User login
A new papule and “age spots”
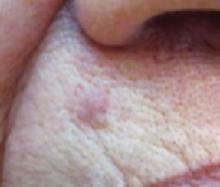
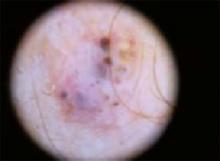
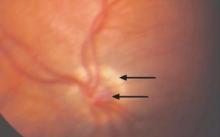
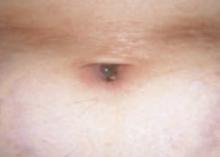
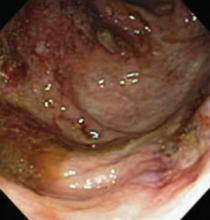
An 87-year-old woman came to the office for evaluation of a lesion above her lip (FIGURE 1) that had “been there a while” and had intermittently been bleeding and crusting for the last few months. On examination, there was a distinct, firm (but not hard) papule with some adjacent erythema. No distinct telangiectasias, ulceration, blood, or crusts were visible with handheld magnification or upon dermoscopy. (see “The digital camera: Another stethoscope for the skin,”)
FIGURE 1
Lesion above lip
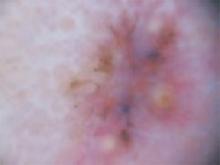
An evaluation of the remainder of the woman’s face revealed 3 more lesions that the patient termed “age spots.” They had been present for quite some time, had not had any notable rapid change, and had not caused her (or a physician in the family) any concern. These “age spots” are depicted in (FIGURE 2A) (left temple), (FIGURE 2B) (forehead), and (FIGURE 2C) (left cheek). Digital photographs were taken through the dermatoscope of the temple, forehead, and cheek lesions (FIGURE 3A, B, AND C).
FIGURE 2A
Digital photos
FIGURE 2B
Digital photos
FIGURE 2C
Digital photos
The 4 lesions are easily identified as worrisome, given that they were pigmented and asymmetric, with a variety of bizarre colors.
The lip. In particular, the lesion above the upper lip (FIGURE 1) clinically presented a wide range of possibilities, including basal cell carcinoma (BCC), milial cyst, nevus, trichoepithelioma, fibrous papule, or any of a variety of adnexal skin neoplasms. Knowing that the lesion was relatively new and had bled and crusted was sufficient to warrant biopsy.
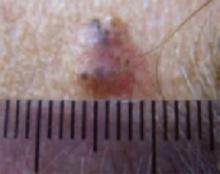
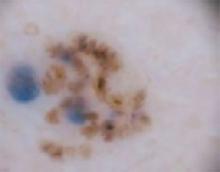
The temple. Dermoscopically, the temple lesion (FIGURE 3A) had blue and brown ovoid structures (also called “blebs” or “blobs”), white areas within the lesion (whiter than normal surrounding skin), a high degree of asymmetry, and distinct telangiectatic vessels. The pink color on dermoscopy was also a cause for concern. The blue ovoid structures plus telangiectasias were highly suggestive of basal cell carcinoma.
FIGURE 3A
Dermoscopy images
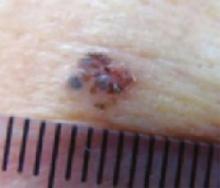
The forehead. Dermoscopy of the forehead lesion (FIGURE 3B) showed leaf-like structures (12 o’clock) and maple-leaf structures (6 o’clock). These alone were highly suggestive of pigmented basal cell carcinoma—but in the absence of distinct telangiectasias, we decided to do a deep incisional biopsy rather than risk potentially “shaving a melanoma.” (If a melanoma is biopsied via a shave technique, the ability to histologically measure its thickness and to stage it according to Clark and Breslow staging is lost.)
FIGURE 3B
Dermoscopy images
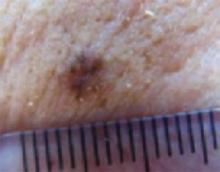
The cheek. Dermoscopically, the lesion on the cheek (FIGURE 3C) also had no obvious telangiectasias but had a “spoke-wheel” structure (6 o’clock) highly suggestive of basal cell carcinoma.
FIGURE 3C
Dermoscopy images
All the lesions—except for the temple lesion, which was biopsied via a shave technique—were biopsied via generous incisional ellipses.
What is your diagnosis?
How would you treat?
Diagnosis: Basal cell carcinoma
Histology confirmed that all 4 lesions were basal cell carcinomas, the most common type of skin malignancy. The temple lesion in Figures 2A AND 3A and the forehead lesion in Figures 2B AND 3B were histologically both pigmented nodular basal cell carcinomas, clinically characterized as pearly papules with pigment. (FIGURE 3A) also demonstrates telangiectasia.
Differential diagnosis: Innocent papule or carcinoma?
The lip lesion, the presenting “symptom,” did not have evident bleeding and crusting on visual or dermoscopic examination. In the absence of a complete history, it could have been “passed off” as an innocent papule, such as a molluscum (though not common in the elderly) or a milial or epidermoid cyst.
Remember that basal cell carcinoma can be subtle. These lesions were missed by a patient and her family—which included a physician within the household—and grew slowly enough that the patient felt they were simply “age spots.” We have seen basal cell carcinomas that patients have indicated have not changed in years—have not bled, ulcerated, or crusted, while symptomatic lesions have been the least impressive, clinically, at the time of the exam. Always maintain a high index of suspicion.
The clinical types of basal cell carcinoma and their dermoscopic findings are summarized in the (TABLE).
TABLE
Clinical types of basal cell carcinoma and dermoscopic findings
| CLINICAL TYPE | DERMOSCOPIC FINDINGS | NOTES |
|---|---|---|
Nodular (including noduloulcerative and cystic)
“Wart” on a supraclavicular area—note pearly translucency of nodular basal cell carcinoma. | Arborizing (tree-like branching telangiectasias)
Dermoscopy of lesion at left, clearly showing arborized telangiectatic vessels. |
|
| Pigmented |
|
|
| Sclerosing, cicatricial, or morpheaform | Arborizing telangiectasias |
|
| Superficial | Arborizing telangiectasias |
|
| *Sensitivity/specificity. Sensitivity is the percentage of basal cell carcinomas that possess the feature. Specificity listed is the percentage of melanomas that lack the feature.1 All discussion of dermoscopic diagnosis of basal cell carcinoma assumes absence of a melanocytic pigment network, the presence of which suggests a melanocytic lesion such as a nevus, lentigo, or melanoma. | ||
| Note: The primary use of dermoscopy is the evaluation of pigmented lesions. Thus, except to aid in visualization of telangiectasias and ulceration, there are no characteristic dermoscopic findings in other types of basal cell carcinoma. Telangiectasias may not be visualized if the dermatoscope is applied with sufficient pressure to blanch them. Basal cell carcinomas may exhibit no definite or suggestive findings by dermoscopy, as was the case with the lip papule on this patient. | ||
Tips for making an accurate diagnosis
Basal cell carcinoma and melanoma can mimic other lesions, so keep these tips in mind:
- The “company” a lesion keeps sometimes can help in diagnosis. A patient may have a group of small “pearly papules,” only one of which may show the typical umbilication that allows a confident diagnosis of molluscum contagiosum, for example. Here, 3 lesions had similar dermoscopic structures, only one of which exhibited telangiectasia. A fourth lesion lacked diagnostic characteristics. The best guess, based on the sum total appearance of all of these lesions, is that all are basal cell carcinomas because of the “company they are keeping”—but note that this is also potentially a trap: missing the single basal cell carcinoma lesion among a field of sebaceous hyperplasia, for instance.
- Don’t focus exclusively on the symptomatic lesion. Do a survey of the general region. For ultraviolet-associated lesions (including basal cell carcinoma), it’s preferable to perform, at minimum, a survey of “high-radiation” areas (face, exposed scalp, neck, ears, and dorsal hands and forearms) for other ultraviolet “damage” (eg, actinic keratoses).
- Be meticulous when examining patients’ backs. Patients may not spot lesions on their backs—especially if they are older and have poor vision.
- Avoid thinking in terms of absolutes like “never” and “always.” The clinical axiom that basal cell carcinomas “never” have hair growing from them is disproved by (FIGURE 3A), which clinically, dermoscopically, and histologically is a basal cell carcinoma lesion. Some of the hair seen here is overlying, loose scalp hair “caught” in the dermoscopic field because of the location of this lesion on the temple adjacent to the hairline. But there are also very distinct hairs seen coming out from areas (at about 6 and 8 o’clock) that are clearly part of the lesion, especially on its periphery.
When in doubt, biopsy
When in doubt about which technique to perform, do an incisional biopsy—preferably excisional, but at least a good sampling of the most worrisome area(s).
Suspected basal cell carcinoma, when the examiner is confident the lesion is not a melanoma, can be further evaluated by superficial shave biopsy. Potential melanoma generally should not be evaluated by the shave technique.
Options for therapy
Therapy options for basal cell carcinoma vary based on location (high-risk vs low-risk locations), histologic type of basal cell carcinoma, patient preference, and local availability of therapy. The primary therapies for basal cell carcinoma are surgical excision (including Mohs surgery) and curettage, often combined with electrodesiccation. 5-flurouracil (5-FU) should not be used because it can treat the surface tumor while deeper tumor proliferates.3 Imiquimod is not approved for facial lesions or nodular basal cell carcinomas.
While dermatoscopes are the true “skin stethoscopes,” most primary care physicians do not have them. Many, however, do have a digital camera. A digital camera with a macro-focus feature can be viewed as another stethoscope for the skin. Pictures allow great magnification on the computer screen, presenting color and detail that may be missed on routine clinical inspection. They allow an unhurried “self–second opinion”; you can evaluate lesions with no motion, breathing, or other distractions, following the office visit.
Digital images may also be important for:
- the patient, who may not be able to see the lesion, because it’s on his back, buttocks, or behind his ears. Consider, too, the older patient who may not be able to see the seborrhea in his eyebrow with his bifocals, but he can see it on the camera’s monitor.
- the medical record (printed or electronically stored).
- the pathologist—when forwarded with the pathology specimen. The images can be helpful in developing a clinical correlation to include in the pathology report.
- the insurance carrier, as indisputable documentation for the clinical rationale for biopsying 4 lesions on 1 visit in the event of a “Dear Bad Doctor” Medicare letter. In fact, if not for the indisputable photographic record, one author (GNF) would have been extremely hesitant to perform 4 biopsies on a Medicare patient in 1 session.
- light and magnification where the 2 may be in short supply, such as a poorly mobile patient in a hospital bed. A camera with flash, auto-focus, and macro mode may allow access to otherwise inaccessible lesions.
ACKNOWLEDGMENTS
Gary N. Fox wishes to acknowledge the assistance of Peggy Elston and Lisa Nichols for their help with portions of this article.
CORRESPONDENCE
Gary N. Fox, MD, 2458 Willesden Green, Toledo, OH. E-mail: [email protected]
1. Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005.
2. Johr R, Soyer HP, Argenziano G, Hofmann-Wellenhof R, Scalvenzi M. Dermoscopy: The Essentials. New York: Mosby; 2004.
3. Premalignant and malignant nonmelanoma skin tumors [chapter 21]. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004.
An 87-year-old woman came to the office for evaluation of a lesion above her lip (FIGURE 1) that had “been there a while” and had intermittently been bleeding and crusting for the last few months. On examination, there was a distinct, firm (but not hard) papule with some adjacent erythema. No distinct telangiectasias, ulceration, blood, or crusts were visible with handheld magnification or upon dermoscopy. (see “The digital camera: Another stethoscope for the skin,”)
FIGURE 1
Lesion above lip
An evaluation of the remainder of the woman’s face revealed 3 more lesions that the patient termed “age spots.” They had been present for quite some time, had not had any notable rapid change, and had not caused her (or a physician in the family) any concern. These “age spots” are depicted in (FIGURE 2A) (left temple), (FIGURE 2B) (forehead), and (FIGURE 2C) (left cheek). Digital photographs were taken through the dermatoscope of the temple, forehead, and cheek lesions (FIGURE 3A, B, AND C).
FIGURE 2A
Digital photos
FIGURE 2B
Digital photos
FIGURE 2C
Digital photos
The 4 lesions are easily identified as worrisome, given that they were pigmented and asymmetric, with a variety of bizarre colors.
The lip. In particular, the lesion above the upper lip (FIGURE 1) clinically presented a wide range of possibilities, including basal cell carcinoma (BCC), milial cyst, nevus, trichoepithelioma, fibrous papule, or any of a variety of adnexal skin neoplasms. Knowing that the lesion was relatively new and had bled and crusted was sufficient to warrant biopsy.
The temple. Dermoscopically, the temple lesion (FIGURE 3A) had blue and brown ovoid structures (also called “blebs” or “blobs”), white areas within the lesion (whiter than normal surrounding skin), a high degree of asymmetry, and distinct telangiectatic vessels. The pink color on dermoscopy was also a cause for concern. The blue ovoid structures plus telangiectasias were highly suggestive of basal cell carcinoma.
FIGURE 3A
Dermoscopy images
The forehead. Dermoscopy of the forehead lesion (FIGURE 3B) showed leaf-like structures (12 o’clock) and maple-leaf structures (6 o’clock). These alone were highly suggestive of pigmented basal cell carcinoma—but in the absence of distinct telangiectasias, we decided to do a deep incisional biopsy rather than risk potentially “shaving a melanoma.” (If a melanoma is biopsied via a shave technique, the ability to histologically measure its thickness and to stage it according to Clark and Breslow staging is lost.)
FIGURE 3B
Dermoscopy images
The cheek. Dermoscopically, the lesion on the cheek (FIGURE 3C) also had no obvious telangiectasias but had a “spoke-wheel” structure (6 o’clock) highly suggestive of basal cell carcinoma.
FIGURE 3C
Dermoscopy images
All the lesions—except for the temple lesion, which was biopsied via a shave technique—were biopsied via generous incisional ellipses.
What is your diagnosis?
How would you treat?
Diagnosis: Basal cell carcinoma
Histology confirmed that all 4 lesions were basal cell carcinomas, the most common type of skin malignancy. The temple lesion in Figures 2A AND 3A and the forehead lesion in Figures 2B AND 3B were histologically both pigmented nodular basal cell carcinomas, clinically characterized as pearly papules with pigment. (FIGURE 3A) also demonstrates telangiectasia.
Differential diagnosis: Innocent papule or carcinoma?
The lip lesion, the presenting “symptom,” did not have evident bleeding and crusting on visual or dermoscopic examination. In the absence of a complete history, it could have been “passed off” as an innocent papule, such as a molluscum (though not common in the elderly) or a milial or epidermoid cyst.
Remember that basal cell carcinoma can be subtle. These lesions were missed by a patient and her family—which included a physician within the household—and grew slowly enough that the patient felt they were simply “age spots.” We have seen basal cell carcinomas that patients have indicated have not changed in years—have not bled, ulcerated, or crusted, while symptomatic lesions have been the least impressive, clinically, at the time of the exam. Always maintain a high index of suspicion.
The clinical types of basal cell carcinoma and their dermoscopic findings are summarized in the (TABLE).
TABLE
Clinical types of basal cell carcinoma and dermoscopic findings
| CLINICAL TYPE | DERMOSCOPIC FINDINGS | NOTES |
|---|---|---|
Nodular (including noduloulcerative and cystic)
“Wart” on a supraclavicular area—note pearly translucency of nodular basal cell carcinoma. | Arborizing (tree-like branching telangiectasias)
Dermoscopy of lesion at left, clearly showing arborized telangiectatic vessels. |
|
| Pigmented |
|
|
| Sclerosing, cicatricial, or morpheaform | Arborizing telangiectasias |
|
| Superficial | Arborizing telangiectasias |
|
| *Sensitivity/specificity. Sensitivity is the percentage of basal cell carcinomas that possess the feature. Specificity listed is the percentage of melanomas that lack the feature.1 All discussion of dermoscopic diagnosis of basal cell carcinoma assumes absence of a melanocytic pigment network, the presence of which suggests a melanocytic lesion such as a nevus, lentigo, or melanoma. | ||
| Note: The primary use of dermoscopy is the evaluation of pigmented lesions. Thus, except to aid in visualization of telangiectasias and ulceration, there are no characteristic dermoscopic findings in other types of basal cell carcinoma. Telangiectasias may not be visualized if the dermatoscope is applied with sufficient pressure to blanch them. Basal cell carcinomas may exhibit no definite or suggestive findings by dermoscopy, as was the case with the lip papule on this patient. | ||
Tips for making an accurate diagnosis
Basal cell carcinoma and melanoma can mimic other lesions, so keep these tips in mind:
- The “company” a lesion keeps sometimes can help in diagnosis. A patient may have a group of small “pearly papules,” only one of which may show the typical umbilication that allows a confident diagnosis of molluscum contagiosum, for example. Here, 3 lesions had similar dermoscopic structures, only one of which exhibited telangiectasia. A fourth lesion lacked diagnostic characteristics. The best guess, based on the sum total appearance of all of these lesions, is that all are basal cell carcinomas because of the “company they are keeping”—but note that this is also potentially a trap: missing the single basal cell carcinoma lesion among a field of sebaceous hyperplasia, for instance.
- Don’t focus exclusively on the symptomatic lesion. Do a survey of the general region. For ultraviolet-associated lesions (including basal cell carcinoma), it’s preferable to perform, at minimum, a survey of “high-radiation” areas (face, exposed scalp, neck, ears, and dorsal hands and forearms) for other ultraviolet “damage” (eg, actinic keratoses).
- Be meticulous when examining patients’ backs. Patients may not spot lesions on their backs—especially if they are older and have poor vision.
- Avoid thinking in terms of absolutes like “never” and “always.” The clinical axiom that basal cell carcinomas “never” have hair growing from them is disproved by (FIGURE 3A), which clinically, dermoscopically, and histologically is a basal cell carcinoma lesion. Some of the hair seen here is overlying, loose scalp hair “caught” in the dermoscopic field because of the location of this lesion on the temple adjacent to the hairline. But there are also very distinct hairs seen coming out from areas (at about 6 and 8 o’clock) that are clearly part of the lesion, especially on its periphery.
When in doubt, biopsy
When in doubt about which technique to perform, do an incisional biopsy—preferably excisional, but at least a good sampling of the most worrisome area(s).
Suspected basal cell carcinoma, when the examiner is confident the lesion is not a melanoma, can be further evaluated by superficial shave biopsy. Potential melanoma generally should not be evaluated by the shave technique.
Options for therapy
Therapy options for basal cell carcinoma vary based on location (high-risk vs low-risk locations), histologic type of basal cell carcinoma, patient preference, and local availability of therapy. The primary therapies for basal cell carcinoma are surgical excision (including Mohs surgery) and curettage, often combined with electrodesiccation. 5-flurouracil (5-FU) should not be used because it can treat the surface tumor while deeper tumor proliferates.3 Imiquimod is not approved for facial lesions or nodular basal cell carcinomas.
While dermatoscopes are the true “skin stethoscopes,” most primary care physicians do not have them. Many, however, do have a digital camera. A digital camera with a macro-focus feature can be viewed as another stethoscope for the skin. Pictures allow great magnification on the computer screen, presenting color and detail that may be missed on routine clinical inspection. They allow an unhurried “self–second opinion”; you can evaluate lesions with no motion, breathing, or other distractions, following the office visit.
Digital images may also be important for:
- the patient, who may not be able to see the lesion, because it’s on his back, buttocks, or behind his ears. Consider, too, the older patient who may not be able to see the seborrhea in his eyebrow with his bifocals, but he can see it on the camera’s monitor.
- the medical record (printed or electronically stored).
- the pathologist—when forwarded with the pathology specimen. The images can be helpful in developing a clinical correlation to include in the pathology report.
- the insurance carrier, as indisputable documentation for the clinical rationale for biopsying 4 lesions on 1 visit in the event of a “Dear Bad Doctor” Medicare letter. In fact, if not for the indisputable photographic record, one author (GNF) would have been extremely hesitant to perform 4 biopsies on a Medicare patient in 1 session.
- light and magnification where the 2 may be in short supply, such as a poorly mobile patient in a hospital bed. A camera with flash, auto-focus, and macro mode may allow access to otherwise inaccessible lesions.
ACKNOWLEDGMENTS
Gary N. Fox wishes to acknowledge the assistance of Peggy Elston and Lisa Nichols for their help with portions of this article.
CORRESPONDENCE
Gary N. Fox, MD, 2458 Willesden Green, Toledo, OH. E-mail: [email protected]
An 87-year-old woman came to the office for evaluation of a lesion above her lip (FIGURE 1) that had “been there a while” and had intermittently been bleeding and crusting for the last few months. On examination, there was a distinct, firm (but not hard) papule with some adjacent erythema. No distinct telangiectasias, ulceration, blood, or crusts were visible with handheld magnification or upon dermoscopy. (see “The digital camera: Another stethoscope for the skin,”)
FIGURE 1
Lesion above lip
An evaluation of the remainder of the woman’s face revealed 3 more lesions that the patient termed “age spots.” They had been present for quite some time, had not had any notable rapid change, and had not caused her (or a physician in the family) any concern. These “age spots” are depicted in (FIGURE 2A) (left temple), (FIGURE 2B) (forehead), and (FIGURE 2C) (left cheek). Digital photographs were taken through the dermatoscope of the temple, forehead, and cheek lesions (FIGURE 3A, B, AND C).
FIGURE 2A
Digital photos
FIGURE 2B
Digital photos
FIGURE 2C
Digital photos
The 4 lesions are easily identified as worrisome, given that they were pigmented and asymmetric, with a variety of bizarre colors.
The lip. In particular, the lesion above the upper lip (FIGURE 1) clinically presented a wide range of possibilities, including basal cell carcinoma (BCC), milial cyst, nevus, trichoepithelioma, fibrous papule, or any of a variety of adnexal skin neoplasms. Knowing that the lesion was relatively new and had bled and crusted was sufficient to warrant biopsy.
The temple. Dermoscopically, the temple lesion (FIGURE 3A) had blue and brown ovoid structures (also called “blebs” or “blobs”), white areas within the lesion (whiter than normal surrounding skin), a high degree of asymmetry, and distinct telangiectatic vessels. The pink color on dermoscopy was also a cause for concern. The blue ovoid structures plus telangiectasias were highly suggestive of basal cell carcinoma.
FIGURE 3A
Dermoscopy images
The forehead. Dermoscopy of the forehead lesion (FIGURE 3B) showed leaf-like structures (12 o’clock) and maple-leaf structures (6 o’clock). These alone were highly suggestive of pigmented basal cell carcinoma—but in the absence of distinct telangiectasias, we decided to do a deep incisional biopsy rather than risk potentially “shaving a melanoma.” (If a melanoma is biopsied via a shave technique, the ability to histologically measure its thickness and to stage it according to Clark and Breslow staging is lost.)
FIGURE 3B
Dermoscopy images
The cheek. Dermoscopically, the lesion on the cheek (FIGURE 3C) also had no obvious telangiectasias but had a “spoke-wheel” structure (6 o’clock) highly suggestive of basal cell carcinoma.
FIGURE 3C
Dermoscopy images
All the lesions—except for the temple lesion, which was biopsied via a shave technique—were biopsied via generous incisional ellipses.
What is your diagnosis?
How would you treat?
Diagnosis: Basal cell carcinoma
Histology confirmed that all 4 lesions were basal cell carcinomas, the most common type of skin malignancy. The temple lesion in Figures 2A AND 3A and the forehead lesion in Figures 2B AND 3B were histologically both pigmented nodular basal cell carcinomas, clinically characterized as pearly papules with pigment. (FIGURE 3A) also demonstrates telangiectasia.
Differential diagnosis: Innocent papule or carcinoma?
The lip lesion, the presenting “symptom,” did not have evident bleeding and crusting on visual or dermoscopic examination. In the absence of a complete history, it could have been “passed off” as an innocent papule, such as a molluscum (though not common in the elderly) or a milial or epidermoid cyst.
Remember that basal cell carcinoma can be subtle. These lesions were missed by a patient and her family—which included a physician within the household—and grew slowly enough that the patient felt they were simply “age spots.” We have seen basal cell carcinomas that patients have indicated have not changed in years—have not bled, ulcerated, or crusted, while symptomatic lesions have been the least impressive, clinically, at the time of the exam. Always maintain a high index of suspicion.
The clinical types of basal cell carcinoma and their dermoscopic findings are summarized in the (TABLE).
TABLE
Clinical types of basal cell carcinoma and dermoscopic findings
| CLINICAL TYPE | DERMOSCOPIC FINDINGS | NOTES |
|---|---|---|
Nodular (including noduloulcerative and cystic)
“Wart” on a supraclavicular area—note pearly translucency of nodular basal cell carcinoma. | Arborizing (tree-like branching telangiectasias)
Dermoscopy of lesion at left, clearly showing arborized telangiectatic vessels. |
|
| Pigmented |
|
|
| Sclerosing, cicatricial, or morpheaform | Arborizing telangiectasias |
|
| Superficial | Arborizing telangiectasias |
|
| *Sensitivity/specificity. Sensitivity is the percentage of basal cell carcinomas that possess the feature. Specificity listed is the percentage of melanomas that lack the feature.1 All discussion of dermoscopic diagnosis of basal cell carcinoma assumes absence of a melanocytic pigment network, the presence of which suggests a melanocytic lesion such as a nevus, lentigo, or melanoma. | ||
| Note: The primary use of dermoscopy is the evaluation of pigmented lesions. Thus, except to aid in visualization of telangiectasias and ulceration, there are no characteristic dermoscopic findings in other types of basal cell carcinoma. Telangiectasias may not be visualized if the dermatoscope is applied with sufficient pressure to blanch them. Basal cell carcinomas may exhibit no definite or suggestive findings by dermoscopy, as was the case with the lip papule on this patient. | ||
Tips for making an accurate diagnosis
Basal cell carcinoma and melanoma can mimic other lesions, so keep these tips in mind:
- The “company” a lesion keeps sometimes can help in diagnosis. A patient may have a group of small “pearly papules,” only one of which may show the typical umbilication that allows a confident diagnosis of molluscum contagiosum, for example. Here, 3 lesions had similar dermoscopic structures, only one of which exhibited telangiectasia. A fourth lesion lacked diagnostic characteristics. The best guess, based on the sum total appearance of all of these lesions, is that all are basal cell carcinomas because of the “company they are keeping”—but note that this is also potentially a trap: missing the single basal cell carcinoma lesion among a field of sebaceous hyperplasia, for instance.
- Don’t focus exclusively on the symptomatic lesion. Do a survey of the general region. For ultraviolet-associated lesions (including basal cell carcinoma), it’s preferable to perform, at minimum, a survey of “high-radiation” areas (face, exposed scalp, neck, ears, and dorsal hands and forearms) for other ultraviolet “damage” (eg, actinic keratoses).
- Be meticulous when examining patients’ backs. Patients may not spot lesions on their backs—especially if they are older and have poor vision.
- Avoid thinking in terms of absolutes like “never” and “always.” The clinical axiom that basal cell carcinomas “never” have hair growing from them is disproved by (FIGURE 3A), which clinically, dermoscopically, and histologically is a basal cell carcinoma lesion. Some of the hair seen here is overlying, loose scalp hair “caught” in the dermoscopic field because of the location of this lesion on the temple adjacent to the hairline. But there are also very distinct hairs seen coming out from areas (at about 6 and 8 o’clock) that are clearly part of the lesion, especially on its periphery.
When in doubt, biopsy
When in doubt about which technique to perform, do an incisional biopsy—preferably excisional, but at least a good sampling of the most worrisome area(s).
Suspected basal cell carcinoma, when the examiner is confident the lesion is not a melanoma, can be further evaluated by superficial shave biopsy. Potential melanoma generally should not be evaluated by the shave technique.
Options for therapy
Therapy options for basal cell carcinoma vary based on location (high-risk vs low-risk locations), histologic type of basal cell carcinoma, patient preference, and local availability of therapy. The primary therapies for basal cell carcinoma are surgical excision (including Mohs surgery) and curettage, often combined with electrodesiccation. 5-flurouracil (5-FU) should not be used because it can treat the surface tumor while deeper tumor proliferates.3 Imiquimod is not approved for facial lesions or nodular basal cell carcinomas.
While dermatoscopes are the true “skin stethoscopes,” most primary care physicians do not have them. Many, however, do have a digital camera. A digital camera with a macro-focus feature can be viewed as another stethoscope for the skin. Pictures allow great magnification on the computer screen, presenting color and detail that may be missed on routine clinical inspection. They allow an unhurried “self–second opinion”; you can evaluate lesions with no motion, breathing, or other distractions, following the office visit.
Digital images may also be important for:
- the patient, who may not be able to see the lesion, because it’s on his back, buttocks, or behind his ears. Consider, too, the older patient who may not be able to see the seborrhea in his eyebrow with his bifocals, but he can see it on the camera’s monitor.
- the medical record (printed or electronically stored).
- the pathologist—when forwarded with the pathology specimen. The images can be helpful in developing a clinical correlation to include in the pathology report.
- the insurance carrier, as indisputable documentation for the clinical rationale for biopsying 4 lesions on 1 visit in the event of a “Dear Bad Doctor” Medicare letter. In fact, if not for the indisputable photographic record, one author (GNF) would have been extremely hesitant to perform 4 biopsies on a Medicare patient in 1 session.
- light and magnification where the 2 may be in short supply, such as a poorly mobile patient in a hospital bed. A camera with flash, auto-focus, and macro mode may allow access to otherwise inaccessible lesions.
ACKNOWLEDGMENTS
Gary N. Fox wishes to acknowledge the assistance of Peggy Elston and Lisa Nichols for their help with portions of this article.
CORRESPONDENCE
Gary N. Fox, MD, 2458 Willesden Green, Toledo, OH. E-mail: [email protected]
1. Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005.
2. Johr R, Soyer HP, Argenziano G, Hofmann-Wellenhof R, Scalvenzi M. Dermoscopy: The Essentials. New York: Mosby; 2004.
3. Premalignant and malignant nonmelanoma skin tumors [chapter 21]. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004.
1. Marghoob AA, Braun RP, Kopf AW, eds. Atlas of Dermoscopy. New York: Taylor & Francis; 2005.
2. Johr R, Soyer HP, Argenziano G, Hofmann-Wellenhof R, Scalvenzi M. Dermoscopy: The Essentials. New York: Mosby; 2004.
3. Premalignant and malignant nonmelanoma skin tumors [chapter 21]. In: Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. New York: Mosby; 2004.
Nodulo-cystic eruption with musculoskeletal pain
A healthy 17-year-old white patient with mild acne was treated with isotretinoin (Accutane), 40 mg/day. After a month of treatment, the acne got worse and the patient complained of polymyalgia and arthralgia.
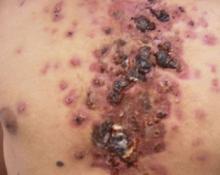
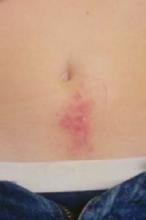
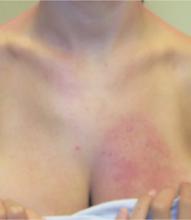
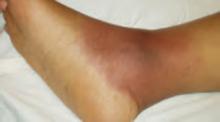
An examination revealed numerous nodules and cysts covered by hemorrhagic crusts on his chest and back (FIGURE). The patient had severe muscular tenderness with gait disability. Leukocytosis and elevated erythrocyte sedimentation rate (ESR) were found; creatinine phosphokinase was within the normal values.
FIGURE
Nodules and cysts on chest and back
What is your diagnosis?
How would you manage this condition?
Diagnosis: Acne fulminans
Acne fulminans is an uncommon complication of acne. It was first described by Burns and Colville in 1959;1 Plevig and Kligman coined the term.2
Signs and symptoms. It is characterized by sudden onset ulcerative crusting cystic acne, mostly on the chest and back. Fever, malaise, nausea, arthralgia, myalgia, and weight loss are common.
Leukocytosis and elevated ESR are usually found. There may also be focal osteolytic lesions.
Thomson and Cunliffe suggest that the term acne fulminans may also be used in cases of severe aggravation of acne without systemic features.3
The cause of acne fulminans is not clear. However, alteration of type III and IV hypersensitivity to Propionibacterium acnes,4 circulating immune complexes,5 and increased cellular immunity6 have been found by some investigators.
Isotretinoin has been identified as a potential trigger.5 Some have suggested that isotretinoin increases the fragility of the pilosebaceous ducts, leading to massive contact with the P acnes antigen.
Management: A treatment and a trigger
The treatment of acne fulminans consists of supportive care and systemic steroids that are gradually reduced over weeks or months, according to the patient’s response. Interestingly, isotretinoin—a trigger for acne fulminans—is also very effective in its treatment.5,7
Outcome: Partial, gradual resolution
Isotretinoin stopped, prednisone started. In this patient’s case, we stopped the isotretinoin and treated him with prednisone, with an initial dose of 40 mg/day. His muscle pain improved.
Dosage tapered. Several attempts to reduce his dosage, however, resulted in a recurrence of pain. Gradual tapering of the steroidal treatment was achieved after 3 months.
Gradual improvement. The acne lesions resolved partially, and gradually, during several months of follow-up.
CORRESPONDENCE
Marcelo H. Grunwald, MD Soroka University Medical Center, Ben Gurion University of the Negev, Beer-Sheva, Israel E-mail: [email protected]
1. Burns RE, Colville JM. Acne conglobata with septicaemia. Arch Dermatol 1959;79:361-363.
2. Seukeran DC, Cuniliffe WJ. Treatment of acne fulminans: a review of 25 cases. Br J Dermatol 1999;141:307-309.
3. Thomson KF, Cuniliffe WJ. Acne fulminans ‘sine fulminans’. Clin Exp Dermatol 2000;25:299-301.
4. Karnoven SL, Rasanen L, Cuniliffe WJ, et al. Delayed hypersensitivity to Propionebacterium acnes in patients with severe nodular acne and acne fulminans. Dermatology 1994;189:344-349.
5. Kellet JK, Beck MH, Chalmers RJG. Erythema nodosum and circulating immune complexes in acne fulminans after treatment with isotretinoin. Br Med J 1985;290:820.-
6. Gowland G, Ward RM, Holland KY, Cuniliffe WJ. Cellular immunity to Propionebacterium acnes in normal population and patients with acne vulgaris. Br J Dermatol 1978;99:43-47.
7. Karvogen SL. Acne fulminans: report of clinical findings and treatment of twenty-four patients. J Am Acad Dermatol 1993;28:572-579.
A healthy 17-year-old white patient with mild acne was treated with isotretinoin (Accutane), 40 mg/day. After a month of treatment, the acne got worse and the patient complained of polymyalgia and arthralgia.
An examination revealed numerous nodules and cysts covered by hemorrhagic crusts on his chest and back (FIGURE). The patient had severe muscular tenderness with gait disability. Leukocytosis and elevated erythrocyte sedimentation rate (ESR) were found; creatinine phosphokinase was within the normal values.
FIGURE
Nodules and cysts on chest and back
What is your diagnosis?
How would you manage this condition?
Diagnosis: Acne fulminans
Acne fulminans is an uncommon complication of acne. It was first described by Burns and Colville in 1959;1 Plevig and Kligman coined the term.2
Signs and symptoms. It is characterized by sudden onset ulcerative crusting cystic acne, mostly on the chest and back. Fever, malaise, nausea, arthralgia, myalgia, and weight loss are common.
Leukocytosis and elevated ESR are usually found. There may also be focal osteolytic lesions.
Thomson and Cunliffe suggest that the term acne fulminans may also be used in cases of severe aggravation of acne without systemic features.3
The cause of acne fulminans is not clear. However, alteration of type III and IV hypersensitivity to Propionibacterium acnes,4 circulating immune complexes,5 and increased cellular immunity6 have been found by some investigators.
Isotretinoin has been identified as a potential trigger.5 Some have suggested that isotretinoin increases the fragility of the pilosebaceous ducts, leading to massive contact with the P acnes antigen.
Management: A treatment and a trigger
The treatment of acne fulminans consists of supportive care and systemic steroids that are gradually reduced over weeks or months, according to the patient’s response. Interestingly, isotretinoin—a trigger for acne fulminans—is also very effective in its treatment.5,7
Outcome: Partial, gradual resolution
Isotretinoin stopped, prednisone started. In this patient’s case, we stopped the isotretinoin and treated him with prednisone, with an initial dose of 40 mg/day. His muscle pain improved.
Dosage tapered. Several attempts to reduce his dosage, however, resulted in a recurrence of pain. Gradual tapering of the steroidal treatment was achieved after 3 months.
Gradual improvement. The acne lesions resolved partially, and gradually, during several months of follow-up.
CORRESPONDENCE
Marcelo H. Grunwald, MD Soroka University Medical Center, Ben Gurion University of the Negev, Beer-Sheva, Israel E-mail: [email protected]
A healthy 17-year-old white patient with mild acne was treated with isotretinoin (Accutane), 40 mg/day. After a month of treatment, the acne got worse and the patient complained of polymyalgia and arthralgia.
An examination revealed numerous nodules and cysts covered by hemorrhagic crusts on his chest and back (FIGURE). The patient had severe muscular tenderness with gait disability. Leukocytosis and elevated erythrocyte sedimentation rate (ESR) were found; creatinine phosphokinase was within the normal values.
FIGURE
Nodules and cysts on chest and back
What is your diagnosis?
How would you manage this condition?
Diagnosis: Acne fulminans
Acne fulminans is an uncommon complication of acne. It was first described by Burns and Colville in 1959;1 Plevig and Kligman coined the term.2
Signs and symptoms. It is characterized by sudden onset ulcerative crusting cystic acne, mostly on the chest and back. Fever, malaise, nausea, arthralgia, myalgia, and weight loss are common.
Leukocytosis and elevated ESR are usually found. There may also be focal osteolytic lesions.
Thomson and Cunliffe suggest that the term acne fulminans may also be used in cases of severe aggravation of acne without systemic features.3
The cause of acne fulminans is not clear. However, alteration of type III and IV hypersensitivity to Propionibacterium acnes,4 circulating immune complexes,5 and increased cellular immunity6 have been found by some investigators.
Isotretinoin has been identified as a potential trigger.5 Some have suggested that isotretinoin increases the fragility of the pilosebaceous ducts, leading to massive contact with the P acnes antigen.
Management: A treatment and a trigger
The treatment of acne fulminans consists of supportive care and systemic steroids that are gradually reduced over weeks or months, according to the patient’s response. Interestingly, isotretinoin—a trigger for acne fulminans—is also very effective in its treatment.5,7
Outcome: Partial, gradual resolution
Isotretinoin stopped, prednisone started. In this patient’s case, we stopped the isotretinoin and treated him with prednisone, with an initial dose of 40 mg/day. His muscle pain improved.
Dosage tapered. Several attempts to reduce his dosage, however, resulted in a recurrence of pain. Gradual tapering of the steroidal treatment was achieved after 3 months.
Gradual improvement. The acne lesions resolved partially, and gradually, during several months of follow-up.
CORRESPONDENCE
Marcelo H. Grunwald, MD Soroka University Medical Center, Ben Gurion University of the Negev, Beer-Sheva, Israel E-mail: [email protected]
1. Burns RE, Colville JM. Acne conglobata with septicaemia. Arch Dermatol 1959;79:361-363.
2. Seukeran DC, Cuniliffe WJ. Treatment of acne fulminans: a review of 25 cases. Br J Dermatol 1999;141:307-309.
3. Thomson KF, Cuniliffe WJ. Acne fulminans ‘sine fulminans’. Clin Exp Dermatol 2000;25:299-301.
4. Karnoven SL, Rasanen L, Cuniliffe WJ, et al. Delayed hypersensitivity to Propionebacterium acnes in patients with severe nodular acne and acne fulminans. Dermatology 1994;189:344-349.
5. Kellet JK, Beck MH, Chalmers RJG. Erythema nodosum and circulating immune complexes in acne fulminans after treatment with isotretinoin. Br Med J 1985;290:820.-
6. Gowland G, Ward RM, Holland KY, Cuniliffe WJ. Cellular immunity to Propionebacterium acnes in normal population and patients with acne vulgaris. Br J Dermatol 1978;99:43-47.
7. Karvogen SL. Acne fulminans: report of clinical findings and treatment of twenty-four patients. J Am Acad Dermatol 1993;28:572-579.
1. Burns RE, Colville JM. Acne conglobata with septicaemia. Arch Dermatol 1959;79:361-363.
2. Seukeran DC, Cuniliffe WJ. Treatment of acne fulminans: a review of 25 cases. Br J Dermatol 1999;141:307-309.
3. Thomson KF, Cuniliffe WJ. Acne fulminans ‘sine fulminans’. Clin Exp Dermatol 2000;25:299-301.
4. Karnoven SL, Rasanen L, Cuniliffe WJ, et al. Delayed hypersensitivity to Propionebacterium acnes in patients with severe nodular acne and acne fulminans. Dermatology 1994;189:344-349.
5. Kellet JK, Beck MH, Chalmers RJG. Erythema nodosum and circulating immune complexes in acne fulminans after treatment with isotretinoin. Br Med J 1985;290:820.-
6. Gowland G, Ward RM, Holland KY, Cuniliffe WJ. Cellular immunity to Propionebacterium acnes in normal population and patients with acne vulgaris. Br J Dermatol 1978;99:43-47.
7. Karvogen SL. Acne fulminans: report of clinical findings and treatment of twenty-four patients. J Am Acad Dermatol 1993;28:572-579.
A toddler with failure to thrive and impaired vision
A 2½-year-old boy came to the office for a routine check-up. His height and weight were below the fifth percentile and he was developmentally delayed. His parents noted that while he could walk unsupported, he could only say “mama” and “dada,” and had just recently started climbing stairs. His previous physician had diagnosed him with hypothyroidism, but he was not taking any medications.
The child’s birth history revealed that he was born to a 20-year-old G1P0 female via Cesarean section secondary to fetal distress at 42 weeks’ gestation. He subsequently spent 1 week in the neonatal intensive care unit due to hypoglycemia.
Following the office visit, the patient was admitted to the children’s hospital to investigate his failure to thrive and possible pituitary dysfunction. An ophthalmologic exam in the hospital showed that the patient was unable to follow objects, but did blink to bright light. Intermittent nystagmus was noted. A fundus exam revealed pale, hypoplastic optic nerves, bilaterally. Both nerves were also positive for the “double ring” sign (FIGURE 1).
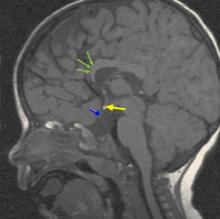
Magnetic resonance imaging (MRI) of the brain (FIGURE 2) revealed hypoplastic genu of the corpus callosum, cavum septum pellucidum, ectopic posterior pituitary, and small anterior pituitary. There was also enhancement of the inferior hypothalamus.
FIGURE 1
Hypoplastic left optic nerve
FIGURE 2
MRI of the brain
What is your diagnosis?
How would you manage the patient?
Diagnosis: Septo-optic dysplasia
Septo-optic dysplasia is a form of optic nerve hypoplasia, the most common congenital optic nerve anomaly, which results from a decreased number of ganglion cell axons.1,2 By definition the syndrome also includes the absence or dysplasia of the septum pellucidum. In many cases, there are other central nervous system abnormalities, such as thinning of the corpus callosum, pituitary malformation, and schizencephaly.3 Most cases of septo-optic dysplasia are diagnosed in infancy and childhood, and there is an equal gender distribution. While rare (6.3 cases per 100,000), septo-optic dysplasia is potentially fatal.1,4
The exact cause of septo-optic dysplasia is unknown; however, both genetic and environmental factors appear to play a role. It has been linked with a defect in the HESX1 gene, young maternal age, maternal insulin-dependent diabetes mellitus, and a variety of toxins.1,5
Poor vision and the “double ring” sign
Most patients present with visual disturbance secondary to optic nerve hypoplasia. Visual acuity may range from 20/20 to no light perception; however, the majority of patients have vision worse than 20/200.1,3 Severe congenital vision loss often leads to sensory nystagmus and strabismus, as well as absent fixation, visual inattentiveness, and an afferent pupillary defect.1,2,6
On examination of the fundus, you may see a “double ring” sign, the appearance of 2 borders around the optic nerve. This outer ring shows a visible sclera at the outer border of a normal-sized nerve and the inner represents the junction between the retinal pigment epithelium and the actual nerve border.1,2,3,7
From CNS abnormalities to pituitary and adrenal problems
While the absence of a septum pellucidum alone has been shown to be clinically insignificant, the presence of other central nervous system abnormalities—such as schizencephaly, cortical heterotopias, or periventricular leukomalacia—can lead to a variety of deficits.8,9 Three-fourths of patients present with seizures, mental retardation, focal neurological deficits, cerebral palsy, or cortical visual loss.1,9
In addition, 15% of patients have some level of pituitary hormone deficiency. Decreased growth hormone is most common, followed by lack of thyroid-stimulating hormone, corticotropic hormone, and vasopressin. Common presentations include failure to thrive, hypoglycemia, prolonged jaundice, thermoregulatory dysfunction, developmental delay, growth retardation, hypotension, recurrent infection, polydipsia, polyuria, and hyper-natremia.1,4,10
Sudden death has been reported in septo-optic dysplasia patients who exhibit hypocortisolism (adrenal insufficiency), diabetes insipidus, and thermo-regulatory dysfunction. Viral illness and other forms of stress are particularly problematic for these patients, as they are unable to mount an appropriate response and are overwhelmed by hypoglycemia, dehydration, shock, and high fevers.4 As a result, clinicians need to be watch for those infants and neonates who are vulnerable to this stress. Extra care should be taken if they require surgery.4,6
Differentiation from a broad swath of possibilities
Due to the variety of presentations, the differential diagnosis for septo-optic dysplasia is very large. It includes all causes of failure to thrive, pituitary dysfunction, neurodevelopmental delay, and congenital vision loss. However, it’s important to maintain a high suspicion for the condition when the combination of the common signs and symptoms are present.
In addition to a full history and physical with dilated fundus exam, one of the most valuable tools in the diagnosis and prognosis of a patient with septo-optic dysplasia is an MRI of the brain with and without contrast, including the pituitary gland. In addition to detecting severely hypoplastic optic nerves, an MRI may also reveal the condition of the septum pellucidum and corpus callosum, cerebral hemispheric abnormalities, and pituitary ectopia. The presence or absence of these findings can help predict the clinical course in relation to pituitary function and neurodevelopmental delay.9,11
Laboratory testing of the hypothalamic-pituitary axis is important, as well. Referrals to both an ophthalmologist and endocrinologist are also necessary.6
Management
In septo-optic dysplasia, the goals of treatment are to preserve the patient’s vision, normalize hormone levels, and assist with neurodevelopmental delays.
Ophthalmology
Patients should be followed closely by an ophthalmologist. That’s because a patient may have a component of delayed maturation of vision, which may improve as he develops. Also, those with unilateral or asymmetric vision loss must be monitored and treated for a resulting amblyopia. Patching of the unaffected eye can aid toward this end.1,7
Endocrinology
Those patients with documented hormonal insufficiency require supplementation. Any patient at risk for developing a deficiency should also be closely monitored so that a change in hormone levels can be quickly recognized and treated. This will help avoid such complications as impaired growth and development—or even death.1,4
Parent education
It’s very important to teach parents about the critical signs and symptoms of dangerous hormone insufficiencies. In patients with cortisol deficiency or diabetes insipidus, febrile illnesses or extreme dehydration require an immediate stress dose of injectable parenteral corticosteroids and hospitalization.3,4 Parents should also be told to monitor their child for the development of other hormone insufficiencies by paying close attention to his growth pattern.1
Outcome
Our patient had deficiencies of growth hormone, thyroid stimulating hormone, and corticotropic hormone. As a result, we started him on supplements, including somatropin, levothyroxine, and hydrocortisone sodium succinate.
Our patient was also diagnosed with oral aversion, so a gastric tube was placed to ensure adequate nutrition. His parents were instructed to follow-up with ophthalmology, neurology, and endocrinology, as well as his primary care physician.
Five months after discharge, the patient had gained 3 kg and adjustments were made to his hormone therapy.
CORRESPONDENCE
Madhu Agarwal, MD Loma Linda University Department of Ophthalmology, 11370 Anderson, Suite 1800, Loma Linda, CA 92354. [email protected]
1. Smith PM, Rismondo V. Diagnosing septo-optic dysplasia. Eyenet 2006;March:37-39.
2. Zeki SM, Hollman AS, Dutton GN. Neuroradiologic features of patients with optic nerve hypoplasia. J Ped Ophthalmol Strabis 1993;29:107-112.
3. Brodsky MC. Congenital optic disc anomalies. In: Yanoff: Ophthalmogy., 2nd ed. St Louis, Mo: Mosby, Inc; 2004:1255-1256.
4. Brodsky MC, Conte FA, Taylor D, et al. Sudden death in septo-optic dysplasia. Report of 5 cases. Arch Ophthalmol 1997;155:66-70.
5. Oliver SCN, Bennett JL. Genetic disorders and the optic nerve: a clinical survey. Ophthalmol Clin N Am 2004;17:435-445.
6. Siatkowski RM, Sanchez JC, Andrade R, Alvarez A. The clinical, neuroradiographic, and endocrinologic profile of patients with bilateral optic nerve hypoplasia. Ophthalmology 1997;104:493-496.
7. Levin AV. Congenital eye anomalies. Pediatric Clin N Am 2003;50:55-76.
8. Williams J, Brodsky MC, Griebel M, et al. Septo-optic dysplasia: The clinical insignificance of an absent septum pellucidum. Develop Med Child Neurol 1993;35:490-501.
9. Brodsky MC, Glasier CM. Optic nerve hypoplasia: Clinical significance of associated central nervous system abnormalities of magnetic resonance imaging. Arch Ophthalmol 1993;111:66-74.
10. Donahue SP, Lavina A, Najjar J. Infantile infection and diabetes insipidus in children with optic nerve hypoplasia. Br J Ophthalmol 2005;89:1275-1277.
11. Brodsky MC, Glasier CM, Pollock SC, et al. Optic nerve hypoplasia: Identification by magnetic resonance imaging. Arch Ophthalmol 1990;108:1562-1568.
A 2½-year-old boy came to the office for a routine check-up. His height and weight were below the fifth percentile and he was developmentally delayed. His parents noted that while he could walk unsupported, he could only say “mama” and “dada,” and had just recently started climbing stairs. His previous physician had diagnosed him with hypothyroidism, but he was not taking any medications.
The child’s birth history revealed that he was born to a 20-year-old G1P0 female via Cesarean section secondary to fetal distress at 42 weeks’ gestation. He subsequently spent 1 week in the neonatal intensive care unit due to hypoglycemia.
Following the office visit, the patient was admitted to the children’s hospital to investigate his failure to thrive and possible pituitary dysfunction. An ophthalmologic exam in the hospital showed that the patient was unable to follow objects, but did blink to bright light. Intermittent nystagmus was noted. A fundus exam revealed pale, hypoplastic optic nerves, bilaterally. Both nerves were also positive for the “double ring” sign (FIGURE 1).
Magnetic resonance imaging (MRI) of the brain (FIGURE 2) revealed hypoplastic genu of the corpus callosum, cavum septum pellucidum, ectopic posterior pituitary, and small anterior pituitary. There was also enhancement of the inferior hypothalamus.
FIGURE 1
Hypoplastic left optic nerve
FIGURE 2
MRI of the brain
What is your diagnosis?
How would you manage the patient?
Diagnosis: Septo-optic dysplasia
Septo-optic dysplasia is a form of optic nerve hypoplasia, the most common congenital optic nerve anomaly, which results from a decreased number of ganglion cell axons.1,2 By definition the syndrome also includes the absence or dysplasia of the septum pellucidum. In many cases, there are other central nervous system abnormalities, such as thinning of the corpus callosum, pituitary malformation, and schizencephaly.3 Most cases of septo-optic dysplasia are diagnosed in infancy and childhood, and there is an equal gender distribution. While rare (6.3 cases per 100,000), septo-optic dysplasia is potentially fatal.1,4
The exact cause of septo-optic dysplasia is unknown; however, both genetic and environmental factors appear to play a role. It has been linked with a defect in the HESX1 gene, young maternal age, maternal insulin-dependent diabetes mellitus, and a variety of toxins.1,5
Poor vision and the “double ring” sign
Most patients present with visual disturbance secondary to optic nerve hypoplasia. Visual acuity may range from 20/20 to no light perception; however, the majority of patients have vision worse than 20/200.1,3 Severe congenital vision loss often leads to sensory nystagmus and strabismus, as well as absent fixation, visual inattentiveness, and an afferent pupillary defect.1,2,6
On examination of the fundus, you may see a “double ring” sign, the appearance of 2 borders around the optic nerve. This outer ring shows a visible sclera at the outer border of a normal-sized nerve and the inner represents the junction between the retinal pigment epithelium and the actual nerve border.1,2,3,7
From CNS abnormalities to pituitary and adrenal problems
While the absence of a septum pellucidum alone has been shown to be clinically insignificant, the presence of other central nervous system abnormalities—such as schizencephaly, cortical heterotopias, or periventricular leukomalacia—can lead to a variety of deficits.8,9 Three-fourths of patients present with seizures, mental retardation, focal neurological deficits, cerebral palsy, or cortical visual loss.1,9
In addition, 15% of patients have some level of pituitary hormone deficiency. Decreased growth hormone is most common, followed by lack of thyroid-stimulating hormone, corticotropic hormone, and vasopressin. Common presentations include failure to thrive, hypoglycemia, prolonged jaundice, thermoregulatory dysfunction, developmental delay, growth retardation, hypotension, recurrent infection, polydipsia, polyuria, and hyper-natremia.1,4,10
Sudden death has been reported in septo-optic dysplasia patients who exhibit hypocortisolism (adrenal insufficiency), diabetes insipidus, and thermo-regulatory dysfunction. Viral illness and other forms of stress are particularly problematic for these patients, as they are unable to mount an appropriate response and are overwhelmed by hypoglycemia, dehydration, shock, and high fevers.4 As a result, clinicians need to be watch for those infants and neonates who are vulnerable to this stress. Extra care should be taken if they require surgery.4,6
Differentiation from a broad swath of possibilities
Due to the variety of presentations, the differential diagnosis for septo-optic dysplasia is very large. It includes all causes of failure to thrive, pituitary dysfunction, neurodevelopmental delay, and congenital vision loss. However, it’s important to maintain a high suspicion for the condition when the combination of the common signs and symptoms are present.
In addition to a full history and physical with dilated fundus exam, one of the most valuable tools in the diagnosis and prognosis of a patient with septo-optic dysplasia is an MRI of the brain with and without contrast, including the pituitary gland. In addition to detecting severely hypoplastic optic nerves, an MRI may also reveal the condition of the septum pellucidum and corpus callosum, cerebral hemispheric abnormalities, and pituitary ectopia. The presence or absence of these findings can help predict the clinical course in relation to pituitary function and neurodevelopmental delay.9,11
Laboratory testing of the hypothalamic-pituitary axis is important, as well. Referrals to both an ophthalmologist and endocrinologist are also necessary.6
Management
In septo-optic dysplasia, the goals of treatment are to preserve the patient’s vision, normalize hormone levels, and assist with neurodevelopmental delays.
Ophthalmology
Patients should be followed closely by an ophthalmologist. That’s because a patient may have a component of delayed maturation of vision, which may improve as he develops. Also, those with unilateral or asymmetric vision loss must be monitored and treated for a resulting amblyopia. Patching of the unaffected eye can aid toward this end.1,7
Endocrinology
Those patients with documented hormonal insufficiency require supplementation. Any patient at risk for developing a deficiency should also be closely monitored so that a change in hormone levels can be quickly recognized and treated. This will help avoid such complications as impaired growth and development—or even death.1,4
Parent education
It’s very important to teach parents about the critical signs and symptoms of dangerous hormone insufficiencies. In patients with cortisol deficiency or diabetes insipidus, febrile illnesses or extreme dehydration require an immediate stress dose of injectable parenteral corticosteroids and hospitalization.3,4 Parents should also be told to monitor their child for the development of other hormone insufficiencies by paying close attention to his growth pattern.1
Outcome
Our patient had deficiencies of growth hormone, thyroid stimulating hormone, and corticotropic hormone. As a result, we started him on supplements, including somatropin, levothyroxine, and hydrocortisone sodium succinate.
Our patient was also diagnosed with oral aversion, so a gastric tube was placed to ensure adequate nutrition. His parents were instructed to follow-up with ophthalmology, neurology, and endocrinology, as well as his primary care physician.
Five months after discharge, the patient had gained 3 kg and adjustments were made to his hormone therapy.
CORRESPONDENCE
Madhu Agarwal, MD Loma Linda University Department of Ophthalmology, 11370 Anderson, Suite 1800, Loma Linda, CA 92354. [email protected]
A 2½-year-old boy came to the office for a routine check-up. His height and weight were below the fifth percentile and he was developmentally delayed. His parents noted that while he could walk unsupported, he could only say “mama” and “dada,” and had just recently started climbing stairs. His previous physician had diagnosed him with hypothyroidism, but he was not taking any medications.
The child’s birth history revealed that he was born to a 20-year-old G1P0 female via Cesarean section secondary to fetal distress at 42 weeks’ gestation. He subsequently spent 1 week in the neonatal intensive care unit due to hypoglycemia.
Following the office visit, the patient was admitted to the children’s hospital to investigate his failure to thrive and possible pituitary dysfunction. An ophthalmologic exam in the hospital showed that the patient was unable to follow objects, but did blink to bright light. Intermittent nystagmus was noted. A fundus exam revealed pale, hypoplastic optic nerves, bilaterally. Both nerves were also positive for the “double ring” sign (FIGURE 1).
Magnetic resonance imaging (MRI) of the brain (FIGURE 2) revealed hypoplastic genu of the corpus callosum, cavum septum pellucidum, ectopic posterior pituitary, and small anterior pituitary. There was also enhancement of the inferior hypothalamus.
FIGURE 1
Hypoplastic left optic nerve
FIGURE 2
MRI of the brain
What is your diagnosis?
How would you manage the patient?
Diagnosis: Septo-optic dysplasia
Septo-optic dysplasia is a form of optic nerve hypoplasia, the most common congenital optic nerve anomaly, which results from a decreased number of ganglion cell axons.1,2 By definition the syndrome also includes the absence or dysplasia of the septum pellucidum. In many cases, there are other central nervous system abnormalities, such as thinning of the corpus callosum, pituitary malformation, and schizencephaly.3 Most cases of septo-optic dysplasia are diagnosed in infancy and childhood, and there is an equal gender distribution. While rare (6.3 cases per 100,000), septo-optic dysplasia is potentially fatal.1,4
The exact cause of septo-optic dysplasia is unknown; however, both genetic and environmental factors appear to play a role. It has been linked with a defect in the HESX1 gene, young maternal age, maternal insulin-dependent diabetes mellitus, and a variety of toxins.1,5
Poor vision and the “double ring” sign
Most patients present with visual disturbance secondary to optic nerve hypoplasia. Visual acuity may range from 20/20 to no light perception; however, the majority of patients have vision worse than 20/200.1,3 Severe congenital vision loss often leads to sensory nystagmus and strabismus, as well as absent fixation, visual inattentiveness, and an afferent pupillary defect.1,2,6
On examination of the fundus, you may see a “double ring” sign, the appearance of 2 borders around the optic nerve. This outer ring shows a visible sclera at the outer border of a normal-sized nerve and the inner represents the junction between the retinal pigment epithelium and the actual nerve border.1,2,3,7
From CNS abnormalities to pituitary and adrenal problems
While the absence of a septum pellucidum alone has been shown to be clinically insignificant, the presence of other central nervous system abnormalities—such as schizencephaly, cortical heterotopias, or periventricular leukomalacia—can lead to a variety of deficits.8,9 Three-fourths of patients present with seizures, mental retardation, focal neurological deficits, cerebral palsy, or cortical visual loss.1,9
In addition, 15% of patients have some level of pituitary hormone deficiency. Decreased growth hormone is most common, followed by lack of thyroid-stimulating hormone, corticotropic hormone, and vasopressin. Common presentations include failure to thrive, hypoglycemia, prolonged jaundice, thermoregulatory dysfunction, developmental delay, growth retardation, hypotension, recurrent infection, polydipsia, polyuria, and hyper-natremia.1,4,10
Sudden death has been reported in septo-optic dysplasia patients who exhibit hypocortisolism (adrenal insufficiency), diabetes insipidus, and thermo-regulatory dysfunction. Viral illness and other forms of stress are particularly problematic for these patients, as they are unable to mount an appropriate response and are overwhelmed by hypoglycemia, dehydration, shock, and high fevers.4 As a result, clinicians need to be watch for those infants and neonates who are vulnerable to this stress. Extra care should be taken if they require surgery.4,6
Differentiation from a broad swath of possibilities
Due to the variety of presentations, the differential diagnosis for septo-optic dysplasia is very large. It includes all causes of failure to thrive, pituitary dysfunction, neurodevelopmental delay, and congenital vision loss. However, it’s important to maintain a high suspicion for the condition when the combination of the common signs and symptoms are present.
In addition to a full history and physical with dilated fundus exam, one of the most valuable tools in the diagnosis and prognosis of a patient with septo-optic dysplasia is an MRI of the brain with and without contrast, including the pituitary gland. In addition to detecting severely hypoplastic optic nerves, an MRI may also reveal the condition of the septum pellucidum and corpus callosum, cerebral hemispheric abnormalities, and pituitary ectopia. The presence or absence of these findings can help predict the clinical course in relation to pituitary function and neurodevelopmental delay.9,11
Laboratory testing of the hypothalamic-pituitary axis is important, as well. Referrals to both an ophthalmologist and endocrinologist are also necessary.6
Management
In septo-optic dysplasia, the goals of treatment are to preserve the patient’s vision, normalize hormone levels, and assist with neurodevelopmental delays.
Ophthalmology
Patients should be followed closely by an ophthalmologist. That’s because a patient may have a component of delayed maturation of vision, which may improve as he develops. Also, those with unilateral or asymmetric vision loss must be monitored and treated for a resulting amblyopia. Patching of the unaffected eye can aid toward this end.1,7
Endocrinology
Those patients with documented hormonal insufficiency require supplementation. Any patient at risk for developing a deficiency should also be closely monitored so that a change in hormone levels can be quickly recognized and treated. This will help avoid such complications as impaired growth and development—or even death.1,4
Parent education
It’s very important to teach parents about the critical signs and symptoms of dangerous hormone insufficiencies. In patients with cortisol deficiency or diabetes insipidus, febrile illnesses or extreme dehydration require an immediate stress dose of injectable parenteral corticosteroids and hospitalization.3,4 Parents should also be told to monitor their child for the development of other hormone insufficiencies by paying close attention to his growth pattern.1
Outcome
Our patient had deficiencies of growth hormone, thyroid stimulating hormone, and corticotropic hormone. As a result, we started him on supplements, including somatropin, levothyroxine, and hydrocortisone sodium succinate.
Our patient was also diagnosed with oral aversion, so a gastric tube was placed to ensure adequate nutrition. His parents were instructed to follow-up with ophthalmology, neurology, and endocrinology, as well as his primary care physician.
Five months after discharge, the patient had gained 3 kg and adjustments were made to his hormone therapy.
CORRESPONDENCE
Madhu Agarwal, MD Loma Linda University Department of Ophthalmology, 11370 Anderson, Suite 1800, Loma Linda, CA 92354. [email protected]
1. Smith PM, Rismondo V. Diagnosing septo-optic dysplasia. Eyenet 2006;March:37-39.
2. Zeki SM, Hollman AS, Dutton GN. Neuroradiologic features of patients with optic nerve hypoplasia. J Ped Ophthalmol Strabis 1993;29:107-112.
3. Brodsky MC. Congenital optic disc anomalies. In: Yanoff: Ophthalmogy., 2nd ed. St Louis, Mo: Mosby, Inc; 2004:1255-1256.
4. Brodsky MC, Conte FA, Taylor D, et al. Sudden death in septo-optic dysplasia. Report of 5 cases. Arch Ophthalmol 1997;155:66-70.
5. Oliver SCN, Bennett JL. Genetic disorders and the optic nerve: a clinical survey. Ophthalmol Clin N Am 2004;17:435-445.
6. Siatkowski RM, Sanchez JC, Andrade R, Alvarez A. The clinical, neuroradiographic, and endocrinologic profile of patients with bilateral optic nerve hypoplasia. Ophthalmology 1997;104:493-496.
7. Levin AV. Congenital eye anomalies. Pediatric Clin N Am 2003;50:55-76.
8. Williams J, Brodsky MC, Griebel M, et al. Septo-optic dysplasia: The clinical insignificance of an absent septum pellucidum. Develop Med Child Neurol 1993;35:490-501.
9. Brodsky MC, Glasier CM. Optic nerve hypoplasia: Clinical significance of associated central nervous system abnormalities of magnetic resonance imaging. Arch Ophthalmol 1993;111:66-74.
10. Donahue SP, Lavina A, Najjar J. Infantile infection and diabetes insipidus in children with optic nerve hypoplasia. Br J Ophthalmol 2005;89:1275-1277.
11. Brodsky MC, Glasier CM, Pollock SC, et al. Optic nerve hypoplasia: Identification by magnetic resonance imaging. Arch Ophthalmol 1990;108:1562-1568.
1. Smith PM, Rismondo V. Diagnosing septo-optic dysplasia. Eyenet 2006;March:37-39.
2. Zeki SM, Hollman AS, Dutton GN. Neuroradiologic features of patients with optic nerve hypoplasia. J Ped Ophthalmol Strabis 1993;29:107-112.
3. Brodsky MC. Congenital optic disc anomalies. In: Yanoff: Ophthalmogy., 2nd ed. St Louis, Mo: Mosby, Inc; 2004:1255-1256.
4. Brodsky MC, Conte FA, Taylor D, et al. Sudden death in septo-optic dysplasia. Report of 5 cases. Arch Ophthalmol 1997;155:66-70.
5. Oliver SCN, Bennett JL. Genetic disorders and the optic nerve: a clinical survey. Ophthalmol Clin N Am 2004;17:435-445.
6. Siatkowski RM, Sanchez JC, Andrade R, Alvarez A. The clinical, neuroradiographic, and endocrinologic profile of patients with bilateral optic nerve hypoplasia. Ophthalmology 1997;104:493-496.
7. Levin AV. Congenital eye anomalies. Pediatric Clin N Am 2003;50:55-76.
8. Williams J, Brodsky MC, Griebel M, et al. Septo-optic dysplasia: The clinical insignificance of an absent septum pellucidum. Develop Med Child Neurol 1993;35:490-501.
9. Brodsky MC, Glasier CM. Optic nerve hypoplasia: Clinical significance of associated central nervous system abnormalities of magnetic resonance imaging. Arch Ophthalmol 1993;111:66-74.
10. Donahue SP, Lavina A, Najjar J. Infantile infection and diabetes insipidus in children with optic nerve hypoplasia. Br J Ophthalmol 2005;89:1275-1277.
11. Brodsky MC, Glasier CM, Pollock SC, et al. Optic nerve hypoplasia: Identification by magnetic resonance imaging. Arch Ophthalmol 1990;108:1562-1568.
Itchy rash near the navel
An 8-year-old boy presented with a pruritic periumbilical rash that he’d had for several months. The rash intensified during the day and improved at night. Some days the rash was much worse than others.
The patient’s mother noted he had recently begun tucking the front of his shirt into his pants. Over-the-counter steroid creams had provided intermittent improvement. He had no significant past medical history and took no other medications.
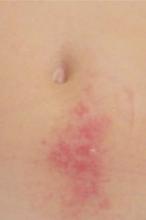
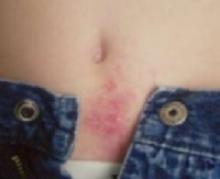
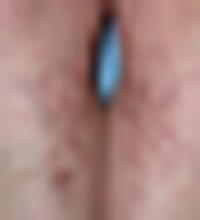
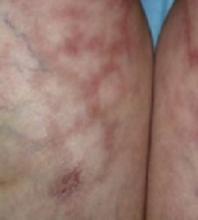
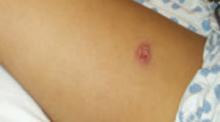
Examination revealed a 4-cm patch of erythema, scaling, and excoriation without a clear border and no central clearing (FIGURES 1 AND 2). The rest of the skin exam was unremarkable.
FIGURE 1
The periumbilical rash
A pruritic rash near the navel, which worsened during the day and improved at night.
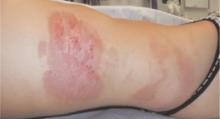
FIGURE 2
A closer look
What is your diagnosis?
What therapy would you advise?
Diagnosis: Blue jean button dermatitis
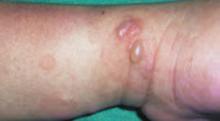
Contact dermatitis secondary to nickel allergy is a type IV hypersensitivity reaction to the nickel contained in many blue jean snaps and buttons (FIGURE 3). The rash is typical of contact dermatitis. Macular erythema, sometimes accompanied by papules or vesicles, is also common.
The border is usually ill-defined, and the lesion may become fissured and excoriated. Long-standing cases may become lichenified and scaly. Occasionally, secondary eruptions on flexor surfaces appear.
FIGURE 3
It all lines up
The child’s nickel allergy rash aligns with the snap and button of his blue jeans.
More common among women
Nickel allergy is relatively common; up to 20% of women and 4% of men are affected.1 This discrepancy is thought to be due to the ways in which individuals become sensitized to nickel.
Many women develop a nickel allergy after getting their ears pierced with nickel-containing studs, whereas men tend to be sensitized by an occupational exposure. Nickel allergy appears to be increasing, which may reflect the more common practice of body piercing in general.
How much nickel is too much?
Sensitized patients will react to a metal if more than 1 part in 10,000 is nickel. Metal objects can be tested for nickel content by using a cotton-tipped applicator to apply a few drops of dimethylglyoxime in ammonium hydroxide to the object. If greater than 1 part per 10,000 is nickel, the cotton will turn pink. Recently, Byer and Morrell found that 10% of blue jeans buttons, and more than 50% of belt buckles, test positive.2
Differential diagnosis
The differential diagnosis for a pruritic periumbilical rash in a child would include psoriasis, scabies, tinea corpus and pityriasis rosea.
Psoriatic lesions
The periumbilical area is a common location for psoriatic lesions. More often involved areas include the scalp and areas of trauma such as the knees, elbows, hands and feet. The lesions of psoriasis tend to have a sharp border and characteristically have silvery white scales associated with them. Psoriasis is often associated with nail abnormalities and arthritis.
Scabies
Scabies is a parasitic infestation with the mite Sarcoptes scabiei. Scabies causes an intensely pruritic rash that is classically worse in the finger and toe webs, along the belt line, the groin and axillary regions and, often, periumbilically.
The rash of scabies consists of linearly arranged papules and vesicles due to a hypersensitivity reaction to the mite’s eggs and feces which are left behind as it burrows through the skin. Excoriation often obscures the typical findings and may be extensive. Treatment of scabies with steroid creams often leads to diffuse erythema and crusting.3
Tinea corpus
Tinea corpus is a dermatophyte infection of the body. These lesions are typically oval and pruritic. Tinea corpus lesions tend to enlarge slowly with a clearly demarcated, slightly raised, erythematous border and central clearing. Satellite lesions may be seen. Applying topical steroids to tinea corpus may result in initial improvement with subsequent flare as the body’s immune response is suppressed.
Pityriasis rosea
Pityriasis rosea is a common pruritic exanthem that can begin as a solitary lesion on the trunk (herald patch). The herald patch is typically an erythematous oval lesion several centimeters in size with a collarette of scales along its border. Typically, within a week numerous other salmon-colored lesions appear on the trunk along Langer’s lines.
The age of peak incidence is during the third decade of life but it does develop in children. Therapy is conservative and targets the control of pruritus.4
Treatment: A simple solution
The mainstay of therapy is avoidance of nickel-containing metals. Buttons can be replaced with plastic ones, or covered with cloth. Patients should not coat buttons with nail polish—it doesn’t work very well.
Stainless steel is a good option for piercings because nickel tends to be tightly bound within the alloy. Topical steroids can be used to speed resolution of the rash and antihistamines may help with pruritus.
Outcome
The patient was placed on a topical steroid (triamcinolone 0.1% ointment). His mother sewed a piece of cloth over the inside of all his pants buttons. The ointment was only given because the mother wanted something that would make the rash go away quickly. Within a week, he was no longer itching and his rash was nearly gone.
CORRESPONDENCE
Dean A. Seehusen, MD, MPH Department of Family and Community Medicine, Eisenhower Army Medical Center, Fort Gordon, GA 30509 E-mail: [email protected]
1. Cerveny KA, Brodell RT. Blue jean button dermatitis. Postgrad Med 2002;112:79-80,83.
2. Byer TT, Morrell DS. Periumbilical contact dermatitis: Blue jeans or belt buckles? Pediatr Dermatol 2004;21:223-226.
3. Flinders DC, DeSchweinitz P. Pediculosis and scabies. Am Fam Physician 2004;69:341-348.
4. Stulberg DL, Wolfrey J. Pityriasis rosea. Am Fam Physician 2004;69:97-92.
An 8-year-old boy presented with a pruritic periumbilical rash that he’d had for several months. The rash intensified during the day and improved at night. Some days the rash was much worse than others.
The patient’s mother noted he had recently begun tucking the front of his shirt into his pants. Over-the-counter steroid creams had provided intermittent improvement. He had no significant past medical history and took no other medications.
Examination revealed a 4-cm patch of erythema, scaling, and excoriation without a clear border and no central clearing (FIGURES 1 AND 2). The rest of the skin exam was unremarkable.
FIGURE 1
The periumbilical rash
A pruritic rash near the navel, which worsened during the day and improved at night.
FIGURE 2
A closer look
What is your diagnosis?
What therapy would you advise?
Diagnosis: Blue jean button dermatitis
Contact dermatitis secondary to nickel allergy is a type IV hypersensitivity reaction to the nickel contained in many blue jean snaps and buttons (FIGURE 3). The rash is typical of contact dermatitis. Macular erythema, sometimes accompanied by papules or vesicles, is also common.
The border is usually ill-defined, and the lesion may become fissured and excoriated. Long-standing cases may become lichenified and scaly. Occasionally, secondary eruptions on flexor surfaces appear.
FIGURE 3
It all lines up
The child’s nickel allergy rash aligns with the snap and button of his blue jeans.
More common among women
Nickel allergy is relatively common; up to 20% of women and 4% of men are affected.1 This discrepancy is thought to be due to the ways in which individuals become sensitized to nickel.
Many women develop a nickel allergy after getting their ears pierced with nickel-containing studs, whereas men tend to be sensitized by an occupational exposure. Nickel allergy appears to be increasing, which may reflect the more common practice of body piercing in general.
How much nickel is too much?
Sensitized patients will react to a metal if more than 1 part in 10,000 is nickel. Metal objects can be tested for nickel content by using a cotton-tipped applicator to apply a few drops of dimethylglyoxime in ammonium hydroxide to the object. If greater than 1 part per 10,000 is nickel, the cotton will turn pink. Recently, Byer and Morrell found that 10% of blue jeans buttons, and more than 50% of belt buckles, test positive.2
Differential diagnosis
The differential diagnosis for a pruritic periumbilical rash in a child would include psoriasis, scabies, tinea corpus and pityriasis rosea.
Psoriatic lesions
The periumbilical area is a common location for psoriatic lesions. More often involved areas include the scalp and areas of trauma such as the knees, elbows, hands and feet. The lesions of psoriasis tend to have a sharp border and characteristically have silvery white scales associated with them. Psoriasis is often associated with nail abnormalities and arthritis.
Scabies
Scabies is a parasitic infestation with the mite Sarcoptes scabiei. Scabies causes an intensely pruritic rash that is classically worse in the finger and toe webs, along the belt line, the groin and axillary regions and, often, periumbilically.
The rash of scabies consists of linearly arranged papules and vesicles due to a hypersensitivity reaction to the mite’s eggs and feces which are left behind as it burrows through the skin. Excoriation often obscures the typical findings and may be extensive. Treatment of scabies with steroid creams often leads to diffuse erythema and crusting.3
Tinea corpus
Tinea corpus is a dermatophyte infection of the body. These lesions are typically oval and pruritic. Tinea corpus lesions tend to enlarge slowly with a clearly demarcated, slightly raised, erythematous border and central clearing. Satellite lesions may be seen. Applying topical steroids to tinea corpus may result in initial improvement with subsequent flare as the body’s immune response is suppressed.
Pityriasis rosea
Pityriasis rosea is a common pruritic exanthem that can begin as a solitary lesion on the trunk (herald patch). The herald patch is typically an erythematous oval lesion several centimeters in size with a collarette of scales along its border. Typically, within a week numerous other salmon-colored lesions appear on the trunk along Langer’s lines.
The age of peak incidence is during the third decade of life but it does develop in children. Therapy is conservative and targets the control of pruritus.4
Treatment: A simple solution
The mainstay of therapy is avoidance of nickel-containing metals. Buttons can be replaced with plastic ones, or covered with cloth. Patients should not coat buttons with nail polish—it doesn’t work very well.
Stainless steel is a good option for piercings because nickel tends to be tightly bound within the alloy. Topical steroids can be used to speed resolution of the rash and antihistamines may help with pruritus.
Outcome
The patient was placed on a topical steroid (triamcinolone 0.1% ointment). His mother sewed a piece of cloth over the inside of all his pants buttons. The ointment was only given because the mother wanted something that would make the rash go away quickly. Within a week, he was no longer itching and his rash was nearly gone.
CORRESPONDENCE
Dean A. Seehusen, MD, MPH Department of Family and Community Medicine, Eisenhower Army Medical Center, Fort Gordon, GA 30509 E-mail: [email protected]
An 8-year-old boy presented with a pruritic periumbilical rash that he’d had for several months. The rash intensified during the day and improved at night. Some days the rash was much worse than others.
The patient’s mother noted he had recently begun tucking the front of his shirt into his pants. Over-the-counter steroid creams had provided intermittent improvement. He had no significant past medical history and took no other medications.
Examination revealed a 4-cm patch of erythema, scaling, and excoriation without a clear border and no central clearing (FIGURES 1 AND 2). The rest of the skin exam was unremarkable.
FIGURE 1
The periumbilical rash
A pruritic rash near the navel, which worsened during the day and improved at night.
FIGURE 2
A closer look
What is your diagnosis?
What therapy would you advise?
Diagnosis: Blue jean button dermatitis
Contact dermatitis secondary to nickel allergy is a type IV hypersensitivity reaction to the nickel contained in many blue jean snaps and buttons (FIGURE 3). The rash is typical of contact dermatitis. Macular erythema, sometimes accompanied by papules or vesicles, is also common.
The border is usually ill-defined, and the lesion may become fissured and excoriated. Long-standing cases may become lichenified and scaly. Occasionally, secondary eruptions on flexor surfaces appear.
FIGURE 3
It all lines up
The child’s nickel allergy rash aligns with the snap and button of his blue jeans.
More common among women
Nickel allergy is relatively common; up to 20% of women and 4% of men are affected.1 This discrepancy is thought to be due to the ways in which individuals become sensitized to nickel.
Many women develop a nickel allergy after getting their ears pierced with nickel-containing studs, whereas men tend to be sensitized by an occupational exposure. Nickel allergy appears to be increasing, which may reflect the more common practice of body piercing in general.
How much nickel is too much?
Sensitized patients will react to a metal if more than 1 part in 10,000 is nickel. Metal objects can be tested for nickel content by using a cotton-tipped applicator to apply a few drops of dimethylglyoxime in ammonium hydroxide to the object. If greater than 1 part per 10,000 is nickel, the cotton will turn pink. Recently, Byer and Morrell found that 10% of blue jeans buttons, and more than 50% of belt buckles, test positive.2
Differential diagnosis
The differential diagnosis for a pruritic periumbilical rash in a child would include psoriasis, scabies, tinea corpus and pityriasis rosea.
Psoriatic lesions
The periumbilical area is a common location for psoriatic lesions. More often involved areas include the scalp and areas of trauma such as the knees, elbows, hands and feet. The lesions of psoriasis tend to have a sharp border and characteristically have silvery white scales associated with them. Psoriasis is often associated with nail abnormalities and arthritis.
Scabies
Scabies is a parasitic infestation with the mite Sarcoptes scabiei. Scabies causes an intensely pruritic rash that is classically worse in the finger and toe webs, along the belt line, the groin and axillary regions and, often, periumbilically.
The rash of scabies consists of linearly arranged papules and vesicles due to a hypersensitivity reaction to the mite’s eggs and feces which are left behind as it burrows through the skin. Excoriation often obscures the typical findings and may be extensive. Treatment of scabies with steroid creams often leads to diffuse erythema and crusting.3
Tinea corpus
Tinea corpus is a dermatophyte infection of the body. These lesions are typically oval and pruritic. Tinea corpus lesions tend to enlarge slowly with a clearly demarcated, slightly raised, erythematous border and central clearing. Satellite lesions may be seen. Applying topical steroids to tinea corpus may result in initial improvement with subsequent flare as the body’s immune response is suppressed.
Pityriasis rosea
Pityriasis rosea is a common pruritic exanthem that can begin as a solitary lesion on the trunk (herald patch). The herald patch is typically an erythematous oval lesion several centimeters in size with a collarette of scales along its border. Typically, within a week numerous other salmon-colored lesions appear on the trunk along Langer’s lines.
The age of peak incidence is during the third decade of life but it does develop in children. Therapy is conservative and targets the control of pruritus.4
Treatment: A simple solution
The mainstay of therapy is avoidance of nickel-containing metals. Buttons can be replaced with plastic ones, or covered with cloth. Patients should not coat buttons with nail polish—it doesn’t work very well.
Stainless steel is a good option for piercings because nickel tends to be tightly bound within the alloy. Topical steroids can be used to speed resolution of the rash and antihistamines may help with pruritus.
Outcome
The patient was placed on a topical steroid (triamcinolone 0.1% ointment). His mother sewed a piece of cloth over the inside of all his pants buttons. The ointment was only given because the mother wanted something that would make the rash go away quickly. Within a week, he was no longer itching and his rash was nearly gone.
CORRESPONDENCE
Dean A. Seehusen, MD, MPH Department of Family and Community Medicine, Eisenhower Army Medical Center, Fort Gordon, GA 30509 E-mail: [email protected]
1. Cerveny KA, Brodell RT. Blue jean button dermatitis. Postgrad Med 2002;112:79-80,83.
2. Byer TT, Morrell DS. Periumbilical contact dermatitis: Blue jeans or belt buckles? Pediatr Dermatol 2004;21:223-226.
3. Flinders DC, DeSchweinitz P. Pediculosis and scabies. Am Fam Physician 2004;69:341-348.
4. Stulberg DL, Wolfrey J. Pityriasis rosea. Am Fam Physician 2004;69:97-92.
1. Cerveny KA, Brodell RT. Blue jean button dermatitis. Postgrad Med 2002;112:79-80,83.
2. Byer TT, Morrell DS. Periumbilical contact dermatitis: Blue jeans or belt buckles? Pediatr Dermatol 2004;21:223-226.
3. Flinders DC, DeSchweinitz P. Pediculosis and scabies. Am Fam Physician 2004;69:341-348.
4. Stulberg DL, Wolfrey J. Pityriasis rosea. Am Fam Physician 2004;69:97-92.
A newborn with a skin lesion on the back
A 24-year-old woman without a family history of congenital anomalies delivered her first baby after a minimally complicated prenatal course. Poor weight gain led to an ultrasound at 33 weeks gestation; this test and others at 13 weeks to determine gestational age and at 20 weeks to evaluate for anatomic abnormalities all had normal results.
Prenatal screenings included a normal alpha feta-protein level. Review of the mother’s medical records suggested compliance with routine prenatal care and vitamins; however, she smoked cigarettes during the pregnancy and occasionally took a pill containing butalbital, acetaminophen, and caffeine for migraine headaches. She did not have gestational diabetes, but late in the pregnancy she developed hypertension, necessitating induction of labor.
She delivered (via cesarean section) a full-term baby girl weighing 6 pounds, 9 ounces. The infant appeared healthy, with Apgar scores of 8 and 9 and a normal physical exam, except for a 2.5-cm appendage overlying the spine (Figures 1 and 2). The 2-day nursery stay was that of a normal neonate, without complications. The pediatric surgeon who was consulted arranged for outpatient follow-up after hospital dismissal.
FIGURE 1
Skin lesion
The small appendage was in the middle of the infant’s lower back.
FIGURE 2
Close-up
What is your diagnosis?
Diagnosis: Pseudotail
Inspection of the lesion at the time of excision revealed fatty tissue within its core, consistent with the diagnosis of pseudotail. Ultrasound of the spine performed before hospital discharge was normal.
Tails and pseudotails
Tails and pseudotails appear similar on physical exam and are differentiated histologically. True tails—remnants of the embryologic tail—contain a core of muscle fibers, nerves, and blood vessels, whereas pseudotails contain primarily fatty tissue.1-3 From a practical and clinical point of view, you do not need to differentiate tails and pseudotails as they both can be associated with skin-covered congenital anomalies of the spine, or occult spinal dysraphism (OSD).
Although tail-like structures rarely occur, when they are present you should consider OSD.3 Two noteworthy literature reviews support this. The first identified 33 tails and pseudotails, of which 10 had OSD.1 An analysis of more recent cases (1960 through 1997) demonstrated OSD in 29 of 59 cases with lesions described as tails.2 A higher incidence of OSD occurred between 1980 and 1997 in this series. The authors attribute the higher incidence to the advent of computed tomography and magnetic resonance imaging (MRI), thereby stressing the importance of spine imaging with the presence of any caudal appendage resembling a tail.
Epidemiology of occult spinal dysraphism
The true incidence of OSD is not known. Congenital anomalies of the central nervous system, second only to birth defects of the cardiovascular system, are reported on birth certificates, but OSD may go unrecognized in the neonatal period. Frequently a benign-appearing midline cutaneous lesion is the only evidence of OSD at birth.4 Although 3% to 8% of newborns with a skin lesion over the spine have OSD,5 50% to 90% of patients with OSD have a skin marker.5-7 Of 2010 newborns in 1 study, 144 had a midline cutaneous lesion, and 5.5% of these had an abnormal ultrasound suggesting OSD.8 Additionally, the presence of more than 1 lesion increases the probability of finding OSD.5,9,10
You should look for underlying OSD in newborns with a suggestive skin lesion, since the embryologic origin of the spinal cord and the skin overlying the spine are the same: the ectoderm layer of cells within the trilaminar (endoderm, mesoderm, ectoderm) early embryo gives rise to both structures. Midline cutaneous lesions with high likelihood for OSD include hypertrichosis (hairy patch), atypical dimple (a dimple >5 mm diameter or >2.5 cm above the anus), hemangioma, lipoma, aplasia cutis, dermoid cyst or sinus, skin tags, tails, and pseudotails.3
Evaluation: The neonate with a midline cutaneous lesion
The incidence of OSD appears low in newborns with midline cutaneous lesions, but irreversible orthopedic, urologic, and neurologic problems from OSD may occur later in life. These complications can be prevented by early surgical intervention; therefore, it is critical that you do not miss a diagnosis of OSD and pursue spine imaging.
The definitive test, an MRI,3,6,9 usually requires sedation of infants,11 making high-resolution ultrasound the recommended choice for initial screening.3-10 Ultrasound is not invasive and may allow for testing in the hospital nursery. After age 3 months, when ossification of the posterior spine limits visualization of the spinal cord by ultrasound, consider an MRI first. If the ultrasound demonstrates OSD or is inconclusive, follow with an MRI for a more specific diagnosis.
Indications for an ultrasound of the newborn’s spine include any one of the following: an abnormal antenatal ultrasound; a midline cutaneous lesion other than a simple dimple; presence of other OSD related anomalies; or an abnormal neurologic exam.5 The authors of this protocol retrospectively applied it to 223 infants who had an ultrasound, and it reduced the number of tests by 50% without missing a single case of OSD.
If you are caring for a newborn infant you should resist the temptation to simply remove a midline cutaneous lesion, including a pseudotail, in the nursery without first searching for OSD. If OSD is present, neurosurgical consultation should be obtained as soon as possible. The optimal time for surgical intervention is within the first 4 months of life.4
Patient outcome
The infant’s pseudotail was removed without consequence and postoperatively the patient has thrived.
CORRESPONDENCE
Timothy J. Benton, MD Assistant Professor, Associate Residency Program Director, Texas Tech University Health Sciences Center at Amarillo, School of Medicine, Department of Family and Community Medicine, 1400 Wallace Boulevard, Amarillo, Texas 79106. E-mail: [email protected]
1. Dao AH, Netsky MG. Human tails and pseudotails. Hum Pathol 1984;56:339-340.
2. Lu FL, Wang PJ, Teng RJ, Yau KI. The human tail. Pediatr Neurol 1998;19:230-233.
3. Drolet B. Cutaneous signs of neural tube dysraphism. Pediatr Clin North Am 2000;47:813-823.
4. Kriss VM, Kriss TC, Desai NS, Warf BC. Occult spinal dysraphism in the infant. Clin Pediatr 1995;34:650-654.
5. Robinson AJ, Russell S, Rimmer S. The value of ultrasonic examination of the lumbar spine in infants with specific reference to cutaneous markers of occult spinal dysraphism. Clin Radiol 2005;60:72-77.
6. Mcatee-smith J, Hebert AA, Rapini RP, Goldberg NS. Skin lesions of the spinal axis and spinal dysraphism. Fifteen cases and a review of the literature. Arch Pediatr Adolesc Med 1994;148:740-748.
7. Dick EA, Patel K, Owens CM, De Bruyn R. Spinal ultrasound in infants. Br J Radiol 2002;75:384-392.
8. Henriques JG, Pianetti G, Henriques KS, Costa P, Gusmao S. Minor skin lesions as markers of occult spinal dysraphisms—prospective study. Surg Neurol 2005;63(suppl 1):s8-s12.
9. Guggisberg D, Hadj-rabia S, Viney C, et al. Skin markers of occult spinal dysraphism in children: a review of 54 cases. Arch Dermatol 2004;140:1109-1115.
10. Kriss VM, Desai NS. Occult spinal dysraphism in neonates: assessment of high-risk cutaneous stigmata on sonography. AJR Am J Roentgenol 1998;171:1687-1692.
11. Keengwe IN, Hegde S, Dearlove O, Wilson B, Yates RW, Sharples A. Structured sedation programme for magnetic resonance imaging examination in children. Anaesthesia 1999;54:1069-1072.
A 24-year-old woman without a family history of congenital anomalies delivered her first baby after a minimally complicated prenatal course. Poor weight gain led to an ultrasound at 33 weeks gestation; this test and others at 13 weeks to determine gestational age and at 20 weeks to evaluate for anatomic abnormalities all had normal results.
Prenatal screenings included a normal alpha feta-protein level. Review of the mother’s medical records suggested compliance with routine prenatal care and vitamins; however, she smoked cigarettes during the pregnancy and occasionally took a pill containing butalbital, acetaminophen, and caffeine for migraine headaches. She did not have gestational diabetes, but late in the pregnancy she developed hypertension, necessitating induction of labor.
She delivered (via cesarean section) a full-term baby girl weighing 6 pounds, 9 ounces. The infant appeared healthy, with Apgar scores of 8 and 9 and a normal physical exam, except for a 2.5-cm appendage overlying the spine (Figures 1 and 2). The 2-day nursery stay was that of a normal neonate, without complications. The pediatric surgeon who was consulted arranged for outpatient follow-up after hospital dismissal.
FIGURE 1
Skin lesion
The small appendage was in the middle of the infant’s lower back.
FIGURE 2
Close-up
What is your diagnosis?
Diagnosis: Pseudotail
Inspection of the lesion at the time of excision revealed fatty tissue within its core, consistent with the diagnosis of pseudotail. Ultrasound of the spine performed before hospital discharge was normal.
Tails and pseudotails
Tails and pseudotails appear similar on physical exam and are differentiated histologically. True tails—remnants of the embryologic tail—contain a core of muscle fibers, nerves, and blood vessels, whereas pseudotails contain primarily fatty tissue.1-3 From a practical and clinical point of view, you do not need to differentiate tails and pseudotails as they both can be associated with skin-covered congenital anomalies of the spine, or occult spinal dysraphism (OSD).
Although tail-like structures rarely occur, when they are present you should consider OSD.3 Two noteworthy literature reviews support this. The first identified 33 tails and pseudotails, of which 10 had OSD.1 An analysis of more recent cases (1960 through 1997) demonstrated OSD in 29 of 59 cases with lesions described as tails.2 A higher incidence of OSD occurred between 1980 and 1997 in this series. The authors attribute the higher incidence to the advent of computed tomography and magnetic resonance imaging (MRI), thereby stressing the importance of spine imaging with the presence of any caudal appendage resembling a tail.
Epidemiology of occult spinal dysraphism
The true incidence of OSD is not known. Congenital anomalies of the central nervous system, second only to birth defects of the cardiovascular system, are reported on birth certificates, but OSD may go unrecognized in the neonatal period. Frequently a benign-appearing midline cutaneous lesion is the only evidence of OSD at birth.4 Although 3% to 8% of newborns with a skin lesion over the spine have OSD,5 50% to 90% of patients with OSD have a skin marker.5-7 Of 2010 newborns in 1 study, 144 had a midline cutaneous lesion, and 5.5% of these had an abnormal ultrasound suggesting OSD.8 Additionally, the presence of more than 1 lesion increases the probability of finding OSD.5,9,10
You should look for underlying OSD in newborns with a suggestive skin lesion, since the embryologic origin of the spinal cord and the skin overlying the spine are the same: the ectoderm layer of cells within the trilaminar (endoderm, mesoderm, ectoderm) early embryo gives rise to both structures. Midline cutaneous lesions with high likelihood for OSD include hypertrichosis (hairy patch), atypical dimple (a dimple >5 mm diameter or >2.5 cm above the anus), hemangioma, lipoma, aplasia cutis, dermoid cyst or sinus, skin tags, tails, and pseudotails.3
Evaluation: The neonate with a midline cutaneous lesion
The incidence of OSD appears low in newborns with midline cutaneous lesions, but irreversible orthopedic, urologic, and neurologic problems from OSD may occur later in life. These complications can be prevented by early surgical intervention; therefore, it is critical that you do not miss a diagnosis of OSD and pursue spine imaging.
The definitive test, an MRI,3,6,9 usually requires sedation of infants,11 making high-resolution ultrasound the recommended choice for initial screening.3-10 Ultrasound is not invasive and may allow for testing in the hospital nursery. After age 3 months, when ossification of the posterior spine limits visualization of the spinal cord by ultrasound, consider an MRI first. If the ultrasound demonstrates OSD or is inconclusive, follow with an MRI for a more specific diagnosis.
Indications for an ultrasound of the newborn’s spine include any one of the following: an abnormal antenatal ultrasound; a midline cutaneous lesion other than a simple dimple; presence of other OSD related anomalies; or an abnormal neurologic exam.5 The authors of this protocol retrospectively applied it to 223 infants who had an ultrasound, and it reduced the number of tests by 50% without missing a single case of OSD.
If you are caring for a newborn infant you should resist the temptation to simply remove a midline cutaneous lesion, including a pseudotail, in the nursery without first searching for OSD. If OSD is present, neurosurgical consultation should be obtained as soon as possible. The optimal time for surgical intervention is within the first 4 months of life.4
Patient outcome
The infant’s pseudotail was removed without consequence and postoperatively the patient has thrived.
CORRESPONDENCE
Timothy J. Benton, MD Assistant Professor, Associate Residency Program Director, Texas Tech University Health Sciences Center at Amarillo, School of Medicine, Department of Family and Community Medicine, 1400 Wallace Boulevard, Amarillo, Texas 79106. E-mail: [email protected]
A 24-year-old woman without a family history of congenital anomalies delivered her first baby after a minimally complicated prenatal course. Poor weight gain led to an ultrasound at 33 weeks gestation; this test and others at 13 weeks to determine gestational age and at 20 weeks to evaluate for anatomic abnormalities all had normal results.
Prenatal screenings included a normal alpha feta-protein level. Review of the mother’s medical records suggested compliance with routine prenatal care and vitamins; however, she smoked cigarettes during the pregnancy and occasionally took a pill containing butalbital, acetaminophen, and caffeine for migraine headaches. She did not have gestational diabetes, but late in the pregnancy she developed hypertension, necessitating induction of labor.
She delivered (via cesarean section) a full-term baby girl weighing 6 pounds, 9 ounces. The infant appeared healthy, with Apgar scores of 8 and 9 and a normal physical exam, except for a 2.5-cm appendage overlying the spine (Figures 1 and 2). The 2-day nursery stay was that of a normal neonate, without complications. The pediatric surgeon who was consulted arranged for outpatient follow-up after hospital dismissal.
FIGURE 1
Skin lesion
The small appendage was in the middle of the infant’s lower back.
FIGURE 2
Close-up
What is your diagnosis?
Diagnosis: Pseudotail
Inspection of the lesion at the time of excision revealed fatty tissue within its core, consistent with the diagnosis of pseudotail. Ultrasound of the spine performed before hospital discharge was normal.
Tails and pseudotails
Tails and pseudotails appear similar on physical exam and are differentiated histologically. True tails—remnants of the embryologic tail—contain a core of muscle fibers, nerves, and blood vessels, whereas pseudotails contain primarily fatty tissue.1-3 From a practical and clinical point of view, you do not need to differentiate tails and pseudotails as they both can be associated with skin-covered congenital anomalies of the spine, or occult spinal dysraphism (OSD).
Although tail-like structures rarely occur, when they are present you should consider OSD.3 Two noteworthy literature reviews support this. The first identified 33 tails and pseudotails, of which 10 had OSD.1 An analysis of more recent cases (1960 through 1997) demonstrated OSD in 29 of 59 cases with lesions described as tails.2 A higher incidence of OSD occurred between 1980 and 1997 in this series. The authors attribute the higher incidence to the advent of computed tomography and magnetic resonance imaging (MRI), thereby stressing the importance of spine imaging with the presence of any caudal appendage resembling a tail.
Epidemiology of occult spinal dysraphism
The true incidence of OSD is not known. Congenital anomalies of the central nervous system, second only to birth defects of the cardiovascular system, are reported on birth certificates, but OSD may go unrecognized in the neonatal period. Frequently a benign-appearing midline cutaneous lesion is the only evidence of OSD at birth.4 Although 3% to 8% of newborns with a skin lesion over the spine have OSD,5 50% to 90% of patients with OSD have a skin marker.5-7 Of 2010 newborns in 1 study, 144 had a midline cutaneous lesion, and 5.5% of these had an abnormal ultrasound suggesting OSD.8 Additionally, the presence of more than 1 lesion increases the probability of finding OSD.5,9,10
You should look for underlying OSD in newborns with a suggestive skin lesion, since the embryologic origin of the spinal cord and the skin overlying the spine are the same: the ectoderm layer of cells within the trilaminar (endoderm, mesoderm, ectoderm) early embryo gives rise to both structures. Midline cutaneous lesions with high likelihood for OSD include hypertrichosis (hairy patch), atypical dimple (a dimple >5 mm diameter or >2.5 cm above the anus), hemangioma, lipoma, aplasia cutis, dermoid cyst or sinus, skin tags, tails, and pseudotails.3
Evaluation: The neonate with a midline cutaneous lesion
The incidence of OSD appears low in newborns with midline cutaneous lesions, but irreversible orthopedic, urologic, and neurologic problems from OSD may occur later in life. These complications can be prevented by early surgical intervention; therefore, it is critical that you do not miss a diagnosis of OSD and pursue spine imaging.
The definitive test, an MRI,3,6,9 usually requires sedation of infants,11 making high-resolution ultrasound the recommended choice for initial screening.3-10 Ultrasound is not invasive and may allow for testing in the hospital nursery. After age 3 months, when ossification of the posterior spine limits visualization of the spinal cord by ultrasound, consider an MRI first. If the ultrasound demonstrates OSD or is inconclusive, follow with an MRI for a more specific diagnosis.
Indications for an ultrasound of the newborn’s spine include any one of the following: an abnormal antenatal ultrasound; a midline cutaneous lesion other than a simple dimple; presence of other OSD related anomalies; or an abnormal neurologic exam.5 The authors of this protocol retrospectively applied it to 223 infants who had an ultrasound, and it reduced the number of tests by 50% without missing a single case of OSD.
If you are caring for a newborn infant you should resist the temptation to simply remove a midline cutaneous lesion, including a pseudotail, in the nursery without first searching for OSD. If OSD is present, neurosurgical consultation should be obtained as soon as possible. The optimal time for surgical intervention is within the first 4 months of life.4
Patient outcome
The infant’s pseudotail was removed without consequence and postoperatively the patient has thrived.
CORRESPONDENCE
Timothy J. Benton, MD Assistant Professor, Associate Residency Program Director, Texas Tech University Health Sciences Center at Amarillo, School of Medicine, Department of Family and Community Medicine, 1400 Wallace Boulevard, Amarillo, Texas 79106. E-mail: [email protected]
1. Dao AH, Netsky MG. Human tails and pseudotails. Hum Pathol 1984;56:339-340.
2. Lu FL, Wang PJ, Teng RJ, Yau KI. The human tail. Pediatr Neurol 1998;19:230-233.
3. Drolet B. Cutaneous signs of neural tube dysraphism. Pediatr Clin North Am 2000;47:813-823.
4. Kriss VM, Kriss TC, Desai NS, Warf BC. Occult spinal dysraphism in the infant. Clin Pediatr 1995;34:650-654.
5. Robinson AJ, Russell S, Rimmer S. The value of ultrasonic examination of the lumbar spine in infants with specific reference to cutaneous markers of occult spinal dysraphism. Clin Radiol 2005;60:72-77.
6. Mcatee-smith J, Hebert AA, Rapini RP, Goldberg NS. Skin lesions of the spinal axis and spinal dysraphism. Fifteen cases and a review of the literature. Arch Pediatr Adolesc Med 1994;148:740-748.
7. Dick EA, Patel K, Owens CM, De Bruyn R. Spinal ultrasound in infants. Br J Radiol 2002;75:384-392.
8. Henriques JG, Pianetti G, Henriques KS, Costa P, Gusmao S. Minor skin lesions as markers of occult spinal dysraphisms—prospective study. Surg Neurol 2005;63(suppl 1):s8-s12.
9. Guggisberg D, Hadj-rabia S, Viney C, et al. Skin markers of occult spinal dysraphism in children: a review of 54 cases. Arch Dermatol 2004;140:1109-1115.
10. Kriss VM, Desai NS. Occult spinal dysraphism in neonates: assessment of high-risk cutaneous stigmata on sonography. AJR Am J Roentgenol 1998;171:1687-1692.
11. Keengwe IN, Hegde S, Dearlove O, Wilson B, Yates RW, Sharples A. Structured sedation programme for magnetic resonance imaging examination in children. Anaesthesia 1999;54:1069-1072.
1. Dao AH, Netsky MG. Human tails and pseudotails. Hum Pathol 1984;56:339-340.
2. Lu FL, Wang PJ, Teng RJ, Yau KI. The human tail. Pediatr Neurol 1998;19:230-233.
3. Drolet B. Cutaneous signs of neural tube dysraphism. Pediatr Clin North Am 2000;47:813-823.
4. Kriss VM, Kriss TC, Desai NS, Warf BC. Occult spinal dysraphism in the infant. Clin Pediatr 1995;34:650-654.
5. Robinson AJ, Russell S, Rimmer S. The value of ultrasonic examination of the lumbar spine in infants with specific reference to cutaneous markers of occult spinal dysraphism. Clin Radiol 2005;60:72-77.
6. Mcatee-smith J, Hebert AA, Rapini RP, Goldberg NS. Skin lesions of the spinal axis and spinal dysraphism. Fifteen cases and a review of the literature. Arch Pediatr Adolesc Med 1994;148:740-748.
7. Dick EA, Patel K, Owens CM, De Bruyn R. Spinal ultrasound in infants. Br J Radiol 2002;75:384-392.
8. Henriques JG, Pianetti G, Henriques KS, Costa P, Gusmao S. Minor skin lesions as markers of occult spinal dysraphisms—prospective study. Surg Neurol 2005;63(suppl 1):s8-s12.
9. Guggisberg D, Hadj-rabia S, Viney C, et al. Skin markers of occult spinal dysraphism in children: a review of 54 cases. Arch Dermatol 2004;140:1109-1115.
10. Kriss VM, Desai NS. Occult spinal dysraphism in neonates: assessment of high-risk cutaneous stigmata on sonography. AJR Am J Roentgenol 1998;171:1687-1692.
11. Keengwe IN, Hegde S, Dearlove O, Wilson B, Yates RW, Sharples A. Structured sedation programme for magnetic resonance imaging examination in children. Anaesthesia 1999;54:1069-1072.
Bilateral lesions on the legs
A 50-year-old woman came to the office for medical advice regarding bilateral erythematous lesions on the inner aspects of both of her legs. She had been aware of the lesions for more than 5 months. She had no other medical complaints, and was not taking any prescribed, over-the-counter, or herbal medications, or using any moisturizers or other medicated topical preparations. She mentioned that she was using a hot water bottle on her legs to keep her warm.
On physical examination, we noted bilateral erythema in a mesh-like distribution on her frontal, inner lower thighs as well as the upper and medial aspects of her calves. There were prominent telangiectatic vessels and “spider veins” surrounding the erythema, but her skin was otherwise normal. The review of her systems showed no problems. Personal and family histories were not contributory.
FIGURE 1
Lesions on the legs
Skin biopsy revealed subepidermal separation accompanied by thinning of the epidermis with loss of rete ridges and the presence of dermal edema. There was an increase in the number of elastic fibers with aberrant disruption. Perivascular infiltration of inflammatory cells was also noted.
FIGURE 2
Close-up
What is your diagnosis?
How would you manage this condition?
Diagnosis: Erythema ab igne
Erythema ab igne is characterized by localized erythema, reticulosis, and hypo-/hyperpigmentation, with telangiectsia and skin atrophy appearing in severe cases. This condition is caused by continuous exposure to sources of heat for prolonged periods of time.1 Patients often do not connect their heat exposure to the rash, so you must recognize the local pattern of erythema ab igne and use your detective skills to identify the source.
History: Old disease, modern causes
Erythema ab igne has been noted for many years, and the sources of heat have changed over time. It used to be seen in women who stayed for long periods in front of open fires or furnaces to cook.2,3 Most of the lesions appeared on the medial side of the thigh and the lower leg.
In modern times it is seen on different parts of the body depending on the heat source that initiated the pathology, the angle of the heat radiation, the morphology of the skin, and the layers of clothing.1 Some of the modern causes of erythema ab igne are repeated application of hot water bottles or heat pads to treat chronic pain, exposure to car heaters and furniture with internal heaters, use of a laptop computer for long periods,4 and cook stoves, for restaurant workers who stand for long periods near the heat.3 Ultrasound physiotherapy was also reported as a cause.5 Recently, a case caused by frequent and prolonged hot bathing was reported.6
Clinical features: Mottled pattern clinically, subtle changes
The pattern of lesions form as a result of multiple exposures to the source of heat. Skin lesions may not appear immediately after the exposure—it might take a month to show up.7 Changes start as a reddish-brown pigmentation distributed as a mottled rash, followed by skin atrophy. Telangiectasias with diffuse hyperpigmentation and subepidermal bullae may also develop.8 Some patients complain of mild pruritus or burning sensation, but most patients are asymptomatic.9 Erythema ab igne should be differentiated from other diseases with skin changes that mimic its presentation (TABLE).
If clinically warranted, perform a biopsy to exclude the possibility of malignant formation. Histopathology results show epidermal atrophy, subepidermal separation, and haziness of the dermoepidermal junction. Dilatation of capillaries and connective tissue disintegration, elastosis, hemosiderin deposition, melanocytosis, and abundance of inflammatory cells are all seen in the dermis.10 Some of these lesions might progress to actinic keratosis, which could be a precursor for squamous cell carcinoma of the skin.11 In some rare cases, Merkel cell carcinoma has developed in areas of erythema ab igne.12
Treatment: Eliminate heat source
The first goal of treatment is to identify the source of heat radiation to avoid further exposure. For mild lesions, no intervention is needed after the heat source is removed; the probability of full resolution is good. In this case, the patient was advised to stop using the hot water bottle on her skin. Over 4 months her lesions started to clear with no further intervention.
Topical meds help with cosmesis
Topical retinoids, vitamin A derivatives, hydroquinone, and 5-fluorouracil can be prescribed to treat abnormal skin pigmentation.13 Laser therapy has been used to even out the skin color.
TABLE
Differential diagnosis for erythema ab igne
| DISEASE | CLINICAL FEATURES |
|---|---|
| Acanthosis nigricans | Velvety, light-brown-to-black markings usually on the neck, under the arms, or in the groin |
| Most often associated with being overweight | |
| More common in people with darker skin pigmentation | |
| May begin at any age | |
| May be inherited as a primary condition or associated with various underlying syndromes | |
| Livedo reticularis | Reticular cyanotic cutaneous discoloration surrounding pale central areas due to dilation of capillary blood vessels and stagnation of blood |
| Occurs mostly on the legs, arms, and trunk | |
| More pronounced in cold weather | |
| Idiopathic condition that may be associated with systemic diseases | |
| Poikiloderma atrophicans vasculare | Circumscribed violaceous erythema |
| Occurs mostly in posterior shoulders, back, buttocks, V-shaped area of anterior neck, and chest | |
| May be asymptomatic or mildly pruritic | |
| May remain stable in size or gradually increase | |
| Numerous atypical lymphocytes are observed around dermal blood vessels and someepidermotropism is observed | |
| A variant of mycosis fungoides |
CORRESPONDENCE
Amor Khachemoune, MD, CWS, Ronald O. Perelman Department of Dermatology, New York University School of Medicine, 530 First Avenue, Suite 7R, New York, NY 10016. E-mail: [email protected]
1. Page EH, Shear NH. Temperature-dependent skin disorders. J Am Acad Dermatol 1988;18:1003-1019.
2. Meffert JJ, Davis BM. Furniture-induced erythema ab igne. J Am Acad Dermatol 1996;34:516-517.
3. Helm TN, Spigel GT, Helm KF. Erythema ab igne caused by a car heater. Cutis 1997;59:81-82.
4. Bilic M, Adams BB. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol 2004;50:973-974.
5. Weber MB, Ponzio HA, Costa FB, Camini L. An Bras Dermatol 2005;80:187-188.
6. Lin SJ, Hsu CJ, Chiu HC. Erythema ab igne caused by frequent hot bathing. Acta Derm Venereol 2002;82:478-479.
7. Galvin SA, Buchness MR. Rectangular reticulate patches on the pretibial areas. Erythema ab igne. Arch Dermatol 1990;126:386-387,389.
8. Flanagan N, Watson R, Sweeney E, Barnes L. Bullous erythema ab igne. Br J Dermatol 1996;134:1159-1160.
9. Shahrad P, Marks R. The wages of warmth: changes in erythema ab igne. Br J Dermatol 1977;97:179-186.
10. Milligan A, Graham-Brown RA. Erythema ab igne affecting the palms. Clin Exp Dermatol 1989;14:168-169.
11. Arrington JH, 3rd, Lockman DS. Thermal keratoses and squamous cell carcinoma in situ associated with erythema ab igne. Arch Dermatol 1979;115:1226-1228.
12. Hewitt JB, Sherif A, Kerr KM, Stankler L. Merkel cell and squamous cell carcinomas arising in erythema ab igne. Br J Dermatol 1993;128:591-592.
13. Sahl WJ, Jr, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol 1992;27:109-110.
A 50-year-old woman came to the office for medical advice regarding bilateral erythematous lesions on the inner aspects of both of her legs. She had been aware of the lesions for more than 5 months. She had no other medical complaints, and was not taking any prescribed, over-the-counter, or herbal medications, or using any moisturizers or other medicated topical preparations. She mentioned that she was using a hot water bottle on her legs to keep her warm.
On physical examination, we noted bilateral erythema in a mesh-like distribution on her frontal, inner lower thighs as well as the upper and medial aspects of her calves. There were prominent telangiectatic vessels and “spider veins” surrounding the erythema, but her skin was otherwise normal. The review of her systems showed no problems. Personal and family histories were not contributory.
FIGURE 1
Lesions on the legs
Skin biopsy revealed subepidermal separation accompanied by thinning of the epidermis with loss of rete ridges and the presence of dermal edema. There was an increase in the number of elastic fibers with aberrant disruption. Perivascular infiltration of inflammatory cells was also noted.
FIGURE 2
Close-up
What is your diagnosis?
How would you manage this condition?
Diagnosis: Erythema ab igne
Erythema ab igne is characterized by localized erythema, reticulosis, and hypo-/hyperpigmentation, with telangiectsia and skin atrophy appearing in severe cases. This condition is caused by continuous exposure to sources of heat for prolonged periods of time.1 Patients often do not connect their heat exposure to the rash, so you must recognize the local pattern of erythema ab igne and use your detective skills to identify the source.
History: Old disease, modern causes
Erythema ab igne has been noted for many years, and the sources of heat have changed over time. It used to be seen in women who stayed for long periods in front of open fires or furnaces to cook.2,3 Most of the lesions appeared on the medial side of the thigh and the lower leg.
In modern times it is seen on different parts of the body depending on the heat source that initiated the pathology, the angle of the heat radiation, the morphology of the skin, and the layers of clothing.1 Some of the modern causes of erythema ab igne are repeated application of hot water bottles or heat pads to treat chronic pain, exposure to car heaters and furniture with internal heaters, use of a laptop computer for long periods,4 and cook stoves, for restaurant workers who stand for long periods near the heat.3 Ultrasound physiotherapy was also reported as a cause.5 Recently, a case caused by frequent and prolonged hot bathing was reported.6
Clinical features: Mottled pattern clinically, subtle changes
The pattern of lesions form as a result of multiple exposures to the source of heat. Skin lesions may not appear immediately after the exposure—it might take a month to show up.7 Changes start as a reddish-brown pigmentation distributed as a mottled rash, followed by skin atrophy. Telangiectasias with diffuse hyperpigmentation and subepidermal bullae may also develop.8 Some patients complain of mild pruritus or burning sensation, but most patients are asymptomatic.9 Erythema ab igne should be differentiated from other diseases with skin changes that mimic its presentation (TABLE).
If clinically warranted, perform a biopsy to exclude the possibility of malignant formation. Histopathology results show epidermal atrophy, subepidermal separation, and haziness of the dermoepidermal junction. Dilatation of capillaries and connective tissue disintegration, elastosis, hemosiderin deposition, melanocytosis, and abundance of inflammatory cells are all seen in the dermis.10 Some of these lesions might progress to actinic keratosis, which could be a precursor for squamous cell carcinoma of the skin.11 In some rare cases, Merkel cell carcinoma has developed in areas of erythema ab igne.12
Treatment: Eliminate heat source
The first goal of treatment is to identify the source of heat radiation to avoid further exposure. For mild lesions, no intervention is needed after the heat source is removed; the probability of full resolution is good. In this case, the patient was advised to stop using the hot water bottle on her skin. Over 4 months her lesions started to clear with no further intervention.
Topical meds help with cosmesis
Topical retinoids, vitamin A derivatives, hydroquinone, and 5-fluorouracil can be prescribed to treat abnormal skin pigmentation.13 Laser therapy has been used to even out the skin color.
TABLE
Differential diagnosis for erythema ab igne
| DISEASE | CLINICAL FEATURES |
|---|---|
| Acanthosis nigricans | Velvety, light-brown-to-black markings usually on the neck, under the arms, or in the groin |
| Most often associated with being overweight | |
| More common in people with darker skin pigmentation | |
| May begin at any age | |
| May be inherited as a primary condition or associated with various underlying syndromes | |
| Livedo reticularis | Reticular cyanotic cutaneous discoloration surrounding pale central areas due to dilation of capillary blood vessels and stagnation of blood |
| Occurs mostly on the legs, arms, and trunk | |
| More pronounced in cold weather | |
| Idiopathic condition that may be associated with systemic diseases | |
| Poikiloderma atrophicans vasculare | Circumscribed violaceous erythema |
| Occurs mostly in posterior shoulders, back, buttocks, V-shaped area of anterior neck, and chest | |
| May be asymptomatic or mildly pruritic | |
| May remain stable in size or gradually increase | |
| Numerous atypical lymphocytes are observed around dermal blood vessels and someepidermotropism is observed | |
| A variant of mycosis fungoides |
CORRESPONDENCE
Amor Khachemoune, MD, CWS, Ronald O. Perelman Department of Dermatology, New York University School of Medicine, 530 First Avenue, Suite 7R, New York, NY 10016. E-mail: [email protected]
A 50-year-old woman came to the office for medical advice regarding bilateral erythematous lesions on the inner aspects of both of her legs. She had been aware of the lesions for more than 5 months. She had no other medical complaints, and was not taking any prescribed, over-the-counter, or herbal medications, or using any moisturizers or other medicated topical preparations. She mentioned that she was using a hot water bottle on her legs to keep her warm.
On physical examination, we noted bilateral erythema in a mesh-like distribution on her frontal, inner lower thighs as well as the upper and medial aspects of her calves. There were prominent telangiectatic vessels and “spider veins” surrounding the erythema, but her skin was otherwise normal. The review of her systems showed no problems. Personal and family histories were not contributory.
FIGURE 1
Lesions on the legs
Skin biopsy revealed subepidermal separation accompanied by thinning of the epidermis with loss of rete ridges and the presence of dermal edema. There was an increase in the number of elastic fibers with aberrant disruption. Perivascular infiltration of inflammatory cells was also noted.
FIGURE 2
Close-up
What is your diagnosis?
How would you manage this condition?
Diagnosis: Erythema ab igne
Erythema ab igne is characterized by localized erythema, reticulosis, and hypo-/hyperpigmentation, with telangiectsia and skin atrophy appearing in severe cases. This condition is caused by continuous exposure to sources of heat for prolonged periods of time.1 Patients often do not connect their heat exposure to the rash, so you must recognize the local pattern of erythema ab igne and use your detective skills to identify the source.
History: Old disease, modern causes
Erythema ab igne has been noted for many years, and the sources of heat have changed over time. It used to be seen in women who stayed for long periods in front of open fires or furnaces to cook.2,3 Most of the lesions appeared on the medial side of the thigh and the lower leg.
In modern times it is seen on different parts of the body depending on the heat source that initiated the pathology, the angle of the heat radiation, the morphology of the skin, and the layers of clothing.1 Some of the modern causes of erythema ab igne are repeated application of hot water bottles or heat pads to treat chronic pain, exposure to car heaters and furniture with internal heaters, use of a laptop computer for long periods,4 and cook stoves, for restaurant workers who stand for long periods near the heat.3 Ultrasound physiotherapy was also reported as a cause.5 Recently, a case caused by frequent and prolonged hot bathing was reported.6
Clinical features: Mottled pattern clinically, subtle changes
The pattern of lesions form as a result of multiple exposures to the source of heat. Skin lesions may not appear immediately after the exposure—it might take a month to show up.7 Changes start as a reddish-brown pigmentation distributed as a mottled rash, followed by skin atrophy. Telangiectasias with diffuse hyperpigmentation and subepidermal bullae may also develop.8 Some patients complain of mild pruritus or burning sensation, but most patients are asymptomatic.9 Erythema ab igne should be differentiated from other diseases with skin changes that mimic its presentation (TABLE).
If clinically warranted, perform a biopsy to exclude the possibility of malignant formation. Histopathology results show epidermal atrophy, subepidermal separation, and haziness of the dermoepidermal junction. Dilatation of capillaries and connective tissue disintegration, elastosis, hemosiderin deposition, melanocytosis, and abundance of inflammatory cells are all seen in the dermis.10 Some of these lesions might progress to actinic keratosis, which could be a precursor for squamous cell carcinoma of the skin.11 In some rare cases, Merkel cell carcinoma has developed in areas of erythema ab igne.12
Treatment: Eliminate heat source
The first goal of treatment is to identify the source of heat radiation to avoid further exposure. For mild lesions, no intervention is needed after the heat source is removed; the probability of full resolution is good. In this case, the patient was advised to stop using the hot water bottle on her skin. Over 4 months her lesions started to clear with no further intervention.
Topical meds help with cosmesis
Topical retinoids, vitamin A derivatives, hydroquinone, and 5-fluorouracil can be prescribed to treat abnormal skin pigmentation.13 Laser therapy has been used to even out the skin color.
TABLE
Differential diagnosis for erythema ab igne
| DISEASE | CLINICAL FEATURES |
|---|---|
| Acanthosis nigricans | Velvety, light-brown-to-black markings usually on the neck, under the arms, or in the groin |
| Most often associated with being overweight | |
| More common in people with darker skin pigmentation | |
| May begin at any age | |
| May be inherited as a primary condition or associated with various underlying syndromes | |
| Livedo reticularis | Reticular cyanotic cutaneous discoloration surrounding pale central areas due to dilation of capillary blood vessels and stagnation of blood |
| Occurs mostly on the legs, arms, and trunk | |
| More pronounced in cold weather | |
| Idiopathic condition that may be associated with systemic diseases | |
| Poikiloderma atrophicans vasculare | Circumscribed violaceous erythema |
| Occurs mostly in posterior shoulders, back, buttocks, V-shaped area of anterior neck, and chest | |
| May be asymptomatic or mildly pruritic | |
| May remain stable in size or gradually increase | |
| Numerous atypical lymphocytes are observed around dermal blood vessels and someepidermotropism is observed | |
| A variant of mycosis fungoides |
CORRESPONDENCE
Amor Khachemoune, MD, CWS, Ronald O. Perelman Department of Dermatology, New York University School of Medicine, 530 First Avenue, Suite 7R, New York, NY 10016. E-mail: [email protected]
1. Page EH, Shear NH. Temperature-dependent skin disorders. J Am Acad Dermatol 1988;18:1003-1019.
2. Meffert JJ, Davis BM. Furniture-induced erythema ab igne. J Am Acad Dermatol 1996;34:516-517.
3. Helm TN, Spigel GT, Helm KF. Erythema ab igne caused by a car heater. Cutis 1997;59:81-82.
4. Bilic M, Adams BB. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol 2004;50:973-974.
5. Weber MB, Ponzio HA, Costa FB, Camini L. An Bras Dermatol 2005;80:187-188.
6. Lin SJ, Hsu CJ, Chiu HC. Erythema ab igne caused by frequent hot bathing. Acta Derm Venereol 2002;82:478-479.
7. Galvin SA, Buchness MR. Rectangular reticulate patches on the pretibial areas. Erythema ab igne. Arch Dermatol 1990;126:386-387,389.
8. Flanagan N, Watson R, Sweeney E, Barnes L. Bullous erythema ab igne. Br J Dermatol 1996;134:1159-1160.
9. Shahrad P, Marks R. The wages of warmth: changes in erythema ab igne. Br J Dermatol 1977;97:179-186.
10. Milligan A, Graham-Brown RA. Erythema ab igne affecting the palms. Clin Exp Dermatol 1989;14:168-169.
11. Arrington JH, 3rd, Lockman DS. Thermal keratoses and squamous cell carcinoma in situ associated with erythema ab igne. Arch Dermatol 1979;115:1226-1228.
12. Hewitt JB, Sherif A, Kerr KM, Stankler L. Merkel cell and squamous cell carcinomas arising in erythema ab igne. Br J Dermatol 1993;128:591-592.
13. Sahl WJ, Jr, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol 1992;27:109-110.
1. Page EH, Shear NH. Temperature-dependent skin disorders. J Am Acad Dermatol 1988;18:1003-1019.
2. Meffert JJ, Davis BM. Furniture-induced erythema ab igne. J Am Acad Dermatol 1996;34:516-517.
3. Helm TN, Spigel GT, Helm KF. Erythema ab igne caused by a car heater. Cutis 1997;59:81-82.
4. Bilic M, Adams BB. Erythema ab igne induced by a laptop computer. J Am Acad Dermatol 2004;50:973-974.
5. Weber MB, Ponzio HA, Costa FB, Camini L. An Bras Dermatol 2005;80:187-188.
6. Lin SJ, Hsu CJ, Chiu HC. Erythema ab igne caused by frequent hot bathing. Acta Derm Venereol 2002;82:478-479.
7. Galvin SA, Buchness MR. Rectangular reticulate patches on the pretibial areas. Erythema ab igne. Arch Dermatol 1990;126:386-387,389.
8. Flanagan N, Watson R, Sweeney E, Barnes L. Bullous erythema ab igne. Br J Dermatol 1996;134:1159-1160.
9. Shahrad P, Marks R. The wages of warmth: changes in erythema ab igne. Br J Dermatol 1977;97:179-186.
10. Milligan A, Graham-Brown RA. Erythema ab igne affecting the palms. Clin Exp Dermatol 1989;14:168-169.
11. Arrington JH, 3rd, Lockman DS. Thermal keratoses and squamous cell carcinoma in situ associated with erythema ab igne. Arch Dermatol 1979;115:1226-1228.
12. Hewitt JB, Sherif A, Kerr KM, Stankler L. Merkel cell and squamous cell carcinomas arising in erythema ab igne. Br J Dermatol 1993;128:591-592.
13. Sahl WJ, Jr, Taira JW. Erythema ab igne: treatment with 5-fluorouracil cream. J Am Acad Dermatol 1992;27:109-110.
Hyperpigmentation and vesicles after beach vacation
A 21-year-old woman traveled to Florida for spring break, accompanied by her fiancé and friends. While there, she developed a raised, pruritic 10-cm×30-cm lesion on her right lateral chest wall. A local pharmacist thought she was having an allergic reaction and recommended topical hydrocortisone cream and oral diphenhydramine (Benadryl). In spite of this therapy the rash progressed.
Five days after returning home, she presented to the emergency department, where she was diagnosed with herpes zoster. The rash was described as multiple vesicles in a dermatomal distribution on the right side of her torso. She was started on famciclovir (Famvir) and given a prescription for hydrocodone with acetaminophen (Vicodin).
Four days later she presented to her primary care physician because she continued to develop areas of hyperpigmentation around her mouth, on the chin, upper chest and breasts, thighs, and forearms. The original lesion on the right lateral thorax had dried, crusted, and was beginning to peel. It was no longer pruritic. The rash had never been painful and she had not taken any hydrocodone. She was most concerned about the disfigurement caused by the rash and wondered if she was having a reaction to the famciclovir. She had suffered no injuries or trauma, and did not engage in substance abuse.
While in Florida she did sun on the beach with friends, but she used sunblock regularly. She had used the same skin products and sunblock for many years. Upon questioning, she recalled drinking citrus beverages while out in the sun. Her history was otherwise unremarkable. Her only prescription medication before onset of the rash was montelukast (Singulair) prescribed for allergic rhinitis.
Examination revealed a bizarre pattern of hyperpigmentation with small punctate lesions on the upper chest, linear lesions on the posterior right arm and thighs, and broad areas of darkened skin on the lower face and chin (FIGURE 1). There was a broad patch of dense hyperpigmentation with desquamation on that right lateral chest wall that appeared to be healing (FIGURE 2).
FIGURE 1
Vesicles on the trunk…
FIGURE 2
…and anterior chest
What is your diagnosis?
Diagnosis: Phytophotodermatitis
This patient has phytophotodermatitis, a phototoxic dermatologic reaction that occurs with exposure to ultraviolet light after contact with certain plants. Hyperpigmentation and vesicle or bullae formation are hallmarks of the process.
The sun-sensitizing chemicals are furocoumarins, found in many plants (TABLE).1 Psoralen, the same substance used in PUVA treatments, is one of the furocoumarins. These agents contact the skin when juice is released from the fruit or stem or by direct contact with leaves.2
This patient remembered eating fresh limes on the beach and posing for a photograph next to her fiancé, when he placed his right hand around her back and on her right thorax (FIGURE 1). Limes have a high concentration of psoralens and are often the culprit in phytophotodermatitis.1,3 It is therefore incumbent on the clinician to know this and ask about lime and plant exposure when suspecting this diagnosis.
Erythema typically develops within 24 hours of sun exposure and contact with furocoumarin-containing plants. Vesicles or bulla may develop within 72 hours. Hyperpigmentation occurs over 1 to 2 weeks and can persist for 6 to 12 months. Hyperpigmentation can appear in bizarre streaks or patterns depending on where the chemicals contacted sun-exposed skin. Areas that have formed bulla typically exfoliate within 10 to 14 days.3
TABLE
Common furocoumarin-containing plants1
| Bitter orange | Lime |
| Carrot | Parsley |
| Celery | Parsnip |
| Fig |
Differential diagnosis
The differential diagnosis for this process includes herpes zoster, atopic dermatitis, allergic contact dermatitis, porphyria cutanea tarda, chemical or thermal burn, and abuse.2,4 While chemical or thermal burn and abuse are suspected with the appropriate history, the remainder of the diagnoses have defining characteristics allowing for proper identification.
Herpes zoster is caused by the reactivation of latent varicella zoster virus. Itching or burning precedes cutaneous eruption of red swollen plaques with vesicles of various sizes, which umbilicate or rupture. These lesions occur in a discreet dermatomal distribution.5
Atopic dermatitis has poorly understood pathophysiology. It typically starts in infancy and causes pruritus, eczematous lesions, xerosis, and lichenification of the skin.5
Allergic contact dermatitis is a delayed hypersensitivity reaction that requires prior sensitization. It is classically described as pruritic papules on an erythematous base.5
Porphyria cutanea tarda is a caused by an inborn enzyme deficiency in the heme biosynthetic pathway that results in mechanical fragility, subepidermal bullae, hypertrichosis, and pigmentation.5
Pathophysiology of phytophotodermatitis
In the presence of ultraviolet A (UVA) irradiation, psoralens cause DNA cross-linkage in the skin. This stimulates increased melanin production with resultant hyper-pigmentation. It may also cause the development of radicals, which damage cell membranes and intracellular contents, resulting in edema, erythema, and the formation of vesicles or bullae.3
Several factors have been implicated in the severity of the phototoxic reaction. Increased humidity, concentration of furocoumarins in plant juice, and UVA intensity all worsened the severity of reactions in one study. The concentration of furocoumarin is variable between species and between different parts of the same plant.2
Diagnosis and treatment
Consider phytophotodermatitis when patients present with hyperpigmentation, often in bizarre streaks on sun-exposed areas, or with vesicles in a nondermatomal distribution. It is a clinical diagnosis based on history and physical examination. Clues in this patient included recent vacation to Florida and consumption of citrus beverages along with the bizarre pattern of rash and sparing of the skin of the upper chest covered by her bathing suit. Any laboratory data obtained are used to exclude other diseases in the differential diagnosis. For example, porphyrin levels may be obtained to rule out porphyria cutanea tarda. If the clinical diagnosis remains in question, a skin biopsy may be performed to confirm the diagnosis.
Treatment involves use of topical steroids such as triamcinolone 0.1% cream applied to the skin two to three times per day, and the application of cool compresses for comfort. Advise patients to use sunscreen to prevent further hyperpigmentation. A topical bleaching agent such as hydroquinone may help reduce hyperpigmentation, although most lesions fade with time1 (strength of recommendation: C, based on usual practice or opinion).
CORRESPONDENCE
Andrea L. Darby-Stewart, MD, Assistant Professor of Family Medicine, Mayo Thunderbird Family Medicine, 13737 North 92nd Street, Scottsdale, AZ 85260. E-mail: [email protected]
1. Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other “lime” disease. J Emer Med 1999;17:235-237.
2. Knudson EA, Kroon S. In vitro and in vivo phototoxicity of furocoumarin-containing plants. Clin Exp Dermatol 1988;13:92-96.
3. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furocoumarins in limes. Am J Contact Dermatitis 2002;13:10-14.
4. Solis RR, Dotson DA, Trizna Z. Phytophotodermatitis: a sometimes difficult diagnosis. Arch Fam Med 2000;9:1195-1196.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. St Louis, Mo: Mosby; 2004.
A 21-year-old woman traveled to Florida for spring break, accompanied by her fiancé and friends. While there, she developed a raised, pruritic 10-cm×30-cm lesion on her right lateral chest wall. A local pharmacist thought she was having an allergic reaction and recommended topical hydrocortisone cream and oral diphenhydramine (Benadryl). In spite of this therapy the rash progressed.
Five days after returning home, she presented to the emergency department, where she was diagnosed with herpes zoster. The rash was described as multiple vesicles in a dermatomal distribution on the right side of her torso. She was started on famciclovir (Famvir) and given a prescription for hydrocodone with acetaminophen (Vicodin).
Four days later she presented to her primary care physician because she continued to develop areas of hyperpigmentation around her mouth, on the chin, upper chest and breasts, thighs, and forearms. The original lesion on the right lateral thorax had dried, crusted, and was beginning to peel. It was no longer pruritic. The rash had never been painful and she had not taken any hydrocodone. She was most concerned about the disfigurement caused by the rash and wondered if she was having a reaction to the famciclovir. She had suffered no injuries or trauma, and did not engage in substance abuse.
While in Florida she did sun on the beach with friends, but she used sunblock regularly. She had used the same skin products and sunblock for many years. Upon questioning, she recalled drinking citrus beverages while out in the sun. Her history was otherwise unremarkable. Her only prescription medication before onset of the rash was montelukast (Singulair) prescribed for allergic rhinitis.
Examination revealed a bizarre pattern of hyperpigmentation with small punctate lesions on the upper chest, linear lesions on the posterior right arm and thighs, and broad areas of darkened skin on the lower face and chin (FIGURE 1). There was a broad patch of dense hyperpigmentation with desquamation on that right lateral chest wall that appeared to be healing (FIGURE 2).
FIGURE 1
Vesicles on the trunk…
FIGURE 2
…and anterior chest
What is your diagnosis?
Diagnosis: Phytophotodermatitis
This patient has phytophotodermatitis, a phototoxic dermatologic reaction that occurs with exposure to ultraviolet light after contact with certain plants. Hyperpigmentation and vesicle or bullae formation are hallmarks of the process.
The sun-sensitizing chemicals are furocoumarins, found in many plants (TABLE).1 Psoralen, the same substance used in PUVA treatments, is one of the furocoumarins. These agents contact the skin when juice is released from the fruit or stem or by direct contact with leaves.2
This patient remembered eating fresh limes on the beach and posing for a photograph next to her fiancé, when he placed his right hand around her back and on her right thorax (FIGURE 1). Limes have a high concentration of psoralens and are often the culprit in phytophotodermatitis.1,3 It is therefore incumbent on the clinician to know this and ask about lime and plant exposure when suspecting this diagnosis.
Erythema typically develops within 24 hours of sun exposure and contact with furocoumarin-containing plants. Vesicles or bulla may develop within 72 hours. Hyperpigmentation occurs over 1 to 2 weeks and can persist for 6 to 12 months. Hyperpigmentation can appear in bizarre streaks or patterns depending on where the chemicals contacted sun-exposed skin. Areas that have formed bulla typically exfoliate within 10 to 14 days.3
TABLE
Common furocoumarin-containing plants1
| Bitter orange | Lime |
| Carrot | Parsley |
| Celery | Parsnip |
| Fig |
Differential diagnosis
The differential diagnosis for this process includes herpes zoster, atopic dermatitis, allergic contact dermatitis, porphyria cutanea tarda, chemical or thermal burn, and abuse.2,4 While chemical or thermal burn and abuse are suspected with the appropriate history, the remainder of the diagnoses have defining characteristics allowing for proper identification.
Herpes zoster is caused by the reactivation of latent varicella zoster virus. Itching or burning precedes cutaneous eruption of red swollen plaques with vesicles of various sizes, which umbilicate or rupture. These lesions occur in a discreet dermatomal distribution.5
Atopic dermatitis has poorly understood pathophysiology. It typically starts in infancy and causes pruritus, eczematous lesions, xerosis, and lichenification of the skin.5
Allergic contact dermatitis is a delayed hypersensitivity reaction that requires prior sensitization. It is classically described as pruritic papules on an erythematous base.5
Porphyria cutanea tarda is a caused by an inborn enzyme deficiency in the heme biosynthetic pathway that results in mechanical fragility, subepidermal bullae, hypertrichosis, and pigmentation.5
Pathophysiology of phytophotodermatitis
In the presence of ultraviolet A (UVA) irradiation, psoralens cause DNA cross-linkage in the skin. This stimulates increased melanin production with resultant hyper-pigmentation. It may also cause the development of radicals, which damage cell membranes and intracellular contents, resulting in edema, erythema, and the formation of vesicles or bullae.3
Several factors have been implicated in the severity of the phototoxic reaction. Increased humidity, concentration of furocoumarins in plant juice, and UVA intensity all worsened the severity of reactions in one study. The concentration of furocoumarin is variable between species and between different parts of the same plant.2
Diagnosis and treatment
Consider phytophotodermatitis when patients present with hyperpigmentation, often in bizarre streaks on sun-exposed areas, or with vesicles in a nondermatomal distribution. It is a clinical diagnosis based on history and physical examination. Clues in this patient included recent vacation to Florida and consumption of citrus beverages along with the bizarre pattern of rash and sparing of the skin of the upper chest covered by her bathing suit. Any laboratory data obtained are used to exclude other diseases in the differential diagnosis. For example, porphyrin levels may be obtained to rule out porphyria cutanea tarda. If the clinical diagnosis remains in question, a skin biopsy may be performed to confirm the diagnosis.
Treatment involves use of topical steroids such as triamcinolone 0.1% cream applied to the skin two to three times per day, and the application of cool compresses for comfort. Advise patients to use sunscreen to prevent further hyperpigmentation. A topical bleaching agent such as hydroquinone may help reduce hyperpigmentation, although most lesions fade with time1 (strength of recommendation: C, based on usual practice or opinion).
CORRESPONDENCE
Andrea L. Darby-Stewart, MD, Assistant Professor of Family Medicine, Mayo Thunderbird Family Medicine, 13737 North 92nd Street, Scottsdale, AZ 85260. E-mail: [email protected]
A 21-year-old woman traveled to Florida for spring break, accompanied by her fiancé and friends. While there, she developed a raised, pruritic 10-cm×30-cm lesion on her right lateral chest wall. A local pharmacist thought she was having an allergic reaction and recommended topical hydrocortisone cream and oral diphenhydramine (Benadryl). In spite of this therapy the rash progressed.
Five days after returning home, she presented to the emergency department, where she was diagnosed with herpes zoster. The rash was described as multiple vesicles in a dermatomal distribution on the right side of her torso. She was started on famciclovir (Famvir) and given a prescription for hydrocodone with acetaminophen (Vicodin).
Four days later she presented to her primary care physician because she continued to develop areas of hyperpigmentation around her mouth, on the chin, upper chest and breasts, thighs, and forearms. The original lesion on the right lateral thorax had dried, crusted, and was beginning to peel. It was no longer pruritic. The rash had never been painful and she had not taken any hydrocodone. She was most concerned about the disfigurement caused by the rash and wondered if she was having a reaction to the famciclovir. She had suffered no injuries or trauma, and did not engage in substance abuse.
While in Florida she did sun on the beach with friends, but she used sunblock regularly. She had used the same skin products and sunblock for many years. Upon questioning, she recalled drinking citrus beverages while out in the sun. Her history was otherwise unremarkable. Her only prescription medication before onset of the rash was montelukast (Singulair) prescribed for allergic rhinitis.
Examination revealed a bizarre pattern of hyperpigmentation with small punctate lesions on the upper chest, linear lesions on the posterior right arm and thighs, and broad areas of darkened skin on the lower face and chin (FIGURE 1). There was a broad patch of dense hyperpigmentation with desquamation on that right lateral chest wall that appeared to be healing (FIGURE 2).
FIGURE 1
Vesicles on the trunk…
FIGURE 2
…and anterior chest
What is your diagnosis?
Diagnosis: Phytophotodermatitis
This patient has phytophotodermatitis, a phototoxic dermatologic reaction that occurs with exposure to ultraviolet light after contact with certain plants. Hyperpigmentation and vesicle or bullae formation are hallmarks of the process.
The sun-sensitizing chemicals are furocoumarins, found in many plants (TABLE).1 Psoralen, the same substance used in PUVA treatments, is one of the furocoumarins. These agents contact the skin when juice is released from the fruit or stem or by direct contact with leaves.2
This patient remembered eating fresh limes on the beach and posing for a photograph next to her fiancé, when he placed his right hand around her back and on her right thorax (FIGURE 1). Limes have a high concentration of psoralens and are often the culprit in phytophotodermatitis.1,3 It is therefore incumbent on the clinician to know this and ask about lime and plant exposure when suspecting this diagnosis.
Erythema typically develops within 24 hours of sun exposure and contact with furocoumarin-containing plants. Vesicles or bulla may develop within 72 hours. Hyperpigmentation occurs over 1 to 2 weeks and can persist for 6 to 12 months. Hyperpigmentation can appear in bizarre streaks or patterns depending on where the chemicals contacted sun-exposed skin. Areas that have formed bulla typically exfoliate within 10 to 14 days.3
TABLE
Common furocoumarin-containing plants1
| Bitter orange | Lime |
| Carrot | Parsley |
| Celery | Parsnip |
| Fig |
Differential diagnosis
The differential diagnosis for this process includes herpes zoster, atopic dermatitis, allergic contact dermatitis, porphyria cutanea tarda, chemical or thermal burn, and abuse.2,4 While chemical or thermal burn and abuse are suspected with the appropriate history, the remainder of the diagnoses have defining characteristics allowing for proper identification.
Herpes zoster is caused by the reactivation of latent varicella zoster virus. Itching or burning precedes cutaneous eruption of red swollen plaques with vesicles of various sizes, which umbilicate or rupture. These lesions occur in a discreet dermatomal distribution.5
Atopic dermatitis has poorly understood pathophysiology. It typically starts in infancy and causes pruritus, eczematous lesions, xerosis, and lichenification of the skin.5
Allergic contact dermatitis is a delayed hypersensitivity reaction that requires prior sensitization. It is classically described as pruritic papules on an erythematous base.5
Porphyria cutanea tarda is a caused by an inborn enzyme deficiency in the heme biosynthetic pathway that results in mechanical fragility, subepidermal bullae, hypertrichosis, and pigmentation.5
Pathophysiology of phytophotodermatitis
In the presence of ultraviolet A (UVA) irradiation, psoralens cause DNA cross-linkage in the skin. This stimulates increased melanin production with resultant hyper-pigmentation. It may also cause the development of radicals, which damage cell membranes and intracellular contents, resulting in edema, erythema, and the formation of vesicles or bullae.3
Several factors have been implicated in the severity of the phototoxic reaction. Increased humidity, concentration of furocoumarins in plant juice, and UVA intensity all worsened the severity of reactions in one study. The concentration of furocoumarin is variable between species and between different parts of the same plant.2
Diagnosis and treatment
Consider phytophotodermatitis when patients present with hyperpigmentation, often in bizarre streaks on sun-exposed areas, or with vesicles in a nondermatomal distribution. It is a clinical diagnosis based on history and physical examination. Clues in this patient included recent vacation to Florida and consumption of citrus beverages along with the bizarre pattern of rash and sparing of the skin of the upper chest covered by her bathing suit. Any laboratory data obtained are used to exclude other diseases in the differential diagnosis. For example, porphyrin levels may be obtained to rule out porphyria cutanea tarda. If the clinical diagnosis remains in question, a skin biopsy may be performed to confirm the diagnosis.
Treatment involves use of topical steroids such as triamcinolone 0.1% cream applied to the skin two to three times per day, and the application of cool compresses for comfort. Advise patients to use sunscreen to prevent further hyperpigmentation. A topical bleaching agent such as hydroquinone may help reduce hyperpigmentation, although most lesions fade with time1 (strength of recommendation: C, based on usual practice or opinion).
CORRESPONDENCE
Andrea L. Darby-Stewart, MD, Assistant Professor of Family Medicine, Mayo Thunderbird Family Medicine, 13737 North 92nd Street, Scottsdale, AZ 85260. E-mail: [email protected]
1. Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other “lime” disease. J Emer Med 1999;17:235-237.
2. Knudson EA, Kroon S. In vitro and in vivo phototoxicity of furocoumarin-containing plants. Clin Exp Dermatol 1988;13:92-96.
3. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furocoumarins in limes. Am J Contact Dermatitis 2002;13:10-14.
4. Solis RR, Dotson DA, Trizna Z. Phytophotodermatitis: a sometimes difficult diagnosis. Arch Fam Med 2000;9:1195-1196.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. St Louis, Mo: Mosby; 2004.
1. Weber IC, Davis CP, Greeson DM. Phytophotodermatitis: the other “lime” disease. J Emer Med 1999;17:235-237.
2. Knudson EA, Kroon S. In vitro and in vivo phototoxicity of furocoumarin-containing plants. Clin Exp Dermatol 1988;13:92-96.
3. Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furocoumarins in limes. Am J Contact Dermatitis 2002;13:10-14.
4. Solis RR, Dotson DA, Trizna Z. Phytophotodermatitis: a sometimes difficult diagnosis. Arch Fam Med 2000;9:1195-1196.
5. Habif TP. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 4th ed. St Louis, Mo: Mosby; 2004.
Blisters during pregnancy—just with the second husband
A 33-year-old Hispanic woman who was 5 months pregnant came to the hospital complaining of nausea and vomiting. She had a history of anticardiolipin antibody syndrome, diagnosed originally in 1993 after 2 spontaneous abortions. She had stopped taking warfarin (Coumadin) at the start of her pregnancy, and had been taking heparin for 3 months.
After 4 days of close monitoring, the patient had labor induced for severe life-threatening pre-eclampsia. One day after induction and delivery of a stillborn fetus, she began to develop painful swelling of both hands and feet along with targetoid, urticarial, edematous, deep pink, slightly dusky papules and plaques on her hands, abdomen, lower extremities, and proximal thighs. Some of the edematous sites began to form vesicles and bullae (FIGURE 1 AND 2). When asked about this eruption, the patient mentioned having a similar rash after delivery of one of her children about 10 years before.
Interestingly, she noted that she only experienced these cutaneous findings during pregnancies with her second husband and not with her first. Biopsies were performed and showed prominent eosinophils in the dermis and a subepidermal vesicle (FIGURE 3).
FIGURE 1
Blisters on the wrist…
FIGURE 2
…and the abdomen
FIGURE 3
Biopsy results
What is your diagnosis?
Diagnosis: Pemphigoid gestationis
The patient had pemphigoid gestationis, also known as herpes gestationis, a rare autoimmune bullous disease of pregnancy and the puerperium.1 Clinically and immunopathologically, pemphigoid gestationis is related to the pemphigoid group of disorders and is not virally mediated.2
In the United States, pemphigoid gestationis has an incidence of 1:10,000 to 1:50,000 pregnancies.3 Clinically, it manifests during the second or third trimester, with a sudden onset of extremely pruritic urticarial papules and plaques usually located around the umbilicus. These lesions often progress to tense vesicles and blisters and spread peripherally to the trunk, often sparing the face, palms, and soles.4 Worsening of the lesions at the time of delivery occurs in 75% of cases, and usually recurs with subsequent pregnancies.5 Occasionally, however, subsequent pregnancies are unaffected, so-called “skip pregnancies.”6 This occurs most often when there has been a change in paternity.7
The exact cause of pemphigoid gestationis is unknown. Investigative efforts lead to the identification of an immunoglobulin G (IgG) autoantibody, which binds to bullous pemphigoid (BP) antigen 2, also called BP180, which is a protein associated with hemidesmosomes of basal keratinocytes.8-10 These hemidesmosomes form the central portion of the dermalepidermal anchoring complex, whose function is to establish a connection between the basal keratinocytes and the upper dermis.11,12 This is critical for maintaining dermal-epidermal adhesion. It is hypothesized that binding of autoantibodies to BP180 initiates an inflammatory reaction, leading to blister formation at the dermal-epidermal junction.13
Pathology and immunology
Histopathologic findings demonstrate subepidermal vesicles, spongiosis, and perivascular lymphocyte, and histiocyte infiltrates with a preponderance of eosinophils.3 The sine qua non of the disease, though, is the demonstration through direct immunofluorescence of complement deposition and IgG in a linear band along the basement membrane.14
There appears to be a genetic predisposition toward the development of pemphigoid gestationis. Associations with human leukocyte antigens (HLAs) DR3 (61%–85%), DR4 (52%), or both (43%–50%) have been reported.3,15,16 Interestingly, 85% of persons with a history of pemphigoid gestationis were found to have anti-HLA antibodies, some of which were directed against paternal HLAs expressed in their placentae.17 These findings raised speculation about a possible immunologic insult against placental antigens during pregnancy. Evidence suggests that circulating autoantibodies in patients with pemphigoid gestationis bind to the dermal-epidermal junction of skin and amnion in which BP180 antigen is also present.18-20
It has been demonstrated that in patients with pemphigoid gestationis the cells of the placenta stroma express abnormal major histocompatibility complex (MHC) class II molecules.21,22 This lead to the proposition of 2 possible mechanisms for the initiation of an autoimmune response in pemphigoid gestationis. The first proposes that placental BP180 is presented to the maternal immune system in association with abnormal MHC molecules, which then trigger the production of autoantibodies that cross-react with the skin. Alternatively, the placental stromal cells may evoke an allogeneic reaction against the BP180 antigen presented by paternal MHC molecules of the placental stroma, which then cross-reacts with the skin.23 The latter theory supports the findings in this patient, who developed pemphigoid gestationis during the 2 pregnancies with her second husband and not during the pregnancies with her first husband.
Differential diagnosis
It is important to differentiate the prebullous stage of pemphigoid gestationis from other pregnancy-related dermatoses. These include polymorphic eruption of pregnancy (PEP), pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, prurigo annularis, intrahepatic cholestasis of pregnancy, and impetigo herpetiformis. Impetigo herpetiformis is not related to bacterial or viral causes, but is rather a manifestation of pustular psoriasis during pregnancy. The target lesions that form in pemphigoid gestationis look just like the target lesions of erythema multiforme.
When there is no blister formation, it is impossible to distinguish pemphigoid gestationis from many of the other cutaneous eruptions of pregnancy. If uncertain, the clinician should perform punch biopsies of the involved skin, with one specimen sent for immunofluoresence studies. The biopsy should not pass directly through a bullae, due to risk of losing the overlying epidermis in the specimen. Do the punch biopsy at the edge of the bulla including some normal skin. Other important laboratory exams to perform would include liver function tests to look for an upward trend associated with intrahepatic cholestasis, and herpes simplex virus antibody testing for the association with erythema multiforme. The cutaneous findings and pertinent tests are listed in the table below in order of increasing potential as a life-threatening dermatosis (TABLE).
TABLE
Differential diagnosis for blisters in pregnancy
| DISEASE | ASSOCIATIONS | DIAGNOSIS | TREATMENT |
|---|---|---|---|
| Polymorphous eruption of pregnancy | Nonspecific pruritic eruption of pregnancy | Biopsy to differentiate from prebullous stage of pemphigoid (herpes) gestationis | Mild to mid-potency topical steroids, oral antihistamines |
| Pruritic urticarial papules and plaques of pregnancy | Occur in stretch marks, spare umbilicus; more often in primigravidas | Unless history is very clear, biopsy to differentiate from prebullous stage of pemphigoid gestationis | Emollients, pulse-dye laser during violaceous stage of striae, topical steroids, oral antihistamines |
| Erythema multiforme | Can involve mucous membranes, targetoid lesions, absence of pruritus, centripetal spread, favors palms/soles | Viral, bacterial, or drug-related eruption. Most often with herpes simplex I or II virus. Biopsy to differentiate from pemphigoid gestationis | Acyclovir, valacyclovir if HSV-related, treatment of bacterial infection, or removal of offending drug |
| Pemphigoid gestationis | Blistering, urticarial papules/plaques, pruritus | Biopsy sent for histologic diagnosis and immunofluorescence | Prednisone for short course starting at 1 mg/kg, then tapering over 2–3 months, topical steroids |
| Intrahepatic cholestasis of pregnancy | +/- jaundice, otherwise no cutaneous findings other than generalized pruritus, risk of preterm birth | Elevation in liver function tests, cholesterol, triglycerides, dark urine, right upper quadrant pain, nausea, greasy stools | Ursodeoxycholic acid, S-adenosyl-L-methionine |
| Impetigo herpetiformis (pustular psoriasis of pregnancy) | Extremely ill with fever, chills, nausea, vascular instability, pustules rather than vesicles | Biopsy if uncertain, pustules sterile, risk of hypocalcemia, hypoparathyroidism | High dose oral steroids or cyclosporine |
Treatment
Pemphigoid gestationis should resolve spontaneously within 2 to 3 months of delivery. Treatment is aimed at preventing new blisters and relieving pruritus, with topical corticosteroids and oral antihistamines in mild cases.2,25 In advanced lesions as seen in this case, 0.3 to 0.5 mg/kg of prednisolone daily is usually sufficient.3,25 Alternative medications include sulfapyridine, dapsone, and cyclosporine, though disease response is variable and their safety is questionable.3
When the skin condition began, the patient was treated with oral antihistamines and topical steroids. On day 2, the diagnosis of pemphigoid gestationis was clear, and she was started on oral prednisone at 60 mg/d, which resulted in rapid symptom improvement in her lesions and swelling. New lesions stopped forming, and systemic steroids were tapered off over the 3 months after delivery. The skin lesions healed and she was given supportive counseling to help her cope with her pregnancy loss.
Conclusion
We have described a rare case of a patient with no cutaneous eruptions during her pregnancies with her first husband, who developed pemphigoid gestationis in 2 pregnancies with her second husband. While it is interesting that our patient also had the anticardiolipin syndrome, most patients do not have both conditions.
Our patient had the classic findings of pemphigoid gestationis with many characteristic lesions (including the umbilicus) making the diagnosis possible before biopsy confirmation. This was fortunate for her because her painful swelling responded quickly to the corticosteroids. When cases are less clinically obvious, biopsy for histopathology and immunofluorescence facilitates differentiation of pemphigoid gestationis from other dermatoses of pregnancy.
CORRESPONDENCE
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Coupe RL. Herpes gestationis. Arch Dermatol 1965;91:633-636.
2. Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol 1999;24:255-259.
3. Al-Fouzan AW, Galadari I, Oumeish I, et al. Herpes gestationis (Pemphigoid gestationis). Clinics Dermatology 2006;24:109-112.
4. Shornick JK. Herpes gestationis. J Am Acad Dermatol 1987;17:539-556.
5. Holmes RC, Black MM, Dann J, et al. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol 1982;106:499-510.
6. Cozzani E, Basso M, Parodi A, Rebora A. Pemphigoid gestationis post partum after changing husband. Intn J Dermatol 2005;44:1057-1058.
7. Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol 1992;26:63-68.
8. Diaz LA, Ratrie H, III, Saunders WS, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest 1990;86:1088-1094.
9. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol 1992;99:243-250.
10. Zillikens D, Giudice GJ. BP180/typeXVIII collagen: its role in acquired and inherited disorders of the dermal-epidermal junction. Arch Dermatol Res 1999;291:187-194.
11. Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol 1996;8:647-656.
12. Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci 1999;20:134-154.
13. Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv Dermatol 2000;16:113-157.
14. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg 1998;17:172-181.
15. Holmes RC, Black MM, Jurecka W, et al. Clues to the aetiology and pathogenesis of herpes gestationis. Br J Dermatol 1983;109:131-139.
16. Shornick JK, Stastny P, Gilliam JN. High frequency of histocompatibility antigens DR3 and DR4 in herpes gestationis. J Clin Invest 1981;68:553-555.
17. Shornick JK, Stastny P, Gilliam JN. Paternal histocompatibility (HLA) antigens and maternal anti-HLA antibodies in herpes gestationis. J Invest Dermatol 1983;81:407-409.
18. Ortonne JP, Hsi BL, Verrando P, et al. Herpes gestationis factor reacts with the amniotic epithelial basement membrane. Br J Dermatol 1987;117:147-154.
19. Kelly SE, Bhogal BS, Wojnarowska F, Black MM. Expression of a pemphigoid gestationis-related antigen by human placenta. Br J Dermatol 1988;118:605-611.
20. Fairley JA, Heintz PW, Neuburg M, et al. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br J Dermatol 1995;133:385-391.
21. Kelly SE, Black MM, Fleming S. Antigen-presenting cells in the skin and placenta in pemphigoid gestationis. Br J Dermatol 1990;122:593-599.
22. Borthwick GM, Holmes RC, Stirrat GM. Abnormal expression of class II MHC antigens in placentae from patients with pemphigoid gestationis. Placenta 1988;9:81-94.
23. Kelly SE, Black MM, Fleming S. Pemphigoid gestationis: a unique mechanism of initiation of an autoimmune response by MHC class II molecules. J Pathol 1989;158:81-82.
24. Borradori L, Saurat JH. Specific dermatoses of pregnancy. Toward a comprehensive view. Arch Dermatol 1994;130:778-780.
25. Shimanovich I, Bröcker EB, Zillikens D. Pemphigoid gestationis: new insights into the pathogenesis lead to novel diagnostic tools. Br J Obstet Gynaecol 2002;109:970-976.
A 33-year-old Hispanic woman who was 5 months pregnant came to the hospital complaining of nausea and vomiting. She had a history of anticardiolipin antibody syndrome, diagnosed originally in 1993 after 2 spontaneous abortions. She had stopped taking warfarin (Coumadin) at the start of her pregnancy, and had been taking heparin for 3 months.
After 4 days of close monitoring, the patient had labor induced for severe life-threatening pre-eclampsia. One day after induction and delivery of a stillborn fetus, she began to develop painful swelling of both hands and feet along with targetoid, urticarial, edematous, deep pink, slightly dusky papules and plaques on her hands, abdomen, lower extremities, and proximal thighs. Some of the edematous sites began to form vesicles and bullae (FIGURE 1 AND 2). When asked about this eruption, the patient mentioned having a similar rash after delivery of one of her children about 10 years before.
Interestingly, she noted that she only experienced these cutaneous findings during pregnancies with her second husband and not with her first. Biopsies were performed and showed prominent eosinophils in the dermis and a subepidermal vesicle (FIGURE 3).
FIGURE 1
Blisters on the wrist…
FIGURE 2
…and the abdomen
FIGURE 3
Biopsy results
What is your diagnosis?
Diagnosis: Pemphigoid gestationis
The patient had pemphigoid gestationis, also known as herpes gestationis, a rare autoimmune bullous disease of pregnancy and the puerperium.1 Clinically and immunopathologically, pemphigoid gestationis is related to the pemphigoid group of disorders and is not virally mediated.2
In the United States, pemphigoid gestationis has an incidence of 1:10,000 to 1:50,000 pregnancies.3 Clinically, it manifests during the second or third trimester, with a sudden onset of extremely pruritic urticarial papules and plaques usually located around the umbilicus. These lesions often progress to tense vesicles and blisters and spread peripherally to the trunk, often sparing the face, palms, and soles.4 Worsening of the lesions at the time of delivery occurs in 75% of cases, and usually recurs with subsequent pregnancies.5 Occasionally, however, subsequent pregnancies are unaffected, so-called “skip pregnancies.”6 This occurs most often when there has been a change in paternity.7
The exact cause of pemphigoid gestationis is unknown. Investigative efforts lead to the identification of an immunoglobulin G (IgG) autoantibody, which binds to bullous pemphigoid (BP) antigen 2, also called BP180, which is a protein associated with hemidesmosomes of basal keratinocytes.8-10 These hemidesmosomes form the central portion of the dermalepidermal anchoring complex, whose function is to establish a connection between the basal keratinocytes and the upper dermis.11,12 This is critical for maintaining dermal-epidermal adhesion. It is hypothesized that binding of autoantibodies to BP180 initiates an inflammatory reaction, leading to blister formation at the dermal-epidermal junction.13
Pathology and immunology
Histopathologic findings demonstrate subepidermal vesicles, spongiosis, and perivascular lymphocyte, and histiocyte infiltrates with a preponderance of eosinophils.3 The sine qua non of the disease, though, is the demonstration through direct immunofluorescence of complement deposition and IgG in a linear band along the basement membrane.14
There appears to be a genetic predisposition toward the development of pemphigoid gestationis. Associations with human leukocyte antigens (HLAs) DR3 (61%–85%), DR4 (52%), or both (43%–50%) have been reported.3,15,16 Interestingly, 85% of persons with a history of pemphigoid gestationis were found to have anti-HLA antibodies, some of which were directed against paternal HLAs expressed in their placentae.17 These findings raised speculation about a possible immunologic insult against placental antigens during pregnancy. Evidence suggests that circulating autoantibodies in patients with pemphigoid gestationis bind to the dermal-epidermal junction of skin and amnion in which BP180 antigen is also present.18-20
It has been demonstrated that in patients with pemphigoid gestationis the cells of the placenta stroma express abnormal major histocompatibility complex (MHC) class II molecules.21,22 This lead to the proposition of 2 possible mechanisms for the initiation of an autoimmune response in pemphigoid gestationis. The first proposes that placental BP180 is presented to the maternal immune system in association with abnormal MHC molecules, which then trigger the production of autoantibodies that cross-react with the skin. Alternatively, the placental stromal cells may evoke an allogeneic reaction against the BP180 antigen presented by paternal MHC molecules of the placental stroma, which then cross-reacts with the skin.23 The latter theory supports the findings in this patient, who developed pemphigoid gestationis during the 2 pregnancies with her second husband and not during the pregnancies with her first husband.
Differential diagnosis
It is important to differentiate the prebullous stage of pemphigoid gestationis from other pregnancy-related dermatoses. These include polymorphic eruption of pregnancy (PEP), pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, prurigo annularis, intrahepatic cholestasis of pregnancy, and impetigo herpetiformis. Impetigo herpetiformis is not related to bacterial or viral causes, but is rather a manifestation of pustular psoriasis during pregnancy. The target lesions that form in pemphigoid gestationis look just like the target lesions of erythema multiforme.
When there is no blister formation, it is impossible to distinguish pemphigoid gestationis from many of the other cutaneous eruptions of pregnancy. If uncertain, the clinician should perform punch biopsies of the involved skin, with one specimen sent for immunofluoresence studies. The biopsy should not pass directly through a bullae, due to risk of losing the overlying epidermis in the specimen. Do the punch biopsy at the edge of the bulla including some normal skin. Other important laboratory exams to perform would include liver function tests to look for an upward trend associated with intrahepatic cholestasis, and herpes simplex virus antibody testing for the association with erythema multiforme. The cutaneous findings and pertinent tests are listed in the table below in order of increasing potential as a life-threatening dermatosis (TABLE).
TABLE
Differential diagnosis for blisters in pregnancy
| DISEASE | ASSOCIATIONS | DIAGNOSIS | TREATMENT |
|---|---|---|---|
| Polymorphous eruption of pregnancy | Nonspecific pruritic eruption of pregnancy | Biopsy to differentiate from prebullous stage of pemphigoid (herpes) gestationis | Mild to mid-potency topical steroids, oral antihistamines |
| Pruritic urticarial papules and plaques of pregnancy | Occur in stretch marks, spare umbilicus; more often in primigravidas | Unless history is very clear, biopsy to differentiate from prebullous stage of pemphigoid gestationis | Emollients, pulse-dye laser during violaceous stage of striae, topical steroids, oral antihistamines |
| Erythema multiforme | Can involve mucous membranes, targetoid lesions, absence of pruritus, centripetal spread, favors palms/soles | Viral, bacterial, or drug-related eruption. Most often with herpes simplex I or II virus. Biopsy to differentiate from pemphigoid gestationis | Acyclovir, valacyclovir if HSV-related, treatment of bacterial infection, or removal of offending drug |
| Pemphigoid gestationis | Blistering, urticarial papules/plaques, pruritus | Biopsy sent for histologic diagnosis and immunofluorescence | Prednisone for short course starting at 1 mg/kg, then tapering over 2–3 months, topical steroids |
| Intrahepatic cholestasis of pregnancy | +/- jaundice, otherwise no cutaneous findings other than generalized pruritus, risk of preterm birth | Elevation in liver function tests, cholesterol, triglycerides, dark urine, right upper quadrant pain, nausea, greasy stools | Ursodeoxycholic acid, S-adenosyl-L-methionine |
| Impetigo herpetiformis (pustular psoriasis of pregnancy) | Extremely ill with fever, chills, nausea, vascular instability, pustules rather than vesicles | Biopsy if uncertain, pustules sterile, risk of hypocalcemia, hypoparathyroidism | High dose oral steroids or cyclosporine |
Treatment
Pemphigoid gestationis should resolve spontaneously within 2 to 3 months of delivery. Treatment is aimed at preventing new blisters and relieving pruritus, with topical corticosteroids and oral antihistamines in mild cases.2,25 In advanced lesions as seen in this case, 0.3 to 0.5 mg/kg of prednisolone daily is usually sufficient.3,25 Alternative medications include sulfapyridine, dapsone, and cyclosporine, though disease response is variable and their safety is questionable.3
When the skin condition began, the patient was treated with oral antihistamines and topical steroids. On day 2, the diagnosis of pemphigoid gestationis was clear, and she was started on oral prednisone at 60 mg/d, which resulted in rapid symptom improvement in her lesions and swelling. New lesions stopped forming, and systemic steroids were tapered off over the 3 months after delivery. The skin lesions healed and she was given supportive counseling to help her cope with her pregnancy loss.
Conclusion
We have described a rare case of a patient with no cutaneous eruptions during her pregnancies with her first husband, who developed pemphigoid gestationis in 2 pregnancies with her second husband. While it is interesting that our patient also had the anticardiolipin syndrome, most patients do not have both conditions.
Our patient had the classic findings of pemphigoid gestationis with many characteristic lesions (including the umbilicus) making the diagnosis possible before biopsy confirmation. This was fortunate for her because her painful swelling responded quickly to the corticosteroids. When cases are less clinically obvious, biopsy for histopathology and immunofluorescence facilitates differentiation of pemphigoid gestationis from other dermatoses of pregnancy.
CORRESPONDENCE
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
A 33-year-old Hispanic woman who was 5 months pregnant came to the hospital complaining of nausea and vomiting. She had a history of anticardiolipin antibody syndrome, diagnosed originally in 1993 after 2 spontaneous abortions. She had stopped taking warfarin (Coumadin) at the start of her pregnancy, and had been taking heparin for 3 months.
After 4 days of close monitoring, the patient had labor induced for severe life-threatening pre-eclampsia. One day after induction and delivery of a stillborn fetus, she began to develop painful swelling of both hands and feet along with targetoid, urticarial, edematous, deep pink, slightly dusky papules and plaques on her hands, abdomen, lower extremities, and proximal thighs. Some of the edematous sites began to form vesicles and bullae (FIGURE 1 AND 2). When asked about this eruption, the patient mentioned having a similar rash after delivery of one of her children about 10 years before.
Interestingly, she noted that she only experienced these cutaneous findings during pregnancies with her second husband and not with her first. Biopsies were performed and showed prominent eosinophils in the dermis and a subepidermal vesicle (FIGURE 3).
FIGURE 1
Blisters on the wrist…
FIGURE 2
…and the abdomen
FIGURE 3
Biopsy results
What is your diagnosis?
Diagnosis: Pemphigoid gestationis
The patient had pemphigoid gestationis, also known as herpes gestationis, a rare autoimmune bullous disease of pregnancy and the puerperium.1 Clinically and immunopathologically, pemphigoid gestationis is related to the pemphigoid group of disorders and is not virally mediated.2
In the United States, pemphigoid gestationis has an incidence of 1:10,000 to 1:50,000 pregnancies.3 Clinically, it manifests during the second or third trimester, with a sudden onset of extremely pruritic urticarial papules and plaques usually located around the umbilicus. These lesions often progress to tense vesicles and blisters and spread peripherally to the trunk, often sparing the face, palms, and soles.4 Worsening of the lesions at the time of delivery occurs in 75% of cases, and usually recurs with subsequent pregnancies.5 Occasionally, however, subsequent pregnancies are unaffected, so-called “skip pregnancies.”6 This occurs most often when there has been a change in paternity.7
The exact cause of pemphigoid gestationis is unknown. Investigative efforts lead to the identification of an immunoglobulin G (IgG) autoantibody, which binds to bullous pemphigoid (BP) antigen 2, also called BP180, which is a protein associated with hemidesmosomes of basal keratinocytes.8-10 These hemidesmosomes form the central portion of the dermalepidermal anchoring complex, whose function is to establish a connection between the basal keratinocytes and the upper dermis.11,12 This is critical for maintaining dermal-epidermal adhesion. It is hypothesized that binding of autoantibodies to BP180 initiates an inflammatory reaction, leading to blister formation at the dermal-epidermal junction.13
Pathology and immunology
Histopathologic findings demonstrate subepidermal vesicles, spongiosis, and perivascular lymphocyte, and histiocyte infiltrates with a preponderance of eosinophils.3 The sine qua non of the disease, though, is the demonstration through direct immunofluorescence of complement deposition and IgG in a linear band along the basement membrane.14
There appears to be a genetic predisposition toward the development of pemphigoid gestationis. Associations with human leukocyte antigens (HLAs) DR3 (61%–85%), DR4 (52%), or both (43%–50%) have been reported.3,15,16 Interestingly, 85% of persons with a history of pemphigoid gestationis were found to have anti-HLA antibodies, some of which were directed against paternal HLAs expressed in their placentae.17 These findings raised speculation about a possible immunologic insult against placental antigens during pregnancy. Evidence suggests that circulating autoantibodies in patients with pemphigoid gestationis bind to the dermal-epidermal junction of skin and amnion in which BP180 antigen is also present.18-20
It has been demonstrated that in patients with pemphigoid gestationis the cells of the placenta stroma express abnormal major histocompatibility complex (MHC) class II molecules.21,22 This lead to the proposition of 2 possible mechanisms for the initiation of an autoimmune response in pemphigoid gestationis. The first proposes that placental BP180 is presented to the maternal immune system in association with abnormal MHC molecules, which then trigger the production of autoantibodies that cross-react with the skin. Alternatively, the placental stromal cells may evoke an allogeneic reaction against the BP180 antigen presented by paternal MHC molecules of the placental stroma, which then cross-reacts with the skin.23 The latter theory supports the findings in this patient, who developed pemphigoid gestationis during the 2 pregnancies with her second husband and not during the pregnancies with her first husband.
Differential diagnosis
It is important to differentiate the prebullous stage of pemphigoid gestationis from other pregnancy-related dermatoses. These include polymorphic eruption of pregnancy (PEP), pruritic urticarial papules and plaques of pregnancy (PUPPP), erythema multiforme, prurigo annularis, intrahepatic cholestasis of pregnancy, and impetigo herpetiformis. Impetigo herpetiformis is not related to bacterial or viral causes, but is rather a manifestation of pustular psoriasis during pregnancy. The target lesions that form in pemphigoid gestationis look just like the target lesions of erythema multiforme.
When there is no blister formation, it is impossible to distinguish pemphigoid gestationis from many of the other cutaneous eruptions of pregnancy. If uncertain, the clinician should perform punch biopsies of the involved skin, with one specimen sent for immunofluoresence studies. The biopsy should not pass directly through a bullae, due to risk of losing the overlying epidermis in the specimen. Do the punch biopsy at the edge of the bulla including some normal skin. Other important laboratory exams to perform would include liver function tests to look for an upward trend associated with intrahepatic cholestasis, and herpes simplex virus antibody testing for the association with erythema multiforme. The cutaneous findings and pertinent tests are listed in the table below in order of increasing potential as a life-threatening dermatosis (TABLE).
TABLE
Differential diagnosis for blisters in pregnancy
| DISEASE | ASSOCIATIONS | DIAGNOSIS | TREATMENT |
|---|---|---|---|
| Polymorphous eruption of pregnancy | Nonspecific pruritic eruption of pregnancy | Biopsy to differentiate from prebullous stage of pemphigoid (herpes) gestationis | Mild to mid-potency topical steroids, oral antihistamines |
| Pruritic urticarial papules and plaques of pregnancy | Occur in stretch marks, spare umbilicus; more often in primigravidas | Unless history is very clear, biopsy to differentiate from prebullous stage of pemphigoid gestationis | Emollients, pulse-dye laser during violaceous stage of striae, topical steroids, oral antihistamines |
| Erythema multiforme | Can involve mucous membranes, targetoid lesions, absence of pruritus, centripetal spread, favors palms/soles | Viral, bacterial, or drug-related eruption. Most often with herpes simplex I or II virus. Biopsy to differentiate from pemphigoid gestationis | Acyclovir, valacyclovir if HSV-related, treatment of bacterial infection, or removal of offending drug |
| Pemphigoid gestationis | Blistering, urticarial papules/plaques, pruritus | Biopsy sent for histologic diagnosis and immunofluorescence | Prednisone for short course starting at 1 mg/kg, then tapering over 2–3 months, topical steroids |
| Intrahepatic cholestasis of pregnancy | +/- jaundice, otherwise no cutaneous findings other than generalized pruritus, risk of preterm birth | Elevation in liver function tests, cholesterol, triglycerides, dark urine, right upper quadrant pain, nausea, greasy stools | Ursodeoxycholic acid, S-adenosyl-L-methionine |
| Impetigo herpetiformis (pustular psoriasis of pregnancy) | Extremely ill with fever, chills, nausea, vascular instability, pustules rather than vesicles | Biopsy if uncertain, pustules sterile, risk of hypocalcemia, hypoparathyroidism | High dose oral steroids or cyclosporine |
Treatment
Pemphigoid gestationis should resolve spontaneously within 2 to 3 months of delivery. Treatment is aimed at preventing new blisters and relieving pruritus, with topical corticosteroids and oral antihistamines in mild cases.2,25 In advanced lesions as seen in this case, 0.3 to 0.5 mg/kg of prednisolone daily is usually sufficient.3,25 Alternative medications include sulfapyridine, dapsone, and cyclosporine, though disease response is variable and their safety is questionable.3
When the skin condition began, the patient was treated with oral antihistamines and topical steroids. On day 2, the diagnosis of pemphigoid gestationis was clear, and she was started on oral prednisone at 60 mg/d, which resulted in rapid symptom improvement in her lesions and swelling. New lesions stopped forming, and systemic steroids were tapered off over the 3 months after delivery. The skin lesions healed and she was given supportive counseling to help her cope with her pregnancy loss.
Conclusion
We have described a rare case of a patient with no cutaneous eruptions during her pregnancies with her first husband, who developed pemphigoid gestationis in 2 pregnancies with her second husband. While it is interesting that our patient also had the anticardiolipin syndrome, most patients do not have both conditions.
Our patient had the classic findings of pemphigoid gestationis with many characteristic lesions (including the umbilicus) making the diagnosis possible before biopsy confirmation. This was fortunate for her because her painful swelling responded quickly to the corticosteroids. When cases are less clinically obvious, biopsy for histopathology and immunofluorescence facilitates differentiation of pemphigoid gestationis from other dermatoses of pregnancy.
CORRESPONDENCE
Richard P. Usatine, MD, University of Texas Health Sciences Center at San Antonio, 7703 Floyd Curl Drive, San Antonio, TX 78229-3900. E-mail: [email protected]
1. Coupe RL. Herpes gestationis. Arch Dermatol 1965;91:633-636.
2. Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol 1999;24:255-259.
3. Al-Fouzan AW, Galadari I, Oumeish I, et al. Herpes gestationis (Pemphigoid gestationis). Clinics Dermatology 2006;24:109-112.
4. Shornick JK. Herpes gestationis. J Am Acad Dermatol 1987;17:539-556.
5. Holmes RC, Black MM, Dann J, et al. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol 1982;106:499-510.
6. Cozzani E, Basso M, Parodi A, Rebora A. Pemphigoid gestationis post partum after changing husband. Intn J Dermatol 2005;44:1057-1058.
7. Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol 1992;26:63-68.
8. Diaz LA, Ratrie H, III, Saunders WS, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest 1990;86:1088-1094.
9. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol 1992;99:243-250.
10. Zillikens D, Giudice GJ. BP180/typeXVIII collagen: its role in acquired and inherited disorders of the dermal-epidermal junction. Arch Dermatol Res 1999;291:187-194.
11. Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol 1996;8:647-656.
12. Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci 1999;20:134-154.
13. Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv Dermatol 2000;16:113-157.
14. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg 1998;17:172-181.
15. Holmes RC, Black MM, Jurecka W, et al. Clues to the aetiology and pathogenesis of herpes gestationis. Br J Dermatol 1983;109:131-139.
16. Shornick JK, Stastny P, Gilliam JN. High frequency of histocompatibility antigens DR3 and DR4 in herpes gestationis. J Clin Invest 1981;68:553-555.
17. Shornick JK, Stastny P, Gilliam JN. Paternal histocompatibility (HLA) antigens and maternal anti-HLA antibodies in herpes gestationis. J Invest Dermatol 1983;81:407-409.
18. Ortonne JP, Hsi BL, Verrando P, et al. Herpes gestationis factor reacts with the amniotic epithelial basement membrane. Br J Dermatol 1987;117:147-154.
19. Kelly SE, Bhogal BS, Wojnarowska F, Black MM. Expression of a pemphigoid gestationis-related antigen by human placenta. Br J Dermatol 1988;118:605-611.
20. Fairley JA, Heintz PW, Neuburg M, et al. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br J Dermatol 1995;133:385-391.
21. Kelly SE, Black MM, Fleming S. Antigen-presenting cells in the skin and placenta in pemphigoid gestationis. Br J Dermatol 1990;122:593-599.
22. Borthwick GM, Holmes RC, Stirrat GM. Abnormal expression of class II MHC antigens in placentae from patients with pemphigoid gestationis. Placenta 1988;9:81-94.
23. Kelly SE, Black MM, Fleming S. Pemphigoid gestationis: a unique mechanism of initiation of an autoimmune response by MHC class II molecules. J Pathol 1989;158:81-82.
24. Borradori L, Saurat JH. Specific dermatoses of pregnancy. Toward a comprehensive view. Arch Dermatol 1994;130:778-780.
25. Shimanovich I, Bröcker EB, Zillikens D. Pemphigoid gestationis: new insights into the pathogenesis lead to novel diagnostic tools. Br J Obstet Gynaecol 2002;109:970-976.
1. Coupe RL. Herpes gestationis. Arch Dermatol 1965;91:633-636.
2. Jenkins RE, Hern S, Black MM. Clinical features and management of 87 patients with pemphigoid gestationis. Clin Exp Dermatol 1999;24:255-259.
3. Al-Fouzan AW, Galadari I, Oumeish I, et al. Herpes gestationis (Pemphigoid gestationis). Clinics Dermatology 2006;24:109-112.
4. Shornick JK. Herpes gestationis. J Am Acad Dermatol 1987;17:539-556.
5. Holmes RC, Black MM, Dann J, et al. A comparative study of toxic erythema of pregnancy and herpes gestationis. Br J Dermatol 1982;106:499-510.
6. Cozzani E, Basso M, Parodi A, Rebora A. Pemphigoid gestationis post partum after changing husband. Intn J Dermatol 2005;44:1057-1058.
7. Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol 1992;26:63-68.
8. Diaz LA, Ratrie H, III, Saunders WS, et al. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest 1990;86:1088-1094.
9. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol 1992;99:243-250.
10. Zillikens D, Giudice GJ. BP180/typeXVIII collagen: its role in acquired and inherited disorders of the dermal-epidermal junction. Arch Dermatol Res 1999;291:187-194.
11. Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol 1996;8:647-656.
12. Zillikens D. Acquired skin disease of hemidesmosomes. J Dermatol Sci 1999;20:134-154.
13. Schmidt E, Zillikens D. Autoimmune and inherited subepidermal blistering diseases: advances in the clinic and the laboratory. Adv Dermatol 2000;16:113-157.
14. Shornick JD. Dermatoses of pregnancy. Semin Cutan Med Surg 1998;17:172-181.
15. Holmes RC, Black MM, Jurecka W, et al. Clues to the aetiology and pathogenesis of herpes gestationis. Br J Dermatol 1983;109:131-139.
16. Shornick JK, Stastny P, Gilliam JN. High frequency of histocompatibility antigens DR3 and DR4 in herpes gestationis. J Clin Invest 1981;68:553-555.
17. Shornick JK, Stastny P, Gilliam JN. Paternal histocompatibility (HLA) antigens and maternal anti-HLA antibodies in herpes gestationis. J Invest Dermatol 1983;81:407-409.
18. Ortonne JP, Hsi BL, Verrando P, et al. Herpes gestationis factor reacts with the amniotic epithelial basement membrane. Br J Dermatol 1987;117:147-154.
19. Kelly SE, Bhogal BS, Wojnarowska F, Black MM. Expression of a pemphigoid gestationis-related antigen by human placenta. Br J Dermatol 1988;118:605-611.
20. Fairley JA, Heintz PW, Neuburg M, et al. Expression pattern of the bullous pemphigoid-180 antigen in normal and neoplastic epithelia. Br J Dermatol 1995;133:385-391.
21. Kelly SE, Black MM, Fleming S. Antigen-presenting cells in the skin and placenta in pemphigoid gestationis. Br J Dermatol 1990;122:593-599.
22. Borthwick GM, Holmes RC, Stirrat GM. Abnormal expression of class II MHC antigens in placentae from patients with pemphigoid gestationis. Placenta 1988;9:81-94.
23. Kelly SE, Black MM, Fleming S. Pemphigoid gestationis: a unique mechanism of initiation of an autoimmune response by MHC class II molecules. J Pathol 1989;158:81-82.
24. Borradori L, Saurat JH. Specific dermatoses of pregnancy. Toward a comprehensive view. Arch Dermatol 1994;130:778-780.
25. Shimanovich I, Bröcker EB, Zillikens D. Pemphigoid gestationis: new insights into the pathogenesis lead to novel diagnostic tools. Br J Obstet Gynaecol 2002;109:970-976.
Skin lesions mimicking septic arthritis
A 46-year-old African American woman was admitted to the hospital with complaints of multiple joint pains associated with skin lesions, which had lasted for 3 weeks. The pains were in her right ankle, left wrist, both great toes, and right thumb. Further systems review revealed that the patient had experienced a fever of 102°F, diarrhea (at times bloody), chills, nausea, and decreased appetite for 5 days along with low back pain for 1 week.
Her medical history was significant for hypertension, hypothyroidism, chronic gastrointestinal symptoms attributed to irritable bowel disease for the past 2 years, and intermittent asymmetrical oligoarthritis for 1 year that had responded to episodic treatment with prednisone. She also had 2 episodes of iritis in the previous 4 years responsive to topical corticosteroids.
On exam she looked sick, was febrile, and had skin lesions in vaginal, axillary, perianal, foot, and thigh areas (FIGURES 1 AND 2). The lesions were 5 to 40 mm in size, exquisitely tender, bullous, pustular, and formed violaceous ulcers with scanty purulent-looking discharge. Her ankle exam was significant for swelling, redness, warmth, tenderness and severe pain on passive and active movements (FIGURE 3). Results of an examination of her spine was within normal limits. The remainder of her physical exam also showed normal results.
FIGURE 1
Ulcer on thigh
FIGURE 2
Lesion on foot
FIGURE 3
Swollen ankle joint
What is your diagnosis?
Differential diagnosis
The primary differential was focused on joint symptoms, and included disseminated gonococcal, bacterial, atypical infections, Behçet’s disease, rheumatoid arthritis, Reiter’s disease, reactive and inflammatory bowel disease (IBD)-related arthritis.
Differential diagnosis for the skin lesions included pyoderma gangrenosum, Sweets syndrome, sexually transmitted diseases, granulomatous skin lesions of inflammatory bowel disease, Reiter’s, Behçet’s, vasculitis (Wegener’s granulamatosis, polyarteritis nodosa, cryoglobulinemia), cancer (lymphoma cutis, leukemia cutis, Langerhans cell histiocytosis), infectious (sporotrichosis, blastomycosis, local fungal), drug-induced, exogenous tissue injury, vaso-occlusive (antiphospholipid-antibody syndrome).3
Further testing was necessary to determine the diagnosis.
Laboratory results
The following laboratory tests were ordered.
Blood tests. Patient’s complete blood count was remarkable for anemia and thrombocytosis; a comprehensive metabolic panel showed hypoalbuminemia. Erythrocyte sedimentation rate was elevated at 120 mm/h and C-reactive protein was elevated at 24.9 mg/L.
A diagnostic tap of the ankle showed fluid with inflammatory changes, and cultures for aerobic, anaerobic, acid fast bacilli, fungus, gonorrhea, and herpes were negative.
Joints. The patient’s joints were suspected to have septic arthritis, both clinically and on magnetic resonance imaging exam; they demonstrated no improvement of symptoms with intravenous antibiotics. Early on, the orthopedic service recommended joint debridement and open drainage.
Surgical findings included epidermolysis with subcutaneous fluid and purulent-looking material. Progressive necrosis of the debrided margins occurred over the next few days.
Workup for possible rheumatological joint disease included antinuclear antibody, rheumatoid factor, cyclic citrullinated peptide antibody (anti-CCP antibody), and human leukocyte antigens (HLA) B27 and B51. Radiographs of involved joints did not show rheumatic joint disease.
Rheumatoid arthritis was doubtful due to lack of sufficient criteria for diagnosis, and negative rheumatoid factor and anti-CCP antibody.5 HLA B27 is positive in 80% of patients with Reiter’s syndrome, 60% of those with reactive arthritis, and 50% of those with IBD arthritis.6 HLA B51, although not diagnostic, has a higher than baseline prevalence in Behçet’s disease.4 Recurrent oral aphthae, a major criteria for Behçet’s, was absent in our patient.
Gastrointestinal. A preliminary gastrointestinal evaluation included stool culture and tests for clostridium difficile toxin, viral antibodies for cytomegalovirus (CMV), parvovirus, and herpes—all results were negative.
Colonoscopy, however, demonstrated colonic ulcers (mainly of the right colon) with skip pattern associated with polypoid lesions, mild terminal ileitis, and normal-looking left colon and rectum (FIGURE 4). Colonic biopsy showed crypt distortion and abscesses, terminal ileitis, absence of granuloma formation, and negative immunostain for CMV. Colonoscopy findings along with colon biopsy were suggestive of Crohn’s disease.
Conclusions from the lab tests. Blood, urine, and skin lesion cultures remained negative. Urethral and vaginal cultures for gonorrhea, chlamydia, herpes virus, and serum test for syphilis, HIV, and hepatitis B and C were all negative. Biopsy of skin lesions showed dermal mixed inflammatory infiltrate predominantly neutrophilic, and no vasculitis or micropathogens. Absence of micropathogens, vasculitis, vascular thrombosis, and lack of neoplastic cells made infectious, vasculitic, vaso-occlusive and malignant ulcers implausible.
There is no specific laboratory or pathological finding for diagnosis of pyoderma gangrenosum. Diagnosis is based on exclusion of other ulcerative conditions. Negative skin, arthrocentesis, joint fluid and tissue cultures, progressive necrosis of debrided tissue margins (pathergy), and skin biopsy suggested a diagnosis of pyoderma gangrenosum.
Joint lesions were considered to be atypical pyoderma gangrenosum. Investigations to diagnose or exclude a concomitant systemic disorder are required in patients with pyoderma gangrenosum. This patient had Crohn’s disease associated with pyoderma gangrenosum.
FIGURE 4
Colonoscopy findings
Diagnosis: Pyoderma gangrenosum
Pyoderma gangrenosum is a rare, ulcerative, neutrophilic dermatosis of uncertain etiology. It occurs in 1 in 100,000 people in the US per year.1 Fifty percent of patients with pyoderma gangrenosum have an underlying disorder such as inflammatory bowel disease, arthritis, hematological malignancy, infections, sarcoidosis, hypogammaglobulinemia, or HIV.2 Pyoderma gangrenosum occurs in 2% of patients with Crohn’s disease.9
Variants of pyoderma gangrenosum
The 2 primary variants are classical and atypical. Four types of lesions are seen.2
Ulcerative. Ulcerative pyoderma gangrenosum a classical form of disease and occurs usually in lower limb and trunk. Lesions begin as pustules or as a pathergic phenomenon (development of ulcer at site of minimal trauma). This progresses to a large violaceous ulcer with undermined borders and surrounding halo of erythema. The lesions are distinctively painful.
Pyoderma gangrenosum may occur on the genitalia and would need to be differentiated from sexually transmitted disease. Extracutaneous sterile neutrophilic abscesses have been reported in lungs, eyes, liver, spleen, bones, heart, central nervous system, and gastrointestinal tract.
Pustular. Present as painful postulations on extensor surfaces of the limbs. Pyostomatitis vegetans is a variant of this form and occurs in the oral cavity.
Bullous/atypical. Bullae most often occur on hands, forearm, or face. Most commonly associated with hematological malignancy.
Vegetative. Chronic, nonpainful superficial ulcer.
Treatment: Topical vs systemic
To date there are no established guidelines for treatment of pyoderma gangrenosum. Treatment of underlying disease often results in improvement of pyoderma gangrenosum.
Treat localized cases topically
Localized pyoderma gangrenosum may initially be treated with corticosteroids (topical or intralesional) or tacrolimus (SOR: B),7 reserving systemic treatment for refractory cases.
Topical care of the lesions along with systemic therapy should be limited to antiseptic or occlusive wound dressing in an effort to minimize secondary bacterial infection.
Surgery not recommended in active disease
Adjuvant surgery such as excision of ulcer, skin grafting, etc, has not been shown to reduce morbidity even when done in conjunction with systemic immunosuppressive therapy. Re-epithelialization procedures like debridements or allografts may be done during disease remission (SOR: C).7
These procedures should be avoided in active disease due to risk of pathergy and worsening of original lesions of pyoderma gangrenosum. Similarly, elective surgery should be avoided during active disease, and when necessary should be done alongside systemic pyoderma gangrenosum therapy.
Refractory cases
In refractory or idiopathic cases, disseminated pyoderma gangrenosum is treated systemically with corticosteroids or cyclosporine alone or in combination (strength of recommendation [SOR]: B).7 In steroid-resistant pyoderma gangrenosum, thalidomide, mycophenolate mofetil, tacrolimus, dapsone, azathioprine, and infliximab may be tried (SOR: C).7
Immunoglobulin, plasmapheresis, and cyclophosphamide have shown some efficacy in patients without systemic disease (SOR: C).7 Tumor necrosis factor-α inhibitor infliximab (Remicade) is considered first-line therapy for pyoderma gangrenosum associated with Crohn’s disease (SOR: B).7
The patient’s outcome
The patient did not improve after initial treatment with high-dose systemic corticosteroids. Infliximab in combination with methotrexate dramatically improved her skin and joint lesions and induced remission of Crohn’s disease. Early on, during the active phase of the disease, she also had surgical debridement of the lesions due to confounding diagnosis.
The patient has continued with infliximab infusions every 2 months and weekly oral methotrexate to maintain remission. Data show that 56% of patients with pyoderma gangrenosum require long-term therapy to prevent recurrences.8
This case is interesting because the bullous lesions of pyoderma gangrenosum appeared over the joints, mimicking septic arthritis and resulting in preventable surgical debridement. A case report with pyoderma gangrenosum lesions predominantly over the joints has not been described in the published literature. Recognition of lesions over the joints is important because treatment remains nonsurgical and surgery may exacerbate these skin lesions.
CORRESPONDENCE
Shashi Mittal, MD, Faculty Director of Research, Baylor Family Medicine Residency at Garland, Suite 340, Clara Barton Blvd, Garland, TX 75042.
1. Jackson JM, Callen JP. Pyoderma gangrenosum. Available at: Emedicine.com. Accessed on March 21, 2006.
2. Wines N, Wines M, Ryman W. Understanding pyoderma gangrenosum: A Review.Available at www.Medscape.com. Accessed on March 21, 2006.
3. Weenig RH, Davis MD, Dahl PR, Su WP. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med 2002;347:1412-1418.
4. Smith EL. Clinical manifestations and diagnosis of Behcet’s disease. Available at www.uptodate.com. Accessed on April 2, 2006.
5. Venables PJW, Maini RN. Diagnosis and differential diagnosis of Rheumatoid arthritis. Available at www.uptodate.com. Accessed on April 2, 2006.
6. Yu DT, Wiesenhutter CW. Course and treatment of Reiter’s syndrome; reactive arthritis and undifferentiated spondyloarthropathy. Available at www.uptodate.com. Accessed on April 2, 2006.
7. Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol 2005;53:273-283.
8. Driesch VD. Pyoderma gangrenosum: a report of 44 cases with follow up. Br J Dermatol 1997;137:1000-1005.
9. Moschella SL. Neutrophilic dermatosis. Available at www.uptodate.com.
A 46-year-old African American woman was admitted to the hospital with complaints of multiple joint pains associated with skin lesions, which had lasted for 3 weeks. The pains were in her right ankle, left wrist, both great toes, and right thumb. Further systems review revealed that the patient had experienced a fever of 102°F, diarrhea (at times bloody), chills, nausea, and decreased appetite for 5 days along with low back pain for 1 week.
Her medical history was significant for hypertension, hypothyroidism, chronic gastrointestinal symptoms attributed to irritable bowel disease for the past 2 years, and intermittent asymmetrical oligoarthritis for 1 year that had responded to episodic treatment with prednisone. She also had 2 episodes of iritis in the previous 4 years responsive to topical corticosteroids.
On exam she looked sick, was febrile, and had skin lesions in vaginal, axillary, perianal, foot, and thigh areas (FIGURES 1 AND 2). The lesions were 5 to 40 mm in size, exquisitely tender, bullous, pustular, and formed violaceous ulcers with scanty purulent-looking discharge. Her ankle exam was significant for swelling, redness, warmth, tenderness and severe pain on passive and active movements (FIGURE 3). Results of an examination of her spine was within normal limits. The remainder of her physical exam also showed normal results.
FIGURE 1
Ulcer on thigh
FIGURE 2
Lesion on foot
FIGURE 3
Swollen ankle joint
What is your diagnosis?
Differential diagnosis
The primary differential was focused on joint symptoms, and included disseminated gonococcal, bacterial, atypical infections, Behçet’s disease, rheumatoid arthritis, Reiter’s disease, reactive and inflammatory bowel disease (IBD)-related arthritis.
Differential diagnosis for the skin lesions included pyoderma gangrenosum, Sweets syndrome, sexually transmitted diseases, granulomatous skin lesions of inflammatory bowel disease, Reiter’s, Behçet’s, vasculitis (Wegener’s granulamatosis, polyarteritis nodosa, cryoglobulinemia), cancer (lymphoma cutis, leukemia cutis, Langerhans cell histiocytosis), infectious (sporotrichosis, blastomycosis, local fungal), drug-induced, exogenous tissue injury, vaso-occlusive (antiphospholipid-antibody syndrome).3
Further testing was necessary to determine the diagnosis.
Laboratory results
The following laboratory tests were ordered.
Blood tests. Patient’s complete blood count was remarkable for anemia and thrombocytosis; a comprehensive metabolic panel showed hypoalbuminemia. Erythrocyte sedimentation rate was elevated at 120 mm/h and C-reactive protein was elevated at 24.9 mg/L.
A diagnostic tap of the ankle showed fluid with inflammatory changes, and cultures for aerobic, anaerobic, acid fast bacilli, fungus, gonorrhea, and herpes were negative.
Joints. The patient’s joints were suspected to have septic arthritis, both clinically and on magnetic resonance imaging exam; they demonstrated no improvement of symptoms with intravenous antibiotics. Early on, the orthopedic service recommended joint debridement and open drainage.
Surgical findings included epidermolysis with subcutaneous fluid and purulent-looking material. Progressive necrosis of the debrided margins occurred over the next few days.
Workup for possible rheumatological joint disease included antinuclear antibody, rheumatoid factor, cyclic citrullinated peptide antibody (anti-CCP antibody), and human leukocyte antigens (HLA) B27 and B51. Radiographs of involved joints did not show rheumatic joint disease.
Rheumatoid arthritis was doubtful due to lack of sufficient criteria for diagnosis, and negative rheumatoid factor and anti-CCP antibody.5 HLA B27 is positive in 80% of patients with Reiter’s syndrome, 60% of those with reactive arthritis, and 50% of those with IBD arthritis.6 HLA B51, although not diagnostic, has a higher than baseline prevalence in Behçet’s disease.4 Recurrent oral aphthae, a major criteria for Behçet’s, was absent in our patient.
Gastrointestinal. A preliminary gastrointestinal evaluation included stool culture and tests for clostridium difficile toxin, viral antibodies for cytomegalovirus (CMV), parvovirus, and herpes—all results were negative.
Colonoscopy, however, demonstrated colonic ulcers (mainly of the right colon) with skip pattern associated with polypoid lesions, mild terminal ileitis, and normal-looking left colon and rectum (FIGURE 4). Colonic biopsy showed crypt distortion and abscesses, terminal ileitis, absence of granuloma formation, and negative immunostain for CMV. Colonoscopy findings along with colon biopsy were suggestive of Crohn’s disease.
Conclusions from the lab tests. Blood, urine, and skin lesion cultures remained negative. Urethral and vaginal cultures for gonorrhea, chlamydia, herpes virus, and serum test for syphilis, HIV, and hepatitis B and C were all negative. Biopsy of skin lesions showed dermal mixed inflammatory infiltrate predominantly neutrophilic, and no vasculitis or micropathogens. Absence of micropathogens, vasculitis, vascular thrombosis, and lack of neoplastic cells made infectious, vasculitic, vaso-occlusive and malignant ulcers implausible.
There is no specific laboratory or pathological finding for diagnosis of pyoderma gangrenosum. Diagnosis is based on exclusion of other ulcerative conditions. Negative skin, arthrocentesis, joint fluid and tissue cultures, progressive necrosis of debrided tissue margins (pathergy), and skin biopsy suggested a diagnosis of pyoderma gangrenosum.
Joint lesions were considered to be atypical pyoderma gangrenosum. Investigations to diagnose or exclude a concomitant systemic disorder are required in patients with pyoderma gangrenosum. This patient had Crohn’s disease associated with pyoderma gangrenosum.
FIGURE 4
Colonoscopy findings
Diagnosis: Pyoderma gangrenosum
Pyoderma gangrenosum is a rare, ulcerative, neutrophilic dermatosis of uncertain etiology. It occurs in 1 in 100,000 people in the US per year.1 Fifty percent of patients with pyoderma gangrenosum have an underlying disorder such as inflammatory bowel disease, arthritis, hematological malignancy, infections, sarcoidosis, hypogammaglobulinemia, or HIV.2 Pyoderma gangrenosum occurs in 2% of patients with Crohn’s disease.9
Variants of pyoderma gangrenosum
The 2 primary variants are classical and atypical. Four types of lesions are seen.2
Ulcerative. Ulcerative pyoderma gangrenosum a classical form of disease and occurs usually in lower limb and trunk. Lesions begin as pustules or as a pathergic phenomenon (development of ulcer at site of minimal trauma). This progresses to a large violaceous ulcer with undermined borders and surrounding halo of erythema. The lesions are distinctively painful.
Pyoderma gangrenosum may occur on the genitalia and would need to be differentiated from sexually transmitted disease. Extracutaneous sterile neutrophilic abscesses have been reported in lungs, eyes, liver, spleen, bones, heart, central nervous system, and gastrointestinal tract.
Pustular. Present as painful postulations on extensor surfaces of the limbs. Pyostomatitis vegetans is a variant of this form and occurs in the oral cavity.
Bullous/atypical. Bullae most often occur on hands, forearm, or face. Most commonly associated with hematological malignancy.
Vegetative. Chronic, nonpainful superficial ulcer.
Treatment: Topical vs systemic
To date there are no established guidelines for treatment of pyoderma gangrenosum. Treatment of underlying disease often results in improvement of pyoderma gangrenosum.
Treat localized cases topically
Localized pyoderma gangrenosum may initially be treated with corticosteroids (topical or intralesional) or tacrolimus (SOR: B),7 reserving systemic treatment for refractory cases.
Topical care of the lesions along with systemic therapy should be limited to antiseptic or occlusive wound dressing in an effort to minimize secondary bacterial infection.
Surgery not recommended in active disease
Adjuvant surgery such as excision of ulcer, skin grafting, etc, has not been shown to reduce morbidity even when done in conjunction with systemic immunosuppressive therapy. Re-epithelialization procedures like debridements or allografts may be done during disease remission (SOR: C).7
These procedures should be avoided in active disease due to risk of pathergy and worsening of original lesions of pyoderma gangrenosum. Similarly, elective surgery should be avoided during active disease, and when necessary should be done alongside systemic pyoderma gangrenosum therapy.
Refractory cases
In refractory or idiopathic cases, disseminated pyoderma gangrenosum is treated systemically with corticosteroids or cyclosporine alone or in combination (strength of recommendation [SOR]: B).7 In steroid-resistant pyoderma gangrenosum, thalidomide, mycophenolate mofetil, tacrolimus, dapsone, azathioprine, and infliximab may be tried (SOR: C).7
Immunoglobulin, plasmapheresis, and cyclophosphamide have shown some efficacy in patients without systemic disease (SOR: C).7 Tumor necrosis factor-α inhibitor infliximab (Remicade) is considered first-line therapy for pyoderma gangrenosum associated with Crohn’s disease (SOR: B).7
The patient’s outcome
The patient did not improve after initial treatment with high-dose systemic corticosteroids. Infliximab in combination with methotrexate dramatically improved her skin and joint lesions and induced remission of Crohn’s disease. Early on, during the active phase of the disease, she also had surgical debridement of the lesions due to confounding diagnosis.
The patient has continued with infliximab infusions every 2 months and weekly oral methotrexate to maintain remission. Data show that 56% of patients with pyoderma gangrenosum require long-term therapy to prevent recurrences.8
This case is interesting because the bullous lesions of pyoderma gangrenosum appeared over the joints, mimicking septic arthritis and resulting in preventable surgical debridement. A case report with pyoderma gangrenosum lesions predominantly over the joints has not been described in the published literature. Recognition of lesions over the joints is important because treatment remains nonsurgical and surgery may exacerbate these skin lesions.
CORRESPONDENCE
Shashi Mittal, MD, Faculty Director of Research, Baylor Family Medicine Residency at Garland, Suite 340, Clara Barton Blvd, Garland, TX 75042.
A 46-year-old African American woman was admitted to the hospital with complaints of multiple joint pains associated with skin lesions, which had lasted for 3 weeks. The pains were in her right ankle, left wrist, both great toes, and right thumb. Further systems review revealed that the patient had experienced a fever of 102°F, diarrhea (at times bloody), chills, nausea, and decreased appetite for 5 days along with low back pain for 1 week.
Her medical history was significant for hypertension, hypothyroidism, chronic gastrointestinal symptoms attributed to irritable bowel disease for the past 2 years, and intermittent asymmetrical oligoarthritis for 1 year that had responded to episodic treatment with prednisone. She also had 2 episodes of iritis in the previous 4 years responsive to topical corticosteroids.
On exam she looked sick, was febrile, and had skin lesions in vaginal, axillary, perianal, foot, and thigh areas (FIGURES 1 AND 2). The lesions were 5 to 40 mm in size, exquisitely tender, bullous, pustular, and formed violaceous ulcers with scanty purulent-looking discharge. Her ankle exam was significant for swelling, redness, warmth, tenderness and severe pain on passive and active movements (FIGURE 3). Results of an examination of her spine was within normal limits. The remainder of her physical exam also showed normal results.
FIGURE 1
Ulcer on thigh
FIGURE 2
Lesion on foot
FIGURE 3
Swollen ankle joint
What is your diagnosis?
Differential diagnosis
The primary differential was focused on joint symptoms, and included disseminated gonococcal, bacterial, atypical infections, Behçet’s disease, rheumatoid arthritis, Reiter’s disease, reactive and inflammatory bowel disease (IBD)-related arthritis.
Differential diagnosis for the skin lesions included pyoderma gangrenosum, Sweets syndrome, sexually transmitted diseases, granulomatous skin lesions of inflammatory bowel disease, Reiter’s, Behçet’s, vasculitis (Wegener’s granulamatosis, polyarteritis nodosa, cryoglobulinemia), cancer (lymphoma cutis, leukemia cutis, Langerhans cell histiocytosis), infectious (sporotrichosis, blastomycosis, local fungal), drug-induced, exogenous tissue injury, vaso-occlusive (antiphospholipid-antibody syndrome).3
Further testing was necessary to determine the diagnosis.
Laboratory results
The following laboratory tests were ordered.
Blood tests. Patient’s complete blood count was remarkable for anemia and thrombocytosis; a comprehensive metabolic panel showed hypoalbuminemia. Erythrocyte sedimentation rate was elevated at 120 mm/h and C-reactive protein was elevated at 24.9 mg/L.
A diagnostic tap of the ankle showed fluid with inflammatory changes, and cultures for aerobic, anaerobic, acid fast bacilli, fungus, gonorrhea, and herpes were negative.
Joints. The patient’s joints were suspected to have septic arthritis, both clinically and on magnetic resonance imaging exam; they demonstrated no improvement of symptoms with intravenous antibiotics. Early on, the orthopedic service recommended joint debridement and open drainage.
Surgical findings included epidermolysis with subcutaneous fluid and purulent-looking material. Progressive necrosis of the debrided margins occurred over the next few days.
Workup for possible rheumatological joint disease included antinuclear antibody, rheumatoid factor, cyclic citrullinated peptide antibody (anti-CCP antibody), and human leukocyte antigens (HLA) B27 and B51. Radiographs of involved joints did not show rheumatic joint disease.
Rheumatoid arthritis was doubtful due to lack of sufficient criteria for diagnosis, and negative rheumatoid factor and anti-CCP antibody.5 HLA B27 is positive in 80% of patients with Reiter’s syndrome, 60% of those with reactive arthritis, and 50% of those with IBD arthritis.6 HLA B51, although not diagnostic, has a higher than baseline prevalence in Behçet’s disease.4 Recurrent oral aphthae, a major criteria for Behçet’s, was absent in our patient.
Gastrointestinal. A preliminary gastrointestinal evaluation included stool culture and tests for clostridium difficile toxin, viral antibodies for cytomegalovirus (CMV), parvovirus, and herpes—all results were negative.
Colonoscopy, however, demonstrated colonic ulcers (mainly of the right colon) with skip pattern associated with polypoid lesions, mild terminal ileitis, and normal-looking left colon and rectum (FIGURE 4). Colonic biopsy showed crypt distortion and abscesses, terminal ileitis, absence of granuloma formation, and negative immunostain for CMV. Colonoscopy findings along with colon biopsy were suggestive of Crohn’s disease.
Conclusions from the lab tests. Blood, urine, and skin lesion cultures remained negative. Urethral and vaginal cultures for gonorrhea, chlamydia, herpes virus, and serum test for syphilis, HIV, and hepatitis B and C were all negative. Biopsy of skin lesions showed dermal mixed inflammatory infiltrate predominantly neutrophilic, and no vasculitis or micropathogens. Absence of micropathogens, vasculitis, vascular thrombosis, and lack of neoplastic cells made infectious, vasculitic, vaso-occlusive and malignant ulcers implausible.
There is no specific laboratory or pathological finding for diagnosis of pyoderma gangrenosum. Diagnosis is based on exclusion of other ulcerative conditions. Negative skin, arthrocentesis, joint fluid and tissue cultures, progressive necrosis of debrided tissue margins (pathergy), and skin biopsy suggested a diagnosis of pyoderma gangrenosum.
Joint lesions were considered to be atypical pyoderma gangrenosum. Investigations to diagnose or exclude a concomitant systemic disorder are required in patients with pyoderma gangrenosum. This patient had Crohn’s disease associated with pyoderma gangrenosum.
FIGURE 4
Colonoscopy findings
Diagnosis: Pyoderma gangrenosum
Pyoderma gangrenosum is a rare, ulcerative, neutrophilic dermatosis of uncertain etiology. It occurs in 1 in 100,000 people in the US per year.1 Fifty percent of patients with pyoderma gangrenosum have an underlying disorder such as inflammatory bowel disease, arthritis, hematological malignancy, infections, sarcoidosis, hypogammaglobulinemia, or HIV.2 Pyoderma gangrenosum occurs in 2% of patients with Crohn’s disease.9
Variants of pyoderma gangrenosum
The 2 primary variants are classical and atypical. Four types of lesions are seen.2
Ulcerative. Ulcerative pyoderma gangrenosum a classical form of disease and occurs usually in lower limb and trunk. Lesions begin as pustules or as a pathergic phenomenon (development of ulcer at site of minimal trauma). This progresses to a large violaceous ulcer with undermined borders and surrounding halo of erythema. The lesions are distinctively painful.
Pyoderma gangrenosum may occur on the genitalia and would need to be differentiated from sexually transmitted disease. Extracutaneous sterile neutrophilic abscesses have been reported in lungs, eyes, liver, spleen, bones, heart, central nervous system, and gastrointestinal tract.
Pustular. Present as painful postulations on extensor surfaces of the limbs. Pyostomatitis vegetans is a variant of this form and occurs in the oral cavity.
Bullous/atypical. Bullae most often occur on hands, forearm, or face. Most commonly associated with hematological malignancy.
Vegetative. Chronic, nonpainful superficial ulcer.
Treatment: Topical vs systemic
To date there are no established guidelines for treatment of pyoderma gangrenosum. Treatment of underlying disease often results in improvement of pyoderma gangrenosum.
Treat localized cases topically
Localized pyoderma gangrenosum may initially be treated with corticosteroids (topical or intralesional) or tacrolimus (SOR: B),7 reserving systemic treatment for refractory cases.
Topical care of the lesions along with systemic therapy should be limited to antiseptic or occlusive wound dressing in an effort to minimize secondary bacterial infection.
Surgery not recommended in active disease
Adjuvant surgery such as excision of ulcer, skin grafting, etc, has not been shown to reduce morbidity even when done in conjunction with systemic immunosuppressive therapy. Re-epithelialization procedures like debridements or allografts may be done during disease remission (SOR: C).7
These procedures should be avoided in active disease due to risk of pathergy and worsening of original lesions of pyoderma gangrenosum. Similarly, elective surgery should be avoided during active disease, and when necessary should be done alongside systemic pyoderma gangrenosum therapy.
Refractory cases
In refractory or idiopathic cases, disseminated pyoderma gangrenosum is treated systemically with corticosteroids or cyclosporine alone or in combination (strength of recommendation [SOR]: B).7 In steroid-resistant pyoderma gangrenosum, thalidomide, mycophenolate mofetil, tacrolimus, dapsone, azathioprine, and infliximab may be tried (SOR: C).7
Immunoglobulin, plasmapheresis, and cyclophosphamide have shown some efficacy in patients without systemic disease (SOR: C).7 Tumor necrosis factor-α inhibitor infliximab (Remicade) is considered first-line therapy for pyoderma gangrenosum associated with Crohn’s disease (SOR: B).7
The patient’s outcome
The patient did not improve after initial treatment with high-dose systemic corticosteroids. Infliximab in combination with methotrexate dramatically improved her skin and joint lesions and induced remission of Crohn’s disease. Early on, during the active phase of the disease, she also had surgical debridement of the lesions due to confounding diagnosis.
The patient has continued with infliximab infusions every 2 months and weekly oral methotrexate to maintain remission. Data show that 56% of patients with pyoderma gangrenosum require long-term therapy to prevent recurrences.8
This case is interesting because the bullous lesions of pyoderma gangrenosum appeared over the joints, mimicking septic arthritis and resulting in preventable surgical debridement. A case report with pyoderma gangrenosum lesions predominantly over the joints has not been described in the published literature. Recognition of lesions over the joints is important because treatment remains nonsurgical and surgery may exacerbate these skin lesions.
CORRESPONDENCE
Shashi Mittal, MD, Faculty Director of Research, Baylor Family Medicine Residency at Garland, Suite 340, Clara Barton Blvd, Garland, TX 75042.
1. Jackson JM, Callen JP. Pyoderma gangrenosum. Available at: Emedicine.com. Accessed on March 21, 2006.
2. Wines N, Wines M, Ryman W. Understanding pyoderma gangrenosum: A Review.Available at www.Medscape.com. Accessed on March 21, 2006.
3. Weenig RH, Davis MD, Dahl PR, Su WP. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med 2002;347:1412-1418.
4. Smith EL. Clinical manifestations and diagnosis of Behcet’s disease. Available at www.uptodate.com. Accessed on April 2, 2006.
5. Venables PJW, Maini RN. Diagnosis and differential diagnosis of Rheumatoid arthritis. Available at www.uptodate.com. Accessed on April 2, 2006.
6. Yu DT, Wiesenhutter CW. Course and treatment of Reiter’s syndrome; reactive arthritis and undifferentiated spondyloarthropathy. Available at www.uptodate.com. Accessed on April 2, 2006.
7. Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol 2005;53:273-283.
8. Driesch VD. Pyoderma gangrenosum: a report of 44 cases with follow up. Br J Dermatol 1997;137:1000-1005.
9. Moschella SL. Neutrophilic dermatosis. Available at www.uptodate.com.
1. Jackson JM, Callen JP. Pyoderma gangrenosum. Available at: Emedicine.com. Accessed on March 21, 2006.
2. Wines N, Wines M, Ryman W. Understanding pyoderma gangrenosum: A Review.Available at www.Medscape.com. Accessed on March 21, 2006.
3. Weenig RH, Davis MD, Dahl PR, Su WP. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med 2002;347:1412-1418.
4. Smith EL. Clinical manifestations and diagnosis of Behcet’s disease. Available at www.uptodate.com. Accessed on April 2, 2006.
5. Venables PJW, Maini RN. Diagnosis and differential diagnosis of Rheumatoid arthritis. Available at www.uptodate.com. Accessed on April 2, 2006.
6. Yu DT, Wiesenhutter CW. Course and treatment of Reiter’s syndrome; reactive arthritis and undifferentiated spondyloarthropathy. Available at www.uptodate.com. Accessed on April 2, 2006.
7. Reichrath J, Bens G, Bonowitz A, Tilgen W. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol 2005;53:273-283.
8. Driesch VD. Pyoderma gangrenosum: a report of 44 cases with follow up. Br J Dermatol 1997;137:1000-1005.
9. Moschella SL. Neutrophilic dermatosis. Available at www.uptodate.com.
Two students, 2 tropical beaches, 2 injured feet
A 21-year-old previously healthy male went to his college health center, reporting that he had injured his foot in Columbia while he was on winter break. Two weeks before this clinic visit, he felt a “scrape” on the bottom of his foot while walking on the beach. One week later, the site began to itch and was progressively enlarging. He had no systemic symptoms and no pain at the site. Physical examination showed a 3-cm-wide indurated filamentous/serpiginous eruption with surrounding erythema, and a crusting central “puncture” (FIGURE 1).
The patient seen immediately following the patient above was a 19-year-old female college student. For the last 1 to 2 weeks she had an enlarging, very pruritic eruption on the sole of her foot that she attributed to a bug bite. She had just returned from winter vacation in Brazil, where she recalled spending a lot of time on the beach. The patient was otherwise healthy and did not have fever, other rash, or joint pain. Examination revealed an indurated, erythematous, serpiginous eruption on the plantar aspect of her left foot (FIGURE 2).
FIGURE 1
Patient 1
FIGURE 2
Patient 2
What is your diagnosis for both cases?
How would you treat them, and advise for prevention of future similar problems?
Diagnosis: Cutaneous larva migrans
Cutaneous larva migrans (CLM, sometimes called the “creeping eruption”) occurs when the third-stage larvae of dog (or cat) hookworm (Ancylostoma braziliense and A caninum, most commonly) invade the skin and fail to penetrate the epidermal basement layer. Humans are not the definitive host for this roundworm. Skin manifestations occur due to a hypersensitivity reaction.
The disease is found most commonly in tropical and subtropical climates. Animals defecate on warm, moist sand and the nematode eggs hatch. The larvae quickly invade bare skin in contact with the sand. The highest incidence of infection in the US occurs in Florida and along the Gulf Coast, but the disease is more common in the Caribbean, Central and South America, and Southeast Asia and Africa.
Many patients report a stinging sensation when the larva penetrates, as did patients 1 and 2, who misinterpreted the pain as a puncture (patient 1) or an insect bite (patient 2). Larvae migrate at a rate of several millimeters per day. Migration produces an intensely pruritic, linear or serpiginous track 2 to 4 mm wide, sometimes with vesicles, as the body reacts to the larva or to its excreta. Because each larva leaves an individual track in its wake, lesions may be simple or quite complex, depending upon the number of infecting organisms. A second morphologic variant has been described with follicular papules and pustules accompanying linear burrows.1 Eventually, the larvae die and the condition resolves even without treatment, but treatment accelerates resolution. The lesions may occur anywhere on the body, but most commonly are found on the feet, buttocks, and hands.
Differential diagnosis
The typical lesion is usually a straightforward diagnosis (once the observer is familiar with the infection), but differential diagnosis includes tinea corporis, granuloma annulare, erythema migrans (Lyme disease), ground itch, larva currens, myiasis, and stings by marine invertebrates. In very rare cases, larvae may migrate to the lungs, causing pneumonitis.2
Management of CLM
Among the various treatments, no randomized trials exist comparing their efficacy. Caumes et al1 recommend a single dose of oral ivermectin 12 mg weekly until cure, or oral albendazole 400 mg twice daily for 3 consecutive days. Others recommend ivermectin 200 mcg/kg, repeated once or twice,3 thiabendazole 500 mg 4 times daily for 5 days,4 or even topical thiabendazole 10% cream,5 although creams may be less effective. Topical thiabendazole is a good alternative for young children to avoid the potential side effects of systemic medications.
Folliculitis from the larva may be relatively treatment-resistant, requiring a longer duration of treatment to effect cure (strength of recommendation [SOR]: C).
Outcome and prevention
Both students were treated with ivermectin 15 mg as a single dose, with prompt resolution of symptoms. They were counseled to wear beach shoes and not to sit directly on sand while on the beach.
Additional reading: Brenner MA, Patel MB. Cutaneous larva migrans: the creeping eruption. Cutis 2003; 72:111—115.
1. Caumes E, Ly F, Bricaire F. Cutaneous larva migrans with folliculitis: report of seven cases and review of the literature. Br J Dermatol 2002;146:314-316.
2. Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med 2004;351:799-807.
3. Bouchaud O, Houze S, Schiemann R, et al. Cutaneous larva migrans in travelers: a prospective study, with assessment of therapy with ivermectin. Clin Infect Dis 2001;31:493-498.
4. O’Quinn JC, Dushin R. Cutaneous larva migrans: case report with current recommendations for treatment. J Am Pod Med Assoc 2005;95:291-294.
5. Blackwell V, Vega-Lopez F. Cutaneous larva migrans: clinical features and management of 44 cases presenting in the returning traveler. Br J Dermatol 2001;145:434-437.
A 21-year-old previously healthy male went to his college health center, reporting that he had injured his foot in Columbia while he was on winter break. Two weeks before this clinic visit, he felt a “scrape” on the bottom of his foot while walking on the beach. One week later, the site began to itch and was progressively enlarging. He had no systemic symptoms and no pain at the site. Physical examination showed a 3-cm-wide indurated filamentous/serpiginous eruption with surrounding erythema, and a crusting central “puncture” (FIGURE 1).
The patient seen immediately following the patient above was a 19-year-old female college student. For the last 1 to 2 weeks she had an enlarging, very pruritic eruption on the sole of her foot that she attributed to a bug bite. She had just returned from winter vacation in Brazil, where she recalled spending a lot of time on the beach. The patient was otherwise healthy and did not have fever, other rash, or joint pain. Examination revealed an indurated, erythematous, serpiginous eruption on the plantar aspect of her left foot (FIGURE 2).
FIGURE 1
Patient 1
FIGURE 2
Patient 2
What is your diagnosis for both cases?
How would you treat them, and advise for prevention of future similar problems?
Diagnosis: Cutaneous larva migrans
Cutaneous larva migrans (CLM, sometimes called the “creeping eruption”) occurs when the third-stage larvae of dog (or cat) hookworm (Ancylostoma braziliense and A caninum, most commonly) invade the skin and fail to penetrate the epidermal basement layer. Humans are not the definitive host for this roundworm. Skin manifestations occur due to a hypersensitivity reaction.
The disease is found most commonly in tropical and subtropical climates. Animals defecate on warm, moist sand and the nematode eggs hatch. The larvae quickly invade bare skin in contact with the sand. The highest incidence of infection in the US occurs in Florida and along the Gulf Coast, but the disease is more common in the Caribbean, Central and South America, and Southeast Asia and Africa.
Many patients report a stinging sensation when the larva penetrates, as did patients 1 and 2, who misinterpreted the pain as a puncture (patient 1) or an insect bite (patient 2). Larvae migrate at a rate of several millimeters per day. Migration produces an intensely pruritic, linear or serpiginous track 2 to 4 mm wide, sometimes with vesicles, as the body reacts to the larva or to its excreta. Because each larva leaves an individual track in its wake, lesions may be simple or quite complex, depending upon the number of infecting organisms. A second morphologic variant has been described with follicular papules and pustules accompanying linear burrows.1 Eventually, the larvae die and the condition resolves even without treatment, but treatment accelerates resolution. The lesions may occur anywhere on the body, but most commonly are found on the feet, buttocks, and hands.
Differential diagnosis
The typical lesion is usually a straightforward diagnosis (once the observer is familiar with the infection), but differential diagnosis includes tinea corporis, granuloma annulare, erythema migrans (Lyme disease), ground itch, larva currens, myiasis, and stings by marine invertebrates. In very rare cases, larvae may migrate to the lungs, causing pneumonitis.2
Management of CLM
Among the various treatments, no randomized trials exist comparing their efficacy. Caumes et al1 recommend a single dose of oral ivermectin 12 mg weekly until cure, or oral albendazole 400 mg twice daily for 3 consecutive days. Others recommend ivermectin 200 mcg/kg, repeated once or twice,3 thiabendazole 500 mg 4 times daily for 5 days,4 or even topical thiabendazole 10% cream,5 although creams may be less effective. Topical thiabendazole is a good alternative for young children to avoid the potential side effects of systemic medications.
Folliculitis from the larva may be relatively treatment-resistant, requiring a longer duration of treatment to effect cure (strength of recommendation [SOR]: C).
Outcome and prevention
Both students were treated with ivermectin 15 mg as a single dose, with prompt resolution of symptoms. They were counseled to wear beach shoes and not to sit directly on sand while on the beach.
Additional reading: Brenner MA, Patel MB. Cutaneous larva migrans: the creeping eruption. Cutis 2003; 72:111—115.
A 21-year-old previously healthy male went to his college health center, reporting that he had injured his foot in Columbia while he was on winter break. Two weeks before this clinic visit, he felt a “scrape” on the bottom of his foot while walking on the beach. One week later, the site began to itch and was progressively enlarging. He had no systemic symptoms and no pain at the site. Physical examination showed a 3-cm-wide indurated filamentous/serpiginous eruption with surrounding erythema, and a crusting central “puncture” (FIGURE 1).
The patient seen immediately following the patient above was a 19-year-old female college student. For the last 1 to 2 weeks she had an enlarging, very pruritic eruption on the sole of her foot that she attributed to a bug bite. She had just returned from winter vacation in Brazil, where she recalled spending a lot of time on the beach. The patient was otherwise healthy and did not have fever, other rash, or joint pain. Examination revealed an indurated, erythematous, serpiginous eruption on the plantar aspect of her left foot (FIGURE 2).
FIGURE 1
Patient 1
FIGURE 2
Patient 2
What is your diagnosis for both cases?
How would you treat them, and advise for prevention of future similar problems?
Diagnosis: Cutaneous larva migrans
Cutaneous larva migrans (CLM, sometimes called the “creeping eruption”) occurs when the third-stage larvae of dog (or cat) hookworm (Ancylostoma braziliense and A caninum, most commonly) invade the skin and fail to penetrate the epidermal basement layer. Humans are not the definitive host for this roundworm. Skin manifestations occur due to a hypersensitivity reaction.
The disease is found most commonly in tropical and subtropical climates. Animals defecate on warm, moist sand and the nematode eggs hatch. The larvae quickly invade bare skin in contact with the sand. The highest incidence of infection in the US occurs in Florida and along the Gulf Coast, but the disease is more common in the Caribbean, Central and South America, and Southeast Asia and Africa.
Many patients report a stinging sensation when the larva penetrates, as did patients 1 and 2, who misinterpreted the pain as a puncture (patient 1) or an insect bite (patient 2). Larvae migrate at a rate of several millimeters per day. Migration produces an intensely pruritic, linear or serpiginous track 2 to 4 mm wide, sometimes with vesicles, as the body reacts to the larva or to its excreta. Because each larva leaves an individual track in its wake, lesions may be simple or quite complex, depending upon the number of infecting organisms. A second morphologic variant has been described with follicular papules and pustules accompanying linear burrows.1 Eventually, the larvae die and the condition resolves even without treatment, but treatment accelerates resolution. The lesions may occur anywhere on the body, but most commonly are found on the feet, buttocks, and hands.
Differential diagnosis
The typical lesion is usually a straightforward diagnosis (once the observer is familiar with the infection), but differential diagnosis includes tinea corporis, granuloma annulare, erythema migrans (Lyme disease), ground itch, larva currens, myiasis, and stings by marine invertebrates. In very rare cases, larvae may migrate to the lungs, causing pneumonitis.2
Management of CLM
Among the various treatments, no randomized trials exist comparing their efficacy. Caumes et al1 recommend a single dose of oral ivermectin 12 mg weekly until cure, or oral albendazole 400 mg twice daily for 3 consecutive days. Others recommend ivermectin 200 mcg/kg, repeated once or twice,3 thiabendazole 500 mg 4 times daily for 5 days,4 or even topical thiabendazole 10% cream,5 although creams may be less effective. Topical thiabendazole is a good alternative for young children to avoid the potential side effects of systemic medications.
Folliculitis from the larva may be relatively treatment-resistant, requiring a longer duration of treatment to effect cure (strength of recommendation [SOR]: C).
Outcome and prevention
Both students were treated with ivermectin 15 mg as a single dose, with prompt resolution of symptoms. They were counseled to wear beach shoes and not to sit directly on sand while on the beach.
Additional reading: Brenner MA, Patel MB. Cutaneous larva migrans: the creeping eruption. Cutis 2003; 72:111—115.
1. Caumes E, Ly F, Bricaire F. Cutaneous larva migrans with folliculitis: report of seven cases and review of the literature. Br J Dermatol 2002;146:314-316.
2. Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med 2004;351:799-807.
3. Bouchaud O, Houze S, Schiemann R, et al. Cutaneous larva migrans in travelers: a prospective study, with assessment of therapy with ivermectin. Clin Infect Dis 2001;31:493-498.
4. O’Quinn JC, Dushin R. Cutaneous larva migrans: case report with current recommendations for treatment. J Am Pod Med Assoc 2005;95:291-294.
5. Blackwell V, Vega-Lopez F. Cutaneous larva migrans: clinical features and management of 44 cases presenting in the returning traveler. Br J Dermatol 2001;145:434-437.
1. Caumes E, Ly F, Bricaire F. Cutaneous larva migrans with folliculitis: report of seven cases and review of the literature. Br J Dermatol 2002;146:314-316.
2. Hotez PJ, Brooker S, Bethony JM, Bottazzi ME, Loukas A, Xiao S. Hookworm infection. N Engl J Med 2004;351:799-807.
3. Bouchaud O, Houze S, Schiemann R, et al. Cutaneous larva migrans in travelers: a prospective study, with assessment of therapy with ivermectin. Clin Infect Dis 2001;31:493-498.
4. O’Quinn JC, Dushin R. Cutaneous larva migrans: case report with current recommendations for treatment. J Am Pod Med Assoc 2005;95:291-294.
5. Blackwell V, Vega-Lopez F. Cutaneous larva migrans: clinical features and management of 44 cases presenting in the returning traveler. Br J Dermatol 2001;145:434-437.