User login
Transition to Tenecteplase From t-PA for Acute Ischemic Stroke at Walter Reed National Military Medical Center
Tissue plasminogen activator (t-PA) has been the standard IV thrombolytic used in acute ischemic stroke treatment since its US Food and Drug Administration (FDA) approval in 1995. Trials have established this drug’s efficacy in the treatment of acute ischemic stroke and the appropriate patient population for therapy.1-3 Published guidelines and experiences have made clear that a written protocol with extensive personnel training is important to deliver this care properly.4
Tenecteplase has been available for use in the treatment of acute myocardial infarction (MI) and studied in acute ischemic strokes since 2000. Recent large multicenter trials have suggested tenecteplase may work better than t-PA in the recanalization of large vessel occlusions (LVOs) and have provided guidance on proper dosing in acute ischemic stroke victims.5-8 Compared with t-PA, tenecteplase has a longer half-life, is more fibrin specific (causing less coagulopathy), and is more resistant to endogenous plasminogen activator inhibitor.9,10 Using tenecteplase for acute ischemic stroke is simpler as a single dose bolus rather than a bolus followed by a 1-hour infusion with t-PA. Immediate mechanical thrombectomy for LVO is less complicated without the 1-hour t-PA infusion.5,6 Tenecteplase use also allows for nonthrombectomy hospitals to accelerate transfer times for patients who need thrombectomy following thrombolysis by eliminating the need for critical care nurse–staffed ambulances for interfacility transfer.11 Tenecteplase also is cheaper: Tenecteplase costs $3748 per vial, whereas t-PA costs $5800 per vial equating to roughly a $2000 savings per patient.12,13 Finally, the pharmacy formulary is simplified by using a single thrombolytic agent for both cardiac and neurologic emergencies.
Tenecteplase does have some drawbacks to consider. Currently, tenecteplase is not approved by the FDA for the indication of acute ischemic stroke, though the drug is endorsed by the American Heart Association stroke guidelines of 2019 as an alternative to t-PA.14 There is no stroke-specific preparation of the drug, leading to potential dosing errors. Therefore, a systematic process to safely transition from t-PA to tenecteplase for acute ischemic stroke was undertaken at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. Here, we report the process required in making a complex switch in thrombolytic medication along with the potential benefits of making this transition.
OBSERVATIONS
The process to implement tenecteplase required extensive training and education for staff physicians, nurses, pharmacists, radiologists, trainees, and the rapid response team. Our institution administered IV thrombolytic drugs up to 25 times annually to acute ischemic stroke victims, meaning we had to train personnel extensively and repeatedly.
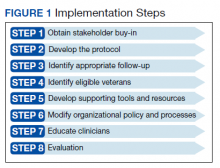
In preparation for the transition to tenecteplase, hospital leadership gathered staff for multidisciplinary administrative meetings that included neurology, emergency medicine, intensive care, pharmacy, radiology, and nursing departments. The purpose of these meetings was to establish a standard operating procedure (SOP) to ensure a safe transition. This process began in May 2020 and involved regular meetings to draft and revise our SOP. Additionally, several leadership and training sessions were held over a 6-month period. Stroke boxes were developed that contained the required evaluation tools, consent forms, medications (tenecteplase and treatments for known complications), dosing cards, and instructions. Final approval of the updated acute ischemic stroke hospital policy was obtained in November 2020 and signed by the above departments.
All inclusion and exclusion criteria were determined to be the same for tenecteplase as they were for t-PA with the notable exception that the WAKE-UP trial protocol would not be supported until further evidence became available.9 The results of the WAKE-UP trial had previously been used at WRNMMC to justify administration of t-PA in patients who awoke with symptoms of acute ischemic stroke, the last known well was unclear or > 4.5 hours, and for whom a magnetic resonance imaging (MRI) of the brain could be obtained rapidly. Based on the WAKE-UP trial, if the MRI scan of the brain in these patients demonstrated restricted diffusion without fluid attenuated inversion recovery (FLAIR) signal changes (diffusion-weighted [DWI]-FLAIR mismatch sign), this indicated that the stroke had likely occurred recently, and it was safe to administer t-PA. This allowed for administration of t-PA outside the standard treatment window of 4.5 hours from last known well, especially in the cases of patients who awoke with symptoms.
Since safety data are not yet available for the use of tenecteplase in this fashion, the WAKE-UP trial protocol was not used as an inclusion criterion. The informed consent form was modified, and the following scenarios were outlined: (1) If the patient or surrogate is immediately available to consent, paper consent will be documented with the additional note that tenecteplase is being used off-label; and (2) If the patient cannot consent and a surrogate is not immediately available, the medicine will be used emergently as long as the neurology resident and attending physicians agree.15
Risk mitigation was considered carefully. The stroke box described above is stocked and maintained by the pharmacy as we have transitioned to using designated pharmacists for the storage and preparation of tenecteplase. We highly recommend the use of designated pharmacists or emergency department pharmacists in this manner to avoid dosing errors.7,16 Since the current pharmacy-provided tenecteplase bottle contains twice the maximum dose indicated for ischemic stroke, only a 5 mL syringe is included in the stroke box to ensure a maximum dose of 25 mg is drawn up after reconstitution. Dosing card charts were made like existing dosing card charts for t-PA to quickly calculate the 0.25 mg/kg dose. In training, the difference in dosing in ischemic stroke was emphasized. Finally, pharmacy has taken responsibility for dosing the medication during stroke codes.
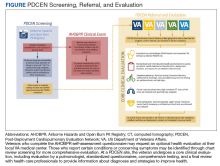
Any medical personnel at WRNMMC can initiate a stroke code by sending a page to the neurology consult service (Figure).
TRANSITION AND RESULTS
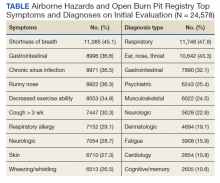
From November 2020 to December 2021, 10 patients have been treated in total at WRNMMC (Table).
CONCLUSIONS
The available evidence supports the transition from t-PA to tenecteplase for acute ischemic stroke. The successful transition required months of preparation involving multidisciplinary meetings between neurology, nursing, pharmacy, radiology, rapid response teams, critical care, and emergency medicine departments. Safeguards must be implemented to avoid a tenecteplase dosing error that can lead to potentially life-threatening adverse effects. The results at WRNMMC thus far are promising for safety and efficacy. Several process improvements are planned: a hospital-wide overhead page will accompany the direct page to neurology; other team members, including radiology and pharmacy, will be included on the acute stroke alert; and a stroke-specific paging application will be implemented to better track real-time stroke metrics and improve flow. These measures mirror processes that are occurring in institutions that treat acute stroke patients.
1. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695-1703. doi:10.1016/S0140-6736(10)60491-6
2. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581- 1587. doi:10.1056/NEJM199512143332401
3. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768-774. doi:10.1016/S0140-6736(04)15692-4
4. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi:10.1161/STR.0b013e318284056a
5. Campbell B, Mitchell P, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. doi:10.1056/nejmoa1716405
6. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi:10.1056/NEJMoa2001123
7. Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, noninferiority trial. Lancet. 2022;400(10347):161-169. doi:10.1016/S0140-6736(22)01054-6
8. Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA. 2020;323(13):1257- 1265. doi:10.1001/jama.2020.1511
9. Warach SJ, Dula AN, Milling TJ Jr. Tenecteplase thrombolysis for acute ischemic stroke. Stroke. 2020;51(11):3440- 3451. doi:10.1161/STROKEAHA.120.029749
10. Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke. 2015;46(12):3543-3546. doi:10.1161/STROKEAHA.115.011290
11. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
12. Potla N, Ganti L. Tenecteplase vs. alteplase for acute ischemic stroke: a systematic review. Int J Emerg Med. 2022;15(1). doi:10.1186/s12245-021-00399-w
13. Warach SJ, Winegar A, Ottenbacher A, Miller C, Gibson D. Abstract WMP52: reduced hospital costs for ischemic stroke treated with tenecteplase. Stroke. 2022;53(suppl 1):AWMP52. doi:10.1161/str.53.suppl_1.WMP52
14. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/str.0000000000000211
15. Faris H, Dewar B, Dowlatshahi D, et al. Ethical justification for deferral of consent in the AcT trial for acute ischemic stroke. Stroke. 2022;53(7):2420-2423. doi:10.1161/strokeaha.122.038760
16. Kvistad CE, Næss H, Helleberg BH, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol. 2022;21(6):511-519. doi:10.1016/S1474-4422(22)00124-7
Tissue plasminogen activator (t-PA) has been the standard IV thrombolytic used in acute ischemic stroke treatment since its US Food and Drug Administration (FDA) approval in 1995. Trials have established this drug’s efficacy in the treatment of acute ischemic stroke and the appropriate patient population for therapy.1-3 Published guidelines and experiences have made clear that a written protocol with extensive personnel training is important to deliver this care properly.4
Tenecteplase has been available for use in the treatment of acute myocardial infarction (MI) and studied in acute ischemic strokes since 2000. Recent large multicenter trials have suggested tenecteplase may work better than t-PA in the recanalization of large vessel occlusions (LVOs) and have provided guidance on proper dosing in acute ischemic stroke victims.5-8 Compared with t-PA, tenecteplase has a longer half-life, is more fibrin specific (causing less coagulopathy), and is more resistant to endogenous plasminogen activator inhibitor.9,10 Using tenecteplase for acute ischemic stroke is simpler as a single dose bolus rather than a bolus followed by a 1-hour infusion with t-PA. Immediate mechanical thrombectomy for LVO is less complicated without the 1-hour t-PA infusion.5,6 Tenecteplase use also allows for nonthrombectomy hospitals to accelerate transfer times for patients who need thrombectomy following thrombolysis by eliminating the need for critical care nurse–staffed ambulances for interfacility transfer.11 Tenecteplase also is cheaper: Tenecteplase costs $3748 per vial, whereas t-PA costs $5800 per vial equating to roughly a $2000 savings per patient.12,13 Finally, the pharmacy formulary is simplified by using a single thrombolytic agent for both cardiac and neurologic emergencies.
Tenecteplase does have some drawbacks to consider. Currently, tenecteplase is not approved by the FDA for the indication of acute ischemic stroke, though the drug is endorsed by the American Heart Association stroke guidelines of 2019 as an alternative to t-PA.14 There is no stroke-specific preparation of the drug, leading to potential dosing errors. Therefore, a systematic process to safely transition from t-PA to tenecteplase for acute ischemic stroke was undertaken at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. Here, we report the process required in making a complex switch in thrombolytic medication along with the potential benefits of making this transition.
OBSERVATIONS
The process to implement tenecteplase required extensive training and education for staff physicians, nurses, pharmacists, radiologists, trainees, and the rapid response team. Our institution administered IV thrombolytic drugs up to 25 times annually to acute ischemic stroke victims, meaning we had to train personnel extensively and repeatedly.
In preparation for the transition to tenecteplase, hospital leadership gathered staff for multidisciplinary administrative meetings that included neurology, emergency medicine, intensive care, pharmacy, radiology, and nursing departments. The purpose of these meetings was to establish a standard operating procedure (SOP) to ensure a safe transition. This process began in May 2020 and involved regular meetings to draft and revise our SOP. Additionally, several leadership and training sessions were held over a 6-month period. Stroke boxes were developed that contained the required evaluation tools, consent forms, medications (tenecteplase and treatments for known complications), dosing cards, and instructions. Final approval of the updated acute ischemic stroke hospital policy was obtained in November 2020 and signed by the above departments.
All inclusion and exclusion criteria were determined to be the same for tenecteplase as they were for t-PA with the notable exception that the WAKE-UP trial protocol would not be supported until further evidence became available.9 The results of the WAKE-UP trial had previously been used at WRNMMC to justify administration of t-PA in patients who awoke with symptoms of acute ischemic stroke, the last known well was unclear or > 4.5 hours, and for whom a magnetic resonance imaging (MRI) of the brain could be obtained rapidly. Based on the WAKE-UP trial, if the MRI scan of the brain in these patients demonstrated restricted diffusion without fluid attenuated inversion recovery (FLAIR) signal changes (diffusion-weighted [DWI]-FLAIR mismatch sign), this indicated that the stroke had likely occurred recently, and it was safe to administer t-PA. This allowed for administration of t-PA outside the standard treatment window of 4.5 hours from last known well, especially in the cases of patients who awoke with symptoms.
Since safety data are not yet available for the use of tenecteplase in this fashion, the WAKE-UP trial protocol was not used as an inclusion criterion. The informed consent form was modified, and the following scenarios were outlined: (1) If the patient or surrogate is immediately available to consent, paper consent will be documented with the additional note that tenecteplase is being used off-label; and (2) If the patient cannot consent and a surrogate is not immediately available, the medicine will be used emergently as long as the neurology resident and attending physicians agree.15
Risk mitigation was considered carefully. The stroke box described above is stocked and maintained by the pharmacy as we have transitioned to using designated pharmacists for the storage and preparation of tenecteplase. We highly recommend the use of designated pharmacists or emergency department pharmacists in this manner to avoid dosing errors.7,16 Since the current pharmacy-provided tenecteplase bottle contains twice the maximum dose indicated for ischemic stroke, only a 5 mL syringe is included in the stroke box to ensure a maximum dose of 25 mg is drawn up after reconstitution. Dosing card charts were made like existing dosing card charts for t-PA to quickly calculate the 0.25 mg/kg dose. In training, the difference in dosing in ischemic stroke was emphasized. Finally, pharmacy has taken responsibility for dosing the medication during stroke codes.
Any medical personnel at WRNMMC can initiate a stroke code by sending a page to the neurology consult service (Figure).
TRANSITION AND RESULTS
From November 2020 to December 2021, 10 patients have been treated in total at WRNMMC (Table).
CONCLUSIONS
The available evidence supports the transition from t-PA to tenecteplase for acute ischemic stroke. The successful transition required months of preparation involving multidisciplinary meetings between neurology, nursing, pharmacy, radiology, rapid response teams, critical care, and emergency medicine departments. Safeguards must be implemented to avoid a tenecteplase dosing error that can lead to potentially life-threatening adverse effects. The results at WRNMMC thus far are promising for safety and efficacy. Several process improvements are planned: a hospital-wide overhead page will accompany the direct page to neurology; other team members, including radiology and pharmacy, will be included on the acute stroke alert; and a stroke-specific paging application will be implemented to better track real-time stroke metrics and improve flow. These measures mirror processes that are occurring in institutions that treat acute stroke patients.
Tissue plasminogen activator (t-PA) has been the standard IV thrombolytic used in acute ischemic stroke treatment since its US Food and Drug Administration (FDA) approval in 1995. Trials have established this drug’s efficacy in the treatment of acute ischemic stroke and the appropriate patient population for therapy.1-3 Published guidelines and experiences have made clear that a written protocol with extensive personnel training is important to deliver this care properly.4
Tenecteplase has been available for use in the treatment of acute myocardial infarction (MI) and studied in acute ischemic strokes since 2000. Recent large multicenter trials have suggested tenecteplase may work better than t-PA in the recanalization of large vessel occlusions (LVOs) and have provided guidance on proper dosing in acute ischemic stroke victims.5-8 Compared with t-PA, tenecteplase has a longer half-life, is more fibrin specific (causing less coagulopathy), and is more resistant to endogenous plasminogen activator inhibitor.9,10 Using tenecteplase for acute ischemic stroke is simpler as a single dose bolus rather than a bolus followed by a 1-hour infusion with t-PA. Immediate mechanical thrombectomy for LVO is less complicated without the 1-hour t-PA infusion.5,6 Tenecteplase use also allows for nonthrombectomy hospitals to accelerate transfer times for patients who need thrombectomy following thrombolysis by eliminating the need for critical care nurse–staffed ambulances for interfacility transfer.11 Tenecteplase also is cheaper: Tenecteplase costs $3748 per vial, whereas t-PA costs $5800 per vial equating to roughly a $2000 savings per patient.12,13 Finally, the pharmacy formulary is simplified by using a single thrombolytic agent for both cardiac and neurologic emergencies.
Tenecteplase does have some drawbacks to consider. Currently, tenecteplase is not approved by the FDA for the indication of acute ischemic stroke, though the drug is endorsed by the American Heart Association stroke guidelines of 2019 as an alternative to t-PA.14 There is no stroke-specific preparation of the drug, leading to potential dosing errors. Therefore, a systematic process to safely transition from t-PA to tenecteplase for acute ischemic stroke was undertaken at Walter Reed National Military Medical Center (WRNMMC) in Bethesda, Maryland. Here, we report the process required in making a complex switch in thrombolytic medication along with the potential benefits of making this transition.
OBSERVATIONS
The process to implement tenecteplase required extensive training and education for staff physicians, nurses, pharmacists, radiologists, trainees, and the rapid response team. Our institution administered IV thrombolytic drugs up to 25 times annually to acute ischemic stroke victims, meaning we had to train personnel extensively and repeatedly.
In preparation for the transition to tenecteplase, hospital leadership gathered staff for multidisciplinary administrative meetings that included neurology, emergency medicine, intensive care, pharmacy, radiology, and nursing departments. The purpose of these meetings was to establish a standard operating procedure (SOP) to ensure a safe transition. This process began in May 2020 and involved regular meetings to draft and revise our SOP. Additionally, several leadership and training sessions were held over a 6-month period. Stroke boxes were developed that contained the required evaluation tools, consent forms, medications (tenecteplase and treatments for known complications), dosing cards, and instructions. Final approval of the updated acute ischemic stroke hospital policy was obtained in November 2020 and signed by the above departments.
All inclusion and exclusion criteria were determined to be the same for tenecteplase as they were for t-PA with the notable exception that the WAKE-UP trial protocol would not be supported until further evidence became available.9 The results of the WAKE-UP trial had previously been used at WRNMMC to justify administration of t-PA in patients who awoke with symptoms of acute ischemic stroke, the last known well was unclear or > 4.5 hours, and for whom a magnetic resonance imaging (MRI) of the brain could be obtained rapidly. Based on the WAKE-UP trial, if the MRI scan of the brain in these patients demonstrated restricted diffusion without fluid attenuated inversion recovery (FLAIR) signal changes (diffusion-weighted [DWI]-FLAIR mismatch sign), this indicated that the stroke had likely occurred recently, and it was safe to administer t-PA. This allowed for administration of t-PA outside the standard treatment window of 4.5 hours from last known well, especially in the cases of patients who awoke with symptoms.
Since safety data are not yet available for the use of tenecteplase in this fashion, the WAKE-UP trial protocol was not used as an inclusion criterion. The informed consent form was modified, and the following scenarios were outlined: (1) If the patient or surrogate is immediately available to consent, paper consent will be documented with the additional note that tenecteplase is being used off-label; and (2) If the patient cannot consent and a surrogate is not immediately available, the medicine will be used emergently as long as the neurology resident and attending physicians agree.15
Risk mitigation was considered carefully. The stroke box described above is stocked and maintained by the pharmacy as we have transitioned to using designated pharmacists for the storage and preparation of tenecteplase. We highly recommend the use of designated pharmacists or emergency department pharmacists in this manner to avoid dosing errors.7,16 Since the current pharmacy-provided tenecteplase bottle contains twice the maximum dose indicated for ischemic stroke, only a 5 mL syringe is included in the stroke box to ensure a maximum dose of 25 mg is drawn up after reconstitution. Dosing card charts were made like existing dosing card charts for t-PA to quickly calculate the 0.25 mg/kg dose. In training, the difference in dosing in ischemic stroke was emphasized. Finally, pharmacy has taken responsibility for dosing the medication during stroke codes.
Any medical personnel at WRNMMC can initiate a stroke code by sending a page to the neurology consult service (Figure).
TRANSITION AND RESULTS
From November 2020 to December 2021, 10 patients have been treated in total at WRNMMC (Table).
CONCLUSIONS
The available evidence supports the transition from t-PA to tenecteplase for acute ischemic stroke. The successful transition required months of preparation involving multidisciplinary meetings between neurology, nursing, pharmacy, radiology, rapid response teams, critical care, and emergency medicine departments. Safeguards must be implemented to avoid a tenecteplase dosing error that can lead to potentially life-threatening adverse effects. The results at WRNMMC thus far are promising for safety and efficacy. Several process improvements are planned: a hospital-wide overhead page will accompany the direct page to neurology; other team members, including radiology and pharmacy, will be included on the acute stroke alert; and a stroke-specific paging application will be implemented to better track real-time stroke metrics and improve flow. These measures mirror processes that are occurring in institutions that treat acute stroke patients.
1. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695-1703. doi:10.1016/S0140-6736(10)60491-6
2. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581- 1587. doi:10.1056/NEJM199512143332401
3. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768-774. doi:10.1016/S0140-6736(04)15692-4
4. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi:10.1161/STR.0b013e318284056a
5. Campbell B, Mitchell P, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. doi:10.1056/nejmoa1716405
6. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi:10.1056/NEJMoa2001123
7. Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, noninferiority trial. Lancet. 2022;400(10347):161-169. doi:10.1016/S0140-6736(22)01054-6
8. Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA. 2020;323(13):1257- 1265. doi:10.1001/jama.2020.1511
9. Warach SJ, Dula AN, Milling TJ Jr. Tenecteplase thrombolysis for acute ischemic stroke. Stroke. 2020;51(11):3440- 3451. doi:10.1161/STROKEAHA.120.029749
10. Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke. 2015;46(12):3543-3546. doi:10.1161/STROKEAHA.115.011290
11. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
12. Potla N, Ganti L. Tenecteplase vs. alteplase for acute ischemic stroke: a systematic review. Int J Emerg Med. 2022;15(1). doi:10.1186/s12245-021-00399-w
13. Warach SJ, Winegar A, Ottenbacher A, Miller C, Gibson D. Abstract WMP52: reduced hospital costs for ischemic stroke treated with tenecteplase. Stroke. 2022;53(suppl 1):AWMP52. doi:10.1161/str.53.suppl_1.WMP52
14. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/str.0000000000000211
15. Faris H, Dewar B, Dowlatshahi D, et al. Ethical justification for deferral of consent in the AcT trial for acute ischemic stroke. Stroke. 2022;53(7):2420-2423. doi:10.1161/strokeaha.122.038760
16. Kvistad CE, Næss H, Helleberg BH, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol. 2022;21(6):511-519. doi:10.1016/S1474-4422(22)00124-7
1. Lees KR, Bluhmki E, von Kummer R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695-1703. doi:10.1016/S0140-6736(10)60491-6
2. National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581- 1587. doi:10.1056/NEJM199512143332401
3. Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004;363(9411):768-774. doi:10.1016/S0140-6736(04)15692-4
4. Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi:10.1161/STR.0b013e318284056a
5. Campbell B, Mitchell P, Churilov L, et al. Tenecteplase versus alteplase before thrombectomy for ischemic stroke. N Engl J Med. 2018;378(17):1573-1582. doi:10.1056/nejmoa1716405
6. Yang P, Zhang Y, Zhang L, et al. Endovascular thrombectomy with or without intravenous alteplase in acute stroke. N Engl J Med. 2020;382(21):1981-1993. doi:10.1056/NEJMoa2001123
7. Menon BK, Buck BH, Singh N, et al. Intravenous tenecteplase compared with alteplase for acute ischaemic stroke in Canada (AcT): a pragmatic, multicentre, open-label, registry-linked, randomised, controlled, noninferiority trial. Lancet. 2022;400(10347):161-169. doi:10.1016/S0140-6736(22)01054-6
8. Campbell BCV, Mitchell PJ, Churilov L, et al. Effect of intravenous tenecteplase dose on cerebral reperfusion before thrombectomy in patients with large vessel occlusion ischemic stroke: the EXTEND-IA TNK part 2 randomized clinical trial. JAMA. 2020;323(13):1257- 1265. doi:10.1001/jama.2020.1511
9. Warach SJ, Dula AN, Milling TJ Jr. Tenecteplase thrombolysis for acute ischemic stroke. Stroke. 2020;51(11):3440- 3451. doi:10.1161/STROKEAHA.120.029749
10. Huang X, Moreton FC, Kalladka D, et al. Coagulation and fibrinolytic activity of tenecteplase and alteplase in acute ischemic stroke. Stroke. 2015;46(12):3543-3546. doi:10.1161/STROKEAHA.115.011290
11. Burgos AM, Saver JL. Evidence that tenecteplase is noninferior to alteplase for acute ischemic stroke: meta-analysis of 5 randomized trials. Stroke. 2019;50(8):2156-2162. doi:10.1161/STROKEAHA.119.025080
12. Potla N, Ganti L. Tenecteplase vs. alteplase for acute ischemic stroke: a systematic review. Int J Emerg Med. 2022;15(1). doi:10.1186/s12245-021-00399-w
13. Warach SJ, Winegar A, Ottenbacher A, Miller C, Gibson D. Abstract WMP52: reduced hospital costs for ischemic stroke treated with tenecteplase. Stroke. 2022;53(suppl 1):AWMP52. doi:10.1161/str.53.suppl_1.WMP52
14. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/str.0000000000000211
15. Faris H, Dewar B, Dowlatshahi D, et al. Ethical justification for deferral of consent in the AcT trial for acute ischemic stroke. Stroke. 2022;53(7):2420-2423. doi:10.1161/strokeaha.122.038760
16. Kvistad CE, Næss H, Helleberg BH, et al. Tenecteplase versus alteplase for the management of acute ischaemic stroke in Norway (NOR-TEST 2, part A): a phase 3, randomised, open-label, blinded endpoint, non-inferiority trial. Lancet Neurol. 2022;21(6):511-519. doi:10.1016/S1474-4422(22)00124-7
A Transdisciplinary Program for Care of Veterans With Neurocognitive Disorders
Dementia is a devastating condition resulting in major functional, emotional, and financial impact on patients, their caregivers, and families. Approximately 6.5 million Americans are living with Alzheimer disease (AD), the most common of many causes of dementia.1 The prevalence of AD could increase to 12.7 million Americans by 2050 as the population ages.1 Studies suggest that dementia, also known as major neurocognitive disorder, is common and underdiagnosed among US veterans, a population with a mean age of 65 years.2 During cognitive screening, memory impairment is present in approximately 20% of veterans aged ≥ 75 years who have not been diagnosed with a neurocognitive disorder.3 In addition, veterans might be particularly vulnerable to dementia at an earlier age than the general population because of vascular risk factors and traumatic brain injuries.4 These concerns highlight the need for effective dementia care programs at US Department of Veterans Affairs (VA) facilities.
The US health care system often does not adequately address the needs of patients with dementia and their caregivers.5 Dementia care requires specialized medical care among collaborating professionals and caregiver and psychosocial interventions and services. However, the US health care system is fragmented with different clinicians and services siloed into separate practices and most dementia care occurring in primary care settings.6 Primary care professionals (PCPs) often are uncomfortable diagnosing and managing dementia because of time constraints, lack of expertise and training, and inability to deal with the range of care needs.7 PCPs do not identify approximately 42% of their patients with dementia and, when recognized, do not adhere to dementia care guidelines and address caregiver needs.8-10 Research indicates that caregiver support improves dementia care by teaching behavioral management skills and caregiver coping strategies, allowing patients to stay at home and delay institutionalization.6,11,12 Clinicians underuse available resources and do not incorporate them in their patient care.10 These community services benefit patients and caregivers and significantly improve the overall quality of care.6
Memory clinics have emerged to address these deficiencies when managing dementia.13 The most effective memory clinics maximize the use of specialists with different expertise in dementia care, particularly integrated programs where disciplines function together rather than independently.1,5,14 Systematic reviews and meta-analyses have documented the effectiveness of collaborative care management programs.11,12,15 Integration of dementia care management is associated with earlier diagnosis and interventions, decreased functional and cognitive symptom severity, decreased or delayed institutionalization, improved quality of life for patients and caregivers, enhanced overall quality of care and cost-effectiveness, and better integration of community services.11,12,14-19 In these programs, designating a dementia care manager (DCM) as the patient’s advocate facilitates the integrated structure, increases the quality of care, helps caregivers, facilitates adherence to dementia practice guidelines, and prevents behavioral and psychological symptoms of dementia (BPSD).1,6,11,12,20,21
The best interprofessional model for dementia care might be the transdisciplinary model that includes a DCM. To meet the specific demands of dementia care, there must be a high level of interprofessional collaboration rather than multiple health care professionals (HCPs) delivering care in isolation—an approach that is time consuming and often difficult to implement.22 Whereas multidisciplinary care refers to delivery of parallel services and interdisciplinary care implies a joint formulation, transdisciplinary care aims to maximize integration of HCPs and their specific expertise and contributions through interactions and discussions that deliver focused input to the lead physician. The transdisciplinary model addresses needs that often are missed and can minimize disparities in the quality of dementia care.23 A DCM is an integral part of our program, facilitating understanding and implementation of the final care plan and providing long-term follow-up and care. We outline a conference-centered transdisciplinary dementia care model with a social worker as DCM (SW-DCM) at our VA medical center.
Program Description
In 2020, the VA Greater Los Angeles Healthcare System (VAGLAHS) in California established a multispecialty clinic dedicated to evaluation and treatment of veterans with memory and neurocognitive disorders and to provide support for their caregivers and families. With the agreement of leadership in mental health, neurology, and geriatrics services on the importance of collaboration for dementia care, the psychiatry and neurology services created a joint Memory and Neurobehavior Clinic, which completed its first 2 years of operation as a full-day program. In recent months, the clinic has scheduled 24 veterans per day, approximately 50% new evaluations and 50% follow-up patients, with wait times of < 2 months. There is a mean of 12 intake or lead physicians who could attend sessions in the morning, afternoon, or both. The general clinic flow consists of a 2-hour intake evaluation of new referrals by the lead physician followed by a clinic conference with transdisciplinary discussion. The DCM then follows up with the veteran/caregiver presenting a final care plan individualized to the veterans, caregivers, and families.
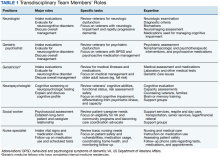
The Memory and Neurobehavior team includes behavioral neurologists, geriatric psychiatrists, neuropsychologists, geriatric fellows, advanced clinical nurses, and social workers who function as the DCM (Table 1).
Procedures
Before the office visit, the coordinating geriatric psychiatrist triages veterans to neurology, psychiatry, or geriatric physicians based on the clinical presentation, history of neurologic signs or symptoms, BPSD or psychiatric history, functional decline, or comorbid medical illnesses. Although veterans often have overlapping concerns, the triage process aims to coordinate the intake evaluations with the most indicated and available specialist with the intention to notify the other specialists during the transdisciplinary conference.
Referrals to the program occur from many sources, notably from primary care (70.8%), mental health (16.7%), and specialty clinics (12.5%). The clinic also receives referrals from the affiliated Veterans Cognitive Assessment and Management Program, which provides dementia evaluation and support via telehealth screening. This VAGLAHS program services a diverse population of veterans: 87% male; 43% aged > 65 years (75% in our clinic); 51% non-Hispanic White; 19% non-Hispanic African American; 16% Hispanic; 4% Asian; and 1% Native American. This population receives care at regional VA medical centers and community-based outpatient clinics over a wide geographic service area.
The initial standardized assessments by intake or lead physicians includes mental status screening with the Montreal Cognitive Assessment (with certified clinicians), the Neurobehavioral Status Examination for a more detailed assessment of cognitive domains, the Columbia-Suicide Severity Rating Scale, the Patient Health Questionnaire for depression screening, and assessment for impairments in instrumental or basic activities of daily living. This initial evaluation aims to apply clinical guidelines and diagnostic criteria for the differential diagnosis of neurocognitive disorders, determine eligibility for cognitive-enhancing medications and techniques, assess for BPSD and the need for nonpharmacologic or pharmacologic interventions, determine functional status, and evaluate the need for supervision, safety concerns, and evidence of neglect or abuse.
As part of its mission, the clinic is charged with implementing the VA Dementia System of Care (DSOC). The stated goals of the DSOC are to provide individualized person-centered dementia care to help veterans experiencing dementia and their caregivers maintain a positive and optimal quality of life and create an environment where VA medical center staff understand the health care needs of veterans with dementia and their caregivers’ role. As part of this initiative, the clinic includes (1) coordination of care through a SW-DCM; (2)
Transdisciplinary Conference
Clinic conferences are held after the veterans are seen. Staff gather to discuss the patient and review management. All team members are present, as well as the head of the clinical clerical staff who can facilitate appointments, make lobby and wait times more bearable for our patients and caregivers, and help manage emergencies. Although this is an in-person conference, the COVID-19 pandemic has allowed us to include staff who screen at remote sites via videoconferencing, similar to other VA programs.24 The Memory and Neurobehavior Clinic has two ≤ 90-minute conferences daily. The lead physicians and their senior attendings present the new intake evaluations (4-6 at each conference session) with a preliminary formulation and questions for discussion. The moderator solicits contributions from the different disciplines, going from one to the next and recording their responses for each veteran. Further specialists are available for consultation through the conference mechanism if necessary. The final assessment is reviewed, a diagnosis is established, and a tailored, individualized care plan for adjusting or optimizing the veteran’s care is presented to the lead physician who makes the final determination. At the close of the conference, the team’s discussion is recorded along with the lead physician’s original detailed intake evaluation. Currently, the records go into the Computerized Patient Record System, but we are making plans to transition to Cerner as it is implemented.
During the discussion, team members review several areas of consideration. If there is neuroimaging, neurologists review the images projected on a large computer screen. Team members also will assess for the need to obtain biomarker studies, such as blood, cerebrospinal fluid, or positron emission tomography. Psychiatrists could review management of BPSD and use of psychotropic agents, and neuropsychologists might consider the need for more precise cognitive testing and whether a capacity assessment is indicated. Social work might bring up the need for a durable power of attorney as well as applicable caregiver and community resources. Geriatric medicine and nursing could provide input into medical management and care and the ability of veterans and caregivers to follow the prescribed regimen. Further areas of discussion include driving safety and restrictions on driving (as required in California) and the presence of guns in the home. Finally, brief education is provided in short 10-to-15-minute lectures covering pertinent topics so staff remain up-to-date in this changing field.
Postconference Continuity
After the conference, the SW-DCM continues to provide support throughout the disease course, helping veterans and their caregivers understand and follow through on the team’s recommendations. The SW-DCM, who is experienced and trained in case management, forms an ongoing relationship with the veterans and their caregivers and remains an advocate for their care. The SW-DCM communicates the final plan by phone and, when necessary, requests the lead physician to call to clarify any poorly understood or technical aspects of the care plan. About 50% of our veterans—primarily those who do not have a neurocognitive disorder or have mild cognitive impairment—return to their PCPs with our care plan consultation; about 25% are already enrolled in geriatric and other programs with long-term follow-up. The assigned SW-DCM follows up with the remaining veterans and caregivers regularly by phone, facilitates communication with other team members, and endeavors to assure postvisit continuity of care and support during advancing stages of the disease. In addition, the SW-DCM can provide supportive counseling and psychotherapy for stressed caregivers, refer to support groups and cognitive rehabilitation programs, and help develop long-term goals and consideration for supervised living environments. The nurse specialist participates with follow-up calls regarding medications and scheduled tests and appointments, clearing up confusion about instructions, avoiding medication errors, and providing education in dementia care. Both social worker and nurse are present throughout the week, reachable by phone, and, in turn, able to contact the clinic physicians for veterans’ needs.
Discussion
Because of the heterogenous medical and psychosocial needs of veterans with dementia and their caregivers, a transdisciplinary team with a dedicated DCM might offer the most effective and efficient model for dementia care. We present a transdisciplinary program that incorporates dementia specialists in a single evaluation by maximizing their time through a conference-centered program. Our program involves neurologists, psychiatrists, geriatricians, psychologists, nurses, and social workers collaborating and communicating to enact effective dementia care. It further meets the goals of the VA-DSOC in implementing individualized patient and caregiver care.
This transdisciplinary model addresses a number of issues, starting with the differential diagnosis of underlying neurologic conditions. Within the transdisciplinary team, the neurologist can provide specific insights into any neurologic findings and illnesses, such as Alzheimer disease and other neurodegenerative dementias, vascular dementia syndromes, normal pressure hydrocephalus, Creutzfeldt-Jakob disease, neurosyphilis, and others. Most veterans with dementia experience BPSD at some point during of their illness. The psychiatrists on the transdisciplinary team can maximize management of BPSD with nonpharmacologic interventions and the fewest and least aversive psychoactive medications. Our program also addresses the need for more precise cognitive evaluation. Neuropsychologists are present and available for administrating neuropsychologic tests and interpreting cognitive performance and any earlier neuropsychologic testing. This model also cares for the caregivers and assesses their needs. The social worker—as well as other members of the team—can provide caregivers with strategies for coping with disruptive and other behaviors related to dementia, counsel them on how to manage the veteran’s functional decline, and aid in establishing a safe living space. Because the social worker serves as a DCM, these coping and adjustment questions occupy significant clinical attention between appointments. This transdisciplinary model places the patient’s illness in the context of their functional status, diagnoses, and medications. The team geriatrician and the nurse specialist are indispensable resources. The clinic conference provides a teaching venue for staff and trainees and a mechanism to discuss new developments in dementia care, such as the increasing need to assess individuals with mild cognitive impairment.25 This model depends on the DCM’s invaluable role in ensuring implementation of the dementia care plan and continuity of care.
Conclusions
We describe effective dementia care with a transdisciplinary team in a conference setting and with the participation of a dedicated DCM.5 To date, this program appears to be an efficient, sustainable application of the limited resources allocated to dementia care. Nevertheless, we are collecting data to compare with performance measures, track use, and assess the programs effects on continuity of care. We look forward to presenting metrics from our program that show improvement in the health care for veterans experiencing a devastating and increasingly common disorder.
1. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700-789. doi:10.1002/alz.12638
2. National Center for Veterans Analysis and Statistics. Profile of veterans: 2016. Accessed October 12, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf
3. Chodosh J, Sultzer DL, Lee ML, et al. Memory impairment among primary care veterans. Aging Ment Health. 2007;11(4):444-450. doi:10.1080/13607860601086272
4. Kennedy E, Panahi S, Stewart IJ, et al. Traumatic brain injury and early onset dementia in post 9-11 veterans. Brain Inj. 2022;36(5):620-627. doi:10.1080/02699052.2022.20338465. Heintz H, Monette P, Epstein-Lubow G, Smith L, Rowlett S, Forester BP. Emerging collaborative care models for dementia care in the primary care setting: a narrative review. Am J Geriatr Psychiatry. 2020;28(3):320-330. doi:10.1016/j.jagp.2019.07.015
6. Reuben DB, Evertson LC, Wenger NS, et al. The University of California at Los Angeles Alzheimer’s and Dementia Care program for comprehensive, coordinated, patient-centered care: preliminary data. J Am Geriatr Soc. 2013;61(12):2214-2218. doi:10.1111/jgs.12562
7. Apesoa-Varano EC, Barker JC, Hinton L. Curing and caring: the work of primary care physicians with dementia patients. Qual Health Res. 2011;21(11):1469-1483. doi:10.1177/1049732311412788
8. Creavin ST, Noel-Storr AH, Langdon RJ, et al. Clinical judgement by primary care physicians for the diagnosis of all-cause dementia or cognitive impairment in symptomatic people. Cochrane Database Syst Rev. 2022;6:CD012558. doi:10.1002/14651858.CD012558.pub2
9. Sivananthan SN, Puyat JH, McGrail KM. Variations in self-reported practice of physicians providing clinical care to individuals with dementia: a systematic review. J Am Geriatr Soc. 2013;61(8):1277-1285. doi:10.1111/jgs.12368
10. Rosen CS, Chow HC, Greenbaum MA, et al. How well are clinicians following dementia practice guidelines? Alzheimer Dis Assoc Disord. 2002;16(1):15-23. doi:10.1097/00002093-200201000-00003
11. Reilly S, Miranda-Castillo C, Malouf R, et al. Case management approaches to home support for people with dementia. Cochrane Database Syst Rev. 2015;1:CD008345. doi:10.1002/14651858.CD008345.pub2
12. Tam-Tham H, Cepoiu-Martin M, Ronksley PE, Maxwell CJ, Hemmelgarn BR. Dementia case management and risk of long-term care placement: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2013;28(9):889-902. doi:10.1002/gps.3906
13. Jolley D, Benbow SM, Grizzell M. Memory clinics. Postgrad Med J. 2006;82(965):199-206. doi:10.1136/pgmj.2005.040592
14. Muhlichen F, Michalowsky B, Radke A, et al. Tasks and activities of an effective collaborative dementia care management program in German primary care. J Alzheimers Dis. 2022;87(4):1615-1625. doi:10.3233/JAD-215656
15. Somme D, Trouve H, Drame M, Gagnon D, Couturier Y, Saint-Jean O. Analysis of case management programs for patients with dementia: a systematic review. Alzheimers Dement. 2012;8(5):426-436. doi:10.1016/j.jalz.2011.06.004
16. Ramakers IH, Verhey FR. Development of memory clinics in the Netherlands: 1998 to 2009. Aging Ment Health. 2011;15(1):34-39. doi:10.1080/13607863.2010.519321
17. LaMantia MA, Alder CA, Callahan CM, et al. The aging brain care medical home: preliminary data. J Am Geriatr Soc. 2015;63(6):1209-1213. doi:10.1111/jgs.13447
18. Rubinsztein JS, van Rensburg MJ, Al-Salihy Z, et al. A memory clinic v. traditional community mental health team service: comparison of costs and quality. BJPsych Bull. 2015;39(1):6-11. doi:10.1192/pb.bp.113.044263
19. Lee L, Hillier LM, Harvey D. Integrating community services into primary care: improving the quality of dementia care. Neurodegener Dis Manag. 2014;4(1):11-21. doi:10.2217/nmt.13.72
20. Bass DM, Judge KS, Snow AL, et al. Caregiver outcomes of partners in dementia care: effect of a care coordination program for veterans with dementia and their family members and friends. J Am Geriatr Soc. 2013;61(8):1377-1386. doi:10.1111/jgs.12362
21. Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148-2157. doi:10.1001/jama.295.18.2148
22. Leggett A, Connell C, Dubin L, et al. Dementia care across a tertiary care health system: what exists now and what needs to change. J Am Med Dir Assoc. 2019;20(10):1307-12 e1. doi:10.1016/j.jamda.2019.04.006
23. Brown AF, Vassar SD, Connor KI, Vickrey BG. Collaborative care management reduces disparities in dementia care quality for caregivers with less education. J Am Geriatr Soc. 2013;61(2):243-251. doi:10.1111/jgs.12079
24. Powers BB, Homer MC, Morone N, Edmonds N, Rossi MI. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
25. Galvin JE, Aisen P, Langbaum JB, et al. Early stages of Alzheimer’s Disease: evolving the care team for optimal patient management. Front Neurol. 2020;11:592302. doi:10.3389/fneur.2020.592302
Dementia is a devastating condition resulting in major functional, emotional, and financial impact on patients, their caregivers, and families. Approximately 6.5 million Americans are living with Alzheimer disease (AD), the most common of many causes of dementia.1 The prevalence of AD could increase to 12.7 million Americans by 2050 as the population ages.1 Studies suggest that dementia, also known as major neurocognitive disorder, is common and underdiagnosed among US veterans, a population with a mean age of 65 years.2 During cognitive screening, memory impairment is present in approximately 20% of veterans aged ≥ 75 years who have not been diagnosed with a neurocognitive disorder.3 In addition, veterans might be particularly vulnerable to dementia at an earlier age than the general population because of vascular risk factors and traumatic brain injuries.4 These concerns highlight the need for effective dementia care programs at US Department of Veterans Affairs (VA) facilities.
The US health care system often does not adequately address the needs of patients with dementia and their caregivers.5 Dementia care requires specialized medical care among collaborating professionals and caregiver and psychosocial interventions and services. However, the US health care system is fragmented with different clinicians and services siloed into separate practices and most dementia care occurring in primary care settings.6 Primary care professionals (PCPs) often are uncomfortable diagnosing and managing dementia because of time constraints, lack of expertise and training, and inability to deal with the range of care needs.7 PCPs do not identify approximately 42% of their patients with dementia and, when recognized, do not adhere to dementia care guidelines and address caregiver needs.8-10 Research indicates that caregiver support improves dementia care by teaching behavioral management skills and caregiver coping strategies, allowing patients to stay at home and delay institutionalization.6,11,12 Clinicians underuse available resources and do not incorporate them in their patient care.10 These community services benefit patients and caregivers and significantly improve the overall quality of care.6
Memory clinics have emerged to address these deficiencies when managing dementia.13 The most effective memory clinics maximize the use of specialists with different expertise in dementia care, particularly integrated programs where disciplines function together rather than independently.1,5,14 Systematic reviews and meta-analyses have documented the effectiveness of collaborative care management programs.11,12,15 Integration of dementia care management is associated with earlier diagnosis and interventions, decreased functional and cognitive symptom severity, decreased or delayed institutionalization, improved quality of life for patients and caregivers, enhanced overall quality of care and cost-effectiveness, and better integration of community services.11,12,14-19 In these programs, designating a dementia care manager (DCM) as the patient’s advocate facilitates the integrated structure, increases the quality of care, helps caregivers, facilitates adherence to dementia practice guidelines, and prevents behavioral and psychological symptoms of dementia (BPSD).1,6,11,12,20,21
The best interprofessional model for dementia care might be the transdisciplinary model that includes a DCM. To meet the specific demands of dementia care, there must be a high level of interprofessional collaboration rather than multiple health care professionals (HCPs) delivering care in isolation—an approach that is time consuming and often difficult to implement.22 Whereas multidisciplinary care refers to delivery of parallel services and interdisciplinary care implies a joint formulation, transdisciplinary care aims to maximize integration of HCPs and their specific expertise and contributions through interactions and discussions that deliver focused input to the lead physician. The transdisciplinary model addresses needs that often are missed and can minimize disparities in the quality of dementia care.23 A DCM is an integral part of our program, facilitating understanding and implementation of the final care plan and providing long-term follow-up and care. We outline a conference-centered transdisciplinary dementia care model with a social worker as DCM (SW-DCM) at our VA medical center.
Program Description
In 2020, the VA Greater Los Angeles Healthcare System (VAGLAHS) in California established a multispecialty clinic dedicated to evaluation and treatment of veterans with memory and neurocognitive disorders and to provide support for their caregivers and families. With the agreement of leadership in mental health, neurology, and geriatrics services on the importance of collaboration for dementia care, the psychiatry and neurology services created a joint Memory and Neurobehavior Clinic, which completed its first 2 years of operation as a full-day program. In recent months, the clinic has scheduled 24 veterans per day, approximately 50% new evaluations and 50% follow-up patients, with wait times of < 2 months. There is a mean of 12 intake or lead physicians who could attend sessions in the morning, afternoon, or both. The general clinic flow consists of a 2-hour intake evaluation of new referrals by the lead physician followed by a clinic conference with transdisciplinary discussion. The DCM then follows up with the veteran/caregiver presenting a final care plan individualized to the veterans, caregivers, and families.
The Memory and Neurobehavior team includes behavioral neurologists, geriatric psychiatrists, neuropsychologists, geriatric fellows, advanced clinical nurses, and social workers who function as the DCM (Table 1).
Procedures
Before the office visit, the coordinating geriatric psychiatrist triages veterans to neurology, psychiatry, or geriatric physicians based on the clinical presentation, history of neurologic signs or symptoms, BPSD or psychiatric history, functional decline, or comorbid medical illnesses. Although veterans often have overlapping concerns, the triage process aims to coordinate the intake evaluations with the most indicated and available specialist with the intention to notify the other specialists during the transdisciplinary conference.
Referrals to the program occur from many sources, notably from primary care (70.8%), mental health (16.7%), and specialty clinics (12.5%). The clinic also receives referrals from the affiliated Veterans Cognitive Assessment and Management Program, which provides dementia evaluation and support via telehealth screening. This VAGLAHS program services a diverse population of veterans: 87% male; 43% aged > 65 years (75% in our clinic); 51% non-Hispanic White; 19% non-Hispanic African American; 16% Hispanic; 4% Asian; and 1% Native American. This population receives care at regional VA medical centers and community-based outpatient clinics over a wide geographic service area.
The initial standardized assessments by intake or lead physicians includes mental status screening with the Montreal Cognitive Assessment (with certified clinicians), the Neurobehavioral Status Examination for a more detailed assessment of cognitive domains, the Columbia-Suicide Severity Rating Scale, the Patient Health Questionnaire for depression screening, and assessment for impairments in instrumental or basic activities of daily living. This initial evaluation aims to apply clinical guidelines and diagnostic criteria for the differential diagnosis of neurocognitive disorders, determine eligibility for cognitive-enhancing medications and techniques, assess for BPSD and the need for nonpharmacologic or pharmacologic interventions, determine functional status, and evaluate the need for supervision, safety concerns, and evidence of neglect or abuse.
As part of its mission, the clinic is charged with implementing the VA Dementia System of Care (DSOC). The stated goals of the DSOC are to provide individualized person-centered dementia care to help veterans experiencing dementia and their caregivers maintain a positive and optimal quality of life and create an environment where VA medical center staff understand the health care needs of veterans with dementia and their caregivers’ role. As part of this initiative, the clinic includes (1) coordination of care through a SW-DCM; (2)
Transdisciplinary Conference
Clinic conferences are held after the veterans are seen. Staff gather to discuss the patient and review management. All team members are present, as well as the head of the clinical clerical staff who can facilitate appointments, make lobby and wait times more bearable for our patients and caregivers, and help manage emergencies. Although this is an in-person conference, the COVID-19 pandemic has allowed us to include staff who screen at remote sites via videoconferencing, similar to other VA programs.24 The Memory and Neurobehavior Clinic has two ≤ 90-minute conferences daily. The lead physicians and their senior attendings present the new intake evaluations (4-6 at each conference session) with a preliminary formulation and questions for discussion. The moderator solicits contributions from the different disciplines, going from one to the next and recording their responses for each veteran. Further specialists are available for consultation through the conference mechanism if necessary. The final assessment is reviewed, a diagnosis is established, and a tailored, individualized care plan for adjusting or optimizing the veteran’s care is presented to the lead physician who makes the final determination. At the close of the conference, the team’s discussion is recorded along with the lead physician’s original detailed intake evaluation. Currently, the records go into the Computerized Patient Record System, but we are making plans to transition to Cerner as it is implemented.
During the discussion, team members review several areas of consideration. If there is neuroimaging, neurologists review the images projected on a large computer screen. Team members also will assess for the need to obtain biomarker studies, such as blood, cerebrospinal fluid, or positron emission tomography. Psychiatrists could review management of BPSD and use of psychotropic agents, and neuropsychologists might consider the need for more precise cognitive testing and whether a capacity assessment is indicated. Social work might bring up the need for a durable power of attorney as well as applicable caregiver and community resources. Geriatric medicine and nursing could provide input into medical management and care and the ability of veterans and caregivers to follow the prescribed regimen. Further areas of discussion include driving safety and restrictions on driving (as required in California) and the presence of guns in the home. Finally, brief education is provided in short 10-to-15-minute lectures covering pertinent topics so staff remain up-to-date in this changing field.
Postconference Continuity
After the conference, the SW-DCM continues to provide support throughout the disease course, helping veterans and their caregivers understand and follow through on the team’s recommendations. The SW-DCM, who is experienced and trained in case management, forms an ongoing relationship with the veterans and their caregivers and remains an advocate for their care. The SW-DCM communicates the final plan by phone and, when necessary, requests the lead physician to call to clarify any poorly understood or technical aspects of the care plan. About 50% of our veterans—primarily those who do not have a neurocognitive disorder or have mild cognitive impairment—return to their PCPs with our care plan consultation; about 25% are already enrolled in geriatric and other programs with long-term follow-up. The assigned SW-DCM follows up with the remaining veterans and caregivers regularly by phone, facilitates communication with other team members, and endeavors to assure postvisit continuity of care and support during advancing stages of the disease. In addition, the SW-DCM can provide supportive counseling and psychotherapy for stressed caregivers, refer to support groups and cognitive rehabilitation programs, and help develop long-term goals and consideration for supervised living environments. The nurse specialist participates with follow-up calls regarding medications and scheduled tests and appointments, clearing up confusion about instructions, avoiding medication errors, and providing education in dementia care. Both social worker and nurse are present throughout the week, reachable by phone, and, in turn, able to contact the clinic physicians for veterans’ needs.
Discussion
Because of the heterogenous medical and psychosocial needs of veterans with dementia and their caregivers, a transdisciplinary team with a dedicated DCM might offer the most effective and efficient model for dementia care. We present a transdisciplinary program that incorporates dementia specialists in a single evaluation by maximizing their time through a conference-centered program. Our program involves neurologists, psychiatrists, geriatricians, psychologists, nurses, and social workers collaborating and communicating to enact effective dementia care. It further meets the goals of the VA-DSOC in implementing individualized patient and caregiver care.
This transdisciplinary model addresses a number of issues, starting with the differential diagnosis of underlying neurologic conditions. Within the transdisciplinary team, the neurologist can provide specific insights into any neurologic findings and illnesses, such as Alzheimer disease and other neurodegenerative dementias, vascular dementia syndromes, normal pressure hydrocephalus, Creutzfeldt-Jakob disease, neurosyphilis, and others. Most veterans with dementia experience BPSD at some point during of their illness. The psychiatrists on the transdisciplinary team can maximize management of BPSD with nonpharmacologic interventions and the fewest and least aversive psychoactive medications. Our program also addresses the need for more precise cognitive evaluation. Neuropsychologists are present and available for administrating neuropsychologic tests and interpreting cognitive performance and any earlier neuropsychologic testing. This model also cares for the caregivers and assesses their needs. The social worker—as well as other members of the team—can provide caregivers with strategies for coping with disruptive and other behaviors related to dementia, counsel them on how to manage the veteran’s functional decline, and aid in establishing a safe living space. Because the social worker serves as a DCM, these coping and adjustment questions occupy significant clinical attention between appointments. This transdisciplinary model places the patient’s illness in the context of their functional status, diagnoses, and medications. The team geriatrician and the nurse specialist are indispensable resources. The clinic conference provides a teaching venue for staff and trainees and a mechanism to discuss new developments in dementia care, such as the increasing need to assess individuals with mild cognitive impairment.25 This model depends on the DCM’s invaluable role in ensuring implementation of the dementia care plan and continuity of care.
Conclusions
We describe effective dementia care with a transdisciplinary team in a conference setting and with the participation of a dedicated DCM.5 To date, this program appears to be an efficient, sustainable application of the limited resources allocated to dementia care. Nevertheless, we are collecting data to compare with performance measures, track use, and assess the programs effects on continuity of care. We look forward to presenting metrics from our program that show improvement in the health care for veterans experiencing a devastating and increasingly common disorder.
Dementia is a devastating condition resulting in major functional, emotional, and financial impact on patients, their caregivers, and families. Approximately 6.5 million Americans are living with Alzheimer disease (AD), the most common of many causes of dementia.1 The prevalence of AD could increase to 12.7 million Americans by 2050 as the population ages.1 Studies suggest that dementia, also known as major neurocognitive disorder, is common and underdiagnosed among US veterans, a population with a mean age of 65 years.2 During cognitive screening, memory impairment is present in approximately 20% of veterans aged ≥ 75 years who have not been diagnosed with a neurocognitive disorder.3 In addition, veterans might be particularly vulnerable to dementia at an earlier age than the general population because of vascular risk factors and traumatic brain injuries.4 These concerns highlight the need for effective dementia care programs at US Department of Veterans Affairs (VA) facilities.
The US health care system often does not adequately address the needs of patients with dementia and their caregivers.5 Dementia care requires specialized medical care among collaborating professionals and caregiver and psychosocial interventions and services. However, the US health care system is fragmented with different clinicians and services siloed into separate practices and most dementia care occurring in primary care settings.6 Primary care professionals (PCPs) often are uncomfortable diagnosing and managing dementia because of time constraints, lack of expertise and training, and inability to deal with the range of care needs.7 PCPs do not identify approximately 42% of their patients with dementia and, when recognized, do not adhere to dementia care guidelines and address caregiver needs.8-10 Research indicates that caregiver support improves dementia care by teaching behavioral management skills and caregiver coping strategies, allowing patients to stay at home and delay institutionalization.6,11,12 Clinicians underuse available resources and do not incorporate them in their patient care.10 These community services benefit patients and caregivers and significantly improve the overall quality of care.6
Memory clinics have emerged to address these deficiencies when managing dementia.13 The most effective memory clinics maximize the use of specialists with different expertise in dementia care, particularly integrated programs where disciplines function together rather than independently.1,5,14 Systematic reviews and meta-analyses have documented the effectiveness of collaborative care management programs.11,12,15 Integration of dementia care management is associated with earlier diagnosis and interventions, decreased functional and cognitive symptom severity, decreased or delayed institutionalization, improved quality of life for patients and caregivers, enhanced overall quality of care and cost-effectiveness, and better integration of community services.11,12,14-19 In these programs, designating a dementia care manager (DCM) as the patient’s advocate facilitates the integrated structure, increases the quality of care, helps caregivers, facilitates adherence to dementia practice guidelines, and prevents behavioral and psychological symptoms of dementia (BPSD).1,6,11,12,20,21
The best interprofessional model for dementia care might be the transdisciplinary model that includes a DCM. To meet the specific demands of dementia care, there must be a high level of interprofessional collaboration rather than multiple health care professionals (HCPs) delivering care in isolation—an approach that is time consuming and often difficult to implement.22 Whereas multidisciplinary care refers to delivery of parallel services and interdisciplinary care implies a joint formulation, transdisciplinary care aims to maximize integration of HCPs and their specific expertise and contributions through interactions and discussions that deliver focused input to the lead physician. The transdisciplinary model addresses needs that often are missed and can minimize disparities in the quality of dementia care.23 A DCM is an integral part of our program, facilitating understanding and implementation of the final care plan and providing long-term follow-up and care. We outline a conference-centered transdisciplinary dementia care model with a social worker as DCM (SW-DCM) at our VA medical center.
Program Description
In 2020, the VA Greater Los Angeles Healthcare System (VAGLAHS) in California established a multispecialty clinic dedicated to evaluation and treatment of veterans with memory and neurocognitive disorders and to provide support for their caregivers and families. With the agreement of leadership in mental health, neurology, and geriatrics services on the importance of collaboration for dementia care, the psychiatry and neurology services created a joint Memory and Neurobehavior Clinic, which completed its first 2 years of operation as a full-day program. In recent months, the clinic has scheduled 24 veterans per day, approximately 50% new evaluations and 50% follow-up patients, with wait times of < 2 months. There is a mean of 12 intake or lead physicians who could attend sessions in the morning, afternoon, or both. The general clinic flow consists of a 2-hour intake evaluation of new referrals by the lead physician followed by a clinic conference with transdisciplinary discussion. The DCM then follows up with the veteran/caregiver presenting a final care plan individualized to the veterans, caregivers, and families.
The Memory and Neurobehavior team includes behavioral neurologists, geriatric psychiatrists, neuropsychologists, geriatric fellows, advanced clinical nurses, and social workers who function as the DCM (Table 1).
Procedures
Before the office visit, the coordinating geriatric psychiatrist triages veterans to neurology, psychiatry, or geriatric physicians based on the clinical presentation, history of neurologic signs or symptoms, BPSD or psychiatric history, functional decline, or comorbid medical illnesses. Although veterans often have overlapping concerns, the triage process aims to coordinate the intake evaluations with the most indicated and available specialist with the intention to notify the other specialists during the transdisciplinary conference.
Referrals to the program occur from many sources, notably from primary care (70.8%), mental health (16.7%), and specialty clinics (12.5%). The clinic also receives referrals from the affiliated Veterans Cognitive Assessment and Management Program, which provides dementia evaluation and support via telehealth screening. This VAGLAHS program services a diverse population of veterans: 87% male; 43% aged > 65 years (75% in our clinic); 51% non-Hispanic White; 19% non-Hispanic African American; 16% Hispanic; 4% Asian; and 1% Native American. This population receives care at regional VA medical centers and community-based outpatient clinics over a wide geographic service area.
The initial standardized assessments by intake or lead physicians includes mental status screening with the Montreal Cognitive Assessment (with certified clinicians), the Neurobehavioral Status Examination for a more detailed assessment of cognitive domains, the Columbia-Suicide Severity Rating Scale, the Patient Health Questionnaire for depression screening, and assessment for impairments in instrumental or basic activities of daily living. This initial evaluation aims to apply clinical guidelines and diagnostic criteria for the differential diagnosis of neurocognitive disorders, determine eligibility for cognitive-enhancing medications and techniques, assess for BPSD and the need for nonpharmacologic or pharmacologic interventions, determine functional status, and evaluate the need for supervision, safety concerns, and evidence of neglect or abuse.
As part of its mission, the clinic is charged with implementing the VA Dementia System of Care (DSOC). The stated goals of the DSOC are to provide individualized person-centered dementia care to help veterans experiencing dementia and their caregivers maintain a positive and optimal quality of life and create an environment where VA medical center staff understand the health care needs of veterans with dementia and their caregivers’ role. As part of this initiative, the clinic includes (1) coordination of care through a SW-DCM; (2)
Transdisciplinary Conference
Clinic conferences are held after the veterans are seen. Staff gather to discuss the patient and review management. All team members are present, as well as the head of the clinical clerical staff who can facilitate appointments, make lobby and wait times more bearable for our patients and caregivers, and help manage emergencies. Although this is an in-person conference, the COVID-19 pandemic has allowed us to include staff who screen at remote sites via videoconferencing, similar to other VA programs.24 The Memory and Neurobehavior Clinic has two ≤ 90-minute conferences daily. The lead physicians and their senior attendings present the new intake evaluations (4-6 at each conference session) with a preliminary formulation and questions for discussion. The moderator solicits contributions from the different disciplines, going from one to the next and recording their responses for each veteran. Further specialists are available for consultation through the conference mechanism if necessary. The final assessment is reviewed, a diagnosis is established, and a tailored, individualized care plan for adjusting or optimizing the veteran’s care is presented to the lead physician who makes the final determination. At the close of the conference, the team’s discussion is recorded along with the lead physician’s original detailed intake evaluation. Currently, the records go into the Computerized Patient Record System, but we are making plans to transition to Cerner as it is implemented.
During the discussion, team members review several areas of consideration. If there is neuroimaging, neurologists review the images projected on a large computer screen. Team members also will assess for the need to obtain biomarker studies, such as blood, cerebrospinal fluid, or positron emission tomography. Psychiatrists could review management of BPSD and use of psychotropic agents, and neuropsychologists might consider the need for more precise cognitive testing and whether a capacity assessment is indicated. Social work might bring up the need for a durable power of attorney as well as applicable caregiver and community resources. Geriatric medicine and nursing could provide input into medical management and care and the ability of veterans and caregivers to follow the prescribed regimen. Further areas of discussion include driving safety and restrictions on driving (as required in California) and the presence of guns in the home. Finally, brief education is provided in short 10-to-15-minute lectures covering pertinent topics so staff remain up-to-date in this changing field.
Postconference Continuity
After the conference, the SW-DCM continues to provide support throughout the disease course, helping veterans and their caregivers understand and follow through on the team’s recommendations. The SW-DCM, who is experienced and trained in case management, forms an ongoing relationship with the veterans and their caregivers and remains an advocate for their care. The SW-DCM communicates the final plan by phone and, when necessary, requests the lead physician to call to clarify any poorly understood or technical aspects of the care plan. About 50% of our veterans—primarily those who do not have a neurocognitive disorder or have mild cognitive impairment—return to their PCPs with our care plan consultation; about 25% are already enrolled in geriatric and other programs with long-term follow-up. The assigned SW-DCM follows up with the remaining veterans and caregivers regularly by phone, facilitates communication with other team members, and endeavors to assure postvisit continuity of care and support during advancing stages of the disease. In addition, the SW-DCM can provide supportive counseling and psychotherapy for stressed caregivers, refer to support groups and cognitive rehabilitation programs, and help develop long-term goals and consideration for supervised living environments. The nurse specialist participates with follow-up calls regarding medications and scheduled tests and appointments, clearing up confusion about instructions, avoiding medication errors, and providing education in dementia care. Both social worker and nurse are present throughout the week, reachable by phone, and, in turn, able to contact the clinic physicians for veterans’ needs.
Discussion
Because of the heterogenous medical and psychosocial needs of veterans with dementia and their caregivers, a transdisciplinary team with a dedicated DCM might offer the most effective and efficient model for dementia care. We present a transdisciplinary program that incorporates dementia specialists in a single evaluation by maximizing their time through a conference-centered program. Our program involves neurologists, psychiatrists, geriatricians, psychologists, nurses, and social workers collaborating and communicating to enact effective dementia care. It further meets the goals of the VA-DSOC in implementing individualized patient and caregiver care.
This transdisciplinary model addresses a number of issues, starting with the differential diagnosis of underlying neurologic conditions. Within the transdisciplinary team, the neurologist can provide specific insights into any neurologic findings and illnesses, such as Alzheimer disease and other neurodegenerative dementias, vascular dementia syndromes, normal pressure hydrocephalus, Creutzfeldt-Jakob disease, neurosyphilis, and others. Most veterans with dementia experience BPSD at some point during of their illness. The psychiatrists on the transdisciplinary team can maximize management of BPSD with nonpharmacologic interventions and the fewest and least aversive psychoactive medications. Our program also addresses the need for more precise cognitive evaluation. Neuropsychologists are present and available for administrating neuropsychologic tests and interpreting cognitive performance and any earlier neuropsychologic testing. This model also cares for the caregivers and assesses their needs. The social worker—as well as other members of the team—can provide caregivers with strategies for coping with disruptive and other behaviors related to dementia, counsel them on how to manage the veteran’s functional decline, and aid in establishing a safe living space. Because the social worker serves as a DCM, these coping and adjustment questions occupy significant clinical attention between appointments. This transdisciplinary model places the patient’s illness in the context of their functional status, diagnoses, and medications. The team geriatrician and the nurse specialist are indispensable resources. The clinic conference provides a teaching venue for staff and trainees and a mechanism to discuss new developments in dementia care, such as the increasing need to assess individuals with mild cognitive impairment.25 This model depends on the DCM’s invaluable role in ensuring implementation of the dementia care plan and continuity of care.
Conclusions
We describe effective dementia care with a transdisciplinary team in a conference setting and with the participation of a dedicated DCM.5 To date, this program appears to be an efficient, sustainable application of the limited resources allocated to dementia care. Nevertheless, we are collecting data to compare with performance measures, track use, and assess the programs effects on continuity of care. We look forward to presenting metrics from our program that show improvement in the health care for veterans experiencing a devastating and increasingly common disorder.
1. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700-789. doi:10.1002/alz.12638
2. National Center for Veterans Analysis and Statistics. Profile of veterans: 2016. Accessed October 12, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf
3. Chodosh J, Sultzer DL, Lee ML, et al. Memory impairment among primary care veterans. Aging Ment Health. 2007;11(4):444-450. doi:10.1080/13607860601086272
4. Kennedy E, Panahi S, Stewart IJ, et al. Traumatic brain injury and early onset dementia in post 9-11 veterans. Brain Inj. 2022;36(5):620-627. doi:10.1080/02699052.2022.20338465. Heintz H, Monette P, Epstein-Lubow G, Smith L, Rowlett S, Forester BP. Emerging collaborative care models for dementia care in the primary care setting: a narrative review. Am J Geriatr Psychiatry. 2020;28(3):320-330. doi:10.1016/j.jagp.2019.07.015
6. Reuben DB, Evertson LC, Wenger NS, et al. The University of California at Los Angeles Alzheimer’s and Dementia Care program for comprehensive, coordinated, patient-centered care: preliminary data. J Am Geriatr Soc. 2013;61(12):2214-2218. doi:10.1111/jgs.12562
7. Apesoa-Varano EC, Barker JC, Hinton L. Curing and caring: the work of primary care physicians with dementia patients. Qual Health Res. 2011;21(11):1469-1483. doi:10.1177/1049732311412788
8. Creavin ST, Noel-Storr AH, Langdon RJ, et al. Clinical judgement by primary care physicians for the diagnosis of all-cause dementia or cognitive impairment in symptomatic people. Cochrane Database Syst Rev. 2022;6:CD012558. doi:10.1002/14651858.CD012558.pub2
9. Sivananthan SN, Puyat JH, McGrail KM. Variations in self-reported practice of physicians providing clinical care to individuals with dementia: a systematic review. J Am Geriatr Soc. 2013;61(8):1277-1285. doi:10.1111/jgs.12368
10. Rosen CS, Chow HC, Greenbaum MA, et al. How well are clinicians following dementia practice guidelines? Alzheimer Dis Assoc Disord. 2002;16(1):15-23. doi:10.1097/00002093-200201000-00003
11. Reilly S, Miranda-Castillo C, Malouf R, et al. Case management approaches to home support for people with dementia. Cochrane Database Syst Rev. 2015;1:CD008345. doi:10.1002/14651858.CD008345.pub2
12. Tam-Tham H, Cepoiu-Martin M, Ronksley PE, Maxwell CJ, Hemmelgarn BR. Dementia case management and risk of long-term care placement: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2013;28(9):889-902. doi:10.1002/gps.3906
13. Jolley D, Benbow SM, Grizzell M. Memory clinics. Postgrad Med J. 2006;82(965):199-206. doi:10.1136/pgmj.2005.040592
14. Muhlichen F, Michalowsky B, Radke A, et al. Tasks and activities of an effective collaborative dementia care management program in German primary care. J Alzheimers Dis. 2022;87(4):1615-1625. doi:10.3233/JAD-215656
15. Somme D, Trouve H, Drame M, Gagnon D, Couturier Y, Saint-Jean O. Analysis of case management programs for patients with dementia: a systematic review. Alzheimers Dement. 2012;8(5):426-436. doi:10.1016/j.jalz.2011.06.004
16. Ramakers IH, Verhey FR. Development of memory clinics in the Netherlands: 1998 to 2009. Aging Ment Health. 2011;15(1):34-39. doi:10.1080/13607863.2010.519321
17. LaMantia MA, Alder CA, Callahan CM, et al. The aging brain care medical home: preliminary data. J Am Geriatr Soc. 2015;63(6):1209-1213. doi:10.1111/jgs.13447
18. Rubinsztein JS, van Rensburg MJ, Al-Salihy Z, et al. A memory clinic v. traditional community mental health team service: comparison of costs and quality. BJPsych Bull. 2015;39(1):6-11. doi:10.1192/pb.bp.113.044263
19. Lee L, Hillier LM, Harvey D. Integrating community services into primary care: improving the quality of dementia care. Neurodegener Dis Manag. 2014;4(1):11-21. doi:10.2217/nmt.13.72
20. Bass DM, Judge KS, Snow AL, et al. Caregiver outcomes of partners in dementia care: effect of a care coordination program for veterans with dementia and their family members and friends. J Am Geriatr Soc. 2013;61(8):1377-1386. doi:10.1111/jgs.12362
21. Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148-2157. doi:10.1001/jama.295.18.2148
22. Leggett A, Connell C, Dubin L, et al. Dementia care across a tertiary care health system: what exists now and what needs to change. J Am Med Dir Assoc. 2019;20(10):1307-12 e1. doi:10.1016/j.jamda.2019.04.006
23. Brown AF, Vassar SD, Connor KI, Vickrey BG. Collaborative care management reduces disparities in dementia care quality for caregivers with less education. J Am Geriatr Soc. 2013;61(2):243-251. doi:10.1111/jgs.12079
24. Powers BB, Homer MC, Morone N, Edmonds N, Rossi MI. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
25. Galvin JE, Aisen P, Langbaum JB, et al. Early stages of Alzheimer’s Disease: evolving the care team for optimal patient management. Front Neurol. 2020;11:592302. doi:10.3389/fneur.2020.592302
1. 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18(4):700-789. doi:10.1002/alz.12638
2. National Center for Veterans Analysis and Statistics. Profile of veterans: 2016. Accessed October 12, 2022. https://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2016.pdf
3. Chodosh J, Sultzer DL, Lee ML, et al. Memory impairment among primary care veterans. Aging Ment Health. 2007;11(4):444-450. doi:10.1080/13607860601086272
4. Kennedy E, Panahi S, Stewart IJ, et al. Traumatic brain injury and early onset dementia in post 9-11 veterans. Brain Inj. 2022;36(5):620-627. doi:10.1080/02699052.2022.20338465. Heintz H, Monette P, Epstein-Lubow G, Smith L, Rowlett S, Forester BP. Emerging collaborative care models for dementia care in the primary care setting: a narrative review. Am J Geriatr Psychiatry. 2020;28(3):320-330. doi:10.1016/j.jagp.2019.07.015
6. Reuben DB, Evertson LC, Wenger NS, et al. The University of California at Los Angeles Alzheimer’s and Dementia Care program for comprehensive, coordinated, patient-centered care: preliminary data. J Am Geriatr Soc. 2013;61(12):2214-2218. doi:10.1111/jgs.12562
7. Apesoa-Varano EC, Barker JC, Hinton L. Curing and caring: the work of primary care physicians with dementia patients. Qual Health Res. 2011;21(11):1469-1483. doi:10.1177/1049732311412788
8. Creavin ST, Noel-Storr AH, Langdon RJ, et al. Clinical judgement by primary care physicians for the diagnosis of all-cause dementia or cognitive impairment in symptomatic people. Cochrane Database Syst Rev. 2022;6:CD012558. doi:10.1002/14651858.CD012558.pub2
9. Sivananthan SN, Puyat JH, McGrail KM. Variations in self-reported practice of physicians providing clinical care to individuals with dementia: a systematic review. J Am Geriatr Soc. 2013;61(8):1277-1285. doi:10.1111/jgs.12368
10. Rosen CS, Chow HC, Greenbaum MA, et al. How well are clinicians following dementia practice guidelines? Alzheimer Dis Assoc Disord. 2002;16(1):15-23. doi:10.1097/00002093-200201000-00003
11. Reilly S, Miranda-Castillo C, Malouf R, et al. Case management approaches to home support for people with dementia. Cochrane Database Syst Rev. 2015;1:CD008345. doi:10.1002/14651858.CD008345.pub2
12. Tam-Tham H, Cepoiu-Martin M, Ronksley PE, Maxwell CJ, Hemmelgarn BR. Dementia case management and risk of long-term care placement: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2013;28(9):889-902. doi:10.1002/gps.3906
13. Jolley D, Benbow SM, Grizzell M. Memory clinics. Postgrad Med J. 2006;82(965):199-206. doi:10.1136/pgmj.2005.040592
14. Muhlichen F, Michalowsky B, Radke A, et al. Tasks and activities of an effective collaborative dementia care management program in German primary care. J Alzheimers Dis. 2022;87(4):1615-1625. doi:10.3233/JAD-215656
15. Somme D, Trouve H, Drame M, Gagnon D, Couturier Y, Saint-Jean O. Analysis of case management programs for patients with dementia: a systematic review. Alzheimers Dement. 2012;8(5):426-436. doi:10.1016/j.jalz.2011.06.004
16. Ramakers IH, Verhey FR. Development of memory clinics in the Netherlands: 1998 to 2009. Aging Ment Health. 2011;15(1):34-39. doi:10.1080/13607863.2010.519321
17. LaMantia MA, Alder CA, Callahan CM, et al. The aging brain care medical home: preliminary data. J Am Geriatr Soc. 2015;63(6):1209-1213. doi:10.1111/jgs.13447
18. Rubinsztein JS, van Rensburg MJ, Al-Salihy Z, et al. A memory clinic v. traditional community mental health team service: comparison of costs and quality. BJPsych Bull. 2015;39(1):6-11. doi:10.1192/pb.bp.113.044263
19. Lee L, Hillier LM, Harvey D. Integrating community services into primary care: improving the quality of dementia care. Neurodegener Dis Manag. 2014;4(1):11-21. doi:10.2217/nmt.13.72
20. Bass DM, Judge KS, Snow AL, et al. Caregiver outcomes of partners in dementia care: effect of a care coordination program for veterans with dementia and their family members and friends. J Am Geriatr Soc. 2013;61(8):1377-1386. doi:10.1111/jgs.12362
21. Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148-2157. doi:10.1001/jama.295.18.2148
22. Leggett A, Connell C, Dubin L, et al. Dementia care across a tertiary care health system: what exists now and what needs to change. J Am Med Dir Assoc. 2019;20(10):1307-12 e1. doi:10.1016/j.jamda.2019.04.006
23. Brown AF, Vassar SD, Connor KI, Vickrey BG. Collaborative care management reduces disparities in dementia care quality for caregivers with less education. J Am Geriatr Soc. 2013;61(2):243-251. doi:10.1111/jgs.12079
24. Powers BB, Homer MC, Morone N, Edmonds N, Rossi MI. Creation of an interprofessional teledementia clinic for rural veterans: preliminary data. J Am Geriatr Soc. 2017;65(5):1092-1099. doi:10.1111/jgs.14839
25. Galvin JE, Aisen P, Langbaum JB, et al. Early stages of Alzheimer’s Disease: evolving the care team for optimal patient management. Front Neurol. 2020;11:592302. doi:10.3389/fneur.2020.592302
Improving Patient Access to the My HealtheVet Electronic Patient Portal for Veterans
Patient portals are secure online website tools that provide patient access to personal health information (PHI). Access to online PHI improves health equity and satisfies the meaningful use objectives of the Medicare electronic health record (EHR) incentive program.1,2 Through patient portals, individuals can access PHI records and current diagnoses, request and reschedule appointments, locate test results, track trends for vital signs and laboratory values, refill medications, and communicate directly with the health care team through secure messaging. This alternative method of communication with the team is associated with increased patient satisfaction.3 Patients reported improved patient engagement in health care self-management and decision making, as well as strengthened relationships with their health care team.4
Background
One well-documented strategy to improve portal use includes the development of a nurse champion to facilitate enrollment during the clinic visit.5 Patient perceptions of portal value increased after education by a health care professional (HCP) and assistance in enrollment to familiarize patients with the platform for ongoing use.5 Use of patient portals has been associated with favorable outcomes in chronic disease management. Patients with diabetes mellitus who regularly use patient portals for prescription refills and secure messaging have demonstrated improved glycemic control, medication adherence, and associated health parameters compared with nonusers.5-7 In patients with congestive heart failure, meaningful patient portal use results in fewer emergency department visits, fewer hospital admissions, lower readmission rates, and reduced unscheduled and no-show visits.8-11
Patient portal access is a quality improvement (QI) measure that meets Medicare and Medicaid meaningful use requirements that is designed to improve collaboration between HCPs and patients through EHRs. Despite legislation, uptake of patient portal access has been slow, especially among older adults.10,12,13 Barriers to patient portal registration and use include patient lack of awareness, perceived or actual digital illiteracy, mistrust in privacy precautions, lack of user-friendly interfaces, lack of internet or technology, HCP bias and workload, and misperceptions of usefulness.9,10,12,14 The HCPs most likely to facilitate the use of patient portals, typically include nurse practitioners (NPs), nurses, and medical residents.10,15 Patient portal platforms promote the partnership of these disciplines with the veteran to help the patient better manage their health. Despite the benefits and widespread integration of patient portals in health care systems, socioeconomic inequalities and HCP attitudes contribute to persistent disparities in its adoption by underserved populations. The veteran population is often faced with additional barriers to health care access with regard to geographic location, advanced age, trauma, disabilities, mental health challenges, and homelessness.10,16 These barriers require unique approaches to maximize the use of technologic advances.17 Advanced age contributes to low rates of patient portal enrollment and lack of digital platform use, thus creating a digital divide.11,12
The digital divide is described as the gap between those persons who use technology including computers and internet, and those persons who do not because of social and geographic barriers.16 It contributes to a growing health disparity in both access to care and quality of care especially for rural veterans. About 25% of the US population lacks fixed broadband at home; these individuals are more likely to be racial minorities, older, widowed, or to have lower levels of education.18,19 Veterans are disproportionately represented in these demographic categories.20 According to the US Department of Veterans Affairs (VA) Office of Rural Health, the percentage of rural veterans enrolled in the VA health care system (58%) is significantly higher than enrollment of urban veterans (38%); additionally, 27% of rural veterans do not access the internet at home.21
My HealtheVet
The VA plays an integral part in increasing virtual access to care, from the introduction of My Healthevet (MHV) in 2003 to the distribution of iPad tablets to vulnerable veterans during the COVID-19 pandemic.22,23 Due to COVID-19, the need for VA patient access to the internet and VA Video-Connect (VVC) telehealth services increased significantly.22 Access to internet and hardware supporting use of VVC and MHV has been facilitated by the Digital Divide Consult, a VA program launched in 2020 to increase access to telehealth services.24 The VA has distributed > 26,000 cellular-enabled tablets and provided > 50,000 veterans with connectivity in collaboration with various private sector companies.22 Patients report that MHV facilitates engagement in health care through improved access to EHRs and expedited communication with the health care team.4
MHV is a secure online tool that provides patients access to PHI. MHV aims to empower veterans to take charge of their health by improving communication with HCPs, setting patient goals, and offering health and well-being resources.25,26 In a study of outpatients at a large urban multisite health care system, < 35% of patients on 16 medical resident panels were enrolled in a patient portal.15 MHV internal national metrics show increasing registration and active users of the patient portal, yet locally, disparities in the use of the portal by rural and older veterans exist.
The Local Problem
A review of the registration process at a rural VA clinic revealed barriers to facilitating the veteran registration process at the point of care. Clinical reminders exist within the EHR to prompt clinicians at the point of care to improve quality of care. At the New England Healthcare System (Veterans Integrated Service Network [VISN] 1), a patient portal clinical reminder prompts staff to encourage veterans to register. Anecdotal data obtained from primary care staff interviews at a rural VA primary care clinic in Vermont revealed low clinician confidence in completing the clinical reminder, a lack of knowledge of MHV, and lack of time to educate veterans about the benefits of MHV.
Despite availability of a registration process at the point of care and clinical staff assigned to provide registration information to the veteran, access to the patient portal among veterans at this clinic remained low. This QI project aimed to increase patient portal enrollment of veterans in MHV in a single NP patient panel of 100 patients from a baseline of 33% by 10% in a 3-month time frame.
Implementation
Before implementing the first Plan-Do-Study-Act (PDSA) cycle, we established the baseline data for 1 patient panel to be 33%. A retrospective review of the NP resident’s panel of 100 revealed 33 veterans were enrolled in MHV, providing a setting for process improvement. Evaluation of potential enrollment data for the panel population revealed unenrolled veterans were primarily aged ≥ 65 years. A rapid cycle QI (RCQI) strategy using the PDSA method was used to identify, implement, and measure changes over a 3-month time frame in 1 NP patient panel.14
The RCQI process included establishing baseline data and 3 PDSA cycles that evaluated the current state of patient access to the electronic patient portal, elucidated patient barriers to registration, assessed the processes for point-of-care enrollment, and developed strategies to improve the process and increase veteran enrollment. The QI project team included an NP resident as the project manager and MHV champion, a clinical faculty mentor at the site, a telehealth coordinator, an MHV coordinator, clinic registered nurse (RN), and clinic licensed nursing assistant (LNA). The RN and LNA additionally served as MHV champions as the project progressed.
PDSA Cycles
The objective for PDSA cycle 1 was to evaluate the process of patient registration and assess the impact on NP workload and clinic workflow over a 4-week period to improve veteran enrollment. Data were collected in a spreadsheet to track the number of veterans enrolled, time frame to enroll, and field notes that the NP resident recorded about the experience. The NP resident was trained in registration methods by the MHV coordinator. Several barriers to the registration process were identified: The process resulted in a change of the clinic visit closure focus, the clinic room was blocked for use by another patient, veterans had difficulty generating a unique username and password, veterans were unfamiliar with basic tablet accessibility and use, and additional time was required if incorrect information was entered. The veterans displayed low confidence in using tablet technology and were unaware of the patient portal or its usefulness. After discussion of the process with the project team, recommendations were made to address challenges, including an RN-led registration process. The first PDSA cycle increased the total patient panel enrollment by 4 veterans to 37%.
In PDSA cycle 2 after the NP visit, patients who agreed to register for the MHV portal were introduced to the tablet. The registration process was completed by the patient with the RN prior to the patient checkout. Once patient registration was completed, the veteran met the MHV coordinator and upgraded to a premium account, which provided full access to portal features. Electronic messaging was tested by the MHV coordinator and veteran to validate patient understanding. Although preloading demographic information improved accessibility issues, time was still required for the RN to orient the veteran to the tablet, provide additional directions, and answer questions.
The registration process reduced NP time commitment but added to the RN time burden and disrupted workflow; and clinic room access continued to be an issue. The wait time for the veteran to register in the clinic remained dependent on the availability of the RN. The decision was to move the registration process to the initial patient rooming assignment in the clinic and was transitioned from RN to LNA, prior to the NP-veteran encounter. Four additional veterans registered in the second PDSA cycle, and total enrollment increased to 41%, an overall 8% increase from baseline.
In the third PDSA cycle the patient enrollment process was managed by the clinic LNA using scripted information about MHV prior to the veteran encounter. A partially preloaded tablet was offered to the veteran to register with MHV during the rooming process, and written and oral instruction were provided to the veteran. The time required for each veteran to register for MHV averaged 10 minutes, and the veteran was able to register while waiting for the NP to enter the room. Typical LNA tasks included greeting patients, updating health records, completing clinical reminders with the veteran, obtaining vital signs, and addressing questions. The LNA introduced the veteran to MHV using scripted information and supported them in registering for MHV prior to the NP-veteran encounter. Registration at point of care during the rooming process was well received by both the LNA and veterans. The LNA reported the process was efficient and did not add excessive time to the LNA workflow. The LNA reported verbal patient satisfaction and registration was facilitated for 6 veterans during the 4-week period.
Registration during point of care was reported as feasible and sustainable by the LNA. Upgrading the patient to a premium MHV account was transitioned to the MHV coordinator. All veterans seen during the 4-week period were approached about registration; if the veteran declined, written at-home step-by-step instructions were provided. A replacement electronic clinical reminder was proposed to the VISN clinical reminders team for review and was pilot tested by the primary care clinical team. The third PDSA cycle increased the total patient panel enrollment to 47%, an overall 14% increase from baseline. Six new veteran users were added during PDSA cycle 3.
Discussion
The project team successfully used a RCQI method with a PDSA strategy to improve patient access to the MHV portal and increased veteran enrollment by 14% on 1 NP resident patient panel. The project evaluated clinic workflow regarding veteran patient portal registration, uncovered inefficiencies, and developed improved processes to increase veteran access to the patient portal. Results were positively impacted through the recognition of inefficiencies and initiation of new processes to engage veterans in the portal registration process. Familiarizing the entire clinical team with the clinical reminder and registration process raised the awareness of a digital divide consult and the utility of the portal in patient care. The project provided an opportunity to evaluate veterans’ digital literacy, digital access to send and receive messages, and to provide coaching as needed. Sequential PDSA cycles employed audit and feedback, information preloading, multimodal teaching strategies (verbal, print, hands-on tablet learning), scripting, staff interviews, time studies, and workflow evaluation to improve processes. An MHV champion led the team, monitored the progress, set deadlines, and effectively communicated project performance.
Limitations
Project limitations included the single-site location, its small sample size, and the short 3-month implementation time frame. The patient panel was representative of other NP resident patient panels at the facility but may not be representative of other VA facilities.
Ethical Considerations
Patient confidentiality was maintained throughout the registration and data collection process. The project team (NP, RN, LNA) received training and written instructions on protection of patient confidentiality by the MHV coordinator prior to assisting veterans with the registration process. Privacy was maintained, no patient identifiers were collected or viewed, and no assistance was provided for username, password, or security questions. The tablet was password protected and secured, used only by the project team when veteran was interested in point-of-care portal registration.
Sustainability
QI projects require ongoing systemic efforts to enhance sustainability.26,27 The project team used the PDSA methodology to stimulate the design of new workflow processes to engage staff and veterans in portal registration. Several actions were taken to promote sustainability for veteran portal registration and improve access to health care for rural and underserved veterans. First, printed instructions and website link are available in the clinic intake and examination rooms. Staff are equipped with patient education discussion points about the portal. A tablet is available in the clinic to encourage veterans to sign up. A clinical reminder is in place to encourage portal registration. A designated super-user is available to help new patient portal users register and navigate the system. Outcomes of the QI project were presented at 2 separate VISN 1 nursing grand rounds and reported to the MHV coordinator and telehealth coordinator to promote dialogue among staff and raise awareness of challenges to veteran MHV access.
Conclusions
Reviewing patient portal registration processes at the local level is essential to improve veteran access. This QI project proposed a realistic and scalable solution to implementing and improving patient enrollment to MHV in primary care clinics. Integrating measurement of patient registration into the daily routine of the clinic empowers the entire clinical team to improve the quality of access to patient portal.
The project team worked together to accomplish a shared goal, using errors as opportunities to improve the process, while using available staff without compromising significant time or resources. Engaging the entire team to audit processes and designating one member of the team as an MHV champion to provide feedback is critical to the sustainability of point-of-care registration in the MHV patient portal. Multifaceted approaches to maximizing the use of technology lessens the digital divide for veterans who are faced with geographical and social barriers to health care access.
Acknowledgments
We thank the Office of Academic Affiliations and the US Department of Veterans Affairs Nursing Academic Partnerships in Graduate Education Nurse Practitioner residency program and clinical faculty and the affiliated University of Vermont faculty mentor/quality improvement coach for the support of the project.
1. Centers for Medicare and Medicaid Services. Promoting interoperability programs. Updated October 6, 2022. Accessed November 3, 2022. https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms
2. American Hospital Association. Goals of the Medicare and Medicaid electronic health records programs. Accessed November 3, 2022. https://www.aha.org/websites/2009-12-11-goals-medicare-and-medicaid-electronic-health-records-programs
3. Rozenblum R, Donzé J, Hockey PM, et al. The impact of medical informatics on patient satisfaction: a USA-based literature review. Int J Med Inform. 2013;82(3):141-158. doi:10.1016/j.ijmedinf.2012.12.008
4. Stewart MT, Hogan TP, Nicklas J, et al. The promise of patient portals for individuals living with chronic illness: qualitative study identifying pathways of patient engagement. J Med Internet Res. 2020;22(7):e17744. Published 2020 Jul 17. doi:10.2196/17744
5. Harris LT, Haneuse SJ, Martin DP, Ralston JD. Diabetes quality of care and outpatient utilization associated with electronic patient-provider messaging: a cross-sectional analysis. Diabetes Care. 2009;32(7):1182-1187. doi:10.2337/dc08-1771
6. Robinson SA, Zocchi MS, Netherton D, et al. Secure messaging, diabetes self-management, and the importance of patient autonomy: a mixed methods study. J Gen Intern Med. 2020;35(10):2955-2962. doi:10.1007/s11606-020-05834-x
7. Zocchi MS, Robinson SA, Ash AS, et al. Patient portal engagement and diabetes management among new portal users in the Veterans Health Administration. J Am Med Inform Assoc. 2021;28(10):2176-2183. doi:10.1093/jamia/ocab115
8. Bao C, Bardhan IR, Singh H, Meyer BA, Kirksey K. Patient-provider engagement and its impact on health outcomes: a longitudinal study of patient portal use. MIS Quarterly. 2020;44(2):699-723. doi:10.25300/MISQ/2020/14180
9. Grossman LV, Masterson Creber RM, Benda NC, Wright D, Vawdrey DK, Ancker JS. Interventions to increase patient portal use in vulnerable populations: a systematic review. J Am Med Informs Assoc. 2019;26(8-9):855-870. doi:10.1093/jamia/ocz023
10. Zhao JY, Song B, Anand E, et al. Barriers, facilitators, and solutions to optimal patient portal and personal health record use: a systematic review of the literature. AMIA Annu Symp Proc. 2018;2017:1913-1922. Published 2018 Apr 16.
11. Zhong X, Park J, Liang M, et al. Characteristics of patients using different patient portal functions and the impact on primary care service utilization and appointment adherence: retrospective observational study. J Med Internet Res. 2020;22(2):e14410. Published 2020 Feb 25. doi:10.2196/14410
12. Krishnaswami A, Beavers C, Dorsch MP, et al. Gerotechnology for older adults with cardiovascular diseases. J Am Coll Cardiol. 2020;76(22):2650-2670. doi:10.1016/j.jacc.2020.09.606
13. Fix GM, Hogan TP, Amante DJ, McInnes DK, Nazi KM, Simon SR. Encouraging patient portal use in the patient-centered medical home: three stakeholder perspectives. J Med Internet Res. 2016;18(11):e308. Published 2016 Nov 22. doi:10.2196/jmir.6488
14. Ancker JS, Nosal S, Hauser D, Way C, Calman N. Access policy and the digital divide in patient access to medical records. Health Policy Technol. 2016;6(3-11). doi:10.1016/j.hlpt.2016.11.004
15. Rhudy C, Broxterman J, Stewart S, et al. Improving patient portal enrolment in an academic resident continuity clinic: quality improvement made simple. BMJ Open Qual. 2019;8(2):e000430. Published 2019 Apr 25. doi:10.1136/bmjoq-2018-000430
16. Kontos E, Blake KD, Chou WY, Prestin A. Predictors of eHealth usage: insights on the digital divide from the Health Information National Trends Survey 2012. J Med Internet Res. 2014;16(7):e172. Published 2014 Jul 16. doi:10.2196/jmir.3117
17. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice. The state of health disparities in the United States. In: Baciu A, Negussie Y, Geller A, et al, eds. Communities in Action: Pathways to Health Equity. National Academies Press (US); January 11, 2017. Accessed November 3, 2022. https://www.ncbi.nlm.nih.gov/books/NBK425848/
18. Pew Research Center. Internet/broadband fact sheet. Updated April 7, 2021. Accessed November 3, 2022. https://www.pewresearch.org/internet/fact-sheet/internet-broadband
19. Roberts ET, Mehrotra A. Assessment of disparities in digital access among Medicare beneficiaries and implications for telemedicine. JAMA Intern Med. 2020;180(10):1386-1389. doi:10.1001/jamainternmed.2020.2666
20. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed November 3, 2022. https://www.va.gov/vetdata/veteran_population.asp
21. US Department of Veterans Affairs, Office of Rural Health. Rural veterans health care challenges. Updated March 31, 2022. Accessed November 3, 2022. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
22. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands veteran access to telehealth with iPad services. Press release. September 15, 2020. Accessed November 3, 2022. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5521
23. Zulman DM, Wong EP, Slightam C, et al. Making connections: National implementation of video telehealth tablets to address access barriers in veterans. JAMIA Open. 2019;2(3):323-329. doi:10.1093/jamiaopen/ooz024
24. Malone NC, Williams MM, Smith Fawzi MC, et al. Mobile health clinics in the United States. Int J Equity Health. 2020;19(1):40. doi:10.1186/s12939-020-1135-7
25. US Department of Veterans Affairs. How to use My HealtheVet. Accessed November 3, 2022. https://www.myhealth.va.gov/mhv-portal-web/how-to-use-mhv
26. US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Whole health for life. 2017. Accessed November 3, 2022. https://www.va.gov/wholehealth/docs/2017-AR-Vet-Facing_FNL-W508.pdf27. Mortimer F, Isherwood J, Wilkinson A, Vaux E. Sustainability in quality improvement: redefining value. Future Healthc J. 2018;5(2):88-93. doi:10.7861/futurehosp.5-2-88
Patient portals are secure online website tools that provide patient access to personal health information (PHI). Access to online PHI improves health equity and satisfies the meaningful use objectives of the Medicare electronic health record (EHR) incentive program.1,2 Through patient portals, individuals can access PHI records and current diagnoses, request and reschedule appointments, locate test results, track trends for vital signs and laboratory values, refill medications, and communicate directly with the health care team through secure messaging. This alternative method of communication with the team is associated with increased patient satisfaction.3 Patients reported improved patient engagement in health care self-management and decision making, as well as strengthened relationships with their health care team.4
Background
One well-documented strategy to improve portal use includes the development of a nurse champion to facilitate enrollment during the clinic visit.5 Patient perceptions of portal value increased after education by a health care professional (HCP) and assistance in enrollment to familiarize patients with the platform for ongoing use.5 Use of patient portals has been associated with favorable outcomes in chronic disease management. Patients with diabetes mellitus who regularly use patient portals for prescription refills and secure messaging have demonstrated improved glycemic control, medication adherence, and associated health parameters compared with nonusers.5-7 In patients with congestive heart failure, meaningful patient portal use results in fewer emergency department visits, fewer hospital admissions, lower readmission rates, and reduced unscheduled and no-show visits.8-11
Patient portal access is a quality improvement (QI) measure that meets Medicare and Medicaid meaningful use requirements that is designed to improve collaboration between HCPs and patients through EHRs. Despite legislation, uptake of patient portal access has been slow, especially among older adults.10,12,13 Barriers to patient portal registration and use include patient lack of awareness, perceived or actual digital illiteracy, mistrust in privacy precautions, lack of user-friendly interfaces, lack of internet or technology, HCP bias and workload, and misperceptions of usefulness.9,10,12,14 The HCPs most likely to facilitate the use of patient portals, typically include nurse practitioners (NPs), nurses, and medical residents.10,15 Patient portal platforms promote the partnership of these disciplines with the veteran to help the patient better manage their health. Despite the benefits and widespread integration of patient portals in health care systems, socioeconomic inequalities and HCP attitudes contribute to persistent disparities in its adoption by underserved populations. The veteran population is often faced with additional barriers to health care access with regard to geographic location, advanced age, trauma, disabilities, mental health challenges, and homelessness.10,16 These barriers require unique approaches to maximize the use of technologic advances.17 Advanced age contributes to low rates of patient portal enrollment and lack of digital platform use, thus creating a digital divide.11,12
The digital divide is described as the gap between those persons who use technology including computers and internet, and those persons who do not because of social and geographic barriers.16 It contributes to a growing health disparity in both access to care and quality of care especially for rural veterans. About 25% of the US population lacks fixed broadband at home; these individuals are more likely to be racial minorities, older, widowed, or to have lower levels of education.18,19 Veterans are disproportionately represented in these demographic categories.20 According to the US Department of Veterans Affairs (VA) Office of Rural Health, the percentage of rural veterans enrolled in the VA health care system (58%) is significantly higher than enrollment of urban veterans (38%); additionally, 27% of rural veterans do not access the internet at home.21
My HealtheVet
The VA plays an integral part in increasing virtual access to care, from the introduction of My Healthevet (MHV) in 2003 to the distribution of iPad tablets to vulnerable veterans during the COVID-19 pandemic.22,23 Due to COVID-19, the need for VA patient access to the internet and VA Video-Connect (VVC) telehealth services increased significantly.22 Access to internet and hardware supporting use of VVC and MHV has been facilitated by the Digital Divide Consult, a VA program launched in 2020 to increase access to telehealth services.24 The VA has distributed > 26,000 cellular-enabled tablets and provided > 50,000 veterans with connectivity in collaboration with various private sector companies.22 Patients report that MHV facilitates engagement in health care through improved access to EHRs and expedited communication with the health care team.4
MHV is a secure online tool that provides patients access to PHI. MHV aims to empower veterans to take charge of their health by improving communication with HCPs, setting patient goals, and offering health and well-being resources.25,26 In a study of outpatients at a large urban multisite health care system, < 35% of patients on 16 medical resident panels were enrolled in a patient portal.15 MHV internal national metrics show increasing registration and active users of the patient portal, yet locally, disparities in the use of the portal by rural and older veterans exist.
The Local Problem
A review of the registration process at a rural VA clinic revealed barriers to facilitating the veteran registration process at the point of care. Clinical reminders exist within the EHR to prompt clinicians at the point of care to improve quality of care. At the New England Healthcare System (Veterans Integrated Service Network [VISN] 1), a patient portal clinical reminder prompts staff to encourage veterans to register. Anecdotal data obtained from primary care staff interviews at a rural VA primary care clinic in Vermont revealed low clinician confidence in completing the clinical reminder, a lack of knowledge of MHV, and lack of time to educate veterans about the benefits of MHV.
Despite availability of a registration process at the point of care and clinical staff assigned to provide registration information to the veteran, access to the patient portal among veterans at this clinic remained low. This QI project aimed to increase patient portal enrollment of veterans in MHV in a single NP patient panel of 100 patients from a baseline of 33% by 10% in a 3-month time frame.
Implementation
Before implementing the first Plan-Do-Study-Act (PDSA) cycle, we established the baseline data for 1 patient panel to be 33%. A retrospective review of the NP resident’s panel of 100 revealed 33 veterans were enrolled in MHV, providing a setting for process improvement. Evaluation of potential enrollment data for the panel population revealed unenrolled veterans were primarily aged ≥ 65 years. A rapid cycle QI (RCQI) strategy using the PDSA method was used to identify, implement, and measure changes over a 3-month time frame in 1 NP patient panel.14
The RCQI process included establishing baseline data and 3 PDSA cycles that evaluated the current state of patient access to the electronic patient portal, elucidated patient barriers to registration, assessed the processes for point-of-care enrollment, and developed strategies to improve the process and increase veteran enrollment. The QI project team included an NP resident as the project manager and MHV champion, a clinical faculty mentor at the site, a telehealth coordinator, an MHV coordinator, clinic registered nurse (RN), and clinic licensed nursing assistant (LNA). The RN and LNA additionally served as MHV champions as the project progressed.
PDSA Cycles
The objective for PDSA cycle 1 was to evaluate the process of patient registration and assess the impact on NP workload and clinic workflow over a 4-week period to improve veteran enrollment. Data were collected in a spreadsheet to track the number of veterans enrolled, time frame to enroll, and field notes that the NP resident recorded about the experience. The NP resident was trained in registration methods by the MHV coordinator. Several barriers to the registration process were identified: The process resulted in a change of the clinic visit closure focus, the clinic room was blocked for use by another patient, veterans had difficulty generating a unique username and password, veterans were unfamiliar with basic tablet accessibility and use, and additional time was required if incorrect information was entered. The veterans displayed low confidence in using tablet technology and were unaware of the patient portal or its usefulness. After discussion of the process with the project team, recommendations were made to address challenges, including an RN-led registration process. The first PDSA cycle increased the total patient panel enrollment by 4 veterans to 37%.
In PDSA cycle 2 after the NP visit, patients who agreed to register for the MHV portal were introduced to the tablet. The registration process was completed by the patient with the RN prior to the patient checkout. Once patient registration was completed, the veteran met the MHV coordinator and upgraded to a premium account, which provided full access to portal features. Electronic messaging was tested by the MHV coordinator and veteran to validate patient understanding. Although preloading demographic information improved accessibility issues, time was still required for the RN to orient the veteran to the tablet, provide additional directions, and answer questions.
The registration process reduced NP time commitment but added to the RN time burden and disrupted workflow; and clinic room access continued to be an issue. The wait time for the veteran to register in the clinic remained dependent on the availability of the RN. The decision was to move the registration process to the initial patient rooming assignment in the clinic and was transitioned from RN to LNA, prior to the NP-veteran encounter. Four additional veterans registered in the second PDSA cycle, and total enrollment increased to 41%, an overall 8% increase from baseline.
In the third PDSA cycle the patient enrollment process was managed by the clinic LNA using scripted information about MHV prior to the veteran encounter. A partially preloaded tablet was offered to the veteran to register with MHV during the rooming process, and written and oral instruction were provided to the veteran. The time required for each veteran to register for MHV averaged 10 minutes, and the veteran was able to register while waiting for the NP to enter the room. Typical LNA tasks included greeting patients, updating health records, completing clinical reminders with the veteran, obtaining vital signs, and addressing questions. The LNA introduced the veteran to MHV using scripted information and supported them in registering for MHV prior to the NP-veteran encounter. Registration at point of care during the rooming process was well received by both the LNA and veterans. The LNA reported the process was efficient and did not add excessive time to the LNA workflow. The LNA reported verbal patient satisfaction and registration was facilitated for 6 veterans during the 4-week period.
Registration during point of care was reported as feasible and sustainable by the LNA. Upgrading the patient to a premium MHV account was transitioned to the MHV coordinator. All veterans seen during the 4-week period were approached about registration; if the veteran declined, written at-home step-by-step instructions were provided. A replacement electronic clinical reminder was proposed to the VISN clinical reminders team for review and was pilot tested by the primary care clinical team. The third PDSA cycle increased the total patient panel enrollment to 47%, an overall 14% increase from baseline. Six new veteran users were added during PDSA cycle 3.
Discussion
The project team successfully used a RCQI method with a PDSA strategy to improve patient access to the MHV portal and increased veteran enrollment by 14% on 1 NP resident patient panel. The project evaluated clinic workflow regarding veteran patient portal registration, uncovered inefficiencies, and developed improved processes to increase veteran access to the patient portal. Results were positively impacted through the recognition of inefficiencies and initiation of new processes to engage veterans in the portal registration process. Familiarizing the entire clinical team with the clinical reminder and registration process raised the awareness of a digital divide consult and the utility of the portal in patient care. The project provided an opportunity to evaluate veterans’ digital literacy, digital access to send and receive messages, and to provide coaching as needed. Sequential PDSA cycles employed audit and feedback, information preloading, multimodal teaching strategies (verbal, print, hands-on tablet learning), scripting, staff interviews, time studies, and workflow evaluation to improve processes. An MHV champion led the team, monitored the progress, set deadlines, and effectively communicated project performance.
Limitations
Project limitations included the single-site location, its small sample size, and the short 3-month implementation time frame. The patient panel was representative of other NP resident patient panels at the facility but may not be representative of other VA facilities.
Ethical Considerations
Patient confidentiality was maintained throughout the registration and data collection process. The project team (NP, RN, LNA) received training and written instructions on protection of patient confidentiality by the MHV coordinator prior to assisting veterans with the registration process. Privacy was maintained, no patient identifiers were collected or viewed, and no assistance was provided for username, password, or security questions. The tablet was password protected and secured, used only by the project team when veteran was interested in point-of-care portal registration.
Sustainability
QI projects require ongoing systemic efforts to enhance sustainability.26,27 The project team used the PDSA methodology to stimulate the design of new workflow processes to engage staff and veterans in portal registration. Several actions were taken to promote sustainability for veteran portal registration and improve access to health care for rural and underserved veterans. First, printed instructions and website link are available in the clinic intake and examination rooms. Staff are equipped with patient education discussion points about the portal. A tablet is available in the clinic to encourage veterans to sign up. A clinical reminder is in place to encourage portal registration. A designated super-user is available to help new patient portal users register and navigate the system. Outcomes of the QI project were presented at 2 separate VISN 1 nursing grand rounds and reported to the MHV coordinator and telehealth coordinator to promote dialogue among staff and raise awareness of challenges to veteran MHV access.
Conclusions
Reviewing patient portal registration processes at the local level is essential to improve veteran access. This QI project proposed a realistic and scalable solution to implementing and improving patient enrollment to MHV in primary care clinics. Integrating measurement of patient registration into the daily routine of the clinic empowers the entire clinical team to improve the quality of access to patient portal.
The project team worked together to accomplish a shared goal, using errors as opportunities to improve the process, while using available staff without compromising significant time or resources. Engaging the entire team to audit processes and designating one member of the team as an MHV champion to provide feedback is critical to the sustainability of point-of-care registration in the MHV patient portal. Multifaceted approaches to maximizing the use of technology lessens the digital divide for veterans who are faced with geographical and social barriers to health care access.
Acknowledgments
We thank the Office of Academic Affiliations and the US Department of Veterans Affairs Nursing Academic Partnerships in Graduate Education Nurse Practitioner residency program and clinical faculty and the affiliated University of Vermont faculty mentor/quality improvement coach for the support of the project.
Patient portals are secure online website tools that provide patient access to personal health information (PHI). Access to online PHI improves health equity and satisfies the meaningful use objectives of the Medicare electronic health record (EHR) incentive program.1,2 Through patient portals, individuals can access PHI records and current diagnoses, request and reschedule appointments, locate test results, track trends for vital signs and laboratory values, refill medications, and communicate directly with the health care team through secure messaging. This alternative method of communication with the team is associated with increased patient satisfaction.3 Patients reported improved patient engagement in health care self-management and decision making, as well as strengthened relationships with their health care team.4
Background
One well-documented strategy to improve portal use includes the development of a nurse champion to facilitate enrollment during the clinic visit.5 Patient perceptions of portal value increased after education by a health care professional (HCP) and assistance in enrollment to familiarize patients with the platform for ongoing use.5 Use of patient portals has been associated with favorable outcomes in chronic disease management. Patients with diabetes mellitus who regularly use patient portals for prescription refills and secure messaging have demonstrated improved glycemic control, medication adherence, and associated health parameters compared with nonusers.5-7 In patients with congestive heart failure, meaningful patient portal use results in fewer emergency department visits, fewer hospital admissions, lower readmission rates, and reduced unscheduled and no-show visits.8-11
Patient portal access is a quality improvement (QI) measure that meets Medicare and Medicaid meaningful use requirements that is designed to improve collaboration between HCPs and patients through EHRs. Despite legislation, uptake of patient portal access has been slow, especially among older adults.10,12,13 Barriers to patient portal registration and use include patient lack of awareness, perceived or actual digital illiteracy, mistrust in privacy precautions, lack of user-friendly interfaces, lack of internet or technology, HCP bias and workload, and misperceptions of usefulness.9,10,12,14 The HCPs most likely to facilitate the use of patient portals, typically include nurse practitioners (NPs), nurses, and medical residents.10,15 Patient portal platforms promote the partnership of these disciplines with the veteran to help the patient better manage their health. Despite the benefits and widespread integration of patient portals in health care systems, socioeconomic inequalities and HCP attitudes contribute to persistent disparities in its adoption by underserved populations. The veteran population is often faced with additional barriers to health care access with regard to geographic location, advanced age, trauma, disabilities, mental health challenges, and homelessness.10,16 These barriers require unique approaches to maximize the use of technologic advances.17 Advanced age contributes to low rates of patient portal enrollment and lack of digital platform use, thus creating a digital divide.11,12
The digital divide is described as the gap between those persons who use technology including computers and internet, and those persons who do not because of social and geographic barriers.16 It contributes to a growing health disparity in both access to care and quality of care especially for rural veterans. About 25% of the US population lacks fixed broadband at home; these individuals are more likely to be racial minorities, older, widowed, or to have lower levels of education.18,19 Veterans are disproportionately represented in these demographic categories.20 According to the US Department of Veterans Affairs (VA) Office of Rural Health, the percentage of rural veterans enrolled in the VA health care system (58%) is significantly higher than enrollment of urban veterans (38%); additionally, 27% of rural veterans do not access the internet at home.21
My HealtheVet
The VA plays an integral part in increasing virtual access to care, from the introduction of My Healthevet (MHV) in 2003 to the distribution of iPad tablets to vulnerable veterans during the COVID-19 pandemic.22,23 Due to COVID-19, the need for VA patient access to the internet and VA Video-Connect (VVC) telehealth services increased significantly.22 Access to internet and hardware supporting use of VVC and MHV has been facilitated by the Digital Divide Consult, a VA program launched in 2020 to increase access to telehealth services.24 The VA has distributed > 26,000 cellular-enabled tablets and provided > 50,000 veterans with connectivity in collaboration with various private sector companies.22 Patients report that MHV facilitates engagement in health care through improved access to EHRs and expedited communication with the health care team.4
MHV is a secure online tool that provides patients access to PHI. MHV aims to empower veterans to take charge of their health by improving communication with HCPs, setting patient goals, and offering health and well-being resources.25,26 In a study of outpatients at a large urban multisite health care system, < 35% of patients on 16 medical resident panels were enrolled in a patient portal.15 MHV internal national metrics show increasing registration and active users of the patient portal, yet locally, disparities in the use of the portal by rural and older veterans exist.
The Local Problem
A review of the registration process at a rural VA clinic revealed barriers to facilitating the veteran registration process at the point of care. Clinical reminders exist within the EHR to prompt clinicians at the point of care to improve quality of care. At the New England Healthcare System (Veterans Integrated Service Network [VISN] 1), a patient portal clinical reminder prompts staff to encourage veterans to register. Anecdotal data obtained from primary care staff interviews at a rural VA primary care clinic in Vermont revealed low clinician confidence in completing the clinical reminder, a lack of knowledge of MHV, and lack of time to educate veterans about the benefits of MHV.
Despite availability of a registration process at the point of care and clinical staff assigned to provide registration information to the veteran, access to the patient portal among veterans at this clinic remained low. This QI project aimed to increase patient portal enrollment of veterans in MHV in a single NP patient panel of 100 patients from a baseline of 33% by 10% in a 3-month time frame.
Implementation
Before implementing the first Plan-Do-Study-Act (PDSA) cycle, we established the baseline data for 1 patient panel to be 33%. A retrospective review of the NP resident’s panel of 100 revealed 33 veterans were enrolled in MHV, providing a setting for process improvement. Evaluation of potential enrollment data for the panel population revealed unenrolled veterans were primarily aged ≥ 65 years. A rapid cycle QI (RCQI) strategy using the PDSA method was used to identify, implement, and measure changes over a 3-month time frame in 1 NP patient panel.14
The RCQI process included establishing baseline data and 3 PDSA cycles that evaluated the current state of patient access to the electronic patient portal, elucidated patient barriers to registration, assessed the processes for point-of-care enrollment, and developed strategies to improve the process and increase veteran enrollment. The QI project team included an NP resident as the project manager and MHV champion, a clinical faculty mentor at the site, a telehealth coordinator, an MHV coordinator, clinic registered nurse (RN), and clinic licensed nursing assistant (LNA). The RN and LNA additionally served as MHV champions as the project progressed.
PDSA Cycles
The objective for PDSA cycle 1 was to evaluate the process of patient registration and assess the impact on NP workload and clinic workflow over a 4-week period to improve veteran enrollment. Data were collected in a spreadsheet to track the number of veterans enrolled, time frame to enroll, and field notes that the NP resident recorded about the experience. The NP resident was trained in registration methods by the MHV coordinator. Several barriers to the registration process were identified: The process resulted in a change of the clinic visit closure focus, the clinic room was blocked for use by another patient, veterans had difficulty generating a unique username and password, veterans were unfamiliar with basic tablet accessibility and use, and additional time was required if incorrect information was entered. The veterans displayed low confidence in using tablet technology and were unaware of the patient portal or its usefulness. After discussion of the process with the project team, recommendations were made to address challenges, including an RN-led registration process. The first PDSA cycle increased the total patient panel enrollment by 4 veterans to 37%.
In PDSA cycle 2 after the NP visit, patients who agreed to register for the MHV portal were introduced to the tablet. The registration process was completed by the patient with the RN prior to the patient checkout. Once patient registration was completed, the veteran met the MHV coordinator and upgraded to a premium account, which provided full access to portal features. Electronic messaging was tested by the MHV coordinator and veteran to validate patient understanding. Although preloading demographic information improved accessibility issues, time was still required for the RN to orient the veteran to the tablet, provide additional directions, and answer questions.
The registration process reduced NP time commitment but added to the RN time burden and disrupted workflow; and clinic room access continued to be an issue. The wait time for the veteran to register in the clinic remained dependent on the availability of the RN. The decision was to move the registration process to the initial patient rooming assignment in the clinic and was transitioned from RN to LNA, prior to the NP-veteran encounter. Four additional veterans registered in the second PDSA cycle, and total enrollment increased to 41%, an overall 8% increase from baseline.
In the third PDSA cycle the patient enrollment process was managed by the clinic LNA using scripted information about MHV prior to the veteran encounter. A partially preloaded tablet was offered to the veteran to register with MHV during the rooming process, and written and oral instruction were provided to the veteran. The time required for each veteran to register for MHV averaged 10 minutes, and the veteran was able to register while waiting for the NP to enter the room. Typical LNA tasks included greeting patients, updating health records, completing clinical reminders with the veteran, obtaining vital signs, and addressing questions. The LNA introduced the veteran to MHV using scripted information and supported them in registering for MHV prior to the NP-veteran encounter. Registration at point of care during the rooming process was well received by both the LNA and veterans. The LNA reported the process was efficient and did not add excessive time to the LNA workflow. The LNA reported verbal patient satisfaction and registration was facilitated for 6 veterans during the 4-week period.
Registration during point of care was reported as feasible and sustainable by the LNA. Upgrading the patient to a premium MHV account was transitioned to the MHV coordinator. All veterans seen during the 4-week period were approached about registration; if the veteran declined, written at-home step-by-step instructions were provided. A replacement electronic clinical reminder was proposed to the VISN clinical reminders team for review and was pilot tested by the primary care clinical team. The third PDSA cycle increased the total patient panel enrollment to 47%, an overall 14% increase from baseline. Six new veteran users were added during PDSA cycle 3.
Discussion
The project team successfully used a RCQI method with a PDSA strategy to improve patient access to the MHV portal and increased veteran enrollment by 14% on 1 NP resident patient panel. The project evaluated clinic workflow regarding veteran patient portal registration, uncovered inefficiencies, and developed improved processes to increase veteran access to the patient portal. Results were positively impacted through the recognition of inefficiencies and initiation of new processes to engage veterans in the portal registration process. Familiarizing the entire clinical team with the clinical reminder and registration process raised the awareness of a digital divide consult and the utility of the portal in patient care. The project provided an opportunity to evaluate veterans’ digital literacy, digital access to send and receive messages, and to provide coaching as needed. Sequential PDSA cycles employed audit and feedback, information preloading, multimodal teaching strategies (verbal, print, hands-on tablet learning), scripting, staff interviews, time studies, and workflow evaluation to improve processes. An MHV champion led the team, monitored the progress, set deadlines, and effectively communicated project performance.
Limitations
Project limitations included the single-site location, its small sample size, and the short 3-month implementation time frame. The patient panel was representative of other NP resident patient panels at the facility but may not be representative of other VA facilities.
Ethical Considerations
Patient confidentiality was maintained throughout the registration and data collection process. The project team (NP, RN, LNA) received training and written instructions on protection of patient confidentiality by the MHV coordinator prior to assisting veterans with the registration process. Privacy was maintained, no patient identifiers were collected or viewed, and no assistance was provided for username, password, or security questions. The tablet was password protected and secured, used only by the project team when veteran was interested in point-of-care portal registration.
Sustainability
QI projects require ongoing systemic efforts to enhance sustainability.26,27 The project team used the PDSA methodology to stimulate the design of new workflow processes to engage staff and veterans in portal registration. Several actions were taken to promote sustainability for veteran portal registration and improve access to health care for rural and underserved veterans. First, printed instructions and website link are available in the clinic intake and examination rooms. Staff are equipped with patient education discussion points about the portal. A tablet is available in the clinic to encourage veterans to sign up. A clinical reminder is in place to encourage portal registration. A designated super-user is available to help new patient portal users register and navigate the system. Outcomes of the QI project were presented at 2 separate VISN 1 nursing grand rounds and reported to the MHV coordinator and telehealth coordinator to promote dialogue among staff and raise awareness of challenges to veteran MHV access.
Conclusions
Reviewing patient portal registration processes at the local level is essential to improve veteran access. This QI project proposed a realistic and scalable solution to implementing and improving patient enrollment to MHV in primary care clinics. Integrating measurement of patient registration into the daily routine of the clinic empowers the entire clinical team to improve the quality of access to patient portal.
The project team worked together to accomplish a shared goal, using errors as opportunities to improve the process, while using available staff without compromising significant time or resources. Engaging the entire team to audit processes and designating one member of the team as an MHV champion to provide feedback is critical to the sustainability of point-of-care registration in the MHV patient portal. Multifaceted approaches to maximizing the use of technology lessens the digital divide for veterans who are faced with geographical and social barriers to health care access.
Acknowledgments
We thank the Office of Academic Affiliations and the US Department of Veterans Affairs Nursing Academic Partnerships in Graduate Education Nurse Practitioner residency program and clinical faculty and the affiliated University of Vermont faculty mentor/quality improvement coach for the support of the project.
1. Centers for Medicare and Medicaid Services. Promoting interoperability programs. Updated October 6, 2022. Accessed November 3, 2022. https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms
2. American Hospital Association. Goals of the Medicare and Medicaid electronic health records programs. Accessed November 3, 2022. https://www.aha.org/websites/2009-12-11-goals-medicare-and-medicaid-electronic-health-records-programs
3. Rozenblum R, Donzé J, Hockey PM, et al. The impact of medical informatics on patient satisfaction: a USA-based literature review. Int J Med Inform. 2013;82(3):141-158. doi:10.1016/j.ijmedinf.2012.12.008
4. Stewart MT, Hogan TP, Nicklas J, et al. The promise of patient portals for individuals living with chronic illness: qualitative study identifying pathways of patient engagement. J Med Internet Res. 2020;22(7):e17744. Published 2020 Jul 17. doi:10.2196/17744
5. Harris LT, Haneuse SJ, Martin DP, Ralston JD. Diabetes quality of care and outpatient utilization associated with electronic patient-provider messaging: a cross-sectional analysis. Diabetes Care. 2009;32(7):1182-1187. doi:10.2337/dc08-1771
6. Robinson SA, Zocchi MS, Netherton D, et al. Secure messaging, diabetes self-management, and the importance of patient autonomy: a mixed methods study. J Gen Intern Med. 2020;35(10):2955-2962. doi:10.1007/s11606-020-05834-x
7. Zocchi MS, Robinson SA, Ash AS, et al. Patient portal engagement and diabetes management among new portal users in the Veterans Health Administration. J Am Med Inform Assoc. 2021;28(10):2176-2183. doi:10.1093/jamia/ocab115
8. Bao C, Bardhan IR, Singh H, Meyer BA, Kirksey K. Patient-provider engagement and its impact on health outcomes: a longitudinal study of patient portal use. MIS Quarterly. 2020;44(2):699-723. doi:10.25300/MISQ/2020/14180
9. Grossman LV, Masterson Creber RM, Benda NC, Wright D, Vawdrey DK, Ancker JS. Interventions to increase patient portal use in vulnerable populations: a systematic review. J Am Med Informs Assoc. 2019;26(8-9):855-870. doi:10.1093/jamia/ocz023
10. Zhao JY, Song B, Anand E, et al. Barriers, facilitators, and solutions to optimal patient portal and personal health record use: a systematic review of the literature. AMIA Annu Symp Proc. 2018;2017:1913-1922. Published 2018 Apr 16.
11. Zhong X, Park J, Liang M, et al. Characteristics of patients using different patient portal functions and the impact on primary care service utilization and appointment adherence: retrospective observational study. J Med Internet Res. 2020;22(2):e14410. Published 2020 Feb 25. doi:10.2196/14410
12. Krishnaswami A, Beavers C, Dorsch MP, et al. Gerotechnology for older adults with cardiovascular diseases. J Am Coll Cardiol. 2020;76(22):2650-2670. doi:10.1016/j.jacc.2020.09.606
13. Fix GM, Hogan TP, Amante DJ, McInnes DK, Nazi KM, Simon SR. Encouraging patient portal use in the patient-centered medical home: three stakeholder perspectives. J Med Internet Res. 2016;18(11):e308. Published 2016 Nov 22. doi:10.2196/jmir.6488
14. Ancker JS, Nosal S, Hauser D, Way C, Calman N. Access policy and the digital divide in patient access to medical records. Health Policy Technol. 2016;6(3-11). doi:10.1016/j.hlpt.2016.11.004
15. Rhudy C, Broxterman J, Stewart S, et al. Improving patient portal enrolment in an academic resident continuity clinic: quality improvement made simple. BMJ Open Qual. 2019;8(2):e000430. Published 2019 Apr 25. doi:10.1136/bmjoq-2018-000430
16. Kontos E, Blake KD, Chou WY, Prestin A. Predictors of eHealth usage: insights on the digital divide from the Health Information National Trends Survey 2012. J Med Internet Res. 2014;16(7):e172. Published 2014 Jul 16. doi:10.2196/jmir.3117
17. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice. The state of health disparities in the United States. In: Baciu A, Negussie Y, Geller A, et al, eds. Communities in Action: Pathways to Health Equity. National Academies Press (US); January 11, 2017. Accessed November 3, 2022. https://www.ncbi.nlm.nih.gov/books/NBK425848/
18. Pew Research Center. Internet/broadband fact sheet. Updated April 7, 2021. Accessed November 3, 2022. https://www.pewresearch.org/internet/fact-sheet/internet-broadband
19. Roberts ET, Mehrotra A. Assessment of disparities in digital access among Medicare beneficiaries and implications for telemedicine. JAMA Intern Med. 2020;180(10):1386-1389. doi:10.1001/jamainternmed.2020.2666
20. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed November 3, 2022. https://www.va.gov/vetdata/veteran_population.asp
21. US Department of Veterans Affairs, Office of Rural Health. Rural veterans health care challenges. Updated March 31, 2022. Accessed November 3, 2022. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
22. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands veteran access to telehealth with iPad services. Press release. September 15, 2020. Accessed November 3, 2022. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5521
23. Zulman DM, Wong EP, Slightam C, et al. Making connections: National implementation of video telehealth tablets to address access barriers in veterans. JAMIA Open. 2019;2(3):323-329. doi:10.1093/jamiaopen/ooz024
24. Malone NC, Williams MM, Smith Fawzi MC, et al. Mobile health clinics in the United States. Int J Equity Health. 2020;19(1):40. doi:10.1186/s12939-020-1135-7
25. US Department of Veterans Affairs. How to use My HealtheVet. Accessed November 3, 2022. https://www.myhealth.va.gov/mhv-portal-web/how-to-use-mhv
26. US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Whole health for life. 2017. Accessed November 3, 2022. https://www.va.gov/wholehealth/docs/2017-AR-Vet-Facing_FNL-W508.pdf27. Mortimer F, Isherwood J, Wilkinson A, Vaux E. Sustainability in quality improvement: redefining value. Future Healthc J. 2018;5(2):88-93. doi:10.7861/futurehosp.5-2-88
1. Centers for Medicare and Medicaid Services. Promoting interoperability programs. Updated October 6, 2022. Accessed November 3, 2022. https://www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms
2. American Hospital Association. Goals of the Medicare and Medicaid electronic health records programs. Accessed November 3, 2022. https://www.aha.org/websites/2009-12-11-goals-medicare-and-medicaid-electronic-health-records-programs
3. Rozenblum R, Donzé J, Hockey PM, et al. The impact of medical informatics on patient satisfaction: a USA-based literature review. Int J Med Inform. 2013;82(3):141-158. doi:10.1016/j.ijmedinf.2012.12.008
4. Stewart MT, Hogan TP, Nicklas J, et al. The promise of patient portals for individuals living with chronic illness: qualitative study identifying pathways of patient engagement. J Med Internet Res. 2020;22(7):e17744. Published 2020 Jul 17. doi:10.2196/17744
5. Harris LT, Haneuse SJ, Martin DP, Ralston JD. Diabetes quality of care and outpatient utilization associated with electronic patient-provider messaging: a cross-sectional analysis. Diabetes Care. 2009;32(7):1182-1187. doi:10.2337/dc08-1771
6. Robinson SA, Zocchi MS, Netherton D, et al. Secure messaging, diabetes self-management, and the importance of patient autonomy: a mixed methods study. J Gen Intern Med. 2020;35(10):2955-2962. doi:10.1007/s11606-020-05834-x
7. Zocchi MS, Robinson SA, Ash AS, et al. Patient portal engagement and diabetes management among new portal users in the Veterans Health Administration. J Am Med Inform Assoc. 2021;28(10):2176-2183. doi:10.1093/jamia/ocab115
8. Bao C, Bardhan IR, Singh H, Meyer BA, Kirksey K. Patient-provider engagement and its impact on health outcomes: a longitudinal study of patient portal use. MIS Quarterly. 2020;44(2):699-723. doi:10.25300/MISQ/2020/14180
9. Grossman LV, Masterson Creber RM, Benda NC, Wright D, Vawdrey DK, Ancker JS. Interventions to increase patient portal use in vulnerable populations: a systematic review. J Am Med Informs Assoc. 2019;26(8-9):855-870. doi:10.1093/jamia/ocz023
10. Zhao JY, Song B, Anand E, et al. Barriers, facilitators, and solutions to optimal patient portal and personal health record use: a systematic review of the literature. AMIA Annu Symp Proc. 2018;2017:1913-1922. Published 2018 Apr 16.
11. Zhong X, Park J, Liang M, et al. Characteristics of patients using different patient portal functions and the impact on primary care service utilization and appointment adherence: retrospective observational study. J Med Internet Res. 2020;22(2):e14410. Published 2020 Feb 25. doi:10.2196/14410
12. Krishnaswami A, Beavers C, Dorsch MP, et al. Gerotechnology for older adults with cardiovascular diseases. J Am Coll Cardiol. 2020;76(22):2650-2670. doi:10.1016/j.jacc.2020.09.606
13. Fix GM, Hogan TP, Amante DJ, McInnes DK, Nazi KM, Simon SR. Encouraging patient portal use in the patient-centered medical home: three stakeholder perspectives. J Med Internet Res. 2016;18(11):e308. Published 2016 Nov 22. doi:10.2196/jmir.6488
14. Ancker JS, Nosal S, Hauser D, Way C, Calman N. Access policy and the digital divide in patient access to medical records. Health Policy Technol. 2016;6(3-11). doi:10.1016/j.hlpt.2016.11.004
15. Rhudy C, Broxterman J, Stewart S, et al. Improving patient portal enrolment in an academic resident continuity clinic: quality improvement made simple. BMJ Open Qual. 2019;8(2):e000430. Published 2019 Apr 25. doi:10.1136/bmjoq-2018-000430
16. Kontos E, Blake KD, Chou WY, Prestin A. Predictors of eHealth usage: insights on the digital divide from the Health Information National Trends Survey 2012. J Med Internet Res. 2014;16(7):e172. Published 2014 Jul 16. doi:10.2196/jmir.3117
17. National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice. The state of health disparities in the United States. In: Baciu A, Negussie Y, Geller A, et al, eds. Communities in Action: Pathways to Health Equity. National Academies Press (US); January 11, 2017. Accessed November 3, 2022. https://www.ncbi.nlm.nih.gov/books/NBK425848/
18. Pew Research Center. Internet/broadband fact sheet. Updated April 7, 2021. Accessed November 3, 2022. https://www.pewresearch.org/internet/fact-sheet/internet-broadband
19. Roberts ET, Mehrotra A. Assessment of disparities in digital access among Medicare beneficiaries and implications for telemedicine. JAMA Intern Med. 2020;180(10):1386-1389. doi:10.1001/jamainternmed.2020.2666
20. US Department of Veterans Affairs, National Center for Veterans Analysis and Statistics. Veteran population. Updated September 7, 2022. Accessed November 3, 2022. https://www.va.gov/vetdata/veteran_population.asp
21. US Department of Veterans Affairs, Office of Rural Health. Rural veterans health care challenges. Updated March 31, 2022. Accessed November 3, 2022. https://www.ruralhealth.va.gov/aboutus/ruralvets.asp
22. US Department of Veterans Affairs, Office of Public and Intergovernmental Affairs. VA expands veteran access to telehealth with iPad services. Press release. September 15, 2020. Accessed November 3, 2022. https://www.va.gov/opa/pressrel/pressrelease.cfm?id=5521
23. Zulman DM, Wong EP, Slightam C, et al. Making connections: National implementation of video telehealth tablets to address access barriers in veterans. JAMIA Open. 2019;2(3):323-329. doi:10.1093/jamiaopen/ooz024
24. Malone NC, Williams MM, Smith Fawzi MC, et al. Mobile health clinics in the United States. Int J Equity Health. 2020;19(1):40. doi:10.1186/s12939-020-1135-7
25. US Department of Veterans Affairs. How to use My HealtheVet. Accessed November 3, 2022. https://www.myhealth.va.gov/mhv-portal-web/how-to-use-mhv
26. US Department of Veterans Affairs, Veterans Health Administration, Office of Patient Centered Care and Cultural Transformation. Whole health for life. 2017. Accessed November 3, 2022. https://www.va.gov/wholehealth/docs/2017-AR-Vet-Facing_FNL-W508.pdf27. Mortimer F, Isherwood J, Wilkinson A, Vaux E. Sustainability in quality improvement: redefining value. Future Healthc J. 2018;5(2):88-93. doi:10.7861/futurehosp.5-2-88
Evaluation of a Pharmacist-Driven Ambulatory Aspirin Deprescribing Protocol
The use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) morbidity and mortality continues to be controversial, particularly for older adults. Recently published, robust randomized controlled trials have revealed less cardiovascular benefit from aspirin for primary prevention compared with previous trials; additionally, an increased risk of major bleeding events has been notably more prevalent in older adults.1-5 These trials have suggested that preventative aspirin use in older adults confers less benefit than other therapies for decreasing atherosclerotic CVD (ASCVD) risk, including blood pressure (BP) control, cholesterol management, and tobacco cessation.1,6
A recent meta-analysis indicated a composite cardiovascular risk reduction in patients aged 53 to 74 years taking aspirin vs no aspirin; however, this benefit was offset with an even greater increased risk of major bleeding.7 This trend was consistent regardless of stratification by 10-year ASCVD risk or presence of diabetes mellitus (DM) diagnosis.7,8 Additionally, the recently published Aspirin in Reducing Events in the Elderly (ASPREE) trial studied the impacts of aspirin use in healthy adults aged ≥ 70 years and aged ≥ 65 years among Black and Hispanic adults.4 The study concluded that the risk of major bleeding with aspirin use was even higher vs the potential cardiovascular benefit in older adults.4
With this emerging evidence, guidelines have been updated to represent the need for risk vs benefit considerations regarding aspirin use for primary prevention in older adults.1,9,10 The most recent guideline update from the American College of Cardiology and American Heart Association (ACC/AHA) recommends against the routine use of aspirin in patients aged > 70 years or those with bleeding risk factors.1 The guideline recommends considering aspirin use for patients ages 40 to 70 years only after a patient-specific risk vs benefit discussion.1 Furthermore, the 2020 American Diabetes Association guideline recommends considering aspirin use for primary prevention in adults with DM between ages 50 and 70 only after a risk vs benefit discussion of patient-specific bleeding risk factors and ASCVD risk-enhancing factors.10
Despite the demonstrated risks for bleeding with the routine use of aspirin, studies indicate that aspirin continues to be used commonly among older adults, often when unnecessary. In the 2017 National Health Interview Survey, about 23% of adults aged > 40 years in the United States without CVD used aspirin daily, and 23% of these did so without recommendation from a health care professional.11 Furthermore, nearly half of adults ages ≥ 70 years and nearly one-quarter of adults with a history of peptic ulcer disease used aspirin daily.11 Although the most recent guidelines from the ACC/AHA do not recommend a 10-year ASCVD risk threshold for therapy, one study illustrated that 12% of older adult patients were inappropriately prescribed aspirin for primary prevention despite a 10-year ASCVD risk of < 6%.1,12 These studies highlight the large proportion of individuals, particularly older adults, who may be inappropriately taking aspirin for primary prevention.
Deprescribing Program
Deprescribing potentially inappropriate medications (PIMs) is particularly important in the older adult population, as these individuals experience a high risk of adverse effects (AEs), polypharmacy, cognitive decline, and falls related to medication use.6,13-17 Evidence suggests that mortality outcomes are improved with the implementation of targeted deprescribing efforts based on patient-specific factors.18 Additionally, deprescribing unnecessary medications may improve adherence to other essential medications and reduce financial burdens.19 Pharmacists play a crucial role among health care professionals in the implementation of deprescribing practices, and studies have shown that physicians are highly accepting of pharmacists’ deprescribing recommendations.13,20-22
Despite the evidence for the benefits of deprescribing, limited data are available regarding the impact and feasibility of a targeted aspirin deprescribing approach by nonphysician practitioners.23 The objective of this study was to implement and evaluate the success of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting.
This aspirin deprescribing protocol was developed by ambulatory care clinical pharmacist or clinical pharmacist practitioners (CPPs), at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. Within the US Department of Veterans Affairs (VA) health care system, CPPs work under a broad scope of practice with the ability to independently prescribe and monitor medications. The protocol was reviewed by physician stakeholders in both primary care and cardiology and a list was generated, including patients from 2 primary care panels aged ≥ 70 years with aspirin on their medication list, either as a prescription or over-the-counter medication, using the VA Information System Technology and Architecture. A CPP or supervised pharmacy intern identified patients from this list who were appropriate for risk/benefit discussions regarding the discontinuation of aspirin. Patients were excluded from the intervention if they had a history of clinical ASCVD, including myocardial infarction (MI), stable or unstable angina, coronary artery disease (CAD), coronary or other arterial revascularization, cerebrovascular accident (CVA), transient ischemic accident (TIA), or peripheral artery disease (PAD), or another documented indication for aspirin use, including pain, flushing (with niacin use), venous thromboembolism prophylaxis, valvular heart disease, or acute or recurrent pericarditis.
After identifying eligible patients, a CPP or pharmacy intern contacted patients by telephone, following a script to guide conversation. All patients were screened for potential appropriate aspirin indications, particularly any history of MI, CAD, CVA, TIA, PAD, or other clinical ASCVD. The patient was asked about their rationale for taking aspirin and patient-specific ASCVD risk-enhancing factors and bleeding risk factors and educated them on lifestyle modalities to reduce ASCVD risk, using the script as a guide. ASCVD risk-enhancing factors included family history of premature MI, inability to achieve BP goal, DM with the inability to achieve blood glucose or hemoglobin A1c goal, tobacco use, or inadequate statin therapy. Bleeding risk factors included a history of gastrointestinal bleed or peptic ulcer disease, concurrent use of medications that increase bleeding risk, chronic kidney disease, or thrombocytopenia.
Through shared decision making with careful consideration of these factors, we reached a conclusion with each patient to either continue or to deprescribe aspirin. Each discussion was documented in the electronic health record (EHR) using a standard documentation template (eAppendix, available at doi:10.12788/fp.0320). The patient’s medication list also was updated to reflect changes in aspirin use. For patients who declined deprescribing, the CPP or pharmacy intern asked the patient for their primary reason for preferring to continue aspirin, which was subsequently categorized as one of the following: no prior concerns with bleeding, concerns about a future cardiovascular event, wishing to discuss further with their primary care practitioner (PCP), or identifying an appropriate use for aspirin not evident through record review. For the patients who wished to further discuss the issue with their PCP before deprescribing, the patient’s PCP was notified of this preference by a record alert to the note documenting the encounter, and the patient was also encouraged to follow up about this issue. A voicemail was left if the patient did not answer requesting a call back, and a second attempt was made within 2 weeks.
Data Collected
We collected data to assess the proportion of patients for whom aspirin for primary prevention was discontinued. For patients who declined deprescribing, we documented the rationale for continuing aspirin. Additionally, the feasibility of implementation was assessed, including pharmacist time spent on each record review and intervention. Descriptive statistics were generated to evaluate baseline characteristics and intervention outcomes. The time to completion of these tasks was summarized with descriptive statistics.
We reviewed 459 patient records, and 110 were determined eligible for risk/benefit discussions.
Patients had various reasons for declining deprescribing, including 8 (28%) who had no prior concerns with bleeding while on aspirin and 6 (21%) who were concerned about a future cardiovascular event. Of those who declined aspirin deprescribing, 6 (21%) wished to further discuss the issue with their PCP. In 9 (31%) patients an alternative appropriate indication for aspirin was identified through discussion. In these cases, the indication for aspirin was documented and updated in the EHR.
Most patients (87%) contacted reported taking low-dose aspirin 81 mg daily, while 10% reported taking higher doses (range, 162-325) and 3% on an as-needed basis. In all 3 patients who agreed to dose reduction, the initial dose of 325 mg daily was reduced to 81 mg daily.
Results of the time-study analysis for each intervention indicated that a pharmacy intern or pharmacist spent about 2 minutes reviewing the record of each patient to determine eligibility for risk/benefit discussions. The 110 patients identified as eligible were 24% of the 459 records reviewed. An average (range) of 12 (6-20) minutes was spent on the telephone call plus documentation for each patient contacted. Additionally, we estimated that CPPs and pharmacy interns spent an approximate combined 12 hours in the development and review of materials for this program, including the protocol, script, and documentation templates. This also included about 1 hour to identify appropriate parameters for, and generate, the eligible patient list.
Discussion
The implementation of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting led to the discontinuation of inappropriate aspirin use in nearly half of older adults contacted. Furthermore, opportunities were identified to update medication lists to reflect previously self-discontinued aspirin for older adults. Just over one-quarter of those contacted declined to discontinue or reduce their aspirin dose. It is hypothesized that with these targeted deprescribing interventions, overall risk reduction for bleeding and polypharmacy will be observed for older adults.1
In addition to deprescribing aspirin, CPPs used shared decision making to initiate risk/benefit discussions and to educate on targeted lifestyle modifications to lower ASCVD risk. While not all patients agreed to discontinue aspirin, all were provided education that may empower them to engage in future discussions with PCPs regarding appropriate aspirin use. Previous pharmacist-led deprescribing initiatives for proton pump inhibitors and other PIMs have indicated that a large percentage of patients who opt to further discuss a deprescribing concern with their PCPs ultimately resulted in deprescribing outcomes.24,25 Additionally, a recent trial examining pharmacist-led deprescribing of 4 common PIMs in older adults compared the impact of pharmacists leading educational interventions directly to patients with pharmacists making deprescribing recommendations to physicians. Deprescribing was more successful when patients were involved in the decision-making process.26
Limitations
Although this quality improvement initiative resulted in the deprescribing of inappropriate aspirin for many older adults, a limitation is the small sample size within a single institution. The population of male veterans also may limit generalizability to nonmale and nonveteran older adults. As the protocol was initiated within a limited number of primary care teams initially, future implementation into additional primary care teams will increase the number of older adults impacted by risk/benefit discussions regarding aspirin use. This work may not be generalizable to other health care systems. Many patients within the VA receive both their primary and specialty care within the system, which facilitates communication and collaboration between primary and specialty practitioners. The protocol may require workflow adjustments for patients receiving care within multiple systems. Additionally, although the deprescribing protocol was created in collaboration with physicians, CPPs within the VA work under a broad scope of practice that includes independent medication prescribing, deprescribing, and monitoring. This may be a consideration when implementing similar protocols at other sites, as collaborative practice agreements may need to be in place.
Future Directions
The time required to complete these interventions was generally feasible, though this intervention would require some workflow alteration to be incorporated routinely into a CPP’s schedule. The telephone calls were completed as isolated interventions and were not incorporated into existing scheduled primary care appointments. In the future, the aspirin deprescribing protocol could be incorporated into existing pharmacist-led primary care appointments. Based on the outcomes of this study, CPPs are leading an initiative to develop an aspirin deprescribing clinical reminder tool, which may be quickly inserted into a progress note within the EHR and may be incorporated into any primary care visit led by a CPP or PCP.
Conclusions
This study demonstrates that a pharmacist-led aspirin deprescribing protocol in the ambulatory care pharmacy setting was successful in the discontinuation of unnecessary aspirin use in older adults. The protocol also provided opportunities for education on ASCVD risk reduction in all older adults reached. These findings highlight the role of pharmacists in deprescribing PIMs for older adults and identifying opportunities to further streamline risk/benefit discussions on aspirin deprescribing potential within primary care visits.
1. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
2. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomized, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
3. Bowman L, Mafham M, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
4. McNeil JJ, Wolfe R, Woods, RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. García Rodríguez LA, Martín-Pérez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long-term low-dose aspirin: a systematic review of observational studies. PloS One. 2016;11(8):e0160046. doi:10.1371/journal.pone.0160046
6. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment): consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83. doi:10.5414/cpp46072
7. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi:10.1001/jama.2018.20578
8. Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16(11):675-686. doi:10.1038/s41569-019-0225-y
9. Bibbins-Domingo K; U.S. Preventative Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(12):836-845. doi:10.7326/M16-0577
10. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi:10.2337/dc20-S002
11. O’Brien CW, Juraschek SP, Wee CC. Prevalence of aspirin use for primary prevention of cardiovascular disease in the United States: results from the 2017 National Health Interview Survey. Ann Intern Med. 2019;171(8):596-598. doi:10.7326/M19-0953
12. Hira RS, Kennedy K, Nambi V, et al. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65(2):111-121. doi:10.1016/j.jacc.2014.10.035
13. Cheong ST, Ng TM, Tan KT. Pharmacist-initiated deprescribing in hospitalized elderly: prevalence and acceptance by physicians. Eur J Hosp Pharm. 2018;25(e1):e35-e39. doi:10.1136/ejhpharm-2017-001251
14. Dyck MJ. Evidence-based administrative guideline: quality improvement in nursing homes. J Gerontol Nurs. 2005;31(2):4-10. doi:10.3928/0098-9134-20050201-04
15. Zullo AR, Gray SL, Holmes HM, Marcum ZA. Screening for medication appropriateness in older adults. Clin Geriatr Med. 2018;34(1):39-54. doi:10.1016/j.cger.2017.09.003
16. American Geriatrics Society. 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
17. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
18. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. doi:10.1111/bcp.12975
19. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. The benefits and harms of deprescribing. Med J Aust. 2014;201(7):386-389. doi:10.5694/mja13.00200
20. Ailabouni NJ, Marcum ZA, Schmader KE, Gray SL. Medication use quality and safety in older adults: 2018 update. J Am Geriatr Soc. 2019;67(12):2458-2462. doi:10.1111/jgs.16243
21. Frank C, Weir E. Deprescribing for older patients. CMAJ. 2014;186(18):1369-1376. doi:10.1503/cmaj.131873
22. Clark CM, LaValley SA, Singh R, Mustafa E, Monte SV, Wahler RG Jr. A pharmacist-led program to facilitate deprescribing in a primary care clinic. J Am Pharm Assoc (2003). 2020;60(1):105-111. doi:10.1016/j.japh.2019.09.011
23. Folks B, Leblanc WG, Staton EW, Pace WD. Reconsidering low-dose aspirin therapy for cardiovascular disease: a study protocol for physician and patient behavioral change. Implement Sci. 2011;6:65. Published 2011 Jun 26. doi:10.1186/1748-5908-6-65
24. Odenthal DR, Philbrick AM, Harris IM. Successful deprescribing of unnecessary proton pump inhibitors in a primary care clinic. J Am Pharm Assoc. 2020;60(1):100-104. doi:10.1016/j.japh.2019.08.012
25. Duncan, P. Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24(1):37-42. doi:10.1136/ejhpharm-2016-000967
26. Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
The use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) morbidity and mortality continues to be controversial, particularly for older adults. Recently published, robust randomized controlled trials have revealed less cardiovascular benefit from aspirin for primary prevention compared with previous trials; additionally, an increased risk of major bleeding events has been notably more prevalent in older adults.1-5 These trials have suggested that preventative aspirin use in older adults confers less benefit than other therapies for decreasing atherosclerotic CVD (ASCVD) risk, including blood pressure (BP) control, cholesterol management, and tobacco cessation.1,6
A recent meta-analysis indicated a composite cardiovascular risk reduction in patients aged 53 to 74 years taking aspirin vs no aspirin; however, this benefit was offset with an even greater increased risk of major bleeding.7 This trend was consistent regardless of stratification by 10-year ASCVD risk or presence of diabetes mellitus (DM) diagnosis.7,8 Additionally, the recently published Aspirin in Reducing Events in the Elderly (ASPREE) trial studied the impacts of aspirin use in healthy adults aged ≥ 70 years and aged ≥ 65 years among Black and Hispanic adults.4 The study concluded that the risk of major bleeding with aspirin use was even higher vs the potential cardiovascular benefit in older adults.4
With this emerging evidence, guidelines have been updated to represent the need for risk vs benefit considerations regarding aspirin use for primary prevention in older adults.1,9,10 The most recent guideline update from the American College of Cardiology and American Heart Association (ACC/AHA) recommends against the routine use of aspirin in patients aged > 70 years or those with bleeding risk factors.1 The guideline recommends considering aspirin use for patients ages 40 to 70 years only after a patient-specific risk vs benefit discussion.1 Furthermore, the 2020 American Diabetes Association guideline recommends considering aspirin use for primary prevention in adults with DM between ages 50 and 70 only after a risk vs benefit discussion of patient-specific bleeding risk factors and ASCVD risk-enhancing factors.10
Despite the demonstrated risks for bleeding with the routine use of aspirin, studies indicate that aspirin continues to be used commonly among older adults, often when unnecessary. In the 2017 National Health Interview Survey, about 23% of adults aged > 40 years in the United States without CVD used aspirin daily, and 23% of these did so without recommendation from a health care professional.11 Furthermore, nearly half of adults ages ≥ 70 years and nearly one-quarter of adults with a history of peptic ulcer disease used aspirin daily.11 Although the most recent guidelines from the ACC/AHA do not recommend a 10-year ASCVD risk threshold for therapy, one study illustrated that 12% of older adult patients were inappropriately prescribed aspirin for primary prevention despite a 10-year ASCVD risk of < 6%.1,12 These studies highlight the large proportion of individuals, particularly older adults, who may be inappropriately taking aspirin for primary prevention.
Deprescribing Program
Deprescribing potentially inappropriate medications (PIMs) is particularly important in the older adult population, as these individuals experience a high risk of adverse effects (AEs), polypharmacy, cognitive decline, and falls related to medication use.6,13-17 Evidence suggests that mortality outcomes are improved with the implementation of targeted deprescribing efforts based on patient-specific factors.18 Additionally, deprescribing unnecessary medications may improve adherence to other essential medications and reduce financial burdens.19 Pharmacists play a crucial role among health care professionals in the implementation of deprescribing practices, and studies have shown that physicians are highly accepting of pharmacists’ deprescribing recommendations.13,20-22
Despite the evidence for the benefits of deprescribing, limited data are available regarding the impact and feasibility of a targeted aspirin deprescribing approach by nonphysician practitioners.23 The objective of this study was to implement and evaluate the success of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting.
This aspirin deprescribing protocol was developed by ambulatory care clinical pharmacist or clinical pharmacist practitioners (CPPs), at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. Within the US Department of Veterans Affairs (VA) health care system, CPPs work under a broad scope of practice with the ability to independently prescribe and monitor medications. The protocol was reviewed by physician stakeholders in both primary care and cardiology and a list was generated, including patients from 2 primary care panels aged ≥ 70 years with aspirin on their medication list, either as a prescription or over-the-counter medication, using the VA Information System Technology and Architecture. A CPP or supervised pharmacy intern identified patients from this list who were appropriate for risk/benefit discussions regarding the discontinuation of aspirin. Patients were excluded from the intervention if they had a history of clinical ASCVD, including myocardial infarction (MI), stable or unstable angina, coronary artery disease (CAD), coronary or other arterial revascularization, cerebrovascular accident (CVA), transient ischemic accident (TIA), or peripheral artery disease (PAD), or another documented indication for aspirin use, including pain, flushing (with niacin use), venous thromboembolism prophylaxis, valvular heart disease, or acute or recurrent pericarditis.
After identifying eligible patients, a CPP or pharmacy intern contacted patients by telephone, following a script to guide conversation. All patients were screened for potential appropriate aspirin indications, particularly any history of MI, CAD, CVA, TIA, PAD, or other clinical ASCVD. The patient was asked about their rationale for taking aspirin and patient-specific ASCVD risk-enhancing factors and bleeding risk factors and educated them on lifestyle modalities to reduce ASCVD risk, using the script as a guide. ASCVD risk-enhancing factors included family history of premature MI, inability to achieve BP goal, DM with the inability to achieve blood glucose or hemoglobin A1c goal, tobacco use, or inadequate statin therapy. Bleeding risk factors included a history of gastrointestinal bleed or peptic ulcer disease, concurrent use of medications that increase bleeding risk, chronic kidney disease, or thrombocytopenia.
Through shared decision making with careful consideration of these factors, we reached a conclusion with each patient to either continue or to deprescribe aspirin. Each discussion was documented in the electronic health record (EHR) using a standard documentation template (eAppendix, available at doi:10.12788/fp.0320). The patient’s medication list also was updated to reflect changes in aspirin use. For patients who declined deprescribing, the CPP or pharmacy intern asked the patient for their primary reason for preferring to continue aspirin, which was subsequently categorized as one of the following: no prior concerns with bleeding, concerns about a future cardiovascular event, wishing to discuss further with their primary care practitioner (PCP), or identifying an appropriate use for aspirin not evident through record review. For the patients who wished to further discuss the issue with their PCP before deprescribing, the patient’s PCP was notified of this preference by a record alert to the note documenting the encounter, and the patient was also encouraged to follow up about this issue. A voicemail was left if the patient did not answer requesting a call back, and a second attempt was made within 2 weeks.
Data Collected
We collected data to assess the proportion of patients for whom aspirin for primary prevention was discontinued. For patients who declined deprescribing, we documented the rationale for continuing aspirin. Additionally, the feasibility of implementation was assessed, including pharmacist time spent on each record review and intervention. Descriptive statistics were generated to evaluate baseline characteristics and intervention outcomes. The time to completion of these tasks was summarized with descriptive statistics.
We reviewed 459 patient records, and 110 were determined eligible for risk/benefit discussions.
Patients had various reasons for declining deprescribing, including 8 (28%) who had no prior concerns with bleeding while on aspirin and 6 (21%) who were concerned about a future cardiovascular event. Of those who declined aspirin deprescribing, 6 (21%) wished to further discuss the issue with their PCP. In 9 (31%) patients an alternative appropriate indication for aspirin was identified through discussion. In these cases, the indication for aspirin was documented and updated in the EHR.
Most patients (87%) contacted reported taking low-dose aspirin 81 mg daily, while 10% reported taking higher doses (range, 162-325) and 3% on an as-needed basis. In all 3 patients who agreed to dose reduction, the initial dose of 325 mg daily was reduced to 81 mg daily.
Results of the time-study analysis for each intervention indicated that a pharmacy intern or pharmacist spent about 2 minutes reviewing the record of each patient to determine eligibility for risk/benefit discussions. The 110 patients identified as eligible were 24% of the 459 records reviewed. An average (range) of 12 (6-20) minutes was spent on the telephone call plus documentation for each patient contacted. Additionally, we estimated that CPPs and pharmacy interns spent an approximate combined 12 hours in the development and review of materials for this program, including the protocol, script, and documentation templates. This also included about 1 hour to identify appropriate parameters for, and generate, the eligible patient list.
Discussion
The implementation of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting led to the discontinuation of inappropriate aspirin use in nearly half of older adults contacted. Furthermore, opportunities were identified to update medication lists to reflect previously self-discontinued aspirin for older adults. Just over one-quarter of those contacted declined to discontinue or reduce their aspirin dose. It is hypothesized that with these targeted deprescribing interventions, overall risk reduction for bleeding and polypharmacy will be observed for older adults.1
In addition to deprescribing aspirin, CPPs used shared decision making to initiate risk/benefit discussions and to educate on targeted lifestyle modifications to lower ASCVD risk. While not all patients agreed to discontinue aspirin, all were provided education that may empower them to engage in future discussions with PCPs regarding appropriate aspirin use. Previous pharmacist-led deprescribing initiatives for proton pump inhibitors and other PIMs have indicated that a large percentage of patients who opt to further discuss a deprescribing concern with their PCPs ultimately resulted in deprescribing outcomes.24,25 Additionally, a recent trial examining pharmacist-led deprescribing of 4 common PIMs in older adults compared the impact of pharmacists leading educational interventions directly to patients with pharmacists making deprescribing recommendations to physicians. Deprescribing was more successful when patients were involved in the decision-making process.26
Limitations
Although this quality improvement initiative resulted in the deprescribing of inappropriate aspirin for many older adults, a limitation is the small sample size within a single institution. The population of male veterans also may limit generalizability to nonmale and nonveteran older adults. As the protocol was initiated within a limited number of primary care teams initially, future implementation into additional primary care teams will increase the number of older adults impacted by risk/benefit discussions regarding aspirin use. This work may not be generalizable to other health care systems. Many patients within the VA receive both their primary and specialty care within the system, which facilitates communication and collaboration between primary and specialty practitioners. The protocol may require workflow adjustments for patients receiving care within multiple systems. Additionally, although the deprescribing protocol was created in collaboration with physicians, CPPs within the VA work under a broad scope of practice that includes independent medication prescribing, deprescribing, and monitoring. This may be a consideration when implementing similar protocols at other sites, as collaborative practice agreements may need to be in place.
Future Directions
The time required to complete these interventions was generally feasible, though this intervention would require some workflow alteration to be incorporated routinely into a CPP’s schedule. The telephone calls were completed as isolated interventions and were not incorporated into existing scheduled primary care appointments. In the future, the aspirin deprescribing protocol could be incorporated into existing pharmacist-led primary care appointments. Based on the outcomes of this study, CPPs are leading an initiative to develop an aspirin deprescribing clinical reminder tool, which may be quickly inserted into a progress note within the EHR and may be incorporated into any primary care visit led by a CPP or PCP.
Conclusions
This study demonstrates that a pharmacist-led aspirin deprescribing protocol in the ambulatory care pharmacy setting was successful in the discontinuation of unnecessary aspirin use in older adults. The protocol also provided opportunities for education on ASCVD risk reduction in all older adults reached. These findings highlight the role of pharmacists in deprescribing PIMs for older adults and identifying opportunities to further streamline risk/benefit discussions on aspirin deprescribing potential within primary care visits.
The use of low-dose aspirin for the primary prevention of cardiovascular disease (CVD) morbidity and mortality continues to be controversial, particularly for older adults. Recently published, robust randomized controlled trials have revealed less cardiovascular benefit from aspirin for primary prevention compared with previous trials; additionally, an increased risk of major bleeding events has been notably more prevalent in older adults.1-5 These trials have suggested that preventative aspirin use in older adults confers less benefit than other therapies for decreasing atherosclerotic CVD (ASCVD) risk, including blood pressure (BP) control, cholesterol management, and tobacco cessation.1,6
A recent meta-analysis indicated a composite cardiovascular risk reduction in patients aged 53 to 74 years taking aspirin vs no aspirin; however, this benefit was offset with an even greater increased risk of major bleeding.7 This trend was consistent regardless of stratification by 10-year ASCVD risk or presence of diabetes mellitus (DM) diagnosis.7,8 Additionally, the recently published Aspirin in Reducing Events in the Elderly (ASPREE) trial studied the impacts of aspirin use in healthy adults aged ≥ 70 years and aged ≥ 65 years among Black and Hispanic adults.4 The study concluded that the risk of major bleeding with aspirin use was even higher vs the potential cardiovascular benefit in older adults.4
With this emerging evidence, guidelines have been updated to represent the need for risk vs benefit considerations regarding aspirin use for primary prevention in older adults.1,9,10 The most recent guideline update from the American College of Cardiology and American Heart Association (ACC/AHA) recommends against the routine use of aspirin in patients aged > 70 years or those with bleeding risk factors.1 The guideline recommends considering aspirin use for patients ages 40 to 70 years only after a patient-specific risk vs benefit discussion.1 Furthermore, the 2020 American Diabetes Association guideline recommends considering aspirin use for primary prevention in adults with DM between ages 50 and 70 only after a risk vs benefit discussion of patient-specific bleeding risk factors and ASCVD risk-enhancing factors.10
Despite the demonstrated risks for bleeding with the routine use of aspirin, studies indicate that aspirin continues to be used commonly among older adults, often when unnecessary. In the 2017 National Health Interview Survey, about 23% of adults aged > 40 years in the United States without CVD used aspirin daily, and 23% of these did so without recommendation from a health care professional.11 Furthermore, nearly half of adults ages ≥ 70 years and nearly one-quarter of adults with a history of peptic ulcer disease used aspirin daily.11 Although the most recent guidelines from the ACC/AHA do not recommend a 10-year ASCVD risk threshold for therapy, one study illustrated that 12% of older adult patients were inappropriately prescribed aspirin for primary prevention despite a 10-year ASCVD risk of < 6%.1,12 These studies highlight the large proportion of individuals, particularly older adults, who may be inappropriately taking aspirin for primary prevention.
Deprescribing Program
Deprescribing potentially inappropriate medications (PIMs) is particularly important in the older adult population, as these individuals experience a high risk of adverse effects (AEs), polypharmacy, cognitive decline, and falls related to medication use.6,13-17 Evidence suggests that mortality outcomes are improved with the implementation of targeted deprescribing efforts based on patient-specific factors.18 Additionally, deprescribing unnecessary medications may improve adherence to other essential medications and reduce financial burdens.19 Pharmacists play a crucial role among health care professionals in the implementation of deprescribing practices, and studies have shown that physicians are highly accepting of pharmacists’ deprescribing recommendations.13,20-22
Despite the evidence for the benefits of deprescribing, limited data are available regarding the impact and feasibility of a targeted aspirin deprescribing approach by nonphysician practitioners.23 The objective of this study was to implement and evaluate the success of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting.
This aspirin deprescribing protocol was developed by ambulatory care clinical pharmacist or clinical pharmacist practitioners (CPPs), at the William S. Middleton Memorial Veterans Hospital in Madison, Wisconsin. Within the US Department of Veterans Affairs (VA) health care system, CPPs work under a broad scope of practice with the ability to independently prescribe and monitor medications. The protocol was reviewed by physician stakeholders in both primary care and cardiology and a list was generated, including patients from 2 primary care panels aged ≥ 70 years with aspirin on their medication list, either as a prescription or over-the-counter medication, using the VA Information System Technology and Architecture. A CPP or supervised pharmacy intern identified patients from this list who were appropriate for risk/benefit discussions regarding the discontinuation of aspirin. Patients were excluded from the intervention if they had a history of clinical ASCVD, including myocardial infarction (MI), stable or unstable angina, coronary artery disease (CAD), coronary or other arterial revascularization, cerebrovascular accident (CVA), transient ischemic accident (TIA), or peripheral artery disease (PAD), or another documented indication for aspirin use, including pain, flushing (with niacin use), venous thromboembolism prophylaxis, valvular heart disease, or acute or recurrent pericarditis.
After identifying eligible patients, a CPP or pharmacy intern contacted patients by telephone, following a script to guide conversation. All patients were screened for potential appropriate aspirin indications, particularly any history of MI, CAD, CVA, TIA, PAD, or other clinical ASCVD. The patient was asked about their rationale for taking aspirin and patient-specific ASCVD risk-enhancing factors and bleeding risk factors and educated them on lifestyle modalities to reduce ASCVD risk, using the script as a guide. ASCVD risk-enhancing factors included family history of premature MI, inability to achieve BP goal, DM with the inability to achieve blood glucose or hemoglobin A1c goal, tobacco use, or inadequate statin therapy. Bleeding risk factors included a history of gastrointestinal bleed or peptic ulcer disease, concurrent use of medications that increase bleeding risk, chronic kidney disease, or thrombocytopenia.
Through shared decision making with careful consideration of these factors, we reached a conclusion with each patient to either continue or to deprescribe aspirin. Each discussion was documented in the electronic health record (EHR) using a standard documentation template (eAppendix, available at doi:10.12788/fp.0320). The patient’s medication list also was updated to reflect changes in aspirin use. For patients who declined deprescribing, the CPP or pharmacy intern asked the patient for their primary reason for preferring to continue aspirin, which was subsequently categorized as one of the following: no prior concerns with bleeding, concerns about a future cardiovascular event, wishing to discuss further with their primary care practitioner (PCP), or identifying an appropriate use for aspirin not evident through record review. For the patients who wished to further discuss the issue with their PCP before deprescribing, the patient’s PCP was notified of this preference by a record alert to the note documenting the encounter, and the patient was also encouraged to follow up about this issue. A voicemail was left if the patient did not answer requesting a call back, and a second attempt was made within 2 weeks.
Data Collected
We collected data to assess the proportion of patients for whom aspirin for primary prevention was discontinued. For patients who declined deprescribing, we documented the rationale for continuing aspirin. Additionally, the feasibility of implementation was assessed, including pharmacist time spent on each record review and intervention. Descriptive statistics were generated to evaluate baseline characteristics and intervention outcomes. The time to completion of these tasks was summarized with descriptive statistics.
We reviewed 459 patient records, and 110 were determined eligible for risk/benefit discussions.
Patients had various reasons for declining deprescribing, including 8 (28%) who had no prior concerns with bleeding while on aspirin and 6 (21%) who were concerned about a future cardiovascular event. Of those who declined aspirin deprescribing, 6 (21%) wished to further discuss the issue with their PCP. In 9 (31%) patients an alternative appropriate indication for aspirin was identified through discussion. In these cases, the indication for aspirin was documented and updated in the EHR.
Most patients (87%) contacted reported taking low-dose aspirin 81 mg daily, while 10% reported taking higher doses (range, 162-325) and 3% on an as-needed basis. In all 3 patients who agreed to dose reduction, the initial dose of 325 mg daily was reduced to 81 mg daily.
Results of the time-study analysis for each intervention indicated that a pharmacy intern or pharmacist spent about 2 minutes reviewing the record of each patient to determine eligibility for risk/benefit discussions. The 110 patients identified as eligible were 24% of the 459 records reviewed. An average (range) of 12 (6-20) minutes was spent on the telephone call plus documentation for each patient contacted. Additionally, we estimated that CPPs and pharmacy interns spent an approximate combined 12 hours in the development and review of materials for this program, including the protocol, script, and documentation templates. This also included about 1 hour to identify appropriate parameters for, and generate, the eligible patient list.
Discussion
The implementation of a pharmacist-driven aspirin deprescribing protocol for older adults in a primary care setting led to the discontinuation of inappropriate aspirin use in nearly half of older adults contacted. Furthermore, opportunities were identified to update medication lists to reflect previously self-discontinued aspirin for older adults. Just over one-quarter of those contacted declined to discontinue or reduce their aspirin dose. It is hypothesized that with these targeted deprescribing interventions, overall risk reduction for bleeding and polypharmacy will be observed for older adults.1
In addition to deprescribing aspirin, CPPs used shared decision making to initiate risk/benefit discussions and to educate on targeted lifestyle modifications to lower ASCVD risk. While not all patients agreed to discontinue aspirin, all were provided education that may empower them to engage in future discussions with PCPs regarding appropriate aspirin use. Previous pharmacist-led deprescribing initiatives for proton pump inhibitors and other PIMs have indicated that a large percentage of patients who opt to further discuss a deprescribing concern with their PCPs ultimately resulted in deprescribing outcomes.24,25 Additionally, a recent trial examining pharmacist-led deprescribing of 4 common PIMs in older adults compared the impact of pharmacists leading educational interventions directly to patients with pharmacists making deprescribing recommendations to physicians. Deprescribing was more successful when patients were involved in the decision-making process.26
Limitations
Although this quality improvement initiative resulted in the deprescribing of inappropriate aspirin for many older adults, a limitation is the small sample size within a single institution. The population of male veterans also may limit generalizability to nonmale and nonveteran older adults. As the protocol was initiated within a limited number of primary care teams initially, future implementation into additional primary care teams will increase the number of older adults impacted by risk/benefit discussions regarding aspirin use. This work may not be generalizable to other health care systems. Many patients within the VA receive both their primary and specialty care within the system, which facilitates communication and collaboration between primary and specialty practitioners. The protocol may require workflow adjustments for patients receiving care within multiple systems. Additionally, although the deprescribing protocol was created in collaboration with physicians, CPPs within the VA work under a broad scope of practice that includes independent medication prescribing, deprescribing, and monitoring. This may be a consideration when implementing similar protocols at other sites, as collaborative practice agreements may need to be in place.
Future Directions
The time required to complete these interventions was generally feasible, though this intervention would require some workflow alteration to be incorporated routinely into a CPP’s schedule. The telephone calls were completed as isolated interventions and were not incorporated into existing scheduled primary care appointments. In the future, the aspirin deprescribing protocol could be incorporated into existing pharmacist-led primary care appointments. Based on the outcomes of this study, CPPs are leading an initiative to develop an aspirin deprescribing clinical reminder tool, which may be quickly inserted into a progress note within the EHR and may be incorporated into any primary care visit led by a CPP or PCP.
Conclusions
This study demonstrates that a pharmacist-led aspirin deprescribing protocol in the ambulatory care pharmacy setting was successful in the discontinuation of unnecessary aspirin use in older adults. The protocol also provided opportunities for education on ASCVD risk reduction in all older adults reached. These findings highlight the role of pharmacists in deprescribing PIMs for older adults and identifying opportunities to further streamline risk/benefit discussions on aspirin deprescribing potential within primary care visits.
1. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
2. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomized, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
3. Bowman L, Mafham M, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
4. McNeil JJ, Wolfe R, Woods, RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. García Rodríguez LA, Martín-Pérez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long-term low-dose aspirin: a systematic review of observational studies. PloS One. 2016;11(8):e0160046. doi:10.1371/journal.pone.0160046
6. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment): consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83. doi:10.5414/cpp46072
7. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi:10.1001/jama.2018.20578
8. Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16(11):675-686. doi:10.1038/s41569-019-0225-y
9. Bibbins-Domingo K; U.S. Preventative Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(12):836-845. doi:10.7326/M16-0577
10. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi:10.2337/dc20-S002
11. O’Brien CW, Juraschek SP, Wee CC. Prevalence of aspirin use for primary prevention of cardiovascular disease in the United States: results from the 2017 National Health Interview Survey. Ann Intern Med. 2019;171(8):596-598. doi:10.7326/M19-0953
12. Hira RS, Kennedy K, Nambi V, et al. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65(2):111-121. doi:10.1016/j.jacc.2014.10.035
13. Cheong ST, Ng TM, Tan KT. Pharmacist-initiated deprescribing in hospitalized elderly: prevalence and acceptance by physicians. Eur J Hosp Pharm. 2018;25(e1):e35-e39. doi:10.1136/ejhpharm-2017-001251
14. Dyck MJ. Evidence-based administrative guideline: quality improvement in nursing homes. J Gerontol Nurs. 2005;31(2):4-10. doi:10.3928/0098-9134-20050201-04
15. Zullo AR, Gray SL, Holmes HM, Marcum ZA. Screening for medication appropriateness in older adults. Clin Geriatr Med. 2018;34(1):39-54. doi:10.1016/j.cger.2017.09.003
16. American Geriatrics Society. 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
17. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
18. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. doi:10.1111/bcp.12975
19. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. The benefits and harms of deprescribing. Med J Aust. 2014;201(7):386-389. doi:10.5694/mja13.00200
20. Ailabouni NJ, Marcum ZA, Schmader KE, Gray SL. Medication use quality and safety in older adults: 2018 update. J Am Geriatr Soc. 2019;67(12):2458-2462. doi:10.1111/jgs.16243
21. Frank C, Weir E. Deprescribing for older patients. CMAJ. 2014;186(18):1369-1376. doi:10.1503/cmaj.131873
22. Clark CM, LaValley SA, Singh R, Mustafa E, Monte SV, Wahler RG Jr. A pharmacist-led program to facilitate deprescribing in a primary care clinic. J Am Pharm Assoc (2003). 2020;60(1):105-111. doi:10.1016/j.japh.2019.09.011
23. Folks B, Leblanc WG, Staton EW, Pace WD. Reconsidering low-dose aspirin therapy for cardiovascular disease: a study protocol for physician and patient behavioral change. Implement Sci. 2011;6:65. Published 2011 Jun 26. doi:10.1186/1748-5908-6-65
24. Odenthal DR, Philbrick AM, Harris IM. Successful deprescribing of unnecessary proton pump inhibitors in a primary care clinic. J Am Pharm Assoc. 2020;60(1):100-104. doi:10.1016/j.japh.2019.08.012
25. Duncan, P. Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24(1):37-42. doi:10.1136/ejhpharm-2016-000967
26. Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
1. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e596-e646. doi:10.1161/CIR.0000000000000678
2. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomized, double-blind, placebo-controlled trial. Lancet. 2018;392(10152):1036-1046. doi:10.1016/S0140-6736(18)31924-X
3. Bowman L, Mafham M, et al; ASCEND Study Collaborative Group. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379(16):1529-1539. doi:10.1056/NEJMoa1804988
4. McNeil JJ, Wolfe R, Woods, RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med. 2018;379(16):1509-1518. doi:10.1056/NEJMoa1805819
5. García Rodríguez LA, Martín-Pérez M, Hennekens CH, Rothwell PM, Lanas A. Bleeding risk with long-term low-dose aspirin: a systematic review of observational studies. PloS One. 2016;11(8):e0160046. doi:10.1371/journal.pone.0160046
6. Gallagher P, Ryan C, Byrne S, Kennedy J, O’Mahony D. STOPP (Screening Tool of Older Person’s Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment): consensus validation. Int J Clin Pharmacol Ther. 2008;46(2):72-83. doi:10.5414/cpp46072
7. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019;321(3):277-287. doi:10.1001/jama.2018.20578
8. Patrono C, Baigent C. Role of aspirin in primary prevention of cardiovascular disease. Nat Rev Cardiol. 2019;16(11):675-686. doi:10.1038/s41569-019-0225-y
9. Bibbins-Domingo K; U.S. Preventative Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2016;164(12):836-845. doi:10.7326/M16-0577
10. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi:10.2337/dc20-S002
11. O’Brien CW, Juraschek SP, Wee CC. Prevalence of aspirin use for primary prevention of cardiovascular disease in the United States: results from the 2017 National Health Interview Survey. Ann Intern Med. 2019;171(8):596-598. doi:10.7326/M19-0953
12. Hira RS, Kennedy K, Nambi V, et al. Frequency and practice-level variation in inappropriate aspirin use for the primary prevention of cardiovascular disease: insights from the National Cardiovascular Disease Registry’s Practice Innovation and Clinical Excellence registry. J Am Coll Cardiol. 2015;65(2):111-121. doi:10.1016/j.jacc.2014.10.035
13. Cheong ST, Ng TM, Tan KT. Pharmacist-initiated deprescribing in hospitalized elderly: prevalence and acceptance by physicians. Eur J Hosp Pharm. 2018;25(e1):e35-e39. doi:10.1136/ejhpharm-2017-001251
14. Dyck MJ. Evidence-based administrative guideline: quality improvement in nursing homes. J Gerontol Nurs. 2005;31(2):4-10. doi:10.3928/0098-9134-20050201-04
15. Zullo AR, Gray SL, Holmes HM, Marcum ZA. Screening for medication appropriateness in older adults. Clin Geriatr Med. 2018;34(1):39-54. doi:10.1016/j.cger.2017.09.003
16. American Geriatrics Society. 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694. doi:10.1111/jgs.15767
17. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med. 2012;28(2):173-186. doi:10.1016/j.cger.2012.01.002
18. Page AT, Clifford RM, Potter K, Schwartz D, Etherton-Beer CD. The feasibility and effect of deprescribing in older adults on mortality and health: a systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(3):583-623. doi:10.1111/bcp.12975
19. Reeve E, Shakib S, Hendrix I, Roberts MS, Wiese MD. The benefits and harms of deprescribing. Med J Aust. 2014;201(7):386-389. doi:10.5694/mja13.00200
20. Ailabouni NJ, Marcum ZA, Schmader KE, Gray SL. Medication use quality and safety in older adults: 2018 update. J Am Geriatr Soc. 2019;67(12):2458-2462. doi:10.1111/jgs.16243
21. Frank C, Weir E. Deprescribing for older patients. CMAJ. 2014;186(18):1369-1376. doi:10.1503/cmaj.131873
22. Clark CM, LaValley SA, Singh R, Mustafa E, Monte SV, Wahler RG Jr. A pharmacist-led program to facilitate deprescribing in a primary care clinic. J Am Pharm Assoc (2003). 2020;60(1):105-111. doi:10.1016/j.japh.2019.09.011
23. Folks B, Leblanc WG, Staton EW, Pace WD. Reconsidering low-dose aspirin therapy for cardiovascular disease: a study protocol for physician and patient behavioral change. Implement Sci. 2011;6:65. Published 2011 Jun 26. doi:10.1186/1748-5908-6-65
24. Odenthal DR, Philbrick AM, Harris IM. Successful deprescribing of unnecessary proton pump inhibitors in a primary care clinic. J Am Pharm Assoc. 2020;60(1):100-104. doi:10.1016/j.japh.2019.08.012
25. Duncan, P. Duerden M, Payne RA. Deprescribing: a primary care perspective. Eur J Hosp Pharm. 2017;24(1):37-42. doi:10.1136/ejhpharm-2016-000967
26. Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA. 2018;320(18):1889-1898. doi:10.1001/jama.2018.16131
Preoperative Insulin Intensification to Improve Day of Surgery Blood Glucose Control
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
Perioperative hyperglycemia, defined as blood glucose levels ≥ 180 mg/dL in the immediate pre- and postoperative period, is associated with increased postoperative morbidity, including infections, preoperative interventions, and in-hospital mortality.1-3 Despite being identified as a barrier to optimal perioperative glycemic control, limited evidence is available on patient or health care practitioner (HCP) adherence to preoperative insulin protocols.4-6
Background
Despite mounting evidence of the advantages of maintaining perioperative glucose levels between 80 and 180 mg/dL, available guidelines vary in their recommendations for long-acting basal insulin dosing.7-10 The Society of Ambulatory Anesthesia suggests using 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery in patients without a history of nocturnal or morning hypoglycemia (category 2A evidence).9 However, the revised 2016 United Kingdom National Health Service consensus guideline recommends using 80% to 100% of the prescribed evening dosage of long-acting basal insulin dose on the night before surgery.7 The 2022 American Diabetes Association references an observational study of patients with type 2 DM (T2DM) treated with evening-only, long-acting glargine insulin, indicating that the optimal basal insulin dose on the evening before surgery is about 75% of the outpatient dose.5,10 However, in a randomized, prospective open trial of patients with DM treated with evening-only long-acting basal insulin, no significant difference was noted in the target day of surgery (DOS) glucose levels among different dosing strategies on the evening before surgery.6 Presently, the optimal dose of long-acting insulin analogs on the evening before surgery is unknown.
Additionally, little is known about the other factors that influence perioperative glycemic control. Several barriers to optimal perioperative care of patients with DM have been identified, including lack of prioritization by HCPs, lack of knowledge about current evidence-based recommendations, and lack of patient information and involvement.4 To determine the effect of patient adherence to preoperative medication instructions on postoperative outcome, a cross-sectional study assessed surgical patients admitted to the postanesthetic care unit (PACU) and found that only 70% of patients with insulin-treated DM took their medications preoperatively. Additionally, 23% of nonadherent patients who omitted their medications either did not understand or forgot preoperative medication management instructions. Preoperative DM medication omission was associated with higher rates of hyperglycemia in the PACU (23.8% vs 3.6%; P = .02).11 Importantly, to our knowledge, the extent of HCP adherence to DM management protocols and the subsequent effect on DOS hyperglycemia has not been examined until now.For patients with DM treated with an evening dose of long-acting basal insulin (ie, either once-daily long-acting basal insulin in the evening or twice-daily long-acting basal insulin, both morning and evening) presenting for elective noncardiac surgery, our aim was to decrease the rate of DOS hyperglycemia from 29% (our baseline) to 15% by intensifying the dose of insulin on the evening before surgery without increasing the rate of hypoglycemia. We also sought to determine the rates of HCP adherence to our insulin protocols as well as patients’ self-reported adherence to HCP instructions over the course of this quality improvement (QI) initiative.
Quality Improvement Program
Our surgical department consists of 11 surgical subspecialties that performed approximately 4400 noncardiac surgeries in 2019. All patients undergoing elective surgery are evaluated in the preoperative clinic, which is staffed by an anesthesiology professional (attending and resident physicians, nurse practitioners, and physician assistants) and internal medicine attending physicians. At the preoperative visit, each patient is evaluated by anesthesiology; medically complex patients may also be referred to an internal medicine professional for further risk stratification and optimization before surgery.
At the preoperative clinic visit, HCPs prepare written patient instructions for the preoperative management of medications, including glucose-lowering medications, based on a DM management protocol that was implemented in 2016 for the preoperative management of insulin, noninsulin injectable agents, and oral hyperglycemic agents. According to this protocol, patients with DM treated with evening long-acting basal insulin (eg, glargine insulin) are instructed to take 50% of their usual evening dose the evening before surgery. A preoperative clinic nurse reviews the final preoperative medication instructions with the patient at the end of the clinic visit. Patients are also instructed to avoid oral intake other than water and necessary medications after midnight before surgery regardless of the time of surgery. On the DOS, the patient’s blood glucose level is measured on arrival to the presurgical area.
Our QI initiative focused only on the dose of self-administered, long-acting basal insulin on the evening before surgery. The effect of the morning of surgery long-acting insulin dose on the DOS glucose levels largely depends on the timing of surgery, which is variable; therefore, we did not target this dose for our initiative. Patients receiving intermediate-acting neutral protamine Hagedorn (NPH) insulin were excluded because our protocol does not recommend a dose reduction for NPH insulin on the evening before surgery.
We developed a comprehensive driver diagram to help elucidate the different factors contributing to DOS hyperglycemia and to guide specific QI interventions.12 Some of the identified contributors to DOS hyperglycemia, such as the length of preoperative fasting and timing of surgery, are unpredictable and were deemed difficult to address preoperatively. Other contributors to DOS hyperglycemia, such as outpatient DM management, often require interventions over several months, which is well beyond the time usually allotted for preoperative evaluation and optimization. On the other hand, immediate preoperative insulin dosing directly affects DOS glycemic control; therefore, improvement of the preoperative insulin management protocol to optimize the dosage on the evening before surgery was considered to be an achievable QI goal with the potential for decreasing the rate of DOS hyperglycemia in patients presenting for elective noncardiac surgery.
We used the Model for Understanding Success in Quality (MUSIQ) as a framework to identify key contextual factors that may affect the success of our QI project.13 Limited resource availability and difficulty with dissemination of protocol changes in the preoperative clinic were determined to be potential barriers to the successful implementation of our QI initiative. Nonetheless, senior leadership support, microsystem QI culture, QI team skills, and physician involvement supported the implementation. The revised Standards for Quality Improvement Reporting Excellence (SQUIRE 2.0) guidelines were followed for this study.14
Interventions
With stakeholder input from anesthesiology, internal medicine, endocrinology, and nursing, we designed an intervention to iteratively change the HCP protocol instructions for long-acting insulin dosing on the evening before surgery. In phase 1 of the study (October 1, 2018, to March 11, 2019), we obtained baseline data on the rates of DOS hyperglycemia (blood glucose ≥ 180 mg/dL) and hypoglycemia (blood glucose < 80 mg/dL), as well as patient and HCP adherence rates to our existing preoperative DM protocol. For phase 2 (March 12, 2019, to July 22, 2019), the preoperative DM management protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with hemoglobin A1c (HbA1c) levels > 8% from 50% of the usual outpatient dose to 100%. Finally, in phase 3 (July 23, 2019, to March 12, 2020), the protocol was changed to increase the dose of long-acting basal insulin on the evening before surgery for patients with HbA1c levels ≤ 8% from 50% of the usual outpatient dose to 75% while sustaining the phase 2 change. Preoperative HCPs were informed of the protocol changes in person and were provided with electronic and hard copies of each new protocol.
Protocol
We used a prospective cohort design of 424 consecutive patients with DM who presented for preoperative evaluation for elective noncardiac surgery between October 1, 2018, and March 12, 2020. For the subset of 195 patients treated with an evening dose of long-acting basal insulin, we examined the effect of intensification of this preoperative basal insulin dose on DOS hyperglycemia and hypoglycemia, HCP adherence to iterative changes of the protocol, and patient adherence to HCP instructions on preoperative medication dosing. The QI project was concluded when elective surgeries were paused due to the COVID-19 pandemic.
We created a standardized preoperative data collection form that included information on the most recent HbA1c, time, dose, and type of patient-administered insulin on the evening before surgery, and DOS blood glucose level. A preoperative clinic nurse completed the standardized preoperative data collection form. The HCP’s preoperative medication instructions and the preoperative data collection forms were gathered for review and data analysis.
The primary outcome was DOS hyperglycemia (blood glucose levels ≥ 180 mg/dL). We monitored the rate of DOS hypoglycemia (blood glucose levels < 80 mg/dL) as a balancing measure to ensure safety with long-acting basal insulin intensification. Although hypoglycemia is defined as a blood glucose level < 70 mg/dL, a target glucose range of 80 mg/dL to 180 mg/dL is recommended during the perioperative period.8 Therefore, we chose a more conservative definition of hypoglycemia (blood glucose levels < 80 mg/dL) to adhere to the recommended perioperative glucose target range.
Process measures included HCP adherence to each protocol change, which was assessed by comparing written preoperative patient instructions to the current protocol. Similarly, patient adherence to HCP-recommended long-acting basal insulin dosing was assessed by comparing written preoperative patient instructions to the patient’s self-reported time and dose of long-acting basal insulin on the evening before surgery. For any discrepancy between the HCP instructions and protocol or HCP-recommended dose and patient self-reported dose of long-acting basal insulin, a detailed chart review was performed to determine the etiology.
Statistical Analysis
We used the statistical process p-control chart to assess the effect of iterative changes to the preoperative long-acting basal insulin protocol on DOS hyperglycemia. The proportion defective (rate of DOS hyperglycemia) was plotted against time to determine whether the observed variations in the rate of DOS hyperglycemia over time were attributable to random common causes or special causes because of our intervention. The lower control limit (LCL) and upper control limit (UCL) define the limits of expected outcome measures in a stable process prior to introducing changes and were set at 3 SDs from the mean to balance the likelihood of type I (false-positive) and type II (false-negative) errors. Because of the variable interval sample sizes, we used the CRITBINOM function of Microsoft Excel to calculate the exact UCL satisfying the 3 SD limits of 0.99865.15 The Shewhart rules (outliers, runs or shifts, trends, sawtooth) were used to analyze the p-control chart to identify special cause signals resulting from our interventions.16 We used the statistical process t-control chart to record the time (days) between the few occurrences of DOS hypoglycemia because cases of hypoglycemia were rare.
Ethical Consideration
The Human Research Protection Program, Associate Chief of Staff for Research and Development, and Quality, Safety, and Values department reviewed this project in accordance with the Veterans Health Administration Program Guide 1200.21 and determined that it was a nonresearch operations activity; thus, approval by an institutional review board was not needed. The authors declare no competing interests.
Patient Outcomes
We prospectively followed 424 consecutive patients with DM undergoing elective noncardiac surgery from the time of the preoperative clinic evaluation until DOS; 195 patients were on evening
A subgroup analysis of DOS glucose levels in insulin-treated patients with preoperative HbA1c levels > 8% did not demonstrate a change in the rate of
Only 7 of 424 (1.7%) patients with DM and 4 of 195 (2.1%) patients treated with evening, long-acting basal insulin had marked hyperglycemia (DOS glucose levels ≥ 300 mg/dL). Only 1 patient who was not on outpatient insulin treatment had surgery canceled for hyperglycemia.
Overall, 89% of the HCPs followed the preoperative insulin protocol. HCP adherence to the protocol decreased to 77% after the phase 2 change, often related to deviations from the protocol or when a prior version was used. By the end of phase 3, HCP adherence returned to the baseline rate (88%). Patient adherence to medication instructions was not affected by protocol changes (86% throughout the study period). Prospective data collection was briefly interrupted between January 18, 2019, and March 5, 2019, while designing our phase 2 intervention. We were unable to track the total number of eligible patients during this time, but were able to identify 8 insulin-treated patients with DM who underwent elective noncardiac surgery and included their data in phase 1.
Discussion
The management and prevention of immediate perioperative hyperglycemia and glycemic variability have attracted attention as evidence has mounted for their association with postoperative morbidity and mortality.1,2,17 Available guidelines for preventing DOS hyperglycemia vary in their recommendations for preoperative insulin management.7-10 Notably, concerns about iatrogenic hypoglycemia often hinder efforts to lower rates of DOS hyperglycemia.4 We successfully implemented an iterative intensification protocol for preoperative long-acting basal insulin doses on the evening before surgery but did not observe a lower rate of hyperglycemia. Importantly, we also did not observe a higher rate of hypoglycemia on the DOS, as observed in a previous study.5
The observational study by Demma and colleagues found that patients receiving 75% of their evening, long-acting basal insulin dose were significantly more likely to achieve target blood glucose levels of 100 to 180 mg/dL than patients receiving no insulin at all (78% vs 0%; P = .001). However, no significant difference was noted when this group was compared with patients receiving 50% of their evening, long-acting basal insulin doses (78% vs 70%; P = .56). This is more clinically pertinent as it is generally accepted that the evening, long-acting insulin dose should not be entirely withheld on the evening before surgery.5
These findings are consistent with our observation that the rate of DOS hyperglycemia did not decrease with intensification of the evening, long-acting insulin dose from 50% to 100% of the prescribed dose in patients with HbA1c levels > 8% (phase 2) and 50% to 75% of the prescribed dose in patients with HbA1c levels ≤ 8% (phase 3). In the study by Demma and colleagues, few patients presented with preoperative hypoglycemia (2.7%) but all had received 100% of their evening, long-acting basal insulin dose, suggesting a significant increase in the rate of hypoglycemia compared with patients receiving lower doses of insulin (P = .01).5 However, long-term DM control as assessed by HbA1c level was available for < 10% of the patients, making it difficult to evaluate the effect of overall DM control on the results.5 In our study, preoperative HbA1c levels were available for 99.5% of the patients and only those with HbA1c levels > 8% received 100% of their evening, long-acting insulin dose on the evening before surgery. Notably, we did not observe a higher rate of hypoglycemia in this patient population, indicating that preoperative insulin dose intensification is safe for this subgroup.
Although HCP adherence to perioperative DM management protocols has been identified as a predominant barrier to the delivery of optimal perioperative DM care, prior studies of various preoperative insulin protocols to reduce perioperative hyperglycemia have not reported HCP adherence to their insulin protocols or its effect on DOS hyperglycemia.4-6 Additionally, patient adherence to HCP instructions is a key factor identified in our driver diagram that may influence DOS hyperglycemia, a hypothesis that is supported by a prior cross-sectional study showing an increased rate of hyperglycemia in the PACU with omission of preoperative DM medication.11 In our study, patient adherence to preoperative medication management instructions was higher than reported previously and remained consistently high regardless of protocol changes, which may explain why patient adherence did not affect the rate of DOS hyperglycemia.
Although not part of our study protocol, our preoperative HCPs routinely prepare written patient instructions for the preoperative management of medications for all patients, which likely explains higher patient adherence to instructions in our study than seen in the previous study where written instructions were only encouraged.11 However, HCP adherence to the protocol decreased after our phase 2 changes and was associated with a transient increase in DOS hyperglycemia rates. The DOS hyperglycemia rates returned to baseline levels with ongoing QI efforts and education to improve HCP adherence to protocol.
Limitations
Our QI initiative had several limitations. Nearly all patients were male veterans with T2DM, and most were older (range, 50-89 years). This limits the generalizability to women, younger patients, and people with type 1 DM. Additionally, our data collection relied on completion and collection of the preoperative form by different HCPs, allowing for sampling bias if some patients with DM undergoing elective noncardiac surgery were missed. Furthermore, although we could verify HCP adherence to the preoperative DM management protocols by reviewing their written instructions, we relied on patients’ self-reported adherence to the preoperative instructions. Finally, we did not evaluate postoperative blood glucose levels because the effect of intraoperative factors such as fluid, insulin, and glucocorticoid administration on postoperative glucose levels are variable. To the best of our knowledge, no other major systematic changes occurred in the preoperative care of patients with DM during the study period.
Conclusions
The findings of our QI initiative suggest that HCP adherence to preoperative DM management protocols may be a key contributor to DOS hyperglycemia and that ensuring HCP adherence may be as important as preoperative insulin dose adjustments. To our knowledge, this is the first study to report rates of HCP adherence to preoperative DM management protocols and its effect on DOS hyperglycemia. We will focus future QI efforts on optimizing HCP adherence to preoperative DM management protocols at our institution.
Acknowledgments
We thank our endocrinology expert, Dr. Kristina Utzschneider, for her guidance in designing this improvement project and our academic research coach, Dr. Helene Starks, for her help in editing the manuscript.
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922
1. van den Boom W, Schroeder RA, Manning MW, Setji TL, Fiestan GO, Dunson DB. Effect of A1c and glucose on postoperative mortality in noncardiac and cardiac surgeries. Diabetes Care. 2018;41(4):782-788. doi:10.2337/dc17-2232
2. Punthakee Z, Iglesias PP, Alonso-Coello P, et al. Association of preoperative glucose concentration with myocardial injury and death after non-cardiac surgery (GlucoVISION): a prospective cohort study. Lancet Diabetes Endocrinol. 2018;6(10):790-797. doi:10.1016/S2213-8587(18)30205-5
3. Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257(1):8-14. doi:10.1097/SLA.0b013e31827b6bbc
4. Hommel I, van Gurp PJ, den Broeder AA, et al. Reactive rather than proactive diabetes management in the perioperative period. Horm Metab Res. 2017;49(7):527-533. doi:10.1055/s-0043-105501
5. Demma LJ, Carlson KT, Duggan EW, Morrow JG 3rd, Umpierrez G. Effect of basal insulin dosage on blood glucose concentration in ambulatory surgery patients with type 2 diabetes. J Clin Anesth. 2017;36:184-188. doi:10.1016/j.jclinane.2016.10.003
6. Rosenblatt SI, Dukatz T, Jahn R, et al. Insulin glargine dosing before next-day surgery: comparing three strategies. J Clin Anesth. 2012;24(8):610-617. doi:10.1016/j.jclinane.2012.02.010
7. Dhatariya K, Levy N, Flanagen D, et al; Joint British Diabetes Societies for Inpatient Care. Management of adults with diabetes undergoing surgery and elective procedures: improving standards. Summary. Published 2011. Revised March 2016. Accessed October 31, 2022. https://www.diabetes.org.uk/resources-s3/2017-09/Surgical%20guideline%202015%20-%20summary%20FINAL%20amended%20Mar%202016.pdf
8. American Diabetes Association. 15. Diabetes care in the hospital: standards of medical care in diabetes–2021. Diabetes Care. 2021;44(suppl 1):S211-S220. doi:10.2337/dc21-S015
9. Joshi GP, Chung F, Vann MA, et al; Society for Ambulatory Anesthesia. Society for Ambulatory Anesthesia consensus statement on perioperative blood glucose management in diabetic patients undergoing ambulatory surgery. Anesth Analg. 2010;111(6):1378-1387. doi:10.1213/ANE.0b013e3181f9c288
10. American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: standards of medical care in diabetes–2022. Diabetes Care. 2021;45(suppl 1):S244-S253. doi:10.2337/dc22-S016
11. Notaras AP, Demetriou E, Galvin J, Ben-Menachem E. A cross-sectional study of preoperative medication adherence and early postoperative recovery. J Clin Anesth. 2016;35:129-135. doi:10.1016/j.jclinane.2016.07.007
12. Bennett B, Provost L. What’s your theory? Driver diagram serves as tool for building and testing theories for improvement. Quality Progress. 2015;48(7):36-43. Accessed August 31, 2022. http://www.apiweb.org/QP_whats-your-theory_201507.pdf
13. Kaplan HC, Provost LP, Froehle CM, Margolis PA. The Model for Understanding Success in Quality (MUSIQ): building a theory of context in healthcare quality improvement. BMJ Qual Saf. 2012;21(1):13-20. doi:10.1136/bmjqs-2011-000010
14. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2016;25(12):986-992. doi:10.1136/bmjqs-2015-004411
15. Duclos A, Voirin N. The p-control chart: a tool for care improvement. Int J Qual Health Care. 2010;22(5):402-407. doi:10.1093/intqhc/mzq037
16. Cheung YY, Jung B, Sohn JH, Ogrinc G. Quality initiatives: statistical control charts: simplifying the analysis of data for quality improvement. Radiographics. 2012;32(7):2113-2126. doi:10.1148/rg.327125713
17. Simha V, Shah P. Perioperative glucose control in patients with diabetes undergoing elective surgery. JAMA. 2019;321(4):399. doi:10.1001/jama.2018.20922
More Than a Health Fair: Preventive Health Care During COVID-19 Vaccine Events
Shortly into the COVID-19 pandemic, Dr. Robert Califf, the commissioner of the US Food and Drug Administration, warned of a coming tsunami of chronic diseases, exacerbated by missed care during the pandemic.1 According to a Centers for Disease Control and Prevention (CDC) survey, more than 30% of adults reported delaying or avoiding routine medical care in the first 6 months of 2020. This rate was highest in people with comorbidities.2 Multiple studies demonstrated declines in hypertension care, hemoglobin A1c testing, mammography, and colon cancer screening.3-5 There has been a resultant increase in colon cancer complications, wounds, and amputations.6,7 The United Kingdom is expected to have a 7.9% to 16.6% increase in future deaths due to breast and colorectal cancer (CRC).8 The World Health Organization estimates an excess 14.9 million people died in 2020 and 2021, either directly from or indirectly related to COVID-19.9
Due to the large-scale conversion from face-to-face care to telehealth modalities, COVID-19 vaccination events offered a unique opportunity to perform preventive health care that requires in-person visits, since most US adults have sought vaccination. However, vaccine events may not reach people most at risk for COVID-19 or chronic disease. Groups of Americans with lower vaccination rates were concerned about driving times and missing work to get the vaccine.10
Distance and travel time may be a particular challenge in Hawaii. Oahu is considered rural by the US Department of Veterans Affairs (VA); some communities are 80 minutes away from the VA Pacific Islands Health Care System (VAPIHCS) main facility. Oahu has approximately 150 veterans experiencing homelessness who may not have transportation to vaccine events. Additionally, VAPIHCS serves veterans that may be at higher risk of not receiving COVID-19 vaccination. Racial and ethnic minority residents have lower vaccination rates, yet are at a higher risk of COVID-19 infection and complications, and through the pandemic, this vaccination gap worsened.11,12 More than 10% of the population of Hawaii is Native Hawaiian or Pacific Islander, and this population is at elevated risk for diabetes mellitus, hypertension, and COVID-19 mortality.13-16
Health Fair Program
The VA provides clinical reminders in its electronic health record (EHR) that are specified by age, gender assigned at birth, and comorbidities. The clinical reminder program is intended to provide clinically relevant reminders for preventive care at the point of care. Veterans with overdue clinical reminders can be identified by name and address, allowing for the creation of health fair events that were directed towards communities with veterans with clinical reminders, including COVID-19 vaccination need. A team of health care professionals from VAPIHCS conceived of a health fair program to increase the reach of vaccine events and include preventive care in partnership with the VAPIHCS Vet Center Program, local communities, U.S.VETS, and the Hawaii Institute of Health Services (HIHS). We sought to determine which services could be offered in community settings; large vaccine events; and at homeless emergency, transitional, or permanent housing. We tracked veterans who received care in the different locations of the directed health fair.
This project was determined to be a quality improvement initiative by the VAPIHCS Office of Research and Development. It was jointly planned by the VAPIHCS pharmacy, infectious diseases, Vet Center Program, and homeless team to make the COVID-19 vaccines available to more rural and to veterans experiencing homelessness, and in response to a decline in facility face-to-face visits. Monthly meetings were held to select sites within zip codes with higher numbers of open clinical reminders and lower vaccination uptake. Informatics developed a list of clinical reminders by zip code for care performed at face-to-face visits.
Partners
The Vet Center Program, suicide prevention coordinator, and the homeless outreach team have a mandate to perform outreach events.17,18 These services collaborate with community partners to locate sites for events. The team was able to leverage these contacts to set up sites for events. The Vet Center Program readjustment counselor and the suicide prevention coordinator provide mental health counseling. The Vet Center counsels on veteran benefits. They supplied a mobile van with WiFi, counseling and examination spaces, and refrigeration, which became the mobile clinic for the preventive care offered at events. The homeless program works with multiple community partners. They contract with HIHS and U.S.VETS to provide emergency and permanent housing for veterans. Each event is reviewed with HIHS and U.S.VETS staff for permission to be on site. The suicide prevention coordinator or the Vet Center readjustment counselor and the homeless team became regular attendees of events. The homeless team provided resources for housing or food insecurity.
Preventive Health Measures
The VA clinical reminder system supports caregivers for both preventive health care and chronic condition management.19 Clinical reminders appear as due in the EHR, and reminder reports can be run by clinical informatics to determine groups of patients who have not had a reminder completed. The following reminders were completed: vaccinations (including COVID-19), CRC screening, diabetic foot check and teaching of foot care, diabetic retinal consultations, laboratory studies (lipids, hemoglobin A1c, microalbumin), mammogram and pap smear referrals, mental health reminders, homeless and food insecurity screening, HIV and hepatitis C testing, and blood pressure (BP) measurement. Health records were reviewed 3 months after each event to determine whether they were completed by the veteran. Additionally, we determined whether BP was controlled (< 130/80 mm Hg).
Settings
Large urban event. The first setting for the health fair was a large vaccination event near the VAPIHCS center in April 2021. Attendance was solicited by VEText, phone calls, and social media advertisements. At check-in, veterans with relevant open clinical reminders were invited to receive preventive health care during the 15-minute monitoring period after the COVID-19 vaccine. The Vet Center Program stationed the mobile van outside the vaccination event, where a physician and a clinical pharmacy specialist (CPS) did assessments, completed reminders, and entered follow-up requests for about 4 hours. A medical support assistant registered veterans who had never signed up for VA health care.
Community Settings. Nine events occurred at least monthly between March and September 2021 at 4 different sites in Oahu. Texts and phone calls were used to solicit attendance; there was no prior publicity on social media. Community events required scheduling resources; this required about 30 hours of medical staff assistant time. Seven sites were visited for about 3 hours each. A physician, pharmacy technician, and CPS conducted assessments, completed reminders, and entered follow-up requests. A medical support assistant registered veterans who had never signed up for VA health care.
Homeless veteran outreach. Five events occurred at 2 homeless veteran housing sites between August 2021 and January 2022. These sites were emergency housing sites (2 events) and transitional and permanent housing (2 events). U.S.VETS and HIHS contacted veterans living in those settings to promote the event. A physician, registered nurse, licensed practical nurse, and CPS conducted assessments, completed reminders, and entered follow-up requests. A medical support assistant registered veterans that had never signed up for VA health care. Each event lasted approximate 3 hours.
Process Quality Improvement
After the CDC changed recommendations to allow concurrent vaccination with the COVID-19 vaccine, we added other vaccinations to the events. This occurred during the course of community events. In June of 2021, there was a health advisory concerning hepatitis A among people experiencing homelessness in Oahu, so hepatitis vaccinations were added for events for veterans.20
Veterans Served
The EHR was used to determine demographics, open clinical reminders, and attendance at follow-up. Simple descriptive statistics were performed in Microsoft Excel. A total of 115 veterans were seen for preventive health visits, and 404 clinical reminders were completed. Seven hundred veterans attended the large centrally located vaccine event and 43 agreed to have a preventive health visit. Thirty-eight veterans had a preventive health visit at homeless outreach events and 34 veterans had a preventive health visit at the community events. Veterans at community
Of the 166 vaccines given, 73 were for COVID-19. Besides vaccination,
Veteran follow-up or completion
Discussion
This program provided evidence that adding preventive screenings to vaccine events may help reach veterans who may have missed important preventive care due to the COVID-19 pandemic. The involvement of clinical informatics service allowed the outreach to be targeted to communities with incomplete clinical reminders. Interventions that could not be completed at the event had high levels of follow-up by veterans with important findings. The presence of a physician or nurse and a CPS allowed for point-of-care testing, as well as entering orders for medication, laboratory tests, and consultations. The attendance by representatives from the Vet Center, suicide prevention, and homeless services allowed counseling regarding benefits, and mental health follow-up. We believe that we were able to reach communities of veterans with unmet preventive needs and had higher risk of severe COVID-19, given the high numbers with open clinical reminders, the number of vaccines provided, and the high percentage of racial and ethnic minority veterans at events in the community. Our program experience provides some evidence that mobile and pop-up vaccination clinics may be beneficial for screening and managing chronic diseases, as proposed elsewhere.21-24
Strengths of this intervention include that we were able to show a high level of follow-up for recommended medical care as well as the results of our interventions. We have found no similar articles that provide data on completion of follow-up appointments after a health fair. A prior study showed only 23% to 63% of participants at a health fair reported having a recommended follow-up discussion with doctors, but the study reported no outcome of completed cancer screenings.25
Limitations
Weaknesses include the fact that health fair events may reach only healthy people, since attendees generally report better health and better health behaviors than nonattendees.26,27 We felt this was more problematic for the large-scale urban event and that offering rural events and events in homeless housing improved the reach. Future efforts will involve the use of social media and mailings to solicit attendance. To improve follow-up, future work will include adding to the events: phlebotomy or expanded point-of-care testing; specialty care telehealth capability; cervical cancer screen self-collection; and tele-retinal services.
Conclusions
This program provided evidence that directed, preventive screening can be performed in outreach settings paired with vaccine events. These vaccination events in rural and homeless settings reached communities with demonstrable COVID-19 vaccination and other preventive care needs. This approach could be used to help veterans catch up on needed preventive care.
Acknowledgments
Veterans Affairs Pacific Islands Health Care System: Anthony Chance, LCSW; Nicholas Chang, PharmD; Andrew Dahlburg, LCSW; Wilminia G. Ellorimo-Gil, RN; Paul Guillory, RN; Wendy D. Joy; Arthur Minor, LCSW; Avalua Smith; Jessica Spurrier, RN. Veterans Health Administration Vet Center Program: Rolly O. Alvarado; Edmond G. DeGuzman; Richard T. Teel. Hawaii Institute for Human Services. U.S.VETS.
1. Califf RM. Avoiding the coming tsunami of common, chronic disease: What the lessons of the COVID-19 pandemic can teach us. Circulation. 2021;143(19):1831-1834. doi:10.1161/CIRCULATIONAHA.121.053461
2. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19-related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. doi:10.15585/mmwr.mm6936a4
3. European Society of Hypertension Corona-virus Disease 19 Task Force. The corona-virus disease 2019 pandemic compromised routine care for hypertension: a survey conducted among excellence centers of the European Society of Hypertension. J Hypertens. 2021;39(1):190-195. doi:10.1097/HJH.0000000000002703
4. Whaley CM, Pera MF, Cantor J, et al. Changes in health services use among commercially insured US populations during the COVID-19 pandemic. JAMA Netw Open. 2020;3(11):e2024984. doi:10.1001/jamanetworkopen.2020.24984
5. Song H, Bergman A, Chen AT, et al. Disruptions in preventive care: mammograms during the COVID-19 pandemic. Health Serv Res. 2021;56(1):95-101. doi:10.1111/1475-6773.13596
6. Shinkwin M, Silva L, Vogel I, et al. COVID-19 and the emergency presentation of colorectal cancer. Colorectal Dis. 2021;23(8):2014-2019. doi:10.1111/codi.15662
7. Rogers LC, Snyder RJ, Joseph WS. Diabetes-related amputations: a pandemic within a pandemic. J Am Podiatr Med Assoc. 2020;20-248. doi:10.7547/20-248
8. Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023-1034. doi:10.1016/S1470-2045(20)30388-0
9. World Health Organization. 14.9 million excess deaths associated with the COVID-19 pandemic in 2020 and 2021. May 5, 2022. Accessed August 31, 2022. https://www.who.int/news/item/05-05-2022-14.9-million-excess-deaths-were-associated-with-the-covid-19-pandemic-in-2020-and-2021
10. Padamsee TJ, Bond RM, Dixon GN, et al. Changes in COVID-19 vaccine hesitancy among Black and White individuals in the US. JAMA Netw Open. 2022;5(1):e2144470. doi:10.1001/jamanetworkopen.2021.44470
11. Barry V, Dasgupta S, Weller DL, et al. Patterns in COVID-19 vaccination coverage, by social vulnerability and urbanicity - United States, December 14, 2020-May 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(22):818-824. doi:10.15585/mmwr.mm7022e1
12. Baack BN, Abad N, Yankey D, et al. COVID-19 vaccination coverage and intent among adults aged 18-39 years - United States, March-May 2021. MMWR Morb Mortal Wkly Rep. 2021;70(25):928-933. doi:10.15585/mmwr.mm7025e2
13. United States Census Bureau. QuickFacts Hawaii. July 7, 2021. Accessed August 31, 2022. https://www.census.gov/quickfacts/HI
14. Hawaii Health Data Warehouse. Diabetes - Adult. November 23, 2021. Updated July 31, 2022. Accessed August 31, 2022. https://hhdw.org/report/indicator/summary/DXDiabetesAA.html
15. Hawaii Health Data Warehouse. High Blood Pressure, Adult. November 23, 2021. Accessed August 31, 2022. https://hhdw.org/report/indicator/summary/DXBPHighAA.html
16. Penaia CS, Morey BN, Thomas KB, et al. Disparities in Native Hawaiian and Pacific Islander COVID-19 mortality: a community-driven data response. Am J Public Health. 2021;111(S2):S49-S52. doi:10.2105/AJPH.2021.306370
17. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1500.02 Readjustment Counseling Services (RCS) Vet Center Program. January 26, 2021. Accessed September 7, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9168
18. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1162.08 Health Care for Veterans Homeless Outreach Services. February 18, 2022. Accessed September 7, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9673
19. US Department of Veterans Affairs. Clinical Reminders Version 2.0. Clinician Guide. October 2006. Accessed August 31, 2022. https://www.va.gov/vdl/documents/clinical/cprs-clinical_reminders/pxrm_2_4_um.pdf
20. Hawaii Department of Health. Hepatitis A Cases on Oahu and Maui. June 21, 2021. Accessed August 31, 2022. https://health.hawaii.gov/docd/files/2021/06/Medical-Advisory-HepA-June-21-2021.pdf
21. Hamel L, Lopes L, Sparks G, et al. KFF COVID-19 vaccine monitor: January 2022. January 28, 2022. Accessed August 31, 2022. https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-january-2022
22. Mast C, Munoz del Rio A. Delayed cancer screenings—a second look. Epic Research Network. July 17, 2020. Accessed August 31, 2022. https://epicresearch.org/articles/delayed-cancer-screenings-a-second-look
23. Shaukat A, Church T. Colorectal cancer screening in the USA in the wake of COVID-19. Lancet Gastroenterol Hepatol. 2020;5(8):726-727. doi:10.1016/S2468-1253(20)30191-6
24. Crespo J, Lazarus JV, Iruzubieta P, García F, García-Samaniego J; Alliance for the elimination of viral hepatitis in Spain. Let’s leverage SARS-CoV2 vaccination to screen for hepatitis C in Spain, in Europe, around the world. J Hepatol. 2021;75(1):224-226. doi:10.1016/j.jhep.2021.03.009
25. Escoffery C, Liang S, Rodgers K, et al. Process evaluation of health fairs promoting cancer screenings. BMC Cancer. 2017;17(1):865. doi:10.1186/s12885-017-3867-3
26. Waller PR, Crow C, Sands D, Becker H. Health related attitudes and health promoting behaviors: differences between health fair attenders and a community group. Am J Health Promot. 1988;3(1):17-32. doi:10.4278/0890-1171-3.1.17
27. Price JH, O’Connell J, Kukulka G. Preventive health behaviors related to the ten leading causes of mortality of health-fair attenders and nonattenders. Psychol Rep. 1985;56(1):131-135. doi:10.2466/pr0.1985.56.1.131
Shortly into the COVID-19 pandemic, Dr. Robert Califf, the commissioner of the US Food and Drug Administration, warned of a coming tsunami of chronic diseases, exacerbated by missed care during the pandemic.1 According to a Centers for Disease Control and Prevention (CDC) survey, more than 30% of adults reported delaying or avoiding routine medical care in the first 6 months of 2020. This rate was highest in people with comorbidities.2 Multiple studies demonstrated declines in hypertension care, hemoglobin A1c testing, mammography, and colon cancer screening.3-5 There has been a resultant increase in colon cancer complications, wounds, and amputations.6,7 The United Kingdom is expected to have a 7.9% to 16.6% increase in future deaths due to breast and colorectal cancer (CRC).8 The World Health Organization estimates an excess 14.9 million people died in 2020 and 2021, either directly from or indirectly related to COVID-19.9
Due to the large-scale conversion from face-to-face care to telehealth modalities, COVID-19 vaccination events offered a unique opportunity to perform preventive health care that requires in-person visits, since most US adults have sought vaccination. However, vaccine events may not reach people most at risk for COVID-19 or chronic disease. Groups of Americans with lower vaccination rates were concerned about driving times and missing work to get the vaccine.10
Distance and travel time may be a particular challenge in Hawaii. Oahu is considered rural by the US Department of Veterans Affairs (VA); some communities are 80 minutes away from the VA Pacific Islands Health Care System (VAPIHCS) main facility. Oahu has approximately 150 veterans experiencing homelessness who may not have transportation to vaccine events. Additionally, VAPIHCS serves veterans that may be at higher risk of not receiving COVID-19 vaccination. Racial and ethnic minority residents have lower vaccination rates, yet are at a higher risk of COVID-19 infection and complications, and through the pandemic, this vaccination gap worsened.11,12 More than 10% of the population of Hawaii is Native Hawaiian or Pacific Islander, and this population is at elevated risk for diabetes mellitus, hypertension, and COVID-19 mortality.13-16
Health Fair Program
The VA provides clinical reminders in its electronic health record (EHR) that are specified by age, gender assigned at birth, and comorbidities. The clinical reminder program is intended to provide clinically relevant reminders for preventive care at the point of care. Veterans with overdue clinical reminders can be identified by name and address, allowing for the creation of health fair events that were directed towards communities with veterans with clinical reminders, including COVID-19 vaccination need. A team of health care professionals from VAPIHCS conceived of a health fair program to increase the reach of vaccine events and include preventive care in partnership with the VAPIHCS Vet Center Program, local communities, U.S.VETS, and the Hawaii Institute of Health Services (HIHS). We sought to determine which services could be offered in community settings; large vaccine events; and at homeless emergency, transitional, or permanent housing. We tracked veterans who received care in the different locations of the directed health fair.
This project was determined to be a quality improvement initiative by the VAPIHCS Office of Research and Development. It was jointly planned by the VAPIHCS pharmacy, infectious diseases, Vet Center Program, and homeless team to make the COVID-19 vaccines available to more rural and to veterans experiencing homelessness, and in response to a decline in facility face-to-face visits. Monthly meetings were held to select sites within zip codes with higher numbers of open clinical reminders and lower vaccination uptake. Informatics developed a list of clinical reminders by zip code for care performed at face-to-face visits.
Partners
The Vet Center Program, suicide prevention coordinator, and the homeless outreach team have a mandate to perform outreach events.17,18 These services collaborate with community partners to locate sites for events. The team was able to leverage these contacts to set up sites for events. The Vet Center Program readjustment counselor and the suicide prevention coordinator provide mental health counseling. The Vet Center counsels on veteran benefits. They supplied a mobile van with WiFi, counseling and examination spaces, and refrigeration, which became the mobile clinic for the preventive care offered at events. The homeless program works with multiple community partners. They contract with HIHS and U.S.VETS to provide emergency and permanent housing for veterans. Each event is reviewed with HIHS and U.S.VETS staff for permission to be on site. The suicide prevention coordinator or the Vet Center readjustment counselor and the homeless team became regular attendees of events. The homeless team provided resources for housing or food insecurity.
Preventive Health Measures
The VA clinical reminder system supports caregivers for both preventive health care and chronic condition management.19 Clinical reminders appear as due in the EHR, and reminder reports can be run by clinical informatics to determine groups of patients who have not had a reminder completed. The following reminders were completed: vaccinations (including COVID-19), CRC screening, diabetic foot check and teaching of foot care, diabetic retinal consultations, laboratory studies (lipids, hemoglobin A1c, microalbumin), mammogram and pap smear referrals, mental health reminders, homeless and food insecurity screening, HIV and hepatitis C testing, and blood pressure (BP) measurement. Health records were reviewed 3 months after each event to determine whether they were completed by the veteran. Additionally, we determined whether BP was controlled (< 130/80 mm Hg).
Settings
Large urban event. The first setting for the health fair was a large vaccination event near the VAPIHCS center in April 2021. Attendance was solicited by VEText, phone calls, and social media advertisements. At check-in, veterans with relevant open clinical reminders were invited to receive preventive health care during the 15-minute monitoring period after the COVID-19 vaccine. The Vet Center Program stationed the mobile van outside the vaccination event, where a physician and a clinical pharmacy specialist (CPS) did assessments, completed reminders, and entered follow-up requests for about 4 hours. A medical support assistant registered veterans who had never signed up for VA health care.
Community Settings. Nine events occurred at least monthly between March and September 2021 at 4 different sites in Oahu. Texts and phone calls were used to solicit attendance; there was no prior publicity on social media. Community events required scheduling resources; this required about 30 hours of medical staff assistant time. Seven sites were visited for about 3 hours each. A physician, pharmacy technician, and CPS conducted assessments, completed reminders, and entered follow-up requests. A medical support assistant registered veterans who had never signed up for VA health care.
Homeless veteran outreach. Five events occurred at 2 homeless veteran housing sites between August 2021 and January 2022. These sites were emergency housing sites (2 events) and transitional and permanent housing (2 events). U.S.VETS and HIHS contacted veterans living in those settings to promote the event. A physician, registered nurse, licensed practical nurse, and CPS conducted assessments, completed reminders, and entered follow-up requests. A medical support assistant registered veterans that had never signed up for VA health care. Each event lasted approximate 3 hours.
Process Quality Improvement
After the CDC changed recommendations to allow concurrent vaccination with the COVID-19 vaccine, we added other vaccinations to the events. This occurred during the course of community events. In June of 2021, there was a health advisory concerning hepatitis A among people experiencing homelessness in Oahu, so hepatitis vaccinations were added for events for veterans.20
Veterans Served
The EHR was used to determine demographics, open clinical reminders, and attendance at follow-up. Simple descriptive statistics were performed in Microsoft Excel. A total of 115 veterans were seen for preventive health visits, and 404 clinical reminders were completed. Seven hundred veterans attended the large centrally located vaccine event and 43 agreed to have a preventive health visit. Thirty-eight veterans had a preventive health visit at homeless outreach events and 34 veterans had a preventive health visit at the community events. Veterans at community
Of the 166 vaccines given, 73 were for COVID-19. Besides vaccination,
Veteran follow-up or completion
Discussion
This program provided evidence that adding preventive screenings to vaccine events may help reach veterans who may have missed important preventive care due to the COVID-19 pandemic. The involvement of clinical informatics service allowed the outreach to be targeted to communities with incomplete clinical reminders. Interventions that could not be completed at the event had high levels of follow-up by veterans with important findings. The presence of a physician or nurse and a CPS allowed for point-of-care testing, as well as entering orders for medication, laboratory tests, and consultations. The attendance by representatives from the Vet Center, suicide prevention, and homeless services allowed counseling regarding benefits, and mental health follow-up. We believe that we were able to reach communities of veterans with unmet preventive needs and had higher risk of severe COVID-19, given the high numbers with open clinical reminders, the number of vaccines provided, and the high percentage of racial and ethnic minority veterans at events in the community. Our program experience provides some evidence that mobile and pop-up vaccination clinics may be beneficial for screening and managing chronic diseases, as proposed elsewhere.21-24
Strengths of this intervention include that we were able to show a high level of follow-up for recommended medical care as well as the results of our interventions. We have found no similar articles that provide data on completion of follow-up appointments after a health fair. A prior study showed only 23% to 63% of participants at a health fair reported having a recommended follow-up discussion with doctors, but the study reported no outcome of completed cancer screenings.25
Limitations
Weaknesses include the fact that health fair events may reach only healthy people, since attendees generally report better health and better health behaviors than nonattendees.26,27 We felt this was more problematic for the large-scale urban event and that offering rural events and events in homeless housing improved the reach. Future efforts will involve the use of social media and mailings to solicit attendance. To improve follow-up, future work will include adding to the events: phlebotomy or expanded point-of-care testing; specialty care telehealth capability; cervical cancer screen self-collection; and tele-retinal services.
Conclusions
This program provided evidence that directed, preventive screening can be performed in outreach settings paired with vaccine events. These vaccination events in rural and homeless settings reached communities with demonstrable COVID-19 vaccination and other preventive care needs. This approach could be used to help veterans catch up on needed preventive care.
Acknowledgments
Veterans Affairs Pacific Islands Health Care System: Anthony Chance, LCSW; Nicholas Chang, PharmD; Andrew Dahlburg, LCSW; Wilminia G. Ellorimo-Gil, RN; Paul Guillory, RN; Wendy D. Joy; Arthur Minor, LCSW; Avalua Smith; Jessica Spurrier, RN. Veterans Health Administration Vet Center Program: Rolly O. Alvarado; Edmond G. DeGuzman; Richard T. Teel. Hawaii Institute for Human Services. U.S.VETS.
Shortly into the COVID-19 pandemic, Dr. Robert Califf, the commissioner of the US Food and Drug Administration, warned of a coming tsunami of chronic diseases, exacerbated by missed care during the pandemic.1 According to a Centers for Disease Control and Prevention (CDC) survey, more than 30% of adults reported delaying or avoiding routine medical care in the first 6 months of 2020. This rate was highest in people with comorbidities.2 Multiple studies demonstrated declines in hypertension care, hemoglobin A1c testing, mammography, and colon cancer screening.3-5 There has been a resultant increase in colon cancer complications, wounds, and amputations.6,7 The United Kingdom is expected to have a 7.9% to 16.6% increase in future deaths due to breast and colorectal cancer (CRC).8 The World Health Organization estimates an excess 14.9 million people died in 2020 and 2021, either directly from or indirectly related to COVID-19.9
Due to the large-scale conversion from face-to-face care to telehealth modalities, COVID-19 vaccination events offered a unique opportunity to perform preventive health care that requires in-person visits, since most US adults have sought vaccination. However, vaccine events may not reach people most at risk for COVID-19 or chronic disease. Groups of Americans with lower vaccination rates were concerned about driving times and missing work to get the vaccine.10
Distance and travel time may be a particular challenge in Hawaii. Oahu is considered rural by the US Department of Veterans Affairs (VA); some communities are 80 minutes away from the VA Pacific Islands Health Care System (VAPIHCS) main facility. Oahu has approximately 150 veterans experiencing homelessness who may not have transportation to vaccine events. Additionally, VAPIHCS serves veterans that may be at higher risk of not receiving COVID-19 vaccination. Racial and ethnic minority residents have lower vaccination rates, yet are at a higher risk of COVID-19 infection and complications, and through the pandemic, this vaccination gap worsened.11,12 More than 10% of the population of Hawaii is Native Hawaiian or Pacific Islander, and this population is at elevated risk for diabetes mellitus, hypertension, and COVID-19 mortality.13-16
Health Fair Program
The VA provides clinical reminders in its electronic health record (EHR) that are specified by age, gender assigned at birth, and comorbidities. The clinical reminder program is intended to provide clinically relevant reminders for preventive care at the point of care. Veterans with overdue clinical reminders can be identified by name and address, allowing for the creation of health fair events that were directed towards communities with veterans with clinical reminders, including COVID-19 vaccination need. A team of health care professionals from VAPIHCS conceived of a health fair program to increase the reach of vaccine events and include preventive care in partnership with the VAPIHCS Vet Center Program, local communities, U.S.VETS, and the Hawaii Institute of Health Services (HIHS). We sought to determine which services could be offered in community settings; large vaccine events; and at homeless emergency, transitional, or permanent housing. We tracked veterans who received care in the different locations of the directed health fair.
This project was determined to be a quality improvement initiative by the VAPIHCS Office of Research and Development. It was jointly planned by the VAPIHCS pharmacy, infectious diseases, Vet Center Program, and homeless team to make the COVID-19 vaccines available to more rural and to veterans experiencing homelessness, and in response to a decline in facility face-to-face visits. Monthly meetings were held to select sites within zip codes with higher numbers of open clinical reminders and lower vaccination uptake. Informatics developed a list of clinical reminders by zip code for care performed at face-to-face visits.
Partners
The Vet Center Program, suicide prevention coordinator, and the homeless outreach team have a mandate to perform outreach events.17,18 These services collaborate with community partners to locate sites for events. The team was able to leverage these contacts to set up sites for events. The Vet Center Program readjustment counselor and the suicide prevention coordinator provide mental health counseling. The Vet Center counsels on veteran benefits. They supplied a mobile van with WiFi, counseling and examination spaces, and refrigeration, which became the mobile clinic for the preventive care offered at events. The homeless program works with multiple community partners. They contract with HIHS and U.S.VETS to provide emergency and permanent housing for veterans. Each event is reviewed with HIHS and U.S.VETS staff for permission to be on site. The suicide prevention coordinator or the Vet Center readjustment counselor and the homeless team became regular attendees of events. The homeless team provided resources for housing or food insecurity.
Preventive Health Measures
The VA clinical reminder system supports caregivers for both preventive health care and chronic condition management.19 Clinical reminders appear as due in the EHR, and reminder reports can be run by clinical informatics to determine groups of patients who have not had a reminder completed. The following reminders were completed: vaccinations (including COVID-19), CRC screening, diabetic foot check and teaching of foot care, diabetic retinal consultations, laboratory studies (lipids, hemoglobin A1c, microalbumin), mammogram and pap smear referrals, mental health reminders, homeless and food insecurity screening, HIV and hepatitis C testing, and blood pressure (BP) measurement. Health records were reviewed 3 months after each event to determine whether they were completed by the veteran. Additionally, we determined whether BP was controlled (< 130/80 mm Hg).
Settings
Large urban event. The first setting for the health fair was a large vaccination event near the VAPIHCS center in April 2021. Attendance was solicited by VEText, phone calls, and social media advertisements. At check-in, veterans with relevant open clinical reminders were invited to receive preventive health care during the 15-minute monitoring period after the COVID-19 vaccine. The Vet Center Program stationed the mobile van outside the vaccination event, where a physician and a clinical pharmacy specialist (CPS) did assessments, completed reminders, and entered follow-up requests for about 4 hours. A medical support assistant registered veterans who had never signed up for VA health care.
Community Settings. Nine events occurred at least monthly between March and September 2021 at 4 different sites in Oahu. Texts and phone calls were used to solicit attendance; there was no prior publicity on social media. Community events required scheduling resources; this required about 30 hours of medical staff assistant time. Seven sites were visited for about 3 hours each. A physician, pharmacy technician, and CPS conducted assessments, completed reminders, and entered follow-up requests. A medical support assistant registered veterans who had never signed up for VA health care.
Homeless veteran outreach. Five events occurred at 2 homeless veteran housing sites between August 2021 and January 2022. These sites were emergency housing sites (2 events) and transitional and permanent housing (2 events). U.S.VETS and HIHS contacted veterans living in those settings to promote the event. A physician, registered nurse, licensed practical nurse, and CPS conducted assessments, completed reminders, and entered follow-up requests. A medical support assistant registered veterans that had never signed up for VA health care. Each event lasted approximate 3 hours.
Process Quality Improvement
After the CDC changed recommendations to allow concurrent vaccination with the COVID-19 vaccine, we added other vaccinations to the events. This occurred during the course of community events. In June of 2021, there was a health advisory concerning hepatitis A among people experiencing homelessness in Oahu, so hepatitis vaccinations were added for events for veterans.20
Veterans Served
The EHR was used to determine demographics, open clinical reminders, and attendance at follow-up. Simple descriptive statistics were performed in Microsoft Excel. A total of 115 veterans were seen for preventive health visits, and 404 clinical reminders were completed. Seven hundred veterans attended the large centrally located vaccine event and 43 agreed to have a preventive health visit. Thirty-eight veterans had a preventive health visit at homeless outreach events and 34 veterans had a preventive health visit at the community events. Veterans at community
Of the 166 vaccines given, 73 were for COVID-19. Besides vaccination,
Veteran follow-up or completion
Discussion
This program provided evidence that adding preventive screenings to vaccine events may help reach veterans who may have missed important preventive care due to the COVID-19 pandemic. The involvement of clinical informatics service allowed the outreach to be targeted to communities with incomplete clinical reminders. Interventions that could not be completed at the event had high levels of follow-up by veterans with important findings. The presence of a physician or nurse and a CPS allowed for point-of-care testing, as well as entering orders for medication, laboratory tests, and consultations. The attendance by representatives from the Vet Center, suicide prevention, and homeless services allowed counseling regarding benefits, and mental health follow-up. We believe that we were able to reach communities of veterans with unmet preventive needs and had higher risk of severe COVID-19, given the high numbers with open clinical reminders, the number of vaccines provided, and the high percentage of racial and ethnic minority veterans at events in the community. Our program experience provides some evidence that mobile and pop-up vaccination clinics may be beneficial for screening and managing chronic diseases, as proposed elsewhere.21-24
Strengths of this intervention include that we were able to show a high level of follow-up for recommended medical care as well as the results of our interventions. We have found no similar articles that provide data on completion of follow-up appointments after a health fair. A prior study showed only 23% to 63% of participants at a health fair reported having a recommended follow-up discussion with doctors, but the study reported no outcome of completed cancer screenings.25
Limitations
Weaknesses include the fact that health fair events may reach only healthy people, since attendees generally report better health and better health behaviors than nonattendees.26,27 We felt this was more problematic for the large-scale urban event and that offering rural events and events in homeless housing improved the reach. Future efforts will involve the use of social media and mailings to solicit attendance. To improve follow-up, future work will include adding to the events: phlebotomy or expanded point-of-care testing; specialty care telehealth capability; cervical cancer screen self-collection; and tele-retinal services.
Conclusions
This program provided evidence that directed, preventive screening can be performed in outreach settings paired with vaccine events. These vaccination events in rural and homeless settings reached communities with demonstrable COVID-19 vaccination and other preventive care needs. This approach could be used to help veterans catch up on needed preventive care.
Acknowledgments
Veterans Affairs Pacific Islands Health Care System: Anthony Chance, LCSW; Nicholas Chang, PharmD; Andrew Dahlburg, LCSW; Wilminia G. Ellorimo-Gil, RN; Paul Guillory, RN; Wendy D. Joy; Arthur Minor, LCSW; Avalua Smith; Jessica Spurrier, RN. Veterans Health Administration Vet Center Program: Rolly O. Alvarado; Edmond G. DeGuzman; Richard T. Teel. Hawaii Institute for Human Services. U.S.VETS.
1. Califf RM. Avoiding the coming tsunami of common, chronic disease: What the lessons of the COVID-19 pandemic can teach us. Circulation. 2021;143(19):1831-1834. doi:10.1161/CIRCULATIONAHA.121.053461
2. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19-related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. doi:10.15585/mmwr.mm6936a4
3. European Society of Hypertension Corona-virus Disease 19 Task Force. The corona-virus disease 2019 pandemic compromised routine care for hypertension: a survey conducted among excellence centers of the European Society of Hypertension. J Hypertens. 2021;39(1):190-195. doi:10.1097/HJH.0000000000002703
4. Whaley CM, Pera MF, Cantor J, et al. Changes in health services use among commercially insured US populations during the COVID-19 pandemic. JAMA Netw Open. 2020;3(11):e2024984. doi:10.1001/jamanetworkopen.2020.24984
5. Song H, Bergman A, Chen AT, et al. Disruptions in preventive care: mammograms during the COVID-19 pandemic. Health Serv Res. 2021;56(1):95-101. doi:10.1111/1475-6773.13596
6. Shinkwin M, Silva L, Vogel I, et al. COVID-19 and the emergency presentation of colorectal cancer. Colorectal Dis. 2021;23(8):2014-2019. doi:10.1111/codi.15662
7. Rogers LC, Snyder RJ, Joseph WS. Diabetes-related amputations: a pandemic within a pandemic. J Am Podiatr Med Assoc. 2020;20-248. doi:10.7547/20-248
8. Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023-1034. doi:10.1016/S1470-2045(20)30388-0
9. World Health Organization. 14.9 million excess deaths associated with the COVID-19 pandemic in 2020 and 2021. May 5, 2022. Accessed August 31, 2022. https://www.who.int/news/item/05-05-2022-14.9-million-excess-deaths-were-associated-with-the-covid-19-pandemic-in-2020-and-2021
10. Padamsee TJ, Bond RM, Dixon GN, et al. Changes in COVID-19 vaccine hesitancy among Black and White individuals in the US. JAMA Netw Open. 2022;5(1):e2144470. doi:10.1001/jamanetworkopen.2021.44470
11. Barry V, Dasgupta S, Weller DL, et al. Patterns in COVID-19 vaccination coverage, by social vulnerability and urbanicity - United States, December 14, 2020-May 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(22):818-824. doi:10.15585/mmwr.mm7022e1
12. Baack BN, Abad N, Yankey D, et al. COVID-19 vaccination coverage and intent among adults aged 18-39 years - United States, March-May 2021. MMWR Morb Mortal Wkly Rep. 2021;70(25):928-933. doi:10.15585/mmwr.mm7025e2
13. United States Census Bureau. QuickFacts Hawaii. July 7, 2021. Accessed August 31, 2022. https://www.census.gov/quickfacts/HI
14. Hawaii Health Data Warehouse. Diabetes - Adult. November 23, 2021. Updated July 31, 2022. Accessed August 31, 2022. https://hhdw.org/report/indicator/summary/DXDiabetesAA.html
15. Hawaii Health Data Warehouse. High Blood Pressure, Adult. November 23, 2021. Accessed August 31, 2022. https://hhdw.org/report/indicator/summary/DXBPHighAA.html
16. Penaia CS, Morey BN, Thomas KB, et al. Disparities in Native Hawaiian and Pacific Islander COVID-19 mortality: a community-driven data response. Am J Public Health. 2021;111(S2):S49-S52. doi:10.2105/AJPH.2021.306370
17. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1500.02 Readjustment Counseling Services (RCS) Vet Center Program. January 26, 2021. Accessed September 7, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9168
18. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1162.08 Health Care for Veterans Homeless Outreach Services. February 18, 2022. Accessed September 7, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9673
19. US Department of Veterans Affairs. Clinical Reminders Version 2.0. Clinician Guide. October 2006. Accessed August 31, 2022. https://www.va.gov/vdl/documents/clinical/cprs-clinical_reminders/pxrm_2_4_um.pdf
20. Hawaii Department of Health. Hepatitis A Cases on Oahu and Maui. June 21, 2021. Accessed August 31, 2022. https://health.hawaii.gov/docd/files/2021/06/Medical-Advisory-HepA-June-21-2021.pdf
21. Hamel L, Lopes L, Sparks G, et al. KFF COVID-19 vaccine monitor: January 2022. January 28, 2022. Accessed August 31, 2022. https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-january-2022
22. Mast C, Munoz del Rio A. Delayed cancer screenings—a second look. Epic Research Network. July 17, 2020. Accessed August 31, 2022. https://epicresearch.org/articles/delayed-cancer-screenings-a-second-look
23. Shaukat A, Church T. Colorectal cancer screening in the USA in the wake of COVID-19. Lancet Gastroenterol Hepatol. 2020;5(8):726-727. doi:10.1016/S2468-1253(20)30191-6
24. Crespo J, Lazarus JV, Iruzubieta P, García F, García-Samaniego J; Alliance for the elimination of viral hepatitis in Spain. Let’s leverage SARS-CoV2 vaccination to screen for hepatitis C in Spain, in Europe, around the world. J Hepatol. 2021;75(1):224-226. doi:10.1016/j.jhep.2021.03.009
25. Escoffery C, Liang S, Rodgers K, et al. Process evaluation of health fairs promoting cancer screenings. BMC Cancer. 2017;17(1):865. doi:10.1186/s12885-017-3867-3
26. Waller PR, Crow C, Sands D, Becker H. Health related attitudes and health promoting behaviors: differences between health fair attenders and a community group. Am J Health Promot. 1988;3(1):17-32. doi:10.4278/0890-1171-3.1.17
27. Price JH, O’Connell J, Kukulka G. Preventive health behaviors related to the ten leading causes of mortality of health-fair attenders and nonattenders. Psychol Rep. 1985;56(1):131-135. doi:10.2466/pr0.1985.56.1.131
1. Califf RM. Avoiding the coming tsunami of common, chronic disease: What the lessons of the COVID-19 pandemic can teach us. Circulation. 2021;143(19):1831-1834. doi:10.1161/CIRCULATIONAHA.121.053461
2. Czeisler MÉ, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19-related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257. doi:10.15585/mmwr.mm6936a4
3. European Society of Hypertension Corona-virus Disease 19 Task Force. The corona-virus disease 2019 pandemic compromised routine care for hypertension: a survey conducted among excellence centers of the European Society of Hypertension. J Hypertens. 2021;39(1):190-195. doi:10.1097/HJH.0000000000002703
4. Whaley CM, Pera MF, Cantor J, et al. Changes in health services use among commercially insured US populations during the COVID-19 pandemic. JAMA Netw Open. 2020;3(11):e2024984. doi:10.1001/jamanetworkopen.2020.24984
5. Song H, Bergman A, Chen AT, et al. Disruptions in preventive care: mammograms during the COVID-19 pandemic. Health Serv Res. 2021;56(1):95-101. doi:10.1111/1475-6773.13596
6. Shinkwin M, Silva L, Vogel I, et al. COVID-19 and the emergency presentation of colorectal cancer. Colorectal Dis. 2021;23(8):2014-2019. doi:10.1111/codi.15662
7. Rogers LC, Snyder RJ, Joseph WS. Diabetes-related amputations: a pandemic within a pandemic. J Am Podiatr Med Assoc. 2020;20-248. doi:10.7547/20-248
8. Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023-1034. doi:10.1016/S1470-2045(20)30388-0
9. World Health Organization. 14.9 million excess deaths associated with the COVID-19 pandemic in 2020 and 2021. May 5, 2022. Accessed August 31, 2022. https://www.who.int/news/item/05-05-2022-14.9-million-excess-deaths-were-associated-with-the-covid-19-pandemic-in-2020-and-2021
10. Padamsee TJ, Bond RM, Dixon GN, et al. Changes in COVID-19 vaccine hesitancy among Black and White individuals in the US. JAMA Netw Open. 2022;5(1):e2144470. doi:10.1001/jamanetworkopen.2021.44470
11. Barry V, Dasgupta S, Weller DL, et al. Patterns in COVID-19 vaccination coverage, by social vulnerability and urbanicity - United States, December 14, 2020-May 1, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(22):818-824. doi:10.15585/mmwr.mm7022e1
12. Baack BN, Abad N, Yankey D, et al. COVID-19 vaccination coverage and intent among adults aged 18-39 years - United States, March-May 2021. MMWR Morb Mortal Wkly Rep. 2021;70(25):928-933. doi:10.15585/mmwr.mm7025e2
13. United States Census Bureau. QuickFacts Hawaii. July 7, 2021. Accessed August 31, 2022. https://www.census.gov/quickfacts/HI
14. Hawaii Health Data Warehouse. Diabetes - Adult. November 23, 2021. Updated July 31, 2022. Accessed August 31, 2022. https://hhdw.org/report/indicator/summary/DXDiabetesAA.html
15. Hawaii Health Data Warehouse. High Blood Pressure, Adult. November 23, 2021. Accessed August 31, 2022. https://hhdw.org/report/indicator/summary/DXBPHighAA.html
16. Penaia CS, Morey BN, Thomas KB, et al. Disparities in Native Hawaiian and Pacific Islander COVID-19 mortality: a community-driven data response. Am J Public Health. 2021;111(S2):S49-S52. doi:10.2105/AJPH.2021.306370
17. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1500.02 Readjustment Counseling Services (RCS) Vet Center Program. January 26, 2021. Accessed September 7, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9168
18. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1162.08 Health Care for Veterans Homeless Outreach Services. February 18, 2022. Accessed September 7, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=9673
19. US Department of Veterans Affairs. Clinical Reminders Version 2.0. Clinician Guide. October 2006. Accessed August 31, 2022. https://www.va.gov/vdl/documents/clinical/cprs-clinical_reminders/pxrm_2_4_um.pdf
20. Hawaii Department of Health. Hepatitis A Cases on Oahu and Maui. June 21, 2021. Accessed August 31, 2022. https://health.hawaii.gov/docd/files/2021/06/Medical-Advisory-HepA-June-21-2021.pdf
21. Hamel L, Lopes L, Sparks G, et al. KFF COVID-19 vaccine monitor: January 2022. January 28, 2022. Accessed August 31, 2022. https://www.kff.org/coronavirus-covid-19/poll-finding/kff-covid-19-vaccine-monitor-january-2022
22. Mast C, Munoz del Rio A. Delayed cancer screenings—a second look. Epic Research Network. July 17, 2020. Accessed August 31, 2022. https://epicresearch.org/articles/delayed-cancer-screenings-a-second-look
23. Shaukat A, Church T. Colorectal cancer screening in the USA in the wake of COVID-19. Lancet Gastroenterol Hepatol. 2020;5(8):726-727. doi:10.1016/S2468-1253(20)30191-6
24. Crespo J, Lazarus JV, Iruzubieta P, García F, García-Samaniego J; Alliance for the elimination of viral hepatitis in Spain. Let’s leverage SARS-CoV2 vaccination to screen for hepatitis C in Spain, in Europe, around the world. J Hepatol. 2021;75(1):224-226. doi:10.1016/j.jhep.2021.03.009
25. Escoffery C, Liang S, Rodgers K, et al. Process evaluation of health fairs promoting cancer screenings. BMC Cancer. 2017;17(1):865. doi:10.1186/s12885-017-3867-3
26. Waller PR, Crow C, Sands D, Becker H. Health related attitudes and health promoting behaviors: differences between health fair attenders and a community group. Am J Health Promot. 1988;3(1):17-32. doi:10.4278/0890-1171-3.1.17
27. Price JH, O’Connell J, Kukulka G. Preventive health behaviors related to the ten leading causes of mortality of health-fair attenders and nonattenders. Psychol Rep. 1985;56(1):131-135. doi:10.2466/pr0.1985.56.1.131
Medications for Opioid Use Disorder Program in a VA Emergency Department
Opioid use disorder (OUD) is a public health crisis significantly affecting veterans. A substantial increase in veterans diagnosed with OUD has occurred, nearly tripling from 25,031 in 2003 to 69,142 in 2017
For patients with active OUD, medications for opioid use disorder (MOUD) reduce the risk of overdose and all-cause mortality.3 In 2009, the US Department of Veterans Affairs (VA) and Department of Defense (DoD) published clinical practice guidelines for substance use disorders that strongly recommended MOUD with either buprenorphine or methadone as a first-line treatment. In 2015 updated guidelines encouraged buprenorphine initiation in primary care settings.4,5 This was followed by an academic detailing campaign designed to encourage VA clinicians to initiate MOUD.1 Despite this institutional support, MOUD remains underutilized within the VA, with widely variable rates of prescribing among VA sites.1
Efforts to further expand MOUD cultivated interest in administering buprenorphine in VA emergency departments (EDs). Patients with OUD often use the ED for same-day care, providing opportunities to initiate buprenorphine in the ED 24 hours, 7 days per week. This has been especially true during the COVID-19 pandemic during which reliable access to usual recovery services has been disrupted and EDs have served as a safety net.6
Buprenorphine’s safety profile and prolonged effect duration make it superior to other MOUD options for ED administration. As a partial opioid agonist, buprenorphine is unlikely to cause significant sedation or respiratory depression compared with full agonists like methadone. This is known as the ceiling effect. Additionally, at higher doses, buprenorphine’s effects can last for about 3 days, potentially obviating the need for repeat dosing. D’Onofrio and colleagues seminal 2015 paper conceptually proved the feasibility and value of initiating buprenorphine in the ED; patients who received ED initiation therapy were more likely to be engaged in addiction treatment 30 days after their visit and have reduced rates of illicit opioid drug use.7 Such ED harm-reduction strategies are increasingly recognized as essential, given that 1 in 20 patients treated for a nonfatal opioid overdose in an ED will die within 1 year of their visit, many within 2 days.8 Finally, a significant barrier faced by physicians wanting to administer or prescribe buprenorphine for patients with OUD has been the special licensing required by the Drug Enforcement Administration Drug Addiction Treatment Act of 2000, also known as an X-waiver. A notable exception to this X-waiver requirement is the 72-hour rule, which allows nonwaivered practitioners to administer (but not prescribe for home use) buprenorphine to a patient to relieve acute withdrawal symptoms for up to 72 hours while arranging for specialist referral.Under the 72-hour rule, ED clinicians have a unique opportunity to treat patients experiencing acute withdrawal symptoms and bridge them to specialty care, without the burden of an X-waiver requirement.
The VA Greater Los Angeles Healthcare System (VAGLAHS), therefore, developed and implemented a program to administer buprenorphine in the ED to bridge patients with OUD to an appointment with substance use disorder (SUD) services. We describe our development, implementation and evaluation of this program protocol as a model for other VA EDs. This project was determined to be quality improvement (nonresearch) by the VAGLAHS Institutional Review Board.
ED MOUD Program
We engaged in a 2-month (January-March 2019) preimplementation process during which we (1) obtained stakeholder buy-in; (2) developed a protocol and supporting resources and tools; (3) worked with stakeholders to enact local organizational policy and process modifications; and (4) educated practitioners.
Appendix 1 provides an overview of MOUD terminology, pharmacology, and regulations. We developed an 8-step program implementation plan for the ED MOUD program (Figure 1).
Obtaining Stakeholder Buy-in
Two ED physician champions (MC, JH) organized all activities. Champions obtained stakeholder buy-in from clinical and administrative leaders as well as from frontline personnel in OUD specialty care, ED, and pharmacy services. ED social workers and clerks who schedule post-ED appointments also were engaged. These stakeholders emphasized the importance of fitting the developed protocol into the existing ED workflows as well as minimizing additional resources required to initiate and maintain the program.
We ascertained that in fiscal year 2018, VAGLAHS had 156 ED visits with International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes related to OUD for 108 unique patients. Based on these data and in consultation with OUD specialty care, we determined that the potential number of referrals to the SUD clinic would be manageable with existing resources. Additionally, there was consensus that most opioid withdrawal patients could be treated in the urgent care portion of our ED since these patients generally do not require special monitoring. This consideration was important for obtaining ED stakeholder buy-in and for planning protocol logistics.
Developing the Protocol
We customized resources created by CalBridge Behavioral Health Navigator Program (CA Bridge), formerly called ED Bridge, a program of the Public Health Institute in Oakland, California, funded through California Department of Health Care Services. CA Bridge offers technical assistance and support for hospitals as well as guidance and tools for establishing processes for EDs providing buprenorphine prescriptions for the management of acute opioid withdrawal and serving as a bridge to follow-up care in SUD clinics.9 We also reviewed protocols described by D’Onofrio and colleagues. With iterative input from stakeholders, we created a protocol concretely delineating each process and corresponding responsible party with the overall aim of removing potential barriers to MOUD initiation and follow-up (Appendix 2).
Identifying Appropriate Follow-up
To operationalize protocol implementation, we built on VA’s Emergency Department Rapid Access Clinic (ED-RAC) process, a mechanism for scheduling appointments for post-ED specialty follow-up care. This process facilitated veterans’ access to urgent specialty care follow-up after ED visits by scheduling appointments prior to ED discharge.10 For the ED MOUD program, we adapted the ED-RAC process to schedule appointments in SUD clinic prior to ED discharge. These appointments allowed patients to be seen by an SUD clinician within 72 hours of ED discharge. This step was critical to working within the 72-hour rule without relying on X-waiver licensing of ED clinicians. Alternatively, as was previous practice, per patient preference, patients were also referred to non-VA residential rehabilitation services if the facility had capacity and patients met criteria for admission.
Identification of Eligible Veterans
Target patients were those primarily presenting with a request for treatment of opioid dependence or withdrawal. Patients were not actively screened for OUD. Clinicians diagnosed and assessed for OUD as per their usual practice. Patients with OUD who presented to the ED for other reasons were assessed, at clinician discretion, for their interest in receiving MOUD. If patients presented in moderate-to-severe withdrawal (eg, Clinical Opiate Withdrawal Scale [COWS] ≥ 8), buprenorphine was initiated in the ED. These patients were subsequently referred to either the local SUD clinic or to a residential treatment center. Patients presenting with a COWS score < 8 were referred to the outpatient SUD clinic or residential treatment centers without initiating buprenorphine from the ED. The SUD clinic or residential treatment centers could offer buprenorphine or other MOUD options. From the ED, prescribing buprenorphine for patients to self-initiate at home was not available as this required an X-waivered prescriber, which were limited during the program time frame.
Support Tools and Resources
To facilitate ED clinicians using the protocol, we worked with a programmer experienced with the Computerized Patient Record System, the VA electronic health record (EHR), to create electronic order menu sets that directed clinicians to the protocol and educational materials (Appendix 3). These menus are readily accessible and embedded into the ED clinician workflow. The menus highlight key elements of the protocol, including indications for initiation, contraindications, recommended dosing with quick orders, and how to obtain follow-up for the patient. Links also are provided to the protocol and patient discharge handouts, including the CA Bridge website.
Organizational Policy and Processes
Before implementing the developed protocol, we worked with stakeholders to modify organizational policies and processes. Our pharmacy agreed to stock buprenorphine in the ED to make it readily available. EHR restrictions that historically prohibited ordering buprenorphine for ED administration by nonwaivered clinicians were modified. Additionally, our chief of staff, pharmacy, and credentialing department agreed that physicians did not need to apply for additional delineated privileges.
Clinician Education
The final preparation step was educating clinicians and other protocol users. The VAGLAHS SUD chief presented a lecture and answered questions about MOUD to core ED faculty about the rising prevalence of OUD and use of buprenorphine as a recommended treatment.
Evaluation
To assess adherence to the developed protocol, we conducted a retrospective health record review of all ED visits March 1 to October 25, 2019, in which the patient had OUD and may have qualified for MOUD. To do this, we identified (1) ED visits with an OUD ICD-10 code as a primary or secondary diagnoses; (2) ED referrals to outpatient SUD treatment; and/or (3) ED visits in which buprenorphine was given or prescribed. We included the latter 2 criteria as application of ICD-10 codes for OUD care was inconsistent. Visits were excluded if patients did not have OUD, had OUD in remission, were already maintained on a stable MOUD regimen and no longer using illicit drugs or craving additional opioids, or were presenting solely for a refill or administration of a missed dose. Patients who relapsed were categorized as unstable. Visits were excluded if the patient was admitted to the hospital or left against medical advice. Patients on MOUD who had relapsed or requested a change in MOUD treatment were included. For all included visits, 2 ED physicians (MC, JH) reviewed the ED clinician and nursing notes, pharmacy and referral records, diagnostic codes, and veteran demographics.
In the evaluation, there were 130 visits with 92 unique veterans meeting inclusion criteria. The final sample included 70 visits with 47 unique veterans (Table 1). Of note, 24 (53%) patients self-identified as homeless or were engaged with VA housing services. Twelve veterans had multiple ED visits (7 patients with 2 visits; 5 patients with ≥ 3 visits). In 30 (43%) visits the veteran’s primary reason for seeking ED care was to obtain treatment for opioid withdrawal or receive MOUD. Type of opiate used was specified in 58% of visits; of these, 69% indicated heroin use and 17% prescription medications. Buprenorphine was initiated in the ED in 18 (26%) visits for 10 veterans. Appendix 4 outlines the clinical course and follow-up after these visits. Some veterans returned to the ED for buprenorphine redosing per the 72-hour rule. SUD clinic appointments were provided in 11 visits, and direct transfer to an inpatient rehabilitation center was arranged in 4 visits. In 42 (60%) visits, across 32 unique veterans, buprenorphine was not given in the ED, but patients were referred for SUD treat
A majority of veterans who received buprenorphine and a referral for an SUD appointment went to their initial SUD follow-up appointment and had ongoing engagement in addiction care 30 days after their index ED visit. Among veterans who did not receive buprenorphine but were referred for SUD treatment, about half went to their SUD appointments and about 1 in 5 had ongoing engagement in addiction care at 30 days after the index ED visit. Of note, 2 patients who received referrals died within 1 year of their index ED visit. The cause of death for one patient was an overdose; the other was unspecified.
DISCUSSION
We developed the ED MOUD program as a bridge to SUD specialty care. Our 8 implementation steps can serve as a model for implementing programs at other VA EDs. We demonstrated feasibility, high follow-up rates, and high retention in treatment.
Patients who received ED buprenorphine initiation were more likely to follow up and had higher rates of ongoing engagement at 30 days than did those who received only a clinic referral. In a similar Canadian study, buprenorphine was initiated in the ED, and patients followed up as a walk-in for addiction services; however, only 54% of patients presented to this initial follow-up.11 Our higher initial follow-up rate may be due to our ability to directly schedule clinic appointments. Our 70% 30-day follow-up rate is comparable, but slightly lower than the 2015 D’Onofrio and colleagues study in which 78% of patients remained engaged at 30 days.7 A possible reason is that in the D’Onofrio and colleagues study, all study physicians obtained X-waiver training and were able to prescribe buprenorphine after ED initiation or for self-initiation at home. X-waiver training was not required of our clinicians, and none of our patients were offered a prescription for self-initiation.
Our program demonstrates that it is feasible to develop a protocol without X-waiver licensing. This program provides a supportive framework for the use of MOUD and allows nonspecialists to gain experience and confidence in using buprenorphine. Any clinician could administer buprenorphine in the ED, and patients could be bridged at later ED visits until follow-up with a specialist. Of note, only a small percentage of the total visits for buprenorphine initiation required multiple daily visits for buprenorphine. Appointments with the specialist were assured to fall within a 72-hour window.
Our program has some limitations. First, the number of patients who were candidates for our ED MOUD program was small. In our 7-month review, only 47 patients were identified as potential candidates for MOUD treatment across 70 visits, and only 10 were initiated in the ED. Second, all patients were not actively screened for OUD. There was potential for missing eligible veterans as inclusion criteria relied on clinicians both recognizing OUD and manually entering a correct diagnostic code. We attempted to mitigate this by also reviewing all ED referrals to the SUD clinic and all patients who received buprenorphine in the ED. In addition, we do not have data on preimplementation rates of follow-up for comparison.
Future Directions
More than half of our patients did not receive ED buprenorphine initiation because they were not in moderate or severe withdrawal (COWS ≥ 8) similar to 57% of patients cited in the D’Onofrio and colleagues study.7 Teaching veterans how to start buprenorphine at home could greatly expand enrollment. However, this requires a prescription from an X-waiver licensed clinician. In 2021, the US Department of Health and Human Services removed the 8-hour training requirement for obtaining an X-waiver.12 However, clinicians are still required to apply for licensing. Eliminating the X-waiver requirement, as proposed by D’Onofrio and colleagues in a 2021 editorial, would have allowed all clinicians to offer home initiation.13
Previous studies suggest that despite the ability to provide a prescription, clinicians may be reluctant to offer home initiation.14–17 In a national VA 2019 survey, many emergency medicine physicians believe that SUD care is not in their scope of practice, as Dieujuste and colleagues described in Federal Practitioner.14 Although it is likely some attitudes have changed with the increased visibility of ED MOUD programs, there is still much work to be done to change perceptions.
Another area for improvement is screening for OUD in the ED to better reveal MOUD candidates. Missed opportunities (neither referral nor treatment offered) occurred in 21% of our visits. D’Onofrio and colleagues identified 66% of patients by screening all ED patients.7 Although universal screening for SUD in routine health care settings has been recommended, 2021 VA guidelines state that there is insufficient evidence to recommend universal screening.18-20 There are also limited data on the best screening tool for OUD in the ED.21 Further research on how to effectively and efficiently identify OUD patients in the ED is needed.
Conclusions
With minimal resource allocation, we started the program to offer MOUD with buprenorphine for patients with OUD at a VA ED and provided addiction treatment follow-up. This program, the first of its kind within VA, can be modeled and expanded to other VA facilities. Given increasing numbers of fatal opioid overdose, and significant adverse impacts of the COVID-19 pandemic on the OUD crisis, developing local and national strategies to treat OUD is essential. Future steps include improved screening and expanding capacity to offer home initiation by increasing the number of X-waiver ED clinicians.6
Acknowledgments
Thank you to Jeffrey Balsam, PharmD, BCPS, Veterans Affairs Greater Los Angeles Clinical Applications Coordinator for his contributions in creating a Computerized Patient Record System opioid use disorder screening tool. Thank you to Gracielle Tan, MD, Veterans Affairs Greater Los Angeles Health Science Specialist for her administrative assistance in manuscript preparation.
1. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: historical perspective, lessons learned, and next steps. Subst Abuse. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
2. Bohnert ASB, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs health system. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
3. Ma J, Bao Y-P, Wang R-J, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1968-1983. doi:10.1038/s41380-018-0094-5
4. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 2.0. US Department of Veterans Affairs; 2009.
5. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 3.0. US Department of Veterans Affairs. 2015. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
6. Hulsey J, Mellis A, Kelly B. COVID-19 pandemic impact on patients, families and individuals in recovery from substance use disorder. Accessed July 7, 2021. https://www.addictionpolicy.org/covid19-report
7. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opiod dependence. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
8. Weiner SG, Baker O, Bernson D, Schuur JD. One-year mortality of patients after emergency department treatment for non-fatal opioid overdose. Ann Emerg Med. 2020;75(1):13-17. doi:10.1016/j.annemergmed.2019.04.020
9. CA Bridge. Updated 2021. Accessed July 1, 2022. https://cabridge.org
10. Penney L, Miake-Lye I, Lewis D, et al. Proceedings from the 11th annual conference on the science of dissemination and implementation: S72 spreading VA’s emergency department-rapid access clinics (ED-RAC) intervention: key factors for success. Implementation Sci. 2019;14(suppl 1). doi:10.1186/s13012-019-0878-2
11. Hu T, Snider-Alder M, Nijmeh L, Pyle A. Buprenorphine/naloxone induction in a Canadian emergency department with rapid access to community-based addictions providers. CJEM. 2019;21(4):492-498. doi:10.1017/cem.2019.24
12. US Department of Health and Human Services. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Federal Register. Accessed July 1, 2022. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
13. D’Onofrio G, Melnick ER, Hawk KF. Improve access to care for opioid use disorder: a call to eliminate the x-waiver requirement now. Ann Emerg Med. 2021;78(2):220-222. doi:10.1016/j.annemergmed.2021.03.023
14. Dieujuste N, Johnson-Koenke R, Celedon M, et al. Provider perceptions of opioid safety measures in VHA emergency department and urgent care centers. Fed Pract. 2021;38(9):412-419. doi:10.12788/fp.0179
15. Hawk KF, D’Onofrio G, Chawarski MC, et al. Barriers and faciliatators to clinician readiness to provide emergency department-initiated buprenorphine. JAMA Netw Open. 2020;3(5):e204561. doi:10.1001/jamanetworkopen.2020.4561
16. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37(9):1787-1790. doi:10.1016/j.ajem.2019.02.025
17. Srivastava A, Kahan M, Leece P, McAndrew A. Buprenorphine unobserved “home” induction: a survey of Ontario’s addiction physicians. Addic Sci Clin Pract. 2019;14(1):18. doi:10.1186/s13722-019-0146-4
18. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 4.0. US Department of Veterans Affairs. 2021. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPG.pdf
19. Patnode CD, Perdue LA, Rushkin M, et al. Screening for unhealthy drug use updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;323(22):2310-2338. doi:10.1001/jama.2019.21381
20. Coles S, Vosooney A. Evidence lacking to support universal unhealthy drug use screening. Am Fam Physician. 2021;103(2):72-73.
21. Sahota PK, Sharstry S, Mukamel DB, et al. Screening emergency department patients for opioid drug use: a qualitative systematic review. Addict Behav. 2018;85:139-146. doi:10.1016/j.addbeh.2018.05.022
Opioid use disorder (OUD) is a public health crisis significantly affecting veterans. A substantial increase in veterans diagnosed with OUD has occurred, nearly tripling from 25,031 in 2003 to 69,142 in 2017
For patients with active OUD, medications for opioid use disorder (MOUD) reduce the risk of overdose and all-cause mortality.3 In 2009, the US Department of Veterans Affairs (VA) and Department of Defense (DoD) published clinical practice guidelines for substance use disorders that strongly recommended MOUD with either buprenorphine or methadone as a first-line treatment. In 2015 updated guidelines encouraged buprenorphine initiation in primary care settings.4,5 This was followed by an academic detailing campaign designed to encourage VA clinicians to initiate MOUD.1 Despite this institutional support, MOUD remains underutilized within the VA, with widely variable rates of prescribing among VA sites.1
Efforts to further expand MOUD cultivated interest in administering buprenorphine in VA emergency departments (EDs). Patients with OUD often use the ED for same-day care, providing opportunities to initiate buprenorphine in the ED 24 hours, 7 days per week. This has been especially true during the COVID-19 pandemic during which reliable access to usual recovery services has been disrupted and EDs have served as a safety net.6
Buprenorphine’s safety profile and prolonged effect duration make it superior to other MOUD options for ED administration. As a partial opioid agonist, buprenorphine is unlikely to cause significant sedation or respiratory depression compared with full agonists like methadone. This is known as the ceiling effect. Additionally, at higher doses, buprenorphine’s effects can last for about 3 days, potentially obviating the need for repeat dosing. D’Onofrio and colleagues seminal 2015 paper conceptually proved the feasibility and value of initiating buprenorphine in the ED; patients who received ED initiation therapy were more likely to be engaged in addiction treatment 30 days after their visit and have reduced rates of illicit opioid drug use.7 Such ED harm-reduction strategies are increasingly recognized as essential, given that 1 in 20 patients treated for a nonfatal opioid overdose in an ED will die within 1 year of their visit, many within 2 days.8 Finally, a significant barrier faced by physicians wanting to administer or prescribe buprenorphine for patients with OUD has been the special licensing required by the Drug Enforcement Administration Drug Addiction Treatment Act of 2000, also known as an X-waiver. A notable exception to this X-waiver requirement is the 72-hour rule, which allows nonwaivered practitioners to administer (but not prescribe for home use) buprenorphine to a patient to relieve acute withdrawal symptoms for up to 72 hours while arranging for specialist referral.Under the 72-hour rule, ED clinicians have a unique opportunity to treat patients experiencing acute withdrawal symptoms and bridge them to specialty care, without the burden of an X-waiver requirement.
The VA Greater Los Angeles Healthcare System (VAGLAHS), therefore, developed and implemented a program to administer buprenorphine in the ED to bridge patients with OUD to an appointment with substance use disorder (SUD) services. We describe our development, implementation and evaluation of this program protocol as a model for other VA EDs. This project was determined to be quality improvement (nonresearch) by the VAGLAHS Institutional Review Board.
ED MOUD Program
We engaged in a 2-month (January-March 2019) preimplementation process during which we (1) obtained stakeholder buy-in; (2) developed a protocol and supporting resources and tools; (3) worked with stakeholders to enact local organizational policy and process modifications; and (4) educated practitioners.
Appendix 1 provides an overview of MOUD terminology, pharmacology, and regulations. We developed an 8-step program implementation plan for the ED MOUD program (Figure 1).
Obtaining Stakeholder Buy-in
Two ED physician champions (MC, JH) organized all activities. Champions obtained stakeholder buy-in from clinical and administrative leaders as well as from frontline personnel in OUD specialty care, ED, and pharmacy services. ED social workers and clerks who schedule post-ED appointments also were engaged. These stakeholders emphasized the importance of fitting the developed protocol into the existing ED workflows as well as minimizing additional resources required to initiate and maintain the program.
We ascertained that in fiscal year 2018, VAGLAHS had 156 ED visits with International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes related to OUD for 108 unique patients. Based on these data and in consultation with OUD specialty care, we determined that the potential number of referrals to the SUD clinic would be manageable with existing resources. Additionally, there was consensus that most opioid withdrawal patients could be treated in the urgent care portion of our ED since these patients generally do not require special monitoring. This consideration was important for obtaining ED stakeholder buy-in and for planning protocol logistics.
Developing the Protocol
We customized resources created by CalBridge Behavioral Health Navigator Program (CA Bridge), formerly called ED Bridge, a program of the Public Health Institute in Oakland, California, funded through California Department of Health Care Services. CA Bridge offers technical assistance and support for hospitals as well as guidance and tools for establishing processes for EDs providing buprenorphine prescriptions for the management of acute opioid withdrawal and serving as a bridge to follow-up care in SUD clinics.9 We also reviewed protocols described by D’Onofrio and colleagues. With iterative input from stakeholders, we created a protocol concretely delineating each process and corresponding responsible party with the overall aim of removing potential barriers to MOUD initiation and follow-up (Appendix 2).
Identifying Appropriate Follow-up
To operationalize protocol implementation, we built on VA’s Emergency Department Rapid Access Clinic (ED-RAC) process, a mechanism for scheduling appointments for post-ED specialty follow-up care. This process facilitated veterans’ access to urgent specialty care follow-up after ED visits by scheduling appointments prior to ED discharge.10 For the ED MOUD program, we adapted the ED-RAC process to schedule appointments in SUD clinic prior to ED discharge. These appointments allowed patients to be seen by an SUD clinician within 72 hours of ED discharge. This step was critical to working within the 72-hour rule without relying on X-waiver licensing of ED clinicians. Alternatively, as was previous practice, per patient preference, patients were also referred to non-VA residential rehabilitation services if the facility had capacity and patients met criteria for admission.
Identification of Eligible Veterans
Target patients were those primarily presenting with a request for treatment of opioid dependence or withdrawal. Patients were not actively screened for OUD. Clinicians diagnosed and assessed for OUD as per their usual practice. Patients with OUD who presented to the ED for other reasons were assessed, at clinician discretion, for their interest in receiving MOUD. If patients presented in moderate-to-severe withdrawal (eg, Clinical Opiate Withdrawal Scale [COWS] ≥ 8), buprenorphine was initiated in the ED. These patients were subsequently referred to either the local SUD clinic or to a residential treatment center. Patients presenting with a COWS score < 8 were referred to the outpatient SUD clinic or residential treatment centers without initiating buprenorphine from the ED. The SUD clinic or residential treatment centers could offer buprenorphine or other MOUD options. From the ED, prescribing buprenorphine for patients to self-initiate at home was not available as this required an X-waivered prescriber, which were limited during the program time frame.
Support Tools and Resources
To facilitate ED clinicians using the protocol, we worked with a programmer experienced with the Computerized Patient Record System, the VA electronic health record (EHR), to create electronic order menu sets that directed clinicians to the protocol and educational materials (Appendix 3). These menus are readily accessible and embedded into the ED clinician workflow. The menus highlight key elements of the protocol, including indications for initiation, contraindications, recommended dosing with quick orders, and how to obtain follow-up for the patient. Links also are provided to the protocol and patient discharge handouts, including the CA Bridge website.
Organizational Policy and Processes
Before implementing the developed protocol, we worked with stakeholders to modify organizational policies and processes. Our pharmacy agreed to stock buprenorphine in the ED to make it readily available. EHR restrictions that historically prohibited ordering buprenorphine for ED administration by nonwaivered clinicians were modified. Additionally, our chief of staff, pharmacy, and credentialing department agreed that physicians did not need to apply for additional delineated privileges.
Clinician Education
The final preparation step was educating clinicians and other protocol users. The VAGLAHS SUD chief presented a lecture and answered questions about MOUD to core ED faculty about the rising prevalence of OUD and use of buprenorphine as a recommended treatment.
Evaluation
To assess adherence to the developed protocol, we conducted a retrospective health record review of all ED visits March 1 to October 25, 2019, in which the patient had OUD and may have qualified for MOUD. To do this, we identified (1) ED visits with an OUD ICD-10 code as a primary or secondary diagnoses; (2) ED referrals to outpatient SUD treatment; and/or (3) ED visits in which buprenorphine was given or prescribed. We included the latter 2 criteria as application of ICD-10 codes for OUD care was inconsistent. Visits were excluded if patients did not have OUD, had OUD in remission, were already maintained on a stable MOUD regimen and no longer using illicit drugs or craving additional opioids, or were presenting solely for a refill or administration of a missed dose. Patients who relapsed were categorized as unstable. Visits were excluded if the patient was admitted to the hospital or left against medical advice. Patients on MOUD who had relapsed or requested a change in MOUD treatment were included. For all included visits, 2 ED physicians (MC, JH) reviewed the ED clinician and nursing notes, pharmacy and referral records, diagnostic codes, and veteran demographics.
In the evaluation, there were 130 visits with 92 unique veterans meeting inclusion criteria. The final sample included 70 visits with 47 unique veterans (Table 1). Of note, 24 (53%) patients self-identified as homeless or were engaged with VA housing services. Twelve veterans had multiple ED visits (7 patients with 2 visits; 5 patients with ≥ 3 visits). In 30 (43%) visits the veteran’s primary reason for seeking ED care was to obtain treatment for opioid withdrawal or receive MOUD. Type of opiate used was specified in 58% of visits; of these, 69% indicated heroin use and 17% prescription medications. Buprenorphine was initiated in the ED in 18 (26%) visits for 10 veterans. Appendix 4 outlines the clinical course and follow-up after these visits. Some veterans returned to the ED for buprenorphine redosing per the 72-hour rule. SUD clinic appointments were provided in 11 visits, and direct transfer to an inpatient rehabilitation center was arranged in 4 visits. In 42 (60%) visits, across 32 unique veterans, buprenorphine was not given in the ED, but patients were referred for SUD treat
A majority of veterans who received buprenorphine and a referral for an SUD appointment went to their initial SUD follow-up appointment and had ongoing engagement in addiction care 30 days after their index ED visit. Among veterans who did not receive buprenorphine but were referred for SUD treatment, about half went to their SUD appointments and about 1 in 5 had ongoing engagement in addiction care at 30 days after the index ED visit. Of note, 2 patients who received referrals died within 1 year of their index ED visit. The cause of death for one patient was an overdose; the other was unspecified.
DISCUSSION
We developed the ED MOUD program as a bridge to SUD specialty care. Our 8 implementation steps can serve as a model for implementing programs at other VA EDs. We demonstrated feasibility, high follow-up rates, and high retention in treatment.
Patients who received ED buprenorphine initiation were more likely to follow up and had higher rates of ongoing engagement at 30 days than did those who received only a clinic referral. In a similar Canadian study, buprenorphine was initiated in the ED, and patients followed up as a walk-in for addiction services; however, only 54% of patients presented to this initial follow-up.11 Our higher initial follow-up rate may be due to our ability to directly schedule clinic appointments. Our 70% 30-day follow-up rate is comparable, but slightly lower than the 2015 D’Onofrio and colleagues study in which 78% of patients remained engaged at 30 days.7 A possible reason is that in the D’Onofrio and colleagues study, all study physicians obtained X-waiver training and were able to prescribe buprenorphine after ED initiation or for self-initiation at home. X-waiver training was not required of our clinicians, and none of our patients were offered a prescription for self-initiation.
Our program demonstrates that it is feasible to develop a protocol without X-waiver licensing. This program provides a supportive framework for the use of MOUD and allows nonspecialists to gain experience and confidence in using buprenorphine. Any clinician could administer buprenorphine in the ED, and patients could be bridged at later ED visits until follow-up with a specialist. Of note, only a small percentage of the total visits for buprenorphine initiation required multiple daily visits for buprenorphine. Appointments with the specialist were assured to fall within a 72-hour window.
Our program has some limitations. First, the number of patients who were candidates for our ED MOUD program was small. In our 7-month review, only 47 patients were identified as potential candidates for MOUD treatment across 70 visits, and only 10 were initiated in the ED. Second, all patients were not actively screened for OUD. There was potential for missing eligible veterans as inclusion criteria relied on clinicians both recognizing OUD and manually entering a correct diagnostic code. We attempted to mitigate this by also reviewing all ED referrals to the SUD clinic and all patients who received buprenorphine in the ED. In addition, we do not have data on preimplementation rates of follow-up for comparison.
Future Directions
More than half of our patients did not receive ED buprenorphine initiation because they were not in moderate or severe withdrawal (COWS ≥ 8) similar to 57% of patients cited in the D’Onofrio and colleagues study.7 Teaching veterans how to start buprenorphine at home could greatly expand enrollment. However, this requires a prescription from an X-waiver licensed clinician. In 2021, the US Department of Health and Human Services removed the 8-hour training requirement for obtaining an X-waiver.12 However, clinicians are still required to apply for licensing. Eliminating the X-waiver requirement, as proposed by D’Onofrio and colleagues in a 2021 editorial, would have allowed all clinicians to offer home initiation.13
Previous studies suggest that despite the ability to provide a prescription, clinicians may be reluctant to offer home initiation.14–17 In a national VA 2019 survey, many emergency medicine physicians believe that SUD care is not in their scope of practice, as Dieujuste and colleagues described in Federal Practitioner.14 Although it is likely some attitudes have changed with the increased visibility of ED MOUD programs, there is still much work to be done to change perceptions.
Another area for improvement is screening for OUD in the ED to better reveal MOUD candidates. Missed opportunities (neither referral nor treatment offered) occurred in 21% of our visits. D’Onofrio and colleagues identified 66% of patients by screening all ED patients.7 Although universal screening for SUD in routine health care settings has been recommended, 2021 VA guidelines state that there is insufficient evidence to recommend universal screening.18-20 There are also limited data on the best screening tool for OUD in the ED.21 Further research on how to effectively and efficiently identify OUD patients in the ED is needed.
Conclusions
With minimal resource allocation, we started the program to offer MOUD with buprenorphine for patients with OUD at a VA ED and provided addiction treatment follow-up. This program, the first of its kind within VA, can be modeled and expanded to other VA facilities. Given increasing numbers of fatal opioid overdose, and significant adverse impacts of the COVID-19 pandemic on the OUD crisis, developing local and national strategies to treat OUD is essential. Future steps include improved screening and expanding capacity to offer home initiation by increasing the number of X-waiver ED clinicians.6
Acknowledgments
Thank you to Jeffrey Balsam, PharmD, BCPS, Veterans Affairs Greater Los Angeles Clinical Applications Coordinator for his contributions in creating a Computerized Patient Record System opioid use disorder screening tool. Thank you to Gracielle Tan, MD, Veterans Affairs Greater Los Angeles Health Science Specialist for her administrative assistance in manuscript preparation.
Opioid use disorder (OUD) is a public health crisis significantly affecting veterans. A substantial increase in veterans diagnosed with OUD has occurred, nearly tripling from 25,031 in 2003 to 69,142 in 2017
For patients with active OUD, medications for opioid use disorder (MOUD) reduce the risk of overdose and all-cause mortality.3 In 2009, the US Department of Veterans Affairs (VA) and Department of Defense (DoD) published clinical practice guidelines for substance use disorders that strongly recommended MOUD with either buprenorphine or methadone as a first-line treatment. In 2015 updated guidelines encouraged buprenorphine initiation in primary care settings.4,5 This was followed by an academic detailing campaign designed to encourage VA clinicians to initiate MOUD.1 Despite this institutional support, MOUD remains underutilized within the VA, with widely variable rates of prescribing among VA sites.1
Efforts to further expand MOUD cultivated interest in administering buprenorphine in VA emergency departments (EDs). Patients with OUD often use the ED for same-day care, providing opportunities to initiate buprenorphine in the ED 24 hours, 7 days per week. This has been especially true during the COVID-19 pandemic during which reliable access to usual recovery services has been disrupted and EDs have served as a safety net.6
Buprenorphine’s safety profile and prolonged effect duration make it superior to other MOUD options for ED administration. As a partial opioid agonist, buprenorphine is unlikely to cause significant sedation or respiratory depression compared with full agonists like methadone. This is known as the ceiling effect. Additionally, at higher doses, buprenorphine’s effects can last for about 3 days, potentially obviating the need for repeat dosing. D’Onofrio and colleagues seminal 2015 paper conceptually proved the feasibility and value of initiating buprenorphine in the ED; patients who received ED initiation therapy were more likely to be engaged in addiction treatment 30 days after their visit and have reduced rates of illicit opioid drug use.7 Such ED harm-reduction strategies are increasingly recognized as essential, given that 1 in 20 patients treated for a nonfatal opioid overdose in an ED will die within 1 year of their visit, many within 2 days.8 Finally, a significant barrier faced by physicians wanting to administer or prescribe buprenorphine for patients with OUD has been the special licensing required by the Drug Enforcement Administration Drug Addiction Treatment Act of 2000, also known as an X-waiver. A notable exception to this X-waiver requirement is the 72-hour rule, which allows nonwaivered practitioners to administer (but not prescribe for home use) buprenorphine to a patient to relieve acute withdrawal symptoms for up to 72 hours while arranging for specialist referral.Under the 72-hour rule, ED clinicians have a unique opportunity to treat patients experiencing acute withdrawal symptoms and bridge them to specialty care, without the burden of an X-waiver requirement.
The VA Greater Los Angeles Healthcare System (VAGLAHS), therefore, developed and implemented a program to administer buprenorphine in the ED to bridge patients with OUD to an appointment with substance use disorder (SUD) services. We describe our development, implementation and evaluation of this program protocol as a model for other VA EDs. This project was determined to be quality improvement (nonresearch) by the VAGLAHS Institutional Review Board.
ED MOUD Program
We engaged in a 2-month (January-March 2019) preimplementation process during which we (1) obtained stakeholder buy-in; (2) developed a protocol and supporting resources and tools; (3) worked with stakeholders to enact local organizational policy and process modifications; and (4) educated practitioners.
Appendix 1 provides an overview of MOUD terminology, pharmacology, and regulations. We developed an 8-step program implementation plan for the ED MOUD program (Figure 1).
Obtaining Stakeholder Buy-in
Two ED physician champions (MC, JH) organized all activities. Champions obtained stakeholder buy-in from clinical and administrative leaders as well as from frontline personnel in OUD specialty care, ED, and pharmacy services. ED social workers and clerks who schedule post-ED appointments also were engaged. These stakeholders emphasized the importance of fitting the developed protocol into the existing ED workflows as well as minimizing additional resources required to initiate and maintain the program.
We ascertained that in fiscal year 2018, VAGLAHS had 156 ED visits with International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes related to OUD for 108 unique patients. Based on these data and in consultation with OUD specialty care, we determined that the potential number of referrals to the SUD clinic would be manageable with existing resources. Additionally, there was consensus that most opioid withdrawal patients could be treated in the urgent care portion of our ED since these patients generally do not require special monitoring. This consideration was important for obtaining ED stakeholder buy-in and for planning protocol logistics.
Developing the Protocol
We customized resources created by CalBridge Behavioral Health Navigator Program (CA Bridge), formerly called ED Bridge, a program of the Public Health Institute in Oakland, California, funded through California Department of Health Care Services. CA Bridge offers technical assistance and support for hospitals as well as guidance and tools for establishing processes for EDs providing buprenorphine prescriptions for the management of acute opioid withdrawal and serving as a bridge to follow-up care in SUD clinics.9 We also reviewed protocols described by D’Onofrio and colleagues. With iterative input from stakeholders, we created a protocol concretely delineating each process and corresponding responsible party with the overall aim of removing potential barriers to MOUD initiation and follow-up (Appendix 2).
Identifying Appropriate Follow-up
To operationalize protocol implementation, we built on VA’s Emergency Department Rapid Access Clinic (ED-RAC) process, a mechanism for scheduling appointments for post-ED specialty follow-up care. This process facilitated veterans’ access to urgent specialty care follow-up after ED visits by scheduling appointments prior to ED discharge.10 For the ED MOUD program, we adapted the ED-RAC process to schedule appointments in SUD clinic prior to ED discharge. These appointments allowed patients to be seen by an SUD clinician within 72 hours of ED discharge. This step was critical to working within the 72-hour rule without relying on X-waiver licensing of ED clinicians. Alternatively, as was previous practice, per patient preference, patients were also referred to non-VA residential rehabilitation services if the facility had capacity and patients met criteria for admission.
Identification of Eligible Veterans
Target patients were those primarily presenting with a request for treatment of opioid dependence or withdrawal. Patients were not actively screened for OUD. Clinicians diagnosed and assessed for OUD as per their usual practice. Patients with OUD who presented to the ED for other reasons were assessed, at clinician discretion, for their interest in receiving MOUD. If patients presented in moderate-to-severe withdrawal (eg, Clinical Opiate Withdrawal Scale [COWS] ≥ 8), buprenorphine was initiated in the ED. These patients were subsequently referred to either the local SUD clinic or to a residential treatment center. Patients presenting with a COWS score < 8 were referred to the outpatient SUD clinic or residential treatment centers without initiating buprenorphine from the ED. The SUD clinic or residential treatment centers could offer buprenorphine or other MOUD options. From the ED, prescribing buprenorphine for patients to self-initiate at home was not available as this required an X-waivered prescriber, which were limited during the program time frame.
Support Tools and Resources
To facilitate ED clinicians using the protocol, we worked with a programmer experienced with the Computerized Patient Record System, the VA electronic health record (EHR), to create electronic order menu sets that directed clinicians to the protocol and educational materials (Appendix 3). These menus are readily accessible and embedded into the ED clinician workflow. The menus highlight key elements of the protocol, including indications for initiation, contraindications, recommended dosing with quick orders, and how to obtain follow-up for the patient. Links also are provided to the protocol and patient discharge handouts, including the CA Bridge website.
Organizational Policy and Processes
Before implementing the developed protocol, we worked with stakeholders to modify organizational policies and processes. Our pharmacy agreed to stock buprenorphine in the ED to make it readily available. EHR restrictions that historically prohibited ordering buprenorphine for ED administration by nonwaivered clinicians were modified. Additionally, our chief of staff, pharmacy, and credentialing department agreed that physicians did not need to apply for additional delineated privileges.
Clinician Education
The final preparation step was educating clinicians and other protocol users. The VAGLAHS SUD chief presented a lecture and answered questions about MOUD to core ED faculty about the rising prevalence of OUD and use of buprenorphine as a recommended treatment.
Evaluation
To assess adherence to the developed protocol, we conducted a retrospective health record review of all ED visits March 1 to October 25, 2019, in which the patient had OUD and may have qualified for MOUD. To do this, we identified (1) ED visits with an OUD ICD-10 code as a primary or secondary diagnoses; (2) ED referrals to outpatient SUD treatment; and/or (3) ED visits in which buprenorphine was given or prescribed. We included the latter 2 criteria as application of ICD-10 codes for OUD care was inconsistent. Visits were excluded if patients did not have OUD, had OUD in remission, were already maintained on a stable MOUD regimen and no longer using illicit drugs or craving additional opioids, or were presenting solely for a refill or administration of a missed dose. Patients who relapsed were categorized as unstable. Visits were excluded if the patient was admitted to the hospital or left against medical advice. Patients on MOUD who had relapsed or requested a change in MOUD treatment were included. For all included visits, 2 ED physicians (MC, JH) reviewed the ED clinician and nursing notes, pharmacy and referral records, diagnostic codes, and veteran demographics.
In the evaluation, there were 130 visits with 92 unique veterans meeting inclusion criteria. The final sample included 70 visits with 47 unique veterans (Table 1). Of note, 24 (53%) patients self-identified as homeless or were engaged with VA housing services. Twelve veterans had multiple ED visits (7 patients with 2 visits; 5 patients with ≥ 3 visits). In 30 (43%) visits the veteran’s primary reason for seeking ED care was to obtain treatment for opioid withdrawal or receive MOUD. Type of opiate used was specified in 58% of visits; of these, 69% indicated heroin use and 17% prescription medications. Buprenorphine was initiated in the ED in 18 (26%) visits for 10 veterans. Appendix 4 outlines the clinical course and follow-up after these visits. Some veterans returned to the ED for buprenorphine redosing per the 72-hour rule. SUD clinic appointments were provided in 11 visits, and direct transfer to an inpatient rehabilitation center was arranged in 4 visits. In 42 (60%) visits, across 32 unique veterans, buprenorphine was not given in the ED, but patients were referred for SUD treat
A majority of veterans who received buprenorphine and a referral for an SUD appointment went to their initial SUD follow-up appointment and had ongoing engagement in addiction care 30 days after their index ED visit. Among veterans who did not receive buprenorphine but were referred for SUD treatment, about half went to their SUD appointments and about 1 in 5 had ongoing engagement in addiction care at 30 days after the index ED visit. Of note, 2 patients who received referrals died within 1 year of their index ED visit. The cause of death for one patient was an overdose; the other was unspecified.
DISCUSSION
We developed the ED MOUD program as a bridge to SUD specialty care. Our 8 implementation steps can serve as a model for implementing programs at other VA EDs. We demonstrated feasibility, high follow-up rates, and high retention in treatment.
Patients who received ED buprenorphine initiation were more likely to follow up and had higher rates of ongoing engagement at 30 days than did those who received only a clinic referral. In a similar Canadian study, buprenorphine was initiated in the ED, and patients followed up as a walk-in for addiction services; however, only 54% of patients presented to this initial follow-up.11 Our higher initial follow-up rate may be due to our ability to directly schedule clinic appointments. Our 70% 30-day follow-up rate is comparable, but slightly lower than the 2015 D’Onofrio and colleagues study in which 78% of patients remained engaged at 30 days.7 A possible reason is that in the D’Onofrio and colleagues study, all study physicians obtained X-waiver training and were able to prescribe buprenorphine after ED initiation or for self-initiation at home. X-waiver training was not required of our clinicians, and none of our patients were offered a prescription for self-initiation.
Our program demonstrates that it is feasible to develop a protocol without X-waiver licensing. This program provides a supportive framework for the use of MOUD and allows nonspecialists to gain experience and confidence in using buprenorphine. Any clinician could administer buprenorphine in the ED, and patients could be bridged at later ED visits until follow-up with a specialist. Of note, only a small percentage of the total visits for buprenorphine initiation required multiple daily visits for buprenorphine. Appointments with the specialist were assured to fall within a 72-hour window.
Our program has some limitations. First, the number of patients who were candidates for our ED MOUD program was small. In our 7-month review, only 47 patients were identified as potential candidates for MOUD treatment across 70 visits, and only 10 were initiated in the ED. Second, all patients were not actively screened for OUD. There was potential for missing eligible veterans as inclusion criteria relied on clinicians both recognizing OUD and manually entering a correct diagnostic code. We attempted to mitigate this by also reviewing all ED referrals to the SUD clinic and all patients who received buprenorphine in the ED. In addition, we do not have data on preimplementation rates of follow-up for comparison.
Future Directions
More than half of our patients did not receive ED buprenorphine initiation because they were not in moderate or severe withdrawal (COWS ≥ 8) similar to 57% of patients cited in the D’Onofrio and colleagues study.7 Teaching veterans how to start buprenorphine at home could greatly expand enrollment. However, this requires a prescription from an X-waiver licensed clinician. In 2021, the US Department of Health and Human Services removed the 8-hour training requirement for obtaining an X-waiver.12 However, clinicians are still required to apply for licensing. Eliminating the X-waiver requirement, as proposed by D’Onofrio and colleagues in a 2021 editorial, would have allowed all clinicians to offer home initiation.13
Previous studies suggest that despite the ability to provide a prescription, clinicians may be reluctant to offer home initiation.14–17 In a national VA 2019 survey, many emergency medicine physicians believe that SUD care is not in their scope of practice, as Dieujuste and colleagues described in Federal Practitioner.14 Although it is likely some attitudes have changed with the increased visibility of ED MOUD programs, there is still much work to be done to change perceptions.
Another area for improvement is screening for OUD in the ED to better reveal MOUD candidates. Missed opportunities (neither referral nor treatment offered) occurred in 21% of our visits. D’Onofrio and colleagues identified 66% of patients by screening all ED patients.7 Although universal screening for SUD in routine health care settings has been recommended, 2021 VA guidelines state that there is insufficient evidence to recommend universal screening.18-20 There are also limited data on the best screening tool for OUD in the ED.21 Further research on how to effectively and efficiently identify OUD patients in the ED is needed.
Conclusions
With minimal resource allocation, we started the program to offer MOUD with buprenorphine for patients with OUD at a VA ED and provided addiction treatment follow-up. This program, the first of its kind within VA, can be modeled and expanded to other VA facilities. Given increasing numbers of fatal opioid overdose, and significant adverse impacts of the COVID-19 pandemic on the OUD crisis, developing local and national strategies to treat OUD is essential. Future steps include improved screening and expanding capacity to offer home initiation by increasing the number of X-waiver ED clinicians.6
Acknowledgments
Thank you to Jeffrey Balsam, PharmD, BCPS, Veterans Affairs Greater Los Angeles Clinical Applications Coordinator for his contributions in creating a Computerized Patient Record System opioid use disorder screening tool. Thank you to Gracielle Tan, MD, Veterans Affairs Greater Los Angeles Health Science Specialist for her administrative assistance in manuscript preparation.
1. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: historical perspective, lessons learned, and next steps. Subst Abuse. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
2. Bohnert ASB, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs health system. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
3. Ma J, Bao Y-P, Wang R-J, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1968-1983. doi:10.1038/s41380-018-0094-5
4. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 2.0. US Department of Veterans Affairs; 2009.
5. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 3.0. US Department of Veterans Affairs. 2015. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
6. Hulsey J, Mellis A, Kelly B. COVID-19 pandemic impact on patients, families and individuals in recovery from substance use disorder. Accessed July 7, 2021. https://www.addictionpolicy.org/covid19-report
7. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opiod dependence. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
8. Weiner SG, Baker O, Bernson D, Schuur JD. One-year mortality of patients after emergency department treatment for non-fatal opioid overdose. Ann Emerg Med. 2020;75(1):13-17. doi:10.1016/j.annemergmed.2019.04.020
9. CA Bridge. Updated 2021. Accessed July 1, 2022. https://cabridge.org
10. Penney L, Miake-Lye I, Lewis D, et al. Proceedings from the 11th annual conference on the science of dissemination and implementation: S72 spreading VA’s emergency department-rapid access clinics (ED-RAC) intervention: key factors for success. Implementation Sci. 2019;14(suppl 1). doi:10.1186/s13012-019-0878-2
11. Hu T, Snider-Alder M, Nijmeh L, Pyle A. Buprenorphine/naloxone induction in a Canadian emergency department with rapid access to community-based addictions providers. CJEM. 2019;21(4):492-498. doi:10.1017/cem.2019.24
12. US Department of Health and Human Services. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Federal Register. Accessed July 1, 2022. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
13. D’Onofrio G, Melnick ER, Hawk KF. Improve access to care for opioid use disorder: a call to eliminate the x-waiver requirement now. Ann Emerg Med. 2021;78(2):220-222. doi:10.1016/j.annemergmed.2021.03.023
14. Dieujuste N, Johnson-Koenke R, Celedon M, et al. Provider perceptions of opioid safety measures in VHA emergency department and urgent care centers. Fed Pract. 2021;38(9):412-419. doi:10.12788/fp.0179
15. Hawk KF, D’Onofrio G, Chawarski MC, et al. Barriers and faciliatators to clinician readiness to provide emergency department-initiated buprenorphine. JAMA Netw Open. 2020;3(5):e204561. doi:10.1001/jamanetworkopen.2020.4561
16. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37(9):1787-1790. doi:10.1016/j.ajem.2019.02.025
17. Srivastava A, Kahan M, Leece P, McAndrew A. Buprenorphine unobserved “home” induction: a survey of Ontario’s addiction physicians. Addic Sci Clin Pract. 2019;14(1):18. doi:10.1186/s13722-019-0146-4
18. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 4.0. US Department of Veterans Affairs. 2021. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPG.pdf
19. Patnode CD, Perdue LA, Rushkin M, et al. Screening for unhealthy drug use updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;323(22):2310-2338. doi:10.1001/jama.2019.21381
20. Coles S, Vosooney A. Evidence lacking to support universal unhealthy drug use screening. Am Fam Physician. 2021;103(2):72-73.
21. Sahota PK, Sharstry S, Mukamel DB, et al. Screening emergency department patients for opioid drug use: a qualitative systematic review. Addict Behav. 2018;85:139-146. doi:10.1016/j.addbeh.2018.05.022
1. Wyse JJ, Gordon AJ, Dobscha SK, et al. Medications for opioid use disorder in the Department of Veterans Affairs (VA) health care system: historical perspective, lessons learned, and next steps. Subst Abuse. 2018;39(2):139-144. doi:10.1080/08897077.2018.1452327
2. Bohnert ASB, Ilgen MA, Galea S, McCarthy JF, Blow FC. Accidental poisoning mortality among patients in the Department of Veterans Affairs health system. Med Care. 2011;49(4):393-396. doi:10.1097/MLR.0b013e318202aa27
3. Ma J, Bao Y-P, Wang R-J, et al. Effects of medication-assisted treatment on mortality among opioids users: a systematic review and meta-analysis. Mol Psychiatry. 2019;24(12):1968-1983. doi:10.1038/s41380-018-0094-5
4. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 2.0. US Department of Veterans Affairs; 2009.
5. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 3.0. US Department of Veterans Affairs. 2015. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPGRevised22216.pdf
6. Hulsey J, Mellis A, Kelly B. COVID-19 pandemic impact on patients, families and individuals in recovery from substance use disorder. Accessed July 7, 2021. https://www.addictionpolicy.org/covid19-report
7. D’Onofrio G, O’Connor PG, Pantalon MV, et al. Emergency department-initiated buprenorphine/naloxone treatment for opiod dependence. JAMA. 2015;313(16):1636-1644. doi:10.1001/jama.2015.3474
8. Weiner SG, Baker O, Bernson D, Schuur JD. One-year mortality of patients after emergency department treatment for non-fatal opioid overdose. Ann Emerg Med. 2020;75(1):13-17. doi:10.1016/j.annemergmed.2019.04.020
9. CA Bridge. Updated 2021. Accessed July 1, 2022. https://cabridge.org
10. Penney L, Miake-Lye I, Lewis D, et al. Proceedings from the 11th annual conference on the science of dissemination and implementation: S72 spreading VA’s emergency department-rapid access clinics (ED-RAC) intervention: key factors for success. Implementation Sci. 2019;14(suppl 1). doi:10.1186/s13012-019-0878-2
11. Hu T, Snider-Alder M, Nijmeh L, Pyle A. Buprenorphine/naloxone induction in a Canadian emergency department with rapid access to community-based addictions providers. CJEM. 2019;21(4):492-498. doi:10.1017/cem.2019.24
12. US Department of Health and Human Services. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. Federal Register. Accessed July 1, 2022. https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorder
13. D’Onofrio G, Melnick ER, Hawk KF. Improve access to care for opioid use disorder: a call to eliminate the x-waiver requirement now. Ann Emerg Med. 2021;78(2):220-222. doi:10.1016/j.annemergmed.2021.03.023
14. Dieujuste N, Johnson-Koenke R, Celedon M, et al. Provider perceptions of opioid safety measures in VHA emergency department and urgent care centers. Fed Pract. 2021;38(9):412-419. doi:10.12788/fp.0179
15. Hawk KF, D’Onofrio G, Chawarski MC, et al. Barriers and faciliatators to clinician readiness to provide emergency department-initiated buprenorphine. JAMA Netw Open. 2020;3(5):e204561. doi:10.1001/jamanetworkopen.2020.4561
16. Lowenstein M, Kilaru A, Perrone J, et al. Barriers and facilitators for emergency department initiation of buprenorphine: a physician survey. Am J Emerg Med. 2019;37(9):1787-1790. doi:10.1016/j.ajem.2019.02.025
17. Srivastava A, Kahan M, Leece P, McAndrew A. Buprenorphine unobserved “home” induction: a survey of Ontario’s addiction physicians. Addic Sci Clin Pract. 2019;14(1):18. doi:10.1186/s13722-019-0146-4
18. The Management of Substance Use Disorders Work Group. VA/DoD Clinical Practice Guideline for the Management of Substance Use Disorders. Version 4.0. US Department of Veterans Affairs. 2021. Accessed July 1, 2022. https://www.healthquality.va.gov/guidelines/MH/sud/VADoDSUDCPG.pdf
19. Patnode CD, Perdue LA, Rushkin M, et al. Screening for unhealthy drug use updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2020;323(22):2310-2338. doi:10.1001/jama.2019.21381
20. Coles S, Vosooney A. Evidence lacking to support universal unhealthy drug use screening. Am Fam Physician. 2021;103(2):72-73.
21. Sahota PK, Sharstry S, Mukamel DB, et al. Screening emergency department patients for opioid drug use: a qualitative systematic review. Addict Behav. 2018;85:139-146. doi:10.1016/j.addbeh.2018.05.022
Implementation of a Virtual Huddle to Support Patient Care During the COVID-19 Pandemic
The COVID-19 pandemic challenged hospital medicine teams to care for patients with complex respiratory needs, comply with evolving protocols, and remain abreast of new therapies.1,2 Pulmonary and critical care medicine (PCCM) faculty grappled with similar issues, acknowledging that their critical care expertise could be beneficial outside of the intensive care unit (ICU). Clinical pharmacists managed the procurement, allocation, and monitoring of complex (and sometimes limited) pharmacologic therapies. Although strategies used by health care systems to prepare and restructure for COVID-19 are reported, processes to enhance multidisciplinary care are limited.3,4 Therefore, we developed the COVID-19 Tele-Huddle Program using video conference to support hospital medicine teams caring for patients with COVID-19 and high disease severity.
Program Description
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, is a 349-bed, level 1A federal health care facility serving more than 113,000 veterans in southeast Texas.5 The COVID-19 Tele-Huddle Program took place over a 4-week period from July 6 to August 2, 2020. By the end of the 4-week period, there was a decline in the number of COVID patient admissions and thus the need for the huddle. Participation in the huddle also declined, likely reflecting the end of the surge and an increase in knowledge about COVID management acquired by the teams. Each COVID-19 Tele-Huddle Program consultation session consisted of at least 1 member from each hospital medicine team, 1 to 2 PCCM faculty members, and 1 to 2 clinical pharmacy specialists (Figure). The consultation team members included 4 PCCM faculty members and 2 clinical pharmacy specialists. The internal medicine (IM) participants included 10 ward teams with a total of 20 interns (PGY1), 12 upper-level residents (PGY2 and PGY 3), and 10 attending physicians.
The COVID-19 Tele-Huddle Program was a daily (including weekends) video conference. The hospital medicine team members joined the huddle from team workrooms, using webcams supplied by the MEDVAMC information technology department. The COVID-19 Tele-Huddle Program consultation team members joined remotely. Each hospital medicine team joined the huddle at a pre-assigned 15- to 30-minute time allotment, which varied based on patient volume. Participation in the huddle was mandatory for the first week and became optional thereafter. This was in recognition of the steep learning curve and provided the teams both basic knowledge of COVID management and a shared understanding of when a multidisciplinary consultation would be critical. Mandatory daily participation was challenging due to the pressures of patient volume during the surge.
COVID-19 patients with high disease severity were discussed during huddles based on specific criteria: all newly admitted COVID-19 patients, patients requiring step-down level of care, those with increasing oxygen requirements, and/or patients requiring authorization of remdesivir therapy, which required clinical pharmacy authorization at MEDVAMC. The hospital medicine teams reported the patients’ oxygen requirements, comorbid medical conditions, current and prior therapies, fluid status, and relevant laboratory values. A dashboard using the Premier Inc. TheraDoc clinical decision support system was developed to display patient vital signs, laboratory values, and medications. The PCCM faculty and clinical pharmacists listened to inpatient medicine teams presentations and used the dashboard and radiographic images to formulate clinical decisions. Discussion of a patient at the huddle did not preclude in-person consultation at any time.
Tele-Huddles were not recorded, and all protected health information discussed was accessed through the electronic health record using a secure network. Data on length of the meeting, number of patients discussed, and management decisions were recorded daily in a spreadsheet. At the end of the 4-week surge, participants in the program completed a survey, which assessed participant demographics, prior experience with COVID-19, and satisfaction with the program based on a series of agree/disagree questions.
Program Metrics
During the COVID-19 Tele-Huddle Program 4-week evaluation period, 323 encounters were discussed with 117 unique patients with COVID-19. A median (IQR) of 5 (4-8) hospital medicine teams discussed 15 (9-18) patients. The COVID-19 Tele-Huddle Program lasted a median (IQR) 74 (53-94) minutes. A mean (SD) 27% (13) of patients with COVID-19 admitted to the acute care services were discussed.
The multidisciplinary team provided 247 chest X-ray interpretations, 82 diagnostic recommendations, 206 therapeutic recommendations, and 32 transition of care recommendations (Table 1). A total of 55 (47%) patients were given remdesivir with first dose authorized by clinical pharmacy and given within a median (IQR) 6 (3-10) hours after the order was placed. Oxygen therapy, including titration and de-escalation of high-flow nasal cannula and noninvasive positive pressure ventilation (NIPPV), was used for 26 (22.2%) patients. Additional interventions included the review of imaging, the assessment of volume status to guide diuretic recommendations, and the discussion of goals of care.
Of the participating IM trainees and attendings, 16 of 37 (43%) completed the user survey (Table 2). Prior experience with COVID-19 patients varied, with 7 of 16 respondents indicating experience with ≥ 5 patients with COVID-19 prior to the intervention period. Respondents believed that the huddle was helpful in management of respiratory issues (13 of 16), management of medications (13 of 16), escalation of care to ICU (10 of 16), and management of nonrespiratory issues (8 of 16) and goals of care (12 of 16). Fifteen of 16 participants strongly agreed or agreed that the COVID-19 Tele-Huddle Program improved their knowledge and confidence in managing patients. One participant commented, “Getting interdisciplinary help on COVID patients has really helped our team feel confident in our decisions about some of these very complex patients.” Another respondent commented, “Reliability was very helpful for planning how to discuss updates with our patients rather than the formal consultative process.”
Discussion
During the unprecedented COVID-19 pandemic, health care systems have been challenged to manage a large volume of patients, often with high disease severity, in non-ICU settings. This surge in cases has placed strain on hospital medicine teams. There is a subset of patients with COVID-19 with high disease severity that may be managed safely by hospital medicine teams, provided the accessibility and support of consultants, such as PCCM faculty and clinical pharmacists.
Huddles are defined as functional groups of people focused on enhancing communication and care coordination to benefit patient safety. While often brief in nature, huddles can encompass a variety of structures, agendas, and outcome measures.6,7 We implemented a modified huddle using video conferencing to provide important aspects of critical care for patients with COVID-19. Face-to-face evaluation of about 15 patients each day would have strained an already burdened PCCM faculty who were providing additional critical care services as part of the surge response. Conversion of in-person consultations to the COVID-19 Tele-Huddle Program allowed for mitigation of COVID-19 transmission risk for additional clinicians, conservation of personal protective equipment, and more effective communication between acute inpatient practitioners and clinical services. The huddle model expedited the authorization and delivery of therapeutics, including remdesivir, which was prescribed for many patients discussed. Clinical pharmacists provided a review of all medications with input on escalation, de-escalation, dosing, drug-drug interactions, and emergency use authorization therapies.
Our experience resonates with previously described advantages of a huddle model, including the reliability of the consultation, empowerment for all members with a de-emphasis on hierarchy and accountability expected by all.8 The huddle provided situational awareness about patients that may require escalation of care to the ICU and/or further goals of care conversations. Assistance with these transitions of care was highly appreciated by the hospital medicine teams who voiced that these decisions were quite challenging. COVID-19 patients at risk for decompensation were referred for in-person consultation and follow-up if required.
addition, the COVID-19 Tele-Huddle Program allowed for a safe and dependable venue for IM trainees and attending physicians to voice questions and concerns about their patients. We observed the development of a shared mental model among all huddle participants, in the face of a steep learning curve on the management of patients with complex respiratory needs. This was reflected in the survey: Most respondents reported improved knowledge and confidence in managing these patients. Situational awareness that arose from the huddle provided the PCCM faculty the opportunity to guide the inpatient ward teams on next steps whether it be escalation to the ICU and/or further goals of care conversations. Facilitation of transitions of care were voiced as challenging decisions faced by the inpatient ward teams, and there was appreciation for additional support from the PCCM faculty in making these difficult decisions.
Challenges and Opportunities
This was a single-center experience caring for veterans. Challenges with having virtual huddles during the COVID-19 surge involved both time for the health care practitioners and technology. This was recognized early by the educational leaders at our facility, and headsets and cameras were purchased for the team rooms and made available as quickly as possible. Another limitation was the unpredictability and variability of patient volume for specific teams that sometimes would affect the efficiency of the huddle. The number of teams who attended the COVID-19 huddle was highest for the first 2 weeks (maximum of 9 teams) but declined to a nadir of 3 at the end of the month. This reflected the increase in knowledge about COVID-19 and respiratory disease that the teams acquired initially as well as a decline in COVID-19 patient admissions over those weeks.
The COVID-19 Tele-Huddle Program model also can be expanded to include other frontline clinicians, including nurses and respiratory therapists. For example, case management huddles were performed in a similar way during the COVID-19 surge to allow for efficient and effective multidisciplinary conversations about patients
Conclusions
Given the rise of telemedicine and availability of video conferencing services, virtual huddles can be implemented in institutions with appropriate staff and remote access to health records. Multidisciplinary consultation services using video conferencing can serve as an adjunct to the traditional, in-person consultation service model for patients with complex needs.
Acknowledgments
The authors acknowledge all of the Baylor Internal Medicine house staff and internal medicine attendings who participated in our huddle and more importantly, cared for our veterans during this COVID-19 surge.
1. Heymann DL, Shindo N; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health?. Lancet. 2020;395(10224):542-545. doi:10.1016/S0140-6736(20)30374-3
2. Dichter JR, Kanter RK, Dries D, et al; Task Force for Mass Critical Care. System-level planning, coordination, and communication: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(suppl 4):e87S-e102S. doi:10.1378/chest.14-0738
3. Chowdhury JM, Patel M, Zheng M, Abramian O, Criner GJ. Mobilization and preparation of a large urban academic center during the COVID-19 pandemic. Ann Am Thorac Soc. 2020;17(8):922-925. doi:10.1513/AnnalsATS.202003-259PS
4. Uppal A, Silvestri DM, Siegler M, et al. Critical care and emergency department response at the epicenter of the COVID-19 pandemic. Health Aff (Millwood). 2020;39(8):1443-1449. doi:10.1377/hlthaff.2020.00901
5. US Department of Veterans Affairs. Michael E. DeBakey VA Medical Center- Houston, Texas. Accessed December 10, 2020. https://www.houston.va.gov/about
6. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. doi:10.1097/HMR.0000000000000009
7. Franklin BJ, Gandhi TK, Bates DW, et al. Impact of multidisciplinary team huddles on patient safety: a systematic review and proposed taxonomy. BMJ Qual Saf. 2020;29(10):1-2. doi:10.1136/bmjqs-2019-009911
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. doi:10.1136/bmjqs-2012-001467
The COVID-19 pandemic challenged hospital medicine teams to care for patients with complex respiratory needs, comply with evolving protocols, and remain abreast of new therapies.1,2 Pulmonary and critical care medicine (PCCM) faculty grappled with similar issues, acknowledging that their critical care expertise could be beneficial outside of the intensive care unit (ICU). Clinical pharmacists managed the procurement, allocation, and monitoring of complex (and sometimes limited) pharmacologic therapies. Although strategies used by health care systems to prepare and restructure for COVID-19 are reported, processes to enhance multidisciplinary care are limited.3,4 Therefore, we developed the COVID-19 Tele-Huddle Program using video conference to support hospital medicine teams caring for patients with COVID-19 and high disease severity.
Program Description
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, is a 349-bed, level 1A federal health care facility serving more than 113,000 veterans in southeast Texas.5 The COVID-19 Tele-Huddle Program took place over a 4-week period from July 6 to August 2, 2020. By the end of the 4-week period, there was a decline in the number of COVID patient admissions and thus the need for the huddle. Participation in the huddle also declined, likely reflecting the end of the surge and an increase in knowledge about COVID management acquired by the teams. Each COVID-19 Tele-Huddle Program consultation session consisted of at least 1 member from each hospital medicine team, 1 to 2 PCCM faculty members, and 1 to 2 clinical pharmacy specialists (Figure). The consultation team members included 4 PCCM faculty members and 2 clinical pharmacy specialists. The internal medicine (IM) participants included 10 ward teams with a total of 20 interns (PGY1), 12 upper-level residents (PGY2 and PGY 3), and 10 attending physicians.
The COVID-19 Tele-Huddle Program was a daily (including weekends) video conference. The hospital medicine team members joined the huddle from team workrooms, using webcams supplied by the MEDVAMC information technology department. The COVID-19 Tele-Huddle Program consultation team members joined remotely. Each hospital medicine team joined the huddle at a pre-assigned 15- to 30-minute time allotment, which varied based on patient volume. Participation in the huddle was mandatory for the first week and became optional thereafter. This was in recognition of the steep learning curve and provided the teams both basic knowledge of COVID management and a shared understanding of when a multidisciplinary consultation would be critical. Mandatory daily participation was challenging due to the pressures of patient volume during the surge.
COVID-19 patients with high disease severity were discussed during huddles based on specific criteria: all newly admitted COVID-19 patients, patients requiring step-down level of care, those with increasing oxygen requirements, and/or patients requiring authorization of remdesivir therapy, which required clinical pharmacy authorization at MEDVAMC. The hospital medicine teams reported the patients’ oxygen requirements, comorbid medical conditions, current and prior therapies, fluid status, and relevant laboratory values. A dashboard using the Premier Inc. TheraDoc clinical decision support system was developed to display patient vital signs, laboratory values, and medications. The PCCM faculty and clinical pharmacists listened to inpatient medicine teams presentations and used the dashboard and radiographic images to formulate clinical decisions. Discussion of a patient at the huddle did not preclude in-person consultation at any time.
Tele-Huddles were not recorded, and all protected health information discussed was accessed through the electronic health record using a secure network. Data on length of the meeting, number of patients discussed, and management decisions were recorded daily in a spreadsheet. At the end of the 4-week surge, participants in the program completed a survey, which assessed participant demographics, prior experience with COVID-19, and satisfaction with the program based on a series of agree/disagree questions.
Program Metrics
During the COVID-19 Tele-Huddle Program 4-week evaluation period, 323 encounters were discussed with 117 unique patients with COVID-19. A median (IQR) of 5 (4-8) hospital medicine teams discussed 15 (9-18) patients. The COVID-19 Tele-Huddle Program lasted a median (IQR) 74 (53-94) minutes. A mean (SD) 27% (13) of patients with COVID-19 admitted to the acute care services were discussed.
The multidisciplinary team provided 247 chest X-ray interpretations, 82 diagnostic recommendations, 206 therapeutic recommendations, and 32 transition of care recommendations (Table 1). A total of 55 (47%) patients were given remdesivir with first dose authorized by clinical pharmacy and given within a median (IQR) 6 (3-10) hours after the order was placed. Oxygen therapy, including titration and de-escalation of high-flow nasal cannula and noninvasive positive pressure ventilation (NIPPV), was used for 26 (22.2%) patients. Additional interventions included the review of imaging, the assessment of volume status to guide diuretic recommendations, and the discussion of goals of care.
Of the participating IM trainees and attendings, 16 of 37 (43%) completed the user survey (Table 2). Prior experience with COVID-19 patients varied, with 7 of 16 respondents indicating experience with ≥ 5 patients with COVID-19 prior to the intervention period. Respondents believed that the huddle was helpful in management of respiratory issues (13 of 16), management of medications (13 of 16), escalation of care to ICU (10 of 16), and management of nonrespiratory issues (8 of 16) and goals of care (12 of 16). Fifteen of 16 participants strongly agreed or agreed that the COVID-19 Tele-Huddle Program improved their knowledge and confidence in managing patients. One participant commented, “Getting interdisciplinary help on COVID patients has really helped our team feel confident in our decisions about some of these very complex patients.” Another respondent commented, “Reliability was very helpful for planning how to discuss updates with our patients rather than the formal consultative process.”
Discussion
During the unprecedented COVID-19 pandemic, health care systems have been challenged to manage a large volume of patients, often with high disease severity, in non-ICU settings. This surge in cases has placed strain on hospital medicine teams. There is a subset of patients with COVID-19 with high disease severity that may be managed safely by hospital medicine teams, provided the accessibility and support of consultants, such as PCCM faculty and clinical pharmacists.
Huddles are defined as functional groups of people focused on enhancing communication and care coordination to benefit patient safety. While often brief in nature, huddles can encompass a variety of structures, agendas, and outcome measures.6,7 We implemented a modified huddle using video conferencing to provide important aspects of critical care for patients with COVID-19. Face-to-face evaluation of about 15 patients each day would have strained an already burdened PCCM faculty who were providing additional critical care services as part of the surge response. Conversion of in-person consultations to the COVID-19 Tele-Huddle Program allowed for mitigation of COVID-19 transmission risk for additional clinicians, conservation of personal protective equipment, and more effective communication between acute inpatient practitioners and clinical services. The huddle model expedited the authorization and delivery of therapeutics, including remdesivir, which was prescribed for many patients discussed. Clinical pharmacists provided a review of all medications with input on escalation, de-escalation, dosing, drug-drug interactions, and emergency use authorization therapies.
Our experience resonates with previously described advantages of a huddle model, including the reliability of the consultation, empowerment for all members with a de-emphasis on hierarchy and accountability expected by all.8 The huddle provided situational awareness about patients that may require escalation of care to the ICU and/or further goals of care conversations. Assistance with these transitions of care was highly appreciated by the hospital medicine teams who voiced that these decisions were quite challenging. COVID-19 patients at risk for decompensation were referred for in-person consultation and follow-up if required.
addition, the COVID-19 Tele-Huddle Program allowed for a safe and dependable venue for IM trainees and attending physicians to voice questions and concerns about their patients. We observed the development of a shared mental model among all huddle participants, in the face of a steep learning curve on the management of patients with complex respiratory needs. This was reflected in the survey: Most respondents reported improved knowledge and confidence in managing these patients. Situational awareness that arose from the huddle provided the PCCM faculty the opportunity to guide the inpatient ward teams on next steps whether it be escalation to the ICU and/or further goals of care conversations. Facilitation of transitions of care were voiced as challenging decisions faced by the inpatient ward teams, and there was appreciation for additional support from the PCCM faculty in making these difficult decisions.
Challenges and Opportunities
This was a single-center experience caring for veterans. Challenges with having virtual huddles during the COVID-19 surge involved both time for the health care practitioners and technology. This was recognized early by the educational leaders at our facility, and headsets and cameras were purchased for the team rooms and made available as quickly as possible. Another limitation was the unpredictability and variability of patient volume for specific teams that sometimes would affect the efficiency of the huddle. The number of teams who attended the COVID-19 huddle was highest for the first 2 weeks (maximum of 9 teams) but declined to a nadir of 3 at the end of the month. This reflected the increase in knowledge about COVID-19 and respiratory disease that the teams acquired initially as well as a decline in COVID-19 patient admissions over those weeks.
The COVID-19 Tele-Huddle Program model also can be expanded to include other frontline clinicians, including nurses and respiratory therapists. For example, case management huddles were performed in a similar way during the COVID-19 surge to allow for efficient and effective multidisciplinary conversations about patients
Conclusions
Given the rise of telemedicine and availability of video conferencing services, virtual huddles can be implemented in institutions with appropriate staff and remote access to health records. Multidisciplinary consultation services using video conferencing can serve as an adjunct to the traditional, in-person consultation service model for patients with complex needs.
Acknowledgments
The authors acknowledge all of the Baylor Internal Medicine house staff and internal medicine attendings who participated in our huddle and more importantly, cared for our veterans during this COVID-19 surge.
The COVID-19 pandemic challenged hospital medicine teams to care for patients with complex respiratory needs, comply with evolving protocols, and remain abreast of new therapies.1,2 Pulmonary and critical care medicine (PCCM) faculty grappled with similar issues, acknowledging that their critical care expertise could be beneficial outside of the intensive care unit (ICU). Clinical pharmacists managed the procurement, allocation, and monitoring of complex (and sometimes limited) pharmacologic therapies. Although strategies used by health care systems to prepare and restructure for COVID-19 are reported, processes to enhance multidisciplinary care are limited.3,4 Therefore, we developed the COVID-19 Tele-Huddle Program using video conference to support hospital medicine teams caring for patients with COVID-19 and high disease severity.
Program Description
The Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, is a 349-bed, level 1A federal health care facility serving more than 113,000 veterans in southeast Texas.5 The COVID-19 Tele-Huddle Program took place over a 4-week period from July 6 to August 2, 2020. By the end of the 4-week period, there was a decline in the number of COVID patient admissions and thus the need for the huddle. Participation in the huddle also declined, likely reflecting the end of the surge and an increase in knowledge about COVID management acquired by the teams. Each COVID-19 Tele-Huddle Program consultation session consisted of at least 1 member from each hospital medicine team, 1 to 2 PCCM faculty members, and 1 to 2 clinical pharmacy specialists (Figure). The consultation team members included 4 PCCM faculty members and 2 clinical pharmacy specialists. The internal medicine (IM) participants included 10 ward teams with a total of 20 interns (PGY1), 12 upper-level residents (PGY2 and PGY 3), and 10 attending physicians.
The COVID-19 Tele-Huddle Program was a daily (including weekends) video conference. The hospital medicine team members joined the huddle from team workrooms, using webcams supplied by the MEDVAMC information technology department. The COVID-19 Tele-Huddle Program consultation team members joined remotely. Each hospital medicine team joined the huddle at a pre-assigned 15- to 30-minute time allotment, which varied based on patient volume. Participation in the huddle was mandatory for the first week and became optional thereafter. This was in recognition of the steep learning curve and provided the teams both basic knowledge of COVID management and a shared understanding of when a multidisciplinary consultation would be critical. Mandatory daily participation was challenging due to the pressures of patient volume during the surge.
COVID-19 patients with high disease severity were discussed during huddles based on specific criteria: all newly admitted COVID-19 patients, patients requiring step-down level of care, those with increasing oxygen requirements, and/or patients requiring authorization of remdesivir therapy, which required clinical pharmacy authorization at MEDVAMC. The hospital medicine teams reported the patients’ oxygen requirements, comorbid medical conditions, current and prior therapies, fluid status, and relevant laboratory values. A dashboard using the Premier Inc. TheraDoc clinical decision support system was developed to display patient vital signs, laboratory values, and medications. The PCCM faculty and clinical pharmacists listened to inpatient medicine teams presentations and used the dashboard and radiographic images to formulate clinical decisions. Discussion of a patient at the huddle did not preclude in-person consultation at any time.
Tele-Huddles were not recorded, and all protected health information discussed was accessed through the electronic health record using a secure network. Data on length of the meeting, number of patients discussed, and management decisions were recorded daily in a spreadsheet. At the end of the 4-week surge, participants in the program completed a survey, which assessed participant demographics, prior experience with COVID-19, and satisfaction with the program based on a series of agree/disagree questions.
Program Metrics
During the COVID-19 Tele-Huddle Program 4-week evaluation period, 323 encounters were discussed with 117 unique patients with COVID-19. A median (IQR) of 5 (4-8) hospital medicine teams discussed 15 (9-18) patients. The COVID-19 Tele-Huddle Program lasted a median (IQR) 74 (53-94) minutes. A mean (SD) 27% (13) of patients with COVID-19 admitted to the acute care services were discussed.
The multidisciplinary team provided 247 chest X-ray interpretations, 82 diagnostic recommendations, 206 therapeutic recommendations, and 32 transition of care recommendations (Table 1). A total of 55 (47%) patients were given remdesivir with first dose authorized by clinical pharmacy and given within a median (IQR) 6 (3-10) hours after the order was placed. Oxygen therapy, including titration and de-escalation of high-flow nasal cannula and noninvasive positive pressure ventilation (NIPPV), was used for 26 (22.2%) patients. Additional interventions included the review of imaging, the assessment of volume status to guide diuretic recommendations, and the discussion of goals of care.
Of the participating IM trainees and attendings, 16 of 37 (43%) completed the user survey (Table 2). Prior experience with COVID-19 patients varied, with 7 of 16 respondents indicating experience with ≥ 5 patients with COVID-19 prior to the intervention period. Respondents believed that the huddle was helpful in management of respiratory issues (13 of 16), management of medications (13 of 16), escalation of care to ICU (10 of 16), and management of nonrespiratory issues (8 of 16) and goals of care (12 of 16). Fifteen of 16 participants strongly agreed or agreed that the COVID-19 Tele-Huddle Program improved their knowledge and confidence in managing patients. One participant commented, “Getting interdisciplinary help on COVID patients has really helped our team feel confident in our decisions about some of these very complex patients.” Another respondent commented, “Reliability was very helpful for planning how to discuss updates with our patients rather than the formal consultative process.”
Discussion
During the unprecedented COVID-19 pandemic, health care systems have been challenged to manage a large volume of patients, often with high disease severity, in non-ICU settings. This surge in cases has placed strain on hospital medicine teams. There is a subset of patients with COVID-19 with high disease severity that may be managed safely by hospital medicine teams, provided the accessibility and support of consultants, such as PCCM faculty and clinical pharmacists.
Huddles are defined as functional groups of people focused on enhancing communication and care coordination to benefit patient safety. While often brief in nature, huddles can encompass a variety of structures, agendas, and outcome measures.6,7 We implemented a modified huddle using video conferencing to provide important aspects of critical care for patients with COVID-19. Face-to-face evaluation of about 15 patients each day would have strained an already burdened PCCM faculty who were providing additional critical care services as part of the surge response. Conversion of in-person consultations to the COVID-19 Tele-Huddle Program allowed for mitigation of COVID-19 transmission risk for additional clinicians, conservation of personal protective equipment, and more effective communication between acute inpatient practitioners and clinical services. The huddle model expedited the authorization and delivery of therapeutics, including remdesivir, which was prescribed for many patients discussed. Clinical pharmacists provided a review of all medications with input on escalation, de-escalation, dosing, drug-drug interactions, and emergency use authorization therapies.
Our experience resonates with previously described advantages of a huddle model, including the reliability of the consultation, empowerment for all members with a de-emphasis on hierarchy and accountability expected by all.8 The huddle provided situational awareness about patients that may require escalation of care to the ICU and/or further goals of care conversations. Assistance with these transitions of care was highly appreciated by the hospital medicine teams who voiced that these decisions were quite challenging. COVID-19 patients at risk for decompensation were referred for in-person consultation and follow-up if required.
addition, the COVID-19 Tele-Huddle Program allowed for a safe and dependable venue for IM trainees and attending physicians to voice questions and concerns about their patients. We observed the development of a shared mental model among all huddle participants, in the face of a steep learning curve on the management of patients with complex respiratory needs. This was reflected in the survey: Most respondents reported improved knowledge and confidence in managing these patients. Situational awareness that arose from the huddle provided the PCCM faculty the opportunity to guide the inpatient ward teams on next steps whether it be escalation to the ICU and/or further goals of care conversations. Facilitation of transitions of care were voiced as challenging decisions faced by the inpatient ward teams, and there was appreciation for additional support from the PCCM faculty in making these difficult decisions.
Challenges and Opportunities
This was a single-center experience caring for veterans. Challenges with having virtual huddles during the COVID-19 surge involved both time for the health care practitioners and technology. This was recognized early by the educational leaders at our facility, and headsets and cameras were purchased for the team rooms and made available as quickly as possible. Another limitation was the unpredictability and variability of patient volume for specific teams that sometimes would affect the efficiency of the huddle. The number of teams who attended the COVID-19 huddle was highest for the first 2 weeks (maximum of 9 teams) but declined to a nadir of 3 at the end of the month. This reflected the increase in knowledge about COVID-19 and respiratory disease that the teams acquired initially as well as a decline in COVID-19 patient admissions over those weeks.
The COVID-19 Tele-Huddle Program model also can be expanded to include other frontline clinicians, including nurses and respiratory therapists. For example, case management huddles were performed in a similar way during the COVID-19 surge to allow for efficient and effective multidisciplinary conversations about patients
Conclusions
Given the rise of telemedicine and availability of video conferencing services, virtual huddles can be implemented in institutions with appropriate staff and remote access to health records. Multidisciplinary consultation services using video conferencing can serve as an adjunct to the traditional, in-person consultation service model for patients with complex needs.
Acknowledgments
The authors acknowledge all of the Baylor Internal Medicine house staff and internal medicine attendings who participated in our huddle and more importantly, cared for our veterans during this COVID-19 surge.
1. Heymann DL, Shindo N; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health?. Lancet. 2020;395(10224):542-545. doi:10.1016/S0140-6736(20)30374-3
2. Dichter JR, Kanter RK, Dries D, et al; Task Force for Mass Critical Care. System-level planning, coordination, and communication: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(suppl 4):e87S-e102S. doi:10.1378/chest.14-0738
3. Chowdhury JM, Patel M, Zheng M, Abramian O, Criner GJ. Mobilization and preparation of a large urban academic center during the COVID-19 pandemic. Ann Am Thorac Soc. 2020;17(8):922-925. doi:10.1513/AnnalsATS.202003-259PS
4. Uppal A, Silvestri DM, Siegler M, et al. Critical care and emergency department response at the epicenter of the COVID-19 pandemic. Health Aff (Millwood). 2020;39(8):1443-1449. doi:10.1377/hlthaff.2020.00901
5. US Department of Veterans Affairs. Michael E. DeBakey VA Medical Center- Houston, Texas. Accessed December 10, 2020. https://www.houston.va.gov/about
6. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. doi:10.1097/HMR.0000000000000009
7. Franklin BJ, Gandhi TK, Bates DW, et al. Impact of multidisciplinary team huddles on patient safety: a systematic review and proposed taxonomy. BMJ Qual Saf. 2020;29(10):1-2. doi:10.1136/bmjqs-2019-009911
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. doi:10.1136/bmjqs-2012-001467
1. Heymann DL, Shindo N; WHO Scientific and Technical Advisory Group for Infectious Hazards. COVID-19: what is next for public health?. Lancet. 2020;395(10224):542-545. doi:10.1016/S0140-6736(20)30374-3
2. Dichter JR, Kanter RK, Dries D, et al; Task Force for Mass Critical Care. System-level planning, coordination, and communication: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014;146(suppl 4):e87S-e102S. doi:10.1378/chest.14-0738
3. Chowdhury JM, Patel M, Zheng M, Abramian O, Criner GJ. Mobilization and preparation of a large urban academic center during the COVID-19 pandemic. Ann Am Thorac Soc. 2020;17(8):922-925. doi:10.1513/AnnalsATS.202003-259PS
4. Uppal A, Silvestri DM, Siegler M, et al. Critical care and emergency department response at the epicenter of the COVID-19 pandemic. Health Aff (Millwood). 2020;39(8):1443-1449. doi:10.1377/hlthaff.2020.00901
5. US Department of Veterans Affairs. Michael E. DeBakey VA Medical Center- Houston, Texas. Accessed December 10, 2020. https://www.houston.va.gov/about
6. Provost SM, Lanham HJ, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev. 2015;40(1):2-12. doi:10.1097/HMR.0000000000000009
7. Franklin BJ, Gandhi TK, Bates DW, et al. Impact of multidisciplinary team huddles on patient safety: a systematic review and proposed taxonomy. BMJ Qual Saf. 2020;29(10):1-2. doi:10.1136/bmjqs-2019-009911
8. Goldenhar LM, Brady PW, Sutcliffe KM, Muething SE. Huddling for high reliability and situation awareness. BMJ Qual Saf. 2013;22(11):899-906. doi:10.1136/bmjqs-2012-001467
Antibiotic Stewardship Improvement Initiative at a Veterans Health Administration Ambulatory Care Center
The negative impact of the unnecessary prescribing of antibiotic is well known. Consequences include exposing patients to antibiotic adverse effects, risk of overgrowth of pathogenetic organisms such as clostridial species, unnecessary cost of drugs, and development of selection of antibiotic-resistant organisms in the populace at large. Acute viral respiratory infections are among the leading causes of inappropriate antibiotic usage.1 In a study of 1000 adults with respiratory tract infections in an outpatient setting, 77% of patients were prescribed antibiotics, and the treatment was inappropriate in 64% of those who received prescriptions.2 Patient expectations and clinician perceptions of these expectations play a role. One study showed that 54% of clinicians felt their patients expected to receive antibiotics for a visit due to an acute respiratory infection (ARI), such as a cough or cold; 26% of patients did in fact have this expectation.3
The US Department of Veterans Affairs (VA) Central Ohio Health Care System is a large ambulatory care facility, with 4 associated community-based outpatient clinics, serving more than 43,000 central Ohio veterans and completing more than 500,000 medical appointments annually. An antimicrobial stewardship program has been in place since 2013. In May 2018, the clinical pharmacist assigned to the program alerted medical leadership that, of 67 patients seen in primary care for ARIs between April 16, 2018, and May 15, 2018, 42 (63%) had been prescribed an antibiotic. Based on this finding, clinical leadership designed a process improvement program aimed at reducing inappropriate antibiotic usage for the treatment of uncomplicated ARls likely due to viral pathogens. Key components were clinician and patient education and the substitution of a symptomatic treatment kit in place of an antibiotic prescription.
Methods
Facility clinical leadership, assisted by Volunteer Services, developed a Viral Illness Support Pack to be dispensed by primary care practitioners (PCPs) to patients presenting with symptoms of viral ARIs. The contents of this support pack are shown in the Figure. Patients were provided with tissues, throat lozenges, lip balm, acetaminophen, hand sanitizer, a surgical mask, patient instructions, and the Antibiotics Aren’t Always the Answer pamphlet.4 The contents of the viral support pack were purchased through Volunteer Services using donated funds. In total, 460 packs were distributed to the primary care patient aligned care teams (PACTs), including the community-based outpatient clinics.
Clinicians and care teams received academic detailing prior to distribution of the viral support packs, stressing the importance of avoiding antibiotics to treat viral illnesses. Viral illness support packs were available for distribution from December 1, 2018, through March 31, 2019. The frequency of antibiotic dispensing to patients coded for ARI during this period was compared with that of the same time period in the previous year. All charts were reviewed for coding accuracy. Patients with illnesses requiring antibiotic treatment, such as pneumonia, exacerbations of chronic obstructive pulmonary disease and chronic bronchitis, and streptococcal pharyngitis, were excluded from the study. Statistical significance was determined using the unpaired t test.
Results
From December 1, 2018, to March 31, 2019, 357 viral support packs were distributed to patients (Table). For the historical control period from December 1, 2017, through March 31, 2018, 508 patients were treated for ARIs. Of these, 295 (58%) received clinically inappropriate antibiotics. In contrast, of the 627 patients treated for ARIs during the study period from December 1, 2018, through March 31, 2019, 310 (49%) received clinically inappropriate antibiotics. The 9% decrease during the period when viral support packs were distributed, compared with the prior year, was statistically significant (P = .02).
Discussion
The decrease in antibiotic prescriptions for ARIs was statistically significant. The success of this project can be attributed to 3 factors: clinician education, patient education, and the option for PCPs to provide symptomatic treatment for these patients rather than prescribe an antibiotic.
The importance of antibiotic stewardship has been emphasized to all PCPs at the VA Central Ohio Health Care System. Antibiotic stewardship has been the subject of grand rounds. Prior to distribution of the viral support pack, the chief of specialty medicine, the project’s champion, spoke to all PCPs. Adequate numbers of viral support packs were distributed to all primary care teams.
In addition to direct clinician-to-patient education at the time of the patients’ visits, educational materials were included in the viral support pack. The Antibiotics Aren’t Always the Answer pamphlet is available from the Centers for Disease Control and Prevention. It covers the importance of antibiotic awareness, discusses what antibiotics do and do not treat, how to stay healthy, and causes of antibiotic resistance. The pamphlet contains the clear message that antibiotics are not only ineffective against viral illness, but also can cause significant undesirable outcomes.
The pamphlet Viral Illness Support Pack Traffic Light Card (eAppendix available online at doi:10.12788/fp.0302) provides important clinical information to the patients about their illness. Patients are instructed to contact their primary care team if they are worse after 3 days of illness; symptoms are not improving after 10 days; or they experience blood in respiratory secretions, chills or generalized aching, and localized pain that is one-sided or significantly worsening. Patients are clearly informed to seek further care if not improving with symptomatic treatment.
The ability to provide patients with symptomatic relief, including throat lozenges, lip balm, and acetaminophen, was felt to be important in the success of the project. Furthermore, this eliminated an extra step of the patient needing to visit the pharmacy.
Limitations
Limitations of the study included starting distribution of the support packs somewhat after the onset of the viral illness season, failure to reach all prescribers for academic detailing at the start of the program, and several instances of temporary unavailability of the support packs in some areas.
Conclusions
Patients with ARIs are often significantly symptomatic and frequently believe that they require an antibiotic for treatment. Clinicians may adjust their behavior in response to their patients’ expectations, stated or unstated. The results of this project demonstrate that the combination of patient education and the ready availability of a nonantibiotic symptomatic treatment option can significantly decrease the unnecessary prescribing of antibiotics for viral illnesses.
Acknowledgments
The authors are grateful to Ms. Traci Washington for assistance in sourcing materials; to Karen Corr, PhD, and Anthony Restuccio, MD, for advice on methods; to Mr. Daniel Pignatelli for assistance with data interpretation; and to Mr. Keith Skidmore, Ms. Crystal Conley, and Ms. Megan Harris for assistance with assembling the Viral Illness Support Packs.
1. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
2. Schroeck JL, Ruh CA, Sellick JA Jr, Ott MC, Mattappallil A, Mergenhagen KA. Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrob Agents Chemother. 2015;59(7):3848-3852. doi:10.1128/AAC.00652-15
3. Francois Watkins LK, Sanchez GV, Albert AP, Roberts RM, Hicks LA. Knowledge and attitudes regarding antibiotic use among adult consumers, adult Hispanic consumers, and health care providers—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2015;64(28):767-770. doi:10.15585/mmwr.mm6428a5
4. Centers for Disease Control and Prevention. Antibiotics Aren’t Always the Answer. Accessed June 28, 2022.www.cdc.gov/antibiotic-use/pdfs/AntibioticsArentAlwaystheAnswer-H.pdf
The negative impact of the unnecessary prescribing of antibiotic is well known. Consequences include exposing patients to antibiotic adverse effects, risk of overgrowth of pathogenetic organisms such as clostridial species, unnecessary cost of drugs, and development of selection of antibiotic-resistant organisms in the populace at large. Acute viral respiratory infections are among the leading causes of inappropriate antibiotic usage.1 In a study of 1000 adults with respiratory tract infections in an outpatient setting, 77% of patients were prescribed antibiotics, and the treatment was inappropriate in 64% of those who received prescriptions.2 Patient expectations and clinician perceptions of these expectations play a role. One study showed that 54% of clinicians felt their patients expected to receive antibiotics for a visit due to an acute respiratory infection (ARI), such as a cough or cold; 26% of patients did in fact have this expectation.3
The US Department of Veterans Affairs (VA) Central Ohio Health Care System is a large ambulatory care facility, with 4 associated community-based outpatient clinics, serving more than 43,000 central Ohio veterans and completing more than 500,000 medical appointments annually. An antimicrobial stewardship program has been in place since 2013. In May 2018, the clinical pharmacist assigned to the program alerted medical leadership that, of 67 patients seen in primary care for ARIs between April 16, 2018, and May 15, 2018, 42 (63%) had been prescribed an antibiotic. Based on this finding, clinical leadership designed a process improvement program aimed at reducing inappropriate antibiotic usage for the treatment of uncomplicated ARls likely due to viral pathogens. Key components were clinician and patient education and the substitution of a symptomatic treatment kit in place of an antibiotic prescription.
Methods
Facility clinical leadership, assisted by Volunteer Services, developed a Viral Illness Support Pack to be dispensed by primary care practitioners (PCPs) to patients presenting with symptoms of viral ARIs. The contents of this support pack are shown in the Figure. Patients were provided with tissues, throat lozenges, lip balm, acetaminophen, hand sanitizer, a surgical mask, patient instructions, and the Antibiotics Aren’t Always the Answer pamphlet.4 The contents of the viral support pack were purchased through Volunteer Services using donated funds. In total, 460 packs were distributed to the primary care patient aligned care teams (PACTs), including the community-based outpatient clinics.
Clinicians and care teams received academic detailing prior to distribution of the viral support packs, stressing the importance of avoiding antibiotics to treat viral illnesses. Viral illness support packs were available for distribution from December 1, 2018, through March 31, 2019. The frequency of antibiotic dispensing to patients coded for ARI during this period was compared with that of the same time period in the previous year. All charts were reviewed for coding accuracy. Patients with illnesses requiring antibiotic treatment, such as pneumonia, exacerbations of chronic obstructive pulmonary disease and chronic bronchitis, and streptococcal pharyngitis, were excluded from the study. Statistical significance was determined using the unpaired t test.
Results
From December 1, 2018, to March 31, 2019, 357 viral support packs were distributed to patients (Table). For the historical control period from December 1, 2017, through March 31, 2018, 508 patients were treated for ARIs. Of these, 295 (58%) received clinically inappropriate antibiotics. In contrast, of the 627 patients treated for ARIs during the study period from December 1, 2018, through March 31, 2019, 310 (49%) received clinically inappropriate antibiotics. The 9% decrease during the period when viral support packs were distributed, compared with the prior year, was statistically significant (P = .02).
Discussion
The decrease in antibiotic prescriptions for ARIs was statistically significant. The success of this project can be attributed to 3 factors: clinician education, patient education, and the option for PCPs to provide symptomatic treatment for these patients rather than prescribe an antibiotic.
The importance of antibiotic stewardship has been emphasized to all PCPs at the VA Central Ohio Health Care System. Antibiotic stewardship has been the subject of grand rounds. Prior to distribution of the viral support pack, the chief of specialty medicine, the project’s champion, spoke to all PCPs. Adequate numbers of viral support packs were distributed to all primary care teams.
In addition to direct clinician-to-patient education at the time of the patients’ visits, educational materials were included in the viral support pack. The Antibiotics Aren’t Always the Answer pamphlet is available from the Centers for Disease Control and Prevention. It covers the importance of antibiotic awareness, discusses what antibiotics do and do not treat, how to stay healthy, and causes of antibiotic resistance. The pamphlet contains the clear message that antibiotics are not only ineffective against viral illness, but also can cause significant undesirable outcomes.
The pamphlet Viral Illness Support Pack Traffic Light Card (eAppendix available online at doi:10.12788/fp.0302) provides important clinical information to the patients about their illness. Patients are instructed to contact their primary care team if they are worse after 3 days of illness; symptoms are not improving after 10 days; or they experience blood in respiratory secretions, chills or generalized aching, and localized pain that is one-sided or significantly worsening. Patients are clearly informed to seek further care if not improving with symptomatic treatment.
The ability to provide patients with symptomatic relief, including throat lozenges, lip balm, and acetaminophen, was felt to be important in the success of the project. Furthermore, this eliminated an extra step of the patient needing to visit the pharmacy.
Limitations
Limitations of the study included starting distribution of the support packs somewhat after the onset of the viral illness season, failure to reach all prescribers for academic detailing at the start of the program, and several instances of temporary unavailability of the support packs in some areas.
Conclusions
Patients with ARIs are often significantly symptomatic and frequently believe that they require an antibiotic for treatment. Clinicians may adjust their behavior in response to their patients’ expectations, stated or unstated. The results of this project demonstrate that the combination of patient education and the ready availability of a nonantibiotic symptomatic treatment option can significantly decrease the unnecessary prescribing of antibiotics for viral illnesses.
Acknowledgments
The authors are grateful to Ms. Traci Washington for assistance in sourcing materials; to Karen Corr, PhD, and Anthony Restuccio, MD, for advice on methods; to Mr. Daniel Pignatelli for assistance with data interpretation; and to Mr. Keith Skidmore, Ms. Crystal Conley, and Ms. Megan Harris for assistance with assembling the Viral Illness Support Packs.
The negative impact of the unnecessary prescribing of antibiotic is well known. Consequences include exposing patients to antibiotic adverse effects, risk of overgrowth of pathogenetic organisms such as clostridial species, unnecessary cost of drugs, and development of selection of antibiotic-resistant organisms in the populace at large. Acute viral respiratory infections are among the leading causes of inappropriate antibiotic usage.1 In a study of 1000 adults with respiratory tract infections in an outpatient setting, 77% of patients were prescribed antibiotics, and the treatment was inappropriate in 64% of those who received prescriptions.2 Patient expectations and clinician perceptions of these expectations play a role. One study showed that 54% of clinicians felt their patients expected to receive antibiotics for a visit due to an acute respiratory infection (ARI), such as a cough or cold; 26% of patients did in fact have this expectation.3
The US Department of Veterans Affairs (VA) Central Ohio Health Care System is a large ambulatory care facility, with 4 associated community-based outpatient clinics, serving more than 43,000 central Ohio veterans and completing more than 500,000 medical appointments annually. An antimicrobial stewardship program has been in place since 2013. In May 2018, the clinical pharmacist assigned to the program alerted medical leadership that, of 67 patients seen in primary care for ARIs between April 16, 2018, and May 15, 2018, 42 (63%) had been prescribed an antibiotic. Based on this finding, clinical leadership designed a process improvement program aimed at reducing inappropriate antibiotic usage for the treatment of uncomplicated ARls likely due to viral pathogens. Key components were clinician and patient education and the substitution of a symptomatic treatment kit in place of an antibiotic prescription.
Methods
Facility clinical leadership, assisted by Volunteer Services, developed a Viral Illness Support Pack to be dispensed by primary care practitioners (PCPs) to patients presenting with symptoms of viral ARIs. The contents of this support pack are shown in the Figure. Patients were provided with tissues, throat lozenges, lip balm, acetaminophen, hand sanitizer, a surgical mask, patient instructions, and the Antibiotics Aren’t Always the Answer pamphlet.4 The contents of the viral support pack were purchased through Volunteer Services using donated funds. In total, 460 packs were distributed to the primary care patient aligned care teams (PACTs), including the community-based outpatient clinics.
Clinicians and care teams received academic detailing prior to distribution of the viral support packs, stressing the importance of avoiding antibiotics to treat viral illnesses. Viral illness support packs were available for distribution from December 1, 2018, through March 31, 2019. The frequency of antibiotic dispensing to patients coded for ARI during this period was compared with that of the same time period in the previous year. All charts were reviewed for coding accuracy. Patients with illnesses requiring antibiotic treatment, such as pneumonia, exacerbations of chronic obstructive pulmonary disease and chronic bronchitis, and streptococcal pharyngitis, were excluded from the study. Statistical significance was determined using the unpaired t test.
Results
From December 1, 2018, to March 31, 2019, 357 viral support packs were distributed to patients (Table). For the historical control period from December 1, 2017, through March 31, 2018, 508 patients were treated for ARIs. Of these, 295 (58%) received clinically inappropriate antibiotics. In contrast, of the 627 patients treated for ARIs during the study period from December 1, 2018, through March 31, 2019, 310 (49%) received clinically inappropriate antibiotics. The 9% decrease during the period when viral support packs were distributed, compared with the prior year, was statistically significant (P = .02).
Discussion
The decrease in antibiotic prescriptions for ARIs was statistically significant. The success of this project can be attributed to 3 factors: clinician education, patient education, and the option for PCPs to provide symptomatic treatment for these patients rather than prescribe an antibiotic.
The importance of antibiotic stewardship has been emphasized to all PCPs at the VA Central Ohio Health Care System. Antibiotic stewardship has been the subject of grand rounds. Prior to distribution of the viral support pack, the chief of specialty medicine, the project’s champion, spoke to all PCPs. Adequate numbers of viral support packs were distributed to all primary care teams.
In addition to direct clinician-to-patient education at the time of the patients’ visits, educational materials were included in the viral support pack. The Antibiotics Aren’t Always the Answer pamphlet is available from the Centers for Disease Control and Prevention. It covers the importance of antibiotic awareness, discusses what antibiotics do and do not treat, how to stay healthy, and causes of antibiotic resistance. The pamphlet contains the clear message that antibiotics are not only ineffective against viral illness, but also can cause significant undesirable outcomes.
The pamphlet Viral Illness Support Pack Traffic Light Card (eAppendix available online at doi:10.12788/fp.0302) provides important clinical information to the patients about their illness. Patients are instructed to contact their primary care team if they are worse after 3 days of illness; symptoms are not improving after 10 days; or they experience blood in respiratory secretions, chills or generalized aching, and localized pain that is one-sided or significantly worsening. Patients are clearly informed to seek further care if not improving with symptomatic treatment.
The ability to provide patients with symptomatic relief, including throat lozenges, lip balm, and acetaminophen, was felt to be important in the success of the project. Furthermore, this eliminated an extra step of the patient needing to visit the pharmacy.
Limitations
Limitations of the study included starting distribution of the support packs somewhat after the onset of the viral illness season, failure to reach all prescribers for academic detailing at the start of the program, and several instances of temporary unavailability of the support packs in some areas.
Conclusions
Patients with ARIs are often significantly symptomatic and frequently believe that they require an antibiotic for treatment. Clinicians may adjust their behavior in response to their patients’ expectations, stated or unstated. The results of this project demonstrate that the combination of patient education and the ready availability of a nonantibiotic symptomatic treatment option can significantly decrease the unnecessary prescribing of antibiotics for viral illnesses.
Acknowledgments
The authors are grateful to Ms. Traci Washington for assistance in sourcing materials; to Karen Corr, PhD, and Anthony Restuccio, MD, for advice on methods; to Mr. Daniel Pignatelli for assistance with data interpretation; and to Mr. Keith Skidmore, Ms. Crystal Conley, and Ms. Megan Harris for assistance with assembling the Viral Illness Support Packs.
1. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
2. Schroeck JL, Ruh CA, Sellick JA Jr, Ott MC, Mattappallil A, Mergenhagen KA. Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrob Agents Chemother. 2015;59(7):3848-3852. doi:10.1128/AAC.00652-15
3. Francois Watkins LK, Sanchez GV, Albert AP, Roberts RM, Hicks LA. Knowledge and attitudes regarding antibiotic use among adult consumers, adult Hispanic consumers, and health care providers—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2015;64(28):767-770. doi:10.15585/mmwr.mm6428a5
4. Centers for Disease Control and Prevention. Antibiotics Aren’t Always the Answer. Accessed June 28, 2022.www.cdc.gov/antibiotic-use/pdfs/AntibioticsArentAlwaystheAnswer-H.pdf
1. Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164(6):425-434. doi:10.7326/M15-1840
2. Schroeck JL, Ruh CA, Sellick JA Jr, Ott MC, Mattappallil A, Mergenhagen KA. Factors associated with antibiotic misuse in outpatient treatment for upper respiratory tract infections. Antimicrob Agents Chemother. 2015;59(7):3848-3852. doi:10.1128/AAC.00652-15
3. Francois Watkins LK, Sanchez GV, Albert AP, Roberts RM, Hicks LA. Knowledge and attitudes regarding antibiotic use among adult consumers, adult Hispanic consumers, and health care providers—United States, 2012-2013. MMWR Morb Mortal Wkly Rep. 2015;64(28):767-770. doi:10.15585/mmwr.mm6428a5
4. Centers for Disease Control and Prevention. Antibiotics Aren’t Always the Answer. Accessed June 28, 2022.www.cdc.gov/antibiotic-use/pdfs/AntibioticsArentAlwaystheAnswer-H.pdf
Postdeployment Respiratory Health: The Roles of the Airborne Hazards and Open Burn Pit Registry and the Post-Deployment Cardiopulmonary Evaluation Network
Case Example
A 37-year-old female never smoker presents to your clinic with progressive dyspnea over the past 15 years. She reports dyspnea on exertion, wheezing, chronic nasal congestion, and difficulty sleeping that started a year after she returned from military deployment to Iraq. She has been unable to exercise, even at low intensity, for the past 5 years, despite being previously active. She has experienced some symptom improvement by taking an albuterol inhaler as needed, loratadine (10 mg), and fluticasone nasal spray (50 mcg). She occasionally uses famotidine for reflux (40 mg). She deployed to Southwest Asia for 12 months (2002-2003) and was primarily stationed in Qayyarah West, an Air Force base in the Mosul district in northern Iraq. She reports exposure during deployment to the fire in the Al-Mishraq sulfur mine, located approximately 25 km north of Qayyarah West, as well as dust storms and burn pits. She currently works as a medical assistant. Her examination is remarkable for normal bronchovesicular breath sounds without any wheezing or crackles on pulmonary evaluation. Her body mass index is 31. You obtain a chest radiograph and spirometry, which are both normal.
The veteran reports feeling frustrated as she has had multiple specialty evaluations in community clinics without receiving a diagnosis, despite worsening symptoms. She reports that she added her information to the Airborne Hazards and Open Burn Pit Registry (AHOBPR). She recently received a letter from the US Department of Veterans Affairs (VA) Post-Deployment Cardiopulmonary Evaluation Network (PDCEN) and is asking you whether she should participate in the PDCEN specialty evaluation. You are not familiar with the military experiences she has described or the programs she asks you about; however, you would like to know more to best care for your patient.
Background
The year 2021 marked the 20th anniversary of the September 11 attacks and the launch of the Global War on Terrorism. Almost 3 million US military personnel have been deployed in support of these operations along with about 300,000 US civilian contractors and thousands of troops from more than 40 nations.1-3
Respiratory hazards associated with deployment to Southwest Asia and Afghanistan are unique and varied. These exposures include blast injuries and a variety of particulate matter sources, such as burn pit combustion byproducts, aeroallergens, and dust storms.7,8,15,16 One air sampling study conducted at 15 deployment sites in Southwest Asia and Afghanistan found mean fine particulate matter (PM2.5) levels were as much as 10 times greater than sampling sites in both rural and urban cities in the United States; all sites sampled exceeded military exposure guidelines (65 µg/m3 for 1 year).17,18 Long-term exposure to PM2.5 has been associated with the development of chronic respiratory and cardiovascular disease; therefore, there has been considerable attention to the respiratory (and nonrespiratory) health of deployed military personnel.19
Concerns regarding the association between deployment and lung disease led to the creation of the national VA Airborne Hazards and Open Burn Pit Registry (AHOBPR) in 2014 and consists of (1) an online questionnaire to document deployment and medical history, exposure concerns, and symptoms; and (2) an optional in-person or virtual clinical health evaluation at the individual’s local VA medical center or military treatment facility (MTF). As of March 2022, more than 300,000 individuals have completed the online questionnaire of which about 30% declined the optional clinical health evaluation.
The clinical evaluation available to AHOBPR participants has not yet been described in the literature. Therefore, our objectives are to examine AHOBPR clinical evaluation data and review its application throughout the VA. In addition, we will also describe a parallel effort by the VA PDCEN, which is to provide comprehensive multiday clinical evaluations for unique AHOBPR participants with unexplained dyspnea and self-reported respiratory disease. A secondary aim of this publication is to disseminate information to health care professionals (HCPs) within and outside of the VA to aid in the referral and evaluation of previously deployed veterans who experience unexplained dyspnea.
AHOBPR Overview
The AHOBPR is an online questionnaire and optional in-person health evaluation that includes 7 major categories targeting deployment history, symptoms, medical history, health concerns, residential history, nonmilitary occupational history, nonmilitary environmental exposures, and health care utilization. The VA Defense Information Repository is used to obtain service dates for the service member/veteran, conflict involvement, and primary location during deployment. The questionnaire portion of the AHOBPR is administered online. It currently is open to all veterans who served in the Southwest Asia theater of operations (including Iraq, Kuwait, and Egypt) any time after August 2, 1990, or Afghanistan, Djibouti, Syria, or Uzbekistan after September 11, 2001. Veterans are eligible for completing the AHOBPR and optional health evaluation at no cost to the veteran regardless of VA benefits or whether they are currently enrolled in VA health care. Though the focus of the present manuscript is to profile a VA program, it is important to note that the US Department of Defense (DoD) is an active partner with the VA in the promotion of the AHOBPR to service members and similarly provides health evaluations for active-duty service members (including activating Reserve and Guard) through their local MTF.
We reviewed and analyzed AHOBPR operations and VA data from 2014 to 2020. Our analyses were limited to veterans seeking evaluation as well as their corresponding symptoms and HCP’s clinical impression from the electronic health record. As of September 20, 2021, 267,125 individuals completed the AHOBPR. The mean age was 43 years (range, 19-84), and the majority were male (86%) and served in the Army (58%). Open-air burn pits (91%), engine maintenance (38.8%), and convoy operations (71.7%) were the most common deployment-related exposures.
The optional in-person AHOBPR health evaluation may be requested by the veteran after completing the online questionnaire and is performed at the veteran’s local VA facility. The evaluation is most often completed by an environmental health clinician or primary care practitioner (PCP). A variety of resources are available to providers for training on this topic, including fact sheets, webinars, monthly calls, conferences, and accredited e-learning.20 As part of the clinical evaluation, the veteran’s chief concerns are assessed and evaluated. At the time of our analysis, 24,578 clinical examinations were performed across 126 VA medical facilities, with considerable geographic variation. Veterans receiving evaluations were predominantly male (89%) with a median age of 46.0 years (IQR, 15). Veterans’ major respiratory concerns included dyspnea (45.1%), decreased exercise ability (34.8%), and cough > 3 weeks (30.3%) (Table). After clinical evaluation by a VA or MTF HCP, 47.8% were found to have a respiratory diagnosis, including asthma (30.1%), COPD (12.8%), and bronchitis (11.9%).
Registry participants who opt to receive the clinical evaluation may benefit directly by undergoing a detailed clinical history and physical examination as well as having the opportunity to document their health concerns. For some, clinicians may need to refer veterans for additional specialty testing beyond this standard AHOBPR clinical evaluation. Although these evaluations can help address some of the veterans’ concerns, a substantial number may have unexplained respiratory symptoms that warrant further investigation.
Post-Deployment Cardiopulmonary Evaluation Network Clinical Evaluation
In May 2019, the VA established the Airborne Hazards and Burn Pits Center of Excellence (AHBPCE). One of the AHBPCE’s objectives is to deliver specialized care and consultation for veterans with concerns about their postdeployment health, including, but not limited to, unexplained dyspnea. To meet this objective, the AHBPCE developed the PDCEN, a national network consisting of specialty HCPs from 5 VA medical centers—located in San Francisco, California; Denver, Colorado; Baltimore, Maryland; Ann Arbor, Michigan; and East Orange, New Jersey. Collectively, the PDCEN has developed a standardized approach for the comprehensive clinical evaluation of unexplained dyspnea that is implemented uniformly across sites. Staff at the PDCEN screen the AHOBPR to identify veterans with features of respiratory disease and invite them to participate in an in-person evaluation at the nearest PDCEN site. Given the specialty expertise (detailed below) within the Network, the PDCEN focuses on complex cases that are resource intensive. To address complex cases of unexplained dyspnea, the PDCEN has developed a core clinical evaluation approach (Figure).
The first step in a veteran’s PDCEN evaluation entails a set of detailed questionnaires that request information about the veteran’s current respiratory, sleep, and mental health symptoms and any associated medical diagnoses. Questionnaires also identify potential exposures to military burn pits, sulfur mine and oil field fires, diesel exhaust fumes, dust storms, urban pollution, explosions/blasts, and chemical weapons. In addition, the questionnaires include deployment geographic location, which may inform future estimates of particulate matter exposure.21 Prior VA and non-VA evaluations and testing of their respiratory concerns are obtained for review. Exposure and health records from the DoD are also reviewed when available.
The next step in the PDCEN evaluation comprises comprehensive testing, including complete pulmonary function testing, methacholine challenge, cardiopulmonary exercise testing, forced oscillometry and exhaled nitric oxide testing, paired high-resolution inspiratory and expiratory chest computed tomography (CT) imaging, sinus CT imaging, direct flexible laryngoscopy, echocardiography, polysomnography, and laboratory blood testing. The testing process is managed by local site coordinators and varies by institution based on availability of each testing modality and subspecialist appointments.
Once testing is completed, the veteran is evaluated by a team of HCPs, including physicians from the disciplines of pulmonary medicine, environmental and occupational health, sleep medicine, otolaryngology and speech pathology, and mental health (when appropriate). After the clinical evaluation has been completed, this team of expert HCPs at each site convenes to provide a final summary review visit intended to be a comprehensive assessment of the veteran’s primary health concerns. The 3 primary objectives of this final review are to inform the veteran of (1) what respiratory and related conditions they have; (2) whether the conditions is/are deployment related; and (3) what treatments and/or follow-up care may enhance their current state of health in partnership with their local HCPs. The PDCEN does not provide ongoing management of any conditions identified during the veteran’s evaluation but communicates findings and recommendations to the veteran and their PCP for long-term care.
Discussion
The AHOBPR was established in response to mounting concerns that service members and veterans were experiencing adverse health effects that might be attributable to deployment-related exposures. Nearly half of all patients currently enrolled in the AHOBPR report dyspnea, and about one-third have decreased exercise tolerance and/or cough. Of those who completed the questionnaire and the subsequent in-person and generalized AHOBPR examination, our interim analysis showed that about half were assigned a respiratory diagnosis. Yet for many veterans, their breathing symptoms remained unexplained or did not respond to treatment.
While the AHOBPR and related examinations address the needs of many veterans, others may require more comprehensive examination. The PDCEN attends to the latter by providing more detailed and comprehensive clinical evaluations of veterans with deployment-related respiratory health concerns and seeks to learn from these evaluations by analyzing data obtained from veterans across sites. As such, the PDCEN hopes not only to improve the health of individual veterans, but also create standard practices for both VA and non-VA community evaluation of veterans exposed to respiratory hazards during deployment.
One of the major challenges in the field of postdeployment respiratory health is the lack of clear universal language or case definitions that encompass the veteran’s clinical concerns. In an influential case series published in 2011, 38 (77%) of 49 soldiers with history of airborne hazard exposure and unexplained exercise intolerance were reported to have histopathology consistent with constrictive bronchiolitis on surgical lung biopsy.14 Subsequent publications have described other histopathologic features in deployed military personnel, including granulomatous inflammation, interstitial lung disease, emphysema, and pleuritis.12-14 Reconciling these findings from surgical lung biopsy with the clinical presentation and noninvasive studies has proved difficult. Therefore, several groups of investigators have proposed terms, including postdeployment respiratory syndrome, deployment-related distal lung disease, and Iraq/Afghanistan War lung injury to describe the increased respiratory symptoms and variety of histopathologic and imaging findings in this population.9,12,22 At present, there remains a lack of consensus on terminology and case definitions as well as the role of military environmental exposures in exacerbating and/or causing these conditions. As HCPs, it is important to appreciate and acknowledge that the ambiguity and controversy pertaining to terminology, causation, and service connections are a common source of frustration experienced by veterans, which are increasingly reflected among reports in popular media and lay press.
A second and related challenge in the field of postdeployment respiratory health that contributes to veteran and HCP frustration is that many of the aforementioned abnormalities described on surgical lung biopsy are not readily identifiable on noninvasive tests, including traditional interpretation of pulmonary function tests or chest CT imaging.12-14,22 Thus, underlying conditions could be overlooked and veterans’ concerns and symptoms may be dismissed or misattributed to other comorbid conditions. While surgical lung biopsies may offer diagnostic clarity in identifying lung disease, there are significant procedural risks of surgical and anesthetic complications. Furthermore, a definitive diagnosis does not necessarily guarantee a clear treatment plan. For example, there are no current therapies approved by the US Food and Drug Administration for the treatment of constrictive bronchiolitis.
Research efforts are underway, including within the PDCEN, to evaluate a more sensitive and noninvasive assessment of the small airways that may even reduce or eliminate the need for surgical lung biopsy. In contrast to traditional pulmonary function testing, which is helpful for evaluation of the larger airways, forced oscillation technique can be used noninvasively, using pressure oscillations to evaluate for diseases of the smaller airways and has been used in the veteran population and in those exposed to dust from the World Trade Center disaster.23-25 Multiple breath washout technique provides a lung clearance index that is determined by the number of lung turnovers it takes to clear the lungs of an inert gas (eg, sulfur hexafluoride, nitrogen). Elevated lung clearance index values suggest ventilation heterogeneity and have been shown to be higher among deployed veterans with dyspnea.26,27 Finally, advanced CT analytic techniques may help identify functional small airways disease and are higher in deployed service members with constrictive bronchiolitis on surgical lung biopsy.28 These innovative noninvasive techniques are experimental but promising, especially as part of a broader evaluation of small airways disease.
AHOBPR clinical evaluations represent an initial step to better understand postdeployment health conditions available to all AHOBPR participants. The PDCEN clinical evaluation extends the AHOBPR evaluation by providing specialty care for certain veterans requiring more comprehensive evaluation while systematically collecting and analyzing clinical data to advance the field. The VA is committed to leveraging these data and all available expertise to provide a clear description of the spectrum of disease in this population and improve our ability to diagnose, follow, and treat respiratory health conditions occurring after deployment to Southwest Asia and Afghanistan.
Case Conclusion
The veteran was referred to a PDCEN site and underwent a comprehensive multidisciplinary evaluation. Pulmonary function testing showed lung volumes and vital capacity within the predicted normal range, mild air trapping, and a low diffusion capacity for carbon monoxide. Methacholine challenge testing was normal; however, forced oscillometry suggested small airways obstruction. A high-resolution CT showed air trapping without parenchymal changes. Cardiopulmonary exercise testing demonstrated a peak exercise capacity within the predicted normal range but low breathing reserve. Otolaryngology evaluation including laryngoscopy suggested chronic nonallergic rhinitis.
At the end of the veteran’s evaluation, a summary review reported nonallergic rhinitis and distal airway obstruction consistent with small airways disease. Both were reported as most likely related to deployment given her significant environmental exposures and the temporal relationship with her deployment and symptom onset as well as lack of other identifiable causes. A more precise histopathologic diagnosis could be firmly established with a surgical lung biopsy, but after shared decision making with a PDCEN HCP, the patient declined to undergo this invasive procedure. After you review the summary review and recommendations from the PDCEN group, you start the veteran on intranasal steroids and a combined inhaled corticosteroid/long-acting β agonist inhaler as well as refer the veteran to pulmonary rehabilitation. After several weeks, she reports an improvement in sleep and nasal symptoms but continues to experience residual exercise intolerance.
This case serves as an example of the significant limitations that a previously active and healthy patient can develop after deployment to Southwest Asia and Afghanistan. Encouraging this veteran to complete the AHOBPR allowed her to be considered for a PDCEN evaluation that provided the opportunity to undergo a comprehensive noninvasive evaluation of her chronic dyspnea. In doing so, she obtained 2 important diagnoses and data from her evaluation will help establish best practices for standardized evaluations of respiratory concerns following deployment. Through the AHOBPR and PDCEN, the VA seeks to better understand postdeployment health conditions, their relationship to military and environmental exposures, and how best to diagnose and treat these conditions.
Acknowledgments
This work was supported by the US Department of Veterans Affairs (VA) Airborne Hazards and Burn Pits Center of Excellence (Public Law 115-929). The authors acknowledge support and contributions from Dr. Eric Shuping and leadership at VA’s Health Outcomes Military Exposures office as well as the New Jersey War Related Illness and Injury Study Center. In addition, we thank Erin McRoberts and Rajeev Swarup for their contributions to the Post-Deployment Cardiopulmonary Evaluation Network. Post-Deployment Cardiopulmonary Evaluation Network members:
Mehrdad Arjomandi, Caroline Davis, Michelle DeLuca, Nancy Eager, Courtney A. Eberhardt, Michael J. Falvo, Timothy Foley, Fiona A.S. Graff, Deborah Heaney, Stella E. Hines, Rachel E. Howard, Nisha Jani, Sheena Kamineni, Silpa Krefft, Mary L. Langlois, Helen Lozier, Simran K. Matharu, Anisa Moore, Lydia Patrick-DeLuca, Edward Pickering, Alexander Rabin, Michelle Robertson, Samantha L. Rogers, Aaron H. Schneider, Anand Shah, Anays Sotolongo, Jennifer H. Therkorn, Rebecca I. Toczylowski, Matthew Watson, Alison D. Wilczynski, Ian W. Wilson, Romi A. Yount.
1. Wenger J, O’Connell C, Cottrell L. Examination of recent deployment experience across the services and components. Exam. RAND Corporation; 2018. Accessed June 27, 2022. doi:10.7249/rr1928
2. Torreon BS. U.S. periods of war and dates of recent conflicts, RS21405. Congressional Research Service; 2017. June 5, 2020. Accessed June 27, 2022. https://crsreports.congress.gov/product/details?prodcode=RS21405
3. Dunigan M, Farmer CM, Burns RM, Hawks A, Setodji CM. Out of the shadows: the health and well-being of private contractors working in conflict environments. RAND Corporation; 2013. Accessed June 27, 2022. https://www.rand.org/pubs/research_reports/RR420.html
4. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71. doi:10.2500/aap.2010.31.3383
5. Pugh MJ, Jaramillo CA, Leung KW, et al. Increasing prevalence of chronic lung disease in veterans of the wars in Iraq and Afghanistan. Mil Med. 2016;181(5):476-481. doi:10.7205/MILMED-D-15-00035
6. Falvo MJ, Osinubi OY, Sotolongo AM, Helmer DA. Airborne hazards exposure and respiratory health of Iraq and Afghanistan veterans. Epidemiol Rev. 2015;37:116-130. doi:10.1093/epirev/mxu009
7. McAndrew LM, Teichman RF, Osinubi OY, Jasien JV, Quigley KS. Environmental exposure and health of Operation Enduring Freedom/Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):665-669. doi:10.1097/JOM.0b013e318255ba1b
8. Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD; Margaret A. K. Ryan for the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170(11):1433-1442. doi:10.1093/aje/kwp287
9. Szema AM, Salihi W, Savary K, Chen JJ. Respiratory symptoms necessitating spirometry among soldiers with Iraq/Afghanistan war lung injury. J Occup Environ Med. 2011;53(9):961-965. doi:10.1097/JOM.0b013e31822c9f05
10. Committee on the Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan; Institute of Medicine. Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan. The National Academies Press; 2011. Accessed June 27, 2022. doi:10.17226/1320911. National Academies of Sciences, Engineering, and Medicine. Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. The National Academies Press; 2020. Accessed June 27, 2022. doi:10.17226/25837
12. Krefft SD, Wolff J, Zell-Baran L, et al. Respiratory diseases in post-9/11 military personnel following Southwest Asia deployment. J Occup Environ Med. 2020;62(5):337-343. doi:10.1097/JOM.0000000000001817
13. Gordetsky J, Kim C, Miller RF, Mehrad M. Non-necrotizing granulomatous pneumonitis and chronic pleuritis in soldiers deployed to Southwest Asia. Histopathology. 2020;77(3):453-459. doi:10.1111/his.14135
14. King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365(3):222-230. doi:10.1056/NEJMoa1101388
15. Helmer DA, Rossignol M, Blatt M, Agarwal R, Teichman R, Lange G. Health and exposure concerns of veterans deployed to Iraq and Afghanistan. J Occup Environ Med. 2007;49(5):475-480. doi:10.1097/JOM.0b013e318042d682
16. Kim YH, Warren SH, Kooter I, et al. Chemistry, lung toxicity and mutagenicity of burn pit smoke-related particulate matter. Part Fibre Toxicol. 2021;18(1):45. Published 2021 Dec 16. doi:10.1186/s12989-021-00435-w
17. Engelbrecht JP, McDonald EV, Gillies JA, Jayanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East—Part 1: ambient sampling. Inhal Toxicol. 2009;21(4):297-326. doi:10.1080/08958370802464273
18. US Army Public Health Command. Technical guide 230: environmental health risk assessment and chemical exposure guidelines for deployed military personnel, 2013 revision. Accessed June 27, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/TG230-DeploymentEHRA-and-MEGs-2013-Revision.pdf
19. Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol. 2012;8(2):166-175. doi:10.1007/s13181-011-0203-1
20. Shuping E, Schneiderman A. Resources on environmental exposures for military veterans. Am Fam Physician. 2020;101(12):709-710.
21. Masri S, Garshick E, Coull BA, Koutrakis P. A novel calibration approach using satellite and visibility observations to estimate fine particulate matter exposures in Southwest Asia and Afghanistan. J Air Waste Manag Assoc. 2017;67(1):86-95. doi:10.1080/10962247.2016.1230079
22. Gutor SS, Richmond BW, Du RH, et al. Postdeployment respiratory syndrome in soldiers with chronic exertional dyspnea. Am J Surg Pathol. 2021;45(12):1587-1596. doi:10.1097/PAS.0000000000001757
23. Goldman MD, Saadeh C, Ross D. Clinical applications of forced oscillation to assess peripheral airway function. Respir Physiol Neurobiol. 2005;148(1-2):179-194. doi:10.1016/j.resp.2005.05.026
24. Butzko RP, Sotolongo AM, Helmer DA, et al. Forced oscillation technique in veterans with preserved spirometry and chronic respiratory symptoms. Respir Physiol Neurobiol. 2019;260:8-16. doi:10.1016/j.resp.2018.11.012
25. Oppenheimer BW, Goldring RM, Herberg ME, et al. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest. 2007;132(4):1275-1282. doi:10.1378/chest.07-0913
26. Zell-Baran LM, Krefft SD, Moore CM, Wolff J, Meehan R, Rose CS. Multiple breath washout: a noninvasive tool for identifying lung disease in symptomatic military deployers. Respir Med. 2021;176:106281. doi:10.1016/j.rmed.2020.106281
27. Krefft SD, Strand M, Smith J, Stroup C, Meehan R, Rose C. Utility of lung clearance index testing as a noninvasive marker of deployment-related lung disease. J Occup Environ Med. 2017;59(8):707-711. doi:10.1097/JOM.000000000000105828. Davis CW, Lopez CL, Bell AJ, et al. The severity of functional small airways disease in military personnel with constrictive bronchiolitis as measured by quantitative CT [published online ahead of print, 2022 May 24]. Am J Respir Crit Care Med. 2022;10.1164/rccm.202201-0153LE. doi:10.1164/rccm.202201-0153LE
Case Example
A 37-year-old female never smoker presents to your clinic with progressive dyspnea over the past 15 years. She reports dyspnea on exertion, wheezing, chronic nasal congestion, and difficulty sleeping that started a year after she returned from military deployment to Iraq. She has been unable to exercise, even at low intensity, for the past 5 years, despite being previously active. She has experienced some symptom improvement by taking an albuterol inhaler as needed, loratadine (10 mg), and fluticasone nasal spray (50 mcg). She occasionally uses famotidine for reflux (40 mg). She deployed to Southwest Asia for 12 months (2002-2003) and was primarily stationed in Qayyarah West, an Air Force base in the Mosul district in northern Iraq. She reports exposure during deployment to the fire in the Al-Mishraq sulfur mine, located approximately 25 km north of Qayyarah West, as well as dust storms and burn pits. She currently works as a medical assistant. Her examination is remarkable for normal bronchovesicular breath sounds without any wheezing or crackles on pulmonary evaluation. Her body mass index is 31. You obtain a chest radiograph and spirometry, which are both normal.
The veteran reports feeling frustrated as she has had multiple specialty evaluations in community clinics without receiving a diagnosis, despite worsening symptoms. She reports that she added her information to the Airborne Hazards and Open Burn Pit Registry (AHOBPR). She recently received a letter from the US Department of Veterans Affairs (VA) Post-Deployment Cardiopulmonary Evaluation Network (PDCEN) and is asking you whether she should participate in the PDCEN specialty evaluation. You are not familiar with the military experiences she has described or the programs she asks you about; however, you would like to know more to best care for your patient.
Background
The year 2021 marked the 20th anniversary of the September 11 attacks and the launch of the Global War on Terrorism. Almost 3 million US military personnel have been deployed in support of these operations along with about 300,000 US civilian contractors and thousands of troops from more than 40 nations.1-3
Respiratory hazards associated with deployment to Southwest Asia and Afghanistan are unique and varied. These exposures include blast injuries and a variety of particulate matter sources, such as burn pit combustion byproducts, aeroallergens, and dust storms.7,8,15,16 One air sampling study conducted at 15 deployment sites in Southwest Asia and Afghanistan found mean fine particulate matter (PM2.5) levels were as much as 10 times greater than sampling sites in both rural and urban cities in the United States; all sites sampled exceeded military exposure guidelines (65 µg/m3 for 1 year).17,18 Long-term exposure to PM2.5 has been associated with the development of chronic respiratory and cardiovascular disease; therefore, there has been considerable attention to the respiratory (and nonrespiratory) health of deployed military personnel.19
Concerns regarding the association between deployment and lung disease led to the creation of the national VA Airborne Hazards and Open Burn Pit Registry (AHOBPR) in 2014 and consists of (1) an online questionnaire to document deployment and medical history, exposure concerns, and symptoms; and (2) an optional in-person or virtual clinical health evaluation at the individual’s local VA medical center or military treatment facility (MTF). As of March 2022, more than 300,000 individuals have completed the online questionnaire of which about 30% declined the optional clinical health evaluation.
The clinical evaluation available to AHOBPR participants has not yet been described in the literature. Therefore, our objectives are to examine AHOBPR clinical evaluation data and review its application throughout the VA. In addition, we will also describe a parallel effort by the VA PDCEN, which is to provide comprehensive multiday clinical evaluations for unique AHOBPR participants with unexplained dyspnea and self-reported respiratory disease. A secondary aim of this publication is to disseminate information to health care professionals (HCPs) within and outside of the VA to aid in the referral and evaluation of previously deployed veterans who experience unexplained dyspnea.
AHOBPR Overview
The AHOBPR is an online questionnaire and optional in-person health evaluation that includes 7 major categories targeting deployment history, symptoms, medical history, health concerns, residential history, nonmilitary occupational history, nonmilitary environmental exposures, and health care utilization. The VA Defense Information Repository is used to obtain service dates for the service member/veteran, conflict involvement, and primary location during deployment. The questionnaire portion of the AHOBPR is administered online. It currently is open to all veterans who served in the Southwest Asia theater of operations (including Iraq, Kuwait, and Egypt) any time after August 2, 1990, or Afghanistan, Djibouti, Syria, or Uzbekistan after September 11, 2001. Veterans are eligible for completing the AHOBPR and optional health evaluation at no cost to the veteran regardless of VA benefits or whether they are currently enrolled in VA health care. Though the focus of the present manuscript is to profile a VA program, it is important to note that the US Department of Defense (DoD) is an active partner with the VA in the promotion of the AHOBPR to service members and similarly provides health evaluations for active-duty service members (including activating Reserve and Guard) through their local MTF.
We reviewed and analyzed AHOBPR operations and VA data from 2014 to 2020. Our analyses were limited to veterans seeking evaluation as well as their corresponding symptoms and HCP’s clinical impression from the electronic health record. As of September 20, 2021, 267,125 individuals completed the AHOBPR. The mean age was 43 years (range, 19-84), and the majority were male (86%) and served in the Army (58%). Open-air burn pits (91%), engine maintenance (38.8%), and convoy operations (71.7%) were the most common deployment-related exposures.
The optional in-person AHOBPR health evaluation may be requested by the veteran after completing the online questionnaire and is performed at the veteran’s local VA facility. The evaluation is most often completed by an environmental health clinician or primary care practitioner (PCP). A variety of resources are available to providers for training on this topic, including fact sheets, webinars, monthly calls, conferences, and accredited e-learning.20 As part of the clinical evaluation, the veteran’s chief concerns are assessed and evaluated. At the time of our analysis, 24,578 clinical examinations were performed across 126 VA medical facilities, with considerable geographic variation. Veterans receiving evaluations were predominantly male (89%) with a median age of 46.0 years (IQR, 15). Veterans’ major respiratory concerns included dyspnea (45.1%), decreased exercise ability (34.8%), and cough > 3 weeks (30.3%) (Table). After clinical evaluation by a VA or MTF HCP, 47.8% were found to have a respiratory diagnosis, including asthma (30.1%), COPD (12.8%), and bronchitis (11.9%).
Registry participants who opt to receive the clinical evaluation may benefit directly by undergoing a detailed clinical history and physical examination as well as having the opportunity to document their health concerns. For some, clinicians may need to refer veterans for additional specialty testing beyond this standard AHOBPR clinical evaluation. Although these evaluations can help address some of the veterans’ concerns, a substantial number may have unexplained respiratory symptoms that warrant further investigation.
Post-Deployment Cardiopulmonary Evaluation Network Clinical Evaluation
In May 2019, the VA established the Airborne Hazards and Burn Pits Center of Excellence (AHBPCE). One of the AHBPCE’s objectives is to deliver specialized care and consultation for veterans with concerns about their postdeployment health, including, but not limited to, unexplained dyspnea. To meet this objective, the AHBPCE developed the PDCEN, a national network consisting of specialty HCPs from 5 VA medical centers—located in San Francisco, California; Denver, Colorado; Baltimore, Maryland; Ann Arbor, Michigan; and East Orange, New Jersey. Collectively, the PDCEN has developed a standardized approach for the comprehensive clinical evaluation of unexplained dyspnea that is implemented uniformly across sites. Staff at the PDCEN screen the AHOBPR to identify veterans with features of respiratory disease and invite them to participate in an in-person evaluation at the nearest PDCEN site. Given the specialty expertise (detailed below) within the Network, the PDCEN focuses on complex cases that are resource intensive. To address complex cases of unexplained dyspnea, the PDCEN has developed a core clinical evaluation approach (Figure).
The first step in a veteran’s PDCEN evaluation entails a set of detailed questionnaires that request information about the veteran’s current respiratory, sleep, and mental health symptoms and any associated medical diagnoses. Questionnaires also identify potential exposures to military burn pits, sulfur mine and oil field fires, diesel exhaust fumes, dust storms, urban pollution, explosions/blasts, and chemical weapons. In addition, the questionnaires include deployment geographic location, which may inform future estimates of particulate matter exposure.21 Prior VA and non-VA evaluations and testing of their respiratory concerns are obtained for review. Exposure and health records from the DoD are also reviewed when available.
The next step in the PDCEN evaluation comprises comprehensive testing, including complete pulmonary function testing, methacholine challenge, cardiopulmonary exercise testing, forced oscillometry and exhaled nitric oxide testing, paired high-resolution inspiratory and expiratory chest computed tomography (CT) imaging, sinus CT imaging, direct flexible laryngoscopy, echocardiography, polysomnography, and laboratory blood testing. The testing process is managed by local site coordinators and varies by institution based on availability of each testing modality and subspecialist appointments.
Once testing is completed, the veteran is evaluated by a team of HCPs, including physicians from the disciplines of pulmonary medicine, environmental and occupational health, sleep medicine, otolaryngology and speech pathology, and mental health (when appropriate). After the clinical evaluation has been completed, this team of expert HCPs at each site convenes to provide a final summary review visit intended to be a comprehensive assessment of the veteran’s primary health concerns. The 3 primary objectives of this final review are to inform the veteran of (1) what respiratory and related conditions they have; (2) whether the conditions is/are deployment related; and (3) what treatments and/or follow-up care may enhance their current state of health in partnership with their local HCPs. The PDCEN does not provide ongoing management of any conditions identified during the veteran’s evaluation but communicates findings and recommendations to the veteran and their PCP for long-term care.
Discussion
The AHOBPR was established in response to mounting concerns that service members and veterans were experiencing adverse health effects that might be attributable to deployment-related exposures. Nearly half of all patients currently enrolled in the AHOBPR report dyspnea, and about one-third have decreased exercise tolerance and/or cough. Of those who completed the questionnaire and the subsequent in-person and generalized AHOBPR examination, our interim analysis showed that about half were assigned a respiratory diagnosis. Yet for many veterans, their breathing symptoms remained unexplained or did not respond to treatment.
While the AHOBPR and related examinations address the needs of many veterans, others may require more comprehensive examination. The PDCEN attends to the latter by providing more detailed and comprehensive clinical evaluations of veterans with deployment-related respiratory health concerns and seeks to learn from these evaluations by analyzing data obtained from veterans across sites. As such, the PDCEN hopes not only to improve the health of individual veterans, but also create standard practices for both VA and non-VA community evaluation of veterans exposed to respiratory hazards during deployment.
One of the major challenges in the field of postdeployment respiratory health is the lack of clear universal language or case definitions that encompass the veteran’s clinical concerns. In an influential case series published in 2011, 38 (77%) of 49 soldiers with history of airborne hazard exposure and unexplained exercise intolerance were reported to have histopathology consistent with constrictive bronchiolitis on surgical lung biopsy.14 Subsequent publications have described other histopathologic features in deployed military personnel, including granulomatous inflammation, interstitial lung disease, emphysema, and pleuritis.12-14 Reconciling these findings from surgical lung biopsy with the clinical presentation and noninvasive studies has proved difficult. Therefore, several groups of investigators have proposed terms, including postdeployment respiratory syndrome, deployment-related distal lung disease, and Iraq/Afghanistan War lung injury to describe the increased respiratory symptoms and variety of histopathologic and imaging findings in this population.9,12,22 At present, there remains a lack of consensus on terminology and case definitions as well as the role of military environmental exposures in exacerbating and/or causing these conditions. As HCPs, it is important to appreciate and acknowledge that the ambiguity and controversy pertaining to terminology, causation, and service connections are a common source of frustration experienced by veterans, which are increasingly reflected among reports in popular media and lay press.
A second and related challenge in the field of postdeployment respiratory health that contributes to veteran and HCP frustration is that many of the aforementioned abnormalities described on surgical lung biopsy are not readily identifiable on noninvasive tests, including traditional interpretation of pulmonary function tests or chest CT imaging.12-14,22 Thus, underlying conditions could be overlooked and veterans’ concerns and symptoms may be dismissed or misattributed to other comorbid conditions. While surgical lung biopsies may offer diagnostic clarity in identifying lung disease, there are significant procedural risks of surgical and anesthetic complications. Furthermore, a definitive diagnosis does not necessarily guarantee a clear treatment plan. For example, there are no current therapies approved by the US Food and Drug Administration for the treatment of constrictive bronchiolitis.
Research efforts are underway, including within the PDCEN, to evaluate a more sensitive and noninvasive assessment of the small airways that may even reduce or eliminate the need for surgical lung biopsy. In contrast to traditional pulmonary function testing, which is helpful for evaluation of the larger airways, forced oscillation technique can be used noninvasively, using pressure oscillations to evaluate for diseases of the smaller airways and has been used in the veteran population and in those exposed to dust from the World Trade Center disaster.23-25 Multiple breath washout technique provides a lung clearance index that is determined by the number of lung turnovers it takes to clear the lungs of an inert gas (eg, sulfur hexafluoride, nitrogen). Elevated lung clearance index values suggest ventilation heterogeneity and have been shown to be higher among deployed veterans with dyspnea.26,27 Finally, advanced CT analytic techniques may help identify functional small airways disease and are higher in deployed service members with constrictive bronchiolitis on surgical lung biopsy.28 These innovative noninvasive techniques are experimental but promising, especially as part of a broader evaluation of small airways disease.
AHOBPR clinical evaluations represent an initial step to better understand postdeployment health conditions available to all AHOBPR participants. The PDCEN clinical evaluation extends the AHOBPR evaluation by providing specialty care for certain veterans requiring more comprehensive evaluation while systematically collecting and analyzing clinical data to advance the field. The VA is committed to leveraging these data and all available expertise to provide a clear description of the spectrum of disease in this population and improve our ability to diagnose, follow, and treat respiratory health conditions occurring after deployment to Southwest Asia and Afghanistan.
Case Conclusion
The veteran was referred to a PDCEN site and underwent a comprehensive multidisciplinary evaluation. Pulmonary function testing showed lung volumes and vital capacity within the predicted normal range, mild air trapping, and a low diffusion capacity for carbon monoxide. Methacholine challenge testing was normal; however, forced oscillometry suggested small airways obstruction. A high-resolution CT showed air trapping without parenchymal changes. Cardiopulmonary exercise testing demonstrated a peak exercise capacity within the predicted normal range but low breathing reserve. Otolaryngology evaluation including laryngoscopy suggested chronic nonallergic rhinitis.
At the end of the veteran’s evaluation, a summary review reported nonallergic rhinitis and distal airway obstruction consistent with small airways disease. Both were reported as most likely related to deployment given her significant environmental exposures and the temporal relationship with her deployment and symptom onset as well as lack of other identifiable causes. A more precise histopathologic diagnosis could be firmly established with a surgical lung biopsy, but after shared decision making with a PDCEN HCP, the patient declined to undergo this invasive procedure. After you review the summary review and recommendations from the PDCEN group, you start the veteran on intranasal steroids and a combined inhaled corticosteroid/long-acting β agonist inhaler as well as refer the veteran to pulmonary rehabilitation. After several weeks, she reports an improvement in sleep and nasal symptoms but continues to experience residual exercise intolerance.
This case serves as an example of the significant limitations that a previously active and healthy patient can develop after deployment to Southwest Asia and Afghanistan. Encouraging this veteran to complete the AHOBPR allowed her to be considered for a PDCEN evaluation that provided the opportunity to undergo a comprehensive noninvasive evaluation of her chronic dyspnea. In doing so, she obtained 2 important diagnoses and data from her evaluation will help establish best practices for standardized evaluations of respiratory concerns following deployment. Through the AHOBPR and PDCEN, the VA seeks to better understand postdeployment health conditions, their relationship to military and environmental exposures, and how best to diagnose and treat these conditions.
Acknowledgments
This work was supported by the US Department of Veterans Affairs (VA) Airborne Hazards and Burn Pits Center of Excellence (Public Law 115-929). The authors acknowledge support and contributions from Dr. Eric Shuping and leadership at VA’s Health Outcomes Military Exposures office as well as the New Jersey War Related Illness and Injury Study Center. In addition, we thank Erin McRoberts and Rajeev Swarup for their contributions to the Post-Deployment Cardiopulmonary Evaluation Network. Post-Deployment Cardiopulmonary Evaluation Network members:
Mehrdad Arjomandi, Caroline Davis, Michelle DeLuca, Nancy Eager, Courtney A. Eberhardt, Michael J. Falvo, Timothy Foley, Fiona A.S. Graff, Deborah Heaney, Stella E. Hines, Rachel E. Howard, Nisha Jani, Sheena Kamineni, Silpa Krefft, Mary L. Langlois, Helen Lozier, Simran K. Matharu, Anisa Moore, Lydia Patrick-DeLuca, Edward Pickering, Alexander Rabin, Michelle Robertson, Samantha L. Rogers, Aaron H. Schneider, Anand Shah, Anays Sotolongo, Jennifer H. Therkorn, Rebecca I. Toczylowski, Matthew Watson, Alison D. Wilczynski, Ian W. Wilson, Romi A. Yount.
Case Example
A 37-year-old female never smoker presents to your clinic with progressive dyspnea over the past 15 years. She reports dyspnea on exertion, wheezing, chronic nasal congestion, and difficulty sleeping that started a year after she returned from military deployment to Iraq. She has been unable to exercise, even at low intensity, for the past 5 years, despite being previously active. She has experienced some symptom improvement by taking an albuterol inhaler as needed, loratadine (10 mg), and fluticasone nasal spray (50 mcg). She occasionally uses famotidine for reflux (40 mg). She deployed to Southwest Asia for 12 months (2002-2003) and was primarily stationed in Qayyarah West, an Air Force base in the Mosul district in northern Iraq. She reports exposure during deployment to the fire in the Al-Mishraq sulfur mine, located approximately 25 km north of Qayyarah West, as well as dust storms and burn pits. She currently works as a medical assistant. Her examination is remarkable for normal bronchovesicular breath sounds without any wheezing or crackles on pulmonary evaluation. Her body mass index is 31. You obtain a chest radiograph and spirometry, which are both normal.
The veteran reports feeling frustrated as she has had multiple specialty evaluations in community clinics without receiving a diagnosis, despite worsening symptoms. She reports that she added her information to the Airborne Hazards and Open Burn Pit Registry (AHOBPR). She recently received a letter from the US Department of Veterans Affairs (VA) Post-Deployment Cardiopulmonary Evaluation Network (PDCEN) and is asking you whether she should participate in the PDCEN specialty evaluation. You are not familiar with the military experiences she has described or the programs she asks you about; however, you would like to know more to best care for your patient.
Background
The year 2021 marked the 20th anniversary of the September 11 attacks and the launch of the Global War on Terrorism. Almost 3 million US military personnel have been deployed in support of these operations along with about 300,000 US civilian contractors and thousands of troops from more than 40 nations.1-3
Respiratory hazards associated with deployment to Southwest Asia and Afghanistan are unique and varied. These exposures include blast injuries and a variety of particulate matter sources, such as burn pit combustion byproducts, aeroallergens, and dust storms.7,8,15,16 One air sampling study conducted at 15 deployment sites in Southwest Asia and Afghanistan found mean fine particulate matter (PM2.5) levels were as much as 10 times greater than sampling sites in both rural and urban cities in the United States; all sites sampled exceeded military exposure guidelines (65 µg/m3 for 1 year).17,18 Long-term exposure to PM2.5 has been associated with the development of chronic respiratory and cardiovascular disease; therefore, there has been considerable attention to the respiratory (and nonrespiratory) health of deployed military personnel.19
Concerns regarding the association between deployment and lung disease led to the creation of the national VA Airborne Hazards and Open Burn Pit Registry (AHOBPR) in 2014 and consists of (1) an online questionnaire to document deployment and medical history, exposure concerns, and symptoms; and (2) an optional in-person or virtual clinical health evaluation at the individual’s local VA medical center or military treatment facility (MTF). As of March 2022, more than 300,000 individuals have completed the online questionnaire of which about 30% declined the optional clinical health evaluation.
The clinical evaluation available to AHOBPR participants has not yet been described in the literature. Therefore, our objectives are to examine AHOBPR clinical evaluation data and review its application throughout the VA. In addition, we will also describe a parallel effort by the VA PDCEN, which is to provide comprehensive multiday clinical evaluations for unique AHOBPR participants with unexplained dyspnea and self-reported respiratory disease. A secondary aim of this publication is to disseminate information to health care professionals (HCPs) within and outside of the VA to aid in the referral and evaluation of previously deployed veterans who experience unexplained dyspnea.
AHOBPR Overview
The AHOBPR is an online questionnaire and optional in-person health evaluation that includes 7 major categories targeting deployment history, symptoms, medical history, health concerns, residential history, nonmilitary occupational history, nonmilitary environmental exposures, and health care utilization. The VA Defense Information Repository is used to obtain service dates for the service member/veteran, conflict involvement, and primary location during deployment. The questionnaire portion of the AHOBPR is administered online. It currently is open to all veterans who served in the Southwest Asia theater of operations (including Iraq, Kuwait, and Egypt) any time after August 2, 1990, or Afghanistan, Djibouti, Syria, or Uzbekistan after September 11, 2001. Veterans are eligible for completing the AHOBPR and optional health evaluation at no cost to the veteran regardless of VA benefits or whether they are currently enrolled in VA health care. Though the focus of the present manuscript is to profile a VA program, it is important to note that the US Department of Defense (DoD) is an active partner with the VA in the promotion of the AHOBPR to service members and similarly provides health evaluations for active-duty service members (including activating Reserve and Guard) through their local MTF.
We reviewed and analyzed AHOBPR operations and VA data from 2014 to 2020. Our analyses were limited to veterans seeking evaluation as well as their corresponding symptoms and HCP’s clinical impression from the electronic health record. As of September 20, 2021, 267,125 individuals completed the AHOBPR. The mean age was 43 years (range, 19-84), and the majority were male (86%) and served in the Army (58%). Open-air burn pits (91%), engine maintenance (38.8%), and convoy operations (71.7%) were the most common deployment-related exposures.
The optional in-person AHOBPR health evaluation may be requested by the veteran after completing the online questionnaire and is performed at the veteran’s local VA facility. The evaluation is most often completed by an environmental health clinician or primary care practitioner (PCP). A variety of resources are available to providers for training on this topic, including fact sheets, webinars, monthly calls, conferences, and accredited e-learning.20 As part of the clinical evaluation, the veteran’s chief concerns are assessed and evaluated. At the time of our analysis, 24,578 clinical examinations were performed across 126 VA medical facilities, with considerable geographic variation. Veterans receiving evaluations were predominantly male (89%) with a median age of 46.0 years (IQR, 15). Veterans’ major respiratory concerns included dyspnea (45.1%), decreased exercise ability (34.8%), and cough > 3 weeks (30.3%) (Table). After clinical evaluation by a VA or MTF HCP, 47.8% were found to have a respiratory diagnosis, including asthma (30.1%), COPD (12.8%), and bronchitis (11.9%).
Registry participants who opt to receive the clinical evaluation may benefit directly by undergoing a detailed clinical history and physical examination as well as having the opportunity to document their health concerns. For some, clinicians may need to refer veterans for additional specialty testing beyond this standard AHOBPR clinical evaluation. Although these evaluations can help address some of the veterans’ concerns, a substantial number may have unexplained respiratory symptoms that warrant further investigation.
Post-Deployment Cardiopulmonary Evaluation Network Clinical Evaluation
In May 2019, the VA established the Airborne Hazards and Burn Pits Center of Excellence (AHBPCE). One of the AHBPCE’s objectives is to deliver specialized care and consultation for veterans with concerns about their postdeployment health, including, but not limited to, unexplained dyspnea. To meet this objective, the AHBPCE developed the PDCEN, a national network consisting of specialty HCPs from 5 VA medical centers—located in San Francisco, California; Denver, Colorado; Baltimore, Maryland; Ann Arbor, Michigan; and East Orange, New Jersey. Collectively, the PDCEN has developed a standardized approach for the comprehensive clinical evaluation of unexplained dyspnea that is implemented uniformly across sites. Staff at the PDCEN screen the AHOBPR to identify veterans with features of respiratory disease and invite them to participate in an in-person evaluation at the nearest PDCEN site. Given the specialty expertise (detailed below) within the Network, the PDCEN focuses on complex cases that are resource intensive. To address complex cases of unexplained dyspnea, the PDCEN has developed a core clinical evaluation approach (Figure).
The first step in a veteran’s PDCEN evaluation entails a set of detailed questionnaires that request information about the veteran’s current respiratory, sleep, and mental health symptoms and any associated medical diagnoses. Questionnaires also identify potential exposures to military burn pits, sulfur mine and oil field fires, diesel exhaust fumes, dust storms, urban pollution, explosions/blasts, and chemical weapons. In addition, the questionnaires include deployment geographic location, which may inform future estimates of particulate matter exposure.21 Prior VA and non-VA evaluations and testing of their respiratory concerns are obtained for review. Exposure and health records from the DoD are also reviewed when available.
The next step in the PDCEN evaluation comprises comprehensive testing, including complete pulmonary function testing, methacholine challenge, cardiopulmonary exercise testing, forced oscillometry and exhaled nitric oxide testing, paired high-resolution inspiratory and expiratory chest computed tomography (CT) imaging, sinus CT imaging, direct flexible laryngoscopy, echocardiography, polysomnography, and laboratory blood testing. The testing process is managed by local site coordinators and varies by institution based on availability of each testing modality and subspecialist appointments.
Once testing is completed, the veteran is evaluated by a team of HCPs, including physicians from the disciplines of pulmonary medicine, environmental and occupational health, sleep medicine, otolaryngology and speech pathology, and mental health (when appropriate). After the clinical evaluation has been completed, this team of expert HCPs at each site convenes to provide a final summary review visit intended to be a comprehensive assessment of the veteran’s primary health concerns. The 3 primary objectives of this final review are to inform the veteran of (1) what respiratory and related conditions they have; (2) whether the conditions is/are deployment related; and (3) what treatments and/or follow-up care may enhance their current state of health in partnership with their local HCPs. The PDCEN does not provide ongoing management of any conditions identified during the veteran’s evaluation but communicates findings and recommendations to the veteran and their PCP for long-term care.
Discussion
The AHOBPR was established in response to mounting concerns that service members and veterans were experiencing adverse health effects that might be attributable to deployment-related exposures. Nearly half of all patients currently enrolled in the AHOBPR report dyspnea, and about one-third have decreased exercise tolerance and/or cough. Of those who completed the questionnaire and the subsequent in-person and generalized AHOBPR examination, our interim analysis showed that about half were assigned a respiratory diagnosis. Yet for many veterans, their breathing symptoms remained unexplained or did not respond to treatment.
While the AHOBPR and related examinations address the needs of many veterans, others may require more comprehensive examination. The PDCEN attends to the latter by providing more detailed and comprehensive clinical evaluations of veterans with deployment-related respiratory health concerns and seeks to learn from these evaluations by analyzing data obtained from veterans across sites. As such, the PDCEN hopes not only to improve the health of individual veterans, but also create standard practices for both VA and non-VA community evaluation of veterans exposed to respiratory hazards during deployment.
One of the major challenges in the field of postdeployment respiratory health is the lack of clear universal language or case definitions that encompass the veteran’s clinical concerns. In an influential case series published in 2011, 38 (77%) of 49 soldiers with history of airborne hazard exposure and unexplained exercise intolerance were reported to have histopathology consistent with constrictive bronchiolitis on surgical lung biopsy.14 Subsequent publications have described other histopathologic features in deployed military personnel, including granulomatous inflammation, interstitial lung disease, emphysema, and pleuritis.12-14 Reconciling these findings from surgical lung biopsy with the clinical presentation and noninvasive studies has proved difficult. Therefore, several groups of investigators have proposed terms, including postdeployment respiratory syndrome, deployment-related distal lung disease, and Iraq/Afghanistan War lung injury to describe the increased respiratory symptoms and variety of histopathologic and imaging findings in this population.9,12,22 At present, there remains a lack of consensus on terminology and case definitions as well as the role of military environmental exposures in exacerbating and/or causing these conditions. As HCPs, it is important to appreciate and acknowledge that the ambiguity and controversy pertaining to terminology, causation, and service connections are a common source of frustration experienced by veterans, which are increasingly reflected among reports in popular media and lay press.
A second and related challenge in the field of postdeployment respiratory health that contributes to veteran and HCP frustration is that many of the aforementioned abnormalities described on surgical lung biopsy are not readily identifiable on noninvasive tests, including traditional interpretation of pulmonary function tests or chest CT imaging.12-14,22 Thus, underlying conditions could be overlooked and veterans’ concerns and symptoms may be dismissed or misattributed to other comorbid conditions. While surgical lung biopsies may offer diagnostic clarity in identifying lung disease, there are significant procedural risks of surgical and anesthetic complications. Furthermore, a definitive diagnosis does not necessarily guarantee a clear treatment plan. For example, there are no current therapies approved by the US Food and Drug Administration for the treatment of constrictive bronchiolitis.
Research efforts are underway, including within the PDCEN, to evaluate a more sensitive and noninvasive assessment of the small airways that may even reduce or eliminate the need for surgical lung biopsy. In contrast to traditional pulmonary function testing, which is helpful for evaluation of the larger airways, forced oscillation technique can be used noninvasively, using pressure oscillations to evaluate for diseases of the smaller airways and has been used in the veteran population and in those exposed to dust from the World Trade Center disaster.23-25 Multiple breath washout technique provides a lung clearance index that is determined by the number of lung turnovers it takes to clear the lungs of an inert gas (eg, sulfur hexafluoride, nitrogen). Elevated lung clearance index values suggest ventilation heterogeneity and have been shown to be higher among deployed veterans with dyspnea.26,27 Finally, advanced CT analytic techniques may help identify functional small airways disease and are higher in deployed service members with constrictive bronchiolitis on surgical lung biopsy.28 These innovative noninvasive techniques are experimental but promising, especially as part of a broader evaluation of small airways disease.
AHOBPR clinical evaluations represent an initial step to better understand postdeployment health conditions available to all AHOBPR participants. The PDCEN clinical evaluation extends the AHOBPR evaluation by providing specialty care for certain veterans requiring more comprehensive evaluation while systematically collecting and analyzing clinical data to advance the field. The VA is committed to leveraging these data and all available expertise to provide a clear description of the spectrum of disease in this population and improve our ability to diagnose, follow, and treat respiratory health conditions occurring after deployment to Southwest Asia and Afghanistan.
Case Conclusion
The veteran was referred to a PDCEN site and underwent a comprehensive multidisciplinary evaluation. Pulmonary function testing showed lung volumes and vital capacity within the predicted normal range, mild air trapping, and a low diffusion capacity for carbon monoxide. Methacholine challenge testing was normal; however, forced oscillometry suggested small airways obstruction. A high-resolution CT showed air trapping without parenchymal changes. Cardiopulmonary exercise testing demonstrated a peak exercise capacity within the predicted normal range but low breathing reserve. Otolaryngology evaluation including laryngoscopy suggested chronic nonallergic rhinitis.
At the end of the veteran’s evaluation, a summary review reported nonallergic rhinitis and distal airway obstruction consistent with small airways disease. Both were reported as most likely related to deployment given her significant environmental exposures and the temporal relationship with her deployment and symptom onset as well as lack of other identifiable causes. A more precise histopathologic diagnosis could be firmly established with a surgical lung biopsy, but after shared decision making with a PDCEN HCP, the patient declined to undergo this invasive procedure. After you review the summary review and recommendations from the PDCEN group, you start the veteran on intranasal steroids and a combined inhaled corticosteroid/long-acting β agonist inhaler as well as refer the veteran to pulmonary rehabilitation. After several weeks, she reports an improvement in sleep and nasal symptoms but continues to experience residual exercise intolerance.
This case serves as an example of the significant limitations that a previously active and healthy patient can develop after deployment to Southwest Asia and Afghanistan. Encouraging this veteran to complete the AHOBPR allowed her to be considered for a PDCEN evaluation that provided the opportunity to undergo a comprehensive noninvasive evaluation of her chronic dyspnea. In doing so, she obtained 2 important diagnoses and data from her evaluation will help establish best practices for standardized evaluations of respiratory concerns following deployment. Through the AHOBPR and PDCEN, the VA seeks to better understand postdeployment health conditions, their relationship to military and environmental exposures, and how best to diagnose and treat these conditions.
Acknowledgments
This work was supported by the US Department of Veterans Affairs (VA) Airborne Hazards and Burn Pits Center of Excellence (Public Law 115-929). The authors acknowledge support and contributions from Dr. Eric Shuping and leadership at VA’s Health Outcomes Military Exposures office as well as the New Jersey War Related Illness and Injury Study Center. In addition, we thank Erin McRoberts and Rajeev Swarup for their contributions to the Post-Deployment Cardiopulmonary Evaluation Network. Post-Deployment Cardiopulmonary Evaluation Network members:
Mehrdad Arjomandi, Caroline Davis, Michelle DeLuca, Nancy Eager, Courtney A. Eberhardt, Michael J. Falvo, Timothy Foley, Fiona A.S. Graff, Deborah Heaney, Stella E. Hines, Rachel E. Howard, Nisha Jani, Sheena Kamineni, Silpa Krefft, Mary L. Langlois, Helen Lozier, Simran K. Matharu, Anisa Moore, Lydia Patrick-DeLuca, Edward Pickering, Alexander Rabin, Michelle Robertson, Samantha L. Rogers, Aaron H. Schneider, Anand Shah, Anays Sotolongo, Jennifer H. Therkorn, Rebecca I. Toczylowski, Matthew Watson, Alison D. Wilczynski, Ian W. Wilson, Romi A. Yount.
1. Wenger J, O’Connell C, Cottrell L. Examination of recent deployment experience across the services and components. Exam. RAND Corporation; 2018. Accessed June 27, 2022. doi:10.7249/rr1928
2. Torreon BS. U.S. periods of war and dates of recent conflicts, RS21405. Congressional Research Service; 2017. June 5, 2020. Accessed June 27, 2022. https://crsreports.congress.gov/product/details?prodcode=RS21405
3. Dunigan M, Farmer CM, Burns RM, Hawks A, Setodji CM. Out of the shadows: the health and well-being of private contractors working in conflict environments. RAND Corporation; 2013. Accessed June 27, 2022. https://www.rand.org/pubs/research_reports/RR420.html
4. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71. doi:10.2500/aap.2010.31.3383
5. Pugh MJ, Jaramillo CA, Leung KW, et al. Increasing prevalence of chronic lung disease in veterans of the wars in Iraq and Afghanistan. Mil Med. 2016;181(5):476-481. doi:10.7205/MILMED-D-15-00035
6. Falvo MJ, Osinubi OY, Sotolongo AM, Helmer DA. Airborne hazards exposure and respiratory health of Iraq and Afghanistan veterans. Epidemiol Rev. 2015;37:116-130. doi:10.1093/epirev/mxu009
7. McAndrew LM, Teichman RF, Osinubi OY, Jasien JV, Quigley KS. Environmental exposure and health of Operation Enduring Freedom/Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):665-669. doi:10.1097/JOM.0b013e318255ba1b
8. Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD; Margaret A. K. Ryan for the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170(11):1433-1442. doi:10.1093/aje/kwp287
9. Szema AM, Salihi W, Savary K, Chen JJ. Respiratory symptoms necessitating spirometry among soldiers with Iraq/Afghanistan war lung injury. J Occup Environ Med. 2011;53(9):961-965. doi:10.1097/JOM.0b013e31822c9f05
10. Committee on the Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan; Institute of Medicine. Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan. The National Academies Press; 2011. Accessed June 27, 2022. doi:10.17226/1320911. National Academies of Sciences, Engineering, and Medicine. Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. The National Academies Press; 2020. Accessed June 27, 2022. doi:10.17226/25837
12. Krefft SD, Wolff J, Zell-Baran L, et al. Respiratory diseases in post-9/11 military personnel following Southwest Asia deployment. J Occup Environ Med. 2020;62(5):337-343. doi:10.1097/JOM.0000000000001817
13. Gordetsky J, Kim C, Miller RF, Mehrad M. Non-necrotizing granulomatous pneumonitis and chronic pleuritis in soldiers deployed to Southwest Asia. Histopathology. 2020;77(3):453-459. doi:10.1111/his.14135
14. King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365(3):222-230. doi:10.1056/NEJMoa1101388
15. Helmer DA, Rossignol M, Blatt M, Agarwal R, Teichman R, Lange G. Health and exposure concerns of veterans deployed to Iraq and Afghanistan. J Occup Environ Med. 2007;49(5):475-480. doi:10.1097/JOM.0b013e318042d682
16. Kim YH, Warren SH, Kooter I, et al. Chemistry, lung toxicity and mutagenicity of burn pit smoke-related particulate matter. Part Fibre Toxicol. 2021;18(1):45. Published 2021 Dec 16. doi:10.1186/s12989-021-00435-w
17. Engelbrecht JP, McDonald EV, Gillies JA, Jayanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East—Part 1: ambient sampling. Inhal Toxicol. 2009;21(4):297-326. doi:10.1080/08958370802464273
18. US Army Public Health Command. Technical guide 230: environmental health risk assessment and chemical exposure guidelines for deployed military personnel, 2013 revision. Accessed June 27, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/TG230-DeploymentEHRA-and-MEGs-2013-Revision.pdf
19. Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol. 2012;8(2):166-175. doi:10.1007/s13181-011-0203-1
20. Shuping E, Schneiderman A. Resources on environmental exposures for military veterans. Am Fam Physician. 2020;101(12):709-710.
21. Masri S, Garshick E, Coull BA, Koutrakis P. A novel calibration approach using satellite and visibility observations to estimate fine particulate matter exposures in Southwest Asia and Afghanistan. J Air Waste Manag Assoc. 2017;67(1):86-95. doi:10.1080/10962247.2016.1230079
22. Gutor SS, Richmond BW, Du RH, et al. Postdeployment respiratory syndrome in soldiers with chronic exertional dyspnea. Am J Surg Pathol. 2021;45(12):1587-1596. doi:10.1097/PAS.0000000000001757
23. Goldman MD, Saadeh C, Ross D. Clinical applications of forced oscillation to assess peripheral airway function. Respir Physiol Neurobiol. 2005;148(1-2):179-194. doi:10.1016/j.resp.2005.05.026
24. Butzko RP, Sotolongo AM, Helmer DA, et al. Forced oscillation technique in veterans with preserved spirometry and chronic respiratory symptoms. Respir Physiol Neurobiol. 2019;260:8-16. doi:10.1016/j.resp.2018.11.012
25. Oppenheimer BW, Goldring RM, Herberg ME, et al. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest. 2007;132(4):1275-1282. doi:10.1378/chest.07-0913
26. Zell-Baran LM, Krefft SD, Moore CM, Wolff J, Meehan R, Rose CS. Multiple breath washout: a noninvasive tool for identifying lung disease in symptomatic military deployers. Respir Med. 2021;176:106281. doi:10.1016/j.rmed.2020.106281
27. Krefft SD, Strand M, Smith J, Stroup C, Meehan R, Rose C. Utility of lung clearance index testing as a noninvasive marker of deployment-related lung disease. J Occup Environ Med. 2017;59(8):707-711. doi:10.1097/JOM.000000000000105828. Davis CW, Lopez CL, Bell AJ, et al. The severity of functional small airways disease in military personnel with constrictive bronchiolitis as measured by quantitative CT [published online ahead of print, 2022 May 24]. Am J Respir Crit Care Med. 2022;10.1164/rccm.202201-0153LE. doi:10.1164/rccm.202201-0153LE
1. Wenger J, O’Connell C, Cottrell L. Examination of recent deployment experience across the services and components. Exam. RAND Corporation; 2018. Accessed June 27, 2022. doi:10.7249/rr1928
2. Torreon BS. U.S. periods of war and dates of recent conflicts, RS21405. Congressional Research Service; 2017. June 5, 2020. Accessed June 27, 2022. https://crsreports.congress.gov/product/details?prodcode=RS21405
3. Dunigan M, Farmer CM, Burns RM, Hawks A, Setodji CM. Out of the shadows: the health and well-being of private contractors working in conflict environments. RAND Corporation; 2013. Accessed June 27, 2022. https://www.rand.org/pubs/research_reports/RR420.html
4. Szema AM, Peters MC, Weissinger KM, Gagliano CA, Chen JJ. New-onset asthma among soldiers serving in Iraq and Afghanistan. Allergy Asthma Proc. 2010;31(5):67-71. doi:10.2500/aap.2010.31.3383
5. Pugh MJ, Jaramillo CA, Leung KW, et al. Increasing prevalence of chronic lung disease in veterans of the wars in Iraq and Afghanistan. Mil Med. 2016;181(5):476-481. doi:10.7205/MILMED-D-15-00035
6. Falvo MJ, Osinubi OY, Sotolongo AM, Helmer DA. Airborne hazards exposure and respiratory health of Iraq and Afghanistan veterans. Epidemiol Rev. 2015;37:116-130. doi:10.1093/epirev/mxu009
7. McAndrew LM, Teichman RF, Osinubi OY, Jasien JV, Quigley KS. Environmental exposure and health of Operation Enduring Freedom/Operation Iraqi Freedom veterans. J Occup Environ Med. 2012;54(6):665-669. doi:10.1097/JOM.0b013e318255ba1b
8. Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD; Margaret A. K. Ryan for the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170(11):1433-1442. doi:10.1093/aje/kwp287
9. Szema AM, Salihi W, Savary K, Chen JJ. Respiratory symptoms necessitating spirometry among soldiers with Iraq/Afghanistan war lung injury. J Occup Environ Med. 2011;53(9):961-965. doi:10.1097/JOM.0b013e31822c9f05
10. Committee on the Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan; Institute of Medicine. Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan. The National Academies Press; 2011. Accessed June 27, 2022. doi:10.17226/1320911. National Academies of Sciences, Engineering, and Medicine. Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. The National Academies Press; 2020. Accessed June 27, 2022. doi:10.17226/25837
12. Krefft SD, Wolff J, Zell-Baran L, et al. Respiratory diseases in post-9/11 military personnel following Southwest Asia deployment. J Occup Environ Med. 2020;62(5):337-343. doi:10.1097/JOM.0000000000001817
13. Gordetsky J, Kim C, Miller RF, Mehrad M. Non-necrotizing granulomatous pneumonitis and chronic pleuritis in soldiers deployed to Southwest Asia. Histopathology. 2020;77(3):453-459. doi:10.1111/his.14135
14. King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365(3):222-230. doi:10.1056/NEJMoa1101388
15. Helmer DA, Rossignol M, Blatt M, Agarwal R, Teichman R, Lange G. Health and exposure concerns of veterans deployed to Iraq and Afghanistan. J Occup Environ Med. 2007;49(5):475-480. doi:10.1097/JOM.0b013e318042d682
16. Kim YH, Warren SH, Kooter I, et al. Chemistry, lung toxicity and mutagenicity of burn pit smoke-related particulate matter. Part Fibre Toxicol. 2021;18(1):45. Published 2021 Dec 16. doi:10.1186/s12989-021-00435-w
17. Engelbrecht JP, McDonald EV, Gillies JA, Jayanty RK, Casuccio G, Gertler AW. Characterizing mineral dusts and other aerosols from the Middle East—Part 1: ambient sampling. Inhal Toxicol. 2009;21(4):297-326. doi:10.1080/08958370802464273
18. US Army Public Health Command. Technical guide 230: environmental health risk assessment and chemical exposure guidelines for deployed military personnel, 2013 revision. Accessed June 27, 2022. https://phc.amedd.army.mil/PHC%20Resource%20Library/TG230-DeploymentEHRA-and-MEGs-2013-Revision.pdf
19. Anderson JO, Thundiyil JG, Stolbach A. Clearing the air: a review of the effects of particulate matter air pollution on human health. J Med Toxicol. 2012;8(2):166-175. doi:10.1007/s13181-011-0203-1
20. Shuping E, Schneiderman A. Resources on environmental exposures for military veterans. Am Fam Physician. 2020;101(12):709-710.
21. Masri S, Garshick E, Coull BA, Koutrakis P. A novel calibration approach using satellite and visibility observations to estimate fine particulate matter exposures in Southwest Asia and Afghanistan. J Air Waste Manag Assoc. 2017;67(1):86-95. doi:10.1080/10962247.2016.1230079
22. Gutor SS, Richmond BW, Du RH, et al. Postdeployment respiratory syndrome in soldiers with chronic exertional dyspnea. Am J Surg Pathol. 2021;45(12):1587-1596. doi:10.1097/PAS.0000000000001757
23. Goldman MD, Saadeh C, Ross D. Clinical applications of forced oscillation to assess peripheral airway function. Respir Physiol Neurobiol. 2005;148(1-2):179-194. doi:10.1016/j.resp.2005.05.026
24. Butzko RP, Sotolongo AM, Helmer DA, et al. Forced oscillation technique in veterans with preserved spirometry and chronic respiratory symptoms. Respir Physiol Neurobiol. 2019;260:8-16. doi:10.1016/j.resp.2018.11.012
25. Oppenheimer BW, Goldring RM, Herberg ME, et al. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest. 2007;132(4):1275-1282. doi:10.1378/chest.07-0913
26. Zell-Baran LM, Krefft SD, Moore CM, Wolff J, Meehan R, Rose CS. Multiple breath washout: a noninvasive tool for identifying lung disease in symptomatic military deployers. Respir Med. 2021;176:106281. doi:10.1016/j.rmed.2020.106281
27. Krefft SD, Strand M, Smith J, Stroup C, Meehan R, Rose C. Utility of lung clearance index testing as a noninvasive marker of deployment-related lung disease. J Occup Environ Med. 2017;59(8):707-711. doi:10.1097/JOM.000000000000105828. Davis CW, Lopez CL, Bell AJ, et al. The severity of functional small airways disease in military personnel with constrictive bronchiolitis as measured by quantitative CT [published online ahead of print, 2022 May 24]. Am J Respir Crit Care Med. 2022;10.1164/rccm.202201-0153LE. doi:10.1164/rccm.202201-0153LE