User login
Moderna vaccine more effective than Pfizer and J&J
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
the Centers for Disease Control and Protection has said.
“Among U.S. adults without immunocompromising conditions, vaccine effectiveness against COVID-19 hospitalization during March 11–Aug. 15, 2021, was higher for the Moderna vaccine (93%) than the Pfizer-BioNTech vaccine (88%) and the Janssen vaccine (71%),” the agency’s Morbidity and Mortality Weekly Report said. Janssen refers to the Johnson & Johnson vaccine.
The CDC said the data could help people make informed decisions.
“Understanding differences in VE [vaccine effectiveness] by vaccine product can guide individual choices and policy recommendations regarding vaccine boosters. All Food and Drug Administration–approved or authorized COVID-19 vaccines provide substantial protection against COVID-19 hospitalization,” the report said.
The study also broke down effectiveness for longer periods. Moderna came out on top again.
After 120 days, the Moderna vaccine provided 92% effectiveness against hospitalization, whereas the Pfizer vaccine’s effectiveness dropped to 77%, the CDC said. There was no similar calculation for the Johnson & Johnson vaccine.
The CDC studied 3,689 adults at 21 hospitals in 18 states who got the two-shot Pfizer or Moderna vaccine or the one-shot Johnson & Johnson vaccine between March and August.
The agency noted some factors that could have come into play.
“Differences in vaccine effectiveness between the Moderna and Pfizer-BioNTech vaccine might be due to higher mRNA content in the Moderna vaccine, differences in timing between doses (3 weeks for Pfizer-BioNTech vs. 4 weeks for Moderna), or possible differences between groups that received each vaccine that were not accounted for in the analysis,” the report said.
The CDC noted limitations in the findings. Children, immunocompromised adults, and vaccine effectiveness against COVID-19 that did not result in hospitalization were not studied.
Other studies have shown all three U.S. vaccines provide a high rate of protection against coronavirus.
A version of this article first appeared on WebMD.com.
FDA grants zanubrutinib an accelerated approval in marginal zone lymphoma
The U.S. Food and Drug Administration has granted an accelerated approval to zanubrutinib (Brukinsa) for the treatment of adult patients with relapsed or refractory (R/R) marginal zone lymphoma (MZL) who have received at least one anti-CD20–based regimen, the drug’s maker BeiGene announced in a press statement.
The drug works as an inhibitor of Bruton’s tyrosine kinase (BTK), which plays a critical role in B-cell–receptor signaling, a driver in the development of marginal zone lymphoma, according to the company.
The new approval comes just 2 weeks after the oral drug received an accelerated approval for the treatment of adult patients with Waldenström’s macroglobulinemia, a rare non-Hodgkin lymphoma. The drug also has an accelerated approval for treating mantle cell lymphoma in patients who have received at least one prior therapy and is being studied in the treatment of chronic lymphocytic leukemia.
The latest indication is based on results from two single-arm clinical trials, with overall response rate (ORR) as the primary endpoint.
In the multicenter, phase 2 MAGNOLIA trial, zanubrutinib “demonstrated impressive overall response and complete remission rates, with responses observed in all MZL subtypes,” said Stephen Opat, MBBS, of Monash University in Melbourne, lead principal investigator of the study. “In addition, this next-generation BTK inhibitor was well-tolerated in these patients, with low rate of discontinuation due to adverse reactions.”
In the MAGNOLIA trial, 66 patients with R/R MZL who had received at least one anti-CD20–based regimen were treated with zanubrutinib. Among the patients were 26 with extranodal subtype, 26 with nodal subtype, 12 with splenic subtype, and 4 with unknown subtype.
The ORR was 56% with a complete response rate of 20%, based on CT scan assessment.
In addition, the ORR was 67% with a complete response rate of 26%, based on PET-CT scan assessment.
The median duration of response (DoR) was not reached at the median follow-up time of 8.3 months, with 85% of responders still in remission at 12 months. Responses were observed in all MZL subtypes.
In an earlier, phase 1/2 trial of the agent, 20 patients were evaluated, including 9 with extranodal subtype, 5 with nodal subtype, and 6 with splenic subtype. Based on CT scan assessment, the ORR was 80% with a complete remission rate of 20%. The median DoR was not reached at the median follow-up time of 31.4 months, with 72% of responders still in remission at 12 months.
The most common (≥30%) adverse reactions, including laboratory abnormalities, in the pooled safety population of 847 patients were decreased neutrophil count, upper respiratory tract infection, decreased platelet count, hemorrhage, decreased lymphocyte count, rash, and musculoskeletal pain, said the company.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted an accelerated approval to zanubrutinib (Brukinsa) for the treatment of adult patients with relapsed or refractory (R/R) marginal zone lymphoma (MZL) who have received at least one anti-CD20–based regimen, the drug’s maker BeiGene announced in a press statement.
The drug works as an inhibitor of Bruton’s tyrosine kinase (BTK), which plays a critical role in B-cell–receptor signaling, a driver in the development of marginal zone lymphoma, according to the company.
The new approval comes just 2 weeks after the oral drug received an accelerated approval for the treatment of adult patients with Waldenström’s macroglobulinemia, a rare non-Hodgkin lymphoma. The drug also has an accelerated approval for treating mantle cell lymphoma in patients who have received at least one prior therapy and is being studied in the treatment of chronic lymphocytic leukemia.
The latest indication is based on results from two single-arm clinical trials, with overall response rate (ORR) as the primary endpoint.
In the multicenter, phase 2 MAGNOLIA trial, zanubrutinib “demonstrated impressive overall response and complete remission rates, with responses observed in all MZL subtypes,” said Stephen Opat, MBBS, of Monash University in Melbourne, lead principal investigator of the study. “In addition, this next-generation BTK inhibitor was well-tolerated in these patients, with low rate of discontinuation due to adverse reactions.”
In the MAGNOLIA trial, 66 patients with R/R MZL who had received at least one anti-CD20–based regimen were treated with zanubrutinib. Among the patients were 26 with extranodal subtype, 26 with nodal subtype, 12 with splenic subtype, and 4 with unknown subtype.
The ORR was 56% with a complete response rate of 20%, based on CT scan assessment.
In addition, the ORR was 67% with a complete response rate of 26%, based on PET-CT scan assessment.
The median duration of response (DoR) was not reached at the median follow-up time of 8.3 months, with 85% of responders still in remission at 12 months. Responses were observed in all MZL subtypes.
In an earlier, phase 1/2 trial of the agent, 20 patients were evaluated, including 9 with extranodal subtype, 5 with nodal subtype, and 6 with splenic subtype. Based on CT scan assessment, the ORR was 80% with a complete remission rate of 20%. The median DoR was not reached at the median follow-up time of 31.4 months, with 72% of responders still in remission at 12 months.
The most common (≥30%) adverse reactions, including laboratory abnormalities, in the pooled safety population of 847 patients were decreased neutrophil count, upper respiratory tract infection, decreased platelet count, hemorrhage, decreased lymphocyte count, rash, and musculoskeletal pain, said the company.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has granted an accelerated approval to zanubrutinib (Brukinsa) for the treatment of adult patients with relapsed or refractory (R/R) marginal zone lymphoma (MZL) who have received at least one anti-CD20–based regimen, the drug’s maker BeiGene announced in a press statement.
The drug works as an inhibitor of Bruton’s tyrosine kinase (BTK), which plays a critical role in B-cell–receptor signaling, a driver in the development of marginal zone lymphoma, according to the company.
The new approval comes just 2 weeks after the oral drug received an accelerated approval for the treatment of adult patients with Waldenström’s macroglobulinemia, a rare non-Hodgkin lymphoma. The drug also has an accelerated approval for treating mantle cell lymphoma in patients who have received at least one prior therapy and is being studied in the treatment of chronic lymphocytic leukemia.
The latest indication is based on results from two single-arm clinical trials, with overall response rate (ORR) as the primary endpoint.
In the multicenter, phase 2 MAGNOLIA trial, zanubrutinib “demonstrated impressive overall response and complete remission rates, with responses observed in all MZL subtypes,” said Stephen Opat, MBBS, of Monash University in Melbourne, lead principal investigator of the study. “In addition, this next-generation BTK inhibitor was well-tolerated in these patients, with low rate of discontinuation due to adverse reactions.”
In the MAGNOLIA trial, 66 patients with R/R MZL who had received at least one anti-CD20–based regimen were treated with zanubrutinib. Among the patients were 26 with extranodal subtype, 26 with nodal subtype, 12 with splenic subtype, and 4 with unknown subtype.
The ORR was 56% with a complete response rate of 20%, based on CT scan assessment.
In addition, the ORR was 67% with a complete response rate of 26%, based on PET-CT scan assessment.
The median duration of response (DoR) was not reached at the median follow-up time of 8.3 months, with 85% of responders still in remission at 12 months. Responses were observed in all MZL subtypes.
In an earlier, phase 1/2 trial of the agent, 20 patients were evaluated, including 9 with extranodal subtype, 5 with nodal subtype, and 6 with splenic subtype. Based on CT scan assessment, the ORR was 80% with a complete remission rate of 20%. The median DoR was not reached at the median follow-up time of 31.4 months, with 72% of responders still in remission at 12 months.
The most common (≥30%) adverse reactions, including laboratory abnormalities, in the pooled safety population of 847 patients were decreased neutrophil count, upper respiratory tract infection, decreased platelet count, hemorrhage, decreased lymphocyte count, rash, and musculoskeletal pain, said the company.
A version of this article first appeared on Medscape.com.
Even those who just test positive at more risk for long COVID: CDC
Long-term symptoms, like those linked with COVID-19, were common in people who had even just a single positive test, new Centers for Disease Control and Prevention data show.
The data show that symptoms in this group – including fatigue, cough, and headache – tended to last for more than a month.
Frequency of symptoms in people with a positive test was 1.5 times higher, compared with people whose tests had always been negative, according to the research published in the CDC’s latest Morbidity and Mortality Weekly Report.
Lead author Valentine Wanga, PhD, with the CDC’s COVID-19 response team, and colleagues conducted a non–probability-based internet panel survey of about 6,000 U.S. adults to assess long-term symptoms often associated with COVID-19 among those who had ever tested positive or always tested negative for COVID-19 between January 2020 and April 2021.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., said in an interview that this research “establishes more securely than before that you don’t have to be hospitalized with COVID in order to develop long COVID symptoms.”
That’s better known among infectious disease experts, he said, but added that “this survey really gives a firm database for that.”
Study results
The study’s results showed that, compared with respondents who had a negative test result, those who received a positive result reported a significantly higher prevalence of any long-term symptom (65.9% vs. 42.9%), fatigue (22.5% vs. 12.0%), change in sense of smell or taste (17.3% vs. 1.7%), shortness of breath (15.5% vs. 5.2%), cough (14.5% vs. 4.9%), and headache (13.8% vs. 9.9%).
More people who had a positive test result (76.2%) reported persistence for more than a month of at least one initially occurring symptom, compared with those whose test results were always negative (69.6%).
The numbers are further proof, Dr. Schaffner said, that COVID not only will be an acute stressor on the health care system but patients with long COVID will need help with managing care for the long term.
“We still don’t know what the COVID virus does that results in these long COVID symptoms,” he said. Vanderbilt and many other institutions have developed “long COVID” centers as a testament to how important the problem is.
Long COVID symptoms are not well understood and most studies have looked at the effects from patients who had been hospitalized with COVID-19.
In this survey, respondents self-reported whether they had ever had a positive SARS-CoV-2 test result (698), always received a negative test result (2,437), or never were tested for SARS-CoV-2 (2,750).
Compared with those who always tested negative, a larger proportion of those who tested positive (28.7% vs. 15.7%) reported believing that receiving a COVID-19 vaccine made their long-term symptoms better. No difference was found in reported beliefs that a vaccine made long-term symptoms worse.
Dr. Schaffner said he found that survey result interesting, but said that is not backed up by current data and would need further study.
“I would treat that with great caution,” he said. “I’m not dismissing it, but you can’t take that at face value. All of us who get sick and those of us who care for people who are sick – if there’s an intervention, we all hope for the best. We’re being optimistic. It’s when you do a randomized, double-blind, placebo-controlled study that you can find out whether your instincts or hopes were correct.”
The authors said that findings can inform public health preparedness, help guide care for people with post-COVID conditions, and help make the case for vaccines.
The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term symptoms, like those linked with COVID-19, were common in people who had even just a single positive test, new Centers for Disease Control and Prevention data show.
The data show that symptoms in this group – including fatigue, cough, and headache – tended to last for more than a month.
Frequency of symptoms in people with a positive test was 1.5 times higher, compared with people whose tests had always been negative, according to the research published in the CDC’s latest Morbidity and Mortality Weekly Report.
Lead author Valentine Wanga, PhD, with the CDC’s COVID-19 response team, and colleagues conducted a non–probability-based internet panel survey of about 6,000 U.S. adults to assess long-term symptoms often associated with COVID-19 among those who had ever tested positive or always tested negative for COVID-19 between January 2020 and April 2021.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., said in an interview that this research “establishes more securely than before that you don’t have to be hospitalized with COVID in order to develop long COVID symptoms.”
That’s better known among infectious disease experts, he said, but added that “this survey really gives a firm database for that.”
Study results
The study’s results showed that, compared with respondents who had a negative test result, those who received a positive result reported a significantly higher prevalence of any long-term symptom (65.9% vs. 42.9%), fatigue (22.5% vs. 12.0%), change in sense of smell or taste (17.3% vs. 1.7%), shortness of breath (15.5% vs. 5.2%), cough (14.5% vs. 4.9%), and headache (13.8% vs. 9.9%).
More people who had a positive test result (76.2%) reported persistence for more than a month of at least one initially occurring symptom, compared with those whose test results were always negative (69.6%).
The numbers are further proof, Dr. Schaffner said, that COVID not only will be an acute stressor on the health care system but patients with long COVID will need help with managing care for the long term.
“We still don’t know what the COVID virus does that results in these long COVID symptoms,” he said. Vanderbilt and many other institutions have developed “long COVID” centers as a testament to how important the problem is.
Long COVID symptoms are not well understood and most studies have looked at the effects from patients who had been hospitalized with COVID-19.
In this survey, respondents self-reported whether they had ever had a positive SARS-CoV-2 test result (698), always received a negative test result (2,437), or never were tested for SARS-CoV-2 (2,750).
Compared with those who always tested negative, a larger proportion of those who tested positive (28.7% vs. 15.7%) reported believing that receiving a COVID-19 vaccine made their long-term symptoms better. No difference was found in reported beliefs that a vaccine made long-term symptoms worse.
Dr. Schaffner said he found that survey result interesting, but said that is not backed up by current data and would need further study.
“I would treat that with great caution,” he said. “I’m not dismissing it, but you can’t take that at face value. All of us who get sick and those of us who care for people who are sick – if there’s an intervention, we all hope for the best. We’re being optimistic. It’s when you do a randomized, double-blind, placebo-controlled study that you can find out whether your instincts or hopes were correct.”
The authors said that findings can inform public health preparedness, help guide care for people with post-COVID conditions, and help make the case for vaccines.
The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term symptoms, like those linked with COVID-19, were common in people who had even just a single positive test, new Centers for Disease Control and Prevention data show.
The data show that symptoms in this group – including fatigue, cough, and headache – tended to last for more than a month.
Frequency of symptoms in people with a positive test was 1.5 times higher, compared with people whose tests had always been negative, according to the research published in the CDC’s latest Morbidity and Mortality Weekly Report.
Lead author Valentine Wanga, PhD, with the CDC’s COVID-19 response team, and colleagues conducted a non–probability-based internet panel survey of about 6,000 U.S. adults to assess long-term symptoms often associated with COVID-19 among those who had ever tested positive or always tested negative for COVID-19 between January 2020 and April 2021.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., said in an interview that this research “establishes more securely than before that you don’t have to be hospitalized with COVID in order to develop long COVID symptoms.”
That’s better known among infectious disease experts, he said, but added that “this survey really gives a firm database for that.”
Study results
The study’s results showed that, compared with respondents who had a negative test result, those who received a positive result reported a significantly higher prevalence of any long-term symptom (65.9% vs. 42.9%), fatigue (22.5% vs. 12.0%), change in sense of smell or taste (17.3% vs. 1.7%), shortness of breath (15.5% vs. 5.2%), cough (14.5% vs. 4.9%), and headache (13.8% vs. 9.9%).
More people who had a positive test result (76.2%) reported persistence for more than a month of at least one initially occurring symptom, compared with those whose test results were always negative (69.6%).
The numbers are further proof, Dr. Schaffner said, that COVID not only will be an acute stressor on the health care system but patients with long COVID will need help with managing care for the long term.
“We still don’t know what the COVID virus does that results in these long COVID symptoms,” he said. Vanderbilt and many other institutions have developed “long COVID” centers as a testament to how important the problem is.
Long COVID symptoms are not well understood and most studies have looked at the effects from patients who had been hospitalized with COVID-19.
In this survey, respondents self-reported whether they had ever had a positive SARS-CoV-2 test result (698), always received a negative test result (2,437), or never were tested for SARS-CoV-2 (2,750).
Compared with those who always tested negative, a larger proportion of those who tested positive (28.7% vs. 15.7%) reported believing that receiving a COVID-19 vaccine made their long-term symptoms better. No difference was found in reported beliefs that a vaccine made long-term symptoms worse.
Dr. Schaffner said he found that survey result interesting, but said that is not backed up by current data and would need further study.
“I would treat that with great caution,” he said. “I’m not dismissing it, but you can’t take that at face value. All of us who get sick and those of us who care for people who are sick – if there’s an intervention, we all hope for the best. We’re being optimistic. It’s when you do a randomized, double-blind, placebo-controlled study that you can find out whether your instincts or hopes were correct.”
The authors said that findings can inform public health preparedness, help guide care for people with post-COVID conditions, and help make the case for vaccines.
The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Three JAK inhibitors get boxed warnings, modified indications
The arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) poses an increased risk of serious cardiac events such as heart attack or stroke, cancer, blood clots, and death, the Food and Drug Administration announced Sept 1.
Manufacturers of this drug along with other Janus kinase (JAK) inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) must update their boxed warnings to include information about these health risks. The FDA made the determination after new study data from Pfizer, which manufacturers Xeljanz, found an association between a lower dose of Xeljanz and increased risk of blood clots and death.
“Recommendations for healthcare professionals will include consideration of the benefits and risks for the individual patient prior to initiating or continuing therapy,” the agency stated.
The FDA is limiting all approved uses of these three medications to patients who have not responded well to tumor necrosis factor (TNF) blockers to ensure their benefits outweigh their risks. Tofacitinib is indicated for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA. The FDA included baricitinib and upadacitinib in the warning because of the similar properties they share with tofacitinib, even though they haven’t been studied as extensively.
“We believe this update will bring important clarity for healthcare plans on the risk/benefit profile of Xeljanz, which is a medicine informed by more clinical data than any other JAK inhibitor,” Pfizer said in a statement.
Investigators for the ORAL Surveillance trial compared two doses of tofacitinib (5 mg twice daily and 10 mg twice daily) with TNF blockers in patients with rheumatoid arthritis who were aged 50 years or older with at least one additional cardiovascular risk factor.
For both dose regimens of tofacitinib, they found an increased risk of major adverse cardiovascular events, malignancies, thrombosis, and death compared with the TNF blocker regimen. In addition, rates of lung cancers and lymphomas were higher with tofacitinib. In trial data released earlier this year, Pfizer revealed that the tofacitinib group had a much higher incidence of adjudicated malignancies compared with the TNF blocker group (1.13 vs. 0.77 per 100 person-years; hazard ratio, 1.48; 95% confidence interval, 1.04-2.09).
Impact on clinical practice
Physicians treating patients who have rheumatoid arthritis with tofacitinib may initially decrease prescriptions following the FDA’s drug safety communication, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy) – particularly those with a principal mechanism of action slightly different from that of tofacitinib, he added.
“Tofacitinib is principally a JAK 1,3 inhibitor at usual concentrations, whereas upadacitinib and baricitinib are JAK 1,2 inhibitors. Thus, I speculate that the tofacitinib prescriptions will go down more than the upadacitinib and baricitinib prescriptions,” he said in an interview.
Some patients may also be worried about taking tofacitinib, particularly those with previous events or predisposing conditions, Dr. Furst noted.
“First and foremost, I think we need to actually look at the data in a publication rather than just an FDA statement before making huge changes in our practice,” he advised.
“I am looking forward to the data finally being published ... It’s interesting that the full data still isn’t really out there beyond the press releases and an abstract. I think there’s a lot more to learn about how these drugs work and who is really at risk for harmful events,” said Alexis R. Ogdie, MD, MSCE, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia.
Pfizer’s data also may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
“I think many rheumatologists have already taken this information in, and begun to incorporate it into their discussions with their patients” since it has been over a year since the first public release of information about the ORAL Surveillance trial, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego. “I don’t know that it will affect the approvals, but it will impact their labels.”
Wariness to prescribing tofacitinib may be lower for patients younger than those in the ORAL Surveillance trial without additional cardiovascular risk factors who are taking tofacitinib for non-RA indications, said gastroenterologist Miguel Regueiro, MD.
“The JAK inhibitor warning by the FDA is an important consideration for any prescriber or patient. The risk of cardiovascular disease and venous thromboembolism with this class of medicine appears higher in older rheumatoid arthritis patients with underlying cardiovascular disease. While the warning applies to all JAK inhibitors and likely the newer selective JAK inhibitors to come, we need to weigh the risk and benefit based on the indication for prescribing,” said Dr. Regueiro, chair of the Digestive Disease and Surgery Institute and of the department of gastroenterology, hepatology and nutrition at the Cleveland Clinic in Ohio.
“I do think that there will be a heightened awareness and wariness for older RA patients and for the prescribers. However, for inflammatory bowel disease (and other non-RA indications), it does not appear that the risk for cardiovascular disease and VTE are significantly increased. To that end, in my own practice, I still use tofacitinib for ulcerative colitis and will do the same for the selective JAK inhibitors to come for IBD. Of course, as with any medication, we need to have discussions with our patients, alert them to potential side effects and have an open line of communication for any questions or concerns.”
Gastroenterologist Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, thought that while patients with RA have many other treatment options besides JAK inhibitors, fewer options available to patients with IBD “may motivate the use of oral [sphingosine-1-phosphate receptor modulator] agents such as ozanimod, although IBD patients are younger and [have fewer] MACE risk factors than RA patients, so absolute risk is very small in the ulcerative colitis population.”
Pfizer’s data may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
The agency’s decision corroborates an earlier 2019 warning about the increased risk of blood clots and of death in patients with ulcerative colitis taking 10 mg tofacitinib twice daily.
The FDA said that two other JAK inhibitors, ruxolitinib (Jakafi) and fedratinib (Inrebic), are not indicated for the treatment of arthritis and other inflammatory conditions, and so are not a part of the updates being required.
Baricitinib, abrocitinib, and upadacitinib are currently under FDA review for treating atopic dermatitis (AD); a topical formulation of the JAK1/2 inhibitor ruxolitinib is under review for treating AD. Reviews for all 4 have been extended. In September 2020, baricitinib was approved for treating moderate to severe AD in Europe, at a dose of 4 mg once a day, with recommendations that the dose can be reduced to 2 mg once a day when the disease is under control, and that the dose may need to be reduced in patients with impaired kidney function, those with an increased risk of infections, and those older than aged 75 years.
In an interview, Jacob Thyssen, MD, PhD, professor of dermatology at the University of Copenhagen, said that in the EU, there has been “extensive education” about cardiovascular risks with baricitinib “and it is my impression that payers and dermatologists in Europe are confident that it is safe to use in AD.” In addition, there has been an emphasis on the differences in cardiovascular risk factors between RA and AD patients, “given that the latter group is generally young and lean.” In the United States, he added, it will be interesting to see which doses of the JAK inhibitors will be approved for AD.
Dr. Thyssen disclosed that he is a speaker, advisory board member and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
*This story was updated 9/3/21 and 9/6/2021.
A version of this article first appeared on Medscape.com.
The arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) poses an increased risk of serious cardiac events such as heart attack or stroke, cancer, blood clots, and death, the Food and Drug Administration announced Sept 1.
Manufacturers of this drug along with other Janus kinase (JAK) inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) must update their boxed warnings to include information about these health risks. The FDA made the determination after new study data from Pfizer, which manufacturers Xeljanz, found an association between a lower dose of Xeljanz and increased risk of blood clots and death.
“Recommendations for healthcare professionals will include consideration of the benefits and risks for the individual patient prior to initiating or continuing therapy,” the agency stated.
The FDA is limiting all approved uses of these three medications to patients who have not responded well to tumor necrosis factor (TNF) blockers to ensure their benefits outweigh their risks. Tofacitinib is indicated for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA. The FDA included baricitinib and upadacitinib in the warning because of the similar properties they share with tofacitinib, even though they haven’t been studied as extensively.
“We believe this update will bring important clarity for healthcare plans on the risk/benefit profile of Xeljanz, which is a medicine informed by more clinical data than any other JAK inhibitor,” Pfizer said in a statement.
Investigators for the ORAL Surveillance trial compared two doses of tofacitinib (5 mg twice daily and 10 mg twice daily) with TNF blockers in patients with rheumatoid arthritis who were aged 50 years or older with at least one additional cardiovascular risk factor.
For both dose regimens of tofacitinib, they found an increased risk of major adverse cardiovascular events, malignancies, thrombosis, and death compared with the TNF blocker regimen. In addition, rates of lung cancers and lymphomas were higher with tofacitinib. In trial data released earlier this year, Pfizer revealed that the tofacitinib group had a much higher incidence of adjudicated malignancies compared with the TNF blocker group (1.13 vs. 0.77 per 100 person-years; hazard ratio, 1.48; 95% confidence interval, 1.04-2.09).
Impact on clinical practice
Physicians treating patients who have rheumatoid arthritis with tofacitinib may initially decrease prescriptions following the FDA’s drug safety communication, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy) – particularly those with a principal mechanism of action slightly different from that of tofacitinib, he added.
“Tofacitinib is principally a JAK 1,3 inhibitor at usual concentrations, whereas upadacitinib and baricitinib are JAK 1,2 inhibitors. Thus, I speculate that the tofacitinib prescriptions will go down more than the upadacitinib and baricitinib prescriptions,” he said in an interview.
Some patients may also be worried about taking tofacitinib, particularly those with previous events or predisposing conditions, Dr. Furst noted.
“First and foremost, I think we need to actually look at the data in a publication rather than just an FDA statement before making huge changes in our practice,” he advised.
“I am looking forward to the data finally being published ... It’s interesting that the full data still isn’t really out there beyond the press releases and an abstract. I think there’s a lot more to learn about how these drugs work and who is really at risk for harmful events,” said Alexis R. Ogdie, MD, MSCE, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia.
Pfizer’s data also may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
“I think many rheumatologists have already taken this information in, and begun to incorporate it into their discussions with their patients” since it has been over a year since the first public release of information about the ORAL Surveillance trial, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego. “I don’t know that it will affect the approvals, but it will impact their labels.”
Wariness to prescribing tofacitinib may be lower for patients younger than those in the ORAL Surveillance trial without additional cardiovascular risk factors who are taking tofacitinib for non-RA indications, said gastroenterologist Miguel Regueiro, MD.
“The JAK inhibitor warning by the FDA is an important consideration for any prescriber or patient. The risk of cardiovascular disease and venous thromboembolism with this class of medicine appears higher in older rheumatoid arthritis patients with underlying cardiovascular disease. While the warning applies to all JAK inhibitors and likely the newer selective JAK inhibitors to come, we need to weigh the risk and benefit based on the indication for prescribing,” said Dr. Regueiro, chair of the Digestive Disease and Surgery Institute and of the department of gastroenterology, hepatology and nutrition at the Cleveland Clinic in Ohio.
“I do think that there will be a heightened awareness and wariness for older RA patients and for the prescribers. However, for inflammatory bowel disease (and other non-RA indications), it does not appear that the risk for cardiovascular disease and VTE are significantly increased. To that end, in my own practice, I still use tofacitinib for ulcerative colitis and will do the same for the selective JAK inhibitors to come for IBD. Of course, as with any medication, we need to have discussions with our patients, alert them to potential side effects and have an open line of communication for any questions or concerns.”
Gastroenterologist Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, thought that while patients with RA have many other treatment options besides JAK inhibitors, fewer options available to patients with IBD “may motivate the use of oral [sphingosine-1-phosphate receptor modulator] agents such as ozanimod, although IBD patients are younger and [have fewer] MACE risk factors than RA patients, so absolute risk is very small in the ulcerative colitis population.”
Pfizer’s data may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
The agency’s decision corroborates an earlier 2019 warning about the increased risk of blood clots and of death in patients with ulcerative colitis taking 10 mg tofacitinib twice daily.
The FDA said that two other JAK inhibitors, ruxolitinib (Jakafi) and fedratinib (Inrebic), are not indicated for the treatment of arthritis and other inflammatory conditions, and so are not a part of the updates being required.
Baricitinib, abrocitinib, and upadacitinib are currently under FDA review for treating atopic dermatitis (AD); a topical formulation of the JAK1/2 inhibitor ruxolitinib is under review for treating AD. Reviews for all 4 have been extended. In September 2020, baricitinib was approved for treating moderate to severe AD in Europe, at a dose of 4 mg once a day, with recommendations that the dose can be reduced to 2 mg once a day when the disease is under control, and that the dose may need to be reduced in patients with impaired kidney function, those with an increased risk of infections, and those older than aged 75 years.
In an interview, Jacob Thyssen, MD, PhD, professor of dermatology at the University of Copenhagen, said that in the EU, there has been “extensive education” about cardiovascular risks with baricitinib “and it is my impression that payers and dermatologists in Europe are confident that it is safe to use in AD.” In addition, there has been an emphasis on the differences in cardiovascular risk factors between RA and AD patients, “given that the latter group is generally young and lean.” In the United States, he added, it will be interesting to see which doses of the JAK inhibitors will be approved for AD.
Dr. Thyssen disclosed that he is a speaker, advisory board member and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
*This story was updated 9/3/21 and 9/6/2021.
A version of this article first appeared on Medscape.com.
The arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR) poses an increased risk of serious cardiac events such as heart attack or stroke, cancer, blood clots, and death, the Food and Drug Administration announced Sept 1.
Manufacturers of this drug along with other Janus kinase (JAK) inhibitors baricitinib (Olumiant) and upadacitinib (Rinvoq) must update their boxed warnings to include information about these health risks. The FDA made the determination after new study data from Pfizer, which manufacturers Xeljanz, found an association between a lower dose of Xeljanz and increased risk of blood clots and death.
“Recommendations for healthcare professionals will include consideration of the benefits and risks for the individual patient prior to initiating or continuing therapy,” the agency stated.
The FDA is limiting all approved uses of these three medications to patients who have not responded well to tumor necrosis factor (TNF) blockers to ensure their benefits outweigh their risks. Tofacitinib is indicated for rheumatoid arthritis, psoriatic arthritis, ulcerative colitis, and polyarticular course juvenile idiopathic arthritis. Baricitinib and upadacitinib are approved only for RA. The FDA included baricitinib and upadacitinib in the warning because of the similar properties they share with tofacitinib, even though they haven’t been studied as extensively.
“We believe this update will bring important clarity for healthcare plans on the risk/benefit profile of Xeljanz, which is a medicine informed by more clinical data than any other JAK inhibitor,” Pfizer said in a statement.
Investigators for the ORAL Surveillance trial compared two doses of tofacitinib (5 mg twice daily and 10 mg twice daily) with TNF blockers in patients with rheumatoid arthritis who were aged 50 years or older with at least one additional cardiovascular risk factor.
For both dose regimens of tofacitinib, they found an increased risk of major adverse cardiovascular events, malignancies, thrombosis, and death compared with the TNF blocker regimen. In addition, rates of lung cancers and lymphomas were higher with tofacitinib. In trial data released earlier this year, Pfizer revealed that the tofacitinib group had a much higher incidence of adjudicated malignancies compared with the TNF blocker group (1.13 vs. 0.77 per 100 person-years; hazard ratio, 1.48; 95% confidence interval, 1.04-2.09).
Impact on clinical practice
Physicians treating patients who have rheumatoid arthritis with tofacitinib may initially decrease prescriptions following the FDA’s drug safety communication, said Daniel E. Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, adjunct professor at the University of Washington, Seattle, and a research professor at the University of Florence (Italy) – particularly those with a principal mechanism of action slightly different from that of tofacitinib, he added.
“Tofacitinib is principally a JAK 1,3 inhibitor at usual concentrations, whereas upadacitinib and baricitinib are JAK 1,2 inhibitors. Thus, I speculate that the tofacitinib prescriptions will go down more than the upadacitinib and baricitinib prescriptions,” he said in an interview.
Some patients may also be worried about taking tofacitinib, particularly those with previous events or predisposing conditions, Dr. Furst noted.
“First and foremost, I think we need to actually look at the data in a publication rather than just an FDA statement before making huge changes in our practice,” he advised.
“I am looking forward to the data finally being published ... It’s interesting that the full data still isn’t really out there beyond the press releases and an abstract. I think there’s a lot more to learn about how these drugs work and who is really at risk for harmful events,” said Alexis R. Ogdie, MD, MSCE, associate professor of medicine and epidemiology at the University of Pennsylvania, Philadelphia.
Pfizer’s data also may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
“I think many rheumatologists have already taken this information in, and begun to incorporate it into their discussions with their patients” since it has been over a year since the first public release of information about the ORAL Surveillance trial, said Arthur Kavanaugh, MD, professor of medicine at the University of California, San Diego. “I don’t know that it will affect the approvals, but it will impact their labels.”
Wariness to prescribing tofacitinib may be lower for patients younger than those in the ORAL Surveillance trial without additional cardiovascular risk factors who are taking tofacitinib for non-RA indications, said gastroenterologist Miguel Regueiro, MD.
“The JAK inhibitor warning by the FDA is an important consideration for any prescriber or patient. The risk of cardiovascular disease and venous thromboembolism with this class of medicine appears higher in older rheumatoid arthritis patients with underlying cardiovascular disease. While the warning applies to all JAK inhibitors and likely the newer selective JAK inhibitors to come, we need to weigh the risk and benefit based on the indication for prescribing,” said Dr. Regueiro, chair of the Digestive Disease and Surgery Institute and of the department of gastroenterology, hepatology and nutrition at the Cleveland Clinic in Ohio.
“I do think that there will be a heightened awareness and wariness for older RA patients and for the prescribers. However, for inflammatory bowel disease (and other non-RA indications), it does not appear that the risk for cardiovascular disease and VTE are significantly increased. To that end, in my own practice, I still use tofacitinib for ulcerative colitis and will do the same for the selective JAK inhibitors to come for IBD. Of course, as with any medication, we need to have discussions with our patients, alert them to potential side effects and have an open line of communication for any questions or concerns.”
Gastroenterologist Stephen Hanauer, MD, professor of medicine at Northwestern University, Chicago, thought that while patients with RA have many other treatment options besides JAK inhibitors, fewer options available to patients with IBD “may motivate the use of oral [sphingosine-1-phosphate receptor modulator] agents such as ozanimod, although IBD patients are younger and [have fewer] MACE risk factors than RA patients, so absolute risk is very small in the ulcerative colitis population.”
Pfizer’s data may be affecting FDA approvals of other JAK inhibitors. This past summer, AbbVie and Eli Lilly stated that the FDA’s ongoing assessment of the safety trial was delaying the agency’s decisions about expanding use of their respective drugs upadacitinib and baricitinib.
The agency’s decision corroborates an earlier 2019 warning about the increased risk of blood clots and of death in patients with ulcerative colitis taking 10 mg tofacitinib twice daily.
The FDA said that two other JAK inhibitors, ruxolitinib (Jakafi) and fedratinib (Inrebic), are not indicated for the treatment of arthritis and other inflammatory conditions, and so are not a part of the updates being required.
Baricitinib, abrocitinib, and upadacitinib are currently under FDA review for treating atopic dermatitis (AD); a topical formulation of the JAK1/2 inhibitor ruxolitinib is under review for treating AD. Reviews for all 4 have been extended. In September 2020, baricitinib was approved for treating moderate to severe AD in Europe, at a dose of 4 mg once a day, with recommendations that the dose can be reduced to 2 mg once a day when the disease is under control, and that the dose may need to be reduced in patients with impaired kidney function, those with an increased risk of infections, and those older than aged 75 years.
In an interview, Jacob Thyssen, MD, PhD, professor of dermatology at the University of Copenhagen, said that in the EU, there has been “extensive education” about cardiovascular risks with baricitinib “and it is my impression that payers and dermatologists in Europe are confident that it is safe to use in AD.” In addition, there has been an emphasis on the differences in cardiovascular risk factors between RA and AD patients, “given that the latter group is generally young and lean.” In the United States, he added, it will be interesting to see which doses of the JAK inhibitors will be approved for AD.
Dr. Thyssen disclosed that he is a speaker, advisory board member and/or investigator for Regeneron, Sanofi-Genzyme, Eli Lilly, Pfizer, LEO Pharma, AbbVie, and Almirall.
*This story was updated 9/3/21 and 9/6/2021.
A version of this article first appeared on Medscape.com.
Children and COVID: Weekly cases top 200,000, vaccinations down
Weekly pediatric cases of COVID-19 exceeded 200,000 for just the second time during the pandemic, while new vaccinations in children continued to decline.
The weekly count has now increased for 9 consecutive weeks, during which time it has risen by over 2,300%, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report. Total cases in children number almost 4.8 million since the pandemic started.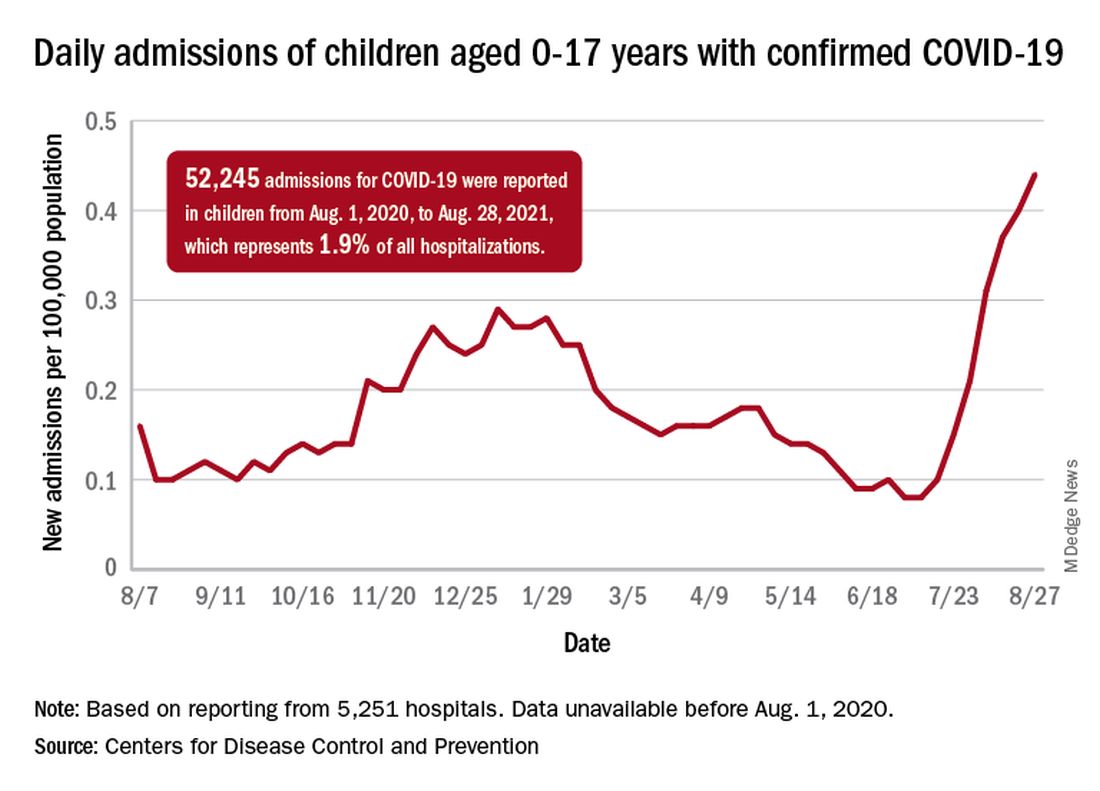
Vaccinations in children are following a different trend. Vaccine initiation has dropped 3 weeks in a row for both of the eligible age groups: First doses administered were down by 29% among 12- to 15-year-olds over that span and by 32% in 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
Since vaccination for children aged 12-15 years started in May, 49% had received at least one dose, and just over 36% were fully vaccinated as of Aug. 30. Among children aged 16-17 years, who have been eligible since December, 57.5% had gotten at least one dose of the vaccine and 46% have completed the two-dose regimen. The total number of children with at least one dose, including those under age 12 who are involved in clinical trials, was about 12 million, the CDC said on its COVID Data Tracker.
Hospitalizations are higher than ever
The recent rise in new child cases has been accompanied by an unprecedented increase in hospitalizations. The daily rate in children aged 0-17 years, which did not surpass 0.30 new admissions per 100,000 population during the worst of the winter surge, had risen to 0.45 per 100,000 by Aug. 26. Since July 4, when the new-admission rate was at its low point of 0.07 per 100,000, hospitalizations in children have jumped by 543%, based on data reported to the CDC by 5,251 hospitals.
A total of 52,245 children were admitted with confirmed COVID-19 from Aug. 1, 2020, when the CDC dataset begins, to Aug. 28, 2021. Those children represent 1.9% of all COVID admissions (2.7 million) in the United States over that period, the CDC said.
Total COVID-related deaths in children are up to 425 in the 48 jurisdictions (45 states, New York City, Puerto Rico, and Guam) that provide mortality data by age, the AAP and the CHA said.
Record-high numbers for the previous 2 reporting weeks – 23 deaths during Aug. 20-26 and 24 deaths during Aug. 13-19, when the previous weekly high was 16 – at least partially reflect the recent addition of South Carolina and New Mexico to the AAP/CHA database, as the two states just started reporting age-related data.
Weekly pediatric cases of COVID-19 exceeded 200,000 for just the second time during the pandemic, while new vaccinations in children continued to decline.
The weekly count has now increased for 9 consecutive weeks, during which time it has risen by over 2,300%, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report. Total cases in children number almost 4.8 million since the pandemic started.
Vaccinations in children are following a different trend. Vaccine initiation has dropped 3 weeks in a row for both of the eligible age groups: First doses administered were down by 29% among 12- to 15-year-olds over that span and by 32% in 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
Since vaccination for children aged 12-15 years started in May, 49% had received at least one dose, and just over 36% were fully vaccinated as of Aug. 30. Among children aged 16-17 years, who have been eligible since December, 57.5% had gotten at least one dose of the vaccine and 46% have completed the two-dose regimen. The total number of children with at least one dose, including those under age 12 who are involved in clinical trials, was about 12 million, the CDC said on its COVID Data Tracker.
Hospitalizations are higher than ever
The recent rise in new child cases has been accompanied by an unprecedented increase in hospitalizations. The daily rate in children aged 0-17 years, which did not surpass 0.30 new admissions per 100,000 population during the worst of the winter surge, had risen to 0.45 per 100,000 by Aug. 26. Since July 4, when the new-admission rate was at its low point of 0.07 per 100,000, hospitalizations in children have jumped by 543%, based on data reported to the CDC by 5,251 hospitals.
A total of 52,245 children were admitted with confirmed COVID-19 from Aug. 1, 2020, when the CDC dataset begins, to Aug. 28, 2021. Those children represent 1.9% of all COVID admissions (2.7 million) in the United States over that period, the CDC said.
Total COVID-related deaths in children are up to 425 in the 48 jurisdictions (45 states, New York City, Puerto Rico, and Guam) that provide mortality data by age, the AAP and the CHA said.
Record-high numbers for the previous 2 reporting weeks – 23 deaths during Aug. 20-26 and 24 deaths during Aug. 13-19, when the previous weekly high was 16 – at least partially reflect the recent addition of South Carolina and New Mexico to the AAP/CHA database, as the two states just started reporting age-related data.
Weekly pediatric cases of COVID-19 exceeded 200,000 for just the second time during the pandemic, while new vaccinations in children continued to decline.
The weekly count has now increased for 9 consecutive weeks, during which time it has risen by over 2,300%, the American Academy of Pediatrics and the Children’s Hospital Association said in their weekly COVID-19 report. Total cases in children number almost 4.8 million since the pandemic started.
Vaccinations in children are following a different trend. Vaccine initiation has dropped 3 weeks in a row for both of the eligible age groups: First doses administered were down by 29% among 12- to 15-year-olds over that span and by 32% in 16- to 17-year-olds, according to data from the Centers for Disease Control and Prevention.
Since vaccination for children aged 12-15 years started in May, 49% had received at least one dose, and just over 36% were fully vaccinated as of Aug. 30. Among children aged 16-17 years, who have been eligible since December, 57.5% had gotten at least one dose of the vaccine and 46% have completed the two-dose regimen. The total number of children with at least one dose, including those under age 12 who are involved in clinical trials, was about 12 million, the CDC said on its COVID Data Tracker.
Hospitalizations are higher than ever
The recent rise in new child cases has been accompanied by an unprecedented increase in hospitalizations. The daily rate in children aged 0-17 years, which did not surpass 0.30 new admissions per 100,000 population during the worst of the winter surge, had risen to 0.45 per 100,000 by Aug. 26. Since July 4, when the new-admission rate was at its low point of 0.07 per 100,000, hospitalizations in children have jumped by 543%, based on data reported to the CDC by 5,251 hospitals.
A total of 52,245 children were admitted with confirmed COVID-19 from Aug. 1, 2020, when the CDC dataset begins, to Aug. 28, 2021. Those children represent 1.9% of all COVID admissions (2.7 million) in the United States over that period, the CDC said.
Total COVID-related deaths in children are up to 425 in the 48 jurisdictions (45 states, New York City, Puerto Rico, and Guam) that provide mortality data by age, the AAP and the CHA said.
Record-high numbers for the previous 2 reporting weeks – 23 deaths during Aug. 20-26 and 24 deaths during Aug. 13-19, when the previous weekly high was 16 – at least partially reflect the recent addition of South Carolina and New Mexico to the AAP/CHA database, as the two states just started reporting age-related data.
EDs saw more benzodiazepine overdoses, but fewer patients overall, in 2020
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.
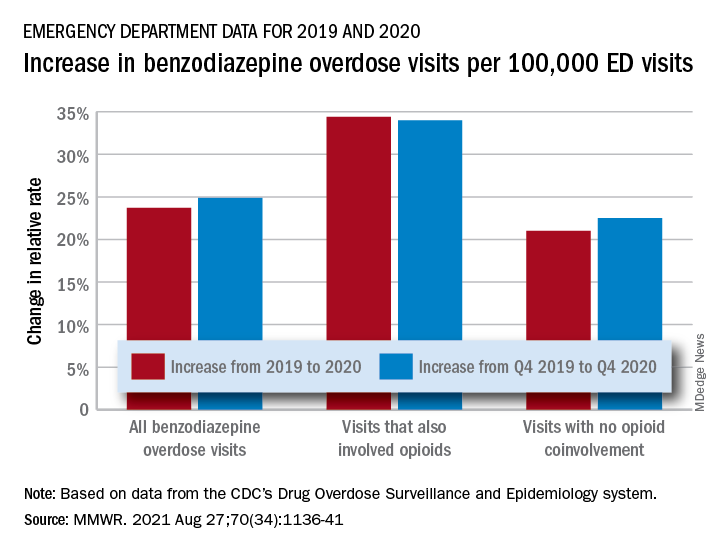
The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
FROM MMWR
CDC panel unanimously backs Pfizer vax, fortifying FDA approval
An independent expert panel within the Centers for Disease Control and Prevention (CDC) has studied the potential benefits and risks of the Pfizer-BioNTech COVID-19 vaccine and voted unanimously to recommend the shots for all Americans ages 16 and older.
The vaccine was fully approved by the U.S. Food and Drug Administration (FDA) last week.
The inoculation is still available to teens ages 12 to 15 under an emergency use authorization from the FDA.
ACIP now sends its recommendation to the CDC Director Rochelle Walensky, MD, for her sign off.
After reviewing the evidence behind the vaccine, panel member Sarah Long, MD, a professor of pediatrics at Drexel University College of Medicine, Philadelphia, said she couldn’t recall another instance where panelists had so much data on which to base their recommendation.
“This vaccine is worthy of the trust of the American people,” she said.
Doctors across the country use vaccines in line with the recommendations made by the ACIP. Their approval typically means that private and government insurers will cover the cost of the shots. In the case of the COVID-19 vaccines, the government is already picking up the tab.
Few surprises
The panel’s independent review of the vaccine’s effectiveness from nine studies held few surprises.
They found the Pfizer vaccine prevented a COVID infection with symptoms about 90%–92% of the time, at least for the first 4 months after the second shot. Protection against hospitalization and death was even higher.
The vaccine was about 89% effective at preventing a COVID infection without symptoms, according to a pooled estimate of five studies.
The data included in the review was updated only through March 13 of this year, however, and does not reflect the impact of further waning of immunity or the impact of the Delta variant.
In making their recommendation, the panel got an update on the safety of the vaccines, which have now been used in the United States for about 9 months.
The rate of anaphylaxis has settled at around five cases for every million shots given, according to the ACIP’s review of the evidence.
Cases of myocarditis and pericarditis were more common after getting a Pfizer-BioNTech vaccine than would be expected to happen naturally in the general population, but the risk was still very rare, and elevated primarily for men younger than age 30.
Out of 17 million second doses of Pfizer-BioNTech vaccines in the United States, there have been 327 confirmed cases of myocarditis reported to the Vaccine Adverse Event Reporting System in people who are younger than age 30. The average hospital stay for a myocarditis cases is 1 to 2 days.
So far, no one in the United States diagnosed with myocarditis after vaccination has died.
What’s more, the risk of myocarditis after vaccination was dwarfed by the risk of myocarditis after a COVID infection. The risk of myocarditis after a COVID infection was 6 to 34 times higher than the risk after receiving an mRNA vaccine.
About 11% of people who get the vaccine experience a serious reaction to the shot, compared with about 3% in the placebo group. Serious reactions were defined as pain; swelling or redness at the injection site that interferes with activity; needing to visit the hospital or ER for pain; tissue necrosis, or having skin slough off; high fever; vomiting that requires hydration; persistent diarrhea; severe headache; or muscle pain/severe joint pain.
“Safe and effective”
After hearing a presentation on the state of the pandemic in the US, some panel members were struck and shaken that 38% of Americans who are eligible are still not fully vaccinated.
Pablo Sanchez, MD, a pediatrician at Nationwide Children’s Hospital in Columbus, Ohio, said, “We’re doing an abysmal job vaccinating the American people. The message has to go out that the vaccines are safe and effective.”
A version of this story first appeared on Medscape.com.
An independent expert panel within the Centers for Disease Control and Prevention (CDC) has studied the potential benefits and risks of the Pfizer-BioNTech COVID-19 vaccine and voted unanimously to recommend the shots for all Americans ages 16 and older.
The vaccine was fully approved by the U.S. Food and Drug Administration (FDA) last week.
The inoculation is still available to teens ages 12 to 15 under an emergency use authorization from the FDA.
ACIP now sends its recommendation to the CDC Director Rochelle Walensky, MD, for her sign off.
After reviewing the evidence behind the vaccine, panel member Sarah Long, MD, a professor of pediatrics at Drexel University College of Medicine, Philadelphia, said she couldn’t recall another instance where panelists had so much data on which to base their recommendation.
“This vaccine is worthy of the trust of the American people,” she said.
Doctors across the country use vaccines in line with the recommendations made by the ACIP. Their approval typically means that private and government insurers will cover the cost of the shots. In the case of the COVID-19 vaccines, the government is already picking up the tab.
Few surprises
The panel’s independent review of the vaccine’s effectiveness from nine studies held few surprises.
They found the Pfizer vaccine prevented a COVID infection with symptoms about 90%–92% of the time, at least for the first 4 months after the second shot. Protection against hospitalization and death was even higher.
The vaccine was about 89% effective at preventing a COVID infection without symptoms, according to a pooled estimate of five studies.
The data included in the review was updated only through March 13 of this year, however, and does not reflect the impact of further waning of immunity or the impact of the Delta variant.
In making their recommendation, the panel got an update on the safety of the vaccines, which have now been used in the United States for about 9 months.
The rate of anaphylaxis has settled at around five cases for every million shots given, according to the ACIP’s review of the evidence.
Cases of myocarditis and pericarditis were more common after getting a Pfizer-BioNTech vaccine than would be expected to happen naturally in the general population, but the risk was still very rare, and elevated primarily for men younger than age 30.
Out of 17 million second doses of Pfizer-BioNTech vaccines in the United States, there have been 327 confirmed cases of myocarditis reported to the Vaccine Adverse Event Reporting System in people who are younger than age 30. The average hospital stay for a myocarditis cases is 1 to 2 days.
So far, no one in the United States diagnosed with myocarditis after vaccination has died.
What’s more, the risk of myocarditis after vaccination was dwarfed by the risk of myocarditis after a COVID infection. The risk of myocarditis after a COVID infection was 6 to 34 times higher than the risk after receiving an mRNA vaccine.
About 11% of people who get the vaccine experience a serious reaction to the shot, compared with about 3% in the placebo group. Serious reactions were defined as pain; swelling or redness at the injection site that interferes with activity; needing to visit the hospital or ER for pain; tissue necrosis, or having skin slough off; high fever; vomiting that requires hydration; persistent diarrhea; severe headache; or muscle pain/severe joint pain.
“Safe and effective”
After hearing a presentation on the state of the pandemic in the US, some panel members were struck and shaken that 38% of Americans who are eligible are still not fully vaccinated.
Pablo Sanchez, MD, a pediatrician at Nationwide Children’s Hospital in Columbus, Ohio, said, “We’re doing an abysmal job vaccinating the American people. The message has to go out that the vaccines are safe and effective.”
A version of this story first appeared on Medscape.com.
An independent expert panel within the Centers for Disease Control and Prevention (CDC) has studied the potential benefits and risks of the Pfizer-BioNTech COVID-19 vaccine and voted unanimously to recommend the shots for all Americans ages 16 and older.
The vaccine was fully approved by the U.S. Food and Drug Administration (FDA) last week.
The inoculation is still available to teens ages 12 to 15 under an emergency use authorization from the FDA.
ACIP now sends its recommendation to the CDC Director Rochelle Walensky, MD, for her sign off.
After reviewing the evidence behind the vaccine, panel member Sarah Long, MD, a professor of pediatrics at Drexel University College of Medicine, Philadelphia, said she couldn’t recall another instance where panelists had so much data on which to base their recommendation.
“This vaccine is worthy of the trust of the American people,” she said.
Doctors across the country use vaccines in line with the recommendations made by the ACIP. Their approval typically means that private and government insurers will cover the cost of the shots. In the case of the COVID-19 vaccines, the government is already picking up the tab.
Few surprises
The panel’s independent review of the vaccine’s effectiveness from nine studies held few surprises.
They found the Pfizer vaccine prevented a COVID infection with symptoms about 90%–92% of the time, at least for the first 4 months after the second shot. Protection against hospitalization and death was even higher.
The vaccine was about 89% effective at preventing a COVID infection without symptoms, according to a pooled estimate of five studies.
The data included in the review was updated only through March 13 of this year, however, and does not reflect the impact of further waning of immunity or the impact of the Delta variant.
In making their recommendation, the panel got an update on the safety of the vaccines, which have now been used in the United States for about 9 months.
The rate of anaphylaxis has settled at around five cases for every million shots given, according to the ACIP’s review of the evidence.
Cases of myocarditis and pericarditis were more common after getting a Pfizer-BioNTech vaccine than would be expected to happen naturally in the general population, but the risk was still very rare, and elevated primarily for men younger than age 30.
Out of 17 million second doses of Pfizer-BioNTech vaccines in the United States, there have been 327 confirmed cases of myocarditis reported to the Vaccine Adverse Event Reporting System in people who are younger than age 30. The average hospital stay for a myocarditis cases is 1 to 2 days.
So far, no one in the United States diagnosed with myocarditis after vaccination has died.
What’s more, the risk of myocarditis after vaccination was dwarfed by the risk of myocarditis after a COVID infection. The risk of myocarditis after a COVID infection was 6 to 34 times higher than the risk after receiving an mRNA vaccine.
About 11% of people who get the vaccine experience a serious reaction to the shot, compared with about 3% in the placebo group. Serious reactions were defined as pain; swelling or redness at the injection site that interferes with activity; needing to visit the hospital or ER for pain; tissue necrosis, or having skin slough off; high fever; vomiting that requires hydration; persistent diarrhea; severe headache; or muscle pain/severe joint pain.
“Safe and effective”
After hearing a presentation on the state of the pandemic in the US, some panel members were struck and shaken that 38% of Americans who are eligible are still not fully vaccinated.
Pablo Sanchez, MD, a pediatrician at Nationwide Children’s Hospital in Columbus, Ohio, said, “We’re doing an abysmal job vaccinating the American people. The message has to go out that the vaccines are safe and effective.”
A version of this story first appeared on Medscape.com.
FDA fully approves Pfizer COVID-19 vaccine
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
The Food and Drug Administration has granted a biological license application, more commonly known as “full approval,” to the Pfizer-BioNTech COVID-19 vaccine.
It is the first COVID-19 vaccine to be fully licensed in the United States. It will be marketed under the trade name Comirnaty.
The approval applies to individuals ages 16 years and older. The vaccine is still available for emergency use for those ages 12-15.
The FDA’s stamp of approval is somewhat anticlimactic, following months of real-world use and millions of doses doled out to the general population. It comes after months of scrutiny by the agency of the clinical trial data.
Still, the approval puts the vaccines on firmer legal footing and is expected to spur a raft of new vaccination requirements by employers, schools, and universities.
“The FDA approval is the gold standard,” President Joe Biden said from the White House. “Those who have been waiting for full approval should go and get your shot now.”
“It could save your life or the lives of those you love,” he said.
Biden also called on businesses to mandate COVID vaccines for their employees.
Indeed, soon after the approval was announced, Defense Secretary Lloyd Austin said the vaccines would be required for all 1.4 million active duty service members.
Public health advocates have seen full approval as an important tool to increase U.S. vaccination rates and had criticized the FDA for taking so long to grant the license.
In a news briefing on the approval, Peter Marks, MD, director of the FDA’s Center for Biologics Evaluation and Research, said the agency had not dragged its feet.
Marks noted that his team had reviewed tens of thousands of pages of clinical trial data -- down to the level of individual patients. They also inspected clinical trial sites and manufacturing facilities, and reviewed information gathered after the vaccines were authorized for use.
“It’s been 97 days since Pfizer completed the role of its [application for approval] and the clock started, which means that we completed this in about 40% of the normal clock time for a submission of this magnitude,” he said. “People worked day and night.”
The agency resisted pressure to speed up its process, saying a thorough review was necessary to ensure public confidence.
“While millions of people have already safely received COVID-19 vaccines, we recognize that for some, the FDA approval of a vaccine may now instill additional confidence to get vaccinated. Today’s milestone puts us one step closer to altering the course of this pandemic in the U.S.,” acting FDA Commissioner Janet Woodcock said in a FDA news release.
Experts agreed the move would increase public confidence.
“I don't expect a big line outside of vaccination sites this afternoon or tomorrow morning, but it will persuade some,” said William Schaffner, MD, a professor of infectious diseases at Vanderbilt University in Nashville.
A recent Kaiser Family Foundation poll found that 3 in 10 unvaccinated adults said they would be more likely to get vaccinated if the vaccines were given full approval.
More importantly, Schaffner said, the FDA’s approval would lay the groundwork for vaccine mandates. “I think those kinds of mandates are going to be necessary to get us up over 80% vaccinated.”
In granting the approval, the agency reviewed a record amount of data from more than 40,000 people who took part in clinical trials. About 12,000 recipients have been followed for at least 6 months, the agency said.
The FDA also reviewed safety data collected since it issued its emergency use authorization for the shots in December.
Based on the results from the clinical trials, the vaccine was 91% effective at preventing COVID-19 disease. But that estimate came from data collected before the Delta variant became widespread.
The most commonly reported side effects in the clinical trials were pain, redness and swelling at the injection site, fatigue, headache, muscle or joint pain, chills, and fever.
The FDA said the vaccine is effective in preventing COVID-19 and potentially serious outcomes, including hospitalization and death.
Based on safety data reviewed since the two-dose vaccine was approved, the FDA said the data demonstrates a higher risk for heart inflammation -- clinically known as myocarditis or pericarditis -- especially within 7 days after the second dose of the shots. The risk is highest for men under age 40, compared to women and older men.
The prescription information includes warnings about these risks. The FDA said the drugmakers must continue to study the risks and long-term effects on people who have myocarditis after vaccination.
A version of this article first appeared on Medscape.com.
This article was updated on 8/24/21.
CDC launches new center to watch for future outbreaks
The Centers for Disease Control and Prevention is setting up a new hub to watch for early warning signs of future infectious outbreaks, the agency announced on Aug. 18.
Epidemiologists learn about emerging outbreaks by tracking information, and the quality of their analysis depends on their access to high-quality data. Gaps in existing systems became obvious during the COVID-19 pandemic as experts were challenged by the crisis.
The new Center for Forecasting and Outbreak Analytics will, in part, work like a meteorological office that tracks weather-related changes, only the center will track possible flareups in infectious disease.
The day after he took office, President Joe Biden pledged to modernize the country’s system for public health data. First funding for the initiative will come from the American Rescue Plan.
“We are excited to have the expertise and ability to model and forecast public health concerns and share information in real-time to activate governmental, private sector, and public actions in anticipation of threats both domestically and abroad,” CDC Director Rochelle Walensky, MD, said in a statement.
Devastating toll of COVID-19
Many world leaders are now responding to the destruction of the health crisis and are investing in new infrastructure. A July report from a G-20 panel calls for $75 billion in international financing for pandemic prevention and preparedness –twice as much as current spending levels.
Testifying in a congressional hearing, epidemiologist Caitlin Rivers, PhD, from the Johns Hopkins Center for Health Security, Baltimore, voiced the importance of never being caught unprepared again.
“We were unprepared to manage the emergence and swift global spread of the novel coronavirus, and we were late to recognize when it reached our shores. Those delays set us on a worse trajectory than we might have otherwise faced,” she said.
Dr. Rivers will join the new center’s leadership team as associate director working alongside Marc Lipsitch, PhD, director for science.
“The new center will meet a longstanding need for a national focal point to analyze data and forecast the trajectory of pandemics with the express goal of informing and improving decisions with the best available evidence,” Dr. Lipsitch said in the CDC’s news release announcing the new center.
Experts will map what data sources are needed to assist disease modelers and public health emergency responders tracking emerging problems that they can share with decision-makers. They will expand tracking capability and data sharing using open-source software and application programming with existing and new data streams from the public health ecosystem and elsewhere.
Dylan George, PhD, who will be the center’s director for operations, said in the CDC news release that the center will provide critical information to communities so they can respond.
“Pandemics threaten our families and communities at speed and scale – our response needs to move at speed and scale, too,” he said.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention is setting up a new hub to watch for early warning signs of future infectious outbreaks, the agency announced on Aug. 18.
Epidemiologists learn about emerging outbreaks by tracking information, and the quality of their analysis depends on their access to high-quality data. Gaps in existing systems became obvious during the COVID-19 pandemic as experts were challenged by the crisis.
The new Center for Forecasting and Outbreak Analytics will, in part, work like a meteorological office that tracks weather-related changes, only the center will track possible flareups in infectious disease.
The day after he took office, President Joe Biden pledged to modernize the country’s system for public health data. First funding for the initiative will come from the American Rescue Plan.
“We are excited to have the expertise and ability to model and forecast public health concerns and share information in real-time to activate governmental, private sector, and public actions in anticipation of threats both domestically and abroad,” CDC Director Rochelle Walensky, MD, said in a statement.
Devastating toll of COVID-19
Many world leaders are now responding to the destruction of the health crisis and are investing in new infrastructure. A July report from a G-20 panel calls for $75 billion in international financing for pandemic prevention and preparedness –twice as much as current spending levels.
Testifying in a congressional hearing, epidemiologist Caitlin Rivers, PhD, from the Johns Hopkins Center for Health Security, Baltimore, voiced the importance of never being caught unprepared again.
“We were unprepared to manage the emergence and swift global spread of the novel coronavirus, and we were late to recognize when it reached our shores. Those delays set us on a worse trajectory than we might have otherwise faced,” she said.
Dr. Rivers will join the new center’s leadership team as associate director working alongside Marc Lipsitch, PhD, director for science.
“The new center will meet a longstanding need for a national focal point to analyze data and forecast the trajectory of pandemics with the express goal of informing and improving decisions with the best available evidence,” Dr. Lipsitch said in the CDC’s news release announcing the new center.
Experts will map what data sources are needed to assist disease modelers and public health emergency responders tracking emerging problems that they can share with decision-makers. They will expand tracking capability and data sharing using open-source software and application programming with existing and new data streams from the public health ecosystem and elsewhere.
Dylan George, PhD, who will be the center’s director for operations, said in the CDC news release that the center will provide critical information to communities so they can respond.
“Pandemics threaten our families and communities at speed and scale – our response needs to move at speed and scale, too,” he said.
A version of this article first appeared on WebMD.com.
The Centers for Disease Control and Prevention is setting up a new hub to watch for early warning signs of future infectious outbreaks, the agency announced on Aug. 18.
Epidemiologists learn about emerging outbreaks by tracking information, and the quality of their analysis depends on their access to high-quality data. Gaps in existing systems became obvious during the COVID-19 pandemic as experts were challenged by the crisis.
The new Center for Forecasting and Outbreak Analytics will, in part, work like a meteorological office that tracks weather-related changes, only the center will track possible flareups in infectious disease.
The day after he took office, President Joe Biden pledged to modernize the country’s system for public health data. First funding for the initiative will come from the American Rescue Plan.
“We are excited to have the expertise and ability to model and forecast public health concerns and share information in real-time to activate governmental, private sector, and public actions in anticipation of threats both domestically and abroad,” CDC Director Rochelle Walensky, MD, said in a statement.
Devastating toll of COVID-19
Many world leaders are now responding to the destruction of the health crisis and are investing in new infrastructure. A July report from a G-20 panel calls for $75 billion in international financing for pandemic prevention and preparedness –twice as much as current spending levels.
Testifying in a congressional hearing, epidemiologist Caitlin Rivers, PhD, from the Johns Hopkins Center for Health Security, Baltimore, voiced the importance of never being caught unprepared again.
“We were unprepared to manage the emergence and swift global spread of the novel coronavirus, and we were late to recognize when it reached our shores. Those delays set us on a worse trajectory than we might have otherwise faced,” she said.
Dr. Rivers will join the new center’s leadership team as associate director working alongside Marc Lipsitch, PhD, director for science.
“The new center will meet a longstanding need for a national focal point to analyze data and forecast the trajectory of pandemics with the express goal of informing and improving decisions with the best available evidence,” Dr. Lipsitch said in the CDC’s news release announcing the new center.
Experts will map what data sources are needed to assist disease modelers and public health emergency responders tracking emerging problems that they can share with decision-makers. They will expand tracking capability and data sharing using open-source software and application programming with existing and new data streams from the public health ecosystem and elsewhere.
Dylan George, PhD, who will be the center’s director for operations, said in the CDC news release that the center will provide critical information to communities so they can respond.
“Pandemics threaten our families and communities at speed and scale – our response needs to move at speed and scale, too,” he said.
A version of this article first appeared on WebMD.com.
Children and COVID: New cases rise to winter levels
Weekly cases of COVID-19 in children topped 100,000 for the first time since early February, according to the American Academy of Pediatrics and the Children’s Hospital Association.
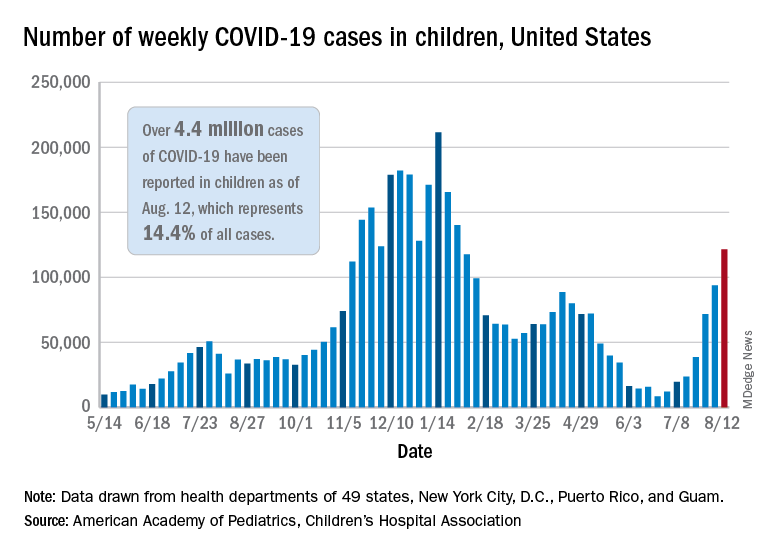
the AAP and CHA said in their weekly COVD-19 report. The recent surge in child COVID has also brought a record high in hospitalizations and shortages of pediatric ICU beds in some areas.
The 121,000 new cases represent an increase of almost 1,400% since June 18-24, when the weekly tally was just 8,447 and at its lowest point in over a year, the AAP/CHA data show.
On the vaccination front in the last week (Aug. 10-16), vaccine initiation for 12- to 17-year-olds was fairly robust but still down slightly, compared with the previous week. Just over 402,000 children aged 12-15 years received a first vaccination, which was down slightly from 411,000 the week before but still higher than any of the 6 weeks from June 22 to Aug. 2, based on data from the Centers for Disease Control and Prevention. Vaccinations were down by a similar margin for 15- to-17-year-olds.
Over 10.9 million children aged 12-17 have had at least one dose of COVID-19 vaccine administered, of whom 8.1 million are fully vaccinated. Among those aged 12-15 years, 44.5% have gotten at least one dose and 31.8% are fully vaccinated, with corresponding figures of 53.9% and 42.5% for 16- and 17-year-olds, according to the CDC’s COVID Data Tracker.
The number of COVID-19 cases reported in children since the start of the pandemic is up to 4.4 million, which makes up 14.4% of all cases in the United States, the AAP and CHA said. Other cumulative figures through Aug. 12 include almost 18,000 hospitalizations – reported by 23 states and New York City – and 378 deaths – reported by 43 states, New York City, Puerto Rico, and Guam.
In the latest edition of their ongoing report, compiled using state data since the summer of 2020, the two groups noted that, “in the summer of 2021, some states have revised cases counts previously reported, begun reporting less frequently, or dropped metrics previously reported.” Among those states are Nebraska, which shut down its online COVID dashboard in late June, and Alabama, which stopped reporting cumulative cases and deaths after July 29.
Weekly cases of COVID-19 in children topped 100,000 for the first time since early February, according to the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVD-19 report. The recent surge in child COVID has also brought a record high in hospitalizations and shortages of pediatric ICU beds in some areas.
The 121,000 new cases represent an increase of almost 1,400% since June 18-24, when the weekly tally was just 8,447 and at its lowest point in over a year, the AAP/CHA data show.
On the vaccination front in the last week (Aug. 10-16), vaccine initiation for 12- to 17-year-olds was fairly robust but still down slightly, compared with the previous week. Just over 402,000 children aged 12-15 years received a first vaccination, which was down slightly from 411,000 the week before but still higher than any of the 6 weeks from June 22 to Aug. 2, based on data from the Centers for Disease Control and Prevention. Vaccinations were down by a similar margin for 15- to-17-year-olds.
Over 10.9 million children aged 12-17 have had at least one dose of COVID-19 vaccine administered, of whom 8.1 million are fully vaccinated. Among those aged 12-15 years, 44.5% have gotten at least one dose and 31.8% are fully vaccinated, with corresponding figures of 53.9% and 42.5% for 16- and 17-year-olds, according to the CDC’s COVID Data Tracker.
The number of COVID-19 cases reported in children since the start of the pandemic is up to 4.4 million, which makes up 14.4% of all cases in the United States, the AAP and CHA said. Other cumulative figures through Aug. 12 include almost 18,000 hospitalizations – reported by 23 states and New York City – and 378 deaths – reported by 43 states, New York City, Puerto Rico, and Guam.
In the latest edition of their ongoing report, compiled using state data since the summer of 2020, the two groups noted that, “in the summer of 2021, some states have revised cases counts previously reported, begun reporting less frequently, or dropped metrics previously reported.” Among those states are Nebraska, which shut down its online COVID dashboard in late June, and Alabama, which stopped reporting cumulative cases and deaths after July 29.
Weekly cases of COVID-19 in children topped 100,000 for the first time since early February, according to the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVD-19 report. The recent surge in child COVID has also brought a record high in hospitalizations and shortages of pediatric ICU beds in some areas.
The 121,000 new cases represent an increase of almost 1,400% since June 18-24, when the weekly tally was just 8,447 and at its lowest point in over a year, the AAP/CHA data show.
On the vaccination front in the last week (Aug. 10-16), vaccine initiation for 12- to 17-year-olds was fairly robust but still down slightly, compared with the previous week. Just over 402,000 children aged 12-15 years received a first vaccination, which was down slightly from 411,000 the week before but still higher than any of the 6 weeks from June 22 to Aug. 2, based on data from the Centers for Disease Control and Prevention. Vaccinations were down by a similar margin for 15- to-17-year-olds.
Over 10.9 million children aged 12-17 have had at least one dose of COVID-19 vaccine administered, of whom 8.1 million are fully vaccinated. Among those aged 12-15 years, 44.5% have gotten at least one dose and 31.8% are fully vaccinated, with corresponding figures of 53.9% and 42.5% for 16- and 17-year-olds, according to the CDC’s COVID Data Tracker.
The number of COVID-19 cases reported in children since the start of the pandemic is up to 4.4 million, which makes up 14.4% of all cases in the United States, the AAP and CHA said. Other cumulative figures through Aug. 12 include almost 18,000 hospitalizations – reported by 23 states and New York City – and 378 deaths – reported by 43 states, New York City, Puerto Rico, and Guam.
In the latest edition of their ongoing report, compiled using state data since the summer of 2020, the two groups noted that, “in the summer of 2021, some states have revised cases counts previously reported, begun reporting less frequently, or dropped metrics previously reported.” Among those states are Nebraska, which shut down its online COVID dashboard in late June, and Alabama, which stopped reporting cumulative cases and deaths after July 29.
