User login
GOC Discussions Among LTC Residents
Hospitalizations of long‐term care (LTC) residents are known to be frequent, costly, often preventable,[1, 2, 3] and potentially associated with negative health outcomes.[4] Often, an advance directive (AD) is made at LTC admission and updated annually when residents are in relatively stable health. An AD is a document that helps to inform a substitute decision maker (SDM) about the consent process for life‐sustaining treatments and is a resource that supports advance care planning (ACP). ACP is a process that allows individuals to consider, express, and plan for future healthcare in the event that they lack capacity to make their own decisions. When an LTC resident's health deteriorates and hospitalization is required, there is an opportunity to update prognosis, discuss risks and benefits of previously held treatment preferences, as well as reassess goals of care (GOC).
Engaging in ACP discussions during relatively stable health can help ensure patient preferences are followed.[5, 6] These discussions, however, are often insufficient, as they involve decision making for hypothetical situations that may not cover all potential scenarios, and may not reflect a patient's reality at the time of health status decline. Discussions held in the moment more authentically reflect the decisions of patients and/or SDM based on the specific needs and clinical realities particular to the patient at that time.[7] GOC discussions, defined in this context as ACP discussions occurring during hospitalization, have the potential to better align patient wishes with care received,[6] improve quality of life and satisfaction,[8, 9, 10] and reduce unwanted extra care.[11, 12] Although in‐the‐moment GOC discussions are recommended for all hospitalized patients who are seriously ill with a high risk of dying,[13] research suggests that this occurs infrequently for elderly patients. A recent multicenter survey of seriously ill hospitalized elderly patients found that only 25% of patients and 32% of family members reported that they had been asked about prior ACP or AD.[14] Another study of hospitalized LTC residents found that resuscitation status and family discussion was documented in only 55% and 42% of admissions, respectively.[15]
Further investigation is required to determine how often LTC patients have GOC discussions, what prompts these discussions, and what are the outcomes. Previous studies have focused on barriers to performing GOC discussions, rather than the factors that are associated with them.[16] By understanding why these discussions currently happen, we can potentially improve how often they occur and the quality of their outcomes.
The objectives of this study were to determine the rate of documented GOC discussions among hospitalized LTC residents, identify factors that were associated with documentation, and examine the association between documentation and outcomes of care.
METHODS
Study Population
We conducted a retrospective chart review of a random convenience sample of hospitalized patients admitted via the emergency department (ED) to the general internal medicine (GIM) service from January 1, 2012 through December 31, 2012, at 2 academic teaching hospitals in Toronto, Canada. Patients were identified through a search of each hospitals' electronic patient record (EPR). Patients were eligible for inclusion if they were (1) a LTC resident and (2) at least 65 years of age. For patients with multiple admissions to the GIM service during the specified 12‐month period, we only included data from the first hospitalization (index hospitalization). The hospital's research ethics board approved this study.
Our primary variable of interest was documentation in the hospital medical record of a discussion between physicians and the patient/family/SDM regarding GOC. A GOC discussion was considered to have taken place if there was documentation of (1) understanding/expectation of treatment options or (2) patient's preferences for life‐sustaining measures. Examples illustrating each criterion are provided in the Supporting Information, Appendix 1, in the online version of this article.
Factors Associated With GOC Documentation
From the EPR, we obtained visit‐level data including age, gender, Canadian Emergency Department Triage and Acuity Scale, vital signs at ED admission including temperature, respiratory rate, oxygen saturation, Glasgow Coma Scale (GCS) and shock index (defined as heart rate divided by systolic blood pressure), admission and discharge dates/times, discharge diagnosis, transfer to intensive care unit (ICU), and hospital use (number of ED visits and hospitalizations to the 2 study hospitals in the 1‐year period prior to index hospitalization).
Trained study personnel (J.W.) used a structured abstraction form to collect data from the hospital medical record that were not available through the EPR, including years living in LTC, contents of LTC AD forms, presence of SDM (identified as immediate family or surrogate with whom the care team communicated), dementia diagnosis (defined as documentation of dementia in the patient's past medical history and/or history of present illness), and measures of functional status. When available, we extracted the AD from LTC; they consisted of 4 levels (level 1: comfort careno transfer to hospital, no cardiopulmonary resuscitation [CPR]; level 2: supportive careadministration of antibiotics and/or other procedures that can be provided within LTC, no transfer to the hospital, no CPR; level 3: transfer to the hospitalno CPR; level 4: aggressive interventiontransfer to hospital for aggressive treatment, CPR).
GOC Documentation in the Discharge Summary
For the subset of patients who survived hospitalization and were discharged back to LTC, we examined whether the ADs ordered during hospitalization were communicated back to LTC via the discharge summary. We additionally assessed if the ADs determined during hospitalization differed from preferences documented prior to hospitalization. Physician orders for ADs were categorized as level 1: comfort measures only, level 3: no CPR, or level 4: full code. LTC level 2 was considered equivalent to physician‐ordered level 3 at admission; a patient with an LTC level 2 with no CPR (level 3) documented during hospitalized would be considered to have no change in the AD. An increase or decrease in the AD was determined by comparing LTC levels 1, 3, and 4 to physician‐ordered level 1, 3, and 4.
Outcomes of GOC Documentation
From the EPR, we obtained visit‐level outcome data including length of stay (LOS), resource intensity weight (RIW) (calculated based on patient case‐mix, severity, age, and procedures performed), visit disposition, number of ED visits and hospitalizations to the 2 study hospitals in the year following index hospitalization, in‐hospital death, and 1‐year mortality. We determined 1‐year mortality by following up with the LTC homes to determine whether the resident had died within the year following index hospitalization; only patients from LTC homes that responded to our request for data were included in 1‐year mortality analyses. We collected physician orders for the AD from chart review.
Statistical Analysis
Patients with and without documented GOC discussions were compared. Descriptive statistics including frequencies and percentages were used to characterize study variables. Differences between the study groups were assessed using Pearson 2/Fisher exact test. Multivariate logistic regression, which included variables that were significant in the bivariate analysis, was used to identify independent predictors of GOC discussion. Adjusted odds ratios (AOR) and 95% confidence intervals (CI) were presented for the logistic model. Patients with missing predictor data were excluded.
We also examined whether there was a correlation between GOC discussion and outcomes of care using Pearson 2/Fisher exact test. Outcomes included orders for the AD, LOS in days (stratified into quartiles), RIW (stratified into quartiles), visit disposition, hospital use in the year following index hospitalization, and 1‐year mortality following discharge back to LTC.
Lastly, to better understand the independent predictors of in‐hospital and 1‐year mortality, we used Pearson 2/Fisher exact test followed by logistic regression that included significant variables from the bivariate analyses.
All analyses were 2‐sided, and a P value of 0.05 was considered statistically significant. We used SPSS version 22.0 (SPSS Inc., Chicago, IL).
RESULTS
We identified a total of 7084 hospitalizations to GIM between January 1, 2012 and December 31, 2012, of which 665 (9.4%) met inclusion criteria of residence in LTC and age 65 years. Of these 665 hospitalizations, 512 were unique patients. We randomly selected a convenience sample of 200 index hospitalizations of the 512 eligible hospitalizations (39%) to perform the chart review.
Predictors of GOC Documentation
Of the 200 randomly sampled charts that were reviewed, 75 (37.5%) had a documented GOC discussion.
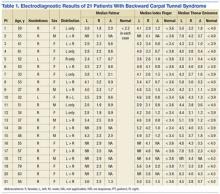
Characteristics of the study patients and results of bivariate analysis of the association between patient characteristics and GOC discussion are summarized in Table 1. No significant differences in demographic and baseline characteristics were seen between patients with and without discussion. However, a number of visit characteristics were found to be significantly associated with discussion. Forty percent of patients in the GOC discussion group had GCS scores 11 compared to 15.2% in the no‐discussion group. Higher respiratory rate, lower oxygen saturation, and ICU transfer were also significantly associated with discussions.
| Goals of Care Discussion Documented in Medical Chart | |||||
|---|---|---|---|---|---|
| No, N = 125 | Yes, N = 75 | P Value | |||
| |||||
| Baseline characteristics | |||||
| Gender, n (%) | 0.88 | ||||
| Male | 48 | (38.4) | 30 | (40.0) | |
| Female | 77 | (61.6) | 45 | (60.0) | |
| Age, y, n (%) | 0.85 | ||||
| 6579 | 36 | (28.8) | 19 | (25.3) | |
| 8084 | 30 | (24.0) | 19 | (25.3) | |
| 8589 | 30 | (24.0) | 16 | (21.3) | |
| 90101 | 29 | (23.2) | 21 | (28.0) | |
| Years living in long‐term care, n (%)* | 0.65 | ||||
| [0, 1) | 28 | (22.4) | 12 | (16.0) | |
| [1, 3) | 31 | (24.8) | 22 | (29.3) | |
| [3, 6) | 33 | (26.4) | 22 | (29.3) | |
| [6, 22) | 25 | (20.0) | 13 | (17.3) | |
| Unknown | 8 | (6.4) | 6 | (8.0) | |
| AD from long‐term care, n (%) | 0.14 | ||||
| Comfort measures only | 2 | (1.6) | 1 | (1.3) | |
| Supportive care with no transfer to hospital | 0 | (0.0) | 3 | (4.0) | |
| Supportive care with transfer to hospital | 70 | (56.0) | 44 | (58.7) | |
| Aggressive care | 53 | (42.4) | 27 | (36.0) | |
| Years since most recent AD signed, n (%)* | 0.12 | ||||
| [0, 1) | 79 | (63.2) | 48 | (64.0) | |
| [1, 2) | 21 | (16.8) | 6 | (8.0) | |
| [2, 6) | 9 | (7.2) | 10 | (13.3) | |
| Unknown | 16 | (12.8) | 11 | (14.7) | |
| Substitute decision maker, n (%) | 0.06 | ||||
| Child | 81 | (64.8) | 44 | (58.7) | |
| Spouse | 9 | (7.2) | 15 | (20.0) | |
| Other | 26 | (20.8) | 13 | (17.3) | |
| Public guardian trustee | 6 | (4.8) | 2 | (2.7) | |
| Unknown | 3 | (2.4) | 1 | (1.3) | |
| Dementia, n (%) | 1.00 | ||||
| No | 47 | (37.6) | 28 | (37.3) | |
| Yes | 78 | (62.4) | 47 | (62.7) | |
| Mobility, n (%) | 0.26 | ||||
| Walk without assistance | 5 | (4.0) | 3 | (4.0) | |
| Walker | 16 | (12.8) | 3 | (4.0) | |
| Wheelchair | 43 | (34.4) | 29 | (38.7) | |
| Bedridden | 7 | (5.6) | 4 | (5.3) | |
| Unknown | 54 | (43.2) | 36 | (48.0) | |
| Continence, n (%) | 0.05 | ||||
| Mostly continent | 16 | (12.8) | 3 | (4.0) | |
| Incontinent | 49 | (39.2) | 34 | (45.3) | |
| Catheter/stoma | 7 | (5.6) | 1 | (1.3) | |
| Unknown | 53 | (42.4) | 37 | (49.3) | |
| Feeding, n (%) | 0.17 | ||||
| Mostly feeds self | 38 | (30.4) | 13 | (17.3) | |
| Needs to be fed | 17 | (13.6) | 14 | (18.7) | |
| Gastrostomy tube | 8 | (6.4) | 5 | (6.7) | |
| Unknown | 62 | (49.6) | 43 | (57.3) | |
| Diet, n (%) | 0.68 | ||||
| Normal | 43 | (34.4) | 16 | (21.3) | |
| Dysphagic | 32 | (25.6) | 15 | (20.0) | |
| Gastrostomy tube | 8 | (6.4) | 5 | (6.7) | |
| Unknown | 42 | (33.6) | 39 | (52.0) | |
| Previous ED visits in last year, n (%) | 0.43 | ||||
| 0 | 70 | (56.0) | 41 | (54.7) | |
| 1 | 35 | (28.0) | 17 | (22.7) | |
| 2+ | 20 | (16.0) | 17 | (22.7) | |
| Previous hospitalizations in last year, n (%) | 0.19 | ||||
| 0 | 98 | (78.4) | 54 | (72.0) | |
| 1 | 23 | (18.4) | 14 | (18.7) | |
| 2+ | 4 | (3.2) | 7 | (9.3) | |
| Visit characteristics | |||||
| Glasgow Coma Scale, n (%) | 0.001 | ||||
| 7 | 4 | (3.2) | 4 | (5.3) | |
| 711 | 15 | (12.0) | 26 | (34.7) | |
| 1213 | 7 | (5.6) | 8 | (10.7) | |
| 1415 | 85 | (68.0) | 32 | (42.7) | |
| Unknown | 14 | (11.2) | 5 | (6.7) | |
| Shock index, n (%) | 0.13 | ||||
| 1 | 105 | (84.0) | 54 | (72.0) | |
| >1 | 19 | (15.2) | 18 | (24.0) | |
| Unknown | 1 | (0.8) | 3 | (4.0) | |
| Respiratory rate, n (%) | 0.02 | ||||
| 20 | 59 | (47.2) | 21 | (28.0) | |
| 20 | 66 | (52.8) | 52 | (69.3) | |
| Unknown | 0 | (0.0) | 2 | (2.7) | |
| Oxygen saturation, n (%) | 0.03 | ||||
| 88 | 2 | (1.6) | 6 | (8.0) | |
| 88 | 122 | (97.6) | 65 | (86.7) | |
| Unknown | 1 | (0.8) | 4 | (5.3) | |
| Temperature, n (%) | 0.09 | ||||
| 38.0 | 100 | (80.0) | 51 | (68.0) | |
| 38.0 | 25 | (20.0) | 23 | (30.7) | |
| Unknown | 0 | (0.0) | 1 | (1.3) | |
| Canadian Triage and Acuity Scale, n (%) | 0.13 | ||||
| Resuscitation | 1 | (0.8) | 3 | (4.0) | |
| Emergent | 70 | (56.0) | 49 | (65.3) | |
| Urgent | 52 | (41.6) | 22 | (29.3) | |
| Less urgent and nonurgent | 2 | (1.6) | 1 | (1.3) | |
| Discharge diagnosis, n (%) | 0.29 | ||||
| Aspiration pneumonia | 12 | (9.6) | 12 | (16.0) | |
| Chronic obstructive pulmonary disease | 15 | (12.0) | 3 | (4.0) | |
| Dehydration/disorders fluid/electrolytes | 9 | (7.2) | 5 | (6.7) | |
| Gastrointestinal hemorrhage | 4 | (3.2) | 3 | (4.0) | |
| Heart failure | 11 | (8.8) | 2 | (2.7) | |
| Infection (other or not identified) | 9 | (7.2) | 9 | (12.0) | |
| Influenza/pneumonia | 14 | (11.2) | 11 | (14.7) | |
| Lower urinary tract infection | 11 | (8.8) | 6 | (8.0) | |
| Other | 40 | (32.0) | 24 | (32.0) | |
| Hospitalization included ICU stay, n (%) | 0.01 | ||||
| No | 124 | (99.2) | 69 | (92.0) | |
| Yes | 1 | (0.8) | 6 | (8.0) | |
When these 4 significant clinical and visit characteristics were tested together in a logistic regression analysis, 2 remained statistically significant (Table 2). Patients with lower GCS scores (GCS 1213 and 711) were more likely to have discussions (AOR: 4.4 [95% CI: 1.4‐13.9] and AOR: 5.9 [95% CI: 2.6‐13.2], respectively) and patients with higher respiratory rates were also more likely to have discussions (AOR: 2.3 [95% CI: 1.1‐4.8]).
| Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Glasgow Coma Scale | 0.001 | ||
| 7 | 1.77 | 0.33‐9.58 | 0.51 |
| 711 | 5.90 | 2.64‐13.22 | 0.001 |
| 1213 | 4.43 | 1.41‐13.91 | 0.01 |
| 1415 | Reference | ||
| Respiration | |||
| 20 | Reference | ||
| 20 | 2.32 | 1.12‐4.78 | 0.02 |
| Oxygen saturation | |||
| 88 | 3.35 | 0.55‐20.56 | 0.19 |
| 88 | Reference | 0.05‐1.83 | |
| Hospitalization included ICU stay | |||
| No | Reference | ||
| Yes | 7.87 | 0.83‐74.73 | 0.07 |
GOC Documentation in the Discharge Summary
For the subset of patients who survived index hospitalization and were discharged back to LTC (176 patients or 88%), we also investigated whether the ADs were documented in the discharge summary back to LTC (data not shown). Of the 42 patients (23.9%) who had a change in the AD (18 patients had an AD increase in care intensity due to hospitalization; 24 had a decrease), only 11 (26%) had this AD change documented in the discharge summary.
Outcomes of GOC Documentation
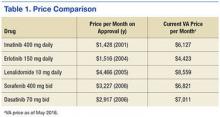
A number of outcomes differed significantly between patients with and without GOC discussions in unadjusted comparisons (Table 3). Patients with discussions had higher rates of orders for no CPR (80% vs 55%) and orders for comfort measures only (7% vs 0%). They also had higher rates of in‐hospital death (29% vs 1%), 1‐year mortality (63% vs 28%), and longer LOS. However, RIW and subsequent hospital use were not found to be significant.
| Variable | Goals of Care Discussion Documented in Medical Chart | ||||
|---|---|---|---|---|---|
| No, N = 125 | Yes, N = 75 | P Value | |||
| |||||
| Physician orders, n (%) | 0.001 | ||||
| Comfort measures only | 0 | (0.0) | 5 | (6.7) | |
| No cardiopulmonary resuscitation | 69 | (55.2) | 60 | (80.0) | |
| Full code | 56 | (44.8) | 10 | (13.3) | |
| Visit disposition, n (%) | 0.001 | ||||
| Long‐term care home | 124 | (99.2) | 52 | (69.3) | |
| Died | 1 | (0.8) | 22 | (29.3) | |
| Transfer to palliative care facility | 0 | (0.0) | 1 | (1.3) | |
| Resource intensity weight, n (%) | 0.43 | ||||
| 0.250.75 | 35 | (28.0) | 19 | (25.3) | |
| 0.761.14 | 29 | (23.2) | 16 | (21.3) | |
| 1.151.60 | 34 | (27.2) | 16 | (21.3) | |
| 1.6125.5 | 27 | (21.6) | 24 | (32.0) | |
| Length of stay, d, n (%) | 0.01 | ||||
| 0.672.97 | 30 | (24.0) | 20 | (26.7) | |
| 2.984.60 | 40 | (32.0) | 10 | (13.3) | |
| 4.618.65 | 30 | (24.0) | 20 | (26.7) | |
| 8.66+ | 25 | (20.0) | 25 | (33.3) | |
| Subsequent emergency department visits in next year, n (% of applicable) | 0.38 | ||||
| 0 | 66 | (53.2) | 32 | (61.5) | |
| 1 | 30 | (24.2) | 13 | (25.0) | |
| 2+ | 28 | (22.6) | 7 | (13.5) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| Subsequent hospitalizations in next year, n (% of applicable) | 0.87 | ||||
| 0 | 87 | (70.2) | 38 | (73.1) | |
| 1 | 24 | (19.4) | 10 | (19.2) | |
| 2+ | 13 | (10.5) | 4 | (7.7) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| 1‐year mortality, n (% of applicable) | 0.001 | ||||
| Alive | 82 | (71.9) | 15 | (37.5) | |
| Dead | 32 | (28.1) | 25 | (62.5) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| Not applicable (unsuccessful follow‐up with long‐term care home) | 10 | 12 | |||
Predictors of In‐hospital Death and 1‐Year Mortality
Given the significant positive associations between discussions and in‐hospital death and 1‐year mortality, we performed separate logistic regression analyses to test whether discussions independently predicted in‐hospital death and 1‐year mortality (Table 4). After adjusting for variables significant in their respective bivariate analyses, patients with discussions continued to have higher odds of in‐hospital death (AOR: 52.0 [95% CI: 6.2‐440.4]) and 1‐year mortality (AOR: 4.1 [95% CI: 1.7‐9.6]). Of note, the presence of dementia had significantly lower adjusted odds of in‐hospital death compared to the reference group of no dementia (AOR: 0.3 [95% CI: 0.1‐0.8]).
| Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| In‐hospital death odds ratios | |||
| Advance directives from long‐term care | 0.91 | ||
| Comfort measures only | Reference | ||
| Supportive care no transfer | 3.43E +18 | 0‐. | 1.00 |
| Transfer to hospital | 3.10E +8 | 0‐. | 1.00 |
| Aggressive care | 4.85E +8 | 0‐. | 1.00 |
| Dementia | |||
| No | Reference | ||
| Yes | .25 | 0.08‐0.79 | 0.02 |
| Previous hospitalizations in last year | 0.05 | ||
| 0 | Reference | ||
| 1 | 0.43 | 0.08‐2.38 | 0.34 |
| 2+ | 6.30 | 1.10‐36.06 | 0.04 |
| Respiration | |||
| 20 | Reference | ||
| 20 | 3.64 | 0.82‐16.24 | 0.09 |
| Documented goals of care discussion | |||
| No | Reference | ||
| Yes | 52.04 | 6.15‐440.40 | 0.001 |
| 1‐year mortality odds ratios | |||
| Oxygen saturation, n (%) | |||
| 88 | 12.15 | 1.18‐124.97 | 0.04 |
| 88 | Reference | ||
| Previous ED visits in last year | 0.06 | ||
| 0 | Reference | ||
| 1 | 3.07 | 1.15‐8.17 | 0.03 |
| 2+ | 3.21 | 0.87‐11.81 | 0.08 |
| Previous hospitalizations in last year | 0.55 | ||
| 0 | Reference | ||
| 1 | 1.66 | 0.57‐4.86 | 0.36 |
| 2+ | 2.52 | 0.30‐20.89 | 0.39 |
| Documented goals of care discussion | |||
| No | Reference | ||
| Yes | 4.07 | 1.73‐9.56 | 0.001 |
DISCUSSION
Our retrospective study of LTC residents admitted to the GIM service showed that these admissions comprised 9.4% of all admissions and that GOC discussions occurred infrequently (37.5%). Our study revealed no differences in baseline patient characteristics associated with discussions, whereas patient acuity at hospital presentation independently contributed to the likelihood of discussions. We found strong associations between documentation and certain outcomes of care, including orders for AD, LOS, in‐hospital death, and 1‐year mortality. No significant associations were found between documentation and subsequent hospital use. Lastly, we found that consistent communication back to the LTC home when there was a change in AD was very poor; only 26% of discharge summaries included this documentation.
Our finding of infrequent GOC discussions during hospitalization aligns with prior studies. A study that identified code status discussions in transcripts of audio‐recorded admission encounters found that code status was discussed in only 24% of seriously ill patient admissions.[17] Furthermore, in a study specific to LTC residents, only 42% of admissions longer than 48 hours had a documented GOC discussion.[15]
We found visit‐level, but not baseline, characteristics were associated with discussions. These findings are supported by a recent study that found that whether GOC discussions took place largely depended on the acute condition presented on admission.[15] Although these results suggest that clinicians are appropriately prioritizing sicker patients who might have the most pressing need for GOC discussions, they also highlight the gap in care for less‐sick patients and the need to broaden clinical practice and consider underlying conditions and functional status. Of note, although the GCS score was found to be significantly associated with discussions, patients in the lowest GCS range did not have significantly different odds of discussions compared to the reference level (highest GCS range). A recent study by You et al. may offer some insight into this finding. They found that patients lacking capacity to make GOC decisions was ranked fifth, whereas lack of SDM availability was eighth among 21 barriers to GOC discussions, as perceived by hospital‐based clinicians.[16]
A major finding of this study was that both in‐hospital and 1‐year mortality were strongly associated with having a GOC discussion, suggesting that patients at higher risk of dying are more likely to have discussions. This is reflected by illness severity measured at initial assessment and by persistence of the association between discussions and mortality after discharge back to LTC. To the best of our knowledge, no previous studies have reported these findings. There are likely some unmeasured clinical factors such as clinical deterioration during hospitalization that contributed to this strong association. Interestingly, in our logistic regression analysis for independent predictors of in‐hospital death, we found that having dementia was associated with lower odds of in‐hospital death. One interpretation of this finding is that perhaps only patients with mild dementia were hospitalized, and those with more advanced dementia had an AD established in LTC that allowed them to remain in their LTC home. This possibility is supported by a systematic review of factors associated with LTC home hospitalization, which found that dementia was shown to be associated with less hospitalization.[18]
For patients who survived hospitalization, we did not find an association between GOC discussions and hospital use in the year following index hospitalization. In both groups, nearly 30% of patients had 1 or more subsequent hospitalizations. This is relevant especially in light of the finding that among patients where GOC discussions resulted in an AD change, only 26% of discharge summaries back to LTC included this documentation. We can only speculate that had these discussions been properly documented, subsequent hospitalizations would have decreased in the GOC group. Previous research has found that omissions of critical information in discharge summaries were common. In a study of hip fracture and stroke patients discharged from a large Midwestern academic medical center in the United States, code status was included in the discharge summary only 7% of the time.[19] The discharge summary is the primary means of sharing patient information between the hospital and LTC home. If GOC discussions are not included in the discharge summary, it is very unlikely that this information will be subsequently updated in the LTC medical record and impact the care the patient receives. A key recommendation for hospital‐based providers is ensuring that GOC discussions are clearly, consistently, and completely documented in the discharge summary so that the care provided is based on the patients' wishes.
Our study has several limitations. Our analysis was based on chart review, and although our analyses take into account a number of patient characteristics, we did not capture other characteristics that might influence GOC discussions such as culture/religion, language barriers, SDM availability, or whether patients clinically deteriorated during the index admission. Additionally, provider‐level predictors, including seniority, previous GOC training, and time available to conduct these discussions, were not captured. We also did not capture the timing or number of occasions that GOC discussions took place during hospitalization. Due to the retrospective nature of our study, we were able to only look at documented GOC discussions. GOC discussions may have happened but were never documented. However, the standard of care is to document these discussions as part of the medical record, and if they are not documented, it can be considered not to have happened and indicates a lower quality of practice. A recent survey of Canadian hospital‐based healthcare providers identified standardized GOC documentation as an effective practice to improve GOC communication.[20] Finally, because our study was conducted in 2 academic hospitals, our results may be less generalizable to other community hospitals. However, our hospitals' catchment areas capture a diverse population, both culturally and in terms of their socioeconomic status.
CONCLUSION
GOC discussions occurred infrequently, appeared to be triggered by illness severity, and were poorly communicated back to LTC. Important outcomes of care, including in‐hospital death and 1‐year mortality, were associated with discussions. This study serves to identify gaps in who might benefit from GOC discussions and illustrates opportunities for improvement including implementing standardized documentation practices.
Disclosures
Hannah J. Wong, PhD, and Robert C. Wu, MD, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Robert C. Wu, MD, Hannah J. Wong, PhD, and Michelle Grinman, MD, were responsible for the conception and design of the study. Robert C. Wu, MD, Hannah J. Wong, PhD, and Jamie Wang were responsible for the acquisition of the data. All of the authors were responsible for the analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the manuscript. Hannah J. Wong, PhD obtained the funding. Hannah J. Wong, PhD, and Robert C. Wu, MD, supervised the study. The authors report no conflicts of interest.
- , , , , . Trends in emergency department visits for ambulatory care sensitive conditions by elderly nursing home residents, 2001 to 2010. JAMA Intern Med. 2014;174(1):156–158.
- , , , . Hospital transfers of nursing home residents with advanced dementia. J Am Geriatr Soc. 2012;60(5):905–909.
- , , , , . Potentially avoidable hospitalizations for elderly long‐stay residents in nursing homes. Med Care. 2013;51(8):673–681.
- , . Reducing unnecessary hospitalizations of nursing home residents. N Engl J Med. 2011;365(13):1165–1167.
- , , . Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211–1218.
- , , , , , . The consistency between treatments provided to nursing facility residents and orders on the physician orders for life‐sustaining treatment form. J Am Geriatr Soc. 2011;59(11):2091–2099.
- , , . What should be the goal of advance care planning? JAMA Intern Med. 2014;174(7):1093–1094.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , et al. Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial. JAMA. 2000;283(11):1437–1444.
- , . Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003.
- , , . Predictors of nursing home residents' time to hospitalization. Health Serv Res. 2011;46(1 pt 1):82–104.
- , , , . Regional variation in the association between advance directives and end‐of‐life Medicare expenditures. JAMA. 2011;306(13):1447–1453.
- , , . Just ask: discussing goals of care with patients in hospital with serious illness. CMAJ. 2014;186(6):425–432.
- , , , et al. Failure to engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med. 2013;173(9):778–787.
- , , , . Hospitalisation of high‐care residents of aged care facilities: are goals of care discussed? Intern Med J. 2013;43(2):144–149.
- , , , et al. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: a multicenter survey of clinicians. JAMA Intern Med. 2015;175(4):549–556.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Predictors of nursing home hospitalization: a review of the literature. Med Care Res Rev. 2008;65(1):3–39.
- , , , , . Provider characteristics, clinical‐work processes and their relationship to discharge summary quality for sub‐acute care patients. J Gen Intern Med. 2012;27(1):78–84.
- , , , . Strategies for effective goals of care discussions and decision‐making: perspectives from a multi‐centre survey of Canadian hospital‐based healthcare providers. BMC Palliat Care. 2015;14:38.
Hospitalizations of long‐term care (LTC) residents are known to be frequent, costly, often preventable,[1, 2, 3] and potentially associated with negative health outcomes.[4] Often, an advance directive (AD) is made at LTC admission and updated annually when residents are in relatively stable health. An AD is a document that helps to inform a substitute decision maker (SDM) about the consent process for life‐sustaining treatments and is a resource that supports advance care planning (ACP). ACP is a process that allows individuals to consider, express, and plan for future healthcare in the event that they lack capacity to make their own decisions. When an LTC resident's health deteriorates and hospitalization is required, there is an opportunity to update prognosis, discuss risks and benefits of previously held treatment preferences, as well as reassess goals of care (GOC).
Engaging in ACP discussions during relatively stable health can help ensure patient preferences are followed.[5, 6] These discussions, however, are often insufficient, as they involve decision making for hypothetical situations that may not cover all potential scenarios, and may not reflect a patient's reality at the time of health status decline. Discussions held in the moment more authentically reflect the decisions of patients and/or SDM based on the specific needs and clinical realities particular to the patient at that time.[7] GOC discussions, defined in this context as ACP discussions occurring during hospitalization, have the potential to better align patient wishes with care received,[6] improve quality of life and satisfaction,[8, 9, 10] and reduce unwanted extra care.[11, 12] Although in‐the‐moment GOC discussions are recommended for all hospitalized patients who are seriously ill with a high risk of dying,[13] research suggests that this occurs infrequently for elderly patients. A recent multicenter survey of seriously ill hospitalized elderly patients found that only 25% of patients and 32% of family members reported that they had been asked about prior ACP or AD.[14] Another study of hospitalized LTC residents found that resuscitation status and family discussion was documented in only 55% and 42% of admissions, respectively.[15]
Further investigation is required to determine how often LTC patients have GOC discussions, what prompts these discussions, and what are the outcomes. Previous studies have focused on barriers to performing GOC discussions, rather than the factors that are associated with them.[16] By understanding why these discussions currently happen, we can potentially improve how often they occur and the quality of their outcomes.
The objectives of this study were to determine the rate of documented GOC discussions among hospitalized LTC residents, identify factors that were associated with documentation, and examine the association between documentation and outcomes of care.
METHODS
Study Population
We conducted a retrospective chart review of a random convenience sample of hospitalized patients admitted via the emergency department (ED) to the general internal medicine (GIM) service from January 1, 2012 through December 31, 2012, at 2 academic teaching hospitals in Toronto, Canada. Patients were identified through a search of each hospitals' electronic patient record (EPR). Patients were eligible for inclusion if they were (1) a LTC resident and (2) at least 65 years of age. For patients with multiple admissions to the GIM service during the specified 12‐month period, we only included data from the first hospitalization (index hospitalization). The hospital's research ethics board approved this study.
Our primary variable of interest was documentation in the hospital medical record of a discussion between physicians and the patient/family/SDM regarding GOC. A GOC discussion was considered to have taken place if there was documentation of (1) understanding/expectation of treatment options or (2) patient's preferences for life‐sustaining measures. Examples illustrating each criterion are provided in the Supporting Information, Appendix 1, in the online version of this article.
Factors Associated With GOC Documentation
From the EPR, we obtained visit‐level data including age, gender, Canadian Emergency Department Triage and Acuity Scale, vital signs at ED admission including temperature, respiratory rate, oxygen saturation, Glasgow Coma Scale (GCS) and shock index (defined as heart rate divided by systolic blood pressure), admission and discharge dates/times, discharge diagnosis, transfer to intensive care unit (ICU), and hospital use (number of ED visits and hospitalizations to the 2 study hospitals in the 1‐year period prior to index hospitalization).
Trained study personnel (J.W.) used a structured abstraction form to collect data from the hospital medical record that were not available through the EPR, including years living in LTC, contents of LTC AD forms, presence of SDM (identified as immediate family or surrogate with whom the care team communicated), dementia diagnosis (defined as documentation of dementia in the patient's past medical history and/or history of present illness), and measures of functional status. When available, we extracted the AD from LTC; they consisted of 4 levels (level 1: comfort careno transfer to hospital, no cardiopulmonary resuscitation [CPR]; level 2: supportive careadministration of antibiotics and/or other procedures that can be provided within LTC, no transfer to the hospital, no CPR; level 3: transfer to the hospitalno CPR; level 4: aggressive interventiontransfer to hospital for aggressive treatment, CPR).
GOC Documentation in the Discharge Summary
For the subset of patients who survived hospitalization and were discharged back to LTC, we examined whether the ADs ordered during hospitalization were communicated back to LTC via the discharge summary. We additionally assessed if the ADs determined during hospitalization differed from preferences documented prior to hospitalization. Physician orders for ADs were categorized as level 1: comfort measures only, level 3: no CPR, or level 4: full code. LTC level 2 was considered equivalent to physician‐ordered level 3 at admission; a patient with an LTC level 2 with no CPR (level 3) documented during hospitalized would be considered to have no change in the AD. An increase or decrease in the AD was determined by comparing LTC levels 1, 3, and 4 to physician‐ordered level 1, 3, and 4.
Outcomes of GOC Documentation
From the EPR, we obtained visit‐level outcome data including length of stay (LOS), resource intensity weight (RIW) (calculated based on patient case‐mix, severity, age, and procedures performed), visit disposition, number of ED visits and hospitalizations to the 2 study hospitals in the year following index hospitalization, in‐hospital death, and 1‐year mortality. We determined 1‐year mortality by following up with the LTC homes to determine whether the resident had died within the year following index hospitalization; only patients from LTC homes that responded to our request for data were included in 1‐year mortality analyses. We collected physician orders for the AD from chart review.
Statistical Analysis
Patients with and without documented GOC discussions were compared. Descriptive statistics including frequencies and percentages were used to characterize study variables. Differences between the study groups were assessed using Pearson 2/Fisher exact test. Multivariate logistic regression, which included variables that were significant in the bivariate analysis, was used to identify independent predictors of GOC discussion. Adjusted odds ratios (AOR) and 95% confidence intervals (CI) were presented for the logistic model. Patients with missing predictor data were excluded.
We also examined whether there was a correlation between GOC discussion and outcomes of care using Pearson 2/Fisher exact test. Outcomes included orders for the AD, LOS in days (stratified into quartiles), RIW (stratified into quartiles), visit disposition, hospital use in the year following index hospitalization, and 1‐year mortality following discharge back to LTC.
Lastly, to better understand the independent predictors of in‐hospital and 1‐year mortality, we used Pearson 2/Fisher exact test followed by logistic regression that included significant variables from the bivariate analyses.
All analyses were 2‐sided, and a P value of 0.05 was considered statistically significant. We used SPSS version 22.0 (SPSS Inc., Chicago, IL).
RESULTS
We identified a total of 7084 hospitalizations to GIM between January 1, 2012 and December 31, 2012, of which 665 (9.4%) met inclusion criteria of residence in LTC and age 65 years. Of these 665 hospitalizations, 512 were unique patients. We randomly selected a convenience sample of 200 index hospitalizations of the 512 eligible hospitalizations (39%) to perform the chart review.
Predictors of GOC Documentation
Of the 200 randomly sampled charts that were reviewed, 75 (37.5%) had a documented GOC discussion.
Characteristics of the study patients and results of bivariate analysis of the association between patient characteristics and GOC discussion are summarized in Table 1. No significant differences in demographic and baseline characteristics were seen between patients with and without discussion. However, a number of visit characteristics were found to be significantly associated with discussion. Forty percent of patients in the GOC discussion group had GCS scores 11 compared to 15.2% in the no‐discussion group. Higher respiratory rate, lower oxygen saturation, and ICU transfer were also significantly associated with discussions.
| Goals of Care Discussion Documented in Medical Chart | |||||
|---|---|---|---|---|---|
| No, N = 125 | Yes, N = 75 | P Value | |||
| |||||
| Baseline characteristics | |||||
| Gender, n (%) | 0.88 | ||||
| Male | 48 | (38.4) | 30 | (40.0) | |
| Female | 77 | (61.6) | 45 | (60.0) | |
| Age, y, n (%) | 0.85 | ||||
| 6579 | 36 | (28.8) | 19 | (25.3) | |
| 8084 | 30 | (24.0) | 19 | (25.3) | |
| 8589 | 30 | (24.0) | 16 | (21.3) | |
| 90101 | 29 | (23.2) | 21 | (28.0) | |
| Years living in long‐term care, n (%)* | 0.65 | ||||
| [0, 1) | 28 | (22.4) | 12 | (16.0) | |
| [1, 3) | 31 | (24.8) | 22 | (29.3) | |
| [3, 6) | 33 | (26.4) | 22 | (29.3) | |
| [6, 22) | 25 | (20.0) | 13 | (17.3) | |
| Unknown | 8 | (6.4) | 6 | (8.0) | |
| AD from long‐term care, n (%) | 0.14 | ||||
| Comfort measures only | 2 | (1.6) | 1 | (1.3) | |
| Supportive care with no transfer to hospital | 0 | (0.0) | 3 | (4.0) | |
| Supportive care with transfer to hospital | 70 | (56.0) | 44 | (58.7) | |
| Aggressive care | 53 | (42.4) | 27 | (36.0) | |
| Years since most recent AD signed, n (%)* | 0.12 | ||||
| [0, 1) | 79 | (63.2) | 48 | (64.0) | |
| [1, 2) | 21 | (16.8) | 6 | (8.0) | |
| [2, 6) | 9 | (7.2) | 10 | (13.3) | |
| Unknown | 16 | (12.8) | 11 | (14.7) | |
| Substitute decision maker, n (%) | 0.06 | ||||
| Child | 81 | (64.8) | 44 | (58.7) | |
| Spouse | 9 | (7.2) | 15 | (20.0) | |
| Other | 26 | (20.8) | 13 | (17.3) | |
| Public guardian trustee | 6 | (4.8) | 2 | (2.7) | |
| Unknown | 3 | (2.4) | 1 | (1.3) | |
| Dementia, n (%) | 1.00 | ||||
| No | 47 | (37.6) | 28 | (37.3) | |
| Yes | 78 | (62.4) | 47 | (62.7) | |
| Mobility, n (%) | 0.26 | ||||
| Walk without assistance | 5 | (4.0) | 3 | (4.0) | |
| Walker | 16 | (12.8) | 3 | (4.0) | |
| Wheelchair | 43 | (34.4) | 29 | (38.7) | |
| Bedridden | 7 | (5.6) | 4 | (5.3) | |
| Unknown | 54 | (43.2) | 36 | (48.0) | |
| Continence, n (%) | 0.05 | ||||
| Mostly continent | 16 | (12.8) | 3 | (4.0) | |
| Incontinent | 49 | (39.2) | 34 | (45.3) | |
| Catheter/stoma | 7 | (5.6) | 1 | (1.3) | |
| Unknown | 53 | (42.4) | 37 | (49.3) | |
| Feeding, n (%) | 0.17 | ||||
| Mostly feeds self | 38 | (30.4) | 13 | (17.3) | |
| Needs to be fed | 17 | (13.6) | 14 | (18.7) | |
| Gastrostomy tube | 8 | (6.4) | 5 | (6.7) | |
| Unknown | 62 | (49.6) | 43 | (57.3) | |
| Diet, n (%) | 0.68 | ||||
| Normal | 43 | (34.4) | 16 | (21.3) | |
| Dysphagic | 32 | (25.6) | 15 | (20.0) | |
| Gastrostomy tube | 8 | (6.4) | 5 | (6.7) | |
| Unknown | 42 | (33.6) | 39 | (52.0) | |
| Previous ED visits in last year, n (%) | 0.43 | ||||
| 0 | 70 | (56.0) | 41 | (54.7) | |
| 1 | 35 | (28.0) | 17 | (22.7) | |
| 2+ | 20 | (16.0) | 17 | (22.7) | |
| Previous hospitalizations in last year, n (%) | 0.19 | ||||
| 0 | 98 | (78.4) | 54 | (72.0) | |
| 1 | 23 | (18.4) | 14 | (18.7) | |
| 2+ | 4 | (3.2) | 7 | (9.3) | |
| Visit characteristics | |||||
| Glasgow Coma Scale, n (%) | 0.001 | ||||
| 7 | 4 | (3.2) | 4 | (5.3) | |
| 711 | 15 | (12.0) | 26 | (34.7) | |
| 1213 | 7 | (5.6) | 8 | (10.7) | |
| 1415 | 85 | (68.0) | 32 | (42.7) | |
| Unknown | 14 | (11.2) | 5 | (6.7) | |
| Shock index, n (%) | 0.13 | ||||
| 1 | 105 | (84.0) | 54 | (72.0) | |
| >1 | 19 | (15.2) | 18 | (24.0) | |
| Unknown | 1 | (0.8) | 3 | (4.0) | |
| Respiratory rate, n (%) | 0.02 | ||||
| 20 | 59 | (47.2) | 21 | (28.0) | |
| 20 | 66 | (52.8) | 52 | (69.3) | |
| Unknown | 0 | (0.0) | 2 | (2.7) | |
| Oxygen saturation, n (%) | 0.03 | ||||
| 88 | 2 | (1.6) | 6 | (8.0) | |
| 88 | 122 | (97.6) | 65 | (86.7) | |
| Unknown | 1 | (0.8) | 4 | (5.3) | |
| Temperature, n (%) | 0.09 | ||||
| 38.0 | 100 | (80.0) | 51 | (68.0) | |
| 38.0 | 25 | (20.0) | 23 | (30.7) | |
| Unknown | 0 | (0.0) | 1 | (1.3) | |
| Canadian Triage and Acuity Scale, n (%) | 0.13 | ||||
| Resuscitation | 1 | (0.8) | 3 | (4.0) | |
| Emergent | 70 | (56.0) | 49 | (65.3) | |
| Urgent | 52 | (41.6) | 22 | (29.3) | |
| Less urgent and nonurgent | 2 | (1.6) | 1 | (1.3) | |
| Discharge diagnosis, n (%) | 0.29 | ||||
| Aspiration pneumonia | 12 | (9.6) | 12 | (16.0) | |
| Chronic obstructive pulmonary disease | 15 | (12.0) | 3 | (4.0) | |
| Dehydration/disorders fluid/electrolytes | 9 | (7.2) | 5 | (6.7) | |
| Gastrointestinal hemorrhage | 4 | (3.2) | 3 | (4.0) | |
| Heart failure | 11 | (8.8) | 2 | (2.7) | |
| Infection (other or not identified) | 9 | (7.2) | 9 | (12.0) | |
| Influenza/pneumonia | 14 | (11.2) | 11 | (14.7) | |
| Lower urinary tract infection | 11 | (8.8) | 6 | (8.0) | |
| Other | 40 | (32.0) | 24 | (32.0) | |
| Hospitalization included ICU stay, n (%) | 0.01 | ||||
| No | 124 | (99.2) | 69 | (92.0) | |
| Yes | 1 | (0.8) | 6 | (8.0) | |
When these 4 significant clinical and visit characteristics were tested together in a logistic regression analysis, 2 remained statistically significant (Table 2). Patients with lower GCS scores (GCS 1213 and 711) were more likely to have discussions (AOR: 4.4 [95% CI: 1.4‐13.9] and AOR: 5.9 [95% CI: 2.6‐13.2], respectively) and patients with higher respiratory rates were also more likely to have discussions (AOR: 2.3 [95% CI: 1.1‐4.8]).
| Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Glasgow Coma Scale | 0.001 | ||
| 7 | 1.77 | 0.33‐9.58 | 0.51 |
| 711 | 5.90 | 2.64‐13.22 | 0.001 |
| 1213 | 4.43 | 1.41‐13.91 | 0.01 |
| 1415 | Reference | ||
| Respiration | |||
| 20 | Reference | ||
| 20 | 2.32 | 1.12‐4.78 | 0.02 |
| Oxygen saturation | |||
| 88 | 3.35 | 0.55‐20.56 | 0.19 |
| 88 | Reference | 0.05‐1.83 | |
| Hospitalization included ICU stay | |||
| No | Reference | ||
| Yes | 7.87 | 0.83‐74.73 | 0.07 |
GOC Documentation in the Discharge Summary
For the subset of patients who survived index hospitalization and were discharged back to LTC (176 patients or 88%), we also investigated whether the ADs were documented in the discharge summary back to LTC (data not shown). Of the 42 patients (23.9%) who had a change in the AD (18 patients had an AD increase in care intensity due to hospitalization; 24 had a decrease), only 11 (26%) had this AD change documented in the discharge summary.
Outcomes of GOC Documentation
A number of outcomes differed significantly between patients with and without GOC discussions in unadjusted comparisons (Table 3). Patients with discussions had higher rates of orders for no CPR (80% vs 55%) and orders for comfort measures only (7% vs 0%). They also had higher rates of in‐hospital death (29% vs 1%), 1‐year mortality (63% vs 28%), and longer LOS. However, RIW and subsequent hospital use were not found to be significant.
| Variable | Goals of Care Discussion Documented in Medical Chart | ||||
|---|---|---|---|---|---|
| No, N = 125 | Yes, N = 75 | P Value | |||
| |||||
| Physician orders, n (%) | 0.001 | ||||
| Comfort measures only | 0 | (0.0) | 5 | (6.7) | |
| No cardiopulmonary resuscitation | 69 | (55.2) | 60 | (80.0) | |
| Full code | 56 | (44.8) | 10 | (13.3) | |
| Visit disposition, n (%) | 0.001 | ||||
| Long‐term care home | 124 | (99.2) | 52 | (69.3) | |
| Died | 1 | (0.8) | 22 | (29.3) | |
| Transfer to palliative care facility | 0 | (0.0) | 1 | (1.3) | |
| Resource intensity weight, n (%) | 0.43 | ||||
| 0.250.75 | 35 | (28.0) | 19 | (25.3) | |
| 0.761.14 | 29 | (23.2) | 16 | (21.3) | |
| 1.151.60 | 34 | (27.2) | 16 | (21.3) | |
| 1.6125.5 | 27 | (21.6) | 24 | (32.0) | |
| Length of stay, d, n (%) | 0.01 | ||||
| 0.672.97 | 30 | (24.0) | 20 | (26.7) | |
| 2.984.60 | 40 | (32.0) | 10 | (13.3) | |
| 4.618.65 | 30 | (24.0) | 20 | (26.7) | |
| 8.66+ | 25 | (20.0) | 25 | (33.3) | |
| Subsequent emergency department visits in next year, n (% of applicable) | 0.38 | ||||
| 0 | 66 | (53.2) | 32 | (61.5) | |
| 1 | 30 | (24.2) | 13 | (25.0) | |
| 2+ | 28 | (22.6) | 7 | (13.5) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| Subsequent hospitalizations in next year, n (% of applicable) | 0.87 | ||||
| 0 | 87 | (70.2) | 38 | (73.1) | |
| 1 | 24 | (19.4) | 10 | (19.2) | |
| 2+ | 13 | (10.5) | 4 | (7.7) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| 1‐year mortality, n (% of applicable) | 0.001 | ||||
| Alive | 82 | (71.9) | 15 | (37.5) | |
| Dead | 32 | (28.1) | 25 | (62.5) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| Not applicable (unsuccessful follow‐up with long‐term care home) | 10 | 12 | |||
Predictors of In‐hospital Death and 1‐Year Mortality
Given the significant positive associations between discussions and in‐hospital death and 1‐year mortality, we performed separate logistic regression analyses to test whether discussions independently predicted in‐hospital death and 1‐year mortality (Table 4). After adjusting for variables significant in their respective bivariate analyses, patients with discussions continued to have higher odds of in‐hospital death (AOR: 52.0 [95% CI: 6.2‐440.4]) and 1‐year mortality (AOR: 4.1 [95% CI: 1.7‐9.6]). Of note, the presence of dementia had significantly lower adjusted odds of in‐hospital death compared to the reference group of no dementia (AOR: 0.3 [95% CI: 0.1‐0.8]).
| Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| In‐hospital death odds ratios | |||
| Advance directives from long‐term care | 0.91 | ||
| Comfort measures only | Reference | ||
| Supportive care no transfer | 3.43E +18 | 0‐. | 1.00 |
| Transfer to hospital | 3.10E +8 | 0‐. | 1.00 |
| Aggressive care | 4.85E +8 | 0‐. | 1.00 |
| Dementia | |||
| No | Reference | ||
| Yes | .25 | 0.08‐0.79 | 0.02 |
| Previous hospitalizations in last year | 0.05 | ||
| 0 | Reference | ||
| 1 | 0.43 | 0.08‐2.38 | 0.34 |
| 2+ | 6.30 | 1.10‐36.06 | 0.04 |
| Respiration | |||
| 20 | Reference | ||
| 20 | 3.64 | 0.82‐16.24 | 0.09 |
| Documented goals of care discussion | |||
| No | Reference | ||
| Yes | 52.04 | 6.15‐440.40 | 0.001 |
| 1‐year mortality odds ratios | |||
| Oxygen saturation, n (%) | |||
| 88 | 12.15 | 1.18‐124.97 | 0.04 |
| 88 | Reference | ||
| Previous ED visits in last year | 0.06 | ||
| 0 | Reference | ||
| 1 | 3.07 | 1.15‐8.17 | 0.03 |
| 2+ | 3.21 | 0.87‐11.81 | 0.08 |
| Previous hospitalizations in last year | 0.55 | ||
| 0 | Reference | ||
| 1 | 1.66 | 0.57‐4.86 | 0.36 |
| 2+ | 2.52 | 0.30‐20.89 | 0.39 |
| Documented goals of care discussion | |||
| No | Reference | ||
| Yes | 4.07 | 1.73‐9.56 | 0.001 |
DISCUSSION
Our retrospective study of LTC residents admitted to the GIM service showed that these admissions comprised 9.4% of all admissions and that GOC discussions occurred infrequently (37.5%). Our study revealed no differences in baseline patient characteristics associated with discussions, whereas patient acuity at hospital presentation independently contributed to the likelihood of discussions. We found strong associations between documentation and certain outcomes of care, including orders for AD, LOS, in‐hospital death, and 1‐year mortality. No significant associations were found between documentation and subsequent hospital use. Lastly, we found that consistent communication back to the LTC home when there was a change in AD was very poor; only 26% of discharge summaries included this documentation.
Our finding of infrequent GOC discussions during hospitalization aligns with prior studies. A study that identified code status discussions in transcripts of audio‐recorded admission encounters found that code status was discussed in only 24% of seriously ill patient admissions.[17] Furthermore, in a study specific to LTC residents, only 42% of admissions longer than 48 hours had a documented GOC discussion.[15]
We found visit‐level, but not baseline, characteristics were associated with discussions. These findings are supported by a recent study that found that whether GOC discussions took place largely depended on the acute condition presented on admission.[15] Although these results suggest that clinicians are appropriately prioritizing sicker patients who might have the most pressing need for GOC discussions, they also highlight the gap in care for less‐sick patients and the need to broaden clinical practice and consider underlying conditions and functional status. Of note, although the GCS score was found to be significantly associated with discussions, patients in the lowest GCS range did not have significantly different odds of discussions compared to the reference level (highest GCS range). A recent study by You et al. may offer some insight into this finding. They found that patients lacking capacity to make GOC decisions was ranked fifth, whereas lack of SDM availability was eighth among 21 barriers to GOC discussions, as perceived by hospital‐based clinicians.[16]
A major finding of this study was that both in‐hospital and 1‐year mortality were strongly associated with having a GOC discussion, suggesting that patients at higher risk of dying are more likely to have discussions. This is reflected by illness severity measured at initial assessment and by persistence of the association between discussions and mortality after discharge back to LTC. To the best of our knowledge, no previous studies have reported these findings. There are likely some unmeasured clinical factors such as clinical deterioration during hospitalization that contributed to this strong association. Interestingly, in our logistic regression analysis for independent predictors of in‐hospital death, we found that having dementia was associated with lower odds of in‐hospital death. One interpretation of this finding is that perhaps only patients with mild dementia were hospitalized, and those with more advanced dementia had an AD established in LTC that allowed them to remain in their LTC home. This possibility is supported by a systematic review of factors associated with LTC home hospitalization, which found that dementia was shown to be associated with less hospitalization.[18]
For patients who survived hospitalization, we did not find an association between GOC discussions and hospital use in the year following index hospitalization. In both groups, nearly 30% of patients had 1 or more subsequent hospitalizations. This is relevant especially in light of the finding that among patients where GOC discussions resulted in an AD change, only 26% of discharge summaries back to LTC included this documentation. We can only speculate that had these discussions been properly documented, subsequent hospitalizations would have decreased in the GOC group. Previous research has found that omissions of critical information in discharge summaries were common. In a study of hip fracture and stroke patients discharged from a large Midwestern academic medical center in the United States, code status was included in the discharge summary only 7% of the time.[19] The discharge summary is the primary means of sharing patient information between the hospital and LTC home. If GOC discussions are not included in the discharge summary, it is very unlikely that this information will be subsequently updated in the LTC medical record and impact the care the patient receives. A key recommendation for hospital‐based providers is ensuring that GOC discussions are clearly, consistently, and completely documented in the discharge summary so that the care provided is based on the patients' wishes.
Our study has several limitations. Our analysis was based on chart review, and although our analyses take into account a number of patient characteristics, we did not capture other characteristics that might influence GOC discussions such as culture/religion, language barriers, SDM availability, or whether patients clinically deteriorated during the index admission. Additionally, provider‐level predictors, including seniority, previous GOC training, and time available to conduct these discussions, were not captured. We also did not capture the timing or number of occasions that GOC discussions took place during hospitalization. Due to the retrospective nature of our study, we were able to only look at documented GOC discussions. GOC discussions may have happened but were never documented. However, the standard of care is to document these discussions as part of the medical record, and if they are not documented, it can be considered not to have happened and indicates a lower quality of practice. A recent survey of Canadian hospital‐based healthcare providers identified standardized GOC documentation as an effective practice to improve GOC communication.[20] Finally, because our study was conducted in 2 academic hospitals, our results may be less generalizable to other community hospitals. However, our hospitals' catchment areas capture a diverse population, both culturally and in terms of their socioeconomic status.
CONCLUSION
GOC discussions occurred infrequently, appeared to be triggered by illness severity, and were poorly communicated back to LTC. Important outcomes of care, including in‐hospital death and 1‐year mortality, were associated with discussions. This study serves to identify gaps in who might benefit from GOC discussions and illustrates opportunities for improvement including implementing standardized documentation practices.
Disclosures
Hannah J. Wong, PhD, and Robert C. Wu, MD, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Robert C. Wu, MD, Hannah J. Wong, PhD, and Michelle Grinman, MD, were responsible for the conception and design of the study. Robert C. Wu, MD, Hannah J. Wong, PhD, and Jamie Wang were responsible for the acquisition of the data. All of the authors were responsible for the analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the manuscript. Hannah J. Wong, PhD obtained the funding. Hannah J. Wong, PhD, and Robert C. Wu, MD, supervised the study. The authors report no conflicts of interest.
Hospitalizations of long‐term care (LTC) residents are known to be frequent, costly, often preventable,[1, 2, 3] and potentially associated with negative health outcomes.[4] Often, an advance directive (AD) is made at LTC admission and updated annually when residents are in relatively stable health. An AD is a document that helps to inform a substitute decision maker (SDM) about the consent process for life‐sustaining treatments and is a resource that supports advance care planning (ACP). ACP is a process that allows individuals to consider, express, and plan for future healthcare in the event that they lack capacity to make their own decisions. When an LTC resident's health deteriorates and hospitalization is required, there is an opportunity to update prognosis, discuss risks and benefits of previously held treatment preferences, as well as reassess goals of care (GOC).
Engaging in ACP discussions during relatively stable health can help ensure patient preferences are followed.[5, 6] These discussions, however, are often insufficient, as they involve decision making for hypothetical situations that may not cover all potential scenarios, and may not reflect a patient's reality at the time of health status decline. Discussions held in the moment more authentically reflect the decisions of patients and/or SDM based on the specific needs and clinical realities particular to the patient at that time.[7] GOC discussions, defined in this context as ACP discussions occurring during hospitalization, have the potential to better align patient wishes with care received,[6] improve quality of life and satisfaction,[8, 9, 10] and reduce unwanted extra care.[11, 12] Although in‐the‐moment GOC discussions are recommended for all hospitalized patients who are seriously ill with a high risk of dying,[13] research suggests that this occurs infrequently for elderly patients. A recent multicenter survey of seriously ill hospitalized elderly patients found that only 25% of patients and 32% of family members reported that they had been asked about prior ACP or AD.[14] Another study of hospitalized LTC residents found that resuscitation status and family discussion was documented in only 55% and 42% of admissions, respectively.[15]
Further investigation is required to determine how often LTC patients have GOC discussions, what prompts these discussions, and what are the outcomes. Previous studies have focused on barriers to performing GOC discussions, rather than the factors that are associated with them.[16] By understanding why these discussions currently happen, we can potentially improve how often they occur and the quality of their outcomes.
The objectives of this study were to determine the rate of documented GOC discussions among hospitalized LTC residents, identify factors that were associated with documentation, and examine the association between documentation and outcomes of care.
METHODS
Study Population
We conducted a retrospective chart review of a random convenience sample of hospitalized patients admitted via the emergency department (ED) to the general internal medicine (GIM) service from January 1, 2012 through December 31, 2012, at 2 academic teaching hospitals in Toronto, Canada. Patients were identified through a search of each hospitals' electronic patient record (EPR). Patients were eligible for inclusion if they were (1) a LTC resident and (2) at least 65 years of age. For patients with multiple admissions to the GIM service during the specified 12‐month period, we only included data from the first hospitalization (index hospitalization). The hospital's research ethics board approved this study.
Our primary variable of interest was documentation in the hospital medical record of a discussion between physicians and the patient/family/SDM regarding GOC. A GOC discussion was considered to have taken place if there was documentation of (1) understanding/expectation of treatment options or (2) patient's preferences for life‐sustaining measures. Examples illustrating each criterion are provided in the Supporting Information, Appendix 1, in the online version of this article.
Factors Associated With GOC Documentation
From the EPR, we obtained visit‐level data including age, gender, Canadian Emergency Department Triage and Acuity Scale, vital signs at ED admission including temperature, respiratory rate, oxygen saturation, Glasgow Coma Scale (GCS) and shock index (defined as heart rate divided by systolic blood pressure), admission and discharge dates/times, discharge diagnosis, transfer to intensive care unit (ICU), and hospital use (number of ED visits and hospitalizations to the 2 study hospitals in the 1‐year period prior to index hospitalization).
Trained study personnel (J.W.) used a structured abstraction form to collect data from the hospital medical record that were not available through the EPR, including years living in LTC, contents of LTC AD forms, presence of SDM (identified as immediate family or surrogate with whom the care team communicated), dementia diagnosis (defined as documentation of dementia in the patient's past medical history and/or history of present illness), and measures of functional status. When available, we extracted the AD from LTC; they consisted of 4 levels (level 1: comfort careno transfer to hospital, no cardiopulmonary resuscitation [CPR]; level 2: supportive careadministration of antibiotics and/or other procedures that can be provided within LTC, no transfer to the hospital, no CPR; level 3: transfer to the hospitalno CPR; level 4: aggressive interventiontransfer to hospital for aggressive treatment, CPR).
GOC Documentation in the Discharge Summary
For the subset of patients who survived hospitalization and were discharged back to LTC, we examined whether the ADs ordered during hospitalization were communicated back to LTC via the discharge summary. We additionally assessed if the ADs determined during hospitalization differed from preferences documented prior to hospitalization. Physician orders for ADs were categorized as level 1: comfort measures only, level 3: no CPR, or level 4: full code. LTC level 2 was considered equivalent to physician‐ordered level 3 at admission; a patient with an LTC level 2 with no CPR (level 3) documented during hospitalized would be considered to have no change in the AD. An increase or decrease in the AD was determined by comparing LTC levels 1, 3, and 4 to physician‐ordered level 1, 3, and 4.
Outcomes of GOC Documentation
From the EPR, we obtained visit‐level outcome data including length of stay (LOS), resource intensity weight (RIW) (calculated based on patient case‐mix, severity, age, and procedures performed), visit disposition, number of ED visits and hospitalizations to the 2 study hospitals in the year following index hospitalization, in‐hospital death, and 1‐year mortality. We determined 1‐year mortality by following up with the LTC homes to determine whether the resident had died within the year following index hospitalization; only patients from LTC homes that responded to our request for data were included in 1‐year mortality analyses. We collected physician orders for the AD from chart review.
Statistical Analysis
Patients with and without documented GOC discussions were compared. Descriptive statistics including frequencies and percentages were used to characterize study variables. Differences between the study groups were assessed using Pearson 2/Fisher exact test. Multivariate logistic regression, which included variables that were significant in the bivariate analysis, was used to identify independent predictors of GOC discussion. Adjusted odds ratios (AOR) and 95% confidence intervals (CI) were presented for the logistic model. Patients with missing predictor data were excluded.
We also examined whether there was a correlation between GOC discussion and outcomes of care using Pearson 2/Fisher exact test. Outcomes included orders for the AD, LOS in days (stratified into quartiles), RIW (stratified into quartiles), visit disposition, hospital use in the year following index hospitalization, and 1‐year mortality following discharge back to LTC.
Lastly, to better understand the independent predictors of in‐hospital and 1‐year mortality, we used Pearson 2/Fisher exact test followed by logistic regression that included significant variables from the bivariate analyses.
All analyses were 2‐sided, and a P value of 0.05 was considered statistically significant. We used SPSS version 22.0 (SPSS Inc., Chicago, IL).
RESULTS
We identified a total of 7084 hospitalizations to GIM between January 1, 2012 and December 31, 2012, of which 665 (9.4%) met inclusion criteria of residence in LTC and age 65 years. Of these 665 hospitalizations, 512 were unique patients. We randomly selected a convenience sample of 200 index hospitalizations of the 512 eligible hospitalizations (39%) to perform the chart review.
Predictors of GOC Documentation
Of the 200 randomly sampled charts that were reviewed, 75 (37.5%) had a documented GOC discussion.
Characteristics of the study patients and results of bivariate analysis of the association between patient characteristics and GOC discussion are summarized in Table 1. No significant differences in demographic and baseline characteristics were seen between patients with and without discussion. However, a number of visit characteristics were found to be significantly associated with discussion. Forty percent of patients in the GOC discussion group had GCS scores 11 compared to 15.2% in the no‐discussion group. Higher respiratory rate, lower oxygen saturation, and ICU transfer were also significantly associated with discussions.
| Goals of Care Discussion Documented in Medical Chart | |||||
|---|---|---|---|---|---|
| No, N = 125 | Yes, N = 75 | P Value | |||
| |||||
| Baseline characteristics | |||||
| Gender, n (%) | 0.88 | ||||
| Male | 48 | (38.4) | 30 | (40.0) | |
| Female | 77 | (61.6) | 45 | (60.0) | |
| Age, y, n (%) | 0.85 | ||||
| 6579 | 36 | (28.8) | 19 | (25.3) | |
| 8084 | 30 | (24.0) | 19 | (25.3) | |
| 8589 | 30 | (24.0) | 16 | (21.3) | |
| 90101 | 29 | (23.2) | 21 | (28.0) | |
| Years living in long‐term care, n (%)* | 0.65 | ||||
| [0, 1) | 28 | (22.4) | 12 | (16.0) | |
| [1, 3) | 31 | (24.8) | 22 | (29.3) | |
| [3, 6) | 33 | (26.4) | 22 | (29.3) | |
| [6, 22) | 25 | (20.0) | 13 | (17.3) | |
| Unknown | 8 | (6.4) | 6 | (8.0) | |
| AD from long‐term care, n (%) | 0.14 | ||||
| Comfort measures only | 2 | (1.6) | 1 | (1.3) | |
| Supportive care with no transfer to hospital | 0 | (0.0) | 3 | (4.0) | |
| Supportive care with transfer to hospital | 70 | (56.0) | 44 | (58.7) | |
| Aggressive care | 53 | (42.4) | 27 | (36.0) | |
| Years since most recent AD signed, n (%)* | 0.12 | ||||
| [0, 1) | 79 | (63.2) | 48 | (64.0) | |
| [1, 2) | 21 | (16.8) | 6 | (8.0) | |
| [2, 6) | 9 | (7.2) | 10 | (13.3) | |
| Unknown | 16 | (12.8) | 11 | (14.7) | |
| Substitute decision maker, n (%) | 0.06 | ||||
| Child | 81 | (64.8) | 44 | (58.7) | |
| Spouse | 9 | (7.2) | 15 | (20.0) | |
| Other | 26 | (20.8) | 13 | (17.3) | |
| Public guardian trustee | 6 | (4.8) | 2 | (2.7) | |
| Unknown | 3 | (2.4) | 1 | (1.3) | |
| Dementia, n (%) | 1.00 | ||||
| No | 47 | (37.6) | 28 | (37.3) | |
| Yes | 78 | (62.4) | 47 | (62.7) | |
| Mobility, n (%) | 0.26 | ||||
| Walk without assistance | 5 | (4.0) | 3 | (4.0) | |
| Walker | 16 | (12.8) | 3 | (4.0) | |
| Wheelchair | 43 | (34.4) | 29 | (38.7) | |
| Bedridden | 7 | (5.6) | 4 | (5.3) | |
| Unknown | 54 | (43.2) | 36 | (48.0) | |
| Continence, n (%) | 0.05 | ||||
| Mostly continent | 16 | (12.8) | 3 | (4.0) | |
| Incontinent | 49 | (39.2) | 34 | (45.3) | |
| Catheter/stoma | 7 | (5.6) | 1 | (1.3) | |
| Unknown | 53 | (42.4) | 37 | (49.3) | |
| Feeding, n (%) | 0.17 | ||||
| Mostly feeds self | 38 | (30.4) | 13 | (17.3) | |
| Needs to be fed | 17 | (13.6) | 14 | (18.7) | |
| Gastrostomy tube | 8 | (6.4) | 5 | (6.7) | |
| Unknown | 62 | (49.6) | 43 | (57.3) | |
| Diet, n (%) | 0.68 | ||||
| Normal | 43 | (34.4) | 16 | (21.3) | |
| Dysphagic | 32 | (25.6) | 15 | (20.0) | |
| Gastrostomy tube | 8 | (6.4) | 5 | (6.7) | |
| Unknown | 42 | (33.6) | 39 | (52.0) | |
| Previous ED visits in last year, n (%) | 0.43 | ||||
| 0 | 70 | (56.0) | 41 | (54.7) | |
| 1 | 35 | (28.0) | 17 | (22.7) | |
| 2+ | 20 | (16.0) | 17 | (22.7) | |
| Previous hospitalizations in last year, n (%) | 0.19 | ||||
| 0 | 98 | (78.4) | 54 | (72.0) | |
| 1 | 23 | (18.4) | 14 | (18.7) | |
| 2+ | 4 | (3.2) | 7 | (9.3) | |
| Visit characteristics | |||||
| Glasgow Coma Scale, n (%) | 0.001 | ||||
| 7 | 4 | (3.2) | 4 | (5.3) | |
| 711 | 15 | (12.0) | 26 | (34.7) | |
| 1213 | 7 | (5.6) | 8 | (10.7) | |
| 1415 | 85 | (68.0) | 32 | (42.7) | |
| Unknown | 14 | (11.2) | 5 | (6.7) | |
| Shock index, n (%) | 0.13 | ||||
| 1 | 105 | (84.0) | 54 | (72.0) | |
| >1 | 19 | (15.2) | 18 | (24.0) | |
| Unknown | 1 | (0.8) | 3 | (4.0) | |
| Respiratory rate, n (%) | 0.02 | ||||
| 20 | 59 | (47.2) | 21 | (28.0) | |
| 20 | 66 | (52.8) | 52 | (69.3) | |
| Unknown | 0 | (0.0) | 2 | (2.7) | |
| Oxygen saturation, n (%) | 0.03 | ||||
| 88 | 2 | (1.6) | 6 | (8.0) | |
| 88 | 122 | (97.6) | 65 | (86.7) | |
| Unknown | 1 | (0.8) | 4 | (5.3) | |
| Temperature, n (%) | 0.09 | ||||
| 38.0 | 100 | (80.0) | 51 | (68.0) | |
| 38.0 | 25 | (20.0) | 23 | (30.7) | |
| Unknown | 0 | (0.0) | 1 | (1.3) | |
| Canadian Triage and Acuity Scale, n (%) | 0.13 | ||||
| Resuscitation | 1 | (0.8) | 3 | (4.0) | |
| Emergent | 70 | (56.0) | 49 | (65.3) | |
| Urgent | 52 | (41.6) | 22 | (29.3) | |
| Less urgent and nonurgent | 2 | (1.6) | 1 | (1.3) | |
| Discharge diagnosis, n (%) | 0.29 | ||||
| Aspiration pneumonia | 12 | (9.6) | 12 | (16.0) | |
| Chronic obstructive pulmonary disease | 15 | (12.0) | 3 | (4.0) | |
| Dehydration/disorders fluid/electrolytes | 9 | (7.2) | 5 | (6.7) | |
| Gastrointestinal hemorrhage | 4 | (3.2) | 3 | (4.0) | |
| Heart failure | 11 | (8.8) | 2 | (2.7) | |
| Infection (other or not identified) | 9 | (7.2) | 9 | (12.0) | |
| Influenza/pneumonia | 14 | (11.2) | 11 | (14.7) | |
| Lower urinary tract infection | 11 | (8.8) | 6 | (8.0) | |
| Other | 40 | (32.0) | 24 | (32.0) | |
| Hospitalization included ICU stay, n (%) | 0.01 | ||||
| No | 124 | (99.2) | 69 | (92.0) | |
| Yes | 1 | (0.8) | 6 | (8.0) | |
When these 4 significant clinical and visit characteristics were tested together in a logistic regression analysis, 2 remained statistically significant (Table 2). Patients with lower GCS scores (GCS 1213 and 711) were more likely to have discussions (AOR: 4.4 [95% CI: 1.4‐13.9] and AOR: 5.9 [95% CI: 2.6‐13.2], respectively) and patients with higher respiratory rates were also more likely to have discussions (AOR: 2.3 [95% CI: 1.1‐4.8]).
| Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| Glasgow Coma Scale | 0.001 | ||
| 7 | 1.77 | 0.33‐9.58 | 0.51 |
| 711 | 5.90 | 2.64‐13.22 | 0.001 |
| 1213 | 4.43 | 1.41‐13.91 | 0.01 |
| 1415 | Reference | ||
| Respiration | |||
| 20 | Reference | ||
| 20 | 2.32 | 1.12‐4.78 | 0.02 |
| Oxygen saturation | |||
| 88 | 3.35 | 0.55‐20.56 | 0.19 |
| 88 | Reference | 0.05‐1.83 | |
| Hospitalization included ICU stay | |||
| No | Reference | ||
| Yes | 7.87 | 0.83‐74.73 | 0.07 |
GOC Documentation in the Discharge Summary
For the subset of patients who survived index hospitalization and were discharged back to LTC (176 patients or 88%), we also investigated whether the ADs were documented in the discharge summary back to LTC (data not shown). Of the 42 patients (23.9%) who had a change in the AD (18 patients had an AD increase in care intensity due to hospitalization; 24 had a decrease), only 11 (26%) had this AD change documented in the discharge summary.
Outcomes of GOC Documentation
A number of outcomes differed significantly between patients with and without GOC discussions in unadjusted comparisons (Table 3). Patients with discussions had higher rates of orders for no CPR (80% vs 55%) and orders for comfort measures only (7% vs 0%). They also had higher rates of in‐hospital death (29% vs 1%), 1‐year mortality (63% vs 28%), and longer LOS. However, RIW and subsequent hospital use were not found to be significant.
| Variable | Goals of Care Discussion Documented in Medical Chart | ||||
|---|---|---|---|---|---|
| No, N = 125 | Yes, N = 75 | P Value | |||
| |||||
| Physician orders, n (%) | 0.001 | ||||
| Comfort measures only | 0 | (0.0) | 5 | (6.7) | |
| No cardiopulmonary resuscitation | 69 | (55.2) | 60 | (80.0) | |
| Full code | 56 | (44.8) | 10 | (13.3) | |
| Visit disposition, n (%) | 0.001 | ||||
| Long‐term care home | 124 | (99.2) | 52 | (69.3) | |
| Died | 1 | (0.8) | 22 | (29.3) | |
| Transfer to palliative care facility | 0 | (0.0) | 1 | (1.3) | |
| Resource intensity weight, n (%) | 0.43 | ||||
| 0.250.75 | 35 | (28.0) | 19 | (25.3) | |
| 0.761.14 | 29 | (23.2) | 16 | (21.3) | |
| 1.151.60 | 34 | (27.2) | 16 | (21.3) | |
| 1.6125.5 | 27 | (21.6) | 24 | (32.0) | |
| Length of stay, d, n (%) | 0.01 | ||||
| 0.672.97 | 30 | (24.0) | 20 | (26.7) | |
| 2.984.60 | 40 | (32.0) | 10 | (13.3) | |
| 4.618.65 | 30 | (24.0) | 20 | (26.7) | |
| 8.66+ | 25 | (20.0) | 25 | (33.3) | |
| Subsequent emergency department visits in next year, n (% of applicable) | 0.38 | ||||
| 0 | 66 | (53.2) | 32 | (61.5) | |
| 1 | 30 | (24.2) | 13 | (25.0) | |
| 2+ | 28 | (22.6) | 7 | (13.5) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| Subsequent hospitalizations in next year, n (% of applicable) | 0.87 | ||||
| 0 | 87 | (70.2) | 38 | (73.1) | |
| 1 | 24 | (19.4) | 10 | (19.2) | |
| 2+ | 13 | (10.5) | 4 | (7.7) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| 1‐year mortality, n (% of applicable) | 0.001 | ||||
| Alive | 82 | (71.9) | 15 | (37.5) | |
| Dead | 32 | (28.1) | 25 | (62.5) | |
| Not applicable (died during index hospitalization or transfer to palliative care) | 1 | 23 | |||
| Not applicable (unsuccessful follow‐up with long‐term care home) | 10 | 12 | |||
Predictors of In‐hospital Death and 1‐Year Mortality
Given the significant positive associations between discussions and in‐hospital death and 1‐year mortality, we performed separate logistic regression analyses to test whether discussions independently predicted in‐hospital death and 1‐year mortality (Table 4). After adjusting for variables significant in their respective bivariate analyses, patients with discussions continued to have higher odds of in‐hospital death (AOR: 52.0 [95% CI: 6.2‐440.4]) and 1‐year mortality (AOR: 4.1 [95% CI: 1.7‐9.6]). Of note, the presence of dementia had significantly lower adjusted odds of in‐hospital death compared to the reference group of no dementia (AOR: 0.3 [95% CI: 0.1‐0.8]).
| Characteristic | Adjusted Odds Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|
| |||
| In‐hospital death odds ratios | |||
| Advance directives from long‐term care | 0.91 | ||
| Comfort measures only | Reference | ||
| Supportive care no transfer | 3.43E +18 | 0‐. | 1.00 |
| Transfer to hospital | 3.10E +8 | 0‐. | 1.00 |
| Aggressive care | 4.85E +8 | 0‐. | 1.00 |
| Dementia | |||
| No | Reference | ||
| Yes | .25 | 0.08‐0.79 | 0.02 |
| Previous hospitalizations in last year | 0.05 | ||
| 0 | Reference | ||
| 1 | 0.43 | 0.08‐2.38 | 0.34 |
| 2+ | 6.30 | 1.10‐36.06 | 0.04 |
| Respiration | |||
| 20 | Reference | ||
| 20 | 3.64 | 0.82‐16.24 | 0.09 |
| Documented goals of care discussion | |||
| No | Reference | ||
| Yes | 52.04 | 6.15‐440.40 | 0.001 |
| 1‐year mortality odds ratios | |||
| Oxygen saturation, n (%) | |||
| 88 | 12.15 | 1.18‐124.97 | 0.04 |
| 88 | Reference | ||
| Previous ED visits in last year | 0.06 | ||
| 0 | Reference | ||
| 1 | 3.07 | 1.15‐8.17 | 0.03 |
| 2+ | 3.21 | 0.87‐11.81 | 0.08 |
| Previous hospitalizations in last year | 0.55 | ||
| 0 | Reference | ||
| 1 | 1.66 | 0.57‐4.86 | 0.36 |
| 2+ | 2.52 | 0.30‐20.89 | 0.39 |
| Documented goals of care discussion | |||
| No | Reference | ||
| Yes | 4.07 | 1.73‐9.56 | 0.001 |
DISCUSSION
Our retrospective study of LTC residents admitted to the GIM service showed that these admissions comprised 9.4% of all admissions and that GOC discussions occurred infrequently (37.5%). Our study revealed no differences in baseline patient characteristics associated with discussions, whereas patient acuity at hospital presentation independently contributed to the likelihood of discussions. We found strong associations between documentation and certain outcomes of care, including orders for AD, LOS, in‐hospital death, and 1‐year mortality. No significant associations were found between documentation and subsequent hospital use. Lastly, we found that consistent communication back to the LTC home when there was a change in AD was very poor; only 26% of discharge summaries included this documentation.
Our finding of infrequent GOC discussions during hospitalization aligns with prior studies. A study that identified code status discussions in transcripts of audio‐recorded admission encounters found that code status was discussed in only 24% of seriously ill patient admissions.[17] Furthermore, in a study specific to LTC residents, only 42% of admissions longer than 48 hours had a documented GOC discussion.[15]
We found visit‐level, but not baseline, characteristics were associated with discussions. These findings are supported by a recent study that found that whether GOC discussions took place largely depended on the acute condition presented on admission.[15] Although these results suggest that clinicians are appropriately prioritizing sicker patients who might have the most pressing need for GOC discussions, they also highlight the gap in care for less‐sick patients and the need to broaden clinical practice and consider underlying conditions and functional status. Of note, although the GCS score was found to be significantly associated with discussions, patients in the lowest GCS range did not have significantly different odds of discussions compared to the reference level (highest GCS range). A recent study by You et al. may offer some insight into this finding. They found that patients lacking capacity to make GOC decisions was ranked fifth, whereas lack of SDM availability was eighth among 21 barriers to GOC discussions, as perceived by hospital‐based clinicians.[16]
A major finding of this study was that both in‐hospital and 1‐year mortality were strongly associated with having a GOC discussion, suggesting that patients at higher risk of dying are more likely to have discussions. This is reflected by illness severity measured at initial assessment and by persistence of the association between discussions and mortality after discharge back to LTC. To the best of our knowledge, no previous studies have reported these findings. There are likely some unmeasured clinical factors such as clinical deterioration during hospitalization that contributed to this strong association. Interestingly, in our logistic regression analysis for independent predictors of in‐hospital death, we found that having dementia was associated with lower odds of in‐hospital death. One interpretation of this finding is that perhaps only patients with mild dementia were hospitalized, and those with more advanced dementia had an AD established in LTC that allowed them to remain in their LTC home. This possibility is supported by a systematic review of factors associated with LTC home hospitalization, which found that dementia was shown to be associated with less hospitalization.[18]
For patients who survived hospitalization, we did not find an association between GOC discussions and hospital use in the year following index hospitalization. In both groups, nearly 30% of patients had 1 or more subsequent hospitalizations. This is relevant especially in light of the finding that among patients where GOC discussions resulted in an AD change, only 26% of discharge summaries back to LTC included this documentation. We can only speculate that had these discussions been properly documented, subsequent hospitalizations would have decreased in the GOC group. Previous research has found that omissions of critical information in discharge summaries were common. In a study of hip fracture and stroke patients discharged from a large Midwestern academic medical center in the United States, code status was included in the discharge summary only 7% of the time.[19] The discharge summary is the primary means of sharing patient information between the hospital and LTC home. If GOC discussions are not included in the discharge summary, it is very unlikely that this information will be subsequently updated in the LTC medical record and impact the care the patient receives. A key recommendation for hospital‐based providers is ensuring that GOC discussions are clearly, consistently, and completely documented in the discharge summary so that the care provided is based on the patients' wishes.
Our study has several limitations. Our analysis was based on chart review, and although our analyses take into account a number of patient characteristics, we did not capture other characteristics that might influence GOC discussions such as culture/religion, language barriers, SDM availability, or whether patients clinically deteriorated during the index admission. Additionally, provider‐level predictors, including seniority, previous GOC training, and time available to conduct these discussions, were not captured. We also did not capture the timing or number of occasions that GOC discussions took place during hospitalization. Due to the retrospective nature of our study, we were able to only look at documented GOC discussions. GOC discussions may have happened but were never documented. However, the standard of care is to document these discussions as part of the medical record, and if they are not documented, it can be considered not to have happened and indicates a lower quality of practice. A recent survey of Canadian hospital‐based healthcare providers identified standardized GOC documentation as an effective practice to improve GOC communication.[20] Finally, because our study was conducted in 2 academic hospitals, our results may be less generalizable to other community hospitals. However, our hospitals' catchment areas capture a diverse population, both culturally and in terms of their socioeconomic status.
CONCLUSION
GOC discussions occurred infrequently, appeared to be triggered by illness severity, and were poorly communicated back to LTC. Important outcomes of care, including in‐hospital death and 1‐year mortality, were associated with discussions. This study serves to identify gaps in who might benefit from GOC discussions and illustrates opportunities for improvement including implementing standardized documentation practices.
Disclosures
Hannah J. Wong, PhD, and Robert C. Wu, MD, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Robert C. Wu, MD, Hannah J. Wong, PhD, and Michelle Grinman, MD, were responsible for the conception and design of the study. Robert C. Wu, MD, Hannah J. Wong, PhD, and Jamie Wang were responsible for the acquisition of the data. All of the authors were responsible for the analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the manuscript. Hannah J. Wong, PhD obtained the funding. Hannah J. Wong, PhD, and Robert C. Wu, MD, supervised the study. The authors report no conflicts of interest.
- , , , , . Trends in emergency department visits for ambulatory care sensitive conditions by elderly nursing home residents, 2001 to 2010. JAMA Intern Med. 2014;174(1):156–158.
- , , , . Hospital transfers of nursing home residents with advanced dementia. J Am Geriatr Soc. 2012;60(5):905–909.
- , , , , . Potentially avoidable hospitalizations for elderly long‐stay residents in nursing homes. Med Care. 2013;51(8):673–681.
- , . Reducing unnecessary hospitalizations of nursing home residents. N Engl J Med. 2011;365(13):1165–1167.
- , , . Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211–1218.
- , , , , , . The consistency between treatments provided to nursing facility residents and orders on the physician orders for life‐sustaining treatment form. J Am Geriatr Soc. 2011;59(11):2091–2099.
- , , . What should be the goal of advance care planning? JAMA Intern Med. 2014;174(7):1093–1094.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , et al. Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial. JAMA. 2000;283(11):1437–1444.
- , . Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003.
- , , . Predictors of nursing home residents' time to hospitalization. Health Serv Res. 2011;46(1 pt 1):82–104.
- , , , . Regional variation in the association between advance directives and end‐of‐life Medicare expenditures. JAMA. 2011;306(13):1447–1453.
- , , . Just ask: discussing goals of care with patients in hospital with serious illness. CMAJ. 2014;186(6):425–432.
- , , , et al. Failure to engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med. 2013;173(9):778–787.
- , , , . Hospitalisation of high‐care residents of aged care facilities: are goals of care discussed? Intern Med J. 2013;43(2):144–149.
- , , , et al. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: a multicenter survey of clinicians. JAMA Intern Med. 2015;175(4):549–556.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Predictors of nursing home hospitalization: a review of the literature. Med Care Res Rev. 2008;65(1):3–39.
- , , , , . Provider characteristics, clinical‐work processes and their relationship to discharge summary quality for sub‐acute care patients. J Gen Intern Med. 2012;27(1):78–84.
- , , , . Strategies for effective goals of care discussions and decision‐making: perspectives from a multi‐centre survey of Canadian hospital‐based healthcare providers. BMC Palliat Care. 2015;14:38.
- , , , , . Trends in emergency department visits for ambulatory care sensitive conditions by elderly nursing home residents, 2001 to 2010. JAMA Intern Med. 2014;174(1):156–158.
- , , , . Hospital transfers of nursing home residents with advanced dementia. J Am Geriatr Soc. 2012;60(5):905–909.
- , , , , . Potentially avoidable hospitalizations for elderly long‐stay residents in nursing homes. Med Care. 2013;51(8):673–681.
- , . Reducing unnecessary hospitalizations of nursing home residents. N Engl J Med. 2011;365(13):1165–1167.
- , , . Advance directives and outcomes of surrogate decision making before death. N Engl J Med. 2010;362(13):1211–1218.
- , , , , , . The consistency between treatments provided to nursing facility residents and orders on the physician orders for life‐sustaining treatment form. J Am Geriatr Soc. 2011;59(11):2091–2099.
- , , . What should be the goal of advance care planning? JAMA Intern Med. 2014;174(7):1093–1094.
- , , , et al. Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment. JAMA. 2008;300(14):1665–1673.
- , , , et al. Systematic implementation of an advance directive program in nursing homes: a randomized controlled trial. JAMA. 2000;283(11):1437–1444.
- , . Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med. 2014;174(12):1994–2003.
- , , . Predictors of nursing home residents' time to hospitalization. Health Serv Res. 2011;46(1 pt 1):82–104.
- , , , . Regional variation in the association between advance directives and end‐of‐life Medicare expenditures. JAMA. 2011;306(13):1447–1453.
- , , . Just ask: discussing goals of care with patients in hospital with serious illness. CMAJ. 2014;186(6):425–432.
- , , , et al. Failure to engage hospitalized elderly patients and their families in advance care planning. JAMA Intern Med. 2013;173(9):778–787.
- , , , . Hospitalisation of high‐care residents of aged care facilities: are goals of care discussed? Intern Med J. 2013;43(2):144–149.
- , , , et al. Barriers to goals of care discussions with seriously ill hospitalized patients and their families: a multicenter survey of clinicians. JAMA Intern Med. 2015;175(4):549–556.
- , , , , . Code status discussions between attending hospitalist physicians and medical patients at hospital admission. J Gen Intern Med. 2011;26(4):359–366.
- , , , . Predictors of nursing home hospitalization: a review of the literature. Med Care Res Rev. 2008;65(1):3–39.
- , , , , . Provider characteristics, clinical‐work processes and their relationship to discharge summary quality for sub‐acute care patients. J Gen Intern Med. 2012;27(1):78–84.
- , , , . Strategies for effective goals of care discussions and decision‐making: perspectives from a multi‐centre survey of Canadian hospital‐based healthcare providers. BMC Palliat Care. 2015;14:38.
The Relationship Between Sustained Gripping and the Development of Carpal Tunnel Syndrome
The dominant limb is the limb preferred for performing an activity that requires one hand or for performing the more demanding part of an activity that requires both hands. For example, most playing card dealers use their dominant limb to distribute cards (the more demanding part of the activity) and their nondominant limb to hold the rest of the pack (the less demanding activity). Although a relationship between nocturnal hand paresthesias and daily hand activities has been known for more than a century, it was not until more recently that it was recognized that unilateral carpal tunnel syndrome (CTS) more commonly involves the dominant limb.1,2
Among people with CTS, the dominant limb tends to be affected earlier and, in the setting of bilateral involvement, more severely.3,4 This relationship, however, is not absolute. In 1983, Falck and Aarnio reported that CTS could be more pronounced on the nondominant side whenever upper extremity usage requirements, especially occupational requirements, stressed that limb to a greater extent than they stressed the dominant limb.5
Regarding occupation, particular CTS risk factors and associations have been reported. One study found that the most common work-related risk factor was repetitive bending and twisting of the hands and wrists.6 In another study, the incidence of CTS was almost 10-fold higher among workers performing high force, high repetition jobs than among those performing low force, low repetition jobs.7-10 A meta-analysis identified a strong causal relationship between forceful, repetitive work and development of CTS.11 A more recent and controversial study found no association between heavy use of computers and CTS.12 In 1911, Hart reported an association between repetitive gripping and thenar atrophy.13 Although he misattributed the association to trauma of the recurrent thenar motor branch, 2 of the 3 described patients reported a period of episodic hand paresthesias preceding the development of thenar eminence atrophy and thus more likely had typical CTS.
Background
The present study was prompted by the clinical and electrodiagnostic (EDX) features of a 27-year-old right-hand–dominant man who presented to the EDX laboratory for assessment of bilateral hand paresthesias. The patient reported episodic bilateral hand tingling that was much more pronounced on the left (nondominant) side. Consistent with his report, EDX assessment revealed bilateral CTS that involved the nondominant limb to a much greater extent than that of the dominant limb. As a blackjack dealer, the patient was using his nondominant hand to “tightly grip 2 decks of cards” and the dominant hand to distribute those cards.
Similar history and EDX patterns (bilateral CTS more pronounced on nondominant side) were subsequently noted in 2 other patients, both of whom were using their nondominant limb to perform an activity that required sustained gripping. One of these patients was a minnow counter. He was using his nondominant hand to firmly grip the top of a bucket and the dominant hand to “deal” the fish into separate tanks. The other patient was a mason. He was using his nondominant hand to firmly hold a brick or stone in place and the dominant hand to apply cement. The clinical and EDX features of these 3 patients suggested that sustained gripping might be a significant risk factor for development of CTS. That all 3 of these patients were using their dominant hand for a repetitive activity (dealing) further suggested that, compared with repetitive activity, sustained gripping was more significant as a risk factor for development of CTS.
As unilateral CTS typically occurs on the dominant side, and bilateral CTS typically is more pronounced on the dominant side, the term backward CTS is applied to cases in which unilateral CTS occurs on the nondominant side or bilateral CTS involves the nondominant side to a greater extent than the dominant side.
Although many investigators have purported an association between CTS and a particular upper extremity activity, their conclusions are limited by use of poorly validated symptom surveys, use of faulty epidemiologic methods, selection of a specific basis for clinical diagnosis (eg, isolated hand pain), or lack of EDX confirmation. Associations between a particular activity and development of CTS are best addressed by studies that include both clinical and EDX assessments and that fully characterize the individual hand usage patterns.
Methods
This study identified the upper extremity usage patterns associated with development of CTS among patients found in the EDX laboratory to have backward CTS (unilateral CTS in nondominant limb or bilateral CTS involving nondominant limb more than dominant limb). Thus, whenever patients who were referred to the EDX laboratory for upper extremity studies were noted to have backward CTS, an extensive upper extremity usage assessment was immediately performed. Both the EDX studies and the upper extremity usage assessments were performed by the author during the same encounter.
All patients had initial screening sensory and motor nerve conduction studies performed: median sensory, recording the second digit; ulnar sensory, recording the fifth digit; superficial radial, recording the dorsum of hand; median motor, recording the thenar eminence; and ulnar motor, recording the hypothenar eminence. As CTS was suspected in all cases, median and ulnar palmar nerve conduction studies were performed as well. All these studies were performed using previously reported techniques, and all collected values were compared with EMG laboratory control values.14,15 In all patients, the median nerve conduction studies were performed bilaterally. Approval from an ethics board or an institutional review board was not needed because this study did not involve personal information or identifiable images.
To avoid identifying small, chance asymmetries related to hypothyroidism and other conditions that produce bilateral CTS, the author predefined the degree of asymmetry required for study inclusion to identify only large asymmetries. Because the EDX manifestations of CTS typically reflect features of demyelination before those of axon loss, the required asymmetries were predefined using peak sensory and distal motor latency values. For study inclusion, the median nerve latency value recorded from the nondominant limb needed to exceed the value recorded from the dominant limb by 0.6 msec for the median palmar responses, 1.0 msec for the median digital sensory responses, or 1.0 msec for the median motor responses.
Excluded from the study were patients who reported being ambidextrous, those who had changed hand dominance at any age and for any reason, those with a history of upper extremity trauma or surgery, and those with EDX findings indicating a concomitant neuromuscular disorder. In addition, patients with diabetes mellitus or any other condition associated with bilateral CTS were excluded.
Results
From the approximately 2,000 upper extremity EDX studies performed over a 30-month period, the author identified 21 patients who met the inclusion criteria (Table 1). Of these 21 patients, 15 (71%) had bilateral CTS and 6 (29%) had unilateral CTS. Sixteen of the 21 patients used their nondominant hand, through a significant portion of the day, to perform an activity that required sustained gripping (Table 2).
Of these 16 patients, 14 reported that the sustained gripping activity was related to their occupation: pipe fitter (4 patients), card dealer (4), professional driver (2), grocery store clerk (1), wire stripper (1), bakery worker (1), and motel room cleaner (1). In their jobs, the pipe fitters were continually cutting pipe during their entire 8-hour shift—using the nondominant hand to tightly grip a pipe while using the dominant hand to direct an electrically powered blade through it. Of the card dealers, 1 was a professional playing card dealer (not the dealer whose case prompted this study), 1 distributed store coupons into containers, and 2 distributed pieces of mail into bins (referred to as casing the mail). All the card dealers used their nondominant hand to tightly grip items that the dominant limb distributed. The professional drivers used their nondominant hand to grip the steering wheel. The grocery store clerk used her nondominant hand to grip shopping items while moving them across a barcode detector. The wire stripper used her nondominant hand to tightly grip bundles of wire while holding a tool in the dominant hand to snip or strip them. The bakery worker continually used her nondominant hand to squeeze off pieces of dough from a mound. And the motel room cleaner used her nondominant hand to grip the side of a bathtub while scrubbing the tub with her dominant hand (she estimated she cleaned bathtubs for about 25% of her 8-hour shift).
Of the 2 patients who reported sustained gripping unrelated to occupation, 1 was baby-sitting her grandson 5 days per week. She carried him, grasping his buttock with her nondominant hand, while performing her daily activities. She estimated she carried the child a minimum of 2 hours a day. After several weeks, she noted episodic tingling in the nondominant hand, yet she continued carrying him for another 7 months, at which point she sought medical care. The other patient, a student in a stress relief class, was instructed to repetitively open and tightly close her nondominant hand for 10 minutes 4 or more times per day. After several weeks, she noted episodic tingling in the exercised, nondominant hand.
Of the 5 patients who denied performing an activity that required sustained gripping, 2 used their nondominant limb to enter data into a computer while turning pages with the dominant limb. A piano teacher, used her nondominant limb to strike piano keys while sitting to the right of her pupils; and a typist, consistently slept with the dorsal aspect of the nondominant hand pressed into her cheek, resulting in sustained wrist flexion throughout the night. One patient could not identify an activity performed with her nondominant limb both frequently and for prolonged periods.
Discussion
As with other syndromic disorders, CTS is associated with several clinical features, the presence of which correlates with the severity of median nerve involvement. During the earliest stage of CTS, episodic hand tingling (a positive symptom) is commonly reported. This tingling typically is more pronounced at night and during relaxation. In addition, many patients come to recognize that their hand tingling is precipitated by activities that involve sustained upper extremity elevation (eg, driving with a limb resting on upper portion of steering wheel; reading with upper extremities maintained in forward abduction) and that lowering a symptomatic limb relieves its tingling.
With progression, negative symptoms appear (eg, numbness and then weakness and wasting). Unfortunately, as the negative symptoms replace the positive ones, affected individuals may become less symptomatic and mistakenly believe their condition is improving. Features of autonomic fiber involvement may also be present but are less reliably elicited. Isolated hand pain is an uncommon manifestation of CTS because pain more commonly occurs later in the course and for this reason tends to be accompanied by other features of CTS.
The clinical features of CTS correlate with its underlying pathology. As demyelination precedes axon disruption pathologically, the clinical features of demyelination (episodic paresthesias) precede those of axon loss (numbness, weakness, wasting). However, clinical features may go unrecognized or be dismissed by the patient. Moreover, there is substantial variation in type, intensity, and frequency of symptoms.16,17
The EDX features of CTS correlate with its underlying pathology and pathophysiology. As demyelination (loss of insulation) increases the capacitance of the membrane and increases internodal current leakage, conduction velocity is reduced. As severity worsens and pathology changes from predominantly demyelination to predominantly axon loss, the individual nerve fiber action potentials, which make up the compound responses being recorded, are lost. As a result the amplitude and negative area under the curve values decrease. Thus, the EDX features of demyelination (eg, prolonged latencies) precede those of axon loss (eg, amplitude, negative area under the curve reduction).
As with other focal mononeuropathies, the sensory responses tend to be affected earlier and to a greater degree than do the motor responses. Consequently, the EDX features of CTS typically follow a standard progression. The median palmar responses are involved sooner and to a greater degree than the median sensory responses recorded from the digits, which in turn tend to be involved earlier and to a greater degree than are the median motor responses.
Awareness of this relationship dictates the severity of the lesion and helps in the recognition of a cool limb and in the avoidance of a false-positive study interpretation. In a cool limb, the fingers are cooler than the wrists. Thus, the peak latency of the median digital sensory response is delayed to a greater extent than the ipsilateral median palmar response (the opposite of the CTS pattern). Accordingly, whenever this pattern is identified, the hand must be warmed or rewarmed and the studies repeated. The hand is also warmed or rewarmed whenever the median motor response is delayed out of proportion to that of the median palmar response.
Conclusion
Cases of CTS mainly in the nondominant limb provide an opportunity to identify particular limb usage patterns that might be associated with CTS. Of the present study’s 21 affected patients, 16 were using their nondominant limb to perform activities that required sustained gripping. Fourteen of the 16 activities were related to occupation. These findings strongly suggest an association between activities that require sustained gripping and development of CTS.
That the card dealers simultaneously used their nondominant hand for sustained gripping and the dominant hand for the repetitive activity of dealing suggests that sustained gripping is a stronger risk factor than repetitive activity for the development of CTS—an unanticipated finding. Interestingly, in a 2001 study that suggested repetitive activity might not be a CTS risk factor, there was a higher incidence of CTS among computer users working with a mouse—an activity that requires sustained gripping.12
Episodic hand tingling during mouse use likely reflects impaired blood flow to the median nerve, which occurs when carpal tunnel pressure approaches or exceeds 20 to 30 mm Hg.18 Placement of a hand on a mouse increases intracarpal pressure from 3 to 5 mm Hg (wrist in neutral position) to 16 to 21 mm Hg, whereas mouse use increases intracarpal pressure to 28 to 33 mm Hg.18-20
1. Ormerod JA. On a peculiar numbness and paresis of the hands. St Barts Hosp Rep. 1883;19:17-26.
2. Rosenbaum RB, Ochoa JL. Carpal Tunnel Syndrome and Other Disorders of the Median Nerve. 2nd ed. Boston, MA: Butterworth-Heineman; 2002.
3. Gainer JV Jr, Nugent GR. Carpal tunnel syndrome: report of 430 operations. South Med J. 1977;70(3):325-328.
4. Reinstein L. Hand dominance in carpal tunnel syndrome. Arch Phys Med Rehabil. 1981;62(5):202-203.
5. Falck B, Aarnio P. Left-sided carpal tunnel syndrome in butchers. Scand J Work Environ Health. 1983;9(3):291-297.
6. Tanaka S, Wild DK, Seligman PJ, Halperin WE, Behrens VJ, Putz-Anerson V. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among U.S. workers: analysis of the Occupational Health Supplement data of 1988 National Health Interview Survey. Am J Ind Med. 1995;27(4):451-470.
7. Silverstein BA, Fine LJ, Armstrong TJ. Occupational factors and carpal tunnel syndrome. Am J Ind Med. 1987;11(3):343-358.
8. de Krom MC, Kester AD, Knipschild PG, Spaans F. Risk factors for carpal tunnel syndrome. Am J Epidemiol. 1990;132(6):1102-1110.
9. Hales TR, Bernard BP. Epidemiology of work-related musculoskeletal disorders. Orthop Clin North Am. 1996;27(4):679-709.
10. Roquelaure Y, Ha C, Pelier-Cady MC, et al. Work increases the incidence of carpal tunnel syndrome in the general population. Muscle Nerve. 2008;37(4):477-482.
11. Stock SR. Workplace ergonomic factors and the development of musculoskeletal disorders of the neck and upper limbs: a meta-analysis. Am J Ind Med. 1991;19(1):87-107.
12. Stevens JC, Witt JC, Smith BE, Weaver AL. The frequency of carpal tunnel syndrome in computer users at a medical facility. Neurology. 2001;56(11):1568-1570.
13. Hart JR. The thenar and hypothenar types of neural atrophy of the hand. Am J Med Sci. 1911;141:224-241.
14. Ferrante MA, Parry GJ, Wilbourn AJ. Sensory nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
15. Litchy WJ, Miller RG, Shields RW. Motor nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
16. Nunez F, Vranceanu AM, Ring D. Determinants of pain in patients with carpal tunnel syndrome. Clin Orthop Relat Res. 2010;468(12):3328-3332.
17. van Suchtelen M, Beck SJ, Gruber JS, Ring D. Progression of carpal tunnel syndrome according to electrodiagnostic testing in nonoperatively treated patients. Arch Bone Jt Surg. 2014;2(3):185-191.
18. Ghasemi-Rad M, Nosair E, Vegh A, et al. A handy review of carpal tunnel syndrome: from anatomy to diagnosis and treatment. World J Radiol. 2014;6(6):284-300.
19. Rydevik B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood flow. An in vivo study on rabbit tibial nerve. J Hand Surg Am. 1981;6(1):3-12.
20. Keir PJ, Bach JM, Rempel D. Effects of computer mouse design and task on carpal tunnel pressure. Ergonomics. 1999;42(10):1350-1360.=
The dominant limb is the limb preferred for performing an activity that requires one hand or for performing the more demanding part of an activity that requires both hands. For example, most playing card dealers use their dominant limb to distribute cards (the more demanding part of the activity) and their nondominant limb to hold the rest of the pack (the less demanding activity). Although a relationship between nocturnal hand paresthesias and daily hand activities has been known for more than a century, it was not until more recently that it was recognized that unilateral carpal tunnel syndrome (CTS) more commonly involves the dominant limb.1,2
Among people with CTS, the dominant limb tends to be affected earlier and, in the setting of bilateral involvement, more severely.3,4 This relationship, however, is not absolute. In 1983, Falck and Aarnio reported that CTS could be more pronounced on the nondominant side whenever upper extremity usage requirements, especially occupational requirements, stressed that limb to a greater extent than they stressed the dominant limb.5
Regarding occupation, particular CTS risk factors and associations have been reported. One study found that the most common work-related risk factor was repetitive bending and twisting of the hands and wrists.6 In another study, the incidence of CTS was almost 10-fold higher among workers performing high force, high repetition jobs than among those performing low force, low repetition jobs.7-10 A meta-analysis identified a strong causal relationship between forceful, repetitive work and development of CTS.11 A more recent and controversial study found no association between heavy use of computers and CTS.12 In 1911, Hart reported an association between repetitive gripping and thenar atrophy.13 Although he misattributed the association to trauma of the recurrent thenar motor branch, 2 of the 3 described patients reported a period of episodic hand paresthesias preceding the development of thenar eminence atrophy and thus more likely had typical CTS.
Background
The present study was prompted by the clinical and electrodiagnostic (EDX) features of a 27-year-old right-hand–dominant man who presented to the EDX laboratory for assessment of bilateral hand paresthesias. The patient reported episodic bilateral hand tingling that was much more pronounced on the left (nondominant) side. Consistent with his report, EDX assessment revealed bilateral CTS that involved the nondominant limb to a much greater extent than that of the dominant limb. As a blackjack dealer, the patient was using his nondominant hand to “tightly grip 2 decks of cards” and the dominant hand to distribute those cards.
Similar history and EDX patterns (bilateral CTS more pronounced on nondominant side) were subsequently noted in 2 other patients, both of whom were using their nondominant limb to perform an activity that required sustained gripping. One of these patients was a minnow counter. He was using his nondominant hand to firmly grip the top of a bucket and the dominant hand to “deal” the fish into separate tanks. The other patient was a mason. He was using his nondominant hand to firmly hold a brick or stone in place and the dominant hand to apply cement. The clinical and EDX features of these 3 patients suggested that sustained gripping might be a significant risk factor for development of CTS. That all 3 of these patients were using their dominant hand for a repetitive activity (dealing) further suggested that, compared with repetitive activity, sustained gripping was more significant as a risk factor for development of CTS.
As unilateral CTS typically occurs on the dominant side, and bilateral CTS typically is more pronounced on the dominant side, the term backward CTS is applied to cases in which unilateral CTS occurs on the nondominant side or bilateral CTS involves the nondominant side to a greater extent than the dominant side.
Although many investigators have purported an association between CTS and a particular upper extremity activity, their conclusions are limited by use of poorly validated symptom surveys, use of faulty epidemiologic methods, selection of a specific basis for clinical diagnosis (eg, isolated hand pain), or lack of EDX confirmation. Associations between a particular activity and development of CTS are best addressed by studies that include both clinical and EDX assessments and that fully characterize the individual hand usage patterns.
Methods
This study identified the upper extremity usage patterns associated with development of CTS among patients found in the EDX laboratory to have backward CTS (unilateral CTS in nondominant limb or bilateral CTS involving nondominant limb more than dominant limb). Thus, whenever patients who were referred to the EDX laboratory for upper extremity studies were noted to have backward CTS, an extensive upper extremity usage assessment was immediately performed. Both the EDX studies and the upper extremity usage assessments were performed by the author during the same encounter.
All patients had initial screening sensory and motor nerve conduction studies performed: median sensory, recording the second digit; ulnar sensory, recording the fifth digit; superficial radial, recording the dorsum of hand; median motor, recording the thenar eminence; and ulnar motor, recording the hypothenar eminence. As CTS was suspected in all cases, median and ulnar palmar nerve conduction studies were performed as well. All these studies were performed using previously reported techniques, and all collected values were compared with EMG laboratory control values.14,15 In all patients, the median nerve conduction studies were performed bilaterally. Approval from an ethics board or an institutional review board was not needed because this study did not involve personal information or identifiable images.
To avoid identifying small, chance asymmetries related to hypothyroidism and other conditions that produce bilateral CTS, the author predefined the degree of asymmetry required for study inclusion to identify only large asymmetries. Because the EDX manifestations of CTS typically reflect features of demyelination before those of axon loss, the required asymmetries were predefined using peak sensory and distal motor latency values. For study inclusion, the median nerve latency value recorded from the nondominant limb needed to exceed the value recorded from the dominant limb by 0.6 msec for the median palmar responses, 1.0 msec for the median digital sensory responses, or 1.0 msec for the median motor responses.
Excluded from the study were patients who reported being ambidextrous, those who had changed hand dominance at any age and for any reason, those with a history of upper extremity trauma or surgery, and those with EDX findings indicating a concomitant neuromuscular disorder. In addition, patients with diabetes mellitus or any other condition associated with bilateral CTS were excluded.
Results
From the approximately 2,000 upper extremity EDX studies performed over a 30-month period, the author identified 21 patients who met the inclusion criteria (Table 1). Of these 21 patients, 15 (71%) had bilateral CTS and 6 (29%) had unilateral CTS. Sixteen of the 21 patients used their nondominant hand, through a significant portion of the day, to perform an activity that required sustained gripping (Table 2).
Of these 16 patients, 14 reported that the sustained gripping activity was related to their occupation: pipe fitter (4 patients), card dealer (4), professional driver (2), grocery store clerk (1), wire stripper (1), bakery worker (1), and motel room cleaner (1). In their jobs, the pipe fitters were continually cutting pipe during their entire 8-hour shift—using the nondominant hand to tightly grip a pipe while using the dominant hand to direct an electrically powered blade through it. Of the card dealers, 1 was a professional playing card dealer (not the dealer whose case prompted this study), 1 distributed store coupons into containers, and 2 distributed pieces of mail into bins (referred to as casing the mail). All the card dealers used their nondominant hand to tightly grip items that the dominant limb distributed. The professional drivers used their nondominant hand to grip the steering wheel. The grocery store clerk used her nondominant hand to grip shopping items while moving them across a barcode detector. The wire stripper used her nondominant hand to tightly grip bundles of wire while holding a tool in the dominant hand to snip or strip them. The bakery worker continually used her nondominant hand to squeeze off pieces of dough from a mound. And the motel room cleaner used her nondominant hand to grip the side of a bathtub while scrubbing the tub with her dominant hand (she estimated she cleaned bathtubs for about 25% of her 8-hour shift).
Of the 2 patients who reported sustained gripping unrelated to occupation, 1 was baby-sitting her grandson 5 days per week. She carried him, grasping his buttock with her nondominant hand, while performing her daily activities. She estimated she carried the child a minimum of 2 hours a day. After several weeks, she noted episodic tingling in the nondominant hand, yet she continued carrying him for another 7 months, at which point she sought medical care. The other patient, a student in a stress relief class, was instructed to repetitively open and tightly close her nondominant hand for 10 minutes 4 or more times per day. After several weeks, she noted episodic tingling in the exercised, nondominant hand.
Of the 5 patients who denied performing an activity that required sustained gripping, 2 used their nondominant limb to enter data into a computer while turning pages with the dominant limb. A piano teacher, used her nondominant limb to strike piano keys while sitting to the right of her pupils; and a typist, consistently slept with the dorsal aspect of the nondominant hand pressed into her cheek, resulting in sustained wrist flexion throughout the night. One patient could not identify an activity performed with her nondominant limb both frequently and for prolonged periods.
Discussion
As with other syndromic disorders, CTS is associated with several clinical features, the presence of which correlates with the severity of median nerve involvement. During the earliest stage of CTS, episodic hand tingling (a positive symptom) is commonly reported. This tingling typically is more pronounced at night and during relaxation. In addition, many patients come to recognize that their hand tingling is precipitated by activities that involve sustained upper extremity elevation (eg, driving with a limb resting on upper portion of steering wheel; reading with upper extremities maintained in forward abduction) and that lowering a symptomatic limb relieves its tingling.
With progression, negative symptoms appear (eg, numbness and then weakness and wasting). Unfortunately, as the negative symptoms replace the positive ones, affected individuals may become less symptomatic and mistakenly believe their condition is improving. Features of autonomic fiber involvement may also be present but are less reliably elicited. Isolated hand pain is an uncommon manifestation of CTS because pain more commonly occurs later in the course and for this reason tends to be accompanied by other features of CTS.
The clinical features of CTS correlate with its underlying pathology. As demyelination precedes axon disruption pathologically, the clinical features of demyelination (episodic paresthesias) precede those of axon loss (numbness, weakness, wasting). However, clinical features may go unrecognized or be dismissed by the patient. Moreover, there is substantial variation in type, intensity, and frequency of symptoms.16,17
The EDX features of CTS correlate with its underlying pathology and pathophysiology. As demyelination (loss of insulation) increases the capacitance of the membrane and increases internodal current leakage, conduction velocity is reduced. As severity worsens and pathology changes from predominantly demyelination to predominantly axon loss, the individual nerve fiber action potentials, which make up the compound responses being recorded, are lost. As a result the amplitude and negative area under the curve values decrease. Thus, the EDX features of demyelination (eg, prolonged latencies) precede those of axon loss (eg, amplitude, negative area under the curve reduction).
As with other focal mononeuropathies, the sensory responses tend to be affected earlier and to a greater degree than do the motor responses. Consequently, the EDX features of CTS typically follow a standard progression. The median palmar responses are involved sooner and to a greater degree than the median sensory responses recorded from the digits, which in turn tend to be involved earlier and to a greater degree than are the median motor responses.
Awareness of this relationship dictates the severity of the lesion and helps in the recognition of a cool limb and in the avoidance of a false-positive study interpretation. In a cool limb, the fingers are cooler than the wrists. Thus, the peak latency of the median digital sensory response is delayed to a greater extent than the ipsilateral median palmar response (the opposite of the CTS pattern). Accordingly, whenever this pattern is identified, the hand must be warmed or rewarmed and the studies repeated. The hand is also warmed or rewarmed whenever the median motor response is delayed out of proportion to that of the median palmar response.
Conclusion
Cases of CTS mainly in the nondominant limb provide an opportunity to identify particular limb usage patterns that might be associated with CTS. Of the present study’s 21 affected patients, 16 were using their nondominant limb to perform activities that required sustained gripping. Fourteen of the 16 activities were related to occupation. These findings strongly suggest an association between activities that require sustained gripping and development of CTS.
That the card dealers simultaneously used their nondominant hand for sustained gripping and the dominant hand for the repetitive activity of dealing suggests that sustained gripping is a stronger risk factor than repetitive activity for the development of CTS—an unanticipated finding. Interestingly, in a 2001 study that suggested repetitive activity might not be a CTS risk factor, there was a higher incidence of CTS among computer users working with a mouse—an activity that requires sustained gripping.12
Episodic hand tingling during mouse use likely reflects impaired blood flow to the median nerve, which occurs when carpal tunnel pressure approaches or exceeds 20 to 30 mm Hg.18 Placement of a hand on a mouse increases intracarpal pressure from 3 to 5 mm Hg (wrist in neutral position) to 16 to 21 mm Hg, whereas mouse use increases intracarpal pressure to 28 to 33 mm Hg.18-20
The dominant limb is the limb preferred for performing an activity that requires one hand or for performing the more demanding part of an activity that requires both hands. For example, most playing card dealers use their dominant limb to distribute cards (the more demanding part of the activity) and their nondominant limb to hold the rest of the pack (the less demanding activity). Although a relationship between nocturnal hand paresthesias and daily hand activities has been known for more than a century, it was not until more recently that it was recognized that unilateral carpal tunnel syndrome (CTS) more commonly involves the dominant limb.1,2
Among people with CTS, the dominant limb tends to be affected earlier and, in the setting of bilateral involvement, more severely.3,4 This relationship, however, is not absolute. In 1983, Falck and Aarnio reported that CTS could be more pronounced on the nondominant side whenever upper extremity usage requirements, especially occupational requirements, stressed that limb to a greater extent than they stressed the dominant limb.5
Regarding occupation, particular CTS risk factors and associations have been reported. One study found that the most common work-related risk factor was repetitive bending and twisting of the hands and wrists.6 In another study, the incidence of CTS was almost 10-fold higher among workers performing high force, high repetition jobs than among those performing low force, low repetition jobs.7-10 A meta-analysis identified a strong causal relationship between forceful, repetitive work and development of CTS.11 A more recent and controversial study found no association between heavy use of computers and CTS.12 In 1911, Hart reported an association between repetitive gripping and thenar atrophy.13 Although he misattributed the association to trauma of the recurrent thenar motor branch, 2 of the 3 described patients reported a period of episodic hand paresthesias preceding the development of thenar eminence atrophy and thus more likely had typical CTS.
Background
The present study was prompted by the clinical and electrodiagnostic (EDX) features of a 27-year-old right-hand–dominant man who presented to the EDX laboratory for assessment of bilateral hand paresthesias. The patient reported episodic bilateral hand tingling that was much more pronounced on the left (nondominant) side. Consistent with his report, EDX assessment revealed bilateral CTS that involved the nondominant limb to a much greater extent than that of the dominant limb. As a blackjack dealer, the patient was using his nondominant hand to “tightly grip 2 decks of cards” and the dominant hand to distribute those cards.
Similar history and EDX patterns (bilateral CTS more pronounced on nondominant side) were subsequently noted in 2 other patients, both of whom were using their nondominant limb to perform an activity that required sustained gripping. One of these patients was a minnow counter. He was using his nondominant hand to firmly grip the top of a bucket and the dominant hand to “deal” the fish into separate tanks. The other patient was a mason. He was using his nondominant hand to firmly hold a brick or stone in place and the dominant hand to apply cement. The clinical and EDX features of these 3 patients suggested that sustained gripping might be a significant risk factor for development of CTS. That all 3 of these patients were using their dominant hand for a repetitive activity (dealing) further suggested that, compared with repetitive activity, sustained gripping was more significant as a risk factor for development of CTS.
As unilateral CTS typically occurs on the dominant side, and bilateral CTS typically is more pronounced on the dominant side, the term backward CTS is applied to cases in which unilateral CTS occurs on the nondominant side or bilateral CTS involves the nondominant side to a greater extent than the dominant side.
Although many investigators have purported an association between CTS and a particular upper extremity activity, their conclusions are limited by use of poorly validated symptom surveys, use of faulty epidemiologic methods, selection of a specific basis for clinical diagnosis (eg, isolated hand pain), or lack of EDX confirmation. Associations between a particular activity and development of CTS are best addressed by studies that include both clinical and EDX assessments and that fully characterize the individual hand usage patterns.
Methods
This study identified the upper extremity usage patterns associated with development of CTS among patients found in the EDX laboratory to have backward CTS (unilateral CTS in nondominant limb or bilateral CTS involving nondominant limb more than dominant limb). Thus, whenever patients who were referred to the EDX laboratory for upper extremity studies were noted to have backward CTS, an extensive upper extremity usage assessment was immediately performed. Both the EDX studies and the upper extremity usage assessments were performed by the author during the same encounter.
All patients had initial screening sensory and motor nerve conduction studies performed: median sensory, recording the second digit; ulnar sensory, recording the fifth digit; superficial radial, recording the dorsum of hand; median motor, recording the thenar eminence; and ulnar motor, recording the hypothenar eminence. As CTS was suspected in all cases, median and ulnar palmar nerve conduction studies were performed as well. All these studies were performed using previously reported techniques, and all collected values were compared with EMG laboratory control values.14,15 In all patients, the median nerve conduction studies were performed bilaterally. Approval from an ethics board or an institutional review board was not needed because this study did not involve personal information or identifiable images.
To avoid identifying small, chance asymmetries related to hypothyroidism and other conditions that produce bilateral CTS, the author predefined the degree of asymmetry required for study inclusion to identify only large asymmetries. Because the EDX manifestations of CTS typically reflect features of demyelination before those of axon loss, the required asymmetries were predefined using peak sensory and distal motor latency values. For study inclusion, the median nerve latency value recorded from the nondominant limb needed to exceed the value recorded from the dominant limb by 0.6 msec for the median palmar responses, 1.0 msec for the median digital sensory responses, or 1.0 msec for the median motor responses.
Excluded from the study were patients who reported being ambidextrous, those who had changed hand dominance at any age and for any reason, those with a history of upper extremity trauma or surgery, and those with EDX findings indicating a concomitant neuromuscular disorder. In addition, patients with diabetes mellitus or any other condition associated with bilateral CTS were excluded.
Results
From the approximately 2,000 upper extremity EDX studies performed over a 30-month period, the author identified 21 patients who met the inclusion criteria (Table 1). Of these 21 patients, 15 (71%) had bilateral CTS and 6 (29%) had unilateral CTS. Sixteen of the 21 patients used their nondominant hand, through a significant portion of the day, to perform an activity that required sustained gripping (Table 2).
Of these 16 patients, 14 reported that the sustained gripping activity was related to their occupation: pipe fitter (4 patients), card dealer (4), professional driver (2), grocery store clerk (1), wire stripper (1), bakery worker (1), and motel room cleaner (1). In their jobs, the pipe fitters were continually cutting pipe during their entire 8-hour shift—using the nondominant hand to tightly grip a pipe while using the dominant hand to direct an electrically powered blade through it. Of the card dealers, 1 was a professional playing card dealer (not the dealer whose case prompted this study), 1 distributed store coupons into containers, and 2 distributed pieces of mail into bins (referred to as casing the mail). All the card dealers used their nondominant hand to tightly grip items that the dominant limb distributed. The professional drivers used their nondominant hand to grip the steering wheel. The grocery store clerk used her nondominant hand to grip shopping items while moving them across a barcode detector. The wire stripper used her nondominant hand to tightly grip bundles of wire while holding a tool in the dominant hand to snip or strip them. The bakery worker continually used her nondominant hand to squeeze off pieces of dough from a mound. And the motel room cleaner used her nondominant hand to grip the side of a bathtub while scrubbing the tub with her dominant hand (she estimated she cleaned bathtubs for about 25% of her 8-hour shift).
Of the 2 patients who reported sustained gripping unrelated to occupation, 1 was baby-sitting her grandson 5 days per week. She carried him, grasping his buttock with her nondominant hand, while performing her daily activities. She estimated she carried the child a minimum of 2 hours a day. After several weeks, she noted episodic tingling in the nondominant hand, yet she continued carrying him for another 7 months, at which point she sought medical care. The other patient, a student in a stress relief class, was instructed to repetitively open and tightly close her nondominant hand for 10 minutes 4 or more times per day. After several weeks, she noted episodic tingling in the exercised, nondominant hand.
Of the 5 patients who denied performing an activity that required sustained gripping, 2 used their nondominant limb to enter data into a computer while turning pages with the dominant limb. A piano teacher, used her nondominant limb to strike piano keys while sitting to the right of her pupils; and a typist, consistently slept with the dorsal aspect of the nondominant hand pressed into her cheek, resulting in sustained wrist flexion throughout the night. One patient could not identify an activity performed with her nondominant limb both frequently and for prolonged periods.
Discussion
As with other syndromic disorders, CTS is associated with several clinical features, the presence of which correlates with the severity of median nerve involvement. During the earliest stage of CTS, episodic hand tingling (a positive symptom) is commonly reported. This tingling typically is more pronounced at night and during relaxation. In addition, many patients come to recognize that their hand tingling is precipitated by activities that involve sustained upper extremity elevation (eg, driving with a limb resting on upper portion of steering wheel; reading with upper extremities maintained in forward abduction) and that lowering a symptomatic limb relieves its tingling.
With progression, negative symptoms appear (eg, numbness and then weakness and wasting). Unfortunately, as the negative symptoms replace the positive ones, affected individuals may become less symptomatic and mistakenly believe their condition is improving. Features of autonomic fiber involvement may also be present but are less reliably elicited. Isolated hand pain is an uncommon manifestation of CTS because pain more commonly occurs later in the course and for this reason tends to be accompanied by other features of CTS.
The clinical features of CTS correlate with its underlying pathology. As demyelination precedes axon disruption pathologically, the clinical features of demyelination (episodic paresthesias) precede those of axon loss (numbness, weakness, wasting). However, clinical features may go unrecognized or be dismissed by the patient. Moreover, there is substantial variation in type, intensity, and frequency of symptoms.16,17
The EDX features of CTS correlate with its underlying pathology and pathophysiology. As demyelination (loss of insulation) increases the capacitance of the membrane and increases internodal current leakage, conduction velocity is reduced. As severity worsens and pathology changes from predominantly demyelination to predominantly axon loss, the individual nerve fiber action potentials, which make up the compound responses being recorded, are lost. As a result the amplitude and negative area under the curve values decrease. Thus, the EDX features of demyelination (eg, prolonged latencies) precede those of axon loss (eg, amplitude, negative area under the curve reduction).
As with other focal mononeuropathies, the sensory responses tend to be affected earlier and to a greater degree than do the motor responses. Consequently, the EDX features of CTS typically follow a standard progression. The median palmar responses are involved sooner and to a greater degree than the median sensory responses recorded from the digits, which in turn tend to be involved earlier and to a greater degree than are the median motor responses.
Awareness of this relationship dictates the severity of the lesion and helps in the recognition of a cool limb and in the avoidance of a false-positive study interpretation. In a cool limb, the fingers are cooler than the wrists. Thus, the peak latency of the median digital sensory response is delayed to a greater extent than the ipsilateral median palmar response (the opposite of the CTS pattern). Accordingly, whenever this pattern is identified, the hand must be warmed or rewarmed and the studies repeated. The hand is also warmed or rewarmed whenever the median motor response is delayed out of proportion to that of the median palmar response.
Conclusion
Cases of CTS mainly in the nondominant limb provide an opportunity to identify particular limb usage patterns that might be associated with CTS. Of the present study’s 21 affected patients, 16 were using their nondominant limb to perform activities that required sustained gripping. Fourteen of the 16 activities were related to occupation. These findings strongly suggest an association between activities that require sustained gripping and development of CTS.
That the card dealers simultaneously used their nondominant hand for sustained gripping and the dominant hand for the repetitive activity of dealing suggests that sustained gripping is a stronger risk factor than repetitive activity for the development of CTS—an unanticipated finding. Interestingly, in a 2001 study that suggested repetitive activity might not be a CTS risk factor, there was a higher incidence of CTS among computer users working with a mouse—an activity that requires sustained gripping.12
Episodic hand tingling during mouse use likely reflects impaired blood flow to the median nerve, which occurs when carpal tunnel pressure approaches or exceeds 20 to 30 mm Hg.18 Placement of a hand on a mouse increases intracarpal pressure from 3 to 5 mm Hg (wrist in neutral position) to 16 to 21 mm Hg, whereas mouse use increases intracarpal pressure to 28 to 33 mm Hg.18-20
1. Ormerod JA. On a peculiar numbness and paresis of the hands. St Barts Hosp Rep. 1883;19:17-26.
2. Rosenbaum RB, Ochoa JL. Carpal Tunnel Syndrome and Other Disorders of the Median Nerve. 2nd ed. Boston, MA: Butterworth-Heineman; 2002.
3. Gainer JV Jr, Nugent GR. Carpal tunnel syndrome: report of 430 operations. South Med J. 1977;70(3):325-328.
4. Reinstein L. Hand dominance in carpal tunnel syndrome. Arch Phys Med Rehabil. 1981;62(5):202-203.
5. Falck B, Aarnio P. Left-sided carpal tunnel syndrome in butchers. Scand J Work Environ Health. 1983;9(3):291-297.
6. Tanaka S, Wild DK, Seligman PJ, Halperin WE, Behrens VJ, Putz-Anerson V. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among U.S. workers: analysis of the Occupational Health Supplement data of 1988 National Health Interview Survey. Am J Ind Med. 1995;27(4):451-470.
7. Silverstein BA, Fine LJ, Armstrong TJ. Occupational factors and carpal tunnel syndrome. Am J Ind Med. 1987;11(3):343-358.
8. de Krom MC, Kester AD, Knipschild PG, Spaans F. Risk factors for carpal tunnel syndrome. Am J Epidemiol. 1990;132(6):1102-1110.
9. Hales TR, Bernard BP. Epidemiology of work-related musculoskeletal disorders. Orthop Clin North Am. 1996;27(4):679-709.
10. Roquelaure Y, Ha C, Pelier-Cady MC, et al. Work increases the incidence of carpal tunnel syndrome in the general population. Muscle Nerve. 2008;37(4):477-482.
11. Stock SR. Workplace ergonomic factors and the development of musculoskeletal disorders of the neck and upper limbs: a meta-analysis. Am J Ind Med. 1991;19(1):87-107.
12. Stevens JC, Witt JC, Smith BE, Weaver AL. The frequency of carpal tunnel syndrome in computer users at a medical facility. Neurology. 2001;56(11):1568-1570.
13. Hart JR. The thenar and hypothenar types of neural atrophy of the hand. Am J Med Sci. 1911;141:224-241.
14. Ferrante MA, Parry GJ, Wilbourn AJ. Sensory nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
15. Litchy WJ, Miller RG, Shields RW. Motor nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
16. Nunez F, Vranceanu AM, Ring D. Determinants of pain in patients with carpal tunnel syndrome. Clin Orthop Relat Res. 2010;468(12):3328-3332.
17. van Suchtelen M, Beck SJ, Gruber JS, Ring D. Progression of carpal tunnel syndrome according to electrodiagnostic testing in nonoperatively treated patients. Arch Bone Jt Surg. 2014;2(3):185-191.
18. Ghasemi-Rad M, Nosair E, Vegh A, et al. A handy review of carpal tunnel syndrome: from anatomy to diagnosis and treatment. World J Radiol. 2014;6(6):284-300.
19. Rydevik B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood flow. An in vivo study on rabbit tibial nerve. J Hand Surg Am. 1981;6(1):3-12.
20. Keir PJ, Bach JM, Rempel D. Effects of computer mouse design and task on carpal tunnel pressure. Ergonomics. 1999;42(10):1350-1360.=
1. Ormerod JA. On a peculiar numbness and paresis of the hands. St Barts Hosp Rep. 1883;19:17-26.
2. Rosenbaum RB, Ochoa JL. Carpal Tunnel Syndrome and Other Disorders of the Median Nerve. 2nd ed. Boston, MA: Butterworth-Heineman; 2002.
3. Gainer JV Jr, Nugent GR. Carpal tunnel syndrome: report of 430 operations. South Med J. 1977;70(3):325-328.
4. Reinstein L. Hand dominance in carpal tunnel syndrome. Arch Phys Med Rehabil. 1981;62(5):202-203.
5. Falck B, Aarnio P. Left-sided carpal tunnel syndrome in butchers. Scand J Work Environ Health. 1983;9(3):291-297.
6. Tanaka S, Wild DK, Seligman PJ, Halperin WE, Behrens VJ, Putz-Anerson V. Prevalence and work-relatedness of self-reported carpal tunnel syndrome among U.S. workers: analysis of the Occupational Health Supplement data of 1988 National Health Interview Survey. Am J Ind Med. 1995;27(4):451-470.
7. Silverstein BA, Fine LJ, Armstrong TJ. Occupational factors and carpal tunnel syndrome. Am J Ind Med. 1987;11(3):343-358.
8. de Krom MC, Kester AD, Knipschild PG, Spaans F. Risk factors for carpal tunnel syndrome. Am J Epidemiol. 1990;132(6):1102-1110.
9. Hales TR, Bernard BP. Epidemiology of work-related musculoskeletal disorders. Orthop Clin North Am. 1996;27(4):679-709.
10. Roquelaure Y, Ha C, Pelier-Cady MC, et al. Work increases the incidence of carpal tunnel syndrome in the general population. Muscle Nerve. 2008;37(4):477-482.
11. Stock SR. Workplace ergonomic factors and the development of musculoskeletal disorders of the neck and upper limbs: a meta-analysis. Am J Ind Med. 1991;19(1):87-107.
12. Stevens JC, Witt JC, Smith BE, Weaver AL. The frequency of carpal tunnel syndrome in computer users at a medical facility. Neurology. 2001;56(11):1568-1570.
13. Hart JR. The thenar and hypothenar types of neural atrophy of the hand. Am J Med Sci. 1911;141:224-241.
14. Ferrante MA, Parry GJ, Wilbourn AJ. Sensory nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
15. Litchy WJ, Miller RG, Shields RW. Motor nerve conduction studies. Paper presented at: 51st Annual Meeting of the American Academy of Neurology; April 1999; Toronto, Canada.
16. Nunez F, Vranceanu AM, Ring D. Determinants of pain in patients with carpal tunnel syndrome. Clin Orthop Relat Res. 2010;468(12):3328-3332.
17. van Suchtelen M, Beck SJ, Gruber JS, Ring D. Progression of carpal tunnel syndrome according to electrodiagnostic testing in nonoperatively treated patients. Arch Bone Jt Surg. 2014;2(3):185-191.
18. Ghasemi-Rad M, Nosair E, Vegh A, et al. A handy review of carpal tunnel syndrome: from anatomy to diagnosis and treatment. World J Radiol. 2014;6(6):284-300.
19. Rydevik B, Lundborg G, Bagge U. Effects of graded compression on intraneural blood flow. An in vivo study on rabbit tibial nerve. J Hand Surg Am. 1981;6(1):3-12.
20. Keir PJ, Bach JM, Rempel D. Effects of computer mouse design and task on carpal tunnel pressure. Ergonomics. 1999;42(10):1350-1360.=
The Cost of Oncology Drugs: A Pharmacy Perspective, Part 2
The Cost of Oncology Drugs: A Pharmacy Perspective, Part 1, appeared in the Federal Practitioner February 2016 special issue “Best Practices in Hematology and Oncology” and can be accessed here.
Health care costs are the fastest growing financial segment of the U.S. economy. The cost of medications, especially those for treating cancer, is the leading cause of increased health care spending.1 Until recently, the discussion of the high costs of cancer treatment was rarely made public.
Part 1 of this article focused on the emerging discussion of the financial impact of high-cost drugs in the U.S. Part 2 will focus on the drivers of increasing oncology drug costs and the challenges high-cost medications pose for the VA. The article also will review the role of the VA Pharmacy Benefits Management Service (PBM) in evaluating new oncology agents. Also presented are the clinical guidance tools designed to aid the clinician in the cost-effective use of these agents and results of a nationwide survey of VA oncology pharmacists regarding the use of cost-containment strategies.
Cost Drivers
Many factors are driving increased oncology drug costs within the VA. Although the cost of individual drugs has the largest impact on the accelerating cost of treating each patient, other clinical and social factors may play a role.
Increasing Cost of Individual Drugs
Drug pricing is not announced until after FDA approval. Oncology drugs at the high end of the cost spectrum are rarely curative and often add only weeks or months to overall survival (OS), the gold standard. Current clinical trial design often uses progression free survival (PFS) as the primary endpoint, which makes the use of traditional pharmacoeconomic determinations of value difficult. In addition, many new drugs are first in class and/or have narrow indications that preclude competition from other drugs. Although addressing the issue of the market price for drugs seems to be one that is not controllable, there is increasing demand for drug pricing reform.2
Many believe drug prices should be linked directly to clinical benefit. In a recent article, Goldstein and colleagues proposed establishing a value-based price for necitumumab based on clinical benefit, prior to FDA approval.3 When this analysis was done, necitumumab was pending FDA approval in combination with cisplatin and gemcitabine for the treatment of squamous carcinoma of the lung. Using clinical data from the SQUIRE trial on which FDA approval was based, the addition of necitumumab to the chemotherapy regimen led to an incremental survival benefit of 0.15 life-years and 0.11 quality-adjusted life-years (QALY).4 Using a Markov model to evaluate cost-effectiveness, these authors established that the price of necitumumab should be from $563 to $1,309 per cycle. Necitumumab was approved by the FDA on November 24, 2015, with the VA acquisition cost, as of May 2016, at $6,100 per cycle.
Lack of Generic Products
The approval of generic alternatives for targeted oncology agents should reduce the cost of treating oncology patients. However, since imatinib was approved in May 2001, no single targeted agent had become available as a generic until February 1, 2016, when generic imatinib was made available in the U.S. following approval by the FDA. Currently, generic imatinib is not used in the VA due to lack of Federal Supply Schedule (FSS) contract pricing, which leads to a generic cost that is much higher than the brand-name drug, Gleevec ($6,127 per month vs $9,472 per month for the generic). The reality is that many older agents have steadily increased in price, outpacing inflation (Table 1).5
Aging U.S. Population
Advancing age is the most common risk factor for cancer, leading to an increase in the incidence and treatment of cancer. Because many newer agents are considered easier to tolerate than are traditional cytotoxic chemotherapy, clinicians have become more comfortable treating elderly patients, and geriatric oncology has become an established subspecialty within oncology.
Changing Treatment Paradigms
The use of targeted therapies is changing the paradigm from the acute treatment of cancer to chronic cancer management. Most targeted therapies are continued until disease progression or toxicity, leading to chronic, open-ended treatment. This approach is in contrast to older treatment approaches such as chemotherapy, which is often given for a limited duration followed by observation. When successful, chronic treatment with targeted agents can lead to unanticipated high costs. The following current cases at the VA San Diego Healthcare System illustrate this point:
- Renal cell carcinoma: 68-year-old man diagnosed in 2005 with a recurrence in 2012
- High-dose interleukin-2 (2 cycles); sunitinib (3.3 years); pazopanib (2 months); everolimus (2 months); sorafenib (3 months); axitinib (7 months)
- Cutaneous T-cell lymphoma: 68-year-old man started romidepsin September 22, 2010
The rate of FDA approval for oncology drugs has been accelerating rapidly in the past 15 years. Sequential therapies beyond second-line therapy are common as more agents become available. Table 2 shows FDA approval for all cancer drugs by decade.
As researchers continue to better understand the many pathways involved with the development and progression of cancer, they are beginning to combine multiple targeted agents to augment response rates, prolong survival, and reduce the potential for resistance. Recent combination regimens approved by the FDA include dabrafenib plus trametinib (January 2014), and ipilimumab plus nivolumab (October 2015), both for the treatment of melanoma. In November 2015, ixazomib was FDA approved to be used in combination with lenalidomide for multiple myeloma. Many more combination regimens are currently in clinical trials, and more combinations are expected to receive FDA approval. It is easy to see how the combination of multiple expensive agents with the prospect of prolonged therapy has the potential to increase the cost of many regimens to well over $100,000 per year.
Maintenance therapy is used to prolong PFS for patients receiving an excellent response to primary therapy. For example, VA costs for maintenance regimens include lenalidomide 10 mg daily: $8,314 for 28 days equals $216,177 for 2 years; bortezomib 1.3 mg/m2 (2.6 mg) q: 2 weeks equals $60,730 for 2 years (includes waste as bortezomib 3.5-mg vials do not a contain preservative and must be discarded within 8 hours of preparation); and rituximab 800 mg q: 2 months equals $47,635 for 2 years.
Until recently, immunotherapy for cancer was limited to melanoma and renal cell carcinoma using interleukin-2 (aldesleukin) and interferon alfa. However, the immergence of new immunotherapies, such as anti-PD-1 and anti-CTLA-4 monoclonal antibodies, have expanded the role of immunotherapy to many other, more common, malignancies, such as lung cancer, breast cancer, prostate cancer, head and neck cancer, and many more.
Most randomized clinical trials study drugs as second- or occasionally third-line therapy. However, many patients continue to be treated beyond the third-line setting, often without evidence-based data to support potential benefit. Patients often place value on treatments unlikely to work so as not to give up hope. These “hopeful gambles,” even with the potential of significant toxicity and decreased quality of life (QOL), are common in cancer treatment.6 In addition, oncologists often overestimate the clinical benefit when considering additional therapy in this setting.7
Influx of New Patients
Outside the VHA setting, the financial burden of cancer treatment has led to an influx of new patients transferring care to the VHA to reduce out-of-pocket expenses. Because private insurance copays for oral agents are increasing, many reaching 20% to 30%, out-of-pocket expenses for medications can reach several thousand dollars per month. Patients often change insurance plans due to changing jobs or to decrease cost, or employers may change plans to save money, which may significantly alter or discontinue coverage. Patients often request that the VA provide medication while continuing to see only their private oncologist. This practice should be discouraged because the VA, without clinical involvement, may supply drugs for inappropriate indications. In addition, VA providers writing prescriptions for medications without personally following patients may be liable for poor outcomes.
VA PBM Services
Prior to 1995, the VA was a much criticized and poorly performing health care system that had experienced significant budget cuts, forcing many veterans to seek care outside the VA. Then beginning in 1995, a remarkable transformation occurred, which modernized and improved the VA into a system that consistently outperforms the private sector in quality of care, patient safety, patient satisfaction, all at a lower cost.8 The story of the VA’s transformation has been well chronicled by Phillip Longman.9
Under the direction of VA Under Secretary for Health Kenneth Kizer, MD, MPH, VA established PBM Clinical Services to develop and maintain the National Drug Formulary, create clinical guidance documents, and manage drug costs and utilization. A recent article by Heron and Geraci examined the functions and role of the VA PBM in controlling oncology drug costs.10 The following is a brief review of several documents and VA PBM responsibilities as reviewed by Heron and Geraci.
VA National Formulary
Prior to the establishment of the VA National Formulary in 1995, each VA maintained its own formulary, which led to extreme variability in drug access across the country. When a patient accessed care at different VAMCs, it was common for the patient’s medications to be changed based on the specific facility formulary. This practice led to many potential problems, such as lack of clinical benefit and potentially increased or new toxicities, and led to extra hospital visits for monitoring and adjustment of medications.
In contrast, the VA National Formulary now offers a uniform pharmacy benefit to all veterans by reducing variation in access to drugs. In addition, using preferred agents in each drug class provides VA with additional leverage when contracting with drug suppliers to reduce prices across the entire VA system.
Many oncology agents are not included on the VA National Formulary due to cost and the potential for off-label use. However, the formulary status of oncology agents in no way limits access or the availability of any oncology drug for appropriate patients. In fact, nonformulary approval requests work as a mechanism for review to ensure that these agents are used properly in the subset of patients who are most likely to benefit.
The PBM assesses all new oncology drugs for value and potential use within the VA, as well as cost impact. Following this assessment, various clinical guidance documents may be developed that are intended to guide clinicians in the proper use of medications for veterans. All documents prepared by the PBM undergo an extensive peer review by the Medical Advisory Panel and other experts in the field.
Drug Monographs
A drug monograph is a comprehensive, evidence-based drug review that summarizes efficacy and safety based on clinical trial data published in peer-reviewed journals, abstracts, and/or FDA Medical Review transcripts. Cost-effectiveness analysis is included if available.
Criteria for Use
Criteria for Use (CFU) are developed for drugs considered to be at high risk for inappropriate use or with safety concerns. The purpose of the CFU is to select patients most likely to benefit from these agents by using clinical criteria, which may qualify or eliminate a patient for treatment. National CFUs are available on the national PBM website. Local CFUs are often written and shared among oncology pharmacists via the VA oncology pharmacist listserv.
Abbreviated Reviews
Similar to drug monographs, abbreviated reviews are much shorter and focus on the relevant clinical sections of the drug monograph necessary for clinical or formulary decision making.
National Acquisition Center
The National Acquisition Center (NAC) is the pharmaceutical contracting mechanism for the VA and works closely with the PBM.5 The NAC pursues significant drug price reductions for the VA based on many strategies. Public Law 102-585 ensures that certain government agencies, including the VA, receive special discounts on pharmaceuticals, which is at least a 24% discount from the nonfederal Average Manufacturer Price. This is known as the Federal Supply Schedule (FSS) and/or Big 4 pricing. In addition, bulk purchases and performance-based incentive agreements can lead to substantial local discounts. By working with specific drug distribution and warehouse contractors, the NAC assures ready access to drugs for VA patients. The NAC also allows for an efficient drug inventory process, thus reducing inventory management costs.
Guidance Documents
In 2012, the VA Oncology Field Advisory Committee (FAC) created the High Cost Oncology Drug Work Group to address the impact of high-cost oncology drugs within the VA.11 This work group was composed of VA oncologists and pharmacists whose efforts resulted in 5 guidance documents designed to reduce drug costs by optimizing therapy and reducing waste: (1) Dose Rounding in Oncology; (2) Oral Anticancer Drugs Dispensing and Monitoring; (3) Oncology Drug Table: Recommended Dispensing and Monitoring; (4) Chemotherapy Review Committee Process; and (5) Determining Clinical Benefit of High Cost Oncology Drugs. Reviews of 2 of these documents follows.
Determining Clinical Benefit of High Cost Oncology Drugs provides a decision tool to aid members of the oncology health care team in optimizing patient outcomes while attempting to obtain the greatest value from innovative therapies. When a high-cost or off-label request is made for a particular patient, using this process encourages thoughtful and evidence-based use of the drug by considering all clinical evidence in addition to the FDA-approved indication. Finally, a drug’s safety profile in relation to the indication, therapeutic goal, and specific patient characteristics and desires are integrated into a final decision to determine the appropriateness of the therapeutic intervention for the patient.
Oncology Drug Table: Recommended Dispensing and Monitoring contains a list of oral oncology drugs and includes recommendations for dispensing amount, adverse effects, laboratory monitoring, formulary status, approval requirements, and monthly cost of each agent based on the current NAC pricing.5 Cost awareness is critical when comparing alternative treatment options to minimize cost when treatments with similar benefits are considered. Most VA oncologists do not have easy access to the cost of various treatments and can be surprised about how expensive many common regimens cost. The costs listed in this document are updated about every 3 months.
Conclusion
Using newer, expensive targeted oncology agents in a cost-effective manner must be a proactive, collaborative, and multidisciplinary process. Pharmacists should not be solely responsible for monitoring and controlling high-cost treatments. Well-informed, evidence-based decisions are needed to ensure expensive agents are used in the subset of patients who are most likely to benefit. Clinical tools addressing value should be used to aid in appropriate and cost-effective treatment plans using drug monographs and CFUs, VHA Guidance on Determining Clinical Benefit of High Cost Oncology Drugs, and the Oral Chemotherapy Dispensing and Monitoring Reference, among other resources. Due to the subjective nature of value in medicine, agreeing on policy will have many challenges, such as how to place a value on various gains in overall survival, progression free survival, response rates, and QOL.
eAppendix
1. Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626-633.
2. Kantarjian H, Steensma D, Rius Sanjuan J, Eishaug A, Light D. High cancer drug prices in the United States: reasons and proposed solutions. J Oncol Pract. 2014;10(4):e208-e211.
3. Goldstein DA, Chen Q, Ayer T, et al. Necitumumab in metastatic squamous cell lung cancer: establishing a value-based cost. JAMA Oncol. 2015;1(9):1293-1300.
4. Thatcher N, Hirsch FR, Luft AV, et al; SQUIRE Investigators. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16(7):763-774.
5. U.S. Department of Veterans Affairs, National Acquisition Center, Pharmaceutical Catalog Search. U.S. Department of Veterans Affairs, National Acquisition Center website. http://www1.va.gov/nac/index.cfm?template=Search_Pharmaceutical_Catalog. Updated June 13, 2016. Accessed June 13, 2016.
6. Lakdawalla DN, Romley JA, Sanchez Y, Maclean JR, Penrod JR, Philipson T. How cancer patients value hope and the implications for cost-effectiveness assessments of high-cost cancer therapies. Health Aff (Millwood). 2012;31(4):676-682.
7. Ubel PA, Berry SR, Nadler E, et al. In a survey, marked inconsistency in how oncologists judged value of high-cost cancer drugs in relation to gains in survival. Health Aff (Millwood). 2012;31(4):709-717.
8. Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Ann Intern Med. 2004;141(12):938-945. 9. Longman P. Best Care Anywhere: Why VA Health Care Would Work for Everyone. 3rd ed. San Francisco, CA: Berrett-Koehler Publishers; 2012. 10. Heron BB, Geraci MC. Controlling the cost of oncology drugs within the VA: a national perspective. Fed Pract. 2015;32(suppl 1):18S-22S.
11. U.S. Department of Veterans Affairs. Pharmacy Benefits Management Services Intranet, Documents and Lists. https://vaww.cmopnational.va.gov/cmop/PBM/Clinical%20Guidance/Forms/AllItems.aspx. Accessed May 19, 2016.
The Cost of Oncology Drugs: A Pharmacy Perspective, Part 1, appeared in the Federal Practitioner February 2016 special issue “Best Practices in Hematology and Oncology” and can be accessed here.
Health care costs are the fastest growing financial segment of the U.S. economy. The cost of medications, especially those for treating cancer, is the leading cause of increased health care spending.1 Until recently, the discussion of the high costs of cancer treatment was rarely made public.
Part 1 of this article focused on the emerging discussion of the financial impact of high-cost drugs in the U.S. Part 2 will focus on the drivers of increasing oncology drug costs and the challenges high-cost medications pose for the VA. The article also will review the role of the VA Pharmacy Benefits Management Service (PBM) in evaluating new oncology agents. Also presented are the clinical guidance tools designed to aid the clinician in the cost-effective use of these agents and results of a nationwide survey of VA oncology pharmacists regarding the use of cost-containment strategies.
Cost Drivers
Many factors are driving increased oncology drug costs within the VA. Although the cost of individual drugs has the largest impact on the accelerating cost of treating each patient, other clinical and social factors may play a role.
Increasing Cost of Individual Drugs
Drug pricing is not announced until after FDA approval. Oncology drugs at the high end of the cost spectrum are rarely curative and often add only weeks or months to overall survival (OS), the gold standard. Current clinical trial design often uses progression free survival (PFS) as the primary endpoint, which makes the use of traditional pharmacoeconomic determinations of value difficult. In addition, many new drugs are first in class and/or have narrow indications that preclude competition from other drugs. Although addressing the issue of the market price for drugs seems to be one that is not controllable, there is increasing demand for drug pricing reform.2
Many believe drug prices should be linked directly to clinical benefit. In a recent article, Goldstein and colleagues proposed establishing a value-based price for necitumumab based on clinical benefit, prior to FDA approval.3 When this analysis was done, necitumumab was pending FDA approval in combination with cisplatin and gemcitabine for the treatment of squamous carcinoma of the lung. Using clinical data from the SQUIRE trial on which FDA approval was based, the addition of necitumumab to the chemotherapy regimen led to an incremental survival benefit of 0.15 life-years and 0.11 quality-adjusted life-years (QALY).4 Using a Markov model to evaluate cost-effectiveness, these authors established that the price of necitumumab should be from $563 to $1,309 per cycle. Necitumumab was approved by the FDA on November 24, 2015, with the VA acquisition cost, as of May 2016, at $6,100 per cycle.
Lack of Generic Products
The approval of generic alternatives for targeted oncology agents should reduce the cost of treating oncology patients. However, since imatinib was approved in May 2001, no single targeted agent had become available as a generic until February 1, 2016, when generic imatinib was made available in the U.S. following approval by the FDA. Currently, generic imatinib is not used in the VA due to lack of Federal Supply Schedule (FSS) contract pricing, which leads to a generic cost that is much higher than the brand-name drug, Gleevec ($6,127 per month vs $9,472 per month for the generic). The reality is that many older agents have steadily increased in price, outpacing inflation (Table 1).5
Aging U.S. Population
Advancing age is the most common risk factor for cancer, leading to an increase in the incidence and treatment of cancer. Because many newer agents are considered easier to tolerate than are traditional cytotoxic chemotherapy, clinicians have become more comfortable treating elderly patients, and geriatric oncology has become an established subspecialty within oncology.
Changing Treatment Paradigms
The use of targeted therapies is changing the paradigm from the acute treatment of cancer to chronic cancer management. Most targeted therapies are continued until disease progression or toxicity, leading to chronic, open-ended treatment. This approach is in contrast to older treatment approaches such as chemotherapy, which is often given for a limited duration followed by observation. When successful, chronic treatment with targeted agents can lead to unanticipated high costs. The following current cases at the VA San Diego Healthcare System illustrate this point:
- Renal cell carcinoma: 68-year-old man diagnosed in 2005 with a recurrence in 2012
- High-dose interleukin-2 (2 cycles); sunitinib (3.3 years); pazopanib (2 months); everolimus (2 months); sorafenib (3 months); axitinib (7 months)
- Cutaneous T-cell lymphoma: 68-year-old man started romidepsin September 22, 2010
The rate of FDA approval for oncology drugs has been accelerating rapidly in the past 15 years. Sequential therapies beyond second-line therapy are common as more agents become available. Table 2 shows FDA approval for all cancer drugs by decade.
As researchers continue to better understand the many pathways involved with the development and progression of cancer, they are beginning to combine multiple targeted agents to augment response rates, prolong survival, and reduce the potential for resistance. Recent combination regimens approved by the FDA include dabrafenib plus trametinib (January 2014), and ipilimumab plus nivolumab (October 2015), both for the treatment of melanoma. In November 2015, ixazomib was FDA approved to be used in combination with lenalidomide for multiple myeloma. Many more combination regimens are currently in clinical trials, and more combinations are expected to receive FDA approval. It is easy to see how the combination of multiple expensive agents with the prospect of prolonged therapy has the potential to increase the cost of many regimens to well over $100,000 per year.
Maintenance therapy is used to prolong PFS for patients receiving an excellent response to primary therapy. For example, VA costs for maintenance regimens include lenalidomide 10 mg daily: $8,314 for 28 days equals $216,177 for 2 years; bortezomib 1.3 mg/m2 (2.6 mg) q: 2 weeks equals $60,730 for 2 years (includes waste as bortezomib 3.5-mg vials do not a contain preservative and must be discarded within 8 hours of preparation); and rituximab 800 mg q: 2 months equals $47,635 for 2 years.
Until recently, immunotherapy for cancer was limited to melanoma and renal cell carcinoma using interleukin-2 (aldesleukin) and interferon alfa. However, the immergence of new immunotherapies, such as anti-PD-1 and anti-CTLA-4 monoclonal antibodies, have expanded the role of immunotherapy to many other, more common, malignancies, such as lung cancer, breast cancer, prostate cancer, head and neck cancer, and many more.
Most randomized clinical trials study drugs as second- or occasionally third-line therapy. However, many patients continue to be treated beyond the third-line setting, often without evidence-based data to support potential benefit. Patients often place value on treatments unlikely to work so as not to give up hope. These “hopeful gambles,” even with the potential of significant toxicity and decreased quality of life (QOL), are common in cancer treatment.6 In addition, oncologists often overestimate the clinical benefit when considering additional therapy in this setting.7
Influx of New Patients
Outside the VHA setting, the financial burden of cancer treatment has led to an influx of new patients transferring care to the VHA to reduce out-of-pocket expenses. Because private insurance copays for oral agents are increasing, many reaching 20% to 30%, out-of-pocket expenses for medications can reach several thousand dollars per month. Patients often change insurance plans due to changing jobs or to decrease cost, or employers may change plans to save money, which may significantly alter or discontinue coverage. Patients often request that the VA provide medication while continuing to see only their private oncologist. This practice should be discouraged because the VA, without clinical involvement, may supply drugs for inappropriate indications. In addition, VA providers writing prescriptions for medications without personally following patients may be liable for poor outcomes.
VA PBM Services
Prior to 1995, the VA was a much criticized and poorly performing health care system that had experienced significant budget cuts, forcing many veterans to seek care outside the VA. Then beginning in 1995, a remarkable transformation occurred, which modernized and improved the VA into a system that consistently outperforms the private sector in quality of care, patient safety, patient satisfaction, all at a lower cost.8 The story of the VA’s transformation has been well chronicled by Phillip Longman.9
Under the direction of VA Under Secretary for Health Kenneth Kizer, MD, MPH, VA established PBM Clinical Services to develop and maintain the National Drug Formulary, create clinical guidance documents, and manage drug costs and utilization. A recent article by Heron and Geraci examined the functions and role of the VA PBM in controlling oncology drug costs.10 The following is a brief review of several documents and VA PBM responsibilities as reviewed by Heron and Geraci.
VA National Formulary
Prior to the establishment of the VA National Formulary in 1995, each VA maintained its own formulary, which led to extreme variability in drug access across the country. When a patient accessed care at different VAMCs, it was common for the patient’s medications to be changed based on the specific facility formulary. This practice led to many potential problems, such as lack of clinical benefit and potentially increased or new toxicities, and led to extra hospital visits for monitoring and adjustment of medications.
In contrast, the VA National Formulary now offers a uniform pharmacy benefit to all veterans by reducing variation in access to drugs. In addition, using preferred agents in each drug class provides VA with additional leverage when contracting with drug suppliers to reduce prices across the entire VA system.
Many oncology agents are not included on the VA National Formulary due to cost and the potential for off-label use. However, the formulary status of oncology agents in no way limits access or the availability of any oncology drug for appropriate patients. In fact, nonformulary approval requests work as a mechanism for review to ensure that these agents are used properly in the subset of patients who are most likely to benefit.
The PBM assesses all new oncology drugs for value and potential use within the VA, as well as cost impact. Following this assessment, various clinical guidance documents may be developed that are intended to guide clinicians in the proper use of medications for veterans. All documents prepared by the PBM undergo an extensive peer review by the Medical Advisory Panel and other experts in the field.
Drug Monographs
A drug monograph is a comprehensive, evidence-based drug review that summarizes efficacy and safety based on clinical trial data published in peer-reviewed journals, abstracts, and/or FDA Medical Review transcripts. Cost-effectiveness analysis is included if available.
Criteria for Use
Criteria for Use (CFU) are developed for drugs considered to be at high risk for inappropriate use or with safety concerns. The purpose of the CFU is to select patients most likely to benefit from these agents by using clinical criteria, which may qualify or eliminate a patient for treatment. National CFUs are available on the national PBM website. Local CFUs are often written and shared among oncology pharmacists via the VA oncology pharmacist listserv.
Abbreviated Reviews
Similar to drug monographs, abbreviated reviews are much shorter and focus on the relevant clinical sections of the drug monograph necessary for clinical or formulary decision making.
National Acquisition Center
The National Acquisition Center (NAC) is the pharmaceutical contracting mechanism for the VA and works closely with the PBM.5 The NAC pursues significant drug price reductions for the VA based on many strategies. Public Law 102-585 ensures that certain government agencies, including the VA, receive special discounts on pharmaceuticals, which is at least a 24% discount from the nonfederal Average Manufacturer Price. This is known as the Federal Supply Schedule (FSS) and/or Big 4 pricing. In addition, bulk purchases and performance-based incentive agreements can lead to substantial local discounts. By working with specific drug distribution and warehouse contractors, the NAC assures ready access to drugs for VA patients. The NAC also allows for an efficient drug inventory process, thus reducing inventory management costs.
Guidance Documents
In 2012, the VA Oncology Field Advisory Committee (FAC) created the High Cost Oncology Drug Work Group to address the impact of high-cost oncology drugs within the VA.11 This work group was composed of VA oncologists and pharmacists whose efforts resulted in 5 guidance documents designed to reduce drug costs by optimizing therapy and reducing waste: (1) Dose Rounding in Oncology; (2) Oral Anticancer Drugs Dispensing and Monitoring; (3) Oncology Drug Table: Recommended Dispensing and Monitoring; (4) Chemotherapy Review Committee Process; and (5) Determining Clinical Benefit of High Cost Oncology Drugs. Reviews of 2 of these documents follows.
Determining Clinical Benefit of High Cost Oncology Drugs provides a decision tool to aid members of the oncology health care team in optimizing patient outcomes while attempting to obtain the greatest value from innovative therapies. When a high-cost or off-label request is made for a particular patient, using this process encourages thoughtful and evidence-based use of the drug by considering all clinical evidence in addition to the FDA-approved indication. Finally, a drug’s safety profile in relation to the indication, therapeutic goal, and specific patient characteristics and desires are integrated into a final decision to determine the appropriateness of the therapeutic intervention for the patient.
Oncology Drug Table: Recommended Dispensing and Monitoring contains a list of oral oncology drugs and includes recommendations for dispensing amount, adverse effects, laboratory monitoring, formulary status, approval requirements, and monthly cost of each agent based on the current NAC pricing.5 Cost awareness is critical when comparing alternative treatment options to minimize cost when treatments with similar benefits are considered. Most VA oncologists do not have easy access to the cost of various treatments and can be surprised about how expensive many common regimens cost. The costs listed in this document are updated about every 3 months.
Conclusion
Using newer, expensive targeted oncology agents in a cost-effective manner must be a proactive, collaborative, and multidisciplinary process. Pharmacists should not be solely responsible for monitoring and controlling high-cost treatments. Well-informed, evidence-based decisions are needed to ensure expensive agents are used in the subset of patients who are most likely to benefit. Clinical tools addressing value should be used to aid in appropriate and cost-effective treatment plans using drug monographs and CFUs, VHA Guidance on Determining Clinical Benefit of High Cost Oncology Drugs, and the Oral Chemotherapy Dispensing and Monitoring Reference, among other resources. Due to the subjective nature of value in medicine, agreeing on policy will have many challenges, such as how to place a value on various gains in overall survival, progression free survival, response rates, and QOL.
eAppendix
The Cost of Oncology Drugs: A Pharmacy Perspective, Part 1, appeared in the Federal Practitioner February 2016 special issue “Best Practices in Hematology and Oncology” and can be accessed here.
Health care costs are the fastest growing financial segment of the U.S. economy. The cost of medications, especially those for treating cancer, is the leading cause of increased health care spending.1 Until recently, the discussion of the high costs of cancer treatment was rarely made public.
Part 1 of this article focused on the emerging discussion of the financial impact of high-cost drugs in the U.S. Part 2 will focus on the drivers of increasing oncology drug costs and the challenges high-cost medications pose for the VA. The article also will review the role of the VA Pharmacy Benefits Management Service (PBM) in evaluating new oncology agents. Also presented are the clinical guidance tools designed to aid the clinician in the cost-effective use of these agents and results of a nationwide survey of VA oncology pharmacists regarding the use of cost-containment strategies.
Cost Drivers
Many factors are driving increased oncology drug costs within the VA. Although the cost of individual drugs has the largest impact on the accelerating cost of treating each patient, other clinical and social factors may play a role.
Increasing Cost of Individual Drugs
Drug pricing is not announced until after FDA approval. Oncology drugs at the high end of the cost spectrum are rarely curative and often add only weeks or months to overall survival (OS), the gold standard. Current clinical trial design often uses progression free survival (PFS) as the primary endpoint, which makes the use of traditional pharmacoeconomic determinations of value difficult. In addition, many new drugs are first in class and/or have narrow indications that preclude competition from other drugs. Although addressing the issue of the market price for drugs seems to be one that is not controllable, there is increasing demand for drug pricing reform.2
Many believe drug prices should be linked directly to clinical benefit. In a recent article, Goldstein and colleagues proposed establishing a value-based price for necitumumab based on clinical benefit, prior to FDA approval.3 When this analysis was done, necitumumab was pending FDA approval in combination with cisplatin and gemcitabine for the treatment of squamous carcinoma of the lung. Using clinical data from the SQUIRE trial on which FDA approval was based, the addition of necitumumab to the chemotherapy regimen led to an incremental survival benefit of 0.15 life-years and 0.11 quality-adjusted life-years (QALY).4 Using a Markov model to evaluate cost-effectiveness, these authors established that the price of necitumumab should be from $563 to $1,309 per cycle. Necitumumab was approved by the FDA on November 24, 2015, with the VA acquisition cost, as of May 2016, at $6,100 per cycle.
Lack of Generic Products
The approval of generic alternatives for targeted oncology agents should reduce the cost of treating oncology patients. However, since imatinib was approved in May 2001, no single targeted agent had become available as a generic until February 1, 2016, when generic imatinib was made available in the U.S. following approval by the FDA. Currently, generic imatinib is not used in the VA due to lack of Federal Supply Schedule (FSS) contract pricing, which leads to a generic cost that is much higher than the brand-name drug, Gleevec ($6,127 per month vs $9,472 per month for the generic). The reality is that many older agents have steadily increased in price, outpacing inflation (Table 1).5
Aging U.S. Population
Advancing age is the most common risk factor for cancer, leading to an increase in the incidence and treatment of cancer. Because many newer agents are considered easier to tolerate than are traditional cytotoxic chemotherapy, clinicians have become more comfortable treating elderly patients, and geriatric oncology has become an established subspecialty within oncology.
Changing Treatment Paradigms
The use of targeted therapies is changing the paradigm from the acute treatment of cancer to chronic cancer management. Most targeted therapies are continued until disease progression or toxicity, leading to chronic, open-ended treatment. This approach is in contrast to older treatment approaches such as chemotherapy, which is often given for a limited duration followed by observation. When successful, chronic treatment with targeted agents can lead to unanticipated high costs. The following current cases at the VA San Diego Healthcare System illustrate this point:
- Renal cell carcinoma: 68-year-old man diagnosed in 2005 with a recurrence in 2012
- High-dose interleukin-2 (2 cycles); sunitinib (3.3 years); pazopanib (2 months); everolimus (2 months); sorafenib (3 months); axitinib (7 months)
- Cutaneous T-cell lymphoma: 68-year-old man started romidepsin September 22, 2010
The rate of FDA approval for oncology drugs has been accelerating rapidly in the past 15 years. Sequential therapies beyond second-line therapy are common as more agents become available. Table 2 shows FDA approval for all cancer drugs by decade.
As researchers continue to better understand the many pathways involved with the development and progression of cancer, they are beginning to combine multiple targeted agents to augment response rates, prolong survival, and reduce the potential for resistance. Recent combination regimens approved by the FDA include dabrafenib plus trametinib (January 2014), and ipilimumab plus nivolumab (October 2015), both for the treatment of melanoma. In November 2015, ixazomib was FDA approved to be used in combination with lenalidomide for multiple myeloma. Many more combination regimens are currently in clinical trials, and more combinations are expected to receive FDA approval. It is easy to see how the combination of multiple expensive agents with the prospect of prolonged therapy has the potential to increase the cost of many regimens to well over $100,000 per year.
Maintenance therapy is used to prolong PFS for patients receiving an excellent response to primary therapy. For example, VA costs for maintenance regimens include lenalidomide 10 mg daily: $8,314 for 28 days equals $216,177 for 2 years; bortezomib 1.3 mg/m2 (2.6 mg) q: 2 weeks equals $60,730 for 2 years (includes waste as bortezomib 3.5-mg vials do not a contain preservative and must be discarded within 8 hours of preparation); and rituximab 800 mg q: 2 months equals $47,635 for 2 years.
Until recently, immunotherapy for cancer was limited to melanoma and renal cell carcinoma using interleukin-2 (aldesleukin) and interferon alfa. However, the immergence of new immunotherapies, such as anti-PD-1 and anti-CTLA-4 monoclonal antibodies, have expanded the role of immunotherapy to many other, more common, malignancies, such as lung cancer, breast cancer, prostate cancer, head and neck cancer, and many more.
Most randomized clinical trials study drugs as second- or occasionally third-line therapy. However, many patients continue to be treated beyond the third-line setting, often without evidence-based data to support potential benefit. Patients often place value on treatments unlikely to work so as not to give up hope. These “hopeful gambles,” even with the potential of significant toxicity and decreased quality of life (QOL), are common in cancer treatment.6 In addition, oncologists often overestimate the clinical benefit when considering additional therapy in this setting.7
Influx of New Patients
Outside the VHA setting, the financial burden of cancer treatment has led to an influx of new patients transferring care to the VHA to reduce out-of-pocket expenses. Because private insurance copays for oral agents are increasing, many reaching 20% to 30%, out-of-pocket expenses for medications can reach several thousand dollars per month. Patients often change insurance plans due to changing jobs or to decrease cost, or employers may change plans to save money, which may significantly alter or discontinue coverage. Patients often request that the VA provide medication while continuing to see only their private oncologist. This practice should be discouraged because the VA, without clinical involvement, may supply drugs for inappropriate indications. In addition, VA providers writing prescriptions for medications without personally following patients may be liable for poor outcomes.
VA PBM Services
Prior to 1995, the VA was a much criticized and poorly performing health care system that had experienced significant budget cuts, forcing many veterans to seek care outside the VA. Then beginning in 1995, a remarkable transformation occurred, which modernized and improved the VA into a system that consistently outperforms the private sector in quality of care, patient safety, patient satisfaction, all at a lower cost.8 The story of the VA’s transformation has been well chronicled by Phillip Longman.9
Under the direction of VA Under Secretary for Health Kenneth Kizer, MD, MPH, VA established PBM Clinical Services to develop and maintain the National Drug Formulary, create clinical guidance documents, and manage drug costs and utilization. A recent article by Heron and Geraci examined the functions and role of the VA PBM in controlling oncology drug costs.10 The following is a brief review of several documents and VA PBM responsibilities as reviewed by Heron and Geraci.
VA National Formulary
Prior to the establishment of the VA National Formulary in 1995, each VA maintained its own formulary, which led to extreme variability in drug access across the country. When a patient accessed care at different VAMCs, it was common for the patient’s medications to be changed based on the specific facility formulary. This practice led to many potential problems, such as lack of clinical benefit and potentially increased or new toxicities, and led to extra hospital visits for monitoring and adjustment of medications.
In contrast, the VA National Formulary now offers a uniform pharmacy benefit to all veterans by reducing variation in access to drugs. In addition, using preferred agents in each drug class provides VA with additional leverage when contracting with drug suppliers to reduce prices across the entire VA system.
Many oncology agents are not included on the VA National Formulary due to cost and the potential for off-label use. However, the formulary status of oncology agents in no way limits access or the availability of any oncology drug for appropriate patients. In fact, nonformulary approval requests work as a mechanism for review to ensure that these agents are used properly in the subset of patients who are most likely to benefit.
The PBM assesses all new oncology drugs for value and potential use within the VA, as well as cost impact. Following this assessment, various clinical guidance documents may be developed that are intended to guide clinicians in the proper use of medications for veterans. All documents prepared by the PBM undergo an extensive peer review by the Medical Advisory Panel and other experts in the field.
Drug Monographs
A drug monograph is a comprehensive, evidence-based drug review that summarizes efficacy and safety based on clinical trial data published in peer-reviewed journals, abstracts, and/or FDA Medical Review transcripts. Cost-effectiveness analysis is included if available.
Criteria for Use
Criteria for Use (CFU) are developed for drugs considered to be at high risk for inappropriate use or with safety concerns. The purpose of the CFU is to select patients most likely to benefit from these agents by using clinical criteria, which may qualify or eliminate a patient for treatment. National CFUs are available on the national PBM website. Local CFUs are often written and shared among oncology pharmacists via the VA oncology pharmacist listserv.
Abbreviated Reviews
Similar to drug monographs, abbreviated reviews are much shorter and focus on the relevant clinical sections of the drug monograph necessary for clinical or formulary decision making.
National Acquisition Center
The National Acquisition Center (NAC) is the pharmaceutical contracting mechanism for the VA and works closely with the PBM.5 The NAC pursues significant drug price reductions for the VA based on many strategies. Public Law 102-585 ensures that certain government agencies, including the VA, receive special discounts on pharmaceuticals, which is at least a 24% discount from the nonfederal Average Manufacturer Price. This is known as the Federal Supply Schedule (FSS) and/or Big 4 pricing. In addition, bulk purchases and performance-based incentive agreements can lead to substantial local discounts. By working with specific drug distribution and warehouse contractors, the NAC assures ready access to drugs for VA patients. The NAC also allows for an efficient drug inventory process, thus reducing inventory management costs.
Guidance Documents
In 2012, the VA Oncology Field Advisory Committee (FAC) created the High Cost Oncology Drug Work Group to address the impact of high-cost oncology drugs within the VA.11 This work group was composed of VA oncologists and pharmacists whose efforts resulted in 5 guidance documents designed to reduce drug costs by optimizing therapy and reducing waste: (1) Dose Rounding in Oncology; (2) Oral Anticancer Drugs Dispensing and Monitoring; (3) Oncology Drug Table: Recommended Dispensing and Monitoring; (4) Chemotherapy Review Committee Process; and (5) Determining Clinical Benefit of High Cost Oncology Drugs. Reviews of 2 of these documents follows.
Determining Clinical Benefit of High Cost Oncology Drugs provides a decision tool to aid members of the oncology health care team in optimizing patient outcomes while attempting to obtain the greatest value from innovative therapies. When a high-cost or off-label request is made for a particular patient, using this process encourages thoughtful and evidence-based use of the drug by considering all clinical evidence in addition to the FDA-approved indication. Finally, a drug’s safety profile in relation to the indication, therapeutic goal, and specific patient characteristics and desires are integrated into a final decision to determine the appropriateness of the therapeutic intervention for the patient.
Oncology Drug Table: Recommended Dispensing and Monitoring contains a list of oral oncology drugs and includes recommendations for dispensing amount, adverse effects, laboratory monitoring, formulary status, approval requirements, and monthly cost of each agent based on the current NAC pricing.5 Cost awareness is critical when comparing alternative treatment options to minimize cost when treatments with similar benefits are considered. Most VA oncologists do not have easy access to the cost of various treatments and can be surprised about how expensive many common regimens cost. The costs listed in this document are updated about every 3 months.
Conclusion
Using newer, expensive targeted oncology agents in a cost-effective manner must be a proactive, collaborative, and multidisciplinary process. Pharmacists should not be solely responsible for monitoring and controlling high-cost treatments. Well-informed, evidence-based decisions are needed to ensure expensive agents are used in the subset of patients who are most likely to benefit. Clinical tools addressing value should be used to aid in appropriate and cost-effective treatment plans using drug monographs and CFUs, VHA Guidance on Determining Clinical Benefit of High Cost Oncology Drugs, and the Oral Chemotherapy Dispensing and Monitoring Reference, among other resources. Due to the subjective nature of value in medicine, agreeing on policy will have many challenges, such as how to place a value on various gains in overall survival, progression free survival, response rates, and QOL.
eAppendix
1. Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626-633.
2. Kantarjian H, Steensma D, Rius Sanjuan J, Eishaug A, Light D. High cancer drug prices in the United States: reasons and proposed solutions. J Oncol Pract. 2014;10(4):e208-e211.
3. Goldstein DA, Chen Q, Ayer T, et al. Necitumumab in metastatic squamous cell lung cancer: establishing a value-based cost. JAMA Oncol. 2015;1(9):1293-1300.
4. Thatcher N, Hirsch FR, Luft AV, et al; SQUIRE Investigators. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16(7):763-774.
5. U.S. Department of Veterans Affairs, National Acquisition Center, Pharmaceutical Catalog Search. U.S. Department of Veterans Affairs, National Acquisition Center website. http://www1.va.gov/nac/index.cfm?template=Search_Pharmaceutical_Catalog. Updated June 13, 2016. Accessed June 13, 2016.
6. Lakdawalla DN, Romley JA, Sanchez Y, Maclean JR, Penrod JR, Philipson T. How cancer patients value hope and the implications for cost-effectiveness assessments of high-cost cancer therapies. Health Aff (Millwood). 2012;31(4):676-682.
7. Ubel PA, Berry SR, Nadler E, et al. In a survey, marked inconsistency in how oncologists judged value of high-cost cancer drugs in relation to gains in survival. Health Aff (Millwood). 2012;31(4):709-717.
8. Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Ann Intern Med. 2004;141(12):938-945. 9. Longman P. Best Care Anywhere: Why VA Health Care Would Work for Everyone. 3rd ed. San Francisco, CA: Berrett-Koehler Publishers; 2012. 10. Heron BB, Geraci MC. Controlling the cost of oncology drugs within the VA: a national perspective. Fed Pract. 2015;32(suppl 1):18S-22S.
11. U.S. Department of Veterans Affairs. Pharmacy Benefits Management Services Intranet, Documents and Lists. https://vaww.cmopnational.va.gov/cmop/PBM/Clinical%20Guidance/Forms/AllItems.aspx. Accessed May 19, 2016.
1. Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. N Engl J Med. 2009;360(6):626-633.
2. Kantarjian H, Steensma D, Rius Sanjuan J, Eishaug A, Light D. High cancer drug prices in the United States: reasons and proposed solutions. J Oncol Pract. 2014;10(4):e208-e211.
3. Goldstein DA, Chen Q, Ayer T, et al. Necitumumab in metastatic squamous cell lung cancer: establishing a value-based cost. JAMA Oncol. 2015;1(9):1293-1300.
4. Thatcher N, Hirsch FR, Luft AV, et al; SQUIRE Investigators. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2015;16(7):763-774.
5. U.S. Department of Veterans Affairs, National Acquisition Center, Pharmaceutical Catalog Search. U.S. Department of Veterans Affairs, National Acquisition Center website. http://www1.va.gov/nac/index.cfm?template=Search_Pharmaceutical_Catalog. Updated June 13, 2016. Accessed June 13, 2016.
6. Lakdawalla DN, Romley JA, Sanchez Y, Maclean JR, Penrod JR, Philipson T. How cancer patients value hope and the implications for cost-effectiveness assessments of high-cost cancer therapies. Health Aff (Millwood). 2012;31(4):676-682.
7. Ubel PA, Berry SR, Nadler E, et al. In a survey, marked inconsistency in how oncologists judged value of high-cost cancer drugs in relation to gains in survival. Health Aff (Millwood). 2012;31(4):709-717.
8. Asch SM, McGlynn EA, Hogan MM, et al. Comparison of quality of care for patients in the Veterans Health Administration and patients in a national sample. Ann Intern Med. 2004;141(12):938-945. 9. Longman P. Best Care Anywhere: Why VA Health Care Would Work for Everyone. 3rd ed. San Francisco, CA: Berrett-Koehler Publishers; 2012. 10. Heron BB, Geraci MC. Controlling the cost of oncology drugs within the VA: a national perspective. Fed Pract. 2015;32(suppl 1):18S-22S.
11. U.S. Department of Veterans Affairs. Pharmacy Benefits Management Services Intranet, Documents and Lists. https://vaww.cmopnational.va.gov/cmop/PBM/Clinical%20Guidance/Forms/AllItems.aspx. Accessed May 19, 2016.