User login
Blood biomarkers predict TBI disability and mortality
, new research suggests.
In new data from the TRACK-TBI study group, high levels of glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) proteins found in glial cells and neurons, respectively, correlated with death and severe injury. Investigators note that measuring these biomarkers may give a more accurate assessment of a patient’s prognosis following TBI.
This study is the “first report of the accuracy of a blood test that can be obtained rapidly on the day of injury to predict neurological recovery at 6 months after injury,” lead author Frederick Korley, MD, PhD, associate professor of emergency medicine at the University of Michigan, Ann Arbor, said in a news release.
The findings were published online in the Lancet Neurology.
Added value
The researchers measured GFAP and UCH-L1 in blood samples taken from 1,696 patients with TBI on the day of their injury, and they assessed patient recovery 6 months later.
The markers were measured using the i-STAT TBI Plasma test (Abbott Labs). The test was approved in 2021 by the U.S. Food and Drug Administration to determine which patients with mild TBI should undergo computed tomography scans.
About two-thirds of the study population were men, and the average age was 39 years. All patients were evaluated at Level I trauma centers for injuries caused primarily by traffic accidents or falls.
Six months following injury, 7% of the patients had died and 14% had an unfavorable outcome, ranging from vegetative state to severe disability requiring daily support. In addition, 67% had incomplete recovery, ranging from moderate disabilities requiring assistance outside of the home to minor disabling neurological or psychological deficits.
Day-of-injury GFAP and UCH-L1 levels had a high probability of predicting death (87% for GFAP and 89% for UCH-L1) and severe disability (86% for both GFAP and UCH-L1) at 6 months, the investigators reported.
The biomarkers were less accurate in predicting incomplete recovery (62% for GFAP and 61% for UCH-L1).
The researchers also assessed the added value of combining the blood biomarkers to current TBI prognostic models that take into account variables such as age, motor score, pupil reactivity, and CT characteristics.
In patients with a Glasgow Coma Scale (GCS) score of 3-12, adding GFAP and UCH-L1 alone or combined to each of the three International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) models significantly increased their accuracy for predicting death (range, 90%-94%) and unfavorable outcome (range, 83%-89%).
In patients with milder TBI (GCS score, 13-15), adding GFAP and UCH-L1 to the UPFRONT prognostic model modestly increased accuracy for predicting incomplete recovery (69%).
‘Important’ findings
Commenting on the study, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University, St. Louis, said this “critical” study shows that these biomarkers can “predict key outcomes,” including mortality and severe disability. “Thus, in conjunction with clinical evaluations and related data such as neuroimaging, these tests may warrant translation to broader clinical practice, particularly in acute settings,” said Dr. Raji, who was not involved in the research.
Also weighing in, Heidi Fusco, MD, assistant director of the traumatic brain injury program at NYU Langone Rusk Rehabilitation, said the findings are “important.”
“Prognosis after brain injury often is based on the initial presentation, ongoing clinical exams, and neuroimaging; and the addition of biomarkers would contribute to creating a more objective prognostic model,” Dr. Fusco said.
She noted “it’s unclear” whether clinical hospital laboratories would be able to accommodate this type of laboratory drawing.
“It is imperative that clinicians still use the patient history [and] clinical and radiological exam when making clinical decisions for a patient and not just lab values. It would be best to incorporate the GFAP and UCH-L1 into a preexisting prognostic model,” Dr. Fusco said.
The study was funded by the U.S. National Institutes of Health, the National Institute of Neurologic Disorders and Stroke, the U.S. Department of Defense, One Mind, and U.S. Army Medical Research and Development Command. Dr. Korley reported having previously consulted for Abbott Laboratories and has received research funding from Abbott Laboratories, which makes the assays used in the study. Dr. Raji is a consultant for Brainreader ApS and Neurevolution. Dr. Fusco has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In new data from the TRACK-TBI study group, high levels of glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) proteins found in glial cells and neurons, respectively, correlated with death and severe injury. Investigators note that measuring these biomarkers may give a more accurate assessment of a patient’s prognosis following TBI.
This study is the “first report of the accuracy of a blood test that can be obtained rapidly on the day of injury to predict neurological recovery at 6 months after injury,” lead author Frederick Korley, MD, PhD, associate professor of emergency medicine at the University of Michigan, Ann Arbor, said in a news release.
The findings were published online in the Lancet Neurology.
Added value
The researchers measured GFAP and UCH-L1 in blood samples taken from 1,696 patients with TBI on the day of their injury, and they assessed patient recovery 6 months later.
The markers were measured using the i-STAT TBI Plasma test (Abbott Labs). The test was approved in 2021 by the U.S. Food and Drug Administration to determine which patients with mild TBI should undergo computed tomography scans.
About two-thirds of the study population were men, and the average age was 39 years. All patients were evaluated at Level I trauma centers for injuries caused primarily by traffic accidents or falls.
Six months following injury, 7% of the patients had died and 14% had an unfavorable outcome, ranging from vegetative state to severe disability requiring daily support. In addition, 67% had incomplete recovery, ranging from moderate disabilities requiring assistance outside of the home to minor disabling neurological or psychological deficits.
Day-of-injury GFAP and UCH-L1 levels had a high probability of predicting death (87% for GFAP and 89% for UCH-L1) and severe disability (86% for both GFAP and UCH-L1) at 6 months, the investigators reported.
The biomarkers were less accurate in predicting incomplete recovery (62% for GFAP and 61% for UCH-L1).
The researchers also assessed the added value of combining the blood biomarkers to current TBI prognostic models that take into account variables such as age, motor score, pupil reactivity, and CT characteristics.
In patients with a Glasgow Coma Scale (GCS) score of 3-12, adding GFAP and UCH-L1 alone or combined to each of the three International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) models significantly increased their accuracy for predicting death (range, 90%-94%) and unfavorable outcome (range, 83%-89%).
In patients with milder TBI (GCS score, 13-15), adding GFAP and UCH-L1 to the UPFRONT prognostic model modestly increased accuracy for predicting incomplete recovery (69%).
‘Important’ findings
Commenting on the study, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University, St. Louis, said this “critical” study shows that these biomarkers can “predict key outcomes,” including mortality and severe disability. “Thus, in conjunction with clinical evaluations and related data such as neuroimaging, these tests may warrant translation to broader clinical practice, particularly in acute settings,” said Dr. Raji, who was not involved in the research.
Also weighing in, Heidi Fusco, MD, assistant director of the traumatic brain injury program at NYU Langone Rusk Rehabilitation, said the findings are “important.”
“Prognosis after brain injury often is based on the initial presentation, ongoing clinical exams, and neuroimaging; and the addition of biomarkers would contribute to creating a more objective prognostic model,” Dr. Fusco said.
She noted “it’s unclear” whether clinical hospital laboratories would be able to accommodate this type of laboratory drawing.
“It is imperative that clinicians still use the patient history [and] clinical and radiological exam when making clinical decisions for a patient and not just lab values. It would be best to incorporate the GFAP and UCH-L1 into a preexisting prognostic model,” Dr. Fusco said.
The study was funded by the U.S. National Institutes of Health, the National Institute of Neurologic Disorders and Stroke, the U.S. Department of Defense, One Mind, and U.S. Army Medical Research and Development Command. Dr. Korley reported having previously consulted for Abbott Laboratories and has received research funding from Abbott Laboratories, which makes the assays used in the study. Dr. Raji is a consultant for Brainreader ApS and Neurevolution. Dr. Fusco has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
In new data from the TRACK-TBI study group, high levels of glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) proteins found in glial cells and neurons, respectively, correlated with death and severe injury. Investigators note that measuring these biomarkers may give a more accurate assessment of a patient’s prognosis following TBI.
This study is the “first report of the accuracy of a blood test that can be obtained rapidly on the day of injury to predict neurological recovery at 6 months after injury,” lead author Frederick Korley, MD, PhD, associate professor of emergency medicine at the University of Michigan, Ann Arbor, said in a news release.
The findings were published online in the Lancet Neurology.
Added value
The researchers measured GFAP and UCH-L1 in blood samples taken from 1,696 patients with TBI on the day of their injury, and they assessed patient recovery 6 months later.
The markers were measured using the i-STAT TBI Plasma test (Abbott Labs). The test was approved in 2021 by the U.S. Food and Drug Administration to determine which patients with mild TBI should undergo computed tomography scans.
About two-thirds of the study population were men, and the average age was 39 years. All patients were evaluated at Level I trauma centers for injuries caused primarily by traffic accidents or falls.
Six months following injury, 7% of the patients had died and 14% had an unfavorable outcome, ranging from vegetative state to severe disability requiring daily support. In addition, 67% had incomplete recovery, ranging from moderate disabilities requiring assistance outside of the home to minor disabling neurological or psychological deficits.
Day-of-injury GFAP and UCH-L1 levels had a high probability of predicting death (87% for GFAP and 89% for UCH-L1) and severe disability (86% for both GFAP and UCH-L1) at 6 months, the investigators reported.
The biomarkers were less accurate in predicting incomplete recovery (62% for GFAP and 61% for UCH-L1).
The researchers also assessed the added value of combining the blood biomarkers to current TBI prognostic models that take into account variables such as age, motor score, pupil reactivity, and CT characteristics.
In patients with a Glasgow Coma Scale (GCS) score of 3-12, adding GFAP and UCH-L1 alone or combined to each of the three International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT) models significantly increased their accuracy for predicting death (range, 90%-94%) and unfavorable outcome (range, 83%-89%).
In patients with milder TBI (GCS score, 13-15), adding GFAP and UCH-L1 to the UPFRONT prognostic model modestly increased accuracy for predicting incomplete recovery (69%).
‘Important’ findings
Commenting on the study, Cyrus A. Raji, MD, PhD, assistant professor of radiology and neurology, Washington University, St. Louis, said this “critical” study shows that these biomarkers can “predict key outcomes,” including mortality and severe disability. “Thus, in conjunction with clinical evaluations and related data such as neuroimaging, these tests may warrant translation to broader clinical practice, particularly in acute settings,” said Dr. Raji, who was not involved in the research.
Also weighing in, Heidi Fusco, MD, assistant director of the traumatic brain injury program at NYU Langone Rusk Rehabilitation, said the findings are “important.”
“Prognosis after brain injury often is based on the initial presentation, ongoing clinical exams, and neuroimaging; and the addition of biomarkers would contribute to creating a more objective prognostic model,” Dr. Fusco said.
She noted “it’s unclear” whether clinical hospital laboratories would be able to accommodate this type of laboratory drawing.
“It is imperative that clinicians still use the patient history [and] clinical and radiological exam when making clinical decisions for a patient and not just lab values. It would be best to incorporate the GFAP and UCH-L1 into a preexisting prognostic model,” Dr. Fusco said.
The study was funded by the U.S. National Institutes of Health, the National Institute of Neurologic Disorders and Stroke, the U.S. Department of Defense, One Mind, and U.S. Army Medical Research and Development Command. Dr. Korley reported having previously consulted for Abbott Laboratories and has received research funding from Abbott Laboratories, which makes the assays used in the study. Dr. Raji is a consultant for Brainreader ApS and Neurevolution. Dr. Fusco has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET NEUROLOGY
Digital therapy may ‘rewire’ the brain to improve tinnitus
, new research suggests. In a randomized controlled trial, results at 12 weeks showed patients with tinnitus reported clinically meaningful reductions in ratings of annoyance, inability to ignore, unpleasantness, and loudness after using a digital polytherapeutic app prototype that focuses on relief, relaxation, and attention-focused retraining. In addition, their improvements were significantly greater than for the control group, which received a common white noise app.
Researchers called the results “promising” for a condition that has no cure and few successful treatments. “What this therapy does is essentially rewire the brain in a way that de-emphasizes the sound of the tinnitus to a background noise that has no meaning or relevance to the listener,” lead author Grant Searchfield, PhD, associate professor of audiology at the University of Auckland, New Zealand, said in a press release.
The findings were published online in Frontiers in Neurology.
Worldwide problem
A recent study showed more than 740 million adults worldwide (nearly 15% of the population) have experienced at least one symptom of tinnitus – and about 120 million are severely affected. Tinnitus is the perception of a ringing, buzzing, whistling, or hissing noise in one or both ears when no external source of the sound is present. Often caused by damage to the auditory system, tinnitus can also be a symptom of a wide range of medical conditions and has been identified as a side effect of COVID-19 vaccination. In its most severe form, which is associated with hearing loss, tinnitus can also affect a patient’s mental, emotional, and social health.
For the current study, participants with tinnitus were randomly assigned to a popular app that uses white noise (control group, n = 30) or to the UpSilent app (n = 31). The UpSilent group received a smartphone app, Bluetooth bone conduction headphones, a Bluetooth neck pillow speaker for sleep, and written counseling materials. Participants in the control group received a widely available app called “White Noise” and in-ear wired headphones.
‘Quicker and more effective’
Both groups reported reductions in ratings of annoyance, inability to ignore, unpleasantness, and loudness at 12 weeks. But significantly more of the UpSilent group reported clinically meaningful improvement compared with the control group (65% vs. 43%, respectively; P = .049).
“Earlier trials have found white noise, goal-based counseling, goal-oriented games, and other technology-based therapies are effective for some people some of the time,” Dr. Searchfield said. “This is quicker and more effective, taking 12 weeks rather than 12 months for more individuals to gain some control,” he added.
The investigators noted that the study was not designed to determine which of the app’s functions of passive listening, active listening, or counseling contributed to symptom improvement.
The next step will be to refine the prototype and proceed to larger local and international trials with a view toward approval by the U.S. Food and Drug Administration, they reported.
The researchers hope the app will be clinically available in about 6 months.
The study was funded by Return on Science, Auckland UniServices. Dr. Searchfield is a founder and scientific officer for TrueSilence, a spinout company of the University of Auckland, and has a financial interest in TrueSilence. His coauthor has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. In a randomized controlled trial, results at 12 weeks showed patients with tinnitus reported clinically meaningful reductions in ratings of annoyance, inability to ignore, unpleasantness, and loudness after using a digital polytherapeutic app prototype that focuses on relief, relaxation, and attention-focused retraining. In addition, their improvements were significantly greater than for the control group, which received a common white noise app.
Researchers called the results “promising” for a condition that has no cure and few successful treatments. “What this therapy does is essentially rewire the brain in a way that de-emphasizes the sound of the tinnitus to a background noise that has no meaning or relevance to the listener,” lead author Grant Searchfield, PhD, associate professor of audiology at the University of Auckland, New Zealand, said in a press release.
The findings were published online in Frontiers in Neurology.
Worldwide problem
A recent study showed more than 740 million adults worldwide (nearly 15% of the population) have experienced at least one symptom of tinnitus – and about 120 million are severely affected. Tinnitus is the perception of a ringing, buzzing, whistling, or hissing noise in one or both ears when no external source of the sound is present. Often caused by damage to the auditory system, tinnitus can also be a symptom of a wide range of medical conditions and has been identified as a side effect of COVID-19 vaccination. In its most severe form, which is associated with hearing loss, tinnitus can also affect a patient’s mental, emotional, and social health.
For the current study, participants with tinnitus were randomly assigned to a popular app that uses white noise (control group, n = 30) or to the UpSilent app (n = 31). The UpSilent group received a smartphone app, Bluetooth bone conduction headphones, a Bluetooth neck pillow speaker for sleep, and written counseling materials. Participants in the control group received a widely available app called “White Noise” and in-ear wired headphones.
‘Quicker and more effective’
Both groups reported reductions in ratings of annoyance, inability to ignore, unpleasantness, and loudness at 12 weeks. But significantly more of the UpSilent group reported clinically meaningful improvement compared with the control group (65% vs. 43%, respectively; P = .049).
“Earlier trials have found white noise, goal-based counseling, goal-oriented games, and other technology-based therapies are effective for some people some of the time,” Dr. Searchfield said. “This is quicker and more effective, taking 12 weeks rather than 12 months for more individuals to gain some control,” he added.
The investigators noted that the study was not designed to determine which of the app’s functions of passive listening, active listening, or counseling contributed to symptom improvement.
The next step will be to refine the prototype and proceed to larger local and international trials with a view toward approval by the U.S. Food and Drug Administration, they reported.
The researchers hope the app will be clinically available in about 6 months.
The study was funded by Return on Science, Auckland UniServices. Dr. Searchfield is a founder and scientific officer for TrueSilence, a spinout company of the University of Auckland, and has a financial interest in TrueSilence. His coauthor has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests. In a randomized controlled trial, results at 12 weeks showed patients with tinnitus reported clinically meaningful reductions in ratings of annoyance, inability to ignore, unpleasantness, and loudness after using a digital polytherapeutic app prototype that focuses on relief, relaxation, and attention-focused retraining. In addition, their improvements were significantly greater than for the control group, which received a common white noise app.
Researchers called the results “promising” for a condition that has no cure and few successful treatments. “What this therapy does is essentially rewire the brain in a way that de-emphasizes the sound of the tinnitus to a background noise that has no meaning or relevance to the listener,” lead author Grant Searchfield, PhD, associate professor of audiology at the University of Auckland, New Zealand, said in a press release.
The findings were published online in Frontiers in Neurology.
Worldwide problem
A recent study showed more than 740 million adults worldwide (nearly 15% of the population) have experienced at least one symptom of tinnitus – and about 120 million are severely affected. Tinnitus is the perception of a ringing, buzzing, whistling, or hissing noise in one or both ears when no external source of the sound is present. Often caused by damage to the auditory system, tinnitus can also be a symptom of a wide range of medical conditions and has been identified as a side effect of COVID-19 vaccination. In its most severe form, which is associated with hearing loss, tinnitus can also affect a patient’s mental, emotional, and social health.
For the current study, participants with tinnitus were randomly assigned to a popular app that uses white noise (control group, n = 30) or to the UpSilent app (n = 31). The UpSilent group received a smartphone app, Bluetooth bone conduction headphones, a Bluetooth neck pillow speaker for sleep, and written counseling materials. Participants in the control group received a widely available app called “White Noise” and in-ear wired headphones.
‘Quicker and more effective’
Both groups reported reductions in ratings of annoyance, inability to ignore, unpleasantness, and loudness at 12 weeks. But significantly more of the UpSilent group reported clinically meaningful improvement compared with the control group (65% vs. 43%, respectively; P = .049).
“Earlier trials have found white noise, goal-based counseling, goal-oriented games, and other technology-based therapies are effective for some people some of the time,” Dr. Searchfield said. “This is quicker and more effective, taking 12 weeks rather than 12 months for more individuals to gain some control,” he added.
The investigators noted that the study was not designed to determine which of the app’s functions of passive listening, active listening, or counseling contributed to symptom improvement.
The next step will be to refine the prototype and proceed to larger local and international trials with a view toward approval by the U.S. Food and Drug Administration, they reported.
The researchers hope the app will be clinically available in about 6 months.
The study was funded by Return on Science, Auckland UniServices. Dr. Searchfield is a founder and scientific officer for TrueSilence, a spinout company of the University of Auckland, and has a financial interest in TrueSilence. His coauthor has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM FRONTIERS IN NEUROLOGY
Incomplete recovery common 6 months after mild TBI
, new data from the TRACK-TBI study shows.
“Seeing that more than half of the GCS [Glasgow Coma Score] 15, CT-negative TBI cohort in our study were not back to their preinjury baseline at 6 months was surprising and impacts the millions of Americans who suffer from concussions annually,” said lead author Debbie Madhok, MD, with department of emergency medicine, University of California, San Francisco.
“These results highlight the importance of improving care pathways for concussion, particularly from the emergency department,” Dr. Madhok said.
The findings were published online in JAMA Network Open.
The short- and long-term outcomes in the large group of patients who come into the ED with TBI, a GCS of 15, and without acute intracranial traumatic injury (defined as a negative head CT scan) remain poorly understood, the investigators noted. To investigate further, they evaluated outcomes at 2 weeks and 6 months in 991 of these patients (mean age, 38 years; 64% men) from the TRACK-TBI study.
Among the 751 (76%) participants followed up at 2 weeks after the injury, only 204 (27%) had functional recovery – with a Glasgow Outcome Scale-Extended (GOS-E) score of 8. The remaining 547 (73%) had incomplete recovery (GOS-E scores < 8).
Among the 659 patients (66%) followed up at 6 months after the injury, 287 (44%) had functional recovery and 372 (56%) had incomplete recovery.
Most patients who failed to recover completely reported they had not returned to their preinjury life (88%). They described trouble returning to social activities outside the home and disruptions in family relationships and friendships.
The researchers noted that the study population had a high rate of preinjury psychiatric comorbidities, and these patients were more likely to have incomplete recovery than those without psychiatric comorbidities. This aligns with results from previous studies, they added.
The investigators also noted that patients with mild TBI without acute intracranial trauma are typically managed by ED personnel.
“These findings highlight the importance of ED clinicians being aware of the risk of incomplete recovery for patients with a mild TBI (that is, GCS score of 15 and negative head CT scan) and providing accurate education and timely referral information before ED discharge,” they wrote.
The study was funded by grants from the National Foundation of Emergency Medicine, the National Institute of Neurological Disorders and Stroke, and the U.S. Department of Defense Traumatic Brain Injury Endpoints Development Initiative. Dr. Madhok has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new data from the TRACK-TBI study shows.
“Seeing that more than half of the GCS [Glasgow Coma Score] 15, CT-negative TBI cohort in our study were not back to their preinjury baseline at 6 months was surprising and impacts the millions of Americans who suffer from concussions annually,” said lead author Debbie Madhok, MD, with department of emergency medicine, University of California, San Francisco.
“These results highlight the importance of improving care pathways for concussion, particularly from the emergency department,” Dr. Madhok said.
The findings were published online in JAMA Network Open.
The short- and long-term outcomes in the large group of patients who come into the ED with TBI, a GCS of 15, and without acute intracranial traumatic injury (defined as a negative head CT scan) remain poorly understood, the investigators noted. To investigate further, they evaluated outcomes at 2 weeks and 6 months in 991 of these patients (mean age, 38 years; 64% men) from the TRACK-TBI study.
Among the 751 (76%) participants followed up at 2 weeks after the injury, only 204 (27%) had functional recovery – with a Glasgow Outcome Scale-Extended (GOS-E) score of 8. The remaining 547 (73%) had incomplete recovery (GOS-E scores < 8).
Among the 659 patients (66%) followed up at 6 months after the injury, 287 (44%) had functional recovery and 372 (56%) had incomplete recovery.
Most patients who failed to recover completely reported they had not returned to their preinjury life (88%). They described trouble returning to social activities outside the home and disruptions in family relationships and friendships.
The researchers noted that the study population had a high rate of preinjury psychiatric comorbidities, and these patients were more likely to have incomplete recovery than those without psychiatric comorbidities. This aligns with results from previous studies, they added.
The investigators also noted that patients with mild TBI without acute intracranial trauma are typically managed by ED personnel.
“These findings highlight the importance of ED clinicians being aware of the risk of incomplete recovery for patients with a mild TBI (that is, GCS score of 15 and negative head CT scan) and providing accurate education and timely referral information before ED discharge,” they wrote.
The study was funded by grants from the National Foundation of Emergency Medicine, the National Institute of Neurological Disorders and Stroke, and the U.S. Department of Defense Traumatic Brain Injury Endpoints Development Initiative. Dr. Madhok has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new data from the TRACK-TBI study shows.
“Seeing that more than half of the GCS [Glasgow Coma Score] 15, CT-negative TBI cohort in our study were not back to their preinjury baseline at 6 months was surprising and impacts the millions of Americans who suffer from concussions annually,” said lead author Debbie Madhok, MD, with department of emergency medicine, University of California, San Francisco.
“These results highlight the importance of improving care pathways for concussion, particularly from the emergency department,” Dr. Madhok said.
The findings were published online in JAMA Network Open.
The short- and long-term outcomes in the large group of patients who come into the ED with TBI, a GCS of 15, and without acute intracranial traumatic injury (defined as a negative head CT scan) remain poorly understood, the investigators noted. To investigate further, they evaluated outcomes at 2 weeks and 6 months in 991 of these patients (mean age, 38 years; 64% men) from the TRACK-TBI study.
Among the 751 (76%) participants followed up at 2 weeks after the injury, only 204 (27%) had functional recovery – with a Glasgow Outcome Scale-Extended (GOS-E) score of 8. The remaining 547 (73%) had incomplete recovery (GOS-E scores < 8).
Among the 659 patients (66%) followed up at 6 months after the injury, 287 (44%) had functional recovery and 372 (56%) had incomplete recovery.
Most patients who failed to recover completely reported they had not returned to their preinjury life (88%). They described trouble returning to social activities outside the home and disruptions in family relationships and friendships.
The researchers noted that the study population had a high rate of preinjury psychiatric comorbidities, and these patients were more likely to have incomplete recovery than those without psychiatric comorbidities. This aligns with results from previous studies, they added.
The investigators also noted that patients with mild TBI without acute intracranial trauma are typically managed by ED personnel.
“These findings highlight the importance of ED clinicians being aware of the risk of incomplete recovery for patients with a mild TBI (that is, GCS score of 15 and negative head CT scan) and providing accurate education and timely referral information before ED discharge,” they wrote.
The study was funded by grants from the National Foundation of Emergency Medicine, the National Institute of Neurological Disorders and Stroke, and the U.S. Department of Defense Traumatic Brain Injury Endpoints Development Initiative. Dr. Madhok has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Mechanistic link between herpes virus, Alzheimer’s revealed?
, new research suggests.
“Our results suggest one pathway to Alzheimer’s disease, caused by a VZV infection which creates inflammatory triggers that awaken HSV in the brain,” lead author Dana Cairns, PhD, research associate, department of biomedical engineering at Tufts University, Boston, said in a news release.
The findings were published online in Journal of Alzheimer’s Disease.
‘One-two punch’
Previous research has suggested a correlation between HSV-1 and AD and involvement of VZV. However, the sequence of events that the viruses create to set the disease in motion has been unclear.
“We think we now have evidence of those events,” co–senior author David Kaplan, PhD, chair of the department of biomedical engineering at Tufts, said in the release.
Working with co–senior author Ruth Itzhaki, PhD, University of Oxford, United Kingdom, the researchers infected human-induced neural stem cells (hiNSCs) and 3D brain tissue models with HSV-1 and/or VZV. Dr. Itzhaki was one of the first to hypothesize a connection between herpes virus and AD.
The investigators found that HSV-1 infection of hiNSCs induces amyloid-beta and P-tau accumulation: the main components of AD plaques and neurofibrillary tangles, respectively.
On the other hand, VZV infection of cultured hiNSCs did not lead to amyloid-beta and P-tau accumulation but instead resulted in gliosis and increased levels of proinflammatory cytokines.
“Strikingly,” VZV infection of cells quiescently infected with HSV-1 caused reactivation of HSV-1, leading to AD-like changes, including amyloid-beta and P-tau accumulation, the investigators report.
This suggests that VZV is unlikely to be a direct cause of AD but rather acts indirectly via reactivation of HSV-1, they add.
Similar findings emerged in similar experiments using 3D human brain tissue models.
“It’s a one-two punch of two viruses that are very common and usually harmless, but the lab studies suggest that if a new exposure to VZV wakes up dormant HSV-1, they could cause trouble,” Dr. Cairns said.
The researchers note that vaccination against VZV has been shown previously to reduce risk for dementia. It is possible, they add, that the vaccine is helping to stop the cycle of viral reactivation, inflammation, and neuronal damage.
‘A first step’
Heather M. Snyder, PhD, vice president of Medical & Scientific Relations at the Alzheimer’s Association, said that the study “is using artificial systems with the goal of more clearly and more deeply understanding” the assessed associations.
She added that although it is a first step, it may provide valuable direction for follow-up research.
“This is preliminary work that first needs replication, validation, and further development to understand if any association that is uncovered between viruses and Alzheimer’s/dementia has a mechanistic link,” said Dr. Snyder.
She noted that several past studies have sought to help the research field better understand the links between different viruses and Alzheimer’s and other forms of dementia.
“There have been some challenges in evaluating these associations in our current model systems or in individuals for a number of reasons,” said Dr. Snyder.
However, “the COVID-19 pandemic has created an opportunity to examine and investigate the relationships between different viruses and Alzheimer’s and other dementias by following individuals in more common and well-established ways,” she added.
She reported that her organization is “leading and working with a large global network of studies and investigators to address some of these questions” from during and after the COVID pandemic.
“The lessons we learn and share may inform our understanding of how other viruses are, or are not, connected to Alzheimer’s and other dementia,” Dr. Snyder said.
More information on the Alzheimer’s Association International Cohort Study of Chronic Neurological Sequelae of SARS-CoV-2 is available online.
The study was funded by the National Institutes of Health. Dr. Cairns, Dr. Kaplan, Dr. Itzhaki, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
“Our results suggest one pathway to Alzheimer’s disease, caused by a VZV infection which creates inflammatory triggers that awaken HSV in the brain,” lead author Dana Cairns, PhD, research associate, department of biomedical engineering at Tufts University, Boston, said in a news release.
The findings were published online in Journal of Alzheimer’s Disease.
‘One-two punch’
Previous research has suggested a correlation between HSV-1 and AD and involvement of VZV. However, the sequence of events that the viruses create to set the disease in motion has been unclear.
“We think we now have evidence of those events,” co–senior author David Kaplan, PhD, chair of the department of biomedical engineering at Tufts, said in the release.
Working with co–senior author Ruth Itzhaki, PhD, University of Oxford, United Kingdom, the researchers infected human-induced neural stem cells (hiNSCs) and 3D brain tissue models with HSV-1 and/or VZV. Dr. Itzhaki was one of the first to hypothesize a connection between herpes virus and AD.
The investigators found that HSV-1 infection of hiNSCs induces amyloid-beta and P-tau accumulation: the main components of AD plaques and neurofibrillary tangles, respectively.
On the other hand, VZV infection of cultured hiNSCs did not lead to amyloid-beta and P-tau accumulation but instead resulted in gliosis and increased levels of proinflammatory cytokines.
“Strikingly,” VZV infection of cells quiescently infected with HSV-1 caused reactivation of HSV-1, leading to AD-like changes, including amyloid-beta and P-tau accumulation, the investigators report.
This suggests that VZV is unlikely to be a direct cause of AD but rather acts indirectly via reactivation of HSV-1, they add.
Similar findings emerged in similar experiments using 3D human brain tissue models.
“It’s a one-two punch of two viruses that are very common and usually harmless, but the lab studies suggest that if a new exposure to VZV wakes up dormant HSV-1, they could cause trouble,” Dr. Cairns said.
The researchers note that vaccination against VZV has been shown previously to reduce risk for dementia. It is possible, they add, that the vaccine is helping to stop the cycle of viral reactivation, inflammation, and neuronal damage.
‘A first step’
Heather M. Snyder, PhD, vice president of Medical & Scientific Relations at the Alzheimer’s Association, said that the study “is using artificial systems with the goal of more clearly and more deeply understanding” the assessed associations.
She added that although it is a first step, it may provide valuable direction for follow-up research.
“This is preliminary work that first needs replication, validation, and further development to understand if any association that is uncovered between viruses and Alzheimer’s/dementia has a mechanistic link,” said Dr. Snyder.
She noted that several past studies have sought to help the research field better understand the links between different viruses and Alzheimer’s and other forms of dementia.
“There have been some challenges in evaluating these associations in our current model systems or in individuals for a number of reasons,” said Dr. Snyder.
However, “the COVID-19 pandemic has created an opportunity to examine and investigate the relationships between different viruses and Alzheimer’s and other dementias by following individuals in more common and well-established ways,” she added.
She reported that her organization is “leading and working with a large global network of studies and investigators to address some of these questions” from during and after the COVID pandemic.
“The lessons we learn and share may inform our understanding of how other viruses are, or are not, connected to Alzheimer’s and other dementia,” Dr. Snyder said.
More information on the Alzheimer’s Association International Cohort Study of Chronic Neurological Sequelae of SARS-CoV-2 is available online.
The study was funded by the National Institutes of Health. Dr. Cairns, Dr. Kaplan, Dr. Itzhaki, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research suggests.
“Our results suggest one pathway to Alzheimer’s disease, caused by a VZV infection which creates inflammatory triggers that awaken HSV in the brain,” lead author Dana Cairns, PhD, research associate, department of biomedical engineering at Tufts University, Boston, said in a news release.
The findings were published online in Journal of Alzheimer’s Disease.
‘One-two punch’
Previous research has suggested a correlation between HSV-1 and AD and involvement of VZV. However, the sequence of events that the viruses create to set the disease in motion has been unclear.
“We think we now have evidence of those events,” co–senior author David Kaplan, PhD, chair of the department of biomedical engineering at Tufts, said in the release.
Working with co–senior author Ruth Itzhaki, PhD, University of Oxford, United Kingdom, the researchers infected human-induced neural stem cells (hiNSCs) and 3D brain tissue models with HSV-1 and/or VZV. Dr. Itzhaki was one of the first to hypothesize a connection between herpes virus and AD.
The investigators found that HSV-1 infection of hiNSCs induces amyloid-beta and P-tau accumulation: the main components of AD plaques and neurofibrillary tangles, respectively.
On the other hand, VZV infection of cultured hiNSCs did not lead to amyloid-beta and P-tau accumulation but instead resulted in gliosis and increased levels of proinflammatory cytokines.
“Strikingly,” VZV infection of cells quiescently infected with HSV-1 caused reactivation of HSV-1, leading to AD-like changes, including amyloid-beta and P-tau accumulation, the investigators report.
This suggests that VZV is unlikely to be a direct cause of AD but rather acts indirectly via reactivation of HSV-1, they add.
Similar findings emerged in similar experiments using 3D human brain tissue models.
“It’s a one-two punch of two viruses that are very common and usually harmless, but the lab studies suggest that if a new exposure to VZV wakes up dormant HSV-1, they could cause trouble,” Dr. Cairns said.
The researchers note that vaccination against VZV has been shown previously to reduce risk for dementia. It is possible, they add, that the vaccine is helping to stop the cycle of viral reactivation, inflammation, and neuronal damage.
‘A first step’
Heather M. Snyder, PhD, vice president of Medical & Scientific Relations at the Alzheimer’s Association, said that the study “is using artificial systems with the goal of more clearly and more deeply understanding” the assessed associations.
She added that although it is a first step, it may provide valuable direction for follow-up research.
“This is preliminary work that first needs replication, validation, and further development to understand if any association that is uncovered between viruses and Alzheimer’s/dementia has a mechanistic link,” said Dr. Snyder.
She noted that several past studies have sought to help the research field better understand the links between different viruses and Alzheimer’s and other forms of dementia.
“There have been some challenges in evaluating these associations in our current model systems or in individuals for a number of reasons,” said Dr. Snyder.
However, “the COVID-19 pandemic has created an opportunity to examine and investigate the relationships between different viruses and Alzheimer’s and other dementias by following individuals in more common and well-established ways,” she added.
She reported that her organization is “leading and working with a large global network of studies and investigators to address some of these questions” from during and after the COVID pandemic.
“The lessons we learn and share may inform our understanding of how other viruses are, or are not, connected to Alzheimer’s and other dementia,” Dr. Snyder said.
More information on the Alzheimer’s Association International Cohort Study of Chronic Neurological Sequelae of SARS-CoV-2 is available online.
The study was funded by the National Institutes of Health. Dr. Cairns, Dr. Kaplan, Dr. Itzhaki, and Dr. Snyder have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF ALZHEIMER’S DISEASE
Drug-resistant epilepsy needs earlier surgical referral
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
, according to expert consensus recommendations from the International League Against Epilepsy (ILAE) published in the journal Epilepsia.
Comprehensive epilepsy care
Such a referral is not ”a commitment to undergo brain surgery,” wrote the authors of the new recommendations study, but surgical evaluations offer patients an opportunity to learn about the range of therapies available to them and to have their diagnosis verified, as well as learning about the cause and type of epilepsy they have, even if they ultimately do not pursue surgery.
”In fact, most patients with drug-resistant epilepsy do not end up undergoing surgery after referral, but still benefit from comprehensive epilepsy care improving quality of life and lowering mortality,” wrote lead author Lara Jehi, MD, professor of neurology and epilepsy specialist at Cleveland Clinic, and her colleagues. “A better characterization of the epilepsy can also help optimize medical therapy and address somatic, cognitive, behavioral, and psychiatric comorbidities.”
Is the diagnosis correct?
They noted that about one-third of patients referred to epilepsy centers with an apparent diagnosis of drug-resistant epilepsy actually have psychogenic nonepileptic seizures (PNES) – not epilepsy – and an early, accurate diagnosis of PNES can ensure they receive psychotherapy, stop taking antiseizure medications, and have better outcomes.
“These recommendations are necessary, as the delay to surgery and the overall underutilization of surgery have not improved much over the last 20 years,” said Selim R. Benbadis, MD, professor of neurology and director of the comprehensive epilepsy program at the University of South Florida and Tampa General Hospital. “Comprehensive epilepsy centers offer more than surgery, including correct and precise diagnosis, drug options, three [Food and Drug Administration]–approved neurostimulation options, and more,” said Dr. Benbadis, who was not involved in the development of these recommendations.
Consensus recommendations
On behalf of the the ILAE’s Surgical Therapies Commission, the authors used the Delphi consensus process to develop expert consensus recommendations on when to refer patients with epilepsy to surgery. They conducted three Delphi rounds on 51 clinical scenarios with 61 epileptologists (38% of participants), epilepsy neurosurgeons (34%), neurologists (23%), neuropsychiatrists (2%), and neuropsychologists (3%) from 28 countries. Most of clinicians focused on adults (39%) or adults and children (41%) while 20% focused only on pediatric epilepsy.
The physicians involved had a median 22 years of practice and represented all six ILAE regions: 30% from North America, 28% from Europe, 18% from Asia/Oceania, 13% from Latin America, 7% from the Eastern Mediterranean, and 4% from Africa.
The result of these rounds were three key recommendations arising from the consensus of experts consulted. First, every patient up to 70 years old who has drug-resistant epilepsy should be offered the option of a surgical evaluation as soon as it’s apparent that they have drug resistance. The option for surgical evaluation should be provided independent of their sex or socioeconomic status and regardless of how long they have had epilepsy, their seizure type, their epilepsy type, localization, and their comorbidities, ”including severe psychiatric comorbidity like psychogenic nonepileptic seizures (PNES) or substance abuse if patients are cooperative with management,” the authors wrote.
”Resective surgery can improve quality of life and cognitive outcomes and is the only treatment demonstrated to improve survival and reverse excess mortality attributed to drug-resistant epilepsy,” the authors wrote. Evidence supports that surgical evaluation is the most cost-effective approach to treating drug-resistant epilepsy, they added. Yet, it still takes about 20 years with epilepsy before an adult patient might be referred, ”and the neurology community remains ambivalent due to ongoing barriers and misconceptions about epilepsy surgery,” they wrote.
The second recommendation is to consider a surgical referral for older patients with drug-resistant epilepsy who have no surgical contraindication. Physicians can also consider a referral for patients of any age who are seizure free while taking one to two antiseizure drugs but who have a brain lesion in the noneloquent cortex.
The third recommendation is not to offer surgery if a patient has an active substance dependency and is not cooperative with management.
“Although there is some evidence that seizure outcomes are no different in individuals with active substance use disorder who have epilepsy surgery, the literature suggests increased perioperative surgical and anesthetic risk in this cohort,” the authors wrote. ”Patients with active substance abuse are more likely to be nonadherent with their seizure medications, and to leave the hospital against medical advice.”
One area where the participants did not reach consensus was regarding whether to refer patients who did not become seizure-free after trying just one “tolerated and appropriately chosen” antiseizure medication. Half (49%) said they would be unlikely to refer or would never refer that patient while 44% said they would likely or always refer them, and 7% weren’t sure.
The ‘next level’ of epilepsy care
“Similar recommendations have been published before, by the National Association of Epilepsy Centers, more than once, and have not changed the referral patterns,” Dr. Benbadis said. “They are not implemented by the average general neurologist.” While there are many reasons for this, one with a relativity simple fix is to adjust the language doctors use to when talking with patients about getting an evaluation, Dr. Benbadis said. ”The key is to rephrase: Instead of referrals ‘for surgery,’ which can be scary to many neurologists and patients, we should use more general terms, like referrals for the ‘next level of care by epilepsy specialists,’ ” said Dr. Benbadis, who advocated for this change in terminology in a 2019 editorial. Such language is less frightening and can ease patients’ concerns about going to an epilepsy center where they can learn about more options than just surgery.
Further, surgical options have expanded in recent years, including the development of laser interstitial thermal therapy and neuromodulation. “Identifying candidacy for any of these approaches starts with a surgical referral, so a timely evaluation is key,” the authors wrote.
Referral delays persist
Despite the strong evidence for timely referrals, delays have persisted for decades, said Dr. Benbadis, echoing what the authors describe. ”Despite the results of two randomized controlled trials showing that surgery for temporal lobe epilepsy in adults, and resective surgery in children, is superior to continued antiseizure medications both in terms of seizure freedom and improved quality of life, the mean epilepsy duration to temporal lobe resection has persisted at over 20 years,” the authors wrote. ”Although drug resistance is reached with a mean latency of 9 years in epilepsy surgery candidates, these patients have experienced a decade of unabating seizures with detrimental effects including cognitive and psychiatric comorbidities, poor psychosocial outcomes, potential injuries, and risk of death.”
Surgery is not a ‘dangerous last resort’
The authors point out a variety of likely reasons for these delays, including patients experiencing temporary remissions with a new drug, lack of adequate health care access, overestimating surgery risks, and underestimating the seriousness and risk of death from ongoing seizures.
Dr. Benbadis agreed, referring to a “combination of lack of knowledge and unrealistic views about surgery outcomes and complications.” Patients and their neurologists think surgery is a “dangerous last resort, fraught with complications, and they don’t know the outcome, so it’s mainly that they are not very well-educated about epilepsy surgery,” he said. Complacency about a patient’s infrequent seizures plays a role as well, he added. “Their patient is having one seizure every 2 months, and they might say, ‘well, that’s okay, that’s not that bad,’ but it is when we can cure it.”
Similar factors are barriers to epilepsy surgery: “lack of knowledge or misconceptions about surgical risks, negative behaviors, or cultural issues and access issues.”
Another major barrier, both within neurology and throughout medicine in general, is that large academic centers that accept referrals, including epilepsy centers, have poor communication, follow-up, and scheduling, Dr. Benbadis said.
The authors provided a table with suggestions on potential solutions to those barriers, including identifying online resources to help doctors identify possible surgery candidates, such as www.toolsforepilepsy.com, and a range of educational resources. Ways to improve access and cost include mobile clinics, telehealth, coordinating with an epilepsy organization, and employing a multidisciplinary team that includes a social worker to help with support such as transportation and health insurance.
FROM EPILEPSIA
How retraining your brain could help with lower back pain
Are you among the hundreds of millions of people worldwide with low back pain? If so, you may be familiar with standard treatments like surgery, shots, medications, and spinal manipulations. But new research suggests the solution for the world’s leading cause of disability may lie in fixing how the brain and the body communicate.
Setting out to challenge traditional treatments for chronic back pain, scientists across Australia, Europe, and the United States came together to test the effectiveness of altering how neural networks recognize pain for new research published this week in JAMA.
The randomized clinical trial recruited two groups of 138 participants with chronic low back pain, testing one group with a novel method called graded sensorimotor retraining intervention (RESOLVE) and the other with things like mock laser therapy and noninvasive brain stimulation.
The researchers found the RESOLVE 12-week training course resulted in a statistically significant improvement in pain intensity at 18 weeks.
“What we observed in our trial was a clinically meaningful effect on pain intensity and a clinically meaningful effect on disability. People were happier, they reported their backs felt better, and their quality of life was better,” the study’s lead author, James McAuley, PhD, said in a statement. “This is the first new treatment of its kind for back pain.”
Brainy talk
Communication between your brain and back changes over time when you have chronic lower back pain, leading the brain to interpret signals from the back differently and change how you move. It is thought that these neural changes make recovery from pain slower and more complicated , according to Neuroscience Research Australia (NeuRA), a nonprofit research institute in Sydney.
“Over time, the back becomes less fit, and the way the back and brain communicate is disrupted in ways that seem to reinforce the notion that the back is vulnerable and needs protecting,” said Dr. McAuley, a professor at the University of New South Wales, Sydney, and a NeuRA senior research scientist. “The treatment we devised aims to break this self-sustaining cycle.”
RESOLVE treatment focuses on improving this transformed brain-back communication by slowly retraining the body and the brain without the use of opioids or surgery. People in the study have reported improved quality of life 1 year later, according to Dr. McAuley.
The researchers said the pain improvement was “modest,” and the method will need to be tested on other patients and conditions. They hope to introduce this new treatment to doctors and physiotherapists within the next 6-9 months and have already enlisted partner organizations to start this process, according to NeuRA.
A version of this article first appeared on Webmd.com.
Are you among the hundreds of millions of people worldwide with low back pain? If so, you may be familiar with standard treatments like surgery, shots, medications, and spinal manipulations. But new research suggests the solution for the world’s leading cause of disability may lie in fixing how the brain and the body communicate.
Setting out to challenge traditional treatments for chronic back pain, scientists across Australia, Europe, and the United States came together to test the effectiveness of altering how neural networks recognize pain for new research published this week in JAMA.
The randomized clinical trial recruited two groups of 138 participants with chronic low back pain, testing one group with a novel method called graded sensorimotor retraining intervention (RESOLVE) and the other with things like mock laser therapy and noninvasive brain stimulation.
The researchers found the RESOLVE 12-week training course resulted in a statistically significant improvement in pain intensity at 18 weeks.
“What we observed in our trial was a clinically meaningful effect on pain intensity and a clinically meaningful effect on disability. People were happier, they reported their backs felt better, and their quality of life was better,” the study’s lead author, James McAuley, PhD, said in a statement. “This is the first new treatment of its kind for back pain.”
Brainy talk
Communication between your brain and back changes over time when you have chronic lower back pain, leading the brain to interpret signals from the back differently and change how you move. It is thought that these neural changes make recovery from pain slower and more complicated , according to Neuroscience Research Australia (NeuRA), a nonprofit research institute in Sydney.
“Over time, the back becomes less fit, and the way the back and brain communicate is disrupted in ways that seem to reinforce the notion that the back is vulnerable and needs protecting,” said Dr. McAuley, a professor at the University of New South Wales, Sydney, and a NeuRA senior research scientist. “The treatment we devised aims to break this self-sustaining cycle.”
RESOLVE treatment focuses on improving this transformed brain-back communication by slowly retraining the body and the brain without the use of opioids or surgery. People in the study have reported improved quality of life 1 year later, according to Dr. McAuley.
The researchers said the pain improvement was “modest,” and the method will need to be tested on other patients and conditions. They hope to introduce this new treatment to doctors and physiotherapists within the next 6-9 months and have already enlisted partner organizations to start this process, according to NeuRA.
A version of this article first appeared on Webmd.com.
Are you among the hundreds of millions of people worldwide with low back pain? If so, you may be familiar with standard treatments like surgery, shots, medications, and spinal manipulations. But new research suggests the solution for the world’s leading cause of disability may lie in fixing how the brain and the body communicate.
Setting out to challenge traditional treatments for chronic back pain, scientists across Australia, Europe, and the United States came together to test the effectiveness of altering how neural networks recognize pain for new research published this week in JAMA.
The randomized clinical trial recruited two groups of 138 participants with chronic low back pain, testing one group with a novel method called graded sensorimotor retraining intervention (RESOLVE) and the other with things like mock laser therapy and noninvasive brain stimulation.
The researchers found the RESOLVE 12-week training course resulted in a statistically significant improvement in pain intensity at 18 weeks.
“What we observed in our trial was a clinically meaningful effect on pain intensity and a clinically meaningful effect on disability. People were happier, they reported their backs felt better, and their quality of life was better,” the study’s lead author, James McAuley, PhD, said in a statement. “This is the first new treatment of its kind for back pain.”
Brainy talk
Communication between your brain and back changes over time when you have chronic lower back pain, leading the brain to interpret signals from the back differently and change how you move. It is thought that these neural changes make recovery from pain slower and more complicated , according to Neuroscience Research Australia (NeuRA), a nonprofit research institute in Sydney.
“Over time, the back becomes less fit, and the way the back and brain communicate is disrupted in ways that seem to reinforce the notion that the back is vulnerable and needs protecting,” said Dr. McAuley, a professor at the University of New South Wales, Sydney, and a NeuRA senior research scientist. “The treatment we devised aims to break this self-sustaining cycle.”
RESOLVE treatment focuses on improving this transformed brain-back communication by slowly retraining the body and the brain without the use of opioids or surgery. People in the study have reported improved quality of life 1 year later, according to Dr. McAuley.
The researchers said the pain improvement was “modest,” and the method will need to be tested on other patients and conditions. They hope to introduce this new treatment to doctors and physiotherapists within the next 6-9 months and have already enlisted partner organizations to start this process, according to NeuRA.
A version of this article first appeared on Webmd.com.
Waking up at night could be your brain boosting your memory
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
We tend to think a good night’s sleep should be uninterrupted, but surprising new research from the University of Copenhagen suggests just the opposite:
The study, done on mice, found that the stress transmitter noradrenaline wakes up the brain many times a night. These “microarousals” were linked to memory consolidation, meaning they help you remember the previous day’s events. In fact, the more “awake” you are during a microarousal, the better the memory boost, suggests the research, which was published in Nature Neuroscience.
“Every time I wake up in the middle of the night now, I think – ah, nice, I probably just had great memory-boosting sleep,” said study author Celia Kjaerby, PhD, an assistant professor at the university’s Center for Translational Neuromedicine.
The findings add insight to what happens in the brain during sleep and may help pave the way for new treatments for those who have sleep disorders.
Waves of noradrenaline
Previous research has suggested that noradrenaline – a hormone that increases during stress but also helps you stay focused – is inactive during sleep. So, the researchers were surprised to see high levels of it in the brains of the sleeping rodents.
“I still remember seeing the first traces showing the brain activity of the norepinephrine stress system during sleep. We could not believe our eyes,” Dr. Kjaerby said. “Everyone had thought the system would be quiet. And now we have found out that it completely controls the microarchitecture of sleep.”
Those noradrenaline levels rise and fall like waves every 30 seconds during non-REM (NREM) sleep. At each “peak” the brain is briefly awake, and at each “valley” it is asleep. Typically, these awakenings are so brief that the sleeping subject does not notice. But the higher the rise, the longer the awakening – and the more likely the sleeper may notice.
During the valleys, or when norepinephrine drops, so-called sleep spindles occur.
“These are short oscillatory bursts of brain activity linked to memory consolidation,” Dr. Kjaerby said. Occasionally there is a “deep valley,” lasting 3-5 minutes, leading to more sleep spindles. The mice with the most deep valleys also had the best memories, the researchers noted.
“We have shown that the amount of these super-boosts of sleep spindles, and not REM sleep, defines how well you remember the experiences you had prior to going to sleep,” said Dr. Kjaerby.
Deep valleys were followed by longer awakenings, the researchers observed. So, the longer the valley, the longer the awakening – and the better the memory boost. This means that, though restless sleep is not good, waking up briefly may be a natural part of memory-related sleep phases and may even mean you’ve slept well.
What happens in our brains when we sleep: Piecing it together
The findings fit with previous clinical data that shows we wake up roughly 100-plus times a night, mostly during NREM sleep stage 2 (the spindle-rich sleep stage), Dr. Kjaerby said.
Still, more research on these small awakenings is needed, Dr. Kjaerby said, noting that professor Maiken Nedergaard, MD, another author of this study, has found that the brain cleans up waste products through a rinsing fluid system.
“It remains a puzzle why the fluid system is so active when we sleep,” Dr. Kjaerby said. “We believe these short awakenings could potentially be the key to answering this question.”
A version of this article first appeared on WebMD.com.
FROM NATURE NEUROSCIENCE
Smell loss may be a biomarker of Alzheimer’s disease risk
, according to new research findings.
Olfactory dysfunction is common in late life and well documented among people with Alzheimer’s disease. However, it was unknown whether faster olfactory decline predicts either onset of Alzheimer’s disease or structural brain changes associated with Alzheimer’s disease.
In a study published online in Alzheimer’s and Dementia, Jayant M. Pinto, MD, and his colleagues at the University of Chicago Medical Center reported that among older adults with normal cognition at baseline, people who experienced rapid loss of sense of smell were more likely to be subsequently diagnosed with mild cognitive impairment (MCI) or dementia, compared with those who did not.
Participants were recruited from Rush University’s Memory and Aging Project, a longitudinal cohort of older adults who undergo yearly cognitive and sensory exams, including a scratch test of 12 common smells to identify. The Rush study “was ahead of the curve in looking at smell,” Dr. Pinto said in an interview. “It gave us a very valuable resource with which to attack these questions.”
Dr. Pinto has long investigated links between smell and accelerated aging; in 2014 his group published the finding that olfactory dysfunction could predict death within 5 years in older adults, and in 2018 they reported that olfactory dysfunction could predict dementia.
Smell and cognition over time
For the current study, Dr. Pinto said, “we were able to look at the question not just using a single point in time, but a more granular trajectory of smell loss. Measuring change year by year showed that the faster people’s sense of smell declined, the more likely they were to be diagnosed with MCI or Alzheimer’s disease.”
Dr. Pinto and his colleagues evaluated results from 515 adults (mean age 76.6, 78% female, 94% White) with no cognitive impairment and at least 3 years of normal results on smell tests at baseline. The subjects were followed for a mean 8 years. One hundred subjects (19%) were diagnosed with MCI or dementia by the end of the study period. A subset of the cohort (n = 121) underwent structural magnetic resonance imaging (MRI) between their final smell tests and the study’s end. Of these, most still had normal cognition; 17 individuals had MCI.
Patients’ individual trajectories of smell loss were mapped as slopes. After adjusting for expected differences in age and sex, the investigators found steeper decline associated with greater risk of incident MCI or dementia (odds ratio, 1.89; 95% confidence interval, 1.26-2.90; P < .01). The risk was comparable to that of carrying an apo E ε4 allele, the key risk variant for late-onset Alzheimer’s disease, but was independent of apo E status. The association was strongest among subjects younger than 76 years.
Olfactory decline and brain volume
Dr. Pinto and his colleagues, including lead author Rachel R. Pacyna, a 4th-year medical student at the University of Chicago, also sought to identify brain volume changes corresponding with olfactory decline and Alzheimer’s disease. The researchers hypothesized that certain brain regions not seen affected in Alzheimer’s disease would remain unchanged regardless of olfactory status, but that regions associated with smell and Alzheimer’s disease would see smaller volumes linked with olfactory decline.
Faster olfactory decline did predict lower gray matter volume in olfactory regions, even after controlling for apo E status and other known risk factors. Conversely, cognitively unimpaired patients undergoing MRI saw more gray matter volume in primary olfactory and temporal brain regions, compared with those with cognitive symptoms.
Taken together, the findings suggest that “change in sense of smell is better than looking at sense of smell at one time point,” Dr. Pinto commented. “There are other reasons people have impaired sense of smell: car accidents, COVID, other viruses and infections. But if you identify on a time course those who are starting to lose it faster, these are the people on whom we need to focus.”
Not yet diagnostic
More work needs to be done to establish thresholds for smell loss that could be useful in clinical or investigative settings as a marker of dementia risk, Dr. Pinto acknowledged. “Everyone gets their hearing tested; everyone gets their vision tested. It’s not as easy to get your sense of smell tested. But this study is telling people that if we were to start measuring it routinely, we could actually use it.”
Smell testing “could become a component of a diagnostic battery that includes things like genotyping and cerebrospinal fluid markers, but adds a little more information. It could be useful in clinical prevention trials to identify people at the highest risk, as smell loss presents quite a few years before MCI or Alzheimer’s disease.”
The investigators acknowledged that their findings need to be replicated in more diverse cohorts that better represent the Alzheimer’s population in the United States. Another limitation of their study, they said, was that the method used to calculate the rate of olfactory decline “was based on slope of measured time points assuming linearity, which may oversimplify the complexity of olfactory changes in normal aging and during the preclinical Alzheimer’s disease period.” The study was funded by the National Institutes of Health. Dr. Pinto disclosed receiving consulting fees from Sanofi/Regeneron, Optinose, and Genentech not related to this work.
, according to new research findings.
Olfactory dysfunction is common in late life and well documented among people with Alzheimer’s disease. However, it was unknown whether faster olfactory decline predicts either onset of Alzheimer’s disease or structural brain changes associated with Alzheimer’s disease.
In a study published online in Alzheimer’s and Dementia, Jayant M. Pinto, MD, and his colleagues at the University of Chicago Medical Center reported that among older adults with normal cognition at baseline, people who experienced rapid loss of sense of smell were more likely to be subsequently diagnosed with mild cognitive impairment (MCI) or dementia, compared with those who did not.
Participants were recruited from Rush University’s Memory and Aging Project, a longitudinal cohort of older adults who undergo yearly cognitive and sensory exams, including a scratch test of 12 common smells to identify. The Rush study “was ahead of the curve in looking at smell,” Dr. Pinto said in an interview. “It gave us a very valuable resource with which to attack these questions.”
Dr. Pinto has long investigated links between smell and accelerated aging; in 2014 his group published the finding that olfactory dysfunction could predict death within 5 years in older adults, and in 2018 they reported that olfactory dysfunction could predict dementia.
Smell and cognition over time
For the current study, Dr. Pinto said, “we were able to look at the question not just using a single point in time, but a more granular trajectory of smell loss. Measuring change year by year showed that the faster people’s sense of smell declined, the more likely they were to be diagnosed with MCI or Alzheimer’s disease.”
Dr. Pinto and his colleagues evaluated results from 515 adults (mean age 76.6, 78% female, 94% White) with no cognitive impairment and at least 3 years of normal results on smell tests at baseline. The subjects were followed for a mean 8 years. One hundred subjects (19%) were diagnosed with MCI or dementia by the end of the study period. A subset of the cohort (n = 121) underwent structural magnetic resonance imaging (MRI) between their final smell tests and the study’s end. Of these, most still had normal cognition; 17 individuals had MCI.
Patients’ individual trajectories of smell loss were mapped as slopes. After adjusting for expected differences in age and sex, the investigators found steeper decline associated with greater risk of incident MCI or dementia (odds ratio, 1.89; 95% confidence interval, 1.26-2.90; P < .01). The risk was comparable to that of carrying an apo E ε4 allele, the key risk variant for late-onset Alzheimer’s disease, but was independent of apo E status. The association was strongest among subjects younger than 76 years.
Olfactory decline and brain volume
Dr. Pinto and his colleagues, including lead author Rachel R. Pacyna, a 4th-year medical student at the University of Chicago, also sought to identify brain volume changes corresponding with olfactory decline and Alzheimer’s disease. The researchers hypothesized that certain brain regions not seen affected in Alzheimer’s disease would remain unchanged regardless of olfactory status, but that regions associated with smell and Alzheimer’s disease would see smaller volumes linked with olfactory decline.
Faster olfactory decline did predict lower gray matter volume in olfactory regions, even after controlling for apo E status and other known risk factors. Conversely, cognitively unimpaired patients undergoing MRI saw more gray matter volume in primary olfactory and temporal brain regions, compared with those with cognitive symptoms.
Taken together, the findings suggest that “change in sense of smell is better than looking at sense of smell at one time point,” Dr. Pinto commented. “There are other reasons people have impaired sense of smell: car accidents, COVID, other viruses and infections. But if you identify on a time course those who are starting to lose it faster, these are the people on whom we need to focus.”
Not yet diagnostic
More work needs to be done to establish thresholds for smell loss that could be useful in clinical or investigative settings as a marker of dementia risk, Dr. Pinto acknowledged. “Everyone gets their hearing tested; everyone gets their vision tested. It’s not as easy to get your sense of smell tested. But this study is telling people that if we were to start measuring it routinely, we could actually use it.”
Smell testing “could become a component of a diagnostic battery that includes things like genotyping and cerebrospinal fluid markers, but adds a little more information. It could be useful in clinical prevention trials to identify people at the highest risk, as smell loss presents quite a few years before MCI or Alzheimer’s disease.”
The investigators acknowledged that their findings need to be replicated in more diverse cohorts that better represent the Alzheimer’s population in the United States. Another limitation of their study, they said, was that the method used to calculate the rate of olfactory decline “was based on slope of measured time points assuming linearity, which may oversimplify the complexity of olfactory changes in normal aging and during the preclinical Alzheimer’s disease period.” The study was funded by the National Institutes of Health. Dr. Pinto disclosed receiving consulting fees from Sanofi/Regeneron, Optinose, and Genentech not related to this work.
, according to new research findings.
Olfactory dysfunction is common in late life and well documented among people with Alzheimer’s disease. However, it was unknown whether faster olfactory decline predicts either onset of Alzheimer’s disease or structural brain changes associated with Alzheimer’s disease.
In a study published online in Alzheimer’s and Dementia, Jayant M. Pinto, MD, and his colleagues at the University of Chicago Medical Center reported that among older adults with normal cognition at baseline, people who experienced rapid loss of sense of smell were more likely to be subsequently diagnosed with mild cognitive impairment (MCI) or dementia, compared with those who did not.
Participants were recruited from Rush University’s Memory and Aging Project, a longitudinal cohort of older adults who undergo yearly cognitive and sensory exams, including a scratch test of 12 common smells to identify. The Rush study “was ahead of the curve in looking at smell,” Dr. Pinto said in an interview. “It gave us a very valuable resource with which to attack these questions.”
Dr. Pinto has long investigated links between smell and accelerated aging; in 2014 his group published the finding that olfactory dysfunction could predict death within 5 years in older adults, and in 2018 they reported that olfactory dysfunction could predict dementia.
Smell and cognition over time
For the current study, Dr. Pinto said, “we were able to look at the question not just using a single point in time, but a more granular trajectory of smell loss. Measuring change year by year showed that the faster people’s sense of smell declined, the more likely they were to be diagnosed with MCI or Alzheimer’s disease.”
Dr. Pinto and his colleagues evaluated results from 515 adults (mean age 76.6, 78% female, 94% White) with no cognitive impairment and at least 3 years of normal results on smell tests at baseline. The subjects were followed for a mean 8 years. One hundred subjects (19%) were diagnosed with MCI or dementia by the end of the study period. A subset of the cohort (n = 121) underwent structural magnetic resonance imaging (MRI) between their final smell tests and the study’s end. Of these, most still had normal cognition; 17 individuals had MCI.
Patients’ individual trajectories of smell loss were mapped as slopes. After adjusting for expected differences in age and sex, the investigators found steeper decline associated with greater risk of incident MCI or dementia (odds ratio, 1.89; 95% confidence interval, 1.26-2.90; P < .01). The risk was comparable to that of carrying an apo E ε4 allele, the key risk variant for late-onset Alzheimer’s disease, but was independent of apo E status. The association was strongest among subjects younger than 76 years.
Olfactory decline and brain volume
Dr. Pinto and his colleagues, including lead author Rachel R. Pacyna, a 4th-year medical student at the University of Chicago, also sought to identify brain volume changes corresponding with olfactory decline and Alzheimer’s disease. The researchers hypothesized that certain brain regions not seen affected in Alzheimer’s disease would remain unchanged regardless of olfactory status, but that regions associated with smell and Alzheimer’s disease would see smaller volumes linked with olfactory decline.
Faster olfactory decline did predict lower gray matter volume in olfactory regions, even after controlling for apo E status and other known risk factors. Conversely, cognitively unimpaired patients undergoing MRI saw more gray matter volume in primary olfactory and temporal brain regions, compared with those with cognitive symptoms.
Taken together, the findings suggest that “change in sense of smell is better than looking at sense of smell at one time point,” Dr. Pinto commented. “There are other reasons people have impaired sense of smell: car accidents, COVID, other viruses and infections. But if you identify on a time course those who are starting to lose it faster, these are the people on whom we need to focus.”
Not yet diagnostic
More work needs to be done to establish thresholds for smell loss that could be useful in clinical or investigative settings as a marker of dementia risk, Dr. Pinto acknowledged. “Everyone gets their hearing tested; everyone gets their vision tested. It’s not as easy to get your sense of smell tested. But this study is telling people that if we were to start measuring it routinely, we could actually use it.”
Smell testing “could become a component of a diagnostic battery that includes things like genotyping and cerebrospinal fluid markers, but adds a little more information. It could be useful in clinical prevention trials to identify people at the highest risk, as smell loss presents quite a few years before MCI or Alzheimer’s disease.”
The investigators acknowledged that their findings need to be replicated in more diverse cohorts that better represent the Alzheimer’s population in the United States. Another limitation of their study, they said, was that the method used to calculate the rate of olfactory decline “was based on slope of measured time points assuming linearity, which may oversimplify the complexity of olfactory changes in normal aging and during the preclinical Alzheimer’s disease period.” The study was funded by the National Institutes of Health. Dr. Pinto disclosed receiving consulting fees from Sanofi/Regeneron, Optinose, and Genentech not related to this work.
FROM ALZHEIMER’S & DEMENTIA
Body-brain neuroinflammation loop may cause chronic ME/CFS, long COVID symptoms
ME/CFS has been established as resulting from infections, environmental exposures, stressors, and surgery. Similarities have been drawn during the COVID-19 pandemic between ME/CFS and a large subgroup of patients with post-acute sequelae of SARS-CoV-2 infection – also known as post-COVID conditions, or long COVID – who continue to have viral fatigue and other lingering symptoms after their infection resolves.
What has been less clearly understood, the researchers said, is the reason behind why ME/CFS and other postviral fatigue tends to be chronic and can sometime develop into a lifelong condition.
“These diseases are very closely related, and it is clear the biological basis of long COVID is unequivocally connected to the original COVID infection – so there should no longer be any debate and doubt about the fact that postviral fatigue syndromes like ME/CFS are biologically based and involve much disturbed physiology,” Warren Tate, MSc, PhD, emeritus professor in the department of biochemistry at the University of Otago in Dunedin, New Zealand, stated in a press release.
Their hypothesis, set forth in a study published in Frontiers of Neurology, proposes that the systemic immune/inflammatory response that occurs after an infection or stressful event does not revolve, which results in a “fluctuating chronic neuroinflammation that sustains and controls the complex neurological symptoms of ME/CFS and long COVID and facilitates frequent more serious relapses in response to life stress, as evidenced from a comprehensive disruption to the cellular molecular biology and body’s physiological pathways.”
Dr. Tate and colleagues said that it is still unclear how the neuroinflammation occurs, why it’s persistent in ME/CFS, and how it causes symptoms associated with ME/CFS. In their hypothesis, “abnormal signaling or transport of molecules/cells occurs through one or both of neurovascular pathways and/or a dysfunctional blood brain barrier,” they said, noting “the normally separate and contained brain/CNS compartment in the healthy person becomes more porous.” The neurological symptoms associated with ME/CFS occur due to strong signals sent because of persistent “inflammatory signals or immune cells/molecules migrating into the brain,” they explained.
This results in a continuous loop where the central nervous system sends signals back to the body through the hypothalamus/paraventricular nucleus and the brain stem. “The resulting symptoms and the neurologically driven ‘sickness response’ for the ME/CFS patient would persist, preventing healing and a return to the preinfectious/stress-related state,” Dr. Tate and colleagues said.
Lingering inflammation may be the culprit
Commenting on the study, Achillefs Ntranos, MD, a board-certified neurologist in private practice in Scarsdale, N.Y., who was not involved with the research, said previous studies have shown that long COVID is linked to chronic activation of microglia in the brain, which has also been seen to activate in patients with ME/CFS.
“The hypothesis that lingering inflammation in the brain is the culprit behind the neurological symptoms of long COVID and ME/CFS is valid,” he said. “If these cells remain activated in the brain, they can cause a state of increased and lingering inflammation, which can interfere with the function of neurons, thus producing neurological symptoms. Since the neurological symptoms are similar between these entities, the mechanisms that produce them might also be similar.”
While the exact cause of ME/CFS is still unclear, it is often tied to the aftereffects of a flu-like illness, Dr. Ntranos said. “This has led researchers to propose that it arises after a viral infection, with many different types of viruses being associated with it. Other ways researchers think ME/CFS is being brought on after a viral illness is via changes in the immune system, such as chronic production of cytokines, neuroinflammation, and disruption of the hypothalamic-pituitary-adrenal axis, which regulates the body’s response to stress,” he explained.
While a newer condition, long COVID is not all that different from ME/CFS, Dr. Ntranos noted, sharing the catalyst of a viral infection and core neurological symptoms such as fatigue, postexertional malaise, a “brain fog” that makes thinking or concentrating difficult, sleep problems, and lightheadedness, but there are differences that set it apart from ME/CFS.
“Long COVID is unique in having additional symptoms that are specific to the SARS-CoV-2 virus, such as respiratory and cardiovascular symptoms and loss of smell and taste. However most central nervous system effects are the same between these two entities,” he said.
Dr. Ntranos said long COVID’s neurological symptoms are similar to that of multiple sclerosis (MS), such as “brain fog” and postexertional malaise. “Since MS only affects the brain and spinal cord, there are no symptoms from other organ systems, such as the lungs, heart, or digestive system, contrary to long COVID. Furthermore, MS rarely affects smell and taste, making these symptoms unique to COVID,” he said.
However, he pointed out that brain fog and fatigue symptoms on their own can be nonspecific and attributed to many different conditions, such as obstructive sleep apnea, migraines, depression, anxiety, thyroid problems, vitamin deficiencies, dehydration, sleep disorders, and side effects of medications.
“More research needs to be done to understand how these cells are being activated, how they interfere with neuronal function, and why they remain in that state in some people, who then go on to develop fatigue and brain fog,” he said.
This study was funded by the Healthcare Otago Charitable Trust, the Associated New Zealand Myalgic Encephalomyelitis Society, and donations from families of patients with ME/CFS. The authors and Dr. Ntranos report no relevant financial disclosures.
ME/CFS has been established as resulting from infections, environmental exposures, stressors, and surgery. Similarities have been drawn during the COVID-19 pandemic between ME/CFS and a large subgroup of patients with post-acute sequelae of SARS-CoV-2 infection – also known as post-COVID conditions, or long COVID – who continue to have viral fatigue and other lingering symptoms after their infection resolves.
What has been less clearly understood, the researchers said, is the reason behind why ME/CFS and other postviral fatigue tends to be chronic and can sometime develop into a lifelong condition.
“These diseases are very closely related, and it is clear the biological basis of long COVID is unequivocally connected to the original COVID infection – so there should no longer be any debate and doubt about the fact that postviral fatigue syndromes like ME/CFS are biologically based and involve much disturbed physiology,” Warren Tate, MSc, PhD, emeritus professor in the department of biochemistry at the University of Otago in Dunedin, New Zealand, stated in a press release.
Their hypothesis, set forth in a study published in Frontiers of Neurology, proposes that the systemic immune/inflammatory response that occurs after an infection or stressful event does not revolve, which results in a “fluctuating chronic neuroinflammation that sustains and controls the complex neurological symptoms of ME/CFS and long COVID and facilitates frequent more serious relapses in response to life stress, as evidenced from a comprehensive disruption to the cellular molecular biology and body’s physiological pathways.”
Dr. Tate and colleagues said that it is still unclear how the neuroinflammation occurs, why it’s persistent in ME/CFS, and how it causes symptoms associated with ME/CFS. In their hypothesis, “abnormal signaling or transport of molecules/cells occurs through one or both of neurovascular pathways and/or a dysfunctional blood brain barrier,” they said, noting “the normally separate and contained brain/CNS compartment in the healthy person becomes more porous.” The neurological symptoms associated with ME/CFS occur due to strong signals sent because of persistent “inflammatory signals or immune cells/molecules migrating into the brain,” they explained.
This results in a continuous loop where the central nervous system sends signals back to the body through the hypothalamus/paraventricular nucleus and the brain stem. “The resulting symptoms and the neurologically driven ‘sickness response’ for the ME/CFS patient would persist, preventing healing and a return to the preinfectious/stress-related state,” Dr. Tate and colleagues said.
Lingering inflammation may be the culprit
Commenting on the study, Achillefs Ntranos, MD, a board-certified neurologist in private practice in Scarsdale, N.Y., who was not involved with the research, said previous studies have shown that long COVID is linked to chronic activation of microglia in the brain, which has also been seen to activate in patients with ME/CFS.
“The hypothesis that lingering inflammation in the brain is the culprit behind the neurological symptoms of long COVID and ME/CFS is valid,” he said. “If these cells remain activated in the brain, they can cause a state of increased and lingering inflammation, which can interfere with the function of neurons, thus producing neurological symptoms. Since the neurological symptoms are similar between these entities, the mechanisms that produce them might also be similar.”
While the exact cause of ME/CFS is still unclear, it is often tied to the aftereffects of a flu-like illness, Dr. Ntranos said. “This has led researchers to propose that it arises after a viral infection, with many different types of viruses being associated with it. Other ways researchers think ME/CFS is being brought on after a viral illness is via changes in the immune system, such as chronic production of cytokines, neuroinflammation, and disruption of the hypothalamic-pituitary-adrenal axis, which regulates the body’s response to stress,” he explained.
While a newer condition, long COVID is not all that different from ME/CFS, Dr. Ntranos noted, sharing the catalyst of a viral infection and core neurological symptoms such as fatigue, postexertional malaise, a “brain fog” that makes thinking or concentrating difficult, sleep problems, and lightheadedness, but there are differences that set it apart from ME/CFS.
“Long COVID is unique in having additional symptoms that are specific to the SARS-CoV-2 virus, such as respiratory and cardiovascular symptoms and loss of smell and taste. However most central nervous system effects are the same between these two entities,” he said.
Dr. Ntranos said long COVID’s neurological symptoms are similar to that of multiple sclerosis (MS), such as “brain fog” and postexertional malaise. “Since MS only affects the brain and spinal cord, there are no symptoms from other organ systems, such as the lungs, heart, or digestive system, contrary to long COVID. Furthermore, MS rarely affects smell and taste, making these symptoms unique to COVID,” he said.
However, he pointed out that brain fog and fatigue symptoms on their own can be nonspecific and attributed to many different conditions, such as obstructive sleep apnea, migraines, depression, anxiety, thyroid problems, vitamin deficiencies, dehydration, sleep disorders, and side effects of medications.
“More research needs to be done to understand how these cells are being activated, how they interfere with neuronal function, and why they remain in that state in some people, who then go on to develop fatigue and brain fog,” he said.
This study was funded by the Healthcare Otago Charitable Trust, the Associated New Zealand Myalgic Encephalomyelitis Society, and donations from families of patients with ME/CFS. The authors and Dr. Ntranos report no relevant financial disclosures.
ME/CFS has been established as resulting from infections, environmental exposures, stressors, and surgery. Similarities have been drawn during the COVID-19 pandemic between ME/CFS and a large subgroup of patients with post-acute sequelae of SARS-CoV-2 infection – also known as post-COVID conditions, or long COVID – who continue to have viral fatigue and other lingering symptoms after their infection resolves.
What has been less clearly understood, the researchers said, is the reason behind why ME/CFS and other postviral fatigue tends to be chronic and can sometime develop into a lifelong condition.
“These diseases are very closely related, and it is clear the biological basis of long COVID is unequivocally connected to the original COVID infection – so there should no longer be any debate and doubt about the fact that postviral fatigue syndromes like ME/CFS are biologically based and involve much disturbed physiology,” Warren Tate, MSc, PhD, emeritus professor in the department of biochemistry at the University of Otago in Dunedin, New Zealand, stated in a press release.
Their hypothesis, set forth in a study published in Frontiers of Neurology, proposes that the systemic immune/inflammatory response that occurs after an infection or stressful event does not revolve, which results in a “fluctuating chronic neuroinflammation that sustains and controls the complex neurological symptoms of ME/CFS and long COVID and facilitates frequent more serious relapses in response to life stress, as evidenced from a comprehensive disruption to the cellular molecular biology and body’s physiological pathways.”
Dr. Tate and colleagues said that it is still unclear how the neuroinflammation occurs, why it’s persistent in ME/CFS, and how it causes symptoms associated with ME/CFS. In their hypothesis, “abnormal signaling or transport of molecules/cells occurs through one or both of neurovascular pathways and/or a dysfunctional blood brain barrier,” they said, noting “the normally separate and contained brain/CNS compartment in the healthy person becomes more porous.” The neurological symptoms associated with ME/CFS occur due to strong signals sent because of persistent “inflammatory signals or immune cells/molecules migrating into the brain,” they explained.
This results in a continuous loop where the central nervous system sends signals back to the body through the hypothalamus/paraventricular nucleus and the brain stem. “The resulting symptoms and the neurologically driven ‘sickness response’ for the ME/CFS patient would persist, preventing healing and a return to the preinfectious/stress-related state,” Dr. Tate and colleagues said.
Lingering inflammation may be the culprit
Commenting on the study, Achillefs Ntranos, MD, a board-certified neurologist in private practice in Scarsdale, N.Y., who was not involved with the research, said previous studies have shown that long COVID is linked to chronic activation of microglia in the brain, which has also been seen to activate in patients with ME/CFS.
“The hypothesis that lingering inflammation in the brain is the culprit behind the neurological symptoms of long COVID and ME/CFS is valid,” he said. “If these cells remain activated in the brain, they can cause a state of increased and lingering inflammation, which can interfere with the function of neurons, thus producing neurological symptoms. Since the neurological symptoms are similar between these entities, the mechanisms that produce them might also be similar.”
While the exact cause of ME/CFS is still unclear, it is often tied to the aftereffects of a flu-like illness, Dr. Ntranos said. “This has led researchers to propose that it arises after a viral infection, with many different types of viruses being associated with it. Other ways researchers think ME/CFS is being brought on after a viral illness is via changes in the immune system, such as chronic production of cytokines, neuroinflammation, and disruption of the hypothalamic-pituitary-adrenal axis, which regulates the body’s response to stress,” he explained.
While a newer condition, long COVID is not all that different from ME/CFS, Dr. Ntranos noted, sharing the catalyst of a viral infection and core neurological symptoms such as fatigue, postexertional malaise, a “brain fog” that makes thinking or concentrating difficult, sleep problems, and lightheadedness, but there are differences that set it apart from ME/CFS.
“Long COVID is unique in having additional symptoms that are specific to the SARS-CoV-2 virus, such as respiratory and cardiovascular symptoms and loss of smell and taste. However most central nervous system effects are the same between these two entities,” he said.
Dr. Ntranos said long COVID’s neurological symptoms are similar to that of multiple sclerosis (MS), such as “brain fog” and postexertional malaise. “Since MS only affects the brain and spinal cord, there are no symptoms from other organ systems, such as the lungs, heart, or digestive system, contrary to long COVID. Furthermore, MS rarely affects smell and taste, making these symptoms unique to COVID,” he said.
However, he pointed out that brain fog and fatigue symptoms on their own can be nonspecific and attributed to many different conditions, such as obstructive sleep apnea, migraines, depression, anxiety, thyroid problems, vitamin deficiencies, dehydration, sleep disorders, and side effects of medications.
“More research needs to be done to understand how these cells are being activated, how they interfere with neuronal function, and why they remain in that state in some people, who then go on to develop fatigue and brain fog,” he said.
This study was funded by the Healthcare Otago Charitable Trust, the Associated New Zealand Myalgic Encephalomyelitis Society, and donations from families of patients with ME/CFS. The authors and Dr. Ntranos report no relevant financial disclosures.
FROM FRONTIERS IN NEUROLOGY
Parkinson’s disease: Is copper culpable?
, according to investigators. The techniques used in this research also may enable rapid identification of blood-borne cofactors driving abnormal protein development in a range of other neurodegenerative diseases, reported lead author Olena Synhaivska, MSc, of the Swiss Federal Laboratories for Materials Science and Technology, Dübendorf, Switzerland.

“While alpha‑synuclein oligomers are the known neurotoxic species in Parkinson’s disease, the development of effective anti–Parkinson’s disease drugs requires targeting of specific structures arising in the early stages of alpha‑synuclein phase transitions or the nucleation-dependent elongation of oligomers into protofibrils,” the investigators wrote in ACS Chemical Neuroscience. “In parallel, advanced methods are required to routinely characterize the size and morphology of intermediary nano- and microstructures formed during self-assembly and aggregation in the presence of aqueous metal ions to track disease progression in, for example, a blood test, to provide effective personalized patient care.”
Pathologic aggregation of alpha‑synuclein
To better understand the relationship between copper and alpha‑synuclein, the investigators used liquid-based atomic force microscopy to observe the protein in solution over 10 days as it transitioned from a simple monomer to a complex, three-dimensional aggregate. Protein aggregation occurred in the absence or presence of copper; however, when incubated in solution with Cu2+ ions, alpha‑synuclein aggregated faster, predominantly forming annular (ring-shaped) structures that were not observed in the absence of copper.
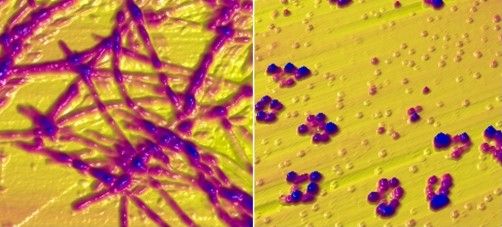
These annular oligomers are noteworthy because they are cytotoxic, and they nucleate formation of alpha‑synuclein filaments, meaning they could serve as early therapeutic targets, according to the investigators.
The above experiments were supported by Raman spectroscopy, which confirmed the various superstructures of alpha‑synuclein formed with or without copper. In addition, the investigators used molecular dynamics computer simulations to map “the dimensions, supramolecular packing interactions, and thermodynamic stabilities” involved in aggregation.
These findings “could potentially serve as guidelines for better understanding protein aggregated states in body fluids from individuals who have been exposed to environmental metals over their lifetime,” the investigators wrote. “The nanoscale imaging, chemical spectroscopy, and integrated modeling-measurement methodologies presented here may inform rapid screening of other potential blood-borne cofactors, for example, other biometals, heavy metals, physiological amino acids, and metabolites, in directing and potentially rerouting intrinsically disordered protein aggregation in the initiation and pathology of neurodegenerative diseases.”
What is copper’s role in Parkinson’s disease pathogenesis?
In a joint written comment, Vikram Khurana MD, PhD, and Richard Krolewski MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, said, “This study is important in that it demonstrates that the presence of copper can accelerate and alter the aggregation of wild type alpha‑synuclein. We know that pathologic aggregation of alpha‑synuclein is critical for diseases like Parkinson’s disease known as synucleinopathies – so any insight into how this is happening at the biophysical level has potential implications for altering that process.”
While Dr. Khurana and Dr. Krolewski praised the elegance of the study, including the techniques used to observe alpha‑synuclein aggregation in near real-time, they suggested that more work is needed to determine relevance for patients with Parkinson’s disease.
“It is not clear whether this process is happening in cells, how alpha‑synuclein fibrils might be directly exposed to copper intracellularly (with most of the copper being bound to proteins), and the relevance of the copper concentrations used here are in question,” they said. “Substantially more cell biology and in vivo modeling would be needed to further evaluate the connection of copper specifically to synucleinopathy. All this notwithstanding, the findings are exciting and intriguing and definitely warrant follow-up.”
In the meantime, an increasing number of studies, including a recent preprint by Dr. Khurana and Dr. Krolewski, are strengthening the case for a link between copper exposure and Parkinson’s disease pathogenesis. This body of evidence, they noted, “now spans epidemiology, cell biology, and biophysics.”
Their study, which tested 53 pesticides associated with Parkinson’s disease in patient-derived pluripotent stem cells, found that 2 out of 10 pesticides causing cell death were copper compounds.
“Ongoing work will explore the mechanism of this cell death and investigate ways to mitigate it,” said Dr. Khurana and Dr. Krolewski. “Our hope is that this line of research will raise public awareness about these and other pesticides to reduce potential harm from their use and highlight protective approaches. The study by Dr. Synhaivska and colleagues now raises the possibility of new mechanisms.”
The study by Dr. Synhaivska and colleagues was supported by grants from the Swiss National Science Foundation and the Science Foundation Ireland. The investigators disclosed no conflicts of interest. Dr. Krolewski has been retained as an expert consultant for plaintiffs in a lawsuit on the role of pesticides in Parkinson’s disease causation.
, according to investigators. The techniques used in this research also may enable rapid identification of blood-borne cofactors driving abnormal protein development in a range of other neurodegenerative diseases, reported lead author Olena Synhaivska, MSc, of the Swiss Federal Laboratories for Materials Science and Technology, Dübendorf, Switzerland.

“While alpha‑synuclein oligomers are the known neurotoxic species in Parkinson’s disease, the development of effective anti–Parkinson’s disease drugs requires targeting of specific structures arising in the early stages of alpha‑synuclein phase transitions or the nucleation-dependent elongation of oligomers into protofibrils,” the investigators wrote in ACS Chemical Neuroscience. “In parallel, advanced methods are required to routinely characterize the size and morphology of intermediary nano- and microstructures formed during self-assembly and aggregation in the presence of aqueous metal ions to track disease progression in, for example, a blood test, to provide effective personalized patient care.”
Pathologic aggregation of alpha‑synuclein
To better understand the relationship between copper and alpha‑synuclein, the investigators used liquid-based atomic force microscopy to observe the protein in solution over 10 days as it transitioned from a simple monomer to a complex, three-dimensional aggregate. Protein aggregation occurred in the absence or presence of copper; however, when incubated in solution with Cu2+ ions, alpha‑synuclein aggregated faster, predominantly forming annular (ring-shaped) structures that were not observed in the absence of copper.

These annular oligomers are noteworthy because they are cytotoxic, and they nucleate formation of alpha‑synuclein filaments, meaning they could serve as early therapeutic targets, according to the investigators.
The above experiments were supported by Raman spectroscopy, which confirmed the various superstructures of alpha‑synuclein formed with or without copper. In addition, the investigators used molecular dynamics computer simulations to map “the dimensions, supramolecular packing interactions, and thermodynamic stabilities” involved in aggregation.
These findings “could potentially serve as guidelines for better understanding protein aggregated states in body fluids from individuals who have been exposed to environmental metals over their lifetime,” the investigators wrote. “The nanoscale imaging, chemical spectroscopy, and integrated modeling-measurement methodologies presented here may inform rapid screening of other potential blood-borne cofactors, for example, other biometals, heavy metals, physiological amino acids, and metabolites, in directing and potentially rerouting intrinsically disordered protein aggregation in the initiation and pathology of neurodegenerative diseases.”
What is copper’s role in Parkinson’s disease pathogenesis?
In a joint written comment, Vikram Khurana MD, PhD, and Richard Krolewski MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, said, “This study is important in that it demonstrates that the presence of copper can accelerate and alter the aggregation of wild type alpha‑synuclein. We know that pathologic aggregation of alpha‑synuclein is critical for diseases like Parkinson’s disease known as synucleinopathies – so any insight into how this is happening at the biophysical level has potential implications for altering that process.”
While Dr. Khurana and Dr. Krolewski praised the elegance of the study, including the techniques used to observe alpha‑synuclein aggregation in near real-time, they suggested that more work is needed to determine relevance for patients with Parkinson’s disease.
“It is not clear whether this process is happening in cells, how alpha‑synuclein fibrils might be directly exposed to copper intracellularly (with most of the copper being bound to proteins), and the relevance of the copper concentrations used here are in question,” they said. “Substantially more cell biology and in vivo modeling would be needed to further evaluate the connection of copper specifically to synucleinopathy. All this notwithstanding, the findings are exciting and intriguing and definitely warrant follow-up.”
In the meantime, an increasing number of studies, including a recent preprint by Dr. Khurana and Dr. Krolewski, are strengthening the case for a link between copper exposure and Parkinson’s disease pathogenesis. This body of evidence, they noted, “now spans epidemiology, cell biology, and biophysics.”
Their study, which tested 53 pesticides associated with Parkinson’s disease in patient-derived pluripotent stem cells, found that 2 out of 10 pesticides causing cell death were copper compounds.
“Ongoing work will explore the mechanism of this cell death and investigate ways to mitigate it,” said Dr. Khurana and Dr. Krolewski. “Our hope is that this line of research will raise public awareness about these and other pesticides to reduce potential harm from their use and highlight protective approaches. The study by Dr. Synhaivska and colleagues now raises the possibility of new mechanisms.”
The study by Dr. Synhaivska and colleagues was supported by grants from the Swiss National Science Foundation and the Science Foundation Ireland. The investigators disclosed no conflicts of interest. Dr. Krolewski has been retained as an expert consultant for plaintiffs in a lawsuit on the role of pesticides in Parkinson’s disease causation.
, according to investigators. The techniques used in this research also may enable rapid identification of blood-borne cofactors driving abnormal protein development in a range of other neurodegenerative diseases, reported lead author Olena Synhaivska, MSc, of the Swiss Federal Laboratories for Materials Science and Technology, Dübendorf, Switzerland.

“While alpha‑synuclein oligomers are the known neurotoxic species in Parkinson’s disease, the development of effective anti–Parkinson’s disease drugs requires targeting of specific structures arising in the early stages of alpha‑synuclein phase transitions or the nucleation-dependent elongation of oligomers into protofibrils,” the investigators wrote in ACS Chemical Neuroscience. “In parallel, advanced methods are required to routinely characterize the size and morphology of intermediary nano- and microstructures formed during self-assembly and aggregation in the presence of aqueous metal ions to track disease progression in, for example, a blood test, to provide effective personalized patient care.”
Pathologic aggregation of alpha‑synuclein
To better understand the relationship between copper and alpha‑synuclein, the investigators used liquid-based atomic force microscopy to observe the protein in solution over 10 days as it transitioned from a simple monomer to a complex, three-dimensional aggregate. Protein aggregation occurred in the absence or presence of copper; however, when incubated in solution with Cu2+ ions, alpha‑synuclein aggregated faster, predominantly forming annular (ring-shaped) structures that were not observed in the absence of copper.

These annular oligomers are noteworthy because they are cytotoxic, and they nucleate formation of alpha‑synuclein filaments, meaning they could serve as early therapeutic targets, according to the investigators.
The above experiments were supported by Raman spectroscopy, which confirmed the various superstructures of alpha‑synuclein formed with or without copper. In addition, the investigators used molecular dynamics computer simulations to map “the dimensions, supramolecular packing interactions, and thermodynamic stabilities” involved in aggregation.
These findings “could potentially serve as guidelines for better understanding protein aggregated states in body fluids from individuals who have been exposed to environmental metals over their lifetime,” the investigators wrote. “The nanoscale imaging, chemical spectroscopy, and integrated modeling-measurement methodologies presented here may inform rapid screening of other potential blood-borne cofactors, for example, other biometals, heavy metals, physiological amino acids, and metabolites, in directing and potentially rerouting intrinsically disordered protein aggregation in the initiation and pathology of neurodegenerative diseases.”
What is copper’s role in Parkinson’s disease pathogenesis?
In a joint written comment, Vikram Khurana MD, PhD, and Richard Krolewski MD, PhD, of Brigham and Women’s Hospital and Harvard Medical School, Boston, said, “This study is important in that it demonstrates that the presence of copper can accelerate and alter the aggregation of wild type alpha‑synuclein. We know that pathologic aggregation of alpha‑synuclein is critical for diseases like Parkinson’s disease known as synucleinopathies – so any insight into how this is happening at the biophysical level has potential implications for altering that process.”
While Dr. Khurana and Dr. Krolewski praised the elegance of the study, including the techniques used to observe alpha‑synuclein aggregation in near real-time, they suggested that more work is needed to determine relevance for patients with Parkinson’s disease.
“It is not clear whether this process is happening in cells, how alpha‑synuclein fibrils might be directly exposed to copper intracellularly (with most of the copper being bound to proteins), and the relevance of the copper concentrations used here are in question,” they said. “Substantially more cell biology and in vivo modeling would be needed to further evaluate the connection of copper specifically to synucleinopathy. All this notwithstanding, the findings are exciting and intriguing and definitely warrant follow-up.”
In the meantime, an increasing number of studies, including a recent preprint by Dr. Khurana and Dr. Krolewski, are strengthening the case for a link between copper exposure and Parkinson’s disease pathogenesis. This body of evidence, they noted, “now spans epidemiology, cell biology, and biophysics.”
Their study, which tested 53 pesticides associated with Parkinson’s disease in patient-derived pluripotent stem cells, found that 2 out of 10 pesticides causing cell death were copper compounds.
“Ongoing work will explore the mechanism of this cell death and investigate ways to mitigate it,” said Dr. Khurana and Dr. Krolewski. “Our hope is that this line of research will raise public awareness about these and other pesticides to reduce potential harm from their use and highlight protective approaches. The study by Dr. Synhaivska and colleagues now raises the possibility of new mechanisms.”
The study by Dr. Synhaivska and colleagues was supported by grants from the Swiss National Science Foundation and the Science Foundation Ireland. The investigators disclosed no conflicts of interest. Dr. Krolewski has been retained as an expert consultant for plaintiffs in a lawsuit on the role of pesticides in Parkinson’s disease causation.
FROM ACS CHEMICAL NEUROSCIENCE



