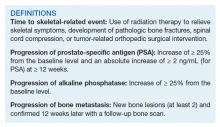
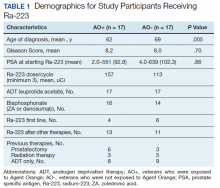
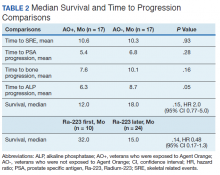
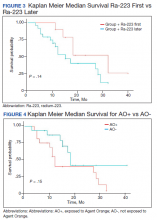
User login
Major breakthrough? Average 10% weight loss with semaglutide
In a phase 3 trial where all participants received intensive behavior therapy, investigational 2.4-mg once-weekly subcutaneous semaglutide (Novo Nordisk) resulted in a 10.3% greater average weight loss than placebo over a period of 68 weeks.
If approved, this medication could be a “potential major breakthrough” in obesity management, the investigators suggested. But other experts urged caution, as cost and uptake are important considerations.
‘Potential weight loss that patients would be happy with’
Thomas A. Wadden, PhD, presented results from the study of 611 adults with overweight or obesity but no diabetes at the virtual ObesityWeek® Interactive 2020 meeting.
“Perhaps even more impressive was the finding that 75% of patients lost 10% or more of baseline body weight,” said Dr. Wadden, of the department of psychiatry at the University of Pennsylvania, Philadelphia.
Moreover, in this trial of semaglutide, a glucagonlike peptide–1 (GLP-1) receptor agonist that is approved for treating type 2 diabetes at a weekly subcutaneous dose of 1 mg, but is being investigated at the higher dose for weight loss – 55% of patients lost ≥15% of their initial weight, and 36% lost ≥20% of their initial weight.
“These large categorical weight losses – particularly of 15% and 20% of initial weight – are potentially a major breakthrough in the management of obesity,” Dr. Wadden said in an interview.
Weight losses of this size, he added, “should confer greater improvements in cardiometabolic risk factors (such as hypertension, sleep apnea, and type 2 diabetes) as compared with losses of 5%-10% achieved with current behavioral or pharmacological approaches.” And patients are generally not satisfied with losses of less than 10% of initial weight when participating in intensive behavior programs or taking weight-loss medications.
Now, “the larger categorical weight losses will mean that a greater number of patients with obesity will be able to achieve a weight loss with which they are ... happy,” Dr. Wadden said in an interview.
According to Louis J. Aronne, MD, Weill Professor of Metabolic Research, Weill Cornell Medicine, New York, who is an investigator for another trial of semaglutide: “Even though it has the same mechanism of action [as liraglutide], the weight loss is two or more times greater [with semaglutide]. In my opinion, it’s really going to be a major advance in the treatment of obesity.”
In the discussion that followed the virtual presentation, one attendee asked about potential weight regain if a patient stopped taking the drug. Based on experience with another subcutaneous injectable GLP-1 receptor agonist, liraglutide (Saxenda), already approved for obesity, it may be that taking medicine for chronic overweight may become like taking a statin for elevated cholesterol, said Dr. Wadden.
Novo Nordisk has now completed the four trials in the STEP (Semaglutide Treatment Effect in People With Obesity) global phase 3 clinical development program, and plans to file applications with the Food and Drug Administration later this year and with the European Medicines Agency in early 2021 for review of semaglutide 2.4 mg for weight management.
“Fundamental issues need to be figured out”
Invited to comment, Scott Kahan, MD, said: “This is impressive data, confirming that semaglutide, particularly when used in concert with evidence-based counseling, is a highly effective agent for obesity management.”
However, “the real question, though, is what comes next,” stressed Dr. Kahan, director of the National Center for Weight and Wellness, Washington, DC.
“Will it be approved by the U.S. FDA? I believe so,” he said in an interview. “Yet we already have several effective obesity medications approved over the past decade – all of which are rarely used and therefore make little impact for patients in the real world.”
“Will there be insurance coverage, and therefore practical access for those who could most benefit?” he continued. “Will prescribers counsel their patients about obesity management, including the use of effective medications? Will patients utilize available options?”
“These and other fundamental issues must be figured out before we anoint any treatment option as a meaningful step forward, let alone a transformative development,” according to Dr. Kahan.
Similarly, Irl B. Hirsch, MD, stressed that, should this medication be approved for weight loss, cost would be a major factor in its uptake.
“I’m old enough to recall when we started using lovastatin in the late 1980s,” Dr. Hirsch, professor of medicine, University of Washington Medicine Diabetes Institute, Seattle, said in an interview.
“We used it without the type of evidence of statin use we have today. A pill, but in those days the statins were expensive. But over time, the evidence for statins grew and over the next 15 years it was quite clear that for both primary prevention (for those with diabetes) and secondary intervention these drugs needed to be used by millions of people. These recommendations became easier once the drugs became generic.
“Will the same thing happen for GLP-1 agonists? The problem is we need both ‘hard-outcome data’ [such as 3-point major adverse cardiovascular events] and more reasonable cost before we see this expanding to an entire population.
“In the future perhaps we could have a biosimilar GLP-1 agonist that would be more affordable than what we pay now, but even before that we need agreement from our reimbursement thought leaders that our society should reimburse these agents.
“My thinking now is the cost-benefit could be favorable, but this is all dependent on what happens to the cost of the drugs over time,” he said.
Additive effect of intensive behavior therapy plus medication
Dr. Wadden explained that intensive behavioral therapy “provides 14 or more counseling sessions in 6 months to modify diet and physical activity, through the patients’ use of behavioral strategies (such as keeping daily food and activity diaries).”
Such programs typically produce mean weight loss of 5%-8% of initial weight; less frequent (e.g., monthly) programs typically produce weight loss of only 1%-3%.
Prior studies suggest that intensive behavioral therapy and medication have additive effects. To investigate this, Dr. Wadden and colleagues randomized 611 adults (81% women) who were a mean age of 46 years and had a mean body mass index of 38 kg/m2.
All participants received 30 intensive behavior therapy sessions provided by a registered dietitian (or other qualified provider), which typically lasted 20-30 minutes and were given weekly for 12 weeks, every other week for the next 12 weeks, and then monthly.
The dietitian gave participants behavioral strategies to help them adhere to diet and physical activity goals.
During the first 8 weeks, participants were provided with a 1,000-1,200 kcal/day meal replacement diet that included liquid shakes, meal bars, and prepared entrees designed to facilitate a large initial weight loss.
They then transitioned to a diet of conventional foods (of their choosing), with a goal of 1,200-1,800 kcal/day based on body weight.
The physical activity goal was 100 minutes/week of walking or other aerobic activity in the first month, building up to 200 minutes/week by month 6.
‘More effective than current FDA-approved weight-loss medications’
At week 68, mean body weight decreased from baseline by 16.0% in the semaglutide group versus 5.7% in the placebo group (P < .0001).
In this trial, where all participants received extensive intensive behavior therapy, more participants had weight loss ≥5%, ≥10%, ≥15%, and ≥20% of their initial weight with semaglutide versus placebo (87% vs. 48%; 75% vs. 27%; 56% vs. 13%; 36% vs. 4%, respectively; all P < .0001).
From baseline to week 68, the proportion of participants with prediabetes decreased from 48% to 7% in the semaglutide group and from 53% to 26% in the placebo group.
Patients who received semaglutide had greater improvements in lipids, too.
Although the weight loss was 10.3% (10.6 kg) greater with semaglutide, Dr. Wadden noted, “additional studies have shown this net benefit to be as great as 11%-12%, which would make semaglutide 2.4 mg more effective than current [FDA-approved] weight-loss medications.”
“Naltrexone-bupropion (Contrave) with lifestyle counseling, for example,” he continued, “produces a loss that is 5 kg greater than lifestyle counseling plus placebo, liraglutide 3.0 mg (Saxenda) a loss 5.3 kg greater than placebo, and phentermine-topiramate (Qsymia) a loss that is 8.8 kg greater than placebo.”
Semaglutide was well tolerated. Gastrointestinal adverse events, the most common type, occurred in 83% of patients in the semaglutide group and 63% of patients in the placebo group.
Nausea, as well as constipation and diarrhea, are common in medications that increase GLP-1 levels, Dr. Wadden noted. Side effects can be managed by slowly increasing the medication dose over 4 months.
Dr. Wadden expects that, if approved, semaglutide 2.4 mg subcutaneous once-weekly will be recommended as an adjunct to a reduced calorie diet and increased physical activity. Additional studies suggest that monthly counseling should be sufficient to obtain similar weight losses as those seen in the current trial, which had more intensive counseling.
As well as being approved as a weekly subcutaneous injectable treatment for type 2 diabetes, semaglutide is also approved as an once-daily oral agent for the same indication (Rybelsus, Novo Nordisk) in doses of 7 mg and 14 mg to improve glycemic control along with diet and exercise. It is the first GLP-1 agonist available in tablet form.
Dr. Wadden serves on scientific advisory boards for Novo Nordisk and WW (formerly Weight Watchers), and has received grant support, on behalf of the University of Pennsylvania, from Novo Nordisk. Dr. Aronne is an investigator in a long-term trial of semaglutide and has served on scientific advisory boards for Novo Nordisk in the past. He also has other industry relationships that are not related to semaglutide.
A version of this article originally appeared on Medscape.com.
In a phase 3 trial where all participants received intensive behavior therapy, investigational 2.4-mg once-weekly subcutaneous semaglutide (Novo Nordisk) resulted in a 10.3% greater average weight loss than placebo over a period of 68 weeks.
If approved, this medication could be a “potential major breakthrough” in obesity management, the investigators suggested. But other experts urged caution, as cost and uptake are important considerations.
‘Potential weight loss that patients would be happy with’
Thomas A. Wadden, PhD, presented results from the study of 611 adults with overweight or obesity but no diabetes at the virtual ObesityWeek® Interactive 2020 meeting.
“Perhaps even more impressive was the finding that 75% of patients lost 10% or more of baseline body weight,” said Dr. Wadden, of the department of psychiatry at the University of Pennsylvania, Philadelphia.
Moreover, in this trial of semaglutide, a glucagonlike peptide–1 (GLP-1) receptor agonist that is approved for treating type 2 diabetes at a weekly subcutaneous dose of 1 mg, but is being investigated at the higher dose for weight loss – 55% of patients lost ≥15% of their initial weight, and 36% lost ≥20% of their initial weight.
“These large categorical weight losses – particularly of 15% and 20% of initial weight – are potentially a major breakthrough in the management of obesity,” Dr. Wadden said in an interview.
Weight losses of this size, he added, “should confer greater improvements in cardiometabolic risk factors (such as hypertension, sleep apnea, and type 2 diabetes) as compared with losses of 5%-10% achieved with current behavioral or pharmacological approaches.” And patients are generally not satisfied with losses of less than 10% of initial weight when participating in intensive behavior programs or taking weight-loss medications.
Now, “the larger categorical weight losses will mean that a greater number of patients with obesity will be able to achieve a weight loss with which they are ... happy,” Dr. Wadden said in an interview.
According to Louis J. Aronne, MD, Weill Professor of Metabolic Research, Weill Cornell Medicine, New York, who is an investigator for another trial of semaglutide: “Even though it has the same mechanism of action [as liraglutide], the weight loss is two or more times greater [with semaglutide]. In my opinion, it’s really going to be a major advance in the treatment of obesity.”
In the discussion that followed the virtual presentation, one attendee asked about potential weight regain if a patient stopped taking the drug. Based on experience with another subcutaneous injectable GLP-1 receptor agonist, liraglutide (Saxenda), already approved for obesity, it may be that taking medicine for chronic overweight may become like taking a statin for elevated cholesterol, said Dr. Wadden.
Novo Nordisk has now completed the four trials in the STEP (Semaglutide Treatment Effect in People With Obesity) global phase 3 clinical development program, and plans to file applications with the Food and Drug Administration later this year and with the European Medicines Agency in early 2021 for review of semaglutide 2.4 mg for weight management.
“Fundamental issues need to be figured out”
Invited to comment, Scott Kahan, MD, said: “This is impressive data, confirming that semaglutide, particularly when used in concert with evidence-based counseling, is a highly effective agent for obesity management.”
However, “the real question, though, is what comes next,” stressed Dr. Kahan, director of the National Center for Weight and Wellness, Washington, DC.
“Will it be approved by the U.S. FDA? I believe so,” he said in an interview. “Yet we already have several effective obesity medications approved over the past decade – all of which are rarely used and therefore make little impact for patients in the real world.”
“Will there be insurance coverage, and therefore practical access for those who could most benefit?” he continued. “Will prescribers counsel their patients about obesity management, including the use of effective medications? Will patients utilize available options?”
“These and other fundamental issues must be figured out before we anoint any treatment option as a meaningful step forward, let alone a transformative development,” according to Dr. Kahan.
Similarly, Irl B. Hirsch, MD, stressed that, should this medication be approved for weight loss, cost would be a major factor in its uptake.
“I’m old enough to recall when we started using lovastatin in the late 1980s,” Dr. Hirsch, professor of medicine, University of Washington Medicine Diabetes Institute, Seattle, said in an interview.
“We used it without the type of evidence of statin use we have today. A pill, but in those days the statins were expensive. But over time, the evidence for statins grew and over the next 15 years it was quite clear that for both primary prevention (for those with diabetes) and secondary intervention these drugs needed to be used by millions of people. These recommendations became easier once the drugs became generic.
“Will the same thing happen for GLP-1 agonists? The problem is we need both ‘hard-outcome data’ [such as 3-point major adverse cardiovascular events] and more reasonable cost before we see this expanding to an entire population.
“In the future perhaps we could have a biosimilar GLP-1 agonist that would be more affordable than what we pay now, but even before that we need agreement from our reimbursement thought leaders that our society should reimburse these agents.
“My thinking now is the cost-benefit could be favorable, but this is all dependent on what happens to the cost of the drugs over time,” he said.
Additive effect of intensive behavior therapy plus medication
Dr. Wadden explained that intensive behavioral therapy “provides 14 or more counseling sessions in 6 months to modify diet and physical activity, through the patients’ use of behavioral strategies (such as keeping daily food and activity diaries).”
Such programs typically produce mean weight loss of 5%-8% of initial weight; less frequent (e.g., monthly) programs typically produce weight loss of only 1%-3%.
Prior studies suggest that intensive behavioral therapy and medication have additive effects. To investigate this, Dr. Wadden and colleagues randomized 611 adults (81% women) who were a mean age of 46 years and had a mean body mass index of 38 kg/m2.
All participants received 30 intensive behavior therapy sessions provided by a registered dietitian (or other qualified provider), which typically lasted 20-30 minutes and were given weekly for 12 weeks, every other week for the next 12 weeks, and then monthly.
The dietitian gave participants behavioral strategies to help them adhere to diet and physical activity goals.
During the first 8 weeks, participants were provided with a 1,000-1,200 kcal/day meal replacement diet that included liquid shakes, meal bars, and prepared entrees designed to facilitate a large initial weight loss.
They then transitioned to a diet of conventional foods (of their choosing), with a goal of 1,200-1,800 kcal/day based on body weight.
The physical activity goal was 100 minutes/week of walking or other aerobic activity in the first month, building up to 200 minutes/week by month 6.
‘More effective than current FDA-approved weight-loss medications’
At week 68, mean body weight decreased from baseline by 16.0% in the semaglutide group versus 5.7% in the placebo group (P < .0001).
In this trial, where all participants received extensive intensive behavior therapy, more participants had weight loss ≥5%, ≥10%, ≥15%, and ≥20% of their initial weight with semaglutide versus placebo (87% vs. 48%; 75% vs. 27%; 56% vs. 13%; 36% vs. 4%, respectively; all P < .0001).
From baseline to week 68, the proportion of participants with prediabetes decreased from 48% to 7% in the semaglutide group and from 53% to 26% in the placebo group.
Patients who received semaglutide had greater improvements in lipids, too.
Although the weight loss was 10.3% (10.6 kg) greater with semaglutide, Dr. Wadden noted, “additional studies have shown this net benefit to be as great as 11%-12%, which would make semaglutide 2.4 mg more effective than current [FDA-approved] weight-loss medications.”
“Naltrexone-bupropion (Contrave) with lifestyle counseling, for example,” he continued, “produces a loss that is 5 kg greater than lifestyle counseling plus placebo, liraglutide 3.0 mg (Saxenda) a loss 5.3 kg greater than placebo, and phentermine-topiramate (Qsymia) a loss that is 8.8 kg greater than placebo.”
Semaglutide was well tolerated. Gastrointestinal adverse events, the most common type, occurred in 83% of patients in the semaglutide group and 63% of patients in the placebo group.
Nausea, as well as constipation and diarrhea, are common in medications that increase GLP-1 levels, Dr. Wadden noted. Side effects can be managed by slowly increasing the medication dose over 4 months.
Dr. Wadden expects that, if approved, semaglutide 2.4 mg subcutaneous once-weekly will be recommended as an adjunct to a reduced calorie diet and increased physical activity. Additional studies suggest that monthly counseling should be sufficient to obtain similar weight losses as those seen in the current trial, which had more intensive counseling.
As well as being approved as a weekly subcutaneous injectable treatment for type 2 diabetes, semaglutide is also approved as an once-daily oral agent for the same indication (Rybelsus, Novo Nordisk) in doses of 7 mg and 14 mg to improve glycemic control along with diet and exercise. It is the first GLP-1 agonist available in tablet form.
Dr. Wadden serves on scientific advisory boards for Novo Nordisk and WW (formerly Weight Watchers), and has received grant support, on behalf of the University of Pennsylvania, from Novo Nordisk. Dr. Aronne is an investigator in a long-term trial of semaglutide and has served on scientific advisory boards for Novo Nordisk in the past. He also has other industry relationships that are not related to semaglutide.
A version of this article originally appeared on Medscape.com.
In a phase 3 trial where all participants received intensive behavior therapy, investigational 2.4-mg once-weekly subcutaneous semaglutide (Novo Nordisk) resulted in a 10.3% greater average weight loss than placebo over a period of 68 weeks.
If approved, this medication could be a “potential major breakthrough” in obesity management, the investigators suggested. But other experts urged caution, as cost and uptake are important considerations.
‘Potential weight loss that patients would be happy with’
Thomas A. Wadden, PhD, presented results from the study of 611 adults with overweight or obesity but no diabetes at the virtual ObesityWeek® Interactive 2020 meeting.
“Perhaps even more impressive was the finding that 75% of patients lost 10% or more of baseline body weight,” said Dr. Wadden, of the department of psychiatry at the University of Pennsylvania, Philadelphia.
Moreover, in this trial of semaglutide, a glucagonlike peptide–1 (GLP-1) receptor agonist that is approved for treating type 2 diabetes at a weekly subcutaneous dose of 1 mg, but is being investigated at the higher dose for weight loss – 55% of patients lost ≥15% of their initial weight, and 36% lost ≥20% of their initial weight.
“These large categorical weight losses – particularly of 15% and 20% of initial weight – are potentially a major breakthrough in the management of obesity,” Dr. Wadden said in an interview.
Weight losses of this size, he added, “should confer greater improvements in cardiometabolic risk factors (such as hypertension, sleep apnea, and type 2 diabetes) as compared with losses of 5%-10% achieved with current behavioral or pharmacological approaches.” And patients are generally not satisfied with losses of less than 10% of initial weight when participating in intensive behavior programs or taking weight-loss medications.
Now, “the larger categorical weight losses will mean that a greater number of patients with obesity will be able to achieve a weight loss with which they are ... happy,” Dr. Wadden said in an interview.
According to Louis J. Aronne, MD, Weill Professor of Metabolic Research, Weill Cornell Medicine, New York, who is an investigator for another trial of semaglutide: “Even though it has the same mechanism of action [as liraglutide], the weight loss is two or more times greater [with semaglutide]. In my opinion, it’s really going to be a major advance in the treatment of obesity.”
In the discussion that followed the virtual presentation, one attendee asked about potential weight regain if a patient stopped taking the drug. Based on experience with another subcutaneous injectable GLP-1 receptor agonist, liraglutide (Saxenda), already approved for obesity, it may be that taking medicine for chronic overweight may become like taking a statin for elevated cholesterol, said Dr. Wadden.
Novo Nordisk has now completed the four trials in the STEP (Semaglutide Treatment Effect in People With Obesity) global phase 3 clinical development program, and plans to file applications with the Food and Drug Administration later this year and with the European Medicines Agency in early 2021 for review of semaglutide 2.4 mg for weight management.
“Fundamental issues need to be figured out”
Invited to comment, Scott Kahan, MD, said: “This is impressive data, confirming that semaglutide, particularly when used in concert with evidence-based counseling, is a highly effective agent for obesity management.”
However, “the real question, though, is what comes next,” stressed Dr. Kahan, director of the National Center for Weight and Wellness, Washington, DC.
“Will it be approved by the U.S. FDA? I believe so,” he said in an interview. “Yet we already have several effective obesity medications approved over the past decade – all of which are rarely used and therefore make little impact for patients in the real world.”
“Will there be insurance coverage, and therefore practical access for those who could most benefit?” he continued. “Will prescribers counsel their patients about obesity management, including the use of effective medications? Will patients utilize available options?”
“These and other fundamental issues must be figured out before we anoint any treatment option as a meaningful step forward, let alone a transformative development,” according to Dr. Kahan.
Similarly, Irl B. Hirsch, MD, stressed that, should this medication be approved for weight loss, cost would be a major factor in its uptake.
“I’m old enough to recall when we started using lovastatin in the late 1980s,” Dr. Hirsch, professor of medicine, University of Washington Medicine Diabetes Institute, Seattle, said in an interview.
“We used it without the type of evidence of statin use we have today. A pill, but in those days the statins were expensive. But over time, the evidence for statins grew and over the next 15 years it was quite clear that for both primary prevention (for those with diabetes) and secondary intervention these drugs needed to be used by millions of people. These recommendations became easier once the drugs became generic.
“Will the same thing happen for GLP-1 agonists? The problem is we need both ‘hard-outcome data’ [such as 3-point major adverse cardiovascular events] and more reasonable cost before we see this expanding to an entire population.
“In the future perhaps we could have a biosimilar GLP-1 agonist that would be more affordable than what we pay now, but even before that we need agreement from our reimbursement thought leaders that our society should reimburse these agents.
“My thinking now is the cost-benefit could be favorable, but this is all dependent on what happens to the cost of the drugs over time,” he said.
Additive effect of intensive behavior therapy plus medication
Dr. Wadden explained that intensive behavioral therapy “provides 14 or more counseling sessions in 6 months to modify diet and physical activity, through the patients’ use of behavioral strategies (such as keeping daily food and activity diaries).”
Such programs typically produce mean weight loss of 5%-8% of initial weight; less frequent (e.g., monthly) programs typically produce weight loss of only 1%-3%.
Prior studies suggest that intensive behavioral therapy and medication have additive effects. To investigate this, Dr. Wadden and colleagues randomized 611 adults (81% women) who were a mean age of 46 years and had a mean body mass index of 38 kg/m2.
All participants received 30 intensive behavior therapy sessions provided by a registered dietitian (or other qualified provider), which typically lasted 20-30 minutes and were given weekly for 12 weeks, every other week for the next 12 weeks, and then monthly.
The dietitian gave participants behavioral strategies to help them adhere to diet and physical activity goals.
During the first 8 weeks, participants were provided with a 1,000-1,200 kcal/day meal replacement diet that included liquid shakes, meal bars, and prepared entrees designed to facilitate a large initial weight loss.
They then transitioned to a diet of conventional foods (of their choosing), with a goal of 1,200-1,800 kcal/day based on body weight.
The physical activity goal was 100 minutes/week of walking or other aerobic activity in the first month, building up to 200 minutes/week by month 6.
‘More effective than current FDA-approved weight-loss medications’
At week 68, mean body weight decreased from baseline by 16.0% in the semaglutide group versus 5.7% in the placebo group (P < .0001).
In this trial, where all participants received extensive intensive behavior therapy, more participants had weight loss ≥5%, ≥10%, ≥15%, and ≥20% of their initial weight with semaglutide versus placebo (87% vs. 48%; 75% vs. 27%; 56% vs. 13%; 36% vs. 4%, respectively; all P < .0001).
From baseline to week 68, the proportion of participants with prediabetes decreased from 48% to 7% in the semaglutide group and from 53% to 26% in the placebo group.
Patients who received semaglutide had greater improvements in lipids, too.
Although the weight loss was 10.3% (10.6 kg) greater with semaglutide, Dr. Wadden noted, “additional studies have shown this net benefit to be as great as 11%-12%, which would make semaglutide 2.4 mg more effective than current [FDA-approved] weight-loss medications.”
“Naltrexone-bupropion (Contrave) with lifestyle counseling, for example,” he continued, “produces a loss that is 5 kg greater than lifestyle counseling plus placebo, liraglutide 3.0 mg (Saxenda) a loss 5.3 kg greater than placebo, and phentermine-topiramate (Qsymia) a loss that is 8.8 kg greater than placebo.”
Semaglutide was well tolerated. Gastrointestinal adverse events, the most common type, occurred in 83% of patients in the semaglutide group and 63% of patients in the placebo group.
Nausea, as well as constipation and diarrhea, are common in medications that increase GLP-1 levels, Dr. Wadden noted. Side effects can be managed by slowly increasing the medication dose over 4 months.
Dr. Wadden expects that, if approved, semaglutide 2.4 mg subcutaneous once-weekly will be recommended as an adjunct to a reduced calorie diet and increased physical activity. Additional studies suggest that monthly counseling should be sufficient to obtain similar weight losses as those seen in the current trial, which had more intensive counseling.
As well as being approved as a weekly subcutaneous injectable treatment for type 2 diabetes, semaglutide is also approved as an once-daily oral agent for the same indication (Rybelsus, Novo Nordisk) in doses of 7 mg and 14 mg to improve glycemic control along with diet and exercise. It is the first GLP-1 agonist available in tablet form.
Dr. Wadden serves on scientific advisory boards for Novo Nordisk and WW (formerly Weight Watchers), and has received grant support, on behalf of the University of Pennsylvania, from Novo Nordisk. Dr. Aronne is an investigator in a long-term trial of semaglutide and has served on scientific advisory boards for Novo Nordisk in the past. He also has other industry relationships that are not related to semaglutide.
A version of this article originally appeared on Medscape.com.
FDA grants emergency use authorization to Lilly’s antibody COVID-19 therapy
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
The monoclonal antibody therapy has emergency authorization for treating patients who have tested positive for SARS-CoV-2 infection and who are considered to be at high risk for progression to severe COVID-19 or hospitalization. To be eligible for treatment with bamlanivimab, patients must be at least 12 years of age and weigh at least 40 kg (approximately 88 lb). The agency notes that this includes patients aged 65 years and older or people with certain chronic conditions.
Bamlanivimab is not authorized for use in patients who are hospitalized or who require oxygen therapy because of COVID-19. The FDA’s action comes less than 2 weeks after Eli Lilly halted the ACTIV-3 study of the therapy for severe, hospitalized COVID-19 patients after evidence showed that adding the antibody therapy to standard care did not improve outcomes over standard care alone for patients with advanced COVID-19.
The government contract with Eli Lilly involves the purchase of 300,000 doses through December, with the option to procure another 650,000 doses through June 2021.
Because of Operation Warp Speed, “we have supplies to distribute now. Product distribution will begin this week,” US Health & Human Services (HHS) Secretary Alex Azar said at a news conference today.
“We talked about building the bridge to safe and effective vaccines” for COVID-19, Azar added. “With this therapeutic, the bridge is taking shape.”
Bamlanivimab 700 mg will be administered as a 1-hour infusion followed by a 1-hour observation period for detecting any infusion-related side effects. The authorized dose is 700 mg, which was on the lower end of the dose range evaluated in studies.
During the press conference, a reporter asked whether the lower dose was chosen in order that more doses of the antibody could be made available. “The lower dose is a rational choice in this situation because we don’t want to give more of a drug than you need,” said Janet Woodcock, MD, the therapeutics lead for Operation Warp Speed. “I think we could probably go lower.”
Bamlanivimab works by attaching to the virus and blocking its entry into the cells and possibly by helping the patients’ immune system clear the virus, said Woodcock, who is also director of the FDA’s Center for Drug Evaluation and Research.
“The goal is to treat high-risk people as soon as possible after they show symptoms and are diagnosed,” she added.
Infusions an initial challenge?
There could be some logistic challenges at first because the antibody is administered via infusion. “We expect there will initially be a challenge in administering ... these infusions and setting up infusion centers,” Woodcock said.
Outpatient intravenous infusions are normally performed at infusion centers for patients with cancer and immune disorders, she noted. “You really don’t want them mixing with people who have COVID-19 disease, so we will need to set up separate sites.”
Bamlanivimab will be provided free of cost to patients, Azar said. Patients should be aware that coinsurance may be required for the infusion.
“Fair and equitable” distribution planned
During phase 1 of distribution, the agent will first be allocated to hospitals and hospital-affiliated locations only, John Redd, MD, MPH, chief medical officer, Office of the Assistant Secretary for Preparedness and Response at HHS, said at the press conference.
During phase 2, “there will be expanded distribution to outpatient sites,” he said. In an effort to keep the process transparent, a new website features the latest updates on the distribution of bamlanivimab.
Allocation will be based on two factors: the number of new cases reported in a state or territory in the prior 7 days, and rates of COVID-19 hospitalization during the same period.
Asked why the government would determine distribution of the antibody on the basis of the number of hospitalized patients when the indication includes prevention of admission, Woodcock replied that hospitalization is a surrogate measure that can reflect risk factors in a particular state population, such as obesity, diabetes, or the proportion of older people.
Furthermore, the confirmed cases are a “leading indicator,” she said, that can help identify a steep rise in COVID-19 cases that could indicate more hospitalizations are likely soon. “We don’t want to miss that.”
Data underlying the EUA decision
A decrease in hospitalizations or emergency department visits within 28 days of treatment in preclinical studies was “the most important evidence that bamlanivimab may be effective,” the agency noted in the press release announcing the EUA. Among patients at high risk for progression, 3% required such interventions, compared with 10% of placebo-treated patients.
Potential side effects of bamlanivimab include anaphylaxis, infusion-related reactions, nausea, diarrhea, dizziness, headache, itching, and vomiting.
“As illustrated by today’s action, the FDA remains committed to expediting the development and availability of potential COVID-19 treatments and providing sick patients timely access to new therapies where appropriate,” FDA Commissioner Stephen M. Hahn, MD, said in the news release.
Healthcare providers can download a detailed FDA fact sheet on the EUA for bamlanivimab, which includes dosing instructions.
This article first appeared on Medscape.com.
Continued Dosing of Oritavancin for Complicated Gram-Positive Infections
Oritavancin is a lipoglycopeptide antibiotic. The US Food and Drug Administration (FDA) approved oritavancin in 2014 for adults with acute bacterial skin and skin structure infections (ABSSSI).1 The antibiotic is currently FDA approved for infections caused by Gram-positive organisms, including methicillin-resistant and methicillinsusceptible Staphylococcus aureus (MRSA, MSSA), a variety of Streptococcus species, and vancomycin-susceptible Enterococcus faecalis (VSE). Oritavancin demonstrates concentrationdependent bactericidal activity and has a half-life of 245 hours. This half-life allows for treatment of ABSSSI with a single 1,200 mg IV dose, which has been shown to be noninferior to vancomycin dosed twice daily for 7 to 10 days.1-3
Proposal for Expanded Uses
Although the approved indication for oritavancin is narrow, in vitro studies have shown that oritavancin also has activity against vancomycin-resistant enterococci (VRE), and rabbit studies have demonstrated its excellent bone penetration.4,5 These findings have raised the question of whether oritavancin can be safely and effectively used for infections such as endocarditis, osteomyelitis, and bacteremia, which are often caused by invasive Grampositive organisms. These types of invasive infections, particularly when MRSA is implicated, generally require IV antibiotic therapy for several weeks, often with vancomycin.6
To avoid long hospital stays solely for antibiotic administration, health care practitioners will often use outpatient parenteral antimicrobial therapy (OPAT). However, using OPAT presents many challenges due to the need for frequent dosing, the risk of peripheral or central-line infections, and therapeutic drug monitoring when using vancomycin; additionally, administration and line care oftentimes require caregiver support, which may not be present for all patients.7 Concerns also have been raised regarding the use of OPAT in patients with a history of IV drug use due to the potential increased risk of line infections or line abuse. Few studies have explored OPAT in this population, and the Infectious Diseases Society of America OPAT guidelines recommend that the decision to use OPAT should be made on a case-by-case basis.7 Thus, patients who are deemed inappropriate for OPAT oftentimes remain hospitalized or reside briefly in nursing facilities solely for antibiotic administration
Oritavancin’s long half-life and potent activity against Gram-positive organisms has led to increased interest in off-label use of infrequent dosing intervals, such as weekly, to treat complicated and invasive infections. Weekly rather than daily dosing would allow for less burdensome antibiotic administration regimens and shorter hospital stays especially for patients who are not candidates for OPAT.
Efficacy of Continued Dosing
This proposed weekly dosing pattern, referred to as continued dosing or a multiple-dose regimen, has gained traction in the literature. To date, no randomized controlled trials have been conducted to assess oritavancin’s efficacy in off-label indications or continued dosing, but several case reports and retrospective cohort analyses show promising outcomes.8-16 In an analysis of data from the Clinical and Historic Registry and Orbactiv Medical Evaluation (CHROME) patient registry, 32 patients received multiple doses of oritavancin for complicated Gram-positive infections with a 93.8% overall clinical success rate, including success rates of 90.9% (10/11) for general bone and joint infections and 87.5% (7/8) for patients diagnosed specifically with osteomyelitis.8
Patients received between 2 and 10 doses of 1,200 mg IV given every 6 to 14 days. Johnson and colleagues report using oritavancin 1,200 mg IV every other day for 3 doses followed by 1,200 mg IV once weekly for a patient with daptomycin- and vancomycin-resistant Enterococcus endocarditis, resulting in negative blood cultures while on therapy.9 However, source control via valve replacement and postoperative oritavancin 1,200 mg IV twice weekly for 10 weeks was required to fully clear the infection.
Schulz and colleagues published a retrospective cohort analysis of 17 patients who received multiple doses of oritavancin for complicated bacterial infections, including osteomyelitis, pneumonia, and bacteremia.10 They reported 100% of patients were either successfully cured or had demonstrable improvements in their infections by using a 1,200 mg IV loading dose followed by 800 mg IV if the second dose was given within 7 days or 1,200 mg IV if the second dose was given more than 10 days later. Patients received between 2 and 18 total doses, with 6 out of 17 (35%) receiving only 2 doses. One patient who received 18 doses was an outlier, as her treatment goal was palliative suppression due to an infected endovascular graft that could not be removed.
In a published case series, 1 of 10 patients receiving oritavancin for invasive Grampositive infections received multiple doses of oritavancin for an MSSA deep tissue infection.11 The 3 total doses (strength not reported) were separated by 19 days and 14 days and resulted in cure. Several case reports and a retrospective chart review study specifically show the effectiveness of oritavancin for osteomyelitis caused by MSSA, MRSA, and VRE.12-16 However, dosing strategies varied widely after the initial 1,200 mg IV loading dose.
Drug Interactions, Safety, and Tolerability
Oritavancin has minimal drug-drug interactions, the most notable being with anticoagulants. 1 Use of IV heparin within 120 hours of oritavancin administration can falsely elevate activated partial thromboplastin time (aPTT) levels; therefore, heparin should not be monitored with aPTT during this period. Oritavancin also can artificially prolong international normalized ratio (INR) values for up to 12 hours, and dose adjustments based on INRs during this window are not recommended. Of note, factor Xa laboratory monitoring is unaffected by oritavancin, as it does not depend on phospholipid reagents as do aPTT and INR measurements.
Oritavancin has been shown to be well tolerated when dosed according to both the package insert and continued dosing strategies. The most common adverse effects (AEs) (≥ 3%), occurring at similar rates to vancomycin, are nausea, vomiting, diarrhea, headache, and limb and subcutaneous abscesses.1 Infusion reactions also have been reported, although they are usually reversible on slowing or stopping the infusion. It is worth noting that the use of oritavancin for osteomyelitis is not recommended in the product labeling, as an increased rate of osteomyelitis was observed in the oritavancin vs IV vancomycin groups for the treatment of patients with acute bacterial skin and skin structure infection (SOLO) trials (0.6% in oritavancin group vs 0.1% in vancomycin group, statistical significance not reported).17 However, it was postulated that these osteomyelitis cases were likely present, yet not recognized, at baseline and were not the result of administering oritavancin. This conclusion is further corroborated by previously presented research demonstrating successful cure of osteomyelitis with continued dosing strategies.12-16
Many patients receiving multiple doses of oritavancin did not experience AEs or laboratory abnormalities.13,15 Four of 17 patients (24%) in one retrospective review experienced AEs, including infusion reactions, anemia, and leukopenia; all were reversible on discontinuation of oritavancin, and contributions of other antibiotics in some cases could not be ruled out.10 One patient experienced taste disturbance for several hours after each infusion, and a second had documented hearing loss after 3 doses of oritavancin in a 33-day period, though she had received 6 weeks of IV vancomycin prior to oritavancin.11,12 A patient treated for daptomycin- and vancomycinresistant Enterococcus faecium prosthetic valve endocarditis experienced nausea, anorexia, and minor liver function test (LFT) abnormalities after cumulative oritavancin exposure over 18 weeks.9 On discontinuation of the drug, nausea and anorexia improved, and LFTs normalized 11 months later. Overall, AEs reported with continued dosing of oritavancin have been minimal and largely reversible, mimicking the AEs in the product labeling for traditional dosing. This suggests that using a continued dosing strategy may not result in worse or more frequent AEs, though randomized controlled trials are needed to fully ascertain these preliminary findings.
Conclusions
The literature supporting the use of oritavancin beyond single-dose administration for ABSSSI is growing. Continued dosing regimens have been well tolerated and have resulted in clinical cure for many patients with barriers to first-line treatment and complicated or invasive infections. While randomized controlled trials are needed to concretely demonstrate the efficacy and safety of continued dosing of oritavancin, it may fill an important treatment niche in this era of growing antibiotic resistance and increasing complexity of patient cases.
1. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2019.
2. Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370(23):2180-2190. doi:10.1056/NEJMoa1310422
3. Corey GR, Good S, Jiang H, et al. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60(2):254-262. doi:10.1093/cid/ciu778
4. Sweeney D, Stoneburner A, Shinabarger DL, et al. Comparative in vitro activity of oritavancin and other agents against vancomycin-susceptible and -resistant enterococci. J Antimicrob Chemother. 2017;72(2):622-624. doi.10.1093/jac/dkw451
5. Lehoux D, Ostiguy V, Vadieux C, et al. Oritavancin pharmacokinetics and bone penetration in rabbits. Antimicrob Agents Chemother. 2015;59(10):6501-6505. doi:10.1128/AAC.00981-15
6. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55. doi:10.1093/cid/ciq146
7. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):e1-e35. doi:10.1093/cid/ciy745
8. Redell M, Seirra-Hoffman M, Assi Maha, et al. The CHROME study, a real-world experience of single- and multiple-dose oritavancin for treatment of gram-positive infections. Open Forum Infect Dis. 2019;6(11):ofz479. doi:10.1093/ofid/ofz479
9. Johnson JA, Feeney ER, Kubiak DW, Corey GR. Prolonged use of oritavancin for vancomycin-resistant Enterococcus faecium prosthetic valve endocarditis. Open Forum Infect Dis. 2015;2(4):ofv156. doi:10.1093/ofid/ofv156
10. Schulz LT, Dworkin E, Dela-Pena J, Rose WE. Multipledose oritavancin evaluation in a retrospective cohort of patients with complicated infections. Pharmacotherapy. 2018;38(1):152-159. doi:10.1002/phar.2057
11. Stewart CL, Turner MS, Frens JJ, Snider CB, Smith JR. Real-world experience with oritavancin therapy in invasive gram-positive infections. Infect Dis Ther. 2017;6(2):277-289. doi:10.1007/s40121-017-0156-z
12. Delaportas DJ, Estrada SJ, Darmelio M. Successful treatment of methicillin susceptible Staphylococcus aureus osteomyelitis with oritavancin. Pharmacotherapy. 2017;37(8):e90-e92. doi:10.1002/phar.1957
13. Chastain DB, Davis A. Treatment of chronic osteomyelitis with multidose oritavancin: a case series and literature review. Int J Antimicrob Agents. 2019;53(4):429-434. doi:10.1016/j.ijantimicag.2018.11.023
14. Dahesh S, Wong B, Nizet V, Sakoulas G, Tran TT, Aitken SL. Treatment of multidrug-resistant vancomycinresistant Enterococcus faecium hardware-associated vertebral osteomyelitis with oritavancin plus ampicillin. Antimicrob Agents Chemother. 2019;63(7):e02622-18. doi:10.1128/AAC.02622-18
15. Foster RA, Philavong KP, Weissman S, Tang X, Bookstaver PB. Oritavancin for the treatment of daptomycin nonsusceptible vancomycin-resistant Enterococci osteomyelitis. Infect Dis Clin Pract. 2018;26(2):97-99. doi:10.1097/IPC.0000000000000517
16. Ruggero M, Ziegler M, Tebas P, Binkley A, Kelly B. Successful treatment of methicillin-resistant Staphylococcus aureus vertebral osteomyelitis with outpatient oritavancin therapy. Infect Dis Clin Pract. 2018;26(3):141-144. doi:10.1097/IPC.0000000000000599
17. Corey GR, Loutit J, Moeck G, et al. Single intravenous dose of oritavancin for treatment of acute skin and skin structure infections caused by gram-positive bacteria: summary of safety analysis from the phase 3 SOLO studies. Antimicrob Agents Chemother. 2018;62(4):e01919- 17. doi:10.1128/AAC.01919-17
Oritavancin is a lipoglycopeptide antibiotic. The US Food and Drug Administration (FDA) approved oritavancin in 2014 for adults with acute bacterial skin and skin structure infections (ABSSSI).1 The antibiotic is currently FDA approved for infections caused by Gram-positive organisms, including methicillin-resistant and methicillinsusceptible Staphylococcus aureus (MRSA, MSSA), a variety of Streptococcus species, and vancomycin-susceptible Enterococcus faecalis (VSE). Oritavancin demonstrates concentrationdependent bactericidal activity and has a half-life of 245 hours. This half-life allows for treatment of ABSSSI with a single 1,200 mg IV dose, which has been shown to be noninferior to vancomycin dosed twice daily for 7 to 10 days.1-3
Proposal for Expanded Uses
Although the approved indication for oritavancin is narrow, in vitro studies have shown that oritavancin also has activity against vancomycin-resistant enterococci (VRE), and rabbit studies have demonstrated its excellent bone penetration.4,5 These findings have raised the question of whether oritavancin can be safely and effectively used for infections such as endocarditis, osteomyelitis, and bacteremia, which are often caused by invasive Grampositive organisms. These types of invasive infections, particularly when MRSA is implicated, generally require IV antibiotic therapy for several weeks, often with vancomycin.6
To avoid long hospital stays solely for antibiotic administration, health care practitioners will often use outpatient parenteral antimicrobial therapy (OPAT). However, using OPAT presents many challenges due to the need for frequent dosing, the risk of peripheral or central-line infections, and therapeutic drug monitoring when using vancomycin; additionally, administration and line care oftentimes require caregiver support, which may not be present for all patients.7 Concerns also have been raised regarding the use of OPAT in patients with a history of IV drug use due to the potential increased risk of line infections or line abuse. Few studies have explored OPAT in this population, and the Infectious Diseases Society of America OPAT guidelines recommend that the decision to use OPAT should be made on a case-by-case basis.7 Thus, patients who are deemed inappropriate for OPAT oftentimes remain hospitalized or reside briefly in nursing facilities solely for antibiotic administration
Oritavancin’s long half-life and potent activity against Gram-positive organisms has led to increased interest in off-label use of infrequent dosing intervals, such as weekly, to treat complicated and invasive infections. Weekly rather than daily dosing would allow for less burdensome antibiotic administration regimens and shorter hospital stays especially for patients who are not candidates for OPAT.
Efficacy of Continued Dosing
This proposed weekly dosing pattern, referred to as continued dosing or a multiple-dose regimen, has gained traction in the literature. To date, no randomized controlled trials have been conducted to assess oritavancin’s efficacy in off-label indications or continued dosing, but several case reports and retrospective cohort analyses show promising outcomes.8-16 In an analysis of data from the Clinical and Historic Registry and Orbactiv Medical Evaluation (CHROME) patient registry, 32 patients received multiple doses of oritavancin for complicated Gram-positive infections with a 93.8% overall clinical success rate, including success rates of 90.9% (10/11) for general bone and joint infections and 87.5% (7/8) for patients diagnosed specifically with osteomyelitis.8
Patients received between 2 and 10 doses of 1,200 mg IV given every 6 to 14 days. Johnson and colleagues report using oritavancin 1,200 mg IV every other day for 3 doses followed by 1,200 mg IV once weekly for a patient with daptomycin- and vancomycin-resistant Enterococcus endocarditis, resulting in negative blood cultures while on therapy.9 However, source control via valve replacement and postoperative oritavancin 1,200 mg IV twice weekly for 10 weeks was required to fully clear the infection.
Schulz and colleagues published a retrospective cohort analysis of 17 patients who received multiple doses of oritavancin for complicated bacterial infections, including osteomyelitis, pneumonia, and bacteremia.10 They reported 100% of patients were either successfully cured or had demonstrable improvements in their infections by using a 1,200 mg IV loading dose followed by 800 mg IV if the second dose was given within 7 days or 1,200 mg IV if the second dose was given more than 10 days later. Patients received between 2 and 18 total doses, with 6 out of 17 (35%) receiving only 2 doses. One patient who received 18 doses was an outlier, as her treatment goal was palliative suppression due to an infected endovascular graft that could not be removed.
In a published case series, 1 of 10 patients receiving oritavancin for invasive Grampositive infections received multiple doses of oritavancin for an MSSA deep tissue infection.11 The 3 total doses (strength not reported) were separated by 19 days and 14 days and resulted in cure. Several case reports and a retrospective chart review study specifically show the effectiveness of oritavancin for osteomyelitis caused by MSSA, MRSA, and VRE.12-16 However, dosing strategies varied widely after the initial 1,200 mg IV loading dose.
Drug Interactions, Safety, and Tolerability
Oritavancin has minimal drug-drug interactions, the most notable being with anticoagulants. 1 Use of IV heparin within 120 hours of oritavancin administration can falsely elevate activated partial thromboplastin time (aPTT) levels; therefore, heparin should not be monitored with aPTT during this period. Oritavancin also can artificially prolong international normalized ratio (INR) values for up to 12 hours, and dose adjustments based on INRs during this window are not recommended. Of note, factor Xa laboratory monitoring is unaffected by oritavancin, as it does not depend on phospholipid reagents as do aPTT and INR measurements.
Oritavancin has been shown to be well tolerated when dosed according to both the package insert and continued dosing strategies. The most common adverse effects (AEs) (≥ 3%), occurring at similar rates to vancomycin, are nausea, vomiting, diarrhea, headache, and limb and subcutaneous abscesses.1 Infusion reactions also have been reported, although they are usually reversible on slowing or stopping the infusion. It is worth noting that the use of oritavancin for osteomyelitis is not recommended in the product labeling, as an increased rate of osteomyelitis was observed in the oritavancin vs IV vancomycin groups for the treatment of patients with acute bacterial skin and skin structure infection (SOLO) trials (0.6% in oritavancin group vs 0.1% in vancomycin group, statistical significance not reported).17 However, it was postulated that these osteomyelitis cases were likely present, yet not recognized, at baseline and were not the result of administering oritavancin. This conclusion is further corroborated by previously presented research demonstrating successful cure of osteomyelitis with continued dosing strategies.12-16
Many patients receiving multiple doses of oritavancin did not experience AEs or laboratory abnormalities.13,15 Four of 17 patients (24%) in one retrospective review experienced AEs, including infusion reactions, anemia, and leukopenia; all were reversible on discontinuation of oritavancin, and contributions of other antibiotics in some cases could not be ruled out.10 One patient experienced taste disturbance for several hours after each infusion, and a second had documented hearing loss after 3 doses of oritavancin in a 33-day period, though she had received 6 weeks of IV vancomycin prior to oritavancin.11,12 A patient treated for daptomycin- and vancomycinresistant Enterococcus faecium prosthetic valve endocarditis experienced nausea, anorexia, and minor liver function test (LFT) abnormalities after cumulative oritavancin exposure over 18 weeks.9 On discontinuation of the drug, nausea and anorexia improved, and LFTs normalized 11 months later. Overall, AEs reported with continued dosing of oritavancin have been minimal and largely reversible, mimicking the AEs in the product labeling for traditional dosing. This suggests that using a continued dosing strategy may not result in worse or more frequent AEs, though randomized controlled trials are needed to fully ascertain these preliminary findings.
Conclusions
The literature supporting the use of oritavancin beyond single-dose administration for ABSSSI is growing. Continued dosing regimens have been well tolerated and have resulted in clinical cure for many patients with barriers to first-line treatment and complicated or invasive infections. While randomized controlled trials are needed to concretely demonstrate the efficacy and safety of continued dosing of oritavancin, it may fill an important treatment niche in this era of growing antibiotic resistance and increasing complexity of patient cases.
Oritavancin is a lipoglycopeptide antibiotic. The US Food and Drug Administration (FDA) approved oritavancin in 2014 for adults with acute bacterial skin and skin structure infections (ABSSSI).1 The antibiotic is currently FDA approved for infections caused by Gram-positive organisms, including methicillin-resistant and methicillinsusceptible Staphylococcus aureus (MRSA, MSSA), a variety of Streptococcus species, and vancomycin-susceptible Enterococcus faecalis (VSE). Oritavancin demonstrates concentrationdependent bactericidal activity and has a half-life of 245 hours. This half-life allows for treatment of ABSSSI with a single 1,200 mg IV dose, which has been shown to be noninferior to vancomycin dosed twice daily for 7 to 10 days.1-3
Proposal for Expanded Uses
Although the approved indication for oritavancin is narrow, in vitro studies have shown that oritavancin also has activity against vancomycin-resistant enterococci (VRE), and rabbit studies have demonstrated its excellent bone penetration.4,5 These findings have raised the question of whether oritavancin can be safely and effectively used for infections such as endocarditis, osteomyelitis, and bacteremia, which are often caused by invasive Grampositive organisms. These types of invasive infections, particularly when MRSA is implicated, generally require IV antibiotic therapy for several weeks, often with vancomycin.6
To avoid long hospital stays solely for antibiotic administration, health care practitioners will often use outpatient parenteral antimicrobial therapy (OPAT). However, using OPAT presents many challenges due to the need for frequent dosing, the risk of peripheral or central-line infections, and therapeutic drug monitoring when using vancomycin; additionally, administration and line care oftentimes require caregiver support, which may not be present for all patients.7 Concerns also have been raised regarding the use of OPAT in patients with a history of IV drug use due to the potential increased risk of line infections or line abuse. Few studies have explored OPAT in this population, and the Infectious Diseases Society of America OPAT guidelines recommend that the decision to use OPAT should be made on a case-by-case basis.7 Thus, patients who are deemed inappropriate for OPAT oftentimes remain hospitalized or reside briefly in nursing facilities solely for antibiotic administration
Oritavancin’s long half-life and potent activity against Gram-positive organisms has led to increased interest in off-label use of infrequent dosing intervals, such as weekly, to treat complicated and invasive infections. Weekly rather than daily dosing would allow for less burdensome antibiotic administration regimens and shorter hospital stays especially for patients who are not candidates for OPAT.
Efficacy of Continued Dosing
This proposed weekly dosing pattern, referred to as continued dosing or a multiple-dose regimen, has gained traction in the literature. To date, no randomized controlled trials have been conducted to assess oritavancin’s efficacy in off-label indications or continued dosing, but several case reports and retrospective cohort analyses show promising outcomes.8-16 In an analysis of data from the Clinical and Historic Registry and Orbactiv Medical Evaluation (CHROME) patient registry, 32 patients received multiple doses of oritavancin for complicated Gram-positive infections with a 93.8% overall clinical success rate, including success rates of 90.9% (10/11) for general bone and joint infections and 87.5% (7/8) for patients diagnosed specifically with osteomyelitis.8
Patients received between 2 and 10 doses of 1,200 mg IV given every 6 to 14 days. Johnson and colleagues report using oritavancin 1,200 mg IV every other day for 3 doses followed by 1,200 mg IV once weekly for a patient with daptomycin- and vancomycin-resistant Enterococcus endocarditis, resulting in negative blood cultures while on therapy.9 However, source control via valve replacement and postoperative oritavancin 1,200 mg IV twice weekly for 10 weeks was required to fully clear the infection.
Schulz and colleagues published a retrospective cohort analysis of 17 patients who received multiple doses of oritavancin for complicated bacterial infections, including osteomyelitis, pneumonia, and bacteremia.10 They reported 100% of patients were either successfully cured or had demonstrable improvements in their infections by using a 1,200 mg IV loading dose followed by 800 mg IV if the second dose was given within 7 days or 1,200 mg IV if the second dose was given more than 10 days later. Patients received between 2 and 18 total doses, with 6 out of 17 (35%) receiving only 2 doses. One patient who received 18 doses was an outlier, as her treatment goal was palliative suppression due to an infected endovascular graft that could not be removed.
In a published case series, 1 of 10 patients receiving oritavancin for invasive Grampositive infections received multiple doses of oritavancin for an MSSA deep tissue infection.11 The 3 total doses (strength not reported) were separated by 19 days and 14 days and resulted in cure. Several case reports and a retrospective chart review study specifically show the effectiveness of oritavancin for osteomyelitis caused by MSSA, MRSA, and VRE.12-16 However, dosing strategies varied widely after the initial 1,200 mg IV loading dose.
Drug Interactions, Safety, and Tolerability
Oritavancin has minimal drug-drug interactions, the most notable being with anticoagulants. 1 Use of IV heparin within 120 hours of oritavancin administration can falsely elevate activated partial thromboplastin time (aPTT) levels; therefore, heparin should not be monitored with aPTT during this period. Oritavancin also can artificially prolong international normalized ratio (INR) values for up to 12 hours, and dose adjustments based on INRs during this window are not recommended. Of note, factor Xa laboratory monitoring is unaffected by oritavancin, as it does not depend on phospholipid reagents as do aPTT and INR measurements.
Oritavancin has been shown to be well tolerated when dosed according to both the package insert and continued dosing strategies. The most common adverse effects (AEs) (≥ 3%), occurring at similar rates to vancomycin, are nausea, vomiting, diarrhea, headache, and limb and subcutaneous abscesses.1 Infusion reactions also have been reported, although they are usually reversible on slowing or stopping the infusion. It is worth noting that the use of oritavancin for osteomyelitis is not recommended in the product labeling, as an increased rate of osteomyelitis was observed in the oritavancin vs IV vancomycin groups for the treatment of patients with acute bacterial skin and skin structure infection (SOLO) trials (0.6% in oritavancin group vs 0.1% in vancomycin group, statistical significance not reported).17 However, it was postulated that these osteomyelitis cases were likely present, yet not recognized, at baseline and were not the result of administering oritavancin. This conclusion is further corroborated by previously presented research demonstrating successful cure of osteomyelitis with continued dosing strategies.12-16
Many patients receiving multiple doses of oritavancin did not experience AEs or laboratory abnormalities.13,15 Four of 17 patients (24%) in one retrospective review experienced AEs, including infusion reactions, anemia, and leukopenia; all were reversible on discontinuation of oritavancin, and contributions of other antibiotics in some cases could not be ruled out.10 One patient experienced taste disturbance for several hours after each infusion, and a second had documented hearing loss after 3 doses of oritavancin in a 33-day period, though she had received 6 weeks of IV vancomycin prior to oritavancin.11,12 A patient treated for daptomycin- and vancomycinresistant Enterococcus faecium prosthetic valve endocarditis experienced nausea, anorexia, and minor liver function test (LFT) abnormalities after cumulative oritavancin exposure over 18 weeks.9 On discontinuation of the drug, nausea and anorexia improved, and LFTs normalized 11 months later. Overall, AEs reported with continued dosing of oritavancin have been minimal and largely reversible, mimicking the AEs in the product labeling for traditional dosing. This suggests that using a continued dosing strategy may not result in worse or more frequent AEs, though randomized controlled trials are needed to fully ascertain these preliminary findings.
Conclusions
The literature supporting the use of oritavancin beyond single-dose administration for ABSSSI is growing. Continued dosing regimens have been well tolerated and have resulted in clinical cure for many patients with barriers to first-line treatment and complicated or invasive infections. While randomized controlled trials are needed to concretely demonstrate the efficacy and safety of continued dosing of oritavancin, it may fill an important treatment niche in this era of growing antibiotic resistance and increasing complexity of patient cases.
1. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2019.
2. Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370(23):2180-2190. doi:10.1056/NEJMoa1310422
3. Corey GR, Good S, Jiang H, et al. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60(2):254-262. doi:10.1093/cid/ciu778
4. Sweeney D, Stoneburner A, Shinabarger DL, et al. Comparative in vitro activity of oritavancin and other agents against vancomycin-susceptible and -resistant enterococci. J Antimicrob Chemother. 2017;72(2):622-624. doi.10.1093/jac/dkw451
5. Lehoux D, Ostiguy V, Vadieux C, et al. Oritavancin pharmacokinetics and bone penetration in rabbits. Antimicrob Agents Chemother. 2015;59(10):6501-6505. doi:10.1128/AAC.00981-15
6. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55. doi:10.1093/cid/ciq146
7. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):e1-e35. doi:10.1093/cid/ciy745
8. Redell M, Seirra-Hoffman M, Assi Maha, et al. The CHROME study, a real-world experience of single- and multiple-dose oritavancin for treatment of gram-positive infections. Open Forum Infect Dis. 2019;6(11):ofz479. doi:10.1093/ofid/ofz479
9. Johnson JA, Feeney ER, Kubiak DW, Corey GR. Prolonged use of oritavancin for vancomycin-resistant Enterococcus faecium prosthetic valve endocarditis. Open Forum Infect Dis. 2015;2(4):ofv156. doi:10.1093/ofid/ofv156
10. Schulz LT, Dworkin E, Dela-Pena J, Rose WE. Multipledose oritavancin evaluation in a retrospective cohort of patients with complicated infections. Pharmacotherapy. 2018;38(1):152-159. doi:10.1002/phar.2057
11. Stewart CL, Turner MS, Frens JJ, Snider CB, Smith JR. Real-world experience with oritavancin therapy in invasive gram-positive infections. Infect Dis Ther. 2017;6(2):277-289. doi:10.1007/s40121-017-0156-z
12. Delaportas DJ, Estrada SJ, Darmelio M. Successful treatment of methicillin susceptible Staphylococcus aureus osteomyelitis with oritavancin. Pharmacotherapy. 2017;37(8):e90-e92. doi:10.1002/phar.1957
13. Chastain DB, Davis A. Treatment of chronic osteomyelitis with multidose oritavancin: a case series and literature review. Int J Antimicrob Agents. 2019;53(4):429-434. doi:10.1016/j.ijantimicag.2018.11.023
14. Dahesh S, Wong B, Nizet V, Sakoulas G, Tran TT, Aitken SL. Treatment of multidrug-resistant vancomycinresistant Enterococcus faecium hardware-associated vertebral osteomyelitis with oritavancin plus ampicillin. Antimicrob Agents Chemother. 2019;63(7):e02622-18. doi:10.1128/AAC.02622-18
15. Foster RA, Philavong KP, Weissman S, Tang X, Bookstaver PB. Oritavancin for the treatment of daptomycin nonsusceptible vancomycin-resistant Enterococci osteomyelitis. Infect Dis Clin Pract. 2018;26(2):97-99. doi:10.1097/IPC.0000000000000517
16. Ruggero M, Ziegler M, Tebas P, Binkley A, Kelly B. Successful treatment of methicillin-resistant Staphylococcus aureus vertebral osteomyelitis with outpatient oritavancin therapy. Infect Dis Clin Pract. 2018;26(3):141-144. doi:10.1097/IPC.0000000000000599
17. Corey GR, Loutit J, Moeck G, et al. Single intravenous dose of oritavancin for treatment of acute skin and skin structure infections caused by gram-positive bacteria: summary of safety analysis from the phase 3 SOLO studies. Antimicrob Agents Chemother. 2018;62(4):e01919- 17. doi:10.1128/AAC.01919-17
1. Orbactiv [package insert]. Parsippany, NJ: The Medicines Company; 2019.
2. Corey GR, Kabler H, Mehra P, et al. Single-dose oritavancin in the treatment of acute bacterial skin infections. N Engl J Med. 2014;370(23):2180-2190. doi:10.1056/NEJMoa1310422
3. Corey GR, Good S, Jiang H, et al. Single-dose oritavancin versus 7-10 days of vancomycin in the treatment of gram-positive acute bacterial skin and skin structure infections: the SOLO II noninferiority study. Clin Infect Dis. 2015;60(2):254-262. doi:10.1093/cid/ciu778
4. Sweeney D, Stoneburner A, Shinabarger DL, et al. Comparative in vitro activity of oritavancin and other agents against vancomycin-susceptible and -resistant enterococci. J Antimicrob Chemother. 2017;72(2):622-624. doi.10.1093/jac/dkw451
5. Lehoux D, Ostiguy V, Vadieux C, et al. Oritavancin pharmacokinetics and bone penetration in rabbits. Antimicrob Agents Chemother. 2015;59(10):6501-6505. doi:10.1128/AAC.00981-15
6. Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52(3):e18-e55. doi:10.1093/cid/ciq146
7. Norris AH, Shrestha NK, Allison GM, et al. 2018 Infectious Diseases Society of America clinical practice guideline for the management of outpatient parenteral antimicrobial therapy. Clin Infect Dis. 2019;68(1):e1-e35. doi:10.1093/cid/ciy745
8. Redell M, Seirra-Hoffman M, Assi Maha, et al. The CHROME study, a real-world experience of single- and multiple-dose oritavancin for treatment of gram-positive infections. Open Forum Infect Dis. 2019;6(11):ofz479. doi:10.1093/ofid/ofz479
9. Johnson JA, Feeney ER, Kubiak DW, Corey GR. Prolonged use of oritavancin for vancomycin-resistant Enterococcus faecium prosthetic valve endocarditis. Open Forum Infect Dis. 2015;2(4):ofv156. doi:10.1093/ofid/ofv156
10. Schulz LT, Dworkin E, Dela-Pena J, Rose WE. Multipledose oritavancin evaluation in a retrospective cohort of patients with complicated infections. Pharmacotherapy. 2018;38(1):152-159. doi:10.1002/phar.2057
11. Stewart CL, Turner MS, Frens JJ, Snider CB, Smith JR. Real-world experience with oritavancin therapy in invasive gram-positive infections. Infect Dis Ther. 2017;6(2):277-289. doi:10.1007/s40121-017-0156-z
12. Delaportas DJ, Estrada SJ, Darmelio M. Successful treatment of methicillin susceptible Staphylococcus aureus osteomyelitis with oritavancin. Pharmacotherapy. 2017;37(8):e90-e92. doi:10.1002/phar.1957
13. Chastain DB, Davis A. Treatment of chronic osteomyelitis with multidose oritavancin: a case series and literature review. Int J Antimicrob Agents. 2019;53(4):429-434. doi:10.1016/j.ijantimicag.2018.11.023
14. Dahesh S, Wong B, Nizet V, Sakoulas G, Tran TT, Aitken SL. Treatment of multidrug-resistant vancomycinresistant Enterococcus faecium hardware-associated vertebral osteomyelitis with oritavancin plus ampicillin. Antimicrob Agents Chemother. 2019;63(7):e02622-18. doi:10.1128/AAC.02622-18
15. Foster RA, Philavong KP, Weissman S, Tang X, Bookstaver PB. Oritavancin for the treatment of daptomycin nonsusceptible vancomycin-resistant Enterococci osteomyelitis. Infect Dis Clin Pract. 2018;26(2):97-99. doi:10.1097/IPC.0000000000000517
16. Ruggero M, Ziegler M, Tebas P, Binkley A, Kelly B. Successful treatment of methicillin-resistant Staphylococcus aureus vertebral osteomyelitis with outpatient oritavancin therapy. Infect Dis Clin Pract. 2018;26(3):141-144. doi:10.1097/IPC.0000000000000599
17. Corey GR, Loutit J, Moeck G, et al. Single intravenous dose of oritavancin for treatment of acute skin and skin structure infections caused by gram-positive bacteria: summary of safety analysis from the phase 3 SOLO studies. Antimicrob Agents Chemother. 2018;62(4):e01919- 17. doi:10.1128/AAC.01919-17
The Effect of Radium-223 Therapy in Agent Orange-Related Prostate Carcinoma
Patients with metastatic castrate resistant prostate carcinoma (CRPC) have several treatment options, including radium-223 dichloride (Ra-223) radionuclide therapy, abiraterone, enzalutamide, and cabazitaxel. Ra-223 therapy has been reported to increase median survival in patients with bone metastatic prostate carcinoma.1,2 However, ERA 223 trial data showed an increase of bone fractures with combination of Ra-223 and abiraterone.3
Agent Orange (AO) exposure has been studied as a potential risk factor for development of prostate carcinoma. AO was a commercially manufactured defoliate that was sprayed extensively during the Vietnam War. Due to a side product of chemical manufacturing, AO was contaminated with the toxin 2,3,7,8-tetrachlorodibenzo-p-dioxin, a putative carcinogen. These dioxins can enter the food chain through soil contamination. There is enough evidence to link AO to hematologic malignancies and several solid tumors, including prostate carcinoma.4 Although no real estimates exist for what percentage of Vietnam veterans experienced AO exposure, Surveillance, Epidemiology, and End Results data showed that about 3 million veterans served in Southeast Asia where AO was used extensively in the combat theater. AO has been reported to be positively associated with a 52% increase in risk of prostate carcinoma detection at initial prostate biopsy.5
There has been no reported study of treatment efficacy in veterans with AO-related prostate carcinoma. We present a retrospective study of Ra-223 and other therapies in metastatic CRPC. The purpose of this study was to compare response to therapy and survival in veterans exposed to agent orange (AO+) vs veterans who were not exposed to (AO-) in a single US Department of Veteran Affairs (VA) medical center.
Methods
This was a retrospective study of veterans with metastatic CRPC to bones who received Ra-223 radionuclide therapy with standard dose of 50 kBq per kg of body weight and other sequential therapies at VA Pittsburgh Healthcare System (VAPHS) from January 2014 to January 2019. The purpose of this study was to measure difference in treatment outcome between AO+ veterans and AO- veterans.
Eligibility Criteria
All veterans had a history that included bone metastasis CRPC. They could have 2 to 3 small lymphadenopathies but not visceral metastasis. They received a minimum of 3 cycles and a maximum of 6 cycles of Ra-223 therapy, which was given in 4-week intervals. Pretreatment criteria was hemoglobin > 10 g/dL, platelet > 100
Statistics
Time to study was calculated from the initiation of Ra-223 therapy. Time to skeletal-related events (SRE), progression of prostate specific antigen (PSA), bone metastasis, and alkaline phosphatase (ALP) were calculated in months, using unpaired t test with 2-tailed P value. Median survival was calculated in months by Kaplan Meier R log-rank test Definition).
Results
Forty-eight veterans with bone metastasis CRPC received Ra-223 therapy. Of those, 34 veterans were eligible for this retrospective study: 17 AO+ veterans and 17 AO- veterans. Mean age of diagnosis was 62 years (AO+) and 69 years (AO-) (P = .005). Mean Gleason score was 8.2 (AO+) and 8.0 (AO-) (P = .705). Veterans received initial therapy at diagnosis of prostate carcinoma, including radical prostatectomy (6 AO+ and 3 AO-), localized radiation therapy (3 AO+ and 5 AO-), and ADT (8 AO+ and 9 AO-) (Table 1).
Mean PSA at the initiation of Ra-223 therapy for AO+ was 92.8 (range, 2-551) and for AO- was 102.3 (range, 4-639; P = .86). Mean Ra-223 dose per cycle for AO+ and AO- was 157 uCi and 113 uCi, respectively. All 34 veterans received ADT (leuprolide acetate), and 30 veterans (16 AO+ and 14 AO-) received bisphosphonates (zoledronic acid or denosumab). A total of 10 veterans (29%) received Ra-223 as a first-line therapy (4 AO+ and 6 AO-), and 24 veterans (71%) received Ra-223 after hormonal or chemotherapy (13 AO+ and 11 AO-).
There were 12 SRE (8 AO+ and 4 AO-). Mean time to SRE for AO+ was 10.6 months and AO- was 10.3 months (P = .93). Three veterans received concurrent Ra-223 and abiraterone (participated in ERA 223 trial). Two AO+ veterans experienced SRE at 7 months and 11 months, respectively. Mean time to PSA progression for AO+ was 5.4 months and for AO- was 6.8 months (P = .28). Mean time to bone progression for AO+ and for AO- were 7.6 months and 10.1 months, respectively (P = .16). Mean time to ALP progression for AO+ and AO- were 6.3 months and 8.7 months, respectively (P = .05). (Table 2). The treatment pattern of AO+ and AO- is depicted on a swimmer plot (Figures 1 and 2).
Twenty veterans (58%) had died: 13 AO+ and 7 AO- veterans. Median survival for Ra-223 first and Ra-223 later was was 32 months and 15 months, respectively (P = .14; hazard ratio [HR], 0.48). Overall median survival for AO+ veterans and AO- veterans were 12 months and 18 months, respectively (P = .15; HR, 2.0) (Figures 3 and 4).
Discussions
There has been no reported VA study of using Ra-223 and other therapies (hormonal and chemotherapy) in veterans exposed to AO. This is the first retrospective study to compare the response and survival between AO+ and AO- veterans. Even though this study featured a small sample, it is interesting to note the difference between those 2 populations. There was 1 prior study in veterans with prostate carcinoma using radiotherapy (brachytherapy) in early-stage disease. Everly and colleagues reported that AO+ veterans were less likely to remain biochemically controlled compared with AO- and nonveteran patients with prostate carcinoma.4
Ansbaugh and colleagues reported that AO was associated with a 75% increase in the risk of Gleason ≥ 7 and a 110% increase in Gleason ≥ 8. AO+ veterans are at risk for the detection of high-grade prostate carcinoma. They also tend to have an average age of diagnosis that is 4 to 5 years younger than AO- veterans.6
Our study revealed that AO+ veterans were diagnosed at a younger age (mean 62 years) compared with that of AO- veterans (mean 69 years, P = .005). We also proved that AO veterans have a higher mean Gleason score (8.2) compared with that of AO- veterans (8.0). Veterans received therapy at the time of diagnosis of prostate carcinoma with either radical prostatectomy, radiation therapy, or ADT with leuprolide acetate. Mean PSA at the start of Ra-223 therapy for AO+ was 92.8 (range, 2-551); for AO- was 102.3 (range, 4-639), which is not statistically significant.
Ra-223, an
In a phase 3, randomized, double-blind, placebo-controlled study by Parker and colleagues (ALSYMPCA study), 921 patients who had received, were not eligible to receive, or declined docetaxel, in a 2:1 ratio, were randomized to receive 6 injections of Ra-223 or matching placebo.2 Ra-223 significantly improved overall survival (OS) (median, 14.9 months vs 11.3 months) compared with that of placebo. Ra-223 also prolonged the time to the first symptomatic SRE (median, 15.6 months vs 9.8 months), the time to an increase in the total ALP level (median 7.4 months vs 3.8 months), and the time to an increase in the PSA level (median 3.6 months vs 3.4 months).2
In our study, the mean time to SRE for AO+ was 10.6 months and AO- was 10.3 months (P = .93). Mean time to PSA progression for AO+ was 5.4 months and for AO- was 6.8 months (P = .28). Mean time to bone progression for AO+ and for AO- were 7.6 months and 10.1 months respectively (P = .16). Mean time to ALP progression for AO+ and AO- were 6.3 months and 8.7 months respectively (P = .05). There is a trend of shorter PSA progression, bone progression, and ALP progression in AO+ veterans, though these were not statistically significant due to small sample population. In our study the median survival in for AO- was 18 months and for AO+ was 12 months, which is comparable with median survival of 14.9 months from the ALSYMPCA study.
There were 12 veterans who developed SREs. All received radiation therapy due to bone progression or impending fracture. AO+ veterans developed more SREs (n = 8) when compared with AO- veterans (n = 4). There were more AO- veterans alive (n = 10) than there were AO+ veterans (n = 4). The plausible explanation of this may be due to the aggressive pattern of prostate carcinoma in AO+ veterans (younger age and higher Gleason score).
VAPHS participated in the ERA trial between 2014 and 2016. The trial enrolled 806 patients who were randomly assigned to receive first-line Ra-223 or placebo in addition to abiraterone acetate plus prednisone.3 The study was unblinded prematurely after more fractures and deaths were noted in the Ra-223 and abiraterone group than there were in the placebo and abiraterone group. Median symptomatic SRE was 22.3 months in the Ra-223 group and 26.0 months in the placebo group. Fractures (any grade) occurred in 29% in the Ra-223 group and 11% in the placebo group. It was suggested that Ra-223 could contribute to the risk of osteoporotic fractures in patients with bone metastatic prostate carcinoma. Median OS was 30.7 months in the Ra-223 group and 33.3 months in the placebo group.
We enrolled 3 veterans in the ERA clinical trial. Two AO+ veterans had SREs at 7 months and 11 months. In our study, the median OS for Ra-223 first line was 32 months, which is comparable with median survival of 30.7 months from ERA-223 study. Median survival for Ra-223 later was only 15 months. We recommend veterans with at least 2 to 3-bone metastasis receive Ra-223 in the first-line setting rather than second- or third-line setting. In this retrospective study with Ra-223 and other therapies, we proved that AO+ veterans have a worse response and OS when compared with that of AO- veterans.
Conclusions
This is the first VA study to compare the efficacy of Ra-223 and other therapies in metastatic CRPC between AO+ and AO- veterans. AO+ veterans were diagnosed at a younger age and had higher Gleason scores. There was no statistical difference between AO+ and AO- veterans in terms of time to SRE, PSA progression, and bone and ALP progression even though there was a trend of shorter duration in AO+ veterans. There was no median survival difference between veterans who received Ra-223 first vs Ra-223 later as well as between AO+ and AO- veterans, but there was a trend of worse survival in veteran who received Ra-223 later and AO+ veterans.
This study showed that AO+ veterans have a shorter duration of response to therapy and shorter median survival compared with that of AO- veterans. We recommend that veterans should get Ra-223 in the first-line setting rather than after hormonal therapy and chemotherapy because their marrows are still intact. We need to investigate further whether veterans that exposed to carcinogen 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) may have different molecular biology and as such may cause inferior efficacy in the treatment of prostate carcinoma.
1. Shore ND. Radium-223 dichloride for metastatic castration-resistant prostate cancer: the urologist’s perspective. Urology. 2015;85(4):717-724. doi:10.1016/j.urology.2014.11.031
2. Parker C, Nilsson S, Heinrich D, et al; ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. doi:10.1056/NEJMoa1213755
3. Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomized, double-blind, placebo-controlled, phase 3 trial [published correction appears in Lancet Oncol. 2019 Oct;20(10):e559]. Lancet Oncol. 2019;20(3):408-419. doi:10.1016/S1470-2045(18)30860-X
4. Everly L, Merrick GS, Allen ZA, et al. Prostate cancer control and survival in Vietnam veterans exposed to Agent Orange. Brachytherapy. 2009;8(1):57-62. doi: 10.1016/j.brachy.2008.08.001
5. Altekruse S. SEER Cancer Statistics Review, 1975-2017 Bethesda, MD: National Cancer Institute. 2009. 6. Ansbaugh N, Shannon J, Mori M, Farris PE, Garzotto M. Agent Orange as a risk factor for high-grade prostate cancer. Cancer. 2013;119(13):2399-2404. doi:10.1002/cncr.27941
7. Jadvar H, Quinn DI. Targeted α-particle therapy of bone metastases in prostate cancer. Clin Nucl Med. 2013;38(12):966-971. doi:10.1097/RLU.0000000000000290
Patients with metastatic castrate resistant prostate carcinoma (CRPC) have several treatment options, including radium-223 dichloride (Ra-223) radionuclide therapy, abiraterone, enzalutamide, and cabazitaxel. Ra-223 therapy has been reported to increase median survival in patients with bone metastatic prostate carcinoma.1,2 However, ERA 223 trial data showed an increase of bone fractures with combination of Ra-223 and abiraterone.3
Agent Orange (AO) exposure has been studied as a potential risk factor for development of prostate carcinoma. AO was a commercially manufactured defoliate that was sprayed extensively during the Vietnam War. Due to a side product of chemical manufacturing, AO was contaminated with the toxin 2,3,7,8-tetrachlorodibenzo-p-dioxin, a putative carcinogen. These dioxins can enter the food chain through soil contamination. There is enough evidence to link AO to hematologic malignancies and several solid tumors, including prostate carcinoma.4 Although no real estimates exist for what percentage of Vietnam veterans experienced AO exposure, Surveillance, Epidemiology, and End Results data showed that about 3 million veterans served in Southeast Asia where AO was used extensively in the combat theater. AO has been reported to be positively associated with a 52% increase in risk of prostate carcinoma detection at initial prostate biopsy.5
There has been no reported study of treatment efficacy in veterans with AO-related prostate carcinoma. We present a retrospective study of Ra-223 and other therapies in metastatic CRPC. The purpose of this study was to compare response to therapy and survival in veterans exposed to agent orange (AO+) vs veterans who were not exposed to (AO-) in a single US Department of Veteran Affairs (VA) medical center.
Methods
This was a retrospective study of veterans with metastatic CRPC to bones who received Ra-223 radionuclide therapy with standard dose of 50 kBq per kg of body weight and other sequential therapies at VA Pittsburgh Healthcare System (VAPHS) from January 2014 to January 2019. The purpose of this study was to measure difference in treatment outcome between AO+ veterans and AO- veterans.
Eligibility Criteria
All veterans had a history that included bone metastasis CRPC. They could have 2 to 3 small lymphadenopathies but not visceral metastasis. They received a minimum of 3 cycles and a maximum of 6 cycles of Ra-223 therapy, which was given in 4-week intervals. Pretreatment criteria was hemoglobin > 10 g/dL, platelet > 100
Statistics
Time to study was calculated from the initiation of Ra-223 therapy. Time to skeletal-related events (SRE), progression of prostate specific antigen (PSA), bone metastasis, and alkaline phosphatase (ALP) were calculated in months, using unpaired t test with 2-tailed P value. Median survival was calculated in months by Kaplan Meier R log-rank test Definition).
Results
Forty-eight veterans with bone metastasis CRPC received Ra-223 therapy. Of those, 34 veterans were eligible for this retrospective study: 17 AO+ veterans and 17 AO- veterans. Mean age of diagnosis was 62 years (AO+) and 69 years (AO-) (P = .005). Mean Gleason score was 8.2 (AO+) and 8.0 (AO-) (P = .705). Veterans received initial therapy at diagnosis of prostate carcinoma, including radical prostatectomy (6 AO+ and 3 AO-), localized radiation therapy (3 AO+ and 5 AO-), and ADT (8 AO+ and 9 AO-) (Table 1).
Mean PSA at the initiation of Ra-223 therapy for AO+ was 92.8 (range, 2-551) and for AO- was 102.3 (range, 4-639; P = .86). Mean Ra-223 dose per cycle for AO+ and AO- was 157 uCi and 113 uCi, respectively. All 34 veterans received ADT (leuprolide acetate), and 30 veterans (16 AO+ and 14 AO-) received bisphosphonates (zoledronic acid or denosumab). A total of 10 veterans (29%) received Ra-223 as a first-line therapy (4 AO+ and 6 AO-), and 24 veterans (71%) received Ra-223 after hormonal or chemotherapy (13 AO+ and 11 AO-).
There were 12 SRE (8 AO+ and 4 AO-). Mean time to SRE for AO+ was 10.6 months and AO- was 10.3 months (P = .93). Three veterans received concurrent Ra-223 and abiraterone (participated in ERA 223 trial). Two AO+ veterans experienced SRE at 7 months and 11 months, respectively. Mean time to PSA progression for AO+ was 5.4 months and for AO- was 6.8 months (P = .28). Mean time to bone progression for AO+ and for AO- were 7.6 months and 10.1 months, respectively (P = .16). Mean time to ALP progression for AO+ and AO- were 6.3 months and 8.7 months, respectively (P = .05). (Table 2). The treatment pattern of AO+ and AO- is depicted on a swimmer plot (Figures 1 and 2).
Twenty veterans (58%) had died: 13 AO+ and 7 AO- veterans. Median survival for Ra-223 first and Ra-223 later was was 32 months and 15 months, respectively (P = .14; hazard ratio [HR], 0.48). Overall median survival for AO+ veterans and AO- veterans were 12 months and 18 months, respectively (P = .15; HR, 2.0) (Figures 3 and 4).
Discussions
There has been no reported VA study of using Ra-223 and other therapies (hormonal and chemotherapy) in veterans exposed to AO. This is the first retrospective study to compare the response and survival between AO+ and AO- veterans. Even though this study featured a small sample, it is interesting to note the difference between those 2 populations. There was 1 prior study in veterans with prostate carcinoma using radiotherapy (brachytherapy) in early-stage disease. Everly and colleagues reported that AO+ veterans were less likely to remain biochemically controlled compared with AO- and nonveteran patients with prostate carcinoma.4
Ansbaugh and colleagues reported that AO was associated with a 75% increase in the risk of Gleason ≥ 7 and a 110% increase in Gleason ≥ 8. AO+ veterans are at risk for the detection of high-grade prostate carcinoma. They also tend to have an average age of diagnosis that is 4 to 5 years younger than AO- veterans.6
Our study revealed that AO+ veterans were diagnosed at a younger age (mean 62 years) compared with that of AO- veterans (mean 69 years, P = .005). We also proved that AO veterans have a higher mean Gleason score (8.2) compared with that of AO- veterans (8.0). Veterans received therapy at the time of diagnosis of prostate carcinoma with either radical prostatectomy, radiation therapy, or ADT with leuprolide acetate. Mean PSA at the start of Ra-223 therapy for AO+ was 92.8 (range, 2-551); for AO- was 102.3 (range, 4-639), which is not statistically significant.
Ra-223, an
In a phase 3, randomized, double-blind, placebo-controlled study by Parker and colleagues (ALSYMPCA study), 921 patients who had received, were not eligible to receive, or declined docetaxel, in a 2:1 ratio, were randomized to receive 6 injections of Ra-223 or matching placebo.2 Ra-223 significantly improved overall survival (OS) (median, 14.9 months vs 11.3 months) compared with that of placebo. Ra-223 also prolonged the time to the first symptomatic SRE (median, 15.6 months vs 9.8 months), the time to an increase in the total ALP level (median 7.4 months vs 3.8 months), and the time to an increase in the PSA level (median 3.6 months vs 3.4 months).2
In our study, the mean time to SRE for AO+ was 10.6 months and AO- was 10.3 months (P = .93). Mean time to PSA progression for AO+ was 5.4 months and for AO- was 6.8 months (P = .28). Mean time to bone progression for AO+ and for AO- were 7.6 months and 10.1 months respectively (P = .16). Mean time to ALP progression for AO+ and AO- were 6.3 months and 8.7 months respectively (P = .05). There is a trend of shorter PSA progression, bone progression, and ALP progression in AO+ veterans, though these were not statistically significant due to small sample population. In our study the median survival in for AO- was 18 months and for AO+ was 12 months, which is comparable with median survival of 14.9 months from the ALSYMPCA study.
There were 12 veterans who developed SREs. All received radiation therapy due to bone progression or impending fracture. AO+ veterans developed more SREs (n = 8) when compared with AO- veterans (n = 4). There were more AO- veterans alive (n = 10) than there were AO+ veterans (n = 4). The plausible explanation of this may be due to the aggressive pattern of prostate carcinoma in AO+ veterans (younger age and higher Gleason score).
VAPHS participated in the ERA trial between 2014 and 2016. The trial enrolled 806 patients who were randomly assigned to receive first-line Ra-223 or placebo in addition to abiraterone acetate plus prednisone.3 The study was unblinded prematurely after more fractures and deaths were noted in the Ra-223 and abiraterone group than there were in the placebo and abiraterone group. Median symptomatic SRE was 22.3 months in the Ra-223 group and 26.0 months in the placebo group. Fractures (any grade) occurred in 29% in the Ra-223 group and 11% in the placebo group. It was suggested that Ra-223 could contribute to the risk of osteoporotic fractures in patients with bone metastatic prostate carcinoma. Median OS was 30.7 months in the Ra-223 group and 33.3 months in the placebo group.
We enrolled 3 veterans in the ERA clinical trial. Two AO+ veterans had SREs at 7 months and 11 months. In our study, the median OS for Ra-223 first line was 32 months, which is comparable with median survival of 30.7 months from ERA-223 study. Median survival for Ra-223 later was only 15 months. We recommend veterans with at least 2 to 3-bone metastasis receive Ra-223 in the first-line setting rather than second- or third-line setting. In this retrospective study with Ra-223 and other therapies, we proved that AO+ veterans have a worse response and OS when compared with that of AO- veterans.
Conclusions
This is the first VA study to compare the efficacy of Ra-223 and other therapies in metastatic CRPC between AO+ and AO- veterans. AO+ veterans were diagnosed at a younger age and had higher Gleason scores. There was no statistical difference between AO+ and AO- veterans in terms of time to SRE, PSA progression, and bone and ALP progression even though there was a trend of shorter duration in AO+ veterans. There was no median survival difference between veterans who received Ra-223 first vs Ra-223 later as well as between AO+ and AO- veterans, but there was a trend of worse survival in veteran who received Ra-223 later and AO+ veterans.
This study showed that AO+ veterans have a shorter duration of response to therapy and shorter median survival compared with that of AO- veterans. We recommend that veterans should get Ra-223 in the first-line setting rather than after hormonal therapy and chemotherapy because their marrows are still intact. We need to investigate further whether veterans that exposed to carcinogen 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) may have different molecular biology and as such may cause inferior efficacy in the treatment of prostate carcinoma.
Patients with metastatic castrate resistant prostate carcinoma (CRPC) have several treatment options, including radium-223 dichloride (Ra-223) radionuclide therapy, abiraterone, enzalutamide, and cabazitaxel. Ra-223 therapy has been reported to increase median survival in patients with bone metastatic prostate carcinoma.1,2 However, ERA 223 trial data showed an increase of bone fractures with combination of Ra-223 and abiraterone.3
Agent Orange (AO) exposure has been studied as a potential risk factor for development of prostate carcinoma. AO was a commercially manufactured defoliate that was sprayed extensively during the Vietnam War. Due to a side product of chemical manufacturing, AO was contaminated with the toxin 2,3,7,8-tetrachlorodibenzo-p-dioxin, a putative carcinogen. These dioxins can enter the food chain through soil contamination. There is enough evidence to link AO to hematologic malignancies and several solid tumors, including prostate carcinoma.4 Although no real estimates exist for what percentage of Vietnam veterans experienced AO exposure, Surveillance, Epidemiology, and End Results data showed that about 3 million veterans served in Southeast Asia where AO was used extensively in the combat theater. AO has been reported to be positively associated with a 52% increase in risk of prostate carcinoma detection at initial prostate biopsy.5
There has been no reported study of treatment efficacy in veterans with AO-related prostate carcinoma. We present a retrospective study of Ra-223 and other therapies in metastatic CRPC. The purpose of this study was to compare response to therapy and survival in veterans exposed to agent orange (AO+) vs veterans who were not exposed to (AO-) in a single US Department of Veteran Affairs (VA) medical center.
Methods
This was a retrospective study of veterans with metastatic CRPC to bones who received Ra-223 radionuclide therapy with standard dose of 50 kBq per kg of body weight and other sequential therapies at VA Pittsburgh Healthcare System (VAPHS) from January 2014 to January 2019. The purpose of this study was to measure difference in treatment outcome between AO+ veterans and AO- veterans.
Eligibility Criteria
All veterans had a history that included bone metastasis CRPC. They could have 2 to 3 small lymphadenopathies but not visceral metastasis. They received a minimum of 3 cycles and a maximum of 6 cycles of Ra-223 therapy, which was given in 4-week intervals. Pretreatment criteria was hemoglobin > 10 g/dL, platelet > 100
Statistics
Time to study was calculated from the initiation of Ra-223 therapy. Time to skeletal-related events (SRE), progression of prostate specific antigen (PSA), bone metastasis, and alkaline phosphatase (ALP) were calculated in months, using unpaired t test with 2-tailed P value. Median survival was calculated in months by Kaplan Meier R log-rank test Definition).
Results
Forty-eight veterans with bone metastasis CRPC received Ra-223 therapy. Of those, 34 veterans were eligible for this retrospective study: 17 AO+ veterans and 17 AO- veterans. Mean age of diagnosis was 62 years (AO+) and 69 years (AO-) (P = .005). Mean Gleason score was 8.2 (AO+) and 8.0 (AO-) (P = .705). Veterans received initial therapy at diagnosis of prostate carcinoma, including radical prostatectomy (6 AO+ and 3 AO-), localized radiation therapy (3 AO+ and 5 AO-), and ADT (8 AO+ and 9 AO-) (Table 1).
Mean PSA at the initiation of Ra-223 therapy for AO+ was 92.8 (range, 2-551) and for AO- was 102.3 (range, 4-639; P = .86). Mean Ra-223 dose per cycle for AO+ and AO- was 157 uCi and 113 uCi, respectively. All 34 veterans received ADT (leuprolide acetate), and 30 veterans (16 AO+ and 14 AO-) received bisphosphonates (zoledronic acid or denosumab). A total of 10 veterans (29%) received Ra-223 as a first-line therapy (4 AO+ and 6 AO-), and 24 veterans (71%) received Ra-223 after hormonal or chemotherapy (13 AO+ and 11 AO-).
There were 12 SRE (8 AO+ and 4 AO-). Mean time to SRE for AO+ was 10.6 months and AO- was 10.3 months (P = .93). Three veterans received concurrent Ra-223 and abiraterone (participated in ERA 223 trial). Two AO+ veterans experienced SRE at 7 months and 11 months, respectively. Mean time to PSA progression for AO+ was 5.4 months and for AO- was 6.8 months (P = .28). Mean time to bone progression for AO+ and for AO- were 7.6 months and 10.1 months, respectively (P = .16). Mean time to ALP progression for AO+ and AO- were 6.3 months and 8.7 months, respectively (P = .05). (Table 2). The treatment pattern of AO+ and AO- is depicted on a swimmer plot (Figures 1 and 2).
Twenty veterans (58%) had died: 13 AO+ and 7 AO- veterans. Median survival for Ra-223 first and Ra-223 later was was 32 months and 15 months, respectively (P = .14; hazard ratio [HR], 0.48). Overall median survival for AO+ veterans and AO- veterans were 12 months and 18 months, respectively (P = .15; HR, 2.0) (Figures 3 and 4).
Discussions
There has been no reported VA study of using Ra-223 and other therapies (hormonal and chemotherapy) in veterans exposed to AO. This is the first retrospective study to compare the response and survival between AO+ and AO- veterans. Even though this study featured a small sample, it is interesting to note the difference between those 2 populations. There was 1 prior study in veterans with prostate carcinoma using radiotherapy (brachytherapy) in early-stage disease. Everly and colleagues reported that AO+ veterans were less likely to remain biochemically controlled compared with AO- and nonveteran patients with prostate carcinoma.4
Ansbaugh and colleagues reported that AO was associated with a 75% increase in the risk of Gleason ≥ 7 and a 110% increase in Gleason ≥ 8. AO+ veterans are at risk for the detection of high-grade prostate carcinoma. They also tend to have an average age of diagnosis that is 4 to 5 years younger than AO- veterans.6
Our study revealed that AO+ veterans were diagnosed at a younger age (mean 62 years) compared with that of AO- veterans (mean 69 years, P = .005). We also proved that AO veterans have a higher mean Gleason score (8.2) compared with that of AO- veterans (8.0). Veterans received therapy at the time of diagnosis of prostate carcinoma with either radical prostatectomy, radiation therapy, or ADT with leuprolide acetate. Mean PSA at the start of Ra-223 therapy for AO+ was 92.8 (range, 2-551); for AO- was 102.3 (range, 4-639), which is not statistically significant.
Ra-223, an
In a phase 3, randomized, double-blind, placebo-controlled study by Parker and colleagues (ALSYMPCA study), 921 patients who had received, were not eligible to receive, or declined docetaxel, in a 2:1 ratio, were randomized to receive 6 injections of Ra-223 or matching placebo.2 Ra-223 significantly improved overall survival (OS) (median, 14.9 months vs 11.3 months) compared with that of placebo. Ra-223 also prolonged the time to the first symptomatic SRE (median, 15.6 months vs 9.8 months), the time to an increase in the total ALP level (median 7.4 months vs 3.8 months), and the time to an increase in the PSA level (median 3.6 months vs 3.4 months).2
In our study, the mean time to SRE for AO+ was 10.6 months and AO- was 10.3 months (P = .93). Mean time to PSA progression for AO+ was 5.4 months and for AO- was 6.8 months (P = .28). Mean time to bone progression for AO+ and for AO- were 7.6 months and 10.1 months respectively (P = .16). Mean time to ALP progression for AO+ and AO- were 6.3 months and 8.7 months respectively (P = .05). There is a trend of shorter PSA progression, bone progression, and ALP progression in AO+ veterans, though these were not statistically significant due to small sample population. In our study the median survival in for AO- was 18 months and for AO+ was 12 months, which is comparable with median survival of 14.9 months from the ALSYMPCA study.
There were 12 veterans who developed SREs. All received radiation therapy due to bone progression or impending fracture. AO+ veterans developed more SREs (n = 8) when compared with AO- veterans (n = 4). There were more AO- veterans alive (n = 10) than there were AO+ veterans (n = 4). The plausible explanation of this may be due to the aggressive pattern of prostate carcinoma in AO+ veterans (younger age and higher Gleason score).
VAPHS participated in the ERA trial between 2014 and 2016. The trial enrolled 806 patients who were randomly assigned to receive first-line Ra-223 or placebo in addition to abiraterone acetate plus prednisone.3 The study was unblinded prematurely after more fractures and deaths were noted in the Ra-223 and abiraterone group than there were in the placebo and abiraterone group. Median symptomatic SRE was 22.3 months in the Ra-223 group and 26.0 months in the placebo group. Fractures (any grade) occurred in 29% in the Ra-223 group and 11% in the placebo group. It was suggested that Ra-223 could contribute to the risk of osteoporotic fractures in patients with bone metastatic prostate carcinoma. Median OS was 30.7 months in the Ra-223 group and 33.3 months in the placebo group.
We enrolled 3 veterans in the ERA clinical trial. Two AO+ veterans had SREs at 7 months and 11 months. In our study, the median OS for Ra-223 first line was 32 months, which is comparable with median survival of 30.7 months from ERA-223 study. Median survival for Ra-223 later was only 15 months. We recommend veterans with at least 2 to 3-bone metastasis receive Ra-223 in the first-line setting rather than second- or third-line setting. In this retrospective study with Ra-223 and other therapies, we proved that AO+ veterans have a worse response and OS when compared with that of AO- veterans.
Conclusions
This is the first VA study to compare the efficacy of Ra-223 and other therapies in metastatic CRPC between AO+ and AO- veterans. AO+ veterans were diagnosed at a younger age and had higher Gleason scores. There was no statistical difference between AO+ and AO- veterans in terms of time to SRE, PSA progression, and bone and ALP progression even though there was a trend of shorter duration in AO+ veterans. There was no median survival difference between veterans who received Ra-223 first vs Ra-223 later as well as between AO+ and AO- veterans, but there was a trend of worse survival in veteran who received Ra-223 later and AO+ veterans.
This study showed that AO+ veterans have a shorter duration of response to therapy and shorter median survival compared with that of AO- veterans. We recommend that veterans should get Ra-223 in the first-line setting rather than after hormonal therapy and chemotherapy because their marrows are still intact. We need to investigate further whether veterans that exposed to carcinogen 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) may have different molecular biology and as such may cause inferior efficacy in the treatment of prostate carcinoma.
1. Shore ND. Radium-223 dichloride for metastatic castration-resistant prostate cancer: the urologist’s perspective. Urology. 2015;85(4):717-724. doi:10.1016/j.urology.2014.11.031
2. Parker C, Nilsson S, Heinrich D, et al; ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. doi:10.1056/NEJMoa1213755
3. Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomized, double-blind, placebo-controlled, phase 3 trial [published correction appears in Lancet Oncol. 2019 Oct;20(10):e559]. Lancet Oncol. 2019;20(3):408-419. doi:10.1016/S1470-2045(18)30860-X
4. Everly L, Merrick GS, Allen ZA, et al. Prostate cancer control and survival in Vietnam veterans exposed to Agent Orange. Brachytherapy. 2009;8(1):57-62. doi: 10.1016/j.brachy.2008.08.001
5. Altekruse S. SEER Cancer Statistics Review, 1975-2017 Bethesda, MD: National Cancer Institute. 2009. 6. Ansbaugh N, Shannon J, Mori M, Farris PE, Garzotto M. Agent Orange as a risk factor for high-grade prostate cancer. Cancer. 2013;119(13):2399-2404. doi:10.1002/cncr.27941
7. Jadvar H, Quinn DI. Targeted α-particle therapy of bone metastases in prostate cancer. Clin Nucl Med. 2013;38(12):966-971. doi:10.1097/RLU.0000000000000290
1. Shore ND. Radium-223 dichloride for metastatic castration-resistant prostate cancer: the urologist’s perspective. Urology. 2015;85(4):717-724. doi:10.1016/j.urology.2014.11.031
2. Parker C, Nilsson S, Heinrich D, et al; ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. doi:10.1056/NEJMoa1213755
3. Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomized, double-blind, placebo-controlled, phase 3 trial [published correction appears in Lancet Oncol. 2019 Oct;20(10):e559]. Lancet Oncol. 2019;20(3):408-419. doi:10.1016/S1470-2045(18)30860-X
4. Everly L, Merrick GS, Allen ZA, et al. Prostate cancer control and survival in Vietnam veterans exposed to Agent Orange. Brachytherapy. 2009;8(1):57-62. doi: 10.1016/j.brachy.2008.08.001
5. Altekruse S. SEER Cancer Statistics Review, 1975-2017 Bethesda, MD: National Cancer Institute. 2009. 6. Ansbaugh N, Shannon J, Mori M, Farris PE, Garzotto M. Agent Orange as a risk factor for high-grade prostate cancer. Cancer. 2013;119(13):2399-2404. doi:10.1002/cncr.27941
7. Jadvar H, Quinn DI. Targeted α-particle therapy of bone metastases in prostate cancer. Clin Nucl Med. 2013;38(12):966-971. doi:10.1097/RLU.0000000000000290
Choosing pharmacotherapy for bipolar disorder requires a risk-benefit analysis
When selecting pharmacotherapy for patients with bipolar disorder, clinical and prognostic correlates will ultimately influence what treatments make the most sense for a patient – but the process is a balancing act, according to Joseph F. Goldberg, MD.
“Everything we do in medicine in general, and psychiatry, and bipolar disorder in particular is a risk-benefit analysis,” Dr. Goldberg said at the virtual Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “Everything has its side effects. We’re always balancing risks and benefits.”
Patients with bipolar disorder often present with three common subtypes of the illness: Those who have associated psychosis, comorbid anxiety disorders, and comorbid ADHD. “These are three common presentations of the many, many kinds of presentations,” said Dr. Goldberg, clinical professor of psychiatry at the Icahn School of Medicine at Mount Sinai in New York.
Bipolar disorder with associated psychosis
In the case of bipolar I disorder, more than 50% of manic episodes have some element of psychosis, with as many as 10% of patients showing signs of delusions 2 years after an episode, Dr. Goldberg explained. In these patients, mania relapse is predicted by mood-incongruent psychosis – a condition usually associated with schizophrenia, he said.
“If [they] have unusual beliefs and ideas, and they’re not consistent with a particular mood state, we sometimes clinically think this sounds more like a primary psychotic process,” he said. “Maybe, but not necessarily. So just because the patient may say, ‘The FBI is after me,’ or, ‘My thoughts are being read over the Internet,’ and they don’t connect that with a grandiose theme, it doesn’t negate a diagnosis of bipolar disorder.”
Psychotic mania is also associated with comorbid anxiety disorder. About half of patients with bipolar I disorder will also experience impairments of attention, executive functioning, and verbal memory separately from ADHD. “The cognitive symptoms of bipolar disorder that are part of what’s inherited doesn’t seem to be the case, that there’s a clear greater degree of neuropsychological impairment in psychotic than nonpsychotic mania,” Dr. Goldberg said.
Lithium has a poor response in the presence of psychosis in patients with bipolar disorder but performs better when the patient receives it alongside an antipsychotic. “Lithium does have value in psychotic mania,” Dr. Goldberg said. “Psychosis would be a negative prognostic sign, and certainly an indication for including an antipsychotic.”
In contrast to lithium, divalproex has shown evidence in reducing manic and psychotic symptoms similarly to haloperidol. “Divalproex may reduce mania symptoms, whether or not it’s helping psychosis. You’d think you have to get both reduced at the same time, but actually can see that even if there’s baseline psychosis, that does not diminish the chance of seeing a reduction in core mania symptoms,” Dr. Goldberg said.
Carbamazepine may also be advantageous to use over lithium when patients present with delusions, and a combination of carbamazepine and lithium may be comparable to haloperidol in combination with lithium when treating psychotic mania. “What we do know is, at least in some studies, there may be some greater value in treating psychotic mania with carbamazepine as compared to lithium, particularly when there are delusions present, more so than hallucinations or formal thought disorder,” Dr. Goldberg said.
In patients with bipolar disorder and associated psychotic mania, clinicians should avoid dopamine agonists such as amphetamine and pramipexole, as well as ketamine. While some evidence has shown that second-generation antipsychotics work to treat bipolar depression, “there’s not really an evidence base to suggest that first-generation antipsychotics are protective against depression,” Dr. Goldberg said.
Bipolar disorder with anxiety
An association exists between comorbid anxiety disorders in patients with bipolar disorder and having a younger age of onset, in people who are less likely to recover from an initial mood episode, in people with poorer quality of life and role functioning, and in people who are less euthymic and more likely to attempt suicide, Dr. Goldberg said.
In addition, some patients may demonstrate symptoms of anxiety that aren’t part of the DSM-5 criteria for an anxiety disorder. Dr. Goldberg said he asks his patients to specify what they mean when they say they feel anxious.
“I always ask patients to tell me in very basic terms what [they] mean by anxiety. If they say, ‘I just I can’t sit still; I’m very fidgety,’ maybe that’s akathisia,” he said. “Or maybe if they say they’re very anxious, what they mean is they have so much energy they can’t contain it. This is mania or hypomania that they’re misconstruing as anxiety. We have to be very diligent and vigilant in clarifying the language here.”
To treat comorbid anxiety in patients with bipolar disorder, consider adjunctive olanzapine or lamotrigine, as both have evidence of anxiolytic efficacy. “Olanzapine does count as an antianxiety agent. Would you use it just as an antianxiety agent? Probably not in and of itself, but there’s other compelling reasons to use it,” he said. Before assuming you need to add another medication to address anxiety in a patient, “step back and think perhaps their anxiety symptoms will in themselves remit with olanzapine,” he said. , he added.
Divalproex is another option for patients that has anxiolytic efficacy. “In the context of bipolar depression, divalproex does have antianxiety properties,” Dr. Goldberg said. Other anxiolytic options include lurasidone, cariprazine, quetiapine, and combination olanzapine–fluoxetine.
Bipolar disorder and ADHD
Among patients with bipolar disorder and comorbid adult ADHD, cognitive dysfunction inherent to bipolar disorder may be difficult to distinguish from signs of ADHD, Dr. Goldberg explained, with about 20% of people with bipolar I disorder and about 30% of people with bipolar II disorder have deficits of attentional processing, verbal memory, and executive functioning.
“Some researchers are very intrigued by the notion that cognitive problems and attentional problems aren’t necessarily a sign of [ADHD] comorbidities. They might be, but they may just be part of the endophenotype or the non-overt, genetically driven phenomenology that makes bipolar disorder so heterogeneous,” he said.
Patients with bipolar disorder and comorbid ADHD are more likely to have mania than depression, the condition is more common in men, and these patients are more likely to have substance use problems, increased risk of suicide attempts, problems in school, lower socioeconomic status, greater unemployment history, higher divorce rates, and low family history of bipolar disorder. Clinicians should check a patient’s history if they suspect comorbid adult ADHD in their patients with bipolar disorder, as there is a good chance evidence of ADHD will be present around the time of adolescence.
“You don’t wake up with [ADHD] at age 40, at least that’s not the prevailing perspective,” Dr. Goldberg said.
Focus on the ADHD symptoms that do not overlap with bipolar disorder, such as nondiscrete, chronic symptoms; lack of psychosis and suicidality; no evidence of grandiose beliefs; lack of hypersexuality; and depression that is not prominent. “You really need to go back in time and get some clarity as to the longitudinal course. If this was present earlier on and it persists into adulthood and it’s not better accounted for by either what we think of as the cognitive pervasive problems that emerge in bipolar disorder, or in relatives as a collaborator for attentional problems and bipolar disorder, we can then contemplate [whether] there’s a plausible basis for using a stimulant or [other ADHD] treatment,” he said.
In patients who are found to have adult comorbid ADHD and are nonmanic and nonpsychotic, stimulants do have an effect. Studies suggest that amphetamines such as adjunctive lisdexamfetamine added to a mood stabilizer show an improvement in ADHD symptoms after 4 weeks (Hum Psychopharmacol. 2013; 28[5]:421-7).
Adjunctive methylphenidate added to a mood stabilizer has also shown evidence of not causing treatment-emergent mania. “If you’re going to use methylphenidate, make sure it’s in the context of an antimanic mood stabilizer,” Dr. Goldberg said. In one study, methylphenidate without a mood stabilizer caused an increase in manic episodes within 3 months (Am J Psychiatry. 2017 Apr 1;174:341-8).
“All may pose safe and effective evidence-based, albeit provisional, but evidence-based options to consider in targeting the attentional symptoms in patients with bipolar disorder,” Dr. Goldberg said.
He reported that he has been a consultant for BioXcel Therapeutics, Medscape/WebMD, Otsuka, and Sage Therapeutics. In addition, Dr. Goldberg is on the speakers bureau for Allergan, Neurocrine, Otsuka, and Sunovion; and receives royalties from American Psychiatric Publishing. Global Academy and this news organization are owned by the same parent company.
When selecting pharmacotherapy for patients with bipolar disorder, clinical and prognostic correlates will ultimately influence what treatments make the most sense for a patient – but the process is a balancing act, according to Joseph F. Goldberg, MD.
“Everything we do in medicine in general, and psychiatry, and bipolar disorder in particular is a risk-benefit analysis,” Dr. Goldberg said at the virtual Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “Everything has its side effects. We’re always balancing risks and benefits.”
Patients with bipolar disorder often present with three common subtypes of the illness: Those who have associated psychosis, comorbid anxiety disorders, and comorbid ADHD. “These are three common presentations of the many, many kinds of presentations,” said Dr. Goldberg, clinical professor of psychiatry at the Icahn School of Medicine at Mount Sinai in New York.
Bipolar disorder with associated psychosis
In the case of bipolar I disorder, more than 50% of manic episodes have some element of psychosis, with as many as 10% of patients showing signs of delusions 2 years after an episode, Dr. Goldberg explained. In these patients, mania relapse is predicted by mood-incongruent psychosis – a condition usually associated with schizophrenia, he said.
“If [they] have unusual beliefs and ideas, and they’re not consistent with a particular mood state, we sometimes clinically think this sounds more like a primary psychotic process,” he said. “Maybe, but not necessarily. So just because the patient may say, ‘The FBI is after me,’ or, ‘My thoughts are being read over the Internet,’ and they don’t connect that with a grandiose theme, it doesn’t negate a diagnosis of bipolar disorder.”
Psychotic mania is also associated with comorbid anxiety disorder. About half of patients with bipolar I disorder will also experience impairments of attention, executive functioning, and verbal memory separately from ADHD. “The cognitive symptoms of bipolar disorder that are part of what’s inherited doesn’t seem to be the case, that there’s a clear greater degree of neuropsychological impairment in psychotic than nonpsychotic mania,” Dr. Goldberg said.
Lithium has a poor response in the presence of psychosis in patients with bipolar disorder but performs better when the patient receives it alongside an antipsychotic. “Lithium does have value in psychotic mania,” Dr. Goldberg said. “Psychosis would be a negative prognostic sign, and certainly an indication for including an antipsychotic.”
In contrast to lithium, divalproex has shown evidence in reducing manic and psychotic symptoms similarly to haloperidol. “Divalproex may reduce mania symptoms, whether or not it’s helping psychosis. You’d think you have to get both reduced at the same time, but actually can see that even if there’s baseline psychosis, that does not diminish the chance of seeing a reduction in core mania symptoms,” Dr. Goldberg said.
Carbamazepine may also be advantageous to use over lithium when patients present with delusions, and a combination of carbamazepine and lithium may be comparable to haloperidol in combination with lithium when treating psychotic mania. “What we do know is, at least in some studies, there may be some greater value in treating psychotic mania with carbamazepine as compared to lithium, particularly when there are delusions present, more so than hallucinations or formal thought disorder,” Dr. Goldberg said.
In patients with bipolar disorder and associated psychotic mania, clinicians should avoid dopamine agonists such as amphetamine and pramipexole, as well as ketamine. While some evidence has shown that second-generation antipsychotics work to treat bipolar depression, “there’s not really an evidence base to suggest that first-generation antipsychotics are protective against depression,” Dr. Goldberg said.
Bipolar disorder with anxiety
An association exists between comorbid anxiety disorders in patients with bipolar disorder and having a younger age of onset, in people who are less likely to recover from an initial mood episode, in people with poorer quality of life and role functioning, and in people who are less euthymic and more likely to attempt suicide, Dr. Goldberg said.
In addition, some patients may demonstrate symptoms of anxiety that aren’t part of the DSM-5 criteria for an anxiety disorder. Dr. Goldberg said he asks his patients to specify what they mean when they say they feel anxious.
“I always ask patients to tell me in very basic terms what [they] mean by anxiety. If they say, ‘I just I can’t sit still; I’m very fidgety,’ maybe that’s akathisia,” he said. “Or maybe if they say they’re very anxious, what they mean is they have so much energy they can’t contain it. This is mania or hypomania that they’re misconstruing as anxiety. We have to be very diligent and vigilant in clarifying the language here.”
To treat comorbid anxiety in patients with bipolar disorder, consider adjunctive olanzapine or lamotrigine, as both have evidence of anxiolytic efficacy. “Olanzapine does count as an antianxiety agent. Would you use it just as an antianxiety agent? Probably not in and of itself, but there’s other compelling reasons to use it,” he said. Before assuming you need to add another medication to address anxiety in a patient, “step back and think perhaps their anxiety symptoms will in themselves remit with olanzapine,” he said. , he added.
Divalproex is another option for patients that has anxiolytic efficacy. “In the context of bipolar depression, divalproex does have antianxiety properties,” Dr. Goldberg said. Other anxiolytic options include lurasidone, cariprazine, quetiapine, and combination olanzapine–fluoxetine.
Bipolar disorder and ADHD
Among patients with bipolar disorder and comorbid adult ADHD, cognitive dysfunction inherent to bipolar disorder may be difficult to distinguish from signs of ADHD, Dr. Goldberg explained, with about 20% of people with bipolar I disorder and about 30% of people with bipolar II disorder have deficits of attentional processing, verbal memory, and executive functioning.
“Some researchers are very intrigued by the notion that cognitive problems and attentional problems aren’t necessarily a sign of [ADHD] comorbidities. They might be, but they may just be part of the endophenotype or the non-overt, genetically driven phenomenology that makes bipolar disorder so heterogeneous,” he said.
Patients with bipolar disorder and comorbid ADHD are more likely to have mania than depression, the condition is more common in men, and these patients are more likely to have substance use problems, increased risk of suicide attempts, problems in school, lower socioeconomic status, greater unemployment history, higher divorce rates, and low family history of bipolar disorder. Clinicians should check a patient’s history if they suspect comorbid adult ADHD in their patients with bipolar disorder, as there is a good chance evidence of ADHD will be present around the time of adolescence.
“You don’t wake up with [ADHD] at age 40, at least that’s not the prevailing perspective,” Dr. Goldberg said.
Focus on the ADHD symptoms that do not overlap with bipolar disorder, such as nondiscrete, chronic symptoms; lack of psychosis and suicidality; no evidence of grandiose beliefs; lack of hypersexuality; and depression that is not prominent. “You really need to go back in time and get some clarity as to the longitudinal course. If this was present earlier on and it persists into adulthood and it’s not better accounted for by either what we think of as the cognitive pervasive problems that emerge in bipolar disorder, or in relatives as a collaborator for attentional problems and bipolar disorder, we can then contemplate [whether] there’s a plausible basis for using a stimulant or [other ADHD] treatment,” he said.
In patients who are found to have adult comorbid ADHD and are nonmanic and nonpsychotic, stimulants do have an effect. Studies suggest that amphetamines such as adjunctive lisdexamfetamine added to a mood stabilizer show an improvement in ADHD symptoms after 4 weeks (Hum Psychopharmacol. 2013; 28[5]:421-7).
Adjunctive methylphenidate added to a mood stabilizer has also shown evidence of not causing treatment-emergent mania. “If you’re going to use methylphenidate, make sure it’s in the context of an antimanic mood stabilizer,” Dr. Goldberg said. In one study, methylphenidate without a mood stabilizer caused an increase in manic episodes within 3 months (Am J Psychiatry. 2017 Apr 1;174:341-8).
“All may pose safe and effective evidence-based, albeit provisional, but evidence-based options to consider in targeting the attentional symptoms in patients with bipolar disorder,” Dr. Goldberg said.
He reported that he has been a consultant for BioXcel Therapeutics, Medscape/WebMD, Otsuka, and Sage Therapeutics. In addition, Dr. Goldberg is on the speakers bureau for Allergan, Neurocrine, Otsuka, and Sunovion; and receives royalties from American Psychiatric Publishing. Global Academy and this news organization are owned by the same parent company.
When selecting pharmacotherapy for patients with bipolar disorder, clinical and prognostic correlates will ultimately influence what treatments make the most sense for a patient – but the process is a balancing act, according to Joseph F. Goldberg, MD.
“Everything we do in medicine in general, and psychiatry, and bipolar disorder in particular is a risk-benefit analysis,” Dr. Goldberg said at the virtual Psychopharmacology Update presented by Current Psychiatry and Global Academy for Medical Education. “Everything has its side effects. We’re always balancing risks and benefits.”
Patients with bipolar disorder often present with three common subtypes of the illness: Those who have associated psychosis, comorbid anxiety disorders, and comorbid ADHD. “These are three common presentations of the many, many kinds of presentations,” said Dr. Goldberg, clinical professor of psychiatry at the Icahn School of Medicine at Mount Sinai in New York.
Bipolar disorder with associated psychosis
In the case of bipolar I disorder, more than 50% of manic episodes have some element of psychosis, with as many as 10% of patients showing signs of delusions 2 years after an episode, Dr. Goldberg explained. In these patients, mania relapse is predicted by mood-incongruent psychosis – a condition usually associated with schizophrenia, he said.
“If [they] have unusual beliefs and ideas, and they’re not consistent with a particular mood state, we sometimes clinically think this sounds more like a primary psychotic process,” he said. “Maybe, but not necessarily. So just because the patient may say, ‘The FBI is after me,’ or, ‘My thoughts are being read over the Internet,’ and they don’t connect that with a grandiose theme, it doesn’t negate a diagnosis of bipolar disorder.”
Psychotic mania is also associated with comorbid anxiety disorder. About half of patients with bipolar I disorder will also experience impairments of attention, executive functioning, and verbal memory separately from ADHD. “The cognitive symptoms of bipolar disorder that are part of what’s inherited doesn’t seem to be the case, that there’s a clear greater degree of neuropsychological impairment in psychotic than nonpsychotic mania,” Dr. Goldberg said.
Lithium has a poor response in the presence of psychosis in patients with bipolar disorder but performs better when the patient receives it alongside an antipsychotic. “Lithium does have value in psychotic mania,” Dr. Goldberg said. “Psychosis would be a negative prognostic sign, and certainly an indication for including an antipsychotic.”
In contrast to lithium, divalproex has shown evidence in reducing manic and psychotic symptoms similarly to haloperidol. “Divalproex may reduce mania symptoms, whether or not it’s helping psychosis. You’d think you have to get both reduced at the same time, but actually can see that even if there’s baseline psychosis, that does not diminish the chance of seeing a reduction in core mania symptoms,” Dr. Goldberg said.
Carbamazepine may also be advantageous to use over lithium when patients present with delusions, and a combination of carbamazepine and lithium may be comparable to haloperidol in combination with lithium when treating psychotic mania. “What we do know is, at least in some studies, there may be some greater value in treating psychotic mania with carbamazepine as compared to lithium, particularly when there are delusions present, more so than hallucinations or formal thought disorder,” Dr. Goldberg said.
In patients with bipolar disorder and associated psychotic mania, clinicians should avoid dopamine agonists such as amphetamine and pramipexole, as well as ketamine. While some evidence has shown that second-generation antipsychotics work to treat bipolar depression, “there’s not really an evidence base to suggest that first-generation antipsychotics are protective against depression,” Dr. Goldberg said.
Bipolar disorder with anxiety
An association exists between comorbid anxiety disorders in patients with bipolar disorder and having a younger age of onset, in people who are less likely to recover from an initial mood episode, in people with poorer quality of life and role functioning, and in people who are less euthymic and more likely to attempt suicide, Dr. Goldberg said.
In addition, some patients may demonstrate symptoms of anxiety that aren’t part of the DSM-5 criteria for an anxiety disorder. Dr. Goldberg said he asks his patients to specify what they mean when they say they feel anxious.
“I always ask patients to tell me in very basic terms what [they] mean by anxiety. If they say, ‘I just I can’t sit still; I’m very fidgety,’ maybe that’s akathisia,” he said. “Or maybe if they say they’re very anxious, what they mean is they have so much energy they can’t contain it. This is mania or hypomania that they’re misconstruing as anxiety. We have to be very diligent and vigilant in clarifying the language here.”
To treat comorbid anxiety in patients with bipolar disorder, consider adjunctive olanzapine or lamotrigine, as both have evidence of anxiolytic efficacy. “Olanzapine does count as an antianxiety agent. Would you use it just as an antianxiety agent? Probably not in and of itself, but there’s other compelling reasons to use it,” he said. Before assuming you need to add another medication to address anxiety in a patient, “step back and think perhaps their anxiety symptoms will in themselves remit with olanzapine,” he said. , he added.
Divalproex is another option for patients that has anxiolytic efficacy. “In the context of bipolar depression, divalproex does have antianxiety properties,” Dr. Goldberg said. Other anxiolytic options include lurasidone, cariprazine, quetiapine, and combination olanzapine–fluoxetine.
Bipolar disorder and ADHD
Among patients with bipolar disorder and comorbid adult ADHD, cognitive dysfunction inherent to bipolar disorder may be difficult to distinguish from signs of ADHD, Dr. Goldberg explained, with about 20% of people with bipolar I disorder and about 30% of people with bipolar II disorder have deficits of attentional processing, verbal memory, and executive functioning.
“Some researchers are very intrigued by the notion that cognitive problems and attentional problems aren’t necessarily a sign of [ADHD] comorbidities. They might be, but they may just be part of the endophenotype or the non-overt, genetically driven phenomenology that makes bipolar disorder so heterogeneous,” he said.
Patients with bipolar disorder and comorbid ADHD are more likely to have mania than depression, the condition is more common in men, and these patients are more likely to have substance use problems, increased risk of suicide attempts, problems in school, lower socioeconomic status, greater unemployment history, higher divorce rates, and low family history of bipolar disorder. Clinicians should check a patient’s history if they suspect comorbid adult ADHD in their patients with bipolar disorder, as there is a good chance evidence of ADHD will be present around the time of adolescence.
“You don’t wake up with [ADHD] at age 40, at least that’s not the prevailing perspective,” Dr. Goldberg said.
Focus on the ADHD symptoms that do not overlap with bipolar disorder, such as nondiscrete, chronic symptoms; lack of psychosis and suicidality; no evidence of grandiose beliefs; lack of hypersexuality; and depression that is not prominent. “You really need to go back in time and get some clarity as to the longitudinal course. If this was present earlier on and it persists into adulthood and it’s not better accounted for by either what we think of as the cognitive pervasive problems that emerge in bipolar disorder, or in relatives as a collaborator for attentional problems and bipolar disorder, we can then contemplate [whether] there’s a plausible basis for using a stimulant or [other ADHD] treatment,” he said.
In patients who are found to have adult comorbid ADHD and are nonmanic and nonpsychotic, stimulants do have an effect. Studies suggest that amphetamines such as adjunctive lisdexamfetamine added to a mood stabilizer show an improvement in ADHD symptoms after 4 weeks (Hum Psychopharmacol. 2013; 28[5]:421-7).
Adjunctive methylphenidate added to a mood stabilizer has also shown evidence of not causing treatment-emergent mania. “If you’re going to use methylphenidate, make sure it’s in the context of an antimanic mood stabilizer,” Dr. Goldberg said. In one study, methylphenidate without a mood stabilizer caused an increase in manic episodes within 3 months (Am J Psychiatry. 2017 Apr 1;174:341-8).
“All may pose safe and effective evidence-based, albeit provisional, but evidence-based options to consider in targeting the attentional symptoms in patients with bipolar disorder,” Dr. Goldberg said.
He reported that he has been a consultant for BioXcel Therapeutics, Medscape/WebMD, Otsuka, and Sage Therapeutics. In addition, Dr. Goldberg is on the speakers bureau for Allergan, Neurocrine, Otsuka, and Sunovion; and receives royalties from American Psychiatric Publishing. Global Academy and this news organization are owned by the same parent company.
FROM PSYCHOPHARMACOLOGY UPDATE
How cannabis-based therapeutics could help fight COVID inflammation
Plagued by false starts, a few dashed hopes, but with perhaps a glimmer of light on the horizon, the race to find an effective treatment for COVID-19 continues. At last count, more than 300 treatments and 200 vaccines were in preclinical or clinical development (not to mention the numerous existing agents that are being evaluated for repurposing).
There is also a renewed interest in cannabinoid therapeutics — in particular, the nonpsychoactive agent cannabidiol (CBD) and the prospect of its modulating inflammatory and other disease-associated clinical indices, including SARS-CoV-2–induced viral load, hyperinflammation, the cytokine storm, and acute respiratory distress syndrome (ARDS).
Long hobbled by regulatory, political, and financial barriers, CBD’s potential ability to knock back COVID-19–related inflammation might just open doors that have been closed for years to CBD researchers.
Why CBD and why now?
CBD and the resulting therapeutics have been plagued by a complicated association with recreational cannabis use. It’s been just 2 years since CBD-based therapeutics moved into mainstream medicine — the US Food and Drug Administration (FDA) approved Epidiolex oral solution for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, and in August, the FDA approved it for tuberous sclerosis complex.
CBD’s mechanism of action has not been fully elucidated, but on the basis of its role in immune responses — well described in research spanning more than two decades — it›s not surprising that cannabinoid researchers have thrown their hats into the COVID-19 drug development ring.
The anti-inflammatory potential of CBD is substantial and appears to be related to the fact that it shares 20 protein targets common to inflammation-related pathways, Jenny Wilkerson, PhD, research assistant professor at the University of Florida School of Pharmacy, Gainesville, Florida, explained to Medscape Medical News.
Among the various trials that are currently recruiting or are underway is one that is slated for completion this fall. CANDIDATE (Cannabidiol for COVID-19 Patients With Mild-to-Moderate COVID-19) is a randomized, controlled, double-blind study led by Brazilian researchers at the University of São Paulo. The study, which began recruitment this past August, enrolled 100 patients, 50 in the active treatment group (who received capsulated CBD 300 mg daily for 14 days plus pharmacologic therapy [antipyretics] and clinical measures) and 50 who received placebo.
The primary outcome is intended to help clarify the potential role of oral CBD for preventing COVID-19 disease progression, modifying disease-associated clinical indices, and modulating inflammatory parameters, such as the cytokine storm, according to lead investigator Jose Alexandre de Souza Crippa, MD, PhD, professor of neuropsychology at the Ribeirao Preto Medical School at the University of São Paulo in Brazil, in the description of the study on clinicaltrials.gov. Crippa declined to provide any additional information about the trial in an email to Medscape Medical News.
Calming or preventing the storm
While Crippa and colleagues wrap up their CBD trial in South America, several North American and Canadian researchers are seeking to clarify and address one of the most therapeutically challenging aspects of SARS-CoV-2 infection — the lung macrophage–orchestrated hyperinflammatory response.
Although hyperinflammation is not unique to SARS-CoV-2 infection, disease severity and COVID-19–related mortality have been linked to this rapid and prolonged surge of inflammatory cytokines (eg, interleukin 6 [IL-6], IL-10, tumor necrosis factors [TNF], and chemokines) and the cytokine storm.
“When you stimulate CB2 receptors (involved in fighting inflammation), you get a release of the same inflammatory cytokines that are involved in COVID,” Cecilia Costiniuk, MD, associate professor and researcher at the Research Institute of the McGill University Health Center, Montreal, Canada, told Medscape Medical News.
“So, if you can act on this receptor, you might be able to reduce the release of those damaging cytokines that are causing ARDS, lung damage, etc,” she explained. Targeting these inflammatory mediators has been a key strategy in research aimed at reducing COVID-19 severity and related mortality, which is where CBD comes into play.
“CBD is a very powerful immune regulator. It keeps the [immune] engine on, but it doesn’t push the gas pedal, and it doesn’t push the brake completely,” Babak Baban, PhD, professor and immunologist at the Dental College of Georgia at Augusta University, told Medscape Medical News.
To explore the effectiveness of CBD in reducing hyperactivated inflammatory reactions, Baban and colleagues examined the potential of CBD to ameliorate ARDS in a murine model. The group divided wild-type male mice into sham, control, and treatment groups.
The sham group received intranasal phosphate buffered saline; the treatment and control groups received a polyriboinosinic:polycytidylic acid (poly I:C) double-stranded RNA analogue (100 mcg daily for 3 days) to simulate the cytokine storm and clinical ARDS symptoms.
Following the second poly I:C dose, the treatment group received CBD 5 mg/kg intraperitoneally every other day for 6 days. The mice were sacrificed on day 8.
The study results, published in July in Cannabis and Cannabinoid Research, first confirmed that the poly I:C model simulated the cytokine storm in ARDS, reducing blood oxygen saturation by as much as 10% (from ±81.6% to ±72.2%).
Intraperitoneally administered CBD appeared to reverse these ARDS-like trends. “We observed a significant improvement in severe lymphopenia, a mild decline in the ratio of neutrophils to T cells, and significant reductions in levels of [inflammatory and immune factors] IL-6, IFN-gamma [interferon gamma], and in TNF-alpha after the second CBD dose,” Baban said.
There was also a marked downregulation in infiltrating neutrophils and macrophages in the lung, leading to partial restoration of lung morphology and structure. The investigators write that this suggests “a counter inflammatory role for CBD to limit ARDS progression.”
Additional findings from a follow-up study published in mid-October “provide strong data that CBD may partially assert its beneficial and protective impact through its regulation of the apelin peptide,” wrote Baban in an email to Medscape Medical News.
“Apelin may also be a reliable biomarker for early diagnosis of ARDS in general, and in COVID-19 in particular,” he wrote.
Questions remain concerning dose response and whether CBD alone or in combination with other phytocannabinoids is more effective for treating COVID-19. Timing is likewise unclear.
Baban explained that as a result of the biphasic nature of COVID-19, the “sweet spot” appears to be just before the innate immune response progresses into an inflammation-driven response and fibrotic lung damage occurs.
But Wilkerson isn’t as convinced. She said that as with a thermostat, the endocannabinoid system needs tweaking to get it in the right place, that is, to achieve immune homeostasis. The COVID cytokine storm is highly unpredictable, she added, saying, “Right now, the timing for controlling the COVID cytokine storm is really a moving target.”
Is safety a concern?
Safety questions are expected to arise, especially in relation to COVID-19. CBD is not risk free, and one size does not fit all. Human CBD studies report gastrointestinal and somnolent effects, as well as drug-drug interactions.
Findings from a recent systematic review of randomized, controlled CBD trials support overall tolerability, suggesting that serious adverse events are rare. Such events are believed to be related to drug-drug interactions rather than to CBD itself. On the flip side, it is nonintoxicating, and there does not appear to be potential for abuse.
“It’s generally well tolerated,” Wilkerson said. “There’ve now been several clinical trials in numerous patient population settings where basically the only time you really start to have issues is where you have patients on very select agents. But this is where a pharmacist would come into play.”
Costiniuk agreed: “Just because it’s cannabis, it doesn’t mean that there’s going to be strange or unusual effects; these people [ie, those with severe COVID-19] are in the hospital and monitored very closely.”
Delving into the weeds: What’s next?
Although non-COVID-19 cannabinoid researchers have encountered regulatory roadblocks, several research groups that have had the prescience to dive in at the right time are gaining momentum.
Baban’s team has connected with one of the nation’s few academic laboratories authorized to work with the SARS-CoV-2 virus and are awaiting protocol approval so that they can reproduce their research, this time using two CBD formulations (injectable and inhaled).
If findings are positive, they will move forward quickly to meet with the FDA, Baban said, adding that the team is also collaborating with two organizations to conduct human clinical trials in hopes of pushing up timing.
The initial article caught the eye of the World Health Organization, which included it in its global literature on the coronavirus resource section.
Israeli researchers have also been quite busy. InnoCan Pharma and Tel Aviv University are collaborating to explore the potential for CBD-loaded exosomes (minute extracellular particles that mediate intracellular communication, including via innate and adaptive immune responses). The group plans to use these loaded exosomes to target and facilitate recovery of COVID-19–damaged lung cells.
From a broader perspective, the prospects for harnessing cannabinoids for immune modulation will be more thoroughly explored in a special issue of Cannabis and Cannabinoid Research, which has extended its current call for papers, studies, abstracts, and conference proceedings until the end of December.
Like many of the therapeutic strategies under investigation for the treatment of COVID-19, studies in CBD may continue to raise more questions than answers.
Still, Wilkerson is optimistic. “Taken together, these studies along with countless others suggest that the complex pharmacophore of Cannabis sativa may hold therapeutic utility to treat lung inflammation, such as what is seen in a COVID-19 cytokine storm,» she told Medscape Medical News. “I’m very excited to see what comes out of the research.”
Baban, Wilkerson, and Costiniuk have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Plagued by false starts, a few dashed hopes, but with perhaps a glimmer of light on the horizon, the race to find an effective treatment for COVID-19 continues. At last count, more than 300 treatments and 200 vaccines were in preclinical or clinical development (not to mention the numerous existing agents that are being evaluated for repurposing).
There is also a renewed interest in cannabinoid therapeutics — in particular, the nonpsychoactive agent cannabidiol (CBD) and the prospect of its modulating inflammatory and other disease-associated clinical indices, including SARS-CoV-2–induced viral load, hyperinflammation, the cytokine storm, and acute respiratory distress syndrome (ARDS).
Long hobbled by regulatory, political, and financial barriers, CBD’s potential ability to knock back COVID-19–related inflammation might just open doors that have been closed for years to CBD researchers.
Why CBD and why now?
CBD and the resulting therapeutics have been plagued by a complicated association with recreational cannabis use. It’s been just 2 years since CBD-based therapeutics moved into mainstream medicine — the US Food and Drug Administration (FDA) approved Epidiolex oral solution for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, and in August, the FDA approved it for tuberous sclerosis complex.
CBD’s mechanism of action has not been fully elucidated, but on the basis of its role in immune responses — well described in research spanning more than two decades — it›s not surprising that cannabinoid researchers have thrown their hats into the COVID-19 drug development ring.
The anti-inflammatory potential of CBD is substantial and appears to be related to the fact that it shares 20 protein targets common to inflammation-related pathways, Jenny Wilkerson, PhD, research assistant professor at the University of Florida School of Pharmacy, Gainesville, Florida, explained to Medscape Medical News.
Among the various trials that are currently recruiting or are underway is one that is slated for completion this fall. CANDIDATE (Cannabidiol for COVID-19 Patients With Mild-to-Moderate COVID-19) is a randomized, controlled, double-blind study led by Brazilian researchers at the University of São Paulo. The study, which began recruitment this past August, enrolled 100 patients, 50 in the active treatment group (who received capsulated CBD 300 mg daily for 14 days plus pharmacologic therapy [antipyretics] and clinical measures) and 50 who received placebo.
The primary outcome is intended to help clarify the potential role of oral CBD for preventing COVID-19 disease progression, modifying disease-associated clinical indices, and modulating inflammatory parameters, such as the cytokine storm, according to lead investigator Jose Alexandre de Souza Crippa, MD, PhD, professor of neuropsychology at the Ribeirao Preto Medical School at the University of São Paulo in Brazil, in the description of the study on clinicaltrials.gov. Crippa declined to provide any additional information about the trial in an email to Medscape Medical News.
Calming or preventing the storm
While Crippa and colleagues wrap up their CBD trial in South America, several North American and Canadian researchers are seeking to clarify and address one of the most therapeutically challenging aspects of SARS-CoV-2 infection — the lung macrophage–orchestrated hyperinflammatory response.
Although hyperinflammation is not unique to SARS-CoV-2 infection, disease severity and COVID-19–related mortality have been linked to this rapid and prolonged surge of inflammatory cytokines (eg, interleukin 6 [IL-6], IL-10, tumor necrosis factors [TNF], and chemokines) and the cytokine storm.
“When you stimulate CB2 receptors (involved in fighting inflammation), you get a release of the same inflammatory cytokines that are involved in COVID,” Cecilia Costiniuk, MD, associate professor and researcher at the Research Institute of the McGill University Health Center, Montreal, Canada, told Medscape Medical News.
“So, if you can act on this receptor, you might be able to reduce the release of those damaging cytokines that are causing ARDS, lung damage, etc,” she explained. Targeting these inflammatory mediators has been a key strategy in research aimed at reducing COVID-19 severity and related mortality, which is where CBD comes into play.
“CBD is a very powerful immune regulator. It keeps the [immune] engine on, but it doesn’t push the gas pedal, and it doesn’t push the brake completely,” Babak Baban, PhD, professor and immunologist at the Dental College of Georgia at Augusta University, told Medscape Medical News.
To explore the effectiveness of CBD in reducing hyperactivated inflammatory reactions, Baban and colleagues examined the potential of CBD to ameliorate ARDS in a murine model. The group divided wild-type male mice into sham, control, and treatment groups.
The sham group received intranasal phosphate buffered saline; the treatment and control groups received a polyriboinosinic:polycytidylic acid (poly I:C) double-stranded RNA analogue (100 mcg daily for 3 days) to simulate the cytokine storm and clinical ARDS symptoms.
Following the second poly I:C dose, the treatment group received CBD 5 mg/kg intraperitoneally every other day for 6 days. The mice were sacrificed on day 8.
The study results, published in July in Cannabis and Cannabinoid Research, first confirmed that the poly I:C model simulated the cytokine storm in ARDS, reducing blood oxygen saturation by as much as 10% (from ±81.6% to ±72.2%).
Intraperitoneally administered CBD appeared to reverse these ARDS-like trends. “We observed a significant improvement in severe lymphopenia, a mild decline in the ratio of neutrophils to T cells, and significant reductions in levels of [inflammatory and immune factors] IL-6, IFN-gamma [interferon gamma], and in TNF-alpha after the second CBD dose,” Baban said.
There was also a marked downregulation in infiltrating neutrophils and macrophages in the lung, leading to partial restoration of lung morphology and structure. The investigators write that this suggests “a counter inflammatory role for CBD to limit ARDS progression.”
Additional findings from a follow-up study published in mid-October “provide strong data that CBD may partially assert its beneficial and protective impact through its regulation of the apelin peptide,” wrote Baban in an email to Medscape Medical News.
“Apelin may also be a reliable biomarker for early diagnosis of ARDS in general, and in COVID-19 in particular,” he wrote.
Questions remain concerning dose response and whether CBD alone or in combination with other phytocannabinoids is more effective for treating COVID-19. Timing is likewise unclear.
Baban explained that as a result of the biphasic nature of COVID-19, the “sweet spot” appears to be just before the innate immune response progresses into an inflammation-driven response and fibrotic lung damage occurs.
But Wilkerson isn’t as convinced. She said that as with a thermostat, the endocannabinoid system needs tweaking to get it in the right place, that is, to achieve immune homeostasis. The COVID cytokine storm is highly unpredictable, she added, saying, “Right now, the timing for controlling the COVID cytokine storm is really a moving target.”
Is safety a concern?
Safety questions are expected to arise, especially in relation to COVID-19. CBD is not risk free, and one size does not fit all. Human CBD studies report gastrointestinal and somnolent effects, as well as drug-drug interactions.
Findings from a recent systematic review of randomized, controlled CBD trials support overall tolerability, suggesting that serious adverse events are rare. Such events are believed to be related to drug-drug interactions rather than to CBD itself. On the flip side, it is nonintoxicating, and there does not appear to be potential for abuse.
“It’s generally well tolerated,” Wilkerson said. “There’ve now been several clinical trials in numerous patient population settings where basically the only time you really start to have issues is where you have patients on very select agents. But this is where a pharmacist would come into play.”
Costiniuk agreed: “Just because it’s cannabis, it doesn’t mean that there’s going to be strange or unusual effects; these people [ie, those with severe COVID-19] are in the hospital and monitored very closely.”
Delving into the weeds: What’s next?
Although non-COVID-19 cannabinoid researchers have encountered regulatory roadblocks, several research groups that have had the prescience to dive in at the right time are gaining momentum.
Baban’s team has connected with one of the nation’s few academic laboratories authorized to work with the SARS-CoV-2 virus and are awaiting protocol approval so that they can reproduce their research, this time using two CBD formulations (injectable and inhaled).
If findings are positive, they will move forward quickly to meet with the FDA, Baban said, adding that the team is also collaborating with two organizations to conduct human clinical trials in hopes of pushing up timing.
The initial article caught the eye of the World Health Organization, which included it in its global literature on the coronavirus resource section.
Israeli researchers have also been quite busy. InnoCan Pharma and Tel Aviv University are collaborating to explore the potential for CBD-loaded exosomes (minute extracellular particles that mediate intracellular communication, including via innate and adaptive immune responses). The group plans to use these loaded exosomes to target and facilitate recovery of COVID-19–damaged lung cells.
From a broader perspective, the prospects for harnessing cannabinoids for immune modulation will be more thoroughly explored in a special issue of Cannabis and Cannabinoid Research, which has extended its current call for papers, studies, abstracts, and conference proceedings until the end of December.
Like many of the therapeutic strategies under investigation for the treatment of COVID-19, studies in CBD may continue to raise more questions than answers.
Still, Wilkerson is optimistic. “Taken together, these studies along with countless others suggest that the complex pharmacophore of Cannabis sativa may hold therapeutic utility to treat lung inflammation, such as what is seen in a COVID-19 cytokine storm,» she told Medscape Medical News. “I’m very excited to see what comes out of the research.”
Baban, Wilkerson, and Costiniuk have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Plagued by false starts, a few dashed hopes, but with perhaps a glimmer of light on the horizon, the race to find an effective treatment for COVID-19 continues. At last count, more than 300 treatments and 200 vaccines were in preclinical or clinical development (not to mention the numerous existing agents that are being evaluated for repurposing).
There is also a renewed interest in cannabinoid therapeutics — in particular, the nonpsychoactive agent cannabidiol (CBD) and the prospect of its modulating inflammatory and other disease-associated clinical indices, including SARS-CoV-2–induced viral load, hyperinflammation, the cytokine storm, and acute respiratory distress syndrome (ARDS).
Long hobbled by regulatory, political, and financial barriers, CBD’s potential ability to knock back COVID-19–related inflammation might just open doors that have been closed for years to CBD researchers.
Why CBD and why now?
CBD and the resulting therapeutics have been plagued by a complicated association with recreational cannabis use. It’s been just 2 years since CBD-based therapeutics moved into mainstream medicine — the US Food and Drug Administration (FDA) approved Epidiolex oral solution for the treatment of Lennox-Gastaut syndrome and Dravet syndrome, and in August, the FDA approved it for tuberous sclerosis complex.
CBD’s mechanism of action has not been fully elucidated, but on the basis of its role in immune responses — well described in research spanning more than two decades — it›s not surprising that cannabinoid researchers have thrown their hats into the COVID-19 drug development ring.
The anti-inflammatory potential of CBD is substantial and appears to be related to the fact that it shares 20 protein targets common to inflammation-related pathways, Jenny Wilkerson, PhD, research assistant professor at the University of Florida School of Pharmacy, Gainesville, Florida, explained to Medscape Medical News.
Among the various trials that are currently recruiting or are underway is one that is slated for completion this fall. CANDIDATE (Cannabidiol for COVID-19 Patients With Mild-to-Moderate COVID-19) is a randomized, controlled, double-blind study led by Brazilian researchers at the University of São Paulo. The study, which began recruitment this past August, enrolled 100 patients, 50 in the active treatment group (who received capsulated CBD 300 mg daily for 14 days plus pharmacologic therapy [antipyretics] and clinical measures) and 50 who received placebo.
The primary outcome is intended to help clarify the potential role of oral CBD for preventing COVID-19 disease progression, modifying disease-associated clinical indices, and modulating inflammatory parameters, such as the cytokine storm, according to lead investigator Jose Alexandre de Souza Crippa, MD, PhD, professor of neuropsychology at the Ribeirao Preto Medical School at the University of São Paulo in Brazil, in the description of the study on clinicaltrials.gov. Crippa declined to provide any additional information about the trial in an email to Medscape Medical News.
Calming or preventing the storm
While Crippa and colleagues wrap up their CBD trial in South America, several North American and Canadian researchers are seeking to clarify and address one of the most therapeutically challenging aspects of SARS-CoV-2 infection — the lung macrophage–orchestrated hyperinflammatory response.
Although hyperinflammation is not unique to SARS-CoV-2 infection, disease severity and COVID-19–related mortality have been linked to this rapid and prolonged surge of inflammatory cytokines (eg, interleukin 6 [IL-6], IL-10, tumor necrosis factors [TNF], and chemokines) and the cytokine storm.
“When you stimulate CB2 receptors (involved in fighting inflammation), you get a release of the same inflammatory cytokines that are involved in COVID,” Cecilia Costiniuk, MD, associate professor and researcher at the Research Institute of the McGill University Health Center, Montreal, Canada, told Medscape Medical News.
“So, if you can act on this receptor, you might be able to reduce the release of those damaging cytokines that are causing ARDS, lung damage, etc,” she explained. Targeting these inflammatory mediators has been a key strategy in research aimed at reducing COVID-19 severity and related mortality, which is where CBD comes into play.
“CBD is a very powerful immune regulator. It keeps the [immune] engine on, but it doesn’t push the gas pedal, and it doesn’t push the brake completely,” Babak Baban, PhD, professor and immunologist at the Dental College of Georgia at Augusta University, told Medscape Medical News.
To explore the effectiveness of CBD in reducing hyperactivated inflammatory reactions, Baban and colleagues examined the potential of CBD to ameliorate ARDS in a murine model. The group divided wild-type male mice into sham, control, and treatment groups.
The sham group received intranasal phosphate buffered saline; the treatment and control groups received a polyriboinosinic:polycytidylic acid (poly I:C) double-stranded RNA analogue (100 mcg daily for 3 days) to simulate the cytokine storm and clinical ARDS symptoms.
Following the second poly I:C dose, the treatment group received CBD 5 mg/kg intraperitoneally every other day for 6 days. The mice were sacrificed on day 8.
The study results, published in July in Cannabis and Cannabinoid Research, first confirmed that the poly I:C model simulated the cytokine storm in ARDS, reducing blood oxygen saturation by as much as 10% (from ±81.6% to ±72.2%).
Intraperitoneally administered CBD appeared to reverse these ARDS-like trends. “We observed a significant improvement in severe lymphopenia, a mild decline in the ratio of neutrophils to T cells, and significant reductions in levels of [inflammatory and immune factors] IL-6, IFN-gamma [interferon gamma], and in TNF-alpha after the second CBD dose,” Baban said.
There was also a marked downregulation in infiltrating neutrophils and macrophages in the lung, leading to partial restoration of lung morphology and structure. The investigators write that this suggests “a counter inflammatory role for CBD to limit ARDS progression.”
Additional findings from a follow-up study published in mid-October “provide strong data that CBD may partially assert its beneficial and protective impact through its regulation of the apelin peptide,” wrote Baban in an email to Medscape Medical News.
“Apelin may also be a reliable biomarker for early diagnosis of ARDS in general, and in COVID-19 in particular,” he wrote.
Questions remain concerning dose response and whether CBD alone or in combination with other phytocannabinoids is more effective for treating COVID-19. Timing is likewise unclear.
Baban explained that as a result of the biphasic nature of COVID-19, the “sweet spot” appears to be just before the innate immune response progresses into an inflammation-driven response and fibrotic lung damage occurs.
But Wilkerson isn’t as convinced. She said that as with a thermostat, the endocannabinoid system needs tweaking to get it in the right place, that is, to achieve immune homeostasis. The COVID cytokine storm is highly unpredictable, she added, saying, “Right now, the timing for controlling the COVID cytokine storm is really a moving target.”
Is safety a concern?
Safety questions are expected to arise, especially in relation to COVID-19. CBD is not risk free, and one size does not fit all. Human CBD studies report gastrointestinal and somnolent effects, as well as drug-drug interactions.
Findings from a recent systematic review of randomized, controlled CBD trials support overall tolerability, suggesting that serious adverse events are rare. Such events are believed to be related to drug-drug interactions rather than to CBD itself. On the flip side, it is nonintoxicating, and there does not appear to be potential for abuse.
“It’s generally well tolerated,” Wilkerson said. “There’ve now been several clinical trials in numerous patient population settings where basically the only time you really start to have issues is where you have patients on very select agents. But this is where a pharmacist would come into play.”
Costiniuk agreed: “Just because it’s cannabis, it doesn’t mean that there’s going to be strange or unusual effects; these people [ie, those with severe COVID-19] are in the hospital and monitored very closely.”
Delving into the weeds: What’s next?
Although non-COVID-19 cannabinoid researchers have encountered regulatory roadblocks, several research groups that have had the prescience to dive in at the right time are gaining momentum.
Baban’s team has connected with one of the nation’s few academic laboratories authorized to work with the SARS-CoV-2 virus and are awaiting protocol approval so that they can reproduce their research, this time using two CBD formulations (injectable and inhaled).
If findings are positive, they will move forward quickly to meet with the FDA, Baban said, adding that the team is also collaborating with two organizations to conduct human clinical trials in hopes of pushing up timing.
The initial article caught the eye of the World Health Organization, which included it in its global literature on the coronavirus resource section.
Israeli researchers have also been quite busy. InnoCan Pharma and Tel Aviv University are collaborating to explore the potential for CBD-loaded exosomes (minute extracellular particles that mediate intracellular communication, including via innate and adaptive immune responses). The group plans to use these loaded exosomes to target and facilitate recovery of COVID-19–damaged lung cells.
From a broader perspective, the prospects for harnessing cannabinoids for immune modulation will be more thoroughly explored in a special issue of Cannabis and Cannabinoid Research, which has extended its current call for papers, studies, abstracts, and conference proceedings until the end of December.
Like many of the therapeutic strategies under investigation for the treatment of COVID-19, studies in CBD may continue to raise more questions than answers.
Still, Wilkerson is optimistic. “Taken together, these studies along with countless others suggest that the complex pharmacophore of Cannabis sativa may hold therapeutic utility to treat lung inflammation, such as what is seen in a COVID-19 cytokine storm,» she told Medscape Medical News. “I’m very excited to see what comes out of the research.”
Baban, Wilkerson, and Costiniuk have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Sublingual apomorphine alleviates off episodes in Parkinson’s disease
, long-term follow-up of a phase 3 study has shown. Besides the usual adverse effects with apomorphine, the sublingual film was associated with more oral adverse effects than seen with the injectable drug. However, it may have some advantages over subcutaneous apomorphine injections in terms of administration during off episodes.
The study was presented at the Movement Disorder Society 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
For example, the new formulation is more convenient than carrying an injection. It comes in a small, tear-open packet that contains a medication strip patients place under their tongues.
“When a patient is in the off state, depending on how off they are, they could have a little difficulty opening the strip [packet], but anyone can open the strip for them,” said lead author Rajesh Pahwa, MD, professor of neurology and chief of the Parkinson and Movement Disorder Division at the University of Kansas Medical Center in Kansas City. “On the other hand with the subcutaneous, they have to give the injection themselves and a stranger or someone is not going to help them with that.”
Open-label safety and efficacy study
The aims of this open-label, 48-week follow-up were to add new patients to assess safety and tolerability over the long term and to see if continued benefit from a previous 12-week double-blind study was still present at 1 year for patients in the earlier study.
This multicenter study (NCT02542696) included “rollover” patients (n = 78 for safety; n = 70 for efficacy) from the previous phase 2/3 double-blind trial, as well as new patients with no prior exposure to apomorphine sublingual film (n = 347 for safety; n = 275 for efficacy).
New patients experienced one or more off episodes per day with a daily off time of 2 hours or more per day while on stable doses of levodopa/carbidopa. All had clinically meaningful responses to levodopa/carbidopa and were judged by the investigator to be Stage 1-3 by modified Hoehn and Yahr scale rating during ON periods.
Rollover patients completed the prior study and had no major changes in their anti-Parkinson’s medications since then. Mouth cankers or sores were exclusion criteria for either group. New subjects could not have received subcutaneous apomorphine within 7 days of a screening visit.
The demographics and baseline characteristics of the new and rollover groups were similar (approximately 64 years; 65%-71% male; 96% White; 8.3-9.6 years since diagnosis; 3.9 to 4.1 off episodes/day, and total mean daily levodopa dose of 1120 to 1478 mg).
Assessing only the group of new patients, the investigators reported that 80% had a Hoehn and Yahr score of 2 or 2.5 when in the ON state and a Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III predose score of 41.8.
At the beginning of this study, patients in an off period received titrated doses of 10-35 mg of sublingual apomorphine in 5 mg increments during sequential office visits until they achieved a tolerable full ON within 45 minutes of a dose. They then entered a 48-week safety and efficacy phase, during which they self-administered the drug at home up to five times daily for off episodes with a minimum of 2 hours between doses. The investigators could adjust the doses for safety or lack of efficacy.
Two-thirds of new patients and three-quarters of rollovers received doses in the 10-20 mg range. The highest dose in the study of 35 mg was used by only 8%-9% of patients, but the highest approved and marketed dose is 30 mg.
Long-term benefits
Onset of efficacy was achieved by 15 minutes after dose for both new and rollover patients, and maximal efficacy occurred by 30 minutes. Results were very similar at 24, 36, and 48 weeks. The investigators did not perform statistical analyses.
Across study weeks 1, 12, 24, 36, and 48, between 77% and 92% of new patients and between 65% and 77% of rollover patients self-reported full ON within 30 minutes. “The long-term benefits are maintained over a year as far as the speed of onset and the duration,” Dr. Pahwa said.
Treatment-emergent adverse events occurred in about half of the new and the rollover patient groups in the titration phase and in 71%-81% of patients during the long-term safety phase. Nearly all were mild to moderate in severity.
A large number of participants withdrew from this long-term safety phase because of adverse events – 90 (33%) of new enrollees and 16 (23%) of rollover patients. Only 4% dropped out for lack of efficacy, all in the new enrollee group. Because the sublingual formulation is delivered under the tongue, patients in that group had more oral side effects, Dr. Pahwa said. Otherwise, “the side effects were very similar to the subcutaneous delivery.”
Treatment-emergent adverse events specific to sublingual apomorphine included oral mucosal erythema, lip or tongue swelling, and mouth ulceration (6% to 7% of patients each). Occurring less often were glossodynia, oral candidiasis, stomatitis, and tongue ulceration (2% each).
These were in addition to adverse events typically occurring with subcutaneous apomorphine, which are nausea, falls, dizziness, somnolence, dyskinesia, syncope, and yawning.
There are no head-to-head comparisons of sublingual versus subcutaneous delivery of apomorphine. But based on experience, Dr. Pahwa said, “With the subcutaneous, you have a slightly faster onset of action compared to the sublingual. However, sublingual has a slightly longer duration of benefit.”
He predicted that patients may prefer using an injection for a faster benefit or a sublingual for a slightly longer benefit.
More therapeutic options are welcome
Commenting on the study, Ray Dorsey, MD, professor of neurology at the University of Rochester (N.Y.), said that, for people with more advanced Parkinson’s disease “there’s usually a caregiver who’s injecting someone with an off period, as opposed to sublingual, which seems like a much easier way of administering a drug, especially for people with motor fluctuations.”
He noted that adverse events that led to premature discontinuation from the study “are concerning about the overall tolerability of the drug, which also will be determined in clinical practice, and will likely influence its overall utility.”
However, more therapeutic options are welcome because “the number of people with advanced Parkinson’s disease is going to grow and grow substantially,” he said. “So having therapies that help people with more advanced Parkinson’s disease ... many of whom don’t reach the clinic ... are going to be increasingly important.”
The study was supported by Sunovion. Dr. Pahwa and Dr. Dorsey reported conflicts of interest with numerous sources in industry.
A version of this article originally appeared on Medscape.com.
, long-term follow-up of a phase 3 study has shown. Besides the usual adverse effects with apomorphine, the sublingual film was associated with more oral adverse effects than seen with the injectable drug. However, it may have some advantages over subcutaneous apomorphine injections in terms of administration during off episodes.
The study was presented at the Movement Disorder Society 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
For example, the new formulation is more convenient than carrying an injection. It comes in a small, tear-open packet that contains a medication strip patients place under their tongues.
“When a patient is in the off state, depending on how off they are, they could have a little difficulty opening the strip [packet], but anyone can open the strip for them,” said lead author Rajesh Pahwa, MD, professor of neurology and chief of the Parkinson and Movement Disorder Division at the University of Kansas Medical Center in Kansas City. “On the other hand with the subcutaneous, they have to give the injection themselves and a stranger or someone is not going to help them with that.”
Open-label safety and efficacy study
The aims of this open-label, 48-week follow-up were to add new patients to assess safety and tolerability over the long term and to see if continued benefit from a previous 12-week double-blind study was still present at 1 year for patients in the earlier study.
This multicenter study (NCT02542696) included “rollover” patients (n = 78 for safety; n = 70 for efficacy) from the previous phase 2/3 double-blind trial, as well as new patients with no prior exposure to apomorphine sublingual film (n = 347 for safety; n = 275 for efficacy).
New patients experienced one or more off episodes per day with a daily off time of 2 hours or more per day while on stable doses of levodopa/carbidopa. All had clinically meaningful responses to levodopa/carbidopa and were judged by the investigator to be Stage 1-3 by modified Hoehn and Yahr scale rating during ON periods.
Rollover patients completed the prior study and had no major changes in their anti-Parkinson’s medications since then. Mouth cankers or sores were exclusion criteria for either group. New subjects could not have received subcutaneous apomorphine within 7 days of a screening visit.
The demographics and baseline characteristics of the new and rollover groups were similar (approximately 64 years; 65%-71% male; 96% White; 8.3-9.6 years since diagnosis; 3.9 to 4.1 off episodes/day, and total mean daily levodopa dose of 1120 to 1478 mg).
Assessing only the group of new patients, the investigators reported that 80% had a Hoehn and Yahr score of 2 or 2.5 when in the ON state and a Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III predose score of 41.8.
At the beginning of this study, patients in an off period received titrated doses of 10-35 mg of sublingual apomorphine in 5 mg increments during sequential office visits until they achieved a tolerable full ON within 45 minutes of a dose. They then entered a 48-week safety and efficacy phase, during which they self-administered the drug at home up to five times daily for off episodes with a minimum of 2 hours between doses. The investigators could adjust the doses for safety or lack of efficacy.
Two-thirds of new patients and three-quarters of rollovers received doses in the 10-20 mg range. The highest dose in the study of 35 mg was used by only 8%-9% of patients, but the highest approved and marketed dose is 30 mg.
Long-term benefits
Onset of efficacy was achieved by 15 minutes after dose for both new and rollover patients, and maximal efficacy occurred by 30 minutes. Results were very similar at 24, 36, and 48 weeks. The investigators did not perform statistical analyses.
Across study weeks 1, 12, 24, 36, and 48, between 77% and 92% of new patients and between 65% and 77% of rollover patients self-reported full ON within 30 minutes. “The long-term benefits are maintained over a year as far as the speed of onset and the duration,” Dr. Pahwa said.
Treatment-emergent adverse events occurred in about half of the new and the rollover patient groups in the titration phase and in 71%-81% of patients during the long-term safety phase. Nearly all were mild to moderate in severity.
A large number of participants withdrew from this long-term safety phase because of adverse events – 90 (33%) of new enrollees and 16 (23%) of rollover patients. Only 4% dropped out for lack of efficacy, all in the new enrollee group. Because the sublingual formulation is delivered under the tongue, patients in that group had more oral side effects, Dr. Pahwa said. Otherwise, “the side effects were very similar to the subcutaneous delivery.”
Treatment-emergent adverse events specific to sublingual apomorphine included oral mucosal erythema, lip or tongue swelling, and mouth ulceration (6% to 7% of patients each). Occurring less often were glossodynia, oral candidiasis, stomatitis, and tongue ulceration (2% each).
These were in addition to adverse events typically occurring with subcutaneous apomorphine, which are nausea, falls, dizziness, somnolence, dyskinesia, syncope, and yawning.
There are no head-to-head comparisons of sublingual versus subcutaneous delivery of apomorphine. But based on experience, Dr. Pahwa said, “With the subcutaneous, you have a slightly faster onset of action compared to the sublingual. However, sublingual has a slightly longer duration of benefit.”
He predicted that patients may prefer using an injection for a faster benefit or a sublingual for a slightly longer benefit.
More therapeutic options are welcome
Commenting on the study, Ray Dorsey, MD, professor of neurology at the University of Rochester (N.Y.), said that, for people with more advanced Parkinson’s disease “there’s usually a caregiver who’s injecting someone with an off period, as opposed to sublingual, which seems like a much easier way of administering a drug, especially for people with motor fluctuations.”
He noted that adverse events that led to premature discontinuation from the study “are concerning about the overall tolerability of the drug, which also will be determined in clinical practice, and will likely influence its overall utility.”
However, more therapeutic options are welcome because “the number of people with advanced Parkinson’s disease is going to grow and grow substantially,” he said. “So having therapies that help people with more advanced Parkinson’s disease ... many of whom don’t reach the clinic ... are going to be increasingly important.”
The study was supported by Sunovion. Dr. Pahwa and Dr. Dorsey reported conflicts of interest with numerous sources in industry.
A version of this article originally appeared on Medscape.com.
, long-term follow-up of a phase 3 study has shown. Besides the usual adverse effects with apomorphine, the sublingual film was associated with more oral adverse effects than seen with the injectable drug. However, it may have some advantages over subcutaneous apomorphine injections in terms of administration during off episodes.
The study was presented at the Movement Disorder Society 23rd International Congress of Parkinson’s Disease and Movement Disorders (Virtual) 2020.
For example, the new formulation is more convenient than carrying an injection. It comes in a small, tear-open packet that contains a medication strip patients place under their tongues.
“When a patient is in the off state, depending on how off they are, they could have a little difficulty opening the strip [packet], but anyone can open the strip for them,” said lead author Rajesh Pahwa, MD, professor of neurology and chief of the Parkinson and Movement Disorder Division at the University of Kansas Medical Center in Kansas City. “On the other hand with the subcutaneous, they have to give the injection themselves and a stranger or someone is not going to help them with that.”
Open-label safety and efficacy study
The aims of this open-label, 48-week follow-up were to add new patients to assess safety and tolerability over the long term and to see if continued benefit from a previous 12-week double-blind study was still present at 1 year for patients in the earlier study.
This multicenter study (NCT02542696) included “rollover” patients (n = 78 for safety; n = 70 for efficacy) from the previous phase 2/3 double-blind trial, as well as new patients with no prior exposure to apomorphine sublingual film (n = 347 for safety; n = 275 for efficacy).
New patients experienced one or more off episodes per day with a daily off time of 2 hours or more per day while on stable doses of levodopa/carbidopa. All had clinically meaningful responses to levodopa/carbidopa and were judged by the investigator to be Stage 1-3 by modified Hoehn and Yahr scale rating during ON periods.
Rollover patients completed the prior study and had no major changes in their anti-Parkinson’s medications since then. Mouth cankers or sores were exclusion criteria for either group. New subjects could not have received subcutaneous apomorphine within 7 days of a screening visit.
The demographics and baseline characteristics of the new and rollover groups were similar (approximately 64 years; 65%-71% male; 96% White; 8.3-9.6 years since diagnosis; 3.9 to 4.1 off episodes/day, and total mean daily levodopa dose of 1120 to 1478 mg).
Assessing only the group of new patients, the investigators reported that 80% had a Hoehn and Yahr score of 2 or 2.5 when in the ON state and a Movement Disorder Society–Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III predose score of 41.8.
At the beginning of this study, patients in an off period received titrated doses of 10-35 mg of sublingual apomorphine in 5 mg increments during sequential office visits until they achieved a tolerable full ON within 45 minutes of a dose. They then entered a 48-week safety and efficacy phase, during which they self-administered the drug at home up to five times daily for off episodes with a minimum of 2 hours between doses. The investigators could adjust the doses for safety or lack of efficacy.
Two-thirds of new patients and three-quarters of rollovers received doses in the 10-20 mg range. The highest dose in the study of 35 mg was used by only 8%-9% of patients, but the highest approved and marketed dose is 30 mg.
Long-term benefits
Onset of efficacy was achieved by 15 minutes after dose for both new and rollover patients, and maximal efficacy occurred by 30 minutes. Results were very similar at 24, 36, and 48 weeks. The investigators did not perform statistical analyses.
Across study weeks 1, 12, 24, 36, and 48, between 77% and 92% of new patients and between 65% and 77% of rollover patients self-reported full ON within 30 minutes. “The long-term benefits are maintained over a year as far as the speed of onset and the duration,” Dr. Pahwa said.
Treatment-emergent adverse events occurred in about half of the new and the rollover patient groups in the titration phase and in 71%-81% of patients during the long-term safety phase. Nearly all were mild to moderate in severity.
A large number of participants withdrew from this long-term safety phase because of adverse events – 90 (33%) of new enrollees and 16 (23%) of rollover patients. Only 4% dropped out for lack of efficacy, all in the new enrollee group. Because the sublingual formulation is delivered under the tongue, patients in that group had more oral side effects, Dr. Pahwa said. Otherwise, “the side effects were very similar to the subcutaneous delivery.”
Treatment-emergent adverse events specific to sublingual apomorphine included oral mucosal erythema, lip or tongue swelling, and mouth ulceration (6% to 7% of patients each). Occurring less often were glossodynia, oral candidiasis, stomatitis, and tongue ulceration (2% each).
These were in addition to adverse events typically occurring with subcutaneous apomorphine, which are nausea, falls, dizziness, somnolence, dyskinesia, syncope, and yawning.
There are no head-to-head comparisons of sublingual versus subcutaneous delivery of apomorphine. But based on experience, Dr. Pahwa said, “With the subcutaneous, you have a slightly faster onset of action compared to the sublingual. However, sublingual has a slightly longer duration of benefit.”
He predicted that patients may prefer using an injection for a faster benefit or a sublingual for a slightly longer benefit.
More therapeutic options are welcome
Commenting on the study, Ray Dorsey, MD, professor of neurology at the University of Rochester (N.Y.), said that, for people with more advanced Parkinson’s disease “there’s usually a caregiver who’s injecting someone with an off period, as opposed to sublingual, which seems like a much easier way of administering a drug, especially for people with motor fluctuations.”
He noted that adverse events that led to premature discontinuation from the study “are concerning about the overall tolerability of the drug, which also will be determined in clinical practice, and will likely influence its overall utility.”
However, more therapeutic options are welcome because “the number of people with advanced Parkinson’s disease is going to grow and grow substantially,” he said. “So having therapies that help people with more advanced Parkinson’s disease ... many of whom don’t reach the clinic ... are going to be increasingly important.”
The study was supported by Sunovion. Dr. Pahwa and Dr. Dorsey reported conflicts of interest with numerous sources in industry.
A version of this article originally appeared on Medscape.com.
FROM MOVEMENT DISORDERS SOCIETY 2020
Biologics may protect psoriasis patients against severe COVID-19
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
presented at the virtual annual congress of the European Academy of Dermatology and Venereology.
“Biologics seem to be very protective against severe, poor-prognosis COVID-19, but they do not prevent infection with the virus,” reported Giovanni Damiani, MD, a dermatologist at the University of Milan.
This apparent protective effect of biologic agents against severe and even fatal COVID-19 is all the more impressive because the psoriasis patients included in the Italian study – as is true of those elsewhere throughout the world – had relatively high rates of obesity, smoking, and chronic obstructive pulmonary disease, known risk factors for severe COVID-19, he added.
He presented a case-control study including 1,193 adult psoriasis patients on biologics or apremilast (Otezla) at Milan’s San Donato Hospital during the period from Feb. 21 to April 9, 2020. The control group comprised more than 10 million individuals, the entire adult population of the Lombardy region, of which Milan is the capital. This was the hardest-hit area in all of Italy during the first wave of COVID-19.
Twenty-two of the 1,193 psoriasis patients experienced confirmed COVID-19 during the study period. Seventeen were quarantined at home because their disease was mild. Five were hospitalized. But no psoriasis patients were placed in intensive care, and none died.
Psoriasis patients on biologics were significantly more likely than the general Lombardian population to test positive for COVID-19, with an unadjusted odds ratio of 3.43. They were at 9.05-fold increased risk of home quarantine for mild disease, and at 3.59-fold greater risk than controls for hospitalization for COVID-19. However, they were not at significantly increased risk of ICU admission. And while they actually had a 59% relative risk reduction for death, this didn’t achieve statistical significance.
Forty-five percent of the psoriasis patients were on an interleukin-17 (IL-17) inhibitor, 22% were on a tumor necrosis factor–alpha inhibitor, and 20% were taking an IL-12/23 inhibitor. Of note, none of 77 patients on apremilast developed COVID-19, even though it is widely considered a less potent psoriasis therapy than the injectable monoclonal antibody biologics.
The French experience
Anne-Claire Fougerousse, MD, and her French coinvestigators conducted a study designed to address a different question: Is it safe to start psoriasis patients on biologics or older conventional systemic agents such as methotrexate during the pandemic?
She presented a French national cross-sectional study of 1,418 adult psoriasis patients on a biologic or standard systemic therapy during a snapshot in time near the peak of the first wave of the pandemic in France: the period from April 27 to May 7, 2020. The group included 1,188 psoriasis patients on maintenance therapy and 230 who had initiated systemic treatment within the past 4 months. More than one-third of the patients had at least one risk factor for severe COVID-19.
Although testing wasn’t available to confirm all cases, 54 patients developed probable COVID-19 during the study period. Only five required hospitalization. None died. The two hospitalized psoriasis patients admitted to an ICU had obesity as a risk factor for severe COVID-19, as did another of the five hospitalized patients, reported Dr. Fougerousse, a dermatologist at the Bégin Military Teaching Hospital in Saint-Mandé, France. Hospitalization for COVID-19 was required in 0.43% of the French treatment initiators, not significantly different from the 0.34% rate in patients on maintenance systemic therapy. A study limitation was the lack of a control group.
Nonetheless, the data did answer the investigators’ main question: “This is the first data showing no increased incidence of severe COVID-19 in psoriasis patients receiving systemic therapy in the treatment initiation period compared to those on maintenance therapy. This may now allow physicians to initiate conventional systemic or biologic therapy in patients with severe psoriasis on a case-by-case basis in the context of the persistent COVID-19 pandemic,” Dr. Fougerousse concluded.
Proposed mechanism of benefit
The Italian study findings that biologics boost the risk of infection with the SARS-CoV-2 virus in psoriasis patients while potentially protecting them against ICU admission and death are backed by a biologically plausible albeit as yet unproven mechanism of action, Dr. Damiani asserted.
He elaborated: A vast body of high-quality clinical trials data demonstrates that these targeted immunosuppressive agents are associated with modestly increased risk of viral infections, including both skin and respiratory tract infections. So there is no reason to suppose these agents would offer protection against the first phase of COVID-19, involving SARS-CoV-2 infection, nor protect against the second (pulmonary phase), whose hallmarks are dyspnea with or without hypoxia. But progression to the third phase, involving hyperinflammation and hypercoagulation – dubbed the cytokine storm – could be a different matter.
“Of particular interest was that our patients on IL-17 inhibitors displayed a really great outcome. Interleukin-17 has procoagulant and prothrombotic effects, organizes bronchoalveolar remodeling, has a profibrotic effect, induces mitochondrial dysfunction, and encourages dendritic cell migration in peribronchial lymph nodes. Therefore, by antagonizing this interleukin, we may have a better prognosis, although further studies are needed to be certain,” Dr. Damiani commented.
Publication of his preliminary findings drew the attention of a group of highly respected thought leaders in psoriasis, including James G. Krueger, MD, head of the laboratory for investigative dermatology and codirector of the center for clinical and investigative science at Rockefeller University, New York.
The Italian report prompted them to analyze data from the phase 4, double-blind, randomized ObePso-S study investigating the effects of the IL-17 inhibitor secukinumab (Cosentyx) on systemic inflammatory markers and gene expression in psoriasis patients. The investigators demonstrated that IL-17–mediated inflammation in psoriasis patients was associated with increased expression of the angiotensin-converting enzyme 2 (ACE2) receptor in lesional skin, and that treatment with secukinumab dropped ACE2 expression to levels seen in nonlesional skin. Given that ACE2 is the chief portal of entry for SARS-CoV-2 and that IL-17 exerts systemic proinflammatory effects, it’s plausible that inhibition of IL-17–mediated inflammation via dampening of ACE2 expression in noncutaneous epithelia “could prove to be advantageous in patients with psoriasis who are at risk for SARS-CoV-2 infection,” according to Dr. Krueger and his coinvestigators in the Journal of Allergy and Clinical Immunology.
Dr. Damiani and Dr. Fougerousse reported having no financial conflicts regarding their studies. The secukinumab/ACE2 receptor study was funded by Novartis.
FROM THE EADV CONGRESS
Two COVID-19 outpatient antibody drugs show encouraging results
Two COVID-19 antibody treatments, one developed by Regeneron and the other by Eli Lilly, show promise in the outpatient setting in results released on Oct. 28.
Regeneron, in a randomized, double-blind trial, is assessing the effect of adding its investigational antibody cocktail REGN-COV2 to usual standard of care in comparison with adding placebo to standard of care. A descriptive analysis from the first 275 patients was previously reported. The data described on Oct. 28, which involve an additional 524 patients, show that the trial met all of the first nine endpoints.
Regeneron announced prospective results from its phase 2/3 trial showing REGN-COV2 significantly reduced viral load and patient medical visits, which included hospitalizations, visits to an emergency department, visits for urgent care, and/or physician office/telemedicine visits.
Interest in the cocktail spiked after President Donald Trump extolled its benefits after it was used in his own COVID-19 treatment earlier in October.
Trump received the highest dose of the drug, 8 g, but, according to a Regeneron news release announcing the latest findings, “results showed no significant difference in virologic or clinical efficacy between the REGN-COV2 high dose (8 grams) and low dose (2.4 grams).”
The company described further results of the industry-funded study in the release: “On the primary endpoint, the average daily change in viral load through day 7 (mean time-weighted average change from baseline) in patients with high viral load (defined as greater than107 copies/mL) was a 0.68 log10 copies/mL greater reduction with REGN-COV2 compared to placebo (combined dose groups; P < .0001). There was a 1.08 log greater reduction with REGN-COV2 treatment by day 5, which corresponds to REGN-COV2 patients having, on average, a greater than 10-fold reduction in viral load, compared to placebo.”
The treatment appears to be most effective in patients most at risk, whether because of high viral load, ineffective baseline antibody immune response, or preexisting conditions, according to the researchers.
According to the press release, these results have not been peer reviewed but have been submitted to the US Food and Drug Administration, which is reviewing a potential emergency use authorization for the treatment in high-risk adults with mild to moderate COVID-19.
Operation Warp Speed, the Trump administration’s treatment and vaccine program, contracted in July with Regeneron for up to 300,000 doses of its antibody cocktail.
Lilly treatment shows drop in hospitalizations, symptoms
Another treatment, also given in the outpatient setting, shows promise as well.
Patients recently diagnosed with mild to moderate COVID-19 who received Eli Lilly’s antibody treatment LY-CoV555 had fewer hospitalizations and symptoms compared with a group that received placebo, an interim analysis of a phase 2 trial indicates.
Peter Chen, MD, with the Department of Medicine, Women’s Guild Lung Institute at Cedars-Sinai Medical Center, Los Angeles, California, and colleagues found that the most profound effects were in the high-risk groups.
The interim findings of the BLAZE-1 study, which was funded by Eli Lilly, were published online October 28 in The New England Journal of Medicine.
Researchers randomly assigned 452 patients to receive an intravenous infusion of LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo.
In the interim analysis, the researchers found that for the entire population, more than 99.97% of viral RNA was eliminated.
For patients who received the 2800-mg dose, the difference from placebo in the decrease from baseline was −0.53 (95% CI, −0.98 to −0.08; P = .02), for a log viral load that was lower by a factor of 3.4. Benefit over placebo was not significant with the other doses.
At day 29, according to the investigators, the percentage of patients hospitalized with COVID-19 was 1.6% (5 of 309 patients) in the treatment group compared with 6.3% (9 of 143 patients) in the placebo group.
Data indicate that the safety profile was similar whether patients received the active treatment or placebo.
“If these results are confirmed in additional analyses in this trial, LY-CoV555 could become a useful treatment for emergency use in patients with recently diagnosed Covid-19,” the authors write.
Deborah Fuller, PhD, professor in the Department of Microbiology at the University of Washington School of Medicine in Seattle, told Medscape Medical News the findings are «exciting» but only part of the treatment solution.
“What’s remarkable about these two studies and others I’ve seen,” she said, “is how consistent they are in terms of the window of time they will be effective, and that’s because they are just targeting the virus itself. They do not have an effect on the inflammation unless they stop the replication early enough.”
The treatments are effective when they are given near the time of diagnosis, she pointed out.
“Once the virus has started that inflammatory cascade in your body, then that train has left the station and you have to deal with the inflammation,” Fuller said.
She says future treatments will likely have to include both the antiviral and anti-inflammatory properties, and physicians will have to assess what’s best, given the stage of the the patient’s disease.
The trial of REGN-COV2 is funded by Regeneron. The BLAZE-1 study is funded by Eli Lilly. Many of the authors have financial ties to Eli Lilly. Fuller has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Two COVID-19 antibody treatments, one developed by Regeneron and the other by Eli Lilly, show promise in the outpatient setting in results released on Oct. 28.
Regeneron, in a randomized, double-blind trial, is assessing the effect of adding its investigational antibody cocktail REGN-COV2 to usual standard of care in comparison with adding placebo to standard of care. A descriptive analysis from the first 275 patients was previously reported. The data described on Oct. 28, which involve an additional 524 patients, show that the trial met all of the first nine endpoints.
Regeneron announced prospective results from its phase 2/3 trial showing REGN-COV2 significantly reduced viral load and patient medical visits, which included hospitalizations, visits to an emergency department, visits for urgent care, and/or physician office/telemedicine visits.
Interest in the cocktail spiked after President Donald Trump extolled its benefits after it was used in his own COVID-19 treatment earlier in October.
Trump received the highest dose of the drug, 8 g, but, according to a Regeneron news release announcing the latest findings, “results showed no significant difference in virologic or clinical efficacy between the REGN-COV2 high dose (8 grams) and low dose (2.4 grams).”
The company described further results of the industry-funded study in the release: “On the primary endpoint, the average daily change in viral load through day 7 (mean time-weighted average change from baseline) in patients with high viral load (defined as greater than107 copies/mL) was a 0.68 log10 copies/mL greater reduction with REGN-COV2 compared to placebo (combined dose groups; P < .0001). There was a 1.08 log greater reduction with REGN-COV2 treatment by day 5, which corresponds to REGN-COV2 patients having, on average, a greater than 10-fold reduction in viral load, compared to placebo.”
The treatment appears to be most effective in patients most at risk, whether because of high viral load, ineffective baseline antibody immune response, or preexisting conditions, according to the researchers.
According to the press release, these results have not been peer reviewed but have been submitted to the US Food and Drug Administration, which is reviewing a potential emergency use authorization for the treatment in high-risk adults with mild to moderate COVID-19.
Operation Warp Speed, the Trump administration’s treatment and vaccine program, contracted in July with Regeneron for up to 300,000 doses of its antibody cocktail.
Lilly treatment shows drop in hospitalizations, symptoms
Another treatment, also given in the outpatient setting, shows promise as well.
Patients recently diagnosed with mild to moderate COVID-19 who received Eli Lilly’s antibody treatment LY-CoV555 had fewer hospitalizations and symptoms compared with a group that received placebo, an interim analysis of a phase 2 trial indicates.
Peter Chen, MD, with the Department of Medicine, Women’s Guild Lung Institute at Cedars-Sinai Medical Center, Los Angeles, California, and colleagues found that the most profound effects were in the high-risk groups.
The interim findings of the BLAZE-1 study, which was funded by Eli Lilly, were published online October 28 in The New England Journal of Medicine.
Researchers randomly assigned 452 patients to receive an intravenous infusion of LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo.
In the interim analysis, the researchers found that for the entire population, more than 99.97% of viral RNA was eliminated.
For patients who received the 2800-mg dose, the difference from placebo in the decrease from baseline was −0.53 (95% CI, −0.98 to −0.08; P = .02), for a log viral load that was lower by a factor of 3.4. Benefit over placebo was not significant with the other doses.
At day 29, according to the investigators, the percentage of patients hospitalized with COVID-19 was 1.6% (5 of 309 patients) in the treatment group compared with 6.3% (9 of 143 patients) in the placebo group.
Data indicate that the safety profile was similar whether patients received the active treatment or placebo.
“If these results are confirmed in additional analyses in this trial, LY-CoV555 could become a useful treatment for emergency use in patients with recently diagnosed Covid-19,” the authors write.
Deborah Fuller, PhD, professor in the Department of Microbiology at the University of Washington School of Medicine in Seattle, told Medscape Medical News the findings are «exciting» but only part of the treatment solution.
“What’s remarkable about these two studies and others I’ve seen,” she said, “is how consistent they are in terms of the window of time they will be effective, and that’s because they are just targeting the virus itself. They do not have an effect on the inflammation unless they stop the replication early enough.”
The treatments are effective when they are given near the time of diagnosis, she pointed out.
“Once the virus has started that inflammatory cascade in your body, then that train has left the station and you have to deal with the inflammation,” Fuller said.
She says future treatments will likely have to include both the antiviral and anti-inflammatory properties, and physicians will have to assess what’s best, given the stage of the the patient’s disease.
The trial of REGN-COV2 is funded by Regeneron. The BLAZE-1 study is funded by Eli Lilly. Many of the authors have financial ties to Eli Lilly. Fuller has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Two COVID-19 antibody treatments, one developed by Regeneron and the other by Eli Lilly, show promise in the outpatient setting in results released on Oct. 28.
Regeneron, in a randomized, double-blind trial, is assessing the effect of adding its investigational antibody cocktail REGN-COV2 to usual standard of care in comparison with adding placebo to standard of care. A descriptive analysis from the first 275 patients was previously reported. The data described on Oct. 28, which involve an additional 524 patients, show that the trial met all of the first nine endpoints.
Regeneron announced prospective results from its phase 2/3 trial showing REGN-COV2 significantly reduced viral load and patient medical visits, which included hospitalizations, visits to an emergency department, visits for urgent care, and/or physician office/telemedicine visits.
Interest in the cocktail spiked after President Donald Trump extolled its benefits after it was used in his own COVID-19 treatment earlier in October.
Trump received the highest dose of the drug, 8 g, but, according to a Regeneron news release announcing the latest findings, “results showed no significant difference in virologic or clinical efficacy between the REGN-COV2 high dose (8 grams) and low dose (2.4 grams).”
The company described further results of the industry-funded study in the release: “On the primary endpoint, the average daily change in viral load through day 7 (mean time-weighted average change from baseline) in patients with high viral load (defined as greater than107 copies/mL) was a 0.68 log10 copies/mL greater reduction with REGN-COV2 compared to placebo (combined dose groups; P < .0001). There was a 1.08 log greater reduction with REGN-COV2 treatment by day 5, which corresponds to REGN-COV2 patients having, on average, a greater than 10-fold reduction in viral load, compared to placebo.”
The treatment appears to be most effective in patients most at risk, whether because of high viral load, ineffective baseline antibody immune response, or preexisting conditions, according to the researchers.
According to the press release, these results have not been peer reviewed but have been submitted to the US Food and Drug Administration, which is reviewing a potential emergency use authorization for the treatment in high-risk adults with mild to moderate COVID-19.
Operation Warp Speed, the Trump administration’s treatment and vaccine program, contracted in July with Regeneron for up to 300,000 doses of its antibody cocktail.
Lilly treatment shows drop in hospitalizations, symptoms
Another treatment, also given in the outpatient setting, shows promise as well.
Patients recently diagnosed with mild to moderate COVID-19 who received Eli Lilly’s antibody treatment LY-CoV555 had fewer hospitalizations and symptoms compared with a group that received placebo, an interim analysis of a phase 2 trial indicates.
Peter Chen, MD, with the Department of Medicine, Women’s Guild Lung Institute at Cedars-Sinai Medical Center, Los Angeles, California, and colleagues found that the most profound effects were in the high-risk groups.
The interim findings of the BLAZE-1 study, which was funded by Eli Lilly, were published online October 28 in The New England Journal of Medicine.
Researchers randomly assigned 452 patients to receive an intravenous infusion of LY-CoV555 in one of three doses (700 mg, 2800 mg, or 7000 mg) or placebo.
In the interim analysis, the researchers found that for the entire population, more than 99.97% of viral RNA was eliminated.
For patients who received the 2800-mg dose, the difference from placebo in the decrease from baseline was −0.53 (95% CI, −0.98 to −0.08; P = .02), for a log viral load that was lower by a factor of 3.4. Benefit over placebo was not significant with the other doses.
At day 29, according to the investigators, the percentage of patients hospitalized with COVID-19 was 1.6% (5 of 309 patients) in the treatment group compared with 6.3% (9 of 143 patients) in the placebo group.
Data indicate that the safety profile was similar whether patients received the active treatment or placebo.
“If these results are confirmed in additional analyses in this trial, LY-CoV555 could become a useful treatment for emergency use in patients with recently diagnosed Covid-19,” the authors write.
Deborah Fuller, PhD, professor in the Department of Microbiology at the University of Washington School of Medicine in Seattle, told Medscape Medical News the findings are «exciting» but only part of the treatment solution.
“What’s remarkable about these two studies and others I’ve seen,” she said, “is how consistent they are in terms of the window of time they will be effective, and that’s because they are just targeting the virus itself. They do not have an effect on the inflammation unless they stop the replication early enough.”
The treatments are effective when they are given near the time of diagnosis, she pointed out.
“Once the virus has started that inflammatory cascade in your body, then that train has left the station and you have to deal with the inflammation,” Fuller said.
She says future treatments will likely have to include both the antiviral and anti-inflammatory properties, and physicians will have to assess what’s best, given the stage of the the patient’s disease.
The trial of REGN-COV2 is funded by Regeneron. The BLAZE-1 study is funded by Eli Lilly. Many of the authors have financial ties to Eli Lilly. Fuller has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Real-world results with checkpoint inhibitors found inferior to trial results
according to research published in JCO Clinical Cancer Informatics.
However, the research also suggests that real-world patients who receive ICIs achieve longer survival than patients on standard-of-care medications.
“Patients receiving ICIs in real-world practice may differ from those enrolled in trials in a variety of ways, including age, race, performance status, and comorbidity burden,” said study author Jerry S.H. Lee, PhD, of the University of Southern California, Los Angeles.
Dr. Lee noted that only 3%-4% of cancer patients participate in clinical trials. In fact, more than half of patients with melanoma and nearly three-quarters of those with non–small cell lung cancer (NSCLC) do not meet criteria for eligibility in clinical trials, he said.
To examine the discrepancies between real-world practice and clinical trials and to better understand which patients receive ICIs in clinical practice, Dr. Lee and colleagues conducted a retrospective analysis using electronic health record data from Veterans Administration (VA) facilities nationwide.
The researchers identified 11,888 cancer patients who were treated with ICIs. The cohort included patients who are underrepresented in pivotal clinical trials, including older, non-White, and/or higher disease-burdened patients.
The majority of patients were treated for NSCLC (51.1%), followed by melanoma (14.4%), renal cell carcinoma (RCC; 8.1%), squamous cell carcinoma of the head and neck (6.8%), urothelial cancer (6.4%), hepatocellular carcinoma (4.5%), and other less common cancer types (8.8%).
Overall survival by indication
In general, median overall survival (OS) in the VA cohort was inferior to median OS reported in clinical trials. However, patients treated with first-line nivolumab for melanoma and second-line pembrolizumab or nivolumab for NSCLC had similar OS in the real-world and trial data.
The researchers did not report exact OS numbers from clinical trials. However, they did report the exact numbers from the VA cohort and show OS differences between the VA cohort and clinical trials graphically.
Among patients in the VA cohort, the median OS was:
- 25.5 months in melanoma patients on first-line nivolumab
- 16.3 months in RCC patients receiving nivolumab in the second line or higher
- 14 months in RCC patients on first-line ipilimumab and nivolumab
- 10.6 months in NSCLC patients on first-line pembrolizumab
- 9.9 months in NSCLC patients receiving pembrolizumab or nivolumab in the second line or higher
- 9.1 months in NSCLC patients on first-line pembrolizumab and platinum-based chemotherapy
- 6.7 months in urothelial cancer patients receiving ICIs in the second line or higher.
A number of factors may have contributed to the shorter OS observed in the VA cohort, according to the researchers. The VA cohort is predominantly male, is older, and has a higher degree of comorbidity, compared with patients in clinical trials.
In addition, no data are available to determine the cause for discontinuation of therapy, and VA patients may have received ICIs after failing multiple lines of previous therapy, while clinical trials may limit patients to only one or two previous lines of therapy.
After stratifying VA patients by frailty status, the OS among non-frail patients was more similar to the OS reported in clinical trials.
“Real-world outcomes from the VA were more similar when adjusted for frailty, which shows the importance of patient diversity in clinical trials,” Dr. Lee said. He added that the definition of frailty among VA patients included potential injury during combat and therefore differs from a generic frailty definition.
ICIs vs. standard care
The researchers also found that VA patients treated with ICIs had longer OS, compared with a cohort of VA patients receiving standard-of-care therapies.
The median OS was as follows:
- In melanoma patients on first-line treatment – 39.29 months with nivolumab and 5.75 months with chemotherapy (P < .001).
- In RCC patients on first-line treatment – 14.01 months with ipilimumab plus nivolumab and 8.63 months with targeted therapy (P = .051).
- In RCC patients on second-line or greater treatment – 12.43 months with nivolumab and 8.09 months with everolimus (P < .001).
- In NSCLC patients on first-line therapy – 8.88 months with pembrolizumab and 6.38 months with a platinum doublet (P < .001).
- In NSCLC patients on first-line combination therapy – 10.59 months with pembrolizumab plus platinum chemotherapy and 6.38 months with a platinum doublet (P < .001).
- In NSCLC patients on second-line or greater therapy – 10.06 months with pembrolizumab or nivolumab and 6.41 months with docetaxel (P < .001).
- In urothelial cancer patients on second-line or greater therapy – 7.66 months with an ICI and 6.31 months with chemotherapy (P = .043).
Help for treatment decisions
“The real-world survival outcomes not only indicate the breadth of indications but also represent patients who tend not to be eligible for immunotherapy trials, based on their health status,” Dr. Lee said. “We hope this dataset of national-level experience provides practicing oncologists evidence to help patients and family members in the process of decision-making about therapy.”
Real-world data can also inform oncologists who face decisions on whether to prescribe or withhold ICIs and patients who face the financial burden of paying for ICIs, he said.
This dataset will be continually updated. The researchers have already added another 10,000 VA patients who have received immunotherapies in the year since the trial began.
“In a longitudinal way, we plan to examine what causes differences in outcomes and continue to find ways to extend care to veterans with a balance of high quality of life,” Dr. Lee said.
“Patients who participate in clinical trials are, on average, younger and healthier than the general population,” said Bora Youn, PhD, a senior biostatistician at Biogen in Cambridge, Mass., who was not involved in this study.
“In the case of immunotherapies, those with poor performance status and autoimmune conditions are often excluded from trials,” Dr. Youn added. “In the real world, these patients can also receive treatments, and clinicians often need to extrapolate the results from clinical trials. It is therefore important to collect real-world data to understand the effectiveness and safety of these therapies in patients with limited evidence.”
Dr. Youn led a real-world study, published in Cancer, of 1,256 Medicare recipients who were diagnosed with NSCLC and received ICI therapy.
“We found that factors associated with poor prognosis in general, such as squamous histology and failure of aggressive prior treatment, are also predictive of decreased survival among those who initiated immunotherapies. Yet, OS of older patients was relatively comparable to those observed in clinical trials,” Dr. Youn said.
“Understanding the real-world effectiveness of these treatments will help improve the evidence base, especially for those underrepresented in clinical trials. These studies can also help identify patients who are most likely to benefit from immunotherapies,” Dr. Youn added.
This study was supported by the VA Office of Research and Development Cooperative Studies Program. Dr. Lee and Dr. Youn disclosed no conflicts of interest.
SOURCE: Jennifer La et al. JCO Clinical Cancer Informatics. 2020:4:918-28.
according to research published in JCO Clinical Cancer Informatics.
However, the research also suggests that real-world patients who receive ICIs achieve longer survival than patients on standard-of-care medications.
“Patients receiving ICIs in real-world practice may differ from those enrolled in trials in a variety of ways, including age, race, performance status, and comorbidity burden,” said study author Jerry S.H. Lee, PhD, of the University of Southern California, Los Angeles.
Dr. Lee noted that only 3%-4% of cancer patients participate in clinical trials. In fact, more than half of patients with melanoma and nearly three-quarters of those with non–small cell lung cancer (NSCLC) do not meet criteria for eligibility in clinical trials, he said.
To examine the discrepancies between real-world practice and clinical trials and to better understand which patients receive ICIs in clinical practice, Dr. Lee and colleagues conducted a retrospective analysis using electronic health record data from Veterans Administration (VA) facilities nationwide.
The researchers identified 11,888 cancer patients who were treated with ICIs. The cohort included patients who are underrepresented in pivotal clinical trials, including older, non-White, and/or higher disease-burdened patients.
The majority of patients were treated for NSCLC (51.1%), followed by melanoma (14.4%), renal cell carcinoma (RCC; 8.1%), squamous cell carcinoma of the head and neck (6.8%), urothelial cancer (6.4%), hepatocellular carcinoma (4.5%), and other less common cancer types (8.8%).
Overall survival by indication
In general, median overall survival (OS) in the VA cohort was inferior to median OS reported in clinical trials. However, patients treated with first-line nivolumab for melanoma and second-line pembrolizumab or nivolumab for NSCLC had similar OS in the real-world and trial data.
The researchers did not report exact OS numbers from clinical trials. However, they did report the exact numbers from the VA cohort and show OS differences between the VA cohort and clinical trials graphically.
Among patients in the VA cohort, the median OS was:
- 25.5 months in melanoma patients on first-line nivolumab
- 16.3 months in RCC patients receiving nivolumab in the second line or higher
- 14 months in RCC patients on first-line ipilimumab and nivolumab
- 10.6 months in NSCLC patients on first-line pembrolizumab
- 9.9 months in NSCLC patients receiving pembrolizumab or nivolumab in the second line or higher
- 9.1 months in NSCLC patients on first-line pembrolizumab and platinum-based chemotherapy
- 6.7 months in urothelial cancer patients receiving ICIs in the second line or higher.
A number of factors may have contributed to the shorter OS observed in the VA cohort, according to the researchers. The VA cohort is predominantly male, is older, and has a higher degree of comorbidity, compared with patients in clinical trials.
In addition, no data are available to determine the cause for discontinuation of therapy, and VA patients may have received ICIs after failing multiple lines of previous therapy, while clinical trials may limit patients to only one or two previous lines of therapy.
After stratifying VA patients by frailty status, the OS among non-frail patients was more similar to the OS reported in clinical trials.
“Real-world outcomes from the VA were more similar when adjusted for frailty, which shows the importance of patient diversity in clinical trials,” Dr. Lee said. He added that the definition of frailty among VA patients included potential injury during combat and therefore differs from a generic frailty definition.
ICIs vs. standard care
The researchers also found that VA patients treated with ICIs had longer OS, compared with a cohort of VA patients receiving standard-of-care therapies.
The median OS was as follows:
- In melanoma patients on first-line treatment – 39.29 months with nivolumab and 5.75 months with chemotherapy (P < .001).
- In RCC patients on first-line treatment – 14.01 months with ipilimumab plus nivolumab and 8.63 months with targeted therapy (P = .051).
- In RCC patients on second-line or greater treatment – 12.43 months with nivolumab and 8.09 months with everolimus (P < .001).
- In NSCLC patients on first-line therapy – 8.88 months with pembrolizumab and 6.38 months with a platinum doublet (P < .001).
- In NSCLC patients on first-line combination therapy – 10.59 months with pembrolizumab plus platinum chemotherapy and 6.38 months with a platinum doublet (P < .001).
- In NSCLC patients on second-line or greater therapy – 10.06 months with pembrolizumab or nivolumab and 6.41 months with docetaxel (P < .001).
- In urothelial cancer patients on second-line or greater therapy – 7.66 months with an ICI and 6.31 months with chemotherapy (P = .043).
Help for treatment decisions
“The real-world survival outcomes not only indicate the breadth of indications but also represent patients who tend not to be eligible for immunotherapy trials, based on their health status,” Dr. Lee said. “We hope this dataset of national-level experience provides practicing oncologists evidence to help patients and family members in the process of decision-making about therapy.”
Real-world data can also inform oncologists who face decisions on whether to prescribe or withhold ICIs and patients who face the financial burden of paying for ICIs, he said.
This dataset will be continually updated. The researchers have already added another 10,000 VA patients who have received immunotherapies in the year since the trial began.
“In a longitudinal way, we plan to examine what causes differences in outcomes and continue to find ways to extend care to veterans with a balance of high quality of life,” Dr. Lee said.
“Patients who participate in clinical trials are, on average, younger and healthier than the general population,” said Bora Youn, PhD, a senior biostatistician at Biogen in Cambridge, Mass., who was not involved in this study.
“In the case of immunotherapies, those with poor performance status and autoimmune conditions are often excluded from trials,” Dr. Youn added. “In the real world, these patients can also receive treatments, and clinicians often need to extrapolate the results from clinical trials. It is therefore important to collect real-world data to understand the effectiveness and safety of these therapies in patients with limited evidence.”
Dr. Youn led a real-world study, published in Cancer, of 1,256 Medicare recipients who were diagnosed with NSCLC and received ICI therapy.
“We found that factors associated with poor prognosis in general, such as squamous histology and failure of aggressive prior treatment, are also predictive of decreased survival among those who initiated immunotherapies. Yet, OS of older patients was relatively comparable to those observed in clinical trials,” Dr. Youn said.
“Understanding the real-world effectiveness of these treatments will help improve the evidence base, especially for those underrepresented in clinical trials. These studies can also help identify patients who are most likely to benefit from immunotherapies,” Dr. Youn added.
This study was supported by the VA Office of Research and Development Cooperative Studies Program. Dr. Lee and Dr. Youn disclosed no conflicts of interest.
SOURCE: Jennifer La et al. JCO Clinical Cancer Informatics. 2020:4:918-28.
according to research published in JCO Clinical Cancer Informatics.
However, the research also suggests that real-world patients who receive ICIs achieve longer survival than patients on standard-of-care medications.
“Patients receiving ICIs in real-world practice may differ from those enrolled in trials in a variety of ways, including age, race, performance status, and comorbidity burden,” said study author Jerry S.H. Lee, PhD, of the University of Southern California, Los Angeles.
Dr. Lee noted that only 3%-4% of cancer patients participate in clinical trials. In fact, more than half of patients with melanoma and nearly three-quarters of those with non–small cell lung cancer (NSCLC) do not meet criteria for eligibility in clinical trials, he said.
To examine the discrepancies between real-world practice and clinical trials and to better understand which patients receive ICIs in clinical practice, Dr. Lee and colleagues conducted a retrospective analysis using electronic health record data from Veterans Administration (VA) facilities nationwide.
The researchers identified 11,888 cancer patients who were treated with ICIs. The cohort included patients who are underrepresented in pivotal clinical trials, including older, non-White, and/or higher disease-burdened patients.
The majority of patients were treated for NSCLC (51.1%), followed by melanoma (14.4%), renal cell carcinoma (RCC; 8.1%), squamous cell carcinoma of the head and neck (6.8%), urothelial cancer (6.4%), hepatocellular carcinoma (4.5%), and other less common cancer types (8.8%).
Overall survival by indication
In general, median overall survival (OS) in the VA cohort was inferior to median OS reported in clinical trials. However, patients treated with first-line nivolumab for melanoma and second-line pembrolizumab or nivolumab for NSCLC had similar OS in the real-world and trial data.
The researchers did not report exact OS numbers from clinical trials. However, they did report the exact numbers from the VA cohort and show OS differences between the VA cohort and clinical trials graphically.
Among patients in the VA cohort, the median OS was:
- 25.5 months in melanoma patients on first-line nivolumab
- 16.3 months in RCC patients receiving nivolumab in the second line or higher
- 14 months in RCC patients on first-line ipilimumab and nivolumab
- 10.6 months in NSCLC patients on first-line pembrolizumab
- 9.9 months in NSCLC patients receiving pembrolizumab or nivolumab in the second line or higher
- 9.1 months in NSCLC patients on first-line pembrolizumab and platinum-based chemotherapy
- 6.7 months in urothelial cancer patients receiving ICIs in the second line or higher.
A number of factors may have contributed to the shorter OS observed in the VA cohort, according to the researchers. The VA cohort is predominantly male, is older, and has a higher degree of comorbidity, compared with patients in clinical trials.
In addition, no data are available to determine the cause for discontinuation of therapy, and VA patients may have received ICIs after failing multiple lines of previous therapy, while clinical trials may limit patients to only one or two previous lines of therapy.
After stratifying VA patients by frailty status, the OS among non-frail patients was more similar to the OS reported in clinical trials.
“Real-world outcomes from the VA were more similar when adjusted for frailty, which shows the importance of patient diversity in clinical trials,” Dr. Lee said. He added that the definition of frailty among VA patients included potential injury during combat and therefore differs from a generic frailty definition.
ICIs vs. standard care
The researchers also found that VA patients treated with ICIs had longer OS, compared with a cohort of VA patients receiving standard-of-care therapies.
The median OS was as follows:
- In melanoma patients on first-line treatment – 39.29 months with nivolumab and 5.75 months with chemotherapy (P < .001).
- In RCC patients on first-line treatment – 14.01 months with ipilimumab plus nivolumab and 8.63 months with targeted therapy (P = .051).
- In RCC patients on second-line or greater treatment – 12.43 months with nivolumab and 8.09 months with everolimus (P < .001).
- In NSCLC patients on first-line therapy – 8.88 months with pembrolizumab and 6.38 months with a platinum doublet (P < .001).
- In NSCLC patients on first-line combination therapy – 10.59 months with pembrolizumab plus platinum chemotherapy and 6.38 months with a platinum doublet (P < .001).
- In NSCLC patients on second-line or greater therapy – 10.06 months with pembrolizumab or nivolumab and 6.41 months with docetaxel (P < .001).
- In urothelial cancer patients on second-line or greater therapy – 7.66 months with an ICI and 6.31 months with chemotherapy (P = .043).
Help for treatment decisions
“The real-world survival outcomes not only indicate the breadth of indications but also represent patients who tend not to be eligible for immunotherapy trials, based on their health status,” Dr. Lee said. “We hope this dataset of national-level experience provides practicing oncologists evidence to help patients and family members in the process of decision-making about therapy.”
Real-world data can also inform oncologists who face decisions on whether to prescribe or withhold ICIs and patients who face the financial burden of paying for ICIs, he said.
This dataset will be continually updated. The researchers have already added another 10,000 VA patients who have received immunotherapies in the year since the trial began.
“In a longitudinal way, we plan to examine what causes differences in outcomes and continue to find ways to extend care to veterans with a balance of high quality of life,” Dr. Lee said.
“Patients who participate in clinical trials are, on average, younger and healthier than the general population,” said Bora Youn, PhD, a senior biostatistician at Biogen in Cambridge, Mass., who was not involved in this study.
“In the case of immunotherapies, those with poor performance status and autoimmune conditions are often excluded from trials,” Dr. Youn added. “In the real world, these patients can also receive treatments, and clinicians often need to extrapolate the results from clinical trials. It is therefore important to collect real-world data to understand the effectiveness and safety of these therapies in patients with limited evidence.”
Dr. Youn led a real-world study, published in Cancer, of 1,256 Medicare recipients who were diagnosed with NSCLC and received ICI therapy.
“We found that factors associated with poor prognosis in general, such as squamous histology and failure of aggressive prior treatment, are also predictive of decreased survival among those who initiated immunotherapies. Yet, OS of older patients was relatively comparable to those observed in clinical trials,” Dr. Youn said.
“Understanding the real-world effectiveness of these treatments will help improve the evidence base, especially for those underrepresented in clinical trials. These studies can also help identify patients who are most likely to benefit from immunotherapies,” Dr. Youn added.
This study was supported by the VA Office of Research and Development Cooperative Studies Program. Dr. Lee and Dr. Youn disclosed no conflicts of interest.
SOURCE: Jennifer La et al. JCO Clinical Cancer Informatics. 2020:4:918-28.
FROM JCO CLINICAL CANCER INFORMATICS