User login
Itchy Papules and Plaques on the Dorsal Hands
The Diagnosis: Neutrophilic Dermatosis of the Dorsal Hands
Neutrophilic dermatosis of the dorsal hands (NDDH) is considered to be an uncommon localized variant of Sweet syndrome (SS). The term pustular vasculitis originally was used to describe this condition by Strutton et al1 in 1995 due to the presence of leukocytoclastic vasculitis on histology. In 2000, Galaria et al2 suggested this eruption was a localized variant of SS based on clinical presentations that demonstrated associated fever and lack of necrotizing vasculitis and proposed the term neutrophilic dermatosis of the dorsal hands to describe the condition. Cases of similar cutaneous eruptions on the hands associated with fever, leukocytosis, elevated erythrocyte sedimentation rate, and leukocytoclasis have since been reported.3-5 Some authors have concluded that these eruptions, previously termed atypical pyoderma gangrenosum and pustular vasculitis of the hands, represent a single disease entity and should be designated as NDDH.3,4
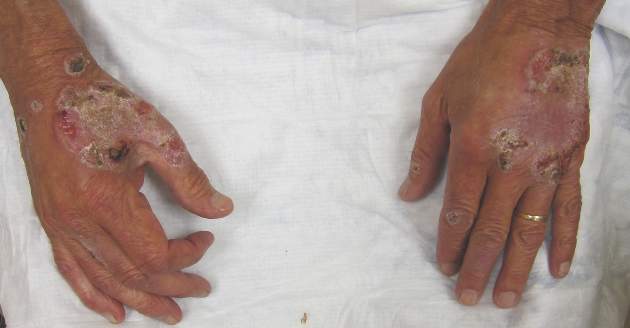
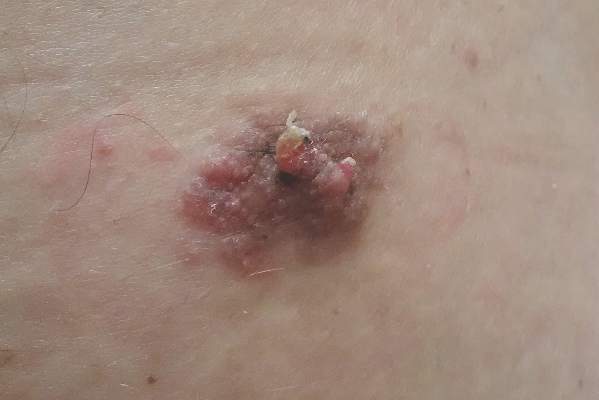
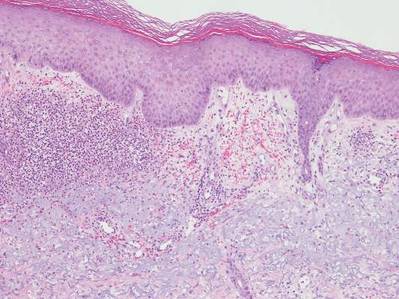
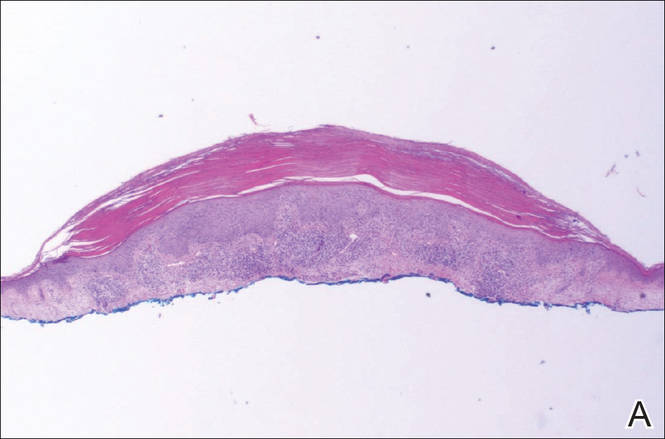
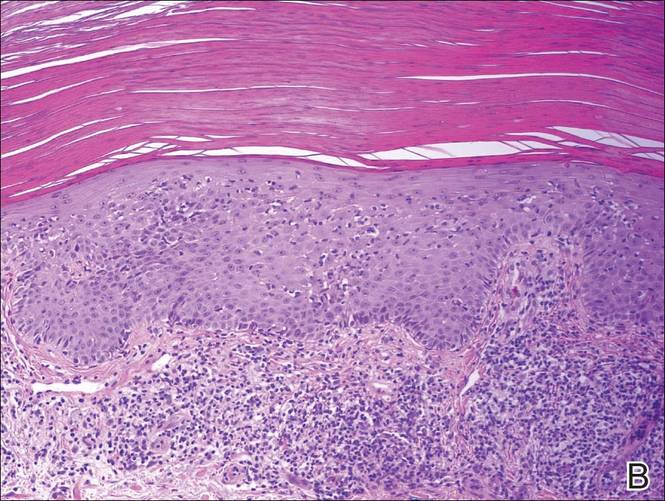
Neutrophilic dermatosis of the dorsal hands characteristically presents with hemorrhagic pustular ulcerations limited to or predominantly located on the dorsal hands, as seen in our patient. Histopathologically, NDDH demonstrates a neutrophil-predominant infiltrate of the upper dermis and marked papillary dermal edema; a punch biopsy specimen from our patient was consistent with these features (Figure). Two punch biopsies were performed and were negative for fungus and acid-fast bacteria and positive for methicillin-sensitive Staphylococcus aureus. Vasculitis, if present, is more commonly seen in eruptions of longer duration (ie, months to years) and is thought to be secondary to the dense neutrophilic infiltrate and not a primary vasculitis.3,6,7 Similar to classic SS, NDDH is inherently responsive to corticosteroid therapy. Successful treatment also has been reported with dapsone, colchicine, sulfapyridine, potassium iodide, intralesional and topical corticosteroids, and topical tacrolimus.2-8 Oral minocycline has shown variable results.3,4
Numerous case series have demonstrated that a majority of cases of NDDH are associated with hematologic or solid organ malignancies, myelodysplastic syndrome (MDS), inflammatory bowel disease, or other underlying systemic diseases.3,5,9 It is important for dermatologists to recognize NDDH, distinguish it from localized infection, and perform the appropriate workup (eg, basic laboratory tests [complete blood count, complete metabolic panel], age-appropriate malignancy screening, colonoscopy, bone marrow biopsy) to exclude associated systemic diseases.
Our patient demonstrated characteristic clinical and histopathologic findings of NDDH in association with early MDS and possible common bile duct (CBD) malignancy. The lesions showed a rapid response to topical corticosteroid therapy. The initial differential diagnoses included NDDH or other neutrophilic dermatosis, phototoxic drug eruption, and atypical mycobacterial or fungal infection (cultures were negative in our patient). Physical examination and histopathologic findings along with the patient’s clinical course and rapid response to topical corticosteroid therapy supported the diagnosis of NDDH. Our patient’s multiple comorbidities, including macrocytic anemia, MDS, and potential CBD malignancy, presented a therapeutic challenge. Oral dapsone, an ideal steroid-sparing agent for neutrophilic dermatoses including NDDH, was avoided given its associated hematologic side effects including hemolysis, methemoglobinemia, and possible agranulocytosis. To date, the patient has not received any further treatment for MDS or the CBD mass and continues regular follow-up with hematology, gastroenterology, and dermatology.
This case highlights the importance of including NDDH in the differential diagnosis of papules and plaques on the hands, especially in patients with known malignancies, and emphasizes the association of neutrophilic dermatoses with malignancy and systemic disease.
- Strutton G, Weedon D, Robertson I. Pustular vasculitis of the hands. J Am Acad Dermatol. 1995;32:192-198.
- Galaria NA, Junkins-Hopkins JM, Kligman D, et al. Neutrophilic dermatosis of the dorsal hands: pustular vasculitis revisited. J Am Acad Dermatol. 2000;43:870-874.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- DiCaudo DJ, Connolly SM. Neutrophilic dermatosis (pustular vasculitis) of the dorsal hands. Arch Dermatol. 2002;138:361-365.
- Weening RH, Bruce AJ, McEvoy MT, et al. Neutrophilic dermatosis of the hands: four new cases and review of the literature. Int J Dermatol. 2004;43:95-102.
- Malone JC, Slone SP, Wills-Frank LA, et al. Vascular inflammation (vasculitis) in Sweet syndrome: a clinicopathologic study of 28 biopsy specimens from 21 patients. Arch Dermatol. 2002;138:345-349.
- Cohen PR. Skin lesions of Sweet syndrome and its dorsal hand variant contain vasculitis: an oxymoron or an epiphenomenon? Arch Dermatol. 2002;138:400-403.
- Del Pozo J, Sacristán F, Martínez W, et al. Neutrophilic dermatosis of the hands: presentation of eight cases and review of the literature. J Dermatol. 2007;34:243-247.
- Callen JP. Neutrophilic dermatoses. Dermatol Clin. 2002;20:409-419.
The Diagnosis: Neutrophilic Dermatosis of the Dorsal Hands
Neutrophilic dermatosis of the dorsal hands (NDDH) is considered to be an uncommon localized variant of Sweet syndrome (SS). The term pustular vasculitis originally was used to describe this condition by Strutton et al1 in 1995 due to the presence of leukocytoclastic vasculitis on histology. In 2000, Galaria et al2 suggested this eruption was a localized variant of SS based on clinical presentations that demonstrated associated fever and lack of necrotizing vasculitis and proposed the term neutrophilic dermatosis of the dorsal hands to describe the condition. Cases of similar cutaneous eruptions on the hands associated with fever, leukocytosis, elevated erythrocyte sedimentation rate, and leukocytoclasis have since been reported.3-5 Some authors have concluded that these eruptions, previously termed atypical pyoderma gangrenosum and pustular vasculitis of the hands, represent a single disease entity and should be designated as NDDH.3,4
Neutrophilic dermatosis of the dorsal hands characteristically presents with hemorrhagic pustular ulcerations limited to or predominantly located on the dorsal hands, as seen in our patient. Histopathologically, NDDH demonstrates a neutrophil-predominant infiltrate of the upper dermis and marked papillary dermal edema; a punch biopsy specimen from our patient was consistent with these features (Figure). Two punch biopsies were performed and were negative for fungus and acid-fast bacteria and positive for methicillin-sensitive Staphylococcus aureus. Vasculitis, if present, is more commonly seen in eruptions of longer duration (ie, months to years) and is thought to be secondary to the dense neutrophilic infiltrate and not a primary vasculitis.3,6,7 Similar to classic SS, NDDH is inherently responsive to corticosteroid therapy. Successful treatment also has been reported with dapsone, colchicine, sulfapyridine, potassium iodide, intralesional and topical corticosteroids, and topical tacrolimus.2-8 Oral minocycline has shown variable results.3,4

Numerous case series have demonstrated that a majority of cases of NDDH are associated with hematologic or solid organ malignancies, myelodysplastic syndrome (MDS), inflammatory bowel disease, or other underlying systemic diseases.3,5,9 It is important for dermatologists to recognize NDDH, distinguish it from localized infection, and perform the appropriate workup (eg, basic laboratory tests [complete blood count, complete metabolic panel], age-appropriate malignancy screening, colonoscopy, bone marrow biopsy) to exclude associated systemic diseases.
Our patient demonstrated characteristic clinical and histopathologic findings of NDDH in association with early MDS and possible common bile duct (CBD) malignancy. The lesions showed a rapid response to topical corticosteroid therapy. The initial differential diagnoses included NDDH or other neutrophilic dermatosis, phototoxic drug eruption, and atypical mycobacterial or fungal infection (cultures were negative in our patient). Physical examination and histopathologic findings along with the patient’s clinical course and rapid response to topical corticosteroid therapy supported the diagnosis of NDDH. Our patient’s multiple comorbidities, including macrocytic anemia, MDS, and potential CBD malignancy, presented a therapeutic challenge. Oral dapsone, an ideal steroid-sparing agent for neutrophilic dermatoses including NDDH, was avoided given its associated hematologic side effects including hemolysis, methemoglobinemia, and possible agranulocytosis. To date, the patient has not received any further treatment for MDS or the CBD mass and continues regular follow-up with hematology, gastroenterology, and dermatology.
This case highlights the importance of including NDDH in the differential diagnosis of papules and plaques on the hands, especially in patients with known malignancies, and emphasizes the association of neutrophilic dermatoses with malignancy and systemic disease.
The Diagnosis: Neutrophilic Dermatosis of the Dorsal Hands
Neutrophilic dermatosis of the dorsal hands (NDDH) is considered to be an uncommon localized variant of Sweet syndrome (SS). The term pustular vasculitis originally was used to describe this condition by Strutton et al1 in 1995 due to the presence of leukocytoclastic vasculitis on histology. In 2000, Galaria et al2 suggested this eruption was a localized variant of SS based on clinical presentations that demonstrated associated fever and lack of necrotizing vasculitis and proposed the term neutrophilic dermatosis of the dorsal hands to describe the condition. Cases of similar cutaneous eruptions on the hands associated with fever, leukocytosis, elevated erythrocyte sedimentation rate, and leukocytoclasis have since been reported.3-5 Some authors have concluded that these eruptions, previously termed atypical pyoderma gangrenosum and pustular vasculitis of the hands, represent a single disease entity and should be designated as NDDH.3,4
Neutrophilic dermatosis of the dorsal hands characteristically presents with hemorrhagic pustular ulcerations limited to or predominantly located on the dorsal hands, as seen in our patient. Histopathologically, NDDH demonstrates a neutrophil-predominant infiltrate of the upper dermis and marked papillary dermal edema; a punch biopsy specimen from our patient was consistent with these features (Figure). Two punch biopsies were performed and were negative for fungus and acid-fast bacteria and positive for methicillin-sensitive Staphylococcus aureus. Vasculitis, if present, is more commonly seen in eruptions of longer duration (ie, months to years) and is thought to be secondary to the dense neutrophilic infiltrate and not a primary vasculitis.3,6,7 Similar to classic SS, NDDH is inherently responsive to corticosteroid therapy. Successful treatment also has been reported with dapsone, colchicine, sulfapyridine, potassium iodide, intralesional and topical corticosteroids, and topical tacrolimus.2-8 Oral minocycline has shown variable results.3,4

Numerous case series have demonstrated that a majority of cases of NDDH are associated with hematologic or solid organ malignancies, myelodysplastic syndrome (MDS), inflammatory bowel disease, or other underlying systemic diseases.3,5,9 It is important for dermatologists to recognize NDDH, distinguish it from localized infection, and perform the appropriate workup (eg, basic laboratory tests [complete blood count, complete metabolic panel], age-appropriate malignancy screening, colonoscopy, bone marrow biopsy) to exclude associated systemic diseases.
Our patient demonstrated characteristic clinical and histopathologic findings of NDDH in association with early MDS and possible common bile duct (CBD) malignancy. The lesions showed a rapid response to topical corticosteroid therapy. The initial differential diagnoses included NDDH or other neutrophilic dermatosis, phototoxic drug eruption, and atypical mycobacterial or fungal infection (cultures were negative in our patient). Physical examination and histopathologic findings along with the patient’s clinical course and rapid response to topical corticosteroid therapy supported the diagnosis of NDDH. Our patient’s multiple comorbidities, including macrocytic anemia, MDS, and potential CBD malignancy, presented a therapeutic challenge. Oral dapsone, an ideal steroid-sparing agent for neutrophilic dermatoses including NDDH, was avoided given its associated hematologic side effects including hemolysis, methemoglobinemia, and possible agranulocytosis. To date, the patient has not received any further treatment for MDS or the CBD mass and continues regular follow-up with hematology, gastroenterology, and dermatology.
This case highlights the importance of including NDDH in the differential diagnosis of papules and plaques on the hands, especially in patients with known malignancies, and emphasizes the association of neutrophilic dermatoses with malignancy and systemic disease.
- Strutton G, Weedon D, Robertson I. Pustular vasculitis of the hands. J Am Acad Dermatol. 1995;32:192-198.
- Galaria NA, Junkins-Hopkins JM, Kligman D, et al. Neutrophilic dermatosis of the dorsal hands: pustular vasculitis revisited. J Am Acad Dermatol. 2000;43:870-874.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- DiCaudo DJ, Connolly SM. Neutrophilic dermatosis (pustular vasculitis) of the dorsal hands. Arch Dermatol. 2002;138:361-365.
- Weening RH, Bruce AJ, McEvoy MT, et al. Neutrophilic dermatosis of the hands: four new cases and review of the literature. Int J Dermatol. 2004;43:95-102.
- Malone JC, Slone SP, Wills-Frank LA, et al. Vascular inflammation (vasculitis) in Sweet syndrome: a clinicopathologic study of 28 biopsy specimens from 21 patients. Arch Dermatol. 2002;138:345-349.
- Cohen PR. Skin lesions of Sweet syndrome and its dorsal hand variant contain vasculitis: an oxymoron or an epiphenomenon? Arch Dermatol. 2002;138:400-403.
- Del Pozo J, Sacristán F, Martínez W, et al. Neutrophilic dermatosis of the hands: presentation of eight cases and review of the literature. J Dermatol. 2007;34:243-247.
- Callen JP. Neutrophilic dermatoses. Dermatol Clin. 2002;20:409-419.
- Strutton G, Weedon D, Robertson I. Pustular vasculitis of the hands. J Am Acad Dermatol. 1995;32:192-198.
- Galaria NA, Junkins-Hopkins JM, Kligman D, et al. Neutrophilic dermatosis of the dorsal hands: pustular vasculitis revisited. J Am Acad Dermatol. 2000;43:870-874.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- DiCaudo DJ, Connolly SM. Neutrophilic dermatosis (pustular vasculitis) of the dorsal hands. Arch Dermatol. 2002;138:361-365.
- Weening RH, Bruce AJ, McEvoy MT, et al. Neutrophilic dermatosis of the hands: four new cases and review of the literature. Int J Dermatol. 2004;43:95-102.
- Malone JC, Slone SP, Wills-Frank LA, et al. Vascular inflammation (vasculitis) in Sweet syndrome: a clinicopathologic study of 28 biopsy specimens from 21 patients. Arch Dermatol. 2002;138:345-349.
- Cohen PR. Skin lesions of Sweet syndrome and its dorsal hand variant contain vasculitis: an oxymoron or an epiphenomenon? Arch Dermatol. 2002;138:400-403.
- Del Pozo J, Sacristán F, Martínez W, et al. Neutrophilic dermatosis of the hands: presentation of eight cases and review of the literature. J Dermatol. 2007;34:243-247.
- Callen JP. Neutrophilic dermatoses. Dermatol Clin. 2002;20:409-419.
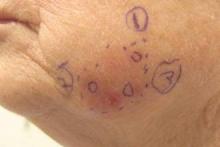
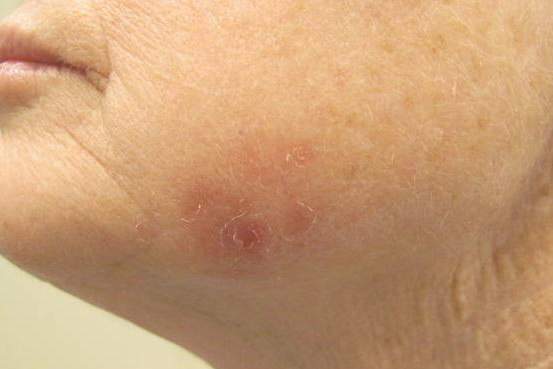
A 69-year-old man presented with tender, itchy papules and plaques on the bilateral dorsal hands of 2 months’ duration. The plaques had started as small papules that gradually enlarged and then became ulcerated. The patient denied prior trauma or constitutional symptoms. Laboratory testing revealed macrocytic anemia, thrombocytosis, and hypoalbuminemia. A complete blood count and complete metabolic panel were otherwise unremarkable. A recent bone marrow biopsy for macrocytic anemia performed prior to the current presentation suggested early myelodysplastic syndrome, and endoscopic retrograde cholangiopancreatography revealed a large mass in the common bile duct that was suspicious for malignancy. Two punch biopsies were performed and were negative for fungus and acid-fast bacteria and positive for methicillin-sensitive Staphylococcus aureus. Treatment with topical clobetasol 0.05% twice daily was initiated with complete healing of the plaques on the hands after 2 weeks of use; however, the patient continued to develop new ulcerated papulonodules distally.
Erythematous Scaly Papules on the Shins and Calves
The Diagnosis: Hyperkeratosis Lenticularis Perstans
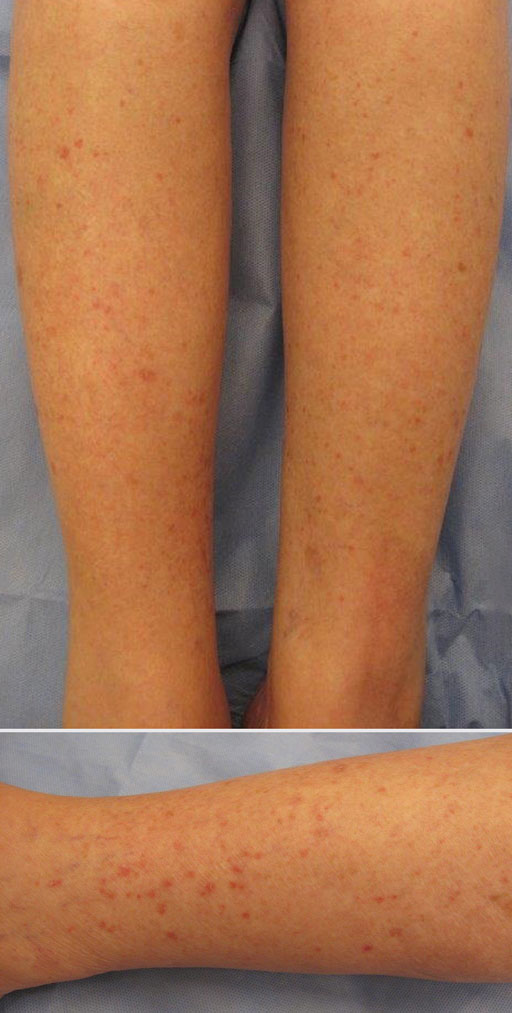
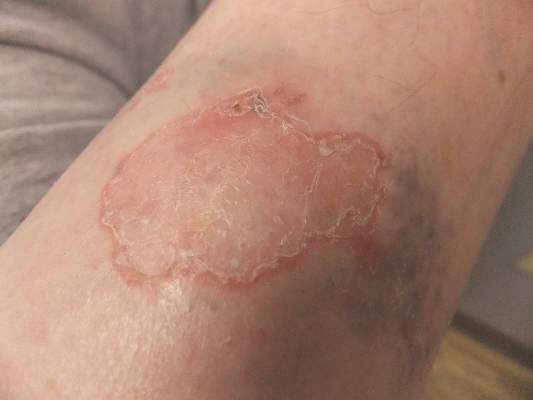
A shave biopsy of a lesion on the right leg was performed. Histopathology revealed a discrete papule with overlying compact hyperkeratosis. There was parakeratosis with an absent granular layer and a lichenoid lymphocytic infiltrate within the papillary dermis (Figure). Given the clinical context, these changes were consistent with a diagnosis of hyperkeratosis lenticularis perstans (HLP), also known as Flegel disease.
The patient was started on tretinoin cream 0.1% nightly for 3 months and triamcinolone ointment 0.1% as needed for pruritus but showed no clinical response. Given the benign nature of the condition and because the lesions were asymptomatic, additional treatment options were not pursued.
Originally described by Flegel1 in 1958, HLP is a rare skin disorder commonly seen in white individuals with onset in the fourth or fifth decades of life.1,2 While most cases are sporadic,3-6 HLP also has been associated with autosomal dominant inheritance.7-10
Patients with HLP typically present with multiple 1- to 5-mm reddish-brown, hyperkeratotic, scaly papules that reveal a moist, erythematous base with pinpoint bleeding upon removal of the scale. Lesions usually are distributed symmetrically and most commonly present on the extensor surfaces of the lower legs and dorsal feet.1,2,7 Lesions also may appear on the extensor surfaces of the arms, pinna, periocular region, antecubital and popliteal fossae, and oral mucosa and also may present as pits on the palms and soles.2,4,7,8 Furthermore, unilateral and localized variants of HLP have been described.11,12 Hyperkeratosis lenticularis perstans usually is asymptomatic but can present with mild pruritus or burning.3,5,13
The etiology and pathogenesis of HLP are unknown. Exposure to UV light has been implicated as an inciting factor14; however, reports of spontaneous resolution in the summer13 and upon treatment with psoralen plus UVA therapy15 make the role of UV light unclear. Furthermore, investigators disagree as to whether the primary pathogenic event in HLP is an inflammatory process or one of abnormal keratinization.1,3,7,10 Fernandez-Flores and Manjon16 suggested HLP is an inflammatory process with periods of exacerbations and remissions after finding mounds of parakeratosis with neutrophils arranged in different strata in the stratum corneum.
Histologically, compact hyperkeratosis usually is noted, often with associated parakeratosis, epidermal atrophy with thinning or absence of the granular layer, and a bandlike lymphohistiocytic infiltrate in the papillary dermis.1-3 Histopathologic differences between recent-onset versus longstanding lesions have been found, with old lesions lacking an inflammatory infiltrate.3 Furthermore, new lesions often show abnormalities in quantity and/or morphology of membrane-coating granules, also known as Odland bodies, in keratinocytes on electron microscopy,3,10,17 while old lesions do not.3 Odland bodies are involved in normal desquamation, leading some to speculate on their role in HLP.10 Currently, it is unclear whether abnormalities in these organelles cause the retention hyperkeratosis seen in HLP or if such abnormalities are a secondary phenomenon.3,17
There are questionable associations between HLP and diabetes mellitus type 2, hyperthyroidism, basal and squamous cell carcinomas of the skin, and gastrointestinal malignancy.4,9,18 Our patient had a history of basal cell carcinoma on the face, diet-controlled diabetes mellitus, and hypothyroidism. Given the high prevalence of these diseases in the general population, however, it is difficult to ascertain whether a true association with HLP exists.
While HLP can slowly progress to involve additional body sites, it is overall a benign condition that does not require treatment. Therapeutic options are based on case reports, with no single treatment showing a consistent response. From review of the literature, therapies that have been most effective include dermabrasion, excision,19 topical 5-fluorouracil,2,17,20 and oral retinoids.8 Hyperkeratosis lenticularis perstans generally is resistant to topical steroids, retinoids, and vitamin D3 analogs, although success with betamethasone dipropionate,5 isotretinoin
gel 0.05%,11 and calcipotriol have been reported.6 A case of HLP with clinical response to psoralen plus UVA therapy also has been described.15
- Flegel H. Hyperkeratosis lenticularis perstans. Hautarzt. 1958;9:363-364.
- Pearson LH, Smith JG, Chalker DK. Hyperkeratosis lenticularis perstans (Flegel’s disease). J Am Acad Dermatol. 1987;16:190-195.
- Ando K, Hattori H, Yamauchi Y. Histopathological differences between early and old lesions of hyperkeratosis lenticularis perstans (Flegel’s disease). Am J Dermatopathol. 2006;28:122-126.
- Fernández-Crehuet P, Rodríguez-Rey E, Ríos-Martín JJ, et al. Hyperkeratosis lenticularis perstans, or Flegel disease, with palmoplantar involvement. Actas Dermosifiliogr. 2009;100:157-159.
- Sterneberg-Vos H, van Marion AM, Frank J, et al. Hyperkeratosis lenticularis perstans (Flegel’s disease)—successful treatment with topical corticosteroids. Int J Dermatol. 2008;47:38-41.
- Bayramgürler D, Apaydin R, Dökmeci S, et al. Flegel’s disease: treatment with topical calcipotriol. Clin Exp Dermatol. 2002;27:161-162.
- Price ML, Jones EW, MacDonald DM. A clinicopathological study of Flegel’s disease (hyperkeratosis lenticularis perstans). Br J Dermatol. 1987;116:681-691.
- Krishnan A, Kar S. Photoletter to the editor: hyperkeratosis lenticularis perstans (Flegel’s disease) with unusual clinical presentation. response to isotretinoin therapy. J Dermatol Case Rep. 2012;6:93-95.
- Beveridge GW, Langlands AO. Familial hyperkeratosis lenticularis perstans associated with tumours of the skin. Br J Dermatol. 1973;88:453-458.
- Frenk E, Tapernoux B. Hyperkeratosis lenticularis perstans (Flegel): a biological model for keratinization occurring in the absence of Odland bodies? Dermatologica. 1976;153:253-262.
- Miranda-Romero A, Sánchez Sambucety P, Bajo del Pozo C, et al. Unilateral hyperkeratosis lenticularis perstans (Flegel's disease). J Am Acad Dermatol. 1998;39:655-657.
- Gutiérrez MC, Hasson A, Arias MD, et al. Localized hyperkeratosis lenticularis perstans (Flegel's disease). Cutis. 1991;48:201-204.
- Fathy S, Azadeh B. Hyperkeratosis lenticularis perstans. Int J Dermatol. 1988;27:120-121.
- Rosdahl I, Rosen K. Hyperkeratosis lenticularis perstans: report on two cases. Acta Derm Venerol. 1985;65:562-564.
- Cooper SM, George S. Flegel's disease treated with psoralen ultraviolet A. Br J Dermatol. 2000;142:340-342.
- Fernandez-Flores A, Manjon JA. Morphological evidence of periodical exacerbation of hyperkeratosis lenticularis perstans. Acta Dermatovenerol Croat. 2009;17:16-19.
- Langer K, Zonzits E, Konrad K. Hyperkeratosis lenticularis perstans (Flegel's disease). ultrastructural study of lesional and perilesional skin and therapeutic trial of topical tretinoin versus 5-fluorouracil. J Am Acad Dermatol. 1992;27:812-816.
- Ishibashi A, Tsuboi R, Fujita K. Familial hyperkeratosis lenticularis perstans. associated with cancers of the digestive organs. J Dermatol. 1984;11:407-409.
- Cunha Filho RR, Almeida Jr HL. Hyperkeratosis lenticularis perstans. An Bras Dermatol. 2011;86(4 suppl 1):S76-S77.
- Blaheta HJ, Metzler G, Rassner G, et al. Hyperkeratosis lenticularis perstans (Flegel's disease)—lack of response to treatment with tacalcitol and calcipotriol. Dermatology. 2001;202:255-258.
The Diagnosis: Hyperkeratosis Lenticularis Perstans
A shave biopsy of a lesion on the right leg was performed. Histopathology revealed a discrete papule with overlying compact hyperkeratosis. There was parakeratosis with an absent granular layer and a lichenoid lymphocytic infiltrate within the papillary dermis (Figure). Given the clinical context, these changes were consistent with a diagnosis of hyperkeratosis lenticularis perstans (HLP), also known as Flegel disease.


The patient was started on tretinoin cream 0.1% nightly for 3 months and triamcinolone ointment 0.1% as needed for pruritus but showed no clinical response. Given the benign nature of the condition and because the lesions were asymptomatic, additional treatment options were not pursued.
Originally described by Flegel1 in 1958, HLP is a rare skin disorder commonly seen in white individuals with onset in the fourth or fifth decades of life.1,2 While most cases are sporadic,3-6 HLP also has been associated with autosomal dominant inheritance.7-10
Patients with HLP typically present with multiple 1- to 5-mm reddish-brown, hyperkeratotic, scaly papules that reveal a moist, erythematous base with pinpoint bleeding upon removal of the scale. Lesions usually are distributed symmetrically and most commonly present on the extensor surfaces of the lower legs and dorsal feet.1,2,7 Lesions also may appear on the extensor surfaces of the arms, pinna, periocular region, antecubital and popliteal fossae, and oral mucosa and also may present as pits on the palms and soles.2,4,7,8 Furthermore, unilateral and localized variants of HLP have been described.11,12 Hyperkeratosis lenticularis perstans usually is asymptomatic but can present with mild pruritus or burning.3,5,13
The etiology and pathogenesis of HLP are unknown. Exposure to UV light has been implicated as an inciting factor14; however, reports of spontaneous resolution in the summer13 and upon treatment with psoralen plus UVA therapy15 make the role of UV light unclear. Furthermore, investigators disagree as to whether the primary pathogenic event in HLP is an inflammatory process or one of abnormal keratinization.1,3,7,10 Fernandez-Flores and Manjon16 suggested HLP is an inflammatory process with periods of exacerbations and remissions after finding mounds of parakeratosis with neutrophils arranged in different strata in the stratum corneum.
Histologically, compact hyperkeratosis usually is noted, often with associated parakeratosis, epidermal atrophy with thinning or absence of the granular layer, and a bandlike lymphohistiocytic infiltrate in the papillary dermis.1-3 Histopathologic differences between recent-onset versus longstanding lesions have been found, with old lesions lacking an inflammatory infiltrate.3 Furthermore, new lesions often show abnormalities in quantity and/or morphology of membrane-coating granules, also known as Odland bodies, in keratinocytes on electron microscopy,3,10,17 while old lesions do not.3 Odland bodies are involved in normal desquamation, leading some to speculate on their role in HLP.10 Currently, it is unclear whether abnormalities in these organelles cause the retention hyperkeratosis seen in HLP or if such abnormalities are a secondary phenomenon.3,17
There are questionable associations between HLP and diabetes mellitus type 2, hyperthyroidism, basal and squamous cell carcinomas of the skin, and gastrointestinal malignancy.4,9,18 Our patient had a history of basal cell carcinoma on the face, diet-controlled diabetes mellitus, and hypothyroidism. Given the high prevalence of these diseases in the general population, however, it is difficult to ascertain whether a true association with HLP exists.
While HLP can slowly progress to involve additional body sites, it is overall a benign condition that does not require treatment. Therapeutic options are based on case reports, with no single treatment showing a consistent response. From review of the literature, therapies that have been most effective include dermabrasion, excision,19 topical 5-fluorouracil,2,17,20 and oral retinoids.8 Hyperkeratosis lenticularis perstans generally is resistant to topical steroids, retinoids, and vitamin D3 analogs, although success with betamethasone dipropionate,5 isotretinoin
gel 0.05%,11 and calcipotriol have been reported.6 A case of HLP with clinical response to psoralen plus UVA therapy also has been described.15
The Diagnosis: Hyperkeratosis Lenticularis Perstans
A shave biopsy of a lesion on the right leg was performed. Histopathology revealed a discrete papule with overlying compact hyperkeratosis. There was parakeratosis with an absent granular layer and a lichenoid lymphocytic infiltrate within the papillary dermis (Figure). Given the clinical context, these changes were consistent with a diagnosis of hyperkeratosis lenticularis perstans (HLP), also known as Flegel disease.


The patient was started on tretinoin cream 0.1% nightly for 3 months and triamcinolone ointment 0.1% as needed for pruritus but showed no clinical response. Given the benign nature of the condition and because the lesions were asymptomatic, additional treatment options were not pursued.
Originally described by Flegel1 in 1958, HLP is a rare skin disorder commonly seen in white individuals with onset in the fourth or fifth decades of life.1,2 While most cases are sporadic,3-6 HLP also has been associated with autosomal dominant inheritance.7-10
Patients with HLP typically present with multiple 1- to 5-mm reddish-brown, hyperkeratotic, scaly papules that reveal a moist, erythematous base with pinpoint bleeding upon removal of the scale. Lesions usually are distributed symmetrically and most commonly present on the extensor surfaces of the lower legs and dorsal feet.1,2,7 Lesions also may appear on the extensor surfaces of the arms, pinna, periocular region, antecubital and popliteal fossae, and oral mucosa and also may present as pits on the palms and soles.2,4,7,8 Furthermore, unilateral and localized variants of HLP have been described.11,12 Hyperkeratosis lenticularis perstans usually is asymptomatic but can present with mild pruritus or burning.3,5,13
The etiology and pathogenesis of HLP are unknown. Exposure to UV light has been implicated as an inciting factor14; however, reports of spontaneous resolution in the summer13 and upon treatment with psoralen plus UVA therapy15 make the role of UV light unclear. Furthermore, investigators disagree as to whether the primary pathogenic event in HLP is an inflammatory process or one of abnormal keratinization.1,3,7,10 Fernandez-Flores and Manjon16 suggested HLP is an inflammatory process with periods of exacerbations and remissions after finding mounds of parakeratosis with neutrophils arranged in different strata in the stratum corneum.
Histologically, compact hyperkeratosis usually is noted, often with associated parakeratosis, epidermal atrophy with thinning or absence of the granular layer, and a bandlike lymphohistiocytic infiltrate in the papillary dermis.1-3 Histopathologic differences between recent-onset versus longstanding lesions have been found, with old lesions lacking an inflammatory infiltrate.3 Furthermore, new lesions often show abnormalities in quantity and/or morphology of membrane-coating granules, also known as Odland bodies, in keratinocytes on electron microscopy,3,10,17 while old lesions do not.3 Odland bodies are involved in normal desquamation, leading some to speculate on their role in HLP.10 Currently, it is unclear whether abnormalities in these organelles cause the retention hyperkeratosis seen in HLP or if such abnormalities are a secondary phenomenon.3,17
There are questionable associations between HLP and diabetes mellitus type 2, hyperthyroidism, basal and squamous cell carcinomas of the skin, and gastrointestinal malignancy.4,9,18 Our patient had a history of basal cell carcinoma on the face, diet-controlled diabetes mellitus, and hypothyroidism. Given the high prevalence of these diseases in the general population, however, it is difficult to ascertain whether a true association with HLP exists.
While HLP can slowly progress to involve additional body sites, it is overall a benign condition that does not require treatment. Therapeutic options are based on case reports, with no single treatment showing a consistent response. From review of the literature, therapies that have been most effective include dermabrasion, excision,19 topical 5-fluorouracil,2,17,20 and oral retinoids.8 Hyperkeratosis lenticularis perstans generally is resistant to topical steroids, retinoids, and vitamin D3 analogs, although success with betamethasone dipropionate,5 isotretinoin
gel 0.05%,11 and calcipotriol have been reported.6 A case of HLP with clinical response to psoralen plus UVA therapy also has been described.15
- Flegel H. Hyperkeratosis lenticularis perstans. Hautarzt. 1958;9:363-364.
- Pearson LH, Smith JG, Chalker DK. Hyperkeratosis lenticularis perstans (Flegel’s disease). J Am Acad Dermatol. 1987;16:190-195.
- Ando K, Hattori H, Yamauchi Y. Histopathological differences between early and old lesions of hyperkeratosis lenticularis perstans (Flegel’s disease). Am J Dermatopathol. 2006;28:122-126.
- Fernández-Crehuet P, Rodríguez-Rey E, Ríos-Martín JJ, et al. Hyperkeratosis lenticularis perstans, or Flegel disease, with palmoplantar involvement. Actas Dermosifiliogr. 2009;100:157-159.
- Sterneberg-Vos H, van Marion AM, Frank J, et al. Hyperkeratosis lenticularis perstans (Flegel’s disease)—successful treatment with topical corticosteroids. Int J Dermatol. 2008;47:38-41.
- Bayramgürler D, Apaydin R, Dökmeci S, et al. Flegel’s disease: treatment with topical calcipotriol. Clin Exp Dermatol. 2002;27:161-162.
- Price ML, Jones EW, MacDonald DM. A clinicopathological study of Flegel’s disease (hyperkeratosis lenticularis perstans). Br J Dermatol. 1987;116:681-691.
- Krishnan A, Kar S. Photoletter to the editor: hyperkeratosis lenticularis perstans (Flegel’s disease) with unusual clinical presentation. response to isotretinoin therapy. J Dermatol Case Rep. 2012;6:93-95.
- Beveridge GW, Langlands AO. Familial hyperkeratosis lenticularis perstans associated with tumours of the skin. Br J Dermatol. 1973;88:453-458.
- Frenk E, Tapernoux B. Hyperkeratosis lenticularis perstans (Flegel): a biological model for keratinization occurring in the absence of Odland bodies? Dermatologica. 1976;153:253-262.
- Miranda-Romero A, Sánchez Sambucety P, Bajo del Pozo C, et al. Unilateral hyperkeratosis lenticularis perstans (Flegel's disease). J Am Acad Dermatol. 1998;39:655-657.
- Gutiérrez MC, Hasson A, Arias MD, et al. Localized hyperkeratosis lenticularis perstans (Flegel's disease). Cutis. 1991;48:201-204.
- Fathy S, Azadeh B. Hyperkeratosis lenticularis perstans. Int J Dermatol. 1988;27:120-121.
- Rosdahl I, Rosen K. Hyperkeratosis lenticularis perstans: report on two cases. Acta Derm Venerol. 1985;65:562-564.
- Cooper SM, George S. Flegel's disease treated with psoralen ultraviolet A. Br J Dermatol. 2000;142:340-342.
- Fernandez-Flores A, Manjon JA. Morphological evidence of periodical exacerbation of hyperkeratosis lenticularis perstans. Acta Dermatovenerol Croat. 2009;17:16-19.
- Langer K, Zonzits E, Konrad K. Hyperkeratosis lenticularis perstans (Flegel's disease). ultrastructural study of lesional and perilesional skin and therapeutic trial of topical tretinoin versus 5-fluorouracil. J Am Acad Dermatol. 1992;27:812-816.
- Ishibashi A, Tsuboi R, Fujita K. Familial hyperkeratosis lenticularis perstans. associated with cancers of the digestive organs. J Dermatol. 1984;11:407-409.
- Cunha Filho RR, Almeida Jr HL. Hyperkeratosis lenticularis perstans. An Bras Dermatol. 2011;86(4 suppl 1):S76-S77.
- Blaheta HJ, Metzler G, Rassner G, et al. Hyperkeratosis lenticularis perstans (Flegel's disease)—lack of response to treatment with tacalcitol and calcipotriol. Dermatology. 2001;202:255-258.
- Flegel H. Hyperkeratosis lenticularis perstans. Hautarzt. 1958;9:363-364.
- Pearson LH, Smith JG, Chalker DK. Hyperkeratosis lenticularis perstans (Flegel’s disease). J Am Acad Dermatol. 1987;16:190-195.
- Ando K, Hattori H, Yamauchi Y. Histopathological differences between early and old lesions of hyperkeratosis lenticularis perstans (Flegel’s disease). Am J Dermatopathol. 2006;28:122-126.
- Fernández-Crehuet P, Rodríguez-Rey E, Ríos-Martín JJ, et al. Hyperkeratosis lenticularis perstans, or Flegel disease, with palmoplantar involvement. Actas Dermosifiliogr. 2009;100:157-159.
- Sterneberg-Vos H, van Marion AM, Frank J, et al. Hyperkeratosis lenticularis perstans (Flegel’s disease)—successful treatment with topical corticosteroids. Int J Dermatol. 2008;47:38-41.
- Bayramgürler D, Apaydin R, Dökmeci S, et al. Flegel’s disease: treatment with topical calcipotriol. Clin Exp Dermatol. 2002;27:161-162.
- Price ML, Jones EW, MacDonald DM. A clinicopathological study of Flegel’s disease (hyperkeratosis lenticularis perstans). Br J Dermatol. 1987;116:681-691.
- Krishnan A, Kar S. Photoletter to the editor: hyperkeratosis lenticularis perstans (Flegel’s disease) with unusual clinical presentation. response to isotretinoin therapy. J Dermatol Case Rep. 2012;6:93-95.
- Beveridge GW, Langlands AO. Familial hyperkeratosis lenticularis perstans associated with tumours of the skin. Br J Dermatol. 1973;88:453-458.
- Frenk E, Tapernoux B. Hyperkeratosis lenticularis perstans (Flegel): a biological model for keratinization occurring in the absence of Odland bodies? Dermatologica. 1976;153:253-262.
- Miranda-Romero A, Sánchez Sambucety P, Bajo del Pozo C, et al. Unilateral hyperkeratosis lenticularis perstans (Flegel's disease). J Am Acad Dermatol. 1998;39:655-657.
- Gutiérrez MC, Hasson A, Arias MD, et al. Localized hyperkeratosis lenticularis perstans (Flegel's disease). Cutis. 1991;48:201-204.
- Fathy S, Azadeh B. Hyperkeratosis lenticularis perstans. Int J Dermatol. 1988;27:120-121.
- Rosdahl I, Rosen K. Hyperkeratosis lenticularis perstans: report on two cases. Acta Derm Venerol. 1985;65:562-564.
- Cooper SM, George S. Flegel's disease treated with psoralen ultraviolet A. Br J Dermatol. 2000;142:340-342.
- Fernandez-Flores A, Manjon JA. Morphological evidence of periodical exacerbation of hyperkeratosis lenticularis perstans. Acta Dermatovenerol Croat. 2009;17:16-19.
- Langer K, Zonzits E, Konrad K. Hyperkeratosis lenticularis perstans (Flegel's disease). ultrastructural study of lesional and perilesional skin and therapeutic trial of topical tretinoin versus 5-fluorouracil. J Am Acad Dermatol. 1992;27:812-816.
- Ishibashi A, Tsuboi R, Fujita K. Familial hyperkeratosis lenticularis perstans. associated with cancers of the digestive organs. J Dermatol. 1984;11:407-409.
- Cunha Filho RR, Almeida Jr HL. Hyperkeratosis lenticularis perstans. An Bras Dermatol. 2011;86(4 suppl 1):S76-S77.
- Blaheta HJ, Metzler G, Rassner G, et al. Hyperkeratosis lenticularis perstans (Flegel's disease)—lack of response to treatment with tacalcitol and calcipotriol. Dermatology. 2001;202:255-258.

What Is Your Diagnosis? Herpes Zoster
The Diagnosis: Herpes Zoster
Herpes zoster is a common infection that clinically presents with moderate to intense pain in the involved dermatome (1–3 days before the outbreak1) followed by a unilateral dermatomal eruption. The most common sites of involvement include the trunk (dermatomes T3–L2) and upper face (dermatome V1).2 Herpes zoster characteristically begins as erythematous macules and papules that progress to vesicles and sometimes pustules, which subsequently crust over (7–10 days after the initial outbreak).1 Regional lymphadenopathy is present in most cases. Due to the classic presentation of herpes zoster, clinical diagnosis is the mainstay for most cases. Although it can present in any age group, herpes zoster is most commonly associated with advancing age.3
During the course of primary varicella infection, the herpes virus spreads from infected lesions to the contiguous endings of sensory nerves and travels to the dorsal root ganglion cells where it remains latent.1 It is hypothesized that cell-mediated immunity suppresses viral activity and maintains viral latency. A decline in varicella zoster virus–specific cell-mediated immunity can result in reactivation of the latent virus.1 The virus is then transported to the skin from the dorsal root ganglion via myelinated nerves, which terminate at the isthmus of hair follicles, and subsequent infection of the folliculosebaceous unit occurs.4
Laboratory tests that can assist in the diagnosis of herpes zoster, especially atypical cases, include Tzanck smear and viral culture of the vesicle fluid.1 When vesicles are not present, biopsy of the lesion followed by immunohistochemical staining and polymerase chain reaction assay can aid in the diagnosis. The differential diagnosis for our patient included pseudolymphoma, herpes simplex virus, lymphomatoid papulosis, sarcoidosis, trigeminal trophic syndrome, and Sweet syndrome.
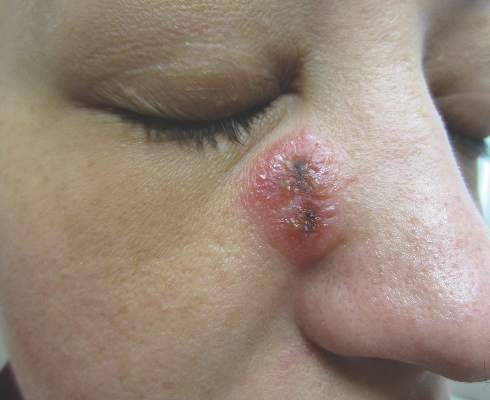
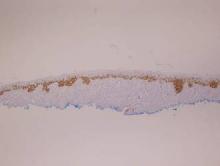
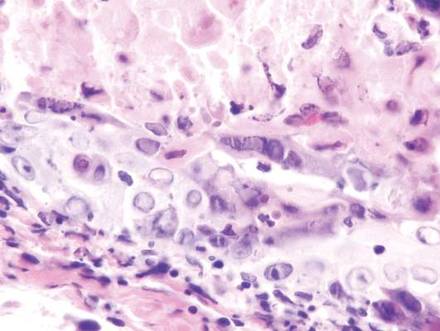
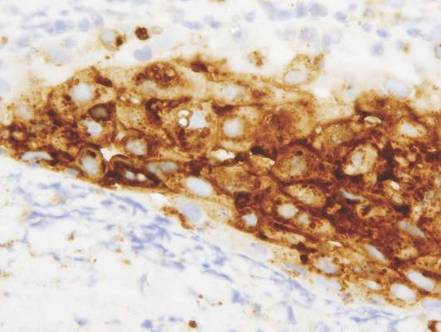
Vesicles were not present in our patient, but the dermatomal nature of the eruption and the pain she experienced made the clinical scenario suspicious for herpes zoster. A 4-mm punch biopsy of a single folliculosebaceous unit revealed herpetic, cytopathic features including prominent keratinocyte necrosis involving sebaceous, isthmic, and infundibular epithelium; ballooning of epithelial cells with steel gray nuclei; and multinucleation with nuclear molding (Figure 1). Strong nuclear and cytoplasmic staining was seen in the affected keratinocytes under anti–varicella zoster virus immunohistochemical analysis (Figure 2). Staining for herpes simplex virus types 1 and 2 was negative. Within days of starting valacyclovir 1000 mg (every 8 hours for 1 week), the patient’s symptoms resolved.
| 
| |
|
|
Atypical presentations of herpes zoster (eg, presentations that are not completely dermatomal) are becoming more common. Herpetic infections should always be in the differential diagnosis for cutaneous ulcerations. Misdiagnosis of herpes zoster due to atypical features is common and can delay prompt and adequate treatment.
- Rockley PF, Tyring SK. Pathophysiology and clinical manifestations of varicella zoster virus infections. Int J Dermatol. 1994;33:227-232.
- Chen TM, George S, Woodruff CA, et al. Clinical manifestations of varicella-zoster virus infection. Dermatol Clin. 2002;20:267-282.
- Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9-20.
- Walsh N, Boutilier R, Glasgow D, et al. Exclusive involvement of folliculosebaceous units by herpes: a reflection of early herpes zoster. Am J Dermatopathol. 2005;27:189-194.
The Diagnosis: Herpes Zoster
Herpes zoster is a common infection that clinically presents with moderate to intense pain in the involved dermatome (1–3 days before the outbreak1) followed by a unilateral dermatomal eruption. The most common sites of involvement include the trunk (dermatomes T3–L2) and upper face (dermatome V1).2 Herpes zoster characteristically begins as erythematous macules and papules that progress to vesicles and sometimes pustules, which subsequently crust over (7–10 days after the initial outbreak).1 Regional lymphadenopathy is present in most cases. Due to the classic presentation of herpes zoster, clinical diagnosis is the mainstay for most cases. Although it can present in any age group, herpes zoster is most commonly associated with advancing age.3
During the course of primary varicella infection, the herpes virus spreads from infected lesions to the contiguous endings of sensory nerves and travels to the dorsal root ganglion cells where it remains latent.1 It is hypothesized that cell-mediated immunity suppresses viral activity and maintains viral latency. A decline in varicella zoster virus–specific cell-mediated immunity can result in reactivation of the latent virus.1 The virus is then transported to the skin from the dorsal root ganglion via myelinated nerves, which terminate at the isthmus of hair follicles, and subsequent infection of the folliculosebaceous unit occurs.4
Laboratory tests that can assist in the diagnosis of herpes zoster, especially atypical cases, include Tzanck smear and viral culture of the vesicle fluid.1 When vesicles are not present, biopsy of the lesion followed by immunohistochemical staining and polymerase chain reaction assay can aid in the diagnosis. The differential diagnosis for our patient included pseudolymphoma, herpes simplex virus, lymphomatoid papulosis, sarcoidosis, trigeminal trophic syndrome, and Sweet syndrome.
Vesicles were not present in our patient, but the dermatomal nature of the eruption and the pain she experienced made the clinical scenario suspicious for herpes zoster. A 4-mm punch biopsy of a single folliculosebaceous unit revealed herpetic, cytopathic features including prominent keratinocyte necrosis involving sebaceous, isthmic, and infundibular epithelium; ballooning of epithelial cells with steel gray nuclei; and multinucleation with nuclear molding (Figure 1). Strong nuclear and cytoplasmic staining was seen in the affected keratinocytes under anti–varicella zoster virus immunohistochemical analysis (Figure 2). Staining for herpes simplex virus types 1 and 2 was negative. Within days of starting valacyclovir 1000 mg (every 8 hours for 1 week), the patient’s symptoms resolved.

| 
| |
|
|
Atypical presentations of herpes zoster (eg, presentations that are not completely dermatomal) are becoming more common. Herpetic infections should always be in the differential diagnosis for cutaneous ulcerations. Misdiagnosis of herpes zoster due to atypical features is common and can delay prompt and adequate treatment.
The Diagnosis: Herpes Zoster
Herpes zoster is a common infection that clinically presents with moderate to intense pain in the involved dermatome (1–3 days before the outbreak1) followed by a unilateral dermatomal eruption. The most common sites of involvement include the trunk (dermatomes T3–L2) and upper face (dermatome V1).2 Herpes zoster characteristically begins as erythematous macules and papules that progress to vesicles and sometimes pustules, which subsequently crust over (7–10 days after the initial outbreak).1 Regional lymphadenopathy is present in most cases. Due to the classic presentation of herpes zoster, clinical diagnosis is the mainstay for most cases. Although it can present in any age group, herpes zoster is most commonly associated with advancing age.3
During the course of primary varicella infection, the herpes virus spreads from infected lesions to the contiguous endings of sensory nerves and travels to the dorsal root ganglion cells where it remains latent.1 It is hypothesized that cell-mediated immunity suppresses viral activity and maintains viral latency. A decline in varicella zoster virus–specific cell-mediated immunity can result in reactivation of the latent virus.1 The virus is then transported to the skin from the dorsal root ganglion via myelinated nerves, which terminate at the isthmus of hair follicles, and subsequent infection of the folliculosebaceous unit occurs.4
Laboratory tests that can assist in the diagnosis of herpes zoster, especially atypical cases, include Tzanck smear and viral culture of the vesicle fluid.1 When vesicles are not present, biopsy of the lesion followed by immunohistochemical staining and polymerase chain reaction assay can aid in the diagnosis. The differential diagnosis for our patient included pseudolymphoma, herpes simplex virus, lymphomatoid papulosis, sarcoidosis, trigeminal trophic syndrome, and Sweet syndrome.
Vesicles were not present in our patient, but the dermatomal nature of the eruption and the pain she experienced made the clinical scenario suspicious for herpes zoster. A 4-mm punch biopsy of a single folliculosebaceous unit revealed herpetic, cytopathic features including prominent keratinocyte necrosis involving sebaceous, isthmic, and infundibular epithelium; ballooning of epithelial cells with steel gray nuclei; and multinucleation with nuclear molding (Figure 1). Strong nuclear and cytoplasmic staining was seen in the affected keratinocytes under anti–varicella zoster virus immunohistochemical analysis (Figure 2). Staining for herpes simplex virus types 1 and 2 was negative. Within days of starting valacyclovir 1000 mg (every 8 hours for 1 week), the patient’s symptoms resolved.

| 
| |
|
|
Atypical presentations of herpes zoster (eg, presentations that are not completely dermatomal) are becoming more common. Herpetic infections should always be in the differential diagnosis for cutaneous ulcerations. Misdiagnosis of herpes zoster due to atypical features is common and can delay prompt and adequate treatment.
- Rockley PF, Tyring SK. Pathophysiology and clinical manifestations of varicella zoster virus infections. Int J Dermatol. 1994;33:227-232.
- Chen TM, George S, Woodruff CA, et al. Clinical manifestations of varicella-zoster virus infection. Dermatol Clin. 2002;20:267-282.
- Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9-20.
- Walsh N, Boutilier R, Glasgow D, et al. Exclusive involvement of folliculosebaceous units by herpes: a reflection of early herpes zoster. Am J Dermatopathol. 2005;27:189-194.
- Rockley PF, Tyring SK. Pathophysiology and clinical manifestations of varicella zoster virus infections. Int J Dermatol. 1994;33:227-232.
- Chen TM, George S, Woodruff CA, et al. Clinical manifestations of varicella-zoster virus infection. Dermatol Clin. 2002;20:267-282.
- Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9-20.
- Walsh N, Boutilier R, Glasgow D, et al. Exclusive involvement of folliculosebaceous units by herpes: a reflection of early herpes zoster. Am J Dermatopathol. 2005;27:189-194.
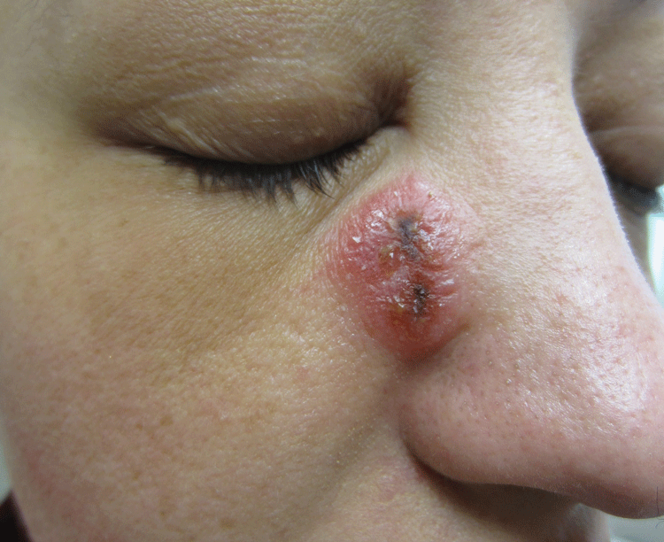
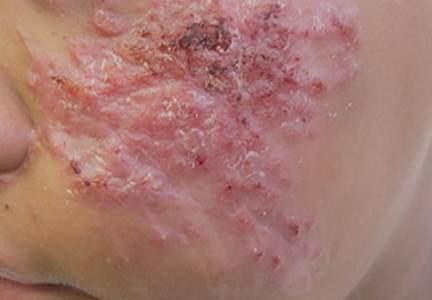
A 32-year-old woman with no remarkable medical history presented with a progressively worsening erythematous and edematous plaque on the right cheek of 8 days’ duration. She had previously been treated by her primary care physician with cephalexin for 3 days and trimethoprim-sulfamethoxazole for 2 days, which resulted in “flattening” of the plaque, but the lesion did not resolve. She was referred to our dermatology clinic for further evaluation. She denied any trauma to the cheek or scratching of the lesion. On physical examination, a 2-cm pink, erythematous, edematous plaque with a central eschar was noted on the right cheek with crusting of the right nasal wall.
Scaly Plaque With Pustules and Anonychia on the Middle Finger
The Diagnosis: Acrodermatitis Continua of Hallopeau
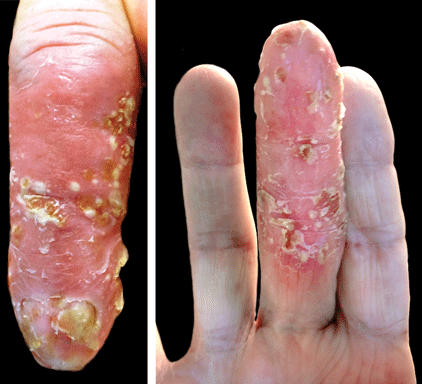
Acrodermatitis continua of Hallopeau (ACH) is considered to be a form of acropustular psoriasis that presents as a sterile, pustular eruption initially affecting the fingertips and/or toes.1 The slow-growing pustules typically progress locally and can lead to onychodystrophy and/or osteolysis of the underlying bone.2,3 Most commonly affecting adult women, ACH often begins following local trauma to or infection of a single digit.4 As the disease progresses proximally, the small pustules burst, leaving a shiny, erythematous surface on which new pustules can develop. These pustules have a tendency to amalgamate, leading to the characteristic clinical finding of lakes of pus. Pustules frequently appear on the nail matrix and nail bed presenting as severe onychodystrophy and ultimately anonychia.5,6 Rarely, ACH can be associated with generalized pustular psoriasis as well as conjunctivitis, balanitis, and fissuring or annulus migrans of the tongue.2,7
Diagnosis can be established based on clinical findings, biopsy, and bacterial and fungal cultures revealing sterile pustules.8,9 Histologic findings are similar to those seen in pustular psoriasis, demonstrating subcorneal neutrophilic pustules, Munro microabscesses, and dilated blood vessels with lymphocytic infiltrate in the papillary dermis.10
Due to the refractory nature of the disease, there are no recommended guidelines for treatment of ACH. Most successful treatment regimens consist of topical psoriasis medications combined with systemic psoriatic therapies such as cyclosporine, methotrexate, acitretin, or biologic therapy.8,11-16 Our patient achieved satisfactory clinical improvement with clobetasol propionate ointment 0.05% twice daily alternating with calcipotriene cream 0.005% twice daily.
- Suchanek J. Relation of Hallopeau’s acrodermatitis continua to psoriasis. Przegl Dermatol. 1951;1:165-181.
- Adam BA, Loh CL. Acropustulosis (acrodermatitis continua) with resorption of terminal phalanges. Med J Malaysia. 1972;27:30-32.
- Mrowietz U. Pustular eruptions of palms and soles. In: Wolff K, Goldsmith LS, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2007:215-218.
- Yerushalmi J, Grunwald MH, Hallel-Halevy D, et al. Chronic pustular eruption of the thumbs. diagnosis: acrodermatitis continue of Hallopeau (ACH). Arch Dermatol. 2000:136:925-930.
- Granelli U. Impetigo herpetiformis; acrodermatitis continue of Hallopeau and pustular psoriasis; etiology and pathogenesis and differential diagnosis. Minerva Dermatol. 1956;31:120-126.
- Mobini N, Toussaint S, Kamino H. Noninfectious erythematous, papular, and squamous diseases. In: Elder DE, Elenitsas R, Johnson B, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005:174-210.
- Radcliff-Crocker H. Diseases of the Skin: Their Descriptions, Pathology, Diagnosis and Treatment. Philadelphia, PA: P. Blakiston, Son, & Co; 1888.
- Sehgal VN, Verma P, Sharma S, et al. Review: acrodermatitis continua of Hallopeau: evolution of treatment options. Int J Dermatol. 2011;50:1195-1211.
- Post CF, Hopper ME. Dermatitis repens: a report of two cases with bacteriologic studies. AMA Arc Derm Syphilol. 1951;63:220-223.
- Sehgal VN, Sharma S. The significance of Gram’s stain smear, potassium hydroxide mount, culture and microscopic pathology in the diagnosis of acrodermatitis continua of Hallopeau. Skinmed. 2011;9:260-261.
- Mosser G, Pillekamp H, Peter RU. Suppurative acrodermatitis continua of Hallopeau. a differential diagnosis of paronychia. Dtsch Med Wochenschr. 1998;123:386-390.
- Piquero-Casals J, Fonseca de Mello AP, Dal Coleto C, et al. Using oral tetracycline and topical betamethasone valerate to treat acrodermatitis continua of Hallopeau. Cutis. 2002;70:106-108.
- Tsuji T, Nishimura M. Topically administered fluorouracil in acrodermatitis continua of Hallopeau. Arch Dermatol. 1991;127:27-28.
- Van de Kerkhof PCM. In vivo effects of vitamin D3 analogs. J Dermatolog Treat. 1998;(suppl 3):S25-S29.
- Kokelj F, Plozzer C, Trevisan G. Uselessness of topical calcipotriol as monotherapy for acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2001;81:153.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
The Diagnosis: Acrodermatitis Continua of Hallopeau
Acrodermatitis continua of Hallopeau (ACH) is considered to be a form of acropustular psoriasis that presents as a sterile, pustular eruption initially affecting the fingertips and/or toes.1 The slow-growing pustules typically progress locally and can lead to onychodystrophy and/or osteolysis of the underlying bone.2,3 Most commonly affecting adult women, ACH often begins following local trauma to or infection of a single digit.4 As the disease progresses proximally, the small pustules burst, leaving a shiny, erythematous surface on which new pustules can develop. These pustules have a tendency to amalgamate, leading to the characteristic clinical finding of lakes of pus. Pustules frequently appear on the nail matrix and nail bed presenting as severe onychodystrophy and ultimately anonychia.5,6 Rarely, ACH can be associated with generalized pustular psoriasis as well as conjunctivitis, balanitis, and fissuring or annulus migrans of the tongue.2,7
Diagnosis can be established based on clinical findings, biopsy, and bacterial and fungal cultures revealing sterile pustules.8,9 Histologic findings are similar to those seen in pustular psoriasis, demonstrating subcorneal neutrophilic pustules, Munro microabscesses, and dilated blood vessels with lymphocytic infiltrate in the papillary dermis.10
Due to the refractory nature of the disease, there are no recommended guidelines for treatment of ACH. Most successful treatment regimens consist of topical psoriasis medications combined with systemic psoriatic therapies such as cyclosporine, methotrexate, acitretin, or biologic therapy.8,11-16 Our patient achieved satisfactory clinical improvement with clobetasol propionate ointment 0.05% twice daily alternating with calcipotriene cream 0.005% twice daily.
The Diagnosis: Acrodermatitis Continua of Hallopeau
Acrodermatitis continua of Hallopeau (ACH) is considered to be a form of acropustular psoriasis that presents as a sterile, pustular eruption initially affecting the fingertips and/or toes.1 The slow-growing pustules typically progress locally and can lead to onychodystrophy and/or osteolysis of the underlying bone.2,3 Most commonly affecting adult women, ACH often begins following local trauma to or infection of a single digit.4 As the disease progresses proximally, the small pustules burst, leaving a shiny, erythematous surface on which new pustules can develop. These pustules have a tendency to amalgamate, leading to the characteristic clinical finding of lakes of pus. Pustules frequently appear on the nail matrix and nail bed presenting as severe onychodystrophy and ultimately anonychia.5,6 Rarely, ACH can be associated with generalized pustular psoriasis as well as conjunctivitis, balanitis, and fissuring or annulus migrans of the tongue.2,7
Diagnosis can be established based on clinical findings, biopsy, and bacterial and fungal cultures revealing sterile pustules.8,9 Histologic findings are similar to those seen in pustular psoriasis, demonstrating subcorneal neutrophilic pustules, Munro microabscesses, and dilated blood vessels with lymphocytic infiltrate in the papillary dermis.10
Due to the refractory nature of the disease, there are no recommended guidelines for treatment of ACH. Most successful treatment regimens consist of topical psoriasis medications combined with systemic psoriatic therapies such as cyclosporine, methotrexate, acitretin, or biologic therapy.8,11-16 Our patient achieved satisfactory clinical improvement with clobetasol propionate ointment 0.05% twice daily alternating with calcipotriene cream 0.005% twice daily.
- Suchanek J. Relation of Hallopeau’s acrodermatitis continua to psoriasis. Przegl Dermatol. 1951;1:165-181.
- Adam BA, Loh CL. Acropustulosis (acrodermatitis continua) with resorption of terminal phalanges. Med J Malaysia. 1972;27:30-32.
- Mrowietz U. Pustular eruptions of palms and soles. In: Wolff K, Goldsmith LS, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2007:215-218.
- Yerushalmi J, Grunwald MH, Hallel-Halevy D, et al. Chronic pustular eruption of the thumbs. diagnosis: acrodermatitis continue of Hallopeau (ACH). Arch Dermatol. 2000:136:925-930.
- Granelli U. Impetigo herpetiformis; acrodermatitis continue of Hallopeau and pustular psoriasis; etiology and pathogenesis and differential diagnosis. Minerva Dermatol. 1956;31:120-126.
- Mobini N, Toussaint S, Kamino H. Noninfectious erythematous, papular, and squamous diseases. In: Elder DE, Elenitsas R, Johnson B, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005:174-210.
- Radcliff-Crocker H. Diseases of the Skin: Their Descriptions, Pathology, Diagnosis and Treatment. Philadelphia, PA: P. Blakiston, Son, & Co; 1888.
- Sehgal VN, Verma P, Sharma S, et al. Review: acrodermatitis continua of Hallopeau: evolution of treatment options. Int J Dermatol. 2011;50:1195-1211.
- Post CF, Hopper ME. Dermatitis repens: a report of two cases with bacteriologic studies. AMA Arc Derm Syphilol. 1951;63:220-223.
- Sehgal VN, Sharma S. The significance of Gram’s stain smear, potassium hydroxide mount, culture and microscopic pathology in the diagnosis of acrodermatitis continua of Hallopeau. Skinmed. 2011;9:260-261.
- Mosser G, Pillekamp H, Peter RU. Suppurative acrodermatitis continua of Hallopeau. a differential diagnosis of paronychia. Dtsch Med Wochenschr. 1998;123:386-390.
- Piquero-Casals J, Fonseca de Mello AP, Dal Coleto C, et al. Using oral tetracycline and topical betamethasone valerate to treat acrodermatitis continua of Hallopeau. Cutis. 2002;70:106-108.
- Tsuji T, Nishimura M. Topically administered fluorouracil in acrodermatitis continua of Hallopeau. Arch Dermatol. 1991;127:27-28.
- Van de Kerkhof PCM. In vivo effects of vitamin D3 analogs. J Dermatolog Treat. 1998;(suppl 3):S25-S29.
- Kokelj F, Plozzer C, Trevisan G. Uselessness of topical calcipotriol as monotherapy for acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2001;81:153.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Suchanek J. Relation of Hallopeau’s acrodermatitis continua to psoriasis. Przegl Dermatol. 1951;1:165-181.
- Adam BA, Loh CL. Acropustulosis (acrodermatitis continua) with resorption of terminal phalanges. Med J Malaysia. 1972;27:30-32.
- Mrowietz U. Pustular eruptions of palms and soles. In: Wolff K, Goldsmith LS, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 7th ed. New York, NY: McGraw-Hill; 2007:215-218.
- Yerushalmi J, Grunwald MH, Hallel-Halevy D, et al. Chronic pustular eruption of the thumbs. diagnosis: acrodermatitis continue of Hallopeau (ACH). Arch Dermatol. 2000:136:925-930.
- Granelli U. Impetigo herpetiformis; acrodermatitis continue of Hallopeau and pustular psoriasis; etiology and pathogenesis and differential diagnosis. Minerva Dermatol. 1956;31:120-126.
- Mobini N, Toussaint S, Kamino H. Noninfectious erythematous, papular, and squamous diseases. In: Elder DE, Elenitsas R, Johnson B, et al, eds. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, PA: Lippincott, Williams & Wilkins; 2005:174-210.
- Radcliff-Crocker H. Diseases of the Skin: Their Descriptions, Pathology, Diagnosis and Treatment. Philadelphia, PA: P. Blakiston, Son, & Co; 1888.
- Sehgal VN, Verma P, Sharma S, et al. Review: acrodermatitis continua of Hallopeau: evolution of treatment options. Int J Dermatol. 2011;50:1195-1211.
- Post CF, Hopper ME. Dermatitis repens: a report of two cases with bacteriologic studies. AMA Arc Derm Syphilol. 1951;63:220-223.
- Sehgal VN, Sharma S. The significance of Gram’s stain smear, potassium hydroxide mount, culture and microscopic pathology in the diagnosis of acrodermatitis continua of Hallopeau. Skinmed. 2011;9:260-261.
- Mosser G, Pillekamp H, Peter RU. Suppurative acrodermatitis continua of Hallopeau. a differential diagnosis of paronychia. Dtsch Med Wochenschr. 1998;123:386-390.
- Piquero-Casals J, Fonseca de Mello AP, Dal Coleto C, et al. Using oral tetracycline and topical betamethasone valerate to treat acrodermatitis continua of Hallopeau. Cutis. 2002;70:106-108.
- Tsuji T, Nishimura M. Topically administered fluorouracil in acrodermatitis continua of Hallopeau. Arch Dermatol. 1991;127:27-28.
- Van de Kerkhof PCM. In vivo effects of vitamin D3 analogs. J Dermatolog Treat. 1998;(suppl 3):S25-S29.
- Kokelj F, Plozzer C, Trevisan G. Uselessness of topical calcipotriol as monotherapy for acrodermatitis continua of Hallopeau. Acta Derm Venereol. 2001;81:153.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
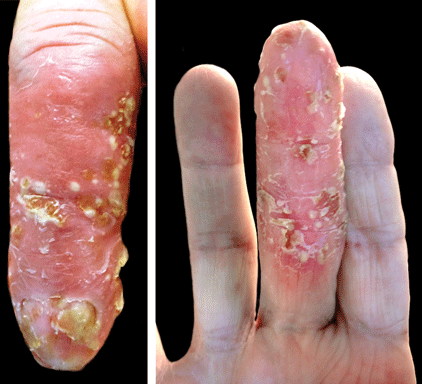
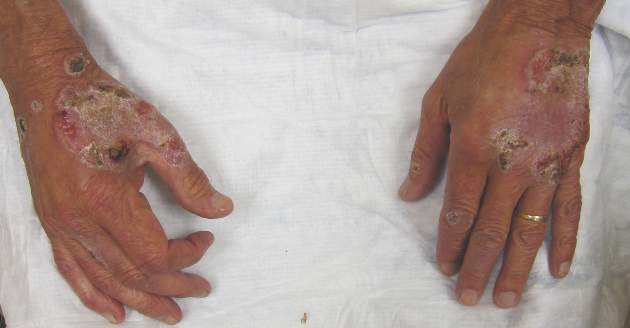
A 69-year-old man presented to our dermatology clinic with a persistent rash on the right middle finger of 5 years’ duration (left). Physical examination revealed a well-demarcated scaly plaque with pustules and anonychia localized to the right middle finger (right). Fungal and bacterial cultures revealed sterile pustules. The patient was successfully treated with an occluded superpotent topical steroid alternating with a topical vitamin D analogue.
What Is Your Diagnosis? Tinea Corporis
The Diagnosis: Tinea Corporis
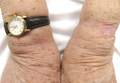
Although contact dermatitis from the metal on the back of the watch was suspected, many modern wrist watches are made with stainless steel rather than nickel, which is a common contact allergen; therefore, other diagnoses were considered in the differential including irritant contact dermatitis, psoriasis, and tinea infection. A potassium hydroxide (KOH) preparation was performed to rule out tinea infection. Unexpectedly, the KOH preparation was positive for fungal hyphae, confirming a diagnosis of tinea corporis. The patient was treated with clotrimazole cream 1% twice daily for 3 weeks during which time the rash completely resolved.
We present this case to stress the importance of performing KOH preparations even when the likelihood of tinea infection seems remote. At our institution, we teach our residents, “If it’s scaly, scrape it.” This adage has served us well. Tinea corporis may be mistaken for many other skin diseases, including eczema, psoriasis, and seborrheic dermatitis.1 A KOH preparation often is a helpful tool in confirming the diagnosis and should be performed when a dermatophyte infection is suspected. The KOH preparation is the most sensitive diagnostic test used to confirm dermatophyte infection, with 90% of infections showing positive results.2,3
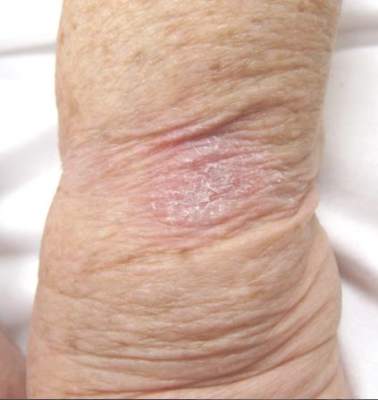
Tinea infections may occur anywhere on the body, but areas that are prone to excessive heat and/or moisture are particularly susceptible.4 Dermatophyte infections typically present as annular, scaly, pruritic patches or plaques often with central clearing and an active border.1 In our patient, the lesion showed characteristics that were suggestive of a dermatophyte infection but was somewhat atypical in appearance, as it lacked central clearing (Figure). The 3 genera of dermatophytes—Trichophyton, Microsporum, and Epidermophyton—are common causes of fungal infections.2 The pathogenesis of dermatophytosis is the synthesis of keratinases that digest keratin and sustain the presence of the fungi. Local factors such as sweating and occlusion facilitate the activity of these organisms.2 In our case, the pathogenesis was believed to be due to the entrapment of moisture behind the patient’s watch, creating a favorable environment for fungal growth.
- Ely JW, Rosenfeld S, Seabury SM. Diagnosis and management of tinea infections. Am Fam Physician. 2014:90:702-710.
- Wolff K, Saavedra AP, Fitzpatrick TB. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill Medical; 2013.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis (published online ahead of print June 22, 2010). Dermatol Res Pract. doi:10.1155/2010/764843.
- Gupta AK, Chaudhry M, Elewski B. Tinea corporis, tinea cruris, tinea nigra and piedra. Dermatol Clin. 2003;21:395-400.
The Diagnosis: Tinea Corporis
Although contact dermatitis from the metal on the back of the watch was suspected, many modern wrist watches are made with stainless steel rather than nickel, which is a common contact allergen; therefore, other diagnoses were considered in the differential including irritant contact dermatitis, psoriasis, and tinea infection. A potassium hydroxide (KOH) preparation was performed to rule out tinea infection. Unexpectedly, the KOH preparation was positive for fungal hyphae, confirming a diagnosis of tinea corporis. The patient was treated with clotrimazole cream 1% twice daily for 3 weeks during which time the rash completely resolved.

We present this case to stress the importance of performing KOH preparations even when the likelihood of tinea infection seems remote. At our institution, we teach our residents, “If it’s scaly, scrape it.” This adage has served us well. Tinea corporis may be mistaken for many other skin diseases, including eczema, psoriasis, and seborrheic dermatitis.1 A KOH preparation often is a helpful tool in confirming the diagnosis and should be performed when a dermatophyte infection is suspected. The KOH preparation is the most sensitive diagnostic test used to confirm dermatophyte infection, with 90% of infections showing positive results.2,3
Tinea infections may occur anywhere on the body, but areas that are prone to excessive heat and/or moisture are particularly susceptible.4 Dermatophyte infections typically present as annular, scaly, pruritic patches or plaques often with central clearing and an active border.1 In our patient, the lesion showed characteristics that were suggestive of a dermatophyte infection but was somewhat atypical in appearance, as it lacked central clearing (Figure). The 3 genera of dermatophytes—Trichophyton, Microsporum, and Epidermophyton—are common causes of fungal infections.2 The pathogenesis of dermatophytosis is the synthesis of keratinases that digest keratin and sustain the presence of the fungi. Local factors such as sweating and occlusion facilitate the activity of these organisms.2 In our case, the pathogenesis was believed to be due to the entrapment of moisture behind the patient’s watch, creating a favorable environment for fungal growth.
The Diagnosis: Tinea Corporis
Although contact dermatitis from the metal on the back of the watch was suspected, many modern wrist watches are made with stainless steel rather than nickel, which is a common contact allergen; therefore, other diagnoses were considered in the differential including irritant contact dermatitis, psoriasis, and tinea infection. A potassium hydroxide (KOH) preparation was performed to rule out tinea infection. Unexpectedly, the KOH preparation was positive for fungal hyphae, confirming a diagnosis of tinea corporis. The patient was treated with clotrimazole cream 1% twice daily for 3 weeks during which time the rash completely resolved.

We present this case to stress the importance of performing KOH preparations even when the likelihood of tinea infection seems remote. At our institution, we teach our residents, “If it’s scaly, scrape it.” This adage has served us well. Tinea corporis may be mistaken for many other skin diseases, including eczema, psoriasis, and seborrheic dermatitis.1 A KOH preparation often is a helpful tool in confirming the diagnosis and should be performed when a dermatophyte infection is suspected. The KOH preparation is the most sensitive diagnostic test used to confirm dermatophyte infection, with 90% of infections showing positive results.2,3
Tinea infections may occur anywhere on the body, but areas that are prone to excessive heat and/or moisture are particularly susceptible.4 Dermatophyte infections typically present as annular, scaly, pruritic patches or plaques often with central clearing and an active border.1 In our patient, the lesion showed characteristics that were suggestive of a dermatophyte infection but was somewhat atypical in appearance, as it lacked central clearing (Figure). The 3 genera of dermatophytes—Trichophyton, Microsporum, and Epidermophyton—are common causes of fungal infections.2 The pathogenesis of dermatophytosis is the synthesis of keratinases that digest keratin and sustain the presence of the fungi. Local factors such as sweating and occlusion facilitate the activity of these organisms.2 In our case, the pathogenesis was believed to be due to the entrapment of moisture behind the patient’s watch, creating a favorable environment for fungal growth.
- Ely JW, Rosenfeld S, Seabury SM. Diagnosis and management of tinea infections. Am Fam Physician. 2014:90:702-710.
- Wolff K, Saavedra AP, Fitzpatrick TB. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill Medical; 2013.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis (published online ahead of print June 22, 2010). Dermatol Res Pract. doi:10.1155/2010/764843.
- Gupta AK, Chaudhry M, Elewski B. Tinea corporis, tinea cruris, tinea nigra and piedra. Dermatol Clin. 2003;21:395-400.
- Ely JW, Rosenfeld S, Seabury SM. Diagnosis and management of tinea infections. Am Fam Physician. 2014:90:702-710.
- Wolff K, Saavedra AP, Fitzpatrick TB. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill Medical; 2013.
- Levitt JO, Levitt BH, Akhavan A, et al. The sensitivity and specificity of potassium hydroxide smear and fungal culture relative to clinical assessment in the evaluation of tinea pedis: a pooled analysis (published online ahead of print June 22, 2010). Dermatol Res Pract. doi:10.1155/2010/764843.
- Gupta AK, Chaudhry M, Elewski B. Tinea corporis, tinea cruris, tinea nigra and piedra. Dermatol Clin. 2003;21:395-400.
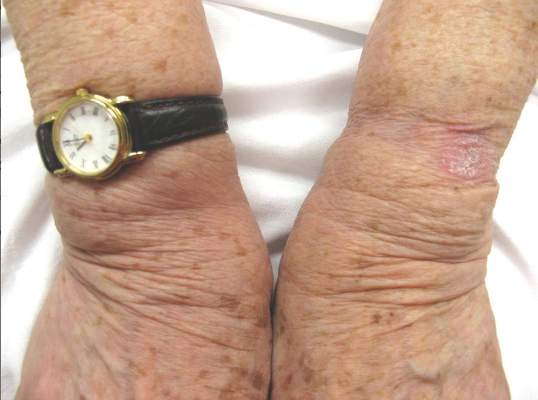
An 81-year-old woman presented with a 2-cm erythematous, scaly, pruritic rash on the left dorsal wrist localized to the skin under her watch. The patient first noticed the lesion 2 months prior. She moved the watch to the right wrist a few days prior to presentation and no symptoms developed in that location. No other areas of the skin were affected. She had no known allergies and was otherwise in good health.
Linear Bluish Black Papules on the Shoulder
The Diagnosis: Agminated Blue Nevus
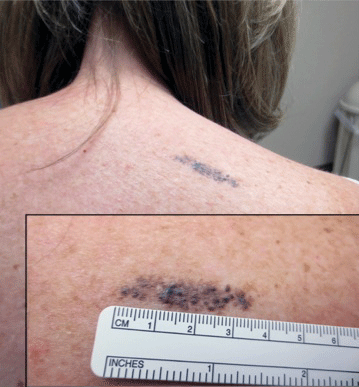
Agminated blue nevus is a rare melanocytic nevus that characteristically presents as a group of multiple small, bluish papules occurring in a well-circumscribed area.1 It also has been referred to as a plaque-type nevus2,3 and an eruptive blue nevus.4 Originally described by Upshaw et al2 in 1947, agminated blue nevi may be congenital or arise in early childhood and almost always occur on the trunk. The skin between the papules often is unaffected or sometimes may show bluish or brown pigmentation.4 Agminated blue nevi usually are smaller than 10 cm in diameter; however, rare cases have measured up to 24 cm.1,4-6 The incidence of agminated blue nevi is 2 times higher in males than in females.1
|
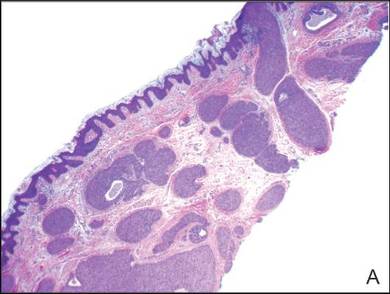
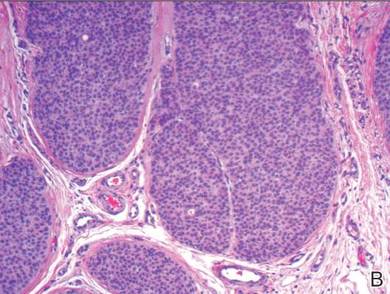
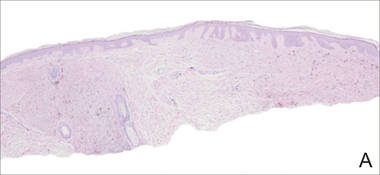
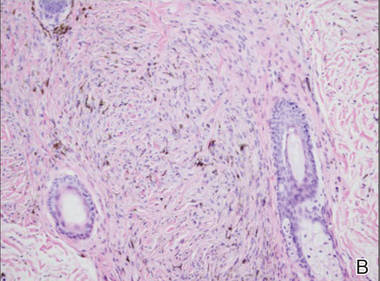
A biopsy of the lesion revealed pigmented dendritic melanocytes admixed with melanophages, forming fascicles and bundles (A)(H&E, original magnification ×10). In some areas the dermis was uninvolved. Higher magnification showed dendritic and epithelioid melanocytes extending down along the adnexal structures (B)(H&E, original magnification ×40). |
Histopathologically, agminated blue nevi typically demonstrate the features of common and/or cellular blue nevi. Cytologic atypia and mitoses are rare.1 The degree of cellularity and pigmentation of the lesions is variable, and the presence of subcutaneous cellular nodules also has been described.5
In our patient, histologic evaluation revealed foci of diffuse dermal spindle cell proliferation composed of heavily pigmented dendritic melanocytes admixed with melanophages in a fibrotic stroma (Figure, A). The dermis was uninvolved in some areas and the melanocytes were epithelioid and formed fascicles and bundles that extended down adnexal structures in other areas (Figure, B). Junctional involvement of melanocytes, cellular atypia, and mitoses were not identified. Our case demonstrated a combination of histologic findings of a cellular blue nevus as well as features reminiscent of a deep penetrating nevus. The differential diagnosis of agminated blue nevus includes agminated Spitz nevus arising in a speckled lentiginous nevus,7 dermal melanocytosis, melanoma, and pilar neurocristic hamartoma. Pilar neurocristic hamartomas may resemble plaque-type blue nevi; however, the former show a predilection for the scalp, histologically demonstrate features that overlap with blue nevi and congenital nevi, and are associated with neural structures that show Schwannian differentiation.8 Agminated blue nevi usually are characterized by a benign clinical course, but few cases describing malignant changes with development of malignant melanoma have been reported.9,10 Therefore, recognition of the clinical and histopathologic spectrum of agminated blue nevus is critical in order to avoid diagnostic pitfalls and confusion with melanoma.
- Vélez A, del-Río E, Martín-de-Hijas C, et al. Agminated blue nevi: case report and review of the literature. Dermatology. 1993;186:144-148.
- Upshaw BY, Ghormley RK, Montgomery H. Extensive blue nevus of Jadassohn-Tièche; report of a case. Surgery. 1947;22:761-765.
- Pittman JL, Fisher BK. Plaque-type blue nevus. Arch Dermatol. 1976;112:1127-1128.
- Hendricks WM. Eruptive blue nevi. J Am Acad Dermatol.1981;4:50-53.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Shenfield HT, Maize JC. Multiple and agminated blue nevi. J Dermatol Surg Oncol. 1980;6:725-728.
- Misago N, Narisawa Y, Kohda H. A combination of speckled lentiginous nevus with patch-type blue nevus. J Dermatol. 1993;20:643-647.
- Bevona C, Tannous Z, Tsao H. Dermal melanocytic proliferation with features of a plaque-type blue nevus and neurocristic hamartoma. J Am Acad Dermatol. 2003;49:924-929.
- Yeh I, Fang Y, Busam KJ. Melanoma arising in a large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2012;36:1258-1263.
- Zattra E, Salmaso R, Montesco MC, et al. Large plaque type blue nevus with subcutaneous cellular nodules. Eur J Dermatol. 2009;19:287-288.
The Diagnosis: Agminated Blue Nevus
Agminated blue nevus is a rare melanocytic nevus that characteristically presents as a group of multiple small, bluish papules occurring in a well-circumscribed area.1 It also has been referred to as a plaque-type nevus2,3 and an eruptive blue nevus.4 Originally described by Upshaw et al2 in 1947, agminated blue nevi may be congenital or arise in early childhood and almost always occur on the trunk. The skin between the papules often is unaffected or sometimes may show bluish or brown pigmentation.4 Agminated blue nevi usually are smaller than 10 cm in diameter; however, rare cases have measured up to 24 cm.1,4-6 The incidence of agminated blue nevi is 2 times higher in males than in females.1


|
A biopsy of the lesion revealed pigmented dendritic melanocytes admixed with melanophages, forming fascicles and bundles (A)(H&E, original magnification ×10). In some areas the dermis was uninvolved. Higher magnification showed dendritic and epithelioid melanocytes extending down along the adnexal structures (B)(H&E, original magnification ×40). |
Histopathologically, agminated blue nevi typically demonstrate the features of common and/or cellular blue nevi. Cytologic atypia and mitoses are rare.1 The degree of cellularity and pigmentation of the lesions is variable, and the presence of subcutaneous cellular nodules also has been described.5
In our patient, histologic evaluation revealed foci of diffuse dermal spindle cell proliferation composed of heavily pigmented dendritic melanocytes admixed with melanophages in a fibrotic stroma (Figure, A). The dermis was uninvolved in some areas and the melanocytes were epithelioid and formed fascicles and bundles that extended down adnexal structures in other areas (Figure, B). Junctional involvement of melanocytes, cellular atypia, and mitoses were not identified. Our case demonstrated a combination of histologic findings of a cellular blue nevus as well as features reminiscent of a deep penetrating nevus. The differential diagnosis of agminated blue nevus includes agminated Spitz nevus arising in a speckled lentiginous nevus,7 dermal melanocytosis, melanoma, and pilar neurocristic hamartoma. Pilar neurocristic hamartomas may resemble plaque-type blue nevi; however, the former show a predilection for the scalp, histologically demonstrate features that overlap with blue nevi and congenital nevi, and are associated with neural structures that show Schwannian differentiation.8 Agminated blue nevi usually are characterized by a benign clinical course, but few cases describing malignant changes with development of malignant melanoma have been reported.9,10 Therefore, recognition of the clinical and histopathologic spectrum of agminated blue nevus is critical in order to avoid diagnostic pitfalls and confusion with melanoma.
The Diagnosis: Agminated Blue Nevus
Agminated blue nevus is a rare melanocytic nevus that characteristically presents as a group of multiple small, bluish papules occurring in a well-circumscribed area.1 It also has been referred to as a plaque-type nevus2,3 and an eruptive blue nevus.4 Originally described by Upshaw et al2 in 1947, agminated blue nevi may be congenital or arise in early childhood and almost always occur on the trunk. The skin between the papules often is unaffected or sometimes may show bluish or brown pigmentation.4 Agminated blue nevi usually are smaller than 10 cm in diameter; however, rare cases have measured up to 24 cm.1,4-6 The incidence of agminated blue nevi is 2 times higher in males than in females.1


|
A biopsy of the lesion revealed pigmented dendritic melanocytes admixed with melanophages, forming fascicles and bundles (A)(H&E, original magnification ×10). In some areas the dermis was uninvolved. Higher magnification showed dendritic and epithelioid melanocytes extending down along the adnexal structures (B)(H&E, original magnification ×40). |
Histopathologically, agminated blue nevi typically demonstrate the features of common and/or cellular blue nevi. Cytologic atypia and mitoses are rare.1 The degree of cellularity and pigmentation of the lesions is variable, and the presence of subcutaneous cellular nodules also has been described.5
In our patient, histologic evaluation revealed foci of diffuse dermal spindle cell proliferation composed of heavily pigmented dendritic melanocytes admixed with melanophages in a fibrotic stroma (Figure, A). The dermis was uninvolved in some areas and the melanocytes were epithelioid and formed fascicles and bundles that extended down adnexal structures in other areas (Figure, B). Junctional involvement of melanocytes, cellular atypia, and mitoses were not identified. Our case demonstrated a combination of histologic findings of a cellular blue nevus as well as features reminiscent of a deep penetrating nevus. The differential diagnosis of agminated blue nevus includes agminated Spitz nevus arising in a speckled lentiginous nevus,7 dermal melanocytosis, melanoma, and pilar neurocristic hamartoma. Pilar neurocristic hamartomas may resemble plaque-type blue nevi; however, the former show a predilection for the scalp, histologically demonstrate features that overlap with blue nevi and congenital nevi, and are associated with neural structures that show Schwannian differentiation.8 Agminated blue nevi usually are characterized by a benign clinical course, but few cases describing malignant changes with development of malignant melanoma have been reported.9,10 Therefore, recognition of the clinical and histopathologic spectrum of agminated blue nevus is critical in order to avoid diagnostic pitfalls and confusion with melanoma.
- Vélez A, del-Río E, Martín-de-Hijas C, et al. Agminated blue nevi: case report and review of the literature. Dermatology. 1993;186:144-148.
- Upshaw BY, Ghormley RK, Montgomery H. Extensive blue nevus of Jadassohn-Tièche; report of a case. Surgery. 1947;22:761-765.
- Pittman JL, Fisher BK. Plaque-type blue nevus. Arch Dermatol. 1976;112:1127-1128.
- Hendricks WM. Eruptive blue nevi. J Am Acad Dermatol.1981;4:50-53.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Shenfield HT, Maize JC. Multiple and agminated blue nevi. J Dermatol Surg Oncol. 1980;6:725-728.
- Misago N, Narisawa Y, Kohda H. A combination of speckled lentiginous nevus with patch-type blue nevus. J Dermatol. 1993;20:643-647.
- Bevona C, Tannous Z, Tsao H. Dermal melanocytic proliferation with features of a plaque-type blue nevus and neurocristic hamartoma. J Am Acad Dermatol. 2003;49:924-929.
- Yeh I, Fang Y, Busam KJ. Melanoma arising in a large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2012;36:1258-1263.
- Zattra E, Salmaso R, Montesco MC, et al. Large plaque type blue nevus with subcutaneous cellular nodules. Eur J Dermatol. 2009;19:287-288.
- Vélez A, del-Río E, Martín-de-Hijas C, et al. Agminated blue nevi: case report and review of the literature. Dermatology. 1993;186:144-148.
- Upshaw BY, Ghormley RK, Montgomery H. Extensive blue nevus of Jadassohn-Tièche; report of a case. Surgery. 1947;22:761-765.
- Pittman JL, Fisher BK. Plaque-type blue nevus. Arch Dermatol. 1976;112:1127-1128.
- Hendricks WM. Eruptive blue nevi. J Am Acad Dermatol.1981;4:50-53.
- Busam KJ, Woodruff JM, Erlandson RA, et al. Large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2000;24:92-99.
- Shenfield HT, Maize JC. Multiple and agminated blue nevi. J Dermatol Surg Oncol. 1980;6:725-728.
- Misago N, Narisawa Y, Kohda H. A combination of speckled lentiginous nevus with patch-type blue nevus. J Dermatol. 1993;20:643-647.
- Bevona C, Tannous Z, Tsao H. Dermal melanocytic proliferation with features of a plaque-type blue nevus and neurocristic hamartoma. J Am Acad Dermatol. 2003;49:924-929.
- Yeh I, Fang Y, Busam KJ. Melanoma arising in a large plaque-type blue nevus with subcutaneous cellular nodules. Am J Surg Pathol. 2012;36:1258-1263.
- Zattra E, Salmaso R, Montesco MC, et al. Large plaque type blue nevus with subcutaneous cellular nodules. Eur J Dermatol. 2009;19:287-288.
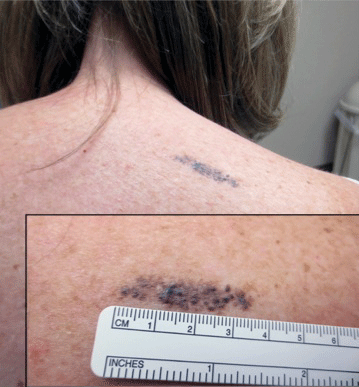
A 57-year-old woman presented with an asymptomatic, unchanging, 3.5×0.7-cm linear plaque on the right shoulder composed of dozens of clustered, bluish black papules that had been present for several decades. The skin between the papules was unaffected. The patient’s medical and family histories were unremarkable. A deep shave biopsy from the center of the plaque was performed.
Sessile Pink Plaque on the Lower Back
The Diagnosis: Eccrine Poroma
A shave biopsy of the lesion was performed for definitive diagnosis and demonstrated a well-circumscribed tumor with cords and broad columns composed of uniform basaloid cells extending into the dermis and in areas connecting to the overlying epidermis (Figure). There also were small ducts and cysts admixed in the tumor columns that were embedded in a tumor stroma rich in blood vessels. A diagnosis of eccrine poroma was made based on these characteristic histologic features.
|
Biopsy revealed a basaloid tumor originating from the epidermis and extending into the dermis (A)(H&E, original magnification ×4). On higher magnification, ducts were evident amongst the tumor cells and a vascular rich stroma was revealed (B)(H&E, original magnification ×10). |
First described by Pinkus et al1 in 1956, eccrine poroma is a benign neoplasm of cells from the intraepidermal ductal portion of the eccrine sweat gland. Eccrine poroma (along with hidroacanthoma simplex, dermal duct tumor, and poroid hidradenoma) is one of the poroid neoplasms, which account for approximately 10% of all primary sweat gland tumors.2 Eccrine poroma usually is seen in patients over 40 years of age without any predilection for race or sex.
Characteristically, eccrine poromas clinically manifest as solitary, firm, sharply demarcated papules or nodules that may be sessile or pedunculated and rarely exceed 2 cm in diameter. This entity classically presents on acral, non–hair-bearing areas (eg, palms and soles). Eccrine poromas have a wide range of clinical appearances that can lead to broad differential diagnoses3 and have been described as flesh-colored,3 pink to red,4 purple,5 and pigmented3,4 papules or nodules depending on features such as blood vessel proliferation and pigment deposition.
Eccrine poromas also have been reported on hair-bearing areas of the body, including the head,3 neck,3,6 chest,4,6 hip,7 and pubic area,8 despite the paucity of eccrine glands in these areas on the body. These findings suggest that these neoplasms may not be purely eccrine in origin. The wide range of clinical presentations of eccrine poromas has prompted investigation into further classification and delineation of this neoplasm.3 The occurrence of eccrine poromas on areas of the skin known to have few eccrine glands suggests that eccrine poromas may not be purely comprised of eccrine ducts and instead may be of apocrine origin.3,9,10 Histologic features of eccrine poromas that suggest apocrine origination include sebaceous and follicular differentiation (eg, folliculocentric distribution), the association with the follicular infundibulum, and the presence of follicular germ cells.3,9,10 Thus, apocrine gland involvement in eccrine poromas may account for their appearance in anatomic areas that do not have high concentrations of eccrine glands, such as the trunk and pubic area.
Based on these findings, eccrine poromas may therefore be of eccrine and/or apocrine origin; however, the nomenclature of this neoplasm remains confusing and possibly misleading, as the term eccrine poroma continues to be accepted even in instances in which the differentiation appears to be largely apocrine. The terms poroma and eccrine poroma often are used interchangeably, which contributes to the confusion by failing to acknowledge the possibility of apocrine influence and possibly causing the clinician to exclude eccrine poromas from the differential diagnosis in areas that do not have high concentrations of eccrine glands.
Because of their high degree of clinical variability, characteristic acral location, and misleading nomenclature, eccrine poromas often are mistakenly confused with a long list of other cutaneous neoplasms, including hemangiomas, pyogenic granulomas, melanocytic nevi, warts, cysts, and other adnexal neoplasms.3 In our case, the lesion was abnormally large and was clinically concerning for an unusual sebaceous nevus. Its location on the lower back is not commonly noted and should remind the clinician of the possibility of apocrine differentiation. Clinicians should be aware of the wide phenotypic diversity of eccrine poromas, and therefore they should consider this diagnosis in their differential diagnosis for solitary papules or nodules occurring in any anatomic area.
- Pinkus H, Rogin JR, Goldman P. Eccrine poroma: tumors exhibiting features of the epidermal sweat duct unit. Arch Dermatol. 1956;74:511-521.
- Pylyser K, Dewolf-Peeters C, Marlen K. The histology of eccrine poromas: a study of 14 cases. Dermatologica. 1983;167:243-249.
- Moore TO, Orman HL, Orman SK, et al. Poromas of the head and neck. J Am Acad Dermatol. 2001;44:48-52.
- Agarwal S, Kumar B, Sharma N. Nodule on the chest. eccrine poroma. Indian J Dermatol Venereol Leprol. 2009;75:639.
- Ackerman AB, Abenoza P. Neoplasms With Eccrine Differentiation. Philadelphia, PA: Lea & Febinger; 1990:113-185.
- Okun M, Ansell H. Eccrine poroma. report of three cases, two with an unusual location. Arch Dermatol. 1963;88:561-566.
- Sarma DP, Zaman SU, Santos EE, et al. Poroma of the hip and buttock. Dermatol Online J. 2009;15:10.
- Altamura D, Piccolo D, Lozzi GP, et al. Eccrine poroma in an unusual site: a clinical and dermoscopic simulator of amelanotic melanoma. J Am Acad Dermatol. 2005;53:539-541.
- Groben PA, Hitchcock MG, Leshin B, et al. Apocrine poroma, a distinctive case in a patient with nevoid BCC. Am J Dermatopathol. 1992;21:31-33.
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9.
The Diagnosis: Eccrine Poroma
A shave biopsy of the lesion was performed for definitive diagnosis and demonstrated a well-circumscribed tumor with cords and broad columns composed of uniform basaloid cells extending into the dermis and in areas connecting to the overlying epidermis (Figure). There also were small ducts and cysts admixed in the tumor columns that were embedded in a tumor stroma rich in blood vessels. A diagnosis of eccrine poroma was made based on these characteristic histologic features.
|
Biopsy revealed a basaloid tumor originating from the epidermis and extending into the dermis (A)(H&E, original magnification ×4). On higher magnification, ducts were evident amongst the tumor cells and a vascular rich stroma was revealed (B)(H&E, original magnification ×10). |
First described by Pinkus et al1 in 1956, eccrine poroma is a benign neoplasm of cells from the intraepidermal ductal portion of the eccrine sweat gland. Eccrine poroma (along with hidroacanthoma simplex, dermal duct tumor, and poroid hidradenoma) is one of the poroid neoplasms, which account for approximately 10% of all primary sweat gland tumors.2 Eccrine poroma usually is seen in patients over 40 years of age without any predilection for race or sex.
Characteristically, eccrine poromas clinically manifest as solitary, firm, sharply demarcated papules or nodules that may be sessile or pedunculated and rarely exceed 2 cm in diameter. This entity classically presents on acral, non–hair-bearing areas (eg, palms and soles). Eccrine poromas have a wide range of clinical appearances that can lead to broad differential diagnoses3 and have been described as flesh-colored,3 pink to red,4 purple,5 and pigmented3,4 papules or nodules depending on features such as blood vessel proliferation and pigment deposition.
Eccrine poromas also have been reported on hair-bearing areas of the body, including the head,3 neck,3,6 chest,4,6 hip,7 and pubic area,8 despite the paucity of eccrine glands in these areas on the body. These findings suggest that these neoplasms may not be purely eccrine in origin. The wide range of clinical presentations of eccrine poromas has prompted investigation into further classification and delineation of this neoplasm.3 The occurrence of eccrine poromas on areas of the skin known to have few eccrine glands suggests that eccrine poromas may not be purely comprised of eccrine ducts and instead may be of apocrine origin.3,9,10 Histologic features of eccrine poromas that suggest apocrine origination include sebaceous and follicular differentiation (eg, folliculocentric distribution), the association with the follicular infundibulum, and the presence of follicular germ cells.3,9,10 Thus, apocrine gland involvement in eccrine poromas may account for their appearance in anatomic areas that do not have high concentrations of eccrine glands, such as the trunk and pubic area.
Based on these findings, eccrine poromas may therefore be of eccrine and/or apocrine origin; however, the nomenclature of this neoplasm remains confusing and possibly misleading, as the term eccrine poroma continues to be accepted even in instances in which the differentiation appears to be largely apocrine. The terms poroma and eccrine poroma often are used interchangeably, which contributes to the confusion by failing to acknowledge the possibility of apocrine influence and possibly causing the clinician to exclude eccrine poromas from the differential diagnosis in areas that do not have high concentrations of eccrine glands.
Because of their high degree of clinical variability, characteristic acral location, and misleading nomenclature, eccrine poromas often are mistakenly confused with a long list of other cutaneous neoplasms, including hemangiomas, pyogenic granulomas, melanocytic nevi, warts, cysts, and other adnexal neoplasms.3 In our case, the lesion was abnormally large and was clinically concerning for an unusual sebaceous nevus. Its location on the lower back is not commonly noted and should remind the clinician of the possibility of apocrine differentiation. Clinicians should be aware of the wide phenotypic diversity of eccrine poromas, and therefore they should consider this diagnosis in their differential diagnosis for solitary papules or nodules occurring in any anatomic area.
The Diagnosis: Eccrine Poroma
A shave biopsy of the lesion was performed for definitive diagnosis and demonstrated a well-circumscribed tumor with cords and broad columns composed of uniform basaloid cells extending into the dermis and in areas connecting to the overlying epidermis (Figure). There also were small ducts and cysts admixed in the tumor columns that were embedded in a tumor stroma rich in blood vessels. A diagnosis of eccrine poroma was made based on these characteristic histologic features.
|
Biopsy revealed a basaloid tumor originating from the epidermis and extending into the dermis (A)(H&E, original magnification ×4). On higher magnification, ducts were evident amongst the tumor cells and a vascular rich stroma was revealed (B)(H&E, original magnification ×10). |
First described by Pinkus et al1 in 1956, eccrine poroma is a benign neoplasm of cells from the intraepidermal ductal portion of the eccrine sweat gland. Eccrine poroma (along with hidroacanthoma simplex, dermal duct tumor, and poroid hidradenoma) is one of the poroid neoplasms, which account for approximately 10% of all primary sweat gland tumors.2 Eccrine poroma usually is seen in patients over 40 years of age without any predilection for race or sex.
Characteristically, eccrine poromas clinically manifest as solitary, firm, sharply demarcated papules or nodules that may be sessile or pedunculated and rarely exceed 2 cm in diameter. This entity classically presents on acral, non–hair-bearing areas (eg, palms and soles). Eccrine poromas have a wide range of clinical appearances that can lead to broad differential diagnoses3 and have been described as flesh-colored,3 pink to red,4 purple,5 and pigmented3,4 papules or nodules depending on features such as blood vessel proliferation and pigment deposition.
Eccrine poromas also have been reported on hair-bearing areas of the body, including the head,3 neck,3,6 chest,4,6 hip,7 and pubic area,8 despite the paucity of eccrine glands in these areas on the body. These findings suggest that these neoplasms may not be purely eccrine in origin. The wide range of clinical presentations of eccrine poromas has prompted investigation into further classification and delineation of this neoplasm.3 The occurrence of eccrine poromas on areas of the skin known to have few eccrine glands suggests that eccrine poromas may not be purely comprised of eccrine ducts and instead may be of apocrine origin.3,9,10 Histologic features of eccrine poromas that suggest apocrine origination include sebaceous and follicular differentiation (eg, folliculocentric distribution), the association with the follicular infundibulum, and the presence of follicular germ cells.3,9,10 Thus, apocrine gland involvement in eccrine poromas may account for their appearance in anatomic areas that do not have high concentrations of eccrine glands, such as the trunk and pubic area.
Based on these findings, eccrine poromas may therefore be of eccrine and/or apocrine origin; however, the nomenclature of this neoplasm remains confusing and possibly misleading, as the term eccrine poroma continues to be accepted even in instances in which the differentiation appears to be largely apocrine. The terms poroma and eccrine poroma often are used interchangeably, which contributes to the confusion by failing to acknowledge the possibility of apocrine influence and possibly causing the clinician to exclude eccrine poromas from the differential diagnosis in areas that do not have high concentrations of eccrine glands.
Because of their high degree of clinical variability, characteristic acral location, and misleading nomenclature, eccrine poromas often are mistakenly confused with a long list of other cutaneous neoplasms, including hemangiomas, pyogenic granulomas, melanocytic nevi, warts, cysts, and other adnexal neoplasms.3 In our case, the lesion was abnormally large and was clinically concerning for an unusual sebaceous nevus. Its location on the lower back is not commonly noted and should remind the clinician of the possibility of apocrine differentiation. Clinicians should be aware of the wide phenotypic diversity of eccrine poromas, and therefore they should consider this diagnosis in their differential diagnosis for solitary papules or nodules occurring in any anatomic area.
- Pinkus H, Rogin JR, Goldman P. Eccrine poroma: tumors exhibiting features of the epidermal sweat duct unit. Arch Dermatol. 1956;74:511-521.
- Pylyser K, Dewolf-Peeters C, Marlen K. The histology of eccrine poromas: a study of 14 cases. Dermatologica. 1983;167:243-249.
- Moore TO, Orman HL, Orman SK, et al. Poromas of the head and neck. J Am Acad Dermatol. 2001;44:48-52.
- Agarwal S, Kumar B, Sharma N. Nodule on the chest. eccrine poroma. Indian J Dermatol Venereol Leprol. 2009;75:639.
- Ackerman AB, Abenoza P. Neoplasms With Eccrine Differentiation. Philadelphia, PA: Lea & Febinger; 1990:113-185.
- Okun M, Ansell H. Eccrine poroma. report of three cases, two with an unusual location. Arch Dermatol. 1963;88:561-566.
- Sarma DP, Zaman SU, Santos EE, et al. Poroma of the hip and buttock. Dermatol Online J. 2009;15:10.
- Altamura D, Piccolo D, Lozzi GP, et al. Eccrine poroma in an unusual site: a clinical and dermoscopic simulator of amelanotic melanoma. J Am Acad Dermatol. 2005;53:539-541.
- Groben PA, Hitchcock MG, Leshin B, et al. Apocrine poroma, a distinctive case in a patient with nevoid BCC. Am J Dermatopathol. 1992;21:31-33.
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9.
- Pinkus H, Rogin JR, Goldman P. Eccrine poroma: tumors exhibiting features of the epidermal sweat duct unit. Arch Dermatol. 1956;74:511-521.
- Pylyser K, Dewolf-Peeters C, Marlen K. The histology of eccrine poromas: a study of 14 cases. Dermatologica. 1983;167:243-249.
- Moore TO, Orman HL, Orman SK, et al. Poromas of the head and neck. J Am Acad Dermatol. 2001;44:48-52.
- Agarwal S, Kumar B, Sharma N. Nodule on the chest. eccrine poroma. Indian J Dermatol Venereol Leprol. 2009;75:639.
- Ackerman AB, Abenoza P. Neoplasms With Eccrine Differentiation. Philadelphia, PA: Lea & Febinger; 1990:113-185.
- Okun M, Ansell H. Eccrine poroma. report of three cases, two with an unusual location. Arch Dermatol. 1963;88:561-566.
- Sarma DP, Zaman SU, Santos EE, et al. Poroma of the hip and buttock. Dermatol Online J. 2009;15:10.
- Altamura D, Piccolo D, Lozzi GP, et al. Eccrine poroma in an unusual site: a clinical and dermoscopic simulator of amelanotic melanoma. J Am Acad Dermatol. 2005;53:539-541.
- Groben PA, Hitchcock MG, Leshin B, et al. Apocrine poroma, a distinctive case in a patient with nevoid BCC. Am J Dermatopathol. 1992;21:31-33.
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9.
A 47-year-old man presented with an asymptomatic, 2.5×1.5-cm, sessile pink plaque with a coalescing papular texture on the lower back of 30 years’ duration. The patient reported that 2 papillated papules with peripheral rims of dark crust had developed in the center of the lesion over the past 6 months. His personal and family histories were unremarkable.
Solitary Lesion on the Left Ankle
The Diagnosis: Porokeratosis of Mibelli
There are 5 variants of porokeratosis: disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, porokeratosis of Mibelli, porokeratosis palmaris et plantaris disseminata, and punctate porokeratosis. The most common type is DSAP,1 which is characterized by multiple lesions on the body, particularly in sun-exposed areas. The distinguishing feature of porokeratosis is the cornoid lamella, which is made up of parakeratotic cells extending through the stratum corneum. There also is a thin or absent granular layer beneath it (Figure).2
Patients generally present in the third and fourth decades of life.1 Risk factors for porokeratosis include sun exposure, immunosuppression, and genetics.2-4 Overexpression of the protein p53 in porokeratosis lesions has been demonstrated in studies investigating the genetics of porokeratosis.5,6 A study of Chinese families with DSAP identified 3 different loci associated with DSAP: DSAP1, DSAP2, and DSAP3.2 The progression to cancer has been noted in all types of porokeratosis lesions. Malignancies include squamous cell carcinoma, Bowen disease, and basal cell carcinoma.7,8
Many treatments have been tried for DSAP including cryotherapy, topical 5-fluorouracil, photodynamic therapy, and topical imiquimod with varying success.1 Our patient was treated with cryotherapy but had side effects from treatment including cellulitis and local infections with ulceration before finally healing.
Interestingly, our patient had a single lesion with pathology findings most consistent with DSAP at a later age. Although the pathology suggested DSAP, the size and solitary lesion was more consistent with porokeratosis of Mibelli. Porokeratosis of Mibelli can occur concurrently with DSAP,9 but we have not seen other lesions in this patient. We have educated our patient to be aware of other lesions that may occur in the future. Due to risk for malignant conversion, it is generally viewed as beneficial to treat patients who present with porokeratosis lesions. Our patient’s lesion ultimately cleared and he has not developed new lesions at 1-year follow-up.
Although DSAP generally presents in the third and fourth decades of life and porokeratosis of Mibelli during childhood, it is important to educate both dermatologists and primary care physicians to be aware of the possibility of both diagnoses in the elderly population.
- Rouhani P, Fischer M, Meehan S, et al. Disseminated superficial actinic porokeratosis. Dermatol Online J. 2012;18:24.
- Murase J, Gilliam AC, et al. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Lederman JS, Sober AJ, Lederman GS. Immunosuppression: a cause of porokeratosis? J Am Acad Dermatol. 1985;13:75-79.
- Hernandez MH, Lai CH, Mallory SB. Disseminated porokeratosis associated with chronic renal failure: a new type of disseminated porokeratosis? Arch Dermatol. 2000;136:1568-1569.
- Magee JW, McCalmont TH, LeBoit PE. Overexpression of p53 tumor suppressor protein in porokeratosis. Arch Dermatol. 1994;130:187-190.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536-538.
- Mehta V, Balachandran C. Simultaneous co-occurrence of porokeratosis of Mibelli with disseminated superficial actinic porokeratosis. Indian J Dermatol. 2009;54:390-391.
The Diagnosis: Porokeratosis of Mibelli
There are 5 variants of porokeratosis: disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, porokeratosis of Mibelli, porokeratosis palmaris et plantaris disseminata, and punctate porokeratosis. The most common type is DSAP,1 which is characterized by multiple lesions on the body, particularly in sun-exposed areas. The distinguishing feature of porokeratosis is the cornoid lamella, which is made up of parakeratotic cells extending through the stratum corneum. There also is a thin or absent granular layer beneath it (Figure).2
Patients generally present in the third and fourth decades of life.1 Risk factors for porokeratosis include sun exposure, immunosuppression, and genetics.2-4 Overexpression of the protein p53 in porokeratosis lesions has been demonstrated in studies investigating the genetics of porokeratosis.5,6 A study of Chinese families with DSAP identified 3 different loci associated with DSAP: DSAP1, DSAP2, and DSAP3.2 The progression to cancer has been noted in all types of porokeratosis lesions. Malignancies include squamous cell carcinoma, Bowen disease, and basal cell carcinoma.7,8
Many treatments have been tried for DSAP including cryotherapy, topical 5-fluorouracil, photodynamic therapy, and topical imiquimod with varying success.1 Our patient was treated with cryotherapy but had side effects from treatment including cellulitis and local infections with ulceration before finally healing.
Interestingly, our patient had a single lesion with pathology findings most consistent with DSAP at a later age. Although the pathology suggested DSAP, the size and solitary lesion was more consistent with porokeratosis of Mibelli. Porokeratosis of Mibelli can occur concurrently with DSAP,9 but we have not seen other lesions in this patient. We have educated our patient to be aware of other lesions that may occur in the future. Due to risk for malignant conversion, it is generally viewed as beneficial to treat patients who present with porokeratosis lesions. Our patient’s lesion ultimately cleared and he has not developed new lesions at 1-year follow-up.
Although DSAP generally presents in the third and fourth decades of life and porokeratosis of Mibelli during childhood, it is important to educate both dermatologists and primary care physicians to be aware of the possibility of both diagnoses in the elderly population.
The Diagnosis: Porokeratosis of Mibelli
There are 5 variants of porokeratosis: disseminated superficial actinic porokeratosis (DSAP), linear porokeratosis, porokeratosis of Mibelli, porokeratosis palmaris et plantaris disseminata, and punctate porokeratosis. The most common type is DSAP,1 which is characterized by multiple lesions on the body, particularly in sun-exposed areas. The distinguishing feature of porokeratosis is the cornoid lamella, which is made up of parakeratotic cells extending through the stratum corneum. There also is a thin or absent granular layer beneath it (Figure).2
Patients generally present in the third and fourth decades of life.1 Risk factors for porokeratosis include sun exposure, immunosuppression, and genetics.2-4 Overexpression of the protein p53 in porokeratosis lesions has been demonstrated in studies investigating the genetics of porokeratosis.5,6 A study of Chinese families with DSAP identified 3 different loci associated with DSAP: DSAP1, DSAP2, and DSAP3.2 The progression to cancer has been noted in all types of porokeratosis lesions. Malignancies include squamous cell carcinoma, Bowen disease, and basal cell carcinoma.7,8
Many treatments have been tried for DSAP including cryotherapy, topical 5-fluorouracil, photodynamic therapy, and topical imiquimod with varying success.1 Our patient was treated with cryotherapy but had side effects from treatment including cellulitis and local infections with ulceration before finally healing.
Interestingly, our patient had a single lesion with pathology findings most consistent with DSAP at a later age. Although the pathology suggested DSAP, the size and solitary lesion was more consistent with porokeratosis of Mibelli. Porokeratosis of Mibelli can occur concurrently with DSAP,9 but we have not seen other lesions in this patient. We have educated our patient to be aware of other lesions that may occur in the future. Due to risk for malignant conversion, it is generally viewed as beneficial to treat patients who present with porokeratosis lesions. Our patient’s lesion ultimately cleared and he has not developed new lesions at 1-year follow-up.
Although DSAP generally presents in the third and fourth decades of life and porokeratosis of Mibelli during childhood, it is important to educate both dermatologists and primary care physicians to be aware of the possibility of both diagnoses in the elderly population.
- Rouhani P, Fischer M, Meehan S, et al. Disseminated superficial actinic porokeratosis. Dermatol Online J. 2012;18:24.
- Murase J, Gilliam AC, et al. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Lederman JS, Sober AJ, Lederman GS. Immunosuppression: a cause of porokeratosis? J Am Acad Dermatol. 1985;13:75-79.
- Hernandez MH, Lai CH, Mallory SB. Disseminated porokeratosis associated with chronic renal failure: a new type of disseminated porokeratosis? Arch Dermatol. 2000;136:1568-1569.
- Magee JW, McCalmont TH, LeBoit PE. Overexpression of p53 tumor suppressor protein in porokeratosis. Arch Dermatol. 1994;130:187-190.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536-538.
- Mehta V, Balachandran C. Simultaneous co-occurrence of porokeratosis of Mibelli with disseminated superficial actinic porokeratosis. Indian J Dermatol. 2009;54:390-391.
- Rouhani P, Fischer M, Meehan S, et al. Disseminated superficial actinic porokeratosis. Dermatol Online J. 2012;18:24.
- Murase J, Gilliam AC, et al. Disseminated superficial actinic porokeratosis co-existing with linear and verrucous porokeratosis in an elderly woman: update on the genetics and clinical expression of porokeratosis. J Am Acad Dermatol. 2010;63:886-891.
- Lederman JS, Sober AJ, Lederman GS. Immunosuppression: a cause of porokeratosis? J Am Acad Dermatol. 1985;13:75-79.
- Hernandez MH, Lai CH, Mallory SB. Disseminated porokeratosis associated with chronic renal failure: a new type of disseminated porokeratosis? Arch Dermatol. 2000;136:1568-1569.
- Magee JW, McCalmont TH, LeBoit PE. Overexpression of p53 tumor suppressor protein in porokeratosis. Arch Dermatol. 1994;130:187-190.
- Arranz-Salas I, Sanz-Trelles A, Ojeda DB. p53 alterations in porokeratosis. J Cutan Pathol. 2003;30:455-458.
- Curnow P, Foley P, Baker C. Multiple squamous cell carcinomas complicating linear porokeratosis. Australas J Dermatol. 2003;44:136-139.
- Lee HR, Han TY, Son SJ, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536-538.
- Mehta V, Balachandran C. Simultaneous co-occurrence of porokeratosis of Mibelli with disseminated superficial actinic porokeratosis. Indian J Dermatol. 2009;54:390-391.

A 69-year-old white man presented with a solitary lesion on the left ankle. His medical history included hypertension and arthritis. He resided in Florida for 11 years but denied tanning and has had sensitive skin throughout his life. He had no other notable skin conditions, except for nummular eczema. He did not have a family history of skin cancer. Physical examination showed the single lesion on the left ankle.
What Is Your Diagnosis? Fixed Cutaneous Sporotrichosis
The Diagnosis: Fixed Cutaneous Sporotrichosis
On further questioning at our dermatology clinic, the patient reported having landed face-first into rocks and gravel during the all-terrain vehicle accident. After his medical history was noted and a physical examination was completed, bacterial and fungal cultures of the wound were taken. The fungal culture was positive for Sporothrix schenckii. The patient was prescribed itraconazole 200 mg 3 times daily for 3 days, then 200 mg twice daily for an additional 4 weeks after the lesions completely resolved. An ophthalmologist was immediately consulted to rule out sinus and periorbital involvement. After computed tomography revealed possible preseptal cellulitis with frontal sinus involvement, the patient was admitted and intravenous amphotericin B was administered. Following consultations with infectious disease specialists and radiologists, amphotericin B was discontinued and the patient was discharged on itraconazole 200 mg twice daily with close monitoring. At 3-month follow-up, the sporotrichosis infection had completely cleared (Figure).
Deep fungal infections comprise 2 distinct groups: systemic and subcutaneous mycoses. Individuals with subcutaneous mycoses present with skin involvement as the primary feature. Sporotrichosis is the most common cause of this type of mycosis1 and is caused by the dimorphic fungus S schenckii, an environmental saprophyte often residing in soil. Sporothrix schenckii exists as mold in a natural environment but exists as yeast in host tissue, thus causing ensuing infection.
Epidemiology
Sporotrichosis occurs worldwide but most frequently in temperate tropical and subtropical regions. The majority of cases are reported in Mexico and Central and South America1; however, cases have been seen in the southern United States, Japan, and Australia.2 In the United States, sporotrichosis is most commonly found in river valleys of the Midwest.
Sporothrix schenckii is most commonly isolated in hay, sphagnum moss, thorny plants, and soil, but it also has been described in other manifold host environments. Unusual origins of inoculation include an old and rust-stained camping tent in Mexico,3 crawl space joists of a house in Indiana,4 and hay bales used as props in a haunted house in Oklahoma.5
The incidence of infection is primarily sporadic; however, outbreaks among individuals who share a common environment favorable for the growth of S schenckii are at risk. Those identified to be at risk include rose gardeners, berry pickers, those who work in tree nurseries, horticulturists, landscapers, and miners.
Pathogenesis
As a dimorphic fungus, infection occurs when a conidium in the mold phase is introduced into the skin, usually by traumatic skin injury, and is converted to the yeast form in vivo. Distribution of infection by this organism is most commonly localized to the cutaneous, subcutaneous, and lymphocutaneous regions in healthy hosts but can involve visceral and osteoarticular structures in immunocompromised hosts.1,6 Pulmonary and disseminated forms are rare but can occur when S schenckii conidia are inhaled. Zoonotic transmission of the fungus also can occur with exposure to infected animals. Sporothrix schenckii has been reported to occur in cats, dogs, horses, donkeys, squirrels, armadillos, and dolphins.7-11
Pathology
Sporothrix schenckii is typically not visualized on microscopic examination due to the small number of microorganisms present; however, cultures grow rapidly (3–5 days) on Sabouraud agar. The fungus most commonly develops as white or off-white compact colonies that progressively darken with age, transitioning to gray and then black.1 Microscopically, the hyphae produce oval or pyriform conidia, which are assembled in a typical bouquetlike manner. Conversion of the organism to yeast on enriched medium such as brain-heart infusion agar or blood-cysteine-glucose agar confirms the diagnosis.
Acute lesions typically show a nonspecific mixed infiltrate, but established lesions may reveal granulomatous formation and neutrophilic microabscesses.1,2 Asteroid bodies, which are cigar-shaped yeasts surrounded by eosinophilic coronae radiata, may be found. Organisms are sparsely distributed within the lesions, necessitating a thorough examination of the culture for identification.
Clinical Features
Sporotrichosis has 3 main classifications: lymphocutaneous, fixed cutaneous, and disseminated. Lymphocutaneous sporotrichosis is the most common form of the infection.2 The disease presents with a small indurated papule occurring approximately 7 to 30 days after inoculation into the skin. The papule slowly enlarges, forms a nodule, and then frequently ulcerates. Over time, draining lymphatics become edematous and inflammatory, and a chain of secondary nodules begins to appear proximal to the initial lesion. The primary and secondary nodules may continue to ulcerate; alternately, they may heal or become chronic.
In fixed cutaneous sporotrichosis, the infection remains localized to one region and a granuloma may develop, which also may ulcerate. Satellite nodules may appear along the periphery of the lesion. Lymphatic spread is not observed in this form of the disease.
The disseminated form is a result of hematogenous spread from the primary inoculation site and typically occurs in an immunocompromised host. This form can present as pulmonary disease, sinusitis, and meningitis.1
Differential Diagnosis
The differential diagnosis for sporotrichosis includes atypical mycobacteria, nocardiosis, blastomycosis, pyogenic bacteria, leishmaniasis, tularemia, and tuberculosis.
Treatment
Treatment of sporotrichosis is always required. A saturated solution of potassium iodide has classically been used; however, it is frequently associated with side effects and can be problematic to administer.12 Given its low cost and traditional efficacy, it may still be used in some parts of the world.
Currently, the treatment of choice for fixed cutaneous and lymphocutaneous sporotrichosis is itraconazole 100 to 200 mg once daily for 3 to 6 months.1 The recommended treatment of osteoarticular sporotrichosis is itraconazole, but prolonged therapy is required.
Heat therapy is an alternative treatment option, as certain strains of S schenckii do not grow at temperatures higher than 35°C. Hot compresses must be used for at least 1 hour a day for several months, which may affect patient compliance.
Immunocompromised patients often have disseminated infection and require lifelong suppressive therapy with itraconazole and may require initial treatment with amphotericin B.13
Conclusion
Subcutaneous sporotrichosis can develop in patients with a traumatic injury involving vegetation, soil, or animals. Although some patients may develop more invasive disease, most infections in immunocompetent patients will resolve after 3 to 6 months of itraconazole 100 to 200 mg once daily.1
- De Araujo T, Marques AC, Kerdel F. Sporotrichosis. Int J Dermatol. 2001;40:737-742.
- Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 2. 6th ed. New York, NY: McGraw-Hill; 2003.
- Campos P, Arenas R, Coronado H. Epidemic cutaneous sporotrichosis. Int J Dermatol. 1994;33:38-41.
- Dillon GP, Lehmann PF, Talanin NY. Handyperson’s hazard: crawl space sporotrichosis. JAMA. 1995;274: 1673-1674.
- Dooley DP, Bostic PS, Beckius ML. Spook house sporotrichosis: a point-source outbreak of sporotrichosis associated with hay bale props in a Halloween haunted house. Arch Int Med. 1997;157:1885-1887.
- Kauffman CA. Sporotrichosis. Clin Infect Dis. 1999;29:231-236.
- Migaki G, Font RL, Kaplan W, et al. Sporotrichosis in a Pacific white-sided dolphin (Lagenorhynchus obliquidens). Am J Vet Res. 1978;39:1916-1919.
- Crothers SL, White SD, Ihrke PJ, et al. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007). Vet Dermatol. 2009;20:249-259.
- Saravanakumar PS, Eslami P, Zar FA. Lymphocutaneous sporotrichosis associated with a squirrel bite: case reports and review. Clin Infect Dis. 1996;23:647-648.
- Wenker CJ, Kaufman L, Bacciarini LN, et al. Sporotrichosis in a nine-banded armadillo (Dasypus novemcinctus). J Zoo Wildl Med. 1998;29:474-478.
- Barros MB, Schubach Ade O, do Valle AC, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529-535.
- Kauffman CA. Old and new therapies for sporotrichosis. Clin Infect Dis. 1995;21:981-985.
- Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the managements of patients with sporotrichosis. Clin Infect Dis. 2000;30:684-687.
The Diagnosis: Fixed Cutaneous Sporotrichosis
On further questioning at our dermatology clinic, the patient reported having landed face-first into rocks and gravel during the all-terrain vehicle accident. After his medical history was noted and a physical examination was completed, bacterial and fungal cultures of the wound were taken. The fungal culture was positive for Sporothrix schenckii. The patient was prescribed itraconazole 200 mg 3 times daily for 3 days, then 200 mg twice daily for an additional 4 weeks after the lesions completely resolved. An ophthalmologist was immediately consulted to rule out sinus and periorbital involvement. After computed tomography revealed possible preseptal cellulitis with frontal sinus involvement, the patient was admitted and intravenous amphotericin B was administered. Following consultations with infectious disease specialists and radiologists, amphotericin B was discontinued and the patient was discharged on itraconazole 200 mg twice daily with close monitoring. At 3-month follow-up, the sporotrichosis infection had completely cleared (Figure).
Deep fungal infections comprise 2 distinct groups: systemic and subcutaneous mycoses. Individuals with subcutaneous mycoses present with skin involvement as the primary feature. Sporotrichosis is the most common cause of this type of mycosis1 and is caused by the dimorphic fungus S schenckii, an environmental saprophyte often residing in soil. Sporothrix schenckii exists as mold in a natural environment but exists as yeast in host tissue, thus causing ensuing infection.
Epidemiology
Sporotrichosis occurs worldwide but most frequently in temperate tropical and subtropical regions. The majority of cases are reported in Mexico and Central and South America1; however, cases have been seen in the southern United States, Japan, and Australia.2 In the United States, sporotrichosis is most commonly found in river valleys of the Midwest.
Sporothrix schenckii is most commonly isolated in hay, sphagnum moss, thorny plants, and soil, but it also has been described in other manifold host environments. Unusual origins of inoculation include an old and rust-stained camping tent in Mexico,3 crawl space joists of a house in Indiana,4 and hay bales used as props in a haunted house in Oklahoma.5
The incidence of infection is primarily sporadic; however, outbreaks among individuals who share a common environment favorable for the growth of S schenckii are at risk. Those identified to be at risk include rose gardeners, berry pickers, those who work in tree nurseries, horticulturists, landscapers, and miners.
Pathogenesis
As a dimorphic fungus, infection occurs when a conidium in the mold phase is introduced into the skin, usually by traumatic skin injury, and is converted to the yeast form in vivo. Distribution of infection by this organism is most commonly localized to the cutaneous, subcutaneous, and lymphocutaneous regions in healthy hosts but can involve visceral and osteoarticular structures in immunocompromised hosts.1,6 Pulmonary and disseminated forms are rare but can occur when S schenckii conidia are inhaled. Zoonotic transmission of the fungus also can occur with exposure to infected animals. Sporothrix schenckii has been reported to occur in cats, dogs, horses, donkeys, squirrels, armadillos, and dolphins.7-11
Pathology
Sporothrix schenckii is typically not visualized on microscopic examination due to the small number of microorganisms present; however, cultures grow rapidly (3–5 days) on Sabouraud agar. The fungus most commonly develops as white or off-white compact colonies that progressively darken with age, transitioning to gray and then black.1 Microscopically, the hyphae produce oval or pyriform conidia, which are assembled in a typical bouquetlike manner. Conversion of the organism to yeast on enriched medium such as brain-heart infusion agar or blood-cysteine-glucose agar confirms the diagnosis.
Acute lesions typically show a nonspecific mixed infiltrate, but established lesions may reveal granulomatous formation and neutrophilic microabscesses.1,2 Asteroid bodies, which are cigar-shaped yeasts surrounded by eosinophilic coronae radiata, may be found. Organisms are sparsely distributed within the lesions, necessitating a thorough examination of the culture for identification.
Clinical Features
Sporotrichosis has 3 main classifications: lymphocutaneous, fixed cutaneous, and disseminated. Lymphocutaneous sporotrichosis is the most common form of the infection.2 The disease presents with a small indurated papule occurring approximately 7 to 30 days after inoculation into the skin. The papule slowly enlarges, forms a nodule, and then frequently ulcerates. Over time, draining lymphatics become edematous and inflammatory, and a chain of secondary nodules begins to appear proximal to the initial lesion. The primary and secondary nodules may continue to ulcerate; alternately, they may heal or become chronic.
In fixed cutaneous sporotrichosis, the infection remains localized to one region and a granuloma may develop, which also may ulcerate. Satellite nodules may appear along the periphery of the lesion. Lymphatic spread is not observed in this form of the disease.
The disseminated form is a result of hematogenous spread from the primary inoculation site and typically occurs in an immunocompromised host. This form can present as pulmonary disease, sinusitis, and meningitis.1
Differential Diagnosis
The differential diagnosis for sporotrichosis includes atypical mycobacteria, nocardiosis, blastomycosis, pyogenic bacteria, leishmaniasis, tularemia, and tuberculosis.
Treatment
Treatment of sporotrichosis is always required. A saturated solution of potassium iodide has classically been used; however, it is frequently associated with side effects and can be problematic to administer.12 Given its low cost and traditional efficacy, it may still be used in some parts of the world.
Currently, the treatment of choice for fixed cutaneous and lymphocutaneous sporotrichosis is itraconazole 100 to 200 mg once daily for 3 to 6 months.1 The recommended treatment of osteoarticular sporotrichosis is itraconazole, but prolonged therapy is required.
Heat therapy is an alternative treatment option, as certain strains of S schenckii do not grow at temperatures higher than 35°C. Hot compresses must be used for at least 1 hour a day for several months, which may affect patient compliance.
Immunocompromised patients often have disseminated infection and require lifelong suppressive therapy with itraconazole and may require initial treatment with amphotericin B.13
Conclusion
Subcutaneous sporotrichosis can develop in patients with a traumatic injury involving vegetation, soil, or animals. Although some patients may develop more invasive disease, most infections in immunocompetent patients will resolve after 3 to 6 months of itraconazole 100 to 200 mg once daily.1
The Diagnosis: Fixed Cutaneous Sporotrichosis
On further questioning at our dermatology clinic, the patient reported having landed face-first into rocks and gravel during the all-terrain vehicle accident. After his medical history was noted and a physical examination was completed, bacterial and fungal cultures of the wound were taken. The fungal culture was positive for Sporothrix schenckii. The patient was prescribed itraconazole 200 mg 3 times daily for 3 days, then 200 mg twice daily for an additional 4 weeks after the lesions completely resolved. An ophthalmologist was immediately consulted to rule out sinus and periorbital involvement. After computed tomography revealed possible preseptal cellulitis with frontal sinus involvement, the patient was admitted and intravenous amphotericin B was administered. Following consultations with infectious disease specialists and radiologists, amphotericin B was discontinued and the patient was discharged on itraconazole 200 mg twice daily with close monitoring. At 3-month follow-up, the sporotrichosis infection had completely cleared (Figure).
Deep fungal infections comprise 2 distinct groups: systemic and subcutaneous mycoses. Individuals with subcutaneous mycoses present with skin involvement as the primary feature. Sporotrichosis is the most common cause of this type of mycosis1 and is caused by the dimorphic fungus S schenckii, an environmental saprophyte often residing in soil. Sporothrix schenckii exists as mold in a natural environment but exists as yeast in host tissue, thus causing ensuing infection.
Epidemiology
Sporotrichosis occurs worldwide but most frequently in temperate tropical and subtropical regions. The majority of cases are reported in Mexico and Central and South America1; however, cases have been seen in the southern United States, Japan, and Australia.2 In the United States, sporotrichosis is most commonly found in river valleys of the Midwest.
Sporothrix schenckii is most commonly isolated in hay, sphagnum moss, thorny plants, and soil, but it also has been described in other manifold host environments. Unusual origins of inoculation include an old and rust-stained camping tent in Mexico,3 crawl space joists of a house in Indiana,4 and hay bales used as props in a haunted house in Oklahoma.5
The incidence of infection is primarily sporadic; however, outbreaks among individuals who share a common environment favorable for the growth of S schenckii are at risk. Those identified to be at risk include rose gardeners, berry pickers, those who work in tree nurseries, horticulturists, landscapers, and miners.
Pathogenesis
As a dimorphic fungus, infection occurs when a conidium in the mold phase is introduced into the skin, usually by traumatic skin injury, and is converted to the yeast form in vivo. Distribution of infection by this organism is most commonly localized to the cutaneous, subcutaneous, and lymphocutaneous regions in healthy hosts but can involve visceral and osteoarticular structures in immunocompromised hosts.1,6 Pulmonary and disseminated forms are rare but can occur when S schenckii conidia are inhaled. Zoonotic transmission of the fungus also can occur with exposure to infected animals. Sporothrix schenckii has been reported to occur in cats, dogs, horses, donkeys, squirrels, armadillos, and dolphins.7-11
Pathology
Sporothrix schenckii is typically not visualized on microscopic examination due to the small number of microorganisms present; however, cultures grow rapidly (3–5 days) on Sabouraud agar. The fungus most commonly develops as white or off-white compact colonies that progressively darken with age, transitioning to gray and then black.1 Microscopically, the hyphae produce oval or pyriform conidia, which are assembled in a typical bouquetlike manner. Conversion of the organism to yeast on enriched medium such as brain-heart infusion agar or blood-cysteine-glucose agar confirms the diagnosis.
Acute lesions typically show a nonspecific mixed infiltrate, but established lesions may reveal granulomatous formation and neutrophilic microabscesses.1,2 Asteroid bodies, which are cigar-shaped yeasts surrounded by eosinophilic coronae radiata, may be found. Organisms are sparsely distributed within the lesions, necessitating a thorough examination of the culture for identification.
Clinical Features
Sporotrichosis has 3 main classifications: lymphocutaneous, fixed cutaneous, and disseminated. Lymphocutaneous sporotrichosis is the most common form of the infection.2 The disease presents with a small indurated papule occurring approximately 7 to 30 days after inoculation into the skin. The papule slowly enlarges, forms a nodule, and then frequently ulcerates. Over time, draining lymphatics become edematous and inflammatory, and a chain of secondary nodules begins to appear proximal to the initial lesion. The primary and secondary nodules may continue to ulcerate; alternately, they may heal or become chronic.
In fixed cutaneous sporotrichosis, the infection remains localized to one region and a granuloma may develop, which also may ulcerate. Satellite nodules may appear along the periphery of the lesion. Lymphatic spread is not observed in this form of the disease.
The disseminated form is a result of hematogenous spread from the primary inoculation site and typically occurs in an immunocompromised host. This form can present as pulmonary disease, sinusitis, and meningitis.1
Differential Diagnosis
The differential diagnosis for sporotrichosis includes atypical mycobacteria, nocardiosis, blastomycosis, pyogenic bacteria, leishmaniasis, tularemia, and tuberculosis.
Treatment
Treatment of sporotrichosis is always required. A saturated solution of potassium iodide has classically been used; however, it is frequently associated with side effects and can be problematic to administer.12 Given its low cost and traditional efficacy, it may still be used in some parts of the world.
Currently, the treatment of choice for fixed cutaneous and lymphocutaneous sporotrichosis is itraconazole 100 to 200 mg once daily for 3 to 6 months.1 The recommended treatment of osteoarticular sporotrichosis is itraconazole, but prolonged therapy is required.
Heat therapy is an alternative treatment option, as certain strains of S schenckii do not grow at temperatures higher than 35°C. Hot compresses must be used for at least 1 hour a day for several months, which may affect patient compliance.
Immunocompromised patients often have disseminated infection and require lifelong suppressive therapy with itraconazole and may require initial treatment with amphotericin B.13
Conclusion
Subcutaneous sporotrichosis can develop in patients with a traumatic injury involving vegetation, soil, or animals. Although some patients may develop more invasive disease, most infections in immunocompetent patients will resolve after 3 to 6 months of itraconazole 100 to 200 mg once daily.1
- De Araujo T, Marques AC, Kerdel F. Sporotrichosis. Int J Dermatol. 2001;40:737-742.
- Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 2. 6th ed. New York, NY: McGraw-Hill; 2003.
- Campos P, Arenas R, Coronado H. Epidemic cutaneous sporotrichosis. Int J Dermatol. 1994;33:38-41.
- Dillon GP, Lehmann PF, Talanin NY. Handyperson’s hazard: crawl space sporotrichosis. JAMA. 1995;274: 1673-1674.
- Dooley DP, Bostic PS, Beckius ML. Spook house sporotrichosis: a point-source outbreak of sporotrichosis associated with hay bale props in a Halloween haunted house. Arch Int Med. 1997;157:1885-1887.
- Kauffman CA. Sporotrichosis. Clin Infect Dis. 1999;29:231-236.
- Migaki G, Font RL, Kaplan W, et al. Sporotrichosis in a Pacific white-sided dolphin (Lagenorhynchus obliquidens). Am J Vet Res. 1978;39:1916-1919.
- Crothers SL, White SD, Ihrke PJ, et al. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007). Vet Dermatol. 2009;20:249-259.
- Saravanakumar PS, Eslami P, Zar FA. Lymphocutaneous sporotrichosis associated with a squirrel bite: case reports and review. Clin Infect Dis. 1996;23:647-648.
- Wenker CJ, Kaufman L, Bacciarini LN, et al. Sporotrichosis in a nine-banded armadillo (Dasypus novemcinctus). J Zoo Wildl Med. 1998;29:474-478.
- Barros MB, Schubach Ade O, do Valle AC, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529-535.
- Kauffman CA. Old and new therapies for sporotrichosis. Clin Infect Dis. 1995;21:981-985.
- Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the managements of patients with sporotrichosis. Clin Infect Dis. 2000;30:684-687.
- De Araujo T, Marques AC, Kerdel F. Sporotrichosis. Int J Dermatol. 2001;40:737-742.
- Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. Vol 2. 6th ed. New York, NY: McGraw-Hill; 2003.
- Campos P, Arenas R, Coronado H. Epidemic cutaneous sporotrichosis. Int J Dermatol. 1994;33:38-41.
- Dillon GP, Lehmann PF, Talanin NY. Handyperson’s hazard: crawl space sporotrichosis. JAMA. 1995;274: 1673-1674.
- Dooley DP, Bostic PS, Beckius ML. Spook house sporotrichosis: a point-source outbreak of sporotrichosis associated with hay bale props in a Halloween haunted house. Arch Int Med. 1997;157:1885-1887.
- Kauffman CA. Sporotrichosis. Clin Infect Dis. 1999;29:231-236.
- Migaki G, Font RL, Kaplan W, et al. Sporotrichosis in a Pacific white-sided dolphin (Lagenorhynchus obliquidens). Am J Vet Res. 1978;39:1916-1919.
- Crothers SL, White SD, Ihrke PJ, et al. Sporotrichosis: a retrospective evaluation of 23 cases seen in northern California (1987-2007). Vet Dermatol. 2009;20:249-259.
- Saravanakumar PS, Eslami P, Zar FA. Lymphocutaneous sporotrichosis associated with a squirrel bite: case reports and review. Clin Infect Dis. 1996;23:647-648.
- Wenker CJ, Kaufman L, Bacciarini LN, et al. Sporotrichosis in a nine-banded armadillo (Dasypus novemcinctus). J Zoo Wildl Med. 1998;29:474-478.
- Barros MB, Schubach Ade O, do Valle AC, et al. Cat-transmitted sporotrichosis epidemic in Rio de Janeiro, Brazil: description of a series of cases. Clin Infect Dis. 2004;38:529-535.
- Kauffman CA. Old and new therapies for sporotrichosis. Clin Infect Dis. 1995;21:981-985.
- Kauffman CA, Hajjeh R, Chapman SW. Practice guidelines for the managements of patients with sporotrichosis. Clin Infect Dis. 2000;30:684-687.
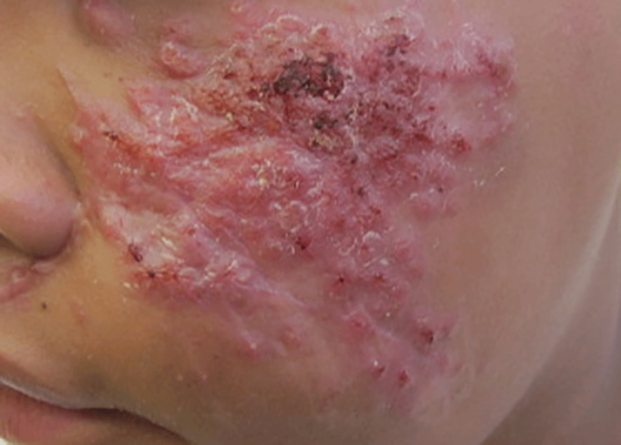
A 13-year-old adolescent boy presented with erythematous, tender, scaly, indurated nodules coalescing into plaques on the left cheek and periocular region. He denied any vision changes, the extraocular muscles were intact, and he was afebrile. Two weeks prior to presentation, the patient was hospitalized after an all-terrain vehicle accident that resulted in an extensive midfacial avulsion of the left cheek. The wound was cleaned and repaired by an otorhinolaryngologist. Three days later, he developed swelling and erythema of the left cheek, which was treated by his primary care provider with oral cephalexin, then trimethoprim-sulfamethoxazole for postsurgical wound infection. After completing his antibiotic course, he noticed continued worsening of the wound with increased edema, erythema, and tenderness. He was then referred to our clinic for further evaluation.
Erythematous Scaly Patch on the Jawline
The Diagnosis: Amelanotic Melanoma In Situ
Histopathology revealed a broad asymmetric melanocytic proliferation at the dermoepidermal junction, consisting both of singly dispersed cells as well as randomly positioned nests (Figure 1). The single cells demonstrated junctional confluence and extension along adnexal structures highlighted by melan-A stain (Figure 2). The melanocytes were markedly atypical with enlarged and hyperchromatic nuclei containing multiple nucleoli. No dermal involvement was seen. There was papillary dermal fibrosis and an active host lymphocytic response. Based on these findings, a diagnosis of amelanotic melanoma in situ was made.
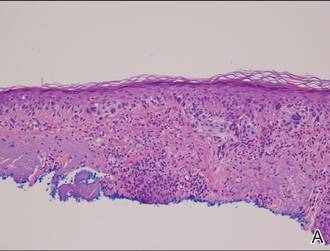
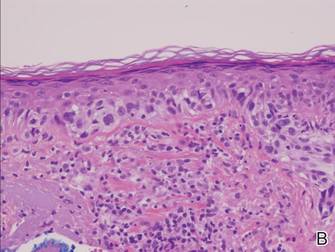
|
Figure 1. Histopathology revealed confluence of atypical melanocytes at the dermoepidermal junction and pagetoid scatter of melanocytes to the spinous layer (A)(H&E, original magnification ×4). Higher-power magnification highlighted the atypia of the individual melanocytes (B)(H&E, original magnification ×10). |
Subsequent scouting punch biopsies at the superior, anterior, and posterior aspects of the lesion were performed (Figure 3). All 3 revealed a similar nested and single cell proliferation at the dermoepidermal junction, confirming residual amelanotic melanoma in situ. The patient was referred to the otolaryngology department and underwent wide local excision with 5-mm margins and reconstructive repair.
Amelanotic melanoma comprises 2% to 8% of cutaneous melanomas. It is more common in fair-skinned elderly women with an average age of diagnosis of 61.8 years. Because features typically associated with melanoma such as asymmetry, border irregularity, and color variegation often are absent, amelanotic melanoma represents a notable diagnostic challenge for clinicians. Lesions can present nonspecifically as erythematous macules, papules, patches, or plaques and can have associated pruritus and scale.1,2
Clinical misdiagnoses for amelanotic melanoma include Bowen disease, basal cell carcinoma, actinic keratosis, lichenoid keratosis, intradermal nevus, dermatofibroma, inflamed seborrheic keratosis, nummular dermatitis, pyogenic granuloma, and granuloma annulare.1-6 There have been few case reports of amelanotic melanoma in situ, with most being the lentigo maligna variant that were initially clinically diagnosed as superficial basal cell carcinoma, Bowen disease, or dermatitis.7,8 In one case report, an amelanotic lentigo maligna was incidentally discovered after performing a mapping shave biopsy on what was normal-appearing skin.9
Dermoscopic evidence of vascular structures in lesions, including the presence of dotted vessels, milky red areas, and/or serpentine (linear irregular) vessels, may be the only clues to suggest amelanotic melanoma before biopsy. However, these findings are nonspecific and can be seen in other benign and malignant skin conditions.2
Complete surgical excision is the standard treatment of amelanotic melanoma in situ given its potential for invasion. However, the lack of pigment can make margins difficult to define. Because of its ability to detect disease beyond visual margins, Mohs micrographic surgery may have better cure rates than conventional excision.4 Prognosis for amelanotic melanoma is the same as other melanomas of equal thickness and location, though delay in diagnosis can adversely affect outcomes. Furthermore, amelanotic melanoma in situ can rapidly progress to invasive melanoma.3,5 Thus it is important to maintain clinical suspicion for amelanotic melanoma in fair-skinned elderly women presenting with a persistent or recurring erythematous scaly lesion on sun-exposed skin.
- Rahbari H, Nabai H, Mehregan AH, et al. Amelanotic lentigo maligna melanoma: a diagnostic conundrum— presentation of four new cases. Cancer. 1996;77:2052-2057.
- Jaimes N, Braun RP, Thomas L, et al. Clinical and dermoscopic characteristics of amelanotic melanomas that are not of the nodular subtype. J Eur Acad Dermatol Venereol. 2012;26:591-596.
- Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol. 2000;42:731-734.
- Conrad N, Jackson B, Goldberg L. Amelanotic lentigo maligna melanoma: a unique case presentation. Dermatol Surg. 1999;25:408-411.
- Cliff S, Otter M, Holden CA. Amelanotic lentigo maligna melanoma of the face: a case report and review of the literature. Clin Exp Dermatol. 1997;22:177-179.
- Dalton SR, Fillman EP, Altman CE, et al. Atypical junctional melanocytic proliferations in benign lichenoid keratosis. Hum Pathol. 2003;34:706-709.
- Paver K, Stewart M, Kossard S, et al. Amelanotic lentigo maligna. Australas J Dermatol. 1981;22:106-108.
- Lewis JE. Lentigo maligna presenting as an eczematous lesion. Cutis. 1987;40:357-359.
- Perera E, Mellick N, Teng P, et al. A clinically invisible melanoma. Australas J Dermatol. 2014;55:e58-e59.
The Diagnosis: Amelanotic Melanoma In Situ
Histopathology revealed a broad asymmetric melanocytic proliferation at the dermoepidermal junction, consisting both of singly dispersed cells as well as randomly positioned nests (Figure 1). The single cells demonstrated junctional confluence and extension along adnexal structures highlighted by melan-A stain (Figure 2). The melanocytes were markedly atypical with enlarged and hyperchromatic nuclei containing multiple nucleoli. No dermal involvement was seen. There was papillary dermal fibrosis and an active host lymphocytic response. Based on these findings, a diagnosis of amelanotic melanoma in situ was made.


|
Figure 1. Histopathology revealed confluence of atypical melanocytes at the dermoepidermal junction and pagetoid scatter of melanocytes to the spinous layer (A)(H&E, original magnification ×4). Higher-power magnification highlighted the atypia of the individual melanocytes (B)(H&E, original magnification ×10). |
Subsequent scouting punch biopsies at the superior, anterior, and posterior aspects of the lesion were performed (Figure 3). All 3 revealed a similar nested and single cell proliferation at the dermoepidermal junction, confirming residual amelanotic melanoma in situ. The patient was referred to the otolaryngology department and underwent wide local excision with 5-mm margins and reconstructive repair.
Amelanotic melanoma comprises 2% to 8% of cutaneous melanomas. It is more common in fair-skinned elderly women with an average age of diagnosis of 61.8 years. Because features typically associated with melanoma such as asymmetry, border irregularity, and color variegation often are absent, amelanotic melanoma represents a notable diagnostic challenge for clinicians. Lesions can present nonspecifically as erythematous macules, papules, patches, or plaques and can have associated pruritus and scale.1,2
Clinical misdiagnoses for amelanotic melanoma include Bowen disease, basal cell carcinoma, actinic keratosis, lichenoid keratosis, intradermal nevus, dermatofibroma, inflamed seborrheic keratosis, nummular dermatitis, pyogenic granuloma, and granuloma annulare.1-6 There have been few case reports of amelanotic melanoma in situ, with most being the lentigo maligna variant that were initially clinically diagnosed as superficial basal cell carcinoma, Bowen disease, or dermatitis.7,8 In one case report, an amelanotic lentigo maligna was incidentally discovered after performing a mapping shave biopsy on what was normal-appearing skin.9
Dermoscopic evidence of vascular structures in lesions, including the presence of dotted vessels, milky red areas, and/or serpentine (linear irregular) vessels, may be the only clues to suggest amelanotic melanoma before biopsy. However, these findings are nonspecific and can be seen in other benign and malignant skin conditions.2
Complete surgical excision is the standard treatment of amelanotic melanoma in situ given its potential for invasion. However, the lack of pigment can make margins difficult to define. Because of its ability to detect disease beyond visual margins, Mohs micrographic surgery may have better cure rates than conventional excision.4 Prognosis for amelanotic melanoma is the same as other melanomas of equal thickness and location, though delay in diagnosis can adversely affect outcomes. Furthermore, amelanotic melanoma in situ can rapidly progress to invasive melanoma.3,5 Thus it is important to maintain clinical suspicion for amelanotic melanoma in fair-skinned elderly women presenting with a persistent or recurring erythematous scaly lesion on sun-exposed skin.
The Diagnosis: Amelanotic Melanoma In Situ
Histopathology revealed a broad asymmetric melanocytic proliferation at the dermoepidermal junction, consisting both of singly dispersed cells as well as randomly positioned nests (Figure 1). The single cells demonstrated junctional confluence and extension along adnexal structures highlighted by melan-A stain (Figure 2). The melanocytes were markedly atypical with enlarged and hyperchromatic nuclei containing multiple nucleoli. No dermal involvement was seen. There was papillary dermal fibrosis and an active host lymphocytic response. Based on these findings, a diagnosis of amelanotic melanoma in situ was made.


|
Figure 1. Histopathology revealed confluence of atypical melanocytes at the dermoepidermal junction and pagetoid scatter of melanocytes to the spinous layer (A)(H&E, original magnification ×4). Higher-power magnification highlighted the atypia of the individual melanocytes (B)(H&E, original magnification ×10). |
Subsequent scouting punch biopsies at the superior, anterior, and posterior aspects of the lesion were performed (Figure 3). All 3 revealed a similar nested and single cell proliferation at the dermoepidermal junction, confirming residual amelanotic melanoma in situ. The patient was referred to the otolaryngology department and underwent wide local excision with 5-mm margins and reconstructive repair.
Amelanotic melanoma comprises 2% to 8% of cutaneous melanomas. It is more common in fair-skinned elderly women with an average age of diagnosis of 61.8 years. Because features typically associated with melanoma such as asymmetry, border irregularity, and color variegation often are absent, amelanotic melanoma represents a notable diagnostic challenge for clinicians. Lesions can present nonspecifically as erythematous macules, papules, patches, or plaques and can have associated pruritus and scale.1,2
Clinical misdiagnoses for amelanotic melanoma include Bowen disease, basal cell carcinoma, actinic keratosis, lichenoid keratosis, intradermal nevus, dermatofibroma, inflamed seborrheic keratosis, nummular dermatitis, pyogenic granuloma, and granuloma annulare.1-6 There have been few case reports of amelanotic melanoma in situ, with most being the lentigo maligna variant that were initially clinically diagnosed as superficial basal cell carcinoma, Bowen disease, or dermatitis.7,8 In one case report, an amelanotic lentigo maligna was incidentally discovered after performing a mapping shave biopsy on what was normal-appearing skin.9
Dermoscopic evidence of vascular structures in lesions, including the presence of dotted vessels, milky red areas, and/or serpentine (linear irregular) vessels, may be the only clues to suggest amelanotic melanoma before biopsy. However, these findings are nonspecific and can be seen in other benign and malignant skin conditions.2
Complete surgical excision is the standard treatment of amelanotic melanoma in situ given its potential for invasion. However, the lack of pigment can make margins difficult to define. Because of its ability to detect disease beyond visual margins, Mohs micrographic surgery may have better cure rates than conventional excision.4 Prognosis for amelanotic melanoma is the same as other melanomas of equal thickness and location, though delay in diagnosis can adversely affect outcomes. Furthermore, amelanotic melanoma in situ can rapidly progress to invasive melanoma.3,5 Thus it is important to maintain clinical suspicion for amelanotic melanoma in fair-skinned elderly women presenting with a persistent or recurring erythematous scaly lesion on sun-exposed skin.
- Rahbari H, Nabai H, Mehregan AH, et al. Amelanotic lentigo maligna melanoma: a diagnostic conundrum— presentation of four new cases. Cancer. 1996;77:2052-2057.
- Jaimes N, Braun RP, Thomas L, et al. Clinical and dermoscopic characteristics of amelanotic melanomas that are not of the nodular subtype. J Eur Acad Dermatol Venereol. 2012;26:591-596.
- Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol. 2000;42:731-734.
- Conrad N, Jackson B, Goldberg L. Amelanotic lentigo maligna melanoma: a unique case presentation. Dermatol Surg. 1999;25:408-411.
- Cliff S, Otter M, Holden CA. Amelanotic lentigo maligna melanoma of the face: a case report and review of the literature. Clin Exp Dermatol. 1997;22:177-179.
- Dalton SR, Fillman EP, Altman CE, et al. Atypical junctional melanocytic proliferations in benign lichenoid keratosis. Hum Pathol. 2003;34:706-709.
- Paver K, Stewart M, Kossard S, et al. Amelanotic lentigo maligna. Australas J Dermatol. 1981;22:106-108.
- Lewis JE. Lentigo maligna presenting as an eczematous lesion. Cutis. 1987;40:357-359.
- Perera E, Mellick N, Teng P, et al. A clinically invisible melanoma. Australas J Dermatol. 2014;55:e58-e59.
- Rahbari H, Nabai H, Mehregan AH, et al. Amelanotic lentigo maligna melanoma: a diagnostic conundrum— presentation of four new cases. Cancer. 1996;77:2052-2057.
- Jaimes N, Braun RP, Thomas L, et al. Clinical and dermoscopic characteristics of amelanotic melanomas that are not of the nodular subtype. J Eur Acad Dermatol Venereol. 2012;26:591-596.
- Koch SE, Lange JR. Amelanotic melanoma: the great masquerader. J Am Acad Dermatol. 2000;42:731-734.
- Conrad N, Jackson B, Goldberg L. Amelanotic lentigo maligna melanoma: a unique case presentation. Dermatol Surg. 1999;25:408-411.
- Cliff S, Otter M, Holden CA. Amelanotic lentigo maligna melanoma of the face: a case report and review of the literature. Clin Exp Dermatol. 1997;22:177-179.
- Dalton SR, Fillman EP, Altman CE, et al. Atypical junctional melanocytic proliferations in benign lichenoid keratosis. Hum Pathol. 2003;34:706-709.
- Paver K, Stewart M, Kossard S, et al. Amelanotic lentigo maligna. Australas J Dermatol. 1981;22:106-108.
- Lewis JE. Lentigo maligna presenting as an eczematous lesion. Cutis. 1987;40:357-359.
- Perera E, Mellick N, Teng P, et al. A clinically invisible melanoma. Australas J Dermatol. 2014;55:e58-e59.

Atypia of the individual melanocytes.
Melan-A stain highlighted the density and confluence of melanocytes within the epidermis.
Three scouting punch biopsies were performed along the periphery of the lesion.
A 70-year-old white woman with a history of basal cell carcinoma presented with a 2.7×1.9-cm ill-defined, erythematous, scaly patch along the left side of the jawline. Ten months prior to presentation, the lesion appeared as a grayish macule that was clinically diagnosed as a pigmented actinic keratosis and was treated with cryotherapy with resolution noted at 6-month follow-up. Differential diagnosis of the current lesion included actinic keratosis, lichenoid keratosis, and superficial basal cell carcinoma. A shave biopsy was performed.