User login
Lung Cancer Screening: Translating Research Into Practice (FULL)
According to the National Lung Screening Trial (NLST), use of low-dose computed tomography (LDCT) reduced lung cancer deaths by 20%. That finding led the U.S. Preventive Services Task Force (USPSTF) to recommend screening for high-risk individuals (current and former heavy smokers). And that, in turn, led to hospitals nationwide setting up lung cancer screening programs—a number that rose “dramatically,” according to the National Cancer Institute (NCI), after the Centers for Medicare and Medicaid Services decided to cover LDCT screening for high-risk Medicare beneficiaries.
But how does the screening recommendation pan out in real life? Primary care physicians and pulmonologists alike were concerned about the workability of putting LDCT into real-life practice. And not without basis: Published experience with implementation of lung cancer screening (LCS) is limited, say VHA researchers. Their 3-year demonstration project bears out the concerns, they add.
When they designed the study, the researchers wanted to see how feasible LCS would be in terms of resources and effort, whether patients would take part, what their clinical experience might be, and what type of findings the screenings might produce. The researchers found that establishing and sustaining a screening program requires “significant clinical effort for as-yet uncertain patient benefit.” They also found “wide variation” in both processes and patient experiences among the 8 VA hospitals in the study. Moreover, they found that, although most patients had findings that required follow-up, few had early-stage lung cancers.
Of the 2,106 screened patients in a JAMA Internal Medicine study, 1,257 had nodules. More than half of those required tracking, and 2% required further evaluation, but the findings were not cancer. Just 1.5% had lung cancer. Scans of 857 patients (40.7%) also revealed a variety of incidental findings, such as emphysema, other pulmonary abnormalities, and coronary artery calcification.
The researchers say that implementing a comprehensive program that followed recommendations was “challenging and complex,” requiring new tools and processes for staff as well as for dedicated patient coordination. As an example, they say creating electronic tools to capture the necessary clinical data in real time that met the needs of the screening coordinators proved to be difficult, “even with the VHA’s highly regarded electronic medical record.”
Also, finding out who actually had a smoking history of > 30 pack-years of smoking per the USPSTF recommendation was not easy. Lead investigator Linda Kinsinger, MD, MPH, points out, “People who smoke don’t track that sort of thing as closely as you think they would, and they don’t smoke at the same level for years and years.”
The researchers estimate that nearly 900,000 veterans meet the initial screening criteria for age, smoking history, and medical history, but they caution that accurately identifying the patients and discussing risks and benefits will take “significant effort” for primary care teams. Even if that number were reduced by 16% to account for longer medical contraindications, the number of veterans who might be candidates for annual LCS would be “substantial.” And based on the researchers’ experience, a bit more than half the candidates will agree to be screened.
Although screening programs are a complex endeavor, the researchers say, the results of their study can help the VHA plan for broader implementation of comprehensive screening programs.
“What [the VHA] is reporting is the initial experience for almost everybody,” said Lynn Tanoue, MD, director of the Lung Screening and Nodule Program at Yale Cancer Center in New Haven, Connecticut, in an interview with the NCI. “Until people really started doing lung cancer screening and began to understand the challenges of doing it properly, you couldn’t have known what it was going to be like.” But she adds, “The data from NLST were very clear. We should accept that there is benefit and choose the right population to screen.”
However, although the LDCT screening can find signs of early lung cancer, a biopsy is often necessary. Researchers from Boston University suggest an effective, much less invasive approach: analyzing gene expression in nasal cell samples.
The researchers collected and analyzed nasal cell samples from 505 current and former smokers for gene expression. They found 535 genes that were expressed differently between patients who were diagnosed with lung cancer and those whose lesions were benign.
Comparing those data with data from bronchial airway samples from the same patients, the researchers found changes that were similar between the nose and lung samples of patients with lung cancer, suggesting that smoking might cause similar genetic changes throughout the entire airway.
The researchers used the 30 most prominent changes to create a biomarker panel and tested it in 130 other patients. Compared with a clinical risk factor model that considered age, smoking status, and other factors, the biomarker panel was better at predicting lung cancer. Combining the 2 models further improved detection. “We find that nasal gene expression contains information about the presence of cancer that is independent of standard clinical risk factors,” said one of the co-lead investigators.
Lung cancer screening is still experiencing “growing pains,” the NCI says. And the need for screening is both acute and chronic: A 2015 study found that only 4% of people who meet the criteria for screening actually undergo screening.
These studies not only open avenues for better screening and diagnosis, but also highlight the need for better patient education.
Click here to read the digital edition.
Kinsinger LS, Anderson C, Kim J, et al. JAMA Intern Med. 2017;177(3):399-406.
National Cancer Institute. https://www.cancer.gov/types/lung/research/nlst. Updated September 8, 2014. Accessed April 25, 2017.
National Institutes of Health. https://www.nih.gov/news-events/nih-research-matters/noninvasive-strategies-lung-cancer-testing. Published March 7, 2017. Accessed April
25, 2017.
AEGIS Study Team. J. Natl Cancer Inst. 2017;109(7).
National Cancer Institute. https://www.cancer.gov/news-events/cancer-currents-blog/2017/lung-cancer-screening-challenges. Published February 27, 2017. Accessed April
25, 2017.
According to the National Lung Screening Trial (NLST), use of low-dose computed tomography (LDCT) reduced lung cancer deaths by 20%. That finding led the U.S. Preventive Services Task Force (USPSTF) to recommend screening for high-risk individuals (current and former heavy smokers). And that, in turn, led to hospitals nationwide setting up lung cancer screening programs—a number that rose “dramatically,” according to the National Cancer Institute (NCI), after the Centers for Medicare and Medicaid Services decided to cover LDCT screening for high-risk Medicare beneficiaries.
But how does the screening recommendation pan out in real life? Primary care physicians and pulmonologists alike were concerned about the workability of putting LDCT into real-life practice. And not without basis: Published experience with implementation of lung cancer screening (LCS) is limited, say VHA researchers. Their 3-year demonstration project bears out the concerns, they add.
When they designed the study, the researchers wanted to see how feasible LCS would be in terms of resources and effort, whether patients would take part, what their clinical experience might be, and what type of findings the screenings might produce. The researchers found that establishing and sustaining a screening program requires “significant clinical effort for as-yet uncertain patient benefit.” They also found “wide variation” in both processes and patient experiences among the 8 VA hospitals in the study. Moreover, they found that, although most patients had findings that required follow-up, few had early-stage lung cancers.
Of the 2,106 screened patients in a JAMA Internal Medicine study, 1,257 had nodules. More than half of those required tracking, and 2% required further evaluation, but the findings were not cancer. Just 1.5% had lung cancer. Scans of 857 patients (40.7%) also revealed a variety of incidental findings, such as emphysema, other pulmonary abnormalities, and coronary artery calcification.
The researchers say that implementing a comprehensive program that followed recommendations was “challenging and complex,” requiring new tools and processes for staff as well as for dedicated patient coordination. As an example, they say creating electronic tools to capture the necessary clinical data in real time that met the needs of the screening coordinators proved to be difficult, “even with the VHA’s highly regarded electronic medical record.”
Also, finding out who actually had a smoking history of > 30 pack-years of smoking per the USPSTF recommendation was not easy. Lead investigator Linda Kinsinger, MD, MPH, points out, “People who smoke don’t track that sort of thing as closely as you think they would, and they don’t smoke at the same level for years and years.”
The researchers estimate that nearly 900,000 veterans meet the initial screening criteria for age, smoking history, and medical history, but they caution that accurately identifying the patients and discussing risks and benefits will take “significant effort” for primary care teams. Even if that number were reduced by 16% to account for longer medical contraindications, the number of veterans who might be candidates for annual LCS would be “substantial.” And based on the researchers’ experience, a bit more than half the candidates will agree to be screened.
Although screening programs are a complex endeavor, the researchers say, the results of their study can help the VHA plan for broader implementation of comprehensive screening programs.
“What [the VHA] is reporting is the initial experience for almost everybody,” said Lynn Tanoue, MD, director of the Lung Screening and Nodule Program at Yale Cancer Center in New Haven, Connecticut, in an interview with the NCI. “Until people really started doing lung cancer screening and began to understand the challenges of doing it properly, you couldn’t have known what it was going to be like.” But she adds, “The data from NLST were very clear. We should accept that there is benefit and choose the right population to screen.”
However, although the LDCT screening can find signs of early lung cancer, a biopsy is often necessary. Researchers from Boston University suggest an effective, much less invasive approach: analyzing gene expression in nasal cell samples.
The researchers collected and analyzed nasal cell samples from 505 current and former smokers for gene expression. They found 535 genes that were expressed differently between patients who were diagnosed with lung cancer and those whose lesions were benign.
Comparing those data with data from bronchial airway samples from the same patients, the researchers found changes that were similar between the nose and lung samples of patients with lung cancer, suggesting that smoking might cause similar genetic changes throughout the entire airway.
The researchers used the 30 most prominent changes to create a biomarker panel and tested it in 130 other patients. Compared with a clinical risk factor model that considered age, smoking status, and other factors, the biomarker panel was better at predicting lung cancer. Combining the 2 models further improved detection. “We find that nasal gene expression contains information about the presence of cancer that is independent of standard clinical risk factors,” said one of the co-lead investigators.
Lung cancer screening is still experiencing “growing pains,” the NCI says. And the need for screening is both acute and chronic: A 2015 study found that only 4% of people who meet the criteria for screening actually undergo screening.
These studies not only open avenues for better screening and diagnosis, but also highlight the need for better patient education.
Click here to read the digital edition.
According to the National Lung Screening Trial (NLST), use of low-dose computed tomography (LDCT) reduced lung cancer deaths by 20%. That finding led the U.S. Preventive Services Task Force (USPSTF) to recommend screening for high-risk individuals (current and former heavy smokers). And that, in turn, led to hospitals nationwide setting up lung cancer screening programs—a number that rose “dramatically,” according to the National Cancer Institute (NCI), after the Centers for Medicare and Medicaid Services decided to cover LDCT screening for high-risk Medicare beneficiaries.
But how does the screening recommendation pan out in real life? Primary care physicians and pulmonologists alike were concerned about the workability of putting LDCT into real-life practice. And not without basis: Published experience with implementation of lung cancer screening (LCS) is limited, say VHA researchers. Their 3-year demonstration project bears out the concerns, they add.
When they designed the study, the researchers wanted to see how feasible LCS would be in terms of resources and effort, whether patients would take part, what their clinical experience might be, and what type of findings the screenings might produce. The researchers found that establishing and sustaining a screening program requires “significant clinical effort for as-yet uncertain patient benefit.” They also found “wide variation” in both processes and patient experiences among the 8 VA hospitals in the study. Moreover, they found that, although most patients had findings that required follow-up, few had early-stage lung cancers.
Of the 2,106 screened patients in a JAMA Internal Medicine study, 1,257 had nodules. More than half of those required tracking, and 2% required further evaluation, but the findings were not cancer. Just 1.5% had lung cancer. Scans of 857 patients (40.7%) also revealed a variety of incidental findings, such as emphysema, other pulmonary abnormalities, and coronary artery calcification.
The researchers say that implementing a comprehensive program that followed recommendations was “challenging and complex,” requiring new tools and processes for staff as well as for dedicated patient coordination. As an example, they say creating electronic tools to capture the necessary clinical data in real time that met the needs of the screening coordinators proved to be difficult, “even with the VHA’s highly regarded electronic medical record.”
Also, finding out who actually had a smoking history of > 30 pack-years of smoking per the USPSTF recommendation was not easy. Lead investigator Linda Kinsinger, MD, MPH, points out, “People who smoke don’t track that sort of thing as closely as you think they would, and they don’t smoke at the same level for years and years.”
The researchers estimate that nearly 900,000 veterans meet the initial screening criteria for age, smoking history, and medical history, but they caution that accurately identifying the patients and discussing risks and benefits will take “significant effort” for primary care teams. Even if that number were reduced by 16% to account for longer medical contraindications, the number of veterans who might be candidates for annual LCS would be “substantial.” And based on the researchers’ experience, a bit more than half the candidates will agree to be screened.
Although screening programs are a complex endeavor, the researchers say, the results of their study can help the VHA plan for broader implementation of comprehensive screening programs.
“What [the VHA] is reporting is the initial experience for almost everybody,” said Lynn Tanoue, MD, director of the Lung Screening and Nodule Program at Yale Cancer Center in New Haven, Connecticut, in an interview with the NCI. “Until people really started doing lung cancer screening and began to understand the challenges of doing it properly, you couldn’t have known what it was going to be like.” But she adds, “The data from NLST were very clear. We should accept that there is benefit and choose the right population to screen.”
However, although the LDCT screening can find signs of early lung cancer, a biopsy is often necessary. Researchers from Boston University suggest an effective, much less invasive approach: analyzing gene expression in nasal cell samples.
The researchers collected and analyzed nasal cell samples from 505 current and former smokers for gene expression. They found 535 genes that were expressed differently between patients who were diagnosed with lung cancer and those whose lesions were benign.
Comparing those data with data from bronchial airway samples from the same patients, the researchers found changes that were similar between the nose and lung samples of patients with lung cancer, suggesting that smoking might cause similar genetic changes throughout the entire airway.
The researchers used the 30 most prominent changes to create a biomarker panel and tested it in 130 other patients. Compared with a clinical risk factor model that considered age, smoking status, and other factors, the biomarker panel was better at predicting lung cancer. Combining the 2 models further improved detection. “We find that nasal gene expression contains information about the presence of cancer that is independent of standard clinical risk factors,” said one of the co-lead investigators.
Lung cancer screening is still experiencing “growing pains,” the NCI says. And the need for screening is both acute and chronic: A 2015 study found that only 4% of people who meet the criteria for screening actually undergo screening.
These studies not only open avenues for better screening and diagnosis, but also highlight the need for better patient education.
Click here to read the digital edition.
Kinsinger LS, Anderson C, Kim J, et al. JAMA Intern Med. 2017;177(3):399-406.
National Cancer Institute. https://www.cancer.gov/types/lung/research/nlst. Updated September 8, 2014. Accessed April 25, 2017.
National Institutes of Health. https://www.nih.gov/news-events/nih-research-matters/noninvasive-strategies-lung-cancer-testing. Published March 7, 2017. Accessed April
25, 2017.
AEGIS Study Team. J. Natl Cancer Inst. 2017;109(7).
National Cancer Institute. https://www.cancer.gov/news-events/cancer-currents-blog/2017/lung-cancer-screening-challenges. Published February 27, 2017. Accessed April
25, 2017.
Kinsinger LS, Anderson C, Kim J, et al. JAMA Intern Med. 2017;177(3):399-406.
National Cancer Institute. https://www.cancer.gov/types/lung/research/nlst. Updated September 8, 2014. Accessed April 25, 2017.
National Institutes of Health. https://www.nih.gov/news-events/nih-research-matters/noninvasive-strategies-lung-cancer-testing. Published March 7, 2017. Accessed April
25, 2017.
AEGIS Study Team. J. Natl Cancer Inst. 2017;109(7).
National Cancer Institute. https://www.cancer.gov/news-events/cancer-currents-blog/2017/lung-cancer-screening-challenges. Published February 27, 2017. Accessed April
25, 2017.
Enhanced Melanoma Diagnosis With Multispectral Digital Skin Lesion Analysis
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
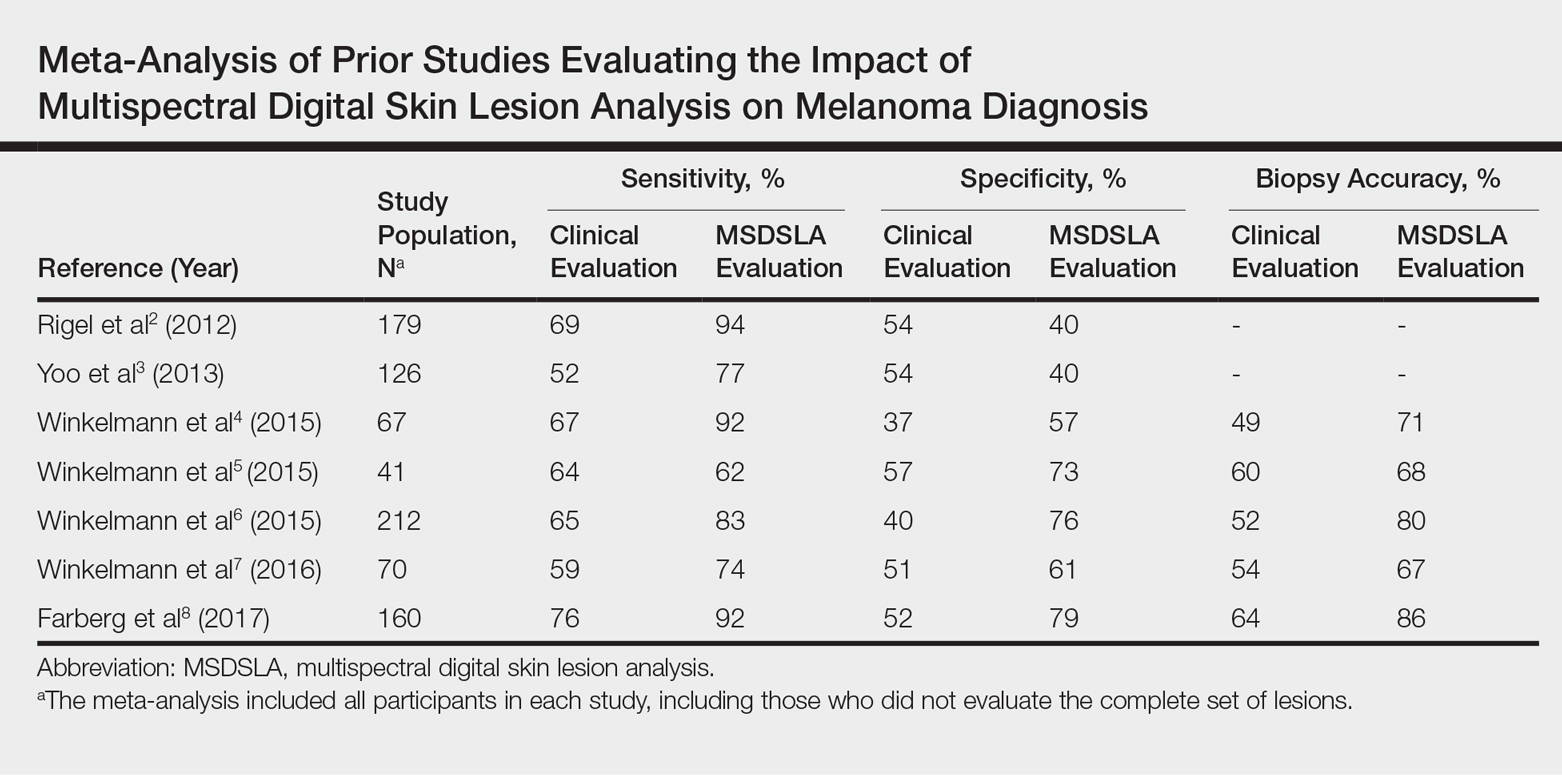
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods
Results
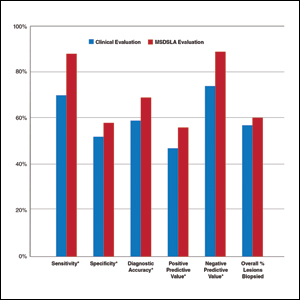
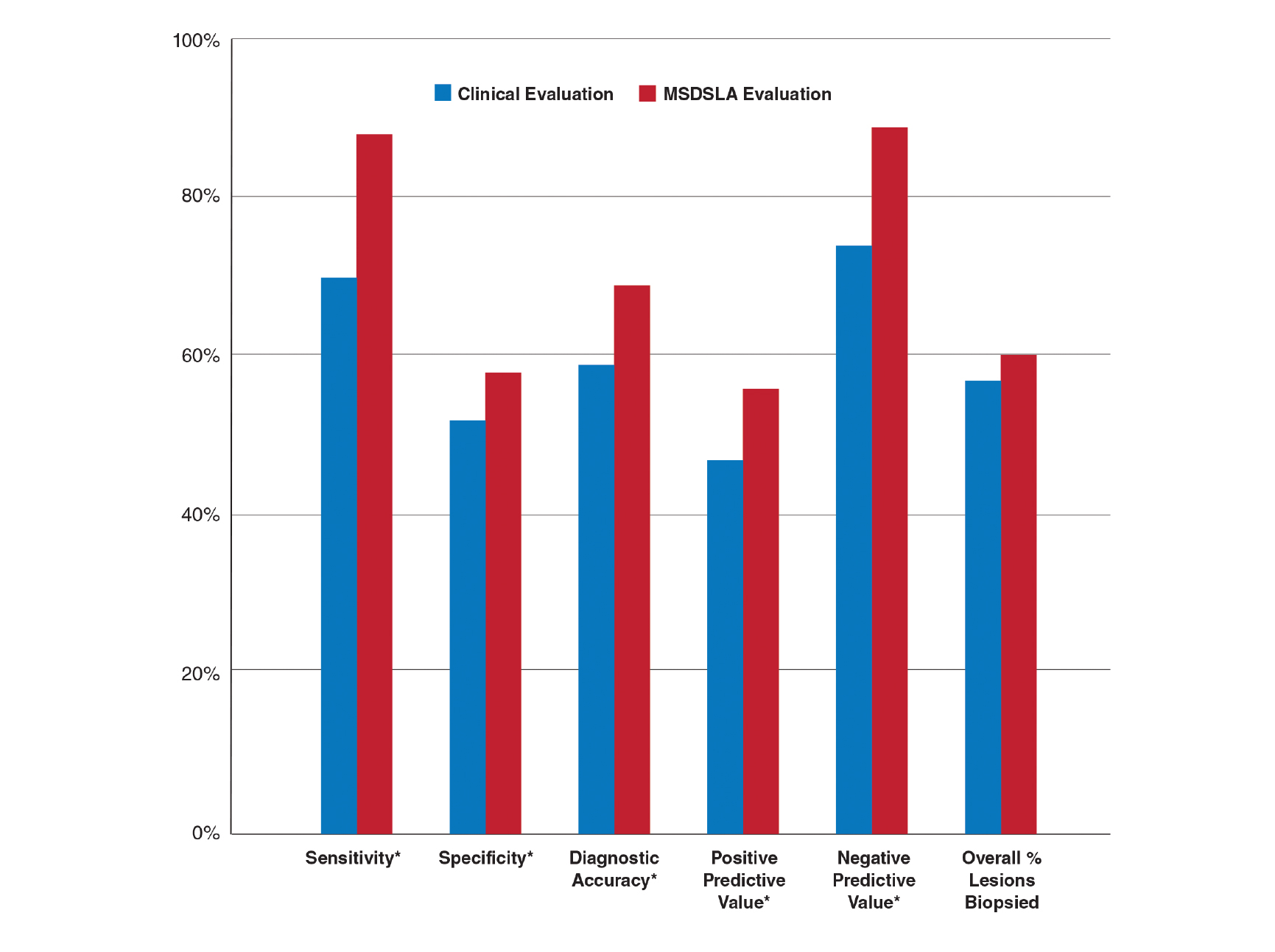
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.
Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods

Results
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.

Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
Early detection of melanoma, which is known to improve survival rates, remains a challenge for dermatologists. Suspicious pigmented lesions typically are evaluated via clinical examination and dermoscopy; however, new technologies are being developed to provide additional objective information for clinicians to incorporate into their biopsy decisions.
Multispectral digital skin lesion analysis (MSDSLA) uses 10 bands of visible and near-infrared light (430–950 nm) to image and analyze pigmented skin lesions (PSLs) down to 2.5 mm below the skin surface and measures the distribution of melanin using 75 unique algorithms to determine the degree of the morphologic disorder. Using a logical regression model previously validated on a set of 1632 PSLs, the probability of melanoma and probability of being a melanoma/PSL of high-risk malignant potential are then provided to the clinician.1
In this study, we analyzed aggregate data from 7 prior studies2-8 to better determine how MSDSLA impacts the biopsy decisions of dermatologists and nondermatologists following clinical examination and dermoscopic evaluation of PSLs.
Methods

Results
Overall sensitivity for the detection of melanoma or other high-grade PSLs improved from 70% on clinical and dermoscopic evaluation to 88% after MSDSLA information was provided (P<.0001), and specificity increased from 52% to 58% (P<.001). Diagnostic accuracy also improved from 59% on clinical evaluation to 69% after review of MSDSLA findings (P<.0001). The positive predictive value of biopsy decisions was 47% following clinical evaluation, which improved to 56% after evaluation of MSDSLA findings (P<.001), and the negative predictive value increased from 74% to 89% (P<.0001). The overall percentage of lesions selected for biopsy did not significantly change following MSDSLA data integration (57% vs 60%)(Figure). Given that similar numbers of lesions were biopsied with improved sensitivity and specificity, the integration of MSDSLA data into the biopsy decision led to an improved biopsy ratio (ratio of melanomas biopsied to total biopsies) and fewer unnecessary biopsies.

Comment
Our broad analysis further supported the findings of prior studies that decisions to biopsy clinically suspicious PSLs are more sensitive, specific, and accurate when practitioners are provided MSDSLA information following clinical examination.2-8
Given the evolution in health care economics, it is clear that greater emphasis will continue to be placed on superior, evidence-based, effective care. The reported diagnostic sensitivities and specificities of clinical evaluation and dermoscopy for melanoma detection vary widely throughout the literature, with sensitivities ranging from 58% to over 90% and specificities ranging from 77% to 99%.9-11
Our study had several limitations. For this analysis to be more representative of lesion biopsy selection in the clinical setting, biopsy sensitivity (correctly identifying lesions appropriate for biopsy) vs melanoma sensitivity (identifying a lesion as melanoma) was used.13 The overall sensitivity found was within the range of prior studies,2-8 but this approach may have potentially led to a lower specificity due to an increased number of lesions biopsied. Additionally, the melanomas selected for these studies were early (malignant melanoma in situ or mean thickness of invasive malignant melanoma of 0.3 mm), and the nonmelanomas (including low-grade dysplastic nevi) were not necessarily diagnostically straightforward. This may have led to the clinical and dermoscopic sensitivity and specificity noted being lower than in some prior studies.9-11
The risk of missing a melanoma with MSDSLA devices has led manufacturers to strive for a high sensitivity for their devices, leading to lower specificity as a consequence. For this reason and other ambiguous practical considerations (eg, device and patient costs, difficulty with insurance reimbursement), the adoption of this technology into routine clinical practice has remained relatively static; however, using enhanced diagnostic technologies such as MSDSLA may help with more accurate identification of high-risk PSLs, thereby leading to earlier detection and overall less expensive, more cost-effective treatment of melanoma.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
- Monheit G, Cognetta AB, Ferris L, et al. The performance of MelaFind: a prospective multicenter study. Arch Dermatol. 2011;147:188-194.
- Rigel DS, Roy M, Yoo J, et al. Impact of guidance from a computer-aided multispectral digital skin lesion analysis device on decision to biopsy lesions clinically suggestive of melanoma. Arch Dermatol. 2012;148:541-543.
- Yoo J, Rigel DS, Roy M, et al. Impact of guidance from a multispectral digital skin lesion analysis device on dermatology residents decisions to biopsy lesions clinically suggestive of melanoma. J Am Acad Dermatol. 2013;68:AB152.
- Winkelmann RR, Yoo J, Tucker N, et al. Impact of guidance provided by a multispectral digital skin lesion analysis device following dermoscopy on decisions to biopsy atypical melanocytic lesions. J Clin Aesthet Dermatol. 2015;8:21-24.
- Winkelmann RR, Hauschild A, Tucker N, et al. The impact of multispectral digital skin lesion analysis on German dermatologist decisions to biopsy atypical pigmented lesions with clinical characteristics of melanoma. J Clin Aesthet Dermatol. 2015;8:27-29.
- Winkelmann RR, Tucker N, White R, et al. Pigmented skin lesion biopsies after computer-aided multispectral digital skin lesion analysis. J Am Osteopath Assoc. 2015;115:666-669.
- Winkelmann RR, Farberg AS, Tucker N, et al. Enhancement of international dermatologists’ pigmented skin lesion biopsy decisions following dermoscopy with subsequent integration of multispectral digital skin lesion analysis [published online July 1, 2016]. J Clin Aesthet Dermatol. 2016;9:53-55.
- Farberg AS, Winkelmann RR, Tucker N, et al. The impact of quantitative data provided by a multi-spectral digital skin lesion analysis device on dermatologists’ decisions to biopsy pigmented lesions [published online September 1, 2017]. J Clin Aesthet Dermatol. 2017;10:24-26.
- Wolf IH, Smolle J, Soyer HP, et al. Sensitivity in the clinical diagnosis of malignant melanoma. Melanoma Res. 1998;8:425-429.
- Kittler H, Pehamberger H, Wolff K, et al. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159-165.
- Ascierto PA, Palmieri G, Celentano E, et al. Sensitivity and specificity of epiluminescence microscopy: evaluation on a sample of 2731 excised cutaneous pigmented lesions: the Melanoma Cooperative Study. Br J Dermatol. 2000;142:893-898.
- Carli P, Nardini P, Crocetti E, et al. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14:403-407.
- Friedman RJ, Gutkowicz-Krusin D, Farber MJ, et al. The diagnostic performance of expert dermoscopists vs a computer-vision system on small-diameter melanomas. Arch Dermatol. 2008;144:476-482.
Practice Points
- Multispectral digital skin lesion analysis (MSDSLA) can be a valuable tool in the evaluation of pigmented skin lesions (PSLs).
- MSDSLA may help to better identify high-risk PSLs and improve cost of care.
Mohs Micrographic Surgery for Digital Melanoma and Nonmelanoma Skin Cancers
Mohs micrographic surgery (MMS) is a specialized surgical technique for the treatment of melanoma and nonmelanoma skin cancers (NMSCs).1-3 The procedure involves surgical excision, histopathologic examination, precise mapping of malignant tissue, and wound management. Indications for MMS in skin cancer patients include recurring lesions, lesions in high-risk anatomic locations, aggressive histologic subtypes (ie, morpheaform, micronodular, infiltrative, high-grade, poorly differentiated), perineural invasion, large lesion size (>2 cm in diameter), poorly defined lateral or vertical clinical borders, rapid growth of the lesion, immunocompromised status, and sites of positive margins on prior excision. The therapeutic advantages of MMS include tissue conservation and optimal margin control in cosmetically or functionally sensitive areas, such as acral sites (eg, hands, feet, digits).1,3
The intricacies of the nail apparatus complicate diagnostic biopsy and precise delineation of peripheral margins in digital skin cancers; thus, early diagnosis and intraoperative histologic examination of the margins are essential. Traditionally, the surgical approach to subungual cutaneous tumors such as melanoma has included digital amputation4; however, a study of the treatment of subungual melanoma revealed no difference in survival based on the level of amputation, therefore advocating for less radical treatment.4
Interestingly, MMS for cutaneous tumors localized to the digits is not frequently reviewed in the dermatologic literature. We present a retrospective case series evaluating the clinical outcomes of digital melanoma and NMSCs treated with MMS.
Methods
A retrospective chart review was performed at a private dermatology practice to identify patients who underwent MMS for melanoma or NMSC localized to the digits from January 2009 to December 2014. All patients were treated in the office by 1 Mohs surgeon (A.H.) and were evaluated before and after MMS. Data were collected from the electronic medical record of the practice, including patient demographics, histopathologic diagnosis, tumor status (primary or recurrent lesion), anatomic site of the tumor, preoperative and postoperative size of the lesion, number of MMS stages, surgical repair technique, postoperative complications, and follow-up period.
Results
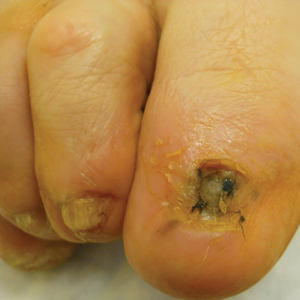
Twenty-seven patients (13 male, 14 female) with a total of 28 lesions (malignant melanoma or NMSC) localized to the digits were identified (Table). The mean age at the time of MMS was 64.07 years.
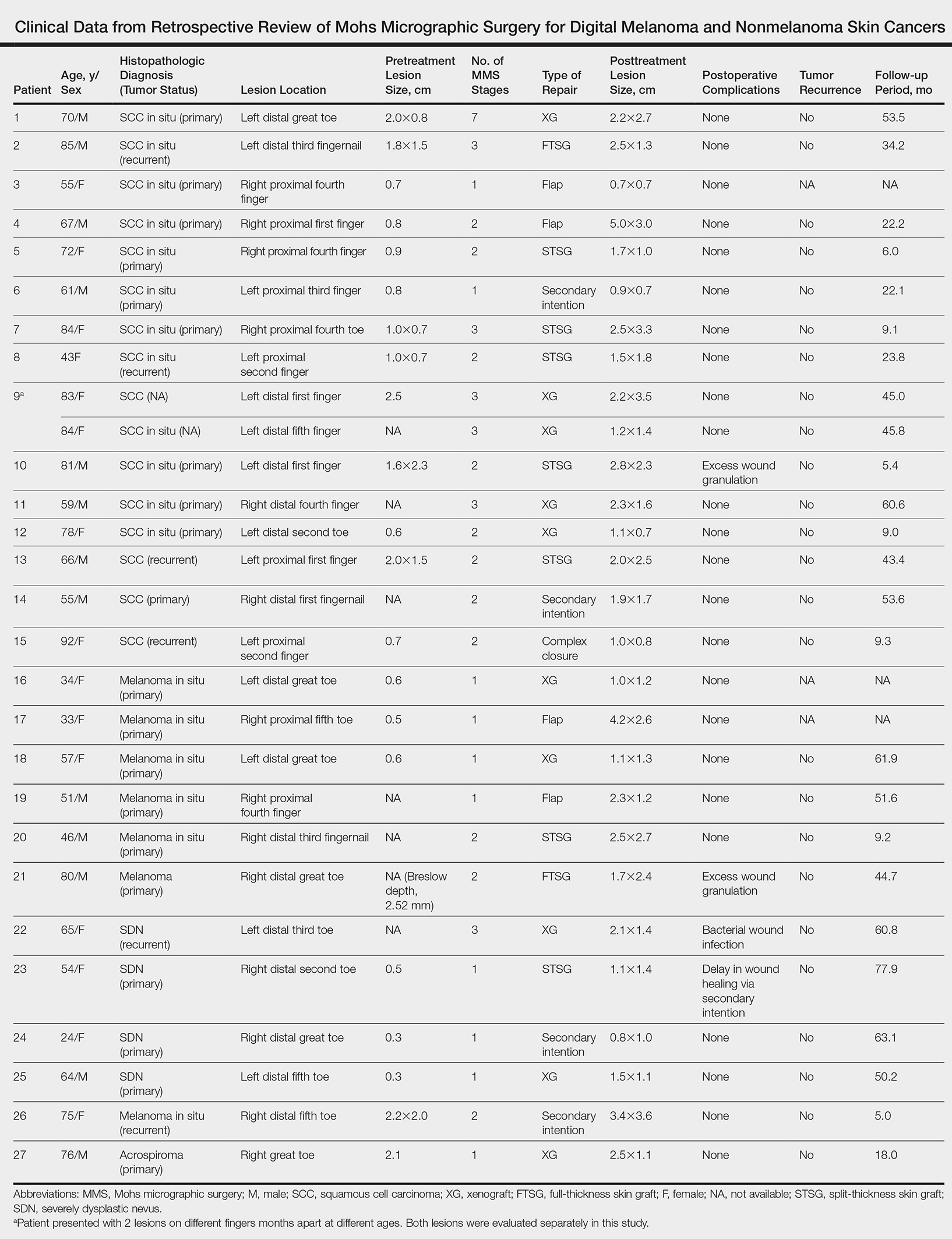
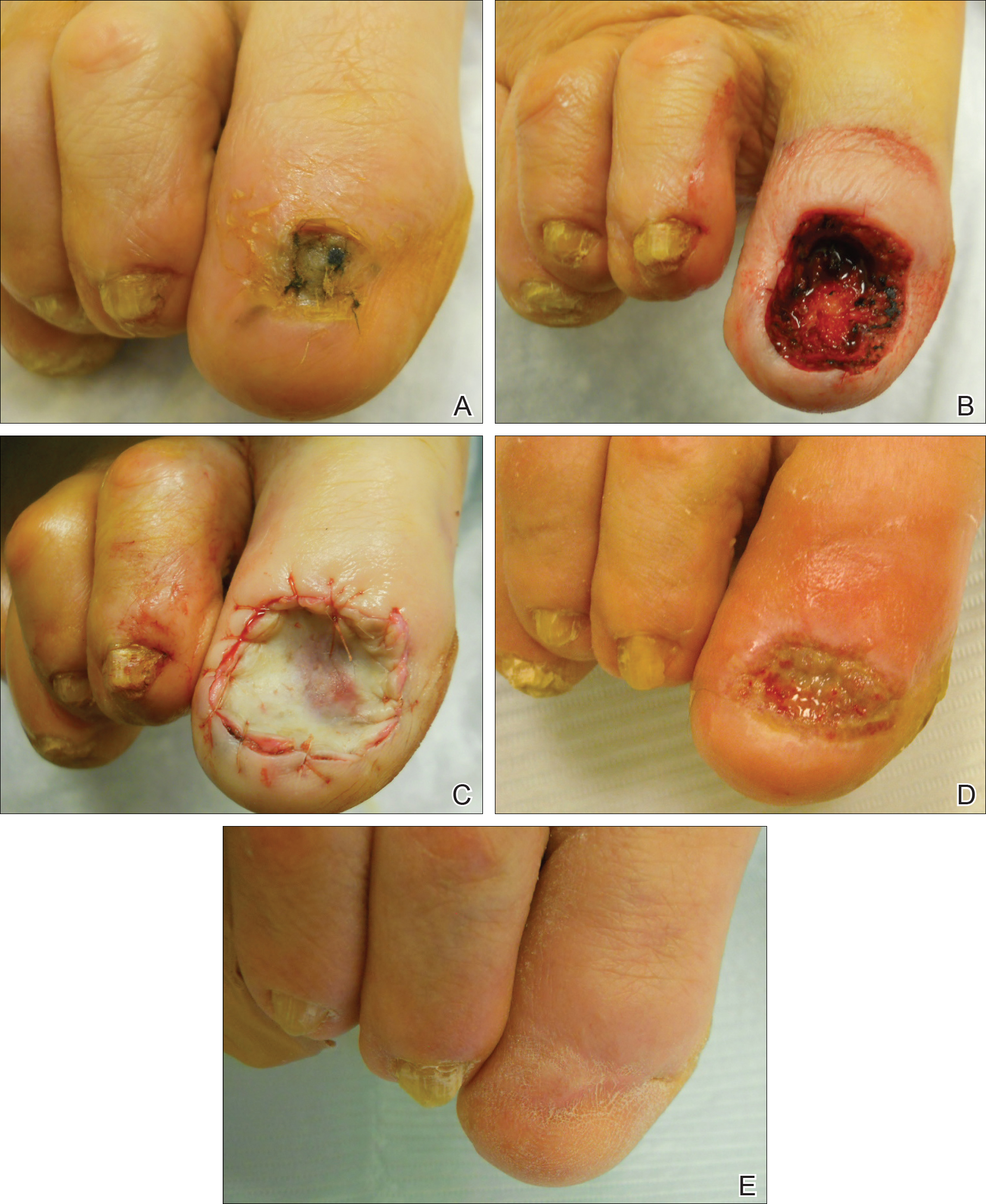
Surgical techniques used for repair following MMS included xenograft (10/28 [35.71%]); split-thickness skin graft (7/28 [25.0%]); secondary intention (4/28 [14.29%]); flap (4/28 [14.29%]); full-thickness skin graft (2/28 [7.14%]); and complex closure (1/28 [3.57%]). Clinical preoperative, operative, and postoperative photos from Patient 21 in this series are shown here (Figure). Two patients required bony phalanx resection due to invasion of the tumor into the periosteum: 1 had a malignant melanoma (Breslow depth, 2.52 mm); the other had an SCC. In addition, following removal of a severely dysplastic nevus, debulked tissue revealed melanoma in 1 patient.
Postoperative complications were noted in 4 (14.29%) of 28 MMS procedures, including bacterial wound infection (3.57%), excess granulation tissue that required wound debridement (7.14%), and delay in wound healing (3.57%). Follow-up data were available for 25 of the 28 MMS procedures (mean follow-up, 35.4 months), during which no recurrences were observed.
Comment
Mohs micrographic surgery is a specialized technique used in the treatment of cutaneous tumors, including basal cell carcinoma, SCC, melanoma in situ, atypical fibroxanthoma, dermatofibrosarcoma protuberans, sebaceous carcinoma, microcystic adnexal carcinoma, and Merkel cell carcinoma, among other cutaneous tumors.1-3 Mohs micrographic surgery provides the advantage of tissue conservation as well as optimal margin control in cosmetically or functionally sensitive areas while providing a higher cure rate than surgical excision. During the procedure, the surgical margin is examined histologically, thus ensuring definitive removal of the tumor but minimal loss of surrounding normal tissue.1-3 Mohs micrographic surgery is particularly useful for treating lesions on acral sites (eg, hands, feet, and digits).3-5
The treatment of digital skin cancers has evolved over the past 50 years with advancements resulting in more precise, tissue-sparing methods, in contrast to previous treatments such as amputation and wide local excision.6 More specifically, traditional digital amputation for the treatment of subungual melanoma has been reevaluated in multiple studies, which did not demonstrate a statistically significant difference in survival based on the level of amputation, thereby favoring less radical treatment.4,6 Moehrle et al7 found no statistical difference in recurrence rate when comparing patients with digital melanomas treated with partial amputation and those treated with digit-sparing surgery with limited excision and histologic evaluation of margins. Additionally, in a study conducted by Lazar et al,8 no recurrence of 13 subungual malignancies treated with MMS that utilized a full-thickness graft was reported at 4-year follow-up. In a large retrospective series of digital melanomas treated with MMS, Terushkin et al5 reported that 96.5% (55/57) of patients with primary melanomas that were treated with MMS avoided amputation, and the 5- and 10-year melanoma-specific survival rates for all patients treated with MMS were 95.0% and 82.6%, respectively.
In our study, cutaneous malignancies were located most often on the fingers, and the most common skin cancer identified was SCC in situ. The literature has shown that SCC in situ and SCC are the most common cutaneous neoplasms of the digits and nail unit.9 The most common specific anatomic site of cutaneous malignancy in our study was the great toe, followed by the fourth finger. A study conducted by Tan et al9 revealed that the great toe was the most common location of melanoma of the nail bed and subungual region, followed by the thumb. In contrast, primary subungual SCCs occur most frequently on the finger, with rare cases involving the toes.10
The etiology of digital SCC may involve extensive sun exposure, chronic trauma and wounds, and viral infection.9,11 More specifically, the dermatologic literature provides evidence of human papillomavirus (HPV) type 16 involvement in the pathogenesis of digital and periungual SCC. A genital-digital mechanism of spread has been implicated.11,12 An increased recurrence rate of HPV-associated digital SCCs has been reported following MMS, likely secondary to residual postsurgical HPV infection.11,12
Maintaining function and cosmesis of the hands, feet, and digits following MMS can be challenging, sometimes requiring skin grafts and flaps to close the defect. In the 28 MMS procedures evaluated in our study, 19 (67.9%) surgical defects were repaired with a graft (ie, split-thickness skin graft, full-thickness skin graft, xenograft), 4 (14.3%) with a flap (advancement and rotation), 4 (14.3%) by secondary intention, and 1 (3.6%) with primary complex closure.
Surgical grafts can be categorized based on the origin of the graft.2,13 Autografts, derived from the patient’s skin, are the most frequently used dermatologic graft and can be further categorized as full-thickness skin grafts, which include the epidermis and the entire dermis, thus preserving adnexal structures, and split-thickness skin grafts, which include the epidermis and partial dermis.2,13
A cross-sectional survey of fellowship-trained Mohs surgeons revealed that more than two-thirds of repairs for cutaneous acral cancers were performed using a primary closure technique, and one-fourth of closures were performed using secondary intention.15 Of the less frequently utilized skin-graft repairs, more were for acral lesions on the legs than on the arms.14 The type of procedure and graft used is dependent on multiple variables, including the anatomic location of the lesion and final size of the defect following MMS.2 Similarly, the use of specific types of sutures depends on the anatomic location of the lesion, relative thickness of the skin, degree of tension, and desired cosmetic result.15 The expertise of a hand surgeon may be required, particularly in cases in which the extensor tendon of the distal interphalangeal joint is compromised, manifested by a droopy fingertip when the hand is held horizontally. Additionally, special attention should be paid to removing the entire nail matrix before skin grafting for subungual tumors to avoid nail growth under the skin graft.
Evaluation of debulked tissue from digital skin cancers proved to be important in our study. In Patient 21, debulked tissue revealed melanoma following removal of a severely dysplastic nevus. This finding emphasizes the importance of complete excision of such lesions, as remaining underlying portions of the lesion can reveal residual tumor of the same or different histopathology.
In a prospective study, MMS was shown to have a low rate (0.91%; 95% confidence interval, 0.38%-1.45%) of surgical site infection in the absence of prophylactic antibiotics.16 The highest rates of surgical site infection were closely associated with flap closure. In our study, most patients had an uncomplicated and successful postoperative recovery. Only 1 (3.57%) of the 28 MMS procedures (Patient 22) was complicated by a bacterial wound infection postoperatively. The lesion removed in this case was a severely dysplastic melanocytic nevus on the toe. Infection resolved after a course of oral antibiotics, but the underlying cause of the wound infection in the patient was unclear. Other postoperative complications in our study included delayed wound healing and excess granulation tissue requiring wound debridement.
There are limited data in the dermatologic literature regarding outcomes following MMS for the treatment of cutaneous malignancies localized to the digits.
Additional limitations of this case review include its single-center and retrospective design, the small sample size, and 1 Mohs surgeon having performed all surgeries.
Conclusion
This study provides further evidence of the benefit of MMS for the treatment of malignant melanoma and NMSCs of the digits. This procedure provides margin-controlled excision of these malignant neoplasms while preserving maximal normal tissue, thereby providing patients with improved postoperative function and cosmesis. Long-term follow-up data demonstrating a lack of tumor recurrence underscores the assertion that MMS is safe and effective for the treatment of skin cancer of the digits.
- Dim-Jamora KC, Perone JB. Management of cutaneous tumors with mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
- McLeod MP, Choudhary S, Alqubaisy YA, et al. Indications for Mohs micrographic surgery. In: Nouri K, ed. Mohs Micrographic Surgery. New York, NY: Springer; 2012:5-13.
- Loosemore MP, Morales-Burgos A, Goldberg LH. Acral lentiginous melanoma of the toe treated using Mohs surgery with sparing of the digit and subsequent reconstruction using split-thickness skin graft. Dermatol Surg. 2013;39:136-138.
- Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg. 2007;60:635-638.
- Terushkin V, Brodland DG, Sharon DJ, et al. Digit-sparing Mohs surgery for melanoma. Dermatol Surg. 2016;42:83-93.
- Viola KV, Jhaveri MB, Soulos PR, et al. Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148:473-477.
- Moehrle M, Metzger S, Schippert W. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003;29:366-374.
- Lazar A, Abimelec P, Dumontier C, et al. Full thickness skin graft from nail unit reconstruction. J Hand Surg Br. 2005;30:194-198.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential for histologic reports. Am J Surg Pathol. 2007;31:1902-1912.
- Nasca MR, Innocenzi D, Micali G. Subungual squamous cell carcinoma of the toe: report on three cases. Dermatol Surg. 2004;30:345-348.
- Dika E, Piraccini BM, Balestri RR, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
- Alam M, Caldwell JB, Eliezri YD. Human papillomavirus-associated digital squamous cell carcinoma: literature review and report of 21 new cases. J Am Acad Dermatol. 2003;48:385-393.
- Filho L, Anselmo J, Dadalti P, et al. Skin grafts in cutaneous oncology. Braz Ann Dermatol. 2006;81:465-472.
- Raimer DW, Group AR, Petitt MS, et al. Porcine xenograft biosynthetic wound dressings for the management of postoperative Mohs wounds. Dermatol Online J. 2011;17:1.
- Alam M, Helenowksi IB, Cohen JL, et al. Association between type of reconstruction after Mohs micrographic surgery and surgeon-, patient-, and tumor-specific features: a cross-sectional study. Dermatol Surg. 2013;39:51-55.
- Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
Mohs micrographic surgery (MMS) is a specialized surgical technique for the treatment of melanoma and nonmelanoma skin cancers (NMSCs).1-3 The procedure involves surgical excision, histopathologic examination, precise mapping of malignant tissue, and wound management. Indications for MMS in skin cancer patients include recurring lesions, lesions in high-risk anatomic locations, aggressive histologic subtypes (ie, morpheaform, micronodular, infiltrative, high-grade, poorly differentiated), perineural invasion, large lesion size (>2 cm in diameter), poorly defined lateral or vertical clinical borders, rapid growth of the lesion, immunocompromised status, and sites of positive margins on prior excision. The therapeutic advantages of MMS include tissue conservation and optimal margin control in cosmetically or functionally sensitive areas, such as acral sites (eg, hands, feet, digits).1,3
The intricacies of the nail apparatus complicate diagnostic biopsy and precise delineation of peripheral margins in digital skin cancers; thus, early diagnosis and intraoperative histologic examination of the margins are essential. Traditionally, the surgical approach to subungual cutaneous tumors such as melanoma has included digital amputation4; however, a study of the treatment of subungual melanoma revealed no difference in survival based on the level of amputation, therefore advocating for less radical treatment.4
Interestingly, MMS for cutaneous tumors localized to the digits is not frequently reviewed in the dermatologic literature. We present a retrospective case series evaluating the clinical outcomes of digital melanoma and NMSCs treated with MMS.
Methods
A retrospective chart review was performed at a private dermatology practice to identify patients who underwent MMS for melanoma or NMSC localized to the digits from January 2009 to December 2014. All patients were treated in the office by 1 Mohs surgeon (A.H.) and were evaluated before and after MMS. Data were collected from the electronic medical record of the practice, including patient demographics, histopathologic diagnosis, tumor status (primary or recurrent lesion), anatomic site of the tumor, preoperative and postoperative size of the lesion, number of MMS stages, surgical repair technique, postoperative complications, and follow-up period.
Results
Twenty-seven patients (13 male, 14 female) with a total of 28 lesions (malignant melanoma or NMSC) localized to the digits were identified (Table). The mean age at the time of MMS was 64.07 years.

Surgical techniques used for repair following MMS included xenograft (10/28 [35.71%]); split-thickness skin graft (7/28 [25.0%]); secondary intention (4/28 [14.29%]); flap (4/28 [14.29%]); full-thickness skin graft (2/28 [7.14%]); and complex closure (1/28 [3.57%]). Clinical preoperative, operative, and postoperative photos from Patient 21 in this series are shown here (Figure). Two patients required bony phalanx resection due to invasion of the tumor into the periosteum: 1 had a malignant melanoma (Breslow depth, 2.52 mm); the other had an SCC. In addition, following removal of a severely dysplastic nevus, debulked tissue revealed melanoma in 1 patient.

Postoperative complications were noted in 4 (14.29%) of 28 MMS procedures, including bacterial wound infection (3.57%), excess granulation tissue that required wound debridement (7.14%), and delay in wound healing (3.57%). Follow-up data were available for 25 of the 28 MMS procedures (mean follow-up, 35.4 months), during which no recurrences were observed.
Comment
Mohs micrographic surgery is a specialized technique used in the treatment of cutaneous tumors, including basal cell carcinoma, SCC, melanoma in situ, atypical fibroxanthoma, dermatofibrosarcoma protuberans, sebaceous carcinoma, microcystic adnexal carcinoma, and Merkel cell carcinoma, among other cutaneous tumors.1-3 Mohs micrographic surgery provides the advantage of tissue conservation as well as optimal margin control in cosmetically or functionally sensitive areas while providing a higher cure rate than surgical excision. During the procedure, the surgical margin is examined histologically, thus ensuring definitive removal of the tumor but minimal loss of surrounding normal tissue.1-3 Mohs micrographic surgery is particularly useful for treating lesions on acral sites (eg, hands, feet, and digits).3-5
The treatment of digital skin cancers has evolved over the past 50 years with advancements resulting in more precise, tissue-sparing methods, in contrast to previous treatments such as amputation and wide local excision.6 More specifically, traditional digital amputation for the treatment of subungual melanoma has been reevaluated in multiple studies, which did not demonstrate a statistically significant difference in survival based on the level of amputation, thereby favoring less radical treatment.4,6 Moehrle et al7 found no statistical difference in recurrence rate when comparing patients with digital melanomas treated with partial amputation and those treated with digit-sparing surgery with limited excision and histologic evaluation of margins. Additionally, in a study conducted by Lazar et al,8 no recurrence of 13 subungual malignancies treated with MMS that utilized a full-thickness graft was reported at 4-year follow-up. In a large retrospective series of digital melanomas treated with MMS, Terushkin et al5 reported that 96.5% (55/57) of patients with primary melanomas that were treated with MMS avoided amputation, and the 5- and 10-year melanoma-specific survival rates for all patients treated with MMS were 95.0% and 82.6%, respectively.
In our study, cutaneous malignancies were located most often on the fingers, and the most common skin cancer identified was SCC in situ. The literature has shown that SCC in situ and SCC are the most common cutaneous neoplasms of the digits and nail unit.9 The most common specific anatomic site of cutaneous malignancy in our study was the great toe, followed by the fourth finger. A study conducted by Tan et al9 revealed that the great toe was the most common location of melanoma of the nail bed and subungual region, followed by the thumb. In contrast, primary subungual SCCs occur most frequently on the finger, with rare cases involving the toes.10
The etiology of digital SCC may involve extensive sun exposure, chronic trauma and wounds, and viral infection.9,11 More specifically, the dermatologic literature provides evidence of human papillomavirus (HPV) type 16 involvement in the pathogenesis of digital and periungual SCC. A genital-digital mechanism of spread has been implicated.11,12 An increased recurrence rate of HPV-associated digital SCCs has been reported following MMS, likely secondary to residual postsurgical HPV infection.11,12
Maintaining function and cosmesis of the hands, feet, and digits following MMS can be challenging, sometimes requiring skin grafts and flaps to close the defect. In the 28 MMS procedures evaluated in our study, 19 (67.9%) surgical defects were repaired with a graft (ie, split-thickness skin graft, full-thickness skin graft, xenograft), 4 (14.3%) with a flap (advancement and rotation), 4 (14.3%) by secondary intention, and 1 (3.6%) with primary complex closure.
Surgical grafts can be categorized based on the origin of the graft.2,13 Autografts, derived from the patient’s skin, are the most frequently used dermatologic graft and can be further categorized as full-thickness skin grafts, which include the epidermis and the entire dermis, thus preserving adnexal structures, and split-thickness skin grafts, which include the epidermis and partial dermis.2,13
A cross-sectional survey of fellowship-trained Mohs surgeons revealed that more than two-thirds of repairs for cutaneous acral cancers were performed using a primary closure technique, and one-fourth of closures were performed using secondary intention.15 Of the less frequently utilized skin-graft repairs, more were for acral lesions on the legs than on the arms.14 The type of procedure and graft used is dependent on multiple variables, including the anatomic location of the lesion and final size of the defect following MMS.2 Similarly, the use of specific types of sutures depends on the anatomic location of the lesion, relative thickness of the skin, degree of tension, and desired cosmetic result.15 The expertise of a hand surgeon may be required, particularly in cases in which the extensor tendon of the distal interphalangeal joint is compromised, manifested by a droopy fingertip when the hand is held horizontally. Additionally, special attention should be paid to removing the entire nail matrix before skin grafting for subungual tumors to avoid nail growth under the skin graft.
Evaluation of debulked tissue from digital skin cancers proved to be important in our study. In Patient 21, debulked tissue revealed melanoma following removal of a severely dysplastic nevus. This finding emphasizes the importance of complete excision of such lesions, as remaining underlying portions of the lesion can reveal residual tumor of the same or different histopathology.
In a prospective study, MMS was shown to have a low rate (0.91%; 95% confidence interval, 0.38%-1.45%) of surgical site infection in the absence of prophylactic antibiotics.16 The highest rates of surgical site infection were closely associated with flap closure. In our study, most patients had an uncomplicated and successful postoperative recovery. Only 1 (3.57%) of the 28 MMS procedures (Patient 22) was complicated by a bacterial wound infection postoperatively. The lesion removed in this case was a severely dysplastic melanocytic nevus on the toe. Infection resolved after a course of oral antibiotics, but the underlying cause of the wound infection in the patient was unclear. Other postoperative complications in our study included delayed wound healing and excess granulation tissue requiring wound debridement.
There are limited data in the dermatologic literature regarding outcomes following MMS for the treatment of cutaneous malignancies localized to the digits.
Additional limitations of this case review include its single-center and retrospective design, the small sample size, and 1 Mohs surgeon having performed all surgeries.
Conclusion
This study provides further evidence of the benefit of MMS for the treatment of malignant melanoma and NMSCs of the digits. This procedure provides margin-controlled excision of these malignant neoplasms while preserving maximal normal tissue, thereby providing patients with improved postoperative function and cosmesis. Long-term follow-up data demonstrating a lack of tumor recurrence underscores the assertion that MMS is safe and effective for the treatment of skin cancer of the digits.
Mohs micrographic surgery (MMS) is a specialized surgical technique for the treatment of melanoma and nonmelanoma skin cancers (NMSCs).1-3 The procedure involves surgical excision, histopathologic examination, precise mapping of malignant tissue, and wound management. Indications for MMS in skin cancer patients include recurring lesions, lesions in high-risk anatomic locations, aggressive histologic subtypes (ie, morpheaform, micronodular, infiltrative, high-grade, poorly differentiated), perineural invasion, large lesion size (>2 cm in diameter), poorly defined lateral or vertical clinical borders, rapid growth of the lesion, immunocompromised status, and sites of positive margins on prior excision. The therapeutic advantages of MMS include tissue conservation and optimal margin control in cosmetically or functionally sensitive areas, such as acral sites (eg, hands, feet, digits).1,3
The intricacies of the nail apparatus complicate diagnostic biopsy and precise delineation of peripheral margins in digital skin cancers; thus, early diagnosis and intraoperative histologic examination of the margins are essential. Traditionally, the surgical approach to subungual cutaneous tumors such as melanoma has included digital amputation4; however, a study of the treatment of subungual melanoma revealed no difference in survival based on the level of amputation, therefore advocating for less radical treatment.4
Interestingly, MMS for cutaneous tumors localized to the digits is not frequently reviewed in the dermatologic literature. We present a retrospective case series evaluating the clinical outcomes of digital melanoma and NMSCs treated with MMS.
Methods
A retrospective chart review was performed at a private dermatology practice to identify patients who underwent MMS for melanoma or NMSC localized to the digits from January 2009 to December 2014. All patients were treated in the office by 1 Mohs surgeon (A.H.) and were evaluated before and after MMS. Data were collected from the electronic medical record of the practice, including patient demographics, histopathologic diagnosis, tumor status (primary or recurrent lesion), anatomic site of the tumor, preoperative and postoperative size of the lesion, number of MMS stages, surgical repair technique, postoperative complications, and follow-up period.
Results
Twenty-seven patients (13 male, 14 female) with a total of 28 lesions (malignant melanoma or NMSC) localized to the digits were identified (Table). The mean age at the time of MMS was 64.07 years.

Surgical techniques used for repair following MMS included xenograft (10/28 [35.71%]); split-thickness skin graft (7/28 [25.0%]); secondary intention (4/28 [14.29%]); flap (4/28 [14.29%]); full-thickness skin graft (2/28 [7.14%]); and complex closure (1/28 [3.57%]). Clinical preoperative, operative, and postoperative photos from Patient 21 in this series are shown here (Figure). Two patients required bony phalanx resection due to invasion of the tumor into the periosteum: 1 had a malignant melanoma (Breslow depth, 2.52 mm); the other had an SCC. In addition, following removal of a severely dysplastic nevus, debulked tissue revealed melanoma in 1 patient.

Postoperative complications were noted in 4 (14.29%) of 28 MMS procedures, including bacterial wound infection (3.57%), excess granulation tissue that required wound debridement (7.14%), and delay in wound healing (3.57%). Follow-up data were available for 25 of the 28 MMS procedures (mean follow-up, 35.4 months), during which no recurrences were observed.
Comment
Mohs micrographic surgery is a specialized technique used in the treatment of cutaneous tumors, including basal cell carcinoma, SCC, melanoma in situ, atypical fibroxanthoma, dermatofibrosarcoma protuberans, sebaceous carcinoma, microcystic adnexal carcinoma, and Merkel cell carcinoma, among other cutaneous tumors.1-3 Mohs micrographic surgery provides the advantage of tissue conservation as well as optimal margin control in cosmetically or functionally sensitive areas while providing a higher cure rate than surgical excision. During the procedure, the surgical margin is examined histologically, thus ensuring definitive removal of the tumor but minimal loss of surrounding normal tissue.1-3 Mohs micrographic surgery is particularly useful for treating lesions on acral sites (eg, hands, feet, and digits).3-5
The treatment of digital skin cancers has evolved over the past 50 years with advancements resulting in more precise, tissue-sparing methods, in contrast to previous treatments such as amputation and wide local excision.6 More specifically, traditional digital amputation for the treatment of subungual melanoma has been reevaluated in multiple studies, which did not demonstrate a statistically significant difference in survival based on the level of amputation, thereby favoring less radical treatment.4,6 Moehrle et al7 found no statistical difference in recurrence rate when comparing patients with digital melanomas treated with partial amputation and those treated with digit-sparing surgery with limited excision and histologic evaluation of margins. Additionally, in a study conducted by Lazar et al,8 no recurrence of 13 subungual malignancies treated with MMS that utilized a full-thickness graft was reported at 4-year follow-up. In a large retrospective series of digital melanomas treated with MMS, Terushkin et al5 reported that 96.5% (55/57) of patients with primary melanomas that were treated with MMS avoided amputation, and the 5- and 10-year melanoma-specific survival rates for all patients treated with MMS were 95.0% and 82.6%, respectively.
In our study, cutaneous malignancies were located most often on the fingers, and the most common skin cancer identified was SCC in situ. The literature has shown that SCC in situ and SCC are the most common cutaneous neoplasms of the digits and nail unit.9 The most common specific anatomic site of cutaneous malignancy in our study was the great toe, followed by the fourth finger. A study conducted by Tan et al9 revealed that the great toe was the most common location of melanoma of the nail bed and subungual region, followed by the thumb. In contrast, primary subungual SCCs occur most frequently on the finger, with rare cases involving the toes.10
The etiology of digital SCC may involve extensive sun exposure, chronic trauma and wounds, and viral infection.9,11 More specifically, the dermatologic literature provides evidence of human papillomavirus (HPV) type 16 involvement in the pathogenesis of digital and periungual SCC. A genital-digital mechanism of spread has been implicated.11,12 An increased recurrence rate of HPV-associated digital SCCs has been reported following MMS, likely secondary to residual postsurgical HPV infection.11,12
Maintaining function and cosmesis of the hands, feet, and digits following MMS can be challenging, sometimes requiring skin grafts and flaps to close the defect. In the 28 MMS procedures evaluated in our study, 19 (67.9%) surgical defects were repaired with a graft (ie, split-thickness skin graft, full-thickness skin graft, xenograft), 4 (14.3%) with a flap (advancement and rotation), 4 (14.3%) by secondary intention, and 1 (3.6%) with primary complex closure.
Surgical grafts can be categorized based on the origin of the graft.2,13 Autografts, derived from the patient’s skin, are the most frequently used dermatologic graft and can be further categorized as full-thickness skin grafts, which include the epidermis and the entire dermis, thus preserving adnexal structures, and split-thickness skin grafts, which include the epidermis and partial dermis.2,13
A cross-sectional survey of fellowship-trained Mohs surgeons revealed that more than two-thirds of repairs for cutaneous acral cancers were performed using a primary closure technique, and one-fourth of closures were performed using secondary intention.15 Of the less frequently utilized skin-graft repairs, more were for acral lesions on the legs than on the arms.14 The type of procedure and graft used is dependent on multiple variables, including the anatomic location of the lesion and final size of the defect following MMS.2 Similarly, the use of specific types of sutures depends on the anatomic location of the lesion, relative thickness of the skin, degree of tension, and desired cosmetic result.15 The expertise of a hand surgeon may be required, particularly in cases in which the extensor tendon of the distal interphalangeal joint is compromised, manifested by a droopy fingertip when the hand is held horizontally. Additionally, special attention should be paid to removing the entire nail matrix before skin grafting for subungual tumors to avoid nail growth under the skin graft.
Evaluation of debulked tissue from digital skin cancers proved to be important in our study. In Patient 21, debulked tissue revealed melanoma following removal of a severely dysplastic nevus. This finding emphasizes the importance of complete excision of such lesions, as remaining underlying portions of the lesion can reveal residual tumor of the same or different histopathology.
In a prospective study, MMS was shown to have a low rate (0.91%; 95% confidence interval, 0.38%-1.45%) of surgical site infection in the absence of prophylactic antibiotics.16 The highest rates of surgical site infection were closely associated with flap closure. In our study, most patients had an uncomplicated and successful postoperative recovery. Only 1 (3.57%) of the 28 MMS procedures (Patient 22) was complicated by a bacterial wound infection postoperatively. The lesion removed in this case was a severely dysplastic melanocytic nevus on the toe. Infection resolved after a course of oral antibiotics, but the underlying cause of the wound infection in the patient was unclear. Other postoperative complications in our study included delayed wound healing and excess granulation tissue requiring wound debridement.
There are limited data in the dermatologic literature regarding outcomes following MMS for the treatment of cutaneous malignancies localized to the digits.
Additional limitations of this case review include its single-center and retrospective design, the small sample size, and 1 Mohs surgeon having performed all surgeries.
Conclusion
This study provides further evidence of the benefit of MMS for the treatment of malignant melanoma and NMSCs of the digits. This procedure provides margin-controlled excision of these malignant neoplasms while preserving maximal normal tissue, thereby providing patients with improved postoperative function and cosmesis. Long-term follow-up data demonstrating a lack of tumor recurrence underscores the assertion that MMS is safe and effective for the treatment of skin cancer of the digits.
- Dim-Jamora KC, Perone JB. Management of cutaneous tumors with mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
- McLeod MP, Choudhary S, Alqubaisy YA, et al. Indications for Mohs micrographic surgery. In: Nouri K, ed. Mohs Micrographic Surgery. New York, NY: Springer; 2012:5-13.
- Loosemore MP, Morales-Burgos A, Goldberg LH. Acral lentiginous melanoma of the toe treated using Mohs surgery with sparing of the digit and subsequent reconstruction using split-thickness skin graft. Dermatol Surg. 2013;39:136-138.
- Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg. 2007;60:635-638.
- Terushkin V, Brodland DG, Sharon DJ, et al. Digit-sparing Mohs surgery for melanoma. Dermatol Surg. 2016;42:83-93.
- Viola KV, Jhaveri MB, Soulos PR, et al. Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148:473-477.
- Moehrle M, Metzger S, Schippert W. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003;29:366-374.
- Lazar A, Abimelec P, Dumontier C, et al. Full thickness skin graft from nail unit reconstruction. J Hand Surg Br. 2005;30:194-198.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential for histologic reports. Am J Surg Pathol. 2007;31:1902-1912.
- Nasca MR, Innocenzi D, Micali G. Subungual squamous cell carcinoma of the toe: report on three cases. Dermatol Surg. 2004;30:345-348.
- Dika E, Piraccini BM, Balestri RR, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
- Alam M, Caldwell JB, Eliezri YD. Human papillomavirus-associated digital squamous cell carcinoma: literature review and report of 21 new cases. J Am Acad Dermatol. 2003;48:385-393.
- Filho L, Anselmo J, Dadalti P, et al. Skin grafts in cutaneous oncology. Braz Ann Dermatol. 2006;81:465-472.
- Raimer DW, Group AR, Petitt MS, et al. Porcine xenograft biosynthetic wound dressings for the management of postoperative Mohs wounds. Dermatol Online J. 2011;17:1.
- Alam M, Helenowksi IB, Cohen JL, et al. Association between type of reconstruction after Mohs micrographic surgery and surgeon-, patient-, and tumor-specific features: a cross-sectional study. Dermatol Surg. 2013;39:51-55.
- Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
- Dim-Jamora KC, Perone JB. Management of cutaneous tumors with mohs micrographic surgery. Semin Plast Surg. 2008;22:247-256.
- McLeod MP, Choudhary S, Alqubaisy YA, et al. Indications for Mohs micrographic surgery. In: Nouri K, ed. Mohs Micrographic Surgery. New York, NY: Springer; 2012:5-13.
- Loosemore MP, Morales-Burgos A, Goldberg LH. Acral lentiginous melanoma of the toe treated using Mohs surgery with sparing of the digit and subsequent reconstruction using split-thickness skin graft. Dermatol Surg. 2013;39:136-138.
- Rayatt SS, Dancey AL, Davison PM. Thumb subungual melanoma: is amputation necessary? J Plast Reconstr Aesthet Surg. 2007;60:635-638.
- Terushkin V, Brodland DG, Sharon DJ, et al. Digit-sparing Mohs surgery for melanoma. Dermatol Surg. 2016;42:83-93.
- Viola KV, Jhaveri MB, Soulos PR, et al. Mohs micrographic surgery and surgical excision for nonmelanoma skin cancer treatment in the Medicare population. Arch Dermatol. 2012;148:473-477.
- Moehrle M, Metzger S, Schippert W. “Functional” surgery in subungual melanoma. Dermatol Surg. 2003;29:366-374.
- Lazar A, Abimelec P, Dumontier C, et al. Full thickness skin graft from nail unit reconstruction. J Hand Surg Br. 2005;30:194-198.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential for histologic reports. Am J Surg Pathol. 2007;31:1902-1912.
- Nasca MR, Innocenzi D, Micali G. Subungual squamous cell carcinoma of the toe: report on three cases. Dermatol Surg. 2004;30:345-348.
- Dika E, Piraccini BM, Balestri RR, et al. Mohs surgery for squamous cell carcinoma of the nail: report of 15 cases. our experience and a long-term follow-up. Br J Dermatol. 2012;167:1310-1314.
- Alam M, Caldwell JB, Eliezri YD. Human papillomavirus-associated digital squamous cell carcinoma: literature review and report of 21 new cases. J Am Acad Dermatol. 2003;48:385-393.
- Filho L, Anselmo J, Dadalti P, et al. Skin grafts in cutaneous oncology. Braz Ann Dermatol. 2006;81:465-472.
- Raimer DW, Group AR, Petitt MS, et al. Porcine xenograft biosynthetic wound dressings for the management of postoperative Mohs wounds. Dermatol Online J. 2011;17:1.
- Alam M, Helenowksi IB, Cohen JL, et al. Association between type of reconstruction after Mohs micrographic surgery and surgeon-, patient-, and tumor-specific features: a cross-sectional study. Dermatol Surg. 2013;39:51-55.
- Rogers HD, Desciak EB, Marcus RP, et al. Prospective study of wound infections in Mohs micrographic surgery using clean surgical technique in the absence of prophylactic antibiotics. J Am Acad Dermatol. 2010;63:842-851.
Practice Points
- Melanoma and nonmelanoma skin cancers of the digits traditionally have been treated with wide local surgical excision and even amputation.
- Conservative tissue sparing techniques such as Mohs micrographic surgery can be used to treat digital skin cancers with high cure rates and improved functional and cosmetic results.
Improving Teamwork and Patient Outcomes with Daily Structured Interdisciplinary Bedside Rounds: A Multimethod Evaluation
Evidence has emerged over the last decade of the importance of the front line patient care team in improving quality and safety of patient care.1-3 Improving collaboration and workflow is thought to increase reliability of care delivery.1 One promising method to improve collaboration is the interdisciplinary ward round (IDR), whereby medical, nursing, and allied health staff attend ward rounds together. IDRs have been shown to reduce the average cost and length of hospital stay,4,5 although a recent systematic review found inconsistent improvements across studies.6 Using the term “interdisciplinary,” however, does not necessarily imply the inclusion of all disciplines necessary for patient care. The challenge of conducting interdisciplinary rounds is considerable in today’s busy clinical environment: health professionals who are spread across multiple locations within the hospital, and who have competing hospital responsibilities and priorities, must come together at the same time and for a set period each day. A survey with respondents from Australia, the United States, and Canada found that only 65% of rounds labelled “interdisciplinary” included a physician.7
While IDRs are not new, structured IDRs involve the purposeful inclusion of all disciplinary groups relevant to a patient’s care, alongside a checklist tool to aid comprehensive but concise daily assessment of progress and treatment planning. Novel, structured IDR interventions have been tested recently in various settings, resulting in improved teamwork, hospital performance, and patient outcomes in the US, including the Structured Interdisciplinary Bedside Round (SIBR) model.8-12
The aim of this study was to assess the impact of the new structure and the associated practice changes on interprofessional working and a set of key patient and hospital outcome measures. As part of the intervention, the hospital established an Acute Medical Unit (AMU) based on the Accountable Care Unit model.13
METHODS
Description of the Intervention
The AMU brought together 2 existing medical wards, a general medical ward and a 48-hour turnaround Medical Assessment Unit (MAU), into 1 geographical location with 26 beds. Prior to the merger, the MAU and general medical ward had separate and distinct cultures and workflows. The MAU was staffed with experienced nurses; nurses worked within a patient allocation model, the workload was shared, and relationships were collegial. In contrast, the medical ward was more typical of the remainder of the hospital: nurses had a heavy workload, managed a large group of longer-term complex patients, and they used a team-based nursing model of care in which senior nurses supervised junior staff. It was decided that because of the seniority of the MAU staff, they should be in charge of the combined AMU, and the patient allocation model of care would be used to facilitate SIBR.
Consultants, junior doctors, nurses, and allied health professionals (including a pharmacist, physiotherapist, occupational therapist, and social worker) were geographically aligned to the new ward, allowing them to participate as a team in daily structured ward rounds. Rounds are scheduled at the same time each day to enable family participation. The ward round is coordinated by a registrar or intern, with input from patient, family, nursing staff, pharmacy, allied health, and other doctors (intern, registrar, and consultant) based on the unit. The patient load is distributed between 2 rounds: 1 scheduled for 10
Data Collection Strategy
The study was set in an AMU in a large tertiary care hospital in regional Australia and used a convergent parallel multimethod approach14 to evaluate the implementation and effect of SIBR in the AMU. The study population consisted of 32 clinicians employed at the study hospital: (1) the leadership team involved in the development and implementation of the intervention and (2) members of clinical staff who were part of the AMU team.
Qualitative Data
Qualitative measures consisted of semistructured interviews. We utilized multiple strategies to recruit interviewees, including a snowball technique, criterion sampling,15 and emergent sampling, so that we could seek the views of both the leadership team responsible for the implementation and “frontline” clinical staff whose daily work was directly affected by it. Everyone who was initially recruited agreed to be interviewed, and additional frontline staff asked to be interviewed once they realized that we were asking about how staff experienced the changes in practice.
The research team developed a semistructured interview guide based on an understanding of the merger of the 2 units as well as an understanding of changes in practice of the rounds (provided in Appendix 1). The questions were pilot tested on a separate unit and revised. Questions were structured into 5 topic areas: planning and implementation of AMU/SIBR model, changes in work practices because of the new model, team functioning, job satisfaction, and perceived impact of the new model on patients and families. All interviews were audio-recorded and transcribed verbatim for analysis.
Quantitative Data
Quantitative data were collected on patient outcome measures: length of stay (LOS), discharge date and time, mode of separation (including death), primary diagnostic category, total hospital stay cost and “clinical response calls,” and patient demographic data (age, gender, and Patient Clinical Complexity Level [PCCL]). The PCCL is a standard measure used in Australian public inpatient facilities and is calculated for each episode of care.16 It measures the cumulative effect of a patient’s complications and/or comorbidities and takes an integer value between 0 (no clinical complexity effect) and 4 (catastrophic clinical complexity effect).
Data regarding LOS, diagnosis (Australian Refined Diagnosis Related Groups [AR-DRG], version 7), discharge date, and mode of separation (including death) were obtained from the New South Wales Ministry of Health’s Health Information Exchange for patients discharged during the year prior to the intervention through 1 year after the implementation of the intervention. The total hospital stay cost for these individuals was obtained from the local Health Service Organizational Performance Management unit. Inclusion criteria were inpatients aged over 15 years experiencing acute episodes of care; patients with a primary diagnostic category of mental diseases and disorders were excluded. LOS was calculated based on ward stay. AMU data were compared with the remaining hospital ward data (the control group). Data on “clinical response calls” per month per ward were also obtained for the 12 months prior to intervention and the 12 months of the intervention.
Analysis
Qualitative Analysis
Qualitative data analysis consisted of a hybrid form of textual analysis, combining inductive and deductive logics.17,18 Initially, 3 researchers (J.P., J.J., and R.C.W.) independently coded the interview data inductively to identify themes. Discrepancies were resolved through discussion until consensus was reached. Then, to further facilitate analysis, the researchers deductively imposed a matrix categorization, consisting of 4 a priori categories: context/conditions, practices/processes, professional interactions, and consequences.19,20 Additional a priori categories were used to sort the themes further in terms of experiences prior to, during, and following implementation of the intervention. To compare changes in those different time periods, we wanted to know what themes were related to implementation and whether those themes continued to be applicable to sustainability of the changes.
Quantitative analysis. Distribution of continuous data was examined by using the one-sample Kolmogorov-Smirnov test. We compared pre-SIBR (baseline) measures using the Student t test for normally distributed data, the Mann-Whitney U z test for nonparametric data (denoted as M-W U z), and χ2 tests for categorical data. Changes in monthly “clinical response calls” between the AMU and the control wards over time were explored by using analysis of variance (ANOVA). Changes in LOS and cost of stay from the year prior to the intervention to the first year of the intervention were analyzed by using generalized linear models, which are a form of linear regression. Factors, or independent variables, included in the models were time period (before or during intervention), ward (AMU or control), an interaction term (time by ward), patient age, gender, primary diagnosis (major diagnostic categories of the AR-DRG version 7.0), and acuity (PCCL). The estimated marginal means for cost of stay for the 12-month period prior to the intervention and for the first 12 months of the intervention were produced. All statistical analyses were performed by using IBM SPSS version 21 (IBM Corp., Armonk, New York) and with alpha set at P < .05.
RESULTS
Qualitative Evaluation of the Intervention
Participants.
Three researchers (RCW, JP, and JJ) conducted in-person, semistructured interviews with 32 clinicians (9 male, 23 female) during a 3-day period. The duration of the interviews ranged from 19 minutes to 68 minutes. Participants consisted of 8 doctors, 18 nurses, 5 allied health professionals, and an administrator. Ten of the participants were involved in the leadership group that drove the planning and implementation of SIBR and the AMU.
Themes
Context and Conditions of Work
Practices and Processes
Participants perceived postintervention work processes to be more efficient. A primary example was a near-universal approval of the time saved from not “chasing” other professionals now that they were predictably available on the ward. More timely decision-making was thought to result from this predicted availability and associated improvements in communication.
The SIBR enforced a workflow on all staff, who felt there was less flexibility to work autonomously (doctors) or according to patients’ needs (nurses). More junior staff expressed anxiety about delayed completion of discharge-related administrative tasks because of the midday completion of the round. Allied health professionals who had commitments in other areas of the hospital often faced a dilemma about how to prioritize SIBR attendance and activities on other wards. This was managed differently depending on the specific allied health profession and the individuals within that profession.
Professional Interactions
In terms of interprofessional dynamics on the AMU, the implementation of SIBR resulted in a shift in power between the doctors and the nurses. In the old ward, doctors largely controlled the timing of medical rounding processes. In the new AMU, doctors had to relinquish some control over the timing of personal workflow to comply with the requirements of SIBR. Furthermore, there was evidence that this had some impact on traditional hierarchical models of communication and created a more level playing field, as nonmedical professionals felt more empowered to voice their thoughts during and outside of rounds.
The rounds provided much greater visibility of the “big picture” and each profession’s role within it; this allowed each clinician to adjust their work to fit in and take account of others. The process was not instantaneous, and trust developed over a period of weeks. Better communication meant fewer misunderstandings, and workload dropped.
The participation of allied health professionals in the round enhanced clinician interprofessional skills and knowledge. The more inclusive approach facilitated greater trust between clinical disciplines and a development of increased confidence among nursing, allied health, and administrative professionals.
In contrast to the positive impacts of the new model of care on communication and relationships within the AMU, interdepartmental relationships were seen to have suffered. The processes and practices of the new AMU are different to those in the other hospital departments, resulting in some isolation of the unit and difficulties interacting with other areas of the hospital. For example, the trade-offs that allied health professionals made to participate in SIBR often came at the expense of other units or departments.
Consequences
All interviewees lauded the benefits of the SIBR intervention for patients. Patients were perceived to be better informed and more respected, and they benefited from greater perceived timeliness of treatment and discharge, easier access to doctors, better continuity of treatment and outcomes, improved nurse knowledge of their circumstances, and fewer gaps in their care. Clinicians spoke directly to the patient during SIBR, rather than consulting with professional colleagues over the patient’s head. Some staff felt that doctors were now thinking of patients as “people” rather than “a set of symptoms.” Nurses discovered that informed patients are easier to manage.
Staff members were prepared to compromise on their own needs in the interests of the patient. The emphasis on the patient during rounds resulted in improved advocacy behaviors of clinicians. The nurses became more empowered and able to show greater initiative. Families appeared to find it much easier to access the doctors and obtain information about the patient, resulting in less distress and a greater sense of control and trust in the process.
Quantitative Evaluation of the Intervention
Hospital Outcomes
Patient demographics did not change over time within either the AMU or control wards. However, there were significant increases in Patient Clinical Complexity Level (PCCL) ratings for both the AMU (44.7% to 40.3%; P<0.05) and the control wards (65.2% to 61.6%; P < .001). There was not a statistically significant shift over time in median LoS on the ward prior to (2.16 days, IQR 3.07) and during SIBR in the AMU (2.15 days; IQR 3.28), while LoS increased in the control (pre-SIBR: 1.67, 2.34; during SIBR 1.73, 2.40; M-W U z = -2.46, P = .014). Mortality rates were stable across time for both the AMU (pre-SIBR 2.6% [95% confidence interval {CI}, 1.9-3.5]; during SIBR 2.8% [95% CI, 2.1-3.7]) and the control (pre-SIBR 1.3% [95% CI, 1.0-1.5]; during SIBR 1.2% [95% CI, 1.0-1.4]).
The total number of “clinical response calls” or “flags” per month dropped significantly from pre-SIBR to during SIBR for the AMU from a mean of 63.1 (standard deviation 15.1) to 31.5 (10.8), but remained relatively stable in the control (pre-SIBR 72.5 [17.6]; during SIBR 74.0 [28.3]), and this difference was statistically significant (F (1,44) = 9.03; P = .004). There was no change in monthly “red flags” or “rapid response calls” over time (AMU: 10.5 [3.6] to 9.1 [4.7]; control: 40.3 [11.7] to 41.8 [10.8]). The change in total “clinical response calls” over time was attributable to the “yellow flags” or the decline in “calls for clinical review” in the AMU (from 52.6 [13.5] to 22.4 [9.2]). The average monthly “yellow flags” remained stable in the control (pre-SIBR 32.2 [11.6]; during SIBR 32.3 [22.4]). The AMU and the control wards differed significantly in how the number of monthly “calls for clinical review” changed from pre-SIBR to during SIBR (F (1,44) = 12.18; P = .001).
The 2 main outcome measures, LOS and costs, were analyzed to determine whether changes over time differed between the AMU and the control wards after accounting for age, gender, and PCCL. There was no statistically significant difference between the AMU and control wards in terms of change in LOS over time (Wald χ2 = 1.05; degrees of freedom [df] = 1; P = .31). There was a statistically significant interaction for cost of stay, indicating that ward types differed in how they changed over time (with a drop in cost over time observed in the AMU and an increase observed in the control) (Wald χ2 = 6.34; df = 1; P = .012.
DISCUSSION
We report on the implementation of an AMU model of care, including the reorganization of a nursing unit, implementation of IDR, and geographical localization. Our study design allowed a more comprehensive assessment of the implementation of system redesign to include provider perceptions and clinical outcomes.
The 2 very different cultures of the old wards that were combined into the AMU, as well as the fact that the teams had not previously worked together, made the merger of the 2 wards difficult. Historically, the 2 teams had worked in very different ways, and this created barriers to implementation. The SIBR also demanded new ways of working closely with other disciplines, which disrupted older clinical cultures and relationships. While organizational culture is often discussed, and even measured, the full impact of cultural factors when making workplace changes is frequently underestimated.21 The development of a new culture takes time, and it can lag organizational structural changes by months or even years.22 As our interviewees expressed, often emotionally, there was a sense of loss during the merger of the 2 units. While this is a potential consequence of any large organizational change, it could be addressed during the planning stages, prior to implementation, by acknowledging and perhaps honoring what is being left behind. It is safe to assume that future units implementing the rounding intervention will not fully realize commensurate levels of culture change until well after the structural and process changes are finalized, and only then if explicit effort is made to engender cultural change.
Overall, however, the interviewees perceived that the SIBR intervention led to improved teamwork and team functioning. These improvements were thought to benefit task performance and patient safety. Our study is consistent with other research in the literature that reported that greater staff empowerment and commitment is associated with interdisciplinary patient care interventions in front line caregiving teams.23,24 The perception of a more equal nurse-physician relationship resulted in improved job satisfaction, better interprofessional relationships, and perceived improvements in patient care. A flatter power gradient across professions and increased interdisciplinary teamwork has been shown to be associated with improved patient outcomes.25,26
Changes to clinician workflow can significantly impact the introduction of new models of care. A mandated time each day for structured rounds meant less flexibility in workflow for clinicians and made greater demands on their time management and communication skills. Furthermore, the need for human resource negotiations with nurse representatives was an unexpected component of successfully introducing the changes to workflow. Once the benefits of saved time and better communication became evident, changes to workflow were generally accepted. These challenges can be managed if stakeholders are engaged and supportive of the changes.13
Finally, our findings emphasize the importance of combining qualitative and quantitative data when evaluating an intervention. In this case, the qualitative outcomes that include “intangible” positive effects, such as cultural change and improved staff understanding of one another’s roles, might encourage us to continue with the SIBR intervention, which would allow more time to see if the trend of reduced LOS identified in the statistical analysis would translate to a significant effect over time.
We are unable to identify which aspects of the intervention led to the greatest impact on our outcomes. A recent study found that interdisciplinary rounds had no impact on patients’ perceptions of shared decision-making or care satisfaction.27 Although our findings indicated many potential benefits for patients, we were not able to interview patients or their carers to confirm these findings. In addition, we do not have any patient-centered outcomes, which would be important to consider in future work. Although our data on clinical response calls might be seen as a proxy for adverse events, we do not have data on adverse events or errors, and these are important to consider in future work. Finally, our findings are based on data from a single institution.
CONCLUSIONS
While there were some criticisms, participants expressed overwhelmingly positive reactions to the SIBR. The biggest reported benefit was perceived improved communication and understanding between and within the clinical professions, and between clinicians and patients. Improved communication was perceived to have fostered improved teamwork and team functioning, with most respondents feeling that they were a valued part of the new team. Improved teamwork was thought to contribute to improved task performance and led interviewees to perceive a higher level of patient safety. This research highlights the need for multimethod evaluations that address contextual factors as well as clinical outcomes.
Acknowledgments
The authors would like to acknowledge the clinicians and staff members who participated in this study. We would also like to acknowledge the support from the NSW Clinical Excellence Commission, in particular, Dr. Peter Kennedy, Mr. Wilson Yeung, Ms. Tracy Clarke, and Mr. Allan Zhang, and also from Ms. Karen Storey and Mr. Steve Shea of the Organisational Performance Management team at the Orange Health Service.
Disclosures
None of the authors had conflicts of interest in relation to the conduct or reporting of this study, with the exception that the lead author’s institution, the Australian Institute of Health Innovation, received a small grant from the New South Wales Clinical Excellence Commission to conduct the work. Ethics approval for the research was granted by the Greater Western Area Health Service Human Research Ethics Committee (HREC/13/GWAHS/22). All interviewees consented to participate in the study. For patient data, consent was not obtained, but presented data are anonymized. The full dataset is available from the corresponding author with restrictions. This research was funded by the NSW Clinical Excellence Commission, who also encouraged submission of the article for publication. The funding source did not have any role in conduct or reporting of the study. R.C.W., J.P., and J.J. conceptualized and conducted the qualitative component of the study, including method, data collection, data analysis, and writing of the manuscript. G.L., C.H., and H.D. conceptualized the quantitative component of the study, including method, data collection, data analysis, and writing of the manuscript. G.S. contributed to conceptualization of the study, and significantly contributed to the revision of the manuscript. All authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. As the lead author, R.C.W. affirms that the manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
1. Johnson JK, Batalden PB. Educating health professionals to improve care within the clinical microsystem. McLaughlin and Kaluzny’s Continuous Quality Improvement In Health Care. Burlington: Jones & Bartlett Learning; 2013.
2. Mohr JJ, Batalden P, Barach PB. Integrating patient safety into the clinical microsystem. Qual Saf Health Care. 2004;13:ii34-ii38. PubMed
3. Sanchez JA, Barach PR. High reliability organizations and surgical microsystems: re-engineering surgical care. Surg Clin North Am. 2012;92:1-14. PubMed
4. Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36:AS4-AS12. PubMed
5. O’Mahony S, Mazur E, Charney P, Wang Y, Fine J. Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J Gen Intern Med. 2007;22:1073-1079. PubMed
6. Pannick S, Beveridge I, Wachter RM, Sevdalis N. Improving the quality and safety of care on the medical ward: a review and synthesis of the evidence base. Eur J Intern Med. 2014;25:874-887. PubMed
7. Halm MA, Gagner S, Goering M, Sabo J, Smith M, Zaccagnini M. Interdisciplinary rounds: impact on patients, families, and staff. Clin Nurse Spec. 2003;17:133-142. PubMed
8. Stein J, Murphy D, Payne C, et al. A remedy for fragmented hospital care. Harvard Business Review. 2013.
9. O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2010;171:678-684. PubMed
10. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6:88-93. PubMed
11. O’Leary KJ, Ritter CD, Wheeler H, Szekendi MK, Brinton TS, Williams MV. Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2011;19:117-121. PubMed
12. O’Leary KJ, Creden AJ, Slade ME, et al. Implementation of unit-based interventions to improve teamwork and patient safety on a medical service. Am J Med Qual. 2014;30:409-416. PubMed
13. Stein J, Payne C, Methvin A, et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10:36-40. PubMed
14. Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. Thousand Oaks: SAGE Publications; 2013.
15. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Pol Ment Health. 2015;42:533-544. PubMed
16. Australian Consortium for Classification Development (ACCD). Review of the AR-DRG classification Case Complexity Process: Final Report; 2014.
http://ihpa.gov.au/internet/ihpa/publishing.nsf/Content/admitted-acute. Accessed September 21, 2015.
17. Lofland J, Lofland LH. Analyzing Social Settings. Belmont: Wadsworth Publishing Company; 2006.
18. Miles MB, Huberman AM, Saldaña J. Qualitative Data Analysis: A Methods Sourcebook. Los Angeles: SAGE Publications; 2014.
19. Corbin J, Strauss A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Thousand Oaks: SAGE Publications; 2008.
20. Corbin JM, Strauss A. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13:3-21.
21. O’Leary KJ, Johnson JK, Auerbach AD. Do interdisciplinary rounds improve patient outcomes? only if they improve teamwork. J Hosp Med. 2016;11:524-525. PubMed
22. Clay-Williams R. Restructuring and the resilient organisation: implications for health care. In: Hollnagel E, Braithwaite J, Wears R, editors. Resilient health care. Surrey: Ashgate Publishing Limited; 2013.
23. Williams I, Dickinson H, Robinson S, Allen C. Clinical microsystems and the NHS: a sustainable method for improvement? J Health Organ and Manag. 2009;23:119-132. PubMed
24. Nelson EC, Godfrey MM, Batalden PB, et al. Clinical microsystems, part 1. The building blocks of health systems. Jt Comm J Qual Patient Saf. 2008;34:367-378. PubMed
25. Chisholm-Burns MA, Lee JK, Spivey CA, et al. US pharmacists’ effect as team members on patient care: systematic review and meta-analyses. Med Care. 2010;48:923-933. PubMed
26. Zwarenstein M, Goldman J, Reeves S. Interprofessional collaboration: effects of practice-based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;3:CD000072. PubMed
27. O’Leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. 2015;25:921-928. PubMed
Evidence has emerged over the last decade of the importance of the front line patient care team in improving quality and safety of patient care.1-3 Improving collaboration and workflow is thought to increase reliability of care delivery.1 One promising method to improve collaboration is the interdisciplinary ward round (IDR), whereby medical, nursing, and allied health staff attend ward rounds together. IDRs have been shown to reduce the average cost and length of hospital stay,4,5 although a recent systematic review found inconsistent improvements across studies.6 Using the term “interdisciplinary,” however, does not necessarily imply the inclusion of all disciplines necessary for patient care. The challenge of conducting interdisciplinary rounds is considerable in today’s busy clinical environment: health professionals who are spread across multiple locations within the hospital, and who have competing hospital responsibilities and priorities, must come together at the same time and for a set period each day. A survey with respondents from Australia, the United States, and Canada found that only 65% of rounds labelled “interdisciplinary” included a physician.7
While IDRs are not new, structured IDRs involve the purposeful inclusion of all disciplinary groups relevant to a patient’s care, alongside a checklist tool to aid comprehensive but concise daily assessment of progress and treatment planning. Novel, structured IDR interventions have been tested recently in various settings, resulting in improved teamwork, hospital performance, and patient outcomes in the US, including the Structured Interdisciplinary Bedside Round (SIBR) model.8-12
The aim of this study was to assess the impact of the new structure and the associated practice changes on interprofessional working and a set of key patient and hospital outcome measures. As part of the intervention, the hospital established an Acute Medical Unit (AMU) based on the Accountable Care Unit model.13
METHODS
Description of the Intervention
The AMU brought together 2 existing medical wards, a general medical ward and a 48-hour turnaround Medical Assessment Unit (MAU), into 1 geographical location with 26 beds. Prior to the merger, the MAU and general medical ward had separate and distinct cultures and workflows. The MAU was staffed with experienced nurses; nurses worked within a patient allocation model, the workload was shared, and relationships were collegial. In contrast, the medical ward was more typical of the remainder of the hospital: nurses had a heavy workload, managed a large group of longer-term complex patients, and they used a team-based nursing model of care in which senior nurses supervised junior staff. It was decided that because of the seniority of the MAU staff, they should be in charge of the combined AMU, and the patient allocation model of care would be used to facilitate SIBR.
Consultants, junior doctors, nurses, and allied health professionals (including a pharmacist, physiotherapist, occupational therapist, and social worker) were geographically aligned to the new ward, allowing them to participate as a team in daily structured ward rounds. Rounds are scheduled at the same time each day to enable family participation. The ward round is coordinated by a registrar or intern, with input from patient, family, nursing staff, pharmacy, allied health, and other doctors (intern, registrar, and consultant) based on the unit. The patient load is distributed between 2 rounds: 1 scheduled for 10
Data Collection Strategy
The study was set in an AMU in a large tertiary care hospital in regional Australia and used a convergent parallel multimethod approach14 to evaluate the implementation and effect of SIBR in the AMU. The study population consisted of 32 clinicians employed at the study hospital: (1) the leadership team involved in the development and implementation of the intervention and (2) members of clinical staff who were part of the AMU team.
Qualitative Data
Qualitative measures consisted of semistructured interviews. We utilized multiple strategies to recruit interviewees, including a snowball technique, criterion sampling,15 and emergent sampling, so that we could seek the views of both the leadership team responsible for the implementation and “frontline” clinical staff whose daily work was directly affected by it. Everyone who was initially recruited agreed to be interviewed, and additional frontline staff asked to be interviewed once they realized that we were asking about how staff experienced the changes in practice.
The research team developed a semistructured interview guide based on an understanding of the merger of the 2 units as well as an understanding of changes in practice of the rounds (provided in Appendix 1). The questions were pilot tested on a separate unit and revised. Questions were structured into 5 topic areas: planning and implementation of AMU/SIBR model, changes in work practices because of the new model, team functioning, job satisfaction, and perceived impact of the new model on patients and families. All interviews were audio-recorded and transcribed verbatim for analysis.
Quantitative Data
Quantitative data were collected on patient outcome measures: length of stay (LOS), discharge date and time, mode of separation (including death), primary diagnostic category, total hospital stay cost and “clinical response calls,” and patient demographic data (age, gender, and Patient Clinical Complexity Level [PCCL]). The PCCL is a standard measure used in Australian public inpatient facilities and is calculated for each episode of care.16 It measures the cumulative effect of a patient’s complications and/or comorbidities and takes an integer value between 0 (no clinical complexity effect) and 4 (catastrophic clinical complexity effect).
Data regarding LOS, diagnosis (Australian Refined Diagnosis Related Groups [AR-DRG], version 7), discharge date, and mode of separation (including death) were obtained from the New South Wales Ministry of Health’s Health Information Exchange for patients discharged during the year prior to the intervention through 1 year after the implementation of the intervention. The total hospital stay cost for these individuals was obtained from the local Health Service Organizational Performance Management unit. Inclusion criteria were inpatients aged over 15 years experiencing acute episodes of care; patients with a primary diagnostic category of mental diseases and disorders were excluded. LOS was calculated based on ward stay. AMU data were compared with the remaining hospital ward data (the control group). Data on “clinical response calls” per month per ward were also obtained for the 12 months prior to intervention and the 12 months of the intervention.
Analysis
Qualitative Analysis
Qualitative data analysis consisted of a hybrid form of textual analysis, combining inductive and deductive logics.17,18 Initially, 3 researchers (J.P., J.J., and R.C.W.) independently coded the interview data inductively to identify themes. Discrepancies were resolved through discussion until consensus was reached. Then, to further facilitate analysis, the researchers deductively imposed a matrix categorization, consisting of 4 a priori categories: context/conditions, practices/processes, professional interactions, and consequences.19,20 Additional a priori categories were used to sort the themes further in terms of experiences prior to, during, and following implementation of the intervention. To compare changes in those different time periods, we wanted to know what themes were related to implementation and whether those themes continued to be applicable to sustainability of the changes.
Quantitative analysis. Distribution of continuous data was examined by using the one-sample Kolmogorov-Smirnov test. We compared pre-SIBR (baseline) measures using the Student t test for normally distributed data, the Mann-Whitney U z test for nonparametric data (denoted as M-W U z), and χ2 tests for categorical data. Changes in monthly “clinical response calls” between the AMU and the control wards over time were explored by using analysis of variance (ANOVA). Changes in LOS and cost of stay from the year prior to the intervention to the first year of the intervention were analyzed by using generalized linear models, which are a form of linear regression. Factors, or independent variables, included in the models were time period (before or during intervention), ward (AMU or control), an interaction term (time by ward), patient age, gender, primary diagnosis (major diagnostic categories of the AR-DRG version 7.0), and acuity (PCCL). The estimated marginal means for cost of stay for the 12-month period prior to the intervention and for the first 12 months of the intervention were produced. All statistical analyses were performed by using IBM SPSS version 21 (IBM Corp., Armonk, New York) and with alpha set at P < .05.
RESULTS
Qualitative Evaluation of the Intervention
Participants.
Three researchers (RCW, JP, and JJ) conducted in-person, semistructured interviews with 32 clinicians (9 male, 23 female) during a 3-day period. The duration of the interviews ranged from 19 minutes to 68 minutes. Participants consisted of 8 doctors, 18 nurses, 5 allied health professionals, and an administrator. Ten of the participants were involved in the leadership group that drove the planning and implementation of SIBR and the AMU.
Themes
Context and Conditions of Work
Practices and Processes
Participants perceived postintervention work processes to be more efficient. A primary example was a near-universal approval of the time saved from not “chasing” other professionals now that they were predictably available on the ward. More timely decision-making was thought to result from this predicted availability and associated improvements in communication.
The SIBR enforced a workflow on all staff, who felt there was less flexibility to work autonomously (doctors) or according to patients’ needs (nurses). More junior staff expressed anxiety about delayed completion of discharge-related administrative tasks because of the midday completion of the round. Allied health professionals who had commitments in other areas of the hospital often faced a dilemma about how to prioritize SIBR attendance and activities on other wards. This was managed differently depending on the specific allied health profession and the individuals within that profession.
Professional Interactions
In terms of interprofessional dynamics on the AMU, the implementation of SIBR resulted in a shift in power between the doctors and the nurses. In the old ward, doctors largely controlled the timing of medical rounding processes. In the new AMU, doctors had to relinquish some control over the timing of personal workflow to comply with the requirements of SIBR. Furthermore, there was evidence that this had some impact on traditional hierarchical models of communication and created a more level playing field, as nonmedical professionals felt more empowered to voice their thoughts during and outside of rounds.
The rounds provided much greater visibility of the “big picture” and each profession’s role within it; this allowed each clinician to adjust their work to fit in and take account of others. The process was not instantaneous, and trust developed over a period of weeks. Better communication meant fewer misunderstandings, and workload dropped.
The participation of allied health professionals in the round enhanced clinician interprofessional skills and knowledge. The more inclusive approach facilitated greater trust between clinical disciplines and a development of increased confidence among nursing, allied health, and administrative professionals.
In contrast to the positive impacts of the new model of care on communication and relationships within the AMU, interdepartmental relationships were seen to have suffered. The processes and practices of the new AMU are different to those in the other hospital departments, resulting in some isolation of the unit and difficulties interacting with other areas of the hospital. For example, the trade-offs that allied health professionals made to participate in SIBR often came at the expense of other units or departments.
Consequences
All interviewees lauded the benefits of the SIBR intervention for patients. Patients were perceived to be better informed and more respected, and they benefited from greater perceived timeliness of treatment and discharge, easier access to doctors, better continuity of treatment and outcomes, improved nurse knowledge of their circumstances, and fewer gaps in their care. Clinicians spoke directly to the patient during SIBR, rather than consulting with professional colleagues over the patient’s head. Some staff felt that doctors were now thinking of patients as “people” rather than “a set of symptoms.” Nurses discovered that informed patients are easier to manage.
Staff members were prepared to compromise on their own needs in the interests of the patient. The emphasis on the patient during rounds resulted in improved advocacy behaviors of clinicians. The nurses became more empowered and able to show greater initiative. Families appeared to find it much easier to access the doctors and obtain information about the patient, resulting in less distress and a greater sense of control and trust in the process.
Quantitative Evaluation of the Intervention
Hospital Outcomes
Patient demographics did not change over time within either the AMU or control wards. However, there were significant increases in Patient Clinical Complexity Level (PCCL) ratings for both the AMU (44.7% to 40.3%; P<0.05) and the control wards (65.2% to 61.6%; P < .001). There was not a statistically significant shift over time in median LoS on the ward prior to (2.16 days, IQR 3.07) and during SIBR in the AMU (2.15 days; IQR 3.28), while LoS increased in the control (pre-SIBR: 1.67, 2.34; during SIBR 1.73, 2.40; M-W U z = -2.46, P = .014). Mortality rates were stable across time for both the AMU (pre-SIBR 2.6% [95% confidence interval {CI}, 1.9-3.5]; during SIBR 2.8% [95% CI, 2.1-3.7]) and the control (pre-SIBR 1.3% [95% CI, 1.0-1.5]; during SIBR 1.2% [95% CI, 1.0-1.4]).
The total number of “clinical response calls” or “flags” per month dropped significantly from pre-SIBR to during SIBR for the AMU from a mean of 63.1 (standard deviation 15.1) to 31.5 (10.8), but remained relatively stable in the control (pre-SIBR 72.5 [17.6]; during SIBR 74.0 [28.3]), and this difference was statistically significant (F (1,44) = 9.03; P = .004). There was no change in monthly “red flags” or “rapid response calls” over time (AMU: 10.5 [3.6] to 9.1 [4.7]; control: 40.3 [11.7] to 41.8 [10.8]). The change in total “clinical response calls” over time was attributable to the “yellow flags” or the decline in “calls for clinical review” in the AMU (from 52.6 [13.5] to 22.4 [9.2]). The average monthly “yellow flags” remained stable in the control (pre-SIBR 32.2 [11.6]; during SIBR 32.3 [22.4]). The AMU and the control wards differed significantly in how the number of monthly “calls for clinical review” changed from pre-SIBR to during SIBR (F (1,44) = 12.18; P = .001).
The 2 main outcome measures, LOS and costs, were analyzed to determine whether changes over time differed between the AMU and the control wards after accounting for age, gender, and PCCL. There was no statistically significant difference between the AMU and control wards in terms of change in LOS over time (Wald χ2 = 1.05; degrees of freedom [df] = 1; P = .31). There was a statistically significant interaction for cost of stay, indicating that ward types differed in how they changed over time (with a drop in cost over time observed in the AMU and an increase observed in the control) (Wald χ2 = 6.34; df = 1; P = .012.
DISCUSSION
We report on the implementation of an AMU model of care, including the reorganization of a nursing unit, implementation of IDR, and geographical localization. Our study design allowed a more comprehensive assessment of the implementation of system redesign to include provider perceptions and clinical outcomes.
The 2 very different cultures of the old wards that were combined into the AMU, as well as the fact that the teams had not previously worked together, made the merger of the 2 wards difficult. Historically, the 2 teams had worked in very different ways, and this created barriers to implementation. The SIBR also demanded new ways of working closely with other disciplines, which disrupted older clinical cultures and relationships. While organizational culture is often discussed, and even measured, the full impact of cultural factors when making workplace changes is frequently underestimated.21 The development of a new culture takes time, and it can lag organizational structural changes by months or even years.22 As our interviewees expressed, often emotionally, there was a sense of loss during the merger of the 2 units. While this is a potential consequence of any large organizational change, it could be addressed during the planning stages, prior to implementation, by acknowledging and perhaps honoring what is being left behind. It is safe to assume that future units implementing the rounding intervention will not fully realize commensurate levels of culture change until well after the structural and process changes are finalized, and only then if explicit effort is made to engender cultural change.
Overall, however, the interviewees perceived that the SIBR intervention led to improved teamwork and team functioning. These improvements were thought to benefit task performance and patient safety. Our study is consistent with other research in the literature that reported that greater staff empowerment and commitment is associated with interdisciplinary patient care interventions in front line caregiving teams.23,24 The perception of a more equal nurse-physician relationship resulted in improved job satisfaction, better interprofessional relationships, and perceived improvements in patient care. A flatter power gradient across professions and increased interdisciplinary teamwork has been shown to be associated with improved patient outcomes.25,26
Changes to clinician workflow can significantly impact the introduction of new models of care. A mandated time each day for structured rounds meant less flexibility in workflow for clinicians and made greater demands on their time management and communication skills. Furthermore, the need for human resource negotiations with nurse representatives was an unexpected component of successfully introducing the changes to workflow. Once the benefits of saved time and better communication became evident, changes to workflow were generally accepted. These challenges can be managed if stakeholders are engaged and supportive of the changes.13
Finally, our findings emphasize the importance of combining qualitative and quantitative data when evaluating an intervention. In this case, the qualitative outcomes that include “intangible” positive effects, such as cultural change and improved staff understanding of one another’s roles, might encourage us to continue with the SIBR intervention, which would allow more time to see if the trend of reduced LOS identified in the statistical analysis would translate to a significant effect over time.
We are unable to identify which aspects of the intervention led to the greatest impact on our outcomes. A recent study found that interdisciplinary rounds had no impact on patients’ perceptions of shared decision-making or care satisfaction.27 Although our findings indicated many potential benefits for patients, we were not able to interview patients or their carers to confirm these findings. In addition, we do not have any patient-centered outcomes, which would be important to consider in future work. Although our data on clinical response calls might be seen as a proxy for adverse events, we do not have data on adverse events or errors, and these are important to consider in future work. Finally, our findings are based on data from a single institution.
CONCLUSIONS
While there were some criticisms, participants expressed overwhelmingly positive reactions to the SIBR. The biggest reported benefit was perceived improved communication and understanding between and within the clinical professions, and between clinicians and patients. Improved communication was perceived to have fostered improved teamwork and team functioning, with most respondents feeling that they were a valued part of the new team. Improved teamwork was thought to contribute to improved task performance and led interviewees to perceive a higher level of patient safety. This research highlights the need for multimethod evaluations that address contextual factors as well as clinical outcomes.
Acknowledgments
The authors would like to acknowledge the clinicians and staff members who participated in this study. We would also like to acknowledge the support from the NSW Clinical Excellence Commission, in particular, Dr. Peter Kennedy, Mr. Wilson Yeung, Ms. Tracy Clarke, and Mr. Allan Zhang, and also from Ms. Karen Storey and Mr. Steve Shea of the Organisational Performance Management team at the Orange Health Service.
Disclosures
None of the authors had conflicts of interest in relation to the conduct or reporting of this study, with the exception that the lead author’s institution, the Australian Institute of Health Innovation, received a small grant from the New South Wales Clinical Excellence Commission to conduct the work. Ethics approval for the research was granted by the Greater Western Area Health Service Human Research Ethics Committee (HREC/13/GWAHS/22). All interviewees consented to participate in the study. For patient data, consent was not obtained, but presented data are anonymized. The full dataset is available from the corresponding author with restrictions. This research was funded by the NSW Clinical Excellence Commission, who also encouraged submission of the article for publication. The funding source did not have any role in conduct or reporting of the study. R.C.W., J.P., and J.J. conceptualized and conducted the qualitative component of the study, including method, data collection, data analysis, and writing of the manuscript. G.L., C.H., and H.D. conceptualized the quantitative component of the study, including method, data collection, data analysis, and writing of the manuscript. G.S. contributed to conceptualization of the study, and significantly contributed to the revision of the manuscript. All authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. As the lead author, R.C.W. affirms that the manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
Evidence has emerged over the last decade of the importance of the front line patient care team in improving quality and safety of patient care.1-3 Improving collaboration and workflow is thought to increase reliability of care delivery.1 One promising method to improve collaboration is the interdisciplinary ward round (IDR), whereby medical, nursing, and allied health staff attend ward rounds together. IDRs have been shown to reduce the average cost and length of hospital stay,4,5 although a recent systematic review found inconsistent improvements across studies.6 Using the term “interdisciplinary,” however, does not necessarily imply the inclusion of all disciplines necessary for patient care. The challenge of conducting interdisciplinary rounds is considerable in today’s busy clinical environment: health professionals who are spread across multiple locations within the hospital, and who have competing hospital responsibilities and priorities, must come together at the same time and for a set period each day. A survey with respondents from Australia, the United States, and Canada found that only 65% of rounds labelled “interdisciplinary” included a physician.7
While IDRs are not new, structured IDRs involve the purposeful inclusion of all disciplinary groups relevant to a patient’s care, alongside a checklist tool to aid comprehensive but concise daily assessment of progress and treatment planning. Novel, structured IDR interventions have been tested recently in various settings, resulting in improved teamwork, hospital performance, and patient outcomes in the US, including the Structured Interdisciplinary Bedside Round (SIBR) model.8-12
The aim of this study was to assess the impact of the new structure and the associated practice changes on interprofessional working and a set of key patient and hospital outcome measures. As part of the intervention, the hospital established an Acute Medical Unit (AMU) based on the Accountable Care Unit model.13
METHODS
Description of the Intervention
The AMU brought together 2 existing medical wards, a general medical ward and a 48-hour turnaround Medical Assessment Unit (MAU), into 1 geographical location with 26 beds. Prior to the merger, the MAU and general medical ward had separate and distinct cultures and workflows. The MAU was staffed with experienced nurses; nurses worked within a patient allocation model, the workload was shared, and relationships were collegial. In contrast, the medical ward was more typical of the remainder of the hospital: nurses had a heavy workload, managed a large group of longer-term complex patients, and they used a team-based nursing model of care in which senior nurses supervised junior staff. It was decided that because of the seniority of the MAU staff, they should be in charge of the combined AMU, and the patient allocation model of care would be used to facilitate SIBR.
Consultants, junior doctors, nurses, and allied health professionals (including a pharmacist, physiotherapist, occupational therapist, and social worker) were geographically aligned to the new ward, allowing them to participate as a team in daily structured ward rounds. Rounds are scheduled at the same time each day to enable family participation. The ward round is coordinated by a registrar or intern, with input from patient, family, nursing staff, pharmacy, allied health, and other doctors (intern, registrar, and consultant) based on the unit. The patient load is distributed between 2 rounds: 1 scheduled for 10
Data Collection Strategy
The study was set in an AMU in a large tertiary care hospital in regional Australia and used a convergent parallel multimethod approach14 to evaluate the implementation and effect of SIBR in the AMU. The study population consisted of 32 clinicians employed at the study hospital: (1) the leadership team involved in the development and implementation of the intervention and (2) members of clinical staff who were part of the AMU team.
Qualitative Data
Qualitative measures consisted of semistructured interviews. We utilized multiple strategies to recruit interviewees, including a snowball technique, criterion sampling,15 and emergent sampling, so that we could seek the views of both the leadership team responsible for the implementation and “frontline” clinical staff whose daily work was directly affected by it. Everyone who was initially recruited agreed to be interviewed, and additional frontline staff asked to be interviewed once they realized that we were asking about how staff experienced the changes in practice.
The research team developed a semistructured interview guide based on an understanding of the merger of the 2 units as well as an understanding of changes in practice of the rounds (provided in Appendix 1). The questions were pilot tested on a separate unit and revised. Questions were structured into 5 topic areas: planning and implementation of AMU/SIBR model, changes in work practices because of the new model, team functioning, job satisfaction, and perceived impact of the new model on patients and families. All interviews were audio-recorded and transcribed verbatim for analysis.
Quantitative Data
Quantitative data were collected on patient outcome measures: length of stay (LOS), discharge date and time, mode of separation (including death), primary diagnostic category, total hospital stay cost and “clinical response calls,” and patient demographic data (age, gender, and Patient Clinical Complexity Level [PCCL]). The PCCL is a standard measure used in Australian public inpatient facilities and is calculated for each episode of care.16 It measures the cumulative effect of a patient’s complications and/or comorbidities and takes an integer value between 0 (no clinical complexity effect) and 4 (catastrophic clinical complexity effect).
Data regarding LOS, diagnosis (Australian Refined Diagnosis Related Groups [AR-DRG], version 7), discharge date, and mode of separation (including death) were obtained from the New South Wales Ministry of Health’s Health Information Exchange for patients discharged during the year prior to the intervention through 1 year after the implementation of the intervention. The total hospital stay cost for these individuals was obtained from the local Health Service Organizational Performance Management unit. Inclusion criteria were inpatients aged over 15 years experiencing acute episodes of care; patients with a primary diagnostic category of mental diseases and disorders were excluded. LOS was calculated based on ward stay. AMU data were compared with the remaining hospital ward data (the control group). Data on “clinical response calls” per month per ward were also obtained for the 12 months prior to intervention and the 12 months of the intervention.
Analysis
Qualitative Analysis
Qualitative data analysis consisted of a hybrid form of textual analysis, combining inductive and deductive logics.17,18 Initially, 3 researchers (J.P., J.J., and R.C.W.) independently coded the interview data inductively to identify themes. Discrepancies were resolved through discussion until consensus was reached. Then, to further facilitate analysis, the researchers deductively imposed a matrix categorization, consisting of 4 a priori categories: context/conditions, practices/processes, professional interactions, and consequences.19,20 Additional a priori categories were used to sort the themes further in terms of experiences prior to, during, and following implementation of the intervention. To compare changes in those different time periods, we wanted to know what themes were related to implementation and whether those themes continued to be applicable to sustainability of the changes.
Quantitative analysis. Distribution of continuous data was examined by using the one-sample Kolmogorov-Smirnov test. We compared pre-SIBR (baseline) measures using the Student t test for normally distributed data, the Mann-Whitney U z test for nonparametric data (denoted as M-W U z), and χ2 tests for categorical data. Changes in monthly “clinical response calls” between the AMU and the control wards over time were explored by using analysis of variance (ANOVA). Changes in LOS and cost of stay from the year prior to the intervention to the first year of the intervention were analyzed by using generalized linear models, which are a form of linear regression. Factors, or independent variables, included in the models were time period (before or during intervention), ward (AMU or control), an interaction term (time by ward), patient age, gender, primary diagnosis (major diagnostic categories of the AR-DRG version 7.0), and acuity (PCCL). The estimated marginal means for cost of stay for the 12-month period prior to the intervention and for the first 12 months of the intervention were produced. All statistical analyses were performed by using IBM SPSS version 21 (IBM Corp., Armonk, New York) and with alpha set at P < .05.
RESULTS
Qualitative Evaluation of the Intervention
Participants.
Three researchers (RCW, JP, and JJ) conducted in-person, semistructured interviews with 32 clinicians (9 male, 23 female) during a 3-day period. The duration of the interviews ranged from 19 minutes to 68 minutes. Participants consisted of 8 doctors, 18 nurses, 5 allied health professionals, and an administrator. Ten of the participants were involved in the leadership group that drove the planning and implementation of SIBR and the AMU.
Themes
Context and Conditions of Work
Practices and Processes
Participants perceived postintervention work processes to be more efficient. A primary example was a near-universal approval of the time saved from not “chasing” other professionals now that they were predictably available on the ward. More timely decision-making was thought to result from this predicted availability and associated improvements in communication.
The SIBR enforced a workflow on all staff, who felt there was less flexibility to work autonomously (doctors) or according to patients’ needs (nurses). More junior staff expressed anxiety about delayed completion of discharge-related administrative tasks because of the midday completion of the round. Allied health professionals who had commitments in other areas of the hospital often faced a dilemma about how to prioritize SIBR attendance and activities on other wards. This was managed differently depending on the specific allied health profession and the individuals within that profession.
Professional Interactions
In terms of interprofessional dynamics on the AMU, the implementation of SIBR resulted in a shift in power between the doctors and the nurses. In the old ward, doctors largely controlled the timing of medical rounding processes. In the new AMU, doctors had to relinquish some control over the timing of personal workflow to comply with the requirements of SIBR. Furthermore, there was evidence that this had some impact on traditional hierarchical models of communication and created a more level playing field, as nonmedical professionals felt more empowered to voice their thoughts during and outside of rounds.
The rounds provided much greater visibility of the “big picture” and each profession’s role within it; this allowed each clinician to adjust their work to fit in and take account of others. The process was not instantaneous, and trust developed over a period of weeks. Better communication meant fewer misunderstandings, and workload dropped.
The participation of allied health professionals in the round enhanced clinician interprofessional skills and knowledge. The more inclusive approach facilitated greater trust between clinical disciplines and a development of increased confidence among nursing, allied health, and administrative professionals.
In contrast to the positive impacts of the new model of care on communication and relationships within the AMU, interdepartmental relationships were seen to have suffered. The processes and practices of the new AMU are different to those in the other hospital departments, resulting in some isolation of the unit and difficulties interacting with other areas of the hospital. For example, the trade-offs that allied health professionals made to participate in SIBR often came at the expense of other units or departments.
Consequences
All interviewees lauded the benefits of the SIBR intervention for patients. Patients were perceived to be better informed and more respected, and they benefited from greater perceived timeliness of treatment and discharge, easier access to doctors, better continuity of treatment and outcomes, improved nurse knowledge of their circumstances, and fewer gaps in their care. Clinicians spoke directly to the patient during SIBR, rather than consulting with professional colleagues over the patient’s head. Some staff felt that doctors were now thinking of patients as “people” rather than “a set of symptoms.” Nurses discovered that informed patients are easier to manage.
Staff members were prepared to compromise on their own needs in the interests of the patient. The emphasis on the patient during rounds resulted in improved advocacy behaviors of clinicians. The nurses became more empowered and able to show greater initiative. Families appeared to find it much easier to access the doctors and obtain information about the patient, resulting in less distress and a greater sense of control and trust in the process.
Quantitative Evaluation of the Intervention
Hospital Outcomes
Patient demographics did not change over time within either the AMU or control wards. However, there were significant increases in Patient Clinical Complexity Level (PCCL) ratings for both the AMU (44.7% to 40.3%; P<0.05) and the control wards (65.2% to 61.6%; P < .001). There was not a statistically significant shift over time in median LoS on the ward prior to (2.16 days, IQR 3.07) and during SIBR in the AMU (2.15 days; IQR 3.28), while LoS increased in the control (pre-SIBR: 1.67, 2.34; during SIBR 1.73, 2.40; M-W U z = -2.46, P = .014). Mortality rates were stable across time for both the AMU (pre-SIBR 2.6% [95% confidence interval {CI}, 1.9-3.5]; during SIBR 2.8% [95% CI, 2.1-3.7]) and the control (pre-SIBR 1.3% [95% CI, 1.0-1.5]; during SIBR 1.2% [95% CI, 1.0-1.4]).
The total number of “clinical response calls” or “flags” per month dropped significantly from pre-SIBR to during SIBR for the AMU from a mean of 63.1 (standard deviation 15.1) to 31.5 (10.8), but remained relatively stable in the control (pre-SIBR 72.5 [17.6]; during SIBR 74.0 [28.3]), and this difference was statistically significant (F (1,44) = 9.03; P = .004). There was no change in monthly “red flags” or “rapid response calls” over time (AMU: 10.5 [3.6] to 9.1 [4.7]; control: 40.3 [11.7] to 41.8 [10.8]). The change in total “clinical response calls” over time was attributable to the “yellow flags” or the decline in “calls for clinical review” in the AMU (from 52.6 [13.5] to 22.4 [9.2]). The average monthly “yellow flags” remained stable in the control (pre-SIBR 32.2 [11.6]; during SIBR 32.3 [22.4]). The AMU and the control wards differed significantly in how the number of monthly “calls for clinical review” changed from pre-SIBR to during SIBR (F (1,44) = 12.18; P = .001).
The 2 main outcome measures, LOS and costs, were analyzed to determine whether changes over time differed between the AMU and the control wards after accounting for age, gender, and PCCL. There was no statistically significant difference between the AMU and control wards in terms of change in LOS over time (Wald χ2 = 1.05; degrees of freedom [df] = 1; P = .31). There was a statistically significant interaction for cost of stay, indicating that ward types differed in how they changed over time (with a drop in cost over time observed in the AMU and an increase observed in the control) (Wald χ2 = 6.34; df = 1; P = .012.
DISCUSSION
We report on the implementation of an AMU model of care, including the reorganization of a nursing unit, implementation of IDR, and geographical localization. Our study design allowed a more comprehensive assessment of the implementation of system redesign to include provider perceptions and clinical outcomes.
The 2 very different cultures of the old wards that were combined into the AMU, as well as the fact that the teams had not previously worked together, made the merger of the 2 wards difficult. Historically, the 2 teams had worked in very different ways, and this created barriers to implementation. The SIBR also demanded new ways of working closely with other disciplines, which disrupted older clinical cultures and relationships. While organizational culture is often discussed, and even measured, the full impact of cultural factors when making workplace changes is frequently underestimated.21 The development of a new culture takes time, and it can lag organizational structural changes by months or even years.22 As our interviewees expressed, often emotionally, there was a sense of loss during the merger of the 2 units. While this is a potential consequence of any large organizational change, it could be addressed during the planning stages, prior to implementation, by acknowledging and perhaps honoring what is being left behind. It is safe to assume that future units implementing the rounding intervention will not fully realize commensurate levels of culture change until well after the structural and process changes are finalized, and only then if explicit effort is made to engender cultural change.
Overall, however, the interviewees perceived that the SIBR intervention led to improved teamwork and team functioning. These improvements were thought to benefit task performance and patient safety. Our study is consistent with other research in the literature that reported that greater staff empowerment and commitment is associated with interdisciplinary patient care interventions in front line caregiving teams.23,24 The perception of a more equal nurse-physician relationship resulted in improved job satisfaction, better interprofessional relationships, and perceived improvements in patient care. A flatter power gradient across professions and increased interdisciplinary teamwork has been shown to be associated with improved patient outcomes.25,26
Changes to clinician workflow can significantly impact the introduction of new models of care. A mandated time each day for structured rounds meant less flexibility in workflow for clinicians and made greater demands on their time management and communication skills. Furthermore, the need for human resource negotiations with nurse representatives was an unexpected component of successfully introducing the changes to workflow. Once the benefits of saved time and better communication became evident, changes to workflow were generally accepted. These challenges can be managed if stakeholders are engaged and supportive of the changes.13
Finally, our findings emphasize the importance of combining qualitative and quantitative data when evaluating an intervention. In this case, the qualitative outcomes that include “intangible” positive effects, such as cultural change and improved staff understanding of one another’s roles, might encourage us to continue with the SIBR intervention, which would allow more time to see if the trend of reduced LOS identified in the statistical analysis would translate to a significant effect over time.
We are unable to identify which aspects of the intervention led to the greatest impact on our outcomes. A recent study found that interdisciplinary rounds had no impact on patients’ perceptions of shared decision-making or care satisfaction.27 Although our findings indicated many potential benefits for patients, we were not able to interview patients or their carers to confirm these findings. In addition, we do not have any patient-centered outcomes, which would be important to consider in future work. Although our data on clinical response calls might be seen as a proxy for adverse events, we do not have data on adverse events or errors, and these are important to consider in future work. Finally, our findings are based on data from a single institution.
CONCLUSIONS
While there were some criticisms, participants expressed overwhelmingly positive reactions to the SIBR. The biggest reported benefit was perceived improved communication and understanding between and within the clinical professions, and between clinicians and patients. Improved communication was perceived to have fostered improved teamwork and team functioning, with most respondents feeling that they were a valued part of the new team. Improved teamwork was thought to contribute to improved task performance and led interviewees to perceive a higher level of patient safety. This research highlights the need for multimethod evaluations that address contextual factors as well as clinical outcomes.
Acknowledgments
The authors would like to acknowledge the clinicians and staff members who participated in this study. We would also like to acknowledge the support from the NSW Clinical Excellence Commission, in particular, Dr. Peter Kennedy, Mr. Wilson Yeung, Ms. Tracy Clarke, and Mr. Allan Zhang, and also from Ms. Karen Storey and Mr. Steve Shea of the Organisational Performance Management team at the Orange Health Service.
Disclosures
None of the authors had conflicts of interest in relation to the conduct or reporting of this study, with the exception that the lead author’s institution, the Australian Institute of Health Innovation, received a small grant from the New South Wales Clinical Excellence Commission to conduct the work. Ethics approval for the research was granted by the Greater Western Area Health Service Human Research Ethics Committee (HREC/13/GWAHS/22). All interviewees consented to participate in the study. For patient data, consent was not obtained, but presented data are anonymized. The full dataset is available from the corresponding author with restrictions. This research was funded by the NSW Clinical Excellence Commission, who also encouraged submission of the article for publication. The funding source did not have any role in conduct or reporting of the study. R.C.W., J.P., and J.J. conceptualized and conducted the qualitative component of the study, including method, data collection, data analysis, and writing of the manuscript. G.L., C.H., and H.D. conceptualized the quantitative component of the study, including method, data collection, data analysis, and writing of the manuscript. G.S. contributed to conceptualization of the study, and significantly contributed to the revision of the manuscript. All authors, external and internal, had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. As the lead author, R.C.W. affirms that the manuscript is an honest, accurate, and transparent account of the study being reported, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned have been explained.
1. Johnson JK, Batalden PB. Educating health professionals to improve care within the clinical microsystem. McLaughlin and Kaluzny’s Continuous Quality Improvement In Health Care. Burlington: Jones & Bartlett Learning; 2013.
2. Mohr JJ, Batalden P, Barach PB. Integrating patient safety into the clinical microsystem. Qual Saf Health Care. 2004;13:ii34-ii38. PubMed
3. Sanchez JA, Barach PR. High reliability organizations and surgical microsystems: re-engineering surgical care. Surg Clin North Am. 2012;92:1-14. PubMed
4. Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36:AS4-AS12. PubMed
5. O’Mahony S, Mazur E, Charney P, Wang Y, Fine J. Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J Gen Intern Med. 2007;22:1073-1079. PubMed
6. Pannick S, Beveridge I, Wachter RM, Sevdalis N. Improving the quality and safety of care on the medical ward: a review and synthesis of the evidence base. Eur J Intern Med. 2014;25:874-887. PubMed
7. Halm MA, Gagner S, Goering M, Sabo J, Smith M, Zaccagnini M. Interdisciplinary rounds: impact on patients, families, and staff. Clin Nurse Spec. 2003;17:133-142. PubMed
8. Stein J, Murphy D, Payne C, et al. A remedy for fragmented hospital care. Harvard Business Review. 2013.
9. O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2010;171:678-684. PubMed
10. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6:88-93. PubMed
11. O’Leary KJ, Ritter CD, Wheeler H, Szekendi MK, Brinton TS, Williams MV. Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2011;19:117-121. PubMed
12. O’Leary KJ, Creden AJ, Slade ME, et al. Implementation of unit-based interventions to improve teamwork and patient safety on a medical service. Am J Med Qual. 2014;30:409-416. PubMed
13. Stein J, Payne C, Methvin A, et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10:36-40. PubMed
14. Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. Thousand Oaks: SAGE Publications; 2013.
15. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Pol Ment Health. 2015;42:533-544. PubMed
16. Australian Consortium for Classification Development (ACCD). Review of the AR-DRG classification Case Complexity Process: Final Report; 2014.
http://ihpa.gov.au/internet/ihpa/publishing.nsf/Content/admitted-acute. Accessed September 21, 2015.
17. Lofland J, Lofland LH. Analyzing Social Settings. Belmont: Wadsworth Publishing Company; 2006.
18. Miles MB, Huberman AM, Saldaña J. Qualitative Data Analysis: A Methods Sourcebook. Los Angeles: SAGE Publications; 2014.
19. Corbin J, Strauss A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Thousand Oaks: SAGE Publications; 2008.
20. Corbin JM, Strauss A. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13:3-21.
21. O’Leary KJ, Johnson JK, Auerbach AD. Do interdisciplinary rounds improve patient outcomes? only if they improve teamwork. J Hosp Med. 2016;11:524-525. PubMed
22. Clay-Williams R. Restructuring and the resilient organisation: implications for health care. In: Hollnagel E, Braithwaite J, Wears R, editors. Resilient health care. Surrey: Ashgate Publishing Limited; 2013.
23. Williams I, Dickinson H, Robinson S, Allen C. Clinical microsystems and the NHS: a sustainable method for improvement? J Health Organ and Manag. 2009;23:119-132. PubMed
24. Nelson EC, Godfrey MM, Batalden PB, et al. Clinical microsystems, part 1. The building blocks of health systems. Jt Comm J Qual Patient Saf. 2008;34:367-378. PubMed
25. Chisholm-Burns MA, Lee JK, Spivey CA, et al. US pharmacists’ effect as team members on patient care: systematic review and meta-analyses. Med Care. 2010;48:923-933. PubMed
26. Zwarenstein M, Goldman J, Reeves S. Interprofessional collaboration: effects of practice-based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;3:CD000072. PubMed
27. O’Leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. 2015;25:921-928. PubMed
1. Johnson JK, Batalden PB. Educating health professionals to improve care within the clinical microsystem. McLaughlin and Kaluzny’s Continuous Quality Improvement In Health Care. Burlington: Jones & Bartlett Learning; 2013.
2. Mohr JJ, Batalden P, Barach PB. Integrating patient safety into the clinical microsystem. Qual Saf Health Care. 2004;13:ii34-ii38. PubMed
3. Sanchez JA, Barach PR. High reliability organizations and surgical microsystems: re-engineering surgical care. Surg Clin North Am. 2012;92:1-14. PubMed
4. Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement. Med Care. 1998;36:AS4-AS12. PubMed
5. O’Mahony S, Mazur E, Charney P, Wang Y, Fine J. Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay. J Gen Intern Med. 2007;22:1073-1079. PubMed
6. Pannick S, Beveridge I, Wachter RM, Sevdalis N. Improving the quality and safety of care on the medical ward: a review and synthesis of the evidence base. Eur J Intern Med. 2014;25:874-887. PubMed
7. Halm MA, Gagner S, Goering M, Sabo J, Smith M, Zaccagnini M. Interdisciplinary rounds: impact on patients, families, and staff. Clin Nurse Spec. 2003;17:133-142. PubMed
8. Stein J, Murphy D, Payne C, et al. A remedy for fragmented hospital care. Harvard Business Review. 2013.
9. O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2010;171:678-684. PubMed
10. O’Leary KJ, Haviley C, Slade ME, Shah HM, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit. J Hosp Med. 2011;6:88-93. PubMed
11. O’Leary KJ, Ritter CD, Wheeler H, Szekendi MK, Brinton TS, Williams MV. Teamwork on inpatient medical units: assessing attitudes and barriers. Qual Saf Health Care. 2011;19:117-121. PubMed
12. O’Leary KJ, Creden AJ, Slade ME, et al. Implementation of unit-based interventions to improve teamwork and patient safety on a medical service. Am J Med Qual. 2014;30:409-416. PubMed
13. Stein J, Payne C, Methvin A, et al. Reorganizing a hospital ward as an accountable care unit. J Hosp Med. 2015;10:36-40. PubMed
14. Creswell JW. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches. Thousand Oaks: SAGE Publications; 2013.
15. Palinkas LA, Horwitz SM, Green CA, Wisdom JP, Duan N, Hoagwood K. Purposeful sampling for qualitative data collection and analysis in mixed method implementation research. Adm Pol Ment Health. 2015;42:533-544. PubMed
16. Australian Consortium for Classification Development (ACCD). Review of the AR-DRG classification Case Complexity Process: Final Report; 2014.
http://ihpa.gov.au/internet/ihpa/publishing.nsf/Content/admitted-acute. Accessed September 21, 2015.
17. Lofland J, Lofland LH. Analyzing Social Settings. Belmont: Wadsworth Publishing Company; 2006.
18. Miles MB, Huberman AM, Saldaña J. Qualitative Data Analysis: A Methods Sourcebook. Los Angeles: SAGE Publications; 2014.
19. Corbin J, Strauss A. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Thousand Oaks: SAGE Publications; 2008.
20. Corbin JM, Strauss A. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13:3-21.
21. O’Leary KJ, Johnson JK, Auerbach AD. Do interdisciplinary rounds improve patient outcomes? only if they improve teamwork. J Hosp Med. 2016;11:524-525. PubMed
22. Clay-Williams R. Restructuring and the resilient organisation: implications for health care. In: Hollnagel E, Braithwaite J, Wears R, editors. Resilient health care. Surrey: Ashgate Publishing Limited; 2013.
23. Williams I, Dickinson H, Robinson S, Allen C. Clinical microsystems and the NHS: a sustainable method for improvement? J Health Organ and Manag. 2009;23:119-132. PubMed
24. Nelson EC, Godfrey MM, Batalden PB, et al. Clinical microsystems, part 1. The building blocks of health systems. Jt Comm J Qual Patient Saf. 2008;34:367-378. PubMed
25. Chisholm-Burns MA, Lee JK, Spivey CA, et al. US pharmacists’ effect as team members on patient care: systematic review and meta-analyses. Med Care. 2010;48:923-933. PubMed
26. Zwarenstein M, Goldman J, Reeves S. Interprofessional collaboration: effects of practice-based interventions on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2009;3:CD000072. PubMed
27. O’Leary KJ, Killarney A, Hansen LO, et al. Effect of patient-centred bedside rounds on hospitalised patients’ decision control, activation and satisfaction with care. BMJ Qual Saf. 2015;25:921-928. PubMed
© 2018 Society of Hospital Medicine
Proximal Humerus Fracture 3-D Modeling
ABSTRACT
The objective of this study is to determine the reproducibility and feasibility of using 3-dimensional (3-D) computer simulation of proximal humerus fracture computed tomography (CT) scans for fracture reduction. We hypothesized that anatomic reconstruction with 3-D models would be anatomically accurate and reproducible.
Preoperative CT scans of 28 patients with 3- and 4-part (AO classification 11-B1, 11-B2, 11-C1, 11-C2) proximal humerus fractures who were treated by hemiarthroplasty were converted into 3-D computer models. The displaced fractured fragments were anatomically reduced with computer simulation by 2 fellowship-trained shoulder surgeons, and measurements were made of the reconstructed proximal humerus.
The measurements of the reconstructed models had very good to excellent interobserver and intraobserver reliability. The reconstructions of these humerus fractures showed interclass correlation coefficients ranging from 0.71 to 0.93 between 1 observer and from 0.82 to 0.98 between 2 different observers. The fracture reduction was judged against normal proximal humerus geometry to determine reduction accuracy.
The 3-D modeling techniques used to reconstruct 3- and 4-part proximal humerus fractures were reliable and accurate. This technique of modeling and reconstructing proximal humerus fractures could be used to enhance the preoperative planning of open reduction and internal fixation or hemiarthroplasty for 3- and 4-part proximal humerus fractures.
The treatment of proximal humerus fractures is influenced by multiple factors, including patient age, associated injuries, bone quality, and fracture pattern. Three- and 4-part fractures are among the more severe of these fractures, which may result in vascular compromise to the humeral head, leading to avascular necrosis. Surgical goals for the management of these fractures are to optimize functional outcomes by re-creating a stable construct with a functional rotator cuff by open reduction and internal fixation (ORIF), hemiarthroplasty with tuberosity ORIF, or reverse shoulder replacement. Achieving a good outcome following hemiarthroplasty is dependent on many factors, including anatomic tuberosity healing and component positioning.1,2,3 Repairing the greater tuberosity in a near-anatomic position has been shown to greatly affect the results of hemiarthroplasty for fracture.3,4
Continue to: Three-dimensional (3-D) modeling...
Three-dimensional (3-D) modeling is increasingly being used in preoperative planning of shoulder arthroplasty and determining proper proximal humeral fracture treatment. 5 However, no studies have examined the reconstruction of a fractured proximal humerus into native anatomy using computer simulation. The purpose of this study is to determine the accuracy and reliability of anatomically reconstructing the preinjury proximal humerus using 3-D computer models created from postinjury computed tomography (CT) scans. The results of this study could lead to useful techniques employing CT–based models for patient-specific preoperative planning of proximal humeral fracture ORIF and during tuberosity reduction and fixation during hemiarthroplasty for fracture. We hypothesize that it is feasible to reconstruct the original anatomy of the proximal humerus by using 3-D computer modeling of proximal humerus fractures with high reliability based on interobserver and intraobserver review.
METHODS
After Institutional Review Board approval was obtained, we reviewed the medical records of consecutive patients with a diagnosis of proximal humeral fracture and the treatment codes for hemiarthroplasty from 2000 to 2013. Inclusion criteria included 3- and 4-part fractures (AO classifications 11-B1, 11-B2, 11-C1, 11-C2). CT scans with insufficient quality to differentiate bone from soft tissue (inadequate signal-to-noise ratio) were excluded from the study. A total of 28 patients with adequate CT scans met the criteria for inclusion in this study.
The CT scan protocol included 0.5-mm axial cuts with inclusion of the proximal humerus in the Digital Imaging and Communications in Medicine format. These CT scans were converted into patient-specific 3-D computer models of the shoulder using Mimics software (Materialise Inc.). The use of this software to produce anatomically accurate models has previously been verified in a shoulder model.6,7 The tuberosity fragments were then individually separated from each other using the voxel-selecting capabilities of 3-D software and manipulated with translation and rotation for anatomic reduction (Figures 1A-1D, Figure 2).
The de-identified anatomically reconstructed shoulder models were then uploaded into Materialise’s Magics rapid prototyping software, and a user-defined humeral Cartesian coordinate system was defined with anatomic landmarks as reference points to standardize the position of each model (Figure 3).8,9
A series of measurements were made on these models to assess the validity and reliability of the reassembly. The bicipital groove at the anatomic neck was used to measure humeral head version as described by Kummer and colleagues.10 The head-shaft angle, humeral head-greater tuberosity distance, humeral head-bicipital groove angle, and posterior and medial humeral head offset were measured directly on the reconstructed humerus.
Continue to: Two fellowship-trained shoulder...
Two fellowship-trained shoulder surgeons independently reassembled these fracture fragments via computer simulation. Interobserver reliability testing was conducted on these reconstructions by measuring the geometry between the 2 different surgeons’ reconstructions. Intraobserver reliability testing was conducted by 1 surgeon repeating the reconstructions with 4-week intervals between trials and measuring the geometry between the 2 different trials. The average dimensions of the reconstructed proximal humerus fractures were compared with the geometry of normal humeri reported in previously conducted anatomic studies.11,12,13
STATISTICS
The measured dimensions of the 28 reassembled proximal humeri models were averaged across all trials between the 2 fellowship-trained surgeons and compared with the range of normal dimensions of a healthy proximal humerus using the 2 one-sided tests (TOST) method for equivalence between 2 means given a range. The interobserver and intraobserver reliabilities were quantified using the interclass correlation coefficient. An excellent correlation was defined as a correlation coefficient >0.81; very good was defined as 0.61 to 0.80; and good was defined as 0.41 to 0.60.
RESULTS
Of the patients studied, 9 (32.1%) were male, and the average age at the time of CT scanning was 72 years. Of the 28 patients with fracture, 18 (64.2%) had 3-part fractures (AO classifications 11-B1, 11-B2), and 10 (35.8%) had 4-part fractures (AO classifications 11-C1, 11-C2). When examining the location of the intertubercular fracture line, we found that 13 (46.4%) fractures went through the bicipital groove. Of the remaining fracture lines, 9 (32.1%) extended into the greater tuberosity and 6 (21.4%) extended into the lesser tuberosity.
All users were able to reconstruct all 28 fractures using this technique. The average measured dimensions fell within the range of dimensions of a normal healthy proximal humerus specified in the literature to within a 95% confidence interval using the TOST for equivalence, in which we compared measured values with ranges reported in the literature (Table).11,12,13
Table. Dimensions of Proximal Humerus Geometry
| Normal Parameters | Average Dimensions From Trials | Dimensions From Literature |
| Head shaft angle | 43.5° ± 1° | 42.5° ± 12.5° |
| Head to greater tuberosity distance | 4.9 mm ± 0.4 mm | 8 mm ± 3.2 mm |
Head to bicipital groove angle (anatomic neck) | 26.4° ± 2° | 27.3° ± 14° |
| Posterior humeral head offset | 1.6 mm ± 0.3 mm | 4 mm ± 6 mm |
| Medial humeral head offset | 4.5 mm ± 0.3 mm | 9 mm ± 5 mm |
The reconstructions of these humerus fractures showed intraclass correlation coefficients ranging from 0.71 to 0.93 in 1 observer and interclass correlation coefficients from 0.82 to 0.98 between 2 different observers (Table).
DISCUSSION
This study demonstrates that it is feasible to reliably and accurately reconstruct the original anatomy of the proximal humerus by using 3-D computer modeling of proximal humerus fractures. Poor outcomes after hemiarthroplasty for proximal humerus fractures are mostly related to tuberosity malpositioning, resorption, or failure of fixation and resultant dysfunction of the rotator cuff.14,15,16 These studies highlight the importance of accurate tuberosity reduction during surgical care of these fractures.
Continue to: The 3-D computer model...
The 3-D computer model reconstruction of 3- and 4-part proximal humerus fractures were reliable and valid. The interclass correlation coefficients showed very good to excellent interobserver and intraobserver reliability for all measurements conducted. The averaged dimensions from all trials fell within the appropriate range of dimensions for a normal healthy humerus reported in the literature, as verified by the TOST method.11,12,13 The 3-D modeling capabilities demonstrated in this study allowed a greater understanding of the fracture patterns present in 3- and 4-part (AO classifications 11-B1, 11-B2, 11-C1, 11-C2) humerus fractures.
Overreduction of greater tuberosity to create cortical overlap with the lateral shaft may be used to promote bony union. As a result of this distalization, there may be extra strains placed on the rotator cuff, making the patient more prone to rotator cuff tear, as well as improperly balancing the dynamic stabilizers of the shoulder. Poor clinical outcomes in hemiarthroplasty for proximal humerus fractures have been correlated with a greater tuberosity placed distal relative to the humeral head by 1 cm in a study2 and by 2 cm in another.3
This study has several limitations. The first is the assumption that our injured patients had preinjury proximal humerus geometry within the range of normal dimensions of a healthy humerus. Unfortunately, because we were unable to obtain CT scans of the contralateral shoulder, we had to use standard proximal humerus geometry as the control. Another limitation, inherent in the technique, is that only cortical and dense trabecular bone was modeled, so that comminuted or osteoporotic bone was not well modeled. This study did not correlate the findings from these models with clinical outcomes. A prospective study is needed to evaluate the impact of this 3-D modeling on fracture reductions and clinical outcomes.
This study demonstrates that patient-specific modeling of proximal humerus fracture 3-D CT scans may help surgeons reliably and accurately reconstruct fractures. This technique may have utility in the preoperative planning of tuberosity fracture reduction and hemiarthroplasty. It gives surgeons the ability to visualize fracture fragments, and the process of reconstructing the fragments may help surgeons understand the required maneuvers for reduction at the time of surgery. This technique also provides dimensions of the patient’s native humerus, thus potentially improving the anatomic accuracy of the reduction or hemiarthroplasty reconstruction. With the new trend toward patient-specific instrumentation, this study also provides a means of planning the size of the humeral prostheses as well as the version relative to the biceps groove and intertubercular fracture line.
CONCLUSION
This study demonstrates the feasibility of using 3-D computer modeling of complex proximal humerus fractures in anatomic reconstruction. These techniques of computer-simulated 3-D models are valid and reliable. We believe that this technique of modeling and reconstructing proximal humerus fractures could be used to enhance the preoperative planning of hemiarthroplasty for 3- and 4-part proximal humerus fractures by providing improved understanding of the patient’s native humeral geometry and tuberosity reduction.
1. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Mole D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412. doi:10.1067/mse.2002.124527.
2. Mighell MA, Kolm GP, Collinge CA, Frankle MA. Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):569-577. doi:10.1016/S1058274603002131.
3. Greiner SH, Kaab MJ, Kroning I, Scheibel M, Perka C. Reconstruction of humeral length and centering of the prosthetic head in hemiarthroplasty for proximal humeral fractures. J Shoulder Elbow Surg. 2008;17(5):709-714. doi:10.1016/j.jse.2008.03.004.
4. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24. doi:10.1016/j.jse.2006.05.008.
5. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491. doi:10.1016/j.jse.2007.09.006.
6. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832. doi:10.1016/j.jse.2008.01.141.
7. Yongpravat C, Kim HM, Gardner TR, Bigliani LU, Levine WN, Ahmad CS. Glenoid implant orientation and cement failure in total shoulder arthroplasty: a finite element analysis. J Shoulder Elbow Surg. 2013;22(7):940-947. doi:10.1016/j.jse.2012.09.007.
8. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-865. doi:10.1302/0301-620X.79B5.0790857.
9. Wu G, van der Helm FC, Veeger HE, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38(5):981-992.
10. Kummer FJ, Perkins R, Zuckerman JD. The use of the bicipital groove for alignment of the humeral stem in shoulder arthroplasty. J Shoulder Elbow Surg. 1998;7(2):144-146. doi:10.1016/S1058-2746(98)90225-7.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326. doi:10.1016/S1058-2746(96)80060-7.
13. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(1 Suppl S):99S-104S. doi:10.1016/j.jse.2004.09.025.
14. Prakash U, McGurty DW, Dent JA. Hemiarthroplasty for severe fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):428-430. doi:10.1067/mse.2002.126615.
15. Robinson CM, Page RS, Hill RM, Sanders DL, Court-Brown CM, Wakefield AE. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am. 2003;85-A(7):1215-1223.
16. Zyto K, Wallace WA, Frostick SP, Preston BJ. Outcome after hemiarthroplasty for three- and four-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1998;7(2):85-89. doi:10.1016/S1058-2746(98)90215-4.
ABSTRACT
The objective of this study is to determine the reproducibility and feasibility of using 3-dimensional (3-D) computer simulation of proximal humerus fracture computed tomography (CT) scans for fracture reduction. We hypothesized that anatomic reconstruction with 3-D models would be anatomically accurate and reproducible.
Preoperative CT scans of 28 patients with 3- and 4-part (AO classification 11-B1, 11-B2, 11-C1, 11-C2) proximal humerus fractures who were treated by hemiarthroplasty were converted into 3-D computer models. The displaced fractured fragments were anatomically reduced with computer simulation by 2 fellowship-trained shoulder surgeons, and measurements were made of the reconstructed proximal humerus.
The measurements of the reconstructed models had very good to excellent interobserver and intraobserver reliability. The reconstructions of these humerus fractures showed interclass correlation coefficients ranging from 0.71 to 0.93 between 1 observer and from 0.82 to 0.98 between 2 different observers. The fracture reduction was judged against normal proximal humerus geometry to determine reduction accuracy.
The 3-D modeling techniques used to reconstruct 3- and 4-part proximal humerus fractures were reliable and accurate. This technique of modeling and reconstructing proximal humerus fractures could be used to enhance the preoperative planning of open reduction and internal fixation or hemiarthroplasty for 3- and 4-part proximal humerus fractures.
The treatment of proximal humerus fractures is influenced by multiple factors, including patient age, associated injuries, bone quality, and fracture pattern. Three- and 4-part fractures are among the more severe of these fractures, which may result in vascular compromise to the humeral head, leading to avascular necrosis. Surgical goals for the management of these fractures are to optimize functional outcomes by re-creating a stable construct with a functional rotator cuff by open reduction and internal fixation (ORIF), hemiarthroplasty with tuberosity ORIF, or reverse shoulder replacement. Achieving a good outcome following hemiarthroplasty is dependent on many factors, including anatomic tuberosity healing and component positioning.1,2,3 Repairing the greater tuberosity in a near-anatomic position has been shown to greatly affect the results of hemiarthroplasty for fracture.3,4
Continue to: Three-dimensional (3-D) modeling...
Three-dimensional (3-D) modeling is increasingly being used in preoperative planning of shoulder arthroplasty and determining proper proximal humeral fracture treatment. 5 However, no studies have examined the reconstruction of a fractured proximal humerus into native anatomy using computer simulation. The purpose of this study is to determine the accuracy and reliability of anatomically reconstructing the preinjury proximal humerus using 3-D computer models created from postinjury computed tomography (CT) scans. The results of this study could lead to useful techniques employing CT–based models for patient-specific preoperative planning of proximal humeral fracture ORIF and during tuberosity reduction and fixation during hemiarthroplasty for fracture. We hypothesize that it is feasible to reconstruct the original anatomy of the proximal humerus by using 3-D computer modeling of proximal humerus fractures with high reliability based on interobserver and intraobserver review.
METHODS
After Institutional Review Board approval was obtained, we reviewed the medical records of consecutive patients with a diagnosis of proximal humeral fracture and the treatment codes for hemiarthroplasty from 2000 to 2013. Inclusion criteria included 3- and 4-part fractures (AO classifications 11-B1, 11-B2, 11-C1, 11-C2). CT scans with insufficient quality to differentiate bone from soft tissue (inadequate signal-to-noise ratio) were excluded from the study. A total of 28 patients with adequate CT scans met the criteria for inclusion in this study.
The CT scan protocol included 0.5-mm axial cuts with inclusion of the proximal humerus in the Digital Imaging and Communications in Medicine format. These CT scans were converted into patient-specific 3-D computer models of the shoulder using Mimics software (Materialise Inc.). The use of this software to produce anatomically accurate models has previously been verified in a shoulder model.6,7 The tuberosity fragments were then individually separated from each other using the voxel-selecting capabilities of 3-D software and manipulated with translation and rotation for anatomic reduction (Figures 1A-1D, Figure 2).
The de-identified anatomically reconstructed shoulder models were then uploaded into Materialise’s Magics rapid prototyping software, and a user-defined humeral Cartesian coordinate system was defined with anatomic landmarks as reference points to standardize the position of each model (Figure 3).8,9
A series of measurements were made on these models to assess the validity and reliability of the reassembly. The bicipital groove at the anatomic neck was used to measure humeral head version as described by Kummer and colleagues.10 The head-shaft angle, humeral head-greater tuberosity distance, humeral head-bicipital groove angle, and posterior and medial humeral head offset were measured directly on the reconstructed humerus.
Continue to: Two fellowship-trained shoulder...
Two fellowship-trained shoulder surgeons independently reassembled these fracture fragments via computer simulation. Interobserver reliability testing was conducted on these reconstructions by measuring the geometry between the 2 different surgeons’ reconstructions. Intraobserver reliability testing was conducted by 1 surgeon repeating the reconstructions with 4-week intervals between trials and measuring the geometry between the 2 different trials. The average dimensions of the reconstructed proximal humerus fractures were compared with the geometry of normal humeri reported in previously conducted anatomic studies.11,12,13
STATISTICS
The measured dimensions of the 28 reassembled proximal humeri models were averaged across all trials between the 2 fellowship-trained surgeons and compared with the range of normal dimensions of a healthy proximal humerus using the 2 one-sided tests (TOST) method for equivalence between 2 means given a range. The interobserver and intraobserver reliabilities were quantified using the interclass correlation coefficient. An excellent correlation was defined as a correlation coefficient >0.81; very good was defined as 0.61 to 0.80; and good was defined as 0.41 to 0.60.
RESULTS
Of the patients studied, 9 (32.1%) were male, and the average age at the time of CT scanning was 72 years. Of the 28 patients with fracture, 18 (64.2%) had 3-part fractures (AO classifications 11-B1, 11-B2), and 10 (35.8%) had 4-part fractures (AO classifications 11-C1, 11-C2). When examining the location of the intertubercular fracture line, we found that 13 (46.4%) fractures went through the bicipital groove. Of the remaining fracture lines, 9 (32.1%) extended into the greater tuberosity and 6 (21.4%) extended into the lesser tuberosity.
All users were able to reconstruct all 28 fractures using this technique. The average measured dimensions fell within the range of dimensions of a normal healthy proximal humerus specified in the literature to within a 95% confidence interval using the TOST for equivalence, in which we compared measured values with ranges reported in the literature (Table).11,12,13
Table. Dimensions of Proximal Humerus Geometry
| Normal Parameters | Average Dimensions From Trials | Dimensions From Literature |
| Head shaft angle | 43.5° ± 1° | 42.5° ± 12.5° |
| Head to greater tuberosity distance | 4.9 mm ± 0.4 mm | 8 mm ± 3.2 mm |
Head to bicipital groove angle (anatomic neck) | 26.4° ± 2° | 27.3° ± 14° |
| Posterior humeral head offset | 1.6 mm ± 0.3 mm | 4 mm ± 6 mm |
| Medial humeral head offset | 4.5 mm ± 0.3 mm | 9 mm ± 5 mm |
The reconstructions of these humerus fractures showed intraclass correlation coefficients ranging from 0.71 to 0.93 in 1 observer and interclass correlation coefficients from 0.82 to 0.98 between 2 different observers (Table).
DISCUSSION
This study demonstrates that it is feasible to reliably and accurately reconstruct the original anatomy of the proximal humerus by using 3-D computer modeling of proximal humerus fractures. Poor outcomes after hemiarthroplasty for proximal humerus fractures are mostly related to tuberosity malpositioning, resorption, or failure of fixation and resultant dysfunction of the rotator cuff.14,15,16 These studies highlight the importance of accurate tuberosity reduction during surgical care of these fractures.
Continue to: The 3-D computer model...
The 3-D computer model reconstruction of 3- and 4-part proximal humerus fractures were reliable and valid. The interclass correlation coefficients showed very good to excellent interobserver and intraobserver reliability for all measurements conducted. The averaged dimensions from all trials fell within the appropriate range of dimensions for a normal healthy humerus reported in the literature, as verified by the TOST method.11,12,13 The 3-D modeling capabilities demonstrated in this study allowed a greater understanding of the fracture patterns present in 3- and 4-part (AO classifications 11-B1, 11-B2, 11-C1, 11-C2) humerus fractures.
Overreduction of greater tuberosity to create cortical overlap with the lateral shaft may be used to promote bony union. As a result of this distalization, there may be extra strains placed on the rotator cuff, making the patient more prone to rotator cuff tear, as well as improperly balancing the dynamic stabilizers of the shoulder. Poor clinical outcomes in hemiarthroplasty for proximal humerus fractures have been correlated with a greater tuberosity placed distal relative to the humeral head by 1 cm in a study2 and by 2 cm in another.3
This study has several limitations. The first is the assumption that our injured patients had preinjury proximal humerus geometry within the range of normal dimensions of a healthy humerus. Unfortunately, because we were unable to obtain CT scans of the contralateral shoulder, we had to use standard proximal humerus geometry as the control. Another limitation, inherent in the technique, is that only cortical and dense trabecular bone was modeled, so that comminuted or osteoporotic bone was not well modeled. This study did not correlate the findings from these models with clinical outcomes. A prospective study is needed to evaluate the impact of this 3-D modeling on fracture reductions and clinical outcomes.
This study demonstrates that patient-specific modeling of proximal humerus fracture 3-D CT scans may help surgeons reliably and accurately reconstruct fractures. This technique may have utility in the preoperative planning of tuberosity fracture reduction and hemiarthroplasty. It gives surgeons the ability to visualize fracture fragments, and the process of reconstructing the fragments may help surgeons understand the required maneuvers for reduction at the time of surgery. This technique also provides dimensions of the patient’s native humerus, thus potentially improving the anatomic accuracy of the reduction or hemiarthroplasty reconstruction. With the new trend toward patient-specific instrumentation, this study also provides a means of planning the size of the humeral prostheses as well as the version relative to the biceps groove and intertubercular fracture line.
CONCLUSION
This study demonstrates the feasibility of using 3-D computer modeling of complex proximal humerus fractures in anatomic reconstruction. These techniques of computer-simulated 3-D models are valid and reliable. We believe that this technique of modeling and reconstructing proximal humerus fractures could be used to enhance the preoperative planning of hemiarthroplasty for 3- and 4-part proximal humerus fractures by providing improved understanding of the patient’s native humeral geometry and tuberosity reduction.
ABSTRACT
The objective of this study is to determine the reproducibility and feasibility of using 3-dimensional (3-D) computer simulation of proximal humerus fracture computed tomography (CT) scans for fracture reduction. We hypothesized that anatomic reconstruction with 3-D models would be anatomically accurate and reproducible.
Preoperative CT scans of 28 patients with 3- and 4-part (AO classification 11-B1, 11-B2, 11-C1, 11-C2) proximal humerus fractures who were treated by hemiarthroplasty were converted into 3-D computer models. The displaced fractured fragments were anatomically reduced with computer simulation by 2 fellowship-trained shoulder surgeons, and measurements were made of the reconstructed proximal humerus.
The measurements of the reconstructed models had very good to excellent interobserver and intraobserver reliability. The reconstructions of these humerus fractures showed interclass correlation coefficients ranging from 0.71 to 0.93 between 1 observer and from 0.82 to 0.98 between 2 different observers. The fracture reduction was judged against normal proximal humerus geometry to determine reduction accuracy.
The 3-D modeling techniques used to reconstruct 3- and 4-part proximal humerus fractures were reliable and accurate. This technique of modeling and reconstructing proximal humerus fractures could be used to enhance the preoperative planning of open reduction and internal fixation or hemiarthroplasty for 3- and 4-part proximal humerus fractures.
The treatment of proximal humerus fractures is influenced by multiple factors, including patient age, associated injuries, bone quality, and fracture pattern. Three- and 4-part fractures are among the more severe of these fractures, which may result in vascular compromise to the humeral head, leading to avascular necrosis. Surgical goals for the management of these fractures are to optimize functional outcomes by re-creating a stable construct with a functional rotator cuff by open reduction and internal fixation (ORIF), hemiarthroplasty with tuberosity ORIF, or reverse shoulder replacement. Achieving a good outcome following hemiarthroplasty is dependent on many factors, including anatomic tuberosity healing and component positioning.1,2,3 Repairing the greater tuberosity in a near-anatomic position has been shown to greatly affect the results of hemiarthroplasty for fracture.3,4
Continue to: Three-dimensional (3-D) modeling...
Three-dimensional (3-D) modeling is increasingly being used in preoperative planning of shoulder arthroplasty and determining proper proximal humeral fracture treatment. 5 However, no studies have examined the reconstruction of a fractured proximal humerus into native anatomy using computer simulation. The purpose of this study is to determine the accuracy and reliability of anatomically reconstructing the preinjury proximal humerus using 3-D computer models created from postinjury computed tomography (CT) scans. The results of this study could lead to useful techniques employing CT–based models for patient-specific preoperative planning of proximal humeral fracture ORIF and during tuberosity reduction and fixation during hemiarthroplasty for fracture. We hypothesize that it is feasible to reconstruct the original anatomy of the proximal humerus by using 3-D computer modeling of proximal humerus fractures with high reliability based on interobserver and intraobserver review.
METHODS
After Institutional Review Board approval was obtained, we reviewed the medical records of consecutive patients with a diagnosis of proximal humeral fracture and the treatment codes for hemiarthroplasty from 2000 to 2013. Inclusion criteria included 3- and 4-part fractures (AO classifications 11-B1, 11-B2, 11-C1, 11-C2). CT scans with insufficient quality to differentiate bone from soft tissue (inadequate signal-to-noise ratio) were excluded from the study. A total of 28 patients with adequate CT scans met the criteria for inclusion in this study.
The CT scan protocol included 0.5-mm axial cuts with inclusion of the proximal humerus in the Digital Imaging and Communications in Medicine format. These CT scans were converted into patient-specific 3-D computer models of the shoulder using Mimics software (Materialise Inc.). The use of this software to produce anatomically accurate models has previously been verified in a shoulder model.6,7 The tuberosity fragments were then individually separated from each other using the voxel-selecting capabilities of 3-D software and manipulated with translation and rotation for anatomic reduction (Figures 1A-1D, Figure 2).
The de-identified anatomically reconstructed shoulder models were then uploaded into Materialise’s Magics rapid prototyping software, and a user-defined humeral Cartesian coordinate system was defined with anatomic landmarks as reference points to standardize the position of each model (Figure 3).8,9
A series of measurements were made on these models to assess the validity and reliability of the reassembly. The bicipital groove at the anatomic neck was used to measure humeral head version as described by Kummer and colleagues.10 The head-shaft angle, humeral head-greater tuberosity distance, humeral head-bicipital groove angle, and posterior and medial humeral head offset were measured directly on the reconstructed humerus.
Continue to: Two fellowship-trained shoulder...
Two fellowship-trained shoulder surgeons independently reassembled these fracture fragments via computer simulation. Interobserver reliability testing was conducted on these reconstructions by measuring the geometry between the 2 different surgeons’ reconstructions. Intraobserver reliability testing was conducted by 1 surgeon repeating the reconstructions with 4-week intervals between trials and measuring the geometry between the 2 different trials. The average dimensions of the reconstructed proximal humerus fractures were compared with the geometry of normal humeri reported in previously conducted anatomic studies.11,12,13
STATISTICS
The measured dimensions of the 28 reassembled proximal humeri models were averaged across all trials between the 2 fellowship-trained surgeons and compared with the range of normal dimensions of a healthy proximal humerus using the 2 one-sided tests (TOST) method for equivalence between 2 means given a range. The interobserver and intraobserver reliabilities were quantified using the interclass correlation coefficient. An excellent correlation was defined as a correlation coefficient >0.81; very good was defined as 0.61 to 0.80; and good was defined as 0.41 to 0.60.
RESULTS
Of the patients studied, 9 (32.1%) were male, and the average age at the time of CT scanning was 72 years. Of the 28 patients with fracture, 18 (64.2%) had 3-part fractures (AO classifications 11-B1, 11-B2), and 10 (35.8%) had 4-part fractures (AO classifications 11-C1, 11-C2). When examining the location of the intertubercular fracture line, we found that 13 (46.4%) fractures went through the bicipital groove. Of the remaining fracture lines, 9 (32.1%) extended into the greater tuberosity and 6 (21.4%) extended into the lesser tuberosity.
All users were able to reconstruct all 28 fractures using this technique. The average measured dimensions fell within the range of dimensions of a normal healthy proximal humerus specified in the literature to within a 95% confidence interval using the TOST for equivalence, in which we compared measured values with ranges reported in the literature (Table).11,12,13
Table. Dimensions of Proximal Humerus Geometry
| Normal Parameters | Average Dimensions From Trials | Dimensions From Literature |
| Head shaft angle | 43.5° ± 1° | 42.5° ± 12.5° |
| Head to greater tuberosity distance | 4.9 mm ± 0.4 mm | 8 mm ± 3.2 mm |
Head to bicipital groove angle (anatomic neck) | 26.4° ± 2° | 27.3° ± 14° |
| Posterior humeral head offset | 1.6 mm ± 0.3 mm | 4 mm ± 6 mm |
| Medial humeral head offset | 4.5 mm ± 0.3 mm | 9 mm ± 5 mm |
The reconstructions of these humerus fractures showed intraclass correlation coefficients ranging from 0.71 to 0.93 in 1 observer and interclass correlation coefficients from 0.82 to 0.98 between 2 different observers (Table).
DISCUSSION
This study demonstrates that it is feasible to reliably and accurately reconstruct the original anatomy of the proximal humerus by using 3-D computer modeling of proximal humerus fractures. Poor outcomes after hemiarthroplasty for proximal humerus fractures are mostly related to tuberosity malpositioning, resorption, or failure of fixation and resultant dysfunction of the rotator cuff.14,15,16 These studies highlight the importance of accurate tuberosity reduction during surgical care of these fractures.
Continue to: The 3-D computer model...
The 3-D computer model reconstruction of 3- and 4-part proximal humerus fractures were reliable and valid. The interclass correlation coefficients showed very good to excellent interobserver and intraobserver reliability for all measurements conducted. The averaged dimensions from all trials fell within the appropriate range of dimensions for a normal healthy humerus reported in the literature, as verified by the TOST method.11,12,13 The 3-D modeling capabilities demonstrated in this study allowed a greater understanding of the fracture patterns present in 3- and 4-part (AO classifications 11-B1, 11-B2, 11-C1, 11-C2) humerus fractures.
Overreduction of greater tuberosity to create cortical overlap with the lateral shaft may be used to promote bony union. As a result of this distalization, there may be extra strains placed on the rotator cuff, making the patient more prone to rotator cuff tear, as well as improperly balancing the dynamic stabilizers of the shoulder. Poor clinical outcomes in hemiarthroplasty for proximal humerus fractures have been correlated with a greater tuberosity placed distal relative to the humeral head by 1 cm in a study2 and by 2 cm in another.3
This study has several limitations. The first is the assumption that our injured patients had preinjury proximal humerus geometry within the range of normal dimensions of a healthy humerus. Unfortunately, because we were unable to obtain CT scans of the contralateral shoulder, we had to use standard proximal humerus geometry as the control. Another limitation, inherent in the technique, is that only cortical and dense trabecular bone was modeled, so that comminuted or osteoporotic bone was not well modeled. This study did not correlate the findings from these models with clinical outcomes. A prospective study is needed to evaluate the impact of this 3-D modeling on fracture reductions and clinical outcomes.
This study demonstrates that patient-specific modeling of proximal humerus fracture 3-D CT scans may help surgeons reliably and accurately reconstruct fractures. This technique may have utility in the preoperative planning of tuberosity fracture reduction and hemiarthroplasty. It gives surgeons the ability to visualize fracture fragments, and the process of reconstructing the fragments may help surgeons understand the required maneuvers for reduction at the time of surgery. This technique also provides dimensions of the patient’s native humerus, thus potentially improving the anatomic accuracy of the reduction or hemiarthroplasty reconstruction. With the new trend toward patient-specific instrumentation, this study also provides a means of planning the size of the humeral prostheses as well as the version relative to the biceps groove and intertubercular fracture line.
CONCLUSION
This study demonstrates the feasibility of using 3-D computer modeling of complex proximal humerus fractures in anatomic reconstruction. These techniques of computer-simulated 3-D models are valid and reliable. We believe that this technique of modeling and reconstructing proximal humerus fractures could be used to enhance the preoperative planning of hemiarthroplasty for 3- and 4-part proximal humerus fractures by providing improved understanding of the patient’s native humeral geometry and tuberosity reduction.
1. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Mole D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412. doi:10.1067/mse.2002.124527.
2. Mighell MA, Kolm GP, Collinge CA, Frankle MA. Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):569-577. doi:10.1016/S1058274603002131.
3. Greiner SH, Kaab MJ, Kroning I, Scheibel M, Perka C. Reconstruction of humeral length and centering of the prosthetic head in hemiarthroplasty for proximal humeral fractures. J Shoulder Elbow Surg. 2008;17(5):709-714. doi:10.1016/j.jse.2008.03.004.
4. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24. doi:10.1016/j.jse.2006.05.008.
5. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491. doi:10.1016/j.jse.2007.09.006.
6. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832. doi:10.1016/j.jse.2008.01.141.
7. Yongpravat C, Kim HM, Gardner TR, Bigliani LU, Levine WN, Ahmad CS. Glenoid implant orientation and cement failure in total shoulder arthroplasty: a finite element analysis. J Shoulder Elbow Surg. 2013;22(7):940-947. doi:10.1016/j.jse.2012.09.007.
8. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-865. doi:10.1302/0301-620X.79B5.0790857.
9. Wu G, van der Helm FC, Veeger HE, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38(5):981-992.
10. Kummer FJ, Perkins R, Zuckerman JD. The use of the bicipital groove for alignment of the humeral stem in shoulder arthroplasty. J Shoulder Elbow Surg. 1998;7(2):144-146. doi:10.1016/S1058-2746(98)90225-7.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326. doi:10.1016/S1058-2746(96)80060-7.
13. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(1 Suppl S):99S-104S. doi:10.1016/j.jse.2004.09.025.
14. Prakash U, McGurty DW, Dent JA. Hemiarthroplasty for severe fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):428-430. doi:10.1067/mse.2002.126615.
15. Robinson CM, Page RS, Hill RM, Sanders DL, Court-Brown CM, Wakefield AE. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am. 2003;85-A(7):1215-1223.
16. Zyto K, Wallace WA, Frostick SP, Preston BJ. Outcome after hemiarthroplasty for three- and four-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1998;7(2):85-89. doi:10.1016/S1058-2746(98)90215-4.
1. Boileau P, Krishnan SG, Tinsi L, Walch G, Coste JS, Mole D. Tuberosity malposition and migration: reasons for poor outcomes after hemiarthroplasty for displaced fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):401-412. doi:10.1067/mse.2002.124527.
2. Mighell MA, Kolm GP, Collinge CA, Frankle MA. Outcomes of hemiarthroplasty for fractures of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):569-577. doi:10.1016/S1058274603002131.
3. Greiner SH, Kaab MJ, Kroning I, Scheibel M, Perka C. Reconstruction of humeral length and centering of the prosthetic head in hemiarthroplasty for proximal humeral fractures. J Shoulder Elbow Surg. 2008;17(5):709-714. doi:10.1016/j.jse.2008.03.004.
4. Smith AM, Mardones RM, Sperling JW, Cofield RH. Early complications of operatively treated proximal humeral fractures. J Shoulder Elbow Surg. 2007;16(1):14-24. doi:10.1016/j.jse.2006.05.008.
5. Scalise JJ, Codsi MJ, Bryan J, Iannotti JP. The three-dimensional glenoid vault model can estimate normal glenoid version in osteoarthritis. J Shoulder Elbow Surg. 2008;17(3):487-491. doi:10.1016/j.jse.2007.09.006.
6. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832. doi:10.1016/j.jse.2008.01.141.
7. Yongpravat C, Kim HM, Gardner TR, Bigliani LU, Levine WN, Ahmad CS. Glenoid implant orientation and cement failure in total shoulder arthroplasty: a finite element analysis. J Shoulder Elbow Surg. 2013;22(7):940-947. doi:10.1016/j.jse.2012.09.007.
8. Boileau P, Walch G. The three-dimensional geometry of the proximal humerus. Implications for surgical technique and prosthetic design. J Bone Joint Surg Br. 1997;79(5):857-865. doi:10.1302/0301-620X.79B5.0790857.
9. Wu G, van der Helm FC, Veeger HE, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion--Part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38(5):981-992.
10. Kummer FJ, Perkins R, Zuckerman JD. The use of the bicipital groove for alignment of the humeral stem in shoulder arthroplasty. J Shoulder Elbow Surg. 1998;7(2):144-146. doi:10.1016/S1058-2746(98)90225-7.
11. Iannotti JP, Gabriel JP, Schneck SL, Evans BG, Misra S. The normal glenohumeral relationships. An anatomical study of one hundred and forty shoulders. J Bone Joint Surg Am. 1992;74(4):491-500.
12. Pearl ML, Volk AG. Coronal plane geometry of the proximal humerus relevant to prosthetic arthroplasty. J Shoulder Elbow Surg. 1996;5(4):320-326. doi:10.1016/S1058-2746(96)80060-7.
13. Pearl ML. Proximal humeral anatomy in shoulder arthroplasty: Implications for prosthetic design and surgical technique. J Shoulder Elbow Surg. 2005;14(1 Suppl S):99S-104S. doi:10.1016/j.jse.2004.09.025.
14. Prakash U, McGurty DW, Dent JA. Hemiarthroplasty for severe fractures of the proximal humerus. J Shoulder Elbow Surg. 2002;11(5):428-430. doi:10.1067/mse.2002.126615.
15. Robinson CM, Page RS, Hill RM, Sanders DL, Court-Brown CM, Wakefield AE. Primary hemiarthroplasty for treatment of proximal humeral fractures. J Bone Joint Surg Am. 2003;85-A(7):1215-1223.
16. Zyto K, Wallace WA, Frostick SP, Preston BJ. Outcome after hemiarthroplasty for three- and four-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1998;7(2):85-89. doi:10.1016/S1058-2746(98)90215-4.
TAKE-HOME POINTS
- Proximal humerus fractures may be better understood with 3-D CT imaging.
- 3-D computer modeling of complex proximal humerus fractures allows an understanding of tuebroisty reduction durring ORIF or hemiarthroplasty.
- 3-D modeling enhances preoperative planning for hemiarthroplasty implant size and position relative to the repaired tuberosity fragments.
- 3-D modeling of fracture reduction can help surgeons understand the patient’s native humeral geometry and anatomy.
- Preoperative evaluation of fracture characteristics and fragment reduction help surgeons better understand surgical solutions.
A Practical Guide to Urine Drug Monitoring
Urine drug monitoring (UDM) is an important tool to screen adherence and identify possible misuse and abuse in patients on opioid therapy.1 Various guidelines for opioid therapy emphasize the importance of UDM as a standard of care.2-6 Routine and random monitoring is recommended for all patients on long-term opioid therapy prior to initiation and throughout duration of therapy.1-3 The recommended UDM frequency varies based on individual risk assessment and clinical judgment. Similar to any other diagnostic or monitoring test, the goal for UDM should be to guide therapy and improve patient care (Box). Inappropriate interpretation of the results and failure to order definitive testing when necessary may adversely affect patient care.
Urine Drug Monitoring
Sample Collection
Urine drug testing generally requires a minimum of 30 mL of urine (depending on the kit type) collected in a private restroom. In the authors’ experience, the sample collection most often is unobserved in clinical practice. Most laboratories keep urine samples for a limited time, often 7 days. Therefor, if results are unexpected, health care providers must notify the laboratory in a timely manner to order definitive testing if indicated.
Specimen Validity Testing
Attempts to dilute, adulterate, and substitute urine may be detected by visual inspection and laboratory validity testing. Validity testing of urine specimens includes temperature, specific gravity, pH, urine creatinine, and presence of adulterants (Tables 1 and 2).7-9
The combination of specific gravity and urinary creatinine may help screen for dilution or substitution. Dilution may occur precollection by consumption of excess amounts of fluids or postcollection by adding fluid to the specimen. Other causes of diluted urine should be considered, such as renal tubular dysfunction or diuretic use. Household adulterants include vinegar, detergent, sodium chloride, hydrogen peroxide, eye/nose drops, soda, or ammonia.10 There are numerous commercially available adulterants, including Klear, UrinAid, Urine Luck, Stealth Synthetics, Whizzies, and Clear Choice. The active ingredients of some include peroxide/peroxidase, sodium or potassium nitrate, pyridinium chlorochromate, or glutaraldehyde. There are laboratory tests to detect the presence of these adulterants. Whenever in doubt, it is advisable that health care providers (HCPs) contact their laboratory to investigate tampering. Another approach if tampering is suspected is to collect blood samples. Although this method is more expensive and invasive, it eliminates means of tampering. Hair follicle testing is an option as well.
Types of Urine Drug Monitoring
There are 2 general types of UDM: Presumptive by immunoassay (IA) and confirmatory testing by chromatography. Simply, UDM by IA commonly referred to as urine drug screening (UDS), serves as the differential assessments, whereas chromatography is the definitive assessment. This article reviews the clinical utility and limitations of the 2 types of UDM, including false positives and false negatives, and when to order more tests.
Immunoassay
The IA drug test uses antibodies to detect the presence of selected drugs and/or their metabolites based on a predetermined cutoff threshold.8 Immunoassay monitoring is the initial qualitative test to identify the presence of drug classes in the urine based on a detection threshold. Typically, UDM by IA is performed as an initial evaluation of potential appropriate use, misuse, nonuse, or abuse of medications. It also can detect the presence of illegal substances or unprescribed medications. Immunoassay is relatively quick, inexpensive, and sensitive; however, because it lacks specificity, it can result in various false positives and false negatives.
Immunoassay tests also are subject to varying windows of detection depending on the substance ingested (Table 3).
The cutoff levels listed in Table 1 are consistent with testing for employment but not necessarily for aberrant behavior in patients receiving long-term opioid therapy. These cutoffs lower the risk of false positives and provide better accuracy with clinical monitoring. For example, a level of 2,000 ng/mL is listed for both test types in Table 4, but for clinical testing, the IA cutoff is 3,000 ng/mL, and gas chromatography/mass spectrometry (GC-MS) can detect even trace amounts of opioid and their metabolites.
The opiate panel with IA tests for opium alkaloids and/or their metabolites, including morphine and codeine.7-9 Heroin is a semisynthetic opioid that is metabolized to diacetyl morphine and ultimately is detected as morphine.7,8 Other semisynthetic opioids, such as hydrocodone and oxycodone, may or may not be detected by the opiate IA depending on the dose and assay.
Benzodiazepine IAs often are designed to detect nordiazepam, oxazepam, and temazepam, all of which are metabolites of diazepam. However, benzodiazepine IAs also can detect other drugs that are structurally similar to benzodiazepines.11,12 This means that benzodiazepines are detected based on their ability to cross-react with the IA test. Lorazepam and clonazepam have low cross-reactivity and are generally not detected on benzodiazepine IA.12,13 Therefore, it is not uncommon for patients on lorazepam or clonazepam to test negative for benzodiazepines on this IA. If these patients do test positive at low doses, it could be a concern that they are taking a different benzodiazepine instead of, or in addition to, the prescribed medication.
Amphetamines and methamphetamine are simple molecules that are difficult to develop specific antibodies for; therefore, they carry a high false-positive rate with IA testing.8 It is important to note that methylphenidate is not detected by the amphetamine IA as it is not an amphetamine.8 The IA for cocaine tests specifically for benzoylecgonine, a metabolite specific to cocaine and has no cross-reactivity.8,12,14
False positives. Due to the lack of specificity of UDM by IA, false positives are common; with the exception of cocaine. Clinicians must obtain a comprehensive medication history of the patient, including over-the-counter medications, herbals, and supplements. Table 6 lists common sources of false positives with UDM by IA.1,8,9
False negatives. A variety of factors can cause false-negative results, includingthe cross-reactivity of the antibody in the IA, the cutoff concentration that yields a positive result, and/or the time between drug ingestion. As discussed previously, the opiate panel tests for metabolites of morphine, codeine, and heroin, which consequently may lead to semisynthetic/synthetic opioids not being detected.8,11 For example, a patient who was prescribed hydrocodone/acetaminophen 5 mg/325 mg 4 times a day, tests negative for opiates by IA. The negative result is not unexpected because the dose of semisynthetic opioid is too low for detection by IA.
Chromatography
Chromatography generally is reserved for confirmatory or definitive testing when the initial UDM by IA results are unexpected.1 Unlike IA, chromatography can detect the presence of specific drugs and/or metabolites. Types of chromatography testing include GC/MS, liquid chromatography tandem mass spectrometry (LC/MS/MS), and high-performance liquid chromatography.9 Depending on the specific test, chromatography uses a gas or liquid carrier medium to separate the urine sample’s compounds by their molecular interactions with the carrier medium (mainly by different polarities). During this separation process, all the individual compounds are fed into a mass spectrometer, that ionizes the compounds and detects fragments by using their mass-to-charge ratios. This process allows for the identification of distinct compounds based on their molecular fingerprints.
Gas chromatography/mass spectrometry has remained the standard test for confirmatory testing.1,8 However, it is important to note that LC/MS/MS has been gaining favor over GC/MS. Using LC/MS/MS requires less urine volume to conduct an analysis, and the analysis has a second analytical separation step, thus it is expected to have a lower susceptibility to false results caused by concomitant use of other medications.15,16
Regardless of the test medium, quantitative confirmation through chromatography offers several advantages over IA. It is more accurate, as it can identify small quantities of specific drugs and confirm their presence in urine.8 Also, although there are still cutoff limits associated with chromatography, the specific cutoffs are much lower in value than those in IA tests.Finally, a study conducted in 2010 by Pesce and colleagues found that IA testing was associated with varying rates of false-negative results compared with those of LC-MS/MS.17 Specifically, false-negative rates associated with IA were found to be 22%, 50%, and 23.4% for benzodiazepines, cocaine, and propoxyphene, respectively.17 Unfortunately, chromatography testing methods take longer to produce results and are costly compared with those of IA.Thus, chromatography testing methods typically are reserved for when the IA produces unexpected results. Conversely, IA can be done at point of care with in-office readable cups or strips, or sent out for a 24-hour to 48-hour turnaround time.7,8
Alcohol Testing
Health care providers also could screen for alcohol misuse, which can compromise safe opioid use. Alcohol can accelerate the release of certain sustained-release formulations, causing “dose dumping.”18 Furthermore, alcohol also can increase the risk of opioid-induced respiratory depression. Many laboratories include ethanol that is measured using an enzymatic reaction and generally detected 12 hours after alcohol use.7-9 Urinary ethanol is not an optimal marker for assessing alcohol use. Ethyl glucuronide (EtG) and ethyl sulfate (EtS) are 2 minor metabolites of ethanol formed by UDP-glucuronosyltransferase.19 These markers can be detected for up to 80 hours after alcohol consumption. Markers for prolonged and/or heavy drinking include but are not limited to phosphatidylethanol, γ-glutamyltransferase, or carbohydrate-deficient transferrin.20
Pharmacokinetics/Pharmacogenetics
Pharmacokinetics is what the body does with the drug and is measured by absorption, distribution, metabolism, and elimination.16 Pharmacokinetics ultimately determines the fate of how much and how fast a drug and/or metabolites end up in the urine. It is important to understand the pharmacokinetics to interpret the results of UDM by chromatography as the reported results include parent drugs and metabolites.
Some metabolites of medications available commercially could be mistaken as if the patient were taking a medication that was not prescribed. For example, hydromorphone is a metabolite of hydrocodone and oxymorphone is a metabolite of oxycodone, both of which are commercially available as stand-alone prescriptions. Likewise, oxazepam is commercially available as is temazepam, and both are metabolites of diazepam. Also, it is important to consider patient’s body habitus, which affects volume of distribution, meaning more drug is stored in the periphery and may have a longer detection window.21 Patients with renal and/or hepatic impairment can have reduced clearance of the medications.
It is equally important to consider the role that pharmacogenetic polymorphism can play in UDM, as polymorphisms may impact results.1,8 For example, consider a patient on extended-release oxycodone 30 mg twice daily. Oxycodone is metabolized via cytochrome (CYP) P450 enzyme 3A4 into noroxycodone and, to a much lesser extent, by CYP2D6 into oxymorphone. In this case, if tested by chromatography, the patient’s urine level of oxycodone should be higher than that of either metabolite; specifically, the urine level of noroxycodone should be higher than that of oxymorphone. If there are only concentrations of oxycodone found in the urine with no metabolites, the possible explanations are either the patient dissolved oxycodone into the urine sample without ingestion or the patient may have poor activity of CYP2D6 and CYP3A4 isoenzymes; the latter of which can be confirmed by pharmacogenetic testing. Notwithstanding, drug-drug interactions with CYP inhibitors can produce the same outcome.
Conclusion
Urine drug monitoring is an important tool for substance misuse or abuse and adherence to the prescribed regimen. The most commonly used test is UDM by IA due to its low cost and quick results. However, it comes with an array of false-positive and false-negative results. Clinicians should seek definitive results by confirmatory testing prior to making changes that alter patient care, and all results should include discussions with the patient.
Clinical pharmacy specialists are generally an excellent and often untapped resource to provide guidance for interpretation of both IA and chromatographic testing. Clinical pharmacy specialists have an excellent understanding of the physical and medicinal chemistry properties of laboratory testing, a vast understanding of drug metabolites and interactions that might increase or decrease drug concentrations might account for possible false positives and false negatives, and they can help decipher unexpected results.
Finally, it is important to consider that UDM is done for patients and not to patients, with the ultimate goal of improving the safety of the patient and the public. Unexpected results should be discussed with patients to identify the underlying reasons, which may then warrant further intervention, such as definitive testing and ultimate referral to a substance abuse treatment program. Simply sending a discharge or medication discontinuation letter to a patient can create a confrontational situation rather than an educational opportunity for both patient and provider.
1. Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician. 2012;15(3)(suppl):ES119-ES133.
2. US Department of Defense, US Department of Veteran Affairs, The Opioid Therapy for Chronic Pain Working Group. VA/DoD clinical practice guideline for opioid therapy in chronic pain. Version 3.0. Washington, DC: Veterans Health Administration and Department of Defense; 2017.
3. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
4. Cheung CW, Qiu Q, Choi SW, Moore B, Goucke R, Irwin M. Chronic opioid therapy for chronic non-cancer pain: a review and comparison of treatment guidelines. Pain Physician. 2014;17(5):401-414.
5. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
6. Manchikanti L, Abdi S, Atluri S, et al; American Society of Interventional Pain Physicians. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2 – guidance. Pain Physician. 2012;15(3)(suppl):S67-S116.
7. Hammet-Stabler CA, Webster LR. A clinical guide to urine drug testing. CME certified monograph. http://ccoe.rbhs.rutgers.edu/online/ARCHIVE/endurings/09MC07.pdf. Published May 2008. Accessed March 23, 2018.
8. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76.
9. Gourlay DL, Heit HA, Caplan YH. Urine drug testing in clinical practice. The art and science of patient care. Edition 6. https://www.remitigate.com/wp-content/uploads/2015/11 /Urine-Drug-Testing-in-Clinical-Practice-Ed6_2015-08.pdf. Published August 31, 2015. Accessed March 23, 2018.
10. Jamison RL, Oliver RE. Disorders of urinary concentration and dilution. Am J Med. 1982;72(2):308-322.
11. Valentine JL, Middleton R, Sparks C. Identification of urinary benzodiazepines and their metabolites: comparison of automated HPLC and GC-MS after immunoassay screening of clinical specimens. J Anal Toxicol. 1996;20(6):416-424.
12. Raouf M, Fudin J. Benzodiazepine Metabolism and Pharmacokinetics. http://paindr.com/wp-content/up loads/2015/10/Revised-BZD_-9-30.pdf. Published September 30, 2015. Accessed March 23, 2018. 13. DRI Benzodiazepine Assay [package insert]. Fremont, CA: Mircogenics Corp; 2012.
14. Carney S, Wolf CE, Tarnai-Moak L, Poklis A. Evaluation of two enzyme immunoassays for the detection of the cocaine metabolite benzoylecgonine in 1,398 urine specimens. J Clin Lab Anal. 2012;26(3):130-135.
15. Mikel C, Pesce A, West C. A tale of two drug testing technologies: GC-MS and LC-MS/MS. Pain Physician. 2010;13(1):91- 92.
16. Stout PR, Bynum ND, Mitchell JM, Baylor MR, Ropero-Miller JD. A comparison of the validity of gas chromatography- mass spectrometry and liquid chromatography- tandem mass spectrometry analysis of urine samples for morphine, codeine, 6-acetylmorphine, and benzoylecgonine. J Anal Toxicol. 2009;33(8):398-408.
17. Pesce A, Rosenthal M, West R, et al. An evaluation of the diagnostic accuracy of liquid chromatography-tandem mass spectroscopy versus immunoassay drug testing in pain patients. Pain Physician. 2010;13(3):273-281.
18. Gudin J, Mogali S, Jones J, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med. 2013;125(4):115–130.
19. Böttcher M, Beck O, Helander A. Evaluation of a new immunoassay for urinary ethyl glucuronide testing. Alcohol Alcohol. 2008;43(1):46-48.
20. Peterson K. Biomarkers for alcohol use and abuse—a summary. Alcohol Res Health. 2004-2005;28(1):30-37.
21. Sera LC, McPherson ML.Pharmacokinetics and pharmacodynamic changes associated with aging and implications for drug therapy. Clin Geriatr Med. 2012;28(2):273-286.
Urine drug monitoring (UDM) is an important tool to screen adherence and identify possible misuse and abuse in patients on opioid therapy.1 Various guidelines for opioid therapy emphasize the importance of UDM as a standard of care.2-6 Routine and random monitoring is recommended for all patients on long-term opioid therapy prior to initiation and throughout duration of therapy.1-3 The recommended UDM frequency varies based on individual risk assessment and clinical judgment. Similar to any other diagnostic or monitoring test, the goal for UDM should be to guide therapy and improve patient care (Box). Inappropriate interpretation of the results and failure to order definitive testing when necessary may adversely affect patient care.
Urine Drug Monitoring
Sample Collection
Urine drug testing generally requires a minimum of 30 mL of urine (depending on the kit type) collected in a private restroom. In the authors’ experience, the sample collection most often is unobserved in clinical practice. Most laboratories keep urine samples for a limited time, often 7 days. Therefor, if results are unexpected, health care providers must notify the laboratory in a timely manner to order definitive testing if indicated.
Specimen Validity Testing
Attempts to dilute, adulterate, and substitute urine may be detected by visual inspection and laboratory validity testing. Validity testing of urine specimens includes temperature, specific gravity, pH, urine creatinine, and presence of adulterants (Tables 1 and 2).7-9
The combination of specific gravity and urinary creatinine may help screen for dilution or substitution. Dilution may occur precollection by consumption of excess amounts of fluids or postcollection by adding fluid to the specimen. Other causes of diluted urine should be considered, such as renal tubular dysfunction or diuretic use. Household adulterants include vinegar, detergent, sodium chloride, hydrogen peroxide, eye/nose drops, soda, or ammonia.10 There are numerous commercially available adulterants, including Klear, UrinAid, Urine Luck, Stealth Synthetics, Whizzies, and Clear Choice. The active ingredients of some include peroxide/peroxidase, sodium or potassium nitrate, pyridinium chlorochromate, or glutaraldehyde. There are laboratory tests to detect the presence of these adulterants. Whenever in doubt, it is advisable that health care providers (HCPs) contact their laboratory to investigate tampering. Another approach if tampering is suspected is to collect blood samples. Although this method is more expensive and invasive, it eliminates means of tampering. Hair follicle testing is an option as well.
Types of Urine Drug Monitoring
There are 2 general types of UDM: Presumptive by immunoassay (IA) and confirmatory testing by chromatography. Simply, UDM by IA commonly referred to as urine drug screening (UDS), serves as the differential assessments, whereas chromatography is the definitive assessment. This article reviews the clinical utility and limitations of the 2 types of UDM, including false positives and false negatives, and when to order more tests.
Immunoassay
The IA drug test uses antibodies to detect the presence of selected drugs and/or their metabolites based on a predetermined cutoff threshold.8 Immunoassay monitoring is the initial qualitative test to identify the presence of drug classes in the urine based on a detection threshold. Typically, UDM by IA is performed as an initial evaluation of potential appropriate use, misuse, nonuse, or abuse of medications. It also can detect the presence of illegal substances or unprescribed medications. Immunoassay is relatively quick, inexpensive, and sensitive; however, because it lacks specificity, it can result in various false positives and false negatives.
Immunoassay tests also are subject to varying windows of detection depending on the substance ingested (Table 3).
The cutoff levels listed in Table 1 are consistent with testing for employment but not necessarily for aberrant behavior in patients receiving long-term opioid therapy. These cutoffs lower the risk of false positives and provide better accuracy with clinical monitoring. For example, a level of 2,000 ng/mL is listed for both test types in Table 4, but for clinical testing, the IA cutoff is 3,000 ng/mL, and gas chromatography/mass spectrometry (GC-MS) can detect even trace amounts of opioid and their metabolites.
The opiate panel with IA tests for opium alkaloids and/or their metabolites, including morphine and codeine.7-9 Heroin is a semisynthetic opioid that is metabolized to diacetyl morphine and ultimately is detected as morphine.7,8 Other semisynthetic opioids, such as hydrocodone and oxycodone, may or may not be detected by the opiate IA depending on the dose and assay.
Benzodiazepine IAs often are designed to detect nordiazepam, oxazepam, and temazepam, all of which are metabolites of diazepam. However, benzodiazepine IAs also can detect other drugs that are structurally similar to benzodiazepines.11,12 This means that benzodiazepines are detected based on their ability to cross-react with the IA test. Lorazepam and clonazepam have low cross-reactivity and are generally not detected on benzodiazepine IA.12,13 Therefore, it is not uncommon for patients on lorazepam or clonazepam to test negative for benzodiazepines on this IA. If these patients do test positive at low doses, it could be a concern that they are taking a different benzodiazepine instead of, or in addition to, the prescribed medication.
Amphetamines and methamphetamine are simple molecules that are difficult to develop specific antibodies for; therefore, they carry a high false-positive rate with IA testing.8 It is important to note that methylphenidate is not detected by the amphetamine IA as it is not an amphetamine.8 The IA for cocaine tests specifically for benzoylecgonine, a metabolite specific to cocaine and has no cross-reactivity.8,12,14
False positives. Due to the lack of specificity of UDM by IA, false positives are common; with the exception of cocaine. Clinicians must obtain a comprehensive medication history of the patient, including over-the-counter medications, herbals, and supplements. Table 6 lists common sources of false positives with UDM by IA.1,8,9
False negatives. A variety of factors can cause false-negative results, includingthe cross-reactivity of the antibody in the IA, the cutoff concentration that yields a positive result, and/or the time between drug ingestion. As discussed previously, the opiate panel tests for metabolites of morphine, codeine, and heroin, which consequently may lead to semisynthetic/synthetic opioids not being detected.8,11 For example, a patient who was prescribed hydrocodone/acetaminophen 5 mg/325 mg 4 times a day, tests negative for opiates by IA. The negative result is not unexpected because the dose of semisynthetic opioid is too low for detection by IA.
Chromatography
Chromatography generally is reserved for confirmatory or definitive testing when the initial UDM by IA results are unexpected.1 Unlike IA, chromatography can detect the presence of specific drugs and/or metabolites. Types of chromatography testing include GC/MS, liquid chromatography tandem mass spectrometry (LC/MS/MS), and high-performance liquid chromatography.9 Depending on the specific test, chromatography uses a gas or liquid carrier medium to separate the urine sample’s compounds by their molecular interactions with the carrier medium (mainly by different polarities). During this separation process, all the individual compounds are fed into a mass spectrometer, that ionizes the compounds and detects fragments by using their mass-to-charge ratios. This process allows for the identification of distinct compounds based on their molecular fingerprints.
Gas chromatography/mass spectrometry has remained the standard test for confirmatory testing.1,8 However, it is important to note that LC/MS/MS has been gaining favor over GC/MS. Using LC/MS/MS requires less urine volume to conduct an analysis, and the analysis has a second analytical separation step, thus it is expected to have a lower susceptibility to false results caused by concomitant use of other medications.15,16
Regardless of the test medium, quantitative confirmation through chromatography offers several advantages over IA. It is more accurate, as it can identify small quantities of specific drugs and confirm their presence in urine.8 Also, although there are still cutoff limits associated with chromatography, the specific cutoffs are much lower in value than those in IA tests.Finally, a study conducted in 2010 by Pesce and colleagues found that IA testing was associated with varying rates of false-negative results compared with those of LC-MS/MS.17 Specifically, false-negative rates associated with IA were found to be 22%, 50%, and 23.4% for benzodiazepines, cocaine, and propoxyphene, respectively.17 Unfortunately, chromatography testing methods take longer to produce results and are costly compared with those of IA.Thus, chromatography testing methods typically are reserved for when the IA produces unexpected results. Conversely, IA can be done at point of care with in-office readable cups or strips, or sent out for a 24-hour to 48-hour turnaround time.7,8
Alcohol Testing
Health care providers also could screen for alcohol misuse, which can compromise safe opioid use. Alcohol can accelerate the release of certain sustained-release formulations, causing “dose dumping.”18 Furthermore, alcohol also can increase the risk of opioid-induced respiratory depression. Many laboratories include ethanol that is measured using an enzymatic reaction and generally detected 12 hours after alcohol use.7-9 Urinary ethanol is not an optimal marker for assessing alcohol use. Ethyl glucuronide (EtG) and ethyl sulfate (EtS) are 2 minor metabolites of ethanol formed by UDP-glucuronosyltransferase.19 These markers can be detected for up to 80 hours after alcohol consumption. Markers for prolonged and/or heavy drinking include but are not limited to phosphatidylethanol, γ-glutamyltransferase, or carbohydrate-deficient transferrin.20
Pharmacokinetics/Pharmacogenetics
Pharmacokinetics is what the body does with the drug and is measured by absorption, distribution, metabolism, and elimination.16 Pharmacokinetics ultimately determines the fate of how much and how fast a drug and/or metabolites end up in the urine. It is important to understand the pharmacokinetics to interpret the results of UDM by chromatography as the reported results include parent drugs and metabolites.
Some metabolites of medications available commercially could be mistaken as if the patient were taking a medication that was not prescribed. For example, hydromorphone is a metabolite of hydrocodone and oxymorphone is a metabolite of oxycodone, both of which are commercially available as stand-alone prescriptions. Likewise, oxazepam is commercially available as is temazepam, and both are metabolites of diazepam. Also, it is important to consider patient’s body habitus, which affects volume of distribution, meaning more drug is stored in the periphery and may have a longer detection window.21 Patients with renal and/or hepatic impairment can have reduced clearance of the medications.
It is equally important to consider the role that pharmacogenetic polymorphism can play in UDM, as polymorphisms may impact results.1,8 For example, consider a patient on extended-release oxycodone 30 mg twice daily. Oxycodone is metabolized via cytochrome (CYP) P450 enzyme 3A4 into noroxycodone and, to a much lesser extent, by CYP2D6 into oxymorphone. In this case, if tested by chromatography, the patient’s urine level of oxycodone should be higher than that of either metabolite; specifically, the urine level of noroxycodone should be higher than that of oxymorphone. If there are only concentrations of oxycodone found in the urine with no metabolites, the possible explanations are either the patient dissolved oxycodone into the urine sample without ingestion or the patient may have poor activity of CYP2D6 and CYP3A4 isoenzymes; the latter of which can be confirmed by pharmacogenetic testing. Notwithstanding, drug-drug interactions with CYP inhibitors can produce the same outcome.
Conclusion
Urine drug monitoring is an important tool for substance misuse or abuse and adherence to the prescribed regimen. The most commonly used test is UDM by IA due to its low cost and quick results. However, it comes with an array of false-positive and false-negative results. Clinicians should seek definitive results by confirmatory testing prior to making changes that alter patient care, and all results should include discussions with the patient.
Clinical pharmacy specialists are generally an excellent and often untapped resource to provide guidance for interpretation of both IA and chromatographic testing. Clinical pharmacy specialists have an excellent understanding of the physical and medicinal chemistry properties of laboratory testing, a vast understanding of drug metabolites and interactions that might increase or decrease drug concentrations might account for possible false positives and false negatives, and they can help decipher unexpected results.
Finally, it is important to consider that UDM is done for patients and not to patients, with the ultimate goal of improving the safety of the patient and the public. Unexpected results should be discussed with patients to identify the underlying reasons, which may then warrant further intervention, such as definitive testing and ultimate referral to a substance abuse treatment program. Simply sending a discharge or medication discontinuation letter to a patient can create a confrontational situation rather than an educational opportunity for both patient and provider.
Urine drug monitoring (UDM) is an important tool to screen adherence and identify possible misuse and abuse in patients on opioid therapy.1 Various guidelines for opioid therapy emphasize the importance of UDM as a standard of care.2-6 Routine and random monitoring is recommended for all patients on long-term opioid therapy prior to initiation and throughout duration of therapy.1-3 The recommended UDM frequency varies based on individual risk assessment and clinical judgment. Similar to any other diagnostic or monitoring test, the goal for UDM should be to guide therapy and improve patient care (Box). Inappropriate interpretation of the results and failure to order definitive testing when necessary may adversely affect patient care.
Urine Drug Monitoring
Sample Collection
Urine drug testing generally requires a minimum of 30 mL of urine (depending on the kit type) collected in a private restroom. In the authors’ experience, the sample collection most often is unobserved in clinical practice. Most laboratories keep urine samples for a limited time, often 7 days. Therefor, if results are unexpected, health care providers must notify the laboratory in a timely manner to order definitive testing if indicated.
Specimen Validity Testing
Attempts to dilute, adulterate, and substitute urine may be detected by visual inspection and laboratory validity testing. Validity testing of urine specimens includes temperature, specific gravity, pH, urine creatinine, and presence of adulterants (Tables 1 and 2).7-9
The combination of specific gravity and urinary creatinine may help screen for dilution or substitution. Dilution may occur precollection by consumption of excess amounts of fluids or postcollection by adding fluid to the specimen. Other causes of diluted urine should be considered, such as renal tubular dysfunction or diuretic use. Household adulterants include vinegar, detergent, sodium chloride, hydrogen peroxide, eye/nose drops, soda, or ammonia.10 There are numerous commercially available adulterants, including Klear, UrinAid, Urine Luck, Stealth Synthetics, Whizzies, and Clear Choice. The active ingredients of some include peroxide/peroxidase, sodium or potassium nitrate, pyridinium chlorochromate, or glutaraldehyde. There are laboratory tests to detect the presence of these adulterants. Whenever in doubt, it is advisable that health care providers (HCPs) contact their laboratory to investigate tampering. Another approach if tampering is suspected is to collect blood samples. Although this method is more expensive and invasive, it eliminates means of tampering. Hair follicle testing is an option as well.
Types of Urine Drug Monitoring
There are 2 general types of UDM: Presumptive by immunoassay (IA) and confirmatory testing by chromatography. Simply, UDM by IA commonly referred to as urine drug screening (UDS), serves as the differential assessments, whereas chromatography is the definitive assessment. This article reviews the clinical utility and limitations of the 2 types of UDM, including false positives and false negatives, and when to order more tests.
Immunoassay
The IA drug test uses antibodies to detect the presence of selected drugs and/or their metabolites based on a predetermined cutoff threshold.8 Immunoassay monitoring is the initial qualitative test to identify the presence of drug classes in the urine based on a detection threshold. Typically, UDM by IA is performed as an initial evaluation of potential appropriate use, misuse, nonuse, or abuse of medications. It also can detect the presence of illegal substances or unprescribed medications. Immunoassay is relatively quick, inexpensive, and sensitive; however, because it lacks specificity, it can result in various false positives and false negatives.
Immunoassay tests also are subject to varying windows of detection depending on the substance ingested (Table 3).
The cutoff levels listed in Table 1 are consistent with testing for employment but not necessarily for aberrant behavior in patients receiving long-term opioid therapy. These cutoffs lower the risk of false positives and provide better accuracy with clinical monitoring. For example, a level of 2,000 ng/mL is listed for both test types in Table 4, but for clinical testing, the IA cutoff is 3,000 ng/mL, and gas chromatography/mass spectrometry (GC-MS) can detect even trace amounts of opioid and their metabolites.
The opiate panel with IA tests for opium alkaloids and/or their metabolites, including morphine and codeine.7-9 Heroin is a semisynthetic opioid that is metabolized to diacetyl morphine and ultimately is detected as morphine.7,8 Other semisynthetic opioids, such as hydrocodone and oxycodone, may or may not be detected by the opiate IA depending on the dose and assay.
Benzodiazepine IAs often are designed to detect nordiazepam, oxazepam, and temazepam, all of which are metabolites of diazepam. However, benzodiazepine IAs also can detect other drugs that are structurally similar to benzodiazepines.11,12 This means that benzodiazepines are detected based on their ability to cross-react with the IA test. Lorazepam and clonazepam have low cross-reactivity and are generally not detected on benzodiazepine IA.12,13 Therefore, it is not uncommon for patients on lorazepam or clonazepam to test negative for benzodiazepines on this IA. If these patients do test positive at low doses, it could be a concern that they are taking a different benzodiazepine instead of, or in addition to, the prescribed medication.
Amphetamines and methamphetamine are simple molecules that are difficult to develop specific antibodies for; therefore, they carry a high false-positive rate with IA testing.8 It is important to note that methylphenidate is not detected by the amphetamine IA as it is not an amphetamine.8 The IA for cocaine tests specifically for benzoylecgonine, a metabolite specific to cocaine and has no cross-reactivity.8,12,14
False positives. Due to the lack of specificity of UDM by IA, false positives are common; with the exception of cocaine. Clinicians must obtain a comprehensive medication history of the patient, including over-the-counter medications, herbals, and supplements. Table 6 lists common sources of false positives with UDM by IA.1,8,9
False negatives. A variety of factors can cause false-negative results, includingthe cross-reactivity of the antibody in the IA, the cutoff concentration that yields a positive result, and/or the time between drug ingestion. As discussed previously, the opiate panel tests for metabolites of morphine, codeine, and heroin, which consequently may lead to semisynthetic/synthetic opioids not being detected.8,11 For example, a patient who was prescribed hydrocodone/acetaminophen 5 mg/325 mg 4 times a day, tests negative for opiates by IA. The negative result is not unexpected because the dose of semisynthetic opioid is too low for detection by IA.
Chromatography
Chromatography generally is reserved for confirmatory or definitive testing when the initial UDM by IA results are unexpected.1 Unlike IA, chromatography can detect the presence of specific drugs and/or metabolites. Types of chromatography testing include GC/MS, liquid chromatography tandem mass spectrometry (LC/MS/MS), and high-performance liquid chromatography.9 Depending on the specific test, chromatography uses a gas or liquid carrier medium to separate the urine sample’s compounds by their molecular interactions with the carrier medium (mainly by different polarities). During this separation process, all the individual compounds are fed into a mass spectrometer, that ionizes the compounds and detects fragments by using their mass-to-charge ratios. This process allows for the identification of distinct compounds based on their molecular fingerprints.
Gas chromatography/mass spectrometry has remained the standard test for confirmatory testing.1,8 However, it is important to note that LC/MS/MS has been gaining favor over GC/MS. Using LC/MS/MS requires less urine volume to conduct an analysis, and the analysis has a second analytical separation step, thus it is expected to have a lower susceptibility to false results caused by concomitant use of other medications.15,16
Regardless of the test medium, quantitative confirmation through chromatography offers several advantages over IA. It is more accurate, as it can identify small quantities of specific drugs and confirm their presence in urine.8 Also, although there are still cutoff limits associated with chromatography, the specific cutoffs are much lower in value than those in IA tests.Finally, a study conducted in 2010 by Pesce and colleagues found that IA testing was associated with varying rates of false-negative results compared with those of LC-MS/MS.17 Specifically, false-negative rates associated with IA were found to be 22%, 50%, and 23.4% for benzodiazepines, cocaine, and propoxyphene, respectively.17 Unfortunately, chromatography testing methods take longer to produce results and are costly compared with those of IA.Thus, chromatography testing methods typically are reserved for when the IA produces unexpected results. Conversely, IA can be done at point of care with in-office readable cups or strips, or sent out for a 24-hour to 48-hour turnaround time.7,8
Alcohol Testing
Health care providers also could screen for alcohol misuse, which can compromise safe opioid use. Alcohol can accelerate the release of certain sustained-release formulations, causing “dose dumping.”18 Furthermore, alcohol also can increase the risk of opioid-induced respiratory depression. Many laboratories include ethanol that is measured using an enzymatic reaction and generally detected 12 hours after alcohol use.7-9 Urinary ethanol is not an optimal marker for assessing alcohol use. Ethyl glucuronide (EtG) and ethyl sulfate (EtS) are 2 minor metabolites of ethanol formed by UDP-glucuronosyltransferase.19 These markers can be detected for up to 80 hours after alcohol consumption. Markers for prolonged and/or heavy drinking include but are not limited to phosphatidylethanol, γ-glutamyltransferase, or carbohydrate-deficient transferrin.20
Pharmacokinetics/Pharmacogenetics
Pharmacokinetics is what the body does with the drug and is measured by absorption, distribution, metabolism, and elimination.16 Pharmacokinetics ultimately determines the fate of how much and how fast a drug and/or metabolites end up in the urine. It is important to understand the pharmacokinetics to interpret the results of UDM by chromatography as the reported results include parent drugs and metabolites.
Some metabolites of medications available commercially could be mistaken as if the patient were taking a medication that was not prescribed. For example, hydromorphone is a metabolite of hydrocodone and oxymorphone is a metabolite of oxycodone, both of which are commercially available as stand-alone prescriptions. Likewise, oxazepam is commercially available as is temazepam, and both are metabolites of diazepam. Also, it is important to consider patient’s body habitus, which affects volume of distribution, meaning more drug is stored in the periphery and may have a longer detection window.21 Patients with renal and/or hepatic impairment can have reduced clearance of the medications.
It is equally important to consider the role that pharmacogenetic polymorphism can play in UDM, as polymorphisms may impact results.1,8 For example, consider a patient on extended-release oxycodone 30 mg twice daily. Oxycodone is metabolized via cytochrome (CYP) P450 enzyme 3A4 into noroxycodone and, to a much lesser extent, by CYP2D6 into oxymorphone. In this case, if tested by chromatography, the patient’s urine level of oxycodone should be higher than that of either metabolite; specifically, the urine level of noroxycodone should be higher than that of oxymorphone. If there are only concentrations of oxycodone found in the urine with no metabolites, the possible explanations are either the patient dissolved oxycodone into the urine sample without ingestion or the patient may have poor activity of CYP2D6 and CYP3A4 isoenzymes; the latter of which can be confirmed by pharmacogenetic testing. Notwithstanding, drug-drug interactions with CYP inhibitors can produce the same outcome.
Conclusion
Urine drug monitoring is an important tool for substance misuse or abuse and adherence to the prescribed regimen. The most commonly used test is UDM by IA due to its low cost and quick results. However, it comes with an array of false-positive and false-negative results. Clinicians should seek definitive results by confirmatory testing prior to making changes that alter patient care, and all results should include discussions with the patient.
Clinical pharmacy specialists are generally an excellent and often untapped resource to provide guidance for interpretation of both IA and chromatographic testing. Clinical pharmacy specialists have an excellent understanding of the physical and medicinal chemistry properties of laboratory testing, a vast understanding of drug metabolites and interactions that might increase or decrease drug concentrations might account for possible false positives and false negatives, and they can help decipher unexpected results.
Finally, it is important to consider that UDM is done for patients and not to patients, with the ultimate goal of improving the safety of the patient and the public. Unexpected results should be discussed with patients to identify the underlying reasons, which may then warrant further intervention, such as definitive testing and ultimate referral to a substance abuse treatment program. Simply sending a discharge or medication discontinuation letter to a patient can create a confrontational situation rather than an educational opportunity for both patient and provider.
1. Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician. 2012;15(3)(suppl):ES119-ES133.
2. US Department of Defense, US Department of Veteran Affairs, The Opioid Therapy for Chronic Pain Working Group. VA/DoD clinical practice guideline for opioid therapy in chronic pain. Version 3.0. Washington, DC: Veterans Health Administration and Department of Defense; 2017.
3. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
4. Cheung CW, Qiu Q, Choi SW, Moore B, Goucke R, Irwin M. Chronic opioid therapy for chronic non-cancer pain: a review and comparison of treatment guidelines. Pain Physician. 2014;17(5):401-414.
5. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
6. Manchikanti L, Abdi S, Atluri S, et al; American Society of Interventional Pain Physicians. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2 – guidance. Pain Physician. 2012;15(3)(suppl):S67-S116.
7. Hammet-Stabler CA, Webster LR. A clinical guide to urine drug testing. CME certified monograph. http://ccoe.rbhs.rutgers.edu/online/ARCHIVE/endurings/09MC07.pdf. Published May 2008. Accessed March 23, 2018.
8. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76.
9. Gourlay DL, Heit HA, Caplan YH. Urine drug testing in clinical practice. The art and science of patient care. Edition 6. https://www.remitigate.com/wp-content/uploads/2015/11 /Urine-Drug-Testing-in-Clinical-Practice-Ed6_2015-08.pdf. Published August 31, 2015. Accessed March 23, 2018.
10. Jamison RL, Oliver RE. Disorders of urinary concentration and dilution. Am J Med. 1982;72(2):308-322.
11. Valentine JL, Middleton R, Sparks C. Identification of urinary benzodiazepines and their metabolites: comparison of automated HPLC and GC-MS after immunoassay screening of clinical specimens. J Anal Toxicol. 1996;20(6):416-424.
12. Raouf M, Fudin J. Benzodiazepine Metabolism and Pharmacokinetics. http://paindr.com/wp-content/up loads/2015/10/Revised-BZD_-9-30.pdf. Published September 30, 2015. Accessed March 23, 2018. 13. DRI Benzodiazepine Assay [package insert]. Fremont, CA: Mircogenics Corp; 2012.
14. Carney S, Wolf CE, Tarnai-Moak L, Poklis A. Evaluation of two enzyme immunoassays for the detection of the cocaine metabolite benzoylecgonine in 1,398 urine specimens. J Clin Lab Anal. 2012;26(3):130-135.
15. Mikel C, Pesce A, West C. A tale of two drug testing technologies: GC-MS and LC-MS/MS. Pain Physician. 2010;13(1):91- 92.
16. Stout PR, Bynum ND, Mitchell JM, Baylor MR, Ropero-Miller JD. A comparison of the validity of gas chromatography- mass spectrometry and liquid chromatography- tandem mass spectrometry analysis of urine samples for morphine, codeine, 6-acetylmorphine, and benzoylecgonine. J Anal Toxicol. 2009;33(8):398-408.
17. Pesce A, Rosenthal M, West R, et al. An evaluation of the diagnostic accuracy of liquid chromatography-tandem mass spectroscopy versus immunoassay drug testing in pain patients. Pain Physician. 2010;13(3):273-281.
18. Gudin J, Mogali S, Jones J, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med. 2013;125(4):115–130.
19. Böttcher M, Beck O, Helander A. Evaluation of a new immunoassay for urinary ethyl glucuronide testing. Alcohol Alcohol. 2008;43(1):46-48.
20. Peterson K. Biomarkers for alcohol use and abuse—a summary. Alcohol Res Health. 2004-2005;28(1):30-37.
21. Sera LC, McPherson ML.Pharmacokinetics and pharmacodynamic changes associated with aging and implications for drug therapy. Clin Geriatr Med. 2012;28(2):273-286.
1. Owen GT, Burton AW, Schade CM, Passik S. Urine drug testing: current recommendations and best practices. Pain Physician. 2012;15(3)(suppl):ES119-ES133.
2. US Department of Defense, US Department of Veteran Affairs, The Opioid Therapy for Chronic Pain Working Group. VA/DoD clinical practice guideline for opioid therapy in chronic pain. Version 3.0. Washington, DC: Veterans Health Administration and Department of Defense; 2017.
3. Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
4. Cheung CW, Qiu Q, Choi SW, Moore B, Goucke R, Irwin M. Chronic opioid therapy for chronic non-cancer pain: a review and comparison of treatment guidelines. Pain Physician. 2014;17(5):401-414.
5. Chou R, Fanciullo GJ, Fine PG, et al; American Pain Society-American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
6. Manchikanti L, Abdi S, Atluri S, et al; American Society of Interventional Pain Physicians. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part 2 – guidance. Pain Physician. 2012;15(3)(suppl):S67-S116.
7. Hammet-Stabler CA, Webster LR. A clinical guide to urine drug testing. CME certified monograph. http://ccoe.rbhs.rutgers.edu/online/ARCHIVE/endurings/09MC07.pdf. Published May 2008. Accessed March 23, 2018.
8. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76.
9. Gourlay DL, Heit HA, Caplan YH. Urine drug testing in clinical practice. The art and science of patient care. Edition 6. https://www.remitigate.com/wp-content/uploads/2015/11 /Urine-Drug-Testing-in-Clinical-Practice-Ed6_2015-08.pdf. Published August 31, 2015. Accessed March 23, 2018.
10. Jamison RL, Oliver RE. Disorders of urinary concentration and dilution. Am J Med. 1982;72(2):308-322.
11. Valentine JL, Middleton R, Sparks C. Identification of urinary benzodiazepines and their metabolites: comparison of automated HPLC and GC-MS after immunoassay screening of clinical specimens. J Anal Toxicol. 1996;20(6):416-424.
12. Raouf M, Fudin J. Benzodiazepine Metabolism and Pharmacokinetics. http://paindr.com/wp-content/up loads/2015/10/Revised-BZD_-9-30.pdf. Published September 30, 2015. Accessed March 23, 2018. 13. DRI Benzodiazepine Assay [package insert]. Fremont, CA: Mircogenics Corp; 2012.
14. Carney S, Wolf CE, Tarnai-Moak L, Poklis A. Evaluation of two enzyme immunoassays for the detection of the cocaine metabolite benzoylecgonine in 1,398 urine specimens. J Clin Lab Anal. 2012;26(3):130-135.
15. Mikel C, Pesce A, West C. A tale of two drug testing technologies: GC-MS and LC-MS/MS. Pain Physician. 2010;13(1):91- 92.
16. Stout PR, Bynum ND, Mitchell JM, Baylor MR, Ropero-Miller JD. A comparison of the validity of gas chromatography- mass spectrometry and liquid chromatography- tandem mass spectrometry analysis of urine samples for morphine, codeine, 6-acetylmorphine, and benzoylecgonine. J Anal Toxicol. 2009;33(8):398-408.
17. Pesce A, Rosenthal M, West R, et al. An evaluation of the diagnostic accuracy of liquid chromatography-tandem mass spectroscopy versus immunoassay drug testing in pain patients. Pain Physician. 2010;13(3):273-281.
18. Gudin J, Mogali S, Jones J, Comer SD. Risks, management, and monitoring of combination opioid, benzodiazepines, and/or alcohol use. Postgrad Med. 2013;125(4):115–130.
19. Böttcher M, Beck O, Helander A. Evaluation of a new immunoassay for urinary ethyl glucuronide testing. Alcohol Alcohol. 2008;43(1):46-48.
20. Peterson K. Biomarkers for alcohol use and abuse—a summary. Alcohol Res Health. 2004-2005;28(1):30-37.
21. Sera LC, McPherson ML.Pharmacokinetics and pharmacodynamic changes associated with aging and implications for drug therapy. Clin Geriatr Med. 2012;28(2):273-286.
Short-Term Storage of Platelet-Rich Plasma at Room Temperature Does Not Affect Growth Factor or Catabolic Cytokine Concentration
ABSTRACT
The aim of this study was to provide clinical recommendations about the use of platelet-rich plasma (PRP) that was subjected to short-term storage at room temperature. We determined bioactive growth factor and cytokine concentrations as indicators of platelet and white blood cell degranulation in blood and PRP. Additionally, this study sought to validate the use of manual, direct smear analysis as an alternative to automated methods for platelet quantification in PRP.
Blood was used to generate low-leukocyte PRP (Llo PRP) or high-leukocyte PRP (Lhi PRP). Blood was either processed immediately or kept at room temperature for 2 or 4 hours prior to generation of PRP, which was then held at room temperature for 0, 1, 2, or 4 hours. Subsequently, bioactive transforming growth factor beta-1 and matrix metalloproteinase-9 were measured by ELISA (enzyme-linked immunosorbent assay). Manual and automated platelet counts were performed on all blood and PRP samples.
There were no differences in growth factor or cytokine concentration when blood or Llo PRP or Lhi PRP was retained at room temperature for up to 4 hours. Manual, direct smear analysis for platelet quantification was not different from the use of automated machine counting for PRP samples, but in the starting blood samples, manual platelet counts were significantly higher than those generated using automated technology.
When there is a delay of up to 4 hours in the generation of PRP from blood or in the application of PRP to the patient, bioactive growth factor and cytokine concentrations remain stable in both blood and PRP. A manual direct counting method is a simple, cost-effective, and valid method to measure the contents of the PRP product being delivered to the patient.
Platelet-rich plasma (PRP) is used to promote healing in many areas of medicine, such as dental surgery,1,2 soft-tissue injury,3,4 orthopedic surgery,5,6 wound healing,7 and veterinary medicine.8,9 Despite its extensive use, there are still questions about the clinical efficacy of PRP.10-12 Due to biological heterogeneity between patients13,14 and differences between available manufacturing kits,13,15 PRP can be highly variable between patients. There are classification schemes to categorize the various types of PRP,16-18 which can be divided broadly into low-leukocyte PRP (Llo PRP) and high-leukocyte PRP (Lhi PRP). PRP can be used as a point of care therapy, prepared and used immediately, or it can be used during a surgical procedure. In some institutions, blood is drawn by a phlebotomist, processed in the hospital laboratory, and then delivered to the operating room. In other instances, PRP is generated patient-side by the primary attending physician’s team, who draws the blood and processes it for immediate use.5,19 Delays at any step in these various scenarios could lead to the blood or the resultant PRP remaining at room temperature from minutes to several hours prior to administration to the patient. This variability in PRP protocols between clinical and surgical settings adds to concerns regarding the stability and efficacy of the biologic.
Continue to: When performing clinical or research...
When performing clinical or research studies using PRP, it is important to report the contents of the PRP delivered to the patient. By documenting the cellular content of the PRP delivered to the patient, the common questions of optimal platelet dose and the importance of leukocytes in PRP can begin to be answered. There are some known factors that contribute to PRP variability, such as patient biology and operator technique, but there are many other unknown factors. In some instances, there is a failure to generate PRP, defined as a lower platelet count in the PRP preparation than in the starting blood sample.13,14 To measure the platelet and cellular contents of the starting blood and PRP, samples can be submitted to a clinical pathology laboratory for a complete blood count, which adds cost to the patient above the typically unreimbursed cost of the PRP injection itself. An alternative method for measuring platelet concentrations is the use of direct smear analysis on glass slides. The use of direct smears to measure platelet concentration is well validated for blood,20,21 but the use of direct smears of PRP for determining platelet concentrations has not been previously validated. The use of manual platelet counts would provide an alternative to automated platelet counting for clinical and preclinical research studies to characterize the type of PRP administered to the patient.
The primary aim of this study was to determine if retention of blood or PRP at room temperature for various time intervals had an effect on final growth factor or catabolic cytokine concentration. Bioactive transforming growth factor-β1 (TGF-β1) and matrix metalloproteinase-9 (MMP-9) were measured as representatives of growth factors and catabolic cytokines, respectively. The secondary aim was to identify if manual platelet counts were an accurate reflection of automated counts. The outcomes of these experiments should provide immediately relevant information for the clinical application of PRP.
MATERIALS AND METHODS
Blood Collection and Generation of PRP
Under Institutional Review Board approval, blood (105 mL) was collected from healthy human volunteers (N = 5) into a syringe containing acid citrate dextrose anticoagulant to a final concentration of 10% acid citrate dextrose. Three 15-mL aliquots of blood were used to generate Llo PRP (Autologous Conditioned Plasma Double Syringe, Arthrex) and three 20-mL aliquots were used to generate Lhi PRP (SmartPReP 2, Harvest Technologies) (Figure 1).
Automated and Manual Counts
Automated complete blood counts were performed by a board certified clinical pathologist in the clinical pathology department of Cornell University on all blood, Llo PRP, and Lhi PRP samples. A manual platelet count, using a modified Giemsa stain,22 was performed on smears of all blood and PRP samples (Video). Slides were scanned at 10x magnification to identify an area where many red blood cells were present while maintaining a clear field of view (Figure 2A). The magnification was then increased to 100x using oil immersion, and the total number of platelets was counted in 10 fields of view (Figure 2B).
Growth Factor and Catabolic Cytokine Measurements
Blood and PRP samples were thawed for ELISA (enzyme-linked immunosorbent assay) analysis. TGF-β1 concentration was determined using the TGF-β1 Emax ImmunoAssay System (Promega Corporation), which measures biologically active TGF-β1. We chose TGF-β1 because it is commonly measured in PRP studies as an anabolic cytokine with multiple effects on tissue healing. The functions of TGF-β1 include stimulation of undifferentiated mesenchymal cell proliferation; regulation of endothelial, fibroblast, and osteoblast mitogenesis; coordination of collagen synthesis; promotion of endothelial chemotaxis and angiogenesis; activation of extracellular matrix synthesis in cartilage; and reduction of the catabolic activity of interluekin-1 and MMPs.23-25 In addition, TGF-β1 concentration strongly correlates with platelet concentration.26 MMP-9 concentration was determined using the MMP-9 Biotrak Activity Assay (GE Healthcare Biosciences) which measures both active and pro- forms of MMP-9. In PRP, MMP-9 was measured as an indicator of white blood cell (WBC) concentration.26 A catabolic cytokine capable of degrading collagen,13,27 MMP-9 has been linked to poor healing.28 For both assays, samples were measured in duplicate using a multiple detection plate reader (Tecan Safire).
Continue to: Statistical Analysis...
Statistical Analysis
Data were tested for the normal distribution to determine the appropriate statistical test. Manual and automated platelet counts were compared to each other in whole blood, Llo PRP, and Lhi PRP samples using a paired t test. Bioactive TGF-β1 concentrations in blood, Llo PRP, and Lhi PRP, were compared using a Kruskal-Wallis one-way analysis of variance (ANOVA) with Dunn’s all-pairwise comparison. Bioactive and pro-MMP-9 concentrations measured in the retained blood or PRP samples were compared using a one-way ANOVA with Tukey’s all-pairwise comparison. Statistical analyses were performed using Statistix 9 software (Analytical Software). A P value of <0.05 was considered significant.
RESULTS
Validation of PRP
PRP, as defined by an increase in platelet concentration in PRP compared with blood, was successfully generated in all samples by both systems. There was an average 1.98 ± 0.14-fold increase in platelet concentration in Llo PRP and an average 3.06 ± 0.24-fold increase in Lhi PRP. Platelet concentration was significantly higher in Lhi PRP than in Llo PRP (P = 0.001). Compared to whole blood, WBC concentration was 0.47 ± 0.07-fold lower in Llo PRP and 1.98 ± 0.14-fold greater in Lhi PRP. Similar to platelets, WBCs were significantly greater in Lhi PRP than in Llo PRP (P = 0.02).
Bioactive TGF-β1 and MMP-9 Concentration in Blood Retained at Room Temperature
To reflect the clinical situation where blood would be drawn from a patient, but there would be a delay in processing the blood to generate PRP, blood samples were retained at room temperature for up to 4 hours prior to analysis. Neither bioactive TGF-β1 (Figure 3) nor bioactive/pro-MMP-9 concentrations (Figure 4) changed significantly over time when blood was retained at room temperature prior to centrifugation to generate PRP.
Bioactive TGF-β1 and MMP-9 Concentration in PRP Retained at Room Temperature
In order to mimic the clinical situation where PRP would be generated but might sit out prior to being administered to the patient, PRP samples were retained at room temperature for up to 4 hours prior to analysis. In these samples, bioactive TGF-β1 concentrations were not significantly different between PRP products analyzed immediately and those samples retained at room temperature for up to 4 hours (Figure 5). 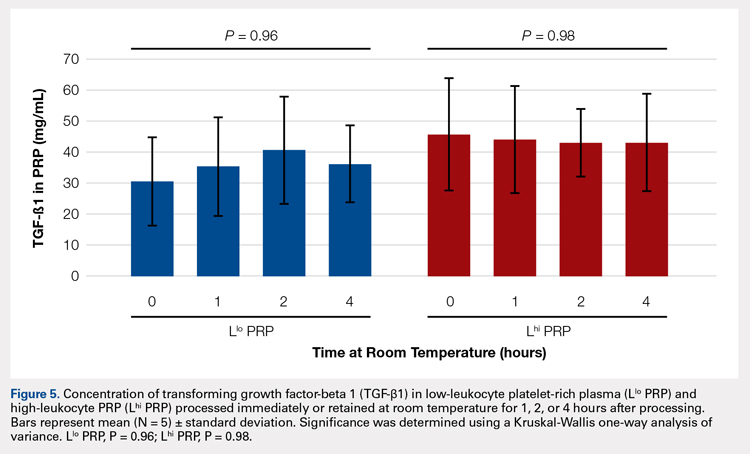
Automatic vs Manual Platelet Count
Manual platelet counts were compared to automated platelet counts to determine if a manual platelet smear analysis could be a reliable method for analyzing PRP in clinical and pre-clinical studies. There was a significant difference between the automated and manual platelet counts in blood samples (Table) (P = 0.05, N = 5) with the manual platelet count having a higher average (99.1 thou/uL) platelet concentration than automated counts. Platelet clumping was identified in 2 automated counts, which falsely decreased platelet concentration by an unknown quantity. Manual platelet counts for both Llo PRP (n = 30) and Lhi PRP (n = 30) were not different from automated platelet counts. Platelet clumping was not reported on any manual platelet counts performed on PRP samples.
Table. Platelet Concentrations of Whole Blood, Llo PRP, and Lhi PRP (N = 5)
| |||||
| Automated Count | Manual Count | P Value | ||
| Mean ± SD | Range | Mean ± SD | Range |
|
Blood | 111.8 ± 59.5 | 54-202 | 210.9 ± 59.4 | 144-297 | 0.05 |
Llo PRP | 421.4 ± 132.8 | 319-620 | 410.1 ± 94.2 | 318-543 | 0.61 |
Lhi PRP | 634.4 ± 88.8 | 517-766 | 635.4 ± 176.6 | 491-933 | 0.99 |
A paired t test was performed to compare results obtained from an automated platelet count and those obtained from a manual count.
Abbreviations: Lhi PRP, high-leukocyte platelet-rich plasma; Llo PRP, low-leukocyte platelet-rich plasma; SD, standard deviation.
Continue to:The primary aim of this study...
DISCUSSION
The primary aim of this study was to improve the clinical use of PRP by characterizing changes that might occur due to extended preparation times. Physicians commonly question the stability of blood or PRP if it is retained at room temperature prior to being administered to the patient. Clinical recommendations to optimize PRP preparation can be derived from a better understanding of the stability of platelets and WBCs, which contribute to the anabolic and catabolic cytokines in PRP.
The results of this study suggest that platelets and WBCs remain stable in blood and both Llo PRP and Lhi PRP for up to 4 hours. The use of bioactive ELISAs to measure TGF-β1 and MMP-9 allows for determination of stability of the PRP product retained at room temperature for up to 4 hours. This provides a time buffer to allow for delays from either institutional logistics or unanticipated clinical delays, without adverse effects on the generation of the final PRP product. As with all biologics, there are many factors that contribute to variability, but a relatively short delay of up to 4 hours in either generation of PRP from blood or in administration of PRP to the patient does not appear to contribute to that variability. Similar studies have been performed on equine PRP and suggest that growth factor concentrations remain stable for up to 6 hours after preparation of PRP29 and in human PRP, which implies that although samples degrade over time, platelet integrity might be acceptable for clinical use for up to 5 days after preparation, particularly if stored in oxygen.30 In contrast to this study, neither of the previously published reports used assays to measure biological activity in the stored PRP. Regardless of the variability between the studies with respect to the type of PRP evaluated and the outcome measures used, all of the studies support the concept that PRP can be stored at room temperature for at least a few hours before clinical use.
Centrifugation of blood does not guarantee the generation of PRP.13,14 In some cases, platelet counts in PRP are similar to or even less than that in the starting whole blood sample. To determine whether a clinical outcome is attributed to PRP, it is vital to know the platelet concentration and, arguably, the WBC concentration in the blood used to generate PRP and in the PRP sample administered to the patient. The platelet concentration in blood and PRP samples can be quantified using automated or manual methods. The use of automated methods can add significant cost to a study or procedure. Manually evaluating a blood smear is an accepted, though more time consuming, method of analyzing cellular components of a blood sample. Depending on the standard operating procedure of the laboratory, manual smears are often done in conjunction with an automated count. This identifies abnormalities in cellular shape or size, or platelet clumping, which are not consistently recognized by automated methods. Manually evaluating a blood smear does take some training, but the material cost is very low, which has added value for clinical or preclinical research studies. Interestingly, the results of this study indicate that manual platelet counts in blood may be more accurate than the count generated from an automated counter because the automated platelet counts were falsely low due to platelet clumping. Platelet clumping can occur as early as 1 hour after blood collection, regardless of the type of anticoagulant used.31
LIMITATIONS
The sample size of this study was small. However, variability in PRP has been well documented in multiple other studies using slightly larger sample sizes.13,14,16 Another potential limitation of this study could be that only one growth factor, TGF-β1, and one catabolic cytokine, MMP-9, were used as surrogate measures to represent platelet and WBC stability, respectively. We chose TGF-β1 because it is correlated with platelet concentrations14,15,26 and MMP-9 because it is an indicator of catabolic factors in PRP that have been correlated with WBC concentrations.26
CONCLUSION
This study illustrated that growth factor and cytokine concentrations in both Llo PRP and Lhi PRP are stable for up to 4 hours. The clinical implications of these results suggest that if the generation or administration of PRP is delayed by up to 4 hours, the resultant PRP retains its bioactivity and is acceptable for clinical application. However, given the known variability of PRP generated due to patient and manufacturer variability,13,14 it is still important to ensure that the product is indeed PRP, with an increase in platelet number over the starting sample of blood. This validation can be performed with a simple and cost-effective manual smear analysis of blood and PRP. The results of this study provide information that can be immediately translated into clinical, surgical, and research practices.
1. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: Literature review. Tissue Eng Part B Rev. 2008;14(3):249-258. doi:10.1089/ten.teb.2008.0062.
2. Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93-103.
3. Monto RR. Platelet rich plasma treatment for chronic achilles tendinosis. Foot Ankle Int. 2012;33(5):379-385. doi:10.3113/FAI.2012.0379.
4. Owens RF, Ginnetti J, Conti SF, Latona C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot ankle Int. 2011;32(11):1032-1039. doi:10.3113/FAI.2011.1032.
5. Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245-251. doi:10.1177/0363546506294078.
6. Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? Knee Surg Sports Traumatol Arthrosc. 2009;17(6):676-682. doi:10.1007/s00167-009-0762-8.
7. Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84(5):434-439. doi:10.1177/154405910508400507.
8. Bosch G, René van Weeren P, Barneveld A, van Schie HTM. Computerised analysis of standardised ultrasonographic images to monitor the repair of surgically created core lesions in equine superficial digital flexor tendons following treatment with intratendinous platelet rich plasma or placebo. Vet J. 2011;187(1):92-98. doi:10.1016/j.tvjl.2009.10.014.
9. Torricelli P, Fini M, Filardo G, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35(10):1569-1576. doi:10.1007/s00264-011-1237-3.
10. Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3-4):165-174. doi:10.1007/s12178-008-9032-5.
11. Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298-307. doi:10.2106/JBJS.K.00154.
12. Vannini F, Di Matteo B, Filardo G, Kon E, Marcacci M, Giannini S. Platelet-rich plasma for foot and ankle pathologies: a systematic review. Foot Ankle Surg. 2014;20(1):2-9. doi:10.1016/j.fas.2013.08.001.
13. Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429-439. doi:10.1016/j.arthro.2011.10.018.
14. Mazzocca AD, McCarthy MBR, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316. doi:10.2106/JBJS.K.00430.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271. doi:10.1177/0363546510387517.
16. Arnoczky SP, Sheibani-Rad S, Shebani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21(4):180-185. doi:10.1097/JSA.0b013e3182999712.
17. Dohan Ehrenfest DM, Bielecki T, Corso M Del, Inchingolo F, Sammartino G. Shedding light in the controversial terminology for platelet-rich products: Platelet-rich plasma (PRP), platelet-rich fibrin (PRF), platelet-leukocyte gel (PLG), preparation rich in growth factors (PRGF), classification and commercialism. J Biomed Mater Res Part A. 2010;95A(4):1280-1282. doi:10.1002/jbm.a.32894.
18. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158-167. doi:10.1016/j.tibtech.2008.11.009.
19. Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174-187.
20. Malok M, Titchener EH, Bridgers C, Lee BY, Bamberg R. Comparison of two platelet count estimation methodologies for peripheral blood smears. Clin Lab Sci. 2007;20(3):154-160.
21. Gulati G, Uppal G, Florea AD, Gong J. Detection of platelet clumps on peripheral blood smears by CellaVision DM96 System and Microscopic Review. Lab Med. 2014;45(4):368-371. doi:10.1309/LM604RQVKVLRFXOR.
22. Gulati G, Song J, Florea AD, Gong J. Purpose and criteria for blood smear scan, blood smear examination, and blood smear review. Ann Lab Med. 2013;33(1):1-7. doi:10.3343/alm.2013.33.1.1.
23. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585-601. doi:10.1111/j.1524-475X.2008.00410.x.
24. Crane D, Everts P. Platelet rich plasma (PRP) matrix grafts. Pract Pain Manag. 2008;8(1):12-26.
25. Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706-2715. doi:10.1007/s11999-011-1857-3.
26. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140. doi:10.1177/0363546511417792.
27. Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93(3):411-422.
28. Watelet JB, Demetter P, Claeys C, Van Cauwenberge P, Cuvelier C, Bachert C. Neutrophil-derived metalloproteinase-9 predicts healing quality after sinus surgery. Laryngoscope. 2005;115(1):56-61. doi:10.1097/01.mlg.0000150674.30237.3f.
29. Hauschild G, Geburek F, Gosheger G, et al. Short term storage stability at room temperature of two different platelet-rich plasma preparations from equine donors and potential impact on growth factor concentrations. BMC Vet Res. 2017;13(1):7. doi:10.1186/s12917-016-0920-4.
30. Moore GW, Maloney JC, Archer RA, et al. Platelet-rich plasma for tissue regeneration can be stored at room temperature for at least five days. Br J Biomed Sci. 2017;74(2):71-77. doi:10.1080/09674845.2016.1233792.
31. McShine RL, Sibinga S, Brozovic B. Differences between the effects of EDTA and citrate anticoagulants on platelet count and mean platelet volume. Clin Lab Haematol. 1990;12(3):277-285.
ABSTRACT
The aim of this study was to provide clinical recommendations about the use of platelet-rich plasma (PRP) that was subjected to short-term storage at room temperature. We determined bioactive growth factor and cytokine concentrations as indicators of platelet and white blood cell degranulation in blood and PRP. Additionally, this study sought to validate the use of manual, direct smear analysis as an alternative to automated methods for platelet quantification in PRP.
Blood was used to generate low-leukocyte PRP (Llo PRP) or high-leukocyte PRP (Lhi PRP). Blood was either processed immediately or kept at room temperature for 2 or 4 hours prior to generation of PRP, which was then held at room temperature for 0, 1, 2, or 4 hours. Subsequently, bioactive transforming growth factor beta-1 and matrix metalloproteinase-9 were measured by ELISA (enzyme-linked immunosorbent assay). Manual and automated platelet counts were performed on all blood and PRP samples.
There were no differences in growth factor or cytokine concentration when blood or Llo PRP or Lhi PRP was retained at room temperature for up to 4 hours. Manual, direct smear analysis for platelet quantification was not different from the use of automated machine counting for PRP samples, but in the starting blood samples, manual platelet counts were significantly higher than those generated using automated technology.
When there is a delay of up to 4 hours in the generation of PRP from blood or in the application of PRP to the patient, bioactive growth factor and cytokine concentrations remain stable in both blood and PRP. A manual direct counting method is a simple, cost-effective, and valid method to measure the contents of the PRP product being delivered to the patient.
Platelet-rich plasma (PRP) is used to promote healing in many areas of medicine, such as dental surgery,1,2 soft-tissue injury,3,4 orthopedic surgery,5,6 wound healing,7 and veterinary medicine.8,9 Despite its extensive use, there are still questions about the clinical efficacy of PRP.10-12 Due to biological heterogeneity between patients13,14 and differences between available manufacturing kits,13,15 PRP can be highly variable between patients. There are classification schemes to categorize the various types of PRP,16-18 which can be divided broadly into low-leukocyte PRP (Llo PRP) and high-leukocyte PRP (Lhi PRP). PRP can be used as a point of care therapy, prepared and used immediately, or it can be used during a surgical procedure. In some institutions, blood is drawn by a phlebotomist, processed in the hospital laboratory, and then delivered to the operating room. In other instances, PRP is generated patient-side by the primary attending physician’s team, who draws the blood and processes it for immediate use.5,19 Delays at any step in these various scenarios could lead to the blood or the resultant PRP remaining at room temperature from minutes to several hours prior to administration to the patient. This variability in PRP protocols between clinical and surgical settings adds to concerns regarding the stability and efficacy of the biologic.
Continue to: When performing clinical or research...
When performing clinical or research studies using PRP, it is important to report the contents of the PRP delivered to the patient. By documenting the cellular content of the PRP delivered to the patient, the common questions of optimal platelet dose and the importance of leukocytes in PRP can begin to be answered. There are some known factors that contribute to PRP variability, such as patient biology and operator technique, but there are many other unknown factors. In some instances, there is a failure to generate PRP, defined as a lower platelet count in the PRP preparation than in the starting blood sample.13,14 To measure the platelet and cellular contents of the starting blood and PRP, samples can be submitted to a clinical pathology laboratory for a complete blood count, which adds cost to the patient above the typically unreimbursed cost of the PRP injection itself. An alternative method for measuring platelet concentrations is the use of direct smear analysis on glass slides. The use of direct smears to measure platelet concentration is well validated for blood,20,21 but the use of direct smears of PRP for determining platelet concentrations has not been previously validated. The use of manual platelet counts would provide an alternative to automated platelet counting for clinical and preclinical research studies to characterize the type of PRP administered to the patient.
The primary aim of this study was to determine if retention of blood or PRP at room temperature for various time intervals had an effect on final growth factor or catabolic cytokine concentration. Bioactive transforming growth factor-β1 (TGF-β1) and matrix metalloproteinase-9 (MMP-9) were measured as representatives of growth factors and catabolic cytokines, respectively. The secondary aim was to identify if manual platelet counts were an accurate reflection of automated counts. The outcomes of these experiments should provide immediately relevant information for the clinical application of PRP.
MATERIALS AND METHODS
Blood Collection and Generation of PRP
Under Institutional Review Board approval, blood (105 mL) was collected from healthy human volunteers (N = 5) into a syringe containing acid citrate dextrose anticoagulant to a final concentration of 10% acid citrate dextrose. Three 15-mL aliquots of blood were used to generate Llo PRP (Autologous Conditioned Plasma Double Syringe, Arthrex) and three 20-mL aliquots were used to generate Lhi PRP (SmartPReP 2, Harvest Technologies) (Figure 1).
Automated and Manual Counts
Automated complete blood counts were performed by a board certified clinical pathologist in the clinical pathology department of Cornell University on all blood, Llo PRP, and Lhi PRP samples. A manual platelet count, using a modified Giemsa stain,22 was performed on smears of all blood and PRP samples (Video). Slides were scanned at 10x magnification to identify an area where many red blood cells were present while maintaining a clear field of view (Figure 2A). The magnification was then increased to 100x using oil immersion, and the total number of platelets was counted in 10 fields of view (Figure 2B).
Growth Factor and Catabolic Cytokine Measurements
Blood and PRP samples were thawed for ELISA (enzyme-linked immunosorbent assay) analysis. TGF-β1 concentration was determined using the TGF-β1 Emax ImmunoAssay System (Promega Corporation), which measures biologically active TGF-β1. We chose TGF-β1 because it is commonly measured in PRP studies as an anabolic cytokine with multiple effects on tissue healing. The functions of TGF-β1 include stimulation of undifferentiated mesenchymal cell proliferation; regulation of endothelial, fibroblast, and osteoblast mitogenesis; coordination of collagen synthesis; promotion of endothelial chemotaxis and angiogenesis; activation of extracellular matrix synthesis in cartilage; and reduction of the catabolic activity of interluekin-1 and MMPs.23-25 In addition, TGF-β1 concentration strongly correlates with platelet concentration.26 MMP-9 concentration was determined using the MMP-9 Biotrak Activity Assay (GE Healthcare Biosciences) which measures both active and pro- forms of MMP-9. In PRP, MMP-9 was measured as an indicator of white blood cell (WBC) concentration.26 A catabolic cytokine capable of degrading collagen,13,27 MMP-9 has been linked to poor healing.28 For both assays, samples were measured in duplicate using a multiple detection plate reader (Tecan Safire).
Continue to: Statistical Analysis...
Statistical Analysis
Data were tested for the normal distribution to determine the appropriate statistical test. Manual and automated platelet counts were compared to each other in whole blood, Llo PRP, and Lhi PRP samples using a paired t test. Bioactive TGF-β1 concentrations in blood, Llo PRP, and Lhi PRP, were compared using a Kruskal-Wallis one-way analysis of variance (ANOVA) with Dunn’s all-pairwise comparison. Bioactive and pro-MMP-9 concentrations measured in the retained blood or PRP samples were compared using a one-way ANOVA with Tukey’s all-pairwise comparison. Statistical analyses were performed using Statistix 9 software (Analytical Software). A P value of <0.05 was considered significant.
RESULTS
Validation of PRP
PRP, as defined by an increase in platelet concentration in PRP compared with blood, was successfully generated in all samples by both systems. There was an average 1.98 ± 0.14-fold increase in platelet concentration in Llo PRP and an average 3.06 ± 0.24-fold increase in Lhi PRP. Platelet concentration was significantly higher in Lhi PRP than in Llo PRP (P = 0.001). Compared to whole blood, WBC concentration was 0.47 ± 0.07-fold lower in Llo PRP and 1.98 ± 0.14-fold greater in Lhi PRP. Similar to platelets, WBCs were significantly greater in Lhi PRP than in Llo PRP (P = 0.02).
Bioactive TGF-β1 and MMP-9 Concentration in Blood Retained at Room Temperature
To reflect the clinical situation where blood would be drawn from a patient, but there would be a delay in processing the blood to generate PRP, blood samples were retained at room temperature for up to 4 hours prior to analysis. Neither bioactive TGF-β1 (Figure 3) nor bioactive/pro-MMP-9 concentrations (Figure 4) changed significantly over time when blood was retained at room temperature prior to centrifugation to generate PRP.
Bioactive TGF-β1 and MMP-9 Concentration in PRP Retained at Room Temperature
In order to mimic the clinical situation where PRP would be generated but might sit out prior to being administered to the patient, PRP samples were retained at room temperature for up to 4 hours prior to analysis. In these samples, bioactive TGF-β1 concentrations were not significantly different between PRP products analyzed immediately and those samples retained at room temperature for up to 4 hours (Figure 5). 
Automatic vs Manual Platelet Count
Manual platelet counts were compared to automated platelet counts to determine if a manual platelet smear analysis could be a reliable method for analyzing PRP in clinical and pre-clinical studies. There was a significant difference between the automated and manual platelet counts in blood samples (Table) (P = 0.05, N = 5) with the manual platelet count having a higher average (99.1 thou/uL) platelet concentration than automated counts. Platelet clumping was identified in 2 automated counts, which falsely decreased platelet concentration by an unknown quantity. Manual platelet counts for both Llo PRP (n = 30) and Lhi PRP (n = 30) were not different from automated platelet counts. Platelet clumping was not reported on any manual platelet counts performed on PRP samples.
Table. Platelet Concentrations of Whole Blood, Llo PRP, and Lhi PRP (N = 5)
| |||||
| Automated Count | Manual Count | P Value | ||
| Mean ± SD | Range | Mean ± SD | Range |
|
Blood | 111.8 ± 59.5 | 54-202 | 210.9 ± 59.4 | 144-297 | 0.05 |
Llo PRP | 421.4 ± 132.8 | 319-620 | 410.1 ± 94.2 | 318-543 | 0.61 |
Lhi PRP | 634.4 ± 88.8 | 517-766 | 635.4 ± 176.6 | 491-933 | 0.99 |
A paired t test was performed to compare results obtained from an automated platelet count and those obtained from a manual count.
Abbreviations: Lhi PRP, high-leukocyte platelet-rich plasma; Llo PRP, low-leukocyte platelet-rich plasma; SD, standard deviation.
Continue to:The primary aim of this study...
DISCUSSION
The primary aim of this study was to improve the clinical use of PRP by characterizing changes that might occur due to extended preparation times. Physicians commonly question the stability of blood or PRP if it is retained at room temperature prior to being administered to the patient. Clinical recommendations to optimize PRP preparation can be derived from a better understanding of the stability of platelets and WBCs, which contribute to the anabolic and catabolic cytokines in PRP.
The results of this study suggest that platelets and WBCs remain stable in blood and both Llo PRP and Lhi PRP for up to 4 hours. The use of bioactive ELISAs to measure TGF-β1 and MMP-9 allows for determination of stability of the PRP product retained at room temperature for up to 4 hours. This provides a time buffer to allow for delays from either institutional logistics or unanticipated clinical delays, without adverse effects on the generation of the final PRP product. As with all biologics, there are many factors that contribute to variability, but a relatively short delay of up to 4 hours in either generation of PRP from blood or in administration of PRP to the patient does not appear to contribute to that variability. Similar studies have been performed on equine PRP and suggest that growth factor concentrations remain stable for up to 6 hours after preparation of PRP29 and in human PRP, which implies that although samples degrade over time, platelet integrity might be acceptable for clinical use for up to 5 days after preparation, particularly if stored in oxygen.30 In contrast to this study, neither of the previously published reports used assays to measure biological activity in the stored PRP. Regardless of the variability between the studies with respect to the type of PRP evaluated and the outcome measures used, all of the studies support the concept that PRP can be stored at room temperature for at least a few hours before clinical use.
Centrifugation of blood does not guarantee the generation of PRP.13,14 In some cases, platelet counts in PRP are similar to or even less than that in the starting whole blood sample. To determine whether a clinical outcome is attributed to PRP, it is vital to know the platelet concentration and, arguably, the WBC concentration in the blood used to generate PRP and in the PRP sample administered to the patient. The platelet concentration in blood and PRP samples can be quantified using automated or manual methods. The use of automated methods can add significant cost to a study or procedure. Manually evaluating a blood smear is an accepted, though more time consuming, method of analyzing cellular components of a blood sample. Depending on the standard operating procedure of the laboratory, manual smears are often done in conjunction with an automated count. This identifies abnormalities in cellular shape or size, or platelet clumping, which are not consistently recognized by automated methods. Manually evaluating a blood smear does take some training, but the material cost is very low, which has added value for clinical or preclinical research studies. Interestingly, the results of this study indicate that manual platelet counts in blood may be more accurate than the count generated from an automated counter because the automated platelet counts were falsely low due to platelet clumping. Platelet clumping can occur as early as 1 hour after blood collection, regardless of the type of anticoagulant used.31
LIMITATIONS
The sample size of this study was small. However, variability in PRP has been well documented in multiple other studies using slightly larger sample sizes.13,14,16 Another potential limitation of this study could be that only one growth factor, TGF-β1, and one catabolic cytokine, MMP-9, were used as surrogate measures to represent platelet and WBC stability, respectively. We chose TGF-β1 because it is correlated with platelet concentrations14,15,26 and MMP-9 because it is an indicator of catabolic factors in PRP that have been correlated with WBC concentrations.26
CONCLUSION
This study illustrated that growth factor and cytokine concentrations in both Llo PRP and Lhi PRP are stable for up to 4 hours. The clinical implications of these results suggest that if the generation or administration of PRP is delayed by up to 4 hours, the resultant PRP retains its bioactivity and is acceptable for clinical application. However, given the known variability of PRP generated due to patient and manufacturer variability,13,14 it is still important to ensure that the product is indeed PRP, with an increase in platelet number over the starting sample of blood. This validation can be performed with a simple and cost-effective manual smear analysis of blood and PRP. The results of this study provide information that can be immediately translated into clinical, surgical, and research practices.
ABSTRACT
The aim of this study was to provide clinical recommendations about the use of platelet-rich plasma (PRP) that was subjected to short-term storage at room temperature. We determined bioactive growth factor and cytokine concentrations as indicators of platelet and white blood cell degranulation in blood and PRP. Additionally, this study sought to validate the use of manual, direct smear analysis as an alternative to automated methods for platelet quantification in PRP.
Blood was used to generate low-leukocyte PRP (Llo PRP) or high-leukocyte PRP (Lhi PRP). Blood was either processed immediately or kept at room temperature for 2 or 4 hours prior to generation of PRP, which was then held at room temperature for 0, 1, 2, or 4 hours. Subsequently, bioactive transforming growth factor beta-1 and matrix metalloproteinase-9 were measured by ELISA (enzyme-linked immunosorbent assay). Manual and automated platelet counts were performed on all blood and PRP samples.
There were no differences in growth factor or cytokine concentration when blood or Llo PRP or Lhi PRP was retained at room temperature for up to 4 hours. Manual, direct smear analysis for platelet quantification was not different from the use of automated machine counting for PRP samples, but in the starting blood samples, manual platelet counts were significantly higher than those generated using automated technology.
When there is a delay of up to 4 hours in the generation of PRP from blood or in the application of PRP to the patient, bioactive growth factor and cytokine concentrations remain stable in both blood and PRP. A manual direct counting method is a simple, cost-effective, and valid method to measure the contents of the PRP product being delivered to the patient.
Platelet-rich plasma (PRP) is used to promote healing in many areas of medicine, such as dental surgery,1,2 soft-tissue injury,3,4 orthopedic surgery,5,6 wound healing,7 and veterinary medicine.8,9 Despite its extensive use, there are still questions about the clinical efficacy of PRP.10-12 Due to biological heterogeneity between patients13,14 and differences between available manufacturing kits,13,15 PRP can be highly variable between patients. There are classification schemes to categorize the various types of PRP,16-18 which can be divided broadly into low-leukocyte PRP (Llo PRP) and high-leukocyte PRP (Lhi PRP). PRP can be used as a point of care therapy, prepared and used immediately, or it can be used during a surgical procedure. In some institutions, blood is drawn by a phlebotomist, processed in the hospital laboratory, and then delivered to the operating room. In other instances, PRP is generated patient-side by the primary attending physician’s team, who draws the blood and processes it for immediate use.5,19 Delays at any step in these various scenarios could lead to the blood or the resultant PRP remaining at room temperature from minutes to several hours prior to administration to the patient. This variability in PRP protocols between clinical and surgical settings adds to concerns regarding the stability and efficacy of the biologic.
Continue to: When performing clinical or research...
When performing clinical or research studies using PRP, it is important to report the contents of the PRP delivered to the patient. By documenting the cellular content of the PRP delivered to the patient, the common questions of optimal platelet dose and the importance of leukocytes in PRP can begin to be answered. There are some known factors that contribute to PRP variability, such as patient biology and operator technique, but there are many other unknown factors. In some instances, there is a failure to generate PRP, defined as a lower platelet count in the PRP preparation than in the starting blood sample.13,14 To measure the platelet and cellular contents of the starting blood and PRP, samples can be submitted to a clinical pathology laboratory for a complete blood count, which adds cost to the patient above the typically unreimbursed cost of the PRP injection itself. An alternative method for measuring platelet concentrations is the use of direct smear analysis on glass slides. The use of direct smears to measure platelet concentration is well validated for blood,20,21 but the use of direct smears of PRP for determining platelet concentrations has not been previously validated. The use of manual platelet counts would provide an alternative to automated platelet counting for clinical and preclinical research studies to characterize the type of PRP administered to the patient.
The primary aim of this study was to determine if retention of blood or PRP at room temperature for various time intervals had an effect on final growth factor or catabolic cytokine concentration. Bioactive transforming growth factor-β1 (TGF-β1) and matrix metalloproteinase-9 (MMP-9) were measured as representatives of growth factors and catabolic cytokines, respectively. The secondary aim was to identify if manual platelet counts were an accurate reflection of automated counts. The outcomes of these experiments should provide immediately relevant information for the clinical application of PRP.
MATERIALS AND METHODS
Blood Collection and Generation of PRP
Under Institutional Review Board approval, blood (105 mL) was collected from healthy human volunteers (N = 5) into a syringe containing acid citrate dextrose anticoagulant to a final concentration of 10% acid citrate dextrose. Three 15-mL aliquots of blood were used to generate Llo PRP (Autologous Conditioned Plasma Double Syringe, Arthrex) and three 20-mL aliquots were used to generate Lhi PRP (SmartPReP 2, Harvest Technologies) (Figure 1).
Automated and Manual Counts
Automated complete blood counts were performed by a board certified clinical pathologist in the clinical pathology department of Cornell University on all blood, Llo PRP, and Lhi PRP samples. A manual platelet count, using a modified Giemsa stain,22 was performed on smears of all blood and PRP samples (Video). Slides were scanned at 10x magnification to identify an area where many red blood cells were present while maintaining a clear field of view (Figure 2A). The magnification was then increased to 100x using oil immersion, and the total number of platelets was counted in 10 fields of view (Figure 2B).
Growth Factor and Catabolic Cytokine Measurements
Blood and PRP samples were thawed for ELISA (enzyme-linked immunosorbent assay) analysis. TGF-β1 concentration was determined using the TGF-β1 Emax ImmunoAssay System (Promega Corporation), which measures biologically active TGF-β1. We chose TGF-β1 because it is commonly measured in PRP studies as an anabolic cytokine with multiple effects on tissue healing. The functions of TGF-β1 include stimulation of undifferentiated mesenchymal cell proliferation; regulation of endothelial, fibroblast, and osteoblast mitogenesis; coordination of collagen synthesis; promotion of endothelial chemotaxis and angiogenesis; activation of extracellular matrix synthesis in cartilage; and reduction of the catabolic activity of interluekin-1 and MMPs.23-25 In addition, TGF-β1 concentration strongly correlates with platelet concentration.26 MMP-9 concentration was determined using the MMP-9 Biotrak Activity Assay (GE Healthcare Biosciences) which measures both active and pro- forms of MMP-9. In PRP, MMP-9 was measured as an indicator of white blood cell (WBC) concentration.26 A catabolic cytokine capable of degrading collagen,13,27 MMP-9 has been linked to poor healing.28 For both assays, samples were measured in duplicate using a multiple detection plate reader (Tecan Safire).
Continue to: Statistical Analysis...
Statistical Analysis
Data were tested for the normal distribution to determine the appropriate statistical test. Manual and automated platelet counts were compared to each other in whole blood, Llo PRP, and Lhi PRP samples using a paired t test. Bioactive TGF-β1 concentrations in blood, Llo PRP, and Lhi PRP, were compared using a Kruskal-Wallis one-way analysis of variance (ANOVA) with Dunn’s all-pairwise comparison. Bioactive and pro-MMP-9 concentrations measured in the retained blood or PRP samples were compared using a one-way ANOVA with Tukey’s all-pairwise comparison. Statistical analyses were performed using Statistix 9 software (Analytical Software). A P value of <0.05 was considered significant.
RESULTS
Validation of PRP
PRP, as defined by an increase in platelet concentration in PRP compared with blood, was successfully generated in all samples by both systems. There was an average 1.98 ± 0.14-fold increase in platelet concentration in Llo PRP and an average 3.06 ± 0.24-fold increase in Lhi PRP. Platelet concentration was significantly higher in Lhi PRP than in Llo PRP (P = 0.001). Compared to whole blood, WBC concentration was 0.47 ± 0.07-fold lower in Llo PRP and 1.98 ± 0.14-fold greater in Lhi PRP. Similar to platelets, WBCs were significantly greater in Lhi PRP than in Llo PRP (P = 0.02).
Bioactive TGF-β1 and MMP-9 Concentration in Blood Retained at Room Temperature
To reflect the clinical situation where blood would be drawn from a patient, but there would be a delay in processing the blood to generate PRP, blood samples were retained at room temperature for up to 4 hours prior to analysis. Neither bioactive TGF-β1 (Figure 3) nor bioactive/pro-MMP-9 concentrations (Figure 4) changed significantly over time when blood was retained at room temperature prior to centrifugation to generate PRP.
Bioactive TGF-β1 and MMP-9 Concentration in PRP Retained at Room Temperature
In order to mimic the clinical situation where PRP would be generated but might sit out prior to being administered to the patient, PRP samples were retained at room temperature for up to 4 hours prior to analysis. In these samples, bioactive TGF-β1 concentrations were not significantly different between PRP products analyzed immediately and those samples retained at room temperature for up to 4 hours (Figure 5). 
Automatic vs Manual Platelet Count
Manual platelet counts were compared to automated platelet counts to determine if a manual platelet smear analysis could be a reliable method for analyzing PRP in clinical and pre-clinical studies. There was a significant difference between the automated and manual platelet counts in blood samples (Table) (P = 0.05, N = 5) with the manual platelet count having a higher average (99.1 thou/uL) platelet concentration than automated counts. Platelet clumping was identified in 2 automated counts, which falsely decreased platelet concentration by an unknown quantity. Manual platelet counts for both Llo PRP (n = 30) and Lhi PRP (n = 30) were not different from automated platelet counts. Platelet clumping was not reported on any manual platelet counts performed on PRP samples.
Table. Platelet Concentrations of Whole Blood, Llo PRP, and Lhi PRP (N = 5)
| |||||
| Automated Count | Manual Count | P Value | ||
| Mean ± SD | Range | Mean ± SD | Range |
|
Blood | 111.8 ± 59.5 | 54-202 | 210.9 ± 59.4 | 144-297 | 0.05 |
Llo PRP | 421.4 ± 132.8 | 319-620 | 410.1 ± 94.2 | 318-543 | 0.61 |
Lhi PRP | 634.4 ± 88.8 | 517-766 | 635.4 ± 176.6 | 491-933 | 0.99 |
A paired t test was performed to compare results obtained from an automated platelet count and those obtained from a manual count.
Abbreviations: Lhi PRP, high-leukocyte platelet-rich plasma; Llo PRP, low-leukocyte platelet-rich plasma; SD, standard deviation.
Continue to:The primary aim of this study...
DISCUSSION
The primary aim of this study was to improve the clinical use of PRP by characterizing changes that might occur due to extended preparation times. Physicians commonly question the stability of blood or PRP if it is retained at room temperature prior to being administered to the patient. Clinical recommendations to optimize PRP preparation can be derived from a better understanding of the stability of platelets and WBCs, which contribute to the anabolic and catabolic cytokines in PRP.
The results of this study suggest that platelets and WBCs remain stable in blood and both Llo PRP and Lhi PRP for up to 4 hours. The use of bioactive ELISAs to measure TGF-β1 and MMP-9 allows for determination of stability of the PRP product retained at room temperature for up to 4 hours. This provides a time buffer to allow for delays from either institutional logistics or unanticipated clinical delays, without adverse effects on the generation of the final PRP product. As with all biologics, there are many factors that contribute to variability, but a relatively short delay of up to 4 hours in either generation of PRP from blood or in administration of PRP to the patient does not appear to contribute to that variability. Similar studies have been performed on equine PRP and suggest that growth factor concentrations remain stable for up to 6 hours after preparation of PRP29 and in human PRP, which implies that although samples degrade over time, platelet integrity might be acceptable for clinical use for up to 5 days after preparation, particularly if stored in oxygen.30 In contrast to this study, neither of the previously published reports used assays to measure biological activity in the stored PRP. Regardless of the variability between the studies with respect to the type of PRP evaluated and the outcome measures used, all of the studies support the concept that PRP can be stored at room temperature for at least a few hours before clinical use.
Centrifugation of blood does not guarantee the generation of PRP.13,14 In some cases, platelet counts in PRP are similar to or even less than that in the starting whole blood sample. To determine whether a clinical outcome is attributed to PRP, it is vital to know the platelet concentration and, arguably, the WBC concentration in the blood used to generate PRP and in the PRP sample administered to the patient. The platelet concentration in blood and PRP samples can be quantified using automated or manual methods. The use of automated methods can add significant cost to a study or procedure. Manually evaluating a blood smear is an accepted, though more time consuming, method of analyzing cellular components of a blood sample. Depending on the standard operating procedure of the laboratory, manual smears are often done in conjunction with an automated count. This identifies abnormalities in cellular shape or size, or platelet clumping, which are not consistently recognized by automated methods. Manually evaluating a blood smear does take some training, but the material cost is very low, which has added value for clinical or preclinical research studies. Interestingly, the results of this study indicate that manual platelet counts in blood may be more accurate than the count generated from an automated counter because the automated platelet counts were falsely low due to platelet clumping. Platelet clumping can occur as early as 1 hour after blood collection, regardless of the type of anticoagulant used.31
LIMITATIONS
The sample size of this study was small. However, variability in PRP has been well documented in multiple other studies using slightly larger sample sizes.13,14,16 Another potential limitation of this study could be that only one growth factor, TGF-β1, and one catabolic cytokine, MMP-9, were used as surrogate measures to represent platelet and WBC stability, respectively. We chose TGF-β1 because it is correlated with platelet concentrations14,15,26 and MMP-9 because it is an indicator of catabolic factors in PRP that have been correlated with WBC concentrations.26
CONCLUSION
This study illustrated that growth factor and cytokine concentrations in both Llo PRP and Lhi PRP are stable for up to 4 hours. The clinical implications of these results suggest that if the generation or administration of PRP is delayed by up to 4 hours, the resultant PRP retains its bioactivity and is acceptable for clinical application. However, given the known variability of PRP generated due to patient and manufacturer variability,13,14 it is still important to ensure that the product is indeed PRP, with an increase in platelet number over the starting sample of blood. This validation can be performed with a simple and cost-effective manual smear analysis of blood and PRP. The results of this study provide information that can be immediately translated into clinical, surgical, and research practices.
1. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: Literature review. Tissue Eng Part B Rev. 2008;14(3):249-258. doi:10.1089/ten.teb.2008.0062.
2. Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93-103.
3. Monto RR. Platelet rich plasma treatment for chronic achilles tendinosis. Foot Ankle Int. 2012;33(5):379-385. doi:10.3113/FAI.2012.0379.
4. Owens RF, Ginnetti J, Conti SF, Latona C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot ankle Int. 2011;32(11):1032-1039. doi:10.3113/FAI.2011.1032.
5. Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245-251. doi:10.1177/0363546506294078.
6. Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? Knee Surg Sports Traumatol Arthrosc. 2009;17(6):676-682. doi:10.1007/s00167-009-0762-8.
7. Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84(5):434-439. doi:10.1177/154405910508400507.
8. Bosch G, René van Weeren P, Barneveld A, van Schie HTM. Computerised analysis of standardised ultrasonographic images to monitor the repair of surgically created core lesions in equine superficial digital flexor tendons following treatment with intratendinous platelet rich plasma or placebo. Vet J. 2011;187(1):92-98. doi:10.1016/j.tvjl.2009.10.014.
9. Torricelli P, Fini M, Filardo G, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35(10):1569-1576. doi:10.1007/s00264-011-1237-3.
10. Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3-4):165-174. doi:10.1007/s12178-008-9032-5.
11. Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298-307. doi:10.2106/JBJS.K.00154.
12. Vannini F, Di Matteo B, Filardo G, Kon E, Marcacci M, Giannini S. Platelet-rich plasma for foot and ankle pathologies: a systematic review. Foot Ankle Surg. 2014;20(1):2-9. doi:10.1016/j.fas.2013.08.001.
13. Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429-439. doi:10.1016/j.arthro.2011.10.018.
14. Mazzocca AD, McCarthy MBR, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316. doi:10.2106/JBJS.K.00430.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271. doi:10.1177/0363546510387517.
16. Arnoczky SP, Sheibani-Rad S, Shebani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21(4):180-185. doi:10.1097/JSA.0b013e3182999712.
17. Dohan Ehrenfest DM, Bielecki T, Corso M Del, Inchingolo F, Sammartino G. Shedding light in the controversial terminology for platelet-rich products: Platelet-rich plasma (PRP), platelet-rich fibrin (PRF), platelet-leukocyte gel (PLG), preparation rich in growth factors (PRGF), classification and commercialism. J Biomed Mater Res Part A. 2010;95A(4):1280-1282. doi:10.1002/jbm.a.32894.
18. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158-167. doi:10.1016/j.tibtech.2008.11.009.
19. Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174-187.
20. Malok M, Titchener EH, Bridgers C, Lee BY, Bamberg R. Comparison of two platelet count estimation methodologies for peripheral blood smears. Clin Lab Sci. 2007;20(3):154-160.
21. Gulati G, Uppal G, Florea AD, Gong J. Detection of platelet clumps on peripheral blood smears by CellaVision DM96 System and Microscopic Review. Lab Med. 2014;45(4):368-371. doi:10.1309/LM604RQVKVLRFXOR.
22. Gulati G, Song J, Florea AD, Gong J. Purpose and criteria for blood smear scan, blood smear examination, and blood smear review. Ann Lab Med. 2013;33(1):1-7. doi:10.3343/alm.2013.33.1.1.
23. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585-601. doi:10.1111/j.1524-475X.2008.00410.x.
24. Crane D, Everts P. Platelet rich plasma (PRP) matrix grafts. Pract Pain Manag. 2008;8(1):12-26.
25. Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706-2715. doi:10.1007/s11999-011-1857-3.
26. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140. doi:10.1177/0363546511417792.
27. Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93(3):411-422.
28. Watelet JB, Demetter P, Claeys C, Van Cauwenberge P, Cuvelier C, Bachert C. Neutrophil-derived metalloproteinase-9 predicts healing quality after sinus surgery. Laryngoscope. 2005;115(1):56-61. doi:10.1097/01.mlg.0000150674.30237.3f.
29. Hauschild G, Geburek F, Gosheger G, et al. Short term storage stability at room temperature of two different platelet-rich plasma preparations from equine donors and potential impact on growth factor concentrations. BMC Vet Res. 2017;13(1):7. doi:10.1186/s12917-016-0920-4.
30. Moore GW, Maloney JC, Archer RA, et al. Platelet-rich plasma for tissue regeneration can be stored at room temperature for at least five days. Br J Biomed Sci. 2017;74(2):71-77. doi:10.1080/09674845.2016.1233792.
31. McShine RL, Sibinga S, Brozovic B. Differences between the effects of EDTA and citrate anticoagulants on platelet count and mean platelet volume. Clin Lab Haematol. 1990;12(3):277-285.
1. Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: Literature review. Tissue Eng Part B Rev. 2008;14(3):249-258. doi:10.1089/ten.teb.2008.0062.
2. Sánchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants. 2003;18(1):93-103.
3. Monto RR. Platelet rich plasma treatment for chronic achilles tendinosis. Foot Ankle Int. 2012;33(5):379-385. doi:10.3113/FAI.2012.0379.
4. Owens RF, Ginnetti J, Conti SF, Latona C. Clinical and magnetic resonance imaging outcomes following platelet rich plasma injection for chronic midsubstance Achilles tendinopathy. Foot ankle Int. 2011;32(11):1032-1039. doi:10.3113/FAI.2011.1032.
5. Sánchez M, Anitua E, Azofra J, Andía I, Padilla S, Mujika I. Comparison of surgically repaired achilles tendon tears using platelet-rich fibrin matrices. Am J Sports Med. 2007;35(2):245-251. doi:10.1177/0363546506294078.
6. Silva A, Sampaio R. Anatomic ACL reconstruction: does the platelet-rich plasma accelerate tendon healing? Knee Surg Sports Traumatol Arthrosc. 2009;17(6):676-682. doi:10.1007/s00167-009-0762-8.
7. Fréchette JP, Martineau I, Gagnon G. Platelet-rich plasmas: growth factor content and roles in wound healing. J Dent Res. 2005;84(5):434-439. doi:10.1177/154405910508400507.
8. Bosch G, René van Weeren P, Barneveld A, van Schie HTM. Computerised analysis of standardised ultrasonographic images to monitor the repair of surgically created core lesions in equine superficial digital flexor tendons following treatment with intratendinous platelet rich plasma or placebo. Vet J. 2011;187(1):92-98. doi:10.1016/j.tvjl.2009.10.014.
9. Torricelli P, Fini M, Filardo G, et al. Regenerative medicine for the treatment of musculoskeletal overuse injuries in competition horses. Int Orthop. 2011;35(10):1569-1576. doi:10.1007/s00264-011-1237-3.
10. Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008;1(3-4):165-174. doi:10.1007/s12178-008-9032-5.
11. Sheth U, Simunovic N, Klein G, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94(4):298-307. doi:10.2106/JBJS.K.00154.
12. Vannini F, Di Matteo B, Filardo G, Kon E, Marcacci M, Giannini S. Platelet-rich plasma for foot and ankle pathologies: a systematic review. Foot Ankle Surg. 2014;20(1):2-9. doi:10.1016/j.fas.2013.08.001.
13. Boswell SG, Cole BJ, Sundman EA, Karas V, Fortier LA. Platelet-rich plasma: a milieu of bioactive factors. Arthroscopy. 2012;28(3):429-439. doi:10.1016/j.arthro.2011.10.018.
14. Mazzocca AD, McCarthy MBR, Chowaniec DM, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94(4):308-316. doi:10.2106/JBJS.K.00430.
15. Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39(2):266-271. doi:10.1177/0363546510387517.
16. Arnoczky SP, Sheibani-Rad S, Shebani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc. 2013;21(4):180-185. doi:10.1097/JSA.0b013e3182999712.
17. Dohan Ehrenfest DM, Bielecki T, Corso M Del, Inchingolo F, Sammartino G. Shedding light in the controversial terminology for platelet-rich products: Platelet-rich plasma (PRP), platelet-rich fibrin (PRF), platelet-leukocyte gel (PLG), preparation rich in growth factors (PRGF), classification and commercialism. J Biomed Mater Res Part A. 2010;95A(4):1280-1282. doi:10.1002/jbm.a.32894.
18. Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009;27(3):158-167. doi:10.1016/j.tibtech.2008.11.009.
19. Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol. 2006;38(2):174-187.
20. Malok M, Titchener EH, Bridgers C, Lee BY, Bamberg R. Comparison of two platelet count estimation methodologies for peripheral blood smears. Clin Lab Sci. 2007;20(3):154-160.
21. Gulati G, Uppal G, Florea AD, Gong J. Detection of platelet clumps on peripheral blood smears by CellaVision DM96 System and Microscopic Review. Lab Med. 2014;45(4):368-371. doi:10.1309/LM604RQVKVLRFXOR.
22. Gulati G, Song J, Florea AD, Gong J. Purpose and criteria for blood smear scan, blood smear examination, and blood smear review. Ann Lab Med. 2013;33(1):1-7. doi:10.3343/alm.2013.33.1.1.
23. Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16(5):585-601. doi:10.1111/j.1524-475X.2008.00410.x.
24. Crane D, Everts P. Platelet rich plasma (PRP) matrix grafts. Pract Pain Manag. 2008;8(1):12-26.
25. Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ. The role of growth factors in cartilage repair. Clin Orthop Relat Res. 2011;469(10):2706-2715. doi:10.1007/s11999-011-1857-3.
26. Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39(10):2135-2140. doi:10.1177/0363546511417792.
27. Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998;93(3):411-422.
28. Watelet JB, Demetter P, Claeys C, Van Cauwenberge P, Cuvelier C, Bachert C. Neutrophil-derived metalloproteinase-9 predicts healing quality after sinus surgery. Laryngoscope. 2005;115(1):56-61. doi:10.1097/01.mlg.0000150674.30237.3f.
29. Hauschild G, Geburek F, Gosheger G, et al. Short term storage stability at room temperature of two different platelet-rich plasma preparations from equine donors and potential impact on growth factor concentrations. BMC Vet Res. 2017;13(1):7. doi:10.1186/s12917-016-0920-4.
30. Moore GW, Maloney JC, Archer RA, et al. Platelet-rich plasma for tissue regeneration can be stored at room temperature for at least five days. Br J Biomed Sci. 2017;74(2):71-77. doi:10.1080/09674845.2016.1233792.
31. McShine RL, Sibinga S, Brozovic B. Differences between the effects of EDTA and citrate anticoagulants on platelet count and mean platelet volume. Clin Lab Haematol. 1990;12(3):277-285.
TAKE-HOME POINTS
- Blood can be stored at room temperature for up to 4 hours before making PRP without loss in activity.
- PRP can be stored at room temperature for up to 4 hours before administration to a patient without loss in activity.
- Manual, direct smear analysis is as accurate as automated counting for measuring platelet concentration in PRP.
Using the Child and Adolescent Service Intensity Instrument (CASII) as an Outcome Measure
From the Jewish Family and Children’s Service, Phoenix, AZ (Dr. Henderson) and Consult-Stat, Macungie, PA (Ms. Wasser, Dr. Wasser).
Abstract
- Background: The reliability and validity of the Child and Adolescent Service Intensity Instrument (CASII) as a tool to help determine needed level of care for children with behavioral health needs has previously been established.
- Objective: To determine the utility of the CASII as an outcome measure.
- Methods: A sample consisting of all clients (n = 8465) admitted to service at an outpatient beha
vioral health facility from 2013 through 2016 were studied. CASII was administered at admission and discharge and ratings were compared with paired t-tests within demographic and diagnosis groups. - Results: Mean CASII composite ratings decreased between admission and discharge in the entire cohort as well as within gender, age group, and multiple diagnosis groups tested.
- Conclusion: CASII was useful as an outcome measure in our relatively low to moderate acuity population.
Keywords: outcomes, evidence based practice, child psychology, outpatient research.
The primary goal of mental health services is to provide interventions that result in a reduction of problematic symptomatology [1]; therefore, evaluation of those interventions is important for both the client as well as the stakeholders of the organization providing them. Health care payment reforms require tracking quality measures, and such measures directly influence the development, administration, and monitoring of mental health programs as well as specific treatment modalities [2,3]. Organizations are more likely to benefit when outcomes measures are relayed quantitatively [4]. In addition, clients are becoming more informed regarding the quality of care, and outcomes assessments can inform clients that programs are delivering the most efficacious therapies based on current evidence-based practice standards.
Developing outcomes assessments in behavioral health is challenging [5–7]. There are numerous potential outcome domains that can be assessed as well as different ways of measuring them. Futher, evaluating treatment can be expensive, with components including developing a tool, training staff to administer the tool, ensuring the necessary technical support to store and process the data, interpretation of the data, compiling reports, and communicating results to clients and providers [5]. Being mindful of these components and their associated costs, our organization considered whether a tool we currently use to assess the appropriate intensity of service needed for an individual could also be used as an outcome measure.
Therapeutic methods for children in our organization consist of a “system of care” approach designed by a treatment team that incorporates varied methods depending on the needs of the child. The primary goal is to prevent children with traumatic-based disorders from developing continuing disorders associated with their experiences, such as substance use and chronic health and mental health disorders. Our organization currently uses the CASII (Child and Adolescent Service Intensity Instrument) to assess the appropriate level of intensity of service needed by the child. The CASII incorporates holistic information on the child, within the context of his/her family and social ecology, assessing across 6 dimensions: risk of harm (including trauma issues), functional status, co-occurring conditions, recovery environment, resiliency/response to services, and involvement in services.
In order to comply with the call to consider outcomes measurement and evidence based practice as an integral component of children’s mental health services, this study was performed. It examines the use of the CASII as an outcomes measure based on the rationale that a decreased level of care upon discharge would correlate with a positive outcome by proxy.
Methods
CASII Instrument
The CASII is a decision support tool to help the service provider determine the intensity of services that a child should have to adequately address their behavioral health needs. The CASII has a strong evidence base supporting its reliability and validity [8], and has gained wide usage in a range of health care settings over the past 13 years [9–11].
As mentioned, the CASII assesses the client across 6 key dimensions: risk of harm (including trauma issues), functional status, co-occurring conditions, recovery environment, resiliency/response to services, and involvement in services. Each dimension is scored along a 5-point rating scale, and a total or composite rating is calculated by adding the scores for each dimension. The composite rating corresponds with the level of service intensity needed. There are 7 levels of service intensity, ranging from Level 0 (corresponding with a composite rating of 9 or less) to Level 6 (corresponding with a composite rating of 28 or more) (Table 1).
Study Sample
The sample consisted of all clients (n = 8465) admitted to service from 2013 through 2016 to our facility. Our facility is an outpatient facility offering counseling, mental health assessment and treatment, early childhood trauma assessment, child crisis interventions and rehabilitation for domestic violence, child abuse and neglect, and substance abuse. All clients between the ages of 6 and 17 are assessed with the CASII on admission and then at 6-month intervals until discharge from the program. Being discharged from the program of care prompts the completion of the discharge CASII. If the client had been rated within the 30 days prior to discharge the most recent CASII is used as the discharge measure.
Data Analysis
Data for all admissions from 2013 and 2016 were extracted from the organization’s computer system into an Excel file. The data collected included gender, calendar year of admission to the program, age, and diagnosis group based on the discharge diagnosis given by the mental health team, and whether the client was a participant in the Youth in Transition (YIT) program (program for older clients that includes life skills training in addition to standard therapy). The CASII composite rating at baseline and discharge as well as ratings for each of the 6 dimensions assessed with the CASII were also collected.
We used SPSS (v25.01) software for statistical analysis. Analysis included paired (pre-post) t-tests that were applied to the entire cohort as well as within gender, age group, participation in the YIT program, and diagnosis groups. Diagnosis groups were included only if the frequency of cases within the group was large enough to meet the sample size requirements of central limit theorem (in general, n > 25), with 2 exceptions: schizophrenia spectrum was included because of the rarity of the diagnosis (n = 11) and neurodevelopmental disorders (also n = 11) was included because there was no violation of the equal variance assumption as well as interest to the investigators. In addition to the paired analysis, we used group t tests to determine if there were severity differences between groups at baseline. Lastly, we assessed change from admission to discharge for each of the 6 dimensions that make up the composite rating.
We designated the 7 levels of care defined by the CASII as continuous in nature, and therefore computations of means and standard deviations (SD) are appropriate for assessment. The interpretation of the CASII composite rating and the level of care as a continuous variable has also been reported in the literature [11,12].
The research and analysis was viewed as exploratory in nature and a P value less than 0.05 was considered statistically significant. There was no correction for multiple comparisons applied to the data in order to not mask any observed differences in the data. All analyses were 2-tailed. If any individual had a missing value for either an admission or discharge CASII assessment they were excluded from the statistical analysis.
Results
There were 8465 clients admitted from 2013 and 2016. The sample was predominantly male (54.5%), and the majority fell into the older 12–17 year old cohort (54.0%). Admissions were evenly distributed across the 4 years that we studied, with the lowest percentage in 2013 at 23.4% and the highest in 2014 at 26.0%. Discharge diagnosis was available for the majority of the cohort. The top 5 most frequent diagnosis groups were adjustment disorders (n = 807, 18.3%), ADHD (n = 798, 18.1%), child neglect (n = 775, 17.6%), mood disorders (n = 602, 13.6%), and impulse disorders (n = 262, 5.9%). There were 232 (2.7%) clients that participated in the YIT program. Table 2
At admission, several groups had higher mean composite ratings. Males had higher ratings (in need of higher level of service intensity) than females (P < 0.001), 12–17 year olds had a significantly higher acuity level than 6–11 year olds (P < 0.001), and clients in the YIT program had a higher acuity level than those not in the YIT program (P = 0.001). Baseline acuity levels for primary discharge diagnosis for selected groups are shown in the Figure.
When analyzing the entire cohort for which data were available (n = 6944), the mean CASII composite rating dropped from 13.23 (± 4.35 SD) to 12.04 (± 3.84 SD), P < 0.001. Excluding youth that participated in YIT, the mean CASII score dropped from 13.21 (± 4.33) at admission to 13.17 (± 4.52) at discharge. Mean composite rating for clients participating in the YIT program dropped from 14.31 (± 5.12) at admission to 13.17 (± 4.52) at discharge (P = 0.022). For diagnosis groups, statistically significant reduction in mean CASII composite rating was observed for all groups except neurodevelopmentall disorders (P = 0.166). The results for all groups and diagnosis cohorts can be found in Table 3.
As noted, the CASII assesses the client across 6 dimensions, each of which is scored along a 5-point rating scale, and the composite rating is calculated by adding the scores for each dimension. Table 4 shows the change in mean dimension scores from baseline to discharge for these dimensions. Mean scores improved significantly (all P < 0.001).
Discussion
Organizations that provide mental health services are burdened with a complicated milieu of providing the best care possible in a complicated system of assessment, reimbursement, admissions/discharges, and a variety of other tasks. Using multiple measures complicates assessment and increases costs because of training staff, developing and interpreting the tool results, data storage and more comprehensive analysis and communication of results back to stakeholders and staff. Complicated measures are often times not understood by the staff and those responsible for care, nor are measures understood by the clients and their families. While a wide array of psychometric assessment tools exist, most are applicable to only specific diagnosis groups or illnesses.
Our study showed that the CASII may be used to monitor progress and reassess the level of service intensity needed, and therefore may be useful as an outcome measure. There are benefits in having a single score as an outcome measure. A single score for each client is quick and easy to understand by board members, staff of the organization as well as clients outside of the organization such as funders, client, press etc. Also the use of a single score is cost effective as costs for interpretation, training and communication within and outside of the organization are reduced.
A number of limitations must be mentioned. Although a change in score represents a change in client condition, this change in condition can have a wide variety of explanations. Change can be related to the therapy received, to changes in the client’s environment, support services, and many other factors. Our research did not allow us to discern what aspects of care may have reduced level of service intensity needed at discharge. In addition, our study involved clients of low and moderate acuity. The study does not address if CASII would be sensitive to change in upper acuity ranges. Therefore, our findings may not be generalizable in these settings.
Tolan and Dodge [10] called for the enhancement or an elevation in the assessment of psychology as a matter of public policy. An approach that involves all levels of scientific inquiry including economics, political science and other sciences is desperately needed. Assessment of the type presented in this article, even if instruments such as the CASII are not used, can help to shape that policy by providing unquestionably accurate assessment of a client’s condition which demonstrates the need for that support. Further research looking at specific attributes of therapy and the client’s condition and environment may be helpful in applying CASII composite ratings and dimension scores as outcome measures.
Corresponding author: Dr. Lorrie Henderson, Jewish Family and Children’s Service, 4747 North 7th St., Suite 100, Phoenix, AZ 850142.
Financial disclosures: None.
1. Thornicroft G, Slade M. New trends in assessing the outcomes of mental health interventions. World Psychiatry 2014;13:118.
2. England MJ, Butler AS, Gonzalez ML, editors. Psychosocial interventions for mental and substance use disorders: a framework for establishing evidence-based standards. Committee on Developing Evidence-Based Standards for Psychosocial Interventions for Mental Disorders; Board on Health Sciences Policy; Institute of Medicine. Washington (DC): National Academies Press; 2015 Sep 18.
3. Schurer Coldiron J, Hensley SW, Bruns EJ, Paragoris R. Putting the outcomes‐based principle into action part one: a guide for wraparound care coordinators; The National Technical Assistance Network for Children’s Behavioral Health. 2016. Available at: https://nwi.pdx.edu/pdf/Putting-the-Outcomes-Based-Principle-Into-Action.pdf.
4. Lachar D, Randle S, Harper R, et al. The brief psychiatric rating scale for children (BPRS-C): Validity and reliability of an anchored version. J Am Acad Child Adol Psychiatry 2001;40:333–40.
5. Sperry L, Brill PL, Howard KI, Grissom GR. Treatment outcomes in psychotherapy and psychiatric interventions. Philadelphia: Brunner/Mazel; 1996.
6. Burlingame GM, Lambert MJ, Reisinger CW, et al. Pragmatics of tracking mental health outcomes in a managed care setting. J Ment Health Adm 1995;22:226–36.
7. Henderson L, McIlhaney K, Wasser T. Measuring outcomes of multiple diagnosis groups in residential treatment using the brief psychiatric rating scale for children (BPRS-C). Children Youth Serv Rev 2008:24:243–59.
8. Fallon T Jr, Pumariega A, Sowers W, et al. A level of care instrument for children’s systems of care: Construction, reliability and validity. J Child Fam Studies 2006:15:143–155.
9. Minnesota Department of Human Services announcement. DHS updates requirement for standardized outcome measures for children’s mental health. #17-53-01. 27 Feb 2017.
10. Tolan P, Dodge K. Children’s mental health as a primary care and concern: a system for comprehensive support and service. Am Psychol 2005;60:601–14.
11. Child and Adolescent Service Intensity Instrument (CASII) Overview for Anthem Connecticut Members. Accessed at www11.anthem.com/provider/ct/f3/s9/t1/pw_e205607.pdf?refer=ahpprovider.
12. Chenven M, Dominguez E, Grimes K, et al. CASII: Child and adolescent Service Intensity Instrument Background information and Initial Data Analysis. American Academy of Child and Adolescent Psychiatry Work Group June 2001.
From the Jewish Family and Children’s Service, Phoenix, AZ (Dr. Henderson) and Consult-Stat, Macungie, PA (Ms. Wasser, Dr. Wasser).
Abstract
- Background: The reliability and validity of the Child and Adolescent Service Intensity Instrument (CASII) as a tool to help determine needed level of care for children with behavioral health needs has previously been established.
- Objective: To determine the utility of the CASII as an outcome measure.
- Methods: A sample consisting of all clients (n = 8465) admitted to service at an outpatient beha
vioral health facility from 2013 through 2016 were studied. CASII was administered at admission and discharge and ratings were compared with paired t-tests within demographic and diagnosis groups. - Results: Mean CASII composite ratings decreased between admission and discharge in the entire cohort as well as within gender, age group, and multiple diagnosis groups tested.
- Conclusion: CASII was useful as an outcome measure in our relatively low to moderate acuity population.
Keywords: outcomes, evidence based practice, child psychology, outpatient research.
The primary goal of mental health services is to provide interventions that result in a reduction of problematic symptomatology [1]; therefore, evaluation of those interventions is important for both the client as well as the stakeholders of the organization providing them. Health care payment reforms require tracking quality measures, and such measures directly influence the development, administration, and monitoring of mental health programs as well as specific treatment modalities [2,3]. Organizations are more likely to benefit when outcomes measures are relayed quantitatively [4]. In addition, clients are becoming more informed regarding the quality of care, and outcomes assessments can inform clients that programs are delivering the most efficacious therapies based on current evidence-based practice standards.
Developing outcomes assessments in behavioral health is challenging [5–7]. There are numerous potential outcome domains that can be assessed as well as different ways of measuring them. Futher, evaluating treatment can be expensive, with components including developing a tool, training staff to administer the tool, ensuring the necessary technical support to store and process the data, interpretation of the data, compiling reports, and communicating results to clients and providers [5]. Being mindful of these components and their associated costs, our organization considered whether a tool we currently use to assess the appropriate intensity of service needed for an individual could also be used as an outcome measure.
Therapeutic methods for children in our organization consist of a “system of care” approach designed by a treatment team that incorporates varied methods depending on the needs of the child. The primary goal is to prevent children with traumatic-based disorders from developing continuing disorders associated with their experiences, such as substance use and chronic health and mental health disorders. Our organization currently uses the CASII (Child and Adolescent Service Intensity Instrument) to assess the appropriate level of intensity of service needed by the child. The CASII incorporates holistic information on the child, within the context of his/her family and social ecology, assessing across 6 dimensions: risk of harm (including trauma issues), functional status, co-occurring conditions, recovery environment, resiliency/response to services, and involvement in services.
In order to comply with the call to consider outcomes measurement and evidence based practice as an integral component of children’s mental health services, this study was performed. It examines the use of the CASII as an outcomes measure based on the rationale that a decreased level of care upon discharge would correlate with a positive outcome by proxy.
Methods
CASII Instrument
The CASII is a decision support tool to help the service provider determine the intensity of services that a child should have to adequately address their behavioral health needs. The CASII has a strong evidence base supporting its reliability and validity [8], and has gained wide usage in a range of health care settings over the past 13 years [9–11].
As mentioned, the CASII assesses the client across 6 key dimensions: risk of harm (including trauma issues), functional status, co-occurring conditions, recovery environment, resiliency/response to services, and involvement in services. Each dimension is scored along a 5-point rating scale, and a total or composite rating is calculated by adding the scores for each dimension. The composite rating corresponds with the level of service intensity needed. There are 7 levels of service intensity, ranging from Level 0 (corresponding with a composite rating of 9 or less) to Level 6 (corresponding with a composite rating of 28 or more) (Table 1).
Study Sample
The sample consisted of all clients (n = 8465) admitted to service from 2013 through 2016 to our facility. Our facility is an outpatient facility offering counseling, mental health assessment and treatment, early childhood trauma assessment, child crisis interventions and rehabilitation for domestic violence, child abuse and neglect, and substance abuse. All clients between the ages of 6 and 17 are assessed with the CASII on admission and then at 6-month intervals until discharge from the program. Being discharged from the program of care prompts the completion of the discharge CASII. If the client had been rated within the 30 days prior to discharge the most recent CASII is used as the discharge measure.
Data Analysis
Data for all admissions from 2013 and 2016 were extracted from the organization’s computer system into an Excel file. The data collected included gender, calendar year of admission to the program, age, and diagnosis group based on the discharge diagnosis given by the mental health team, and whether the client was a participant in the Youth in Transition (YIT) program (program for older clients that includes life skills training in addition to standard therapy). The CASII composite rating at baseline and discharge as well as ratings for each of the 6 dimensions assessed with the CASII were also collected.
We used SPSS (v25.01) software for statistical analysis. Analysis included paired (pre-post) t-tests that were applied to the entire cohort as well as within gender, age group, participation in the YIT program, and diagnosis groups. Diagnosis groups were included only if the frequency of cases within the group was large enough to meet the sample size requirements of central limit theorem (in general, n > 25), with 2 exceptions: schizophrenia spectrum was included because of the rarity of the diagnosis (n = 11) and neurodevelopmental disorders (also n = 11) was included because there was no violation of the equal variance assumption as well as interest to the investigators. In addition to the paired analysis, we used group t tests to determine if there were severity differences between groups at baseline. Lastly, we assessed change from admission to discharge for each of the 6 dimensions that make up the composite rating.
We designated the 7 levels of care defined by the CASII as continuous in nature, and therefore computations of means and standard deviations (SD) are appropriate for assessment. The interpretation of the CASII composite rating and the level of care as a continuous variable has also been reported in the literature [11,12].
The research and analysis was viewed as exploratory in nature and a P value less than 0.05 was considered statistically significant. There was no correction for multiple comparisons applied to the data in order to not mask any observed differences in the data. All analyses were 2-tailed. If any individual had a missing value for either an admission or discharge CASII assessment they were excluded from the statistical analysis.
Results
There were 8465 clients admitted from 2013 and 2016. The sample was predominantly male (54.5%), and the majority fell into the older 12–17 year old cohort (54.0%). Admissions were evenly distributed across the 4 years that we studied, with the lowest percentage in 2013 at 23.4% and the highest in 2014 at 26.0%. Discharge diagnosis was available for the majority of the cohort. The top 5 most frequent diagnosis groups were adjustment disorders (n = 807, 18.3%), ADHD (n = 798, 18.1%), child neglect (n = 775, 17.6%), mood disorders (n = 602, 13.6%), and impulse disorders (n = 262, 5.9%). There were 232 (2.7%) clients that participated in the YIT program. Table 2
At admission, several groups had higher mean composite ratings. Males had higher ratings (in need of higher level of service intensity) than females (P < 0.001), 12–17 year olds had a significantly higher acuity level than 6–11 year olds (P < 0.001), and clients in the YIT program had a higher acuity level than those not in the YIT program (P = 0.001). Baseline acuity levels for primary discharge diagnosis for selected groups are shown in the Figure.
When analyzing the entire cohort for which data were available (n = 6944), the mean CASII composite rating dropped from 13.23 (± 4.35 SD) to 12.04 (± 3.84 SD), P < 0.001. Excluding youth that participated in YIT, the mean CASII score dropped from 13.21 (± 4.33) at admission to 13.17 (± 4.52) at discharge. Mean composite rating for clients participating in the YIT program dropped from 14.31 (± 5.12) at admission to 13.17 (± 4.52) at discharge (P = 0.022). For diagnosis groups, statistically significant reduction in mean CASII composite rating was observed for all groups except neurodevelopmentall disorders (P = 0.166). The results for all groups and diagnosis cohorts can be found in Table 3.
As noted, the CASII assesses the client across 6 dimensions, each of which is scored along a 5-point rating scale, and the composite rating is calculated by adding the scores for each dimension. Table 4 shows the change in mean dimension scores from baseline to discharge for these dimensions. Mean scores improved significantly (all P < 0.001).
Discussion
Organizations that provide mental health services are burdened with a complicated milieu of providing the best care possible in a complicated system of assessment, reimbursement, admissions/discharges, and a variety of other tasks. Using multiple measures complicates assessment and increases costs because of training staff, developing and interpreting the tool results, data storage and more comprehensive analysis and communication of results back to stakeholders and staff. Complicated measures are often times not understood by the staff and those responsible for care, nor are measures understood by the clients and their families. While a wide array of psychometric assessment tools exist, most are applicable to only specific diagnosis groups or illnesses.
Our study showed that the CASII may be used to monitor progress and reassess the level of service intensity needed, and therefore may be useful as an outcome measure. There are benefits in having a single score as an outcome measure. A single score for each client is quick and easy to understand by board members, staff of the organization as well as clients outside of the organization such as funders, client, press etc. Also the use of a single score is cost effective as costs for interpretation, training and communication within and outside of the organization are reduced.
A number of limitations must be mentioned. Although a change in score represents a change in client condition, this change in condition can have a wide variety of explanations. Change can be related to the therapy received, to changes in the client’s environment, support services, and many other factors. Our research did not allow us to discern what aspects of care may have reduced level of service intensity needed at discharge. In addition, our study involved clients of low and moderate acuity. The study does not address if CASII would be sensitive to change in upper acuity ranges. Therefore, our findings may not be generalizable in these settings.
Tolan and Dodge [10] called for the enhancement or an elevation in the assessment of psychology as a matter of public policy. An approach that involves all levels of scientific inquiry including economics, political science and other sciences is desperately needed. Assessment of the type presented in this article, even if instruments such as the CASII are not used, can help to shape that policy by providing unquestionably accurate assessment of a client’s condition which demonstrates the need for that support. Further research looking at specific attributes of therapy and the client’s condition and environment may be helpful in applying CASII composite ratings and dimension scores as outcome measures.
Corresponding author: Dr. Lorrie Henderson, Jewish Family and Children’s Service, 4747 North 7th St., Suite 100, Phoenix, AZ 850142.
Financial disclosures: None.
From the Jewish Family and Children’s Service, Phoenix, AZ (Dr. Henderson) and Consult-Stat, Macungie, PA (Ms. Wasser, Dr. Wasser).
Abstract
- Background: The reliability and validity of the Child and Adolescent Service Intensity Instrument (CASII) as a tool to help determine needed level of care for children with behavioral health needs has previously been established.
- Objective: To determine the utility of the CASII as an outcome measure.
- Methods: A sample consisting of all clients (n = 8465) admitted to service at an outpatient beha
vioral health facility from 2013 through 2016 were studied. CASII was administered at admission and discharge and ratings were compared with paired t-tests within demographic and diagnosis groups. - Results: Mean CASII composite ratings decreased between admission and discharge in the entire cohort as well as within gender, age group, and multiple diagnosis groups tested.
- Conclusion: CASII was useful as an outcome measure in our relatively low to moderate acuity population.
Keywords: outcomes, evidence based practice, child psychology, outpatient research.
The primary goal of mental health services is to provide interventions that result in a reduction of problematic symptomatology [1]; therefore, evaluation of those interventions is important for both the client as well as the stakeholders of the organization providing them. Health care payment reforms require tracking quality measures, and such measures directly influence the development, administration, and monitoring of mental health programs as well as specific treatment modalities [2,3]. Organizations are more likely to benefit when outcomes measures are relayed quantitatively [4]. In addition, clients are becoming more informed regarding the quality of care, and outcomes assessments can inform clients that programs are delivering the most efficacious therapies based on current evidence-based practice standards.
Developing outcomes assessments in behavioral health is challenging [5–7]. There are numerous potential outcome domains that can be assessed as well as different ways of measuring them. Futher, evaluating treatment can be expensive, with components including developing a tool, training staff to administer the tool, ensuring the necessary technical support to store and process the data, interpretation of the data, compiling reports, and communicating results to clients and providers [5]. Being mindful of these components and their associated costs, our organization considered whether a tool we currently use to assess the appropriate intensity of service needed for an individual could also be used as an outcome measure.
Therapeutic methods for children in our organization consist of a “system of care” approach designed by a treatment team that incorporates varied methods depending on the needs of the child. The primary goal is to prevent children with traumatic-based disorders from developing continuing disorders associated with their experiences, such as substance use and chronic health and mental health disorders. Our organization currently uses the CASII (Child and Adolescent Service Intensity Instrument) to assess the appropriate level of intensity of service needed by the child. The CASII incorporates holistic information on the child, within the context of his/her family and social ecology, assessing across 6 dimensions: risk of harm (including trauma issues), functional status, co-occurring conditions, recovery environment, resiliency/response to services, and involvement in services.
In order to comply with the call to consider outcomes measurement and evidence based practice as an integral component of children’s mental health services, this study was performed. It examines the use of the CASII as an outcomes measure based on the rationale that a decreased level of care upon discharge would correlate with a positive outcome by proxy.
Methods
CASII Instrument
The CASII is a decision support tool to help the service provider determine the intensity of services that a child should have to adequately address their behavioral health needs. The CASII has a strong evidence base supporting its reliability and validity [8], and has gained wide usage in a range of health care settings over the past 13 years [9–11].
As mentioned, the CASII assesses the client across 6 key dimensions: risk of harm (including trauma issues), functional status, co-occurring conditions, recovery environment, resiliency/response to services, and involvement in services. Each dimension is scored along a 5-point rating scale, and a total or composite rating is calculated by adding the scores for each dimension. The composite rating corresponds with the level of service intensity needed. There are 7 levels of service intensity, ranging from Level 0 (corresponding with a composite rating of 9 or less) to Level 6 (corresponding with a composite rating of 28 or more) (Table 1).
Study Sample
The sample consisted of all clients (n = 8465) admitted to service from 2013 through 2016 to our facility. Our facility is an outpatient facility offering counseling, mental health assessment and treatment, early childhood trauma assessment, child crisis interventions and rehabilitation for domestic violence, child abuse and neglect, and substance abuse. All clients between the ages of 6 and 17 are assessed with the CASII on admission and then at 6-month intervals until discharge from the program. Being discharged from the program of care prompts the completion of the discharge CASII. If the client had been rated within the 30 days prior to discharge the most recent CASII is used as the discharge measure.
Data Analysis
Data for all admissions from 2013 and 2016 were extracted from the organization’s computer system into an Excel file. The data collected included gender, calendar year of admission to the program, age, and diagnosis group based on the discharge diagnosis given by the mental health team, and whether the client was a participant in the Youth in Transition (YIT) program (program for older clients that includes life skills training in addition to standard therapy). The CASII composite rating at baseline and discharge as well as ratings for each of the 6 dimensions assessed with the CASII were also collected.
We used SPSS (v25.01) software for statistical analysis. Analysis included paired (pre-post) t-tests that were applied to the entire cohort as well as within gender, age group, participation in the YIT program, and diagnosis groups. Diagnosis groups were included only if the frequency of cases within the group was large enough to meet the sample size requirements of central limit theorem (in general, n > 25), with 2 exceptions: schizophrenia spectrum was included because of the rarity of the diagnosis (n = 11) and neurodevelopmental disorders (also n = 11) was included because there was no violation of the equal variance assumption as well as interest to the investigators. In addition to the paired analysis, we used group t tests to determine if there were severity differences between groups at baseline. Lastly, we assessed change from admission to discharge for each of the 6 dimensions that make up the composite rating.
We designated the 7 levels of care defined by the CASII as continuous in nature, and therefore computations of means and standard deviations (SD) are appropriate for assessment. The interpretation of the CASII composite rating and the level of care as a continuous variable has also been reported in the literature [11,12].
The research and analysis was viewed as exploratory in nature and a P value less than 0.05 was considered statistically significant. There was no correction for multiple comparisons applied to the data in order to not mask any observed differences in the data. All analyses were 2-tailed. If any individual had a missing value for either an admission or discharge CASII assessment they were excluded from the statistical analysis.
Results
There were 8465 clients admitted from 2013 and 2016. The sample was predominantly male (54.5%), and the majority fell into the older 12–17 year old cohort (54.0%). Admissions were evenly distributed across the 4 years that we studied, with the lowest percentage in 2013 at 23.4% and the highest in 2014 at 26.0%. Discharge diagnosis was available for the majority of the cohort. The top 5 most frequent diagnosis groups were adjustment disorders (n = 807, 18.3%), ADHD (n = 798, 18.1%), child neglect (n = 775, 17.6%), mood disorders (n = 602, 13.6%), and impulse disorders (n = 262, 5.9%). There were 232 (2.7%) clients that participated in the YIT program. Table 2
At admission, several groups had higher mean composite ratings. Males had higher ratings (in need of higher level of service intensity) than females (P < 0.001), 12–17 year olds had a significantly higher acuity level than 6–11 year olds (P < 0.001), and clients in the YIT program had a higher acuity level than those not in the YIT program (P = 0.001). Baseline acuity levels for primary discharge diagnosis for selected groups are shown in the Figure.
When analyzing the entire cohort for which data were available (n = 6944), the mean CASII composite rating dropped from 13.23 (± 4.35 SD) to 12.04 (± 3.84 SD), P < 0.001. Excluding youth that participated in YIT, the mean CASII score dropped from 13.21 (± 4.33) at admission to 13.17 (± 4.52) at discharge. Mean composite rating for clients participating in the YIT program dropped from 14.31 (± 5.12) at admission to 13.17 (± 4.52) at discharge (P = 0.022). For diagnosis groups, statistically significant reduction in mean CASII composite rating was observed for all groups except neurodevelopmentall disorders (P = 0.166). The results for all groups and diagnosis cohorts can be found in Table 3.
As noted, the CASII assesses the client across 6 dimensions, each of which is scored along a 5-point rating scale, and the composite rating is calculated by adding the scores for each dimension. Table 4 shows the change in mean dimension scores from baseline to discharge for these dimensions. Mean scores improved significantly (all P < 0.001).
Discussion
Organizations that provide mental health services are burdened with a complicated milieu of providing the best care possible in a complicated system of assessment, reimbursement, admissions/discharges, and a variety of other tasks. Using multiple measures complicates assessment and increases costs because of training staff, developing and interpreting the tool results, data storage and more comprehensive analysis and communication of results back to stakeholders and staff. Complicated measures are often times not understood by the staff and those responsible for care, nor are measures understood by the clients and their families. While a wide array of psychometric assessment tools exist, most are applicable to only specific diagnosis groups or illnesses.
Our study showed that the CASII may be used to monitor progress and reassess the level of service intensity needed, and therefore may be useful as an outcome measure. There are benefits in having a single score as an outcome measure. A single score for each client is quick and easy to understand by board members, staff of the organization as well as clients outside of the organization such as funders, client, press etc. Also the use of a single score is cost effective as costs for interpretation, training and communication within and outside of the organization are reduced.
A number of limitations must be mentioned. Although a change in score represents a change in client condition, this change in condition can have a wide variety of explanations. Change can be related to the therapy received, to changes in the client’s environment, support services, and many other factors. Our research did not allow us to discern what aspects of care may have reduced level of service intensity needed at discharge. In addition, our study involved clients of low and moderate acuity. The study does not address if CASII would be sensitive to change in upper acuity ranges. Therefore, our findings may not be generalizable in these settings.
Tolan and Dodge [10] called for the enhancement or an elevation in the assessment of psychology as a matter of public policy. An approach that involves all levels of scientific inquiry including economics, political science and other sciences is desperately needed. Assessment of the type presented in this article, even if instruments such as the CASII are not used, can help to shape that policy by providing unquestionably accurate assessment of a client’s condition which demonstrates the need for that support. Further research looking at specific attributes of therapy and the client’s condition and environment may be helpful in applying CASII composite ratings and dimension scores as outcome measures.
Corresponding author: Dr. Lorrie Henderson, Jewish Family and Children’s Service, 4747 North 7th St., Suite 100, Phoenix, AZ 850142.
Financial disclosures: None.
1. Thornicroft G, Slade M. New trends in assessing the outcomes of mental health interventions. World Psychiatry 2014;13:118.
2. England MJ, Butler AS, Gonzalez ML, editors. Psychosocial interventions for mental and substance use disorders: a framework for establishing evidence-based standards. Committee on Developing Evidence-Based Standards for Psychosocial Interventions for Mental Disorders; Board on Health Sciences Policy; Institute of Medicine. Washington (DC): National Academies Press; 2015 Sep 18.
3. Schurer Coldiron J, Hensley SW, Bruns EJ, Paragoris R. Putting the outcomes‐based principle into action part one: a guide for wraparound care coordinators; The National Technical Assistance Network for Children’s Behavioral Health. 2016. Available at: https://nwi.pdx.edu/pdf/Putting-the-Outcomes-Based-Principle-Into-Action.pdf.
4. Lachar D, Randle S, Harper R, et al. The brief psychiatric rating scale for children (BPRS-C): Validity and reliability of an anchored version. J Am Acad Child Adol Psychiatry 2001;40:333–40.
5. Sperry L, Brill PL, Howard KI, Grissom GR. Treatment outcomes in psychotherapy and psychiatric interventions. Philadelphia: Brunner/Mazel; 1996.
6. Burlingame GM, Lambert MJ, Reisinger CW, et al. Pragmatics of tracking mental health outcomes in a managed care setting. J Ment Health Adm 1995;22:226–36.
7. Henderson L, McIlhaney K, Wasser T. Measuring outcomes of multiple diagnosis groups in residential treatment using the brief psychiatric rating scale for children (BPRS-C). Children Youth Serv Rev 2008:24:243–59.
8. Fallon T Jr, Pumariega A, Sowers W, et al. A level of care instrument for children’s systems of care: Construction, reliability and validity. J Child Fam Studies 2006:15:143–155.
9. Minnesota Department of Human Services announcement. DHS updates requirement for standardized outcome measures for children’s mental health. #17-53-01. 27 Feb 2017.
10. Tolan P, Dodge K. Children’s mental health as a primary care and concern: a system for comprehensive support and service. Am Psychol 2005;60:601–14.
11. Child and Adolescent Service Intensity Instrument (CASII) Overview for Anthem Connecticut Members. Accessed at www11.anthem.com/provider/ct/f3/s9/t1/pw_e205607.pdf?refer=ahpprovider.
12. Chenven M, Dominguez E, Grimes K, et al. CASII: Child and adolescent Service Intensity Instrument Background information and Initial Data Analysis. American Academy of Child and Adolescent Psychiatry Work Group June 2001.
1. Thornicroft G, Slade M. New trends in assessing the outcomes of mental health interventions. World Psychiatry 2014;13:118.
2. England MJ, Butler AS, Gonzalez ML, editors. Psychosocial interventions for mental and substance use disorders: a framework for establishing evidence-based standards. Committee on Developing Evidence-Based Standards for Psychosocial Interventions for Mental Disorders; Board on Health Sciences Policy; Institute of Medicine. Washington (DC): National Academies Press; 2015 Sep 18.
3. Schurer Coldiron J, Hensley SW, Bruns EJ, Paragoris R. Putting the outcomes‐based principle into action part one: a guide for wraparound care coordinators; The National Technical Assistance Network for Children’s Behavioral Health. 2016. Available at: https://nwi.pdx.edu/pdf/Putting-the-Outcomes-Based-Principle-Into-Action.pdf.
4. Lachar D, Randle S, Harper R, et al. The brief psychiatric rating scale for children (BPRS-C): Validity and reliability of an anchored version. J Am Acad Child Adol Psychiatry 2001;40:333–40.
5. Sperry L, Brill PL, Howard KI, Grissom GR. Treatment outcomes in psychotherapy and psychiatric interventions. Philadelphia: Brunner/Mazel; 1996.
6. Burlingame GM, Lambert MJ, Reisinger CW, et al. Pragmatics of tracking mental health outcomes in a managed care setting. J Ment Health Adm 1995;22:226–36.
7. Henderson L, McIlhaney K, Wasser T. Measuring outcomes of multiple diagnosis groups in residential treatment using the brief psychiatric rating scale for children (BPRS-C). Children Youth Serv Rev 2008:24:243–59.
8. Fallon T Jr, Pumariega A, Sowers W, et al. A level of care instrument for children’s systems of care: Construction, reliability and validity. J Child Fam Studies 2006:15:143–155.
9. Minnesota Department of Human Services announcement. DHS updates requirement for standardized outcome measures for children’s mental health. #17-53-01. 27 Feb 2017.
10. Tolan P, Dodge K. Children’s mental health as a primary care and concern: a system for comprehensive support and service. Am Psychol 2005;60:601–14.
11. Child and Adolescent Service Intensity Instrument (CASII) Overview for Anthem Connecticut Members. Accessed at www11.anthem.com/provider/ct/f3/s9/t1/pw_e205607.pdf?refer=ahpprovider.
12. Chenven M, Dominguez E, Grimes K, et al. CASII: Child and adolescent Service Intensity Instrument Background information and Initial Data Analysis. American Academy of Child and Adolescent Psychiatry Work Group June 2001.
Gone Fishing: A Unique Histologic Pattern in Cutaneous Angiosarcoma
Cutaneous angiosarcoma is a rare malignant tumor of vascular endothelial cells that has the propensity to arise in various clinical settings. This tumor predominantly occurs in the head and neck region in elderly patients, but it also has been reported to develop postradiotherapy or in the setting of chronic lymphedema in the extremities.1-3 In all settings, the diagnosis carries a very poor prognosis with a high likelihood of local recurrence and rapid dissemination. The mortality rate typically is 80% or higher.2,4-6
Making the correct clinical diagnosis of cutaneous angiosarcoma may be difficult given the variety of patient symptoms and clinical appearances that can be demonstrated on presentation. Lesions can appear as bluish or violaceous plaques, macules, or nodules, and ulceration may be present in some advanced cases.5,7 Clinical misdiagnosis is common, as cutaneous angiosarcomas may be mistaken for infectious processes, benign vascular malformations, and other cutaneous malignancies.1 Biopsy often is delayed given the initial benign appearance of the lesions, and this frequently results in aggressive and extensive disease at the time of diagnosis, which is unfortunate given that small tumor size has been shown to be one of the only favorable prognostic indicators in cutaneous angiosarcoma.1,2,6,8
Microscopically, diagnosis of cutaneous angiosarcoma can present a challenge, as the histology varies between a well-differentiated vascular neoplasm and a considerably anaplastic and poorly differentiated malignancy. On low power, some areas may appear as benign hemangiomas with other areas showing frank sarcomatous features.9 As a result, these tumors can be mistaken for a variety of other diseases including melanomas, carcinomas, or other vascular tumors.6,8,9 Previously, electron microscopy has been utilized on undifferentiated tumors to help distinguish cutaneous angiosarcomas from other potential diagnoses. The atypical tumor cells of cutaneous angiosarcoma display common features of endothelial cells (eg, pinocytotic vesicles, tubulated bodies).7 Historically, it has been noted that the histologic findings and tumor grade provide little evidence regarding the aggressiveness of the tumor, and all cutaneous angiosarcoma diagnoses receive a poor prognosis.6,8
Classically, the histologic findings of cutaneous angiosarcoma include a highly infiltrative neoplasm forming irregular vascular channels that penetrate through the cutaneous soft tissues and frequently extend into the subcutaneous fat. The vascular spaces are lined by hyperchromatic endothelial cells with varying degrees of atypia.1,2,4,6,7,10 Occasionally, prominent endothelial cells lining a papillary structure within the lumen of the neoformed vessel may also be observed. Currently, immunohistochemical staining for MYC, Ki-67, D2-40, and various other markers complement the histologic findings to aid in the diagnosis of cutaneous angiosarcoma.11,12 An additional diagnostic clue that has been described in cases of postirradiation cutaneous angiosarcoma shows free-floating or tufted pleomorphic spindle cells within the vascular lumen (Figure). This finding has been described as “fish in the creek.”11 In this study, we aimed to determine the frequency and subsequent diagnostic utility of the fish-in-the-creek finding in cases of cutaneous angiosarcoma.

Methods
A natural language search of our institutional archives over a 20-year period (1997–2017) using the term angiosarcoma was performed. Fifteen cases of cutaneous angiosarcoma were identified. Fifteen additional benign and malignant vascular tumors with cutaneous angiosarco
Results
The histologic pattern of fish in the creek was identified in all 15 cases of cutaneous angiosarcoma and was absent in the other 15 malignancies examined in this study. This finding shows the potential for the fish-in-the-creek pattern to be used as an additional diagnostic tool for dermatopathologists.
Comment
Cutaneous angiosarcoma is a rare but aggressive malignancy that proves difficult to diagnose both clinically and histologically as well as to treat effectively.1,5-8 Our results indicate that fish in the creek may be a useful and salient histologic feature in cutaneous angiosarcoma. It is important to recognize, however, that this finding should not be the sole feature upon which a diagnosis of cutaneous angiosarcoma is made, as it requires corroboration with positivity of MYC and D2-40 as well as a high Ki-67 proliferation index (>20%).11,12 Finding a fish-in-the-creek pattern should prompt dermatopathologists to consider a diagnosis of cutaneous angiosarcoma in the appropriate clinical and histologic settings.
The chief limitation of this study was the small sample size, with only 15 cases of cutaneous angiosarcoma available in the last 20 years at our institution. The limited sample size did not allow us to make claims on sensitivity and specificity regarding this histologic feature; however, with a larger sample size, the true diagnostic potential could be elucidated. Although the pathologists were blinded to the original diagnoses as they examined it for fish in the creek, it is possible they were able to make the correct diagnosis based on other histopathologic clues and therefore were biased.
Although the fish-in-the-creek pattern is present in cutaneous angiosarcoma, there may be other mimickers to consider. Intraluminal papillary projections lined by endothelial cells may be sectioned in a manner imitating this finding.3 In such a case, these endothelial cells must be differentiated from the free-floating or tufted spindle cells in order to have a positive finding for fish in the creek. There can be confusion if the biopsy cuts through a section of spindled cells, resulting in difficulty differentiating cutaneous angiosarcoma from other spindle tumors such as spindle cell melanoma or spindle cell squamous cell carcinoma.6 In such cases, immunohistochemistry may be helpful, as spindle cell melanoma would stain positive for S100 and SOX10 and spindle cell squamous cell carcinoma would stain positive for p63 and cytokeratin.
Various treatment strategies for cutaneous angiosarcoma have been employed, with the majority still resulting in poor outcomes.2,4-6 The recommended treatment is radical surgical excision of the primary tumor with lymph node clearance if possible. Following excision, the patient should undergo high-dose, wide-field radiotherapy to the region.5,8 Cutaneous angiosarcomas also have the ability to spread extensively through the dermis and can result in subclinical or clinically obvious widespread disease with multifocal or satellite lesions present. Distant metastases occur most frequently in the cervical lymph nodes and lungs.7 In cases where the disease is too extensive for surgery, palliative radiation monotherapy can be used.5,6
As atypical vascular lesions are considered to be a precursor to cutaneous angiosarcoma, it is important to note that the fish-in-the-creek feature was absent in all 6 of the atypical vascular lesions observed in the study. The differentiation generally is made based on MYC, which is present in cutaneous angiosarcomas and absent in atypical vascular lesions.10 The feature of fish in the creek may now be an additional clue for dermatopathologists to differentiate between angiosarcomas and other similar-appearing tumors.
Conclusion
Our study aimed to highlight an important histologic feature of cutaneous angiosarcomas that can aid in the diagnosis of this deceptive malignancy. Our findings warrant further study of the fish-in-the-creek histologic pattern in a larger sample size to determine its success as a diagnostic tool for cutaneous angiosarcomas. As noted previously, tumor grade does not impact survival outcome, but small tumor size has been one of the only features found to result in a more favorable prognosis.1,6,8 Future studies to identify a correlation between the histologic finding of fish in the creek and disease outcome in cutaneous angiosarcoma may be helpful to determine if these histologic findings provide prognostic significance in cases of cutaneous angiosarcoma.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Woodward AH, Ivins JC, Soule EH. Lymphangiosarcoma arising in chronic lymphedematous extremities. Cancer. 1972;30:562-572.
- Calonje E, Brenn T, McKee PH, et al. McKee’s Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier Saunders; 2012.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Rosai J, Sumner HW, Kostianovsky M, et al. Angiosarcoma of the skin: a clinicopathologic and fine structural study. Hum Pathol. 1976;7:83-109.
- Pawlik TM, Paulino AF, Mcginn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Haustein UF. Angiosarcoma of the face and scalp. Int J Dermatol. 1991;30:851-856.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. Edinburgh, Scotland: Saunders Elsevier; 2014.
- Requena L, Kutzner H. Cutaneous Soft Tissue Tumors. Philadelphia, PA: Wolters Kluwer; 2015.
- Cuda J, Mirzamani N, Kantipudi R, et al. Diagnostic utility of Fli-1 and D2-40 in distinguishing atypical fibroxanthoma from angiosarcoma. Am J Dermatopathol. 2013;35:316-318.
Cutaneous angiosarcoma is a rare malignant tumor of vascular endothelial cells that has the propensity to arise in various clinical settings. This tumor predominantly occurs in the head and neck region in elderly patients, but it also has been reported to develop postradiotherapy or in the setting of chronic lymphedema in the extremities.1-3 In all settings, the diagnosis carries a very poor prognosis with a high likelihood of local recurrence and rapid dissemination. The mortality rate typically is 80% or higher.2,4-6
Making the correct clinical diagnosis of cutaneous angiosarcoma may be difficult given the variety of patient symptoms and clinical appearances that can be demonstrated on presentation. Lesions can appear as bluish or violaceous plaques, macules, or nodules, and ulceration may be present in some advanced cases.5,7 Clinical misdiagnosis is common, as cutaneous angiosarcomas may be mistaken for infectious processes, benign vascular malformations, and other cutaneous malignancies.1 Biopsy often is delayed given the initial benign appearance of the lesions, and this frequently results in aggressive and extensive disease at the time of diagnosis, which is unfortunate given that small tumor size has been shown to be one of the only favorable prognostic indicators in cutaneous angiosarcoma.1,2,6,8
Microscopically, diagnosis of cutaneous angiosarcoma can present a challenge, as the histology varies between a well-differentiated vascular neoplasm and a considerably anaplastic and poorly differentiated malignancy. On low power, some areas may appear as benign hemangiomas with other areas showing frank sarcomatous features.9 As a result, these tumors can be mistaken for a variety of other diseases including melanomas, carcinomas, or other vascular tumors.6,8,9 Previously, electron microscopy has been utilized on undifferentiated tumors to help distinguish cutaneous angiosarcomas from other potential diagnoses. The atypical tumor cells of cutaneous angiosarcoma display common features of endothelial cells (eg, pinocytotic vesicles, tubulated bodies).7 Historically, it has been noted that the histologic findings and tumor grade provide little evidence regarding the aggressiveness of the tumor, and all cutaneous angiosarcoma diagnoses receive a poor prognosis.6,8
Classically, the histologic findings of cutaneous angiosarcoma include a highly infiltrative neoplasm forming irregular vascular channels that penetrate through the cutaneous soft tissues and frequently extend into the subcutaneous fat. The vascular spaces are lined by hyperchromatic endothelial cells with varying degrees of atypia.1,2,4,6,7,10 Occasionally, prominent endothelial cells lining a papillary structure within the lumen of the neoformed vessel may also be observed. Currently, immunohistochemical staining for MYC, Ki-67, D2-40, and various other markers complement the histologic findings to aid in the diagnosis of cutaneous angiosarcoma.11,12 An additional diagnostic clue that has been described in cases of postirradiation cutaneous angiosarcoma shows free-floating or tufted pleomorphic spindle cells within the vascular lumen (Figure). This finding has been described as “fish in the creek.”11 In this study, we aimed to determine the frequency and subsequent diagnostic utility of the fish-in-the-creek finding in cases of cutaneous angiosarcoma.

Methods
A natural language search of our institutional archives over a 20-year period (1997–2017) using the term angiosarcoma was performed. Fifteen cases of cutaneous angiosarcoma were identified. Fifteen additional benign and malignant vascular tumors with cutaneous angiosarco
Results
The histologic pattern of fish in the creek was identified in all 15 cases of cutaneous angiosarcoma and was absent in the other 15 malignancies examined in this study. This finding shows the potential for the fish-in-the-creek pattern to be used as an additional diagnostic tool for dermatopathologists.
Comment
Cutaneous angiosarcoma is a rare but aggressive malignancy that proves difficult to diagnose both clinically and histologically as well as to treat effectively.1,5-8 Our results indicate that fish in the creek may be a useful and salient histologic feature in cutaneous angiosarcoma. It is important to recognize, however, that this finding should not be the sole feature upon which a diagnosis of cutaneous angiosarcoma is made, as it requires corroboration with positivity of MYC and D2-40 as well as a high Ki-67 proliferation index (>20%).11,12 Finding a fish-in-the-creek pattern should prompt dermatopathologists to consider a diagnosis of cutaneous angiosarcoma in the appropriate clinical and histologic settings.
The chief limitation of this study was the small sample size, with only 15 cases of cutaneous angiosarcoma available in the last 20 years at our institution. The limited sample size did not allow us to make claims on sensitivity and specificity regarding this histologic feature; however, with a larger sample size, the true diagnostic potential could be elucidated. Although the pathologists were blinded to the original diagnoses as they examined it for fish in the creek, it is possible they were able to make the correct diagnosis based on other histopathologic clues and therefore were biased.
Although the fish-in-the-creek pattern is present in cutaneous angiosarcoma, there may be other mimickers to consider. Intraluminal papillary projections lined by endothelial cells may be sectioned in a manner imitating this finding.3 In such a case, these endothelial cells must be differentiated from the free-floating or tufted spindle cells in order to have a positive finding for fish in the creek. There can be confusion if the biopsy cuts through a section of spindled cells, resulting in difficulty differentiating cutaneous angiosarcoma from other spindle tumors such as spindle cell melanoma or spindle cell squamous cell carcinoma.6 In such cases, immunohistochemistry may be helpful, as spindle cell melanoma would stain positive for S100 and SOX10 and spindle cell squamous cell carcinoma would stain positive for p63 and cytokeratin.
Various treatment strategies for cutaneous angiosarcoma have been employed, with the majority still resulting in poor outcomes.2,4-6 The recommended treatment is radical surgical excision of the primary tumor with lymph node clearance if possible. Following excision, the patient should undergo high-dose, wide-field radiotherapy to the region.5,8 Cutaneous angiosarcomas also have the ability to spread extensively through the dermis and can result in subclinical or clinically obvious widespread disease with multifocal or satellite lesions present. Distant metastases occur most frequently in the cervical lymph nodes and lungs.7 In cases where the disease is too extensive for surgery, palliative radiation monotherapy can be used.5,6
As atypical vascular lesions are considered to be a precursor to cutaneous angiosarcoma, it is important to note that the fish-in-the-creek feature was absent in all 6 of the atypical vascular lesions observed in the study. The differentiation generally is made based on MYC, which is present in cutaneous angiosarcomas and absent in atypical vascular lesions.10 The feature of fish in the creek may now be an additional clue for dermatopathologists to differentiate between angiosarcomas and other similar-appearing tumors.
Conclusion
Our study aimed to highlight an important histologic feature of cutaneous angiosarcomas that can aid in the diagnosis of this deceptive malignancy. Our findings warrant further study of the fish-in-the-creek histologic pattern in a larger sample size to determine its success as a diagnostic tool for cutaneous angiosarcomas. As noted previously, tumor grade does not impact survival outcome, but small tumor size has been one of the only features found to result in a more favorable prognosis.1,6,8 Future studies to identify a correlation between the histologic finding of fish in the creek and disease outcome in cutaneous angiosarcoma may be helpful to determine if these histologic findings provide prognostic significance in cases of cutaneous angiosarcoma.
Cutaneous angiosarcoma is a rare malignant tumor of vascular endothelial cells that has the propensity to arise in various clinical settings. This tumor predominantly occurs in the head and neck region in elderly patients, but it also has been reported to develop postradiotherapy or in the setting of chronic lymphedema in the extremities.1-3 In all settings, the diagnosis carries a very poor prognosis with a high likelihood of local recurrence and rapid dissemination. The mortality rate typically is 80% or higher.2,4-6
Making the correct clinical diagnosis of cutaneous angiosarcoma may be difficult given the variety of patient symptoms and clinical appearances that can be demonstrated on presentation. Lesions can appear as bluish or violaceous plaques, macules, or nodules, and ulceration may be present in some advanced cases.5,7 Clinical misdiagnosis is common, as cutaneous angiosarcomas may be mistaken for infectious processes, benign vascular malformations, and other cutaneous malignancies.1 Biopsy often is delayed given the initial benign appearance of the lesions, and this frequently results in aggressive and extensive disease at the time of diagnosis, which is unfortunate given that small tumor size has been shown to be one of the only favorable prognostic indicators in cutaneous angiosarcoma.1,2,6,8
Microscopically, diagnosis of cutaneous angiosarcoma can present a challenge, as the histology varies between a well-differentiated vascular neoplasm and a considerably anaplastic and poorly differentiated malignancy. On low power, some areas may appear as benign hemangiomas with other areas showing frank sarcomatous features.9 As a result, these tumors can be mistaken for a variety of other diseases including melanomas, carcinomas, or other vascular tumors.6,8,9 Previously, electron microscopy has been utilized on undifferentiated tumors to help distinguish cutaneous angiosarcomas from other potential diagnoses. The atypical tumor cells of cutaneous angiosarcoma display common features of endothelial cells (eg, pinocytotic vesicles, tubulated bodies).7 Historically, it has been noted that the histologic findings and tumor grade provide little evidence regarding the aggressiveness of the tumor, and all cutaneous angiosarcoma diagnoses receive a poor prognosis.6,8
Classically, the histologic findings of cutaneous angiosarcoma include a highly infiltrative neoplasm forming irregular vascular channels that penetrate through the cutaneous soft tissues and frequently extend into the subcutaneous fat. The vascular spaces are lined by hyperchromatic endothelial cells with varying degrees of atypia.1,2,4,6,7,10 Occasionally, prominent endothelial cells lining a papillary structure within the lumen of the neoformed vessel may also be observed. Currently, immunohistochemical staining for MYC, Ki-67, D2-40, and various other markers complement the histologic findings to aid in the diagnosis of cutaneous angiosarcoma.11,12 An additional diagnostic clue that has been described in cases of postirradiation cutaneous angiosarcoma shows free-floating or tufted pleomorphic spindle cells within the vascular lumen (Figure). This finding has been described as “fish in the creek.”11 In this study, we aimed to determine the frequency and subsequent diagnostic utility of the fish-in-the-creek finding in cases of cutaneous angiosarcoma.

Methods
A natural language search of our institutional archives over a 20-year period (1997–2017) using the term angiosarcoma was performed. Fifteen cases of cutaneous angiosarcoma were identified. Fifteen additional benign and malignant vascular tumors with cutaneous angiosarco
Results
The histologic pattern of fish in the creek was identified in all 15 cases of cutaneous angiosarcoma and was absent in the other 15 malignancies examined in this study. This finding shows the potential for the fish-in-the-creek pattern to be used as an additional diagnostic tool for dermatopathologists.
Comment
Cutaneous angiosarcoma is a rare but aggressive malignancy that proves difficult to diagnose both clinically and histologically as well as to treat effectively.1,5-8 Our results indicate that fish in the creek may be a useful and salient histologic feature in cutaneous angiosarcoma. It is important to recognize, however, that this finding should not be the sole feature upon which a diagnosis of cutaneous angiosarcoma is made, as it requires corroboration with positivity of MYC and D2-40 as well as a high Ki-67 proliferation index (>20%).11,12 Finding a fish-in-the-creek pattern should prompt dermatopathologists to consider a diagnosis of cutaneous angiosarcoma in the appropriate clinical and histologic settings.
The chief limitation of this study was the small sample size, with only 15 cases of cutaneous angiosarcoma available in the last 20 years at our institution. The limited sample size did not allow us to make claims on sensitivity and specificity regarding this histologic feature; however, with a larger sample size, the true diagnostic potential could be elucidated. Although the pathologists were blinded to the original diagnoses as they examined it for fish in the creek, it is possible they were able to make the correct diagnosis based on other histopathologic clues and therefore were biased.
Although the fish-in-the-creek pattern is present in cutaneous angiosarcoma, there may be other mimickers to consider. Intraluminal papillary projections lined by endothelial cells may be sectioned in a manner imitating this finding.3 In such a case, these endothelial cells must be differentiated from the free-floating or tufted spindle cells in order to have a positive finding for fish in the creek. There can be confusion if the biopsy cuts through a section of spindled cells, resulting in difficulty differentiating cutaneous angiosarcoma from other spindle tumors such as spindle cell melanoma or spindle cell squamous cell carcinoma.6 In such cases, immunohistochemistry may be helpful, as spindle cell melanoma would stain positive for S100 and SOX10 and spindle cell squamous cell carcinoma would stain positive for p63 and cytokeratin.
Various treatment strategies for cutaneous angiosarcoma have been employed, with the majority still resulting in poor outcomes.2,4-6 The recommended treatment is radical surgical excision of the primary tumor with lymph node clearance if possible. Following excision, the patient should undergo high-dose, wide-field radiotherapy to the region.5,8 Cutaneous angiosarcomas also have the ability to spread extensively through the dermis and can result in subclinical or clinically obvious widespread disease with multifocal or satellite lesions present. Distant metastases occur most frequently in the cervical lymph nodes and lungs.7 In cases where the disease is too extensive for surgery, palliative radiation monotherapy can be used.5,6
As atypical vascular lesions are considered to be a precursor to cutaneous angiosarcoma, it is important to note that the fish-in-the-creek feature was absent in all 6 of the atypical vascular lesions observed in the study. The differentiation generally is made based on MYC, which is present in cutaneous angiosarcomas and absent in atypical vascular lesions.10 The feature of fish in the creek may now be an additional clue for dermatopathologists to differentiate between angiosarcomas and other similar-appearing tumors.
Conclusion
Our study aimed to highlight an important histologic feature of cutaneous angiosarcomas that can aid in the diagnosis of this deceptive malignancy. Our findings warrant further study of the fish-in-the-creek histologic pattern in a larger sample size to determine its success as a diagnostic tool for cutaneous angiosarcomas. As noted previously, tumor grade does not impact survival outcome, but small tumor size has been one of the only features found to result in a more favorable prognosis.1,6,8 Future studies to identify a correlation between the histologic finding of fish in the creek and disease outcome in cutaneous angiosarcoma may be helpful to determine if these histologic findings provide prognostic significance in cases of cutaneous angiosarcoma.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Woodward AH, Ivins JC, Soule EH. Lymphangiosarcoma arising in chronic lymphedematous extremities. Cancer. 1972;30:562-572.
- Calonje E, Brenn T, McKee PH, et al. McKee’s Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier Saunders; 2012.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Rosai J, Sumner HW, Kostianovsky M, et al. Angiosarcoma of the skin: a clinicopathologic and fine structural study. Hum Pathol. 1976;7:83-109.
- Pawlik TM, Paulino AF, Mcginn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Haustein UF. Angiosarcoma of the face and scalp. Int J Dermatol. 1991;30:851-856.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. Edinburgh, Scotland: Saunders Elsevier; 2014.
- Requena L, Kutzner H. Cutaneous Soft Tissue Tumors. Philadelphia, PA: Wolters Kluwer; 2015.
- Cuda J, Mirzamani N, Kantipudi R, et al. Diagnostic utility of Fli-1 and D2-40 in distinguishing atypical fibroxanthoma from angiosarcoma. Am J Dermatopathol. 2013;35:316-318.
- Aust MR, Olsen KD, Lewis JE, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Ann Otol Rhinol Laryngol. 1997;106:943-951.
- Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59:1046-1057.
- Woodward AH, Ivins JC, Soule EH. Lymphangiosarcoma arising in chronic lymphedematous extremities. Cancer. 1972;30:562-572.
- Calonje E, Brenn T, McKee PH, et al. McKee’s Pathology of the Skin. 4th ed. Edinburgh, Scotland: Elsevier Saunders; 2012.
- Morrison WH, Byers RM, Garden AS, et al. Cutaneous angiosarcoma of the head and neck. a therapeutic dilemma. Cancer. 1995;76:319-327.
- Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44:1106-1113.
- Rosai J, Sumner HW, Kostianovsky M, et al. Angiosarcoma of the skin: a clinicopathologic and fine structural study. Hum Pathol. 1976;7:83-109.
- Pawlik TM, Paulino AF, Mcginn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98:1716-1726.
- Haustein UF. Angiosarcoma of the face and scalp. Int J Dermatol. 1991;30:851-856.
- Elston DM, Ferringer T, Ko C, et al. Dermatopathology. 2nd ed. Edinburgh, Scotland: Saunders Elsevier; 2014.
- Requena L, Kutzner H. Cutaneous Soft Tissue Tumors. Philadelphia, PA: Wolters Kluwer; 2015.
- Cuda J, Mirzamani N, Kantipudi R, et al. Diagnostic utility of Fli-1 and D2-40 in distinguishing atypical fibroxanthoma from angiosarcoma. Am J Dermatopathol. 2013;35:316-318.
Practice Points
- The histologic finding of “fish in the creek” is characterized by free-floating or tufted pleomorphic spindle cells within the vascular lumen.
- Fish in the creek has only been demonstrated in cutaneous angiosarcoma when compared to histologic findings of other similar vascular malignancies.
- The fish-in-the-creek finding may be an additional diagnostic tool in cases of cutaneous angiosarcoma.
Getting the hypertension Dx right: Patient positioning matters
ABSTRACT
Purpose This study evaluated the effect of patient positioning on the diagnosis of hypertension in a clinic setting and the importance of following guidelines for measuring blood pressure (BP).
Methods In the trial part of this study, we recorded BP measurements by an aneroid sphygmomanometer with patients seated first on an examination table, a commonly observed practice, and second in the standard seated position as defined by the American Heart Association. Two measurements were obtained in each position for 204 patients, and we determined the difference between the average readings in the 2 positions. Factored into the comparison was an estimation of inherent variance of the device and observer achieved by repeated measurements on a healthy individual.
Results This investigation included an initial observational study of 25 regional primary care offices, the results of which showed frequent lack of adherence with accepted guidelines in patient positioning during BP measurement. The overall systolic and diastolic BPs were more than 2 mm Hg lower in the standard seated position compared with the examination table position (P<.001). Noncompliance with the position guideline resulted in misclassification of 15 patients (7.4%) as prehypertensive, when, in fact, they were normotensive. Misclassification of hypertension occurred in 12 patients (5.9%), when, in fact, they were normotensive. Logistic regression using relevant clinical factors did not identify those individuals who were misclassified.
Conclusion This study underscores the importance of patient positioning on BP determinations in order to accurately diagnose hypertension.
The high prevalence of hypertension and its burden of disease in the United States and worldwide are well known.1 Hypertension is a major risk factor for coronary heart disease, congestive heart failure, ischemic and hemorrhagic stroke, chronic kidney disease, and peripheral arterial disease.2 Among all risk factors, hypertension ranked first worldwide in disability-adjusted life-years.3 However, misclassification of an individual’s blood pressure (BP) as prehypertension or hypertension also confers significant health and financial burdens due to unnecessary medical encounters, testing, and treatment, and to increased cost of insurance coverage and out-of-pocket expenses. A correct assessment of BP in the outpatient setting depends on accurate measurement technique.
The diagnosis of hypertension is based on indirect measurement of BP using in-office, ambulatory, or home monitoring. Although office BP measurement is less than ideal, it is used most often to diagnose and monitor hypertension. Furthermore, most published trials of treatment recommendations are based on office BP measurements.4
Automated oscillometric and aneroid sphygmomanometers are common BP measurement devices. Proper technique is particularly important with the aneroid sphygmomanometer to obtain consistent and accurate results.5 Good training and an ability to hear the Korotkoff sounds are crucial.
Expert consensus groups such as the American Heart Association (AHA) publish recommendations for proper technique in reliably measuring BP,6-8 and they emphasize the importance of patient positioning during BP measurement. The individual should be seated comfortably in a chair with both arms and back supported, legs uncrossed, and feet flat on the floor. We’ll refer to this as the “standard position.” Although the proper technique for measuring BP has been widely advocated, a recent literature review for the US Preventive Services Task Force concluded that surprisingly few studies are available on the diagnostic accuracy of office BP practices.9
One paper evaluated the effect of leg crossing on accuracy of BP measurement. No subjects were reclassified as hypertensive, but the study lacked statistical rigor.10 Another study found variable BP readings regardless of body position.11
The purpose of our study was to compare BP measurement in 2 positions: the standard position described above, and the examination table position in which the patient is seated on the edge of the table with back, arms, and feet unsupported.
METHODS
We conducted our literature search across several scientific and medical literature databases, including PubMed, ScienceDirect, and CINAHL. Only English-language articles were reviewed.
We followed the BP measurement guidelines of the AHA. Prior to beginning the study, we provided instructions in proper BP measurement technique to the nurses who would obtain the data. The minimum sample size of patients needed to identify a difference of at least 2 mm Hg was 26, as estimated by power analysis. This was calculated using an alpha of .05 and a beta of .13.
The study population consisted of patients presenting consecutively to a teaching family medicine center. Adult patients, ages 18 and older, were informed about the study and invited to participate. Those who agreed were asked to read and sign an informed consent approved by a regional institutional review board for human subjects. We excluded patients who declined participation for any reason, who were in severe pain or distress that may have prevented them from completing the protocol, or who had limited mobility that could interfere with climbing onto the examination table. Patients considered for the study totaled 250, 28 of whom were ineligible. Another 18 patients declined participation, leaving 204 who completed the protocol.
Before testing began, we estimated the standard deviation of each aneroid sphygmomanometer and the assigned observer by repeatedly measuring the BP of a healthy normotensive individual sitting in the standard position. We obtained 46 measurements over 2 days to avoid subject and operator fatigue. Standard deviation for systolic BP was 3.6 mm Hg; for diastolic it was 3.8 mm Hg.
During testing, nurses recorded BP for each patient twice in the examination table position and twice in the standard position. They entered data into an Excel workbook for subsequent analysis. All examination rooms were equipped with newly purchased aneroid sphygmomanometers, and the appropriate cuff size was selected for each patient. Patients were instructed to remain quiet during the measurements. Patients sat first on the edge of the examination table. After a 5-minute rest, BP was measured twice in the same arm. Measurements were separated by 1 to 2 minutes. Patients then sat in the chair and rested another 2 minutes before BP was again measured twice in the same arm. The arms and back were supported in the chair and the stethoscope placed at heart level.
As per protocol, we obtained 4 BP readings on each patient and calculated the difference between the average systolic and diastolic BP values from the 2 positions. The standard error of the mean of this difference was determined using the equation, where Sd is the standard deviation of the aneroid sphygmomanometer and observer.12 A one-sided, 95% confidence upper bound for the standard error of the difference is 1.65 × SEd. We compared patient-specific differences against this upper bound to identify significant systolic and diastolic BP changes due to positioning. If the patient’s BP difference exceeded the upper bound, it was attributed to the positional change and not to variation inherent to the sphygmomanometer and observer.
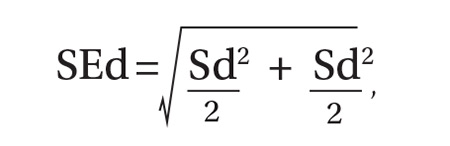
As an example, consider a patient whose average systolic BP readings from the examination-table and standard positions, respectively, were 128 mm Hg and 120 mm Hg. Assuming an SEd of 3.55 and an upper bound of 5.86, the observed 8 mm Hg difference in average systolic BPs would be considered significant. The amount of random variation from the sphygmomanometer and observer would not be expected to exceed 5.86 mm Hg.
In accordance with accepted standards, prehypertension was defined as a BP between 120-139/80-89 mm Hg, and hypertension was defined as a BP ≥140/90 mm Hg.4 BP below 120/80 mm Hg was considered normal. We calculated each patient’s average systolic and diastolic BP values in the 2 positions and thereby classified the individual as normotensive, prehypertensive, or hypertensive. We regarded as misclassified any patient whose BP showed significant lowering between the examination-table and standard positions resulting in a change of classification from prehypertensive or hypertensive to normotensive. For example, a patient with an examination-table position average reading of 126/85 mm Hg and a standard position average reading of 118/78 mm Hg would have been misclassified as prehypertensive.
We reviewed charts and gathered data, including subject age, sex, obesity (defined as a body mass index of ≥30 kg/m2), and history of diabetes, hypertension, or smoking. Other than age, all data were binary. We performed logistic regression analysis using the Excel Add-in Real Statistics Resource Pack software (Release 4.3)13 to determine if these factors could predict significant lowering of BP due to positional change.
Our associated observational study. We also conducted a separate observational study of 25 regional primary care offices to evaluate compliance with the AHA guidelines for measuring BP. The office nurses taking measurements were not informed of the study’s purpose to prevent deviation from their common practice.
Data on 9 guideline criteria were collected to assess supervision of patients before and during measurements, including having the patient sit in a chair in quiet and comfortable surroundings with arms and back supported and feet on the ground. We also noted the type of BP measuring device used. Additionally, observers assessed the technique of the individuals using a manual device, including cuff placement and deflation rate. The observations were conducted during a clinic visit by a medical student knowledgeable in the AHA guidelines for measuring BP by automated oscillometric or aneroid sphygmomanometric devices. We conducted the study over a 2-week period in the second quarter of 2016.
RESULTS
Power analysis performed prior to the study showed that a minimum of 26 patients would be needed to predict a 2 mm Hg difference between BPs obtained in the 2 positions. Of the 204 patients used in the logistic regression analysis, 78 were men and 126 were women. Ages ranged from 18 to 101 years, yielding a mean of 54. One-hundred sixteen had previously received a diagnosis of hypertension, 39 had diabetes, 92 were obese, 22 were current smokers, and 68 were former smokers.
TABLE 1 shows the means and ranges of systolic and diastolic BP for both study positions. With this study population, mean BP recorded in the examination-table position decreased in the standard position by 2.1 and 2.2 mm Hg for systolic and diastolic BP, respectively (P<.001).
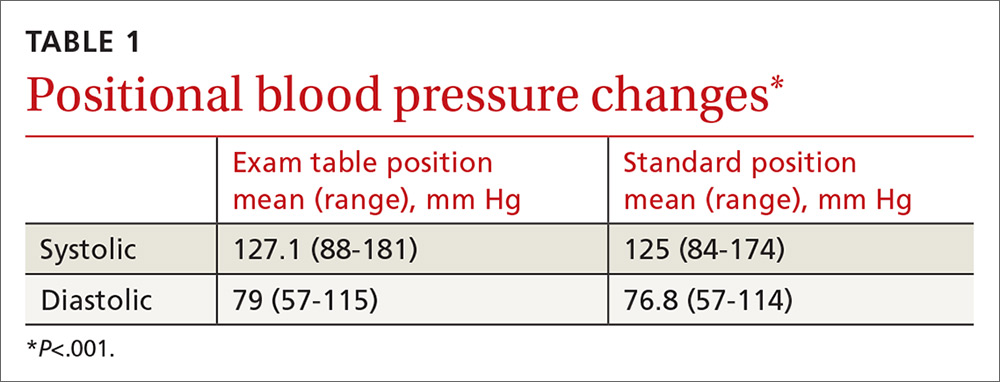
Significant BP lowering—as defined by a one-sided 95% confidence upper bound for the standard error of differences between study positions—was determined to be 5.86 and 6.22 mm Hg for systolic and diastolic pressures, respectively. Significant lowering of BP and misclassification due to positioning are summarized in TABLE 2. Significant lowering of mean systolic or diastolic BP with positional change from table to chair occurred in 62 subjects (30.4%). Misclassification of prehypertension occurred in 7.4% of subjects, and misclassification of hypertension occurred in 5.9%.
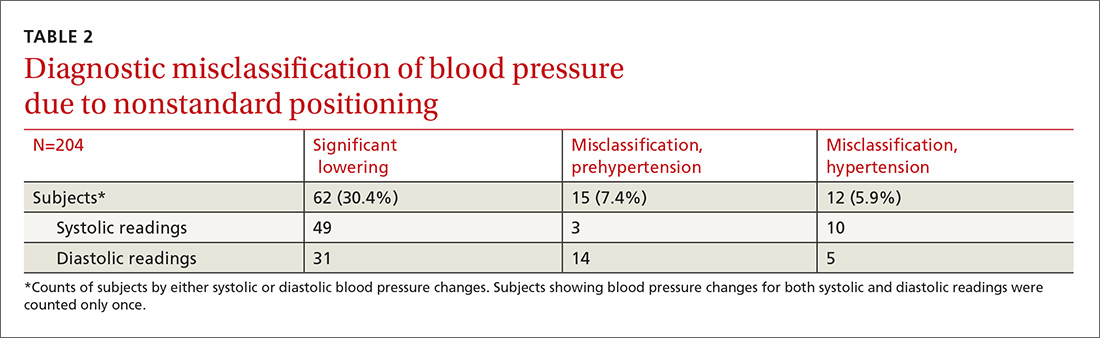
Logistic regression using patient age, sex, obesity, and history of diabetes, hypertension, and smoking as independent factors did not predict significant BP lowering with positional change.
Our observational study revealed that proper positioning in a chair was followed in only 10 of the 25 offices. In the remaining offices, patients were seated on the examination table. A 5-minute rest period before measuring BP was allowed in only 10 of the 25 offices. An automated oscillometric device was used in only 2 of the 25 offices.
DISCUSSION
In this study, 27 subjects (13.2%) were misclassified as prehypertensive or hypertensive as a result of deviating from the standard position in obtaining BP. Although the standard position is universally recommended, the guideline is not always followed in clinical practice.14
One study by Villegas et al found that 60% of physicians and nurses working in a major hospital were measuring BP inaccurately.15 In our initial observational study, 60% of primary care practices visited did not adhere to the recommended patient positioning. These medical offices are located in the community surrounding our facility and are operated by the same health care organization. The misclassification of prehypertension and hypertension observed in our prospective comparison of BP recordings in table and chair positions is, therefore, likely to occur to some degree at these practices, as well.
Similar diagnostic misclassifications have been reported in other medical settings. In a published survey of 114 medical offices, McKay and coworkers noted frequent inconsistencies with published guidelines in measuring BP.16
Common clinical demographic data obtained during this study showed no association with the positional BP change. Increased muscle tension due to lack of body support while sitting on the edge of the examination table could be the cause of elevated BP for this subgroup of individuals. Measuring muscle tension of the arms and back while seated on an exam table and chair was beyond the scope of this study.
In clinical practice, different types of BP measuring devices are used. Calibration and quality control of these devices is often lacking.17 Before starting our study, we determined the statistical variance of the aneroid sphygmomanometers and found it to approximate the manufacturer’s precision specification. Guidelines recommend using the mean of 2 BP readings as representing the patient’s BP for a given clinic visit. Additional readings are recommended if there is more than a 5 mm Hg difference between the initial 2 readings.4
In our study, we used sampling statistics of the BP readings and clinical guideline BP ranges in making diagnostic determinations. The inability to identify those patients whose BP will be affected by positional change highlights the importance of following standard BP measurement guidelines for all patients.
Study limitations. Positional change in BP from examination table to chair lacks a comparison to BP changes in positioning from chair to table. If similar BP changes in the reverse sequence were to be observed, this would add support to the hypothesis that muscle tension of the unsupported body is a cause of BP elevation in certain individuals. We believe, however, that the sequence of BP measurements (from table to chair) did not have a significant impact because all patients were allowed to rest in each position before the BP was measured. The BP was therefore measured in a steady-state in both positions.
Additionally, BP measurement by aneroid sphygmomanometry is highly dependent on observer skill and hearing ability. Furthermore, a disproportionate number of BP measurements recorded in the study ended in zero, suggesting terminal digit bias by the observer. These sources of error may be avoided using an automated oscillometric measuring device.18 Automated devices also allow for repeated independent measurements that minimize the white-coat effect. However, there are also limitations to the accuracy of oscillometric equipment. This is especially true when recording BP in the elderly, a group whose stiff arterial walls may cause erroneous measurements.19
Guideline justification. Nonadherence to standard positioning when measuring BP leads to certain individuals being misclassified as prehypertensive or hypertensive. Misclassification in turn leads to unnecessary medical encounters, testing, and treatment. Misdiagnosis is also likely to increase the cost of an individual’s insurance coverage and out-of-pocket health care expenses.
CORRESPONDENCE
Roy N. Morcos, MD, St. Elizabeth Family Medicine Residency Program, 8423 Market Street, Suite 101, Boardman, Ohio 44512; [email protected].
1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217-223.
2. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224-2260.
3. Murray CJ, Lopez AD. Measuring the global burden of disease. New Engl J Med. 2013;369:448-457.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
5. Bailey RH, Bauer JH. A review of common errors in the indirect measurement of blood pressure. Sphygmomanometry. Arch Intern Med. 1993;153:2741-2748.
6. Padwal RS, Hemmelgarn BR, McAlister FA, et al. The 2007 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1- blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2007;23:529-538.
7. Campbell NR, Chockalingam A, Fodor JG, et al. Accurate, reproducible measurement of blood pressure. CMAJ. 1990;143:19-24.
8. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens. 2005;7:102-109.
9. Piper MA, Evans CV, Burda BU, et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192-204.
10. Peters GL, Binder SK, Campbell NR. The effect of crossing legs on blood pressure: a randomized single-blind cross-over study. Blood Press Monit. 1999;4:97-101.
11. Cicolini G, Pizzi C, Palma E, et al. Differences in blood pressure by body position (supine, Fowler’s, and sitting) in hypertensive subjects. Am J Hypertens. 2011;24:1073-1079.
12. Daniel WW, Cross CL. Biostatistics: A Foundation for Analysis in the Health Sciences (10th Edition). Hoboken, NJ: John Wiley & Sons; 2013.
13. Zaiontz C. Real statistics using Excel. Available at: http://www.real-statistics.com/. Accessed February 20, 2018.
14. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
15. Villegas I, Arias IC, Botero A, et al. Evaluation of the technique used by health-care workers for taking blood pressure. Hypertension. 1995;26:1204-1206.
16. McKay DW, Campbell NR, Parab LS, et al. Clinical assessment of blood pressure. J Hum Hypertens. 1990;4:639-645.
17. Jones DW, Appel LJ, Sheps SG, et al. Measuring blood pressure accurately: new and persistent challenges. JAMA. 2003;289:1027-1030.
18. Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Can J Cardiol. 2016;32:569-588.
19. Raamat R, Talts J, Jagomägi K, et al. Errors of oscillometric blood pressure measurement as predicted by simulation. Blood Press Monit. 2011;16:238-245.
ABSTRACT
Purpose This study evaluated the effect of patient positioning on the diagnosis of hypertension in a clinic setting and the importance of following guidelines for measuring blood pressure (BP).
Methods In the trial part of this study, we recorded BP measurements by an aneroid sphygmomanometer with patients seated first on an examination table, a commonly observed practice, and second in the standard seated position as defined by the American Heart Association. Two measurements were obtained in each position for 204 patients, and we determined the difference between the average readings in the 2 positions. Factored into the comparison was an estimation of inherent variance of the device and observer achieved by repeated measurements on a healthy individual.
Results This investigation included an initial observational study of 25 regional primary care offices, the results of which showed frequent lack of adherence with accepted guidelines in patient positioning during BP measurement. The overall systolic and diastolic BPs were more than 2 mm Hg lower in the standard seated position compared with the examination table position (P<.001). Noncompliance with the position guideline resulted in misclassification of 15 patients (7.4%) as prehypertensive, when, in fact, they were normotensive. Misclassification of hypertension occurred in 12 patients (5.9%), when, in fact, they were normotensive. Logistic regression using relevant clinical factors did not identify those individuals who were misclassified.
Conclusion This study underscores the importance of patient positioning on BP determinations in order to accurately diagnose hypertension.
The high prevalence of hypertension and its burden of disease in the United States and worldwide are well known.1 Hypertension is a major risk factor for coronary heart disease, congestive heart failure, ischemic and hemorrhagic stroke, chronic kidney disease, and peripheral arterial disease.2 Among all risk factors, hypertension ranked first worldwide in disability-adjusted life-years.3 However, misclassification of an individual’s blood pressure (BP) as prehypertension or hypertension also confers significant health and financial burdens due to unnecessary medical encounters, testing, and treatment, and to increased cost of insurance coverage and out-of-pocket expenses. A correct assessment of BP in the outpatient setting depends on accurate measurement technique.
The diagnosis of hypertension is based on indirect measurement of BP using in-office, ambulatory, or home monitoring. Although office BP measurement is less than ideal, it is used most often to diagnose and monitor hypertension. Furthermore, most published trials of treatment recommendations are based on office BP measurements.4
Automated oscillometric and aneroid sphygmomanometers are common BP measurement devices. Proper technique is particularly important with the aneroid sphygmomanometer to obtain consistent and accurate results.5 Good training and an ability to hear the Korotkoff sounds are crucial.
Expert consensus groups such as the American Heart Association (AHA) publish recommendations for proper technique in reliably measuring BP,6-8 and they emphasize the importance of patient positioning during BP measurement. The individual should be seated comfortably in a chair with both arms and back supported, legs uncrossed, and feet flat on the floor. We’ll refer to this as the “standard position.” Although the proper technique for measuring BP has been widely advocated, a recent literature review for the US Preventive Services Task Force concluded that surprisingly few studies are available on the diagnostic accuracy of office BP practices.9
One paper evaluated the effect of leg crossing on accuracy of BP measurement. No subjects were reclassified as hypertensive, but the study lacked statistical rigor.10 Another study found variable BP readings regardless of body position.11
The purpose of our study was to compare BP measurement in 2 positions: the standard position described above, and the examination table position in which the patient is seated on the edge of the table with back, arms, and feet unsupported.
METHODS
We conducted our literature search across several scientific and medical literature databases, including PubMed, ScienceDirect, and CINAHL. Only English-language articles were reviewed.
We followed the BP measurement guidelines of the AHA. Prior to beginning the study, we provided instructions in proper BP measurement technique to the nurses who would obtain the data. The minimum sample size of patients needed to identify a difference of at least 2 mm Hg was 26, as estimated by power analysis. This was calculated using an alpha of .05 and a beta of .13.
The study population consisted of patients presenting consecutively to a teaching family medicine center. Adult patients, ages 18 and older, were informed about the study and invited to participate. Those who agreed were asked to read and sign an informed consent approved by a regional institutional review board for human subjects. We excluded patients who declined participation for any reason, who were in severe pain or distress that may have prevented them from completing the protocol, or who had limited mobility that could interfere with climbing onto the examination table. Patients considered for the study totaled 250, 28 of whom were ineligible. Another 18 patients declined participation, leaving 204 who completed the protocol.
Before testing began, we estimated the standard deviation of each aneroid sphygmomanometer and the assigned observer by repeatedly measuring the BP of a healthy normotensive individual sitting in the standard position. We obtained 46 measurements over 2 days to avoid subject and operator fatigue. Standard deviation for systolic BP was 3.6 mm Hg; for diastolic it was 3.8 mm Hg.
During testing, nurses recorded BP for each patient twice in the examination table position and twice in the standard position. They entered data into an Excel workbook for subsequent analysis. All examination rooms were equipped with newly purchased aneroid sphygmomanometers, and the appropriate cuff size was selected for each patient. Patients were instructed to remain quiet during the measurements. Patients sat first on the edge of the examination table. After a 5-minute rest, BP was measured twice in the same arm. Measurements were separated by 1 to 2 minutes. Patients then sat in the chair and rested another 2 minutes before BP was again measured twice in the same arm. The arms and back were supported in the chair and the stethoscope placed at heart level.
As per protocol, we obtained 4 BP readings on each patient and calculated the difference between the average systolic and diastolic BP values from the 2 positions. The standard error of the mean of this difference was determined using the equation, where Sd is the standard deviation of the aneroid sphygmomanometer and observer.12 A one-sided, 95% confidence upper bound for the standard error of the difference is 1.65 × SEd. We compared patient-specific differences against this upper bound to identify significant systolic and diastolic BP changes due to positioning. If the patient’s BP difference exceeded the upper bound, it was attributed to the positional change and not to variation inherent to the sphygmomanometer and observer.
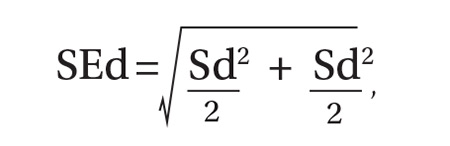
As an example, consider a patient whose average systolic BP readings from the examination-table and standard positions, respectively, were 128 mm Hg and 120 mm Hg. Assuming an SEd of 3.55 and an upper bound of 5.86, the observed 8 mm Hg difference in average systolic BPs would be considered significant. The amount of random variation from the sphygmomanometer and observer would not be expected to exceed 5.86 mm Hg.
In accordance with accepted standards, prehypertension was defined as a BP between 120-139/80-89 mm Hg, and hypertension was defined as a BP ≥140/90 mm Hg.4 BP below 120/80 mm Hg was considered normal. We calculated each patient’s average systolic and diastolic BP values in the 2 positions and thereby classified the individual as normotensive, prehypertensive, or hypertensive. We regarded as misclassified any patient whose BP showed significant lowering between the examination-table and standard positions resulting in a change of classification from prehypertensive or hypertensive to normotensive. For example, a patient with an examination-table position average reading of 126/85 mm Hg and a standard position average reading of 118/78 mm Hg would have been misclassified as prehypertensive.
We reviewed charts and gathered data, including subject age, sex, obesity (defined as a body mass index of ≥30 kg/m2), and history of diabetes, hypertension, or smoking. Other than age, all data were binary. We performed logistic regression analysis using the Excel Add-in Real Statistics Resource Pack software (Release 4.3)13 to determine if these factors could predict significant lowering of BP due to positional change.
Our associated observational study. We also conducted a separate observational study of 25 regional primary care offices to evaluate compliance with the AHA guidelines for measuring BP. The office nurses taking measurements were not informed of the study’s purpose to prevent deviation from their common practice.
Data on 9 guideline criteria were collected to assess supervision of patients before and during measurements, including having the patient sit in a chair in quiet and comfortable surroundings with arms and back supported and feet on the ground. We also noted the type of BP measuring device used. Additionally, observers assessed the technique of the individuals using a manual device, including cuff placement and deflation rate. The observations were conducted during a clinic visit by a medical student knowledgeable in the AHA guidelines for measuring BP by automated oscillometric or aneroid sphygmomanometric devices. We conducted the study over a 2-week period in the second quarter of 2016.
RESULTS
Power analysis performed prior to the study showed that a minimum of 26 patients would be needed to predict a 2 mm Hg difference between BPs obtained in the 2 positions. Of the 204 patients used in the logistic regression analysis, 78 were men and 126 were women. Ages ranged from 18 to 101 years, yielding a mean of 54. One-hundred sixteen had previously received a diagnosis of hypertension, 39 had diabetes, 92 were obese, 22 were current smokers, and 68 were former smokers.
TABLE 1 shows the means and ranges of systolic and diastolic BP for both study positions. With this study population, mean BP recorded in the examination-table position decreased in the standard position by 2.1 and 2.2 mm Hg for systolic and diastolic BP, respectively (P<.001).

Significant BP lowering—as defined by a one-sided 95% confidence upper bound for the standard error of differences between study positions—was determined to be 5.86 and 6.22 mm Hg for systolic and diastolic pressures, respectively. Significant lowering of BP and misclassification due to positioning are summarized in TABLE 2. Significant lowering of mean systolic or diastolic BP with positional change from table to chair occurred in 62 subjects (30.4%). Misclassification of prehypertension occurred in 7.4% of subjects, and misclassification of hypertension occurred in 5.9%.

Logistic regression using patient age, sex, obesity, and history of diabetes, hypertension, and smoking as independent factors did not predict significant BP lowering with positional change.
Our observational study revealed that proper positioning in a chair was followed in only 10 of the 25 offices. In the remaining offices, patients were seated on the examination table. A 5-minute rest period before measuring BP was allowed in only 10 of the 25 offices. An automated oscillometric device was used in only 2 of the 25 offices.
DISCUSSION
In this study, 27 subjects (13.2%) were misclassified as prehypertensive or hypertensive as a result of deviating from the standard position in obtaining BP. Although the standard position is universally recommended, the guideline is not always followed in clinical practice.14
One study by Villegas et al found that 60% of physicians and nurses working in a major hospital were measuring BP inaccurately.15 In our initial observational study, 60% of primary care practices visited did not adhere to the recommended patient positioning. These medical offices are located in the community surrounding our facility and are operated by the same health care organization. The misclassification of prehypertension and hypertension observed in our prospective comparison of BP recordings in table and chair positions is, therefore, likely to occur to some degree at these practices, as well.
Similar diagnostic misclassifications have been reported in other medical settings. In a published survey of 114 medical offices, McKay and coworkers noted frequent inconsistencies with published guidelines in measuring BP.16
Common clinical demographic data obtained during this study showed no association with the positional BP change. Increased muscle tension due to lack of body support while sitting on the edge of the examination table could be the cause of elevated BP for this subgroup of individuals. Measuring muscle tension of the arms and back while seated on an exam table and chair was beyond the scope of this study.
In clinical practice, different types of BP measuring devices are used. Calibration and quality control of these devices is often lacking.17 Before starting our study, we determined the statistical variance of the aneroid sphygmomanometers and found it to approximate the manufacturer’s precision specification. Guidelines recommend using the mean of 2 BP readings as representing the patient’s BP for a given clinic visit. Additional readings are recommended if there is more than a 5 mm Hg difference between the initial 2 readings.4
In our study, we used sampling statistics of the BP readings and clinical guideline BP ranges in making diagnostic determinations. The inability to identify those patients whose BP will be affected by positional change highlights the importance of following standard BP measurement guidelines for all patients.
Study limitations. Positional change in BP from examination table to chair lacks a comparison to BP changes in positioning from chair to table. If similar BP changes in the reverse sequence were to be observed, this would add support to the hypothesis that muscle tension of the unsupported body is a cause of BP elevation in certain individuals. We believe, however, that the sequence of BP measurements (from table to chair) did not have a significant impact because all patients were allowed to rest in each position before the BP was measured. The BP was therefore measured in a steady-state in both positions.
Additionally, BP measurement by aneroid sphygmomanometry is highly dependent on observer skill and hearing ability. Furthermore, a disproportionate number of BP measurements recorded in the study ended in zero, suggesting terminal digit bias by the observer. These sources of error may be avoided using an automated oscillometric measuring device.18 Automated devices also allow for repeated independent measurements that minimize the white-coat effect. However, there are also limitations to the accuracy of oscillometric equipment. This is especially true when recording BP in the elderly, a group whose stiff arterial walls may cause erroneous measurements.19
Guideline justification. Nonadherence to standard positioning when measuring BP leads to certain individuals being misclassified as prehypertensive or hypertensive. Misclassification in turn leads to unnecessary medical encounters, testing, and treatment. Misdiagnosis is also likely to increase the cost of an individual’s insurance coverage and out-of-pocket health care expenses.
CORRESPONDENCE
Roy N. Morcos, MD, St. Elizabeth Family Medicine Residency Program, 8423 Market Street, Suite 101, Boardman, Ohio 44512; [email protected].
ABSTRACT
Purpose This study evaluated the effect of patient positioning on the diagnosis of hypertension in a clinic setting and the importance of following guidelines for measuring blood pressure (BP).
Methods In the trial part of this study, we recorded BP measurements by an aneroid sphygmomanometer with patients seated first on an examination table, a commonly observed practice, and second in the standard seated position as defined by the American Heart Association. Two measurements were obtained in each position for 204 patients, and we determined the difference between the average readings in the 2 positions. Factored into the comparison was an estimation of inherent variance of the device and observer achieved by repeated measurements on a healthy individual.
Results This investigation included an initial observational study of 25 regional primary care offices, the results of which showed frequent lack of adherence with accepted guidelines in patient positioning during BP measurement. The overall systolic and diastolic BPs were more than 2 mm Hg lower in the standard seated position compared with the examination table position (P<.001). Noncompliance with the position guideline resulted in misclassification of 15 patients (7.4%) as prehypertensive, when, in fact, they were normotensive. Misclassification of hypertension occurred in 12 patients (5.9%), when, in fact, they were normotensive. Logistic regression using relevant clinical factors did not identify those individuals who were misclassified.
Conclusion This study underscores the importance of patient positioning on BP determinations in order to accurately diagnose hypertension.
The high prevalence of hypertension and its burden of disease in the United States and worldwide are well known.1 Hypertension is a major risk factor for coronary heart disease, congestive heart failure, ischemic and hemorrhagic stroke, chronic kidney disease, and peripheral arterial disease.2 Among all risk factors, hypertension ranked first worldwide in disability-adjusted life-years.3 However, misclassification of an individual’s blood pressure (BP) as prehypertension or hypertension also confers significant health and financial burdens due to unnecessary medical encounters, testing, and treatment, and to increased cost of insurance coverage and out-of-pocket expenses. A correct assessment of BP in the outpatient setting depends on accurate measurement technique.
The diagnosis of hypertension is based on indirect measurement of BP using in-office, ambulatory, or home monitoring. Although office BP measurement is less than ideal, it is used most often to diagnose and monitor hypertension. Furthermore, most published trials of treatment recommendations are based on office BP measurements.4
Automated oscillometric and aneroid sphygmomanometers are common BP measurement devices. Proper technique is particularly important with the aneroid sphygmomanometer to obtain consistent and accurate results.5 Good training and an ability to hear the Korotkoff sounds are crucial.
Expert consensus groups such as the American Heart Association (AHA) publish recommendations for proper technique in reliably measuring BP,6-8 and they emphasize the importance of patient positioning during BP measurement. The individual should be seated comfortably in a chair with both arms and back supported, legs uncrossed, and feet flat on the floor. We’ll refer to this as the “standard position.” Although the proper technique for measuring BP has been widely advocated, a recent literature review for the US Preventive Services Task Force concluded that surprisingly few studies are available on the diagnostic accuracy of office BP practices.9
One paper evaluated the effect of leg crossing on accuracy of BP measurement. No subjects were reclassified as hypertensive, but the study lacked statistical rigor.10 Another study found variable BP readings regardless of body position.11
The purpose of our study was to compare BP measurement in 2 positions: the standard position described above, and the examination table position in which the patient is seated on the edge of the table with back, arms, and feet unsupported.
METHODS
We conducted our literature search across several scientific and medical literature databases, including PubMed, ScienceDirect, and CINAHL. Only English-language articles were reviewed.
We followed the BP measurement guidelines of the AHA. Prior to beginning the study, we provided instructions in proper BP measurement technique to the nurses who would obtain the data. The minimum sample size of patients needed to identify a difference of at least 2 mm Hg was 26, as estimated by power analysis. This was calculated using an alpha of .05 and a beta of .13.
The study population consisted of patients presenting consecutively to a teaching family medicine center. Adult patients, ages 18 and older, were informed about the study and invited to participate. Those who agreed were asked to read and sign an informed consent approved by a regional institutional review board for human subjects. We excluded patients who declined participation for any reason, who were in severe pain or distress that may have prevented them from completing the protocol, or who had limited mobility that could interfere with climbing onto the examination table. Patients considered for the study totaled 250, 28 of whom were ineligible. Another 18 patients declined participation, leaving 204 who completed the protocol.
Before testing began, we estimated the standard deviation of each aneroid sphygmomanometer and the assigned observer by repeatedly measuring the BP of a healthy normotensive individual sitting in the standard position. We obtained 46 measurements over 2 days to avoid subject and operator fatigue. Standard deviation for systolic BP was 3.6 mm Hg; for diastolic it was 3.8 mm Hg.
During testing, nurses recorded BP for each patient twice in the examination table position and twice in the standard position. They entered data into an Excel workbook for subsequent analysis. All examination rooms were equipped with newly purchased aneroid sphygmomanometers, and the appropriate cuff size was selected for each patient. Patients were instructed to remain quiet during the measurements. Patients sat first on the edge of the examination table. After a 5-minute rest, BP was measured twice in the same arm. Measurements were separated by 1 to 2 minutes. Patients then sat in the chair and rested another 2 minutes before BP was again measured twice in the same arm. The arms and back were supported in the chair and the stethoscope placed at heart level.
As per protocol, we obtained 4 BP readings on each patient and calculated the difference between the average systolic and diastolic BP values from the 2 positions. The standard error of the mean of this difference was determined using the equation, where Sd is the standard deviation of the aneroid sphygmomanometer and observer.12 A one-sided, 95% confidence upper bound for the standard error of the difference is 1.65 × SEd. We compared patient-specific differences against this upper bound to identify significant systolic and diastolic BP changes due to positioning. If the patient’s BP difference exceeded the upper bound, it was attributed to the positional change and not to variation inherent to the sphygmomanometer and observer.
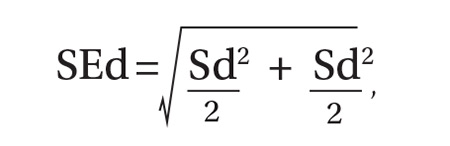
As an example, consider a patient whose average systolic BP readings from the examination-table and standard positions, respectively, were 128 mm Hg and 120 mm Hg. Assuming an SEd of 3.55 and an upper bound of 5.86, the observed 8 mm Hg difference in average systolic BPs would be considered significant. The amount of random variation from the sphygmomanometer and observer would not be expected to exceed 5.86 mm Hg.
In accordance with accepted standards, prehypertension was defined as a BP between 120-139/80-89 mm Hg, and hypertension was defined as a BP ≥140/90 mm Hg.4 BP below 120/80 mm Hg was considered normal. We calculated each patient’s average systolic and diastolic BP values in the 2 positions and thereby classified the individual as normotensive, prehypertensive, or hypertensive. We regarded as misclassified any patient whose BP showed significant lowering between the examination-table and standard positions resulting in a change of classification from prehypertensive or hypertensive to normotensive. For example, a patient with an examination-table position average reading of 126/85 mm Hg and a standard position average reading of 118/78 mm Hg would have been misclassified as prehypertensive.
We reviewed charts and gathered data, including subject age, sex, obesity (defined as a body mass index of ≥30 kg/m2), and history of diabetes, hypertension, or smoking. Other than age, all data were binary. We performed logistic regression analysis using the Excel Add-in Real Statistics Resource Pack software (Release 4.3)13 to determine if these factors could predict significant lowering of BP due to positional change.
Our associated observational study. We also conducted a separate observational study of 25 regional primary care offices to evaluate compliance with the AHA guidelines for measuring BP. The office nurses taking measurements were not informed of the study’s purpose to prevent deviation from their common practice.
Data on 9 guideline criteria were collected to assess supervision of patients before and during measurements, including having the patient sit in a chair in quiet and comfortable surroundings with arms and back supported and feet on the ground. We also noted the type of BP measuring device used. Additionally, observers assessed the technique of the individuals using a manual device, including cuff placement and deflation rate. The observations were conducted during a clinic visit by a medical student knowledgeable in the AHA guidelines for measuring BP by automated oscillometric or aneroid sphygmomanometric devices. We conducted the study over a 2-week period in the second quarter of 2016.
RESULTS
Power analysis performed prior to the study showed that a minimum of 26 patients would be needed to predict a 2 mm Hg difference between BPs obtained in the 2 positions. Of the 204 patients used in the logistic regression analysis, 78 were men and 126 were women. Ages ranged from 18 to 101 years, yielding a mean of 54. One-hundred sixteen had previously received a diagnosis of hypertension, 39 had diabetes, 92 were obese, 22 were current smokers, and 68 were former smokers.
TABLE 1 shows the means and ranges of systolic and diastolic BP for both study positions. With this study population, mean BP recorded in the examination-table position decreased in the standard position by 2.1 and 2.2 mm Hg for systolic and diastolic BP, respectively (P<.001).

Significant BP lowering—as defined by a one-sided 95% confidence upper bound for the standard error of differences between study positions—was determined to be 5.86 and 6.22 mm Hg for systolic and diastolic pressures, respectively. Significant lowering of BP and misclassification due to positioning are summarized in TABLE 2. Significant lowering of mean systolic or diastolic BP with positional change from table to chair occurred in 62 subjects (30.4%). Misclassification of prehypertension occurred in 7.4% of subjects, and misclassification of hypertension occurred in 5.9%.

Logistic regression using patient age, sex, obesity, and history of diabetes, hypertension, and smoking as independent factors did not predict significant BP lowering with positional change.
Our observational study revealed that proper positioning in a chair was followed in only 10 of the 25 offices. In the remaining offices, patients were seated on the examination table. A 5-minute rest period before measuring BP was allowed in only 10 of the 25 offices. An automated oscillometric device was used in only 2 of the 25 offices.
DISCUSSION
In this study, 27 subjects (13.2%) were misclassified as prehypertensive or hypertensive as a result of deviating from the standard position in obtaining BP. Although the standard position is universally recommended, the guideline is not always followed in clinical practice.14
One study by Villegas et al found that 60% of physicians and nurses working in a major hospital were measuring BP inaccurately.15 In our initial observational study, 60% of primary care practices visited did not adhere to the recommended patient positioning. These medical offices are located in the community surrounding our facility and are operated by the same health care organization. The misclassification of prehypertension and hypertension observed in our prospective comparison of BP recordings in table and chair positions is, therefore, likely to occur to some degree at these practices, as well.
Similar diagnostic misclassifications have been reported in other medical settings. In a published survey of 114 medical offices, McKay and coworkers noted frequent inconsistencies with published guidelines in measuring BP.16
Common clinical demographic data obtained during this study showed no association with the positional BP change. Increased muscle tension due to lack of body support while sitting on the edge of the examination table could be the cause of elevated BP for this subgroup of individuals. Measuring muscle tension of the arms and back while seated on an exam table and chair was beyond the scope of this study.
In clinical practice, different types of BP measuring devices are used. Calibration and quality control of these devices is often lacking.17 Before starting our study, we determined the statistical variance of the aneroid sphygmomanometers and found it to approximate the manufacturer’s precision specification. Guidelines recommend using the mean of 2 BP readings as representing the patient’s BP for a given clinic visit. Additional readings are recommended if there is more than a 5 mm Hg difference between the initial 2 readings.4
In our study, we used sampling statistics of the BP readings and clinical guideline BP ranges in making diagnostic determinations. The inability to identify those patients whose BP will be affected by positional change highlights the importance of following standard BP measurement guidelines for all patients.
Study limitations. Positional change in BP from examination table to chair lacks a comparison to BP changes in positioning from chair to table. If similar BP changes in the reverse sequence were to be observed, this would add support to the hypothesis that muscle tension of the unsupported body is a cause of BP elevation in certain individuals. We believe, however, that the sequence of BP measurements (from table to chair) did not have a significant impact because all patients were allowed to rest in each position before the BP was measured. The BP was therefore measured in a steady-state in both positions.
Additionally, BP measurement by aneroid sphygmomanometry is highly dependent on observer skill and hearing ability. Furthermore, a disproportionate number of BP measurements recorded in the study ended in zero, suggesting terminal digit bias by the observer. These sources of error may be avoided using an automated oscillometric measuring device.18 Automated devices also allow for repeated independent measurements that minimize the white-coat effect. However, there are also limitations to the accuracy of oscillometric equipment. This is especially true when recording BP in the elderly, a group whose stiff arterial walls may cause erroneous measurements.19
Guideline justification. Nonadherence to standard positioning when measuring BP leads to certain individuals being misclassified as prehypertensive or hypertensive. Misclassification in turn leads to unnecessary medical encounters, testing, and treatment. Misdiagnosis is also likely to increase the cost of an individual’s insurance coverage and out-of-pocket health care expenses.
CORRESPONDENCE
Roy N. Morcos, MD, St. Elizabeth Family Medicine Residency Program, 8423 Market Street, Suite 101, Boardman, Ohio 44512; [email protected].
1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217-223.
2. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224-2260.
3. Murray CJ, Lopez AD. Measuring the global burden of disease. New Engl J Med. 2013;369:448-457.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
5. Bailey RH, Bauer JH. A review of common errors in the indirect measurement of blood pressure. Sphygmomanometry. Arch Intern Med. 1993;153:2741-2748.
6. Padwal RS, Hemmelgarn BR, McAlister FA, et al. The 2007 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1- blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2007;23:529-538.
7. Campbell NR, Chockalingam A, Fodor JG, et al. Accurate, reproducible measurement of blood pressure. CMAJ. 1990;143:19-24.
8. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens. 2005;7:102-109.
9. Piper MA, Evans CV, Burda BU, et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192-204.
10. Peters GL, Binder SK, Campbell NR. The effect of crossing legs on blood pressure: a randomized single-blind cross-over study. Blood Press Monit. 1999;4:97-101.
11. Cicolini G, Pizzi C, Palma E, et al. Differences in blood pressure by body position (supine, Fowler’s, and sitting) in hypertensive subjects. Am J Hypertens. 2011;24:1073-1079.
12. Daniel WW, Cross CL. Biostatistics: A Foundation for Analysis in the Health Sciences (10th Edition). Hoboken, NJ: John Wiley & Sons; 2013.
13. Zaiontz C. Real statistics using Excel. Available at: http://www.real-statistics.com/. Accessed February 20, 2018.
14. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
15. Villegas I, Arias IC, Botero A, et al. Evaluation of the technique used by health-care workers for taking blood pressure. Hypertension. 1995;26:1204-1206.
16. McKay DW, Campbell NR, Parab LS, et al. Clinical assessment of blood pressure. J Hum Hypertens. 1990;4:639-645.
17. Jones DW, Appel LJ, Sheps SG, et al. Measuring blood pressure accurately: new and persistent challenges. JAMA. 2003;289:1027-1030.
18. Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Can J Cardiol. 2016;32:569-588.
19. Raamat R, Talts J, Jagomägi K, et al. Errors of oscillometric blood pressure measurement as predicted by simulation. Blood Press Monit. 2011;16:238-245.
1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217-223.
2. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224-2260.
3. Murray CJ, Lopez AD. Measuring the global burden of disease. New Engl J Med. 2013;369:448-457.
4. Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572.
5. Bailey RH, Bauer JH. A review of common errors in the indirect measurement of blood pressure. Sphygmomanometry. Arch Intern Med. 1993;153:2741-2748.
6. Padwal RS, Hemmelgarn BR, McAlister FA, et al. The 2007 Canadian Hypertension Education Program recommendations for the management of hypertension: part 1- blood pressure measurement, diagnosis and assessment of risk. Can J Cardiol. 2007;23:529-538.
7. Campbell NR, Chockalingam A, Fodor JG, et al. Accurate, reproducible measurement of blood pressure. CMAJ. 1990;143:19-24.
8. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens. 2005;7:102-109.
9. Piper MA, Evans CV, Burda BU, et al. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192-204.
10. Peters GL, Binder SK, Campbell NR. The effect of crossing legs on blood pressure: a randomized single-blind cross-over study. Blood Press Monit. 1999;4:97-101.
11. Cicolini G, Pizzi C, Palma E, et al. Differences in blood pressure by body position (supine, Fowler’s, and sitting) in hypertensive subjects. Am J Hypertens. 2011;24:1073-1079.
12. Daniel WW, Cross CL. Biostatistics: A Foundation for Analysis in the Health Sciences (10th Edition). Hoboken, NJ: John Wiley & Sons; 2013.
13. Zaiontz C. Real statistics using Excel. Available at: http://www.real-statistics.com/. Accessed February 20, 2018.
14. Burgess SE, MacLaughlin EJ, Smith PA, et al. Blood pressure rising: differences between current clinical and recommended measurement techniques. J Am Soc Hypertens. 2011;5:484-488.
15. Villegas I, Arias IC, Botero A, et al. Evaluation of the technique used by health-care workers for taking blood pressure. Hypertension. 1995;26:1204-1206.
16. McKay DW, Campbell NR, Parab LS, et al. Clinical assessment of blood pressure. J Hum Hypertens. 1990;4:639-645.
17. Jones DW, Appel LJ, Sheps SG, et al. Measuring blood pressure accurately: new and persistent challenges. JAMA. 2003;289:1027-1030.
18. Leung AA, Nerenberg K, Daskalopoulou SS, et al. Hypertension Canada’s 2016 Canadian Hypertension Education Program Guidelines for Blood Pressure Measurement, Diagnosis, Assessment of Risk, Prevention, and Treatment of Hypertension. Can J Cardiol. 2016;32:569-588.
19. Raamat R, Talts J, Jagomägi K, et al. Errors of oscillometric blood pressure measurement as predicted by simulation. Blood Press Monit. 2011;16:238-245.