User login
Finding meaning in ‘Lean’?
Using systems improvement strategies to support the Quadruple Aim
General background on well-being and burnout
With burnout increasingly recognized as a shared responsibility that requires addressing organizational drivers while supporting individuals to be well,1-4 practical strategies and examples of successful implementation of systems interventions to address burnout will be helpful for service directors to support their staff. The Charter on Physician Well-being, recently developed through collaborative input from multiple organizations, defines guiding principles and key commitments at the societal, organizational, interpersonal, and individual levels and may be a useful framework for organizations that are developing well-being initiatives.5
The charter advocates including physician well-being as a quality improvement metric for health systems, aligned with the concept of the Quadruple Aim of optimizing patient care by enhancing provider experience, promoting high-value care, and improving population health.6 Identifying areas of alignment between the charter’s recommendations and systems improvement strategies that seek to optimize efficiency and reduce waste, such as Lean Management, may help physician leaders to contextualize well-being initiatives more easily within ongoing systems improvement efforts. In this perspective, we provide one division’s experience using the Charter to assess successes and identify additional areas of improvement for well-being initiatives developed using Lean Management methodology.
Past and current state of affairs
In 2011, the division of hospital medicine at Zuckerberg San Francisco General Hospital was established and has seen continual expansion in terms of direct patient care, medical education, and hospital leadership.
In 2015, the division of hospital medicine experienced leadership transitions, faculty attrition, and insufficient recruitment resulting in staffing shortages, service line closure, schedule instability, and ultimately, low morale. A baseline survey conducted using the 2-Item Maslach Burnout Inventory. This survey, which uses one item in the domain of emotional exhaustion and one item in the domain of depersonalization, has shown good correlation with the full Maslach Burnout Inventory.7 At baseline, approximately one-third of the division’s physicians experienced burnout.
In response, a subsequent retreat focused on the three greatest areas of concern identified by the survey: scheduling, faculty development, and well-being.
Like many health systems, the hospital has adopted Lean as its preferred systems-improvement framework. The retreat was structured around the principles of Lean philosophy, and was designed to emulate that of a consolidated Kaizen workshop.
“Kaizen” in Japanese means “change for the better.” A typical Kaizen workshop revolves around rapid problem-solving over the course of 3-5 days, in which a team of people come together to identify and implement significant improvements for a selected process. To this end, the retreat was divided into subgroups for each area of concern. In turn, each subgroup mapped out existing workflows (“value stream”), identified areas of waste and non–value added time, and generated ideas of what an idealized process would be. Next, a root-cause analysis was performed and subsequent interventions (“countermeasures”) developed to address each problem. At the conclusion of the retreat, each subgroup shared a summary of their findings with the larger group.
Moving forward, this information served as a guiding framework for service and division leadership to run small tests of change. We enacted a series of countermeasures over the course of several years, and multiple cycles of improvement work addressed the three areas of concern. We developed an A3 report (a Lean project management tool that incorporates the plan-do-study-act cycle, organizes strategic efforts, and tracks progress on a single page) to summarize and present these initiatives to the Performance Improvement and Patient Safety Committee of the hospital executive leadership team. This structure illustrated alignment with the hospital’s core values (“true north”) of “developing people” and “care experience.”
In 2018, interval surveys demonstrated a gradual reduction of burnout to approximately one-fifth of division physicians as measured by the 2-item Maslach Burnout Inventory.
Initiatives in faculty well-being
The Charter of Physician Well-being outlines a framework to promote well-being among doctors by maximizing a sense of fulfillment and minimizing the harms of burnout. It shares this responsibility among societal, organizational, and interpersonal and individual commitments.5
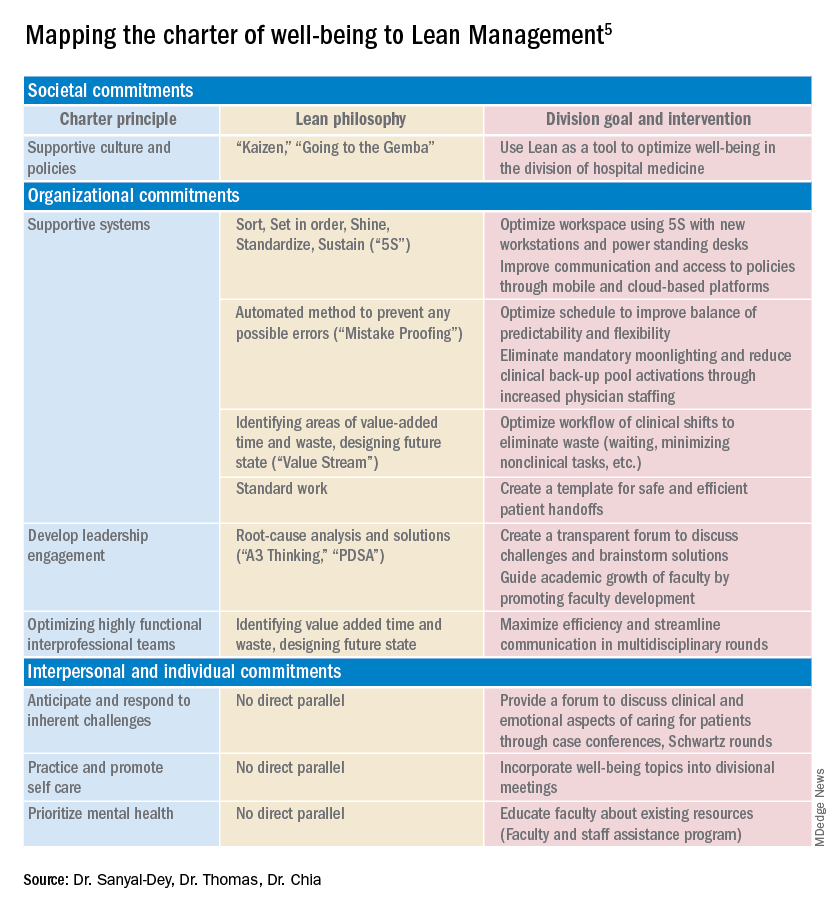
As illustrated above, we used principles of Lean Management to prospectively create initiatives to improve well-being in our division. Lean in health care is designed to optimize primarily the patient experience; its implementation has subsequently demonstrated mixed provider and staff experiences,8,9 and many providers are skeptical of Lean’s potential to improve their own well-being. If, however, Lean is aligned with best practice frameworks for well-being such as those outline in the charter, it may also help to meet the Quadruple Aim of optimizing both provider well-being and patient experience. To further test this hypothesis, we retrospectively categorized our Lean-based interventions into the commitments described by the charter to identify areas of alignment and gaps that were not initially addressed using Lean Management (Table).
Organizational commitments5Supportive systems
We optimized scheduling and enhanced physician staffing by budgeting for a physician staffing buffer each academic year in order to minimize mandatory moonlighting and jeopardy pool activations that result from operating on a thin staffing margin when expected personal leave and reductions in clinical effort occur. Furthermore, we revised scheduling principles to balance patient continuity and individual time off requests while setting limits on the maximum duration of clinical stretches and instituting mandatory minimum time off between them.
Leadership engagement
We initiated monthly operations meetings as a forum to discuss challenges, brainstorm solutions, and message new initiatives with group input. For example, as a result of these meetings, we designed and implemented an additional service line to address the high census, revised the distribution of new patient admissions to level-load clinical shifts, and established a maximum number of weekends worked per month and year. This approach aligns with recommendations to use participatory leadership strategies to enhance physician well-being.10 Engaging both executive level and service level management to focus on burnout and other related well-being metrics is necessary for sustaining such work.
Interprofessional teamwork
We revised multidisciplinary rounds with social work, utilization management, and physical therapy to maximize efficiency and streamline communication by developing standard approaches for each patient presentation.
Interpersonal and individual commitments5Address emotional challenges of physician work
Although these commitments did not have a direct corollary with Lean philosophy, some of these needs were identified by our physician group at our annual retreats. As a result, we initiated a monthly faculty-led noon conference series focused on the clinical challenges of caring for vulnerable populations, a particular source of distress in our practice setting, and revised the division schedule to encourage attendance at the hospital’s Schwartz rounds.
Mental health and self-care
We organized focus groups and faculty development sessions on provider well-being and burnout and dealing with challenging patients and invited the Faculty and Staff Assistance Program, our institution’s mental health service provider, to our weekly division meeting.
Future directions
After using Lean Management as an approach to prospectively improve physician well-being, we were able to use the Charter on Physician Well-being retrospectively as a “checklist” to identify additional gaps for targeted intervention to ensure all commitments are sufficiently addressed.
Overall, we found that, not surprisingly, Lean Management aligned best with the organizational commitments in the charter. Reviewing the organizational commitments, we found our biggest remaining challenges are in building supportive systems, namely ensuring sustainable workloads, offloading and delegating nonphysician tasks, and minimizing the burden of documentation and administration.
Reviewing the societal commitments helped us to identify opportunities for future directions that we may not have otherwise considered. As a safety-net institution, we benefit from a strong sense of mission and shared values within our hospital and division. However, we recognize the need to continue to be vigilant to ensure that our physicians perceive that their own values are aligned with the division’s stated mission. Devoting a Kaizen-style retreat to well-being likely helped, and allocating divisional resources to a well-being committee indirectly helped, to foster a culture of well-being; however, we could more deliberately identify local policies that may benefit from advocacy or revision. Although our faculty identified interventions to improve interpersonal and individual drivers of well-being, these charter commitments did not have direct parallels in Lean philosophy, and organizations may need to deliberately seek to address these commitments outside of a Lean approach. Specifically, by reviewing the charter, we identified opportunities to provide additional resources for peer support and protected time for mental health care and self-care.
Conclusion
Lean Management can be an effective strategy to address many of the organizational commitments outlined in the Charter on Physician Well-being. This approach may be particularly effective for solving local challenges with systems and workflows. Those who use Lean as a primary method to approach systems improvement in support of the Quadruple Aim may need to use additional strategies to address societal and interpersonal and individual commitments outlined in the charter.
Dr. Sanyal-Dey is visiting associate clinical professor of medicine at Zuckerberg San Francisco General Hospital and director of client services, LeanTaaS. Dr. Thomas is associate clinical professor of medicine at Zuckerberg San Francisco General Hospital. Dr. Chia is associate professor of clinical medicine at Zuckerberg San Francisco General Hospital.
References
1. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016;388(10057):2272-81.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-46.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Shanafelt T et al. Building a program on well-being: Key design considerations to meet the unique needs of each organization. Acad Med. 2019 Feb;94(2):156-161.
5. Thomas LR et al. Charter on physician well-being. JAMA. 2018;319(15):1541-42.
6. Bodenheimer T, Sinsky C. From triple to quadruple aim: Care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-6.
7. West CP et al. Concurrent Validity of Single-Item Measures of Emotional Exhaustion and Depersonalization in Burnout Assessment. J Gen Intern Med. 2012;27(11):1445-52.
8. Hung DY et al. Experiences of primary care physicians and staff following lean workflow redesign. BMC Health Serv Res. 2018 Apr 10;18(1):274.
9. Zibrowski E et al. Easier and faster is not always better: Grounded theory of the impact of large-scale system transformation on the clinical work of emergency medicine nurses and physicians. JMIR Hum Factors. 2018. doi: 10.2196/11013.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90(4):432-40.
Using systems improvement strategies to support the Quadruple Aim
Using systems improvement strategies to support the Quadruple Aim
General background on well-being and burnout
With burnout increasingly recognized as a shared responsibility that requires addressing organizational drivers while supporting individuals to be well,1-4 practical strategies and examples of successful implementation of systems interventions to address burnout will be helpful for service directors to support their staff. The Charter on Physician Well-being, recently developed through collaborative input from multiple organizations, defines guiding principles and key commitments at the societal, organizational, interpersonal, and individual levels and may be a useful framework for organizations that are developing well-being initiatives.5
The charter advocates including physician well-being as a quality improvement metric for health systems, aligned with the concept of the Quadruple Aim of optimizing patient care by enhancing provider experience, promoting high-value care, and improving population health.6 Identifying areas of alignment between the charter’s recommendations and systems improvement strategies that seek to optimize efficiency and reduce waste, such as Lean Management, may help physician leaders to contextualize well-being initiatives more easily within ongoing systems improvement efforts. In this perspective, we provide one division’s experience using the Charter to assess successes and identify additional areas of improvement for well-being initiatives developed using Lean Management methodology.
Past and current state of affairs
In 2011, the division of hospital medicine at Zuckerberg San Francisco General Hospital was established and has seen continual expansion in terms of direct patient care, medical education, and hospital leadership.
In 2015, the division of hospital medicine experienced leadership transitions, faculty attrition, and insufficient recruitment resulting in staffing shortages, service line closure, schedule instability, and ultimately, low morale. A baseline survey conducted using the 2-Item Maslach Burnout Inventory. This survey, which uses one item in the domain of emotional exhaustion and one item in the domain of depersonalization, has shown good correlation with the full Maslach Burnout Inventory.7 At baseline, approximately one-third of the division’s physicians experienced burnout.
In response, a subsequent retreat focused on the three greatest areas of concern identified by the survey: scheduling, faculty development, and well-being.
Like many health systems, the hospital has adopted Lean as its preferred systems-improvement framework. The retreat was structured around the principles of Lean philosophy, and was designed to emulate that of a consolidated Kaizen workshop.
“Kaizen” in Japanese means “change for the better.” A typical Kaizen workshop revolves around rapid problem-solving over the course of 3-5 days, in which a team of people come together to identify and implement significant improvements for a selected process. To this end, the retreat was divided into subgroups for each area of concern. In turn, each subgroup mapped out existing workflows (“value stream”), identified areas of waste and non–value added time, and generated ideas of what an idealized process would be. Next, a root-cause analysis was performed and subsequent interventions (“countermeasures”) developed to address each problem. At the conclusion of the retreat, each subgroup shared a summary of their findings with the larger group.
Moving forward, this information served as a guiding framework for service and division leadership to run small tests of change. We enacted a series of countermeasures over the course of several years, and multiple cycles of improvement work addressed the three areas of concern. We developed an A3 report (a Lean project management tool that incorporates the plan-do-study-act cycle, organizes strategic efforts, and tracks progress on a single page) to summarize and present these initiatives to the Performance Improvement and Patient Safety Committee of the hospital executive leadership team. This structure illustrated alignment with the hospital’s core values (“true north”) of “developing people” and “care experience.”
In 2018, interval surveys demonstrated a gradual reduction of burnout to approximately one-fifth of division physicians as measured by the 2-item Maslach Burnout Inventory.
Initiatives in faculty well-being
The Charter of Physician Well-being outlines a framework to promote well-being among doctors by maximizing a sense of fulfillment and minimizing the harms of burnout. It shares this responsibility among societal, organizational, and interpersonal and individual commitments.5

As illustrated above, we used principles of Lean Management to prospectively create initiatives to improve well-being in our division. Lean in health care is designed to optimize primarily the patient experience; its implementation has subsequently demonstrated mixed provider and staff experiences,8,9 and many providers are skeptical of Lean’s potential to improve their own well-being. If, however, Lean is aligned with best practice frameworks for well-being such as those outline in the charter, it may also help to meet the Quadruple Aim of optimizing both provider well-being and patient experience. To further test this hypothesis, we retrospectively categorized our Lean-based interventions into the commitments described by the charter to identify areas of alignment and gaps that were not initially addressed using Lean Management (Table).
Organizational commitments5Supportive systems
We optimized scheduling and enhanced physician staffing by budgeting for a physician staffing buffer each academic year in order to minimize mandatory moonlighting and jeopardy pool activations that result from operating on a thin staffing margin when expected personal leave and reductions in clinical effort occur. Furthermore, we revised scheduling principles to balance patient continuity and individual time off requests while setting limits on the maximum duration of clinical stretches and instituting mandatory minimum time off between them.
Leadership engagement
We initiated monthly operations meetings as a forum to discuss challenges, brainstorm solutions, and message new initiatives with group input. For example, as a result of these meetings, we designed and implemented an additional service line to address the high census, revised the distribution of new patient admissions to level-load clinical shifts, and established a maximum number of weekends worked per month and year. This approach aligns with recommendations to use participatory leadership strategies to enhance physician well-being.10 Engaging both executive level and service level management to focus on burnout and other related well-being metrics is necessary for sustaining such work.
Interprofessional teamwork
We revised multidisciplinary rounds with social work, utilization management, and physical therapy to maximize efficiency and streamline communication by developing standard approaches for each patient presentation.
Interpersonal and individual commitments5Address emotional challenges of physician work
Although these commitments did not have a direct corollary with Lean philosophy, some of these needs were identified by our physician group at our annual retreats. As a result, we initiated a monthly faculty-led noon conference series focused on the clinical challenges of caring for vulnerable populations, a particular source of distress in our practice setting, and revised the division schedule to encourage attendance at the hospital’s Schwartz rounds.
Mental health and self-care
We organized focus groups and faculty development sessions on provider well-being and burnout and dealing with challenging patients and invited the Faculty and Staff Assistance Program, our institution’s mental health service provider, to our weekly division meeting.
Future directions
After using Lean Management as an approach to prospectively improve physician well-being, we were able to use the Charter on Physician Well-being retrospectively as a “checklist” to identify additional gaps for targeted intervention to ensure all commitments are sufficiently addressed.
Overall, we found that, not surprisingly, Lean Management aligned best with the organizational commitments in the charter. Reviewing the organizational commitments, we found our biggest remaining challenges are in building supportive systems, namely ensuring sustainable workloads, offloading and delegating nonphysician tasks, and minimizing the burden of documentation and administration.
Reviewing the societal commitments helped us to identify opportunities for future directions that we may not have otherwise considered. As a safety-net institution, we benefit from a strong sense of mission and shared values within our hospital and division. However, we recognize the need to continue to be vigilant to ensure that our physicians perceive that their own values are aligned with the division’s stated mission. Devoting a Kaizen-style retreat to well-being likely helped, and allocating divisional resources to a well-being committee indirectly helped, to foster a culture of well-being; however, we could more deliberately identify local policies that may benefit from advocacy or revision. Although our faculty identified interventions to improve interpersonal and individual drivers of well-being, these charter commitments did not have direct parallels in Lean philosophy, and organizations may need to deliberately seek to address these commitments outside of a Lean approach. Specifically, by reviewing the charter, we identified opportunities to provide additional resources for peer support and protected time for mental health care and self-care.
Conclusion
Lean Management can be an effective strategy to address many of the organizational commitments outlined in the Charter on Physician Well-being. This approach may be particularly effective for solving local challenges with systems and workflows. Those who use Lean as a primary method to approach systems improvement in support of the Quadruple Aim may need to use additional strategies to address societal and interpersonal and individual commitments outlined in the charter.
Dr. Sanyal-Dey is visiting associate clinical professor of medicine at Zuckerberg San Francisco General Hospital and director of client services, LeanTaaS. Dr. Thomas is associate clinical professor of medicine at Zuckerberg San Francisco General Hospital. Dr. Chia is associate professor of clinical medicine at Zuckerberg San Francisco General Hospital.
References
1. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016;388(10057):2272-81.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-46.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Shanafelt T et al. Building a program on well-being: Key design considerations to meet the unique needs of each organization. Acad Med. 2019 Feb;94(2):156-161.
5. Thomas LR et al. Charter on physician well-being. JAMA. 2018;319(15):1541-42.
6. Bodenheimer T, Sinsky C. From triple to quadruple aim: Care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-6.
7. West CP et al. Concurrent Validity of Single-Item Measures of Emotional Exhaustion and Depersonalization in Burnout Assessment. J Gen Intern Med. 2012;27(11):1445-52.
8. Hung DY et al. Experiences of primary care physicians and staff following lean workflow redesign. BMC Health Serv Res. 2018 Apr 10;18(1):274.
9. Zibrowski E et al. Easier and faster is not always better: Grounded theory of the impact of large-scale system transformation on the clinical work of emergency medicine nurses and physicians. JMIR Hum Factors. 2018. doi: 10.2196/11013.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90(4):432-40.
General background on well-being and burnout
With burnout increasingly recognized as a shared responsibility that requires addressing organizational drivers while supporting individuals to be well,1-4 practical strategies and examples of successful implementation of systems interventions to address burnout will be helpful for service directors to support their staff. The Charter on Physician Well-being, recently developed through collaborative input from multiple organizations, defines guiding principles and key commitments at the societal, organizational, interpersonal, and individual levels and may be a useful framework for organizations that are developing well-being initiatives.5
The charter advocates including physician well-being as a quality improvement metric for health systems, aligned with the concept of the Quadruple Aim of optimizing patient care by enhancing provider experience, promoting high-value care, and improving population health.6 Identifying areas of alignment between the charter’s recommendations and systems improvement strategies that seek to optimize efficiency and reduce waste, such as Lean Management, may help physician leaders to contextualize well-being initiatives more easily within ongoing systems improvement efforts. In this perspective, we provide one division’s experience using the Charter to assess successes and identify additional areas of improvement for well-being initiatives developed using Lean Management methodology.
Past and current state of affairs
In 2011, the division of hospital medicine at Zuckerberg San Francisco General Hospital was established and has seen continual expansion in terms of direct patient care, medical education, and hospital leadership.
In 2015, the division of hospital medicine experienced leadership transitions, faculty attrition, and insufficient recruitment resulting in staffing shortages, service line closure, schedule instability, and ultimately, low morale. A baseline survey conducted using the 2-Item Maslach Burnout Inventory. This survey, which uses one item in the domain of emotional exhaustion and one item in the domain of depersonalization, has shown good correlation with the full Maslach Burnout Inventory.7 At baseline, approximately one-third of the division’s physicians experienced burnout.
In response, a subsequent retreat focused on the three greatest areas of concern identified by the survey: scheduling, faculty development, and well-being.
Like many health systems, the hospital has adopted Lean as its preferred systems-improvement framework. The retreat was structured around the principles of Lean philosophy, and was designed to emulate that of a consolidated Kaizen workshop.
“Kaizen” in Japanese means “change for the better.” A typical Kaizen workshop revolves around rapid problem-solving over the course of 3-5 days, in which a team of people come together to identify and implement significant improvements for a selected process. To this end, the retreat was divided into subgroups for each area of concern. In turn, each subgroup mapped out existing workflows (“value stream”), identified areas of waste and non–value added time, and generated ideas of what an idealized process would be. Next, a root-cause analysis was performed and subsequent interventions (“countermeasures”) developed to address each problem. At the conclusion of the retreat, each subgroup shared a summary of their findings with the larger group.
Moving forward, this information served as a guiding framework for service and division leadership to run small tests of change. We enacted a series of countermeasures over the course of several years, and multiple cycles of improvement work addressed the three areas of concern. We developed an A3 report (a Lean project management tool that incorporates the plan-do-study-act cycle, organizes strategic efforts, and tracks progress on a single page) to summarize and present these initiatives to the Performance Improvement and Patient Safety Committee of the hospital executive leadership team. This structure illustrated alignment with the hospital’s core values (“true north”) of “developing people” and “care experience.”
In 2018, interval surveys demonstrated a gradual reduction of burnout to approximately one-fifth of division physicians as measured by the 2-item Maslach Burnout Inventory.
Initiatives in faculty well-being
The Charter of Physician Well-being outlines a framework to promote well-being among doctors by maximizing a sense of fulfillment and minimizing the harms of burnout. It shares this responsibility among societal, organizational, and interpersonal and individual commitments.5

As illustrated above, we used principles of Lean Management to prospectively create initiatives to improve well-being in our division. Lean in health care is designed to optimize primarily the patient experience; its implementation has subsequently demonstrated mixed provider and staff experiences,8,9 and many providers are skeptical of Lean’s potential to improve their own well-being. If, however, Lean is aligned with best practice frameworks for well-being such as those outline in the charter, it may also help to meet the Quadruple Aim of optimizing both provider well-being and patient experience. To further test this hypothesis, we retrospectively categorized our Lean-based interventions into the commitments described by the charter to identify areas of alignment and gaps that were not initially addressed using Lean Management (Table).
Organizational commitments5Supportive systems
We optimized scheduling and enhanced physician staffing by budgeting for a physician staffing buffer each academic year in order to minimize mandatory moonlighting and jeopardy pool activations that result from operating on a thin staffing margin when expected personal leave and reductions in clinical effort occur. Furthermore, we revised scheduling principles to balance patient continuity and individual time off requests while setting limits on the maximum duration of clinical stretches and instituting mandatory minimum time off between them.
Leadership engagement
We initiated monthly operations meetings as a forum to discuss challenges, brainstorm solutions, and message new initiatives with group input. For example, as a result of these meetings, we designed and implemented an additional service line to address the high census, revised the distribution of new patient admissions to level-load clinical shifts, and established a maximum number of weekends worked per month and year. This approach aligns with recommendations to use participatory leadership strategies to enhance physician well-being.10 Engaging both executive level and service level management to focus on burnout and other related well-being metrics is necessary for sustaining such work.
Interprofessional teamwork
We revised multidisciplinary rounds with social work, utilization management, and physical therapy to maximize efficiency and streamline communication by developing standard approaches for each patient presentation.
Interpersonal and individual commitments5Address emotional challenges of physician work
Although these commitments did not have a direct corollary with Lean philosophy, some of these needs were identified by our physician group at our annual retreats. As a result, we initiated a monthly faculty-led noon conference series focused on the clinical challenges of caring for vulnerable populations, a particular source of distress in our practice setting, and revised the division schedule to encourage attendance at the hospital’s Schwartz rounds.
Mental health and self-care
We organized focus groups and faculty development sessions on provider well-being and burnout and dealing with challenging patients and invited the Faculty and Staff Assistance Program, our institution’s mental health service provider, to our weekly division meeting.
Future directions
After using Lean Management as an approach to prospectively improve physician well-being, we were able to use the Charter on Physician Well-being retrospectively as a “checklist” to identify additional gaps for targeted intervention to ensure all commitments are sufficiently addressed.
Overall, we found that, not surprisingly, Lean Management aligned best with the organizational commitments in the charter. Reviewing the organizational commitments, we found our biggest remaining challenges are in building supportive systems, namely ensuring sustainable workloads, offloading and delegating nonphysician tasks, and minimizing the burden of documentation and administration.
Reviewing the societal commitments helped us to identify opportunities for future directions that we may not have otherwise considered. As a safety-net institution, we benefit from a strong sense of mission and shared values within our hospital and division. However, we recognize the need to continue to be vigilant to ensure that our physicians perceive that their own values are aligned with the division’s stated mission. Devoting a Kaizen-style retreat to well-being likely helped, and allocating divisional resources to a well-being committee indirectly helped, to foster a culture of well-being; however, we could more deliberately identify local policies that may benefit from advocacy or revision. Although our faculty identified interventions to improve interpersonal and individual drivers of well-being, these charter commitments did not have direct parallels in Lean philosophy, and organizations may need to deliberately seek to address these commitments outside of a Lean approach. Specifically, by reviewing the charter, we identified opportunities to provide additional resources for peer support and protected time for mental health care and self-care.
Conclusion
Lean Management can be an effective strategy to address many of the organizational commitments outlined in the Charter on Physician Well-being. This approach may be particularly effective for solving local challenges with systems and workflows. Those who use Lean as a primary method to approach systems improvement in support of the Quadruple Aim may need to use additional strategies to address societal and interpersonal and individual commitments outlined in the charter.
Dr. Sanyal-Dey is visiting associate clinical professor of medicine at Zuckerberg San Francisco General Hospital and director of client services, LeanTaaS. Dr. Thomas is associate clinical professor of medicine at Zuckerberg San Francisco General Hospital. Dr. Chia is associate professor of clinical medicine at Zuckerberg San Francisco General Hospital.
References
1. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016;388(10057):2272-81.
2. Shanafelt TD, Noseworthy JH. Executive leadership and physician: Nine organizational strategies to promote engagement and reduce burnout. Mayo Clin Proc. 2017;92(1):129-46.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Shanafelt T et al. Building a program on well-being: Key design considerations to meet the unique needs of each organization. Acad Med. 2019 Feb;94(2):156-161.
5. Thomas LR et al. Charter on physician well-being. JAMA. 2018;319(15):1541-42.
6. Bodenheimer T, Sinsky C. From triple to quadruple aim: Care of the patient requires care of the provider. Ann Fam Med. 2014;12(6):573-6.
7. West CP et al. Concurrent Validity of Single-Item Measures of Emotional Exhaustion and Depersonalization in Burnout Assessment. J Gen Intern Med. 2012;27(11):1445-52.
8. Hung DY et al. Experiences of primary care physicians and staff following lean workflow redesign. BMC Health Serv Res. 2018 Apr 10;18(1):274.
9. Zibrowski E et al. Easier and faster is not always better: Grounded theory of the impact of large-scale system transformation on the clinical work of emergency medicine nurses and physicians. JMIR Hum Factors. 2018. doi: 10.2196/11013.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015;90(4):432-40.
DOACs show safety benefit in early stages of CKD
Background: Chronic kidney disease (CKD) is both a prothrombotic state and a condition with an elevated bleeding risk that increases in a linear fashion as estimated glomerular filtration rate (eGFR) decreases. These features of the disease along with the exclusion of patients with CKD from most anticoagulation trials have resulted in uncertainty about overall risks and benefits of anticoagulant use in this population.
Study design: Systematic review and meta-analysis.
Setting: Variable across included trials.
Synopsis: Forty-five randomized, controlled trials of anticoagulation covering a broad range of anticoagulants, doses, indications, and methodologies were included in this meta-analysis, representing 34,082 patients with CKD or end-stage kidney disease.
The most compelling data were seen in the management of atrial fibrillation in early-stage CKD (five studies representing 11,332 patients) in which high-dose DOACs were associated with a lower risk for stroke or systemic embolism (risk ratio, 0.79; 95% confidence interval, 0.66-0.92), hemorrhagic stroke (RR, 0.48; 95% CI, 0.30-0.76), and all-cause death (RR, 0.88; 95% CI, 0.78-0.99). Overall stroke reduction was primarily hemorrhagic, and DOACs were equivalent to vitamin K antagonists (VKAs) for ischemic stroke risk.
The analysis also suggests that, in CKD, DOACs may reduce major bleeding when compared with VKAs across a variety of indications, though that finding was not statistically significant.
Efficacy of DOACs, compared with VKAs, in treatment of venous thromboembolism was uncertain, and patients with end-stage kidney disease and advanced CKD (creatinine clearance, less than 25 mL/min) were excluded from all trials comparing DOACs with VKAs, with limited overall data in these populations.
Bottom line: For patients with atrial fibrillation and early-stage CKD, direct oral anticoagulants show a promising risk-benefit profile when compared with vitamin K antagonists. Very few data are available on the safety and efficacy of anticoagulants in patients with advanced CKD and end-stage kidney disease.
Citation: Ha JT et al. Benefits and harms of oral anticoagulant therapy in chronic kidney disease. Ann Intern Med. 2019 Aug 6;171(3):181-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.
Background: Chronic kidney disease (CKD) is both a prothrombotic state and a condition with an elevated bleeding risk that increases in a linear fashion as estimated glomerular filtration rate (eGFR) decreases. These features of the disease along with the exclusion of patients with CKD from most anticoagulation trials have resulted in uncertainty about overall risks and benefits of anticoagulant use in this population.
Study design: Systematic review and meta-analysis.
Setting: Variable across included trials.
Synopsis: Forty-five randomized, controlled trials of anticoagulation covering a broad range of anticoagulants, doses, indications, and methodologies were included in this meta-analysis, representing 34,082 patients with CKD or end-stage kidney disease.
The most compelling data were seen in the management of atrial fibrillation in early-stage CKD (five studies representing 11,332 patients) in which high-dose DOACs were associated with a lower risk for stroke or systemic embolism (risk ratio, 0.79; 95% confidence interval, 0.66-0.92), hemorrhagic stroke (RR, 0.48; 95% CI, 0.30-0.76), and all-cause death (RR, 0.88; 95% CI, 0.78-0.99). Overall stroke reduction was primarily hemorrhagic, and DOACs were equivalent to vitamin K antagonists (VKAs) for ischemic stroke risk.
The analysis also suggests that, in CKD, DOACs may reduce major bleeding when compared with VKAs across a variety of indications, though that finding was not statistically significant.
Efficacy of DOACs, compared with VKAs, in treatment of venous thromboembolism was uncertain, and patients with end-stage kidney disease and advanced CKD (creatinine clearance, less than 25 mL/min) were excluded from all trials comparing DOACs with VKAs, with limited overall data in these populations.
Bottom line: For patients with atrial fibrillation and early-stage CKD, direct oral anticoagulants show a promising risk-benefit profile when compared with vitamin K antagonists. Very few data are available on the safety and efficacy of anticoagulants in patients with advanced CKD and end-stage kidney disease.
Citation: Ha JT et al. Benefits and harms of oral anticoagulant therapy in chronic kidney disease. Ann Intern Med. 2019 Aug 6;171(3):181-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.
Background: Chronic kidney disease (CKD) is both a prothrombotic state and a condition with an elevated bleeding risk that increases in a linear fashion as estimated glomerular filtration rate (eGFR) decreases. These features of the disease along with the exclusion of patients with CKD from most anticoagulation trials have resulted in uncertainty about overall risks and benefits of anticoagulant use in this population.
Study design: Systematic review and meta-analysis.
Setting: Variable across included trials.
Synopsis: Forty-five randomized, controlled trials of anticoagulation covering a broad range of anticoagulants, doses, indications, and methodologies were included in this meta-analysis, representing 34,082 patients with CKD or end-stage kidney disease.
The most compelling data were seen in the management of atrial fibrillation in early-stage CKD (five studies representing 11,332 patients) in which high-dose DOACs were associated with a lower risk for stroke or systemic embolism (risk ratio, 0.79; 95% confidence interval, 0.66-0.92), hemorrhagic stroke (RR, 0.48; 95% CI, 0.30-0.76), and all-cause death (RR, 0.88; 95% CI, 0.78-0.99). Overall stroke reduction was primarily hemorrhagic, and DOACs were equivalent to vitamin K antagonists (VKAs) for ischemic stroke risk.
The analysis also suggests that, in CKD, DOACs may reduce major bleeding when compared with VKAs across a variety of indications, though that finding was not statistically significant.
Efficacy of DOACs, compared with VKAs, in treatment of venous thromboembolism was uncertain, and patients with end-stage kidney disease and advanced CKD (creatinine clearance, less than 25 mL/min) were excluded from all trials comparing DOACs with VKAs, with limited overall data in these populations.
Bottom line: For patients with atrial fibrillation and early-stage CKD, direct oral anticoagulants show a promising risk-benefit profile when compared with vitamin K antagonists. Very few data are available on the safety and efficacy of anticoagulants in patients with advanced CKD and end-stage kidney disease.
Citation: Ha JT et al. Benefits and harms of oral anticoagulant therapy in chronic kidney disease. Ann Intern Med. 2019 Aug 6;171(3):181-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.
Overdiagnosis and overtreatment of COPD appears rampant
Background: COPD is a highly morbid disease, and there is a need for a better understanding of the true prevalence. Little is known regarding overdiagnosis of COPD. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), airflow limitation by spirometry is a key criteria for diagnosis.
Study design: Population-based survey.
Setting: Altogether, 23 sites in 20 countries worldwide were included.
Synopsis: The Burden of Obstructive Lung Disease (BOLD) study recruited community-dwelling adults who underwent questionnaires, as well as spirometry. Of the 16,717 participants, 919 self-reported a COPD diagnosis. Of these, more than half were found to not meet obstructive lung disease criteria on spirometry, and therefore were misdiagnosed: 62% when defined as forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) ratio less than the lower limit of normal and 55% when using the GOLD definition of FEV1/FVC less than 0.7. After patients with reported asthma were excluded, 34% of participants with false-positive COPD were found to be treated with respiratory medications as outpatients.
Overdiagnosis of COPD was noted to be more prevalent in high-income countries than they were in low- to middle-income countries (4.9% versus 1.9% of the participants sampled).
The self-reporting of the diagnosis of COPD is a limitation of the study because it may have artificially inflated the rate of false positives.
Bottom line: Patient-reported diagnoses of COPD should be taken with a degree of caution because of high rates of overdiagnosis and overtreatment.
Citation: Sator L et al. Overdiagnosis of COPD in subjects with unobstructed spirometry. Chest. 2019 Aug;156(2):277-88.
Dr. Gordon is a hospitalist at Maine Medical Center in Portland.
Background: COPD is a highly morbid disease, and there is a need for a better understanding of the true prevalence. Little is known regarding overdiagnosis of COPD. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), airflow limitation by spirometry is a key criteria for diagnosis.
Study design: Population-based survey.
Setting: Altogether, 23 sites in 20 countries worldwide were included.
Synopsis: The Burden of Obstructive Lung Disease (BOLD) study recruited community-dwelling adults who underwent questionnaires, as well as spirometry. Of the 16,717 participants, 919 self-reported a COPD diagnosis. Of these, more than half were found to not meet obstructive lung disease criteria on spirometry, and therefore were misdiagnosed: 62% when defined as forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) ratio less than the lower limit of normal and 55% when using the GOLD definition of FEV1/FVC less than 0.7. After patients with reported asthma were excluded, 34% of participants with false-positive COPD were found to be treated with respiratory medications as outpatients.
Overdiagnosis of COPD was noted to be more prevalent in high-income countries than they were in low- to middle-income countries (4.9% versus 1.9% of the participants sampled).
The self-reporting of the diagnosis of COPD is a limitation of the study because it may have artificially inflated the rate of false positives.
Bottom line: Patient-reported diagnoses of COPD should be taken with a degree of caution because of high rates of overdiagnosis and overtreatment.
Citation: Sator L et al. Overdiagnosis of COPD in subjects with unobstructed spirometry. Chest. 2019 Aug;156(2):277-88.
Dr. Gordon is a hospitalist at Maine Medical Center in Portland.
Background: COPD is a highly morbid disease, and there is a need for a better understanding of the true prevalence. Little is known regarding overdiagnosis of COPD. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), airflow limitation by spirometry is a key criteria for diagnosis.
Study design: Population-based survey.
Setting: Altogether, 23 sites in 20 countries worldwide were included.
Synopsis: The Burden of Obstructive Lung Disease (BOLD) study recruited community-dwelling adults who underwent questionnaires, as well as spirometry. Of the 16,717 participants, 919 self-reported a COPD diagnosis. Of these, more than half were found to not meet obstructive lung disease criteria on spirometry, and therefore were misdiagnosed: 62% when defined as forced expiratory volume in 1 second to forced vital capacity (FEV1/FVC) ratio less than the lower limit of normal and 55% when using the GOLD definition of FEV1/FVC less than 0.7. After patients with reported asthma were excluded, 34% of participants with false-positive COPD were found to be treated with respiratory medications as outpatients.
Overdiagnosis of COPD was noted to be more prevalent in high-income countries than they were in low- to middle-income countries (4.9% versus 1.9% of the participants sampled).
The self-reporting of the diagnosis of COPD is a limitation of the study because it may have artificially inflated the rate of false positives.
Bottom line: Patient-reported diagnoses of COPD should be taken with a degree of caution because of high rates of overdiagnosis and overtreatment.
Citation: Sator L et al. Overdiagnosis of COPD in subjects with unobstructed spirometry. Chest. 2019 Aug;156(2):277-88.
Dr. Gordon is a hospitalist at Maine Medical Center in Portland.
A standardized approach to postop management of DOACs in AFib
Clinical question: Is it safe to adopt a standardized approach to direct oral anticoagulant (DOAC) interruption for patients with atrial fibrillation (AFib) who are undergoing elective surgeries/procedures?
Background: At present, perioperative management of DOACs for patients with AFib has significant variation, and robust data are absent. Points of controversy include: The length of time to hold DOACs before and after the procedure, whether to bridge with heparin, and whether to measure coagulation function studies prior to the procedure.
Study design: Prospective cohort study.
Setting: Conducted in Canada, the United States, and Europe.
Synopsis: The PAUSE study included adults with atrial fibrillation who were long-term users of either apixaban, dabigatran, or rivaroxaban and were scheduled for an elective procedure (n = 3,007). Patients were placed on a standardized DOAC interruption schedule based on whether their procedure had high bleeding risk (held for 2 days prior; resumed 2-3 days after) or low bleeding risk (held for 1 day prior; resumed 1 day after).
The primary clinical outcomes were major bleeding and arterial thromboembolism. Authors determined safety by comparing to expected outcome rates derived from research on perioperative warfarin management.
They found that all three drugs were associated with acceptable rates of arterial thromboembolism (apixaban 0.2%, dabigatran 0.6%, rivaroxaban 0.4%). The rates of major bleeding observed with each drug (apixaban 0.6% low-risk procedures, 3% high-risk procedures; dabigatran 0.9% both low- and high-risk procedures; and rivaroxaban 1.3% low-risk procedures, 3% high-risk procedures) were similar to those in the BRIDGE trial (patients on warfarin who were not bridged perioperatively). However, it must still be noted that only dabigatran met the authors’ predetermined definition of safety for major bleeding.
Limitations include the lack of true control rates for major bleeding and stroke, the relatively low mean CHADS2-Va2Sc of 3.3-3.5, and that greater than 95% of patients were white.
Bottom line: For patients with moderate-risk atrial fibrillation, a standardized approach to DOAC interruption in the perioperative period that omits bridging along with coagulation function testing appears safe in this preliminary study.
Citation: Douketis JD et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2431.
Dr. Gordon is a hospitalist at Maine Medical Center in Portland.
Clinical question: Is it safe to adopt a standardized approach to direct oral anticoagulant (DOAC) interruption for patients with atrial fibrillation (AFib) who are undergoing elective surgeries/procedures?
Background: At present, perioperative management of DOACs for patients with AFib has significant variation, and robust data are absent. Points of controversy include: The length of time to hold DOACs before and after the procedure, whether to bridge with heparin, and whether to measure coagulation function studies prior to the procedure.
Study design: Prospective cohort study.
Setting: Conducted in Canada, the United States, and Europe.
Synopsis: The PAUSE study included adults with atrial fibrillation who were long-term users of either apixaban, dabigatran, or rivaroxaban and were scheduled for an elective procedure (n = 3,007). Patients were placed on a standardized DOAC interruption schedule based on whether their procedure had high bleeding risk (held for 2 days prior; resumed 2-3 days after) or low bleeding risk (held for 1 day prior; resumed 1 day after).
The primary clinical outcomes were major bleeding and arterial thromboembolism. Authors determined safety by comparing to expected outcome rates derived from research on perioperative warfarin management.
They found that all three drugs were associated with acceptable rates of arterial thromboembolism (apixaban 0.2%, dabigatran 0.6%, rivaroxaban 0.4%). The rates of major bleeding observed with each drug (apixaban 0.6% low-risk procedures, 3% high-risk procedures; dabigatran 0.9% both low- and high-risk procedures; and rivaroxaban 1.3% low-risk procedures, 3% high-risk procedures) were similar to those in the BRIDGE trial (patients on warfarin who were not bridged perioperatively). However, it must still be noted that only dabigatran met the authors’ predetermined definition of safety for major bleeding.
Limitations include the lack of true control rates for major bleeding and stroke, the relatively low mean CHADS2-Va2Sc of 3.3-3.5, and that greater than 95% of patients were white.
Bottom line: For patients with moderate-risk atrial fibrillation, a standardized approach to DOAC interruption in the perioperative period that omits bridging along with coagulation function testing appears safe in this preliminary study.
Citation: Douketis JD et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2431.
Dr. Gordon is a hospitalist at Maine Medical Center in Portland.
Clinical question: Is it safe to adopt a standardized approach to direct oral anticoagulant (DOAC) interruption for patients with atrial fibrillation (AFib) who are undergoing elective surgeries/procedures?
Background: At present, perioperative management of DOACs for patients with AFib has significant variation, and robust data are absent. Points of controversy include: The length of time to hold DOACs before and after the procedure, whether to bridge with heparin, and whether to measure coagulation function studies prior to the procedure.
Study design: Prospective cohort study.
Setting: Conducted in Canada, the United States, and Europe.
Synopsis: The PAUSE study included adults with atrial fibrillation who were long-term users of either apixaban, dabigatran, or rivaroxaban and were scheduled for an elective procedure (n = 3,007). Patients were placed on a standardized DOAC interruption schedule based on whether their procedure had high bleeding risk (held for 2 days prior; resumed 2-3 days after) or low bleeding risk (held for 1 day prior; resumed 1 day after).
The primary clinical outcomes were major bleeding and arterial thromboembolism. Authors determined safety by comparing to expected outcome rates derived from research on perioperative warfarin management.
They found that all three drugs were associated with acceptable rates of arterial thromboembolism (apixaban 0.2%, dabigatran 0.6%, rivaroxaban 0.4%). The rates of major bleeding observed with each drug (apixaban 0.6% low-risk procedures, 3% high-risk procedures; dabigatran 0.9% both low- and high-risk procedures; and rivaroxaban 1.3% low-risk procedures, 3% high-risk procedures) were similar to those in the BRIDGE trial (patients on warfarin who were not bridged perioperatively). However, it must still be noted that only dabigatran met the authors’ predetermined definition of safety for major bleeding.
Limitations include the lack of true control rates for major bleeding and stroke, the relatively low mean CHADS2-Va2Sc of 3.3-3.5, and that greater than 95% of patients were white.
Bottom line: For patients with moderate-risk atrial fibrillation, a standardized approach to DOAC interruption in the perioperative period that omits bridging along with coagulation function testing appears safe in this preliminary study.
Citation: Douketis JD et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2431.
Dr. Gordon is a hospitalist at Maine Medical Center in Portland.
How can hospitalists change the status quo?
Lean framework for efficiency and empathy of care
“My census is too high.”
“I don’t have enough time to talk to patients.”
“These are outside our scope of practice.”
These are statements that I have heard from colleagues over the last fourteen years as a hospitalist. Back in 1996, when Dr. Bob Wachter coined the term ‘hospitalist,’ we were still in our infancy – the scope of what we could do had yet to be fully realized. Our focus was on providing care for hospitalized patients and improving quality of clinical care and patient safety. As health care organizations began to see the potential for our field, the demands on our services grew. We grew to comanage patients with our surgical colleagues, worked on patient satisfaction, facilitated transitions of care, and attempted to reduce readmissions – all of which improved patient care and the bottom line for our organizations.
Somewhere along the way, we were expected to staff high patient volumes to add more value, but this always seemed to come with compromise in another aspect of care or our own well-being. After all, there are only so many hours in the day and a limit on what one individual can accomplish in that time.
One of the reasons I love hospital medicine is the novelty of what we do – we are creative thinkers. We have the capacity to innovate solutions to hospital problems based on our expertise as frontline providers for our patients. Hospitalists of every discipline staff a large majority of inpatients, which makes our collective experience significant to the management of inpatient health care. We are often the ones tasked with executing improvement projects, but how often are we involved in their design? I know that we collectively have an enormous opportunity to improve our health care practice, both for ourselves, our patients, and the institutions we work for. But more than just being a voice of advocacy, we need to understand how to positively influence the health care structures that allow us to deliver quality patient care.
It is no surprise that the inefficiencies we deal with in our hospitals are many – daily workflow interruptions, delays in results, scheduling issues, communication difficulties. These are not unique to any one institution. The pandemic added more to that plate – PPE deficiencies, patient volume triage, and resource management are examples. Hospitals often contract consultants to help solve these problems, and many utilize a variety of frameworks to improve these system processes. The Lean framework is one of these, and it originated in the manufacturing industry to eliminate waste in systems in the pursuit of efficiency.
In my business training and prior hospital medicine leadership roles, I was educated in Lean thinking and methodologies for improving quality and applied its principles to projects for improving workflow. Last year I attended a virtual conference on ‘Lean Innovation during the pandemic’ for New York region hospitals, and it again highlighted how the Lean management methodology can help improve patient care but importantly, our workflow as clinicians. This got me thinking. Why is Lean well accepted in business and manufacturing circles, but less so in health care?
I think the answer is twofold – knowledge and people.
What is Lean and how can it help us?
The ‘Toyota Production System’-based philosophy has 14 core principles that help eliminate waste in systems in pursuit of efficiency. These principles are the “Toyota Way.” They center around two pillars: continuous improvement and respect for people. The cornerstone of this management methodology is based on efficient processes, developing employees to add value to the organization and continuous improvement through problem-solving and organizational learning.
Lean is often implemented with Six Sigma methodology. Six Sigma has its origins in Motorola. While Lean cuts waste in our systems to provide value, Six Sigma uses DMAIC (Define, Measure, Analyze, Improve, Control) to reduce variation in our processes. When done in its entirety, Lean Six Sigma methodology adds value by increasing efficiency, reducing cost, and improving our everyday work.
Statistical principles suggest that 80% of consequences comes from 20% of causes. Lean methodology and tools allow us to systematically identify root causes for the problems we face and help narrow it down to the ‘vital few.’ In other words, fixing these would give us the most bang for our buck. As hospitalists, we are able to do this better than most because we work in these hospital processes everyday – we truly know the strengths and weaknesses of our systems.
As a hospitalist, I would love for the process of seeing patients in hospitals to be more efficient, less variable, and be more cost-effective for my institution. By eliminating the time wasted performing unnecessary and redundant tasks in my everyday work, I can reallocate that time to patient care – the very reason I chose a career in medicine.
We, the people
There are two common rebuttals I hear for adopting Lean Six Sigma methodology in health care. A frequent misconception is that Lean is all about reducing staff or time with patients. The second is that manufacturing methodologies do not work for a service profession. For instance, an article published on Reuters Events (www.reutersevents.com/supplychain/supply-chain/end-just-time) talks about Lean JIT (Just In Time) inventory as a culprit for creating a supply chain deficit during COVID-19. It is not entirely without merit. However, if done the correct way, Lean is all about involving the frontline worker to create a workflow that would work best for them.
Reducing the waste in our processes and empowering our frontline doctors to be creative in finding solutions naturally leads to cost reduction. The cornerstone of Lean is creating a continuously learning organization and putting your employees at the forefront. I think it is important that Lean principles be utilized within health care – but we cannot push to fix every problem in our systems to perfection at a significant expense to the physician and other health care staff.
Why HM can benefit from Lean
There is no hard and fast rule about the way health care should adopt Lean thinking. It is a way of thinking that aims to balance purpose, people, and process – extremes of inventory management may not be necessary to be successful in health care. Lean tools alone would not create results. John Shook, chairman of Lean Global Network, has said that the social side of Lean needs to be in balance with the technical side. In other words, rigidity and efficiency is good, but so is encouraging creativity and flexibility in thinking within the workforce.
In the crisis created by the novel coronavirus, many hospitals in New York state, including my own, turned to Lean to respond quickly and effectively to the challenges. Lean principles helped them problem-solve and develop strategies to both recover from the pandemic surge and adapt to future problems that could occur. Geographic clustering of patients, PPE supply, OR shut down and ramp up, emergency management offices at the peak of the pandemic, telehealth streamlining, and post-COVID-19 care planning are some areas where the application of Lean resulted in successful responses to the challenges that 2020 brought to our work.
As Warren Bennis said, ‘The manager accepts the status quo; the leader challenges it.’ As hospitalists, we can lead the way our hospitals provide care. Lean is not just a way for hospitals to cut costs (although it helps quite a bit there). Its processes and philosophies could enable hospitalists to maximize potential, efficiency, quality of care, and allow for a balanced work environment. When applied in a manner that focuses on continuous improvement (and is cognizant of its limitations), it has the potential to increase the capability of our service lines and streamline our processes and workday for greater efficiency. As a specialty, we stand to benefit by taking the lead role in choosing how best to improve how we work. We should think outside the box. What better time to do this than now?
Dr. Kanikkannan is a practicing hospitalist and assistant professor of medicine at Albany (N.Y) Medical College. She is a former hospitalist medical director and has served on SHM’s national committees, and is a certified Lean Six Sigma black belt and MBA candidate.
Lean framework for efficiency and empathy of care
Lean framework for efficiency and empathy of care
“My census is too high.”
“I don’t have enough time to talk to patients.”
“These are outside our scope of practice.”
These are statements that I have heard from colleagues over the last fourteen years as a hospitalist. Back in 1996, when Dr. Bob Wachter coined the term ‘hospitalist,’ we were still in our infancy – the scope of what we could do had yet to be fully realized. Our focus was on providing care for hospitalized patients and improving quality of clinical care and patient safety. As health care organizations began to see the potential for our field, the demands on our services grew. We grew to comanage patients with our surgical colleagues, worked on patient satisfaction, facilitated transitions of care, and attempted to reduce readmissions – all of which improved patient care and the bottom line for our organizations.
Somewhere along the way, we were expected to staff high patient volumes to add more value, but this always seemed to come with compromise in another aspect of care or our own well-being. After all, there are only so many hours in the day and a limit on what one individual can accomplish in that time.
One of the reasons I love hospital medicine is the novelty of what we do – we are creative thinkers. We have the capacity to innovate solutions to hospital problems based on our expertise as frontline providers for our patients. Hospitalists of every discipline staff a large majority of inpatients, which makes our collective experience significant to the management of inpatient health care. We are often the ones tasked with executing improvement projects, but how often are we involved in their design? I know that we collectively have an enormous opportunity to improve our health care practice, both for ourselves, our patients, and the institutions we work for. But more than just being a voice of advocacy, we need to understand how to positively influence the health care structures that allow us to deliver quality patient care.
It is no surprise that the inefficiencies we deal with in our hospitals are many – daily workflow interruptions, delays in results, scheduling issues, communication difficulties. These are not unique to any one institution. The pandemic added more to that plate – PPE deficiencies, patient volume triage, and resource management are examples. Hospitals often contract consultants to help solve these problems, and many utilize a variety of frameworks to improve these system processes. The Lean framework is one of these, and it originated in the manufacturing industry to eliminate waste in systems in the pursuit of efficiency.
In my business training and prior hospital medicine leadership roles, I was educated in Lean thinking and methodologies for improving quality and applied its principles to projects for improving workflow. Last year I attended a virtual conference on ‘Lean Innovation during the pandemic’ for New York region hospitals, and it again highlighted how the Lean management methodology can help improve patient care but importantly, our workflow as clinicians. This got me thinking. Why is Lean well accepted in business and manufacturing circles, but less so in health care?
I think the answer is twofold – knowledge and people.
What is Lean and how can it help us?
The ‘Toyota Production System’-based philosophy has 14 core principles that help eliminate waste in systems in pursuit of efficiency. These principles are the “Toyota Way.” They center around two pillars: continuous improvement and respect for people. The cornerstone of this management methodology is based on efficient processes, developing employees to add value to the organization and continuous improvement through problem-solving and organizational learning.
Lean is often implemented with Six Sigma methodology. Six Sigma has its origins in Motorola. While Lean cuts waste in our systems to provide value, Six Sigma uses DMAIC (Define, Measure, Analyze, Improve, Control) to reduce variation in our processes. When done in its entirety, Lean Six Sigma methodology adds value by increasing efficiency, reducing cost, and improving our everyday work.
Statistical principles suggest that 80% of consequences comes from 20% of causes. Lean methodology and tools allow us to systematically identify root causes for the problems we face and help narrow it down to the ‘vital few.’ In other words, fixing these would give us the most bang for our buck. As hospitalists, we are able to do this better than most because we work in these hospital processes everyday – we truly know the strengths and weaknesses of our systems.
As a hospitalist, I would love for the process of seeing patients in hospitals to be more efficient, less variable, and be more cost-effective for my institution. By eliminating the time wasted performing unnecessary and redundant tasks in my everyday work, I can reallocate that time to patient care – the very reason I chose a career in medicine.
We, the people
There are two common rebuttals I hear for adopting Lean Six Sigma methodology in health care. A frequent misconception is that Lean is all about reducing staff or time with patients. The second is that manufacturing methodologies do not work for a service profession. For instance, an article published on Reuters Events (www.reutersevents.com/supplychain/supply-chain/end-just-time) talks about Lean JIT (Just In Time) inventory as a culprit for creating a supply chain deficit during COVID-19. It is not entirely without merit. However, if done the correct way, Lean is all about involving the frontline worker to create a workflow that would work best for them.
Reducing the waste in our processes and empowering our frontline doctors to be creative in finding solutions naturally leads to cost reduction. The cornerstone of Lean is creating a continuously learning organization and putting your employees at the forefront. I think it is important that Lean principles be utilized within health care – but we cannot push to fix every problem in our systems to perfection at a significant expense to the physician and other health care staff.
Why HM can benefit from Lean
There is no hard and fast rule about the way health care should adopt Lean thinking. It is a way of thinking that aims to balance purpose, people, and process – extremes of inventory management may not be necessary to be successful in health care. Lean tools alone would not create results. John Shook, chairman of Lean Global Network, has said that the social side of Lean needs to be in balance with the technical side. In other words, rigidity and efficiency is good, but so is encouraging creativity and flexibility in thinking within the workforce.
In the crisis created by the novel coronavirus, many hospitals in New York state, including my own, turned to Lean to respond quickly and effectively to the challenges. Lean principles helped them problem-solve and develop strategies to both recover from the pandemic surge and adapt to future problems that could occur. Geographic clustering of patients, PPE supply, OR shut down and ramp up, emergency management offices at the peak of the pandemic, telehealth streamlining, and post-COVID-19 care planning are some areas where the application of Lean resulted in successful responses to the challenges that 2020 brought to our work.
As Warren Bennis said, ‘The manager accepts the status quo; the leader challenges it.’ As hospitalists, we can lead the way our hospitals provide care. Lean is not just a way for hospitals to cut costs (although it helps quite a bit there). Its processes and philosophies could enable hospitalists to maximize potential, efficiency, quality of care, and allow for a balanced work environment. When applied in a manner that focuses on continuous improvement (and is cognizant of its limitations), it has the potential to increase the capability of our service lines and streamline our processes and workday for greater efficiency. As a specialty, we stand to benefit by taking the lead role in choosing how best to improve how we work. We should think outside the box. What better time to do this than now?
Dr. Kanikkannan is a practicing hospitalist and assistant professor of medicine at Albany (N.Y) Medical College. She is a former hospitalist medical director and has served on SHM’s national committees, and is a certified Lean Six Sigma black belt and MBA candidate.
“My census is too high.”
“I don’t have enough time to talk to patients.”
“These are outside our scope of practice.”
These are statements that I have heard from colleagues over the last fourteen years as a hospitalist. Back in 1996, when Dr. Bob Wachter coined the term ‘hospitalist,’ we were still in our infancy – the scope of what we could do had yet to be fully realized. Our focus was on providing care for hospitalized patients and improving quality of clinical care and patient safety. As health care organizations began to see the potential for our field, the demands on our services grew. We grew to comanage patients with our surgical colleagues, worked on patient satisfaction, facilitated transitions of care, and attempted to reduce readmissions – all of which improved patient care and the bottom line for our organizations.
Somewhere along the way, we were expected to staff high patient volumes to add more value, but this always seemed to come with compromise in another aspect of care or our own well-being. After all, there are only so many hours in the day and a limit on what one individual can accomplish in that time.
One of the reasons I love hospital medicine is the novelty of what we do – we are creative thinkers. We have the capacity to innovate solutions to hospital problems based on our expertise as frontline providers for our patients. Hospitalists of every discipline staff a large majority of inpatients, which makes our collective experience significant to the management of inpatient health care. We are often the ones tasked with executing improvement projects, but how often are we involved in their design? I know that we collectively have an enormous opportunity to improve our health care practice, both for ourselves, our patients, and the institutions we work for. But more than just being a voice of advocacy, we need to understand how to positively influence the health care structures that allow us to deliver quality patient care.
It is no surprise that the inefficiencies we deal with in our hospitals are many – daily workflow interruptions, delays in results, scheduling issues, communication difficulties. These are not unique to any one institution. The pandemic added more to that plate – PPE deficiencies, patient volume triage, and resource management are examples. Hospitals often contract consultants to help solve these problems, and many utilize a variety of frameworks to improve these system processes. The Lean framework is one of these, and it originated in the manufacturing industry to eliminate waste in systems in the pursuit of efficiency.
In my business training and prior hospital medicine leadership roles, I was educated in Lean thinking and methodologies for improving quality and applied its principles to projects for improving workflow. Last year I attended a virtual conference on ‘Lean Innovation during the pandemic’ for New York region hospitals, and it again highlighted how the Lean management methodology can help improve patient care but importantly, our workflow as clinicians. This got me thinking. Why is Lean well accepted in business and manufacturing circles, but less so in health care?
I think the answer is twofold – knowledge and people.
What is Lean and how can it help us?
The ‘Toyota Production System’-based philosophy has 14 core principles that help eliminate waste in systems in pursuit of efficiency. These principles are the “Toyota Way.” They center around two pillars: continuous improvement and respect for people. The cornerstone of this management methodology is based on efficient processes, developing employees to add value to the organization and continuous improvement through problem-solving and organizational learning.
Lean is often implemented with Six Sigma methodology. Six Sigma has its origins in Motorola. While Lean cuts waste in our systems to provide value, Six Sigma uses DMAIC (Define, Measure, Analyze, Improve, Control) to reduce variation in our processes. When done in its entirety, Lean Six Sigma methodology adds value by increasing efficiency, reducing cost, and improving our everyday work.
Statistical principles suggest that 80% of consequences comes from 20% of causes. Lean methodology and tools allow us to systematically identify root causes for the problems we face and help narrow it down to the ‘vital few.’ In other words, fixing these would give us the most bang for our buck. As hospitalists, we are able to do this better than most because we work in these hospital processes everyday – we truly know the strengths and weaknesses of our systems.
As a hospitalist, I would love for the process of seeing patients in hospitals to be more efficient, less variable, and be more cost-effective for my institution. By eliminating the time wasted performing unnecessary and redundant tasks in my everyday work, I can reallocate that time to patient care – the very reason I chose a career in medicine.
We, the people
There are two common rebuttals I hear for adopting Lean Six Sigma methodology in health care. A frequent misconception is that Lean is all about reducing staff or time with patients. The second is that manufacturing methodologies do not work for a service profession. For instance, an article published on Reuters Events (www.reutersevents.com/supplychain/supply-chain/end-just-time) talks about Lean JIT (Just In Time) inventory as a culprit for creating a supply chain deficit during COVID-19. It is not entirely without merit. However, if done the correct way, Lean is all about involving the frontline worker to create a workflow that would work best for them.
Reducing the waste in our processes and empowering our frontline doctors to be creative in finding solutions naturally leads to cost reduction. The cornerstone of Lean is creating a continuously learning organization and putting your employees at the forefront. I think it is important that Lean principles be utilized within health care – but we cannot push to fix every problem in our systems to perfection at a significant expense to the physician and other health care staff.
Why HM can benefit from Lean
There is no hard and fast rule about the way health care should adopt Lean thinking. It is a way of thinking that aims to balance purpose, people, and process – extremes of inventory management may not be necessary to be successful in health care. Lean tools alone would not create results. John Shook, chairman of Lean Global Network, has said that the social side of Lean needs to be in balance with the technical side. In other words, rigidity and efficiency is good, but so is encouraging creativity and flexibility in thinking within the workforce.
In the crisis created by the novel coronavirus, many hospitals in New York state, including my own, turned to Lean to respond quickly and effectively to the challenges. Lean principles helped them problem-solve and develop strategies to both recover from the pandemic surge and adapt to future problems that could occur. Geographic clustering of patients, PPE supply, OR shut down and ramp up, emergency management offices at the peak of the pandemic, telehealth streamlining, and post-COVID-19 care planning are some areas where the application of Lean resulted in successful responses to the challenges that 2020 brought to our work.
As Warren Bennis said, ‘The manager accepts the status quo; the leader challenges it.’ As hospitalists, we can lead the way our hospitals provide care. Lean is not just a way for hospitals to cut costs (although it helps quite a bit there). Its processes and philosophies could enable hospitalists to maximize potential, efficiency, quality of care, and allow for a balanced work environment. When applied in a manner that focuses on continuous improvement (and is cognizant of its limitations), it has the potential to increase the capability of our service lines and streamline our processes and workday for greater efficiency. As a specialty, we stand to benefit by taking the lead role in choosing how best to improve how we work. We should think outside the box. What better time to do this than now?
Dr. Kanikkannan is a practicing hospitalist and assistant professor of medicine at Albany (N.Y) Medical College. She is a former hospitalist medical director and has served on SHM’s national committees, and is a certified Lean Six Sigma black belt and MBA candidate.
Eosinophilia-guided treatment cuts corticosteroid exposure in COPD exacerbations
Background: Corticosteroids in the setting of an acute exacerbation of improve COPD symptoms but do not affect the decline in lung function, rate of repeat exacerbations after a month, or mortality. There is concern regarding the cumulative adverse effects over time. Limited prior research suggests that a patient’s blood eosinophil count may be useful for determining the necessity of steroids for treatment of exacerbation.
Study design: Randomized, controlled, open-label trial.
Setting: Respiratory departments of three university hospitals in Denmark.
Synopsis: A total of 318 patients admitted for COPD exacerbation were randomized to standard or eosinophilia-guided therapy. On day 1, all patients received 80 mg of IV methylprednisolone. The standard-therapy group then received 37.5 mg of oral prednisolone for 4 more days. In contrast, the eosinophilia-guided group received prednisolone only if their blood eosinophil count was 300 cells/mcL or greater.
The primary outcome of days alive and out of the hospital within 14 days after recruitment was similar between groups (9 days; P = .34), along with the secondary outcome of treatment failure (26%; P = .90). Importantly, the cumulative steroid dose in the eosinophilia-guided group was lower than that of the control group at days 5, 30, and 90 (P less than or equal to .0002). Additionally, the control arm had worsening of baseline diabetes within 30 days and was more likely to require antibiotics for infections within 90 days.
Although not statistically significant, a trend was noted toward increased readmission for COPD exacerbations or death at 30 days in the eosinophilia-guided group (25% vs. 17% of control; P = .10). Future work will need to further study this trend.
Bottom line: Eosinophilia-guided treatment of COPD exacerbations reduced the cumulative exposure of steroid therapy, thereby decreasing side effects, although further study of safety profile is warranted.
Citation: Sivapalan P et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): A multicenter, randomized, controlled, open-label, non-inferiority trial. Lancet Respir Med. 2019 Aug;7(8): 699-709.
Dr. Dupuis is a hospitalist at Maine Medical Center in Portland.
Background: Corticosteroids in the setting of an acute exacerbation of improve COPD symptoms but do not affect the decline in lung function, rate of repeat exacerbations after a month, or mortality. There is concern regarding the cumulative adverse effects over time. Limited prior research suggests that a patient’s blood eosinophil count may be useful for determining the necessity of steroids for treatment of exacerbation.
Study design: Randomized, controlled, open-label trial.
Setting: Respiratory departments of three university hospitals in Denmark.
Synopsis: A total of 318 patients admitted for COPD exacerbation were randomized to standard or eosinophilia-guided therapy. On day 1, all patients received 80 mg of IV methylprednisolone. The standard-therapy group then received 37.5 mg of oral prednisolone for 4 more days. In contrast, the eosinophilia-guided group received prednisolone only if their blood eosinophil count was 300 cells/mcL or greater.
The primary outcome of days alive and out of the hospital within 14 days after recruitment was similar between groups (9 days; P = .34), along with the secondary outcome of treatment failure (26%; P = .90). Importantly, the cumulative steroid dose in the eosinophilia-guided group was lower than that of the control group at days 5, 30, and 90 (P less than or equal to .0002). Additionally, the control arm had worsening of baseline diabetes within 30 days and was more likely to require antibiotics for infections within 90 days.
Although not statistically significant, a trend was noted toward increased readmission for COPD exacerbations or death at 30 days in the eosinophilia-guided group (25% vs. 17% of control; P = .10). Future work will need to further study this trend.
Bottom line: Eosinophilia-guided treatment of COPD exacerbations reduced the cumulative exposure of steroid therapy, thereby decreasing side effects, although further study of safety profile is warranted.
Citation: Sivapalan P et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): A multicenter, randomized, controlled, open-label, non-inferiority trial. Lancet Respir Med. 2019 Aug;7(8): 699-709.
Dr. Dupuis is a hospitalist at Maine Medical Center in Portland.
Background: Corticosteroids in the setting of an acute exacerbation of improve COPD symptoms but do not affect the decline in lung function, rate of repeat exacerbations after a month, or mortality. There is concern regarding the cumulative adverse effects over time. Limited prior research suggests that a patient’s blood eosinophil count may be useful for determining the necessity of steroids for treatment of exacerbation.
Study design: Randomized, controlled, open-label trial.
Setting: Respiratory departments of three university hospitals in Denmark.
Synopsis: A total of 318 patients admitted for COPD exacerbation were randomized to standard or eosinophilia-guided therapy. On day 1, all patients received 80 mg of IV methylprednisolone. The standard-therapy group then received 37.5 mg of oral prednisolone for 4 more days. In contrast, the eosinophilia-guided group received prednisolone only if their blood eosinophil count was 300 cells/mcL or greater.
The primary outcome of days alive and out of the hospital within 14 days after recruitment was similar between groups (9 days; P = .34), along with the secondary outcome of treatment failure (26%; P = .90). Importantly, the cumulative steroid dose in the eosinophilia-guided group was lower than that of the control group at days 5, 30, and 90 (P less than or equal to .0002). Additionally, the control arm had worsening of baseline diabetes within 30 days and was more likely to require antibiotics for infections within 90 days.
Although not statistically significant, a trend was noted toward increased readmission for COPD exacerbations or death at 30 days in the eosinophilia-guided group (25% vs. 17% of control; P = .10). Future work will need to further study this trend.
Bottom line: Eosinophilia-guided treatment of COPD exacerbations reduced the cumulative exposure of steroid therapy, thereby decreasing side effects, although further study of safety profile is warranted.
Citation: Sivapalan P et al. Eosinophil-guided corticosteroid therapy in patients admitted to hospital with COPD exacerbation (CORTICO-COP): A multicenter, randomized, controlled, open-label, non-inferiority trial. Lancet Respir Med. 2019 Aug;7(8): 699-709.
Dr. Dupuis is a hospitalist at Maine Medical Center in Portland.
Complications and death within 30 days after noncardiac surgery
Background: There have been advances in perioperative care and technology for adults, but at the same time the patient population is increasingly medically complex. We do not know the current mortality risk of noncardiac surgery in adults.
Study design: Prospective cohort study.
Setting: Twenty-eight academic centers in 14 countries in North America, South America, Asia, Europe, Africa, and Australia. At least four academic centers represented each of these continents, except Africa, with one center reporting there.
Synopsis: The VISION study included 40,004 inpatients, aged 45 years and older, followed for 30-day mortality after noncardiac surgery. One-third of surgeries were considered low risk. A startling 99.1% of patients completed the study. Mortality rate was 1.8%, with 71% of patients dying during the index admission and 29% dying after discharge.
Nine events were independently associated with postoperative death, but the top three – major bleeding, myocardial injury after noncardiac surgery (MINS), and sepsis – accounted for 45% of the attributable fraction. These, on average, occurred within 1-6 days after surgery. The other events (infection, kidney injury with dialysis, stroke, venous thromboembolism, new atrial fibrillation, and congestive heart failure) constituted less than 3% of the attributable fraction. Findings suggest that closer monitoring in the hospital and post discharge might improve survival after noncardiac surgery.
Limitations for hospitalists include that patients were younger and less medically complex than our typically comanaged patients: More than half of patients were aged 45-64, less than 10% had chronic kidney disease stage 3b or greater, and only 20% had diabetes mellitus.
Bottom line: Postoperative and postdischarge bleeding, myocardial injury after noncardiac surgery, and sepsis are major risk factors for 30-day mortality in adults undergoing noncardiac surgery. Closer postoperative monitoring for these conditions should be explored.
Citation: The Vision Study Investigators (Spence J et al.) Association between complications and death within 30 days after noncardiac surgery. CMAJ. 2019 Jul 29;191(30):E830-7.
Dr. Brouillette is a med-peds hospitalist at Maine Medical Center in Portland.
Background: There have been advances in perioperative care and technology for adults, but at the same time the patient population is increasingly medically complex. We do not know the current mortality risk of noncardiac surgery in adults.
Study design: Prospective cohort study.
Setting: Twenty-eight academic centers in 14 countries in North America, South America, Asia, Europe, Africa, and Australia. At least four academic centers represented each of these continents, except Africa, with one center reporting there.
Synopsis: The VISION study included 40,004 inpatients, aged 45 years and older, followed for 30-day mortality after noncardiac surgery. One-third of surgeries were considered low risk. A startling 99.1% of patients completed the study. Mortality rate was 1.8%, with 71% of patients dying during the index admission and 29% dying after discharge.
Nine events were independently associated with postoperative death, but the top three – major bleeding, myocardial injury after noncardiac surgery (MINS), and sepsis – accounted for 45% of the attributable fraction. These, on average, occurred within 1-6 days after surgery. The other events (infection, kidney injury with dialysis, stroke, venous thromboembolism, new atrial fibrillation, and congestive heart failure) constituted less than 3% of the attributable fraction. Findings suggest that closer monitoring in the hospital and post discharge might improve survival after noncardiac surgery.
Limitations for hospitalists include that patients were younger and less medically complex than our typically comanaged patients: More than half of patients were aged 45-64, less than 10% had chronic kidney disease stage 3b or greater, and only 20% had diabetes mellitus.
Bottom line: Postoperative and postdischarge bleeding, myocardial injury after noncardiac surgery, and sepsis are major risk factors for 30-day mortality in adults undergoing noncardiac surgery. Closer postoperative monitoring for these conditions should be explored.
Citation: The Vision Study Investigators (Spence J et al.) Association between complications and death within 30 days after noncardiac surgery. CMAJ. 2019 Jul 29;191(30):E830-7.
Dr. Brouillette is a med-peds hospitalist at Maine Medical Center in Portland.
Background: There have been advances in perioperative care and technology for adults, but at the same time the patient population is increasingly medically complex. We do not know the current mortality risk of noncardiac surgery in adults.
Study design: Prospective cohort study.
Setting: Twenty-eight academic centers in 14 countries in North America, South America, Asia, Europe, Africa, and Australia. At least four academic centers represented each of these continents, except Africa, with one center reporting there.
Synopsis: The VISION study included 40,004 inpatients, aged 45 years and older, followed for 30-day mortality after noncardiac surgery. One-third of surgeries were considered low risk. A startling 99.1% of patients completed the study. Mortality rate was 1.8%, with 71% of patients dying during the index admission and 29% dying after discharge.
Nine events were independently associated with postoperative death, but the top three – major bleeding, myocardial injury after noncardiac surgery (MINS), and sepsis – accounted for 45% of the attributable fraction. These, on average, occurred within 1-6 days after surgery. The other events (infection, kidney injury with dialysis, stroke, venous thromboembolism, new atrial fibrillation, and congestive heart failure) constituted less than 3% of the attributable fraction. Findings suggest that closer monitoring in the hospital and post discharge might improve survival after noncardiac surgery.
Limitations for hospitalists include that patients were younger and less medically complex than our typically comanaged patients: More than half of patients were aged 45-64, less than 10% had chronic kidney disease stage 3b or greater, and only 20% had diabetes mellitus.
Bottom line: Postoperative and postdischarge bleeding, myocardial injury after noncardiac surgery, and sepsis are major risk factors for 30-day mortality in adults undergoing noncardiac surgery. Closer postoperative monitoring for these conditions should be explored.
Citation: The Vision Study Investigators (Spence J et al.) Association between complications and death within 30 days after noncardiac surgery. CMAJ. 2019 Jul 29;191(30):E830-7.
Dr. Brouillette is a med-peds hospitalist at Maine Medical Center in Portland.
The importance of community pediatric hospital medicine
According to data from the American Academy of Pediatrics, over 2,000 physicians – or approximately 70% of all physicians practicing pediatric hospital medicine – do so in a community hospital. Like all areas of hospital medicine, community pediatric hospital medicine (CPHM) strives to fulfill one of our field’s central tenets – providing high-quality, evidence-based care to our patients.
A phrase often used among CPHM practitioners is that, “if you’ve seen one CPHM program, you’ve seen one CPHM program.” Every CPHM program is different. While this phrase may seem rather simplistic, it quite accurately portrays a unique aspect of our place in the hospital medicine field. CPHM programs usually require their practitioners to perform a broader range of roles and responsibilities than our colleagues who practice in university or children’s hospitals. Typically, these roles are aligned with the unique needs of each hospital within which we practice and the communities we serve. Factors such as the distance to a tertiary care referral center, access to subspecialists, availability and expertise of ancillary services for children, and the particular needs of each community further shape the role that CPHM practitioners may be asked to play.
In 2014, the AAP section on hospital medicine’s subcommittee on community hospitalists surveyed all CPHM programs to understand the unique roles that practitioners play within their institutions. Under the leadership of Clota Snow, MD, and Jacques Corriveau, MD, the aim was to contact every hospital in the country using the American Hospital Directory to see if they had a PHM program and to identify what roles the program was responsible for within their hospital.
Of the 535 programs identified, the primary responsibilities included inpatient care (85%), ED consultations (76%) and newborn nursery care (73%). Other common roles not typically associated with a university-based hospitalist’s responsibilities included delivery room attendance/neonatal resuscitations (44%), neonatal ICU management (47%) and subspecialty or surgical comanagement (52%). In some communities, even pediatric ICU management, sedation, and patient transport are part of our role. Because of the large breadth of roles that a CPHM practitioner may cover, we have often been referred to as “pediatric hospital-based generalists.”
Ideally, the presence of a pediatric hospitalist in a community hospital allows children to obtain high-quality, evidence-based care within their home communities. Most hospitalized children do not require direct access to subspecialists or all the pediatric-specific resources only available within a university or children’s hospital. Thus, if these resources are not required for the child’s care, CPHM practitioners can provide the care that a child needs in a setting that is less disruptive to the family and typically more cost effective.
CPHM physicians are often drawn to a career in a community hospital because it allows them to use their entire skill set to care for children with a wide variety of conditions. As they are often the only physicians in an adult hospital with a full understanding of the unique aspects of care that children require, it is important that they be comfortable in their role of managing the majority of pediatric care independently. Yet they also need to understand the limitations of their own ability, as well as their institution’s level of expertise in pediatric-specific care. They must be confident and vocal advocates for pediatric-specific needs throughout their institution and its numerous committees, and form close working relationships with colleagues and administrators in the different fields with whom we share care of our patients (e.g., ED, obstetrics, radiology, trauma, and other medical and surgical subspecialties).
CPHM physicians are particularly well suited to partner with local outpatient providers as well as tertiary care physicians to provide coordinated transitions between the inpatient and outpatient management of a child’s illness. In addition, a CPHM physician can often bring a unique and valuable perspective of the particular ethnic, cultural, and socioeconomic diversity of their community, as well as its available resources, to facilitate a greater level of engagement with the child’s needs and ultimate success of their care.
The 2014 survey of CPHM programs identified several major challenges to recruitment and career satisfaction as a CPHM physician. These include a lack of access to subspecialists, a lack of pediatric-specific ancillary services and the perception that our importance as community hospital providers was not valued as much in the PHM community as PHM physicians working in a university/children’s hospital setting. With the recent recognition of PHM as an official subspecialty by the American Board of Pediatrics, the concern has intensified within our field that a two-tiered system will develop with some PHM physicians being board certified and others not.
While the development of board subspecialization was not meant to limit the pool of providers available to staff community hospital sites, there is nowhere near the number of fellowship trained physicians to provide an adequate workforce to staff CPHM programs. This means that many CPHM physicians will not be board certified in pediatric hospital medicine but does not mean that CPHM programs will be unable to provide high-quality local care that benefits children and their families, including safe care for children who require the skills that an immediately available CPHM physician can provide.
Many pediatric residency programs do not currently provide their trainees with exposure to community hospital medicine. Further, with increased sub-specialization throughout pediatrics, fewer residents are developing the necessary skill set to perform roles integral to a caring for children in community hospitals such as stabilization of a critically ill child prior to transport and complex neonatal resuscitation.
A career in CPHM provides physicians with the opportunity to work together with a close-knit group to provide exceptional care to children and to advocate for the medical needs of children in their hospital and their community. The AAP’s subcommittee has made it a priority to engage physicians during all parts of their pediatric training about why a career in CPHM is exciting, fulfilling and a great life, as well as continuing to educate training programs at every level – as well as the larger PHM community – about why CPHM is a valuable and important part of pediatric medicine.
Dr. Welsh is a clinical associate professor of pediatrics at the Stanford (Calif.) University in the division of pediatric hospital medicine. He has practiced community pediatric hospital medicine for over 27 years in Washington state and the San Francisco Bay Area. He is the chair of the working group of the Future of Community Pediatric Hospital Medicine for the AAP section on hospital medicine’s subcommittee on community hospitalists.
According to data from the American Academy of Pediatrics, over 2,000 physicians – or approximately 70% of all physicians practicing pediatric hospital medicine – do so in a community hospital. Like all areas of hospital medicine, community pediatric hospital medicine (CPHM) strives to fulfill one of our field’s central tenets – providing high-quality, evidence-based care to our patients.
A phrase often used among CPHM practitioners is that, “if you’ve seen one CPHM program, you’ve seen one CPHM program.” Every CPHM program is different. While this phrase may seem rather simplistic, it quite accurately portrays a unique aspect of our place in the hospital medicine field. CPHM programs usually require their practitioners to perform a broader range of roles and responsibilities than our colleagues who practice in university or children’s hospitals. Typically, these roles are aligned with the unique needs of each hospital within which we practice and the communities we serve. Factors such as the distance to a tertiary care referral center, access to subspecialists, availability and expertise of ancillary services for children, and the particular needs of each community further shape the role that CPHM practitioners may be asked to play.
In 2014, the AAP section on hospital medicine’s subcommittee on community hospitalists surveyed all CPHM programs to understand the unique roles that practitioners play within their institutions. Under the leadership of Clota Snow, MD, and Jacques Corriveau, MD, the aim was to contact every hospital in the country using the American Hospital Directory to see if they had a PHM program and to identify what roles the program was responsible for within their hospital.
Of the 535 programs identified, the primary responsibilities included inpatient care (85%), ED consultations (76%) and newborn nursery care (73%). Other common roles not typically associated with a university-based hospitalist’s responsibilities included delivery room attendance/neonatal resuscitations (44%), neonatal ICU management (47%) and subspecialty or surgical comanagement (52%). In some communities, even pediatric ICU management, sedation, and patient transport are part of our role. Because of the large breadth of roles that a CPHM practitioner may cover, we have often been referred to as “pediatric hospital-based generalists.”
Ideally, the presence of a pediatric hospitalist in a community hospital allows children to obtain high-quality, evidence-based care within their home communities. Most hospitalized children do not require direct access to subspecialists or all the pediatric-specific resources only available within a university or children’s hospital. Thus, if these resources are not required for the child’s care, CPHM practitioners can provide the care that a child needs in a setting that is less disruptive to the family and typically more cost effective.
CPHM physicians are often drawn to a career in a community hospital because it allows them to use their entire skill set to care for children with a wide variety of conditions. As they are often the only physicians in an adult hospital with a full understanding of the unique aspects of care that children require, it is important that they be comfortable in their role of managing the majority of pediatric care independently. Yet they also need to understand the limitations of their own ability, as well as their institution’s level of expertise in pediatric-specific care. They must be confident and vocal advocates for pediatric-specific needs throughout their institution and its numerous committees, and form close working relationships with colleagues and administrators in the different fields with whom we share care of our patients (e.g., ED, obstetrics, radiology, trauma, and other medical and surgical subspecialties).
CPHM physicians are particularly well suited to partner with local outpatient providers as well as tertiary care physicians to provide coordinated transitions between the inpatient and outpatient management of a child’s illness. In addition, a CPHM physician can often bring a unique and valuable perspective of the particular ethnic, cultural, and socioeconomic diversity of their community, as well as its available resources, to facilitate a greater level of engagement with the child’s needs and ultimate success of their care.
The 2014 survey of CPHM programs identified several major challenges to recruitment and career satisfaction as a CPHM physician. These include a lack of access to subspecialists, a lack of pediatric-specific ancillary services and the perception that our importance as community hospital providers was not valued as much in the PHM community as PHM physicians working in a university/children’s hospital setting. With the recent recognition of PHM as an official subspecialty by the American Board of Pediatrics, the concern has intensified within our field that a two-tiered system will develop with some PHM physicians being board certified and others not.
While the development of board subspecialization was not meant to limit the pool of providers available to staff community hospital sites, there is nowhere near the number of fellowship trained physicians to provide an adequate workforce to staff CPHM programs. This means that many CPHM physicians will not be board certified in pediatric hospital medicine but does not mean that CPHM programs will be unable to provide high-quality local care that benefits children and their families, including safe care for children who require the skills that an immediately available CPHM physician can provide.
Many pediatric residency programs do not currently provide their trainees with exposure to community hospital medicine. Further, with increased sub-specialization throughout pediatrics, fewer residents are developing the necessary skill set to perform roles integral to a caring for children in community hospitals such as stabilization of a critically ill child prior to transport and complex neonatal resuscitation.
A career in CPHM provides physicians with the opportunity to work together with a close-knit group to provide exceptional care to children and to advocate for the medical needs of children in their hospital and their community. The AAP’s subcommittee has made it a priority to engage physicians during all parts of their pediatric training about why a career in CPHM is exciting, fulfilling and a great life, as well as continuing to educate training programs at every level – as well as the larger PHM community – about why CPHM is a valuable and important part of pediatric medicine.
Dr. Welsh is a clinical associate professor of pediatrics at the Stanford (Calif.) University in the division of pediatric hospital medicine. He has practiced community pediatric hospital medicine for over 27 years in Washington state and the San Francisco Bay Area. He is the chair of the working group of the Future of Community Pediatric Hospital Medicine for the AAP section on hospital medicine’s subcommittee on community hospitalists.
According to data from the American Academy of Pediatrics, over 2,000 physicians – or approximately 70% of all physicians practicing pediatric hospital medicine – do so in a community hospital. Like all areas of hospital medicine, community pediatric hospital medicine (CPHM) strives to fulfill one of our field’s central tenets – providing high-quality, evidence-based care to our patients.
A phrase often used among CPHM practitioners is that, “if you’ve seen one CPHM program, you’ve seen one CPHM program.” Every CPHM program is different. While this phrase may seem rather simplistic, it quite accurately portrays a unique aspect of our place in the hospital medicine field. CPHM programs usually require their practitioners to perform a broader range of roles and responsibilities than our colleagues who practice in university or children’s hospitals. Typically, these roles are aligned with the unique needs of each hospital within which we practice and the communities we serve. Factors such as the distance to a tertiary care referral center, access to subspecialists, availability and expertise of ancillary services for children, and the particular needs of each community further shape the role that CPHM practitioners may be asked to play.
In 2014, the AAP section on hospital medicine’s subcommittee on community hospitalists surveyed all CPHM programs to understand the unique roles that practitioners play within their institutions. Under the leadership of Clota Snow, MD, and Jacques Corriveau, MD, the aim was to contact every hospital in the country using the American Hospital Directory to see if they had a PHM program and to identify what roles the program was responsible for within their hospital.
Of the 535 programs identified, the primary responsibilities included inpatient care (85%), ED consultations (76%) and newborn nursery care (73%). Other common roles not typically associated with a university-based hospitalist’s responsibilities included delivery room attendance/neonatal resuscitations (44%), neonatal ICU management (47%) and subspecialty or surgical comanagement (52%). In some communities, even pediatric ICU management, sedation, and patient transport are part of our role. Because of the large breadth of roles that a CPHM practitioner may cover, we have often been referred to as “pediatric hospital-based generalists.”
Ideally, the presence of a pediatric hospitalist in a community hospital allows children to obtain high-quality, evidence-based care within their home communities. Most hospitalized children do not require direct access to subspecialists or all the pediatric-specific resources only available within a university or children’s hospital. Thus, if these resources are not required for the child’s care, CPHM practitioners can provide the care that a child needs in a setting that is less disruptive to the family and typically more cost effective.
CPHM physicians are often drawn to a career in a community hospital because it allows them to use their entire skill set to care for children with a wide variety of conditions. As they are often the only physicians in an adult hospital with a full understanding of the unique aspects of care that children require, it is important that they be comfortable in their role of managing the majority of pediatric care independently. Yet they also need to understand the limitations of their own ability, as well as their institution’s level of expertise in pediatric-specific care. They must be confident and vocal advocates for pediatric-specific needs throughout their institution and its numerous committees, and form close working relationships with colleagues and administrators in the different fields with whom we share care of our patients (e.g., ED, obstetrics, radiology, trauma, and other medical and surgical subspecialties).
CPHM physicians are particularly well suited to partner with local outpatient providers as well as tertiary care physicians to provide coordinated transitions between the inpatient and outpatient management of a child’s illness. In addition, a CPHM physician can often bring a unique and valuable perspective of the particular ethnic, cultural, and socioeconomic diversity of their community, as well as its available resources, to facilitate a greater level of engagement with the child’s needs and ultimate success of their care.
The 2014 survey of CPHM programs identified several major challenges to recruitment and career satisfaction as a CPHM physician. These include a lack of access to subspecialists, a lack of pediatric-specific ancillary services and the perception that our importance as community hospital providers was not valued as much in the PHM community as PHM physicians working in a university/children’s hospital setting. With the recent recognition of PHM as an official subspecialty by the American Board of Pediatrics, the concern has intensified within our field that a two-tiered system will develop with some PHM physicians being board certified and others not.
While the development of board subspecialization was not meant to limit the pool of providers available to staff community hospital sites, there is nowhere near the number of fellowship trained physicians to provide an adequate workforce to staff CPHM programs. This means that many CPHM physicians will not be board certified in pediatric hospital medicine but does not mean that CPHM programs will be unable to provide high-quality local care that benefits children and their families, including safe care for children who require the skills that an immediately available CPHM physician can provide.
Many pediatric residency programs do not currently provide their trainees with exposure to community hospital medicine. Further, with increased sub-specialization throughout pediatrics, fewer residents are developing the necessary skill set to perform roles integral to a caring for children in community hospitals such as stabilization of a critically ill child prior to transport and complex neonatal resuscitation.
A career in CPHM provides physicians with the opportunity to work together with a close-knit group to provide exceptional care to children and to advocate for the medical needs of children in their hospital and their community. The AAP’s subcommittee has made it a priority to engage physicians during all parts of their pediatric training about why a career in CPHM is exciting, fulfilling and a great life, as well as continuing to educate training programs at every level – as well as the larger PHM community – about why CPHM is a valuable and important part of pediatric medicine.
Dr. Welsh is a clinical associate professor of pediatrics at the Stanford (Calif.) University in the division of pediatric hospital medicine. He has practiced community pediatric hospital medicine for over 27 years in Washington state and the San Francisco Bay Area. He is the chair of the working group of the Future of Community Pediatric Hospital Medicine for the AAP section on hospital medicine’s subcommittee on community hospitalists.
Social isolation at the time of social distancing
Implications of loneliness and suggested management strategies in hospitalized patients with COVID-19
During a busy morning of rounds, our patient, Mrs. M., appeared distraught. She was diagnosed with COVID-19 2 weeks prior and remained inpatient because of medicosocial reasons. Since admission she remained on the same ward, in the same room, cared for by the same group of providers donned in masks, gowns, gloves, and face shields. The personal protective equipment helped to shield us from the virus, but it also shielded Mrs. M. from us.
During initial interaction, Mrs. M. appeared anxious, tearful, and detached. It seemed that she recognized a new voice; however, she did not express much interest in engaging during the visit. When she realized that she was not being discharged, Mrs. M. appeared to lose further interest. She wanted to go home. Her outpatient dialysis arrangements were not complete, and that precluded hospital discharge. Prescribed anxiolytics were doing little to relieve her symptoms.
The next day, Mrs. M. continued to ask if she could go home. She stated that there was nothing for her to do while in the hospital. She was tired of watching TV, she was unable to call her friends, and was not able to see her family. Because of COVID-19 status, Mrs. M was not permitted to leave her hospital room, and she was transported to the dialysis unit via stretcher, being unable to walk. The more we talked, the more engaged Mrs. M. had become. When it was time to complete the encounter, Mrs. M. started pleading with us to “stay a little longer, please don’t leave.”
Throughout her hospitalization, Mrs. M. had an extremely limited number of human encounters. Those encounters were fragmented and brief, centered on the infection mitigation. The chaplain was not permitted to enter her room, and she was unwilling to use the phone. The subspecialty consultants utilized telemedicine visits. As a result, Mrs. M. felt isolated and lonely. Social distancing in the hospital makes human interactions particularly challenging and contributes to the development of isolation, loneliness, and fear.
Loneliness is real
Loneliness is the “subjective experience of involuntary social isolation.”1 As the COVID-19 pandemic began to entrap the world in early 2020, many people have faced new challenges – loneliness and its impact on physical and mental health. The prevalence of loneliness nearly tripled in the early months of the pandemic, leading to psychological distress and reopening conversations on ethical issues.2
Ethical implications of loneliness
Social distancing challenges all four main ethical principles: autonomy, beneficence, nonmaleficence, and justice. How do we reconcile these principles from the standpoint of each affected individual, their caregivers, health care providers, and public health at large? How can we continue to mitigate the spread of COVID-19, but also remain attentive to our patients who are still in need of human interactions to recover and thrive?
Social distancing is important, but so is social interaction. What strategies do we have in place to combat loneliness? How do we help our hospitalized patients who feel connected to the “outside world?” Is battling loneliness worth the risks of additional exposure to COVID-19? These dilemmas cannot be easily resolved. However, it is important for us to recognize the negative impacts of loneliness and identify measures to help our patients.
In our mission to fulfill the beneficence and nonmaleficence principles of caring for patients affected by COVID-19, patients like Mrs. M. lose much of their autonomy during hospital admission. Despite our best efforts, our isolated patients during the pandemic, remain alone, which further heightens their feeling of loneliness.
Clinical implications of loneliness
With the advancements in technology, our capabilities to substitute personal human interactions have grown exponentially. The use of telemedicine, video- and audio-conferencing communications have changed the landscape of our capacities to exchange information. This could be a blessing and a curse. While the use of digital platforms for virtual communication is tempting, we should preserve human interactions as much as possible, particularly when caring for patients affected by COVID-19. Interpersonal “connectedness” plays a crucial role in providing psychological and psychotherapeutic support, particularly when the number of human encounters is already limited.
Social distancing requirements have magnified loneliness. Several studies demonstrate that the perception of loneliness leads to poor health outcomes, including lower immunity, increased peripheral vascular resistance,3 and higher overall mortality.4 Loneliness can lead to functional impairment, such as poor social skills, and even increased inflammation.5 The negative emotional impact of SARS-CoV-2 echoes the experiences of patients affected by the severe acute respiratory syndrome (SARS) outbreak in 2003. However, with COVID-19, we are witnessing the amplified effects of loneliness on a global scale. The majority of affected patients during the 2003 SARS outbreak in Canada reported loneliness, fear, aggression, and boredom: They had concerns about the impacts of the infection on loved ones, and psychological support was required for many patients with mild to moderate SARS disease.6
Nonpharmacological management strategies for battling loneliness
Utilization of early supportive services has been well described in literature and includes extending additional resources such as books, newspapers and, most importantly, additional in-person time to our patients.6 Maintaining rapport with patients’ families is also helpful in reducing anxiety and fear. The following measures have been suggested to prevent the negative impacts of loneliness and should be considered when caring for hospitalized patients diagnosed with COVID-19.7
- Screen patients for depression and delirium and utilize delirium prevention measures throughout the hospitalization.
- Educate patients about the signs and symptoms of loneliness, fear, and anxiety.
- Extend additional resources to patients, including books, magazines, and newspapers.
- Keep the patient’s cell or hospital phone within their reach.
- Adequately manage pain and prevent insomnia.
- Communicate frequently, utilizing audio- and visual-teleconferencing platforms that simultaneously include the patient and their loved ones.
- For patients who continue to exhibit feelings of loneliness despite the above interventions, consider consultations with psychiatry to offer additional coping strategies.
- Ensure a multidisciplinary approach when applicable – proactive consultation with the members of a palliative care team, ethics, spiritual health, social and ancillary services.
It is important to recognize how vulnerable our patients are. Diagnosed with COVID-19, and caught in the midst of the current pandemic, not only do they suffer from the physical effects of this novel disease, but they also have to endure prolonged confinement, social isolation, and uncertainty – all wrapped in a cloak of loneliness and fear.
With our main focus being on the management of a largely unknown viral illness, patients’ personal experiences can be easily overlooked. It is vital for us as health care providers on the front lines to recognize, reflect, and reform to ease our patients’ journey through COVID-19.
Dr. Burklin is an assistant professor of medicine, division of hospital medicine, at the department of medicine, Emory University, Atlanta. Dr. Wiley is an assistant professor of medicine, division of infectious disease, at the department of Medicine, Emory University, Atlanta.
References
1. Schlomann A et al. Use of information and communication technology (ICT) devices among the oldest-old: Loneliness, anomie, and autonomy. Innov Aging. 2020 Jan 1;4(2):igz050.
2. McGinty E et al. Psychological distress and loneliness reported by U.S. adults in 2018 and April 2020. JAMA. 2020 Jun 3. doi: 10.1001/jama.2020.9740. 3. Wang J et al. Associations between loneliness and perceived social support and outcomes of mental health problems: A systematic review. BMC Psychiatry. 2018 May 29;18(1):156.
4. Luo Y et al. Loneliness, health, and mortality in old age: A national longitudinal study. Soc Sci Med. 2012 Mar;74(6):907-14.
5. Smith KJ et al. The association between loneliness, social isolation, and inflammation: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020 Feb 21; 112:519-41.
6. Maunder R et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003 May 13;168(10):1245-51.
7. Masi CM et al. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011 Aug;15(3):219-66.
Implications of loneliness and suggested management strategies in hospitalized patients with COVID-19
Implications of loneliness and suggested management strategies in hospitalized patients with COVID-19
During a busy morning of rounds, our patient, Mrs. M., appeared distraught. She was diagnosed with COVID-19 2 weeks prior and remained inpatient because of medicosocial reasons. Since admission she remained on the same ward, in the same room, cared for by the same group of providers donned in masks, gowns, gloves, and face shields. The personal protective equipment helped to shield us from the virus, but it also shielded Mrs. M. from us.
During initial interaction, Mrs. M. appeared anxious, tearful, and detached. It seemed that she recognized a new voice; however, she did not express much interest in engaging during the visit. When she realized that she was not being discharged, Mrs. M. appeared to lose further interest. She wanted to go home. Her outpatient dialysis arrangements were not complete, and that precluded hospital discharge. Prescribed anxiolytics were doing little to relieve her symptoms.
The next day, Mrs. M. continued to ask if she could go home. She stated that there was nothing for her to do while in the hospital. She was tired of watching TV, she was unable to call her friends, and was not able to see her family. Because of COVID-19 status, Mrs. M was not permitted to leave her hospital room, and she was transported to the dialysis unit via stretcher, being unable to walk. The more we talked, the more engaged Mrs. M. had become. When it was time to complete the encounter, Mrs. M. started pleading with us to “stay a little longer, please don’t leave.”
Throughout her hospitalization, Mrs. M. had an extremely limited number of human encounters. Those encounters were fragmented and brief, centered on the infection mitigation. The chaplain was not permitted to enter her room, and she was unwilling to use the phone. The subspecialty consultants utilized telemedicine visits. As a result, Mrs. M. felt isolated and lonely. Social distancing in the hospital makes human interactions particularly challenging and contributes to the development of isolation, loneliness, and fear.
Loneliness is real
Loneliness is the “subjective experience of involuntary social isolation.”1 As the COVID-19 pandemic began to entrap the world in early 2020, many people have faced new challenges – loneliness and its impact on physical and mental health. The prevalence of loneliness nearly tripled in the early months of the pandemic, leading to psychological distress and reopening conversations on ethical issues.2
Ethical implications of loneliness
Social distancing challenges all four main ethical principles: autonomy, beneficence, nonmaleficence, and justice. How do we reconcile these principles from the standpoint of each affected individual, their caregivers, health care providers, and public health at large? How can we continue to mitigate the spread of COVID-19, but also remain attentive to our patients who are still in need of human interactions to recover and thrive?
Social distancing is important, but so is social interaction. What strategies do we have in place to combat loneliness? How do we help our hospitalized patients who feel connected to the “outside world?” Is battling loneliness worth the risks of additional exposure to COVID-19? These dilemmas cannot be easily resolved. However, it is important for us to recognize the negative impacts of loneliness and identify measures to help our patients.
In our mission to fulfill the beneficence and nonmaleficence principles of caring for patients affected by COVID-19, patients like Mrs. M. lose much of their autonomy during hospital admission. Despite our best efforts, our isolated patients during the pandemic, remain alone, which further heightens their feeling of loneliness.
Clinical implications of loneliness
With the advancements in technology, our capabilities to substitute personal human interactions have grown exponentially. The use of telemedicine, video- and audio-conferencing communications have changed the landscape of our capacities to exchange information. This could be a blessing and a curse. While the use of digital platforms for virtual communication is tempting, we should preserve human interactions as much as possible, particularly when caring for patients affected by COVID-19. Interpersonal “connectedness” plays a crucial role in providing psychological and psychotherapeutic support, particularly when the number of human encounters is already limited.
Social distancing requirements have magnified loneliness. Several studies demonstrate that the perception of loneliness leads to poor health outcomes, including lower immunity, increased peripheral vascular resistance,3 and higher overall mortality.4 Loneliness can lead to functional impairment, such as poor social skills, and even increased inflammation.5 The negative emotional impact of SARS-CoV-2 echoes the experiences of patients affected by the severe acute respiratory syndrome (SARS) outbreak in 2003. However, with COVID-19, we are witnessing the amplified effects of loneliness on a global scale. The majority of affected patients during the 2003 SARS outbreak in Canada reported loneliness, fear, aggression, and boredom: They had concerns about the impacts of the infection on loved ones, and psychological support was required for many patients with mild to moderate SARS disease.6
Nonpharmacological management strategies for battling loneliness
Utilization of early supportive services has been well described in literature and includes extending additional resources such as books, newspapers and, most importantly, additional in-person time to our patients.6 Maintaining rapport with patients’ families is also helpful in reducing anxiety and fear. The following measures have been suggested to prevent the negative impacts of loneliness and should be considered when caring for hospitalized patients diagnosed with COVID-19.7
- Screen patients for depression and delirium and utilize delirium prevention measures throughout the hospitalization.
- Educate patients about the signs and symptoms of loneliness, fear, and anxiety.
- Extend additional resources to patients, including books, magazines, and newspapers.
- Keep the patient’s cell or hospital phone within their reach.
- Adequately manage pain and prevent insomnia.
- Communicate frequently, utilizing audio- and visual-teleconferencing platforms that simultaneously include the patient and their loved ones.
- For patients who continue to exhibit feelings of loneliness despite the above interventions, consider consultations with psychiatry to offer additional coping strategies.
- Ensure a multidisciplinary approach when applicable – proactive consultation with the members of a palliative care team, ethics, spiritual health, social and ancillary services.
It is important to recognize how vulnerable our patients are. Diagnosed with COVID-19, and caught in the midst of the current pandemic, not only do they suffer from the physical effects of this novel disease, but they also have to endure prolonged confinement, social isolation, and uncertainty – all wrapped in a cloak of loneliness and fear.
With our main focus being on the management of a largely unknown viral illness, patients’ personal experiences can be easily overlooked. It is vital for us as health care providers on the front lines to recognize, reflect, and reform to ease our patients’ journey through COVID-19.
Dr. Burklin is an assistant professor of medicine, division of hospital medicine, at the department of medicine, Emory University, Atlanta. Dr. Wiley is an assistant professor of medicine, division of infectious disease, at the department of Medicine, Emory University, Atlanta.
References
1. Schlomann A et al. Use of information and communication technology (ICT) devices among the oldest-old: Loneliness, anomie, and autonomy. Innov Aging. 2020 Jan 1;4(2):igz050.
2. McGinty E et al. Psychological distress and loneliness reported by U.S. adults in 2018 and April 2020. JAMA. 2020 Jun 3. doi: 10.1001/jama.2020.9740. 3. Wang J et al. Associations between loneliness and perceived social support and outcomes of mental health problems: A systematic review. BMC Psychiatry. 2018 May 29;18(1):156.
4. Luo Y et al. Loneliness, health, and mortality in old age: A national longitudinal study. Soc Sci Med. 2012 Mar;74(6):907-14.
5. Smith KJ et al. The association between loneliness, social isolation, and inflammation: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020 Feb 21; 112:519-41.
6. Maunder R et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003 May 13;168(10):1245-51.
7. Masi CM et al. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011 Aug;15(3):219-66.
During a busy morning of rounds, our patient, Mrs. M., appeared distraught. She was diagnosed with COVID-19 2 weeks prior and remained inpatient because of medicosocial reasons. Since admission she remained on the same ward, in the same room, cared for by the same group of providers donned in masks, gowns, gloves, and face shields. The personal protective equipment helped to shield us from the virus, but it also shielded Mrs. M. from us.
During initial interaction, Mrs. M. appeared anxious, tearful, and detached. It seemed that she recognized a new voice; however, she did not express much interest in engaging during the visit. When she realized that she was not being discharged, Mrs. M. appeared to lose further interest. She wanted to go home. Her outpatient dialysis arrangements were not complete, and that precluded hospital discharge. Prescribed anxiolytics were doing little to relieve her symptoms.
The next day, Mrs. M. continued to ask if she could go home. She stated that there was nothing for her to do while in the hospital. She was tired of watching TV, she was unable to call her friends, and was not able to see her family. Because of COVID-19 status, Mrs. M was not permitted to leave her hospital room, and she was transported to the dialysis unit via stretcher, being unable to walk. The more we talked, the more engaged Mrs. M. had become. When it was time to complete the encounter, Mrs. M. started pleading with us to “stay a little longer, please don’t leave.”
Throughout her hospitalization, Mrs. M. had an extremely limited number of human encounters. Those encounters were fragmented and brief, centered on the infection mitigation. The chaplain was not permitted to enter her room, and she was unwilling to use the phone. The subspecialty consultants utilized telemedicine visits. As a result, Mrs. M. felt isolated and lonely. Social distancing in the hospital makes human interactions particularly challenging and contributes to the development of isolation, loneliness, and fear.
Loneliness is real
Loneliness is the “subjective experience of involuntary social isolation.”1 As the COVID-19 pandemic began to entrap the world in early 2020, many people have faced new challenges – loneliness and its impact on physical and mental health. The prevalence of loneliness nearly tripled in the early months of the pandemic, leading to psychological distress and reopening conversations on ethical issues.2
Ethical implications of loneliness
Social distancing challenges all four main ethical principles: autonomy, beneficence, nonmaleficence, and justice. How do we reconcile these principles from the standpoint of each affected individual, their caregivers, health care providers, and public health at large? How can we continue to mitigate the spread of COVID-19, but also remain attentive to our patients who are still in need of human interactions to recover and thrive?
Social distancing is important, but so is social interaction. What strategies do we have in place to combat loneliness? How do we help our hospitalized patients who feel connected to the “outside world?” Is battling loneliness worth the risks of additional exposure to COVID-19? These dilemmas cannot be easily resolved. However, it is important for us to recognize the negative impacts of loneliness and identify measures to help our patients.
In our mission to fulfill the beneficence and nonmaleficence principles of caring for patients affected by COVID-19, patients like Mrs. M. lose much of their autonomy during hospital admission. Despite our best efforts, our isolated patients during the pandemic, remain alone, which further heightens their feeling of loneliness.
Clinical implications of loneliness
With the advancements in technology, our capabilities to substitute personal human interactions have grown exponentially. The use of telemedicine, video- and audio-conferencing communications have changed the landscape of our capacities to exchange information. This could be a blessing and a curse. While the use of digital platforms for virtual communication is tempting, we should preserve human interactions as much as possible, particularly when caring for patients affected by COVID-19. Interpersonal “connectedness” plays a crucial role in providing psychological and psychotherapeutic support, particularly when the number of human encounters is already limited.
Social distancing requirements have magnified loneliness. Several studies demonstrate that the perception of loneliness leads to poor health outcomes, including lower immunity, increased peripheral vascular resistance,3 and higher overall mortality.4 Loneliness can lead to functional impairment, such as poor social skills, and even increased inflammation.5 The negative emotional impact of SARS-CoV-2 echoes the experiences of patients affected by the severe acute respiratory syndrome (SARS) outbreak in 2003. However, with COVID-19, we are witnessing the amplified effects of loneliness on a global scale. The majority of affected patients during the 2003 SARS outbreak in Canada reported loneliness, fear, aggression, and boredom: They had concerns about the impacts of the infection on loved ones, and psychological support was required for many patients with mild to moderate SARS disease.6
Nonpharmacological management strategies for battling loneliness
Utilization of early supportive services has been well described in literature and includes extending additional resources such as books, newspapers and, most importantly, additional in-person time to our patients.6 Maintaining rapport with patients’ families is also helpful in reducing anxiety and fear. The following measures have been suggested to prevent the negative impacts of loneliness and should be considered when caring for hospitalized patients diagnosed with COVID-19.7
- Screen patients for depression and delirium and utilize delirium prevention measures throughout the hospitalization.
- Educate patients about the signs and symptoms of loneliness, fear, and anxiety.
- Extend additional resources to patients, including books, magazines, and newspapers.
- Keep the patient’s cell or hospital phone within their reach.
- Adequately manage pain and prevent insomnia.
- Communicate frequently, utilizing audio- and visual-teleconferencing platforms that simultaneously include the patient and their loved ones.
- For patients who continue to exhibit feelings of loneliness despite the above interventions, consider consultations with psychiatry to offer additional coping strategies.
- Ensure a multidisciplinary approach when applicable – proactive consultation with the members of a palliative care team, ethics, spiritual health, social and ancillary services.
It is important to recognize how vulnerable our patients are. Diagnosed with COVID-19, and caught in the midst of the current pandemic, not only do they suffer from the physical effects of this novel disease, but they also have to endure prolonged confinement, social isolation, and uncertainty – all wrapped in a cloak of loneliness and fear.
With our main focus being on the management of a largely unknown viral illness, patients’ personal experiences can be easily overlooked. It is vital for us as health care providers on the front lines to recognize, reflect, and reform to ease our patients’ journey through COVID-19.
Dr. Burklin is an assistant professor of medicine, division of hospital medicine, at the department of medicine, Emory University, Atlanta. Dr. Wiley is an assistant professor of medicine, division of infectious disease, at the department of Medicine, Emory University, Atlanta.
References
1. Schlomann A et al. Use of information and communication technology (ICT) devices among the oldest-old: Loneliness, anomie, and autonomy. Innov Aging. 2020 Jan 1;4(2):igz050.
2. McGinty E et al. Psychological distress and loneliness reported by U.S. adults in 2018 and April 2020. JAMA. 2020 Jun 3. doi: 10.1001/jama.2020.9740. 3. Wang J et al. Associations between loneliness and perceived social support and outcomes of mental health problems: A systematic review. BMC Psychiatry. 2018 May 29;18(1):156.
4. Luo Y et al. Loneliness, health, and mortality in old age: A national longitudinal study. Soc Sci Med. 2012 Mar;74(6):907-14.
5. Smith KJ et al. The association between loneliness, social isolation, and inflammation: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020 Feb 21; 112:519-41.
6. Maunder R et al. The immediate psychological and occupational impact of the 2003 SARS outbreak in a teaching hospital. CMAJ. 2003 May 13;168(10):1245-51.
7. Masi CM et al. A meta-analysis of interventions to reduce loneliness. Pers Soc Psychol Rev. 2011 Aug;15(3):219-66.
Racism in medicine: Implicit and explicit
With the shootings of Breonna Taylor, George Floyd, and other Black citizens setting off protests and unrest, race was at the forefront of national conversation in the United States – along with COVID-19 – over the past year.
“We’ve heard things like, ‘We’re in a post-racial society,’ but I think 2020 in particular has emphasized that we’re not,” said Gregory Johnson, MD, SFHM, chief medical officer of hospital medicine at Sound Physicians, a national physician practice. “Racism is very present in our lives, it’s very present in our world, and it is absolutely present in medicine.”
Yes, race is still an issue in the U.S. as we head into 2021, though this may have come as something of a surprise to people who do not live with racism daily.
“If you have a brain, you have bias, and that bias will likely apply to race as well,” Dr. Johnson said. “When we’re talking about institutional racism, the educational system and the media have led us to create presumptions and prejudices that we don’t necessarily recognize off the top because they’ve just been a part of the fabric of who we are as we’ve grown up.”
The term “racism” has extremely negative connotations because there’s character judgment attached to it, but to say someone is racist or racially insensitive does not equate them with being a Klansman, said Dr. Johnson. “I think we as people have to acknowledge that, yes, it’s possible for me to be racist and I might not be 100% aware of it. It’s being open to the possibility – or rather probability – that you are and then taking steps to figure out how you can address that, so you can limit it. And that requires constant self-evaluation and work,” he said.
Racism in the medical environment
Institutional racism is evident before students are even accepted into medical school, said Areeba Kara, MD, SFHM, associate professor of clinical medicine at Indiana University, Indianapolis, and a hospitalist at IU Health Physicians.
Mean MCAT scores are lower for applicants traditionally underrepresented in medicine (UIM) compared to the scores of well-represented groups.1 “Lower scores are associated with lower acceptance rates into medical school,” Dr. Kara said. “These differences reflect unequal educational opportunities rooted in centuries of legal discrimination.”
Racism is apparent in both the hidden medical education curriculum and in lessons implicitly taught to students, said Ndidi Unaka, MD, MEd, associate program director of the pediatric residency training program at Cincinnati Children’s Hospital.
“These lessons inform the way in which we as physicians see our patients, each other, and how we practice,” she said. “We reinforce race-based medicine and shape clinical decision making through flawed guidelines and practices, which exacerbates health inequities. We teach that race – rather than racism – is a risk factor for poor health outcomes. Our students and trainees watch as we assume the worst of our patients from marginalized communities of color.”
Terms describing patients of color, such as “difficult,” “non-compliant,” or “frequent flyer” are thrown around and sometimes, instead of finding out why, “we view these states of being as static, root causes for poor outcomes rather than symptoms of social conditions and obstacles that impact overall health and wellbeing,” Dr. Unaka said.
Leadership opportunities
Though hospital medicine is a growing field, Dr. Kara noted that the 2020 State of Hospital Medicine Report found that only 5.5% of hospital medical group leaders were Black, and just 2.2% were Hispanic/Latino.2 “I think these numbers speak for themselves,” she said.
Dr. Unaka said that the lack of UIM hospitalists and physician leaders creates fewer opportunities for “race-concordant mentorship relationships.” It also forces UIM physicians to shoulder more responsibilities – often obligations that do little to help them move forward in their careers – all in the name of diversity. And when UIM physicians are given leadership opportunities, Dr. Unaka said they are often unsure as to whether their appointments are genuine or just a hollow gesture made for the sake of diversity.
Dr. Johnson pointed out that Black and Latinx populations primarily get their care from hospital-based specialties, yet this is not reflected in the number of UIM practitioners in leadership roles. He said race and ethnicity, as well as gender, need to be factors when individuals are evaluated for leadership opportunities – for the individual’s sake, as well as for the community he or she is serving.
“When we can evaluate for unconscious bias and factor in that diverse groups tend to have better outcomes, whether it’s business or clinical outcomes, it’s one of the opportunities that we collectively have in the specialty to improve what we’re delivering for hospitals and, more importantly, for patients,” he said.
Relationships with colleagues and patients
Racism creeps into interactions and relationships with others as well, whether it’s between clinicians, clinician to patient, or patient to clinician. Sometimes it’s blatant; often it’s subtle.
A common, recurring example Dr. Unaka has experienced in the clinician to clinician relationship is being confused for other Black physicians, making her feel invisible. “The everyday verbal, nonverbal, and environmental slights, snubs, or insults from colleagues are frequent and contribute to feelings of exclusion, isolation, and exhaustion,” she said. Despite this, she is still expected to “address microaggressions and other forms of interpersonal racism and find ways to move through professional spaces in spite of the trauma, fear, and stress associated with my reality and lived experiences.” She said that clinicians who remain silent on the topic of racism participate in the violence and contribute to the disillusionment of UIM physicians.
Dr. Kara said that the discrimination from the health care team is the hardest to deal with. In the clinician to clinician relationship, there is a sense among UIM physicians that they’re being watched more closely and “have to prove themselves at every single turn.” Unfortunately, this comes from the environment, which tends to be adversarial rather than supportive and nurturing, she said.
“There are lots of opportunities for racism or racial insensitivity to crop up from clinician to clinician,” said Dr. Johnson. When he started his career as a physician after his training, Dr. Johnson was informed that his colleagues were watching him because they were not sure about his clinical skills. The fact that he was a former chief resident and board certified in two specialties did not seem to make any difference.
Patients refusing care from UIM physicians or expressing disapproval – both verbal and nonverbal – of such care, happens all too often. “It’s easier for me to excuse patients and their families as we often meet them on their worst days,” said Dr. Kara. Still, “understanding my oath to care for people and do no harm, but at the same time, recognizing that this is an individual that is rejecting my care without having any idea of who I am as a physician is frustrating,” Dr. Johnson acknowledged.
Then there’s the complex clinician to patient relationship, which research clearly shows contributes to health disparities.3 For one thing, the physician workforce does not reflect the patient population, Dr. Unaka said. “We cannot ignore the lack of race concordance between patients and clinicians, nor can the continued misplacement of blame for medical mistrust be at the feet of our patients,” she said.
Dr. Unaka feels that clinicians need to accept both that health inequities exist and that frontline physicians themselves contribute to the inequities. “Our diagnostic and therapeutic decisions are not immune to bias and are influenced by our deeply held beliefs about specific populations,” she said. “And the health care system that our patients navigate is no different than other systems, settings, and environments that are marred by racism in all its forms.”
Systemic racism greatly impacts patient care, said Dr. Kara. She pointed to several examples: Research showing that race concordance between patients and providers in an emergency department setting led to better pain control with fewer analgesics.4 The high maternal and infant mortality rates amongst Black women and children.5 Evidence of poorer outcomes in sepsis patients with limited English proficiency.6 “There are plenty more,” she said. “We need to be asking ourselves what we are going to do about it.”
Moving forward
That racial biases are steeped so thoroughly into our culture and consciousness means that moving beyond them is a continual, purposeful work in progress. But it is work that is critical for everyone, and certainly necessary for those who care for their fellow human beings when they are in a vulnerable state.
Health care systems need to move toward equity – giving everyone what they need to thrive – rather than focusing on equality – giving everyone the same thing, said Jenny Baenziger, MD, assistant professor of clinical medicine and pediatrics at Indiana University, Indianapolis, and associate director of education at IU Center for Global Health. “We know that minoritized patients are going to need more attention, more advocacy, more sensitivity, and more creative solutions in order to help them achieve health in a world that is often stacked against them,” she said.
“The unique needs of each patient, family unit, and/or population must be taken into consideration,” said Dr. Unaka. She said hospitalists need to embrace creative approaches that can better serve the specific needs of patients. Equitable practices should be the default, which means data transparency, thoroughly dissecting hospital processes to find existing inequities, giving stakeholders – especially patients and families of color – a voice, and tearing down oppressive systems that contribute to poor health outcomes and oppression, she said.
“It’s time for us to talk about racism openly,” said Dr. Kara. “Believe your colleagues when they share their fears and treat each other with respect. We should be actively learning about and celebrating our diversity.” She encourages finding out what your institution is doing on this front and getting involved.
Dr. Johnson believes that first and foremost, hospitalists need to be exposed to the data on health care disparities. “The next step is asking what we as hospitalists, or any other specialty, can do to intervene and improve in those areas,” he said. Focusing on unconscious bias training is important, he said, so clinicians can see what biases they might be bringing into the hospital and to the bedside. Maintaining a diverse workforce and bringing UIM physicians into leadership roles to encourage diversity of ideas and approaches are also critical to promoting equity, he said.
“You cannot fix what you cannot face,” said Dr. Unaka. Education on how racism impacts patients and colleagues is essential, she believes, as is advocacy for changing inequitable health system policies. She recommends expanding social and professional circles. “Diverse social groups allow us to consider the perspectives of others; diverse professional groups allow us to ask better research questions and practice better medicine.”
Start by developing the ability to question personal assumptions and pinpoint implicit biases, suggested Dr. Baenziger. “Asking for feedback can be scary and difficult, but we should take a deep breath and do it anyway,” she said. “Simply ask your team, ‘I’ve been thinking a lot about racial equity and disparities. How can I do better at my interactions with people of color? What are my blind spots?’” Dr. Baenziger said that “to help us remember how beautifully complicated and diverse people are,” all health care professionals need to watch Nigerian novelist Chimamanda Ngozi Adichie’s TED talk “The Danger of a Single Story.”
Dr. Baenziger also stressed the importance of conversations about “places where race is built into our clinical assessments, like eGFR,” as well as being aware that many of the research studies that are used to support everyday clinical decisions didn’t include people of color. She also encouraged clinicians to consider how and when they include race in their notes.7 “Is it really helpful to make sure people know right away that you are treating a ‘46-year-old Hispanic male’ or can the fact that he is Hispanic be saved for the social history section with other important details of his life such as being a father, veteran, and mechanic?” she asked.
“Racism is real and very much a part of our history. We can no longer be in denial regarding the racism that exists in medicine and the impact it has on our patients,” Dr. Unaka said. “As a profession, we cannot hide behind our espoused core values. We must live up to them.”
References
1. Lucey CR, Saguil, A. The Consequences of Structural Racism on MCAT Scores and Medical School Admissions: The Past Is Prologue. Acad Med. 2020 Mar;95(3):351-356. doi: 10.1097/ACM.0000000000002939.
2. Flores L. Increasing racial diversity in hospital medicine’s leadership ranks. The Hospitalist. 2020 Oct 21.
3. Smedley BD, et al, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Institute of Medicine Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Washington: National Academies Press; 2003.
4. Heins A, et al. Physician Race/Ethnicity Predicts Successful Emergency Department Analgesia. J Pain. 2010 July;11(7):692-697. doi: 10.1016/j.jpain.2009.10.017.
5. U.S. Department of Health and Human Serves, Office of Minority Health. Infant Mortality and African Americans. 2019 Nov 8. minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=23.
6. Jacobs ZG, et al. The Association between Limited English Proficiency and Sepsis Mortality. J Hosp Med. 2020;3;140-146. Published Online First 2019 Nov 20. doi:10.12788/jhm.3334.
7. Finucane TE. Mention of a Patient’s “Race” in Clinical Presentations. Virtual Mentor. 2014;16(6):423-427. doi: 10.1001/virtualmentor.2014.16.6.ecas1-1406.
With the shootings of Breonna Taylor, George Floyd, and other Black citizens setting off protests and unrest, race was at the forefront of national conversation in the United States – along with COVID-19 – over the past year.
“We’ve heard things like, ‘We’re in a post-racial society,’ but I think 2020 in particular has emphasized that we’re not,” said Gregory Johnson, MD, SFHM, chief medical officer of hospital medicine at Sound Physicians, a national physician practice. “Racism is very present in our lives, it’s very present in our world, and it is absolutely present in medicine.”
Yes, race is still an issue in the U.S. as we head into 2021, though this may have come as something of a surprise to people who do not live with racism daily.
“If you have a brain, you have bias, and that bias will likely apply to race as well,” Dr. Johnson said. “When we’re talking about institutional racism, the educational system and the media have led us to create presumptions and prejudices that we don’t necessarily recognize off the top because they’ve just been a part of the fabric of who we are as we’ve grown up.”
The term “racism” has extremely negative connotations because there’s character judgment attached to it, but to say someone is racist or racially insensitive does not equate them with being a Klansman, said Dr. Johnson. “I think we as people have to acknowledge that, yes, it’s possible for me to be racist and I might not be 100% aware of it. It’s being open to the possibility – or rather probability – that you are and then taking steps to figure out how you can address that, so you can limit it. And that requires constant self-evaluation and work,” he said.
Racism in the medical environment
Institutional racism is evident before students are even accepted into medical school, said Areeba Kara, MD, SFHM, associate professor of clinical medicine at Indiana University, Indianapolis, and a hospitalist at IU Health Physicians.
Mean MCAT scores are lower for applicants traditionally underrepresented in medicine (UIM) compared to the scores of well-represented groups.1 “Lower scores are associated with lower acceptance rates into medical school,” Dr. Kara said. “These differences reflect unequal educational opportunities rooted in centuries of legal discrimination.”
Racism is apparent in both the hidden medical education curriculum and in lessons implicitly taught to students, said Ndidi Unaka, MD, MEd, associate program director of the pediatric residency training program at Cincinnati Children’s Hospital.
“These lessons inform the way in which we as physicians see our patients, each other, and how we practice,” she said. “We reinforce race-based medicine and shape clinical decision making through flawed guidelines and practices, which exacerbates health inequities. We teach that race – rather than racism – is a risk factor for poor health outcomes. Our students and trainees watch as we assume the worst of our patients from marginalized communities of color.”
Terms describing patients of color, such as “difficult,” “non-compliant,” or “frequent flyer” are thrown around and sometimes, instead of finding out why, “we view these states of being as static, root causes for poor outcomes rather than symptoms of social conditions and obstacles that impact overall health and wellbeing,” Dr. Unaka said.
Leadership opportunities
Though hospital medicine is a growing field, Dr. Kara noted that the 2020 State of Hospital Medicine Report found that only 5.5% of hospital medical group leaders were Black, and just 2.2% were Hispanic/Latino.2 “I think these numbers speak for themselves,” she said.
Dr. Unaka said that the lack of UIM hospitalists and physician leaders creates fewer opportunities for “race-concordant mentorship relationships.” It also forces UIM physicians to shoulder more responsibilities – often obligations that do little to help them move forward in their careers – all in the name of diversity. And when UIM physicians are given leadership opportunities, Dr. Unaka said they are often unsure as to whether their appointments are genuine or just a hollow gesture made for the sake of diversity.
Dr. Johnson pointed out that Black and Latinx populations primarily get their care from hospital-based specialties, yet this is not reflected in the number of UIM practitioners in leadership roles. He said race and ethnicity, as well as gender, need to be factors when individuals are evaluated for leadership opportunities – for the individual’s sake, as well as for the community he or she is serving.
“When we can evaluate for unconscious bias and factor in that diverse groups tend to have better outcomes, whether it’s business or clinical outcomes, it’s one of the opportunities that we collectively have in the specialty to improve what we’re delivering for hospitals and, more importantly, for patients,” he said.
Relationships with colleagues and patients
Racism creeps into interactions and relationships with others as well, whether it’s between clinicians, clinician to patient, or patient to clinician. Sometimes it’s blatant; often it’s subtle.
A common, recurring example Dr. Unaka has experienced in the clinician to clinician relationship is being confused for other Black physicians, making her feel invisible. “The everyday verbal, nonverbal, and environmental slights, snubs, or insults from colleagues are frequent and contribute to feelings of exclusion, isolation, and exhaustion,” she said. Despite this, she is still expected to “address microaggressions and other forms of interpersonal racism and find ways to move through professional spaces in spite of the trauma, fear, and stress associated with my reality and lived experiences.” She said that clinicians who remain silent on the topic of racism participate in the violence and contribute to the disillusionment of UIM physicians.
Dr. Kara said that the discrimination from the health care team is the hardest to deal with. In the clinician to clinician relationship, there is a sense among UIM physicians that they’re being watched more closely and “have to prove themselves at every single turn.” Unfortunately, this comes from the environment, which tends to be adversarial rather than supportive and nurturing, she said.
“There are lots of opportunities for racism or racial insensitivity to crop up from clinician to clinician,” said Dr. Johnson. When he started his career as a physician after his training, Dr. Johnson was informed that his colleagues were watching him because they were not sure about his clinical skills. The fact that he was a former chief resident and board certified in two specialties did not seem to make any difference.
Patients refusing care from UIM physicians or expressing disapproval – both verbal and nonverbal – of such care, happens all too often. “It’s easier for me to excuse patients and their families as we often meet them on their worst days,” said Dr. Kara. Still, “understanding my oath to care for people and do no harm, but at the same time, recognizing that this is an individual that is rejecting my care without having any idea of who I am as a physician is frustrating,” Dr. Johnson acknowledged.
Then there’s the complex clinician to patient relationship, which research clearly shows contributes to health disparities.3 For one thing, the physician workforce does not reflect the patient population, Dr. Unaka said. “We cannot ignore the lack of race concordance between patients and clinicians, nor can the continued misplacement of blame for medical mistrust be at the feet of our patients,” she said.
Dr. Unaka feels that clinicians need to accept both that health inequities exist and that frontline physicians themselves contribute to the inequities. “Our diagnostic and therapeutic decisions are not immune to bias and are influenced by our deeply held beliefs about specific populations,” she said. “And the health care system that our patients navigate is no different than other systems, settings, and environments that are marred by racism in all its forms.”
Systemic racism greatly impacts patient care, said Dr. Kara. She pointed to several examples: Research showing that race concordance between patients and providers in an emergency department setting led to better pain control with fewer analgesics.4 The high maternal and infant mortality rates amongst Black women and children.5 Evidence of poorer outcomes in sepsis patients with limited English proficiency.6 “There are plenty more,” she said. “We need to be asking ourselves what we are going to do about it.”
Moving forward
That racial biases are steeped so thoroughly into our culture and consciousness means that moving beyond them is a continual, purposeful work in progress. But it is work that is critical for everyone, and certainly necessary for those who care for their fellow human beings when they are in a vulnerable state.
Health care systems need to move toward equity – giving everyone what they need to thrive – rather than focusing on equality – giving everyone the same thing, said Jenny Baenziger, MD, assistant professor of clinical medicine and pediatrics at Indiana University, Indianapolis, and associate director of education at IU Center for Global Health. “We know that minoritized patients are going to need more attention, more advocacy, more sensitivity, and more creative solutions in order to help them achieve health in a world that is often stacked against them,” she said.
“The unique needs of each patient, family unit, and/or population must be taken into consideration,” said Dr. Unaka. She said hospitalists need to embrace creative approaches that can better serve the specific needs of patients. Equitable practices should be the default, which means data transparency, thoroughly dissecting hospital processes to find existing inequities, giving stakeholders – especially patients and families of color – a voice, and tearing down oppressive systems that contribute to poor health outcomes and oppression, she said.
“It’s time for us to talk about racism openly,” said Dr. Kara. “Believe your colleagues when they share their fears and treat each other with respect. We should be actively learning about and celebrating our diversity.” She encourages finding out what your institution is doing on this front and getting involved.
Dr. Johnson believes that first and foremost, hospitalists need to be exposed to the data on health care disparities. “The next step is asking what we as hospitalists, or any other specialty, can do to intervene and improve in those areas,” he said. Focusing on unconscious bias training is important, he said, so clinicians can see what biases they might be bringing into the hospital and to the bedside. Maintaining a diverse workforce and bringing UIM physicians into leadership roles to encourage diversity of ideas and approaches are also critical to promoting equity, he said.
“You cannot fix what you cannot face,” said Dr. Unaka. Education on how racism impacts patients and colleagues is essential, she believes, as is advocacy for changing inequitable health system policies. She recommends expanding social and professional circles. “Diverse social groups allow us to consider the perspectives of others; diverse professional groups allow us to ask better research questions and practice better medicine.”
Start by developing the ability to question personal assumptions and pinpoint implicit biases, suggested Dr. Baenziger. “Asking for feedback can be scary and difficult, but we should take a deep breath and do it anyway,” she said. “Simply ask your team, ‘I’ve been thinking a lot about racial equity and disparities. How can I do better at my interactions with people of color? What are my blind spots?’” Dr. Baenziger said that “to help us remember how beautifully complicated and diverse people are,” all health care professionals need to watch Nigerian novelist Chimamanda Ngozi Adichie’s TED talk “The Danger of a Single Story.”
Dr. Baenziger also stressed the importance of conversations about “places where race is built into our clinical assessments, like eGFR,” as well as being aware that many of the research studies that are used to support everyday clinical decisions didn’t include people of color. She also encouraged clinicians to consider how and when they include race in their notes.7 “Is it really helpful to make sure people know right away that you are treating a ‘46-year-old Hispanic male’ or can the fact that he is Hispanic be saved for the social history section with other important details of his life such as being a father, veteran, and mechanic?” she asked.
“Racism is real and very much a part of our history. We can no longer be in denial regarding the racism that exists in medicine and the impact it has on our patients,” Dr. Unaka said. “As a profession, we cannot hide behind our espoused core values. We must live up to them.”
References
1. Lucey CR, Saguil, A. The Consequences of Structural Racism on MCAT Scores and Medical School Admissions: The Past Is Prologue. Acad Med. 2020 Mar;95(3):351-356. doi: 10.1097/ACM.0000000000002939.
2. Flores L. Increasing racial diversity in hospital medicine’s leadership ranks. The Hospitalist. 2020 Oct 21.
3. Smedley BD, et al, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Institute of Medicine Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Washington: National Academies Press; 2003.
4. Heins A, et al. Physician Race/Ethnicity Predicts Successful Emergency Department Analgesia. J Pain. 2010 July;11(7):692-697. doi: 10.1016/j.jpain.2009.10.017.
5. U.S. Department of Health and Human Serves, Office of Minority Health. Infant Mortality and African Americans. 2019 Nov 8. minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=23.
6. Jacobs ZG, et al. The Association between Limited English Proficiency and Sepsis Mortality. J Hosp Med. 2020;3;140-146. Published Online First 2019 Nov 20. doi:10.12788/jhm.3334.
7. Finucane TE. Mention of a Patient’s “Race” in Clinical Presentations. Virtual Mentor. 2014;16(6):423-427. doi: 10.1001/virtualmentor.2014.16.6.ecas1-1406.
With the shootings of Breonna Taylor, George Floyd, and other Black citizens setting off protests and unrest, race was at the forefront of national conversation in the United States – along with COVID-19 – over the past year.
“We’ve heard things like, ‘We’re in a post-racial society,’ but I think 2020 in particular has emphasized that we’re not,” said Gregory Johnson, MD, SFHM, chief medical officer of hospital medicine at Sound Physicians, a national physician practice. “Racism is very present in our lives, it’s very present in our world, and it is absolutely present in medicine.”
Yes, race is still an issue in the U.S. as we head into 2021, though this may have come as something of a surprise to people who do not live with racism daily.
“If you have a brain, you have bias, and that bias will likely apply to race as well,” Dr. Johnson said. “When we’re talking about institutional racism, the educational system and the media have led us to create presumptions and prejudices that we don’t necessarily recognize off the top because they’ve just been a part of the fabric of who we are as we’ve grown up.”
The term “racism” has extremely negative connotations because there’s character judgment attached to it, but to say someone is racist or racially insensitive does not equate them with being a Klansman, said Dr. Johnson. “I think we as people have to acknowledge that, yes, it’s possible for me to be racist and I might not be 100% aware of it. It’s being open to the possibility – or rather probability – that you are and then taking steps to figure out how you can address that, so you can limit it. And that requires constant self-evaluation and work,” he said.
Racism in the medical environment
Institutional racism is evident before students are even accepted into medical school, said Areeba Kara, MD, SFHM, associate professor of clinical medicine at Indiana University, Indianapolis, and a hospitalist at IU Health Physicians.
Mean MCAT scores are lower for applicants traditionally underrepresented in medicine (UIM) compared to the scores of well-represented groups.1 “Lower scores are associated with lower acceptance rates into medical school,” Dr. Kara said. “These differences reflect unequal educational opportunities rooted in centuries of legal discrimination.”
Racism is apparent in both the hidden medical education curriculum and in lessons implicitly taught to students, said Ndidi Unaka, MD, MEd, associate program director of the pediatric residency training program at Cincinnati Children’s Hospital.
“These lessons inform the way in which we as physicians see our patients, each other, and how we practice,” she said. “We reinforce race-based medicine and shape clinical decision making through flawed guidelines and practices, which exacerbates health inequities. We teach that race – rather than racism – is a risk factor for poor health outcomes. Our students and trainees watch as we assume the worst of our patients from marginalized communities of color.”
Terms describing patients of color, such as “difficult,” “non-compliant,” or “frequent flyer” are thrown around and sometimes, instead of finding out why, “we view these states of being as static, root causes for poor outcomes rather than symptoms of social conditions and obstacles that impact overall health and wellbeing,” Dr. Unaka said.
Leadership opportunities
Though hospital medicine is a growing field, Dr. Kara noted that the 2020 State of Hospital Medicine Report found that only 5.5% of hospital medical group leaders were Black, and just 2.2% were Hispanic/Latino.2 “I think these numbers speak for themselves,” she said.
Dr. Unaka said that the lack of UIM hospitalists and physician leaders creates fewer opportunities for “race-concordant mentorship relationships.” It also forces UIM physicians to shoulder more responsibilities – often obligations that do little to help them move forward in their careers – all in the name of diversity. And when UIM physicians are given leadership opportunities, Dr. Unaka said they are often unsure as to whether their appointments are genuine or just a hollow gesture made for the sake of diversity.
Dr. Johnson pointed out that Black and Latinx populations primarily get their care from hospital-based specialties, yet this is not reflected in the number of UIM practitioners in leadership roles. He said race and ethnicity, as well as gender, need to be factors when individuals are evaluated for leadership opportunities – for the individual’s sake, as well as for the community he or she is serving.
“When we can evaluate for unconscious bias and factor in that diverse groups tend to have better outcomes, whether it’s business or clinical outcomes, it’s one of the opportunities that we collectively have in the specialty to improve what we’re delivering for hospitals and, more importantly, for patients,” he said.
Relationships with colleagues and patients
Racism creeps into interactions and relationships with others as well, whether it’s between clinicians, clinician to patient, or patient to clinician. Sometimes it’s blatant; often it’s subtle.
A common, recurring example Dr. Unaka has experienced in the clinician to clinician relationship is being confused for other Black physicians, making her feel invisible. “The everyday verbal, nonverbal, and environmental slights, snubs, or insults from colleagues are frequent and contribute to feelings of exclusion, isolation, and exhaustion,” she said. Despite this, she is still expected to “address microaggressions and other forms of interpersonal racism and find ways to move through professional spaces in spite of the trauma, fear, and stress associated with my reality and lived experiences.” She said that clinicians who remain silent on the topic of racism participate in the violence and contribute to the disillusionment of UIM physicians.
Dr. Kara said that the discrimination from the health care team is the hardest to deal with. In the clinician to clinician relationship, there is a sense among UIM physicians that they’re being watched more closely and “have to prove themselves at every single turn.” Unfortunately, this comes from the environment, which tends to be adversarial rather than supportive and nurturing, she said.
“There are lots of opportunities for racism or racial insensitivity to crop up from clinician to clinician,” said Dr. Johnson. When he started his career as a physician after his training, Dr. Johnson was informed that his colleagues were watching him because they were not sure about his clinical skills. The fact that he was a former chief resident and board certified in two specialties did not seem to make any difference.
Patients refusing care from UIM physicians or expressing disapproval – both verbal and nonverbal – of such care, happens all too often. “It’s easier for me to excuse patients and their families as we often meet them on their worst days,” said Dr. Kara. Still, “understanding my oath to care for people and do no harm, but at the same time, recognizing that this is an individual that is rejecting my care without having any idea of who I am as a physician is frustrating,” Dr. Johnson acknowledged.
Then there’s the complex clinician to patient relationship, which research clearly shows contributes to health disparities.3 For one thing, the physician workforce does not reflect the patient population, Dr. Unaka said. “We cannot ignore the lack of race concordance between patients and clinicians, nor can the continued misplacement of blame for medical mistrust be at the feet of our patients,” she said.
Dr. Unaka feels that clinicians need to accept both that health inequities exist and that frontline physicians themselves contribute to the inequities. “Our diagnostic and therapeutic decisions are not immune to bias and are influenced by our deeply held beliefs about specific populations,” she said. “And the health care system that our patients navigate is no different than other systems, settings, and environments that are marred by racism in all its forms.”
Systemic racism greatly impacts patient care, said Dr. Kara. She pointed to several examples: Research showing that race concordance between patients and providers in an emergency department setting led to better pain control with fewer analgesics.4 The high maternal and infant mortality rates amongst Black women and children.5 Evidence of poorer outcomes in sepsis patients with limited English proficiency.6 “There are plenty more,” she said. “We need to be asking ourselves what we are going to do about it.”
Moving forward
That racial biases are steeped so thoroughly into our culture and consciousness means that moving beyond them is a continual, purposeful work in progress. But it is work that is critical for everyone, and certainly necessary for those who care for their fellow human beings when they are in a vulnerable state.
Health care systems need to move toward equity – giving everyone what they need to thrive – rather than focusing on equality – giving everyone the same thing, said Jenny Baenziger, MD, assistant professor of clinical medicine and pediatrics at Indiana University, Indianapolis, and associate director of education at IU Center for Global Health. “We know that minoritized patients are going to need more attention, more advocacy, more sensitivity, and more creative solutions in order to help them achieve health in a world that is often stacked against them,” she said.
“The unique needs of each patient, family unit, and/or population must be taken into consideration,” said Dr. Unaka. She said hospitalists need to embrace creative approaches that can better serve the specific needs of patients. Equitable practices should be the default, which means data transparency, thoroughly dissecting hospital processes to find existing inequities, giving stakeholders – especially patients and families of color – a voice, and tearing down oppressive systems that contribute to poor health outcomes and oppression, she said.
“It’s time for us to talk about racism openly,” said Dr. Kara. “Believe your colleagues when they share their fears and treat each other with respect. We should be actively learning about and celebrating our diversity.” She encourages finding out what your institution is doing on this front and getting involved.
Dr. Johnson believes that first and foremost, hospitalists need to be exposed to the data on health care disparities. “The next step is asking what we as hospitalists, or any other specialty, can do to intervene and improve in those areas,” he said. Focusing on unconscious bias training is important, he said, so clinicians can see what biases they might be bringing into the hospital and to the bedside. Maintaining a diverse workforce and bringing UIM physicians into leadership roles to encourage diversity of ideas and approaches are also critical to promoting equity, he said.
“You cannot fix what you cannot face,” said Dr. Unaka. Education on how racism impacts patients and colleagues is essential, she believes, as is advocacy for changing inequitable health system policies. She recommends expanding social and professional circles. “Diverse social groups allow us to consider the perspectives of others; diverse professional groups allow us to ask better research questions and practice better medicine.”
Start by developing the ability to question personal assumptions and pinpoint implicit biases, suggested Dr. Baenziger. “Asking for feedback can be scary and difficult, but we should take a deep breath and do it anyway,” she said. “Simply ask your team, ‘I’ve been thinking a lot about racial equity and disparities. How can I do better at my interactions with people of color? What are my blind spots?’” Dr. Baenziger said that “to help us remember how beautifully complicated and diverse people are,” all health care professionals need to watch Nigerian novelist Chimamanda Ngozi Adichie’s TED talk “The Danger of a Single Story.”
Dr. Baenziger also stressed the importance of conversations about “places where race is built into our clinical assessments, like eGFR,” as well as being aware that many of the research studies that are used to support everyday clinical decisions didn’t include people of color. She also encouraged clinicians to consider how and when they include race in their notes.7 “Is it really helpful to make sure people know right away that you are treating a ‘46-year-old Hispanic male’ or can the fact that he is Hispanic be saved for the social history section with other important details of his life such as being a father, veteran, and mechanic?” she asked.
“Racism is real and very much a part of our history. We can no longer be in denial regarding the racism that exists in medicine and the impact it has on our patients,” Dr. Unaka said. “As a profession, we cannot hide behind our espoused core values. We must live up to them.”
References
1. Lucey CR, Saguil, A. The Consequences of Structural Racism on MCAT Scores and Medical School Admissions: The Past Is Prologue. Acad Med. 2020 Mar;95(3):351-356. doi: 10.1097/ACM.0000000000002939.
2. Flores L. Increasing racial diversity in hospital medicine’s leadership ranks. The Hospitalist. 2020 Oct 21.
3. Smedley BD, et al, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Institute of Medicine Committee on Understanding and Eliminating Racial and Ethnic Disparities in Health Care. Washington: National Academies Press; 2003.
4. Heins A, et al. Physician Race/Ethnicity Predicts Successful Emergency Department Analgesia. J Pain. 2010 July;11(7):692-697. doi: 10.1016/j.jpain.2009.10.017.
5. U.S. Department of Health and Human Serves, Office of Minority Health. Infant Mortality and African Americans. 2019 Nov 8. minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=23.
6. Jacobs ZG, et al. The Association between Limited English Proficiency and Sepsis Mortality. J Hosp Med. 2020;3;140-146. Published Online First 2019 Nov 20. doi:10.12788/jhm.3334.
7. Finucane TE. Mention of a Patient’s “Race” in Clinical Presentations. Virtual Mentor. 2014;16(6):423-427. doi: 10.1001/virtualmentor.2014.16.6.ecas1-1406.
























