User login
Why mRNA COVID vaccines are preferred (and why patients should be reassured)
On December 16, 2021, the Advisory Committee on Immunization Practices (ACIP) voted to preferentially recommend messenger RNA (mRNA) vaccines over the Johnson & Johnson/Janssen (J&J) COVID-19 (Ad.26.COV2.S) adenovirus vector vaccine for prevention of COVID-19.1 The mRNA vaccines include Pfizer-BioNTech COVID-19 (BNT162b2) and Moderna COVID-19 (mRNA-1273).
The reason for this preferential recommendation is a rare but serious adverse reaction—thrombosis with thrombocytopenia (TTS) —that has been associated with the J&J vaccine. As of December 8, 2021, more than 16.9 million doses of the J&J COVID-19 vaccine have been given in the United States. The CDC has identified 57 confirmed reports of people who received this vaccine and later developed TTS.2 The known incidence of TTS is thus 1 per ~ 300,000 doses, although the rate may actually be higher.2 All cases have been documented as having occurred after administration of the J&J primary single-dose vaccine; none have been documented (so far) after the booster—although the number of booster doses of the J&J COVID-19 vaccine has been small.
Women between the ages of 30 and 50 years have the highest risk for TTS, with rates of 1 per 94,000 in those ages 30-39 and 1 per 111,000 for those ages 40-49.2,3 All those with TTS have been hospitalized, and 9 have died.2,3 While this adverse reaction is rare, the seriousness of it led the ACIP to state a preference for the mRNA vaccines.
The significance of the recommendation:
- Unless a person has a contraindication to an mRNA vaccine, they should receive 1 of these 2 vaccines for their primary series and boosters.
- The only “Mix and Match” that should occur with boosters is to follow a J&J/Janssen COVID-19 vaccine with an mRNA booster. At this time, booster doses following a 2-dose mRNA primary series should be with an mRNA vaccine.
- The recommendation is for adults ages 18 and older; however, the J&J/Janssen COVID-19 vaccine is not yet approved for younger age-groups.
- The J&J/Janssen COVID-19 vaccine remains an option for those who cannot receive an mRNA vaccine, but it should be administered only after full informed consent.
The J&J/Janssen COVID-19 vaccine initially looked promising a year ago because of its single-dose primary series and its much less stringent storage requirements. However, things have not quite panned out for the vaccine. Its effectiveness after a single dose has proven to be significantly inferior to the 2-dose mRNA vaccines, and it has now been associated with a very serious, albeit rare, adverse reaction.
The major take-home point for physicians to pass on to their patients is that the nation’s system for monitoring vaccine safety works. It can pick up serious adverse reactions that occur at a rate as low as 1/300,000. This should be reassuring.
1. CDC. CDC Endorses ACIP’s Updated COVID-19 Vaccine Recommendations [press release]. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html
2. CDC. Selected Adverse Events Reported after COVID-19 Vaccination. December 20, 2021. Accessed December 22, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
3. See I. Updates on thrombosis with thrombocytopenia syndrome (TTS). Presented to the Advisory Committee on Immunization Practices. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf
On December 16, 2021, the Advisory Committee on Immunization Practices (ACIP) voted to preferentially recommend messenger RNA (mRNA) vaccines over the Johnson & Johnson/Janssen (J&J) COVID-19 (Ad.26.COV2.S) adenovirus vector vaccine for prevention of COVID-19.1 The mRNA vaccines include Pfizer-BioNTech COVID-19 (BNT162b2) and Moderna COVID-19 (mRNA-1273).
The reason for this preferential recommendation is a rare but serious adverse reaction—thrombosis with thrombocytopenia (TTS) —that has been associated with the J&J vaccine. As of December 8, 2021, more than 16.9 million doses of the J&J COVID-19 vaccine have been given in the United States. The CDC has identified 57 confirmed reports of people who received this vaccine and later developed TTS.2 The known incidence of TTS is thus 1 per ~ 300,000 doses, although the rate may actually be higher.2 All cases have been documented as having occurred after administration of the J&J primary single-dose vaccine; none have been documented (so far) after the booster—although the number of booster doses of the J&J COVID-19 vaccine has been small.
Women between the ages of 30 and 50 years have the highest risk for TTS, with rates of 1 per 94,000 in those ages 30-39 and 1 per 111,000 for those ages 40-49.2,3 All those with TTS have been hospitalized, and 9 have died.2,3 While this adverse reaction is rare, the seriousness of it led the ACIP to state a preference for the mRNA vaccines.
The significance of the recommendation:
- Unless a person has a contraindication to an mRNA vaccine, they should receive 1 of these 2 vaccines for their primary series and boosters.
- The only “Mix and Match” that should occur with boosters is to follow a J&J/Janssen COVID-19 vaccine with an mRNA booster. At this time, booster doses following a 2-dose mRNA primary series should be with an mRNA vaccine.
- The recommendation is for adults ages 18 and older; however, the J&J/Janssen COVID-19 vaccine is not yet approved for younger age-groups.
- The J&J/Janssen COVID-19 vaccine remains an option for those who cannot receive an mRNA vaccine, but it should be administered only after full informed consent.
The J&J/Janssen COVID-19 vaccine initially looked promising a year ago because of its single-dose primary series and its much less stringent storage requirements. However, things have not quite panned out for the vaccine. Its effectiveness after a single dose has proven to be significantly inferior to the 2-dose mRNA vaccines, and it has now been associated with a very serious, albeit rare, adverse reaction.
The major take-home point for physicians to pass on to their patients is that the nation’s system for monitoring vaccine safety works. It can pick up serious adverse reactions that occur at a rate as low as 1/300,000. This should be reassuring.
On December 16, 2021, the Advisory Committee on Immunization Practices (ACIP) voted to preferentially recommend messenger RNA (mRNA) vaccines over the Johnson & Johnson/Janssen (J&J) COVID-19 (Ad.26.COV2.S) adenovirus vector vaccine for prevention of COVID-19.1 The mRNA vaccines include Pfizer-BioNTech COVID-19 (BNT162b2) and Moderna COVID-19 (mRNA-1273).
The reason for this preferential recommendation is a rare but serious adverse reaction—thrombosis with thrombocytopenia (TTS) —that has been associated with the J&J vaccine. As of December 8, 2021, more than 16.9 million doses of the J&J COVID-19 vaccine have been given in the United States. The CDC has identified 57 confirmed reports of people who received this vaccine and later developed TTS.2 The known incidence of TTS is thus 1 per ~ 300,000 doses, although the rate may actually be higher.2 All cases have been documented as having occurred after administration of the J&J primary single-dose vaccine; none have been documented (so far) after the booster—although the number of booster doses of the J&J COVID-19 vaccine has been small.
Women between the ages of 30 and 50 years have the highest risk for TTS, with rates of 1 per 94,000 in those ages 30-39 and 1 per 111,000 for those ages 40-49.2,3 All those with TTS have been hospitalized, and 9 have died.2,3 While this adverse reaction is rare, the seriousness of it led the ACIP to state a preference for the mRNA vaccines.
The significance of the recommendation:
- Unless a person has a contraindication to an mRNA vaccine, they should receive 1 of these 2 vaccines for their primary series and boosters.
- The only “Mix and Match” that should occur with boosters is to follow a J&J/Janssen COVID-19 vaccine with an mRNA booster. At this time, booster doses following a 2-dose mRNA primary series should be with an mRNA vaccine.
- The recommendation is for adults ages 18 and older; however, the J&J/Janssen COVID-19 vaccine is not yet approved for younger age-groups.
- The J&J/Janssen COVID-19 vaccine remains an option for those who cannot receive an mRNA vaccine, but it should be administered only after full informed consent.
The J&J/Janssen COVID-19 vaccine initially looked promising a year ago because of its single-dose primary series and its much less stringent storage requirements. However, things have not quite panned out for the vaccine. Its effectiveness after a single dose has proven to be significantly inferior to the 2-dose mRNA vaccines, and it has now been associated with a very serious, albeit rare, adverse reaction.
The major take-home point for physicians to pass on to their patients is that the nation’s system for monitoring vaccine safety works. It can pick up serious adverse reactions that occur at a rate as low as 1/300,000. This should be reassuring.
1. CDC. CDC Endorses ACIP’s Updated COVID-19 Vaccine Recommendations [press release]. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html
2. CDC. Selected Adverse Events Reported after COVID-19 Vaccination. December 20, 2021. Accessed December 22, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
3. See I. Updates on thrombosis with thrombocytopenia syndrome (TTS). Presented to the Advisory Committee on Immunization Practices. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf
1. CDC. CDC Endorses ACIP’s Updated COVID-19 Vaccine Recommendations [press release]. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/media/releases/2021/s1216-covid-19-vaccines.html
2. CDC. Selected Adverse Events Reported after COVID-19 Vaccination. December 20, 2021. Accessed December 22, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html
3. See I. Updates on thrombosis with thrombocytopenia syndrome (TTS). Presented to the Advisory Committee on Immunization Practices. December 16, 2021. Accessed December 22, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-12-16/02-COVID-See-508.pdf
2021 CDC guidelines on sexually transmitted infections
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.

Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1

Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
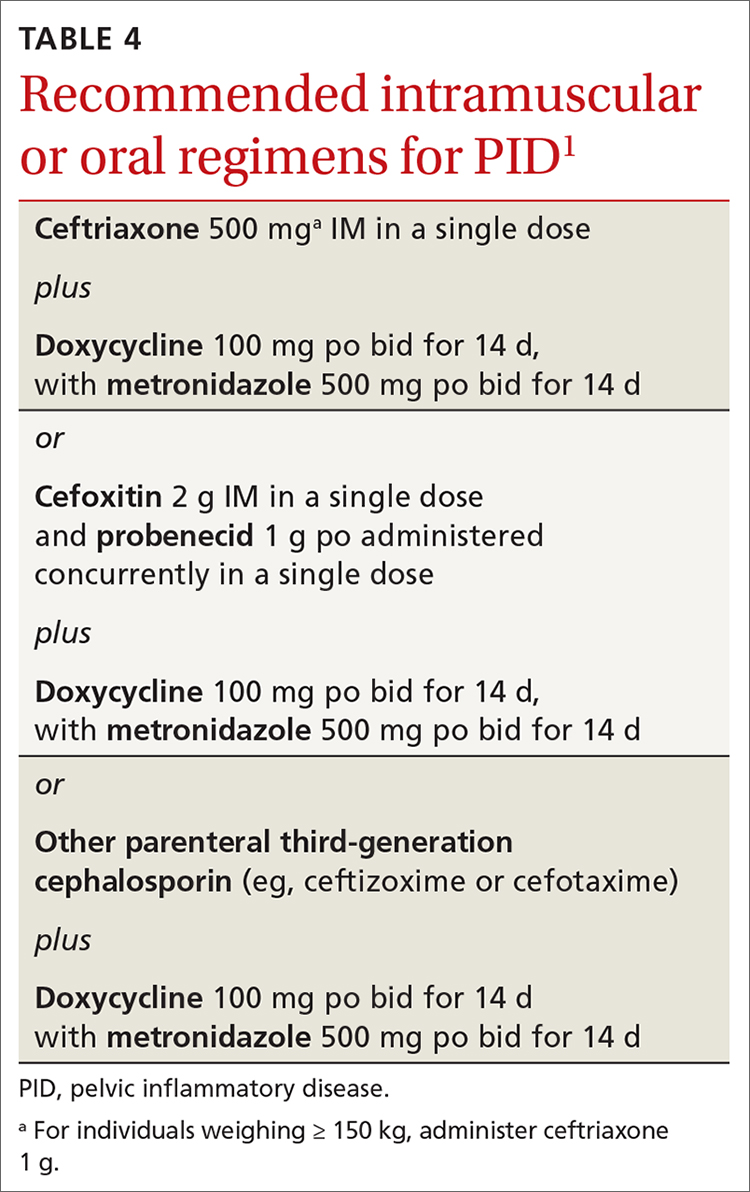
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.
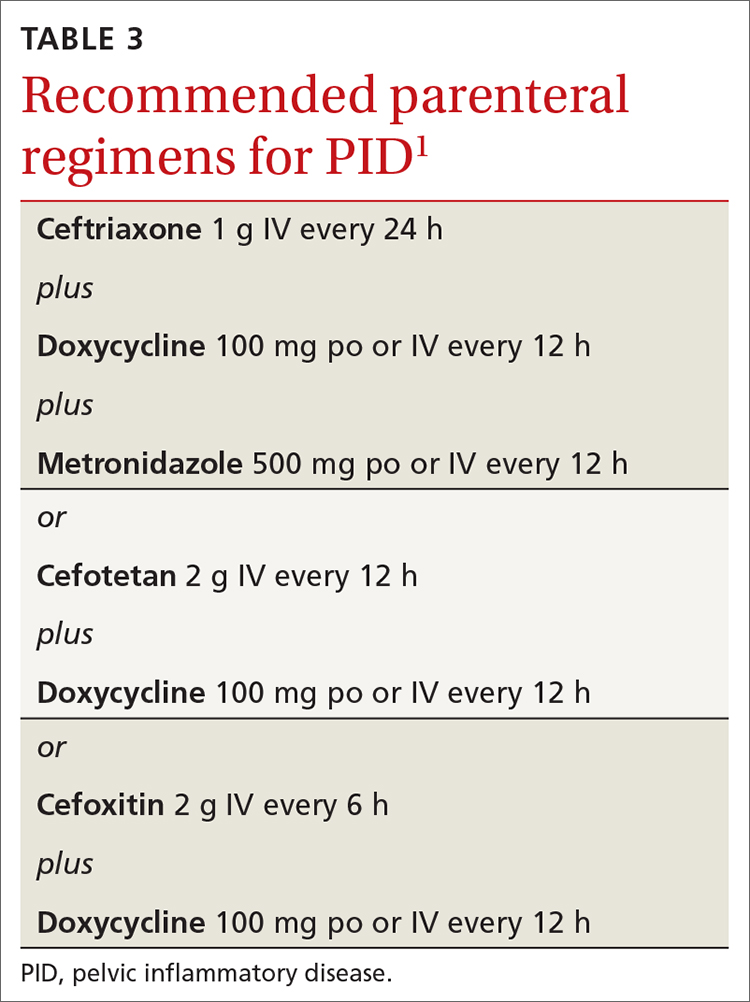
To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.
Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.

Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1

Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.

To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.

Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
In July 2021, the Centers for Disease Control and Prevention (CDC) published its updated guidelines on the diagnosis, treatment, and prevention of sexually transmitted infections (STIs).1 These guidelines were last published in 2015.2 Family physicians should be familiar with these guidelines as they are considered the standard of care for the treatment and prevention of STIs.
To revise the guidelines, the CDC convened a large panel that included CDC staff and subject matter experts from around the country. Using methodology borrowed from the US Preventive Services Task Force (USPSTF),3 the panel developed key questions and completed systematic reviews using a standard approach. The evidence behind key recommendations was ranked as high, medium, or low. However, the specific recommendations presented in the published guidelines appear without strength-of-recommendation descriptions or rankings of the levels of evidence supporting them.
The CDC approach to STI control involves 5 strategies (TABLE 1),1 which family physicians can implement as follows:
- Elicit an accurate sexual history.
- Discuss with patients and advise them on preventive interventions including barrier methods, microbicides, vaccines, and HIV pre-exposure prophylaxis.
- Order recommended screening tests for specific STIs from all sites of potential infection.
- Recognize the signs and symptoms of STIs and order recommended tests for confirmation.
- Treat confirmed infections using current recommended medications.
- Seek to advise, evaluate, and treat sex partners of those with documented STIs, and offer expedited partner therapy if allowed by state law.
- Perform recommended follow-up services for treated individuals.

Details on each of these strategies can be found in the new guidelines and are described for each specific pathogen and for specific demographic groups. Recommendations on screening for asymptomatic STIs can be found on the USPSTF website.4
The first step leading to targeted prevention strategies such as behavioral counseling, vaccination, and screening involves taking an accurate and complete sexual history. The CDC offers a 5-step process it calls the “5 Ps approach” to gathering needed information (TABLE 2).1

Major updates on the treatment of specific infections
Gonorrhea
The current recommendation for treating uncomplicated gonococcal infections of the cervix, urethra, pharynx, and rectum in adults and adolescents weighing < 150 kg is ceftriaxone 500 mg intramuscularly (IM) as a single dose; give 1 g for those weighing ≥ 150 kg.1 If co-infection with chlamydia has not been ruled out, co-treatment with doxycycline 100 mg po twice a day for 7 days is also recommended.1
This differs from the first-line treatment recommended in the previous guideline, which was dual therapy with ceftriaxone 250 mg IM and azithromycin 1 g po as a single dose, regardless of testing results for chlamydia.2 The higher dose for ceftriaxone now recommended is due to a gradual decrease in gonorrhea susceptibility to cephalosporins in recent years, although complete resistance remains rare. The move away from universal dual therapy reflects a concern about antibiotic stewardship and the potential effects of antibiotics on the microbiome. The elimination of azithromycin from recommended first-line therapies is due to a 10-fold increase in the proportion of bacterium isolates demonstrating reduced susceptibility, as measured by minimal inhibitory concentrations in the past few years.
Continue to: If ceftriaxone...
If ceftriaxone is unavailable, there are 2 alternative regimens: gentamicin 240 mg IM in a single dose, plus azithromycin 2 g po in a single dose; or cefixime 800 mg po in a single dose.1 However, these alternatives are not recommended for gonococcal infection of the pharynx, for which ceftriaxone should be used.
Counsel those treated for gonorrhea to avoid sexual activity for 7 days after treatment and until all sex partners have been treated. Because of the high rates of asymptomatic infections, tell patients to refer those with whom they have had sexual contact during the previous 60 days for evaluation, testing, and presumptive treatment.
Following treatment with the recommended dose of ceftriaxone, performing a test of cure is not recommended, with 1 exception: those with confirmed pharyngeal infection should be tested to confirm treatment success 7 to 14 days after being treated. However, all those treated for gonorrhea should be seen again in 3 months and retested to rule out reinfection, regardless of whether they think their sex partners have been adequately treated.
Chlamydia
The recommended first-line therapy for chlamydia is now doxycycline 100 mg twice a day for 7 days, which has proven to be superior to azithromycin (which was recommended as first-line therapy in 2015) for urogenital chlamydia in men and anal chlamydia in both men and women.1,2 Alternatives to doxycycline include azithromycin 1 g po as a single dose or levofloxacin 500 mg po once a day for 7 days.1 No test of cure is recommended; but as with gonorrhea, retesting at 3 months is recommended because of the risk for re-infection.
Instruct patients treated for chlamydia to avoid sexual intercourse for 7 days after therapy is initiated or until symptoms, if present, have resolved. To reduce the chances of reinfection, advise treated individuals to abstain from sexual intercourse until all of their sex partners have been treated.
Continue to: Sex partners...
Sex partners in the 60 days prior to the patient’s onset of symptoms or diagnosis should be advised to seek evaluation, testing, and presumptive treatment.
Trichomonas
The recommended first-line treatment for trichomonas now differs for men and women: metronidazole 2 g po as a single dose for men, and metronidazole 500 mg po twice a day for 7 days for women.1 Tinidazole 2 g po as a single dose is an alternative for both men and women. Previously, the single metronidazole dose was recommended for men and women,2 but there is now evidence that the 7-day course is markedly superior in achieving a cure in women.
No test of cure is recommended, but women should be retested at 3 months because of a high rate of re-infection. Current sex partners should be treated presumptively, and treated patients and their partners should avoid sex until all current sex partners have been treated. Consider expedited partner therapy if allowed by state law.
Bacterial vaginosis
First-line treatment recommendations for bacterial vaginosis (BV) have not changed: metronidazole 500 mg po twice a day for 7 days, or metronidazole gel 0.75% intravaginally daily for 5 days, or clindamycin cream 2% intravaginally at bedtime for 7 days. Advise women to avoid sexual activity or to use condoms for the duration of the treatment regimen.
A test of cure is not recommended if symptoms resolve, and no treatment or evaluation of sex partners is recommended. The guidelines describe several treatment options for women who have frequent, recurrent BV. To help prevent recurrences, they additionally suggest treating male partners with metronidazole 400 mg po twice a day and with 2% clindamycin cream applied to the penis twice a day, both for 7 days.
Continue to: Pelvic inflammatory disease
Pelvic inflammatory disease
Recommended regimens for treating pelvic inflammatory disease (PID) have changed (TABLES 3 and 4).1 Women with mild or moderate PID can be treated with intramuscular or oral regimens, as outcomes with these regimens are equivalent to those seen with intravenous treatments. The nonintravenous options all include 3 antibiotics: a cephalosporin, doxycycline, and metronidazole.

To minimize disease transmission, instruct women to avoid sex until therapy is complete, their symptoms have resolved, and sex partners have been treated. Sex partners of those with PID in the 60 days prior to the onset of symptoms should be evaluated, tested, and presumptively treated for chlamydia and gonorrhea.

Follow through on public health procedures
STIs are an important set of diseases from a public health perspective. Family physicians have the opportunity to assist with the prevention and control of these infections through screening, making accurate diagnoses, and applying recommended treatments. When you suspect that a patient has an STI, test for the most common ones: gonorrhea, chlamydia, HIV, and syphilis. Report all confirmed diagnoses to the local public health department and be prepared to refer patients’ sexual contacts to the local public health department or to provide contact evaluation and treatment.
Vaccines against STIs include hepatitis B vaccine, human papillomavirus vaccine, and hepatitis A vaccine. Offer these vaccines to all previously unvaccinated adolescents and young adults as per recommendations from the Advisory Committee on Immunization Practices.5
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
1. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70:1-187.
2. Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64:1-137.
3. USPSTF. Methods and processes. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes
4. USPSTF. Recommendations. Infectious diseases. Accessed November 17, 2021. https://uspreventiveservicestaskforce.org/uspstf/topic_search_results?topic_status=P&category%5B%5D=18&searchterm=
5. CDC. Advisory Committee on Immunization Practices. COVID-19 ACIP vaccine recommendations. Accessed October 18, 2021. www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html
Influenza vaccine update, 2021-22
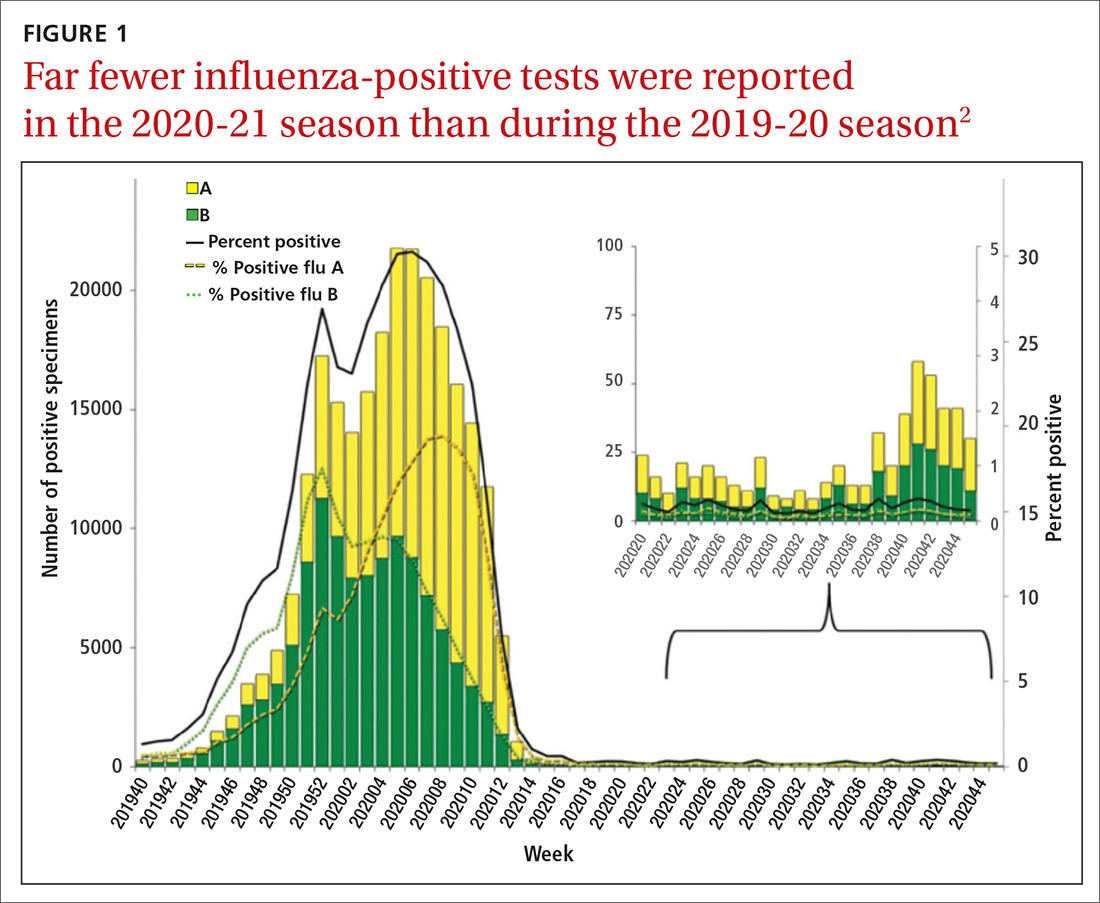
During the 2020-2021 influenza season, fewer cases of influenza were reported than in any previous year since 1997, when data were first recorded.1FIGURE 12 shows the dramatic decline in the number of influenza-positive clinical samples reported to the Centers for Disease Control and Prevention (CDC) during the 2020-2021 influenza season compared with the 2019-2020 season. There was only one pediatric death attributed to influenza in 2020-2021, compared with a mean of 177 per year in the previous 3 seasons.
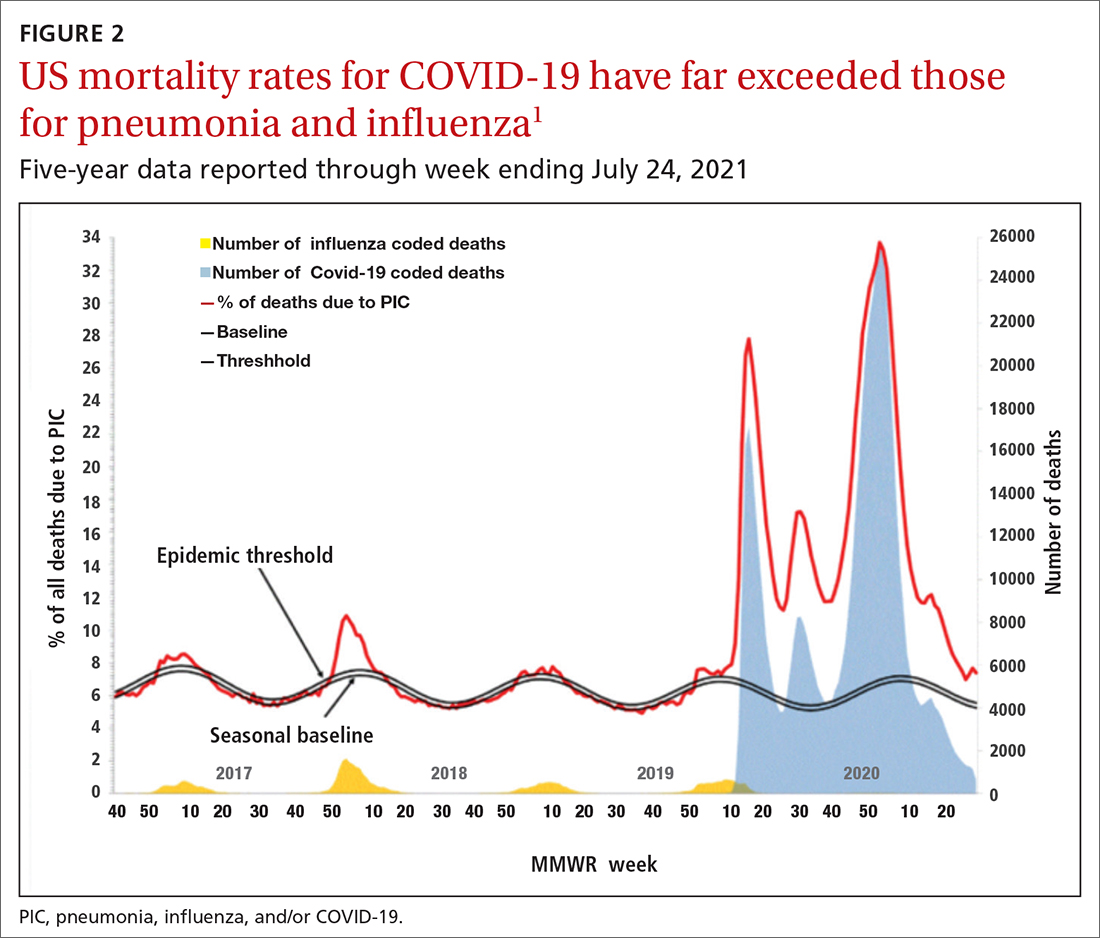
Deaths attributed to pneumonia and influenza were recorded over a recent 5-year period, with COVID-19 added in early mid-2020 (FIGURE 2).1 Total cumulative deaths for 2020-2021 were extremely high, mostly due to COVID-19. Of the relatively few influenza cases last season, 37.5% were caused by influenza A and 62.5% by influenza B. The extremely low incidence of influenza precludes determining influenza vaccine effectiveness for last season.1
In addition, other common respiratory pathogens—parainfluenza, adenoviruses, rhinoviruses, enteroviruses, and common coronaviruses—circulated last winter at historic lows.3 All of these historic lows can be attributed to the measures taken to mitigate the effect of the COVID-19 pandemic, including masks, social distancing, closure of certain venues that normally attract large crowds, and the closure of schools with a resulting increase in schooling at home. With the anticipated relaxation of these measures in 2021-2022, we can expect more influenza and other respiratory ailments due to common pathogens.
Updates to influenza vaccine recommendations
At its June 2021 meeting, the Advisory Committee on Immunization Practices (ACIP) approved the influenza vaccine recommendations for the 2021-2022 season.4 The central recommendation is unchanged: Everyone ≥ 6 months of age should receive a vaccine unless they have a contraindication. Updates to the previous recommendations include the content of the 2021 vaccines, the specific vaccines that will be available for different age groups, the timing of vaccine administration, advice on co-administration with COVID-19 vaccines, and the list of contraindications and precautions based on vaccine type.4
Viral composition of US vaccines for the 2021-22 season
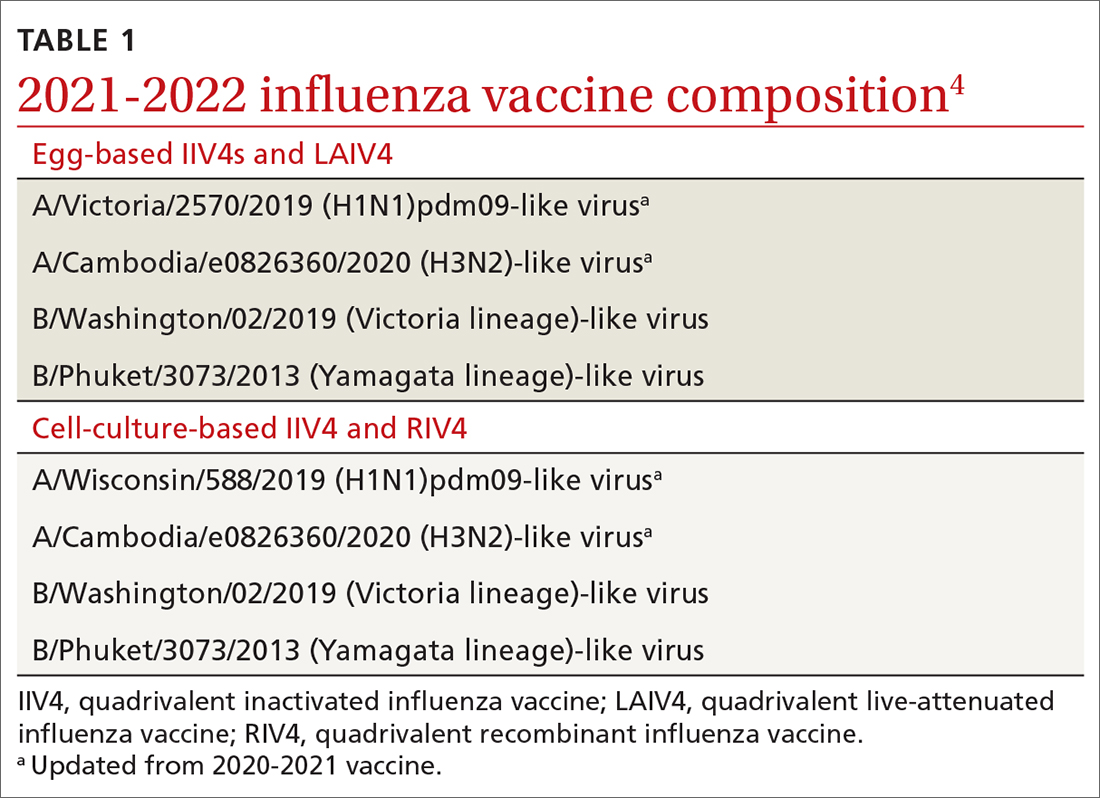
The antigens that will be included in the 2021-2022 influenza vaccines are listed in TABLE 1.4 The B strains are the same as last year; the A strains have been updated. The H3N2 strain is the same in all vaccines, but the H1N1 strain differs based on whether the vaccine is egg based or non-egg based. The advantage of non-egg-based vaccines is that the production process does not take as long and can be delayed in an attempt to better reflect the influenza stains in worldwide circulation.
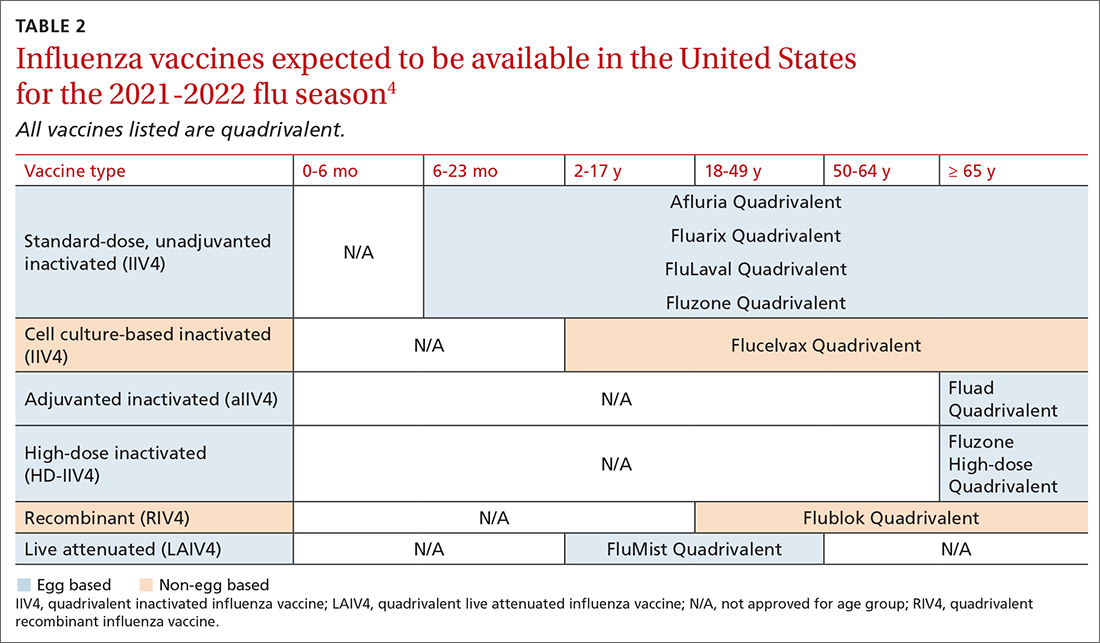
The influenza vaccines expected to be available for the 2021-22 season
TABLE 24 lists the influenza vaccines approved for use in the United States and the ages for which they are approved.4 All products for 2021-2022 will be quadrivalent, containing 2 type-A and 2 type-B antigens. The only change in age indications is that cell culture–based inactivated influenza vaccine (ccIIV4) (Flucelvax Quadrivalent) is now approved for use starting at age 2 years; previously it was approved starting at age 4 years.4
Timing of vaccination
The onsets and peaks of influenza disease occur at different times each year and can also vary by geographic location. An analysis of 36 influenza seasons starting in 1982 showed that peak activity occurred most frequently in February (15 seasons), followed by December (7 seasons), and January and March (6 seasons each).5 Only once did peak activity occur in October and once in November. This information, along with observational studies showing the waning of influenza vaccine effectiveness after 5 to 6 months, especially in the elderly, informed the ACIP decision to modify their recommendation on the timing of vaccination. The recommendation now states that vaccine should be administered by the end of October and that July and August would have been too early, especially for older adults.
Continue to: Children ages 6 months...
Children ages 6 months through 8 years who have not been vaccinated previously require 2 doses separated by at least 4 weeks, and the first dose should be administered early enough to allow for the second by the end of October.4 Children who require only 1 dose can also receive the vaccine as soon as it is available, as there is less evidence that vaccine effectiveness wanes in children.
Earlier administration is also recommended for pregnant women in their third trimester. Delaying vaccination in this group could result in postpartum administration of the vaccine, thereby depriving infants of protection against influenza illness during their first 6 months after birth.4
Co-administration of influenza and COVID-19 vaccines
Current guidance from the CDC states that COVID-19 vaccines can be co-administered with other vaccines including influenza vaccines.6 However, there are no data by which to judge the efficacy of each vaccine in coadministration or the potential for increased adverse reactions. ACIP advises caution on 2 points: (1) physicians should watch for updated guidance as more information becomes available, and (2) there is the potential for increased reactogenicity after co-administration, especially with the more reactogenic influenza vaccines: adjuvanted inactivated influenza vaccine (aIIV4) and high-dose inactivated influenza vaccine (HD-IIV4). Moreover, these vaccines and the co-administered COVID-19 vaccine should be injected into different limbs.
Contraindications and precautions
Serious allergic reactions to influenza vaccines are rare—about 1.3 incidents per million doses administered.7 However, a previous severe allergic reaction to a particular vaccine or to any component of the vaccine is a contraindication for use of that vaccine. In addition, a history of severe allergic reaction to any influenza vaccine is a contraindication for all egg-based vaccines.
There are 2 precautions for all influenza vaccines: a concurrent moderate or severe acute illness (with or without fever), and a history of Guillain-Barré syndrome within 6 weeks of receiving any influenza vaccine. An additional precaution for ccIIV4 and recombinant influenza vaccine (RIV4) is a history of severe allergic reaction after administration of any other influenza vaccine. Administration of RIV4 or ccIIV4 to someone with such a history should occur in a medical setting and be supervised by someone who can recognize and treat a severe reaction.
Continue to: Live attenuated influenza vaccine...
Live attenuated influenza vaccine (LAIV) continues to have a considerably longer list of contraindications, which can be found in the published recommendations for 2021-2022.8
Final advice
The upcoming influenza season has the potential to be clinically challenging with the possibility of co-existing high rates of both COVID-19 and influenza. Recommend both influenza and COVID-19 vaccination to patients. Also, be sure to encourage and practice other preventive measures such as masking in crowds, frequent hand washing, isolation when sick, respiratory hygiene, and (for physicians) selected prescribing of influenza antiviral medications and meticulous office-based infection control practices.9
1. CDC. Weekly U.S. influenza surveillance report. Accessed September 23, 2021. www.cdc.gov/flu/weekly/index.htm
2. CDC. Weekly archives. Accessed September 23, 2021. www.cdc.gov/flu/weekly/weeklyarchives2020-2021/WhoNPHL45.html
3. Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic — United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2021;70:1013-1019.
4. Grohskopf L. WG considerations and proposed influenza vaccine recommendations, 2021-22. Presented at the June 24, 2021, meeting of the Advisory Committee on Immunization Practices. Accessed September 23, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-influenza-grohskopf-508.pdf
5. CDC. The flu season. Accessed September 23, 2021. www.cdc.gov/flu/about/season/flu-season.htm
6. CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Accessed September 23, 2021. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fclinical-considerations.html#Coadministration
7. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
8. Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Morb Mortal Wkly Rep. 2021;70:1-28.
9. CDC. Prevent flu. Accessed September 23, 2021. www.cdc.gov/flu/prevent/index.html
During the 2020-2021 influenza season, fewer cases of influenza were reported than in any previous year since 1997, when data were first recorded.1FIGURE 12 shows the dramatic decline in the number of influenza-positive clinical samples reported to the Centers for Disease Control and Prevention (CDC) during the 2020-2021 influenza season compared with the 2019-2020 season. There was only one pediatric death attributed to influenza in 2020-2021, compared with a mean of 177 per year in the previous 3 seasons.

Deaths attributed to pneumonia and influenza were recorded over a recent 5-year period, with COVID-19 added in early mid-2020 (FIGURE 2).1 Total cumulative deaths for 2020-2021 were extremely high, mostly due to COVID-19. Of the relatively few influenza cases last season, 37.5% were caused by influenza A and 62.5% by influenza B. The extremely low incidence of influenza precludes determining influenza vaccine effectiveness for last season.1

In addition, other common respiratory pathogens—parainfluenza, adenoviruses, rhinoviruses, enteroviruses, and common coronaviruses—circulated last winter at historic lows.3 All of these historic lows can be attributed to the measures taken to mitigate the effect of the COVID-19 pandemic, including masks, social distancing, closure of certain venues that normally attract large crowds, and the closure of schools with a resulting increase in schooling at home. With the anticipated relaxation of these measures in 2021-2022, we can expect more influenza and other respiratory ailments due to common pathogens.
Updates to influenza vaccine recommendations
At its June 2021 meeting, the Advisory Committee on Immunization Practices (ACIP) approved the influenza vaccine recommendations for the 2021-2022 season.4 The central recommendation is unchanged: Everyone ≥ 6 months of age should receive a vaccine unless they have a contraindication. Updates to the previous recommendations include the content of the 2021 vaccines, the specific vaccines that will be available for different age groups, the timing of vaccine administration, advice on co-administration with COVID-19 vaccines, and the list of contraindications and precautions based on vaccine type.4
Viral composition of US vaccines for the 2021-22 season
The antigens that will be included in the 2021-2022 influenza vaccines are listed in TABLE 1.4 The B strains are the same as last year; the A strains have been updated. The H3N2 strain is the same in all vaccines, but the H1N1 strain differs based on whether the vaccine is egg based or non-egg based. The advantage of non-egg-based vaccines is that the production process does not take as long and can be delayed in an attempt to better reflect the influenza stains in worldwide circulation.

The influenza vaccines expected to be available for the 2021-22 season
TABLE 24 lists the influenza vaccines approved for use in the United States and the ages for which they are approved.4 All products for 2021-2022 will be quadrivalent, containing 2 type-A and 2 type-B antigens. The only change in age indications is that cell culture–based inactivated influenza vaccine (ccIIV4) (Flucelvax Quadrivalent) is now approved for use starting at age 2 years; previously it was approved starting at age 4 years.4

Timing of vaccination
The onsets and peaks of influenza disease occur at different times each year and can also vary by geographic location. An analysis of 36 influenza seasons starting in 1982 showed that peak activity occurred most frequently in February (15 seasons), followed by December (7 seasons), and January and March (6 seasons each).5 Only once did peak activity occur in October and once in November. This information, along with observational studies showing the waning of influenza vaccine effectiveness after 5 to 6 months, especially in the elderly, informed the ACIP decision to modify their recommendation on the timing of vaccination. The recommendation now states that vaccine should be administered by the end of October and that July and August would have been too early, especially for older adults.
Continue to: Children ages 6 months...
Children ages 6 months through 8 years who have not been vaccinated previously require 2 doses separated by at least 4 weeks, and the first dose should be administered early enough to allow for the second by the end of October.4 Children who require only 1 dose can also receive the vaccine as soon as it is available, as there is less evidence that vaccine effectiveness wanes in children.
Earlier administration is also recommended for pregnant women in their third trimester. Delaying vaccination in this group could result in postpartum administration of the vaccine, thereby depriving infants of protection against influenza illness during their first 6 months after birth.4
Co-administration of influenza and COVID-19 vaccines
Current guidance from the CDC states that COVID-19 vaccines can be co-administered with other vaccines including influenza vaccines.6 However, there are no data by which to judge the efficacy of each vaccine in coadministration or the potential for increased adverse reactions. ACIP advises caution on 2 points: (1) physicians should watch for updated guidance as more information becomes available, and (2) there is the potential for increased reactogenicity after co-administration, especially with the more reactogenic influenza vaccines: adjuvanted inactivated influenza vaccine (aIIV4) and high-dose inactivated influenza vaccine (HD-IIV4). Moreover, these vaccines and the co-administered COVID-19 vaccine should be injected into different limbs.
Contraindications and precautions
Serious allergic reactions to influenza vaccines are rare—about 1.3 incidents per million doses administered.7 However, a previous severe allergic reaction to a particular vaccine or to any component of the vaccine is a contraindication for use of that vaccine. In addition, a history of severe allergic reaction to any influenza vaccine is a contraindication for all egg-based vaccines.
There are 2 precautions for all influenza vaccines: a concurrent moderate or severe acute illness (with or without fever), and a history of Guillain-Barré syndrome within 6 weeks of receiving any influenza vaccine. An additional precaution for ccIIV4 and recombinant influenza vaccine (RIV4) is a history of severe allergic reaction after administration of any other influenza vaccine. Administration of RIV4 or ccIIV4 to someone with such a history should occur in a medical setting and be supervised by someone who can recognize and treat a severe reaction.
Continue to: Live attenuated influenza vaccine...
Live attenuated influenza vaccine (LAIV) continues to have a considerably longer list of contraindications, which can be found in the published recommendations for 2021-2022.8
Final advice
The upcoming influenza season has the potential to be clinically challenging with the possibility of co-existing high rates of both COVID-19 and influenza. Recommend both influenza and COVID-19 vaccination to patients. Also, be sure to encourage and practice other preventive measures such as masking in crowds, frequent hand washing, isolation when sick, respiratory hygiene, and (for physicians) selected prescribing of influenza antiviral medications and meticulous office-based infection control practices.9
During the 2020-2021 influenza season, fewer cases of influenza were reported than in any previous year since 1997, when data were first recorded.1FIGURE 12 shows the dramatic decline in the number of influenza-positive clinical samples reported to the Centers for Disease Control and Prevention (CDC) during the 2020-2021 influenza season compared with the 2019-2020 season. There was only one pediatric death attributed to influenza in 2020-2021, compared with a mean of 177 per year in the previous 3 seasons.

Deaths attributed to pneumonia and influenza were recorded over a recent 5-year period, with COVID-19 added in early mid-2020 (FIGURE 2).1 Total cumulative deaths for 2020-2021 were extremely high, mostly due to COVID-19. Of the relatively few influenza cases last season, 37.5% were caused by influenza A and 62.5% by influenza B. The extremely low incidence of influenza precludes determining influenza vaccine effectiveness for last season.1

In addition, other common respiratory pathogens—parainfluenza, adenoviruses, rhinoviruses, enteroviruses, and common coronaviruses—circulated last winter at historic lows.3 All of these historic lows can be attributed to the measures taken to mitigate the effect of the COVID-19 pandemic, including masks, social distancing, closure of certain venues that normally attract large crowds, and the closure of schools with a resulting increase in schooling at home. With the anticipated relaxation of these measures in 2021-2022, we can expect more influenza and other respiratory ailments due to common pathogens.
Updates to influenza vaccine recommendations
At its June 2021 meeting, the Advisory Committee on Immunization Practices (ACIP) approved the influenza vaccine recommendations for the 2021-2022 season.4 The central recommendation is unchanged: Everyone ≥ 6 months of age should receive a vaccine unless they have a contraindication. Updates to the previous recommendations include the content of the 2021 vaccines, the specific vaccines that will be available for different age groups, the timing of vaccine administration, advice on co-administration with COVID-19 vaccines, and the list of contraindications and precautions based on vaccine type.4
Viral composition of US vaccines for the 2021-22 season
The antigens that will be included in the 2021-2022 influenza vaccines are listed in TABLE 1.4 The B strains are the same as last year; the A strains have been updated. The H3N2 strain is the same in all vaccines, but the H1N1 strain differs based on whether the vaccine is egg based or non-egg based. The advantage of non-egg-based vaccines is that the production process does not take as long and can be delayed in an attempt to better reflect the influenza stains in worldwide circulation.

The influenza vaccines expected to be available for the 2021-22 season
TABLE 24 lists the influenza vaccines approved for use in the United States and the ages for which they are approved.4 All products for 2021-2022 will be quadrivalent, containing 2 type-A and 2 type-B antigens. The only change in age indications is that cell culture–based inactivated influenza vaccine (ccIIV4) (Flucelvax Quadrivalent) is now approved for use starting at age 2 years; previously it was approved starting at age 4 years.4

Timing of vaccination
The onsets and peaks of influenza disease occur at different times each year and can also vary by geographic location. An analysis of 36 influenza seasons starting in 1982 showed that peak activity occurred most frequently in February (15 seasons), followed by December (7 seasons), and January and March (6 seasons each).5 Only once did peak activity occur in October and once in November. This information, along with observational studies showing the waning of influenza vaccine effectiveness after 5 to 6 months, especially in the elderly, informed the ACIP decision to modify their recommendation on the timing of vaccination. The recommendation now states that vaccine should be administered by the end of October and that July and August would have been too early, especially for older adults.
Continue to: Children ages 6 months...
Children ages 6 months through 8 years who have not been vaccinated previously require 2 doses separated by at least 4 weeks, and the first dose should be administered early enough to allow for the second by the end of October.4 Children who require only 1 dose can also receive the vaccine as soon as it is available, as there is less evidence that vaccine effectiveness wanes in children.
Earlier administration is also recommended for pregnant women in their third trimester. Delaying vaccination in this group could result in postpartum administration of the vaccine, thereby depriving infants of protection against influenza illness during their first 6 months after birth.4
Co-administration of influenza and COVID-19 vaccines
Current guidance from the CDC states that COVID-19 vaccines can be co-administered with other vaccines including influenza vaccines.6 However, there are no data by which to judge the efficacy of each vaccine in coadministration or the potential for increased adverse reactions. ACIP advises caution on 2 points: (1) physicians should watch for updated guidance as more information becomes available, and (2) there is the potential for increased reactogenicity after co-administration, especially with the more reactogenic influenza vaccines: adjuvanted inactivated influenza vaccine (aIIV4) and high-dose inactivated influenza vaccine (HD-IIV4). Moreover, these vaccines and the co-administered COVID-19 vaccine should be injected into different limbs.
Contraindications and precautions
Serious allergic reactions to influenza vaccines are rare—about 1.3 incidents per million doses administered.7 However, a previous severe allergic reaction to a particular vaccine or to any component of the vaccine is a contraindication for use of that vaccine. In addition, a history of severe allergic reaction to any influenza vaccine is a contraindication for all egg-based vaccines.
There are 2 precautions for all influenza vaccines: a concurrent moderate or severe acute illness (with or without fever), and a history of Guillain-Barré syndrome within 6 weeks of receiving any influenza vaccine. An additional precaution for ccIIV4 and recombinant influenza vaccine (RIV4) is a history of severe allergic reaction after administration of any other influenza vaccine. Administration of RIV4 or ccIIV4 to someone with such a history should occur in a medical setting and be supervised by someone who can recognize and treat a severe reaction.
Continue to: Live attenuated influenza vaccine...
Live attenuated influenza vaccine (LAIV) continues to have a considerably longer list of contraindications, which can be found in the published recommendations for 2021-2022.8
Final advice
The upcoming influenza season has the potential to be clinically challenging with the possibility of co-existing high rates of both COVID-19 and influenza. Recommend both influenza and COVID-19 vaccination to patients. Also, be sure to encourage and practice other preventive measures such as masking in crowds, frequent hand washing, isolation when sick, respiratory hygiene, and (for physicians) selected prescribing of influenza antiviral medications and meticulous office-based infection control practices.9
1. CDC. Weekly U.S. influenza surveillance report. Accessed September 23, 2021. www.cdc.gov/flu/weekly/index.htm
2. CDC. Weekly archives. Accessed September 23, 2021. www.cdc.gov/flu/weekly/weeklyarchives2020-2021/WhoNPHL45.html
3. Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic — United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2021;70:1013-1019.
4. Grohskopf L. WG considerations and proposed influenza vaccine recommendations, 2021-22. Presented at the June 24, 2021, meeting of the Advisory Committee on Immunization Practices. Accessed September 23, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-influenza-grohskopf-508.pdf
5. CDC. The flu season. Accessed September 23, 2021. www.cdc.gov/flu/about/season/flu-season.htm
6. CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Accessed September 23, 2021. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fclinical-considerations.html#Coadministration
7. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
8. Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Morb Mortal Wkly Rep. 2021;70:1-28.
9. CDC. Prevent flu. Accessed September 23, 2021. www.cdc.gov/flu/prevent/index.html
1. CDC. Weekly U.S. influenza surveillance report. Accessed September 23, 2021. www.cdc.gov/flu/weekly/index.htm
2. CDC. Weekly archives. Accessed September 23, 2021. www.cdc.gov/flu/weekly/weeklyarchives2020-2021/WhoNPHL45.html
3. Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic — United States, 2020-2021. MMWR Morb Mortal Wkly Rep. 2021;70:1013-1019.
4. Grohskopf L. WG considerations and proposed influenza vaccine recommendations, 2021-22. Presented at the June 24, 2021, meeting of the Advisory Committee on Immunization Practices. Accessed September 23, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-06/03-influenza-grohskopf-508.pdf
5. CDC. The flu season. Accessed September 23, 2021. www.cdc.gov/flu/about/season/flu-season.htm
6. CDC. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. Accessed September 23, 2021. www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fclinical-considerations.html#Coadministration
7. McNeil MM, Weintraub ES, Duffy J, et al. Risk of anaphylaxis after vaccination in children and adults. J Allergy Clin Immunol. 2016;137:868-878.
8. Grohskopf LA, Alyanak E, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2021–22 influenza season. MMWR Morb Mortal Wkly Rep. 2021;70:1-28.
9. CDC. Prevent flu. Accessed September 23, 2021. www.cdc.gov/flu/prevent/index.html
How to proceed when it comes to vitamin D
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.

Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.
What should the family physician do?
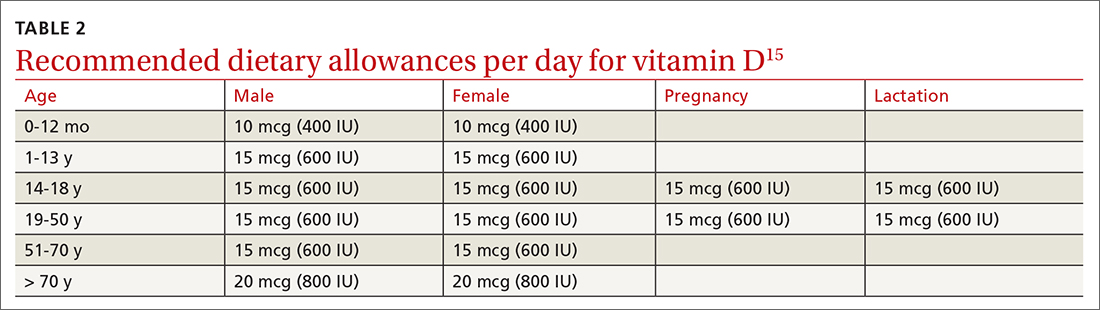
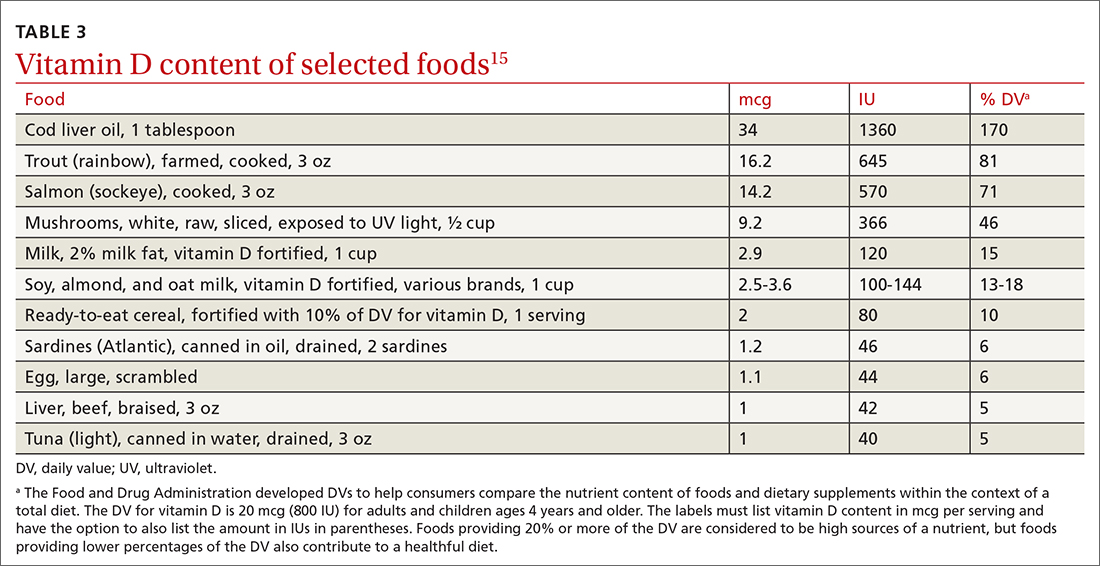
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15
Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.

Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.

What should the family physician do?
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15

Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
In April 2021, the US Preventive Services Task Force (USPSTF) published an updated recommendation on screening for vitamin D deficiency in adults. It reaffirmed an “I” statement first made in 2014: evidence is insufficient to balance the benefits and harms of screening.1 This recommendation applies to asymptomatic, community-dwelling, nonpregnant adults without conditions treatable with vitamin D. It’s important to remember that screening refers to testing asymptomatic individuals to detect a condition early before it causes illness. Testing performed to determine whether symptoms are evidence of an underlying condition is not screening but diagnostic testing.
The Task Force statement explains the problems they found with the current level of knowledge about screening for vitamin D deficiency. First, while 25-hydroxyvitamin D [25(OH)D] is considered the best test for vitamin D levels, it is hard to measure accurately and test results vary by the method used and laboratories doing the testing. There also is uncertainty about how best to measure vitamin D status in different racial and ethnic groups, especially those with dark skin pigmentation. In addition, 25(OH)D in the blood is predominantly the bound form, with only 10% to 15% being unbound and bioavailable. Current tests do not determine the amount of bound vs unbound 25(OH)D.1-3
There is no consensus about the optimal blood level of vitamin D or the level that defines deficiency. The Institute of Medicine (now the National Academy of Medicine—NAM) stated that serum 25(OH)D levels ≥ 20 ng/mL are adequate to meet the metabolic needs of 97.5% of people, and that levels of 12 to 20 ng/mL pose a risk of deficiency, with levels < 12 considered to be very low.4 The Endocrine Society defines deficiency as < 20 ng/mL and insufficiency as 21 to 29 ng/mL.5
The rate of testing for vitamin D deficiency in primary care in unknown, but there is evidence that since 2000, it has increased 80 fold at least among those with Medicare.6 Data from the 2011-2014 National Health and Nutrition Examination Survey showed that 5% of the population had 25(OH)D levels < 12 ng/mL and 18% had levels between 12 and 19 ng/mL.7 Some have estimated that as many as half of all adults would be considered vitamin D deficient or insufficient using current less conservative definitions, with higher rates in racial/ethnic minorities.2,8
There are no firm data on the frequency, or benefits, of screening for vitamin D levels in asymptomatic adults (and treating those found to have vitamin D deficiency). The Task Force looked for indirect evidence by examining the effect of treating vitamin D deficiency in a number of conditions and found that for some, there was adequate evidence of no benefit and for others there was inadequate evidence for possible benefits.9 No benefit was found for incidence of fractures, type 2 diabetes, and overall mortality.9 Inadequate evidence was found for incidence of cancer, cardiovascular disease, scores on measures of depression and physical functioning, and urinary tract infections in those with impaired fasting glucose.9
Known risk factors for low vitamin D levels include low vitamin D intake, older age, obesity, low UVB exposure or absorption due to long winter seasons in northern latitudes, sun avoidance, and dark skin pigmentation.1 In addition, certain medical conditions contribute to, or are caused by, low vitamin D levels—eg, osteoporosis, chronic kidney disease, malabsorption syndromes, and medication use (ie, glucocorticoids).1-3
The Task Force recommendation on screening for vitamin D deficiency differs from those of some other organizations. However, none recommend universal population-based screening. The Endocrine Society and the American Association of Clinical Endocrinologists recommend screening but only in those at risk for vitamin D deficiency.5,10 The American Academy of Family Physicians endorses the USPSTF recommendation.11
Continue to: Specific USPSTF topics related to vitamin D
Specific USPSTF topics related to vitamin D
The Task Force has specifically addressed 3 topics pertaining to vitamin D.

Prevention of falls in the elderly. In 2018 the Task Force recommended against the use of vitamin D to prevent falls in community-dwelling adults ≥ 65 years.12 This reversed its 2012 recommendation advising vitamin D supplementation to prevent falls. The Task Force re-examined the old evidence and looked at newer studies and concluded that their previous conclusion was wrong and that the evidence showed no benefit from vitamin D in preventing falls in the elderly. The reversal of a prior recommendation is rare for the USPSTF because of the rigor of its evidence reviews and its policy of not making a recommendation unless solid evidence for or against exists.
Prevention of cardiovascular disease and cancer. The Task Force concludes that current evidence is insufficient to assess the balance of benefits and harms in the use of single- or paired-nutrient supplements to prevent cardiovascular disease or cancer.13 (The exceptions are beta-carotene and vitamin E, which the Task Force recommends against.) This statement is consistent with the lack of evidence the Task Force found regarding prevention of these conditions by vitamin D supplementation in those who are vitamin D deficient.
Prevention of fractures in men and in premenopausal and postmenopausal women. For men and premenopausal women, the Task Force concludes that evidence is insufficient to assess the benefits and harms of vitamin D and calcium supplementation, alone or in combination, to prevent fractures.14 For prevention of fractures in postmenopausal women, there are 2 recommendations. The first one advises against the use of ≤ 400 IU of vitamin D and ≤ 1000 mg of calcium because the evidence indicates ineffectiveness. The second one is another “I” statement for the use of doses > 400 IU of vitamin D and > 1000 mg of calcium. These 3 recommendations apply to adults who live in the community and not in nursing homes or other institutional care facilities; they do not apply to those who have osteoporosis.

What should the family physician do?
Encourage all patients to take the recommended dietary allowances (RDA) of vitamin D. The RDA is the average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%-98%) healthy individuals. Most professional organizations recommend that adults ≥ 50 years consume 800 to 1000 IU of vitamin D daily. TABLE 2 lists the RDA for vitamin D by age and sex.15 The amount of vitamin D in selected food products is listed in TABLE 3.15 Some increase in levels of vitamin D can occur as a result of sun exposure, but current practices of sun avoidance make it difficult to achieve a significant contribution to vitamin D requirements.15

Continue to: Alternatives to universal screening
Alternatives to universal screening. Screening for vitamin D deficiency might benefit some patients, although there is no evidence to support it. Universal screening will likely lead to overdiagnosis and overtreatment based on what is essentially a poorly understood blood test. This was the concern expressed by the NAM.4,16 An editorial accompanying publication of the recent USPSTF recommendation suggested not measuring vitamin D levels but instead advising patients to consume the age-based RDA of vitamin D.3 For those at increased risk for vitamin D deficiency, advise a higher dose of vitamin D (eg, 2000 IU/d, which is still lower than the upper daily limit).3
Other options are to screen for vitamin D deficiency only in those at high risk for low vitamin D levels, and to test for vitamin D deficiency in those with symptoms associated with deficiency such as bone pain and muscle weakness. These options would be consistent with recommendations from the Endocrine Society.5 Some have recommended that if testing is ordered, it should be performed by a laboratory that uses liquid chromatography-mass spectrometry because it is the criterion standard.2
Treatment options. Vitamin D deficiency can be treated with either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). These treatments can also be recommended for those whose diets may not provide the RDA for vitamin D. Both are readily available over the counter and by prescription. The Task Force found that the harms of treating vitamin D deficiency with vitamin D at recommended doses are small to none.1 There is possibly a small increase in kidney stones with the combined use of 1000 mg/d calcium and 10 mcg (400 IU)/d vitamin D.17 Large doses of vitamin D can cause toxicity including marked hypercalcemia, nausea, vomiting, muscle weakness, neuropsychiatric disturbances, pain, loss of appetite, dehydration, polyuria, excessive thirst, and kidney stones.15A cautious evidence-based approach would be to selectively screen for vitamin D deficiency, conduct diagnostic testing when indicated, and advise vitamin D supplementation as needed.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
1. USPSTF. Screening for vitamin D deficiency in adults: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1436-1442.
2. Michos ED, Kalyani RR, Segal JB. Why USPSTF still finds insufficient evidence to support screening for vitamin D deficiency. JAMA Netw Open. 2021;4:e213627.
3. Burnett-Bowie AAM, Cappola AR. The USPSTF 2021 recommendations on screening for asymptomatic vitamin D deficiency in adults: the challenge for clinicians continues. JAMA. 2021;325:1401-1402.
4. Institute of Medicine. Dietary reference intakes for calcium and vitamin D. National Academies Press; 2011. Accessed May 22, 2021. https://pubmed.ncbi.nlm.nih.gov/21796828/
5. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinolgy Metab. 2011;96:1911-1930.
6. Shahangian S, Alspach TD, Astles JR, et al. Trends in laboratory test volumes for Medicare part B reimbursements, 2000-2010. Arch Pathol Lab Med. 2014;138:189-203.
7. Herrick KA, Storandt RJ, Afful J, et al. Vitamin D status in the United States, 2011-2014. Am J Clin Nutr. 2019;110:150-157.
8. Forrest KYZ, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48-54.
9. Kahwati LC, LeBlanc E, Weber RP, et al. Screening for vitamin D deficiency in adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2021;325:1443-1463.
10. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis-2016. Endocr Pract. 2016;22(supp 4):1-42.
11. AAFP. Clinical preventive services. Accessed May 22, 2021. www.aafp.org/family-physician/patient-care/clinical-recommendations/aafp-cps.html
12. USPSTF. Falls prevention in community-dwelling older adults: interventions. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
13. USPSTF. Vitamin supplementation to prevent cancer and CVD: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-supplementation-to-prevent-cancer-and-cvd-counseling
14. USPSTF. Vitamin D, calcium, or combined supplementation for the primary prevention of fractures in community-dwelling adults: preventive medication. Accessed May 22, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/vitamin-d-calcium-or-combined-supplementation-for-the-primary-prevention-of-fractures-in-adults-preventive-medication
15. NIH. Vitamin D. Accessed May 22, 2021. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
16. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:53-58.
17. Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669-683.
A review of the latest USPSTF recommendations
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.
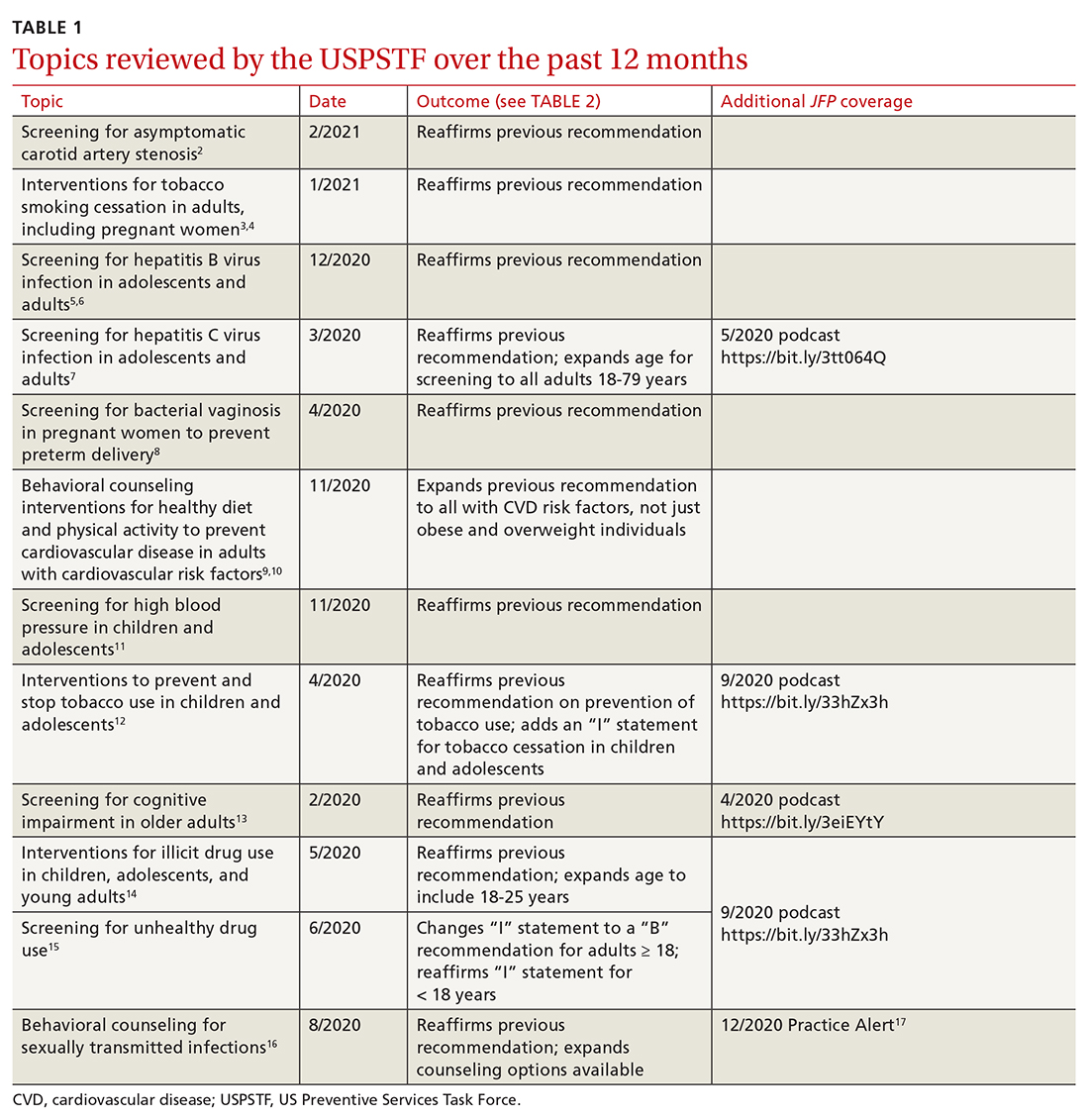
This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.

Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.

This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.

Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
Since the last Practice Alert update on recommendations made by the US Preventive Services Task Force,1 the Task Force has completed work on 12 topics (TABLE 1).2-17 Five of these topics have been discussed in JFP audio recordings, and the links are provided in TABLE 1.

This latest Task Force endeavor resulted in 18 recommendations (TABLE 2), all of which reaffirm previous recommendations on these topics and expand the scope of 2. There were 2 “A” recommendations, 6 “B” recommendations, 2 “D” recommendations, and 8 “I” statements, indicating that there was insufficient evidence to assess effectiveness or harms. The willingness to make “I” statements when there is little or no evidence on the intervention being assessed distinguishes the USPSTF from other clinical guideline committees.

Screening for carotid artery stenosis
One of the “D” recommendations this past year reaffirms the prior recommendation against screening for carotid artery stenosis in asymptomatic adults—ie, those without a history of transient ischemic attack, stroke, or neurologic signs or symptoms that might be caused by carotid artery stenosis.2 The screening tests the Task Force researched included carotid duplex ultrasonography (DUS), magnetic resonance angiography, and computed tomography angiography. The Task Force did not look at the value of auscultation for carotid bruits because it has been proven to be inaccurate and they do not consider it to be a useful screening tool.
The Task Force based its “D” recommendation on a lack of evidence for any benefit in detecting asymptomatic carotid artery stenosis, and on evidence that screening can lead to harms through false-positive tests and potential complications from carotid endarterectomy and carotid artery angioplasty and stenting. In its clinical considerations, the Task Force emphasized the primary prevention of atherosclerotic disease by focusing on the following actions:
- screening for high blood pressure in adults
- encouraging tobacco smoking cessation in adults
- promoting a healthy diet and physical activity in adults with cardiovascular risk factors
- recommending aspirin use to prevent cardiovascular disease and colorectal cancer
- advising statin use for the primary prevention of cardiovascular disease in adults ages 45 to 75 years who have 1 or more risk factors (hyperlipidemia, diabetes, hypertension, smoking) and those with a 10-year risk of a cardiovascular event of 10% or greater.
This “D” recommendation differs from recommendations made by other professional organizations, some of which recommend testing with DUS for asymptomatic patients with a carotid bruit, and others that recommend DUS screening in patients with multiple risk factors for stroke and in those with known peripheral artery disease or other cardiovascular disease.18,19
Smoking cessation in adults
Smoking tobacco is the leading preventable cause of death in the United States, causing about 480,000 deaths annually.3 Smoking during pregnancy increases the risk of complications including miscarriage, congenital anomalies, stillbirth, fetal growth restriction, preterm birth, and placental abruption.
The Task Force published recommendations earlier this year advising all clinicians to ask all adult patients about tobacco use; and, for those who smoke, to provide (or refer them to) smoking cessation behavioral therapy. The Task Force also recommends prescribing pharmacotherapy approved by the Food and Drug Administration (FDA) for smoking cessation for nonpregnant adults. (There is a lack of information to assess the harms and benefits of smoking cessation pharmacotherapy during pregnancy.)
Continue to: FDA-approved medications...
FDA-approved medications for treating tobacco smoking dependence are nicotine replacement therapy (NRT), bupropion hydrochloride, and varenicline.3 NRT is available in transdermal patches, lozenges, gum, inhalers, and nasal sprays.
In addition, the Task Force indicates that there is insufficient evidence to assess the benefits and harms of e-cigarettes when used as a method of achieving smoking cessation: “Few randomized trials have evaluated the effectiveness of e-cigarettes to increase tobacco smoking cessation in nonpregnant adults, and no trials have evaluated e-cigarettes for tobacco smoking cessation in pregnant persons.”4
Hepatitis B infection screening
The Task Force reaffirmed a previous recommendation to screen for hepatitis B virus (HBV) infection only in adults who are at high risk,5 rather than universal screening that it recommends for hepatitis C virus infection (HCV).7 (See: https://bit.ly/3tt064Q). The Task Force has a separate recommendation to screen all pregnant women for hepatitis B at the first prenatal visit.6
Those at high risk for hepatitis B who should be screened include individuals born in countries or regions of the world with a hepatitis B surface antigen (HBsAg) prevalence ≥ 2% and individuals born in the United States who have not received HBV vaccine and whose parents were born in regions with an HBsAg prevalence ≥ 8%.5 (A table listing countries with HBsAg ≥ 8%—as well as those in lower prevalence categories—is included with the recommendation.5)
HBV screening should also be offered to other high-risk groups that have a prevalence of positive HBsAg ≥ 2%: those who have injected drugs in the past or are currently injecting drugs; men who have sex with men; individuals with HIV; and sex partners, needle-sharing contacts, and household contacts of people known to be HBsAg positive.5
Continue to: It is estimated that...
It is estimated that > 860,000 people in the United States have chronic HBV infection and that close to two-thirds of them are unaware of their infection.5 The screening test for HBV is highly accurate; sensitivity and specificity are both > 98%.5 While there is no direct evidence that screening, detecting, and treating asymptomatic HBV infection reduces morbidity and mortality, the Task Force felt that the evidence for improvement in multiple outcomes in those with HBV when treated with antiviral regimens was sufficient to support the recommendation.
Screening for bacterial vaginosis in pregnancy
While bacterial vaginosis (BV) is associated with a two-fold risk of preterm delivery, treating BV during pregnancy does not seem to reduce this risk, indicating that some other variable is involved.8 In addition, studies that looked at screening for, and treatment of, asymptomatic BV in pregnant women at high risk for preterm delivery (defined primarily as those with a previous preterm delivery) have shown inconsistent results. There is the potential for harm in treating BV in pregnancy, chiefly involving gastrointestinal upset caused by metronidazole or clindamycin.
Given that there are no benefits—and some harms—resulting from treatment, the Task Force recommends against screening for BV in non-high-risk pregnant women. A lack of sufficient information to assess any potential benefits to screening in high-risk pregnancies led the Task Force to an “I” statement on this question.8
Behavioral counseling on healthy diet, exercise for adults with CV risks
Cardiovascular disease (CVD) remains the number one cause of death in the United States. The major risk factors for CVD, which can be modified, are high blood pressure, hyperlipidemia, diabetes, smoking, obesity or overweight, and lack of physical activity.
The Task Force has previously recommended intensive behavioral interventions to improve nutrition and physical activity in those who are overweight/obese and in those with abnormal blood glucose levels,9 and has addressed smoking prevention and cessation.4 This new recommendation applies to those with other CVD risks such as high blood pressure and/or hyperlipidemia and those with an estimated 10-year CVD risk of ≥ 7.5%.10
Continue to: Behavioral interventions...
Behavioral interventions included in the Task Force analysis employed a median of 12 contacts and an estimated 6 hours of contact time over 6 to 18 months.10 Most interventions involved motivational interviewing and instruction on behavioral change methods. These interventions can be provided by primary care clinicians, as well as a wide range of other trained professionals. The Affordable Care Act dictates that all “A” and “B” recommendations must be provided by commercial health plans at no out-of-pocket expense for the patient.
Nutritional advice should include reductions in saturated fats, salt, and sugars and increases in fruits, vegetables, and whole grains. The Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) diet are often recommended.10 Physical activity counseling should advocate for 90 to 180 minutes per week of moderate to vigorous activity.
This new recommendation, along with the previous ones pertaining to behavioral interventions for lifestyle changes, make it clear that intensive interventions are needed to achieve meaningful change. Simple advice from a clinician will have little to no effect.
Task Force reviews evidence on HTN, smoking cessation in young people
In 2020 the Task Force completed reviews of evidence relevant to screening for high blood pressure11 and
The 2 “I” statements are in disagreement with recommendations of other professional organizations. The American Academy of Pediatrics (AAP) and the American Heart Association recommend routine screening for high blood pressure starting at age 3 years. And the AAP recommends screening teenagers for tobacco use and offering tobacco dependence treatment, referral, or both (including pharmacotherapy) when indicated. E-cigarettes are not recommended as a treatment for tobacco dependence.20
Continue to: The difference between...
The difference between the methods used by the Task Force and other guideline-producing organizations becomes apparent when it comes to recommendations pertaining to children and adolescents, for whom long-term outcome-oriented studies on prevention issues are rare. The Task Force is unwilling to make recommendations when evidence does not exist. The AAP often makes recommendations based on expert opinion consensus in such situations. One notable part of each Task Force recommendation statement is a discussion of what other organizations recommend on the same topic so that these differences can be openly described.
Better Task Force funding could expand topic coverage
It is worth revisiting 2 issues that were pointed out in last year’s USPSTF summary in this column.1 First, the Task Force methods are robust and evidence based, and recommendations therefore are rarely changed once they are made at an “A”, “B”, or “D” level. Second, Task Force resources are finite, and thus, the group is currently unable to update previous recommendations with greater frequency or to consider many new topics. In the past 2 years, the Task Force has developed recommendations on only 2 completely new topics. Hopefully, its budget can be expanded so that new topics can be added in the future.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
1. Campos-Outcalt D. USPSTF roundup. J Fam Pract. 2020;69:201-204.
2. USPSTF. Screening for asymptomatic carotid artery stenosis. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/carotid-artery-stenosis-screening
3. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. Accessed April 30, 2021. www.uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-use-in-adults-and-pregnant-women-counseling-and-interventions
4. USPSTF. Interventions for tobacco smoking cessation in adults, including pregnant persons. JAMA. 2021;325:265-279.
5. USPSTF. Screening for Hepatitis B virus infection in adolescents and adults. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-screening
6. USPSTF. Hepatitis B virus infection in pregnant women: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-b-virus-infection-in-pregnant-women-screening
7. USPSTF. Hepatitis C virus infection in adolescents and adults: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening
8. USPSTF; Owens DK, Davidson KW, Krisk AH, et al. Screening for bacterial vaginosis in pregnant persons to prevent preterm delivery: US Preventive Services Task Force recommendation statement. JAMA. 2020;323:1286-1292.
9. Behavioral counseling to promote a healthful diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;161:587-593.
10. USPSTF. Behavioral counseling interventions to promote a healthy and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324:2069-2075.
11. USPSTF. High blood pressure in children and adolescents: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/blood-pressure-in-children-and-adolescents-hypertension-screening
12. USPSTF. Prevention and cessation of tobacco use in children and adolescents: primary care interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/tobacco-and-nicotine-use-prevention-in-children-and-adolescents-primary-care-interventions
13. USPSTF. Cognitive impairment in older adults: screening. Accessed March 26, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/cognitive-impairment-in-older-adults-screening
14. USPSTF. Illicit drug use in children, adolescents, and young adults: primary care-based interventions. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-primary-care-interventions-for-children-and-adolescents
15. USPSTF. Unhealthy drug use: screening. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/drug-use-illicit-screening
16. USPSTF. Sexually transmitted infections: behavioral counseling. Accessed April 30, 2021. https://uspreventiveservicestaskforce.org/uspstf/recommendation/sexually-transmitted-infections-behavioral-counseling.
17. Campos-Outcalt D. USPSTF update on sexually transmitted infections. J Fam Pract. 2020;69:514-517.
18. Brott TG, Halperin JL, Abbara S, et al; ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Catheter Cardiovasc Interv. 2013;81:E76-E123.
19. Ricotta JJ, Aburahma A, Ascher E, et al; Society for Vascular Surgery. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1-e31.
20. Farber HJ, Walley SC, Groner JA, et al; Section on Tobacco Control. Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke. Pediatrics. 2015;136:1008-1017.
ACIP recommendations for COVID-19 vaccines—and more
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).
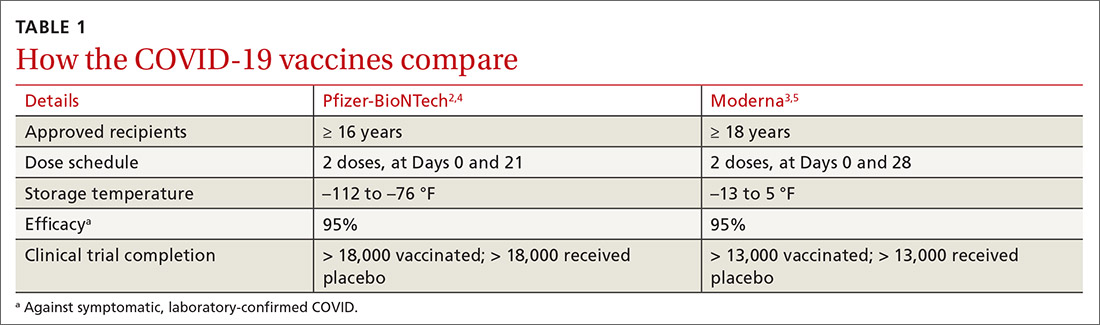
In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).
Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.
Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.
The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9
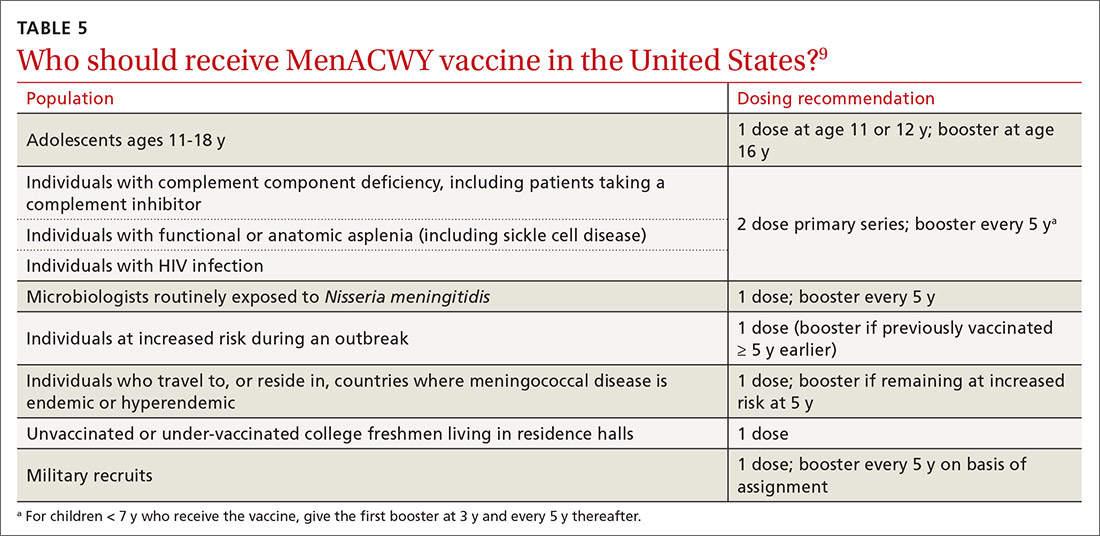
Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).

In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).

Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.

Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.

The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9

Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
The year 2020 was challenging for public health agencies and especially for the Centers for Disease Control and Prevention (CDC) and its Advisory Committee on Immunization Practices (ACIP). In a normal year, the ACIP meets in person 3 times for a total of 6 days of deliberations. In 2020, there were 10 meetings (all but 1 using Zoom) covering 14 days. Much of the time was dedicated to the COVID-19 pandemic, the vaccines being developed to prevent COVID-19, and the prioritization of those who should receive the vaccines first.
The ACIP also made recommendations for the use of influenza vaccines in the 2020-2021 season, approved the adult and pediatric immunization schedules for 2021, and approved the use of 2 new vaccines, one to protect against meningococcal meningitis and the other to prevent Ebola virus disease. The influenza recommendations were covered in the October 2020 Practice Alert,1 and the immunization schedules can be found on the CDC website at www.cdc.gov/vaccines/schedules/hcp/index.html.
COVID-19 vaccines
Two COVID-19 vaccines have been approved for use in the United States. The first was the Pfizer-BioNTech COVID-19 vaccine, approved by the Food and Drug Administration (FDA) on December 11 and recommended for use by the ACIP on December 12.2 The second vaccine, from Moderna, was approved by the FDA on December 18 and recommended by the ACIP on December 19.3 Both were approved by the FDA under an Emergency Use Authorization (EUA) and were approved by the ACIP for use while the EUA is in effect. Both vaccines must eventually undergo regular approval by the FDA and will be reconsidered by the ACIP regarding use in non–public health emergency conditions. A description of the EUA process and measures taken to assure efficacy and safety, before and after approval, were discussed in the September 2020 audiocast.
Both COVID-19 vaccines consist of nucleoside-modified mRNA encapsulated with lipid nanoparticles, which encode for a spike glycoprotein of SARS-CoV-2, the virus that causes COVID-19. Both vaccines require 2 doses (separated by 3 weeks for the Pfizer vaccine and 4 weeks for the Moderna vaccine) and are approved for use only in adults and older adolescents (ages ≥ 16 years for the Pfizer vaccine and ≥ 18 years for the Moderna vaccine) (TABLE 12-5).

In anticipation of vaccine shortages immediately after approval for use and a high demand for the vaccine, the ACIP developed a list of high-priority groups who should receive the vaccine in ranked order.6 States are encouraged, but not required, to follow this priority list (TABLE 26).

Caveats with usage. Both COVID-19 vaccines are very reactogenic, causing local and systemic adverse effects that patients should be warned about (TABLE 37,8). These reactions are usually mild to moderate and last 24 hours or less. Acetaminophen can alleviate these symptoms but should not be used to prevent them. In addition, both vaccines have stringent cold-storage requirements; once the vaccines are thawed, they must be used within a defined time-period.

Neither vaccine is listed as preferred. And they are not interchangeable; both recommended doses should be completed with the same vaccine. More details about the use of these vaccines were discussed in the January 2021 audiocast (www.mdedge.com/familymedicine/article/234239/coronavirus-updates/covid-19-vaccines-rollout-risks-and-reason-still) and can be located on the CDC website (www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html; www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html).
Continue to: Much remains unknown...
Much remains unknown regarding the use of these COVID-19 vaccines:
- What is their duration of protection, and will booster doses be needed?
- Will they protect against asymptomatic infection and carrier states, and thereby prevent transmission?
- Can they be co-administered with other vaccines?
- Will they be efficacious and safe to use during pregnancy and breastfeeding?
These issues will need to be addressed before they are recommended for non–public health emergency use.
Quadrivalent meningococcal conjugate vaccine (MenACWY)
In June 2020, the ACIP added a third quadrivalent meningococcal conjugate vaccine to its recommended list of vaccines that are FDA-approved for meningococcal disease (TABLE 49). The new vaccine fills a void left by the meningococcal polysaccharide vaccine (MPSV4), which is no longer marketed in the United States. MPSV4 was previously the only meningococcal vaccine approved for individuals 55 years and older.

The new vaccine, MenACWY-TT (MenQuadfi), is approved for those ages 2 years and older, including those > 55 years. It is anticipated that MenQuadfi will, in the near future, be licensed and approved for individuals 6 months and older and will replace MenACWY-D (Menactra). (Both are manufactured by Sanofi Pasteur.)
Groups for whom a MenACWY vaccine is recommended are listed in TABLE 5.9 A full description of current, updated recommendations for the prevention of meningococcal disease is also available.9

Continue to: Ebola virus (EBOV) vaccine
Ebola virus (EBOV) vaccine
A vaccine to prevent Ebola virus disease (EVD) is available by special request in the United States. Recombinant vesicular stomatitis virus-based Ebola virus vaccine, abbreviated as rVSVΔG-ZEBOV-GP (brand name, ERVBO) is manufactured by Merck and received approval by the FDA on December 19, 2019, for use in those ages 18 years and older. It is a live, attenuated vaccine.
The ACIP has recommended pre-exposure vaccination with rVSVΔG-ZEBOV-GP for adults 18 years or older who are at risk of exposure to EBOV while responding to an outbreak of EVD; while working as health care personnel at a federally designated Ebola Treatment Center; or while working at biosafety-level 4 facilities.10 The vaccine is protective against just 1 of 4 EBOV species, Zaire ebolavirus, which has been the cause of most reported EVD outbreaks, including the 2 largest EVD outbreaks in history that occurred in West Africa and the Republic of Congo.
It is estimated that EBOV outbreaks have infected more than 31,000 people and resulted in more than 12,000 deaths worldwide.11 Only 11 people infected with EBOV have been treated in the United States, all related to the 2014-2016 large outbreaks in West Africa. Nine of these cases were imported and only 1 resulted in transmission, to 2 people.10 The mammalian species that are suspected as intermediate hosts for EBOV are not present in the United States, which prevents EBOV from becoming endemic here.
The rVSVΔG-ZEBOV-GP vaccine was tested in a large trial in Africa during the 2014 outbreak. Its effectiveness was 100% (95% confidence interval, 63.5%-100%). The most common adverse effects were injection site pain, swelling, and redness. Mild-to-moderate systemic symptoms can occur within the first 2 days following vaccination, and include headache (37%), fever (34%), muscle pain (33%), fatigue (19%), joint pain (18%), nausea (8%), arthritis (5%), rash (4%), and
Since the vaccine contains a live virus that causes stomatitis in animals, it is possible that the virus could be transmitted to humans and other animals through close contact. Accordingly, the CDC has published some precautions including, but not limited to, not donating blood and, for 6 weeks after vaccination, avoiding contact with those who are immunosuppressed.10 The vaccine is not commercially available in the United States and must be obtained from the CDC. Information on requesting the vaccine is available at www.cdc.gov/vhf/ebola/clinicians/vaccine/.
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
1. Campos-Outcalt D. Prospects and challenges for the upcoming influenza season. J Fam Pract 2020;69:406-411.
2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1922-1924.
3. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine-United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1653-1656.
4. CDC. Pfizer-BioNTech COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
5. CDC. Moderna COVID-19 vaccine. Accessed February 17, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/moderna/index.html#:~:text=How%20to%20Store%20the%20Moderna%20COVID%2D19%20Vaccine&text=Vaccine%20may%20be%20stored%20in,for%20this%20vaccine%20is%20tighter
6. Dooling K, Marin M, Wallace M, et al. The Advisory Committee on Immunization Practices’ updated interim recommendation for allocation of COVID-19 Vaccine—United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69:1657-1660.
7. FDA. Fact sheet for healthcare providers administering vaccine. [Pfizer–BioNTech]. Accessed February 17, 2021. www.fda.gov/media/144413/download
8. FDA. Fact sheet for healthcare providers administering vaccine. [Moderna]. Accessed February 17, 2021. www.fda.gov/media/144637/download
9. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69:1-41.
10. Choi MJ, Cossaboom CM, Whitesell AN, et al. Use of Ebola vaccine: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020. MMWR Recomm Rep. 2021;70:1-12.
11. CDC. Ebola background. Accessed February 17, 2021. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-02/Ebola-02-Choi-508.pdf
AT PRESS TIME
The US Food and Drug Administration issued an Emergency Use Authorization for a third COVID-19 vaccine. The single-dose vaccine was developed by the Janssen Pharmaceutical Companies of Johnson & Johnson. For more information, go to www.mdedge.com/familymedicine