User login
Toe Nodule Obliterating the Nail Bed
The Diagnosis: Superficial Acral Fibromyxoma
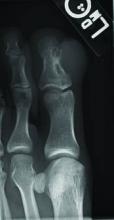
Superficial acral fibromyxoma (SAF) was first described in 2001 by Fetsch et al.1 Subsequently, the term digital fibromyxoma was proposed in 2012 by Hollmann et al2 to describe a distinctive, slow-growing, soft-tissue tumor with a predilection for the periungual or subungual regions of the fingers and toes. The benign growth typically presents as a painless or tender nodule in middle-aged adults with a slight male predominance (1.3:1 ratio).1,2 In a case series (N=124) described by Hollmann et al,2 9 of 25 patients (36%) who had imaging studies showed bone involvement by an erosive or lytic lesion. Reports of SAF with bone involvement also have been described in the radiologic and orthopedic surgery literature.3,4 Radiographically, the soft-tissue invasion of the bone is demonstrated by scalloping on plain radiographs (Figure 1).3
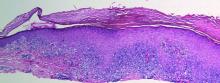
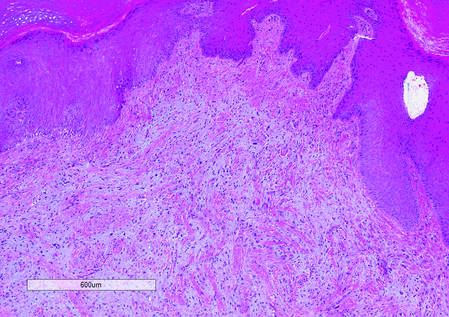
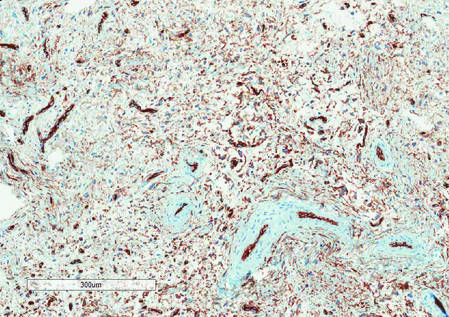
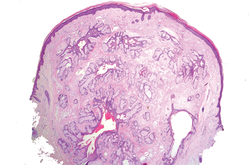
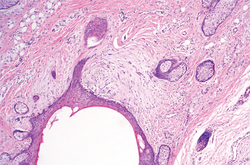
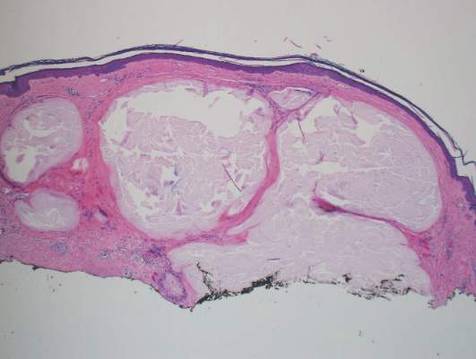
Histologically, SAFs are moderately cellular with spindled or stellate fibroblastlike cells within a myxoid or collagenous matrix (Figure 2).1 The vasculature is mildly accentuated and an increase in mast cells usually is observed. The nuclei have a low degree of atypia with few mitotic figures, and the stellate cells exhibit positive immunohistochemical staining for CD34 (Figure 3), epithelial membrane antigen, and CD99.1 Hollmann et al2 found that 66 of 95 tumors (69.5%) infiltrated the dermal collagen, 26 (27.4%) infiltrated fat, and 3 (3.2%) invaded bone. Of the 47 cases that were evaluated on follow-up, 10 tumors (21.3%) recurred locally (all near the nail unit of the fingers or toes) after a mean interval of 27 months. Although invasion of underlying tissues and recurrence of the tumor has been demonstrated, this growth is considered benign. The histologic differential diagnosis includes neurofibroma, myxoma, fibroma, low-grade fibromyxoid sarcoma, dermatofibroma, superficial angiomyxoma, and dermatofibrosarcoma protuberans.2
The primary treatment of SAF is local excision. The incidence of local recurrence found in the case series by Hollmann et al2 was directly linked to positive margins after the first excision (10/47 [21.3%] recurrent lesions had positive margins). To date, there are no known reports of metastatic disease in SAF.2 Our case manifested with a late recurrence of the tumor and bone involvement requiring surgical excision, which illustrates the role of adjuvant imaging and close follow-up following excision of any soft-tissue tumors of the fingers and toes that have been histologically confirmed as SAF, particularly those of the periungual region.
|
|
|
|
1. Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma (a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes.) Hum Pathol. 2001;32:704-714.
2. Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
3. Varikatt W, Soper J, Simmon G, et al. Superficial acral fibromyxoma: a report of two cases with radiological findings. Skeletal Radiol. 2008;37:499-503.
4. Oteo-Alvaro A, Meizoso T, Scarpellini A, et al. Superficial acral fibromyxoma of the toe, with erosion of the distal phalanx. a clinical report. Arch Orthop Trauma Surg. 2008;128:271-274.
The Diagnosis: Superficial Acral Fibromyxoma
Superficial acral fibromyxoma (SAF) was first described in 2001 by Fetsch et al.1 Subsequently, the term digital fibromyxoma was proposed in 2012 by Hollmann et al2 to describe a distinctive, slow-growing, soft-tissue tumor with a predilection for the periungual or subungual regions of the fingers and toes. The benign growth typically presents as a painless or tender nodule in middle-aged adults with a slight male predominance (1.3:1 ratio).1,2 In a case series (N=124) described by Hollmann et al,2 9 of 25 patients (36%) who had imaging studies showed bone involvement by an erosive or lytic lesion. Reports of SAF with bone involvement also have been described in the radiologic and orthopedic surgery literature.3,4 Radiographically, the soft-tissue invasion of the bone is demonstrated by scalloping on plain radiographs (Figure 1).3
Histologically, SAFs are moderately cellular with spindled or stellate fibroblastlike cells within a myxoid or collagenous matrix (Figure 2).1 The vasculature is mildly accentuated and an increase in mast cells usually is observed. The nuclei have a low degree of atypia with few mitotic figures, and the stellate cells exhibit positive immunohistochemical staining for CD34 (Figure 3), epithelial membrane antigen, and CD99.1 Hollmann et al2 found that 66 of 95 tumors (69.5%) infiltrated the dermal collagen, 26 (27.4%) infiltrated fat, and 3 (3.2%) invaded bone. Of the 47 cases that were evaluated on follow-up, 10 tumors (21.3%) recurred locally (all near the nail unit of the fingers or toes) after a mean interval of 27 months. Although invasion of underlying tissues and recurrence of the tumor has been demonstrated, this growth is considered benign. The histologic differential diagnosis includes neurofibroma, myxoma, fibroma, low-grade fibromyxoid sarcoma, dermatofibroma, superficial angiomyxoma, and dermatofibrosarcoma protuberans.2
The primary treatment of SAF is local excision. The incidence of local recurrence found in the case series by Hollmann et al2 was directly linked to positive margins after the first excision (10/47 [21.3%] recurrent lesions had positive margins). To date, there are no known reports of metastatic disease in SAF.2 Our case manifested with a late recurrence of the tumor and bone involvement requiring surgical excision, which illustrates the role of adjuvant imaging and close follow-up following excision of any soft-tissue tumors of the fingers and toes that have been histologically confirmed as SAF, particularly those of the periungual region.

|
|

|
|
The Diagnosis: Superficial Acral Fibromyxoma
Superficial acral fibromyxoma (SAF) was first described in 2001 by Fetsch et al.1 Subsequently, the term digital fibromyxoma was proposed in 2012 by Hollmann et al2 to describe a distinctive, slow-growing, soft-tissue tumor with a predilection for the periungual or subungual regions of the fingers and toes. The benign growth typically presents as a painless or tender nodule in middle-aged adults with a slight male predominance (1.3:1 ratio).1,2 In a case series (N=124) described by Hollmann et al,2 9 of 25 patients (36%) who had imaging studies showed bone involvement by an erosive or lytic lesion. Reports of SAF with bone involvement also have been described in the radiologic and orthopedic surgery literature.3,4 Radiographically, the soft-tissue invasion of the bone is demonstrated by scalloping on plain radiographs (Figure 1).3
Histologically, SAFs are moderately cellular with spindled or stellate fibroblastlike cells within a myxoid or collagenous matrix (Figure 2).1 The vasculature is mildly accentuated and an increase in mast cells usually is observed. The nuclei have a low degree of atypia with few mitotic figures, and the stellate cells exhibit positive immunohistochemical staining for CD34 (Figure 3), epithelial membrane antigen, and CD99.1 Hollmann et al2 found that 66 of 95 tumors (69.5%) infiltrated the dermal collagen, 26 (27.4%) infiltrated fat, and 3 (3.2%) invaded bone. Of the 47 cases that were evaluated on follow-up, 10 tumors (21.3%) recurred locally (all near the nail unit of the fingers or toes) after a mean interval of 27 months. Although invasion of underlying tissues and recurrence of the tumor has been demonstrated, this growth is considered benign. The histologic differential diagnosis includes neurofibroma, myxoma, fibroma, low-grade fibromyxoid sarcoma, dermatofibroma, superficial angiomyxoma, and dermatofibrosarcoma protuberans.2
The primary treatment of SAF is local excision. The incidence of local recurrence found in the case series by Hollmann et al2 was directly linked to positive margins after the first excision (10/47 [21.3%] recurrent lesions had positive margins). To date, there are no known reports of metastatic disease in SAF.2 Our case manifested with a late recurrence of the tumor and bone involvement requiring surgical excision, which illustrates the role of adjuvant imaging and close follow-up following excision of any soft-tissue tumors of the fingers and toes that have been histologically confirmed as SAF, particularly those of the periungual region.

|
|

|
|
1. Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma (a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes.) Hum Pathol. 2001;32:704-714.
2. Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
3. Varikatt W, Soper J, Simmon G, et al. Superficial acral fibromyxoma: a report of two cases with radiological findings. Skeletal Radiol. 2008;37:499-503.
4. Oteo-Alvaro A, Meizoso T, Scarpellini A, et al. Superficial acral fibromyxoma of the toe, with erosion of the distal phalanx. a clinical report. Arch Orthop Trauma Surg. 2008;128:271-274.
1. Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma (a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes.) Hum Pathol. 2001;32:704-714.
2. Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
3. Varikatt W, Soper J, Simmon G, et al. Superficial acral fibromyxoma: a report of two cases with radiological findings. Skeletal Radiol. 2008;37:499-503.
4. Oteo-Alvaro A, Meizoso T, Scarpellini A, et al. Superficial acral fibromyxoma of the toe, with erosion of the distal phalanx. a clinical report. Arch Orthop Trauma Surg. 2008;128:271-274.
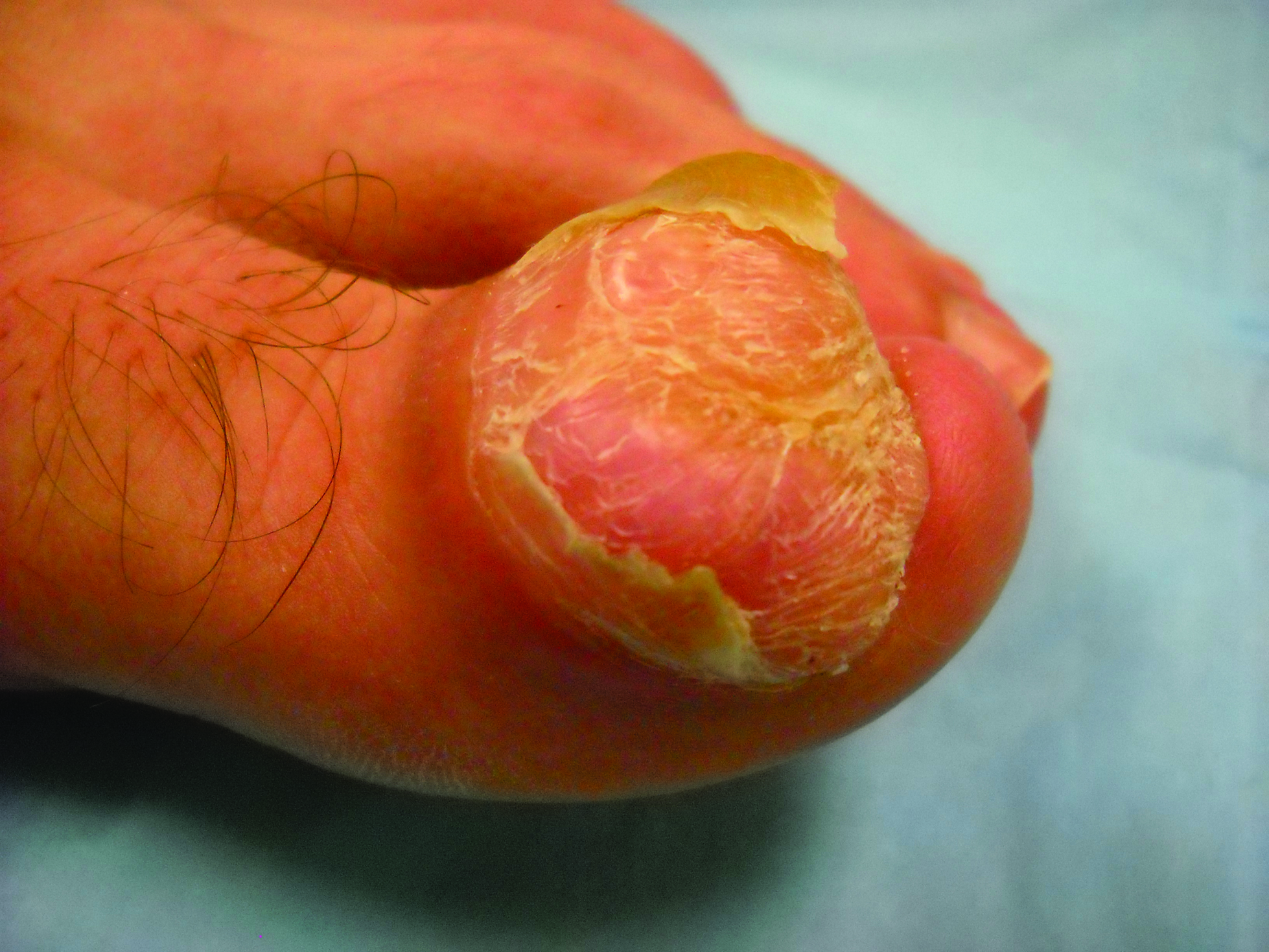
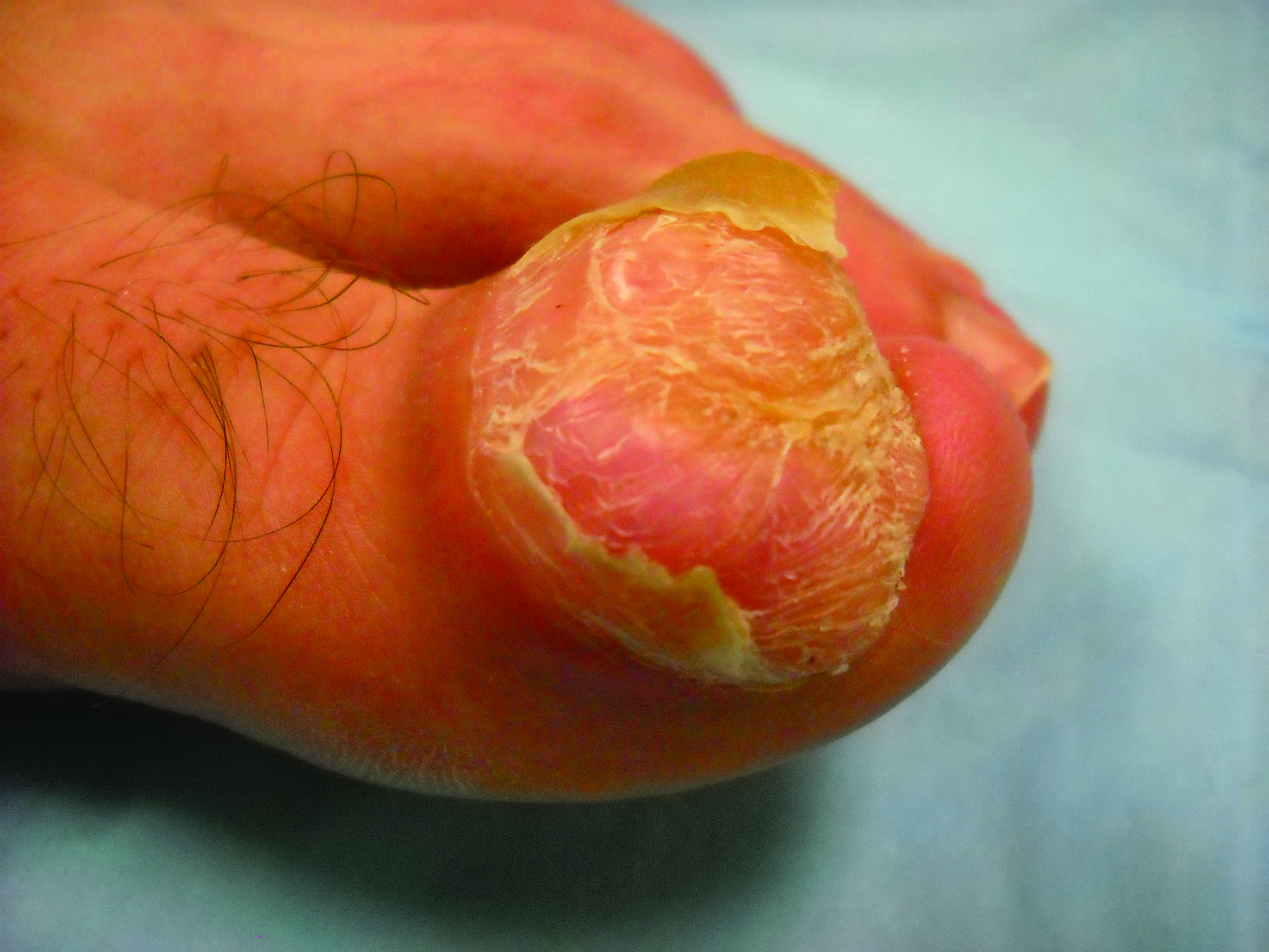
A generally healthy 30-year-old man presented with a 3-cm exophytic, yellowish red, subungual nodule of the left great toe of 1 year’s duration that was obliterating the nail plate. Ten years prior, a similar nodule in the same location was removed via laser by a podiatrist. Medical records were not retrievable, but the patient reported that he was told the excised lesion was a benign tumor. Plain radiographs were performed at the current presentation and demonstrated an inferior cortical lucency of the distal phalanx as well as a lucency over the nail bed region with extension of calcification to the soft tissues. Magnetic resonance imaging showed a mass with a proximal to distal maximum dimension of 2.1 cm that involved the dorsal surface of the proximal phalanx. Magnetic resonance imaging also demonstrated bone erosion from the overlying mass. A 4-mm incisional punch biopsy was performed prior to surgical excision.
Dome-Shaped Papule With a Bloody Crust
The Diagnosis: Congenital Folliculosebaceous Cystic Hamartoma
Folliculosebaceous cystic hamartoma (FSCH) is a rare skin condition that is either congenital or acquired. It presents as a slow-growing and flesh-colored papulonodular lesion1 that mainly occurs on the head and neck. Involvement of the nipples, perineum, back, forearms, genital areas, and subcutaneous tissue also has been reported but usually indicates a larger lesion.1,2
Histologically, FSCH is considered a hamartoma composed of both ectodermal and mesodermal elements.1 Folliculosebaceous cystic hamartoma is a more complex lesion composed of infundibulocystic structures connected to maloriented folliculosebaceous units surrounded by whorls of highly vascularized fibrous stroma and adipocytes. Clefts between fibroepithelial units and surrounding stroma usually are present.1
Epithelial components contribute to the adnexal and folliculosebaceous cystic proliferations, and mesenchymal elements include vascular tissue, adipose tissue, and fibroblast-rich stroma.1,2 Acquired lesions arising in adults have been described,1-5 but the congenital presentation of FSCH in infancy is rare.
Histopathologically, some variations of FSCH are mainly composed of epithelial components while others are composed of nonepithelial components. Nonepithelial components include neural proliferation, muscle components, vascular proliferation, and mucin deposition.1-4 In some cases, FSCH may coexist with other diseases, such as nevus lipomatosus cutaneous superficialis and neurofibromatosis type I.4,5
In our case, histopathology showed several dermal infundibulocystic structures that were lined by stratified squamous epithelium and contained horny material (Figure 1). Numerous immature sebaceous lobules and rudimentary hair follicles emanated from some of the cyst walls. Mesenchymal changes around the fibroepithelial units included fibrillary bundles of collagen, clusters of adipocytes, and an increased number of small venules (Figure 2). In addition, the stroma adjacent to the malformed perifollicle contained some amount of mucin. Prominent clefts formed between fibroepithelial units and the surrounding altered stroma.
| 
| |
|
The differential diagnosis mainly includes sebaceous trichofolliculoma, molluscum contagiosum, dermoid cysts, pilomatrixoma, Spitz nevus, and nevus lipomatosus superficialis. The differential diagnosis between FSCH and sebaceous trichofolliculoma is challenging. Both lesions show an infundibular cyst and surrounding sebaceous nodules. According to Plewig,6 trichofolliculoma has a wide spectrum ranging from low to high differentiation represented by trichofolliculoma, sebaceous trichofolliculoma, and FSCH, respectively. It is not difficult to distinguish FSCH from other diseases according to its peculiar histopathologic features.
The clinicopathologic features of our case were similar to those of reported FSCH cases, except for the following unique characteristics: congenital lesion, lack of terminal hair, and no sebaceous material extrusion. These features of hair and sebaceous material may be correlated with the patient’s age and hormonal level.1 Androgen may play a key role in sebaceous gland development at puberty, which leads to sebaceous gland hyperplasia and hypertrophy. Therefore, slight pressure from the lesions can make ivory-white sebaceous material discharge. Hence, the dermatologist and pediatrician must be poised and sensitive while making an initial diagnosis of FSCH.
1. Kimura T, Miyazawa H, Aoyagi T, et al. Folliculosebaceous cystic hamartoma: a distinctive malformation of the skin. Am J Dermatopathol. 1991;13:213-220.
2. Moriki M, Ito T, Hirakawa S, et al. Folliculosebaceous cystic hamartoma presenting as a subcutaneous nodule on the thigh. J Dermatol. 2013;40:483-484.
3. Aloi F, Tomasini C, Pippione M. Folliculosebaceous cystic hamartoma with perifollicular mucinosis. Am J Dermatopathol. 1996;18:58-62.
4. Brasanac D, Boricic I. Giant nevus lipomatosus superficialis with multiple folliculosebaceous cystic hamartomas and dermoid cysts. J Eur Acad Dermatol Venereol. 2005;19:84-86.
5. Noh S, Kwon JE, Lee KG, et al. Folliculosebaceous cystic hamartoma in a patient with neurofibromatosis type I. Ann Dermatol. 2011;23(suppl 2):S185-S187.
6. Plewig G. In discussion of: Leserbrief zu Zheng LQ, Han XC, Huang Y, Li HW. Several acneiform papules and nodules on the neck. diagnosis: folliculosebaceous cystic hamartoma. J Dtsch Dermatol Ges. 2014;12:824-825.
The Diagnosis: Congenital Folliculosebaceous Cystic Hamartoma
Folliculosebaceous cystic hamartoma (FSCH) is a rare skin condition that is either congenital or acquired. It presents as a slow-growing and flesh-colored papulonodular lesion1 that mainly occurs on the head and neck. Involvement of the nipples, perineum, back, forearms, genital areas, and subcutaneous tissue also has been reported but usually indicates a larger lesion.1,2
Histologically, FSCH is considered a hamartoma composed of both ectodermal and mesodermal elements.1 Folliculosebaceous cystic hamartoma is a more complex lesion composed of infundibulocystic structures connected to maloriented folliculosebaceous units surrounded by whorls of highly vascularized fibrous stroma and adipocytes. Clefts between fibroepithelial units and surrounding stroma usually are present.1
Epithelial components contribute to the adnexal and folliculosebaceous cystic proliferations, and mesenchymal elements include vascular tissue, adipose tissue, and fibroblast-rich stroma.1,2 Acquired lesions arising in adults have been described,1-5 but the congenital presentation of FSCH in infancy is rare.
Histopathologically, some variations of FSCH are mainly composed of epithelial components while others are composed of nonepithelial components. Nonepithelial components include neural proliferation, muscle components, vascular proliferation, and mucin deposition.1-4 In some cases, FSCH may coexist with other diseases, such as nevus lipomatosus cutaneous superficialis and neurofibromatosis type I.4,5
In our case, histopathology showed several dermal infundibulocystic structures that were lined by stratified squamous epithelium and contained horny material (Figure 1). Numerous immature sebaceous lobules and rudimentary hair follicles emanated from some of the cyst walls. Mesenchymal changes around the fibroepithelial units included fibrillary bundles of collagen, clusters of adipocytes, and an increased number of small venules (Figure 2). In addition, the stroma adjacent to the malformed perifollicle contained some amount of mucin. Prominent clefts formed between fibroepithelial units and the surrounding altered stroma.

| 
| |
|
The differential diagnosis mainly includes sebaceous trichofolliculoma, molluscum contagiosum, dermoid cysts, pilomatrixoma, Spitz nevus, and nevus lipomatosus superficialis. The differential diagnosis between FSCH and sebaceous trichofolliculoma is challenging. Both lesions show an infundibular cyst and surrounding sebaceous nodules. According to Plewig,6 trichofolliculoma has a wide spectrum ranging from low to high differentiation represented by trichofolliculoma, sebaceous trichofolliculoma, and FSCH, respectively. It is not difficult to distinguish FSCH from other diseases according to its peculiar histopathologic features.
The clinicopathologic features of our case were similar to those of reported FSCH cases, except for the following unique characteristics: congenital lesion, lack of terminal hair, and no sebaceous material extrusion. These features of hair and sebaceous material may be correlated with the patient’s age and hormonal level.1 Androgen may play a key role in sebaceous gland development at puberty, which leads to sebaceous gland hyperplasia and hypertrophy. Therefore, slight pressure from the lesions can make ivory-white sebaceous material discharge. Hence, the dermatologist and pediatrician must be poised and sensitive while making an initial diagnosis of FSCH.
The Diagnosis: Congenital Folliculosebaceous Cystic Hamartoma
Folliculosebaceous cystic hamartoma (FSCH) is a rare skin condition that is either congenital or acquired. It presents as a slow-growing and flesh-colored papulonodular lesion1 that mainly occurs on the head and neck. Involvement of the nipples, perineum, back, forearms, genital areas, and subcutaneous tissue also has been reported but usually indicates a larger lesion.1,2
Histologically, FSCH is considered a hamartoma composed of both ectodermal and mesodermal elements.1 Folliculosebaceous cystic hamartoma is a more complex lesion composed of infundibulocystic structures connected to maloriented folliculosebaceous units surrounded by whorls of highly vascularized fibrous stroma and adipocytes. Clefts between fibroepithelial units and surrounding stroma usually are present.1
Epithelial components contribute to the adnexal and folliculosebaceous cystic proliferations, and mesenchymal elements include vascular tissue, adipose tissue, and fibroblast-rich stroma.1,2 Acquired lesions arising in adults have been described,1-5 but the congenital presentation of FSCH in infancy is rare.
Histopathologically, some variations of FSCH are mainly composed of epithelial components while others are composed of nonepithelial components. Nonepithelial components include neural proliferation, muscle components, vascular proliferation, and mucin deposition.1-4 In some cases, FSCH may coexist with other diseases, such as nevus lipomatosus cutaneous superficialis and neurofibromatosis type I.4,5
In our case, histopathology showed several dermal infundibulocystic structures that were lined by stratified squamous epithelium and contained horny material (Figure 1). Numerous immature sebaceous lobules and rudimentary hair follicles emanated from some of the cyst walls. Mesenchymal changes around the fibroepithelial units included fibrillary bundles of collagen, clusters of adipocytes, and an increased number of small venules (Figure 2). In addition, the stroma adjacent to the malformed perifollicle contained some amount of mucin. Prominent clefts formed between fibroepithelial units and the surrounding altered stroma.

| 
| |
|
The differential diagnosis mainly includes sebaceous trichofolliculoma, molluscum contagiosum, dermoid cysts, pilomatrixoma, Spitz nevus, and nevus lipomatosus superficialis. The differential diagnosis between FSCH and sebaceous trichofolliculoma is challenging. Both lesions show an infundibular cyst and surrounding sebaceous nodules. According to Plewig,6 trichofolliculoma has a wide spectrum ranging from low to high differentiation represented by trichofolliculoma, sebaceous trichofolliculoma, and FSCH, respectively. It is not difficult to distinguish FSCH from other diseases according to its peculiar histopathologic features.
The clinicopathologic features of our case were similar to those of reported FSCH cases, except for the following unique characteristics: congenital lesion, lack of terminal hair, and no sebaceous material extrusion. These features of hair and sebaceous material may be correlated with the patient’s age and hormonal level.1 Androgen may play a key role in sebaceous gland development at puberty, which leads to sebaceous gland hyperplasia and hypertrophy. Therefore, slight pressure from the lesions can make ivory-white sebaceous material discharge. Hence, the dermatologist and pediatrician must be poised and sensitive while making an initial diagnosis of FSCH.
1. Kimura T, Miyazawa H, Aoyagi T, et al. Folliculosebaceous cystic hamartoma: a distinctive malformation of the skin. Am J Dermatopathol. 1991;13:213-220.
2. Moriki M, Ito T, Hirakawa S, et al. Folliculosebaceous cystic hamartoma presenting as a subcutaneous nodule on the thigh. J Dermatol. 2013;40:483-484.
3. Aloi F, Tomasini C, Pippione M. Folliculosebaceous cystic hamartoma with perifollicular mucinosis. Am J Dermatopathol. 1996;18:58-62.
4. Brasanac D, Boricic I. Giant nevus lipomatosus superficialis with multiple folliculosebaceous cystic hamartomas and dermoid cysts. J Eur Acad Dermatol Venereol. 2005;19:84-86.
5. Noh S, Kwon JE, Lee KG, et al. Folliculosebaceous cystic hamartoma in a patient with neurofibromatosis type I. Ann Dermatol. 2011;23(suppl 2):S185-S187.
6. Plewig G. In discussion of: Leserbrief zu Zheng LQ, Han XC, Huang Y, Li HW. Several acneiform papules and nodules on the neck. diagnosis: folliculosebaceous cystic hamartoma. J Dtsch Dermatol Ges. 2014;12:824-825.
1. Kimura T, Miyazawa H, Aoyagi T, et al. Folliculosebaceous cystic hamartoma: a distinctive malformation of the skin. Am J Dermatopathol. 1991;13:213-220.
2. Moriki M, Ito T, Hirakawa S, et al. Folliculosebaceous cystic hamartoma presenting as a subcutaneous nodule on the thigh. J Dermatol. 2013;40:483-484.
3. Aloi F, Tomasini C, Pippione M. Folliculosebaceous cystic hamartoma with perifollicular mucinosis. Am J Dermatopathol. 1996;18:58-62.
4. Brasanac D, Boricic I. Giant nevus lipomatosus superficialis with multiple folliculosebaceous cystic hamartomas and dermoid cysts. J Eur Acad Dermatol Venereol. 2005;19:84-86.
5. Noh S, Kwon JE, Lee KG, et al. Folliculosebaceous cystic hamartoma in a patient with neurofibromatosis type I. Ann Dermatol. 2011;23(suppl 2):S185-S187.
6. Plewig G. In discussion of: Leserbrief zu Zheng LQ, Han XC, Huang Y, Li HW. Several acneiform papules and nodules on the neck. diagnosis: folliculosebaceous cystic hamartoma. J Dtsch Dermatol Ges. 2014;12:824-825.

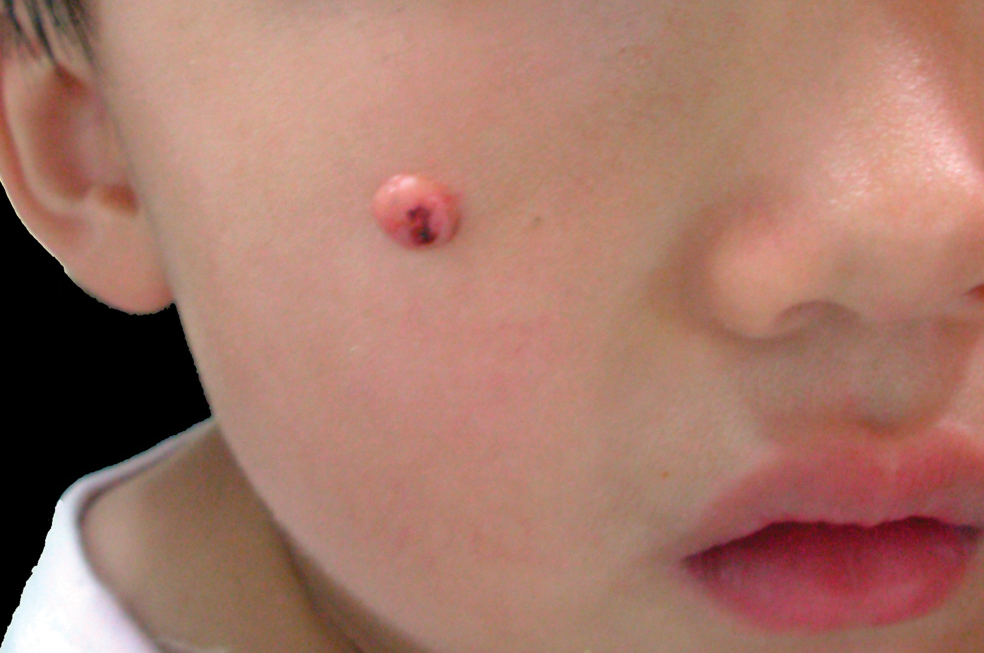
A 3-year-old girl was referred to our clinic for a lesion on the face that had been present since birth and had enlarged slowly with slight itching. Physical examination revealed a 1.0×1.0-cm, sessile, flesh-colored, sharply demarcated, and dome-shaped papule with a bloody crust. It was firm and slightly painful to palpation. Dilated hair follicle–like orifices and thick central terminal hair were not found. Sebaceous material was not discharged. There was no notable family history or evidence of systemic disease. The lesion was surgically removed for cosmetic reasons and further histopathologic examination was performed.
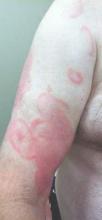
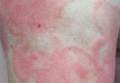
Confluent Erythematous Plaques on the Palm
The Diagnosis: Palmoplantar Lichen Planus
A skin biopsy from a lesion on the inner wrist showed an interface pattern with a dense bandlike infiltrate obscuring the dermoepidermal junction coupled with a superficial perivascular infiltrate (Figure). At higher magnification (×10), the histologic features included compact orthokeratosis, wedge-shaped hypergranulosis, vacuolar degeneration of the basal layer, basal dyskeratosis, a dense lymphohistiocytic infiltrate obscuring the basement membrane, and melanophages in the papillary dermis.
Lichen planus (LP) is a common inflammatory disease of the skin presenting with flat-topped, violaceous, polygonal papules with fine white lines (Wickham striae) on the surface. It is recognized and diagnosed clinically by its characteristic appearance. Common areas of LP presentation include the shins, inner thighs, genitalia, trunk, volar aspect of the wrists, and oral mucosa.
Palmoplantar LP can present as erythematous plaques, punctuate keratosis, diffuse keratoderma, or ulcerated lesions. The most common concern among patients with LP is pruritus. One-fourth of patients with LP may present with lesions on the palms and soles, but diffuse palmoplantar hyperkeratosis is rare.1 Lesions typically heal in 1 to 8 months, with an average of 3 months. Palmoplantar LP recurs within 1 year after stopping treatment in one-third of patients.1
The cause of LP is unknown, but the pathophysiology is beginning to be understood. Cytotoxic CD8+ T cells stimulate apoptosis of the keratinocytes. The induction of this mechanism may be due to a self-antigen in a genetically predisposed patient. The evidence for LP being an autoimmune disease is supported by the high female predominance and the association of LP with other autoimmune diseases.2 Patients with LP have an increased chance of coexisting hepatitis C virus. In a cross-sectional study of 303 patients, Lodi et al3 found that approximately 20% of LP patients were hepatitis C virus seropositive.
Treatment options for LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive agents.4 Our patient initially was treated with oral cyclosporine 100 mg every morning and oral methotrexate at a dose of 7.5 mg weekly. She also was treated with clobetasol ointment 0.05%. After 3 months, cyclosporine was discontinued. Methotrexate was maintained. At 5 months’ followup there was marked improvement of both clinical and symptomatic concerns with only residual palmoplantar erythema.
The differential diagnosis for pruritic palmoplantar hyperkeratosis is large. The most common differential diagnoses include hyperkeratotic eczema, psoriasis, secondary syphilis, and hereditary palmoplantar keratoderma. Lichen planus should be considered in the differential diagnosis of palmoplantar hyperkeratosis. A skin biopsy may be needed, as palmoplantar LP often has an atypical presentation.5
1. Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
2. Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28:100-108.
3. Lodi G, Giuliani M, Majorana A, et al. Lichen planus and hepatitis C virus: a multicentre study of patients with oral lesions and a systematic review. Br J Dermatol. 2004;151:1172-1181.
4. Karakatsanis G, Patsatsi A, Kastoridou C, et al. Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
5. Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
The Diagnosis: Palmoplantar Lichen Planus
A skin biopsy from a lesion on the inner wrist showed an interface pattern with a dense bandlike infiltrate obscuring the dermoepidermal junction coupled with a superficial perivascular infiltrate (Figure). At higher magnification (×10), the histologic features included compact orthokeratosis, wedge-shaped hypergranulosis, vacuolar degeneration of the basal layer, basal dyskeratosis, a dense lymphohistiocytic infiltrate obscuring the basement membrane, and melanophages in the papillary dermis.
Lichen planus (LP) is a common inflammatory disease of the skin presenting with flat-topped, violaceous, polygonal papules with fine white lines (Wickham striae) on the surface. It is recognized and diagnosed clinically by its characteristic appearance. Common areas of LP presentation include the shins, inner thighs, genitalia, trunk, volar aspect of the wrists, and oral mucosa.
Palmoplantar LP can present as erythematous plaques, punctuate keratosis, diffuse keratoderma, or ulcerated lesions. The most common concern among patients with LP is pruritus. One-fourth of patients with LP may present with lesions on the palms and soles, but diffuse palmoplantar hyperkeratosis is rare.1 Lesions typically heal in 1 to 8 months, with an average of 3 months. Palmoplantar LP recurs within 1 year after stopping treatment in one-third of patients.1
The cause of LP is unknown, but the pathophysiology is beginning to be understood. Cytotoxic CD8+ T cells stimulate apoptosis of the keratinocytes. The induction of this mechanism may be due to a self-antigen in a genetically predisposed patient. The evidence for LP being an autoimmune disease is supported by the high female predominance and the association of LP with other autoimmune diseases.2 Patients with LP have an increased chance of coexisting hepatitis C virus. In a cross-sectional study of 303 patients, Lodi et al3 found that approximately 20% of LP patients were hepatitis C virus seropositive.
Treatment options for LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive agents.4 Our patient initially was treated with oral cyclosporine 100 mg every morning and oral methotrexate at a dose of 7.5 mg weekly. She also was treated with clobetasol ointment 0.05%. After 3 months, cyclosporine was discontinued. Methotrexate was maintained. At 5 months’ followup there was marked improvement of both clinical and symptomatic concerns with only residual palmoplantar erythema.
The differential diagnosis for pruritic palmoplantar hyperkeratosis is large. The most common differential diagnoses include hyperkeratotic eczema, psoriasis, secondary syphilis, and hereditary palmoplantar keratoderma. Lichen planus should be considered in the differential diagnosis of palmoplantar hyperkeratosis. A skin biopsy may be needed, as palmoplantar LP often has an atypical presentation.5
The Diagnosis: Palmoplantar Lichen Planus
A skin biopsy from a lesion on the inner wrist showed an interface pattern with a dense bandlike infiltrate obscuring the dermoepidermal junction coupled with a superficial perivascular infiltrate (Figure). At higher magnification (×10), the histologic features included compact orthokeratosis, wedge-shaped hypergranulosis, vacuolar degeneration of the basal layer, basal dyskeratosis, a dense lymphohistiocytic infiltrate obscuring the basement membrane, and melanophages in the papillary dermis.
Lichen planus (LP) is a common inflammatory disease of the skin presenting with flat-topped, violaceous, polygonal papules with fine white lines (Wickham striae) on the surface. It is recognized and diagnosed clinically by its characteristic appearance. Common areas of LP presentation include the shins, inner thighs, genitalia, trunk, volar aspect of the wrists, and oral mucosa.
Palmoplantar LP can present as erythematous plaques, punctuate keratosis, diffuse keratoderma, or ulcerated lesions. The most common concern among patients with LP is pruritus. One-fourth of patients with LP may present with lesions on the palms and soles, but diffuse palmoplantar hyperkeratosis is rare.1 Lesions typically heal in 1 to 8 months, with an average of 3 months. Palmoplantar LP recurs within 1 year after stopping treatment in one-third of patients.1
The cause of LP is unknown, but the pathophysiology is beginning to be understood. Cytotoxic CD8+ T cells stimulate apoptosis of the keratinocytes. The induction of this mechanism may be due to a self-antigen in a genetically predisposed patient. The evidence for LP being an autoimmune disease is supported by the high female predominance and the association of LP with other autoimmune diseases.2 Patients with LP have an increased chance of coexisting hepatitis C virus. In a cross-sectional study of 303 patients, Lodi et al3 found that approximately 20% of LP patients were hepatitis C virus seropositive.
Treatment options for LP include topical and systemic steroids, tazarotene, acitretin, and immunosuppressive agents.4 Our patient initially was treated with oral cyclosporine 100 mg every morning and oral methotrexate at a dose of 7.5 mg weekly. She also was treated with clobetasol ointment 0.05%. After 3 months, cyclosporine was discontinued. Methotrexate was maintained. At 5 months’ followup there was marked improvement of both clinical and symptomatic concerns with only residual palmoplantar erythema.
The differential diagnosis for pruritic palmoplantar hyperkeratosis is large. The most common differential diagnoses include hyperkeratotic eczema, psoriasis, secondary syphilis, and hereditary palmoplantar keratoderma. Lichen planus should be considered in the differential diagnosis of palmoplantar hyperkeratosis. A skin biopsy may be needed, as palmoplantar LP often has an atypical presentation.5
1. Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
2. Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28:100-108.
3. Lodi G, Giuliani M, Majorana A, et al. Lichen planus and hepatitis C virus: a multicentre study of patients with oral lesions and a systematic review. Br J Dermatol. 2004;151:1172-1181.
4. Karakatsanis G, Patsatsi A, Kastoridou C, et al. Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
5. Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
1. Sánchez-Pérez J, Rios Buceta L, Fraga J, et al. Lichen planus with lesions on the palms and/or soles: prevalence and clinicopathological study of 36 patients. Br J Dermatol. 2000;142:310-314.
2. Farhi D, Dupin N. Pathophysiology, etiologic factors, and clinical management of oral lichen planus, part I: facts and controversies. Clin Dermatol. 2010;28:100-108.
3. Lodi G, Giuliani M, Majorana A, et al. Lichen planus and hepatitis C virus: a multicentre study of patients with oral lesions and a systematic review. Br J Dermatol. 2004;151:1172-1181.
4. Karakatsanis G, Patsatsi A, Kastoridou C, et al. Palmoplantar lichen planus with umbilicated papules: an atypical case with rapid therapeutic response to cyclosporin. J Eur Acad Dermatol Venereol. 2007;21:1006-1007.
5. Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
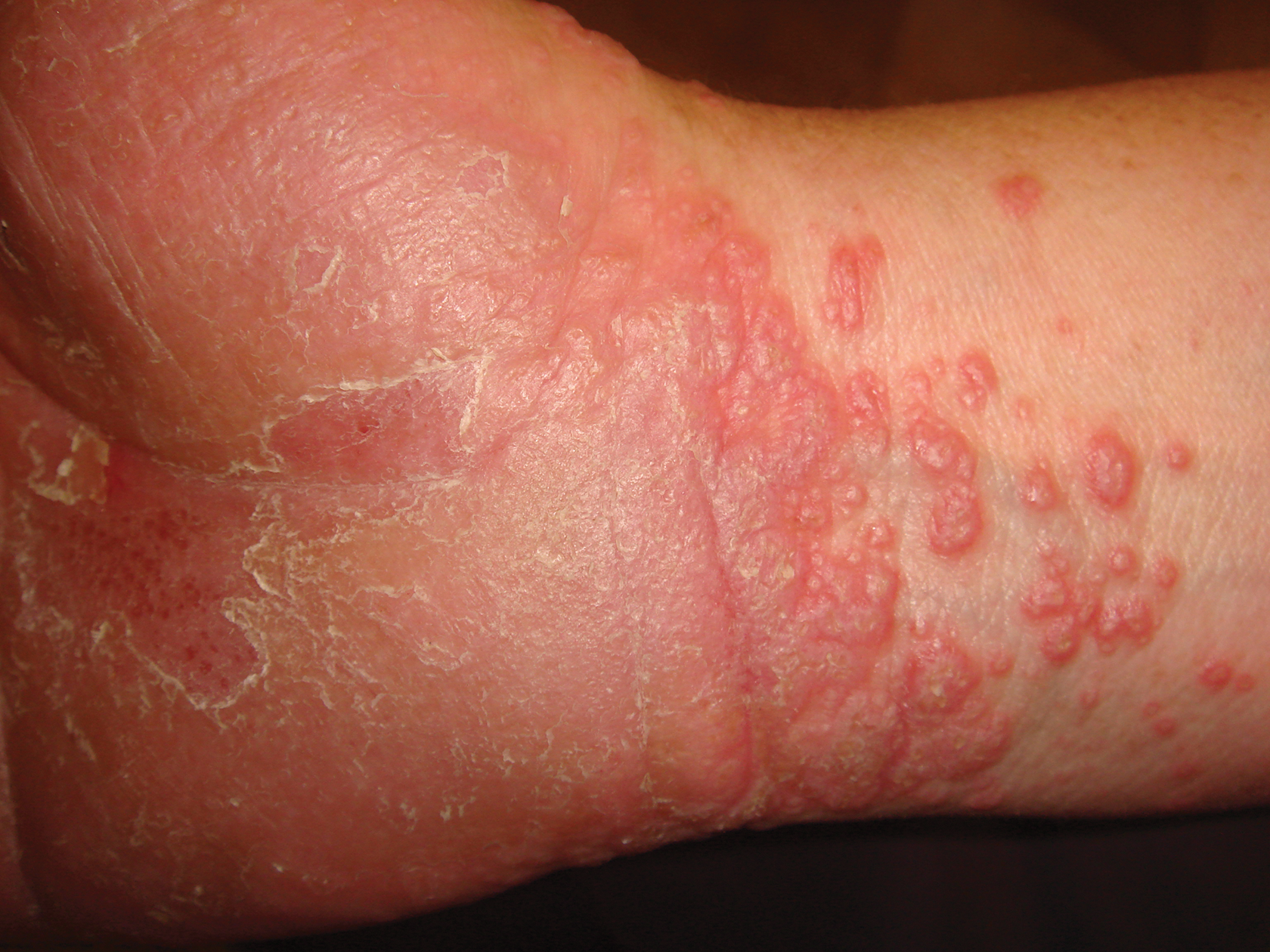
A 45-year-old woman was referred to dermatology by her general internist for the management of a pruritic rash on the hands and feet that was unresponsive to topical steroid creams. The pruritus also was unresponsive to hydroxyzine and aspirin. Erythematous plaques were present on the palms and soles. Physical examination revealed thickened volar skin with a yellowish surface. There were individual papules with atrophic tops at the edge of the plaques on the inner wrists. The patient’s medical history was otherwise unremarkable. Blood tests for glucose and liver function did not reveal any abnormalities.
Multiple Superficial White Nodules on the Bilateral Helical Rims
The Diagnosis: Bilateral Auricular Tophaceous Gout
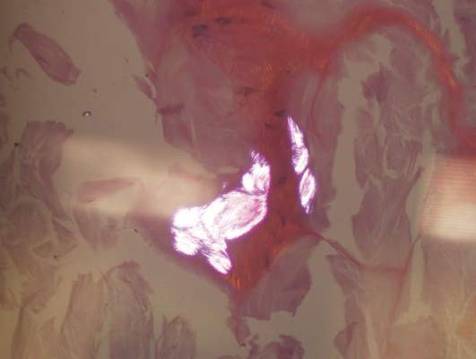
Histopathologic evaluation with hematoxylin and eosin staining demonstrated clusters of abundant granular amorphous material within the subcutaneous tissue (Figure 1). The overlying epidermis and dermis were unremarkable. The granular amorphous material demonstrated numerous monosodium urate crystals under polarized light (Figure 2). At a return visit following the biopsy results, the patient reported a history of a single episode of monoarticular gouty arthritis involving the right hallux approximately 6 months after the onset of the skin lesions. With the added clinical history and the biopsy results, his serum uric acid level was obtained and was found to be elevated at 9.2 mg/dL (reference range, 3.5–8 mg/dL).
|
|
In our patient, the clinical differential diagnosis included calcium deposits, weathering nodules, and tophaceous gout. The differential diagnosis of auricular lesions is broad, and benign lesions may mimic cancerous entities such as basal cell carcinoma and squamous cell carcinoma.1 Therefore a detailed history, thorough physical examination, and tissue sampling are key to establishing the correct diagnosis. Our patient’s history of monoarticular gouty arthritis was only elucidated after a diagnosis of bilateral auricular tophaceous gout was made based on the biopsy results.
Subcutaneous tophi represent a chronic state of hyperuricemia and tend to manifest after long-standing polyarthritis and repeated acute gout attacks.2-5 These lesions develop in approximately 50% of gout patients and usually occur an average of 11.6 years after the onset of disease.2 There is a subset of individuals that are at higher risk for developing tophi, including elderly and female patients, diuretic and chronic nonsteroidal anti-inflammatory drug users, patients with a history of cyclosporine therapy, and patients with underlying chronic renal insufficiency.2,6,7 The most commonly affected tissues are those with poor blood supply and lower temperatures, such as the ear helix and first metacarpal joint.4 The auricle is the most common site of tophi on the head and neck. Tophi of the helices are generally asymptomatic and nontender; however, tophi can become large, inflamed, and ulcerated, causing pressure and discomfort.2 Combination treatment with dietary modification and antihyperuricemic therapy (eg, allopurinol) has been shown to reduce the size of lesions and prevent future tophi formation. However, these results may take months, warranting excision of large and symptomatic lesions.4,8
Our case is unusual in that the onset of the auricular lesions predated the articular gout by 6 months. Gouty tophi as the initial presentation of hyperuricemia is rare; however, tophi formation without concomitant arthritis has been reported.2,3,7,9 Wernick et al7 described 6 patients presenting with tophi before the onset of inflammatory arthritis that they attributed to changes in active inflammation by age (eg, elderly patients were more commonly immunosuppressed), chronic illnesses, and anti-inflammatory medications (eg, nonsteroidal anti-inflammatory drugs). Another possible explanation for this atypical presentation is misdiagnosis caused by other forms of arthritis (eg, rheumatoid arthritis, osteoarthritis) masking acute gout episodes. It also has been reported that monosodium urate crystals can be found in synovial fluid with no inflammation and therefore no symptoms.7
Tophi, although rare, may be the sole clinical manifestation of underlying gouty disease. It is important to be aware of this atypical presentation to prevent misdiagnosis and provide appropriate treatment.
- Dompmartin A. Nodules of the external ear [in French]. Ann Dermatol Venereol. 1999;126:261-266.
- Griffin G, Munns J, Fullen D, et al. Auricular tophi as the initial presentation of gout. Otolaryngol Head Neck Surg. 2009;141:153-154.
- Koley S, Salodkar A, Choudhary S, et al. Tophi as first manifestation of gout. Indian J Dermatol Venerol. 2010;76:393-393-396.
- Moriwaki Y. Tophaceous gout [in Japanese]. Nihon Rinsho. 2008;66:711-716.
- Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76:801-808.
- Hollingworth P, Scott JT, Burry HC. Nonarticular gout: hyperuricemia and tophus formation without gouty arthritis. Arthritis Rheum. 1983;26:98-101.
- Wernick R, Winkler C, Campbell S. Tophi as the initial manifestation of gout. report of six cases and review of the literature. Arch Intern Med. 1992;152:873-876.
- Caldas CA, Fuller R. Excellent response to the clinical treatment of tophaceous gout. Clin Rheumatol. 2009;26:1553-1555.
- Iglesias A, Londono JC, Saaibi DL, et al. Gout nodulosis: widespread subcutaneous deposits without gout. Arthritis Care Res. 1996;9:74-77.
The Diagnosis: Bilateral Auricular Tophaceous Gout
Histopathologic evaluation with hematoxylin and eosin staining demonstrated clusters of abundant granular amorphous material within the subcutaneous tissue (Figure 1). The overlying epidermis and dermis were unremarkable. The granular amorphous material demonstrated numerous monosodium urate crystals under polarized light (Figure 2). At a return visit following the biopsy results, the patient reported a history of a single episode of monoarticular gouty arthritis involving the right hallux approximately 6 months after the onset of the skin lesions. With the added clinical history and the biopsy results, his serum uric acid level was obtained and was found to be elevated at 9.2 mg/dL (reference range, 3.5–8 mg/dL).

|

|
In our patient, the clinical differential diagnosis included calcium deposits, weathering nodules, and tophaceous gout. The differential diagnosis of auricular lesions is broad, and benign lesions may mimic cancerous entities such as basal cell carcinoma and squamous cell carcinoma.1 Therefore a detailed history, thorough physical examination, and tissue sampling are key to establishing the correct diagnosis. Our patient’s history of monoarticular gouty arthritis was only elucidated after a diagnosis of bilateral auricular tophaceous gout was made based on the biopsy results.
Subcutaneous tophi represent a chronic state of hyperuricemia and tend to manifest after long-standing polyarthritis and repeated acute gout attacks.2-5 These lesions develop in approximately 50% of gout patients and usually occur an average of 11.6 years after the onset of disease.2 There is a subset of individuals that are at higher risk for developing tophi, including elderly and female patients, diuretic and chronic nonsteroidal anti-inflammatory drug users, patients with a history of cyclosporine therapy, and patients with underlying chronic renal insufficiency.2,6,7 The most commonly affected tissues are those with poor blood supply and lower temperatures, such as the ear helix and first metacarpal joint.4 The auricle is the most common site of tophi on the head and neck. Tophi of the helices are generally asymptomatic and nontender; however, tophi can become large, inflamed, and ulcerated, causing pressure and discomfort.2 Combination treatment with dietary modification and antihyperuricemic therapy (eg, allopurinol) has been shown to reduce the size of lesions and prevent future tophi formation. However, these results may take months, warranting excision of large and symptomatic lesions.4,8
Our case is unusual in that the onset of the auricular lesions predated the articular gout by 6 months. Gouty tophi as the initial presentation of hyperuricemia is rare; however, tophi formation without concomitant arthritis has been reported.2,3,7,9 Wernick et al7 described 6 patients presenting with tophi before the onset of inflammatory arthritis that they attributed to changes in active inflammation by age (eg, elderly patients were more commonly immunosuppressed), chronic illnesses, and anti-inflammatory medications (eg, nonsteroidal anti-inflammatory drugs). Another possible explanation for this atypical presentation is misdiagnosis caused by other forms of arthritis (eg, rheumatoid arthritis, osteoarthritis) masking acute gout episodes. It also has been reported that monosodium urate crystals can be found in synovial fluid with no inflammation and therefore no symptoms.7
Tophi, although rare, may be the sole clinical manifestation of underlying gouty disease. It is important to be aware of this atypical presentation to prevent misdiagnosis and provide appropriate treatment.
The Diagnosis: Bilateral Auricular Tophaceous Gout
Histopathologic evaluation with hematoxylin and eosin staining demonstrated clusters of abundant granular amorphous material within the subcutaneous tissue (Figure 1). The overlying epidermis and dermis were unremarkable. The granular amorphous material demonstrated numerous monosodium urate crystals under polarized light (Figure 2). At a return visit following the biopsy results, the patient reported a history of a single episode of monoarticular gouty arthritis involving the right hallux approximately 6 months after the onset of the skin lesions. With the added clinical history and the biopsy results, his serum uric acid level was obtained and was found to be elevated at 9.2 mg/dL (reference range, 3.5–8 mg/dL).

|

|
In our patient, the clinical differential diagnosis included calcium deposits, weathering nodules, and tophaceous gout. The differential diagnosis of auricular lesions is broad, and benign lesions may mimic cancerous entities such as basal cell carcinoma and squamous cell carcinoma.1 Therefore a detailed history, thorough physical examination, and tissue sampling are key to establishing the correct diagnosis. Our patient’s history of monoarticular gouty arthritis was only elucidated after a diagnosis of bilateral auricular tophaceous gout was made based on the biopsy results.
Subcutaneous tophi represent a chronic state of hyperuricemia and tend to manifest after long-standing polyarthritis and repeated acute gout attacks.2-5 These lesions develop in approximately 50% of gout patients and usually occur an average of 11.6 years after the onset of disease.2 There is a subset of individuals that are at higher risk for developing tophi, including elderly and female patients, diuretic and chronic nonsteroidal anti-inflammatory drug users, patients with a history of cyclosporine therapy, and patients with underlying chronic renal insufficiency.2,6,7 The most commonly affected tissues are those with poor blood supply and lower temperatures, such as the ear helix and first metacarpal joint.4 The auricle is the most common site of tophi on the head and neck. Tophi of the helices are generally asymptomatic and nontender; however, tophi can become large, inflamed, and ulcerated, causing pressure and discomfort.2 Combination treatment with dietary modification and antihyperuricemic therapy (eg, allopurinol) has been shown to reduce the size of lesions and prevent future tophi formation. However, these results may take months, warranting excision of large and symptomatic lesions.4,8
Our case is unusual in that the onset of the auricular lesions predated the articular gout by 6 months. Gouty tophi as the initial presentation of hyperuricemia is rare; however, tophi formation without concomitant arthritis has been reported.2,3,7,9 Wernick et al7 described 6 patients presenting with tophi before the onset of inflammatory arthritis that they attributed to changes in active inflammation by age (eg, elderly patients were more commonly immunosuppressed), chronic illnesses, and anti-inflammatory medications (eg, nonsteroidal anti-inflammatory drugs). Another possible explanation for this atypical presentation is misdiagnosis caused by other forms of arthritis (eg, rheumatoid arthritis, osteoarthritis) masking acute gout episodes. It also has been reported that monosodium urate crystals can be found in synovial fluid with no inflammation and therefore no symptoms.7
Tophi, although rare, may be the sole clinical manifestation of underlying gouty disease. It is important to be aware of this atypical presentation to prevent misdiagnosis and provide appropriate treatment.
- Dompmartin A. Nodules of the external ear [in French]. Ann Dermatol Venereol. 1999;126:261-266.
- Griffin G, Munns J, Fullen D, et al. Auricular tophi as the initial presentation of gout. Otolaryngol Head Neck Surg. 2009;141:153-154.
- Koley S, Salodkar A, Choudhary S, et al. Tophi as first manifestation of gout. Indian J Dermatol Venerol. 2010;76:393-393-396.
- Moriwaki Y. Tophaceous gout [in Japanese]. Nihon Rinsho. 2008;66:711-716.
- Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76:801-808.
- Hollingworth P, Scott JT, Burry HC. Nonarticular gout: hyperuricemia and tophus formation without gouty arthritis. Arthritis Rheum. 1983;26:98-101.
- Wernick R, Winkler C, Campbell S. Tophi as the initial manifestation of gout. report of six cases and review of the literature. Arch Intern Med. 1992;152:873-876.
- Caldas CA, Fuller R. Excellent response to the clinical treatment of tophaceous gout. Clin Rheumatol. 2009;26:1553-1555.
- Iglesias A, Londono JC, Saaibi DL, et al. Gout nodulosis: widespread subcutaneous deposits without gout. Arthritis Care Res. 1996;9:74-77.
- Dompmartin A. Nodules of the external ear [in French]. Ann Dermatol Venereol. 1999;126:261-266.
- Griffin G, Munns J, Fullen D, et al. Auricular tophi as the initial presentation of gout. Otolaryngol Head Neck Surg. 2009;141:153-154.
- Koley S, Salodkar A, Choudhary S, et al. Tophi as first manifestation of gout. Indian J Dermatol Venerol. 2010;76:393-393-396.
- Moriwaki Y. Tophaceous gout [in Japanese]. Nihon Rinsho. 2008;66:711-716.
- Eggebeen AT. Gout: an update. Am Fam Physician. 2007;76:801-808.
- Hollingworth P, Scott JT, Burry HC. Nonarticular gout: hyperuricemia and tophus formation without gouty arthritis. Arthritis Rheum. 1983;26:98-101.
- Wernick R, Winkler C, Campbell S. Tophi as the initial manifestation of gout. report of six cases and review of the literature. Arch Intern Med. 1992;152:873-876.
- Caldas CA, Fuller R. Excellent response to the clinical treatment of tophaceous gout. Clin Rheumatol. 2009;26:1553-1555.
- Iglesias A, Londono JC, Saaibi DL, et al. Gout nodulosis: widespread subcutaneous deposits without gout. Arthritis Care Res. 1996;9:74-77.
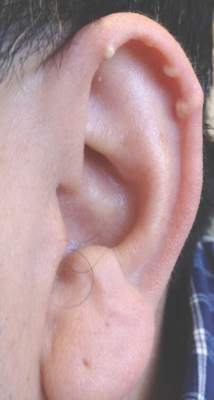
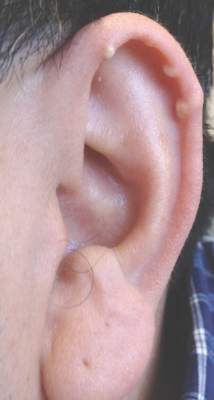
A 40-year-old man presented for evaluation of multiple small nodules on the bilateral auricles primarily involving the helices of 1 year’s duration. The lesions were nontender with no associated bleeding, burning, or pruritus. He denied any trauma to these sites and denied any systemic symptoms including fever, chills, joint pain, or weight loss. His medical history was remarkable for type 2 diabetes mellitus. He had no history of similar skin lesions or renal disease and denied any alcohol intake. He also denied taking any over-the-counter or prescription medications. Physical examination revealed several 1- to 4-mm superficial white dermal nodules located on the bilateral helical rims. The lesions were firm and well circumscribed and the surrounding skin showed mild erythema. Shave biopsies of the nodules were performed.
An Eruption While on Total Parenteral Nutrition
The Diagnosis: Acquired Acrodermatitis Enteropathica
Acquired acrodermatitis enteropathica (AAE) is a rare disorder caused by severe zinc deficiency. Although acrodermatitis enteropathica is an autosomal-recessive disorder that typically manifests in infancy, AAE also can result from poor zinc intake, impaired absorption, or accelerated losses. There are reports of AAE in patients with zinc-deficient diets,1 eating disorders,2 bariatric and other gastrointestinal surgeries,3 malabsorptive diseases,4 and nephrotic syndrome.5
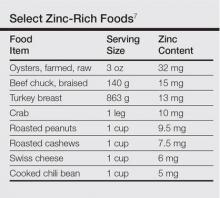
Zinc plays an important role in DNA and RNA synthesis, reactive oxygen species attenuation, and energy metabolism, allowing for proper wound healing, skin differentiation, and proliferation.6 Zinc is found in most foods, but animal protein contains higher concentrations (Table).7 Approximately 85% of zinc is stored in muscles and bones, with only a small amount of accessible zinc available in the liver. Liver stores can be depleted as quickly as 1 week.8 Total parenteral nutrition without trace element supplementation can quickly predispose patients to AAE.

|

|
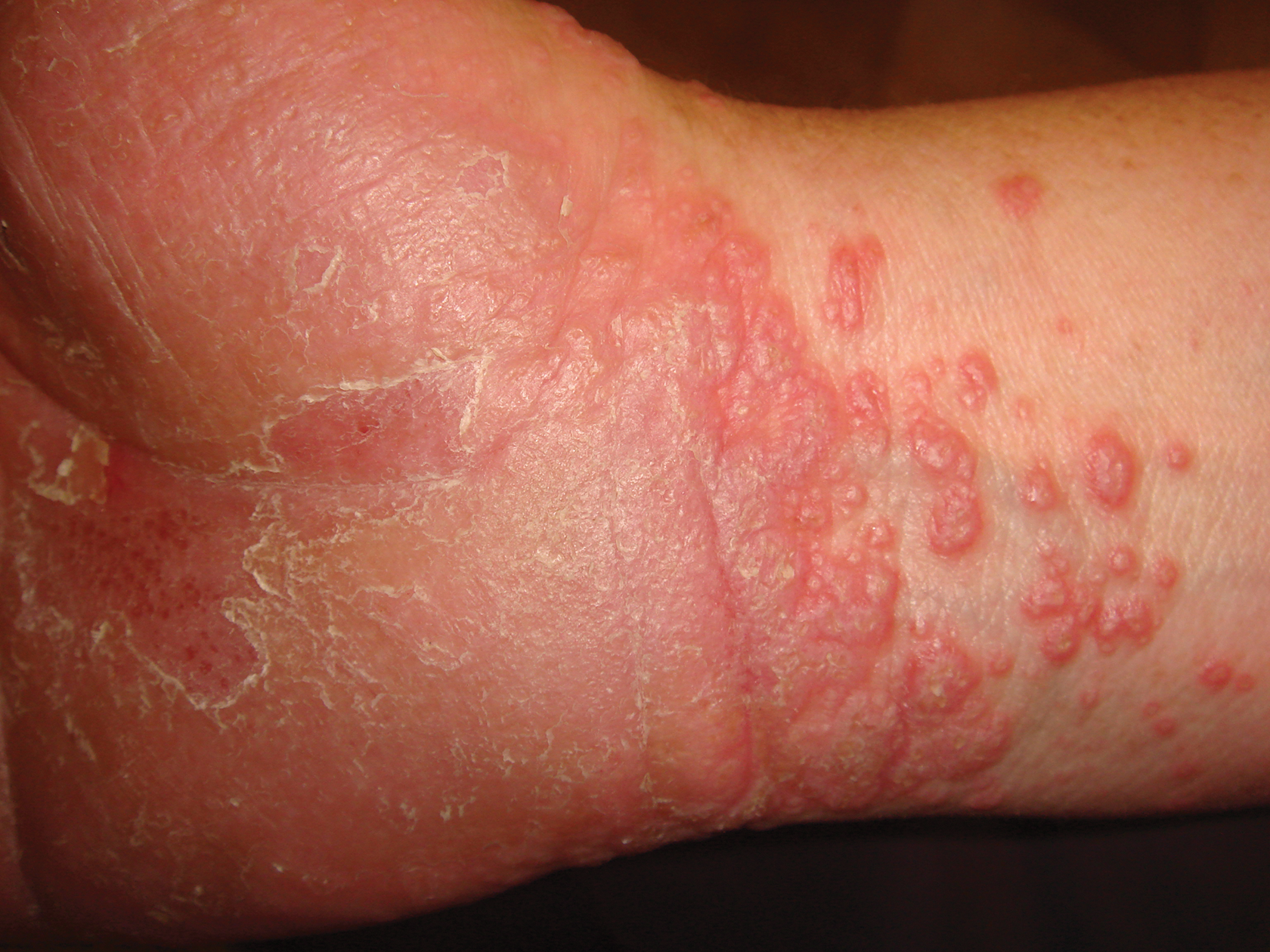
Diagnosis of this condition requires triangulation of clinical presentation, histopathology examination, and laboratory findings. Acrodermatitis enteropathica typically is characterized by dermatitis, diarrhea, and epidermal appendage findings. In its early stages, the dermatitis often manifests with angular cheilitis and paronychia.9 Patients then develop erythema, erosions, and occasionally vesicles or psoriasiform plaques in periorificial, perineal, and acral sites (Figure 1). Epidermal appendage effects include generalized alopecia and thinning nails with white transverse ridges. Although dermatologic and gastrointestinal manifestations are the most obvious, severe AAE may cause other symptoms, including mental slowing, hypogonadism, and impaired immune function.9
Histopathology of AAE skin lesions is similar to other nutritional deficiencies. Early changes are more specific to deficiency dermatitis and include cytoplasmic pallor and ballooning degeneration of keratinocytes in the stratum spinosum and granulosum.9 Necrolysis results in confluent keratinocyte necrosis developing into subcorneal bulla. Later in the disease course, the presentation becomes psoriasiform with keratinocyte dyskeratosis and confluent parakeratosis10 (Figure 2). Dermal edema with dilated tortuous vessels and a neutrophilic infiltrate may be present throughout disease progression.
Common laboratory abnormalities used to confirm zinc deficiency are decreased plasma zinc and alkaline phosphatase levels. Plasma zinc levels should be drawn after fasting because zinc levels decrease after food intake.9 Concurrent albumin levels should be drawn to correct for low levels caused by hypoalbuminemia. Acquired acrodermatitis enteropathica has been seen in patients with only mildly decreased plasma zinc levels or even zinc levels within reference range.11 Alkaline phosphatase metalloenzyme synthesis requires zinc and a decreased level suggests zinc deficiency even with a plasma zinc level within reference range. Alkaline phosphatase levels usually can be ascertained in a matter of hours, while the zinc levels take much longer to result.
Acquired acrodermatitis enteropathica is treated with oral elemental zinc supplementation at 1 to 2 mg/kg daily.12 Diarrhea typically resolves within 24 hours, but skin lesions heal in 1 to 2 weeks or longer. Although there is no consensus on when to discontinue zinc replacement therapy, therapy generally is not lifelong. Once the patient is zinc replete and the inciting factor has resolved, patients can discontinue supplementation without risk for recurrence.
Trace elements had not been added to our patient’s total parenteral nutrition prior to admission. Basic nutrition laboratory results and zinc levels returned markedly low: 14 μg/dL (reference range, 60–120 μg/dL). Alkaline phosphatase, a zinc-dependent protein, also was low at 12 U/L (reference range, 40–150 U/L). We added trace elements and vitamins and began empiric zinc replacement with 440 mg oral zinc sulfate daily (100 mg elemental zinc). Cephalexin was prescribed for impetiginized skin lesions. The patient noted skin improvement after 3 days on zinc replacement therapy.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Kim ST, Kang JS, Baek JW, et al. Acrodermatitis enteropathica with anorexia nervosa. J Dermatol. 2010;37:726-729.
- Bae-Harboe YS, Solky A, Masterpol KS. A case of acquired zinc deficiency. Dermatol Online J. 2012;18:1.
- Krasovec M, Frenk E. Acrodermatitis enteropathica secondary to Crohn’s disease. Dermatol Basel Switz. 1996;193:361-363.
- Reichel M, Mauro TM, Ziboh VA, et al. Acrodermatitis enteropathica in a patient with the acquired immunodeficiency syndrome. Arch Dermatol. 1992;128:415-417.
- Perafan-Riveros C, Franca LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- National Nutrient Database for Standard Reference, Release 28. United States Department of Agriculture, Agricultural Research Service website. http://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=309&nutrient2=&nutrient3=&subset=0&fg=&sort=f&measureby=m. Accessed December 14, 2015.
- McPherson RA, Pincus MR. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia, PA: Saunders Elsevier; 2011.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Kumar P, Lal NR, Mondal A, et al. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
The Diagnosis: Acquired Acrodermatitis Enteropathica
Acquired acrodermatitis enteropathica (AAE) is a rare disorder caused by severe zinc deficiency. Although acrodermatitis enteropathica is an autosomal-recessive disorder that typically manifests in infancy, AAE also can result from poor zinc intake, impaired absorption, or accelerated losses. There are reports of AAE in patients with zinc-deficient diets,1 eating disorders,2 bariatric and other gastrointestinal surgeries,3 malabsorptive diseases,4 and nephrotic syndrome.5
Zinc plays an important role in DNA and RNA synthesis, reactive oxygen species attenuation, and energy metabolism, allowing for proper wound healing, skin differentiation, and proliferation.6 Zinc is found in most foods, but animal protein contains higher concentrations (Table).7 Approximately 85% of zinc is stored in muscles and bones, with only a small amount of accessible zinc available in the liver. Liver stores can be depleted as quickly as 1 week.8 Total parenteral nutrition without trace element supplementation can quickly predispose patients to AAE.

|

|
Diagnosis of this condition requires triangulation of clinical presentation, histopathology examination, and laboratory findings. Acrodermatitis enteropathica typically is characterized by dermatitis, diarrhea, and epidermal appendage findings. In its early stages, the dermatitis often manifests with angular cheilitis and paronychia.9 Patients then develop erythema, erosions, and occasionally vesicles or psoriasiform plaques in periorificial, perineal, and acral sites (Figure 1). Epidermal appendage effects include generalized alopecia and thinning nails with white transverse ridges. Although dermatologic and gastrointestinal manifestations are the most obvious, severe AAE may cause other symptoms, including mental slowing, hypogonadism, and impaired immune function.9
Histopathology of AAE skin lesions is similar to other nutritional deficiencies. Early changes are more specific to deficiency dermatitis and include cytoplasmic pallor and ballooning degeneration of keratinocytes in the stratum spinosum and granulosum.9 Necrolysis results in confluent keratinocyte necrosis developing into subcorneal bulla. Later in the disease course, the presentation becomes psoriasiform with keratinocyte dyskeratosis and confluent parakeratosis10 (Figure 2). Dermal edema with dilated tortuous vessels and a neutrophilic infiltrate may be present throughout disease progression.
Common laboratory abnormalities used to confirm zinc deficiency are decreased plasma zinc and alkaline phosphatase levels. Plasma zinc levels should be drawn after fasting because zinc levels decrease after food intake.9 Concurrent albumin levels should be drawn to correct for low levels caused by hypoalbuminemia. Acquired acrodermatitis enteropathica has been seen in patients with only mildly decreased plasma zinc levels or even zinc levels within reference range.11 Alkaline phosphatase metalloenzyme synthesis requires zinc and a decreased level suggests zinc deficiency even with a plasma zinc level within reference range. Alkaline phosphatase levels usually can be ascertained in a matter of hours, while the zinc levels take much longer to result.
Acquired acrodermatitis enteropathica is treated with oral elemental zinc supplementation at 1 to 2 mg/kg daily.12 Diarrhea typically resolves within 24 hours, but skin lesions heal in 1 to 2 weeks or longer. Although there is no consensus on when to discontinue zinc replacement therapy, therapy generally is not lifelong. Once the patient is zinc replete and the inciting factor has resolved, patients can discontinue supplementation without risk for recurrence.
Trace elements had not been added to our patient’s total parenteral nutrition prior to admission. Basic nutrition laboratory results and zinc levels returned markedly low: 14 μg/dL (reference range, 60–120 μg/dL). Alkaline phosphatase, a zinc-dependent protein, also was low at 12 U/L (reference range, 40–150 U/L). We added trace elements and vitamins and began empiric zinc replacement with 440 mg oral zinc sulfate daily (100 mg elemental zinc). Cephalexin was prescribed for impetiginized skin lesions. The patient noted skin improvement after 3 days on zinc replacement therapy.
The Diagnosis: Acquired Acrodermatitis Enteropathica
Acquired acrodermatitis enteropathica (AAE) is a rare disorder caused by severe zinc deficiency. Although acrodermatitis enteropathica is an autosomal-recessive disorder that typically manifests in infancy, AAE also can result from poor zinc intake, impaired absorption, or accelerated losses. There are reports of AAE in patients with zinc-deficient diets,1 eating disorders,2 bariatric and other gastrointestinal surgeries,3 malabsorptive diseases,4 and nephrotic syndrome.5
Zinc plays an important role in DNA and RNA synthesis, reactive oxygen species attenuation, and energy metabolism, allowing for proper wound healing, skin differentiation, and proliferation.6 Zinc is found in most foods, but animal protein contains higher concentrations (Table).7 Approximately 85% of zinc is stored in muscles and bones, with only a small amount of accessible zinc available in the liver. Liver stores can be depleted as quickly as 1 week.8 Total parenteral nutrition without trace element supplementation can quickly predispose patients to AAE.

|

|
Diagnosis of this condition requires triangulation of clinical presentation, histopathology examination, and laboratory findings. Acrodermatitis enteropathica typically is characterized by dermatitis, diarrhea, and epidermal appendage findings. In its early stages, the dermatitis often manifests with angular cheilitis and paronychia.9 Patients then develop erythema, erosions, and occasionally vesicles or psoriasiform plaques in periorificial, perineal, and acral sites (Figure 1). Epidermal appendage effects include generalized alopecia and thinning nails with white transverse ridges. Although dermatologic and gastrointestinal manifestations are the most obvious, severe AAE may cause other symptoms, including mental slowing, hypogonadism, and impaired immune function.9
Histopathology of AAE skin lesions is similar to other nutritional deficiencies. Early changes are more specific to deficiency dermatitis and include cytoplasmic pallor and ballooning degeneration of keratinocytes in the stratum spinosum and granulosum.9 Necrolysis results in confluent keratinocyte necrosis developing into subcorneal bulla. Later in the disease course, the presentation becomes psoriasiform with keratinocyte dyskeratosis and confluent parakeratosis10 (Figure 2). Dermal edema with dilated tortuous vessels and a neutrophilic infiltrate may be present throughout disease progression.
Common laboratory abnormalities used to confirm zinc deficiency are decreased plasma zinc and alkaline phosphatase levels. Plasma zinc levels should be drawn after fasting because zinc levels decrease after food intake.9 Concurrent albumin levels should be drawn to correct for low levels caused by hypoalbuminemia. Acquired acrodermatitis enteropathica has been seen in patients with only mildly decreased plasma zinc levels or even zinc levels within reference range.11 Alkaline phosphatase metalloenzyme synthesis requires zinc and a decreased level suggests zinc deficiency even with a plasma zinc level within reference range. Alkaline phosphatase levels usually can be ascertained in a matter of hours, while the zinc levels take much longer to result.
Acquired acrodermatitis enteropathica is treated with oral elemental zinc supplementation at 1 to 2 mg/kg daily.12 Diarrhea typically resolves within 24 hours, but skin lesions heal in 1 to 2 weeks or longer. Although there is no consensus on when to discontinue zinc replacement therapy, therapy generally is not lifelong. Once the patient is zinc replete and the inciting factor has resolved, patients can discontinue supplementation without risk for recurrence.
Trace elements had not been added to our patient’s total parenteral nutrition prior to admission. Basic nutrition laboratory results and zinc levels returned markedly low: 14 μg/dL (reference range, 60–120 μg/dL). Alkaline phosphatase, a zinc-dependent protein, also was low at 12 U/L (reference range, 40–150 U/L). We added trace elements and vitamins and began empiric zinc replacement with 440 mg oral zinc sulfate daily (100 mg elemental zinc). Cephalexin was prescribed for impetiginized skin lesions. The patient noted skin improvement after 3 days on zinc replacement therapy.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Kim ST, Kang JS, Baek JW, et al. Acrodermatitis enteropathica with anorexia nervosa. J Dermatol. 2010;37:726-729.
- Bae-Harboe YS, Solky A, Masterpol KS. A case of acquired zinc deficiency. Dermatol Online J. 2012;18:1.
- Krasovec M, Frenk E. Acrodermatitis enteropathica secondary to Crohn’s disease. Dermatol Basel Switz. 1996;193:361-363.
- Reichel M, Mauro TM, Ziboh VA, et al. Acrodermatitis enteropathica in a patient with the acquired immunodeficiency syndrome. Arch Dermatol. 1992;128:415-417.
- Perafan-Riveros C, Franca LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- National Nutrient Database for Standard Reference, Release 28. United States Department of Agriculture, Agricultural Research Service website. http://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=309&nutrient2=&nutrient3=&subset=0&fg=&sort=f&measureby=m. Accessed December 14, 2015.
- McPherson RA, Pincus MR. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia, PA: Saunders Elsevier; 2011.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Kumar P, Lal NR, Mondal A, et al. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
- Saritha M, Gupta D, Chandrashekar L, et al. Acquired zinc deficiency in an adult female. Indian J Dermatol. 2012;57:492-494.
- Kim ST, Kang JS, Baek JW, et al. Acrodermatitis enteropathica with anorexia nervosa. J Dermatol. 2010;37:726-729.
- Bae-Harboe YS, Solky A, Masterpol KS. A case of acquired zinc deficiency. Dermatol Online J. 2012;18:1.
- Krasovec M, Frenk E. Acrodermatitis enteropathica secondary to Crohn’s disease. Dermatol Basel Switz. 1996;193:361-363.
- Reichel M, Mauro TM, Ziboh VA, et al. Acrodermatitis enteropathica in a patient with the acquired immunodeficiency syndrome. Arch Dermatol. 1992;128:415-417.
- Perafan-Riveros C, Franca LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- National Nutrient Database for Standard Reference, Release 28. United States Department of Agriculture, Agricultural Research Service website. http://ndb.nal.usda.gov/ndb/nutrients/report/nutrientsfrm?max=25&offset=0&totCount=0&nutrient1=309&nutrient2=&nutrient3=&subset=0&fg=&sort=f&measureby=m. Accessed December 14, 2015.
- McPherson RA, Pincus MR. Henry’s Clinical Diagnosis and Management by Laboratory Methods. 22nd ed. Philadelphia, PA: Saunders Elsevier; 2011.
- Maverakis E, Fung MA, Lynch PJ, et al. Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol. 2007;56:116-124.
- Gonzalez JR, Botet MV, Sanchez JL. The histopathology of acrodermatitis enteropathica. Am J Dermatopathol. 1982;4:303-311.
- Macdonald JB, Connolly SM, DiCaudo DJ. Think zinc deficiency: acquired acrodermatitis enteropathica due to poor diet and common medications. Arch Dermatol. 2012;148:961-963.
- Kumar P, Lal NR, Mondal A, et al. Zinc and skin: a brief summary. Dermatol Online J. 2012;18:1.
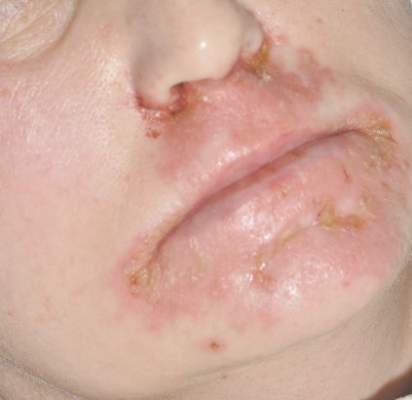
A 47-year-old woman with a history of bulimia and gastroparesis who had been on total parenteral nutrition for 8 weeks presented with a painful, perioral, perineal, and acral eruption of 7 weeks’ duration. Additionally, she had experienced diarrhea, vomiting, and a 13.5-kg weight loss in the last 4 months. Physical examination revealed perioral and perineal, well-demarcated, erythematous, scaly plaques with yellow crusting. She had edematous crusted erosions on the bilateral palms and soles and psoriasiform plaques along the right arm and flank. Punch biopsies (4 mm) from the right inguinal fold and right elbow were obtained.
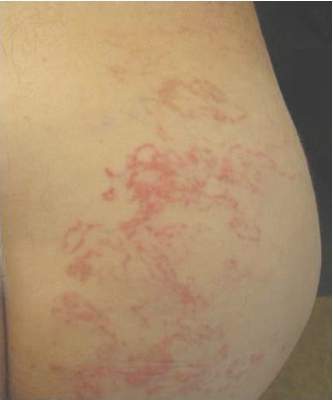
Acute Serpiginous Rash
The Diagnosis: Cutaneous Larva Migrans
Three punch biopsies were obtained. Spongiotic dermatitis with eosinophils was seen. There was a single specimen of tissue that showed a possible intraepidermal larva with a tract in the epidermis. The differential diagnosis included allergic contact dermatitis and arthropod bite eruption, among others, but clinical correlation made cutaneous larva migrans (CLM) the likely diagnosis.
The patient was treated empirically with albendazole 400 mg once daily for 3 days. In addition, he was prescribed triamcinolone for symptomatic relief and remained asymptomatic for 8 weeks at which time he presented again to the dermatology clinic with a similar rash in the same distribution. He was treated with a repeat course of albendazole and further educated on the etiology of the infection. The patient has not exhibited a recurrence after treatment of the second episode of CLM.
Cutaneous larva migrans is a dermatosis of the skin caused by the larvae of parasitic nematodes from the hookworm family, most commonly Ancylostoma caninum and Ancylostoma braziliense.1,2 These hookworms thrive in warm moist climates and are most frequently found in tropical coastal regions. They normally inhabit the intestines of animals such as dogs and cats and are transmitted to soil and sand via feces. Humans become accidental hosts through contact with the contaminated sand or soil3; however, the larvae are unable to penetrate deeper than the upper dermis of the skin in humans, subsequently limiting the infection. Because humans are accidental hosts, the larvae are unable to complete their life cycle and larval death occurs within weeks to months after the initial infection3; thus treatment may be unnecessary unless complications arise.
Cutaneous larva migrans is most commonly observed in travelers or inhabitants of tropical coastal regions but can occur anywhere in the world.1 Clinically, CLM presents as an enlarging, intensely pruritic, erythematous linear or serpiginous tract,3 most commonly on the hands, feet, abdomen, and buttocks.1 Complications may include allergic reactions, secondary bacterial infections, and hookworm folliculitis.4 Although rare, migration to the intestinal tract5 and/or hematological spread with Löffler syndrome has been described.6 Although this dermatological disease has been well described in the medical literature, it is not well recognized by Western physicians and is consequently either not diagnosed or misdiagnosed, leading to delays in treatment.4 Although the infection is usually self-limiting without treatment, the risk for prolonged active disease may occur, with 1 reported case lasting up to 18 months.4,5 The first indicator of CLM is intense pruritus localized to the site of infection.4 As the larvae migrate or creep, they create a lesion that may appear edematous with vesiculobullous lesions that are either serpiginous or linear.4 The differential diagnosis may include fungal infection, bacterial infection, and atypical herpes simplex infections; however, the key finding in CLM is the presence of undulating tracts localized to the borders of the lesion.2 Patients may report experiencing a stinging sensation prior to the formation of the erythematous scaly papule,5 which is attributed to the initial penetration of the larva into the skin. This development, accompanied with a history of travel to tropical or subtropical regions, should elicit CLM as a likely diagnosis. Because hookworms are a type of helminth, they likely elicit an eosinophilic immune response and thus peripheral eosinophilia may be present.5
Effective treatment of CLM is accomplished with oral albendazole 400 mg once daily for 3 to 7 days.2,7 Alternatively, oral ivermectin, topical thiabendazole, and cryosurgery can be used,2 though albendazole currently is the preferred treatment of CLM.7
- Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med. 2004;351:799-807.
- Roest MA, Ratnavel R. Cutaneous larva migrans contracted in England: a reminder. Clin Exp Dermatol. 2001;26:389-390.
- Blackwell V, Vega-Lopez F. Cutaneous larva migrans: clinical features and management of 44 cases presenting in the returning traveller. Br J Dermatol. 2001;145:434-437.
- Hochedez P, Caumes E. Hookworm-related cutaneous larva migrans. J Travel Med. 2007;14:326-333.
- Bravo F, Sanchez MR. New and re-emerging cutaneous infectious diseases in Latin America and other geographic areas. Dermatol Clin. 2003;21:655-668, viii.
- Guill MA, Odom RB. Larva migrans complicated by Loeffler’s syndrome. Arch Dermatol. 1978;114:1525-1526.
- Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814.
The Diagnosis: Cutaneous Larva Migrans
Three punch biopsies were obtained. Spongiotic dermatitis with eosinophils was seen. There was a single specimen of tissue that showed a possible intraepidermal larva with a tract in the epidermis. The differential diagnosis included allergic contact dermatitis and arthropod bite eruption, among others, but clinical correlation made cutaneous larva migrans (CLM) the likely diagnosis.
The patient was treated empirically with albendazole 400 mg once daily for 3 days. In addition, he was prescribed triamcinolone for symptomatic relief and remained asymptomatic for 8 weeks at which time he presented again to the dermatology clinic with a similar rash in the same distribution. He was treated with a repeat course of albendazole and further educated on the etiology of the infection. The patient has not exhibited a recurrence after treatment of the second episode of CLM.
Cutaneous larva migrans is a dermatosis of the skin caused by the larvae of parasitic nematodes from the hookworm family, most commonly Ancylostoma caninum and Ancylostoma braziliense.1,2 These hookworms thrive in warm moist climates and are most frequently found in tropical coastal regions. They normally inhabit the intestines of animals such as dogs and cats and are transmitted to soil and sand via feces. Humans become accidental hosts through contact with the contaminated sand or soil3; however, the larvae are unable to penetrate deeper than the upper dermis of the skin in humans, subsequently limiting the infection. Because humans are accidental hosts, the larvae are unable to complete their life cycle and larval death occurs within weeks to months after the initial infection3; thus treatment may be unnecessary unless complications arise.
Cutaneous larva migrans is most commonly observed in travelers or inhabitants of tropical coastal regions but can occur anywhere in the world.1 Clinically, CLM presents as an enlarging, intensely pruritic, erythematous linear or serpiginous tract,3 most commonly on the hands, feet, abdomen, and buttocks.1 Complications may include allergic reactions, secondary bacterial infections, and hookworm folliculitis.4 Although rare, migration to the intestinal tract5 and/or hematological spread with Löffler syndrome has been described.6 Although this dermatological disease has been well described in the medical literature, it is not well recognized by Western physicians and is consequently either not diagnosed or misdiagnosed, leading to delays in treatment.4 Although the infection is usually self-limiting without treatment, the risk for prolonged active disease may occur, with 1 reported case lasting up to 18 months.4,5 The first indicator of CLM is intense pruritus localized to the site of infection.4 As the larvae migrate or creep, they create a lesion that may appear edematous with vesiculobullous lesions that are either serpiginous or linear.4 The differential diagnosis may include fungal infection, bacterial infection, and atypical herpes simplex infections; however, the key finding in CLM is the presence of undulating tracts localized to the borders of the lesion.2 Patients may report experiencing a stinging sensation prior to the formation of the erythematous scaly papule,5 which is attributed to the initial penetration of the larva into the skin. This development, accompanied with a history of travel to tropical or subtropical regions, should elicit CLM as a likely diagnosis. Because hookworms are a type of helminth, they likely elicit an eosinophilic immune response and thus peripheral eosinophilia may be present.5
Effective treatment of CLM is accomplished with oral albendazole 400 mg once daily for 3 to 7 days.2,7 Alternatively, oral ivermectin, topical thiabendazole, and cryosurgery can be used,2 though albendazole currently is the preferred treatment of CLM.7
The Diagnosis: Cutaneous Larva Migrans
Three punch biopsies were obtained. Spongiotic dermatitis with eosinophils was seen. There was a single specimen of tissue that showed a possible intraepidermal larva with a tract in the epidermis. The differential diagnosis included allergic contact dermatitis and arthropod bite eruption, among others, but clinical correlation made cutaneous larva migrans (CLM) the likely diagnosis.
The patient was treated empirically with albendazole 400 mg once daily for 3 days. In addition, he was prescribed triamcinolone for symptomatic relief and remained asymptomatic for 8 weeks at which time he presented again to the dermatology clinic with a similar rash in the same distribution. He was treated with a repeat course of albendazole and further educated on the etiology of the infection. The patient has not exhibited a recurrence after treatment of the second episode of CLM.
Cutaneous larva migrans is a dermatosis of the skin caused by the larvae of parasitic nematodes from the hookworm family, most commonly Ancylostoma caninum and Ancylostoma braziliense.1,2 These hookworms thrive in warm moist climates and are most frequently found in tropical coastal regions. They normally inhabit the intestines of animals such as dogs and cats and are transmitted to soil and sand via feces. Humans become accidental hosts through contact with the contaminated sand or soil3; however, the larvae are unable to penetrate deeper than the upper dermis of the skin in humans, subsequently limiting the infection. Because humans are accidental hosts, the larvae are unable to complete their life cycle and larval death occurs within weeks to months after the initial infection3; thus treatment may be unnecessary unless complications arise.
Cutaneous larva migrans is most commonly observed in travelers or inhabitants of tropical coastal regions but can occur anywhere in the world.1 Clinically, CLM presents as an enlarging, intensely pruritic, erythematous linear or serpiginous tract,3 most commonly on the hands, feet, abdomen, and buttocks.1 Complications may include allergic reactions, secondary bacterial infections, and hookworm folliculitis.4 Although rare, migration to the intestinal tract5 and/or hematological spread with Löffler syndrome has been described.6 Although this dermatological disease has been well described in the medical literature, it is not well recognized by Western physicians and is consequently either not diagnosed or misdiagnosed, leading to delays in treatment.4 Although the infection is usually self-limiting without treatment, the risk for prolonged active disease may occur, with 1 reported case lasting up to 18 months.4,5 The first indicator of CLM is intense pruritus localized to the site of infection.4 As the larvae migrate or creep, they create a lesion that may appear edematous with vesiculobullous lesions that are either serpiginous or linear.4 The differential diagnosis may include fungal infection, bacterial infection, and atypical herpes simplex infections; however, the key finding in CLM is the presence of undulating tracts localized to the borders of the lesion.2 Patients may report experiencing a stinging sensation prior to the formation of the erythematous scaly papule,5 which is attributed to the initial penetration of the larva into the skin. This development, accompanied with a history of travel to tropical or subtropical regions, should elicit CLM as a likely diagnosis. Because hookworms are a type of helminth, they likely elicit an eosinophilic immune response and thus peripheral eosinophilia may be present.5
Effective treatment of CLM is accomplished with oral albendazole 400 mg once daily for 3 to 7 days.2,7 Alternatively, oral ivermectin, topical thiabendazole, and cryosurgery can be used,2 though albendazole currently is the preferred treatment of CLM.7
- Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med. 2004;351:799-807.
- Roest MA, Ratnavel R. Cutaneous larva migrans contracted in England: a reminder. Clin Exp Dermatol. 2001;26:389-390.
- Blackwell V, Vega-Lopez F. Cutaneous larva migrans: clinical features and management of 44 cases presenting in the returning traveller. Br J Dermatol. 2001;145:434-437.
- Hochedez P, Caumes E. Hookworm-related cutaneous larva migrans. J Travel Med. 2007;14:326-333.
- Bravo F, Sanchez MR. New and re-emerging cutaneous infectious diseases in Latin America and other geographic areas. Dermatol Clin. 2003;21:655-668, viii.
- Guill MA, Odom RB. Larva migrans complicated by Loeffler’s syndrome. Arch Dermatol. 1978;114:1525-1526.
- Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814.
- Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med. 2004;351:799-807.
- Roest MA, Ratnavel R. Cutaneous larva migrans contracted in England: a reminder. Clin Exp Dermatol. 2001;26:389-390.
- Blackwell V, Vega-Lopez F. Cutaneous larva migrans: clinical features and management of 44 cases presenting in the returning traveller. Br J Dermatol. 2001;145:434-437.
- Hochedez P, Caumes E. Hookworm-related cutaneous larva migrans. J Travel Med. 2007;14:326-333.
- Bravo F, Sanchez MR. New and re-emerging cutaneous infectious diseases in Latin America and other geographic areas. Dermatol Clin. 2003;21:655-668, viii.
- Guill MA, Odom RB. Larva migrans complicated by Loeffler’s syndrome. Arch Dermatol. 1978;114:1525-1526.
- Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis. 2000;30:811-814.
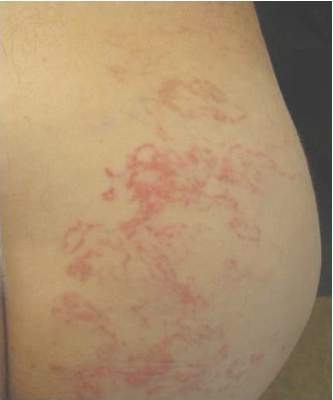
A 62-year-old man presented to the dermatology clinic with a severely pruritic and painful rash of 1 week’s duration. The rash began as an erythematous papule on the right buttock but had spread in a serpiginous manner to the groin and left buttock. The patient stated that he could see the rash spreading in a serpiginous manner over a matter of hours. The patient’s medical history was unremarkable and a review of symptoms was otherwise negative. Physical examination revealed an erythematous serpiginous eruption that was most prominent on the right buttock but extended to the left buttock and down the right leg. He also exhibited several erythematous papules with excoriations in that region.
What Is Your Diagnosis? Stinkbug Staining
The Diagnosis: Stinkbug Staining
After discussing management options with the patient including biopsy, we decided that we would photograph the lesion and follow-up in clinic. While dressing, the patient discovered the source of the pigment, a stinkbug, stuck to the corresponding area of the sock.
The brown marmorated stinkbug (Halyomorpha halys)(Figure) is a member of the Pentatomidae family. This insect is native to East Asia and has become an invasive species in the United States. Their presence has recently increased in the eastern United States and they have become an important agricultural pest as well as a household nuisance. Stinkbugs most commonly interact with humans during the fall and winter months when they enter homes because of cooler temperatures outdoors. They can fit into many unexpected places because of their thin profile.1

Stinkbugs earned their name because of their defensive release of a malodorous chemical. This chemical is comprised of trans-2-decenal and trans-2-octenal, which are both aldehydes and are chemically related to formaldehyde. Based on the material safety data sheet, trans-2-decenal also may be responsible for the orange-brown color seen on the patient’s skin.2 Contact dermatitis caused by direct excretion of this chemical onto human skin has been reported3; anecdotal reports of irritation in agricultural workers have been noted. Stinkbugs are becoming a more common household and agricultural pest and should be recognized as possible causes of some presentations in the dermatology clinic.
- Nielsen AL, Hamilton GC. Seasonal occurrence and impact of Halyomorpha halys (Hemiptera: Pentatomidae) in tree fruit. J Econ Entomol. 2009;102:1133-1140.
- Material safety data sheet: trans-2-Decenal. https://fscimage.fishersci.com/msds/45077.htm. Published October 24, 1998. Updated November 20, 2008. Accessed January 11, 2016.
- Anderson BE, Miller JJ, Adams DR. Irritant contact dermatitis to the brown marmorated stink bug, Halyomorpha halys. Dermatitis. 2012;23:170-172.
The Diagnosis: Stinkbug Staining
After discussing management options with the patient including biopsy, we decided that we would photograph the lesion and follow-up in clinic. While dressing, the patient discovered the source of the pigment, a stinkbug, stuck to the corresponding area of the sock.
The brown marmorated stinkbug (Halyomorpha halys)(Figure) is a member of the Pentatomidae family. This insect is native to East Asia and has become an invasive species in the United States. Their presence has recently increased in the eastern United States and they have become an important agricultural pest as well as a household nuisance. Stinkbugs most commonly interact with humans during the fall and winter months when they enter homes because of cooler temperatures outdoors. They can fit into many unexpected places because of their thin profile.1

Stinkbugs earned their name because of their defensive release of a malodorous chemical. This chemical is comprised of trans-2-decenal and trans-2-octenal, which are both aldehydes and are chemically related to formaldehyde. Based on the material safety data sheet, trans-2-decenal also may be responsible for the orange-brown color seen on the patient’s skin.2 Contact dermatitis caused by direct excretion of this chemical onto human skin has been reported3; anecdotal reports of irritation in agricultural workers have been noted. Stinkbugs are becoming a more common household and agricultural pest and should be recognized as possible causes of some presentations in the dermatology clinic.
The Diagnosis: Stinkbug Staining
After discussing management options with the patient including biopsy, we decided that we would photograph the lesion and follow-up in clinic. While dressing, the patient discovered the source of the pigment, a stinkbug, stuck to the corresponding area of the sock.
The brown marmorated stinkbug (Halyomorpha halys)(Figure) is a member of the Pentatomidae family. This insect is native to East Asia and has become an invasive species in the United States. Their presence has recently increased in the eastern United States and they have become an important agricultural pest as well as a household nuisance. Stinkbugs most commonly interact with humans during the fall and winter months when they enter homes because of cooler temperatures outdoors. They can fit into many unexpected places because of their thin profile.1

Stinkbugs earned their name because of their defensive release of a malodorous chemical. This chemical is comprised of trans-2-decenal and trans-2-octenal, which are both aldehydes and are chemically related to formaldehyde. Based on the material safety data sheet, trans-2-decenal also may be responsible for the orange-brown color seen on the patient’s skin.2 Contact dermatitis caused by direct excretion of this chemical onto human skin has been reported3; anecdotal reports of irritation in agricultural workers have been noted. Stinkbugs are becoming a more common household and agricultural pest and should be recognized as possible causes of some presentations in the dermatology clinic.
- Nielsen AL, Hamilton GC. Seasonal occurrence and impact of Halyomorpha halys (Hemiptera: Pentatomidae) in tree fruit. J Econ Entomol. 2009;102:1133-1140.
- Material safety data sheet: trans-2-Decenal. https://fscimage.fishersci.com/msds/45077.htm. Published October 24, 1998. Updated November 20, 2008. Accessed January 11, 2016.
- Anderson BE, Miller JJ, Adams DR. Irritant contact dermatitis to the brown marmorated stink bug, Halyomorpha halys. Dermatitis. 2012;23:170-172.
- Nielsen AL, Hamilton GC. Seasonal occurrence and impact of Halyomorpha halys (Hemiptera: Pentatomidae) in tree fruit. J Econ Entomol. 2009;102:1133-1140.
- Material safety data sheet: trans-2-Decenal. https://fscimage.fishersci.com/msds/45077.htm. Published October 24, 1998. Updated November 20, 2008. Accessed January 11, 2016.
- Anderson BE, Miller JJ, Adams DR. Irritant contact dermatitis to the brown marmorated stink bug, Halyomorpha halys. Dermatitis. 2012;23:170-172.
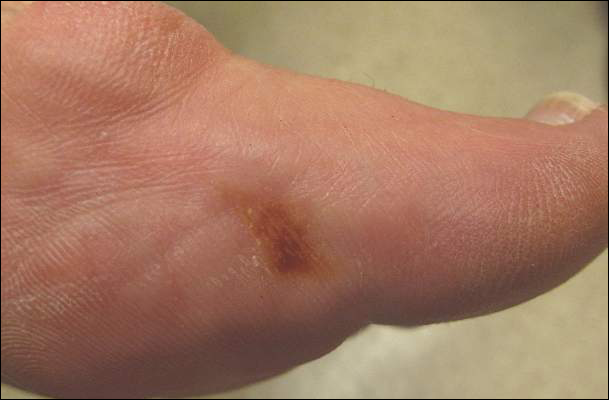
A 56-year-old woman presented at the clinic for a total-body skin examination. A pigmented lesion was found on the medial aspect of the left first toe during the examination. The patient did not recognize this spot as a long-standing nevus. The area was scrubbed vigorously with an alcohol swab, which did not change the pigment. Clinically the lesion was concerning for an atypical nevus. Dermoscopic examination showed an unusual pattern with pigment deposition in ridges and on furrows.
Erythematous Eruption on the Left Leg
The Diagnosis: Bullous Henoch-Schönlein Purpura
Laboratory tests in this patient showed no abnormalities for complete blood cell count, immunoglobulins, anti–double-stranded DNA, antinuclear antibody, p–antineutrophil cytoplasmic antibodies, lupus anticoagulant, Sjögren antibodies, liver enzymes, and erythrocyte sedimentation rate. Urinalysis was normal. Punch biopsies were obtained and a histologic examination showed an intense inflammatory infiltrate of neutrophils around blood vessels within the dermis (Figure). These blood vessels showed swollen endothelium and narrowing of the vessel lumina with leukocytoclasia. Direct immunofluorescence revealed granular IgA, C3, fibrin, and weak IgM deposits in blood vessels in the papillary dermis consistent with Henoch-Schönlein purpura (HSP).
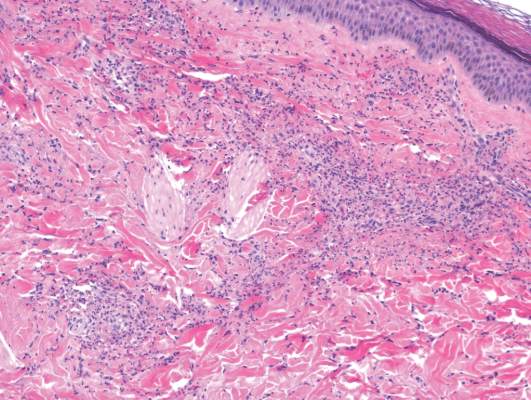
Henoch-Schönlein purpura is the most common vasculitis in children.1-6 However, its bullous variant is rare, with few pediatric cases reported. Bullous HSP affects arterioles through an IgA-mediated pathway.1-6 It is believed that the bullae are formed secondary to neutrophilic release of matrix metalloproteinase 9 (MMP-9), which degrades extracellular collagen.2 Additionally, bullous fluid from HSP has been noted to have markedly elevated levels of soluble CD23, a form of the CD23 B-cell surface receptor used in antibody feedback regulation and B-cell recruitment, which also has been found to be elevated in the fluid of bullous pemphigoid, suggesting a similar pathogenesis of exaggerated humoral immunity.3
The most common sign of HSP is palpable purpura; however, other cutaneous findings can be present including targetoid plaques, macules, papules, petechiae, and bullae that may become hemorrhagic, ulcerated, necrotic, or scarred.1-6 Bullae appear in the most dependent parts of the body, such as the feet and lower legs. Hydrostatic pressure may play a role in the pathogenesis of this phenomenon.1 When other classic signs of HSP are absent, the presence of bullae clouds the diagnosis and creates controversy regarding treatment, as there is a dearth of literature on proper therapy for severe cutaneous manifestations of HSP.6
Our patient was treated with morphine for pain management along with topical mupirocin and nonadherent dressings for wound care. She also received pulse intravenous methylprednisolone 2 mg/kg daily for 3 days and then was transitioned to oral prednisone 1 mg/kg daily, which was tapered over 3 weeks after discharge. This regimen resulted in resolution of symptoms with rapid regression of bullae and subsequent postinflammatory hyperpigmentation. Prior reports have noted that the presence of bullae does not alter the prognosis or predict probability of renal involvement of this self-limited disease, leading to controversy in determining if treatment offers more favorable outcomes.1,3 One study suggested that steroids only improve symptoms, arthralgia, and abdominal pain, but they do not aid in the resolution of cutaneous lesions or prevent the progression of renal disease.3 Contrarily, others have suggested that the presence of bullae and renal disease is an indication to start treatment.6 This claim is based on the mechanistic finding that immunosuppression with corticosteroids decreases inflammation by inhibiting activator protein 1, a transcription factor for MMP-9, thereby reducing MMP-9 activity and the formation of bullae.4 Clinical anecdotes, including our own, that demonstrate dramatic improvement of hemorrhagic bullae with the administration of corticosteroids substantiate this mechanism. Through the inhibition of neutrophil interactions and IgA production, other anti-inflammatory and immunosuppressive medications such as colchicine, dapsone, and azathioprine also have been reported to aid in resolution of the cutaneous lesions.1,5,6 Although there is a clear drawback to the lack of controlled trials and prospective studies regarding the treatment of bullous HSP, it is nearly impossible to expect such studies to be carried out given the rare and unpredictable nature of the disease. For now, claims derived from case series and case reports guide our understanding of treatment efficacy.
Acknowledgment—Quiz photograph courtesy of Steve Taylor, BS, Phoenix, Arizona.
- Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature [published online July 16, 2009]. Rheumatol Int. 2010;30:1355-1359. doi:10.1007/s00296-009-1055-8.
- Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiologic role of gelatinate (MMP-9). Dermatology. 1990;197:62-64.
- Bansal AS, Dwivedi N, Adsett M. Serum and blister fluid cytokines and complement proteins in a patient with Henoch Schönlein purpura associated with a bullous skin rash. Australas J Dermatol. 1997;38:190-192.
- Aljada A, Ghanim H, Mohanty P, et al. Hydrocortisone suppresses intranuclear activator-protein-1 (AP-1) binding activity in mononuclear cells and plasma matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9). J Clin Endocrinol Metab. 2001;86:5988-5991.
- Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
- den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch Schönlein purpura as indication to start systemic prednisone [published online January 5, 2009]. Acta Paediatr. 2010;99:781-783. doi:10.1111/j.1651-2227.2009.01650.x.
The Diagnosis: Bullous Henoch-Schönlein Purpura
Laboratory tests in this patient showed no abnormalities for complete blood cell count, immunoglobulins, anti–double-stranded DNA, antinuclear antibody, p–antineutrophil cytoplasmic antibodies, lupus anticoagulant, Sjögren antibodies, liver enzymes, and erythrocyte sedimentation rate. Urinalysis was normal. Punch biopsies were obtained and a histologic examination showed an intense inflammatory infiltrate of neutrophils around blood vessels within the dermis (Figure). These blood vessels showed swollen endothelium and narrowing of the vessel lumina with leukocytoclasia. Direct immunofluorescence revealed granular IgA, C3, fibrin, and weak IgM deposits in blood vessels in the papillary dermis consistent with Henoch-Schönlein purpura (HSP).

Henoch-Schönlein purpura is the most common vasculitis in children.1-6 However, its bullous variant is rare, with few pediatric cases reported. Bullous HSP affects arterioles through an IgA-mediated pathway.1-6 It is believed that the bullae are formed secondary to neutrophilic release of matrix metalloproteinase 9 (MMP-9), which degrades extracellular collagen.2 Additionally, bullous fluid from HSP has been noted to have markedly elevated levels of soluble CD23, a form of the CD23 B-cell surface receptor used in antibody feedback regulation and B-cell recruitment, which also has been found to be elevated in the fluid of bullous pemphigoid, suggesting a similar pathogenesis of exaggerated humoral immunity.3
The most common sign of HSP is palpable purpura; however, other cutaneous findings can be present including targetoid plaques, macules, papules, petechiae, and bullae that may become hemorrhagic, ulcerated, necrotic, or scarred.1-6 Bullae appear in the most dependent parts of the body, such as the feet and lower legs. Hydrostatic pressure may play a role in the pathogenesis of this phenomenon.1 When other classic signs of HSP are absent, the presence of bullae clouds the diagnosis and creates controversy regarding treatment, as there is a dearth of literature on proper therapy for severe cutaneous manifestations of HSP.6
Our patient was treated with morphine for pain management along with topical mupirocin and nonadherent dressings for wound care. She also received pulse intravenous methylprednisolone 2 mg/kg daily for 3 days and then was transitioned to oral prednisone 1 mg/kg daily, which was tapered over 3 weeks after discharge. This regimen resulted in resolution of symptoms with rapid regression of bullae and subsequent postinflammatory hyperpigmentation. Prior reports have noted that the presence of bullae does not alter the prognosis or predict probability of renal involvement of this self-limited disease, leading to controversy in determining if treatment offers more favorable outcomes.1,3 One study suggested that steroids only improve symptoms, arthralgia, and abdominal pain, but they do not aid in the resolution of cutaneous lesions or prevent the progression of renal disease.3 Contrarily, others have suggested that the presence of bullae and renal disease is an indication to start treatment.6 This claim is based on the mechanistic finding that immunosuppression with corticosteroids decreases inflammation by inhibiting activator protein 1, a transcription factor for MMP-9, thereby reducing MMP-9 activity and the formation of bullae.4 Clinical anecdotes, including our own, that demonstrate dramatic improvement of hemorrhagic bullae with the administration of corticosteroids substantiate this mechanism. Through the inhibition of neutrophil interactions and IgA production, other anti-inflammatory and immunosuppressive medications such as colchicine, dapsone, and azathioprine also have been reported to aid in resolution of the cutaneous lesions.1,5,6 Although there is a clear drawback to the lack of controlled trials and prospective studies regarding the treatment of bullous HSP, it is nearly impossible to expect such studies to be carried out given the rare and unpredictable nature of the disease. For now, claims derived from case series and case reports guide our understanding of treatment efficacy.
Acknowledgment—Quiz photograph courtesy of Steve Taylor, BS, Phoenix, Arizona.
The Diagnosis: Bullous Henoch-Schönlein Purpura
Laboratory tests in this patient showed no abnormalities for complete blood cell count, immunoglobulins, anti–double-stranded DNA, antinuclear antibody, p–antineutrophil cytoplasmic antibodies, lupus anticoagulant, Sjögren antibodies, liver enzymes, and erythrocyte sedimentation rate. Urinalysis was normal. Punch biopsies were obtained and a histologic examination showed an intense inflammatory infiltrate of neutrophils around blood vessels within the dermis (Figure). These blood vessels showed swollen endothelium and narrowing of the vessel lumina with leukocytoclasia. Direct immunofluorescence revealed granular IgA, C3, fibrin, and weak IgM deposits in blood vessels in the papillary dermis consistent with Henoch-Schönlein purpura (HSP).

Henoch-Schönlein purpura is the most common vasculitis in children.1-6 However, its bullous variant is rare, with few pediatric cases reported. Bullous HSP affects arterioles through an IgA-mediated pathway.1-6 It is believed that the bullae are formed secondary to neutrophilic release of matrix metalloproteinase 9 (MMP-9), which degrades extracellular collagen.2 Additionally, bullous fluid from HSP has been noted to have markedly elevated levels of soluble CD23, a form of the CD23 B-cell surface receptor used in antibody feedback regulation and B-cell recruitment, which also has been found to be elevated in the fluid of bullous pemphigoid, suggesting a similar pathogenesis of exaggerated humoral immunity.3
The most common sign of HSP is palpable purpura; however, other cutaneous findings can be present including targetoid plaques, macules, papules, petechiae, and bullae that may become hemorrhagic, ulcerated, necrotic, or scarred.1-6 Bullae appear in the most dependent parts of the body, such as the feet and lower legs. Hydrostatic pressure may play a role in the pathogenesis of this phenomenon.1 When other classic signs of HSP are absent, the presence of bullae clouds the diagnosis and creates controversy regarding treatment, as there is a dearth of literature on proper therapy for severe cutaneous manifestations of HSP.6
Our patient was treated with morphine for pain management along with topical mupirocin and nonadherent dressings for wound care. She also received pulse intravenous methylprednisolone 2 mg/kg daily for 3 days and then was transitioned to oral prednisone 1 mg/kg daily, which was tapered over 3 weeks after discharge. This regimen resulted in resolution of symptoms with rapid regression of bullae and subsequent postinflammatory hyperpigmentation. Prior reports have noted that the presence of bullae does not alter the prognosis or predict probability of renal involvement of this self-limited disease, leading to controversy in determining if treatment offers more favorable outcomes.1,3 One study suggested that steroids only improve symptoms, arthralgia, and abdominal pain, but they do not aid in the resolution of cutaneous lesions or prevent the progression of renal disease.3 Contrarily, others have suggested that the presence of bullae and renal disease is an indication to start treatment.6 This claim is based on the mechanistic finding that immunosuppression with corticosteroids decreases inflammation by inhibiting activator protein 1, a transcription factor for MMP-9, thereby reducing MMP-9 activity and the formation of bullae.4 Clinical anecdotes, including our own, that demonstrate dramatic improvement of hemorrhagic bullae with the administration of corticosteroids substantiate this mechanism. Through the inhibition of neutrophil interactions and IgA production, other anti-inflammatory and immunosuppressive medications such as colchicine, dapsone, and azathioprine also have been reported to aid in resolution of the cutaneous lesions.1,5,6 Although there is a clear drawback to the lack of controlled trials and prospective studies regarding the treatment of bullous HSP, it is nearly impossible to expect such studies to be carried out given the rare and unpredictable nature of the disease. For now, claims derived from case series and case reports guide our understanding of treatment efficacy.
Acknowledgment—Quiz photograph courtesy of Steve Taylor, BS, Phoenix, Arizona.
- Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature [published online July 16, 2009]. Rheumatol Int. 2010;30:1355-1359. doi:10.1007/s00296-009-1055-8.
- Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiologic role of gelatinate (MMP-9). Dermatology. 1990;197:62-64.
- Bansal AS, Dwivedi N, Adsett M. Serum and blister fluid cytokines and complement proteins in a patient with Henoch Schönlein purpura associated with a bullous skin rash. Australas J Dermatol. 1997;38:190-192.
- Aljada A, Ghanim H, Mohanty P, et al. Hydrocortisone suppresses intranuclear activator-protein-1 (AP-1) binding activity in mononuclear cells and plasma matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9). J Clin Endocrinol Metab. 2001;86:5988-5991.
- Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
- den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch Schönlein purpura as indication to start systemic prednisone [published online January 5, 2009]. Acta Paediatr. 2010;99:781-783. doi:10.1111/j.1651-2227.2009.01650.x.
- Trapani S, Mariotti P, Resti M, et al. Severe hemorrhagic bullous lesions in Henoch Schönlein purpura: three pediatric cases and review of the literature [published online July 16, 2009]. Rheumatol Int. 2010;30:1355-1359. doi:10.1007/s00296-009-1055-8.
- Kobayashi T, Sakuraoka K, Iwamoto M, et al. A case of anaphylactoid purpura with multiple blister formation: possible pathophysiologic role of gelatinate (MMP-9). Dermatology. 1990;197:62-64.
- Bansal AS, Dwivedi N, Adsett M. Serum and blister fluid cytokines and complement proteins in a patient with Henoch Schönlein purpura associated with a bullous skin rash. Australas J Dermatol. 1997;38:190-192.
- Aljada A, Ghanim H, Mohanty P, et al. Hydrocortisone suppresses intranuclear activator-protein-1 (AP-1) binding activity in mononuclear cells and plasma matrix metalloproteinase 2 and 9 (MMP-2 and MMP-9). J Clin Endocrinol Metab. 2001;86:5988-5991.
- Iqbal H, Evans A. Dapsone therapy for Henoch-Schönlein purpura: a case series. Arch Dis Child. 2005;90:985-986.
- den Boer SL, Pasmans SG, Wulffraat NM, et al. Bullous lesions in Henoch Schönlein purpura as indication to start systemic prednisone [published online January 5, 2009]. Acta Paediatr. 2010;99:781-783. doi:10.1111/j.1651-2227.2009.01650.x.

A 12-year-old girl presented with an erythematous eruption that had started on the left leg approximately 1 week prior with subsequent spread to the abdomen and arms. She had associated knee pain, myalgia, abdominal pain, nausea, and nonbloody and nonbilious emesis. Her medical history was notable for methicillin-resistant Staphylococcus aureus abscesses, the most recent of which was treated with trimethoprim-sulfamethoxazole; treatment was completed 5 days before the onset of the rash. Family history was notable for her paternal aunt who died of systemic lupus erythematosus. Physical examination showed erythematous macules and purpuric papules with central vesiculation extending up the thighs and lower abdomen associated with edema of the lower extremities and pain after palpation. Tense bullae also were present.
Subcutaneous Tortuous Nodules on the Posterior Lower Extremity
The Diagnosis: Plexiform Neurofibroma as a Manifestation of Neurofibromatosis Type I
Physical examination revealed a large 10×8-cm subcutaneous nodule that was boggy and resembled a bag of worms on palpation. It was covered by slightly hyperpigmented skin. He also had numerous (>20) café au lait spots measuring 2 to 3 cm across the body and several others on the axillae. There were no gross eye findings. Otherwise the examination was unremarkable on the rest of the body. The patient’s paternal grandfather and aunt had similar macules and multiple nodules. The patient had mild to moderate learning difficulties. He was subsequently referred for genetic and ophthalmology evaluation.
Plexiform neurofibromas are usually benign nerve sheath tumors that are elongated and are multinodular, forming when the tumor involves either multiple trunks of a plexus or multiple fascicles of a large nerve such as the sciatic. Some plexiform neurofibromas resemble a bag of worms; others produce a massive ropy enlargement of the nerve.1,2 Plexiform neurofibromas are associated with cases of neurofibromatosis type I (NFI) and are themselves one of the diagnostic criteria for NFI.1
Plexiform neurofibromas are benign tumors that are the result of a genetic mutation in which loss of heterozygosity occurs, as is the case with the other predominant neoplasms of NFI, that results in unrestricted cell growth.3,4 Some patients have a loss of heterozygosity of this tumor suppression gene with overgrowth of neurofibromatosis on a Blaschko segment. One study in mouse models showed that stromal mast cells were involved in promoting inflammation and increasing tumor growth by mediation of mitogenic signals involved in vascular ingrowth, collagen deposition, and cellular proliferation.5 Plexiform neurofibromas are a presenting feature in 30% of NFI cases within the first year of life. They are extensive nerve sheath tumors with an unpredictable growth pattern that can involve multiple fascicles (ie, large nerves and their branches). Five percent become malignant and the transformation is often heralded by rapid growth and pain.6 If malignant transformation is suspected, biopsy is diagnostic. Magnetic resonance imaging with and without contrast can categorize them into 3 growth categories: superficial, displacing, and invasive.7 Because plexiform neurofibromas are rare tumors, it previously was common practice to delay surgical intervention until disfigurement or disability arose. Complete surgical resection at more advanced stages is nearly impossible given the networklike growth pattern that commonly encapsulates vital structures.8,9 Therefore, surgery has been used in the past for debulking the large growths that eventually will recur. A study of 9 small superficial plexiform neurofibromas in children aged 3 to 15 years documented treatment with early surgical resection, which showed complete resection and no relapse at 4 years. This study showed a promising strategy to prevent future extension of these fast-growing tumors into vital structures.8 There also are current clinical trials investigating sirolimus and peginterferon alfa-2b in patients with more invasive plexiform neurofibromas that are unable to undergo surgical resection due to encapsulation or proximity to essential anatomical structures (registered at www.clinicaltrials.gov with the identifiers NCT00652990 and NCT00678951, respectively).
Pain, development of a neurologic deficit, or enlargement of a preexisting plexiform neurofibroma may signal a malignant peripheral nerve sheath tumor (MPNST) and require immediate evaluation.10 Examination by magnetic resonance imaging and positron emission tomography is useful in distinguishing benign and MPNSTs,8,11,12 but definitive differentiation can only be made by histologic examination of the tumor. Complete surgical excision, when possible, is the only treatment that offers the possibility of cure of MPNSTs. Adjuvant chemotherapy or radiotherapy also is sometimes used, though benefit has not been clearly established.8,9,13,14
Death certificate and population-based studies have shown that approximately 10% of patients with NFI have a reduced life expectancy due to MPNSTs; indeed, these tumors arising from plexiform neurofibromas are the main cause of death in adults with NFI. In 2003, Mautner et al7 studied 50 individuals with NFI. The objective was to establish magnetic resonance imaging criteria for MPNST and to test their usefulness in detecting early malignant change in plexiform neurofibromas. This study found that MPNST in patients with NFI frequently showed inhomogeneous contrast enhancement. This inhomogeneity was due to necrosis and hemorrhage, as shown by macroscopic and histologic analysis of amputated limbs in 2 patients within the study. The investigators found it to be possible to detect malignant transformation at an early stage in patients with no overt clinical signs of progression.7 Careful follow-up will determine how frequently early malignancy can be detected and if it is worthwhile carrying out magnetic resonance imaging at defined intervals.2,7,10,15,16
- Friedman JM. Neurofibromatosis 1. In: Pagon RA, ed. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993. http://www.ncbi.nlm.nih.gov/books/NBK1109/. Updated September 4, 2014. Accessed April 6, 2015.
- Friedman JM, Riccardi VM. Clinical epidemiological features. In: Friedman JM, Gutmann DH, MacCollin M, et al, eds. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. Baltimore, MD: Johns Hopkins University Press; 1999:29-86.
- Bausch B, Borozdin W, Mautner VF, et al; European-American Phaeochromocytoma Registry Study Group. Germline NF1 mutational spectra and loss-of-heterozygosity analyses in patients with phaeochromocytoma and neurofibromatosis type 1. J Clin Endocrinol Metab. 2007;92:2784-2792.
- Bottillo I, Ahlquist T, Brekke H, et al. Germline and somatic NF1 mutations in sporadic and NF1-associated malignant peripheral nerve sheath tumours. J Pathol. 2009;217:693-701.
- Staser K, Yang FC, Clapp DW. Pathogenesis of plexiform neurofibroma: tumor-stromal/hematopoietic interactions in tumor progression. Ann Rev Pathol. 2012;7:469-495.
- Murphey MD, Smith WS, Smith SE, et al. From the archives of the AFIP: imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlation. Radiographics. 1999;19:1253-1280.
- Mautner VF, Friedrich RE, von Deimling A, et al. Malignant peripheral nerve sheath tumours in neurofibromatosis type 1: MRI supports the diagnosis of malignant plexiform neurofibroma. Neuroradiology. 2003;45:618-625.
- Friedrich RE, Schmelzle R, Hartmann M, et al. Resection of small plexiform neurofibromas in neurofibromatosis type 1 children. World J Surg Oncol. 2005;3:6.
- Gottfried ON, Viskochil DH, Fults DW, et al. Molecular, genetic, and cellular pathogenesis of neurofibromas and surgical implications. Neurosurgery. 2006;58:1-16.
- Valeyrie-Allanore L, Ismaïli N, Bastuji-Garin S, et al. Symptoms associated with malignancy of peripheral nerve sheath tumours: a retrospective study of 69 patients with neurofibromatosis 1. Br J Dermatol. 2005;153:79-82.
- Bensaid B, Giammarile F, Mognetti T, et al. Utility of 18 FDG positron emission tomography in detection of sarcomatous transformation in neurofibromatosis type 1. Ann Dermatol Venereol. 2007;134:735-741.
- Ferner RE, Lucas JD, O’Doherty MJ, et al. Evaluation of (18)fluorodeoxyglucose positron emission tomography ((18)FDG PET) in the detection of malignant peripheral nerve sheath tumours arising from within plexiform neurofibromas in neurofibromatosis 1. J Neurol Neurosurg Psychiatry. 2000;68:3353-3357.
- Baujat B, Krastinova-Lolov D, Blumen M, et al. Radiofrequency in the treatment of craniofacial plexiform neurofibromatosis: a pilot study. Plast Reconstr Surg. 2006;117:1261-1268.
- Hummel T, Anyane-Yeboa A, Mo J, et al. Response of NF1-related plexiform neurofibroma to high-dose carboplatin. Pediatr Blood Cancer. 2011;56:488-490.
- Feldmann R, Schuierer G, Wessel A, et al. Development of MRI T2 hyperintensities and cognitive functioning in patients with neurofibromatosis type 1. Acta Paediatr. 2010;99:1657-1660.
- Blazo MA, Lewis RA, Chintagumpala MM, et al. Outcomes of systematic screening for optic pathway tumors in children with neurofibromatosis type 1. Am J Med Genet A. 2004;127A:224-229.
The Diagnosis: Plexiform Neurofibroma as a Manifestation of Neurofibromatosis Type I
Physical examination revealed a large 10×8-cm subcutaneous nodule that was boggy and resembled a bag of worms on palpation. It was covered by slightly hyperpigmented skin. He also had numerous (>20) café au lait spots measuring 2 to 3 cm across the body and several others on the axillae. There were no gross eye findings. Otherwise the examination was unremarkable on the rest of the body. The patient’s paternal grandfather and aunt had similar macules and multiple nodules. The patient had mild to moderate learning difficulties. He was subsequently referred for genetic and ophthalmology evaluation.
Plexiform neurofibromas are usually benign nerve sheath tumors that are elongated and are multinodular, forming when the tumor involves either multiple trunks of a plexus or multiple fascicles of a large nerve such as the sciatic. Some plexiform neurofibromas resemble a bag of worms; others produce a massive ropy enlargement of the nerve.1,2 Plexiform neurofibromas are associated with cases of neurofibromatosis type I (NFI) and are themselves one of the diagnostic criteria for NFI.1
Plexiform neurofibromas are benign tumors that are the result of a genetic mutation in which loss of heterozygosity occurs, as is the case with the other predominant neoplasms of NFI, that results in unrestricted cell growth.3,4 Some patients have a loss of heterozygosity of this tumor suppression gene with overgrowth of neurofibromatosis on a Blaschko segment. One study in mouse models showed that stromal mast cells were involved in promoting inflammation and increasing tumor growth by mediation of mitogenic signals involved in vascular ingrowth, collagen deposition, and cellular proliferation.5 Plexiform neurofibromas are a presenting feature in 30% of NFI cases within the first year of life. They are extensive nerve sheath tumors with an unpredictable growth pattern that can involve multiple fascicles (ie, large nerves and their branches). Five percent become malignant and the transformation is often heralded by rapid growth and pain.6 If malignant transformation is suspected, biopsy is diagnostic. Magnetic resonance imaging with and without contrast can categorize them into 3 growth categories: superficial, displacing, and invasive.7 Because plexiform neurofibromas are rare tumors, it previously was common practice to delay surgical intervention until disfigurement or disability arose. Complete surgical resection at more advanced stages is nearly impossible given the networklike growth pattern that commonly encapsulates vital structures.8,9 Therefore, surgery has been used in the past for debulking the large growths that eventually will recur. A study of 9 small superficial plexiform neurofibromas in children aged 3 to 15 years documented treatment with early surgical resection, which showed complete resection and no relapse at 4 years. This study showed a promising strategy to prevent future extension of these fast-growing tumors into vital structures.8 There also are current clinical trials investigating sirolimus and peginterferon alfa-2b in patients with more invasive plexiform neurofibromas that are unable to undergo surgical resection due to encapsulation or proximity to essential anatomical structures (registered at www.clinicaltrials.gov with the identifiers NCT00652990 and NCT00678951, respectively).
Pain, development of a neurologic deficit, or enlargement of a preexisting plexiform neurofibroma may signal a malignant peripheral nerve sheath tumor (MPNST) and require immediate evaluation.10 Examination by magnetic resonance imaging and positron emission tomography is useful in distinguishing benign and MPNSTs,8,11,12 but definitive differentiation can only be made by histologic examination of the tumor. Complete surgical excision, when possible, is the only treatment that offers the possibility of cure of MPNSTs. Adjuvant chemotherapy or radiotherapy also is sometimes used, though benefit has not been clearly established.8,9,13,14
Death certificate and population-based studies have shown that approximately 10% of patients with NFI have a reduced life expectancy due to MPNSTs; indeed, these tumors arising from plexiform neurofibromas are the main cause of death in adults with NFI. In 2003, Mautner et al7 studied 50 individuals with NFI. The objective was to establish magnetic resonance imaging criteria for MPNST and to test their usefulness in detecting early malignant change in plexiform neurofibromas. This study found that MPNST in patients with NFI frequently showed inhomogeneous contrast enhancement. This inhomogeneity was due to necrosis and hemorrhage, as shown by macroscopic and histologic analysis of amputated limbs in 2 patients within the study. The investigators found it to be possible to detect malignant transformation at an early stage in patients with no overt clinical signs of progression.7 Careful follow-up will determine how frequently early malignancy can be detected and if it is worthwhile carrying out magnetic resonance imaging at defined intervals.2,7,10,15,16
The Diagnosis: Plexiform Neurofibroma as a Manifestation of Neurofibromatosis Type I
Physical examination revealed a large 10×8-cm subcutaneous nodule that was boggy and resembled a bag of worms on palpation. It was covered by slightly hyperpigmented skin. He also had numerous (>20) café au lait spots measuring 2 to 3 cm across the body and several others on the axillae. There were no gross eye findings. Otherwise the examination was unremarkable on the rest of the body. The patient’s paternal grandfather and aunt had similar macules and multiple nodules. The patient had mild to moderate learning difficulties. He was subsequently referred for genetic and ophthalmology evaluation.
Plexiform neurofibromas are usually benign nerve sheath tumors that are elongated and are multinodular, forming when the tumor involves either multiple trunks of a plexus or multiple fascicles of a large nerve such as the sciatic. Some plexiform neurofibromas resemble a bag of worms; others produce a massive ropy enlargement of the nerve.1,2 Plexiform neurofibromas are associated with cases of neurofibromatosis type I (NFI) and are themselves one of the diagnostic criteria for NFI.1
Plexiform neurofibromas are benign tumors that are the result of a genetic mutation in which loss of heterozygosity occurs, as is the case with the other predominant neoplasms of NFI, that results in unrestricted cell growth.3,4 Some patients have a loss of heterozygosity of this tumor suppression gene with overgrowth of neurofibromatosis on a Blaschko segment. One study in mouse models showed that stromal mast cells were involved in promoting inflammation and increasing tumor growth by mediation of mitogenic signals involved in vascular ingrowth, collagen deposition, and cellular proliferation.5 Plexiform neurofibromas are a presenting feature in 30% of NFI cases within the first year of life. They are extensive nerve sheath tumors with an unpredictable growth pattern that can involve multiple fascicles (ie, large nerves and their branches). Five percent become malignant and the transformation is often heralded by rapid growth and pain.6 If malignant transformation is suspected, biopsy is diagnostic. Magnetic resonance imaging with and without contrast can categorize them into 3 growth categories: superficial, displacing, and invasive.7 Because plexiform neurofibromas are rare tumors, it previously was common practice to delay surgical intervention until disfigurement or disability arose. Complete surgical resection at more advanced stages is nearly impossible given the networklike growth pattern that commonly encapsulates vital structures.8,9 Therefore, surgery has been used in the past for debulking the large growths that eventually will recur. A study of 9 small superficial plexiform neurofibromas in children aged 3 to 15 years documented treatment with early surgical resection, which showed complete resection and no relapse at 4 years. This study showed a promising strategy to prevent future extension of these fast-growing tumors into vital structures.8 There also are current clinical trials investigating sirolimus and peginterferon alfa-2b in patients with more invasive plexiform neurofibromas that are unable to undergo surgical resection due to encapsulation or proximity to essential anatomical structures (registered at www.clinicaltrials.gov with the identifiers NCT00652990 and NCT00678951, respectively).
Pain, development of a neurologic deficit, or enlargement of a preexisting plexiform neurofibroma may signal a malignant peripheral nerve sheath tumor (MPNST) and require immediate evaluation.10 Examination by magnetic resonance imaging and positron emission tomography is useful in distinguishing benign and MPNSTs,8,11,12 but definitive differentiation can only be made by histologic examination of the tumor. Complete surgical excision, when possible, is the only treatment that offers the possibility of cure of MPNSTs. Adjuvant chemotherapy or radiotherapy also is sometimes used, though benefit has not been clearly established.8,9,13,14
Death certificate and population-based studies have shown that approximately 10% of patients with NFI have a reduced life expectancy due to MPNSTs; indeed, these tumors arising from plexiform neurofibromas are the main cause of death in adults with NFI. In 2003, Mautner et al7 studied 50 individuals with NFI. The objective was to establish magnetic resonance imaging criteria for MPNST and to test their usefulness in detecting early malignant change in plexiform neurofibromas. This study found that MPNST in patients with NFI frequently showed inhomogeneous contrast enhancement. This inhomogeneity was due to necrosis and hemorrhage, as shown by macroscopic and histologic analysis of amputated limbs in 2 patients within the study. The investigators found it to be possible to detect malignant transformation at an early stage in patients with no overt clinical signs of progression.7 Careful follow-up will determine how frequently early malignancy can be detected and if it is worthwhile carrying out magnetic resonance imaging at defined intervals.2,7,10,15,16
- Friedman JM. Neurofibromatosis 1. In: Pagon RA, ed. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993. http://www.ncbi.nlm.nih.gov/books/NBK1109/. Updated September 4, 2014. Accessed April 6, 2015.
- Friedman JM, Riccardi VM. Clinical epidemiological features. In: Friedman JM, Gutmann DH, MacCollin M, et al, eds. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. Baltimore, MD: Johns Hopkins University Press; 1999:29-86.
- Bausch B, Borozdin W, Mautner VF, et al; European-American Phaeochromocytoma Registry Study Group. Germline NF1 mutational spectra and loss-of-heterozygosity analyses in patients with phaeochromocytoma and neurofibromatosis type 1. J Clin Endocrinol Metab. 2007;92:2784-2792.
- Bottillo I, Ahlquist T, Brekke H, et al. Germline and somatic NF1 mutations in sporadic and NF1-associated malignant peripheral nerve sheath tumours. J Pathol. 2009;217:693-701.
- Staser K, Yang FC, Clapp DW. Pathogenesis of plexiform neurofibroma: tumor-stromal/hematopoietic interactions in tumor progression. Ann Rev Pathol. 2012;7:469-495.
- Murphey MD, Smith WS, Smith SE, et al. From the archives of the AFIP: imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlation. Radiographics. 1999;19:1253-1280.
- Mautner VF, Friedrich RE, von Deimling A, et al. Malignant peripheral nerve sheath tumours in neurofibromatosis type 1: MRI supports the diagnosis of malignant plexiform neurofibroma. Neuroradiology. 2003;45:618-625.
- Friedrich RE, Schmelzle R, Hartmann M, et al. Resection of small plexiform neurofibromas in neurofibromatosis type 1 children. World J Surg Oncol. 2005;3:6.
- Gottfried ON, Viskochil DH, Fults DW, et al. Molecular, genetic, and cellular pathogenesis of neurofibromas and surgical implications. Neurosurgery. 2006;58:1-16.
- Valeyrie-Allanore L, Ismaïli N, Bastuji-Garin S, et al. Symptoms associated with malignancy of peripheral nerve sheath tumours: a retrospective study of 69 patients with neurofibromatosis 1. Br J Dermatol. 2005;153:79-82.
- Bensaid B, Giammarile F, Mognetti T, et al. Utility of 18 FDG positron emission tomography in detection of sarcomatous transformation in neurofibromatosis type 1. Ann Dermatol Venereol. 2007;134:735-741.
- Ferner RE, Lucas JD, O’Doherty MJ, et al. Evaluation of (18)fluorodeoxyglucose positron emission tomography ((18)FDG PET) in the detection of malignant peripheral nerve sheath tumours arising from within plexiform neurofibromas in neurofibromatosis 1. J Neurol Neurosurg Psychiatry. 2000;68:3353-3357.
- Baujat B, Krastinova-Lolov D, Blumen M, et al. Radiofrequency in the treatment of craniofacial plexiform neurofibromatosis: a pilot study. Plast Reconstr Surg. 2006;117:1261-1268.
- Hummel T, Anyane-Yeboa A, Mo J, et al. Response of NF1-related plexiform neurofibroma to high-dose carboplatin. Pediatr Blood Cancer. 2011;56:488-490.
- Feldmann R, Schuierer G, Wessel A, et al. Development of MRI T2 hyperintensities and cognitive functioning in patients with neurofibromatosis type 1. Acta Paediatr. 2010;99:1657-1660.
- Blazo MA, Lewis RA, Chintagumpala MM, et al. Outcomes of systematic screening for optic pathway tumors in children with neurofibromatosis type 1. Am J Med Genet A. 2004;127A:224-229.
- Friedman JM. Neurofibromatosis 1. In: Pagon RA, ed. GeneReviews. Seattle, WA: University of Washington, Seattle; 1993. http://www.ncbi.nlm.nih.gov/books/NBK1109/. Updated September 4, 2014. Accessed April 6, 2015.
- Friedman JM, Riccardi VM. Clinical epidemiological features. In: Friedman JM, Gutmann DH, MacCollin M, et al, eds. Neurofibromatosis: Phenotype, Natural History, and Pathogenesis. Baltimore, MD: Johns Hopkins University Press; 1999:29-86.
- Bausch B, Borozdin W, Mautner VF, et al; European-American Phaeochromocytoma Registry Study Group. Germline NF1 mutational spectra and loss-of-heterozygosity analyses in patients with phaeochromocytoma and neurofibromatosis type 1. J Clin Endocrinol Metab. 2007;92:2784-2792.
- Bottillo I, Ahlquist T, Brekke H, et al. Germline and somatic NF1 mutations in sporadic and NF1-associated malignant peripheral nerve sheath tumours. J Pathol. 2009;217:693-701.
- Staser K, Yang FC, Clapp DW. Pathogenesis of plexiform neurofibroma: tumor-stromal/hematopoietic interactions in tumor progression. Ann Rev Pathol. 2012;7:469-495.
- Murphey MD, Smith WS, Smith SE, et al. From the archives of the AFIP: imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlation. Radiographics. 1999;19:1253-1280.
- Mautner VF, Friedrich RE, von Deimling A, et al. Malignant peripheral nerve sheath tumours in neurofibromatosis type 1: MRI supports the diagnosis of malignant plexiform neurofibroma. Neuroradiology. 2003;45:618-625.
- Friedrich RE, Schmelzle R, Hartmann M, et al. Resection of small plexiform neurofibromas in neurofibromatosis type 1 children. World J Surg Oncol. 2005;3:6.
- Gottfried ON, Viskochil DH, Fults DW, et al. Molecular, genetic, and cellular pathogenesis of neurofibromas and surgical implications. Neurosurgery. 2006;58:1-16.
- Valeyrie-Allanore L, Ismaïli N, Bastuji-Garin S, et al. Symptoms associated with malignancy of peripheral nerve sheath tumours: a retrospective study of 69 patients with neurofibromatosis 1. Br J Dermatol. 2005;153:79-82.
- Bensaid B, Giammarile F, Mognetti T, et al. Utility of 18 FDG positron emission tomography in detection of sarcomatous transformation in neurofibromatosis type 1. Ann Dermatol Venereol. 2007;134:735-741.
- Ferner RE, Lucas JD, O’Doherty MJ, et al. Evaluation of (18)fluorodeoxyglucose positron emission tomography ((18)FDG PET) in the detection of malignant peripheral nerve sheath tumours arising from within plexiform neurofibromas in neurofibromatosis 1. J Neurol Neurosurg Psychiatry. 2000;68:3353-3357.
- Baujat B, Krastinova-Lolov D, Blumen M, et al. Radiofrequency in the treatment of craniofacial plexiform neurofibromatosis: a pilot study. Plast Reconstr Surg. 2006;117:1261-1268.
- Hummel T, Anyane-Yeboa A, Mo J, et al. Response of NF1-related plexiform neurofibroma to high-dose carboplatin. Pediatr Blood Cancer. 2011;56:488-490.
- Feldmann R, Schuierer G, Wessel A, et al. Development of MRI T2 hyperintensities and cognitive functioning in patients with neurofibromatosis type 1. Acta Paediatr. 2010;99:1657-1660.
- Blazo MA, Lewis RA, Chintagumpala MM, et al. Outcomes of systematic screening for optic pathway tumors in children with neurofibromatosis type 1. Am J Med Genet A. 2004;127A:224-229.
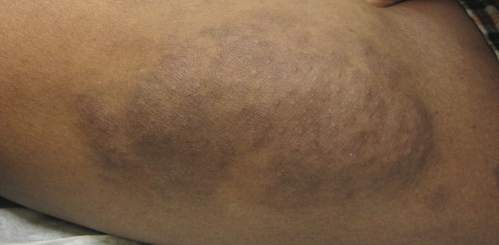
An 11-year-old boy presented for evaluation of a 10×8-cm tortuous lesion on the right posterior leg. Although it had been present since birth, the patient’s mother reported recent growth of the lesion. The lesion was noted to occasionally become irritated and pruritic. The patient’s history was remarkable for asthma.
What Is Your Diagnosis? Mycosis Fungoides
The Diagnosis: Mycosis Fungoides
Physical examination revealed erythematous polycyclic and arcuate plaques with fine overlying scale on the right arm and shoulder (Figure 1). Mild wrinkling and telangiectasias were noted on the skin surrounding the lesions. Laboratory tests showed normal values for antinuclear antibodies, anti–Sjögren syndrome–related antigen A, and anti–Sjögren syndrome–related antigen B.
A skin biopsy of a plaque on the right upper arm showed enlarged pleomorphic lymphocytes arranged along the basal layer and in focal collections within the epidermis (Figure 2). Within the dermis were wiry bundles of collagen, a sparse superficial and patchy infiltrate of lymphocytes, and scattered large mononuclear cells (Figure 3). Immunoperoxidase staining revealed large intraepidermal lymphocytes positive for CD4 (Figure 4A) and CD5. Notably, these lymphocytes also stained positive for CD30 (Figure 4B). Staining for CD8, CD1a, CD56, and anaplastic lymphoma kinase was negative, with aberrant loss of CD3. The morphology and pattern of immunoreactivity supported the diagnosis of mycosis fungoides (MF).
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma.1 Its progression is classified in 3 stages: (1) early (patch) stage, (2) plaque stage, and (3) tumor stage. Conclusive diagnosis of early stage MF often is difficult due to its clinical features that are similar to more common benign dermatoses (eg, atopic dermatitis, psoriasis, lichen planus), leading to shortcomings in determining prognosis and selecting an appropriate treatment regimen. With this diagnositic difficulty in mind, guidelines have been created to aid in the diagnosis of early stage MF.2
Clinical features consistent with early stage MF include multiple erythematous, well-demarcated lesions with varying shapes that typically are greater than 5 cm in diameter.2 Lesions usually are flat or thinly elevated and may exhibit slight scaling. As was noted in our patient, poikiloderma of the surrounding skin is fairly specific for early stage MF, as it is not a feature associated with common clinical mimics of MF (eg, atopic dermatitis, psoriasis, lichen planus). The distribution of skin lesions in non–sun-exposed areas is common. The eruption is persistent, though it may wax and wane in severity.2
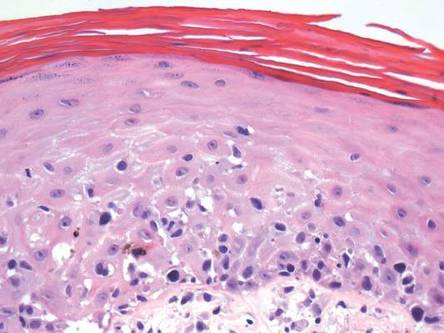
| 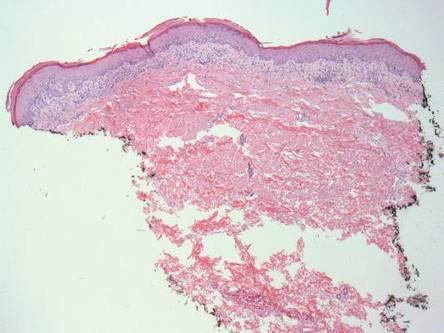
| |
|
|
Histopathologic examination is necessary to confirm a diagnosis of MF. Typically, early stage MF is marked by enlarged T lymphocytes within the epidermis as well as the papillary and superficial reticular dermis. Cerebriform nuclei are a key finding in the diagnosis of MF. Lymphocytes frequently are arranged linearly along the basal layer of the epidermis. Within the epidermis, clusters of atypical lymphocytes (Pautrier microabscesses) without spongiosis are uncommon but are a characteristic finding of MF if present.1 Papillary dermal fibrosis also may be evident.2
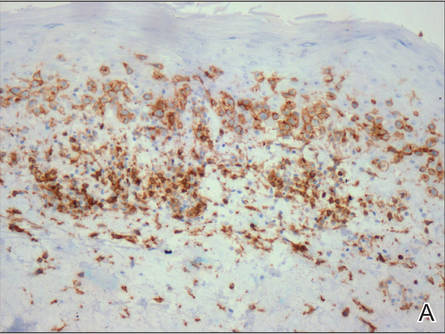
| 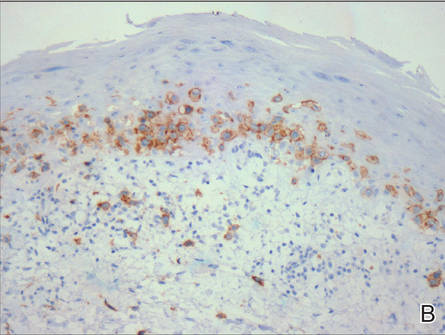
| |
Figure 4. Large intraepidermal lymphocytes were highlighted on CD4 (A) and CD30 immunostaining (B)(original magnification ×200 and ×200). | ||
Immunostaining typically reveals positivity for CD3 and CD4, as well as for lymphocyte antigens CD2 and CD5.1 CD30 positivity in early stage MF rarely has been reported in the literature.3,4 Such cases appear histologically similarly to CD30‒negative cases in other respects. One study showed that the presence of CD30-positive lymphocytes does not alter the clinical course of MF.3 Another study found that, while epidermal CD30-postive lymphocytes had no prognostic relevance, an increased percentage of dermal CD30-positive cells was linked to a higher stage at diagnosis and worse overall prognosis.5 Pathogenesis underlying CD30 positivity in early MF is unknown. It is important to note that CD30-positive cells commonly are seen in lymphomatoid papulosis and anaplastic large cell lymphoma, as well as a variety of nonneoplastic conditions.3,6,7
- Smoller BR. Mycosis fungoides: what do/do not we know? J Cutan Pathol. 2008;35(suppl 2):35-39.
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
- Wu H, Telang GH, Lessin SR, et al. Mycosis fungoides with CD30-positive cells in the epidermis. Am J Dermatopathol. 2000;22:212-216.
- Ohtani T, Kikuchi K, Koizumi H, et al. A case of CD30+ large-cell transformation in a patient with unilesional patch-stage mycosis fungoides. Int J Dermatol. 2009;48:623-626.
- Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33:1860-1868.
- Resnik KS, Kutzner H. Of lymphocytes and cutaneous epithelium: keratoacanthomatous hyperplasia in CD30+ lymphoproliferative disorders and CD30+ cells associated with keratoacanthoma. Am J Dermatopathol. 2010;32:314-315.
- Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J Cutan Pathol. 2006;33(suppl 1):58-70.
The Diagnosis: Mycosis Fungoides
Physical examination revealed erythematous polycyclic and arcuate plaques with fine overlying scale on the right arm and shoulder (Figure 1). Mild wrinkling and telangiectasias were noted on the skin surrounding the lesions. Laboratory tests showed normal values for antinuclear antibodies, anti–Sjögren syndrome–related antigen A, and anti–Sjögren syndrome–related antigen B.
A skin biopsy of a plaque on the right upper arm showed enlarged pleomorphic lymphocytes arranged along the basal layer and in focal collections within the epidermis (Figure 2). Within the dermis were wiry bundles of collagen, a sparse superficial and patchy infiltrate of lymphocytes, and scattered large mononuclear cells (Figure 3). Immunoperoxidase staining revealed large intraepidermal lymphocytes positive for CD4 (Figure 4A) and CD5. Notably, these lymphocytes also stained positive for CD30 (Figure 4B). Staining for CD8, CD1a, CD56, and anaplastic lymphoma kinase was negative, with aberrant loss of CD3. The morphology and pattern of immunoreactivity supported the diagnosis of mycosis fungoides (MF).
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma.1 Its progression is classified in 3 stages: (1) early (patch) stage, (2) plaque stage, and (3) tumor stage. Conclusive diagnosis of early stage MF often is difficult due to its clinical features that are similar to more common benign dermatoses (eg, atopic dermatitis, psoriasis, lichen planus), leading to shortcomings in determining prognosis and selecting an appropriate treatment regimen. With this diagnositic difficulty in mind, guidelines have been created to aid in the diagnosis of early stage MF.2
Clinical features consistent with early stage MF include multiple erythematous, well-demarcated lesions with varying shapes that typically are greater than 5 cm in diameter.2 Lesions usually are flat or thinly elevated and may exhibit slight scaling. As was noted in our patient, poikiloderma of the surrounding skin is fairly specific for early stage MF, as it is not a feature associated with common clinical mimics of MF (eg, atopic dermatitis, psoriasis, lichen planus). The distribution of skin lesions in non–sun-exposed areas is common. The eruption is persistent, though it may wax and wane in severity.2

| 
| |
|
|
Histopathologic examination is necessary to confirm a diagnosis of MF. Typically, early stage MF is marked by enlarged T lymphocytes within the epidermis as well as the papillary and superficial reticular dermis. Cerebriform nuclei are a key finding in the diagnosis of MF. Lymphocytes frequently are arranged linearly along the basal layer of the epidermis. Within the epidermis, clusters of atypical lymphocytes (Pautrier microabscesses) without spongiosis are uncommon but are a characteristic finding of MF if present.1 Papillary dermal fibrosis also may be evident.2

| 
| |
Figure 4. Large intraepidermal lymphocytes were highlighted on CD4 (A) and CD30 immunostaining (B)(original magnification ×200 and ×200). | ||
Immunostaining typically reveals positivity for CD3 and CD4, as well as for lymphocyte antigens CD2 and CD5.1 CD30 positivity in early stage MF rarely has been reported in the literature.3,4 Such cases appear histologically similarly to CD30‒negative cases in other respects. One study showed that the presence of CD30-positive lymphocytes does not alter the clinical course of MF.3 Another study found that, while epidermal CD30-postive lymphocytes had no prognostic relevance, an increased percentage of dermal CD30-positive cells was linked to a higher stage at diagnosis and worse overall prognosis.5 Pathogenesis underlying CD30 positivity in early MF is unknown. It is important to note that CD30-positive cells commonly are seen in lymphomatoid papulosis and anaplastic large cell lymphoma, as well as a variety of nonneoplastic conditions.3,6,7
The Diagnosis: Mycosis Fungoides
Physical examination revealed erythematous polycyclic and arcuate plaques with fine overlying scale on the right arm and shoulder (Figure 1). Mild wrinkling and telangiectasias were noted on the skin surrounding the lesions. Laboratory tests showed normal values for antinuclear antibodies, anti–Sjögren syndrome–related antigen A, and anti–Sjögren syndrome–related antigen B.
A skin biopsy of a plaque on the right upper arm showed enlarged pleomorphic lymphocytes arranged along the basal layer and in focal collections within the epidermis (Figure 2). Within the dermis were wiry bundles of collagen, a sparse superficial and patchy infiltrate of lymphocytes, and scattered large mononuclear cells (Figure 3). Immunoperoxidase staining revealed large intraepidermal lymphocytes positive for CD4 (Figure 4A) and CD5. Notably, these lymphocytes also stained positive for CD30 (Figure 4B). Staining for CD8, CD1a, CD56, and anaplastic lymphoma kinase was negative, with aberrant loss of CD3. The morphology and pattern of immunoreactivity supported the diagnosis of mycosis fungoides (MF).
Mycosis fungoides is the most common form of cutaneous T-cell lymphoma.1 Its progression is classified in 3 stages: (1) early (patch) stage, (2) plaque stage, and (3) tumor stage. Conclusive diagnosis of early stage MF often is difficult due to its clinical features that are similar to more common benign dermatoses (eg, atopic dermatitis, psoriasis, lichen planus), leading to shortcomings in determining prognosis and selecting an appropriate treatment regimen. With this diagnositic difficulty in mind, guidelines have been created to aid in the diagnosis of early stage MF.2
Clinical features consistent with early stage MF include multiple erythematous, well-demarcated lesions with varying shapes that typically are greater than 5 cm in diameter.2 Lesions usually are flat or thinly elevated and may exhibit slight scaling. As was noted in our patient, poikiloderma of the surrounding skin is fairly specific for early stage MF, as it is not a feature associated with common clinical mimics of MF (eg, atopic dermatitis, psoriasis, lichen planus). The distribution of skin lesions in non–sun-exposed areas is common. The eruption is persistent, though it may wax and wane in severity.2

| 
| |
|
|
Histopathologic examination is necessary to confirm a diagnosis of MF. Typically, early stage MF is marked by enlarged T lymphocytes within the epidermis as well as the papillary and superficial reticular dermis. Cerebriform nuclei are a key finding in the diagnosis of MF. Lymphocytes frequently are arranged linearly along the basal layer of the epidermis. Within the epidermis, clusters of atypical lymphocytes (Pautrier microabscesses) without spongiosis are uncommon but are a characteristic finding of MF if present.1 Papillary dermal fibrosis also may be evident.2

| 
| |
Figure 4. Large intraepidermal lymphocytes were highlighted on CD4 (A) and CD30 immunostaining (B)(original magnification ×200 and ×200). | ||
Immunostaining typically reveals positivity for CD3 and CD4, as well as for lymphocyte antigens CD2 and CD5.1 CD30 positivity in early stage MF rarely has been reported in the literature.3,4 Such cases appear histologically similarly to CD30‒negative cases in other respects. One study showed that the presence of CD30-positive lymphocytes does not alter the clinical course of MF.3 Another study found that, while epidermal CD30-postive lymphocytes had no prognostic relevance, an increased percentage of dermal CD30-positive cells was linked to a higher stage at diagnosis and worse overall prognosis.5 Pathogenesis underlying CD30 positivity in early MF is unknown. It is important to note that CD30-positive cells commonly are seen in lymphomatoid papulosis and anaplastic large cell lymphoma, as well as a variety of nonneoplastic conditions.3,6,7
- Smoller BR. Mycosis fungoides: what do/do not we know? J Cutan Pathol. 2008;35(suppl 2):35-39.
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
- Wu H, Telang GH, Lessin SR, et al. Mycosis fungoides with CD30-positive cells in the epidermis. Am J Dermatopathol. 2000;22:212-216.
- Ohtani T, Kikuchi K, Koizumi H, et al. A case of CD30+ large-cell transformation in a patient with unilesional patch-stage mycosis fungoides. Int J Dermatol. 2009;48:623-626.
- Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33:1860-1868.
- Resnik KS, Kutzner H. Of lymphocytes and cutaneous epithelium: keratoacanthomatous hyperplasia in CD30+ lymphoproliferative disorders and CD30+ cells associated with keratoacanthoma. Am J Dermatopathol. 2010;32:314-315.
- Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J Cutan Pathol. 2006;33(suppl 1):58-70.
- Smoller BR. Mycosis fungoides: what do/do not we know? J Cutan Pathol. 2008;35(suppl 2):35-39.
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063.
- Wu H, Telang GH, Lessin SR, et al. Mycosis fungoides with CD30-positive cells in the epidermis. Am J Dermatopathol. 2000;22:212-216.
- Ohtani T, Kikuchi K, Koizumi H, et al. A case of CD30+ large-cell transformation in a patient with unilesional patch-stage mycosis fungoides. Int J Dermatol. 2009;48:623-626.
- Edinger JT, Clark BZ, Pucevich BE, et al. CD30 expression and proliferative fraction in nontransformed mycosis fungoides. Am J Surg Pathol. 2009;33:1860-1868.
- Resnik KS, Kutzner H. Of lymphocytes and cutaneous epithelium: keratoacanthomatous hyperplasia in CD30+ lymphoproliferative disorders and CD30+ cells associated with keratoacanthoma. Am J Dermatopathol. 2010;32:314-315.
- Kempf W. CD30+ lymphoproliferative disorders: histopathology, differential diagnosis, new variants, and simulators. J Cutan Pathol. 2006;33(suppl 1):58-70.
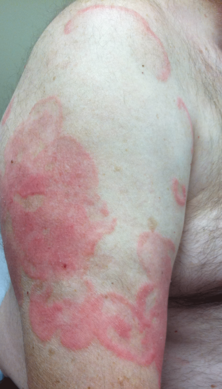
An otherwise healthy 62-year-old man presented for evaluation of multiple scaly erythematous plaques on the right upper arm and shoulder of 10 years’ duration. The patient reported a burning sensation but no exacerbation of the lesions upon sun exposure. He previously had been treated for a presumed clinical diagnosis of erythema annulare centrifugum but experienced only modest improvement with topical corticosteroids and tacrolimus ointment 0.1%. Previous trials of systemic antifungals also yielded minimal benefit.