User login
Surgical Specimens and Margins
We have attended grand rounds presentations at which students announce that Mohs micrographic surgery evaluates 100% of the surgical margin, whereas standard excision samples 1% to 2% of the margin; we have even fielded questions from neighbors who have come across this information on the internet.1-5 This statement describes a best-case scenario for Mohs surgery and a worst-case scenario for standard excision. We believe that it is important for clinicians to have a more nuanced understanding of how simple excisions are processed so that they can have pertinent discussions with patients, especially now that there is increasing access to personal health information along with increased agency in patient decision-making.
Margins for Mohs Surgery
Theoretically, Mohs surgery should sample all true surgical margins by complete circumferential, peripheral, and deep-margin assessment. Unfortunately, some sections are not cut full face—sections may not always sample a complete surface—when technicians make an error or lack expertise. Some sections may have small tissue folds or small gaps that prevent complete visualization. We estimate that the Mohs sections we review in consultation that are prepared by private practice Mohs surgeons in our communities visualize approximately 98% of surgical margins on average. Incomplete sections contribute to the rare tumor recurrences after Mohs surgery of approximately 2% to 3%.6
Standard Excision Margins
When we obtained the references cited in articles asserting that
Here is a simple example to show that more margin is accessed in some cases. Consider this hypothetical situation: If a tumor can be readily visualized grossly and housed entirely within an imaginary cuboid (rectangular) prism that is removed in an elliptical specimen with a length of 6 cm, a width of 2 cm, and a height of 1 cm (Figure), then standard sectioning assesses a greater margin.
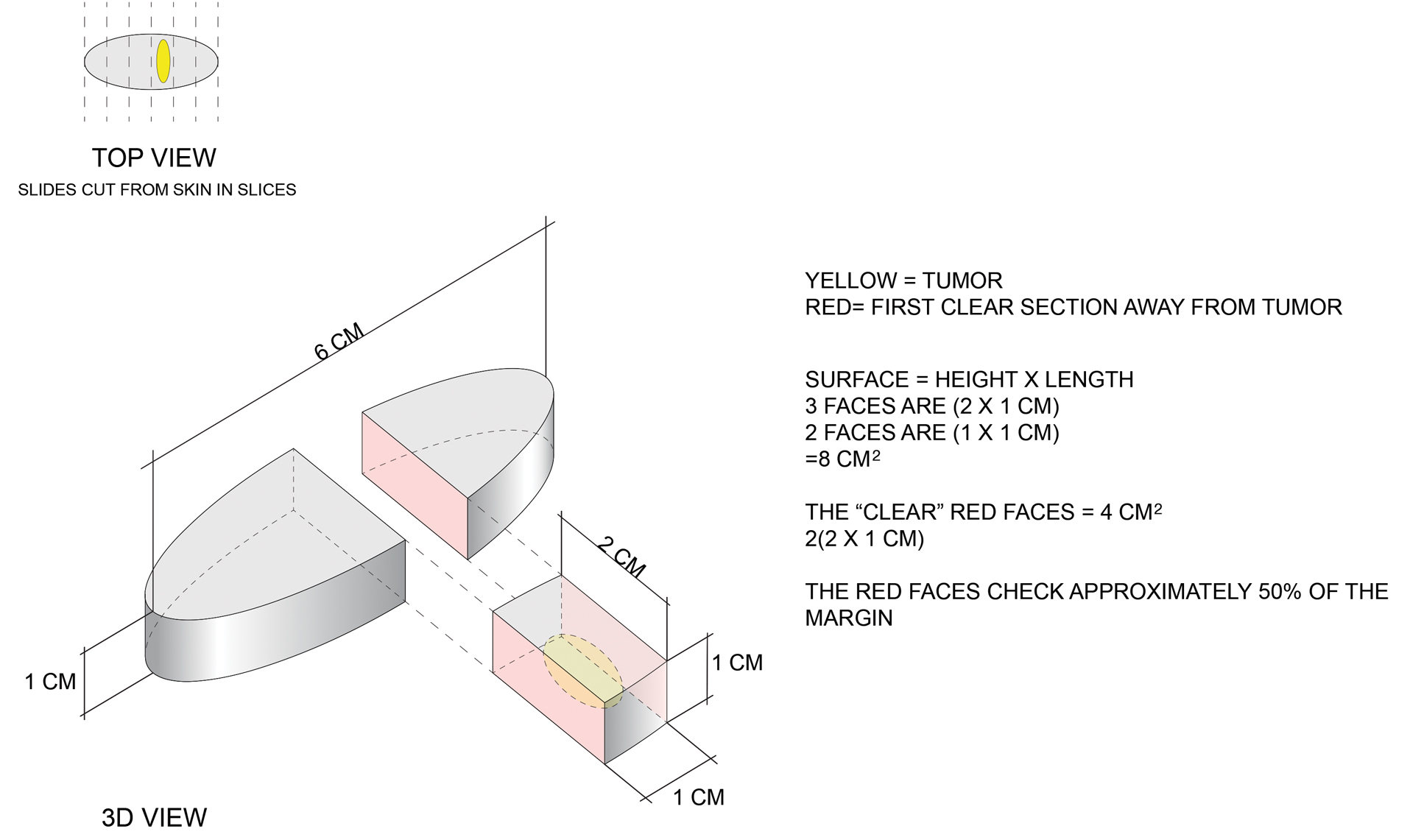
Bread-loaf sectioning would be expected to examine the complete surface of 2 sides (faces) of the cuboid. Assessing 2 of the 5 clinically relevant sides provides information for approximately 50% of the margins, as sections in the next parallel plane can be expected to be clear after the first clear section is identified. The clinically useful information is not limited to the sum of the widths of sections. Encountering a clear plane typically indicates that there will be no tumor in more distal parallel planes. Warne et al6 developed a formula that can accurately predict the percentage of the margin evaluated by proxy that considers the curvature of the ellipse.
Comparing Standard Excision and Mohs Surgery
Mohs surgery consistently results in the best outcomes, but standard excision is effective, too. Standard excision is relatively simple, requires less equipment, is less time consuming, and can provide good value when resources are finite. Data on recurrence of basal cell carcinoma after simple excision are limited, but the recurrence rate is reported to be approximately 3%.7,8 A meta-analysis found that the recurrence rate of basal cell carcinoma treated with standard excision was 0.4%, 1.6%, 2.6%, and 4% with 5-mm, 4-mm, 3-mm, and 2-mm surgical margins, respectively.9
Mohs surgery is the best, most effective, and most tissue-sparing technique for certain nonmelanoma skin cancers. This observation is reflected in guidelines worldwide.10 The adequacy of standard approaches to margin evaluation depends on the capabilities and focus of the laboratory team. Dermatopathologists often are called to the laboratory to decide which technique will be best for a particular case.11 Technicians are trained to take more sections in areas where abnormalities are seen, and some laboratories take photographs of specimens or provide sketches for correlation. Dermatopathologists also routinely request additional sections in areas where visible tumor extends close to surgical margins on microscopic examination.
It is not simply a matter of knowing how much of the margin is sampled but if the most pertinent areas are adequately sampled. Simple sectioning can work well and be cost effective. Many clinicians are unaware of how tissue processing can vary from laboratory to laboratory. There are no uniformly accepted standards for how tissue should be processed. Assiduous and thoughtful evaluation of specimens can affect results. As with any service, some laboratories provide more detailed and conscientious care while others focus more on immediate costs. Clinicians should understand how their specimens are processed by discussing margin evaluation with their dermatopathologist.
Final Thoughts
Used appropriately, Mohs surgery is an excellent technique that can provide outstanding results. Standard excision also has an important place in the dermatologist’s armamentarium and typically provides information about more than 1% to 2% of the margin. Understanding the techniques used to process specimens is critical to delivering the best possible care.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271. doi:10.1016/j.mayocp.2017.04.009
- Thomas RM, Amonette RA. Mohs micrographic surgery. Am Fam Physician. 1988;37:135-142.
- Buker JL, Amonette RA. Micrographic surgery. Clin Dermatol. 1992:10:309-315. doi:10.1016/0738-081x(92)90074-9
- Kauvar ANB. Mohs: the gold standard. The Skin Cancer Foundation website. Updated March 9, 2021. Accessed June 15, 2022. https://www.skincancer.org/treatment-resources/mohs-surgery/mohs-the-gold-standard/
- van Delft LCJ, Nelemans PJ, van Loo E, et al. The illusion of conventional histological resection margin control. Br J Dermatol. 2019;180:1240-1241. doi:10.1111/bjd.17510
- Warne MM, Klawonn MM, Brodell RT. Bread loaf sections provide useful information on more than 0.5% of surgical margins [published July 5, 2022]. Br J Dermatol. doi:10.1111/bjd.21740
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985-988. doi:10.1001/archderm.140.8.985
- Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766-1772. doi:10.1016/S0140-6736(04)17399-6
- Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a basal cell carcinoma: a meta-analysis of theliterature. Plast Reconstr Surg. 2010;126:1222-1231. doi:10.1097/PRS.0b013e3181ea450d
- Nahhas AF, Scarbrough CA, Trotter S. A review of the global guidelines on surgical margins for nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2017;10:37-46.
- Rapini RP. Comparison of methods for checking surgical margins. J Am Acad Dermatol. 1990; 23:288-294. doi:10.1016/0190-9622(90)70212-z
We have attended grand rounds presentations at which students announce that Mohs micrographic surgery evaluates 100% of the surgical margin, whereas standard excision samples 1% to 2% of the margin; we have even fielded questions from neighbors who have come across this information on the internet.1-5 This statement describes a best-case scenario for Mohs surgery and a worst-case scenario for standard excision. We believe that it is important for clinicians to have a more nuanced understanding of how simple excisions are processed so that they can have pertinent discussions with patients, especially now that there is increasing access to personal health information along with increased agency in patient decision-making.
Margins for Mohs Surgery
Theoretically, Mohs surgery should sample all true surgical margins by complete circumferential, peripheral, and deep-margin assessment. Unfortunately, some sections are not cut full face—sections may not always sample a complete surface—when technicians make an error or lack expertise. Some sections may have small tissue folds or small gaps that prevent complete visualization. We estimate that the Mohs sections we review in consultation that are prepared by private practice Mohs surgeons in our communities visualize approximately 98% of surgical margins on average. Incomplete sections contribute to the rare tumor recurrences after Mohs surgery of approximately 2% to 3%.6
Standard Excision Margins
When we obtained the references cited in articles asserting that
Here is a simple example to show that more margin is accessed in some cases. Consider this hypothetical situation: If a tumor can be readily visualized grossly and housed entirely within an imaginary cuboid (rectangular) prism that is removed in an elliptical specimen with a length of 6 cm, a width of 2 cm, and a height of 1 cm (Figure), then standard sectioning assesses a greater margin.

Bread-loaf sectioning would be expected to examine the complete surface of 2 sides (faces) of the cuboid. Assessing 2 of the 5 clinically relevant sides provides information for approximately 50% of the margins, as sections in the next parallel plane can be expected to be clear after the first clear section is identified. The clinically useful information is not limited to the sum of the widths of sections. Encountering a clear plane typically indicates that there will be no tumor in more distal parallel planes. Warne et al6 developed a formula that can accurately predict the percentage of the margin evaluated by proxy that considers the curvature of the ellipse.
Comparing Standard Excision and Mohs Surgery
Mohs surgery consistently results in the best outcomes, but standard excision is effective, too. Standard excision is relatively simple, requires less equipment, is less time consuming, and can provide good value when resources are finite. Data on recurrence of basal cell carcinoma after simple excision are limited, but the recurrence rate is reported to be approximately 3%.7,8 A meta-analysis found that the recurrence rate of basal cell carcinoma treated with standard excision was 0.4%, 1.6%, 2.6%, and 4% with 5-mm, 4-mm, 3-mm, and 2-mm surgical margins, respectively.9
Mohs surgery is the best, most effective, and most tissue-sparing technique for certain nonmelanoma skin cancers. This observation is reflected in guidelines worldwide.10 The adequacy of standard approaches to margin evaluation depends on the capabilities and focus of the laboratory team. Dermatopathologists often are called to the laboratory to decide which technique will be best for a particular case.11 Technicians are trained to take more sections in areas where abnormalities are seen, and some laboratories take photographs of specimens or provide sketches for correlation. Dermatopathologists also routinely request additional sections in areas where visible tumor extends close to surgical margins on microscopic examination.
It is not simply a matter of knowing how much of the margin is sampled but if the most pertinent areas are adequately sampled. Simple sectioning can work well and be cost effective. Many clinicians are unaware of how tissue processing can vary from laboratory to laboratory. There are no uniformly accepted standards for how tissue should be processed. Assiduous and thoughtful evaluation of specimens can affect results. As with any service, some laboratories provide more detailed and conscientious care while others focus more on immediate costs. Clinicians should understand how their specimens are processed by discussing margin evaluation with their dermatopathologist.
Final Thoughts
Used appropriately, Mohs surgery is an excellent technique that can provide outstanding results. Standard excision also has an important place in the dermatologist’s armamentarium and typically provides information about more than 1% to 2% of the margin. Understanding the techniques used to process specimens is critical to delivering the best possible care.
We have attended grand rounds presentations at which students announce that Mohs micrographic surgery evaluates 100% of the surgical margin, whereas standard excision samples 1% to 2% of the margin; we have even fielded questions from neighbors who have come across this information on the internet.1-5 This statement describes a best-case scenario for Mohs surgery and a worst-case scenario for standard excision. We believe that it is important for clinicians to have a more nuanced understanding of how simple excisions are processed so that they can have pertinent discussions with patients, especially now that there is increasing access to personal health information along with increased agency in patient decision-making.
Margins for Mohs Surgery
Theoretically, Mohs surgery should sample all true surgical margins by complete circumferential, peripheral, and deep-margin assessment. Unfortunately, some sections are not cut full face—sections may not always sample a complete surface—when technicians make an error or lack expertise. Some sections may have small tissue folds or small gaps that prevent complete visualization. We estimate that the Mohs sections we review in consultation that are prepared by private practice Mohs surgeons in our communities visualize approximately 98% of surgical margins on average. Incomplete sections contribute to the rare tumor recurrences after Mohs surgery of approximately 2% to 3%.6
Standard Excision Margins
When we obtained the references cited in articles asserting that
Here is a simple example to show that more margin is accessed in some cases. Consider this hypothetical situation: If a tumor can be readily visualized grossly and housed entirely within an imaginary cuboid (rectangular) prism that is removed in an elliptical specimen with a length of 6 cm, a width of 2 cm, and a height of 1 cm (Figure), then standard sectioning assesses a greater margin.

Bread-loaf sectioning would be expected to examine the complete surface of 2 sides (faces) of the cuboid. Assessing 2 of the 5 clinically relevant sides provides information for approximately 50% of the margins, as sections in the next parallel plane can be expected to be clear after the first clear section is identified. The clinically useful information is not limited to the sum of the widths of sections. Encountering a clear plane typically indicates that there will be no tumor in more distal parallel planes. Warne et al6 developed a formula that can accurately predict the percentage of the margin evaluated by proxy that considers the curvature of the ellipse.
Comparing Standard Excision and Mohs Surgery
Mohs surgery consistently results in the best outcomes, but standard excision is effective, too. Standard excision is relatively simple, requires less equipment, is less time consuming, and can provide good value when resources are finite. Data on recurrence of basal cell carcinoma after simple excision are limited, but the recurrence rate is reported to be approximately 3%.7,8 A meta-analysis found that the recurrence rate of basal cell carcinoma treated with standard excision was 0.4%, 1.6%, 2.6%, and 4% with 5-mm, 4-mm, 3-mm, and 2-mm surgical margins, respectively.9
Mohs surgery is the best, most effective, and most tissue-sparing technique for certain nonmelanoma skin cancers. This observation is reflected in guidelines worldwide.10 The adequacy of standard approaches to margin evaluation depends on the capabilities and focus of the laboratory team. Dermatopathologists often are called to the laboratory to decide which technique will be best for a particular case.11 Technicians are trained to take more sections in areas where abnormalities are seen, and some laboratories take photographs of specimens or provide sketches for correlation. Dermatopathologists also routinely request additional sections in areas where visible tumor extends close to surgical margins on microscopic examination.
It is not simply a matter of knowing how much of the margin is sampled but if the most pertinent areas are adequately sampled. Simple sectioning can work well and be cost effective. Many clinicians are unaware of how tissue processing can vary from laboratory to laboratory. There are no uniformly accepted standards for how tissue should be processed. Assiduous and thoughtful evaluation of specimens can affect results. As with any service, some laboratories provide more detailed and conscientious care while others focus more on immediate costs. Clinicians should understand how their specimens are processed by discussing margin evaluation with their dermatopathologist.
Final Thoughts
Used appropriately, Mohs surgery is an excellent technique that can provide outstanding results. Standard excision also has an important place in the dermatologist’s armamentarium and typically provides information about more than 1% to 2% of the margin. Understanding the techniques used to process specimens is critical to delivering the best possible care.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271. doi:10.1016/j.mayocp.2017.04.009
- Thomas RM, Amonette RA. Mohs micrographic surgery. Am Fam Physician. 1988;37:135-142.
- Buker JL, Amonette RA. Micrographic surgery. Clin Dermatol. 1992:10:309-315. doi:10.1016/0738-081x(92)90074-9
- Kauvar ANB. Mohs: the gold standard. The Skin Cancer Foundation website. Updated March 9, 2021. Accessed June 15, 2022. https://www.skincancer.org/treatment-resources/mohs-surgery/mohs-the-gold-standard/
- van Delft LCJ, Nelemans PJ, van Loo E, et al. The illusion of conventional histological resection margin control. Br J Dermatol. 2019;180:1240-1241. doi:10.1111/bjd.17510
- Warne MM, Klawonn MM, Brodell RT. Bread loaf sections provide useful information on more than 0.5% of surgical margins [published July 5, 2022]. Br J Dermatol. doi:10.1111/bjd.21740
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985-988. doi:10.1001/archderm.140.8.985
- Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766-1772. doi:10.1016/S0140-6736(04)17399-6
- Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a basal cell carcinoma: a meta-analysis of theliterature. Plast Reconstr Surg. 2010;126:1222-1231. doi:10.1097/PRS.0b013e3181ea450d
- Nahhas AF, Scarbrough CA, Trotter S. A review of the global guidelines on surgical margins for nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2017;10:37-46.
- Rapini RP. Comparison of methods for checking surgical margins. J Am Acad Dermatol. 1990; 23:288-294. doi:10.1016/0190-9622(90)70212-z
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271. doi:10.1016/j.mayocp.2017.04.009
- Thomas RM, Amonette RA. Mohs micrographic surgery. Am Fam Physician. 1988;37:135-142.
- Buker JL, Amonette RA. Micrographic surgery. Clin Dermatol. 1992:10:309-315. doi:10.1016/0738-081x(92)90074-9
- Kauvar ANB. Mohs: the gold standard. The Skin Cancer Foundation website. Updated March 9, 2021. Accessed June 15, 2022. https://www.skincancer.org/treatment-resources/mohs-surgery/mohs-the-gold-standard/
- van Delft LCJ, Nelemans PJ, van Loo E, et al. The illusion of conventional histological resection margin control. Br J Dermatol. 2019;180:1240-1241. doi:10.1111/bjd.17510
- Warne MM, Klawonn MM, Brodell RT. Bread loaf sections provide useful information on more than 0.5% of surgical margins [published July 5, 2022]. Br J Dermatol. doi:10.1111/bjd.21740
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985-988. doi:10.1001/archderm.140.8.985
- Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766-1772. doi:10.1016/S0140-6736(04)17399-6
- Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a basal cell carcinoma: a meta-analysis of theliterature. Plast Reconstr Surg. 2010;126:1222-1231. doi:10.1097/PRS.0b013e3181ea450d
- Nahhas AF, Scarbrough CA, Trotter S. A review of the global guidelines on surgical margins for nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2017;10:37-46.
- Rapini RP. Comparison of methods for checking surgical margins. J Am Acad Dermatol. 1990; 23:288-294. doi:10.1016/0190-9622(90)70212-z
Practice Points
- Margin analysis in simple excisions can provide useful information by proxy about more than the 1% of the margin often quoted in the literature.
- Simple excisions of uncomplicated keratinocytic carcinomas are associated with high cure rates.
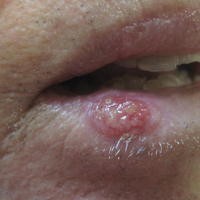
Eroded Plaque on the Lower Lip
The Diagnosis: Squamous Cell Carcinoma
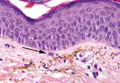
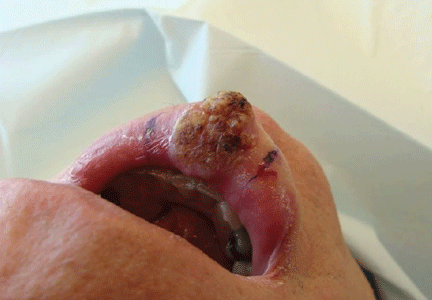
The initial clinical presentation suggested a diagnosis of herpes simplex labialis. The patient reported no response to topical acyclovir, and because the plaque persisted, a biopsy was performed. Pathology demonstrated squamous cell carcinoma (SCC) that was moderately well differentiated and invasive (Figure).

Approximately 38% of all oral SCCs in the United States occur on the lower lip and typically are solar-related cancers developing within the epidermis.1 Oral lesions initially may be asymptomatic and may not be of concern to the patient; however, it is important to recognize SCC early, as invasive lesions have the potential to metastasize. Some factors that increase the chance for the development of metastases include tumor size larger than 2 cm; location on the ear, lip, or other sites on the head and neck; and history of prior unsuccessful treatment.2 Any solitary ulcer, lump, wound, or lesion that will not heal and persists for more than 3 weeks should be regarded as cancer until proven otherwise. Although few oral SCCs are detected by clinicians at an early stage, diagnostic aids such as vital staining and molecular markers in tissues and saliva may be implemented.3 Toluidine blue is a simple, fast, and inexpensive technique that stains the nuclear material of malignant lesions, but not normal mucosa, and may be a worthwhile diagnostic adjunct to clinical inspection.4
Our patient presented with a lesion that clinically looked herpetic, though he reported no prodromal signs of tingling, burning, or pain before the occurrence of the lesion. Due to the persistence of the lesion and lack of response to treatment, a biopsy was indicated. The differential diagnoses include aphthous ulcers, which may occasionally extend on to the vermilion border of the lip and exhibit nondiagnostic histology.5 Bullous oral lichen planus is the least common variant of oral lichen planus, is unlikely to present as a solitary lesion, and is rarely seen on the lips. Histologically, the lesion demonstrated lichenoid inflammation.6 Solitary keratoacanthoma, though histologically similar to SCC, typically presents as a rapidly growing crateriform nodule without erosion or ulceration.7 The differential diagnoses are summarized in the Table.

The patient underwent wide excision with repair by mucosal advancement flap. He continues to be regularly seen in the clinic for monitoring of other skin cancers and is doing well. Clinicians encountering any wound or ulcer that does not show signs of healing should be wary of underlying malignancy and be prompted to perform a biopsy.
- Fehrenbach MJ. Extraoral and intraoral clinical assessment. In: Darby ML, Walsh MM, eds. Dental Hygiene: Theory and Practice. 4th ed. St Louis, MO: Elsevier; 2014:214-233.
- Hawrot A, Alam M, Ratner D. Squamous cell carcinoma. Curr Probl Dermatol. 2003;15:91-133.
- Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45:301-308.
- Chhabra N, Chhabra S, Sapra N. Diagnostic modalities for squamous cell carcinoma: an extensive review of literature considering toluidine blue as a useful adjunct. J Oral Maxillofac Surg. 2015;14:188-200.
- Porter SR, Scully C, Pedersen A. Recurrent aphthous stomatitis. Crit Rev Oral Biol Med. 2003;9:1499-1505.
- Bricker SL. Oral lichen planus: a review. Semin Dermatol. 1994;13:87-90.
- Cabrijan L, Lipozencic´ J, Batinac T, et al. Differences between keratoacanthoma and squamous cell carcinoma using TGF-alpha. Coll Antropol. 2013;37:147-150.
- Douglas GD, Couch RB. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970;104:289-295.
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:976-983.
- van Tuyll van Serooskerken AM, van Marion AM, de Zwart-Storm E, et al. Lichen planus with bullous manifestation on the lip. Int J Dermatol. 2007;46(suppl 3):25-26.
- Messadi DV, Younai F. Apthous ulcers. Dermatol Ther. 2010;23:281-290.
- Ko CJ. Keratoacanthoma: facts and controversies. Clin Dermatol. 2010;28:254-261.
The Diagnosis: Squamous Cell Carcinoma
The initial clinical presentation suggested a diagnosis of herpes simplex labialis. The patient reported no response to topical acyclovir, and because the plaque persisted, a biopsy was performed. Pathology demonstrated squamous cell carcinoma (SCC) that was moderately well differentiated and invasive (Figure).

Approximately 38% of all oral SCCs in the United States occur on the lower lip and typically are solar-related cancers developing within the epidermis.1 Oral lesions initially may be asymptomatic and may not be of concern to the patient; however, it is important to recognize SCC early, as invasive lesions have the potential to metastasize. Some factors that increase the chance for the development of metastases include tumor size larger than 2 cm; location on the ear, lip, or other sites on the head and neck; and history of prior unsuccessful treatment.2 Any solitary ulcer, lump, wound, or lesion that will not heal and persists for more than 3 weeks should be regarded as cancer until proven otherwise. Although few oral SCCs are detected by clinicians at an early stage, diagnostic aids such as vital staining and molecular markers in tissues and saliva may be implemented.3 Toluidine blue is a simple, fast, and inexpensive technique that stains the nuclear material of malignant lesions, but not normal mucosa, and may be a worthwhile diagnostic adjunct to clinical inspection.4
Our patient presented with a lesion that clinically looked herpetic, though he reported no prodromal signs of tingling, burning, or pain before the occurrence of the lesion. Due to the persistence of the lesion and lack of response to treatment, a biopsy was indicated. The differential diagnoses include aphthous ulcers, which may occasionally extend on to the vermilion border of the lip and exhibit nondiagnostic histology.5 Bullous oral lichen planus is the least common variant of oral lichen planus, is unlikely to present as a solitary lesion, and is rarely seen on the lips. Histologically, the lesion demonstrated lichenoid inflammation.6 Solitary keratoacanthoma, though histologically similar to SCC, typically presents as a rapidly growing crateriform nodule without erosion or ulceration.7 The differential diagnoses are summarized in the Table.

The patient underwent wide excision with repair by mucosal advancement flap. He continues to be regularly seen in the clinic for monitoring of other skin cancers and is doing well. Clinicians encountering any wound or ulcer that does not show signs of healing should be wary of underlying malignancy and be prompted to perform a biopsy.
The Diagnosis: Squamous Cell Carcinoma
The initial clinical presentation suggested a diagnosis of herpes simplex labialis. The patient reported no response to topical acyclovir, and because the plaque persisted, a biopsy was performed. Pathology demonstrated squamous cell carcinoma (SCC) that was moderately well differentiated and invasive (Figure).

Approximately 38% of all oral SCCs in the United States occur on the lower lip and typically are solar-related cancers developing within the epidermis.1 Oral lesions initially may be asymptomatic and may not be of concern to the patient; however, it is important to recognize SCC early, as invasive lesions have the potential to metastasize. Some factors that increase the chance for the development of metastases include tumor size larger than 2 cm; location on the ear, lip, or other sites on the head and neck; and history of prior unsuccessful treatment.2 Any solitary ulcer, lump, wound, or lesion that will not heal and persists for more than 3 weeks should be regarded as cancer until proven otherwise. Although few oral SCCs are detected by clinicians at an early stage, diagnostic aids such as vital staining and molecular markers in tissues and saliva may be implemented.3 Toluidine blue is a simple, fast, and inexpensive technique that stains the nuclear material of malignant lesions, but not normal mucosa, and may be a worthwhile diagnostic adjunct to clinical inspection.4
Our patient presented with a lesion that clinically looked herpetic, though he reported no prodromal signs of tingling, burning, or pain before the occurrence of the lesion. Due to the persistence of the lesion and lack of response to treatment, a biopsy was indicated. The differential diagnoses include aphthous ulcers, which may occasionally extend on to the vermilion border of the lip and exhibit nondiagnostic histology.5 Bullous oral lichen planus is the least common variant of oral lichen planus, is unlikely to present as a solitary lesion, and is rarely seen on the lips. Histologically, the lesion demonstrated lichenoid inflammation.6 Solitary keratoacanthoma, though histologically similar to SCC, typically presents as a rapidly growing crateriform nodule without erosion or ulceration.7 The differential diagnoses are summarized in the Table.

The patient underwent wide excision with repair by mucosal advancement flap. He continues to be regularly seen in the clinic for monitoring of other skin cancers and is doing well. Clinicians encountering any wound or ulcer that does not show signs of healing should be wary of underlying malignancy and be prompted to perform a biopsy.
- Fehrenbach MJ. Extraoral and intraoral clinical assessment. In: Darby ML, Walsh MM, eds. Dental Hygiene: Theory and Practice. 4th ed. St Louis, MO: Elsevier; 2014:214-233.
- Hawrot A, Alam M, Ratner D. Squamous cell carcinoma. Curr Probl Dermatol. 2003;15:91-133.
- Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45:301-308.
- Chhabra N, Chhabra S, Sapra N. Diagnostic modalities for squamous cell carcinoma: an extensive review of literature considering toluidine blue as a useful adjunct. J Oral Maxillofac Surg. 2015;14:188-200.
- Porter SR, Scully C, Pedersen A. Recurrent aphthous stomatitis. Crit Rev Oral Biol Med. 2003;9:1499-1505.
- Bricker SL. Oral lichen planus: a review. Semin Dermatol. 1994;13:87-90.
- Cabrijan L, Lipozencic´ J, Batinac T, et al. Differences between keratoacanthoma and squamous cell carcinoma using TGF-alpha. Coll Antropol. 2013;37:147-150.
- Douglas GD, Couch RB. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970;104:289-295.
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:976-983.
- van Tuyll van Serooskerken AM, van Marion AM, de Zwart-Storm E, et al. Lichen planus with bullous manifestation on the lip. Int J Dermatol. 2007;46(suppl 3):25-26.
- Messadi DV, Younai F. Apthous ulcers. Dermatol Ther. 2010;23:281-290.
- Ko CJ. Keratoacanthoma: facts and controversies. Clin Dermatol. 2010;28:254-261.
- Fehrenbach MJ. Extraoral and intraoral clinical assessment. In: Darby ML, Walsh MM, eds. Dental Hygiene: Theory and Practice. 4th ed. St Louis, MO: Elsevier; 2014:214-233.
- Hawrot A, Alam M, Ratner D. Squamous cell carcinoma. Curr Probl Dermatol. 2003;15:91-133.
- Scully C, Bagan J. Oral squamous cell carcinoma overview. Oral Oncol. 2009;45:301-308.
- Chhabra N, Chhabra S, Sapra N. Diagnostic modalities for squamous cell carcinoma: an extensive review of literature considering toluidine blue as a useful adjunct. J Oral Maxillofac Surg. 2015;14:188-200.
- Porter SR, Scully C, Pedersen A. Recurrent aphthous stomatitis. Crit Rev Oral Biol Med. 2003;9:1499-1505.
- Bricker SL. Oral lichen planus: a review. Semin Dermatol. 1994;13:87-90.
- Cabrijan L, Lipozencic´ J, Batinac T, et al. Differences between keratoacanthoma and squamous cell carcinoma using TGF-alpha. Coll Antropol. 2013;37:147-150.
- Douglas GD, Couch RB. A prospective study of chronic herpes simplex virus infection and recurrent herpes labialis in humans. J Immunol. 1970;104:289-295.
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:976-983.
- van Tuyll van Serooskerken AM, van Marion AM, de Zwart-Storm E, et al. Lichen planus with bullous manifestation on the lip. Int J Dermatol. 2007;46(suppl 3):25-26.
- Messadi DV, Younai F. Apthous ulcers. Dermatol Ther. 2010;23:281-290.
- Ko CJ. Keratoacanthoma: facts and controversies. Clin Dermatol. 2010;28:254-261.
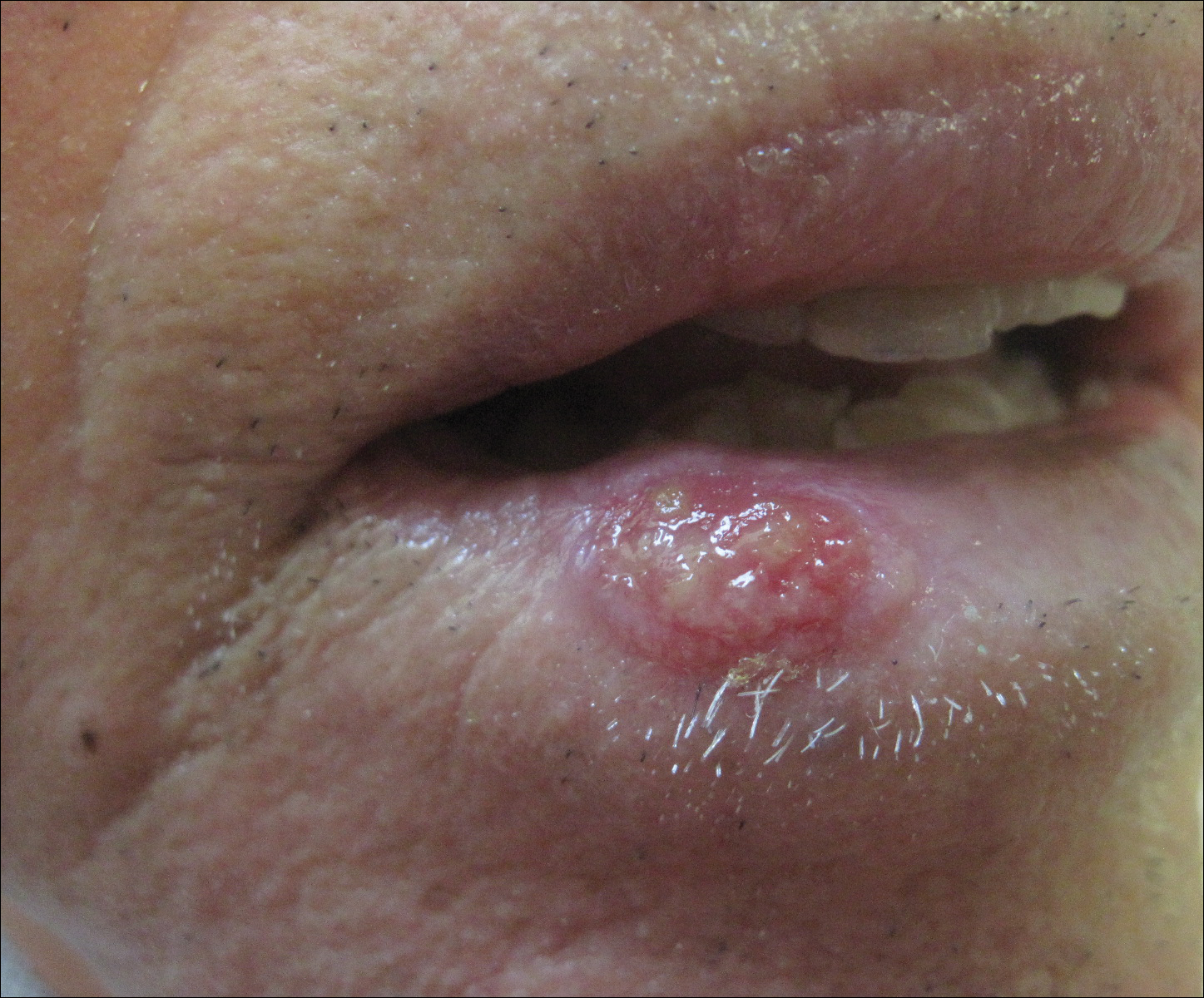
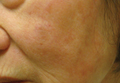
An 83-year-old man presented with a new-onset 1.2-cm eroded plaque on the vermilion border of the right lower lip that reportedly developed 2 weeks prior and was increasing in size. The plaque was moist and was composed of confluent glistening papules. Medical history was notable for the presence of both basal cell and squamous cell carcinomas.
Firm Plaques and Nodules Over the Body
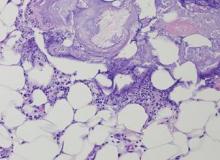
The Diagnosis: Pancreatic Panniculitis
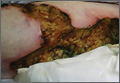
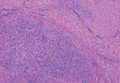
The biopsy specimen revealed necrosis of the panniculus with “ghost” cells (Figure). Calcification was encountered. Changes of vasculitis were not identified and fungal organisms were not noted. The histopathologic findings supported a diagnosis of pancreatic panniculitis.
Pancreatic panniculitis has been associated with pancreatitis, pancreatic carcinoma, pancreatic pseudocysts, congenital abnormalities of the pancreas, and drug-induced pancreatitis.1 Skin lesions may herald a diagnosis of pancreatic disease in an outpatient and should prompt thorough clinical evaluation when encountered in an outpatient setting. Our patient first developed tender nodules on the left shin 2 to 3 weeks prior to presentation. She reported that her initial nodules were flesh colored but then became erythematous and tender over 1 week’s time. The patient’s history was remarkable for ovarian cancer. She had been hospitalized 2 weeks prior to presentation for abdominal pain and ascites. Imaging studies revealed a cystic lesion in the head of the pancreas. The pancreas was traumatized during a peritoneal tap. Her nodules developed shortly thereafter and were distributed on the arms, legs, back, and abdomen.
Pancreatic tumors or inflammation are thought to trigger pancreatic panniculitis by releasing enzymes. Pancreatic enzymes such as lipase are thought to play a role in the development of pancreatic panniculitis by entering the vascular system and leading to fat necrosis.2,3 Biopsy reveals necrosis of adipocytes in the center of fat lobules.4 Ghost cells result from hydrolytic activity of enzymes on the fat cells followed by calcium deposition. A report indicates that fungal infection or gout also can cause changes that mimic pancreatic panniculitis.5
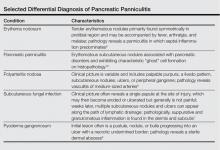
Other entities in the differential diagnosis can be excluded by biopsy. Polyarteritis nodosa is a vasculitis. Although panniculitis may be seen in polyarteritis as a secondary phenomenon, lesions are centered around blood vessels and often eventuate in ulceration.6 Subcutaneous fungal infection typically reveals organisms on periodic acid–Schiff stain.7 Pyoderma gangrenosum is associated with ulceration and a neutrophilic infiltrate that is often centered around a central pilosebaceous unit in developing lesions.8 Erythema nodosum is a panniculitis in which septal inflammation predominates.9 These differential diagnoses of pancreatic panniculitis are summarized in the Table.
Pancreatic panniculitis can be associated with acute arthritis and inflammation of periarticular fat.10 Treatment of pancreatic panniculitis is usually focused on the underlying pancreatic disease.11,12 Our patient benefited from analgesic therapy and her lesions improved on follow-up. Clinicians encountering a patient with new tender nodules should be prompted to perform a biopsy. When histopathologic evaluation reveals ghosted adipocytes, pancreatic panniculitis should be suspected and clinical evaluation undertaken.
1. Garcia-Romero D, Vanaclocha F. Pancreatic panniculitis. Dermatol Clin. 2008;26:465-470.
2. Berman B, Conteas C, Smith B, et al. Fatal pancreatitis presenting with subcutaneous fat necrosis. J Am Acad Dermatol. 1987;17:359-364.
3. Dhawan SS, Jimenez-Acosta F, Poppiti RJ Jr, et al. Subcutaneous fat necrosis associated with pancreatitis: histochemical and electron microscopic findings. Am J Gastroenterol. 1990;85:1025-1028.
4. Cannon JR, Pitha JV, Everett MA. Subcutaneous fat necrosis in pancreatitis. J Cutan Pathol. 1979;6:501-506.
5. Requena L, Sitthinamsuwan P, Santonja C, et al. Cutaneous and mucosal mucormycosis mimicking pancreatic panniculitis and gouty panniculitis. J Am Acad Dermatol. 2012;66:975-984.
6. Grattan CEH. Polyarteritis nodosa. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 1. 3rd ed. China: Elsevier Saunders; 2012:405-407.
7. Millett CR, Halpern AV, Heymann WR. Subcutaneous mycoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier Saunders; 2012:1266-1273.
8. Moschella SL, Davis MDP. Pyoderma gangrenosum. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 1. 3rd ed. China: Elsevier Saunders; 2012:427-431.
9. Patterson JW. Erythema nodosum. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier Saunders; 2012:1641-1645.
10. Patterson JW. Pancreatic panniculitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier Saunders; 2012:1649-1650.
11. Requena L, Sanchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
12. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
The Diagnosis: Pancreatic Panniculitis
The biopsy specimen revealed necrosis of the panniculus with “ghost” cells (Figure). Calcification was encountered. Changes of vasculitis were not identified and fungal organisms were not noted. The histopathologic findings supported a diagnosis of pancreatic panniculitis.
Pancreatic panniculitis has been associated with pancreatitis, pancreatic carcinoma, pancreatic pseudocysts, congenital abnormalities of the pancreas, and drug-induced pancreatitis.1 Skin lesions may herald a diagnosis of pancreatic disease in an outpatient and should prompt thorough clinical evaluation when encountered in an outpatient setting. Our patient first developed tender nodules on the left shin 2 to 3 weeks prior to presentation. She reported that her initial nodules were flesh colored but then became erythematous and tender over 1 week’s time. The patient’s history was remarkable for ovarian cancer. She had been hospitalized 2 weeks prior to presentation for abdominal pain and ascites. Imaging studies revealed a cystic lesion in the head of the pancreas. The pancreas was traumatized during a peritoneal tap. Her nodules developed shortly thereafter and were distributed on the arms, legs, back, and abdomen.
Pancreatic tumors or inflammation are thought to trigger pancreatic panniculitis by releasing enzymes. Pancreatic enzymes such as lipase are thought to play a role in the development of pancreatic panniculitis by entering the vascular system and leading to fat necrosis.2,3 Biopsy reveals necrosis of adipocytes in the center of fat lobules.4 Ghost cells result from hydrolytic activity of enzymes on the fat cells followed by calcium deposition. A report indicates that fungal infection or gout also can cause changes that mimic pancreatic panniculitis.5
Other entities in the differential diagnosis can be excluded by biopsy. Polyarteritis nodosa is a vasculitis. Although panniculitis may be seen in polyarteritis as a secondary phenomenon, lesions are centered around blood vessels and often eventuate in ulceration.6 Subcutaneous fungal infection typically reveals organisms on periodic acid–Schiff stain.7 Pyoderma gangrenosum is associated with ulceration and a neutrophilic infiltrate that is often centered around a central pilosebaceous unit in developing lesions.8 Erythema nodosum is a panniculitis in which septal inflammation predominates.9 These differential diagnoses of pancreatic panniculitis are summarized in the Table.
Pancreatic panniculitis can be associated with acute arthritis and inflammation of periarticular fat.10 Treatment of pancreatic panniculitis is usually focused on the underlying pancreatic disease.11,12 Our patient benefited from analgesic therapy and her lesions improved on follow-up. Clinicians encountering a patient with new tender nodules should be prompted to perform a biopsy. When histopathologic evaluation reveals ghosted adipocytes, pancreatic panniculitis should be suspected and clinical evaluation undertaken.
The Diagnosis: Pancreatic Panniculitis
The biopsy specimen revealed necrosis of the panniculus with “ghost” cells (Figure). Calcification was encountered. Changes of vasculitis were not identified and fungal organisms were not noted. The histopathologic findings supported a diagnosis of pancreatic panniculitis.
Pancreatic panniculitis has been associated with pancreatitis, pancreatic carcinoma, pancreatic pseudocysts, congenital abnormalities of the pancreas, and drug-induced pancreatitis.1 Skin lesions may herald a diagnosis of pancreatic disease in an outpatient and should prompt thorough clinical evaluation when encountered in an outpatient setting. Our patient first developed tender nodules on the left shin 2 to 3 weeks prior to presentation. She reported that her initial nodules were flesh colored but then became erythematous and tender over 1 week’s time. The patient’s history was remarkable for ovarian cancer. She had been hospitalized 2 weeks prior to presentation for abdominal pain and ascites. Imaging studies revealed a cystic lesion in the head of the pancreas. The pancreas was traumatized during a peritoneal tap. Her nodules developed shortly thereafter and were distributed on the arms, legs, back, and abdomen.
Pancreatic tumors or inflammation are thought to trigger pancreatic panniculitis by releasing enzymes. Pancreatic enzymes such as lipase are thought to play a role in the development of pancreatic panniculitis by entering the vascular system and leading to fat necrosis.2,3 Biopsy reveals necrosis of adipocytes in the center of fat lobules.4 Ghost cells result from hydrolytic activity of enzymes on the fat cells followed by calcium deposition. A report indicates that fungal infection or gout also can cause changes that mimic pancreatic panniculitis.5
Other entities in the differential diagnosis can be excluded by biopsy. Polyarteritis nodosa is a vasculitis. Although panniculitis may be seen in polyarteritis as a secondary phenomenon, lesions are centered around blood vessels and often eventuate in ulceration.6 Subcutaneous fungal infection typically reveals organisms on periodic acid–Schiff stain.7 Pyoderma gangrenosum is associated with ulceration and a neutrophilic infiltrate that is often centered around a central pilosebaceous unit in developing lesions.8 Erythema nodosum is a panniculitis in which septal inflammation predominates.9 These differential diagnoses of pancreatic panniculitis are summarized in the Table.
Pancreatic panniculitis can be associated with acute arthritis and inflammation of periarticular fat.10 Treatment of pancreatic panniculitis is usually focused on the underlying pancreatic disease.11,12 Our patient benefited from analgesic therapy and her lesions improved on follow-up. Clinicians encountering a patient with new tender nodules should be prompted to perform a biopsy. When histopathologic evaluation reveals ghosted adipocytes, pancreatic panniculitis should be suspected and clinical evaluation undertaken.
1. Garcia-Romero D, Vanaclocha F. Pancreatic panniculitis. Dermatol Clin. 2008;26:465-470.
2. Berman B, Conteas C, Smith B, et al. Fatal pancreatitis presenting with subcutaneous fat necrosis. J Am Acad Dermatol. 1987;17:359-364.
3. Dhawan SS, Jimenez-Acosta F, Poppiti RJ Jr, et al. Subcutaneous fat necrosis associated with pancreatitis: histochemical and electron microscopic findings. Am J Gastroenterol. 1990;85:1025-1028.
4. Cannon JR, Pitha JV, Everett MA. Subcutaneous fat necrosis in pancreatitis. J Cutan Pathol. 1979;6:501-506.
5. Requena L, Sitthinamsuwan P, Santonja C, et al. Cutaneous and mucosal mucormycosis mimicking pancreatic panniculitis and gouty panniculitis. J Am Acad Dermatol. 2012;66:975-984.
6. Grattan CEH. Polyarteritis nodosa. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 1. 3rd ed. China: Elsevier Saunders; 2012:405-407.
7. Millett CR, Halpern AV, Heymann WR. Subcutaneous mycoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier Saunders; 2012:1266-1273.
8. Moschella SL, Davis MDP. Pyoderma gangrenosum. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 1. 3rd ed. China: Elsevier Saunders; 2012:427-431.
9. Patterson JW. Erythema nodosum. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier Saunders; 2012:1641-1645.
10. Patterson JW. Pancreatic panniculitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier Saunders; 2012:1649-1650.
11. Requena L, Sanchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
12. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
1. Garcia-Romero D, Vanaclocha F. Pancreatic panniculitis. Dermatol Clin. 2008;26:465-470.
2. Berman B, Conteas C, Smith B, et al. Fatal pancreatitis presenting with subcutaneous fat necrosis. J Am Acad Dermatol. 1987;17:359-364.
3. Dhawan SS, Jimenez-Acosta F, Poppiti RJ Jr, et al. Subcutaneous fat necrosis associated with pancreatitis: histochemical and electron microscopic findings. Am J Gastroenterol. 1990;85:1025-1028.
4. Cannon JR, Pitha JV, Everett MA. Subcutaneous fat necrosis in pancreatitis. J Cutan Pathol. 1979;6:501-506.
5. Requena L, Sitthinamsuwan P, Santonja C, et al. Cutaneous and mucosal mucormycosis mimicking pancreatic panniculitis and gouty panniculitis. J Am Acad Dermatol. 2012;66:975-984.
6. Grattan CEH. Polyarteritis nodosa. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 1. 3rd ed. China: Elsevier Saunders; 2012:405-407.
7. Millett CR, Halpern AV, Heymann WR. Subcutaneous mycoses. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier Saunders; 2012:1266-1273.
8. Moschella SL, Davis MDP. Pyoderma gangrenosum. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 1. 3rd ed. China: Elsevier Saunders; 2012:427-431.
9. Patterson JW. Erythema nodosum. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier Saunders; 2012:1641-1645.
10. Patterson JW. Pancreatic panniculitis. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. Vol 2. 3rd ed. China: Elsevier Saunders; 2012:1649-1650.
11. Requena L, Sanchez Yus E. Panniculitis. part II. mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325-361.
12. Dahl PR, Su WP, Cullimore KC, et al. Pancreatic panniculitis. J Am Acad Dermatol. 1995;33:413-417.
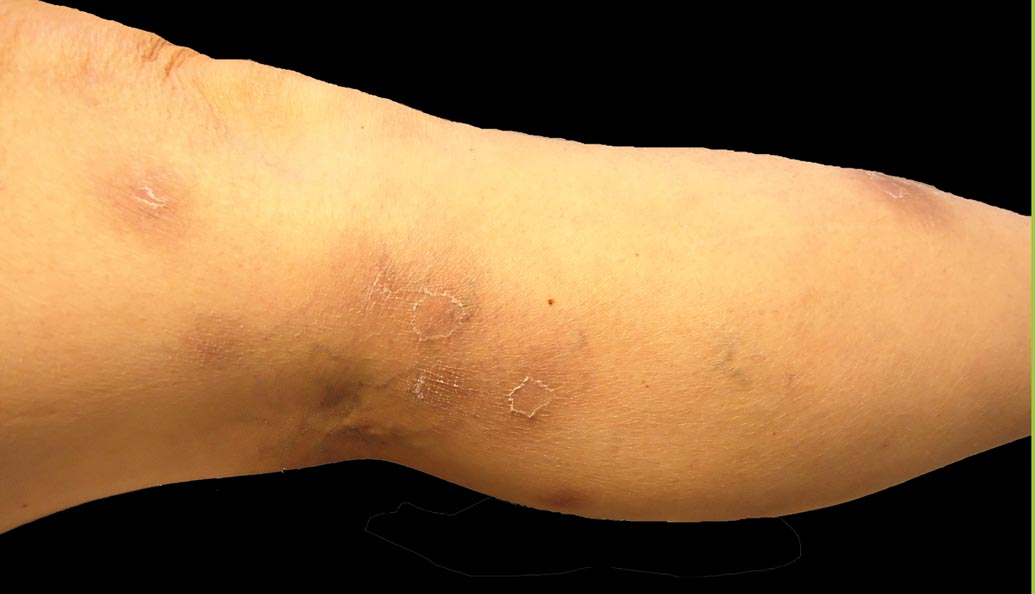
A 52-year-old woman presented with painful erythematous nodules of 2 weeks’ duration that began as a single lesion on the left shin and spread rapidly to involve the trunk, arms, and legs. A punch biopsy was performed. Pertinent history included a recent hospitalization for drainage of malignant ascites secondary to metastatic ovarian cancer. The lesions did not drain and were not pruritic. The patient did not have a history of fever, night sweats, nausea, headache, neurologic change, muscle aching, or recent weight loss.
Friable Nodule on the Back
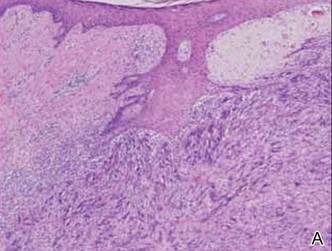
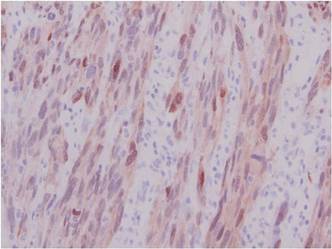
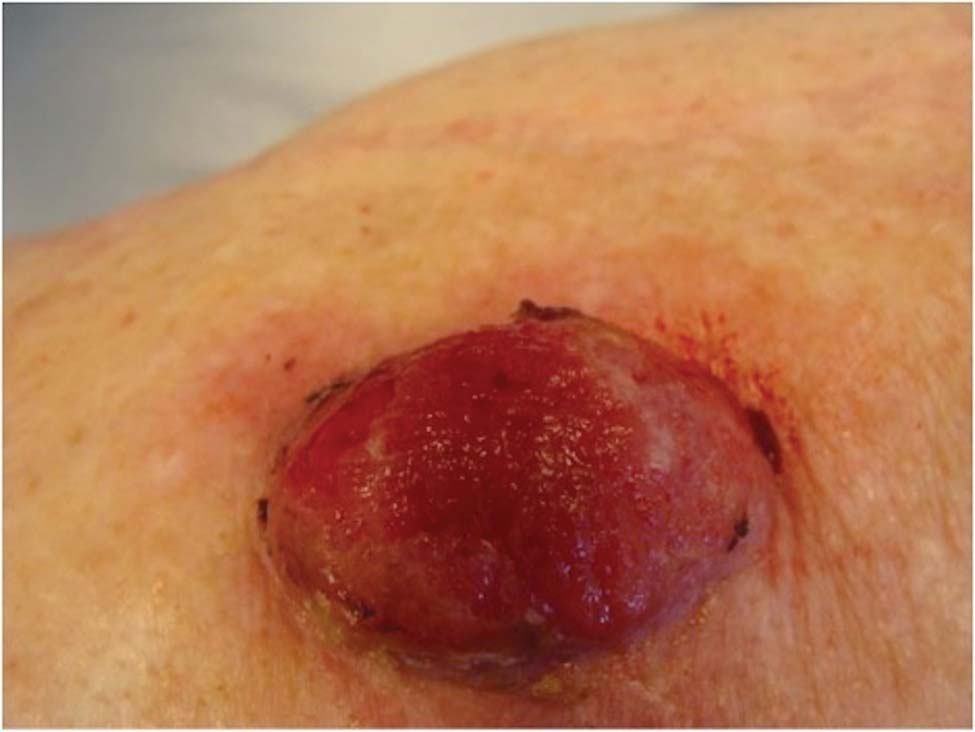
The Diagnosis: Spindle Cell Malignant Melanoma With Perineural Invasion
The incidence of melanoma has steadily increased in the United States since the 1930s when the incidence was reported at 1.0 per 100,000.1 In 1973 melanoma incidence was 6.8 per 100,000, and by 2007 the rate increased to 20.1 per 100,000.2 The American Cancer Society projects 73,870 new cases of melanoma in 2015, with melanoma as the fifth most common cancer in males and the seventh most common in females.3 Melanoma-related deaths are projected to be 9940. The lifetime probability of developing melanoma is 1 in 34 for males and 1 in 53 for females. Twice as many males are estimated to have melanoma-related deaths compared to females. The 5-year relative survival rate is 93% for white individuals and 75% for black individuals.3
Spindle cell melanoma is a rare variant of melanoma that was originally described by Conley et al4 in 1971. The lesion represents 2% to 4% of all melanomas and presents in older patients on sun-exposed skin as a pink or variably pigmented nodule measuring an average of 2 cm.5 Males are affected more than females, and prominent neural invasion is present in 30% to 100% of cases.6-10 Neural invasion can result in nerve palsies and/or dysesthesia. Because half of these lesions are amelanotic, they are often clinically misdiagnosed prior to biopsy.11
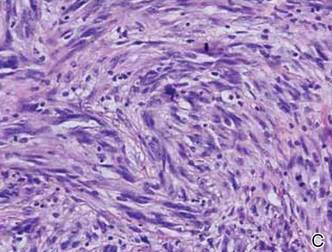
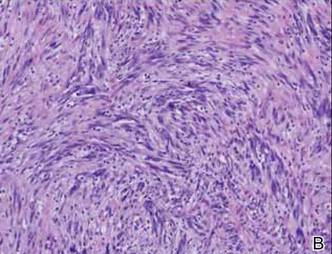
Histopathologically, these lesions can be quite challenging. Spindle cell melanoma histologically is an intradermal lesion composed of spindle cells distributed in bundles, fascicles, or nests, or singly between collagen fibers of the dermis. Other spindle tumors such as spindle cell squamous cell carcinoma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, angiosarcoma, and leiomyosarcoma have a similar presentation with hematoxylin and eosin stain. The diagnostic process for spindle cell tumors is greatly aided by immunohistologic analysis. Spindle cell melanoma usually shows immunoreactivity with S-100. Human melanoma black 45, CD57, and neuron-specific enolase usually do not stain, and CD68 has been demonstrated in a minority of cases.
The initial biopsy specimen in our case displayed a dense dermal atypical spindle cell proliferation with hematoxylin and eosin stain. The differential diagnosis of the proliferation included desmoplastic melanoma, spindle cell squamous cell carcinoma, leiomyosarcoma, angiosarcoma, and atypical fibroxanthoma. Immunostains were used to further study the lesional biopsy. Cytokeratin 34bE12, CK5/6, cerium ammonium molybdate 5.2, CK7, epithelial membrane antigen, CK18, high-molecular-weight cytokeratin, S-100, Melan-A, human melanoma black 45, smooth muscle actin, desmin, CD68, CD34, CD10, and p63 were studied. The atypical dermal spindle cells were positive for S-100. S-100 and Melan-A highlighted an increased number of single melanocytes at the dermoepidermal junction. Other markers were negative.
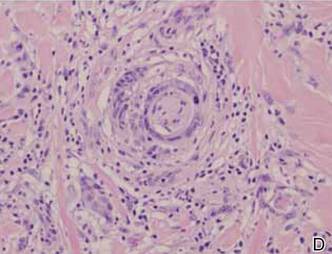
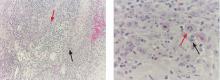
A 1.0-cm wide excision to fascia was performed. Routine hematoxylin and eosin stain showed atypical dermal spindle cells with perineural invasion (Figure 1). The malignant spindle cells were S-100 positive (Figure 2). The spindle cells were negative for high-molecular-weight cytokeratin, CK5/6, p63, Melan-A, A103, microphthalmia, and tyrosinase. These findings confirm melanoma of the spindle cell type.
|
The lesion was a Clark level V melanoma with a Breslow thickness of at least 12 mm. Perineural invasion was noted and the mitotic index was 7 cells/mm2. Vascular and lymphatic invasion was negative and ulceration was present. Tumor-infiltrating lymphocytes were brisk, resulting in a pathologic staging of pT4NXMX.
On initial histologic study with hematoxylin and eosin stain, spindle cell melanoma lesions tend to be generally quite thick. Manganoni et al12 reported in their series a Breslow thickness ranging from 2.1 to 12 mm with a mean of 5.8 mm.
Treatment in our case involved a second wide incision to fascia with an additional 2-cm margin. The role of sentinel lymph node biopsy (SLNB) remains undefined. Patients with spindle cell melanoma have a lower frequency of positive sentinel lymph nodes than nondesmoplastic melanomas.13 As such, the need to perform SLNB has not been determined.13-15 Our patient had a history of breast cancer. The lesion appeared on the left side of the back and she pre-viously had a complete axillary lymphadenectomy on the left axillae. She declined SLNB. A systematic workup by the oncology service was negative. Continued follow-up has revealed no recurrent disease and recent workup was negative for metastatic disease.
Many studies report the increased incidence of local recurrence after excision for spindle cell melanoma as compared to non–spindle cell melanoma,16-18 which is likely related to perineural invasion as in our patient.
Spindle cell melanoma is a rare tumor that is often amelanotic and difficult to diagnose clinically. Routine hematoxylin and eosin staining shows a dermal spindle cell tumor. Immunohistochemical study is of great aid in defining the tumor. The clinician and pathologist must work together to correctly diagnose and treat this lesion.
1. Mikkilineni R, Weinstock MA. Epidemiology. In: Sober AJ, Haluska FG, eds. Atlas of Clinical Oncology: Skin Cancer. London, England: BC Decker; 2001:1-15.
2. Rigel D. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29:204-209.
3. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed February 10, 2015.
4. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of malignant melanoma. Cancer. 1971;28:914-936.
5. Repertinger SK, Teruya B, Sarma DP. Common spindle cell malignant neoplasms of the skin: differential diagnosis and review of the literature. Internet J Dermatol. 2009;7. https://ispub.com/IJD/7/2/11747. Accessed February 10, 2015.
6. Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradial. 2004;25:5-11.
7. Cruz J, Reis-Filho JS, Lopes JM. Malignant peripheral nerve sheath tumor-like primary cutaneous melanoma. J Clin Pathol. 2004;57:218-220.
8. Tsao H, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. Semin Cutan Med Surg. 1997;16:45-47.
9. Kossard S, Doherty E, Murray E. Neurotropic melanoma. a variant of desmoplastic melanoma. Arch Dermatol. 1997;7:907-912.
10. Bruijn JA, Salasche S, Sober AJ, et al. Desmoplastic melanoma: clinicopathologic aspects of six cases. Dermatology. 1992;185:3-8.
11. Jesitus J. Desmoplastic melanoma. Dermatology Times. March 2009:1-2.
12. Manganoni AM, Farisoglio C, Bassissi S, et al. Desmoplastic melanoma: report of 5 cases. Dermatol Res Pract. 2009;2009:679010.
13. Gyorki DE, Busam K, Panageas K, et al. Sentinel lymph node biopsy for patients with desmoplastic melanoma. Ann Surg Oncol. 2003;10:403-407.
14. Livestro DP, Muzikansky A, Kaine EM, et al. of desmoplastic melanoma: a case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739-6746.
15. Pawlik TM, Ross MI, Prieto VG, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106:900-906.
16. Smithers HM, McLeod GR, Little JH. Desmoplastic melanoma: patterns of recurrence. World J Surg. 1992;16:186-190.
17. McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 2004;36:445-451.
18. Bruijn JA, Mihm MC Jr, Barnhill RL. Desmoplastic melanoma. Histopathology. 1992;20:197-205.
The Diagnosis: Spindle Cell Malignant Melanoma With Perineural Invasion
The incidence of melanoma has steadily increased in the United States since the 1930s when the incidence was reported at 1.0 per 100,000.1 In 1973 melanoma incidence was 6.8 per 100,000, and by 2007 the rate increased to 20.1 per 100,000.2 The American Cancer Society projects 73,870 new cases of melanoma in 2015, with melanoma as the fifth most common cancer in males and the seventh most common in females.3 Melanoma-related deaths are projected to be 9940. The lifetime probability of developing melanoma is 1 in 34 for males and 1 in 53 for females. Twice as many males are estimated to have melanoma-related deaths compared to females. The 5-year relative survival rate is 93% for white individuals and 75% for black individuals.3
Spindle cell melanoma is a rare variant of melanoma that was originally described by Conley et al4 in 1971. The lesion represents 2% to 4% of all melanomas and presents in older patients on sun-exposed skin as a pink or variably pigmented nodule measuring an average of 2 cm.5 Males are affected more than females, and prominent neural invasion is present in 30% to 100% of cases.6-10 Neural invasion can result in nerve palsies and/or dysesthesia. Because half of these lesions are amelanotic, they are often clinically misdiagnosed prior to biopsy.11
Histopathologically, these lesions can be quite challenging. Spindle cell melanoma histologically is an intradermal lesion composed of spindle cells distributed in bundles, fascicles, or nests, or singly between collagen fibers of the dermis. Other spindle tumors such as spindle cell squamous cell carcinoma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, angiosarcoma, and leiomyosarcoma have a similar presentation with hematoxylin and eosin stain. The diagnostic process for spindle cell tumors is greatly aided by immunohistologic analysis. Spindle cell melanoma usually shows immunoreactivity with S-100. Human melanoma black 45, CD57, and neuron-specific enolase usually do not stain, and CD68 has been demonstrated in a minority of cases.
The initial biopsy specimen in our case displayed a dense dermal atypical spindle cell proliferation with hematoxylin and eosin stain. The differential diagnosis of the proliferation included desmoplastic melanoma, spindle cell squamous cell carcinoma, leiomyosarcoma, angiosarcoma, and atypical fibroxanthoma. Immunostains were used to further study the lesional biopsy. Cytokeratin 34bE12, CK5/6, cerium ammonium molybdate 5.2, CK7, epithelial membrane antigen, CK18, high-molecular-weight cytokeratin, S-100, Melan-A, human melanoma black 45, smooth muscle actin, desmin, CD68, CD34, CD10, and p63 were studied. The atypical dermal spindle cells were positive for S-100. S-100 and Melan-A highlighted an increased number of single melanocytes at the dermoepidermal junction. Other markers were negative.
A 1.0-cm wide excision to fascia was performed. Routine hematoxylin and eosin stain showed atypical dermal spindle cells with perineural invasion (Figure 1). The malignant spindle cells were S-100 positive (Figure 2). The spindle cells were negative for high-molecular-weight cytokeratin, CK5/6, p63, Melan-A, A103, microphthalmia, and tyrosinase. These findings confirm melanoma of the spindle cell type.
|
The lesion was a Clark level V melanoma with a Breslow thickness of at least 12 mm. Perineural invasion was noted and the mitotic index was 7 cells/mm2. Vascular and lymphatic invasion was negative and ulceration was present. Tumor-infiltrating lymphocytes were brisk, resulting in a pathologic staging of pT4NXMX.
On initial histologic study with hematoxylin and eosin stain, spindle cell melanoma lesions tend to be generally quite thick. Manganoni et al12 reported in their series a Breslow thickness ranging from 2.1 to 12 mm with a mean of 5.8 mm.
Treatment in our case involved a second wide incision to fascia with an additional 2-cm margin. The role of sentinel lymph node biopsy (SLNB) remains undefined. Patients with spindle cell melanoma have a lower frequency of positive sentinel lymph nodes than nondesmoplastic melanomas.13 As such, the need to perform SLNB has not been determined.13-15 Our patient had a history of breast cancer. The lesion appeared on the left side of the back and she pre-viously had a complete axillary lymphadenectomy on the left axillae. She declined SLNB. A systematic workup by the oncology service was negative. Continued follow-up has revealed no recurrent disease and recent workup was negative for metastatic disease.
Many studies report the increased incidence of local recurrence after excision for spindle cell melanoma as compared to non–spindle cell melanoma,16-18 which is likely related to perineural invasion as in our patient.
Spindle cell melanoma is a rare tumor that is often amelanotic and difficult to diagnose clinically. Routine hematoxylin and eosin staining shows a dermal spindle cell tumor. Immunohistochemical study is of great aid in defining the tumor. The clinician and pathologist must work together to correctly diagnose and treat this lesion.
The Diagnosis: Spindle Cell Malignant Melanoma With Perineural Invasion
The incidence of melanoma has steadily increased in the United States since the 1930s when the incidence was reported at 1.0 per 100,000.1 In 1973 melanoma incidence was 6.8 per 100,000, and by 2007 the rate increased to 20.1 per 100,000.2 The American Cancer Society projects 73,870 new cases of melanoma in 2015, with melanoma as the fifth most common cancer in males and the seventh most common in females.3 Melanoma-related deaths are projected to be 9940. The lifetime probability of developing melanoma is 1 in 34 for males and 1 in 53 for females. Twice as many males are estimated to have melanoma-related deaths compared to females. The 5-year relative survival rate is 93% for white individuals and 75% for black individuals.3
Spindle cell melanoma is a rare variant of melanoma that was originally described by Conley et al4 in 1971. The lesion represents 2% to 4% of all melanomas and presents in older patients on sun-exposed skin as a pink or variably pigmented nodule measuring an average of 2 cm.5 Males are affected more than females, and prominent neural invasion is present in 30% to 100% of cases.6-10 Neural invasion can result in nerve palsies and/or dysesthesia. Because half of these lesions are amelanotic, they are often clinically misdiagnosed prior to biopsy.11
Histopathologically, these lesions can be quite challenging. Spindle cell melanoma histologically is an intradermal lesion composed of spindle cells distributed in bundles, fascicles, or nests, or singly between collagen fibers of the dermis. Other spindle tumors such as spindle cell squamous cell carcinoma, atypical fibroxanthoma, dermatofibrosarcoma protuberans, angiosarcoma, and leiomyosarcoma have a similar presentation with hematoxylin and eosin stain. The diagnostic process for spindle cell tumors is greatly aided by immunohistologic analysis. Spindle cell melanoma usually shows immunoreactivity with S-100. Human melanoma black 45, CD57, and neuron-specific enolase usually do not stain, and CD68 has been demonstrated in a minority of cases.
The initial biopsy specimen in our case displayed a dense dermal atypical spindle cell proliferation with hematoxylin and eosin stain. The differential diagnosis of the proliferation included desmoplastic melanoma, spindle cell squamous cell carcinoma, leiomyosarcoma, angiosarcoma, and atypical fibroxanthoma. Immunostains were used to further study the lesional biopsy. Cytokeratin 34bE12, CK5/6, cerium ammonium molybdate 5.2, CK7, epithelial membrane antigen, CK18, high-molecular-weight cytokeratin, S-100, Melan-A, human melanoma black 45, smooth muscle actin, desmin, CD68, CD34, CD10, and p63 were studied. The atypical dermal spindle cells were positive for S-100. S-100 and Melan-A highlighted an increased number of single melanocytes at the dermoepidermal junction. Other markers were negative.
A 1.0-cm wide excision to fascia was performed. Routine hematoxylin and eosin stain showed atypical dermal spindle cells with perineural invasion (Figure 1). The malignant spindle cells were S-100 positive (Figure 2). The spindle cells were negative for high-molecular-weight cytokeratin, CK5/6, p63, Melan-A, A103, microphthalmia, and tyrosinase. These findings confirm melanoma of the spindle cell type.
|
The lesion was a Clark level V melanoma with a Breslow thickness of at least 12 mm. Perineural invasion was noted and the mitotic index was 7 cells/mm2. Vascular and lymphatic invasion was negative and ulceration was present. Tumor-infiltrating lymphocytes were brisk, resulting in a pathologic staging of pT4NXMX.
On initial histologic study with hematoxylin and eosin stain, spindle cell melanoma lesions tend to be generally quite thick. Manganoni et al12 reported in their series a Breslow thickness ranging from 2.1 to 12 mm with a mean of 5.8 mm.
Treatment in our case involved a second wide incision to fascia with an additional 2-cm margin. The role of sentinel lymph node biopsy (SLNB) remains undefined. Patients with spindle cell melanoma have a lower frequency of positive sentinel lymph nodes than nondesmoplastic melanomas.13 As such, the need to perform SLNB has not been determined.13-15 Our patient had a history of breast cancer. The lesion appeared on the left side of the back and she pre-viously had a complete axillary lymphadenectomy on the left axillae. She declined SLNB. A systematic workup by the oncology service was negative. Continued follow-up has revealed no recurrent disease and recent workup was negative for metastatic disease.
Many studies report the increased incidence of local recurrence after excision for spindle cell melanoma as compared to non–spindle cell melanoma,16-18 which is likely related to perineural invasion as in our patient.
Spindle cell melanoma is a rare tumor that is often amelanotic and difficult to diagnose clinically. Routine hematoxylin and eosin staining shows a dermal spindle cell tumor. Immunohistochemical study is of great aid in defining the tumor. The clinician and pathologist must work together to correctly diagnose and treat this lesion.
1. Mikkilineni R, Weinstock MA. Epidemiology. In: Sober AJ, Haluska FG, eds. Atlas of Clinical Oncology: Skin Cancer. London, England: BC Decker; 2001:1-15.
2. Rigel D. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29:204-209.
3. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed February 10, 2015.
4. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of malignant melanoma. Cancer. 1971;28:914-936.
5. Repertinger SK, Teruya B, Sarma DP. Common spindle cell malignant neoplasms of the skin: differential diagnosis and review of the literature. Internet J Dermatol. 2009;7. https://ispub.com/IJD/7/2/11747. Accessed February 10, 2015.
6. Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradial. 2004;25:5-11.
7. Cruz J, Reis-Filho JS, Lopes JM. Malignant peripheral nerve sheath tumor-like primary cutaneous melanoma. J Clin Pathol. 2004;57:218-220.
8. Tsao H, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. Semin Cutan Med Surg. 1997;16:45-47.
9. Kossard S, Doherty E, Murray E. Neurotropic melanoma. a variant of desmoplastic melanoma. Arch Dermatol. 1997;7:907-912.
10. Bruijn JA, Salasche S, Sober AJ, et al. Desmoplastic melanoma: clinicopathologic aspects of six cases. Dermatology. 1992;185:3-8.
11. Jesitus J. Desmoplastic melanoma. Dermatology Times. March 2009:1-2.
12. Manganoni AM, Farisoglio C, Bassissi S, et al. Desmoplastic melanoma: report of 5 cases. Dermatol Res Pract. 2009;2009:679010.
13. Gyorki DE, Busam K, Panageas K, et al. Sentinel lymph node biopsy for patients with desmoplastic melanoma. Ann Surg Oncol. 2003;10:403-407.
14. Livestro DP, Muzikansky A, Kaine EM, et al. of desmoplastic melanoma: a case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739-6746.
15. Pawlik TM, Ross MI, Prieto VG, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106:900-906.
16. Smithers HM, McLeod GR, Little JH. Desmoplastic melanoma: patterns of recurrence. World J Surg. 1992;16:186-190.
17. McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 2004;36:445-451.
18. Bruijn JA, Mihm MC Jr, Barnhill RL. Desmoplastic melanoma. Histopathology. 1992;20:197-205.
1. Mikkilineni R, Weinstock MA. Epidemiology. In: Sober AJ, Haluska FG, eds. Atlas of Clinical Oncology: Skin Cancer. London, England: BC Decker; 2001:1-15.
2. Rigel D. Epidemiology of melanoma. Semin Cutan Med Surg. 2010;29:204-209.
3. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. Accessed February 10, 2015.
4. Conley J, Lattes R, Orr W. Desmoplastic malignant melanoma (a rare variant of malignant melanoma. Cancer. 1971;28:914-936.
5. Repertinger SK, Teruya B, Sarma DP. Common spindle cell malignant neoplasms of the skin: differential diagnosis and review of the literature. Internet J Dermatol. 2009;7. https://ispub.com/IJD/7/2/11747. Accessed February 10, 2015.
6. Chang PC, Fischbein NJ, McCalmont TH, et al. Perineural spread of malignant melanoma of the head and neck: clinical and imaging features. AJNR Am J Neuroradial. 2004;25:5-11.
7. Cruz J, Reis-Filho JS, Lopes JM. Malignant peripheral nerve sheath tumor-like primary cutaneous melanoma. J Clin Pathol. 2004;57:218-220.
8. Tsao H, Sober AJ, Barnhill RL. Desmoplastic neurotropic melanoma. Semin Cutan Med Surg. 1997;16:45-47.
9. Kossard S, Doherty E, Murray E. Neurotropic melanoma. a variant of desmoplastic melanoma. Arch Dermatol. 1997;7:907-912.
10. Bruijn JA, Salasche S, Sober AJ, et al. Desmoplastic melanoma: clinicopathologic aspects of six cases. Dermatology. 1992;185:3-8.
11. Jesitus J. Desmoplastic melanoma. Dermatology Times. March 2009:1-2.
12. Manganoni AM, Farisoglio C, Bassissi S, et al. Desmoplastic melanoma: report of 5 cases. Dermatol Res Pract. 2009;2009:679010.
13. Gyorki DE, Busam K, Panageas K, et al. Sentinel lymph node biopsy for patients with desmoplastic melanoma. Ann Surg Oncol. 2003;10:403-407.
14. Livestro DP, Muzikansky A, Kaine EM, et al. of desmoplastic melanoma: a case-control comparison with other melanomas. J Clin Oncol. 2005;23:6739-6746.
15. Pawlik TM, Ross MI, Prieto VG, et al. Assessment of the role of sentinel lymph node biopsy for primary cutaneous desmoplastic melanoma. Cancer. 2006;106:900-906.
16. Smithers HM, McLeod GR, Little JH. Desmoplastic melanoma: patterns of recurrence. World J Surg. 1992;16:186-190.
17. McCarthy SW, Scolyer RA, Palmer AA. Desmoplastic melanoma: a diagnostic trap for the unwary. Pathology. 2004;36:445-451.
18. Bruijn JA, Mihm MC Jr, Barnhill RL. Desmoplastic melanoma. Histopathology. 1992;20:197-205.
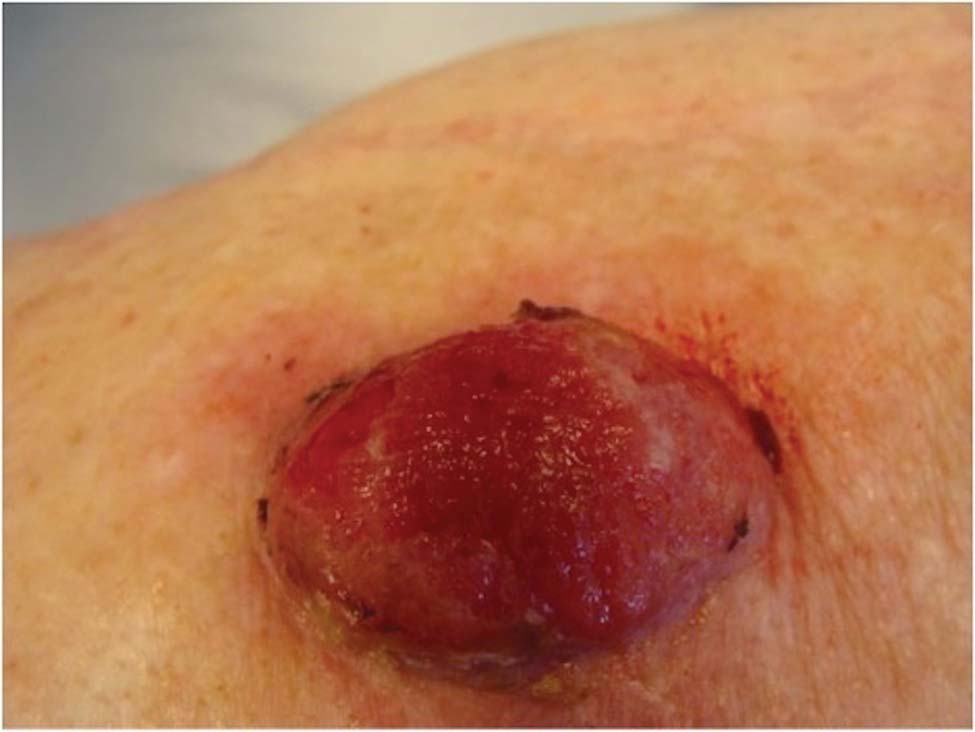
A 78-year-old woman presented with a large friable, sharply demarcated nodule of 3 months’ duration on the left side of the back. The lesion occasionally bled but was otherwise asymptomatic. There was no perilesional paresthesia. The patient’s medical history included hypertension, depression, chronic obstructive pulmonary disease, breast cancer, osteoporosis, and aortic valve disease.
Localized Argyria With Pseudo-ochronosis
Localized cutaneous argyria often presents as asymptomatic black or blue-gray pigmented macules in areas of the skin exposed to silver-containing compounds.1 Silver may enter the skin by traumatic implantation or absorption via eccrine sweat glands.2 Our patient witnessed a gun fight several years ago while on a mission trip and sustained multiple shrapnel wounds.
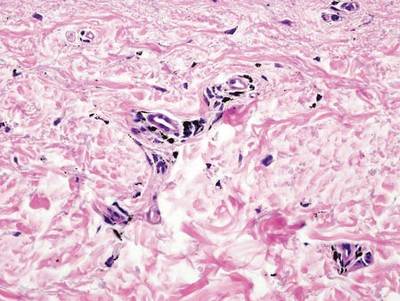
As in our patient, hyperpigmentation may appear years following initial exposure. Over time, incident light reduces colorless silver salts and compounds to black elemental silver.3 It also has been suggested that metallic silver granules stimulate tyrosine kinase activity, leading to locally increased melanin production.4 Together, these processes result in the clinical appearance of a blue-black macule. Despite its long-standing association with silver, this appearance also has been noted with deposition of other metals.5 Histologically, metal deposits can be seen as black granules surrounding eccrine glands, blood vessels, and elastic fibers on higher magnification.6 Granules also may be found in sebaceous glands and arrector pili muscle fibers. These findings do not distinguish from generalized argyria due to increased serum silver levels; however, some cases of localized cutaneous argyria have demonstrated spheroid black globules with surrounding collagen necrosis,1 which have not been reported with generalized disease. Localized cutaneous argyria also may be associated with ocher pigmentation of thickened collagen fibers, resembling changes typically found in alkaptonuria, an inherited deficiency of homogentisic acid oxidase (an enzyme involved in tyrosine metabolism).7 The resulting buildup of metabolic intermediates leads to ochronosis, a deposition of ocher-pigmented intermediates in connective tissue throughout the body. In the skin, ocher pigmentation occurs in elastic fibers of the reticular dermis.1 Grossly, these changes result in a blue-gray discoloration of the skin due to a light-scattering phenomenon known as the Tyndall effect. Exogenous ochronosis also can occur, most commonly from the topical application of hydroquinone or other skin-lightening compounds.1,5 Ocher pigmentation occurring in the setting of localized cutaneous argyria is referred to as pseudo-ochronosis, a finding first described by Robinson-Bostom et al.1 The etiology of this condition is poorly understood, but Robinson-Bostom et al1 noted the appearance of dark metal granules surrounding collagen bundles and hypothesized that metal aggregates surrounding collagen bundles in pseudo-ochronosis cause a homogenized appearance under light microscopy. Yellow-brown, swollen, homogenized collagen bundles can be visualized in the reticular dermis with surrounding deposition of metal granules (Figures 1 and 2).1 Typical patterns of granule deposition in localized argyria also are present.
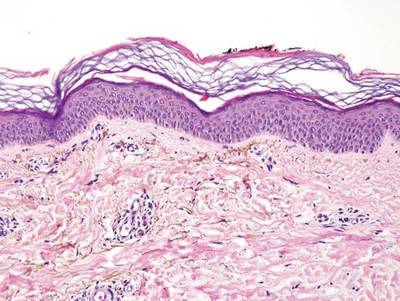

A blue nevus is a collection of proliferating dermal melanocytes. Many histologic subtypes exist and there may be extensive variability in the extent of sclerosis, cellular architecture, and tissue cellularity between each variant.8 Blue nevi commonly present as blue-black hyperpigmentation in the dermis and subcutaneous tissue.9 Histologically, they are characterized by slender, bipolar, dendritic melanocytes in a sclerotic stroma (Figure 3).8 Melanocytes are highly pigmented and contain small monomorphic nuclei. Lesions are relatively homogenous and typically are restricted to the dermis with epidermal sparing.9 Dark granules and ocher fibers are absent.
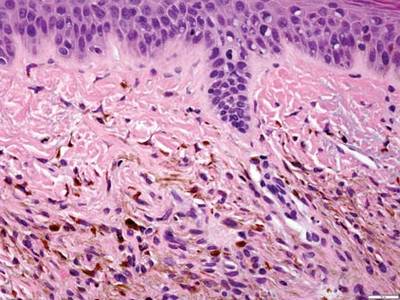
Long-term use of hydroxychloroquine or other antimalarials may cause a macular pattern of blue-gray hyperpigmentation.10 Biopsy specimens typically reveal coarse, yellow-brown pigment granules primarily affecting the superficial dermis (Figure 4). Granules are found both extracellularly and within macrophages. Fontana-Masson silver staining may identify melanin, as hydroxychloroquine-melanin binding may contribute to patterns of hyperpigmentation.10 Hemosiderin often is present in cases of hydroxychloroquine pigmentation. Preceding ecchymosis appears to favor the deposition of hydroxychloroquine in the skin.11 The absence of dark metal granules helps distinguish hydroxychloroquine pigmentation from argyria.
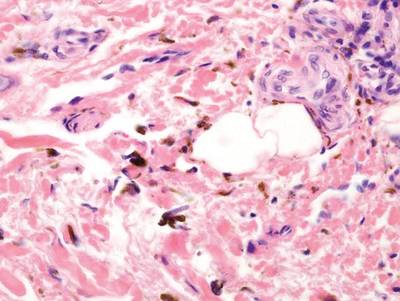
Regressed melanomas may appear clinically as gray macules. These lesions arise in cases of malignant melanoma that spontaneously regress without treatment. Spontaneous regression occurs in 10% to 35% of cases depending on tumor subtype.12 Lesions can have a variable appearance based on the degree of regression. Partial regression is demonstrated by mixed melanosis and fibrosis in the dermis (Figure 5).13,14 Melanin is housed within melanophages present in a variably expanded papillary dermis. Tumors in early stages of regression can be surrounded by an inflammatory infiltrate, which becomes diminished at later stages. However, a few exceptional cases have been noted with extensive inflammatory infiltrate and no residual tumor.14 Completely regressed lesions typically appear as a band of dermal melanophages in the absence of inflammation or melanocytic atypia.15 The finding of regressed melanoma should prompt further investigation including sentinel lymph node biopsy, as it may be associated with metastasis.
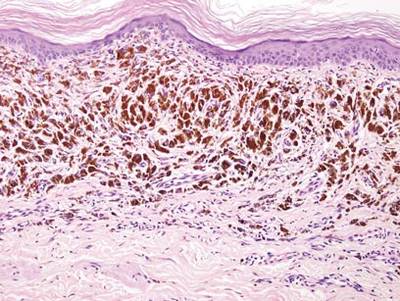
Tattooing occurs following traumatic penetration of the skin with impregnation of pigmented foreign material into deep dermal layers.16 Histologic examination usually reveals clumps of fine particulate material in the dermis (Figure 6). The color of the pigment depends on the agent used. For example, graphite appears as black particles that may be confused with localized cutaneous argyria. Distinction can be made using elemental identification techniques such as energy-dispersive X-ray spectroscopy.1 The intensity of the pigment in granules found in tattoos or localized cutaneous argyria will fail to diminish with the application of melanin bleach.6
- Robinson-Bostom L, Pomerantz D, Wilkel C, et al. Localized argyria with pseudo-ochronosis. J Am Acad Dermatol. 2002;46:222-227.
- Tajirian AL, Campbell RM, Robinson-Bostom L. Localized argyria after exposure to aerosolized solder. Cutis. 2006;78:305-308.
- Shelley WB, Shelley ED, Burmeister V. Argyria: the intradermal photograph, a manifestation of passive photosensitivity. J Am Acad Dermatol. 1987;16:211-217.
- Buckley WR, Terhaar CJ. The skin as an excretory organ in argyria. Trans St Johns Hosp Dermatol Soc. 1973;59:39-44.
- Shimizu I, Dill SW, McBean J, et al. Metal-induced granule deposition with pseudo-ochronosis. J Am Acad Dermatol. 2010;63:357-359.
- Rackoff EMJ, Benbenisty KM, Maize JC, et al. Localized cutaneous argyria from an acupuncture needle clini-cally concerning for metastatic melanoma. Cutis. 2007;80:423-426.
- Fernandez-Canon JM, Granadino B, Beltran-Valero de Bernabe D, et al. The molecular basis of alkaptonuria. Nat Genet. 1996;14:5-6.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Granter SR, McKee PH, Calonje E, et al. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called ‘malignant blue nevus.’ Am J Surg Pathol. 2001;25:316.
- Puri PK, Lountzis NI, Tyler W, et al. Hydroxychloroquine-induced hyperpigmentation: the staining pattern. J Cutan Pathol. 2008;35:1134-1137.
- Jallouli M, Francès C, Piette JC, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case-control study. JAMA Dermatol. 2013;149:935-940.
- Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992;20:315-322.
- LeBoit PE. Melanosis and its meanings. Am J Dermatopathol. 2002;24:369-372.
- Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
- Yang CH, Yeh JT, Shen SC, et al. Regressed subungual melanoma simulating cellular blue nevus: managed with sentinel lymph node biopsy. Dermatol Surg. 2006;32:577-581.
- Apfelberg DB, Manchester GH. Decorative and traumatic tattoo biophysics and removal. Clin Plast Surg. 1987;14:243-251.
Localized cutaneous argyria often presents as asymptomatic black or blue-gray pigmented macules in areas of the skin exposed to silver-containing compounds.1 Silver may enter the skin by traumatic implantation or absorption via eccrine sweat glands.2 Our patient witnessed a gun fight several years ago while on a mission trip and sustained multiple shrapnel wounds.
As in our patient, hyperpigmentation may appear years following initial exposure. Over time, incident light reduces colorless silver salts and compounds to black elemental silver.3 It also has been suggested that metallic silver granules stimulate tyrosine kinase activity, leading to locally increased melanin production.4 Together, these processes result in the clinical appearance of a blue-black macule. Despite its long-standing association with silver, this appearance also has been noted with deposition of other metals.5 Histologically, metal deposits can be seen as black granules surrounding eccrine glands, blood vessels, and elastic fibers on higher magnification.6 Granules also may be found in sebaceous glands and arrector pili muscle fibers. These findings do not distinguish from generalized argyria due to increased serum silver levels; however, some cases of localized cutaneous argyria have demonstrated spheroid black globules with surrounding collagen necrosis,1 which have not been reported with generalized disease. Localized cutaneous argyria also may be associated with ocher pigmentation of thickened collagen fibers, resembling changes typically found in alkaptonuria, an inherited deficiency of homogentisic acid oxidase (an enzyme involved in tyrosine metabolism).7 The resulting buildup of metabolic intermediates leads to ochronosis, a deposition of ocher-pigmented intermediates in connective tissue throughout the body. In the skin, ocher pigmentation occurs in elastic fibers of the reticular dermis.1 Grossly, these changes result in a blue-gray discoloration of the skin due to a light-scattering phenomenon known as the Tyndall effect. Exogenous ochronosis also can occur, most commonly from the topical application of hydroquinone or other skin-lightening compounds.1,5 Ocher pigmentation occurring in the setting of localized cutaneous argyria is referred to as pseudo-ochronosis, a finding first described by Robinson-Bostom et al.1 The etiology of this condition is poorly understood, but Robinson-Bostom et al1 noted the appearance of dark metal granules surrounding collagen bundles and hypothesized that metal aggregates surrounding collagen bundles in pseudo-ochronosis cause a homogenized appearance under light microscopy. Yellow-brown, swollen, homogenized collagen bundles can be visualized in the reticular dermis with surrounding deposition of metal granules (Figures 1 and 2).1 Typical patterns of granule deposition in localized argyria also are present.


A blue nevus is a collection of proliferating dermal melanocytes. Many histologic subtypes exist and there may be extensive variability in the extent of sclerosis, cellular architecture, and tissue cellularity between each variant.8 Blue nevi commonly present as blue-black hyperpigmentation in the dermis and subcutaneous tissue.9 Histologically, they are characterized by slender, bipolar, dendritic melanocytes in a sclerotic stroma (Figure 3).8 Melanocytes are highly pigmented and contain small monomorphic nuclei. Lesions are relatively homogenous and typically are restricted to the dermis with epidermal sparing.9 Dark granules and ocher fibers are absent.

Long-term use of hydroxychloroquine or other antimalarials may cause a macular pattern of blue-gray hyperpigmentation.10 Biopsy specimens typically reveal coarse, yellow-brown pigment granules primarily affecting the superficial dermis (Figure 4). Granules are found both extracellularly and within macrophages. Fontana-Masson silver staining may identify melanin, as hydroxychloroquine-melanin binding may contribute to patterns of hyperpigmentation.10 Hemosiderin often is present in cases of hydroxychloroquine pigmentation. Preceding ecchymosis appears to favor the deposition of hydroxychloroquine in the skin.11 The absence of dark metal granules helps distinguish hydroxychloroquine pigmentation from argyria.

Regressed melanomas may appear clinically as gray macules. These lesions arise in cases of malignant melanoma that spontaneously regress without treatment. Spontaneous regression occurs in 10% to 35% of cases depending on tumor subtype.12 Lesions can have a variable appearance based on the degree of regression. Partial regression is demonstrated by mixed melanosis and fibrosis in the dermis (Figure 5).13,14 Melanin is housed within melanophages present in a variably expanded papillary dermis. Tumors in early stages of regression can be surrounded by an inflammatory infiltrate, which becomes diminished at later stages. However, a few exceptional cases have been noted with extensive inflammatory infiltrate and no residual tumor.14 Completely regressed lesions typically appear as a band of dermal melanophages in the absence of inflammation or melanocytic atypia.15 The finding of regressed melanoma should prompt further investigation including sentinel lymph node biopsy, as it may be associated with metastasis.

Tattooing occurs following traumatic penetration of the skin with impregnation of pigmented foreign material into deep dermal layers.16 Histologic examination usually reveals clumps of fine particulate material in the dermis (Figure 6). The color of the pigment depends on the agent used. For example, graphite appears as black particles that may be confused with localized cutaneous argyria. Distinction can be made using elemental identification techniques such as energy-dispersive X-ray spectroscopy.1 The intensity of the pigment in granules found in tattoos or localized cutaneous argyria will fail to diminish with the application of melanin bleach.6

Localized cutaneous argyria often presents as asymptomatic black or blue-gray pigmented macules in areas of the skin exposed to silver-containing compounds.1 Silver may enter the skin by traumatic implantation or absorption via eccrine sweat glands.2 Our patient witnessed a gun fight several years ago while on a mission trip and sustained multiple shrapnel wounds.
As in our patient, hyperpigmentation may appear years following initial exposure. Over time, incident light reduces colorless silver salts and compounds to black elemental silver.3 It also has been suggested that metallic silver granules stimulate tyrosine kinase activity, leading to locally increased melanin production.4 Together, these processes result in the clinical appearance of a blue-black macule. Despite its long-standing association with silver, this appearance also has been noted with deposition of other metals.5 Histologically, metal deposits can be seen as black granules surrounding eccrine glands, blood vessels, and elastic fibers on higher magnification.6 Granules also may be found in sebaceous glands and arrector pili muscle fibers. These findings do not distinguish from generalized argyria due to increased serum silver levels; however, some cases of localized cutaneous argyria have demonstrated spheroid black globules with surrounding collagen necrosis,1 which have not been reported with generalized disease. Localized cutaneous argyria also may be associated with ocher pigmentation of thickened collagen fibers, resembling changes typically found in alkaptonuria, an inherited deficiency of homogentisic acid oxidase (an enzyme involved in tyrosine metabolism).7 The resulting buildup of metabolic intermediates leads to ochronosis, a deposition of ocher-pigmented intermediates in connective tissue throughout the body. In the skin, ocher pigmentation occurs in elastic fibers of the reticular dermis.1 Grossly, these changes result in a blue-gray discoloration of the skin due to a light-scattering phenomenon known as the Tyndall effect. Exogenous ochronosis also can occur, most commonly from the topical application of hydroquinone or other skin-lightening compounds.1,5 Ocher pigmentation occurring in the setting of localized cutaneous argyria is referred to as pseudo-ochronosis, a finding first described by Robinson-Bostom et al.1 The etiology of this condition is poorly understood, but Robinson-Bostom et al1 noted the appearance of dark metal granules surrounding collagen bundles and hypothesized that metal aggregates surrounding collagen bundles in pseudo-ochronosis cause a homogenized appearance under light microscopy. Yellow-brown, swollen, homogenized collagen bundles can be visualized in the reticular dermis with surrounding deposition of metal granules (Figures 1 and 2).1 Typical patterns of granule deposition in localized argyria also are present.


A blue nevus is a collection of proliferating dermal melanocytes. Many histologic subtypes exist and there may be extensive variability in the extent of sclerosis, cellular architecture, and tissue cellularity between each variant.8 Blue nevi commonly present as blue-black hyperpigmentation in the dermis and subcutaneous tissue.9 Histologically, they are characterized by slender, bipolar, dendritic melanocytes in a sclerotic stroma (Figure 3).8 Melanocytes are highly pigmented and contain small monomorphic nuclei. Lesions are relatively homogenous and typically are restricted to the dermis with epidermal sparing.9 Dark granules and ocher fibers are absent.

Long-term use of hydroxychloroquine or other antimalarials may cause a macular pattern of blue-gray hyperpigmentation.10 Biopsy specimens typically reveal coarse, yellow-brown pigment granules primarily affecting the superficial dermis (Figure 4). Granules are found both extracellularly and within macrophages. Fontana-Masson silver staining may identify melanin, as hydroxychloroquine-melanin binding may contribute to patterns of hyperpigmentation.10 Hemosiderin often is present in cases of hydroxychloroquine pigmentation. Preceding ecchymosis appears to favor the deposition of hydroxychloroquine in the skin.11 The absence of dark metal granules helps distinguish hydroxychloroquine pigmentation from argyria.

Regressed melanomas may appear clinically as gray macules. These lesions arise in cases of malignant melanoma that spontaneously regress without treatment. Spontaneous regression occurs in 10% to 35% of cases depending on tumor subtype.12 Lesions can have a variable appearance based on the degree of regression. Partial regression is demonstrated by mixed melanosis and fibrosis in the dermis (Figure 5).13,14 Melanin is housed within melanophages present in a variably expanded papillary dermis. Tumors in early stages of regression can be surrounded by an inflammatory infiltrate, which becomes diminished at later stages. However, a few exceptional cases have been noted with extensive inflammatory infiltrate and no residual tumor.14 Completely regressed lesions typically appear as a band of dermal melanophages in the absence of inflammation or melanocytic atypia.15 The finding of regressed melanoma should prompt further investigation including sentinel lymph node biopsy, as it may be associated with metastasis.

Tattooing occurs following traumatic penetration of the skin with impregnation of pigmented foreign material into deep dermal layers.16 Histologic examination usually reveals clumps of fine particulate material in the dermis (Figure 6). The color of the pigment depends on the agent used. For example, graphite appears as black particles that may be confused with localized cutaneous argyria. Distinction can be made using elemental identification techniques such as energy-dispersive X-ray spectroscopy.1 The intensity of the pigment in granules found in tattoos or localized cutaneous argyria will fail to diminish with the application of melanin bleach.6

- Robinson-Bostom L, Pomerantz D, Wilkel C, et al. Localized argyria with pseudo-ochronosis. J Am Acad Dermatol. 2002;46:222-227.
- Tajirian AL, Campbell RM, Robinson-Bostom L. Localized argyria after exposure to aerosolized solder. Cutis. 2006;78:305-308.
- Shelley WB, Shelley ED, Burmeister V. Argyria: the intradermal photograph, a manifestation of passive photosensitivity. J Am Acad Dermatol. 1987;16:211-217.
- Buckley WR, Terhaar CJ. The skin as an excretory organ in argyria. Trans St Johns Hosp Dermatol Soc. 1973;59:39-44.
- Shimizu I, Dill SW, McBean J, et al. Metal-induced granule deposition with pseudo-ochronosis. J Am Acad Dermatol. 2010;63:357-359.
- Rackoff EMJ, Benbenisty KM, Maize JC, et al. Localized cutaneous argyria from an acupuncture needle clini-cally concerning for metastatic melanoma. Cutis. 2007;80:423-426.
- Fernandez-Canon JM, Granadino B, Beltran-Valero de Bernabe D, et al. The molecular basis of alkaptonuria. Nat Genet. 1996;14:5-6.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Granter SR, McKee PH, Calonje E, et al. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called ‘malignant blue nevus.’ Am J Surg Pathol. 2001;25:316.
- Puri PK, Lountzis NI, Tyler W, et al. Hydroxychloroquine-induced hyperpigmentation: the staining pattern. J Cutan Pathol. 2008;35:1134-1137.
- Jallouli M, Francès C, Piette JC, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case-control study. JAMA Dermatol. 2013;149:935-940.
- Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992;20:315-322.
- LeBoit PE. Melanosis and its meanings. Am J Dermatopathol. 2002;24:369-372.
- Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
- Yang CH, Yeh JT, Shen SC, et al. Regressed subungual melanoma simulating cellular blue nevus: managed with sentinel lymph node biopsy. Dermatol Surg. 2006;32:577-581.
- Apfelberg DB, Manchester GH. Decorative and traumatic tattoo biophysics and removal. Clin Plast Surg. 1987;14:243-251.
- Robinson-Bostom L, Pomerantz D, Wilkel C, et al. Localized argyria with pseudo-ochronosis. J Am Acad Dermatol. 2002;46:222-227.
- Tajirian AL, Campbell RM, Robinson-Bostom L. Localized argyria after exposure to aerosolized solder. Cutis. 2006;78:305-308.
- Shelley WB, Shelley ED, Burmeister V. Argyria: the intradermal photograph, a manifestation of passive photosensitivity. J Am Acad Dermatol. 1987;16:211-217.
- Buckley WR, Terhaar CJ. The skin as an excretory organ in argyria. Trans St Johns Hosp Dermatol Soc. 1973;59:39-44.
- Shimizu I, Dill SW, McBean J, et al. Metal-induced granule deposition with pseudo-ochronosis. J Am Acad Dermatol. 2010;63:357-359.
- Rackoff EMJ, Benbenisty KM, Maize JC, et al. Localized cutaneous argyria from an acupuncture needle clini-cally concerning for metastatic melanoma. Cutis. 2007;80:423-426.
- Fernandez-Canon JM, Granadino B, Beltran-Valero de Bernabe D, et al. The molecular basis of alkaptonuria. Nat Genet. 1996;14:5-6.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Granter SR, McKee PH, Calonje E, et al. Melanoma associated with blue nevus and melanoma mimicking cellular blue nevus: a clinicopathologic study of 10 cases on the spectrum of so-called ‘malignant blue nevus.’ Am J Surg Pathol. 2001;25:316.
- Puri PK, Lountzis NI, Tyler W, et al. Hydroxychloroquine-induced hyperpigmentation: the staining pattern. J Cutan Pathol. 2008;35:1134-1137.
- Jallouli M, Francès C, Piette JC, et al. Hydroxychloroquine-induced pigmentation in patients with systemic lupus erythematosus: a case-control study. JAMA Dermatol. 2013;149:935-940.
- Blessing K, McLaren KM. Histological regression in primary cutaneous melanoma: recognition, prevalence and significance. Histopathology. 1992;20:315-322.
- LeBoit PE. Melanosis and its meanings. Am J Dermatopathol. 2002;24:369-372.
- Emanuel PO, Mannion M, Phelps RG. Complete regression of primary malignant melanoma. Am J Dermatopathol. 2008;30:178-181.
- Yang CH, Yeh JT, Shen SC, et al. Regressed subungual melanoma simulating cellular blue nevus: managed with sentinel lymph node biopsy. Dermatol Surg. 2006;32:577-581.
- Apfelberg DB, Manchester GH. Decorative and traumatic tattoo biophysics and removal. Clin Plast Surg. 1987;14:243-251.
What Is Your Diagnosis? Calciphylaxis
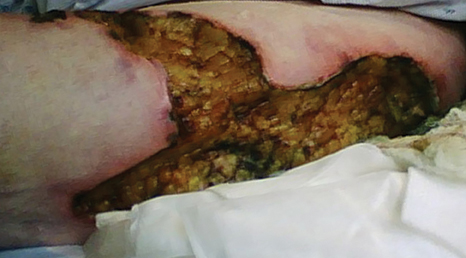
A 62-year-old woman who was obese with idiopathic thrombocytopenic purpura; a history of diabetes mellitus, hypertension, and acute renal insufficiency; recent treatment with high-dose (2 mg/kg) glucocorticosteroids; and sepsis presented with ulcers on the thighs and buttocks after being transferred in a patient lift. The ulcers had developed in areas of pressure corresponding to placement in the patient lift.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare disorder that most commonly affects patients with impaired renal failure. Calciphylaxis, known as calcific uremic arteriolopathy,1,2 involves calcification of small- and medium-sized blood vessels,3 which may lead to necrosis of the dermis, subcutaneous tissue, muscles, fascia, and internal organs.4-6 Calciphylaxis usually occurs in fatty areas such as the calves, thighs (Figure 1), abdomen, and buttocks.3,7,8 Although calciphylaxis most commonly is associated with severe renal disease,1,3 pharmacologic agents such as glucocorticoids, high-dose vitamin D, warfarin, iron salts, insulin injections, calcium-based phosphate binders, and chemotherapeutic agents also may precipitate this condition.9,10 Diagnostic biopsies typically reveal fat necrosis, inflammation, and associated small vessel calcification (Figures 2 and 3).
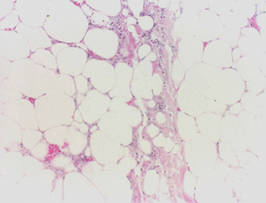
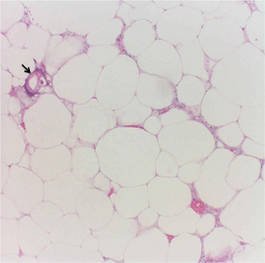
Our patient may have been predisposed to calciphylaxis due to her history of obesity, diabetes mellitus, and hypertension, and recent high-dose (2 mg/kg) glucocorticosteroid treatment. Five months prior to the current presentation, the patient was admitted to the hospital for treatment of sepsis with high-dose glucocorticoids for presumed idiopathic thrombocytopenic purpura. She also developed several ecchymotic regions on her skin at that time. Additionally, she developed acute renal insufficiency resulting from septic shock that improved over several weeks. Interestingly, 2 months after full recovery of her renal function, which was marked by normal creatinine levels and glomerular filtration rate, she developed a violaceous rash over the thighs and buttocks in the distribution of the straps from a patient lift that was used to transfer her from a bed into a wheelchair while residing at a physical rehabilitation facility. In the time after recovery of renal function, the eruption progressed to necrosis over 2 weeks in the areas of skin that came into contact with the lift’s straps. The patient’s renal function and parathyroid hormone, ionized calcium, phosphate, and vitamin D levels were all within reference range at the time of diagnosis.
On biopsy, characteristic findings established the diagnosis of calciphylaxis. Furthermore, the pattern of ulceration in pressure areas associated with the patient lift indicated that infection was not the precipitating event. A von Kossa stain highlighted the calcium deposition in small blood vessels in areas of panniculitis (Figure 4). Atherosclerosis may be associated with vascular calcification but does not involve acute small blood vessel calcification, and other ulcerative disorders in the differential diagnosis (eg, pyoderma gangrenosum) would not be associated with small vessel vasculitis.
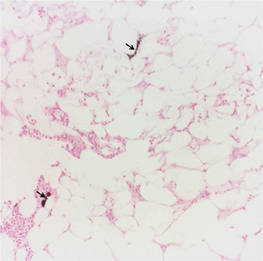
Treatment of calciphylaxis must be individualized. It is important to treat any underlying disease and remove any precipitating factors. In the case of severe renal disease, a multidisciplinary approach typically is necessary.3 Correction of serum calcium and phosphate levels, rapid and safe rehydration, dialysis, and intravenous bisphosphonates all may be needed in patients with chronic kidney disease.11 Surgical management of the nonhealing necrotic skin lesions via surgical debridement and skin grafting has been reported to improve overall morbidity and survival rates.12 For patients with calciphylaxis from hyperparathyroidism, parathyroidectomy has been used but has demonstrated minimal effects on survival rates.9 Currently, there is no standard therapy in place for treatment of calciphylaxis but several are under investigation. Most notably, sodium thiosulfate, an inorganic salt that promotes dissolution of calcium deposits by chelating calcium, has shown rapid improvement when other treatments have failed.13-16 In patients with marginal decreases in blood flow and ischemia of skin, hyperbaric oxygen therapy has been found to resolve cutaneous ulcers of calciphylaxis.17,18 Patients with renal disease who develop calciphylaxis have benefited from treatment with cinacalcet, a calcimemetic that lowers parathyroid hormone levels and improves calcium phosphorus metabolism in patients undergoing dialysis.13,14
Our patient was treated with 2 weeks of broad-spectrum antibiotics along with extensive wound care and vacuum-assisted closure of surgically debrided tissue on her thighs and buttocks. Glucocorticoid therapy was rapidly tapered. Three months after the initial presentation, the patient was placed in outpatient wound care with evaluation for hyperbaric oxygen therapy.
Calciphylaxis is a rare condition with a high mortality rate, most often due to septicemia from secondary infection of skin lesions.19 The exact pathogenic mechanisms are vague but may be the result of several precipitating factors.10 Local wound care and therapeutics should be used synergistically and individualized for each patient with calciphylaxis.
- Brandenburg VM, Cozzolino M, Ketteler M. Calciphylaxis: a still unmet challenge. J Nephrol. 2011;24:142-148.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Ng AT, Peng DH. Calciphylaxis. Dermatol Ther. 2011;24:256-262.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:1267-1276.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Mazhar AR, Johnson RJ, Gillen D, et al. Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int. 2001;60:324-332.
- Scheinman PL, Helm KF, Fairley JA. Acral necrosis in a patient with chronic renal failure. calciphylaxis. Arch Dermatol. 1991;127:248-249, 251-252.
- Hafner J, Keusch G, Wahl C, et al. Calciphylaxis: a syndrome of skin necrosis and acral gangrene in chronic renal failure. Vasa. 1998;27:137-143.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome [published online ahead of print December 1, 2006]. J Am Acad Dermatol. 2007;56:569-579.
- Guldbakke KK, Khachemoune A. Calciphylaxis. Int J Dermatol. 2007;46:231-238.
- Hanafusa T, Yamaguchi Y, Tani M, et al. Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol. 2007;57:1021-1025.
- Janigan DT, Hirsch DJ, Klassen GA, et al. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis. 2000;35:588-597.
- Robinson MR, Augustine JJ, Korman NJ. Cinacalcet for the treatment of calciphylaxis. Arch Dermatol. 2007;143:152-154.
- Sharma A, Burkitt-Wright E, Rustom R. Cinacalcet as an adjunct in successful treatment of calciphylaxis. Br J Dermatol. 2006;155:1295-1297.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Guerra G, Shah RC, Ross EA. Rapid resolution of calciphylaxis with intravenous sodium thiosulfate and continuous venovenous haemofiltration using low calcium replacement fluid: case report [published online ahead of print May 3, 2005]. Nephrol Dial Transplant. 2005;20:1260-1262.
- Podymow T, Wherrett C, Burns KD. Hyperbaricoxygen in the treatment of calciphylaxis: a case series. Nephrol Dial Transplant. 2001;16:2176-2180.
- Basile C, Montanaro A, Masi M, et al. Hyperbaric oxygen therapy for calcific uremic arteriolopathy: a case series. J Nephrol. 2002;15:676-680.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.

A 62-year-old woman who was obese with idiopathic thrombocytopenic purpura; a history of diabetes mellitus, hypertension, and acute renal insufficiency; recent treatment with high-dose (2 mg/kg) glucocorticosteroids; and sepsis presented with ulcers on the thighs and buttocks after being transferred in a patient lift. The ulcers had developed in areas of pressure corresponding to placement in the patient lift.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare disorder that most commonly affects patients with impaired renal failure. Calciphylaxis, known as calcific uremic arteriolopathy,1,2 involves calcification of small- and medium-sized blood vessels,3 which may lead to necrosis of the dermis, subcutaneous tissue, muscles, fascia, and internal organs.4-6 Calciphylaxis usually occurs in fatty areas such as the calves, thighs (Figure 1), abdomen, and buttocks.3,7,8 Although calciphylaxis most commonly is associated with severe renal disease,1,3 pharmacologic agents such as glucocorticoids, high-dose vitamin D, warfarin, iron salts, insulin injections, calcium-based phosphate binders, and chemotherapeutic agents also may precipitate this condition.9,10 Diagnostic biopsies typically reveal fat necrosis, inflammation, and associated small vessel calcification (Figures 2 and 3).



Our patient may have been predisposed to calciphylaxis due to her history of obesity, diabetes mellitus, and hypertension, and recent high-dose (2 mg/kg) glucocorticosteroid treatment. Five months prior to the current presentation, the patient was admitted to the hospital for treatment of sepsis with high-dose glucocorticoids for presumed idiopathic thrombocytopenic purpura. She also developed several ecchymotic regions on her skin at that time. Additionally, she developed acute renal insufficiency resulting from septic shock that improved over several weeks. Interestingly, 2 months after full recovery of her renal function, which was marked by normal creatinine levels and glomerular filtration rate, she developed a violaceous rash over the thighs and buttocks in the distribution of the straps from a patient lift that was used to transfer her from a bed into a wheelchair while residing at a physical rehabilitation facility. In the time after recovery of renal function, the eruption progressed to necrosis over 2 weeks in the areas of skin that came into contact with the lift’s straps. The patient’s renal function and parathyroid hormone, ionized calcium, phosphate, and vitamin D levels were all within reference range at the time of diagnosis.
On biopsy, characteristic findings established the diagnosis of calciphylaxis. Furthermore, the pattern of ulceration in pressure areas associated with the patient lift indicated that infection was not the precipitating event. A von Kossa stain highlighted the calcium deposition in small blood vessels in areas of panniculitis (Figure 4). Atherosclerosis may be associated with vascular calcification but does not involve acute small blood vessel calcification, and other ulcerative disorders in the differential diagnosis (eg, pyoderma gangrenosum) would not be associated with small vessel vasculitis.

Treatment of calciphylaxis must be individualized. It is important to treat any underlying disease and remove any precipitating factors. In the case of severe renal disease, a multidisciplinary approach typically is necessary.3 Correction of serum calcium and phosphate levels, rapid and safe rehydration, dialysis, and intravenous bisphosphonates all may be needed in patients with chronic kidney disease.11 Surgical management of the nonhealing necrotic skin lesions via surgical debridement and skin grafting has been reported to improve overall morbidity and survival rates.12 For patients with calciphylaxis from hyperparathyroidism, parathyroidectomy has been used but has demonstrated minimal effects on survival rates.9 Currently, there is no standard therapy in place for treatment of calciphylaxis but several are under investigation. Most notably, sodium thiosulfate, an inorganic salt that promotes dissolution of calcium deposits by chelating calcium, has shown rapid improvement when other treatments have failed.13-16 In patients with marginal decreases in blood flow and ischemia of skin, hyperbaric oxygen therapy has been found to resolve cutaneous ulcers of calciphylaxis.17,18 Patients with renal disease who develop calciphylaxis have benefited from treatment with cinacalcet, a calcimemetic that lowers parathyroid hormone levels and improves calcium phosphorus metabolism in patients undergoing dialysis.13,14
Our patient was treated with 2 weeks of broad-spectrum antibiotics along with extensive wound care and vacuum-assisted closure of surgically debrided tissue on her thighs and buttocks. Glucocorticoid therapy was rapidly tapered. Three months after the initial presentation, the patient was placed in outpatient wound care with evaluation for hyperbaric oxygen therapy.
Calciphylaxis is a rare condition with a high mortality rate, most often due to septicemia from secondary infection of skin lesions.19 The exact pathogenic mechanisms are vague but may be the result of several precipitating factors.10 Local wound care and therapeutics should be used synergistically and individualized for each patient with calciphylaxis.

A 62-year-old woman who was obese with idiopathic thrombocytopenic purpura; a history of diabetes mellitus, hypertension, and acute renal insufficiency; recent treatment with high-dose (2 mg/kg) glucocorticosteroids; and sepsis presented with ulcers on the thighs and buttocks after being transferred in a patient lift. The ulcers had developed in areas of pressure corresponding to placement in the patient lift.
The Diagnosis: Calciphylaxis
Calciphylaxis is a rare disorder that most commonly affects patients with impaired renal failure. Calciphylaxis, known as calcific uremic arteriolopathy,1,2 involves calcification of small- and medium-sized blood vessels,3 which may lead to necrosis of the dermis, subcutaneous tissue, muscles, fascia, and internal organs.4-6 Calciphylaxis usually occurs in fatty areas such as the calves, thighs (Figure 1), abdomen, and buttocks.3,7,8 Although calciphylaxis most commonly is associated with severe renal disease,1,3 pharmacologic agents such as glucocorticoids, high-dose vitamin D, warfarin, iron salts, insulin injections, calcium-based phosphate binders, and chemotherapeutic agents also may precipitate this condition.9,10 Diagnostic biopsies typically reveal fat necrosis, inflammation, and associated small vessel calcification (Figures 2 and 3).



Our patient may have been predisposed to calciphylaxis due to her history of obesity, diabetes mellitus, and hypertension, and recent high-dose (2 mg/kg) glucocorticosteroid treatment. Five months prior to the current presentation, the patient was admitted to the hospital for treatment of sepsis with high-dose glucocorticoids for presumed idiopathic thrombocytopenic purpura. She also developed several ecchymotic regions on her skin at that time. Additionally, she developed acute renal insufficiency resulting from septic shock that improved over several weeks. Interestingly, 2 months after full recovery of her renal function, which was marked by normal creatinine levels and glomerular filtration rate, she developed a violaceous rash over the thighs and buttocks in the distribution of the straps from a patient lift that was used to transfer her from a bed into a wheelchair while residing at a physical rehabilitation facility. In the time after recovery of renal function, the eruption progressed to necrosis over 2 weeks in the areas of skin that came into contact with the lift’s straps. The patient’s renal function and parathyroid hormone, ionized calcium, phosphate, and vitamin D levels were all within reference range at the time of diagnosis.
On biopsy, characteristic findings established the diagnosis of calciphylaxis. Furthermore, the pattern of ulceration in pressure areas associated with the patient lift indicated that infection was not the precipitating event. A von Kossa stain highlighted the calcium deposition in small blood vessels in areas of panniculitis (Figure 4). Atherosclerosis may be associated with vascular calcification but does not involve acute small blood vessel calcification, and other ulcerative disorders in the differential diagnosis (eg, pyoderma gangrenosum) would not be associated with small vessel vasculitis.

Treatment of calciphylaxis must be individualized. It is important to treat any underlying disease and remove any precipitating factors. In the case of severe renal disease, a multidisciplinary approach typically is necessary.3 Correction of serum calcium and phosphate levels, rapid and safe rehydration, dialysis, and intravenous bisphosphonates all may be needed in patients with chronic kidney disease.11 Surgical management of the nonhealing necrotic skin lesions via surgical debridement and skin grafting has been reported to improve overall morbidity and survival rates.12 For patients with calciphylaxis from hyperparathyroidism, parathyroidectomy has been used but has demonstrated minimal effects on survival rates.9 Currently, there is no standard therapy in place for treatment of calciphylaxis but several are under investigation. Most notably, sodium thiosulfate, an inorganic salt that promotes dissolution of calcium deposits by chelating calcium, has shown rapid improvement when other treatments have failed.13-16 In patients with marginal decreases in blood flow and ischemia of skin, hyperbaric oxygen therapy has been found to resolve cutaneous ulcers of calciphylaxis.17,18 Patients with renal disease who develop calciphylaxis have benefited from treatment with cinacalcet, a calcimemetic that lowers parathyroid hormone levels and improves calcium phosphorus metabolism in patients undergoing dialysis.13,14
Our patient was treated with 2 weeks of broad-spectrum antibiotics along with extensive wound care and vacuum-assisted closure of surgically debrided tissue on her thighs and buttocks. Glucocorticoid therapy was rapidly tapered. Three months after the initial presentation, the patient was placed in outpatient wound care with evaluation for hyperbaric oxygen therapy.
Calciphylaxis is a rare condition with a high mortality rate, most often due to septicemia from secondary infection of skin lesions.19 The exact pathogenic mechanisms are vague but may be the result of several precipitating factors.10 Local wound care and therapeutics should be used synergistically and individualized for each patient with calciphylaxis.
- Brandenburg VM, Cozzolino M, Ketteler M. Calciphylaxis: a still unmet challenge. J Nephrol. 2011;24:142-148.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Ng AT, Peng DH. Calciphylaxis. Dermatol Ther. 2011;24:256-262.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:1267-1276.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Mazhar AR, Johnson RJ, Gillen D, et al. Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int. 2001;60:324-332.
- Scheinman PL, Helm KF, Fairley JA. Acral necrosis in a patient with chronic renal failure. calciphylaxis. Arch Dermatol. 1991;127:248-249, 251-252.
- Hafner J, Keusch G, Wahl C, et al. Calciphylaxis: a syndrome of skin necrosis and acral gangrene in chronic renal failure. Vasa. 1998;27:137-143.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome [published online ahead of print December 1, 2006]. J Am Acad Dermatol. 2007;56:569-579.
- Guldbakke KK, Khachemoune A. Calciphylaxis. Int J Dermatol. 2007;46:231-238.
- Hanafusa T, Yamaguchi Y, Tani M, et al. Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol. 2007;57:1021-1025.
- Janigan DT, Hirsch DJ, Klassen GA, et al. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis. 2000;35:588-597.
- Robinson MR, Augustine JJ, Korman NJ. Cinacalcet for the treatment of calciphylaxis. Arch Dermatol. 2007;143:152-154.
- Sharma A, Burkitt-Wright E, Rustom R. Cinacalcet as an adjunct in successful treatment of calciphylaxis. Br J Dermatol. 2006;155:1295-1297.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Guerra G, Shah RC, Ross EA. Rapid resolution of calciphylaxis with intravenous sodium thiosulfate and continuous venovenous haemofiltration using low calcium replacement fluid: case report [published online ahead of print May 3, 2005]. Nephrol Dial Transplant. 2005;20:1260-1262.
- Podymow T, Wherrett C, Burns KD. Hyperbaricoxygen in the treatment of calciphylaxis: a case series. Nephrol Dial Transplant. 2001;16:2176-2180.
- Basile C, Montanaro A, Masi M, et al. Hyperbaric oxygen therapy for calcific uremic arteriolopathy: a case series. J Nephrol. 2002;15:676-680.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
- Brandenburg VM, Cozzolino M, Ketteler M. Calciphylaxis: a still unmet challenge. J Nephrol. 2011;24:142-148.
- Hafner J, Keusch G, Wahl C, et al. Uremic small-artery disease with medial calcification and intimal hyperplasia (so-called calciphylaxis): a complication of chronic renal failure and benefit from parathyroidectomy. J Am Acad Dermatol. 1995;33:954-962.
- Ng AT, Peng DH. Calciphylaxis. Dermatol Ther. 2011;24:256-262.
- Ahmed S, O’Neill KD, Hood AF, et al. Calciphylaxis associated with hyperphosphatemia and increased osteopontin expression by vascular smooth muscle cells. Am J Kidney Dis. 2001;37:1267-1276.
- Bleyer AJ, Choi M, Igwemezie B, et al. A case control study of proximal calciphylaxis. Am J Kidney Dis. 1998;32:376-383.
- Mazhar AR, Johnson RJ, Gillen D, et al. Risk factors and mortality associated with calciphylaxis in end-stage renal disease. Kidney Int. 2001;60:324-332.
- Scheinman PL, Helm KF, Fairley JA. Acral necrosis in a patient with chronic renal failure. calciphylaxis. Arch Dermatol. 1991;127:248-249, 251-252.
- Hafner J, Keusch G, Wahl C, et al. Calciphylaxis: a syndrome of skin necrosis and acral gangrene in chronic renal failure. Vasa. 1998;27:137-143.
- Weenig RH, Sewell LD, Davis MD, et al. Calciphylaxis: natural history, risk factor analysis, and outcome [published online ahead of print December 1, 2006]. J Am Acad Dermatol. 2007;56:569-579.
- Guldbakke KK, Khachemoune A. Calciphylaxis. Int J Dermatol. 2007;46:231-238.
- Hanafusa T, Yamaguchi Y, Tani M, et al. Intractable wounds caused by calcific uremic arteriolopathy treated with bisphosphonates. J Am Acad Dermatol. 2007;57:1021-1025.
- Janigan DT, Hirsch DJ, Klassen GA, et al. Calcified subcutaneous arterioles with infarcts of the subcutis and skin (“calciphylaxis”) in chronic renal failure. Am J Kidney Dis. 2000;35:588-597.
- Robinson MR, Augustine JJ, Korman NJ. Cinacalcet for the treatment of calciphylaxis. Arch Dermatol. 2007;143:152-154.
- Sharma A, Burkitt-Wright E, Rustom R. Cinacalcet as an adjunct in successful treatment of calciphylaxis. Br J Dermatol. 2006;155:1295-1297.
- Cicone JS, Petronis JB, Embert CD, et al. Successful treatment of calciphylaxis with intravenous sodium thiosulfate. Am J Kidney Dis. 2004;43:1104-1108.
- Guerra G, Shah RC, Ross EA. Rapid resolution of calciphylaxis with intravenous sodium thiosulfate and continuous venovenous haemofiltration using low calcium replacement fluid: case report [published online ahead of print May 3, 2005]. Nephrol Dial Transplant. 2005;20:1260-1262.
- Podymow T, Wherrett C, Burns KD. Hyperbaricoxygen in the treatment of calciphylaxis: a case series. Nephrol Dial Transplant. 2001;16:2176-2180.
- Basile C, Montanaro A, Masi M, et al. Hyperbaric oxygen therapy for calcific uremic arteriolopathy: a case series. J Nephrol. 2002;15:676-680.
- Fine A, Zacharias J. Calciphylaxis is usually non-ulcerating: risk factors, outcome and therapy. Kidney Int. 2002;61:2210-2217.
Superficial Plantar Fibromatosis
What Is Your Diagnosis? Hansen Disease (Leprosy)
A rapidly growing crusted nodule on the lip
A 76-year-old man presented with a rapidly growing, indurated, crusted nodule on his lower lip (Figure 1). This combination—a rapidly growing nodule on a sun-exposed surface in an older patient—pointed to a diagnosis of squamous cell carcinoma, and biopsy study of the affected area confirmed this diagnosis (Figure 2). Clinical examination, computed tomography, and ultrasonography of the neck revealed no lymph node involvement; the tumor was staged as T2N0M0. Mohs microscopically controlled surgery was performed to remove the tumor with clear margins, and the patient has done well.
DIFFERENTIAL DIAGNOSIS
Malignant melanoma, Merkel cell carcinoma, and deep fungal infection can also cause a rapidly growing crusted nodule on the lower lip, but this typically is not an initial presentation for these conditions.
Malignant melanoma
Malignant melanoma should be considered for any rapidly growing cutaneous tumor, especially on sun-damaged skin. Most melanoma lesions have variations in pigment and may contain shades of blue, brown, red, pink, and white. Amelanotic melanomas may mimic squamous cell carcinomas, but the histologic features of atypical keratinocytes in this patient ruled out that diagnosis. Biopsy of amelanotic melanoma reveals nests of melanocytes, usually associated with an in situ component in the overlying epithelium.
Merkel cell carcinoma
Merkel cell carcinoma can mimic squamous cell carcinoma or can even arise in association with squamous cell carcinoma. Histologic examination allows for differentiation. In difficult cases, cytokeratin staining with cytokeratin 20 and CAM 5.2 can clarify the diagnosis, because Merkel cell carcinomas exhibit a characteristic paranuclear dot-like pattern not evident in squamous cell carcinoma.
Deep fungal infection
Colonization by Candida organisms was noted within the overlying crust in this patient’s lesion, but fungal organisms were not noted in the epidermis or dermis. Pseudoepitheliomatous hyperplasia can be prominent in deep fungal infection, but the marked cytologic atypia in this case excluded deep fungal infection.
Mucosal neuroma
Mucosal neuromas are typically smooth-surfaced, soft lesions on the lip. They may be associated with multiple endocrine neoplasia syndromes. Biopsy study reveals that they are composed of delicate spindle cells that lack atypia.
SQUAMOUS CELL CARCINOMA
Squamous cell carcinoma is one of the most common types of nonmelanoma skin cancer and is associated with increased sun exposure and light skin pigmentation.1 Unlike basal cell carcinoma, squamous cell carcinoma has a considerable potential to metastasize.2–4 The current cancer staging manual of the American Joint Commission on Cancer refects recent evidence-based information on staging and prognosis.5–7 Squamous cell carcinoma of the lip carries an increased risk of metastasis,8 and an increase in the number or the size of involved lymph nodes carries a worse prognosis.9 The lower lip is most commonly involved because of increased sun exposure. The most common route for the spread of squamous cell carcinoma of the head and neck is via lymphatics, so careful evaluation of the head and neck is indicated.10
STAGING OUR PATIENT’S LESION
Current staging of cutaneous squamous cell carcinoma takes into account a variety of different features. Our patient’s tumor was not associated with invasion of the maxilla, mandible, orbit, or temporal bone. This alone would have qualified the tumor as a T1 lesion, but its size of 3 cm indicated a higher risk and qualified it as a T2 lesion.
The histologic features of our patient’s lesion also indicate higher risk according to the current staging system.5 The Breslow thickness on histologic examination (measured from the granular cell layer of the epidermis to the deepest portion of the tumor) was 4 mm and the Clark’s level was IV (extension to the reticular dermis). The high-risk features and the location on the lip warrant a classification as T2 and carry a worse prognosis.
Wedge excision of the lower lip and Mohs surgery are accepted treatment options. The patient chose Mohs surgery and has done well, with excellent cosmetic and functional outcome. Radiation therapy can be useful as an adjuvant when lymph nodes are involved,11 but this was not necessary in our patient. Careful long-term follow up is warranted, as these patients are at higher risk of developing other, separate tumors.
- Schwartz RA. Squamous cell carcinoma. In:Schwartz RA, editor. Skin Cancer: Recognition and Management, 2nd ed. Malden, MA: Blackwell Publishing; 2008:47–65.
- D’Souza J, Clark J. Management of the neck in metastatic cutaneous squamous cell carcinoma of the head and neck. Curr Opin Otolaryngol Head Neck Surg 2011; 19:99–105.
- Zbar RI, Canady JW. MOC-PSSM CME article: Nonmelanoma facial skin malignancy. Plast Reconstr Surg 2008; 121(suppl 1):1–9.
- Morselli P, Masciotra L, Pinto V, Zollino I, Brunelli G, Carinci F. Clinical parameters in T1N0M0 lower lip squamous cell carcinoma. J Craniofac Surg 2007; 18:1079–1082.
- American Joint Commission on Cancer. Cancer Staging Manual. 7th ed. http://www.cancerstaging.org. Accessed February 3, 2013.
- Lardaro T, Shea SM, Sharfman W, Liégeois N, Sober AJ. Improvements in the staging of cutaneous squamous-cell carcinoma in the 7th edition of the AJCC Cancer Staging Manual. Ann Surg Oncol 2010; 17:1979–1980.
- Cutaneous squamous cell carcinoma and other cutaneous carcinomas. In:Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:301–314.
- Frierson HF, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Hum Pathol 1986; 17:346–354.
- Civantos FJ, Moffat FL, goodwin WJ. Lymphatic mapping and sentinel lymphadenectomy for 106 head and neck lesions: contrasts between oral cavity and cutaneous malignancy. Laryngoscope 2006; 112(3 Pt 2 suppl 109):1–15.
- Rowe De, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol 1992; 26:976–990.
- Veness MJ, Palme CE, Smith M, Cakir B, Morgan GJ, Kalnins I. Cutaneous head and neck squamous cell carcinoma metastatic to cervical lymph nodes (nonparotid): a better outcome with surgery and adjuvant radiotherapy. Laryngoscope 2003; 113:1827–1833.
A 76-year-old man presented with a rapidly growing, indurated, crusted nodule on his lower lip (Figure 1). This combination—a rapidly growing nodule on a sun-exposed surface in an older patient—pointed to a diagnosis of squamous cell carcinoma, and biopsy study of the affected area confirmed this diagnosis (Figure 2). Clinical examination, computed tomography, and ultrasonography of the neck revealed no lymph node involvement; the tumor was staged as T2N0M0. Mohs microscopically controlled surgery was performed to remove the tumor with clear margins, and the patient has done well.
DIFFERENTIAL DIAGNOSIS
Malignant melanoma, Merkel cell carcinoma, and deep fungal infection can also cause a rapidly growing crusted nodule on the lower lip, but this typically is not an initial presentation for these conditions.
Malignant melanoma
Malignant melanoma should be considered for any rapidly growing cutaneous tumor, especially on sun-damaged skin. Most melanoma lesions have variations in pigment and may contain shades of blue, brown, red, pink, and white. Amelanotic melanomas may mimic squamous cell carcinomas, but the histologic features of atypical keratinocytes in this patient ruled out that diagnosis. Biopsy of amelanotic melanoma reveals nests of melanocytes, usually associated with an in situ component in the overlying epithelium.
Merkel cell carcinoma
Merkel cell carcinoma can mimic squamous cell carcinoma or can even arise in association with squamous cell carcinoma. Histologic examination allows for differentiation. In difficult cases, cytokeratin staining with cytokeratin 20 and CAM 5.2 can clarify the diagnosis, because Merkel cell carcinomas exhibit a characteristic paranuclear dot-like pattern not evident in squamous cell carcinoma.
Deep fungal infection
Colonization by Candida organisms was noted within the overlying crust in this patient’s lesion, but fungal organisms were not noted in the epidermis or dermis. Pseudoepitheliomatous hyperplasia can be prominent in deep fungal infection, but the marked cytologic atypia in this case excluded deep fungal infection.
Mucosal neuroma
Mucosal neuromas are typically smooth-surfaced, soft lesions on the lip. They may be associated with multiple endocrine neoplasia syndromes. Biopsy study reveals that they are composed of delicate spindle cells that lack atypia.
SQUAMOUS CELL CARCINOMA
Squamous cell carcinoma is one of the most common types of nonmelanoma skin cancer and is associated with increased sun exposure and light skin pigmentation.1 Unlike basal cell carcinoma, squamous cell carcinoma has a considerable potential to metastasize.2–4 The current cancer staging manual of the American Joint Commission on Cancer refects recent evidence-based information on staging and prognosis.5–7 Squamous cell carcinoma of the lip carries an increased risk of metastasis,8 and an increase in the number or the size of involved lymph nodes carries a worse prognosis.9 The lower lip is most commonly involved because of increased sun exposure. The most common route for the spread of squamous cell carcinoma of the head and neck is via lymphatics, so careful evaluation of the head and neck is indicated.10
STAGING OUR PATIENT’S LESION
Current staging of cutaneous squamous cell carcinoma takes into account a variety of different features. Our patient’s tumor was not associated with invasion of the maxilla, mandible, orbit, or temporal bone. This alone would have qualified the tumor as a T1 lesion, but its size of 3 cm indicated a higher risk and qualified it as a T2 lesion.
The histologic features of our patient’s lesion also indicate higher risk according to the current staging system.5 The Breslow thickness on histologic examination (measured from the granular cell layer of the epidermis to the deepest portion of the tumor) was 4 mm and the Clark’s level was IV (extension to the reticular dermis). The high-risk features and the location on the lip warrant a classification as T2 and carry a worse prognosis.
Wedge excision of the lower lip and Mohs surgery are accepted treatment options. The patient chose Mohs surgery and has done well, with excellent cosmetic and functional outcome. Radiation therapy can be useful as an adjuvant when lymph nodes are involved,11 but this was not necessary in our patient. Careful long-term follow up is warranted, as these patients are at higher risk of developing other, separate tumors.
A 76-year-old man presented with a rapidly growing, indurated, crusted nodule on his lower lip (Figure 1). This combination—a rapidly growing nodule on a sun-exposed surface in an older patient—pointed to a diagnosis of squamous cell carcinoma, and biopsy study of the affected area confirmed this diagnosis (Figure 2). Clinical examination, computed tomography, and ultrasonography of the neck revealed no lymph node involvement; the tumor was staged as T2N0M0. Mohs microscopically controlled surgery was performed to remove the tumor with clear margins, and the patient has done well.
DIFFERENTIAL DIAGNOSIS
Malignant melanoma, Merkel cell carcinoma, and deep fungal infection can also cause a rapidly growing crusted nodule on the lower lip, but this typically is not an initial presentation for these conditions.
Malignant melanoma
Malignant melanoma should be considered for any rapidly growing cutaneous tumor, especially on sun-damaged skin. Most melanoma lesions have variations in pigment and may contain shades of blue, brown, red, pink, and white. Amelanotic melanomas may mimic squamous cell carcinomas, but the histologic features of atypical keratinocytes in this patient ruled out that diagnosis. Biopsy of amelanotic melanoma reveals nests of melanocytes, usually associated with an in situ component in the overlying epithelium.
Merkel cell carcinoma
Merkel cell carcinoma can mimic squamous cell carcinoma or can even arise in association with squamous cell carcinoma. Histologic examination allows for differentiation. In difficult cases, cytokeratin staining with cytokeratin 20 and CAM 5.2 can clarify the diagnosis, because Merkel cell carcinomas exhibit a characteristic paranuclear dot-like pattern not evident in squamous cell carcinoma.
Deep fungal infection
Colonization by Candida organisms was noted within the overlying crust in this patient’s lesion, but fungal organisms were not noted in the epidermis or dermis. Pseudoepitheliomatous hyperplasia can be prominent in deep fungal infection, but the marked cytologic atypia in this case excluded deep fungal infection.
Mucosal neuroma
Mucosal neuromas are typically smooth-surfaced, soft lesions on the lip. They may be associated with multiple endocrine neoplasia syndromes. Biopsy study reveals that they are composed of delicate spindle cells that lack atypia.
SQUAMOUS CELL CARCINOMA
Squamous cell carcinoma is one of the most common types of nonmelanoma skin cancer and is associated with increased sun exposure and light skin pigmentation.1 Unlike basal cell carcinoma, squamous cell carcinoma has a considerable potential to metastasize.2–4 The current cancer staging manual of the American Joint Commission on Cancer refects recent evidence-based information on staging and prognosis.5–7 Squamous cell carcinoma of the lip carries an increased risk of metastasis,8 and an increase in the number or the size of involved lymph nodes carries a worse prognosis.9 The lower lip is most commonly involved because of increased sun exposure. The most common route for the spread of squamous cell carcinoma of the head and neck is via lymphatics, so careful evaluation of the head and neck is indicated.10
STAGING OUR PATIENT’S LESION
Current staging of cutaneous squamous cell carcinoma takes into account a variety of different features. Our patient’s tumor was not associated with invasion of the maxilla, mandible, orbit, or temporal bone. This alone would have qualified the tumor as a T1 lesion, but its size of 3 cm indicated a higher risk and qualified it as a T2 lesion.
The histologic features of our patient’s lesion also indicate higher risk according to the current staging system.5 The Breslow thickness on histologic examination (measured from the granular cell layer of the epidermis to the deepest portion of the tumor) was 4 mm and the Clark’s level was IV (extension to the reticular dermis). The high-risk features and the location on the lip warrant a classification as T2 and carry a worse prognosis.
Wedge excision of the lower lip and Mohs surgery are accepted treatment options. The patient chose Mohs surgery and has done well, with excellent cosmetic and functional outcome. Radiation therapy can be useful as an adjuvant when lymph nodes are involved,11 but this was not necessary in our patient. Careful long-term follow up is warranted, as these patients are at higher risk of developing other, separate tumors.
- Schwartz RA. Squamous cell carcinoma. In:Schwartz RA, editor. Skin Cancer: Recognition and Management, 2nd ed. Malden, MA: Blackwell Publishing; 2008:47–65.
- D’Souza J, Clark J. Management of the neck in metastatic cutaneous squamous cell carcinoma of the head and neck. Curr Opin Otolaryngol Head Neck Surg 2011; 19:99–105.
- Zbar RI, Canady JW. MOC-PSSM CME article: Nonmelanoma facial skin malignancy. Plast Reconstr Surg 2008; 121(suppl 1):1–9.
- Morselli P, Masciotra L, Pinto V, Zollino I, Brunelli G, Carinci F. Clinical parameters in T1N0M0 lower lip squamous cell carcinoma. J Craniofac Surg 2007; 18:1079–1082.
- American Joint Commission on Cancer. Cancer Staging Manual. 7th ed. http://www.cancerstaging.org. Accessed February 3, 2013.
- Lardaro T, Shea SM, Sharfman W, Liégeois N, Sober AJ. Improvements in the staging of cutaneous squamous-cell carcinoma in the 7th edition of the AJCC Cancer Staging Manual. Ann Surg Oncol 2010; 17:1979–1980.
- Cutaneous squamous cell carcinoma and other cutaneous carcinomas. In:Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:301–314.
- Frierson HF, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Hum Pathol 1986; 17:346–354.
- Civantos FJ, Moffat FL, goodwin WJ. Lymphatic mapping and sentinel lymphadenectomy for 106 head and neck lesions: contrasts between oral cavity and cutaneous malignancy. Laryngoscope 2006; 112(3 Pt 2 suppl 109):1–15.
- Rowe De, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol 1992; 26:976–990.
- Veness MJ, Palme CE, Smith M, Cakir B, Morgan GJ, Kalnins I. Cutaneous head and neck squamous cell carcinoma metastatic to cervical lymph nodes (nonparotid): a better outcome with surgery and adjuvant radiotherapy. Laryngoscope 2003; 113:1827–1833.
- Schwartz RA. Squamous cell carcinoma. In:Schwartz RA, editor. Skin Cancer: Recognition and Management, 2nd ed. Malden, MA: Blackwell Publishing; 2008:47–65.
- D’Souza J, Clark J. Management of the neck in metastatic cutaneous squamous cell carcinoma of the head and neck. Curr Opin Otolaryngol Head Neck Surg 2011; 19:99–105.
- Zbar RI, Canady JW. MOC-PSSM CME article: Nonmelanoma facial skin malignancy. Plast Reconstr Surg 2008; 121(suppl 1):1–9.
- Morselli P, Masciotra L, Pinto V, Zollino I, Brunelli G, Carinci F. Clinical parameters in T1N0M0 lower lip squamous cell carcinoma. J Craniofac Surg 2007; 18:1079–1082.
- American Joint Commission on Cancer. Cancer Staging Manual. 7th ed. http://www.cancerstaging.org. Accessed February 3, 2013.
- Lardaro T, Shea SM, Sharfman W, Liégeois N, Sober AJ. Improvements in the staging of cutaneous squamous-cell carcinoma in the 7th edition of the AJCC Cancer Staging Manual. Ann Surg Oncol 2010; 17:1979–1980.
- Cutaneous squamous cell carcinoma and other cutaneous carcinomas. In:Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:301–314.
- Frierson HF, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Hum Pathol 1986; 17:346–354.
- Civantos FJ, Moffat FL, goodwin WJ. Lymphatic mapping and sentinel lymphadenectomy for 106 head and neck lesions: contrasts between oral cavity and cutaneous malignancy. Laryngoscope 2006; 112(3 Pt 2 suppl 109):1–15.
- Rowe De, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol 1992; 26:976–990.
- Veness MJ, Palme CE, Smith M, Cakir B, Morgan GJ, Kalnins I. Cutaneous head and neck squamous cell carcinoma metastatic to cervical lymph nodes (nonparotid): a better outcome with surgery and adjuvant radiotherapy. Laryngoscope 2003; 113:1827–1833.