User login
Children and COVID: Weekly cases dropped by 57% in September
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.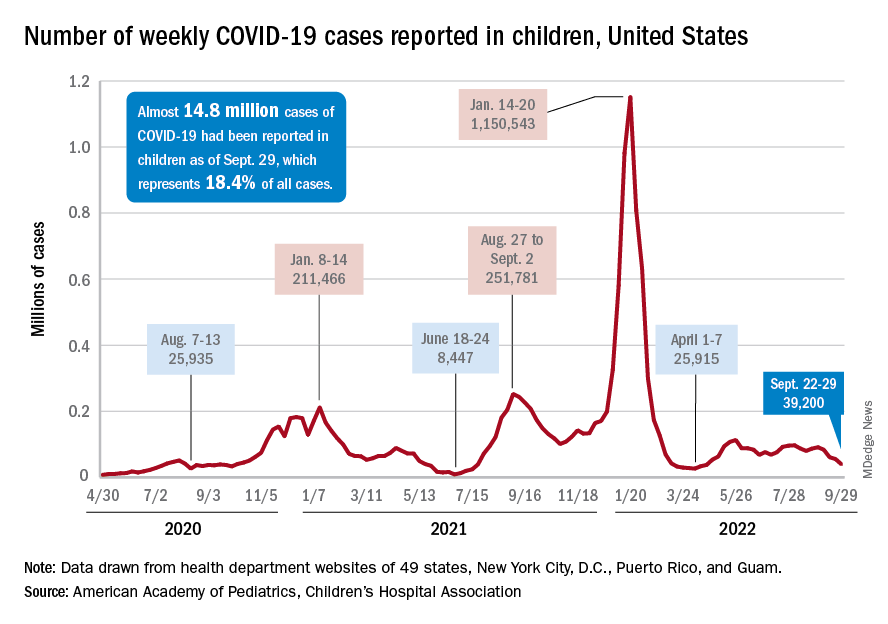
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
The last full week of September brought a 4th straight week of declines in the number of new COVID-19 cases reported among children, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, with the month of September bringing a decline of about 57% in reported cases for the 45 states and territories that are still releasing pediatric COVID data on their health department websites, the AAP and CHA said in their joint weekly report.
New cases dropped in all four regions after the Northeast and West had seen increases the previous week, and the distribution of cases for the latest week was fairly even, with the Midwest and Northeast right around 10,000, the South slightly over 10,000, and the West under 10,000 by about the same amount. At the state level, the largest increases – around 1.5% – over the last 2 weeks occurred in Kentucky and Nevada, the AAP/CHA data show.
The cumulative number of COVID-19 cases in children was almost 14.8 million as of Sept. 29, with children representing 18.4% of all cases since the pandemic began, the AAP and CHA said. The Centers for Disease Control and Prevention, which is able to use a uniform age range of 0-17 years, puts total cases at 15.2 million and the proportion of child cases at 17.4%. Total deaths in children from COVID as of Oct. 3 were 1,745, the CDC reported.
New vaccinations, in the meantime, are being added in numbers only slightly higher than new cases. Initial COVID vaccinations for the week of Sept. 22-28 were about 44,000 for children under 5 years of age (down from 51,000 the week before), 24,000 for children aged 5-11 years (down from 28,000), and 17,000 for those aged 12-17 (down from 18,000), the AAP said in its weekly vaccination report.
To look at it another way, the total proportion of children under 5 years of age who had received at least one dose of COVID vaccine as of Sept. 28 was 6.5%, compared with 6.4% on Sept. 21, while the corresponding rates for children aged 5-11 and 12-17 were unchanged at 38.5% and 70.9%. The 12- to 17-year-olds, in fact, have been stuck at 70.9% since Sept. 13, according to data from the CDC.
In a recent study published in Vaccine, investigators attributed the discrepancies between age groups at least partly to the acceptance of misinformation about vaccine safety in general and the COVID-19 vaccines in particular.
“All of the misconceptions we studied focused in one way or another on the safety of vaccination, and that explains why people’s misbeliefs about vaccinating kids are so highly related to their concerns about vaccines in general. Unfortunately, those concerns weigh even more heavily when adults consider vaccinating children,” lead author Dan Romer, PhD, of the University of Pennsylvania, Philadelphia, said in a written statement.
FDA approves HIV-1 treatment ibalizumab for 30-second IV push
The Food and Drug Administration has approved the HIV-1 medication ibalizumab-uiyk (Trogarzo, Theratechnologies) for administration by intravenous push.
Ibalizumab-uiyk, a long-acting monoclonal antibody, was first approved by the FDA in 2018 for the treatment of adults with multidrug-resistant HIV-1. It is used in combination with other antiretroviral drugs.
Prior to this approval, the drug was administered intravenously as a single 2,000-mg loading dose, followed by an 800-mg maintenance dose every 2 weeks by a trained medical professional. The intravenous infusion is given over 15-30 minutes, according to the Trogarzo website. Now, the maintenance dose can be administered by intravenous push, a method where the undiluted medication is delivered intravenously by injection, in just 30 seconds.
for patients and their health care providers, possibly allowing for more clinics to administer this treatment,” said Christian Marsolais, PhD, the chief medical officer of Theratechnologies, in an Oct. 3 press release.
The FDA approval of the intravenous push method was based on a clinical study which found that ibalizumab administered via intravenous push had similar safety and pharmacokinetic profiles as the intravenous infusion method. So far, 350 individuals have received ibalizumab as a part of the clinical development program, including 19 people who received the medication via intravenous push. The medication is also being studied for administration via intramuscular injection, the press release said.
The most common side effects of ibalizumab include diarrhea, dizziness, nausea, and rash. Severe adverse events have been reported in two patients: one who developed immune reconstitution inflammatory syndrome and another who reported a severe rash.
While multidrug-resistant HIV that would require ibalizumab is not very common – one study found it occurred in fewer than 2% of people with HIV in Western Europe – it is a “very difficult problem because we need to treat these patients to try to achieve virologic suppression,” Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, noted in an email. While providers generally try to use nonintravenous medications when possible, ibalizumab is an important medication for people with multidrug-resistant HIV and limited treatment options.
“One barrier to administration was the need for IV infusion over 15-30 minutes,” Dr. Gandhi added. “The ability to give this medication as an IV push is an important breakthrough, as we could give this medication more readily for the relatively low number of individuals who will need it.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the HIV-1 medication ibalizumab-uiyk (Trogarzo, Theratechnologies) for administration by intravenous push.
Ibalizumab-uiyk, a long-acting monoclonal antibody, was first approved by the FDA in 2018 for the treatment of adults with multidrug-resistant HIV-1. It is used in combination with other antiretroviral drugs.
Prior to this approval, the drug was administered intravenously as a single 2,000-mg loading dose, followed by an 800-mg maintenance dose every 2 weeks by a trained medical professional. The intravenous infusion is given over 15-30 minutes, according to the Trogarzo website. Now, the maintenance dose can be administered by intravenous push, a method where the undiluted medication is delivered intravenously by injection, in just 30 seconds.
for patients and their health care providers, possibly allowing for more clinics to administer this treatment,” said Christian Marsolais, PhD, the chief medical officer of Theratechnologies, in an Oct. 3 press release.
The FDA approval of the intravenous push method was based on a clinical study which found that ibalizumab administered via intravenous push had similar safety and pharmacokinetic profiles as the intravenous infusion method. So far, 350 individuals have received ibalizumab as a part of the clinical development program, including 19 people who received the medication via intravenous push. The medication is also being studied for administration via intramuscular injection, the press release said.
The most common side effects of ibalizumab include diarrhea, dizziness, nausea, and rash. Severe adverse events have been reported in two patients: one who developed immune reconstitution inflammatory syndrome and another who reported a severe rash.
While multidrug-resistant HIV that would require ibalizumab is not very common – one study found it occurred in fewer than 2% of people with HIV in Western Europe – it is a “very difficult problem because we need to treat these patients to try to achieve virologic suppression,” Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, noted in an email. While providers generally try to use nonintravenous medications when possible, ibalizumab is an important medication for people with multidrug-resistant HIV and limited treatment options.
“One barrier to administration was the need for IV infusion over 15-30 minutes,” Dr. Gandhi added. “The ability to give this medication as an IV push is an important breakthrough, as we could give this medication more readily for the relatively low number of individuals who will need it.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the HIV-1 medication ibalizumab-uiyk (Trogarzo, Theratechnologies) for administration by intravenous push.
Ibalizumab-uiyk, a long-acting monoclonal antibody, was first approved by the FDA in 2018 for the treatment of adults with multidrug-resistant HIV-1. It is used in combination with other antiretroviral drugs.
Prior to this approval, the drug was administered intravenously as a single 2,000-mg loading dose, followed by an 800-mg maintenance dose every 2 weeks by a trained medical professional. The intravenous infusion is given over 15-30 minutes, according to the Trogarzo website. Now, the maintenance dose can be administered by intravenous push, a method where the undiluted medication is delivered intravenously by injection, in just 30 seconds.
for patients and their health care providers, possibly allowing for more clinics to administer this treatment,” said Christian Marsolais, PhD, the chief medical officer of Theratechnologies, in an Oct. 3 press release.
The FDA approval of the intravenous push method was based on a clinical study which found that ibalizumab administered via intravenous push had similar safety and pharmacokinetic profiles as the intravenous infusion method. So far, 350 individuals have received ibalizumab as a part of the clinical development program, including 19 people who received the medication via intravenous push. The medication is also being studied for administration via intramuscular injection, the press release said.
The most common side effects of ibalizumab include diarrhea, dizziness, nausea, and rash. Severe adverse events have been reported in two patients: one who developed immune reconstitution inflammatory syndrome and another who reported a severe rash.
While multidrug-resistant HIV that would require ibalizumab is not very common – one study found it occurred in fewer than 2% of people with HIV in Western Europe – it is a “very difficult problem because we need to treat these patients to try to achieve virologic suppression,” Monica Gandhi, MD, MPH, associate chief of the division of HIV, infectious diseases, and global medicine at the University of California, San Francisco, noted in an email. While providers generally try to use nonintravenous medications when possible, ibalizumab is an important medication for people with multidrug-resistant HIV and limited treatment options.
“One barrier to administration was the need for IV infusion over 15-30 minutes,” Dr. Gandhi added. “The ability to give this medication as an IV push is an important breakthrough, as we could give this medication more readily for the relatively low number of individuals who will need it.”
A version of this article first appeared on Medscape.com.
FDA approves futibatinib (Lytgobi) for certain biliary tract cancers
Futibatinib was granted priority review and breakthrough designation, the agency said in its announcement.
Futibatinib is indicated for use in adult patients with previously treated, unresectable, locally advanced, or metastatic intrahepatic cholangiocarcinoma harboring fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements.
The FDA noted that efficacy was evaluated in a multicenter, open-label, single-arm trial (known as TAS-120-101 [NCT02052778]), which involved 103 patients with such tumors. The presence of FGFR2 fusions or other rearrangements was determined using next-generation sequencing.
All the patients in this trial received futibatinib (20 mg orally once daily) until disease progression or unacceptable toxicity.
The overall response rate was 42% (95% confidence interval, 32%-52%), and all of the 43 patients who responded achieved partial responses.
The median duration of response was 9.7 months (95% CI, 7.6-17.1).
The most common adverse reactions that occurred in 20% or more of patients were nail toxicity, musculoskeletal pain, constipation, diarrhea, fatigue, dry mouth, alopecia, stomatitis, abdominal pain, dry skin, arthralgia, dysgeusia, dry eye, nausea, decreased appetite, urinary tract infection, palmar-plantar erythrodysesthesia syndrome, and vomiting.
The manufacturer noted in its announcement that futibatinib covalently binds to FGFR2 and inhibits the signaling pathway. The other approved FGFR inhibitors are reversible ATP-competitive inhibitors.
The company also provided some background information on the cancer.
As a whole, cholangiocarcinoma is an aggressive cancer of the bile ducts. It is diagnosed in approximately 8000 individuals each year in the United States, the company noted.
These cases include both intrahepatic (inside the liver) and extrahepatic (outside the liver) forms of the disease. Approximately 20% of patients diagnosed with cholangiocarcinoma have the intrahepatic form of the disease. Among these 20%, approximately 10%-16% of patients have FGFR2 gene rearrangements, including fusions, which promote tumor proliferation.
Futibatinib is “a key example of the potential of precision medicine in iCCA [intrahepatic cholangiocarcinoma] and represents another advance in the treatment of this rare and challenging disease,” said medical oncologist Lipika Goyal, MD, MPhil, of the Massachusetts General Hospital Cancer Center, Boston, and lead investigator of the pivotal study that supported the approval.
“I am encouraged that treatment options continue to expand and evolve for this disease through the dedicated efforts of many over several years,” she commented in the company’s press release.
A version of this article first appeared on Medscape.com.
Futibatinib was granted priority review and breakthrough designation, the agency said in its announcement.
Futibatinib is indicated for use in adult patients with previously treated, unresectable, locally advanced, or metastatic intrahepatic cholangiocarcinoma harboring fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements.
The FDA noted that efficacy was evaluated in a multicenter, open-label, single-arm trial (known as TAS-120-101 [NCT02052778]), which involved 103 patients with such tumors. The presence of FGFR2 fusions or other rearrangements was determined using next-generation sequencing.
All the patients in this trial received futibatinib (20 mg orally once daily) until disease progression or unacceptable toxicity.
The overall response rate was 42% (95% confidence interval, 32%-52%), and all of the 43 patients who responded achieved partial responses.
The median duration of response was 9.7 months (95% CI, 7.6-17.1).
The most common adverse reactions that occurred in 20% or more of patients were nail toxicity, musculoskeletal pain, constipation, diarrhea, fatigue, dry mouth, alopecia, stomatitis, abdominal pain, dry skin, arthralgia, dysgeusia, dry eye, nausea, decreased appetite, urinary tract infection, palmar-plantar erythrodysesthesia syndrome, and vomiting.
The manufacturer noted in its announcement that futibatinib covalently binds to FGFR2 and inhibits the signaling pathway. The other approved FGFR inhibitors are reversible ATP-competitive inhibitors.
The company also provided some background information on the cancer.
As a whole, cholangiocarcinoma is an aggressive cancer of the bile ducts. It is diagnosed in approximately 8000 individuals each year in the United States, the company noted.
These cases include both intrahepatic (inside the liver) and extrahepatic (outside the liver) forms of the disease. Approximately 20% of patients diagnosed with cholangiocarcinoma have the intrahepatic form of the disease. Among these 20%, approximately 10%-16% of patients have FGFR2 gene rearrangements, including fusions, which promote tumor proliferation.
Futibatinib is “a key example of the potential of precision medicine in iCCA [intrahepatic cholangiocarcinoma] and represents another advance in the treatment of this rare and challenging disease,” said medical oncologist Lipika Goyal, MD, MPhil, of the Massachusetts General Hospital Cancer Center, Boston, and lead investigator of the pivotal study that supported the approval.
“I am encouraged that treatment options continue to expand and evolve for this disease through the dedicated efforts of many over several years,” she commented in the company’s press release.
A version of this article first appeared on Medscape.com.
Futibatinib was granted priority review and breakthrough designation, the agency said in its announcement.
Futibatinib is indicated for use in adult patients with previously treated, unresectable, locally advanced, or metastatic intrahepatic cholangiocarcinoma harboring fibroblast growth factor receptor 2 (FGFR2) gene fusions or other rearrangements.
The FDA noted that efficacy was evaluated in a multicenter, open-label, single-arm trial (known as TAS-120-101 [NCT02052778]), which involved 103 patients with such tumors. The presence of FGFR2 fusions or other rearrangements was determined using next-generation sequencing.
All the patients in this trial received futibatinib (20 mg orally once daily) until disease progression or unacceptable toxicity.
The overall response rate was 42% (95% confidence interval, 32%-52%), and all of the 43 patients who responded achieved partial responses.
The median duration of response was 9.7 months (95% CI, 7.6-17.1).
The most common adverse reactions that occurred in 20% or more of patients were nail toxicity, musculoskeletal pain, constipation, diarrhea, fatigue, dry mouth, alopecia, stomatitis, abdominal pain, dry skin, arthralgia, dysgeusia, dry eye, nausea, decreased appetite, urinary tract infection, palmar-plantar erythrodysesthesia syndrome, and vomiting.
The manufacturer noted in its announcement that futibatinib covalently binds to FGFR2 and inhibits the signaling pathway. The other approved FGFR inhibitors are reversible ATP-competitive inhibitors.
The company also provided some background information on the cancer.
As a whole, cholangiocarcinoma is an aggressive cancer of the bile ducts. It is diagnosed in approximately 8000 individuals each year in the United States, the company noted.
These cases include both intrahepatic (inside the liver) and extrahepatic (outside the liver) forms of the disease. Approximately 20% of patients diagnosed with cholangiocarcinoma have the intrahepatic form of the disease. Among these 20%, approximately 10%-16% of patients have FGFR2 gene rearrangements, including fusions, which promote tumor proliferation.
Futibatinib is “a key example of the potential of precision medicine in iCCA [intrahepatic cholangiocarcinoma] and represents another advance in the treatment of this rare and challenging disease,” said medical oncologist Lipika Goyal, MD, MPhil, of the Massachusetts General Hospital Cancer Center, Boston, and lead investigator of the pivotal study that supported the approval.
“I am encouraged that treatment options continue to expand and evolve for this disease through the dedicated efforts of many over several years,” she commented in the company’s press release.
A version of this article first appeared on Medscape.com.
FDA approves dupilumab for treatment of prurigo nodularis
The according to a press release from the manufacturers.
Recent studies of dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, show significant improvements in both itchiness and lesion counts, compared with placebo, in adults with prurigo nodularis (PN).
Approval was based on data from two randomized, controlled trials, PRIME and PRIME2, comparing dupilumab with placebo in 311 adults with uncontrolled PN, according to the release issued by Regeneron and Sanofi. Dupilumab is administered via a 300 mg subcutaneous injection every 2 weeks after a loading dose.
The primary endpoint in PRIME and PRIME 2 was a clinically meaningful improvement in itch from baseline as measured by at least a 4-point reduction in the Worst Itch Numeric Rating Scale, a 0-10 scale, at 24 and 12 weeks, respectively. In the studies, 60% and 58% of patients treated with dupilumab met the primary endpoint at 24 weeks, compared with 18% and 20% of those on placebo. At 24 weeks, 48% and 45% of patients on dupilumab achieved clear or almost clear skin, another study endpoint, compared with 18% and 16% among those on placebo.*
In PRIME and PRIME2, 44% and 37% of patients on dupilumab met the primary endpoint at 12 weeks versus16% and 22% among those on placebo.
Safety profiles were similar to those seen in other dupilumab studies, according to the release. The most common adverse events in the two studies combined were nasopharyngitis, reported in 5% of those on dupilumab versus 2% of those on placebo; conjunctivitis in 4% versus 1%; herpes infection in 3% versus 0; dizziness in 3% vs. 1%; muscle pain in 3% versus 1%; and diarrhea in 3% versus 1%.
Phase 3 data on dupilumab for PN were recently presented at the annual congress of the European Academy of Dermatology and Venereology.
A regulatory submission for dupilumab for treating PN is in progress at the European Medicines Agency, and submissions are planned to regulatory agencies in additional countries later in 2022, according to the company press release.
Dupilumab is currently approved in the United States for atopic dermatitis in children aged 6 months and older and adults with moderate to severe atopic dermatitis and in children and adults aged 6 years and older with moderate to severe eosinophilic or oral steroid-dependent asthma, as well as for the treatment of chronic rhinosinusitis with nasal polyposis in adults, and for the treatment of eosinophilic esophagitis in adults and children aged 12 years and older, weighing at least 40 kg. Dupilumab is under clinical development for the treatment of chronic spontaneous urticaria and bullous pemphigoid, according to the manufacturers.
The studies were supported by Regeneron and Sanofi.
A version of this article first appeared on Medscape.com.
*Correction, 9/30/22: An earlier version of this article misstated results of one endpoint.
The according to a press release from the manufacturers.
Recent studies of dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, show significant improvements in both itchiness and lesion counts, compared with placebo, in adults with prurigo nodularis (PN).
Approval was based on data from two randomized, controlled trials, PRIME and PRIME2, comparing dupilumab with placebo in 311 adults with uncontrolled PN, according to the release issued by Regeneron and Sanofi. Dupilumab is administered via a 300 mg subcutaneous injection every 2 weeks after a loading dose.
The primary endpoint in PRIME and PRIME 2 was a clinically meaningful improvement in itch from baseline as measured by at least a 4-point reduction in the Worst Itch Numeric Rating Scale, a 0-10 scale, at 24 and 12 weeks, respectively. In the studies, 60% and 58% of patients treated with dupilumab met the primary endpoint at 24 weeks, compared with 18% and 20% of those on placebo. At 24 weeks, 48% and 45% of patients on dupilumab achieved clear or almost clear skin, another study endpoint, compared with 18% and 16% among those on placebo.*
In PRIME and PRIME2, 44% and 37% of patients on dupilumab met the primary endpoint at 12 weeks versus16% and 22% among those on placebo.
Safety profiles were similar to those seen in other dupilumab studies, according to the release. The most common adverse events in the two studies combined were nasopharyngitis, reported in 5% of those on dupilumab versus 2% of those on placebo; conjunctivitis in 4% versus 1%; herpes infection in 3% versus 0; dizziness in 3% vs. 1%; muscle pain in 3% versus 1%; and diarrhea in 3% versus 1%.
Phase 3 data on dupilumab for PN were recently presented at the annual congress of the European Academy of Dermatology and Venereology.
A regulatory submission for dupilumab for treating PN is in progress at the European Medicines Agency, and submissions are planned to regulatory agencies in additional countries later in 2022, according to the company press release.
Dupilumab is currently approved in the United States for atopic dermatitis in children aged 6 months and older and adults with moderate to severe atopic dermatitis and in children and adults aged 6 years and older with moderate to severe eosinophilic or oral steroid-dependent asthma, as well as for the treatment of chronic rhinosinusitis with nasal polyposis in adults, and for the treatment of eosinophilic esophagitis in adults and children aged 12 years and older, weighing at least 40 kg. Dupilumab is under clinical development for the treatment of chronic spontaneous urticaria and bullous pemphigoid, according to the manufacturers.
The studies were supported by Regeneron and Sanofi.
A version of this article first appeared on Medscape.com.
*Correction, 9/30/22: An earlier version of this article misstated results of one endpoint.
The according to a press release from the manufacturers.
Recent studies of dupilumab (Dupixent), which inhibits the signaling of the interleukin-4 and IL-13 pathways, show significant improvements in both itchiness and lesion counts, compared with placebo, in adults with prurigo nodularis (PN).
Approval was based on data from two randomized, controlled trials, PRIME and PRIME2, comparing dupilumab with placebo in 311 adults with uncontrolled PN, according to the release issued by Regeneron and Sanofi. Dupilumab is administered via a 300 mg subcutaneous injection every 2 weeks after a loading dose.
The primary endpoint in PRIME and PRIME 2 was a clinically meaningful improvement in itch from baseline as measured by at least a 4-point reduction in the Worst Itch Numeric Rating Scale, a 0-10 scale, at 24 and 12 weeks, respectively. In the studies, 60% and 58% of patients treated with dupilumab met the primary endpoint at 24 weeks, compared with 18% and 20% of those on placebo. At 24 weeks, 48% and 45% of patients on dupilumab achieved clear or almost clear skin, another study endpoint, compared with 18% and 16% among those on placebo.*
In PRIME and PRIME2, 44% and 37% of patients on dupilumab met the primary endpoint at 12 weeks versus16% and 22% among those on placebo.
Safety profiles were similar to those seen in other dupilumab studies, according to the release. The most common adverse events in the two studies combined were nasopharyngitis, reported in 5% of those on dupilumab versus 2% of those on placebo; conjunctivitis in 4% versus 1%; herpes infection in 3% versus 0; dizziness in 3% vs. 1%; muscle pain in 3% versus 1%; and diarrhea in 3% versus 1%.
Phase 3 data on dupilumab for PN were recently presented at the annual congress of the European Academy of Dermatology and Venereology.
A regulatory submission for dupilumab for treating PN is in progress at the European Medicines Agency, and submissions are planned to regulatory agencies in additional countries later in 2022, according to the company press release.
Dupilumab is currently approved in the United States for atopic dermatitis in children aged 6 months and older and adults with moderate to severe atopic dermatitis and in children and adults aged 6 years and older with moderate to severe eosinophilic or oral steroid-dependent asthma, as well as for the treatment of chronic rhinosinusitis with nasal polyposis in adults, and for the treatment of eosinophilic esophagitis in adults and children aged 12 years and older, weighing at least 40 kg. Dupilumab is under clinical development for the treatment of chronic spontaneous urticaria and bullous pemphigoid, according to the manufacturers.
The studies were supported by Regeneron and Sanofi.
A version of this article first appeared on Medscape.com.
*Correction, 9/30/22: An earlier version of this article misstated results of one endpoint.
Children and COVID: September slowdown continues
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.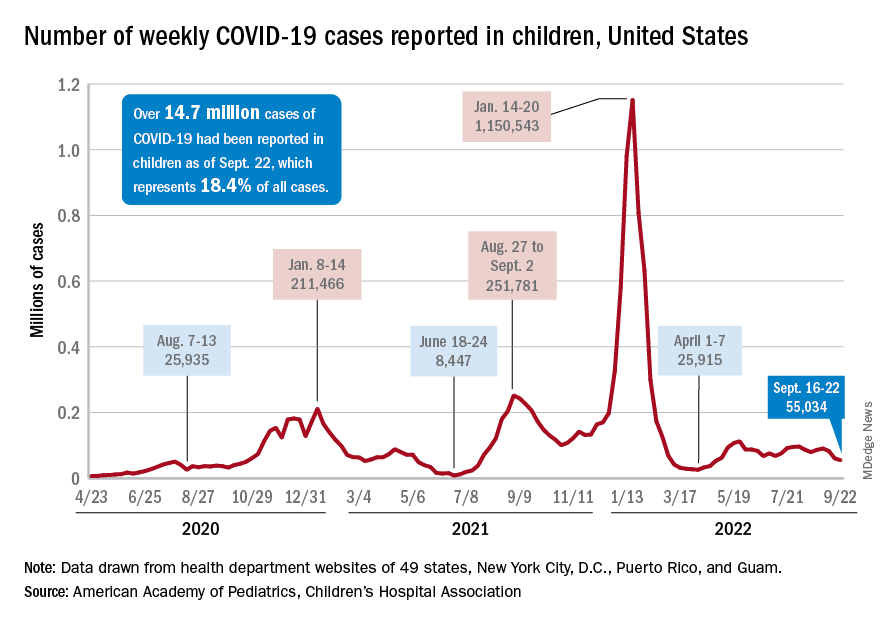
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
New COVID-19 cases and hospital admissions in children continue to decline, while the slow pace of vaccinations has not deterred manufacturers from seeking new emergency authorizations.
Since reaching a post-Omicron peak of 112,000 in late May, the number of weekly cases has fluctuated, with no stretch of increases or decreases lasting more than 4 weeks or the weekly count rising above 97,000 or falling lower than the current 55,000, according to state-level data collected by the American Academy of Pediatrics and the Children’s Hospital Association.
New admissions with confirmed COVID for children aged 0-17 years, which did not follow that pattern and instead continued to rise through the spring and early summer, have been largely decreasing in recent weeks and had fallen to 0.27 per 100,000 population as of Sept. 21 after peaking at 0.46 per 100,000 in late July, the Centers for Disease Control and Prevention reported. A similar decline has been seen for emergency department visits since late August.
The biggest vaccination news of the week came from Moderna and Pfizer and BioNTech, which are each seeking emergency authorization from the Food and Drug Administration for bivalent vaccine boosters that target both the original COVID strain and the BA.4 and BA.5 strains of Omicron.
“Pfizer’s booster would be for children 5 to 11 who have completed a primary vaccination series [and] Moderna’s updated boosters would be for children ages 6 to 17 who have completed a primary vaccination series,” WebMD said.
Although almost 61% of children aged 12-17 years are already fully vaccinated, that is not the case among those aged 5-11, of whom only 31.4% have completed the initial vaccine regimen. Since becoming eligible in June, just 1.9% of children under 5 years of age have been fully vaccinated and 6.3% have received at least one dose, the CDC said on its COVID Data Tracker. The latest data put the already boosted child populations at 28.8% for 12- to 17-year-olds and 14.8% in those aged 5-11.
About 51,000 children under age 5 years received their initial COVID vaccination during the week of Sept. 15-21, and the trend for that measure is one of gradual decline since July. Among the older children that same week, there were 28,000 initial vaccinations in the 5- to 11-year-olds and 18,000 for those aged 12-17, and activity in both age groups has largely stagnated since the spring, according to a separate AAP report based on CDC data.
FDA okays terlipressin (Terlivaz) injection for hepatorenal syndrome
The Food and Drug Administration has approved terlipressin (Terlivaz), the first and only drug approved for patients with hepatorenal syndrome (HRS).
HRS is characterized by progressive deterioration in kidney function in people with advanced liver disease.
Terlipressin is an injectable synthetic vasopressin analogue indicated for patients with HRS who are experiencing rapid deterioration of kidney function (type 1 HRS). The condition affects an estimated 35,000 Americans annually.
The safety and efficacy of terlipressin for type 1 HRS was assessed in the phase 3 CONFIRM trial, which involved 300 patients in the United States and Canada.
Patients received an injection of terlipressin (0.85 mg) or placebo every 6 hours for a maximum of 14 days. The dose was adjusted on the basis of changes in kidney function.
Twenty-nine percent of patients in the terlipressin group experienced improvement in kidney function, vs. 16% in the placebo group.
The CONFIRM trial met its primary endpoint of verified HRS reversal, defined as renal function improvement, avoidance of dialysis, and short-term survival (P = .012).
To achieve this endpoint, patients had to have two consecutive serum creatinine (SCr) values of ≤ 1.5 mg/dL at least 2 hours apart by day 14 or be discharged from the hospital.
The most commonly observed adverse reactions that occurred in at least 4% of patients treated with terlipressin were abdominal pain (19.5%), nausea (16%), respiratory failure (15.5%), diarrhea (13%), and dyspnea (12.5%).
Results of the CONFIRM trial were published in The New England Journal of Medicine.
“Diagnosing and treating HRS can be challenging, and every minute counts when managing patients who have it,” said Steven Romano, MD, executive vice president and chief scientific officer at Mallinckrodt, which makes the drug.
“Terlivaz gives physicians the first FDA-approved option for treating HRS patients with rapid reduction in kidney function that may help them improve kidney function and lessen the associated need for renal replacement therapy, such as dialysis,” Dr. Romano said.
The company plans to launch the product in the coming weeks.
The application for terlipressin for HRS was granted priority review and fast-track status, as well as orphan drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved terlipressin (Terlivaz), the first and only drug approved for patients with hepatorenal syndrome (HRS).
HRS is characterized by progressive deterioration in kidney function in people with advanced liver disease.
Terlipressin is an injectable synthetic vasopressin analogue indicated for patients with HRS who are experiencing rapid deterioration of kidney function (type 1 HRS). The condition affects an estimated 35,000 Americans annually.
The safety and efficacy of terlipressin for type 1 HRS was assessed in the phase 3 CONFIRM trial, which involved 300 patients in the United States and Canada.
Patients received an injection of terlipressin (0.85 mg) or placebo every 6 hours for a maximum of 14 days. The dose was adjusted on the basis of changes in kidney function.
Twenty-nine percent of patients in the terlipressin group experienced improvement in kidney function, vs. 16% in the placebo group.
The CONFIRM trial met its primary endpoint of verified HRS reversal, defined as renal function improvement, avoidance of dialysis, and short-term survival (P = .012).
To achieve this endpoint, patients had to have two consecutive serum creatinine (SCr) values of ≤ 1.5 mg/dL at least 2 hours apart by day 14 or be discharged from the hospital.
The most commonly observed adverse reactions that occurred in at least 4% of patients treated with terlipressin were abdominal pain (19.5%), nausea (16%), respiratory failure (15.5%), diarrhea (13%), and dyspnea (12.5%).
Results of the CONFIRM trial were published in The New England Journal of Medicine.
“Diagnosing and treating HRS can be challenging, and every minute counts when managing patients who have it,” said Steven Romano, MD, executive vice president and chief scientific officer at Mallinckrodt, which makes the drug.
“Terlivaz gives physicians the first FDA-approved option for treating HRS patients with rapid reduction in kidney function that may help them improve kidney function and lessen the associated need for renal replacement therapy, such as dialysis,” Dr. Romano said.
The company plans to launch the product in the coming weeks.
The application for terlipressin for HRS was granted priority review and fast-track status, as well as orphan drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved terlipressin (Terlivaz), the first and only drug approved for patients with hepatorenal syndrome (HRS).
HRS is characterized by progressive deterioration in kidney function in people with advanced liver disease.
Terlipressin is an injectable synthetic vasopressin analogue indicated for patients with HRS who are experiencing rapid deterioration of kidney function (type 1 HRS). The condition affects an estimated 35,000 Americans annually.
The safety and efficacy of terlipressin for type 1 HRS was assessed in the phase 3 CONFIRM trial, which involved 300 patients in the United States and Canada.
Patients received an injection of terlipressin (0.85 mg) or placebo every 6 hours for a maximum of 14 days. The dose was adjusted on the basis of changes in kidney function.
Twenty-nine percent of patients in the terlipressin group experienced improvement in kidney function, vs. 16% in the placebo group.
The CONFIRM trial met its primary endpoint of verified HRS reversal, defined as renal function improvement, avoidance of dialysis, and short-term survival (P = .012).
To achieve this endpoint, patients had to have two consecutive serum creatinine (SCr) values of ≤ 1.5 mg/dL at least 2 hours apart by day 14 or be discharged from the hospital.
The most commonly observed adverse reactions that occurred in at least 4% of patients treated with terlipressin were abdominal pain (19.5%), nausea (16%), respiratory failure (15.5%), diarrhea (13%), and dyspnea (12.5%).
Results of the CONFIRM trial were published in The New England Journal of Medicine.
“Diagnosing and treating HRS can be challenging, and every minute counts when managing patients who have it,” said Steven Romano, MD, executive vice president and chief scientific officer at Mallinckrodt, which makes the drug.
“Terlivaz gives physicians the first FDA-approved option for treating HRS patients with rapid reduction in kidney function that may help them improve kidney function and lessen the associated need for renal replacement therapy, such as dialysis,” Dr. Romano said.
The company plans to launch the product in the coming weeks.
The application for terlipressin for HRS was granted priority review and fast-track status, as well as orphan drug designation, which provides incentives to assist and encourage the development of drugs for rare diseases.
A version of this article first appeared on Medscape.com.
FDA OKs sodium thiosulfate injection to reduce ototoxicity risk in children with cancer
This approval makes sodium thiosulfate the first and only treatment FDA-approved in this area.
“Historically, there have been no approved treatments for preventing cisplatin-induced hearing loss,” said David R. Freyer, DO, of Children’s Hospital Los Angeles and primary investigator of one of the two trials, COG ACCL0431. The FDA’s approval “addresses an enormous unmet need for many children and young adults.”
The approval was based on safety and efficacy data from two multicenter open-label, randomized controlled phase 3 trials – SIOPEL 6 and COG ACCL0431 – comparing sodium thiosulfate plus a cisplatin-based regimen to a cisplatin-based regimen alone in pediatric patients. SIOPEL 6 included patients with standard risk hepatoblastoma, and COG ACCL0431 included pediatric patients with solid tumors.
In both studies, the incidence of hearing loss was significantly lower in the sodium thiosulfate group, compared with the cisplatin-only group. In SIOPEL 6, hearing loss of grade 1 or higher occurred in 33% of children (18 of 55) in the cisplatin–sodium thiosulfate group and 63% (29 of 46) in the cisplatin-only group, indicating a 48% lower incidence of hearing loss for those receiving sodium thiosulfate. In COG ACCL0431, hearing loss was identified in 28.6% of patients (14 of 49) receiving sodium thiosulfate, compared with 56.4% (31 of 55) in the control group, indicating a 69% lower risk for hearing loss in the sodium thiosulfate group.
The FDA reported the same overall trend but highlighted slightly different figures. In SIOPEL 6, hearing loss incidence occurred in 39% of patients (24 of 61) in the sodium thiosulfate arm versus 68% (36 of 53) in the control group; in COG ACCL0431, hearing loss incidence occurred among 44% of patients (17 of 39) in the sodium thiosulfate group versus 58% (22 of 38) in the control group.
The recommended dose is based on surface area according to body weight. Sodium thiosulfate is administered as an intravenous infusion over 15 minutes following cisplatin infusions that are 1 to 6 hours in duration.
Serious adverse reactions occurred in 40% of patients who received cisplatin–sodium thiosulfate in SIOPEL 6 and 36% of these patients in COG ACCL0431. The most common adverse reactions in the trials included vomiting, infection, nausea, decreased hemoglobin, hypernatremia, and hypokalemia.
A version of this article first appeared on Medscape.com.
This approval makes sodium thiosulfate the first and only treatment FDA-approved in this area.
“Historically, there have been no approved treatments for preventing cisplatin-induced hearing loss,” said David R. Freyer, DO, of Children’s Hospital Los Angeles and primary investigator of one of the two trials, COG ACCL0431. The FDA’s approval “addresses an enormous unmet need for many children and young adults.”
The approval was based on safety and efficacy data from two multicenter open-label, randomized controlled phase 3 trials – SIOPEL 6 and COG ACCL0431 – comparing sodium thiosulfate plus a cisplatin-based regimen to a cisplatin-based regimen alone in pediatric patients. SIOPEL 6 included patients with standard risk hepatoblastoma, and COG ACCL0431 included pediatric patients with solid tumors.
In both studies, the incidence of hearing loss was significantly lower in the sodium thiosulfate group, compared with the cisplatin-only group. In SIOPEL 6, hearing loss of grade 1 or higher occurred in 33% of children (18 of 55) in the cisplatin–sodium thiosulfate group and 63% (29 of 46) in the cisplatin-only group, indicating a 48% lower incidence of hearing loss for those receiving sodium thiosulfate. In COG ACCL0431, hearing loss was identified in 28.6% of patients (14 of 49) receiving sodium thiosulfate, compared with 56.4% (31 of 55) in the control group, indicating a 69% lower risk for hearing loss in the sodium thiosulfate group.
The FDA reported the same overall trend but highlighted slightly different figures. In SIOPEL 6, hearing loss incidence occurred in 39% of patients (24 of 61) in the sodium thiosulfate arm versus 68% (36 of 53) in the control group; in COG ACCL0431, hearing loss incidence occurred among 44% of patients (17 of 39) in the sodium thiosulfate group versus 58% (22 of 38) in the control group.
The recommended dose is based on surface area according to body weight. Sodium thiosulfate is administered as an intravenous infusion over 15 minutes following cisplatin infusions that are 1 to 6 hours in duration.
Serious adverse reactions occurred in 40% of patients who received cisplatin–sodium thiosulfate in SIOPEL 6 and 36% of these patients in COG ACCL0431. The most common adverse reactions in the trials included vomiting, infection, nausea, decreased hemoglobin, hypernatremia, and hypokalemia.
A version of this article first appeared on Medscape.com.
This approval makes sodium thiosulfate the first and only treatment FDA-approved in this area.
“Historically, there have been no approved treatments for preventing cisplatin-induced hearing loss,” said David R. Freyer, DO, of Children’s Hospital Los Angeles and primary investigator of one of the two trials, COG ACCL0431. The FDA’s approval “addresses an enormous unmet need for many children and young adults.”
The approval was based on safety and efficacy data from two multicenter open-label, randomized controlled phase 3 trials – SIOPEL 6 and COG ACCL0431 – comparing sodium thiosulfate plus a cisplatin-based regimen to a cisplatin-based regimen alone in pediatric patients. SIOPEL 6 included patients with standard risk hepatoblastoma, and COG ACCL0431 included pediatric patients with solid tumors.
In both studies, the incidence of hearing loss was significantly lower in the sodium thiosulfate group, compared with the cisplatin-only group. In SIOPEL 6, hearing loss of grade 1 or higher occurred in 33% of children (18 of 55) in the cisplatin–sodium thiosulfate group and 63% (29 of 46) in the cisplatin-only group, indicating a 48% lower incidence of hearing loss for those receiving sodium thiosulfate. In COG ACCL0431, hearing loss was identified in 28.6% of patients (14 of 49) receiving sodium thiosulfate, compared with 56.4% (31 of 55) in the control group, indicating a 69% lower risk for hearing loss in the sodium thiosulfate group.
The FDA reported the same overall trend but highlighted slightly different figures. In SIOPEL 6, hearing loss incidence occurred in 39% of patients (24 of 61) in the sodium thiosulfate arm versus 68% (36 of 53) in the control group; in COG ACCL0431, hearing loss incidence occurred among 44% of patients (17 of 39) in the sodium thiosulfate group versus 58% (22 of 38) in the control group.
The recommended dose is based on surface area according to body weight. Sodium thiosulfate is administered as an intravenous infusion over 15 minutes following cisplatin infusions that are 1 to 6 hours in duration.
Serious adverse reactions occurred in 40% of patients who received cisplatin–sodium thiosulfate in SIOPEL 6 and 36% of these patients in COG ACCL0431. The most common adverse reactions in the trials included vomiting, infection, nausea, decreased hemoglobin, hypernatremia, and hypokalemia.
A version of this article first appeared on Medscape.com.
FDA warns against cooking chicken in NyQuil
Called the “sleepy chicken challenge,” the trend tells people to cook chicken in NyQuil or similar over-the-counter cough and cold medications, which include ingredients such as acetaminophen, dextromethorphan, and doxylamine.
“The challenge sounds silly and unappetizing – and it is. But it could also be very unsafe,” the FDA said. “Boiling a medication can make it much more concentrated and change its properties in other ways.”
Even if someone doesn’t plan to eat the chicken, inhaling the vapors of the medication while it cooks could cause high levels of the drug to enter the body.
“It could also hurt your lungs,” the FDA said. “Put simply: Someone could take a dangerously high amount of the cough and cold medicine without even realizing it.”
This isn’t the first time that social media challenges involving medicine have gone viral. In a 2020 TikTok challenge, people were encouraged to take large doses of the allergy medicine diphenhydramine, called the “Benadryl challenge,” to cause hallucinations. The FDA received several reports of teens who were hospitalized or died, and it issued a warning about taking high doses of the drug.
“These video challenges, which often target youths, can harm people – and even cause death,” the FDA said. “Nonprescription (also called over-the-counter or OTC) drugs are readily available in many homes, making these challenges even more risky.”
In the latest warning, the FDA provided several ways for parents to make it less likely for children to do the social media challenges, such as locking up prescription and over-the-counter medications to prevent accidental overdoses. The FDA also encouraged parents and guardians to have open conversations with their children.
“Sit down with your children and discuss the dangers of misusing drugs and how social media trends can lead to real, sometimes irreversible, damage,” the FDA said. “Remind your children that overdoses can occur with OTC drugs as well as with prescription drugs.”
Following the FDA warning, the American Academy of Pediatrics also issued an advisory about social media trends. Some challenges, such as the ALS ice bucket challenge or the mannequin challenge, can be fun and positive activities. But medication-related challenges, such as the sleepy chicken and Benadryl challenges, can cause serious heart problems, seizures, coma, and even death.
“Teens’ brains are still developing. The part of the brain that handles rational thought, the prefrontal cortex, is not fully developed until the mid-20s,” the American Academy of Pediatrics said. “This means teens are naturally more impulsive and likely to act before thinking through all of the ramifications.”
Social media rewards outrageous behavior, it wrote, and the more outrageous the behavior, the more likely someone will get more engagement online.
“It’s a quick moving, impulsive environment, and the fear of losing out is real for teens,” the academy said. “What they will focus on is that a popular kid in class did this and got hundreds of likes and comments.”
The academy suggested that parents and guardians talk with teens about which challenges are trending on social media and at school.
“Sometimes kids are more willing to talk about their peers than themselves,” it said. “Asking questions about school trends, friends and fads may yield more answers than direct questions about their own activities.”
A version of this article first appeared on WebMD.com.
Called the “sleepy chicken challenge,” the trend tells people to cook chicken in NyQuil or similar over-the-counter cough and cold medications, which include ingredients such as acetaminophen, dextromethorphan, and doxylamine.
“The challenge sounds silly and unappetizing – and it is. But it could also be very unsafe,” the FDA said. “Boiling a medication can make it much more concentrated and change its properties in other ways.”
Even if someone doesn’t plan to eat the chicken, inhaling the vapors of the medication while it cooks could cause high levels of the drug to enter the body.
“It could also hurt your lungs,” the FDA said. “Put simply: Someone could take a dangerously high amount of the cough and cold medicine without even realizing it.”
This isn’t the first time that social media challenges involving medicine have gone viral. In a 2020 TikTok challenge, people were encouraged to take large doses of the allergy medicine diphenhydramine, called the “Benadryl challenge,” to cause hallucinations. The FDA received several reports of teens who were hospitalized or died, and it issued a warning about taking high doses of the drug.
“These video challenges, which often target youths, can harm people – and even cause death,” the FDA said. “Nonprescription (also called over-the-counter or OTC) drugs are readily available in many homes, making these challenges even more risky.”
In the latest warning, the FDA provided several ways for parents to make it less likely for children to do the social media challenges, such as locking up prescription and over-the-counter medications to prevent accidental overdoses. The FDA also encouraged parents and guardians to have open conversations with their children.
“Sit down with your children and discuss the dangers of misusing drugs and how social media trends can lead to real, sometimes irreversible, damage,” the FDA said. “Remind your children that overdoses can occur with OTC drugs as well as with prescription drugs.”
Following the FDA warning, the American Academy of Pediatrics also issued an advisory about social media trends. Some challenges, such as the ALS ice bucket challenge or the mannequin challenge, can be fun and positive activities. But medication-related challenges, such as the sleepy chicken and Benadryl challenges, can cause serious heart problems, seizures, coma, and even death.
“Teens’ brains are still developing. The part of the brain that handles rational thought, the prefrontal cortex, is not fully developed until the mid-20s,” the American Academy of Pediatrics said. “This means teens are naturally more impulsive and likely to act before thinking through all of the ramifications.”
Social media rewards outrageous behavior, it wrote, and the more outrageous the behavior, the more likely someone will get more engagement online.
“It’s a quick moving, impulsive environment, and the fear of losing out is real for teens,” the academy said. “What they will focus on is that a popular kid in class did this and got hundreds of likes and comments.”
The academy suggested that parents and guardians talk with teens about which challenges are trending on social media and at school.
“Sometimes kids are more willing to talk about their peers than themselves,” it said. “Asking questions about school trends, friends and fads may yield more answers than direct questions about their own activities.”
A version of this article first appeared on WebMD.com.
Called the “sleepy chicken challenge,” the trend tells people to cook chicken in NyQuil or similar over-the-counter cough and cold medications, which include ingredients such as acetaminophen, dextromethorphan, and doxylamine.
“The challenge sounds silly and unappetizing – and it is. But it could also be very unsafe,” the FDA said. “Boiling a medication can make it much more concentrated and change its properties in other ways.”
Even if someone doesn’t plan to eat the chicken, inhaling the vapors of the medication while it cooks could cause high levels of the drug to enter the body.
“It could also hurt your lungs,” the FDA said. “Put simply: Someone could take a dangerously high amount of the cough and cold medicine without even realizing it.”
This isn’t the first time that social media challenges involving medicine have gone viral. In a 2020 TikTok challenge, people were encouraged to take large doses of the allergy medicine diphenhydramine, called the “Benadryl challenge,” to cause hallucinations. The FDA received several reports of teens who were hospitalized or died, and it issued a warning about taking high doses of the drug.
“These video challenges, which often target youths, can harm people – and even cause death,” the FDA said. “Nonprescription (also called over-the-counter or OTC) drugs are readily available in many homes, making these challenges even more risky.”
In the latest warning, the FDA provided several ways for parents to make it less likely for children to do the social media challenges, such as locking up prescription and over-the-counter medications to prevent accidental overdoses. The FDA also encouraged parents and guardians to have open conversations with their children.
“Sit down with your children and discuss the dangers of misusing drugs and how social media trends can lead to real, sometimes irreversible, damage,” the FDA said. “Remind your children that overdoses can occur with OTC drugs as well as with prescription drugs.”
Following the FDA warning, the American Academy of Pediatrics also issued an advisory about social media trends. Some challenges, such as the ALS ice bucket challenge or the mannequin challenge, can be fun and positive activities. But medication-related challenges, such as the sleepy chicken and Benadryl challenges, can cause serious heart problems, seizures, coma, and even death.
“Teens’ brains are still developing. The part of the brain that handles rational thought, the prefrontal cortex, is not fully developed until the mid-20s,” the American Academy of Pediatrics said. “This means teens are naturally more impulsive and likely to act before thinking through all of the ramifications.”
Social media rewards outrageous behavior, it wrote, and the more outrageous the behavior, the more likely someone will get more engagement online.
“It’s a quick moving, impulsive environment, and the fear of losing out is real for teens,” the academy said. “What they will focus on is that a popular kid in class did this and got hundreds of likes and comments.”
The academy suggested that parents and guardians talk with teens about which challenges are trending on social media and at school.
“Sometimes kids are more willing to talk about their peers than themselves,” it said. “Asking questions about school trends, friends and fads may yield more answers than direct questions about their own activities.”
A version of this article first appeared on WebMD.com.
Children and COVID: Weekly cases drop to lowest level since April
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.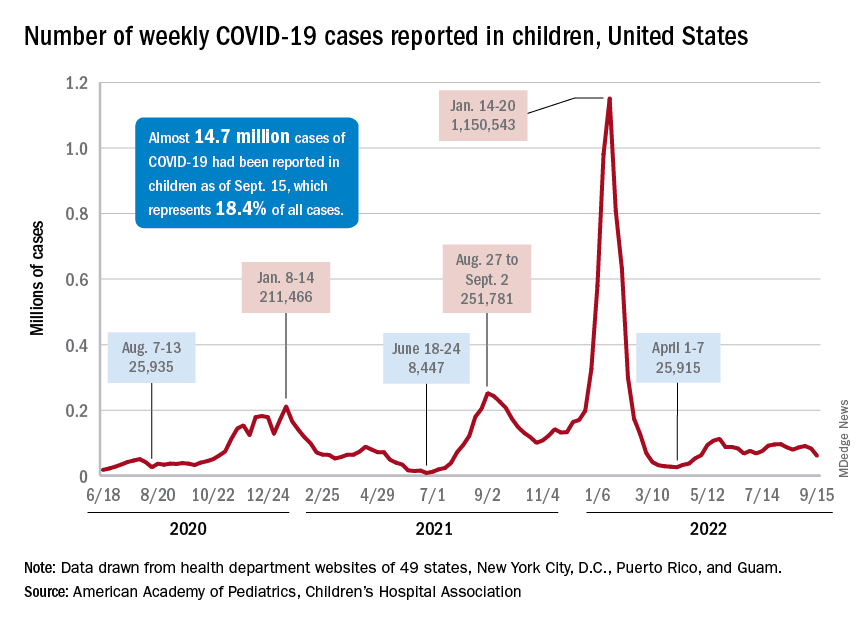
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.
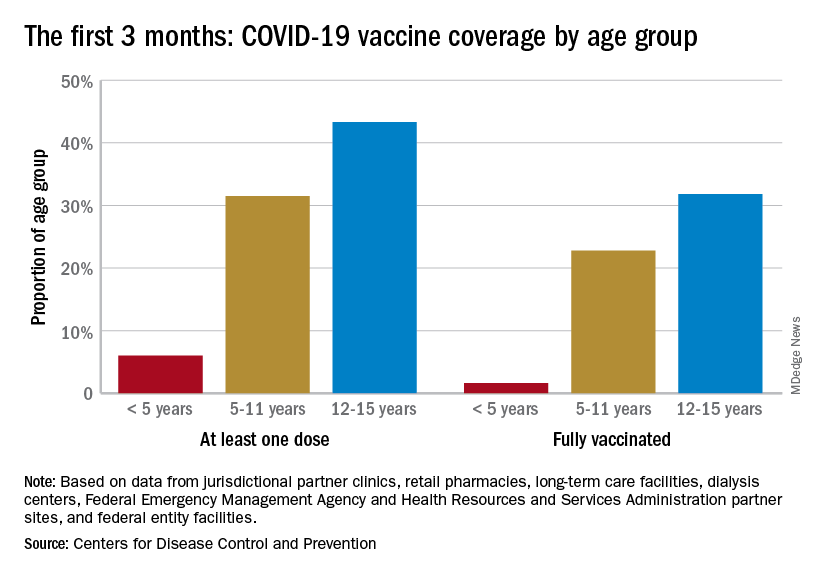
In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.

In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
A hefty decline in new COVID-19 cases among children resulted in the lowest weekly total since late April, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
, making for 2 consecutive weeks of declines after almost 91,000 cases were recorded for the week ending Sept. 1, the AAP and CHA said in their latest COVID report of state-level data.
The last time the weekly count was under 60,000 came during the week of April 22-28, when 53,000 were reported by state and territorial health departments in the midst of a 7-week stretch of rising cases. Since that streak ended in mid-May, however, “reported weekly cases have plateaued, fluctuating between a low, now of 60,300 cases and a high of about 112,000,” the AAP noted.
Emergency department visits and hospital admissions, which showed less fluctuation over the summer and more steady rise and fall, have both dropped in recent weeks and are now approaching late May/early June rates, according to data from the Centers for Disease Control and Prevention.
On Sept. 15, for example, ED visits for children under 12 years with diagnosed COVID were just 2.2% of all visits, lower than at any time since May 19 and down from a summer high of 6.8% in late July. Hospital admissions for children aged 0-17 years also rose steadily through June and July, reaching 0.46 per 100,000 population on July 30, but have since slipped to 0.29 per 100,000 as of Sept. 17, the CDC said on its COVID Data Tracker.
Vaccination continues to be a tough sell
Vaccination activity among the most recently eligible age group, in the meantime, remains tepid. Just 6.0% of children under age 5 had received at least one dose of COVID-19 vaccine as of Sept. 13, about 3 months since its final approval in June, and 1.6% were fully vaccinated. For the two older groups of children with separate vaccine approvals, 31.5% of those aged 5-11 years and 43.3% of those aged 12-15 had received at least one dose 3 months after their vaccinations began, the CDC data show.

In the 2 weeks ending Sept. 14, almost 59,000 children under age 5 received their initial COVID-19 vaccine dose, as did 28,000 5- to 11-year-olds and 14,000 children aged 12-17. Children under age 5 years represented almost 20% of all Americans getting a first dose during Sept. 1-14, compared with 9.7% for those aged 5-11 and 4.8% for the 12- to 17-year-olds, the CDC said.
At the state level, children under age 5 years in the District of Columbia, where 28% have received at least one dose, and Vermont, at 24%, are the most likely to be vaccinated. The states with the lowest rates in this age group are Alabama, Louisiana, and Mississippi, all of which are at 2%. Vermont and D.C. have the highest rates for ages 5-11 at 70% each, and Alabama (17%) is the lowest, while D.C. (100%), Rhode Island (99%), and Massachusetts (99%) are highest for children aged 12-17 years and Wyoming (41%) is the lowest, the AAP said in a separate report.
Children and COVID: New cases took a downturn in September
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.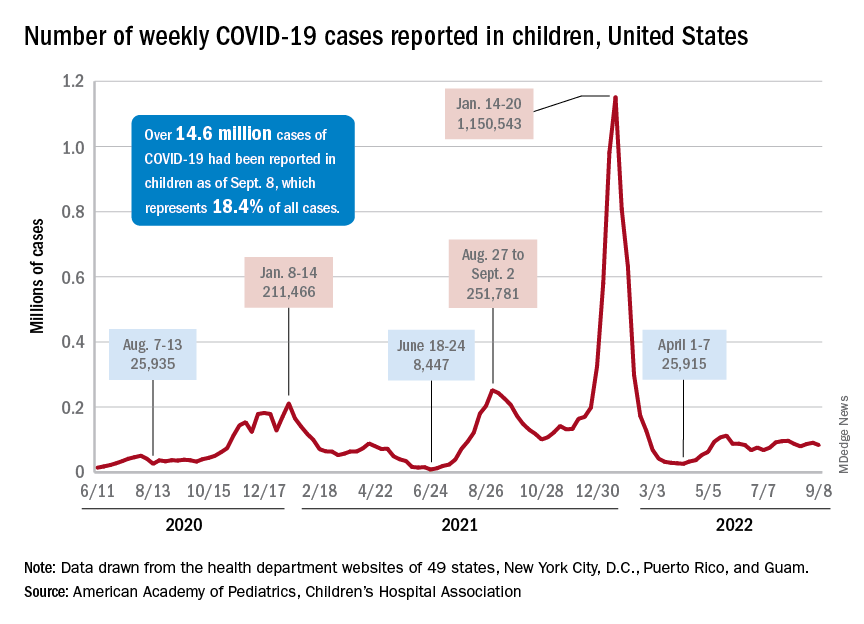
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.
After 2 weeks of increases in the number of new COVID-19 cases in children – a trend that just happened to coincide with the start of a new school year – there were fewer cases reported during the first full week of September, according to the American Academy of Pediatrics and the Children’s Hospital Association.
, the AAP and CHA said in their weekly COVID-19 report, noting also that seven states and the District of Columbia no longer update their online dashboards while others publish new data less often than every week.
The drop in new cases was accompanied by declines in emergency department visits and hospital admissions, both of which had shown some signs of resurgence in mid- to late August. The brief rise in ED visits seemed to be age-related, occurring in those aged 12 years and older but not in younger children, whose ED visit rate fell steadily through August. Through the first week of September, however, 7-day averages were down for both those aged 12-15 and for 16- to 17-year-olds, the Centers for Disease Control and Prevention reported.
The rate of new hospital admissions of children with confirmed COVID-19, available only for ages 0-17 years, has declined every day since Aug. 28, when it reached 0.44 per 100,000 population after a week of climbing, the CDC said on its COVID Data Tracker.
Cumulatively, about 156,000 children were hospitalized with COVID from Aug. 1, 2020 to Sept. 10, 2022, according to the CDC, which puts the total number of pediatric cases at just over 15 million and deaths at 1,778. Those last two figures represent 17.4% and about 0.4% of all U.S. cases and deaths. The AAP and CHA estimate that about 14.6 million child cases have been reported so far, which is 18.4% of cases in all ages.
Vaccinations are slowly adding up
On the prevention side of the health care system’s response to COVID, the CDC’s cumulative numbers looked like this as of Sept. 6:
- 1.1 million children under age 5 (about 5.8% of the age group) had received at least one dose of vaccine, and 280,000 (1.4%) were fully vaccinated.
- Almost 11 million (38.2%) children aged 5-11 had gotten one dose, and 8.9 million (31.1%) were fully vaccinated.
- 17.9 million (70.8%) children aged 12-17 had received at least one dose, and 15.3 million (60.5%) were fully vaccinated.
Over the 14 days ending Sept. 7, children aged 2-4 years made up the largest group (21.4%) of Americans getting their first vaccine doses, while those aged 5-11 years were the third largest age group at 16.7% of all vaccinees (25- to 49-year-olds were second). The situation was reversed for vaccine completion over the last 2 weeks: Those aged 5-11 were first at 24.7%, and the 2- to 4-year-olds were third at 16.7% (those aged 25-49 were second again), according to the COVID Data Tracker.