User login
Cumulative Irritation Comparison of Adapalene Gel and Solution With 2 Tazarotene Gels and 3 Tretinoin Formulations
Adapalene (Differin®) is a naphthoic-acid derivative with retinoid activity that is effective in the treatment of mild to moderate acne vulgaris.1-4 Adapalene, in both gel and cream formulations, at the marketed and approved concentration of 0.1%, is better tolerated than most tretinoin formulations, including tretinoin microsphere gel 0.1% (Retin-A Micro®) and tretinoin cream 0.025% (Avita®).5-10 The cumulative irritancy assay (patch test) is designed to assess the irritation potential of topically applied materials. Irritation results obtained from this type of assay are due to direct damage to the epidermal cells, and no immunologic (allergic) mechanism is involved. Results of this standard assay are widely accepted to be indicators of irritation. This study compared the irritation potential of adapalene gel and solution with several retinoid and retinoidlike products containing either tazarotene or tretinoin.back to top
METHODS This cumulative irritancy study was conducted as a single-center, randomized, controlled, investigator/evaluator, double-blind, intraindividual comparison involving healthy subjects meeting specific inclusion-exclusion criteria. The cumulative irritancy assay, a 21-day patch test, was designed to assess the irritation potential of topically applied dermatologic materials under stressful conditions (ie, occlusion).11 A total of 42 subjects (6 males and 36 females) ranging in age from 22.9 to 74.8 years were enrolled and evaluated. All subjects received adapalene gel 0.1%, adapalene solution 0.1%, tazarotene gel 0.1%, tazarotene gel 0.05%, tretinoin microsphere gel 0.1%, tretinoin cream 0.025%, tretinoin gel 0.025%, and white petrolatum (negative control). Approximately 0.2 g of each of the 7 test products and negative control was applied to 8 sites on the upper area of the back according to a predefined randomization list. Application was made under occlusive conditions for 24 hours (4 times per week) and 72 hours (once weekly) for 3 weeks. At each study visit, skin reactions (erythema scores±other local reactions) were assessed by the same trained board-certified physician evaluator during the study, 15 to 30 minutes after removal of the product, using the grading scale for erythema (Table 1).
| View this table | Table 1. Erythema Grading Scale |
In addition, other concomitant cutaneous reactions (eg, dryness, cracking, peeling) on test sites were noted, including adhesive reactions. The principal safety criterion was the mean cumulative irritancy index (MCII) assessed by clinical evaluation of the erythema at each test site. Evaluation of the test product application sites was conducted by the same investigator/evaluator throughout the study. The sites were scored at baseline (day 1) and at each study visit, week 1 (days 2 through 5, inclusively), week 2 (days 8 through 12, inclusively), week 3 (days 15 through 19, inclusively), and week 4 (day 22). The backs of the subjects were photographed before each reading. When an irritation reaction related to the product was graded 3 for any site, product application was discontinued for the incriminated sites. When an irritation reaction related to the adhesive prohibited the wearing of a patch at a particular site, all patch applications were discontinued for the subject. However, the subject was not discontinued from treatment unless, in the investigator’s/evaluator’s opinion, there was a safety concern. At that time, an adverse event form would have been completed. All subjects were informed in accordance with the International Conference on Harmonization guidelines and Good Clinical Practices. A written consent form, approved by the Institutional Review Board, was supplied by the investigator and was understood and signed by each subject before inclusion in the study. back to top
Statistical Methodology Sample Size, Design, and Randomization—A standard sample size for this type of cumulative irritancy clinical study is 25 subjects. To account for the multiplicity of comparisons, planned enrollment was estimated at 48 subjects. Enrollment was completed at 42 subjects, with the consent of the sponsor. On initiation, each of the 8 products was applied to one of the zones (Z1–Z8) according to the predefined randomization schedule. This randomization schedule was generated by the RANUNI routine of SAS using 8x8 Latin squares. Statistically Analyzed Variables—For evaluating the cutaneous tolerance, a cumulative irritancy index (CII) was calculated for each treatment and for each subject, as follows: CII=sum of irritation score/number of readings. The following conventions were applied for the CII calculation: baseline (day 1) score was excluded from the calculation. When the irritation reaction was rated 3 for any site, the product application was discontinued for the incriminated sites, and a score of 3 was assigned to the remaining readings (last observation carried forward). When a subject missed a scheduled visit, the scores of the sites from the next visit were assigned to the previously missed visit. Individual CII scores were averaged across subjects to obtain an MCII score for each treatment. MCII scores were submitted to an analysis of variance with effects for subject, zone, and formulation. To adjust for multiple comparisons, MCII score was compared, and formulations were classified using the Tukey multiple comparisons test performed at the 1% and 5% significance levels. According to MCII values, each test product could be classified into the irritation classes (Table 2).
| View this table | Table 2. Irritation Classification* |
results Of the 42 subjects enrolled, 38 subjects (90.5%) completed the study. Demographic data are presented in Table 3. Results are summarized in Table 4 and Figure 1. Figure 2 shows a clinical photograph of typical irritation observed during the study.
| View this table | Table 3. Demographic Data |
| View this table | Table 4. Summary of Mean Cumulative Irritancy Index (MCII) Statistical Comparisons |
In the study, the reasons for treatment discontinuation were not always due to an erythema score of 3 but also because of other clinical aspects of severe intolerance, such as epidermal peeling with subsequent superficial erosion (without severe erythema). Figure 3 shows the number of subjects who discontinued wearing the patches due to an irritation score of 3.
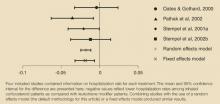
Adapalene gel and solution 0.1% were each significantly less irritating during sustained use than tazarotene gels 0.05% and 0.1%, tretinoin microsphere gel 0.1%, and tretinoin cream 0.025%. Although tretinoin gel 0.1% MCII was numerically superior to both adapalene gel and solution MCIIs, no statistically significant difference could be depicted between the 3 products. Repeated applications of adapalene gel or solution resulted in levels of irritation that were not significantly different from the white petrolatum control. back to top
- Verschoore M, Langner A, Wolska H, et al. Vehicle controlled study of CD 271 lotion in the topical treatment of acne vulgaris. J Invest Dermatol. 1993;100:221A.
- Verschoore M, Langner A, Wolska H, et al. Efficacy and safety of CD 271 alcoholic gels in the topical treatment of acne vulgaris. Br J Dermatol. 1991;124:368-371.
- Bernard BA. Adapalene, a new chemical entity with retinoid activity. Skin Pharmacol. 1993;6(suppl 1):61-69.
- Shroot B, Michel S. Pharmacology and chemistry of adapalene. J Am Acad Dermatol. 1997;36:S96-S103.
- Verschoore M, Poncet M, Czernielewski J, et al. Adapalene 0.1% gel has low skin-irritation potential. J Am Acad Dermatol. 1997;36:S104-S109.
- Caron D, Sorba V, Kerrouche N, et al. Split-face comparison of adapalene 0.1% gel and tretinoin 0.025% gel in acne patients. J Am Acad Dermatol. 1997;36:S110-S112.
- Cunliffe WJ, Caputo R, Dreno B, et al. Clinical efficacy and safety comparison of adapalene gel and tretinoin gel in the treatment of acne vulgaris. Europe and U.S. multicenter trials. J Am Acad Dermatol. 1997;36:S126-S134.
- Shalita A, Weiss JS, Chalker DK, et al. A comparison of the efficacy and safety of adapalene gel 0.1% and tretinoin gel 0.025% in the treatment of acne vulgaris: a multicenter trial. J Am Acad Dermatol. 1996;34:482-485.
- Thiboutot D, Gold MH, Jarratt MT, et al. Randomized controlled trial of tolerability, safety, and efficacy of adapalene gel 0.1% and tretinoin microsphere gel 0.1% for the treatment of acne vulgaris. Cutis. 2001;68(suppl 4):10-19.
- Egan N, Loesche MC, Baker MM. Randomized, controlled, bilateral (split-face) comparison trial of the tolerability and patient preference of adapalene gel 0.1% and tretinoin microsphere gel 0.1% for the treatment of acne vulgaris. Cutis. 2001;68(suppl 4):20-24.
- Berger RS, Bowman JP. A reappraisal of the 21-day Cumulative Irritation Test in Man. J Toxicol Cutan Ocul Toxicol. 1982;1:109-115.
Adapalene (Differin®) is a naphthoic-acid derivative with retinoid activity that is effective in the treatment of mild to moderate acne vulgaris.1-4 Adapalene, in both gel and cream formulations, at the marketed and approved concentration of 0.1%, is better tolerated than most tretinoin formulations, including tretinoin microsphere gel 0.1% (Retin-A Micro®) and tretinoin cream 0.025% (Avita®).5-10 The cumulative irritancy assay (patch test) is designed to assess the irritation potential of topically applied materials. Irritation results obtained from this type of assay are due to direct damage to the epidermal cells, and no immunologic (allergic) mechanism is involved. Results of this standard assay are widely accepted to be indicators of irritation. This study compared the irritation potential of adapalene gel and solution with several retinoid and retinoidlike products containing either tazarotene or tretinoin.back to top
METHODS This cumulative irritancy study was conducted as a single-center, randomized, controlled, investigator/evaluator, double-blind, intraindividual comparison involving healthy subjects meeting specific inclusion-exclusion criteria. The cumulative irritancy assay, a 21-day patch test, was designed to assess the irritation potential of topically applied dermatologic materials under stressful conditions (ie, occlusion).11 A total of 42 subjects (6 males and 36 females) ranging in age from 22.9 to 74.8 years were enrolled and evaluated. All subjects received adapalene gel 0.1%, adapalene solution 0.1%, tazarotene gel 0.1%, tazarotene gel 0.05%, tretinoin microsphere gel 0.1%, tretinoin cream 0.025%, tretinoin gel 0.025%, and white petrolatum (negative control). Approximately 0.2 g of each of the 7 test products and negative control was applied to 8 sites on the upper area of the back according to a predefined randomization list. Application was made under occlusive conditions for 24 hours (4 times per week) and 72 hours (once weekly) for 3 weeks. At each study visit, skin reactions (erythema scores±other local reactions) were assessed by the same trained board-certified physician evaluator during the study, 15 to 30 minutes after removal of the product, using the grading scale for erythema (Table 1).
| View this table | Table 1. Erythema Grading Scale |
In addition, other concomitant cutaneous reactions (eg, dryness, cracking, peeling) on test sites were noted, including adhesive reactions. The principal safety criterion was the mean cumulative irritancy index (MCII) assessed by clinical evaluation of the erythema at each test site. Evaluation of the test product application sites was conducted by the same investigator/evaluator throughout the study. The sites were scored at baseline (day 1) and at each study visit, week 1 (days 2 through 5, inclusively), week 2 (days 8 through 12, inclusively), week 3 (days 15 through 19, inclusively), and week 4 (day 22). The backs of the subjects were photographed before each reading. When an irritation reaction related to the product was graded 3 for any site, product application was discontinued for the incriminated sites. When an irritation reaction related to the adhesive prohibited the wearing of a patch at a particular site, all patch applications were discontinued for the subject. However, the subject was not discontinued from treatment unless, in the investigator’s/evaluator’s opinion, there was a safety concern. At that time, an adverse event form would have been completed. All subjects were informed in accordance with the International Conference on Harmonization guidelines and Good Clinical Practices. A written consent form, approved by the Institutional Review Board, was supplied by the investigator and was understood and signed by each subject before inclusion in the study. back to top
Statistical Methodology Sample Size, Design, and Randomization—A standard sample size for this type of cumulative irritancy clinical study is 25 subjects. To account for the multiplicity of comparisons, planned enrollment was estimated at 48 subjects. Enrollment was completed at 42 subjects, with the consent of the sponsor. On initiation, each of the 8 products was applied to one of the zones (Z1–Z8) according to the predefined randomization schedule. This randomization schedule was generated by the RANUNI routine of SAS using 8x8 Latin squares. Statistically Analyzed Variables—For evaluating the cutaneous tolerance, a cumulative irritancy index (CII) was calculated for each treatment and for each subject, as follows: CII=sum of irritation score/number of readings. The following conventions were applied for the CII calculation: baseline (day 1) score was excluded from the calculation. When the irritation reaction was rated 3 for any site, the product application was discontinued for the incriminated sites, and a score of 3 was assigned to the remaining readings (last observation carried forward). When a subject missed a scheduled visit, the scores of the sites from the next visit were assigned to the previously missed visit. Individual CII scores were averaged across subjects to obtain an MCII score for each treatment. MCII scores were submitted to an analysis of variance with effects for subject, zone, and formulation. To adjust for multiple comparisons, MCII score was compared, and formulations were classified using the Tukey multiple comparisons test performed at the 1% and 5% significance levels. According to MCII values, each test product could be classified into the irritation classes (Table 2).
| View this table | Table 2. Irritation Classification* |
results Of the 42 subjects enrolled, 38 subjects (90.5%) completed the study. Demographic data are presented in Table 3. Results are summarized in Table 4 and Figure 1. Figure 2 shows a clinical photograph of typical irritation observed during the study.
| View this table | Table 3. Demographic Data |
| View this table | Table 4. Summary of Mean Cumulative Irritancy Index (MCII) Statistical Comparisons |
In the study, the reasons for treatment discontinuation were not always due to an erythema score of 3 but also because of other clinical aspects of severe intolerance, such as epidermal peeling with subsequent superficial erosion (without severe erythema). Figure 3 shows the number of subjects who discontinued wearing the patches due to an irritation score of 3.
Adapalene gel and solution 0.1% were each significantly less irritating during sustained use than tazarotene gels 0.05% and 0.1%, tretinoin microsphere gel 0.1%, and tretinoin cream 0.025%. Although tretinoin gel 0.1% MCII was numerically superior to both adapalene gel and solution MCIIs, no statistically significant difference could be depicted between the 3 products. Repeated applications of adapalene gel or solution resulted in levels of irritation that were not significantly different from the white petrolatum control. back to top
Adapalene (Differin®) is a naphthoic-acid derivative with retinoid activity that is effective in the treatment of mild to moderate acne vulgaris.1-4 Adapalene, in both gel and cream formulations, at the marketed and approved concentration of 0.1%, is better tolerated than most tretinoin formulations, including tretinoin microsphere gel 0.1% (Retin-A Micro®) and tretinoin cream 0.025% (Avita®).5-10 The cumulative irritancy assay (patch test) is designed to assess the irritation potential of topically applied materials. Irritation results obtained from this type of assay are due to direct damage to the epidermal cells, and no immunologic (allergic) mechanism is involved. Results of this standard assay are widely accepted to be indicators of irritation. This study compared the irritation potential of adapalene gel and solution with several retinoid and retinoidlike products containing either tazarotene or tretinoin.back to top
METHODS This cumulative irritancy study was conducted as a single-center, randomized, controlled, investigator/evaluator, double-blind, intraindividual comparison involving healthy subjects meeting specific inclusion-exclusion criteria. The cumulative irritancy assay, a 21-day patch test, was designed to assess the irritation potential of topically applied dermatologic materials under stressful conditions (ie, occlusion).11 A total of 42 subjects (6 males and 36 females) ranging in age from 22.9 to 74.8 years were enrolled and evaluated. All subjects received adapalene gel 0.1%, adapalene solution 0.1%, tazarotene gel 0.1%, tazarotene gel 0.05%, tretinoin microsphere gel 0.1%, tretinoin cream 0.025%, tretinoin gel 0.025%, and white petrolatum (negative control). Approximately 0.2 g of each of the 7 test products and negative control was applied to 8 sites on the upper area of the back according to a predefined randomization list. Application was made under occlusive conditions for 24 hours (4 times per week) and 72 hours (once weekly) for 3 weeks. At each study visit, skin reactions (erythema scores±other local reactions) were assessed by the same trained board-certified physician evaluator during the study, 15 to 30 minutes after removal of the product, using the grading scale for erythema (Table 1).
| View this table | Table 1. Erythema Grading Scale |
In addition, other concomitant cutaneous reactions (eg, dryness, cracking, peeling) on test sites were noted, including adhesive reactions. The principal safety criterion was the mean cumulative irritancy index (MCII) assessed by clinical evaluation of the erythema at each test site. Evaluation of the test product application sites was conducted by the same investigator/evaluator throughout the study. The sites were scored at baseline (day 1) and at each study visit, week 1 (days 2 through 5, inclusively), week 2 (days 8 through 12, inclusively), week 3 (days 15 through 19, inclusively), and week 4 (day 22). The backs of the subjects were photographed before each reading. When an irritation reaction related to the product was graded 3 for any site, product application was discontinued for the incriminated sites. When an irritation reaction related to the adhesive prohibited the wearing of a patch at a particular site, all patch applications were discontinued for the subject. However, the subject was not discontinued from treatment unless, in the investigator’s/evaluator’s opinion, there was a safety concern. At that time, an adverse event form would have been completed. All subjects were informed in accordance with the International Conference on Harmonization guidelines and Good Clinical Practices. A written consent form, approved by the Institutional Review Board, was supplied by the investigator and was understood and signed by each subject before inclusion in the study. back to top
Statistical Methodology Sample Size, Design, and Randomization—A standard sample size for this type of cumulative irritancy clinical study is 25 subjects. To account for the multiplicity of comparisons, planned enrollment was estimated at 48 subjects. Enrollment was completed at 42 subjects, with the consent of the sponsor. On initiation, each of the 8 products was applied to one of the zones (Z1–Z8) according to the predefined randomization schedule. This randomization schedule was generated by the RANUNI routine of SAS using 8x8 Latin squares. Statistically Analyzed Variables—For evaluating the cutaneous tolerance, a cumulative irritancy index (CII) was calculated for each treatment and for each subject, as follows: CII=sum of irritation score/number of readings. The following conventions were applied for the CII calculation: baseline (day 1) score was excluded from the calculation. When the irritation reaction was rated 3 for any site, the product application was discontinued for the incriminated sites, and a score of 3 was assigned to the remaining readings (last observation carried forward). When a subject missed a scheduled visit, the scores of the sites from the next visit were assigned to the previously missed visit. Individual CII scores were averaged across subjects to obtain an MCII score for each treatment. MCII scores were submitted to an analysis of variance with effects for subject, zone, and formulation. To adjust for multiple comparisons, MCII score was compared, and formulations were classified using the Tukey multiple comparisons test performed at the 1% and 5% significance levels. According to MCII values, each test product could be classified into the irritation classes (Table 2).
| View this table | Table 2. Irritation Classification* |
results Of the 42 subjects enrolled, 38 subjects (90.5%) completed the study. Demographic data are presented in Table 3. Results are summarized in Table 4 and Figure 1. Figure 2 shows a clinical photograph of typical irritation observed during the study.
| View this table | Table 3. Demographic Data |
| View this table | Table 4. Summary of Mean Cumulative Irritancy Index (MCII) Statistical Comparisons |
In the study, the reasons for treatment discontinuation were not always due to an erythema score of 3 but also because of other clinical aspects of severe intolerance, such as epidermal peeling with subsequent superficial erosion (without severe erythema). Figure 3 shows the number of subjects who discontinued wearing the patches due to an irritation score of 3.
Adapalene gel and solution 0.1% were each significantly less irritating during sustained use than tazarotene gels 0.05% and 0.1%, tretinoin microsphere gel 0.1%, and tretinoin cream 0.025%. Although tretinoin gel 0.1% MCII was numerically superior to both adapalene gel and solution MCIIs, no statistically significant difference could be depicted between the 3 products. Repeated applications of adapalene gel or solution resulted in levels of irritation that were not significantly different from the white petrolatum control. back to top
- Verschoore M, Langner A, Wolska H, et al. Vehicle controlled study of CD 271 lotion in the topical treatment of acne vulgaris. J Invest Dermatol. 1993;100:221A.
- Verschoore M, Langner A, Wolska H, et al. Efficacy and safety of CD 271 alcoholic gels in the topical treatment of acne vulgaris. Br J Dermatol. 1991;124:368-371.
- Bernard BA. Adapalene, a new chemical entity with retinoid activity. Skin Pharmacol. 1993;6(suppl 1):61-69.
- Shroot B, Michel S. Pharmacology and chemistry of adapalene. J Am Acad Dermatol. 1997;36:S96-S103.
- Verschoore M, Poncet M, Czernielewski J, et al. Adapalene 0.1% gel has low skin-irritation potential. J Am Acad Dermatol. 1997;36:S104-S109.
- Caron D, Sorba V, Kerrouche N, et al. Split-face comparison of adapalene 0.1% gel and tretinoin 0.025% gel in acne patients. J Am Acad Dermatol. 1997;36:S110-S112.
- Cunliffe WJ, Caputo R, Dreno B, et al. Clinical efficacy and safety comparison of adapalene gel and tretinoin gel in the treatment of acne vulgaris. Europe and U.S. multicenter trials. J Am Acad Dermatol. 1997;36:S126-S134.
- Shalita A, Weiss JS, Chalker DK, et al. A comparison of the efficacy and safety of adapalene gel 0.1% and tretinoin gel 0.025% in the treatment of acne vulgaris: a multicenter trial. J Am Acad Dermatol. 1996;34:482-485.
- Thiboutot D, Gold MH, Jarratt MT, et al. Randomized controlled trial of tolerability, safety, and efficacy of adapalene gel 0.1% and tretinoin microsphere gel 0.1% for the treatment of acne vulgaris. Cutis. 2001;68(suppl 4):10-19.
- Egan N, Loesche MC, Baker MM. Randomized, controlled, bilateral (split-face) comparison trial of the tolerability and patient preference of adapalene gel 0.1% and tretinoin microsphere gel 0.1% for the treatment of acne vulgaris. Cutis. 2001;68(suppl 4):20-24.
- Berger RS, Bowman JP. A reappraisal of the 21-day Cumulative Irritation Test in Man. J Toxicol Cutan Ocul Toxicol. 1982;1:109-115.
- Verschoore M, Langner A, Wolska H, et al. Vehicle controlled study of CD 271 lotion in the topical treatment of acne vulgaris. J Invest Dermatol. 1993;100:221A.
- Verschoore M, Langner A, Wolska H, et al. Efficacy and safety of CD 271 alcoholic gels in the topical treatment of acne vulgaris. Br J Dermatol. 1991;124:368-371.
- Bernard BA. Adapalene, a new chemical entity with retinoid activity. Skin Pharmacol. 1993;6(suppl 1):61-69.
- Shroot B, Michel S. Pharmacology and chemistry of adapalene. J Am Acad Dermatol. 1997;36:S96-S103.
- Verschoore M, Poncet M, Czernielewski J, et al. Adapalene 0.1% gel has low skin-irritation potential. J Am Acad Dermatol. 1997;36:S104-S109.
- Caron D, Sorba V, Kerrouche N, et al. Split-face comparison of adapalene 0.1% gel and tretinoin 0.025% gel in acne patients. J Am Acad Dermatol. 1997;36:S110-S112.
- Cunliffe WJ, Caputo R, Dreno B, et al. Clinical efficacy and safety comparison of adapalene gel and tretinoin gel in the treatment of acne vulgaris. Europe and U.S. multicenter trials. J Am Acad Dermatol. 1997;36:S126-S134.
- Shalita A, Weiss JS, Chalker DK, et al. A comparison of the efficacy and safety of adapalene gel 0.1% and tretinoin gel 0.025% in the treatment of acne vulgaris: a multicenter trial. J Am Acad Dermatol. 1996;34:482-485.
- Thiboutot D, Gold MH, Jarratt MT, et al. Randomized controlled trial of tolerability, safety, and efficacy of adapalene gel 0.1% and tretinoin microsphere gel 0.1% for the treatment of acne vulgaris. Cutis. 2001;68(suppl 4):10-19.
- Egan N, Loesche MC, Baker MM. Randomized, controlled, bilateral (split-face) comparison trial of the tolerability and patient preference of adapalene gel 0.1% and tretinoin microsphere gel 0.1% for the treatment of acne vulgaris. Cutis. 2001;68(suppl 4):20-24.
- Berger RS, Bowman JP. A reappraisal of the 21-day Cumulative Irritation Test in Man. J Toxicol Cutan Ocul Toxicol. 1982;1:109-115.
Efficacy and Safety of a New Triple-Combination Agent for the Treatment of Facial Melasma
Cutaneous melasma is a relatively common dermatologic disease, occurring most commonly in Asian and Hispanic women of childbearing years.1-5 Exposure to solar UV radiation is the most important environmental factor in the pathogenesis of melasma.2,3 Therapy for melasma remains a challenge. Pharmacologic treatments are the mainstay.2,6,7 Hydroquinone, azelaic acid, tretinoin, and topical corticosteroids have been used as monotherapy7-11 or in various combinations.12-15 Kligman and Willis15 found that monotherapy with hydroquinone, tretinoin, or the topical corticosteroid dexamethasone did not produce substantial hypopigmentation within a 3-month treatment period. However, they did observe satisfactory results with a combination of tretinoin 0.1%, hydroquinone 5.0%, and dexamethasone 0.1% in a hydrophilic ointment.15 Furthermore, Kligman and Willis,15 as well as other researchers, have noted efficacy and safety benefits with use of hydroquinone, tretinoin, and various topical corticosteroids. In experimental and clinical studies, the use of tretinoin and other retinoids has been found to abrogate the epidermal atrophy that can occur with topical corticosteroids.16,17 This could be due to the ability of tretinoin and other retinoids to induce hyperplasia of epidermal cells and to induce dermal collagen synthesis.16,17 The objective of the 2 well-controlled trials featured in this article was to compare the efficacy and safety of the combination of hydroquinone, tretinoin, and the fluorinated topical corticosteroid fluocinolone acetonide, in a hydrophilic cream formulation, with 3 dual-combination products in the clearing of melasma. back to top
METHODS Study Design—The 2 pivotal trials used similar multicenter, randomized, investigator-blind, active-control, parallel-group protocols. Thirteen centers were involved in these trials. Both studies compared a triple-combination hydrophilic cream vehicle containing tretinoin 0.05%, hydroquinone 4.0%, and fluocinolone acetonide 0.01% (RA+HQ+FA) with the dual-combination products tretinoin plus hydroquinone (RA+HQ), tretinoin plus fluocinolone acetonide (RA+FA), and hydroquinone plus fluocinolone acetonide (HQ+FA). All products involved the same drug concentrations and vehicle. All formulations were used once daily at night. A total of 641 adult patients were randomized to the various treatment groups. Objective evaluation of melasma severity at baseline and at various points after treatment involved investigator assessment of global improvement from baseline using an 8-point scale (0=completely clear to 7=worse) at each follow-up visit. A baseline photograph was used for comparison. Patient Population—Patients enrolled in the study were predominantly white women (aged 21 to 75 years) with Fitzpatrick skin types I through IV. For enrollment into the study, all patients had to demonstrate a stable hyperpigmentation on the face for at least 3 months’ duration, macular lesions that were neither depressed nor atrophic, and melasma severity scores of at least 2 (ie, hyperpigmentation that was at least moderately darker than the surrounding normal skin). There were no significant differences in demographic parameters or skin phototypes among patients in each of the 4 treatment groups. The degree of hyperpigmentation in all patients was moderate to severe. Efficacy and Safety Analysis—The primary efficacy end point involved the investigators’ assessment of the proportion of intent-to-treat patients in each treatment group who achieved complete clearing at week 8. The secondary end point (secondary success) involved the proportion of intent-to-treat patients in each treatment group who achieved complete clearing (score=0) or near-complete clearing (ie, mild residual hyperpigmentation, score=1) by week 8 (Table 1).
| View this table | Table 1. Melasma Severity Rating Scale Used in Primary and Secondary Efficacy Analysis |
All patients randomized to the various treatment groups were analyzed for adverse events. Statistical analysis involved the Cochran-Mantel-Haenszel test, stratified by center. back to top
RESULTS Efficacy—Significantly more of the patients treated with RA+HQ+FA (26.1%) experienced complete clearing compared with each of the dual-therapy groups at week 8 (9.5% for RA+HQ, 1.9% for RA+FA, and 2.5% for HQ+FA, P
- Grimes PE. Melasma: etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-1457.
- Mosher DB, Fitzpatrick TB, Ortonne J-P, et al. Hypomelanoses and hypermelanoses. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 1. New York, NY: McGraw-Hill; 1999:945-1017.
- Barankin B, Silver SG, Carruthera A. The skin in pregnancy. J Cut Med Surg. 2002;6:236-240.
- Sober AF, Fitzpatrick TB. Disturbances of pigmentation. section I. mechanisms of pigmentation in man. In: Moschella SL, Pillsbury DM, Hurley HJ Jr, eds. Dermatology. Vol 2. Philadelphia, Pa: WB Saunders Co; 1975:1085.
- Vasquez M, Maldonado H, Benmaman C, et al. Melasma in men. Int J Dermatol. 1988;27:25-27.
- Pathak MA, Fitzpatrick TB, Kraus EW. Usefulness of retinoic acid in the treatment of melasma. J Am Acad Dermatol. 1986;15:894-899.
- Giannotti B, Melli MC. Current approaches to the treatment of melasma. Clin Drug Invest. 1995;10(suppl 2):57-64.
- Griffiths CEM, Finkel LJ, Ditre CM, et al. Topical tretinoin (retinoic acid) improves melasma. a vehicle-controlled, clinical trial. Br J Dermatol. 1993;129:415-421.
- Verallo-Rowell VM, Verallo V, Graupe K, et al. Double-blind comparison of azelaic acid and hydroquinone in the treatment of melasma. Acta Derm Venereol. 1989;143(suppl):58-61.
- Sanchez JL, Vazquez M. A hydroquinone solution in the treatment of melasma. Int J Dermatol. 1982;20:55-58.
- Kimbrough-Green CK, Griffiths CEM, Finkel LJ, et al. Topical retinoic acid (tretinoin) for melasma in black patients. Arch Dermatol. 1994;130:727-733.
- Gano SE, Garcia RL. Topical tretinoin, hydroquinone, and betamethasone valerate in the therapy of melasma. Cutis. 1979;23:239-241.
- Kang WH, Chun SC, Lee S. Intermittent therapy for melasma in Asian patients with combined topical agents (retinoic acid, hydroquinone and hydrocortisone): clinical and histological studies. J Dermatol. 1998;25:587-596.
- Katsambas A, Antoniou CH. Melasma: classification and treatment. J Eur Acad Dermatol Venereol. 1995;4:217-223.
- Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111:40-48.
- Kligman LH, Schwartz E, Lesnik RH, et al. Topical tretinoin prevents corticosteroid-induced atrophy without lessening the anti-inflammatory effect. Curr Probl Dermatol. 1993;21:79-88.
- McMichael AJ, Griffiths CE, Talwar HS, et al. Concurrent application of tretinoin (retinoic acid) partially protects against corticosteroid-induced epidermal atrophy. Br J Dermatol. 1996;135:60-64.
- Sanchez PN, Pathak MA, Sato S, et al. Melasma: a clinical, light microscopic, ultrastructural, and immunofluorescence study. J Am Acad Dermatol. 1981;4:698-710.
- Denton CR, Lerner AB,
Cutaneous melasma is a relatively common dermatologic disease, occurring most commonly in Asian and Hispanic women of childbearing years.1-5 Exposure to solar UV radiation is the most important environmental factor in the pathogenesis of melasma.2,3 Therapy for melasma remains a challenge. Pharmacologic treatments are the mainstay.2,6,7 Hydroquinone, azelaic acid, tretinoin, and topical corticosteroids have been used as monotherapy7-11 or in various combinations.12-15 Kligman and Willis15 found that monotherapy with hydroquinone, tretinoin, or the topical corticosteroid dexamethasone did not produce substantial hypopigmentation within a 3-month treatment period. However, they did observe satisfactory results with a combination of tretinoin 0.1%, hydroquinone 5.0%, and dexamethasone 0.1% in a hydrophilic ointment.15 Furthermore, Kligman and Willis,15 as well as other researchers, have noted efficacy and safety benefits with use of hydroquinone, tretinoin, and various topical corticosteroids. In experimental and clinical studies, the use of tretinoin and other retinoids has been found to abrogate the epidermal atrophy that can occur with topical corticosteroids.16,17 This could be due to the ability of tretinoin and other retinoids to induce hyperplasia of epidermal cells and to induce dermal collagen synthesis.16,17 The objective of the 2 well-controlled trials featured in this article was to compare the efficacy and safety of the combination of hydroquinone, tretinoin, and the fluorinated topical corticosteroid fluocinolone acetonide, in a hydrophilic cream formulation, with 3 dual-combination products in the clearing of melasma. back to top
METHODS Study Design—The 2 pivotal trials used similar multicenter, randomized, investigator-blind, active-control, parallel-group protocols. Thirteen centers were involved in these trials. Both studies compared a triple-combination hydrophilic cream vehicle containing tretinoin 0.05%, hydroquinone 4.0%, and fluocinolone acetonide 0.01% (RA+HQ+FA) with the dual-combination products tretinoin plus hydroquinone (RA+HQ), tretinoin plus fluocinolone acetonide (RA+FA), and hydroquinone plus fluocinolone acetonide (HQ+FA). All products involved the same drug concentrations and vehicle. All formulations were used once daily at night. A total of 641 adult patients were randomized to the various treatment groups. Objective evaluation of melasma severity at baseline and at various points after treatment involved investigator assessment of global improvement from baseline using an 8-point scale (0=completely clear to 7=worse) at each follow-up visit. A baseline photograph was used for comparison. Patient Population—Patients enrolled in the study were predominantly white women (aged 21 to 75 years) with Fitzpatrick skin types I through IV. For enrollment into the study, all patients had to demonstrate a stable hyperpigmentation on the face for at least 3 months’ duration, macular lesions that were neither depressed nor atrophic, and melasma severity scores of at least 2 (ie, hyperpigmentation that was at least moderately darker than the surrounding normal skin). There were no significant differences in demographic parameters or skin phototypes among patients in each of the 4 treatment groups. The degree of hyperpigmentation in all patients was moderate to severe. Efficacy and Safety Analysis—The primary efficacy end point involved the investigators’ assessment of the proportion of intent-to-treat patients in each treatment group who achieved complete clearing at week 8. The secondary end point (secondary success) involved the proportion of intent-to-treat patients in each treatment group who achieved complete clearing (score=0) or near-complete clearing (ie, mild residual hyperpigmentation, score=1) by week 8 (Table 1).
| View this table | Table 1. Melasma Severity Rating Scale Used in Primary and Secondary Efficacy Analysis |
All patients randomized to the various treatment groups were analyzed for adverse events. Statistical analysis involved the Cochran-Mantel-Haenszel test, stratified by center. back to top
RESULTS Efficacy—Significantly more of the patients treated with RA+HQ+FA (26.1%) experienced complete clearing compared with each of the dual-therapy groups at week 8 (9.5% for RA+HQ, 1.9% for RA+FA, and 2.5% for HQ+FA, P
Cutaneous melasma is a relatively common dermatologic disease, occurring most commonly in Asian and Hispanic women of childbearing years.1-5 Exposure to solar UV radiation is the most important environmental factor in the pathogenesis of melasma.2,3 Therapy for melasma remains a challenge. Pharmacologic treatments are the mainstay.2,6,7 Hydroquinone, azelaic acid, tretinoin, and topical corticosteroids have been used as monotherapy7-11 or in various combinations.12-15 Kligman and Willis15 found that monotherapy with hydroquinone, tretinoin, or the topical corticosteroid dexamethasone did not produce substantial hypopigmentation within a 3-month treatment period. However, they did observe satisfactory results with a combination of tretinoin 0.1%, hydroquinone 5.0%, and dexamethasone 0.1% in a hydrophilic ointment.15 Furthermore, Kligman and Willis,15 as well as other researchers, have noted efficacy and safety benefits with use of hydroquinone, tretinoin, and various topical corticosteroids. In experimental and clinical studies, the use of tretinoin and other retinoids has been found to abrogate the epidermal atrophy that can occur with topical corticosteroids.16,17 This could be due to the ability of tretinoin and other retinoids to induce hyperplasia of epidermal cells and to induce dermal collagen synthesis.16,17 The objective of the 2 well-controlled trials featured in this article was to compare the efficacy and safety of the combination of hydroquinone, tretinoin, and the fluorinated topical corticosteroid fluocinolone acetonide, in a hydrophilic cream formulation, with 3 dual-combination products in the clearing of melasma. back to top
METHODS Study Design—The 2 pivotal trials used similar multicenter, randomized, investigator-blind, active-control, parallel-group protocols. Thirteen centers were involved in these trials. Both studies compared a triple-combination hydrophilic cream vehicle containing tretinoin 0.05%, hydroquinone 4.0%, and fluocinolone acetonide 0.01% (RA+HQ+FA) with the dual-combination products tretinoin plus hydroquinone (RA+HQ), tretinoin plus fluocinolone acetonide (RA+FA), and hydroquinone plus fluocinolone acetonide (HQ+FA). All products involved the same drug concentrations and vehicle. All formulations were used once daily at night. A total of 641 adult patients were randomized to the various treatment groups. Objective evaluation of melasma severity at baseline and at various points after treatment involved investigator assessment of global improvement from baseline using an 8-point scale (0=completely clear to 7=worse) at each follow-up visit. A baseline photograph was used for comparison. Patient Population—Patients enrolled in the study were predominantly white women (aged 21 to 75 years) with Fitzpatrick skin types I through IV. For enrollment into the study, all patients had to demonstrate a stable hyperpigmentation on the face for at least 3 months’ duration, macular lesions that were neither depressed nor atrophic, and melasma severity scores of at least 2 (ie, hyperpigmentation that was at least moderately darker than the surrounding normal skin). There were no significant differences in demographic parameters or skin phototypes among patients in each of the 4 treatment groups. The degree of hyperpigmentation in all patients was moderate to severe. Efficacy and Safety Analysis—The primary efficacy end point involved the investigators’ assessment of the proportion of intent-to-treat patients in each treatment group who achieved complete clearing at week 8. The secondary end point (secondary success) involved the proportion of intent-to-treat patients in each treatment group who achieved complete clearing (score=0) or near-complete clearing (ie, mild residual hyperpigmentation, score=1) by week 8 (Table 1).
| View this table | Table 1. Melasma Severity Rating Scale Used in Primary and Secondary Efficacy Analysis |
All patients randomized to the various treatment groups were analyzed for adverse events. Statistical analysis involved the Cochran-Mantel-Haenszel test, stratified by center. back to top
RESULTS Efficacy—Significantly more of the patients treated with RA+HQ+FA (26.1%) experienced complete clearing compared with each of the dual-therapy groups at week 8 (9.5% for RA+HQ, 1.9% for RA+FA, and 2.5% for HQ+FA, P
- Grimes PE. Melasma: etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-1457.
- Mosher DB, Fitzpatrick TB, Ortonne J-P, et al. Hypomelanoses and hypermelanoses. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 1. New York, NY: McGraw-Hill; 1999:945-1017.
- Barankin B, Silver SG, Carruthera A. The skin in pregnancy. J Cut Med Surg. 2002;6:236-240.
- Sober AF, Fitzpatrick TB. Disturbances of pigmentation. section I. mechanisms of pigmentation in man. In: Moschella SL, Pillsbury DM, Hurley HJ Jr, eds. Dermatology. Vol 2. Philadelphia, Pa: WB Saunders Co; 1975:1085.
- Vasquez M, Maldonado H, Benmaman C, et al. Melasma in men. Int J Dermatol. 1988;27:25-27.
- Pathak MA, Fitzpatrick TB, Kraus EW. Usefulness of retinoic acid in the treatment of melasma. J Am Acad Dermatol. 1986;15:894-899.
- Giannotti B, Melli MC. Current approaches to the treatment of melasma. Clin Drug Invest. 1995;10(suppl 2):57-64.
- Griffiths CEM, Finkel LJ, Ditre CM, et al. Topical tretinoin (retinoic acid) improves melasma. a vehicle-controlled, clinical trial. Br J Dermatol. 1993;129:415-421.
- Verallo-Rowell VM, Verallo V, Graupe K, et al. Double-blind comparison of azelaic acid and hydroquinone in the treatment of melasma. Acta Derm Venereol. 1989;143(suppl):58-61.
- Sanchez JL, Vazquez M. A hydroquinone solution in the treatment of melasma. Int J Dermatol. 1982;20:55-58.
- Kimbrough-Green CK, Griffiths CEM, Finkel LJ, et al. Topical retinoic acid (tretinoin) for melasma in black patients. Arch Dermatol. 1994;130:727-733.
- Gano SE, Garcia RL. Topical tretinoin, hydroquinone, and betamethasone valerate in the therapy of melasma. Cutis. 1979;23:239-241.
- Kang WH, Chun SC, Lee S. Intermittent therapy for melasma in Asian patients with combined topical agents (retinoic acid, hydroquinone and hydrocortisone): clinical and histological studies. J Dermatol. 1998;25:587-596.
- Katsambas A, Antoniou CH. Melasma: classification and treatment. J Eur Acad Dermatol Venereol. 1995;4:217-223.
- Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111:40-48.
- Kligman LH, Schwartz E, Lesnik RH, et al. Topical tretinoin prevents corticosteroid-induced atrophy without lessening the anti-inflammatory effect. Curr Probl Dermatol. 1993;21:79-88.
- McMichael AJ, Griffiths CE, Talwar HS, et al. Concurrent application of tretinoin (retinoic acid) partially protects against corticosteroid-induced epidermal atrophy. Br J Dermatol. 1996;135:60-64.
- Sanchez PN, Pathak MA, Sato S, et al. Melasma: a clinical, light microscopic, ultrastructural, and immunofluorescence study. J Am Acad Dermatol. 1981;4:698-710.
- Denton CR, Lerner AB,
- Grimes PE. Melasma: etiologic and therapeutic considerations. Arch Dermatol. 1995;131:1453-1457.
- Mosher DB, Fitzpatrick TB, Ortonne J-P, et al. Hypomelanoses and hypermelanoses. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 1. New York, NY: McGraw-Hill; 1999:945-1017.
- Barankin B, Silver SG, Carruthera A. The skin in pregnancy. J Cut Med Surg. 2002;6:236-240.
- Sober AF, Fitzpatrick TB. Disturbances of pigmentation. section I. mechanisms of pigmentation in man. In: Moschella SL, Pillsbury DM, Hurley HJ Jr, eds. Dermatology. Vol 2. Philadelphia, Pa: WB Saunders Co; 1975:1085.
- Vasquez M, Maldonado H, Benmaman C, et al. Melasma in men. Int J Dermatol. 1988;27:25-27.
- Pathak MA, Fitzpatrick TB, Kraus EW. Usefulness of retinoic acid in the treatment of melasma. J Am Acad Dermatol. 1986;15:894-899.
- Giannotti B, Melli MC. Current approaches to the treatment of melasma. Clin Drug Invest. 1995;10(suppl 2):57-64.
- Griffiths CEM, Finkel LJ, Ditre CM, et al. Topical tretinoin (retinoic acid) improves melasma. a vehicle-controlled, clinical trial. Br J Dermatol. 1993;129:415-421.
- Verallo-Rowell VM, Verallo V, Graupe K, et al. Double-blind comparison of azelaic acid and hydroquinone in the treatment of melasma. Acta Derm Venereol. 1989;143(suppl):58-61.
- Sanchez JL, Vazquez M. A hydroquinone solution in the treatment of melasma. Int J Dermatol. 1982;20:55-58.
- Kimbrough-Green CK, Griffiths CEM, Finkel LJ, et al. Topical retinoic acid (tretinoin) for melasma in black patients. Arch Dermatol. 1994;130:727-733.
- Gano SE, Garcia RL. Topical tretinoin, hydroquinone, and betamethasone valerate in the therapy of melasma. Cutis. 1979;23:239-241.
- Kang WH, Chun SC, Lee S. Intermittent therapy for melasma in Asian patients with combined topical agents (retinoic acid, hydroquinone and hydrocortisone): clinical and histological studies. J Dermatol. 1998;25:587-596.
- Katsambas A, Antoniou CH. Melasma: classification and treatment. J Eur Acad Dermatol Venereol. 1995;4:217-223.
- Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975;111:40-48.
- Kligman LH, Schwartz E, Lesnik RH, et al. Topical tretinoin prevents corticosteroid-induced atrophy without lessening the anti-inflammatory effect. Curr Probl Dermatol. 1993;21:79-88.
- McMichael AJ, Griffiths CE, Talwar HS, et al. Concurrent application of tretinoin (retinoic acid) partially protects against corticosteroid-induced epidermal atrophy. Br J Dermatol. 1996;135:60-64.
- Sanchez PN, Pathak MA, Sato S, et al. Melasma: a clinical, light microscopic, ultrastructural, and immunofluorescence study. J Am Acad Dermatol. 1981;4:698-710.
- Denton CR, Lerner AB,
Video study of physician selection: Preferences in the face of diversity
Objectives: To determine whether a diverse group of people would predominantly choose a white male physician regardless of group member’s sex and ethnicity when given a choice among 6 actor-portrayed video doctors (males and females of Latino, European, and African descent) and whether further exposure would alter initial selections.
Study Design: Participants selected a video doctor after viewing a brief introduction and again after viewing the delivery of a prevention message.
Population: Three hundred ninety-five participants recruited at a shopping mall in the San Francisco Bay Area (61% female, 39% male; 30% Asian American, 29% European American, 26% Latino, 8% African American, and 7% other).
Outcomes Measured: Initial and final video doctor selections; ratings of video doctors on interpersonal qualities.
Results: Most participants (85% of females and 63% of males) initially chose a female video doctor (P<.001) and even more did so at final selection. Approximately half initially chose a same-race video doctor (66% of European Americans, 51% of Latinos, and 50% of African Americans), but fewer did so at final selection (56% of European Americans, 44% of Latinos, and 52% of African Americans). In addition, at final selection 57% of Asian Americans and other-ethnicity participants chose a non–European American video doctor.
Conclusions: Many healthcare consumers will accept physicians of both sexes and of different races. After observing the video doctors demonstrate a professional and warm affect, participants became even more receptive to choosing a video doctor of a different race. Video doctor technology holds promise for increasing our understanding of patients’ preferences.
As the physician workforce diversifies,1,2 the question of patients’ preferences for physicians by sex and race becomes increasingly important. Early investigations suggested that many patients, especially males, prefer same-sex physicians across a variety of clinical complaints,3-5 but subsequent studies found these preferences to be more limited,6-9 except for sex-specific health problems (eg, gynecologic examinations and sexual health issues).10
A more recent study examining patients’ actual selections of physicians in a large health maintenance organization showed that most patients of both sexes chose a male physician.11 Whether these findings reflect actual patients’ preferences is debatable, however, because patients’ choices may have been influenced by the greater availability of male physicians on the panel.
Compared with sex, even less is known about preferences for physicians’ race, a topic that is complicated by patient and physician attributes such as language, religion, ethnicity, immigration status, acculturation, and multiracial identities. One recent survey on minority health care found that approximately one fourth of African American and Latino patients who had chosen same-race physicians reported explicitly considering the physician’s race or ethnicity in making their selection.12
In this study, we examined people’s choices when asked to select a male or female African American, Latino, or European American actor-portrayed “video doctor” to be their physician. Choices were examined at 2 time points: after viewing a brief introduction and after viewing the delivery of a brief health advice message. Our research questions were: After gaining a first impression, will patients choose a male of European descent regardless of their own sex and race? Will exposure to the video doctors’ deliveries of a brief health advice message alter these preferences? The video doctor methodology allowed us to offer participants a verisimilar experience of choosing a physician from a diverse panel and to avoid the limitations of availability and access inherent in real-life choices.
Methods
Video doctor filming and editing
We selected 6 actors of similar age (45 years) and attractiveness: 1 female and 1 male African American, Latino, and European American. We used the term Latino to represent a racial identity characterized by dark hair and a medium complexion. The fictitious surnames of the Latino and Latina video doctors also indicated their ethnicity.
When producing the video doctor presentations, we held constant the script, the setting (a doctor’s office), and the clothing. Two segments were produced for each video doctor: a brief introduction in which the doctor used a fictitious name assigned by the researchers to say, for example, “Hi, I’m Dr. Ann Johnson,” and a 45-second health advice message about eating 5 fruits and/or vegetables a day (chosen because of the neutral and universally relevant nature of this topic). The health message contained key elements known to enhance effectiveness of brief interventions.13 The actors’ deliveries of the message were standardized to include interpersonal elements associated with patient-centered health care and positive patient behavior change—for example, warmth, friendliness, empathy, and a nonjudgmental, respectful, and collaborative affect.14,15 (A full description of our procedures is available in Appendix A at www.jfponline.com.)
To balance the video doctors with respect to any possible order effect, we created 18 video presentations showing the video doctors in different orders. We obtained the sequences by creating 6 x 6 Latin squares containing all 720 possible orders and then randomly selecting 3 Latin squares and using the 18 orders contained therein. By delivering 1 of the 18 orders to each group of 22 to 24 participants, we obtained nearly perfect balance in the ordering of the video doctors.
Participants
Individuals at a shopping mall in the San Francisco Bay Area aged >18 years and able to read and write English were invited to watch a short video and rate doctors for a healthcare research project. Four hundred people participated; 395 completed questionnaires. Participants were told that their responses were anonymous, and each questionnaire was marked only with the group number. Study procedures were approved by the Committee on Human Research at the University of California at San Francisco.
FIGURE
Video doctors
Study design and procedures
After viewing brief introductions of each video doctor (Figure), participants were asked: “If you were to choose 1 of these doctors to be your doctor, which would you pick?” They were then instructed to write the number of their choice on the questionnaire.
Participants then viewed the message from each video doctor about eating 5 fruits and vegetables a day. After each presentation, participants rated the video doctor by circling a number on 7-point scales, where a response of 7 indicated the following qualities: very professional, very knowledgeable, excellent communication skills, respectfulness, genuine/authentic, warm/friendly, and pleasant facial expressions.14,15 Participants also rated each video doctor on a 7-point scale for how likely they would be to increase their fruit and vegetable consumption, how interested they might be in choosing this person as their doctor, and how comfortable they might be in talking with this person about personal health matters such as sexual, alcohol, and drug-using behaviors.
After viewing and rating all 6 video doctors, participants again viewed the 6 head shots together and answered the following question: “Now that you’ve heard each video doctor, which one would you pick to be your doctor?” To conclude, participants answered demographic questions, turned in their booklets, and received a $20 gift certificate.
Statistical analysis
Differences in the initial preferences for the sex and race of the video doctors by the sex and race of the participants were studied by using standard 2-way tables, with Fisher exact tests for 2 × 2 tables and χ2 tests for larger tables. Multivariable analysis of sex preferences for the video doctor was done with logistic regression to test the effect of participants’ demographic variables. Matched pair analysis, with an exact version of the McNemar test, was used to assess whether participants’ tendency to choose a same-sex or a same-race video doctor changed from their initial to their final selection.
From each participant’s ratings of the video doctors, an assessment score was generated by averaging the 10 scaled ratings. The clustered assessment scores were analyzed with a normal linear mixed model analysis with a random effect to represent participant scoring tendency and fixed effects to account for the differential mean score for the preferred vs nonpreferred video doctors and differences in mean score depending on the order in which the video doctor was scored. All analyses were performed in Stata 6.0. (More detailed on the methods is found in Appendix A at www.jfponline.com.)
Results
Demographics
Participants were diverse in sex (61% female, 39% male), ethnicity (30% Asian American, 29% European American, 26% Latino, 8% African American, and 7% other), age (11% were 18 to 19 years old, 24% were 20 to 29, 18% were 30 to 39, 17% were 40 to 49, 13% were 50 to 59, 8% were 60 to 69, and 9% were 70 to 87), and education (9% had less than a high school education, 34% had a high school diploma or graduation equivalency diploma, 26% had some college, 22% were college graduates, and 9% had graduate degrees).
Initial preferences for video doctors
Initial sex preference. The strong preference for a female video doctor was significantly different from the 50% preference for each sex that would be expected in the absence of any sex preference (P<.0001). Most females (85%) and males (63%) selected a female video doctor (difference between males and females significant at P<.001; Table 1). The percentages of sex preference by race were not significantly different from one another (P=.36).
Multivariable logistic regression confirmed the relation between participants’ sex and the sex preference of the video doctor but showed no convincing evidence of differences in sex preference related to race (P=.73), age (P=.15), schooling (P=.23), marital status (P=.13), or employment status (P=.19).
Initial race preference. For their initial video doctor selection, 53% of participants chose a European American, 29% chose a Latino, and 18% chose an African American. This pattern of preference was significantly different from the 33.3% for each race that would be expected in the absence of a racial preference (P<.001; Table 2).
Video doctor racial preferences differed significantly by race of the participant (P<.0001), with a preference for the same race. A substantial number of participants, however, chose a different-race video doctor. Racial preferences were similar across male and female participants (P=.98).
TABLE 1
Initial and final video doctor selections by sex
| Initial selection | Final selection | |||
|---|---|---|---|---|
| Participants | Female video doctor | Male doctor video | Female video doctor | Male video doctor |
| Female (n=240) | 85% | 15% | 88% | 12% |
| Male (n=155) | 63% | 37% | 71% | 29% |
| Overall (n=395) | 76% | 24% | 82% | 18% |
TABLE 2
Initial and final video doctor selections by race
| Initial selection | Final selection | |||||
|---|---|---|---|---|---|---|
| Participants | African American | Latino | European American | African American | Latino | European American |
| African American (n=30) | 50% | 17% | 33% | 52% | 19% | 29% |
| Latino (n=101) | 12% | 51% | 37% | 20% | 44% | 36% |
| European American (n=113) | 15% | 19% | 66% | 23% | 21% | 56% |
| Asian American or “other” (n=145) | 18% | 25% | 57% | 20% | 37% | 43% |
| Overall (n=389) | 18% | 29% | 53% | 23% | 32% | 44% |
Final preferences for video doctors
Final sex preference. The preference for a female video doctor increased across female and male participants (P<.001; Table 1). The net shift among males from male to female video doctor was significant (P=.014). More female participants shifted from male to female (9%) than from female to male (4%), although the difference was not statistically significant (P=.10).
Final race preference. Forty-eight percent of African American participants, 56% of Latino participants, and 44% of European Americans chose a different-race video doctor. Among Asian and other-race participants, a sizable shift occurred so that only 43% selected a European American video doctor (Table 2).
Between the initial and final selections, 3% of African American participants shifted to a video doctor of a different race, whereas 7% shifted to an African American video doctor. Eleven percent of Latino participants shifted to a different-race video doctor, whereas 6% shifted to a Latino video doctor. Among European American participants, 22% shifted to a different-race video doctor, whereas 12% shifted to a European American video doctor. With the exception of African American participants, there was a significant net shift from same- to different-race choice (P=.036). Many Asian and other-race participants shifted from a European American video doctor to a non–European American video doctor (14% net).
Assessment scores
The 3 female video doctors, who were chosen by more participants than were the 3 male video doctors at the initial and final selections, also received higher mean assessment scores (Table 3). On particular items, the highest score was 6.001 (of a possible 7), received by the European American female for the question: “How professional is this doctor?” The lowest score was 3.590 received by the European American male for the question: “If this person were your doctor, how comfortable might you be in talking with this person about personal health matters?”
TABLE 3
Selection of video doctor by sex and race
| Video doctor’s name* (ethnicity/sex) | Initial selection of video doctor | Mean assessment score | Final selection of video doctor† |
|---|---|---|---|
| Dr. Ann Johnson (European American/female) | 43% | 5.49 | 38% |
| Dr. Renee Garcia (Latina/female) | 22% | 5.32 | 26% |
| Dr. Terry Williams (African American/female) | 12% | 5.13 | 17% |
| Dr. Mark Benson (European American/male) | 10% | 4.31 | 6% |
| Dr. Glen Martinez (Latino/male) | 7% | 4.33 | 6% |
| Dr. Calvin Butler (African American/male) | 6% | 4.84 | 6% |
| *Fictitious names were assigned by the researchers. | |||
| †Figures do not add to 100% due to rounding. | |||
Association of preferences and ratings. Analysis of the mean assessment scores showed a substantial rating tendency among participants, by which they tended to give all 6 video doctors relatively high or low scores. Our analysis indicated that 34.9% (95% confidence interval [CI], 30.4–39.5) of the variance in assessment scores is explained by rating tendency.
We also found that participants tended to increase their scores as they proceeded through the sequence of doctors. Compared with the first video doctor, the second through the sixth video doctors received increases in mean scores of 0.15 (P=.016), 0.16 (P=.011), 0.29 (P<.001), 0.43 (P<.001), and 0.60 (P<.001), respectively. These results showed the importance of using multiple presentation orders to balance the order effect.
After adjusting for the order effect and the respondent rating tendency, the mean assessment scores given to video doctors selected at the initial stage were an average of 0.7 points higher than scores given to the other video doctors (P<.001, 95% CI, 0.56–0.81). At the final selection, the chosen video doctor scored on average 1.04 units higher on the assessment scores than did the other video doctors (P<.001, 95% CI, 0.94–1.1). Thus, the selection made based on the video doctors’ images and brief introductions alone was significantly associated with the subsequent assessment, and the final selection of video doctor was even more strongly associated with the assessment.
Discussion
More participants preferred same-race physicians at the initial selection (66% of European Americans, 51% of Latinos, and 50% of African Americans). This effect was not as large as one might expect, however, because a substantial minority of subjects in each racial category selected a different-race video doctor at the initial selection and a majority of Latinos selected a different-race video doctor at the final selection.
After viewing the delivery of the prevention message, more in each group, except for African Americans, chose a video doctor of a different race. In addition, at final selection, 57% of Asian and other-race participants chose a non– European American video doctor. With regard to sex, most males and females chose a female video doctor at the initial selection, and even more did so at the final selection. These data suggested that many healthcare consumers are in concordance with the recent shift toward a more diverse population of physicians and that the white male physician may no longer be viewed as the stereotypical medical professional.
The qualities patients seek in a doctor
The assessment scores for the video doctors indicated that participants were choosing, both on first impressions and after further exposure, video doctors who they perceived to possess the qualities associated with patient-centered care.21,22 Although the overall ranking of the 6 video doctors was unchanged from initial to final selection, after viewing the delivery of the prevention message, many participants altered their choices: more males and females chose a female video doctor; more European American and Latino participants shifted from same-race to different-race video doctors; and more Asian and other-race participants shifted from European American to non–European American video doctors.
These findings suggested that, even in brief meetings with physicians, patients respond to a combination of patient-centered qualities and that this combination may carry more weight than the physician’s sex and race. In other words, from the point of view of the public at large, physicians of both sexes and all races can possess the desired physician qualities, and people may be receptive to any physician who exhibits these qualities.
Preference for a female doctor
Our finding that men and women in our sample preferred a female video doctor contrasts with sex preference findings from previous studies,3,6,8,10,11 although in general studies on sex preference of physicians have shown inconsistent findings. The female preference finding in our study may represent evolving positive attitudes toward and increasing familiarity with female physicians. From 1971 to 1991, the percentage of women first-year medical school students rose from 13.7% to 39.8%.14
The strong female preference also may represent sex stereotyping. Patients reported that they desire physicians who are sensitive to their needs and circumstances, deliver a warm and empathic style of care,15 invite participation in decision making,16 engage in emotionally focused talk, and provide health information within patients’ social, emotional, and cultural contexts.17 Other studies found that women, when compared with men, provide a style of care that approximates these patient-centered characteristics.18-20
Our participants, many of whom preferred female video doctors even at first, may have strongly associated a patient-centered, empathic style with being female. The particular female actors we chose also may have been better able to exhibit, regardless of our efforts to standardize, the combination of professional and personal skills most desired in a doctor.
Racial preferences
The preference for a same-race video doctor may have several origins. People may feel more familiar and comfortable with race-concordant relationships in general and may believe that a physician of one’s own race can better attend to specific health concerns. Same-race preference also may arise from the desire to avoid a racially prejudiced physician. Racially concordant as opposed to discordant care has been associated with increased patient satisfaction and use of health care services and with higher ratings from patients regarding their level of participation during physician visits.16,23
As indicated in our study and others, African Americans express a stronger preference than do individuals from other racial groups for receiving care from physicians of their own race.23 To support patients in exercising their racial preferences, some health care professional organizations, such as the National Medical Association, have provided a toll-free number that patients can call to locate a local African American physician.
Limitations of the study
The study had several limitations. We may not have successfully held constant the actors’ personalities and acting abilities. Future video doctor studies about patients’ acceptance regarding physicians’ race and sex could address this drawback by including multiple video doctors in each sex and race category.
Because only English-speaking participants were included in the study, we do not know whether Latinos who spoke only Spanish would have chosen differently. Our study also used a convenience sample in a San Francisco Bay Area shopping mall, and our results may not be generalizable to other populations.
We were unable to study the same-race preferences of the Asian participants in our sample. Because more than 10% of physicians practicing in the United States are of Asian ancestry, patients’ receptivity to Asian physicians and Asian patients’ preference for a same-race physician would be important research topics. Diversity of language and culture among various Asian and other ethnicities also could be addressed with a well-designed video doctor study. The absence of an Asian video doctor, however, did allow us to examine the selections made by participants when no same-race video doctor was available.
Strengths of the study
A major strength of our study was that participants represented both sexes and a range of ages, races, and education levels. In addition, the video technology allowed participants to select a video doctor based on a verisimilar experience and without the constraints of availability and access found in real-life choices. All our study participants accepted the survey questions and responded to the video doctor as a “real” physician.
Video doctor technology does allow for holding constant certain variables such as age, appearance, message content, and style of delivery, an advantage that cannot be achieved in real encounters between patients and physicians.
Challenges for the future
Some of our most crucial health care challenges are providing access to quality care and equal career opportunities for those who seek to practice medicine. Our results supported the growing diversity of the population of physicians, and emphasized that many patients will choose physicians, regardless of their sex and race, who appear professional, competent, and caring. Medical schools need to continue the trend toward teaching patient-centered, empathic care and recruiting and retaining minority physicians to rectify current imbalances. In addition, practicing physicians can take note that providing quality care for patients of all cultural backgrounds may be an easier task than they think—the common language of compassion may transcend our differences.
Future studies could use video doctor technology to confirm our findings and to further investigate patients’ preferences and attitudes about various dimensions of the relationship between patient and physician. As the patient population and the physician workforce diversify, and as managed care organizations continue to strive to increase patient satisfaction and retention, information about patient preferences could inform the future of health care delivery.
Acknowledgments
We thank Scott Ludwig for his excellent casting of actors, directing, and video production; and Annabelle Ison for designing subject recruitment materials. We also thank our video doctors, the staff of Tanforan Park Shopping Center in San Bruno, CA, and the mall visitors who volunteered to participate in the study.
Corresponding author
Barbara Gerbert, PhD, University of California at San Francisco, 350 Parnassus Avenue, Suite 905, San Francisco, CA 94117. E-mail: [email protected].
1. US Census Bureau Detailed occupation by race, Hispanic origin and sex: 1990. Available at: http://censtats.census.gov/ cgi-bin/eeo/eeojobs.pl. Accessed on June 9, 2003.
2. Johnson LMI ed. Minority Student Opportunities in United States Medical Schools. 15th ed. Washington, DC: Association of American Medical Colleges; 2000.
3. Ackerman-Ross SF, Sochat N. Close encounters of the medical kind: attitudes toward male and female physicians. Soc Sci Med 1980;14A:61-64.
4. Engleman E. Attitudes toward women physicians: a study of 500 clinic patients. West J Med 1974;120:95-100.
5. Challacombe C. Do women patients need women doctors? Practitioner 1983;227:848-850.
6. Fennema K, Meyer D, Owen N. Sex of physician: patients’ p and stereotypes. J Fam Pract 1990;30:441-446.
7. Weyrauch KF, Boiko PE, Alvin B. Patient sex role and preference for a male or female physician. J Fam Pract 1990;30:559-562.
8. Kerssens JJ, Bensing JM, Andela MG. Patient preference for genders of health professionals. Soc Sci Med 1997;44:1531-1540.
9. Graffy J. Patient choice in practice with men and women general practitioners. Br J Gen Pract 1990;40:13-15.
10. Elstad JI. Women’s priorities regarding physician behavior and their preference for a female physician. Women Health 1994;21(4):1-17.
11. Schmittdiel MA, Grumbach K, Selby JV, Quesenberry CP. Effect of physician and patient gender concordance on patient satisfaction and preventive care practices. J Gen Intern Med 2000;15:761-769.
12. Saha S, Taggart SH, Komaromy M, Bindman AB. Do patients choose physicians of their own race? Health Aff (Millwood). 2000;19(4):76-83.
13. Miller W, Sanchez V. Motivating young adults for treatment and lifestyle change. In: Howard G, ed. Issues in Alcohol Use and Misuse by Young Adults. Notre Dame, Ind: University of Notre Dame Press; 1994;55-82.
14. Jonas H, Etzel S, Baransky B. Educational programs in the US medical schools. JAMA 1992;268:1083-1090.
15. Rollnick S, Mason P, Butler C. Health Behavior Change: A Guide for Practitioners. Edinburgh: Churchill Livingstone; 1999.
16. Cooper-Patrick L, Gallo JJ, Gonzales JJ, Vu HT, Nelson C, Ford DE. Race, gender, and partnership in the patient–physician relationship. JAMA 1999;282:583-589.
17. Roter D, Hall J. Why physicians’ gender matters in the shaping of the patient-physician relationship. J Womens Health 1998;7:1093-1097.
18. Roter D, Lipkin M, Korsgaard A. Sex differences in patients’ and physicians’ communication during primary care medical visits. Med Care 1991;29:1083-1093.
19. Hall J, Irish J, Roter D, Ehrich C, Miller L. Gender in medical encounters: an analysis of physician and patient communication in a primary care setting. Health Psychol 1994;13:384-392.
20. Elderkin-Thompson V, Waitzkin H. Differences in clinical communication by gender. J Gen Intern Med 1999;14:112-121.
21. Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behaviors. New York: Guilford Press; 1991.
22. Stewart M. Effective physician–patient communication and health outcomes. CMAJ 1995;152:1423-1433.
23. Saha S, Komaromy M, Koepsell TD, Bindman AB. Patient–physician racial concordance and the perceived quality and use of health care. Arch Intern Med 1999;159:997-1004.
Objectives: To determine whether a diverse group of people would predominantly choose a white male physician regardless of group member’s sex and ethnicity when given a choice among 6 actor-portrayed video doctors (males and females of Latino, European, and African descent) and whether further exposure would alter initial selections.
Study Design: Participants selected a video doctor after viewing a brief introduction and again after viewing the delivery of a prevention message.
Population: Three hundred ninety-five participants recruited at a shopping mall in the San Francisco Bay Area (61% female, 39% male; 30% Asian American, 29% European American, 26% Latino, 8% African American, and 7% other).
Outcomes Measured: Initial and final video doctor selections; ratings of video doctors on interpersonal qualities.
Results: Most participants (85% of females and 63% of males) initially chose a female video doctor (P<.001) and even more did so at final selection. Approximately half initially chose a same-race video doctor (66% of European Americans, 51% of Latinos, and 50% of African Americans), but fewer did so at final selection (56% of European Americans, 44% of Latinos, and 52% of African Americans). In addition, at final selection 57% of Asian Americans and other-ethnicity participants chose a non–European American video doctor.
Conclusions: Many healthcare consumers will accept physicians of both sexes and of different races. After observing the video doctors demonstrate a professional and warm affect, participants became even more receptive to choosing a video doctor of a different race. Video doctor technology holds promise for increasing our understanding of patients’ preferences.
As the physician workforce diversifies,1,2 the question of patients’ preferences for physicians by sex and race becomes increasingly important. Early investigations suggested that many patients, especially males, prefer same-sex physicians across a variety of clinical complaints,3-5 but subsequent studies found these preferences to be more limited,6-9 except for sex-specific health problems (eg, gynecologic examinations and sexual health issues).10
A more recent study examining patients’ actual selections of physicians in a large health maintenance organization showed that most patients of both sexes chose a male physician.11 Whether these findings reflect actual patients’ preferences is debatable, however, because patients’ choices may have been influenced by the greater availability of male physicians on the panel.
Compared with sex, even less is known about preferences for physicians’ race, a topic that is complicated by patient and physician attributes such as language, religion, ethnicity, immigration status, acculturation, and multiracial identities. One recent survey on minority health care found that approximately one fourth of African American and Latino patients who had chosen same-race physicians reported explicitly considering the physician’s race or ethnicity in making their selection.12
In this study, we examined people’s choices when asked to select a male or female African American, Latino, or European American actor-portrayed “video doctor” to be their physician. Choices were examined at 2 time points: after viewing a brief introduction and after viewing the delivery of a brief health advice message. Our research questions were: After gaining a first impression, will patients choose a male of European descent regardless of their own sex and race? Will exposure to the video doctors’ deliveries of a brief health advice message alter these preferences? The video doctor methodology allowed us to offer participants a verisimilar experience of choosing a physician from a diverse panel and to avoid the limitations of availability and access inherent in real-life choices.
Methods
Video doctor filming and editing
We selected 6 actors of similar age (45 years) and attractiveness: 1 female and 1 male African American, Latino, and European American. We used the term Latino to represent a racial identity characterized by dark hair and a medium complexion. The fictitious surnames of the Latino and Latina video doctors also indicated their ethnicity.
When producing the video doctor presentations, we held constant the script, the setting (a doctor’s office), and the clothing. Two segments were produced for each video doctor: a brief introduction in which the doctor used a fictitious name assigned by the researchers to say, for example, “Hi, I’m Dr. Ann Johnson,” and a 45-second health advice message about eating 5 fruits and/or vegetables a day (chosen because of the neutral and universally relevant nature of this topic). The health message contained key elements known to enhance effectiveness of brief interventions.13 The actors’ deliveries of the message were standardized to include interpersonal elements associated with patient-centered health care and positive patient behavior change—for example, warmth, friendliness, empathy, and a nonjudgmental, respectful, and collaborative affect.14,15 (A full description of our procedures is available in Appendix A at www.jfponline.com.)
To balance the video doctors with respect to any possible order effect, we created 18 video presentations showing the video doctors in different orders. We obtained the sequences by creating 6 x 6 Latin squares containing all 720 possible orders and then randomly selecting 3 Latin squares and using the 18 orders contained therein. By delivering 1 of the 18 orders to each group of 22 to 24 participants, we obtained nearly perfect balance in the ordering of the video doctors.
Participants
Individuals at a shopping mall in the San Francisco Bay Area aged >18 years and able to read and write English were invited to watch a short video and rate doctors for a healthcare research project. Four hundred people participated; 395 completed questionnaires. Participants were told that their responses were anonymous, and each questionnaire was marked only with the group number. Study procedures were approved by the Committee on Human Research at the University of California at San Francisco.
FIGURE
Video doctors
Study design and procedures
After viewing brief introductions of each video doctor (Figure), participants were asked: “If you were to choose 1 of these doctors to be your doctor, which would you pick?” They were then instructed to write the number of their choice on the questionnaire.
Participants then viewed the message from each video doctor about eating 5 fruits and vegetables a day. After each presentation, participants rated the video doctor by circling a number on 7-point scales, where a response of 7 indicated the following qualities: very professional, very knowledgeable, excellent communication skills, respectfulness, genuine/authentic, warm/friendly, and pleasant facial expressions.14,15 Participants also rated each video doctor on a 7-point scale for how likely they would be to increase their fruit and vegetable consumption, how interested they might be in choosing this person as their doctor, and how comfortable they might be in talking with this person about personal health matters such as sexual, alcohol, and drug-using behaviors.
After viewing and rating all 6 video doctors, participants again viewed the 6 head shots together and answered the following question: “Now that you’ve heard each video doctor, which one would you pick to be your doctor?” To conclude, participants answered demographic questions, turned in their booklets, and received a $20 gift certificate.
Statistical analysis
Differences in the initial preferences for the sex and race of the video doctors by the sex and race of the participants were studied by using standard 2-way tables, with Fisher exact tests for 2 × 2 tables and χ2 tests for larger tables. Multivariable analysis of sex preferences for the video doctor was done with logistic regression to test the effect of participants’ demographic variables. Matched pair analysis, with an exact version of the McNemar test, was used to assess whether participants’ tendency to choose a same-sex or a same-race video doctor changed from their initial to their final selection.
From each participant’s ratings of the video doctors, an assessment score was generated by averaging the 10 scaled ratings. The clustered assessment scores were analyzed with a normal linear mixed model analysis with a random effect to represent participant scoring tendency and fixed effects to account for the differential mean score for the preferred vs nonpreferred video doctors and differences in mean score depending on the order in which the video doctor was scored. All analyses were performed in Stata 6.0. (More detailed on the methods is found in Appendix A at www.jfponline.com.)
Results
Demographics
Participants were diverse in sex (61% female, 39% male), ethnicity (30% Asian American, 29% European American, 26% Latino, 8% African American, and 7% other), age (11% were 18 to 19 years old, 24% were 20 to 29, 18% were 30 to 39, 17% were 40 to 49, 13% were 50 to 59, 8% were 60 to 69, and 9% were 70 to 87), and education (9% had less than a high school education, 34% had a high school diploma or graduation equivalency diploma, 26% had some college, 22% were college graduates, and 9% had graduate degrees).
Initial preferences for video doctors
Initial sex preference. The strong preference for a female video doctor was significantly different from the 50% preference for each sex that would be expected in the absence of any sex preference (P<.0001). Most females (85%) and males (63%) selected a female video doctor (difference between males and females significant at P<.001; Table 1). The percentages of sex preference by race were not significantly different from one another (P=.36).
Multivariable logistic regression confirmed the relation between participants’ sex and the sex preference of the video doctor but showed no convincing evidence of differences in sex preference related to race (P=.73), age (P=.15), schooling (P=.23), marital status (P=.13), or employment status (P=.19).
Initial race preference. For their initial video doctor selection, 53% of participants chose a European American, 29% chose a Latino, and 18% chose an African American. This pattern of preference was significantly different from the 33.3% for each race that would be expected in the absence of a racial preference (P<.001; Table 2).
Video doctor racial preferences differed significantly by race of the participant (P<.0001), with a preference for the same race. A substantial number of participants, however, chose a different-race video doctor. Racial preferences were similar across male and female participants (P=.98).
TABLE 1
Initial and final video doctor selections by sex
| Initial selection | Final selection | |||
|---|---|---|---|---|
| Participants | Female video doctor | Male doctor video | Female video doctor | Male video doctor |
| Female (n=240) | 85% | 15% | 88% | 12% |
| Male (n=155) | 63% | 37% | 71% | 29% |
| Overall (n=395) | 76% | 24% | 82% | 18% |
TABLE 2
Initial and final video doctor selections by race
| Initial selection | Final selection | |||||
|---|---|---|---|---|---|---|
| Participants | African American | Latino | European American | African American | Latino | European American |
| African American (n=30) | 50% | 17% | 33% | 52% | 19% | 29% |
| Latino (n=101) | 12% | 51% | 37% | 20% | 44% | 36% |
| European American (n=113) | 15% | 19% | 66% | 23% | 21% | 56% |
| Asian American or “other” (n=145) | 18% | 25% | 57% | 20% | 37% | 43% |
| Overall (n=389) | 18% | 29% | 53% | 23% | 32% | 44% |
Final preferences for video doctors
Final sex preference. The preference for a female video doctor increased across female and male participants (P<.001; Table 1). The net shift among males from male to female video doctor was significant (P=.014). More female participants shifted from male to female (9%) than from female to male (4%), although the difference was not statistically significant (P=.10).
Final race preference. Forty-eight percent of African American participants, 56% of Latino participants, and 44% of European Americans chose a different-race video doctor. Among Asian and other-race participants, a sizable shift occurred so that only 43% selected a European American video doctor (Table 2).
Between the initial and final selections, 3% of African American participants shifted to a video doctor of a different race, whereas 7% shifted to an African American video doctor. Eleven percent of Latino participants shifted to a different-race video doctor, whereas 6% shifted to a Latino video doctor. Among European American participants, 22% shifted to a different-race video doctor, whereas 12% shifted to a European American video doctor. With the exception of African American participants, there was a significant net shift from same- to different-race choice (P=.036). Many Asian and other-race participants shifted from a European American video doctor to a non–European American video doctor (14% net).
Assessment scores
The 3 female video doctors, who were chosen by more participants than were the 3 male video doctors at the initial and final selections, also received higher mean assessment scores (Table 3). On particular items, the highest score was 6.001 (of a possible 7), received by the European American female for the question: “How professional is this doctor?” The lowest score was 3.590 received by the European American male for the question: “If this person were your doctor, how comfortable might you be in talking with this person about personal health matters?”
TABLE 3
Selection of video doctor by sex and race
| Video doctor’s name* (ethnicity/sex) | Initial selection of video doctor | Mean assessment score | Final selection of video doctor† |
|---|---|---|---|
| Dr. Ann Johnson (European American/female) | 43% | 5.49 | 38% |
| Dr. Renee Garcia (Latina/female) | 22% | 5.32 | 26% |
| Dr. Terry Williams (African American/female) | 12% | 5.13 | 17% |
| Dr. Mark Benson (European American/male) | 10% | 4.31 | 6% |
| Dr. Glen Martinez (Latino/male) | 7% | 4.33 | 6% |
| Dr. Calvin Butler (African American/male) | 6% | 4.84 | 6% |
| *Fictitious names were assigned by the researchers. | |||
| †Figures do not add to 100% due to rounding. | |||
Association of preferences and ratings. Analysis of the mean assessment scores showed a substantial rating tendency among participants, by which they tended to give all 6 video doctors relatively high or low scores. Our analysis indicated that 34.9% (95% confidence interval [CI], 30.4–39.5) of the variance in assessment scores is explained by rating tendency.
We also found that participants tended to increase their scores as they proceeded through the sequence of doctors. Compared with the first video doctor, the second through the sixth video doctors received increases in mean scores of 0.15 (P=.016), 0.16 (P=.011), 0.29 (P<.001), 0.43 (P<.001), and 0.60 (P<.001), respectively. These results showed the importance of using multiple presentation orders to balance the order effect.
After adjusting for the order effect and the respondent rating tendency, the mean assessment scores given to video doctors selected at the initial stage were an average of 0.7 points higher than scores given to the other video doctors (P<.001, 95% CI, 0.56–0.81). At the final selection, the chosen video doctor scored on average 1.04 units higher on the assessment scores than did the other video doctors (P<.001, 95% CI, 0.94–1.1). Thus, the selection made based on the video doctors’ images and brief introductions alone was significantly associated with the subsequent assessment, and the final selection of video doctor was even more strongly associated with the assessment.
Discussion
More participants preferred same-race physicians at the initial selection (66% of European Americans, 51% of Latinos, and 50% of African Americans). This effect was not as large as one might expect, however, because a substantial minority of subjects in each racial category selected a different-race video doctor at the initial selection and a majority of Latinos selected a different-race video doctor at the final selection.
After viewing the delivery of the prevention message, more in each group, except for African Americans, chose a video doctor of a different race. In addition, at final selection, 57% of Asian and other-race participants chose a non– European American video doctor. With regard to sex, most males and females chose a female video doctor at the initial selection, and even more did so at the final selection. These data suggested that many healthcare consumers are in concordance with the recent shift toward a more diverse population of physicians and that the white male physician may no longer be viewed as the stereotypical medical professional.
The qualities patients seek in a doctor
The assessment scores for the video doctors indicated that participants were choosing, both on first impressions and after further exposure, video doctors who they perceived to possess the qualities associated with patient-centered care.21,22 Although the overall ranking of the 6 video doctors was unchanged from initial to final selection, after viewing the delivery of the prevention message, many participants altered their choices: more males and females chose a female video doctor; more European American and Latino participants shifted from same-race to different-race video doctors; and more Asian and other-race participants shifted from European American to non–European American video doctors.
These findings suggested that, even in brief meetings with physicians, patients respond to a combination of patient-centered qualities and that this combination may carry more weight than the physician’s sex and race. In other words, from the point of view of the public at large, physicians of both sexes and all races can possess the desired physician qualities, and people may be receptive to any physician who exhibits these qualities.
Preference for a female doctor
Our finding that men and women in our sample preferred a female video doctor contrasts with sex preference findings from previous studies,3,6,8,10,11 although in general studies on sex preference of physicians have shown inconsistent findings. The female preference finding in our study may represent evolving positive attitudes toward and increasing familiarity with female physicians. From 1971 to 1991, the percentage of women first-year medical school students rose from 13.7% to 39.8%.14
The strong female preference also may represent sex stereotyping. Patients reported that they desire physicians who are sensitive to their needs and circumstances, deliver a warm and empathic style of care,15 invite participation in decision making,16 engage in emotionally focused talk, and provide health information within patients’ social, emotional, and cultural contexts.17 Other studies found that women, when compared with men, provide a style of care that approximates these patient-centered characteristics.18-20
Our participants, many of whom preferred female video doctors even at first, may have strongly associated a patient-centered, empathic style with being female. The particular female actors we chose also may have been better able to exhibit, regardless of our efforts to standardize, the combination of professional and personal skills most desired in a doctor.
Racial preferences
The preference for a same-race video doctor may have several origins. People may feel more familiar and comfortable with race-concordant relationships in general and may believe that a physician of one’s own race can better attend to specific health concerns. Same-race preference also may arise from the desire to avoid a racially prejudiced physician. Racially concordant as opposed to discordant care has been associated with increased patient satisfaction and use of health care services and with higher ratings from patients regarding their level of participation during physician visits.16,23
As indicated in our study and others, African Americans express a stronger preference than do individuals from other racial groups for receiving care from physicians of their own race.23 To support patients in exercising their racial preferences, some health care professional organizations, such as the National Medical Association, have provided a toll-free number that patients can call to locate a local African American physician.
Limitations of the study
The study had several limitations. We may not have successfully held constant the actors’ personalities and acting abilities. Future video doctor studies about patients’ acceptance regarding physicians’ race and sex could address this drawback by including multiple video doctors in each sex and race category.
Because only English-speaking participants were included in the study, we do not know whether Latinos who spoke only Spanish would have chosen differently. Our study also used a convenience sample in a San Francisco Bay Area shopping mall, and our results may not be generalizable to other populations.
We were unable to study the same-race preferences of the Asian participants in our sample. Because more than 10% of physicians practicing in the United States are of Asian ancestry, patients’ receptivity to Asian physicians and Asian patients’ preference for a same-race physician would be important research topics. Diversity of language and culture among various Asian and other ethnicities also could be addressed with a well-designed video doctor study. The absence of an Asian video doctor, however, did allow us to examine the selections made by participants when no same-race video doctor was available.
Strengths of the study
A major strength of our study was that participants represented both sexes and a range of ages, races, and education levels. In addition, the video technology allowed participants to select a video doctor based on a verisimilar experience and without the constraints of availability and access found in real-life choices. All our study participants accepted the survey questions and responded to the video doctor as a “real” physician.
Video doctor technology does allow for holding constant certain variables such as age, appearance, message content, and style of delivery, an advantage that cannot be achieved in real encounters between patients and physicians.
Challenges for the future
Some of our most crucial health care challenges are providing access to quality care and equal career opportunities for those who seek to practice medicine. Our results supported the growing diversity of the population of physicians, and emphasized that many patients will choose physicians, regardless of their sex and race, who appear professional, competent, and caring. Medical schools need to continue the trend toward teaching patient-centered, empathic care and recruiting and retaining minority physicians to rectify current imbalances. In addition, practicing physicians can take note that providing quality care for patients of all cultural backgrounds may be an easier task than they think—the common language of compassion may transcend our differences.
Future studies could use video doctor technology to confirm our findings and to further investigate patients’ preferences and attitudes about various dimensions of the relationship between patient and physician. As the patient population and the physician workforce diversify, and as managed care organizations continue to strive to increase patient satisfaction and retention, information about patient preferences could inform the future of health care delivery.
Acknowledgments
We thank Scott Ludwig for his excellent casting of actors, directing, and video production; and Annabelle Ison for designing subject recruitment materials. We also thank our video doctors, the staff of Tanforan Park Shopping Center in San Bruno, CA, and the mall visitors who volunteered to participate in the study.
Corresponding author
Barbara Gerbert, PhD, University of California at San Francisco, 350 Parnassus Avenue, Suite 905, San Francisco, CA 94117. E-mail: [email protected].
Objectives: To determine whether a diverse group of people would predominantly choose a white male physician regardless of group member’s sex and ethnicity when given a choice among 6 actor-portrayed video doctors (males and females of Latino, European, and African descent) and whether further exposure would alter initial selections.
Study Design: Participants selected a video doctor after viewing a brief introduction and again after viewing the delivery of a prevention message.
Population: Three hundred ninety-five participants recruited at a shopping mall in the San Francisco Bay Area (61% female, 39% male; 30% Asian American, 29% European American, 26% Latino, 8% African American, and 7% other).
Outcomes Measured: Initial and final video doctor selections; ratings of video doctors on interpersonal qualities.
Results: Most participants (85% of females and 63% of males) initially chose a female video doctor (P<.001) and even more did so at final selection. Approximately half initially chose a same-race video doctor (66% of European Americans, 51% of Latinos, and 50% of African Americans), but fewer did so at final selection (56% of European Americans, 44% of Latinos, and 52% of African Americans). In addition, at final selection 57% of Asian Americans and other-ethnicity participants chose a non–European American video doctor.
Conclusions: Many healthcare consumers will accept physicians of both sexes and of different races. After observing the video doctors demonstrate a professional and warm affect, participants became even more receptive to choosing a video doctor of a different race. Video doctor technology holds promise for increasing our understanding of patients’ preferences.
As the physician workforce diversifies,1,2 the question of patients’ preferences for physicians by sex and race becomes increasingly important. Early investigations suggested that many patients, especially males, prefer same-sex physicians across a variety of clinical complaints,3-5 but subsequent studies found these preferences to be more limited,6-9 except for sex-specific health problems (eg, gynecologic examinations and sexual health issues).10
A more recent study examining patients’ actual selections of physicians in a large health maintenance organization showed that most patients of both sexes chose a male physician.11 Whether these findings reflect actual patients’ preferences is debatable, however, because patients’ choices may have been influenced by the greater availability of male physicians on the panel.
Compared with sex, even less is known about preferences for physicians’ race, a topic that is complicated by patient and physician attributes such as language, religion, ethnicity, immigration status, acculturation, and multiracial identities. One recent survey on minority health care found that approximately one fourth of African American and Latino patients who had chosen same-race physicians reported explicitly considering the physician’s race or ethnicity in making their selection.12
In this study, we examined people’s choices when asked to select a male or female African American, Latino, or European American actor-portrayed “video doctor” to be their physician. Choices were examined at 2 time points: after viewing a brief introduction and after viewing the delivery of a brief health advice message. Our research questions were: After gaining a first impression, will patients choose a male of European descent regardless of their own sex and race? Will exposure to the video doctors’ deliveries of a brief health advice message alter these preferences? The video doctor methodology allowed us to offer participants a verisimilar experience of choosing a physician from a diverse panel and to avoid the limitations of availability and access inherent in real-life choices.
Methods
Video doctor filming and editing
We selected 6 actors of similar age (45 years) and attractiveness: 1 female and 1 male African American, Latino, and European American. We used the term Latino to represent a racial identity characterized by dark hair and a medium complexion. The fictitious surnames of the Latino and Latina video doctors also indicated their ethnicity.
When producing the video doctor presentations, we held constant the script, the setting (a doctor’s office), and the clothing. Two segments were produced for each video doctor: a brief introduction in which the doctor used a fictitious name assigned by the researchers to say, for example, “Hi, I’m Dr. Ann Johnson,” and a 45-second health advice message about eating 5 fruits and/or vegetables a day (chosen because of the neutral and universally relevant nature of this topic). The health message contained key elements known to enhance effectiveness of brief interventions.13 The actors’ deliveries of the message were standardized to include interpersonal elements associated with patient-centered health care and positive patient behavior change—for example, warmth, friendliness, empathy, and a nonjudgmental, respectful, and collaborative affect.14,15 (A full description of our procedures is available in Appendix A at www.jfponline.com.)
To balance the video doctors with respect to any possible order effect, we created 18 video presentations showing the video doctors in different orders. We obtained the sequences by creating 6 x 6 Latin squares containing all 720 possible orders and then randomly selecting 3 Latin squares and using the 18 orders contained therein. By delivering 1 of the 18 orders to each group of 22 to 24 participants, we obtained nearly perfect balance in the ordering of the video doctors.
Participants
Individuals at a shopping mall in the San Francisco Bay Area aged >18 years and able to read and write English were invited to watch a short video and rate doctors for a healthcare research project. Four hundred people participated; 395 completed questionnaires. Participants were told that their responses were anonymous, and each questionnaire was marked only with the group number. Study procedures were approved by the Committee on Human Research at the University of California at San Francisco.
FIGURE
Video doctors
Study design and procedures
After viewing brief introductions of each video doctor (Figure), participants were asked: “If you were to choose 1 of these doctors to be your doctor, which would you pick?” They were then instructed to write the number of their choice on the questionnaire.
Participants then viewed the message from each video doctor about eating 5 fruits and vegetables a day. After each presentation, participants rated the video doctor by circling a number on 7-point scales, where a response of 7 indicated the following qualities: very professional, very knowledgeable, excellent communication skills, respectfulness, genuine/authentic, warm/friendly, and pleasant facial expressions.14,15 Participants also rated each video doctor on a 7-point scale for how likely they would be to increase their fruit and vegetable consumption, how interested they might be in choosing this person as their doctor, and how comfortable they might be in talking with this person about personal health matters such as sexual, alcohol, and drug-using behaviors.
After viewing and rating all 6 video doctors, participants again viewed the 6 head shots together and answered the following question: “Now that you’ve heard each video doctor, which one would you pick to be your doctor?” To conclude, participants answered demographic questions, turned in their booklets, and received a $20 gift certificate.
Statistical analysis
Differences in the initial preferences for the sex and race of the video doctors by the sex and race of the participants were studied by using standard 2-way tables, with Fisher exact tests for 2 × 2 tables and χ2 tests for larger tables. Multivariable analysis of sex preferences for the video doctor was done with logistic regression to test the effect of participants’ demographic variables. Matched pair analysis, with an exact version of the McNemar test, was used to assess whether participants’ tendency to choose a same-sex or a same-race video doctor changed from their initial to their final selection.
From each participant’s ratings of the video doctors, an assessment score was generated by averaging the 10 scaled ratings. The clustered assessment scores were analyzed with a normal linear mixed model analysis with a random effect to represent participant scoring tendency and fixed effects to account for the differential mean score for the preferred vs nonpreferred video doctors and differences in mean score depending on the order in which the video doctor was scored. All analyses were performed in Stata 6.0. (More detailed on the methods is found in Appendix A at www.jfponline.com.)
Results
Demographics
Participants were diverse in sex (61% female, 39% male), ethnicity (30% Asian American, 29% European American, 26% Latino, 8% African American, and 7% other), age (11% were 18 to 19 years old, 24% were 20 to 29, 18% were 30 to 39, 17% were 40 to 49, 13% were 50 to 59, 8% were 60 to 69, and 9% were 70 to 87), and education (9% had less than a high school education, 34% had a high school diploma or graduation equivalency diploma, 26% had some college, 22% were college graduates, and 9% had graduate degrees).
Initial preferences for video doctors
Initial sex preference. The strong preference for a female video doctor was significantly different from the 50% preference for each sex that would be expected in the absence of any sex preference (P<.0001). Most females (85%) and males (63%) selected a female video doctor (difference between males and females significant at P<.001; Table 1). The percentages of sex preference by race were not significantly different from one another (P=.36).
Multivariable logistic regression confirmed the relation between participants’ sex and the sex preference of the video doctor but showed no convincing evidence of differences in sex preference related to race (P=.73), age (P=.15), schooling (P=.23), marital status (P=.13), or employment status (P=.19).
Initial race preference. For their initial video doctor selection, 53% of participants chose a European American, 29% chose a Latino, and 18% chose an African American. This pattern of preference was significantly different from the 33.3% for each race that would be expected in the absence of a racial preference (P<.001; Table 2).
Video doctor racial preferences differed significantly by race of the participant (P<.0001), with a preference for the same race. A substantial number of participants, however, chose a different-race video doctor. Racial preferences were similar across male and female participants (P=.98).
TABLE 1
Initial and final video doctor selections by sex
| Initial selection | Final selection | |||
|---|---|---|---|---|
| Participants | Female video doctor | Male doctor video | Female video doctor | Male video doctor |
| Female (n=240) | 85% | 15% | 88% | 12% |
| Male (n=155) | 63% | 37% | 71% | 29% |
| Overall (n=395) | 76% | 24% | 82% | 18% |
TABLE 2
Initial and final video doctor selections by race
| Initial selection | Final selection | |||||
|---|---|---|---|---|---|---|
| Participants | African American | Latino | European American | African American | Latino | European American |
| African American (n=30) | 50% | 17% | 33% | 52% | 19% | 29% |
| Latino (n=101) | 12% | 51% | 37% | 20% | 44% | 36% |
| European American (n=113) | 15% | 19% | 66% | 23% | 21% | 56% |
| Asian American or “other” (n=145) | 18% | 25% | 57% | 20% | 37% | 43% |
| Overall (n=389) | 18% | 29% | 53% | 23% | 32% | 44% |
Final preferences for video doctors
Final sex preference. The preference for a female video doctor increased across female and male participants (P<.001; Table 1). The net shift among males from male to female video doctor was significant (P=.014). More female participants shifted from male to female (9%) than from female to male (4%), although the difference was not statistically significant (P=.10).
Final race preference. Forty-eight percent of African American participants, 56% of Latino participants, and 44% of European Americans chose a different-race video doctor. Among Asian and other-race participants, a sizable shift occurred so that only 43% selected a European American video doctor (Table 2).
Between the initial and final selections, 3% of African American participants shifted to a video doctor of a different race, whereas 7% shifted to an African American video doctor. Eleven percent of Latino participants shifted to a different-race video doctor, whereas 6% shifted to a Latino video doctor. Among European American participants, 22% shifted to a different-race video doctor, whereas 12% shifted to a European American video doctor. With the exception of African American participants, there was a significant net shift from same- to different-race choice (P=.036). Many Asian and other-race participants shifted from a European American video doctor to a non–European American video doctor (14% net).
Assessment scores
The 3 female video doctors, who were chosen by more participants than were the 3 male video doctors at the initial and final selections, also received higher mean assessment scores (Table 3). On particular items, the highest score was 6.001 (of a possible 7), received by the European American female for the question: “How professional is this doctor?” The lowest score was 3.590 received by the European American male for the question: “If this person were your doctor, how comfortable might you be in talking with this person about personal health matters?”
TABLE 3
Selection of video doctor by sex and race
| Video doctor’s name* (ethnicity/sex) | Initial selection of video doctor | Mean assessment score | Final selection of video doctor† |
|---|---|---|---|
| Dr. Ann Johnson (European American/female) | 43% | 5.49 | 38% |
| Dr. Renee Garcia (Latina/female) | 22% | 5.32 | 26% |
| Dr. Terry Williams (African American/female) | 12% | 5.13 | 17% |
| Dr. Mark Benson (European American/male) | 10% | 4.31 | 6% |
| Dr. Glen Martinez (Latino/male) | 7% | 4.33 | 6% |
| Dr. Calvin Butler (African American/male) | 6% | 4.84 | 6% |
| *Fictitious names were assigned by the researchers. | |||
| †Figures do not add to 100% due to rounding. | |||
Association of preferences and ratings. Analysis of the mean assessment scores showed a substantial rating tendency among participants, by which they tended to give all 6 video doctors relatively high or low scores. Our analysis indicated that 34.9% (95% confidence interval [CI], 30.4–39.5) of the variance in assessment scores is explained by rating tendency.
We also found that participants tended to increase their scores as they proceeded through the sequence of doctors. Compared with the first video doctor, the second through the sixth video doctors received increases in mean scores of 0.15 (P=.016), 0.16 (P=.011), 0.29 (P<.001), 0.43 (P<.001), and 0.60 (P<.001), respectively. These results showed the importance of using multiple presentation orders to balance the order effect.
After adjusting for the order effect and the respondent rating tendency, the mean assessment scores given to video doctors selected at the initial stage were an average of 0.7 points higher than scores given to the other video doctors (P<.001, 95% CI, 0.56–0.81). At the final selection, the chosen video doctor scored on average 1.04 units higher on the assessment scores than did the other video doctors (P<.001, 95% CI, 0.94–1.1). Thus, the selection made based on the video doctors’ images and brief introductions alone was significantly associated with the subsequent assessment, and the final selection of video doctor was even more strongly associated with the assessment.
Discussion
More participants preferred same-race physicians at the initial selection (66% of European Americans, 51% of Latinos, and 50% of African Americans). This effect was not as large as one might expect, however, because a substantial minority of subjects in each racial category selected a different-race video doctor at the initial selection and a majority of Latinos selected a different-race video doctor at the final selection.
After viewing the delivery of the prevention message, more in each group, except for African Americans, chose a video doctor of a different race. In addition, at final selection, 57% of Asian and other-race participants chose a non– European American video doctor. With regard to sex, most males and females chose a female video doctor at the initial selection, and even more did so at the final selection. These data suggested that many healthcare consumers are in concordance with the recent shift toward a more diverse population of physicians and that the white male physician may no longer be viewed as the stereotypical medical professional.
The qualities patients seek in a doctor
The assessment scores for the video doctors indicated that participants were choosing, both on first impressions and after further exposure, video doctors who they perceived to possess the qualities associated with patient-centered care.21,22 Although the overall ranking of the 6 video doctors was unchanged from initial to final selection, after viewing the delivery of the prevention message, many participants altered their choices: more males and females chose a female video doctor; more European American and Latino participants shifted from same-race to different-race video doctors; and more Asian and other-race participants shifted from European American to non–European American video doctors.
These findings suggested that, even in brief meetings with physicians, patients respond to a combination of patient-centered qualities and that this combination may carry more weight than the physician’s sex and race. In other words, from the point of view of the public at large, physicians of both sexes and all races can possess the desired physician qualities, and people may be receptive to any physician who exhibits these qualities.
Preference for a female doctor
Our finding that men and women in our sample preferred a female video doctor contrasts with sex preference findings from previous studies,3,6,8,10,11 although in general studies on sex preference of physicians have shown inconsistent findings. The female preference finding in our study may represent evolving positive attitudes toward and increasing familiarity with female physicians. From 1971 to 1991, the percentage of women first-year medical school students rose from 13.7% to 39.8%.14
The strong female preference also may represent sex stereotyping. Patients reported that they desire physicians who are sensitive to their needs and circumstances, deliver a warm and empathic style of care,15 invite participation in decision making,16 engage in emotionally focused talk, and provide health information within patients’ social, emotional, and cultural contexts.17 Other studies found that women, when compared with men, provide a style of care that approximates these patient-centered characteristics.18-20
Our participants, many of whom preferred female video doctors even at first, may have strongly associated a patient-centered, empathic style with being female. The particular female actors we chose also may have been better able to exhibit, regardless of our efforts to standardize, the combination of professional and personal skills most desired in a doctor.
Racial preferences
The preference for a same-race video doctor may have several origins. People may feel more familiar and comfortable with race-concordant relationships in general and may believe that a physician of one’s own race can better attend to specific health concerns. Same-race preference also may arise from the desire to avoid a racially prejudiced physician. Racially concordant as opposed to discordant care has been associated with increased patient satisfaction and use of health care services and with higher ratings from patients regarding their level of participation during physician visits.16,23
As indicated in our study and others, African Americans express a stronger preference than do individuals from other racial groups for receiving care from physicians of their own race.23 To support patients in exercising their racial preferences, some health care professional organizations, such as the National Medical Association, have provided a toll-free number that patients can call to locate a local African American physician.
Limitations of the study
The study had several limitations. We may not have successfully held constant the actors’ personalities and acting abilities. Future video doctor studies about patients’ acceptance regarding physicians’ race and sex could address this drawback by including multiple video doctors in each sex and race category.
Because only English-speaking participants were included in the study, we do not know whether Latinos who spoke only Spanish would have chosen differently. Our study also used a convenience sample in a San Francisco Bay Area shopping mall, and our results may not be generalizable to other populations.
We were unable to study the same-race preferences of the Asian participants in our sample. Because more than 10% of physicians practicing in the United States are of Asian ancestry, patients’ receptivity to Asian physicians and Asian patients’ preference for a same-race physician would be important research topics. Diversity of language and culture among various Asian and other ethnicities also could be addressed with a well-designed video doctor study. The absence of an Asian video doctor, however, did allow us to examine the selections made by participants when no same-race video doctor was available.
Strengths of the study
A major strength of our study was that participants represented both sexes and a range of ages, races, and education levels. In addition, the video technology allowed participants to select a video doctor based on a verisimilar experience and without the constraints of availability and access found in real-life choices. All our study participants accepted the survey questions and responded to the video doctor as a “real” physician.
Video doctor technology does allow for holding constant certain variables such as age, appearance, message content, and style of delivery, an advantage that cannot be achieved in real encounters between patients and physicians.
Challenges for the future
Some of our most crucial health care challenges are providing access to quality care and equal career opportunities for those who seek to practice medicine. Our results supported the growing diversity of the population of physicians, and emphasized that many patients will choose physicians, regardless of their sex and race, who appear professional, competent, and caring. Medical schools need to continue the trend toward teaching patient-centered, empathic care and recruiting and retaining minority physicians to rectify current imbalances. In addition, practicing physicians can take note that providing quality care for patients of all cultural backgrounds may be an easier task than they think—the common language of compassion may transcend our differences.
Future studies could use video doctor technology to confirm our findings and to further investigate patients’ preferences and attitudes about various dimensions of the relationship between patient and physician. As the patient population and the physician workforce diversify, and as managed care organizations continue to strive to increase patient satisfaction and retention, information about patient preferences could inform the future of health care delivery.
Acknowledgments
We thank Scott Ludwig for his excellent casting of actors, directing, and video production; and Annabelle Ison for designing subject recruitment materials. We also thank our video doctors, the staff of Tanforan Park Shopping Center in San Bruno, CA, and the mall visitors who volunteered to participate in the study.
Corresponding author
Barbara Gerbert, PhD, University of California at San Francisco, 350 Parnassus Avenue, Suite 905, San Francisco, CA 94117. E-mail: [email protected].
1. US Census Bureau Detailed occupation by race, Hispanic origin and sex: 1990. Available at: http://censtats.census.gov/ cgi-bin/eeo/eeojobs.pl. Accessed on June 9, 2003.
2. Johnson LMI ed. Minority Student Opportunities in United States Medical Schools. 15th ed. Washington, DC: Association of American Medical Colleges; 2000.
3. Ackerman-Ross SF, Sochat N. Close encounters of the medical kind: attitudes toward male and female physicians. Soc Sci Med 1980;14A:61-64.
4. Engleman E. Attitudes toward women physicians: a study of 500 clinic patients. West J Med 1974;120:95-100.
5. Challacombe C. Do women patients need women doctors? Practitioner 1983;227:848-850.
6. Fennema K, Meyer D, Owen N. Sex of physician: patients’ p and stereotypes. J Fam Pract 1990;30:441-446.
7. Weyrauch KF, Boiko PE, Alvin B. Patient sex role and preference for a male or female physician. J Fam Pract 1990;30:559-562.
8. Kerssens JJ, Bensing JM, Andela MG. Patient preference for genders of health professionals. Soc Sci Med 1997;44:1531-1540.
9. Graffy J. Patient choice in practice with men and women general practitioners. Br J Gen Pract 1990;40:13-15.
10. Elstad JI. Women’s priorities regarding physician behavior and their preference for a female physician. Women Health 1994;21(4):1-17.
11. Schmittdiel MA, Grumbach K, Selby JV, Quesenberry CP. Effect of physician and patient gender concordance on patient satisfaction and preventive care practices. J Gen Intern Med 2000;15:761-769.
12. Saha S, Taggart SH, Komaromy M, Bindman AB. Do patients choose physicians of their own race? Health Aff (Millwood). 2000;19(4):76-83.
13. Miller W, Sanchez V. Motivating young adults for treatment and lifestyle change. In: Howard G, ed. Issues in Alcohol Use and Misuse by Young Adults. Notre Dame, Ind: University of Notre Dame Press; 1994;55-82.
14. Jonas H, Etzel S, Baransky B. Educational programs in the US medical schools. JAMA 1992;268:1083-1090.
15. Rollnick S, Mason P, Butler C. Health Behavior Change: A Guide for Practitioners. Edinburgh: Churchill Livingstone; 1999.
16. Cooper-Patrick L, Gallo JJ, Gonzales JJ, Vu HT, Nelson C, Ford DE. Race, gender, and partnership in the patient–physician relationship. JAMA 1999;282:583-589.
17. Roter D, Hall J. Why physicians’ gender matters in the shaping of the patient-physician relationship. J Womens Health 1998;7:1093-1097.
18. Roter D, Lipkin M, Korsgaard A. Sex differences in patients’ and physicians’ communication during primary care medical visits. Med Care 1991;29:1083-1093.
19. Hall J, Irish J, Roter D, Ehrich C, Miller L. Gender in medical encounters: an analysis of physician and patient communication in a primary care setting. Health Psychol 1994;13:384-392.
20. Elderkin-Thompson V, Waitzkin H. Differences in clinical communication by gender. J Gen Intern Med 1999;14:112-121.
21. Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behaviors. New York: Guilford Press; 1991.
22. Stewart M. Effective physician–patient communication and health outcomes. CMAJ 1995;152:1423-1433.
23. Saha S, Komaromy M, Koepsell TD, Bindman AB. Patient–physician racial concordance and the perceived quality and use of health care. Arch Intern Med 1999;159:997-1004.
1. US Census Bureau Detailed occupation by race, Hispanic origin and sex: 1990. Available at: http://censtats.census.gov/ cgi-bin/eeo/eeojobs.pl. Accessed on June 9, 2003.
2. Johnson LMI ed. Minority Student Opportunities in United States Medical Schools. 15th ed. Washington, DC: Association of American Medical Colleges; 2000.
3. Ackerman-Ross SF, Sochat N. Close encounters of the medical kind: attitudes toward male and female physicians. Soc Sci Med 1980;14A:61-64.
4. Engleman E. Attitudes toward women physicians: a study of 500 clinic patients. West J Med 1974;120:95-100.
5. Challacombe C. Do women patients need women doctors? Practitioner 1983;227:848-850.
6. Fennema K, Meyer D, Owen N. Sex of physician: patients’ p and stereotypes. J Fam Pract 1990;30:441-446.
7. Weyrauch KF, Boiko PE, Alvin B. Patient sex role and preference for a male or female physician. J Fam Pract 1990;30:559-562.
8. Kerssens JJ, Bensing JM, Andela MG. Patient preference for genders of health professionals. Soc Sci Med 1997;44:1531-1540.
9. Graffy J. Patient choice in practice with men and women general practitioners. Br J Gen Pract 1990;40:13-15.
10. Elstad JI. Women’s priorities regarding physician behavior and their preference for a female physician. Women Health 1994;21(4):1-17.
11. Schmittdiel MA, Grumbach K, Selby JV, Quesenberry CP. Effect of physician and patient gender concordance on patient satisfaction and preventive care practices. J Gen Intern Med 2000;15:761-769.
12. Saha S, Taggart SH, Komaromy M, Bindman AB. Do patients choose physicians of their own race? Health Aff (Millwood). 2000;19(4):76-83.
13. Miller W, Sanchez V. Motivating young adults for treatment and lifestyle change. In: Howard G, ed. Issues in Alcohol Use and Misuse by Young Adults. Notre Dame, Ind: University of Notre Dame Press; 1994;55-82.
14. Jonas H, Etzel S, Baransky B. Educational programs in the US medical schools. JAMA 1992;268:1083-1090.
15. Rollnick S, Mason P, Butler C. Health Behavior Change: A Guide for Practitioners. Edinburgh: Churchill Livingstone; 1999.
16. Cooper-Patrick L, Gallo JJ, Gonzales JJ, Vu HT, Nelson C, Ford DE. Race, gender, and partnership in the patient–physician relationship. JAMA 1999;282:583-589.
17. Roter D, Hall J. Why physicians’ gender matters in the shaping of the patient-physician relationship. J Womens Health 1998;7:1093-1097.
18. Roter D, Lipkin M, Korsgaard A. Sex differences in patients’ and physicians’ communication during primary care medical visits. Med Care 1991;29:1083-1093.
19. Hall J, Irish J, Roter D, Ehrich C, Miller L. Gender in medical encounters: an analysis of physician and patient communication in a primary care setting. Health Psychol 1994;13:384-392.
20. Elderkin-Thompson V, Waitzkin H. Differences in clinical communication by gender. J Gen Intern Med 1999;14:112-121.
21. Miller WR, Rollnick S. Motivational Interviewing: Preparing People to Change Addictive Behaviors. New York: Guilford Press; 1991.
22. Stewart M. Effective physician–patient communication and health outcomes. CMAJ 1995;152:1423-1433.
23. Saha S, Komaromy M, Koepsell TD, Bindman AB. Patient–physician racial concordance and the perceived quality and use of health care. Arch Intern Med 1999;159:997-1004.
The Effects of an Estrogen and Glycolic Acid Cream on the Facial Skin of Postmenopausal Women: A Randomized Histologic Study
Herbs for serum cholesterol reduction
- There is some evidence from randomized clinical trials that guggul (Commiphora mukul), fenugreek (Trigonella foenum-graecum), artichoke (Cynara scolymus), yarrow (Achillea wilhelmsii), holy basil (Ocimum sanctum), red yeast (Monascus purpureus) rice, eggplant (Solanum melongena), and arjun (Terminalia arjuna) reduce serum cholesterol.
- The evidence is not conclusive for any of the products, although preliminary clinical trials seem promising; further research is warranted.
- Safety profiles from clinical trials appear encouraging, but the long-term safety has not been established; herb-drug interactions may be possible with milk thistle (Silybum marianum), Asian ginseng (Panax ginseng), guggul, and fenugreek.
- It is important for physicians to discuss the use of complementary and alternative therapies with their patients.
- Objectives To systematically review the clinical evidence for herbal medicinal products in the treatment of hypercholesterolemia.
- Study Design A systematic review of randomized clinical trials of herbal medicinal products used to lower serum cholesterol. Systematic literature searches were conducted in 6 electronic data-bases. The reference lists of all papers and our files were searched for more relevant publications. Experts in the field and manufacturers of identified herbal medicinal products were contacted for published and unpublished data. No language restrictions were imposed.
- Outcomes Measured All randomized clinical trials of serum cholesterol reduction, in which mono-preparations of herbal medicinal products were administered as supplements to human subjects, were included.
- Results Twenty-five randomized clinical trials involving 11 herbal medicinal products were identified. Guggul (Commiphora mukul), fenugreek (Trigonella foenum-graecum), red yeast rice, and artichoke (Cynara scolymus) have been most extensively studied and have demonstrated reductions in total serum cholesterol levels of between10% and 33%. The methodological quality as assessed by the Jadad score was less than 3 (maximum, 5) for 13 of the 25 trials.
- Conclusions Many herbal medicinal products have potential hypocholesterolemic activity and encouraging safety profiles. However, only a limited amount of clinical research exists to support their efficacy. Further research is warranted to establish the value of these extracts in the treatment of hypercholesterolemia.
Two recent surveys of patients undergoing cardiac surgery reported that 75% (263 of 376) and 81% (224 of 246) of patients currently use some form of complementary medicine (including herbs, vitamins, supplements, megavitamins, prayer, relaxation, spiritual healing, massage, imagery, and lifestyle and diet modifications).1,2
Many herbal medicinal products are promoted for hypercholesterolemia, including some of the top-selling supplements. It is therefore vital to establish both the efficacy of these herbal supplements in reducing serum cholesterol levels and their relative safety. This review is an attempt to systematically summarize the evidence from randomized clinical trials for the efficacy and safety of lipid-lowering herbal medicinal products.
Methods
Identification of clinical trials
To identify clinical trials involving herbal medicinal products with hypocholesterolemic properties, we conducted systematic literature searches in the following electronic databases (all from their inception to May 2001): MEDLINE (via PubMed), EMBASE, CINAHL, AMED (Alternative and Allied Medicine Database, British Library Medical Information Centre), the Cochrane Library (Issue 2, 2001), and CISCOM (Research Council for Complementary Medicine, London, UK). The search strategy is summarized in Appendix A (available online at http://www.jfponline.com).
Further relevant papers were located by hand-searching the reference lists of all papers and departmental files. In addition, experts in the field and manufacturers were contacted to provide published and unpublished material.
Inclusion and exclusion criteria
Only randomized clinical trials investigating serum cholesterol reduction of monopreparations of herbal medicinal products administered as supplements were included. These could be placebo-controlled or equivalent trials. All retrieved data including uncontrolled trials, case reports, and preclinical and observational studies were reviewed for safety data. No language restrictions were imposed.
Data extraction and quality assessment
All articles were read in full. Data relating to sample size, study design, intervention and control, treatment duration, primary outcome measures, and results were extracted by the first author and validated by the second. The methodological quality of each trial was assessed using the Jadad scoring system,3 which ranges from 0 (poorest) to 5 (highest). A score of 3 or above indicates reasonable methodological quality.
Results
We identified 11 herbal medicinal products investigated for hypocholesterolemic properties in randomized clinical trials: guggul (Commiphora mukul), artichoke (Cynara scolymus), garlic (Allium sativum), fenugreek (Trigonella foenum- graecum), red yeast (Monascus purpureus) rice, Asian ginseng (Panax ginseng), yarrow (Achillea wilhelmsii), eggplant (Solanum melongena), holy basil (Ocimum sanctum), milk thistle (Silybum marianum), and arjun (Terminalia arjuna).
The efficacy and safety of garlic has been reviewed extensively elsewhere4–6 and is therefore not discussed in this paper. Details of all identified studies are shown in Tables 1–5 (and Table W1, available online at http://www.jfponline.com). Guggul, fenugreek, red yeast rice, and artichoke have been studied most extensively; randomized clinical trials of these herbal medicinal products with a Jadad score of 3 or above are discussed in more depth in Appendix B (available online at http://www.jfponline.com). Table 6 summarizes the adverse events experienced by subjects within these clinical trials and potential herb-drug interactions identified from systematic reviews.
Guggul (Commiphora mukul)
Six randomized clinical trials of guggul, involving 388 patients with different diagnoses, were identified.7-12 Five were conducted in India and 1 in the United States; 4 were placebo-controlled; and 1 compared guggul with 2 reference compounds. The results suggest reductions in total serum cholesterol from 10% to 27% compared with baseline levels (Tables 1 and W1).
High-density lipoprotein (HDL) cholesterol levels were measured in 3 of the studies.7- 9 A significant increase was seen after 8 weeks of treatment in 1 study9; in the others, no significant differences were seen.7,8 A statistically significant decrease in lipid peroxide levels was reported in 1 study, with no corresponding change in the placebo-treated group.7
Several mild adverse events were reported during these trials, including rash, nausea, vomiting, eructation, hiccup, headache, loose stools, restlessness, and apprehension, although information regarding adverse events experienced during placebo administration was not always provided. A potential drug interaction with propranolol and diltiazem was investigated in a randomized crossover trial of 17 healthy volunteers, in which guggul was found to significantly reduce the peak plasma concentration of both drugs.13
Fenugreek (Trigonella foenum-graecum)
Fenugreek seeds. Five randomized clinical trials were identified, involving 140 patients; all but 1 trial was conducted in India.14-17 Although the methodological quality of the studies was considered generally poor in 4 of the trials, statistically significant reductions in total serum cholesterol of between 15% and 33% compared with baseline were demonstrated (Table 2).
Fenugreek leaves. In a single-blind study of 20 healthy male volunteers, Abdel-Barry and colleagues found a nonsignificant decrease of 9% in total serum cholesterol after a single dose of an aqueous extract made from fenugreek leaves (40 mg/kg) compared with a reduction of 2.8% after dilute coffee extract (placebo) (Table 2). 18
Within all the identified studies of fenugreek, patients reported mild gastrointestinal symptoms such as increased flatulence, nausea, fullness, and diarrhea during fenugreek treatment, but none was severe enough to necessitate withdrawal from the study. A 14% reduction in serum potassium was noted in healthy volunteers after a single dose of an aqueous extract of fenugreek leaves. 18
Red yeast rice
Red yeast rice is produced by solid-state fermentation of washed and cooked rice using the fungus Monascus purpureus. It has been used in Asia as a food preservative and colorant and for its medicinal properties since the Tang Dynasty (ad 800). It is available in capsules that contain a pulverized powder of fermented rice and yeast.
Four randomized clinical trials of the lipid-lowering effects of red yeast rice conducted in patients (n=695) with hyperlipidemia were identified (Table 3).19-22 In all studies, statistically significant reductions (16% to 31%) in total serum cholesterol compared with placebo or control or baseline were seen.
Adverse events experienced in clinical trials included stomachache, heartburn, dizziness, and flatulence. No changes in liver function tests were demonstrated. There was 1 case report of a 26-year-old man who used red yeast rice in preparing sausages and developed anaphylaxis due to immediate sensitivity to M purpureus.23 Whether this is relevant to the oral administration of red yeast rice capsules is not clear.
Artichoke (Cynara scolymus)
The choleretic effect of the leaf extract of artichoke has been studied widely, but only 2 randomized clinical trials of its hypocholesterolemic effects, involving 187 patients, were identified (Table 4).24,25 One trial (n=44 healthy volunteers), published in abstract form only, found no significant difference in lipid levels compared with placebo, although post hoc subanalyses revealed some reductions in total serum cholesterol in patients with baseline levels above 5.4 mmol/L; these results should be interpreted with some caution. Reductions in total cholesterol of 18.5% and 8.6% were reported in the other, larger trial after artichoke and placebo treatments, respectively.
No adverse events were reported during either study. Three post-marketing surveillance studies were located: one included 417 patients and reported excellent tolerability in 95%; in the second (203 patients) no adverse reactions were reported; and the third (553 patients) described mild adverse events in 1.3% of patients (flatulence, hunger, and weakness).24-28
Discussion
Many different herbal medicinal products have been identified with potential lipid-lowering properties (Table 5), but the evidence for each herb is limited. The largest amount of published literature exists for guggul, fenugreek, red yeast rice, and artichoke, with reductions in total serum cholesterol ranging from 10% to 33%.
Although HDL and low-density lipoprotein (LDL) cholesterol were not measured in all the studies, increases in HDL and decreases in LDL levels were seen with guggul, red yeast rice, and yarrow, and decreases in LDL levels were seen in studies of fenugreek, arjun, and artichoke.
Safety
Few adverse events or drug interactions were reported in clinical trials of any of the 11 herbs identified. Many are used extensively in traditional medicine and culinary practices around the world, which supports their relative safety.
However, the long-term safety for use as herbal medicinal products has not been established. Long-term exposure of large numbers of patients within a formal setting would be necessary to determine safety, although difficulties associated with all herbal medicinal products exist, such as the inability to identify active ingredients and the potential for adulteration and misbranded products. No direct or indirect evidence exists for herb-drug interactions for fenugreek, guggul, Asian ginseng, and milk thistle (Table 6).
Study limitations
Although differences in study design, methodological quality, statistical methods, and subject populations create problems with interpretation of these figures, they appear to compare favorably with studies of garlic; the most recent meta-analysis suggested an average effect size of 4% to 6%.6 Studies of conventional therapeutic options for hypercholesterolemia (eg, statins) have demonstrated reductions of 20% to 30% in serum cholesterol.29
Several shortcomings of the review need to be addressed. First, although attempts were made to obtain data from unpublished trials by contacting authors and manufacturers, none were located. There is evidence to suggest that studies with significant positive results are more likely to be published,30 and this may be more pronounced with unfamiliar herbal therapies.
Second, because much of this research has been conducted in India and China, our extensive search strategy may not have located all the published material.
Third, there were several weaknesses with the original trials; of the 25 randomized clinical trials of herbal medicinal products for serum cholesterol reduction identified, only 12 scored 3 or more points on the Jadad scale. The most frequent methodological flaws were conduct of single-blind or open studies and incomplete reporting of methods of randomization, blinding, and subject withdrawals.
Conclusions
Evidence suggests that physicians do not ask their patients about complementary and alternative therapies and that patients are reticent to discuss these treatments with their physicians.31- 34 Surveys indicate widespread use of complementary and alternative therapies among patients undergoing cardiac surgery.1,2 Although no equivalent surveys have been conducted for patients with hypercholesterolemia, in light of the relatively large number of herbal medicinal products with potential lipid-lowering properties available, it seems prudent for physicians to explore this area in their clinical decision-making process.
In conclusion, although 11 herbal medicinal products were identified with potential hypo-cholesterolemic activity, the evidence supporting individual plants is limited. In addition to lowering cholesterol, several of the herbs may exert beneficial effects in cardiovascular disease by elevating HDL levels and inhibiting lipid oxidation. The safety profiles of the products in question seems to be encouraging. Further research is therefore warranted to establish the therapeutic value of these herbs in the treatment of hypercholesterolemia.
Acknowledgments
The authors thank Jongbae Park, Barbara Wider, and Francesca Borelli, Complementary Medicine, Peninsula Medical School, Universities of Exeter and Plymouth, for translation of papers from Chinese, Italian, and French, to and Esther Prati, Pharmaton, Lugano, for assistance with locating relevant articles. JTC received a research fellowship from Pharmaton SA, Lugano, Switzerland.
1. Ai AL, Bolling SF. The use of complementary and alternative therapies among middle-aged and older cardiac patients. Am J Med Qual 2002;17:21-27.
2. Liu EH, Turner LM, Lin SX, et al. Use of alternative medicine by patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 2000;120:335-341.
3. Jadad AR, Moore A, Carroll D, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12.
4. Warshafsky S, Kamer RS, Sivak SL. Effect of garlic on total serum cholesterol. A meta-analysis. Arch Intern Med 1993;119:599-605.
5. Silagy C, Neil A. Garlic as a lipid lowering agent: a meta-analysis. J R Coll Physicians Lond 1994;28:39-45.
6. Stevinson C, Pittler MH, Ernst E. Garlic for treating hypercholesterolemia. A meta-analysis of randomised clinical trials. Ann Intern Med 2000;133:420-429.
7. Singh RB, Niaz MA, Ghosh S. Hypolipidemic and antioxidant effects of Commiphora mukul as an adjunct to dietary therapy in patients with hypercholesterolemia. Cardiovasc Drugs Ther 1994;8:659-664.
8. Szapary PO, Wolfe ML, Bloedon LT, Fair MB, Berezich DJ, Cirigliano AJ, Rader DJ. A double blind, randomised, placebo controlled clinical trial of standardized guggul extract in patients with hypercholesterolemia. Complement Ther Med 2002;10:112.
9. Verma SK, Bordia A. Effect of Commiphora mukul (gum guggulu) in patients with hyperlipidemia with special reference to HDL cholesterol. Indian J Med Res 1988;87:356-360.
10. Malhotra SC, Ahuja MMS. Comparative hypolipidaemic effectiveness of gum guggulu (Commiphora mukul) fraction ‘A’, ethyl-P-Chlorophenoxyisobutyrate and Ciba-13437-Su. Indian J Med Res 1971;59:1621-1632.
11. Kuppurajan K, Rajagopalan SS, Koteswara Rao T, Sitaraman R. Effect of guggulu (Commiphora mukul–Engl) on serum, lipids in obese, hypercholesterolemic and hyperlipemic cases. J Assoc Physicians India 1978;26:367-373.
12. Bordia A, Chuttani SK. Effect of gum guggulu on fibrinolysis and platelet adhesiveness in coronary heart disease. Indian J Med Res 1979;70:992-996.
13. Dalvi SS, Nayak VK, Pohujani SM, Desai NK, Kshirsagar NA, Gupta KC. Effect of gugulipid on bioavailability of diltiazem and propranolol. J Assoc Physicians India 1994;42:454-455.
14. Singh RB, Niaz MA, Rastogi V, Singh N, Postiglione A, Rastogi SS. Hypolipidemic and antioxidant effects of fenugreek seeds and triphala as adjuncts to dietary therapy in patients with mild to moderate hypercholesterolemia. Perfusion 1998;11:124-130.
15. Prasanna M. Hypolipidemic effect of fenugreek: a clinical study. Indian J Pharmacol 2000;32:34-36.
16. Sharma RD, Raghuram TC. Hypoglycaemic effect of fenugreek seeds in non-insulin dependent diabetic subjects. Nutr Res 1990;10:731-739.
17. Sharma RD, Raghuram TC, Rao NS. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur J Clin Nutr 1990;44:301-306.
18. Abdel-Barry JA, Abdel-Hassan IA, Jawad AM, Al-Hakiem MHH. Hypoglycaemic effect of aqueous extract of the leaves of Trigonella foenum-graecum in healthy volunteers. East Mediterr Health J 2000;6:83-88.
19. Keithley J, Swanson B, Sha B, Zeller J, Kessler HA, Smith KY. A pilot study of the safety and efficacy of Cholestin in treating HIV-related dyslipidemia. Nutrition 2002;18:201-204.
20. Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VLW. Cholesterol lowering effects of a proprietary Chinese red yeast rice dietary supplement. Am J Clin Nutr 1999;69:231-236.
21. Shen Z, Yu P, Su M, et al. A prospective study of Zhitai capsules in the treatment of primary hyperlipidemia. Natl Med J China 1996;76:156-157.
22. Wang J, Lu Z, Chi J, et al. Multicenter clinical trial of the serum lipid-lowering effects of a Monascus purpureus (red yeast) rice preparation from traditional chinese medicine. Curr Ther Res 1997;58:964-978.
23. Wigger-Alberti W, Bauer A, Hipler UC, Elsner P. Anaphylaxis due to Monascus purpureus fermented rice (red yeast rice). Allergy 1999;54:1328-1336.
24. Englisch W, Beckers C, Unkauf M, Ruepp M, Zinserling V. Efficacy of artichoke dry extract in patients with hyperlipoproteinemia. Arzneimittelforschung 2000;50:260-265.
25. Petrowicz O, Gebhardt R, Donner M, Schwandt P, Kraft K. Effects of artichoke leaf extract (ALE) on lipoprotein metabolism in vitro and in vivo. Atherosclerosis 1997;129:147.
26. Fintelmann V. Antidyspeptic and lipid lowering effect of an extract from artichoke leaves. results of clinical trials on efficacy and tolerability of Hepar SL in 553 patients. Z Allg Med 1996;72:3-19.
27. Fintelmann V, Wegener T. Langzeitanwendung von artischockenblattertrockenextrakt (Hepar-SL forte) bei dyspeptischem symptomkomplex. Presented at: Phytotherapie Kongress 1997;November 27-28, 1997; Wurzburg, Germany.
28. Held C. Von der 1. Deutsch-ungarischen Phytopharmaka-konferenz 1991; 20 November, Budapest. Z Klin Med 1992;47:92.-
29. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-1389.
30. Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet 1991;337:867-872.
31. Adler SR, Fosket JR. Disclosing complementary and alternative medicine use in the medical encounter: a qualitative study in women with breast cancer. J Fam Pract 1999;48:453.
32. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. Results of a follow-up national survey. JAMA 1998;280:1569-1575.
33. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. N Engl J Med 1993;328:246-252.
34. Abbot NC, Ernst E. Patients’ opinions about complementary medicine. Forsch Komplementarmed 1997;4:164-168.
35. Guimaraes PR, Galvao AMP, Batista CM, et al. Eggplant (Solanum melongena) infusion has a modest and transitory effect on hypercholesterolemic subjects. Braz J Med Biol Res 2000;33:1027-1036.
36. Sotaniemi EA, Haapakoski E, Rautio A. Ginseng therapy in non-insulin dependent diabetic patients. Diabetes Care 1995;18:1373-1375.
37. Petronelli A, Roda E, Briganti M, Labate AMM, Barbara L. Effeto della somministrazione di silimarina sui livelli dei lipidi sierici. Clin Ter 1981;99:471-482.
38. Gupta R, Singhal S, Goyle A, Sharma VN. Antioxidant and hypocholesterolaemic effects of Terminalia arjuna tree bark powder: a randomised placebo controlled trial. J Assoc Physicians India 2001;49:231-235.
39. Agarwal P, Rai V, Singh RB. Randomised placebo-controlled, single blind trial of holy basil leaves in patients with non insulin dependent diabetes mellitus. Int J Clin Pharmacol Ther 1996;34:406-409.
40. Asgary S, Naderi GH, Sarrafzadegan N, Mohammadifard N, Mostafavi S, Vakili R. Antihypertensive and antihyperlipidemic effects of Achillea wilhelmsii. Drugs Exp Clin Res 2000;26:89-93.
41. Fugh-Berman A. Herb-drug interactions. Lancet 2000;355:134-138.
42. Ernst E. Possible interactions between synthetic and herbal medicinal products. Part 1: a systematic review of the indirect evidence. Perfusion 2000;13:4-15.
43. Ernst E. Interactions between synthetic and herbal medicinal products. Part 2: a systematic review of the direct evidence. Perfusion 2000;13:60-70.
44. Thompson Coon J, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf 2002;25:323-44.
- There is some evidence from randomized clinical trials that guggul (Commiphora mukul), fenugreek (Trigonella foenum-graecum), artichoke (Cynara scolymus), yarrow (Achillea wilhelmsii), holy basil (Ocimum sanctum), red yeast (Monascus purpureus) rice, eggplant (Solanum melongena), and arjun (Terminalia arjuna) reduce serum cholesterol.
- The evidence is not conclusive for any of the products, although preliminary clinical trials seem promising; further research is warranted.
- Safety profiles from clinical trials appear encouraging, but the long-term safety has not been established; herb-drug interactions may be possible with milk thistle (Silybum marianum), Asian ginseng (Panax ginseng), guggul, and fenugreek.
- It is important for physicians to discuss the use of complementary and alternative therapies with their patients.
- Objectives To systematically review the clinical evidence for herbal medicinal products in the treatment of hypercholesterolemia.
- Study Design A systematic review of randomized clinical trials of herbal medicinal products used to lower serum cholesterol. Systematic literature searches were conducted in 6 electronic data-bases. The reference lists of all papers and our files were searched for more relevant publications. Experts in the field and manufacturers of identified herbal medicinal products were contacted for published and unpublished data. No language restrictions were imposed.
- Outcomes Measured All randomized clinical trials of serum cholesterol reduction, in which mono-preparations of herbal medicinal products were administered as supplements to human subjects, were included.
- Results Twenty-five randomized clinical trials involving 11 herbal medicinal products were identified. Guggul (Commiphora mukul), fenugreek (Trigonella foenum-graecum), red yeast rice, and artichoke (Cynara scolymus) have been most extensively studied and have demonstrated reductions in total serum cholesterol levels of between10% and 33%. The methodological quality as assessed by the Jadad score was less than 3 (maximum, 5) for 13 of the 25 trials.
- Conclusions Many herbal medicinal products have potential hypocholesterolemic activity and encouraging safety profiles. However, only a limited amount of clinical research exists to support their efficacy. Further research is warranted to establish the value of these extracts in the treatment of hypercholesterolemia.
Two recent surveys of patients undergoing cardiac surgery reported that 75% (263 of 376) and 81% (224 of 246) of patients currently use some form of complementary medicine (including herbs, vitamins, supplements, megavitamins, prayer, relaxation, spiritual healing, massage, imagery, and lifestyle and diet modifications).1,2
Many herbal medicinal products are promoted for hypercholesterolemia, including some of the top-selling supplements. It is therefore vital to establish both the efficacy of these herbal supplements in reducing serum cholesterol levels and their relative safety. This review is an attempt to systematically summarize the evidence from randomized clinical trials for the efficacy and safety of lipid-lowering herbal medicinal products.
Methods
Identification of clinical trials
To identify clinical trials involving herbal medicinal products with hypocholesterolemic properties, we conducted systematic literature searches in the following electronic databases (all from their inception to May 2001): MEDLINE (via PubMed), EMBASE, CINAHL, AMED (Alternative and Allied Medicine Database, British Library Medical Information Centre), the Cochrane Library (Issue 2, 2001), and CISCOM (Research Council for Complementary Medicine, London, UK). The search strategy is summarized in Appendix A (available online at http://www.jfponline.com).
Further relevant papers were located by hand-searching the reference lists of all papers and departmental files. In addition, experts in the field and manufacturers were contacted to provide published and unpublished material.
Inclusion and exclusion criteria
Only randomized clinical trials investigating serum cholesterol reduction of monopreparations of herbal medicinal products administered as supplements were included. These could be placebo-controlled or equivalent trials. All retrieved data including uncontrolled trials, case reports, and preclinical and observational studies were reviewed for safety data. No language restrictions were imposed.
Data extraction and quality assessment
All articles were read in full. Data relating to sample size, study design, intervention and control, treatment duration, primary outcome measures, and results were extracted by the first author and validated by the second. The methodological quality of each trial was assessed using the Jadad scoring system,3 which ranges from 0 (poorest) to 5 (highest). A score of 3 or above indicates reasonable methodological quality.
Results
We identified 11 herbal medicinal products investigated for hypocholesterolemic properties in randomized clinical trials: guggul (Commiphora mukul), artichoke (Cynara scolymus), garlic (Allium sativum), fenugreek (Trigonella foenum- graecum), red yeast (Monascus purpureus) rice, Asian ginseng (Panax ginseng), yarrow (Achillea wilhelmsii), eggplant (Solanum melongena), holy basil (Ocimum sanctum), milk thistle (Silybum marianum), and arjun (Terminalia arjuna).
The efficacy and safety of garlic has been reviewed extensively elsewhere4–6 and is therefore not discussed in this paper. Details of all identified studies are shown in Tables 1–5 (and Table W1, available online at http://www.jfponline.com). Guggul, fenugreek, red yeast rice, and artichoke have been studied most extensively; randomized clinical trials of these herbal medicinal products with a Jadad score of 3 or above are discussed in more depth in Appendix B (available online at http://www.jfponline.com). Table 6 summarizes the adverse events experienced by subjects within these clinical trials and potential herb-drug interactions identified from systematic reviews.
Guggul (Commiphora mukul)
Six randomized clinical trials of guggul, involving 388 patients with different diagnoses, were identified.7-12 Five were conducted in India and 1 in the United States; 4 were placebo-controlled; and 1 compared guggul with 2 reference compounds. The results suggest reductions in total serum cholesterol from 10% to 27% compared with baseline levels (Tables 1 and W1).
High-density lipoprotein (HDL) cholesterol levels were measured in 3 of the studies.7- 9 A significant increase was seen after 8 weeks of treatment in 1 study9; in the others, no significant differences were seen.7,8 A statistically significant decrease in lipid peroxide levels was reported in 1 study, with no corresponding change in the placebo-treated group.7
Several mild adverse events were reported during these trials, including rash, nausea, vomiting, eructation, hiccup, headache, loose stools, restlessness, and apprehension, although information regarding adverse events experienced during placebo administration was not always provided. A potential drug interaction with propranolol and diltiazem was investigated in a randomized crossover trial of 17 healthy volunteers, in which guggul was found to significantly reduce the peak plasma concentration of both drugs.13
Fenugreek (Trigonella foenum-graecum)
Fenugreek seeds. Five randomized clinical trials were identified, involving 140 patients; all but 1 trial was conducted in India.14-17 Although the methodological quality of the studies was considered generally poor in 4 of the trials, statistically significant reductions in total serum cholesterol of between 15% and 33% compared with baseline were demonstrated (Table 2).
Fenugreek leaves. In a single-blind study of 20 healthy male volunteers, Abdel-Barry and colleagues found a nonsignificant decrease of 9% in total serum cholesterol after a single dose of an aqueous extract made from fenugreek leaves (40 mg/kg) compared with a reduction of 2.8% after dilute coffee extract (placebo) (Table 2). 18
Within all the identified studies of fenugreek, patients reported mild gastrointestinal symptoms such as increased flatulence, nausea, fullness, and diarrhea during fenugreek treatment, but none was severe enough to necessitate withdrawal from the study. A 14% reduction in serum potassium was noted in healthy volunteers after a single dose of an aqueous extract of fenugreek leaves. 18
Red yeast rice
Red yeast rice is produced by solid-state fermentation of washed and cooked rice using the fungus Monascus purpureus. It has been used in Asia as a food preservative and colorant and for its medicinal properties since the Tang Dynasty (ad 800). It is available in capsules that contain a pulverized powder of fermented rice and yeast.
Four randomized clinical trials of the lipid-lowering effects of red yeast rice conducted in patients (n=695) with hyperlipidemia were identified (Table 3).19-22 In all studies, statistically significant reductions (16% to 31%) in total serum cholesterol compared with placebo or control or baseline were seen.
Adverse events experienced in clinical trials included stomachache, heartburn, dizziness, and flatulence. No changes in liver function tests were demonstrated. There was 1 case report of a 26-year-old man who used red yeast rice in preparing sausages and developed anaphylaxis due to immediate sensitivity to M purpureus.23 Whether this is relevant to the oral administration of red yeast rice capsules is not clear.
Artichoke (Cynara scolymus)
The choleretic effect of the leaf extract of artichoke has been studied widely, but only 2 randomized clinical trials of its hypocholesterolemic effects, involving 187 patients, were identified (Table 4).24,25 One trial (n=44 healthy volunteers), published in abstract form only, found no significant difference in lipid levels compared with placebo, although post hoc subanalyses revealed some reductions in total serum cholesterol in patients with baseline levels above 5.4 mmol/L; these results should be interpreted with some caution. Reductions in total cholesterol of 18.5% and 8.6% were reported in the other, larger trial after artichoke and placebo treatments, respectively.
No adverse events were reported during either study. Three post-marketing surveillance studies were located: one included 417 patients and reported excellent tolerability in 95%; in the second (203 patients) no adverse reactions were reported; and the third (553 patients) described mild adverse events in 1.3% of patients (flatulence, hunger, and weakness).24-28
Discussion
Many different herbal medicinal products have been identified with potential lipid-lowering properties (Table 5), but the evidence for each herb is limited. The largest amount of published literature exists for guggul, fenugreek, red yeast rice, and artichoke, with reductions in total serum cholesterol ranging from 10% to 33%.
Although HDL and low-density lipoprotein (LDL) cholesterol were not measured in all the studies, increases in HDL and decreases in LDL levels were seen with guggul, red yeast rice, and yarrow, and decreases in LDL levels were seen in studies of fenugreek, arjun, and artichoke.
Safety
Few adverse events or drug interactions were reported in clinical trials of any of the 11 herbs identified. Many are used extensively in traditional medicine and culinary practices around the world, which supports their relative safety.
However, the long-term safety for use as herbal medicinal products has not been established. Long-term exposure of large numbers of patients within a formal setting would be necessary to determine safety, although difficulties associated with all herbal medicinal products exist, such as the inability to identify active ingredients and the potential for adulteration and misbranded products. No direct or indirect evidence exists for herb-drug interactions for fenugreek, guggul, Asian ginseng, and milk thistle (Table 6).
Study limitations
Although differences in study design, methodological quality, statistical methods, and subject populations create problems with interpretation of these figures, they appear to compare favorably with studies of garlic; the most recent meta-analysis suggested an average effect size of 4% to 6%.6 Studies of conventional therapeutic options for hypercholesterolemia (eg, statins) have demonstrated reductions of 20% to 30% in serum cholesterol.29
Several shortcomings of the review need to be addressed. First, although attempts were made to obtain data from unpublished trials by contacting authors and manufacturers, none were located. There is evidence to suggest that studies with significant positive results are more likely to be published,30 and this may be more pronounced with unfamiliar herbal therapies.
Second, because much of this research has been conducted in India and China, our extensive search strategy may not have located all the published material.
Third, there were several weaknesses with the original trials; of the 25 randomized clinical trials of herbal medicinal products for serum cholesterol reduction identified, only 12 scored 3 or more points on the Jadad scale. The most frequent methodological flaws were conduct of single-blind or open studies and incomplete reporting of methods of randomization, blinding, and subject withdrawals.
Conclusions
Evidence suggests that physicians do not ask their patients about complementary and alternative therapies and that patients are reticent to discuss these treatments with their physicians.31- 34 Surveys indicate widespread use of complementary and alternative therapies among patients undergoing cardiac surgery.1,2 Although no equivalent surveys have been conducted for patients with hypercholesterolemia, in light of the relatively large number of herbal medicinal products with potential lipid-lowering properties available, it seems prudent for physicians to explore this area in their clinical decision-making process.
In conclusion, although 11 herbal medicinal products were identified with potential hypo-cholesterolemic activity, the evidence supporting individual plants is limited. In addition to lowering cholesterol, several of the herbs may exert beneficial effects in cardiovascular disease by elevating HDL levels and inhibiting lipid oxidation. The safety profiles of the products in question seems to be encouraging. Further research is therefore warranted to establish the therapeutic value of these herbs in the treatment of hypercholesterolemia.
Acknowledgments
The authors thank Jongbae Park, Barbara Wider, and Francesca Borelli, Complementary Medicine, Peninsula Medical School, Universities of Exeter and Plymouth, for translation of papers from Chinese, Italian, and French, to and Esther Prati, Pharmaton, Lugano, for assistance with locating relevant articles. JTC received a research fellowship from Pharmaton SA, Lugano, Switzerland.
- There is some evidence from randomized clinical trials that guggul (Commiphora mukul), fenugreek (Trigonella foenum-graecum), artichoke (Cynara scolymus), yarrow (Achillea wilhelmsii), holy basil (Ocimum sanctum), red yeast (Monascus purpureus) rice, eggplant (Solanum melongena), and arjun (Terminalia arjuna) reduce serum cholesterol.
- The evidence is not conclusive for any of the products, although preliminary clinical trials seem promising; further research is warranted.
- Safety profiles from clinical trials appear encouraging, but the long-term safety has not been established; herb-drug interactions may be possible with milk thistle (Silybum marianum), Asian ginseng (Panax ginseng), guggul, and fenugreek.
- It is important for physicians to discuss the use of complementary and alternative therapies with their patients.
- Objectives To systematically review the clinical evidence for herbal medicinal products in the treatment of hypercholesterolemia.
- Study Design A systematic review of randomized clinical trials of herbal medicinal products used to lower serum cholesterol. Systematic literature searches were conducted in 6 electronic data-bases. The reference lists of all papers and our files were searched for more relevant publications. Experts in the field and manufacturers of identified herbal medicinal products were contacted for published and unpublished data. No language restrictions were imposed.
- Outcomes Measured All randomized clinical trials of serum cholesterol reduction, in which mono-preparations of herbal medicinal products were administered as supplements to human subjects, were included.
- Results Twenty-five randomized clinical trials involving 11 herbal medicinal products were identified. Guggul (Commiphora mukul), fenugreek (Trigonella foenum-graecum), red yeast rice, and artichoke (Cynara scolymus) have been most extensively studied and have demonstrated reductions in total serum cholesterol levels of between10% and 33%. The methodological quality as assessed by the Jadad score was less than 3 (maximum, 5) for 13 of the 25 trials.
- Conclusions Many herbal medicinal products have potential hypocholesterolemic activity and encouraging safety profiles. However, only a limited amount of clinical research exists to support their efficacy. Further research is warranted to establish the value of these extracts in the treatment of hypercholesterolemia.
Two recent surveys of patients undergoing cardiac surgery reported that 75% (263 of 376) and 81% (224 of 246) of patients currently use some form of complementary medicine (including herbs, vitamins, supplements, megavitamins, prayer, relaxation, spiritual healing, massage, imagery, and lifestyle and diet modifications).1,2
Many herbal medicinal products are promoted for hypercholesterolemia, including some of the top-selling supplements. It is therefore vital to establish both the efficacy of these herbal supplements in reducing serum cholesterol levels and their relative safety. This review is an attempt to systematically summarize the evidence from randomized clinical trials for the efficacy and safety of lipid-lowering herbal medicinal products.
Methods
Identification of clinical trials
To identify clinical trials involving herbal medicinal products with hypocholesterolemic properties, we conducted systematic literature searches in the following electronic databases (all from their inception to May 2001): MEDLINE (via PubMed), EMBASE, CINAHL, AMED (Alternative and Allied Medicine Database, British Library Medical Information Centre), the Cochrane Library (Issue 2, 2001), and CISCOM (Research Council for Complementary Medicine, London, UK). The search strategy is summarized in Appendix A (available online at http://www.jfponline.com).
Further relevant papers were located by hand-searching the reference lists of all papers and departmental files. In addition, experts in the field and manufacturers were contacted to provide published and unpublished material.
Inclusion and exclusion criteria
Only randomized clinical trials investigating serum cholesterol reduction of monopreparations of herbal medicinal products administered as supplements were included. These could be placebo-controlled or equivalent trials. All retrieved data including uncontrolled trials, case reports, and preclinical and observational studies were reviewed for safety data. No language restrictions were imposed.
Data extraction and quality assessment
All articles were read in full. Data relating to sample size, study design, intervention and control, treatment duration, primary outcome measures, and results were extracted by the first author and validated by the second. The methodological quality of each trial was assessed using the Jadad scoring system,3 which ranges from 0 (poorest) to 5 (highest). A score of 3 or above indicates reasonable methodological quality.
Results
We identified 11 herbal medicinal products investigated for hypocholesterolemic properties in randomized clinical trials: guggul (Commiphora mukul), artichoke (Cynara scolymus), garlic (Allium sativum), fenugreek (Trigonella foenum- graecum), red yeast (Monascus purpureus) rice, Asian ginseng (Panax ginseng), yarrow (Achillea wilhelmsii), eggplant (Solanum melongena), holy basil (Ocimum sanctum), milk thistle (Silybum marianum), and arjun (Terminalia arjuna).
The efficacy and safety of garlic has been reviewed extensively elsewhere4–6 and is therefore not discussed in this paper. Details of all identified studies are shown in Tables 1–5 (and Table W1, available online at http://www.jfponline.com). Guggul, fenugreek, red yeast rice, and artichoke have been studied most extensively; randomized clinical trials of these herbal medicinal products with a Jadad score of 3 or above are discussed in more depth in Appendix B (available online at http://www.jfponline.com). Table 6 summarizes the adverse events experienced by subjects within these clinical trials and potential herb-drug interactions identified from systematic reviews.
Guggul (Commiphora mukul)
Six randomized clinical trials of guggul, involving 388 patients with different diagnoses, were identified.7-12 Five were conducted in India and 1 in the United States; 4 were placebo-controlled; and 1 compared guggul with 2 reference compounds. The results suggest reductions in total serum cholesterol from 10% to 27% compared with baseline levels (Tables 1 and W1).
High-density lipoprotein (HDL) cholesterol levels were measured in 3 of the studies.7- 9 A significant increase was seen after 8 weeks of treatment in 1 study9; in the others, no significant differences were seen.7,8 A statistically significant decrease in lipid peroxide levels was reported in 1 study, with no corresponding change in the placebo-treated group.7
Several mild adverse events were reported during these trials, including rash, nausea, vomiting, eructation, hiccup, headache, loose stools, restlessness, and apprehension, although information regarding adverse events experienced during placebo administration was not always provided. A potential drug interaction with propranolol and diltiazem was investigated in a randomized crossover trial of 17 healthy volunteers, in which guggul was found to significantly reduce the peak plasma concentration of both drugs.13
Fenugreek (Trigonella foenum-graecum)
Fenugreek seeds. Five randomized clinical trials were identified, involving 140 patients; all but 1 trial was conducted in India.14-17 Although the methodological quality of the studies was considered generally poor in 4 of the trials, statistically significant reductions in total serum cholesterol of between 15% and 33% compared with baseline were demonstrated (Table 2).
Fenugreek leaves. In a single-blind study of 20 healthy male volunteers, Abdel-Barry and colleagues found a nonsignificant decrease of 9% in total serum cholesterol after a single dose of an aqueous extract made from fenugreek leaves (40 mg/kg) compared with a reduction of 2.8% after dilute coffee extract (placebo) (Table 2). 18
Within all the identified studies of fenugreek, patients reported mild gastrointestinal symptoms such as increased flatulence, nausea, fullness, and diarrhea during fenugreek treatment, but none was severe enough to necessitate withdrawal from the study. A 14% reduction in serum potassium was noted in healthy volunteers after a single dose of an aqueous extract of fenugreek leaves. 18
Red yeast rice
Red yeast rice is produced by solid-state fermentation of washed and cooked rice using the fungus Monascus purpureus. It has been used in Asia as a food preservative and colorant and for its medicinal properties since the Tang Dynasty (ad 800). It is available in capsules that contain a pulverized powder of fermented rice and yeast.
Four randomized clinical trials of the lipid-lowering effects of red yeast rice conducted in patients (n=695) with hyperlipidemia were identified (Table 3).19-22 In all studies, statistically significant reductions (16% to 31%) in total serum cholesterol compared with placebo or control or baseline were seen.
Adverse events experienced in clinical trials included stomachache, heartburn, dizziness, and flatulence. No changes in liver function tests were demonstrated. There was 1 case report of a 26-year-old man who used red yeast rice in preparing sausages and developed anaphylaxis due to immediate sensitivity to M purpureus.23 Whether this is relevant to the oral administration of red yeast rice capsules is not clear.
Artichoke (Cynara scolymus)
The choleretic effect of the leaf extract of artichoke has been studied widely, but only 2 randomized clinical trials of its hypocholesterolemic effects, involving 187 patients, were identified (Table 4).24,25 One trial (n=44 healthy volunteers), published in abstract form only, found no significant difference in lipid levels compared with placebo, although post hoc subanalyses revealed some reductions in total serum cholesterol in patients with baseline levels above 5.4 mmol/L; these results should be interpreted with some caution. Reductions in total cholesterol of 18.5% and 8.6% were reported in the other, larger trial after artichoke and placebo treatments, respectively.
No adverse events were reported during either study. Three post-marketing surveillance studies were located: one included 417 patients and reported excellent tolerability in 95%; in the second (203 patients) no adverse reactions were reported; and the third (553 patients) described mild adverse events in 1.3% of patients (flatulence, hunger, and weakness).24-28
Discussion
Many different herbal medicinal products have been identified with potential lipid-lowering properties (Table 5), but the evidence for each herb is limited. The largest amount of published literature exists for guggul, fenugreek, red yeast rice, and artichoke, with reductions in total serum cholesterol ranging from 10% to 33%.
Although HDL and low-density lipoprotein (LDL) cholesterol were not measured in all the studies, increases in HDL and decreases in LDL levels were seen with guggul, red yeast rice, and yarrow, and decreases in LDL levels were seen in studies of fenugreek, arjun, and artichoke.
Safety
Few adverse events or drug interactions were reported in clinical trials of any of the 11 herbs identified. Many are used extensively in traditional medicine and culinary practices around the world, which supports their relative safety.
However, the long-term safety for use as herbal medicinal products has not been established. Long-term exposure of large numbers of patients within a formal setting would be necessary to determine safety, although difficulties associated with all herbal medicinal products exist, such as the inability to identify active ingredients and the potential for adulteration and misbranded products. No direct or indirect evidence exists for herb-drug interactions for fenugreek, guggul, Asian ginseng, and milk thistle (Table 6).
Study limitations
Although differences in study design, methodological quality, statistical methods, and subject populations create problems with interpretation of these figures, they appear to compare favorably with studies of garlic; the most recent meta-analysis suggested an average effect size of 4% to 6%.6 Studies of conventional therapeutic options for hypercholesterolemia (eg, statins) have demonstrated reductions of 20% to 30% in serum cholesterol.29
Several shortcomings of the review need to be addressed. First, although attempts were made to obtain data from unpublished trials by contacting authors and manufacturers, none were located. There is evidence to suggest that studies with significant positive results are more likely to be published,30 and this may be more pronounced with unfamiliar herbal therapies.
Second, because much of this research has been conducted in India and China, our extensive search strategy may not have located all the published material.
Third, there were several weaknesses with the original trials; of the 25 randomized clinical trials of herbal medicinal products for serum cholesterol reduction identified, only 12 scored 3 or more points on the Jadad scale. The most frequent methodological flaws were conduct of single-blind or open studies and incomplete reporting of methods of randomization, blinding, and subject withdrawals.
Conclusions
Evidence suggests that physicians do not ask their patients about complementary and alternative therapies and that patients are reticent to discuss these treatments with their physicians.31- 34 Surveys indicate widespread use of complementary and alternative therapies among patients undergoing cardiac surgery.1,2 Although no equivalent surveys have been conducted for patients with hypercholesterolemia, in light of the relatively large number of herbal medicinal products with potential lipid-lowering properties available, it seems prudent for physicians to explore this area in their clinical decision-making process.
In conclusion, although 11 herbal medicinal products were identified with potential hypo-cholesterolemic activity, the evidence supporting individual plants is limited. In addition to lowering cholesterol, several of the herbs may exert beneficial effects in cardiovascular disease by elevating HDL levels and inhibiting lipid oxidation. The safety profiles of the products in question seems to be encouraging. Further research is therefore warranted to establish the therapeutic value of these herbs in the treatment of hypercholesterolemia.
Acknowledgments
The authors thank Jongbae Park, Barbara Wider, and Francesca Borelli, Complementary Medicine, Peninsula Medical School, Universities of Exeter and Plymouth, for translation of papers from Chinese, Italian, and French, to and Esther Prati, Pharmaton, Lugano, for assistance with locating relevant articles. JTC received a research fellowship from Pharmaton SA, Lugano, Switzerland.
1. Ai AL, Bolling SF. The use of complementary and alternative therapies among middle-aged and older cardiac patients. Am J Med Qual 2002;17:21-27.
2. Liu EH, Turner LM, Lin SX, et al. Use of alternative medicine by patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 2000;120:335-341.
3. Jadad AR, Moore A, Carroll D, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12.
4. Warshafsky S, Kamer RS, Sivak SL. Effect of garlic on total serum cholesterol. A meta-analysis. Arch Intern Med 1993;119:599-605.
5. Silagy C, Neil A. Garlic as a lipid lowering agent: a meta-analysis. J R Coll Physicians Lond 1994;28:39-45.
6. Stevinson C, Pittler MH, Ernst E. Garlic for treating hypercholesterolemia. A meta-analysis of randomised clinical trials. Ann Intern Med 2000;133:420-429.
7. Singh RB, Niaz MA, Ghosh S. Hypolipidemic and antioxidant effects of Commiphora mukul as an adjunct to dietary therapy in patients with hypercholesterolemia. Cardiovasc Drugs Ther 1994;8:659-664.
8. Szapary PO, Wolfe ML, Bloedon LT, Fair MB, Berezich DJ, Cirigliano AJ, Rader DJ. A double blind, randomised, placebo controlled clinical trial of standardized guggul extract in patients with hypercholesterolemia. Complement Ther Med 2002;10:112.
9. Verma SK, Bordia A. Effect of Commiphora mukul (gum guggulu) in patients with hyperlipidemia with special reference to HDL cholesterol. Indian J Med Res 1988;87:356-360.
10. Malhotra SC, Ahuja MMS. Comparative hypolipidaemic effectiveness of gum guggulu (Commiphora mukul) fraction ‘A’, ethyl-P-Chlorophenoxyisobutyrate and Ciba-13437-Su. Indian J Med Res 1971;59:1621-1632.
11. Kuppurajan K, Rajagopalan SS, Koteswara Rao T, Sitaraman R. Effect of guggulu (Commiphora mukul–Engl) on serum, lipids in obese, hypercholesterolemic and hyperlipemic cases. J Assoc Physicians India 1978;26:367-373.
12. Bordia A, Chuttani SK. Effect of gum guggulu on fibrinolysis and platelet adhesiveness in coronary heart disease. Indian J Med Res 1979;70:992-996.
13. Dalvi SS, Nayak VK, Pohujani SM, Desai NK, Kshirsagar NA, Gupta KC. Effect of gugulipid on bioavailability of diltiazem and propranolol. J Assoc Physicians India 1994;42:454-455.
14. Singh RB, Niaz MA, Rastogi V, Singh N, Postiglione A, Rastogi SS. Hypolipidemic and antioxidant effects of fenugreek seeds and triphala as adjuncts to dietary therapy in patients with mild to moderate hypercholesterolemia. Perfusion 1998;11:124-130.
15. Prasanna M. Hypolipidemic effect of fenugreek: a clinical study. Indian J Pharmacol 2000;32:34-36.
16. Sharma RD, Raghuram TC. Hypoglycaemic effect of fenugreek seeds in non-insulin dependent diabetic subjects. Nutr Res 1990;10:731-739.
17. Sharma RD, Raghuram TC, Rao NS. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur J Clin Nutr 1990;44:301-306.
18. Abdel-Barry JA, Abdel-Hassan IA, Jawad AM, Al-Hakiem MHH. Hypoglycaemic effect of aqueous extract of the leaves of Trigonella foenum-graecum in healthy volunteers. East Mediterr Health J 2000;6:83-88.
19. Keithley J, Swanson B, Sha B, Zeller J, Kessler HA, Smith KY. A pilot study of the safety and efficacy of Cholestin in treating HIV-related dyslipidemia. Nutrition 2002;18:201-204.
20. Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VLW. Cholesterol lowering effects of a proprietary Chinese red yeast rice dietary supplement. Am J Clin Nutr 1999;69:231-236.
21. Shen Z, Yu P, Su M, et al. A prospective study of Zhitai capsules in the treatment of primary hyperlipidemia. Natl Med J China 1996;76:156-157.
22. Wang J, Lu Z, Chi J, et al. Multicenter clinical trial of the serum lipid-lowering effects of a Monascus purpureus (red yeast) rice preparation from traditional chinese medicine. Curr Ther Res 1997;58:964-978.
23. Wigger-Alberti W, Bauer A, Hipler UC, Elsner P. Anaphylaxis due to Monascus purpureus fermented rice (red yeast rice). Allergy 1999;54:1328-1336.
24. Englisch W, Beckers C, Unkauf M, Ruepp M, Zinserling V. Efficacy of artichoke dry extract in patients with hyperlipoproteinemia. Arzneimittelforschung 2000;50:260-265.
25. Petrowicz O, Gebhardt R, Donner M, Schwandt P, Kraft K. Effects of artichoke leaf extract (ALE) on lipoprotein metabolism in vitro and in vivo. Atherosclerosis 1997;129:147.
26. Fintelmann V. Antidyspeptic and lipid lowering effect of an extract from artichoke leaves. results of clinical trials on efficacy and tolerability of Hepar SL in 553 patients. Z Allg Med 1996;72:3-19.
27. Fintelmann V, Wegener T. Langzeitanwendung von artischockenblattertrockenextrakt (Hepar-SL forte) bei dyspeptischem symptomkomplex. Presented at: Phytotherapie Kongress 1997;November 27-28, 1997; Wurzburg, Germany.
28. Held C. Von der 1. Deutsch-ungarischen Phytopharmaka-konferenz 1991; 20 November, Budapest. Z Klin Med 1992;47:92.-
29. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-1389.
30. Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet 1991;337:867-872.
31. Adler SR, Fosket JR. Disclosing complementary and alternative medicine use in the medical encounter: a qualitative study in women with breast cancer. J Fam Pract 1999;48:453.
32. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. Results of a follow-up national survey. JAMA 1998;280:1569-1575.
33. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. N Engl J Med 1993;328:246-252.
34. Abbot NC, Ernst E. Patients’ opinions about complementary medicine. Forsch Komplementarmed 1997;4:164-168.
35. Guimaraes PR, Galvao AMP, Batista CM, et al. Eggplant (Solanum melongena) infusion has a modest and transitory effect on hypercholesterolemic subjects. Braz J Med Biol Res 2000;33:1027-1036.
36. Sotaniemi EA, Haapakoski E, Rautio A. Ginseng therapy in non-insulin dependent diabetic patients. Diabetes Care 1995;18:1373-1375.
37. Petronelli A, Roda E, Briganti M, Labate AMM, Barbara L. Effeto della somministrazione di silimarina sui livelli dei lipidi sierici. Clin Ter 1981;99:471-482.
38. Gupta R, Singhal S, Goyle A, Sharma VN. Antioxidant and hypocholesterolaemic effects of Terminalia arjuna tree bark powder: a randomised placebo controlled trial. J Assoc Physicians India 2001;49:231-235.
39. Agarwal P, Rai V, Singh RB. Randomised placebo-controlled, single blind trial of holy basil leaves in patients with non insulin dependent diabetes mellitus. Int J Clin Pharmacol Ther 1996;34:406-409.
40. Asgary S, Naderi GH, Sarrafzadegan N, Mohammadifard N, Mostafavi S, Vakili R. Antihypertensive and antihyperlipidemic effects of Achillea wilhelmsii. Drugs Exp Clin Res 2000;26:89-93.
41. Fugh-Berman A. Herb-drug interactions. Lancet 2000;355:134-138.
42. Ernst E. Possible interactions between synthetic and herbal medicinal products. Part 1: a systematic review of the indirect evidence. Perfusion 2000;13:4-15.
43. Ernst E. Interactions between synthetic and herbal medicinal products. Part 2: a systematic review of the direct evidence. Perfusion 2000;13:60-70.
44. Thompson Coon J, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf 2002;25:323-44.
1. Ai AL, Bolling SF. The use of complementary and alternative therapies among middle-aged and older cardiac patients. Am J Med Qual 2002;17:21-27.
2. Liu EH, Turner LM, Lin SX, et al. Use of alternative medicine by patients undergoing cardiac surgery. J Thorac Cardiovasc Surg 2000;120:335-341.
3. Jadad AR, Moore A, Carroll D, et al. Assessing the quality of reports of randomised clinical trials: is blinding necessary? Control Clin Trials 1996;17:1-12.
4. Warshafsky S, Kamer RS, Sivak SL. Effect of garlic on total serum cholesterol. A meta-analysis. Arch Intern Med 1993;119:599-605.
5. Silagy C, Neil A. Garlic as a lipid lowering agent: a meta-analysis. J R Coll Physicians Lond 1994;28:39-45.
6. Stevinson C, Pittler MH, Ernst E. Garlic for treating hypercholesterolemia. A meta-analysis of randomised clinical trials. Ann Intern Med 2000;133:420-429.
7. Singh RB, Niaz MA, Ghosh S. Hypolipidemic and antioxidant effects of Commiphora mukul as an adjunct to dietary therapy in patients with hypercholesterolemia. Cardiovasc Drugs Ther 1994;8:659-664.
8. Szapary PO, Wolfe ML, Bloedon LT, Fair MB, Berezich DJ, Cirigliano AJ, Rader DJ. A double blind, randomised, placebo controlled clinical trial of standardized guggul extract in patients with hypercholesterolemia. Complement Ther Med 2002;10:112.
9. Verma SK, Bordia A. Effect of Commiphora mukul (gum guggulu) in patients with hyperlipidemia with special reference to HDL cholesterol. Indian J Med Res 1988;87:356-360.
10. Malhotra SC, Ahuja MMS. Comparative hypolipidaemic effectiveness of gum guggulu (Commiphora mukul) fraction ‘A’, ethyl-P-Chlorophenoxyisobutyrate and Ciba-13437-Su. Indian J Med Res 1971;59:1621-1632.
11. Kuppurajan K, Rajagopalan SS, Koteswara Rao T, Sitaraman R. Effect of guggulu (Commiphora mukul–Engl) on serum, lipids in obese, hypercholesterolemic and hyperlipemic cases. J Assoc Physicians India 1978;26:367-373.
12. Bordia A, Chuttani SK. Effect of gum guggulu on fibrinolysis and platelet adhesiveness in coronary heart disease. Indian J Med Res 1979;70:992-996.
13. Dalvi SS, Nayak VK, Pohujani SM, Desai NK, Kshirsagar NA, Gupta KC. Effect of gugulipid on bioavailability of diltiazem and propranolol. J Assoc Physicians India 1994;42:454-455.
14. Singh RB, Niaz MA, Rastogi V, Singh N, Postiglione A, Rastogi SS. Hypolipidemic and antioxidant effects of fenugreek seeds and triphala as adjuncts to dietary therapy in patients with mild to moderate hypercholesterolemia. Perfusion 1998;11:124-130.
15. Prasanna M. Hypolipidemic effect of fenugreek: a clinical study. Indian J Pharmacol 2000;32:34-36.
16. Sharma RD, Raghuram TC. Hypoglycaemic effect of fenugreek seeds in non-insulin dependent diabetic subjects. Nutr Res 1990;10:731-739.
17. Sharma RD, Raghuram TC, Rao NS. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur J Clin Nutr 1990;44:301-306.
18. Abdel-Barry JA, Abdel-Hassan IA, Jawad AM, Al-Hakiem MHH. Hypoglycaemic effect of aqueous extract of the leaves of Trigonella foenum-graecum in healthy volunteers. East Mediterr Health J 2000;6:83-88.
19. Keithley J, Swanson B, Sha B, Zeller J, Kessler HA, Smith KY. A pilot study of the safety and efficacy of Cholestin in treating HIV-related dyslipidemia. Nutrition 2002;18:201-204.
20. Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VLW. Cholesterol lowering effects of a proprietary Chinese red yeast rice dietary supplement. Am J Clin Nutr 1999;69:231-236.
21. Shen Z, Yu P, Su M, et al. A prospective study of Zhitai capsules in the treatment of primary hyperlipidemia. Natl Med J China 1996;76:156-157.
22. Wang J, Lu Z, Chi J, et al. Multicenter clinical trial of the serum lipid-lowering effects of a Monascus purpureus (red yeast) rice preparation from traditional chinese medicine. Curr Ther Res 1997;58:964-978.
23. Wigger-Alberti W, Bauer A, Hipler UC, Elsner P. Anaphylaxis due to Monascus purpureus fermented rice (red yeast rice). Allergy 1999;54:1328-1336.
24. Englisch W, Beckers C, Unkauf M, Ruepp M, Zinserling V. Efficacy of artichoke dry extract in patients with hyperlipoproteinemia. Arzneimittelforschung 2000;50:260-265.
25. Petrowicz O, Gebhardt R, Donner M, Schwandt P, Kraft K. Effects of artichoke leaf extract (ALE) on lipoprotein metabolism in vitro and in vivo. Atherosclerosis 1997;129:147.
26. Fintelmann V. Antidyspeptic and lipid lowering effect of an extract from artichoke leaves. results of clinical trials on efficacy and tolerability of Hepar SL in 553 patients. Z Allg Med 1996;72:3-19.
27. Fintelmann V, Wegener T. Langzeitanwendung von artischockenblattertrockenextrakt (Hepar-SL forte) bei dyspeptischem symptomkomplex. Presented at: Phytotherapie Kongress 1997;November 27-28, 1997; Wurzburg, Germany.
28. Held C. Von der 1. Deutsch-ungarischen Phytopharmaka-konferenz 1991; 20 November, Budapest. Z Klin Med 1992;47:92.-
29. Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383-1389.
30. Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet 1991;337:867-872.
31. Adler SR, Fosket JR. Disclosing complementary and alternative medicine use in the medical encounter: a qualitative study in women with breast cancer. J Fam Pract 1999;48:453.
32. Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990-1997. Results of a follow-up national survey. JAMA 1998;280:1569-1575.
33. Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. N Engl J Med 1993;328:246-252.
34. Abbot NC, Ernst E. Patients’ opinions about complementary medicine. Forsch Komplementarmed 1997;4:164-168.
35. Guimaraes PR, Galvao AMP, Batista CM, et al. Eggplant (Solanum melongena) infusion has a modest and transitory effect on hypercholesterolemic subjects. Braz J Med Biol Res 2000;33:1027-1036.
36. Sotaniemi EA, Haapakoski E, Rautio A. Ginseng therapy in non-insulin dependent diabetic patients. Diabetes Care 1995;18:1373-1375.
37. Petronelli A, Roda E, Briganti M, Labate AMM, Barbara L. Effeto della somministrazione di silimarina sui livelli dei lipidi sierici. Clin Ter 1981;99:471-482.
38. Gupta R, Singhal S, Goyle A, Sharma VN. Antioxidant and hypocholesterolaemic effects of Terminalia arjuna tree bark powder: a randomised placebo controlled trial. J Assoc Physicians India 2001;49:231-235.
39. Agarwal P, Rai V, Singh RB. Randomised placebo-controlled, single blind trial of holy basil leaves in patients with non insulin dependent diabetes mellitus. Int J Clin Pharmacol Ther 1996;34:406-409.
40. Asgary S, Naderi GH, Sarrafzadegan N, Mohammadifard N, Mostafavi S, Vakili R. Antihypertensive and antihyperlipidemic effects of Achillea wilhelmsii. Drugs Exp Clin Res 2000;26:89-93.
41. Fugh-Berman A. Herb-drug interactions. Lancet 2000;355:134-138.
42. Ernst E. Possible interactions between synthetic and herbal medicinal products. Part 1: a systematic review of the indirect evidence. Perfusion 2000;13:4-15.
43. Ernst E. Interactions between synthetic and herbal medicinal products. Part 2: a systematic review of the direct evidence. Perfusion 2000;13:60-70.
44. Thompson Coon J, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf 2002;25:323-44.
Treatment of Molluscum Contagiosum With the Pulsed Dye Laser Over a 28-Month Period
Asthma: Resource use and costs for inhaled corticosteroid vs leukotriene modifier treatment—a meta-analysis
Objective To compare the effects of inhaled corticosteroid treatment with leukotriene modifier treatment on medical resource use and costs for asthma patients.
Study design Meta-analysis combining results from published and unpublished studies.
Data sources Studies were identified from the MEDLINE and EMBASE databases and the GlaxoSmithKline internal database study registers. Two independent reviewers evaluated the identified studies; studies meeting specified inclusion criteria were abstracted and summarized by meta-analysis with a random effects model.
Outcomes measured Hospitalization rate, emergency department visit rate, emergency department costs, drug costs, total asthma-related costs, and total medical care costs.
Results Patients taking inhaled corticosteroids had:
a significantly lower annual rate of hospitalization than those taking leukotriene modifiers (2.2% vs 4.3%, respectively;P<.05)
a greater decline in hospitalization rate (before vs after therapy initiation) than those taking leukotriene modifiers (decline of 2.4% vs 0.55%; P<.01)
a lower annual rate of emergency department visits than those taking leukotriene modifiers (6.2% vs 7.7%;P<.005).
lower total asthma-related medical costs than those taking leukotriene modifiers (P<.05) and a 17% reduction in overall total medical care costs (P not significant).
Conclusions Patients with asthma treated with inhaled corticosteroids had significantly fewer asthma-related hospitalizations and emergency department visits and lower total asthma-related health care costs than patients treated with leukotriene modifiers. These meta-analysis findings are consistent with results from randomized controlled trials showing improvements in lung function for patients taking inhaled corticosteroids as opposed to leukotriene modifiers.
Although many medications are available for patients with asthma, inhaled corticosteroids are generally the preferred treatment.1-4 Multiple studies have demonstrated that inhaled corticosteroid therapy improves patient outcomes.1 Inhaled corticosteroids have been shown to decrease costs5 and use of medical care resources.6-8
More recently, leukotriene modifiers have been introduced for asthma treatment. This class of medication has bronchodilator and anti-inflammatory effects.9 Although multiple studies have indicated improved outcomes and decreased costs associated with leukotriene modifier therapy incertain patient populations,10,11 its role in asthmamanagement is uncertain.9
Several studies have compared the clinical outcomes of these therapies12-15 and their impact on medical care resource use and costs.16,17 However, these studies were not powered specifically to detect significant differences in resource use or costs.
We performed a meta-analysis to (1) compare the rate of hospitalization among patients with asthma treated with inhaled corticosteroids vs those treated with leukotriene modifiers and (2) evaluate other resource use rates and costs for these patients.
Methods
The meta-analysis consisted of a literature search, the development of inclusion criteria, form development, and literature review.
Literature search
We searched the MEDLINE, EMBASE, Cochrane Collaboration Study Registry, and GlaxoSmithKline databases and consulted experts in this field. The GlaxoSmithKline database consists of studies sponsored by GlaxoSmithKline that met companywide minimum quality thresholds and were published in full or abstract form.
We also contacted the manufacturers of leukotriene modifiers available in the United States, AstraZeneca and Merck, to request published and unpublished information on studies comparing leukotriene modifiers with inhaled corticosteroids. To provide results corresponding to current treatment patterns, only studies from 1991 to 2001 were included. Published and unpublished materials were included.18,19
Inclusion criteria
Studies were included in the meta-analysis if they met the following criteria.
Population. Patients with diagnosed asthma. Only studies that did not restrict analyses to severe asthma patients or children were included.
Study design. Prospective and retrospective comparative studies of patients receiving inhaled corticosteroid or leukotriene modifier monotherapy (no other controller therapy) in the same study. Studies were required to have defined inclusion and exclusion criteria, defined number of patients in each study arm, defined treatment protocol (ie, medications and doses used), and separate results for each medication.
Only studies presenting primary research (hence excluding review articles and metaanalyses) were included. Only studies presenting data for at least 6 months on all participants were included.
Outcomes. Hospitalization visit rates and costs, emergency department visit rates and costs, pharmacy costs, total asthma-related costs, and total medical care costs. Because resource use patterns and medical care cost information differs substantially between countries, we only included US studies.
Study process
Each identified article was evaluated by 2 independent reviewers (KMS and MG); any differences were discussed with a project leader to reach a consensus. Documents selected for inclusion were then reviewed by the 2 reviewers, and differences in data abstraction were resolved before inclusion.
Analysis
We used the Q statistic20 to assess heterogeneity and, when appropriate, combined data from included studies with the use of a random effects model. Random effects methodology was used to assess the impact of inhaled corticosteroid vs leukotriene modifier therapy on the overall asthma population, not just the subpopulation of patients participating in included studies.21
Two sets of meta-analyses were performed with the abstracted data. First, the specified outcome measures were compared for patients taking inhaled corticosteroid vs leukotriene modifier therapy. Second, the impact (before vs after) of each treatment initiation was compared for each outcome.
Assessment of statistically significant differences between meta-analysis results was performed with the Student t test, with an Α of .05. Charges were used in the included studies as proxies for costs. These costs were inflated to 2000 values by using the medical care component of the consumer price index before inclusion.
Results
We identified 49 documents and reviewed them for inclusion in the meta-analysis; 6 (12.2%) met the inclusion criteria (Table 1). Five were retrospective cohort studies; only 1 study was identified as a prospective trial comparing inhaled corticosteroid and leukotriene modifier therapies and including results on resource use or medical care costs.16,17,22-25 All 6 studies were performed with support from GlaxoSmithKline.
Forty-three documents were excluded due to 1 or more of the following criteria: lack of primary results (9 documents, 21%); did not contain resource use rate or cost outcomes (22 documents, 51%); did not provide at least 6 months’ worth of data on resource use or cost outcomes (5 documents, 12%); did not meet the defined inclusion and exclusion criteria (primarily studies not including both inhaled corticosteroid and leukotriene modifiers or those restricted to patient clinical subgroups; 10 documents, 34%); or did not define the number of patients included in the study (1 document, 3%).
Because few studies presented data on the specified outcomes, we were unable to assess asthma-specific costs for subcategories of resource use. Too few studies included data on hospitalization costs (either asthma-specific or overall) to include in the analysis. Therefore, meta-analysis was performed on overall (ie, all causes) emergency department, pharmacy, and total medical care costs.
TABLE 1
Characteristics of studies included in the meta-analysis
| Duration (mo) | |||||
|---|---|---|---|---|---|
| Study | LOE* | Before therapy | After therapy | Treatment (N) | Comparison (N) |
| Oates and Gothard22 | 2b | 9 | 9 | Inhaled corticosteroids (546)† | Leukotriene modifiers (152)‡ |
| Pathak et al23 | 2b | 9 | 9 | Fluticasone propionate (284) | Leukotriene modifiers (497)‡ |
| Stanford et al24 | 1b | - | 6 | Fluticasone propionate (271) | Montelukast (262) |
| Stempel et al16 | 2b | 9 | 12 | Fluticasone propionate (602) | Zafirlukast (309) |
| Stempel et al17 | 2b | 9 | 9 | Fluticasone propionate (559) | Montelukast (382) |
| White et al25 | 2b | 9 | 9 | Inhaled corticosteroids (1305)† | Leukotriene modifiers (109)‡ |
| *LOE, level of evidence. For an explanation of levels of evidence. | |||||
| †Results were presented for all inhaled corticosteroids combined. | |||||
| ‡Results were presented for all leukotriene modifiers combined. | |||||
Primary analysis
The primary objective of this study was to evaluate the impact of inhaled corticosteroid and leukotriene modifier treatment on the mean annual hospitalization rate. Four of the 6 included studies contained information on hospitalization rate for each treatment. Results from the primary analysis are presented in Table 2.
Patients taking inhaled corticosteroids had a significantly lower annual rate of hospitalization than did patients taking leukotriene modifiers (2.23% vs 4.30%, respectively; P<.005). The absolute risk reduction was 2.07% (number needed to treat=48 for 1 year).
The difference in annual hospitalization visit rates for each study in the primary analysis is presented in the Figure, where negative values reflect lower hospitalization rates among patients taking inhaled corticosteroids than among those taking leukotriene modifiers. Two studies16,23 had statistically significant differences in hospitalization rates, whereas the differences in the other 2 studies were not statistically significant (P<.05). Combining studies with the use of a random effects model (the default methodology for this analysis) or a fixed effects model produced similar results. The Q statistic indicated no significant heterogeneity (P=.43).
TABLE 2
Meta-analysis results for inhaled corticosteroid vs leukotriene modifier therapy*
| Inhaled corticosteroid vs leukotriene modifier patients † | ||||
|---|---|---|---|---|
| Inhaled corticosteroids | Leukotriene modifiers | Absolute difference | Relative difference | |
| Annual asthma hospitalizations‡ | 2.23% (1.69-2.78) | 4.30% (3.53-5.07) | -1.79% (-2.45 to -1.14) | -42.88% (-55.95 to -29.80) |
| Annual rate of visits to the emergency department due to asthma§ | 6.19% (4.84-7.53) | 7.74% (6.30-9.19) | -1.53% (-1.78 to -1.28) | -21.35% (-25.31 to -17.38) |
| Total annual costs of visits to the emergency department | $93 (38-148) | $73 (52-94) | $21(-17 to 59) | 1.00% (-38 to 40) |
| Total annual drug costs§ | $807 (548-1065) | $1062 (812-1312) | -$258 (-308 to -208) | -27.20% (-33.2 to -21.3) |
| Annual asthma-related cost‡ | $882 (613-1150) | $1393 (1143-1643) | $513 (-392 to -634) | -38.01% (-47.4 to -28.8) |
| Total annual cost | $5254 (4474-6033) | $7140 (4970-9311) | -$1918 (-3509 to -327) | -17.20% (-30.9 to -3.5) |
| *Data are presented as mean (95% confidence interval). | ||||
| †Absolute and relative differences were determined from meta-analyses of the absolute and relative differences for each included study. | ||||
| ‡Inhaled corticosteroid vs leukotriene modifier significant at P<.05. | ||||
| §Inhaled corticosteroid vs leukotriene modifier significant at P<.005. | ||||
FIGURE
Difference in hospitalization rates (mean, confidence interval)
Secondary outcomes
Results of secondary analyses for 5 study outcomes (annual visits to the emergency department due to asthma, total emergency department costs, total drug costs, total asthma-related costs, and overall total cost) are presented in Table 2.
Mean annual rates of visits to the emergency department and total annual drug costs were significantly higher for patients taking leukotriene modifiers than for those taking inhaled corticosteroids (P<.005 for each). Patients taking leukotriene modifiers had lower annual costs for visits to the emergency department than did those taking inhaled corticosteroids, although this difference was not statistically significant. The higher rate and lower cost of emergency department visits for patients taking leukotriene modifiers suggest that medical resources were used less at each visit as compared with those for patients taking inhaled corticosteroids.
Total asthma-related costs for patients taking inhaled corticosteroids were significantly lower than those for patients taking leukotriene modifiers (P<.05). Patients taking inhaled corticosteroids also incurred decreased annual total (allcause) medical care costs. Although this difference was qualitatively large (approximately $1900, or a decrease of over 17%), it did not reach statistical significance.
Pre- vs post-initiation of therapy
In addition to comparing the impact of the 2 therapies on resource use and costs, we were interested in the impact of initiating each therapy on the study outcomes. We therefore assessed resource use rates and costs before and after treatment initiation. These patients may have been receiving asthma therapies (or no asthma therapy) other than inhaled corticosteroids or leukotriene modifiers. The number of studies presenting data on costs was too small to allow comparison.
Results of this within-group analysis are presented in Table 3. For patients taking inhaled corticosteroids, hospitalization rates and emergency department visit rates decreased significantly after treatment initiation compared with the preinitiation values (P<.005 and P<.05, respectively). The decreases for patients taking leukotriene modifiers upon treatment initiation were smaller and not statistically significant.
Both groups showed similar small decreases in annual emergency department costs, neither of which was significant. The increases in annual total drug costs and annual total medical care costs after treatment initiation were significant for both groups of patients (all at P<.005). However, both increases were greater for patients taking leukotriene modifiers; the increase in drug costs was statistically significant (P<.001).
TABLE 3
Resource utilization and costs before and after therapy initiation for inhaled corticosteroids vs leukotriene modifiers*
| Before vs after treatment, mean (95% confidence interval) | |||
|---|---|---|---|
| Inhaled corticosteroid patients | Leukotriene modifier patients | Absolute difference | |
| Annual hospitalization rate | -2.37%† (-2.89 to -1.86) | -0.55% (-1.18 to 0.08) | -1.91%‡(-2.45 to -1.36) |
| Annual rate of visits to the emergency department due to asthma | -4.44%§ (-5.98 to -2.90) | -2.06% (-3.61 to -0.51) | -2.47%||(-3.09 to -1.86) |
| Total annual costs of the emergency despartment | -$5 (-21 to 10) | -$15 (-44 to 15) | $9 (-33 to 51) |
| Total annual drug costs | $415†(312-517) | $579† (472-686) | -$167||(-192 to 142) |
| Total annual medical care costs | $641† (113-1169) | $1712† (927-3529) | -$1080 (-2802 to 643) |
| *Post-therapy initiation values for inhaled corticosteroids and leukotriene modifiers are presented in Table 1. | |||
| †Before vs after treatment initiation significant at P<.005. | |||
| ‡Before vs after treatment initiation outcomes for inhaled corticosteroids vs leukotriene modifiers significant at P<.01. | |||
| §Before vs after treatment initiation significant at P<.05. | |||
| ||Before vs after treatment initiation outcomes for inhaled corticosteroids vs leukotriene modifiers significant at P<.001. | |||
Discussion
In this study, we used meta-analysis to combine data across studies and determine more robust estimates of the impact of inhaled corticosteroid vs leukotriene modifier therapy on medical resource use rates and costs. The primary analysis indicated that annual hospitalization rates among patients taking inhaled corticosteroids are significantly lower that those taking leukotriene modifiers. Other resource use rates and costs evaluated in this study also generally showed decreased values for patients taking inhaled corticosteroids.
Although meta-analysis generally has been used for clinical outcome measures, it is a highly appropriate method for resource use and cost outcomes. In general, studies of the impact of a particular treatment are powered to assess safety and efficacy or effectiveness; there is often insufficient power to detect differences in resource use or costs in any one study. Due to substantial variation in treatment patterns, the variance associated with resource use rates (and associated costs) may be substantially higher than that for clinical measures; such a wide variance adds to the difficulties in assessing differences for nonclinical outcomes.
Limitations
This study has a number of limitations. Only a few studies met inclusion criteria for the meta-analysis; the analysis should be replicated as additional studies become available. Data were abstracted from the included studies without modification (except for inflating costs to 2000 values when necessary). As in all meta-analyses, any problems present in the original data are present in the combined data; limitations of the original data are not addressed by this method.
Among the studies evaluated for the metaanalysis were a number published only as abstracts. Inclusion of unpublished literature in meta-analyses is controversial; however, several sources18,19 now recommend inclusion of published and unpublished studies. Egger and Smith26 found that studies with significant results are more likely to be published than are studies with nonsignificant results, leading to publication bias. Studies with significant results also may be more likely to be published in indexed journals, leading to “database bias.” As such, inclusion of unpublished studies is important to produce unbiased results.
Five of the 6 studies that met the inclusion criteria were observational, retrospective cohort analyses. Whereas many meta-analyses focus solely on prospective, randomized clinical trials, several have included retrospective data.27,28 Retrospective analyses and observational data have a number of limitations, in particular the lack of randomization that can lead to differences in characteristics of specified treatment groups. Further, as discussed by Egger et al,29 metaanalyses based on observational studies may involve bias and confounding.
However, observational data and retrospective analyses also have the advantage of reflecting “real world” treatment patterns and broader patient groups that increase the generalizability of the data, whereas clinical trials may include protocol-driven utilization and selected patient groups. Clinical trials also may occur in specialized health care settings, whereas observational (cohort) data may be more applicable to clinical practice. Due to these factors, meta-analysis of observational data has become common.29
The pre-initiation vs post-initiation analysis indicated that values for each treatment group provide information on the similarities between treatment groups before initiation of controller therapy. Even though the treatment groups were not randomized to each therapy and we have no means to ensure compatibility between groups, having similar rates of resource use between groups provides some evidence regarding similarity. Nonetheless, given the limitations of the retrospective data and meta-analysis in general, it will be important to validate the results of this meta-analysis in the future with naturalistic prospective studies.
Despite these limitations, this study provides important information on the impact of asthma therapies on resource use and costs. Specifically, the resource use and cost outcomes assessed in this study were lower for inhaled corticosteroid patients than for leukotriene modifier patients. This study also illustrates the usefulness of metaanalysis in evaluating resource use and costs. By selecting and combining outcomes across studies in a standardized, rigorous, and transparent manner, the effects of different therapies can be evaluated with greater precision.
Acknowledgments
We thank John O’Donnell and Layne Gothard for their assistance with this manuscript.
Corresponding author
Michael T. Halpern, MD, PhD, Principal Scientist, Exponent, Inc., 1800 Diagonal Road, Alexandria, VA 22314. E-mail: [email protected]
1. Janson S. National asthma education and prevention program, expert panel report. II: overview and application to primary care. Prim Care Pract 1998;2:578-588.
2. Lalloo UG, Bateman ED, Feldman C, et al. Guideline for the management of chronic asthma in adults—2000 update. South African Pulmonology Society Adult Asthma Working Group. S Afr Med J 2000;90:540-541-544-552.
3. Podell RN. National guidelines for the management of asthma in adults. Am Fam Physician 1992;46:1189-1196.
4. Veninga CC, Lagerlov P, Wahlstrom R, et al. Evaluating an educational intervention to improve the treatment of asthma in four European countries. Drug Education Project Group. Am J Respir Crit Care Med 1999;160:1254-1262.
5. Ozminkowski RJ, Wang S, Marder WD, Azzolini J, Schutt D. Cost implications for the use of inhaled anti-inflammatory medications in the treatment of asthma. Pharmacoeconomics 2000;18:253-264.
6. Paltiel AD, Fuhlbrigge AL, Kitch BT, et al. Cost-effectiveness of inhaled corticosteroids in adults with mild-to-moderate asthma: results from the asthma policy model. J Allergy Clin Immunol 2001;108:39-49.
7. Adams RJ, Fuhlbrigge A, Finkelstein JA, et al. Impact of inhaled antiinflammatory therapy on hospitalization and emergency department visits for children with asthma. Pediatrics 2001;107:706-711.
8. Donahue JG, Weiss ST, Livingston JM, Goetsch MA, Greineder DK, Platt R. Inhaled steroids and the risk of hospitalization for asthma. JAMA 1997;277:887-891.
9. Dempsey OJ. Leukotriene receptor antagonist therapy. Postgrad Med J 2000;76:767-773.
10. Klingman D, Bielory L, Wang Y, et al. Asthma outcome changes associated with use of the leukotriene-receptor antagonist zafirlukast. Manag Care Interface 2001;14:62-66.
11. Price DB, Ben-Joseph RH, Zhang Q. Changes in asthma drug therapy costs for patients receiving chronic montelukast therapy in the U.K. Resp Med 2001;95:83-89.
12. Bleecker ER, Welch MJ, Weinstein SF, et al. Low-dose inhaled fluticasone propionate versus oral zafirlukast in the treatment of persistent asthma. J Allergy Clin Immunol 2000;105:1123-1129.
13. Ind PW. Inhaled corticosteroids versus anti-leukotrienes: a literature review on the clinical effects. Allergy 1999;54(suppl 50):43-46.
14. Malmstrom K, Rodriguez-Gomez G, Guerra J, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med 1999;130:487-495.
15. Busse W, Nelson H, Wolfe J, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. J Allergy Clin Immunol 1999;103:1075-1080.
16. Stempel DA, Meyer JW, Stanford RH, Yancey SW. One-year claims analysis comparing inhaled fluticasone propionate with zafirlukast for the treatment of asthma. J Allergy Clin Immunol 2001;107:94-98.
17. Stempel DA, Mauskopf J, McLaughlin T, Yazdani C, Stanford RH. Comparison of asthma costs in patients starting fluticasone propionate compared to patients starting montelukast. Respir Med 2001;95:227-234.
18. Cook DJ, Guyatt GH, Ryan G, et al. Should unpublished data be included in meta-analyses? Current convictions and controversies. JAMA 1993;269:2749-2753.
19. McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 2000;356:1228-1231.
20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-188.
21. Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care 1990;6:5-30.
22. Oates V, Gothard L. PEER Study: New Starts on Inhaled Corticosteroids or Leukotriene Modifiers in an Asthmatic Population. Advanced Paradigm and GlaxoWellcome, Inc. July 19, 2000. Information available from GlaxoSmithKline at 1-800-825-5249.
23. Pathak DS, Davis EA, Stanford RH. Economic impact of asthma therapy with fluticasone propionate, montelukast, or zafirlukast in a managed care population. Pharmacotherapy 2002;22:166-174.
24. Stanford R, Davis A, Edwards L, Kalberg C, Rickard K. The costs and efficacy of fluticasone propionate 88 mcg twice daily and montelukast 10 mg once daily in patients needing single controller therapy. Chest 2001;120:225S.-
25. White TJ, Gothard L, Fontes CL, Juzba M, Berenbeim DM, Gilderman AM. A Retrospective Administrative Healthcare Claims Analysis to Describe and Assess Pharmaceutical and Medical Resource Utilization within the PacifiCare CA Healthplan PROJECT 2: Longitudinal Analysis of Newly Diagnosed Asthmatics. Prescription Solutions/PacifiCare Health Systems and GlaxoWellcome Inc. November 27, 2000. Information available from GlaxoSmithKline at 1-800-825-5249.
26. Egger M, Smith GD. Meta-analysis bias in location and selection of studies. BMJ 1998;316:61-66.
27. Ebell MH. Prearrest predictors of survival following inhospital cardiopulmonary resuscitation: a meta-analysis. J Fam Pract 1992;34:551-558.
28. Poynard T, Moussalli J, Ratziu V, et al. Is antiviral treatment (IFN alpha and/or ribavirin) justified in cirrhosis related to hepatitis C virus? Societe Royale Belge de Gastroenterologie. Acta Gastroenterol Belg 1998;61:431-437.
29. Egger M, Schneider M, Smith GD. Meta-analysis spurious precision? Meta-analysis of observational studies. BMJ 1998;316:140-144.
Objective To compare the effects of inhaled corticosteroid treatment with leukotriene modifier treatment on medical resource use and costs for asthma patients.
Study design Meta-analysis combining results from published and unpublished studies.
Data sources Studies were identified from the MEDLINE and EMBASE databases and the GlaxoSmithKline internal database study registers. Two independent reviewers evaluated the identified studies; studies meeting specified inclusion criteria were abstracted and summarized by meta-analysis with a random effects model.
Outcomes measured Hospitalization rate, emergency department visit rate, emergency department costs, drug costs, total asthma-related costs, and total medical care costs.
Results Patients taking inhaled corticosteroids had:
a significantly lower annual rate of hospitalization than those taking leukotriene modifiers (2.2% vs 4.3%, respectively;P<.05)
a greater decline in hospitalization rate (before vs after therapy initiation) than those taking leukotriene modifiers (decline of 2.4% vs 0.55%; P<.01)
a lower annual rate of emergency department visits than those taking leukotriene modifiers (6.2% vs 7.7%;P<.005).
lower total asthma-related medical costs than those taking leukotriene modifiers (P<.05) and a 17% reduction in overall total medical care costs (P not significant).
Conclusions Patients with asthma treated with inhaled corticosteroids had significantly fewer asthma-related hospitalizations and emergency department visits and lower total asthma-related health care costs than patients treated with leukotriene modifiers. These meta-analysis findings are consistent with results from randomized controlled trials showing improvements in lung function for patients taking inhaled corticosteroids as opposed to leukotriene modifiers.
Although many medications are available for patients with asthma, inhaled corticosteroids are generally the preferred treatment.1-4 Multiple studies have demonstrated that inhaled corticosteroid therapy improves patient outcomes.1 Inhaled corticosteroids have been shown to decrease costs5 and use of medical care resources.6-8
More recently, leukotriene modifiers have been introduced for asthma treatment. This class of medication has bronchodilator and anti-inflammatory effects.9 Although multiple studies have indicated improved outcomes and decreased costs associated with leukotriene modifier therapy incertain patient populations,10,11 its role in asthmamanagement is uncertain.9
Several studies have compared the clinical outcomes of these therapies12-15 and their impact on medical care resource use and costs.16,17 However, these studies were not powered specifically to detect significant differences in resource use or costs.
We performed a meta-analysis to (1) compare the rate of hospitalization among patients with asthma treated with inhaled corticosteroids vs those treated with leukotriene modifiers and (2) evaluate other resource use rates and costs for these patients.
Methods
The meta-analysis consisted of a literature search, the development of inclusion criteria, form development, and literature review.
Literature search
We searched the MEDLINE, EMBASE, Cochrane Collaboration Study Registry, and GlaxoSmithKline databases and consulted experts in this field. The GlaxoSmithKline database consists of studies sponsored by GlaxoSmithKline that met companywide minimum quality thresholds and were published in full or abstract form.
We also contacted the manufacturers of leukotriene modifiers available in the United States, AstraZeneca and Merck, to request published and unpublished information on studies comparing leukotriene modifiers with inhaled corticosteroids. To provide results corresponding to current treatment patterns, only studies from 1991 to 2001 were included. Published and unpublished materials were included.18,19
Inclusion criteria
Studies were included in the meta-analysis if they met the following criteria.
Population. Patients with diagnosed asthma. Only studies that did not restrict analyses to severe asthma patients or children were included.
Study design. Prospective and retrospective comparative studies of patients receiving inhaled corticosteroid or leukotriene modifier monotherapy (no other controller therapy) in the same study. Studies were required to have defined inclusion and exclusion criteria, defined number of patients in each study arm, defined treatment protocol (ie, medications and doses used), and separate results for each medication.
Only studies presenting primary research (hence excluding review articles and metaanalyses) were included. Only studies presenting data for at least 6 months on all participants were included.
Outcomes. Hospitalization visit rates and costs, emergency department visit rates and costs, pharmacy costs, total asthma-related costs, and total medical care costs. Because resource use patterns and medical care cost information differs substantially between countries, we only included US studies.
Study process
Each identified article was evaluated by 2 independent reviewers (KMS and MG); any differences were discussed with a project leader to reach a consensus. Documents selected for inclusion were then reviewed by the 2 reviewers, and differences in data abstraction were resolved before inclusion.
Analysis
We used the Q statistic20 to assess heterogeneity and, when appropriate, combined data from included studies with the use of a random effects model. Random effects methodology was used to assess the impact of inhaled corticosteroid vs leukotriene modifier therapy on the overall asthma population, not just the subpopulation of patients participating in included studies.21
Two sets of meta-analyses were performed with the abstracted data. First, the specified outcome measures were compared for patients taking inhaled corticosteroid vs leukotriene modifier therapy. Second, the impact (before vs after) of each treatment initiation was compared for each outcome.
Assessment of statistically significant differences between meta-analysis results was performed with the Student t test, with an Α of .05. Charges were used in the included studies as proxies for costs. These costs were inflated to 2000 values by using the medical care component of the consumer price index before inclusion.
Results
We identified 49 documents and reviewed them for inclusion in the meta-analysis; 6 (12.2%) met the inclusion criteria (Table 1). Five were retrospective cohort studies; only 1 study was identified as a prospective trial comparing inhaled corticosteroid and leukotriene modifier therapies and including results on resource use or medical care costs.16,17,22-25 All 6 studies were performed with support from GlaxoSmithKline.
Forty-three documents were excluded due to 1 or more of the following criteria: lack of primary results (9 documents, 21%); did not contain resource use rate or cost outcomes (22 documents, 51%); did not provide at least 6 months’ worth of data on resource use or cost outcomes (5 documents, 12%); did not meet the defined inclusion and exclusion criteria (primarily studies not including both inhaled corticosteroid and leukotriene modifiers or those restricted to patient clinical subgroups; 10 documents, 34%); or did not define the number of patients included in the study (1 document, 3%).
Because few studies presented data on the specified outcomes, we were unable to assess asthma-specific costs for subcategories of resource use. Too few studies included data on hospitalization costs (either asthma-specific or overall) to include in the analysis. Therefore, meta-analysis was performed on overall (ie, all causes) emergency department, pharmacy, and total medical care costs.
TABLE 1
Characteristics of studies included in the meta-analysis
| Duration (mo) | |||||
|---|---|---|---|---|---|
| Study | LOE* | Before therapy | After therapy | Treatment (N) | Comparison (N) |
| Oates and Gothard22 | 2b | 9 | 9 | Inhaled corticosteroids (546)† | Leukotriene modifiers (152)‡ |
| Pathak et al23 | 2b | 9 | 9 | Fluticasone propionate (284) | Leukotriene modifiers (497)‡ |
| Stanford et al24 | 1b | - | 6 | Fluticasone propionate (271) | Montelukast (262) |
| Stempel et al16 | 2b | 9 | 12 | Fluticasone propionate (602) | Zafirlukast (309) |
| Stempel et al17 | 2b | 9 | 9 | Fluticasone propionate (559) | Montelukast (382) |
| White et al25 | 2b | 9 | 9 | Inhaled corticosteroids (1305)† | Leukotriene modifiers (109)‡ |
| *LOE, level of evidence. For an explanation of levels of evidence. | |||||
| †Results were presented for all inhaled corticosteroids combined. | |||||
| ‡Results were presented for all leukotriene modifiers combined. | |||||
Primary analysis
The primary objective of this study was to evaluate the impact of inhaled corticosteroid and leukotriene modifier treatment on the mean annual hospitalization rate. Four of the 6 included studies contained information on hospitalization rate for each treatment. Results from the primary analysis are presented in Table 2.
Patients taking inhaled corticosteroids had a significantly lower annual rate of hospitalization than did patients taking leukotriene modifiers (2.23% vs 4.30%, respectively; P<.005). The absolute risk reduction was 2.07% (number needed to treat=48 for 1 year).
The difference in annual hospitalization visit rates for each study in the primary analysis is presented in the Figure, where negative values reflect lower hospitalization rates among patients taking inhaled corticosteroids than among those taking leukotriene modifiers. Two studies16,23 had statistically significant differences in hospitalization rates, whereas the differences in the other 2 studies were not statistically significant (P<.05). Combining studies with the use of a random effects model (the default methodology for this analysis) or a fixed effects model produced similar results. The Q statistic indicated no significant heterogeneity (P=.43).
TABLE 2
Meta-analysis results for inhaled corticosteroid vs leukotriene modifier therapy*
| Inhaled corticosteroid vs leukotriene modifier patients † | ||||
|---|---|---|---|---|
| Inhaled corticosteroids | Leukotriene modifiers | Absolute difference | Relative difference | |
| Annual asthma hospitalizations‡ | 2.23% (1.69-2.78) | 4.30% (3.53-5.07) | -1.79% (-2.45 to -1.14) | -42.88% (-55.95 to -29.80) |
| Annual rate of visits to the emergency department due to asthma§ | 6.19% (4.84-7.53) | 7.74% (6.30-9.19) | -1.53% (-1.78 to -1.28) | -21.35% (-25.31 to -17.38) |
| Total annual costs of visits to the emergency department | $93 (38-148) | $73 (52-94) | $21(-17 to 59) | 1.00% (-38 to 40) |
| Total annual drug costs§ | $807 (548-1065) | $1062 (812-1312) | -$258 (-308 to -208) | -27.20% (-33.2 to -21.3) |
| Annual asthma-related cost‡ | $882 (613-1150) | $1393 (1143-1643) | $513 (-392 to -634) | -38.01% (-47.4 to -28.8) |
| Total annual cost | $5254 (4474-6033) | $7140 (4970-9311) | -$1918 (-3509 to -327) | -17.20% (-30.9 to -3.5) |
| *Data are presented as mean (95% confidence interval). | ||||
| †Absolute and relative differences were determined from meta-analyses of the absolute and relative differences for each included study. | ||||
| ‡Inhaled corticosteroid vs leukotriene modifier significant at P<.05. | ||||
| §Inhaled corticosteroid vs leukotriene modifier significant at P<.005. | ||||
FIGURE
Difference in hospitalization rates (mean, confidence interval)
Secondary outcomes
Results of secondary analyses for 5 study outcomes (annual visits to the emergency department due to asthma, total emergency department costs, total drug costs, total asthma-related costs, and overall total cost) are presented in Table 2.
Mean annual rates of visits to the emergency department and total annual drug costs were significantly higher for patients taking leukotriene modifiers than for those taking inhaled corticosteroids (P<.005 for each). Patients taking leukotriene modifiers had lower annual costs for visits to the emergency department than did those taking inhaled corticosteroids, although this difference was not statistically significant. The higher rate and lower cost of emergency department visits for patients taking leukotriene modifiers suggest that medical resources were used less at each visit as compared with those for patients taking inhaled corticosteroids.
Total asthma-related costs for patients taking inhaled corticosteroids were significantly lower than those for patients taking leukotriene modifiers (P<.05). Patients taking inhaled corticosteroids also incurred decreased annual total (allcause) medical care costs. Although this difference was qualitatively large (approximately $1900, or a decrease of over 17%), it did not reach statistical significance.
Pre- vs post-initiation of therapy
In addition to comparing the impact of the 2 therapies on resource use and costs, we were interested in the impact of initiating each therapy on the study outcomes. We therefore assessed resource use rates and costs before and after treatment initiation. These patients may have been receiving asthma therapies (or no asthma therapy) other than inhaled corticosteroids or leukotriene modifiers. The number of studies presenting data on costs was too small to allow comparison.
Results of this within-group analysis are presented in Table 3. For patients taking inhaled corticosteroids, hospitalization rates and emergency department visit rates decreased significantly after treatment initiation compared with the preinitiation values (P<.005 and P<.05, respectively). The decreases for patients taking leukotriene modifiers upon treatment initiation were smaller and not statistically significant.
Both groups showed similar small decreases in annual emergency department costs, neither of which was significant. The increases in annual total drug costs and annual total medical care costs after treatment initiation were significant for both groups of patients (all at P<.005). However, both increases were greater for patients taking leukotriene modifiers; the increase in drug costs was statistically significant (P<.001).
TABLE 3
Resource utilization and costs before and after therapy initiation for inhaled corticosteroids vs leukotriene modifiers*
| Before vs after treatment, mean (95% confidence interval) | |||
|---|---|---|---|
| Inhaled corticosteroid patients | Leukotriene modifier patients | Absolute difference | |
| Annual hospitalization rate | -2.37%† (-2.89 to -1.86) | -0.55% (-1.18 to 0.08) | -1.91%‡(-2.45 to -1.36) |
| Annual rate of visits to the emergency department due to asthma | -4.44%§ (-5.98 to -2.90) | -2.06% (-3.61 to -0.51) | -2.47%||(-3.09 to -1.86) |
| Total annual costs of the emergency despartment | -$5 (-21 to 10) | -$15 (-44 to 15) | $9 (-33 to 51) |
| Total annual drug costs | $415†(312-517) | $579† (472-686) | -$167||(-192 to 142) |
| Total annual medical care costs | $641† (113-1169) | $1712† (927-3529) | -$1080 (-2802 to 643) |
| *Post-therapy initiation values for inhaled corticosteroids and leukotriene modifiers are presented in Table 1. | |||
| †Before vs after treatment initiation significant at P<.005. | |||
| ‡Before vs after treatment initiation outcomes for inhaled corticosteroids vs leukotriene modifiers significant at P<.01. | |||
| §Before vs after treatment initiation significant at P<.05. | |||
| ||Before vs after treatment initiation outcomes for inhaled corticosteroids vs leukotriene modifiers significant at P<.001. | |||
Discussion
In this study, we used meta-analysis to combine data across studies and determine more robust estimates of the impact of inhaled corticosteroid vs leukotriene modifier therapy on medical resource use rates and costs. The primary analysis indicated that annual hospitalization rates among patients taking inhaled corticosteroids are significantly lower that those taking leukotriene modifiers. Other resource use rates and costs evaluated in this study also generally showed decreased values for patients taking inhaled corticosteroids.
Although meta-analysis generally has been used for clinical outcome measures, it is a highly appropriate method for resource use and cost outcomes. In general, studies of the impact of a particular treatment are powered to assess safety and efficacy or effectiveness; there is often insufficient power to detect differences in resource use or costs in any one study. Due to substantial variation in treatment patterns, the variance associated with resource use rates (and associated costs) may be substantially higher than that for clinical measures; such a wide variance adds to the difficulties in assessing differences for nonclinical outcomes.
Limitations
This study has a number of limitations. Only a few studies met inclusion criteria for the meta-analysis; the analysis should be replicated as additional studies become available. Data were abstracted from the included studies without modification (except for inflating costs to 2000 values when necessary). As in all meta-analyses, any problems present in the original data are present in the combined data; limitations of the original data are not addressed by this method.
Among the studies evaluated for the metaanalysis were a number published only as abstracts. Inclusion of unpublished literature in meta-analyses is controversial; however, several sources18,19 now recommend inclusion of published and unpublished studies. Egger and Smith26 found that studies with significant results are more likely to be published than are studies with nonsignificant results, leading to publication bias. Studies with significant results also may be more likely to be published in indexed journals, leading to “database bias.” As such, inclusion of unpublished studies is important to produce unbiased results.
Five of the 6 studies that met the inclusion criteria were observational, retrospective cohort analyses. Whereas many meta-analyses focus solely on prospective, randomized clinical trials, several have included retrospective data.27,28 Retrospective analyses and observational data have a number of limitations, in particular the lack of randomization that can lead to differences in characteristics of specified treatment groups. Further, as discussed by Egger et al,29 metaanalyses based on observational studies may involve bias and confounding.
However, observational data and retrospective analyses also have the advantage of reflecting “real world” treatment patterns and broader patient groups that increase the generalizability of the data, whereas clinical trials may include protocol-driven utilization and selected patient groups. Clinical trials also may occur in specialized health care settings, whereas observational (cohort) data may be more applicable to clinical practice. Due to these factors, meta-analysis of observational data has become common.29
The pre-initiation vs post-initiation analysis indicated that values for each treatment group provide information on the similarities between treatment groups before initiation of controller therapy. Even though the treatment groups were not randomized to each therapy and we have no means to ensure compatibility between groups, having similar rates of resource use between groups provides some evidence regarding similarity. Nonetheless, given the limitations of the retrospective data and meta-analysis in general, it will be important to validate the results of this meta-analysis in the future with naturalistic prospective studies.
Despite these limitations, this study provides important information on the impact of asthma therapies on resource use and costs. Specifically, the resource use and cost outcomes assessed in this study were lower for inhaled corticosteroid patients than for leukotriene modifier patients. This study also illustrates the usefulness of metaanalysis in evaluating resource use and costs. By selecting and combining outcomes across studies in a standardized, rigorous, and transparent manner, the effects of different therapies can be evaluated with greater precision.
Acknowledgments
We thank John O’Donnell and Layne Gothard for their assistance with this manuscript.
Corresponding author
Michael T. Halpern, MD, PhD, Principal Scientist, Exponent, Inc., 1800 Diagonal Road, Alexandria, VA 22314. E-mail: [email protected]
Objective To compare the effects of inhaled corticosteroid treatment with leukotriene modifier treatment on medical resource use and costs for asthma patients.
Study design Meta-analysis combining results from published and unpublished studies.
Data sources Studies were identified from the MEDLINE and EMBASE databases and the GlaxoSmithKline internal database study registers. Two independent reviewers evaluated the identified studies; studies meeting specified inclusion criteria were abstracted and summarized by meta-analysis with a random effects model.
Outcomes measured Hospitalization rate, emergency department visit rate, emergency department costs, drug costs, total asthma-related costs, and total medical care costs.
Results Patients taking inhaled corticosteroids had:
a significantly lower annual rate of hospitalization than those taking leukotriene modifiers (2.2% vs 4.3%, respectively;P<.05)
a greater decline in hospitalization rate (before vs after therapy initiation) than those taking leukotriene modifiers (decline of 2.4% vs 0.55%; P<.01)
a lower annual rate of emergency department visits than those taking leukotriene modifiers (6.2% vs 7.7%;P<.005).
lower total asthma-related medical costs than those taking leukotriene modifiers (P<.05) and a 17% reduction in overall total medical care costs (P not significant).
Conclusions Patients with asthma treated with inhaled corticosteroids had significantly fewer asthma-related hospitalizations and emergency department visits and lower total asthma-related health care costs than patients treated with leukotriene modifiers. These meta-analysis findings are consistent with results from randomized controlled trials showing improvements in lung function for patients taking inhaled corticosteroids as opposed to leukotriene modifiers.
Although many medications are available for patients with asthma, inhaled corticosteroids are generally the preferred treatment.1-4 Multiple studies have demonstrated that inhaled corticosteroid therapy improves patient outcomes.1 Inhaled corticosteroids have been shown to decrease costs5 and use of medical care resources.6-8
More recently, leukotriene modifiers have been introduced for asthma treatment. This class of medication has bronchodilator and anti-inflammatory effects.9 Although multiple studies have indicated improved outcomes and decreased costs associated with leukotriene modifier therapy incertain patient populations,10,11 its role in asthmamanagement is uncertain.9
Several studies have compared the clinical outcomes of these therapies12-15 and their impact on medical care resource use and costs.16,17 However, these studies were not powered specifically to detect significant differences in resource use or costs.
We performed a meta-analysis to (1) compare the rate of hospitalization among patients with asthma treated with inhaled corticosteroids vs those treated with leukotriene modifiers and (2) evaluate other resource use rates and costs for these patients.
Methods
The meta-analysis consisted of a literature search, the development of inclusion criteria, form development, and literature review.
Literature search
We searched the MEDLINE, EMBASE, Cochrane Collaboration Study Registry, and GlaxoSmithKline databases and consulted experts in this field. The GlaxoSmithKline database consists of studies sponsored by GlaxoSmithKline that met companywide minimum quality thresholds and were published in full or abstract form.
We also contacted the manufacturers of leukotriene modifiers available in the United States, AstraZeneca and Merck, to request published and unpublished information on studies comparing leukotriene modifiers with inhaled corticosteroids. To provide results corresponding to current treatment patterns, only studies from 1991 to 2001 were included. Published and unpublished materials were included.18,19
Inclusion criteria
Studies were included in the meta-analysis if they met the following criteria.
Population. Patients with diagnosed asthma. Only studies that did not restrict analyses to severe asthma patients or children were included.
Study design. Prospective and retrospective comparative studies of patients receiving inhaled corticosteroid or leukotriene modifier monotherapy (no other controller therapy) in the same study. Studies were required to have defined inclusion and exclusion criteria, defined number of patients in each study arm, defined treatment protocol (ie, medications and doses used), and separate results for each medication.
Only studies presenting primary research (hence excluding review articles and metaanalyses) were included. Only studies presenting data for at least 6 months on all participants were included.
Outcomes. Hospitalization visit rates and costs, emergency department visit rates and costs, pharmacy costs, total asthma-related costs, and total medical care costs. Because resource use patterns and medical care cost information differs substantially between countries, we only included US studies.
Study process
Each identified article was evaluated by 2 independent reviewers (KMS and MG); any differences were discussed with a project leader to reach a consensus. Documents selected for inclusion were then reviewed by the 2 reviewers, and differences in data abstraction were resolved before inclusion.
Analysis
We used the Q statistic20 to assess heterogeneity and, when appropriate, combined data from included studies with the use of a random effects model. Random effects methodology was used to assess the impact of inhaled corticosteroid vs leukotriene modifier therapy on the overall asthma population, not just the subpopulation of patients participating in included studies.21
Two sets of meta-analyses were performed with the abstracted data. First, the specified outcome measures were compared for patients taking inhaled corticosteroid vs leukotriene modifier therapy. Second, the impact (before vs after) of each treatment initiation was compared for each outcome.
Assessment of statistically significant differences between meta-analysis results was performed with the Student t test, with an Α of .05. Charges were used in the included studies as proxies for costs. These costs were inflated to 2000 values by using the medical care component of the consumer price index before inclusion.
Results
We identified 49 documents and reviewed them for inclusion in the meta-analysis; 6 (12.2%) met the inclusion criteria (Table 1). Five were retrospective cohort studies; only 1 study was identified as a prospective trial comparing inhaled corticosteroid and leukotriene modifier therapies and including results on resource use or medical care costs.16,17,22-25 All 6 studies were performed with support from GlaxoSmithKline.
Forty-three documents were excluded due to 1 or more of the following criteria: lack of primary results (9 documents, 21%); did not contain resource use rate or cost outcomes (22 documents, 51%); did not provide at least 6 months’ worth of data on resource use or cost outcomes (5 documents, 12%); did not meet the defined inclusion and exclusion criteria (primarily studies not including both inhaled corticosteroid and leukotriene modifiers or those restricted to patient clinical subgroups; 10 documents, 34%); or did not define the number of patients included in the study (1 document, 3%).
Because few studies presented data on the specified outcomes, we were unable to assess asthma-specific costs for subcategories of resource use. Too few studies included data on hospitalization costs (either asthma-specific or overall) to include in the analysis. Therefore, meta-analysis was performed on overall (ie, all causes) emergency department, pharmacy, and total medical care costs.
TABLE 1
Characteristics of studies included in the meta-analysis
| Duration (mo) | |||||
|---|---|---|---|---|---|
| Study | LOE* | Before therapy | After therapy | Treatment (N) | Comparison (N) |
| Oates and Gothard22 | 2b | 9 | 9 | Inhaled corticosteroids (546)† | Leukotriene modifiers (152)‡ |
| Pathak et al23 | 2b | 9 | 9 | Fluticasone propionate (284) | Leukotriene modifiers (497)‡ |
| Stanford et al24 | 1b | - | 6 | Fluticasone propionate (271) | Montelukast (262) |
| Stempel et al16 | 2b | 9 | 12 | Fluticasone propionate (602) | Zafirlukast (309) |
| Stempel et al17 | 2b | 9 | 9 | Fluticasone propionate (559) | Montelukast (382) |
| White et al25 | 2b | 9 | 9 | Inhaled corticosteroids (1305)† | Leukotriene modifiers (109)‡ |
| *LOE, level of evidence. For an explanation of levels of evidence. | |||||
| †Results were presented for all inhaled corticosteroids combined. | |||||
| ‡Results were presented for all leukotriene modifiers combined. | |||||
Primary analysis
The primary objective of this study was to evaluate the impact of inhaled corticosteroid and leukotriene modifier treatment on the mean annual hospitalization rate. Four of the 6 included studies contained information on hospitalization rate for each treatment. Results from the primary analysis are presented in Table 2.
Patients taking inhaled corticosteroids had a significantly lower annual rate of hospitalization than did patients taking leukotriene modifiers (2.23% vs 4.30%, respectively; P<.005). The absolute risk reduction was 2.07% (number needed to treat=48 for 1 year).
The difference in annual hospitalization visit rates for each study in the primary analysis is presented in the Figure, where negative values reflect lower hospitalization rates among patients taking inhaled corticosteroids than among those taking leukotriene modifiers. Two studies16,23 had statistically significant differences in hospitalization rates, whereas the differences in the other 2 studies were not statistically significant (P<.05). Combining studies with the use of a random effects model (the default methodology for this analysis) or a fixed effects model produced similar results. The Q statistic indicated no significant heterogeneity (P=.43).
TABLE 2
Meta-analysis results for inhaled corticosteroid vs leukotriene modifier therapy*
| Inhaled corticosteroid vs leukotriene modifier patients † | ||||
|---|---|---|---|---|
| Inhaled corticosteroids | Leukotriene modifiers | Absolute difference | Relative difference | |
| Annual asthma hospitalizations‡ | 2.23% (1.69-2.78) | 4.30% (3.53-5.07) | -1.79% (-2.45 to -1.14) | -42.88% (-55.95 to -29.80) |
| Annual rate of visits to the emergency department due to asthma§ | 6.19% (4.84-7.53) | 7.74% (6.30-9.19) | -1.53% (-1.78 to -1.28) | -21.35% (-25.31 to -17.38) |
| Total annual costs of visits to the emergency department | $93 (38-148) | $73 (52-94) | $21(-17 to 59) | 1.00% (-38 to 40) |
| Total annual drug costs§ | $807 (548-1065) | $1062 (812-1312) | -$258 (-308 to -208) | -27.20% (-33.2 to -21.3) |
| Annual asthma-related cost‡ | $882 (613-1150) | $1393 (1143-1643) | $513 (-392 to -634) | -38.01% (-47.4 to -28.8) |
| Total annual cost | $5254 (4474-6033) | $7140 (4970-9311) | -$1918 (-3509 to -327) | -17.20% (-30.9 to -3.5) |
| *Data are presented as mean (95% confidence interval). | ||||
| †Absolute and relative differences were determined from meta-analyses of the absolute and relative differences for each included study. | ||||
| ‡Inhaled corticosteroid vs leukotriene modifier significant at P<.05. | ||||
| §Inhaled corticosteroid vs leukotriene modifier significant at P<.005. | ||||
FIGURE
Difference in hospitalization rates (mean, confidence interval)
Secondary outcomes
Results of secondary analyses for 5 study outcomes (annual visits to the emergency department due to asthma, total emergency department costs, total drug costs, total asthma-related costs, and overall total cost) are presented in Table 2.
Mean annual rates of visits to the emergency department and total annual drug costs were significantly higher for patients taking leukotriene modifiers than for those taking inhaled corticosteroids (P<.005 for each). Patients taking leukotriene modifiers had lower annual costs for visits to the emergency department than did those taking inhaled corticosteroids, although this difference was not statistically significant. The higher rate and lower cost of emergency department visits for patients taking leukotriene modifiers suggest that medical resources were used less at each visit as compared with those for patients taking inhaled corticosteroids.
Total asthma-related costs for patients taking inhaled corticosteroids were significantly lower than those for patients taking leukotriene modifiers (P<.05). Patients taking inhaled corticosteroids also incurred decreased annual total (allcause) medical care costs. Although this difference was qualitatively large (approximately $1900, or a decrease of over 17%), it did not reach statistical significance.
Pre- vs post-initiation of therapy
In addition to comparing the impact of the 2 therapies on resource use and costs, we were interested in the impact of initiating each therapy on the study outcomes. We therefore assessed resource use rates and costs before and after treatment initiation. These patients may have been receiving asthma therapies (or no asthma therapy) other than inhaled corticosteroids or leukotriene modifiers. The number of studies presenting data on costs was too small to allow comparison.
Results of this within-group analysis are presented in Table 3. For patients taking inhaled corticosteroids, hospitalization rates and emergency department visit rates decreased significantly after treatment initiation compared with the preinitiation values (P<.005 and P<.05, respectively). The decreases for patients taking leukotriene modifiers upon treatment initiation were smaller and not statistically significant.
Both groups showed similar small decreases in annual emergency department costs, neither of which was significant. The increases in annual total drug costs and annual total medical care costs after treatment initiation were significant for both groups of patients (all at P<.005). However, both increases were greater for patients taking leukotriene modifiers; the increase in drug costs was statistically significant (P<.001).
TABLE 3
Resource utilization and costs before and after therapy initiation for inhaled corticosteroids vs leukotriene modifiers*
| Before vs after treatment, mean (95% confidence interval) | |||
|---|---|---|---|
| Inhaled corticosteroid patients | Leukotriene modifier patients | Absolute difference | |
| Annual hospitalization rate | -2.37%† (-2.89 to -1.86) | -0.55% (-1.18 to 0.08) | -1.91%‡(-2.45 to -1.36) |
| Annual rate of visits to the emergency department due to asthma | -4.44%§ (-5.98 to -2.90) | -2.06% (-3.61 to -0.51) | -2.47%||(-3.09 to -1.86) |
| Total annual costs of the emergency despartment | -$5 (-21 to 10) | -$15 (-44 to 15) | $9 (-33 to 51) |
| Total annual drug costs | $415†(312-517) | $579† (472-686) | -$167||(-192 to 142) |
| Total annual medical care costs | $641† (113-1169) | $1712† (927-3529) | -$1080 (-2802 to 643) |
| *Post-therapy initiation values for inhaled corticosteroids and leukotriene modifiers are presented in Table 1. | |||
| †Before vs after treatment initiation significant at P<.005. | |||
| ‡Before vs after treatment initiation outcomes for inhaled corticosteroids vs leukotriene modifiers significant at P<.01. | |||
| §Before vs after treatment initiation significant at P<.05. | |||
| ||Before vs after treatment initiation outcomes for inhaled corticosteroids vs leukotriene modifiers significant at P<.001. | |||
Discussion
In this study, we used meta-analysis to combine data across studies and determine more robust estimates of the impact of inhaled corticosteroid vs leukotriene modifier therapy on medical resource use rates and costs. The primary analysis indicated that annual hospitalization rates among patients taking inhaled corticosteroids are significantly lower that those taking leukotriene modifiers. Other resource use rates and costs evaluated in this study also generally showed decreased values for patients taking inhaled corticosteroids.
Although meta-analysis generally has been used for clinical outcome measures, it is a highly appropriate method for resource use and cost outcomes. In general, studies of the impact of a particular treatment are powered to assess safety and efficacy or effectiveness; there is often insufficient power to detect differences in resource use or costs in any one study. Due to substantial variation in treatment patterns, the variance associated with resource use rates (and associated costs) may be substantially higher than that for clinical measures; such a wide variance adds to the difficulties in assessing differences for nonclinical outcomes.
Limitations
This study has a number of limitations. Only a few studies met inclusion criteria for the meta-analysis; the analysis should be replicated as additional studies become available. Data were abstracted from the included studies without modification (except for inflating costs to 2000 values when necessary). As in all meta-analyses, any problems present in the original data are present in the combined data; limitations of the original data are not addressed by this method.
Among the studies evaluated for the metaanalysis were a number published only as abstracts. Inclusion of unpublished literature in meta-analyses is controversial; however, several sources18,19 now recommend inclusion of published and unpublished studies. Egger and Smith26 found that studies with significant results are more likely to be published than are studies with nonsignificant results, leading to publication bias. Studies with significant results also may be more likely to be published in indexed journals, leading to “database bias.” As such, inclusion of unpublished studies is important to produce unbiased results.
Five of the 6 studies that met the inclusion criteria were observational, retrospective cohort analyses. Whereas many meta-analyses focus solely on prospective, randomized clinical trials, several have included retrospective data.27,28 Retrospective analyses and observational data have a number of limitations, in particular the lack of randomization that can lead to differences in characteristics of specified treatment groups. Further, as discussed by Egger et al,29 metaanalyses based on observational studies may involve bias and confounding.
However, observational data and retrospective analyses also have the advantage of reflecting “real world” treatment patterns and broader patient groups that increase the generalizability of the data, whereas clinical trials may include protocol-driven utilization and selected patient groups. Clinical trials also may occur in specialized health care settings, whereas observational (cohort) data may be more applicable to clinical practice. Due to these factors, meta-analysis of observational data has become common.29
The pre-initiation vs post-initiation analysis indicated that values for each treatment group provide information on the similarities between treatment groups before initiation of controller therapy. Even though the treatment groups were not randomized to each therapy and we have no means to ensure compatibility between groups, having similar rates of resource use between groups provides some evidence regarding similarity. Nonetheless, given the limitations of the retrospective data and meta-analysis in general, it will be important to validate the results of this meta-analysis in the future with naturalistic prospective studies.
Despite these limitations, this study provides important information on the impact of asthma therapies on resource use and costs. Specifically, the resource use and cost outcomes assessed in this study were lower for inhaled corticosteroid patients than for leukotriene modifier patients. This study also illustrates the usefulness of metaanalysis in evaluating resource use and costs. By selecting and combining outcomes across studies in a standardized, rigorous, and transparent manner, the effects of different therapies can be evaluated with greater precision.
Acknowledgments
We thank John O’Donnell and Layne Gothard for their assistance with this manuscript.
Corresponding author
Michael T. Halpern, MD, PhD, Principal Scientist, Exponent, Inc., 1800 Diagonal Road, Alexandria, VA 22314. E-mail: [email protected]
1. Janson S. National asthma education and prevention program, expert panel report. II: overview and application to primary care. Prim Care Pract 1998;2:578-588.
2. Lalloo UG, Bateman ED, Feldman C, et al. Guideline for the management of chronic asthma in adults—2000 update. South African Pulmonology Society Adult Asthma Working Group. S Afr Med J 2000;90:540-541-544-552.
3. Podell RN. National guidelines for the management of asthma in adults. Am Fam Physician 1992;46:1189-1196.
4. Veninga CC, Lagerlov P, Wahlstrom R, et al. Evaluating an educational intervention to improve the treatment of asthma in four European countries. Drug Education Project Group. Am J Respir Crit Care Med 1999;160:1254-1262.
5. Ozminkowski RJ, Wang S, Marder WD, Azzolini J, Schutt D. Cost implications for the use of inhaled anti-inflammatory medications in the treatment of asthma. Pharmacoeconomics 2000;18:253-264.
6. Paltiel AD, Fuhlbrigge AL, Kitch BT, et al. Cost-effectiveness of inhaled corticosteroids in adults with mild-to-moderate asthma: results from the asthma policy model. J Allergy Clin Immunol 2001;108:39-49.
7. Adams RJ, Fuhlbrigge A, Finkelstein JA, et al. Impact of inhaled antiinflammatory therapy on hospitalization and emergency department visits for children with asthma. Pediatrics 2001;107:706-711.
8. Donahue JG, Weiss ST, Livingston JM, Goetsch MA, Greineder DK, Platt R. Inhaled steroids and the risk of hospitalization for asthma. JAMA 1997;277:887-891.
9. Dempsey OJ. Leukotriene receptor antagonist therapy. Postgrad Med J 2000;76:767-773.
10. Klingman D, Bielory L, Wang Y, et al. Asthma outcome changes associated with use of the leukotriene-receptor antagonist zafirlukast. Manag Care Interface 2001;14:62-66.
11. Price DB, Ben-Joseph RH, Zhang Q. Changes in asthma drug therapy costs for patients receiving chronic montelukast therapy in the U.K. Resp Med 2001;95:83-89.
12. Bleecker ER, Welch MJ, Weinstein SF, et al. Low-dose inhaled fluticasone propionate versus oral zafirlukast in the treatment of persistent asthma. J Allergy Clin Immunol 2000;105:1123-1129.
13. Ind PW. Inhaled corticosteroids versus anti-leukotrienes: a literature review on the clinical effects. Allergy 1999;54(suppl 50):43-46.
14. Malmstrom K, Rodriguez-Gomez G, Guerra J, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med 1999;130:487-495.
15. Busse W, Nelson H, Wolfe J, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. J Allergy Clin Immunol 1999;103:1075-1080.
16. Stempel DA, Meyer JW, Stanford RH, Yancey SW. One-year claims analysis comparing inhaled fluticasone propionate with zafirlukast for the treatment of asthma. J Allergy Clin Immunol 2001;107:94-98.
17. Stempel DA, Mauskopf J, McLaughlin T, Yazdani C, Stanford RH. Comparison of asthma costs in patients starting fluticasone propionate compared to patients starting montelukast. Respir Med 2001;95:227-234.
18. Cook DJ, Guyatt GH, Ryan G, et al. Should unpublished data be included in meta-analyses? Current convictions and controversies. JAMA 1993;269:2749-2753.
19. McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 2000;356:1228-1231.
20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-188.
21. Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care 1990;6:5-30.
22. Oates V, Gothard L. PEER Study: New Starts on Inhaled Corticosteroids or Leukotriene Modifiers in an Asthmatic Population. Advanced Paradigm and GlaxoWellcome, Inc. July 19, 2000. Information available from GlaxoSmithKline at 1-800-825-5249.
23. Pathak DS, Davis EA, Stanford RH. Economic impact of asthma therapy with fluticasone propionate, montelukast, or zafirlukast in a managed care population. Pharmacotherapy 2002;22:166-174.
24. Stanford R, Davis A, Edwards L, Kalberg C, Rickard K. The costs and efficacy of fluticasone propionate 88 mcg twice daily and montelukast 10 mg once daily in patients needing single controller therapy. Chest 2001;120:225S.-
25. White TJ, Gothard L, Fontes CL, Juzba M, Berenbeim DM, Gilderman AM. A Retrospective Administrative Healthcare Claims Analysis to Describe and Assess Pharmaceutical and Medical Resource Utilization within the PacifiCare CA Healthplan PROJECT 2: Longitudinal Analysis of Newly Diagnosed Asthmatics. Prescription Solutions/PacifiCare Health Systems and GlaxoWellcome Inc. November 27, 2000. Information available from GlaxoSmithKline at 1-800-825-5249.
26. Egger M, Smith GD. Meta-analysis bias in location and selection of studies. BMJ 1998;316:61-66.
27. Ebell MH. Prearrest predictors of survival following inhospital cardiopulmonary resuscitation: a meta-analysis. J Fam Pract 1992;34:551-558.
28. Poynard T, Moussalli J, Ratziu V, et al. Is antiviral treatment (IFN alpha and/or ribavirin) justified in cirrhosis related to hepatitis C virus? Societe Royale Belge de Gastroenterologie. Acta Gastroenterol Belg 1998;61:431-437.
29. Egger M, Schneider M, Smith GD. Meta-analysis spurious precision? Meta-analysis of observational studies. BMJ 1998;316:140-144.
1. Janson S. National asthma education and prevention program, expert panel report. II: overview and application to primary care. Prim Care Pract 1998;2:578-588.
2. Lalloo UG, Bateman ED, Feldman C, et al. Guideline for the management of chronic asthma in adults—2000 update. South African Pulmonology Society Adult Asthma Working Group. S Afr Med J 2000;90:540-541-544-552.
3. Podell RN. National guidelines for the management of asthma in adults. Am Fam Physician 1992;46:1189-1196.
4. Veninga CC, Lagerlov P, Wahlstrom R, et al. Evaluating an educational intervention to improve the treatment of asthma in four European countries. Drug Education Project Group. Am J Respir Crit Care Med 1999;160:1254-1262.
5. Ozminkowski RJ, Wang S, Marder WD, Azzolini J, Schutt D. Cost implications for the use of inhaled anti-inflammatory medications in the treatment of asthma. Pharmacoeconomics 2000;18:253-264.
6. Paltiel AD, Fuhlbrigge AL, Kitch BT, et al. Cost-effectiveness of inhaled corticosteroids in adults with mild-to-moderate asthma: results from the asthma policy model. J Allergy Clin Immunol 2001;108:39-49.
7. Adams RJ, Fuhlbrigge A, Finkelstein JA, et al. Impact of inhaled antiinflammatory therapy on hospitalization and emergency department visits for children with asthma. Pediatrics 2001;107:706-711.
8. Donahue JG, Weiss ST, Livingston JM, Goetsch MA, Greineder DK, Platt R. Inhaled steroids and the risk of hospitalization for asthma. JAMA 1997;277:887-891.
9. Dempsey OJ. Leukotriene receptor antagonist therapy. Postgrad Med J 2000;76:767-773.
10. Klingman D, Bielory L, Wang Y, et al. Asthma outcome changes associated with use of the leukotriene-receptor antagonist zafirlukast. Manag Care Interface 2001;14:62-66.
11. Price DB, Ben-Joseph RH, Zhang Q. Changes in asthma drug therapy costs for patients receiving chronic montelukast therapy in the U.K. Resp Med 2001;95:83-89.
12. Bleecker ER, Welch MJ, Weinstein SF, et al. Low-dose inhaled fluticasone propionate versus oral zafirlukast in the treatment of persistent asthma. J Allergy Clin Immunol 2000;105:1123-1129.
13. Ind PW. Inhaled corticosteroids versus anti-leukotrienes: a literature review on the clinical effects. Allergy 1999;54(suppl 50):43-46.
14. Malmstrom K, Rodriguez-Gomez G, Guerra J, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med 1999;130:487-495.
15. Busse W, Nelson H, Wolfe J, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. J Allergy Clin Immunol 1999;103:1075-1080.
16. Stempel DA, Meyer JW, Stanford RH, Yancey SW. One-year claims analysis comparing inhaled fluticasone propionate with zafirlukast for the treatment of asthma. J Allergy Clin Immunol 2001;107:94-98.
17. Stempel DA, Mauskopf J, McLaughlin T, Yazdani C, Stanford RH. Comparison of asthma costs in patients starting fluticasone propionate compared to patients starting montelukast. Respir Med 2001;95:227-234.
18. Cook DJ, Guyatt GH, Ryan G, et al. Should unpublished data be included in meta-analyses? Current convictions and controversies. JAMA 1993;269:2749-2753.
19. McAuley L, Pham B, Tugwell P, Moher D. Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 2000;356:1228-1231.
20. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-188.
21. Laird NM, Mosteller F. Some statistical methods for combining experimental results. Int J Technol Assess Health Care 1990;6:5-30.
22. Oates V, Gothard L. PEER Study: New Starts on Inhaled Corticosteroids or Leukotriene Modifiers in an Asthmatic Population. Advanced Paradigm and GlaxoWellcome, Inc. July 19, 2000. Information available from GlaxoSmithKline at 1-800-825-5249.
23. Pathak DS, Davis EA, Stanford RH. Economic impact of asthma therapy with fluticasone propionate, montelukast, or zafirlukast in a managed care population. Pharmacotherapy 2002;22:166-174.
24. Stanford R, Davis A, Edwards L, Kalberg C, Rickard K. The costs and efficacy of fluticasone propionate 88 mcg twice daily and montelukast 10 mg once daily in patients needing single controller therapy. Chest 2001;120:225S.-
25. White TJ, Gothard L, Fontes CL, Juzba M, Berenbeim DM, Gilderman AM. A Retrospective Administrative Healthcare Claims Analysis to Describe and Assess Pharmaceutical and Medical Resource Utilization within the PacifiCare CA Healthplan PROJECT 2: Longitudinal Analysis of Newly Diagnosed Asthmatics. Prescription Solutions/PacifiCare Health Systems and GlaxoWellcome Inc. November 27, 2000. Information available from GlaxoSmithKline at 1-800-825-5249.
26. Egger M, Smith GD. Meta-analysis bias in location and selection of studies. BMJ 1998;316:61-66.
27. Ebell MH. Prearrest predictors of survival following inhospital cardiopulmonary resuscitation: a meta-analysis. J Fam Pract 1992;34:551-558.
28. Poynard T, Moussalli J, Ratziu V, et al. Is antiviral treatment (IFN alpha and/or ribavirin) justified in cirrhosis related to hepatitis C virus? Societe Royale Belge de Gastroenterologie. Acta Gastroenterol Belg 1998;61:431-437.
29. Egger M, Schneider M, Smith GD. Meta-analysis spurious precision? Meta-analysis of observational studies. BMJ 1998;316:140-144.
Remote diagnosis of cervical neoplasia: 2 types of telecolposcopy compared with cervicography
- Computer-based telecolposcopy and network telecolposcopy detected more cervical neoplasia than cervicography.
- Computer-based telecolposcopy could provide many women with greater access to expert diagnostic services.
Telemedicine enables doctors in rural areas or areas with poor medical service to consult with experts at distant locations. Telecolposcopy and cervicography both enable remote diagnoses of the cervix. The 2 methods differ in equipment, operations, image format, timeliness of consultation, and probably cost. However, these diagnostic approaches have not been compared previously. The purpose of this study was to compare the accuracy of telecolposcopy and cervicography with on-site colposcopy in the remote evaluation of women with potential cervical neoplasia.
Telecolposcopy and cervicography
Telecolposcopy involves a distant expert colposcopist’s evaluation of women with potential lower genital tract neoplasia.1 Existing telemedicine network and computer systems provide an audiovisual interface between local colposcopists and expert colposcopists at other locations.2 For health systems already using computer or video networks, telecolposcopic consultation can be implemented with only small additional charges per examination.2 Telecolposcopy services may improve health care access for women in medically underserved areas.1
Cervicography is distant evaluation of 2 photographs taken of the cervix following 5% acetic acid application.3 A special 35-mm camera is used to take these images. The end product, developed at a central processing center, resembles a low-magnification colposcopic photograph. Certified evaluators interpret these images, classifying them as negative, atypical, or positive. Cervicography is used primarily as an adjunct test to the Papanicolaou (Pap) smear.4 It has also been evaluated as an intermediate triage test for evaluating women with mildly abnormal Pap smear results.5-8
Methods
Women aged 18 years or older who came to 1 of 2 rural clinic sites for a colposcopic examination were enrolled in the trial after signing an institutional review board–approved informed consent document. We included women with a recent abnormal Pap smear report or a lower genital tract finding that required further evaluation by colposcopy. The exclusion criteria were pregnancy, severe cervicitis, heavy menses, refusal to participate, or technical problems with the telecolposcopy or cervicography equipment.
Both clinics were part of the Medical College of Georgia Telemedicine Network. This system uses sophisticated telecommunications equipment to provide distant consultation services to clinicians practicing in rural areas of the state.1 Small change-coupled device cameras were attached to the colposcopes at the 2 clinics.
For network telecolposcopy, images were transmitted using the network’s existing hardware and high-speed telecommunication lines. For computer telecolposcopy, personal computers (DIMS, DenVu, Tucson, Ariz) were also used to capture and transmit images to a computer at the Telemedicine Center. These digitized images were transmitted by modem via telephone lines.2 Cerviscopes (35-mm cameras) supplied by the manufacturer (NTL Worldwide, Fenton, Mo) were used to acquire cervigrams (photographs).
Pertinent clinicians received appropriate training to take cervigrams. Certified evaluators interpreted the images according to company protocol and returned a standardized report to the investigators at a later date.
Study design
The study design has been described in detail previously.1,2 Briefly, subjects were initially examined by 1 of 3 on-site, university-based expert colposcopists, who took 2 cervigrams of each patient, and then conducted a colposcopic examination independently.
A local clinician then completed another colposcopic examination, including histologic sampling, if indicated. This examination was observed simultaneously by another expert at a telemedicine center. Prior to obtaining histologic samples or using dilute Lugol’s iodine solution, the local clinician captured 2 cervical images (low and high magnification) using the computer telemedicine system. These images were then transmitted to the expert at the telemedicine center for independent interpretation.
A third expert colposcopist interpreted the video and computer images at a later time. However, these third interpretations were not considered in this report. Colposcopists were blinded to each other’s clinical diagnoses. However, all colposcopists were informed of the subject’s referral cervical cytology results and other pertinent history.
Data analysis
Each subject had 2 observations using each of the 3 colposcopy methods (on-site, network, and computer-based), and a single observation using cervicography. On-site colposcopy, consisting of the observations of the on-site expert and local colposcopist, was considered for reference purposes. Agreement with histologic results was calculated for each method, across all histologic diagnoses together and separately by diagnosis.
Sensitivity and specificity estimates were calculated using 2 definitions of disease: (1) normal versus any other histologic diagnosis, and (2) normal or cervical intraepithelial neoplasia 1 (CIN 1) versus any more severe diagnosis. The primary analysis model was complete block analysis of variance, with subjects included as blocks in the analysis to account for the multiple observations on the same subjects. Nonparametric comparisons of proportions of agreement with histology, sensitivity, and specificity among the methods were made using permutation tests. Post-hoc comparisons were made using a Tukey test; 95% confidence intervals (CIs) were calculated for all point estimates. Adjustment for dependence among multiple observations per subject was made by basing these tests and CIs on least-squares means.
The available sample sizes for all analyses were adequate to ensure approximate normality of the estimated means. Power to detect, at Α=.05, a difference in agreement of 15% between cervigram and the other evaluation methods, was estimated using Monte Carlo simulations. Data were simulated using the observed levels of agreement for on-site, network, and computer telecolposcopy, and specifying a difference of 15% between cervicography agreement and the maximum of the other methods’ agreement. Power estimates were based on analysis of 1000 simulations. SAS release 8.02 was used for all calculations (SAS, Inc, Cary, NC).
Results
A total of 264 subjects were enrolled in the trial, but the total number of subjects considered differed depending on the various analyses of interest. The demographic data of this study cohort have been published previously.1
Briefly, the subjects’ mean age was 31.7 years and mean parity was 2.1. Subjects presented with a wide range of prior cervical cytology results: 20.4% normal, 29.2% atypical squamous cells of undetermined significance, 40.4% low-grade squamous intraepithelial lesion, 7.3% high-grade squamous intraepithelial lesion, and 2.7% atypical glandular cells of undetermined significance. Histology results included all levels of CIN (52.9% CIN 1 and 13.4% CIN 2 or 3), and endocervical histologic sampling results were reported as both positive and negative for neoplasia.
The agreement between telecolposcopic/cervicography impressions and histology were estimated (Table 1). Data for on-site colposcopy was also considered for reference purposes.
When all histologic diagnoses were considered, there was no statistically significant difference in the rates of agreement for colposcopy, the 2 types of telecolposcopy, and cervicography. This was also true if only cases of CIN 1 were examined.
However, a statistically significant difference was noted between agreement rates for computer-based telecolposcopy (63.95%) and on-site colposcopy (47.7%, P=.03, Tukey test) for normal histology. A statistically significant difference was also found between agreement rates for on-site colposcopy (50.0%) and cervicography (19.1%, P=.04, Tukey test) for women with biopsy-proven CIN 2 or 3. If all histologic diagnoses were considered, the study provided 85% power to detect a difference in agreement of 15% among the evaluation methods.
We also estimated the sensitivity and specificity of the four diagnostic methods to detect cervical neoplasia (Table 2). A statistically significant difference was found in observed sensitivity between on-site colposcopy (47.7%) and cervicography (18.2%, P=.04, Tukey test) when a positive threshold of at least CIN 2 was considered. The difference was not significant, however, if the lower positive test threshold of at least CIN 1 was considered.
A statistically significant difference in specificity was noted between computer-based telecolposcopy (64.0%) and on-site colposcopy (47.7%, P=.03, Tukey test) at a positive threshold of at least CIN 1. The study provided a power of 71% and 60% to detect differences of 15% in sensitivity and specificity, respectively, using the CIN 1 threshold. Using CIN 2 as the positive threshold, the power to detect this 15% difference was 24% and 81% for sensitivity and specificity, respectively.
TABLE 1
Colposcopic, telecolposcopic, and cervicographic agreement with histology
| Histologya | On-site colposcopyb | Network telecolposcopyc | Computer-based telecolposcopyd | Cervicographye | Pf |
|---|---|---|---|---|---|
| All diagnoses | |||||
| % | 56.9 | 53.5 | 55.5 | 52.4 | .66 |
| n/Ng | 165/290 | 155/290 | 161/290 | 76/145 | |
| 95% CIh | 52.0–61.8 | 48.5–58.3 | 50.6–60.4 | 45.5–59.4 | |
| Normal | |||||
| % | 47.7 | 48.8 | 63.95 | 58.1 | .03I |
| n/N | 41/86 | 42/86 | 55/86 | 25/43 | |
| 95% CI | 39.1–56.2 | 40.3–57.4 | 55.4–72.5 | 46.0–70.2 | |
| CIN 1 | |||||
| % | 64.4 | 58.8 | 56.9 | 58.8 | .47 |
| n/N | 103/160 | 94/160 | 91/160 | 47/80 | |
| 95% CI | 57.7–71.1 | 52.0–65.5 | 50.2–63.6 | 49.3–68.2 | |
| CIN 2/3 | |||||
| % | 50.0 | 45.2 | 35.7 | 19.1 | .04j |
| n/N | 21/42 | 19/42 | 15/42 | 4/21 | |
| 95% CI | 36.6–63.4 | 31.9–58.6 | 22.3–49.1 | 0.1–38.0 | |
| a. Cervical biopsy result. | |||||
| b. Colposcopy conducted at rural site by site expert and local colposcopist. | |||||
| c. Colposcopy observed by 2 distant experts at telemedicine center using telemedicine network equipment. | |||||
| d. Colposcopy observed by 2 distant experts at telemedicine center using computer-based system. | |||||
| e. Cervicography interpreted by a single cervical evaluator. | |||||
| f. P value from permutation test. | |||||
| g. The numerator is the number of observations in agreement with histology; the denominator is the number of observations with 2 per subject for on-site, network, and computer-based, 1 observation per subject for cervicography. | |||||
| h. 95% confidence intervals based on normal approximation, adjusted for repeated measures. | |||||
| i. Computer-based > on-site, Tukey’s test. | |||||
| j. On-site > cervicography, Tukey’s test. | |||||
| CI, confidence interval; CIN, cervical intraepithelial neoplasia | |||||
TABLE 2
Sensitivity and specificity of tests to detect cervical neoplasia
| Positive thresholda | Assessment device | Sensitivity | Specificity | LR+b | LR-c |
|---|---|---|---|---|---|
| CIN 1 | On-site colposcopyd | 1.2 | 0.8 | ||
| % (95% CI)f | 60.8 (54.8–66.7) | 47.7 (39.1–56.2) | |||
| n/Ne | 124/204 | 41/86 | |||
| Network telecolposcopyg | 1.1 | 0.9 | |||
| % (95% CI) | 55.4 (49.6–61.2) | 48.8 (40.3–57.4) | |||
| n/N | 113/204 | 42/86 | |||
| Computer-based telecolposcopyh | 1.4 | 0.8 | |||
| % (95% CI) | 52.0 (46.0–57.9) | 64.0(55.4–72.5) | |||
| n/N | 106/204 | 55/86 | |||
| Cervicographyi | 1.2 | 0.9 | |||
| % (95% CI) | 50.0 (41.6–58.4) | 58.1 (46.0–70.2) | |||
| n/N | 51/102 | 25/43 | |||
| P j | .1 | .3k | |||
| CIN 2 | On-site colposcopy | 1.2 | 0.9 | ||
| % (95% CI) | 47.7 (34.9–60.5) | 58.5 (53.2–63.8) | |||
| n/N | 21/44 | 144/246 | |||
| Network telecolposcopy | 1.0 | 1.0 | |||
| % (95% CI) | 43.2 (30.4–56.0) | 55.3 (50.0–60.6) | |||
| n/N | 19/44 | 136/246 | |||
| Computer-based telecolposcopy | 0.8 | 1.1 | |||
| % (95% CI) | 34.1 (21.3–46.9) | 59.4 (54.0–64.7) | |||
| n/N | 15/44 | 146/246 | |||
| Cervicography | 0.4 | 1.4 | |||
| % (95% CI) | 18.2 (0.1–36.3) | 58.5 (51.0–66.0) | |||
| n/N | 4/22 | 72/123 | |||
| P | .049l | .74 | |||
| a. Threshold considered positive (ie, disease vs nondisease). | |||||
| b. Likelihood ratio of positive test = sensitivity / (1 - specificity). | |||||
| c. Likelihood ratio of negative test = (1 - sensitivity) / specificity. | |||||
| d. Colposcopy conducted at rural site by site expert and local colposcopist. | |||||
| e. The numerator is the number of observations that led to correct diagnosis; the denominator is the number of observations with 2 per subject for on-site, network, and computer-based, 1 observation per subject for cervicography. | |||||
| f. 95% confidence intervals based on normal approximation, adjusted for repeated measures. | |||||
| g. Colposcopy observed by 2 distant experts at telemedicine center using existing telemedicine network equipment. | |||||
| h. Colposcopy observed by 2 distant experts at telemedicine center using computer-based system. | |||||
| i. Cervicography interpreted by a single certified evaluator. | |||||
| j. P from permutation test. | |||||
| k. Computer-based > on-site, Tukey test. | |||||
| l. On-site > cervicography Tukey test. | |||||
| CI, confidence interval; LR+, positive likelihood ratio; LR-, negative likelihood ratio; CIN, cervical intraepithelial neoplasia. | |||||
Discussion
Until recently, cervicography had been the only type of remote diagnostic system available for the evaluation of women with potential lower genital tract neoplasia. With the advent of telemedicine during the past decade, expert-level health care has now become more readily available to patients previously isolated from this important resource.
The future of telecolposcopy
Because of its nature, telecolposcopy may also be well suited to assist in the evaluation and management of women with lower genital tract neoplasia. Computer-based telecolposcopy has the potential to support clinical sites located wherever standard telephone service exists. Cellular telephone systems now broaden access to nearglobal availability. Soon, assuming sufficient funding is obtained, the provision of expertenhanced colposcopy may become a reality for all women. However, universal availability may be irrelevant if computer-based telecolposcopy performs at a substandard level.
Telecolposcopy vs cervicography
We have demonstrated that telecolposcopy was at least as effective as cervicography for detecting cervical cancer precursors. Although the difference was not statistically significant, both network and computer-based telecolposcopy systems detected a higher percentage of women with CIN 2 or 3 than cervicography.
Our results also included on-site colposcopy. As anticipated, on-site colposcopy had the greatest sensitivity for disease detection at either positive test thresholds (at least CIN 1 and CIN 2). Ability to manipulate the cervix, stereoscopic viewing, longitudinal observation after 5% acetic acid application, and better resolution of the cervical epithelium and vascularity all favor on-site colposcopic diagnoses. Of the 2 telecolposcopy systems, network telecolposcopy had a slightly, but not significantly, greater sensitivity for detecting cervical cancer precursors compared with computer-based telecolposcopy.
Expert colposcopists’ accuracy with interpretation of network (real-time) cervical images was similar to that for on-site colposcopy, as might be expected. Network telecolposcopy might be equated with remote video colposcopy. Previously we have shown that traditional optical colposcopy is equivalent to video colposcopy with respect to colposcopic/histologic agreement.9
Comparison of telecolposcopy systems
The computer-based telecolposcopy system used in our study was, in all fairness, more similar to cervicography. Each method involves evaluation of 2 static images. Computer-based telecolposcopy provides 2 digitized images, but of a low- and high-power magnification view of the cervix. In comparison, cervicography produces dual low-power magnification celluloid images (2 x 2 slides) of the cervix. The provision of a high-power cervical image may explain the better sensitivity of computer-based telecolposcopy. This one feature may be more valuable than the better image resolution obtained from cervicography. However, computer-based resolution appears to be sufficient to render diagnoses at a level equivalent to or better than cervicography.
These 2 “static” systems differ in other aspects as well. First, computer-based systems are nonproprietary. Several systems are commercially available and other colposcopists have devised their own unique systems using modifications of off-the-shelf technology. Although not available at the initiation of our trial, computerbased systems now have the capability of capturing short video streams. These video segments should help improve the diagnostic ability of consulting colposcopists as demonstrated by our study.
Second, computer-based telecolposcopy can provide instantaneous consultation as opposed to cervicography, which generally takes a minimum of several weeks to receive a report. Computerbased telecolposcopy also allows interaction between the on-site provider and remote expert.
Third, cervicography is a screening test adjunct. The computer-based system was used as a colposcopy diagnostic adjunct. However, colposcopy could easily be adapted to provide the function of cervicography. A simple handheld miniature change-coupled device camera and light source could potentially replace a more expensive colposcope and video camera, or video colposcope. With an average laptop computer (with appropriate software) and cellular phone, health care providers of potentially all women in the world could have access to expert-level cervical evaluation services.
Finally, computer-based telecolposcopy images and associated data automatically become part of a modern electronic medical record. This format is more conducive to the direction toward which contemporary medicine is rapidly shifting. Consequently, computer-based telecolposcopy may offer clinicians superior, modern diagnostic services not previously available to women.
Acknowledgments
Special thanks to Dr. Debra Crawley and Diane Watson, MSN, for rural site participation.
1. Ferris DG, Macfee MS, Miller JA, Crawley D, Watson D. The efficacy of telecolposcopy compared with traditional colposcopy. Obstet Gynecol 2002;99:248-254.
2. Ferris DG, Bishai DM, Macfee MS, Litaker MS, Dickman ED, Miller JA. Telemedicine network telecolposcopy compared with computer-based telecolposcopy. Ann Fam Med 2003;accepted, pending publication.
3. Stafl A. Cervicography a new method for cervical cancer detection. Am J Obstet Gynecol 1981;139:815-825.
4. Ferris DG, Payne P, Frisch LE, Milner FH, di Paola FM, Petry LJ. Cervicography: adjunctive cervical cancer screening by primary care clinicians. J Fam Pract 1993;37:158-164.
5. Ferris DG, Payne P, Frisch LE. Cervicography: an intermediate triage test for the evaluation of cervical atypia. J Fam Pract 1993;37:463-468.
6. Ferris DG, Schiffman M, Litaker MS. Cervicography for triage of women with mildly abnormal cervical cytology results. Am J Obstet Gynecol 2001;185:939-943.
7. Schneider DL, Herrero R, Bratti C, et al. Cervicography screening for cervical cancer among 8460 women in a high-risk population. Am J Obstet Gynecol 1999;180:290-298.
8. Eskridge C, Begneaud WP, Landwehr C. Cervicography combined with repeat Papanicolaou test as a triage for low grade cytologic abnormalities. Obstet Gynecol 1998;92:351-355.
9. Ferris DG, Ho TH, Guijon F, et al. A comparison of colposcopy using optical and video colposcopes. Journal of Lower Genital Tract Disease 2000;2:65-71.
- Computer-based telecolposcopy and network telecolposcopy detected more cervical neoplasia than cervicography.
- Computer-based telecolposcopy could provide many women with greater access to expert diagnostic services.
Telemedicine enables doctors in rural areas or areas with poor medical service to consult with experts at distant locations. Telecolposcopy and cervicography both enable remote diagnoses of the cervix. The 2 methods differ in equipment, operations, image format, timeliness of consultation, and probably cost. However, these diagnostic approaches have not been compared previously. The purpose of this study was to compare the accuracy of telecolposcopy and cervicography with on-site colposcopy in the remote evaluation of women with potential cervical neoplasia.
Telecolposcopy and cervicography
Telecolposcopy involves a distant expert colposcopist’s evaluation of women with potential lower genital tract neoplasia.1 Existing telemedicine network and computer systems provide an audiovisual interface between local colposcopists and expert colposcopists at other locations.2 For health systems already using computer or video networks, telecolposcopic consultation can be implemented with only small additional charges per examination.2 Telecolposcopy services may improve health care access for women in medically underserved areas.1
Cervicography is distant evaluation of 2 photographs taken of the cervix following 5% acetic acid application.3 A special 35-mm camera is used to take these images. The end product, developed at a central processing center, resembles a low-magnification colposcopic photograph. Certified evaluators interpret these images, classifying them as negative, atypical, or positive. Cervicography is used primarily as an adjunct test to the Papanicolaou (Pap) smear.4 It has also been evaluated as an intermediate triage test for evaluating women with mildly abnormal Pap smear results.5-8
Methods
Women aged 18 years or older who came to 1 of 2 rural clinic sites for a colposcopic examination were enrolled in the trial after signing an institutional review board–approved informed consent document. We included women with a recent abnormal Pap smear report or a lower genital tract finding that required further evaluation by colposcopy. The exclusion criteria were pregnancy, severe cervicitis, heavy menses, refusal to participate, or technical problems with the telecolposcopy or cervicography equipment.
Both clinics were part of the Medical College of Georgia Telemedicine Network. This system uses sophisticated telecommunications equipment to provide distant consultation services to clinicians practicing in rural areas of the state.1 Small change-coupled device cameras were attached to the colposcopes at the 2 clinics.
For network telecolposcopy, images were transmitted using the network’s existing hardware and high-speed telecommunication lines. For computer telecolposcopy, personal computers (DIMS, DenVu, Tucson, Ariz) were also used to capture and transmit images to a computer at the Telemedicine Center. These digitized images were transmitted by modem via telephone lines.2 Cerviscopes (35-mm cameras) supplied by the manufacturer (NTL Worldwide, Fenton, Mo) were used to acquire cervigrams (photographs).
Pertinent clinicians received appropriate training to take cervigrams. Certified evaluators interpreted the images according to company protocol and returned a standardized report to the investigators at a later date.
Study design
The study design has been described in detail previously.1,2 Briefly, subjects were initially examined by 1 of 3 on-site, university-based expert colposcopists, who took 2 cervigrams of each patient, and then conducted a colposcopic examination independently.
A local clinician then completed another colposcopic examination, including histologic sampling, if indicated. This examination was observed simultaneously by another expert at a telemedicine center. Prior to obtaining histologic samples or using dilute Lugol’s iodine solution, the local clinician captured 2 cervical images (low and high magnification) using the computer telemedicine system. These images were then transmitted to the expert at the telemedicine center for independent interpretation.
A third expert colposcopist interpreted the video and computer images at a later time. However, these third interpretations were not considered in this report. Colposcopists were blinded to each other’s clinical diagnoses. However, all colposcopists were informed of the subject’s referral cervical cytology results and other pertinent history.
Data analysis
Each subject had 2 observations using each of the 3 colposcopy methods (on-site, network, and computer-based), and a single observation using cervicography. On-site colposcopy, consisting of the observations of the on-site expert and local colposcopist, was considered for reference purposes. Agreement with histologic results was calculated for each method, across all histologic diagnoses together and separately by diagnosis.
Sensitivity and specificity estimates were calculated using 2 definitions of disease: (1) normal versus any other histologic diagnosis, and (2) normal or cervical intraepithelial neoplasia 1 (CIN 1) versus any more severe diagnosis. The primary analysis model was complete block analysis of variance, with subjects included as blocks in the analysis to account for the multiple observations on the same subjects. Nonparametric comparisons of proportions of agreement with histology, sensitivity, and specificity among the methods were made using permutation tests. Post-hoc comparisons were made using a Tukey test; 95% confidence intervals (CIs) were calculated for all point estimates. Adjustment for dependence among multiple observations per subject was made by basing these tests and CIs on least-squares means.
The available sample sizes for all analyses were adequate to ensure approximate normality of the estimated means. Power to detect, at Α=.05, a difference in agreement of 15% between cervigram and the other evaluation methods, was estimated using Monte Carlo simulations. Data were simulated using the observed levels of agreement for on-site, network, and computer telecolposcopy, and specifying a difference of 15% between cervicography agreement and the maximum of the other methods’ agreement. Power estimates were based on analysis of 1000 simulations. SAS release 8.02 was used for all calculations (SAS, Inc, Cary, NC).
Results
A total of 264 subjects were enrolled in the trial, but the total number of subjects considered differed depending on the various analyses of interest. The demographic data of this study cohort have been published previously.1
Briefly, the subjects’ mean age was 31.7 years and mean parity was 2.1. Subjects presented with a wide range of prior cervical cytology results: 20.4% normal, 29.2% atypical squamous cells of undetermined significance, 40.4% low-grade squamous intraepithelial lesion, 7.3% high-grade squamous intraepithelial lesion, and 2.7% atypical glandular cells of undetermined significance. Histology results included all levels of CIN (52.9% CIN 1 and 13.4% CIN 2 or 3), and endocervical histologic sampling results were reported as both positive and negative for neoplasia.
The agreement between telecolposcopic/cervicography impressions and histology were estimated (Table 1). Data for on-site colposcopy was also considered for reference purposes.
When all histologic diagnoses were considered, there was no statistically significant difference in the rates of agreement for colposcopy, the 2 types of telecolposcopy, and cervicography. This was also true if only cases of CIN 1 were examined.
However, a statistically significant difference was noted between agreement rates for computer-based telecolposcopy (63.95%) and on-site colposcopy (47.7%, P=.03, Tukey test) for normal histology. A statistically significant difference was also found between agreement rates for on-site colposcopy (50.0%) and cervicography (19.1%, P=.04, Tukey test) for women with biopsy-proven CIN 2 or 3. If all histologic diagnoses were considered, the study provided 85% power to detect a difference in agreement of 15% among the evaluation methods.
We also estimated the sensitivity and specificity of the four diagnostic methods to detect cervical neoplasia (Table 2). A statistically significant difference was found in observed sensitivity between on-site colposcopy (47.7%) and cervicography (18.2%, P=.04, Tukey test) when a positive threshold of at least CIN 2 was considered. The difference was not significant, however, if the lower positive test threshold of at least CIN 1 was considered.
A statistically significant difference in specificity was noted between computer-based telecolposcopy (64.0%) and on-site colposcopy (47.7%, P=.03, Tukey test) at a positive threshold of at least CIN 1. The study provided a power of 71% and 60% to detect differences of 15% in sensitivity and specificity, respectively, using the CIN 1 threshold. Using CIN 2 as the positive threshold, the power to detect this 15% difference was 24% and 81% for sensitivity and specificity, respectively.
TABLE 1
Colposcopic, telecolposcopic, and cervicographic agreement with histology
| Histologya | On-site colposcopyb | Network telecolposcopyc | Computer-based telecolposcopyd | Cervicographye | Pf |
|---|---|---|---|---|---|
| All diagnoses | |||||
| % | 56.9 | 53.5 | 55.5 | 52.4 | .66 |
| n/Ng | 165/290 | 155/290 | 161/290 | 76/145 | |
| 95% CIh | 52.0–61.8 | 48.5–58.3 | 50.6–60.4 | 45.5–59.4 | |
| Normal | |||||
| % | 47.7 | 48.8 | 63.95 | 58.1 | .03I |
| n/N | 41/86 | 42/86 | 55/86 | 25/43 | |
| 95% CI | 39.1–56.2 | 40.3–57.4 | 55.4–72.5 | 46.0–70.2 | |
| CIN 1 | |||||
| % | 64.4 | 58.8 | 56.9 | 58.8 | .47 |
| n/N | 103/160 | 94/160 | 91/160 | 47/80 | |
| 95% CI | 57.7–71.1 | 52.0–65.5 | 50.2–63.6 | 49.3–68.2 | |
| CIN 2/3 | |||||
| % | 50.0 | 45.2 | 35.7 | 19.1 | .04j |
| n/N | 21/42 | 19/42 | 15/42 | 4/21 | |
| 95% CI | 36.6–63.4 | 31.9–58.6 | 22.3–49.1 | 0.1–38.0 | |
| a. Cervical biopsy result. | |||||
| b. Colposcopy conducted at rural site by site expert and local colposcopist. | |||||
| c. Colposcopy observed by 2 distant experts at telemedicine center using telemedicine network equipment. | |||||
| d. Colposcopy observed by 2 distant experts at telemedicine center using computer-based system. | |||||
| e. Cervicography interpreted by a single cervical evaluator. | |||||
| f. P value from permutation test. | |||||
| g. The numerator is the number of observations in agreement with histology; the denominator is the number of observations with 2 per subject for on-site, network, and computer-based, 1 observation per subject for cervicography. | |||||
| h. 95% confidence intervals based on normal approximation, adjusted for repeated measures. | |||||
| i. Computer-based > on-site, Tukey’s test. | |||||
| j. On-site > cervicography, Tukey’s test. | |||||
| CI, confidence interval; CIN, cervical intraepithelial neoplasia | |||||
TABLE 2
Sensitivity and specificity of tests to detect cervical neoplasia
| Positive thresholda | Assessment device | Sensitivity | Specificity | LR+b | LR-c |
|---|---|---|---|---|---|
| CIN 1 | On-site colposcopyd | 1.2 | 0.8 | ||
| % (95% CI)f | 60.8 (54.8–66.7) | 47.7 (39.1–56.2) | |||
| n/Ne | 124/204 | 41/86 | |||
| Network telecolposcopyg | 1.1 | 0.9 | |||
| % (95% CI) | 55.4 (49.6–61.2) | 48.8 (40.3–57.4) | |||
| n/N | 113/204 | 42/86 | |||
| Computer-based telecolposcopyh | 1.4 | 0.8 | |||
| % (95% CI) | 52.0 (46.0–57.9) | 64.0(55.4–72.5) | |||
| n/N | 106/204 | 55/86 | |||
| Cervicographyi | 1.2 | 0.9 | |||
| % (95% CI) | 50.0 (41.6–58.4) | 58.1 (46.0–70.2) | |||
| n/N | 51/102 | 25/43 | |||
| P j | .1 | .3k | |||
| CIN 2 | On-site colposcopy | 1.2 | 0.9 | ||
| % (95% CI) | 47.7 (34.9–60.5) | 58.5 (53.2–63.8) | |||
| n/N | 21/44 | 144/246 | |||
| Network telecolposcopy | 1.0 | 1.0 | |||
| % (95% CI) | 43.2 (30.4–56.0) | 55.3 (50.0–60.6) | |||
| n/N | 19/44 | 136/246 | |||
| Computer-based telecolposcopy | 0.8 | 1.1 | |||
| % (95% CI) | 34.1 (21.3–46.9) | 59.4 (54.0–64.7) | |||
| n/N | 15/44 | 146/246 | |||
| Cervicography | 0.4 | 1.4 | |||
| % (95% CI) | 18.2 (0.1–36.3) | 58.5 (51.0–66.0) | |||
| n/N | 4/22 | 72/123 | |||
| P | .049l | .74 | |||
| a. Threshold considered positive (ie, disease vs nondisease). | |||||
| b. Likelihood ratio of positive test = sensitivity / (1 - specificity). | |||||
| c. Likelihood ratio of negative test = (1 - sensitivity) / specificity. | |||||
| d. Colposcopy conducted at rural site by site expert and local colposcopist. | |||||
| e. The numerator is the number of observations that led to correct diagnosis; the denominator is the number of observations with 2 per subject for on-site, network, and computer-based, 1 observation per subject for cervicography. | |||||
| f. 95% confidence intervals based on normal approximation, adjusted for repeated measures. | |||||
| g. Colposcopy observed by 2 distant experts at telemedicine center using existing telemedicine network equipment. | |||||
| h. Colposcopy observed by 2 distant experts at telemedicine center using computer-based system. | |||||
| i. Cervicography interpreted by a single certified evaluator. | |||||
| j. P from permutation test. | |||||
| k. Computer-based > on-site, Tukey test. | |||||
| l. On-site > cervicography Tukey test. | |||||
| CI, confidence interval; LR+, positive likelihood ratio; LR-, negative likelihood ratio; CIN, cervical intraepithelial neoplasia. | |||||
Discussion
Until recently, cervicography had been the only type of remote diagnostic system available for the evaluation of women with potential lower genital tract neoplasia. With the advent of telemedicine during the past decade, expert-level health care has now become more readily available to patients previously isolated from this important resource.
The future of telecolposcopy
Because of its nature, telecolposcopy may also be well suited to assist in the evaluation and management of women with lower genital tract neoplasia. Computer-based telecolposcopy has the potential to support clinical sites located wherever standard telephone service exists. Cellular telephone systems now broaden access to nearglobal availability. Soon, assuming sufficient funding is obtained, the provision of expertenhanced colposcopy may become a reality for all women. However, universal availability may be irrelevant if computer-based telecolposcopy performs at a substandard level.
Telecolposcopy vs cervicography
We have demonstrated that telecolposcopy was at least as effective as cervicography for detecting cervical cancer precursors. Although the difference was not statistically significant, both network and computer-based telecolposcopy systems detected a higher percentage of women with CIN 2 or 3 than cervicography.
Our results also included on-site colposcopy. As anticipated, on-site colposcopy had the greatest sensitivity for disease detection at either positive test thresholds (at least CIN 1 and CIN 2). Ability to manipulate the cervix, stereoscopic viewing, longitudinal observation after 5% acetic acid application, and better resolution of the cervical epithelium and vascularity all favor on-site colposcopic diagnoses. Of the 2 telecolposcopy systems, network telecolposcopy had a slightly, but not significantly, greater sensitivity for detecting cervical cancer precursors compared with computer-based telecolposcopy.
Expert colposcopists’ accuracy with interpretation of network (real-time) cervical images was similar to that for on-site colposcopy, as might be expected. Network telecolposcopy might be equated with remote video colposcopy. Previously we have shown that traditional optical colposcopy is equivalent to video colposcopy with respect to colposcopic/histologic agreement.9
Comparison of telecolposcopy systems
The computer-based telecolposcopy system used in our study was, in all fairness, more similar to cervicography. Each method involves evaluation of 2 static images. Computer-based telecolposcopy provides 2 digitized images, but of a low- and high-power magnification view of the cervix. In comparison, cervicography produces dual low-power magnification celluloid images (2 x 2 slides) of the cervix. The provision of a high-power cervical image may explain the better sensitivity of computer-based telecolposcopy. This one feature may be more valuable than the better image resolution obtained from cervicography. However, computer-based resolution appears to be sufficient to render diagnoses at a level equivalent to or better than cervicography.
These 2 “static” systems differ in other aspects as well. First, computer-based systems are nonproprietary. Several systems are commercially available and other colposcopists have devised their own unique systems using modifications of off-the-shelf technology. Although not available at the initiation of our trial, computerbased systems now have the capability of capturing short video streams. These video segments should help improve the diagnostic ability of consulting colposcopists as demonstrated by our study.
Second, computer-based telecolposcopy can provide instantaneous consultation as opposed to cervicography, which generally takes a minimum of several weeks to receive a report. Computerbased telecolposcopy also allows interaction between the on-site provider and remote expert.
Third, cervicography is a screening test adjunct. The computer-based system was used as a colposcopy diagnostic adjunct. However, colposcopy could easily be adapted to provide the function of cervicography. A simple handheld miniature change-coupled device camera and light source could potentially replace a more expensive colposcope and video camera, or video colposcope. With an average laptop computer (with appropriate software) and cellular phone, health care providers of potentially all women in the world could have access to expert-level cervical evaluation services.
Finally, computer-based telecolposcopy images and associated data automatically become part of a modern electronic medical record. This format is more conducive to the direction toward which contemporary medicine is rapidly shifting. Consequently, computer-based telecolposcopy may offer clinicians superior, modern diagnostic services not previously available to women.
Acknowledgments
Special thanks to Dr. Debra Crawley and Diane Watson, MSN, for rural site participation.
- Computer-based telecolposcopy and network telecolposcopy detected more cervical neoplasia than cervicography.
- Computer-based telecolposcopy could provide many women with greater access to expert diagnostic services.
Telemedicine enables doctors in rural areas or areas with poor medical service to consult with experts at distant locations. Telecolposcopy and cervicography both enable remote diagnoses of the cervix. The 2 methods differ in equipment, operations, image format, timeliness of consultation, and probably cost. However, these diagnostic approaches have not been compared previously. The purpose of this study was to compare the accuracy of telecolposcopy and cervicography with on-site colposcopy in the remote evaluation of women with potential cervical neoplasia.
Telecolposcopy and cervicography
Telecolposcopy involves a distant expert colposcopist’s evaluation of women with potential lower genital tract neoplasia.1 Existing telemedicine network and computer systems provide an audiovisual interface between local colposcopists and expert colposcopists at other locations.2 For health systems already using computer or video networks, telecolposcopic consultation can be implemented with only small additional charges per examination.2 Telecolposcopy services may improve health care access for women in medically underserved areas.1
Cervicography is distant evaluation of 2 photographs taken of the cervix following 5% acetic acid application.3 A special 35-mm camera is used to take these images. The end product, developed at a central processing center, resembles a low-magnification colposcopic photograph. Certified evaluators interpret these images, classifying them as negative, atypical, or positive. Cervicography is used primarily as an adjunct test to the Papanicolaou (Pap) smear.4 It has also been evaluated as an intermediate triage test for evaluating women with mildly abnormal Pap smear results.5-8
Methods
Women aged 18 years or older who came to 1 of 2 rural clinic sites for a colposcopic examination were enrolled in the trial after signing an institutional review board–approved informed consent document. We included women with a recent abnormal Pap smear report or a lower genital tract finding that required further evaluation by colposcopy. The exclusion criteria were pregnancy, severe cervicitis, heavy menses, refusal to participate, or technical problems with the telecolposcopy or cervicography equipment.
Both clinics were part of the Medical College of Georgia Telemedicine Network. This system uses sophisticated telecommunications equipment to provide distant consultation services to clinicians practicing in rural areas of the state.1 Small change-coupled device cameras were attached to the colposcopes at the 2 clinics.
For network telecolposcopy, images were transmitted using the network’s existing hardware and high-speed telecommunication lines. For computer telecolposcopy, personal computers (DIMS, DenVu, Tucson, Ariz) were also used to capture and transmit images to a computer at the Telemedicine Center. These digitized images were transmitted by modem via telephone lines.2 Cerviscopes (35-mm cameras) supplied by the manufacturer (NTL Worldwide, Fenton, Mo) were used to acquire cervigrams (photographs).
Pertinent clinicians received appropriate training to take cervigrams. Certified evaluators interpreted the images according to company protocol and returned a standardized report to the investigators at a later date.
Study design
The study design has been described in detail previously.1,2 Briefly, subjects were initially examined by 1 of 3 on-site, university-based expert colposcopists, who took 2 cervigrams of each patient, and then conducted a colposcopic examination independently.
A local clinician then completed another colposcopic examination, including histologic sampling, if indicated. This examination was observed simultaneously by another expert at a telemedicine center. Prior to obtaining histologic samples or using dilute Lugol’s iodine solution, the local clinician captured 2 cervical images (low and high magnification) using the computer telemedicine system. These images were then transmitted to the expert at the telemedicine center for independent interpretation.
A third expert colposcopist interpreted the video and computer images at a later time. However, these third interpretations were not considered in this report. Colposcopists were blinded to each other’s clinical diagnoses. However, all colposcopists were informed of the subject’s referral cervical cytology results and other pertinent history.
Data analysis
Each subject had 2 observations using each of the 3 colposcopy methods (on-site, network, and computer-based), and a single observation using cervicography. On-site colposcopy, consisting of the observations of the on-site expert and local colposcopist, was considered for reference purposes. Agreement with histologic results was calculated for each method, across all histologic diagnoses together and separately by diagnosis.
Sensitivity and specificity estimates were calculated using 2 definitions of disease: (1) normal versus any other histologic diagnosis, and (2) normal or cervical intraepithelial neoplasia 1 (CIN 1) versus any more severe diagnosis. The primary analysis model was complete block analysis of variance, with subjects included as blocks in the analysis to account for the multiple observations on the same subjects. Nonparametric comparisons of proportions of agreement with histology, sensitivity, and specificity among the methods were made using permutation tests. Post-hoc comparisons were made using a Tukey test; 95% confidence intervals (CIs) were calculated for all point estimates. Adjustment for dependence among multiple observations per subject was made by basing these tests and CIs on least-squares means.
The available sample sizes for all analyses were adequate to ensure approximate normality of the estimated means. Power to detect, at Α=.05, a difference in agreement of 15% between cervigram and the other evaluation methods, was estimated using Monte Carlo simulations. Data were simulated using the observed levels of agreement for on-site, network, and computer telecolposcopy, and specifying a difference of 15% between cervicography agreement and the maximum of the other methods’ agreement. Power estimates were based on analysis of 1000 simulations. SAS release 8.02 was used for all calculations (SAS, Inc, Cary, NC).
Results
A total of 264 subjects were enrolled in the trial, but the total number of subjects considered differed depending on the various analyses of interest. The demographic data of this study cohort have been published previously.1
Briefly, the subjects’ mean age was 31.7 years and mean parity was 2.1. Subjects presented with a wide range of prior cervical cytology results: 20.4% normal, 29.2% atypical squamous cells of undetermined significance, 40.4% low-grade squamous intraepithelial lesion, 7.3% high-grade squamous intraepithelial lesion, and 2.7% atypical glandular cells of undetermined significance. Histology results included all levels of CIN (52.9% CIN 1 and 13.4% CIN 2 or 3), and endocervical histologic sampling results were reported as both positive and negative for neoplasia.
The agreement between telecolposcopic/cervicography impressions and histology were estimated (Table 1). Data for on-site colposcopy was also considered for reference purposes.
When all histologic diagnoses were considered, there was no statistically significant difference in the rates of agreement for colposcopy, the 2 types of telecolposcopy, and cervicography. This was also true if only cases of CIN 1 were examined.
However, a statistically significant difference was noted between agreement rates for computer-based telecolposcopy (63.95%) and on-site colposcopy (47.7%, P=.03, Tukey test) for normal histology. A statistically significant difference was also found between agreement rates for on-site colposcopy (50.0%) and cervicography (19.1%, P=.04, Tukey test) for women with biopsy-proven CIN 2 or 3. If all histologic diagnoses were considered, the study provided 85% power to detect a difference in agreement of 15% among the evaluation methods.
We also estimated the sensitivity and specificity of the four diagnostic methods to detect cervical neoplasia (Table 2). A statistically significant difference was found in observed sensitivity between on-site colposcopy (47.7%) and cervicography (18.2%, P=.04, Tukey test) when a positive threshold of at least CIN 2 was considered. The difference was not significant, however, if the lower positive test threshold of at least CIN 1 was considered.
A statistically significant difference in specificity was noted between computer-based telecolposcopy (64.0%) and on-site colposcopy (47.7%, P=.03, Tukey test) at a positive threshold of at least CIN 1. The study provided a power of 71% and 60% to detect differences of 15% in sensitivity and specificity, respectively, using the CIN 1 threshold. Using CIN 2 as the positive threshold, the power to detect this 15% difference was 24% and 81% for sensitivity and specificity, respectively.
TABLE 1
Colposcopic, telecolposcopic, and cervicographic agreement with histology
| Histologya | On-site colposcopyb | Network telecolposcopyc | Computer-based telecolposcopyd | Cervicographye | Pf |
|---|---|---|---|---|---|
| All diagnoses | |||||
| % | 56.9 | 53.5 | 55.5 | 52.4 | .66 |
| n/Ng | 165/290 | 155/290 | 161/290 | 76/145 | |
| 95% CIh | 52.0–61.8 | 48.5–58.3 | 50.6–60.4 | 45.5–59.4 | |
| Normal | |||||
| % | 47.7 | 48.8 | 63.95 | 58.1 | .03I |
| n/N | 41/86 | 42/86 | 55/86 | 25/43 | |
| 95% CI | 39.1–56.2 | 40.3–57.4 | 55.4–72.5 | 46.0–70.2 | |
| CIN 1 | |||||
| % | 64.4 | 58.8 | 56.9 | 58.8 | .47 |
| n/N | 103/160 | 94/160 | 91/160 | 47/80 | |
| 95% CI | 57.7–71.1 | 52.0–65.5 | 50.2–63.6 | 49.3–68.2 | |
| CIN 2/3 | |||||
| % | 50.0 | 45.2 | 35.7 | 19.1 | .04j |
| n/N | 21/42 | 19/42 | 15/42 | 4/21 | |
| 95% CI | 36.6–63.4 | 31.9–58.6 | 22.3–49.1 | 0.1–38.0 | |
| a. Cervical biopsy result. | |||||
| b. Colposcopy conducted at rural site by site expert and local colposcopist. | |||||
| c. Colposcopy observed by 2 distant experts at telemedicine center using telemedicine network equipment. | |||||
| d. Colposcopy observed by 2 distant experts at telemedicine center using computer-based system. | |||||
| e. Cervicography interpreted by a single cervical evaluator. | |||||
| f. P value from permutation test. | |||||
| g. The numerator is the number of observations in agreement with histology; the denominator is the number of observations with 2 per subject for on-site, network, and computer-based, 1 observation per subject for cervicography. | |||||
| h. 95% confidence intervals based on normal approximation, adjusted for repeated measures. | |||||
| i. Computer-based > on-site, Tukey’s test. | |||||
| j. On-site > cervicography, Tukey’s test. | |||||
| CI, confidence interval; CIN, cervical intraepithelial neoplasia | |||||
TABLE 2
Sensitivity and specificity of tests to detect cervical neoplasia
| Positive thresholda | Assessment device | Sensitivity | Specificity | LR+b | LR-c |
|---|---|---|---|---|---|
| CIN 1 | On-site colposcopyd | 1.2 | 0.8 | ||
| % (95% CI)f | 60.8 (54.8–66.7) | 47.7 (39.1–56.2) | |||
| n/Ne | 124/204 | 41/86 | |||
| Network telecolposcopyg | 1.1 | 0.9 | |||
| % (95% CI) | 55.4 (49.6–61.2) | 48.8 (40.3–57.4) | |||
| n/N | 113/204 | 42/86 | |||
| Computer-based telecolposcopyh | 1.4 | 0.8 | |||
| % (95% CI) | 52.0 (46.0–57.9) | 64.0(55.4–72.5) | |||
| n/N | 106/204 | 55/86 | |||
| Cervicographyi | 1.2 | 0.9 | |||
| % (95% CI) | 50.0 (41.6–58.4) | 58.1 (46.0–70.2) | |||
| n/N | 51/102 | 25/43 | |||
| P j | .1 | .3k | |||
| CIN 2 | On-site colposcopy | 1.2 | 0.9 | ||
| % (95% CI) | 47.7 (34.9–60.5) | 58.5 (53.2–63.8) | |||
| n/N | 21/44 | 144/246 | |||
| Network telecolposcopy | 1.0 | 1.0 | |||
| % (95% CI) | 43.2 (30.4–56.0) | 55.3 (50.0–60.6) | |||
| n/N | 19/44 | 136/246 | |||
| Computer-based telecolposcopy | 0.8 | 1.1 | |||
| % (95% CI) | 34.1 (21.3–46.9) | 59.4 (54.0–64.7) | |||
| n/N | 15/44 | 146/246 | |||
| Cervicography | 0.4 | 1.4 | |||
| % (95% CI) | 18.2 (0.1–36.3) | 58.5 (51.0–66.0) | |||
| n/N | 4/22 | 72/123 | |||
| P | .049l | .74 | |||
| a. Threshold considered positive (ie, disease vs nondisease). | |||||
| b. Likelihood ratio of positive test = sensitivity / (1 - specificity). | |||||
| c. Likelihood ratio of negative test = (1 - sensitivity) / specificity. | |||||
| d. Colposcopy conducted at rural site by site expert and local colposcopist. | |||||
| e. The numerator is the number of observations that led to correct diagnosis; the denominator is the number of observations with 2 per subject for on-site, network, and computer-based, 1 observation per subject for cervicography. | |||||
| f. 95% confidence intervals based on normal approximation, adjusted for repeated measures. | |||||
| g. Colposcopy observed by 2 distant experts at telemedicine center using existing telemedicine network equipment. | |||||
| h. Colposcopy observed by 2 distant experts at telemedicine center using computer-based system. | |||||
| i. Cervicography interpreted by a single certified evaluator. | |||||
| j. P from permutation test. | |||||
| k. Computer-based > on-site, Tukey test. | |||||
| l. On-site > cervicography Tukey test. | |||||
| CI, confidence interval; LR+, positive likelihood ratio; LR-, negative likelihood ratio; CIN, cervical intraepithelial neoplasia. | |||||
Discussion
Until recently, cervicography had been the only type of remote diagnostic system available for the evaluation of women with potential lower genital tract neoplasia. With the advent of telemedicine during the past decade, expert-level health care has now become more readily available to patients previously isolated from this important resource.
The future of telecolposcopy
Because of its nature, telecolposcopy may also be well suited to assist in the evaluation and management of women with lower genital tract neoplasia. Computer-based telecolposcopy has the potential to support clinical sites located wherever standard telephone service exists. Cellular telephone systems now broaden access to nearglobal availability. Soon, assuming sufficient funding is obtained, the provision of expertenhanced colposcopy may become a reality for all women. However, universal availability may be irrelevant if computer-based telecolposcopy performs at a substandard level.
Telecolposcopy vs cervicography
We have demonstrated that telecolposcopy was at least as effective as cervicography for detecting cervical cancer precursors. Although the difference was not statistically significant, both network and computer-based telecolposcopy systems detected a higher percentage of women with CIN 2 or 3 than cervicography.
Our results also included on-site colposcopy. As anticipated, on-site colposcopy had the greatest sensitivity for disease detection at either positive test thresholds (at least CIN 1 and CIN 2). Ability to manipulate the cervix, stereoscopic viewing, longitudinal observation after 5% acetic acid application, and better resolution of the cervical epithelium and vascularity all favor on-site colposcopic diagnoses. Of the 2 telecolposcopy systems, network telecolposcopy had a slightly, but not significantly, greater sensitivity for detecting cervical cancer precursors compared with computer-based telecolposcopy.
Expert colposcopists’ accuracy with interpretation of network (real-time) cervical images was similar to that for on-site colposcopy, as might be expected. Network telecolposcopy might be equated with remote video colposcopy. Previously we have shown that traditional optical colposcopy is equivalent to video colposcopy with respect to colposcopic/histologic agreement.9
Comparison of telecolposcopy systems
The computer-based telecolposcopy system used in our study was, in all fairness, more similar to cervicography. Each method involves evaluation of 2 static images. Computer-based telecolposcopy provides 2 digitized images, but of a low- and high-power magnification view of the cervix. In comparison, cervicography produces dual low-power magnification celluloid images (2 x 2 slides) of the cervix. The provision of a high-power cervical image may explain the better sensitivity of computer-based telecolposcopy. This one feature may be more valuable than the better image resolution obtained from cervicography. However, computer-based resolution appears to be sufficient to render diagnoses at a level equivalent to or better than cervicography.
These 2 “static” systems differ in other aspects as well. First, computer-based systems are nonproprietary. Several systems are commercially available and other colposcopists have devised their own unique systems using modifications of off-the-shelf technology. Although not available at the initiation of our trial, computerbased systems now have the capability of capturing short video streams. These video segments should help improve the diagnostic ability of consulting colposcopists as demonstrated by our study.
Second, computer-based telecolposcopy can provide instantaneous consultation as opposed to cervicography, which generally takes a minimum of several weeks to receive a report. Computerbased telecolposcopy also allows interaction between the on-site provider and remote expert.
Third, cervicography is a screening test adjunct. The computer-based system was used as a colposcopy diagnostic adjunct. However, colposcopy could easily be adapted to provide the function of cervicography. A simple handheld miniature change-coupled device camera and light source could potentially replace a more expensive colposcope and video camera, or video colposcope. With an average laptop computer (with appropriate software) and cellular phone, health care providers of potentially all women in the world could have access to expert-level cervical evaluation services.
Finally, computer-based telecolposcopy images and associated data automatically become part of a modern electronic medical record. This format is more conducive to the direction toward which contemporary medicine is rapidly shifting. Consequently, computer-based telecolposcopy may offer clinicians superior, modern diagnostic services not previously available to women.
Acknowledgments
Special thanks to Dr. Debra Crawley and Diane Watson, MSN, for rural site participation.
1. Ferris DG, Macfee MS, Miller JA, Crawley D, Watson D. The efficacy of telecolposcopy compared with traditional colposcopy. Obstet Gynecol 2002;99:248-254.
2. Ferris DG, Bishai DM, Macfee MS, Litaker MS, Dickman ED, Miller JA. Telemedicine network telecolposcopy compared with computer-based telecolposcopy. Ann Fam Med 2003;accepted, pending publication.
3. Stafl A. Cervicography a new method for cervical cancer detection. Am J Obstet Gynecol 1981;139:815-825.
4. Ferris DG, Payne P, Frisch LE, Milner FH, di Paola FM, Petry LJ. Cervicography: adjunctive cervical cancer screening by primary care clinicians. J Fam Pract 1993;37:158-164.
5. Ferris DG, Payne P, Frisch LE. Cervicography: an intermediate triage test for the evaluation of cervical atypia. J Fam Pract 1993;37:463-468.
6. Ferris DG, Schiffman M, Litaker MS. Cervicography for triage of women with mildly abnormal cervical cytology results. Am J Obstet Gynecol 2001;185:939-943.
7. Schneider DL, Herrero R, Bratti C, et al. Cervicography screening for cervical cancer among 8460 women in a high-risk population. Am J Obstet Gynecol 1999;180:290-298.
8. Eskridge C, Begneaud WP, Landwehr C. Cervicography combined with repeat Papanicolaou test as a triage for low grade cytologic abnormalities. Obstet Gynecol 1998;92:351-355.
9. Ferris DG, Ho TH, Guijon F, et al. A comparison of colposcopy using optical and video colposcopes. Journal of Lower Genital Tract Disease 2000;2:65-71.
1. Ferris DG, Macfee MS, Miller JA, Crawley D, Watson D. The efficacy of telecolposcopy compared with traditional colposcopy. Obstet Gynecol 2002;99:248-254.
2. Ferris DG, Bishai DM, Macfee MS, Litaker MS, Dickman ED, Miller JA. Telemedicine network telecolposcopy compared with computer-based telecolposcopy. Ann Fam Med 2003;accepted, pending publication.
3. Stafl A. Cervicography a new method for cervical cancer detection. Am J Obstet Gynecol 1981;139:815-825.
4. Ferris DG, Payne P, Frisch LE, Milner FH, di Paola FM, Petry LJ. Cervicography: adjunctive cervical cancer screening by primary care clinicians. J Fam Pract 1993;37:158-164.
5. Ferris DG, Payne P, Frisch LE. Cervicography: an intermediate triage test for the evaluation of cervical atypia. J Fam Pract 1993;37:463-468.
6. Ferris DG, Schiffman M, Litaker MS. Cervicography for triage of women with mildly abnormal cervical cytology results. Am J Obstet Gynecol 2001;185:939-943.
7. Schneider DL, Herrero R, Bratti C, et al. Cervicography screening for cervical cancer among 8460 women in a high-risk population. Am J Obstet Gynecol 1999;180:290-298.
8. Eskridge C, Begneaud WP, Landwehr C. Cervicography combined with repeat Papanicolaou test as a triage for low grade cytologic abnormalities. Obstet Gynecol 1998;92:351-355.
9. Ferris DG, Ho TH, Guijon F, et al. A comparison of colposcopy using optical and video colposcopes. Journal of Lower Genital Tract Disease 2000;2:65-71.
Valacyclovir for Prevention of Recurrent Herpes Labialis: 2 Double-Blind, Placebo-Controlled Studies
Patient safety after hours: Time for action
Associate Editor, Journal of Family Practice.
About: “After-hours telephone triage affects patient safety,”
Will all of you who enjoy taking after-hours calls please stand up?
What? Everyone is still sitting? That’s what I thought. Although taking calls after hours is not one of our favorite duties, after-hours care is a crucial component of primary health care. The recent Institute of Medicine report, Crossing the Quality Chasm.,1 cited 6 characteristics essential for a high-quality health care system for the 21st century:
- safe
- effective
- efficient
- equitable
- timely
- patient-centered.
After-hours call coverage systems should pass muster on all 6 qualities. Do they?
Telephone triage after hours not up to standard
Hildebrandt, Westfall, and Smith provide evidence that the after-hours primary care call systems in the United States are not up to standard.2 They investigated call coverage systems of 91 primary care practices in the Denver area by phoning the office numbers, following the recorded instructions, and asking how calls were managed when they spoke to a live person. More than two thirds of the offices used answering services to triage calls, and 93% of these required patients to decide whether the condition was serious enough to warrant contacting the physician on call (this is correct!).
I suppose one could call this approach “patient-centered,” but I suspect this strategy is more to lessen the burden of the on-call physician rather than to promote safe and effective patientcentered care.
The investigators then reviewed reports of all calls not forwarded to the physician on call from 1 of these 91 practices. (A list of calls not forwarded to the physician on call is routinely forwarded to the office the next day by fax.) The physician reviewers in this study judged 50% of these calls to be potentially serious; the patients should have been referred immediately to the physician on call. Clearly, our patients are not making good decisions about the potentially serious nature of their complaints.
To be fair, only 10% of all calls were not forwarded to the on-call physician. Further, the researchers did not investigate each case to determine whether the delay in contact resulted in any untoward events that might have been prevented by immediate referral to the on-call doctor. Perhaps all of the patients needing immediate attention found appropriate care on their own by going to an emergency department or urgent care center. Further research is needed to explore the extent to which medical errors related to afterhours call procedures contribute to adverse patient outcomes.
The Institute of Medicine’s report, To Err is Human,. reminds us that the best way to prevent errors is by improving care systems rather than by attributing personal blame.3 If systems are inadequate for the job, then even the best-intentioned practitioner will provide suboptimal care. Hildebrandt and associates spotted a weakness in the system, a latent error that is easily correctable.
Solutions
What is the solution? I agree with the authors: all after-hours calls for clinical questions should be referred in a timely manner to a clinician. The clinician may be a physician, a physician assistant, or a nurse practitioner. After-hours call systems should be monitored periodically to ensure the systems are safe, effective, efficient, equitable, timely, and patient-centered. Patient complaints about suboptimal after-hours care should be investigated promptly. Continuous quality improvement principles should be applied to assess and improve after-hours care systems, just as we use them to improve office care.
I see no reason to wait. Check out your own after-hours coverage system today to ensure that all clinical calls reach the attention of a competent clinician as soon as possible. You might get another call or two each night you are on call, but I believe the gain will be worth the pain.
1. Committee on Quality and Health Care in America, Institute of Medicine. Crossing the Quality Chasm.. Washington, DC: National Academy Press; 2001.
2. Hildebrandt DE, Westfall JM, Smith PC. After-hours phone calls to physicians: barriers that may affect patient safety. J Fam Pract 2003;222-227.
3. Kohn LT, Corrigan JM, Donaldson MS. To Err Is Human: Building a Safer Health System.. Washington, DC: National Academy Press; 2000.
Associate Editor, Journal of Family Practice.
About: “After-hours telephone triage affects patient safety,”
Will all of you who enjoy taking after-hours calls please stand up?
What? Everyone is still sitting? That’s what I thought. Although taking calls after hours is not one of our favorite duties, after-hours care is a crucial component of primary health care. The recent Institute of Medicine report, Crossing the Quality Chasm.,1 cited 6 characteristics essential for a high-quality health care system for the 21st century:
- safe
- effective
- efficient
- equitable
- timely
- patient-centered.
After-hours call coverage systems should pass muster on all 6 qualities. Do they?
Telephone triage after hours not up to standard
Hildebrandt, Westfall, and Smith provide evidence that the after-hours primary care call systems in the United States are not up to standard.2 They investigated call coverage systems of 91 primary care practices in the Denver area by phoning the office numbers, following the recorded instructions, and asking how calls were managed when they spoke to a live person. More than two thirds of the offices used answering services to triage calls, and 93% of these required patients to decide whether the condition was serious enough to warrant contacting the physician on call (this is correct!).
I suppose one could call this approach “patient-centered,” but I suspect this strategy is more to lessen the burden of the on-call physician rather than to promote safe and effective patientcentered care.
The investigators then reviewed reports of all calls not forwarded to the physician on call from 1 of these 91 practices. (A list of calls not forwarded to the physician on call is routinely forwarded to the office the next day by fax.) The physician reviewers in this study judged 50% of these calls to be potentially serious; the patients should have been referred immediately to the physician on call. Clearly, our patients are not making good decisions about the potentially serious nature of their complaints.
To be fair, only 10% of all calls were not forwarded to the on-call physician. Further, the researchers did not investigate each case to determine whether the delay in contact resulted in any untoward events that might have been prevented by immediate referral to the on-call doctor. Perhaps all of the patients needing immediate attention found appropriate care on their own by going to an emergency department or urgent care center. Further research is needed to explore the extent to which medical errors related to afterhours call procedures contribute to adverse patient outcomes.
The Institute of Medicine’s report, To Err is Human,. reminds us that the best way to prevent errors is by improving care systems rather than by attributing personal blame.3 If systems are inadequate for the job, then even the best-intentioned practitioner will provide suboptimal care. Hildebrandt and associates spotted a weakness in the system, a latent error that is easily correctable.
Solutions
What is the solution? I agree with the authors: all after-hours calls for clinical questions should be referred in a timely manner to a clinician. The clinician may be a physician, a physician assistant, or a nurse practitioner. After-hours call systems should be monitored periodically to ensure the systems are safe, effective, efficient, equitable, timely, and patient-centered. Patient complaints about suboptimal after-hours care should be investigated promptly. Continuous quality improvement principles should be applied to assess and improve after-hours care systems, just as we use them to improve office care.
I see no reason to wait. Check out your own after-hours coverage system today to ensure that all clinical calls reach the attention of a competent clinician as soon as possible. You might get another call or two each night you are on call, but I believe the gain will be worth the pain.
Associate Editor, Journal of Family Practice.
About: “After-hours telephone triage affects patient safety,”
Will all of you who enjoy taking after-hours calls please stand up?
What? Everyone is still sitting? That’s what I thought. Although taking calls after hours is not one of our favorite duties, after-hours care is a crucial component of primary health care. The recent Institute of Medicine report, Crossing the Quality Chasm.,1 cited 6 characteristics essential for a high-quality health care system for the 21st century:
- safe
- effective
- efficient
- equitable
- timely
- patient-centered.
After-hours call coverage systems should pass muster on all 6 qualities. Do they?
Telephone triage after hours not up to standard
Hildebrandt, Westfall, and Smith provide evidence that the after-hours primary care call systems in the United States are not up to standard.2 They investigated call coverage systems of 91 primary care practices in the Denver area by phoning the office numbers, following the recorded instructions, and asking how calls were managed when they spoke to a live person. More than two thirds of the offices used answering services to triage calls, and 93% of these required patients to decide whether the condition was serious enough to warrant contacting the physician on call (this is correct!).
I suppose one could call this approach “patient-centered,” but I suspect this strategy is more to lessen the burden of the on-call physician rather than to promote safe and effective patientcentered care.
The investigators then reviewed reports of all calls not forwarded to the physician on call from 1 of these 91 practices. (A list of calls not forwarded to the physician on call is routinely forwarded to the office the next day by fax.) The physician reviewers in this study judged 50% of these calls to be potentially serious; the patients should have been referred immediately to the physician on call. Clearly, our patients are not making good decisions about the potentially serious nature of their complaints.
To be fair, only 10% of all calls were not forwarded to the on-call physician. Further, the researchers did not investigate each case to determine whether the delay in contact resulted in any untoward events that might have been prevented by immediate referral to the on-call doctor. Perhaps all of the patients needing immediate attention found appropriate care on their own by going to an emergency department or urgent care center. Further research is needed to explore the extent to which medical errors related to afterhours call procedures contribute to adverse patient outcomes.
The Institute of Medicine’s report, To Err is Human,. reminds us that the best way to prevent errors is by improving care systems rather than by attributing personal blame.3 If systems are inadequate for the job, then even the best-intentioned practitioner will provide suboptimal care. Hildebrandt and associates spotted a weakness in the system, a latent error that is easily correctable.
Solutions
What is the solution? I agree with the authors: all after-hours calls for clinical questions should be referred in a timely manner to a clinician. The clinician may be a physician, a physician assistant, or a nurse practitioner. After-hours call systems should be monitored periodically to ensure the systems are safe, effective, efficient, equitable, timely, and patient-centered. Patient complaints about suboptimal after-hours care should be investigated promptly. Continuous quality improvement principles should be applied to assess and improve after-hours care systems, just as we use them to improve office care.
I see no reason to wait. Check out your own after-hours coverage system today to ensure that all clinical calls reach the attention of a competent clinician as soon as possible. You might get another call or two each night you are on call, but I believe the gain will be worth the pain.
1. Committee on Quality and Health Care in America, Institute of Medicine. Crossing the Quality Chasm.. Washington, DC: National Academy Press; 2001.
2. Hildebrandt DE, Westfall JM, Smith PC. After-hours phone calls to physicians: barriers that may affect patient safety. J Fam Pract 2003;222-227.
3. Kohn LT, Corrigan JM, Donaldson MS. To Err Is Human: Building a Safer Health System.. Washington, DC: National Academy Press; 2000.
1. Committee on Quality and Health Care in America, Institute of Medicine. Crossing the Quality Chasm.. Washington, DC: National Academy Press; 2001.
2. Hildebrandt DE, Westfall JM, Smith PC. After-hours phone calls to physicians: barriers that may affect patient safety. J Fam Pract 2003;222-227.
3. Kohn LT, Corrigan JM, Donaldson MS. To Err Is Human: Building a Safer Health System.. Washington, DC: National Academy Press; 2000.