User login
Safest, most effective medications for spine-related pain in older adults?
, a new comprehensive literature review suggests.
Investigators assessed the evidence for medications used for this indication in older adults by reviewing 138 double-blind, placebo-controlled trials.
Among their key findings and recommendations: Acetaminophen has a favorable safety profile for spine-related pain but nonsteroidal anti-inflammatory drugs (NSAIDs) have greater efficacy.
However, NSAIDs should be used in lower doses in the short term, with gastrointestinal precaution, the researchers note.
Corticosteroids have the least evidence for treating nonspecific back pain, they add.
“Most older people experience neck or low back pain at some point, bothersome enough to see their doctor,” coinvestigator Michael Perloff, MD, PhD, department of neurology, Boston University, said in a news release.
“Our findings provide a helpful medication guide for physicians to use for spine pain in an older population that can have a complex medical history,” Dr. Perloff added.
The results were published online in Drugs and Aging.
Recommendations, warnings
With the graying of the U.S. population, spine-related pain is increasingly common, the investigators note.
Medications play an important role in pain management, but their use has limitations in the elderly, owing to reduced liver and renal function, comorbid medical problems, and polypharmacy.
Other key findings from the literature review include that, although the nerve pain medications gabapentin and pregabalin may cause dizziness or difficulty walking, they also have some demonstrated benefit for neck and back nerve pain, such as sciatica, in older adults.
These agents should be used in lower doses with smaller dose adjustments, the researchers note.
They caution that the muscle relaxants carisoprodol, chlorzoxazone, cyclobenzaprine, metaxalone, methocarbamol, and orphenadrine should be avoided in older adults because of their association with risk for sedation and falls.
‘Rational therapeutic choices’
Three other muscle relaxants – tizanidine, baclofen, and dantrolene – may be helpful for neck and back pain. The most evidence favors tizanidine and baclofen. These should be used in reduced doses. Tizanidine should be avoided in patients with liver disease, and for patients with kidney disease, the dosing of baclofen should be reduced, the investigators write.
Other findings include the following:
- Older tricyclic antidepressants should typically be avoided in this population because of their side effects, but nortriptyline and desipramine may be better tolerated for neck and back nerve pain at lower doses.
- Newer antidepressants, particularly the selective serotonin-norepinephrine reuptake inhibitor duloxetine, have a better safety profile and good efficacy for spine-related nerve pain.
- Traditional opioids are typically avoided in the treatment of spine-related pain in older adults, owing to their associated risks.
However, low-dose opioid therapy may be helpful for severe refractory pain, with close monitoring of patients, the researchers note.
Weaker opioids, such as tramadol, may be better tolerated by older patients. They work well when combined with acetaminophen, but they carry the risk for sedation, upset stomach, and constipation.
“Medications used at the correct dose, for the correct diagnosis, adjusting for preexisting medical problems can result in better use of treatments for spine pain,” coinvestigator Jonathan Fu, MD, also with the department of neurology, Boston University, said in the release.
“Rational therapeutic choices should be targeted to spine pain diagnosis, such as NSAIDs and acetaminophen for arthritic and myofascial-based complaints, gabapentinoids or duloxetine for neuropathic and radicular symptoms, antispastic agents for myofascial-based pain, and combination therapy for mixed etiologies,” the investigators write.
They also emphasize that medications should be coupled with physical therapy and exercise programs, as well as treatment of the underlying degenerative disease process and medical illness, while keeping in mind the need for possible interventions and/or corrective surgery.
The research had no specific funding. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new comprehensive literature review suggests.
Investigators assessed the evidence for medications used for this indication in older adults by reviewing 138 double-blind, placebo-controlled trials.
Among their key findings and recommendations: Acetaminophen has a favorable safety profile for spine-related pain but nonsteroidal anti-inflammatory drugs (NSAIDs) have greater efficacy.
However, NSAIDs should be used in lower doses in the short term, with gastrointestinal precaution, the researchers note.
Corticosteroids have the least evidence for treating nonspecific back pain, they add.
“Most older people experience neck or low back pain at some point, bothersome enough to see their doctor,” coinvestigator Michael Perloff, MD, PhD, department of neurology, Boston University, said in a news release.
“Our findings provide a helpful medication guide for physicians to use for spine pain in an older population that can have a complex medical history,” Dr. Perloff added.
The results were published online in Drugs and Aging.
Recommendations, warnings
With the graying of the U.S. population, spine-related pain is increasingly common, the investigators note.
Medications play an important role in pain management, but their use has limitations in the elderly, owing to reduced liver and renal function, comorbid medical problems, and polypharmacy.
Other key findings from the literature review include that, although the nerve pain medications gabapentin and pregabalin may cause dizziness or difficulty walking, they also have some demonstrated benefit for neck and back nerve pain, such as sciatica, in older adults.
These agents should be used in lower doses with smaller dose adjustments, the researchers note.
They caution that the muscle relaxants carisoprodol, chlorzoxazone, cyclobenzaprine, metaxalone, methocarbamol, and orphenadrine should be avoided in older adults because of their association with risk for sedation and falls.
‘Rational therapeutic choices’
Three other muscle relaxants – tizanidine, baclofen, and dantrolene – may be helpful for neck and back pain. The most evidence favors tizanidine and baclofen. These should be used in reduced doses. Tizanidine should be avoided in patients with liver disease, and for patients with kidney disease, the dosing of baclofen should be reduced, the investigators write.
Other findings include the following:
- Older tricyclic antidepressants should typically be avoided in this population because of their side effects, but nortriptyline and desipramine may be better tolerated for neck and back nerve pain at lower doses.
- Newer antidepressants, particularly the selective serotonin-norepinephrine reuptake inhibitor duloxetine, have a better safety profile and good efficacy for spine-related nerve pain.
- Traditional opioids are typically avoided in the treatment of spine-related pain in older adults, owing to their associated risks.
However, low-dose opioid therapy may be helpful for severe refractory pain, with close monitoring of patients, the researchers note.
Weaker opioids, such as tramadol, may be better tolerated by older patients. They work well when combined with acetaminophen, but they carry the risk for sedation, upset stomach, and constipation.
“Medications used at the correct dose, for the correct diagnosis, adjusting for preexisting medical problems can result in better use of treatments for spine pain,” coinvestigator Jonathan Fu, MD, also with the department of neurology, Boston University, said in the release.
“Rational therapeutic choices should be targeted to spine pain diagnosis, such as NSAIDs and acetaminophen for arthritic and myofascial-based complaints, gabapentinoids or duloxetine for neuropathic and radicular symptoms, antispastic agents for myofascial-based pain, and combination therapy for mixed etiologies,” the investigators write.
They also emphasize that medications should be coupled with physical therapy and exercise programs, as well as treatment of the underlying degenerative disease process and medical illness, while keeping in mind the need for possible interventions and/or corrective surgery.
The research had no specific funding. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new comprehensive literature review suggests.
Investigators assessed the evidence for medications used for this indication in older adults by reviewing 138 double-blind, placebo-controlled trials.
Among their key findings and recommendations: Acetaminophen has a favorable safety profile for spine-related pain but nonsteroidal anti-inflammatory drugs (NSAIDs) have greater efficacy.
However, NSAIDs should be used in lower doses in the short term, with gastrointestinal precaution, the researchers note.
Corticosteroids have the least evidence for treating nonspecific back pain, they add.
“Most older people experience neck or low back pain at some point, bothersome enough to see their doctor,” coinvestigator Michael Perloff, MD, PhD, department of neurology, Boston University, said in a news release.
“Our findings provide a helpful medication guide for physicians to use for spine pain in an older population that can have a complex medical history,” Dr. Perloff added.
The results were published online in Drugs and Aging.
Recommendations, warnings
With the graying of the U.S. population, spine-related pain is increasingly common, the investigators note.
Medications play an important role in pain management, but their use has limitations in the elderly, owing to reduced liver and renal function, comorbid medical problems, and polypharmacy.
Other key findings from the literature review include that, although the nerve pain medications gabapentin and pregabalin may cause dizziness or difficulty walking, they also have some demonstrated benefit for neck and back nerve pain, such as sciatica, in older adults.
These agents should be used in lower doses with smaller dose adjustments, the researchers note.
They caution that the muscle relaxants carisoprodol, chlorzoxazone, cyclobenzaprine, metaxalone, methocarbamol, and orphenadrine should be avoided in older adults because of their association with risk for sedation and falls.
‘Rational therapeutic choices’
Three other muscle relaxants – tizanidine, baclofen, and dantrolene – may be helpful for neck and back pain. The most evidence favors tizanidine and baclofen. These should be used in reduced doses. Tizanidine should be avoided in patients with liver disease, and for patients with kidney disease, the dosing of baclofen should be reduced, the investigators write.
Other findings include the following:
- Older tricyclic antidepressants should typically be avoided in this population because of their side effects, but nortriptyline and desipramine may be better tolerated for neck and back nerve pain at lower doses.
- Newer antidepressants, particularly the selective serotonin-norepinephrine reuptake inhibitor duloxetine, have a better safety profile and good efficacy for spine-related nerve pain.
- Traditional opioids are typically avoided in the treatment of spine-related pain in older adults, owing to their associated risks.
However, low-dose opioid therapy may be helpful for severe refractory pain, with close monitoring of patients, the researchers note.
Weaker opioids, such as tramadol, may be better tolerated by older patients. They work well when combined with acetaminophen, but they carry the risk for sedation, upset stomach, and constipation.
“Medications used at the correct dose, for the correct diagnosis, adjusting for preexisting medical problems can result in better use of treatments for spine pain,” coinvestigator Jonathan Fu, MD, also with the department of neurology, Boston University, said in the release.
“Rational therapeutic choices should be targeted to spine pain diagnosis, such as NSAIDs and acetaminophen for arthritic and myofascial-based complaints, gabapentinoids or duloxetine for neuropathic and radicular symptoms, antispastic agents for myofascial-based pain, and combination therapy for mixed etiologies,” the investigators write.
They also emphasize that medications should be coupled with physical therapy and exercise programs, as well as treatment of the underlying degenerative disease process and medical illness, while keeping in mind the need for possible interventions and/or corrective surgery.
The research had no specific funding. The investigators have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DRUGS AND AGING
Alcohol’s detrimental impact on the brain explained?
Results of a large observational study suggest brain iron accumulation is a “plausible pathway” through which alcohol negatively affects cognition, study Anya Topiwala, MD, PhD, senior clinical researcher, Nuffield Department of Population Health, University of Oxford, England, said in an interview.
Study participants who drank 56 grams of alcohol a week had higher brain iron levels. The U.K. guideline for “low risk” alcohol consumption is less than 14 units weekly, or 112 grams.
“We are finding harmful associations with iron within those low-risk alcohol intake guidelines,” said Dr. Topiwala.
The study was published online in PLOS Medicine.
Early intervention opportunity?
Previous research suggests higher brain iron may be involved in the pathophysiology of Alzheimer’s and Parkinson’s diseases. However, it’s unclear whether deposition plays a role in alcohol’s effect on the brain and if it does, whether this could present an opportunity for early intervention with, for example, chelating agents.
The study included 20,729 participants in the UK Biobank study, which recruited volunteers from 2006 to 2010. Participants had a mean age of 54.8 years, and 48.6% were female.
Participants self-identified as current, never, or previous alcohol consumers. For current drinkers, researchers calculated the total weekly number of U.K. units of alcohol consumed. One unit is 8 grams. A standard drink in the United States is 14 grams. They categorized weekly consumption into quintiles and used the lowest quintile as the reference category.
Participants underwent MRI to determine brain iron levels. Areas of interest were deep brain structures in the basal ganglia.
Mean weekly alcohol consumption was 17.7 units, which is higher than U.K. guidelines for low-risk consumption. “Half of the sample were drinking above what is recommended,” said Dr. Topiwala.
Alcohol consumption was associated with markers of higher iron in the bilateral putamen (beta, 0.08 standard deviation; 95% confidence interval, 0.06-0.09; P < .001), caudate (beta, 0.05; 95% CI, 0.04-0.07; P < .001), and substantia nigra (beta, 0.03; 95% CI; 0.02-0.05; P < .001).
Poorer performance
Drinking more than 7 units (56 grams) weekly was associated with higher susceptibility for all brain regions, except the thalamus.
Controlling for menopause status did not alter associations between alcohol and susceptibility for any brain region. This was also the case when excluding blood pressure and cholesterol as covariates.
There were significant interactions with age in the bilateral putamen and caudate but not with sex, smoking, or Townsend Deprivation Index, which includes such factors as unemployment and living conditions.
To gather data on liver iron levels, participants underwent abdominal imaging at the same time as brain imaging. Dr. Topiwala explained that the liver is a primary storage center for iron, so it was used as “a kind of surrogate marker” of iron in the body.
The researchers showed an indirect effect of alcohol through systemic iron. A 1 SD increase in weekly alcohol consumption was associated with a 0.05 mg/g (95% CI, 0.02-0.07; P < .001) increase in liver iron. In addition, a 1 mg/g increase in liver iron was associated with a 0.44 (95% CI, 0.35-0.52; P < .001) SD increase in left putamen susceptibility.
In this sample, 32% (95% CI, 22-49; P < .001) of alcohol’s total effect on left putamen susceptibility was mediated via higher systemic iron levels.
To minimize the impact of other factors influencing the association between alcohol consumption and brain iron – and the possibility that people with more brain iron drink more – researchers used Mendelian randomization that considers genetically predicted alcohol intake. This analysis supported findings of associations between alcohol consumption and brain iron.
Participants completed a cognitive battery, which included trail-making tests that reflect executive function, puzzle tests that assess fluid intelligence or logic and reasoning, and task-based tests using the “Snap” card game to measure reaction time.
Investigators found the more iron that was present in certain brain regions, the poorer participants’ cognitive performance.
Patients should know about the risks of moderate alcohol intake so they can make decisions about drinking, said Dr. Topiwala. “They should be aware that 14 units of alcohol per week is not a zero risk.”
Novel research
Commenting for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, noted the study’s large size as a strength of the research.
She noted previous research has shown an association between higher iron levels and alcohol dependence and worse cognitive function, but the potential connection of brain iron levels, moderate alcohol consumption, and cognition has not been studied to date.
“This paper aims to look at whether there is a potential biological link between moderate alcohol consumption and cognition through iron-related pathways.”
The authors suggest more work is needed to understand whether alcohol consumption impacts iron-related biologies to affect downstream cognition, said Dr. Snyder. “Although this study does not answer that question, it does highlight some important questions.”
Study authors received funding from Wellcome Trust, UK Medical Research Council, National Institute for Health Research (NIHR) Oxford Biomedical Research Centre, BHF Centre of Research Excellence, British Heart Foundation, NIHR Cambridge Biomedical Research Centre, U.S. Department of Veterans Affairs, China Scholarship Council, and Li Ka Shing Centre for Health Information and Discovery. Dr. Topiwala has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large observational study suggest brain iron accumulation is a “plausible pathway” through which alcohol negatively affects cognition, study Anya Topiwala, MD, PhD, senior clinical researcher, Nuffield Department of Population Health, University of Oxford, England, said in an interview.
Study participants who drank 56 grams of alcohol a week had higher brain iron levels. The U.K. guideline for “low risk” alcohol consumption is less than 14 units weekly, or 112 grams.
“We are finding harmful associations with iron within those low-risk alcohol intake guidelines,” said Dr. Topiwala.
The study was published online in PLOS Medicine.
Early intervention opportunity?
Previous research suggests higher brain iron may be involved in the pathophysiology of Alzheimer’s and Parkinson’s diseases. However, it’s unclear whether deposition plays a role in alcohol’s effect on the brain and if it does, whether this could present an opportunity for early intervention with, for example, chelating agents.
The study included 20,729 participants in the UK Biobank study, which recruited volunteers from 2006 to 2010. Participants had a mean age of 54.8 years, and 48.6% were female.
Participants self-identified as current, never, or previous alcohol consumers. For current drinkers, researchers calculated the total weekly number of U.K. units of alcohol consumed. One unit is 8 grams. A standard drink in the United States is 14 grams. They categorized weekly consumption into quintiles and used the lowest quintile as the reference category.
Participants underwent MRI to determine brain iron levels. Areas of interest were deep brain structures in the basal ganglia.
Mean weekly alcohol consumption was 17.7 units, which is higher than U.K. guidelines for low-risk consumption. “Half of the sample were drinking above what is recommended,” said Dr. Topiwala.
Alcohol consumption was associated with markers of higher iron in the bilateral putamen (beta, 0.08 standard deviation; 95% confidence interval, 0.06-0.09; P < .001), caudate (beta, 0.05; 95% CI, 0.04-0.07; P < .001), and substantia nigra (beta, 0.03; 95% CI; 0.02-0.05; P < .001).
Poorer performance
Drinking more than 7 units (56 grams) weekly was associated with higher susceptibility for all brain regions, except the thalamus.
Controlling for menopause status did not alter associations between alcohol and susceptibility for any brain region. This was also the case when excluding blood pressure and cholesterol as covariates.
There were significant interactions with age in the bilateral putamen and caudate but not with sex, smoking, or Townsend Deprivation Index, which includes such factors as unemployment and living conditions.
To gather data on liver iron levels, participants underwent abdominal imaging at the same time as brain imaging. Dr. Topiwala explained that the liver is a primary storage center for iron, so it was used as “a kind of surrogate marker” of iron in the body.
The researchers showed an indirect effect of alcohol through systemic iron. A 1 SD increase in weekly alcohol consumption was associated with a 0.05 mg/g (95% CI, 0.02-0.07; P < .001) increase in liver iron. In addition, a 1 mg/g increase in liver iron was associated with a 0.44 (95% CI, 0.35-0.52; P < .001) SD increase in left putamen susceptibility.
In this sample, 32% (95% CI, 22-49; P < .001) of alcohol’s total effect on left putamen susceptibility was mediated via higher systemic iron levels.
To minimize the impact of other factors influencing the association between alcohol consumption and brain iron – and the possibility that people with more brain iron drink more – researchers used Mendelian randomization that considers genetically predicted alcohol intake. This analysis supported findings of associations between alcohol consumption and brain iron.
Participants completed a cognitive battery, which included trail-making tests that reflect executive function, puzzle tests that assess fluid intelligence or logic and reasoning, and task-based tests using the “Snap” card game to measure reaction time.
Investigators found the more iron that was present in certain brain regions, the poorer participants’ cognitive performance.
Patients should know about the risks of moderate alcohol intake so they can make decisions about drinking, said Dr. Topiwala. “They should be aware that 14 units of alcohol per week is not a zero risk.”
Novel research
Commenting for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, noted the study’s large size as a strength of the research.
She noted previous research has shown an association between higher iron levels and alcohol dependence and worse cognitive function, but the potential connection of brain iron levels, moderate alcohol consumption, and cognition has not been studied to date.
“This paper aims to look at whether there is a potential biological link between moderate alcohol consumption and cognition through iron-related pathways.”
The authors suggest more work is needed to understand whether alcohol consumption impacts iron-related biologies to affect downstream cognition, said Dr. Snyder. “Although this study does not answer that question, it does highlight some important questions.”
Study authors received funding from Wellcome Trust, UK Medical Research Council, National Institute for Health Research (NIHR) Oxford Biomedical Research Centre, BHF Centre of Research Excellence, British Heart Foundation, NIHR Cambridge Biomedical Research Centre, U.S. Department of Veterans Affairs, China Scholarship Council, and Li Ka Shing Centre for Health Information and Discovery. Dr. Topiwala has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a large observational study suggest brain iron accumulation is a “plausible pathway” through which alcohol negatively affects cognition, study Anya Topiwala, MD, PhD, senior clinical researcher, Nuffield Department of Population Health, University of Oxford, England, said in an interview.
Study participants who drank 56 grams of alcohol a week had higher brain iron levels. The U.K. guideline for “low risk” alcohol consumption is less than 14 units weekly, or 112 grams.
“We are finding harmful associations with iron within those low-risk alcohol intake guidelines,” said Dr. Topiwala.
The study was published online in PLOS Medicine.
Early intervention opportunity?
Previous research suggests higher brain iron may be involved in the pathophysiology of Alzheimer’s and Parkinson’s diseases. However, it’s unclear whether deposition plays a role in alcohol’s effect on the brain and if it does, whether this could present an opportunity for early intervention with, for example, chelating agents.
The study included 20,729 participants in the UK Biobank study, which recruited volunteers from 2006 to 2010. Participants had a mean age of 54.8 years, and 48.6% were female.
Participants self-identified as current, never, or previous alcohol consumers. For current drinkers, researchers calculated the total weekly number of U.K. units of alcohol consumed. One unit is 8 grams. A standard drink in the United States is 14 grams. They categorized weekly consumption into quintiles and used the lowest quintile as the reference category.
Participants underwent MRI to determine brain iron levels. Areas of interest were deep brain structures in the basal ganglia.
Mean weekly alcohol consumption was 17.7 units, which is higher than U.K. guidelines for low-risk consumption. “Half of the sample were drinking above what is recommended,” said Dr. Topiwala.
Alcohol consumption was associated with markers of higher iron in the bilateral putamen (beta, 0.08 standard deviation; 95% confidence interval, 0.06-0.09; P < .001), caudate (beta, 0.05; 95% CI, 0.04-0.07; P < .001), and substantia nigra (beta, 0.03; 95% CI; 0.02-0.05; P < .001).
Poorer performance
Drinking more than 7 units (56 grams) weekly was associated with higher susceptibility for all brain regions, except the thalamus.
Controlling for menopause status did not alter associations between alcohol and susceptibility for any brain region. This was also the case when excluding blood pressure and cholesterol as covariates.
There were significant interactions with age in the bilateral putamen and caudate but not with sex, smoking, or Townsend Deprivation Index, which includes such factors as unemployment and living conditions.
To gather data on liver iron levels, participants underwent abdominal imaging at the same time as brain imaging. Dr. Topiwala explained that the liver is a primary storage center for iron, so it was used as “a kind of surrogate marker” of iron in the body.
The researchers showed an indirect effect of alcohol through systemic iron. A 1 SD increase in weekly alcohol consumption was associated with a 0.05 mg/g (95% CI, 0.02-0.07; P < .001) increase in liver iron. In addition, a 1 mg/g increase in liver iron was associated with a 0.44 (95% CI, 0.35-0.52; P < .001) SD increase in left putamen susceptibility.
In this sample, 32% (95% CI, 22-49; P < .001) of alcohol’s total effect on left putamen susceptibility was mediated via higher systemic iron levels.
To minimize the impact of other factors influencing the association between alcohol consumption and brain iron – and the possibility that people with more brain iron drink more – researchers used Mendelian randomization that considers genetically predicted alcohol intake. This analysis supported findings of associations between alcohol consumption and brain iron.
Participants completed a cognitive battery, which included trail-making tests that reflect executive function, puzzle tests that assess fluid intelligence or logic and reasoning, and task-based tests using the “Snap” card game to measure reaction time.
Investigators found the more iron that was present in certain brain regions, the poorer participants’ cognitive performance.
Patients should know about the risks of moderate alcohol intake so they can make decisions about drinking, said Dr. Topiwala. “They should be aware that 14 units of alcohol per week is not a zero risk.”
Novel research
Commenting for this news organization, Heather Snyder, PhD, vice president of medical and scientific relations, Alzheimer’s Association, noted the study’s large size as a strength of the research.
She noted previous research has shown an association between higher iron levels and alcohol dependence and worse cognitive function, but the potential connection of brain iron levels, moderate alcohol consumption, and cognition has not been studied to date.
“This paper aims to look at whether there is a potential biological link between moderate alcohol consumption and cognition through iron-related pathways.”
The authors suggest more work is needed to understand whether alcohol consumption impacts iron-related biologies to affect downstream cognition, said Dr. Snyder. “Although this study does not answer that question, it does highlight some important questions.”
Study authors received funding from Wellcome Trust, UK Medical Research Council, National Institute for Health Research (NIHR) Oxford Biomedical Research Centre, BHF Centre of Research Excellence, British Heart Foundation, NIHR Cambridge Biomedical Research Centre, U.S. Department of Veterans Affairs, China Scholarship Council, and Li Ka Shing Centre for Health Information and Discovery. Dr. Topiwala has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PLOS MEDICINE
Sleep-deprived physicians less empathetic to patient pain?
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
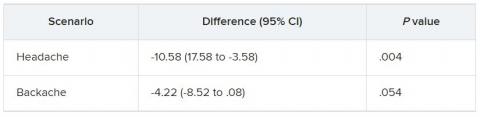
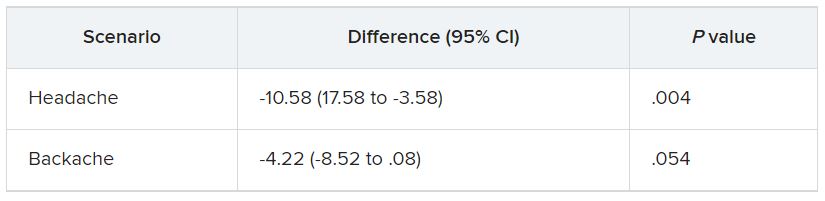
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
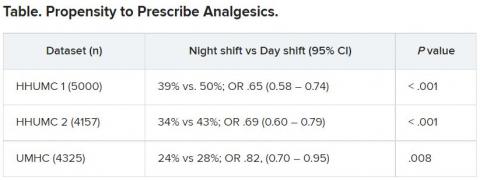
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
In the first of two studies, resident physicians were presented with two hypothetical scenarios involving a patient who complains of pain. They were asked about their likelihood of prescribing pain medication. The test was given to one group of residents who were just starting their day and to another group who were at the end of their night shift after being on call for 26 hours.
Results showed that the night shift residents were less likely than their daytime counterparts to say they would prescribe pain medication to the patients.
In further analysis of discharge notes from more than 13,000 electronic records of patients presenting with pain complaints at hospitals in Israel and the United States, the likelihood of an analgesic being prescribed during the night shift was 11% lower in Israel and 9% lower in the United States, compared with the day shift.
“Pain management is a major challenge, and a doctor’s perception of a patient’s subjective pain is susceptible to bias,” coinvestigator David Gozal, MD, the Marie M. and Harry L. Smith Endowed Chair of Child Health, University of Missouri–Columbia, said in a press release.
“This study demonstrated that night shift work is an important and previously unrecognized source of bias in pain management, likely stemming from impaired perception of pain,” Dr. Gozal added.
The findings were published online in the Proceedings of the National Academy of Sciences.
‘Directional’ differences
Senior investigator Alex Gileles-Hillel, MD, senior pediatric pulmonologist and sleep researcher at Hadassah University Medical Center, Jerusalem, said in an interview that physicians must make “complex assessments of patients’ subjective pain experience” – and the “subjective nature of pain management decisions can give rise to various biases.”
Dr. Gileles-Hillel has previously researched the cognitive toll of night shift work on physicians.
“It’s pretty established, for example, not to drive when sleep deprived because cognition is impaired,” he said. The current study explored whether sleep deprivation could affect areas other than cognition, including emotions and empathy.
The researchers used “two complementary approaches.” First, they administered tests to measure empathy and pain management decisions in 67 resident physicians at Hadassah Medical Centers either following a 26-hour night shift that began at 8:00 a.m. the day before (n = 36) or immediately before starting the workday (n = 31).
There were no significant differences in demographic, sleep, or burnout measures between the two groups, except that night shift physicians had slept less than those in the daytime group (2.93 vs. 5.96 hours).
Participants completed two tasks. In the empathy-for-pain task, they rated their emotional reactions to pictures of individuals in pain. In the empathy accuracy task, they were asked to assess the feelings of videotaped individuals telling emotional stories.
They were then presented with two clinical scenarios: a female patient with a headache and a male patient with a backache. Following that, they were asked to assess the magnitude of the patients’ pain and how likely they would be to prescribe pain medication.
In the empathy-for-pain task, physicians’ empathy scores were significantly lower in the night shift group than in the day group (difference, –0.83; 95% CI, –1.55 to –0.10; P = .026). There were no significant differences between the groups in the empathy accuracy task.
In both scenarios, physicians in the night shift group assessed the patient’s pain as weaker in comparison with physicians in the day group. There was a statistically significant difference in the headache scenario but not the backache scenario.
In the headache scenario, the propensity of the physicians to prescribe analgesics was “directionally lower” but did not reach statistical significance. In the backache scenario, there was no significant difference between the groups’ prescribing propensities.
In both scenarios, pain assessment was positively correlated with the propensity to prescribe analgesics.
Despite the lack of statistical significance, the findings “documented a negative effect of night shift work on physician empathy for pain and a positive association between physician assessment of patient pain and the propensity to prescribe analgesics,” the investigators wrote.
Need for naps?
The researchers then analyzed analgesic prescription patterns drawn from three datasets of discharge notes of patients presenting to the emergency department with pain complaints (n = 13,482) at two branches of Hadassah-Hebrew University Medical Center and the University of Missouri Health Center.
The researchers collected data, including discharge time, medications patients were prescribed upon discharge, and patients’ subjective pain rating on a scale of 0-10 on a visual analogue scale (VAS).
Although patients’ VAS scores did not differ with respect to time or shift, patients were discharged with significantly less prescribed analgesics during the night shift in comparison with the day shift.
No similar differences in prescriptions between night shifts and day shifts were found for nonanalgesic medications, such as for diabetes or blood pressure. This suggests “the effect was specific to pain,” Dr. Gileles-Hillel said.
The pattern remained significant after controlling for potential confounders, including patient and physician variables and emergency department characteristics.
In addition, patients seen during night shifts received fewer analgesics, particularly opioids, than recommended by the World Health Organization for pain management.
“The first study enabled us to measure empathy for pain directly and examine our hypothesis in a controlled environment, while the second enabled us to test the implications by examining real-life pain management decisions,” Dr. Gileles-Hillel said.
“Physicians need to be aware of this,” he noted. “I try to be aware when I’m taking calls [at night] that I’m less empathetic to others and I might be more brief or angry with others.”
On a “house management level, perhaps institutions should try to schedule naps either before or during overnight call. A nap might give a boost and reboot not only to cognitive but also to emotional resources,” Dr. Gileles-Hillel added.
Compromised safety
In a comment, Eti Ben Simon, PhD, a postdoctoral fellow at the Center for Human Sleep Science, University of California, Berkeley, called the study “an important contribution to a growing list of studies that reveal how long night shifts reduce overall safety” for both patients and clinicians.
“It’s time to abandon the notion that the human brain can function as normal after being deprived of sleep for 24 hours,” said Dr. Ben Simon, who was not involved with the research.
“This is especially true in medicine, where we trust others to take care of us and feel our pain. These functions are simply not possible without adequate sleep,” she added.
Also commenting, Kannan Ramar, MD, president of the American Academy of Sleep Medicine, suggested that being cognizant of these findings “may help providers to mitigate this bias” of underprescribing pain medications when treating their patients.
Dr. Ramar, who is also a critical care specialist, pulmonologist, and sleep medicine specialist at Mayo Clinic, Rochester, Minn., was not involved with the research.
He noted that “further studies that systematically evaluate this further in a prospective and blinded way will be important.”
The research was supported in part by grants from the Israel Science Foundation, Joy Ventures, the Recanati Fund at the Jerusalem School of Business at the Hebrew University, and a fellowship from the Azrieli Foundation and received grant support to various investigators from the NIH, the Leda J. Sears Foundation, and the University of Missouri. The investigators, Ramar, and Ben Simon have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
‘Striking’ jump in cost of brand-name epilepsy meds
, a new analysis shows.
After adjustment for inflation, the cost of a 1-year supply of brand-name ASMs grew 277%, while generics became 42% less expensive.
“Our study makes transparent striking trends in brand name prescribing patterns,” the study team wrote.
Since 2010, the costs for brand-name ASMs have “consistently” increased. Costs were particularly boosted by increases in prescriptions for lacosamide (Vimpat), in addition to a “steep increase in the cost per pill, with brand-name drugs costing 10 times more than their generic counterparts,” first author Samuel Waller Terman, MD, of the University of Michigan, Ann Arbor, added in a news release.
The study was published online in Neurology.
Is a 10-fold increase in cost worth it?
To evaluate trends in ASM prescriptions and costs, the researchers used a random sample of 20% of Medicare beneficiaries with coverage from 2008 to 2018. There were 77,000 to 133,000 patients with epilepsy each year.
Over time, likely because of increasing availability of generics, brand-name ASMs made up a smaller proportion of pills prescribed, from 56% in 2008 to 14% in 2018, but still made up 79% of prescription drug costs in 2018.
The annual cost of brand-name ASMs rose from $2,800 in 2008 to $10,700 in 2018, while the cost of generic drugs decreased from $800 to $460 during that time.
An increased number of prescriptions for lacosamide was responsible for 45% of the total increase in brand-name costs.
As of 2018, lacosamide comprised 30% of all brand-name pill supply (followed by pregabalin, at 15%) and 30% of all brand-name costs (followed by clobazam and pregabalin, both at 9%), the investigators reported.
Brand-name antiepileptic drug costs decreased from 2008 to 2010, but after the introduction of lacosamide, total brand-name costs steadily rose from $72 million in 2010 (in 2018 dollars) to $256 million in 2018, they noted.
Because the dataset consists of a 20% random Medicare sample, total Medicare costs for brand-name ASMs for beneficiaries with epilepsy alone likely rose from roughly $360 million in 2010 to $1.3 billion in 2018, they added.
“Clinicians must remain cognizant of this societal cost magnitude when judging whether the 10-fold increased expense per pill for brand name medications is worth the possible benefits,” they wrote.
“While newer-generation drugs have potential advantages such as limited drug interactions and different side effect profiles, there have been conflicting studies on whether they are cost effective,” Dr. Terman noted in a news release.
A barrier to treatment
The authors of an accompanying editorial propose that the problem of prescription drug costs could be solved through a combination of competition and government regulation of prices. Patients and physicians are the most important stakeholders in this issue.
“When something represents 14% of the total use, but contributes 79% of the cost, it would be wise to consider alternatives, assuming that these alternatives are not of lower quality,” wrote Wyatt Bensken, with Case Western Reserve University, Cleveland, and Iván Sánchez Fernández, MD, with Boston Medical Center.
“When there are several ASMs with a similar mechanism of action, similar efficacy, similar safety and tolerability profile, and different costs, it would be unwise to choose the more expensive alternative just because it is newer,” they said.
This study, they added, provides data to “understand, and begin to act, on the challenging problem of the cost of prescription ASMs. After all, what is the point of having a large number of ASMs if their cost severely limits their use?”
A limitation of the study is that only Medicare prescription claims were included, so the results may not apply to younger patients with private insurance.
The study received no direct funding. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis shows.
After adjustment for inflation, the cost of a 1-year supply of brand-name ASMs grew 277%, while generics became 42% less expensive.
“Our study makes transparent striking trends in brand name prescribing patterns,” the study team wrote.
Since 2010, the costs for brand-name ASMs have “consistently” increased. Costs were particularly boosted by increases in prescriptions for lacosamide (Vimpat), in addition to a “steep increase in the cost per pill, with brand-name drugs costing 10 times more than their generic counterparts,” first author Samuel Waller Terman, MD, of the University of Michigan, Ann Arbor, added in a news release.
The study was published online in Neurology.
Is a 10-fold increase in cost worth it?
To evaluate trends in ASM prescriptions and costs, the researchers used a random sample of 20% of Medicare beneficiaries with coverage from 2008 to 2018. There were 77,000 to 133,000 patients with epilepsy each year.
Over time, likely because of increasing availability of generics, brand-name ASMs made up a smaller proportion of pills prescribed, from 56% in 2008 to 14% in 2018, but still made up 79% of prescription drug costs in 2018.
The annual cost of brand-name ASMs rose from $2,800 in 2008 to $10,700 in 2018, while the cost of generic drugs decreased from $800 to $460 during that time.
An increased number of prescriptions for lacosamide was responsible for 45% of the total increase in brand-name costs.
As of 2018, lacosamide comprised 30% of all brand-name pill supply (followed by pregabalin, at 15%) and 30% of all brand-name costs (followed by clobazam and pregabalin, both at 9%), the investigators reported.
Brand-name antiepileptic drug costs decreased from 2008 to 2010, but after the introduction of lacosamide, total brand-name costs steadily rose from $72 million in 2010 (in 2018 dollars) to $256 million in 2018, they noted.
Because the dataset consists of a 20% random Medicare sample, total Medicare costs for brand-name ASMs for beneficiaries with epilepsy alone likely rose from roughly $360 million in 2010 to $1.3 billion in 2018, they added.
“Clinicians must remain cognizant of this societal cost magnitude when judging whether the 10-fold increased expense per pill for brand name medications is worth the possible benefits,” they wrote.
“While newer-generation drugs have potential advantages such as limited drug interactions and different side effect profiles, there have been conflicting studies on whether they are cost effective,” Dr. Terman noted in a news release.
A barrier to treatment
The authors of an accompanying editorial propose that the problem of prescription drug costs could be solved through a combination of competition and government regulation of prices. Patients and physicians are the most important stakeholders in this issue.
“When something represents 14% of the total use, but contributes 79% of the cost, it would be wise to consider alternatives, assuming that these alternatives are not of lower quality,” wrote Wyatt Bensken, with Case Western Reserve University, Cleveland, and Iván Sánchez Fernández, MD, with Boston Medical Center.
“When there are several ASMs with a similar mechanism of action, similar efficacy, similar safety and tolerability profile, and different costs, it would be unwise to choose the more expensive alternative just because it is newer,” they said.
This study, they added, provides data to “understand, and begin to act, on the challenging problem of the cost of prescription ASMs. After all, what is the point of having a large number of ASMs if their cost severely limits their use?”
A limitation of the study is that only Medicare prescription claims were included, so the results may not apply to younger patients with private insurance.
The study received no direct funding. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, a new analysis shows.
After adjustment for inflation, the cost of a 1-year supply of brand-name ASMs grew 277%, while generics became 42% less expensive.
“Our study makes transparent striking trends in brand name prescribing patterns,” the study team wrote.
Since 2010, the costs for brand-name ASMs have “consistently” increased. Costs were particularly boosted by increases in prescriptions for lacosamide (Vimpat), in addition to a “steep increase in the cost per pill, with brand-name drugs costing 10 times more than their generic counterparts,” first author Samuel Waller Terman, MD, of the University of Michigan, Ann Arbor, added in a news release.
The study was published online in Neurology.
Is a 10-fold increase in cost worth it?
To evaluate trends in ASM prescriptions and costs, the researchers used a random sample of 20% of Medicare beneficiaries with coverage from 2008 to 2018. There were 77,000 to 133,000 patients with epilepsy each year.
Over time, likely because of increasing availability of generics, brand-name ASMs made up a smaller proportion of pills prescribed, from 56% in 2008 to 14% in 2018, but still made up 79% of prescription drug costs in 2018.
The annual cost of brand-name ASMs rose from $2,800 in 2008 to $10,700 in 2018, while the cost of generic drugs decreased from $800 to $460 during that time.
An increased number of prescriptions for lacosamide was responsible for 45% of the total increase in brand-name costs.
As of 2018, lacosamide comprised 30% of all brand-name pill supply (followed by pregabalin, at 15%) and 30% of all brand-name costs (followed by clobazam and pregabalin, both at 9%), the investigators reported.
Brand-name antiepileptic drug costs decreased from 2008 to 2010, but after the introduction of lacosamide, total brand-name costs steadily rose from $72 million in 2010 (in 2018 dollars) to $256 million in 2018, they noted.
Because the dataset consists of a 20% random Medicare sample, total Medicare costs for brand-name ASMs for beneficiaries with epilepsy alone likely rose from roughly $360 million in 2010 to $1.3 billion in 2018, they added.
“Clinicians must remain cognizant of this societal cost magnitude when judging whether the 10-fold increased expense per pill for brand name medications is worth the possible benefits,” they wrote.
“While newer-generation drugs have potential advantages such as limited drug interactions and different side effect profiles, there have been conflicting studies on whether they are cost effective,” Dr. Terman noted in a news release.
A barrier to treatment
The authors of an accompanying editorial propose that the problem of prescription drug costs could be solved through a combination of competition and government regulation of prices. Patients and physicians are the most important stakeholders in this issue.
“When something represents 14% of the total use, but contributes 79% of the cost, it would be wise to consider alternatives, assuming that these alternatives are not of lower quality,” wrote Wyatt Bensken, with Case Western Reserve University, Cleveland, and Iván Sánchez Fernández, MD, with Boston Medical Center.
“When there are several ASMs with a similar mechanism of action, similar efficacy, similar safety and tolerability profile, and different costs, it would be unwise to choose the more expensive alternative just because it is newer,” they said.
This study, they added, provides data to “understand, and begin to act, on the challenging problem of the cost of prescription ASMs. After all, what is the point of having a large number of ASMs if their cost severely limits their use?”
A limitation of the study is that only Medicare prescription claims were included, so the results may not apply to younger patients with private insurance.
The study received no direct funding. The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Scientists find brain mechanism behind age-related memory loss
Scientists at Johns Hopkins University have identified a mechanism in the brain behind age-related memory loss, expanding our knowledge of the inner workings of the aging brain and possibly opening the door to new Alzheimer’s treatments.
The researchers looked at the hippocampus, a part of the brain thought to store long-term memories.
Neurons there are responsible for a pair of memory functions – called pattern separation and pattern completion – that work together in young, healthy brains. These functions can swing out of balance with age, impacting memory.
The Johns Hopkins team may have discovered what causes this imbalance. Their findings – reported in a paper in the journal Current Biology – may not only help us improve dementia treatments, but even prevent or delay a loss of thinking skills in the first place, the researchers say.
Pattern separation vs. pattern completion
To understand how the hippocampus changes with age, the researchers looked at rats’ brains. In rats and in humans, pattern separation and pattern completion are present, controlled by neurons in the hippocampus.
As the name suggests, pattern completion is when you take a few details or fragments of information – a few notes of music, or the start of a famous movie quote – and your brain retrieves the full memory. Pattern separation, on the other hand, is being able to tell similar observations or experiences apart (like two visits to the same restaurant) to be stored as separate memories.
These functions occur along a gradient across a tiny region called CA3. That gradient, the study found, disappears with aging, said lead study author Hey-Kyoung Lee, PhD, an assistant research scientist at the university’s Zanvyl Krieger Mind/Brain Institute. “The main consequence of the loss,” Dr. Lee said, “is that pattern completion becomes more dominant in rats as they age.”
What’s happening in the brain
Neurons responsible for pattern completion occupy the “distal” end of CA3, while those in charge of pattern separation reside at the “proximal” end. Dr. Lee said prior studies had not examined the proximal and distal regions separately, as she and her team did in this study.
What was surprising, said Dr. Lee, “was that hyperactivity in aging was observed toward the proximal CA3 region, not the expected distal region.” Contrary to their expectations, that hyperactivity did not enhance function in that area but rather dampened it. Hence: “There is diminished pattern separation and augmented pattern completion,” she said.
– they may recall a certain restaurant they’d been to but not be able to separate what happened during one visit versus another.
Why do some older adults stay sharp?
That memory impairment does not happen to everyone, and it doesn’t happen to all rats either. In fact, the researchers found that some older rats performed spatial-learning tasks as well as young rats did – even though their brains were already beginning to favor pattern completion.
If we can better understand why this happens, we may uncover new therapies for age-related memory loss, Dr. Lee said.
Coauthor Michela Gallagher’s team previously demonstrated that the anti-epilepsy drug levetiracetam improves memory performance by reducing hyperactivity in the hippocampus.
The extra detail this study adds may allow scientists to better aim such drugs in the future, Dr. Lee speculated. “It would give us better control of where we could possibly target the deficits we see.”
A version of this article first appeared on WebMD.com.
Scientists at Johns Hopkins University have identified a mechanism in the brain behind age-related memory loss, expanding our knowledge of the inner workings of the aging brain and possibly opening the door to new Alzheimer’s treatments.
The researchers looked at the hippocampus, a part of the brain thought to store long-term memories.
Neurons there are responsible for a pair of memory functions – called pattern separation and pattern completion – that work together in young, healthy brains. These functions can swing out of balance with age, impacting memory.
The Johns Hopkins team may have discovered what causes this imbalance. Their findings – reported in a paper in the journal Current Biology – may not only help us improve dementia treatments, but even prevent or delay a loss of thinking skills in the first place, the researchers say.
Pattern separation vs. pattern completion
To understand how the hippocampus changes with age, the researchers looked at rats’ brains. In rats and in humans, pattern separation and pattern completion are present, controlled by neurons in the hippocampus.
As the name suggests, pattern completion is when you take a few details or fragments of information – a few notes of music, or the start of a famous movie quote – and your brain retrieves the full memory. Pattern separation, on the other hand, is being able to tell similar observations or experiences apart (like two visits to the same restaurant) to be stored as separate memories.
These functions occur along a gradient across a tiny region called CA3. That gradient, the study found, disappears with aging, said lead study author Hey-Kyoung Lee, PhD, an assistant research scientist at the university’s Zanvyl Krieger Mind/Brain Institute. “The main consequence of the loss,” Dr. Lee said, “is that pattern completion becomes more dominant in rats as they age.”
What’s happening in the brain
Neurons responsible for pattern completion occupy the “distal” end of CA3, while those in charge of pattern separation reside at the “proximal” end. Dr. Lee said prior studies had not examined the proximal and distal regions separately, as she and her team did in this study.
What was surprising, said Dr. Lee, “was that hyperactivity in aging was observed toward the proximal CA3 region, not the expected distal region.” Contrary to their expectations, that hyperactivity did not enhance function in that area but rather dampened it. Hence: “There is diminished pattern separation and augmented pattern completion,” she said.
– they may recall a certain restaurant they’d been to but not be able to separate what happened during one visit versus another.
Why do some older adults stay sharp?
That memory impairment does not happen to everyone, and it doesn’t happen to all rats either. In fact, the researchers found that some older rats performed spatial-learning tasks as well as young rats did – even though their brains were already beginning to favor pattern completion.
If we can better understand why this happens, we may uncover new therapies for age-related memory loss, Dr. Lee said.
Coauthor Michela Gallagher’s team previously demonstrated that the anti-epilepsy drug levetiracetam improves memory performance by reducing hyperactivity in the hippocampus.
The extra detail this study adds may allow scientists to better aim such drugs in the future, Dr. Lee speculated. “It would give us better control of where we could possibly target the deficits we see.”
A version of this article first appeared on WebMD.com.
Scientists at Johns Hopkins University have identified a mechanism in the brain behind age-related memory loss, expanding our knowledge of the inner workings of the aging brain and possibly opening the door to new Alzheimer’s treatments.
The researchers looked at the hippocampus, a part of the brain thought to store long-term memories.
Neurons there are responsible for a pair of memory functions – called pattern separation and pattern completion – that work together in young, healthy brains. These functions can swing out of balance with age, impacting memory.
The Johns Hopkins team may have discovered what causes this imbalance. Their findings – reported in a paper in the journal Current Biology – may not only help us improve dementia treatments, but even prevent or delay a loss of thinking skills in the first place, the researchers say.
Pattern separation vs. pattern completion
To understand how the hippocampus changes with age, the researchers looked at rats’ brains. In rats and in humans, pattern separation and pattern completion are present, controlled by neurons in the hippocampus.
As the name suggests, pattern completion is when you take a few details or fragments of information – a few notes of music, or the start of a famous movie quote – and your brain retrieves the full memory. Pattern separation, on the other hand, is being able to tell similar observations or experiences apart (like two visits to the same restaurant) to be stored as separate memories.
These functions occur along a gradient across a tiny region called CA3. That gradient, the study found, disappears with aging, said lead study author Hey-Kyoung Lee, PhD, an assistant research scientist at the university’s Zanvyl Krieger Mind/Brain Institute. “The main consequence of the loss,” Dr. Lee said, “is that pattern completion becomes more dominant in rats as they age.”
What’s happening in the brain
Neurons responsible for pattern completion occupy the “distal” end of CA3, while those in charge of pattern separation reside at the “proximal” end. Dr. Lee said prior studies had not examined the proximal and distal regions separately, as she and her team did in this study.
What was surprising, said Dr. Lee, “was that hyperactivity in aging was observed toward the proximal CA3 region, not the expected distal region.” Contrary to their expectations, that hyperactivity did not enhance function in that area but rather dampened it. Hence: “There is diminished pattern separation and augmented pattern completion,” she said.
– they may recall a certain restaurant they’d been to but not be able to separate what happened during one visit versus another.
Why do some older adults stay sharp?
That memory impairment does not happen to everyone, and it doesn’t happen to all rats either. In fact, the researchers found that some older rats performed spatial-learning tasks as well as young rats did – even though their brains were already beginning to favor pattern completion.
If we can better understand why this happens, we may uncover new therapies for age-related memory loss, Dr. Lee said.
Coauthor Michela Gallagher’s team previously demonstrated that the anti-epilepsy drug levetiracetam improves memory performance by reducing hyperactivity in the hippocampus.
The extra detail this study adds may allow scientists to better aim such drugs in the future, Dr. Lee speculated. “It would give us better control of where we could possibly target the deficits we see.”
A version of this article first appeared on WebMD.com.
FROM CURRENT BIOLOGY
Can bone density scans help predict dementia risk?
, new research suggests.
In an analysis of more than 900 study participants, women in their 70s with more advanced abdominal aortic calcification (AAC) seen on lateral spine images during dual-energy x-ray absorptiometry (DXA) had a two- to fourfold higher risk for late-life dementia than those with low AAC.
This finding was independent of cardiovascular risk factors and apolipoprotein E (APOE ) genotype.
“While these results are exciting, we now need to undertake further large screening studies in older men and women using this approach to show that the findings are generalizable to older men and can identify people with greater cognitive decline,” coinvestigator Marc Sim, PhD, Edith Cowan University, Joondalup, Australia, said in an interview.
“This will hopefully open the door to studies of early disease-modifying interventions,” Sim said.
The findings were published online in The Lancet Regional Health – Western Pacific.
AAC and cognition
Late-life dementia occurring after age 80 is increasingly common because of both vascular and nonvascular risk factors.
Two recent studies in middle-aged and older men and women showed that AAC identified on bone densitometry was associated with poorer cognition, suggesting it may be related to cognitive decline and increased dementia risk.
This provided the rationale for the current study, Dr. Sim noted.
The researchers assessed AAC using DXA lateral spine images captured in the late 1990s in a prospective cohort of 958 older women who were participating in an osteoporosis study.
AAC was classified into established low, moderate, and extensive categories. At baseline, all women were aged 70 and older, and 45% had low AAC, 36% had moderate AAC, and 19% had extensive AAC.
Over 14.5 years, 150 women (15.7%) had a late-life hospitalization and/or died.
Improved risk prediction
Results showed that, compared with women who had low AAC, women with moderate and extensive AAC were more likely to experience late-life dementia hospitalization (9.3% low, 15.5% moderate, and 18.3% extensive) and death (2.8%, 8.3%, and 9.4%, respectively).
After multivariable adjustment, women with moderate AAC had a two- and threefold increased relative risk for late-life dementia hospitalization or death, compared with their peers who had low AAC.
Women with extensive AAC had a two- and fourfold increase in the adjusted relative risk for late-life dementia hospitalization or death.
“To our knowledge this is the first time it has been shown that AAC from these scans is related to late-life dementia,” Dr. Sim said.
“We demonstrated that AAC improved risk prediction in addition to cardiovascular risk factors and APOE genotype, a genetic risk factor for Alzheimer’s disease, the major form of dementia,” he added.
Dr. Sim noted “these additional lateral spine images” can be taken at the same time that hip and spine bone density tests are done.
“This provides an opportunity to identify AAC in large numbers of people,” he said.
He cautioned, however, that further studies with detailed dementia-related phenotypes, brain imaging, and measures of cognition are needed to confirm whether AAC will add value to dementia risk prediction.
‘Not surprising’
Commenting on the findings for this article, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, Chicago, noted that AAC is a marker of atherosclerosis and is associated with vascular health outcomes.
Therefore, it is “not surprising it would be associated with dementia too. There’s been previous research linking atherosclerosis and Alzheimer’s disease,” Dr. Sexton said.
“What’s novel about this research is that it’s looking at AAC specifically, which can be identified through a relatively simple test that is already in widespread use,” she added.
Dr. Sexton noted that “much more research” is now needed in larger, more diverse populations in order to better understand the link between AAC and dementia – and whether bone density testing may be an appropriate dementia-screening tool.
“The good news is vascular conditions like atherosclerosis can be managed through lifestyle changes like eating a healthy diet and getting regular exercise. And research tells us what’s good for the heart is good for the brain,” Dr. Sexton said.
The study was funded by Kidney Health Australia, Healthway Health Promotion Foundation of Western Australia, Sir Charles Gairdner Hospital Research Advisory Committee Grant, and the National Health and Medical Research Council of Australia. Dr. Sim and Dr. Sexton have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In an analysis of more than 900 study participants, women in their 70s with more advanced abdominal aortic calcification (AAC) seen on lateral spine images during dual-energy x-ray absorptiometry (DXA) had a two- to fourfold higher risk for late-life dementia than those with low AAC.
This finding was independent of cardiovascular risk factors and apolipoprotein E (APOE ) genotype.
“While these results are exciting, we now need to undertake further large screening studies in older men and women using this approach to show that the findings are generalizable to older men and can identify people with greater cognitive decline,” coinvestigator Marc Sim, PhD, Edith Cowan University, Joondalup, Australia, said in an interview.
“This will hopefully open the door to studies of early disease-modifying interventions,” Sim said.
The findings were published online in The Lancet Regional Health – Western Pacific.
AAC and cognition
Late-life dementia occurring after age 80 is increasingly common because of both vascular and nonvascular risk factors.
Two recent studies in middle-aged and older men and women showed that AAC identified on bone densitometry was associated with poorer cognition, suggesting it may be related to cognitive decline and increased dementia risk.
This provided the rationale for the current study, Dr. Sim noted.
The researchers assessed AAC using DXA lateral spine images captured in the late 1990s in a prospective cohort of 958 older women who were participating in an osteoporosis study.
AAC was classified into established low, moderate, and extensive categories. At baseline, all women were aged 70 and older, and 45% had low AAC, 36% had moderate AAC, and 19% had extensive AAC.
Over 14.5 years, 150 women (15.7%) had a late-life hospitalization and/or died.
Improved risk prediction
Results showed that, compared with women who had low AAC, women with moderate and extensive AAC were more likely to experience late-life dementia hospitalization (9.3% low, 15.5% moderate, and 18.3% extensive) and death (2.8%, 8.3%, and 9.4%, respectively).
After multivariable adjustment, women with moderate AAC had a two- and threefold increased relative risk for late-life dementia hospitalization or death, compared with their peers who had low AAC.
Women with extensive AAC had a two- and fourfold increase in the adjusted relative risk for late-life dementia hospitalization or death.
“To our knowledge this is the first time it has been shown that AAC from these scans is related to late-life dementia,” Dr. Sim said.
“We demonstrated that AAC improved risk prediction in addition to cardiovascular risk factors and APOE genotype, a genetic risk factor for Alzheimer’s disease, the major form of dementia,” he added.
Dr. Sim noted “these additional lateral spine images” can be taken at the same time that hip and spine bone density tests are done.
“This provides an opportunity to identify AAC in large numbers of people,” he said.
He cautioned, however, that further studies with detailed dementia-related phenotypes, brain imaging, and measures of cognition are needed to confirm whether AAC will add value to dementia risk prediction.
‘Not surprising’
Commenting on the findings for this article, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, Chicago, noted that AAC is a marker of atherosclerosis and is associated with vascular health outcomes.
Therefore, it is “not surprising it would be associated with dementia too. There’s been previous research linking atherosclerosis and Alzheimer’s disease,” Dr. Sexton said.
“What’s novel about this research is that it’s looking at AAC specifically, which can be identified through a relatively simple test that is already in widespread use,” she added.
Dr. Sexton noted that “much more research” is now needed in larger, more diverse populations in order to better understand the link between AAC and dementia – and whether bone density testing may be an appropriate dementia-screening tool.
“The good news is vascular conditions like atherosclerosis can be managed through lifestyle changes like eating a healthy diet and getting regular exercise. And research tells us what’s good for the heart is good for the brain,” Dr. Sexton said.
The study was funded by Kidney Health Australia, Healthway Health Promotion Foundation of Western Australia, Sir Charles Gairdner Hospital Research Advisory Committee Grant, and the National Health and Medical Research Council of Australia. Dr. Sim and Dr. Sexton have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In an analysis of more than 900 study participants, women in their 70s with more advanced abdominal aortic calcification (AAC) seen on lateral spine images during dual-energy x-ray absorptiometry (DXA) had a two- to fourfold higher risk for late-life dementia than those with low AAC.
This finding was independent of cardiovascular risk factors and apolipoprotein E (APOE ) genotype.
“While these results are exciting, we now need to undertake further large screening studies in older men and women using this approach to show that the findings are generalizable to older men and can identify people with greater cognitive decline,” coinvestigator Marc Sim, PhD, Edith Cowan University, Joondalup, Australia, said in an interview.
“This will hopefully open the door to studies of early disease-modifying interventions,” Sim said.
The findings were published online in The Lancet Regional Health – Western Pacific.
AAC and cognition
Late-life dementia occurring after age 80 is increasingly common because of both vascular and nonvascular risk factors.
Two recent studies in middle-aged and older men and women showed that AAC identified on bone densitometry was associated with poorer cognition, suggesting it may be related to cognitive decline and increased dementia risk.
This provided the rationale for the current study, Dr. Sim noted.
The researchers assessed AAC using DXA lateral spine images captured in the late 1990s in a prospective cohort of 958 older women who were participating in an osteoporosis study.
AAC was classified into established low, moderate, and extensive categories. At baseline, all women were aged 70 and older, and 45% had low AAC, 36% had moderate AAC, and 19% had extensive AAC.
Over 14.5 years, 150 women (15.7%) had a late-life hospitalization and/or died.
Improved risk prediction
Results showed that, compared with women who had low AAC, women with moderate and extensive AAC were more likely to experience late-life dementia hospitalization (9.3% low, 15.5% moderate, and 18.3% extensive) and death (2.8%, 8.3%, and 9.4%, respectively).
After multivariable adjustment, women with moderate AAC had a two- and threefold increased relative risk for late-life dementia hospitalization or death, compared with their peers who had low AAC.
Women with extensive AAC had a two- and fourfold increase in the adjusted relative risk for late-life dementia hospitalization or death.
“To our knowledge this is the first time it has been shown that AAC from these scans is related to late-life dementia,” Dr. Sim said.
“We demonstrated that AAC improved risk prediction in addition to cardiovascular risk factors and APOE genotype, a genetic risk factor for Alzheimer’s disease, the major form of dementia,” he added.
Dr. Sim noted “these additional lateral spine images” can be taken at the same time that hip and spine bone density tests are done.
“This provides an opportunity to identify AAC in large numbers of people,” he said.
He cautioned, however, that further studies with detailed dementia-related phenotypes, brain imaging, and measures of cognition are needed to confirm whether AAC will add value to dementia risk prediction.
‘Not surprising’
Commenting on the findings for this article, Claire Sexton, DPhil, senior director of scientific programs and outreach at the Alzheimer’s Association, Chicago, noted that AAC is a marker of atherosclerosis and is associated with vascular health outcomes.
Therefore, it is “not surprising it would be associated with dementia too. There’s been previous research linking atherosclerosis and Alzheimer’s disease,” Dr. Sexton said.
“What’s novel about this research is that it’s looking at AAC specifically, which can be identified through a relatively simple test that is already in widespread use,” she added.
Dr. Sexton noted that “much more research” is now needed in larger, more diverse populations in order to better understand the link between AAC and dementia – and whether bone density testing may be an appropriate dementia-screening tool.
“The good news is vascular conditions like atherosclerosis can be managed through lifestyle changes like eating a healthy diet and getting regular exercise. And research tells us what’s good for the heart is good for the brain,” Dr. Sexton said.
The study was funded by Kidney Health Australia, Healthway Health Promotion Foundation of Western Australia, Sir Charles Gairdner Hospital Research Advisory Committee Grant, and the National Health and Medical Research Council of Australia. Dr. Sim and Dr. Sexton have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET REGIONAL HEALTH – WESTERN PACIFIC
First-ever Huntington staging system may jump-start drug development for early-stage disease
Researchers liken the Huntington’s disease Integrated Staging System (HD-ISS) to the system currently used to stage cancer. It groups patients according to their underlying biological, clinical, and functional characteristics.
It also includes criteria to biologically define Huntington’s disease stages across the entire disease spectrum, from birth to death, which is something that has not been done before. For now, the HD-ISS is only intended for research, but it could one day be modified for use in the clinic, investigators wrote.
“This systematization is of critical importance to select the most appropriate target population for clinical trials and studies,” said co-investigator Cristina Sampaio, MD, chief medical officer at the CHDI Foundation, Princeton, N.J.
“By providing a methodology to precisely define cases early in the neurodegenerative process, the HD-ISS will be instrumental in conducting trials in the very early disease stages,” Dr. Sampaio added.
The position paper was published in the July issue of the Lancet Neurology.
New approach needed
There is no approved therapy to slow Huntington’s disease progression. Clinical trials currently enroll patients with demonstrable symptoms, which limits the ability to test therapeutics that could delay or prevent neurodegeneration.
Huntington’s disease is rare, occurring in about 2.7 per 100,000 individuals worldwide. It is caused by a mutation in the HTT gene involving a DNA segment known as a CAG trinucleotide repeat.
Currently, Huntington’s disease is diagnosed on the basis of clinical signs that emerge late in the disease course, an approach developed before the discovery of the HTT gene and the development of the genetic test for the CAG mutation.
The disease phase prior to diagnosis has been described as presymptomatic, premanifest, or prodromal. However, the three terms have varying definitions that make it difficult to compare study results across trials.
Because drug development had focused on the overt motor sign phase of the disease, there was no real need for an evidence-based staging system that classified disease phases from birth, the investigators noted.
“Now, the research community and regulators recognize that it is critical to conduct trials early in the disease when no signs or overt symptoms are measurable,” Dr. Sampaio said.
Defining disease stages
Work on the staging system was done through the Huntington’s Disease Regulatory Science Consortium, an international project begun in 2018 among biotech and pharma companies, academic institutions, and nonprofit research and advocacy organizations.
Overall, more than 50 clinicians and researchers were involved in developing the HD-ISS.
Using modeling data from four large observational studies that included patients with Huntington’s disease and control groups, researchers identified four different stages of Huntington’s disease:
- Stage 0: Begins at birth with identification of HTT gene mutations but no detectable pathologic changes.
- Stage 1: Begins when biomarker changes are detected via MRI by a volume decrease in six brain areas.
- Stage 2: Begins when clinical signs of Huntington’s disease are present, as determined through motor and cognitive assessments.
- Stage 3: Begins when functional decline is evident, with worsening on the Independence Scale and the Total Functional Capacity of the Unified Huntington’s Disease Rating Scale.
Applying the HD-ISS to clinical trials requires the collection of information routinely recorded in Huntington’s disease research, as well as some additional data, but researchers say its application is straightforward.
The HD-ISS uses a numerical staging system similar to that used in the U.S. Food and Drug Administration’s guidance for Alzheimer’s disease (AD) and integrates the prodromal, presymptomatic, or premanifest phase of the disease. This distinguishes it from earlier classification systems.
The HD-ISS can be adapted if new Huntington’s disease biomarkers are identified.
“As research results are generated, this will further validate the HD-ISS and potentially lead to the development of a derivative, and possibly simplified, system for clinical practice,” Dr. Sampaio said.
The new system goes further than a more recent proposal from the Movement Disorder Society task force, which addresses earlier stages in Huntington’s disease but doesn’t consider objective biomarker data.
Question of timing
Commenting on the findings, Erin Furr-Stimming, MD, neurologist and director of the Huntington’s Disease Society of America Center of Excellence with McGovern Medical School, UTHealth, Houston, said targeting early-stage disease will be key.
“Similar to more common neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, there is a period of at least a decade when changes are occurring in the nervous system, prior to the manifestation of clinical symptoms and signs significant enough to warrant a clinical diagnosis,” Dr. Furr-Stimming said.
She noted that multiple trials of disease-modifying agents for Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease have failed for a multitude of reasons, “but one consistent question that is relevant to all these diseases is that of timing: Should we intervene and test these therapies earlier?
“The premanifest or prodromal period may be the ideal time to intervene with a disease-modifying therapy, prior to onset of any neurodegeneration,” Dr. Furr-Stimming said.
The CHDI Foundation provided financial support to the Critical Path Institute for the Huntington’s Disease Regulatory Science Consortium, including all working group efforts. Dr. Sampio is an employee of and receives salary from CHDI Management. She has also received consultancy honorariums (unrelated to HD) from Pfizer, Kyowa Kirin, vTv Therapeutics, GW Pharmaceuticals, Neuraly, Neuroderm, Green Valley Pharmaceuticals, and Pinteon Pharmaceuticals. A full list of disclosures for the other researchers is in the original article. Dr. Furr-Stimming reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers liken the Huntington’s disease Integrated Staging System (HD-ISS) to the system currently used to stage cancer. It groups patients according to their underlying biological, clinical, and functional characteristics.
It also includes criteria to biologically define Huntington’s disease stages across the entire disease spectrum, from birth to death, which is something that has not been done before. For now, the HD-ISS is only intended for research, but it could one day be modified for use in the clinic, investigators wrote.
“This systematization is of critical importance to select the most appropriate target population for clinical trials and studies,” said co-investigator Cristina Sampaio, MD, chief medical officer at the CHDI Foundation, Princeton, N.J.
“By providing a methodology to precisely define cases early in the neurodegenerative process, the HD-ISS will be instrumental in conducting trials in the very early disease stages,” Dr. Sampaio added.
The position paper was published in the July issue of the Lancet Neurology.
New approach needed
There is no approved therapy to slow Huntington’s disease progression. Clinical trials currently enroll patients with demonstrable symptoms, which limits the ability to test therapeutics that could delay or prevent neurodegeneration.
Huntington’s disease is rare, occurring in about 2.7 per 100,000 individuals worldwide. It is caused by a mutation in the HTT gene involving a DNA segment known as a CAG trinucleotide repeat.
Currently, Huntington’s disease is diagnosed on the basis of clinical signs that emerge late in the disease course, an approach developed before the discovery of the HTT gene and the development of the genetic test for the CAG mutation.
The disease phase prior to diagnosis has been described as presymptomatic, premanifest, or prodromal. However, the three terms have varying definitions that make it difficult to compare study results across trials.
Because drug development had focused on the overt motor sign phase of the disease, there was no real need for an evidence-based staging system that classified disease phases from birth, the investigators noted.
“Now, the research community and regulators recognize that it is critical to conduct trials early in the disease when no signs or overt symptoms are measurable,” Dr. Sampaio said.
Defining disease stages
Work on the staging system was done through the Huntington’s Disease Regulatory Science Consortium, an international project begun in 2018 among biotech and pharma companies, academic institutions, and nonprofit research and advocacy organizations.
Overall, more than 50 clinicians and researchers were involved in developing the HD-ISS.
Using modeling data from four large observational studies that included patients with Huntington’s disease and control groups, researchers identified four different stages of Huntington’s disease:
- Stage 0: Begins at birth with identification of HTT gene mutations but no detectable pathologic changes.
- Stage 1: Begins when biomarker changes are detected via MRI by a volume decrease in six brain areas.
- Stage 2: Begins when clinical signs of Huntington’s disease are present, as determined through motor and cognitive assessments.
- Stage 3: Begins when functional decline is evident, with worsening on the Independence Scale and the Total Functional Capacity of the Unified Huntington’s Disease Rating Scale.
Applying the HD-ISS to clinical trials requires the collection of information routinely recorded in Huntington’s disease research, as well as some additional data, but researchers say its application is straightforward.
The HD-ISS uses a numerical staging system similar to that used in the U.S. Food and Drug Administration’s guidance for Alzheimer’s disease (AD) and integrates the prodromal, presymptomatic, or premanifest phase of the disease. This distinguishes it from earlier classification systems.
The HD-ISS can be adapted if new Huntington’s disease biomarkers are identified.
“As research results are generated, this will further validate the HD-ISS and potentially lead to the development of a derivative, and possibly simplified, system for clinical practice,” Dr. Sampaio said.
The new system goes further than a more recent proposal from the Movement Disorder Society task force, which addresses earlier stages in Huntington’s disease but doesn’t consider objective biomarker data.
Question of timing
Commenting on the findings, Erin Furr-Stimming, MD, neurologist and director of the Huntington’s Disease Society of America Center of Excellence with McGovern Medical School, UTHealth, Houston, said targeting early-stage disease will be key.
“Similar to more common neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, there is a period of at least a decade when changes are occurring in the nervous system, prior to the manifestation of clinical symptoms and signs significant enough to warrant a clinical diagnosis,” Dr. Furr-Stimming said.
She noted that multiple trials of disease-modifying agents for Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease have failed for a multitude of reasons, “but one consistent question that is relevant to all these diseases is that of timing: Should we intervene and test these therapies earlier?
“The premanifest or prodromal period may be the ideal time to intervene with a disease-modifying therapy, prior to onset of any neurodegeneration,” Dr. Furr-Stimming said.
The CHDI Foundation provided financial support to the Critical Path Institute for the Huntington’s Disease Regulatory Science Consortium, including all working group efforts. Dr. Sampio is an employee of and receives salary from CHDI Management. She has also received consultancy honorariums (unrelated to HD) from Pfizer, Kyowa Kirin, vTv Therapeutics, GW Pharmaceuticals, Neuraly, Neuroderm, Green Valley Pharmaceuticals, and Pinteon Pharmaceuticals. A full list of disclosures for the other researchers is in the original article. Dr. Furr-Stimming reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Researchers liken the Huntington’s disease Integrated Staging System (HD-ISS) to the system currently used to stage cancer. It groups patients according to their underlying biological, clinical, and functional characteristics.
It also includes criteria to biologically define Huntington’s disease stages across the entire disease spectrum, from birth to death, which is something that has not been done before. For now, the HD-ISS is only intended for research, but it could one day be modified for use in the clinic, investigators wrote.
“This systematization is of critical importance to select the most appropriate target population for clinical trials and studies,” said co-investigator Cristina Sampaio, MD, chief medical officer at the CHDI Foundation, Princeton, N.J.
“By providing a methodology to precisely define cases early in the neurodegenerative process, the HD-ISS will be instrumental in conducting trials in the very early disease stages,” Dr. Sampaio added.
The position paper was published in the July issue of the Lancet Neurology.
New approach needed
There is no approved therapy to slow Huntington’s disease progression. Clinical trials currently enroll patients with demonstrable symptoms, which limits the ability to test therapeutics that could delay or prevent neurodegeneration.
Huntington’s disease is rare, occurring in about 2.7 per 100,000 individuals worldwide. It is caused by a mutation in the HTT gene involving a DNA segment known as a CAG trinucleotide repeat.
Currently, Huntington’s disease is diagnosed on the basis of clinical signs that emerge late in the disease course, an approach developed before the discovery of the HTT gene and the development of the genetic test for the CAG mutation.
The disease phase prior to diagnosis has been described as presymptomatic, premanifest, or prodromal. However, the three terms have varying definitions that make it difficult to compare study results across trials.
Because drug development had focused on the overt motor sign phase of the disease, there was no real need for an evidence-based staging system that classified disease phases from birth, the investigators noted.
“Now, the research community and regulators recognize that it is critical to conduct trials early in the disease when no signs or overt symptoms are measurable,” Dr. Sampaio said.
Defining disease stages
Work on the staging system was done through the Huntington’s Disease Regulatory Science Consortium, an international project begun in 2018 among biotech and pharma companies, academic institutions, and nonprofit research and advocacy organizations.
Overall, more than 50 clinicians and researchers were involved in developing the HD-ISS.
Using modeling data from four large observational studies that included patients with Huntington’s disease and control groups, researchers identified four different stages of Huntington’s disease:
- Stage 0: Begins at birth with identification of HTT gene mutations but no detectable pathologic changes.
- Stage 1: Begins when biomarker changes are detected via MRI by a volume decrease in six brain areas.
- Stage 2: Begins when clinical signs of Huntington’s disease are present, as determined through motor and cognitive assessments.
- Stage 3: Begins when functional decline is evident, with worsening on the Independence Scale and the Total Functional Capacity of the Unified Huntington’s Disease Rating Scale.
Applying the HD-ISS to clinical trials requires the collection of information routinely recorded in Huntington’s disease research, as well as some additional data, but researchers say its application is straightforward.
The HD-ISS uses a numerical staging system similar to that used in the U.S. Food and Drug Administration’s guidance for Alzheimer’s disease (AD) and integrates the prodromal, presymptomatic, or premanifest phase of the disease. This distinguishes it from earlier classification systems.
The HD-ISS can be adapted if new Huntington’s disease biomarkers are identified.
“As research results are generated, this will further validate the HD-ISS and potentially lead to the development of a derivative, and possibly simplified, system for clinical practice,” Dr. Sampaio said.
The new system goes further than a more recent proposal from the Movement Disorder Society task force, which addresses earlier stages in Huntington’s disease but doesn’t consider objective biomarker data.
Question of timing
Commenting on the findings, Erin Furr-Stimming, MD, neurologist and director of the Huntington’s Disease Society of America Center of Excellence with McGovern Medical School, UTHealth, Houston, said targeting early-stage disease will be key.
“Similar to more common neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, there is a period of at least a decade when changes are occurring in the nervous system, prior to the manifestation of clinical symptoms and signs significant enough to warrant a clinical diagnosis,” Dr. Furr-Stimming said.
She noted that multiple trials of disease-modifying agents for Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease have failed for a multitude of reasons, “but one consistent question that is relevant to all these diseases is that of timing: Should we intervene and test these therapies earlier?
“The premanifest or prodromal period may be the ideal time to intervene with a disease-modifying therapy, prior to onset of any neurodegeneration,” Dr. Furr-Stimming said.
The CHDI Foundation provided financial support to the Critical Path Institute for the Huntington’s Disease Regulatory Science Consortium, including all working group efforts. Dr. Sampio is an employee of and receives salary from CHDI Management. She has also received consultancy honorariums (unrelated to HD) from Pfizer, Kyowa Kirin, vTv Therapeutics, GW Pharmaceuticals, Neuraly, Neuroderm, Green Valley Pharmaceuticals, and Pinteon Pharmaceuticals. A full list of disclosures for the other researchers is in the original article. Dr. Furr-Stimming reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET NEUROLOGY
Acupuncture deep needling technique points to greater tension headache relief
(TTH), new research suggests. Result of a randomized trial showed that though the majority of participants reported some relief from TTH after 8 weeks of acupuncture treatment, those who received needling at a depth of 12.5-20.0 mm reported the greatest reduction in headache frequency and severity.
At this depth, acupuncture promotes deqi sensation, a feeling of numbness, soreness, heaviness, or irritating pain in the needling site that is considered key to successful acupuncture treatment in traditional Chinese acupuncture theory.
“Our study showed that deqi sensation could enhance the effect of acupuncture in the treatment of chronic TTH, and the effect of acupuncture lasted at least 6 months when the treatment was stopped,” said co-investigator Ying Li, MD, PhD, The Third Hospital/Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, China.
The findings were published online in Neurology.
Deqi sensation key
TTH is the most common type of headache, with a lifetime prevalence of up to 78% in some studies. The pain is often described as throbbing or a vice-like tightness on both sides of the head. TTH is considered chronic when it occurs at least 15 days a month.
Previous studies have suggested that acupuncture can offer relief from headache pain, but specific information on TTH, especially chronic TTH, has been lacking.
To address the issue, researchers designed a parallel-design, patient-and-assessor blinded randomized controlled trial with 218 individuals with a history of chronic TTH. All were untreated with prophylactic treatment in the previous 3 months.
The treatment group (n = 110) received 20 sessions of true acupuncture (TA) over 8 weeks. This included three sessions per week in the first 4 weeks and two sessions per week in the last 4 weeks. The depth of needling at each point ranged from 12.5 to 20 mm, which is needed to achieve deqi sensation.
The control group (n = 108) received superficial acupuncture (SA) on the same schedule as the TA group and at traditional acupuncture points. However, this was done at a maximum depth of 2 mm, which is not deep enough for deqi sensation.
At week 16, 68.2% of the participants receiving TA reported a greater than 50% reduction in monthly headache days, compared with 48.1% of those receiving SA (odds ratio, 2.65; P < .001).
Mean monthly headache days decreased from 20.38 days at baseline to 7.48 days at week 32 in the TA group versus 22.6 days at baseline to 11.94 days in the SA group.
Headache intensity and severity decreased in both groups, although those who achieved deqi sensation reported the most improvement.
Only four patients reported adverse effects, all of which were mild and none requiring treatment.
Patients in both groups reported some pain relief, suggesting that those who are not comfortable with deqi sensation may still benefit from superficial acupuncture, although to a lesser extent, Dr. Li said.
“We assume that the point-specific effect and placebo effect were combined to give the patients relief of headaches,” Dr. Li added. “Further, the effect of deqi sensation added more treatment effect. This might be explained by gate-control theory or other unknown mechanisms.”
Deeper understanding?
Commenting on the research, Jennifer Bickel, MD, a senior member of neurology at Moffit Cancer Center and professor of oncologic sciences at University of South Florida, Tampa, said that the study provides a deeper understanding of acupuncture’s efficacy for chronic TTH, which could aid clinicians who are unfamiliar with the therapy or when and how to refer treatment.
“This study provides a more descriptive outline for what type of acupuncture treatment and duration can be effective for patients so doctors can prep patients on what to expect and so doctors can better assess if patients received appropriate acupuncture for their headaches,” said Dr. Bickel, who was not involved with the research.
However, she noted that the acupuncture sites and techniques did not vary during the trial. Although that makes sense for a controlled study, it may not reflect real-world clinical practice, she added.
“The downside is that the study didn’t fully reflect that most acupuncturists in clinical practice would alter treatments during the 20 sessions based on the patient’s response and accompanying symptoms or comorbidities,” Dr. Bickel said.
The study also lacked information on medication overuse headache or patients’ prior history of TTH treatments.
“This could be helpful to understand which patients in clinical practice are most likely to benefit from treatment,” Dr. Bickel said.
Study authors received funding from the Department of Science and Technology of Sichuan Province and the National Natural Science Foundation of China. Dr. Li, Dr. Bickel, and Dr. Vickers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(TTH), new research suggests. Result of a randomized trial showed that though the majority of participants reported some relief from TTH after 8 weeks of acupuncture treatment, those who received needling at a depth of 12.5-20.0 mm reported the greatest reduction in headache frequency and severity.
At this depth, acupuncture promotes deqi sensation, a feeling of numbness, soreness, heaviness, or irritating pain in the needling site that is considered key to successful acupuncture treatment in traditional Chinese acupuncture theory.
“Our study showed that deqi sensation could enhance the effect of acupuncture in the treatment of chronic TTH, and the effect of acupuncture lasted at least 6 months when the treatment was stopped,” said co-investigator Ying Li, MD, PhD, The Third Hospital/Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, China.
The findings were published online in Neurology.
Deqi sensation key
TTH is the most common type of headache, with a lifetime prevalence of up to 78% in some studies. The pain is often described as throbbing or a vice-like tightness on both sides of the head. TTH is considered chronic when it occurs at least 15 days a month.
Previous studies have suggested that acupuncture can offer relief from headache pain, but specific information on TTH, especially chronic TTH, has been lacking.
To address the issue, researchers designed a parallel-design, patient-and-assessor blinded randomized controlled trial with 218 individuals with a history of chronic TTH. All were untreated with prophylactic treatment in the previous 3 months.
The treatment group (n = 110) received 20 sessions of true acupuncture (TA) over 8 weeks. This included three sessions per week in the first 4 weeks and two sessions per week in the last 4 weeks. The depth of needling at each point ranged from 12.5 to 20 mm, which is needed to achieve deqi sensation.
The control group (n = 108) received superficial acupuncture (SA) on the same schedule as the TA group and at traditional acupuncture points. However, this was done at a maximum depth of 2 mm, which is not deep enough for deqi sensation.
At week 16, 68.2% of the participants receiving TA reported a greater than 50% reduction in monthly headache days, compared with 48.1% of those receiving SA (odds ratio, 2.65; P < .001).
Mean monthly headache days decreased from 20.38 days at baseline to 7.48 days at week 32 in the TA group versus 22.6 days at baseline to 11.94 days in the SA group.
Headache intensity and severity decreased in both groups, although those who achieved deqi sensation reported the most improvement.
Only four patients reported adverse effects, all of which were mild and none requiring treatment.
Patients in both groups reported some pain relief, suggesting that those who are not comfortable with deqi sensation may still benefit from superficial acupuncture, although to a lesser extent, Dr. Li said.
“We assume that the point-specific effect and placebo effect were combined to give the patients relief of headaches,” Dr. Li added. “Further, the effect of deqi sensation added more treatment effect. This might be explained by gate-control theory or other unknown mechanisms.”
Deeper understanding?
Commenting on the research, Jennifer Bickel, MD, a senior member of neurology at Moffit Cancer Center and professor of oncologic sciences at University of South Florida, Tampa, said that the study provides a deeper understanding of acupuncture’s efficacy for chronic TTH, which could aid clinicians who are unfamiliar with the therapy or when and how to refer treatment.
“This study provides a more descriptive outline for what type of acupuncture treatment and duration can be effective for patients so doctors can prep patients on what to expect and so doctors can better assess if patients received appropriate acupuncture for their headaches,” said Dr. Bickel, who was not involved with the research.
However, she noted that the acupuncture sites and techniques did not vary during the trial. Although that makes sense for a controlled study, it may not reflect real-world clinical practice, she added.
“The downside is that the study didn’t fully reflect that most acupuncturists in clinical practice would alter treatments during the 20 sessions based on the patient’s response and accompanying symptoms or comorbidities,” Dr. Bickel said.
The study also lacked information on medication overuse headache or patients’ prior history of TTH treatments.
“This could be helpful to understand which patients in clinical practice are most likely to benefit from treatment,” Dr. Bickel said.
Study authors received funding from the Department of Science and Technology of Sichuan Province and the National Natural Science Foundation of China. Dr. Li, Dr. Bickel, and Dr. Vickers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(TTH), new research suggests. Result of a randomized trial showed that though the majority of participants reported some relief from TTH after 8 weeks of acupuncture treatment, those who received needling at a depth of 12.5-20.0 mm reported the greatest reduction in headache frequency and severity.
At this depth, acupuncture promotes deqi sensation, a feeling of numbness, soreness, heaviness, or irritating pain in the needling site that is considered key to successful acupuncture treatment in traditional Chinese acupuncture theory.
“Our study showed that deqi sensation could enhance the effect of acupuncture in the treatment of chronic TTH, and the effect of acupuncture lasted at least 6 months when the treatment was stopped,” said co-investigator Ying Li, MD, PhD, The Third Hospital/Acupuncture and Tuina School, Chengdu University of Traditional Chinese Medicine, China.
The findings were published online in Neurology.
Deqi sensation key
TTH is the most common type of headache, with a lifetime prevalence of up to 78% in some studies. The pain is often described as throbbing or a vice-like tightness on both sides of the head. TTH is considered chronic when it occurs at least 15 days a month.
Previous studies have suggested that acupuncture can offer relief from headache pain, but specific information on TTH, especially chronic TTH, has been lacking.
To address the issue, researchers designed a parallel-design, patient-and-assessor blinded randomized controlled trial with 218 individuals with a history of chronic TTH. All were untreated with prophylactic treatment in the previous 3 months.
The treatment group (n = 110) received 20 sessions of true acupuncture (TA) over 8 weeks. This included three sessions per week in the first 4 weeks and two sessions per week in the last 4 weeks. The depth of needling at each point ranged from 12.5 to 20 mm, which is needed to achieve deqi sensation.
The control group (n = 108) received superficial acupuncture (SA) on the same schedule as the TA group and at traditional acupuncture points. However, this was done at a maximum depth of 2 mm, which is not deep enough for deqi sensation.
At week 16, 68.2% of the participants receiving TA reported a greater than 50% reduction in monthly headache days, compared with 48.1% of those receiving SA (odds ratio, 2.65; P < .001).
Mean monthly headache days decreased from 20.38 days at baseline to 7.48 days at week 32 in the TA group versus 22.6 days at baseline to 11.94 days in the SA group.
Headache intensity and severity decreased in both groups, although those who achieved deqi sensation reported the most improvement.
Only four patients reported adverse effects, all of which were mild and none requiring treatment.
Patients in both groups reported some pain relief, suggesting that those who are not comfortable with deqi sensation may still benefit from superficial acupuncture, although to a lesser extent, Dr. Li said.
“We assume that the point-specific effect and placebo effect were combined to give the patients relief of headaches,” Dr. Li added. “Further, the effect of deqi sensation added more treatment effect. This might be explained by gate-control theory or other unknown mechanisms.”
Deeper understanding?
Commenting on the research, Jennifer Bickel, MD, a senior member of neurology at Moffit Cancer Center and professor of oncologic sciences at University of South Florida, Tampa, said that the study provides a deeper understanding of acupuncture’s efficacy for chronic TTH, which could aid clinicians who are unfamiliar with the therapy or when and how to refer treatment.
“This study provides a more descriptive outline for what type of acupuncture treatment and duration can be effective for patients so doctors can prep patients on what to expect and so doctors can better assess if patients received appropriate acupuncture for their headaches,” said Dr. Bickel, who was not involved with the research.
However, she noted that the acupuncture sites and techniques did not vary during the trial. Although that makes sense for a controlled study, it may not reflect real-world clinical practice, she added.
“The downside is that the study didn’t fully reflect that most acupuncturists in clinical practice would alter treatments during the 20 sessions based on the patient’s response and accompanying symptoms or comorbidities,” Dr. Bickel said.
The study also lacked information on medication overuse headache or patients’ prior history of TTH treatments.
“This could be helpful to understand which patients in clinical practice are most likely to benefit from treatment,” Dr. Bickel said.
Study authors received funding from the Department of Science and Technology of Sichuan Province and the National Natural Science Foundation of China. Dr. Li, Dr. Bickel, and Dr. Vickers report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
What’s the best time of day to exercise? It depends on your goals
For most of us, the “best” time of day to work out is simple: When we can.
Maybe that’s before or after work. Or when the gym offers free daycare. Or when our favorite instructor teaches our favorite class.
That’s why we call it a “routine.” And if the results are the same, it’s hard to imagine changing it up.
But what if the results aren’t the same?
They may not be, according to a new study from a research team at Skidmore College in Saratoga Springs, N.Y.
Women who worked out in the morning lost more fat, while those who trained in the evening gained more upper-body strength and power. As for men, the performance improvements were similar no matter when they exercised. But those who did so in the evening had a significant drop in blood pressure, among other benefits.
The study is part of a growing body of research showing different results for different times of day among different populations. As it turns out, when you exercise can ultimately have a big effect, not just on strength and fat loss, but also heart health, mood, and quality of sleep.
An accidental discovery
The original goal of the Skidmore study was to test a unique fitness program with a group of healthy, fit, and extremely active adults in early middle age.
The program includes four workouts a week, each with a different focus: strength, steady-pace endurance, high-intensity intervals, and flexibility (traditional stretching combined with yoga and Pilates exercises).
But because the group was so large – 27 women and 20 men completed the 3-month program – they had to split them into morning and evening workout groups.
It wasn’t until researchers looked at the results that they saw the differences between morning and evening exercise, says lead author Paul Arciero, PhD.
Dr. Arciero stressed that participants in every group got leaner and stronger. But the women who worked out in the morning got much bigger reductions in body fat and body-fat percentage than the evening group. Meanwhile, women in the evening group got much bigger gains in upper-body strength, power, and muscular endurance than their morning counterparts.
Among the men, the evening group had significantly larger improvements in blood pressure, cholesterol levels, and the percentage of fat they burned for energy, along with a bigger drop in feelings of fatigue.
Strategic timing for powerful results
Some of these findings are consistent with previous research. For example, a study published in 2021 showed that the ability to exert high effort and express strength and power peaks in the late afternoon, about the same time that your core body temperature is at its highest point.
On the other hand, you’ll probably perform better in the morning when the activity requires a lot of skill and coordination or depends on strategic decision-making.
The findings apply to both men and women.
Performance aside, exercise timing might offer strong health benefits for men with type 2 diabetes, or at high risk for it.
A study showed that men who exercised between 3 p.m. and 6 p.m. saw dramatic improvements in blood sugar management and insulin sensitivity, compared to a group that worked out between 8 a.m. and 10 a.m.
They also lost more fat during the 12-week program, even though they were doing the exact same workouts.
Train consistently, sleep well
When you exercise can affect your sleep quality in many ways, said neuroscientist Jennifer Heisz, PhD, of McMaster University, Hamilton, Ont.
First, she said, “exercise helps you fall asleep faster and sleep deeper at night.” (The only exception is if you exercise so intensely or so close to bedtime that your heart rate is still elevated.)
Second, “exercising at a consistent time every day helps regulate the body’s circadian rhythms.” It doesn’t matter if the exercise is in the morning, evening, or anywhere in between. As long as it’s predictable, it will help you fall asleep and wake up at the same times.
Outdoor exercise is even better, she said. The sun is the most powerful regulator of the circadian clock and works in tandem with physical activity.
Third, exercising at specific times can help you overcome jet lag or adjust to an earlier or later shift at work.
“Exercising at 7 a.m. or between 1 and 4 p.m. helps your circadian clock to ‘fall back’ in time, making it easier to wake up earlier,” Dr. Heisz said. If you need to train your body to wake up later in the morning, try working out between 7 p.m. and 10 p.m.
All exercise is good, but the right timing can make it even better
“The best time to exercise is when you can fit it in,” Dr. Arciero said. “You’ve got to choose the time that fits your lifestyle best.”
But context matters, he noted.
“For someone needing to achieve an improvement in their risk for cardiometabolic disease,” his study shows an advantage to working out later in the day, especially for men. If you’re more focused on building upper-body strength and power, you’ll probably get better results from training in the afternoon or evening.
And for fat loss, the Skidmore study shows better results for women who did morning workouts.
And if you’re still not sure? Try sleeping on it – preferably after your workout.
A version of this article first appeared on WebMD.com.
For most of us, the “best” time of day to work out is simple: When we can.
Maybe that’s before or after work. Or when the gym offers free daycare. Or when our favorite instructor teaches our favorite class.
That’s why we call it a “routine.” And if the results are the same, it’s hard to imagine changing it up.
But what if the results aren’t the same?
They may not be, according to a new study from a research team at Skidmore College in Saratoga Springs, N.Y.
Women who worked out in the morning lost more fat, while those who trained in the evening gained more upper-body strength and power. As for men, the performance improvements were similar no matter when they exercised. But those who did so in the evening had a significant drop in blood pressure, among other benefits.
The study is part of a growing body of research showing different results for different times of day among different populations. As it turns out, when you exercise can ultimately have a big effect, not just on strength and fat loss, but also heart health, mood, and quality of sleep.
An accidental discovery
The original goal of the Skidmore study was to test a unique fitness program with a group of healthy, fit, and extremely active adults in early middle age.
The program includes four workouts a week, each with a different focus: strength, steady-pace endurance, high-intensity intervals, and flexibility (traditional stretching combined with yoga and Pilates exercises).
But because the group was so large – 27 women and 20 men completed the 3-month program – they had to split them into morning and evening workout groups.
It wasn’t until researchers looked at the results that they saw the differences between morning and evening exercise, says lead author Paul Arciero, PhD.
Dr. Arciero stressed that participants in every group got leaner and stronger. But the women who worked out in the morning got much bigger reductions in body fat and body-fat percentage than the evening group. Meanwhile, women in the evening group got much bigger gains in upper-body strength, power, and muscular endurance than their morning counterparts.
Among the men, the evening group had significantly larger improvements in blood pressure, cholesterol levels, and the percentage of fat they burned for energy, along with a bigger drop in feelings of fatigue.
Strategic timing for powerful results
Some of these findings are consistent with previous research. For example, a study published in 2021 showed that the ability to exert high effort and express strength and power peaks in the late afternoon, about the same time that your core body temperature is at its highest point.
On the other hand, you’ll probably perform better in the morning when the activity requires a lot of skill and coordination or depends on strategic decision-making.
The findings apply to both men and women.
Performance aside, exercise timing might offer strong health benefits for men with type 2 diabetes, or at high risk for it.
A study showed that men who exercised between 3 p.m. and 6 p.m. saw dramatic improvements in blood sugar management and insulin sensitivity, compared to a group that worked out between 8 a.m. and 10 a.m.
They also lost more fat during the 12-week program, even though they were doing the exact same workouts.
Train consistently, sleep well
When you exercise can affect your sleep quality in many ways, said neuroscientist Jennifer Heisz, PhD, of McMaster University, Hamilton, Ont.
First, she said, “exercise helps you fall asleep faster and sleep deeper at night.” (The only exception is if you exercise so intensely or so close to bedtime that your heart rate is still elevated.)
Second, “exercising at a consistent time every day helps regulate the body’s circadian rhythms.” It doesn’t matter if the exercise is in the morning, evening, or anywhere in between. As long as it’s predictable, it will help you fall asleep and wake up at the same times.
Outdoor exercise is even better, she said. The sun is the most powerful regulator of the circadian clock and works in tandem with physical activity.
Third, exercising at specific times can help you overcome jet lag or adjust to an earlier or later shift at work.
“Exercising at 7 a.m. or between 1 and 4 p.m. helps your circadian clock to ‘fall back’ in time, making it easier to wake up earlier,” Dr. Heisz said. If you need to train your body to wake up later in the morning, try working out between 7 p.m. and 10 p.m.
All exercise is good, but the right timing can make it even better
“The best time to exercise is when you can fit it in,” Dr. Arciero said. “You’ve got to choose the time that fits your lifestyle best.”
But context matters, he noted.
“For someone needing to achieve an improvement in their risk for cardiometabolic disease,” his study shows an advantage to working out later in the day, especially for men. If you’re more focused on building upper-body strength and power, you’ll probably get better results from training in the afternoon or evening.
And for fat loss, the Skidmore study shows better results for women who did morning workouts.
And if you’re still not sure? Try sleeping on it – preferably after your workout.
A version of this article first appeared on WebMD.com.
For most of us, the “best” time of day to work out is simple: When we can.
Maybe that’s before or after work. Or when the gym offers free daycare. Or when our favorite instructor teaches our favorite class.
That’s why we call it a “routine.” And if the results are the same, it’s hard to imagine changing it up.
But what if the results aren’t the same?
They may not be, according to a new study from a research team at Skidmore College in Saratoga Springs, N.Y.
Women who worked out in the morning lost more fat, while those who trained in the evening gained more upper-body strength and power. As for men, the performance improvements were similar no matter when they exercised. But those who did so in the evening had a significant drop in blood pressure, among other benefits.
The study is part of a growing body of research showing different results for different times of day among different populations. As it turns out, when you exercise can ultimately have a big effect, not just on strength and fat loss, but also heart health, mood, and quality of sleep.
An accidental discovery
The original goal of the Skidmore study was to test a unique fitness program with a group of healthy, fit, and extremely active adults in early middle age.
The program includes four workouts a week, each with a different focus: strength, steady-pace endurance, high-intensity intervals, and flexibility (traditional stretching combined with yoga and Pilates exercises).
But because the group was so large – 27 women and 20 men completed the 3-month program – they had to split them into morning and evening workout groups.
It wasn’t until researchers looked at the results that they saw the differences between morning and evening exercise, says lead author Paul Arciero, PhD.
Dr. Arciero stressed that participants in every group got leaner and stronger. But the women who worked out in the morning got much bigger reductions in body fat and body-fat percentage than the evening group. Meanwhile, women in the evening group got much bigger gains in upper-body strength, power, and muscular endurance than their morning counterparts.
Among the men, the evening group had significantly larger improvements in blood pressure, cholesterol levels, and the percentage of fat they burned for energy, along with a bigger drop in feelings of fatigue.
Strategic timing for powerful results
Some of these findings are consistent with previous research. For example, a study published in 2021 showed that the ability to exert high effort and express strength and power peaks in the late afternoon, about the same time that your core body temperature is at its highest point.
On the other hand, you’ll probably perform better in the morning when the activity requires a lot of skill and coordination or depends on strategic decision-making.
The findings apply to both men and women.
Performance aside, exercise timing might offer strong health benefits for men with type 2 diabetes, or at high risk for it.
A study showed that men who exercised between 3 p.m. and 6 p.m. saw dramatic improvements in blood sugar management and insulin sensitivity, compared to a group that worked out between 8 a.m. and 10 a.m.
They also lost more fat during the 12-week program, even though they were doing the exact same workouts.
Train consistently, sleep well
When you exercise can affect your sleep quality in many ways, said neuroscientist Jennifer Heisz, PhD, of McMaster University, Hamilton, Ont.
First, she said, “exercise helps you fall asleep faster and sleep deeper at night.” (The only exception is if you exercise so intensely or so close to bedtime that your heart rate is still elevated.)
Second, “exercising at a consistent time every day helps regulate the body’s circadian rhythms.” It doesn’t matter if the exercise is in the morning, evening, or anywhere in between. As long as it’s predictable, it will help you fall asleep and wake up at the same times.
Outdoor exercise is even better, she said. The sun is the most powerful regulator of the circadian clock and works in tandem with physical activity.
Third, exercising at specific times can help you overcome jet lag or adjust to an earlier or later shift at work.
“Exercising at 7 a.m. or between 1 and 4 p.m. helps your circadian clock to ‘fall back’ in time, making it easier to wake up earlier,” Dr. Heisz said. If you need to train your body to wake up later in the morning, try working out between 7 p.m. and 10 p.m.
All exercise is good, but the right timing can make it even better
“The best time to exercise is when you can fit it in,” Dr. Arciero said. “You’ve got to choose the time that fits your lifestyle best.”
But context matters, he noted.
“For someone needing to achieve an improvement in their risk for cardiometabolic disease,” his study shows an advantage to working out later in the day, especially for men. If you’re more focused on building upper-body strength and power, you’ll probably get better results from training in the afternoon or evening.
And for fat loss, the Skidmore study shows better results for women who did morning workouts.
And if you’re still not sure? Try sleeping on it – preferably after your workout.
A version of this article first appeared on WebMD.com.
FROM FRONTIERS IN PHYSIOLOGY
How we treat acute pain could be wrong
In a surprising discovery that flies in the face of conventional medicine,
The paper, published in Science Translational Medicine, suggests that inflammation, a normal part of injury recovery, helps resolve acute pain and prevents it from becoming chronic. Blocking that inflammation may interfere with this process, leading to harder-to-treat pain.
“What we’ve been doing for decades not only appears to be wrong, but appears to be 180 degrees wrong,” says senior study author Jeffrey Mogil, PhD, a professor in the department of psychology at McGill University in Montreal. “You should not be blocking inflammation. You should be letting inflammation happen. That’s what stops chronic pain.”
Inflammation: Nature’s pain reliever
Wanting to know why pain goes away for some but drags on (and on) for others, the researchers looked at pain mechanisms in both humans and mice. They found that a type of white blood cell known as a neutrophil seems to play a key role.
“In analyzing the genes of people suffering from lower back pain, we observed active changes in genes over time in people whose pain went away,” says Luda Diatchenko, PhD, a professor in the faculty of medicine and Canada excellence research chair in human pain genetics at McGill. “Changes in the blood cells and their activity seemed to be the most important factor, especially in cells called neutrophils.”
To test this link, the researchers blocked neutrophils in mice and found the pain lasted 2-10 times longer than normal. Anti-inflammatory drugs, despite providing short-term relief, had the same pain-prolonging effect – though injecting neutrophils into the mice seemed to keep that from happening.
The findings are supported by a separate analysis of 500,000 people in the United Kingdom that showed those taking anti-inflammatory drugs to treat their pain were more likely to have pain 2-10 years later.
“Inflammation occurs for a reason,” says Dr. Mogil, “and it looks like it’s dangerous to interfere with it.”
Rethinking how we treat pain
Neutrophils arrive early during inflammation, at the onset of injury – just when many of us reach for pain medication. This research suggests it might be better not to block inflammation, instead letting the neutrophils “do their thing.” Taking an analgesic that alleviates pain without blocking neutrophils, like acetaminophen, may be better than taking an anti-inflammatory drug or steroid, says Dr. Mogil.
Still, while the findings are compelling, clinical trials are needed to directly compare anti-inflammatory drugs to other painkillers, the researchers said. This research may also lay the groundwork for new drug development for chronic pain patients, Dr. Mogil says.
“Our data strongly suggests that neutrophils act like analgesics themselves, which is potentially useful in terms of analgesic development,” Dr. Mogil says. “And of course, we need new analgesics.”
A version of this article first appeared on WebMD.com.
In a surprising discovery that flies in the face of conventional medicine,
The paper, published in Science Translational Medicine, suggests that inflammation, a normal part of injury recovery, helps resolve acute pain and prevents it from becoming chronic. Blocking that inflammation may interfere with this process, leading to harder-to-treat pain.
“What we’ve been doing for decades not only appears to be wrong, but appears to be 180 degrees wrong,” says senior study author Jeffrey Mogil, PhD, a professor in the department of psychology at McGill University in Montreal. “You should not be blocking inflammation. You should be letting inflammation happen. That’s what stops chronic pain.”
Inflammation: Nature’s pain reliever
Wanting to know why pain goes away for some but drags on (and on) for others, the researchers looked at pain mechanisms in both humans and mice. They found that a type of white blood cell known as a neutrophil seems to play a key role.
“In analyzing the genes of people suffering from lower back pain, we observed active changes in genes over time in people whose pain went away,” says Luda Diatchenko, PhD, a professor in the faculty of medicine and Canada excellence research chair in human pain genetics at McGill. “Changes in the blood cells and their activity seemed to be the most important factor, especially in cells called neutrophils.”
To test this link, the researchers blocked neutrophils in mice and found the pain lasted 2-10 times longer than normal. Anti-inflammatory drugs, despite providing short-term relief, had the same pain-prolonging effect – though injecting neutrophils into the mice seemed to keep that from happening.
The findings are supported by a separate analysis of 500,000 people in the United Kingdom that showed those taking anti-inflammatory drugs to treat their pain were more likely to have pain 2-10 years later.
“Inflammation occurs for a reason,” says Dr. Mogil, “and it looks like it’s dangerous to interfere with it.”
Rethinking how we treat pain
Neutrophils arrive early during inflammation, at the onset of injury – just when many of us reach for pain medication. This research suggests it might be better not to block inflammation, instead letting the neutrophils “do their thing.” Taking an analgesic that alleviates pain without blocking neutrophils, like acetaminophen, may be better than taking an anti-inflammatory drug or steroid, says Dr. Mogil.
Still, while the findings are compelling, clinical trials are needed to directly compare anti-inflammatory drugs to other painkillers, the researchers said. This research may also lay the groundwork for new drug development for chronic pain patients, Dr. Mogil says.
“Our data strongly suggests that neutrophils act like analgesics themselves, which is potentially useful in terms of analgesic development,” Dr. Mogil says. “And of course, we need new analgesics.”
A version of this article first appeared on WebMD.com.
In a surprising discovery that flies in the face of conventional medicine,
The paper, published in Science Translational Medicine, suggests that inflammation, a normal part of injury recovery, helps resolve acute pain and prevents it from becoming chronic. Blocking that inflammation may interfere with this process, leading to harder-to-treat pain.
“What we’ve been doing for decades not only appears to be wrong, but appears to be 180 degrees wrong,” says senior study author Jeffrey Mogil, PhD, a professor in the department of psychology at McGill University in Montreal. “You should not be blocking inflammation. You should be letting inflammation happen. That’s what stops chronic pain.”
Inflammation: Nature’s pain reliever
Wanting to know why pain goes away for some but drags on (and on) for others, the researchers looked at pain mechanisms in both humans and mice. They found that a type of white blood cell known as a neutrophil seems to play a key role.
“In analyzing the genes of people suffering from lower back pain, we observed active changes in genes over time in people whose pain went away,” says Luda Diatchenko, PhD, a professor in the faculty of medicine and Canada excellence research chair in human pain genetics at McGill. “Changes in the blood cells and their activity seemed to be the most important factor, especially in cells called neutrophils.”
To test this link, the researchers blocked neutrophils in mice and found the pain lasted 2-10 times longer than normal. Anti-inflammatory drugs, despite providing short-term relief, had the same pain-prolonging effect – though injecting neutrophils into the mice seemed to keep that from happening.
The findings are supported by a separate analysis of 500,000 people in the United Kingdom that showed those taking anti-inflammatory drugs to treat their pain were more likely to have pain 2-10 years later.
“Inflammation occurs for a reason,” says Dr. Mogil, “and it looks like it’s dangerous to interfere with it.”
Rethinking how we treat pain
Neutrophils arrive early during inflammation, at the onset of injury – just when many of us reach for pain medication. This research suggests it might be better not to block inflammation, instead letting the neutrophils “do their thing.” Taking an analgesic that alleviates pain without blocking neutrophils, like acetaminophen, may be better than taking an anti-inflammatory drug or steroid, says Dr. Mogil.
Still, while the findings are compelling, clinical trials are needed to directly compare anti-inflammatory drugs to other painkillers, the researchers said. This research may also lay the groundwork for new drug development for chronic pain patients, Dr. Mogil says.
“Our data strongly suggests that neutrophils act like analgesics themselves, which is potentially useful in terms of analgesic development,” Dr. Mogil says. “And of course, we need new analgesics.”
A version of this article first appeared on WebMD.com.
FROM SCIENCE TRANSLATIONAL MEDICINE