User login
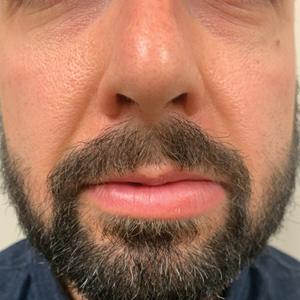
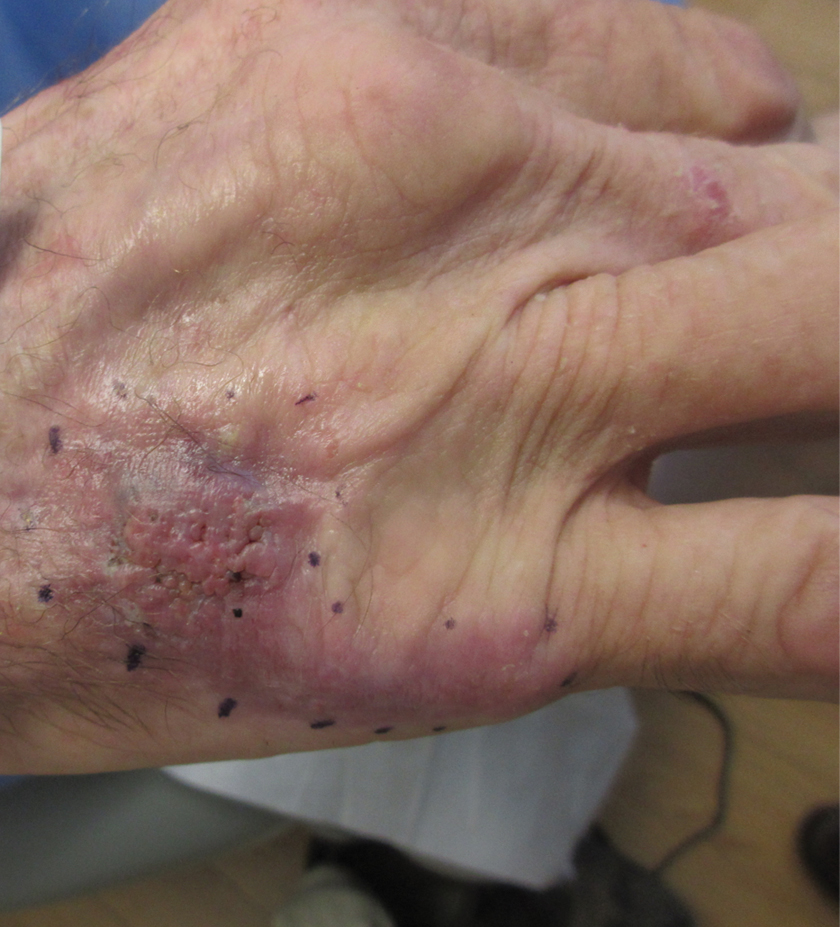
Nonhealing Violaceous Plaque of the Hand Following a Splinter Injury
The Diagnosis: Chromoblastomycosis
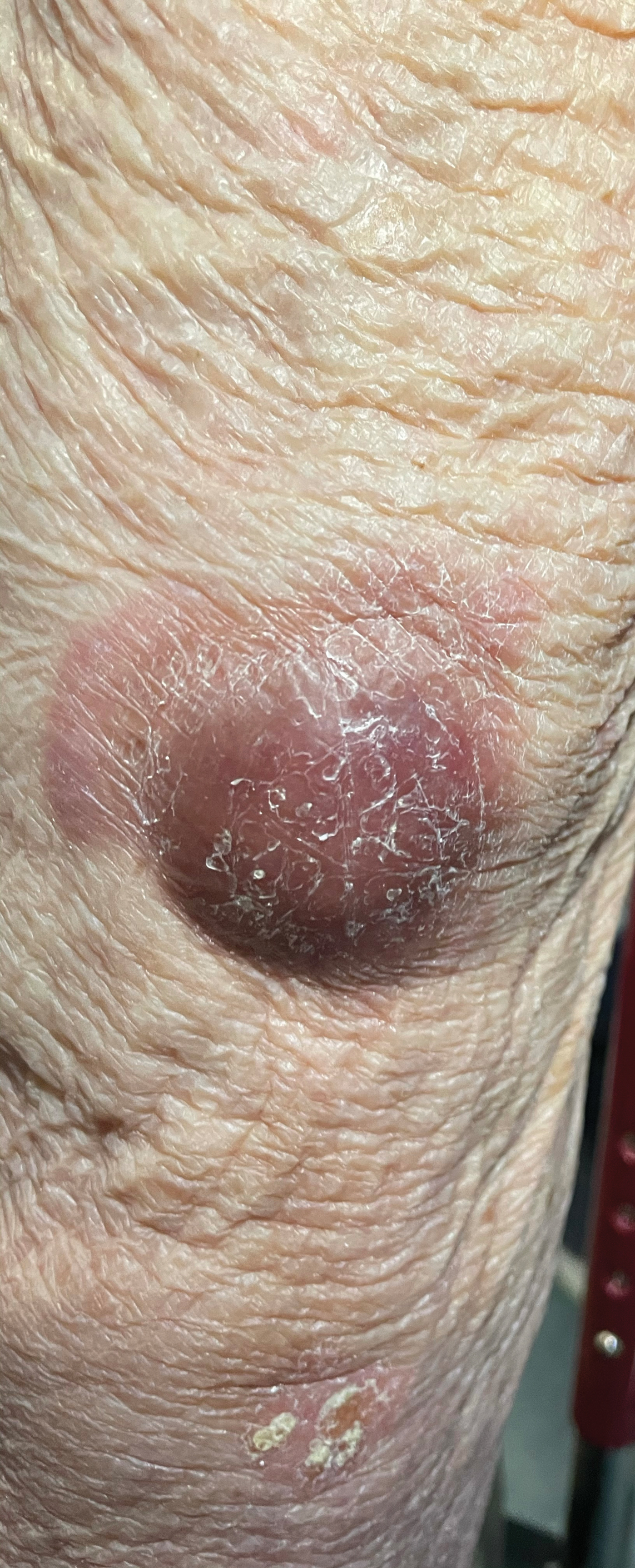
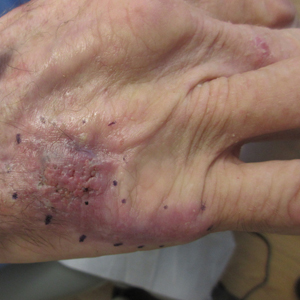
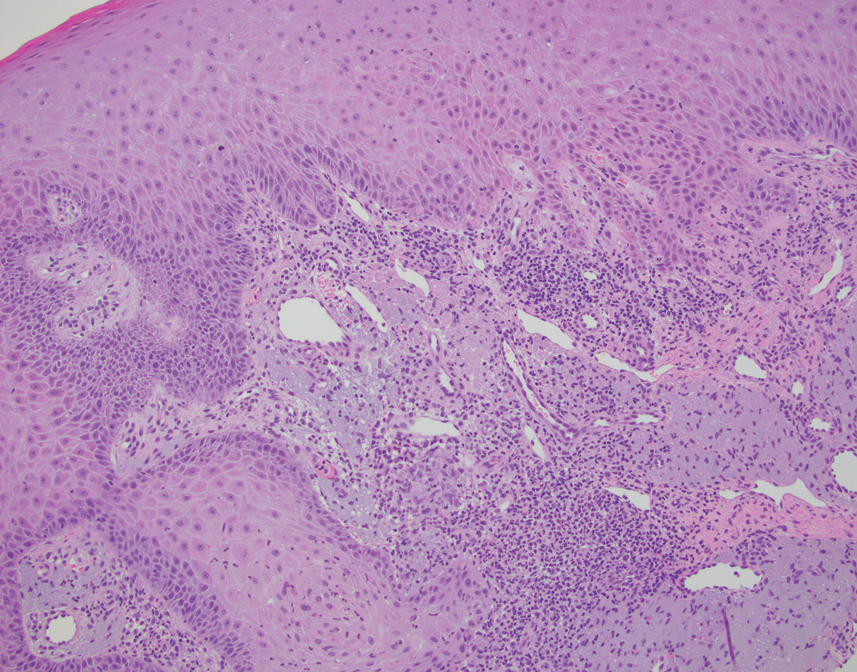
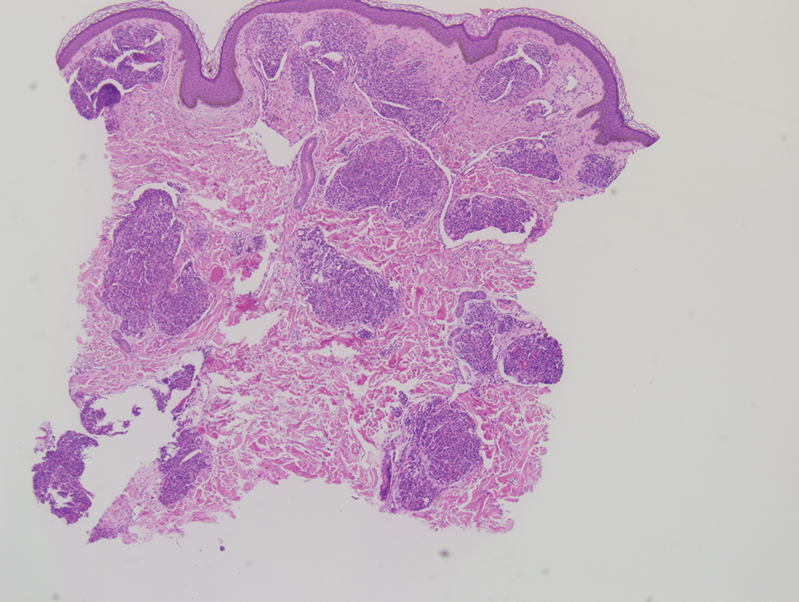
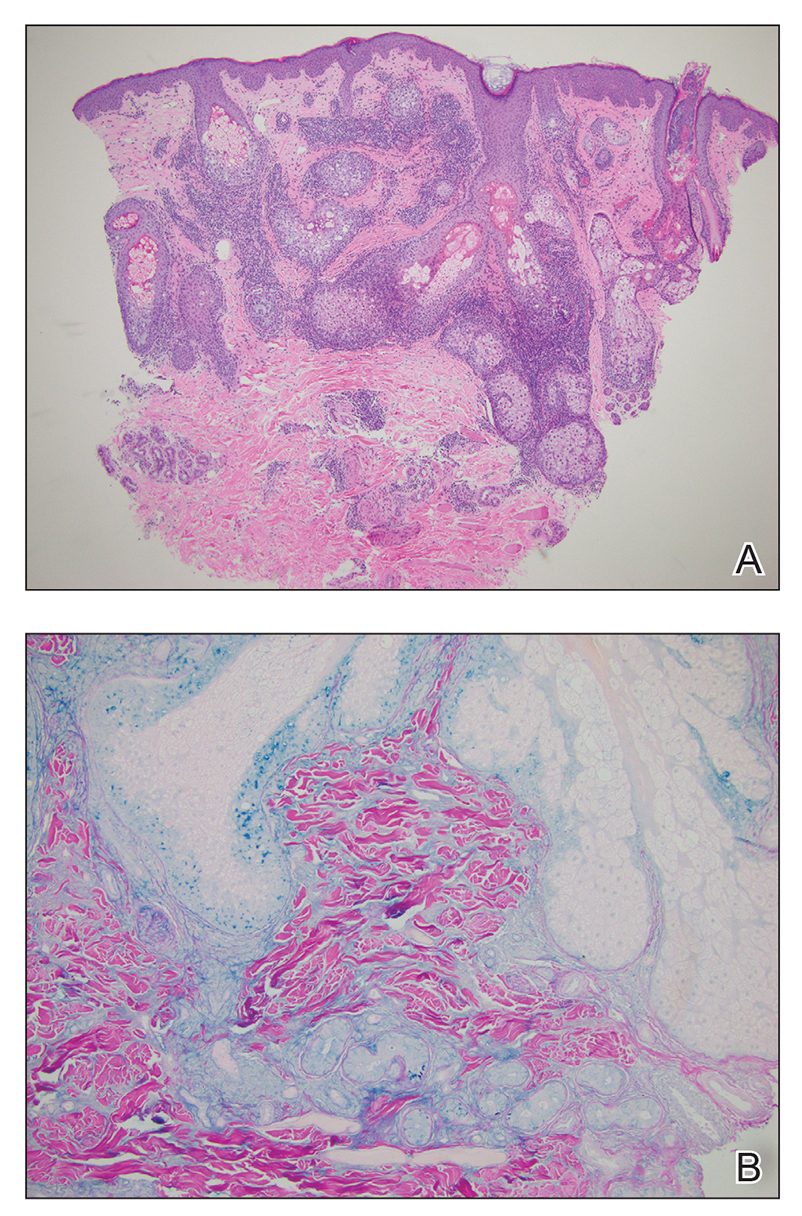
This case highlights the importance of routine skin biopsy and tissue culture when clinical suspicion for mycotic infection is high. Despite nonspecific biopsy results (Figure), a diagnosis of chromoblastomycosis (CBM) was reached based on tissue culture. Surgical excision was not possible in our patient due to the size and location of the lesion. The patient was referred to infectious disease, with the plan to start long-term itraconazole for at least 6 to 12 months.
Cases of CBM were first documented in 1914 and distinguished by the appearance of spherical, brown, muriform cells on skin biopsy—features that now serve as the hallmark of CBM diagnoses.1,2 The implantation mycosis commonly is caused by agents such as Fonsecaea pedrosoi and Fonsecaea monophora of the bantiana-clade, as classified according to molecular phylogeny2; these agents have been isolated from soil, plants, and wood sources in tropical and subtropical regions and are strongly associated with agricultural activities.3
Chromoblastomycosis lesions tend to be asymptomatic with a variable amount of time between inoculation and lesion presentation, delaying medical care by months to years.3 The fungus causes a granulomatous reaction after skin damage, with noticeable pseudoepitheliomatous hyperplasia of the epidermis and granulomas formed by epithelioid and Langerhans cells in the dermis.4 Typically, CBM initially presents as an erythematous macular skin lesion, which then progresses to become more pink, papular, and sometimes pruritic.2 Muriform (sclerotic) bodies, which reflect fungal components, extrude transepidermally and appear as black dots on the lesion’s surface.4 Chromoblastomycosis is limited to the subcutaneous tissue and has been classified into 5 types of lesions: nodular, tumoral, verrucous, scarring, and plaque.2 Diagnosis is established using fungal tests such as potassium hydroxide direct microscopy, which exposes muriform bodies often in combination with dematiaceous hyphae, while fungal culture of F pedrosoi in Sabouraud agar produces velvety dark colonies.3 Although an immune response to CBM infection remains unclear, it has been demonstrated that the response differs based on the severity of the infection. The severe form of CBM produces high levels of IL-10, low levels of IFN-γ, and inefficient T-cell proliferation, while milder forms of CBM display low levels of IL-10, high levels of IFN-γ, and efficient T-cell proliferation.5 Complications of CBM include chronic lymphedema, ankylosis, and secondary bacterial infections, which largely are observed in advanced cases; malignant transformation to squamous cell carcinoma, though rare, also has been observed.6
Several therapeutic methods have been implemented in the treatment of CBM, but lesions often remain refractory, especially in advanced cases.6 Approaches to treatment can be divided into antifungal and physical methods. Commonly employed antifungal agents include itraconazole and terbinafine, which must be taken daily for a period ranging from 6 months to 1 year or longer; flucytosine with or without amphotericin also has been employed.4 Among the physical methods, surgical excision is not suggested due to possible dissemination of disease; other options include cryotherapy, thermotherapy, and laser vaporization.6 The prognosis has improved since the use of extended-spectrum triazoles, but high rates of refractory disease remain unchanged.2
The differential diagnosis includes other infections. Nocardiosis is a bacterial infection in which cutaneous disease can result in actinomycetoma, which presents with grains that are small, round, and stain blue on hematoxylin and eosin with eosinophilic rays at the periphery.7 Although the clinical features and pseudoepitheliomatous hyperplasia seen in CBM can mimic squamous cell carcinoma, the latter would show variable degrees of differentiation, keratinization, nuclear atypia, and architectural atypia with a negative tissue culture.8 Eumycetoma is a fungal infection that typically is not caused by F pedrosoi but rather most commonly Madurella mycetomatis.9 Leishmaniasis is a parasitic infection in which a biopsy of cutaneous lesions often displays parasite-filled histiocytes.10
- Rudolph M. Über die brasilianische “figueira” (vorläufige mitteilung). Arch Schiffs Trop Hyg. 1914;18:498-499.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- Kurien G, Sugumar K, Chandran V. Chromoblastomycosis. StatPearls. StatPearls Publishing; 2021. Accessed June 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470253/
- Mazo Fávero Gimenes V, Da Glória de Souza M, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708-713. doi:10.1016/j.micinf.2005.01.006
- Krzys´ciak PM, Pindycka-Piaszczyn´ska M, Piaszczyn´ski M. Chromoblastomycosis. Postepy Dermatol Alergol. 2014;31:310-321. doi:10.5114/pdia.2014.40949
- Siddig EE, van de Sande WWJ, Fahal AH. Actinomycetoma laboratory-based diagnosis: a mini-review. Trans R Soc Trop Med Hyg. 2021;115:355-363.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll .2017.06.003
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- Saliba M, Shalhoub A, Taraif S, et al. Cutaneous leishmaniasis: an evolving disease with ancient roots. Int J Dermatol. 2019;58:834-843. doi:10.1111/ijd.14451
The Diagnosis: Chromoblastomycosis
This case highlights the importance of routine skin biopsy and tissue culture when clinical suspicion for mycotic infection is high. Despite nonspecific biopsy results (Figure), a diagnosis of chromoblastomycosis (CBM) was reached based on tissue culture. Surgical excision was not possible in our patient due to the size and location of the lesion. The patient was referred to infectious disease, with the plan to start long-term itraconazole for at least 6 to 12 months.

Cases of CBM were first documented in 1914 and distinguished by the appearance of spherical, brown, muriform cells on skin biopsy—features that now serve as the hallmark of CBM diagnoses.1,2 The implantation mycosis commonly is caused by agents such as Fonsecaea pedrosoi and Fonsecaea monophora of the bantiana-clade, as classified according to molecular phylogeny2; these agents have been isolated from soil, plants, and wood sources in tropical and subtropical regions and are strongly associated with agricultural activities.3
Chromoblastomycosis lesions tend to be asymptomatic with a variable amount of time between inoculation and lesion presentation, delaying medical care by months to years.3 The fungus causes a granulomatous reaction after skin damage, with noticeable pseudoepitheliomatous hyperplasia of the epidermis and granulomas formed by epithelioid and Langerhans cells in the dermis.4 Typically, CBM initially presents as an erythematous macular skin lesion, which then progresses to become more pink, papular, and sometimes pruritic.2 Muriform (sclerotic) bodies, which reflect fungal components, extrude transepidermally and appear as black dots on the lesion’s surface.4 Chromoblastomycosis is limited to the subcutaneous tissue and has been classified into 5 types of lesions: nodular, tumoral, verrucous, scarring, and plaque.2 Diagnosis is established using fungal tests such as potassium hydroxide direct microscopy, which exposes muriform bodies often in combination with dematiaceous hyphae, while fungal culture of F pedrosoi in Sabouraud agar produces velvety dark colonies.3 Although an immune response to CBM infection remains unclear, it has been demonstrated that the response differs based on the severity of the infection. The severe form of CBM produces high levels of IL-10, low levels of IFN-γ, and inefficient T-cell proliferation, while milder forms of CBM display low levels of IL-10, high levels of IFN-γ, and efficient T-cell proliferation.5 Complications of CBM include chronic lymphedema, ankylosis, and secondary bacterial infections, which largely are observed in advanced cases; malignant transformation to squamous cell carcinoma, though rare, also has been observed.6
Several therapeutic methods have been implemented in the treatment of CBM, but lesions often remain refractory, especially in advanced cases.6 Approaches to treatment can be divided into antifungal and physical methods. Commonly employed antifungal agents include itraconazole and terbinafine, which must be taken daily for a period ranging from 6 months to 1 year or longer; flucytosine with or without amphotericin also has been employed.4 Among the physical methods, surgical excision is not suggested due to possible dissemination of disease; other options include cryotherapy, thermotherapy, and laser vaporization.6 The prognosis has improved since the use of extended-spectrum triazoles, but high rates of refractory disease remain unchanged.2
The differential diagnosis includes other infections. Nocardiosis is a bacterial infection in which cutaneous disease can result in actinomycetoma, which presents with grains that are small, round, and stain blue on hematoxylin and eosin with eosinophilic rays at the periphery.7 Although the clinical features and pseudoepitheliomatous hyperplasia seen in CBM can mimic squamous cell carcinoma, the latter would show variable degrees of differentiation, keratinization, nuclear atypia, and architectural atypia with a negative tissue culture.8 Eumycetoma is a fungal infection that typically is not caused by F pedrosoi but rather most commonly Madurella mycetomatis.9 Leishmaniasis is a parasitic infection in which a biopsy of cutaneous lesions often displays parasite-filled histiocytes.10
The Diagnosis: Chromoblastomycosis
This case highlights the importance of routine skin biopsy and tissue culture when clinical suspicion for mycotic infection is high. Despite nonspecific biopsy results (Figure), a diagnosis of chromoblastomycosis (CBM) was reached based on tissue culture. Surgical excision was not possible in our patient due to the size and location of the lesion. The patient was referred to infectious disease, with the plan to start long-term itraconazole for at least 6 to 12 months.

Cases of CBM were first documented in 1914 and distinguished by the appearance of spherical, brown, muriform cells on skin biopsy—features that now serve as the hallmark of CBM diagnoses.1,2 The implantation mycosis commonly is caused by agents such as Fonsecaea pedrosoi and Fonsecaea monophora of the bantiana-clade, as classified according to molecular phylogeny2; these agents have been isolated from soil, plants, and wood sources in tropical and subtropical regions and are strongly associated with agricultural activities.3
Chromoblastomycosis lesions tend to be asymptomatic with a variable amount of time between inoculation and lesion presentation, delaying medical care by months to years.3 The fungus causes a granulomatous reaction after skin damage, with noticeable pseudoepitheliomatous hyperplasia of the epidermis and granulomas formed by epithelioid and Langerhans cells in the dermis.4 Typically, CBM initially presents as an erythematous macular skin lesion, which then progresses to become more pink, papular, and sometimes pruritic.2 Muriform (sclerotic) bodies, which reflect fungal components, extrude transepidermally and appear as black dots on the lesion’s surface.4 Chromoblastomycosis is limited to the subcutaneous tissue and has been classified into 5 types of lesions: nodular, tumoral, verrucous, scarring, and plaque.2 Diagnosis is established using fungal tests such as potassium hydroxide direct microscopy, which exposes muriform bodies often in combination with dematiaceous hyphae, while fungal culture of F pedrosoi in Sabouraud agar produces velvety dark colonies.3 Although an immune response to CBM infection remains unclear, it has been demonstrated that the response differs based on the severity of the infection. The severe form of CBM produces high levels of IL-10, low levels of IFN-γ, and inefficient T-cell proliferation, while milder forms of CBM display low levels of IL-10, high levels of IFN-γ, and efficient T-cell proliferation.5 Complications of CBM include chronic lymphedema, ankylosis, and secondary bacterial infections, which largely are observed in advanced cases; malignant transformation to squamous cell carcinoma, though rare, also has been observed.6
Several therapeutic methods have been implemented in the treatment of CBM, but lesions often remain refractory, especially in advanced cases.6 Approaches to treatment can be divided into antifungal and physical methods. Commonly employed antifungal agents include itraconazole and terbinafine, which must be taken daily for a period ranging from 6 months to 1 year or longer; flucytosine with or without amphotericin also has been employed.4 Among the physical methods, surgical excision is not suggested due to possible dissemination of disease; other options include cryotherapy, thermotherapy, and laser vaporization.6 The prognosis has improved since the use of extended-spectrum triazoles, but high rates of refractory disease remain unchanged.2
The differential diagnosis includes other infections. Nocardiosis is a bacterial infection in which cutaneous disease can result in actinomycetoma, which presents with grains that are small, round, and stain blue on hematoxylin and eosin with eosinophilic rays at the periphery.7 Although the clinical features and pseudoepitheliomatous hyperplasia seen in CBM can mimic squamous cell carcinoma, the latter would show variable degrees of differentiation, keratinization, nuclear atypia, and architectural atypia with a negative tissue culture.8 Eumycetoma is a fungal infection that typically is not caused by F pedrosoi but rather most commonly Madurella mycetomatis.9 Leishmaniasis is a parasitic infection in which a biopsy of cutaneous lesions often displays parasite-filled histiocytes.10
- Rudolph M. Über die brasilianische “figueira” (vorläufige mitteilung). Arch Schiffs Trop Hyg. 1914;18:498-499.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- Kurien G, Sugumar K, Chandran V. Chromoblastomycosis. StatPearls. StatPearls Publishing; 2021. Accessed June 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470253/
- Mazo Fávero Gimenes V, Da Glória de Souza M, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708-713. doi:10.1016/j.micinf.2005.01.006
- Krzys´ciak PM, Pindycka-Piaszczyn´ska M, Piaszczyn´ski M. Chromoblastomycosis. Postepy Dermatol Alergol. 2014;31:310-321. doi:10.5114/pdia.2014.40949
- Siddig EE, van de Sande WWJ, Fahal AH. Actinomycetoma laboratory-based diagnosis: a mini-review. Trans R Soc Trop Med Hyg. 2021;115:355-363.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll .2017.06.003
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- Saliba M, Shalhoub A, Taraif S, et al. Cutaneous leishmaniasis: an evolving disease with ancient roots. Int J Dermatol. 2019;58:834-843. doi:10.1111/ijd.14451
- Rudolph M. Über die brasilianische “figueira” (vorläufige mitteilung). Arch Schiffs Trop Hyg. 1914;18:498-499.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- Kurien G, Sugumar K, Chandran V. Chromoblastomycosis. StatPearls. StatPearls Publishing; 2021. Accessed June 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470253/
- Mazo Fávero Gimenes V, Da Glória de Souza M, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708-713. doi:10.1016/j.micinf.2005.01.006
- Krzys´ciak PM, Pindycka-Piaszczyn´ska M, Piaszczyn´ski M. Chromoblastomycosis. Postepy Dermatol Alergol. 2014;31:310-321. doi:10.5114/pdia.2014.40949
- Siddig EE, van de Sande WWJ, Fahal AH. Actinomycetoma laboratory-based diagnosis: a mini-review. Trans R Soc Trop Med Hyg. 2021;115:355-363.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll .2017.06.003
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- Saliba M, Shalhoub A, Taraif S, et al. Cutaneous leishmaniasis: an evolving disease with ancient roots. Int J Dermatol. 2019;58:834-843. doi:10.1111/ijd.14451
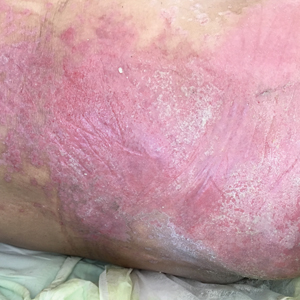
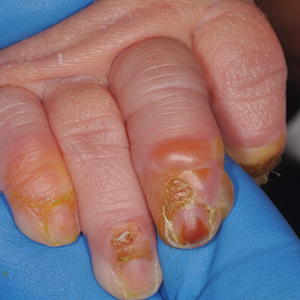
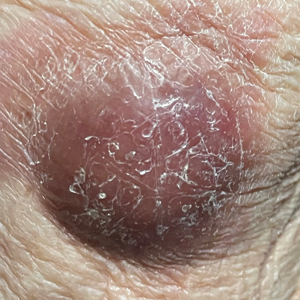
A 70-year-old immunocompetent man presented to the dermatology department with a progressive asymptomatic hand wound of 2 years’ duration following a splinter injury in Belize. Prior treatment included oral antibiotics without improvement. Physical examination revealed a 5.1×3.0 cm, pink to violaceous, nonpurulent plaque with a cobblestonelike appearance on the dorsal aspect of the right hand. Both the initial and a repeat skin biopsy revealed nonspecific changes, including hyperkeratosis, hypergranulosis, acute and chronic inflammation, and vascular ectasia. Grocott-Gomori methenamine-silver staining was negative for fungal organisms. One month after the repeat biopsy, a tissue culture returned positive for the rare Fonsecaea pedrosoi.
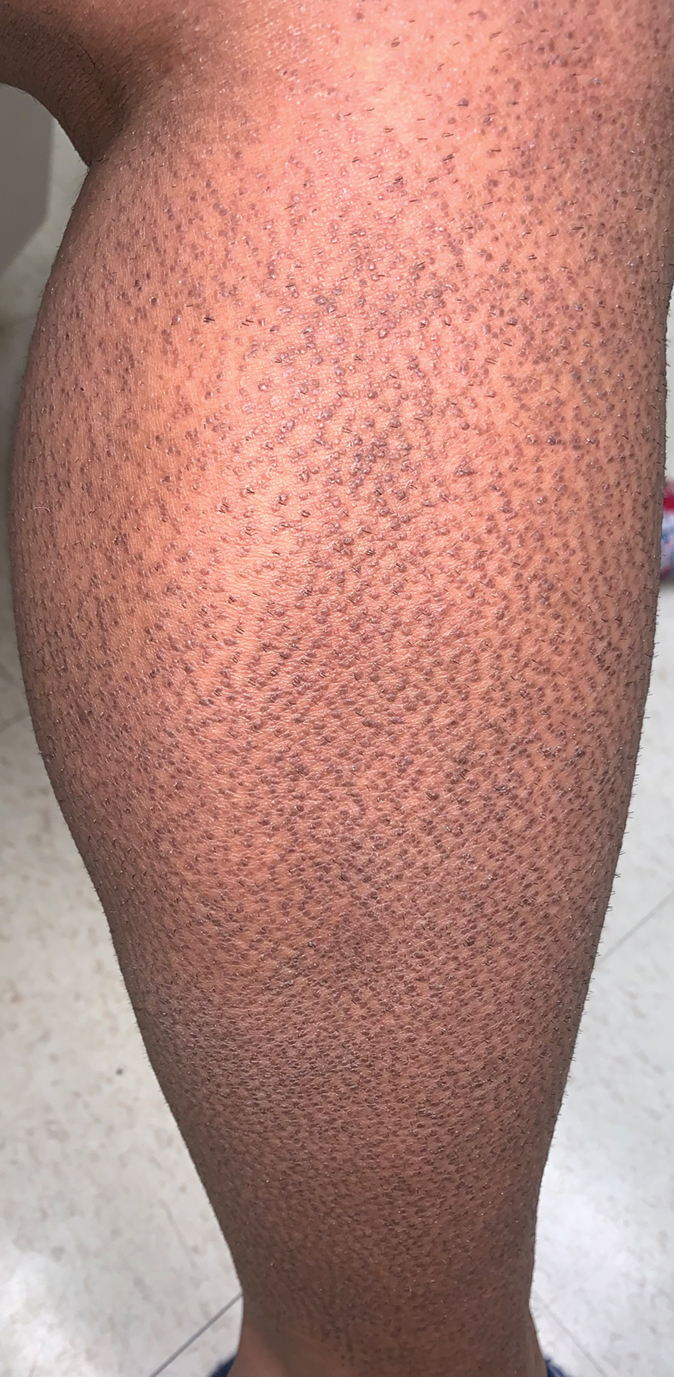
Rippled Macules and Papules on the Legs
The Diagnosis: Cutaneous Amyloidosis
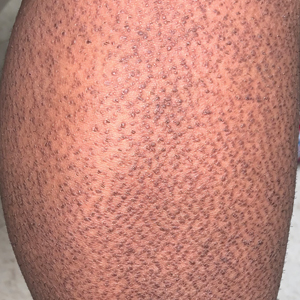
A punch biopsy confirmed the diagnosis of cutaneous amyloidosis, which is characterized by the deposition of amyloid proteins in the skin without systemic involvement. Subtypes of cutaneous amyloidosis include lichenoid, macular, and nodular amyloidosis. A mixed or biphasic amyloidosis can occur when both lichenoid and macular lesions are present.1 Lichenoid and macular amyloidosis generally are characterized by moderate to severe pruritus. Lichenoid amyloidosis favors the shins, calves, ankles, and extensor extremities; macular amyloidosis has a predilection for the interscapular area and less frequently the upper arms, chest, and thighs.2 Atypical variants also have been reported, including amyloidosis cutis dyschromica, poikilodermalike amyloidosis, and bullous amyloidosis, as well as incontinentia pigmenti–like, linear, and nevoid types.3 Macular amyloidosis has been reported to occur in association with progressive systemic sclerosis, primary biliary cirrhosis, systemic lupus erythematosus, paronychia, and multiple endocrine neoplasia type 2.2
Acanthosis nigricans typically presents on the neck and intertriginous areas as velvety hyperpigmented plaques. Confluent and reticulated papillomatosis also appears as slightly elevated papules; however, it occurs in the intermammary region in a reticulated pattern. Ichthyosis vulgaris also may occur on the lower extremities but presents with adherent large scales rather than papules. Keratosis pilaris may present on the proximal lower extremities with smaller, folliculocentric, fleshcolored to pink papules.
Treatment of cutaneous amyloidosis has long been challenging for dermatologists. The primary focus should be treatment of any underlying disease that is causing the pruritus and subsequent manipulation of skin lesions. Topical calcipotriol, phototherapy, oral cyclophosphamide, and Nd:YAG laser have demonstrated beneficial outcomes. IL-31 antibodies may be a potential future treatment.1
1. Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9 2. Rasi A, Khatami A, Javaheri SM. Macular amyloidosis: an assessment of prevalence, sex, and age. Int J Dermatol. 2004;43:898-899. doi:10.1111 /j.1365-4632.2004.01935.x 3. Hamie L, Haddad I, Nasser N, et al. Primary localized cutaneous amyloidosis of keratinocyte origin: an update with emphasis on atypical clinical variants [published online July 21, 2021]. 2021;22:667-680. Am J Clin Dermatol. doi:10.1007/s40257-021-00620-9
The Diagnosis: Cutaneous Amyloidosis
A punch biopsy confirmed the diagnosis of cutaneous amyloidosis, which is characterized by the deposition of amyloid proteins in the skin without systemic involvement. Subtypes of cutaneous amyloidosis include lichenoid, macular, and nodular amyloidosis. A mixed or biphasic amyloidosis can occur when both lichenoid and macular lesions are present.1 Lichenoid and macular amyloidosis generally are characterized by moderate to severe pruritus. Lichenoid amyloidosis favors the shins, calves, ankles, and extensor extremities; macular amyloidosis has a predilection for the interscapular area and less frequently the upper arms, chest, and thighs.2 Atypical variants also have been reported, including amyloidosis cutis dyschromica, poikilodermalike amyloidosis, and bullous amyloidosis, as well as incontinentia pigmenti–like, linear, and nevoid types.3 Macular amyloidosis has been reported to occur in association with progressive systemic sclerosis, primary biliary cirrhosis, systemic lupus erythematosus, paronychia, and multiple endocrine neoplasia type 2.2
Acanthosis nigricans typically presents on the neck and intertriginous areas as velvety hyperpigmented plaques. Confluent and reticulated papillomatosis also appears as slightly elevated papules; however, it occurs in the intermammary region in a reticulated pattern. Ichthyosis vulgaris also may occur on the lower extremities but presents with adherent large scales rather than papules. Keratosis pilaris may present on the proximal lower extremities with smaller, folliculocentric, fleshcolored to pink papules.
Treatment of cutaneous amyloidosis has long been challenging for dermatologists. The primary focus should be treatment of any underlying disease that is causing the pruritus and subsequent manipulation of skin lesions. Topical calcipotriol, phototherapy, oral cyclophosphamide, and Nd:YAG laser have demonstrated beneficial outcomes. IL-31 antibodies may be a potential future treatment.1
The Diagnosis: Cutaneous Amyloidosis
A punch biopsy confirmed the diagnosis of cutaneous amyloidosis, which is characterized by the deposition of amyloid proteins in the skin without systemic involvement. Subtypes of cutaneous amyloidosis include lichenoid, macular, and nodular amyloidosis. A mixed or biphasic amyloidosis can occur when both lichenoid and macular lesions are present.1 Lichenoid and macular amyloidosis generally are characterized by moderate to severe pruritus. Lichenoid amyloidosis favors the shins, calves, ankles, and extensor extremities; macular amyloidosis has a predilection for the interscapular area and less frequently the upper arms, chest, and thighs.2 Atypical variants also have been reported, including amyloidosis cutis dyschromica, poikilodermalike amyloidosis, and bullous amyloidosis, as well as incontinentia pigmenti–like, linear, and nevoid types.3 Macular amyloidosis has been reported to occur in association with progressive systemic sclerosis, primary biliary cirrhosis, systemic lupus erythematosus, paronychia, and multiple endocrine neoplasia type 2.2
Acanthosis nigricans typically presents on the neck and intertriginous areas as velvety hyperpigmented plaques. Confluent and reticulated papillomatosis also appears as slightly elevated papules; however, it occurs in the intermammary region in a reticulated pattern. Ichthyosis vulgaris also may occur on the lower extremities but presents with adherent large scales rather than papules. Keratosis pilaris may present on the proximal lower extremities with smaller, folliculocentric, fleshcolored to pink papules.
Treatment of cutaneous amyloidosis has long been challenging for dermatologists. The primary focus should be treatment of any underlying disease that is causing the pruritus and subsequent manipulation of skin lesions. Topical calcipotriol, phototherapy, oral cyclophosphamide, and Nd:YAG laser have demonstrated beneficial outcomes. IL-31 antibodies may be a potential future treatment.1
1. Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9 2. Rasi A, Khatami A, Javaheri SM. Macular amyloidosis: an assessment of prevalence, sex, and age. Int J Dermatol. 2004;43:898-899. doi:10.1111 /j.1365-4632.2004.01935.x 3. Hamie L, Haddad I, Nasser N, et al. Primary localized cutaneous amyloidosis of keratinocyte origin: an update with emphasis on atypical clinical variants [published online July 21, 2021]. 2021;22:667-680. Am J Clin Dermatol. doi:10.1007/s40257-021-00620-9
1. Weidner T, Illing T, Elsner P. Primary localized cutaneous amyloidosis: a systematic treatment review. Am J Clin Dermatol. 2017;18:629-642. doi:10.1007/s40257-017-0278-9 2. Rasi A, Khatami A, Javaheri SM. Macular amyloidosis: an assessment of prevalence, sex, and age. Int J Dermatol. 2004;43:898-899. doi:10.1111 /j.1365-4632.2004.01935.x 3. Hamie L, Haddad I, Nasser N, et al. Primary localized cutaneous amyloidosis of keratinocyte origin: an update with emphasis on atypical clinical variants [published online July 21, 2021]. 2021;22:667-680. Am J Clin Dermatol. doi:10.1007/s40257-021-00620-9
A 34-year-old woman presented to our dermatology clinic with an intensely pruritic rash on the legs of 2 years’ duration. The pruritus had waxed and waned in intensity, and the skin lesions were refractory to treatment with low-potency topical steroids. She had no other chronic medical conditions and was not taking any other medications.
Vascular Plaque in a Pregnant Patient With a History of Breast Cancer
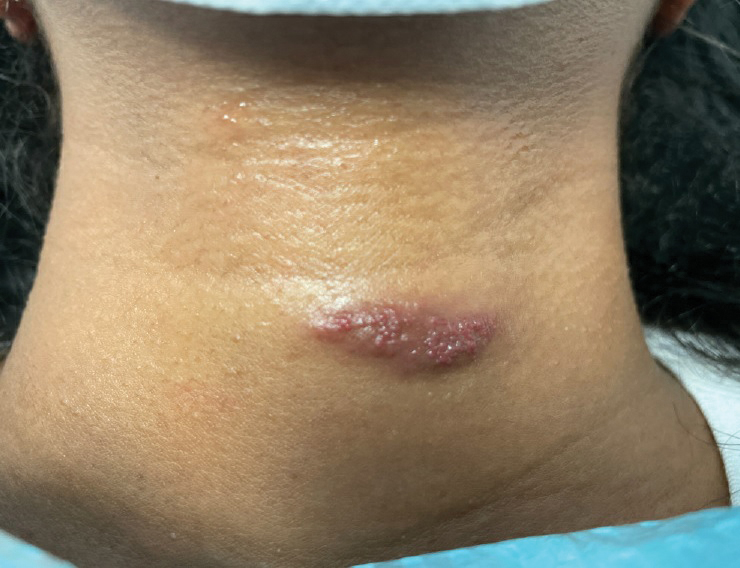
The Diagnosis: Tufted Angioma
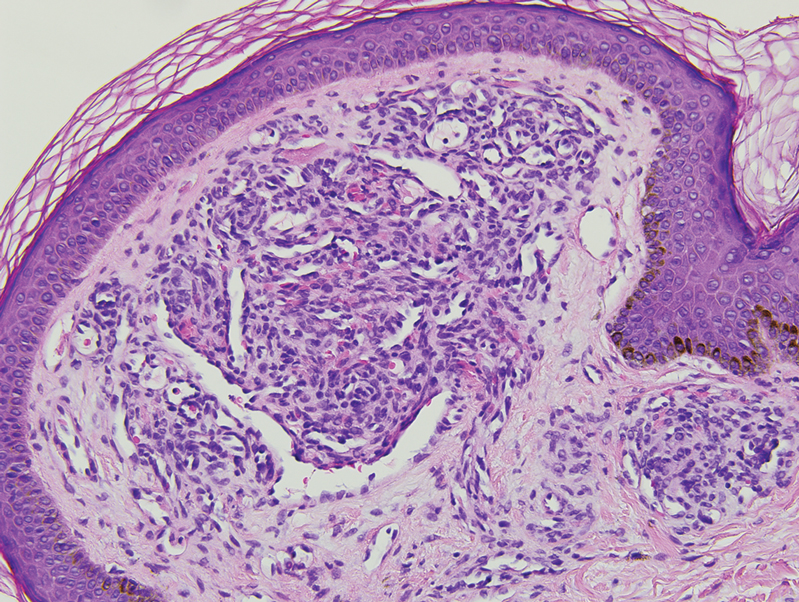
Histopathology revealed discrete lobules of closely packed capillaries with bland endothelial cells throughout the upper and lower dermis (Figure 1). The surrounding crescentlike vessels and lymphatics stained with D2-40 (Figure 2). These histologic findings were consistent with tufted angioma, and the patient elected for observation.
Tufted angiomas are benign vascular lesions named for the tufted appearance of capillaries on histology.1 They commonly present in children, with a lower incidence in adults and rare cases in pregnancy.2 Tufted angiomas typically present as solitary, slowly expanding, erythematous macules, plaques, or nodules on the neck or trunk ranging in size from less than 1 to 10 cm.2-4 They can be histologically distinguished from other vascular tumors, including aggressive malignant neoplasms.1
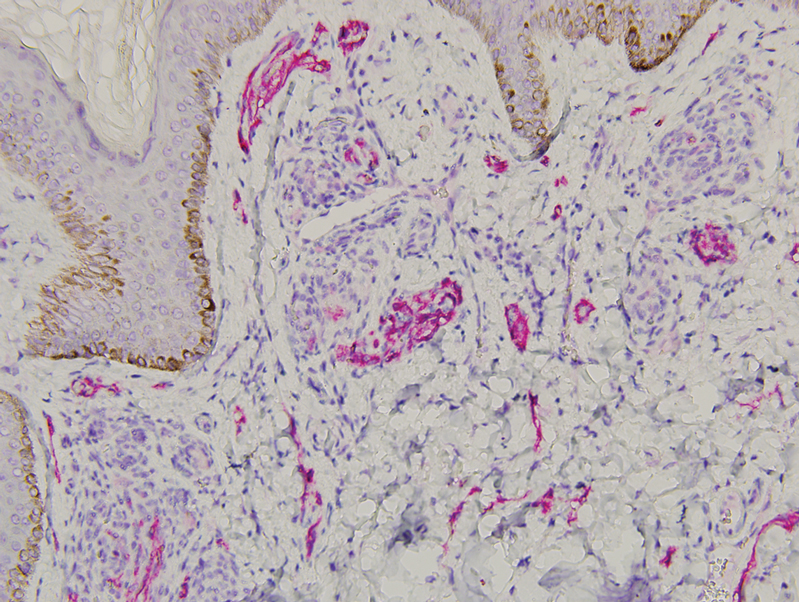
Tufted angiomas are identified by characteristic “cannon ball tufts” of capillaries in the dermis and subcutis at low power.3,5 Distinct cellular lobules may be found bulging into thin-walled vascular channels at the margins of the lobules in the dermis and subcutis (Figure 3).4 The lobules are formed by cells with spindle-shaped nuclei.6 Some mitotic figures may be present, but no cellular atypia is seen.2 The capillaries at the periphery appear as dilated semilunar vessels.4 Dilated lymphatics, which stain with D2-40, can be found at the periphery of the tufted capillaries and throughout the remaining dermis.3,4
Tufted angiomas may arise independently in adults but also have been associated with conditions such as pregnancy. Omori et al7 identified an acquired tufted angioma in pregnancy that was positive for estrogen and progesterone receptors. Reports of tufted angiomas in pregnancy vary; some are multiple lesions, some regress postpartum, and some undergo successful surgical treatment.3,5
Vascular lesions such as tufted angiomas specifically may appear in pregnancy due to a high-volume state with vasodilation and increased vascular proliferation. Although tumor angiogenesis has been linked to specific growth factors and cytokines, it has been hypothesized that the systemic hormones of pregnancy such as human chorionic gonadotropin, estradiol, and progesterone also shift the body to a more angiogenic state.8 In a study of cutaneous changes in pregnant women (N=905), 41% developed a vascular skin change, including spider veins, varicosities, hemangiomas, and granulomas.9 The most common vascular tumor in pregnancy is pyogenic granuloma. Pyogenic granulomas are small, solitary, friable papules that commonly are found on the hands, forearms, face, or in the mouth; histologically they demonstrate dilated capillaries in lobular structures accompanied by larger thick-walled vessels.3,10,11
Tufted angiomas may mimic a variety of other conditions. Epithelioid hemangioma, considered by some to be on the same morphologic spectrum as angiolymphoid hyperplasia with eosinophilia, classically occurs in young adults on the head and in the neck region. It histologically demonstrates a lobular appearance at low power; however, these lobules are made up of vessels with histiocytoid to epithelioid endothelial cells surrounded by a prominent inflammatory infiltrate consisting of lymphocytes and eosinophils.12
Kaposi sarcoma may appear on the neck but most often presents as macules and patches on the extremities that may form nodules with a rubbery consistency. In tufted angiomas, the cellular nodules with dilated channels at the margins bear a resemblance to Kaposi sarcoma or kaposiform hemangioendothelioma; however, in tufted angiomas the lobules are composed of bland spindle cells and slitlike vessels at the periphery.3,13,14 Tufted angiomas are negative for human herpesvirus 8 and typically do not have an associated inflammatory infiltrate with plasma cells.11,15
Moreover, it is important to differentiate tufted angioma from a cutaneous manifestation of an underlying malignancy, which has been described previously in cases of breast cancer.16,17 Our case illustrates a rare vascular tumor arising in the novel context of a pregnant patient with breast cancer. Distinguishing tufted angioma from other benign or malignant vascular tumors is necessary to avoid inappropriate therapeutic interventions.
- Jones EW, Orkin M. Tufted angioma (angioblastoma). a benign progressive angioma, not to be confused with Kaposi’s sarcoma or low-grade angiosarcoma. J Am Acad Dermatol. 1989;20(2 pt 1):214-225.
- Lee B, Chiu M, Soriano T, et al. Adult-onset tufted angioma: a case report and review of the literature. Cutis. 2006;78:341-345.
- Kim YK, Kim HJ, Lee KG. Acquired tufted angioma associated with pregnancy. Clin Exp Dermatol. 1992;17:458-459.
- Feito-Rodriguez M, Sanchez-Orta A, De Lucas R, et al. Congenital tufted angioma: a multicenter retrospective study of 30 cases. Pediatr Dermatol. 2018;35:808-816.
- Pietroletti R, Leardi S, Simi M. Perianal acquired tufted angioma associated with pregnancy: case report. Tech Coloproctol. 2002;6:117-119.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Omori M, Bito T, Nishigori C. Acquired tufted angioma in pregnancy showing expression of estrogen and progesterone receptors. Eur J Dermatol. 2013;23:898-899.
- Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232:R27-R44.
- Fernandes LB, Amaral W. Clinical study of skin changes in low and high risk pregnant women. An Bras Dermatol. 2015;90:822-826.
- Walker JL, Wang AR, Kroumpouzos G, et al. Cutaneous tumors in pregnancy. Clin Dermatol. 2016;34:359-367.
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. In: StatPearls. StatPearls Publishing; 2021.
- Ortins-Pina A, Llamas-Velasco M, Turpin S, et al. FOSB immunoreactivity in endothelia of epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia). J Cutan Pathol. 2018;45:395-402.
- Arai E, Kuramochi A, Tsuchida T, et al. Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol. 2006;33:492-497.
- Grassi S, Carugno A, Vignini M, et al. Adult-onset tufted angiomas associated with an arteriovenous malformation in a renal transplant recipient: case report and review of the literature. Am J Dermatopathol. 2015;37:162-165.
- Lyons LL, North PE, Mac-Moune Lai F, et al. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559-568.
- Putra HP, Djawad K, Nurdin AR. Cutaneous lesions as the first manifestation of breast cancer: a rare case. Pan Afr Med J. 2020;37:383.
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98.
The Diagnosis: Tufted Angioma
Histopathology revealed discrete lobules of closely packed capillaries with bland endothelial cells throughout the upper and lower dermis (Figure 1). The surrounding crescentlike vessels and lymphatics stained with D2-40 (Figure 2). These histologic findings were consistent with tufted angioma, and the patient elected for observation.

Tufted angiomas are benign vascular lesions named for the tufted appearance of capillaries on histology.1 They commonly present in children, with a lower incidence in adults and rare cases in pregnancy.2 Tufted angiomas typically present as solitary, slowly expanding, erythematous macules, plaques, or nodules on the neck or trunk ranging in size from less than 1 to 10 cm.2-4 They can be histologically distinguished from other vascular tumors, including aggressive malignant neoplasms.1

Tufted angiomas are identified by characteristic “cannon ball tufts” of capillaries in the dermis and subcutis at low power.3,5 Distinct cellular lobules may be found bulging into thin-walled vascular channels at the margins of the lobules in the dermis and subcutis (Figure 3).4 The lobules are formed by cells with spindle-shaped nuclei.6 Some mitotic figures may be present, but no cellular atypia is seen.2 The capillaries at the periphery appear as dilated semilunar vessels.4 Dilated lymphatics, which stain with D2-40, can be found at the periphery of the tufted capillaries and throughout the remaining dermis.3,4

Tufted angiomas may arise independently in adults but also have been associated with conditions such as pregnancy. Omori et al7 identified an acquired tufted angioma in pregnancy that was positive for estrogen and progesterone receptors. Reports of tufted angiomas in pregnancy vary; some are multiple lesions, some regress postpartum, and some undergo successful surgical treatment.3,5
Vascular lesions such as tufted angiomas specifically may appear in pregnancy due to a high-volume state with vasodilation and increased vascular proliferation. Although tumor angiogenesis has been linked to specific growth factors and cytokines, it has been hypothesized that the systemic hormones of pregnancy such as human chorionic gonadotropin, estradiol, and progesterone also shift the body to a more angiogenic state.8 In a study of cutaneous changes in pregnant women (N=905), 41% developed a vascular skin change, including spider veins, varicosities, hemangiomas, and granulomas.9 The most common vascular tumor in pregnancy is pyogenic granuloma. Pyogenic granulomas are small, solitary, friable papules that commonly are found on the hands, forearms, face, or in the mouth; histologically they demonstrate dilated capillaries in lobular structures accompanied by larger thick-walled vessels.3,10,11
Tufted angiomas may mimic a variety of other conditions. Epithelioid hemangioma, considered by some to be on the same morphologic spectrum as angiolymphoid hyperplasia with eosinophilia, classically occurs in young adults on the head and in the neck region. It histologically demonstrates a lobular appearance at low power; however, these lobules are made up of vessels with histiocytoid to epithelioid endothelial cells surrounded by a prominent inflammatory infiltrate consisting of lymphocytes and eosinophils.12
Kaposi sarcoma may appear on the neck but most often presents as macules and patches on the extremities that may form nodules with a rubbery consistency. In tufted angiomas, the cellular nodules with dilated channels at the margins bear a resemblance to Kaposi sarcoma or kaposiform hemangioendothelioma; however, in tufted angiomas the lobules are composed of bland spindle cells and slitlike vessels at the periphery.3,13,14 Tufted angiomas are negative for human herpesvirus 8 and typically do not have an associated inflammatory infiltrate with plasma cells.11,15
Moreover, it is important to differentiate tufted angioma from a cutaneous manifestation of an underlying malignancy, which has been described previously in cases of breast cancer.16,17 Our case illustrates a rare vascular tumor arising in the novel context of a pregnant patient with breast cancer. Distinguishing tufted angioma from other benign or malignant vascular tumors is necessary to avoid inappropriate therapeutic interventions.
The Diagnosis: Tufted Angioma
Histopathology revealed discrete lobules of closely packed capillaries with bland endothelial cells throughout the upper and lower dermis (Figure 1). The surrounding crescentlike vessels and lymphatics stained with D2-40 (Figure 2). These histologic findings were consistent with tufted angioma, and the patient elected for observation.

Tufted angiomas are benign vascular lesions named for the tufted appearance of capillaries on histology.1 They commonly present in children, with a lower incidence in adults and rare cases in pregnancy.2 Tufted angiomas typically present as solitary, slowly expanding, erythematous macules, plaques, or nodules on the neck or trunk ranging in size from less than 1 to 10 cm.2-4 They can be histologically distinguished from other vascular tumors, including aggressive malignant neoplasms.1

Tufted angiomas are identified by characteristic “cannon ball tufts” of capillaries in the dermis and subcutis at low power.3,5 Distinct cellular lobules may be found bulging into thin-walled vascular channels at the margins of the lobules in the dermis and subcutis (Figure 3).4 The lobules are formed by cells with spindle-shaped nuclei.6 Some mitotic figures may be present, but no cellular atypia is seen.2 The capillaries at the periphery appear as dilated semilunar vessels.4 Dilated lymphatics, which stain with D2-40, can be found at the periphery of the tufted capillaries and throughout the remaining dermis.3,4

Tufted angiomas may arise independently in adults but also have been associated with conditions such as pregnancy. Omori et al7 identified an acquired tufted angioma in pregnancy that was positive for estrogen and progesterone receptors. Reports of tufted angiomas in pregnancy vary; some are multiple lesions, some regress postpartum, and some undergo successful surgical treatment.3,5
Vascular lesions such as tufted angiomas specifically may appear in pregnancy due to a high-volume state with vasodilation and increased vascular proliferation. Although tumor angiogenesis has been linked to specific growth factors and cytokines, it has been hypothesized that the systemic hormones of pregnancy such as human chorionic gonadotropin, estradiol, and progesterone also shift the body to a more angiogenic state.8 In a study of cutaneous changes in pregnant women (N=905), 41% developed a vascular skin change, including spider veins, varicosities, hemangiomas, and granulomas.9 The most common vascular tumor in pregnancy is pyogenic granuloma. Pyogenic granulomas are small, solitary, friable papules that commonly are found on the hands, forearms, face, or in the mouth; histologically they demonstrate dilated capillaries in lobular structures accompanied by larger thick-walled vessels.3,10,11
Tufted angiomas may mimic a variety of other conditions. Epithelioid hemangioma, considered by some to be on the same morphologic spectrum as angiolymphoid hyperplasia with eosinophilia, classically occurs in young adults on the head and in the neck region. It histologically demonstrates a lobular appearance at low power; however, these lobules are made up of vessels with histiocytoid to epithelioid endothelial cells surrounded by a prominent inflammatory infiltrate consisting of lymphocytes and eosinophils.12
Kaposi sarcoma may appear on the neck but most often presents as macules and patches on the extremities that may form nodules with a rubbery consistency. In tufted angiomas, the cellular nodules with dilated channels at the margins bear a resemblance to Kaposi sarcoma or kaposiform hemangioendothelioma; however, in tufted angiomas the lobules are composed of bland spindle cells and slitlike vessels at the periphery.3,13,14 Tufted angiomas are negative for human herpesvirus 8 and typically do not have an associated inflammatory infiltrate with plasma cells.11,15
Moreover, it is important to differentiate tufted angioma from a cutaneous manifestation of an underlying malignancy, which has been described previously in cases of breast cancer.16,17 Our case illustrates a rare vascular tumor arising in the novel context of a pregnant patient with breast cancer. Distinguishing tufted angioma from other benign or malignant vascular tumors is necessary to avoid inappropriate therapeutic interventions.
- Jones EW, Orkin M. Tufted angioma (angioblastoma). a benign progressive angioma, not to be confused with Kaposi’s sarcoma or low-grade angiosarcoma. J Am Acad Dermatol. 1989;20(2 pt 1):214-225.
- Lee B, Chiu M, Soriano T, et al. Adult-onset tufted angioma: a case report and review of the literature. Cutis. 2006;78:341-345.
- Kim YK, Kim HJ, Lee KG. Acquired tufted angioma associated with pregnancy. Clin Exp Dermatol. 1992;17:458-459.
- Feito-Rodriguez M, Sanchez-Orta A, De Lucas R, et al. Congenital tufted angioma: a multicenter retrospective study of 30 cases. Pediatr Dermatol. 2018;35:808-816.
- Pietroletti R, Leardi S, Simi M. Perianal acquired tufted angioma associated with pregnancy: case report. Tech Coloproctol. 2002;6:117-119.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Omori M, Bito T, Nishigori C. Acquired tufted angioma in pregnancy showing expression of estrogen and progesterone receptors. Eur J Dermatol. 2013;23:898-899.
- Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232:R27-R44.
- Fernandes LB, Amaral W. Clinical study of skin changes in low and high risk pregnant women. An Bras Dermatol. 2015;90:822-826.
- Walker JL, Wang AR, Kroumpouzos G, et al. Cutaneous tumors in pregnancy. Clin Dermatol. 2016;34:359-367.
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. In: StatPearls. StatPearls Publishing; 2021.
- Ortins-Pina A, Llamas-Velasco M, Turpin S, et al. FOSB immunoreactivity in endothelia of epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia). J Cutan Pathol. 2018;45:395-402.
- Arai E, Kuramochi A, Tsuchida T, et al. Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol. 2006;33:492-497.
- Grassi S, Carugno A, Vignini M, et al. Adult-onset tufted angiomas associated with an arteriovenous malformation in a renal transplant recipient: case report and review of the literature. Am J Dermatopathol. 2015;37:162-165.
- Lyons LL, North PE, Mac-Moune Lai F, et al. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559-568.
- Putra HP, Djawad K, Nurdin AR. Cutaneous lesions as the first manifestation of breast cancer: a rare case. Pan Afr Med J. 2020;37:383.
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98.
- Jones EW, Orkin M. Tufted angioma (angioblastoma). a benign progressive angioma, not to be confused with Kaposi’s sarcoma or low-grade angiosarcoma. J Am Acad Dermatol. 1989;20(2 pt 1):214-225.
- Lee B, Chiu M, Soriano T, et al. Adult-onset tufted angioma: a case report and review of the literature. Cutis. 2006;78:341-345.
- Kim YK, Kim HJ, Lee KG. Acquired tufted angioma associated with pregnancy. Clin Exp Dermatol. 1992;17:458-459.
- Feito-Rodriguez M, Sanchez-Orta A, De Lucas R, et al. Congenital tufted angioma: a multicenter retrospective study of 30 cases. Pediatr Dermatol. 2018;35:808-816.
- Pietroletti R, Leardi S, Simi M. Perianal acquired tufted angioma associated with pregnancy: case report. Tech Coloproctol. 2002;6:117-119.
- Osio A, Fraitag S, Hadj-Rabia S, et al. Clinical spectrum of tufted angiomas in childhood: a report of 13 cases and a review of the literature. Arch Dermatol. 2010;146:758-763.
- Omori M, Bito T, Nishigori C. Acquired tufted angioma in pregnancy showing expression of estrogen and progesterone receptors. Eur J Dermatol. 2013;23:898-899.
- Boeldt DS, Bird IM. Vascular adaptation in pregnancy and endothelial dysfunction in preeclampsia. J Endocrinol. 2017;232:R27-R44.
- Fernandes LB, Amaral W. Clinical study of skin changes in low and high risk pregnant women. An Bras Dermatol. 2015;90:822-826.
- Walker JL, Wang AR, Kroumpouzos G, et al. Cutaneous tumors in pregnancy. Clin Dermatol. 2016;34:359-367.
- Sarwal P, Lapumnuaypol K. Pyogenic granuloma. In: StatPearls. StatPearls Publishing; 2021.
- Ortins-Pina A, Llamas-Velasco M, Turpin S, et al. FOSB immunoreactivity in endothelia of epithelioid hemangioma (angiolymphoid hyperplasia with eosinophilia). J Cutan Pathol. 2018;45:395-402.
- Arai E, Kuramochi A, Tsuchida T, et al. Usefulness of D2-40 immunohistochemistry for differentiation between kaposiform hemangioendothelioma and tufted angioma. J Cutan Pathol. 2006;33:492-497.
- Grassi S, Carugno A, Vignini M, et al. Adult-onset tufted angiomas associated with an arteriovenous malformation in a renal transplant recipient: case report and review of the literature. Am J Dermatopathol. 2015;37:162-165.
- Lyons LL, North PE, Mac-Moune Lai F, et al. Kaposiform hemangioendothelioma: a study of 33 cases emphasizing its pathologic, immunophenotypic, and biologic uniqueness from juvenile hemangioma. Am J Surg Pathol. 2004;28:559-568.
- Putra HP, Djawad K, Nurdin AR. Cutaneous lesions as the first manifestation of breast cancer: a rare case. Pan Afr Med J. 2020;37:383.
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98.
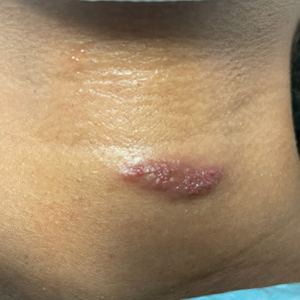
A 31-year-old woman at 34 weeks’ gestation presented with skin discoloration of the anterior neck of 7 months’ duration. Her pregnancy had been complicated by a diagnosis of invasive papillary carcinoma of the breast with unilateral complete mastectomy and negative sentinel lymph node biopsy in the first trimester. The lesion was tender, darkening, and rapidly enlarging. Physical examination demonstrated a linear, violaceous, vascular, and indurated plaque with microvesiculation that was 3.5 cm in width. She had no history of blistering sunburns, frequent UV exposure, or skin cancer.
Persistent Lip Swelling
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.
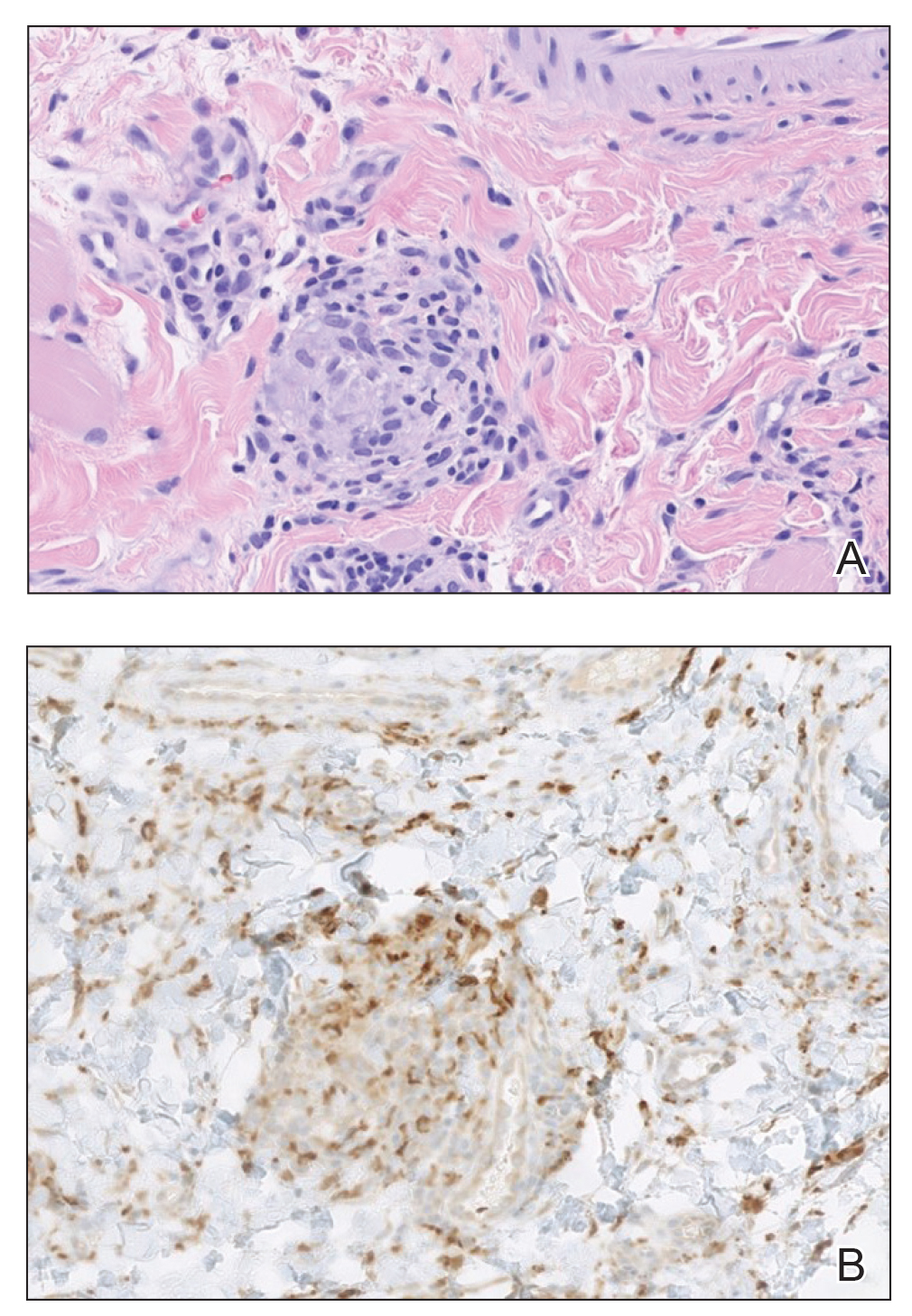
Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.

Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
The Diagnosis: Granulomatous Cheilitis
A punch biopsy of the lip revealed a noncaseating microgranuloma in the submucosa with modest submucosal vascular ectasia and perivascular lymphoplasmacytic infiltrates (Figure). Comprehensive metabolic panel, complete blood cell count, angiotensinconverting enzyme (ACE) levels, and inflammatory markers (ie, erythrocyte sedimentation rate, C-reactive protein) all were within reference range. A serum environmental allergen test was negative except for ragweed. Levels of complements—C1 esterase inhibitor (C1-INH) antigen and function, C1q, C3, and C4—and antinuclear antibodies all were normal. Chest radiography was unremarkable. In lieu of a colonoscopy, a fecal calprotectin obtained by gastroenterology was normal. Given the clinical presentation and histopathologic findings, a diagnosis of granulomatous cheilitis (GC) was made.

Granulomatous cheilitis (also known as Miescher cheilitis) is an idiopathic condition characterized by recurrent or persistent swelling of one or both lips. Granulomatous cheilitis usually is an isolated finding but can occur in the setting of Melkersson-Rosenthal syndrome, which refers to a triad of orofacial swelling, facial paralysis, and fissured tongue. Orofacial granulomatosis is a unifying term for any orofacial swelling associated with histologic findings of noncaseating granulomas without evidence of a systemic disease.
Granulomatous cheilitis is a rare disease that most commonly occurs in young adults without any sex predilection.1 The etiology still is unknown, but genetic predisposition, idiopathic influx of inflammatory cells, sensitivity to food or dental materials, and infections have been implicated.2 Granulomatous cheilitis initially presents as soft, nonerythematous, nontender swelling affecting one or both lips. The first episode usually resolves in hours or days, but the frequency and duration of the attacks may increase until the swelling becomes persistent and indurated.3 Granulomatous cheilitis often is a diagnosis of exclusion. A tissue biopsy may show noncaseating epithelioid and multinucleated giant cells with associated lymphedema and fibrosis4; however, histologic findings may be nonspecific, especially early in the disease course, and may be indistinguishable from those of other granulomatous diseases such as sarcoidosis and Crohn disease (CD).5
Lip swelling may be an oral manifestation of CD. Compared with GC, however, CD more commonly is associated with ulcerations, buccal sulcus involvement, abnormalities in complete blood cell count such as anemia and thrombocytosis, and elevated C-reactive protein and erythrocyte sedimentation rate. Although infrequent, GC may coincide with or precede the onset of CD.6 Thus, a detailed gastrointestinal history and appropriate laboratory tests are needed to rule out undiagnosed CD. Nevertheless, performing a routine colonoscopy in the absence of gastrointestinal symptoms is debated.7,8
Sarcoidosis is a systemic granulomatous disease that can have oral involvement in the form of edema, nodules, or ulcers. Oral sarcoidosis usually occurs in patients with chronic multisystemic sarcoidosis and likely is accompanied by pulmonary manifestations such as hilar adenopathy and infiltrates on chest radiography, which are found in more than 90% of patients with sarcoidosis.9,10 A diagnosis of sarcoidosis is additionally supported by other organ involvement such as the joints, skin, or eyes, as well as elevated ACE and calcium levels.
Foreign bodies are another source of granulomatous inflammation and may present with nonspecific findings of swelling, masses, erythema, pain, or ulceration in oral tissues.11 Foreign body reactions to dental materials, retained sutures, and cosmetic fillers have been reported.12-14 In many cases, the foreign material is evident on biopsy.
Angioedema may mimic GC and should be excluded before more extensive testing is done, as it can result in life-threatening respiratory compromise. Numerous etiologies of angioedema have been identified including allergens, acquired or hereditary C1-INH deficiency, nonsteroidal anti-inflammatory drugs, ACE inhibitors, autoimmune disorders, and chronic infections.15 Patients with angioedema may have abnormalities in C4 and C1-INH levels or report certain medication use, allergen exposure, or family history of unexplained recurrent swellings or gastrointestinal symptoms.
There currently is no established treatment of GC due to the unclear etiology and unpredictable clinical course that can lead to spontaneous remissions or frequent recurrences. Corticosteroids administered systemically, intralesionally, or topically have been the mainstay treatment of GC.2 In particular, intralesional injections have been reported as effective in reducing swelling and preventing recurrences in several studies.16,17 Numerous other treatments have been reported in the literature with inconsistent outcomes, including antibiotics such as minocycline, metronidazole, and roxithromycin; clofazimine; thalidomide; immunomodulators such as tumor necrosis factor inhibitors and methotrexate; fumaric acid esters; and cheiloplasty in severe cases.16 Our patient showed near-complete resolution of the lip swelling after a single intralesional injection of 0.5 cc of triamcinolone acetonide 5 mg/mL. The patient has since received 5 additional maintenance injections of 0.1 to 0.2 cc of triamcinolone acetonide 2.5 to 5 mg/mL spaced 2 to 4 months apart with excellent control of the lip swelling, which the patient feels has resolved. We anticipate that repeated injections and monitoring of recurrences may be required for long-term remission.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
- McCartan BE, Healy CM, McCreary CE, et al. Characteristics of patients with orofacial granulomatosis. Oral Dis. 2011;17:696-704.
- Grave B, McCullough M, Wiesenfeld D. Orofacial granulomatosis—a 20-year review. Oral Dis. 2009;15:46-51.
- Critchlow WA, Chang D. Cheilitis granulomatosa: a review. Head Neck Pathol. 2014;8:209-213.
- Wiesenfeld D, Ferguson MM, Mitchell DN, et al. Oro-facial granulomatosis—a clinical and pathological analysis. Q J Med. 1985;54:101-113.
- Rogers RS 3rd. Melkersson-Rosenthal syndrome and orofacial granulomatosis. Dermatol Clin. 1996;14:371-379.
- Campbell H, Escudier M, Patel P, et al. Distinguishing orofacial granulomatosis from Crohn’s disease: two separate disease entities? Inflamm Bowel Dis. 2011;17:2109-2115.
- Plauth M, Jenss H, Meyle J. Oral manifestations of Crohn’s disease. an analysis of 79 cases. J Clin Gastroenterol. 1991;13:29-37.
- Van der Waal RI, Schulten EA, van der Meij EH, et al. Cheilitis granulomatosa: overview of 13 patients with long-term follow-up— results of management. Int J Dermatol. 2002;41:225-229.
- Bouaziz A, Le Scanff J, Chapelon-Abric C, et al. Oral involvement in sarcoidosis: report of 12 cases. QJM. 2012;105:755-767.
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736-755.
- Alawi F. An update on granulomatous diseases of the oral tissues. Dent Clin North Am. 2013;57:657-671.
- Stewart CM, Watson RE. Experimental oral foreign body reactions. commonly employed dental materials. Oral Surg Oral Med Oral Pathol. 1990;69:713-719.
- Selvig KA, Biagiotti GR, Leknes KN, et al. Oral tissue reactions to suture materials. Int J Periodontics Restorative Dent. 1998;18:474-487.
- Jham BC, Nikitakis NG, Scheper MA, et al. Granulomatous foreignbody reaction involving oral and perioral tissues after injection of biomaterials: a series of 7 cases and review of the literature. J Oral Maxillofac Surg. 2009;67:280-285.
- Zingale LC, Beltrami L, Zanichelli A, et al. Angioedema without urticaria: a large clinical survey. CMAJ. 2006;175:1065-1070.
- Banks T, Gada S. A comprehensive review of current treatments for granulomatous cheilitis. Br J Dermatol. 2012;166:934-937.
- Fedele S, Fung PP, Bamashmous N, et al. Long-term effectiveness of intralesional triamcinolone acetonide therapy in orofacial granulomatosis: an observational cohort study. Br J Dermatol. 2014;170:794-801.
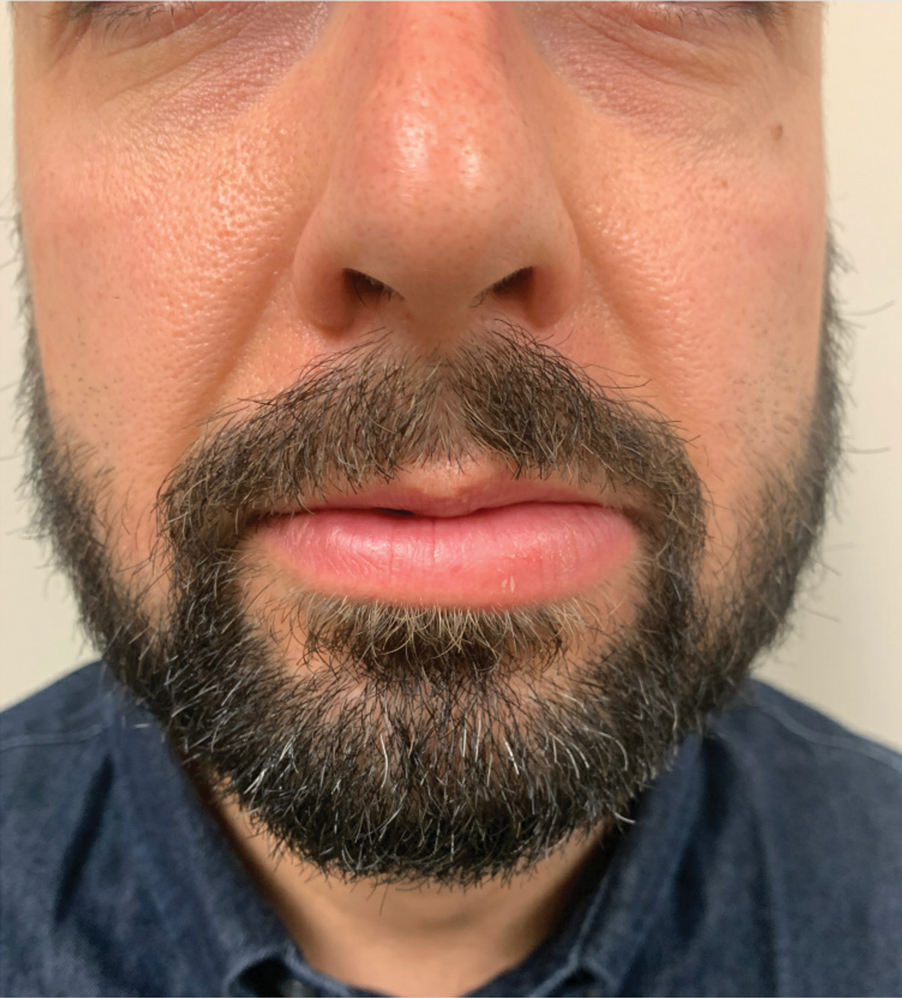
A 36-year-old man with allergic rhinitis presented with lower lip swelling of several months’ duration. The swelling was persistent and predominantly on the left side of the lower lip but occasionally spread to the entire lower lip. The episodes of increased swelling would last for several days and were not associated with any apparent triggers. He denied any pain, pruritus, or dryness. He noted more drooling from the affected side but denied any associated breathing difficulty or throat discomfort. Treatment with an oral antihistamine provided no relief. He denied any recent nonsteroidal anti-inflammatory drug or angiotensinconverting enzyme inhibitor use. His family history was notable for lupus in his maternal grandmother and maternal aunt. He denied any personal or family history of inflammatory bowel disease or recent gastrointestinal tract symptoms. Physical examination revealed nontender edema in the left side of the lower lip with no surface changes. No warmth or erythema were noted. The tongue and the rest of the oral cavity were unremarkable.
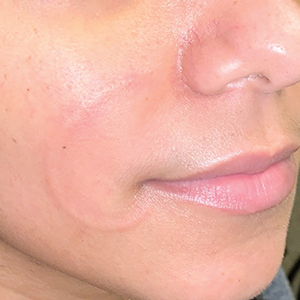
Recurrent Arciform Plaque on the Face
The Diagnosis: Lupus Erythematosus Tumidus
Histopathologic evaluation of a punch biopsy revealed focal dermal mucin deposition and CD123+ discrete clusters of plasmacytoid dendritic cells without interface changes (Figure 1), favoring a diagnosis of lupus erythematosus tumidus (LET) in our patient. There was no clinical improvement in symptoms when she previously was treated with topical antifungals or class III corticosteroid creams. Tacrolimus ointment 0.1% twice daily for 1 month did not result in substantial improvement in the appearance of the plaque, and it spontaneously resolved after 2 to 3 months. She declined treatment with hydroxychloroquine.
Lupus erythematosus tumidus is an uncommon subtype of chronic cutaneous lupus erythematosus with no distinct etiology. It is clinically characterized by edematous, urticarial, single or multiple plaques with a smooth surface affecting sun-exposed areas that can last for months to years.1 In contrast to other variations of chronic cutaneous lupus such as discoid lupus erythematosus, LET lesions lack surface papulosquamous features such as scaling, atrophy, and follicular plugging.1-4 Based solely on histologic findings, LET may be indistinguishable from reticular erythematous mucinosis and Jessner lymphocytic infiltration of the skin (JLIS) due to a similar lack of epidermal involvement and presence of a perivascular lymphocytic infiltrate (Figure 2).
The average age at disease onset is 36 years, nearly the same as that described in discoid lupus erythematosus.4 Lupus erythematosus tumidus has a favorable prognosis and commonly presents without other autoimmune signs, serologic abnormalities, or gender preference, with concomitant systemic lupus erythematosus sometimes reported.5
The absence of clinical and histological epidermal involvement are the most important clues to aid in the diagnosis. It has been postulated that JLIS could be an early cutaneous manifestation of LET.6 The differential diagnosis also may include erythema annulare centrifugum, granuloma annulare, and urticarial vasculitis. Lesions typically respond well to photoprotection, topical corticosteroids, and/or antimalarials. The addition of tacrolimus ointment 0.1% may result in complete regression without recurrence.7
Erythema annulare centrifugum is a reactive erythema that classically begins as a pink papule that gradually enlarges to form an annular erythematous plaque with a fine trailing scale that may recur.8 The histopathology of erythema annulare centrifugum shares features seen in LET, making the diagnosis difficult; however, secondary changes to the epidermis (eg, spongiosis, hyperkeratosis) may be seen. This condition has been associated with lymphoproliferative malignancies.8
Reticular erythematous mucinosis is clinically distinguished from LET, as it presents as reticular, rather than arciform, erythematous macules, papules, or plaques that may be asymptomatic or pruritic.9 Histopathology typically shows more superficial mucin deposition than in LET as well as superficial to mid-dermal perivascular and periadnexal lymphocytic infiltrates. Reticular erythematous mucinosis more frequently is reported in women in their 30s and 40s and has been associated with UV exposure and hormonal triggers, such as oral contraceptive medications and pregnancy.9
Granuloma annulare typically presents as asymptomatic, erythematous, annular plaques or papules in young women.10 There are several histologic subtypes that show focal collagen degeneration, inflammation with palisaded or interstitial histiocytes, and mucin deposition, regardless of clinical presentation. Granuloma annulare has been associated with systemic diseases including type 2 diabetes mellitus and thyroid disease. Localized granuloma annulare most commonly presents on the dorsal aspects of the hands or feet.10
We present a case of LET on the face. Although histologically similar to other dermatoses, LET often lacks epidermal involvement and presents on sun-exposed areas of the body. Jessner lymphocytic infiltration of the skin also should be considered in the differential, as there is an overlap of clinical and histopathological features; JLIS lacks mucin deposits.6 This case reinforces the importance of correlating clinical with histopathologic findings. Our patient was treated with tacrolimus ointment 0.1%, and the plaque eventually resolved in 2 to 3 months without recurrence. This condition should be included in the differential diagnosis of recurring annular plaques on sunexposed areas, particularly in middle-aged adults, even in the absence of systemic involvement.
- Liu E, Daze RP, Moon S. Tumid lupus erythematosus: a rare and distinctive variant of cutaneous lupus erythematosus masquerading as urticarial vasculitis. Cureus. 2020;12:E8305. doi:10.7759/cureus.8305
- Saleh D, Crane JS. Tumid lupus erythematosus. In: StatPearls. StatPearls Publishing; 2020.
- Verma P, Sharma S, Yadav P, et al. Tumid lupus erythematosus: an intriguing dermatopathological connotation treated successfully with topical tacrolimus and hydroxychloroquine combination. Indian J Dermatol. 2014;59:210. doi:10.4103/0019-5154.127716
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448. doi:10.1016/j. autrev.2008.12.010
- Jatwani K, Chugh K, Osholowu OS, et al. Tumid lupus erythematosus and systemic lupus erythematosus: a report on their rare coexistence. Cureus. 2020;12:E7545. doi:10.7759/cureus.7545
- Tomasini D, Mentzel T, Hantschke M, et al. Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner’s lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37:1132-1139. doi:10.1111/j.1600-0560.2010.01587.x
- Patsinakidis N, Kautz O, Gibbs BF, et al. Lupus erythematosus tumidus: clinical perspectives. Clin Cosmet Investig Dermatol. 2019;12:707-719. doi:10.2147/CCID.S166723
- Mu EW, Sanchez M, Mir A, et al. Paraneoplastic erythema annulare centrifugum eruption (PEACE). Dermatol Online J. 2015;21:13030/ qt6053h29n.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335. doi:10.1177/0961203320965702 10. Keimig EL. Granuloma annulare. Dermatol Clin. 2015;33:315-329. doi:10.1016/j.det.2015.03.001
The Diagnosis: Lupus Erythematosus Tumidus
Histopathologic evaluation of a punch biopsy revealed focal dermal mucin deposition and CD123+ discrete clusters of plasmacytoid dendritic cells without interface changes (Figure 1), favoring a diagnosis of lupus erythematosus tumidus (LET) in our patient. There was no clinical improvement in symptoms when she previously was treated with topical antifungals or class III corticosteroid creams. Tacrolimus ointment 0.1% twice daily for 1 month did not result in substantial improvement in the appearance of the plaque, and it spontaneously resolved after 2 to 3 months. She declined treatment with hydroxychloroquine.

Lupus erythematosus tumidus is an uncommon subtype of chronic cutaneous lupus erythematosus with no distinct etiology. It is clinically characterized by edematous, urticarial, single or multiple plaques with a smooth surface affecting sun-exposed areas that can last for months to years.1 In contrast to other variations of chronic cutaneous lupus such as discoid lupus erythematosus, LET lesions lack surface papulosquamous features such as scaling, atrophy, and follicular plugging.1-4 Based solely on histologic findings, LET may be indistinguishable from reticular erythematous mucinosis and Jessner lymphocytic infiltration of the skin (JLIS) due to a similar lack of epidermal involvement and presence of a perivascular lymphocytic infiltrate (Figure 2).

The average age at disease onset is 36 years, nearly the same as that described in discoid lupus erythematosus.4 Lupus erythematosus tumidus has a favorable prognosis and commonly presents without other autoimmune signs, serologic abnormalities, or gender preference, with concomitant systemic lupus erythematosus sometimes reported.5
The absence of clinical and histological epidermal involvement are the most important clues to aid in the diagnosis. It has been postulated that JLIS could be an early cutaneous manifestation of LET.6 The differential diagnosis also may include erythema annulare centrifugum, granuloma annulare, and urticarial vasculitis. Lesions typically respond well to photoprotection, topical corticosteroids, and/or antimalarials. The addition of tacrolimus ointment 0.1% may result in complete regression without recurrence.7
Erythema annulare centrifugum is a reactive erythema that classically begins as a pink papule that gradually enlarges to form an annular erythematous plaque with a fine trailing scale that may recur.8 The histopathology of erythema annulare centrifugum shares features seen in LET, making the diagnosis difficult; however, secondary changes to the epidermis (eg, spongiosis, hyperkeratosis) may be seen. This condition has been associated with lymphoproliferative malignancies.8
Reticular erythematous mucinosis is clinically distinguished from LET, as it presents as reticular, rather than arciform, erythematous macules, papules, or plaques that may be asymptomatic or pruritic.9 Histopathology typically shows more superficial mucin deposition than in LET as well as superficial to mid-dermal perivascular and periadnexal lymphocytic infiltrates. Reticular erythematous mucinosis more frequently is reported in women in their 30s and 40s and has been associated with UV exposure and hormonal triggers, such as oral contraceptive medications and pregnancy.9
Granuloma annulare typically presents as asymptomatic, erythematous, annular plaques or papules in young women.10 There are several histologic subtypes that show focal collagen degeneration, inflammation with palisaded or interstitial histiocytes, and mucin deposition, regardless of clinical presentation. Granuloma annulare has been associated with systemic diseases including type 2 diabetes mellitus and thyroid disease. Localized granuloma annulare most commonly presents on the dorsal aspects of the hands or feet.10
We present a case of LET on the face. Although histologically similar to other dermatoses, LET often lacks epidermal involvement and presents on sun-exposed areas of the body. Jessner lymphocytic infiltration of the skin also should be considered in the differential, as there is an overlap of clinical and histopathological features; JLIS lacks mucin deposits.6 This case reinforces the importance of correlating clinical with histopathologic findings. Our patient was treated with tacrolimus ointment 0.1%, and the plaque eventually resolved in 2 to 3 months without recurrence. This condition should be included in the differential diagnosis of recurring annular plaques on sunexposed areas, particularly in middle-aged adults, even in the absence of systemic involvement.
The Diagnosis: Lupus Erythematosus Tumidus
Histopathologic evaluation of a punch biopsy revealed focal dermal mucin deposition and CD123+ discrete clusters of plasmacytoid dendritic cells without interface changes (Figure 1), favoring a diagnosis of lupus erythematosus tumidus (LET) in our patient. There was no clinical improvement in symptoms when she previously was treated with topical antifungals or class III corticosteroid creams. Tacrolimus ointment 0.1% twice daily for 1 month did not result in substantial improvement in the appearance of the plaque, and it spontaneously resolved after 2 to 3 months. She declined treatment with hydroxychloroquine.

Lupus erythematosus tumidus is an uncommon subtype of chronic cutaneous lupus erythematosus with no distinct etiology. It is clinically characterized by edematous, urticarial, single or multiple plaques with a smooth surface affecting sun-exposed areas that can last for months to years.1 In contrast to other variations of chronic cutaneous lupus such as discoid lupus erythematosus, LET lesions lack surface papulosquamous features such as scaling, atrophy, and follicular plugging.1-4 Based solely on histologic findings, LET may be indistinguishable from reticular erythematous mucinosis and Jessner lymphocytic infiltration of the skin (JLIS) due to a similar lack of epidermal involvement and presence of a perivascular lymphocytic infiltrate (Figure 2).

The average age at disease onset is 36 years, nearly the same as that described in discoid lupus erythematosus.4 Lupus erythematosus tumidus has a favorable prognosis and commonly presents without other autoimmune signs, serologic abnormalities, or gender preference, with concomitant systemic lupus erythematosus sometimes reported.5
The absence of clinical and histological epidermal involvement are the most important clues to aid in the diagnosis. It has been postulated that JLIS could be an early cutaneous manifestation of LET.6 The differential diagnosis also may include erythema annulare centrifugum, granuloma annulare, and urticarial vasculitis. Lesions typically respond well to photoprotection, topical corticosteroids, and/or antimalarials. The addition of tacrolimus ointment 0.1% may result in complete regression without recurrence.7
Erythema annulare centrifugum is a reactive erythema that classically begins as a pink papule that gradually enlarges to form an annular erythematous plaque with a fine trailing scale that may recur.8 The histopathology of erythema annulare centrifugum shares features seen in LET, making the diagnosis difficult; however, secondary changes to the epidermis (eg, spongiosis, hyperkeratosis) may be seen. This condition has been associated with lymphoproliferative malignancies.8
Reticular erythematous mucinosis is clinically distinguished from LET, as it presents as reticular, rather than arciform, erythematous macules, papules, or plaques that may be asymptomatic or pruritic.9 Histopathology typically shows more superficial mucin deposition than in LET as well as superficial to mid-dermal perivascular and periadnexal lymphocytic infiltrates. Reticular erythematous mucinosis more frequently is reported in women in their 30s and 40s and has been associated with UV exposure and hormonal triggers, such as oral contraceptive medications and pregnancy.9
Granuloma annulare typically presents as asymptomatic, erythematous, annular plaques or papules in young women.10 There are several histologic subtypes that show focal collagen degeneration, inflammation with palisaded or interstitial histiocytes, and mucin deposition, regardless of clinical presentation. Granuloma annulare has been associated with systemic diseases including type 2 diabetes mellitus and thyroid disease. Localized granuloma annulare most commonly presents on the dorsal aspects of the hands or feet.10
We present a case of LET on the face. Although histologically similar to other dermatoses, LET often lacks epidermal involvement and presents on sun-exposed areas of the body. Jessner lymphocytic infiltration of the skin also should be considered in the differential, as there is an overlap of clinical and histopathological features; JLIS lacks mucin deposits.6 This case reinforces the importance of correlating clinical with histopathologic findings. Our patient was treated with tacrolimus ointment 0.1%, and the plaque eventually resolved in 2 to 3 months without recurrence. This condition should be included in the differential diagnosis of recurring annular plaques on sunexposed areas, particularly in middle-aged adults, even in the absence of systemic involvement.
- Liu E, Daze RP, Moon S. Tumid lupus erythematosus: a rare and distinctive variant of cutaneous lupus erythematosus masquerading as urticarial vasculitis. Cureus. 2020;12:E8305. doi:10.7759/cureus.8305
- Saleh D, Crane JS. Tumid lupus erythematosus. In: StatPearls. StatPearls Publishing; 2020.
- Verma P, Sharma S, Yadav P, et al. Tumid lupus erythematosus: an intriguing dermatopathological connotation treated successfully with topical tacrolimus and hydroxychloroquine combination. Indian J Dermatol. 2014;59:210. doi:10.4103/0019-5154.127716
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448. doi:10.1016/j. autrev.2008.12.010
- Jatwani K, Chugh K, Osholowu OS, et al. Tumid lupus erythematosus and systemic lupus erythematosus: a report on their rare coexistence. Cureus. 2020;12:E7545. doi:10.7759/cureus.7545
- Tomasini D, Mentzel T, Hantschke M, et al. Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner’s lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37:1132-1139. doi:10.1111/j.1600-0560.2010.01587.x
- Patsinakidis N, Kautz O, Gibbs BF, et al. Lupus erythematosus tumidus: clinical perspectives. Clin Cosmet Investig Dermatol. 2019;12:707-719. doi:10.2147/CCID.S166723
- Mu EW, Sanchez M, Mir A, et al. Paraneoplastic erythema annulare centrifugum eruption (PEACE). Dermatol Online J. 2015;21:13030/ qt6053h29n.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335. doi:10.1177/0961203320965702 10. Keimig EL. Granuloma annulare. Dermatol Clin. 2015;33:315-329. doi:10.1016/j.det.2015.03.001
- Liu E, Daze RP, Moon S. Tumid lupus erythematosus: a rare and distinctive variant of cutaneous lupus erythematosus masquerading as urticarial vasculitis. Cureus. 2020;12:E8305. doi:10.7759/cureus.8305
- Saleh D, Crane JS. Tumid lupus erythematosus. In: StatPearls. StatPearls Publishing; 2020.
- Verma P, Sharma S, Yadav P, et al. Tumid lupus erythematosus: an intriguing dermatopathological connotation treated successfully with topical tacrolimus and hydroxychloroquine combination. Indian J Dermatol. 2014;59:210. doi:10.4103/0019-5154.127716
- Kuhn A, Bein D, Bonsmann G. The 100th anniversary of lupus erythematosus tumidus. Autoimmun Rev. 2009;8:441-448. doi:10.1016/j. autrev.2008.12.010
- Jatwani K, Chugh K, Osholowu OS, et al. Tumid lupus erythematosus and systemic lupus erythematosus: a report on their rare coexistence. Cureus. 2020;12:E7545. doi:10.7759/cureus.7545
- Tomasini D, Mentzel T, Hantschke M, et al. Plasmacytoid dendritic cells: an overview of their presence and distribution in different inflammatory skin diseases, with special emphasis on Jessner’s lymphocytic infiltrate of the skin and cutaneous lupus erythematosus. J Cutan Pathol. 2010;37:1132-1139. doi:10.1111/j.1600-0560.2010.01587.x
- Patsinakidis N, Kautz O, Gibbs BF, et al. Lupus erythematosus tumidus: clinical perspectives. Clin Cosmet Investig Dermatol. 2019;12:707-719. doi:10.2147/CCID.S166723
- Mu EW, Sanchez M, Mir A, et al. Paraneoplastic erythema annulare centrifugum eruption (PEACE). Dermatol Online J. 2015;21:13030/ qt6053h29n.
- Ocanha-Xavier JP, Cola-Senra CO, Xavier-Junior JCC. Reticular erythematous mucinosis: literature review and case report of a 24-year-old patient with systemic erythematosus lupus. Lupus. 2021;30:325-335. doi:10.1177/0961203320965702 10. Keimig EL. Granuloma annulare. Dermatol Clin. 2015;33:315-329. doi:10.1016/j.det.2015.03.001
An otherwise healthy 31-year-old woman presented with a gradual growth of a semiannular, arciform, mildly pruritic plaque around the mouth of 10 years’ duration that recurred biannually, persisted for a few months, and spontaneously remitted without residual scarring. She denied joint pain, muscle aches, sores in the mouth, personal or family history of autoimmune diseases, or other remarkable review of systems. Physical examination revealed a welldefined, edematous, smooth, arciform plaque on the face with no mucous membrane involvement. Laboratory evaluation, including complete blood cell count, comprehensive metabolic panel, and antinuclear antibody titer, was unremarkable. A punch biopsy was obtained.

Retiform Purpura on the Legs
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2
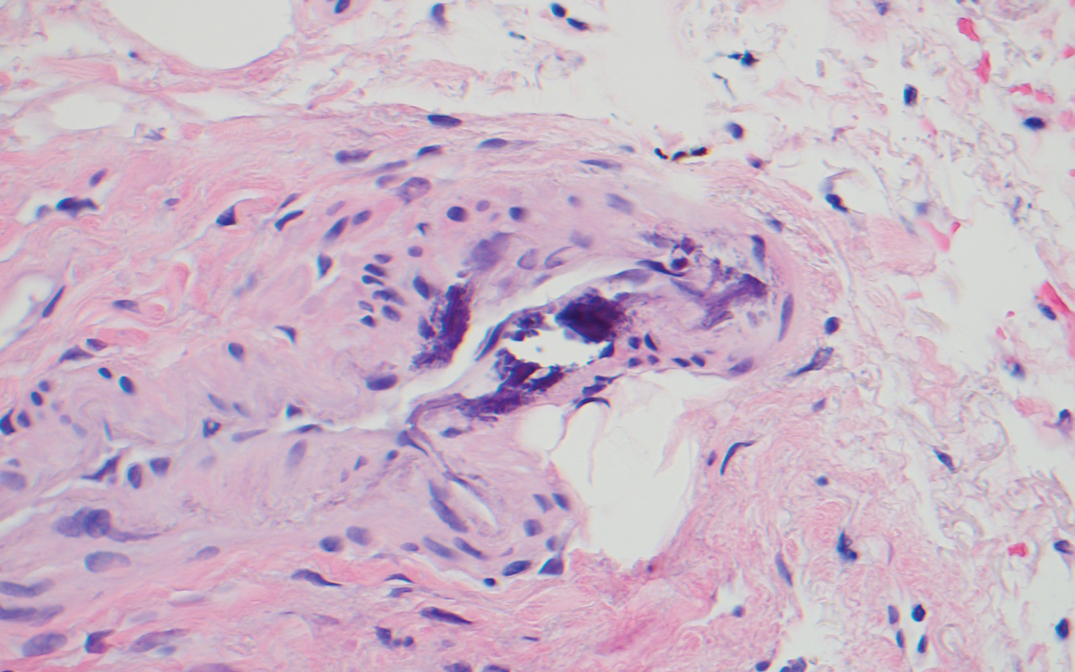
Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2

Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
The Diagnosis: Calciphylaxis
Histopathology revealed epidermal and dermal necrosis, a perivascular neutrophilic infiltrate, and scattered microcalcifications within small- and medium-sized subcutaneous vessels, consistent with a diagnosis of calciphylaxis (Figure). Calciphylaxis (also known as calcific uremic arteriolopathy) is a rare, severe, and often fatal vasculopathy that predominately occurs in patients with end-stage renal failure.1 The pathogenesis of calciphylaxis remains poorly understood; however, it generally is thought that an imbalance in calcium homeostasis in susceptible hosts results in the precipitation of calcium phosphate within vessel walls leading to endothelial damage with subsequent thrombotic vasculopathy and ischemic tissue damage. Acquired and congenital hypercoagulable states have been implicated in the pathogenesis of calciphylaxis.2

Treatment of calciphylaxis is directed at normalizing abnormal calcium metabolism; removing possible exacerbating agents, such as warfarin, systemic corticosteroids, calcium, and iron; and transitioning patients with end-stage renal disease to hemodialysis, if not already initiated. The treatment approach is multifaceted, and numerous therapies usually are attempted simultaneously. Vitamin K supplementation, low-calcium dialysate, non–calcium carbonate phosphate binders, cinacalcet, becaplermin, bisphosphonates, hyperbaric oxygen, and intravenous sodium thiosulfate all have been utilized with some success. Currently, intravenous sodium thiosulfate is the mainstay therapy for the treatment of calciphylaxis.2 Although the mechanism of sodium thiosulfate is not entirely understood, it is known to have anticalcification, vasodilatory, and antioxidant properties.
Retiform purpura clinically is characterized by reticulated, branching, purpuric skin lesions. It occurs following vascular insult by way of vessel lumen occlusion (thrombotic vasculopathy) and less frequently by vessel wall inflammation (vasculitis). The differential diagnosis for retiform purpura includes various causes of microvascular occlusion, including hypercoagulable states and type I cryoglobulinemia, calciphylaxis, infections, autoimmune vasculitic conditions, and embolic causes.3
Cutaneous disease in individuals with antiphospholipid antibodies may present similarly with retiform purpura in the form of necrotizing livedo reticularis, leg ulcers, or widespread cutaneous necrosis. Histopathologic findings include vascular thrombi with partial or complete obstruction of the small- to medium-sized arteries at the dermoepidermal junction, often in the absence of an inflammatory infiltrate.4 True vasculitis is not typical of antiphospholipid syndrome.
Medium vessel vasculitides, such as polyarteritis nodosa, clinically present with livedo reticularis, subcutaneous nodules, and tissue necrosis. Dermatopathologic evaluation of a medium-sized vessel vasculitis would demonstrate a neutrophilic vasculitis involving vessels within the deep dermis and septa of subcutaneous fat.5 Tissue sampling should be deep and wide enough to visualize the pathology, as shallow biopsies may show intraluminal thrombi of the superficial dermal plexus only, while a narrow specimen may result in falsenegative findings due to the focal nature of vessel involvement in conditions such as polyarteritis nodosa.
Type I cryoglobulinemia often is a manifestation of plasma cell dyscrasia and commonly presents with Raynaud phenomenon, livedo reticularis, and acrocyanosis of helices6 ; pathology demonstrates vessel occlusion and erythrocyte extravasation. In contrast, types II and III, also known as mixed cryoglobulinemia, are associated with hepatitis C and autoimmune connective tissue disease. They clinically present as purpuric plaques and nodules that have a propensity to vesiculate and ulcerate.7 Histopathologically, features of leukocytoclastic vasculitis are seen, and direct immunofluorescence demonstrates perivascular granular deposits consisting predominantly of IgM and C3 in the papillary dermis.8
Warfarin therapy, particularly in high initial doses, can induce lesions of cutaneous necrosis, which clinically may resemble the appearance of calciphylaxis. Warfarininduced skin necrosis typically occurs 3 to 5 days after the initiation of therapy and is the result of a temporary prothrombotic state.9 The half-life of antithrombotic protein C is shorter than vitamin K–dependent prothrombotic factors II, X, and IX. Early in warfarin treatment, an acquired state of reduced protein C level exists, which can lead to vessel thrombosis and subsequent cutaneous necrosis. Treatment of warfarin-induced skin necrosis involves cessation of warfarin, supplementation with vitamin K to reverse the effects of warfarin, and the initiation of heparin or low-molecular-weight heparin.9
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
- Hayashi M. Calciphylaxis: diagnosis and clinical features. Clin Exp Nephrol. 2013;17:498-503.
- Strazzula L, Nigwekar SU, Steele D, et al. Intralesional sodium thiosulfate for the treatment of calciphylaxis. JAMA Dermatol. 2013;149:946-949.
- Georgesen C, Fox LP, Harp J. Retiform purpura: a diagnostic approach. J Am Acad Dermatol. 2020;82:783-796.
- Llamas-Velasco M, Alegría V, Santos-Briz Á, et al. Occlusive nonvasculitic vasculopathy. Am J Dermatopathol. 2017;39:637-662.
- Daoud MS, Hutton KP, Gibson LE. Cutaneous periarteritis nodosa: a clinicopathologic study of 79 cases. Br J Dermatol. 1997; 136:706-713.
- Fraser Gibson J, Leventhal JS, King B. Purpuric lesions on acral sites. type I cryoglobulinemia associated with multiple myeloma. JAMA Dermatol. 2015;151:659-660.
- Pakula AS, Garden JM, Roth SI. Mixed cryoglobulinemia and hepatitis C virus infection. J Am Acad Dermatol. 1994;30:143.
- Daoud MS, el-Azhary RA, Gibson LE, et al. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. clinical, pathologic, and immunopathologic study of twelve patients. J Am Acad Dermatol. 1996;34:219-223.
- Nazarian RM, Van Cott EM, Zembowicz A, et al. Warfarin-induced skin necrosis. J Am Acad Dermatol. 2009;61:325-332.
A 70-year-old woman with a medical history of Takayasu arteritis, end-stage renal disease on peritoneal dialysis, coronary artery disease, hypertension, hypothyroidism, and anemia of chronic disease presented to the emergency department with enlarging painful stellate eschars of the legs with associated edema of 3 weeks’ duration. She denied a history of similar-appearing skin lesions. She initially thought the lesions were burns secondary to frequent hot showers for relief of uremic pruritus. For the treatment of these suspected burns prior to hospitalization, she had been applying over-the-counter antibiotic ointments to the affected areas and had completed a 2-week course of oral cephalexin without notable improvement. Physical examination revealed retiform purpura of the legs with large stellate eschars overlying the anteromedial thighs and right medial calf. Computed tomography angiogram of the abdomen and pelvis demonstrated diffuse calcifications of the aortic wall and its associated branches that were most pronounced in the legs without evidence of vessel wall thickening. Punch biopsies were performed, and nephrology, rheumatology, and wound care services were consulted.
Painful Fungating Perianal Mass
The Diagnosis: Condyloma Latum
A punch biopsy of the perianal mass revealed epidermal acanthosis with elongated slender rete ridges, scattered intraepidermal neutrophils, and a dense dermal inflammatory infiltrate (Figure, A) with a prominent plasma cell component (Figure, B). A treponemal immunohistochemical stain revealed numerous coiled spirochetes concentrated in the lower epidermis (Figure, C). Serologic test results including rapid plasma reagin (titer 1:1024) and Treponema pallidum antibody were reactive, confirming the diagnosis of secondary syphilis with condyloma latum. The patient was treated with intramuscular penicillin G with resolution of the lesion 2 weeks later.
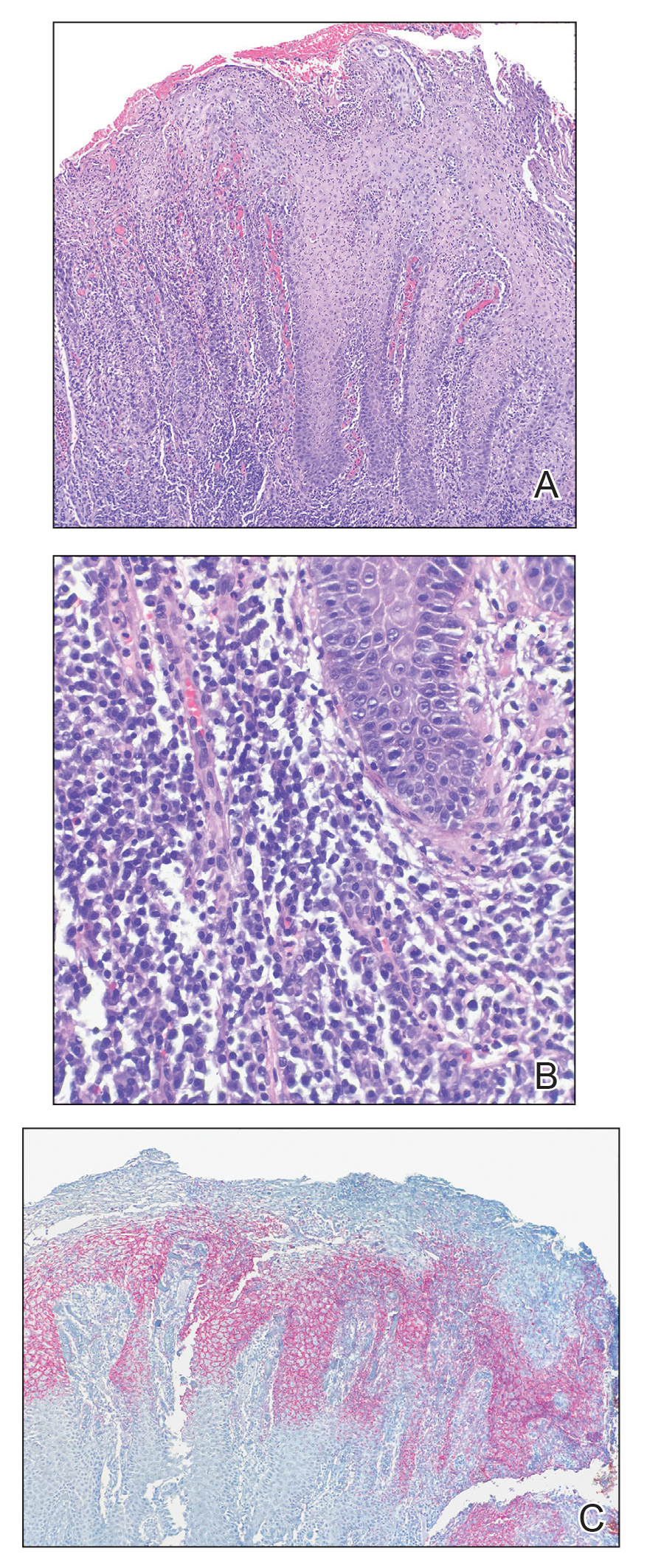
Syphilis, a sexually transmitted infection caused by the spirochete T pallidum, reached historically low rates in the United States in the early 2000s due to the widespread use of penicillin and effective public health efforts.1 However, the rates of primary and secondary syphilis infections recently have markedly increased, resulting in the current epidemic of syphilis in the United States and Europe.1,2 Its wide variety of clinical and histopathologic manifestations make recognition challenging and lend it the moniker “the great imitator.”
Secondary syphilis results from the systemic spread of T pallidum and classically is characterized by the triad of a skin rash that frequently involves the palms and soles, mucosal ulceration such as condyloma latum, and lymphadenopathy.2,3 However, condyloma latum may represent the only manifestation of secondary syphilis in a subset of patients,4 as observed in our patient.
In the 2 months prior to diagnosis, our patient was evaluated at multiple emergency departments and primary care clinics, receiving diagnoses of condyloma acuminatum, genital herpes simplex virus, hemorrhoids, and suspicion for malignancy—entities that comprise the differential diagnosis for condyloma latum.2,5 Despite some degree of overlap in patient populations, risk factors, and presentations between these diagnostic considerations, recognition of certain clinical features, in addition to histopathologic evaluation, may facilitate navigation of this differential diagnosis.
Primary and secondary syphilis infections have been predominantly observed in men, mostly men who have sex with men and/or those who are infected with HIV.1 Condyloma acuminata, genital herpes simplex virus, and chancroid also are seen in younger individuals, more commonly in those with multiple sexual partners, but show a more even gender distribution and are not restricted to those partaking in anal intercourse. The clinical presentation of condyloma latum can be differentiated by its painless, flat, smooth, and commonly hypopigmented appearance, often with associated surface erosion and a gray exudate, in contrast to condyloma acuminatum, which typically presents as nontender, flesh-colored or hyperpigmented, exophytic papules that may coalesce into plaques.2,3,6 Genital herpes simplex virus infection presents with multiple small papulovesicular lesions with ulceration, most commonly on the tip or shaft of the penis, though perianal lesions may be seen in men who have sex with men.7 Similarly, chancroid presents with painful necrotizing genital ulcers most commonly on the penis, though perianal lesions also may be seen.8 Hemorrhoids classically are seen in middle-aged adults with a history of constipation, present with rectal bleeding, and may be associated with pain in the setting of thrombosis or ulceration.9 Finally, perianal squamous cell carcinoma primarily occurs in older adults, typically in the sixth decade of life. Verrucous carcinoma most commonly arises in the oropharynx or anogenital region in sites of chronic irritation and presents as a slow-growing exophytic mass. Classic squamous cell carcinoma most commonly occurs in association with human papillomavirus infection and presents with scaly erythematous papules or plaques.10
Our case highlighted the clinical difficulty in recognizing condyloma latum, as this lesion remained undiagnosed for 2 months, and our patient presumptively was treated for multiple perianal pathologies prior to a biopsy being performed. Due to the clinical similarity of various perianal lesions, the diagnosis of condyloma latum should be considered, and serologic studies should be performed in fitting clinical contexts, especially in light of recently rising rates of syphilis infection.1,2
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Tayal S, Shaban F, Dasgupta K, et al. A case of syphilitic anal condylomata lata mimicking malignancy. Int J Surg Case Rep. 2015; 17:69-71.
- Aung PP, Wimmer DB, Lester TR, et al. Perianal condylomata lata mimicking carcinoma. J Cutan Pathol. 2022;49:209-214.
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21.
- Bruins FG, van Deudekom FJ, de Vries HJ. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259.
- Leslie SW, Sajjad H, Kumar S. Genital warts. In: StatPearls. StatPearls Publishing; 2021.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Irizarry L, Velasquez J, Wray AA. Chancroid. In: StatPearls. StatPearls Publishing; 2022.
- Mounsey AL, Halladay J, Sadiq TS. Hemorrhoids. Am Fam Physician. 2011;84:204-210.
- Abbass MA, Valente MA. Premalignant and malignant perianal lesions. Clin Colon Rectal Surg. 2019;32:386-393.
The Diagnosis: Condyloma Latum
A punch biopsy of the perianal mass revealed epidermal acanthosis with elongated slender rete ridges, scattered intraepidermal neutrophils, and a dense dermal inflammatory infiltrate (Figure, A) with a prominent plasma cell component (Figure, B). A treponemal immunohistochemical stain revealed numerous coiled spirochetes concentrated in the lower epidermis (Figure, C). Serologic test results including rapid plasma reagin (titer 1:1024) and Treponema pallidum antibody were reactive, confirming the diagnosis of secondary syphilis with condyloma latum. The patient was treated with intramuscular penicillin G with resolution of the lesion 2 weeks later.

Syphilis, a sexually transmitted infection caused by the spirochete T pallidum, reached historically low rates in the United States in the early 2000s due to the widespread use of penicillin and effective public health efforts.1 However, the rates of primary and secondary syphilis infections recently have markedly increased, resulting in the current epidemic of syphilis in the United States and Europe.1,2 Its wide variety of clinical and histopathologic manifestations make recognition challenging and lend it the moniker “the great imitator.”
Secondary syphilis results from the systemic spread of T pallidum and classically is characterized by the triad of a skin rash that frequently involves the palms and soles, mucosal ulceration such as condyloma latum, and lymphadenopathy.2,3 However, condyloma latum may represent the only manifestation of secondary syphilis in a subset of patients,4 as observed in our patient.
In the 2 months prior to diagnosis, our patient was evaluated at multiple emergency departments and primary care clinics, receiving diagnoses of condyloma acuminatum, genital herpes simplex virus, hemorrhoids, and suspicion for malignancy—entities that comprise the differential diagnosis for condyloma latum.2,5 Despite some degree of overlap in patient populations, risk factors, and presentations between these diagnostic considerations, recognition of certain clinical features, in addition to histopathologic evaluation, may facilitate navigation of this differential diagnosis.
Primary and secondary syphilis infections have been predominantly observed in men, mostly men who have sex with men and/or those who are infected with HIV.1 Condyloma acuminata, genital herpes simplex virus, and chancroid also are seen in younger individuals, more commonly in those with multiple sexual partners, but show a more even gender distribution and are not restricted to those partaking in anal intercourse. The clinical presentation of condyloma latum can be differentiated by its painless, flat, smooth, and commonly hypopigmented appearance, often with associated surface erosion and a gray exudate, in contrast to condyloma acuminatum, which typically presents as nontender, flesh-colored or hyperpigmented, exophytic papules that may coalesce into plaques.2,3,6 Genital herpes simplex virus infection presents with multiple small papulovesicular lesions with ulceration, most commonly on the tip or shaft of the penis, though perianal lesions may be seen in men who have sex with men.7 Similarly, chancroid presents with painful necrotizing genital ulcers most commonly on the penis, though perianal lesions also may be seen.8 Hemorrhoids classically are seen in middle-aged adults with a history of constipation, present with rectal bleeding, and may be associated with pain in the setting of thrombosis or ulceration.9 Finally, perianal squamous cell carcinoma primarily occurs in older adults, typically in the sixth decade of life. Verrucous carcinoma most commonly arises in the oropharynx or anogenital region in sites of chronic irritation and presents as a slow-growing exophytic mass. Classic squamous cell carcinoma most commonly occurs in association with human papillomavirus infection and presents with scaly erythematous papules or plaques.10
Our case highlighted the clinical difficulty in recognizing condyloma latum, as this lesion remained undiagnosed for 2 months, and our patient presumptively was treated for multiple perianal pathologies prior to a biopsy being performed. Due to the clinical similarity of various perianal lesions, the diagnosis of condyloma latum should be considered, and serologic studies should be performed in fitting clinical contexts, especially in light of recently rising rates of syphilis infection.1,2
The Diagnosis: Condyloma Latum
A punch biopsy of the perianal mass revealed epidermal acanthosis with elongated slender rete ridges, scattered intraepidermal neutrophils, and a dense dermal inflammatory infiltrate (Figure, A) with a prominent plasma cell component (Figure, B). A treponemal immunohistochemical stain revealed numerous coiled spirochetes concentrated in the lower epidermis (Figure, C). Serologic test results including rapid plasma reagin (titer 1:1024) and Treponema pallidum antibody were reactive, confirming the diagnosis of secondary syphilis with condyloma latum. The patient was treated with intramuscular penicillin G with resolution of the lesion 2 weeks later.

Syphilis, a sexually transmitted infection caused by the spirochete T pallidum, reached historically low rates in the United States in the early 2000s due to the widespread use of penicillin and effective public health efforts.1 However, the rates of primary and secondary syphilis infections recently have markedly increased, resulting in the current epidemic of syphilis in the United States and Europe.1,2 Its wide variety of clinical and histopathologic manifestations make recognition challenging and lend it the moniker “the great imitator.”
Secondary syphilis results from the systemic spread of T pallidum and classically is characterized by the triad of a skin rash that frequently involves the palms and soles, mucosal ulceration such as condyloma latum, and lymphadenopathy.2,3 However, condyloma latum may represent the only manifestation of secondary syphilis in a subset of patients,4 as observed in our patient.
In the 2 months prior to diagnosis, our patient was evaluated at multiple emergency departments and primary care clinics, receiving diagnoses of condyloma acuminatum, genital herpes simplex virus, hemorrhoids, and suspicion for malignancy—entities that comprise the differential diagnosis for condyloma latum.2,5 Despite some degree of overlap in patient populations, risk factors, and presentations between these diagnostic considerations, recognition of certain clinical features, in addition to histopathologic evaluation, may facilitate navigation of this differential diagnosis.
Primary and secondary syphilis infections have been predominantly observed in men, mostly men who have sex with men and/or those who are infected with HIV.1 Condyloma acuminata, genital herpes simplex virus, and chancroid also are seen in younger individuals, more commonly in those with multiple sexual partners, but show a more even gender distribution and are not restricted to those partaking in anal intercourse. The clinical presentation of condyloma latum can be differentiated by its painless, flat, smooth, and commonly hypopigmented appearance, often with associated surface erosion and a gray exudate, in contrast to condyloma acuminatum, which typically presents as nontender, flesh-colored or hyperpigmented, exophytic papules that may coalesce into plaques.2,3,6 Genital herpes simplex virus infection presents with multiple small papulovesicular lesions with ulceration, most commonly on the tip or shaft of the penis, though perianal lesions may be seen in men who have sex with men.7 Similarly, chancroid presents with painful necrotizing genital ulcers most commonly on the penis, though perianal lesions also may be seen.8 Hemorrhoids classically are seen in middle-aged adults with a history of constipation, present with rectal bleeding, and may be associated with pain in the setting of thrombosis or ulceration.9 Finally, perianal squamous cell carcinoma primarily occurs in older adults, typically in the sixth decade of life. Verrucous carcinoma most commonly arises in the oropharynx or anogenital region in sites of chronic irritation and presents as a slow-growing exophytic mass. Classic squamous cell carcinoma most commonly occurs in association with human papillomavirus infection and presents with scaly erythematous papules or plaques.10
Our case highlighted the clinical difficulty in recognizing condyloma latum, as this lesion remained undiagnosed for 2 months, and our patient presumptively was treated for multiple perianal pathologies prior to a biopsy being performed. Due to the clinical similarity of various perianal lesions, the diagnosis of condyloma latum should be considered, and serologic studies should be performed in fitting clinical contexts, especially in light of recently rising rates of syphilis infection.1,2
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Tayal S, Shaban F, Dasgupta K, et al. A case of syphilitic anal condylomata lata mimicking malignancy. Int J Surg Case Rep. 2015; 17:69-71.
- Aung PP, Wimmer DB, Lester TR, et al. Perianal condylomata lata mimicking carcinoma. J Cutan Pathol. 2022;49:209-214.
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21.
- Bruins FG, van Deudekom FJ, de Vries HJ. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259.
- Leslie SW, Sajjad H, Kumar S. Genital warts. In: StatPearls. StatPearls Publishing; 2021.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Irizarry L, Velasquez J, Wray AA. Chancroid. In: StatPearls. StatPearls Publishing; 2022.
- Mounsey AL, Halladay J, Sadiq TS. Hemorrhoids. Am Fam Physician. 2011;84:204-210.
- Abbass MA, Valente MA. Premalignant and malignant perianal lesions. Clin Colon Rectal Surg. 2019;32:386-393.
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854.
- Tayal S, Shaban F, Dasgupta K, et al. A case of syphilitic anal condylomata lata mimicking malignancy. Int J Surg Case Rep. 2015; 17:69-71.
- Aung PP, Wimmer DB, Lester TR, et al. Perianal condylomata lata mimicking carcinoma. J Cutan Pathol. 2022;49:209-214.
- Pourang A, Fung MA, Tartar D, et al. Condyloma lata in secondary syphilis. JAAD Case Rep. 2021;10:18-21.
- Bruins FG, van Deudekom FJ, de Vries HJ. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259.
- Leslie SW, Sajjad H, Kumar S. Genital warts. In: StatPearls. StatPearls Publishing; 2021.
- Groves MJ. Genital herpes: a review. Am Fam Physician. 2016; 93:928-934.
- Irizarry L, Velasquez J, Wray AA. Chancroid. In: StatPearls. StatPearls Publishing; 2022.
- Mounsey AL, Halladay J, Sadiq TS. Hemorrhoids. Am Fam Physician. 2011;84:204-210.
- Abbass MA, Valente MA. Premalignant and malignant perianal lesions. Clin Colon Rectal Surg. 2019;32:386-393.
A 21-year-old man presented to our clinic with rectal pain of 2 months’ duration that occurred in association with bowel movements and rectal bleeding in the setting of constipation. The patient’s symptoms had persisted despite multiple clinical encounters and treatment with sulfamethoxazole-trimethoprim, clotrimazole, valacyclovir, topical hydrocortisone and pramoxine, topical lidocaine, imiquimod, and psyllium seed. The patient denied engaging in receptive anal intercourse and had no notable medical or surgical history. Physical examination revealed a 6-cm hypopigmented fungating mass on the left gluteal cleft just external to the anal verge; there were no other abnormal findings. The patient denied any other systemic symptoms.
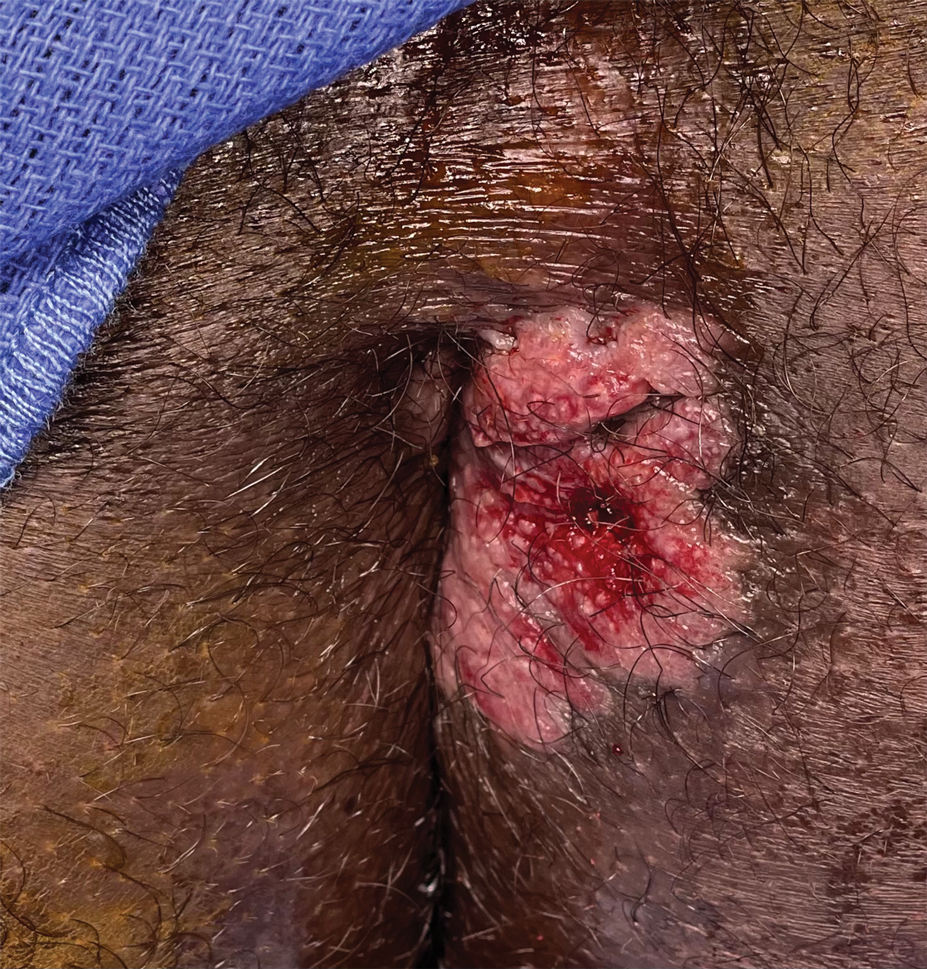
Erythematous Plaque on the Groin and Buttocks
The Diagnosis: Pseudomonas Pyoderma
A skin swab confirmed the presence of a ciprofloxacinsusceptible Pseudomonas aeruginosa strain. Our patient received oral ciprofloxacin 500 mg twice daily for 10 days with remarkable clinical improvement. The remaining skin lesion was successfully treated with more frequent diaper changes and the use of topical corticosteroids and emollients.
The topographical location, cutaneous morphology, clinical context, and sometimes the type of exudate are fundamental for the diagnosis of eruptions in intertriginous areas. Cutaneous Candida infections are common in these locations. They classically present as markedly erythematous plaques that occasionally are erosive, accompanied by satellite papules and pustules.1 Tinea cruris is a dermatophyte infection of the groin, proximal medial thighs, perineum, and buttocks. It usually presents as an erythematous patch that spreads centrifugally with partial central clearing and a slightly elevated, scaly border. Although candidiasis was higher on the differential, it was less likely, as our patient had a concomitant exudate inconsistent with Candida infections. Also, the lack of response to antifungal agents made hypotheses of fungal infections improbable.1
Inverse psoriasis is a variant of psoriasis identified by the development of well-demarcated, nonscaly, shiny plaques on body folds.2 Psoriasis is a chronic disease with several other cutaneous manifestations, such as nail and scalp involvement, as well as erythematous scaly plaques on the extensor surfaces of the limbs. The absence of a history of psoriasis, lack of other cutaneous manifestations, and no response to topical corticosteroids made the diagnosis of inverse psoriasis unlikely in our patient.
Erythrasma is a common superficial cutaneous infection caused by Corynebacterium minutissimum, a grampositive bacillus. It typically presents as an intertriginous eruption characterized by small erythematous to brown patches or thin plaques with fine scaling and sharp borders.3 Erythrasma displays a coral red fluorescence on Wood lamp examination that can be useful in the distinction from other causes of intertrigo.1 Although this examination had not been performed in our patient, the striking exudate made erythrasma less likely, and the culture performed on skin swab material would help to rule out this diagnosis.
Pseudomonas aeruginosa is a gram-negative strict aerobic bacillus of ubiquitous distribution with a preference for humid environments.4,5 Pseudomonas aeruginosa infections were first reported in the 19th century by physicians who noticed a peculiar odorous condition that caused a blue-green discoloration on bandages. This coloration explains the species name aeruginosa which is derived from the Latin word for copper rust.4 It comes from several water-soluble pigments produced by this microorganism, the most prevalent of which are pyocyanin and pyoverdine. Pyocyanin has a greenish-blue color and is nonfluorescent, while pyoverdine is green-yellowish and fluoresces under Wood light.5 Other pigments, such as pyorubin and pyomelanin, can be produced by some Pseudomonas strains.4
Pseudomonas aeruginosa has become one of the main pathogens involved in hospital-acquired infections,6 especially in immunocompromised patients.6,7 It is a frequent cause of respiratory infections in patients with cystic fibrosis, as it is present in the airways of up to 70% of these patients in adulthood.7 Also, due to a variety of adaptive mechanisms with the development of resistance to a range of antibiotics, P aeruginosa has become a worldwide public health problem and is involved in several life-threatening nosocomial infections.7,8
Cutaneous P aeruginosa infections range from superficial to deep tissue involvement and can affect both immunocompromised and immunocompetent individuals.9 They are classified as primary when they originate directly from the skin or secondary when they occur in the context of bacteremia. Primary infections mostly are mild and often are seen in healthy individuals; they usually occur by inoculation and predominate in moist areas where skin breakdown is frequent. Secondary infections typically affect immunocompromised individuals and portend a poor prognosis.5,9
Denominated as Pseudomonas pyoderma, the superficial skin infection by P aeruginosa is described as a condition where the epidermis has a moth-eaten appearance with macerated or eroded borders.10 A blue-greenish exudate and a grape juice odor often are present. This infection usually occurs as a complication of several skin conditions such as tinea pedis, eczema, burns, wounds, and ulcers.5,10
We believe that our patient developed Pseudomonas pyoderma as a complication of diaper dermatitis. His extended hospital stay with the use of different antibiotic regimens for the treatment of several infectious complications may have contributed to the development of infection by P aeruginosa.11 Despite its great clinical relevance, there are few studies in the literature on primary skin infections caused by P aeruginosa, and clinical descriptions with images are rare. Our patient had a nonspecific noneczematous dermatitis, and the projections on the periphery of the lesion resembled the moth-eaten appearance of the classic description of Pseudomonas pyoderma.5,10 The presence of a greenish exudate should promptly raise suspicion for this entity. We believe that the presentation of this case can illustrate this finding and help physicians to recognize this infection.
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Micali G, Verzi AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019; 12:953-959.
- Somerville DA. Erythrasma in normal young adults. J Med Microbiol. 1970;3:57-64.
- D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Vol 2. 8th ed. Elsevier; 2015:2518-2531.
- Silvestre JF, Betlloch MI. Cutaneous manifestations due to Pseudomonas infection. Int J Dermatol. 1999;38:419-431.
- Young LS, Armstrong D. Pseudomonas aeruginosa infections. CRC Crit Rev Clin Lab Sci. 1972;3:291-347.
- Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: device-associated module. Am J Infect Control. 2020;48:423-432.
- Wu DC, Chan WW, Metelitsa AI, et al. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol. 2011;12:157-169.
- Hall JH, Callaway JL, Tindall JP, et al. Pseudomonas aeruginosa in dermatology. Arch Dermatol. 1968;97:312-324.
- Merchant S, Proudfoot EM, Quadri HN, et al. Risk factors for Pseudomonas aeruginosa infections in Asia-Pacific and consequences of inappropriate initial antimicrobial therapy: a systematic literature review and meta-analysis. J Glob Antimicrob Resist. 2018;14:33-44.
The Diagnosis: Pseudomonas Pyoderma
A skin swab confirmed the presence of a ciprofloxacinsusceptible Pseudomonas aeruginosa strain. Our patient received oral ciprofloxacin 500 mg twice daily for 10 days with remarkable clinical improvement. The remaining skin lesion was successfully treated with more frequent diaper changes and the use of topical corticosteroids and emollients.
The topographical location, cutaneous morphology, clinical context, and sometimes the type of exudate are fundamental for the diagnosis of eruptions in intertriginous areas. Cutaneous Candida infections are common in these locations. They classically present as markedly erythematous plaques that occasionally are erosive, accompanied by satellite papules and pustules.1 Tinea cruris is a dermatophyte infection of the groin, proximal medial thighs, perineum, and buttocks. It usually presents as an erythematous patch that spreads centrifugally with partial central clearing and a slightly elevated, scaly border. Although candidiasis was higher on the differential, it was less likely, as our patient had a concomitant exudate inconsistent with Candida infections. Also, the lack of response to antifungal agents made hypotheses of fungal infections improbable.1
Inverse psoriasis is a variant of psoriasis identified by the development of well-demarcated, nonscaly, shiny plaques on body folds.2 Psoriasis is a chronic disease with several other cutaneous manifestations, such as nail and scalp involvement, as well as erythematous scaly plaques on the extensor surfaces of the limbs. The absence of a history of psoriasis, lack of other cutaneous manifestations, and no response to topical corticosteroids made the diagnosis of inverse psoriasis unlikely in our patient.
Erythrasma is a common superficial cutaneous infection caused by Corynebacterium minutissimum, a grampositive bacillus. It typically presents as an intertriginous eruption characterized by small erythematous to brown patches or thin plaques with fine scaling and sharp borders.3 Erythrasma displays a coral red fluorescence on Wood lamp examination that can be useful in the distinction from other causes of intertrigo.1 Although this examination had not been performed in our patient, the striking exudate made erythrasma less likely, and the culture performed on skin swab material would help to rule out this diagnosis.
Pseudomonas aeruginosa is a gram-negative strict aerobic bacillus of ubiquitous distribution with a preference for humid environments.4,5 Pseudomonas aeruginosa infections were first reported in the 19th century by physicians who noticed a peculiar odorous condition that caused a blue-green discoloration on bandages. This coloration explains the species name aeruginosa which is derived from the Latin word for copper rust.4 It comes from several water-soluble pigments produced by this microorganism, the most prevalent of which are pyocyanin and pyoverdine. Pyocyanin has a greenish-blue color and is nonfluorescent, while pyoverdine is green-yellowish and fluoresces under Wood light.5 Other pigments, such as pyorubin and pyomelanin, can be produced by some Pseudomonas strains.4
Pseudomonas aeruginosa has become one of the main pathogens involved in hospital-acquired infections,6 especially in immunocompromised patients.6,7 It is a frequent cause of respiratory infections in patients with cystic fibrosis, as it is present in the airways of up to 70% of these patients in adulthood.7 Also, due to a variety of adaptive mechanisms with the development of resistance to a range of antibiotics, P aeruginosa has become a worldwide public health problem and is involved in several life-threatening nosocomial infections.7,8
Cutaneous P aeruginosa infections range from superficial to deep tissue involvement and can affect both immunocompromised and immunocompetent individuals.9 They are classified as primary when they originate directly from the skin or secondary when they occur in the context of bacteremia. Primary infections mostly are mild and often are seen in healthy individuals; they usually occur by inoculation and predominate in moist areas where skin breakdown is frequent. Secondary infections typically affect immunocompromised individuals and portend a poor prognosis.5,9
Denominated as Pseudomonas pyoderma, the superficial skin infection by P aeruginosa is described as a condition where the epidermis has a moth-eaten appearance with macerated or eroded borders.10 A blue-greenish exudate and a grape juice odor often are present. This infection usually occurs as a complication of several skin conditions such as tinea pedis, eczema, burns, wounds, and ulcers.5,10
We believe that our patient developed Pseudomonas pyoderma as a complication of diaper dermatitis. His extended hospital stay with the use of different antibiotic regimens for the treatment of several infectious complications may have contributed to the development of infection by P aeruginosa.11 Despite its great clinical relevance, there are few studies in the literature on primary skin infections caused by P aeruginosa, and clinical descriptions with images are rare. Our patient had a nonspecific noneczematous dermatitis, and the projections on the periphery of the lesion resembled the moth-eaten appearance of the classic description of Pseudomonas pyoderma.5,10 The presence of a greenish exudate should promptly raise suspicion for this entity. We believe that the presentation of this case can illustrate this finding and help physicians to recognize this infection.
The Diagnosis: Pseudomonas Pyoderma
A skin swab confirmed the presence of a ciprofloxacinsusceptible Pseudomonas aeruginosa strain. Our patient received oral ciprofloxacin 500 mg twice daily for 10 days with remarkable clinical improvement. The remaining skin lesion was successfully treated with more frequent diaper changes and the use of topical corticosteroids and emollients.
The topographical location, cutaneous morphology, clinical context, and sometimes the type of exudate are fundamental for the diagnosis of eruptions in intertriginous areas. Cutaneous Candida infections are common in these locations. They classically present as markedly erythematous plaques that occasionally are erosive, accompanied by satellite papules and pustules.1 Tinea cruris is a dermatophyte infection of the groin, proximal medial thighs, perineum, and buttocks. It usually presents as an erythematous patch that spreads centrifugally with partial central clearing and a slightly elevated, scaly border. Although candidiasis was higher on the differential, it was less likely, as our patient had a concomitant exudate inconsistent with Candida infections. Also, the lack of response to antifungal agents made hypotheses of fungal infections improbable.1
Inverse psoriasis is a variant of psoriasis identified by the development of well-demarcated, nonscaly, shiny plaques on body folds.2 Psoriasis is a chronic disease with several other cutaneous manifestations, such as nail and scalp involvement, as well as erythematous scaly plaques on the extensor surfaces of the limbs. The absence of a history of psoriasis, lack of other cutaneous manifestations, and no response to topical corticosteroids made the diagnosis of inverse psoriasis unlikely in our patient.
Erythrasma is a common superficial cutaneous infection caused by Corynebacterium minutissimum, a grampositive bacillus. It typically presents as an intertriginous eruption characterized by small erythematous to brown patches or thin plaques with fine scaling and sharp borders.3 Erythrasma displays a coral red fluorescence on Wood lamp examination that can be useful in the distinction from other causes of intertrigo.1 Although this examination had not been performed in our patient, the striking exudate made erythrasma less likely, and the culture performed on skin swab material would help to rule out this diagnosis.
Pseudomonas aeruginosa is a gram-negative strict aerobic bacillus of ubiquitous distribution with a preference for humid environments.4,5 Pseudomonas aeruginosa infections were first reported in the 19th century by physicians who noticed a peculiar odorous condition that caused a blue-green discoloration on bandages. This coloration explains the species name aeruginosa which is derived from the Latin word for copper rust.4 It comes from several water-soluble pigments produced by this microorganism, the most prevalent of which are pyocyanin and pyoverdine. Pyocyanin has a greenish-blue color and is nonfluorescent, while pyoverdine is green-yellowish and fluoresces under Wood light.5 Other pigments, such as pyorubin and pyomelanin, can be produced by some Pseudomonas strains.4
Pseudomonas aeruginosa has become one of the main pathogens involved in hospital-acquired infections,6 especially in immunocompromised patients.6,7 It is a frequent cause of respiratory infections in patients with cystic fibrosis, as it is present in the airways of up to 70% of these patients in adulthood.7 Also, due to a variety of adaptive mechanisms with the development of resistance to a range of antibiotics, P aeruginosa has become a worldwide public health problem and is involved in several life-threatening nosocomial infections.7,8
Cutaneous P aeruginosa infections range from superficial to deep tissue involvement and can affect both immunocompromised and immunocompetent individuals.9 They are classified as primary when they originate directly from the skin or secondary when they occur in the context of bacteremia. Primary infections mostly are mild and often are seen in healthy individuals; they usually occur by inoculation and predominate in moist areas where skin breakdown is frequent. Secondary infections typically affect immunocompromised individuals and portend a poor prognosis.5,9
Denominated as Pseudomonas pyoderma, the superficial skin infection by P aeruginosa is described as a condition where the epidermis has a moth-eaten appearance with macerated or eroded borders.10 A blue-greenish exudate and a grape juice odor often are present. This infection usually occurs as a complication of several skin conditions such as tinea pedis, eczema, burns, wounds, and ulcers.5,10
We believe that our patient developed Pseudomonas pyoderma as a complication of diaper dermatitis. His extended hospital stay with the use of different antibiotic regimens for the treatment of several infectious complications may have contributed to the development of infection by P aeruginosa.11 Despite its great clinical relevance, there are few studies in the literature on primary skin infections caused by P aeruginosa, and clinical descriptions with images are rare. Our patient had a nonspecific noneczematous dermatitis, and the projections on the periphery of the lesion resembled the moth-eaten appearance of the classic description of Pseudomonas pyoderma.5,10 The presence of a greenish exudate should promptly raise suspicion for this entity. We believe that the presentation of this case can illustrate this finding and help physicians to recognize this infection.
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Micali G, Verzi AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019; 12:953-959.
- Somerville DA. Erythrasma in normal young adults. J Med Microbiol. 1970;3:57-64.
- D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Vol 2. 8th ed. Elsevier; 2015:2518-2531.
- Silvestre JF, Betlloch MI. Cutaneous manifestations due to Pseudomonas infection. Int J Dermatol. 1999;38:419-431.
- Young LS, Armstrong D. Pseudomonas aeruginosa infections. CRC Crit Rev Clin Lab Sci. 1972;3:291-347.
- Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: device-associated module. Am J Infect Control. 2020;48:423-432.
- Wu DC, Chan WW, Metelitsa AI, et al. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol. 2011;12:157-169.
- Hall JH, Callaway JL, Tindall JP, et al. Pseudomonas aeruginosa in dermatology. Arch Dermatol. 1968;97:312-324.
- Merchant S, Proudfoot EM, Quadri HN, et al. Risk factors for Pseudomonas aeruginosa infections in Asia-Pacific and consequences of inappropriate initial antimicrobial therapy: a systematic literature review and meta-analysis. J Glob Antimicrob Resist. 2018;14:33-44.
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Micali G, Verzi AE, Giuffrida G, et al. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Investig Dermatol. 2019; 12:953-959.
- Somerville DA. Erythrasma in normal young adults. J Med Microbiol. 1970;3:57-64.
- D’Agata E. Pseudomonas aeruginosa and other Pseudomonas species. In: Bennett JE, Dolin R, Blaser MJ, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. Vol 2. 8th ed. Elsevier; 2015:2518-2531.
- Silvestre JF, Betlloch MI. Cutaneous manifestations due to Pseudomonas infection. Int J Dermatol. 1999;38:419-431.
- Young LS, Armstrong D. Pseudomonas aeruginosa infections. CRC Crit Rev Clin Lab Sci. 1972;3:291-347.
- Moradali MF, Ghods S, Rehm BH. Pseudomonas aeruginosa lifestyle: a paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:39.
- Rosenthal VD, Bat-Erdene I, Gupta D, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: device-associated module. Am J Infect Control. 2020;48:423-432.
- Wu DC, Chan WW, Metelitsa AI, et al. Pseudomonas skin infection: clinical features, epidemiology, and management. Am J Clin Dermatol. 2011;12:157-169.
- Hall JH, Callaway JL, Tindall JP, et al. Pseudomonas aeruginosa in dermatology. Arch Dermatol. 1968;97:312-324.
- Merchant S, Proudfoot EM, Quadri HN, et al. Risk factors for Pseudomonas aeruginosa infections in Asia-Pacific and consequences of inappropriate initial antimicrobial therapy: a systematic literature review and meta-analysis. J Glob Antimicrob Resist. 2018;14:33-44.
A 68-year-old man presented with an extensive erythematous plaque of 3 weeks’ duration that started in the groin and spread to the buttocks. It was associated with pruritus and a burning sensation. He was admitted to the palliative care unit 1 year prior for the management of terminal lung cancer. Despite the use of topical corticosteroids and antifungals, the lesions gradually worsened with dissemination to the back. Physical examination revealed an erythematous macerated plaque that extended from the buttocks and groin region to the scapular area (top). Its borders had an eroded appearance with projections compatible with radial spread (bottom). A greenish exudate soaked the diaper and sheets. No other cutaneous lesions were noted.
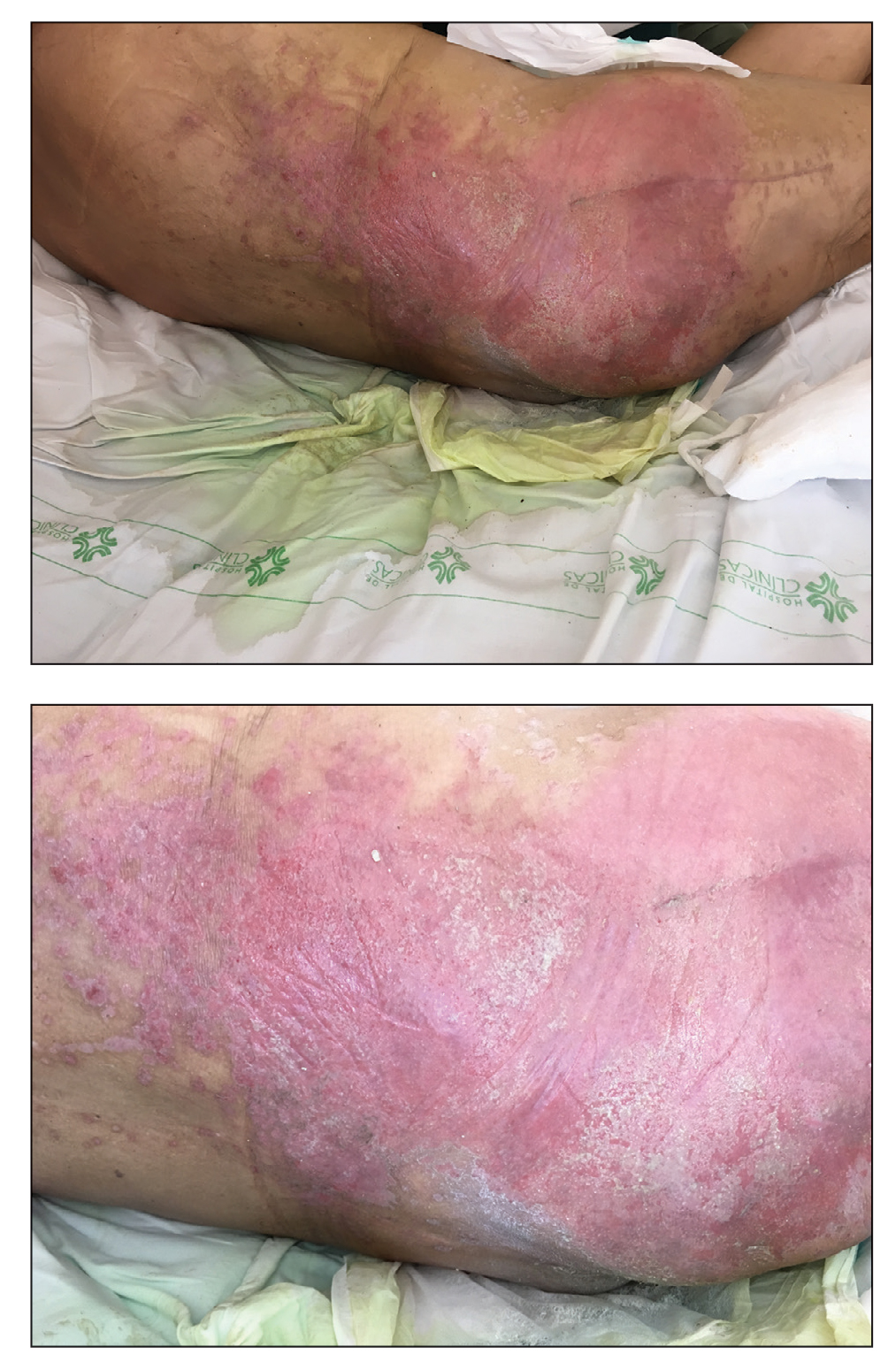
Blistering Lesions in a Newborn
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
A 4-day-old infant boy presented with blisters on the skin. He was born at 36 weeks’ gestation by cesarean delivery to a nulliparous mother who received appropriate prenatal care. On day 2 of life, the patient developed bullae with breakdown of the skin on the bilateral heels and on the skin surrounding intravenous injection sites. Similar blisters subsequently developed on the fingers (top), thighs, groin, and toes (bottom), sparing the oral mucosa and trunk. He remained afebrile and stable and was started on ampicillin, gentamicin, and acyclovir with continued development of blisters. Two weeks later he developed painful ulcers on the tongue that bled upon scraping.
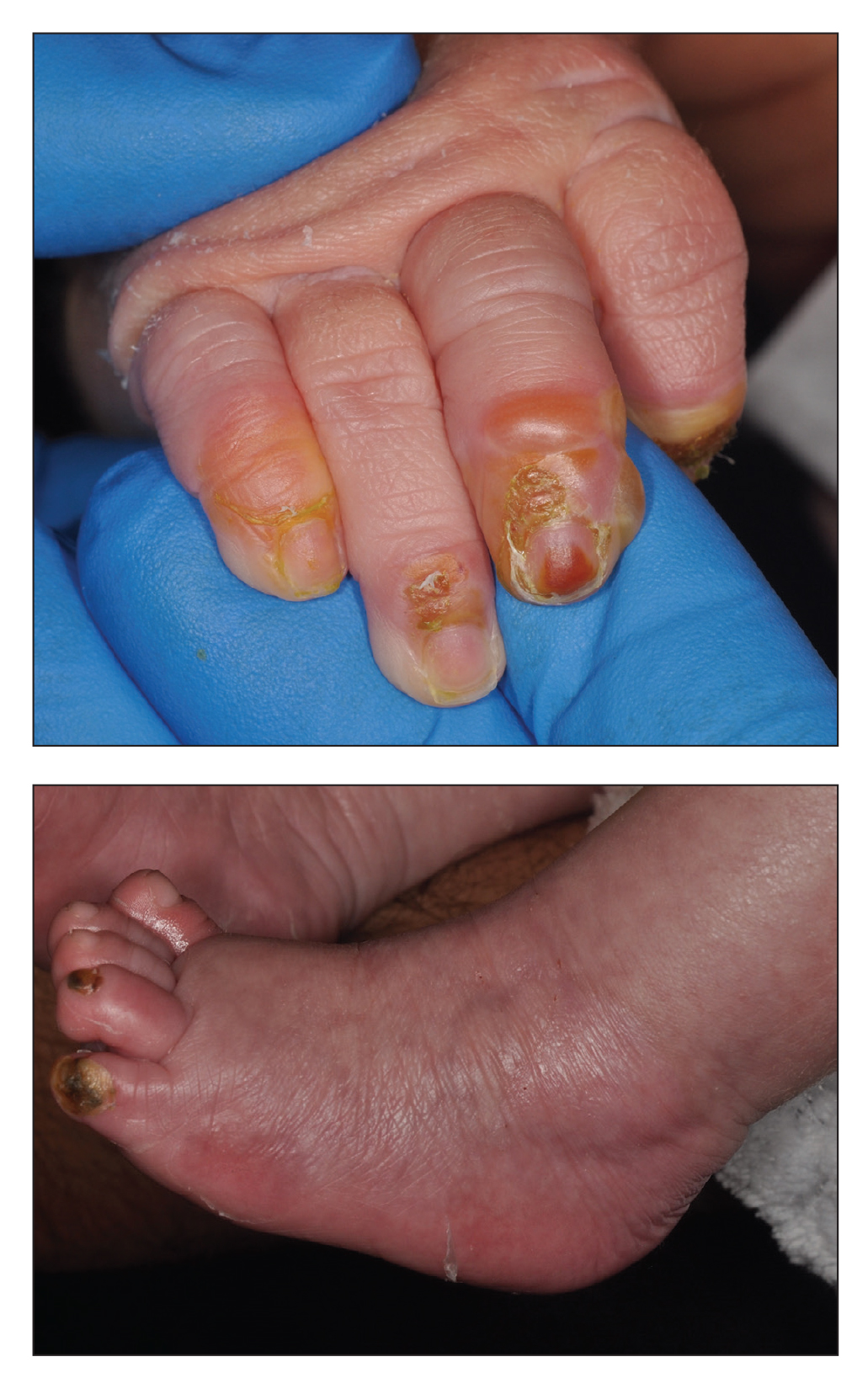
Violaceous Nodules on the Lower Leg
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.
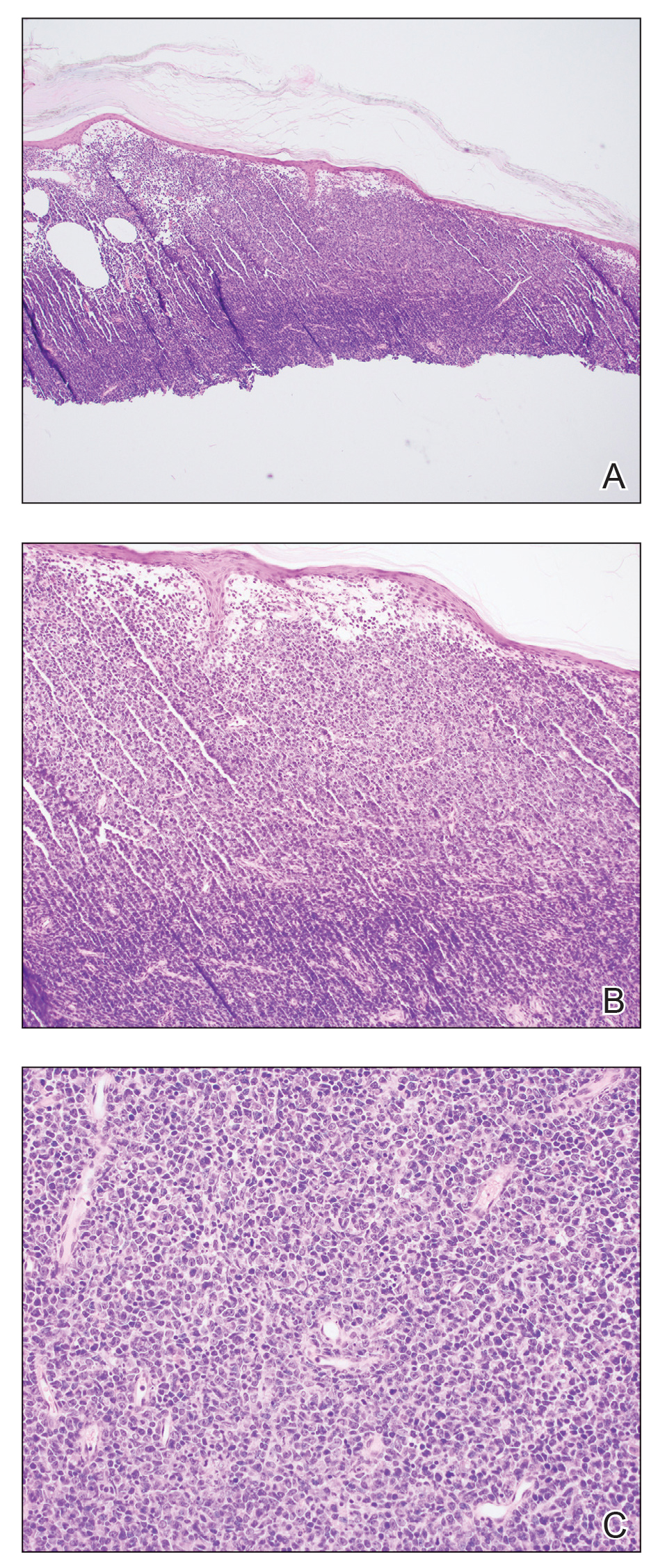
Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
A 79-year-old man presented to the dermatology clinic with 4 enlarging, asymptomatic, violaceous, desquamating nodules on the left pretibial region and calf of 3 months’ duration. He denied any constitutional symptoms such as night sweats or weight loss. His medical history included a malignant melanoma on the left ear that was excised 5 years prior. He also had a history of peripheral edema, hypertension, and rheumatoid arthritis, as well as a 50-pack-year history of smoking. Physical examination revealed 2 large nodules measuring 3.0×3.0 cm each and 2 smaller nodules measuring 1.0×1.0 cm each. There was no appreciable lymphadenopathy.