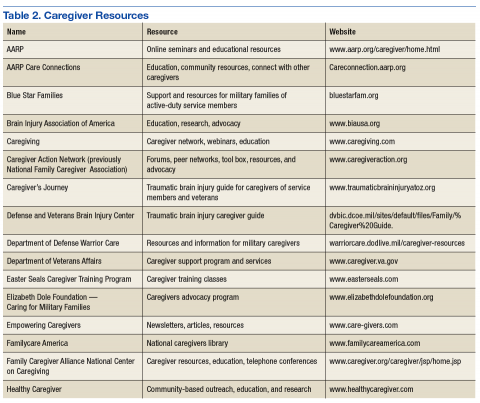
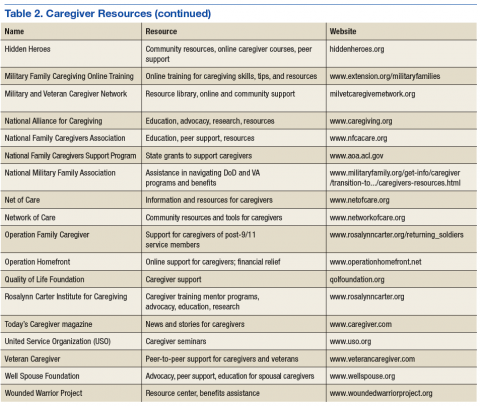
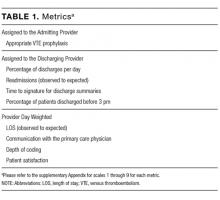
User login
Clinical Decision-Making: Observing the Smartphone UserAn Observational Study in Predicting Acute Surgical Patients’ Suitability for Discharge
The value placed on bedside clinical observation in the decision-making process of a patient’s illness has been diminished by today’s armamentarium of sophisticated technology. Increasing reliance is now placed on the result of nonspecific tests in preference to bedside clinical judgement in the diagnostic and management process. While diagnostic investigations have undoubtedly provided great advancements in medical care, they come at time and financial costs. Physicians should therefore continue to be encouraged to make clinical decisions based on their bedside assessment.
With hospital overcrowding a significant problem within the healthcare system and the expectation that it will worsen with an ageing population, identifying factors that predict patient suitability for discharge has become an important focus for clinicians.1,2 There exists a paucity of literature predicting discharge suitability of general surgical patients admitted through the emergency department (ED). Furthermore, despite the extensive research into the effectiveness of discharge planning,3 little research has been conducted to describe positive predictive indicators for discharge. Observations made during surgical rounds have led the authors to consider that individuals who are using a smartphone during their bedside assessment may be clinically well enough for discharge.
The aim of this study was to assess whether the clinical assessment of an acute surgical patient could be usefully augmented by the observation of the active use of smartphones (the smartphone sign) and whether this could be used as a surrogate marker to indicate a patient’s well-being and suitability for same-day discharge from the hospital in acute surgical patients.
METHODS
Design and Setting
This was a prospective observational study performed over 2 periods at a tertiary hospital in South Australia, Australia. At our institution, acute surgical patients are admitted to the acute surgical unit (ASU) from the ED by junior surgical doctors. Patients are then reviewed by the on-call surgical consultant, who implements management plans or advises discharge on 2 occasions per day.
Participants
All patients admitted under the ASU were considered eligible for the study. Exclusion criteria included patients that (i) required immediate surgical intervention (defined as time of review to theatre of less than 4 hours) and (ii) had immediate admission to the intensive care unit.
Consultant surgeons are employed within a general surgical subspecialty, including upper gastrointestinal, hepatobiliary, breast and endocrine, and colorectal. All surgeons from each team partake in the general surgery on-call roster. Each surgeon was included at least once within the observation periods. Experience of consultant surgeons ranged from 5 years of postfellowship experience to surgeons with more than 30 years of experience, with the majority having more than 10 years of postfellowship experience.
Patients were stratified into 2 distinct cohorts upon consultant review: smartphone positive (spP) was defined as a patient who was using a smartphone or who had their phone on their bed; a patient was classified as smartphone negative (spN) if they did not fulfil these criteria. The presence or absence of a smartphone was recorded by the authors, who were present on consultant ward rounds but not involved in the decision-making process of patient care. In order to minimize bias, only 1 surgeon (PGD) was aware that the study was being conducted and all patients were blinded to the study. Additional information that was collected included patient demographics, requirement for surgery, and length of stay (LOS). A patient who was discharged on the same day as the consultant review was considered to be discharged on day 1, all other patients were considered to have LOS greater than 1 day. Requirement for surgery was defined as a patient who underwent a surgical procedure in an operating suite. Thirty-day unplanned readmission rates for all patients were examined. Readmission to another public hospital within the state was also included within the readmission data.
Observation Periods
An initial 4-week pilot study was conducted to assess for a possible association between spP and same-day discharge. A second 8-week study period was undertaken 1 year later accounting for the employment of the authors at the study’s institution. Unless stated, the results described are the accumulation of both study periods.
Statistical Analysis
As this is the first study of its kind, no prior estimates of numbers were known. After 2 weeks of data collection, data were analyzed in order to provide an estimate of the total number of patients required to provide a statistically valid result (α = 0.05; power = 0.80). Sample size was calculated to be 40 subjects. It was agreed that in order to make the study as robust as possible, data should be collected for the 2 observation periods.
Demographic data are presented as means with standard deviations (SDs) or frequencies with percentages. A 2-sample Student t test was used to compare the age of spP and spN patients. A χ2 test and logistic regressions were used to assess the association between smartphone status and patient demographics, LOS, and requirement for surgery. Results are presented as odds ratios (ORs) with 95% confidence intervals (CIs). A P value of <0.05 was considered significant. All data were analyzed by using R 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
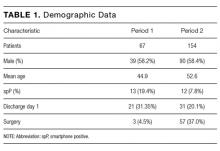
During the 2 observation periods, a total of 227 eligible surgical admissions were observed with complete data for 221 patients. Six patients were excluded as their smartphone status was not recorded. The study sample represents our population of interest within an ASU, and we had complete data for 97.4% of participants with a 100% follow-up. There was no significant effect of study between the 2 observation periods (χ2 = 140.19; P = 0.10). The mean age of patients was 50.24 years. Further demographic data are presented in Table 1. Twenty-five (11.3%) patients were spP and 196 (88.7%) were spN. Fifty-two (23.5%) patients were discharged home on day 1, and 169 (76.5%) had admissions longer than 1 day (see Figure). Sixty (27%) patients underwent surgery during their admission. Twenty-two patients had unplanned readmissions; only 1 of these patients had been observed to be spP.
There was a statistically significant difference in ages between the spP and spN groups (t = 8.40; P < 0.0005), with the average age of spP patients being 31.84 years compared with 52.58 years for spN patients. There was no statistical difference between gender and smartphone status (χ2 = 1.78; P = 0.18; Table 2).
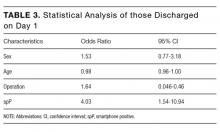
For those patients discharged home on day 1, there was a statistically significant association with being spP (χ2 = 14.55, P = 0.0001). Patients who were spP were 5.29 times more likely to be discharged on day 1 (95% CI, 2.24-12.84). Of the variables analyzed, only gender failed to demonstrate an effect on discharge home on day 1 (Table 3). Overall, the presence of a smartphone was found to have a sensitivity of 56.0% (95% CI, 34.93-75.60) and a specificity of 80.6% (95% CI, 74.37-85.90) in regard to same-day discharge. However, it was found to have a negative predictive value of 93.49% (95% CI, 88.65-96.71).
When examining readmission rates, only 4% of spP patients were readmitted versus 10.7% of spN patients. Accounting for variables, spP patients were 4 times less likely to be readmitted, though this was not statistically significant (OR 4.02; 95% CI, 0.43-37.2; P = 0.22). Furthermore, when examining only those patients discharged on day 1, smartphone status was not a predictor of readmission (OR 0.94; 95% CI, 0.06-15.2; P = 0 .97).
To mitigate the effect of age, analysis was conducted excluding those aged over 55 years (the previous retirement age in Australia), leaving 131 patients for analysis. The average age of spP patients was 31.8 years (SD 10.0) compared with 36.7 years (SD 10.9) for spN patients, representing a significant difference (t = 2.14; P = 0.04); 51.1% of patients were male, 19.1% of patients were spP, 26.0% of patients proceeded to an operation, the oldest spP was 51 years, and 29.0% of patients were discharged home on day 1. There was no difference in gender and smartphone status (χ2 = 0.33; P = 0.6). When analyzing those discharged on day 1, again spP patients were more likely to be discharged home (χ2 = 9.4; P = 0.002), and spP patients were 3.6 times more likely to be discharged home on day 1.
There were 4 spP patients who underwent an operation. Two patients had an incision and drainage of a perianal abscess, 1 patient underwent a laparotomy for an internal hernia after recently undergoing a Roux-en-Y gastric bypass at another hospital, and the final patient underwent a laparoscopic appendicectomy. One of these patients was still discharged home on day 1.
DISCUSSION
As J. A. Lindsay4 said, “For one mistake made for not knowing, ten mistakes are made for not looking.” At medical school, we are taught the finer techniques of the physical examination in order to support our diagnosis made from the history. It is not until we are experienced clinicians do we develop the clinical acumen and ability to tell an unwell patient from a well patient at a glance—colloquially known as the “end of the bed” assessment. In the pretechnology era, a well patient could frequently be seen reading their book, eg, the “novel-sign.” With the advent of the smartphone and electronic devices upon which novels can be read, statuses updated, and locations “checked into” (ie, the modern “vital signs”), the book sign may be a thing of the past. However, the ability for the clinician to assess a patient’s wellness is still crucial, and the value of any additional “physical signs” need to be estimated.
We observed a cohort of patients through a busy ASU in a tertiary hospital in South Australia, Australia. Acute surgical patients admitted to the hospital who were observed to be on their phones upon consultant review were more than 5 times likely to be discharged that same day. To the best of our knowledge, this is the first study to prospectively collect data to assess a frequently used but unevaluated clinical observation.
The use of a smartphone can tell us a lot about an individual’s physiology. We can assume the individual’s airway and breathing are adequate, allowing enough oxygen to reach the lungs and subsequently circulate. The individual is usually sitting up in bed and thus has an adequate blood pressure and blood oxygenation that can maintain cerebral perfusion. They have the cognitive and cerebral processing in place to function the device, and we can examine their cerebellar function by looking for fine-motor movements.
Mobile phone ownership is pervasive within Australia,5 with a conservative estimated 85.7% of the population (20.57 million people of a total population of approximately 24 million) owning a mobile phone and an estimated 50% to 79% of mobile phone ownership being of a smartphone.6,7 This ownership is not just limited to the young, with 74% of Australians over 65 owning or using a mobile phone.8 Despite this high phone ownership among those over 65, it is still significantly less than their younger counterparts and may be one reason for the absence of spP in those older than 51 years. A key point in the study is that overall phone ownership was not known, and, thus, it is not possible to determine the proportion of spN patients who were negative because they did not own a phone. However, based on general population data, the incidence of spP patients was well below that seen in the community (11.3%)5 and even when excluding those over 55, the percentage of spP patients only rose to 19.1%. Unsurprisingly, increasing age was associated with a decreased likelihood of being spP (P < 0.0005), as younger people are more likely to own a phone.8 There was no association with gender (P = 0.18). There are a number of explanations that may explain the lower than expected percentage of spP patients, including the inability for the patient to gather their possessions during a medical emergency, patients storing their phones prior to doctor review (72%-85% of Australians report talking on phones in public places to be rude or intrusive5), but more importantly, that our hypothesis that patients were too unwell to use their device appears to hold true.
There are potential alternate reasons other than smartphone status that may account for patients being discharged home on day 1. While there was no association seen with gender, the need for an operation prolonged a patient’s stay (OR 1.64; 95% CI, 0.046-0.46), and there was a trend seen with increasing age (OR 0.98; 95% CI, 0.96-1.00). Neither of these 2 demographics are unsurprising: increasing age is associated with increasing medical comorbidities and thus complexity; even the simplest of operations require a postprocedure observation period, automatically increasing their LOS. Additionally, measured demographics are limited and there may be further unmeasured reasons that account for earlier discharge.
The other key component to this study is the value of the physical examination, albeit only assessing 1 component: the general inspection. In their review of the value of the physical examination of the cardiovascular system, Elder et al. highlight an important point: in traditional teaching, the value of a physical sign is compared with a diagnostic reference, typically imaging or an invasive test.9 They argue that this definition undervalues the physical examination and list other values aside from accuracy including accessibility, contribution to clinical care beyond diagnoses, cost effectiveness, patients’ safety, patients’ perceptions, and pedagogic value; and they argue that the physical examination should always be considered in regard to the clinical context—in this case, the newly admitted general surgical patient.
The assessment of the presence or absence of a smartphone is readily performed upon general inspection and is easily visible; general inspection of the patient and failure to observe the clinical sign when present are 2 of the greatest errors associated with physical examination.10 Furthermore, given its unique status as a physical sign, the authors’ opinion and experience is that it is readily teachable. McGee states, “…a fundamental lesson [in regards to teaching] is that the diagnosis of many clinical problems, despite modern testing, still depends primarily on what the clinician sees, hears, and feels.”11 In their article, Paley et al. found that more than 80% of patients admitted from the ED under internal medicine could be accurately diagnosed based largely on history and examination alone and concluded that basic clinical skills are sufficient for achieving an accurate diagnosis in most cases.12 Although Paley et al. were assisted with basic tests (such as electrocardiogram and basic haematological and biochemistry results), the point of clinical skills is not lost. Furthermore, this assessment was made in a group of patients generally considered to be complex in contrast to the “standard” appendicitis or cholecystitis patient that makes up a significant proportion of general surgical patients.
There are a number of limitations to this study, however, including smartphones that may have been missed during the observational period. Potential confounding variables such as socioeconomic status and the overall smartphone ownership of our subjects were not known. We did not ask all admitted patients whether they owned a phone or whether they had a phone in their possession. Knowledge of those who owned phones but were not in possession of them could strengthen our argument that spN patients were not using their phone because they were unwell, rather than just not having access to it.
However, this study has a number of strengths, including a large sample size and data that were prospectively collected by a method and in a setting that was the same for all participants. Clear and appropriate definitions were used, which minimizes misclassification bias. Participants and decision makers were blinded to the study, and potentially confounding variables such as age and sex were accounted for.
Assessing the suitability for discharge from the hospital is a decision encountered by hospital-based clinicians every day. These skills are not taught, but are rather learned as a junior doctor acquires experience. It is unlikely that protocols will be developed to aid identification of potential discharges from an acute surgical ward; acute surgical conditions are too varied and dynamic to be able to pool all data. We continue to rely on our own and fellow colleagues’ (doctors, nurses, and other staff
CONCLUSION
While these observations might appear to be rather a simplistic way of trying to quantify whether or not a patient is fit for discharge, any clues that hint towards a patient’s well-being should be taken into account when making an overall assessment. The active use of a smartphone is one such measure.
Acknowledgments
The authors thank Emma Knight and Nancy Briggs from the Data Management & Analysis Centre, Discipline of Public Health, University of Adelaide.
Disclosure
No author nor the institution received any payment or services from a third party for any aspect of the submitted work and report no conflict of interest. There are no reported financial relationships with any entities by any of the authors. There are no patents pending based upon this publication. There are no relationships or activities that readers could perceive to have influenced, or give the appearance of influencing, the submitted work. The corresponding author is not in receipt of a research scholarship. The paper is not based on a previous communication.
1. Sprivulis PC, Da Silva JA, Jacobs IG, Frazer AR, Jelinek GA. The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments. Med J Aust. 2006;184(5):208-212. PubMed
2. Shepherd T. Hospital Overcrowding kills as many as our road toll. The Advertiser. November 23, 2010. Available from: http://www.adelaidenow.com.au/news/south-australia/hospital-overcrowding-kills-as-many-as-our-road-toll/news-story/3389668c23b8b141f1d335b096ced416. Accessed February 2, 2017.
3. Shepperd S, Lannin NA, Clemson LM, McCluskey A, Cameron ID, Barras SL. Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;Jan 31(1):CD000313. PubMed
4. Breathnach CS, Moynihan JB. James Alexander Lindsay (1856–1931), and his clinical axioms and aphorisms. Ulster Med J. 2012;81(3):149-153. PubMed
5. Enhanced Media Metrics Australia. Product Insights Report. Digital Australia: A snapshot of attitudes and usage. August 2013. Ipsos Australia. North Sydney, Australia. Report available from: https://emma.com.au/wp-content/uploads/2013/10/digital.pdf
6. Australian Communications and Media Authority. Communications report 2013-24. Melbounre: Commonwealth of Australia; 2014. http://www.acma.gov.au/~/media/Research%20and%20Analysis/Publication/Comms%20Report%202013%2014/PDF/Communications%20report%20201314_LOW-RES%20FOR%20WEB%20pdf.pdf
7. Drumm J, Johnston S. Mobile Consumer Survery 2015—The Australian Cut. Deloitte. Australia; 2015. Deloitte Touche Tohmatsu. Sydney, Australia. file:///C:/Users/user/Desktop/deloitte-au-tmt-mobile-consumer-survey-2015-291015.pdf
8. Older Australians Resist Cutting the Cord: Australian Communications and Media Authority. 2014. http://www.acma.gov.au/theACMA/engage-blogs/engage-blogs/Research-snapshots/Older-Australians-resist-cutting-the-cord. Accessed February 23, 2017.
9. Elder A, Japp A, Verghese A. How valuable is physical examination of the cardiovascular system? BMJ. 2016;354:i3309. PubMed
10. Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JP. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med. 2015;128(12):1322-1324.e3. PubMed
11. McGee S. A piece of my mind. Bedside teaching rounds reconsidered. JAMA. 2014;311(19):1971-1972. PubMed
12. Paley L, Zornitzki T, Cohen J, Friedman J, Kozak N, Schattner A. Utility of clinical examination in the diagnosis of emergency department patients admitted to the department of medicine of an academic hospital. Arch Intern Med. 2011;171(15):1394-1396. PubMed
The value placed on bedside clinical observation in the decision-making process of a patient’s illness has been diminished by today’s armamentarium of sophisticated technology. Increasing reliance is now placed on the result of nonspecific tests in preference to bedside clinical judgement in the diagnostic and management process. While diagnostic investigations have undoubtedly provided great advancements in medical care, they come at time and financial costs. Physicians should therefore continue to be encouraged to make clinical decisions based on their bedside assessment.
With hospital overcrowding a significant problem within the healthcare system and the expectation that it will worsen with an ageing population, identifying factors that predict patient suitability for discharge has become an important focus for clinicians.1,2 There exists a paucity of literature predicting discharge suitability of general surgical patients admitted through the emergency department (ED). Furthermore, despite the extensive research into the effectiveness of discharge planning,3 little research has been conducted to describe positive predictive indicators for discharge. Observations made during surgical rounds have led the authors to consider that individuals who are using a smartphone during their bedside assessment may be clinically well enough for discharge.
The aim of this study was to assess whether the clinical assessment of an acute surgical patient could be usefully augmented by the observation of the active use of smartphones (the smartphone sign) and whether this could be used as a surrogate marker to indicate a patient’s well-being and suitability for same-day discharge from the hospital in acute surgical patients.
METHODS
Design and Setting
This was a prospective observational study performed over 2 periods at a tertiary hospital in South Australia, Australia. At our institution, acute surgical patients are admitted to the acute surgical unit (ASU) from the ED by junior surgical doctors. Patients are then reviewed by the on-call surgical consultant, who implements management plans or advises discharge on 2 occasions per day.
Participants
All patients admitted under the ASU were considered eligible for the study. Exclusion criteria included patients that (i) required immediate surgical intervention (defined as time of review to theatre of less than 4 hours) and (ii) had immediate admission to the intensive care unit.
Consultant surgeons are employed within a general surgical subspecialty, including upper gastrointestinal, hepatobiliary, breast and endocrine, and colorectal. All surgeons from each team partake in the general surgery on-call roster. Each surgeon was included at least once within the observation periods. Experience of consultant surgeons ranged from 5 years of postfellowship experience to surgeons with more than 30 years of experience, with the majority having more than 10 years of postfellowship experience.
Patients were stratified into 2 distinct cohorts upon consultant review: smartphone positive (spP) was defined as a patient who was using a smartphone or who had their phone on their bed; a patient was classified as smartphone negative (spN) if they did not fulfil these criteria. The presence or absence of a smartphone was recorded by the authors, who were present on consultant ward rounds but not involved in the decision-making process of patient care. In order to minimize bias, only 1 surgeon (PGD) was aware that the study was being conducted and all patients were blinded to the study. Additional information that was collected included patient demographics, requirement for surgery, and length of stay (LOS). A patient who was discharged on the same day as the consultant review was considered to be discharged on day 1, all other patients were considered to have LOS greater than 1 day. Requirement for surgery was defined as a patient who underwent a surgical procedure in an operating suite. Thirty-day unplanned readmission rates for all patients were examined. Readmission to another public hospital within the state was also included within the readmission data.
Observation Periods
An initial 4-week pilot study was conducted to assess for a possible association between spP and same-day discharge. A second 8-week study period was undertaken 1 year later accounting for the employment of the authors at the study’s institution. Unless stated, the results described are the accumulation of both study periods.
Statistical Analysis
As this is the first study of its kind, no prior estimates of numbers were known. After 2 weeks of data collection, data were analyzed in order to provide an estimate of the total number of patients required to provide a statistically valid result (α = 0.05; power = 0.80). Sample size was calculated to be 40 subjects. It was agreed that in order to make the study as robust as possible, data should be collected for the 2 observation periods.
Demographic data are presented as means with standard deviations (SDs) or frequencies with percentages. A 2-sample Student t test was used to compare the age of spP and spN patients. A χ2 test and logistic regressions were used to assess the association between smartphone status and patient demographics, LOS, and requirement for surgery. Results are presented as odds ratios (ORs) with 95% confidence intervals (CIs). A P value of <0.05 was considered significant. All data were analyzed by using R 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
During the 2 observation periods, a total of 227 eligible surgical admissions were observed with complete data for 221 patients. Six patients were excluded as their smartphone status was not recorded. The study sample represents our population of interest within an ASU, and we had complete data for 97.4% of participants with a 100% follow-up. There was no significant effect of study between the 2 observation periods (χ2 = 140.19; P = 0.10). The mean age of patients was 50.24 years. Further demographic data are presented in Table 1. Twenty-five (11.3%) patients were spP and 196 (88.7%) were spN. Fifty-two (23.5%) patients were discharged home on day 1, and 169 (76.5%) had admissions longer than 1 day (see Figure). Sixty (27%) patients underwent surgery during their admission. Twenty-two patients had unplanned readmissions; only 1 of these patients had been observed to be spP.
There was a statistically significant difference in ages between the spP and spN groups (t = 8.40; P < 0.0005), with the average age of spP patients being 31.84 years compared with 52.58 years for spN patients. There was no statistical difference between gender and smartphone status (χ2 = 1.78; P = 0.18; Table 2).
For those patients discharged home on day 1, there was a statistically significant association with being spP (χ2 = 14.55, P = 0.0001). Patients who were spP were 5.29 times more likely to be discharged on day 1 (95% CI, 2.24-12.84). Of the variables analyzed, only gender failed to demonstrate an effect on discharge home on day 1 (Table 3). Overall, the presence of a smartphone was found to have a sensitivity of 56.0% (95% CI, 34.93-75.60) and a specificity of 80.6% (95% CI, 74.37-85.90) in regard to same-day discharge. However, it was found to have a negative predictive value of 93.49% (95% CI, 88.65-96.71).
When examining readmission rates, only 4% of spP patients were readmitted versus 10.7% of spN patients. Accounting for variables, spP patients were 4 times less likely to be readmitted, though this was not statistically significant (OR 4.02; 95% CI, 0.43-37.2; P = 0.22). Furthermore, when examining only those patients discharged on day 1, smartphone status was not a predictor of readmission (OR 0.94; 95% CI, 0.06-15.2; P = 0 .97).
To mitigate the effect of age, analysis was conducted excluding those aged over 55 years (the previous retirement age in Australia), leaving 131 patients for analysis. The average age of spP patients was 31.8 years (SD 10.0) compared with 36.7 years (SD 10.9) for spN patients, representing a significant difference (t = 2.14; P = 0.04); 51.1% of patients were male, 19.1% of patients were spP, 26.0% of patients proceeded to an operation, the oldest spP was 51 years, and 29.0% of patients were discharged home on day 1. There was no difference in gender and smartphone status (χ2 = 0.33; P = 0.6). When analyzing those discharged on day 1, again spP patients were more likely to be discharged home (χ2 = 9.4; P = 0.002), and spP patients were 3.6 times more likely to be discharged home on day 1.
There were 4 spP patients who underwent an operation. Two patients had an incision and drainage of a perianal abscess, 1 patient underwent a laparotomy for an internal hernia after recently undergoing a Roux-en-Y gastric bypass at another hospital, and the final patient underwent a laparoscopic appendicectomy. One of these patients was still discharged home on day 1.
DISCUSSION
As J. A. Lindsay4 said, “For one mistake made for not knowing, ten mistakes are made for not looking.” At medical school, we are taught the finer techniques of the physical examination in order to support our diagnosis made from the history. It is not until we are experienced clinicians do we develop the clinical acumen and ability to tell an unwell patient from a well patient at a glance—colloquially known as the “end of the bed” assessment. In the pretechnology era, a well patient could frequently be seen reading their book, eg, the “novel-sign.” With the advent of the smartphone and electronic devices upon which novels can be read, statuses updated, and locations “checked into” (ie, the modern “vital signs”), the book sign may be a thing of the past. However, the ability for the clinician to assess a patient’s wellness is still crucial, and the value of any additional “physical signs” need to be estimated.
We observed a cohort of patients through a busy ASU in a tertiary hospital in South Australia, Australia. Acute surgical patients admitted to the hospital who were observed to be on their phones upon consultant review were more than 5 times likely to be discharged that same day. To the best of our knowledge, this is the first study to prospectively collect data to assess a frequently used but unevaluated clinical observation.
The use of a smartphone can tell us a lot about an individual’s physiology. We can assume the individual’s airway and breathing are adequate, allowing enough oxygen to reach the lungs and subsequently circulate. The individual is usually sitting up in bed and thus has an adequate blood pressure and blood oxygenation that can maintain cerebral perfusion. They have the cognitive and cerebral processing in place to function the device, and we can examine their cerebellar function by looking for fine-motor movements.
Mobile phone ownership is pervasive within Australia,5 with a conservative estimated 85.7% of the population (20.57 million people of a total population of approximately 24 million) owning a mobile phone and an estimated 50% to 79% of mobile phone ownership being of a smartphone.6,7 This ownership is not just limited to the young, with 74% of Australians over 65 owning or using a mobile phone.8 Despite this high phone ownership among those over 65, it is still significantly less than their younger counterparts and may be one reason for the absence of spP in those older than 51 years. A key point in the study is that overall phone ownership was not known, and, thus, it is not possible to determine the proportion of spN patients who were negative because they did not own a phone. However, based on general population data, the incidence of spP patients was well below that seen in the community (11.3%)5 and even when excluding those over 55, the percentage of spP patients only rose to 19.1%. Unsurprisingly, increasing age was associated with a decreased likelihood of being spP (P < 0.0005), as younger people are more likely to own a phone.8 There was no association with gender (P = 0.18). There are a number of explanations that may explain the lower than expected percentage of spP patients, including the inability for the patient to gather their possessions during a medical emergency, patients storing their phones prior to doctor review (72%-85% of Australians report talking on phones in public places to be rude or intrusive5), but more importantly, that our hypothesis that patients were too unwell to use their device appears to hold true.
There are potential alternate reasons other than smartphone status that may account for patients being discharged home on day 1. While there was no association seen with gender, the need for an operation prolonged a patient’s stay (OR 1.64; 95% CI, 0.046-0.46), and there was a trend seen with increasing age (OR 0.98; 95% CI, 0.96-1.00). Neither of these 2 demographics are unsurprising: increasing age is associated with increasing medical comorbidities and thus complexity; even the simplest of operations require a postprocedure observation period, automatically increasing their LOS. Additionally, measured demographics are limited and there may be further unmeasured reasons that account for earlier discharge.
The other key component to this study is the value of the physical examination, albeit only assessing 1 component: the general inspection. In their review of the value of the physical examination of the cardiovascular system, Elder et al. highlight an important point: in traditional teaching, the value of a physical sign is compared with a diagnostic reference, typically imaging or an invasive test.9 They argue that this definition undervalues the physical examination and list other values aside from accuracy including accessibility, contribution to clinical care beyond diagnoses, cost effectiveness, patients’ safety, patients’ perceptions, and pedagogic value; and they argue that the physical examination should always be considered in regard to the clinical context—in this case, the newly admitted general surgical patient.
The assessment of the presence or absence of a smartphone is readily performed upon general inspection and is easily visible; general inspection of the patient and failure to observe the clinical sign when present are 2 of the greatest errors associated with physical examination.10 Furthermore, given its unique status as a physical sign, the authors’ opinion and experience is that it is readily teachable. McGee states, “…a fundamental lesson [in regards to teaching] is that the diagnosis of many clinical problems, despite modern testing, still depends primarily on what the clinician sees, hears, and feels.”11 In their article, Paley et al. found that more than 80% of patients admitted from the ED under internal medicine could be accurately diagnosed based largely on history and examination alone and concluded that basic clinical skills are sufficient for achieving an accurate diagnosis in most cases.12 Although Paley et al. were assisted with basic tests (such as electrocardiogram and basic haematological and biochemistry results), the point of clinical skills is not lost. Furthermore, this assessment was made in a group of patients generally considered to be complex in contrast to the “standard” appendicitis or cholecystitis patient that makes up a significant proportion of general surgical patients.
There are a number of limitations to this study, however, including smartphones that may have been missed during the observational period. Potential confounding variables such as socioeconomic status and the overall smartphone ownership of our subjects were not known. We did not ask all admitted patients whether they owned a phone or whether they had a phone in their possession. Knowledge of those who owned phones but were not in possession of them could strengthen our argument that spN patients were not using their phone because they were unwell, rather than just not having access to it.
However, this study has a number of strengths, including a large sample size and data that were prospectively collected by a method and in a setting that was the same for all participants. Clear and appropriate definitions were used, which minimizes misclassification bias. Participants and decision makers were blinded to the study, and potentially confounding variables such as age and sex were accounted for.
Assessing the suitability for discharge from the hospital is a decision encountered by hospital-based clinicians every day. These skills are not taught, but are rather learned as a junior doctor acquires experience. It is unlikely that protocols will be developed to aid identification of potential discharges from an acute surgical ward; acute surgical conditions are too varied and dynamic to be able to pool all data. We continue to rely on our own and fellow colleagues’ (doctors, nurses, and other staff
CONCLUSION
While these observations might appear to be rather a simplistic way of trying to quantify whether or not a patient is fit for discharge, any clues that hint towards a patient’s well-being should be taken into account when making an overall assessment. The active use of a smartphone is one such measure.
Acknowledgments
The authors thank Emma Knight and Nancy Briggs from the Data Management & Analysis Centre, Discipline of Public Health, University of Adelaide.
Disclosure
No author nor the institution received any payment or services from a third party for any aspect of the submitted work and report no conflict of interest. There are no reported financial relationships with any entities by any of the authors. There are no patents pending based upon this publication. There are no relationships or activities that readers could perceive to have influenced, or give the appearance of influencing, the submitted work. The corresponding author is not in receipt of a research scholarship. The paper is not based on a previous communication.
The value placed on bedside clinical observation in the decision-making process of a patient’s illness has been diminished by today’s armamentarium of sophisticated technology. Increasing reliance is now placed on the result of nonspecific tests in preference to bedside clinical judgement in the diagnostic and management process. While diagnostic investigations have undoubtedly provided great advancements in medical care, they come at time and financial costs. Physicians should therefore continue to be encouraged to make clinical decisions based on their bedside assessment.
With hospital overcrowding a significant problem within the healthcare system and the expectation that it will worsen with an ageing population, identifying factors that predict patient suitability for discharge has become an important focus for clinicians.1,2 There exists a paucity of literature predicting discharge suitability of general surgical patients admitted through the emergency department (ED). Furthermore, despite the extensive research into the effectiveness of discharge planning,3 little research has been conducted to describe positive predictive indicators for discharge. Observations made during surgical rounds have led the authors to consider that individuals who are using a smartphone during their bedside assessment may be clinically well enough for discharge.
The aim of this study was to assess whether the clinical assessment of an acute surgical patient could be usefully augmented by the observation of the active use of smartphones (the smartphone sign) and whether this could be used as a surrogate marker to indicate a patient’s well-being and suitability for same-day discharge from the hospital in acute surgical patients.
METHODS
Design and Setting
This was a prospective observational study performed over 2 periods at a tertiary hospital in South Australia, Australia. At our institution, acute surgical patients are admitted to the acute surgical unit (ASU) from the ED by junior surgical doctors. Patients are then reviewed by the on-call surgical consultant, who implements management plans or advises discharge on 2 occasions per day.
Participants
All patients admitted under the ASU were considered eligible for the study. Exclusion criteria included patients that (i) required immediate surgical intervention (defined as time of review to theatre of less than 4 hours) and (ii) had immediate admission to the intensive care unit.
Consultant surgeons are employed within a general surgical subspecialty, including upper gastrointestinal, hepatobiliary, breast and endocrine, and colorectal. All surgeons from each team partake in the general surgery on-call roster. Each surgeon was included at least once within the observation periods. Experience of consultant surgeons ranged from 5 years of postfellowship experience to surgeons with more than 30 years of experience, with the majority having more than 10 years of postfellowship experience.
Patients were stratified into 2 distinct cohorts upon consultant review: smartphone positive (spP) was defined as a patient who was using a smartphone or who had their phone on their bed; a patient was classified as smartphone negative (spN) if they did not fulfil these criteria. The presence or absence of a smartphone was recorded by the authors, who were present on consultant ward rounds but not involved in the decision-making process of patient care. In order to minimize bias, only 1 surgeon (PGD) was aware that the study was being conducted and all patients were blinded to the study. Additional information that was collected included patient demographics, requirement for surgery, and length of stay (LOS). A patient who was discharged on the same day as the consultant review was considered to be discharged on day 1, all other patients were considered to have LOS greater than 1 day. Requirement for surgery was defined as a patient who underwent a surgical procedure in an operating suite. Thirty-day unplanned readmission rates for all patients were examined. Readmission to another public hospital within the state was also included within the readmission data.
Observation Periods
An initial 4-week pilot study was conducted to assess for a possible association between spP and same-day discharge. A second 8-week study period was undertaken 1 year later accounting for the employment of the authors at the study’s institution. Unless stated, the results described are the accumulation of both study periods.
Statistical Analysis
As this is the first study of its kind, no prior estimates of numbers were known. After 2 weeks of data collection, data were analyzed in order to provide an estimate of the total number of patients required to provide a statistically valid result (α = 0.05; power = 0.80). Sample size was calculated to be 40 subjects. It was agreed that in order to make the study as robust as possible, data should be collected for the 2 observation periods.
Demographic data are presented as means with standard deviations (SDs) or frequencies with percentages. A 2-sample Student t test was used to compare the age of spP and spN patients. A χ2 test and logistic regressions were used to assess the association between smartphone status and patient demographics, LOS, and requirement for surgery. Results are presented as odds ratios (ORs) with 95% confidence intervals (CIs). A P value of <0.05 was considered significant. All data were analyzed by using R 3.2.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
During the 2 observation periods, a total of 227 eligible surgical admissions were observed with complete data for 221 patients. Six patients were excluded as their smartphone status was not recorded. The study sample represents our population of interest within an ASU, and we had complete data for 97.4% of participants with a 100% follow-up. There was no significant effect of study between the 2 observation periods (χ2 = 140.19; P = 0.10). The mean age of patients was 50.24 years. Further demographic data are presented in Table 1. Twenty-five (11.3%) patients were spP and 196 (88.7%) were spN. Fifty-two (23.5%) patients were discharged home on day 1, and 169 (76.5%) had admissions longer than 1 day (see Figure). Sixty (27%) patients underwent surgery during their admission. Twenty-two patients had unplanned readmissions; only 1 of these patients had been observed to be spP.
There was a statistically significant difference in ages between the spP and spN groups (t = 8.40; P < 0.0005), with the average age of spP patients being 31.84 years compared with 52.58 years for spN patients. There was no statistical difference between gender and smartphone status (χ2 = 1.78; P = 0.18; Table 2).
For those patients discharged home on day 1, there was a statistically significant association with being spP (χ2 = 14.55, P = 0.0001). Patients who were spP were 5.29 times more likely to be discharged on day 1 (95% CI, 2.24-12.84). Of the variables analyzed, only gender failed to demonstrate an effect on discharge home on day 1 (Table 3). Overall, the presence of a smartphone was found to have a sensitivity of 56.0% (95% CI, 34.93-75.60) and a specificity of 80.6% (95% CI, 74.37-85.90) in regard to same-day discharge. However, it was found to have a negative predictive value of 93.49% (95% CI, 88.65-96.71).
When examining readmission rates, only 4% of spP patients were readmitted versus 10.7% of spN patients. Accounting for variables, spP patients were 4 times less likely to be readmitted, though this was not statistically significant (OR 4.02; 95% CI, 0.43-37.2; P = 0.22). Furthermore, when examining only those patients discharged on day 1, smartphone status was not a predictor of readmission (OR 0.94; 95% CI, 0.06-15.2; P = 0 .97).
To mitigate the effect of age, analysis was conducted excluding those aged over 55 years (the previous retirement age in Australia), leaving 131 patients for analysis. The average age of spP patients was 31.8 years (SD 10.0) compared with 36.7 years (SD 10.9) for spN patients, representing a significant difference (t = 2.14; P = 0.04); 51.1% of patients were male, 19.1% of patients were spP, 26.0% of patients proceeded to an operation, the oldest spP was 51 years, and 29.0% of patients were discharged home on day 1. There was no difference in gender and smartphone status (χ2 = 0.33; P = 0.6). When analyzing those discharged on day 1, again spP patients were more likely to be discharged home (χ2 = 9.4; P = 0.002), and spP patients were 3.6 times more likely to be discharged home on day 1.
There were 4 spP patients who underwent an operation. Two patients had an incision and drainage of a perianal abscess, 1 patient underwent a laparotomy for an internal hernia after recently undergoing a Roux-en-Y gastric bypass at another hospital, and the final patient underwent a laparoscopic appendicectomy. One of these patients was still discharged home on day 1.
DISCUSSION
As J. A. Lindsay4 said, “For one mistake made for not knowing, ten mistakes are made for not looking.” At medical school, we are taught the finer techniques of the physical examination in order to support our diagnosis made from the history. It is not until we are experienced clinicians do we develop the clinical acumen and ability to tell an unwell patient from a well patient at a glance—colloquially known as the “end of the bed” assessment. In the pretechnology era, a well patient could frequently be seen reading their book, eg, the “novel-sign.” With the advent of the smartphone and electronic devices upon which novels can be read, statuses updated, and locations “checked into” (ie, the modern “vital signs”), the book sign may be a thing of the past. However, the ability for the clinician to assess a patient’s wellness is still crucial, and the value of any additional “physical signs” need to be estimated.
We observed a cohort of patients through a busy ASU in a tertiary hospital in South Australia, Australia. Acute surgical patients admitted to the hospital who were observed to be on their phones upon consultant review were more than 5 times likely to be discharged that same day. To the best of our knowledge, this is the first study to prospectively collect data to assess a frequently used but unevaluated clinical observation.
The use of a smartphone can tell us a lot about an individual’s physiology. We can assume the individual’s airway and breathing are adequate, allowing enough oxygen to reach the lungs and subsequently circulate. The individual is usually sitting up in bed and thus has an adequate blood pressure and blood oxygenation that can maintain cerebral perfusion. They have the cognitive and cerebral processing in place to function the device, and we can examine their cerebellar function by looking for fine-motor movements.
Mobile phone ownership is pervasive within Australia,5 with a conservative estimated 85.7% of the population (20.57 million people of a total population of approximately 24 million) owning a mobile phone and an estimated 50% to 79% of mobile phone ownership being of a smartphone.6,7 This ownership is not just limited to the young, with 74% of Australians over 65 owning or using a mobile phone.8 Despite this high phone ownership among those over 65, it is still significantly less than their younger counterparts and may be one reason for the absence of spP in those older than 51 years. A key point in the study is that overall phone ownership was not known, and, thus, it is not possible to determine the proportion of spN patients who were negative because they did not own a phone. However, based on general population data, the incidence of spP patients was well below that seen in the community (11.3%)5 and even when excluding those over 55, the percentage of spP patients only rose to 19.1%. Unsurprisingly, increasing age was associated with a decreased likelihood of being spP (P < 0.0005), as younger people are more likely to own a phone.8 There was no association with gender (P = 0.18). There are a number of explanations that may explain the lower than expected percentage of spP patients, including the inability for the patient to gather their possessions during a medical emergency, patients storing their phones prior to doctor review (72%-85% of Australians report talking on phones in public places to be rude or intrusive5), but more importantly, that our hypothesis that patients were too unwell to use their device appears to hold true.
There are potential alternate reasons other than smartphone status that may account for patients being discharged home on day 1. While there was no association seen with gender, the need for an operation prolonged a patient’s stay (OR 1.64; 95% CI, 0.046-0.46), and there was a trend seen with increasing age (OR 0.98; 95% CI, 0.96-1.00). Neither of these 2 demographics are unsurprising: increasing age is associated with increasing medical comorbidities and thus complexity; even the simplest of operations require a postprocedure observation period, automatically increasing their LOS. Additionally, measured demographics are limited and there may be further unmeasured reasons that account for earlier discharge.
The other key component to this study is the value of the physical examination, albeit only assessing 1 component: the general inspection. In their review of the value of the physical examination of the cardiovascular system, Elder et al. highlight an important point: in traditional teaching, the value of a physical sign is compared with a diagnostic reference, typically imaging or an invasive test.9 They argue that this definition undervalues the physical examination and list other values aside from accuracy including accessibility, contribution to clinical care beyond diagnoses, cost effectiveness, patients’ safety, patients’ perceptions, and pedagogic value; and they argue that the physical examination should always be considered in regard to the clinical context—in this case, the newly admitted general surgical patient.
The assessment of the presence or absence of a smartphone is readily performed upon general inspection and is easily visible; general inspection of the patient and failure to observe the clinical sign when present are 2 of the greatest errors associated with physical examination.10 Furthermore, given its unique status as a physical sign, the authors’ opinion and experience is that it is readily teachable. McGee states, “…a fundamental lesson [in regards to teaching] is that the diagnosis of many clinical problems, despite modern testing, still depends primarily on what the clinician sees, hears, and feels.”11 In their article, Paley et al. found that more than 80% of patients admitted from the ED under internal medicine could be accurately diagnosed based largely on history and examination alone and concluded that basic clinical skills are sufficient for achieving an accurate diagnosis in most cases.12 Although Paley et al. were assisted with basic tests (such as electrocardiogram and basic haematological and biochemistry results), the point of clinical skills is not lost. Furthermore, this assessment was made in a group of patients generally considered to be complex in contrast to the “standard” appendicitis or cholecystitis patient that makes up a significant proportion of general surgical patients.
There are a number of limitations to this study, however, including smartphones that may have been missed during the observational period. Potential confounding variables such as socioeconomic status and the overall smartphone ownership of our subjects were not known. We did not ask all admitted patients whether they owned a phone or whether they had a phone in their possession. Knowledge of those who owned phones but were not in possession of them could strengthen our argument that spN patients were not using their phone because they were unwell, rather than just not having access to it.
However, this study has a number of strengths, including a large sample size and data that were prospectively collected by a method and in a setting that was the same for all participants. Clear and appropriate definitions were used, which minimizes misclassification bias. Participants and decision makers were blinded to the study, and potentially confounding variables such as age and sex were accounted for.
Assessing the suitability for discharge from the hospital is a decision encountered by hospital-based clinicians every day. These skills are not taught, but are rather learned as a junior doctor acquires experience. It is unlikely that protocols will be developed to aid identification of potential discharges from an acute surgical ward; acute surgical conditions are too varied and dynamic to be able to pool all data. We continue to rely on our own and fellow colleagues’ (doctors, nurses, and other staff
CONCLUSION
While these observations might appear to be rather a simplistic way of trying to quantify whether or not a patient is fit for discharge, any clues that hint towards a patient’s well-being should be taken into account when making an overall assessment. The active use of a smartphone is one such measure.
Acknowledgments
The authors thank Emma Knight and Nancy Briggs from the Data Management & Analysis Centre, Discipline of Public Health, University of Adelaide.
Disclosure
No author nor the institution received any payment or services from a third party for any aspect of the submitted work and report no conflict of interest. There are no reported financial relationships with any entities by any of the authors. There are no patents pending based upon this publication. There are no relationships or activities that readers could perceive to have influenced, or give the appearance of influencing, the submitted work. The corresponding author is not in receipt of a research scholarship. The paper is not based on a previous communication.
1. Sprivulis PC, Da Silva JA, Jacobs IG, Frazer AR, Jelinek GA. The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments. Med J Aust. 2006;184(5):208-212. PubMed
2. Shepherd T. Hospital Overcrowding kills as many as our road toll. The Advertiser. November 23, 2010. Available from: http://www.adelaidenow.com.au/news/south-australia/hospital-overcrowding-kills-as-many-as-our-road-toll/news-story/3389668c23b8b141f1d335b096ced416. Accessed February 2, 2017.
3. Shepperd S, Lannin NA, Clemson LM, McCluskey A, Cameron ID, Barras SL. Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;Jan 31(1):CD000313. PubMed
4. Breathnach CS, Moynihan JB. James Alexander Lindsay (1856–1931), and his clinical axioms and aphorisms. Ulster Med J. 2012;81(3):149-153. PubMed
5. Enhanced Media Metrics Australia. Product Insights Report. Digital Australia: A snapshot of attitudes and usage. August 2013. Ipsos Australia. North Sydney, Australia. Report available from: https://emma.com.au/wp-content/uploads/2013/10/digital.pdf
6. Australian Communications and Media Authority. Communications report 2013-24. Melbounre: Commonwealth of Australia; 2014. http://www.acma.gov.au/~/media/Research%20and%20Analysis/Publication/Comms%20Report%202013%2014/PDF/Communications%20report%20201314_LOW-RES%20FOR%20WEB%20pdf.pdf
7. Drumm J, Johnston S. Mobile Consumer Survery 2015—The Australian Cut. Deloitte. Australia; 2015. Deloitte Touche Tohmatsu. Sydney, Australia. file:///C:/Users/user/Desktop/deloitte-au-tmt-mobile-consumer-survey-2015-291015.pdf
8. Older Australians Resist Cutting the Cord: Australian Communications and Media Authority. 2014. http://www.acma.gov.au/theACMA/engage-blogs/engage-blogs/Research-snapshots/Older-Australians-resist-cutting-the-cord. Accessed February 23, 2017.
9. Elder A, Japp A, Verghese A. How valuable is physical examination of the cardiovascular system? BMJ. 2016;354:i3309. PubMed
10. Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JP. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med. 2015;128(12):1322-1324.e3. PubMed
11. McGee S. A piece of my mind. Bedside teaching rounds reconsidered. JAMA. 2014;311(19):1971-1972. PubMed
12. Paley L, Zornitzki T, Cohen J, Friedman J, Kozak N, Schattner A. Utility of clinical examination in the diagnosis of emergency department patients admitted to the department of medicine of an academic hospital. Arch Intern Med. 2011;171(15):1394-1396. PubMed
1. Sprivulis PC, Da Silva JA, Jacobs IG, Frazer AR, Jelinek GA. The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments. Med J Aust. 2006;184(5):208-212. PubMed
2. Shepherd T. Hospital Overcrowding kills as many as our road toll. The Advertiser. November 23, 2010. Available from: http://www.adelaidenow.com.au/news/south-australia/hospital-overcrowding-kills-as-many-as-our-road-toll/news-story/3389668c23b8b141f1d335b096ced416. Accessed February 2, 2017.
3. Shepperd S, Lannin NA, Clemson LM, McCluskey A, Cameron ID, Barras SL. Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;Jan 31(1):CD000313. PubMed
4. Breathnach CS, Moynihan JB. James Alexander Lindsay (1856–1931), and his clinical axioms and aphorisms. Ulster Med J. 2012;81(3):149-153. PubMed
5. Enhanced Media Metrics Australia. Product Insights Report. Digital Australia: A snapshot of attitudes and usage. August 2013. Ipsos Australia. North Sydney, Australia. Report available from: https://emma.com.au/wp-content/uploads/2013/10/digital.pdf
6. Australian Communications and Media Authority. Communications report 2013-24. Melbounre: Commonwealth of Australia; 2014. http://www.acma.gov.au/~/media/Research%20and%20Analysis/Publication/Comms%20Report%202013%2014/PDF/Communications%20report%20201314_LOW-RES%20FOR%20WEB%20pdf.pdf
7. Drumm J, Johnston S. Mobile Consumer Survery 2015—The Australian Cut. Deloitte. Australia; 2015. Deloitte Touche Tohmatsu. Sydney, Australia. file:///C:/Users/user/Desktop/deloitte-au-tmt-mobile-consumer-survey-2015-291015.pdf
8. Older Australians Resist Cutting the Cord: Australian Communications and Media Authority. 2014. http://www.acma.gov.au/theACMA/engage-blogs/engage-blogs/Research-snapshots/Older-Australians-resist-cutting-the-cord. Accessed February 23, 2017.
9. Elder A, Japp A, Verghese A. How valuable is physical examination of the cardiovascular system? BMJ. 2016;354:i3309. PubMed
10. Verghese A, Charlton B, Kassirer JP, Ramsey M, Ioannidis JP. Inadequacies of physical examination as a cause of medical errors and adverse events: a collection of vignettes. Am J Med. 2015;128(12):1322-1324.e3. PubMed
11. McGee S. A piece of my mind. Bedside teaching rounds reconsidered. JAMA. 2014;311(19):1971-1972. PubMed
12. Paley L, Zornitzki T, Cohen J, Friedman J, Kozak N, Schattner A. Utility of clinical examination in the diagnosis of emergency department patients admitted to the department of medicine of an academic hospital. Arch Intern Med. 2011;171(15):1394-1396. PubMed
© 2018 Society of Hospital Medicine
Total Hip Arthroplasty and Hemiarthroplasty: US National Trends in the Treatment of Femoral Neck Fractures
Take-Home Points
- An increasing number of THAs and HAs were performed over time for FNF.
- HA patients tended to be older.
- Hospitalization and blood transfusion rates were higher for THA.
- Hospital size affected the rate of HAs, while hospital location affected the rate of THAs.
- A larger proportion of THA patients had private insurance.
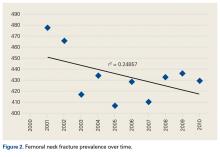
Femoral neck fractures (FNFs) are a common source of morbidity and mortality worldwide. The increasing number of FNFs in the United States is attributed to increases in number of US residents >65 years old, the average life span, and the incidence of osteoporosis.1 Three hundred forty thousand hip fractures occurred in the United States in 1996, and the number is expected to double by 2050.2 By that year, an estimated 6.3 million hip fractures will occur worldwide.3 Given the 1-year mortality rate of 14% to 36%, optimizing the management of these fractures is an important public health issue that must be addressed.4
Treatment is based on preoperative ambulatory status, cognitive function, comorbidities, fracture type and displacement, and other factors. In physiologically elderly patients with displaced fractures, surgical treatment usually involves either hemiarthroplasty (HA) or total hip arthroplasty (THA). There is controversy regarding which modality is the preferred treatment.
Proponents of HA point to a higher rate of dislocation for FNFs treated with THAs,5,6 attributed to increased range of motion.7 Proponents of THA point to superior short-term clinical results and fewer complications, especially in mobile, independent patients.8
We conducted a study to assess recent US national trends in performing THA and HA for FNFs and to evaluate perioperative outcomes for each treatment group.
Materials and Methods
Data for this study were obtained from the National Center for Health Statistics (NCHS) National Hospital Discharge Survey (NHDS) and were imported into Microsoft Office Excel 2010.9 The NHDS examines patient discharges from various hospitals across the US, including federal, military, and Veterans Administration hospitals.9 Only short-stay hospitals (mean stay, <30 days) and hospitals with a general specialty are included in the survey. Each year, about 1% of all hospital admissions from across the US are abstracted and weighted to provide nationwide estimates. The information collected from each hospital record includes age, sex, race, marital status, discharge month, discharge status, days of care, hospital location, hospital size (number of beds), hospital type (proprietary or for-profit, government, nonprofit/church), and up to 15 discharge diagnoses and 8 procedures performed during admission.9
International Classification of Diseases, Ninth Revision (ICD-9) procedure codes were used to search the NHDS for patients admitted after FNF for each year from 2001 through 2010. These codes were then used to identify patients within this group who underwent THA or HA. We also collected data on patient demographics, hospitalization duration, discharge disposition, in-hospital adverse events (deep vein thrombosis [DVT], pulmonary embolism [PE], blood transfusion, mortality), form of primary medical insurance, number of hospital beds (0-99, 100-199, 200-299, 300-499, ≥500), hospital type (proprietary, government, nonprofit/church), and hospital region (Northeast, Midwest, South, West).
Trends were evaluated by linear regression with the Pearson correlation coefficient (r). Statistical comparisons were made using the Student t test for continuous data, and both the Fisher exact test and the χ2 test for categorical variables. Significance level was set at P < .05. All analyses were performed with IBM SPSS Statistics 22.
Results
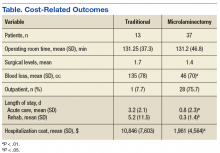
Hospital stay was longer (P < .01) for THA patients (7.7 days; range, 1-312 days) than for HA patients (6.7 days; range, 1-118 days), and blood transfusion rate was higher (P = .02) for THA patients (30.4%) than for HA patients (25.7%), but the groups did not differ in their rates of DVT (THA, 1.2%; HA, 0.80%, P = .50), PE (THA, 0.52%; HA, 0.72%, P = .52), or mortality (THA, 1.8%; HA, 2.9%; P = .16). Discharge disposition varied with surgical status (P < .01): 23.2% of THA patients and 11.6% of HA patients were discharged directly home after their inpatient stay, and 76.8% of THA patients and 88.4% of HA patients were discharged or transferred to a short- or long-term care facility.
Private medical insurance provided coverage for 14.3% of THAs and 9.1% of HAs, and Medicare provided coverage for 80.9% of THAs and 86.0% of HAs (P < .01).
Discussion
The NHDS data showed a preference for HA over THA in the treatment of FNFs and suggested THA was favored for younger, healthier patients while HA was reserved for older patients with more comorbidities. Despite being younger and healthier, the THA group had higher transfusion rates and longer hospitalizations, possibly because of the increased complexity of THA procedures, which generally involve more operative time and increased blood loss. The resultant higher transfusion rate for THAs likely contributed to longer hospitalizations for FNFs. However, the THA and HA groups did not differ in their rates of DVT, PE, or mortality.
Multiple studies have noted no differences in mortality, infection, or general complications between THA and HA for FNF.8,10,11 THA patients have better functional outcomes, including Harris and Oxford hip scores and walking distance, but higher dislocation rates,8,10-12 and HA patients are at higher risk for reoperation because of progressive acetabular erosion.8,10,11
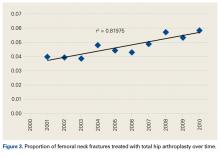
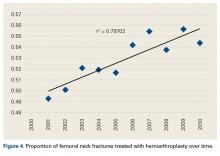
We noted an increase in use of both THA and HA for FNF over the study period (2001-2010). In a review of operative treatment for FNF by surgeons applying for the American Board of Orthopaedic Surgery certification between 1999 and 2011, Miller and colleagues13 found a similar increase in the THA rate over time, but decreases in the HA and internal fixation rates, with candidates in the “adult reconstruction” subspecialty showing a particularly strong trend toward THA use.
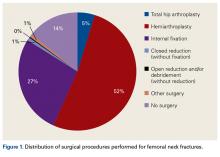
These findings reflect a general propensity toward femoral head replacement rather than preservation through open reduction and internal fixation (ORIF). Recent studies have found that ORIF carries a 39% to 43% rate of fixation failure and need for secondary revision, as well as risks of avascular necrosis, malunion, and nonunion.1,14-16 This need for secondary surgery makes ORIF ultimately less cost-effective than either THA or HA.16,17 Most authors would recommend arthroplasty for FNF in elderly patients with normal mental function1,16,18 and would reserve ORIF for young patients with good bone stock, joint space preservation, and reducible noncomminuted fractures.1,19
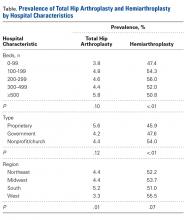
Our study results suggest that smaller hospitals (<100 beds) tend to have lower rates of HA (P < .01, significant) and THA (P = .10, not significant; Table), possibly because FNF patients who present to these hospitals may be referred elsewhere because of regional differences in the availability of orthopedic traumatologists and arthroplasty subspecialists. Surgeon volume affects postoperative outcomes and may play a role in referral patterns.20 Ames and colleagues20 found that HA performed for FNF by surgeons with high-volume THA experience (vs non-hip-arthroplasty surgeons) had lower rates of dislocation, superficial infection, and mortality.
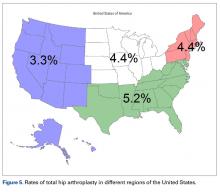
Regional differences were significant for THA alone, with the highest THA rates in the South (5.2%) and the lowest in the West (3.3%; Figure 5). There were no clear regional trends for HA. Possible explanations include a propensity toward a more aggressive approach in these regions, increased regional prevalence of acetabular disease, regional surgeon preferences, and regional differences in patient characteristics (eg, increased prevalence of obesity in the South).21
HA rates were highest for nonprofit/church hospitals and lowest for proprietary hospitals, whereas THA rates did not differ by hospital type. Possible explanations include an older, less mobile nonprofit/church patient cohort that is more amenable to HA, and surgeon preference.
THA patients were more likely to be covered by private medical insurance than by Medicare—a finding in agreement with Hochfelder and colleagues,22 who found that, compared with federal insurance and self-pay patients, private insurance patients were 41% more likely to undergo THA than HA or internal fixation for FNF. We think that the age difference between our THA and HA groups contributed to the insurance variability in our study.
Our study had several limitations. It was conducted to examine the rates of THA and HA after FNF, not to survey treatment types, including ORIF and nonoperative management. The NHDS database does not provide information on HA implant type (unipolar, bipolar), use or nonuse of cement with HA, or surgical approach. Surgical approach could influence the rate of postoperative dislocation, an outcome measure that was not examined in this study. Last, the NHDS database tracks admissions and discharges, not patients. When a patient is discharged, collection of information on the patient’s postoperative course stops; a patient who returns even only 1 day later is recorded as a new or unique patient. Therefore, intermediate or long-term outcome information is unavailable, which likely led to an underrepresentation of DVT, PE, and mortality after these THA and HA procedures.
There was a trend toward femoral head replacement rather than ORIF in the treatment of FNF. Cognitively functional and independent elderly patients, and patients with osteoarthritis or rheumatoid arthritis, may benefit from THA, whereas HA may be better suited to cognitively dysfunctional patients.23,24 The NHDS reflects an increasing trend toward arthroplasty over ORIF, but the exact treatment choice is affected by hospital type, size, location and surgeon preference, training, and subspecialization.
1. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287-293.
2. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
3. Kannus P, Parkkari J, Sievänen H, Heinonen A, Vuori I, Järvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 suppl):57S-63S.
4. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525.
5. Papandrea RF, Froimson MI. Total hip arthroplasty after acute displaced femoral neck fractures. Am J Orthop. 1996;25(2):85-88.
6. Burgers PT, Van Geene AR, Van den Bekerom MP, et al. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures in the healthy elderly: a meta-analysis and systematic review of randomized trials. Int Orthop. 2012;36(8):1549-1560.
7. Skinner P, Riley D, Ellery J, Beaumont A, Coumine R, Shafighian B. Displaced subcapital fractures of the femur: a prospective randomized comparison of internal fixation, hemiarthroplasty and total hip replacement. Injury. 1989;20(5):291-293.
8. Baker RP, Squires B, Gargan MF, Bannister GC. Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(12):2583-2589.
9. Centers for Disease Control and Prevention, National Center for Health Statistics. National Hospital Discharge Survey. http://www.cdc.gov/nchs/nhds/about_nhds.htm. Last updated December 6, 2011. Accessed December 10, 2013.
10. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplasty. 2012;27(4):583-590.
11. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235-2243.
12. Hopley C, Stengel D, Ekkernkamp A, Wich M. Primary total hip arthroplasty versus hemiarthroplasty for displaced intracapsular hip fractures in older patients: systematic review. BMJ. 2010;340:c2332.
13. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2014;96(17):e149.
14. Rogmark C, Carlsson A, Johnell O, Sernbo I. A prospective randomised trial of internal fixation versus arthroplasty for displaced fractures of the neck of the femur. Functional outcome for 450 patients at two years. J Bone Joint Surg Br. 2002;84(2):183-188.
15. Bhandari M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. J Bone Joint Surg Am. 2003;85(9):1673-1681.
16. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
17. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop Relat Res. 2001;(383):229-242.
18. Johansson T, Jacobsson SA, Ivarsson I, Knutsson A, Wahlström O. Internal fixation versus total hip arthroplasty in the treatment of displaced femoral neck fractures: a prospective randomized study of 100 hips. Acta Orthop Scand. 2000;71(6):597-602.
19. Shah AK, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop Relat Res. 2002;(399):28-34.
20. Ames JB, Lurie JD, Tomek IM, Zhou W, Koval KJ. Does surgeon volume for total hip arthroplasty affect outcomes after hemiarthroplasty for femoral neck fracture? Am J Orthop. 2010;39(8):E84-E89.
21. Le A, Judd SE, Allison DB, et al. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity. 2014;22(1):300-306.
22. Hochfelder JP, Khatib ON, Glait SA, Slover JD. Femoral neck fractures in New York state. Is the rate of THA increasing, and do race or payer influence decision making? J Orthop Trauma. 2014;28(7):422-426.
23. Lowe JA, Crist BD, Bhandari M, Ferguson TA. Optimal treatment of femoral neck fractures according to patient’s physiologic age: an evidence-based review. Orthop Clin North Am. 2010;41(2):157-166.
24. Callaghan JJ, Liu SS, Haidukewych GJ. Subcapital fractures: a changing paradigm. J Bone Joint Surg Br. 2012;94(11 suppl A):19-21.
Take-Home Points
- An increasing number of THAs and HAs were performed over time for FNF.
- HA patients tended to be older.
- Hospitalization and blood transfusion rates were higher for THA.
- Hospital size affected the rate of HAs, while hospital location affected the rate of THAs.
- A larger proportion of THA patients had private insurance.
Femoral neck fractures (FNFs) are a common source of morbidity and mortality worldwide. The increasing number of FNFs in the United States is attributed to increases in number of US residents >65 years old, the average life span, and the incidence of osteoporosis.1 Three hundred forty thousand hip fractures occurred in the United States in 1996, and the number is expected to double by 2050.2 By that year, an estimated 6.3 million hip fractures will occur worldwide.3 Given the 1-year mortality rate of 14% to 36%, optimizing the management of these fractures is an important public health issue that must be addressed.4
Treatment is based on preoperative ambulatory status, cognitive function, comorbidities, fracture type and displacement, and other factors. In physiologically elderly patients with displaced fractures, surgical treatment usually involves either hemiarthroplasty (HA) or total hip arthroplasty (THA). There is controversy regarding which modality is the preferred treatment.
Proponents of HA point to a higher rate of dislocation for FNFs treated with THAs,5,6 attributed to increased range of motion.7 Proponents of THA point to superior short-term clinical results and fewer complications, especially in mobile, independent patients.8
We conducted a study to assess recent US national trends in performing THA and HA for FNFs and to evaluate perioperative outcomes for each treatment group.
Materials and Methods
Data for this study were obtained from the National Center for Health Statistics (NCHS) National Hospital Discharge Survey (NHDS) and were imported into Microsoft Office Excel 2010.9 The NHDS examines patient discharges from various hospitals across the US, including federal, military, and Veterans Administration hospitals.9 Only short-stay hospitals (mean stay, <30 days) and hospitals with a general specialty are included in the survey. Each year, about 1% of all hospital admissions from across the US are abstracted and weighted to provide nationwide estimates. The information collected from each hospital record includes age, sex, race, marital status, discharge month, discharge status, days of care, hospital location, hospital size (number of beds), hospital type (proprietary or for-profit, government, nonprofit/church), and up to 15 discharge diagnoses and 8 procedures performed during admission.9
International Classification of Diseases, Ninth Revision (ICD-9) procedure codes were used to search the NHDS for patients admitted after FNF for each year from 2001 through 2010. These codes were then used to identify patients within this group who underwent THA or HA. We also collected data on patient demographics, hospitalization duration, discharge disposition, in-hospital adverse events (deep vein thrombosis [DVT], pulmonary embolism [PE], blood transfusion, mortality), form of primary medical insurance, number of hospital beds (0-99, 100-199, 200-299, 300-499, ≥500), hospital type (proprietary, government, nonprofit/church), and hospital region (Northeast, Midwest, South, West).
Trends were evaluated by linear regression with the Pearson correlation coefficient (r). Statistical comparisons were made using the Student t test for continuous data, and both the Fisher exact test and the χ2 test for categorical variables. Significance level was set at P < .05. All analyses were performed with IBM SPSS Statistics 22.
Results
Hospital stay was longer (P < .01) for THA patients (7.7 days; range, 1-312 days) than for HA patients (6.7 days; range, 1-118 days), and blood transfusion rate was higher (P = .02) for THA patients (30.4%) than for HA patients (25.7%), but the groups did not differ in their rates of DVT (THA, 1.2%; HA, 0.80%, P = .50), PE (THA, 0.52%; HA, 0.72%, P = .52), or mortality (THA, 1.8%; HA, 2.9%; P = .16). Discharge disposition varied with surgical status (P < .01): 23.2% of THA patients and 11.6% of HA patients were discharged directly home after their inpatient stay, and 76.8% of THA patients and 88.4% of HA patients were discharged or transferred to a short- or long-term care facility.
Private medical insurance provided coverage for 14.3% of THAs and 9.1% of HAs, and Medicare provided coverage for 80.9% of THAs and 86.0% of HAs (P < .01).
Discussion
The NHDS data showed a preference for HA over THA in the treatment of FNFs and suggested THA was favored for younger, healthier patients while HA was reserved for older patients with more comorbidities. Despite being younger and healthier, the THA group had higher transfusion rates and longer hospitalizations, possibly because of the increased complexity of THA procedures, which generally involve more operative time and increased blood loss. The resultant higher transfusion rate for THAs likely contributed to longer hospitalizations for FNFs. However, the THA and HA groups did not differ in their rates of DVT, PE, or mortality.
Multiple studies have noted no differences in mortality, infection, or general complications between THA and HA for FNF.8,10,11 THA patients have better functional outcomes, including Harris and Oxford hip scores and walking distance, but higher dislocation rates,8,10-12 and HA patients are at higher risk for reoperation because of progressive acetabular erosion.8,10,11
We noted an increase in use of both THA and HA for FNF over the study period (2001-2010). In a review of operative treatment for FNF by surgeons applying for the American Board of Orthopaedic Surgery certification between 1999 and 2011, Miller and colleagues13 found a similar increase in the THA rate over time, but decreases in the HA and internal fixation rates, with candidates in the “adult reconstruction” subspecialty showing a particularly strong trend toward THA use.
These findings reflect a general propensity toward femoral head replacement rather than preservation through open reduction and internal fixation (ORIF). Recent studies have found that ORIF carries a 39% to 43% rate of fixation failure and need for secondary revision, as well as risks of avascular necrosis, malunion, and nonunion.1,14-16 This need for secondary surgery makes ORIF ultimately less cost-effective than either THA or HA.16,17 Most authors would recommend arthroplasty for FNF in elderly patients with normal mental function1,16,18 and would reserve ORIF for young patients with good bone stock, joint space preservation, and reducible noncomminuted fractures.1,19
Our study results suggest that smaller hospitals (<100 beds) tend to have lower rates of HA (P < .01, significant) and THA (P = .10, not significant; Table), possibly because FNF patients who present to these hospitals may be referred elsewhere because of regional differences in the availability of orthopedic traumatologists and arthroplasty subspecialists. Surgeon volume affects postoperative outcomes and may play a role in referral patterns.20 Ames and colleagues20 found that HA performed for FNF by surgeons with high-volume THA experience (vs non-hip-arthroplasty surgeons) had lower rates of dislocation, superficial infection, and mortality.
Regional differences were significant for THA alone, with the highest THA rates in the South (5.2%) and the lowest in the West (3.3%; Figure 5). There were no clear regional trends for HA. Possible explanations include a propensity toward a more aggressive approach in these regions, increased regional prevalence of acetabular disease, regional surgeon preferences, and regional differences in patient characteristics (eg, increased prevalence of obesity in the South).21
HA rates were highest for nonprofit/church hospitals and lowest for proprietary hospitals, whereas THA rates did not differ by hospital type. Possible explanations include an older, less mobile nonprofit/church patient cohort that is more amenable to HA, and surgeon preference.
THA patients were more likely to be covered by private medical insurance than by Medicare—a finding in agreement with Hochfelder and colleagues,22 who found that, compared with federal insurance and self-pay patients, private insurance patients were 41% more likely to undergo THA than HA or internal fixation for FNF. We think that the age difference between our THA and HA groups contributed to the insurance variability in our study.
Our study had several limitations. It was conducted to examine the rates of THA and HA after FNF, not to survey treatment types, including ORIF and nonoperative management. The NHDS database does not provide information on HA implant type (unipolar, bipolar), use or nonuse of cement with HA, or surgical approach. Surgical approach could influence the rate of postoperative dislocation, an outcome measure that was not examined in this study. Last, the NHDS database tracks admissions and discharges, not patients. When a patient is discharged, collection of information on the patient’s postoperative course stops; a patient who returns even only 1 day later is recorded as a new or unique patient. Therefore, intermediate or long-term outcome information is unavailable, which likely led to an underrepresentation of DVT, PE, and mortality after these THA and HA procedures.
There was a trend toward femoral head replacement rather than ORIF in the treatment of FNF. Cognitively functional and independent elderly patients, and patients with osteoarthritis or rheumatoid arthritis, may benefit from THA, whereas HA may be better suited to cognitively dysfunctional patients.23,24 The NHDS reflects an increasing trend toward arthroplasty over ORIF, but the exact treatment choice is affected by hospital type, size, location and surgeon preference, training, and subspecialization.
Take-Home Points
- An increasing number of THAs and HAs were performed over time for FNF.
- HA patients tended to be older.
- Hospitalization and blood transfusion rates were higher for THA.
- Hospital size affected the rate of HAs, while hospital location affected the rate of THAs.
- A larger proportion of THA patients had private insurance.
Femoral neck fractures (FNFs) are a common source of morbidity and mortality worldwide. The increasing number of FNFs in the United States is attributed to increases in number of US residents >65 years old, the average life span, and the incidence of osteoporosis.1 Three hundred forty thousand hip fractures occurred in the United States in 1996, and the number is expected to double by 2050.2 By that year, an estimated 6.3 million hip fractures will occur worldwide.3 Given the 1-year mortality rate of 14% to 36%, optimizing the management of these fractures is an important public health issue that must be addressed.4
Treatment is based on preoperative ambulatory status, cognitive function, comorbidities, fracture type and displacement, and other factors. In physiologically elderly patients with displaced fractures, surgical treatment usually involves either hemiarthroplasty (HA) or total hip arthroplasty (THA). There is controversy regarding which modality is the preferred treatment.
Proponents of HA point to a higher rate of dislocation for FNFs treated with THAs,5,6 attributed to increased range of motion.7 Proponents of THA point to superior short-term clinical results and fewer complications, especially in mobile, independent patients.8
We conducted a study to assess recent US national trends in performing THA and HA for FNFs and to evaluate perioperative outcomes for each treatment group.
Materials and Methods
Data for this study were obtained from the National Center for Health Statistics (NCHS) National Hospital Discharge Survey (NHDS) and were imported into Microsoft Office Excel 2010.9 The NHDS examines patient discharges from various hospitals across the US, including federal, military, and Veterans Administration hospitals.9 Only short-stay hospitals (mean stay, <30 days) and hospitals with a general specialty are included in the survey. Each year, about 1% of all hospital admissions from across the US are abstracted and weighted to provide nationwide estimates. The information collected from each hospital record includes age, sex, race, marital status, discharge month, discharge status, days of care, hospital location, hospital size (number of beds), hospital type (proprietary or for-profit, government, nonprofit/church), and up to 15 discharge diagnoses and 8 procedures performed during admission.9
International Classification of Diseases, Ninth Revision (ICD-9) procedure codes were used to search the NHDS for patients admitted after FNF for each year from 2001 through 2010. These codes were then used to identify patients within this group who underwent THA or HA. We also collected data on patient demographics, hospitalization duration, discharge disposition, in-hospital adverse events (deep vein thrombosis [DVT], pulmonary embolism [PE], blood transfusion, mortality), form of primary medical insurance, number of hospital beds (0-99, 100-199, 200-299, 300-499, ≥500), hospital type (proprietary, government, nonprofit/church), and hospital region (Northeast, Midwest, South, West).
Trends were evaluated by linear regression with the Pearson correlation coefficient (r). Statistical comparisons were made using the Student t test for continuous data, and both the Fisher exact test and the χ2 test for categorical variables. Significance level was set at P < .05. All analyses were performed with IBM SPSS Statistics 22.
Results
Hospital stay was longer (P < .01) for THA patients (7.7 days; range, 1-312 days) than for HA patients (6.7 days; range, 1-118 days), and blood transfusion rate was higher (P = .02) for THA patients (30.4%) than for HA patients (25.7%), but the groups did not differ in their rates of DVT (THA, 1.2%; HA, 0.80%, P = .50), PE (THA, 0.52%; HA, 0.72%, P = .52), or mortality (THA, 1.8%; HA, 2.9%; P = .16). Discharge disposition varied with surgical status (P < .01): 23.2% of THA patients and 11.6% of HA patients were discharged directly home after their inpatient stay, and 76.8% of THA patients and 88.4% of HA patients were discharged or transferred to a short- or long-term care facility.
Private medical insurance provided coverage for 14.3% of THAs and 9.1% of HAs, and Medicare provided coverage for 80.9% of THAs and 86.0% of HAs (P < .01).
Discussion
The NHDS data showed a preference for HA over THA in the treatment of FNFs and suggested THA was favored for younger, healthier patients while HA was reserved for older patients with more comorbidities. Despite being younger and healthier, the THA group had higher transfusion rates and longer hospitalizations, possibly because of the increased complexity of THA procedures, which generally involve more operative time and increased blood loss. The resultant higher transfusion rate for THAs likely contributed to longer hospitalizations for FNFs. However, the THA and HA groups did not differ in their rates of DVT, PE, or mortality.
Multiple studies have noted no differences in mortality, infection, or general complications between THA and HA for FNF.8,10,11 THA patients have better functional outcomes, including Harris and Oxford hip scores and walking distance, but higher dislocation rates,8,10-12 and HA patients are at higher risk for reoperation because of progressive acetabular erosion.8,10,11
We noted an increase in use of both THA and HA for FNF over the study period (2001-2010). In a review of operative treatment for FNF by surgeons applying for the American Board of Orthopaedic Surgery certification between 1999 and 2011, Miller and colleagues13 found a similar increase in the THA rate over time, but decreases in the HA and internal fixation rates, with candidates in the “adult reconstruction” subspecialty showing a particularly strong trend toward THA use.
These findings reflect a general propensity toward femoral head replacement rather than preservation through open reduction and internal fixation (ORIF). Recent studies have found that ORIF carries a 39% to 43% rate of fixation failure and need for secondary revision, as well as risks of avascular necrosis, malunion, and nonunion.1,14-16 This need for secondary surgery makes ORIF ultimately less cost-effective than either THA or HA.16,17 Most authors would recommend arthroplasty for FNF in elderly patients with normal mental function1,16,18 and would reserve ORIF for young patients with good bone stock, joint space preservation, and reducible noncomminuted fractures.1,19
Our study results suggest that smaller hospitals (<100 beds) tend to have lower rates of HA (P < .01, significant) and THA (P = .10, not significant; Table), possibly because FNF patients who present to these hospitals may be referred elsewhere because of regional differences in the availability of orthopedic traumatologists and arthroplasty subspecialists. Surgeon volume affects postoperative outcomes and may play a role in referral patterns.20 Ames and colleagues20 found that HA performed for FNF by surgeons with high-volume THA experience (vs non-hip-arthroplasty surgeons) had lower rates of dislocation, superficial infection, and mortality.
Regional differences were significant for THA alone, with the highest THA rates in the South (5.2%) and the lowest in the West (3.3%; Figure 5). There were no clear regional trends for HA. Possible explanations include a propensity toward a more aggressive approach in these regions, increased regional prevalence of acetabular disease, regional surgeon preferences, and regional differences in patient characteristics (eg, increased prevalence of obesity in the South).21
HA rates were highest for nonprofit/church hospitals and lowest for proprietary hospitals, whereas THA rates did not differ by hospital type. Possible explanations include an older, less mobile nonprofit/church patient cohort that is more amenable to HA, and surgeon preference.
THA patients were more likely to be covered by private medical insurance than by Medicare—a finding in agreement with Hochfelder and colleagues,22 who found that, compared with federal insurance and self-pay patients, private insurance patients were 41% more likely to undergo THA than HA or internal fixation for FNF. We think that the age difference between our THA and HA groups contributed to the insurance variability in our study.
Our study had several limitations. It was conducted to examine the rates of THA and HA after FNF, not to survey treatment types, including ORIF and nonoperative management. The NHDS database does not provide information on HA implant type (unipolar, bipolar), use or nonuse of cement with HA, or surgical approach. Surgical approach could influence the rate of postoperative dislocation, an outcome measure that was not examined in this study. Last, the NHDS database tracks admissions and discharges, not patients. When a patient is discharged, collection of information on the patient’s postoperative course stops; a patient who returns even only 1 day later is recorded as a new or unique patient. Therefore, intermediate or long-term outcome information is unavailable, which likely led to an underrepresentation of DVT, PE, and mortality after these THA and HA procedures.
There was a trend toward femoral head replacement rather than ORIF in the treatment of FNF. Cognitively functional and independent elderly patients, and patients with osteoarthritis or rheumatoid arthritis, may benefit from THA, whereas HA may be better suited to cognitively dysfunctional patients.23,24 The NHDS reflects an increasing trend toward arthroplasty over ORIF, but the exact treatment choice is affected by hospital type, size, location and surgeon preference, training, and subspecialization.
1. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287-293.
2. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
3. Kannus P, Parkkari J, Sievänen H, Heinonen A, Vuori I, Järvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 suppl):57S-63S.
4. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525.
5. Papandrea RF, Froimson MI. Total hip arthroplasty after acute displaced femoral neck fractures. Am J Orthop. 1996;25(2):85-88.
6. Burgers PT, Van Geene AR, Van den Bekerom MP, et al. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures in the healthy elderly: a meta-analysis and systematic review of randomized trials. Int Orthop. 2012;36(8):1549-1560.
7. Skinner P, Riley D, Ellery J, Beaumont A, Coumine R, Shafighian B. Displaced subcapital fractures of the femur: a prospective randomized comparison of internal fixation, hemiarthroplasty and total hip replacement. Injury. 1989;20(5):291-293.
8. Baker RP, Squires B, Gargan MF, Bannister GC. Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(12):2583-2589.
9. Centers for Disease Control and Prevention, National Center for Health Statistics. National Hospital Discharge Survey. http://www.cdc.gov/nchs/nhds/about_nhds.htm. Last updated December 6, 2011. Accessed December 10, 2013.
10. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplasty. 2012;27(4):583-590.
11. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235-2243.
12. Hopley C, Stengel D, Ekkernkamp A, Wich M. Primary total hip arthroplasty versus hemiarthroplasty for displaced intracapsular hip fractures in older patients: systematic review. BMJ. 2010;340:c2332.
13. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2014;96(17):e149.
14. Rogmark C, Carlsson A, Johnell O, Sernbo I. A prospective randomised trial of internal fixation versus arthroplasty for displaced fractures of the neck of the femur. Functional outcome for 450 patients at two years. J Bone Joint Surg Br. 2002;84(2):183-188.
15. Bhandari M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. J Bone Joint Surg Am. 2003;85(9):1673-1681.
16. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
17. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop Relat Res. 2001;(383):229-242.
18. Johansson T, Jacobsson SA, Ivarsson I, Knutsson A, Wahlström O. Internal fixation versus total hip arthroplasty in the treatment of displaced femoral neck fractures: a prospective randomized study of 100 hips. Acta Orthop Scand. 2000;71(6):597-602.
19. Shah AK, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop Relat Res. 2002;(399):28-34.
20. Ames JB, Lurie JD, Tomek IM, Zhou W, Koval KJ. Does surgeon volume for total hip arthroplasty affect outcomes after hemiarthroplasty for femoral neck fracture? Am J Orthop. 2010;39(8):E84-E89.
21. Le A, Judd SE, Allison DB, et al. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity. 2014;22(1):300-306.
22. Hochfelder JP, Khatib ON, Glait SA, Slover JD. Femoral neck fractures in New York state. Is the rate of THA increasing, and do race or payer influence decision making? J Orthop Trauma. 2014;28(7):422-426.
23. Lowe JA, Crist BD, Bhandari M, Ferguson TA. Optimal treatment of femoral neck fractures according to patient’s physiologic age: an evidence-based review. Orthop Clin North Am. 2010;41(2):157-166.
24. Callaghan JJ, Liu SS, Haidukewych GJ. Subcapital fractures: a changing paradigm. J Bone Joint Surg Br. 2012;94(11 suppl A):19-21.
1. Macaulay W, Pagnotto MR, Iorio R, Mont MA, Saleh KJ. Displaced femoral neck fractures in the elderly: hemiarthroplasty versus total hip arthroplasty. J Am Acad Orthop Surg. 2006;14(5):287-293.
2. Miyamoto RG, Kaplan KM, Levine BR, Egol KA, Zuckerman JD. Surgical management of hip fractures: an evidence-based review of the literature. I: femoral neck fractures. J Am Acad Orthop Surg. 2008;16(10):596-607.
3. Kannus P, Parkkari J, Sievänen H, Heinonen A, Vuori I, Järvinen M. Epidemiology of hip fractures. Bone. 1996;18(1 suppl):57S-63S.
4. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519-1525.
5. Papandrea RF, Froimson MI. Total hip arthroplasty after acute displaced femoral neck fractures. Am J Orthop. 1996;25(2):85-88.
6. Burgers PT, Van Geene AR, Van den Bekerom MP, et al. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures in the healthy elderly: a meta-analysis and systematic review of randomized trials. Int Orthop. 2012;36(8):1549-1560.
7. Skinner P, Riley D, Ellery J, Beaumont A, Coumine R, Shafighian B. Displaced subcapital fractures of the femur: a prospective randomized comparison of internal fixation, hemiarthroplasty and total hip replacement. Injury. 1989;20(5):291-293.
8. Baker RP, Squires B, Gargan MF, Bannister GC. Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(12):2583-2589.
9. Centers for Disease Control and Prevention, National Center for Health Statistics. National Hospital Discharge Survey. http://www.cdc.gov/nchs/nhds/about_nhds.htm. Last updated December 6, 2011. Accessed December 10, 2013.
10. Zi-Sheng A, You-Shui G, Zhi-Zhen J, Ting Y, Chang-Qing Z. Hemiarthroplasty vs primary total hip arthroplasty for displaced fractures of the femoral neck in the elderly: a meta-analysis. J Arthroplasty. 2012;27(4):583-590.
11. Yu L, Wang Y, Chen J. Total hip arthroplasty versus hemiarthroplasty for displaced femoral neck fractures: meta-analysis of randomized trials. Clin Orthop Relat Res. 2012;470(8):2235-2243.
12. Hopley C, Stengel D, Ekkernkamp A, Wich M. Primary total hip arthroplasty versus hemiarthroplasty for displaced intracapsular hip fractures in older patients: systematic review. BMJ. 2010;340:c2332.
13. Miller BJ, Callaghan JJ, Cram P, Karam M, Marsh JL, Noiseux NO. Changing trends in the treatment of femoral neck fractures: a review of the American Board of Orthopaedic Surgery database. J Bone Joint Surg Am. 2014;96(17):e149.
14. Rogmark C, Carlsson A, Johnell O, Sernbo I. A prospective randomised trial of internal fixation versus arthroplasty for displaced fractures of the neck of the femur. Functional outcome for 450 patients at two years. J Bone Joint Surg Br. 2002;84(2):183-188.
15. Bhandari M, Devereaux PJ, Swiontkowski MF, et al. Internal fixation compared with arthroplasty for displaced fractures of the femoral neck. A meta-analysis. J Bone Joint Surg Am. 2003;85(9):1673-1681.
16. Keating JF, Grant A, Masson M, Scott NW, Forbes JF. Randomized comparison of reduction and fixation, bipolar hemiarthroplasty, and total hip arthroplasty. Treatment of displaced intracapsular hip fractures in healthy older patients. J Bone Joint Surg Am. 2006;88(2):249-260.
17. Iorio R, Healy WL, Lemos DW, Appleby D, Lucchesi CA, Saleh KJ. Displaced femoral neck fractures in the elderly: outcomes and cost effectiveness. Clin Orthop Relat Res. 2001;(383):229-242.
18. Johansson T, Jacobsson SA, Ivarsson I, Knutsson A, Wahlström O. Internal fixation versus total hip arthroplasty in the treatment of displaced femoral neck fractures: a prospective randomized study of 100 hips. Acta Orthop Scand. 2000;71(6):597-602.
19. Shah AK, Eissler J, Radomisli T. Algorithms for the treatment of femoral neck fractures. Clin Orthop Relat Res. 2002;(399):28-34.
20. Ames JB, Lurie JD, Tomek IM, Zhou W, Koval KJ. Does surgeon volume for total hip arthroplasty affect outcomes after hemiarthroplasty for femoral neck fracture? Am J Orthop. 2010;39(8):E84-E89.
21. Le A, Judd SE, Allison DB, et al. The geographic distribution of obesity in the US and the potential regional differences in misreporting of obesity. Obesity. 2014;22(1):300-306.
22. Hochfelder JP, Khatib ON, Glait SA, Slover JD. Femoral neck fractures in New York state. Is the rate of THA increasing, and do race or payer influence decision making? J Orthop Trauma. 2014;28(7):422-426.
23. Lowe JA, Crist BD, Bhandari M, Ferguson TA. Optimal treatment of femoral neck fractures according to patient’s physiologic age: an evidence-based review. Orthop Clin North Am. 2010;41(2):157-166.
24. Callaghan JJ, Liu SS, Haidukewych GJ. Subcapital fractures: a changing paradigm. J Bone Joint Surg Br. 2012;94(11 suppl A):19-21.
Management of Isolated Greater Tuberosity Fractures: A Systematic Review
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
Take-Home Points
- Fractures of the greater tuberosity are often mismanaged.
- Comprehension of greater tuberosity fractures involves classification into nonoperative and operative treatment, displacement >5mm or <5 mm, and open vs arthroscopic surgery.
- Nearly a third of patients may suffer concomitant anterior glenohumeral instability.
- Stiffness is the most common postoperative complication.
- Surgery is associated with high patient satisfaction and low rates of complications and reoperations.
Although proximal humerus fractures are common in the elderly, isolated fractures of the greater tuberosity occur less often. Management depends on several factors, including fracture pattern and displacement.1,2 Nondisplaced fractures are often successfully managed with sling immobilization and early range of motion.3,4 Although surgical intervention improves outcomes in displaced greater tuberosity fractures, the ideal surgical treatment is less clear.5
Displaced greater tuberosity fractures may require surgery for prevention of subacromial impingement and range-of-motion deficits.2 Superior fracture displacement results in decreased shoulder abduction, and posterior displacement can limit external rotation.6 Although the greater tuberosity can displace in any direction, posterosuperior displacement has the worst outcomes.1 The exact surgery-warranting displacement amount ranges from 3 mm to 10 mm but is yet to be clearly elucidated.5,6 Less displacement is tolerated by young overhead athletes, and more displacement by older less active patients.5,7,8 Surgical options for isolated greater tuberosity fractures include fragment excision, open reduction and internal fixation (ORIF), closed reduction with percutaneous fixation, and arthroscopically assisted reduction with internal fixation.3,9,10
We conducted a study to determine the management patterns for isolated greater tuberosity fractures. We hypothesized that greater tuberosity fractures displaced <5 mm may be managed nonoperatively and that greater tuberosity fractures displaced >5 mm require surgical fixation.
Methods
Search Strategy
We performed this systematic review according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist11 and registered it (CRD42014010691) with the PROSPERO international prospective register of systematic reviews. Literature searches using the PubMed/Medline database and the Cochrane Central Register of Clinical Trials were completed in August 2014. There were no date or year restrictions. Key words were used to capture all English- language studies with level I to IV evidence (Oxford Centre for Evidence-Based Medicine) and reported clinical or radiographic outcomes. Initial exclusion criteria were cadaveric, biomechanical, histologic, and kinematic results. An electronic search algorithm with key words and a series of NOT phrases was designed to match our exclusion criteria:
((((((((((((((((((((((((((((((((((((((((((((((((((greater[Title/Abstract]) AND tuberosity [Title/Abstract] OR tubercle [Title/Abstract]) AND fracture[Title/Abstract]) AND proximal[Title/Abstract] AND (English[lang]))) NOT intramedullary[Title] AND (English[lang]))) NOT nonunion[Title] AND (English[lang]))) NOT malunion[Title] AND (English[lang]))) NOT biomechanical[Title/Abstract] AND (English[lang]))) NOT cadaveric[Title/Abstract] AND (English[lang]))) NOT cadaver[Title/Abstract] AND (English[lang]))) NOT ((basic[Title/Abstract]) AND science[Title/Abstract] AND (English[lang])) AND (English[lang]))) NOT revision[Title] AND (English[lang]))) NOT pediatric[Title] AND (English[lang]))) NOT physeal[Title] AND (English[lang]))) NOT children[Title] AND (English[lang]))) NOT instability[Title] AND (English[lang]))) NOT imaging[Title])) NOT salter[Title])) NOT physis[Title])) NOT shaft[Title])) NOT distal[Title])) NOT clavicle[Title])) NOT scapula[Title])) NOT ((diaphysis[Title]) AND diaphyseal[Title]))) NOT infection[Title])) NOT laboratory[Title/Abstract])) NOT metastatic[Title/Abstract])) NOT (((((((malignancy[Title/Abstract]) OR malignant[Title/Abstract]) OR tumor[Title/Abstract]) OR oncologic[Title/Abstract]) OR cyst[Title/Abstract]) OR aneurysmal[Title/Abstract]) OR unicameral[Title/Abstract]).
Study Selection
Data Extraction
We extracted data from the 13 studies that met the eligibility criteria. Details of study design, sample size, and patient demographics, including age, sex, and hand dominance, were recorded, as were mechanism of injury and concomitant anterior shoulder instability. To capture the most patients, we noted radiographic fracture displacement categorically rather than continuously; patients were divided into 2 displacement groups (<5 mm, >5 mm). Most studies did not define degree of comminution or specific direction of displacement per fracture, so these variables were not included in the data analysis. Nonoperative management and operative management were studied. We abstracted surgical factors, such as approach, method, fixation type (screws or sutures), and technique (suture anchors or transosseous tunnels). Clinical outcomes included physical examination findings, functional assessment results (patient satisfaction; Constant and University of California Los Angeles [UCLA] shoulder scores), and the number of revisions. Radiologic outcomes, retrieved from radiographs or computed tomography scans, focused on loss of reduction (as determined by the respective authors), malunion, nonunion, and heterotopic ossification. Each study’s methodologic quality and bias were evaluated with the 15-item Modified Coleman Methodology Score (MCMS), which was described by Cowan and colleagues.23 The MCMS has been used to assess randomized and nonrandomized patient trials.24,25 Its scaled potential score ranges from 0 to 100 (85-100, excellent; 70-84, good; 55-69, fair; <55, poor).
Statistical Analysis
We report our data as weighted means (SDs). A mean was calculated for each study that reported a respective data point, and each mean was then weighed according to its study sample size. This calculation was performed by multiplying a study’s individual mean by the number of patients enrolled in that study and dividing the sum of these weighted data points by the number of eligible patients in all relevant studies. The result was that the nonweighted means from studies with smaller sample sizes did not carry as much weight as the nonweighted means from larger studies. We compared 3 paired groups: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic). Regarding all patient, surgery, and outcomes data, unpaired Student t tests were used for continuous variables and 2-tailed Fisher exact tests for categorical variables with α = 0.05 (SPSS Version 18; IBM).
Results
Postoperative physical examination findings were underreported so that surgical groups could be compared. Of all the surgical studies, 4 reported postoperative forward elevation (mean, 160°; SD, 9.8°) and external rotation (mean, 46.4°; SD 26.3°).14,15,18,22 No malunions and only 1 nonunion were reported in all 13 studies. No deaths or other serious medical complications were reported. Patients with anterior instability more often underwent surgery than were treated nonoperatively (39.2% vs 12.0%; P < .01) and more often had fractures displaced >5 mm than <5 mm (44.3% vs 14.5%; P < .01).
Fisher exact tests were used to perform isolated comparisons of screws and sutures as well as suture anchors and transosseous tunnels. Patients with screw fixation were significantly (P = .051) less likely to require reoperation (0/56; 0%) than patients with suture fixation (8/100; 8.0%). Screw fixation also led to significantly less stiffness (0% vs 12.0%; P < .01) but trended toward a higher rate of superficial infection (3.6% vs 0%; P = .13). There was no statistical difference in nerve injury rates between screws and sutures (1.8% vs 3.0%; P = 1.0). There were no significant differences in reoperations, stiffness, superficial infections, or nerve injuries between suture anchor and transosseous tunnel constructs.
For all 13 studies, mean (SD) MCMS was 41.1 (8.6).
Discussion
Five percent of all fractures involve the proximal humerus, and 20% of proximal humerus fractures are isolated greater tuberosity fractures.26,27 In his classic 1970 article, Neer6 formulated the 4-part proximal humerus fracture classification and defined greater tuberosity fracture “parts” using the same criteria as for other fracture “parts.” Neer6 recommended nonoperative management for isolated greater tuberosity fractures displaced <1 cm but did not present evidence corroborating his recommendation. More recent cutoffs for nonoperative management include 5 mm (general population) and 3 mm (athletes).7,17
In the present systematic review of greater tuberosity fractures, 3 separate comparisons were made: treatment type (nonoperative vs operative), fracture displacement amount (<5 mm vs >5 mm), and surgery type (open vs arthroscopic).
Treatment Type. Only 4 studies reported data on nonoperative treatment outcomes.5,12,16,17 Of these 4 studies, 2 found successful outcomes for fractures displaced <5 mm.12,17 Platzer and colleagues17 found good or excellent results in 97% of 135 shoulders after 4 years. Good results were defined with shoulder scores of ≥80 (Constant), <8 (Vienna), and >28 (UCLA), and excellent results were defined with maximum scores on 2 of the 3 systems. Platzer and colleagues17 also found nonsignificantly worse shoulder scores with superior displacement of 3 mm to 5 mm and recommended surgery for overhead athletes in this group. Rath and colleagues12 described a successful 3-phase rehabilitation protocol of sling immobilization for 3 weeks, pendulum exercises for 3 weeks, and active exercises thereafter. By an average of 31 months, patient satisfaction scores improved to 9.5 from 4.2 (10-point scale), though the authors cautioned that pain and decreased motion lasted 8 months on average. Conservative treatment was far less successful in the 2 studies of fractures displaced >5 mm.5,16 Keene and colleagues16 reported unsatisfactory results in all 4 patients with fractures displaced >1.5 cm. In a study separate from their 2005 analysis,17 Platzer and colleagues5 in 2008 evaluated displaced fractures and found function and patient satisfaction were inferior after nonoperative treatment than after surgery. The studies by Keene and colleagues16 and Platzer and colleagues5 support the finding of an overall lower patient satisfaction rate in nonoperative patients.
Fracture Displacement Amount. Only 2 arthroscopic studies and no open studies addressed surgery for fractures displaced <5 mm. Fewer than 16% of these fractures were managed operatively, and <1% required reoperation. By contrast, almost all fractures displaced >5 mm were managed operatively, and 3.6% required reoperation. Radiographic loss of reduction was more common in fractures displaced <5 mm, primarily because they were managed without fixation. Radiographic loss of reduction was reported in only 9 operatively treated patients, none of whom was symptomatic enough to require another surgery.5 Reoperations were most commonly performed for stiffness, which itself was significantly more common in fractures displaced >5 mm. Bhatia and colleagues14 reported the highest reoperation rate (14.3%; 3/21), but they studied more complex, comminuted fractures of the greater tuberosity. Two of their 3 reoperations were biceps tenodeses for inflamed, stiff tenosynovitis, and the third patient had a foreign body giant cell reaction to suture material. Fewer than 1% of patients with operatively managed displaced fractures required revision ORIF, and <2% developed a superficial infection or postoperative nerve palsy.19,22 For displaced greater tuberosity fractures, surgery is highly successful overall, complication rates are very low, and 90% of patients report being satisfied.
Surgery Type. Patients were divided into 2 groups. In the nonarthroscopic group, open and percutaneous approaches were used. All studies that described a percutaneous approach used screw fixation5,21; in addition, 32 patients were treated with screws through an open approach.2,5 The other open and arthroscopic studies used suture fixation. Interestingly, no studies reported on clinical outcomes of fragment excision. There were no statistically significant differences in rates of reoperation, stiffness, infection, or neurologic injury between the arthroscopic and nonarthroscopic groups. Patient satisfaction scores were slightly higher in the nonarthroscopic group (91.0% vs 87.8%), but the difference was not statistically significant.
With surgical techniques isolated, there were no significant differences between suture anchors and transosseous tunnel constructs, but screws performed significantly better than suture techniques. Compared with suture fixation, screw fixation led to significantly fewer cases of stiffness and reoperation, which suggests surgeons need to give screws more consideration in the operative management of these fractures. However, the number of patients treated with screws was smaller than the number treated with suture fixation; it is possible the differences between these cohorts would be eliminated if there were more patients in the screw cohort. In addition, screw fixation was universally performed with an open or percutaneous approach and trended toward a higher infection rate. As screw and suture techniques have low rates of complications and reoperations, we recommend leaving fixation choice to the surgeon.
Anterior shoulder instability has been associated with greater tuberosity fractures.1,8,19 The supraspinatus, infraspinatus, and teres minor muscles all insert into the greater tuberosity and resist anterior translation of the proximal humerus. Loss of this dynamic muscle stabilization is amplified by tuberosity fracture displacement: Anterior shoulder instability was significantly more common in fractures displaced >5 mm (44.3%) vs <5 mm (14.5%). In turn, glenohumeral instability was more common in patients treated with surgery, specifically open surgery, because displaced fractures may not be as easily accessed with arthroscopic techniques. No studies reported concomitant labral repair or capsular plication techniques.
This systematic review was limited by the studies analyzed. All but 1 study5 had level IV evidence. Mean (SD) MCMS was 41.8 (8.6). Any MCMS score <54 indicates a poor methodology level, but this scoring system is designed for randomized controlled trials,23 and there were none in this study. Physical examination findings, such as range of motion, were underreported. In addition, radiographic parameters were not consistently described but rather were determined by the respective authors’ subjective interpretations of malunion, nonunion, and loss of reduction. Publication bias is present in that we excluded non- English language studies and medical conference abstracts and may have omitted potentially eligible studies not discoverable with our search methodology. Performance bias is a factor in any systematic review with multiple surgeons and wide variation in surgical technique.
Conclusion
Greater tuberosity fractures displaced <5 mm may be safely managed nonoperatively, as there are no reports of nonoperatively managed fractures that subsequently required surgery. Nonoperative treatment was initially associated with low patient satisfaction, but only because displaced fractures were conservatively managed in early studies.5,16 Fractures displaced >5 mm respond well to operative fixation with screws, suture anchors, or transosseous suture tunnels. Stiffness is the most common postoperative complication (<6%), followed by heterotopic ossification, transient neurapraxias, and superficial infection. There are no discernible differences in outcome between open and arthroscopic techniques, but screw fixation may lead to significantly fewer cases of stiffness and reoperation in comparison with suture constructs.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
1. Verdano MA, Aliani D, Pellegrini A, Baudi P, Pedrazzi G, Ceccarelli F. Isolated fractures of the greater tuberosity in proximal humerus: does the direction of displacement influence functional outcome? An analysis of displacement in greater tuberosity fractures. Acta Biomed. 2013;84(3):219-228.
2. Yin B, Moen TC, Thompson SA, Bigliani LU, Ahmad CS, Levine WN. Operative treatment of isolated greater tuberosity fractures: retrospective review of clinical and functional outcomes. Orthopedics. 2012;35(6):e807-e814.
3. Green A, Izzi J. Isolated fractures of the greater tuberosity of the proximal humerus. J Shoulder Elbow Surg. 2003;12(6):641-649.
4. Norouzi M, Naderi MN, Komasi MH, Sharifzadeh SR, Shahrezaei M, Eajazi A. Clinical results of using the proximal humeral internal locking system plate for internal fixation of displaced proximal humeral fractures. Am J Orthop. 2012;41(5):E64-E68.
5. Platzer P, Thalhammer G, Oberleitner G, et al. Displaced fractures of the greater tuberosity: a comparison of operative and nonoperative treatment. J Trauma. 2008;65(4):843-848.
6. Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077-1089.
7. Park TS, Choi IY, Kim YH, Park MR, Shon JH, Kim SI. A new suggestion for the treatment of minimally displaced fractures of the greater tuberosity of the proximal humerus. Bull Hosp Jt Dis. 1997;56(3):171-176.
8. McLaughlin HL. Dislocation of the shoulder with tuberosity fracture. Surg Clin North Am. 1963;43:1615-1620.
9. DeBottis D, Anavian J, Green A. Surgical management of isolated greater tuberosity fractures of the proximal humerus. Orthop Clin North Am. 2014;45(2):207-218.
10. Monga P, Verma R, Sharma VK. Closed reduction and external fixation for displaced proximal humeral fractures. J Orthop Surg (Hong Kong). 2009;17(2):142-145.
11. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012.
12. Rath E, Alkrinawi N, Levy O, Debbi R, Amar E, Atoun E. Minimally displaced fractures of the greater tuberosity: outcome of non-operative treatment. J Shoulder Elbow Surg. 2013;22(10):e8-e11.
13. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. J Bone Joint Surg Am. 2007;89(8):1700-1709.
14. Bhatia DN, van Rooyen KS, Toit du DF, de Beer JF. Surgical treatment of comminuted, displaced fractures of the greater tuberosity of the proximal humerus: a new technique of double-row suture-anchor fixation and long-term results. Injury. 2006;37(10):946-952.
15. Flatow EL, Cuomo F, Maday MG, Miller SR, McIlveen SJ, Bigliani LU. Open reduction and internal fixation of two-part displaced fractures of the greater tuberosity of the proximal part of the humerus. J Bone Joint Surg Am. 1991;73(8):1213-1218.
16. Keene JS, Huizenga RE, Engber WD, Rogers SC. Proximal humeral fractures: a correlation of residual deformity with long-term function. Orthopedics. 1983;6(2):173-178.
17. Platzer P, Kutscha-Lissberg F, Lehr S, Vecsei V, Gaebler C. The influence of displacement on shoulder function in patients with minimally displaced fractures of the greater tuberosity. Injury. 2005;36(10):1185-1189.
18. Park SE, Ji JH, Shafi M, Jung JJ, Gil HJ, Lee HH. Arthroscopic management of occult greater tuberosity fracture of the shoulder. Eur J Orthop Surg Traumatol. 2014;24(4):475-482.
19. Dimakopoulos P, Panagopoulos A, Kasimatis G, Syggelos SA, Lambiris E. Anterior traumatic shoulder dislocation associated with displaced greater tuberosity fracture: the necessity of operative treatment. J Orthop Trauma. 2007;21(2):104-112.
20. Kim SH, Ha KI. Arthroscopic treatment of symptomatic shoulders with minimally displaced greater tuberosity fracture. Arthroscopy. 2000;16(7):695-700.
21. Chen CY, Chao EK, Tu YK, Ueng SW, Shih CH. Closed management and percutaneous fixation of unstable proximal humerus fractures. J Trauma. 1998;45(6):1039-1045.
22. Ji JH, Shafi M, Song IS, Kim YY, McFarland EG, Moon CY. Arthroscopic fixation technique for comminuted, displaced greater tuberosity fracture. Arthroscopy. 2010;26(5):600-609.
23. Cowan J, Lozano-Calderón S, Ring D. Quality of prospective controlled randomized trials. Analysis of trials of treatment for lateral epicondylitis as an example. J Bone Joint Surg Am. 2007;89(8):1693-1699.
24. Harris JD, Siston RA, Pan X, Flanigan DC. Autologous chondrocyte implantation: a systematic review. J Bone Joint Surg Am. 2010;92(12):2220-2233.
25. Harris JD, Siston RA, Brophy RH, Lattermann C, Carey JL, Flanigan DC. Failures, re-operations, and complications after autologous chondrocyte implantation—a systematic review. Osteoarthritis Cartilage. 2011;19(7):779-791.
26. Chun JM, Groh GI, Rockwood CA. Two-part fractures of the proximal humerus. J Shoulder Elbow Surg. 1994;3(5):273-287.
27. Gruson KI, Ruchelsman DE, Tejwani NC. Isolated tuberosity fractures of the proximal humeral: current concepts. Injury. 2008;39(3):284-298.
Gorham Disease
Take-Home Points
- Gorham disease is a rare condition that manifests as an acute, spontaneous osteolysis.
- There is no clear hereditary pattern of transmission. Bones of any type or location can be affected.
- Imaging studies are nonspecific, but show permeative osteolysis involving the subcortical and intramedullary regions and typically affect regional, contiguous bones, without adjacent sclerosis, somewhat resembling osteoporosis.
- Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
- There is no single or combined treatment modality that is considered as the gold standard. Surgical treatment includes resection of the lesion and reconstruction. Also, antiosteoclastic medication can be used.
Gorham disease, a rare condition of unknown etiology, manifests as acute, spontaneous osteolysis associated with benign hemangiomatosis or lymphangiomatosis, which presents as skeletal lucency on radiographs, prompting the classic eponym of vanishing bone disease.1-6 There is no evidence supporting the idea that osteoclasts are present in any meaningful amount in the resorption areas or that local reparative osteogenesis occurs.4,6
Jackson and colleagues first described idiopathic osteolysis in 1838,1,2 and Gorham and Stout3 introduced the syndrome to the orthopedic community in 1955. Since then, few strides have been made in identifying the disease origin.1,2,4 Diagnosis is possible only after meticulous work-up has excluded neoplastic and infectious etiologies.7,8
Clinical Presentation
Gorham disease affects patients ranging widely in age, from 2 months to 78 years, but typically presents in those under 40 years. There is a questionable predilection for males but no correlation with ethnicity or geographic region. There is no clear hereditary pattern of transmission.7 Although the bones of the head, neck, and upper extremities are involved in most cases, bone of any type or location can be affected.6 Pelvic bones seem to be involved least often.6,7
Initial clinical presentation varies considerably but typically involves prolonged soreness in the affected region and, rarely, acute pathologic fracture.1,2,4 The nonspecific nature of complaints, lack of markers of systemic illness, and rarity of the disease contribute to delayed diagnosis.1,2
Imaging
Computed tomography (CT) better defines the severity and extent of these changes.
Magnetic resonance imaging shows an infiltrative and irregular T2 hyperintense signal throughout regions of bone affected by osteolysis, but this finding is not characteristic. There is heterogeneous enhancement on postcontrast sequences, and, though masslike enhancement is absent, signal abnormalities may extend into adjacent soft tissues.
Bone scintigraphy using technetium-99m is similarly nonspecific, typically revealing radiotracer uptake that is consistent with bony reaction to an underlying osteolytic process (Figure 4) but turning negative with ongoing resorption.
Positron emission tomography/CT typically shows foci of increased metabolic activity in the areas of osteolysis.10
Diagnosis
There have been 8 histologic and clinical criteria described to diagnose Gorham disease: (1) biopsy positive for presence of angiomatous tissue, (2) complete absence of any cellular atypia, (3) lack of osteoclastic response and lack of dystrophic calcifications, (4) evidence of progressive resorption of native bone, (5) no evidence of expansive or ulcerative lesion, (6) lack of visceral involvement, (7) osteolytic radiographic pattern, and (8) no concrete diagnosis after hereditary, metabolic, neoplastic, immunologic, and infectious work-up.4-6 These criteria confirm that the diagnosis can be rendered only after exclusion of neoplastic and infectious etiologies through clinical and laboratory work-up, imaging studies, and tissue sampling.
Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
The differential diagnosis includes infection (osteomyelitis, Brodie abscess), benign tumors (eosinophilic granuloma/Langerhans cell histiocytosis), malignant tumors (Ewing sarcoma and angiosarcoma), inflammatory conditions (eg, apatite- associated destructive arthritis), endocrine disorders (eg, osteolytic hyperparathyroidism), benign non-neoplastic conditions (venous or venolymphatic malformation), and other syndromes that present with osteolysis.1,2 Nevertheless, progressive and unusually substantial bone destruction without evidence of repair is almost pathognomonic for Gorham disease.9
Treatment
Surgical treatment usually includes lesion resection and subsequent reconstruction using combinations of bone grafts (allogenic) and prostheses. Bone graft alone is quickly resorbed and has not been found to be beneficial.1,2,4,20
1. Saify FY, Gosavi SR. Gorham’s disease: a diagnostic challenge. J Oral Maxillofac Pathol. 2014;18(3):411-414.
2. Patel DV. Gorham’s disease or massive osteolysis. Clin Med Res. 2005;3(2):65-74.
3. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
4. Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1983;55(4):331-343.
5. Gulati U, Mohanty S, Dabas J, Chandra N. “Vanishing bone disease” in maxillofacial region: a review and our experience. J Maxillofac Oral Surg. 2015;14(3):548-557.
6. Nikolaou VS, Chytas D, Korres D, Efstathopoulos N. Vanishing bone disease (Gorham-Stout syndrome): a review of a rare entity. World J Orthop. 2014;5(5):694-698.
7. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
8. Dominguez R, Washowich TL. Gorham’s disease or vanishing bone disease: plain film, CT, and MRI findings of two cases. Pediatr Radiol. 1994;24(5):316-318.
9. Kotecha R, Mascarenhas L, Jackson HA, Venkatramani R. Radiological features of Gorham’s disease. Clin Radiol. 2012;67(8):782-788.
10. Dong A, Bai Y, Wang Y, Zuo C. Bone scan, MRI, and FDG PET/CT findings in composite hemangioendothelioma of the manubrium sterni. Clin Nucl Med. 2014;39(2):e180-e183.
11. Baulieu F, De Pinieux G, Maruani A, Vaillant L, Lorette G. Serial lymphoscintigraphic findings in a patient with Gorham’s disease with lymphedema. Lymphology. 2014;47(3):118-122.
12. Manisali M, Ozaksoy D. Gorham disease: correlation of MR findings with histopathologic changes. Eur Radiol. 1998;8(9):1647-1650.
13. Brodszki N, Länsberg JK, Dictor M, et al. A novel treatment approach for paediatric Gorham-Stout syndrome with chylothorax. Acta Paediatr. 2011;100(11):1448-1453.
14. Nir V, Guralnik L, Livnat G, et al. Propranolol as a treatment option in Gorham-Stout syndrome: a case report. Pediatr Pulmonol. 2014;49(4):417-419.
15. Fontanesi J. Radiation therapy in the treatment of Gorham disease. J Pediatr Hematol. 2003;25(10):816-817.
16. Pfleger A, Schwinger W, Maier A, Tauss J, Popper HH, Zach MS. Gorham-Stout syndrome in a male adolescent—case report and review of the literature. J Pediatr Hematol Oncol. 2006;28(4):231-233.
17. Patrick JH. Massive osteolysis complicated by chylothorax successfully treated by pleurodesis. J Bone Joint Surg Br. 1976;58(3):347-349.
18. Hagberg H, Lamberg K, Åström G. α-2b interferon and oral clodronate for Gorham’s disease. Lancet. 1997;350(9094):1822-1823.
19. Takahashi A, Ogawa C, Kanazawa T, et al. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymph-hemangiomatosis: a severe case of Gorham-Stout syndrome. J Pediatr Surg. 2005;40(3):E47-E50.
20. Paley MD, Lloyd CJ, Penfold CN. Total mandibular reconstruction for massive osteolysis of the mandible (Gorham-Stout syndrome). Br J Oral Maxillofac Surg. 2005;43(2):166-168.
21. Avelar RL, Martins VB, Antunes AA, de Oliveira Neto PJ, de Souza Andrade ES. Use of zoledronic acid in the treatment of Gorham’s disease. Int J Pediatr Otorhinolaryngol. 2010;74(3):319-322.
22. Holroyd I, Dillon M, Roberts GJ. Gorham’s disease: a case (including dental presentation) of vanishing bone disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):125-129.
23. Lee S, Finn L, Sze RW, Perkins JA, Sie KC. Gorham Stout syndrome (disappearing bone disease): two additional case reports and a review of the literature. Arch Otolaryngol Head Neck Surg. 2003;129(12):1340-1343.
Take-Home Points
- Gorham disease is a rare condition that manifests as an acute, spontaneous osteolysis.
- There is no clear hereditary pattern of transmission. Bones of any type or location can be affected.
- Imaging studies are nonspecific, but show permeative osteolysis involving the subcortical and intramedullary regions and typically affect regional, contiguous bones, without adjacent sclerosis, somewhat resembling osteoporosis.
- Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
- There is no single or combined treatment modality that is considered as the gold standard. Surgical treatment includes resection of the lesion and reconstruction. Also, antiosteoclastic medication can be used.
Gorham disease, a rare condition of unknown etiology, manifests as acute, spontaneous osteolysis associated with benign hemangiomatosis or lymphangiomatosis, which presents as skeletal lucency on radiographs, prompting the classic eponym of vanishing bone disease.1-6 There is no evidence supporting the idea that osteoclasts are present in any meaningful amount in the resorption areas or that local reparative osteogenesis occurs.4,6
Jackson and colleagues first described idiopathic osteolysis in 1838,1,2 and Gorham and Stout3 introduced the syndrome to the orthopedic community in 1955. Since then, few strides have been made in identifying the disease origin.1,2,4 Diagnosis is possible only after meticulous work-up has excluded neoplastic and infectious etiologies.7,8
Clinical Presentation
Gorham disease affects patients ranging widely in age, from 2 months to 78 years, but typically presents in those under 40 years. There is a questionable predilection for males but no correlation with ethnicity or geographic region. There is no clear hereditary pattern of transmission.7 Although the bones of the head, neck, and upper extremities are involved in most cases, bone of any type or location can be affected.6 Pelvic bones seem to be involved least often.6,7
Initial clinical presentation varies considerably but typically involves prolonged soreness in the affected region and, rarely, acute pathologic fracture.1,2,4 The nonspecific nature of complaints, lack of markers of systemic illness, and rarity of the disease contribute to delayed diagnosis.1,2
Imaging
Computed tomography (CT) better defines the severity and extent of these changes.
Magnetic resonance imaging shows an infiltrative and irregular T2 hyperintense signal throughout regions of bone affected by osteolysis, but this finding is not characteristic. There is heterogeneous enhancement on postcontrast sequences, and, though masslike enhancement is absent, signal abnormalities may extend into adjacent soft tissues.
Bone scintigraphy using technetium-99m is similarly nonspecific, typically revealing radiotracer uptake that is consistent with bony reaction to an underlying osteolytic process (Figure 4) but turning negative with ongoing resorption.
Positron emission tomography/CT typically shows foci of increased metabolic activity in the areas of osteolysis.10
Diagnosis
There have been 8 histologic and clinical criteria described to diagnose Gorham disease: (1) biopsy positive for presence of angiomatous tissue, (2) complete absence of any cellular atypia, (3) lack of osteoclastic response and lack of dystrophic calcifications, (4) evidence of progressive resorption of native bone, (5) no evidence of expansive or ulcerative lesion, (6) lack of visceral involvement, (7) osteolytic radiographic pattern, and (8) no concrete diagnosis after hereditary, metabolic, neoplastic, immunologic, and infectious work-up.4-6 These criteria confirm that the diagnosis can be rendered only after exclusion of neoplastic and infectious etiologies through clinical and laboratory work-up, imaging studies, and tissue sampling.
Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
The differential diagnosis includes infection (osteomyelitis, Brodie abscess), benign tumors (eosinophilic granuloma/Langerhans cell histiocytosis), malignant tumors (Ewing sarcoma and angiosarcoma), inflammatory conditions (eg, apatite- associated destructive arthritis), endocrine disorders (eg, osteolytic hyperparathyroidism), benign non-neoplastic conditions (venous or venolymphatic malformation), and other syndromes that present with osteolysis.1,2 Nevertheless, progressive and unusually substantial bone destruction without evidence of repair is almost pathognomonic for Gorham disease.9
Treatment
Surgical treatment usually includes lesion resection and subsequent reconstruction using combinations of bone grafts (allogenic) and prostheses. Bone graft alone is quickly resorbed and has not been found to be beneficial.1,2,4,20
Take-Home Points
- Gorham disease is a rare condition that manifests as an acute, spontaneous osteolysis.
- There is no clear hereditary pattern of transmission. Bones of any type or location can be affected.
- Imaging studies are nonspecific, but show permeative osteolysis involving the subcortical and intramedullary regions and typically affect regional, contiguous bones, without adjacent sclerosis, somewhat resembling osteoporosis.
- Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
- There is no single or combined treatment modality that is considered as the gold standard. Surgical treatment includes resection of the lesion and reconstruction. Also, antiosteoclastic medication can be used.
Gorham disease, a rare condition of unknown etiology, manifests as acute, spontaneous osteolysis associated with benign hemangiomatosis or lymphangiomatosis, which presents as skeletal lucency on radiographs, prompting the classic eponym of vanishing bone disease.1-6 There is no evidence supporting the idea that osteoclasts are present in any meaningful amount in the resorption areas or that local reparative osteogenesis occurs.4,6
Jackson and colleagues first described idiopathic osteolysis in 1838,1,2 and Gorham and Stout3 introduced the syndrome to the orthopedic community in 1955. Since then, few strides have been made in identifying the disease origin.1,2,4 Diagnosis is possible only after meticulous work-up has excluded neoplastic and infectious etiologies.7,8
Clinical Presentation
Gorham disease affects patients ranging widely in age, from 2 months to 78 years, but typically presents in those under 40 years. There is a questionable predilection for males but no correlation with ethnicity or geographic region. There is no clear hereditary pattern of transmission.7 Although the bones of the head, neck, and upper extremities are involved in most cases, bone of any type or location can be affected.6 Pelvic bones seem to be involved least often.6,7
Initial clinical presentation varies considerably but typically involves prolonged soreness in the affected region and, rarely, acute pathologic fracture.1,2,4 The nonspecific nature of complaints, lack of markers of systemic illness, and rarity of the disease contribute to delayed diagnosis.1,2
Imaging
Computed tomography (CT) better defines the severity and extent of these changes.
Magnetic resonance imaging shows an infiltrative and irregular T2 hyperintense signal throughout regions of bone affected by osteolysis, but this finding is not characteristic. There is heterogeneous enhancement on postcontrast sequences, and, though masslike enhancement is absent, signal abnormalities may extend into adjacent soft tissues.
Bone scintigraphy using technetium-99m is similarly nonspecific, typically revealing radiotracer uptake that is consistent with bony reaction to an underlying osteolytic process (Figure 4) but turning negative with ongoing resorption.
Positron emission tomography/CT typically shows foci of increased metabolic activity in the areas of osteolysis.10
Diagnosis
There have been 8 histologic and clinical criteria described to diagnose Gorham disease: (1) biopsy positive for presence of angiomatous tissue, (2) complete absence of any cellular atypia, (3) lack of osteoclastic response and lack of dystrophic calcifications, (4) evidence of progressive resorption of native bone, (5) no evidence of expansive or ulcerative lesion, (6) lack of visceral involvement, (7) osteolytic radiographic pattern, and (8) no concrete diagnosis after hereditary, metabolic, neoplastic, immunologic, and infectious work-up.4-6 These criteria confirm that the diagnosis can be rendered only after exclusion of neoplastic and infectious etiologies through clinical and laboratory work-up, imaging studies, and tissue sampling.
Tissue biopsy is indicated to rule out other potential etiologies of osteolysis, and the histologic findings help confirm a diagnosis of Gorham disease.
The differential diagnosis includes infection (osteomyelitis, Brodie abscess), benign tumors (eosinophilic granuloma/Langerhans cell histiocytosis), malignant tumors (Ewing sarcoma and angiosarcoma), inflammatory conditions (eg, apatite- associated destructive arthritis), endocrine disorders (eg, osteolytic hyperparathyroidism), benign non-neoplastic conditions (venous or venolymphatic malformation), and other syndromes that present with osteolysis.1,2 Nevertheless, progressive and unusually substantial bone destruction without evidence of repair is almost pathognomonic for Gorham disease.9
Treatment
Surgical treatment usually includes lesion resection and subsequent reconstruction using combinations of bone grafts (allogenic) and prostheses. Bone graft alone is quickly resorbed and has not been found to be beneficial.1,2,4,20
1. Saify FY, Gosavi SR. Gorham’s disease: a diagnostic challenge. J Oral Maxillofac Pathol. 2014;18(3):411-414.
2. Patel DV. Gorham’s disease or massive osteolysis. Clin Med Res. 2005;3(2):65-74.
3. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
4. Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1983;55(4):331-343.
5. Gulati U, Mohanty S, Dabas J, Chandra N. “Vanishing bone disease” in maxillofacial region: a review and our experience. J Maxillofac Oral Surg. 2015;14(3):548-557.
6. Nikolaou VS, Chytas D, Korres D, Efstathopoulos N. Vanishing bone disease (Gorham-Stout syndrome): a review of a rare entity. World J Orthop. 2014;5(5):694-698.
7. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
8. Dominguez R, Washowich TL. Gorham’s disease or vanishing bone disease: plain film, CT, and MRI findings of two cases. Pediatr Radiol. 1994;24(5):316-318.
9. Kotecha R, Mascarenhas L, Jackson HA, Venkatramani R. Radiological features of Gorham’s disease. Clin Radiol. 2012;67(8):782-788.
10. Dong A, Bai Y, Wang Y, Zuo C. Bone scan, MRI, and FDG PET/CT findings in composite hemangioendothelioma of the manubrium sterni. Clin Nucl Med. 2014;39(2):e180-e183.
11. Baulieu F, De Pinieux G, Maruani A, Vaillant L, Lorette G. Serial lymphoscintigraphic findings in a patient with Gorham’s disease with lymphedema. Lymphology. 2014;47(3):118-122.
12. Manisali M, Ozaksoy D. Gorham disease: correlation of MR findings with histopathologic changes. Eur Radiol. 1998;8(9):1647-1650.
13. Brodszki N, Länsberg JK, Dictor M, et al. A novel treatment approach for paediatric Gorham-Stout syndrome with chylothorax. Acta Paediatr. 2011;100(11):1448-1453.
14. Nir V, Guralnik L, Livnat G, et al. Propranolol as a treatment option in Gorham-Stout syndrome: a case report. Pediatr Pulmonol. 2014;49(4):417-419.
15. Fontanesi J. Radiation therapy in the treatment of Gorham disease. J Pediatr Hematol. 2003;25(10):816-817.
16. Pfleger A, Schwinger W, Maier A, Tauss J, Popper HH, Zach MS. Gorham-Stout syndrome in a male adolescent—case report and review of the literature. J Pediatr Hematol Oncol. 2006;28(4):231-233.
17. Patrick JH. Massive osteolysis complicated by chylothorax successfully treated by pleurodesis. J Bone Joint Surg Br. 1976;58(3):347-349.
18. Hagberg H, Lamberg K, Åström G. α-2b interferon and oral clodronate for Gorham’s disease. Lancet. 1997;350(9094):1822-1823.
19. Takahashi A, Ogawa C, Kanazawa T, et al. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymph-hemangiomatosis: a severe case of Gorham-Stout syndrome. J Pediatr Surg. 2005;40(3):E47-E50.
20. Paley MD, Lloyd CJ, Penfold CN. Total mandibular reconstruction for massive osteolysis of the mandible (Gorham-Stout syndrome). Br J Oral Maxillofac Surg. 2005;43(2):166-168.
21. Avelar RL, Martins VB, Antunes AA, de Oliveira Neto PJ, de Souza Andrade ES. Use of zoledronic acid in the treatment of Gorham’s disease. Int J Pediatr Otorhinolaryngol. 2010;74(3):319-322.
22. Holroyd I, Dillon M, Roberts GJ. Gorham’s disease: a case (including dental presentation) of vanishing bone disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):125-129.
23. Lee S, Finn L, Sze RW, Perkins JA, Sie KC. Gorham Stout syndrome (disappearing bone disease): two additional case reports and a review of the literature. Arch Otolaryngol Head Neck Surg. 2003;129(12):1340-1343.
1. Saify FY, Gosavi SR. Gorham’s disease: a diagnostic challenge. J Oral Maxillofac Pathol. 2014;18(3):411-414.
2. Patel DV. Gorham’s disease or massive osteolysis. Clin Med Res. 2005;3(2):65-74.
3. Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37(5):985-1004.
4. Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1983;55(4):331-343.
5. Gulati U, Mohanty S, Dabas J, Chandra N. “Vanishing bone disease” in maxillofacial region: a review and our experience. J Maxillofac Oral Surg. 2015;14(3):548-557.
6. Nikolaou VS, Chytas D, Korres D, Efstathopoulos N. Vanishing bone disease (Gorham-Stout syndrome): a review of a rare entity. World J Orthop. 2014;5(5):694-698.
7. Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81(3):501-506.
8. Dominguez R, Washowich TL. Gorham’s disease or vanishing bone disease: plain film, CT, and MRI findings of two cases. Pediatr Radiol. 1994;24(5):316-318.
9. Kotecha R, Mascarenhas L, Jackson HA, Venkatramani R. Radiological features of Gorham’s disease. Clin Radiol. 2012;67(8):782-788.
10. Dong A, Bai Y, Wang Y, Zuo C. Bone scan, MRI, and FDG PET/CT findings in composite hemangioendothelioma of the manubrium sterni. Clin Nucl Med. 2014;39(2):e180-e183.
11. Baulieu F, De Pinieux G, Maruani A, Vaillant L, Lorette G. Serial lymphoscintigraphic findings in a patient with Gorham’s disease with lymphedema. Lymphology. 2014;47(3):118-122.
12. Manisali M, Ozaksoy D. Gorham disease: correlation of MR findings with histopathologic changes. Eur Radiol. 1998;8(9):1647-1650.
13. Brodszki N, Länsberg JK, Dictor M, et al. A novel treatment approach for paediatric Gorham-Stout syndrome with chylothorax. Acta Paediatr. 2011;100(11):1448-1453.
14. Nir V, Guralnik L, Livnat G, et al. Propranolol as a treatment option in Gorham-Stout syndrome: a case report. Pediatr Pulmonol. 2014;49(4):417-419.
15. Fontanesi J. Radiation therapy in the treatment of Gorham disease. J Pediatr Hematol. 2003;25(10):816-817.
16. Pfleger A, Schwinger W, Maier A, Tauss J, Popper HH, Zach MS. Gorham-Stout syndrome in a male adolescent—case report and review of the literature. J Pediatr Hematol Oncol. 2006;28(4):231-233.
17. Patrick JH. Massive osteolysis complicated by chylothorax successfully treated by pleurodesis. J Bone Joint Surg Br. 1976;58(3):347-349.
18. Hagberg H, Lamberg K, Åström G. α-2b interferon and oral clodronate for Gorham’s disease. Lancet. 1997;350(9094):1822-1823.
19. Takahashi A, Ogawa C, Kanazawa T, et al. Remission induced by interferon alfa in a patient with massive osteolysis and extension of lymph-hemangiomatosis: a severe case of Gorham-Stout syndrome. J Pediatr Surg. 2005;40(3):E47-E50.
20. Paley MD, Lloyd CJ, Penfold CN. Total mandibular reconstruction for massive osteolysis of the mandible (Gorham-Stout syndrome). Br J Oral Maxillofac Surg. 2005;43(2):166-168.
21. Avelar RL, Martins VB, Antunes AA, de Oliveira Neto PJ, de Souza Andrade ES. Use of zoledronic acid in the treatment of Gorham’s disease. Int J Pediatr Otorhinolaryngol. 2010;74(3):319-322.
22. Holroyd I, Dillon M, Roberts GJ. Gorham’s disease: a case (including dental presentation) of vanishing bone disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;89(1):125-129.
23. Lee S, Finn L, Sze RW, Perkins JA, Sie KC. Gorham Stout syndrome (disappearing bone disease): two additional case reports and a review of the literature. Arch Otolaryngol Head Neck Surg. 2003;129(12):1340-1343.
A Needs Review of Caregivers for Adults With Traumatic Brain Injury
Traumatic brain injury (TBI) is a health concern for the U.S. Military Health System (MHS) as well as the VHA. It occurs in both deployed and nondeployed settings; however, Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) and improved reporting mechanisms have dramatically increased TBI diagnoses in active-duty service members. According to the Defense and Veterans Brain Injury Center (DVBIC), more than 370,000 service members have been diagnosed with a TBI since 2000 (Figure).1
Background
The DoD and the VA are collaborating on clinical research studies to identify, understand, and treat the long-term effects of TBI that can affect patients and their families. Most TBIs are mild (mTBIs), also called concussions, and patients typically recover within a few weeks (Table 1). However, some individuals with mTBI experience symptoms that may persist for months or years. A meta-analysis by Perry and colleagues showed that the prevalence or risk of a neurologic disorder, depression, or other mental health issue following mTBI was 67% higher compared with that in uninjured controls.2
Patients with any severity of TBI may require assistance with activities of daily living (ADLs), such as bathing, dressing, managing medications, and feeding. Patients also may need help with instrumental ADLs, such as meal preparation, grocery shopping, household chores, child care, getting to appointments or activities, coordination of educational and vocational services, financial and benefits management, and supportive listening.
Increased injuries have spurred the DoD and VA to coordinate health care to provide a seamless transition for patients between the 2 agencies. However, individuals who sustained a TBI may need various levels of caregiver assistance over time.
TBI and Caregivers
Despite better agency coordination for patients, caregivers can experience stress. Griffin and colleagues found that caregiving responsibilities can compete with other demands on the caregiver, such as work and family, and may negatively impact their health and finances.3,4
Lou and colleagues studied the factors associated with caring for chronically ill family members that may result in stress for the caregivers.5 Along with an unaccounted for economic contribution, caregivers may face lost work time and pay and limitations on work travel and work advancement. Additionally, lost time for leisure, travel, social activities, family obligations, and retirement could result in physical and mental drain on the caregiver. Stress may reach a level at which the caregivers risk psychological distress. The study also noted that families with perceived high stress experience disrupted family functioning. Some TBI caregiver studies sought to understand how best to evaluate and determine the level of caregiver burden, and other studies investigated appropriate interventions.6-9
Health care practitioners within the federal health care system may benefit from a greater awareness of caregiver needs and caregiver resources. Caregiver support can improve outcomes for both the caregiver and care recipient, and many organizations and resources already exist to assist the caregiver. This article reviews recent published literature on TBI caregivers of patients with TBI across civilian, military, and veteran populations and lists caregiver resources for additional information, assistance, and support.
Literature Review
The DVBIC defines the term caregiver as “any family or support person(s) relied upon by the service member or veteran with traumatic brain injury (TBI) who assumes primary responsibility for ensuring the needed level of care and overall well-being of that service member or veteran. A family or family caregiver may include spouse, parents, children, other extended family members, as well as significant others and friends.”3
In the following discussion, findings from military and veteran literature are separated from civilian population findings to highlight similarities and differences between these 2 bodies of research. Several of the studies in the military/veteran cohorts include polytrauma patients with comorbid physical and mental health issues not necessarily found in civilian literature.
Civilian Literature
A 2015 systematic review by Anderson and colleagues on coping and psychological adjustment in TBI caregivers indicated no Class I or Class II studies.10 Four Class III and 3 Class IV studies were found. The authors suggest that more rigorous studies (ie, Class I and II) are needed.
Despite these limitations, peer-reviewed literature indicates that the levels of stress and distress in TBI caregivers are consistent with reports for other diseases. In a civilian population, Carlozzi and colleagues found that TBI caregivers who reported stress, distress, anxiety, and feeling overwhelmed often had concerns for their social, emotional, physical, cognitive health, as well as feelings of loss.5 In addition, caregivers may need to take leaves of absence or leave the workforce entirely to provide for a family member or friend who had a TBI—often leading to financial strain (eg, depleting assets, accumulating debt). These challenges may occur during prime earning years, and the caregiver may lose the ability to resume work if the care receiver requires care for extended periods.11
Kratz and colleagues showed that caregivers of individuals with moderate-to-severe TBI: (1) felt overburdened with responsibilities; (2) lacked personal time and time for self-care; (3) felt their lives were interrupted or lost; (4) grieved the loss of the person with TBI; and (5) endorsed anger, guilt, anxiety, and sadness.12
Perceptions differed between caregiver parents and caregiver partners. Parents expressed feelings of grief and sadness related to the “loss of the person before the TBI.” Parents also reported a sense of guilt and responsibility for their child’s TBI and feelings of being tied down to the individual with TBI. Parents experienced a greater level of stress if the son or daughter with TBI still lived at home. Partners expressed frustration and despair related to their role as sole decision maker and care provider. Partners’ distress also related to the partner relationship and the relationship between children and the individual with TBI.
Verhasghe and colleagues found that partners experience a greater degree of stress than do parents.13 Young families with minimal social support for coping with financial, psychiatric, and medical problems were the most vulnerable to stress. A systematic review by Ennis and colleagues evaluated depression and anxiety in caregiver parents vs spouses.14 Although methods and quality differed in the studies, findings indicated high levels of distress regardless of the type of caregiver.
Anderson and colleagues used the Ways of Coping Questionnaire to evaluate the association between coping and psychological adjustment in caregivers of TBI individuals.10 The use of emotion-focused coping and problem solving was possibly associated with psychological adjustment in caregivers. Verhasghe and colleagues indicated that the nature of the injuries more than the severity of TBI determined the level of stress up to 15 years after the TBI.13 Gender and social and professional support also influenced coping. The review identified the need to develop models of long-term support and care.
An Australian cohort of 79 family caregivers participated in a study by Perlesz and colleagues.15 Participants’ caregiving responsibilities averaged 19.3 months posttrauma. The Family Satisfaction Scale, Beck Depression Inventory, State Anxiety Inventory, and Profile of Mood States were used in this analysis. Male caregivers reported distress in terms of anger and fatigue; female caregivers were at greatest risk of poor psychosocial outcomes. Although findings from primary caregivers indicated that 35% to 49% displayed enough distress to warrant clinical intervention, between 51% and 80% were not psychologically distressed and were satisfied with their families. Data supported previous reports suggesting caregivers are “not universally distressed.”15
Manskow and colleagues followed patients with severe TBI and assessed caregiver burden 1 year later. Using the Caregiver Burden Scale, caregivers reported the highest scores (N = 92) on the General Strain Index followed by the Disappointment Index.16 Bayen and colleagues also studied caregivers of severe TBI patients.17 Objective and subjective caregiver burden data 4 years later indicated 44% of caregivers (N = 98) reported multidimensional burden. Greater burden was associated in caring for individuals who had poorer Glasgow Outcome Scale Extended scores and more severe cognitive disorders.
Military and Veteran Literature
Griffin and colleagues conducted the Family and Caregiver Experience Study (FACES) with caregivers (N = 564) of service members who incurred a TBI.3 According to the caregivers, two-thirds of the patients lost consciousness for more than 30 minutes, which was followed by inpatient rehabilitation care at a VA polytrauma center between 2001 and 2009. The majority of caregivers of TBI patients were female (79%) and aged < 60 years (84%). Parents comprised 62% and spouses 32% of the cohort. Caregivers tended to have some level of education beyond high school (73%), were married (77%), either worked or were enrolled in school (55%), and earned less than $40,000 a year (70%). Common characteristics of the care receivers were male gender (95%), average age 30, high school educated (52%), married (almost 50%), and employed (50%). Forty-five percent of the care receivers were injured 4 to 6 years prior, and 12% were injured 7 or more years prior. The study determined the caregivers’ perception of intensity of care needed and indicated that families as well as clinicians need to plan for some level of long-term support and services.
In addition to the TBI-related caregiving needs, Griffin and colleagues found in a military population that other medical conditions impacted the level of caregiving and strained a marriage.18 Their study found that in a military population between 30% and 50% of marriages of patients with TBI dissolved within the first 10 years after injury. Caregivers may need to learn nursing activities, such as tube feedings, tracheostomy and stoma care, catheter care, wound care, and medication administration. Family stress with caregiving may interfere with the ability to understand information related to the care receivers’ medical care and may require multiple formats to explain care needs. Sander and colleagues associated better emotional functioning in caregivers with greater social integration and occupation outcomes in patients at the postacute rehabilitation program phase (within 6 months of injury).19 However, these outcomes did not continue more than 6 months postinjury.
Intervention and Research Studies
Powell and colleagues used a telephone-based, individualized TBI education intervention along with problem-solving mentoring (10 phone calls at 2-week intervals following patient discharge for moderate-to-severe TBI from a level 1 trauma center) to determine which programs, activities, and coping strategies could decrease caregiver challenges.20 The telephone interventions resulted in better caregiver outcomes than usual care as measured by composite scores on the Bakas Caregiving Outcomes Scale (BCOS) and the Brief Symptom Inventory (BSI-18) at 6 months post-TBI survivor discharge. Dyer and colleagues explored Internet approaches and mobile applications to provide support for caregivers. 18 In a small sample of 10 caregivers, Damianakis and colleagues conducted a 10-session pilot videoconferencing support-group intervention program led by a clinician. Results indicated that the intervention enhanced caregiver coping and problem-solving skills.7
Petranovich and colleagues examined the efficacy of counselor-assisted problem-solving interventions in improving long-term caregiver psychological functioning following TBI in adolescents.21 Their findings support the utility of online interventions in improving long-term caregiver psychological distress, particularly for lower income families. Although this study focused on adolescents, research may indicate merit in an adult population. In relatives of patients with severe TBI, Norup and colleagues associated improvements in health-related quality of life (HRQOL) with improvements in symptoms of anxiety and depression without specific intervention.22
Moriarty and colleagues conducted a randomized controlled trial for veterans who received care at a VA polytrauma center and their family members who participated in a veteran’s in-home program (VIP) intervention.9 The study aimed to evaluate how VIP affected family members’ caregiver burden, depressive symptoms, satisfaction with caregiving, and the program’s acceptability. Eighty-one veterans with a key family member were randomized. Of those, 63 veterans completed a follow-up interview. The intervention consisted of 6 home visits of 1 to 2 hours each and 2 telephone calls from an occupational therapist over 3 to 4 months. Family members were invited to participate during the home visits. The control group received usual clinic care with 2 telephone calls during the study period. All participants received the follow-up interview 3 to 4 months after baseline interviews. The severity of TBI was determined by a review of the electronic medical record using the VA/DoD Clinical Practice Guidelines. Findings of this study indicated that family members in the intervention group showed significantly lower depressive symptom scores and caregiver burden scores.9 Additionally, the veterans in the intervention group exhibited higher community integration and ability to manage their targeted outcomes. Further research may indicate that VIP could assist patients with TBI and caregivers in an active-duty population.
The DVBIC is the executive agent for a congressionally mandated 15-year longitudinal study on TBI incurred by members of the armed services in OEF and OIF. The John Warner National Defense Authorization Act for Fiscal Year 2007 outlined the study. An initial finding identified the need for an HRQOL outcomes assessment specific to TBI caregivers.23 Having these data will allow investigators to fully determine the comprehensive impact of caring for a person who sustained a mild, moderate, severe, or penetrating TBI and to evaluate the effectiveness of interventions designed to address caregivers’ needs. To date, the study has identified the following HRQOL themes generated among caregivers: social health, emotional health, physical/medical health, cognitive functioning, and feelings of loss (related to changing social roles). Carlozzi and colleagues noted that the study also aimed to identify a sensitive outcome measure to evaluate quality of life in the caregivers over time.7
Knowledge Gaps
Ongoing studies focus on caregiving for individuals with various illnesses and needs. Some of the information in each study may be beneficial to TBI caregivers who are not fully aware of resources and interventions. For example, Fortune and colleagues, Hirano and colleagues, and Grover and colleagues are studying caregiver activities involving other diseases to determine, more generally, which programs, activities, and coping strategies can decrease caregiver challenges.24-26 Further, understanding and addressing the needs of these families over many years will provide data that could inform policy, benefits, resources, and needed services (such as the Caregivers and Veterans Omnibus Health Services Act of 2010) and assist with family resilience efforts, including understanding and enhancing family protective and recovery factors.
As studies have indicated, some families do not report family distress when providing care to an individual with TBI. Understanding the factors that influence positive family adjustment is important to capture and perhaps replicate in future studies so that they can lead to effective treatment interventions. Although this review does not discuss caregiver needs for patients with TBI with disorders of consciousness that require more care than most caregivers can provide in the home setting, caregiving for this population deserves attention in future studies. Furthermore, an area that has not received much attention is the impact on children in the household. Children aged < 18 years can assist not only in the care of a disabled adult, but also of younger siblings; also they can help with household activities from housekeeping to meal preparation. Children also may provide physical and emotional support.
The impact of aging caregivers and subsequent needs for their own care as well as the person(s) they are providing care for has not been fully addressed. Areas requiring more research include both the aging caregiver taking care of an aging spouse or relative and the aging parent taking care of a young adult or child. Along with aging, the issue of long-term caregiving needs further development. For example, how do the differences between access to services between caregivers of adults with TBI in the military and those in the civilian sector impact the family/caregiver? Further research may answer questions such as:
- Which tools are most useful in evaluating and determining caregiver stress and burden?
- Are the needs of military and veteran caregivers unique?
- Do polytrauma patients with comorbid diagnoses have unique caregiver needs and trajectories?
- Do TBI caregiver stressors differ from stressors related to other medical conditions or chronic diseases?
- Is there a need for military and veteran TBI-specific caregiver programs?
- Which interventions best help caregivers and for how long?
- Should the approach to intervention depend on variables such as age and gender of the caregivers or relationship to the patient with a TBI (eg, spouse vs parent)?
Methods or processes to inform and update caregivers about available resources also are critically needed. Also, Sabab and colleagues noted the importance of research on the effects of denial as it relates to cognitive, emotional, social impact.27 Denial may impact delays in treatments.
Resource
Many national, state, local, and grassroots organizations provide information and support for persons with illness and/or disabilities. Most clinicians of neurologic, mental health, and cancer have developed various forms of support interventions for those with the disease and their caregivers (Table 2). Highlighted in this section are a few organizations that specifically provide resources for caregivers caring for active-duty service members or veterans with a TBI.
Although a caregiver generally does not receive money from an outside source for services, the DoD may consider the caregiver as a nonmedical attendant for an active-duty service member and provide a temporary stipend. The VA provides several support and service options for caregivers under the Caregiver Support Program, through which more than 300 VA health care professionals provide support to caregivers. The Caregivers and Veterans Omnibus Health Services Act of 2010 authorizes the VA to provide additional VA services for seriously injured post-9/11 veterans and their family caregivers through the Program of Comprehensive Assistance for Family Caregivers (VA Caregiver Support Program). After meeting eligibility criteria, primary caregivers of post-9/11 veterans may receive a monthly stipend (based on the level of care needed) as well as comprehensive caregiver training, referral services, access to health care insurance, mental health services, counseling, and respite care. The Caregiver Support Program offers a toll-free support line and a 24-hour crisis hot line.
In 2014, the Government Accountability Office (GAO) outlined the VA health care improvements needed to manage the demand for the Caregiver Support Program, which are established at VA medical centers.28 The GAO reported that the “VA significantly underestimated caregivers’ demand for services… larger than expected workloads and …delays in approval determinations” with about 500 approved caregivers who are added to the program each month. Original estimates indicated that about 4,000 caregivers would be approved by September 2014; however, by May 2014 about 15,600 caregivers were approved.
In addition to the VA Caregiver Support Program, a variety of state, local, and nonprofit organizations offer support for caregivers. Established in 2012, the Elizabeth Dole Foundation’s program Caring for Military Families “assists caregivers by raising awareness of the caregiver role, leveraging resources and partnerships to provide support, and identifying best practices and solutions to address the challenges caregivers face.” The foundation commissioned the RAND Corporation to “describe the magnitude of military caregiving in the United States, and to identify gaps in programs, policies, and services.” The 2014 RAND report estimated that among the 5.5 million military caregivers in the U.S., 1 million (19.6%) cared for post-9/11 veterans.29 The military caregivers consistently experienced poorer health outcomes, greater strains on family relationships, and more workplace problems than noncaregivers; post-9/11 military caregivers fared worse in those areas.
The Elizabeth Dole Foundation, Hidden Heroes Impact Council Forum advocates for caregiver empowerment, cultural competency awareness, and better policies, programs, and services. The council focuses its efforts on key impact: community support at home, education and training, employment and workplace support, financial and legal issues, interfaith action and ministry council, mental and physical health, and respite care. It aims to raise the money to build awareness and support for military and veterans’ caregivers. The Military and Veteran Caregiver Network is another Elizabeth Dole Foundation initiative. It is an online forum community, peer support group, and peer mentor program structure. A resource library for referrals to local services also is available.
A variety of other organizations, such as United Service Organizations; Easter Seals; Team Red, White and Blue; Operation Homefront; Blue Star Families; state Brain Injury Associations; and support groups for TBI at local hospitals and community centers provide resources to both patients and caregivers. Organizations for caregivers not exclusive to TBI patients include the Caregiver Action Network (formerly National Family Caregiver Association) and the Family Caregiver Alliance. The National Family Caregivers Support Program provides grants to states and territories to develop and provide supportive services to caregivers. Some training for caregivers could include long-term financial planning, legal issues, residential and educational planning, caregiver stress management, the benefits of utilizing support resources, and actions and behaviors that enhance coping strategies. In 2007, DVBIC developed The Traumatic Brain Injury Guide for Caregivers of Service Members and Veterans, which is intended for family caregivers assisting a service member or veteran who sustained a moderate or severe TBI.6 A recent assessment determined the need to update the guide. The Center of Excellence for Medical Multimedia is another source of information for caregivers.
Conclusion
The recent combat conflicts of OEF and OIF have resulted in a dramatic increase in the occurrence of TBI injuries in active-duty service members both in theater and stateside and have highlighted the need for some service members and veterans with a TBI to require ongoing assistance from a caregiver. The levels of assistance and length of time vary greatly, impacted by the severity of the TBI and psychosocial situations.
In response to elevated awareness, several programs and resources have been developed or enhanced to address the specific needs of caregivers. Certain programs and resources are specific for caregivers of military service members and veterans, whereas others benefit caregivers in general. Likewise, some programs are not specific to individuals with TBI.
Caregivers assume many roles in their efforts to support the person with a TBI. They may need to dramatically adjust their lives to serve as a caregiver. Providing adequate resources for the caregivers impacts their ability to continue providing care. Thus, awareness of and access to resources play a critical role in helping to reduce stress, distress, burden (eg, physical, emotional, and financial), and caregiver burnout. Programs and resources often change, making it difficult for health care practitioners to know which programs offer what or even whether they still exist. Therefore, the authors synthesized the current medical literature of the topic of TBI and their caregiver needs as well as current resources for additional information and support.
Ongoing research studies, such as the congressionally mandated 15-year longitudinal study, are examining the impact of caregiving in the military and veteran communities. Future research could identify specific needs of military caregivers, identify gaps in services or programs, and identify interventions that promote resilience. Moreover, research directed at military and veteran caregivers can promote change that will benefit the general population of caregivers. It will be important for health care practitioners to keep abreast of new findings and information to incorporate into care plans for their patients who have had a TBI and their families.
1. Defense and Veterans Brain Injury Center. DoD worldwide numbers for TBI. http://dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Updated October 5, 2017. Accessed October 10, 2017.
2. Perry DC, Sturm VE, Peterson MJ, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg. 2016;124(2):511-526.
3. Defense and Veterans Brain Injury Center. Traumatic brain injury: a guide for caregivers of service members and veterans. https://dvbic.dcoe.mil /sites/default/files/Family%20Caregiver%20Guide.All%20Modules_updated.pdf. Accessed October 10, 2017
4. Griffin JM, Friedemann-Sánchez G, Jensen AC, et al. The invisible side of war: families caring for US service members with traumatic brain injuries and polytrauma. J Head Trauma Rehabil. 2012;27(1):3-13.
5. Lou VW, Kwan CW, Chong ML, Chi I. Associations between secondary caregivers’ supportive behavior and psychological distress of primary spousal caregivers of cognitively intact and impaired elders. Gerontologist. 2015;55(4):584-594.
6. Carlozzi NE, Kratz AL, Sander AM, et al. Health-related quality of life in caregivers of individuals with traumatic brain injury: development of a conceptual model. Arch Phys Med Rehabil. 2015;96(1):105-113.
7. Damianakis T, Tough A, Marziali E, Dawson DR. Therapy online: a web-based video support group for family caregivers of survivors with traumatic brain injury. J Head Trauma Rehabil. 2016;31(4):E12-E20.
8. Dyer EA, Kansagara D, McInnes DK, Freeman M, Woods, S. Mobile applications and internet-based approaches for supporting non-professional caregivers: a systematic review. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0050675. Accessed October 10, 2017.
9. Moriarty H, Winter L, Robinson K, et al. A randomized controlled trial to evaluate the veterans’ in-home program for military veterans with traumatic brain injury and their families: report on impact for family members. PMR. 2016;8(6):495-509.
10. Anderson MI, Simpson GK, Daher M, Matheson L. Chapter 7 the relationship between coping and psychological adjustment in family caregivers of individuals with traumatic brain injury: a systematic review. Annu Rev Nurs Res. 2015;33:219-247.
11. Van Houtven CH, Friedemann-Sanchez G, Clothier B, et al. Is policy well-targeted to remedy financial strain among caregivers of severely injured U.S. service members? Inquiry. 2012-2013;49(4):339-351.
12. Kratz AL, Sander AM, Brickell TA, Lange RT, Carlozzi NE. Traumatic brain injury caregivers: a qualitative analysis of spouse and parent perspectives on quality of life. Neuropsychol Rehabil. 2017;27(1):16-37.13. Verhasghe S, Defloor T, Grypdonck M. Stress and coping among families of patients with traumatic brain injury: a review of the literature. J Clin Nurs. 2005;14(8):1004-1012.
14. Ennis N, Rosenbloom BN, Canzian S, Topolovec-Vranic J. Depression and anxiety in parent versus spouse caregivers of adult patients with traumatic brain injury: a systematic review. Neuropsychol Rehabil. 2013;23(1):1-18.
15. Perlesz A, Kinsella G, Crowe S. Psychological distress and family satisfaction following traumatic brain injury: injured individuals and their primary, secondary, and tertiary carers. J Head Trauma Rehabil. 2000;15(3):909-929.
16. Manskow US, Sigurdardottir S, Røe C, et al. Factors affecting caregiving burden 1 year after severe traumatic brain injury: a prospective nationwide multicenter study. J Head Trauma Rehabil. 2015;30(6):411-423.
17. Bayen E, Jourdan C, Ghout I, et al. Objective and subjective burden of informal caregivers 4 years after a severe traumatic brain injury: results from the Paris-TBI study. J Head Trauma Rehabil. 2016;31(5):E59-E67.
18. Griffin JM, Friedemann-Sanchez G, Hall C, Phelan S, van Ryn M. Families of patients with polytrauma: understanding the evidence and charting a new research agenda. J Rehabil Res Dev. 2009;46(6):879-892.
19. Sander AM, Maestas KL, Sherer M, Malac JF, Nakase-Richardson R. Relationship of caregiver and family functioning to participation outcomes after post-acute rehabilitation for traumatic brain injury: a multicenter investigation. Arch Phys Med Rehabil. 2012;93(5):842-848.
20. Powell JM, Fraser R, Brockway JA, Temkin N, Bell KR. A telehealth approach to caregiver self-management following traumatic brain injury: a randomized control trial. J Head Trauma Rehabil. 2015;31(3):180-190.
21. Petranovich CL, Wade SL, Taylor HG, et al. Long-term caregiver mental health outcomes following a predominately online intervention for adolescents with complicated mild to severe traumatic brain injury. J Pediatr Psychol, 2015;40(7):680-688.
22. Norup A, Kristensen KS, Poulsen I, Mortensen EL. Evaluating clinically significant changes in health-related quality of life: a sample of relatives of patients with severe traumatic brain injury. Neuropsychol Rehabil. 2017;27(2):196-215.
23. John Warner National Defense Authorization Act for Fiscal Year 2007, HR 5122, 109th Cong, 2nd Sess (2006).
24. Fortune DG, Rogan CR, Richards HL. A structured multicomponent group program for carers of people with acquired brain injury: effects on perceived criticism, strain, and psychological distress. Br J Health Psychol. 2016;21(1):224-243.
25. Hirano A, Umegaki H, Suzuki Y, Hayashi T, Kuzuya M. Effects of leisure activities at home on perceived care burden and the endocrine system of caregivers of dementia patients: a randomized controlled study. Int Psychogeriatr. 2016;28(2):261-268.
26. Grover S, Pradyumna, Chakrabarti S. Coping among caregivers of patients with schizophrenia. Ind Psychiatry J. 2015;24(1):5-11.
27. Saban KL, Hogan NS, Hogan TP, Pape TL. He looks normal but…challenges of family caregivers of veterans diagnosed with a traumatic brain injury. Rehabil Nurs. 2015;40(5):277-285.
28. Williamson RB; United States Government Accountability Office. VA health care improvements needed to manage higher-than-expected demand for the family caregiver program. http://www.gao.gov/assets/670/667275.pdf. Published December 3, 2014. Accessed October 10, 2017.
29. Ramchand R, Tanielian T, Fisher MP, et al. Hidden heroes America’s military caregivers. http://www.rand.org/content/dam/rand/pubs/research_reports/RR400/RR499/RAND_RR499.pdf. Published 2014. Accessed October 10, 2017.
Traumatic brain injury (TBI) is a health concern for the U.S. Military Health System (MHS) as well as the VHA. It occurs in both deployed and nondeployed settings; however, Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) and improved reporting mechanisms have dramatically increased TBI diagnoses in active-duty service members. According to the Defense and Veterans Brain Injury Center (DVBIC), more than 370,000 service members have been diagnosed with a TBI since 2000 (Figure).1
Background
The DoD and the VA are collaborating on clinical research studies to identify, understand, and treat the long-term effects of TBI that can affect patients and their families. Most TBIs are mild (mTBIs), also called concussions, and patients typically recover within a few weeks (Table 1). However, some individuals with mTBI experience symptoms that may persist for months or years. A meta-analysis by Perry and colleagues showed that the prevalence or risk of a neurologic disorder, depression, or other mental health issue following mTBI was 67% higher compared with that in uninjured controls.2
Patients with any severity of TBI may require assistance with activities of daily living (ADLs), such as bathing, dressing, managing medications, and feeding. Patients also may need help with instrumental ADLs, such as meal preparation, grocery shopping, household chores, child care, getting to appointments or activities, coordination of educational and vocational services, financial and benefits management, and supportive listening.
Increased injuries have spurred the DoD and VA to coordinate health care to provide a seamless transition for patients between the 2 agencies. However, individuals who sustained a TBI may need various levels of caregiver assistance over time.
TBI and Caregivers
Despite better agency coordination for patients, caregivers can experience stress. Griffin and colleagues found that caregiving responsibilities can compete with other demands on the caregiver, such as work and family, and may negatively impact their health and finances.3,4
Lou and colleagues studied the factors associated with caring for chronically ill family members that may result in stress for the caregivers.5 Along with an unaccounted for economic contribution, caregivers may face lost work time and pay and limitations on work travel and work advancement. Additionally, lost time for leisure, travel, social activities, family obligations, and retirement could result in physical and mental drain on the caregiver. Stress may reach a level at which the caregivers risk psychological distress. The study also noted that families with perceived high stress experience disrupted family functioning. Some TBI caregiver studies sought to understand how best to evaluate and determine the level of caregiver burden, and other studies investigated appropriate interventions.6-9
Health care practitioners within the federal health care system may benefit from a greater awareness of caregiver needs and caregiver resources. Caregiver support can improve outcomes for both the caregiver and care recipient, and many organizations and resources already exist to assist the caregiver. This article reviews recent published literature on TBI caregivers of patients with TBI across civilian, military, and veteran populations and lists caregiver resources for additional information, assistance, and support.
Literature Review
The DVBIC defines the term caregiver as “any family or support person(s) relied upon by the service member or veteran with traumatic brain injury (TBI) who assumes primary responsibility for ensuring the needed level of care and overall well-being of that service member or veteran. A family or family caregiver may include spouse, parents, children, other extended family members, as well as significant others and friends.”3
In the following discussion, findings from military and veteran literature are separated from civilian population findings to highlight similarities and differences between these 2 bodies of research. Several of the studies in the military/veteran cohorts include polytrauma patients with comorbid physical and mental health issues not necessarily found in civilian literature.
Civilian Literature
A 2015 systematic review by Anderson and colleagues on coping and psychological adjustment in TBI caregivers indicated no Class I or Class II studies.10 Four Class III and 3 Class IV studies were found. The authors suggest that more rigorous studies (ie, Class I and II) are needed.
Despite these limitations, peer-reviewed literature indicates that the levels of stress and distress in TBI caregivers are consistent with reports for other diseases. In a civilian population, Carlozzi and colleagues found that TBI caregivers who reported stress, distress, anxiety, and feeling overwhelmed often had concerns for their social, emotional, physical, cognitive health, as well as feelings of loss.5 In addition, caregivers may need to take leaves of absence or leave the workforce entirely to provide for a family member or friend who had a TBI—often leading to financial strain (eg, depleting assets, accumulating debt). These challenges may occur during prime earning years, and the caregiver may lose the ability to resume work if the care receiver requires care for extended periods.11
Kratz and colleagues showed that caregivers of individuals with moderate-to-severe TBI: (1) felt overburdened with responsibilities; (2) lacked personal time and time for self-care; (3) felt their lives were interrupted or lost; (4) grieved the loss of the person with TBI; and (5) endorsed anger, guilt, anxiety, and sadness.12
Perceptions differed between caregiver parents and caregiver partners. Parents expressed feelings of grief and sadness related to the “loss of the person before the TBI.” Parents also reported a sense of guilt and responsibility for their child’s TBI and feelings of being tied down to the individual with TBI. Parents experienced a greater level of stress if the son or daughter with TBI still lived at home. Partners expressed frustration and despair related to their role as sole decision maker and care provider. Partners’ distress also related to the partner relationship and the relationship between children and the individual with TBI.
Verhasghe and colleagues found that partners experience a greater degree of stress than do parents.13 Young families with minimal social support for coping with financial, psychiatric, and medical problems were the most vulnerable to stress. A systematic review by Ennis and colleagues evaluated depression and anxiety in caregiver parents vs spouses.14 Although methods and quality differed in the studies, findings indicated high levels of distress regardless of the type of caregiver.
Anderson and colleagues used the Ways of Coping Questionnaire to evaluate the association between coping and psychological adjustment in caregivers of TBI individuals.10 The use of emotion-focused coping and problem solving was possibly associated with psychological adjustment in caregivers. Verhasghe and colleagues indicated that the nature of the injuries more than the severity of TBI determined the level of stress up to 15 years after the TBI.13 Gender and social and professional support also influenced coping. The review identified the need to develop models of long-term support and care.
An Australian cohort of 79 family caregivers participated in a study by Perlesz and colleagues.15 Participants’ caregiving responsibilities averaged 19.3 months posttrauma. The Family Satisfaction Scale, Beck Depression Inventory, State Anxiety Inventory, and Profile of Mood States were used in this analysis. Male caregivers reported distress in terms of anger and fatigue; female caregivers were at greatest risk of poor psychosocial outcomes. Although findings from primary caregivers indicated that 35% to 49% displayed enough distress to warrant clinical intervention, between 51% and 80% were not psychologically distressed and were satisfied with their families. Data supported previous reports suggesting caregivers are “not universally distressed.”15
Manskow and colleagues followed patients with severe TBI and assessed caregiver burden 1 year later. Using the Caregiver Burden Scale, caregivers reported the highest scores (N = 92) on the General Strain Index followed by the Disappointment Index.16 Bayen and colleagues also studied caregivers of severe TBI patients.17 Objective and subjective caregiver burden data 4 years later indicated 44% of caregivers (N = 98) reported multidimensional burden. Greater burden was associated in caring for individuals who had poorer Glasgow Outcome Scale Extended scores and more severe cognitive disorders.
Military and Veteran Literature
Griffin and colleagues conducted the Family and Caregiver Experience Study (FACES) with caregivers (N = 564) of service members who incurred a TBI.3 According to the caregivers, two-thirds of the patients lost consciousness for more than 30 minutes, which was followed by inpatient rehabilitation care at a VA polytrauma center between 2001 and 2009. The majority of caregivers of TBI patients were female (79%) and aged < 60 years (84%). Parents comprised 62% and spouses 32% of the cohort. Caregivers tended to have some level of education beyond high school (73%), were married (77%), either worked or were enrolled in school (55%), and earned less than $40,000 a year (70%). Common characteristics of the care receivers were male gender (95%), average age 30, high school educated (52%), married (almost 50%), and employed (50%). Forty-five percent of the care receivers were injured 4 to 6 years prior, and 12% were injured 7 or more years prior. The study determined the caregivers’ perception of intensity of care needed and indicated that families as well as clinicians need to plan for some level of long-term support and services.
In addition to the TBI-related caregiving needs, Griffin and colleagues found in a military population that other medical conditions impacted the level of caregiving and strained a marriage.18 Their study found that in a military population between 30% and 50% of marriages of patients with TBI dissolved within the first 10 years after injury. Caregivers may need to learn nursing activities, such as tube feedings, tracheostomy and stoma care, catheter care, wound care, and medication administration. Family stress with caregiving may interfere with the ability to understand information related to the care receivers’ medical care and may require multiple formats to explain care needs. Sander and colleagues associated better emotional functioning in caregivers with greater social integration and occupation outcomes in patients at the postacute rehabilitation program phase (within 6 months of injury).19 However, these outcomes did not continue more than 6 months postinjury.
Intervention and Research Studies
Powell and colleagues used a telephone-based, individualized TBI education intervention along with problem-solving mentoring (10 phone calls at 2-week intervals following patient discharge for moderate-to-severe TBI from a level 1 trauma center) to determine which programs, activities, and coping strategies could decrease caregiver challenges.20 The telephone interventions resulted in better caregiver outcomes than usual care as measured by composite scores on the Bakas Caregiving Outcomes Scale (BCOS) and the Brief Symptom Inventory (BSI-18) at 6 months post-TBI survivor discharge. Dyer and colleagues explored Internet approaches and mobile applications to provide support for caregivers. 18 In a small sample of 10 caregivers, Damianakis and colleagues conducted a 10-session pilot videoconferencing support-group intervention program led by a clinician. Results indicated that the intervention enhanced caregiver coping and problem-solving skills.7
Petranovich and colleagues examined the efficacy of counselor-assisted problem-solving interventions in improving long-term caregiver psychological functioning following TBI in adolescents.21 Their findings support the utility of online interventions in improving long-term caregiver psychological distress, particularly for lower income families. Although this study focused on adolescents, research may indicate merit in an adult population. In relatives of patients with severe TBI, Norup and colleagues associated improvements in health-related quality of life (HRQOL) with improvements in symptoms of anxiety and depression without specific intervention.22
Moriarty and colleagues conducted a randomized controlled trial for veterans who received care at a VA polytrauma center and their family members who participated in a veteran’s in-home program (VIP) intervention.9 The study aimed to evaluate how VIP affected family members’ caregiver burden, depressive symptoms, satisfaction with caregiving, and the program’s acceptability. Eighty-one veterans with a key family member were randomized. Of those, 63 veterans completed a follow-up interview. The intervention consisted of 6 home visits of 1 to 2 hours each and 2 telephone calls from an occupational therapist over 3 to 4 months. Family members were invited to participate during the home visits. The control group received usual clinic care with 2 telephone calls during the study period. All participants received the follow-up interview 3 to 4 months after baseline interviews. The severity of TBI was determined by a review of the electronic medical record using the VA/DoD Clinical Practice Guidelines. Findings of this study indicated that family members in the intervention group showed significantly lower depressive symptom scores and caregiver burden scores.9 Additionally, the veterans in the intervention group exhibited higher community integration and ability to manage their targeted outcomes. Further research may indicate that VIP could assist patients with TBI and caregivers in an active-duty population.
The DVBIC is the executive agent for a congressionally mandated 15-year longitudinal study on TBI incurred by members of the armed services in OEF and OIF. The John Warner National Defense Authorization Act for Fiscal Year 2007 outlined the study. An initial finding identified the need for an HRQOL outcomes assessment specific to TBI caregivers.23 Having these data will allow investigators to fully determine the comprehensive impact of caring for a person who sustained a mild, moderate, severe, or penetrating TBI and to evaluate the effectiveness of interventions designed to address caregivers’ needs. To date, the study has identified the following HRQOL themes generated among caregivers: social health, emotional health, physical/medical health, cognitive functioning, and feelings of loss (related to changing social roles). Carlozzi and colleagues noted that the study also aimed to identify a sensitive outcome measure to evaluate quality of life in the caregivers over time.7
Knowledge Gaps
Ongoing studies focus on caregiving for individuals with various illnesses and needs. Some of the information in each study may be beneficial to TBI caregivers who are not fully aware of resources and interventions. For example, Fortune and colleagues, Hirano and colleagues, and Grover and colleagues are studying caregiver activities involving other diseases to determine, more generally, which programs, activities, and coping strategies can decrease caregiver challenges.24-26 Further, understanding and addressing the needs of these families over many years will provide data that could inform policy, benefits, resources, and needed services (such as the Caregivers and Veterans Omnibus Health Services Act of 2010) and assist with family resilience efforts, including understanding and enhancing family protective and recovery factors.
As studies have indicated, some families do not report family distress when providing care to an individual with TBI. Understanding the factors that influence positive family adjustment is important to capture and perhaps replicate in future studies so that they can lead to effective treatment interventions. Although this review does not discuss caregiver needs for patients with TBI with disorders of consciousness that require more care than most caregivers can provide in the home setting, caregiving for this population deserves attention in future studies. Furthermore, an area that has not received much attention is the impact on children in the household. Children aged < 18 years can assist not only in the care of a disabled adult, but also of younger siblings; also they can help with household activities from housekeeping to meal preparation. Children also may provide physical and emotional support.
The impact of aging caregivers and subsequent needs for their own care as well as the person(s) they are providing care for has not been fully addressed. Areas requiring more research include both the aging caregiver taking care of an aging spouse or relative and the aging parent taking care of a young adult or child. Along with aging, the issue of long-term caregiving needs further development. For example, how do the differences between access to services between caregivers of adults with TBI in the military and those in the civilian sector impact the family/caregiver? Further research may answer questions such as:
- Which tools are most useful in evaluating and determining caregiver stress and burden?
- Are the needs of military and veteran caregivers unique?
- Do polytrauma patients with comorbid diagnoses have unique caregiver needs and trajectories?
- Do TBI caregiver stressors differ from stressors related to other medical conditions or chronic diseases?
- Is there a need for military and veteran TBI-specific caregiver programs?
- Which interventions best help caregivers and for how long?
- Should the approach to intervention depend on variables such as age and gender of the caregivers or relationship to the patient with a TBI (eg, spouse vs parent)?
Methods or processes to inform and update caregivers about available resources also are critically needed. Also, Sabab and colleagues noted the importance of research on the effects of denial as it relates to cognitive, emotional, social impact.27 Denial may impact delays in treatments.
Resource
Many national, state, local, and grassroots organizations provide information and support for persons with illness and/or disabilities. Most clinicians of neurologic, mental health, and cancer have developed various forms of support interventions for those with the disease and their caregivers (Table 2). Highlighted in this section are a few organizations that specifically provide resources for caregivers caring for active-duty service members or veterans with a TBI.
Although a caregiver generally does not receive money from an outside source for services, the DoD may consider the caregiver as a nonmedical attendant for an active-duty service member and provide a temporary stipend. The VA provides several support and service options for caregivers under the Caregiver Support Program, through which more than 300 VA health care professionals provide support to caregivers. The Caregivers and Veterans Omnibus Health Services Act of 2010 authorizes the VA to provide additional VA services for seriously injured post-9/11 veterans and their family caregivers through the Program of Comprehensive Assistance for Family Caregivers (VA Caregiver Support Program). After meeting eligibility criteria, primary caregivers of post-9/11 veterans may receive a monthly stipend (based on the level of care needed) as well as comprehensive caregiver training, referral services, access to health care insurance, mental health services, counseling, and respite care. The Caregiver Support Program offers a toll-free support line and a 24-hour crisis hot line.
In 2014, the Government Accountability Office (GAO) outlined the VA health care improvements needed to manage the demand for the Caregiver Support Program, which are established at VA medical centers.28 The GAO reported that the “VA significantly underestimated caregivers’ demand for services… larger than expected workloads and …delays in approval determinations” with about 500 approved caregivers who are added to the program each month. Original estimates indicated that about 4,000 caregivers would be approved by September 2014; however, by May 2014 about 15,600 caregivers were approved.
In addition to the VA Caregiver Support Program, a variety of state, local, and nonprofit organizations offer support for caregivers. Established in 2012, the Elizabeth Dole Foundation’s program Caring for Military Families “assists caregivers by raising awareness of the caregiver role, leveraging resources and partnerships to provide support, and identifying best practices and solutions to address the challenges caregivers face.” The foundation commissioned the RAND Corporation to “describe the magnitude of military caregiving in the United States, and to identify gaps in programs, policies, and services.” The 2014 RAND report estimated that among the 5.5 million military caregivers in the U.S., 1 million (19.6%) cared for post-9/11 veterans.29 The military caregivers consistently experienced poorer health outcomes, greater strains on family relationships, and more workplace problems than noncaregivers; post-9/11 military caregivers fared worse in those areas.
The Elizabeth Dole Foundation, Hidden Heroes Impact Council Forum advocates for caregiver empowerment, cultural competency awareness, and better policies, programs, and services. The council focuses its efforts on key impact: community support at home, education and training, employment and workplace support, financial and legal issues, interfaith action and ministry council, mental and physical health, and respite care. It aims to raise the money to build awareness and support for military and veterans’ caregivers. The Military and Veteran Caregiver Network is another Elizabeth Dole Foundation initiative. It is an online forum community, peer support group, and peer mentor program structure. A resource library for referrals to local services also is available.
A variety of other organizations, such as United Service Organizations; Easter Seals; Team Red, White and Blue; Operation Homefront; Blue Star Families; state Brain Injury Associations; and support groups for TBI at local hospitals and community centers provide resources to both patients and caregivers. Organizations for caregivers not exclusive to TBI patients include the Caregiver Action Network (formerly National Family Caregiver Association) and the Family Caregiver Alliance. The National Family Caregivers Support Program provides grants to states and territories to develop and provide supportive services to caregivers. Some training for caregivers could include long-term financial planning, legal issues, residential and educational planning, caregiver stress management, the benefits of utilizing support resources, and actions and behaviors that enhance coping strategies. In 2007, DVBIC developed The Traumatic Brain Injury Guide for Caregivers of Service Members and Veterans, which is intended for family caregivers assisting a service member or veteran who sustained a moderate or severe TBI.6 A recent assessment determined the need to update the guide. The Center of Excellence for Medical Multimedia is another source of information for caregivers.
Conclusion
The recent combat conflicts of OEF and OIF have resulted in a dramatic increase in the occurrence of TBI injuries in active-duty service members both in theater and stateside and have highlighted the need for some service members and veterans with a TBI to require ongoing assistance from a caregiver. The levels of assistance and length of time vary greatly, impacted by the severity of the TBI and psychosocial situations.
In response to elevated awareness, several programs and resources have been developed or enhanced to address the specific needs of caregivers. Certain programs and resources are specific for caregivers of military service members and veterans, whereas others benefit caregivers in general. Likewise, some programs are not specific to individuals with TBI.
Caregivers assume many roles in their efforts to support the person with a TBI. They may need to dramatically adjust their lives to serve as a caregiver. Providing adequate resources for the caregivers impacts their ability to continue providing care. Thus, awareness of and access to resources play a critical role in helping to reduce stress, distress, burden (eg, physical, emotional, and financial), and caregiver burnout. Programs and resources often change, making it difficult for health care practitioners to know which programs offer what or even whether they still exist. Therefore, the authors synthesized the current medical literature of the topic of TBI and their caregiver needs as well as current resources for additional information and support.
Ongoing research studies, such as the congressionally mandated 15-year longitudinal study, are examining the impact of caregiving in the military and veteran communities. Future research could identify specific needs of military caregivers, identify gaps in services or programs, and identify interventions that promote resilience. Moreover, research directed at military and veteran caregivers can promote change that will benefit the general population of caregivers. It will be important for health care practitioners to keep abreast of new findings and information to incorporate into care plans for their patients who have had a TBI and their families.
Traumatic brain injury (TBI) is a health concern for the U.S. Military Health System (MHS) as well as the VHA. It occurs in both deployed and nondeployed settings; however, Operation Enduring Freedom (OEF) and Operation Iraqi Freedom (OIF) and improved reporting mechanisms have dramatically increased TBI diagnoses in active-duty service members. According to the Defense and Veterans Brain Injury Center (DVBIC), more than 370,000 service members have been diagnosed with a TBI since 2000 (Figure).1
Background
The DoD and the VA are collaborating on clinical research studies to identify, understand, and treat the long-term effects of TBI that can affect patients and their families. Most TBIs are mild (mTBIs), also called concussions, and patients typically recover within a few weeks (Table 1). However, some individuals with mTBI experience symptoms that may persist for months or years. A meta-analysis by Perry and colleagues showed that the prevalence or risk of a neurologic disorder, depression, or other mental health issue following mTBI was 67% higher compared with that in uninjured controls.2
Patients with any severity of TBI may require assistance with activities of daily living (ADLs), such as bathing, dressing, managing medications, and feeding. Patients also may need help with instrumental ADLs, such as meal preparation, grocery shopping, household chores, child care, getting to appointments or activities, coordination of educational and vocational services, financial and benefits management, and supportive listening.
Increased injuries have spurred the DoD and VA to coordinate health care to provide a seamless transition for patients between the 2 agencies. However, individuals who sustained a TBI may need various levels of caregiver assistance over time.
TBI and Caregivers
Despite better agency coordination for patients, caregivers can experience stress. Griffin and colleagues found that caregiving responsibilities can compete with other demands on the caregiver, such as work and family, and may negatively impact their health and finances.3,4
Lou and colleagues studied the factors associated with caring for chronically ill family members that may result in stress for the caregivers.5 Along with an unaccounted for economic contribution, caregivers may face lost work time and pay and limitations on work travel and work advancement. Additionally, lost time for leisure, travel, social activities, family obligations, and retirement could result in physical and mental drain on the caregiver. Stress may reach a level at which the caregivers risk psychological distress. The study also noted that families with perceived high stress experience disrupted family functioning. Some TBI caregiver studies sought to understand how best to evaluate and determine the level of caregiver burden, and other studies investigated appropriate interventions.6-9
Health care practitioners within the federal health care system may benefit from a greater awareness of caregiver needs and caregiver resources. Caregiver support can improve outcomes for both the caregiver and care recipient, and many organizations and resources already exist to assist the caregiver. This article reviews recent published literature on TBI caregivers of patients with TBI across civilian, military, and veteran populations and lists caregiver resources for additional information, assistance, and support.
Literature Review
The DVBIC defines the term caregiver as “any family or support person(s) relied upon by the service member or veteran with traumatic brain injury (TBI) who assumes primary responsibility for ensuring the needed level of care and overall well-being of that service member or veteran. A family or family caregiver may include spouse, parents, children, other extended family members, as well as significant others and friends.”3
In the following discussion, findings from military and veteran literature are separated from civilian population findings to highlight similarities and differences between these 2 bodies of research. Several of the studies in the military/veteran cohorts include polytrauma patients with comorbid physical and mental health issues not necessarily found in civilian literature.
Civilian Literature
A 2015 systematic review by Anderson and colleagues on coping and psychological adjustment in TBI caregivers indicated no Class I or Class II studies.10 Four Class III and 3 Class IV studies were found. The authors suggest that more rigorous studies (ie, Class I and II) are needed.
Despite these limitations, peer-reviewed literature indicates that the levels of stress and distress in TBI caregivers are consistent with reports for other diseases. In a civilian population, Carlozzi and colleagues found that TBI caregivers who reported stress, distress, anxiety, and feeling overwhelmed often had concerns for their social, emotional, physical, cognitive health, as well as feelings of loss.5 In addition, caregivers may need to take leaves of absence or leave the workforce entirely to provide for a family member or friend who had a TBI—often leading to financial strain (eg, depleting assets, accumulating debt). These challenges may occur during prime earning years, and the caregiver may lose the ability to resume work if the care receiver requires care for extended periods.11
Kratz and colleagues showed that caregivers of individuals with moderate-to-severe TBI: (1) felt overburdened with responsibilities; (2) lacked personal time and time for self-care; (3) felt their lives were interrupted or lost; (4) grieved the loss of the person with TBI; and (5) endorsed anger, guilt, anxiety, and sadness.12
Perceptions differed between caregiver parents and caregiver partners. Parents expressed feelings of grief and sadness related to the “loss of the person before the TBI.” Parents also reported a sense of guilt and responsibility for their child’s TBI and feelings of being tied down to the individual with TBI. Parents experienced a greater level of stress if the son or daughter with TBI still lived at home. Partners expressed frustration and despair related to their role as sole decision maker and care provider. Partners’ distress also related to the partner relationship and the relationship between children and the individual with TBI.
Verhasghe and colleagues found that partners experience a greater degree of stress than do parents.13 Young families with minimal social support for coping with financial, psychiatric, and medical problems were the most vulnerable to stress. A systematic review by Ennis and colleagues evaluated depression and anxiety in caregiver parents vs spouses.14 Although methods and quality differed in the studies, findings indicated high levels of distress regardless of the type of caregiver.
Anderson and colleagues used the Ways of Coping Questionnaire to evaluate the association between coping and psychological adjustment in caregivers of TBI individuals.10 The use of emotion-focused coping and problem solving was possibly associated with psychological adjustment in caregivers. Verhasghe and colleagues indicated that the nature of the injuries more than the severity of TBI determined the level of stress up to 15 years after the TBI.13 Gender and social and professional support also influenced coping. The review identified the need to develop models of long-term support and care.
An Australian cohort of 79 family caregivers participated in a study by Perlesz and colleagues.15 Participants’ caregiving responsibilities averaged 19.3 months posttrauma. The Family Satisfaction Scale, Beck Depression Inventory, State Anxiety Inventory, and Profile of Mood States were used in this analysis. Male caregivers reported distress in terms of anger and fatigue; female caregivers were at greatest risk of poor psychosocial outcomes. Although findings from primary caregivers indicated that 35% to 49% displayed enough distress to warrant clinical intervention, between 51% and 80% were not psychologically distressed and were satisfied with their families. Data supported previous reports suggesting caregivers are “not universally distressed.”15
Manskow and colleagues followed patients with severe TBI and assessed caregiver burden 1 year later. Using the Caregiver Burden Scale, caregivers reported the highest scores (N = 92) on the General Strain Index followed by the Disappointment Index.16 Bayen and colleagues also studied caregivers of severe TBI patients.17 Objective and subjective caregiver burden data 4 years later indicated 44% of caregivers (N = 98) reported multidimensional burden. Greater burden was associated in caring for individuals who had poorer Glasgow Outcome Scale Extended scores and more severe cognitive disorders.
Military and Veteran Literature
Griffin and colleagues conducted the Family and Caregiver Experience Study (FACES) with caregivers (N = 564) of service members who incurred a TBI.3 According to the caregivers, two-thirds of the patients lost consciousness for more than 30 minutes, which was followed by inpatient rehabilitation care at a VA polytrauma center between 2001 and 2009. The majority of caregivers of TBI patients were female (79%) and aged < 60 years (84%). Parents comprised 62% and spouses 32% of the cohort. Caregivers tended to have some level of education beyond high school (73%), were married (77%), either worked or were enrolled in school (55%), and earned less than $40,000 a year (70%). Common characteristics of the care receivers were male gender (95%), average age 30, high school educated (52%), married (almost 50%), and employed (50%). Forty-five percent of the care receivers were injured 4 to 6 years prior, and 12% were injured 7 or more years prior. The study determined the caregivers’ perception of intensity of care needed and indicated that families as well as clinicians need to plan for some level of long-term support and services.
In addition to the TBI-related caregiving needs, Griffin and colleagues found in a military population that other medical conditions impacted the level of caregiving and strained a marriage.18 Their study found that in a military population between 30% and 50% of marriages of patients with TBI dissolved within the first 10 years after injury. Caregivers may need to learn nursing activities, such as tube feedings, tracheostomy and stoma care, catheter care, wound care, and medication administration. Family stress with caregiving may interfere with the ability to understand information related to the care receivers’ medical care and may require multiple formats to explain care needs. Sander and colleagues associated better emotional functioning in caregivers with greater social integration and occupation outcomes in patients at the postacute rehabilitation program phase (within 6 months of injury).19 However, these outcomes did not continue more than 6 months postinjury.
Intervention and Research Studies
Powell and colleagues used a telephone-based, individualized TBI education intervention along with problem-solving mentoring (10 phone calls at 2-week intervals following patient discharge for moderate-to-severe TBI from a level 1 trauma center) to determine which programs, activities, and coping strategies could decrease caregiver challenges.20 The telephone interventions resulted in better caregiver outcomes than usual care as measured by composite scores on the Bakas Caregiving Outcomes Scale (BCOS) and the Brief Symptom Inventory (BSI-18) at 6 months post-TBI survivor discharge. Dyer and colleagues explored Internet approaches and mobile applications to provide support for caregivers. 18 In a small sample of 10 caregivers, Damianakis and colleagues conducted a 10-session pilot videoconferencing support-group intervention program led by a clinician. Results indicated that the intervention enhanced caregiver coping and problem-solving skills.7
Petranovich and colleagues examined the efficacy of counselor-assisted problem-solving interventions in improving long-term caregiver psychological functioning following TBI in adolescents.21 Their findings support the utility of online interventions in improving long-term caregiver psychological distress, particularly for lower income families. Although this study focused on adolescents, research may indicate merit in an adult population. In relatives of patients with severe TBI, Norup and colleagues associated improvements in health-related quality of life (HRQOL) with improvements in symptoms of anxiety and depression without specific intervention.22
Moriarty and colleagues conducted a randomized controlled trial for veterans who received care at a VA polytrauma center and their family members who participated in a veteran’s in-home program (VIP) intervention.9 The study aimed to evaluate how VIP affected family members’ caregiver burden, depressive symptoms, satisfaction with caregiving, and the program’s acceptability. Eighty-one veterans with a key family member were randomized. Of those, 63 veterans completed a follow-up interview. The intervention consisted of 6 home visits of 1 to 2 hours each and 2 telephone calls from an occupational therapist over 3 to 4 months. Family members were invited to participate during the home visits. The control group received usual clinic care with 2 telephone calls during the study period. All participants received the follow-up interview 3 to 4 months after baseline interviews. The severity of TBI was determined by a review of the electronic medical record using the VA/DoD Clinical Practice Guidelines. Findings of this study indicated that family members in the intervention group showed significantly lower depressive symptom scores and caregiver burden scores.9 Additionally, the veterans in the intervention group exhibited higher community integration and ability to manage their targeted outcomes. Further research may indicate that VIP could assist patients with TBI and caregivers in an active-duty population.
The DVBIC is the executive agent for a congressionally mandated 15-year longitudinal study on TBI incurred by members of the armed services in OEF and OIF. The John Warner National Defense Authorization Act for Fiscal Year 2007 outlined the study. An initial finding identified the need for an HRQOL outcomes assessment specific to TBI caregivers.23 Having these data will allow investigators to fully determine the comprehensive impact of caring for a person who sustained a mild, moderate, severe, or penetrating TBI and to evaluate the effectiveness of interventions designed to address caregivers’ needs. To date, the study has identified the following HRQOL themes generated among caregivers: social health, emotional health, physical/medical health, cognitive functioning, and feelings of loss (related to changing social roles). Carlozzi and colleagues noted that the study also aimed to identify a sensitive outcome measure to evaluate quality of life in the caregivers over time.7
Knowledge Gaps
Ongoing studies focus on caregiving for individuals with various illnesses and needs. Some of the information in each study may be beneficial to TBI caregivers who are not fully aware of resources and interventions. For example, Fortune and colleagues, Hirano and colleagues, and Grover and colleagues are studying caregiver activities involving other diseases to determine, more generally, which programs, activities, and coping strategies can decrease caregiver challenges.24-26 Further, understanding and addressing the needs of these families over many years will provide data that could inform policy, benefits, resources, and needed services (such as the Caregivers and Veterans Omnibus Health Services Act of 2010) and assist with family resilience efforts, including understanding and enhancing family protective and recovery factors.
As studies have indicated, some families do not report family distress when providing care to an individual with TBI. Understanding the factors that influence positive family adjustment is important to capture and perhaps replicate in future studies so that they can lead to effective treatment interventions. Although this review does not discuss caregiver needs for patients with TBI with disorders of consciousness that require more care than most caregivers can provide in the home setting, caregiving for this population deserves attention in future studies. Furthermore, an area that has not received much attention is the impact on children in the household. Children aged < 18 years can assist not only in the care of a disabled adult, but also of younger siblings; also they can help with household activities from housekeeping to meal preparation. Children also may provide physical and emotional support.
The impact of aging caregivers and subsequent needs for their own care as well as the person(s) they are providing care for has not been fully addressed. Areas requiring more research include both the aging caregiver taking care of an aging spouse or relative and the aging parent taking care of a young adult or child. Along with aging, the issue of long-term caregiving needs further development. For example, how do the differences between access to services between caregivers of adults with TBI in the military and those in the civilian sector impact the family/caregiver? Further research may answer questions such as:
- Which tools are most useful in evaluating and determining caregiver stress and burden?
- Are the needs of military and veteran caregivers unique?
- Do polytrauma patients with comorbid diagnoses have unique caregiver needs and trajectories?
- Do TBI caregiver stressors differ from stressors related to other medical conditions or chronic diseases?
- Is there a need for military and veteran TBI-specific caregiver programs?
- Which interventions best help caregivers and for how long?
- Should the approach to intervention depend on variables such as age and gender of the caregivers or relationship to the patient with a TBI (eg, spouse vs parent)?
Methods or processes to inform and update caregivers about available resources also are critically needed. Also, Sabab and colleagues noted the importance of research on the effects of denial as it relates to cognitive, emotional, social impact.27 Denial may impact delays in treatments.
Resource
Many national, state, local, and grassroots organizations provide information and support for persons with illness and/or disabilities. Most clinicians of neurologic, mental health, and cancer have developed various forms of support interventions for those with the disease and their caregivers (Table 2). Highlighted in this section are a few organizations that specifically provide resources for caregivers caring for active-duty service members or veterans with a TBI.
Although a caregiver generally does not receive money from an outside source for services, the DoD may consider the caregiver as a nonmedical attendant for an active-duty service member and provide a temporary stipend. The VA provides several support and service options for caregivers under the Caregiver Support Program, through which more than 300 VA health care professionals provide support to caregivers. The Caregivers and Veterans Omnibus Health Services Act of 2010 authorizes the VA to provide additional VA services for seriously injured post-9/11 veterans and their family caregivers through the Program of Comprehensive Assistance for Family Caregivers (VA Caregiver Support Program). After meeting eligibility criteria, primary caregivers of post-9/11 veterans may receive a monthly stipend (based on the level of care needed) as well as comprehensive caregiver training, referral services, access to health care insurance, mental health services, counseling, and respite care. The Caregiver Support Program offers a toll-free support line and a 24-hour crisis hot line.
In 2014, the Government Accountability Office (GAO) outlined the VA health care improvements needed to manage the demand for the Caregiver Support Program, which are established at VA medical centers.28 The GAO reported that the “VA significantly underestimated caregivers’ demand for services… larger than expected workloads and …delays in approval determinations” with about 500 approved caregivers who are added to the program each month. Original estimates indicated that about 4,000 caregivers would be approved by September 2014; however, by May 2014 about 15,600 caregivers were approved.
In addition to the VA Caregiver Support Program, a variety of state, local, and nonprofit organizations offer support for caregivers. Established in 2012, the Elizabeth Dole Foundation’s program Caring for Military Families “assists caregivers by raising awareness of the caregiver role, leveraging resources and partnerships to provide support, and identifying best practices and solutions to address the challenges caregivers face.” The foundation commissioned the RAND Corporation to “describe the magnitude of military caregiving in the United States, and to identify gaps in programs, policies, and services.” The 2014 RAND report estimated that among the 5.5 million military caregivers in the U.S., 1 million (19.6%) cared for post-9/11 veterans.29 The military caregivers consistently experienced poorer health outcomes, greater strains on family relationships, and more workplace problems than noncaregivers; post-9/11 military caregivers fared worse in those areas.
The Elizabeth Dole Foundation, Hidden Heroes Impact Council Forum advocates for caregiver empowerment, cultural competency awareness, and better policies, programs, and services. The council focuses its efforts on key impact: community support at home, education and training, employment and workplace support, financial and legal issues, interfaith action and ministry council, mental and physical health, and respite care. It aims to raise the money to build awareness and support for military and veterans’ caregivers. The Military and Veteran Caregiver Network is another Elizabeth Dole Foundation initiative. It is an online forum community, peer support group, and peer mentor program structure. A resource library for referrals to local services also is available.
A variety of other organizations, such as United Service Organizations; Easter Seals; Team Red, White and Blue; Operation Homefront; Blue Star Families; state Brain Injury Associations; and support groups for TBI at local hospitals and community centers provide resources to both patients and caregivers. Organizations for caregivers not exclusive to TBI patients include the Caregiver Action Network (formerly National Family Caregiver Association) and the Family Caregiver Alliance. The National Family Caregivers Support Program provides grants to states and territories to develop and provide supportive services to caregivers. Some training for caregivers could include long-term financial planning, legal issues, residential and educational planning, caregiver stress management, the benefits of utilizing support resources, and actions and behaviors that enhance coping strategies. In 2007, DVBIC developed The Traumatic Brain Injury Guide for Caregivers of Service Members and Veterans, which is intended for family caregivers assisting a service member or veteran who sustained a moderate or severe TBI.6 A recent assessment determined the need to update the guide. The Center of Excellence for Medical Multimedia is another source of information for caregivers.
Conclusion
The recent combat conflicts of OEF and OIF have resulted in a dramatic increase in the occurrence of TBI injuries in active-duty service members both in theater and stateside and have highlighted the need for some service members and veterans with a TBI to require ongoing assistance from a caregiver. The levels of assistance and length of time vary greatly, impacted by the severity of the TBI and psychosocial situations.
In response to elevated awareness, several programs and resources have been developed or enhanced to address the specific needs of caregivers. Certain programs and resources are specific for caregivers of military service members and veterans, whereas others benefit caregivers in general. Likewise, some programs are not specific to individuals with TBI.
Caregivers assume many roles in their efforts to support the person with a TBI. They may need to dramatically adjust their lives to serve as a caregiver. Providing adequate resources for the caregivers impacts their ability to continue providing care. Thus, awareness of and access to resources play a critical role in helping to reduce stress, distress, burden (eg, physical, emotional, and financial), and caregiver burnout. Programs and resources often change, making it difficult for health care practitioners to know which programs offer what or even whether they still exist. Therefore, the authors synthesized the current medical literature of the topic of TBI and their caregiver needs as well as current resources for additional information and support.
Ongoing research studies, such as the congressionally mandated 15-year longitudinal study, are examining the impact of caregiving in the military and veteran communities. Future research could identify specific needs of military caregivers, identify gaps in services or programs, and identify interventions that promote resilience. Moreover, research directed at military and veteran caregivers can promote change that will benefit the general population of caregivers. It will be important for health care practitioners to keep abreast of new findings and information to incorporate into care plans for their patients who have had a TBI and their families.
1. Defense and Veterans Brain Injury Center. DoD worldwide numbers for TBI. http://dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Updated October 5, 2017. Accessed October 10, 2017.
2. Perry DC, Sturm VE, Peterson MJ, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg. 2016;124(2):511-526.
3. Defense and Veterans Brain Injury Center. Traumatic brain injury: a guide for caregivers of service members and veterans. https://dvbic.dcoe.mil /sites/default/files/Family%20Caregiver%20Guide.All%20Modules_updated.pdf. Accessed October 10, 2017
4. Griffin JM, Friedemann-Sánchez G, Jensen AC, et al. The invisible side of war: families caring for US service members with traumatic brain injuries and polytrauma. J Head Trauma Rehabil. 2012;27(1):3-13.
5. Lou VW, Kwan CW, Chong ML, Chi I. Associations between secondary caregivers’ supportive behavior and psychological distress of primary spousal caregivers of cognitively intact and impaired elders. Gerontologist. 2015;55(4):584-594.
6. Carlozzi NE, Kratz AL, Sander AM, et al. Health-related quality of life in caregivers of individuals with traumatic brain injury: development of a conceptual model. Arch Phys Med Rehabil. 2015;96(1):105-113.
7. Damianakis T, Tough A, Marziali E, Dawson DR. Therapy online: a web-based video support group for family caregivers of survivors with traumatic brain injury. J Head Trauma Rehabil. 2016;31(4):E12-E20.
8. Dyer EA, Kansagara D, McInnes DK, Freeman M, Woods, S. Mobile applications and internet-based approaches for supporting non-professional caregivers: a systematic review. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0050675. Accessed October 10, 2017.
9. Moriarty H, Winter L, Robinson K, et al. A randomized controlled trial to evaluate the veterans’ in-home program for military veterans with traumatic brain injury and their families: report on impact for family members. PMR. 2016;8(6):495-509.
10. Anderson MI, Simpson GK, Daher M, Matheson L. Chapter 7 the relationship between coping and psychological adjustment in family caregivers of individuals with traumatic brain injury: a systematic review. Annu Rev Nurs Res. 2015;33:219-247.
11. Van Houtven CH, Friedemann-Sanchez G, Clothier B, et al. Is policy well-targeted to remedy financial strain among caregivers of severely injured U.S. service members? Inquiry. 2012-2013;49(4):339-351.
12. Kratz AL, Sander AM, Brickell TA, Lange RT, Carlozzi NE. Traumatic brain injury caregivers: a qualitative analysis of spouse and parent perspectives on quality of life. Neuropsychol Rehabil. 2017;27(1):16-37.13. Verhasghe S, Defloor T, Grypdonck M. Stress and coping among families of patients with traumatic brain injury: a review of the literature. J Clin Nurs. 2005;14(8):1004-1012.
14. Ennis N, Rosenbloom BN, Canzian S, Topolovec-Vranic J. Depression and anxiety in parent versus spouse caregivers of adult patients with traumatic brain injury: a systematic review. Neuropsychol Rehabil. 2013;23(1):1-18.
15. Perlesz A, Kinsella G, Crowe S. Psychological distress and family satisfaction following traumatic brain injury: injured individuals and their primary, secondary, and tertiary carers. J Head Trauma Rehabil. 2000;15(3):909-929.
16. Manskow US, Sigurdardottir S, Røe C, et al. Factors affecting caregiving burden 1 year after severe traumatic brain injury: a prospective nationwide multicenter study. J Head Trauma Rehabil. 2015;30(6):411-423.
17. Bayen E, Jourdan C, Ghout I, et al. Objective and subjective burden of informal caregivers 4 years after a severe traumatic brain injury: results from the Paris-TBI study. J Head Trauma Rehabil. 2016;31(5):E59-E67.
18. Griffin JM, Friedemann-Sanchez G, Hall C, Phelan S, van Ryn M. Families of patients with polytrauma: understanding the evidence and charting a new research agenda. J Rehabil Res Dev. 2009;46(6):879-892.
19. Sander AM, Maestas KL, Sherer M, Malac JF, Nakase-Richardson R. Relationship of caregiver and family functioning to participation outcomes after post-acute rehabilitation for traumatic brain injury: a multicenter investigation. Arch Phys Med Rehabil. 2012;93(5):842-848.
20. Powell JM, Fraser R, Brockway JA, Temkin N, Bell KR. A telehealth approach to caregiver self-management following traumatic brain injury: a randomized control trial. J Head Trauma Rehabil. 2015;31(3):180-190.
21. Petranovich CL, Wade SL, Taylor HG, et al. Long-term caregiver mental health outcomes following a predominately online intervention for adolescents with complicated mild to severe traumatic brain injury. J Pediatr Psychol, 2015;40(7):680-688.
22. Norup A, Kristensen KS, Poulsen I, Mortensen EL. Evaluating clinically significant changes in health-related quality of life: a sample of relatives of patients with severe traumatic brain injury. Neuropsychol Rehabil. 2017;27(2):196-215.
23. John Warner National Defense Authorization Act for Fiscal Year 2007, HR 5122, 109th Cong, 2nd Sess (2006).
24. Fortune DG, Rogan CR, Richards HL. A structured multicomponent group program for carers of people with acquired brain injury: effects on perceived criticism, strain, and psychological distress. Br J Health Psychol. 2016;21(1):224-243.
25. Hirano A, Umegaki H, Suzuki Y, Hayashi T, Kuzuya M. Effects of leisure activities at home on perceived care burden and the endocrine system of caregivers of dementia patients: a randomized controlled study. Int Psychogeriatr. 2016;28(2):261-268.
26. Grover S, Pradyumna, Chakrabarti S. Coping among caregivers of patients with schizophrenia. Ind Psychiatry J. 2015;24(1):5-11.
27. Saban KL, Hogan NS, Hogan TP, Pape TL. He looks normal but…challenges of family caregivers of veterans diagnosed with a traumatic brain injury. Rehabil Nurs. 2015;40(5):277-285.
28. Williamson RB; United States Government Accountability Office. VA health care improvements needed to manage higher-than-expected demand for the family caregiver program. http://www.gao.gov/assets/670/667275.pdf. Published December 3, 2014. Accessed October 10, 2017.
29. Ramchand R, Tanielian T, Fisher MP, et al. Hidden heroes America’s military caregivers. http://www.rand.org/content/dam/rand/pubs/research_reports/RR400/RR499/RAND_RR499.pdf. Published 2014. Accessed October 10, 2017.
1. Defense and Veterans Brain Injury Center. DoD worldwide numbers for TBI. http://dvbic.dcoe.mil/dod-worldwide-numbers-tbi. Updated October 5, 2017. Accessed October 10, 2017.
2. Perry DC, Sturm VE, Peterson MJ, et al. Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J Neurosurg. 2016;124(2):511-526.
3. Defense and Veterans Brain Injury Center. Traumatic brain injury: a guide for caregivers of service members and veterans. https://dvbic.dcoe.mil /sites/default/files/Family%20Caregiver%20Guide.All%20Modules_updated.pdf. Accessed October 10, 2017
4. Griffin JM, Friedemann-Sánchez G, Jensen AC, et al. The invisible side of war: families caring for US service members with traumatic brain injuries and polytrauma. J Head Trauma Rehabil. 2012;27(1):3-13.
5. Lou VW, Kwan CW, Chong ML, Chi I. Associations between secondary caregivers’ supportive behavior and psychological distress of primary spousal caregivers of cognitively intact and impaired elders. Gerontologist. 2015;55(4):584-594.
6. Carlozzi NE, Kratz AL, Sander AM, et al. Health-related quality of life in caregivers of individuals with traumatic brain injury: development of a conceptual model. Arch Phys Med Rehabil. 2015;96(1):105-113.
7. Damianakis T, Tough A, Marziali E, Dawson DR. Therapy online: a web-based video support group for family caregivers of survivors with traumatic brain injury. J Head Trauma Rehabil. 2016;31(4):E12-E20.
8. Dyer EA, Kansagara D, McInnes DK, Freeman M, Woods, S. Mobile applications and internet-based approaches for supporting non-professional caregivers: a systematic review. https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0050675. Accessed October 10, 2017.
9. Moriarty H, Winter L, Robinson K, et al. A randomized controlled trial to evaluate the veterans’ in-home program for military veterans with traumatic brain injury and their families: report on impact for family members. PMR. 2016;8(6):495-509.
10. Anderson MI, Simpson GK, Daher M, Matheson L. Chapter 7 the relationship between coping and psychological adjustment in family caregivers of individuals with traumatic brain injury: a systematic review. Annu Rev Nurs Res. 2015;33:219-247.
11. Van Houtven CH, Friedemann-Sanchez G, Clothier B, et al. Is policy well-targeted to remedy financial strain among caregivers of severely injured U.S. service members? Inquiry. 2012-2013;49(4):339-351.
12. Kratz AL, Sander AM, Brickell TA, Lange RT, Carlozzi NE. Traumatic brain injury caregivers: a qualitative analysis of spouse and parent perspectives on quality of life. Neuropsychol Rehabil. 2017;27(1):16-37.13. Verhasghe S, Defloor T, Grypdonck M. Stress and coping among families of patients with traumatic brain injury: a review of the literature. J Clin Nurs. 2005;14(8):1004-1012.
14. Ennis N, Rosenbloom BN, Canzian S, Topolovec-Vranic J. Depression and anxiety in parent versus spouse caregivers of adult patients with traumatic brain injury: a systematic review. Neuropsychol Rehabil. 2013;23(1):1-18.
15. Perlesz A, Kinsella G, Crowe S. Psychological distress and family satisfaction following traumatic brain injury: injured individuals and their primary, secondary, and tertiary carers. J Head Trauma Rehabil. 2000;15(3):909-929.
16. Manskow US, Sigurdardottir S, Røe C, et al. Factors affecting caregiving burden 1 year after severe traumatic brain injury: a prospective nationwide multicenter study. J Head Trauma Rehabil. 2015;30(6):411-423.
17. Bayen E, Jourdan C, Ghout I, et al. Objective and subjective burden of informal caregivers 4 years after a severe traumatic brain injury: results from the Paris-TBI study. J Head Trauma Rehabil. 2016;31(5):E59-E67.
18. Griffin JM, Friedemann-Sanchez G, Hall C, Phelan S, van Ryn M. Families of patients with polytrauma: understanding the evidence and charting a new research agenda. J Rehabil Res Dev. 2009;46(6):879-892.
19. Sander AM, Maestas KL, Sherer M, Malac JF, Nakase-Richardson R. Relationship of caregiver and family functioning to participation outcomes after post-acute rehabilitation for traumatic brain injury: a multicenter investigation. Arch Phys Med Rehabil. 2012;93(5):842-848.
20. Powell JM, Fraser R, Brockway JA, Temkin N, Bell KR. A telehealth approach to caregiver self-management following traumatic brain injury: a randomized control trial. J Head Trauma Rehabil. 2015;31(3):180-190.
21. Petranovich CL, Wade SL, Taylor HG, et al. Long-term caregiver mental health outcomes following a predominately online intervention for adolescents with complicated mild to severe traumatic brain injury. J Pediatr Psychol, 2015;40(7):680-688.
22. Norup A, Kristensen KS, Poulsen I, Mortensen EL. Evaluating clinically significant changes in health-related quality of life: a sample of relatives of patients with severe traumatic brain injury. Neuropsychol Rehabil. 2017;27(2):196-215.
23. John Warner National Defense Authorization Act for Fiscal Year 2007, HR 5122, 109th Cong, 2nd Sess (2006).
24. Fortune DG, Rogan CR, Richards HL. A structured multicomponent group program for carers of people with acquired brain injury: effects on perceived criticism, strain, and psychological distress. Br J Health Psychol. 2016;21(1):224-243.
25. Hirano A, Umegaki H, Suzuki Y, Hayashi T, Kuzuya M. Effects of leisure activities at home on perceived care burden and the endocrine system of caregivers of dementia patients: a randomized controlled study. Int Psychogeriatr. 2016;28(2):261-268.
26. Grover S, Pradyumna, Chakrabarti S. Coping among caregivers of patients with schizophrenia. Ind Psychiatry J. 2015;24(1):5-11.
27. Saban KL, Hogan NS, Hogan TP, Pape TL. He looks normal but…challenges of family caregivers of veterans diagnosed with a traumatic brain injury. Rehabil Nurs. 2015;40(5):277-285.
28. Williamson RB; United States Government Accountability Office. VA health care improvements needed to manage higher-than-expected demand for the family caregiver program. http://www.gao.gov/assets/670/667275.pdf. Published December 3, 2014. Accessed October 10, 2017.
29. Ramchand R, Tanielian T, Fisher MP, et al. Hidden heroes America’s military caregivers. http://www.rand.org/content/dam/rand/pubs/research_reports/RR400/RR499/RAND_RR499.pdf. Published 2014. Accessed October 10, 2017.
Total Knee Arthroplasty Performed With Long-Acting Liposomal Bupivacaine Versus Femoral Nerve Catheter
Take-Home Points
- At our institution, LALB has shortened our hospital stay.
- There is a trend towards decreased opioid consumption with LALB.
- With the opioid epidemic we face today, LALB can be one of many options in our toolbox towards a solution.
- As stated in prior publications, the effectiveness of LALB is definitely technique dependent.
- Additional clinical studies are warranted to better determine the efficacy and cost-effectiveness of LALB.
Almost 1 million total knee arthroplasties (TKAs) are performed in the United States each year, and the number continues to grow.1.2 For patients about to undergo TKA, a significant concern is postoperative pain.3 Fear of postoperative pain is often cited as a reason for delaying surgery.3 Recent literature suggests that patients with poor pain management during the first 48 hours after surgery have a 50% chance of gaining satisfactory long-term pain relief.4 In addition, inadequate postoperative pain management can interfere with participation in and progression of physical rehabilitation, prolong hospital stay, and increase patient dissatisfaction.5 Poorly controlled pain results in decreased range of motion (ROM), strength, stability, and ambulation thereby prolongs hospital stays, and increases costs and overall dissatisfaction with the procedure.
Post-TKA pain management has received much attention in recent years. A multimodal pain management protocol is now a key component of clinical pathways in TKA. Appropriate postoperative pain control lowers postoperative complications and accelerates recovery.6 Pain-caused loss of function makes surgical patients more susceptible to edema, deep vein thrombosis, and pulmonary embolism.4 Various oral and intravenous medications are used to lessen the pain response during the perioperative period. In addition, regional or neuraxial anesthesia is often added to blunt the immediate surgical pain response.7,8 At our institution, TKA traditionally has been performed with femoral nerve catheters (FNCs) for postoperative pain control. Although effective, this method often results in decreased quadriceps musculature function, which delays rehabilitation and increases the fall risk. Recently, there has been a shift toward using local anesthetic infusions about the knee to provide adequate pain relief and restore motor function, which is often sacrificed with use of regional nerve blocks and continuous catheter infusions.9
Many institutions have started using a new long-acting local anesthetic in their multimodal pain management pathways: Exparel (Pacira Pharmaceuticals), a liposomal membrane-bound bupivacaine with sustained release of approximately 72 hours. Several studies have verified the safety of this medication.10 A systemic review of prospective studies revealed that, compared with bupivacaine, long-acting liposomal bupivacaine (LALB) in therapeutic doses had a higher safety margin and a favorable safety profile.10 However, no study has compared the effectiveness of LALB and FNC in a matched TKA cohort with each patient serving as his or her own control.
We recently reviewed our multimodal pain management protocol for any areas in need of improvement and decided to compare the effects of the indwelling FNC protocol that was in use with the effects of injecting the local anesthetic LALB. We conducted a study to compare the 2 methods with respect to pain control, ROM, ability to ambulate, and hospital length of stay (LOS). We hypothesized that the longer acting local anesthetic would provide comparable post-TKA pain control and post-TKA opioid use but would accelerate post-TKA rehabilitation.
Materials and Methods
This retrospective, longitudinal, repeated- measures study was approved by the Greenville Hospital System Institutional Review Board and conducted at the Steadman Hawkins Clinic of the Carolinas, Greenville Health System.
Interventions
Twenty-three patients underwent separately staged bilateral TKAs between 2010 and 2013. For each TKA, a Genesis II implant (Smith & Nephew) was used, and the surgery was performed with the patient under spinal anesthesia. In each case, FNC was used for pain control after the first TKA, and periarticular injection (PAI) of LALB for pain control after the second TKA.
In the first TKAs, FNC-administered ropivacaine 0.2% (2 mg/mL) was maintained at a standard basal rate of 8 mL/h for 48 hours. In the second TKAs, LALB was administered along with bupivacaine/epinephrine. Twenty milliliters of LALB from a single-use vial was diluted in 40 mL of normal (0.9%) saline to obtain a 60-mL solution, and a 25-gauge needle was used to inject this solution into the periarticular soft tissues; another needle was used for PAI of 30 mL of bupivacaine 0.25% with epinephrine.
Continuous passive motion devices were not used. Most patients began therapy on day of surgery. Knee immobilizers were not used in the FNC group.
The same standardized multimodal pain management protocol was used for all TKAs. Non- narcotic medications, including acetaminophen, ketorolac, and celecoxib, were given on a scheduled basis. Tramadol and opioid medications were administered as needed for pain. The attending physician based patient discharge timing on pain control, ability to safely ambulate, and absence of complications.
Outcome Measures
Outcome measures were LOS; extension and flexion at discharge and 3-week follow-up; total ROM (extension plus flexion) at discharge and 3-week follow-up; per-day and total hospital stay morphine -equivalent doses (MEDs); and per-attempt walking distance during gait training.
ROM was measured with a standard goniometer. Flexion was tested with the patient supine and the hip and knee in neutral rotation. The goniometer axis was along the lateral epicondyle of the femur with the proximal arm of the goniometer parallel to the long axis of the femur and pointing at the greater trochanter and with the distal arm parallel to the long axis of the fibula and pointing at the lateral malleolus. The patient was instructed to flex the hip and knee by moving the heel toward the buttock. Expected normal ROM is 135°. The same landmarks were used for extension. The patient was instructed to push the back of the knee toward the plinth/bed, for maximal active extension. The same ROM assessment strategy was used during the hospitalization and at the 3-week follow-up.
Several opioid medications (eg, hydrocodone, oxycodone, tramadol, hydromorphone, morphine) with different dosages were used during hospitalization. Opioid doses were converted to MEDs to permit FNC–LALB comparisons. For each patient, total MEDs were divided by LOS to determine MEDs per day.
Mean per-attempt walking distance was calculated by dividing the total distance walked during hospitalization—the sum of the number of feet walked during each and every attempt, as measured by the treating physical therapist—by the total number of walking attempts.
Data Analysis
A paired-samples t test was used to calculate differences between all outcome measures: LOS; extension and flexion at discharge and 3-month follow-up; per-day and total MEDs; and mean per-attempt walking distance. P < .05 was considered significant. We elected not to adjust our α for a potential familywise error.
Results
Of the 23 patients, 14 were female and 9 were male, and 19 were white and 4 were black. Mean (SD) age was 64.4 (6.4) years for the FNC group and 66.0 (6.0) years for the LALB group. The age difference was not statistically significant.
Discussion
Poor pain control during the post-TKA period may have a significant impact on recovery rate, standard of living, psychological health, and postoperative complications.10 Inadequate postoperative pain control increases postoperative morbidity, hinders physiotherapy, increases anxiety, disrupts sleep patterns, and decreases patient satisfaction.9 There has been increased interest in PAIs. Local anesthetics are additional sources of pain control at surgical sites. However, the half-life of most local anesthetics is short. Soft-tissue infiltration of LALB into a surgical site extends the duration of active analgesia. Our study found that, compared with patients who received FNC, patients who received LALB had comparable pain control, improved knee ROM, and shorter hospital stays. In addition, the LALB group had no reports of quadriceps weakness or falls, both of which are associated with femoral nerve blocks. The FNC group had no reported falls, either. PAIs have the benefit of avoiding the invasiveness of femoral nerve blocks and possible neuritis.
Many complications are associated with or indirectly related to delayed rehabilitation and immobility during the acute post-TKA period. From prolonged hospitalization to need for manipulation, the consequences of inadequate pain control and decreased function can be numerous and costly for patients and the healthcare system. In the present study, LALB use led to a statistically significant overall decrease in mean LOS (LALB group, 2.3 days; FNC, 2.8 days). With LALB, there was a higher likelihood of discharge the day after surgery; 20% of patients in the LALB group and no patients in the FNC group went home that day.
The implication is that inadequate pain control led to decreased motion and decreased progression during postoperative rehabilitation. Local infiltration resulted in increased total ROM (extension plus flexion) at 3-week follow-up (LALB, 116.3°; FNC, 107.2°). In addition, there was an increase in walking distance per day of hospital stay (LALB, 135.9 feet; FNC, 84.2 feet). Furthermore, patients indicated LALB when asked which anesthetic they preferred. To our knowledge, this is the first study to compare LALB and FNC data in a matched TKA cohort with each patient serving as his or her own control.
Our study had several limitations. First was the retrospective design. Second was the small sample size, which made definitive conclusions difficult. However, the statistically significant differences we noted validated our conclusions. A statistically significant difference favoring LALB over FNC was found for total MEDs during hospitalization, but there was no significant difference in per-day MEDs. A possible reason for this difference is that LALB patients had shorter hospital stays, and therefore received fewer doses overall. Another possible reason is the small sample size; whereas a larger study using our protocol may find a statistically significant difference between LALB and FNC, we found only a trend. In the FNC group, anesthetic infiltration occurred with use of a computerized pump, which was removed on postoperative day 2; most of these patients were discharged home that day or the morning of postoperative day 3. As it is possible that some of these patients could have gone home sooner, our LOS data may have been affected. We do not consider this limitation significant, as one of our discharge criteria was 150 feet of ambulation, and most patients who received FNCs could not ambulate that far until after FNC removal. Furthermore, this study compared LALB only with FNC. It is possible that our improved outcomes could have resulted from the PAIs themselves, irrespective of LALB. In a recent TKA study by Bagsby and colleagues,11 pain was controlled better with the less expensive traditional PAI of ropivacaine, epinephrine, and morphine than with the PAI of liposomal bupivacaine. Last, in our study, the experience of undergoing the first TKA may have increased patients’ confidence going into the second TKA and then helped them make faster progress in rehabilitation. Regardless, the promising results of our study and the firsthand use of LALB at our institution led us to modify our intraoperative pain management protocol for surgeons who perform TKA.
As we continue to use LALB, our study numbers will increase, and we may discover other factors that, though now underpowered, will prove to be statistically significant. Additional clinical studies are needed to better determine the efficacy and cost-effectiveness of LALB and other long-acting local anesthetic formulations.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Ruiz D Jr, Koenig L, Dall TM, et al. The direct and indirect costs to society of treatment for end-stage knee osteoarthritis. J Bone Joint Surg Am. 2013;95(16):1473-1480.
3. Trousdale RT, McGrory BJ, Berry DJ, Becker MW, Harmsen WS. Patients’ concerns prior to undergoing total hip and total knee arthroplasty. Mayo Clin Proc. 1999;74(10):978-982.
4. Wells N, Pasero C, McCaffery M. Improving the quality of care through pain assessment and management. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses, Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2008:469-497.
5. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287-333.
6. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084.
7. Stein BE, Srikumaran U, Tan EW, Freehill MT, Wilckens JH. Lower-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(22):e167.
8. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
9. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
10. Portillo J, Kamar N, Melibary S, Quevedo E, Bergese S. Safety of liposome extended-release bupivacaine for postoperative pain control. Front Pharmacol. 2014;5:90.
11. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
Take-Home Points
- At our institution, LALB has shortened our hospital stay.
- There is a trend towards decreased opioid consumption with LALB.
- With the opioid epidemic we face today, LALB can be one of many options in our toolbox towards a solution.
- As stated in prior publications, the effectiveness of LALB is definitely technique dependent.
- Additional clinical studies are warranted to better determine the efficacy and cost-effectiveness of LALB.
Almost 1 million total knee arthroplasties (TKAs) are performed in the United States each year, and the number continues to grow.1.2 For patients about to undergo TKA, a significant concern is postoperative pain.3 Fear of postoperative pain is often cited as a reason for delaying surgery.3 Recent literature suggests that patients with poor pain management during the first 48 hours after surgery have a 50% chance of gaining satisfactory long-term pain relief.4 In addition, inadequate postoperative pain management can interfere with participation in and progression of physical rehabilitation, prolong hospital stay, and increase patient dissatisfaction.5 Poorly controlled pain results in decreased range of motion (ROM), strength, stability, and ambulation thereby prolongs hospital stays, and increases costs and overall dissatisfaction with the procedure.
Post-TKA pain management has received much attention in recent years. A multimodal pain management protocol is now a key component of clinical pathways in TKA. Appropriate postoperative pain control lowers postoperative complications and accelerates recovery.6 Pain-caused loss of function makes surgical patients more susceptible to edema, deep vein thrombosis, and pulmonary embolism.4 Various oral and intravenous medications are used to lessen the pain response during the perioperative period. In addition, regional or neuraxial anesthesia is often added to blunt the immediate surgical pain response.7,8 At our institution, TKA traditionally has been performed with femoral nerve catheters (FNCs) for postoperative pain control. Although effective, this method often results in decreased quadriceps musculature function, which delays rehabilitation and increases the fall risk. Recently, there has been a shift toward using local anesthetic infusions about the knee to provide adequate pain relief and restore motor function, which is often sacrificed with use of regional nerve blocks and continuous catheter infusions.9
Many institutions have started using a new long-acting local anesthetic in their multimodal pain management pathways: Exparel (Pacira Pharmaceuticals), a liposomal membrane-bound bupivacaine with sustained release of approximately 72 hours. Several studies have verified the safety of this medication.10 A systemic review of prospective studies revealed that, compared with bupivacaine, long-acting liposomal bupivacaine (LALB) in therapeutic doses had a higher safety margin and a favorable safety profile.10 However, no study has compared the effectiveness of LALB and FNC in a matched TKA cohort with each patient serving as his or her own control.
We recently reviewed our multimodal pain management protocol for any areas in need of improvement and decided to compare the effects of the indwelling FNC protocol that was in use with the effects of injecting the local anesthetic LALB. We conducted a study to compare the 2 methods with respect to pain control, ROM, ability to ambulate, and hospital length of stay (LOS). We hypothesized that the longer acting local anesthetic would provide comparable post-TKA pain control and post-TKA opioid use but would accelerate post-TKA rehabilitation.
Materials and Methods
This retrospective, longitudinal, repeated- measures study was approved by the Greenville Hospital System Institutional Review Board and conducted at the Steadman Hawkins Clinic of the Carolinas, Greenville Health System.
Interventions
Twenty-three patients underwent separately staged bilateral TKAs between 2010 and 2013. For each TKA, a Genesis II implant (Smith & Nephew) was used, and the surgery was performed with the patient under spinal anesthesia. In each case, FNC was used for pain control after the first TKA, and periarticular injection (PAI) of LALB for pain control after the second TKA.
In the first TKAs, FNC-administered ropivacaine 0.2% (2 mg/mL) was maintained at a standard basal rate of 8 mL/h for 48 hours. In the second TKAs, LALB was administered along with bupivacaine/epinephrine. Twenty milliliters of LALB from a single-use vial was diluted in 40 mL of normal (0.9%) saline to obtain a 60-mL solution, and a 25-gauge needle was used to inject this solution into the periarticular soft tissues; another needle was used for PAI of 30 mL of bupivacaine 0.25% with epinephrine.
Continuous passive motion devices were not used. Most patients began therapy on day of surgery. Knee immobilizers were not used in the FNC group.
The same standardized multimodal pain management protocol was used for all TKAs. Non- narcotic medications, including acetaminophen, ketorolac, and celecoxib, were given on a scheduled basis. Tramadol and opioid medications were administered as needed for pain. The attending physician based patient discharge timing on pain control, ability to safely ambulate, and absence of complications.
Outcome Measures
Outcome measures were LOS; extension and flexion at discharge and 3-week follow-up; total ROM (extension plus flexion) at discharge and 3-week follow-up; per-day and total hospital stay morphine -equivalent doses (MEDs); and per-attempt walking distance during gait training.
ROM was measured with a standard goniometer. Flexion was tested with the patient supine and the hip and knee in neutral rotation. The goniometer axis was along the lateral epicondyle of the femur with the proximal arm of the goniometer parallel to the long axis of the femur and pointing at the greater trochanter and with the distal arm parallel to the long axis of the fibula and pointing at the lateral malleolus. The patient was instructed to flex the hip and knee by moving the heel toward the buttock. Expected normal ROM is 135°. The same landmarks were used for extension. The patient was instructed to push the back of the knee toward the plinth/bed, for maximal active extension. The same ROM assessment strategy was used during the hospitalization and at the 3-week follow-up.
Several opioid medications (eg, hydrocodone, oxycodone, tramadol, hydromorphone, morphine) with different dosages were used during hospitalization. Opioid doses were converted to MEDs to permit FNC–LALB comparisons. For each patient, total MEDs were divided by LOS to determine MEDs per day.
Mean per-attempt walking distance was calculated by dividing the total distance walked during hospitalization—the sum of the number of feet walked during each and every attempt, as measured by the treating physical therapist—by the total number of walking attempts.
Data Analysis
A paired-samples t test was used to calculate differences between all outcome measures: LOS; extension and flexion at discharge and 3-month follow-up; per-day and total MEDs; and mean per-attempt walking distance. P < .05 was considered significant. We elected not to adjust our α for a potential familywise error.
Results
Of the 23 patients, 14 were female and 9 were male, and 19 were white and 4 were black. Mean (SD) age was 64.4 (6.4) years for the FNC group and 66.0 (6.0) years for the LALB group. The age difference was not statistically significant.
Discussion
Poor pain control during the post-TKA period may have a significant impact on recovery rate, standard of living, psychological health, and postoperative complications.10 Inadequate postoperative pain control increases postoperative morbidity, hinders physiotherapy, increases anxiety, disrupts sleep patterns, and decreases patient satisfaction.9 There has been increased interest in PAIs. Local anesthetics are additional sources of pain control at surgical sites. However, the half-life of most local anesthetics is short. Soft-tissue infiltration of LALB into a surgical site extends the duration of active analgesia. Our study found that, compared with patients who received FNC, patients who received LALB had comparable pain control, improved knee ROM, and shorter hospital stays. In addition, the LALB group had no reports of quadriceps weakness or falls, both of which are associated with femoral nerve blocks. The FNC group had no reported falls, either. PAIs have the benefit of avoiding the invasiveness of femoral nerve blocks and possible neuritis.
Many complications are associated with or indirectly related to delayed rehabilitation and immobility during the acute post-TKA period. From prolonged hospitalization to need for manipulation, the consequences of inadequate pain control and decreased function can be numerous and costly for patients and the healthcare system. In the present study, LALB use led to a statistically significant overall decrease in mean LOS (LALB group, 2.3 days; FNC, 2.8 days). With LALB, there was a higher likelihood of discharge the day after surgery; 20% of patients in the LALB group and no patients in the FNC group went home that day.
The implication is that inadequate pain control led to decreased motion and decreased progression during postoperative rehabilitation. Local infiltration resulted in increased total ROM (extension plus flexion) at 3-week follow-up (LALB, 116.3°; FNC, 107.2°). In addition, there was an increase in walking distance per day of hospital stay (LALB, 135.9 feet; FNC, 84.2 feet). Furthermore, patients indicated LALB when asked which anesthetic they preferred. To our knowledge, this is the first study to compare LALB and FNC data in a matched TKA cohort with each patient serving as his or her own control.
Our study had several limitations. First was the retrospective design. Second was the small sample size, which made definitive conclusions difficult. However, the statistically significant differences we noted validated our conclusions. A statistically significant difference favoring LALB over FNC was found for total MEDs during hospitalization, but there was no significant difference in per-day MEDs. A possible reason for this difference is that LALB patients had shorter hospital stays, and therefore received fewer doses overall. Another possible reason is the small sample size; whereas a larger study using our protocol may find a statistically significant difference between LALB and FNC, we found only a trend. In the FNC group, anesthetic infiltration occurred with use of a computerized pump, which was removed on postoperative day 2; most of these patients were discharged home that day or the morning of postoperative day 3. As it is possible that some of these patients could have gone home sooner, our LOS data may have been affected. We do not consider this limitation significant, as one of our discharge criteria was 150 feet of ambulation, and most patients who received FNCs could not ambulate that far until after FNC removal. Furthermore, this study compared LALB only with FNC. It is possible that our improved outcomes could have resulted from the PAIs themselves, irrespective of LALB. In a recent TKA study by Bagsby and colleagues,11 pain was controlled better with the less expensive traditional PAI of ropivacaine, epinephrine, and morphine than with the PAI of liposomal bupivacaine. Last, in our study, the experience of undergoing the first TKA may have increased patients’ confidence going into the second TKA and then helped them make faster progress in rehabilitation. Regardless, the promising results of our study and the firsthand use of LALB at our institution led us to modify our intraoperative pain management protocol for surgeons who perform TKA.
As we continue to use LALB, our study numbers will increase, and we may discover other factors that, though now underpowered, will prove to be statistically significant. Additional clinical studies are needed to better determine the efficacy and cost-effectiveness of LALB and other long-acting local anesthetic formulations.
Take-Home Points
- At our institution, LALB has shortened our hospital stay.
- There is a trend towards decreased opioid consumption with LALB.
- With the opioid epidemic we face today, LALB can be one of many options in our toolbox towards a solution.
- As stated in prior publications, the effectiveness of LALB is definitely technique dependent.
- Additional clinical studies are warranted to better determine the efficacy and cost-effectiveness of LALB.
Almost 1 million total knee arthroplasties (TKAs) are performed in the United States each year, and the number continues to grow.1.2 For patients about to undergo TKA, a significant concern is postoperative pain.3 Fear of postoperative pain is often cited as a reason for delaying surgery.3 Recent literature suggests that patients with poor pain management during the first 48 hours after surgery have a 50% chance of gaining satisfactory long-term pain relief.4 In addition, inadequate postoperative pain management can interfere with participation in and progression of physical rehabilitation, prolong hospital stay, and increase patient dissatisfaction.5 Poorly controlled pain results in decreased range of motion (ROM), strength, stability, and ambulation thereby prolongs hospital stays, and increases costs and overall dissatisfaction with the procedure.
Post-TKA pain management has received much attention in recent years. A multimodal pain management protocol is now a key component of clinical pathways in TKA. Appropriate postoperative pain control lowers postoperative complications and accelerates recovery.6 Pain-caused loss of function makes surgical patients more susceptible to edema, deep vein thrombosis, and pulmonary embolism.4 Various oral and intravenous medications are used to lessen the pain response during the perioperative period. In addition, regional or neuraxial anesthesia is often added to blunt the immediate surgical pain response.7,8 At our institution, TKA traditionally has been performed with femoral nerve catheters (FNCs) for postoperative pain control. Although effective, this method often results in decreased quadriceps musculature function, which delays rehabilitation and increases the fall risk. Recently, there has been a shift toward using local anesthetic infusions about the knee to provide adequate pain relief and restore motor function, which is often sacrificed with use of regional nerve blocks and continuous catheter infusions.9
Many institutions have started using a new long-acting local anesthetic in their multimodal pain management pathways: Exparel (Pacira Pharmaceuticals), a liposomal membrane-bound bupivacaine with sustained release of approximately 72 hours. Several studies have verified the safety of this medication.10 A systemic review of prospective studies revealed that, compared with bupivacaine, long-acting liposomal bupivacaine (LALB) in therapeutic doses had a higher safety margin and a favorable safety profile.10 However, no study has compared the effectiveness of LALB and FNC in a matched TKA cohort with each patient serving as his or her own control.
We recently reviewed our multimodal pain management protocol for any areas in need of improvement and decided to compare the effects of the indwelling FNC protocol that was in use with the effects of injecting the local anesthetic LALB. We conducted a study to compare the 2 methods with respect to pain control, ROM, ability to ambulate, and hospital length of stay (LOS). We hypothesized that the longer acting local anesthetic would provide comparable post-TKA pain control and post-TKA opioid use but would accelerate post-TKA rehabilitation.
Materials and Methods
This retrospective, longitudinal, repeated- measures study was approved by the Greenville Hospital System Institutional Review Board and conducted at the Steadman Hawkins Clinic of the Carolinas, Greenville Health System.
Interventions
Twenty-three patients underwent separately staged bilateral TKAs between 2010 and 2013. For each TKA, a Genesis II implant (Smith & Nephew) was used, and the surgery was performed with the patient under spinal anesthesia. In each case, FNC was used for pain control after the first TKA, and periarticular injection (PAI) of LALB for pain control after the second TKA.
In the first TKAs, FNC-administered ropivacaine 0.2% (2 mg/mL) was maintained at a standard basal rate of 8 mL/h for 48 hours. In the second TKAs, LALB was administered along with bupivacaine/epinephrine. Twenty milliliters of LALB from a single-use vial was diluted in 40 mL of normal (0.9%) saline to obtain a 60-mL solution, and a 25-gauge needle was used to inject this solution into the periarticular soft tissues; another needle was used for PAI of 30 mL of bupivacaine 0.25% with epinephrine.
Continuous passive motion devices were not used. Most patients began therapy on day of surgery. Knee immobilizers were not used in the FNC group.
The same standardized multimodal pain management protocol was used for all TKAs. Non- narcotic medications, including acetaminophen, ketorolac, and celecoxib, were given on a scheduled basis. Tramadol and opioid medications were administered as needed for pain. The attending physician based patient discharge timing on pain control, ability to safely ambulate, and absence of complications.
Outcome Measures
Outcome measures were LOS; extension and flexion at discharge and 3-week follow-up; total ROM (extension plus flexion) at discharge and 3-week follow-up; per-day and total hospital stay morphine -equivalent doses (MEDs); and per-attempt walking distance during gait training.
ROM was measured with a standard goniometer. Flexion was tested with the patient supine and the hip and knee in neutral rotation. The goniometer axis was along the lateral epicondyle of the femur with the proximal arm of the goniometer parallel to the long axis of the femur and pointing at the greater trochanter and with the distal arm parallel to the long axis of the fibula and pointing at the lateral malleolus. The patient was instructed to flex the hip and knee by moving the heel toward the buttock. Expected normal ROM is 135°. The same landmarks were used for extension. The patient was instructed to push the back of the knee toward the plinth/bed, for maximal active extension. The same ROM assessment strategy was used during the hospitalization and at the 3-week follow-up.
Several opioid medications (eg, hydrocodone, oxycodone, tramadol, hydromorphone, morphine) with different dosages were used during hospitalization. Opioid doses were converted to MEDs to permit FNC–LALB comparisons. For each patient, total MEDs were divided by LOS to determine MEDs per day.
Mean per-attempt walking distance was calculated by dividing the total distance walked during hospitalization—the sum of the number of feet walked during each and every attempt, as measured by the treating physical therapist—by the total number of walking attempts.
Data Analysis
A paired-samples t test was used to calculate differences between all outcome measures: LOS; extension and flexion at discharge and 3-month follow-up; per-day and total MEDs; and mean per-attempt walking distance. P < .05 was considered significant. We elected not to adjust our α for a potential familywise error.
Results
Of the 23 patients, 14 were female and 9 were male, and 19 were white and 4 were black. Mean (SD) age was 64.4 (6.4) years for the FNC group and 66.0 (6.0) years for the LALB group. The age difference was not statistically significant.
Discussion
Poor pain control during the post-TKA period may have a significant impact on recovery rate, standard of living, psychological health, and postoperative complications.10 Inadequate postoperative pain control increases postoperative morbidity, hinders physiotherapy, increases anxiety, disrupts sleep patterns, and decreases patient satisfaction.9 There has been increased interest in PAIs. Local anesthetics are additional sources of pain control at surgical sites. However, the half-life of most local anesthetics is short. Soft-tissue infiltration of LALB into a surgical site extends the duration of active analgesia. Our study found that, compared with patients who received FNC, patients who received LALB had comparable pain control, improved knee ROM, and shorter hospital stays. In addition, the LALB group had no reports of quadriceps weakness or falls, both of which are associated with femoral nerve blocks. The FNC group had no reported falls, either. PAIs have the benefit of avoiding the invasiveness of femoral nerve blocks and possible neuritis.
Many complications are associated with or indirectly related to delayed rehabilitation and immobility during the acute post-TKA period. From prolonged hospitalization to need for manipulation, the consequences of inadequate pain control and decreased function can be numerous and costly for patients and the healthcare system. In the present study, LALB use led to a statistically significant overall decrease in mean LOS (LALB group, 2.3 days; FNC, 2.8 days). With LALB, there was a higher likelihood of discharge the day after surgery; 20% of patients in the LALB group and no patients in the FNC group went home that day.
The implication is that inadequate pain control led to decreased motion and decreased progression during postoperative rehabilitation. Local infiltration resulted in increased total ROM (extension plus flexion) at 3-week follow-up (LALB, 116.3°; FNC, 107.2°). In addition, there was an increase in walking distance per day of hospital stay (LALB, 135.9 feet; FNC, 84.2 feet). Furthermore, patients indicated LALB when asked which anesthetic they preferred. To our knowledge, this is the first study to compare LALB and FNC data in a matched TKA cohort with each patient serving as his or her own control.
Our study had several limitations. First was the retrospective design. Second was the small sample size, which made definitive conclusions difficult. However, the statistically significant differences we noted validated our conclusions. A statistically significant difference favoring LALB over FNC was found for total MEDs during hospitalization, but there was no significant difference in per-day MEDs. A possible reason for this difference is that LALB patients had shorter hospital stays, and therefore received fewer doses overall. Another possible reason is the small sample size; whereas a larger study using our protocol may find a statistically significant difference between LALB and FNC, we found only a trend. In the FNC group, anesthetic infiltration occurred with use of a computerized pump, which was removed on postoperative day 2; most of these patients were discharged home that day or the morning of postoperative day 3. As it is possible that some of these patients could have gone home sooner, our LOS data may have been affected. We do not consider this limitation significant, as one of our discharge criteria was 150 feet of ambulation, and most patients who received FNCs could not ambulate that far until after FNC removal. Furthermore, this study compared LALB only with FNC. It is possible that our improved outcomes could have resulted from the PAIs themselves, irrespective of LALB. In a recent TKA study by Bagsby and colleagues,11 pain was controlled better with the less expensive traditional PAI of ropivacaine, epinephrine, and morphine than with the PAI of liposomal bupivacaine. Last, in our study, the experience of undergoing the first TKA may have increased patients’ confidence going into the second TKA and then helped them make faster progress in rehabilitation. Regardless, the promising results of our study and the firsthand use of LALB at our institution led us to modify our intraoperative pain management protocol for surgeons who perform TKA.
As we continue to use LALB, our study numbers will increase, and we may discover other factors that, though now underpowered, will prove to be statistically significant. Additional clinical studies are needed to better determine the efficacy and cost-effectiveness of LALB and other long-acting local anesthetic formulations.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Ruiz D Jr, Koenig L, Dall TM, et al. The direct and indirect costs to society of treatment for end-stage knee osteoarthritis. J Bone Joint Surg Am. 2013;95(16):1473-1480.
3. Trousdale RT, McGrory BJ, Berry DJ, Becker MW, Harmsen WS. Patients’ concerns prior to undergoing total hip and total knee arthroplasty. Mayo Clin Proc. 1999;74(10):978-982.
4. Wells N, Pasero C, McCaffery M. Improving the quality of care through pain assessment and management. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses, Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2008:469-497.
5. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287-333.
6. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084.
7. Stein BE, Srikumaran U, Tan EW, Freehill MT, Wilckens JH. Lower-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(22):e167.
8. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
9. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
10. Portillo J, Kamar N, Melibary S, Quevedo E, Bergese S. Safety of liposome extended-release bupivacaine for postoperative pain control. Front Pharmacol. 2014;5:90.
11. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
1. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
2. Ruiz D Jr, Koenig L, Dall TM, et al. The direct and indirect costs to society of treatment for end-stage knee osteoarthritis. J Bone Joint Surg Am. 2013;95(16):1473-1480.
3. Trousdale RT, McGrory BJ, Berry DJ, Becker MW, Harmsen WS. Patients’ concerns prior to undergoing total hip and total knee arthroplasty. Mayo Clin Proc. 1999;74(10):978-982.
4. Wells N, Pasero C, McCaffery M. Improving the quality of care through pain assessment and management. In: Hughes RG, ed. Patient Safety and Quality: An Evidence-Based Handbook for Nurses, Vol 1. Rockville, MD: Agency for Healthcare Research and Quality; 2008:469-497.
5. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287-333.
6. Parvizi J, Miller AG, Gandhi K. Multimodal pain management after total joint arthroplasty. J Bone Joint Surg Am. 2011;93(11):1075-1084.
7. Stein BE, Srikumaran U, Tan EW, Freehill MT, Wilckens JH. Lower-extremity peripheral nerve blocks in the perioperative pain management of orthopaedic patients: AAOS exhibit selection. J Bone Joint Surg Am. 2012;94(22):e167.
8. Pugely AJ, Martin CT, Gao Y, Mendoza-Lattes S, Callaghan JJ. Differences in short-term complications between spinal and general anesthesia for primary total knee arthroplasty. J Bone Joint Surg Am. 2013;95(3):193-199.
9. Dalury DF, Lieberman JR, MacDonald SJ. Current and innovative pain management techniques in total knee arthroplasty. J Bone Joint Surg Am. 2011;93(20):1938-1943.
10. Portillo J, Kamar N, Melibary S, Quevedo E, Bergese S. Safety of liposome extended-release bupivacaine for postoperative pain control. Front Pharmacol. 2014;5:90.
11. Bagsby DT, Ireland PH, Meneghini RM. Liposomal bupivacaine versus traditional periarticular injection for pain control after total knee arthroplasty. J Arthroplasty. 2014;29(8):1687-1690.
A Method for Attributing Patient-Level Metrics to Rotating Providers in an Inpatient Setting
Hospitalists’ performance is routinely evaluated by third-party payers, employers, and patients. As hospitalist programs mature, there is a need to develop processes to identify, internally measure, and report on individual and group performance. We know from Society of Hospital Medicine (SHM) data that a significant amount of hospitalists’ total compensation is at least partially based on performance. Often this is based at least in part on quality data. In 2006, SHM issued a white paper detailing the key elements of a successful performance monitoring and reporting process.1,2 Recommendations included the identification of meaningful operational and clinical performance metrics, and the ability to monitor and report both group and individual metrics was highlighted as an essential component. There is evidence that comparison of individual provider performance with that of their peers is a necessary element of successful provider dashboards.3 Additionally, regular feedback and a clear, visual presentation of the data are important components of successful provider feedback dashboards.3-6
Much of the literature regarding provider feedback dashboards has been based in the outpatient setting. The majority of these dashboards focus on the management of chronic illnesses (eg, diabetes and hypertension), rates of preventative care services (eg, colonoscopy or mammogram), or avoidance of unnecessary care (eg, antibiotics for sinusitis).4,5 Unlike in the outpatient setting, in which 1 provider often provides a majority of the care for a given episode of care, hospitalized patients are often cared for by multiple providers, challenging the appropriate attribution of patient-level metrics to specific providers. Under the standard approach, an entire hospitalization is attributed to 1 physician, generally the attending of record for the hospitalization, which may be the admitting provider or the discharging provider, depending on the approach used by the hospital. However, assigning responsibility for an entire hospitalization to a provider who may have only seen the patient for a small percentage of a hospitalization may jeopardize the validity of metrics. As provider metrics are increasingly being used for compensation, it is important to ensure that the method for attribution correctly identifies the providers caring for patients. To our knowledge there is no gold standard approach for attributing metrics to providers when patients are cared for by multiple providers, and the standard attending of record–based approach may lack face validity in many cases.
We aimed to develop and operationalize a system to more fairly attribute patient-level data to individual providers across a single hospitalization even when multiple providers cared for the patient. We then compared our methodology to the standard approach, in which the attending of record receives full attribution for each metric, to determine the difference on a provider level between the 2 models.
METHODS
Clinical Setting
The Johns Hopkins Hospital is a 1145-bed, tertiary-care hospital. Over the years of this project, the Johns Hopkins Hospitalist Program was an approximately 20-physician group providing care in a variety of settings, including a dedicated hospitalist floor, where this metrics program was initiated. Hospitalists in this setting work Monday through Friday, with 1 hospitalist and a moonlighter covering on the weekends. Admissions are performed by an admitter, and overnight care is provided by a nocturnist. Initially 17 beds, this unit expanded to 24 beds in June 2012. For the purposes of this article, we included all general medicine patients admitted to this floor between July 1, 2010, and June 30, 2014, who were cared for by hospitalists. During this period, all patients were inpatients; no patients were admitted under observation status. All of these patients were cared for by hospitalists without housestaff or advanced practitioners. Since 2014, the metrics program has been expanded to other hospitalist-run services in the hospital, but for simplicity, we have not presented these more recent data.
Individual Provider Metrics
Metrics were chosen to reflect institutional quality and efficiency priorities. Our choice of metrics was restricted to those that (1) plausibly reflect provider performance, at least in part, and (2) could be accessed in electronic form (without any manual chart review). Whenever possible, we chose metrics with objective data. Additionally, because funding for this effort was provided by the hospital, we sought to ensure that enough of the metrics were related to cost to justify ongoing hospital support of the project. SAS 9.2 (SAS Institute Inc, Cary, NC) was used to calculate metric weights. Specific metrics included American College of Chest Physicians (ACCP)–compliant venous thromboembolism (VTE) prophylaxis,7 observed-to-expected length of stay (LOS) ratio, percentage of discharges per day, discharges before 3
Appropriate prophylaxis for VTE was calculated by using an algorithm embedded within the computerized provider order entry system, which assessed the prescription of ACCP-compliant VTE prophylaxis within 24 hours following admission. This included a risk assessment, and credit was given for no prophylaxis and/or mechanical and/or pharmacologic prophylaxis per the ACCP guidelines.7
Observed-to-expected LOS was defined by using the University HealthSystem Consortium (UHC; now Vizient Inc) expected LOS for the given calendar year. This approach incorporates patient diagnoses, demographics, and other administrative variables to define an expected LOS for each patient.
The percent of patients discharged per day was defined from billing data as the percentage of a provider’s evaluation and management charges that were the final charge of a patient’s stay (regardless of whether a discharge day service was coded).
Discharge prior to 3
Depth of coding was defined as the number of coded diagnoses submitted to the Maryland Health Services Cost Review Commission for determining payment and was viewed as an indicator of the thoroughness of provider documentation.
Patient satisfaction was defined at the patient level (for those patients who turned in patient satisfaction surveys) as the pooled value of the 5 provider questions on the hospital’s patient satisfaction survey administered by Press Ganey: “time the physician spent with you,” “did the physician show concern for your questions/worries,” “did the physician keep you informed,” “friendliness/courtesy of the physician,” and “skill of the physician.”8
Readmission rates were defined as same-hospital readmissions divided by the total number of patients discharged by a given provider, with exclusions based on the Centers for Medicare and Medicaid Services hospital-wide, all-cause readmission measure.1 The expected same-hospital readmission rate was defined for each patient as the observed readmission rate in the entire UHC (Vizient) data set for all patients with the same All Patient Refined Diagnosis Related Group and severity of illness, as we have described previously.9
Communication with the primary care provider was the only self-reported metric used. It was based on a mandatory prompt on the discharge worksheet in the electronic medical record (EMR). Successful communication with the outpatient provider was defined as verbal or electronic communication by the hospitalist with the outpatient provider. Partial (50%) credit was given for providers who attempted but were unsuccessful in communicating with the outpatient provider, for patients for whom the provider had access to the Johns Hopkins EMR system, and for planned admissions without new or important information to convey. No credit was given for providers who indicated that communication was not indicated, who indicated that a patient and/or family would update the provider, or who indicated that the discharge summary would be sufficient.9 Because the discharge worksheet could be initiated at any time during the hospitalization, providers could document communication with the outpatient provider at any point during hospitalization.
Discharge summary turnaround was defined as the average number of days elapsed between the day of discharge and the signing of the discharge summary in the EMR.
Assigning Ownership of Patients to Individual Providers
Using billing data, we assigned ownership of patient care based on the type, timing, and number of charges that occurred during each hospitalization (Figure 1). Eligible charges included all history and physical (codes 99221, 99222, and 99223), subsequent care (codes 99231, 99232, and 99233), and discharge charges (codes 99238 and 99239).
By using a unique identifier assigned for each hospitalization, professional fees submitted by providers were used to identify which provider saw the patient on the admission day, discharge day, as well as subsequent care days. Providers’ productivity, bonus supplements, and policy compliance were determined by using billing data, which encouraged the prompt submittal of charges.
The provider who billed the admission history and physical (codes 99221, 99222, and 99223) within 1 calendar date of the patient’s initial admission was defined as the admitting provider. Patients transferred to the hospitalist service from other services were not assigned an admitting hospitalist. The sole metric assigned to the admitting hospitalist was ACCP-compliant VTE prophylaxis.
The provider who billed the final subsequent care or discharge code (codes 99231, 99232, 99233, 99238, and 99239) within 1 calendar date of discharge was defined as the discharging provider. For hospitalizations characterized by a single provider charge (eg, for patients admitted and discharged on the same day), the provider billing this charge was assigned as both the admitting and discharging physician. Patients upgraded to the intensive care unit (ICU) were not counted as a discharge unless the patient was downgraded and discharged from the hospitalist service. The discharging provider was assigned responsibility for the time of discharge, the percent of patients discharged per day, the discharge summary turnaround time, and hospital readmissions.
Metrics that were assigned to multiple providers for a single hospitalization were termed “provider day–weighted” metrics. The formula for calculating the weight for each provider day–weighted metric was as follows: weight for provider A = [number of daily charges billed by provider A] divided by [LOS +1]. The initial hospital day was counted as day 0. LOS plus 1 was used to recognize that a typical hospitalization will have a charge on the day of admission (day 0) and a charge on the day of discharge such that an LOS of 2 days (eg, a patient admitted on Monday and discharged on Wednesday) will have 3 daily charges. Provider day–weighted metrics included patient satisfaction, communication with the outpatient provider, depth of coding, and observed-to-expected LOS.
Our billing software prevented providers from the same group from billing multiple daily charges, thus ensuring that there were no duplicated charges submitted for a given day.
Presenting Results
Providers were only shown data from the day-weighted approach. For ease of visual interpretation, scores for each metric were scaled ordinally from 1 (worst performance) to 9 (best performance; Table 1). Data were displayed in a dashboard format on a password-protected website for each provider to view his or her own data relative to that of the hospitalist peer group. The dashboard was implemented in this format on July 1, 2011. Data were updated quarterly (Figure 2).
Results were displayed in a polyhedral or spider-web graph (Figure 2). Provider and group metrics were scaled according to predefined benchmarks established for each metric and standardized to a scale ranging from 1 to 9. The scale for each metric was set based on examining historical data and group median performance on the metrics to ensure that there was a range of performance (ie, to avoid having most hospitalists scoring a 1 or 9). Scaling thresholds were periodically adjusted as appropriate to maintain good visual discrimination. Higher scores (creating a larger-volume polygon) are desirable even for metrics such as LOS, for which a low value is desirable. Both a spider-web graph and trends over time were available to the provider (Figure 2). These graphs display a comparison of the individual provider scores for each metric to the hospitalist group average for that metric.
Comparison with the Standard (Attending of Record) Method of Attribution
For the purposes of this report, we sought to determine whether there were meaningful differences between our day-weighted approach versus the standard method of attribution, in which the attending of record is assigned responsibility for each metric that would not have been attributed to the discharging attending under both methods. Our goal was to determine where and whether there was a meaningful difference between the 2 methodologies, recognizing that the degree of difference between these 2 methodologies might vary in other institutions and settings. In our hospital, the attending of record is generally the discharging attending. In order to compare the 2 methodologies, we arbitrarily picked 2015 to retrospectively evaluate the differences between these 2 methods of attribution. We did not display or provide data using the standard methodology to providers at any point; this approach was used only for the purposes of this report. Because these metrics are intended to evaluate relative provider performance, we assigned a percentile to each provider for his or her performance on the given metric using our attribution methodology and then, similarly, assigned a percentile to each provider using the standard methodology. This yielded 2 percentile scores for each provider and each metric. We then compared these percentile ranks for providers in 2 ways: (1) we determined how often providers who scored in the top half of the group for a given metric (above the 50th percentile) also scored in the top half of the group for that metric by using the other calculation method, and (2) we calculated the absolute value of the difference in percentiles between the 2 methods to characterize the impact on a provider’s ranking for that metric that might result from switching to the other method. For instance, if a provider scored at the 20th percentile for the group in patient satisfaction with 1 attribution method and scored at the 40th percentile for the group in patient satisfaction using the other method, the absolute change in percentile would be 20 percentile points. But, this provider would still be below the 50th percentile by both methods (concordant bottom half performance). We did not perform this comparison for metrics assigned to the discharging provider (such as discharge summary turnaround time or readmissions) because the attending of record designation is assigned to the discharging provider at our hospital.
RESULTS
The dashboard was successfully operationalized on July 1, 2011, with displays visible to providers as shown in Figure 2. Consistent with the principles of providing effective performance feedback to providers, the display simultaneously showed providers their individual performance as well as the performance of their peers. Providers were able to view their spider-web plot for prior quarters. Not shown are additional views that allowed providers to see quarterly trends in their data versus their peers across several fiscal years. Also available to providers was their ranking relative to their peers for each metric; specific peers were deidentified in the display.
There was notable discordance between provider rankings between the 2 methodologies, as shown in Table 2. Provider performance above or below the median was concordant 56% to 75% of the time (depending on the particular metric), indicating substantial discordance because top-half or bottom-half concordance would be expected to occur by chance 50% of the time. Although the provider percentile differences between the 2 methods tended to be modest for most providers (the median difference between the methods was 13 to 22 percentile points for the various metrics), there were some providers for whom the method of calculation dramatically impacted their rankings. For 5 of the 6 metrics we examined, at least 1 provider had a 50-percentile or greater change in his or her ranking based on the method used. This indicates that at least some providers would have had markedly different scores relative to their peers had we used the alternative methodology (Table 2). In VTE prophylaxis, for example, at least 1 provider had a 94-percentile change in his or her ranking; similarly, a provider had an 88-perentile change in his or her LOS ranking between the 2 methodologies.
DISCUSSION
We found that it is possible to assign metrics across 1 hospital stay to multiple providers by using billing data. We also found a meaningful discrepancy in how well providers scored (relative to their peers) based on the method used for attribution. These results imply that hospitals should consider attributing performance metrics based on ascribed ownership from billing data and not just from attending of record status.
As hospitalist programs and providers in general are increasingly being asked to develop dashboards to monitor individual and group performance, correctly attributing care to providers is likely to become increasingly important. Experts agree that principles of effective provider performance dashboards include ranking individual provider performance relative to peers, clearly displaying data in an easily accessible format, and ensuring that data can be credibly attributed to the individual provider.3,4,6 However, there appears to be no gold standard method for attribution, especially in the inpatient setting. Our results imply that hospitals should consider attributing performance metrics based on ascribed ownership from billing data and not just from attending of record status.
Several limitations of our findings are important to consider. First, our program is a relatively small, academic group with handoffs that typically occur every 1 to 2 weeks and sometimes with additional handoffs on weekends. Different care patterns and settings might impact the utility of our attribution methodology relative to the standard methodology. Additionally, it is important to note that the relative merits of the different methodologies cannot be ascertained from our comparison. We can demonstrate discordance between the attribution methodologies, but we cannot say that 1 method is correct and the other is flawed. Although we believe that our day-weighted approach feels fairer to providers based on group input and feedback, we did not conduct a formal survey to examine providers’ preferences for the standard versus day-weighted approaches. The appropriateness of a particular attribution method needs to be assessed locally and may vary based on the clinical setting. For instance, on a service in which patients are admitted for procedures, it may make more sense to attribute the outcome of the case to the proceduralist even if that provider did not bill for the patient’s care on a daily basis. Finally, the computational requirements of our methodology are not trivial and require linking billing data with administrative patient-level data, which may be challenging to operationalize in some institutions.
These limitations aside, we believe that our attribution methodology has face validity. For example, a provider might be justifiably frustrated if, using the standard methodology, he or she is charged with the LOS of a patient who had been hospitalized for months, particularly if that patient is discharged shortly after the provider assumes care. Our method addresses this type of misattribution. Particularly when individual provider compensation is based on performance on metrics (as is the case at our institution), optimizing provider attribution to particular patients may be important, and face validity may be required for group buy-in.
In summary, we have demonstrated that it is possible to use billing data to assign ownership of patients to multiple providers over 1 hospital stay. This could be applied to other hospitalist programs as well as other healthcare settings in which multiple providers care for patients during 1 healthcare encounter (eg, ICUs).
Disclosure
The authors declare they have no relevant conflicts of interest.
1. Horwitz L, Partovian C, Lin Z, et al. Hospital-Wide (All-Condition) 30‐Day Risk-Standardized Readmission Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/downloads/MMSHospital-WideAll-ConditionReadmissionRate.pdf. Accessed March 6, 2015.
2. Medicine SoH. Measuring Hospitalist Performance: Metrics, Reports, and Dashboards. 2007; https://www.hospitalmedicine.org/Web/Practice_Management/Products_and_Programs/measure_hosp_perf_metrics_reports_dashboards.aspx. Accessed May 12, 2013.
3. Teleki SS, Shaw R, Damberg CL, McGlynn EA. Providing performance feedback to individual physicians: current practice and emerging lessons. Santa Monica, CA: RAND Corporation; 2006. 1-47. https://www.rand.org/content/dam/rand/pubs/working_papers/2006/RAND_WR381.pdf. Accessed August, 2017.
4. Brehaut JC, Colquhoun HL, Eva KW, et al. Practice Feedback Interventions: 15 Suggestions for Optimizing Effectiveness Practice Feedback Interventions. Ann Intern Med. 2016;164(6):435-441. PubMed
5. Dowding D, Randell R, Gardner P, et al. Dashboards for improving patient care: review of the literature. Int J Med Inform. 2015;84(2):87-100. PubMed
6. Landon BE, Normand S-LT, Blumenthal D, Daley J. Physician clinical performance assessment: prospects and barriers. JAMA. 2003;290(9):1183-1189. PubMed
7. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ. Executive summary: Antit hrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Ann Intern Med. 2012;141(2 suppl):7S-47S. PubMed
8. Siddiqui Z, Qayyum R, Bertram A, et al. Does Provider Self-reporting of Etiquette Behaviors Improve Patient Experience? A Randomized Controlled Trial. J Hosp Med. 2017;12(6):402-406. PubMed
9. Oduyebo I, Lehmann CU, Pollack CE, et al. Association of self-reported hospital discharge handoffs with 30-day readmissions. JAMA Intern Med. 2013;173(8):624-629. PubMed
Hospitalists’ performance is routinely evaluated by third-party payers, employers, and patients. As hospitalist programs mature, there is a need to develop processes to identify, internally measure, and report on individual and group performance. We know from Society of Hospital Medicine (SHM) data that a significant amount of hospitalists’ total compensation is at least partially based on performance. Often this is based at least in part on quality data. In 2006, SHM issued a white paper detailing the key elements of a successful performance monitoring and reporting process.1,2 Recommendations included the identification of meaningful operational and clinical performance metrics, and the ability to monitor and report both group and individual metrics was highlighted as an essential component. There is evidence that comparison of individual provider performance with that of their peers is a necessary element of successful provider dashboards.3 Additionally, regular feedback and a clear, visual presentation of the data are important components of successful provider feedback dashboards.3-6
Much of the literature regarding provider feedback dashboards has been based in the outpatient setting. The majority of these dashboards focus on the management of chronic illnesses (eg, diabetes and hypertension), rates of preventative care services (eg, colonoscopy or mammogram), or avoidance of unnecessary care (eg, antibiotics for sinusitis).4,5 Unlike in the outpatient setting, in which 1 provider often provides a majority of the care for a given episode of care, hospitalized patients are often cared for by multiple providers, challenging the appropriate attribution of patient-level metrics to specific providers. Under the standard approach, an entire hospitalization is attributed to 1 physician, generally the attending of record for the hospitalization, which may be the admitting provider or the discharging provider, depending on the approach used by the hospital. However, assigning responsibility for an entire hospitalization to a provider who may have only seen the patient for a small percentage of a hospitalization may jeopardize the validity of metrics. As provider metrics are increasingly being used for compensation, it is important to ensure that the method for attribution correctly identifies the providers caring for patients. To our knowledge there is no gold standard approach for attributing metrics to providers when patients are cared for by multiple providers, and the standard attending of record–based approach may lack face validity in many cases.
We aimed to develop and operationalize a system to more fairly attribute patient-level data to individual providers across a single hospitalization even when multiple providers cared for the patient. We then compared our methodology to the standard approach, in which the attending of record receives full attribution for each metric, to determine the difference on a provider level between the 2 models.
METHODS
Clinical Setting
The Johns Hopkins Hospital is a 1145-bed, tertiary-care hospital. Over the years of this project, the Johns Hopkins Hospitalist Program was an approximately 20-physician group providing care in a variety of settings, including a dedicated hospitalist floor, where this metrics program was initiated. Hospitalists in this setting work Monday through Friday, with 1 hospitalist and a moonlighter covering on the weekends. Admissions are performed by an admitter, and overnight care is provided by a nocturnist. Initially 17 beds, this unit expanded to 24 beds in June 2012. For the purposes of this article, we included all general medicine patients admitted to this floor between July 1, 2010, and June 30, 2014, who were cared for by hospitalists. During this period, all patients were inpatients; no patients were admitted under observation status. All of these patients were cared for by hospitalists without housestaff or advanced practitioners. Since 2014, the metrics program has been expanded to other hospitalist-run services in the hospital, but for simplicity, we have not presented these more recent data.
Individual Provider Metrics
Metrics were chosen to reflect institutional quality and efficiency priorities. Our choice of metrics was restricted to those that (1) plausibly reflect provider performance, at least in part, and (2) could be accessed in electronic form (without any manual chart review). Whenever possible, we chose metrics with objective data. Additionally, because funding for this effort was provided by the hospital, we sought to ensure that enough of the metrics were related to cost to justify ongoing hospital support of the project. SAS 9.2 (SAS Institute Inc, Cary, NC) was used to calculate metric weights. Specific metrics included American College of Chest Physicians (ACCP)–compliant venous thromboembolism (VTE) prophylaxis,7 observed-to-expected length of stay (LOS) ratio, percentage of discharges per day, discharges before 3
Appropriate prophylaxis for VTE was calculated by using an algorithm embedded within the computerized provider order entry system, which assessed the prescription of ACCP-compliant VTE prophylaxis within 24 hours following admission. This included a risk assessment, and credit was given for no prophylaxis and/or mechanical and/or pharmacologic prophylaxis per the ACCP guidelines.7
Observed-to-expected LOS was defined by using the University HealthSystem Consortium (UHC; now Vizient Inc) expected LOS for the given calendar year. This approach incorporates patient diagnoses, demographics, and other administrative variables to define an expected LOS for each patient.
The percent of patients discharged per day was defined from billing data as the percentage of a provider’s evaluation and management charges that were the final charge of a patient’s stay (regardless of whether a discharge day service was coded).
Discharge prior to 3
Depth of coding was defined as the number of coded diagnoses submitted to the Maryland Health Services Cost Review Commission for determining payment and was viewed as an indicator of the thoroughness of provider documentation.
Patient satisfaction was defined at the patient level (for those patients who turned in patient satisfaction surveys) as the pooled value of the 5 provider questions on the hospital’s patient satisfaction survey administered by Press Ganey: “time the physician spent with you,” “did the physician show concern for your questions/worries,” “did the physician keep you informed,” “friendliness/courtesy of the physician,” and “skill of the physician.”8
Readmission rates were defined as same-hospital readmissions divided by the total number of patients discharged by a given provider, with exclusions based on the Centers for Medicare and Medicaid Services hospital-wide, all-cause readmission measure.1 The expected same-hospital readmission rate was defined for each patient as the observed readmission rate in the entire UHC (Vizient) data set for all patients with the same All Patient Refined Diagnosis Related Group and severity of illness, as we have described previously.9
Communication with the primary care provider was the only self-reported metric used. It was based on a mandatory prompt on the discharge worksheet in the electronic medical record (EMR). Successful communication with the outpatient provider was defined as verbal or electronic communication by the hospitalist with the outpatient provider. Partial (50%) credit was given for providers who attempted but were unsuccessful in communicating with the outpatient provider, for patients for whom the provider had access to the Johns Hopkins EMR system, and for planned admissions without new or important information to convey. No credit was given for providers who indicated that communication was not indicated, who indicated that a patient and/or family would update the provider, or who indicated that the discharge summary would be sufficient.9 Because the discharge worksheet could be initiated at any time during the hospitalization, providers could document communication with the outpatient provider at any point during hospitalization.
Discharge summary turnaround was defined as the average number of days elapsed between the day of discharge and the signing of the discharge summary in the EMR.
Assigning Ownership of Patients to Individual Providers
Using billing data, we assigned ownership of patient care based on the type, timing, and number of charges that occurred during each hospitalization (Figure 1). Eligible charges included all history and physical (codes 99221, 99222, and 99223), subsequent care (codes 99231, 99232, and 99233), and discharge charges (codes 99238 and 99239).
By using a unique identifier assigned for each hospitalization, professional fees submitted by providers were used to identify which provider saw the patient on the admission day, discharge day, as well as subsequent care days. Providers’ productivity, bonus supplements, and policy compliance were determined by using billing data, which encouraged the prompt submittal of charges.
The provider who billed the admission history and physical (codes 99221, 99222, and 99223) within 1 calendar date of the patient’s initial admission was defined as the admitting provider. Patients transferred to the hospitalist service from other services were not assigned an admitting hospitalist. The sole metric assigned to the admitting hospitalist was ACCP-compliant VTE prophylaxis.
The provider who billed the final subsequent care or discharge code (codes 99231, 99232, 99233, 99238, and 99239) within 1 calendar date of discharge was defined as the discharging provider. For hospitalizations characterized by a single provider charge (eg, for patients admitted and discharged on the same day), the provider billing this charge was assigned as both the admitting and discharging physician. Patients upgraded to the intensive care unit (ICU) were not counted as a discharge unless the patient was downgraded and discharged from the hospitalist service. The discharging provider was assigned responsibility for the time of discharge, the percent of patients discharged per day, the discharge summary turnaround time, and hospital readmissions.
Metrics that were assigned to multiple providers for a single hospitalization were termed “provider day–weighted” metrics. The formula for calculating the weight for each provider day–weighted metric was as follows: weight for provider A = [number of daily charges billed by provider A] divided by [LOS +1]. The initial hospital day was counted as day 0. LOS plus 1 was used to recognize that a typical hospitalization will have a charge on the day of admission (day 0) and a charge on the day of discharge such that an LOS of 2 days (eg, a patient admitted on Monday and discharged on Wednesday) will have 3 daily charges. Provider day–weighted metrics included patient satisfaction, communication with the outpatient provider, depth of coding, and observed-to-expected LOS.
Our billing software prevented providers from the same group from billing multiple daily charges, thus ensuring that there were no duplicated charges submitted for a given day.
Presenting Results
Providers were only shown data from the day-weighted approach. For ease of visual interpretation, scores for each metric were scaled ordinally from 1 (worst performance) to 9 (best performance; Table 1). Data were displayed in a dashboard format on a password-protected website for each provider to view his or her own data relative to that of the hospitalist peer group. The dashboard was implemented in this format on July 1, 2011. Data were updated quarterly (Figure 2).
Results were displayed in a polyhedral or spider-web graph (Figure 2). Provider and group metrics were scaled according to predefined benchmarks established for each metric and standardized to a scale ranging from 1 to 9. The scale for each metric was set based on examining historical data and group median performance on the metrics to ensure that there was a range of performance (ie, to avoid having most hospitalists scoring a 1 or 9). Scaling thresholds were periodically adjusted as appropriate to maintain good visual discrimination. Higher scores (creating a larger-volume polygon) are desirable even for metrics such as LOS, for which a low value is desirable. Both a spider-web graph and trends over time were available to the provider (Figure 2). These graphs display a comparison of the individual provider scores for each metric to the hospitalist group average for that metric.
Comparison with the Standard (Attending of Record) Method of Attribution
For the purposes of this report, we sought to determine whether there were meaningful differences between our day-weighted approach versus the standard method of attribution, in which the attending of record is assigned responsibility for each metric that would not have been attributed to the discharging attending under both methods. Our goal was to determine where and whether there was a meaningful difference between the 2 methodologies, recognizing that the degree of difference between these 2 methodologies might vary in other institutions and settings. In our hospital, the attending of record is generally the discharging attending. In order to compare the 2 methodologies, we arbitrarily picked 2015 to retrospectively evaluate the differences between these 2 methods of attribution. We did not display or provide data using the standard methodology to providers at any point; this approach was used only for the purposes of this report. Because these metrics are intended to evaluate relative provider performance, we assigned a percentile to each provider for his or her performance on the given metric using our attribution methodology and then, similarly, assigned a percentile to each provider using the standard methodology. This yielded 2 percentile scores for each provider and each metric. We then compared these percentile ranks for providers in 2 ways: (1) we determined how often providers who scored in the top half of the group for a given metric (above the 50th percentile) also scored in the top half of the group for that metric by using the other calculation method, and (2) we calculated the absolute value of the difference in percentiles between the 2 methods to characterize the impact on a provider’s ranking for that metric that might result from switching to the other method. For instance, if a provider scored at the 20th percentile for the group in patient satisfaction with 1 attribution method and scored at the 40th percentile for the group in patient satisfaction using the other method, the absolute change in percentile would be 20 percentile points. But, this provider would still be below the 50th percentile by both methods (concordant bottom half performance). We did not perform this comparison for metrics assigned to the discharging provider (such as discharge summary turnaround time or readmissions) because the attending of record designation is assigned to the discharging provider at our hospital.
RESULTS
The dashboard was successfully operationalized on July 1, 2011, with displays visible to providers as shown in Figure 2. Consistent with the principles of providing effective performance feedback to providers, the display simultaneously showed providers their individual performance as well as the performance of their peers. Providers were able to view their spider-web plot for prior quarters. Not shown are additional views that allowed providers to see quarterly trends in their data versus their peers across several fiscal years. Also available to providers was their ranking relative to their peers for each metric; specific peers were deidentified in the display.
There was notable discordance between provider rankings between the 2 methodologies, as shown in Table 2. Provider performance above or below the median was concordant 56% to 75% of the time (depending on the particular metric), indicating substantial discordance because top-half or bottom-half concordance would be expected to occur by chance 50% of the time. Although the provider percentile differences between the 2 methods tended to be modest for most providers (the median difference between the methods was 13 to 22 percentile points for the various metrics), there were some providers for whom the method of calculation dramatically impacted their rankings. For 5 of the 6 metrics we examined, at least 1 provider had a 50-percentile or greater change in his or her ranking based on the method used. This indicates that at least some providers would have had markedly different scores relative to their peers had we used the alternative methodology (Table 2). In VTE prophylaxis, for example, at least 1 provider had a 94-percentile change in his or her ranking; similarly, a provider had an 88-perentile change in his or her LOS ranking between the 2 methodologies.
DISCUSSION
We found that it is possible to assign metrics across 1 hospital stay to multiple providers by using billing data. We also found a meaningful discrepancy in how well providers scored (relative to their peers) based on the method used for attribution. These results imply that hospitals should consider attributing performance metrics based on ascribed ownership from billing data and not just from attending of record status.
As hospitalist programs and providers in general are increasingly being asked to develop dashboards to monitor individual and group performance, correctly attributing care to providers is likely to become increasingly important. Experts agree that principles of effective provider performance dashboards include ranking individual provider performance relative to peers, clearly displaying data in an easily accessible format, and ensuring that data can be credibly attributed to the individual provider.3,4,6 However, there appears to be no gold standard method for attribution, especially in the inpatient setting. Our results imply that hospitals should consider attributing performance metrics based on ascribed ownership from billing data and not just from attending of record status.
Several limitations of our findings are important to consider. First, our program is a relatively small, academic group with handoffs that typically occur every 1 to 2 weeks and sometimes with additional handoffs on weekends. Different care patterns and settings might impact the utility of our attribution methodology relative to the standard methodology. Additionally, it is important to note that the relative merits of the different methodologies cannot be ascertained from our comparison. We can demonstrate discordance between the attribution methodologies, but we cannot say that 1 method is correct and the other is flawed. Although we believe that our day-weighted approach feels fairer to providers based on group input and feedback, we did not conduct a formal survey to examine providers’ preferences for the standard versus day-weighted approaches. The appropriateness of a particular attribution method needs to be assessed locally and may vary based on the clinical setting. For instance, on a service in which patients are admitted for procedures, it may make more sense to attribute the outcome of the case to the proceduralist even if that provider did not bill for the patient’s care on a daily basis. Finally, the computational requirements of our methodology are not trivial and require linking billing data with administrative patient-level data, which may be challenging to operationalize in some institutions.
These limitations aside, we believe that our attribution methodology has face validity. For example, a provider might be justifiably frustrated if, using the standard methodology, he or she is charged with the LOS of a patient who had been hospitalized for months, particularly if that patient is discharged shortly after the provider assumes care. Our method addresses this type of misattribution. Particularly when individual provider compensation is based on performance on metrics (as is the case at our institution), optimizing provider attribution to particular patients may be important, and face validity may be required for group buy-in.
In summary, we have demonstrated that it is possible to use billing data to assign ownership of patients to multiple providers over 1 hospital stay. This could be applied to other hospitalist programs as well as other healthcare settings in which multiple providers care for patients during 1 healthcare encounter (eg, ICUs).
Disclosure
The authors declare they have no relevant conflicts of interest.
Hospitalists’ performance is routinely evaluated by third-party payers, employers, and patients. As hospitalist programs mature, there is a need to develop processes to identify, internally measure, and report on individual and group performance. We know from Society of Hospital Medicine (SHM) data that a significant amount of hospitalists’ total compensation is at least partially based on performance. Often this is based at least in part on quality data. In 2006, SHM issued a white paper detailing the key elements of a successful performance monitoring and reporting process.1,2 Recommendations included the identification of meaningful operational and clinical performance metrics, and the ability to monitor and report both group and individual metrics was highlighted as an essential component. There is evidence that comparison of individual provider performance with that of their peers is a necessary element of successful provider dashboards.3 Additionally, regular feedback and a clear, visual presentation of the data are important components of successful provider feedback dashboards.3-6
Much of the literature regarding provider feedback dashboards has been based in the outpatient setting. The majority of these dashboards focus on the management of chronic illnesses (eg, diabetes and hypertension), rates of preventative care services (eg, colonoscopy or mammogram), or avoidance of unnecessary care (eg, antibiotics for sinusitis).4,5 Unlike in the outpatient setting, in which 1 provider often provides a majority of the care for a given episode of care, hospitalized patients are often cared for by multiple providers, challenging the appropriate attribution of patient-level metrics to specific providers. Under the standard approach, an entire hospitalization is attributed to 1 physician, generally the attending of record for the hospitalization, which may be the admitting provider or the discharging provider, depending on the approach used by the hospital. However, assigning responsibility for an entire hospitalization to a provider who may have only seen the patient for a small percentage of a hospitalization may jeopardize the validity of metrics. As provider metrics are increasingly being used for compensation, it is important to ensure that the method for attribution correctly identifies the providers caring for patients. To our knowledge there is no gold standard approach for attributing metrics to providers when patients are cared for by multiple providers, and the standard attending of record–based approach may lack face validity in many cases.
We aimed to develop and operationalize a system to more fairly attribute patient-level data to individual providers across a single hospitalization even when multiple providers cared for the patient. We then compared our methodology to the standard approach, in which the attending of record receives full attribution for each metric, to determine the difference on a provider level between the 2 models.
METHODS
Clinical Setting
The Johns Hopkins Hospital is a 1145-bed, tertiary-care hospital. Over the years of this project, the Johns Hopkins Hospitalist Program was an approximately 20-physician group providing care in a variety of settings, including a dedicated hospitalist floor, where this metrics program was initiated. Hospitalists in this setting work Monday through Friday, with 1 hospitalist and a moonlighter covering on the weekends. Admissions are performed by an admitter, and overnight care is provided by a nocturnist. Initially 17 beds, this unit expanded to 24 beds in June 2012. For the purposes of this article, we included all general medicine patients admitted to this floor between July 1, 2010, and June 30, 2014, who were cared for by hospitalists. During this period, all patients were inpatients; no patients were admitted under observation status. All of these patients were cared for by hospitalists without housestaff or advanced practitioners. Since 2014, the metrics program has been expanded to other hospitalist-run services in the hospital, but for simplicity, we have not presented these more recent data.
Individual Provider Metrics
Metrics were chosen to reflect institutional quality and efficiency priorities. Our choice of metrics was restricted to those that (1) plausibly reflect provider performance, at least in part, and (2) could be accessed in electronic form (without any manual chart review). Whenever possible, we chose metrics with objective data. Additionally, because funding for this effort was provided by the hospital, we sought to ensure that enough of the metrics were related to cost to justify ongoing hospital support of the project. SAS 9.2 (SAS Institute Inc, Cary, NC) was used to calculate metric weights. Specific metrics included American College of Chest Physicians (ACCP)–compliant venous thromboembolism (VTE) prophylaxis,7 observed-to-expected length of stay (LOS) ratio, percentage of discharges per day, discharges before 3
Appropriate prophylaxis for VTE was calculated by using an algorithm embedded within the computerized provider order entry system, which assessed the prescription of ACCP-compliant VTE prophylaxis within 24 hours following admission. This included a risk assessment, and credit was given for no prophylaxis and/or mechanical and/or pharmacologic prophylaxis per the ACCP guidelines.7
Observed-to-expected LOS was defined by using the University HealthSystem Consortium (UHC; now Vizient Inc) expected LOS for the given calendar year. This approach incorporates patient diagnoses, demographics, and other administrative variables to define an expected LOS for each patient.
The percent of patients discharged per day was defined from billing data as the percentage of a provider’s evaluation and management charges that were the final charge of a patient’s stay (regardless of whether a discharge day service was coded).
Discharge prior to 3
Depth of coding was defined as the number of coded diagnoses submitted to the Maryland Health Services Cost Review Commission for determining payment and was viewed as an indicator of the thoroughness of provider documentation.
Patient satisfaction was defined at the patient level (for those patients who turned in patient satisfaction surveys) as the pooled value of the 5 provider questions on the hospital’s patient satisfaction survey administered by Press Ganey: “time the physician spent with you,” “did the physician show concern for your questions/worries,” “did the physician keep you informed,” “friendliness/courtesy of the physician,” and “skill of the physician.”8
Readmission rates were defined as same-hospital readmissions divided by the total number of patients discharged by a given provider, with exclusions based on the Centers for Medicare and Medicaid Services hospital-wide, all-cause readmission measure.1 The expected same-hospital readmission rate was defined for each patient as the observed readmission rate in the entire UHC (Vizient) data set for all patients with the same All Patient Refined Diagnosis Related Group and severity of illness, as we have described previously.9
Communication with the primary care provider was the only self-reported metric used. It was based on a mandatory prompt on the discharge worksheet in the electronic medical record (EMR). Successful communication with the outpatient provider was defined as verbal or electronic communication by the hospitalist with the outpatient provider. Partial (50%) credit was given for providers who attempted but were unsuccessful in communicating with the outpatient provider, for patients for whom the provider had access to the Johns Hopkins EMR system, and for planned admissions without new or important information to convey. No credit was given for providers who indicated that communication was not indicated, who indicated that a patient and/or family would update the provider, or who indicated that the discharge summary would be sufficient.9 Because the discharge worksheet could be initiated at any time during the hospitalization, providers could document communication with the outpatient provider at any point during hospitalization.
Discharge summary turnaround was defined as the average number of days elapsed between the day of discharge and the signing of the discharge summary in the EMR.
Assigning Ownership of Patients to Individual Providers
Using billing data, we assigned ownership of patient care based on the type, timing, and number of charges that occurred during each hospitalization (Figure 1). Eligible charges included all history and physical (codes 99221, 99222, and 99223), subsequent care (codes 99231, 99232, and 99233), and discharge charges (codes 99238 and 99239).
By using a unique identifier assigned for each hospitalization, professional fees submitted by providers were used to identify which provider saw the patient on the admission day, discharge day, as well as subsequent care days. Providers’ productivity, bonus supplements, and policy compliance were determined by using billing data, which encouraged the prompt submittal of charges.
The provider who billed the admission history and physical (codes 99221, 99222, and 99223) within 1 calendar date of the patient’s initial admission was defined as the admitting provider. Patients transferred to the hospitalist service from other services were not assigned an admitting hospitalist. The sole metric assigned to the admitting hospitalist was ACCP-compliant VTE prophylaxis.
The provider who billed the final subsequent care or discharge code (codes 99231, 99232, 99233, 99238, and 99239) within 1 calendar date of discharge was defined as the discharging provider. For hospitalizations characterized by a single provider charge (eg, for patients admitted and discharged on the same day), the provider billing this charge was assigned as both the admitting and discharging physician. Patients upgraded to the intensive care unit (ICU) were not counted as a discharge unless the patient was downgraded and discharged from the hospitalist service. The discharging provider was assigned responsibility for the time of discharge, the percent of patients discharged per day, the discharge summary turnaround time, and hospital readmissions.
Metrics that were assigned to multiple providers for a single hospitalization were termed “provider day–weighted” metrics. The formula for calculating the weight for each provider day–weighted metric was as follows: weight for provider A = [number of daily charges billed by provider A] divided by [LOS +1]. The initial hospital day was counted as day 0. LOS plus 1 was used to recognize that a typical hospitalization will have a charge on the day of admission (day 0) and a charge on the day of discharge such that an LOS of 2 days (eg, a patient admitted on Monday and discharged on Wednesday) will have 3 daily charges. Provider day–weighted metrics included patient satisfaction, communication with the outpatient provider, depth of coding, and observed-to-expected LOS.
Our billing software prevented providers from the same group from billing multiple daily charges, thus ensuring that there were no duplicated charges submitted for a given day.
Presenting Results
Providers were only shown data from the day-weighted approach. For ease of visual interpretation, scores for each metric were scaled ordinally from 1 (worst performance) to 9 (best performance; Table 1). Data were displayed in a dashboard format on a password-protected website for each provider to view his or her own data relative to that of the hospitalist peer group. The dashboard was implemented in this format on July 1, 2011. Data were updated quarterly (Figure 2).
Results were displayed in a polyhedral or spider-web graph (Figure 2). Provider and group metrics were scaled according to predefined benchmarks established for each metric and standardized to a scale ranging from 1 to 9. The scale for each metric was set based on examining historical data and group median performance on the metrics to ensure that there was a range of performance (ie, to avoid having most hospitalists scoring a 1 or 9). Scaling thresholds were periodically adjusted as appropriate to maintain good visual discrimination. Higher scores (creating a larger-volume polygon) are desirable even for metrics such as LOS, for which a low value is desirable. Both a spider-web graph and trends over time were available to the provider (Figure 2). These graphs display a comparison of the individual provider scores for each metric to the hospitalist group average for that metric.
Comparison with the Standard (Attending of Record) Method of Attribution
For the purposes of this report, we sought to determine whether there were meaningful differences between our day-weighted approach versus the standard method of attribution, in which the attending of record is assigned responsibility for each metric that would not have been attributed to the discharging attending under both methods. Our goal was to determine where and whether there was a meaningful difference between the 2 methodologies, recognizing that the degree of difference between these 2 methodologies might vary in other institutions and settings. In our hospital, the attending of record is generally the discharging attending. In order to compare the 2 methodologies, we arbitrarily picked 2015 to retrospectively evaluate the differences between these 2 methods of attribution. We did not display or provide data using the standard methodology to providers at any point; this approach was used only for the purposes of this report. Because these metrics are intended to evaluate relative provider performance, we assigned a percentile to each provider for his or her performance on the given metric using our attribution methodology and then, similarly, assigned a percentile to each provider using the standard methodology. This yielded 2 percentile scores for each provider and each metric. We then compared these percentile ranks for providers in 2 ways: (1) we determined how often providers who scored in the top half of the group for a given metric (above the 50th percentile) also scored in the top half of the group for that metric by using the other calculation method, and (2) we calculated the absolute value of the difference in percentiles between the 2 methods to characterize the impact on a provider’s ranking for that metric that might result from switching to the other method. For instance, if a provider scored at the 20th percentile for the group in patient satisfaction with 1 attribution method and scored at the 40th percentile for the group in patient satisfaction using the other method, the absolute change in percentile would be 20 percentile points. But, this provider would still be below the 50th percentile by both methods (concordant bottom half performance). We did not perform this comparison for metrics assigned to the discharging provider (such as discharge summary turnaround time or readmissions) because the attending of record designation is assigned to the discharging provider at our hospital.
RESULTS
The dashboard was successfully operationalized on July 1, 2011, with displays visible to providers as shown in Figure 2. Consistent with the principles of providing effective performance feedback to providers, the display simultaneously showed providers their individual performance as well as the performance of their peers. Providers were able to view their spider-web plot for prior quarters. Not shown are additional views that allowed providers to see quarterly trends in their data versus their peers across several fiscal years. Also available to providers was their ranking relative to their peers for each metric; specific peers were deidentified in the display.
There was notable discordance between provider rankings between the 2 methodologies, as shown in Table 2. Provider performance above or below the median was concordant 56% to 75% of the time (depending on the particular metric), indicating substantial discordance because top-half or bottom-half concordance would be expected to occur by chance 50% of the time. Although the provider percentile differences between the 2 methods tended to be modest for most providers (the median difference between the methods was 13 to 22 percentile points for the various metrics), there were some providers for whom the method of calculation dramatically impacted their rankings. For 5 of the 6 metrics we examined, at least 1 provider had a 50-percentile or greater change in his or her ranking based on the method used. This indicates that at least some providers would have had markedly different scores relative to their peers had we used the alternative methodology (Table 2). In VTE prophylaxis, for example, at least 1 provider had a 94-percentile change in his or her ranking; similarly, a provider had an 88-perentile change in his or her LOS ranking between the 2 methodologies.
DISCUSSION
We found that it is possible to assign metrics across 1 hospital stay to multiple providers by using billing data. We also found a meaningful discrepancy in how well providers scored (relative to their peers) based on the method used for attribution. These results imply that hospitals should consider attributing performance metrics based on ascribed ownership from billing data and not just from attending of record status.
As hospitalist programs and providers in general are increasingly being asked to develop dashboards to monitor individual and group performance, correctly attributing care to providers is likely to become increasingly important. Experts agree that principles of effective provider performance dashboards include ranking individual provider performance relative to peers, clearly displaying data in an easily accessible format, and ensuring that data can be credibly attributed to the individual provider.3,4,6 However, there appears to be no gold standard method for attribution, especially in the inpatient setting. Our results imply that hospitals should consider attributing performance metrics based on ascribed ownership from billing data and not just from attending of record status.
Several limitations of our findings are important to consider. First, our program is a relatively small, academic group with handoffs that typically occur every 1 to 2 weeks and sometimes with additional handoffs on weekends. Different care patterns and settings might impact the utility of our attribution methodology relative to the standard methodology. Additionally, it is important to note that the relative merits of the different methodologies cannot be ascertained from our comparison. We can demonstrate discordance between the attribution methodologies, but we cannot say that 1 method is correct and the other is flawed. Although we believe that our day-weighted approach feels fairer to providers based on group input and feedback, we did not conduct a formal survey to examine providers’ preferences for the standard versus day-weighted approaches. The appropriateness of a particular attribution method needs to be assessed locally and may vary based on the clinical setting. For instance, on a service in which patients are admitted for procedures, it may make more sense to attribute the outcome of the case to the proceduralist even if that provider did not bill for the patient’s care on a daily basis. Finally, the computational requirements of our methodology are not trivial and require linking billing data with administrative patient-level data, which may be challenging to operationalize in some institutions.
These limitations aside, we believe that our attribution methodology has face validity. For example, a provider might be justifiably frustrated if, using the standard methodology, he or she is charged with the LOS of a patient who had been hospitalized for months, particularly if that patient is discharged shortly after the provider assumes care. Our method addresses this type of misattribution. Particularly when individual provider compensation is based on performance on metrics (as is the case at our institution), optimizing provider attribution to particular patients may be important, and face validity may be required for group buy-in.
In summary, we have demonstrated that it is possible to use billing data to assign ownership of patients to multiple providers over 1 hospital stay. This could be applied to other hospitalist programs as well as other healthcare settings in which multiple providers care for patients during 1 healthcare encounter (eg, ICUs).
Disclosure
The authors declare they have no relevant conflicts of interest.
1. Horwitz L, Partovian C, Lin Z, et al. Hospital-Wide (All-Condition) 30‐Day Risk-Standardized Readmission Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/downloads/MMSHospital-WideAll-ConditionReadmissionRate.pdf. Accessed March 6, 2015.
2. Medicine SoH. Measuring Hospitalist Performance: Metrics, Reports, and Dashboards. 2007; https://www.hospitalmedicine.org/Web/Practice_Management/Products_and_Programs/measure_hosp_perf_metrics_reports_dashboards.aspx. Accessed May 12, 2013.
3. Teleki SS, Shaw R, Damberg CL, McGlynn EA. Providing performance feedback to individual physicians: current practice and emerging lessons. Santa Monica, CA: RAND Corporation; 2006. 1-47. https://www.rand.org/content/dam/rand/pubs/working_papers/2006/RAND_WR381.pdf. Accessed August, 2017.
4. Brehaut JC, Colquhoun HL, Eva KW, et al. Practice Feedback Interventions: 15 Suggestions for Optimizing Effectiveness Practice Feedback Interventions. Ann Intern Med. 2016;164(6):435-441. PubMed
5. Dowding D, Randell R, Gardner P, et al. Dashboards for improving patient care: review of the literature. Int J Med Inform. 2015;84(2):87-100. PubMed
6. Landon BE, Normand S-LT, Blumenthal D, Daley J. Physician clinical performance assessment: prospects and barriers. JAMA. 2003;290(9):1183-1189. PubMed
7. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ. Executive summary: Antit hrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Ann Intern Med. 2012;141(2 suppl):7S-47S. PubMed
8. Siddiqui Z, Qayyum R, Bertram A, et al. Does Provider Self-reporting of Etiquette Behaviors Improve Patient Experience? A Randomized Controlled Trial. J Hosp Med. 2017;12(6):402-406. PubMed
9. Oduyebo I, Lehmann CU, Pollack CE, et al. Association of self-reported hospital discharge handoffs with 30-day readmissions. JAMA Intern Med. 2013;173(8):624-629. PubMed
1. Horwitz L, Partovian C, Lin Z, et al. Hospital-Wide (All-Condition) 30‐Day Risk-Standardized Readmission Measure. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/MMS/downloads/MMSHospital-WideAll-ConditionReadmissionRate.pdf. Accessed March 6, 2015.
2. Medicine SoH. Measuring Hospitalist Performance: Metrics, Reports, and Dashboards. 2007; https://www.hospitalmedicine.org/Web/Practice_Management/Products_and_Programs/measure_hosp_perf_metrics_reports_dashboards.aspx. Accessed May 12, 2013.
3. Teleki SS, Shaw R, Damberg CL, McGlynn EA. Providing performance feedback to individual physicians: current practice and emerging lessons. Santa Monica, CA: RAND Corporation; 2006. 1-47. https://www.rand.org/content/dam/rand/pubs/working_papers/2006/RAND_WR381.pdf. Accessed August, 2017.
4. Brehaut JC, Colquhoun HL, Eva KW, et al. Practice Feedback Interventions: 15 Suggestions for Optimizing Effectiveness Practice Feedback Interventions. Ann Intern Med. 2016;164(6):435-441. PubMed
5. Dowding D, Randell R, Gardner P, et al. Dashboards for improving patient care: review of the literature. Int J Med Inform. 2015;84(2):87-100. PubMed
6. Landon BE, Normand S-LT, Blumenthal D, Daley J. Physician clinical performance assessment: prospects and barriers. JAMA. 2003;290(9):1183-1189. PubMed
7. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ. Executive summary: Antit hrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Ann Intern Med. 2012;141(2 suppl):7S-47S. PubMed
8. Siddiqui Z, Qayyum R, Bertram A, et al. Does Provider Self-reporting of Etiquette Behaviors Improve Patient Experience? A Randomized Controlled Trial. J Hosp Med. 2017;12(6):402-406. PubMed
9. Oduyebo I, Lehmann CU, Pollack CE, et al. Association of self-reported hospital discharge handoffs with 30-day readmissions. JAMA Intern Med. 2013;173(8):624-629. PubMed
© 2017 Society of Hospital Medicine
Characterization and Surgical Management of Metastatic Disease of the Tibia
Take-Home Points
- Metastatic disease of the tibia is a rare but significant event in a subset of patients.
- Cancer histologies with historically “acral” spread may not apply to tibial disease.
- Patients with leg pain and any cancer diagnosis should be worked up for tibial metastases.
- Tibial disease is probably a late manifestation, and early detection may indicate late diagnosis of malignancy.
- The ultimate surgical plan for these patients should be a patient-centered multidisciplinary decision making process.
Metastatic dissemination to bones is common in advanced cancer stages and affects the axial and appendicular skeleton.1-4 The appendicular skeleton bones most often involved are the proximal femur and the proximal humerus.5,6 The tibia is involved third most often but is comparatively rarely affected.4-6 Metastatic involvement distal to the knee or elbow is more typical of advanced disease.1,3 Distal appendicular lesions are called acral metastases, but the term is inconsistently used and may refer to lesions either distal to the knee and elbow or distal to the ankle and wrist. Regardless of terminology, tibia lesions are uncommon and not well described.1,4,7,8
The tibia is the primary weight-bearing leg bone. Metastatic tibia lesions may cause pain and instability and impair mobility. Although distal skeletal dissemination often presents late in advanced disease in patients with relatively poor prognoses, in some cases early surgical intervention is indicated for pain relief, increased mobility, and improved quality of life.4,8-10
Materials and Methods
Our Institutional Review Board approved this single-institution retrospective study. We used proprietary research software (Clinical Looking Glass) to identify eligible patients treated between 2000 and 2013. The software was used to search all radiology and pathology reports for the term tibia or any variation (eg, tibial) and metastasis or any variation (eg, metastatic). The software was then used to search by Current Procedural Terminology code for any patients treated with intramedullary nail (IMN) or another tibial fixation method. This list was cross-referenced with the list of patients originally identified to help ensure that all eligible patients were identified.
Inclusion criteria were known malignancy and imaging or biopsy evidence of a metastatic tibia lesion. Treatment strategies for patients with metastatic disease and patients with multiple myeloma are sometimes considered together because of similar goals and methodologies. We specifically excluded patients with multiple myeloma in order to more accurately characterize the natural history of metastatic disease and the timing of metastatic development and to report on a more homogeneous population. Patients were excluded if their electronic medical records were inadequate in establishing a diagnosis.
Demographic and pathology data were collected directly from the institutional electronic medical records system. Dr. Geller and Dr. Greenbaum used Centricity software (General Electric Healthcare) to review all imaging on medical diagnostic display monitors. If their interpretation differed from that in the radiology report, or if clarification was needed, the study was sent to Dr. Thornhill, the institution’s director of musculoskeletal radiology, for review and interpretation. Investigated radiographic characteristics included location, cortical breakthrough, presence of fracture, and size (if advanced imaging was available). Surgical interventions were recorded from reviews of operative reports and postoperative imaging studies.
Time to metastasis was defined as number of days from diagnosis of malignancy to diagnosis of tibial osseous spread. Date of diagnosis of malignancy was the date that a biopsy or other confirmatory test was performed. In cases in which that date was unavailable, an imaging study consistent with disease or a clinical note documenting the known diagnosis date was used instead. When only month and year (ie, not an exact date) of diagnosis were available, the 15th of the month was used as an estimate. Of the 36 patients, 4 had records insufficient for establishing date of diagnosis. The first date of any imaging study confirming (or suggestive of) a metastatic lesion of the tibia was used as the date of tibial metastasis.
Many patients had osseous lesions at sites other than the tibiae. These lesions were noted on review of imaging studies, screening examinations, and physicians’ clinical notes. Widespread disease was defined as including both axial and appendicular lesions, and lesions of the tibiae.
Tibia lesion presentation was recorded as either symptomatic or incidental. If the tibiae were imaged for pain, including posttraumatic pain, the presentation was symptomatic. If a lesion was identified on staging examination (eg, bone or positron emission tomography scan), or if the tibiae were imaged for another reason, the presentation was incidental.
Results
Demographics
Thirty-six patients had 43 affected tibiae. Sixteen male patients (44.4% of the total) had 19 (44.2%) of the affected tibiae, and 20 female patients (55.6%) had the other 24 affected tibiae (55.8%). Mean age was 63.5 years for all patients (range, 6-95 years), 68.1 years for males, and 59.8 years for females. Of the 36 patients, 32 (88.9%) were over age 40 years (Table). All patients had radiographic evidence of ≥1 tibia lesion, and 6 (16.7%) also had biopsy-proven metastatic disease of the tibia.
Tumor Characteristics
There were 12 different primary neoplasms (Table). The most common were prostate cancer (7 patients, 19.4%; 10 tibiae, 23.3%), breast cancer (7 patients, 19.4%; 9 tibiae, 20.9%), and lung cancer (7 patients, 19.4%; 7 tibiae, 16.3%). For males, the most common diagnoses were prostate cancer (7 cases, 43.8% of males) and diffuse large B-cell lymphoma and lung cancer (3 cases and 18.8% of males each). For females, the most common diagnoses were breast cancer (7 cases, 35.0% of females) and lung cancer (4 cases, 20.0% of females).
Most of the lesions were proximal (31 tibiae, 72.1%), followed by diaphyseal (7, 16.3%) and distal (2, 4.7%) (Table). Three tibiae (7.0%) were entirely involved, but 1 of these was more affected at the distal end. One tibia had 2 lesions, 1 proximal and 1 distal.
Time to Metastasis, Other Osseous Disease
Mean time from diagnosis of malignancy to diagnosis of osseous disease of the tibia was 1282 days (range, 0-3708 days) (Table). Of the 36 patients, 32 (88.9%) had other metastatic lesions, 3 (8.3%) had isolated tibia lesions, and 1 (2.8%) had a medical record insufficient for establishing lesion status (isolated or not). Of the 32 patients with known other osseous metastases, 14 (43.8%) had widespread (axial and additional appendicular) disease, and 3 (9.4%) had additional lesions only distal to the identified tibial metastases.
Clinical Presentation
Of the 36 lesions, 18 (50%) were asymptomatic and were found on screening examinations, 17 (47.2%) presented with pain, and 1 (2.8%) had a presentation that could not be determined from the medical record (Table). Of the 17 painful lesions, 3 (17.6%) were found after a trauma brought attention to the site, and the other 14 (82.4%) were atraumatic in origin.
Of the 10 patients with cortical breakthrough, 8 (80%) had painful lesions, 1 (10%) had a lesion that was found on screening examination, and 1 (10%) had a medical record insufficient for establishing clinical presentation. Of the 8 patients who underwent surgical stabilization, 6 (75%) had painful lesions. Only 1 patient with an asymptomatic tibia lesion underwent surgical intervention (total knee arthroplasty).
Surgical Intervention
Two patients (5.6%) with affected tibiae (4.8%) had pathologic fractures. One fracture (non-small cell lung cancer) was treated with open reduction and internal fixation (periarticular locking plate with cement augmentation), and the other (urothelial cancer) was treated with IMN fixation.
Ten patients (27.8%) with affected tibiae (23.8%) had radiographs that showed cortical breakthrough (Table). Two of the 10 cases were managed nonoperatively, and the patients died before surgical stabilization could be attempted. Of the 8 surgically managed cases, 3 were prophylactically stabilized with IMN (2 of these were augmented with cement, and the third with a screw-plate construct), 2 were treated with periarticular resection and reconstruction (total knee megaprosthesis), 1 was treated with an approach undertaken to address a concomitant distal femoral pathologic fracture, and 1 was treated with total knee arthroplasty undertaken to address lesions at the proximal end of the tibia and the distal end of the femur.
Discussion
We have described a retrospective descriptive study conducted to characterize tibial metastases, their histologies, and the circumstances surrounding diagnosis and surgical management. In all cases, general findings confirmed advanced metastatic disease. In only 3 cases, the tibia lesion was an isolated metastatic lesion.
Sex predilection of tibial metastases remains controversial. One study found males had up to twice as many hand and foot metastases as women,11 but this contrasts with the relatively equal sex ratio found in other studies8,10 and in the present study. We found metastatic disease of the tibia was unsurprisingly concentrated in patients over age 40 years, in whom the vast majority of all cancers develop.12,13 Our study agrees with those that have found most tibia lesions develop in patients in the 6th decade of life on average.8,10 Mean age was 8.3 years higher in our male patients than in our female patients.
Tumor Characteristics
The most common primary neoplasms in our cohort were prostate, breast, and lung cancers, which are among the most common cancers in the United States12,13 and which have a predilection for osseous spread.2,6,9,14 Renal cell carcinoma has been reported to spread to distal (or “acral”) skeletal sites,2-4,9,11,14 but the present study did not identify any patients with this diagnosis. Of our patients with a primary lung cancer for whom a histologic description was available (5/7), all had non-small cell lung cancer. Three patients had a primary malignancy of colorectal cancer, which occasionally metastasizes to the distal skeleton.3,8,11 We identified 3 patients with diffuse large B-cell lymphoma, a histology not widely reported to metastasize to distal skeletal sites.
Metastatic disease of the tibia is most common at the proximal end of the bone.1,10,11,14 Other studies8,10 have found the proximal tibia is affected much more commonly than the tibial diaphysis, and even fewer cases develop at the distal end. Our findings agree with theirs: Proximal lesions outnumber all other lesions combined (Table).
Time to Metastasis
Distal metastases are typical of late-stage metastatic disease,1,3 but quantification of the time from diagnosis of malignancy to presentation of a tibia lesion is not well defined. In our study, time to metastasis was <100 days for some patients (Table). As osseous involvement, especially acral disease, was considered a late-stage manifestation of malignancy, this result was unexpected and most likely represents undiagnosed and untreated malignancy. Six patients in this group were diagnosed with tibial metastases within 30 days, essentially at the same time the primary neoplasm was diagnosed. These findings suggest that a tibia lesion found at time of patient presentation should raise concern for late-stage undiagnosed metastatic cancer.
Other Osseous Disease
The patients identified in this study had advanced malignancy, and most had widespread bony dissemination. Those with the lowest disease burden had isolated tibia lesions or additional metastases only distal to the tibia lesion in the ipsilateral lower extremity. Most of these patients had undergone surgery or were scheduled for it (Table). Most of the patients with appendicular metastases proximal to the tibia lesion had disease of the femora, the most common long bones affected by osseous metastatic disease.5,6 In accordance with orthopedic oncology principles, all other osseous disease should be thoroughly identified and staged before any surgical planning for identified tibia lesions. Ipsilateral distal femoral lesions are of particular importance for patients with proximal tibia lesions, as reconstruction with total knee endoprosthesis can potentially provide a functional reconstructive option after resection of both lesions.
Clinical Presentation
Most of the patients who had cortical breakthrough or required surgical stabilization had painful lesions. Although tibial metastasis is rare, its potential occurrence should raise concerns and be investigated in the patient with tibial pain.
Surgical Intervention
General surgical management of metastatic disease of other long bones has been extensively studied,6,7,9,14 but there are fewer published recommendations regarding specific treatments for metastatic lesions of the tibia. In 2003, Kelly and colleagues8 described an algorithm based on the anatomical location of the lesion, with either internal fixation or IMN fixation representing the preferred management for lesions in the metaphyseal or diaphyseal regions. For epiphyseal or extensive proximal metaphyseal lesions, modular oncology endoprostheses are described as the procedure of choice. Piccioli and colleagues10 in 2013 and Beauchamp and Sim1 in 1988 described a similar operative approach.
It is unknown if the algorithm of Kelly and colleagues8 was referenced during clinical decision-making, but it appears operative management mirrored these principles. Deviations from this general approach in the operative management of the patients in the present study included modifications such as the addition of a screw-plate construct to an IMN for better stability.
Surgical management depends largely on the anatomical location within the bone and on remaining bone stock. Generally, extensive proximal disease is managed with total knee endoprosthesis reconstruction, diaphyseal disease with IMN, and distal disease with internal fixation. Construct augmentation, such as the addition of cement or use of additional hardware, is decided case by case on the basis of desired stability and surrounding bone stock.
Study Limitations
Despite being a larger series, this single-institution study had a relatively small sample size, and its patient demographics and primary malignancies may reflect institutional recruitment bias. In addition, the study was limited by its retrospective design and some incomplete medical records. Eleven patients had only a bone or positron emission tomography scan depicting metastatic disease, limiting characterization of these lesions. One patient lacked radiologic images, and characterizations were based on written reports. As multiple physicians were involved in diagnosis and treatment, there were many inconsistencies in clinical decision-making across the group.
Conclusion
Metastasis to the tibia is a rare but significant event in a subset of patients over the course of their treatment and surveillance. Patients may present with pain secondary to either pathologic or impending pathologic fractures, and in such instances surgical intervention is often needed. Despite the historical reports of “acral” histologies, tibia lesions are not indicative of histology, and biopsy should be considered, especially if management will depend on histology. Patients with lower leg pain and known malignancy should be evaluated to rule out tibial metastasis, but screening examinations may be prudent for asymptomatic patients as well. Increased vigilance may be indicated for those with prostate, breast, or lung cancer. These lesions should be surgically managed case by case using fundamental tenets of both orthopedic fracture care and orthopedic oncology. Ideally, patients should be treated by a multidisciplinary team using a patient-centered approach.
1. Beauchamp CP, Sim FH. Lesions of the tibia. In: Sim FH, ed. Diagnosis and Management of Metastatic Bone Disease: A Multidisciplinary Approach. New York, NY: Raven; 1988:201-212.
2. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 pt 2):6243s-6249s.
3. Healy JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
4. Leeson MC, Makley JT, Carter JR. Metastatic skeletal disease distal to the elbow and knee. Clin Orthop Relat Res. 1986;(206):94-99.
5. De Geeter K, Reynders P, Samson I, Broos PL. Metastatic fractures of the tibia. Acta Orthop Belg. 2001;67(1):54-59.
6. Kelly M, Lee M, Clarkson P, O’Brien PJ. Metastatic disease of the long bones: a review of the health care burden in a major trauma centre. Can J Surg. 2012;55(2):95-98.
7. Jasmin C. Textbook of Bone Metastases. Chichester, England: Wiley; 2005.
8. Kelly CM, Wilkins RM, Eckardt JJ, Ward WG. Treatment of metastatic disease of the tibia. Clin Orthop Relat Res. 2003;(415 suppl):S219-S229.
9. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9(3):509-524.
10. Piccioli A, Maccauro G, Scaramuzzo L, Graci C, Spinelli MS. Surgical treatment of impending and pathological fractures of tibia. Injury. 2013;44(8):1092-1096.
11. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
12. American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013.
13. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
14. Capanna R, Campanacci DA. The treatment of metastases in the appendicular skeleton. J Bone Joint Surg Br. 2001;83(4):471-481.
Take-Home Points
- Metastatic disease of the tibia is a rare but significant event in a subset of patients.
- Cancer histologies with historically “acral” spread may not apply to tibial disease.
- Patients with leg pain and any cancer diagnosis should be worked up for tibial metastases.
- Tibial disease is probably a late manifestation, and early detection may indicate late diagnosis of malignancy.
- The ultimate surgical plan for these patients should be a patient-centered multidisciplinary decision making process.
Metastatic dissemination to bones is common in advanced cancer stages and affects the axial and appendicular skeleton.1-4 The appendicular skeleton bones most often involved are the proximal femur and the proximal humerus.5,6 The tibia is involved third most often but is comparatively rarely affected.4-6 Metastatic involvement distal to the knee or elbow is more typical of advanced disease.1,3 Distal appendicular lesions are called acral metastases, but the term is inconsistently used and may refer to lesions either distal to the knee and elbow or distal to the ankle and wrist. Regardless of terminology, tibia lesions are uncommon and not well described.1,4,7,8
The tibia is the primary weight-bearing leg bone. Metastatic tibia lesions may cause pain and instability and impair mobility. Although distal skeletal dissemination often presents late in advanced disease in patients with relatively poor prognoses, in some cases early surgical intervention is indicated for pain relief, increased mobility, and improved quality of life.4,8-10
Materials and Methods
Our Institutional Review Board approved this single-institution retrospective study. We used proprietary research software (Clinical Looking Glass) to identify eligible patients treated between 2000 and 2013. The software was used to search all radiology and pathology reports for the term tibia or any variation (eg, tibial) and metastasis or any variation (eg, metastatic). The software was then used to search by Current Procedural Terminology code for any patients treated with intramedullary nail (IMN) or another tibial fixation method. This list was cross-referenced with the list of patients originally identified to help ensure that all eligible patients were identified.
Inclusion criteria were known malignancy and imaging or biopsy evidence of a metastatic tibia lesion. Treatment strategies for patients with metastatic disease and patients with multiple myeloma are sometimes considered together because of similar goals and methodologies. We specifically excluded patients with multiple myeloma in order to more accurately characterize the natural history of metastatic disease and the timing of metastatic development and to report on a more homogeneous population. Patients were excluded if their electronic medical records were inadequate in establishing a diagnosis.
Demographic and pathology data were collected directly from the institutional electronic medical records system. Dr. Geller and Dr. Greenbaum used Centricity software (General Electric Healthcare) to review all imaging on medical diagnostic display monitors. If their interpretation differed from that in the radiology report, or if clarification was needed, the study was sent to Dr. Thornhill, the institution’s director of musculoskeletal radiology, for review and interpretation. Investigated radiographic characteristics included location, cortical breakthrough, presence of fracture, and size (if advanced imaging was available). Surgical interventions were recorded from reviews of operative reports and postoperative imaging studies.
Time to metastasis was defined as number of days from diagnosis of malignancy to diagnosis of tibial osseous spread. Date of diagnosis of malignancy was the date that a biopsy or other confirmatory test was performed. In cases in which that date was unavailable, an imaging study consistent with disease or a clinical note documenting the known diagnosis date was used instead. When only month and year (ie, not an exact date) of diagnosis were available, the 15th of the month was used as an estimate. Of the 36 patients, 4 had records insufficient for establishing date of diagnosis. The first date of any imaging study confirming (or suggestive of) a metastatic lesion of the tibia was used as the date of tibial metastasis.
Many patients had osseous lesions at sites other than the tibiae. These lesions were noted on review of imaging studies, screening examinations, and physicians’ clinical notes. Widespread disease was defined as including both axial and appendicular lesions, and lesions of the tibiae.
Tibia lesion presentation was recorded as either symptomatic or incidental. If the tibiae were imaged for pain, including posttraumatic pain, the presentation was symptomatic. If a lesion was identified on staging examination (eg, bone or positron emission tomography scan), or if the tibiae were imaged for another reason, the presentation was incidental.
Results
Demographics
Thirty-six patients had 43 affected tibiae. Sixteen male patients (44.4% of the total) had 19 (44.2%) of the affected tibiae, and 20 female patients (55.6%) had the other 24 affected tibiae (55.8%). Mean age was 63.5 years for all patients (range, 6-95 years), 68.1 years for males, and 59.8 years for females. Of the 36 patients, 32 (88.9%) were over age 40 years (Table). All patients had radiographic evidence of ≥1 tibia lesion, and 6 (16.7%) also had biopsy-proven metastatic disease of the tibia.
Tumor Characteristics
There were 12 different primary neoplasms (Table). The most common were prostate cancer (7 patients, 19.4%; 10 tibiae, 23.3%), breast cancer (7 patients, 19.4%; 9 tibiae, 20.9%), and lung cancer (7 patients, 19.4%; 7 tibiae, 16.3%). For males, the most common diagnoses were prostate cancer (7 cases, 43.8% of males) and diffuse large B-cell lymphoma and lung cancer (3 cases and 18.8% of males each). For females, the most common diagnoses were breast cancer (7 cases, 35.0% of females) and lung cancer (4 cases, 20.0% of females).
Most of the lesions were proximal (31 tibiae, 72.1%), followed by diaphyseal (7, 16.3%) and distal (2, 4.7%) (Table). Three tibiae (7.0%) were entirely involved, but 1 of these was more affected at the distal end. One tibia had 2 lesions, 1 proximal and 1 distal.
Time to Metastasis, Other Osseous Disease
Mean time from diagnosis of malignancy to diagnosis of osseous disease of the tibia was 1282 days (range, 0-3708 days) (Table). Of the 36 patients, 32 (88.9%) had other metastatic lesions, 3 (8.3%) had isolated tibia lesions, and 1 (2.8%) had a medical record insufficient for establishing lesion status (isolated or not). Of the 32 patients with known other osseous metastases, 14 (43.8%) had widespread (axial and additional appendicular) disease, and 3 (9.4%) had additional lesions only distal to the identified tibial metastases.
Clinical Presentation
Of the 36 lesions, 18 (50%) were asymptomatic and were found on screening examinations, 17 (47.2%) presented with pain, and 1 (2.8%) had a presentation that could not be determined from the medical record (Table). Of the 17 painful lesions, 3 (17.6%) were found after a trauma brought attention to the site, and the other 14 (82.4%) were atraumatic in origin.
Of the 10 patients with cortical breakthrough, 8 (80%) had painful lesions, 1 (10%) had a lesion that was found on screening examination, and 1 (10%) had a medical record insufficient for establishing clinical presentation. Of the 8 patients who underwent surgical stabilization, 6 (75%) had painful lesions. Only 1 patient with an asymptomatic tibia lesion underwent surgical intervention (total knee arthroplasty).
Surgical Intervention
Two patients (5.6%) with affected tibiae (4.8%) had pathologic fractures. One fracture (non-small cell lung cancer) was treated with open reduction and internal fixation (periarticular locking plate with cement augmentation), and the other (urothelial cancer) was treated with IMN fixation.
Ten patients (27.8%) with affected tibiae (23.8%) had radiographs that showed cortical breakthrough (Table). Two of the 10 cases were managed nonoperatively, and the patients died before surgical stabilization could be attempted. Of the 8 surgically managed cases, 3 were prophylactically stabilized with IMN (2 of these were augmented with cement, and the third with a screw-plate construct), 2 were treated with periarticular resection and reconstruction (total knee megaprosthesis), 1 was treated with an approach undertaken to address a concomitant distal femoral pathologic fracture, and 1 was treated with total knee arthroplasty undertaken to address lesions at the proximal end of the tibia and the distal end of the femur.
Discussion
We have described a retrospective descriptive study conducted to characterize tibial metastases, their histologies, and the circumstances surrounding diagnosis and surgical management. In all cases, general findings confirmed advanced metastatic disease. In only 3 cases, the tibia lesion was an isolated metastatic lesion.
Sex predilection of tibial metastases remains controversial. One study found males had up to twice as many hand and foot metastases as women,11 but this contrasts with the relatively equal sex ratio found in other studies8,10 and in the present study. We found metastatic disease of the tibia was unsurprisingly concentrated in patients over age 40 years, in whom the vast majority of all cancers develop.12,13 Our study agrees with those that have found most tibia lesions develop in patients in the 6th decade of life on average.8,10 Mean age was 8.3 years higher in our male patients than in our female patients.
Tumor Characteristics
The most common primary neoplasms in our cohort were prostate, breast, and lung cancers, which are among the most common cancers in the United States12,13 and which have a predilection for osseous spread.2,6,9,14 Renal cell carcinoma has been reported to spread to distal (or “acral”) skeletal sites,2-4,9,11,14 but the present study did not identify any patients with this diagnosis. Of our patients with a primary lung cancer for whom a histologic description was available (5/7), all had non-small cell lung cancer. Three patients had a primary malignancy of colorectal cancer, which occasionally metastasizes to the distal skeleton.3,8,11 We identified 3 patients with diffuse large B-cell lymphoma, a histology not widely reported to metastasize to distal skeletal sites.
Metastatic disease of the tibia is most common at the proximal end of the bone.1,10,11,14 Other studies8,10 have found the proximal tibia is affected much more commonly than the tibial diaphysis, and even fewer cases develop at the distal end. Our findings agree with theirs: Proximal lesions outnumber all other lesions combined (Table).
Time to Metastasis
Distal metastases are typical of late-stage metastatic disease,1,3 but quantification of the time from diagnosis of malignancy to presentation of a tibia lesion is not well defined. In our study, time to metastasis was <100 days for some patients (Table). As osseous involvement, especially acral disease, was considered a late-stage manifestation of malignancy, this result was unexpected and most likely represents undiagnosed and untreated malignancy. Six patients in this group were diagnosed with tibial metastases within 30 days, essentially at the same time the primary neoplasm was diagnosed. These findings suggest that a tibia lesion found at time of patient presentation should raise concern for late-stage undiagnosed metastatic cancer.
Other Osseous Disease
The patients identified in this study had advanced malignancy, and most had widespread bony dissemination. Those with the lowest disease burden had isolated tibia lesions or additional metastases only distal to the tibia lesion in the ipsilateral lower extremity. Most of these patients had undergone surgery or were scheduled for it (Table). Most of the patients with appendicular metastases proximal to the tibia lesion had disease of the femora, the most common long bones affected by osseous metastatic disease.5,6 In accordance with orthopedic oncology principles, all other osseous disease should be thoroughly identified and staged before any surgical planning for identified tibia lesions. Ipsilateral distal femoral lesions are of particular importance for patients with proximal tibia lesions, as reconstruction with total knee endoprosthesis can potentially provide a functional reconstructive option after resection of both lesions.
Clinical Presentation
Most of the patients who had cortical breakthrough or required surgical stabilization had painful lesions. Although tibial metastasis is rare, its potential occurrence should raise concerns and be investigated in the patient with tibial pain.
Surgical Intervention
General surgical management of metastatic disease of other long bones has been extensively studied,6,7,9,14 but there are fewer published recommendations regarding specific treatments for metastatic lesions of the tibia. In 2003, Kelly and colleagues8 described an algorithm based on the anatomical location of the lesion, with either internal fixation or IMN fixation representing the preferred management for lesions in the metaphyseal or diaphyseal regions. For epiphyseal or extensive proximal metaphyseal lesions, modular oncology endoprostheses are described as the procedure of choice. Piccioli and colleagues10 in 2013 and Beauchamp and Sim1 in 1988 described a similar operative approach.
It is unknown if the algorithm of Kelly and colleagues8 was referenced during clinical decision-making, but it appears operative management mirrored these principles. Deviations from this general approach in the operative management of the patients in the present study included modifications such as the addition of a screw-plate construct to an IMN for better stability.
Surgical management depends largely on the anatomical location within the bone and on remaining bone stock. Generally, extensive proximal disease is managed with total knee endoprosthesis reconstruction, diaphyseal disease with IMN, and distal disease with internal fixation. Construct augmentation, such as the addition of cement or use of additional hardware, is decided case by case on the basis of desired stability and surrounding bone stock.
Study Limitations
Despite being a larger series, this single-institution study had a relatively small sample size, and its patient demographics and primary malignancies may reflect institutional recruitment bias. In addition, the study was limited by its retrospective design and some incomplete medical records. Eleven patients had only a bone or positron emission tomography scan depicting metastatic disease, limiting characterization of these lesions. One patient lacked radiologic images, and characterizations were based on written reports. As multiple physicians were involved in diagnosis and treatment, there were many inconsistencies in clinical decision-making across the group.
Conclusion
Metastasis to the tibia is a rare but significant event in a subset of patients over the course of their treatment and surveillance. Patients may present with pain secondary to either pathologic or impending pathologic fractures, and in such instances surgical intervention is often needed. Despite the historical reports of “acral” histologies, tibia lesions are not indicative of histology, and biopsy should be considered, especially if management will depend on histology. Patients with lower leg pain and known malignancy should be evaluated to rule out tibial metastasis, but screening examinations may be prudent for asymptomatic patients as well. Increased vigilance may be indicated for those with prostate, breast, or lung cancer. These lesions should be surgically managed case by case using fundamental tenets of both orthopedic fracture care and orthopedic oncology. Ideally, patients should be treated by a multidisciplinary team using a patient-centered approach.
Take-Home Points
- Metastatic disease of the tibia is a rare but significant event in a subset of patients.
- Cancer histologies with historically “acral” spread may not apply to tibial disease.
- Patients with leg pain and any cancer diagnosis should be worked up for tibial metastases.
- Tibial disease is probably a late manifestation, and early detection may indicate late diagnosis of malignancy.
- The ultimate surgical plan for these patients should be a patient-centered multidisciplinary decision making process.
Metastatic dissemination to bones is common in advanced cancer stages and affects the axial and appendicular skeleton.1-4 The appendicular skeleton bones most often involved are the proximal femur and the proximal humerus.5,6 The tibia is involved third most often but is comparatively rarely affected.4-6 Metastatic involvement distal to the knee or elbow is more typical of advanced disease.1,3 Distal appendicular lesions are called acral metastases, but the term is inconsistently used and may refer to lesions either distal to the knee and elbow or distal to the ankle and wrist. Regardless of terminology, tibia lesions are uncommon and not well described.1,4,7,8
The tibia is the primary weight-bearing leg bone. Metastatic tibia lesions may cause pain and instability and impair mobility. Although distal skeletal dissemination often presents late in advanced disease in patients with relatively poor prognoses, in some cases early surgical intervention is indicated for pain relief, increased mobility, and improved quality of life.4,8-10
Materials and Methods
Our Institutional Review Board approved this single-institution retrospective study. We used proprietary research software (Clinical Looking Glass) to identify eligible patients treated between 2000 and 2013. The software was used to search all radiology and pathology reports for the term tibia or any variation (eg, tibial) and metastasis or any variation (eg, metastatic). The software was then used to search by Current Procedural Terminology code for any patients treated with intramedullary nail (IMN) or another tibial fixation method. This list was cross-referenced with the list of patients originally identified to help ensure that all eligible patients were identified.
Inclusion criteria were known malignancy and imaging or biopsy evidence of a metastatic tibia lesion. Treatment strategies for patients with metastatic disease and patients with multiple myeloma are sometimes considered together because of similar goals and methodologies. We specifically excluded patients with multiple myeloma in order to more accurately characterize the natural history of metastatic disease and the timing of metastatic development and to report on a more homogeneous population. Patients were excluded if their electronic medical records were inadequate in establishing a diagnosis.
Demographic and pathology data were collected directly from the institutional electronic medical records system. Dr. Geller and Dr. Greenbaum used Centricity software (General Electric Healthcare) to review all imaging on medical diagnostic display monitors. If their interpretation differed from that in the radiology report, or if clarification was needed, the study was sent to Dr. Thornhill, the institution’s director of musculoskeletal radiology, for review and interpretation. Investigated radiographic characteristics included location, cortical breakthrough, presence of fracture, and size (if advanced imaging was available). Surgical interventions were recorded from reviews of operative reports and postoperative imaging studies.
Time to metastasis was defined as number of days from diagnosis of malignancy to diagnosis of tibial osseous spread. Date of diagnosis of malignancy was the date that a biopsy or other confirmatory test was performed. In cases in which that date was unavailable, an imaging study consistent with disease or a clinical note documenting the known diagnosis date was used instead. When only month and year (ie, not an exact date) of diagnosis were available, the 15th of the month was used as an estimate. Of the 36 patients, 4 had records insufficient for establishing date of diagnosis. The first date of any imaging study confirming (or suggestive of) a metastatic lesion of the tibia was used as the date of tibial metastasis.
Many patients had osseous lesions at sites other than the tibiae. These lesions were noted on review of imaging studies, screening examinations, and physicians’ clinical notes. Widespread disease was defined as including both axial and appendicular lesions, and lesions of the tibiae.
Tibia lesion presentation was recorded as either symptomatic or incidental. If the tibiae were imaged for pain, including posttraumatic pain, the presentation was symptomatic. If a lesion was identified on staging examination (eg, bone or positron emission tomography scan), or if the tibiae were imaged for another reason, the presentation was incidental.
Results
Demographics
Thirty-six patients had 43 affected tibiae. Sixteen male patients (44.4% of the total) had 19 (44.2%) of the affected tibiae, and 20 female patients (55.6%) had the other 24 affected tibiae (55.8%). Mean age was 63.5 years for all patients (range, 6-95 years), 68.1 years for males, and 59.8 years for females. Of the 36 patients, 32 (88.9%) were over age 40 years (Table). All patients had radiographic evidence of ≥1 tibia lesion, and 6 (16.7%) also had biopsy-proven metastatic disease of the tibia.
Tumor Characteristics
There were 12 different primary neoplasms (Table). The most common were prostate cancer (7 patients, 19.4%; 10 tibiae, 23.3%), breast cancer (7 patients, 19.4%; 9 tibiae, 20.9%), and lung cancer (7 patients, 19.4%; 7 tibiae, 16.3%). For males, the most common diagnoses were prostate cancer (7 cases, 43.8% of males) and diffuse large B-cell lymphoma and lung cancer (3 cases and 18.8% of males each). For females, the most common diagnoses were breast cancer (7 cases, 35.0% of females) and lung cancer (4 cases, 20.0% of females).
Most of the lesions were proximal (31 tibiae, 72.1%), followed by diaphyseal (7, 16.3%) and distal (2, 4.7%) (Table). Three tibiae (7.0%) were entirely involved, but 1 of these was more affected at the distal end. One tibia had 2 lesions, 1 proximal and 1 distal.
Time to Metastasis, Other Osseous Disease
Mean time from diagnosis of malignancy to diagnosis of osseous disease of the tibia was 1282 days (range, 0-3708 days) (Table). Of the 36 patients, 32 (88.9%) had other metastatic lesions, 3 (8.3%) had isolated tibia lesions, and 1 (2.8%) had a medical record insufficient for establishing lesion status (isolated or not). Of the 32 patients with known other osseous metastases, 14 (43.8%) had widespread (axial and additional appendicular) disease, and 3 (9.4%) had additional lesions only distal to the identified tibial metastases.
Clinical Presentation
Of the 36 lesions, 18 (50%) were asymptomatic and were found on screening examinations, 17 (47.2%) presented with pain, and 1 (2.8%) had a presentation that could not be determined from the medical record (Table). Of the 17 painful lesions, 3 (17.6%) were found after a trauma brought attention to the site, and the other 14 (82.4%) were atraumatic in origin.
Of the 10 patients with cortical breakthrough, 8 (80%) had painful lesions, 1 (10%) had a lesion that was found on screening examination, and 1 (10%) had a medical record insufficient for establishing clinical presentation. Of the 8 patients who underwent surgical stabilization, 6 (75%) had painful lesions. Only 1 patient with an asymptomatic tibia lesion underwent surgical intervention (total knee arthroplasty).
Surgical Intervention
Two patients (5.6%) with affected tibiae (4.8%) had pathologic fractures. One fracture (non-small cell lung cancer) was treated with open reduction and internal fixation (periarticular locking plate with cement augmentation), and the other (urothelial cancer) was treated with IMN fixation.
Ten patients (27.8%) with affected tibiae (23.8%) had radiographs that showed cortical breakthrough (Table). Two of the 10 cases were managed nonoperatively, and the patients died before surgical stabilization could be attempted. Of the 8 surgically managed cases, 3 were prophylactically stabilized with IMN (2 of these were augmented with cement, and the third with a screw-plate construct), 2 were treated with periarticular resection and reconstruction (total knee megaprosthesis), 1 was treated with an approach undertaken to address a concomitant distal femoral pathologic fracture, and 1 was treated with total knee arthroplasty undertaken to address lesions at the proximal end of the tibia and the distal end of the femur.
Discussion
We have described a retrospective descriptive study conducted to characterize tibial metastases, their histologies, and the circumstances surrounding diagnosis and surgical management. In all cases, general findings confirmed advanced metastatic disease. In only 3 cases, the tibia lesion was an isolated metastatic lesion.
Sex predilection of tibial metastases remains controversial. One study found males had up to twice as many hand and foot metastases as women,11 but this contrasts with the relatively equal sex ratio found in other studies8,10 and in the present study. We found metastatic disease of the tibia was unsurprisingly concentrated in patients over age 40 years, in whom the vast majority of all cancers develop.12,13 Our study agrees with those that have found most tibia lesions develop in patients in the 6th decade of life on average.8,10 Mean age was 8.3 years higher in our male patients than in our female patients.
Tumor Characteristics
The most common primary neoplasms in our cohort were prostate, breast, and lung cancers, which are among the most common cancers in the United States12,13 and which have a predilection for osseous spread.2,6,9,14 Renal cell carcinoma has been reported to spread to distal (or “acral”) skeletal sites,2-4,9,11,14 but the present study did not identify any patients with this diagnosis. Of our patients with a primary lung cancer for whom a histologic description was available (5/7), all had non-small cell lung cancer. Three patients had a primary malignancy of colorectal cancer, which occasionally metastasizes to the distal skeleton.3,8,11 We identified 3 patients with diffuse large B-cell lymphoma, a histology not widely reported to metastasize to distal skeletal sites.
Metastatic disease of the tibia is most common at the proximal end of the bone.1,10,11,14 Other studies8,10 have found the proximal tibia is affected much more commonly than the tibial diaphysis, and even fewer cases develop at the distal end. Our findings agree with theirs: Proximal lesions outnumber all other lesions combined (Table).
Time to Metastasis
Distal metastases are typical of late-stage metastatic disease,1,3 but quantification of the time from diagnosis of malignancy to presentation of a tibia lesion is not well defined. In our study, time to metastasis was <100 days for some patients (Table). As osseous involvement, especially acral disease, was considered a late-stage manifestation of malignancy, this result was unexpected and most likely represents undiagnosed and untreated malignancy. Six patients in this group were diagnosed with tibial metastases within 30 days, essentially at the same time the primary neoplasm was diagnosed. These findings suggest that a tibia lesion found at time of patient presentation should raise concern for late-stage undiagnosed metastatic cancer.
Other Osseous Disease
The patients identified in this study had advanced malignancy, and most had widespread bony dissemination. Those with the lowest disease burden had isolated tibia lesions or additional metastases only distal to the tibia lesion in the ipsilateral lower extremity. Most of these patients had undergone surgery or were scheduled for it (Table). Most of the patients with appendicular metastases proximal to the tibia lesion had disease of the femora, the most common long bones affected by osseous metastatic disease.5,6 In accordance with orthopedic oncology principles, all other osseous disease should be thoroughly identified and staged before any surgical planning for identified tibia lesions. Ipsilateral distal femoral lesions are of particular importance for patients with proximal tibia lesions, as reconstruction with total knee endoprosthesis can potentially provide a functional reconstructive option after resection of both lesions.
Clinical Presentation
Most of the patients who had cortical breakthrough or required surgical stabilization had painful lesions. Although tibial metastasis is rare, its potential occurrence should raise concerns and be investigated in the patient with tibial pain.
Surgical Intervention
General surgical management of metastatic disease of other long bones has been extensively studied,6,7,9,14 but there are fewer published recommendations regarding specific treatments for metastatic lesions of the tibia. In 2003, Kelly and colleagues8 described an algorithm based on the anatomical location of the lesion, with either internal fixation or IMN fixation representing the preferred management for lesions in the metaphyseal or diaphyseal regions. For epiphyseal or extensive proximal metaphyseal lesions, modular oncology endoprostheses are described as the procedure of choice. Piccioli and colleagues10 in 2013 and Beauchamp and Sim1 in 1988 described a similar operative approach.
It is unknown if the algorithm of Kelly and colleagues8 was referenced during clinical decision-making, but it appears operative management mirrored these principles. Deviations from this general approach in the operative management of the patients in the present study included modifications such as the addition of a screw-plate construct to an IMN for better stability.
Surgical management depends largely on the anatomical location within the bone and on remaining bone stock. Generally, extensive proximal disease is managed with total knee endoprosthesis reconstruction, diaphyseal disease with IMN, and distal disease with internal fixation. Construct augmentation, such as the addition of cement or use of additional hardware, is decided case by case on the basis of desired stability and surrounding bone stock.
Study Limitations
Despite being a larger series, this single-institution study had a relatively small sample size, and its patient demographics and primary malignancies may reflect institutional recruitment bias. In addition, the study was limited by its retrospective design and some incomplete medical records. Eleven patients had only a bone or positron emission tomography scan depicting metastatic disease, limiting characterization of these lesions. One patient lacked radiologic images, and characterizations were based on written reports. As multiple physicians were involved in diagnosis and treatment, there were many inconsistencies in clinical decision-making across the group.
Conclusion
Metastasis to the tibia is a rare but significant event in a subset of patients over the course of their treatment and surveillance. Patients may present with pain secondary to either pathologic or impending pathologic fractures, and in such instances surgical intervention is often needed. Despite the historical reports of “acral” histologies, tibia lesions are not indicative of histology, and biopsy should be considered, especially if management will depend on histology. Patients with lower leg pain and known malignancy should be evaluated to rule out tibial metastasis, but screening examinations may be prudent for asymptomatic patients as well. Increased vigilance may be indicated for those with prostate, breast, or lung cancer. These lesions should be surgically managed case by case using fundamental tenets of both orthopedic fracture care and orthopedic oncology. Ideally, patients should be treated by a multidisciplinary team using a patient-centered approach.
1. Beauchamp CP, Sim FH. Lesions of the tibia. In: Sim FH, ed. Diagnosis and Management of Metastatic Bone Disease: A Multidisciplinary Approach. New York, NY: Raven; 1988:201-212.
2. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 pt 2):6243s-6249s.
3. Healy JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
4. Leeson MC, Makley JT, Carter JR. Metastatic skeletal disease distal to the elbow and knee. Clin Orthop Relat Res. 1986;(206):94-99.
5. De Geeter K, Reynders P, Samson I, Broos PL. Metastatic fractures of the tibia. Acta Orthop Belg. 2001;67(1):54-59.
6. Kelly M, Lee M, Clarkson P, O’Brien PJ. Metastatic disease of the long bones: a review of the health care burden in a major trauma centre. Can J Surg. 2012;55(2):95-98.
7. Jasmin C. Textbook of Bone Metastases. Chichester, England: Wiley; 2005.
8. Kelly CM, Wilkins RM, Eckardt JJ, Ward WG. Treatment of metastatic disease of the tibia. Clin Orthop Relat Res. 2003;(415 suppl):S219-S229.
9. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9(3):509-524.
10. Piccioli A, Maccauro G, Scaramuzzo L, Graci C, Spinelli MS. Surgical treatment of impending and pathological fractures of tibia. Injury. 2013;44(8):1092-1096.
11. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
12. American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013.
13. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
14. Capanna R, Campanacci DA. The treatment of metastases in the appendicular skeleton. J Bone Joint Surg Br. 2001;83(4):471-481.
1. Beauchamp CP, Sim FH. Lesions of the tibia. In: Sim FH, ed. Diagnosis and Management of Metastatic Bone Disease: A Multidisciplinary Approach. New York, NY: Raven; 1988:201-212.
2. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 pt 2):6243s-6249s.
3. Healy JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
4. Leeson MC, Makley JT, Carter JR. Metastatic skeletal disease distal to the elbow and knee. Clin Orthop Relat Res. 1986;(206):94-99.
5. De Geeter K, Reynders P, Samson I, Broos PL. Metastatic fractures of the tibia. Acta Orthop Belg. 2001;67(1):54-59.
6. Kelly M, Lee M, Clarkson P, O’Brien PJ. Metastatic disease of the long bones: a review of the health care burden in a major trauma centre. Can J Surg. 2012;55(2):95-98.
7. Jasmin C. Textbook of Bone Metastases. Chichester, England: Wiley; 2005.
8. Kelly CM, Wilkins RM, Eckardt JJ, Ward WG. Treatment of metastatic disease of the tibia. Clin Orthop Relat Res. 2003;(415 suppl):S219-S229.
9. Nielsen OS, Munro AJ, Tannock IF. Bone metastases: pathophysiology and management policy. J Clin Oncol. 1991;9(3):509-524.
10. Piccioli A, Maccauro G, Scaramuzzo L, Graci C, Spinelli MS. Surgical treatment of impending and pathological fractures of tibia. Injury. 2013;44(8):1092-1096.
11. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
12. American Cancer Society. Cancer Facts and Figures 2013. Atlanta, GA: American Cancer Society; 2013.
13. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11-30.
14. Capanna R, Campanacci DA. The treatment of metastases in the appendicular skeleton. J Bone Joint Surg Br. 2001;83(4):471-481.
Lumbar Microlaminectomy vs Traditional Laminectomy
Lumbar spinal stenosis (LSS) is a common debilitating issue in older patients. Open laminectomies traditionally are the standard treatment for LSS; however, minimally invasive surgery (MIS) has recently become a popular option to facilitate recovery and improve efficiency of care regarding spine procedures.
Guiot and colleagues described the technique for an MIS decompressive lumbar laminectomy procedure.1 The surgery may represent an important strategy to improve the efficiency of care for patients with severe LSS. Several authors have reported clinical benefits with the MIS lumbar laminectomy, leading to a significant improvement in the Oswetry Disability Index (ODI) 25 in the degenerative stenosis group in cases of LSS.2-5 In a recent reviewof 13 studies Wong and colleagues concluded that the MIS laminectomy was efficacious in terms of symptomatic relief and patient satisfaction for patients with LSS.6 Further, Rosen and colleaguesfound a significant improvement in the ODI scores and in the Short Form-36 body pain and physical functions scores in patients aged ≥ 75 years.7
Perioperative measures, including blood loss and narcotic consumption, have been shown to significantly decrease with MIS surgery compared with open decompression.8,9 Decreased narcotic use is of particular interest for the geriatric population because it is expected to allow those patients to remain more physically active and mentally agile.10
Also, long-term success is important when assessing the efficacy of new MIS procedures. Oertel and colleagues found that 85% of patients reported long-term success after unilateral laminotomy of bilateral decompression (ULBD).11 These results indicate that a MIS laminectomy is effective in older patients with LSS and neurogenic claudication.
Although there are numerous MIS approaches to alleviating LSS, more research is needed to determine whether it is superior to the open laminectomy.9,12,13 Skovrliand and colleagues reviewed publications comparing ULBD and open laminectomies and determined that currently insufficient evidence exists to define which technique leads to more positive outcomes.14 Thus, the purpose of this study is 2-fold. First, this study adds to the current research by comparing estimated blood loss and length of stay (LOS) for microscopic MIS laminectomy vs traditional laminectomy. Second, this study aims to address the difference in health care costs between the 2 types of surgery in the VHA.
The U.S. health care system is facing several challenges and in particular pressure for cost reduction.15 VA hospitals are not exempt from those challenges, and their operating budgets are influenced by political and economic factors.16 Because of those challenges, cost-effectiveness is gaining importance.7 Future decisions for procedure coverage and reimbursement rates are likely to consider ratios like the cost to quality-adjusted life-years (QALY). Improving this ratio requires a reduction of cost and/or an improvement in outcome.
Minimally invasive spine surgery (MISS) may lower the cost of spine procedures. Wang and colleagues reported that minimally invasive posterior lumbar interbody fusion (PLIF) led to shorter stay and lower blood loss compared with traditional PLIF.17 These improvements led to about $8,000 in savings for a single-level PLIF.17
Lumbar degenerative disease is a frequently encountered condition, and lumbar laminectomy is one of the most frequently performed spine procedures at VA hospitals. Consequently, MISS may be an important strategy for the VA to face systematic challenges. At the Southern Arizona VA Health Care System (SAVAHCS) in Tucson, the authors converted lumbar laminectomies from traditional open surgery to a MIS procedure using a tubular retractor system and a paramedian approach. To the authors’ knowledge, no studies have evaluated outcomes and cost efficiency of MIS surgery at the VA. The results of such a study may be instrumental in choosing which surgery is appropriate in a patient-centered health care model.
Material and Methods
Fifty veterans with severe lumbar stenosis and neurogenic claudication underwent a 1- or 2-level laminectomy at SAVAHCS (Table). A traditional laminectomy was performed for all patients until conversion to the MIS procedure, then all subsequent patients underwent the microlaminectomy. There was 1 female patient in each group. The preoperative magnetic resonance imaging (MRI) of the patients showed severe spinal canal stenosis defined radiographically by the absence of cerebrospinal fluid signal at the affected level on MRI (Figures 1A and 2A) and clinically by the presence of neurogenic claudication.
Procedure
The open laminectomies were performed in a traditional midline approach with removal of the spinous process along with the lamina bilaterally to provide spinal canal decompression (Figure 2).
The patients were given the choice of going home or being admitted. Overall admission costs were determined by the VA hospital following described models.18 The LOS in rehabilitation were determined from the records of the SAVAHCS rehabilitation center.
Results
There was not a significant difference in age between the 2 groups; mean age was 69.7 ± 9.8 years for the traditional laminectomy group and 64.4 ± 8.3 years for the MIS group. Operating room time was just over 2 hours on average in both groups. Blood loss was estimated and reported by the surgeon and the anesthesiologist, based on values from the surgical suction system. Patients in the MIS group lost on average 46 cc ± 70 cc compared with 135 cc ± 78 cc in the traditional group. The average number of operated levels was higher in the traditional group (1.7 ± 0.5) compared with the MIS group (1.4 ± 0.5), but this difference did not reach significance (P > .05).
Length of Stay and Cost
The LOS was lower for the MIS group, and 76% chose to be discharged from the recovery room. After a traditional laminectomy, the average patient’s stay was 3 days in the hospital and 5 days in the rehabilitation center. The average MIS group patient stayed < 1 day in the hospital. There were no readmissions within 30 days and no severe morbidity (including no new neurologic deficits or death) in the MIS cohort.
Only 1 MIS patient needed transfer to the rehabilitation center. The estimated cost of care (hospital and rehabilitation) for the traditional group was $10,846 compared with $1,961 for the MIS group.
Discussion
In the authors’ experience, the use of MISS microlaminectomy for the treatment of LSS seems to have led to shorter hospital stays and faster recoveries. Some of the possible reasons for faster patient mobilization included a reduction in postoperative pain and the absence of a wound drain. Larger dissections with a traditional laminectomy often lead to the placement of a wound drain, which requires an inpatient stay until the wound output reaches a certain threshold. The absence of a drain and the reduction in pain with the MISS approach allowed the providers to focus on early ambulation and discharge planning. The microlaminectomy technique allowed for a proper surgical decompression with less tissue dissection than is required for a traditional laminectomy. Previous studies have shown that the microlaminectomy technique provides significant symptomatic relief.5-7,17
In most cases, the microlaminectomy can be performed on an outpatient basis. The improvement in bed availability is particularly important as surgical procedures may be delayed when hospitals operate at full capacity. Redesigning a procedure typically requiring hospital admission into an outpatient procedure improves availability, allowing for better patient access to health care.19
Other authors have studied opportunities to transform inpatient neurosurgical care into outpatient procedures. For instance, Purzner and colleagues presented a large series of successful outpatient neurosurgical cases, including craniotomies, cervical fusions, and lumbar microdiscectomies.20 The MISS techniques offer a critical option to facilitate postoperative recovery and improve efficiency of care in regards to spine procedures.5,17
Cost-Effectiveness Within the VHA
The VA has been described as one of the best health care systems in the U.S.9 The arguments in favor of the VA system include its integrated computerized system and its resistance to health care cost inflation over the years.21 The $186.5 billion 2018 fiscal year VA budget is surpassed only by the total DoD budget, and it is expected to rise substantially in the near future.22
Redesigning a procedure typically requiring hospital admission into an outpatient procedure improves bed availability and reduces cost.19 The authors have demonstrated that a minimally invasive unilateral paramedian approach for the treatment of lumbar stenosis leads to shorter hospital stay, improved bed availability, and lower cost while allowing for a proper surgical decompression. These clinical results are in accord with previous MIS surgery studies.5,17 The improvement in bed availability is particularly important within the VA system. Elective surgeries occasionally are delayed or cancelled because hospitals operate at full capacity. However, the authors’ outpatient microlaminectomy patients avoid delays or cancellations.
Given that both laminectomy procedures use similar operating room resources (time and material), the lower LOS associated with the microlaminectomy translates in cost saving. At SAVAHCS, acute care hospitalization is estimated at $3,000 per day when accounting for various costs, including nursing, pharmacy, ancillary services, and maintenance. The MIS procedure costs about $9,000 less than the open surgery. Over a 2-year period with 37 MIS patients, SAVAHCS saved about $300,000.
Patient Satisfaction
Patient satisfaction was assessed 1 day after the lumbar microdecompression outpatient surgery. Patients were asked to rate their overall surgical experience on a scale of 1 (worst) to 10 (best). All 24 patients who were contacted following outpatient lumbar microdecompression surgery rated the experience 10. These results indicate that patients do not expect or desire an admission following lumbar surgery, and they may recover comfortably at home. Studies are needed to compare outpatient and inpatient satisfaction ratings.
Conclusion
In this small sample, lumbar microlaminectomy significantly reduced LOS, successfully decompressed the spinal canal, and achieved symptomatic relief. Also, the procedure is associated with a lower blood loss than a traditional laminectomy and may reduce the rate of perioperative morbidity over time. In addition to faster recovery, the reduction in LOS can improve access to care by increasing the availability to inpatient admission.
1. Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine (Phila PA 1976). 2002;27(4):432-438.
2. Rahman M, Summers LE, Richter B, Mimran RI, Jacob RP. Comparison of techniques for decompressive lumbar laminectomy: the minimally invasive versus the “classic” open approach. Minim Invasive Neurosurg. 2008;51(2)100-105.
3. Sasai K, Umeda M, Maruyama T, Wakabayashi E, Iida H. Microsurgical bilateral decompression via a unilateral approach for lumbar spinal canal stenosis including degenerative spondylolisthesis. J Neurosurg Spine. 2008;9(6):554-559.
4. Pao JL, Chen WC, Chen PQ. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur Spine J. 2009;18(5):672-678.
5. Yagi M, Okada, E, Ninomiya K, Kihara M. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J Neurosurg Spine. 2009;10(4):293-299.
6. Wong AP, Smith ZA, Lall RR, Bresnahan LE, Fessler RG. The microendoscopic decompression of lumbar stenosis: a review of the current literature and clinical results. Minim Invasive Surg. 2012;2012:325095.
7. Rosen DS, O’Toole JE, Eichholz KM, et al. Minimally invasive lumbar spinal decompression in the elderly: outcomes of 50 patients aged 75 years and older. Neurosurgery. 2007;60(3):503-509.
8. Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;51(suppl 5):S146-S154.
9. Mobbs RJ, Li J, Sivabalan P, Raley D, Rao PJ. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine. 2014;21(2):179-186.
10. Avila MJ, Walter CM, Baaj AA. Outcomes and complications of minimally invasive surgery of the lumbar spine in the elderly. Cureus. 2016;8(3):e519.
11. Oertel MF, Ryang YM, Korinth MC, Gilsbach JM, Rohde V. Long-term results of microsurgical treatment of lumbar spinal stenosis by unilateral laminotomy for bilateral decompression. Neurosurgery. 2006;59(6):1264-1269.
12. Haddadi K, Ganjeh Qazvini HR. Outcome after surgery of lumbar spinal stenosis: a randomized comparison of bilateral laminotomy, trumpet laminectomy, and conventional laminectomy. Front Surg. 2016;3:199.
13. Watanabe K, Matsumoto M, Ikegami T, et al. Reduced postoperative wound pain after lumbar spinous process-splitting laminectomy for lumbar canal stenosis: a randomized controlled study. J Neurosurg Spine. 2011;14(1):51-58.
14. Skovrlj B, Belton P, Zarzour H, Qureshi SA. Perioperative outcomes in minimally invasive lumbar spine surgery: a systematic review. World J. Orthop. 2015;6(11):996-1005.
15. Hellander I. The deepening crisis in U.S. health care: a review of data. Int J Health Serv. 2011;41(3):575-586.
16. Chokshi DA. Improving health care for veterans—a watershed moment for the VA. N Engl J Med. 2014;371(4):297-299.
17. Wang MY, Cummock MD, Yu Y, Trivedi RA. An analysis of the differences in the acute hospitalization charges following minimally invasive versus open posterior lumbar interbody fusion. J Neurosurg Spine. 2010;12(6):694-699.
18. Barnett PG. Determination of VA health care costs. Med Care Res Rev. 2003;60(suppl 3):S124-S141.
19. Congressional Budget Office. The health care system for veterans: interim report. https://www.cbo.gov/sites/default/files/110th-congress-2007-2008/reports/12-21-va_healthcare.pdf. Published December 2007. Accessed October 13, 2017.
20. Purzner T, Purzner J, Massicotte EM, Bernstein M. Outpatient brain tumor surgery and spinal decompression: a prospective study of 1003 patients. Neurosurgery. 2011;69(1):119-126.
21. Waller D. How veterans’ hospitals became the best in health care. Time Magazine. http://content.time.com/time/magazine/article/0,9171,1376238,00.html. Published August 27, 2006. Accessed October 13, 2017.
22. U.S. Department of Veterans Affairs, Office of Budget. Annual budget submission—office of budget. https://www.va.gov/budget/products.asp. Updated July 12, 2017. Published October 13, 2017. Accessed October 27, 2017.
Lumbar spinal stenosis (LSS) is a common debilitating issue in older patients. Open laminectomies traditionally are the standard treatment for LSS; however, minimally invasive surgery (MIS) has recently become a popular option to facilitate recovery and improve efficiency of care regarding spine procedures.
Guiot and colleagues described the technique for an MIS decompressive lumbar laminectomy procedure.1 The surgery may represent an important strategy to improve the efficiency of care for patients with severe LSS. Several authors have reported clinical benefits with the MIS lumbar laminectomy, leading to a significant improvement in the Oswetry Disability Index (ODI) 25 in the degenerative stenosis group in cases of LSS.2-5 In a recent reviewof 13 studies Wong and colleagues concluded that the MIS laminectomy was efficacious in terms of symptomatic relief and patient satisfaction for patients with LSS.6 Further, Rosen and colleaguesfound a significant improvement in the ODI scores and in the Short Form-36 body pain and physical functions scores in patients aged ≥ 75 years.7
Perioperative measures, including blood loss and narcotic consumption, have been shown to significantly decrease with MIS surgery compared with open decompression.8,9 Decreased narcotic use is of particular interest for the geriatric population because it is expected to allow those patients to remain more physically active and mentally agile.10
Also, long-term success is important when assessing the efficacy of new MIS procedures. Oertel and colleagues found that 85% of patients reported long-term success after unilateral laminotomy of bilateral decompression (ULBD).11 These results indicate that a MIS laminectomy is effective in older patients with LSS and neurogenic claudication.
Although there are numerous MIS approaches to alleviating LSS, more research is needed to determine whether it is superior to the open laminectomy.9,12,13 Skovrliand and colleagues reviewed publications comparing ULBD and open laminectomies and determined that currently insufficient evidence exists to define which technique leads to more positive outcomes.14 Thus, the purpose of this study is 2-fold. First, this study adds to the current research by comparing estimated blood loss and length of stay (LOS) for microscopic MIS laminectomy vs traditional laminectomy. Second, this study aims to address the difference in health care costs between the 2 types of surgery in the VHA.
The U.S. health care system is facing several challenges and in particular pressure for cost reduction.15 VA hospitals are not exempt from those challenges, and their operating budgets are influenced by political and economic factors.16 Because of those challenges, cost-effectiveness is gaining importance.7 Future decisions for procedure coverage and reimbursement rates are likely to consider ratios like the cost to quality-adjusted life-years (QALY). Improving this ratio requires a reduction of cost and/or an improvement in outcome.
Minimally invasive spine surgery (MISS) may lower the cost of spine procedures. Wang and colleagues reported that minimally invasive posterior lumbar interbody fusion (PLIF) led to shorter stay and lower blood loss compared with traditional PLIF.17 These improvements led to about $8,000 in savings for a single-level PLIF.17
Lumbar degenerative disease is a frequently encountered condition, and lumbar laminectomy is one of the most frequently performed spine procedures at VA hospitals. Consequently, MISS may be an important strategy for the VA to face systematic challenges. At the Southern Arizona VA Health Care System (SAVAHCS) in Tucson, the authors converted lumbar laminectomies from traditional open surgery to a MIS procedure using a tubular retractor system and a paramedian approach. To the authors’ knowledge, no studies have evaluated outcomes and cost efficiency of MIS surgery at the VA. The results of such a study may be instrumental in choosing which surgery is appropriate in a patient-centered health care model.
Material and Methods
Fifty veterans with severe lumbar stenosis and neurogenic claudication underwent a 1- or 2-level laminectomy at SAVAHCS (Table). A traditional laminectomy was performed for all patients until conversion to the MIS procedure, then all subsequent patients underwent the microlaminectomy. There was 1 female patient in each group. The preoperative magnetic resonance imaging (MRI) of the patients showed severe spinal canal stenosis defined radiographically by the absence of cerebrospinal fluid signal at the affected level on MRI (Figures 1A and 2A) and clinically by the presence of neurogenic claudication.
Procedure
The open laminectomies were performed in a traditional midline approach with removal of the spinous process along with the lamina bilaterally to provide spinal canal decompression (Figure 2).
The patients were given the choice of going home or being admitted. Overall admission costs were determined by the VA hospital following described models.18 The LOS in rehabilitation were determined from the records of the SAVAHCS rehabilitation center.
Results
There was not a significant difference in age between the 2 groups; mean age was 69.7 ± 9.8 years for the traditional laminectomy group and 64.4 ± 8.3 years for the MIS group. Operating room time was just over 2 hours on average in both groups. Blood loss was estimated and reported by the surgeon and the anesthesiologist, based on values from the surgical suction system. Patients in the MIS group lost on average 46 cc ± 70 cc compared with 135 cc ± 78 cc in the traditional group. The average number of operated levels was higher in the traditional group (1.7 ± 0.5) compared with the MIS group (1.4 ± 0.5), but this difference did not reach significance (P > .05).
Length of Stay and Cost
The LOS was lower for the MIS group, and 76% chose to be discharged from the recovery room. After a traditional laminectomy, the average patient’s stay was 3 days in the hospital and 5 days in the rehabilitation center. The average MIS group patient stayed < 1 day in the hospital. There were no readmissions within 30 days and no severe morbidity (including no new neurologic deficits or death) in the MIS cohort.
Only 1 MIS patient needed transfer to the rehabilitation center. The estimated cost of care (hospital and rehabilitation) for the traditional group was $10,846 compared with $1,961 for the MIS group.
Discussion
In the authors’ experience, the use of MISS microlaminectomy for the treatment of LSS seems to have led to shorter hospital stays and faster recoveries. Some of the possible reasons for faster patient mobilization included a reduction in postoperative pain and the absence of a wound drain. Larger dissections with a traditional laminectomy often lead to the placement of a wound drain, which requires an inpatient stay until the wound output reaches a certain threshold. The absence of a drain and the reduction in pain with the MISS approach allowed the providers to focus on early ambulation and discharge planning. The microlaminectomy technique allowed for a proper surgical decompression with less tissue dissection than is required for a traditional laminectomy. Previous studies have shown that the microlaminectomy technique provides significant symptomatic relief.5-7,17
In most cases, the microlaminectomy can be performed on an outpatient basis. The improvement in bed availability is particularly important as surgical procedures may be delayed when hospitals operate at full capacity. Redesigning a procedure typically requiring hospital admission into an outpatient procedure improves availability, allowing for better patient access to health care.19
Other authors have studied opportunities to transform inpatient neurosurgical care into outpatient procedures. For instance, Purzner and colleagues presented a large series of successful outpatient neurosurgical cases, including craniotomies, cervical fusions, and lumbar microdiscectomies.20 The MISS techniques offer a critical option to facilitate postoperative recovery and improve efficiency of care in regards to spine procedures.5,17
Cost-Effectiveness Within the VHA
The VA has been described as one of the best health care systems in the U.S.9 The arguments in favor of the VA system include its integrated computerized system and its resistance to health care cost inflation over the years.21 The $186.5 billion 2018 fiscal year VA budget is surpassed only by the total DoD budget, and it is expected to rise substantially in the near future.22
Redesigning a procedure typically requiring hospital admission into an outpatient procedure improves bed availability and reduces cost.19 The authors have demonstrated that a minimally invasive unilateral paramedian approach for the treatment of lumbar stenosis leads to shorter hospital stay, improved bed availability, and lower cost while allowing for a proper surgical decompression. These clinical results are in accord with previous MIS surgery studies.5,17 The improvement in bed availability is particularly important within the VA system. Elective surgeries occasionally are delayed or cancelled because hospitals operate at full capacity. However, the authors’ outpatient microlaminectomy patients avoid delays or cancellations.
Given that both laminectomy procedures use similar operating room resources (time and material), the lower LOS associated with the microlaminectomy translates in cost saving. At SAVAHCS, acute care hospitalization is estimated at $3,000 per day when accounting for various costs, including nursing, pharmacy, ancillary services, and maintenance. The MIS procedure costs about $9,000 less than the open surgery. Over a 2-year period with 37 MIS patients, SAVAHCS saved about $300,000.
Patient Satisfaction
Patient satisfaction was assessed 1 day after the lumbar microdecompression outpatient surgery. Patients were asked to rate their overall surgical experience on a scale of 1 (worst) to 10 (best). All 24 patients who were contacted following outpatient lumbar microdecompression surgery rated the experience 10. These results indicate that patients do not expect or desire an admission following lumbar surgery, and they may recover comfortably at home. Studies are needed to compare outpatient and inpatient satisfaction ratings.
Conclusion
In this small sample, lumbar microlaminectomy significantly reduced LOS, successfully decompressed the spinal canal, and achieved symptomatic relief. Also, the procedure is associated with a lower blood loss than a traditional laminectomy and may reduce the rate of perioperative morbidity over time. In addition to faster recovery, the reduction in LOS can improve access to care by increasing the availability to inpatient admission.
Lumbar spinal stenosis (LSS) is a common debilitating issue in older patients. Open laminectomies traditionally are the standard treatment for LSS; however, minimally invasive surgery (MIS) has recently become a popular option to facilitate recovery and improve efficiency of care regarding spine procedures.
Guiot and colleagues described the technique for an MIS decompressive lumbar laminectomy procedure.1 The surgery may represent an important strategy to improve the efficiency of care for patients with severe LSS. Several authors have reported clinical benefits with the MIS lumbar laminectomy, leading to a significant improvement in the Oswetry Disability Index (ODI) 25 in the degenerative stenosis group in cases of LSS.2-5 In a recent reviewof 13 studies Wong and colleagues concluded that the MIS laminectomy was efficacious in terms of symptomatic relief and patient satisfaction for patients with LSS.6 Further, Rosen and colleaguesfound a significant improvement in the ODI scores and in the Short Form-36 body pain and physical functions scores in patients aged ≥ 75 years.7
Perioperative measures, including blood loss and narcotic consumption, have been shown to significantly decrease with MIS surgery compared with open decompression.8,9 Decreased narcotic use is of particular interest for the geriatric population because it is expected to allow those patients to remain more physically active and mentally agile.10
Also, long-term success is important when assessing the efficacy of new MIS procedures. Oertel and colleagues found that 85% of patients reported long-term success after unilateral laminotomy of bilateral decompression (ULBD).11 These results indicate that a MIS laminectomy is effective in older patients with LSS and neurogenic claudication.
Although there are numerous MIS approaches to alleviating LSS, more research is needed to determine whether it is superior to the open laminectomy.9,12,13 Skovrliand and colleagues reviewed publications comparing ULBD and open laminectomies and determined that currently insufficient evidence exists to define which technique leads to more positive outcomes.14 Thus, the purpose of this study is 2-fold. First, this study adds to the current research by comparing estimated blood loss and length of stay (LOS) for microscopic MIS laminectomy vs traditional laminectomy. Second, this study aims to address the difference in health care costs between the 2 types of surgery in the VHA.
The U.S. health care system is facing several challenges and in particular pressure for cost reduction.15 VA hospitals are not exempt from those challenges, and their operating budgets are influenced by political and economic factors.16 Because of those challenges, cost-effectiveness is gaining importance.7 Future decisions for procedure coverage and reimbursement rates are likely to consider ratios like the cost to quality-adjusted life-years (QALY). Improving this ratio requires a reduction of cost and/or an improvement in outcome.
Minimally invasive spine surgery (MISS) may lower the cost of spine procedures. Wang and colleagues reported that minimally invasive posterior lumbar interbody fusion (PLIF) led to shorter stay and lower blood loss compared with traditional PLIF.17 These improvements led to about $8,000 in savings for a single-level PLIF.17
Lumbar degenerative disease is a frequently encountered condition, and lumbar laminectomy is one of the most frequently performed spine procedures at VA hospitals. Consequently, MISS may be an important strategy for the VA to face systematic challenges. At the Southern Arizona VA Health Care System (SAVAHCS) in Tucson, the authors converted lumbar laminectomies from traditional open surgery to a MIS procedure using a tubular retractor system and a paramedian approach. To the authors’ knowledge, no studies have evaluated outcomes and cost efficiency of MIS surgery at the VA. The results of such a study may be instrumental in choosing which surgery is appropriate in a patient-centered health care model.
Material and Methods
Fifty veterans with severe lumbar stenosis and neurogenic claudication underwent a 1- or 2-level laminectomy at SAVAHCS (Table). A traditional laminectomy was performed for all patients until conversion to the MIS procedure, then all subsequent patients underwent the microlaminectomy. There was 1 female patient in each group. The preoperative magnetic resonance imaging (MRI) of the patients showed severe spinal canal stenosis defined radiographically by the absence of cerebrospinal fluid signal at the affected level on MRI (Figures 1A and 2A) and clinically by the presence of neurogenic claudication.
Procedure
The open laminectomies were performed in a traditional midline approach with removal of the spinous process along with the lamina bilaterally to provide spinal canal decompression (Figure 2).
The patients were given the choice of going home or being admitted. Overall admission costs were determined by the VA hospital following described models.18 The LOS in rehabilitation were determined from the records of the SAVAHCS rehabilitation center.
Results
There was not a significant difference in age between the 2 groups; mean age was 69.7 ± 9.8 years for the traditional laminectomy group and 64.4 ± 8.3 years for the MIS group. Operating room time was just over 2 hours on average in both groups. Blood loss was estimated and reported by the surgeon and the anesthesiologist, based on values from the surgical suction system. Patients in the MIS group lost on average 46 cc ± 70 cc compared with 135 cc ± 78 cc in the traditional group. The average number of operated levels was higher in the traditional group (1.7 ± 0.5) compared with the MIS group (1.4 ± 0.5), but this difference did not reach significance (P > .05).
Length of Stay and Cost
The LOS was lower for the MIS group, and 76% chose to be discharged from the recovery room. After a traditional laminectomy, the average patient’s stay was 3 days in the hospital and 5 days in the rehabilitation center. The average MIS group patient stayed < 1 day in the hospital. There were no readmissions within 30 days and no severe morbidity (including no new neurologic deficits or death) in the MIS cohort.
Only 1 MIS patient needed transfer to the rehabilitation center. The estimated cost of care (hospital and rehabilitation) for the traditional group was $10,846 compared with $1,961 for the MIS group.
Discussion
In the authors’ experience, the use of MISS microlaminectomy for the treatment of LSS seems to have led to shorter hospital stays and faster recoveries. Some of the possible reasons for faster patient mobilization included a reduction in postoperative pain and the absence of a wound drain. Larger dissections with a traditional laminectomy often lead to the placement of a wound drain, which requires an inpatient stay until the wound output reaches a certain threshold. The absence of a drain and the reduction in pain with the MISS approach allowed the providers to focus on early ambulation and discharge planning. The microlaminectomy technique allowed for a proper surgical decompression with less tissue dissection than is required for a traditional laminectomy. Previous studies have shown that the microlaminectomy technique provides significant symptomatic relief.5-7,17
In most cases, the microlaminectomy can be performed on an outpatient basis. The improvement in bed availability is particularly important as surgical procedures may be delayed when hospitals operate at full capacity. Redesigning a procedure typically requiring hospital admission into an outpatient procedure improves availability, allowing for better patient access to health care.19
Other authors have studied opportunities to transform inpatient neurosurgical care into outpatient procedures. For instance, Purzner and colleagues presented a large series of successful outpatient neurosurgical cases, including craniotomies, cervical fusions, and lumbar microdiscectomies.20 The MISS techniques offer a critical option to facilitate postoperative recovery and improve efficiency of care in regards to spine procedures.5,17
Cost-Effectiveness Within the VHA
The VA has been described as one of the best health care systems in the U.S.9 The arguments in favor of the VA system include its integrated computerized system and its resistance to health care cost inflation over the years.21 The $186.5 billion 2018 fiscal year VA budget is surpassed only by the total DoD budget, and it is expected to rise substantially in the near future.22
Redesigning a procedure typically requiring hospital admission into an outpatient procedure improves bed availability and reduces cost.19 The authors have demonstrated that a minimally invasive unilateral paramedian approach for the treatment of lumbar stenosis leads to shorter hospital stay, improved bed availability, and lower cost while allowing for a proper surgical decompression. These clinical results are in accord with previous MIS surgery studies.5,17 The improvement in bed availability is particularly important within the VA system. Elective surgeries occasionally are delayed or cancelled because hospitals operate at full capacity. However, the authors’ outpatient microlaminectomy patients avoid delays or cancellations.
Given that both laminectomy procedures use similar operating room resources (time and material), the lower LOS associated with the microlaminectomy translates in cost saving. At SAVAHCS, acute care hospitalization is estimated at $3,000 per day when accounting for various costs, including nursing, pharmacy, ancillary services, and maintenance. The MIS procedure costs about $9,000 less than the open surgery. Over a 2-year period with 37 MIS patients, SAVAHCS saved about $300,000.
Patient Satisfaction
Patient satisfaction was assessed 1 day after the lumbar microdecompression outpatient surgery. Patients were asked to rate their overall surgical experience on a scale of 1 (worst) to 10 (best). All 24 patients who were contacted following outpatient lumbar microdecompression surgery rated the experience 10. These results indicate that patients do not expect or desire an admission following lumbar surgery, and they may recover comfortably at home. Studies are needed to compare outpatient and inpatient satisfaction ratings.
Conclusion
In this small sample, lumbar microlaminectomy significantly reduced LOS, successfully decompressed the spinal canal, and achieved symptomatic relief. Also, the procedure is associated with a lower blood loss than a traditional laminectomy and may reduce the rate of perioperative morbidity over time. In addition to faster recovery, the reduction in LOS can improve access to care by increasing the availability to inpatient admission.
1. Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine (Phila PA 1976). 2002;27(4):432-438.
2. Rahman M, Summers LE, Richter B, Mimran RI, Jacob RP. Comparison of techniques for decompressive lumbar laminectomy: the minimally invasive versus the “classic” open approach. Minim Invasive Neurosurg. 2008;51(2)100-105.
3. Sasai K, Umeda M, Maruyama T, Wakabayashi E, Iida H. Microsurgical bilateral decompression via a unilateral approach for lumbar spinal canal stenosis including degenerative spondylolisthesis. J Neurosurg Spine. 2008;9(6):554-559.
4. Pao JL, Chen WC, Chen PQ. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur Spine J. 2009;18(5):672-678.
5. Yagi M, Okada, E, Ninomiya K, Kihara M. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J Neurosurg Spine. 2009;10(4):293-299.
6. Wong AP, Smith ZA, Lall RR, Bresnahan LE, Fessler RG. The microendoscopic decompression of lumbar stenosis: a review of the current literature and clinical results. Minim Invasive Surg. 2012;2012:325095.
7. Rosen DS, O’Toole JE, Eichholz KM, et al. Minimally invasive lumbar spinal decompression in the elderly: outcomes of 50 patients aged 75 years and older. Neurosurgery. 2007;60(3):503-509.
8. Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;51(suppl 5):S146-S154.
9. Mobbs RJ, Li J, Sivabalan P, Raley D, Rao PJ. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine. 2014;21(2):179-186.
10. Avila MJ, Walter CM, Baaj AA. Outcomes and complications of minimally invasive surgery of the lumbar spine in the elderly. Cureus. 2016;8(3):e519.
11. Oertel MF, Ryang YM, Korinth MC, Gilsbach JM, Rohde V. Long-term results of microsurgical treatment of lumbar spinal stenosis by unilateral laminotomy for bilateral decompression. Neurosurgery. 2006;59(6):1264-1269.
12. Haddadi K, Ganjeh Qazvini HR. Outcome after surgery of lumbar spinal stenosis: a randomized comparison of bilateral laminotomy, trumpet laminectomy, and conventional laminectomy. Front Surg. 2016;3:199.
13. Watanabe K, Matsumoto M, Ikegami T, et al. Reduced postoperative wound pain after lumbar spinous process-splitting laminectomy for lumbar canal stenosis: a randomized controlled study. J Neurosurg Spine. 2011;14(1):51-58.
14. Skovrlj B, Belton P, Zarzour H, Qureshi SA. Perioperative outcomes in minimally invasive lumbar spine surgery: a systematic review. World J. Orthop. 2015;6(11):996-1005.
15. Hellander I. The deepening crisis in U.S. health care: a review of data. Int J Health Serv. 2011;41(3):575-586.
16. Chokshi DA. Improving health care for veterans—a watershed moment for the VA. N Engl J Med. 2014;371(4):297-299.
17. Wang MY, Cummock MD, Yu Y, Trivedi RA. An analysis of the differences in the acute hospitalization charges following minimally invasive versus open posterior lumbar interbody fusion. J Neurosurg Spine. 2010;12(6):694-699.
18. Barnett PG. Determination of VA health care costs. Med Care Res Rev. 2003;60(suppl 3):S124-S141.
19. Congressional Budget Office. The health care system for veterans: interim report. https://www.cbo.gov/sites/default/files/110th-congress-2007-2008/reports/12-21-va_healthcare.pdf. Published December 2007. Accessed October 13, 2017.
20. Purzner T, Purzner J, Massicotte EM, Bernstein M. Outpatient brain tumor surgery and spinal decompression: a prospective study of 1003 patients. Neurosurgery. 2011;69(1):119-126.
21. Waller D. How veterans’ hospitals became the best in health care. Time Magazine. http://content.time.com/time/magazine/article/0,9171,1376238,00.html. Published August 27, 2006. Accessed October 13, 2017.
22. U.S. Department of Veterans Affairs, Office of Budget. Annual budget submission—office of budget. https://www.va.gov/budget/products.asp. Updated July 12, 2017. Published October 13, 2017. Accessed October 27, 2017.
1. Guiot BH, Khoo LT, Fessler RG. A minimally invasive technique for decompression of the lumbar spine. Spine (Phila PA 1976). 2002;27(4):432-438.
2. Rahman M, Summers LE, Richter B, Mimran RI, Jacob RP. Comparison of techniques for decompressive lumbar laminectomy: the minimally invasive versus the “classic” open approach. Minim Invasive Neurosurg. 2008;51(2)100-105.
3. Sasai K, Umeda M, Maruyama T, Wakabayashi E, Iida H. Microsurgical bilateral decompression via a unilateral approach for lumbar spinal canal stenosis including degenerative spondylolisthesis. J Neurosurg Spine. 2008;9(6):554-559.
4. Pao JL, Chen WC, Chen PQ. Clinical outcomes of microendoscopic decompressive laminotomy for degenerative lumbar spinal stenosis. Eur Spine J. 2009;18(5):672-678.
5. Yagi M, Okada, E, Ninomiya K, Kihara M. Postoperative outcome after modified unilateral-approach microendoscopic midline decompression for degenerative spinal stenosis. J Neurosurg Spine. 2009;10(4):293-299.
6. Wong AP, Smith ZA, Lall RR, Bresnahan LE, Fessler RG. The microendoscopic decompression of lumbar stenosis: a review of the current literature and clinical results. Minim Invasive Surg. 2012;2012:325095.
7. Rosen DS, O’Toole JE, Eichholz KM, et al. Minimally invasive lumbar spinal decompression in the elderly: outcomes of 50 patients aged 75 years and older. Neurosurgery. 2007;60(3):503-509.
8. Khoo LT, Fessler RG. Microendoscopic decompressive laminotomy for the treatment of lumbar stenosis. Neurosurgery. 2002;51(suppl 5):S146-S154.
9. Mobbs RJ, Li J, Sivabalan P, Raley D, Rao PJ. Outcomes after decompressive laminectomy for lumbar spinal stenosis: comparison between minimally invasive unilateral laminectomy for bilateral decompression and open laminectomy: clinical article. J Neurosurg Spine. 2014;21(2):179-186.
10. Avila MJ, Walter CM, Baaj AA. Outcomes and complications of minimally invasive surgery of the lumbar spine in the elderly. Cureus. 2016;8(3):e519.
11. Oertel MF, Ryang YM, Korinth MC, Gilsbach JM, Rohde V. Long-term results of microsurgical treatment of lumbar spinal stenosis by unilateral laminotomy for bilateral decompression. Neurosurgery. 2006;59(6):1264-1269.
12. Haddadi K, Ganjeh Qazvini HR. Outcome after surgery of lumbar spinal stenosis: a randomized comparison of bilateral laminotomy, trumpet laminectomy, and conventional laminectomy. Front Surg. 2016;3:199.
13. Watanabe K, Matsumoto M, Ikegami T, et al. Reduced postoperative wound pain after lumbar spinous process-splitting laminectomy for lumbar canal stenosis: a randomized controlled study. J Neurosurg Spine. 2011;14(1):51-58.
14. Skovrlj B, Belton P, Zarzour H, Qureshi SA. Perioperative outcomes in minimally invasive lumbar spine surgery: a systematic review. World J. Orthop. 2015;6(11):996-1005.
15. Hellander I. The deepening crisis in U.S. health care: a review of data. Int J Health Serv. 2011;41(3):575-586.
16. Chokshi DA. Improving health care for veterans—a watershed moment for the VA. N Engl J Med. 2014;371(4):297-299.
17. Wang MY, Cummock MD, Yu Y, Trivedi RA. An analysis of the differences in the acute hospitalization charges following minimally invasive versus open posterior lumbar interbody fusion. J Neurosurg Spine. 2010;12(6):694-699.
18. Barnett PG. Determination of VA health care costs. Med Care Res Rev. 2003;60(suppl 3):S124-S141.
19. Congressional Budget Office. The health care system for veterans: interim report. https://www.cbo.gov/sites/default/files/110th-congress-2007-2008/reports/12-21-va_healthcare.pdf. Published December 2007. Accessed October 13, 2017.
20. Purzner T, Purzner J, Massicotte EM, Bernstein M. Outpatient brain tumor surgery and spinal decompression: a prospective study of 1003 patients. Neurosurgery. 2011;69(1):119-126.
21. Waller D. How veterans’ hospitals became the best in health care. Time Magazine. http://content.time.com/time/magazine/article/0,9171,1376238,00.html. Published August 27, 2006. Accessed October 13, 2017.
22. U.S. Department of Veterans Affairs, Office of Budget. Annual budget submission—office of budget. https://www.va.gov/budget/products.asp. Updated July 12, 2017. Published October 13, 2017. Accessed October 27, 2017.