains resume, surge capacity can be redirected or stood down to protect the competitive markets.
This digital stockpile can complement and be part of the Strategic National Stockpile. Whereas the Strategic National Stockpile is a reserve of physical products that may offset product shortages, the digital stockpile is a reserve of turnkey, transferable designs that may offset supply chain disruptions and production-capacity shortages.
CONCLUSIONS
The success of 3D-printed NP swabs is a specific example of the importance of collaborations across industry, government, innovators, and researchers. More important than a sole product, however, these collaborations demonstrated the potential for game-changing approaches to how public-private partnerships support the continuity of health care operations nationally and prevent the potential for unnecessary loss of life due to capacity and supply chain disruptions.
As the largest health care system in the US, the VA has a unique capability to lead in the assessment of other novel 3D-printed medical devices in partnership with the FDA. The VA has a unique patient-centered perspective on medical device efficacy, and as a government institution, it is a trusted independent source for medical device evaluation. The VA’s role in the evaluation of 3D-printed medical devices will benefit veterans and their families, clinicians, hospitals, and the broader public by providing a gold-standard evaluation for the growing medical 3D-printing industry to follow. By creating new pathways and expectations for how federal agencies maintain crisis preparedness—such as establishing a digital stockpile—we can be equipped to serve the US health care system and minimize the effects of supply chain disruptions.
1. Sacks CA, Kesselheim AS, Fralick M. The shortage of normal saline in the wake of Hurricane Maria. JAMA Intern Med. 2018;178(7):885–886. doi:10.1001/jamainternmed.2018.1936
2. Bauchner H, Fontanarosa PB, Livingston EH. Conserving supply of personal protective equipment–a call for ideas. JAMA. 2020;323(19):1911. doi:10.1001/jama.2020.4770
3. Sinha MS, Bourgeois FT, Sorger PK. Personal protective equipment for COVID-19: distributed fabrication and additive manufacturing. Am J Public Health. 2020;110(8):1162-1164. doi:10.2105/AJPH.2020.305753
4. McCarthy MC, Di Prima M, Cruz P, et al. Trust in the time of Covid-19: 3D printing and additive manufacturing (3DP/AM) as a solution to supply chain gaps. NEJM Catalyst. 2021;2(6). doi:10.1056/CAT.21.0321
5. Ford J, Goldstein T, Trahan S, Neuwirth A, Tatoris K, Decker S. A 3D-printed nasopharyngeal swab for COVID-19 diagnostic testing. 3D Print Med. 2020;6(1):21. Published 2020 Aug 15. doi:10.1186/s41205-020-00076-3
6. Callahan CJ, Lee R, Zulauf K, et al. Open development and clinical validation of multiple 3D-printed sample-collection swabs: rapid resolution of a critical COVID-19 testing bottleneck. Preprint. medRxiv. 2020;2020.04.14.20065094. Published 2020 Apr 17. doi:10.1101/2020.04.14.20065094
7. Decker SJ, Goldstein TA, Ford JM, et al. 3-dimensional printed alternative to the standard synthetic flocked nasopharyngeal swabs used for coronavirus disease 2019 testing. Clin Infect Dis. 2021;73(9):e3027-e3032. doi:10.1093/cid/ciaa1366
8. US Food and Drug Administration. Memorandum of understanding: rapid response to Covid-19 using 3d printing between National Institutes of Health within U.S. Department of Health and Human Services and Food and Drug Administration, U.S. Department of Health and Human Services and Veterans Health Administration within the U.S. Department of Veterans Affairs. March 26, 2020. Accessed August 31, 2023. https://www.fda.gov/about-fda/domestic-mous/mou-225-20-008
9. National Institutes of Health, NIH 3D Print Exchange. Covid 3D trust: trusted repository for users and suppliers through testing. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=search
10. National Institutes of Health, NIH 3D Print Exchange. 3D printed nasal swabs - assessment criteria. August 17, 2020. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=swabassessment
11. National Institutes of Health, NIH 3D Print Exchange. 3D printed nasal swabs - general information. August 17, 2020. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=swabinfo
12. US Department of Defense. Coronavirus: DOD response. December 20, 2022. Accessed August 31, 2023. https://www.defense.gov/Spotlights/Coronavirus-DoD-Response
13. US Department of Health and Human Services, Biomedical Advanced Research and Development Authority. BARDA COVID-19 response. Updated May 25, 2023. Accessed August 31, 2023. https://www.medicalcountermeasures.gov/barda/barda-covid-19-response
14. Green S. Pandemic prevention platform (P3). Accessed August 31, 2023. https://www.darpa.mil/program/pandemic-prevention-platform
15. America Makes. America makes completes successful scenario testing for crisis response program [press release]. May 25, 2021. Accessed August 31, 2023. https://www.americamakes.us/america-makes-completes-successful-scenario-testing-for-crisis-response-program
1. Sacks CA, Kesselheim AS, Fralick M. The shortage of normal saline in the wake of Hurricane Maria. JAMA Intern Med. 2018;178(7):885–886. doi:10.1001/jamainternmed.2018.1936
2. Bauchner H, Fontanarosa PB, Livingston EH. Conserving supply of personal protective equipment–a call for ideas. JAMA. 2020;323(19):1911. doi:10.1001/jama.2020.4770
3. Sinha MS, Bourgeois FT, Sorger PK. Personal protective equipment for COVID-19: distributed fabrication and additive manufacturing. Am J Public Health. 2020;110(8):1162-1164. doi:10.2105/AJPH.2020.305753
4. McCarthy MC, Di Prima M, Cruz P, et al. Trust in the time of Covid-19: 3D printing and additive manufacturing (3DP/AM) as a solution to supply chain gaps. NEJM Catalyst. 2021;2(6). doi:10.1056/CAT.21.0321
5. Ford J, Goldstein T, Trahan S, Neuwirth A, Tatoris K, Decker S. A 3D-printed nasopharyngeal swab for COVID-19 diagnostic testing. 3D Print Med. 2020;6(1):21. Published 2020 Aug 15. doi:10.1186/s41205-020-00076-3
6. Callahan CJ, Lee R, Zulauf K, et al. Open development and clinical validation of multiple 3D-printed sample-collection swabs: rapid resolution of a critical COVID-19 testing bottleneck. Preprint. medRxiv. 2020;2020.04.14.20065094. Published 2020 Apr 17. doi:10.1101/2020.04.14.20065094
7. Decker SJ, Goldstein TA, Ford JM, et al. 3-dimensional printed alternative to the standard synthetic flocked nasopharyngeal swabs used for coronavirus disease 2019 testing. Clin Infect Dis. 2021;73(9):e3027-e3032. doi:10.1093/cid/ciaa1366
8. US Food and Drug Administration. Memorandum of understanding: rapid response to Covid-19 using 3d printing between National Institutes of Health within U.S. Department of Health and Human Services and Food and Drug Administration, U.S. Department of Health and Human Services and Veterans Health Administration within the U.S. Department of Veterans Affairs. March 26, 2020. Accessed August 31, 2023. https://www.fda.gov/about-fda/domestic-mous/mou-225-20-008
9. National Institutes of Health, NIH 3D Print Exchange. Covid 3D trust: trusted repository for users and suppliers through testing. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=search
10. National Institutes of Health, NIH 3D Print Exchange. 3D printed nasal swabs - assessment criteria. August 17, 2020. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=swabassessment
11. National Institutes of Health, NIH 3D Print Exchange. 3D printed nasal swabs - general information. August 17, 2020. Accessed August 31, 2023. https://3d.nih.gov/collections/covid-19-response?tab=swabinfo
12. US Department of Defense. Coronavirus: DOD response. December 20, 2022. Accessed August 31, 2023. https://www.defense.gov/Spotlights/Coronavirus-DoD-Response
13. US Department of Health and Human Services, Biomedical Advanced Research and Development Authority. BARDA COVID-19 response. Updated May 25, 2023. Accessed August 31, 2023. https://www.medicalcountermeasures.gov/barda/barda-covid-19-response
14. Green S. Pandemic prevention platform (P3). Accessed August 31, 2023. https://www.darpa.mil/program/pandemic-prevention-platform
15. America Makes. America makes completes successful scenario testing for crisis response program [press release]. May 25, 2021. Accessed August 31, 2023. https://www.americamakes.us/america-makes-completes-successful-scenario-testing-for-crisis-response-program
VA SHIELD: A Biorepository for Veterans and the Nation
The Veterans Health Administration (VHA) clinicians, clinician-investigators, and investigators perform basic and translational research for the benefit of our nation and are widely recognized for treating patients and discovering cures.1,2 In May 2020, the US Department of Veterans Affairs (VA) launched the VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). The goal of this novel enterprise was to assemble a comprehensive specimen and data repository for emerging life-threatening diseases and to address future challenges. VA SHIELD was specifically charged with creating a biorepository to advance research, improve diagnostic and therapeutic capabilities, and develop strategies for immediate deployment to VA clinical environments. One main objective of VA SHIELD is to harness the clinical and scientific strengths of the VA in order to create a more cohesive collaboration between preexisting clinical research efforts within the VA.
ANATOMY OF VA SHIELD
The charge and scope of VA SHIELD is unique.3 As an entity, this program leverages the strengths of the diverse VHA network, has a broad potential impact on national health care, is positioned to respond rapidly to national and international health-related events, and substantially contributes to clinical research and development. In addition, VA SHIELD upholds VA’s Fourth Mission, which is to contribute to national emergencies and support emergency management, public health, safety, and homeland security efforts.
VA SHIELD is part of the VA Office of Research and Development (ORD). The coordinating center (CC), headquartered in Cleveland, Ohio, is the central operational partner, leading VA SHIELD and interacting with other important VA programs, including laboratory, clinical science, rehabilitation, and health services. The VA SHIELD CC oversees all aspects of operations, including biospecimen collection, creating and enforcing of standard operating procedures, ensuring the quality of the samples, processing research applications, distribution of samples, financing, and progress reports. The CC also initiates and maintains interagency collaborations, convenes stakeholders, and develops strategic plans to address emerging diseases.
The VA SHIELD Executive Steering Committee (ESC) is composed of infectious disease, biorepository, and public health specialists. The ESC provides scientific and programmatic direction to the CC, including operational activities and guidance regarding biorepository priorities and scientific agenda, and measuring and reporting on VA SHIELD accomplishments.
The primary function of the Programmatic and Scientific Review Board (PSRB) is to evaluate incoming research proposals for specimen and data use for feasibility and make recommendations to the VA SHIELD CC. The PSRB evaluates and ensures that data and specimen use align with VA SHIELD ethical, clinical, and scientific objectives.
VA SHIELD IN PRACTICE
VA SHIELD consisted of 11 specimen collection sites (Atlanta, GA; Boise, ID; Bronx, NY; Cincinnati, OH; Cleveland, OH; Durham, NC; Houston, TX; Los Angeles, CA; Mountain Home, TN; Palo Alto, CA; and Tucson, AZ), a data processing center in Boston, MA, and 2 central biorepositories in Palo Alto, CA, and Tucson, AZ. Information flow is a coordinated process among specimen collection sites, data processing centers, and the biorepositories. Initially, each local collection site identifies residual specimens that would have been discarded after clinical laboratory testing. These samples currently account for the majority of biological material within VA SHIELD via a novel collection protocol known as “Sweep,” which allows residual clinical discarded samples as well as samples from patients with new emerging infectious and noninfectious diseases of concern to be collected at the time of first emergence and submitted to VA SHIELD during the course of routine veteran health care.3 These clinical discarded samples are de-identified and transferred from the clinical laboratory to VA SHIELD. The VA Central Institutional Review Board (cIRB) has approved the use of these samples as nonhuman subject research. Biological samples are collected, processed, aliquoted, shipped to, and stored at the central biorepository sites.
The Umbrella amendment to Sweep that has been approved also by the VA cIRB, will allow VA SHIELD sites to prospectively consent veterans and collect biospecimens and additional clinical and self-reported information. The implementation of Umbrella could significantly enhance collection and research. Although Sweep is a onetime collection of samples, the Umbrella protocol will allow the longitudinal collection of samples from the same patient. Additionally, the Umbrella amendment will allow VA SHIELD to accept samples from other preexisting biorepositories or specimen collections.
Central Biorepositories
VA SHIELD has a federated organization with 2 central specimen biorepositories (Palo Alto, CA and Tucson, AZ), and an enterprise data processing center (Boston, MA). The specimen biorepositories receive de-identified specimens that are stored until distribution to approved research projects. The samples and data are linked using an electronic honest broker system to protect privacy, which integrates de-identified specimens with requested clinical and demographic data as needed for approved projects. The honest broker system is operated by independent personnel and does not have vested interest in any studies being performed under VA SHIELD. The integration of sample and associated data is done only as needed when characterization of the donor/participant is necessary byresearch aims or project outcomes. The process is facilitated by a nationally supported laboratory information management system (LIMS), managed by the VA SHIELD data center, that assists with all data requests. The clinical and demographic data are collected from VA electronic health record (EHR), available through VA Corporate Data Warehouse (CDW) and VA Informatics and Computing Infrastructure (VINCI) as needed and integrated with the biorepository samples information for approved VA SHIELD studies. The CDW is the largest longitudinal EHR data collection in the US and has the ability to provide access to national clinical and demographic data.
VA SHIELD interacts with multiple VA programs and other entities (Figure). For example, Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) is a network of 5 VA medical centers supported by the Centers for Disease Control and Prevention.4 Its initial goal was to perform surveillance for acute gastroenteritis. In 2020, SUPERNOVA shifted to conduct surveillance for COVID-19 variants among veterans.5 VA SHIELD also interacts with VHA genomic surveillance and sequencing programs: the VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) and VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), described by Krishnan and colleagues.6
Working Groups
To encourage research projects that use biospecimens, VA SHIELD developed content-oriented research working groups. The goal is to inspire collaborations between VA scientists and prevent redundant or overlapping projects. Currently working groups are focused on long COVID, and COVID-19 neurology, pathogen host response, epidemiology and sequencing, cancer and cancer biomarkers, antimicrobial resistance, and vector-borne diseases. Working groups meet regularly to discuss projects and report progress. Working groups also may consider samples that might benefit VA health research and identify potential veteran populations for future research. Working groups connect VA SHIELD and investigators and guide the collection and use of resources.
Ethical Considerations
We recognize the significant ethical concerns for biobanking of specimens. However, there is no general consensus or guideline that addresses all of the complex ethical issues regarding biobanking.7 To address these ethical concerns, we applied the VA Ethical Framework Principles for Access to and Use of Veteran Data principles to VA SHIELD, including all parties who oversee the access to, sharing of, or the use of data, or who access or use its data.8
Conclusions
The VA has assembled a scientific enterprise dedicated to combating emerging infectious diseases and other threats to our patients. This enterprise has been modeled in its structure and oversight to support VA SHIELD. The establishment of a real-time biorepository and data procurement system linked to clinical samples is a bold step forward to address current and future challenges. Similarly, the integration and cooperation of multiple arms within the VA that transcend disciplines and boundaries promise to shepherd a new era of system-wide investigation. In the future, VA SHIELD will integrate with other existing government agencies to advance mutual scientific agendas. VA SHIELD has established the data and biorepository infrastructure to develop innovative and novel technologies to address future challenges. The alignment of basic science, clinical, and translational research goals under one governance is a significant advancement compared with previous models of research coordination.
VA SHIELD was developed to meet an immediate need; it was also framed to be a research enterprise that harnesses the robust clinical and research environment in VHA. The VA SHIELD infrastructure was conceptualized to harmonize specimen and data collection across the VA, allowing researchers to leverage broader collection efforts. Building a biorepository and data collection system within the largest integrated health care system has the potential to provide a lasting impact on VHA and on our nation’s health.
Acknowledgments
The authors wish to acknowledge Ms. Daphne Swancutt for her contribution as copywriter for this manuscript. The authors wish to acknowledge the VA SHIELD investigators: Mary Cloud Ammons, David Beenhouwer, Sheldon T. Brown, Victoria Davey, Abhinav Diwan, John B. Harley, Mark Holodniy, Vincent C. Marconi, Jonathan Moorman, Emerson B. Padiernos, Ian F. Robey, Maria Rodriguez-Barradas, Jason Wertheim, Christopher W. Woods.
1. Lipshy KA, Itani K, Chu D, et al. Sentinel contributions of US Department of Veterans Affairs surgeons in shaping the face of health care. JAMA Surg. 2021;156(4):380-386. doi:10.1001/jamasurg.2020.6372
2. Zucker S, Crabbe JC, Cooper G 4th, et al. Veterans Administration support for medical research: opinions of the endangered species of physician-scientists. FASEB J. 2004;18(13):1481-1486. doi:10.1096/fj.04-1573lfe
3. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
4. Meites E, Bajema KL, Kambhampati A, et al; SUPERNOVA COVID-19 Surveillance Group. Adapting the Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) for COVID-19 among hospitalized adults: surveillance protocol. Front Public Health. 2021;9:739076. doi:10.3389/fpubh.2021.739076
5. Bajema KL, Dahl RM, Evener SL, et al; SUPERNOVA COVID-19 Surveillance Group; Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans–five Veterans Affairs Medical Centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. doi:10.15585/mmwr.mm7049a2external icon
6. Krishnan J, Woods C, Holodniy M, et al. Nationwide genomic surveillance and response to coronavirus disease 2019 (COVID-19): SeqCURE and SeqFORCE consortiums. Fed Pract. 2023;40(suppl 5):S44-S47. doi:10.12788/fp.0417
7. Ashcroft JW, Macpherson CC. The complex ethical landscape of biobanking. Lancet Public Health. 2019;(6):e274-e275. doi:10.1016/S2468-2667(19)30081-7
8. Principle-Based Ethics Framework for Access to and Use of Veteran Data. Fed Regist. 2022;87(129):40451-40452.
The Veterans Health Administration (VHA) clinicians, clinician-investigators, and investigators perform basic and translational research for the benefit of our nation and are widely recognized for treating patients and discovering cures.1,2 In May 2020, the US Department of Veterans Affairs (VA) launched the VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). The goal of this novel enterprise was to assemble a comprehensive specimen and data repository for emerging life-threatening diseases and to address future challenges. VA SHIELD was specifically charged with creating a biorepository to advance research, improve diagnostic and therapeutic capabilities, and develop strategies for immediate deployment to VA clinical environments. One main objective of VA SHIELD is to harness the clinical and scientific strengths of the VA in order to create a more cohesive collaboration between preexisting clinical research efforts within the VA.
ANATOMY OF VA SHIELD
The charge and scope of VA SHIELD is unique.3 As an entity, this program leverages the strengths of the diverse VHA network, has a broad potential impact on national health care, is positioned to respond rapidly to national and international health-related events, and substantially contributes to clinical research and development. In addition, VA SHIELD upholds VA’s Fourth Mission, which is to contribute to national emergencies and support emergency management, public health, safety, and homeland security efforts.
VA SHIELD is part of the VA Office of Research and Development (ORD). The coordinating center (CC), headquartered in Cleveland, Ohio, is the central operational partner, leading VA SHIELD and interacting with other important VA programs, including laboratory, clinical science, rehabilitation, and health services. The VA SHIELD CC oversees all aspects of operations, including biospecimen collection, creating and enforcing of standard operating procedures, ensuring the quality of the samples, processing research applications, distribution of samples, financing, and progress reports. The CC also initiates and maintains interagency collaborations, convenes stakeholders, and develops strategic plans to address emerging diseases.
The VA SHIELD Executive Steering Committee (ESC) is composed of infectious disease, biorepository, and public health specialists. The ESC provides scientific and programmatic direction to the CC, including operational activities and guidance regarding biorepository priorities and scientific agenda, and measuring and reporting on VA SHIELD accomplishments.
The primary function of the Programmatic and Scientific Review Board (PSRB) is to evaluate incoming research proposals for specimen and data use for feasibility and make recommendations to the VA SHIELD CC. The PSRB evaluates and ensures that data and specimen use align with VA SHIELD ethical, clinical, and scientific objectives.
VA SHIELD IN PRACTICE
VA SHIELD consisted of 11 specimen collection sites (Atlanta, GA; Boise, ID; Bronx, NY; Cincinnati, OH; Cleveland, OH; Durham, NC; Houston, TX; Los Angeles, CA; Mountain Home, TN; Palo Alto, CA; and Tucson, AZ), a data processing center in Boston, MA, and 2 central biorepositories in Palo Alto, CA, and Tucson, AZ. Information flow is a coordinated process among specimen collection sites, data processing centers, and the biorepositories. Initially, each local collection site identifies residual specimens that would have been discarded after clinical laboratory testing. These samples currently account for the majority of biological material within VA SHIELD via a novel collection protocol known as “Sweep,” which allows residual clinical discarded samples as well as samples from patients with new emerging infectious and noninfectious diseases of concern to be collected at the time of first emergence and submitted to VA SHIELD during the course of routine veteran health care.3 These clinical discarded samples are de-identified and transferred from the clinical laboratory to VA SHIELD. The VA Central Institutional Review Board (cIRB) has approved the use of these samples as nonhuman subject research. Biological samples are collected, processed, aliquoted, shipped to, and stored at the central biorepository sites.
The Umbrella amendment to Sweep that has been approved also by the VA cIRB, will allow VA SHIELD sites to prospectively consent veterans and collect biospecimens and additional clinical and self-reported information. The implementation of Umbrella could significantly enhance collection and research. Although Sweep is a onetime collection of samples, the Umbrella protocol will allow the longitudinal collection of samples from the same patient. Additionally, the Umbrella amendment will allow VA SHIELD to accept samples from other preexisting biorepositories or specimen collections.
Central Biorepositories
VA SHIELD has a federated organization with 2 central specimen biorepositories (Palo Alto, CA and Tucson, AZ), and an enterprise data processing center (Boston, MA). The specimen biorepositories receive de-identified specimens that are stored until distribution to approved research projects. The samples and data are linked using an electronic honest broker system to protect privacy, which integrates de-identified specimens with requested clinical and demographic data as needed for approved projects. The honest broker system is operated by independent personnel and does not have vested interest in any studies being performed under VA SHIELD. The integration of sample and associated data is done only as needed when characterization of the donor/participant is necessary byresearch aims or project outcomes. The process is facilitated by a nationally supported laboratory information management system (LIMS), managed by the VA SHIELD data center, that assists with all data requests. The clinical and demographic data are collected from VA electronic health record (EHR), available through VA Corporate Data Warehouse (CDW) and VA Informatics and Computing Infrastructure (VINCI) as needed and integrated with the biorepository samples information for approved VA SHIELD studies. The CDW is the largest longitudinal EHR data collection in the US and has the ability to provide access to national clinical and demographic data.
VA SHIELD interacts with multiple VA programs and other entities (Figure). For example, Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) is a network of 5 VA medical centers supported by the Centers for Disease Control and Prevention.4 Its initial goal was to perform surveillance for acute gastroenteritis. In 2020, SUPERNOVA shifted to conduct surveillance for COVID-19 variants among veterans.5 VA SHIELD also interacts with VHA genomic surveillance and sequencing programs: the VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) and VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), described by Krishnan and colleagues.6
Working Groups
To encourage research projects that use biospecimens, VA SHIELD developed content-oriented research working groups. The goal is to inspire collaborations between VA scientists and prevent redundant or overlapping projects. Currently working groups are focused on long COVID, and COVID-19 neurology, pathogen host response, epidemiology and sequencing, cancer and cancer biomarkers, antimicrobial resistance, and vector-borne diseases. Working groups meet regularly to discuss projects and report progress. Working groups also may consider samples that might benefit VA health research and identify potential veteran populations for future research. Working groups connect VA SHIELD and investigators and guide the collection and use of resources.
Ethical Considerations
We recognize the significant ethical concerns for biobanking of specimens. However, there is no general consensus or guideline that addresses all of the complex ethical issues regarding biobanking.7 To address these ethical concerns, we applied the VA Ethical Framework Principles for Access to and Use of Veteran Data principles to VA SHIELD, including all parties who oversee the access to, sharing of, or the use of data, or who access or use its data.8
Conclusions
The VA has assembled a scientific enterprise dedicated to combating emerging infectious diseases and other threats to our patients. This enterprise has been modeled in its structure and oversight to support VA SHIELD. The establishment of a real-time biorepository and data procurement system linked to clinical samples is a bold step forward to address current and future challenges. Similarly, the integration and cooperation of multiple arms within the VA that transcend disciplines and boundaries promise to shepherd a new era of system-wide investigation. In the future, VA SHIELD will integrate with other existing government agencies to advance mutual scientific agendas. VA SHIELD has established the data and biorepository infrastructure to develop innovative and novel technologies to address future challenges. The alignment of basic science, clinical, and translational research goals under one governance is a significant advancement compared with previous models of research coordination.
VA SHIELD was developed to meet an immediate need; it was also framed to be a research enterprise that harnesses the robust clinical and research environment in VHA. The VA SHIELD infrastructure was conceptualized to harmonize specimen and data collection across the VA, allowing researchers to leverage broader collection efforts. Building a biorepository and data collection system within the largest integrated health care system has the potential to provide a lasting impact on VHA and on our nation’s health.
Acknowledgments
The authors wish to acknowledge Ms. Daphne Swancutt for her contribution as copywriter for this manuscript. The authors wish to acknowledge the VA SHIELD investigators: Mary Cloud Ammons, David Beenhouwer, Sheldon T. Brown, Victoria Davey, Abhinav Diwan, John B. Harley, Mark Holodniy, Vincent C. Marconi, Jonathan Moorman, Emerson B. Padiernos, Ian F. Robey, Maria Rodriguez-Barradas, Jason Wertheim, Christopher W. Woods.
The Veterans Health Administration (VHA) clinicians, clinician-investigators, and investigators perform basic and translational research for the benefit of our nation and are widely recognized for treating patients and discovering cures.1,2 In May 2020, the US Department of Veterans Affairs (VA) launched the VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). The goal of this novel enterprise was to assemble a comprehensive specimen and data repository for emerging life-threatening diseases and to address future challenges. VA SHIELD was specifically charged with creating a biorepository to advance research, improve diagnostic and therapeutic capabilities, and develop strategies for immediate deployment to VA clinical environments. One main objective of VA SHIELD is to harness the clinical and scientific strengths of the VA in order to create a more cohesive collaboration between preexisting clinical research efforts within the VA.
ANATOMY OF VA SHIELD
The charge and scope of VA SHIELD is unique.3 As an entity, this program leverages the strengths of the diverse VHA network, has a broad potential impact on national health care, is positioned to respond rapidly to national and international health-related events, and substantially contributes to clinical research and development. In addition, VA SHIELD upholds VA’s Fourth Mission, which is to contribute to national emergencies and support emergency management, public health, safety, and homeland security efforts.
VA SHIELD is part of the VA Office of Research and Development (ORD). The coordinating center (CC), headquartered in Cleveland, Ohio, is the central operational partner, leading VA SHIELD and interacting with other important VA programs, including laboratory, clinical science, rehabilitation, and health services. The VA SHIELD CC oversees all aspects of operations, including biospecimen collection, creating and enforcing of standard operating procedures, ensuring the quality of the samples, processing research applications, distribution of samples, financing, and progress reports. The CC also initiates and maintains interagency collaborations, convenes stakeholders, and develops strategic plans to address emerging diseases.
The VA SHIELD Executive Steering Committee (ESC) is composed of infectious disease, biorepository, and public health specialists. The ESC provides scientific and programmatic direction to the CC, including operational activities and guidance regarding biorepository priorities and scientific agenda, and measuring and reporting on VA SHIELD accomplishments.
The primary function of the Programmatic and Scientific Review Board (PSRB) is to evaluate incoming research proposals for specimen and data use for feasibility and make recommendations to the VA SHIELD CC. The PSRB evaluates and ensures that data and specimen use align with VA SHIELD ethical, clinical, and scientific objectives.
VA SHIELD IN PRACTICE
VA SHIELD consisted of 11 specimen collection sites (Atlanta, GA; Boise, ID; Bronx, NY; Cincinnati, OH; Cleveland, OH; Durham, NC; Houston, TX; Los Angeles, CA; Mountain Home, TN; Palo Alto, CA; and Tucson, AZ), a data processing center in Boston, MA, and 2 central biorepositories in Palo Alto, CA, and Tucson, AZ. Information flow is a coordinated process among specimen collection sites, data processing centers, and the biorepositories. Initially, each local collection site identifies residual specimens that would have been discarded after clinical laboratory testing. These samples currently account for the majority of biological material within VA SHIELD via a novel collection protocol known as “Sweep,” which allows residual clinical discarded samples as well as samples from patients with new emerging infectious and noninfectious diseases of concern to be collected at the time of first emergence and submitted to VA SHIELD during the course of routine veteran health care.3 These clinical discarded samples are de-identified and transferred from the clinical laboratory to VA SHIELD. The VA Central Institutional Review Board (cIRB) has approved the use of these samples as nonhuman subject research. Biological samples are collected, processed, aliquoted, shipped to, and stored at the central biorepository sites.
The Umbrella amendment to Sweep that has been approved also by the VA cIRB, will allow VA SHIELD sites to prospectively consent veterans and collect biospecimens and additional clinical and self-reported information. The implementation of Umbrella could significantly enhance collection and research. Although Sweep is a onetime collection of samples, the Umbrella protocol will allow the longitudinal collection of samples from the same patient. Additionally, the Umbrella amendment will allow VA SHIELD to accept samples from other preexisting biorepositories or specimen collections.
Central Biorepositories
VA SHIELD has a federated organization with 2 central specimen biorepositories (Palo Alto, CA and Tucson, AZ), and an enterprise data processing center (Boston, MA). The specimen biorepositories receive de-identified specimens that are stored until distribution to approved research projects. The samples and data are linked using an electronic honest broker system to protect privacy, which integrates de-identified specimens with requested clinical and demographic data as needed for approved projects. The honest broker system is operated by independent personnel and does not have vested interest in any studies being performed under VA SHIELD. The integration of sample and associated data is done only as needed when characterization of the donor/participant is necessary byresearch aims or project outcomes. The process is facilitated by a nationally supported laboratory information management system (LIMS), managed by the VA SHIELD data center, that assists with all data requests. The clinical and demographic data are collected from VA electronic health record (EHR), available through VA Corporate Data Warehouse (CDW) and VA Informatics and Computing Infrastructure (VINCI) as needed and integrated with the biorepository samples information for approved VA SHIELD studies. The CDW is the largest longitudinal EHR data collection in the US and has the ability to provide access to national clinical and demographic data.
VA SHIELD interacts with multiple VA programs and other entities (Figure). For example, Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) is a network of 5 VA medical centers supported by the Centers for Disease Control and Prevention.4 Its initial goal was to perform surveillance for acute gastroenteritis. In 2020, SUPERNOVA shifted to conduct surveillance for COVID-19 variants among veterans.5 VA SHIELD also interacts with VHA genomic surveillance and sequencing programs: the VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) and VA Sequencing for Research Clinical and Epidemiology (SeqFORCE), described by Krishnan and colleagues.6
Working Groups
To encourage research projects that use biospecimens, VA SHIELD developed content-oriented research working groups. The goal is to inspire collaborations between VA scientists and prevent redundant or overlapping projects. Currently working groups are focused on long COVID, and COVID-19 neurology, pathogen host response, epidemiology and sequencing, cancer and cancer biomarkers, antimicrobial resistance, and vector-borne diseases. Working groups meet regularly to discuss projects and report progress. Working groups also may consider samples that might benefit VA health research and identify potential veteran populations for future research. Working groups connect VA SHIELD and investigators and guide the collection and use of resources.
Ethical Considerations
We recognize the significant ethical concerns for biobanking of specimens. However, there is no general consensus or guideline that addresses all of the complex ethical issues regarding biobanking.7 To address these ethical concerns, we applied the VA Ethical Framework Principles for Access to and Use of Veteran Data principles to VA SHIELD, including all parties who oversee the access to, sharing of, or the use of data, or who access or use its data.8
Conclusions
The VA has assembled a scientific enterprise dedicated to combating emerging infectious diseases and other threats to our patients. This enterprise has been modeled in its structure and oversight to support VA SHIELD. The establishment of a real-time biorepository and data procurement system linked to clinical samples is a bold step forward to address current and future challenges. Similarly, the integration and cooperation of multiple arms within the VA that transcend disciplines and boundaries promise to shepherd a new era of system-wide investigation. In the future, VA SHIELD will integrate with other existing government agencies to advance mutual scientific agendas. VA SHIELD has established the data and biorepository infrastructure to develop innovative and novel technologies to address future challenges. The alignment of basic science, clinical, and translational research goals under one governance is a significant advancement compared with previous models of research coordination.
VA SHIELD was developed to meet an immediate need; it was also framed to be a research enterprise that harnesses the robust clinical and research environment in VHA. The VA SHIELD infrastructure was conceptualized to harmonize specimen and data collection across the VA, allowing researchers to leverage broader collection efforts. Building a biorepository and data collection system within the largest integrated health care system has the potential to provide a lasting impact on VHA and on our nation’s health.
Acknowledgments
The authors wish to acknowledge Ms. Daphne Swancutt for her contribution as copywriter for this manuscript. The authors wish to acknowledge the VA SHIELD investigators: Mary Cloud Ammons, David Beenhouwer, Sheldon T. Brown, Victoria Davey, Abhinav Diwan, John B. Harley, Mark Holodniy, Vincent C. Marconi, Jonathan Moorman, Emerson B. Padiernos, Ian F. Robey, Maria Rodriguez-Barradas, Jason Wertheim, Christopher W. Woods.
1. Lipshy KA, Itani K, Chu D, et al. Sentinel contributions of US Department of Veterans Affairs surgeons in shaping the face of health care. JAMA Surg. 2021;156(4):380-386. doi:10.1001/jamasurg.2020.6372
2. Zucker S, Crabbe JC, Cooper G 4th, et al. Veterans Administration support for medical research: opinions of the endangered species of physician-scientists. FASEB J. 2004;18(13):1481-1486. doi:10.1096/fj.04-1573lfe
3. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
4. Meites E, Bajema KL, Kambhampati A, et al; SUPERNOVA COVID-19 Surveillance Group. Adapting the Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) for COVID-19 among hospitalized adults: surveillance protocol. Front Public Health. 2021;9:739076. doi:10.3389/fpubh.2021.739076
5. Bajema KL, Dahl RM, Evener SL, et al; SUPERNOVA COVID-19 Surveillance Group; Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans–five Veterans Affairs Medical Centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. doi:10.15585/mmwr.mm7049a2external icon
6. Krishnan J, Woods C, Holodniy M, et al. Nationwide genomic surveillance and response to coronavirus disease 2019 (COVID-19): SeqCURE and SeqFORCE consortiums. Fed Pract. 2023;40(suppl 5):S44-S47. doi:10.12788/fp.0417
7. Ashcroft JW, Macpherson CC. The complex ethical landscape of biobanking. Lancet Public Health. 2019;(6):e274-e275. doi:10.1016/S2468-2667(19)30081-7
8. Principle-Based Ethics Framework for Access to and Use of Veteran Data. Fed Regist. 2022;87(129):40451-40452.
1. Lipshy KA, Itani K, Chu D, et al. Sentinel contributions of US Department of Veterans Affairs surgeons in shaping the face of health care. JAMA Surg. 2021;156(4):380-386. doi:10.1001/jamasurg.2020.6372
2. Zucker S, Crabbe JC, Cooper G 4th, et al. Veterans Administration support for medical research: opinions of the endangered species of physician-scientists. FASEB J. 2004;18(13):1481-1486. doi:10.1096/fj.04-1573lfe
3. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
4. Meites E, Bajema KL, Kambhampati A, et al; SUPERNOVA COVID-19 Surveillance Group. Adapting the Surveillance Platform for Enteric and Respiratory Infectious Organisms at United States Veterans Affairs Medical Centers (SUPERNOVA) for COVID-19 among hospitalized adults: surveillance protocol. Front Public Health. 2021;9:739076. doi:10.3389/fpubh.2021.739076
5. Bajema KL, Dahl RM, Evener SL, et al; SUPERNOVA COVID-19 Surveillance Group; Surveillance Platform for Enteric and Respiratory Infectious Organisms at the VA (SUPERNOVA) COVID-19 Surveillance Group. Comparative effectiveness and antibody responses to Moderna and Pfizer-BioNTech COVID-19 vaccines among hospitalized veterans–five Veterans Affairs Medical Centers, United States, February 1-September 30, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(49):1700-1705. doi:10.15585/mmwr.mm7049a2external icon
6. Krishnan J, Woods C, Holodniy M, et al. Nationwide genomic surveillance and response to coronavirus disease 2019 (COVID-19): SeqCURE and SeqFORCE consortiums. Fed Pract. 2023;40(suppl 5):S44-S47. doi:10.12788/fp.0417
7. Ashcroft JW, Macpherson CC. The complex ethical landscape of biobanking. Lancet Public Health. 2019;(6):e274-e275. doi:10.1016/S2468-2667(19)30081-7
8. Principle-Based Ethics Framework for Access to and Use of Veteran Data. Fed Regist. 2022;87(129):40451-40452.
Nationwide Genomic Surveillance and Response to COVID-19: The VA SeqFORCE and SeqCURE Consortiums
The COVID-19 virus and its associated pandemic have highlighted the urgent need for a national infrastructure to rapidly identify and respond to emerging pathogens. The importance of understanding viral population dynamics through genetic sequencing has become apparent over time, particularly as the vaccine responses, clinical implications, and therapeutic effectiveness of treatments have varied substantially with COVID-19 variants.1,2
As the largest integrated health care system in the US, the US Department of Veterans Affairs (VA) is uniquely situated to help with pandemic detection and response. This article highlights 2 VA programs dedicated to COVID-19 sequencing at the forefront of pandemic response and research: VA Sequencing for Research Clinical and Epidemiology (SeqFORCE) and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) (Table).
VA Seq FORCE
VA SeqFORCE was established March 2021 to facilitate clinical surveillance of COVID-19 variants in the US veteran population and in VA employees. VA SeqFORCE consists of 9 Clinical Laboratory Improvement Amendment (CLIA)–certified laboratories in VA medical centers, including the VA Public Health Reference Laboratory in Palo Alto, California, and 8 Veterans Health Administration (VHA) clinical laboratories (Los Angeles, California; Boise, Idaho; Iowa City, Iowa; Bronx, New York; West Haven, Connecticut; Indianapolis, Indiana; Denver, Colorado; and Orlando, Florida).3 Specimen standards (eg, real-time polymerase chain reaction [RT-PCR] cycle threshold [Ct] ≤ 30, minimum volume, etc) and clinical criteria (eg, COVID-19–related deaths, COVID-19 vaccine escape, etc) for submitting samples to VA SeqFORCE laboratories were established, and logistics for sample sequencing was centralized, including providing centralized instructions for sample preparation and to which VA SeqFORCE laboratory samples should be sent.
These laboratories sequenced samples from patients and employees with COVID-19 to understand patterns of variant evolution, vaccine, antiviral and monoclonal antibody response, health care–associated outbreaks, and COVID-19 transmission. As clinically relevant findings, such as monoclonal antibody treatment failure, emerged with novel viral variants, VA SeqFORCE was well positioned to rapidly detect the emergent variants and inform better clinical care of patients with COVID-19. Other clinical indications identified for sequencing within VA SeqFORCE included outbreak investigation, re-infection with COVID-19 > 90 days but < 6 months after a prior infection, extended hospitalization of > 21 days, death due to COVID-19, infection with a history of recent nondomestic travel, rebound of symptoms after improvement on oral antiviral therapy, and epidemiologic surveillance.
VA SeqFORCE laboratories use a variety of sequencing platforms, although a federated system was developed that electronically linked all laboratories using a software system (PraediGene, Bitscopic) for sample management, COVID-19 variant analytics, and automated result reporting of clade and lineage into the Veterans Health Information Systems and Technology Architecture (VistA) Computerized Patient Record System. In addition, generated nucleic acid sequence alignment through FASTA consensus sequence files have been archived for secondary research analyses. By archiving the consensus sequences, retrospective studies within the VA have the added benefit of being able to clinically annotate investigations into COVID-19 variant patterns. As of August 2023, 43,003 samples containing COVID-19 have been sequenced, and FASTA file and metadata upload are ongoing to the Global Initiative on Sharing Avian Influenza Data, which houses > 15 million COVID-19 files from global submissions.
VA SeqFORCE’s clinical sequencing efforts have created opportunities for multicenter collaboration in variant surveillance. In work from December 2021, investigators from the James J. Peters VA Medical Center in Bronx, New York, collaborated with the VHA Pathology and Laboratory Medicine Services and Public Health national program offices in Washington, DC, to develop an RT-PCR assay to rapidly differentiate Omicron from Delta variants.4 Samples from VA hospitals across the nation were used in this study.
Lessons from VA SeqFORCE have also been cited as inspiration to address COVID-19 clinical problems, including outbreak investigations in hospital settings and beyond. Researchers at the Iowa City VA Health Care System, for example, proposed a novel probabilistic quantitative method for determining genetic-relatedness among COVID-19 viral strains in an outbreak setting.5 They extended the scope of work to develop COVID-19 outbreak screening tools combining publicly available algorithms with targeted sequencing data to identify outbreaks as they arise.6 We expect VA SeqFORCE, in conjunction with its complement VA SeqCURE, will continue to further pandemic surveillance and response.
VA Seq CURE
As the research-focused complement to VA SeqFORCE, VA SeqCURE is dedicated to a broader study of the COVID-19 genome through sequencing. Established January 2021, the VA SeqCURE network consists of 6 research laboratories in Boise, Idaho; Bronx, New York; Cleveland, Ohio; Durham, North Carolina; Iowa City, Iowa; and Temple, Texas.
Samples are collected as a subset of the broader VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD) biorepository sweep protocol for discarded blood and nasal swab specimens of VHA patients hospitalized with COVID-19, as described by Epstein and colleagues.7-9 While VA SeqFORCE sequences samples positive for COVID-19 by RT-PCR with a Ct value of ≤ 30 for diagnostic purposes, VA SeqCURE laboratories sequence more broadly for nondiagnostic purposes, including samples with a Ct value > 30. The 6 VA SeqCURE laboratories generate sequencing data using various platforms, amplification kits, and formats. To ensure maximum quality and metadata on the sequences generated across the different laboratories, a sequence intake pipeline has been developed, adapting the ViralRecon bioinformatics platform.10 This harmonized analysis pipeline accommodates different file formats and performs quality control, alignment, variant calling, lineage assignment, clade assignment, and annotation. As of August 2023, VA SeqCURE has identified viral sequences from 24,107 unique specimens. Annotated COVID-19 sequences with the appropriate metadata will be available to VA researchers through VA SHIELD.
Research projects include descriptive epidemiology of COVID-19 variants in individuals who receive VHA care, COVID-19 vaccine and therapy effectiveness, and the unique distribution of variants and vaccine effectiveness in rural settings.3 True to its core mission, members of the VA SeqCURE consortium have contributed to the COVID-19 viral sequencing literature over the past 2 years. Researchers also are accessing VA SeqCURE to study COVID-19 persistence and rebound among individuals with mild disease taking nirmatrelvir/ritonavir compared with other COVID-19 therapeutics and untreated controls. Finally, COVID-19 samples and their sequences are stored in the VA SHIELD biorepository, which leverages these samples and data to advance scientific understanding of COVID-19 and future emerging infectious diseases.7-9
Important work from investigators at the Central Texas Veterans Health Care System confronted the issue of whole genome sequencing data from COVID-19 samples with low viral loads, a common issue with COVID-19 sequencing. They found that yields of 2 sequencing protocols, which generated high-sequence coverage, were enhanced further by combining the results of both methods.11 This project, which has potentially broad applications for sequencing in research and clinical settings, is an example of VA SeqCURE’s efforts to address the COVID-19 pandemic. The VA SeqCURE program has substantial potential as a large viral sequencing repository with broad geographic and demographic representation, such that future large-scale sequencing analyses may be generated from preexisting nested cohorts within the repository.
NEXT STEPS
Promising new directions of clinical and laboratory-based research are planned for VA SeqFORCE and VA SeqCURE. While the impact of COVID-19 and other viruses with epidemic potential is perhaps most feared in urban settings, evidence suggests that the distribution of COVID-19 in rural settings is unique and associated with worse outcomes.12,13 Given the wide catchment areas of VA hospitals that encompass both rural and urban settings, the VA’s ongoing COVID-19 sequencing programs and repositories are uniquely positioned to understand viral dynamics in areas of differing population density.
While rates of infection, hospitalization, and death resulting from COVID-19 have substantially dropped, the long-term impact of the pandemic is just beginning to be recognized in conditions such as long COVID or postacute COVID-19 syndrome. Long COVID has already proven to be biologically multifaceted, difficult to diagnose, and unpredictable in identifying the most at-risk patients.14-16 Much remains to be determined in our understanding of long COVID, including a unified definition that can effectively be used in clinical settings to diagnose and treat patients. However, research indicates that comorbidities common in veterans, such as diabetes and cardiovascular disease, are associated with worse long-term outcomes.17,18 Collaborations between VA scientists, clinicians, and national cooperative programs (such as a network of VHA long COVID clinics) create an unmatched opportunity for VA SeqFORCE and VA SeqCURE programs to provide insight into a disease likely to become a chronic disease outcome of the pandemic.
With VA SeqFORCE and VA SeqCURE programs, the VA now has infrastructure ready to respond to new infectious diseases. During the mpox outbreak of 2022, the VA Public Health Reference Laboratory received > 80% of all VA mpox samples for orthopox screening and mpox confirmatory testing. A subset of these samples underwent whole genome sequencing with the identification of 10 unique lineages across VA, and > 200 positive and 400 negative samples have been aliquoted and submitted to VA SHIELD for research. Furthermore, the VA SeqFORCE and VA SeqCURE sequencing processes might be adapted to identify outbreaks of multidrug-resistant organisms among VA patients trialed at other institutions.19 We are hopeful that VA SeqFORCE and VA SeqCURE will become invaluable components of health care delivery and infection prevention at the hospital level and beyond.
Finally, the robust data infrastructure and associated repositories of VA SeqFORCE and VA SeqCURE may be leveraged to study noninfectious diseases. Research groups are starting to apply these programs to cancer sequencing. We anticipate that these efforts may have a substantial impact on our understanding of cancer epidemiology and region-specific risk factors for malignancy, given the size and breadth of VA SeqFORCE and VA SeqCURE. Common oncogenic mutations identified through these programs could be targets for precision oncology therapeutics. Similarly, we envision applications of the VA SeqFORCE and VA SeqCURE data infrastructures and repositories toward other precision medicine fields, including pharmacogenomics and nutrition, to tailor interventions to meet the specific individual needs of veterans.
CONCLUSIONS
The productivity of VA SeqFORCE and VA SeqCURE programs over the past 2 years continues to increase in response to the COVID-19 pandemic. We anticipate that they will be vital components in our nation’s responses to infectious threats and beyond.
1. Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146-152. Published 2022 Jan 28. doi:10.15585/mmwr.mm7104e4
2. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303-1312. doi:10.1016/S0140-6736(22)00462-7
3. Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report - annex C. December 5, 2022. Accessed August 28, 2023. https://www.va.gov/HEALTH/docs/VHA-COVID-19-Response-2022-Annex-C.pdf 4. Barasch NJ, Iqbal J, Coombs M, et al. Utilization of a SARS-CoV-2 variant assay for the rapid differentiation of Omicron and Delta. medRxiv. Preprint posted online December 27, 2021. doi:10.1101/2021.12.22.21268195
5. Bilal MY. Similarity Index-probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia (Basel). 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
6. Bilal MY, Klutts JS. Molecular Epidemiological investigations of localized SARS-CoV-2 outbreaks-utility of public algorithms. Epidemiologia (Basel). 2022;3(3):402-411. doi:10.3390/epidemiologia3030031
7. Veterans Health Administration, Office of Research & Development. VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). Updated November 23, 2022. Accessed August 28, 2023. https://www.research.va.gov/programs/shield/about.cfm
8. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
9. Epstein L, Shive C, Garcia AP, et al. VA SHIELD: a biorepository for our veterans and the nation. Fed Pract. 2023;40(suppl 5):S48-S51. doi:10.12788/fp.0424
10. Patel H, Varona S, Monzón S, et al. Version 2.5. nf-core/viralrecon: nf-core/viralrecon v2.5 - Manganese Monkey (2.5). Zenodo. July 13, 2022. doi:10.5281/zenodo.6827984
11. Choi H, Hwang M, Navarathna DH, Xu J, Lukey J, Jinadatha C. Performance of COVIDSeq and swift normalase amplicon SARS-CoV-2 panels for SARS-CoV-2 genome sequencing: practical guide and combining FASTQ strategy. J Clin Microbiol. 2022;60(4):e0002522. doi:10.1128/jcm.00025-22
12. Cuadros DF, Branscum AJ, Mukandavire Z, Miller FD, MacKinnon N. Dynamics of the COVID-19 epidemic in urban and rural areas in the United States. Ann Epidemiol. 2021;59:16-20. doi:10.1016/j.annepidem.2021.04.007
13. Anzalone AJ, Horswell R, Hendricks BM, et al. Higher hospitalization and mortality rates among SARS-CoV-2-infected persons in rural America. J Rural Health. 2023;39(1):39-54. doi:10.1111/jrh.12689
14. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881-895.e20. doi:10.1016/j.cell.2022.01.014
15. Pfaff ER, Girvin AT, Bennett TD, et al. Identifying who has long COVID in the USA: a machine learning approach using N3C data. Lancet Digit Health. 2022;4(7):e532-e541. doi:10.1016/S2589-7500(22)00048-6
16. Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714. doi:10.1038/s41591-022-01909-w
17. Munblit D, O’Hara ME, Akrami A, Perego E, Olliaro P, Needham DM. Long COVID: aiming for a consensus. Lancet Respir Med. 2022;10(7):632-634. doi:10.1016/S2213-2600(22)00135-7
18. Thaweethai T, Jolley SE, Karlson EW, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934-1946. doi:10.1001/jama.2023.8823
19. Sundermann AJ, Chen J, Kumar P, et al. Whole-genome sequencing surveillance and machine learning of the electronic health record for enhanced healthcare outbreak detection. Clin Infect Dis. 2022;75(3):476-482. doi:10.1093/cid/ciab946
The COVID-19 virus and its associated pandemic have highlighted the urgent need for a national infrastructure to rapidly identify and respond to emerging pathogens. The importance of understanding viral population dynamics through genetic sequencing has become apparent over time, particularly as the vaccine responses, clinical implications, and therapeutic effectiveness of treatments have varied substantially with COVID-19 variants.1,2
As the largest integrated health care system in the US, the US Department of Veterans Affairs (VA) is uniquely situated to help with pandemic detection and response. This article highlights 2 VA programs dedicated to COVID-19 sequencing at the forefront of pandemic response and research: VA Sequencing for Research Clinical and Epidemiology (SeqFORCE) and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) (Table).
VA Seq FORCE
VA SeqFORCE was established March 2021 to facilitate clinical surveillance of COVID-19 variants in the US veteran population and in VA employees. VA SeqFORCE consists of 9 Clinical Laboratory Improvement Amendment (CLIA)–certified laboratories in VA medical centers, including the VA Public Health Reference Laboratory in Palo Alto, California, and 8 Veterans Health Administration (VHA) clinical laboratories (Los Angeles, California; Boise, Idaho; Iowa City, Iowa; Bronx, New York; West Haven, Connecticut; Indianapolis, Indiana; Denver, Colorado; and Orlando, Florida).3 Specimen standards (eg, real-time polymerase chain reaction [RT-PCR] cycle threshold [Ct] ≤ 30, minimum volume, etc) and clinical criteria (eg, COVID-19–related deaths, COVID-19 vaccine escape, etc) for submitting samples to VA SeqFORCE laboratories were established, and logistics for sample sequencing was centralized, including providing centralized instructions for sample preparation and to which VA SeqFORCE laboratory samples should be sent.
These laboratories sequenced samples from patients and employees with COVID-19 to understand patterns of variant evolution, vaccine, antiviral and monoclonal antibody response, health care–associated outbreaks, and COVID-19 transmission. As clinically relevant findings, such as monoclonal antibody treatment failure, emerged with novel viral variants, VA SeqFORCE was well positioned to rapidly detect the emergent variants and inform better clinical care of patients with COVID-19. Other clinical indications identified for sequencing within VA SeqFORCE included outbreak investigation, re-infection with COVID-19 > 90 days but < 6 months after a prior infection, extended hospitalization of > 21 days, death due to COVID-19, infection with a history of recent nondomestic travel, rebound of symptoms after improvement on oral antiviral therapy, and epidemiologic surveillance.
VA SeqFORCE laboratories use a variety of sequencing platforms, although a federated system was developed that electronically linked all laboratories using a software system (PraediGene, Bitscopic) for sample management, COVID-19 variant analytics, and automated result reporting of clade and lineage into the Veterans Health Information Systems and Technology Architecture (VistA) Computerized Patient Record System. In addition, generated nucleic acid sequence alignment through FASTA consensus sequence files have been archived for secondary research analyses. By archiving the consensus sequences, retrospective studies within the VA have the added benefit of being able to clinically annotate investigations into COVID-19 variant patterns. As of August 2023, 43,003 samples containing COVID-19 have been sequenced, and FASTA file and metadata upload are ongoing to the Global Initiative on Sharing Avian Influenza Data, which houses > 15 million COVID-19 files from global submissions.
VA SeqFORCE’s clinical sequencing efforts have created opportunities for multicenter collaboration in variant surveillance. In work from December 2021, investigators from the James J. Peters VA Medical Center in Bronx, New York, collaborated with the VHA Pathology and Laboratory Medicine Services and Public Health national program offices in Washington, DC, to develop an RT-PCR assay to rapidly differentiate Omicron from Delta variants.4 Samples from VA hospitals across the nation were used in this study.
Lessons from VA SeqFORCE have also been cited as inspiration to address COVID-19 clinical problems, including outbreak investigations in hospital settings and beyond. Researchers at the Iowa City VA Health Care System, for example, proposed a novel probabilistic quantitative method for determining genetic-relatedness among COVID-19 viral strains in an outbreak setting.5 They extended the scope of work to develop COVID-19 outbreak screening tools combining publicly available algorithms with targeted sequencing data to identify outbreaks as they arise.6 We expect VA SeqFORCE, in conjunction with its complement VA SeqCURE, will continue to further pandemic surveillance and response.
VA Seq CURE
As the research-focused complement to VA SeqFORCE, VA SeqCURE is dedicated to a broader study of the COVID-19 genome through sequencing. Established January 2021, the VA SeqCURE network consists of 6 research laboratories in Boise, Idaho; Bronx, New York; Cleveland, Ohio; Durham, North Carolina; Iowa City, Iowa; and Temple, Texas.
Samples are collected as a subset of the broader VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD) biorepository sweep protocol for discarded blood and nasal swab specimens of VHA patients hospitalized with COVID-19, as described by Epstein and colleagues.7-9 While VA SeqFORCE sequences samples positive for COVID-19 by RT-PCR with a Ct value of ≤ 30 for diagnostic purposes, VA SeqCURE laboratories sequence more broadly for nondiagnostic purposes, including samples with a Ct value > 30. The 6 VA SeqCURE laboratories generate sequencing data using various platforms, amplification kits, and formats. To ensure maximum quality and metadata on the sequences generated across the different laboratories, a sequence intake pipeline has been developed, adapting the ViralRecon bioinformatics platform.10 This harmonized analysis pipeline accommodates different file formats and performs quality control, alignment, variant calling, lineage assignment, clade assignment, and annotation. As of August 2023, VA SeqCURE has identified viral sequences from 24,107 unique specimens. Annotated COVID-19 sequences with the appropriate metadata will be available to VA researchers through VA SHIELD.
Research projects include descriptive epidemiology of COVID-19 variants in individuals who receive VHA care, COVID-19 vaccine and therapy effectiveness, and the unique distribution of variants and vaccine effectiveness in rural settings.3 True to its core mission, members of the VA SeqCURE consortium have contributed to the COVID-19 viral sequencing literature over the past 2 years. Researchers also are accessing VA SeqCURE to study COVID-19 persistence and rebound among individuals with mild disease taking nirmatrelvir/ritonavir compared with other COVID-19 therapeutics and untreated controls. Finally, COVID-19 samples and their sequences are stored in the VA SHIELD biorepository, which leverages these samples and data to advance scientific understanding of COVID-19 and future emerging infectious diseases.7-9
Important work from investigators at the Central Texas Veterans Health Care System confronted the issue of whole genome sequencing data from COVID-19 samples with low viral loads, a common issue with COVID-19 sequencing. They found that yields of 2 sequencing protocols, which generated high-sequence coverage, were enhanced further by combining the results of both methods.11 This project, which has potentially broad applications for sequencing in research and clinical settings, is an example of VA SeqCURE’s efforts to address the COVID-19 pandemic. The VA SeqCURE program has substantial potential as a large viral sequencing repository with broad geographic and demographic representation, such that future large-scale sequencing analyses may be generated from preexisting nested cohorts within the repository.
NEXT STEPS
Promising new directions of clinical and laboratory-based research are planned for VA SeqFORCE and VA SeqCURE. While the impact of COVID-19 and other viruses with epidemic potential is perhaps most feared in urban settings, evidence suggests that the distribution of COVID-19 in rural settings is unique and associated with worse outcomes.12,13 Given the wide catchment areas of VA hospitals that encompass both rural and urban settings, the VA’s ongoing COVID-19 sequencing programs and repositories are uniquely positioned to understand viral dynamics in areas of differing population density.
While rates of infection, hospitalization, and death resulting from COVID-19 have substantially dropped, the long-term impact of the pandemic is just beginning to be recognized in conditions such as long COVID or postacute COVID-19 syndrome. Long COVID has already proven to be biologically multifaceted, difficult to diagnose, and unpredictable in identifying the most at-risk patients.14-16 Much remains to be determined in our understanding of long COVID, including a unified definition that can effectively be used in clinical settings to diagnose and treat patients. However, research indicates that comorbidities common in veterans, such as diabetes and cardiovascular disease, are associated with worse long-term outcomes.17,18 Collaborations between VA scientists, clinicians, and national cooperative programs (such as a network of VHA long COVID clinics) create an unmatched opportunity for VA SeqFORCE and VA SeqCURE programs to provide insight into a disease likely to become a chronic disease outcome of the pandemic.
With VA SeqFORCE and VA SeqCURE programs, the VA now has infrastructure ready to respond to new infectious diseases. During the mpox outbreak of 2022, the VA Public Health Reference Laboratory received > 80% of all VA mpox samples for orthopox screening and mpox confirmatory testing. A subset of these samples underwent whole genome sequencing with the identification of 10 unique lineages across VA, and > 200 positive and 400 negative samples have been aliquoted and submitted to VA SHIELD for research. Furthermore, the VA SeqFORCE and VA SeqCURE sequencing processes might be adapted to identify outbreaks of multidrug-resistant organisms among VA patients trialed at other institutions.19 We are hopeful that VA SeqFORCE and VA SeqCURE will become invaluable components of health care delivery and infection prevention at the hospital level and beyond.
Finally, the robust data infrastructure and associated repositories of VA SeqFORCE and VA SeqCURE may be leveraged to study noninfectious diseases. Research groups are starting to apply these programs to cancer sequencing. We anticipate that these efforts may have a substantial impact on our understanding of cancer epidemiology and region-specific risk factors for malignancy, given the size and breadth of VA SeqFORCE and VA SeqCURE. Common oncogenic mutations identified through these programs could be targets for precision oncology therapeutics. Similarly, we envision applications of the VA SeqFORCE and VA SeqCURE data infrastructures and repositories toward other precision medicine fields, including pharmacogenomics and nutrition, to tailor interventions to meet the specific individual needs of veterans.
CONCLUSIONS
The productivity of VA SeqFORCE and VA SeqCURE programs over the past 2 years continues to increase in response to the COVID-19 pandemic. We anticipate that they will be vital components in our nation’s responses to infectious threats and beyond.
The COVID-19 virus and its associated pandemic have highlighted the urgent need for a national infrastructure to rapidly identify and respond to emerging pathogens. The importance of understanding viral population dynamics through genetic sequencing has become apparent over time, particularly as the vaccine responses, clinical implications, and therapeutic effectiveness of treatments have varied substantially with COVID-19 variants.1,2
As the largest integrated health care system in the US, the US Department of Veterans Affairs (VA) is uniquely situated to help with pandemic detection and response. This article highlights 2 VA programs dedicated to COVID-19 sequencing at the forefront of pandemic response and research: VA Sequencing for Research Clinical and Epidemiology (SeqFORCE) and VA Sequencing Collaborations United for Research and Epidemiology (SeqCURE) (Table).
VA Seq FORCE
VA SeqFORCE was established March 2021 to facilitate clinical surveillance of COVID-19 variants in the US veteran population and in VA employees. VA SeqFORCE consists of 9 Clinical Laboratory Improvement Amendment (CLIA)–certified laboratories in VA medical centers, including the VA Public Health Reference Laboratory in Palo Alto, California, and 8 Veterans Health Administration (VHA) clinical laboratories (Los Angeles, California; Boise, Idaho; Iowa City, Iowa; Bronx, New York; West Haven, Connecticut; Indianapolis, Indiana; Denver, Colorado; and Orlando, Florida).3 Specimen standards (eg, real-time polymerase chain reaction [RT-PCR] cycle threshold [Ct] ≤ 30, minimum volume, etc) and clinical criteria (eg, COVID-19–related deaths, COVID-19 vaccine escape, etc) for submitting samples to VA SeqFORCE laboratories were established, and logistics for sample sequencing was centralized, including providing centralized instructions for sample preparation and to which VA SeqFORCE laboratory samples should be sent.
These laboratories sequenced samples from patients and employees with COVID-19 to understand patterns of variant evolution, vaccine, antiviral and monoclonal antibody response, health care–associated outbreaks, and COVID-19 transmission. As clinically relevant findings, such as monoclonal antibody treatment failure, emerged with novel viral variants, VA SeqFORCE was well positioned to rapidly detect the emergent variants and inform better clinical care of patients with COVID-19. Other clinical indications identified for sequencing within VA SeqFORCE included outbreak investigation, re-infection with COVID-19 > 90 days but < 6 months after a prior infection, extended hospitalization of > 21 days, death due to COVID-19, infection with a history of recent nondomestic travel, rebound of symptoms after improvement on oral antiviral therapy, and epidemiologic surveillance.
VA SeqFORCE laboratories use a variety of sequencing platforms, although a federated system was developed that electronically linked all laboratories using a software system (PraediGene, Bitscopic) for sample management, COVID-19 variant analytics, and automated result reporting of clade and lineage into the Veterans Health Information Systems and Technology Architecture (VistA) Computerized Patient Record System. In addition, generated nucleic acid sequence alignment through FASTA consensus sequence files have been archived for secondary research analyses. By archiving the consensus sequences, retrospective studies within the VA have the added benefit of being able to clinically annotate investigations into COVID-19 variant patterns. As of August 2023, 43,003 samples containing COVID-19 have been sequenced, and FASTA file and metadata upload are ongoing to the Global Initiative on Sharing Avian Influenza Data, which houses > 15 million COVID-19 files from global submissions.
VA SeqFORCE’s clinical sequencing efforts have created opportunities for multicenter collaboration in variant surveillance. In work from December 2021, investigators from the James J. Peters VA Medical Center in Bronx, New York, collaborated with the VHA Pathology and Laboratory Medicine Services and Public Health national program offices in Washington, DC, to develop an RT-PCR assay to rapidly differentiate Omicron from Delta variants.4 Samples from VA hospitals across the nation were used in this study.
Lessons from VA SeqFORCE have also been cited as inspiration to address COVID-19 clinical problems, including outbreak investigations in hospital settings and beyond. Researchers at the Iowa City VA Health Care System, for example, proposed a novel probabilistic quantitative method for determining genetic-relatedness among COVID-19 viral strains in an outbreak setting.5 They extended the scope of work to develop COVID-19 outbreak screening tools combining publicly available algorithms with targeted sequencing data to identify outbreaks as they arise.6 We expect VA SeqFORCE, in conjunction with its complement VA SeqCURE, will continue to further pandemic surveillance and response.
VA Seq CURE
As the research-focused complement to VA SeqFORCE, VA SeqCURE is dedicated to a broader study of the COVID-19 genome through sequencing. Established January 2021, the VA SeqCURE network consists of 6 research laboratories in Boise, Idaho; Bronx, New York; Cleveland, Ohio; Durham, North Carolina; Iowa City, Iowa; and Temple, Texas.
Samples are collected as a subset of the broader VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD) biorepository sweep protocol for discarded blood and nasal swab specimens of VHA patients hospitalized with COVID-19, as described by Epstein and colleagues.7-9 While VA SeqFORCE sequences samples positive for COVID-19 by RT-PCR with a Ct value of ≤ 30 for diagnostic purposes, VA SeqCURE laboratories sequence more broadly for nondiagnostic purposes, including samples with a Ct value > 30. The 6 VA SeqCURE laboratories generate sequencing data using various platforms, amplification kits, and formats. To ensure maximum quality and metadata on the sequences generated across the different laboratories, a sequence intake pipeline has been developed, adapting the ViralRecon bioinformatics platform.10 This harmonized analysis pipeline accommodates different file formats and performs quality control, alignment, variant calling, lineage assignment, clade assignment, and annotation. As of August 2023, VA SeqCURE has identified viral sequences from 24,107 unique specimens. Annotated COVID-19 sequences with the appropriate metadata will be available to VA researchers through VA SHIELD.
Research projects include descriptive epidemiology of COVID-19 variants in individuals who receive VHA care, COVID-19 vaccine and therapy effectiveness, and the unique distribution of variants and vaccine effectiveness in rural settings.3 True to its core mission, members of the VA SeqCURE consortium have contributed to the COVID-19 viral sequencing literature over the past 2 years. Researchers also are accessing VA SeqCURE to study COVID-19 persistence and rebound among individuals with mild disease taking nirmatrelvir/ritonavir compared with other COVID-19 therapeutics and untreated controls. Finally, COVID-19 samples and their sequences are stored in the VA SHIELD biorepository, which leverages these samples and data to advance scientific understanding of COVID-19 and future emerging infectious diseases.7-9
Important work from investigators at the Central Texas Veterans Health Care System confronted the issue of whole genome sequencing data from COVID-19 samples with low viral loads, a common issue with COVID-19 sequencing. They found that yields of 2 sequencing protocols, which generated high-sequence coverage, were enhanced further by combining the results of both methods.11 This project, which has potentially broad applications for sequencing in research and clinical settings, is an example of VA SeqCURE’s efforts to address the COVID-19 pandemic. The VA SeqCURE program has substantial potential as a large viral sequencing repository with broad geographic and demographic representation, such that future large-scale sequencing analyses may be generated from preexisting nested cohorts within the repository.
NEXT STEPS
Promising new directions of clinical and laboratory-based research are planned for VA SeqFORCE and VA SeqCURE. While the impact of COVID-19 and other viruses with epidemic potential is perhaps most feared in urban settings, evidence suggests that the distribution of COVID-19 in rural settings is unique and associated with worse outcomes.12,13 Given the wide catchment areas of VA hospitals that encompass both rural and urban settings, the VA’s ongoing COVID-19 sequencing programs and repositories are uniquely positioned to understand viral dynamics in areas of differing population density.
While rates of infection, hospitalization, and death resulting from COVID-19 have substantially dropped, the long-term impact of the pandemic is just beginning to be recognized in conditions such as long COVID or postacute COVID-19 syndrome. Long COVID has already proven to be biologically multifaceted, difficult to diagnose, and unpredictable in identifying the most at-risk patients.14-16 Much remains to be determined in our understanding of long COVID, including a unified definition that can effectively be used in clinical settings to diagnose and treat patients. However, research indicates that comorbidities common in veterans, such as diabetes and cardiovascular disease, are associated with worse long-term outcomes.17,18 Collaborations between VA scientists, clinicians, and national cooperative programs (such as a network of VHA long COVID clinics) create an unmatched opportunity for VA SeqFORCE and VA SeqCURE programs to provide insight into a disease likely to become a chronic disease outcome of the pandemic.
With VA SeqFORCE and VA SeqCURE programs, the VA now has infrastructure ready to respond to new infectious diseases. During the mpox outbreak of 2022, the VA Public Health Reference Laboratory received > 80% of all VA mpox samples for orthopox screening and mpox confirmatory testing. A subset of these samples underwent whole genome sequencing with the identification of 10 unique lineages across VA, and > 200 positive and 400 negative samples have been aliquoted and submitted to VA SHIELD for research. Furthermore, the VA SeqFORCE and VA SeqCURE sequencing processes might be adapted to identify outbreaks of multidrug-resistant organisms among VA patients trialed at other institutions.19 We are hopeful that VA SeqFORCE and VA SeqCURE will become invaluable components of health care delivery and infection prevention at the hospital level and beyond.
Finally, the robust data infrastructure and associated repositories of VA SeqFORCE and VA SeqCURE may be leveraged to study noninfectious diseases. Research groups are starting to apply these programs to cancer sequencing. We anticipate that these efforts may have a substantial impact on our understanding of cancer epidemiology and region-specific risk factors for malignancy, given the size and breadth of VA SeqFORCE and VA SeqCURE. Common oncogenic mutations identified through these programs could be targets for precision oncology therapeutics. Similarly, we envision applications of the VA SeqFORCE and VA SeqCURE data infrastructures and repositories toward other precision medicine fields, including pharmacogenomics and nutrition, to tailor interventions to meet the specific individual needs of veterans.
CONCLUSIONS
The productivity of VA SeqFORCE and VA SeqCURE programs over the past 2 years continues to increase in response to the COVID-19 pandemic. We anticipate that they will be vital components in our nation’s responses to infectious threats and beyond.
1. Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146-152. Published 2022 Jan 28. doi:10.15585/mmwr.mm7104e4
2. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303-1312. doi:10.1016/S0140-6736(22)00462-7
3. Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report - annex C. December 5, 2022. Accessed August 28, 2023. https://www.va.gov/HEALTH/docs/VHA-COVID-19-Response-2022-Annex-C.pdf 4. Barasch NJ, Iqbal J, Coombs M, et al. Utilization of a SARS-CoV-2 variant assay for the rapid differentiation of Omicron and Delta. medRxiv. Preprint posted online December 27, 2021. doi:10.1101/2021.12.22.21268195
5. Bilal MY. Similarity Index-probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia (Basel). 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
6. Bilal MY, Klutts JS. Molecular Epidemiological investigations of localized SARS-CoV-2 outbreaks-utility of public algorithms. Epidemiologia (Basel). 2022;3(3):402-411. doi:10.3390/epidemiologia3030031
7. Veterans Health Administration, Office of Research & Development. VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). Updated November 23, 2022. Accessed August 28, 2023. https://www.research.va.gov/programs/shield/about.cfm
8. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
9. Epstein L, Shive C, Garcia AP, et al. VA SHIELD: a biorepository for our veterans and the nation. Fed Pract. 2023;40(suppl 5):S48-S51. doi:10.12788/fp.0424
10. Patel H, Varona S, Monzón S, et al. Version 2.5. nf-core/viralrecon: nf-core/viralrecon v2.5 - Manganese Monkey (2.5). Zenodo. July 13, 2022. doi:10.5281/zenodo.6827984
11. Choi H, Hwang M, Navarathna DH, Xu J, Lukey J, Jinadatha C. Performance of COVIDSeq and swift normalase amplicon SARS-CoV-2 panels for SARS-CoV-2 genome sequencing: practical guide and combining FASTQ strategy. J Clin Microbiol. 2022;60(4):e0002522. doi:10.1128/jcm.00025-22
12. Cuadros DF, Branscum AJ, Mukandavire Z, Miller FD, MacKinnon N. Dynamics of the COVID-19 epidemic in urban and rural areas in the United States. Ann Epidemiol. 2021;59:16-20. doi:10.1016/j.annepidem.2021.04.007
13. Anzalone AJ, Horswell R, Hendricks BM, et al. Higher hospitalization and mortality rates among SARS-CoV-2-infected persons in rural America. J Rural Health. 2023;39(1):39-54. doi:10.1111/jrh.12689
14. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881-895.e20. doi:10.1016/j.cell.2022.01.014
15. Pfaff ER, Girvin AT, Bennett TD, et al. Identifying who has long COVID in the USA: a machine learning approach using N3C data. Lancet Digit Health. 2022;4(7):e532-e541. doi:10.1016/S2589-7500(22)00048-6
16. Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714. doi:10.1038/s41591-022-01909-w
17. Munblit D, O’Hara ME, Akrami A, Perego E, Olliaro P, Needham DM. Long COVID: aiming for a consensus. Lancet Respir Med. 2022;10(7):632-634. doi:10.1016/S2213-2600(22)00135-7
18. Thaweethai T, Jolley SE, Karlson EW, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934-1946. doi:10.1001/jama.2023.8823
19. Sundermann AJ, Chen J, Kumar P, et al. Whole-genome sequencing surveillance and machine learning of the electronic health record for enhanced healthcare outbreak detection. Clin Infect Dis. 2022;75(3):476-482. doi:10.1093/cid/ciab946
1. Iuliano AD, Brunkard JM, Boehmer TK, et al. Trends in disease severity and health care utilization during the early Omicron variant period compared with previous SARS-CoV-2 high transmission periods - United States, December 2020-January 2022. MMWR Morb Mortal Wkly Rep. 2022;71(4):146-152. Published 2022 Jan 28. doi:10.15585/mmwr.mm7104e4
2. Nyberg T, Ferguson NM, Nash SG, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303-1312. doi:10.1016/S0140-6736(22)00462-7
3. Veterans Health Administration. Coronavirus Disease 2019 (COVID-19) response report - annex C. December 5, 2022. Accessed August 28, 2023. https://www.va.gov/HEALTH/docs/VHA-COVID-19-Response-2022-Annex-C.pdf 4. Barasch NJ, Iqbal J, Coombs M, et al. Utilization of a SARS-CoV-2 variant assay for the rapid differentiation of Omicron and Delta. medRxiv. Preprint posted online December 27, 2021. doi:10.1101/2021.12.22.21268195
5. Bilal MY. Similarity Index-probabilistic confidence estimation of SARS-CoV-2 strain relatedness in localized outbreaks. Epidemiologia (Basel). 2022;3(2):238-249. doi:10.3390/epidemiologia3020019
6. Bilal MY, Klutts JS. Molecular Epidemiological investigations of localized SARS-CoV-2 outbreaks-utility of public algorithms. Epidemiologia (Basel). 2022;3(3):402-411. doi:10.3390/epidemiologia3030031
7. Veterans Health Administration, Office of Research & Development. VA Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD). Updated November 23, 2022. Accessed August 28, 2023. https://www.research.va.gov/programs/shield/about.cfm
8. Harley JB, Pyarajan S, Partan ES, et al. The US Department of Veterans Affairs Science and Health Initiative to Combat Infectious and Emerging Life-Threatening Diseases (VA SHIELD): a biorepository addressing national health threats. Open Forum Infect Dis. 2022;9(12):ofac641. doi:10.1093/ofid/ofac641
9. Epstein L, Shive C, Garcia AP, et al. VA SHIELD: a biorepository for our veterans and the nation. Fed Pract. 2023;40(suppl 5):S48-S51. doi:10.12788/fp.0424
10. Patel H, Varona S, Monzón S, et al. Version 2.5. nf-core/viralrecon: nf-core/viralrecon v2.5 - Manganese Monkey (2.5). Zenodo. July 13, 2022. doi:10.5281/zenodo.6827984
11. Choi H, Hwang M, Navarathna DH, Xu J, Lukey J, Jinadatha C. Performance of COVIDSeq and swift normalase amplicon SARS-CoV-2 panels for SARS-CoV-2 genome sequencing: practical guide and combining FASTQ strategy. J Clin Microbiol. 2022;60(4):e0002522. doi:10.1128/jcm.00025-22
12. Cuadros DF, Branscum AJ, Mukandavire Z, Miller FD, MacKinnon N. Dynamics of the COVID-19 epidemic in urban and rural areas in the United States. Ann Epidemiol. 2021;59:16-20. doi:10.1016/j.annepidem.2021.04.007
13. Anzalone AJ, Horswell R, Hendricks BM, et al. Higher hospitalization and mortality rates among SARS-CoV-2-infected persons in rural America. J Rural Health. 2023;39(1):39-54. doi:10.1111/jrh.12689
14. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185(5):881-895.e20. doi:10.1016/j.cell.2022.01.014
15. Pfaff ER, Girvin AT, Bennett TD, et al. Identifying who has long COVID in the USA: a machine learning approach using N3C data. Lancet Digit Health. 2022;4(7):e532-e541. doi:10.1016/S2589-7500(22)00048-6
16. Subramanian A, Nirantharakumar K, Hughes S, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. 2022;28(8):1706-1714. doi:10.1038/s41591-022-01909-w
17. Munblit D, O’Hara ME, Akrami A, Perego E, Olliaro P, Needham DM. Long COVID: aiming for a consensus. Lancet Respir Med. 2022;10(7):632-634. doi:10.1016/S2213-2600(22)00135-7
18. Thaweethai T, Jolley SE, Karlson EW, et al. Development of a definition of postacute sequelae of SARS-CoV-2 infection. JAMA. 2023;329(22):1934-1946. doi:10.1001/jama.2023.8823
19. Sundermann AJ, Chen J, Kumar P, et al. Whole-genome sequencing surveillance and machine learning of the electronic health record for enhanced healthcare outbreak detection. Clin Infect Dis. 2022;75(3):476-482. doi:10.1093/cid/ciab946