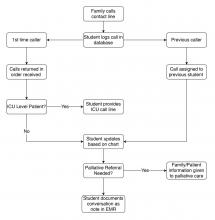
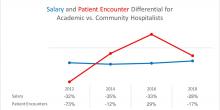
User login
Simplifying the antibiotic selection process
Hospitalists are constantly battling infection.
James Soo Kim, MD, a hospitalist and assistant professor at Emory Healthcare in Atlanta, a presenter of the session “Antibiotics Made Ridiculously Simple” during HM20 Virtual, said that while he has given this talk at previous Society of Hospital Medicine Annual Conferences, the presentation has undergone significant changes over the years as the landscape of infectious disease treatment has shifted.
He hopes attendees of HM20 Virtual will appreciate the changes and encourages those who have attended his presentation in previous years to come see what is new, but admitted newcomers may think the presentation’s title is a bit of a misnomer.
“Despite the title of the talk, there really isn’t any way to make antibiotics ridiculously simple,” he said.
Dr. Kim, who is also an editorial board member for The Hospitalist, said the origin of “Antibiotics Made Ridiculously Simple” took place during his residency, where he had an interest in infectious disease. This interest carried over to his time in fellowship at the Keck School of Medicine of the University of Southern California – and was enough to become board certified in infectious disease by the American Board of Internal Medicine. Infectious disease continues to interest him now as an attending, he said, and since he joined Emory Healthcare in 2012, he has given a version of this presentation every year.
HM20 Virtual attendees will come away from the presentation with an idea of how to choose an antibiotic regimen, Dr. Kim said, including how to select an antibiotic when you’re worried about Pseudomonas, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus or other likely organisms. “There are a variety of drugs out there that have activity against our ‘usual suspects,’ ” he said.
Attendees will also learn to select antibiotic options that have empiric coverage during a shortage of piperacillin/tazobactam (Zosyn), vancomycin, or your preferred drug of choice for treating common infections. He will also review the latest drugs that have been released over the past few years so attendees can add them to their armamentarium.
“I won’t necessarily expect attendees to use everything I talk about, but if you have a patient on service that infectious disease started Vabomere on, you’ll at least have a general idea of what they were worried about,” Dr. Kim said.
One practice pearl he hopes attendees take away from his presentation: Allergies to beta-lactam antibiotics like penicillin (PCN) derivatives are not as common as most providers and patients believe, and not giving these antibiotics to patients can actually decrease the chance that the patient gets appropriate therapy while also increasing the cost of care.
“I hope that my talk changes practice by making people aware of how infrequent true clinically significant PCN cross-reactions are so that patients can get more cost-effective and medically effective therapy,” he said.Dr. Kim reports no relevant financial disclosures.
Antibiotics Made Ridiculously Simple Live Q&A: Tuesday, August 18, 3:30-4:30 p.m.
Hospitalists are constantly battling infection.
James Soo Kim, MD, a hospitalist and assistant professor at Emory Healthcare in Atlanta, a presenter of the session “Antibiotics Made Ridiculously Simple” during HM20 Virtual, said that while he has given this talk at previous Society of Hospital Medicine Annual Conferences, the presentation has undergone significant changes over the years as the landscape of infectious disease treatment has shifted.
He hopes attendees of HM20 Virtual will appreciate the changes and encourages those who have attended his presentation in previous years to come see what is new, but admitted newcomers may think the presentation’s title is a bit of a misnomer.
“Despite the title of the talk, there really isn’t any way to make antibiotics ridiculously simple,” he said.
Dr. Kim, who is also an editorial board member for The Hospitalist, said the origin of “Antibiotics Made Ridiculously Simple” took place during his residency, where he had an interest in infectious disease. This interest carried over to his time in fellowship at the Keck School of Medicine of the University of Southern California – and was enough to become board certified in infectious disease by the American Board of Internal Medicine. Infectious disease continues to interest him now as an attending, he said, and since he joined Emory Healthcare in 2012, he has given a version of this presentation every year.
HM20 Virtual attendees will come away from the presentation with an idea of how to choose an antibiotic regimen, Dr. Kim said, including how to select an antibiotic when you’re worried about Pseudomonas, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus or other likely organisms. “There are a variety of drugs out there that have activity against our ‘usual suspects,’ ” he said.
Attendees will also learn to select antibiotic options that have empiric coverage during a shortage of piperacillin/tazobactam (Zosyn), vancomycin, or your preferred drug of choice for treating common infections. He will also review the latest drugs that have been released over the past few years so attendees can add them to their armamentarium.
“I won’t necessarily expect attendees to use everything I talk about, but if you have a patient on service that infectious disease started Vabomere on, you’ll at least have a general idea of what they were worried about,” Dr. Kim said.
One practice pearl he hopes attendees take away from his presentation: Allergies to beta-lactam antibiotics like penicillin (PCN) derivatives are not as common as most providers and patients believe, and not giving these antibiotics to patients can actually decrease the chance that the patient gets appropriate therapy while also increasing the cost of care.
“I hope that my talk changes practice by making people aware of how infrequent true clinically significant PCN cross-reactions are so that patients can get more cost-effective and medically effective therapy,” he said.Dr. Kim reports no relevant financial disclosures.
Antibiotics Made Ridiculously Simple Live Q&A: Tuesday, August 18, 3:30-4:30 p.m.
Hospitalists are constantly battling infection.
James Soo Kim, MD, a hospitalist and assistant professor at Emory Healthcare in Atlanta, a presenter of the session “Antibiotics Made Ridiculously Simple” during HM20 Virtual, said that while he has given this talk at previous Society of Hospital Medicine Annual Conferences, the presentation has undergone significant changes over the years as the landscape of infectious disease treatment has shifted.
He hopes attendees of HM20 Virtual will appreciate the changes and encourages those who have attended his presentation in previous years to come see what is new, but admitted newcomers may think the presentation’s title is a bit of a misnomer.
“Despite the title of the talk, there really isn’t any way to make antibiotics ridiculously simple,” he said.
Dr. Kim, who is also an editorial board member for The Hospitalist, said the origin of “Antibiotics Made Ridiculously Simple” took place during his residency, where he had an interest in infectious disease. This interest carried over to his time in fellowship at the Keck School of Medicine of the University of Southern California – and was enough to become board certified in infectious disease by the American Board of Internal Medicine. Infectious disease continues to interest him now as an attending, he said, and since he joined Emory Healthcare in 2012, he has given a version of this presentation every year.
HM20 Virtual attendees will come away from the presentation with an idea of how to choose an antibiotic regimen, Dr. Kim said, including how to select an antibiotic when you’re worried about Pseudomonas, methicillin-resistant Staphylococcus aureus, and vancomycin-resistant Enterococcus or other likely organisms. “There are a variety of drugs out there that have activity against our ‘usual suspects,’ ” he said.
Attendees will also learn to select antibiotic options that have empiric coverage during a shortage of piperacillin/tazobactam (Zosyn), vancomycin, or your preferred drug of choice for treating common infections. He will also review the latest drugs that have been released over the past few years so attendees can add them to their armamentarium.
“I won’t necessarily expect attendees to use everything I talk about, but if you have a patient on service that infectious disease started Vabomere on, you’ll at least have a general idea of what they were worried about,” Dr. Kim said.
One practice pearl he hopes attendees take away from his presentation: Allergies to beta-lactam antibiotics like penicillin (PCN) derivatives are not as common as most providers and patients believe, and not giving these antibiotics to patients can actually decrease the chance that the patient gets appropriate therapy while also increasing the cost of care.
“I hope that my talk changes practice by making people aware of how infrequent true clinically significant PCN cross-reactions are so that patients can get more cost-effective and medically effective therapy,” he said.Dr. Kim reports no relevant financial disclosures.
Antibiotics Made Ridiculously Simple Live Q&A: Tuesday, August 18, 3:30-4:30 p.m.
Hospital medicine update highlights research from ‘extended family’
The annual “Update in Hospital Medicine” session will go a step further by highlighting the work and insights of what Dr. Pfeifer affectionately calls the “extended family.”
Scott Kaatz, DO, MSc, SFHM, a hospitalist at Henry Ford Hospital in Detroit, explained that “the Update has a long-standing tradition at the national meeting as an overview of the most impactful or insightful publications relevant to clinicians working in the hospital, which includes internists, pediatricians, obstetricians, family physicians, nurse practitioners, physician assistants, and other specialties.”
Why does the Update embrace such a wide focus? Because there’s a need for a broader perspective, according to Dr. Pfeifer, professor of medicine at the Medical College of Wisconsin, Milwaukee. “The Society of Hospital Medicine Annual Conference has many superb offerings with specific focuses that help attendees fill knowledge and practice gaps and network with individuals with similar interests,” he said. “All of those different offerings highlight something that is very cool about hospital medicine – its diversity. However, it’s also important for us to come together as one big family to support each other and advocate for the larger cause of hospital medicine. With the “Update in Hospital Medicine,” attendees can specifically hear about the clinical changes happening in their “extended family.”
“We will be giving an overview of key new literature across the spectrum of hospital medicine in areas such as sepsis, inclusion/diversity, co-management, and hospital staffing models,” Dr. Kaatz said. “We will also highlight the various different focuses/practices within hospital medicine and the wonderful diversity within the Society of Hospital Medicine. We have coordinated our selection of topics with the Special Interest Groups (SIGs) and the Chapters to make sure we include the voices of our wider membership. This will also allow us to celebrate our diversity by giving shout outs to our SIGs and chapters and showcase the wonderful things going on in hospital medicine, including advances being made by our very own members.”
Dr. Kaatz added that he and Dr. Pfeifer are grateful to the organizers for allowing them to try something new. “Presented papers will reflect the interests of SHM members via a ‘learner needs assessment’ survey,” he said. “Several of the special interest groups and local chapters surveyed their membership and voted on the most impactful papers in the past year. It has been very gratifying to see the level of engagement in our society and to be able to share this important research with a large audience.”
Dr. Pfeifer has no relevant disclosures. Dr. Kaatz discloses research funding to institution (BMS) and consultant/advisory board relationships (BMS, Pfizer and Janssen).
“Update in Hospital Medicine”
The annual “Update in Hospital Medicine” session will go a step further by highlighting the work and insights of what Dr. Pfeifer affectionately calls the “extended family.”
Scott Kaatz, DO, MSc, SFHM, a hospitalist at Henry Ford Hospital in Detroit, explained that “the Update has a long-standing tradition at the national meeting as an overview of the most impactful or insightful publications relevant to clinicians working in the hospital, which includes internists, pediatricians, obstetricians, family physicians, nurse practitioners, physician assistants, and other specialties.”
Why does the Update embrace such a wide focus? Because there’s a need for a broader perspective, according to Dr. Pfeifer, professor of medicine at the Medical College of Wisconsin, Milwaukee. “The Society of Hospital Medicine Annual Conference has many superb offerings with specific focuses that help attendees fill knowledge and practice gaps and network with individuals with similar interests,” he said. “All of those different offerings highlight something that is very cool about hospital medicine – its diversity. However, it’s also important for us to come together as one big family to support each other and advocate for the larger cause of hospital medicine. With the “Update in Hospital Medicine,” attendees can specifically hear about the clinical changes happening in their “extended family.”
“We will be giving an overview of key new literature across the spectrum of hospital medicine in areas such as sepsis, inclusion/diversity, co-management, and hospital staffing models,” Dr. Kaatz said. “We will also highlight the various different focuses/practices within hospital medicine and the wonderful diversity within the Society of Hospital Medicine. We have coordinated our selection of topics with the Special Interest Groups (SIGs) and the Chapters to make sure we include the voices of our wider membership. This will also allow us to celebrate our diversity by giving shout outs to our SIGs and chapters and showcase the wonderful things going on in hospital medicine, including advances being made by our very own members.”
Dr. Kaatz added that he and Dr. Pfeifer are grateful to the organizers for allowing them to try something new. “Presented papers will reflect the interests of SHM members via a ‘learner needs assessment’ survey,” he said. “Several of the special interest groups and local chapters surveyed their membership and voted on the most impactful papers in the past year. It has been very gratifying to see the level of engagement in our society and to be able to share this important research with a large audience.”
Dr. Pfeifer has no relevant disclosures. Dr. Kaatz discloses research funding to institution (BMS) and consultant/advisory board relationships (BMS, Pfizer and Janssen).
“Update in Hospital Medicine”
The annual “Update in Hospital Medicine” session will go a step further by highlighting the work and insights of what Dr. Pfeifer affectionately calls the “extended family.”
Scott Kaatz, DO, MSc, SFHM, a hospitalist at Henry Ford Hospital in Detroit, explained that “the Update has a long-standing tradition at the national meeting as an overview of the most impactful or insightful publications relevant to clinicians working in the hospital, which includes internists, pediatricians, obstetricians, family physicians, nurse practitioners, physician assistants, and other specialties.”
Why does the Update embrace such a wide focus? Because there’s a need for a broader perspective, according to Dr. Pfeifer, professor of medicine at the Medical College of Wisconsin, Milwaukee. “The Society of Hospital Medicine Annual Conference has many superb offerings with specific focuses that help attendees fill knowledge and practice gaps and network with individuals with similar interests,” he said. “All of those different offerings highlight something that is very cool about hospital medicine – its diversity. However, it’s also important for us to come together as one big family to support each other and advocate for the larger cause of hospital medicine. With the “Update in Hospital Medicine,” attendees can specifically hear about the clinical changes happening in their “extended family.”
“We will be giving an overview of key new literature across the spectrum of hospital medicine in areas such as sepsis, inclusion/diversity, co-management, and hospital staffing models,” Dr. Kaatz said. “We will also highlight the various different focuses/practices within hospital medicine and the wonderful diversity within the Society of Hospital Medicine. We have coordinated our selection of topics with the Special Interest Groups (SIGs) and the Chapters to make sure we include the voices of our wider membership. This will also allow us to celebrate our diversity by giving shout outs to our SIGs and chapters and showcase the wonderful things going on in hospital medicine, including advances being made by our very own members.”
Dr. Kaatz added that he and Dr. Pfeifer are grateful to the organizers for allowing them to try something new. “Presented papers will reflect the interests of SHM members via a ‘learner needs assessment’ survey,” he said. “Several of the special interest groups and local chapters surveyed their membership and voted on the most impactful papers in the past year. It has been very gratifying to see the level of engagement in our society and to be able to share this important research with a large audience.”
Dr. Pfeifer has no relevant disclosures. Dr. Kaatz discloses research funding to institution (BMS) and consultant/advisory board relationships (BMS, Pfizer and Janssen).
“Update in Hospital Medicine”
Get updated: Latest ATS/ISDA guidelines for pneumonia
according to Joanna M. Bonsall, MD, PhD, SFHM, chief of hospital medicine at Grady Memorial Hospital and associate professor of medicine at Emory University, both in Atlanta.
Last year, the American Thoracic Society and the Infectious Diseases Society of America updated their clinical guidelines on community-acquired pneumonia (CAP) for the first time since 2007. The guidelines were published in the Oct. 1, 2019 issue of the American Journal of Respiratory and Critical Care Medicine.
CAP is one of the most common reasons for hospitalization in the United States, and it is estimated that CAP comprises over 4.5 million outpatient and ED visits each year, according to the National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey in 2009-2010. It is also the most common cause of death from infection disease, according to the Centers for Disease Control and Prevention.
Dr. Bonsall will present “Updates in Pneumonia” at HM20 Virtual, the virtual annual meeting of the Society of Hospital Medicine; a live question-and-answer session will be held online Aug. 20. In her session, Dr. Bonsall said she plans to cover the new ATS/IDSA guidelines for CAP, which will include what initial testing to order, which empiric antibiotics to use, and how to manage patients at risk for resistant organisms, formerly known as health care–associated pneumonia (HCAP). Dr. Bonsall also will outline the evidence for use of steroids, especially in cases of severe pneumonia, and review the 2016 ATS/IDSA guidelines for hospital-acquired pneumonia with a focus on antibiotic selection.
One major change for 2019: The ATS/IDSA CAP guideline authors issued a strong recommendation to abandon use of the term HCAP as a “distinct clinical entity” when considering antibiotics for patients with CAP. In addition, methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa should only be empirically covered in patients with CAP if they present with locally validated risk factors for either pathogen, according to the guidelines.
“Order pretreatment testing based on severity of illness as well as risk factors for drug-resistant pathogens,” Dr. Bonsall said. Hospitalists also should avoid using procalcitonin levels as a benchmark for whether a patient should be started on antibiotics. Once the recommended antibiotic treatment has been initiated, attendees should use culture results to narrow down the possibilities, especially in cases of drug-resistant pathogens.
The ATS/IDSA guidelines also state that corticosteroids should not be routinely used for patients with nonsevere CAP, but attendees should also be aware of the limitations and interpretations of the evidence, Dr. Bonsall said. Avoiding routine corticosteroid use in patients with severe CAP or in patients with severe influenza pneumonia carries a conditional recommendation with a moderate and low quality of evidence, respectively. In general, cases of CAP should be treated for no more than 5 days, or 3 days of treatment after the patient becomes clinically stable.
Attendees at HM20 Virtual should walk away from the session knowing what testing is necessary and what testing is unnecessary, and how to reduce antibiotic exposure for both broad spectrum use and duration. “At the end of the session, you should feel comfortable using both the CAP and HAP guidelines,” Dr. Bonsall said.
Dr. Bonsall reported no relevant financial disclosures.
Updates in Pneumonia
Live Q&A: Thursday, Aug. 20, 2:15 p.m to 3:15 p.m.
according to Joanna M. Bonsall, MD, PhD, SFHM, chief of hospital medicine at Grady Memorial Hospital and associate professor of medicine at Emory University, both in Atlanta.
Last year, the American Thoracic Society and the Infectious Diseases Society of America updated their clinical guidelines on community-acquired pneumonia (CAP) for the first time since 2007. The guidelines were published in the Oct. 1, 2019 issue of the American Journal of Respiratory and Critical Care Medicine.
CAP is one of the most common reasons for hospitalization in the United States, and it is estimated that CAP comprises over 4.5 million outpatient and ED visits each year, according to the National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey in 2009-2010. It is also the most common cause of death from infection disease, according to the Centers for Disease Control and Prevention.
Dr. Bonsall will present “Updates in Pneumonia” at HM20 Virtual, the virtual annual meeting of the Society of Hospital Medicine; a live question-and-answer session will be held online Aug. 20. In her session, Dr. Bonsall said she plans to cover the new ATS/IDSA guidelines for CAP, which will include what initial testing to order, which empiric antibiotics to use, and how to manage patients at risk for resistant organisms, formerly known as health care–associated pneumonia (HCAP). Dr. Bonsall also will outline the evidence for use of steroids, especially in cases of severe pneumonia, and review the 2016 ATS/IDSA guidelines for hospital-acquired pneumonia with a focus on antibiotic selection.
One major change for 2019: The ATS/IDSA CAP guideline authors issued a strong recommendation to abandon use of the term HCAP as a “distinct clinical entity” when considering antibiotics for patients with CAP. In addition, methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa should only be empirically covered in patients with CAP if they present with locally validated risk factors for either pathogen, according to the guidelines.
“Order pretreatment testing based on severity of illness as well as risk factors for drug-resistant pathogens,” Dr. Bonsall said. Hospitalists also should avoid using procalcitonin levels as a benchmark for whether a patient should be started on antibiotics. Once the recommended antibiotic treatment has been initiated, attendees should use culture results to narrow down the possibilities, especially in cases of drug-resistant pathogens.
The ATS/IDSA guidelines also state that corticosteroids should not be routinely used for patients with nonsevere CAP, but attendees should also be aware of the limitations and interpretations of the evidence, Dr. Bonsall said. Avoiding routine corticosteroid use in patients with severe CAP or in patients with severe influenza pneumonia carries a conditional recommendation with a moderate and low quality of evidence, respectively. In general, cases of CAP should be treated for no more than 5 days, or 3 days of treatment after the patient becomes clinically stable.
Attendees at HM20 Virtual should walk away from the session knowing what testing is necessary and what testing is unnecessary, and how to reduce antibiotic exposure for both broad spectrum use and duration. “At the end of the session, you should feel comfortable using both the CAP and HAP guidelines,” Dr. Bonsall said.
Dr. Bonsall reported no relevant financial disclosures.
Updates in Pneumonia
Live Q&A: Thursday, Aug. 20, 2:15 p.m to 3:15 p.m.
according to Joanna M. Bonsall, MD, PhD, SFHM, chief of hospital medicine at Grady Memorial Hospital and associate professor of medicine at Emory University, both in Atlanta.
Last year, the American Thoracic Society and the Infectious Diseases Society of America updated their clinical guidelines on community-acquired pneumonia (CAP) for the first time since 2007. The guidelines were published in the Oct. 1, 2019 issue of the American Journal of Respiratory and Critical Care Medicine.
CAP is one of the most common reasons for hospitalization in the United States, and it is estimated that CAP comprises over 4.5 million outpatient and ED visits each year, according to the National Ambulatory Medical Care Survey and National Hospital Ambulatory Medical Care Survey in 2009-2010. It is also the most common cause of death from infection disease, according to the Centers for Disease Control and Prevention.
Dr. Bonsall will present “Updates in Pneumonia” at HM20 Virtual, the virtual annual meeting of the Society of Hospital Medicine; a live question-and-answer session will be held online Aug. 20. In her session, Dr. Bonsall said she plans to cover the new ATS/IDSA guidelines for CAP, which will include what initial testing to order, which empiric antibiotics to use, and how to manage patients at risk for resistant organisms, formerly known as health care–associated pneumonia (HCAP). Dr. Bonsall also will outline the evidence for use of steroids, especially in cases of severe pneumonia, and review the 2016 ATS/IDSA guidelines for hospital-acquired pneumonia with a focus on antibiotic selection.
One major change for 2019: The ATS/IDSA CAP guideline authors issued a strong recommendation to abandon use of the term HCAP as a “distinct clinical entity” when considering antibiotics for patients with CAP. In addition, methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa should only be empirically covered in patients with CAP if they present with locally validated risk factors for either pathogen, according to the guidelines.
“Order pretreatment testing based on severity of illness as well as risk factors for drug-resistant pathogens,” Dr. Bonsall said. Hospitalists also should avoid using procalcitonin levels as a benchmark for whether a patient should be started on antibiotics. Once the recommended antibiotic treatment has been initiated, attendees should use culture results to narrow down the possibilities, especially in cases of drug-resistant pathogens.
The ATS/IDSA guidelines also state that corticosteroids should not be routinely used for patients with nonsevere CAP, but attendees should also be aware of the limitations and interpretations of the evidence, Dr. Bonsall said. Avoiding routine corticosteroid use in patients with severe CAP or in patients with severe influenza pneumonia carries a conditional recommendation with a moderate and low quality of evidence, respectively. In general, cases of CAP should be treated for no more than 5 days, or 3 days of treatment after the patient becomes clinically stable.
Attendees at HM20 Virtual should walk away from the session knowing what testing is necessary and what testing is unnecessary, and how to reduce antibiotic exposure for both broad spectrum use and duration. “At the end of the session, you should feel comfortable using both the CAP and HAP guidelines,” Dr. Bonsall said.
Dr. Bonsall reported no relevant financial disclosures.
Updates in Pneumonia
Live Q&A: Thursday, Aug. 20, 2:15 p.m to 3:15 p.m.
Sepsis: Vitamin C, thiamine, glucocorticoids remain controversial
Sepsis is the number one killer in U.S. hospitals. About one in three patient deaths in a hospital are attributable to sepsis, according to the Centers for Disease Control and Prevention, and it is the leading cause of readmission for U.S. hospitals as well.
Patricia Kritek MD, EdM, of the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle, hopes to bring attendees up to speed on sepsis with her presentation, “Put SIRS on the SOFA and Let’s get Septic! Update in Sepsis” at HM20 Virtual.
Each year, approximately 1.7 million American adults develop sepsis, and nearly 270,000 Americans will die from sepsis annually. Although sepsis disproportionately affects young children, older adults, patients with chronic diseases, and those with a weak immune system, the disease can affect anyone.
With that reputation, sepsis is on the forefront of hospitalists’ minds. Hospitalists are traditionally well versed in current sepsis guidelines, but time to treatment is paramount, and it can be difficult to stay up to date on the latest studies in the field.
The title of Dr. Kritek’s presentation hints at the theme: Hospitalists may have learned the systemic inflammatory response syndrome (SIRS) criteria for diagnosing sepsis, but the Sequential Organ Failure Assessment (SOFA) Score developed by the Third International Consensus Definitions for Sepsis and Septic Shock – previously known as the sepsis-related organ failure assessment score – has been the new method since 2016 to assess the clinical outcomes of patients with sepsis.
The quick SOFA score (qSOFA), developed by the Society of Critical Care and European Society of Intensive Care Medicine in 2016 guidelines, further helps hospitalists and other hospital physicians identify those patients at highest risk of mortality from sepsis outside an intensive care unit setting.
Dr. Kritek, who is a board-certified critical care medicine physician, has previously presented this talk at the Society for Hospital Medicine Annual Conference in the past. This year the presentation will include a number of studies that examine what role vitamin C, thiamine, and glucocorticoids have in treating patients with sepsis, she said. For example, it is thought that parenteral administration of vitamin C could raise plasma levels and reduce multiorgan failure. Thiamine could be useful in sepsis treatment because of its role in glucose metabolism and lactate production, while glucocorticoids could help improve the mortality rate of patients with sepsis.
While Dr. Kritek said she is not going to be advocating for the benefit of vitamin C and thiamine during the session, “this is an area of ongoing debate, and we will walk through the most recent data to try to make sense of it,” she said.
Dr. Kritek noted that the role of balanced crystalloids in resuscitation will be discussed versus when to use saline, as well as the potential of new vasopressors for the treatment of septic shock.
“Our goal will be to integrate the most recent literature into day-to-day practice,” Dr. Kritek said.Dr. Kritek reports no conflicts of interest.
“Put SIRS on the SOFA and Let’s get Septic! Update in Sepsis”
Sepsis is the number one killer in U.S. hospitals. About one in three patient deaths in a hospital are attributable to sepsis, according to the Centers for Disease Control and Prevention, and it is the leading cause of readmission for U.S. hospitals as well.
Patricia Kritek MD, EdM, of the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle, hopes to bring attendees up to speed on sepsis with her presentation, “Put SIRS on the SOFA and Let’s get Septic! Update in Sepsis” at HM20 Virtual.
Each year, approximately 1.7 million American adults develop sepsis, and nearly 270,000 Americans will die from sepsis annually. Although sepsis disproportionately affects young children, older adults, patients with chronic diseases, and those with a weak immune system, the disease can affect anyone.
With that reputation, sepsis is on the forefront of hospitalists’ minds. Hospitalists are traditionally well versed in current sepsis guidelines, but time to treatment is paramount, and it can be difficult to stay up to date on the latest studies in the field.
The title of Dr. Kritek’s presentation hints at the theme: Hospitalists may have learned the systemic inflammatory response syndrome (SIRS) criteria for diagnosing sepsis, but the Sequential Organ Failure Assessment (SOFA) Score developed by the Third International Consensus Definitions for Sepsis and Septic Shock – previously known as the sepsis-related organ failure assessment score – has been the new method since 2016 to assess the clinical outcomes of patients with sepsis.
The quick SOFA score (qSOFA), developed by the Society of Critical Care and European Society of Intensive Care Medicine in 2016 guidelines, further helps hospitalists and other hospital physicians identify those patients at highest risk of mortality from sepsis outside an intensive care unit setting.
Dr. Kritek, who is a board-certified critical care medicine physician, has previously presented this talk at the Society for Hospital Medicine Annual Conference in the past. This year the presentation will include a number of studies that examine what role vitamin C, thiamine, and glucocorticoids have in treating patients with sepsis, she said. For example, it is thought that parenteral administration of vitamin C could raise plasma levels and reduce multiorgan failure. Thiamine could be useful in sepsis treatment because of its role in glucose metabolism and lactate production, while glucocorticoids could help improve the mortality rate of patients with sepsis.
While Dr. Kritek said she is not going to be advocating for the benefit of vitamin C and thiamine during the session, “this is an area of ongoing debate, and we will walk through the most recent data to try to make sense of it,” she said.
Dr. Kritek noted that the role of balanced crystalloids in resuscitation will be discussed versus when to use saline, as well as the potential of new vasopressors for the treatment of septic shock.
“Our goal will be to integrate the most recent literature into day-to-day practice,” Dr. Kritek said.Dr. Kritek reports no conflicts of interest.
“Put SIRS on the SOFA and Let’s get Septic! Update in Sepsis”
Sepsis is the number one killer in U.S. hospitals. About one in three patient deaths in a hospital are attributable to sepsis, according to the Centers for Disease Control and Prevention, and it is the leading cause of readmission for U.S. hospitals as well.
Patricia Kritek MD, EdM, of the division of pulmonary, critical care, and sleep medicine at the University of Washington, Seattle, hopes to bring attendees up to speed on sepsis with her presentation, “Put SIRS on the SOFA and Let’s get Septic! Update in Sepsis” at HM20 Virtual.
Each year, approximately 1.7 million American adults develop sepsis, and nearly 270,000 Americans will die from sepsis annually. Although sepsis disproportionately affects young children, older adults, patients with chronic diseases, and those with a weak immune system, the disease can affect anyone.
With that reputation, sepsis is on the forefront of hospitalists’ minds. Hospitalists are traditionally well versed in current sepsis guidelines, but time to treatment is paramount, and it can be difficult to stay up to date on the latest studies in the field.
The title of Dr. Kritek’s presentation hints at the theme: Hospitalists may have learned the systemic inflammatory response syndrome (SIRS) criteria for diagnosing sepsis, but the Sequential Organ Failure Assessment (SOFA) Score developed by the Third International Consensus Definitions for Sepsis and Septic Shock – previously known as the sepsis-related organ failure assessment score – has been the new method since 2016 to assess the clinical outcomes of patients with sepsis.
The quick SOFA score (qSOFA), developed by the Society of Critical Care and European Society of Intensive Care Medicine in 2016 guidelines, further helps hospitalists and other hospital physicians identify those patients at highest risk of mortality from sepsis outside an intensive care unit setting.
Dr. Kritek, who is a board-certified critical care medicine physician, has previously presented this talk at the Society for Hospital Medicine Annual Conference in the past. This year the presentation will include a number of studies that examine what role vitamin C, thiamine, and glucocorticoids have in treating patients with sepsis, she said. For example, it is thought that parenteral administration of vitamin C could raise plasma levels and reduce multiorgan failure. Thiamine could be useful in sepsis treatment because of its role in glucose metabolism and lactate production, while glucocorticoids could help improve the mortality rate of patients with sepsis.
While Dr. Kritek said she is not going to be advocating for the benefit of vitamin C and thiamine during the session, “this is an area of ongoing debate, and we will walk through the most recent data to try to make sense of it,” she said.
Dr. Kritek noted that the role of balanced crystalloids in resuscitation will be discussed versus when to use saline, as well as the potential of new vasopressors for the treatment of septic shock.
“Our goal will be to integrate the most recent literature into day-to-day practice,” Dr. Kritek said.Dr. Kritek reports no conflicts of interest.
“Put SIRS on the SOFA and Let’s get Septic! Update in Sepsis”
Exposing hospital gowns
Bare bottoms, bare minimum
“Don’t let the gown get you down,” was the advice a 26-year-old gentleman with leukemia offered in a study investigating the psychosocial impact of hospital gowns on patients and providers.1 Patients were found to be resigned to their “uncomfortable,” “expos[ing],” “nightmare-[ish]” “uniform,” afraid to even ask to wear more dignifying attire for fear of seeming difficult to providers and potentially harming the therapeutic relationship; one 64-year-old woman with terminal cancer detailed, “I have my own pajamas at home, but I don’t bring them because you can’t wear them here … [wearing a gown] is really not fun, but hey, this is what [providers] have to do, so it’s what you have to do.”1-3
Research has consistently shown that patients are vulnerable to dehumanization and loss of identity in the hospital, often exacerbated by wearing the standard hospital gown.3-8 Case in point, a mixed-methods study revealed that hospital gowns may lead to an increased sense of exposure, discomfort, disempowerment, and embarrassment for patients during a period of potential vulnerability while undergoing medical intervention.8
Hospital gowns strip autonomy from individuals humbly coming to the hospital for help. The gown has become a linchpin of change, initiating the dehumanizing process of “person” to “patient.” One of the main problems with the hospital gown is its exposing nature, often made light of on the wards with the joke, “Do you know who invented the hospital gown?…See-more Hiney!” The joke continued in two Super Bowl LIII commercials for a large academic health care system and insurance provider in Pennsylvania, depicting a construction worker and businessman clad in hospital gowns, mooning their less-than-pleased coworkers, to inform patients of expanded insurance coverage, i.e., “completely covered.” Hospital gowns are also a source of comedic fodder on sitcoms, including “It’s Always Sunny in Philadelphia,” “Man with a Plan,” and “Carol’s Second Act.”
It is common knowledge that hospital gowns are flawed, but very little has been done to change them. Little is known about the origin of hospital gowns, and like their design, their history has many gaps. PubMed, Google, and Wikipedia yield no fruitful insight into the evolution of the hospital gown, and perhaps the best way to understand the hospital gown over time is to watch depictions of patients in television sitcoms, dramas, and movies, ranging from the days of black-and-white into the modern era, and view artistic depictions of hospitals across eras. Case in point, depictions of fourteenth century hospital wards in art show that all patients wore night shirts, under which they also wore some type of underclothing.9 By the end of the 1800s and beginning of the 1900s, pajamas for men became more common as hospital attire.9 Although it is not known who originally invented the traditional hospital gown, the original gown was designed around a century ago with an open back for use on patients admitted the night prior to surgery, who were sedated prior to transfer to the anesthetic room while half-asleep.10
In general, the most common reason that hospitals began to provide, require, or offer clothing to patients was to reduce infection and improve hygiene, as clothing can be ruined by leakage of bodily fluids from various examinations, treatments, and procedures.9 In addition, in certain settings, lifesaving measures require access to the naked body to allow equipment, like a defibrillator, to be connected to the patient; a gown can theoretically be removed quickly.9 For some reason along the way, the simple, open-backed “johnny” gown of the early 20th century became standard of care with minimal meaningful modifications in the last hundred years. One possible explanation for the persistence of the “johnny” gown is that in past eras of medicine, patients in gowns were expected to be bedbound for recovery, keeping their bare bottom under wraps, and this norm became the status quo. Today, ambulation is encouraged in patients as part of venous thromboembolism (VTE) prophylaxis but the gown design has fallen behind.
Modern medicine emphasizes, values, and even advertises evidence-based medicine, patient-centered care, and high-quality care, yet the hospital gown stands as a stark contrast to this pledge to move forward as beacons of change. Hospital gowns have fallen outside of the scope of evidence-based research.11 One may ask why the gown remains decades behind modern medicine, and it appears that this apathy stems from (1) accepting “medical tradition” and choosing to overlook the flaws of the current hospital gown, and (2) believing that changing the hospital gown would cost money, affronting an institution’s almighty bottom-line. Still, several institutions have attempted change, including Hackensack University Medical Center partnering with Cynthia Rowley and Nicole Miller (1999), Cleveland Clinic partnering with Diane von Furstenberg (2010), and Henry Ford Health System of Detroit’s “Model G” gown (2016).12-15
In spite of these efforts to revamp the hospital gown at academic medical centers, change has been neither long lasting nor widely disseminated. Traci Lamar, a professor at the North Carolina State University College of Textiles reasoned that, “There are number of pressures in the hospital environment that influence what they purchase and when they purchase. Cost management, inventory management, storage space. ... There’s more value coming with the apparel item if it also becomes something that replaces or enhances other equipment that’s used in the hospital environment. Like a gown that can also keep an eye on your blood pressure or measure your heart rate.”15
The hospital gown remains a poor attempt at proper attire for human beings, with the most similar evolutionary relative being a hairdresser’s cape. Taken a step further, functionally the hospital gown is most similar to a prison uniform. Although this may seem bold and sensational, one must stop and think about it, considering the parallels. When individuals are admitted to the hospital, they exchange their clothing for a hospital gown, so that they can be easily identified as a “patient” and remain safe in the hospital. When individuals are sentenced to prison, they exchange their clothing for a uniform, so that they can be easily identified as a “prisoner” and remain safe in jail. The problem is, more time, money, and effort has gone into designing prisoners’ garments, who expect a loss of autonomy, than designing patients’ garments, who should never expect a loss of autonomy.
Prison uniforms are designed with safety in mind, ensuring the absence of potential ligatures or improvised weapons. The United Nations even passed an amendment to its Standard Minimum Rules for the Treatment of Prisoners in 2015, prohibiting humiliating clothing and requiring every prisoner who is “not allowed to wear his or her own clothing” to “be provided with an outfit of clothing suitable for the climate and adequate to keep him or her in good health.”16 They also stipulated that prisoners’ clothing could not be degrading or humiliating and was mandated to “be clean and kept in proper condition”.16 Even more compelling, a physician was bequeathed the task of inspecting, and advising the prison director on “the suitability and cleanliness of the prisoners’ clothing and bedding.”16 However, there are no standard minimum rules for hospital patients’ clothing. Hospital gowns have been described as “threadbare,” “one-size-fits-none,” “stained,” and “drafty,” antithetical to both hygiene and the hospital climate – far from “proper condition” (See Figure 1).1
Where are the standard minimum rules for hospital gowns? Patients have admittedly wondered, “What happened to the person who wore this gown before I did?” or worse, “Who died in this gown?” Even more, the current hospital gown can unintentionally put a patient in harms’ way, posing a fall risk for patients with petite frames overwhelmed by the bulk of the gown and also inhibiting fast access to the chest for placement of defibrillation pads in a code. Ironically, prison uniforms have the main things patients have requested: bottoms, modesty, multiple sizes, and … color!1-3
Although jailhouse orange or stripes are unlikely to be high fashion in the hospital, it is important to consider that, through indifference about the current hospital gown, institutions are teaching that it is acceptable for patients to wear this dehumanizing garment analogous to a prison uniform, except less colorful and more exposing. The hospital gown has persisted under the myth of medical tradition, masking the fact that there is neither evidence for the current hospital gown design nor data to support its functional success for patients or providers.3,12,14 Silence speaks volumes, and patients are taught to expect and accept a loss of dignity without questioning this archaic aspect of medical culture. Patients, nurses, and physicians do not challenge the status quo because the hospital gown “is the way it has always been done.” Perceived added-cost and medical tradition have further perpetuated the current open-backed hospital gown because meaningful change would require money.
With that said, “double gowning,” the method hospitals have used to combat lunar eclipses in the hallways and provide a semblance of dignity to patients, is already costing hospitals more money, costs that can be reduced by creating an evidence-based, patient-guided, provider-approved design. As Mike Forbes, the product designer and licensing associate for the Model G gown, argued, “By using two, you’re purchasing two gowns because one doesn’t do the job, which costs money. … If you’re washing twice as many gowns as you need, you’re spending twice as much money as you need on laundry.”17
Thus, improvements can be made without breaking the bank and may even save hospitals money in the long run. For instance, a hospital administrator can order more colors or styles of hospital gowns and bottoms to give patients a choice of what they would prefer to wear: a small piece of autonomy in an environment where minimal autonomy exists. A physician or nurse can not only permit, but also encourage, a patient to wear his or her own attire within reason, for example, a loose-fitting t-shirt and sweatpants from home or pajama pants under a hospital gown. More complex solutions could include a community design contest for a medical center’s new hospital gown print, or even bolder, a community design contest for a medical center’s new inpatient attire. Above all, patients need to know that hospitals and providers care about what patients wear in the hospital. As a terminally ill patient suggested, “maybe all administrators and office staff should have to spend one day in a gown. …They advertise this: ‘We always put the patient first.’ Okay, so then I guess you have to put your money where your mouth is.”3
This new decade offers the opportunity to give patients a sense of dignity back and make concerted, evidence-based efforts towards meaningful and sustainable change in patient attire, be it purchasing more colorful and modest gown options in the present or total redesign in the future. The financial cost may seem burdensome, but the reward would be immensely bountiful. It is time to stop making hospital gown–clad patients’ exposed bottoms the butt of the joke, and the only way to change the punchline is to change the hospital gown. Patients deserve more than the bare minimum and a bare bottom, so hospitals must consider putting their money where their mouth is.
Dr. Lucas is based in the department of pediatrics, University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh. She has a provisional utility patent pending for a novel patient gown. You can contact her at [email protected]. Dr. Dellasega is based in the department of humanities, Penn State University, Hershey.
References
1. Lucas C et al. “Don’t let the gown get you down: How patients and providers perceive hospital gowns.” Abstract published at Hospital Medicine 2019, Mar 24-27, National Harbor, Md., Abstract 322.
2. Lucas C and Dellasega C. “You don’t have to be dying to do comfort measures: Patients’ and physicians’ perceptions of inpatient attire.” Abstract published at ACP Internal Medicine 2019, Apr 11-13, Philadelphia, Abstract.
3. Lucas C and Dellasega C. Finding common threads: How patients, physicians, and nurses perceive the patient gown. Patient Exp J. 2020;7(1):51-64.
4. Detsky A and Krumholtz H. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-70.
5. Krumholz H. Post-hospital syndrome – an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-2.
6. Wellbery C and Chan M. White coat, patient gown. Med Humanit. 2014;40(2):90-6.
7. McDonald E et al. Inpatient attire: An opportunity to improve the patient experience. JAMA Intern Med. 2014;174(11):1865-67.
8. Cogan N et al. Mixed methods study exploring the impact of the hospital gown on recovery and wellbeing: Implications for policy and practice. Lancet. 2019. doi: 10.1016/S0140-6736(19)32829-6.
9. Bergbom I, Pettersson M, and Mattsson E. Patient clothing – practical solution or means of imposing anonymity? J Hosp Med Manage. 2017;3(22):1-6.
10. Who invented the hospital gown? Interweave Healthcare. Accessed Mar 30, 2020.
11. Gordon L and Guttmann S. A user-centered approach to the redesign of the patient hospital gown. Fashion Practice. 2013;5(1):137-51. doi: 10.2752/175693813X13559997788961.
12. Limbong A. “Can a Patient Gown Makeover Move Hospitals to Embrace Change?” NPR. 2018 Feb 11. Accessed Mar 26, 2020.
13. Schiro A. “Patterns: Hospital Style.” New York Times. 1999 June 29. Accessed Mar 26, 2020.
14. Luthra S. “Hospital Gowns Get a Makeover.” The Atlantic. 2015 Apr 4. Accessed Mar 26, 2020.
15. Tien E. “Hospital Gowns Get a Life.” New York Times. 1998 Oct 18. Accessed Mar 26, 2020.
16. McCall-Smith K. United Nations Standard Minimum Rules for the Treatment of Prisoners (Nelson Mandela Rules). Int Leg Materials. 2016;55(6),1180-205.
17. Green C. “Updated hospital gowns a good investment, execs say, restore ‘dignity.’ ” Healthcare Finance. 2015 Aug 3. Accessed Apr 1, 2020.
Bare bottoms, bare minimum
Bare bottoms, bare minimum
“Don’t let the gown get you down,” was the advice a 26-year-old gentleman with leukemia offered in a study investigating the psychosocial impact of hospital gowns on patients and providers.1 Patients were found to be resigned to their “uncomfortable,” “expos[ing],” “nightmare-[ish]” “uniform,” afraid to even ask to wear more dignifying attire for fear of seeming difficult to providers and potentially harming the therapeutic relationship; one 64-year-old woman with terminal cancer detailed, “I have my own pajamas at home, but I don’t bring them because you can’t wear them here … [wearing a gown] is really not fun, but hey, this is what [providers] have to do, so it’s what you have to do.”1-3
Research has consistently shown that patients are vulnerable to dehumanization and loss of identity in the hospital, often exacerbated by wearing the standard hospital gown.3-8 Case in point, a mixed-methods study revealed that hospital gowns may lead to an increased sense of exposure, discomfort, disempowerment, and embarrassment for patients during a period of potential vulnerability while undergoing medical intervention.8
Hospital gowns strip autonomy from individuals humbly coming to the hospital for help. The gown has become a linchpin of change, initiating the dehumanizing process of “person” to “patient.” One of the main problems with the hospital gown is its exposing nature, often made light of on the wards with the joke, “Do you know who invented the hospital gown?…See-more Hiney!” The joke continued in two Super Bowl LIII commercials for a large academic health care system and insurance provider in Pennsylvania, depicting a construction worker and businessman clad in hospital gowns, mooning their less-than-pleased coworkers, to inform patients of expanded insurance coverage, i.e., “completely covered.” Hospital gowns are also a source of comedic fodder on sitcoms, including “It’s Always Sunny in Philadelphia,” “Man with a Plan,” and “Carol’s Second Act.”
It is common knowledge that hospital gowns are flawed, but very little has been done to change them. Little is known about the origin of hospital gowns, and like their design, their history has many gaps. PubMed, Google, and Wikipedia yield no fruitful insight into the evolution of the hospital gown, and perhaps the best way to understand the hospital gown over time is to watch depictions of patients in television sitcoms, dramas, and movies, ranging from the days of black-and-white into the modern era, and view artistic depictions of hospitals across eras. Case in point, depictions of fourteenth century hospital wards in art show that all patients wore night shirts, under which they also wore some type of underclothing.9 By the end of the 1800s and beginning of the 1900s, pajamas for men became more common as hospital attire.9 Although it is not known who originally invented the traditional hospital gown, the original gown was designed around a century ago with an open back for use on patients admitted the night prior to surgery, who were sedated prior to transfer to the anesthetic room while half-asleep.10
In general, the most common reason that hospitals began to provide, require, or offer clothing to patients was to reduce infection and improve hygiene, as clothing can be ruined by leakage of bodily fluids from various examinations, treatments, and procedures.9 In addition, in certain settings, lifesaving measures require access to the naked body to allow equipment, like a defibrillator, to be connected to the patient; a gown can theoretically be removed quickly.9 For some reason along the way, the simple, open-backed “johnny” gown of the early 20th century became standard of care with minimal meaningful modifications in the last hundred years. One possible explanation for the persistence of the “johnny” gown is that in past eras of medicine, patients in gowns were expected to be bedbound for recovery, keeping their bare bottom under wraps, and this norm became the status quo. Today, ambulation is encouraged in patients as part of venous thromboembolism (VTE) prophylaxis but the gown design has fallen behind.
Modern medicine emphasizes, values, and even advertises evidence-based medicine, patient-centered care, and high-quality care, yet the hospital gown stands as a stark contrast to this pledge to move forward as beacons of change. Hospital gowns have fallen outside of the scope of evidence-based research.11 One may ask why the gown remains decades behind modern medicine, and it appears that this apathy stems from (1) accepting “medical tradition” and choosing to overlook the flaws of the current hospital gown, and (2) believing that changing the hospital gown would cost money, affronting an institution’s almighty bottom-line. Still, several institutions have attempted change, including Hackensack University Medical Center partnering with Cynthia Rowley and Nicole Miller (1999), Cleveland Clinic partnering with Diane von Furstenberg (2010), and Henry Ford Health System of Detroit’s “Model G” gown (2016).12-15
In spite of these efforts to revamp the hospital gown at academic medical centers, change has been neither long lasting nor widely disseminated. Traci Lamar, a professor at the North Carolina State University College of Textiles reasoned that, “There are number of pressures in the hospital environment that influence what they purchase and when they purchase. Cost management, inventory management, storage space. ... There’s more value coming with the apparel item if it also becomes something that replaces or enhances other equipment that’s used in the hospital environment. Like a gown that can also keep an eye on your blood pressure or measure your heart rate.”15
The hospital gown remains a poor attempt at proper attire for human beings, with the most similar evolutionary relative being a hairdresser’s cape. Taken a step further, functionally the hospital gown is most similar to a prison uniform. Although this may seem bold and sensational, one must stop and think about it, considering the parallels. When individuals are admitted to the hospital, they exchange their clothing for a hospital gown, so that they can be easily identified as a “patient” and remain safe in the hospital. When individuals are sentenced to prison, they exchange their clothing for a uniform, so that they can be easily identified as a “prisoner” and remain safe in jail. The problem is, more time, money, and effort has gone into designing prisoners’ garments, who expect a loss of autonomy, than designing patients’ garments, who should never expect a loss of autonomy.
Prison uniforms are designed with safety in mind, ensuring the absence of potential ligatures or improvised weapons. The United Nations even passed an amendment to its Standard Minimum Rules for the Treatment of Prisoners in 2015, prohibiting humiliating clothing and requiring every prisoner who is “not allowed to wear his or her own clothing” to “be provided with an outfit of clothing suitable for the climate and adequate to keep him or her in good health.”16 They also stipulated that prisoners’ clothing could not be degrading or humiliating and was mandated to “be clean and kept in proper condition”.16 Even more compelling, a physician was bequeathed the task of inspecting, and advising the prison director on “the suitability and cleanliness of the prisoners’ clothing and bedding.”16 However, there are no standard minimum rules for hospital patients’ clothing. Hospital gowns have been described as “threadbare,” “one-size-fits-none,” “stained,” and “drafty,” antithetical to both hygiene and the hospital climate – far from “proper condition” (See Figure 1).1
Where are the standard minimum rules for hospital gowns? Patients have admittedly wondered, “What happened to the person who wore this gown before I did?” or worse, “Who died in this gown?” Even more, the current hospital gown can unintentionally put a patient in harms’ way, posing a fall risk for patients with petite frames overwhelmed by the bulk of the gown and also inhibiting fast access to the chest for placement of defibrillation pads in a code. Ironically, prison uniforms have the main things patients have requested: bottoms, modesty, multiple sizes, and … color!1-3
Although jailhouse orange or stripes are unlikely to be high fashion in the hospital, it is important to consider that, through indifference about the current hospital gown, institutions are teaching that it is acceptable for patients to wear this dehumanizing garment analogous to a prison uniform, except less colorful and more exposing. The hospital gown has persisted under the myth of medical tradition, masking the fact that there is neither evidence for the current hospital gown design nor data to support its functional success for patients or providers.3,12,14 Silence speaks volumes, and patients are taught to expect and accept a loss of dignity without questioning this archaic aspect of medical culture. Patients, nurses, and physicians do not challenge the status quo because the hospital gown “is the way it has always been done.” Perceived added-cost and medical tradition have further perpetuated the current open-backed hospital gown because meaningful change would require money.
With that said, “double gowning,” the method hospitals have used to combat lunar eclipses in the hallways and provide a semblance of dignity to patients, is already costing hospitals more money, costs that can be reduced by creating an evidence-based, patient-guided, provider-approved design. As Mike Forbes, the product designer and licensing associate for the Model G gown, argued, “By using two, you’re purchasing two gowns because one doesn’t do the job, which costs money. … If you’re washing twice as many gowns as you need, you’re spending twice as much money as you need on laundry.”17
Thus, improvements can be made without breaking the bank and may even save hospitals money in the long run. For instance, a hospital administrator can order more colors or styles of hospital gowns and bottoms to give patients a choice of what they would prefer to wear: a small piece of autonomy in an environment where minimal autonomy exists. A physician or nurse can not only permit, but also encourage, a patient to wear his or her own attire within reason, for example, a loose-fitting t-shirt and sweatpants from home or pajama pants under a hospital gown. More complex solutions could include a community design contest for a medical center’s new hospital gown print, or even bolder, a community design contest for a medical center’s new inpatient attire. Above all, patients need to know that hospitals and providers care about what patients wear in the hospital. As a terminally ill patient suggested, “maybe all administrators and office staff should have to spend one day in a gown. …They advertise this: ‘We always put the patient first.’ Okay, so then I guess you have to put your money where your mouth is.”3
This new decade offers the opportunity to give patients a sense of dignity back and make concerted, evidence-based efforts towards meaningful and sustainable change in patient attire, be it purchasing more colorful and modest gown options in the present or total redesign in the future. The financial cost may seem burdensome, but the reward would be immensely bountiful. It is time to stop making hospital gown–clad patients’ exposed bottoms the butt of the joke, and the only way to change the punchline is to change the hospital gown. Patients deserve more than the bare minimum and a bare bottom, so hospitals must consider putting their money where their mouth is.
Dr. Lucas is based in the department of pediatrics, University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh. She has a provisional utility patent pending for a novel patient gown. You can contact her at [email protected]. Dr. Dellasega is based in the department of humanities, Penn State University, Hershey.
References
1. Lucas C et al. “Don’t let the gown get you down: How patients and providers perceive hospital gowns.” Abstract published at Hospital Medicine 2019, Mar 24-27, National Harbor, Md., Abstract 322.
2. Lucas C and Dellasega C. “You don’t have to be dying to do comfort measures: Patients’ and physicians’ perceptions of inpatient attire.” Abstract published at ACP Internal Medicine 2019, Apr 11-13, Philadelphia, Abstract.
3. Lucas C and Dellasega C. Finding common threads: How patients, physicians, and nurses perceive the patient gown. Patient Exp J. 2020;7(1):51-64.
4. Detsky A and Krumholtz H. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-70.
5. Krumholz H. Post-hospital syndrome – an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-2.
6. Wellbery C and Chan M. White coat, patient gown. Med Humanit. 2014;40(2):90-6.
7. McDonald E et al. Inpatient attire: An opportunity to improve the patient experience. JAMA Intern Med. 2014;174(11):1865-67.
8. Cogan N et al. Mixed methods study exploring the impact of the hospital gown on recovery and wellbeing: Implications for policy and practice. Lancet. 2019. doi: 10.1016/S0140-6736(19)32829-6.
9. Bergbom I, Pettersson M, and Mattsson E. Patient clothing – practical solution or means of imposing anonymity? J Hosp Med Manage. 2017;3(22):1-6.
10. Who invented the hospital gown? Interweave Healthcare. Accessed Mar 30, 2020.
11. Gordon L and Guttmann S. A user-centered approach to the redesign of the patient hospital gown. Fashion Practice. 2013;5(1):137-51. doi: 10.2752/175693813X13559997788961.
12. Limbong A. “Can a Patient Gown Makeover Move Hospitals to Embrace Change?” NPR. 2018 Feb 11. Accessed Mar 26, 2020.
13. Schiro A. “Patterns: Hospital Style.” New York Times. 1999 June 29. Accessed Mar 26, 2020.
14. Luthra S. “Hospital Gowns Get a Makeover.” The Atlantic. 2015 Apr 4. Accessed Mar 26, 2020.
15. Tien E. “Hospital Gowns Get a Life.” New York Times. 1998 Oct 18. Accessed Mar 26, 2020.
16. McCall-Smith K. United Nations Standard Minimum Rules for the Treatment of Prisoners (Nelson Mandela Rules). Int Leg Materials. 2016;55(6),1180-205.
17. Green C. “Updated hospital gowns a good investment, execs say, restore ‘dignity.’ ” Healthcare Finance. 2015 Aug 3. Accessed Apr 1, 2020.
“Don’t let the gown get you down,” was the advice a 26-year-old gentleman with leukemia offered in a study investigating the psychosocial impact of hospital gowns on patients and providers.1 Patients were found to be resigned to their “uncomfortable,” “expos[ing],” “nightmare-[ish]” “uniform,” afraid to even ask to wear more dignifying attire for fear of seeming difficult to providers and potentially harming the therapeutic relationship; one 64-year-old woman with terminal cancer detailed, “I have my own pajamas at home, but I don’t bring them because you can’t wear them here … [wearing a gown] is really not fun, but hey, this is what [providers] have to do, so it’s what you have to do.”1-3
Research has consistently shown that patients are vulnerable to dehumanization and loss of identity in the hospital, often exacerbated by wearing the standard hospital gown.3-8 Case in point, a mixed-methods study revealed that hospital gowns may lead to an increased sense of exposure, discomfort, disempowerment, and embarrassment for patients during a period of potential vulnerability while undergoing medical intervention.8
Hospital gowns strip autonomy from individuals humbly coming to the hospital for help. The gown has become a linchpin of change, initiating the dehumanizing process of “person” to “patient.” One of the main problems with the hospital gown is its exposing nature, often made light of on the wards with the joke, “Do you know who invented the hospital gown?…See-more Hiney!” The joke continued in two Super Bowl LIII commercials for a large academic health care system and insurance provider in Pennsylvania, depicting a construction worker and businessman clad in hospital gowns, mooning their less-than-pleased coworkers, to inform patients of expanded insurance coverage, i.e., “completely covered.” Hospital gowns are also a source of comedic fodder on sitcoms, including “It’s Always Sunny in Philadelphia,” “Man with a Plan,” and “Carol’s Second Act.”
It is common knowledge that hospital gowns are flawed, but very little has been done to change them. Little is known about the origin of hospital gowns, and like their design, their history has many gaps. PubMed, Google, and Wikipedia yield no fruitful insight into the evolution of the hospital gown, and perhaps the best way to understand the hospital gown over time is to watch depictions of patients in television sitcoms, dramas, and movies, ranging from the days of black-and-white into the modern era, and view artistic depictions of hospitals across eras. Case in point, depictions of fourteenth century hospital wards in art show that all patients wore night shirts, under which they also wore some type of underclothing.9 By the end of the 1800s and beginning of the 1900s, pajamas for men became more common as hospital attire.9 Although it is not known who originally invented the traditional hospital gown, the original gown was designed around a century ago with an open back for use on patients admitted the night prior to surgery, who were sedated prior to transfer to the anesthetic room while half-asleep.10
In general, the most common reason that hospitals began to provide, require, or offer clothing to patients was to reduce infection and improve hygiene, as clothing can be ruined by leakage of bodily fluids from various examinations, treatments, and procedures.9 In addition, in certain settings, lifesaving measures require access to the naked body to allow equipment, like a defibrillator, to be connected to the patient; a gown can theoretically be removed quickly.9 For some reason along the way, the simple, open-backed “johnny” gown of the early 20th century became standard of care with minimal meaningful modifications in the last hundred years. One possible explanation for the persistence of the “johnny” gown is that in past eras of medicine, patients in gowns were expected to be bedbound for recovery, keeping their bare bottom under wraps, and this norm became the status quo. Today, ambulation is encouraged in patients as part of venous thromboembolism (VTE) prophylaxis but the gown design has fallen behind.
Modern medicine emphasizes, values, and even advertises evidence-based medicine, patient-centered care, and high-quality care, yet the hospital gown stands as a stark contrast to this pledge to move forward as beacons of change. Hospital gowns have fallen outside of the scope of evidence-based research.11 One may ask why the gown remains decades behind modern medicine, and it appears that this apathy stems from (1) accepting “medical tradition” and choosing to overlook the flaws of the current hospital gown, and (2) believing that changing the hospital gown would cost money, affronting an institution’s almighty bottom-line. Still, several institutions have attempted change, including Hackensack University Medical Center partnering with Cynthia Rowley and Nicole Miller (1999), Cleveland Clinic partnering with Diane von Furstenberg (2010), and Henry Ford Health System of Detroit’s “Model G” gown (2016).12-15
In spite of these efforts to revamp the hospital gown at academic medical centers, change has been neither long lasting nor widely disseminated. Traci Lamar, a professor at the North Carolina State University College of Textiles reasoned that, “There are number of pressures in the hospital environment that influence what they purchase and when they purchase. Cost management, inventory management, storage space. ... There’s more value coming with the apparel item if it also becomes something that replaces or enhances other equipment that’s used in the hospital environment. Like a gown that can also keep an eye on your blood pressure or measure your heart rate.”15
The hospital gown remains a poor attempt at proper attire for human beings, with the most similar evolutionary relative being a hairdresser’s cape. Taken a step further, functionally the hospital gown is most similar to a prison uniform. Although this may seem bold and sensational, one must stop and think about it, considering the parallels. When individuals are admitted to the hospital, they exchange their clothing for a hospital gown, so that they can be easily identified as a “patient” and remain safe in the hospital. When individuals are sentenced to prison, they exchange their clothing for a uniform, so that they can be easily identified as a “prisoner” and remain safe in jail. The problem is, more time, money, and effort has gone into designing prisoners’ garments, who expect a loss of autonomy, than designing patients’ garments, who should never expect a loss of autonomy.
Prison uniforms are designed with safety in mind, ensuring the absence of potential ligatures or improvised weapons. The United Nations even passed an amendment to its Standard Minimum Rules for the Treatment of Prisoners in 2015, prohibiting humiliating clothing and requiring every prisoner who is “not allowed to wear his or her own clothing” to “be provided with an outfit of clothing suitable for the climate and adequate to keep him or her in good health.”16 They also stipulated that prisoners’ clothing could not be degrading or humiliating and was mandated to “be clean and kept in proper condition”.16 Even more compelling, a physician was bequeathed the task of inspecting, and advising the prison director on “the suitability and cleanliness of the prisoners’ clothing and bedding.”16 However, there are no standard minimum rules for hospital patients’ clothing. Hospital gowns have been described as “threadbare,” “one-size-fits-none,” “stained,” and “drafty,” antithetical to both hygiene and the hospital climate – far from “proper condition” (See Figure 1).1
Where are the standard minimum rules for hospital gowns? Patients have admittedly wondered, “What happened to the person who wore this gown before I did?” or worse, “Who died in this gown?” Even more, the current hospital gown can unintentionally put a patient in harms’ way, posing a fall risk for patients with petite frames overwhelmed by the bulk of the gown and also inhibiting fast access to the chest for placement of defibrillation pads in a code. Ironically, prison uniforms have the main things patients have requested: bottoms, modesty, multiple sizes, and … color!1-3
Although jailhouse orange or stripes are unlikely to be high fashion in the hospital, it is important to consider that, through indifference about the current hospital gown, institutions are teaching that it is acceptable for patients to wear this dehumanizing garment analogous to a prison uniform, except less colorful and more exposing. The hospital gown has persisted under the myth of medical tradition, masking the fact that there is neither evidence for the current hospital gown design nor data to support its functional success for patients or providers.3,12,14 Silence speaks volumes, and patients are taught to expect and accept a loss of dignity without questioning this archaic aspect of medical culture. Patients, nurses, and physicians do not challenge the status quo because the hospital gown “is the way it has always been done.” Perceived added-cost and medical tradition have further perpetuated the current open-backed hospital gown because meaningful change would require money.
With that said, “double gowning,” the method hospitals have used to combat lunar eclipses in the hallways and provide a semblance of dignity to patients, is already costing hospitals more money, costs that can be reduced by creating an evidence-based, patient-guided, provider-approved design. As Mike Forbes, the product designer and licensing associate for the Model G gown, argued, “By using two, you’re purchasing two gowns because one doesn’t do the job, which costs money. … If you’re washing twice as many gowns as you need, you’re spending twice as much money as you need on laundry.”17
Thus, improvements can be made without breaking the bank and may even save hospitals money in the long run. For instance, a hospital administrator can order more colors or styles of hospital gowns and bottoms to give patients a choice of what they would prefer to wear: a small piece of autonomy in an environment where minimal autonomy exists. A physician or nurse can not only permit, but also encourage, a patient to wear his or her own attire within reason, for example, a loose-fitting t-shirt and sweatpants from home or pajama pants under a hospital gown. More complex solutions could include a community design contest for a medical center’s new hospital gown print, or even bolder, a community design contest for a medical center’s new inpatient attire. Above all, patients need to know that hospitals and providers care about what patients wear in the hospital. As a terminally ill patient suggested, “maybe all administrators and office staff should have to spend one day in a gown. …They advertise this: ‘We always put the patient first.’ Okay, so then I guess you have to put your money where your mouth is.”3
This new decade offers the opportunity to give patients a sense of dignity back and make concerted, evidence-based efforts towards meaningful and sustainable change in patient attire, be it purchasing more colorful and modest gown options in the present or total redesign in the future. The financial cost may seem burdensome, but the reward would be immensely bountiful. It is time to stop making hospital gown–clad patients’ exposed bottoms the butt of the joke, and the only way to change the punchline is to change the hospital gown. Patients deserve more than the bare minimum and a bare bottom, so hospitals must consider putting their money where their mouth is.
Dr. Lucas is based in the department of pediatrics, University of Pittsburgh Medical Center Children’s Hospital of Pittsburgh. She has a provisional utility patent pending for a novel patient gown. You can contact her at [email protected]. Dr. Dellasega is based in the department of humanities, Penn State University, Hershey.
References
1. Lucas C et al. “Don’t let the gown get you down: How patients and providers perceive hospital gowns.” Abstract published at Hospital Medicine 2019, Mar 24-27, National Harbor, Md., Abstract 322.
2. Lucas C and Dellasega C. “You don’t have to be dying to do comfort measures: Patients’ and physicians’ perceptions of inpatient attire.” Abstract published at ACP Internal Medicine 2019, Apr 11-13, Philadelphia, Abstract.
3. Lucas C and Dellasega C. Finding common threads: How patients, physicians, and nurses perceive the patient gown. Patient Exp J. 2020;7(1):51-64.
4. Detsky A and Krumholtz H. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-70.
5. Krumholz H. Post-hospital syndrome – an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100-2.
6. Wellbery C and Chan M. White coat, patient gown. Med Humanit. 2014;40(2):90-6.
7. McDonald E et al. Inpatient attire: An opportunity to improve the patient experience. JAMA Intern Med. 2014;174(11):1865-67.
8. Cogan N et al. Mixed methods study exploring the impact of the hospital gown on recovery and wellbeing: Implications for policy and practice. Lancet. 2019. doi: 10.1016/S0140-6736(19)32829-6.
9. Bergbom I, Pettersson M, and Mattsson E. Patient clothing – practical solution or means of imposing anonymity? J Hosp Med Manage. 2017;3(22):1-6.
10. Who invented the hospital gown? Interweave Healthcare. Accessed Mar 30, 2020.
11. Gordon L and Guttmann S. A user-centered approach to the redesign of the patient hospital gown. Fashion Practice. 2013;5(1):137-51. doi: 10.2752/175693813X13559997788961.
12. Limbong A. “Can a Patient Gown Makeover Move Hospitals to Embrace Change?” NPR. 2018 Feb 11. Accessed Mar 26, 2020.
13. Schiro A. “Patterns: Hospital Style.” New York Times. 1999 June 29. Accessed Mar 26, 2020.
14. Luthra S. “Hospital Gowns Get a Makeover.” The Atlantic. 2015 Apr 4. Accessed Mar 26, 2020.
15. Tien E. “Hospital Gowns Get a Life.” New York Times. 1998 Oct 18. Accessed Mar 26, 2020.
16. McCall-Smith K. United Nations Standard Minimum Rules for the Treatment of Prisoners (Nelson Mandela Rules). Int Leg Materials. 2016;55(6),1180-205.
17. Green C. “Updated hospital gowns a good investment, execs say, restore ‘dignity.’ ” Healthcare Finance. 2015 Aug 3. Accessed Apr 1, 2020.
Creating a student-staffed family call line to alleviate clinical burden
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
Wave, surge, or tsunami
Different COVID-19 models and predicting inpatient bed capacity
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
Different COVID-19 models and predicting inpatient bed capacity
Different COVID-19 models and predicting inpatient bed capacity
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
Calculations of an academic hospitalist
The term “academic hospitalist” has come to mean more than a mere affiliation to an academic medical center (AMC). Academic hospitalists perform various clinical roles like staffing house staff teams, covering nonteaching services, critical care services, procedure teams, night services, medical consultation, and comanagement services.
Over the last decade, academic hospitalists have successfully managed many nonclinical roles in areas like research, medical unit leadership, faculty development, faculty affairs, quality, safety, informatics, utilization review, clinical documentation, throughput, group management, hospital administration, and educational leadership. The role of an academic hospital is as clear as a chocolate martini these days. Here we present some recent trends in academic hospital medicine.
Compensation
From SHM’s State of Hospital Medicine report (SoHM)2014 to 2018 data, the median compensation for U.S. academic hospitalists has risen by an average of 5.15% every year, although increases vary by rank.1 From 2016 to 2018, clinical instructors saw the most significant growth, 11.23% per year, suggesting a need to remain competitive for junior hospitalists. Compensation also varies by geographic area, with the Southern region reporting the highest compensation. Over the last decade, academic hospitalists received, on average, a 28%-35% lower salary, compared with community hospitalists.
Patient population and census
Lower patient encounters and compensation of the academic hospitalists poses the chicken or the egg dilemma. In the 2018 SoHM report, academic hospitalists had an average of 17% fewer encounters. Of note, AMC patients tend to have higher complexity, as measured by the Case Mix Index (CMI – the average diagnosis-related group weight of a hospital).2 A higher CMI is a surrogate marker for the diagnostic diversity, clinical complexity, and resource needs of the patient population in the hospital.
Productivity and financial metrics
The financial bottom line is a critical aspect, and as a report in the Journal of Hospital Medicine described, all health care executives look at business metrics while making decisions.3 Below are some significant academic and community comparisons from SoHM 2018.
- Collections, encounters, and wRVUs (work relative value units) were highly correlated. All of them were lower for academic hospitalists, corroborating the fact that they see a smaller number of patients. Clinical full-time equivalents (cFTE) is a vernacular of how much of the faculty time is devoted to clinical activities. The academic data from SoHM achieves the same target, as it is standardized to 100% billable clinical activity, so the fact that many academic hospitalists do not work a full-time clinical schedule is not a factor in their lower production.
- Charges had a smaller gap likely because of sicker patients in AMCs. The higher acuity difference can also explain 12% higher wRVU/encounter for academic hospitalists.
- The wRVU/encounter ratio can indicate a few patterns: high acuity of patients in AMCs, higher levels of evaluation and management documentation, or both. As the encounters and charges have the same percentage differences, we would place our bets on the former.
- Compensation per encounter and compensation per wRVU showed that academic hospitalists do get a slight advantage.
CMI and wRVUs
Although the SoHM does not capture information on patient acuity or CMI, we speculate that the relationship between CMI and wRVUs may be more or less linear at lower levels of acuity. However, once level III E/M billing is achieved (assuming there is no critical care provided), wRVUs/encounter plateau, even as acuity continues to increase. This plateau effect may be seen more often in high-acuity AMC settings than in community hospitals.
So, in our opinion, compensation models based solely on wRVU production would not do justice for hospitalists in AMC settings since these models would fail to capture the extra work involved with very-high-acuity patients. SoHM 2018 shows the financial support per wRVU for AMC is $45.81, and for the community is $41.28, an 11% difference. We think the higher financial support per wRVU for academic practices may be related to the lost wRVU potential of caring for very-high-acuity patients.
Conclusion
In an academic setting, hospitalists are reforming the field of hospital medicine and defining the ways we could deliver care. They are the pillars of collaboration, education, research, innovation, quality, and safety. It would be increasingly crucial for academic hospitalist leaders to use comparative metrics from SoHM to advocate for their group. The bottom line can be explained by the title of the qualitative study in JHM referenced above: “Collaboration, not calculation.”3
Dr. Chadha is division chief for the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, scheduling, practice analysis, and operation of the group. He is a first-time member of the practice analysis committee. Ms. Dede is division administrator for the division of hospital medicine at the University of Kentucky Healthcare. She prepares and manages budgets, liaisons with the downstream revenue teams, and contributes to the building of academic compensation models. She is serving in the practice administrators committee for the second year and is currently vice chair of the Executive Council for the Practice Administrators special interest group.
References
1. State of Hospital Medicine Report. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
2. Deloitte Center for Health Solutions. Academic Medical Centers: Joining forces with community providers for broad benefits and positive outcomes. 2015. https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/academic-medical-centers-consolidation.html
3. White AA et al. Collaboration, not calculation: A qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(10):662‐7.
The term “academic hospitalist” has come to mean more than a mere affiliation to an academic medical center (AMC). Academic hospitalists perform various clinical roles like staffing house staff teams, covering nonteaching services, critical care services, procedure teams, night services, medical consultation, and comanagement services.
Over the last decade, academic hospitalists have successfully managed many nonclinical roles in areas like research, medical unit leadership, faculty development, faculty affairs, quality, safety, informatics, utilization review, clinical documentation, throughput, group management, hospital administration, and educational leadership. The role of an academic hospital is as clear as a chocolate martini these days. Here we present some recent trends in academic hospital medicine.
Compensation
From SHM’s State of Hospital Medicine report (SoHM)2014 to 2018 data, the median compensation for U.S. academic hospitalists has risen by an average of 5.15% every year, although increases vary by rank.1 From 2016 to 2018, clinical instructors saw the most significant growth, 11.23% per year, suggesting a need to remain competitive for junior hospitalists. Compensation also varies by geographic area, with the Southern region reporting the highest compensation. Over the last decade, academic hospitalists received, on average, a 28%-35% lower salary, compared with community hospitalists.
Patient population and census
Lower patient encounters and compensation of the academic hospitalists poses the chicken or the egg dilemma. In the 2018 SoHM report, academic hospitalists had an average of 17% fewer encounters. Of note, AMC patients tend to have higher complexity, as measured by the Case Mix Index (CMI – the average diagnosis-related group weight of a hospital).2 A higher CMI is a surrogate marker for the diagnostic diversity, clinical complexity, and resource needs of the patient population in the hospital.
Productivity and financial metrics
The financial bottom line is a critical aspect, and as a report in the Journal of Hospital Medicine described, all health care executives look at business metrics while making decisions.3 Below are some significant academic and community comparisons from SoHM 2018.
- Collections, encounters, and wRVUs (work relative value units) were highly correlated. All of them were lower for academic hospitalists, corroborating the fact that they see a smaller number of patients. Clinical full-time equivalents (cFTE) is a vernacular of how much of the faculty time is devoted to clinical activities. The academic data from SoHM achieves the same target, as it is standardized to 100% billable clinical activity, so the fact that many academic hospitalists do not work a full-time clinical schedule is not a factor in their lower production.
- Charges had a smaller gap likely because of sicker patients in AMCs. The higher acuity difference can also explain 12% higher wRVU/encounter for academic hospitalists.
- The wRVU/encounter ratio can indicate a few patterns: high acuity of patients in AMCs, higher levels of evaluation and management documentation, or both. As the encounters and charges have the same percentage differences, we would place our bets on the former.
- Compensation per encounter and compensation per wRVU showed that academic hospitalists do get a slight advantage.
CMI and wRVUs
Although the SoHM does not capture information on patient acuity or CMI, we speculate that the relationship between CMI and wRVUs may be more or less linear at lower levels of acuity. However, once level III E/M billing is achieved (assuming there is no critical care provided), wRVUs/encounter plateau, even as acuity continues to increase. This plateau effect may be seen more often in high-acuity AMC settings than in community hospitals.
So, in our opinion, compensation models based solely on wRVU production would not do justice for hospitalists in AMC settings since these models would fail to capture the extra work involved with very-high-acuity patients. SoHM 2018 shows the financial support per wRVU for AMC is $45.81, and for the community is $41.28, an 11% difference. We think the higher financial support per wRVU for academic practices may be related to the lost wRVU potential of caring for very-high-acuity patients.
Conclusion
In an academic setting, hospitalists are reforming the field of hospital medicine and defining the ways we could deliver care. They are the pillars of collaboration, education, research, innovation, quality, and safety. It would be increasingly crucial for academic hospitalist leaders to use comparative metrics from SoHM to advocate for their group. The bottom line can be explained by the title of the qualitative study in JHM referenced above: “Collaboration, not calculation.”3
Dr. Chadha is division chief for the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, scheduling, practice analysis, and operation of the group. He is a first-time member of the practice analysis committee. Ms. Dede is division administrator for the division of hospital medicine at the University of Kentucky Healthcare. She prepares and manages budgets, liaisons with the downstream revenue teams, and contributes to the building of academic compensation models. She is serving in the practice administrators committee for the second year and is currently vice chair of the Executive Council for the Practice Administrators special interest group.
References
1. State of Hospital Medicine Report. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
2. Deloitte Center for Health Solutions. Academic Medical Centers: Joining forces with community providers for broad benefits and positive outcomes. 2015. https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/academic-medical-centers-consolidation.html
3. White AA et al. Collaboration, not calculation: A qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(10):662‐7.
The term “academic hospitalist” has come to mean more than a mere affiliation to an academic medical center (AMC). Academic hospitalists perform various clinical roles like staffing house staff teams, covering nonteaching services, critical care services, procedure teams, night services, medical consultation, and comanagement services.
Over the last decade, academic hospitalists have successfully managed many nonclinical roles in areas like research, medical unit leadership, faculty development, faculty affairs, quality, safety, informatics, utilization review, clinical documentation, throughput, group management, hospital administration, and educational leadership. The role of an academic hospital is as clear as a chocolate martini these days. Here we present some recent trends in academic hospital medicine.
Compensation
From SHM’s State of Hospital Medicine report (SoHM)2014 to 2018 data, the median compensation for U.S. academic hospitalists has risen by an average of 5.15% every year, although increases vary by rank.1 From 2016 to 2018, clinical instructors saw the most significant growth, 11.23% per year, suggesting a need to remain competitive for junior hospitalists. Compensation also varies by geographic area, with the Southern region reporting the highest compensation. Over the last decade, academic hospitalists received, on average, a 28%-35% lower salary, compared with community hospitalists.
Patient population and census
Lower patient encounters and compensation of the academic hospitalists poses the chicken or the egg dilemma. In the 2018 SoHM report, academic hospitalists had an average of 17% fewer encounters. Of note, AMC patients tend to have higher complexity, as measured by the Case Mix Index (CMI – the average diagnosis-related group weight of a hospital).2 A higher CMI is a surrogate marker for the diagnostic diversity, clinical complexity, and resource needs of the patient population in the hospital.
Productivity and financial metrics
The financial bottom line is a critical aspect, and as a report in the Journal of Hospital Medicine described, all health care executives look at business metrics while making decisions.3 Below are some significant academic and community comparisons from SoHM 2018.
- Collections, encounters, and wRVUs (work relative value units) were highly correlated. All of them were lower for academic hospitalists, corroborating the fact that they see a smaller number of patients. Clinical full-time equivalents (cFTE) is a vernacular of how much of the faculty time is devoted to clinical activities. The academic data from SoHM achieves the same target, as it is standardized to 100% billable clinical activity, so the fact that many academic hospitalists do not work a full-time clinical schedule is not a factor in their lower production.
- Charges had a smaller gap likely because of sicker patients in AMCs. The higher acuity difference can also explain 12% higher wRVU/encounter for academic hospitalists.
- The wRVU/encounter ratio can indicate a few patterns: high acuity of patients in AMCs, higher levels of evaluation and management documentation, or both. As the encounters and charges have the same percentage differences, we would place our bets on the former.
- Compensation per encounter and compensation per wRVU showed that academic hospitalists do get a slight advantage.
CMI and wRVUs
Although the SoHM does not capture information on patient acuity or CMI, we speculate that the relationship between CMI and wRVUs may be more or less linear at lower levels of acuity. However, once level III E/M billing is achieved (assuming there is no critical care provided), wRVUs/encounter plateau, even as acuity continues to increase. This plateau effect may be seen more often in high-acuity AMC settings than in community hospitals.
So, in our opinion, compensation models based solely on wRVU production would not do justice for hospitalists in AMC settings since these models would fail to capture the extra work involved with very-high-acuity patients. SoHM 2018 shows the financial support per wRVU for AMC is $45.81, and for the community is $41.28, an 11% difference. We think the higher financial support per wRVU for academic practices may be related to the lost wRVU potential of caring for very-high-acuity patients.
Conclusion
In an academic setting, hospitalists are reforming the field of hospital medicine and defining the ways we could deliver care. They are the pillars of collaboration, education, research, innovation, quality, and safety. It would be increasingly crucial for academic hospitalist leaders to use comparative metrics from SoHM to advocate for their group. The bottom line can be explained by the title of the qualitative study in JHM referenced above: “Collaboration, not calculation.”3
Dr. Chadha is division chief for the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, scheduling, practice analysis, and operation of the group. He is a first-time member of the practice analysis committee. Ms. Dede is division administrator for the division of hospital medicine at the University of Kentucky Healthcare. She prepares and manages budgets, liaisons with the downstream revenue teams, and contributes to the building of academic compensation models. She is serving in the practice administrators committee for the second year and is currently vice chair of the Executive Council for the Practice Administrators special interest group.
References
1. State of Hospital Medicine Report. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
2. Deloitte Center for Health Solutions. Academic Medical Centers: Joining forces with community providers for broad benefits and positive outcomes. 2015. https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/academic-medical-centers-consolidation.html
3. White AA et al. Collaboration, not calculation: A qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(10):662‐7.
PHM fellowship changes in response to the COVID-19 pandemic
The COVID-19 surge and pandemic have changed many things in medicine, from how we round on the wards to our increased use of telemedicine in outpatient and inpatient care. This not only changed our interactions with patients, but it also changed our learners’ education. From March to May 2020 during the COVID-19 pandemic, many Pediatric Hospital Medicine (PHM) fellowship directors were required to adjust clinical responsibilities and scholarly activities. These changes led to unique challenges and learning opportunities for fellows and faculty.
Many fellowships were asked to make changes to patient care for patient/provider safety. Because of low censuses, the University of Pittsburgh Medical Center’s Pittsburgh Children’s Hospital closed their observation unit; as a result, Elise Lu, MD, PhD, PHM fellow, spent time rounding on inpatient units instead of the observation unit. At the Children’s Hospital of Los Angeles, PHM fellow Brandon Palmer, MD, said his in-person child abuse elective was switched to a virtual elective. Dr. Adam Cohen, chief PHM fellow at Baylor College of Medicine/Texas Children’s Hospital, both in Houston, offered to care for adult patients and provide telehealth services to pediatric patients, but was never called to participate.
At other programs, fellows experienced greater changes to patient care and systems-based practice. Carlos Plancarte, MD, PHM fellow at Children’s Hospital of Montefiore, New York, provided care for patients up to the age of 30 years as his training hospital began admitting adult patients. Jeremiah Cleveland, MD, PHM fellowship director at Maimonides Children’s Hospital, New York, shared that his fellow’s “away elective” at a pediatric long-term acute care facility was canceled. Dr. Cleveland changed the fellow’s rotation to an infectious disease rotation, which gave her a unique opportunity to evaluate the clinical and nonclinical aspects of pandemic and disaster response.
PHM fellows also experienced changes to their scholarly activities. Matthew Shapiro, MD, a fellow at Anne and Robert H. Lurie Children’s Hospital in Chicago, had to place his quality improvement research on hold and is writing a commentary with his mentors. Marie Pfarr, MD, a fellow at Cincinnati Children’s Hospital and Medical Center, changed her plans from a simulation-based research project to studying compassion fatigue. Many fellows had workshops, platform and/or poster presentations at the Pediatric Academic Societies Meeting and/or the Pediatric Hospital Medicine Conference, both of which were canceled.
Some fellows felt the pandemic provided them an opportunity to learn communication and interpersonal skills. Dr. Shapiro observed his mentors effectively communicating while managing a crisis. Dr. Plancarte shared that he learned that saying “I don’t know” can be helpful when effectively leading a team.
Despite the changes, the COVID-19 pandemic’s most important lesson was the creation of a supportive community. Across the country, PHM fellows were supported by faculty, and faculty by their fellows. Dr. Plancarte observed how his colleagues united during a challenging situation to support each other. Ritu Patel, MD, PHM fellowship director at Kaiser Oakland shared that her fellowship’s weekly informal meetings helped bring her fellowship’s fellows and faculty closer. Joel Forman, MD, PHM fellowship director and vice chair of education at Mount Sinai Kravis Children’s Hospital in New York stressed the importance of camaraderie amongst faculty and learners.
While the pandemic continues, and long after it has passed, fellowship programs have learned that fostering community across training lines is important for both fellows and faculty.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the pediatrics editor for the Hospitalist.
The COVID-19 surge and pandemic have changed many things in medicine, from how we round on the wards to our increased use of telemedicine in outpatient and inpatient care. This not only changed our interactions with patients, but it also changed our learners’ education. From March to May 2020 during the COVID-19 pandemic, many Pediatric Hospital Medicine (PHM) fellowship directors were required to adjust clinical responsibilities and scholarly activities. These changes led to unique challenges and learning opportunities for fellows and faculty.
Many fellowships were asked to make changes to patient care for patient/provider safety. Because of low censuses, the University of Pittsburgh Medical Center’s Pittsburgh Children’s Hospital closed their observation unit; as a result, Elise Lu, MD, PhD, PHM fellow, spent time rounding on inpatient units instead of the observation unit. At the Children’s Hospital of Los Angeles, PHM fellow Brandon Palmer, MD, said his in-person child abuse elective was switched to a virtual elective. Dr. Adam Cohen, chief PHM fellow at Baylor College of Medicine/Texas Children’s Hospital, both in Houston, offered to care for adult patients and provide telehealth services to pediatric patients, but was never called to participate.
At other programs, fellows experienced greater changes to patient care and systems-based practice. Carlos Plancarte, MD, PHM fellow at Children’s Hospital of Montefiore, New York, provided care for patients up to the age of 30 years as his training hospital began admitting adult patients. Jeremiah Cleveland, MD, PHM fellowship director at Maimonides Children’s Hospital, New York, shared that his fellow’s “away elective” at a pediatric long-term acute care facility was canceled. Dr. Cleveland changed the fellow’s rotation to an infectious disease rotation, which gave her a unique opportunity to evaluate the clinical and nonclinical aspects of pandemic and disaster response.
PHM fellows also experienced changes to their scholarly activities. Matthew Shapiro, MD, a fellow at Anne and Robert H. Lurie Children’s Hospital in Chicago, had to place his quality improvement research on hold and is writing a commentary with his mentors. Marie Pfarr, MD, a fellow at Cincinnati Children’s Hospital and Medical Center, changed her plans from a simulation-based research project to studying compassion fatigue. Many fellows had workshops, platform and/or poster presentations at the Pediatric Academic Societies Meeting and/or the Pediatric Hospital Medicine Conference, both of which were canceled.
Some fellows felt the pandemic provided them an opportunity to learn communication and interpersonal skills. Dr. Shapiro observed his mentors effectively communicating while managing a crisis. Dr. Plancarte shared that he learned that saying “I don’t know” can be helpful when effectively leading a team.
Despite the changes, the COVID-19 pandemic’s most important lesson was the creation of a supportive community. Across the country, PHM fellows were supported by faculty, and faculty by their fellows. Dr. Plancarte observed how his colleagues united during a challenging situation to support each other. Ritu Patel, MD, PHM fellowship director at Kaiser Oakland shared that her fellowship’s weekly informal meetings helped bring her fellowship’s fellows and faculty closer. Joel Forman, MD, PHM fellowship director and vice chair of education at Mount Sinai Kravis Children’s Hospital in New York stressed the importance of camaraderie amongst faculty and learners.
While the pandemic continues, and long after it has passed, fellowship programs have learned that fostering community across training lines is important for both fellows and faculty.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the pediatrics editor for the Hospitalist.
The COVID-19 surge and pandemic have changed many things in medicine, from how we round on the wards to our increased use of telemedicine in outpatient and inpatient care. This not only changed our interactions with patients, but it also changed our learners’ education. From March to May 2020 during the COVID-19 pandemic, many Pediatric Hospital Medicine (PHM) fellowship directors were required to adjust clinical responsibilities and scholarly activities. These changes led to unique challenges and learning opportunities for fellows and faculty.
Many fellowships were asked to make changes to patient care for patient/provider safety. Because of low censuses, the University of Pittsburgh Medical Center’s Pittsburgh Children’s Hospital closed their observation unit; as a result, Elise Lu, MD, PhD, PHM fellow, spent time rounding on inpatient units instead of the observation unit. At the Children’s Hospital of Los Angeles, PHM fellow Brandon Palmer, MD, said his in-person child abuse elective was switched to a virtual elective. Dr. Adam Cohen, chief PHM fellow at Baylor College of Medicine/Texas Children’s Hospital, both in Houston, offered to care for adult patients and provide telehealth services to pediatric patients, but was never called to participate.
At other programs, fellows experienced greater changes to patient care and systems-based practice. Carlos Plancarte, MD, PHM fellow at Children’s Hospital of Montefiore, New York, provided care for patients up to the age of 30 years as his training hospital began admitting adult patients. Jeremiah Cleveland, MD, PHM fellowship director at Maimonides Children’s Hospital, New York, shared that his fellow’s “away elective” at a pediatric long-term acute care facility was canceled. Dr. Cleveland changed the fellow’s rotation to an infectious disease rotation, which gave her a unique opportunity to evaluate the clinical and nonclinical aspects of pandemic and disaster response.
PHM fellows also experienced changes to their scholarly activities. Matthew Shapiro, MD, a fellow at Anne and Robert H. Lurie Children’s Hospital in Chicago, had to place his quality improvement research on hold and is writing a commentary with his mentors. Marie Pfarr, MD, a fellow at Cincinnati Children’s Hospital and Medical Center, changed her plans from a simulation-based research project to studying compassion fatigue. Many fellows had workshops, platform and/or poster presentations at the Pediatric Academic Societies Meeting and/or the Pediatric Hospital Medicine Conference, both of which were canceled.
Some fellows felt the pandemic provided them an opportunity to learn communication and interpersonal skills. Dr. Shapiro observed his mentors effectively communicating while managing a crisis. Dr. Plancarte shared that he learned that saying “I don’t know” can be helpful when effectively leading a team.
Despite the changes, the COVID-19 pandemic’s most important lesson was the creation of a supportive community. Across the country, PHM fellows were supported by faculty, and faculty by their fellows. Dr. Plancarte observed how his colleagues united during a challenging situation to support each other. Ritu Patel, MD, PHM fellowship director at Kaiser Oakland shared that her fellowship’s weekly informal meetings helped bring her fellowship’s fellows and faculty closer. Joel Forman, MD, PHM fellowship director and vice chair of education at Mount Sinai Kravis Children’s Hospital in New York stressed the importance of camaraderie amongst faculty and learners.
While the pandemic continues, and long after it has passed, fellowship programs have learned that fostering community across training lines is important for both fellows and faculty.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the pediatrics editor for the Hospitalist.
Risky business: Longer-course prophylactic perioperative antimicrobials
Background: National guidelines recommend that surgical prophylactic antimicrobials be initiated within 1 hour prior to incision and discontinued 24 hours postoperatively. However, the risks and benefits of longer duration of antimicrobials are uncertain.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: After stratification by type of surgery and adjustment for covariates, antibiotic prophylaxis greater than 24 hours was not associated with lower SSI risk.
However, the odds of postoperative AKI increased with each additional day of prophylaxis (adjusted odds ratios, 1.82; 95% confidence interval,1.54-2.16 and aOR, 1.79; 95% CI, 1.27-2.53) with longer than 72 hours prophylaxis for cardiac and noncardiac surgery, respectively). Similarly, C. difficile infections increased with each additional day beyond 24 hours (aOR, 3.65; 95% CI, 2.40-5.55 with more than 72 hours of use).
Bottom line: Each day of perioperative antimicrobial prophylaxis beyond 24 hours increases the risk for postoperative AKI or C. difficile infection without reducing the risk of surgical site infection.
Citation: Branch-Elliman W et al. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 2019 Apr 24. doi: 10.1001/jamasurg.2019.0569.
Dr. Miller is a hospitalist at the University of Colorado at Denver, Aurora.
Background: National guidelines recommend that surgical prophylactic antimicrobials be initiated within 1 hour prior to incision and discontinued 24 hours postoperatively. However, the risks and benefits of longer duration of antimicrobials are uncertain.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: After stratification by type of surgery and adjustment for covariates, antibiotic prophylaxis greater than 24 hours was not associated with lower SSI risk.
However, the odds of postoperative AKI increased with each additional day of prophylaxis (adjusted odds ratios, 1.82; 95% confidence interval,1.54-2.16 and aOR, 1.79; 95% CI, 1.27-2.53) with longer than 72 hours prophylaxis for cardiac and noncardiac surgery, respectively). Similarly, C. difficile infections increased with each additional day beyond 24 hours (aOR, 3.65; 95% CI, 2.40-5.55 with more than 72 hours of use).
Bottom line: Each day of perioperative antimicrobial prophylaxis beyond 24 hours increases the risk for postoperative AKI or C. difficile infection without reducing the risk of surgical site infection.
Citation: Branch-Elliman W et al. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 2019 Apr 24. doi: 10.1001/jamasurg.2019.0569.
Dr. Miller is a hospitalist at the University of Colorado at Denver, Aurora.
Background: National guidelines recommend that surgical prophylactic antimicrobials be initiated within 1 hour prior to incision and discontinued 24 hours postoperatively. However, the risks and benefits of longer duration of antimicrobials are uncertain.
Study design: Retrospective cohort study.
Setting: Veterans Affairs hospitals.
Synopsis: After stratification by type of surgery and adjustment for covariates, antibiotic prophylaxis greater than 24 hours was not associated with lower SSI risk.
However, the odds of postoperative AKI increased with each additional day of prophylaxis (adjusted odds ratios, 1.82; 95% confidence interval,1.54-2.16 and aOR, 1.79; 95% CI, 1.27-2.53) with longer than 72 hours prophylaxis for cardiac and noncardiac surgery, respectively). Similarly, C. difficile infections increased with each additional day beyond 24 hours (aOR, 3.65; 95% CI, 2.40-5.55 with more than 72 hours of use).
Bottom line: Each day of perioperative antimicrobial prophylaxis beyond 24 hours increases the risk for postoperative AKI or C. difficile infection without reducing the risk of surgical site infection.
Citation: Branch-Elliman W et al. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg. 2019 Apr 24. doi: 10.1001/jamasurg.2019.0569.
Dr. Miller is a hospitalist at the University of Colorado at Denver, Aurora.