User login
Antipsychotic administration fails to treat delirium in hospitalized adults
Background: Delirium is a common disorder in hospitalized adults and is associated with poor outcomes. Antipsychotics are used clinically to treat delirium, but benefits and harms remain unclear.
Study design: A systematic review evaluating treatment of delirium in 16 randomized, controlled trials (RCTs) of antipsychotics vs. placebo or other antipsychotics, as well as 10 prospective observational studies reporting harm.
Setting: Data obtained from PubMed, Embase, CENTRAL, CINAHL, and PsycINFO from inception to July 2019 without language restrictions.
Synopsis: For 5,607 adult inpatients, treatment of delirium with haloperidol showed no difference in sedation status, duration of delirium, hospital length of stay, or mortality when compared with second-generation antipsychotics or placebo (low and moderate strength of evidence). Regarding second-generation antipsychotics versus haloperidol, no difference was found in delirium severity and cognitive function (low strength of evidence). Direct comparisons between second-generation antipsychotics showed no difference in mortality.
Limitations include heterogeneous use of agents, routes, dose, and measurement tools, which limits generalization of evidence. Multiple RCTs excluded patients with underlying cardiac and neurologic conditions that likely led to underrepresentation of harm in routine use. Insufficient evidence still exists for multiple clinically relevant outcomes including long-term cognitive function.
Bottom line: Evidence from several studies does not support the use of haloperidol or newer antipsychotics to treat delirium.
Citation: Nikooie R et al. Antipsychotics for delirium treatment in adults: A systematic review. Ann Intern Med. 2019 Oct 1;171(7):485-95.
Dr. Berry is assistant professor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: Delirium is a common disorder in hospitalized adults and is associated with poor outcomes. Antipsychotics are used clinically to treat delirium, but benefits and harms remain unclear.
Study design: A systematic review evaluating treatment of delirium in 16 randomized, controlled trials (RCTs) of antipsychotics vs. placebo or other antipsychotics, as well as 10 prospective observational studies reporting harm.
Setting: Data obtained from PubMed, Embase, CENTRAL, CINAHL, and PsycINFO from inception to July 2019 without language restrictions.
Synopsis: For 5,607 adult inpatients, treatment of delirium with haloperidol showed no difference in sedation status, duration of delirium, hospital length of stay, or mortality when compared with second-generation antipsychotics or placebo (low and moderate strength of evidence). Regarding second-generation antipsychotics versus haloperidol, no difference was found in delirium severity and cognitive function (low strength of evidence). Direct comparisons between second-generation antipsychotics showed no difference in mortality.
Limitations include heterogeneous use of agents, routes, dose, and measurement tools, which limits generalization of evidence. Multiple RCTs excluded patients with underlying cardiac and neurologic conditions that likely led to underrepresentation of harm in routine use. Insufficient evidence still exists for multiple clinically relevant outcomes including long-term cognitive function.
Bottom line: Evidence from several studies does not support the use of haloperidol or newer antipsychotics to treat delirium.
Citation: Nikooie R et al. Antipsychotics for delirium treatment in adults: A systematic review. Ann Intern Med. 2019 Oct 1;171(7):485-95.
Dr. Berry is assistant professor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: Delirium is a common disorder in hospitalized adults and is associated with poor outcomes. Antipsychotics are used clinically to treat delirium, but benefits and harms remain unclear.
Study design: A systematic review evaluating treatment of delirium in 16 randomized, controlled trials (RCTs) of antipsychotics vs. placebo or other antipsychotics, as well as 10 prospective observational studies reporting harm.
Setting: Data obtained from PubMed, Embase, CENTRAL, CINAHL, and PsycINFO from inception to July 2019 without language restrictions.
Synopsis: For 5,607 adult inpatients, treatment of delirium with haloperidol showed no difference in sedation status, duration of delirium, hospital length of stay, or mortality when compared with second-generation antipsychotics or placebo (low and moderate strength of evidence). Regarding second-generation antipsychotics versus haloperidol, no difference was found in delirium severity and cognitive function (low strength of evidence). Direct comparisons between second-generation antipsychotics showed no difference in mortality.
Limitations include heterogeneous use of agents, routes, dose, and measurement tools, which limits generalization of evidence. Multiple RCTs excluded patients with underlying cardiac and neurologic conditions that likely led to underrepresentation of harm in routine use. Insufficient evidence still exists for multiple clinically relevant outcomes including long-term cognitive function.
Bottom line: Evidence from several studies does not support the use of haloperidol or newer antipsychotics to treat delirium.
Citation: Nikooie R et al. Antipsychotics for delirium treatment in adults: A systematic review. Ann Intern Med. 2019 Oct 1;171(7):485-95.
Dr. Berry is assistant professor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Anticoagulation and antiplatelet therapy after GI bleed cut mortality, ischemic events
Background: Resumption of AC or AP therapy for patients following a GIB represents a common clinical challenge. Interruption of these medications following a GIB is associated with increased risk of macrovascular events, thrombosis, morbidity, and death. Prior studies have found inconsistent risk of rebleeding and death with resumption of these therapies following GIB. Little evidence exists for long-term outcomes and optimal timing of AC and AP resumption.
Study design: Retrospective observational cohort study.
Setting: Two general hospitals in Spain.
Synopsis: Overall 871 patients (mean age, 79 years) presenting with GIB on AC or AP therapy were followed for a median of 25 months. A total of 63% of patients experienced one of the following: thrombotic events, recurrent bleeding, or death during follow-up. Resumption of therapy was associated with a twofold risk of rebleeding, but lower rates of ischemic events (hazard ratio, 0.62; 95% confidence interval, 0.4-0.9) and death (HR, 0.60; 95% CI, 0.45-0.80). Early resumption (7 days or less) was associated with more rebleeding (30.6% vs. 23.1%; P = .04), fewer ischemic events (13.6% vs. 20.4%; P = .02%), and no difference in death. Bleeding was more frequent with AC agents, compared with AP agents.
Although resumption of AC or AP following a GIB increased bleeding risk, this may be outweighed by reductions in ischemic events and death if these agents are continued. For hospitalist clinicians, this remains a nuanced and patient-centered decision.
Interpretation is limited by variability in GIB location, agents used, and timing of resumption. Also, the study population included a limited number of elderly patients with multiple comorbidities and high overall death rate.
Bottom line: Resuming AC and AP medications following gastrointestinal bleeding doubled the rebleeding risk but lowered the risk of ischemic events and death, compared with the discontinuation of these medications.
Citation: Sostres C et al. Risk of rebleeding, vascular events and death after gastrointestinal bleeding in anticoagulant and/or antiplatelet users. Aliment Pharmcol Ther. 2019 Oct;50:919-29.
Dr. Berry is assistant professor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: Resumption of AC or AP therapy for patients following a GIB represents a common clinical challenge. Interruption of these medications following a GIB is associated with increased risk of macrovascular events, thrombosis, morbidity, and death. Prior studies have found inconsistent risk of rebleeding and death with resumption of these therapies following GIB. Little evidence exists for long-term outcomes and optimal timing of AC and AP resumption.
Study design: Retrospective observational cohort study.
Setting: Two general hospitals in Spain.
Synopsis: Overall 871 patients (mean age, 79 years) presenting with GIB on AC or AP therapy were followed for a median of 25 months. A total of 63% of patients experienced one of the following: thrombotic events, recurrent bleeding, or death during follow-up. Resumption of therapy was associated with a twofold risk of rebleeding, but lower rates of ischemic events (hazard ratio, 0.62; 95% confidence interval, 0.4-0.9) and death (HR, 0.60; 95% CI, 0.45-0.80). Early resumption (7 days or less) was associated with more rebleeding (30.6% vs. 23.1%; P = .04), fewer ischemic events (13.6% vs. 20.4%; P = .02%), and no difference in death. Bleeding was more frequent with AC agents, compared with AP agents.
Although resumption of AC or AP following a GIB increased bleeding risk, this may be outweighed by reductions in ischemic events and death if these agents are continued. For hospitalist clinicians, this remains a nuanced and patient-centered decision.
Interpretation is limited by variability in GIB location, agents used, and timing of resumption. Also, the study population included a limited number of elderly patients with multiple comorbidities and high overall death rate.
Bottom line: Resuming AC and AP medications following gastrointestinal bleeding doubled the rebleeding risk but lowered the risk of ischemic events and death, compared with the discontinuation of these medications.
Citation: Sostres C et al. Risk of rebleeding, vascular events and death after gastrointestinal bleeding in anticoagulant and/or antiplatelet users. Aliment Pharmcol Ther. 2019 Oct;50:919-29.
Dr. Berry is assistant professor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
Background: Resumption of AC or AP therapy for patients following a GIB represents a common clinical challenge. Interruption of these medications following a GIB is associated with increased risk of macrovascular events, thrombosis, morbidity, and death. Prior studies have found inconsistent risk of rebleeding and death with resumption of these therapies following GIB. Little evidence exists for long-term outcomes and optimal timing of AC and AP resumption.
Study design: Retrospective observational cohort study.
Setting: Two general hospitals in Spain.
Synopsis: Overall 871 patients (mean age, 79 years) presenting with GIB on AC or AP therapy were followed for a median of 25 months. A total of 63% of patients experienced one of the following: thrombotic events, recurrent bleeding, or death during follow-up. Resumption of therapy was associated with a twofold risk of rebleeding, but lower rates of ischemic events (hazard ratio, 0.62; 95% confidence interval, 0.4-0.9) and death (HR, 0.60; 95% CI, 0.45-0.80). Early resumption (7 days or less) was associated with more rebleeding (30.6% vs. 23.1%; P = .04), fewer ischemic events (13.6% vs. 20.4%; P = .02%), and no difference in death. Bleeding was more frequent with AC agents, compared with AP agents.
Although resumption of AC or AP following a GIB increased bleeding risk, this may be outweighed by reductions in ischemic events and death if these agents are continued. For hospitalist clinicians, this remains a nuanced and patient-centered decision.
Interpretation is limited by variability in GIB location, agents used, and timing of resumption. Also, the study population included a limited number of elderly patients with multiple comorbidities and high overall death rate.
Bottom line: Resuming AC and AP medications following gastrointestinal bleeding doubled the rebleeding risk but lowered the risk of ischemic events and death, compared with the discontinuation of these medications.
Citation: Sostres C et al. Risk of rebleeding, vascular events and death after gastrointestinal bleeding in anticoagulant and/or antiplatelet users. Aliment Pharmcol Ther. 2019 Oct;50:919-29.
Dr. Berry is assistant professor of medicine, hospital medicine, at the Rocky Mountain Veterans Affairs Regional Medical Center, Aurora, Colo.
COVID-19 Wellbeing
Resources for hospitalists
SHM is committed to supporting hospitalists and the health care team to safely deliver patient care while maintaining the health and wellbeing of the families and the community they serve. SHM has developed resources for hospitalists as well as compiled a listing of existing resources which you can find on our website. The resources include:
Hospital Medicine COVID-19 Check-in Guide for Self & Peers
This is the first resource produced by SHM’s Wellbeing Taskforce to address the issues of hospitalist burnout and mental health during COVID-19. It is designed to help hospitalists to break the culture of silence around wellbeing, burnout, and mental health during COVID-19 by encouraging open conversation around how they are handling and processing the pandemic. Download the guide at https://bit.ly/3nxikzl.
SHM’s Strategies for Hospitalist Wellbeing Initiatives during COVID-19
This resource was developed based on information shared during an April 2020 webinar on Provider Wellbeing. Included are examples of initiatives currently being implemented by various hospital medicine groups. You can find this resource at https://bit.ly/3seNBKQ.
Webinars
Hear experiences and examples of how hospitalists and hospital medicine grouups are managing their response to the clinical and practice implications of COVID-19. Webinars have included topics related to hospitalist wellbeing. For instance, a recent webinar featured Gail Gazelle, MD, MCC, a physician coach, author, and mentor focused on burnout and resilience. This was a virtual, confidential session created for hospitalists to have a space for honest reflection, support, and the exploration of strategies for navigating the stress and challenges of being on the front lines of the COVID-19 response and in caring for themselves and their families during a pandemic. See upcoming and recorded SHM webinars on the website: www.hospitalmedicine.org/clinical-topics/coronavirus-disease-2019-covid-19-resources-for-hospitalists/webinars.
Other resources not provided directly by SHM include:
Physician Support Line: volunteer psychiatrist-staffed helpline for free and confidential peer support to discuss immediate life stressors. Available 7 days a week, 8:00am-12:00am EST. Contact number: 888-409-0141
Talkspace: virtual therapy tool offering a free month of Unlimited Messaging Plus for health care providers by registering using their NPI. Download app in App Store or Google Play.
National Suicide Prevention Lifeline: free and confidential crisis hotline for anyone available 24/7 across the United States. Contact number: 800-273-8255.
Headspace Meditation App: app-based meditation tool. Premium version (Headspace Plus) available free for health care providers through 2020 by registering using their National Provider Identifier (NPI). Download app in App Store or Google Play.
Tide: A free app that uses natural sounds to help you sleep, relax, focus, and meditate. Tide also listens to your breathing to play an alarm during your lightest sleep phase, waking you up as gently as possible. Their premium service is available to all health care workers. Download app in App Store or Google Play.
Resources for hospitalists
Resources for hospitalists
SHM is committed to supporting hospitalists and the health care team to safely deliver patient care while maintaining the health and wellbeing of the families and the community they serve. SHM has developed resources for hospitalists as well as compiled a listing of existing resources which you can find on our website. The resources include:
Hospital Medicine COVID-19 Check-in Guide for Self & Peers
This is the first resource produced by SHM’s Wellbeing Taskforce to address the issues of hospitalist burnout and mental health during COVID-19. It is designed to help hospitalists to break the culture of silence around wellbeing, burnout, and mental health during COVID-19 by encouraging open conversation around how they are handling and processing the pandemic. Download the guide at https://bit.ly/3nxikzl.
SHM’s Strategies for Hospitalist Wellbeing Initiatives during COVID-19
This resource was developed based on information shared during an April 2020 webinar on Provider Wellbeing. Included are examples of initiatives currently being implemented by various hospital medicine groups. You can find this resource at https://bit.ly/3seNBKQ.
Webinars
Hear experiences and examples of how hospitalists and hospital medicine grouups are managing their response to the clinical and practice implications of COVID-19. Webinars have included topics related to hospitalist wellbeing. For instance, a recent webinar featured Gail Gazelle, MD, MCC, a physician coach, author, and mentor focused on burnout and resilience. This was a virtual, confidential session created for hospitalists to have a space for honest reflection, support, and the exploration of strategies for navigating the stress and challenges of being on the front lines of the COVID-19 response and in caring for themselves and their families during a pandemic. See upcoming and recorded SHM webinars on the website: www.hospitalmedicine.org/clinical-topics/coronavirus-disease-2019-covid-19-resources-for-hospitalists/webinars.
Other resources not provided directly by SHM include:
Physician Support Line: volunteer psychiatrist-staffed helpline for free and confidential peer support to discuss immediate life stressors. Available 7 days a week, 8:00am-12:00am EST. Contact number: 888-409-0141
Talkspace: virtual therapy tool offering a free month of Unlimited Messaging Plus for health care providers by registering using their NPI. Download app in App Store or Google Play.
National Suicide Prevention Lifeline: free and confidential crisis hotline for anyone available 24/7 across the United States. Contact number: 800-273-8255.
Headspace Meditation App: app-based meditation tool. Premium version (Headspace Plus) available free for health care providers through 2020 by registering using their National Provider Identifier (NPI). Download app in App Store or Google Play.
Tide: A free app that uses natural sounds to help you sleep, relax, focus, and meditate. Tide also listens to your breathing to play an alarm during your lightest sleep phase, waking you up as gently as possible. Their premium service is available to all health care workers. Download app in App Store or Google Play.
SHM is committed to supporting hospitalists and the health care team to safely deliver patient care while maintaining the health and wellbeing of the families and the community they serve. SHM has developed resources for hospitalists as well as compiled a listing of existing resources which you can find on our website. The resources include:
Hospital Medicine COVID-19 Check-in Guide for Self & Peers
This is the first resource produced by SHM’s Wellbeing Taskforce to address the issues of hospitalist burnout and mental health during COVID-19. It is designed to help hospitalists to break the culture of silence around wellbeing, burnout, and mental health during COVID-19 by encouraging open conversation around how they are handling and processing the pandemic. Download the guide at https://bit.ly/3nxikzl.
SHM’s Strategies for Hospitalist Wellbeing Initiatives during COVID-19
This resource was developed based on information shared during an April 2020 webinar on Provider Wellbeing. Included are examples of initiatives currently being implemented by various hospital medicine groups. You can find this resource at https://bit.ly/3seNBKQ.
Webinars
Hear experiences and examples of how hospitalists and hospital medicine grouups are managing their response to the clinical and practice implications of COVID-19. Webinars have included topics related to hospitalist wellbeing. For instance, a recent webinar featured Gail Gazelle, MD, MCC, a physician coach, author, and mentor focused on burnout and resilience. This was a virtual, confidential session created for hospitalists to have a space for honest reflection, support, and the exploration of strategies for navigating the stress and challenges of being on the front lines of the COVID-19 response and in caring for themselves and their families during a pandemic. See upcoming and recorded SHM webinars on the website: www.hospitalmedicine.org/clinical-topics/coronavirus-disease-2019-covid-19-resources-for-hospitalists/webinars.
Other resources not provided directly by SHM include:
Physician Support Line: volunteer psychiatrist-staffed helpline for free and confidential peer support to discuss immediate life stressors. Available 7 days a week, 8:00am-12:00am EST. Contact number: 888-409-0141
Talkspace: virtual therapy tool offering a free month of Unlimited Messaging Plus for health care providers by registering using their NPI. Download app in App Store or Google Play.
National Suicide Prevention Lifeline: free and confidential crisis hotline for anyone available 24/7 across the United States. Contact number: 800-273-8255.
Headspace Meditation App: app-based meditation tool. Premium version (Headspace Plus) available free for health care providers through 2020 by registering using their National Provider Identifier (NPI). Download app in App Store or Google Play.
Tide: A free app that uses natural sounds to help you sleep, relax, focus, and meditate. Tide also listens to your breathing to play an alarm during your lightest sleep phase, waking you up as gently as possible. Their premium service is available to all health care workers. Download app in App Store or Google Play.
Hospitalist movers and shakers: January 2021
Daniel Steinberg, MD, SFHM, recently was among 10 medical educators across the county to receive the Accreditation Council for Graduate Medical Education 2021 Parker J. Palmer Courage to Teach Award. Considered the most prestigious award given to graduate medical education program directors, it “recognizes program directors who have fostered innovation and improvement in their residency/fellowship program and served as exemplary role models for residents and fellows.”
Dr. Steinberg was program director for internal medicine residency at Mount Sinai Beth Israel, New York, for 11 years (2009-20) before becoming associate dean for quality and patient safety in graduate medical education in September. He is a professor of medicine and medical education at Icahn School of Medicine at Mount Sinai, New York.
Dr. Steinberg also is a leader within SHM, serving on the education, physicians-in-training, and annual conference committees. He is the course director for SHM Converge 2021.
Ann Sheehy, MD, SFHM, was honored in a virtual ceremony in December 2020 by the University of Wisconsin celebrating Physician Excellence Award winners. She was presented with the Physician Excellence Leadership Award.
Dr. Sheehy is division chief of the division of hospital medicine at the University of Wisconsin–Madison, and chair of the SHM Public Policy Committee.
Donald Schmidt, MD, has been named chief medical officer and vice president of medical affairs at Madonna Rehabilitation Hospitals in Omaha and Lincoln, Neb. He will replace Thomas Stalder, MD, who is retiring. Dr. Schmidt brings 20 years of experience to Madonna Rehabilitation Hospitals, including his most recent post as a hospitalist and medical director of the hospitalist program at Catholic Health Initiatives Health St. Elizabeth (Lincoln, Neb.).
Dr. Schmidt currently serves on the board of directors for OneHealth Nebraska, an independent physicians association.
Ezinne Nwude, MD, recently was presented with the SCP Health Excellence in Leadership Award during the organization’s Medical Leadership Conference. Dr. Nwude is chief of staff and hospitalist at the Medical Center of South Arkansas, El Dorado.
SCP Health coordinates staffing for more than 7,500 providers covering 30 states and is one of the nation’s largest clinical practice management companies. More than 420 medical leaders nationwide were eligible for the award. Dr. Nwude has focused on positive culture and health education since her start at MSCA in 2014. She has been chief of staff since October 2018.
RWJ Barnabas Health (West Orange, N.J.) recently named two new health system leaders from among its hospital medicine ranks, as Christopher Freer, MD, was tabbed as senior vice president for emergency and hospital medicine, and Maninder “Dolly” Abraham, MD, was picked as chief of hospital medicine. The moves were made as RWJBH takes over as the direct employer for Envision Physician Services in Nashville, Tenn.
Dr. Freer was elevated to his new role after spending the past 5 years as RWJBH’s system director for emergency services. He has nearly 3 decades of experience in hospital medicine.
Dr. Abraham comes to his new position after directing the hospitalist program at Saint Barnabas and serving as regional medical director with Envision.
Newman Regional Health (Emporia, Kan.) recently established a partnership with FreeState Healthcare (Wichita, Kan.). FreeState will be responsible for providing hospitalist services to adult inpatients and observation patients at Newman Regional Health during overnights.
Daniel Steinberg, MD, SFHM, recently was among 10 medical educators across the county to receive the Accreditation Council for Graduate Medical Education 2021 Parker J. Palmer Courage to Teach Award. Considered the most prestigious award given to graduate medical education program directors, it “recognizes program directors who have fostered innovation and improvement in their residency/fellowship program and served as exemplary role models for residents and fellows.”
Dr. Steinberg was program director for internal medicine residency at Mount Sinai Beth Israel, New York, for 11 years (2009-20) before becoming associate dean for quality and patient safety in graduate medical education in September. He is a professor of medicine and medical education at Icahn School of Medicine at Mount Sinai, New York.
Dr. Steinberg also is a leader within SHM, serving on the education, physicians-in-training, and annual conference committees. He is the course director for SHM Converge 2021.
Ann Sheehy, MD, SFHM, was honored in a virtual ceremony in December 2020 by the University of Wisconsin celebrating Physician Excellence Award winners. She was presented with the Physician Excellence Leadership Award.
Dr. Sheehy is division chief of the division of hospital medicine at the University of Wisconsin–Madison, and chair of the SHM Public Policy Committee.
Donald Schmidt, MD, has been named chief medical officer and vice president of medical affairs at Madonna Rehabilitation Hospitals in Omaha and Lincoln, Neb. He will replace Thomas Stalder, MD, who is retiring. Dr. Schmidt brings 20 years of experience to Madonna Rehabilitation Hospitals, including his most recent post as a hospitalist and medical director of the hospitalist program at Catholic Health Initiatives Health St. Elizabeth (Lincoln, Neb.).
Dr. Schmidt currently serves on the board of directors for OneHealth Nebraska, an independent physicians association.
Ezinne Nwude, MD, recently was presented with the SCP Health Excellence in Leadership Award during the organization’s Medical Leadership Conference. Dr. Nwude is chief of staff and hospitalist at the Medical Center of South Arkansas, El Dorado.
SCP Health coordinates staffing for more than 7,500 providers covering 30 states and is one of the nation’s largest clinical practice management companies. More than 420 medical leaders nationwide were eligible for the award. Dr. Nwude has focused on positive culture and health education since her start at MSCA in 2014. She has been chief of staff since October 2018.
RWJ Barnabas Health (West Orange, N.J.) recently named two new health system leaders from among its hospital medicine ranks, as Christopher Freer, MD, was tabbed as senior vice president for emergency and hospital medicine, and Maninder “Dolly” Abraham, MD, was picked as chief of hospital medicine. The moves were made as RWJBH takes over as the direct employer for Envision Physician Services in Nashville, Tenn.
Dr. Freer was elevated to his new role after spending the past 5 years as RWJBH’s system director for emergency services. He has nearly 3 decades of experience in hospital medicine.
Dr. Abraham comes to his new position after directing the hospitalist program at Saint Barnabas and serving as regional medical director with Envision.
Newman Regional Health (Emporia, Kan.) recently established a partnership with FreeState Healthcare (Wichita, Kan.). FreeState will be responsible for providing hospitalist services to adult inpatients and observation patients at Newman Regional Health during overnights.
Daniel Steinberg, MD, SFHM, recently was among 10 medical educators across the county to receive the Accreditation Council for Graduate Medical Education 2021 Parker J. Palmer Courage to Teach Award. Considered the most prestigious award given to graduate medical education program directors, it “recognizes program directors who have fostered innovation and improvement in their residency/fellowship program and served as exemplary role models for residents and fellows.”
Dr. Steinberg was program director for internal medicine residency at Mount Sinai Beth Israel, New York, for 11 years (2009-20) before becoming associate dean for quality and patient safety in graduate medical education in September. He is a professor of medicine and medical education at Icahn School of Medicine at Mount Sinai, New York.
Dr. Steinberg also is a leader within SHM, serving on the education, physicians-in-training, and annual conference committees. He is the course director for SHM Converge 2021.
Ann Sheehy, MD, SFHM, was honored in a virtual ceremony in December 2020 by the University of Wisconsin celebrating Physician Excellence Award winners. She was presented with the Physician Excellence Leadership Award.
Dr. Sheehy is division chief of the division of hospital medicine at the University of Wisconsin–Madison, and chair of the SHM Public Policy Committee.
Donald Schmidt, MD, has been named chief medical officer and vice president of medical affairs at Madonna Rehabilitation Hospitals in Omaha and Lincoln, Neb. He will replace Thomas Stalder, MD, who is retiring. Dr. Schmidt brings 20 years of experience to Madonna Rehabilitation Hospitals, including his most recent post as a hospitalist and medical director of the hospitalist program at Catholic Health Initiatives Health St. Elizabeth (Lincoln, Neb.).
Dr. Schmidt currently serves on the board of directors for OneHealth Nebraska, an independent physicians association.
Ezinne Nwude, MD, recently was presented with the SCP Health Excellence in Leadership Award during the organization’s Medical Leadership Conference. Dr. Nwude is chief of staff and hospitalist at the Medical Center of South Arkansas, El Dorado.
SCP Health coordinates staffing for more than 7,500 providers covering 30 states and is one of the nation’s largest clinical practice management companies. More than 420 medical leaders nationwide were eligible for the award. Dr. Nwude has focused on positive culture and health education since her start at MSCA in 2014. She has been chief of staff since October 2018.
RWJ Barnabas Health (West Orange, N.J.) recently named two new health system leaders from among its hospital medicine ranks, as Christopher Freer, MD, was tabbed as senior vice president for emergency and hospital medicine, and Maninder “Dolly” Abraham, MD, was picked as chief of hospital medicine. The moves were made as RWJBH takes over as the direct employer for Envision Physician Services in Nashville, Tenn.
Dr. Freer was elevated to his new role after spending the past 5 years as RWJBH’s system director for emergency services. He has nearly 3 decades of experience in hospital medicine.
Dr. Abraham comes to his new position after directing the hospitalist program at Saint Barnabas and serving as regional medical director with Envision.
Newman Regional Health (Emporia, Kan.) recently established a partnership with FreeState Healthcare (Wichita, Kan.). FreeState will be responsible for providing hospitalist services to adult inpatients and observation patients at Newman Regional Health during overnights.
Pediatric HM highlights from the 2020 State of Hospital Medicine Report
To improve the pediatric data in the State of Hospital Medicine (SoHM) Report, the Practice Analysis Committee (PAC) developed a pediatric task force to recommend content specific to pediatric practice and garner support for survey participation. The pediatric hospital medicine (PHM) community responded with its usual enthusiasm, resulting in a threefold increase in PHM participation (99 groups), making the data from 2020 SoHM Report the most meaningful ever for pediatric practices.
However, data collection for the 2020 SoHM Report concluded in February, just before the face of medical practice and hospital care changed dramatically. A recent report at the virtual Pediatric Hospital Medicine meeting stated that pre–COVID-19 hospital operating margins had already taken a significant decline (from 5% to 2%-3%), putting pressure on pediatric programs in community settings that typically do not generate much revenue. After COVID-19, hospital revenues took an even greater downturn, affecting many hospital-based pediatric programs. While the future direction of many PHM programs remains unclear, the robust nature of the pediatric data in the 2020 SoHM Report defines where we were and where we once again hope to be. In addition, the PAC conducted a supplemental survey designed to assess the impact of COVID-19 on the practice of hospital medicine. Here’s a quick review of PHM highlights from the 2020 SoHM Report, with preliminary findings from the supplemental survey.
Diversity of service and scope of practice: pediatric hospitalist programs continue to provide a wide variety of services beyond care on inpatient wards, with the most common being procedure performance (56.6%), care of healthy newborns (51.5%), and rapid response team (38.4%) coverage. In addition, most PHM programs have a role in comanagement of a wide variety of patient populations, with the greatest presence among the surgical specialties. Approximately 90% of programs report some role in the care of patients admitted to general surgery, orthopedic surgery, and other surgical subspecialties. The role for comanagement with medical specialties remains diverse, with PHM programs routinely having some role in caring for patients hospitalized for neurologic, gastroenterological, cardiac concerns, and others. With the recent decline in hospital revenues affecting PHM practices, one way to ensure program value is to continue to diversify. Based on data from the 2020 SoHM report, broadening of clinical coverage will not require a significant change in practice for most PHM programs.
PHM board certification: With the first certifying exam for PHM taking place just months before SoHM data collection, the survey sought to establish a baseline percentage of providers board certified in PHM. With 98 groups responding, an average of 26.4% of PHM practitioners per group were reported to be board certified. While no difference was seen based on academic status, practitioners in PHM programs employed by a hospital, health system, or integrated delivery system were much more likely to be board certified than those employed by a university or medical school (31% vs. 20%). Regional differences were noted as well, with the East region reporting a much higher median proportion of PHM-certified physicians. It will be interesting to watch the trend in board certification status evolve over the upcoming years.
Anticipated change of budgeted full-time equivalents in the next year/post–COVID-19 analysis: Of the PHM programs responding to the SoHM Survey, 46.5% predicted an increase in budgeted full-time equivalents in the next year, while only 5.1% anticipated a decrease. Expecting this to change in response to COVID-19, the supplemental survey sought to update this information. Of the 30 PHM respondents to the supplemental survey, 41% instituted a temporary hiring freeze because of COVID-19, while 8.3% instituted a hiring freeze felt likely to be permanent. As PHM programs gear up for the next viral season, we wait to see whether the impact of COVID-19 will continue to be reflected in the volume and variety of patients admitted. It is clear that PHM programs will need to remain nimble to stay ahead of the changing landscape of practice in the days ahead. View all data by obtaining access to the 2020 SoHM Report at hospitalmedicine.org/sohm.
Many thanks to pediatric task force members Jack Percelay, MD; Vivien Kon-Ea Sun, MD; Marcos Mestre, MD; Ann Allen, MD; Dimple Khona, MD; Jeff Grill, MD; and Michelle Marks, MD.
Dr. Gage is director of faculty development, pediatric hospital medicine, at Phoenix Children’s Hospital, and associate professor of pediatrics at the University of Arizona, Phoenix.
To improve the pediatric data in the State of Hospital Medicine (SoHM) Report, the Practice Analysis Committee (PAC) developed a pediatric task force to recommend content specific to pediatric practice and garner support for survey participation. The pediatric hospital medicine (PHM) community responded with its usual enthusiasm, resulting in a threefold increase in PHM participation (99 groups), making the data from 2020 SoHM Report the most meaningful ever for pediatric practices.
However, data collection for the 2020 SoHM Report concluded in February, just before the face of medical practice and hospital care changed dramatically. A recent report at the virtual Pediatric Hospital Medicine meeting stated that pre–COVID-19 hospital operating margins had already taken a significant decline (from 5% to 2%-3%), putting pressure on pediatric programs in community settings that typically do not generate much revenue. After COVID-19, hospital revenues took an even greater downturn, affecting many hospital-based pediatric programs. While the future direction of many PHM programs remains unclear, the robust nature of the pediatric data in the 2020 SoHM Report defines where we were and where we once again hope to be. In addition, the PAC conducted a supplemental survey designed to assess the impact of COVID-19 on the practice of hospital medicine. Here’s a quick review of PHM highlights from the 2020 SoHM Report, with preliminary findings from the supplemental survey.
Diversity of service and scope of practice: pediatric hospitalist programs continue to provide a wide variety of services beyond care on inpatient wards, with the most common being procedure performance (56.6%), care of healthy newborns (51.5%), and rapid response team (38.4%) coverage. In addition, most PHM programs have a role in comanagement of a wide variety of patient populations, with the greatest presence among the surgical specialties. Approximately 90% of programs report some role in the care of patients admitted to general surgery, orthopedic surgery, and other surgical subspecialties. The role for comanagement with medical specialties remains diverse, with PHM programs routinely having some role in caring for patients hospitalized for neurologic, gastroenterological, cardiac concerns, and others. With the recent decline in hospital revenues affecting PHM practices, one way to ensure program value is to continue to diversify. Based on data from the 2020 SoHM report, broadening of clinical coverage will not require a significant change in practice for most PHM programs.
PHM board certification: With the first certifying exam for PHM taking place just months before SoHM data collection, the survey sought to establish a baseline percentage of providers board certified in PHM. With 98 groups responding, an average of 26.4% of PHM practitioners per group were reported to be board certified. While no difference was seen based on academic status, practitioners in PHM programs employed by a hospital, health system, or integrated delivery system were much more likely to be board certified than those employed by a university or medical school (31% vs. 20%). Regional differences were noted as well, with the East region reporting a much higher median proportion of PHM-certified physicians. It will be interesting to watch the trend in board certification status evolve over the upcoming years.
Anticipated change of budgeted full-time equivalents in the next year/post–COVID-19 analysis: Of the PHM programs responding to the SoHM Survey, 46.5% predicted an increase in budgeted full-time equivalents in the next year, while only 5.1% anticipated a decrease. Expecting this to change in response to COVID-19, the supplemental survey sought to update this information. Of the 30 PHM respondents to the supplemental survey, 41% instituted a temporary hiring freeze because of COVID-19, while 8.3% instituted a hiring freeze felt likely to be permanent. As PHM programs gear up for the next viral season, we wait to see whether the impact of COVID-19 will continue to be reflected in the volume and variety of patients admitted. It is clear that PHM programs will need to remain nimble to stay ahead of the changing landscape of practice in the days ahead. View all data by obtaining access to the 2020 SoHM Report at hospitalmedicine.org/sohm.
Many thanks to pediatric task force members Jack Percelay, MD; Vivien Kon-Ea Sun, MD; Marcos Mestre, MD; Ann Allen, MD; Dimple Khona, MD; Jeff Grill, MD; and Michelle Marks, MD.
Dr. Gage is director of faculty development, pediatric hospital medicine, at Phoenix Children’s Hospital, and associate professor of pediatrics at the University of Arizona, Phoenix.
To improve the pediatric data in the State of Hospital Medicine (SoHM) Report, the Practice Analysis Committee (PAC) developed a pediatric task force to recommend content specific to pediatric practice and garner support for survey participation. The pediatric hospital medicine (PHM) community responded with its usual enthusiasm, resulting in a threefold increase in PHM participation (99 groups), making the data from 2020 SoHM Report the most meaningful ever for pediatric practices.
However, data collection for the 2020 SoHM Report concluded in February, just before the face of medical practice and hospital care changed dramatically. A recent report at the virtual Pediatric Hospital Medicine meeting stated that pre–COVID-19 hospital operating margins had already taken a significant decline (from 5% to 2%-3%), putting pressure on pediatric programs in community settings that typically do not generate much revenue. After COVID-19, hospital revenues took an even greater downturn, affecting many hospital-based pediatric programs. While the future direction of many PHM programs remains unclear, the robust nature of the pediatric data in the 2020 SoHM Report defines where we were and where we once again hope to be. In addition, the PAC conducted a supplemental survey designed to assess the impact of COVID-19 on the practice of hospital medicine. Here’s a quick review of PHM highlights from the 2020 SoHM Report, with preliminary findings from the supplemental survey.
Diversity of service and scope of practice: pediatric hospitalist programs continue to provide a wide variety of services beyond care on inpatient wards, with the most common being procedure performance (56.6%), care of healthy newborns (51.5%), and rapid response team (38.4%) coverage. In addition, most PHM programs have a role in comanagement of a wide variety of patient populations, with the greatest presence among the surgical specialties. Approximately 90% of programs report some role in the care of patients admitted to general surgery, orthopedic surgery, and other surgical subspecialties. The role for comanagement with medical specialties remains diverse, with PHM programs routinely having some role in caring for patients hospitalized for neurologic, gastroenterological, cardiac concerns, and others. With the recent decline in hospital revenues affecting PHM practices, one way to ensure program value is to continue to diversify. Based on data from the 2020 SoHM report, broadening of clinical coverage will not require a significant change in practice for most PHM programs.
PHM board certification: With the first certifying exam for PHM taking place just months before SoHM data collection, the survey sought to establish a baseline percentage of providers board certified in PHM. With 98 groups responding, an average of 26.4% of PHM practitioners per group were reported to be board certified. While no difference was seen based on academic status, practitioners in PHM programs employed by a hospital, health system, or integrated delivery system were much more likely to be board certified than those employed by a university or medical school (31% vs. 20%). Regional differences were noted as well, with the East region reporting a much higher median proportion of PHM-certified physicians. It will be interesting to watch the trend in board certification status evolve over the upcoming years.
Anticipated change of budgeted full-time equivalents in the next year/post–COVID-19 analysis: Of the PHM programs responding to the SoHM Survey, 46.5% predicted an increase in budgeted full-time equivalents in the next year, while only 5.1% anticipated a decrease. Expecting this to change in response to COVID-19, the supplemental survey sought to update this information. Of the 30 PHM respondents to the supplemental survey, 41% instituted a temporary hiring freeze because of COVID-19, while 8.3% instituted a hiring freeze felt likely to be permanent. As PHM programs gear up for the next viral season, we wait to see whether the impact of COVID-19 will continue to be reflected in the volume and variety of patients admitted. It is clear that PHM programs will need to remain nimble to stay ahead of the changing landscape of practice in the days ahead. View all data by obtaining access to the 2020 SoHM Report at hospitalmedicine.org/sohm.
Many thanks to pediatric task force members Jack Percelay, MD; Vivien Kon-Ea Sun, MD; Marcos Mestre, MD; Ann Allen, MD; Dimple Khona, MD; Jeff Grill, MD; and Michelle Marks, MD.
Dr. Gage is director of faculty development, pediatric hospital medicine, at Phoenix Children’s Hospital, and associate professor of pediatrics at the University of Arizona, Phoenix.
On receiving the COVID-19 vaccine
This moment, for which I am so grateful and fortunate, represents a link in a remarkable chain of events that spans decades and represents the acme of human achievement.
My gratitude starts with scientists who years before this pandemic, perfected the ability to extract DNA from viruses, sequence it, and transcribe it to RNA. From there my gratitude goes to scientists who years ago developed an ingenious animal model for mRNA vaccines. The next link of gratitude is for scientists who at the start of this year quickly identified a deadly novel coronavirus and to scientists who rapidly sequenced its villainous DNA.
Next, I give thanks to scientists who promptly identified the segment of that DNA that codes for the spike proteins that the virus uses to invade our cells. And then I am grateful to the scientists who made the mRNA that corresponds to that specific DNA sequence, and to the scientists who figured out how create a lipid womb to protect that precious mRNA payload during its perilous journey from factory floor to the depths of our deltoid musculature.
I am no less grateful to the brave people who volunteered for the Pfizer trial, taking the risk of being the first humans ever to participate in an mRNA trial with stakes so high, and to the investigators who ran that trial and the scientists at Pfizer, the Food and Drug Administration, and the Western Coalition who reviewed the data and approved the vaccine without bowing to political pressure.
My gratitude extends to the factory workers who manufactured the vaccine in mass quantities, and the workers who manufactured the equipment that those factories rely on, and the pilots of planes and drivers of trucks who transported the vaccine to my hospital in Seattle, and to the workers who made those planes and trucks that carried that precious cargo. And the workers who devised super-cold storage systems and the workers who built those systems, and the people who fed them and clothed them and housed them so that they could do this life-saving work.
And to the leaders at my hospital who devised our immunization plan, and the ethicists who figured out who should go first (thanks Nancy), and the workers who made the glass vials to hold the vaccine, the plastic syringes to deliver it precisely, and surgically sharp needles so that there would be no pain whatsoever when those beautiful little mRNA filled lipid particles got injected into my left deltoid muscle by a highly skilled and compassionate nurse.
From there, the miracle of nature takes hold causing my cells to transcribe that RNA into spike proteins which will trigger my magical B-cells and T-cells to recognize that nasty spike protein as foreign in case it ever shows its ugly head to my respiratory mucosa, where these cells and the antibodies and chemicals they produce would stomp that wretched virus down without me ever knowing it or missing a beat, and keep me safe not only to live and thrive another day but also hopefully prevent me from spreading the virus to those I love and others I don’t even know but pass within just feet of.
For these miracles of nature and the chain of human toil and genius involving innumerable individuals over many years, many whom will never be thanked or recognized, I am truly and forever grateful.
Dr. Aaronson is a hospitalist and chief medical informatics officer at Virginia Mason Medical Center in Seattle.
This moment, for which I am so grateful and fortunate, represents a link in a remarkable chain of events that spans decades and represents the acme of human achievement.
My gratitude starts with scientists who years before this pandemic, perfected the ability to extract DNA from viruses, sequence it, and transcribe it to RNA. From there my gratitude goes to scientists who years ago developed an ingenious animal model for mRNA vaccines. The next link of gratitude is for scientists who at the start of this year quickly identified a deadly novel coronavirus and to scientists who rapidly sequenced its villainous DNA.
Next, I give thanks to scientists who promptly identified the segment of that DNA that codes for the spike proteins that the virus uses to invade our cells. And then I am grateful to the scientists who made the mRNA that corresponds to that specific DNA sequence, and to the scientists who figured out how create a lipid womb to protect that precious mRNA payload during its perilous journey from factory floor to the depths of our deltoid musculature.
I am no less grateful to the brave people who volunteered for the Pfizer trial, taking the risk of being the first humans ever to participate in an mRNA trial with stakes so high, and to the investigators who ran that trial and the scientists at Pfizer, the Food and Drug Administration, and the Western Coalition who reviewed the data and approved the vaccine without bowing to political pressure.
My gratitude extends to the factory workers who manufactured the vaccine in mass quantities, and the workers who manufactured the equipment that those factories rely on, and the pilots of planes and drivers of trucks who transported the vaccine to my hospital in Seattle, and to the workers who made those planes and trucks that carried that precious cargo. And the workers who devised super-cold storage systems and the workers who built those systems, and the people who fed them and clothed them and housed them so that they could do this life-saving work.
And to the leaders at my hospital who devised our immunization plan, and the ethicists who figured out who should go first (thanks Nancy), and the workers who made the glass vials to hold the vaccine, the plastic syringes to deliver it precisely, and surgically sharp needles so that there would be no pain whatsoever when those beautiful little mRNA filled lipid particles got injected into my left deltoid muscle by a highly skilled and compassionate nurse.
From there, the miracle of nature takes hold causing my cells to transcribe that RNA into spike proteins which will trigger my magical B-cells and T-cells to recognize that nasty spike protein as foreign in case it ever shows its ugly head to my respiratory mucosa, where these cells and the antibodies and chemicals they produce would stomp that wretched virus down without me ever knowing it or missing a beat, and keep me safe not only to live and thrive another day but also hopefully prevent me from spreading the virus to those I love and others I don’t even know but pass within just feet of.
For these miracles of nature and the chain of human toil and genius involving innumerable individuals over many years, many whom will never be thanked or recognized, I am truly and forever grateful.
Dr. Aaronson is a hospitalist and chief medical informatics officer at Virginia Mason Medical Center in Seattle.
This moment, for which I am so grateful and fortunate, represents a link in a remarkable chain of events that spans decades and represents the acme of human achievement.
My gratitude starts with scientists who years before this pandemic, perfected the ability to extract DNA from viruses, sequence it, and transcribe it to RNA. From there my gratitude goes to scientists who years ago developed an ingenious animal model for mRNA vaccines. The next link of gratitude is for scientists who at the start of this year quickly identified a deadly novel coronavirus and to scientists who rapidly sequenced its villainous DNA.
Next, I give thanks to scientists who promptly identified the segment of that DNA that codes for the spike proteins that the virus uses to invade our cells. And then I am grateful to the scientists who made the mRNA that corresponds to that specific DNA sequence, and to the scientists who figured out how create a lipid womb to protect that precious mRNA payload during its perilous journey from factory floor to the depths of our deltoid musculature.
I am no less grateful to the brave people who volunteered for the Pfizer trial, taking the risk of being the first humans ever to participate in an mRNA trial with stakes so high, and to the investigators who ran that trial and the scientists at Pfizer, the Food and Drug Administration, and the Western Coalition who reviewed the data and approved the vaccine without bowing to political pressure.
My gratitude extends to the factory workers who manufactured the vaccine in mass quantities, and the workers who manufactured the equipment that those factories rely on, and the pilots of planes and drivers of trucks who transported the vaccine to my hospital in Seattle, and to the workers who made those planes and trucks that carried that precious cargo. And the workers who devised super-cold storage systems and the workers who built those systems, and the people who fed them and clothed them and housed them so that they could do this life-saving work.
And to the leaders at my hospital who devised our immunization plan, and the ethicists who figured out who should go first (thanks Nancy), and the workers who made the glass vials to hold the vaccine, the plastic syringes to deliver it precisely, and surgically sharp needles so that there would be no pain whatsoever when those beautiful little mRNA filled lipid particles got injected into my left deltoid muscle by a highly skilled and compassionate nurse.
From there, the miracle of nature takes hold causing my cells to transcribe that RNA into spike proteins which will trigger my magical B-cells and T-cells to recognize that nasty spike protein as foreign in case it ever shows its ugly head to my respiratory mucosa, where these cells and the antibodies and chemicals they produce would stomp that wretched virus down without me ever knowing it or missing a beat, and keep me safe not only to live and thrive another day but also hopefully prevent me from spreading the virus to those I love and others I don’t even know but pass within just feet of.
For these miracles of nature and the chain of human toil and genius involving innumerable individuals over many years, many whom will never be thanked or recognized, I am truly and forever grateful.
Dr. Aaronson is a hospitalist and chief medical informatics officer at Virginia Mason Medical Center in Seattle.
Limiting antibiotic therapy after surgical drainage for native joint bacterial arthritis
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: Currently the recommended duration of antibiotic therapy for native joint bacterial arthritis is 3-6 weeks based on expert opinion.
Study design: Prospective, unblinded, randomized, noninferiority.
Setting: Single center in Geneva.
Synopsis: In total, 154 patients were randomized to either 2 weeks or 4 weeks of antibiotic regimen selected in consultation with infectious disease specialists after surgical drainage of native joint bacterial arthritis.
The study population was 38% women with a median age of 51 years. Sites of infection were majority hand and wrist arthritis (64%). The most frequent pathogen was Staphylococcus aureus (31%) with no methicillin-resistant strains. There was a low incidence of patients with bacteremia (4%) and chronic immune compromise (10%). Antibiotic regimen varied with 13 different initial intravenous regimens and 11 different oral regimens.
The primary study outcome was rate of recurrent infection within 2 years, which was low with only one recurrence in the 2-week arm and two recurrences in the 4-week arm. This difference was well within the 10% noninferiority margin selected by the authors.
The study was underpowered for nonhand and nonwrist cases, limiting generalizability.
Bottom line: Consider a shorter duration of antibiotic therapy after surgical drainage for native joint bacterial arthritis of the hand and wrist in an otherwise healthy patient.
Citation: Gjika E et al. Two weeks versus four weeks of antibiotic therapy after surgical drainage for native joint bacterial arthritis: a prospective, randomized, non-inferiority trial. Ann Rheum Dis. 2019 Aug;78(8):1114-21.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
The state of inpatient COVID-19 care
A brief evidence-based review of everything we have learned
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.
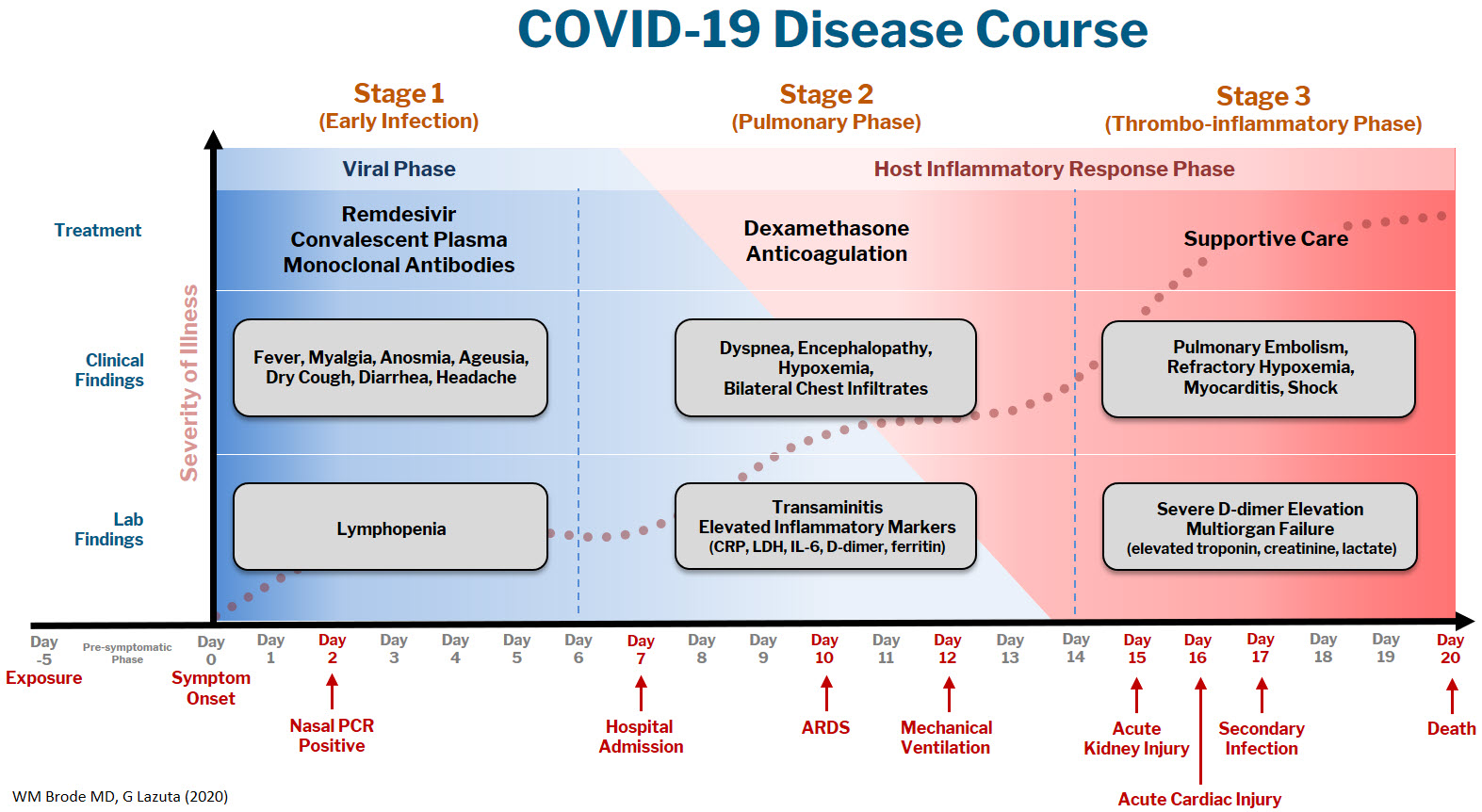
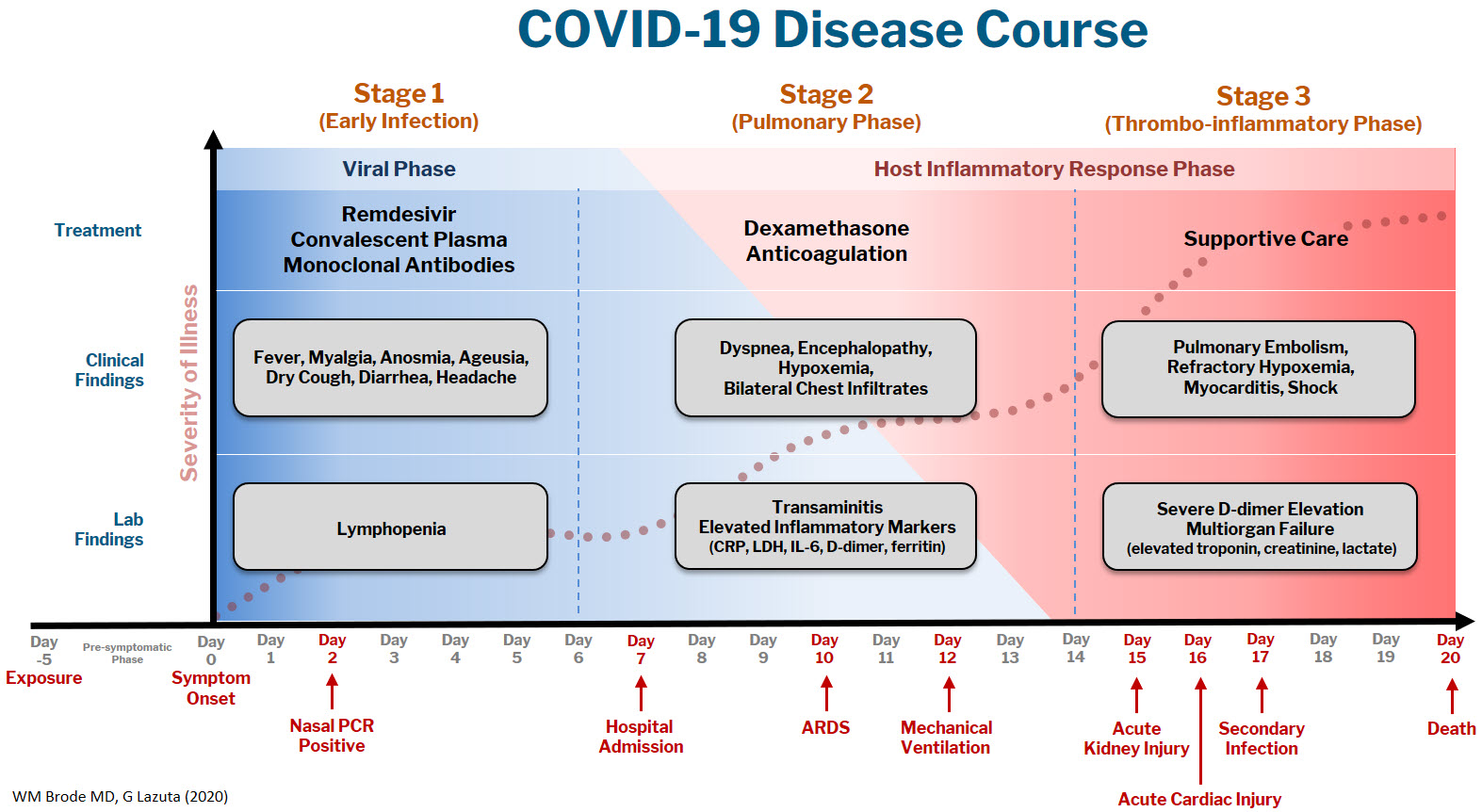
At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
A brief evidence-based review of everything we have learned
A brief evidence-based review of everything we have learned
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.

At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
Evidence on emerging treatments for COVID-19 has been incomplete, often disappointing, and rapidly changing. The concept of a practice-changing press release is as novel as the coronavirus. The pandemic has created an interdependent set of inpatient challenges: keeping up with evolving science and operationalizing clinical workflows, technology, and therapeutics to adapt what we are learning.

At Dell Medical School, we have created a Therapeutics and Informatics Committee to put evidence into practice in real-time, and below is a brief framework of what we have learned to date:
The COVID-19 disease course can be broken down into 3 stages, and workup and interventions should be targeted to those stages.1–3
Stage 1 is the viral phase following a median 5-day pre-symptomatic phase from exposure; this is indistinguishable from an influenza-like illness with the typical fever, cough, GI symptoms, and the more specific anosmia, ageusia, and orthostasis.
Stage 2 is the pulmonary phase where patients develop COVID-19 pneumonia and will have diffuse chest infiltrates on imaging. This stage usually represents the tail end of the viral phase prior to recovery, but for the ~15% of patients who present to the hospital needing admission because of hypoxemia (the definition of severe COVID-19, typically 5-7 days from symptom onset) this phase is characterized by elevated inflammatory markers and an exuberant host-immune response.
Stage 3 is the dreaded thrombo-inflammatory phase, which is a late manifestation usually >10 days from symptom onset and appears to be independent of viral replication. The morbidity and mortality associated with COVID-19 is likely a result of diffuse microthrombosis, and critical disease should no longer be thought of as a “cytokine storm,” but as life-threatening organ dysfunction caused by a dysregulated host response to infection. Unlike sepsis, the predominant pathology is not vasodilation and shock, but a hypercoagulable state with diffuse endothelial damage.4,5
Workup on presentation to the hospital should focus on identifying which phase of illness the patient is in, based on timing of symptom onset, inflammatory markers, and end-organ damage. CBC, CMP, D-dimer, troponin, and CRP are likely sufficient baseline labs in addition to a chest X-ray. There are many risk stratification tools, but to date, the 4C Mortality 4C Deterioration Scores are recommended due to their large derivation cohort and reliance on only 8 practical variables.6
Remdesivir and convalescent plasma (CVP) disrupt viral replication in stages 1 and 2 of the illness. Remdesivir has shown efficacy reducing hospital length of stay and a small trend towards decreasing mortality, especially if given within 10 days of symptom onset, although its effectiveness in general use is very small, if it exists at all.7,8 CVP efficacy has been disappointing and should not be the standard of care: multiple RCTs do not show any clinical benefit, although the Mayo Clinic registry data suggests that high-titer CVP given within 3 days from diagnosis decreases mortality compared to low-titer plasma.9-11 Monoclonal antibodies are theoretically “supercharged” high-titer CVP, but are approved for outpatient use only. Trials for hospitalized patients requiring oxygen were stopped due to futility. By the time the patient is hospitalized, it is probably too late in the disease course for CVP or monoclonal antibodies to be effective.
Dexamethasone is the only treatment with a proven mortality benefit. The RECOVERY trial showed the greatest mortality benefit (number needed to treat [NNT] of 8) in mechanically ventilated patients > 7 days from symptom onset. While there is a benefit to patients requiring any oxygen (NNT of 35), early administration to patients in the viral phase is associated with higher mortality as corticosteroids can reduce viral clearance.12 Corticosteroids should therefore be targeted to a therapeutic window to reduce the dysregulated host immune response and treat ARDS in phases 2 and 3; earlier is not necessarily better.
Incidence of venous thromboembolism (VTE) increases linearly with disease severity (one metanalysis showing a rate of 24% in the ICU13) and autopsy studies demonstrate diffuse microthrombosis even when VTE was not suspected5. Observational studies have shown VTE pharmacoprophylaxis reduces mortality, but the optimal agent, timing, and intensity of regimens is not yet clear.14-15 A recent press release from the NIH reported that full dose prophylactic anticoagulation in moderately ill patients reduced disease progression and trended toward lower mortality. Interestingly, for critically ill patients requiring high-flow nasal cannula (HFNC) or mechanical ventilation, intensified anticoagulation regiments had potential harm, and enrollment was stopped in this cohort.16 This announcement is a hopeful sign that intensified anticoagulation regimens can prevent thrombo-inflammation, but until the data of multiple ongoing trials is published it remains expert opinion only.
The most important treatment remains delivering oxygen with fidelity, correcting the much-observed “silent” or “happy hypoxemic.”17 Given the high mortality associated with mechanical ventilation and that hypoxemia can be out of proportion to respiratory distress, arbitrary thresholds should not be used to decide when to intubate and instead should evaluate work of breathing, hypercapnia, mentation, or progression of end-organ damage rather than a single cutoff.18 High-flow nasal cannula (HFNC) can correct severe hypoxemia in addition to self-proning, and while there is scant outcomes data for this strategy, it has been adopted widely as ICU capacity is strained nationally. A ventilator can add PEEP for alveolar recruitment or perform the work of breathing for a patient, but a patient will receive 100% FiO2 whether it is delivered through the nares on HFNC or 10 inches lower by an endotracheal tube.
In the absence of a single therapeutic cure or breakthrough, caring for a COVID-19 patient requires the hospital system to instead do a thousand things conscientiously and consistently. This is supportive care: most patients will get better with time and attentive evaluation for end-organ complications like myocarditis, encephalopathy, or pressure ulcers. It requires nursing to patient ratios that allows for this type of vigilance, with shared protocols, order sets, and close communication among team members that provides this support. The treatment of COVID-19 continues to evolve, but as we confront rising hospital volumes nationally, it is important to standardize care for patients throughout each of the 3 stages of illness until we find that single breakthrough.
Dr. Brode is a practicing internal medicine physician at Dell Seton Medical Center and assistant professor in the Department of Internal Medicine at Dell Medical School, both in Austin, Texas. He is a clinician educator who emphasizes knowing the patient as a person first, evidence-based diagnosis, and comprehensive care for the patients who are most vulnerable. This article is part of a series originally published in The Hospital Leader, the official blog of SHM.
References
1. Cummings MJ, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. The Lancet. 2020 June 6;395(10239):1763-1770. doi:10.1016/S0140-6736(20)31189-2.
2. Oudkerk M, et al. Diagnosis, prevention, and treatment of thromboembolic complications in COVID-19: Report of the National Institute for Public Health of the Netherlands. Radiology. 2020;297(1):E216-E222. doi:10.1148/radiol.2020201629.
3. Siddiqi HK, and Mehra MR. COVID-19 illness in native and immunosuppressed states: A clinical–therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405-407.
4. Connors JM, and Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033-2040.
5. Ackermann M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020 July 9;383:120-128. doi:10.1056/NEJMoa2015432.
6. Knight SR, et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. doi:10.1136/bmj.m3339.
7. Beigel JH, et al. Remdesivir for the treatment of Covid-19 – Final report. N Engl J Med. 2020;383:1813-1826. doi:10.1056/NEJMoa2007764.
8. Repurposed antiviral drugs for COVID-19: Interim WHO SOLIDARITY trial results. medRxiv. 2020;10.15.20209817. doi:10.1101/2020.10.15.20209817.
9. Agarwal A, et al. Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial). BMJ. 2020;371:m3939.
10. Simonovich VA, et al. A randomized trial of convalescent plasma in Covid-19 severe pneumonia. N Engl J Med. 2020 Nov 24. doi:10.1056/NEJMoa2031304.
11. Joyner MJ, et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N Engl J Med 2021; 384:1015-1027. doi:10.1056/NEJMoa2031893.
12. The RECOVERY Collaborative Group: Dexamethasone in hospitalized patients with Covid-19 – Preliminary report. N Engl J Med. 2020 July 17. doi:10.1056/NEJMoa2021436.
13. Porfidia A, et al. Venous thromboembolism in patients with COVID-19: Systematic review and meta-analysis. Thromb Res. 2020 Dec;196:67-74.
14. Nadkarni GN, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: A single health system study. J Am Coll Cardiol. 2020 Oct 20;76(16):1815-1826. doi:10.1016/j.jacc.2020.08.041.
15. Paranjpe I, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. J Am Coll Cardiol. 2020 Jul 7;76(1):122-124. doi:10.1016/j.jacc.2020.05.001.
16. Full-dose blood thinners decreased need for life support and improved outcome in hospitalized COVID-19 patients. National Institutes of Health. Available at https://www.nih.gov/news-events/news-releases/full-dose-blood-thinners-decreased-need-life-support-improved-outcome-hospitalized-covid-19-patients.
17. Tobin MJ, et al. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020 Aug 1;202(3):356-360. doi:10.1164/rccm.202006-2157CP.
18. Berlin DA, et al. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. doi:10.1056/NEJMcp2009575.
Think twice before intensifying BP regimen in older hospitalized patients
Background: It is common practice for providers to intensify antihypertensive regimen during admission for noncardiac conditions even if a patient has a history of well-controlled blood pressure as an outpatient. Many providers have assumed that these changes will benefit patients; however, this outcome had never been studied.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: The authors analyzed a well-matched retrospective cohort of 4,056 adults aged 65 years or older with hypertension who were admitted for noncardiac conditions including pneumonia, urinary tract infection, and venous thromboembolism. Half of the cohort was discharged with intensification of their antihypertensives, defined as a new antihypertensive medication or an increase of 20% of a prior medication.
Patients discharged with regimen intensification were more likely to be readmitted (hazard ratio, 1.23; 95% confidence interval, 1.07-1.42; number needed to harm = 27), experience a medication-related serious adverse event (HR, 1.42; 95% CI, 1.06-1.88; NNH = 63), and have a cardiovascular event (HR, 1.65; 95% CI, 1.13-2.4) within 30 days of discharge. At 1 year, no significant difference in mortality, cardiovascular events, or systolic BP were noted between the two groups.
A subgroup analysis of patients with poorly controlled blood pressure as outpatients (defined as systolic blood pressure greater than 140 mm Hg) who had their anti-hypertensive medications intensified did not show significant difference in 30-day readmission, severe adverse events, or cardiovascular events.
Limitations of the study include observational design and majority male sex (97.5%) of the study population.
Bottom line: Intensification of antihypertensive regimen among older adults hospitalized for noncardiac conditions with well-controlled blood pressure as an outpatient can potentially cause harm.
Citation: Anderson TS et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.3007.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: It is common practice for providers to intensify antihypertensive regimen during admission for noncardiac conditions even if a patient has a history of well-controlled blood pressure as an outpatient. Many providers have assumed that these changes will benefit patients; however, this outcome had never been studied.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: The authors analyzed a well-matched retrospective cohort of 4,056 adults aged 65 years or older with hypertension who were admitted for noncardiac conditions including pneumonia, urinary tract infection, and venous thromboembolism. Half of the cohort was discharged with intensification of their antihypertensives, defined as a new antihypertensive medication or an increase of 20% of a prior medication.
Patients discharged with regimen intensification were more likely to be readmitted (hazard ratio, 1.23; 95% confidence interval, 1.07-1.42; number needed to harm = 27), experience a medication-related serious adverse event (HR, 1.42; 95% CI, 1.06-1.88; NNH = 63), and have a cardiovascular event (HR, 1.65; 95% CI, 1.13-2.4) within 30 days of discharge. At 1 year, no significant difference in mortality, cardiovascular events, or systolic BP were noted between the two groups.
A subgroup analysis of patients with poorly controlled blood pressure as outpatients (defined as systolic blood pressure greater than 140 mm Hg) who had their anti-hypertensive medications intensified did not show significant difference in 30-day readmission, severe adverse events, or cardiovascular events.
Limitations of the study include observational design and majority male sex (97.5%) of the study population.
Bottom line: Intensification of antihypertensive regimen among older adults hospitalized for noncardiac conditions with well-controlled blood pressure as an outpatient can potentially cause harm.
Citation: Anderson TS et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.3007.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Background: It is common practice for providers to intensify antihypertensive regimen during admission for noncardiac conditions even if a patient has a history of well-controlled blood pressure as an outpatient. Many providers have assumed that these changes will benefit patients; however, this outcome had never been studied.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: The authors analyzed a well-matched retrospective cohort of 4,056 adults aged 65 years or older with hypertension who were admitted for noncardiac conditions including pneumonia, urinary tract infection, and venous thromboembolism. Half of the cohort was discharged with intensification of their antihypertensives, defined as a new antihypertensive medication or an increase of 20% of a prior medication.
Patients discharged with regimen intensification were more likely to be readmitted (hazard ratio, 1.23; 95% confidence interval, 1.07-1.42; number needed to harm = 27), experience a medication-related serious adverse event (HR, 1.42; 95% CI, 1.06-1.88; NNH = 63), and have a cardiovascular event (HR, 1.65; 95% CI, 1.13-2.4) within 30 days of discharge. At 1 year, no significant difference in mortality, cardiovascular events, or systolic BP were noted between the two groups.
A subgroup analysis of patients with poorly controlled blood pressure as outpatients (defined as systolic blood pressure greater than 140 mm Hg) who had their anti-hypertensive medications intensified did not show significant difference in 30-day readmission, severe adverse events, or cardiovascular events.
Limitations of the study include observational design and majority male sex (97.5%) of the study population.
Bottom line: Intensification of antihypertensive regimen among older adults hospitalized for noncardiac conditions with well-controlled blood pressure as an outpatient can potentially cause harm.
Citation: Anderson TS et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019 Aug 19. doi: 10.1001/jamainternmed.2019.3007.
Dr. Zarookian is a hospitalist at Maine Medical Center in Portland and Stephens Memorial Hospital in Norway, Maine.
Covert stroke after noncardiac surgery linked with cognitive decline
Background: Prior studies have established an increased risk of overt stroke after noncardiac surgery, with significant associated morbidity and mortality. Similarly, covert stroke in the nonsurgical population is well described and has been shown to be associated with cognitive decline.
Study design: Prospective cohort study.
Setting: Academic centers in nine countries.
Synopsis: This study evaluated 1,114 patients older than 65 years who were hospitalized for noncardiac surgery, excluding patients with carotid and neurosurgical procedures. All enrolled participants completed diffusion-weight MRI of the brain within 9 days of surgery. Follow-up rates for clinical outcomes (1,112; greater than 99%) were excellent, and the primary outcome measure, follow-up Montreal Cognitive Assessment (MOCA) at 1 year, was defined in 1,001 (90%) of the study subjects.
Covert stroke was detected in 78 (7%) of the study participants. Those with covert stroke had a higher incidence of cognitive decline at 1 year (adjusted odds ratio, 1.98; 95% confidence interval, 1.22-3.2) with an absolute risk increase of 13%. Patients with covert stroke also had a higher rate of delirium within 3 days of surgery (hazard ratio, 2.24; 95% CI, 1.06-4.73) and a higher rate of overt stroke and transient ischemic attack at 1 year (HR, 4.13; 95% CI, 1.14-14.99).
This study helps to establish the incidence of covert stroke after noncardiac surgery and provides support for covert stroke as a risk factor for cognitive impairment.
Bottom line: Covert stroke following noncardiac surgery is common, affecting 1 in 14 patients in this study, and it is associated with an increased risk of cognitive decline, perioperative delirium, and subsequent overt stroke.
Citation: The NeuroVISION Investigators (Mrkobrada M et al.). Perioperative covert stroke in patients undergoing noncardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394(10203):1022-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.
Background: Prior studies have established an increased risk of overt stroke after noncardiac surgery, with significant associated morbidity and mortality. Similarly, covert stroke in the nonsurgical population is well described and has been shown to be associated with cognitive decline.
Study design: Prospective cohort study.
Setting: Academic centers in nine countries.
Synopsis: This study evaluated 1,114 patients older than 65 years who were hospitalized for noncardiac surgery, excluding patients with carotid and neurosurgical procedures. All enrolled participants completed diffusion-weight MRI of the brain within 9 days of surgery. Follow-up rates for clinical outcomes (1,112; greater than 99%) were excellent, and the primary outcome measure, follow-up Montreal Cognitive Assessment (MOCA) at 1 year, was defined in 1,001 (90%) of the study subjects.
Covert stroke was detected in 78 (7%) of the study participants. Those with covert stroke had a higher incidence of cognitive decline at 1 year (adjusted odds ratio, 1.98; 95% confidence interval, 1.22-3.2) with an absolute risk increase of 13%. Patients with covert stroke also had a higher rate of delirium within 3 days of surgery (hazard ratio, 2.24; 95% CI, 1.06-4.73) and a higher rate of overt stroke and transient ischemic attack at 1 year (HR, 4.13; 95% CI, 1.14-14.99).
This study helps to establish the incidence of covert stroke after noncardiac surgery and provides support for covert stroke as a risk factor for cognitive impairment.
Bottom line: Covert stroke following noncardiac surgery is common, affecting 1 in 14 patients in this study, and it is associated with an increased risk of cognitive decline, perioperative delirium, and subsequent overt stroke.
Citation: The NeuroVISION Investigators (Mrkobrada M et al.). Perioperative covert stroke in patients undergoing noncardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394(10203):1022-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.
Background: Prior studies have established an increased risk of overt stroke after noncardiac surgery, with significant associated morbidity and mortality. Similarly, covert stroke in the nonsurgical population is well described and has been shown to be associated with cognitive decline.
Study design: Prospective cohort study.
Setting: Academic centers in nine countries.
Synopsis: This study evaluated 1,114 patients older than 65 years who were hospitalized for noncardiac surgery, excluding patients with carotid and neurosurgical procedures. All enrolled participants completed diffusion-weight MRI of the brain within 9 days of surgery. Follow-up rates for clinical outcomes (1,112; greater than 99%) were excellent, and the primary outcome measure, follow-up Montreal Cognitive Assessment (MOCA) at 1 year, was defined in 1,001 (90%) of the study subjects.
Covert stroke was detected in 78 (7%) of the study participants. Those with covert stroke had a higher incidence of cognitive decline at 1 year (adjusted odds ratio, 1.98; 95% confidence interval, 1.22-3.2) with an absolute risk increase of 13%. Patients with covert stroke also had a higher rate of delirium within 3 days of surgery (hazard ratio, 2.24; 95% CI, 1.06-4.73) and a higher rate of overt stroke and transient ischemic attack at 1 year (HR, 4.13; 95% CI, 1.14-14.99).
This study helps to establish the incidence of covert stroke after noncardiac surgery and provides support for covert stroke as a risk factor for cognitive impairment.
Bottom line: Covert stroke following noncardiac surgery is common, affecting 1 in 14 patients in this study, and it is associated with an increased risk of cognitive decline, perioperative delirium, and subsequent overt stroke.
Citation: The NeuroVISION Investigators (Mrkobrada M et al.). Perioperative covert stroke in patients undergoing noncardiac surgery (NeuroVISION): a prospective cohort study. Lancet. 2019;394(10203):1022-9.
Dr. Herrle is a hospitalist at Maine Medical Center in Portland and at Stephens Memorial Hospital in Norway, Maine.