User login
Implementation of a Patient Medication Disposal Program at a VA Medical Center
Opioid overdoses have quadrupled since 1999, with 78 Americans dying every day of opioid overdoses. More than half of all opioid overdose deaths involve prescription opioids.1 Attacking this problem from both ends—prescribing and disposal—can have a greater impact than focusing on a single strategy.
Background
In 2016, the CDC issued opioid prescription guidelines that included encouraging health care providers to discuss “options for safe disposal of unused opioids.”2 Pharmacies are prohibited from directly taking possession of controlled substances from a user. Historically, the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, recommended that patients follow FDA guidance for household medication disposal, which includes a list of medications that should be flushed down the toilet.3 As more data became available about the negative downstream environmental effects of pharmaceuticals on the water supply, this method of destruction made many patients feel uncomfortable.4,5
The Secure and Responsible Drug Disposal Act of 2010 presented additional options for hospitals and pharmacies to assist the public with medication disposal.6,7 These options offer convenience and anonymity for the end user, reduce potential for diversion, and enhance patient safety by ridding homes of unwanted and expired medications.
Prior to the 2010 act, the only legal methods of controlled substance disposal were via trash disposal, flushing, or delivery to law enforcement, typically at a community-based drug take-back event. These methods were not always convenient, environmentally friendly, or safe for other family members and pets in the home. The Secure and Responsible Drug Disposal Act of 2010 added 2 additional collection options for pharmacies: collection receptacles and mail-back programs.
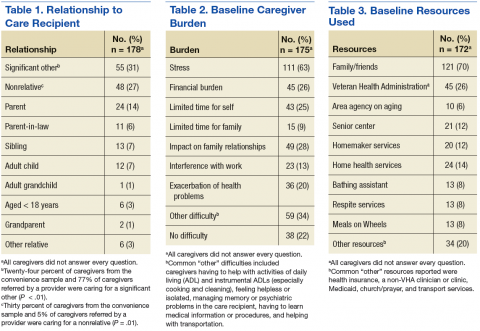
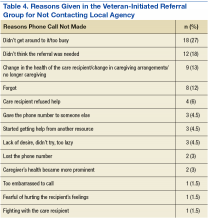
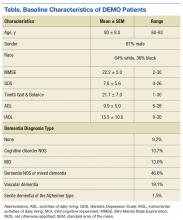
The RLRVAMC treats > 62,000 veterans annually. The RLRVAMC Pharmacy Service had been providing pharmaceutical mail-back envelopes to patients since May 2015 with moderate success (271 lb of medications returned and a 22.8% envelope return rate through September 2016). Although the mail-back envelopes offer at-home convenience, there was no on-site disposal option. It is not uncommon for patients to bring medications to their appointment or the emergency department (ED). When medication reconciliation is performed, some medications are discontinued, and the prescriber wants them to be safely out of the patient’s possession to avoid confusion and/or accidental overdose. The purpose of this project was to offer more disposal options to patients through the addition of an on-site medication collection receptacle that would be in compliance with U.S. Drug Enforcement Agency (DEA) regulations.
Methods
A policy was developed between the RLRVAMC pharmacy and police departments for the management of a medication collection receptacle. Police would oversee the disposal program so that the pharmacy would not have to change its DEA registration to collector status. The 2 access keys to the receptacle were maintained by police and secured within a key accountability system in the police station.
Full liners are removed from the receptacle by 2 police officers and sealed securely according to vendor guidelines. In accordance with DEA regulations, a form is completed that documents the dates the inner liner was acquired, installed, removed, and transferred for destruction as well as the unique identification number and size of the liner, the address of the location where it was installed, and the names and signatures of the 2 employees that witnessed the removal.
Arrangements are made to have a mail courier present during the removal of the liner. Once removed, the liner is immediately sealed and released to the mail courier who transports the liner to the DEA authorized reverse distributor. The reverse distributor is a licensed entity that has the authority to take control of the medications, including controlled substances, for disposal. The liner tracking numbers are kept in a police log book so that delivery can be confirmed and destruction certificates obtained from the vendor’s website at a later date. The records are kept for 3 years.
Funding was obtained for a 38-gallon collection receptacle and 12 liners from Pharmacy Benefits Management Services (PBM). Approval was obtained from RLRVAMC leadership to locate the receptacle in a high-traffic area, anchored to the floor, under video surveillance, and away from the ED entrance (a DEA requirement). Public Affairs promoted the receptacle to veterans. E-mails were sent to staff to provide education on regulatory requirements. Weight and frequency of medications returned were obtained from data collected by the reverse distributor. Descriptive statistics are reported.
Results
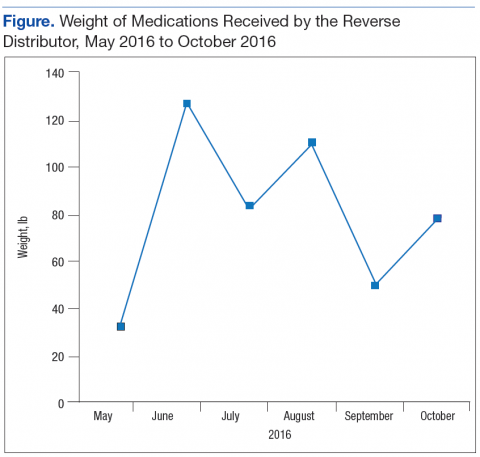
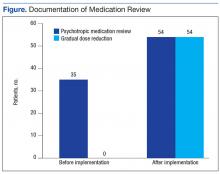
The Federal Supply Schedule cost to procure a DEA-compliant receptacle was $1,450. The additional 12 inner liners cost $2,024.97.8 Staff from Engineering Service were able to install the receptacle at no additional cost to the facility. The collection receptacle was opened to the public in May 2016. From May through October 2016, the facility collected and returned 10 liners to the reverse distributor containing 452 lb of medications. An additional 30 lb of drugs were returned through the mailback envelope program for a total weight of 482 lb over the 6-month period (Figure). The average time between inner liner changes was 2.6 weeks.
Discussion
The most challenging aspect of implementation was identification of a location for the receptacle. The location chosen, an alcove in the hallway between the coffee shop and outpatient pharmacy, was most appropriate. It is a high-traffic area, under video surveillance, and provides easy access for patients. Another challenge was determining the frequency of liner changes. There was no historic data to assist with predicting how quickly the liner would fill up. Initially, Police Service checked the receptacle every week, and it was emptied about every 2 weeks. Aspatients cleaned out their medicine cabinets, the liner needed to be replaced closer to every 4 weeks. An ongoing challenge has been determining how full the liner is without requiring the police to open the receptacle. Consideration is being given to installing a scale in the receptacle under the liner and having the display affixed to the outside of the container.
The receptacle seemed to be the preferred method of disposal, considering that it generated nearly 15 times more waste than did the mail-back envelopes during the same time period. Anecdotal patient feedback has been extremely positive on social media and by word-of-mouth.
Limitations
One limitation of this disposal program is that the specific amount of controlled substance waste vs noncontrolled substance waste cannot be determined since the liner contents are not inventoried. The University of Findlay in Ohio partnered with local law enforcement to host 7 community medication take-back events over a 3-year period, inventoried the drugs, and found that about one-third of the dosing units (eg, tablet or capsule) returned in the analgesic category were controlled substances, suggesting that take-back events may play a role in reducing unauthorized access to prescription painkillers.9 By witnessing the changing of inner liners, it can be anecdotally confirmed that a significant amount of controlled substances were collected and returned at RLRVAMC. These results have been shared with respective VISN leadership, and additional facilities are installing receptacles.
Conclusion
Changes to DEA regulations offer medical centers more options for developing a comprehensive drug disposal program. Implementation of a pharmaceutical take-back program can assist patients with disposal of unwanted and expired medications, promote safety and environmental stewardship, and reduce the risk of diversion.
1. Centers for Disease Control and Prevention. Opioid overdose. http://www.cdc.gov/drugoverdose/index.html. Updated April 16, 2017. Accessed June 5, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
3. U.S. Food and Drug Administration. Disposal of unused medicines: what you should know. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List. Updated April 21, 2017. Accessed June 5, 2017.
4. Li WC. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut. 2014;187:193-201.
5. Boxall AB. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116.
6. Peterson DM. New DEA rules expand options for controlled substance disposal. J Pain Palliat Care Pharmacother. 2015;29(1):22-26.
7. U.S. Department of Justice, Drug Enforcement Administration. Drug disposal information. https:// www.deadiversion.usdoj.gov/drug_disposal/index.html. Accessed June 5, 2016.
8. GSA Advantage! Online shopping. https://www.gsaadvantage.gov. Accessed June 5, 2017.
9. Perry LA, Shinn BW, Stanovich J. Quantification of an ongoing community-based medication take-back program. J Am Pharm Assoc (2003). 2014;54(3):275-279.
Opioid overdoses have quadrupled since 1999, with 78 Americans dying every day of opioid overdoses. More than half of all opioid overdose deaths involve prescription opioids.1 Attacking this problem from both ends—prescribing and disposal—can have a greater impact than focusing on a single strategy.
Background
In 2016, the CDC issued opioid prescription guidelines that included encouraging health care providers to discuss “options for safe disposal of unused opioids.”2 Pharmacies are prohibited from directly taking possession of controlled substances from a user. Historically, the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, recommended that patients follow FDA guidance for household medication disposal, which includes a list of medications that should be flushed down the toilet.3 As more data became available about the negative downstream environmental effects of pharmaceuticals on the water supply, this method of destruction made many patients feel uncomfortable.4,5
The Secure and Responsible Drug Disposal Act of 2010 presented additional options for hospitals and pharmacies to assist the public with medication disposal.6,7 These options offer convenience and anonymity for the end user, reduce potential for diversion, and enhance patient safety by ridding homes of unwanted and expired medications.
Prior to the 2010 act, the only legal methods of controlled substance disposal were via trash disposal, flushing, or delivery to law enforcement, typically at a community-based drug take-back event. These methods were not always convenient, environmentally friendly, or safe for other family members and pets in the home. The Secure and Responsible Drug Disposal Act of 2010 added 2 additional collection options for pharmacies: collection receptacles and mail-back programs.
The RLRVAMC treats > 62,000 veterans annually. The RLRVAMC Pharmacy Service had been providing pharmaceutical mail-back envelopes to patients since May 2015 with moderate success (271 lb of medications returned and a 22.8% envelope return rate through September 2016). Although the mail-back envelopes offer at-home convenience, there was no on-site disposal option. It is not uncommon for patients to bring medications to their appointment or the emergency department (ED). When medication reconciliation is performed, some medications are discontinued, and the prescriber wants them to be safely out of the patient’s possession to avoid confusion and/or accidental overdose. The purpose of this project was to offer more disposal options to patients through the addition of an on-site medication collection receptacle that would be in compliance with U.S. Drug Enforcement Agency (DEA) regulations.
Methods
A policy was developed between the RLRVAMC pharmacy and police departments for the management of a medication collection receptacle. Police would oversee the disposal program so that the pharmacy would not have to change its DEA registration to collector status. The 2 access keys to the receptacle were maintained by police and secured within a key accountability system in the police station.
Full liners are removed from the receptacle by 2 police officers and sealed securely according to vendor guidelines. In accordance with DEA regulations, a form is completed that documents the dates the inner liner was acquired, installed, removed, and transferred for destruction as well as the unique identification number and size of the liner, the address of the location where it was installed, and the names and signatures of the 2 employees that witnessed the removal.
Arrangements are made to have a mail courier present during the removal of the liner. Once removed, the liner is immediately sealed and released to the mail courier who transports the liner to the DEA authorized reverse distributor. The reverse distributor is a licensed entity that has the authority to take control of the medications, including controlled substances, for disposal. The liner tracking numbers are kept in a police log book so that delivery can be confirmed and destruction certificates obtained from the vendor’s website at a later date. The records are kept for 3 years.
Funding was obtained for a 38-gallon collection receptacle and 12 liners from Pharmacy Benefits Management Services (PBM). Approval was obtained from RLRVAMC leadership to locate the receptacle in a high-traffic area, anchored to the floor, under video surveillance, and away from the ED entrance (a DEA requirement). Public Affairs promoted the receptacle to veterans. E-mails were sent to staff to provide education on regulatory requirements. Weight and frequency of medications returned were obtained from data collected by the reverse distributor. Descriptive statistics are reported.
Results
The Federal Supply Schedule cost to procure a DEA-compliant receptacle was $1,450. The additional 12 inner liners cost $2,024.97.8 Staff from Engineering Service were able to install the receptacle at no additional cost to the facility. The collection receptacle was opened to the public in May 2016. From May through October 2016, the facility collected and returned 10 liners to the reverse distributor containing 452 lb of medications. An additional 30 lb of drugs were returned through the mailback envelope program for a total weight of 482 lb over the 6-month period (Figure). The average time between inner liner changes was 2.6 weeks.
Discussion
The most challenging aspect of implementation was identification of a location for the receptacle. The location chosen, an alcove in the hallway between the coffee shop and outpatient pharmacy, was most appropriate. It is a high-traffic area, under video surveillance, and provides easy access for patients. Another challenge was determining the frequency of liner changes. There was no historic data to assist with predicting how quickly the liner would fill up. Initially, Police Service checked the receptacle every week, and it was emptied about every 2 weeks. Aspatients cleaned out their medicine cabinets, the liner needed to be replaced closer to every 4 weeks. An ongoing challenge has been determining how full the liner is without requiring the police to open the receptacle. Consideration is being given to installing a scale in the receptacle under the liner and having the display affixed to the outside of the container.
The receptacle seemed to be the preferred method of disposal, considering that it generated nearly 15 times more waste than did the mail-back envelopes during the same time period. Anecdotal patient feedback has been extremely positive on social media and by word-of-mouth.
Limitations
One limitation of this disposal program is that the specific amount of controlled substance waste vs noncontrolled substance waste cannot be determined since the liner contents are not inventoried. The University of Findlay in Ohio partnered with local law enforcement to host 7 community medication take-back events over a 3-year period, inventoried the drugs, and found that about one-third of the dosing units (eg, tablet or capsule) returned in the analgesic category were controlled substances, suggesting that take-back events may play a role in reducing unauthorized access to prescription painkillers.9 By witnessing the changing of inner liners, it can be anecdotally confirmed that a significant amount of controlled substances were collected and returned at RLRVAMC. These results have been shared with respective VISN leadership, and additional facilities are installing receptacles.
Conclusion
Changes to DEA regulations offer medical centers more options for developing a comprehensive drug disposal program. Implementation of a pharmaceutical take-back program can assist patients with disposal of unwanted and expired medications, promote safety and environmental stewardship, and reduce the risk of diversion.
Opioid overdoses have quadrupled since 1999, with 78 Americans dying every day of opioid overdoses. More than half of all opioid overdose deaths involve prescription opioids.1 Attacking this problem from both ends—prescribing and disposal—can have a greater impact than focusing on a single strategy.
Background
In 2016, the CDC issued opioid prescription guidelines that included encouraging health care providers to discuss “options for safe disposal of unused opioids.”2 Pharmacies are prohibited from directly taking possession of controlled substances from a user. Historically, the Richard L. Roudebush VAMC (RLRVAMC) in Indianapolis, Indiana, recommended that patients follow FDA guidance for household medication disposal, which includes a list of medications that should be flushed down the toilet.3 As more data became available about the negative downstream environmental effects of pharmaceuticals on the water supply, this method of destruction made many patients feel uncomfortable.4,5
The Secure and Responsible Drug Disposal Act of 2010 presented additional options for hospitals and pharmacies to assist the public with medication disposal.6,7 These options offer convenience and anonymity for the end user, reduce potential for diversion, and enhance patient safety by ridding homes of unwanted and expired medications.
Prior to the 2010 act, the only legal methods of controlled substance disposal were via trash disposal, flushing, or delivery to law enforcement, typically at a community-based drug take-back event. These methods were not always convenient, environmentally friendly, or safe for other family members and pets in the home. The Secure and Responsible Drug Disposal Act of 2010 added 2 additional collection options for pharmacies: collection receptacles and mail-back programs.
The RLRVAMC treats > 62,000 veterans annually. The RLRVAMC Pharmacy Service had been providing pharmaceutical mail-back envelopes to patients since May 2015 with moderate success (271 lb of medications returned and a 22.8% envelope return rate through September 2016). Although the mail-back envelopes offer at-home convenience, there was no on-site disposal option. It is not uncommon for patients to bring medications to their appointment or the emergency department (ED). When medication reconciliation is performed, some medications are discontinued, and the prescriber wants them to be safely out of the patient’s possession to avoid confusion and/or accidental overdose. The purpose of this project was to offer more disposal options to patients through the addition of an on-site medication collection receptacle that would be in compliance with U.S. Drug Enforcement Agency (DEA) regulations.
Methods
A policy was developed between the RLRVAMC pharmacy and police departments for the management of a medication collection receptacle. Police would oversee the disposal program so that the pharmacy would not have to change its DEA registration to collector status. The 2 access keys to the receptacle were maintained by police and secured within a key accountability system in the police station.
Full liners are removed from the receptacle by 2 police officers and sealed securely according to vendor guidelines. In accordance with DEA regulations, a form is completed that documents the dates the inner liner was acquired, installed, removed, and transferred for destruction as well as the unique identification number and size of the liner, the address of the location where it was installed, and the names and signatures of the 2 employees that witnessed the removal.
Arrangements are made to have a mail courier present during the removal of the liner. Once removed, the liner is immediately sealed and released to the mail courier who transports the liner to the DEA authorized reverse distributor. The reverse distributor is a licensed entity that has the authority to take control of the medications, including controlled substances, for disposal. The liner tracking numbers are kept in a police log book so that delivery can be confirmed and destruction certificates obtained from the vendor’s website at a later date. The records are kept for 3 years.
Funding was obtained for a 38-gallon collection receptacle and 12 liners from Pharmacy Benefits Management Services (PBM). Approval was obtained from RLRVAMC leadership to locate the receptacle in a high-traffic area, anchored to the floor, under video surveillance, and away from the ED entrance (a DEA requirement). Public Affairs promoted the receptacle to veterans. E-mails were sent to staff to provide education on regulatory requirements. Weight and frequency of medications returned were obtained from data collected by the reverse distributor. Descriptive statistics are reported.
Results
The Federal Supply Schedule cost to procure a DEA-compliant receptacle was $1,450. The additional 12 inner liners cost $2,024.97.8 Staff from Engineering Service were able to install the receptacle at no additional cost to the facility. The collection receptacle was opened to the public in May 2016. From May through October 2016, the facility collected and returned 10 liners to the reverse distributor containing 452 lb of medications. An additional 30 lb of drugs were returned through the mailback envelope program for a total weight of 482 lb over the 6-month period (Figure). The average time between inner liner changes was 2.6 weeks.
Discussion
The most challenging aspect of implementation was identification of a location for the receptacle. The location chosen, an alcove in the hallway between the coffee shop and outpatient pharmacy, was most appropriate. It is a high-traffic area, under video surveillance, and provides easy access for patients. Another challenge was determining the frequency of liner changes. There was no historic data to assist with predicting how quickly the liner would fill up. Initially, Police Service checked the receptacle every week, and it was emptied about every 2 weeks. Aspatients cleaned out their medicine cabinets, the liner needed to be replaced closer to every 4 weeks. An ongoing challenge has been determining how full the liner is without requiring the police to open the receptacle. Consideration is being given to installing a scale in the receptacle under the liner and having the display affixed to the outside of the container.
The receptacle seemed to be the preferred method of disposal, considering that it generated nearly 15 times more waste than did the mail-back envelopes during the same time period. Anecdotal patient feedback has been extremely positive on social media and by word-of-mouth.
Limitations
One limitation of this disposal program is that the specific amount of controlled substance waste vs noncontrolled substance waste cannot be determined since the liner contents are not inventoried. The University of Findlay in Ohio partnered with local law enforcement to host 7 community medication take-back events over a 3-year period, inventoried the drugs, and found that about one-third of the dosing units (eg, tablet or capsule) returned in the analgesic category were controlled substances, suggesting that take-back events may play a role in reducing unauthorized access to prescription painkillers.9 By witnessing the changing of inner liners, it can be anecdotally confirmed that a significant amount of controlled substances were collected and returned at RLRVAMC. These results have been shared with respective VISN leadership, and additional facilities are installing receptacles.
Conclusion
Changes to DEA regulations offer medical centers more options for developing a comprehensive drug disposal program. Implementation of a pharmaceutical take-back program can assist patients with disposal of unwanted and expired medications, promote safety and environmental stewardship, and reduce the risk of diversion.
1. Centers for Disease Control and Prevention. Opioid overdose. http://www.cdc.gov/drugoverdose/index.html. Updated April 16, 2017. Accessed June 5, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
3. U.S. Food and Drug Administration. Disposal of unused medicines: what you should know. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List. Updated April 21, 2017. Accessed June 5, 2017.
4. Li WC. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut. 2014;187:193-201.
5. Boxall AB. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116.
6. Peterson DM. New DEA rules expand options for controlled substance disposal. J Pain Palliat Care Pharmacother. 2015;29(1):22-26.
7. U.S. Department of Justice, Drug Enforcement Administration. Drug disposal information. https:// www.deadiversion.usdoj.gov/drug_disposal/index.html. Accessed June 5, 2016.
8. GSA Advantage! Online shopping. https://www.gsaadvantage.gov. Accessed June 5, 2017.
9. Perry LA, Shinn BW, Stanovich J. Quantification of an ongoing community-based medication take-back program. J Am Pharm Assoc (2003). 2014;54(3):275-279.
1. Centers for Disease Control and Prevention. Opioid overdose. http://www.cdc.gov/drugoverdose/index.html. Updated April 16, 2017. Accessed June 5, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1-49.
3. U.S. Food and Drug Administration. Disposal of unused medicines: what you should know. http://www.fda.gov/Drugs/ResourcesForYou/Consumers/BuyingUsingMedicineSafely/EnsuringSafeUseofMedicine/SafeDisposalofMedicines/ucm186187.htm#Flush_List. Updated April 21, 2017. Accessed June 5, 2017.
4. Li WC. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ Pollut. 2014;187:193-201.
5. Boxall AB. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116.
6. Peterson DM. New DEA rules expand options for controlled substance disposal. J Pain Palliat Care Pharmacother. 2015;29(1):22-26.
7. U.S. Department of Justice, Drug Enforcement Administration. Drug disposal information. https:// www.deadiversion.usdoj.gov/drug_disposal/index.html. Accessed June 5, 2016.
8. GSA Advantage! Online shopping. https://www.gsaadvantage.gov. Accessed June 5, 2017.
9. Perry LA, Shinn BW, Stanovich J. Quantification of an ongoing community-based medication take-back program. J Am Pharm Assoc (2003). 2014;54(3):275-279.
Interprofessional Education in Patient Aligned Care Team Primary Care-Mental Health Integration
Over the past 10 years, the VHA has been a national leader in primary care-mental health integration (PC-MHI) within patient aligned care teams (PACTs).1,2 Studies of the PC-MHI collaborative care model consistently have shown increased access to MH services, higher levels of MH treatment engagement, improved MH treatment outcomes, and high patient and provider satisfaction.3-7 Primary care-mental health integration relies heavily on interprofessional team-based practice with providers from diverse educational and clinical backgrounds who work together to deliver integrated mental and behavioral health services within PACTs. This model requires a unique blending of professional cultures and communication and practice styles.
To sustain PC-MHI in PACT, health care professionals (HCPs) must be well trained to work effectively in interprofessional teams. Across health care organizations, training in collaborative interprofessional team-based practice has been identified as an important and challenging task.8-11
Integrating educational experiences among different HCP learners is an approach to developing competency in interprofessional collaboration early in training. The World Health Organization defined interprofessional education (IPE) as occurring “when students from two or more professions learn about, from, and with each other to enable effective collaboration and improve health outcomes.”9 Fundamental to this definition is the belief that interaction among learners from different disciplines during their training develops competency in subsequent effective collaborative practice. Studies of IPE in MH professional training have found that prelicensure IPE contributes to increased knowledge of roles and responsibilities of different disciplines, improved interprofessional communication and attitudes, and increased willingness to work in teams.12-17
Interprofessional education is a valuable training model, but developing interprofessional learning experiences in a system of diverse and often siloed training programs is difficult. More information about design and implementation of IPE training experiences is needed, particularly in outpatient settings in which integration of traditionally separate discipline-specific care is central to the health care mission. The VA PACT PC-MHI is a strong team-based care model that represents a unique opportunity for training across disciplines in interprofessional collaborative care.
To find innovative approaches to meeting the need for IPE in PACT PC-MHI, the authors developed a new IPE program in PC-MHI at the William S. Middleton Memorial Veterans Hospital (WSMMVH) in Madison, Wisconsin. This article reviews the development, implementation, and first-year evaluation of the training program and discusses the challenges and the IPE areas in need of improvement in PACT PC-MHI.
Methods
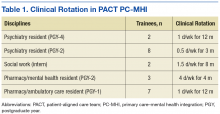
In 2012, the VHA launched phase 1 of the Mental Health Education Expansion Initiative (MHEEI), a collaboration of the Office of Academic Affiliations (OAA), VHA Mental Health Services (VHA-MHS), and the Office of Mental Health Operations (OMHO).18 The MHEEI was intended to “increase expertise in critical areas of need, expand the recruitment pipeline of well-trained, highly qualified health care providers in behavioral and mental health disciplines, and promote the utilization of interprofessional team-based care.”18 In response, WSMMVH organized a planning committee and submitted a funding request through the section of MHEEI called PACT With Integrated Behavioral Health Providers. The planning committee included training program directors and staff from psychiatry, pharmacy, social work, psychology, and primary care. The authors received funding for trainees in psychiatry (postgraduate year 4 [PGY-4]), pharmacy/MH residency (PGY-2), pharmacy/ambulatory care (PGY-1), and social work (interns).
Curriculum Development
The planning committee met regularly for 6 months to develop the organization, learning objectives, educational strategies, and implementation plan for the IPE program. The program was organized as a 4- to 12-month clinical rotation with the PC-MHI team in PACT, combined with 12 months of protected weekly IPE time (Table 1).
Learning Objectives
To better understand the educational needs and foci for learning objectives, the interprofessional planning committee reviewed guidelines on training in integrated care and collaborative team-based practice.2,9,10,19-21 These guidelines were compared with existing training opportunities for each discipline to identify training gaps and needs.
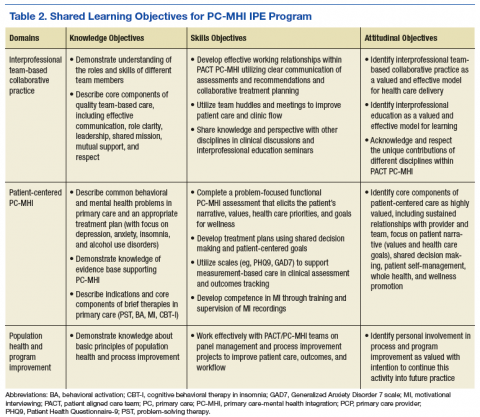
Learning objectives were organized into 3 domains: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Table 2 outlines the shared learning objectives linked to each domain that were common to the psychiatry, pharmacy, and social work disciplines. Although many of the learning objectives were shared among all disciplines, each trainee also had discipline-specific clinical activities and learning objectives. Psychiatry and pharmacy residents focused on primary care psychiatric medication consultation and care management for antidepressant medication starts. Social work interns focused on psychosocial and functional assessment and brief problem-focused psychotherapies. Learning objectives were met through direct veteran care in the primary care clinic as part of the PACT PC-MHI team and through interprofessional learning activities during protected weekly education time.
Implementation
Critical stakeholders in implementing the IPE program involved themselves early and throughout the planning process. Stakeholders included VAMC leadership, primary care and MH service line chiefs and clinic managers, training program directors, and PACT staff. Planning committee members gave presentations on the IPE program at MH service line and PACT meetings in the 2 months before program initiation in order to orient staff to learning objectives, program structure, and impact on PACT PC-MHI operations. Throughout the first year, the planning committee continued to meet every 2 weeks to review progress, solve implementation problems, and revise learning objectives and activities.
Trainee Clinical Activities
A wide range of educational strategies were planned to meet learning objectives across the 3 domains. There was strong emphasis on experiential learning through daily PACT and PC-MHI clinical work, team huddles and meetings, and trainee-led program improvement projects.
Psychiatry and PGY-2 pharmacy/MH residents focused on direct and indirect medication consultation and problem-focused assessments. Their clinical activities included PC-MHI medication evaluation and follow-up visits; chart reviews and e-consults for medication recommendations to PACT providers; reviews of care management data and consultations on veterans enrolled in depression and anxiety care management; “curbside consultations” for providers in PACT huddles and meetings and throughout the clinic day; and “warm handoffs,” same-day initial PC-MHI problem-focused assessments performed on PACT provider request. The residents were part of a pool of staff and trainees who performed these assessments.
PGY-1 pharmacy residents made care management phone calls for antidepressant trials for depression and anxiety. These residents were trained in motivational interviewing (MI). They applied their MI skills during care management calls focused on medication adherence and behavioral interventions for depression (eg, exercise, planning pleasurable activity) and during other clinical rotations, including tobacco cessation and medication management for diabetes and hypertension. Particularly challenging veteran cases from these clinics were cosupervised with medication management and PC-MHIstaff for added consultation on engagement, behavior change, and treatment plan adherence.
Social work interns completed initial PC-MHI psychosocial and functional assessments by phone and directly by same-day warm handoffs from PACT staff. The PC-MHI therapies they provided included problem-solving therapy, behavioral activation, stress management based on cognitive behavioral therapy, and brief alcohol interventions.
Group IPE Activities
All trainees had a weekly protected block of 3 hours during which they came together for group IPE that was designed to elicit active participation; facilitate interprofessional communication; and develop an understanding of and respect for the knowledge, culture, and practice style of the different disciplines.
Trainees participated in a Herrmann Brain Dominance Instrument (HBDI) workshop focused on developing a better understanding of individual differences in thinking and problem solving, with the goal of improving communication and learning within teams.22 In a seminar series on professionalism and boundaries in health care, trainees from each discipline gave a presentation on the traditional structure and content of their discipline’s training and discussed similarities and differences in their disciplines’ professional oaths, codes of ethics, and boundary guidelines.
Motivational interviewing training was conducted early in the year so trainees would be prepared to apply MI skills in their daily PACT PC-MHI clinical work. Motivational inteviewing is a patient-centered approach to engaging patients in health promoting behavior change. It is defined as a “directive, client-centered counseling style for eliciting behavior change by helping clients to explore and resolve ambivalence.”23
Trainees recorded MI sessions with at least 2 live-patient visits and at least 2 simulated-patient interviews (with staff serving as patient actors). The structure of MI training and supervision was deliberately designed to facilitate interprofessional communication and learning. In accord with a group supervision model for MI recorded reviews, the trainees presented their tapes to the entire learning group in the presence of a facilitating supervisor. Trainees had the opportunity to observe different interview styles and exchange feedback within a peer group of interprofessional learners.
Seminars were focused on core PC-MHI clinical content (eg, depression, anxiety, alcohol use disorders) and organized around case-based learning. Trainees divided into small teams in which representatives of each discipline offered their perspective on how to approach planning patient assessment and treatment. During the seminars, the authors engaged trainees as teachers and leaders whenever possible. All trainees presented on a topic in which they had some discipline expertise. For example, social work interns led a seminar on support and social services for victims of domestic violence, and PGY-1 pharmacy/ambulatory care residents led seminars and a panel management project focused on diabetes and depression.
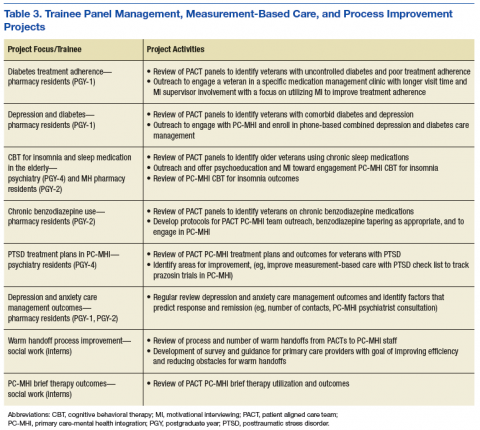
Trainees participated in several PACT PC-MHI projects focused on population- and measurement-based care, panel management, and program improvement (Table 3). Protected IPE time was used to teach trainees about population health principles and different tools for process improvement (eg, Vision-Analysis Team-Aim-Map-Measure-Change-Sustain) and provide a forum in which trainees could share their work with one another.
Evaluations
Several tools were used for trainee and program evaluations. Clinical skills were evaluated during daily supervision. Trainees began the year with PC-MHI staff directly observing all their clinical contacts with veterans. Staff evaluated and offered feedback on trainee clinical interviewing and on assessment and treatment planning skills. Once they were assessed to be ready to see veterans on their own, trainees were advanced by staff to “drop-in” direct supervision: Toward the end of a veteran’s visit, a staff preceptor entered the room to review relevant clinical findings, assessment and finalized treatment planning with the trainee and veteran. When appropriate for trainee competence level, clinical contacts were indirectly supervised: Trainees discussed their assessment and treatment plan with a staff supervisor at the end of the day.
Motivational interviewing recordings were reviewed during group supervision. To objectively evaluate MI skills, supervisors who were VA-certified in MI used the Motivational Interviewing Treatment Integrity (MITI) coding tool to review and code both the live- and simulated-patient recordings.24 The MITI coding involves quantitative and qualitative analysis using standardized coding items.
Quantitative items included percentage of open-ended questions (Proficiency: 50%; competency: 70%); percentage of reflections considered complex reflections, or reflective statements adding substantial meaning or emphasis and conveying a deeper or more complex picture of what patients say (Proficiency: 40%; competency: 50%); reflection-to-question ratio (Proficiency: 1:1; competency: 2:1); and percentage of MI-adherent provider statements (Proficiency: 90%; competency: 100%).
Qualitative coding items were a global rating of therapist “empathy,” which evaluated the extent to which the trainee understood or made an effort to grasp the patient’s perspective, and “MI spirit.” This coding intended to capture the overall competence of the trainee in emphasizing collaboration, patient autonomy, and evocation of the patient experience (Proficiency score: 5; competency score: 6).
The PC-MHI teaching staff met midyear and end of year as a team to complete trainee evaluations focused on the 3 areas of learning objectives: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Patient-centered PC-MHI involves direct observation and supervision of trainee clinical contacts with veterans, including assessment and treatment planning, clinical documentation, and review of live- and simulated-patient MI recordings. Collaborative team-based practice involves review of trainee participation in day-to-day teamwork, huddles, team meetings, and IPE activities. Population health and program improvement involves review of trainee work on a panel measurement-based care management or program improvement project. In each learning objectives area, trainees were rated on a 3-point scale: needs improvement (1); satisfactory (2); achieved (3). Core knowledge about PC-MHI evidence base, structure, and clinical topics was assessed with case-based written examination at midyear and end of year.
Surveys and qualitative interviews were used to assess trainee perceptions about the IPE program. A midyear and end-of-year survey assessed trainee satisfaction and perceived efficacy of the IPE training program in meeting core learning objectives. A midyear survey designed by pharmacy residents as part of their program improvement project evaluated attitudes around interprofessional learning and team practice. Trainees met individually with the PC-MHI IPE director at midyear and end of year to gather qualitative feedback on the IPE program.
Outcomes
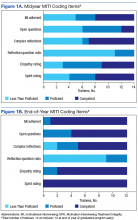
All trainees advanced to the expected level of supervision for clinical contacts (drop-in or indirect clinical supervision). Over the year, there was significant improvement in trainees’ MI skills as measured by MITI coding of at least 2 live-patient or 2 simulated-patient recordings (Figures 1A and 1B). By end of year, most trainees had reached proficiency or competency in several MITI coded items: percentage of open-ended questions (4/12 proficient, 8/12 competent), percentage of complex reflections (2/12 less than proficient, 3/12 proficient, 7/12 competent), MI adherence (1/12 proficient, 11/12 competent), global empathy rating (2/12 proficient, 10/12 competent), and global MI spirit rating (12/12 competent). Average reflection-to-question ratio for the trainee group increased from 0.63 to 0.96 from midyear to end of year, but only 3 of 12 trainees reached the proficiency level of a 1:1 ratio, and no trainee reached the competency level of a 2:1 ratio.
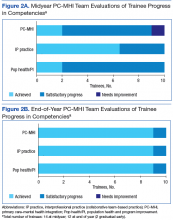
According to the PC-MHI team’s midyear evaluation, most trainees were already making satisfactory progress in the 3 domains of learning objectives for the training program. At end of year, 13 of 14 trainees were assessed as competent in all 3 domains (Figures 2A and B). All trainees passed the midyear and end-of-year written examinations with overall high scores (average score, 82%) demonstrating acquisition of core PC-MHI clinical knowledge.
Trainee evaluations of the IPE program were overall highly favorable at both midyear and at end of year. Trainees rated the program effective or extremely effective in developing their skills in patient-centered care, interprofessional communication, and collaborative team-based practice. They also rated the program highly effective in preparing them to use team-based practice skills in other settings. On a midyear survey, trainees moderately to strongly agreed with several positive beliefs and attitudes about team-based care.
In qualitative interviews at program completion, trainees across disciplines rated the MI training with group supervision as one of their most valuable interprofessional learning experiences. Other highly valued training experiences were PACT PC-MHI panel management projects, team-based clinical case reviews, and cross-disciplinary supervision.
Discussion
This article describes the successful development and implementation of a VA-based IPE program in PACT PC-MHI. Interprofessional clinical training and educational experiences were highly valued, and trainees identified positive attitudes and improved skills related to team-based care. These findings support previous findings that IPE is associated with high satisfaction and positive attitudes toward team-based collaborative practice.12-17 Program implementation presented several challenges: nonsynchronous academic calendars and rotation schedules, cross-disciplinary supervision regulations, variations in clinical and supervisory requirements for accreditation standards, the traditional health care hierarchy, and measurement of the impact of IPE experiences.11,25,26
Rotation schedules and academic calendars varied across the psychiatry, pharmacy, and social work home programs. Organizing different trainee rotation schedules was a significant challenge. Collaboration with training directors and support staff was crucial in planning rotations that offered a longitudinal training experience in PACT PC-MHI. Given the participants’ different starting dates, protected IPE time early in the calendar year was focused on developing clinical skills specific to the pharmacy and psychiatry trainees who would be starting in July, and IPE activities that required the presence of all trainee disciplines (eg, MI training, HBDI) were planned for after the September start of the social work interns.
Cross-disciplinary supervision was highly valued by trainees because it offered exposure to the disciplines’ different communication styles and approaches to clinical assessment and decision making. Throughout the year, however, trainees encountered several obstacles to cross-disciplinary supervision, with respect to coding/billing and home program supervisory policies. The authors worked closely with the VA administration and each training program to develop and revise supervising policies and procedures to meet the necessary administrative and program supervisory requirements for accreditation standards.
In some cases, this work resulted in dual supervision by a preceptor of the same discipline (to meet requirements for coding/billing and home program supervision) and clinical supervision by a preceptor of a different discipline (eg, in team meetings or in clinical case reviews during protected IPE time). Preceptors from each discipline met regularly to discuss challenges in cross-disciplinary supervision, review scope-of-practice issues, share information on discipline-specific training, and revise supervisory procedures.
Interprofessional education activities during weekly protected time were designed to improve collaboration and to challenge trainees to examine traditional hierarchical roles and patterns of communication in health care. An emphasis on case-based learning in small groups encouraged trainees to share perspectives on their discipline-specific approach to assessment and treatment planning. Motivational interviewing training, one of the IPE experiences most valued by trainees and staff, created the opportunity for a truly shared learning environment, as trainees largely started at about the same skill level despite having different educational backgrounds. Group supervision of MI recordings offered trainees the opportunity to learn from each other and develop comfort in offering and receiving interprofessional constructive feedback.
Limitations
There were considerable methodologic limitations in the authors’ efforts to evaluate the impact of this training program on trainee attitudes and skills in collaborative team-based practice. Although trainee surveys revealed highly positive attitudes and beliefs about team-based care as well as perceived competence in collaborative practice, these were not validated surveys, and changes could not be accurately measured over time (trainees were not assessed at baseline). Trainees also were involved in other clinical teams within the VA during the year, and it is difficult to assess the specific impact of their PC-MHI IPE experiences. The PC-MHI staff evaluations of trainee competence in collaborative team-based practice were largely observational and potentially vulnerable to biases, as the staff evaluating trainee competence also were part of the IPE program planning process and invested in its success.
To address these limitations in the future, the authors will use better assessment tools at baseline and end of year to more effectively evaluate the impact of the training program on teamwork skills as well changes in attitudes and beliefs about interprofessional learning and teamwork. The Attitudes Toward Interprofessional Health Care Teams Scale and the Perceptions of Effective Interpersonal Teams Scale should be considered as they have published reliability and validity.27,28 The authors could improve the reliability and depth of trainee evaluations with use of a “360-degree evaluation” model for trainee evaluations that includes other PACT members (beyond PC-MHI staff) as well as veterans.
Assessment of MI skills and competency was limited by use of both live- and simulated-patient recordings. Simulation is a valuable learning tool but often does not accurately represent actual clinical situations and challenges. To appropriately assess MI competence, future MI training should emphasize live-patient recordings over simulated-patient visits. Furthermore, whereas trainees reached competency in several MI coding items, none reached competency in the reflection-to-question ratio, and only about half reached competency in percentage of complex reflections. Future MI training will need to focus on further development of reflection skills.
Trainee intentions to remain involved in program improvement and collaborative team-based care in future professional work were core attitudinal learning objectives, but neither was adequately assessed in end-of-year surveys. Ideally, future evaluations will assess subjective trainee intentions and goals around team-based work and objectively measure future professional choices and activities. For example, it would be interesting to determine whether trainees who participated in this program will choose to practice in an interprofessional team-based model or participate in program improvement activities.
Last, the absence of psychology interns was considered a weakness in the learning environment, resulting in a relatively “prescriber-heavy” balance in discipline perspectives for IPE-focused case discussions and other training. Furthermore, the discipline representation in the IPE program did not exemplify the typical PC-MHI team in most VA clinic settings and community practices in which psychology has a strong presence. Adding psychology trainees was an important goal for the IPE program leadership. In 2015, WSMMVH was awarded additional funding for a PACT PC-MHI predoctoral psychology intern through the phase 4 MHEEI. The PC-MHI track psychology intern joined the IPE program in the 2016-2017 training year.
Conclusion
There is broad consensus that interprofessional team-based practice is crucial for providers in the VA and all health care systems. Primary care-mental health integration is an area of VA care in which interprofessional collaboration is uniquely important to implementation and sustainability of the model. Interprofessional education is an effective approach for preparing HCPs for team-based practice, but implementation is challenging. Several factors crucially contributed to the successful implementation of this program: collaboration of the interprofessional planning team with representation from key stakeholders in different departments and training programs; a well-established PACT PC-MHI team with experience in collaborative team-based care; a curriculum structure that emphasized experiential educational strategies designed to promote interprofessional learning and communication; VA leadership support at the national level (MHEEI funding) and local level; and PACT PC-MHI clinical staff committed to teaching and to the IPE mission.
1. Wray LO, Szymanski BR, Kearney LK, McCarthy JF. Implementation of primary care-mental health integration services in the Veterans Health Administration: program activity and associations with engagement in specialty mental health services. J Clin Psychol Med Settings. 2012;19(1):105-116.
2. Kearney LK, Post EP, Pomerantz AS, Zeiss AM. Applying the interprofessional patient aligned care team in the Department of Veterans Affairs: transforming primary care. Am Psychol. 2014;69(4):399-408.
3. Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of loner-term outcomes. Arch Intern Med. 2006;166(21):2314-2321.
4. Unützer J, Katon W, Callahan CM, et al; IMPACT (Improving Mood-Promoting Access to Collaborative Treatment) Investigators. Collaborative care management in late-life depression in the primary care setting: a randomized control trial. JAMA. 2002;288(22):2836-2845.
5. Katon W, Unützer J. Consultation psychiatry in the medical home and accountable care organizations: achieving the triple aim. Gen Hosp Psychiatry. 2011;3(4):305-310.
6. Bruce ML, Tenhave TR, Reynolds CF III, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291(9):1081-1091.
7. Alexopoulos GS, Reynolds CF III , Bruce ML, et al; PROSPECT Group. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry. 2009;166(8):882-890.
8. Cox M, Cuff P, Brandt B, Reeves S, Zierler B. Measuring the impact of interprofessional education on collaborative practice and patient outcomes. J Interprof Care. 2016;30(1):1-3.
9. World Health Organization, Study Group on Interprofessional Education and Collaborative Practice. Framework for Action on Interprofessional Education and Collaborative Practice. Geneva, Switzerland: World Health Organization; 2010.
10. Interprofessional Education Collaborative Expert Panel. Core Competencies for Interprofessional Collaborative Practice: Report of an Expert Panel. Washington, DC: Interprofessional Education Collaborative; 2011.
11. Blue AV, Mitcham M, Smith T, Raymond J, Greenburg R. Changing the future of health professions: embedding interprofessional education within an academic health center. Acad Med. 2010;85(8):1290-1295.
12. Priest HM, Roberts P, Dent H, Blincoe C, Lawton D, Armstrong C. Interprofessional education and working in mental health: in search of the evidence base. J Nurs Manag. 2008;16(4):474-485.
13. Reeves S. A systematic review of the effects of interprofessional education on staff involved in the care of adults with mental health problems. J Psychiatr Ment Health Nurs. 2001;8(6):533-542.
14. Carpenter J, Barnes D, Dickinson C, Wooff D. Outcomes of interprofessional education for community mental health services in England: the longitudinal evaluation of a postgraduate programme. J Interprof Care. 2006;20(2):145-161.
15. Hammick M, Freeth D, Koppel I, Reeves S, Barr H. A best evidence systematic review of interprofessional education: BEME guide no. 9. Med Teach. 2007;29(8):735-751.
16. Pauzé E, Reeves S. Examining the effects of interprofessional education on mental health providers: findings from an updated systematic review. J Ment Health. 2010;19(3):258-271.
17. Curran V, Heath O, Adey T, et al. An approach to integrating interprofessional education in collaborative mental health care. Acad Psychiatry. 2012;36(2):91-95.
18. Veterans Health Administration. VA Interprofessional Mental Health Education Expansion Initiative, Phase I. Washington, DC; Department of Veterans Affairs; 2012.
19. Dundon M, Dollar K, Schohn M, Lantinga LJ. Primary care–mental health integration: co-located, collaborative care: an operations manual. https://www.mirecc.va.gov/cih-visn2/Documents/Clinical/MH-IPC_CCC_Operations_Manual_Version_2_1.pdf. Published February 2011. Accessed May 19, 2017.
20. McDaniel SH, Grus CL, Cubic BA, et al. Competencies for psychology practice in primary care. Am Psychol. 2014;69(4):409-429.
21. Cowley D, Dunaway K, Forstein M, et al. Teaching psychiatry residents to work at the interface of mental health and primary care. Acad Psychiatry. 2014;38(4):398-404.
22. Herrmann N. The creative brain. J Creat Behav. 1991;25:275-295.
23. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
24. Pierson HM, Hayes SC, Gifford EV, et al. An examination of the Motivational Interviewing Treatment Integrity code. J Subst Abuse Treat. 2007;32(1):11-17.
25. Shaw D, Blue A. Should psychiatry champion interprofessional education? Acad Psychiatry. 2012;36(3):163-166.
26. Gilbert JH. Interprofessional learning and higher education structural barriers. J Interprof Care. 2005;19(suppl 1):87-106.
27. Heinemann GD, Schmitt MH, Farrell PP, Brallier SA. Development of an Attitudes Toward Health Care Teams Scale. Eval Health Prof. 1999;22(1):123-142.
28. Hepburn K, Tsukuda RA, Fasser C. Team Skills Scale. In: Heinemann GD, Zeiss AM, eds. Team Performance in Healthcare: Assessment and Development. New York, NY: Kluwer Academic/Plenum; 2002:159-163.
Over the past 10 years, the VHA has been a national leader in primary care-mental health integration (PC-MHI) within patient aligned care teams (PACTs).1,2 Studies of the PC-MHI collaborative care model consistently have shown increased access to MH services, higher levels of MH treatment engagement, improved MH treatment outcomes, and high patient and provider satisfaction.3-7 Primary care-mental health integration relies heavily on interprofessional team-based practice with providers from diverse educational and clinical backgrounds who work together to deliver integrated mental and behavioral health services within PACTs. This model requires a unique blending of professional cultures and communication and practice styles.
To sustain PC-MHI in PACT, health care professionals (HCPs) must be well trained to work effectively in interprofessional teams. Across health care organizations, training in collaborative interprofessional team-based practice has been identified as an important and challenging task.8-11
Integrating educational experiences among different HCP learners is an approach to developing competency in interprofessional collaboration early in training. The World Health Organization defined interprofessional education (IPE) as occurring “when students from two or more professions learn about, from, and with each other to enable effective collaboration and improve health outcomes.”9 Fundamental to this definition is the belief that interaction among learners from different disciplines during their training develops competency in subsequent effective collaborative practice. Studies of IPE in MH professional training have found that prelicensure IPE contributes to increased knowledge of roles and responsibilities of different disciplines, improved interprofessional communication and attitudes, and increased willingness to work in teams.12-17
Interprofessional education is a valuable training model, but developing interprofessional learning experiences in a system of diverse and often siloed training programs is difficult. More information about design and implementation of IPE training experiences is needed, particularly in outpatient settings in which integration of traditionally separate discipline-specific care is central to the health care mission. The VA PACT PC-MHI is a strong team-based care model that represents a unique opportunity for training across disciplines in interprofessional collaborative care.
To find innovative approaches to meeting the need for IPE in PACT PC-MHI, the authors developed a new IPE program in PC-MHI at the William S. Middleton Memorial Veterans Hospital (WSMMVH) in Madison, Wisconsin. This article reviews the development, implementation, and first-year evaluation of the training program and discusses the challenges and the IPE areas in need of improvement in PACT PC-MHI.
Methods
In 2012, the VHA launched phase 1 of the Mental Health Education Expansion Initiative (MHEEI), a collaboration of the Office of Academic Affiliations (OAA), VHA Mental Health Services (VHA-MHS), and the Office of Mental Health Operations (OMHO).18 The MHEEI was intended to “increase expertise in critical areas of need, expand the recruitment pipeline of well-trained, highly qualified health care providers in behavioral and mental health disciplines, and promote the utilization of interprofessional team-based care.”18 In response, WSMMVH organized a planning committee and submitted a funding request through the section of MHEEI called PACT With Integrated Behavioral Health Providers. The planning committee included training program directors and staff from psychiatry, pharmacy, social work, psychology, and primary care. The authors received funding for trainees in psychiatry (postgraduate year 4 [PGY-4]), pharmacy/MH residency (PGY-2), pharmacy/ambulatory care (PGY-1), and social work (interns).
Curriculum Development
The planning committee met regularly for 6 months to develop the organization, learning objectives, educational strategies, and implementation plan for the IPE program. The program was organized as a 4- to 12-month clinical rotation with the PC-MHI team in PACT, combined with 12 months of protected weekly IPE time (Table 1).
Learning Objectives
To better understand the educational needs and foci for learning objectives, the interprofessional planning committee reviewed guidelines on training in integrated care and collaborative team-based practice.2,9,10,19-21 These guidelines were compared with existing training opportunities for each discipline to identify training gaps and needs.
Learning objectives were organized into 3 domains: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Table 2 outlines the shared learning objectives linked to each domain that were common to the psychiatry, pharmacy, and social work disciplines. Although many of the learning objectives were shared among all disciplines, each trainee also had discipline-specific clinical activities and learning objectives. Psychiatry and pharmacy residents focused on primary care psychiatric medication consultation and care management for antidepressant medication starts. Social work interns focused on psychosocial and functional assessment and brief problem-focused psychotherapies. Learning objectives were met through direct veteran care in the primary care clinic as part of the PACT PC-MHI team and through interprofessional learning activities during protected weekly education time.
Implementation
Critical stakeholders in implementing the IPE program involved themselves early and throughout the planning process. Stakeholders included VAMC leadership, primary care and MH service line chiefs and clinic managers, training program directors, and PACT staff. Planning committee members gave presentations on the IPE program at MH service line and PACT meetings in the 2 months before program initiation in order to orient staff to learning objectives, program structure, and impact on PACT PC-MHI operations. Throughout the first year, the planning committee continued to meet every 2 weeks to review progress, solve implementation problems, and revise learning objectives and activities.
Trainee Clinical Activities
A wide range of educational strategies were planned to meet learning objectives across the 3 domains. There was strong emphasis on experiential learning through daily PACT and PC-MHI clinical work, team huddles and meetings, and trainee-led program improvement projects.
Psychiatry and PGY-2 pharmacy/MH residents focused on direct and indirect medication consultation and problem-focused assessments. Their clinical activities included PC-MHI medication evaluation and follow-up visits; chart reviews and e-consults for medication recommendations to PACT providers; reviews of care management data and consultations on veterans enrolled in depression and anxiety care management; “curbside consultations” for providers in PACT huddles and meetings and throughout the clinic day; and “warm handoffs,” same-day initial PC-MHI problem-focused assessments performed on PACT provider request. The residents were part of a pool of staff and trainees who performed these assessments.
PGY-1 pharmacy residents made care management phone calls for antidepressant trials for depression and anxiety. These residents were trained in motivational interviewing (MI). They applied their MI skills during care management calls focused on medication adherence and behavioral interventions for depression (eg, exercise, planning pleasurable activity) and during other clinical rotations, including tobacco cessation and medication management for diabetes and hypertension. Particularly challenging veteran cases from these clinics were cosupervised with medication management and PC-MHIstaff for added consultation on engagement, behavior change, and treatment plan adherence.
Social work interns completed initial PC-MHI psychosocial and functional assessments by phone and directly by same-day warm handoffs from PACT staff. The PC-MHI therapies they provided included problem-solving therapy, behavioral activation, stress management based on cognitive behavioral therapy, and brief alcohol interventions.
Group IPE Activities
All trainees had a weekly protected block of 3 hours during which they came together for group IPE that was designed to elicit active participation; facilitate interprofessional communication; and develop an understanding of and respect for the knowledge, culture, and practice style of the different disciplines.
Trainees participated in a Herrmann Brain Dominance Instrument (HBDI) workshop focused on developing a better understanding of individual differences in thinking and problem solving, with the goal of improving communication and learning within teams.22 In a seminar series on professionalism and boundaries in health care, trainees from each discipline gave a presentation on the traditional structure and content of their discipline’s training and discussed similarities and differences in their disciplines’ professional oaths, codes of ethics, and boundary guidelines.
Motivational interviewing training was conducted early in the year so trainees would be prepared to apply MI skills in their daily PACT PC-MHI clinical work. Motivational inteviewing is a patient-centered approach to engaging patients in health promoting behavior change. It is defined as a “directive, client-centered counseling style for eliciting behavior change by helping clients to explore and resolve ambivalence.”23
Trainees recorded MI sessions with at least 2 live-patient visits and at least 2 simulated-patient interviews (with staff serving as patient actors). The structure of MI training and supervision was deliberately designed to facilitate interprofessional communication and learning. In accord with a group supervision model for MI recorded reviews, the trainees presented their tapes to the entire learning group in the presence of a facilitating supervisor. Trainees had the opportunity to observe different interview styles and exchange feedback within a peer group of interprofessional learners.
Seminars were focused on core PC-MHI clinical content (eg, depression, anxiety, alcohol use disorders) and organized around case-based learning. Trainees divided into small teams in which representatives of each discipline offered their perspective on how to approach planning patient assessment and treatment. During the seminars, the authors engaged trainees as teachers and leaders whenever possible. All trainees presented on a topic in which they had some discipline expertise. For example, social work interns led a seminar on support and social services for victims of domestic violence, and PGY-1 pharmacy/ambulatory care residents led seminars and a panel management project focused on diabetes and depression.
Trainees participated in several PACT PC-MHI projects focused on population- and measurement-based care, panel management, and program improvement (Table 3). Protected IPE time was used to teach trainees about population health principles and different tools for process improvement (eg, Vision-Analysis Team-Aim-Map-Measure-Change-Sustain) and provide a forum in which trainees could share their work with one another.
Evaluations
Several tools were used for trainee and program evaluations. Clinical skills were evaluated during daily supervision. Trainees began the year with PC-MHI staff directly observing all their clinical contacts with veterans. Staff evaluated and offered feedback on trainee clinical interviewing and on assessment and treatment planning skills. Once they were assessed to be ready to see veterans on their own, trainees were advanced by staff to “drop-in” direct supervision: Toward the end of a veteran’s visit, a staff preceptor entered the room to review relevant clinical findings, assessment and finalized treatment planning with the trainee and veteran. When appropriate for trainee competence level, clinical contacts were indirectly supervised: Trainees discussed their assessment and treatment plan with a staff supervisor at the end of the day.
Motivational interviewing recordings were reviewed during group supervision. To objectively evaluate MI skills, supervisors who were VA-certified in MI used the Motivational Interviewing Treatment Integrity (MITI) coding tool to review and code both the live- and simulated-patient recordings.24 The MITI coding involves quantitative and qualitative analysis using standardized coding items.
Quantitative items included percentage of open-ended questions (Proficiency: 50%; competency: 70%); percentage of reflections considered complex reflections, or reflective statements adding substantial meaning or emphasis and conveying a deeper or more complex picture of what patients say (Proficiency: 40%; competency: 50%); reflection-to-question ratio (Proficiency: 1:1; competency: 2:1); and percentage of MI-adherent provider statements (Proficiency: 90%; competency: 100%).
Qualitative coding items were a global rating of therapist “empathy,” which evaluated the extent to which the trainee understood or made an effort to grasp the patient’s perspective, and “MI spirit.” This coding intended to capture the overall competence of the trainee in emphasizing collaboration, patient autonomy, and evocation of the patient experience (Proficiency score: 5; competency score: 6).
The PC-MHI teaching staff met midyear and end of year as a team to complete trainee evaluations focused on the 3 areas of learning objectives: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Patient-centered PC-MHI involves direct observation and supervision of trainee clinical contacts with veterans, including assessment and treatment planning, clinical documentation, and review of live- and simulated-patient MI recordings. Collaborative team-based practice involves review of trainee participation in day-to-day teamwork, huddles, team meetings, and IPE activities. Population health and program improvement involves review of trainee work on a panel measurement-based care management or program improvement project. In each learning objectives area, trainees were rated on a 3-point scale: needs improvement (1); satisfactory (2); achieved (3). Core knowledge about PC-MHI evidence base, structure, and clinical topics was assessed with case-based written examination at midyear and end of year.
Surveys and qualitative interviews were used to assess trainee perceptions about the IPE program. A midyear and end-of-year survey assessed trainee satisfaction and perceived efficacy of the IPE training program in meeting core learning objectives. A midyear survey designed by pharmacy residents as part of their program improvement project evaluated attitudes around interprofessional learning and team practice. Trainees met individually with the PC-MHI IPE director at midyear and end of year to gather qualitative feedback on the IPE program.
Outcomes
All trainees advanced to the expected level of supervision for clinical contacts (drop-in or indirect clinical supervision). Over the year, there was significant improvement in trainees’ MI skills as measured by MITI coding of at least 2 live-patient or 2 simulated-patient recordings (Figures 1A and 1B). By end of year, most trainees had reached proficiency or competency in several MITI coded items: percentage of open-ended questions (4/12 proficient, 8/12 competent), percentage of complex reflections (2/12 less than proficient, 3/12 proficient, 7/12 competent), MI adherence (1/12 proficient, 11/12 competent), global empathy rating (2/12 proficient, 10/12 competent), and global MI spirit rating (12/12 competent). Average reflection-to-question ratio for the trainee group increased from 0.63 to 0.96 from midyear to end of year, but only 3 of 12 trainees reached the proficiency level of a 1:1 ratio, and no trainee reached the competency level of a 2:1 ratio.
According to the PC-MHI team’s midyear evaluation, most trainees were already making satisfactory progress in the 3 domains of learning objectives for the training program. At end of year, 13 of 14 trainees were assessed as competent in all 3 domains (Figures 2A and B). All trainees passed the midyear and end-of-year written examinations with overall high scores (average score, 82%) demonstrating acquisition of core PC-MHI clinical knowledge.
Trainee evaluations of the IPE program were overall highly favorable at both midyear and at end of year. Trainees rated the program effective or extremely effective in developing their skills in patient-centered care, interprofessional communication, and collaborative team-based practice. They also rated the program highly effective in preparing them to use team-based practice skills in other settings. On a midyear survey, trainees moderately to strongly agreed with several positive beliefs and attitudes about team-based care.
In qualitative interviews at program completion, trainees across disciplines rated the MI training with group supervision as one of their most valuable interprofessional learning experiences. Other highly valued training experiences were PACT PC-MHI panel management projects, team-based clinical case reviews, and cross-disciplinary supervision.
Discussion
This article describes the successful development and implementation of a VA-based IPE program in PACT PC-MHI. Interprofessional clinical training and educational experiences were highly valued, and trainees identified positive attitudes and improved skills related to team-based care. These findings support previous findings that IPE is associated with high satisfaction and positive attitudes toward team-based collaborative practice.12-17 Program implementation presented several challenges: nonsynchronous academic calendars and rotation schedules, cross-disciplinary supervision regulations, variations in clinical and supervisory requirements for accreditation standards, the traditional health care hierarchy, and measurement of the impact of IPE experiences.11,25,26
Rotation schedules and academic calendars varied across the psychiatry, pharmacy, and social work home programs. Organizing different trainee rotation schedules was a significant challenge. Collaboration with training directors and support staff was crucial in planning rotations that offered a longitudinal training experience in PACT PC-MHI. Given the participants’ different starting dates, protected IPE time early in the calendar year was focused on developing clinical skills specific to the pharmacy and psychiatry trainees who would be starting in July, and IPE activities that required the presence of all trainee disciplines (eg, MI training, HBDI) were planned for after the September start of the social work interns.
Cross-disciplinary supervision was highly valued by trainees because it offered exposure to the disciplines’ different communication styles and approaches to clinical assessment and decision making. Throughout the year, however, trainees encountered several obstacles to cross-disciplinary supervision, with respect to coding/billing and home program supervisory policies. The authors worked closely with the VA administration and each training program to develop and revise supervising policies and procedures to meet the necessary administrative and program supervisory requirements for accreditation standards.
In some cases, this work resulted in dual supervision by a preceptor of the same discipline (to meet requirements for coding/billing and home program supervision) and clinical supervision by a preceptor of a different discipline (eg, in team meetings or in clinical case reviews during protected IPE time). Preceptors from each discipline met regularly to discuss challenges in cross-disciplinary supervision, review scope-of-practice issues, share information on discipline-specific training, and revise supervisory procedures.
Interprofessional education activities during weekly protected time were designed to improve collaboration and to challenge trainees to examine traditional hierarchical roles and patterns of communication in health care. An emphasis on case-based learning in small groups encouraged trainees to share perspectives on their discipline-specific approach to assessment and treatment planning. Motivational interviewing training, one of the IPE experiences most valued by trainees and staff, created the opportunity for a truly shared learning environment, as trainees largely started at about the same skill level despite having different educational backgrounds. Group supervision of MI recordings offered trainees the opportunity to learn from each other and develop comfort in offering and receiving interprofessional constructive feedback.
Limitations
There were considerable methodologic limitations in the authors’ efforts to evaluate the impact of this training program on trainee attitudes and skills in collaborative team-based practice. Although trainee surveys revealed highly positive attitudes and beliefs about team-based care as well as perceived competence in collaborative practice, these were not validated surveys, and changes could not be accurately measured over time (trainees were not assessed at baseline). Trainees also were involved in other clinical teams within the VA during the year, and it is difficult to assess the specific impact of their PC-MHI IPE experiences. The PC-MHI staff evaluations of trainee competence in collaborative team-based practice were largely observational and potentially vulnerable to biases, as the staff evaluating trainee competence also were part of the IPE program planning process and invested in its success.
To address these limitations in the future, the authors will use better assessment tools at baseline and end of year to more effectively evaluate the impact of the training program on teamwork skills as well changes in attitudes and beliefs about interprofessional learning and teamwork. The Attitudes Toward Interprofessional Health Care Teams Scale and the Perceptions of Effective Interpersonal Teams Scale should be considered as they have published reliability and validity.27,28 The authors could improve the reliability and depth of trainee evaluations with use of a “360-degree evaluation” model for trainee evaluations that includes other PACT members (beyond PC-MHI staff) as well as veterans.
Assessment of MI skills and competency was limited by use of both live- and simulated-patient recordings. Simulation is a valuable learning tool but often does not accurately represent actual clinical situations and challenges. To appropriately assess MI competence, future MI training should emphasize live-patient recordings over simulated-patient visits. Furthermore, whereas trainees reached competency in several MI coding items, none reached competency in the reflection-to-question ratio, and only about half reached competency in percentage of complex reflections. Future MI training will need to focus on further development of reflection skills.
Trainee intentions to remain involved in program improvement and collaborative team-based care in future professional work were core attitudinal learning objectives, but neither was adequately assessed in end-of-year surveys. Ideally, future evaluations will assess subjective trainee intentions and goals around team-based work and objectively measure future professional choices and activities. For example, it would be interesting to determine whether trainees who participated in this program will choose to practice in an interprofessional team-based model or participate in program improvement activities.
Last, the absence of psychology interns was considered a weakness in the learning environment, resulting in a relatively “prescriber-heavy” balance in discipline perspectives for IPE-focused case discussions and other training. Furthermore, the discipline representation in the IPE program did not exemplify the typical PC-MHI team in most VA clinic settings and community practices in which psychology has a strong presence. Adding psychology trainees was an important goal for the IPE program leadership. In 2015, WSMMVH was awarded additional funding for a PACT PC-MHI predoctoral psychology intern through the phase 4 MHEEI. The PC-MHI track psychology intern joined the IPE program in the 2016-2017 training year.
Conclusion
There is broad consensus that interprofessional team-based practice is crucial for providers in the VA and all health care systems. Primary care-mental health integration is an area of VA care in which interprofessional collaboration is uniquely important to implementation and sustainability of the model. Interprofessional education is an effective approach for preparing HCPs for team-based practice, but implementation is challenging. Several factors crucially contributed to the successful implementation of this program: collaboration of the interprofessional planning team with representation from key stakeholders in different departments and training programs; a well-established PACT PC-MHI team with experience in collaborative team-based care; a curriculum structure that emphasized experiential educational strategies designed to promote interprofessional learning and communication; VA leadership support at the national level (MHEEI funding) and local level; and PACT PC-MHI clinical staff committed to teaching and to the IPE mission.
Over the past 10 years, the VHA has been a national leader in primary care-mental health integration (PC-MHI) within patient aligned care teams (PACTs).1,2 Studies of the PC-MHI collaborative care model consistently have shown increased access to MH services, higher levels of MH treatment engagement, improved MH treatment outcomes, and high patient and provider satisfaction.3-7 Primary care-mental health integration relies heavily on interprofessional team-based practice with providers from diverse educational and clinical backgrounds who work together to deliver integrated mental and behavioral health services within PACTs. This model requires a unique blending of professional cultures and communication and practice styles.
To sustain PC-MHI in PACT, health care professionals (HCPs) must be well trained to work effectively in interprofessional teams. Across health care organizations, training in collaborative interprofessional team-based practice has been identified as an important and challenging task.8-11
Integrating educational experiences among different HCP learners is an approach to developing competency in interprofessional collaboration early in training. The World Health Organization defined interprofessional education (IPE) as occurring “when students from two or more professions learn about, from, and with each other to enable effective collaboration and improve health outcomes.”9 Fundamental to this definition is the belief that interaction among learners from different disciplines during their training develops competency in subsequent effective collaborative practice. Studies of IPE in MH professional training have found that prelicensure IPE contributes to increased knowledge of roles and responsibilities of different disciplines, improved interprofessional communication and attitudes, and increased willingness to work in teams.12-17
Interprofessional education is a valuable training model, but developing interprofessional learning experiences in a system of diverse and often siloed training programs is difficult. More information about design and implementation of IPE training experiences is needed, particularly in outpatient settings in which integration of traditionally separate discipline-specific care is central to the health care mission. The VA PACT PC-MHI is a strong team-based care model that represents a unique opportunity for training across disciplines in interprofessional collaborative care.
To find innovative approaches to meeting the need for IPE in PACT PC-MHI, the authors developed a new IPE program in PC-MHI at the William S. Middleton Memorial Veterans Hospital (WSMMVH) in Madison, Wisconsin. This article reviews the development, implementation, and first-year evaluation of the training program and discusses the challenges and the IPE areas in need of improvement in PACT PC-MHI.
Methods
In 2012, the VHA launched phase 1 of the Mental Health Education Expansion Initiative (MHEEI), a collaboration of the Office of Academic Affiliations (OAA), VHA Mental Health Services (VHA-MHS), and the Office of Mental Health Operations (OMHO).18 The MHEEI was intended to “increase expertise in critical areas of need, expand the recruitment pipeline of well-trained, highly qualified health care providers in behavioral and mental health disciplines, and promote the utilization of interprofessional team-based care.”18 In response, WSMMVH organized a planning committee and submitted a funding request through the section of MHEEI called PACT With Integrated Behavioral Health Providers. The planning committee included training program directors and staff from psychiatry, pharmacy, social work, psychology, and primary care. The authors received funding for trainees in psychiatry (postgraduate year 4 [PGY-4]), pharmacy/MH residency (PGY-2), pharmacy/ambulatory care (PGY-1), and social work (interns).
Curriculum Development
The planning committee met regularly for 6 months to develop the organization, learning objectives, educational strategies, and implementation plan for the IPE program. The program was organized as a 4- to 12-month clinical rotation with the PC-MHI team in PACT, combined with 12 months of protected weekly IPE time (Table 1).
Learning Objectives
To better understand the educational needs and foci for learning objectives, the interprofessional planning committee reviewed guidelines on training in integrated care and collaborative team-based practice.2,9,10,19-21 These guidelines were compared with existing training opportunities for each discipline to identify training gaps and needs.
Learning objectives were organized into 3 domains: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Table 2 outlines the shared learning objectives linked to each domain that were common to the psychiatry, pharmacy, and social work disciplines. Although many of the learning objectives were shared among all disciplines, each trainee also had discipline-specific clinical activities and learning objectives. Psychiatry and pharmacy residents focused on primary care psychiatric medication consultation and care management for antidepressant medication starts. Social work interns focused on psychosocial and functional assessment and brief problem-focused psychotherapies. Learning objectives were met through direct veteran care in the primary care clinic as part of the PACT PC-MHI team and through interprofessional learning activities during protected weekly education time.
Implementation
Critical stakeholders in implementing the IPE program involved themselves early and throughout the planning process. Stakeholders included VAMC leadership, primary care and MH service line chiefs and clinic managers, training program directors, and PACT staff. Planning committee members gave presentations on the IPE program at MH service line and PACT meetings in the 2 months before program initiation in order to orient staff to learning objectives, program structure, and impact on PACT PC-MHI operations. Throughout the first year, the planning committee continued to meet every 2 weeks to review progress, solve implementation problems, and revise learning objectives and activities.
Trainee Clinical Activities
A wide range of educational strategies were planned to meet learning objectives across the 3 domains. There was strong emphasis on experiential learning through daily PACT and PC-MHI clinical work, team huddles and meetings, and trainee-led program improvement projects.
Psychiatry and PGY-2 pharmacy/MH residents focused on direct and indirect medication consultation and problem-focused assessments. Their clinical activities included PC-MHI medication evaluation and follow-up visits; chart reviews and e-consults for medication recommendations to PACT providers; reviews of care management data and consultations on veterans enrolled in depression and anxiety care management; “curbside consultations” for providers in PACT huddles and meetings and throughout the clinic day; and “warm handoffs,” same-day initial PC-MHI problem-focused assessments performed on PACT provider request. The residents were part of a pool of staff and trainees who performed these assessments.
PGY-1 pharmacy residents made care management phone calls for antidepressant trials for depression and anxiety. These residents were trained in motivational interviewing (MI). They applied their MI skills during care management calls focused on medication adherence and behavioral interventions for depression (eg, exercise, planning pleasurable activity) and during other clinical rotations, including tobacco cessation and medication management for diabetes and hypertension. Particularly challenging veteran cases from these clinics were cosupervised with medication management and PC-MHIstaff for added consultation on engagement, behavior change, and treatment plan adherence.
Social work interns completed initial PC-MHI psychosocial and functional assessments by phone and directly by same-day warm handoffs from PACT staff. The PC-MHI therapies they provided included problem-solving therapy, behavioral activation, stress management based on cognitive behavioral therapy, and brief alcohol interventions.
Group IPE Activities
All trainees had a weekly protected block of 3 hours during which they came together for group IPE that was designed to elicit active participation; facilitate interprofessional communication; and develop an understanding of and respect for the knowledge, culture, and practice style of the different disciplines.
Trainees participated in a Herrmann Brain Dominance Instrument (HBDI) workshop focused on developing a better understanding of individual differences in thinking and problem solving, with the goal of improving communication and learning within teams.22 In a seminar series on professionalism and boundaries in health care, trainees from each discipline gave a presentation on the traditional structure and content of their discipline’s training and discussed similarities and differences in their disciplines’ professional oaths, codes of ethics, and boundary guidelines.
Motivational interviewing training was conducted early in the year so trainees would be prepared to apply MI skills in their daily PACT PC-MHI clinical work. Motivational inteviewing is a patient-centered approach to engaging patients in health promoting behavior change. It is defined as a “directive, client-centered counseling style for eliciting behavior change by helping clients to explore and resolve ambivalence.”23
Trainees recorded MI sessions with at least 2 live-patient visits and at least 2 simulated-patient interviews (with staff serving as patient actors). The structure of MI training and supervision was deliberately designed to facilitate interprofessional communication and learning. In accord with a group supervision model for MI recorded reviews, the trainees presented their tapes to the entire learning group in the presence of a facilitating supervisor. Trainees had the opportunity to observe different interview styles and exchange feedback within a peer group of interprofessional learners.
Seminars were focused on core PC-MHI clinical content (eg, depression, anxiety, alcohol use disorders) and organized around case-based learning. Trainees divided into small teams in which representatives of each discipline offered their perspective on how to approach planning patient assessment and treatment. During the seminars, the authors engaged trainees as teachers and leaders whenever possible. All trainees presented on a topic in which they had some discipline expertise. For example, social work interns led a seminar on support and social services for victims of domestic violence, and PGY-1 pharmacy/ambulatory care residents led seminars and a panel management project focused on diabetes and depression.
Trainees participated in several PACT PC-MHI projects focused on population- and measurement-based care, panel management, and program improvement (Table 3). Protected IPE time was used to teach trainees about population health principles and different tools for process improvement (eg, Vision-Analysis Team-Aim-Map-Measure-Change-Sustain) and provide a forum in which trainees could share their work with one another.
Evaluations
Several tools were used for trainee and program evaluations. Clinical skills were evaluated during daily supervision. Trainees began the year with PC-MHI staff directly observing all their clinical contacts with veterans. Staff evaluated and offered feedback on trainee clinical interviewing and on assessment and treatment planning skills. Once they were assessed to be ready to see veterans on their own, trainees were advanced by staff to “drop-in” direct supervision: Toward the end of a veteran’s visit, a staff preceptor entered the room to review relevant clinical findings, assessment and finalized treatment planning with the trainee and veteran. When appropriate for trainee competence level, clinical contacts were indirectly supervised: Trainees discussed their assessment and treatment plan with a staff supervisor at the end of the day.
Motivational interviewing recordings were reviewed during group supervision. To objectively evaluate MI skills, supervisors who were VA-certified in MI used the Motivational Interviewing Treatment Integrity (MITI) coding tool to review and code both the live- and simulated-patient recordings.24 The MITI coding involves quantitative and qualitative analysis using standardized coding items.
Quantitative items included percentage of open-ended questions (Proficiency: 50%; competency: 70%); percentage of reflections considered complex reflections, or reflective statements adding substantial meaning or emphasis and conveying a deeper or more complex picture of what patients say (Proficiency: 40%; competency: 50%); reflection-to-question ratio (Proficiency: 1:1; competency: 2:1); and percentage of MI-adherent provider statements (Proficiency: 90%; competency: 100%).
Qualitative coding items were a global rating of therapist “empathy,” which evaluated the extent to which the trainee understood or made an effort to grasp the patient’s perspective, and “MI spirit.” This coding intended to capture the overall competence of the trainee in emphasizing collaboration, patient autonomy, and evocation of the patient experience (Proficiency score: 5; competency score: 6).
The PC-MHI teaching staff met midyear and end of year as a team to complete trainee evaluations focused on the 3 areas of learning objectives: patient-centered PC-MHI, collaborative team-based practice, and population health and program improvement. Patient-centered PC-MHI involves direct observation and supervision of trainee clinical contacts with veterans, including assessment and treatment planning, clinical documentation, and review of live- and simulated-patient MI recordings. Collaborative team-based practice involves review of trainee participation in day-to-day teamwork, huddles, team meetings, and IPE activities. Population health and program improvement involves review of trainee work on a panel measurement-based care management or program improvement project. In each learning objectives area, trainees were rated on a 3-point scale: needs improvement (1); satisfactory (2); achieved (3). Core knowledge about PC-MHI evidence base, structure, and clinical topics was assessed with case-based written examination at midyear and end of year.
Surveys and qualitative interviews were used to assess trainee perceptions about the IPE program. A midyear and end-of-year survey assessed trainee satisfaction and perceived efficacy of the IPE training program in meeting core learning objectives. A midyear survey designed by pharmacy residents as part of their program improvement project evaluated attitudes around interprofessional learning and team practice. Trainees met individually with the PC-MHI IPE director at midyear and end of year to gather qualitative feedback on the IPE program.
Outcomes
All trainees advanced to the expected level of supervision for clinical contacts (drop-in or indirect clinical supervision). Over the year, there was significant improvement in trainees’ MI skills as measured by MITI coding of at least 2 live-patient or 2 simulated-patient recordings (Figures 1A and 1B). By end of year, most trainees had reached proficiency or competency in several MITI coded items: percentage of open-ended questions (4/12 proficient, 8/12 competent), percentage of complex reflections (2/12 less than proficient, 3/12 proficient, 7/12 competent), MI adherence (1/12 proficient, 11/12 competent), global empathy rating (2/12 proficient, 10/12 competent), and global MI spirit rating (12/12 competent). Average reflection-to-question ratio for the trainee group increased from 0.63 to 0.96 from midyear to end of year, but only 3 of 12 trainees reached the proficiency level of a 1:1 ratio, and no trainee reached the competency level of a 2:1 ratio.
According to the PC-MHI team’s midyear evaluation, most trainees were already making satisfactory progress in the 3 domains of learning objectives for the training program. At end of year, 13 of 14 trainees were assessed as competent in all 3 domains (Figures 2A and B). All trainees passed the midyear and end-of-year written examinations with overall high scores (average score, 82%) demonstrating acquisition of core PC-MHI clinical knowledge.
Trainee evaluations of the IPE program were overall highly favorable at both midyear and at end of year. Trainees rated the program effective or extremely effective in developing their skills in patient-centered care, interprofessional communication, and collaborative team-based practice. They also rated the program highly effective in preparing them to use team-based practice skills in other settings. On a midyear survey, trainees moderately to strongly agreed with several positive beliefs and attitudes about team-based care.
In qualitative interviews at program completion, trainees across disciplines rated the MI training with group supervision as one of their most valuable interprofessional learning experiences. Other highly valued training experiences were PACT PC-MHI panel management projects, team-based clinical case reviews, and cross-disciplinary supervision.
Discussion
This article describes the successful development and implementation of a VA-based IPE program in PACT PC-MHI. Interprofessional clinical training and educational experiences were highly valued, and trainees identified positive attitudes and improved skills related to team-based care. These findings support previous findings that IPE is associated with high satisfaction and positive attitudes toward team-based collaborative practice.12-17 Program implementation presented several challenges: nonsynchronous academic calendars and rotation schedules, cross-disciplinary supervision regulations, variations in clinical and supervisory requirements for accreditation standards, the traditional health care hierarchy, and measurement of the impact of IPE experiences.11,25,26
Rotation schedules and academic calendars varied across the psychiatry, pharmacy, and social work home programs. Organizing different trainee rotation schedules was a significant challenge. Collaboration with training directors and support staff was crucial in planning rotations that offered a longitudinal training experience in PACT PC-MHI. Given the participants’ different starting dates, protected IPE time early in the calendar year was focused on developing clinical skills specific to the pharmacy and psychiatry trainees who would be starting in July, and IPE activities that required the presence of all trainee disciplines (eg, MI training, HBDI) were planned for after the September start of the social work interns.
Cross-disciplinary supervision was highly valued by trainees because it offered exposure to the disciplines’ different communication styles and approaches to clinical assessment and decision making. Throughout the year, however, trainees encountered several obstacles to cross-disciplinary supervision, with respect to coding/billing and home program supervisory policies. The authors worked closely with the VA administration and each training program to develop and revise supervising policies and procedures to meet the necessary administrative and program supervisory requirements for accreditation standards.
In some cases, this work resulted in dual supervision by a preceptor of the same discipline (to meet requirements for coding/billing and home program supervision) and clinical supervision by a preceptor of a different discipline (eg, in team meetings or in clinical case reviews during protected IPE time). Preceptors from each discipline met regularly to discuss challenges in cross-disciplinary supervision, review scope-of-practice issues, share information on discipline-specific training, and revise supervisory procedures.
Interprofessional education activities during weekly protected time were designed to improve collaboration and to challenge trainees to examine traditional hierarchical roles and patterns of communication in health care. An emphasis on case-based learning in small groups encouraged trainees to share perspectives on their discipline-specific approach to assessment and treatment planning. Motivational interviewing training, one of the IPE experiences most valued by trainees and staff, created the opportunity for a truly shared learning environment, as trainees largely started at about the same skill level despite having different educational backgrounds. Group supervision of MI recordings offered trainees the opportunity to learn from each other and develop comfort in offering and receiving interprofessional constructive feedback.
Limitations
There were considerable methodologic limitations in the authors’ efforts to evaluate the impact of this training program on trainee attitudes and skills in collaborative team-based practice. Although trainee surveys revealed highly positive attitudes and beliefs about team-based care as well as perceived competence in collaborative practice, these were not validated surveys, and changes could not be accurately measured over time (trainees were not assessed at baseline). Trainees also were involved in other clinical teams within the VA during the year, and it is difficult to assess the specific impact of their PC-MHI IPE experiences. The PC-MHI staff evaluations of trainee competence in collaborative team-based practice were largely observational and potentially vulnerable to biases, as the staff evaluating trainee competence also were part of the IPE program planning process and invested in its success.
To address these limitations in the future, the authors will use better assessment tools at baseline and end of year to more effectively evaluate the impact of the training program on teamwork skills as well changes in attitudes and beliefs about interprofessional learning and teamwork. The Attitudes Toward Interprofessional Health Care Teams Scale and the Perceptions of Effective Interpersonal Teams Scale should be considered as they have published reliability and validity.27,28 The authors could improve the reliability and depth of trainee evaluations with use of a “360-degree evaluation” model for trainee evaluations that includes other PACT members (beyond PC-MHI staff) as well as veterans.
Assessment of MI skills and competency was limited by use of both live- and simulated-patient recordings. Simulation is a valuable learning tool but often does not accurately represent actual clinical situations and challenges. To appropriately assess MI competence, future MI training should emphasize live-patient recordings over simulated-patient visits. Furthermore, whereas trainees reached competency in several MI coding items, none reached competency in the reflection-to-question ratio, and only about half reached competency in percentage of complex reflections. Future MI training will need to focus on further development of reflection skills.
Trainee intentions to remain involved in program improvement and collaborative team-based care in future professional work were core attitudinal learning objectives, but neither was adequately assessed in end-of-year surveys. Ideally, future evaluations will assess subjective trainee intentions and goals around team-based work and objectively measure future professional choices and activities. For example, it would be interesting to determine whether trainees who participated in this program will choose to practice in an interprofessional team-based model or participate in program improvement activities.
Last, the absence of psychology interns was considered a weakness in the learning environment, resulting in a relatively “prescriber-heavy” balance in discipline perspectives for IPE-focused case discussions and other training. Furthermore, the discipline representation in the IPE program did not exemplify the typical PC-MHI team in most VA clinic settings and community practices in which psychology has a strong presence. Adding psychology trainees was an important goal for the IPE program leadership. In 2015, WSMMVH was awarded additional funding for a PACT PC-MHI predoctoral psychology intern through the phase 4 MHEEI. The PC-MHI track psychology intern joined the IPE program in the 2016-2017 training year.
Conclusion
There is broad consensus that interprofessional team-based practice is crucial for providers in the VA and all health care systems. Primary care-mental health integration is an area of VA care in which interprofessional collaboration is uniquely important to implementation and sustainability of the model. Interprofessional education is an effective approach for preparing HCPs for team-based practice, but implementation is challenging. Several factors crucially contributed to the successful implementation of this program: collaboration of the interprofessional planning team with representation from key stakeholders in different departments and training programs; a well-established PACT PC-MHI team with experience in collaborative team-based care; a curriculum structure that emphasized experiential educational strategies designed to promote interprofessional learning and communication; VA leadership support at the national level (MHEEI funding) and local level; and PACT PC-MHI clinical staff committed to teaching and to the IPE mission.
1. Wray LO, Szymanski BR, Kearney LK, McCarthy JF. Implementation of primary care-mental health integration services in the Veterans Health Administration: program activity and associations with engagement in specialty mental health services. J Clin Psychol Med Settings. 2012;19(1):105-116.
2. Kearney LK, Post EP, Pomerantz AS, Zeiss AM. Applying the interprofessional patient aligned care team in the Department of Veterans Affairs: transforming primary care. Am Psychol. 2014;69(4):399-408.
3. Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of loner-term outcomes. Arch Intern Med. 2006;166(21):2314-2321.
4. Unützer J, Katon W, Callahan CM, et al; IMPACT (Improving Mood-Promoting Access to Collaborative Treatment) Investigators. Collaborative care management in late-life depression in the primary care setting: a randomized control trial. JAMA. 2002;288(22):2836-2845.
5. Katon W, Unützer J. Consultation psychiatry in the medical home and accountable care organizations: achieving the triple aim. Gen Hosp Psychiatry. 2011;3(4):305-310.
6. Bruce ML, Tenhave TR, Reynolds CF III, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291(9):1081-1091.
7. Alexopoulos GS, Reynolds CF III , Bruce ML, et al; PROSPECT Group. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry. 2009;166(8):882-890.
8. Cox M, Cuff P, Brandt B, Reeves S, Zierler B. Measuring the impact of interprofessional education on collaborative practice and patient outcomes. J Interprof Care. 2016;30(1):1-3.
9. World Health Organization, Study Group on Interprofessional Education and Collaborative Practice. Framework for Action on Interprofessional Education and Collaborative Practice. Geneva, Switzerland: World Health Organization; 2010.
10. Interprofessional Education Collaborative Expert Panel. Core Competencies for Interprofessional Collaborative Practice: Report of an Expert Panel. Washington, DC: Interprofessional Education Collaborative; 2011.
11. Blue AV, Mitcham M, Smith T, Raymond J, Greenburg R. Changing the future of health professions: embedding interprofessional education within an academic health center. Acad Med. 2010;85(8):1290-1295.
12. Priest HM, Roberts P, Dent H, Blincoe C, Lawton D, Armstrong C. Interprofessional education and working in mental health: in search of the evidence base. J Nurs Manag. 2008;16(4):474-485.
13. Reeves S. A systematic review of the effects of interprofessional education on staff involved in the care of adults with mental health problems. J Psychiatr Ment Health Nurs. 2001;8(6):533-542.
14. Carpenter J, Barnes D, Dickinson C, Wooff D. Outcomes of interprofessional education for community mental health services in England: the longitudinal evaluation of a postgraduate programme. J Interprof Care. 2006;20(2):145-161.
15. Hammick M, Freeth D, Koppel I, Reeves S, Barr H. A best evidence systematic review of interprofessional education: BEME guide no. 9. Med Teach. 2007;29(8):735-751.
16. Pauzé E, Reeves S. Examining the effects of interprofessional education on mental health providers: findings from an updated systematic review. J Ment Health. 2010;19(3):258-271.
17. Curran V, Heath O, Adey T, et al. An approach to integrating interprofessional education in collaborative mental health care. Acad Psychiatry. 2012;36(2):91-95.
18. Veterans Health Administration. VA Interprofessional Mental Health Education Expansion Initiative, Phase I. Washington, DC; Department of Veterans Affairs; 2012.
19. Dundon M, Dollar K, Schohn M, Lantinga LJ. Primary care–mental health integration: co-located, collaborative care: an operations manual. https://www.mirecc.va.gov/cih-visn2/Documents/Clinical/MH-IPC_CCC_Operations_Manual_Version_2_1.pdf. Published February 2011. Accessed May 19, 2017.
20. McDaniel SH, Grus CL, Cubic BA, et al. Competencies for psychology practice in primary care. Am Psychol. 2014;69(4):409-429.
21. Cowley D, Dunaway K, Forstein M, et al. Teaching psychiatry residents to work at the interface of mental health and primary care. Acad Psychiatry. 2014;38(4):398-404.
22. Herrmann N. The creative brain. J Creat Behav. 1991;25:275-295.
23. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
24. Pierson HM, Hayes SC, Gifford EV, et al. An examination of the Motivational Interviewing Treatment Integrity code. J Subst Abuse Treat. 2007;32(1):11-17.
25. Shaw D, Blue A. Should psychiatry champion interprofessional education? Acad Psychiatry. 2012;36(3):163-166.
26. Gilbert JH. Interprofessional learning and higher education structural barriers. J Interprof Care. 2005;19(suppl 1):87-106.
27. Heinemann GD, Schmitt MH, Farrell PP, Brallier SA. Development of an Attitudes Toward Health Care Teams Scale. Eval Health Prof. 1999;22(1):123-142.
28. Hepburn K, Tsukuda RA, Fasser C. Team Skills Scale. In: Heinemann GD, Zeiss AM, eds. Team Performance in Healthcare: Assessment and Development. New York, NY: Kluwer Academic/Plenum; 2002:159-163.
1. Wray LO, Szymanski BR, Kearney LK, McCarthy JF. Implementation of primary care-mental health integration services in the Veterans Health Administration: program activity and associations with engagement in specialty mental health services. J Clin Psychol Med Settings. 2012;19(1):105-116.
2. Kearney LK, Post EP, Pomerantz AS, Zeiss AM. Applying the interprofessional patient aligned care team in the Department of Veterans Affairs: transforming primary care. Am Psychol. 2014;69(4):399-408.
3. Gilbody S, Bower P, Fletcher J, Richards D, Sutton AJ. Collaborative care for depression: a cumulative meta-analysis and review of loner-term outcomes. Arch Intern Med. 2006;166(21):2314-2321.
4. Unützer J, Katon W, Callahan CM, et al; IMPACT (Improving Mood-Promoting Access to Collaborative Treatment) Investigators. Collaborative care management in late-life depression in the primary care setting: a randomized control trial. JAMA. 2002;288(22):2836-2845.
5. Katon W, Unützer J. Consultation psychiatry in the medical home and accountable care organizations: achieving the triple aim. Gen Hosp Psychiatry. 2011;3(4):305-310.
6. Bruce ML, Tenhave TR, Reynolds CF III, et al. Reducing suicidal ideation and depressive symptoms in depressed older primary care patients: a randomized controlled trial. JAMA. 2004;291(9):1081-1091.
7. Alexopoulos GS, Reynolds CF III , Bruce ML, et al; PROSPECT Group. Reducing suicidal ideation and depression in older primary care patients: 24-month outcomes of the PROSPECT study. Am J Psychiatry. 2009;166(8):882-890.
8. Cox M, Cuff P, Brandt B, Reeves S, Zierler B. Measuring the impact of interprofessional education on collaborative practice and patient outcomes. J Interprof Care. 2016;30(1):1-3.
9. World Health Organization, Study Group on Interprofessional Education and Collaborative Practice. Framework for Action on Interprofessional Education and Collaborative Practice. Geneva, Switzerland: World Health Organization; 2010.
10. Interprofessional Education Collaborative Expert Panel. Core Competencies for Interprofessional Collaborative Practice: Report of an Expert Panel. Washington, DC: Interprofessional Education Collaborative; 2011.
11. Blue AV, Mitcham M, Smith T, Raymond J, Greenburg R. Changing the future of health professions: embedding interprofessional education within an academic health center. Acad Med. 2010;85(8):1290-1295.
12. Priest HM, Roberts P, Dent H, Blincoe C, Lawton D, Armstrong C. Interprofessional education and working in mental health: in search of the evidence base. J Nurs Manag. 2008;16(4):474-485.
13. Reeves S. A systematic review of the effects of interprofessional education on staff involved in the care of adults with mental health problems. J Psychiatr Ment Health Nurs. 2001;8(6):533-542.
14. Carpenter J, Barnes D, Dickinson C, Wooff D. Outcomes of interprofessional education for community mental health services in England: the longitudinal evaluation of a postgraduate programme. J Interprof Care. 2006;20(2):145-161.
15. Hammick M, Freeth D, Koppel I, Reeves S, Barr H. A best evidence systematic review of interprofessional education: BEME guide no. 9. Med Teach. 2007;29(8):735-751.
16. Pauzé E, Reeves S. Examining the effects of interprofessional education on mental health providers: findings from an updated systematic review. J Ment Health. 2010;19(3):258-271.
17. Curran V, Heath O, Adey T, et al. An approach to integrating interprofessional education in collaborative mental health care. Acad Psychiatry. 2012;36(2):91-95.
18. Veterans Health Administration. VA Interprofessional Mental Health Education Expansion Initiative, Phase I. Washington, DC; Department of Veterans Affairs; 2012.
19. Dundon M, Dollar K, Schohn M, Lantinga LJ. Primary care–mental health integration: co-located, collaborative care: an operations manual. https://www.mirecc.va.gov/cih-visn2/Documents/Clinical/MH-IPC_CCC_Operations_Manual_Version_2_1.pdf. Published February 2011. Accessed May 19, 2017.
20. McDaniel SH, Grus CL, Cubic BA, et al. Competencies for psychology practice in primary care. Am Psychol. 2014;69(4):409-429.
21. Cowley D, Dunaway K, Forstein M, et al. Teaching psychiatry residents to work at the interface of mental health and primary care. Acad Psychiatry. 2014;38(4):398-404.
22. Herrmann N. The creative brain. J Creat Behav. 1991;25:275-295.
23. Rollnick S, Miller WR. What is motivational interviewing? Behav Cogn Psychother. 1995;23(4):325-334.
24. Pierson HM, Hayes SC, Gifford EV, et al. An examination of the Motivational Interviewing Treatment Integrity code. J Subst Abuse Treat. 2007;32(1):11-17.
25. Shaw D, Blue A. Should psychiatry champion interprofessional education? Acad Psychiatry. 2012;36(3):163-166.
26. Gilbert JH. Interprofessional learning and higher education structural barriers. J Interprof Care. 2005;19(suppl 1):87-106.
27. Heinemann GD, Schmitt MH, Farrell PP, Brallier SA. Development of an Attitudes Toward Health Care Teams Scale. Eval Health Prof. 1999;22(1):123-142.
28. Hepburn K, Tsukuda RA, Fasser C. Team Skills Scale. In: Heinemann GD, Zeiss AM, eds. Team Performance in Healthcare: Assessment and Development. New York, NY: Kluwer Academic/Plenum; 2002:159-163.
The Design and Implementation of a Home-Based Cardiac Rehabilitation Program
Despite a 30% decline in heart disease mortality from 2001 to 2011, heart disease prevalence is on the rise, responsible for 1 of every 3 deaths in the U.S.1 Cardiac rehabilitation (CR) is an evidence-based, secondary prevention strategy that has been proven effective in preventing future cardiovascular events and decreasing heart disease mortality.2-4 The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) is the leading authority on CR and provides guidelines for CR programs. The AACVPR and the American Heart Association (AHA) published core components for CR programs deemed essential for all CR/secondary prevention programs, including evaluations, interventions, and expected outcomes.5 These core components are aimed at promoting a healthy lifestyle and increasing function and well-being while reducing injury, death, and the reoccurrence of disease.6
In a meta-analysis of 47 trials with 10,794 participants, CR reduced cardiovascular disease (CVD) mortality and hospital admissions by 26% and 18%, respectively.2 Performance measures (Class 1, Level A) recommend the following types of patients should be referred from the inpatient setting: “all patients hospitalized with a primary diagnosis of an acute myocardial infarction (MI) or chronic stable angina, or who during hospitalization have undergone coronary artery bypass graft (CABG) surgery, a percutaneous coronary intervention (PCI), cardiac valve surgery, or cardiac transplantation.”7 However, despite overwhelming evidence and widespread endorsement (Class 1, Level A), service utilization, uptake, and patient adherence to CR programs remain suboptimal. In a U.S. study of claims from > 250,000 Medicare beneficiaries, < 30% of eligible patients participated
This treatment gap is echoed throughout the VHA. Schopfer and colleagues found that only 28% of the 124 VAMCs that provide inpatient care also offer a supervised, facility-based CR program.10 Furthermore, only 10.3% of eligible veterans participated in at least 1 CR session (VA or non-VA). On a systemic level, low patient referral rates and inadequate third-party reimbursement were the most common barriers to participation in CR.10,11 On a patient level, distance was by far the largest barrier to veterans receiving CR. Currently, 74% of the 9.3 million VA-enrolled veterans live at least 1 hour by car from a VA facility that offers CR.9 Within some regions of the VHA, there are no VA facility-based CR programs. For example, VISN 21 has no facility-based CR programs. At the same time, referral of eligible veterans to facility-based CR outside the VA remains low. Prior to April 2013, < 2% of qualified patients residing in VISN 21 were being referred to Non-VA CR programs, making it the VISN with the lowest participation rate for CR.
One potential solution that addresses both systemic and patient barriers to CR utilization is home-based CR. Veterans within the wide geographic area of VISN 21 are referred to San Francisco VAMC (SFVAMC) for ischemic heart disease, cardiovascular revascularization, and cardiac valve surgeries. In 2013, a comprehensive home-based CR program named The Healthy Heart Program was developed based on a successful evidence-based CVD secondary prevention program. The Healthy Heart Program is designed to be a physician-directed, nurse case-managed, customized exercise and lifestyle program that provides a safe and convenient way for veterans to participate in CR. Exercise and disease self-management education are the cornerstones of the Healthy Heart Program. The program’s multidisciplinary team includes physicians, nurses, a dietician, an exercise physiologist, and a health behavior psychologist.
An Alternative Approach
DeBusk and colleagues demonstrated that a physician-directed, nurse-managed, home-based cardiac risk-factor modification program improved smoking cessation, reduced low-density lipoprotein cholesterol, and increased exercise capacity compared with usual care.12 The results of this study helped pave the way for one of the first CR programs with a strong home-based element. The MULTIFIT program was jointly developed by the Stanford Coronary Rehabilitation Program and Kaiser Permanente (Oakland, CA) in 1995. MULTIFIT is a nurse-based care model for CVD prevention.
Further research that evaluated other home-based programs showed similar promise. A Cochrane review demonstrated that home- and facility-based CR programs were equal in cardiac risk factor reduction, reduced hospital readmissions and mortality rates, and improved quality of life (QOL).13 Cost-effectiveness also seemed to be similar in both home- and hospital-based CR
Referrals
To address the problems with referrals that plague other CR programs, staff of the Healthy Heart Program worked closely with interventional cardiology and the cardiothoracic team, including the clinical informatics coordinators, to develop an automatic referral system for CR evaluation. Consults for CR evaluation were embedded within the post-CABG and PCI order sets in the electronic health record. Laboratory troponin alerts were created to alert CR staff of patients with elevated troponins, which identified patients admitted for acute MI. Healthy Heart Program staff members received the referrals once a patient was admitted to the unit following their heart procedure. Early referrals for evaluation allowed staff to begin a chart review of all eligible patients and to follow the patient’s course of recovery. Most consults were generated during hospitalization for one of the indications; however, a minority of consults come from both the cardiology and primary care clinics.
Three Phases of CR
The AACVPR describes the challenges and opportunities found throughout the CR continuum.5 Over the past several decades, the continuum of care was more program centered and service utilization was more isolated. Today, CR is viewed as more process oriented and coordinates care across many professionals and services. Phase 1 inpatient CR begins in the hospital and is a shared responsibility between several services. Shortened hospital stays have led to innovative solutions for early ambulation, risk factor education, and discharge planning, including enrollment into phase 2 CR. Phase 2, also known as early outpatient, should begin within 1 to 2 weeks postevent in healthier patients and can last between 6 and 12 weeks postdischarge. Phase 3 (maintenance phase) should begin immediately at the conclusion of phase 2.
Phase 1
Prior to the advent of the Healthy Heart Program, secondary prevention education was not done at the bedside for SFVAMC patients following cardiac revascularization. The AACVPR recommends patient assessment, mobilization, risk-factor identification and education, and facilitation into outpatient CR as essential components of phase 1 CR.5 The Healthy Heart Program clinician initiates phase 1 CR by examining cardiac risk factor management for all referred patients. Physical and cardiac risk factor assessments are accomplished by completing a detailed chart review and interview with the patient. During this interview with the patient, the clinician evaluates cognitive function and readiness to learn. Staff will interview the patient further to assess the overall patient needs, including availability of social support, resources to maintain optimal health, and the need for secondary preventive education. For the PCI patient, the interview may occur in the hours following their procedure; for the surgical patient, this bedside visit typically occurs postoperative day 3 or 4.
A standardized cardiac risk factor evaluation tool was designed, which also serves as an education form to help guide the conversation on risk factor management. The interactive, patient-centered form includes opportunities to review risk, discuss current laboratory values (eg, lipids and hemoglobin A1c), and establish individualized goals based on patient preference and recommended guidelines. Healthy Heart Program staff assist the patient in formulating achievable goals using the SMART (specific, measurable, attainable, realistic, and time-related) criteria.19 Immediately after a heart event or procedure, patients often feel highly motivated to initiate lifestyle changes.20 However, PCI patients may have a short window of opportunity for learning between their readiness to learn state and before the activities of discharge. Staff use these opportunities as a teachable moment and to increase enrollment into outpatient CR (phase 2).
The provider performs a thorough chart review and bedside consultation to determine whether home-based CR is indicated, feasible, and appropriate. Not every patient that is referred will be enrolled in CR. Patients have the option to opt out. In addition, clinical staff adhere to the program protocol’s exclusion criteria.
Absolute contraindications for home enrollment include unstable angina, staged cardiac procedure (PCI and surgery), complex ventricular arrhythmias, severe or symptomatic aortic stenosis, decompensated heart failure, and uncontrolled hypertension (Table). Patients deemed high risk for home-based CR may be referred to a non-VA facility-based CR program. Risk stratification, using the Canadian Cardiovascular Society Grading of Angina Pectoris, is a continuous process that is used to identify patients who may move from moderate to high risk, both before and during the program.21,22
Phases 2 and 3
Phase 2 of the Healthy Heart Program CR includes physical activity, risk-factor modification, nutritional guidance, psychosocial modification, a return to previous activities, and an improved QOL. Prior to entry into the program, a submaximal exercise test, the 6-minute walk test (6MWT), is used as both a qualifying test and for developing the initial exercise prescription.22 The minimum 6MWT distance needed to qualify is 75 m for postoperative and 150 m for nonsurgical patients. The 6MWT is performed in-hospital for patients who were admitted for stable angina, PCI, and are > 4 days following acute MI.23 Cardiothoracic surgery patients are tested at their first follow-up clinic visit (typically 2-3 weeks postoperatively). The clinician monitors the heart rate with either a wearable device or via inpatient telemetry monitors. This exercise testing also serves as a motivational tool for patients to gain confidence in their ability to begin to exercise at home.
Each participant receives a workbook and a DVD titled An Active Partnership for the Health of Your Heart. A personal health journal is provided for documenting vital signs, activity, and dietary intake. In addition, each participant receives equipment on an as-needed basis, including resistance bands, a weight scale, a blood pressure cuff, a pedometer/heart rate monitoring device, an exercise peddler or stationary bike, and a dietary video. Baseline assessments include the General Anxiety Disorder (GAD-7), Personal Health Questionnaire (PHQ-9) and a nutrition (Rate Your Plate) questionnaire. A cognitive function test (Montreal Cognitive Assessment) is used on an as-needed basis.
Nine 30-minute telephone follow-up sessions are scheduled within a 12-week period (weekly for the first 6 weeks, then biweekly). Topics covered are customized and include exercise; nutrition; medications; smoking cessation; and diabetes, hypertension, and weight management. Via a telephone follow-up session, the program nurses and patients codevelop an electronic individualized treatment plan that is tailored to the patient’s diagnosis, individual goals, and preferences. Clinicians teach participants how to self-monitor exercise, using a continuous heart rate monitoring device (Mio Alpha II or Fuse) and the 6-20 Borg dyspnea rating scale.24 Initially, moderate intensity exercise is prescribed with a target heart rate that is 60% to 75% of the 6MWT peak heart rate and an initial Borg scale target (11-14 on 20 point scale). The program physicians approve the treatment plan at the first patient visit and every 30 days until phase 2 is complete.
Patients who have completed early outpatient phase 2 CR can benefit from continuing to a phase 3 CR program.25 Participants of the Healthy Heart Program automatically are enrolled in phase 3, which is a long-term maintenance program that includes monthly or bimonthly phone calls for up to 1-year posthospital discharge. The goal is to support each veteran’s transition to a long-term healthy lifestyle that includes regular exercise.
Client-Clinician Partnership
The Healthy Heart Program establishes the client-clinician partnership prior to discharge for hospitalized patients. The nurse who initiates phase 1 at the bedside is the primary clinician throughout phases 2 and 3 with the exception of a dietician, psychologist, and/or exercise physiologist who provide follow-up calls as needed. Throughout these weekly follow-up phone sessions, the clinician gains an appreciation of the patient’s understanding of his or her disease, patterns of behavior, desire to change, confidence in being able to change, potential barriers, and responses to obstacles
Tailored Behavioral Change
The clinician’s responsibility is to listen to the patient’s concerns, assess their level of commitment for changing health behaviors, and provide guidance and support at the patient’s current level. The clinician applies the Transtheoretical Model founded on the Stages of Change principals to help understand and provide guidance based on the patient’s feelings about health behavior change.26 People are actively open to changing behaviors by only 20% at any given time.27 Therefore, action-oriented guidance for patients who are in the contemplative stage would not be helpful. This patient-centered approach promotes patients’ self-awareness, participation, and understanding of their decision-making role in their health management. Ultimately, individuals must take ownership of their health care maintenance for sustained behavioral change and medication management, and clinicians should facilitate that process.
Discussion
Secondary prevention strategies for heart disease continue to be underutilized. The Healthy Heart Program aims to improve participation in CR, improve QOL, help patients understand their heart disease, and support these patients psychologically. An advantage of this program is that it begins inpatient CR immediately following the heart event, when many patients often are more receptive to behavioral change support and guidance. Another advantage is that the program breaks down barriers to access, which is especially important in the veteran population. The Healthy Heart Program provides support and guidance for exercise and cardiac risk factor management to patients who otherwise would have not participated in any type of CR program.
A home-based CR program can be adopted independently or in conjunction with a facility-based program to which patients lack access. Furthermore, home-based CR programs function well as a phase 3 maintenance program at the completion of a traditional CR program. Since its inception, the Healthy Heart Program has increased the number of veterans enrolled in cardiac rehabilitation at the SFVAMC dramatically, from < 1% in FY 2012 to > 40% in FY 2015.
Program Limitations
One potential disadvantage of a home-based CR program is patients’ fear of returning to an exercise routine following a cardiac event. In addition, a lack of in-person supervision in home-based CR can lead patients to engage in less intensive activity than in facility-based CR. Other disadvantages include a lack of social support, less patient accountability, and safety concerns for sicker patients. Staff have consulted on several patients who expressed a lack of confidence in their ability to do well in this type of program, where accountability for exercising is self-reported. Staff referred these patients, who had the means to travel, to a non-VA facility-based CR program of their choice. Ideally, patients would have the choice between facility- or home-based programs or be able to choose a hybrid program that would best meet their needs.
Another identified limitation of this program was the lack of group support and in-person interactions with rehabilitation staff. Finally, although this program uses mobile devices with heart rate monitoring technology, these devices currently lack the capability to remotely share data with clinicians. Clinicians are reliant on the patient’s use of a personal health journal and memory. Subjective patient reporting has been found to be overestimated; therefore, more objective methods to measure important clinical outcomes are necessary.28
Conclusion
Facility-based CR is effective but underutilized. Alternative secondary programs are needed to help meet patient needs and overcome patient barriers. One promising approach to increase participation is home-based CR. Home-based CR programs have the potential to increase CR uptake and adherence. Home-based CR optimizes enrollment through evidence-based alternative models due to improved access. The future of CR will become highly individualized and multifaceted as a result of available mobile technologies and Internet-based tools, which will help increase the number of participants and expand the reach of cardiac risk factor management programs beyond the facility-based setting. A home-based program will be a valuable addition to facility-based programs as a stand-alone program or adopted into a hybrid program.
Acknowledgments
This work was funded by the VA Quality Enhancement Research Initiative.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Cicrulation. 2017;135(10):e146-e603.
2. Anderson L, Oldridge N, Thompson DR, Zwisler A, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Systematic Review and Meta-analysis. J Am Coll Card. 2016;67:1-12.
3. Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 1988;260:940-950.
4. Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682-692.
5. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
6. Balady GJ, Williams MA, Ades PA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Association of Cardiovascular and Pulmonary Rehabilitation. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115(10):2675-2682.
7. Thomas R J, King M, Lui K, et al; Writing Committee Members. AACVPR/ACCF/AHA 2010 update: performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: a report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Clinical Performance Measures for Cardiac Rehabilitation). Circulation. 2010;122(13):1342-1350.
8. Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116(15):1653-1662.
9. Balady GJ, Ades PA, Bitner VA, et al; American Heart Association Science Advisory and Coordinating Committee. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124(25):2951-2960.
10. Schopfer DW, Takemoto S, Allsup K, et al. Notice of Retraction and Replacement. Schopfer DW, et al. Cardiac rehabilitation use among veterans with ischemic heart disease. JAMA Intern Med. 2014;174(10):1687-1689. JAMA Intern Med. 2016;176(11):1726-1727.
11. Ferguson EE. Cardiac rehabilitation—an effective and comprehensive but underutilized program to reduce cardiovascular risk in patients with CVD. US Cardiology. 2006;3(2):14-16.
12. DeBusk RF, Miller NH, Superko HR, et al. A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med. 1994;120(9):721-729.
13. Buckingham SA, Taylor RS, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation: abridged Cochrane systematic review and meta-analysis. Open Heart. 2016;3(2):e000463.
14. Taylor RS, Watt A, Dalal HM, et al. Home-based cardiac rehabilitation versus hospital-based rehabilitation: a cost effectiveness analysis. Int J Cardiol. 2007;119(2):196-201.
15. Kotb A, Hsieh S, Wells GA. The effect of telephone support interventions on coronary artery disease (CAD) patient outcomes during cardiac rehabilitation: a systematic review and meta-analysis. PLoS One. 2014;9(5):e96581.
16. Grace SL, McDonald J, Fishman D, Caruso V. Patient preferences for home-based versus hospital-based cardiac rehabilitation. J Cardiopulm Rehabil. 2005;25(1):24-29.
17. Wakefield B, Drwal K, Scherubel M, Klobucar T, Johnson S, Kaboli P. Feasibility and effectiveness of remote, telephone-based delivery of cardiac rehabilitation. Telemed J E Health. 2014;20(1):32-38.
18. Smith SC, Benjamin EJ, Bonow RO, et al; World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458-2473.
19. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manage Rev. 1981;70(11):35-36.
20. Dullaghan L, Lusk L, Donnelly P, McGeough M, Fitzsimons D. Communicating with people who have experienced heart attack. Emerg Nurse. 2013;21(6):33-36.
21. Campeau L. Letter: grading of angina pectoris. Circulation. 1976;54(3):522-523.
22. Fletcher GF, Balady GJ, Armstrong EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104(14):1694-1740.
23. Gibbons RJ, Balady GJ, Bricker JT, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. ACC/AHA 2002 guideline update for exercise testing: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol. 2002;40(8):1531-1540.
24. Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998.
25. Seki E, Watanabe Y, Sunayama S, et al. Effects of phase III cardiac rehabilitation programs on health-related quality of life in elderly patients with coronary artery disease: Juntendo Cardiac Rehabilitation Program (J-CARP). Circ J. 2003;67(1):73-77.
26. The transtheoretical model. Pro-Change Behavior Systems, Inc. http://www.prochange.com/transtheoretical-model-of-behavior-change. Published 2016. Accessed April 6, 2017.
27. Prochaska JO, Ever KE, Castle PH, et al. Enhancing multiple domains of well-being by decreasing multiple health risk behaviors: a randomized clinical trial. Popul Health Manag. 2012;15(5):276-286.
28. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
Despite a 30% decline in heart disease mortality from 2001 to 2011, heart disease prevalence is on the rise, responsible for 1 of every 3 deaths in the U.S.1 Cardiac rehabilitation (CR) is an evidence-based, secondary prevention strategy that has been proven effective in preventing future cardiovascular events and decreasing heart disease mortality.2-4 The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) is the leading authority on CR and provides guidelines for CR programs. The AACVPR and the American Heart Association (AHA) published core components for CR programs deemed essential for all CR/secondary prevention programs, including evaluations, interventions, and expected outcomes.5 These core components are aimed at promoting a healthy lifestyle and increasing function and well-being while reducing injury, death, and the reoccurrence of disease.6
In a meta-analysis of 47 trials with 10,794 participants, CR reduced cardiovascular disease (CVD) mortality and hospital admissions by 26% and 18%, respectively.2 Performance measures (Class 1, Level A) recommend the following types of patients should be referred from the inpatient setting: “all patients hospitalized with a primary diagnosis of an acute myocardial infarction (MI) or chronic stable angina, or who during hospitalization have undergone coronary artery bypass graft (CABG) surgery, a percutaneous coronary intervention (PCI), cardiac valve surgery, or cardiac transplantation.”7 However, despite overwhelming evidence and widespread endorsement (Class 1, Level A), service utilization, uptake, and patient adherence to CR programs remain suboptimal. In a U.S. study of claims from > 250,000 Medicare beneficiaries, < 30% of eligible patients participated
This treatment gap is echoed throughout the VHA. Schopfer and colleagues found that only 28% of the 124 VAMCs that provide inpatient care also offer a supervised, facility-based CR program.10 Furthermore, only 10.3% of eligible veterans participated in at least 1 CR session (VA or non-VA). On a systemic level, low patient referral rates and inadequate third-party reimbursement were the most common barriers to participation in CR.10,11 On a patient level, distance was by far the largest barrier to veterans receiving CR. Currently, 74% of the 9.3 million VA-enrolled veterans live at least 1 hour by car from a VA facility that offers CR.9 Within some regions of the VHA, there are no VA facility-based CR programs. For example, VISN 21 has no facility-based CR programs. At the same time, referral of eligible veterans to facility-based CR outside the VA remains low. Prior to April 2013, < 2% of qualified patients residing in VISN 21 were being referred to Non-VA CR programs, making it the VISN with the lowest participation rate for CR.
One potential solution that addresses both systemic and patient barriers to CR utilization is home-based CR. Veterans within the wide geographic area of VISN 21 are referred to San Francisco VAMC (SFVAMC) for ischemic heart disease, cardiovascular revascularization, and cardiac valve surgeries. In 2013, a comprehensive home-based CR program named The Healthy Heart Program was developed based on a successful evidence-based CVD secondary prevention program. The Healthy Heart Program is designed to be a physician-directed, nurse case-managed, customized exercise and lifestyle program that provides a safe and convenient way for veterans to participate in CR. Exercise and disease self-management education are the cornerstones of the Healthy Heart Program. The program’s multidisciplinary team includes physicians, nurses, a dietician, an exercise physiologist, and a health behavior psychologist.
An Alternative Approach
DeBusk and colleagues demonstrated that a physician-directed, nurse-managed, home-based cardiac risk-factor modification program improved smoking cessation, reduced low-density lipoprotein cholesterol, and increased exercise capacity compared with usual care.12 The results of this study helped pave the way for one of the first CR programs with a strong home-based element. The MULTIFIT program was jointly developed by the Stanford Coronary Rehabilitation Program and Kaiser Permanente (Oakland, CA) in 1995. MULTIFIT is a nurse-based care model for CVD prevention.
Further research that evaluated other home-based programs showed similar promise. A Cochrane review demonstrated that home- and facility-based CR programs were equal in cardiac risk factor reduction, reduced hospital readmissions and mortality rates, and improved quality of life (QOL).13 Cost-effectiveness also seemed to be similar in both home- and hospital-based CR
Referrals
To address the problems with referrals that plague other CR programs, staff of the Healthy Heart Program worked closely with interventional cardiology and the cardiothoracic team, including the clinical informatics coordinators, to develop an automatic referral system for CR evaluation. Consults for CR evaluation were embedded within the post-CABG and PCI order sets in the electronic health record. Laboratory troponin alerts were created to alert CR staff of patients with elevated troponins, which identified patients admitted for acute MI. Healthy Heart Program staff members received the referrals once a patient was admitted to the unit following their heart procedure. Early referrals for evaluation allowed staff to begin a chart review of all eligible patients and to follow the patient’s course of recovery. Most consults were generated during hospitalization for one of the indications; however, a minority of consults come from both the cardiology and primary care clinics.
Three Phases of CR
The AACVPR describes the challenges and opportunities found throughout the CR continuum.5 Over the past several decades, the continuum of care was more program centered and service utilization was more isolated. Today, CR is viewed as more process oriented and coordinates care across many professionals and services. Phase 1 inpatient CR begins in the hospital and is a shared responsibility between several services. Shortened hospital stays have led to innovative solutions for early ambulation, risk factor education, and discharge planning, including enrollment into phase 2 CR. Phase 2, also known as early outpatient, should begin within 1 to 2 weeks postevent in healthier patients and can last between 6 and 12 weeks postdischarge. Phase 3 (maintenance phase) should begin immediately at the conclusion of phase 2.
Phase 1
Prior to the advent of the Healthy Heart Program, secondary prevention education was not done at the bedside for SFVAMC patients following cardiac revascularization. The AACVPR recommends patient assessment, mobilization, risk-factor identification and education, and facilitation into outpatient CR as essential components of phase 1 CR.5 The Healthy Heart Program clinician initiates phase 1 CR by examining cardiac risk factor management for all referred patients. Physical and cardiac risk factor assessments are accomplished by completing a detailed chart review and interview with the patient. During this interview with the patient, the clinician evaluates cognitive function and readiness to learn. Staff will interview the patient further to assess the overall patient needs, including availability of social support, resources to maintain optimal health, and the need for secondary preventive education. For the PCI patient, the interview may occur in the hours following their procedure; for the surgical patient, this bedside visit typically occurs postoperative day 3 or 4.
A standardized cardiac risk factor evaluation tool was designed, which also serves as an education form to help guide the conversation on risk factor management. The interactive, patient-centered form includes opportunities to review risk, discuss current laboratory values (eg, lipids and hemoglobin A1c), and establish individualized goals based on patient preference and recommended guidelines. Healthy Heart Program staff assist the patient in formulating achievable goals using the SMART (specific, measurable, attainable, realistic, and time-related) criteria.19 Immediately after a heart event or procedure, patients often feel highly motivated to initiate lifestyle changes.20 However, PCI patients may have a short window of opportunity for learning between their readiness to learn state and before the activities of discharge. Staff use these opportunities as a teachable moment and to increase enrollment into outpatient CR (phase 2).
The provider performs a thorough chart review and bedside consultation to determine whether home-based CR is indicated, feasible, and appropriate. Not every patient that is referred will be enrolled in CR. Patients have the option to opt out. In addition, clinical staff adhere to the program protocol’s exclusion criteria.
Absolute contraindications for home enrollment include unstable angina, staged cardiac procedure (PCI and surgery), complex ventricular arrhythmias, severe or symptomatic aortic stenosis, decompensated heart failure, and uncontrolled hypertension (Table). Patients deemed high risk for home-based CR may be referred to a non-VA facility-based CR program. Risk stratification, using the Canadian Cardiovascular Society Grading of Angina Pectoris, is a continuous process that is used to identify patients who may move from moderate to high risk, both before and during the program.21,22
Phases 2 and 3
Phase 2 of the Healthy Heart Program CR includes physical activity, risk-factor modification, nutritional guidance, psychosocial modification, a return to previous activities, and an improved QOL. Prior to entry into the program, a submaximal exercise test, the 6-minute walk test (6MWT), is used as both a qualifying test and for developing the initial exercise prescription.22 The minimum 6MWT distance needed to qualify is 75 m for postoperative and 150 m for nonsurgical patients. The 6MWT is performed in-hospital for patients who were admitted for stable angina, PCI, and are > 4 days following acute MI.23 Cardiothoracic surgery patients are tested at their first follow-up clinic visit (typically 2-3 weeks postoperatively). The clinician monitors the heart rate with either a wearable device or via inpatient telemetry monitors. This exercise testing also serves as a motivational tool for patients to gain confidence in their ability to begin to exercise at home.
Each participant receives a workbook and a DVD titled An Active Partnership for the Health of Your Heart. A personal health journal is provided for documenting vital signs, activity, and dietary intake. In addition, each participant receives equipment on an as-needed basis, including resistance bands, a weight scale, a blood pressure cuff, a pedometer/heart rate monitoring device, an exercise peddler or stationary bike, and a dietary video. Baseline assessments include the General Anxiety Disorder (GAD-7), Personal Health Questionnaire (PHQ-9) and a nutrition (Rate Your Plate) questionnaire. A cognitive function test (Montreal Cognitive Assessment) is used on an as-needed basis.
Nine 30-minute telephone follow-up sessions are scheduled within a 12-week period (weekly for the first 6 weeks, then biweekly). Topics covered are customized and include exercise; nutrition; medications; smoking cessation; and diabetes, hypertension, and weight management. Via a telephone follow-up session, the program nurses and patients codevelop an electronic individualized treatment plan that is tailored to the patient’s diagnosis, individual goals, and preferences. Clinicians teach participants how to self-monitor exercise, using a continuous heart rate monitoring device (Mio Alpha II or Fuse) and the 6-20 Borg dyspnea rating scale.24 Initially, moderate intensity exercise is prescribed with a target heart rate that is 60% to 75% of the 6MWT peak heart rate and an initial Borg scale target (11-14 on 20 point scale). The program physicians approve the treatment plan at the first patient visit and every 30 days until phase 2 is complete.
Patients who have completed early outpatient phase 2 CR can benefit from continuing to a phase 3 CR program.25 Participants of the Healthy Heart Program automatically are enrolled in phase 3, which is a long-term maintenance program that includes monthly or bimonthly phone calls for up to 1-year posthospital discharge. The goal is to support each veteran’s transition to a long-term healthy lifestyle that includes regular exercise.
Client-Clinician Partnership
The Healthy Heart Program establishes the client-clinician partnership prior to discharge for hospitalized patients. The nurse who initiates phase 1 at the bedside is the primary clinician throughout phases 2 and 3 with the exception of a dietician, psychologist, and/or exercise physiologist who provide follow-up calls as needed. Throughout these weekly follow-up phone sessions, the clinician gains an appreciation of the patient’s understanding of his or her disease, patterns of behavior, desire to change, confidence in being able to change, potential barriers, and responses to obstacles
Tailored Behavioral Change
The clinician’s responsibility is to listen to the patient’s concerns, assess their level of commitment for changing health behaviors, and provide guidance and support at the patient’s current level. The clinician applies the Transtheoretical Model founded on the Stages of Change principals to help understand and provide guidance based on the patient’s feelings about health behavior change.26 People are actively open to changing behaviors by only 20% at any given time.27 Therefore, action-oriented guidance for patients who are in the contemplative stage would not be helpful. This patient-centered approach promotes patients’ self-awareness, participation, and understanding of their decision-making role in their health management. Ultimately, individuals must take ownership of their health care maintenance for sustained behavioral change and medication management, and clinicians should facilitate that process.
Discussion
Secondary prevention strategies for heart disease continue to be underutilized. The Healthy Heart Program aims to improve participation in CR, improve QOL, help patients understand their heart disease, and support these patients psychologically. An advantage of this program is that it begins inpatient CR immediately following the heart event, when many patients often are more receptive to behavioral change support and guidance. Another advantage is that the program breaks down barriers to access, which is especially important in the veteran population. The Healthy Heart Program provides support and guidance for exercise and cardiac risk factor management to patients who otherwise would have not participated in any type of CR program.
A home-based CR program can be adopted independently or in conjunction with a facility-based program to which patients lack access. Furthermore, home-based CR programs function well as a phase 3 maintenance program at the completion of a traditional CR program. Since its inception, the Healthy Heart Program has increased the number of veterans enrolled in cardiac rehabilitation at the SFVAMC dramatically, from < 1% in FY 2012 to > 40% in FY 2015.
Program Limitations
One potential disadvantage of a home-based CR program is patients’ fear of returning to an exercise routine following a cardiac event. In addition, a lack of in-person supervision in home-based CR can lead patients to engage in less intensive activity than in facility-based CR. Other disadvantages include a lack of social support, less patient accountability, and safety concerns for sicker patients. Staff have consulted on several patients who expressed a lack of confidence in their ability to do well in this type of program, where accountability for exercising is self-reported. Staff referred these patients, who had the means to travel, to a non-VA facility-based CR program of their choice. Ideally, patients would have the choice between facility- or home-based programs or be able to choose a hybrid program that would best meet their needs.
Another identified limitation of this program was the lack of group support and in-person interactions with rehabilitation staff. Finally, although this program uses mobile devices with heart rate monitoring technology, these devices currently lack the capability to remotely share data with clinicians. Clinicians are reliant on the patient’s use of a personal health journal and memory. Subjective patient reporting has been found to be overestimated; therefore, more objective methods to measure important clinical outcomes are necessary.28
Conclusion
Facility-based CR is effective but underutilized. Alternative secondary programs are needed to help meet patient needs and overcome patient barriers. One promising approach to increase participation is home-based CR. Home-based CR programs have the potential to increase CR uptake and adherence. Home-based CR optimizes enrollment through evidence-based alternative models due to improved access. The future of CR will become highly individualized and multifaceted as a result of available mobile technologies and Internet-based tools, which will help increase the number of participants and expand the reach of cardiac risk factor management programs beyond the facility-based setting. A home-based program will be a valuable addition to facility-based programs as a stand-alone program or adopted into a hybrid program.
Acknowledgments
This work was funded by the VA Quality Enhancement Research Initiative.
Despite a 30% decline in heart disease mortality from 2001 to 2011, heart disease prevalence is on the rise, responsible for 1 of every 3 deaths in the U.S.1 Cardiac rehabilitation (CR) is an evidence-based, secondary prevention strategy that has been proven effective in preventing future cardiovascular events and decreasing heart disease mortality.2-4 The American Association of Cardiovascular and Pulmonary Rehabilitation (AACVPR) is the leading authority on CR and provides guidelines for CR programs. The AACVPR and the American Heart Association (AHA) published core components for CR programs deemed essential for all CR/secondary prevention programs, including evaluations, interventions, and expected outcomes.5 These core components are aimed at promoting a healthy lifestyle and increasing function and well-being while reducing injury, death, and the reoccurrence of disease.6
In a meta-analysis of 47 trials with 10,794 participants, CR reduced cardiovascular disease (CVD) mortality and hospital admissions by 26% and 18%, respectively.2 Performance measures (Class 1, Level A) recommend the following types of patients should be referred from the inpatient setting: “all patients hospitalized with a primary diagnosis of an acute myocardial infarction (MI) or chronic stable angina, or who during hospitalization have undergone coronary artery bypass graft (CABG) surgery, a percutaneous coronary intervention (PCI), cardiac valve surgery, or cardiac transplantation.”7 However, despite overwhelming evidence and widespread endorsement (Class 1, Level A), service utilization, uptake, and patient adherence to CR programs remain suboptimal. In a U.S. study of claims from > 250,000 Medicare beneficiaries, < 30% of eligible patients participated
This treatment gap is echoed throughout the VHA. Schopfer and colleagues found that only 28% of the 124 VAMCs that provide inpatient care also offer a supervised, facility-based CR program.10 Furthermore, only 10.3% of eligible veterans participated in at least 1 CR session (VA or non-VA). On a systemic level, low patient referral rates and inadequate third-party reimbursement were the most common barriers to participation in CR.10,11 On a patient level, distance was by far the largest barrier to veterans receiving CR. Currently, 74% of the 9.3 million VA-enrolled veterans live at least 1 hour by car from a VA facility that offers CR.9 Within some regions of the VHA, there are no VA facility-based CR programs. For example, VISN 21 has no facility-based CR programs. At the same time, referral of eligible veterans to facility-based CR outside the VA remains low. Prior to April 2013, < 2% of qualified patients residing in VISN 21 were being referred to Non-VA CR programs, making it the VISN with the lowest participation rate for CR.
One potential solution that addresses both systemic and patient barriers to CR utilization is home-based CR. Veterans within the wide geographic area of VISN 21 are referred to San Francisco VAMC (SFVAMC) for ischemic heart disease, cardiovascular revascularization, and cardiac valve surgeries. In 2013, a comprehensive home-based CR program named The Healthy Heart Program was developed based on a successful evidence-based CVD secondary prevention program. The Healthy Heart Program is designed to be a physician-directed, nurse case-managed, customized exercise and lifestyle program that provides a safe and convenient way for veterans to participate in CR. Exercise and disease self-management education are the cornerstones of the Healthy Heart Program. The program’s multidisciplinary team includes physicians, nurses, a dietician, an exercise physiologist, and a health behavior psychologist.
An Alternative Approach
DeBusk and colleagues demonstrated that a physician-directed, nurse-managed, home-based cardiac risk-factor modification program improved smoking cessation, reduced low-density lipoprotein cholesterol, and increased exercise capacity compared with usual care.12 The results of this study helped pave the way for one of the first CR programs with a strong home-based element. The MULTIFIT program was jointly developed by the Stanford Coronary Rehabilitation Program and Kaiser Permanente (Oakland, CA) in 1995. MULTIFIT is a nurse-based care model for CVD prevention.
Further research that evaluated other home-based programs showed similar promise. A Cochrane review demonstrated that home- and facility-based CR programs were equal in cardiac risk factor reduction, reduced hospital readmissions and mortality rates, and improved quality of life (QOL).13 Cost-effectiveness also seemed to be similar in both home- and hospital-based CR
Referrals
To address the problems with referrals that plague other CR programs, staff of the Healthy Heart Program worked closely with interventional cardiology and the cardiothoracic team, including the clinical informatics coordinators, to develop an automatic referral system for CR evaluation. Consults for CR evaluation were embedded within the post-CABG and PCI order sets in the electronic health record. Laboratory troponin alerts were created to alert CR staff of patients with elevated troponins, which identified patients admitted for acute MI. Healthy Heart Program staff members received the referrals once a patient was admitted to the unit following their heart procedure. Early referrals for evaluation allowed staff to begin a chart review of all eligible patients and to follow the patient’s course of recovery. Most consults were generated during hospitalization for one of the indications; however, a minority of consults come from both the cardiology and primary care clinics.
Three Phases of CR
The AACVPR describes the challenges and opportunities found throughout the CR continuum.5 Over the past several decades, the continuum of care was more program centered and service utilization was more isolated. Today, CR is viewed as more process oriented and coordinates care across many professionals and services. Phase 1 inpatient CR begins in the hospital and is a shared responsibility between several services. Shortened hospital stays have led to innovative solutions for early ambulation, risk factor education, and discharge planning, including enrollment into phase 2 CR. Phase 2, also known as early outpatient, should begin within 1 to 2 weeks postevent in healthier patients and can last between 6 and 12 weeks postdischarge. Phase 3 (maintenance phase) should begin immediately at the conclusion of phase 2.
Phase 1
Prior to the advent of the Healthy Heart Program, secondary prevention education was not done at the bedside for SFVAMC patients following cardiac revascularization. The AACVPR recommends patient assessment, mobilization, risk-factor identification and education, and facilitation into outpatient CR as essential components of phase 1 CR.5 The Healthy Heart Program clinician initiates phase 1 CR by examining cardiac risk factor management for all referred patients. Physical and cardiac risk factor assessments are accomplished by completing a detailed chart review and interview with the patient. During this interview with the patient, the clinician evaluates cognitive function and readiness to learn. Staff will interview the patient further to assess the overall patient needs, including availability of social support, resources to maintain optimal health, and the need for secondary preventive education. For the PCI patient, the interview may occur in the hours following their procedure; for the surgical patient, this bedside visit typically occurs postoperative day 3 or 4.
A standardized cardiac risk factor evaluation tool was designed, which also serves as an education form to help guide the conversation on risk factor management. The interactive, patient-centered form includes opportunities to review risk, discuss current laboratory values (eg, lipids and hemoglobin A1c), and establish individualized goals based on patient preference and recommended guidelines. Healthy Heart Program staff assist the patient in formulating achievable goals using the SMART (specific, measurable, attainable, realistic, and time-related) criteria.19 Immediately after a heart event or procedure, patients often feel highly motivated to initiate lifestyle changes.20 However, PCI patients may have a short window of opportunity for learning between their readiness to learn state and before the activities of discharge. Staff use these opportunities as a teachable moment and to increase enrollment into outpatient CR (phase 2).
The provider performs a thorough chart review and bedside consultation to determine whether home-based CR is indicated, feasible, and appropriate. Not every patient that is referred will be enrolled in CR. Patients have the option to opt out. In addition, clinical staff adhere to the program protocol’s exclusion criteria.
Absolute contraindications for home enrollment include unstable angina, staged cardiac procedure (PCI and surgery), complex ventricular arrhythmias, severe or symptomatic aortic stenosis, decompensated heart failure, and uncontrolled hypertension (Table). Patients deemed high risk for home-based CR may be referred to a non-VA facility-based CR program. Risk stratification, using the Canadian Cardiovascular Society Grading of Angina Pectoris, is a continuous process that is used to identify patients who may move from moderate to high risk, both before and during the program.21,22
Phases 2 and 3
Phase 2 of the Healthy Heart Program CR includes physical activity, risk-factor modification, nutritional guidance, psychosocial modification, a return to previous activities, and an improved QOL. Prior to entry into the program, a submaximal exercise test, the 6-minute walk test (6MWT), is used as both a qualifying test and for developing the initial exercise prescription.22 The minimum 6MWT distance needed to qualify is 75 m for postoperative and 150 m for nonsurgical patients. The 6MWT is performed in-hospital for patients who were admitted for stable angina, PCI, and are > 4 days following acute MI.23 Cardiothoracic surgery patients are tested at their first follow-up clinic visit (typically 2-3 weeks postoperatively). The clinician monitors the heart rate with either a wearable device or via inpatient telemetry monitors. This exercise testing also serves as a motivational tool for patients to gain confidence in their ability to begin to exercise at home.
Each participant receives a workbook and a DVD titled An Active Partnership for the Health of Your Heart. A personal health journal is provided for documenting vital signs, activity, and dietary intake. In addition, each participant receives equipment on an as-needed basis, including resistance bands, a weight scale, a blood pressure cuff, a pedometer/heart rate monitoring device, an exercise peddler or stationary bike, and a dietary video. Baseline assessments include the General Anxiety Disorder (GAD-7), Personal Health Questionnaire (PHQ-9) and a nutrition (Rate Your Plate) questionnaire. A cognitive function test (Montreal Cognitive Assessment) is used on an as-needed basis.
Nine 30-minute telephone follow-up sessions are scheduled within a 12-week period (weekly for the first 6 weeks, then biweekly). Topics covered are customized and include exercise; nutrition; medications; smoking cessation; and diabetes, hypertension, and weight management. Via a telephone follow-up session, the program nurses and patients codevelop an electronic individualized treatment plan that is tailored to the patient’s diagnosis, individual goals, and preferences. Clinicians teach participants how to self-monitor exercise, using a continuous heart rate monitoring device (Mio Alpha II or Fuse) and the 6-20 Borg dyspnea rating scale.24 Initially, moderate intensity exercise is prescribed with a target heart rate that is 60% to 75% of the 6MWT peak heart rate and an initial Borg scale target (11-14 on 20 point scale). The program physicians approve the treatment plan at the first patient visit and every 30 days until phase 2 is complete.
Patients who have completed early outpatient phase 2 CR can benefit from continuing to a phase 3 CR program.25 Participants of the Healthy Heart Program automatically are enrolled in phase 3, which is a long-term maintenance program that includes monthly or bimonthly phone calls for up to 1-year posthospital discharge. The goal is to support each veteran’s transition to a long-term healthy lifestyle that includes regular exercise.
Client-Clinician Partnership
The Healthy Heart Program establishes the client-clinician partnership prior to discharge for hospitalized patients. The nurse who initiates phase 1 at the bedside is the primary clinician throughout phases 2 and 3 with the exception of a dietician, psychologist, and/or exercise physiologist who provide follow-up calls as needed. Throughout these weekly follow-up phone sessions, the clinician gains an appreciation of the patient’s understanding of his or her disease, patterns of behavior, desire to change, confidence in being able to change, potential barriers, and responses to obstacles
Tailored Behavioral Change
The clinician’s responsibility is to listen to the patient’s concerns, assess their level of commitment for changing health behaviors, and provide guidance and support at the patient’s current level. The clinician applies the Transtheoretical Model founded on the Stages of Change principals to help understand and provide guidance based on the patient’s feelings about health behavior change.26 People are actively open to changing behaviors by only 20% at any given time.27 Therefore, action-oriented guidance for patients who are in the contemplative stage would not be helpful. This patient-centered approach promotes patients’ self-awareness, participation, and understanding of their decision-making role in their health management. Ultimately, individuals must take ownership of their health care maintenance for sustained behavioral change and medication management, and clinicians should facilitate that process.
Discussion
Secondary prevention strategies for heart disease continue to be underutilized. The Healthy Heart Program aims to improve participation in CR, improve QOL, help patients understand their heart disease, and support these patients psychologically. An advantage of this program is that it begins inpatient CR immediately following the heart event, when many patients often are more receptive to behavioral change support and guidance. Another advantage is that the program breaks down barriers to access, which is especially important in the veteran population. The Healthy Heart Program provides support and guidance for exercise and cardiac risk factor management to patients who otherwise would have not participated in any type of CR program.
A home-based CR program can be adopted independently or in conjunction with a facility-based program to which patients lack access. Furthermore, home-based CR programs function well as a phase 3 maintenance program at the completion of a traditional CR program. Since its inception, the Healthy Heart Program has increased the number of veterans enrolled in cardiac rehabilitation at the SFVAMC dramatically, from < 1% in FY 2012 to > 40% in FY 2015.
Program Limitations
One potential disadvantage of a home-based CR program is patients’ fear of returning to an exercise routine following a cardiac event. In addition, a lack of in-person supervision in home-based CR can lead patients to engage in less intensive activity than in facility-based CR. Other disadvantages include a lack of social support, less patient accountability, and safety concerns for sicker patients. Staff have consulted on several patients who expressed a lack of confidence in their ability to do well in this type of program, where accountability for exercising is self-reported. Staff referred these patients, who had the means to travel, to a non-VA facility-based CR program of their choice. Ideally, patients would have the choice between facility- or home-based programs or be able to choose a hybrid program that would best meet their needs.
Another identified limitation of this program was the lack of group support and in-person interactions with rehabilitation staff. Finally, although this program uses mobile devices with heart rate monitoring technology, these devices currently lack the capability to remotely share data with clinicians. Clinicians are reliant on the patient’s use of a personal health journal and memory. Subjective patient reporting has been found to be overestimated; therefore, more objective methods to measure important clinical outcomes are necessary.28
Conclusion
Facility-based CR is effective but underutilized. Alternative secondary programs are needed to help meet patient needs and overcome patient barriers. One promising approach to increase participation is home-based CR. Home-based CR programs have the potential to increase CR uptake and adherence. Home-based CR optimizes enrollment through evidence-based alternative models due to improved access. The future of CR will become highly individualized and multifaceted as a result of available mobile technologies and Internet-based tools, which will help increase the number of participants and expand the reach of cardiac risk factor management programs beyond the facility-based setting. A home-based program will be a valuable addition to facility-based programs as a stand-alone program or adopted into a hybrid program.
Acknowledgments
This work was funded by the VA Quality Enhancement Research Initiative.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Cicrulation. 2017;135(10):e146-e603.
2. Anderson L, Oldridge N, Thompson DR, Zwisler A, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Systematic Review and Meta-analysis. J Am Coll Card. 2016;67:1-12.
3. Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 1988;260:940-950.
4. Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682-692.
5. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
6. Balady GJ, Williams MA, Ades PA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Association of Cardiovascular and Pulmonary Rehabilitation. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115(10):2675-2682.
7. Thomas R J, King M, Lui K, et al; Writing Committee Members. AACVPR/ACCF/AHA 2010 update: performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: a report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Clinical Performance Measures for Cardiac Rehabilitation). Circulation. 2010;122(13):1342-1350.
8. Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116(15):1653-1662.
9. Balady GJ, Ades PA, Bitner VA, et al; American Heart Association Science Advisory and Coordinating Committee. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124(25):2951-2960.
10. Schopfer DW, Takemoto S, Allsup K, et al. Notice of Retraction and Replacement. Schopfer DW, et al. Cardiac rehabilitation use among veterans with ischemic heart disease. JAMA Intern Med. 2014;174(10):1687-1689. JAMA Intern Med. 2016;176(11):1726-1727.
11. Ferguson EE. Cardiac rehabilitation—an effective and comprehensive but underutilized program to reduce cardiovascular risk in patients with CVD. US Cardiology. 2006;3(2):14-16.
12. DeBusk RF, Miller NH, Superko HR, et al. A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med. 1994;120(9):721-729.
13. Buckingham SA, Taylor RS, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation: abridged Cochrane systematic review and meta-analysis. Open Heart. 2016;3(2):e000463.
14. Taylor RS, Watt A, Dalal HM, et al. Home-based cardiac rehabilitation versus hospital-based rehabilitation: a cost effectiveness analysis. Int J Cardiol. 2007;119(2):196-201.
15. Kotb A, Hsieh S, Wells GA. The effect of telephone support interventions on coronary artery disease (CAD) patient outcomes during cardiac rehabilitation: a systematic review and meta-analysis. PLoS One. 2014;9(5):e96581.
16. Grace SL, McDonald J, Fishman D, Caruso V. Patient preferences for home-based versus hospital-based cardiac rehabilitation. J Cardiopulm Rehabil. 2005;25(1):24-29.
17. Wakefield B, Drwal K, Scherubel M, Klobucar T, Johnson S, Kaboli P. Feasibility and effectiveness of remote, telephone-based delivery of cardiac rehabilitation. Telemed J E Health. 2014;20(1):32-38.
18. Smith SC, Benjamin EJ, Bonow RO, et al; World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458-2473.
19. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manage Rev. 1981;70(11):35-36.
20. Dullaghan L, Lusk L, Donnelly P, McGeough M, Fitzsimons D. Communicating with people who have experienced heart attack. Emerg Nurse. 2013;21(6):33-36.
21. Campeau L. Letter: grading of angina pectoris. Circulation. 1976;54(3):522-523.
22. Fletcher GF, Balady GJ, Armstrong EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104(14):1694-1740.
23. Gibbons RJ, Balady GJ, Bricker JT, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. ACC/AHA 2002 guideline update for exercise testing: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol. 2002;40(8):1531-1540.
24. Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998.
25. Seki E, Watanabe Y, Sunayama S, et al. Effects of phase III cardiac rehabilitation programs on health-related quality of life in elderly patients with coronary artery disease: Juntendo Cardiac Rehabilitation Program (J-CARP). Circ J. 2003;67(1):73-77.
26. The transtheoretical model. Pro-Change Behavior Systems, Inc. http://www.prochange.com/transtheoretical-model-of-behavior-change. Published 2016. Accessed April 6, 2017.
27. Prochaska JO, Ever KE, Castle PH, et al. Enhancing multiple domains of well-being by decreasing multiple health risk behaviors: a randomized clinical trial. Popul Health Manag. 2012;15(5):276-286.
28. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
1. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Cicrulation. 2017;135(10):e146-e603.
2. Anderson L, Oldridge N, Thompson DR, Zwisler A, Rees K, Martin N, Taylor RS. Exercise-based cardiac rehabilitation for coronary heart disease. Cochrane Systematic Review and Meta-analysis. J Am Coll Card. 2016;67:1-12.
3. Oldridge NB, Guyatt GH, Fischer ME, Rimm AA. Cardiac rehabilitation after myocardial infarction. Combined experience of randomized clinical trials. JAMA. 1988;260:940-950.
4. Taylor RS, Brown A, Ebrahim S, et al. Exercise-based rehabilitation for patients with coronary heart disease: systematic review and meta-analysis of randomized controlled trials. Am J Med. 2004;116(10):682-692.
5. American Association of Cardiovascular and Pulmonary Rehabilitation. Guidelines for Cardiac Rehabilitation and Secondary Prevention Programs. 5th ed. Champaign, IL: Human Kinetics; 2013.
6. Balady GJ, Williams MA, Ades PA, et al; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism; American Association of Cardiovascular and Pulmonary Rehabilitation. Core components of cardiac rehabilitation/secondary prevention programs: 2007 update: a scientific statement from the American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee, the Council on Clinical Cardiology; the Councils on Cardiovascular Nursing, Epidemiology and Prevention, and Nutrition, Physical Activity, and Metabolism; and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation. 2007;115(10):2675-2682.
7. Thomas R J, King M, Lui K, et al; Writing Committee Members. AACVPR/ACCF/AHA 2010 update: performance measures on cardiac rehabilitation for referral to cardiac rehabilitation/secondary prevention services: a report of the American Association of Cardiovascular and Pulmonary Rehabilitation and the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Clinical Performance Measures for Cardiac Rehabilitation). Circulation. 2010;122(13):1342-1350.
8. Suaya JA, Shepard DS, Normand SL, Ades PA, Prottas J, Stason WB. Use of cardiac rehabilitation by Medicare beneficiaries after myocardial infarction or coronary bypass surgery. Circulation. 2007;116(15):1653-1662.
9. Balady GJ, Ades PA, Bitner VA, et al; American Heart Association Science Advisory and Coordinating Committee. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation. 2011;124(25):2951-2960.
10. Schopfer DW, Takemoto S, Allsup K, et al. Notice of Retraction and Replacement. Schopfer DW, et al. Cardiac rehabilitation use among veterans with ischemic heart disease. JAMA Intern Med. 2014;174(10):1687-1689. JAMA Intern Med. 2016;176(11):1726-1727.
11. Ferguson EE. Cardiac rehabilitation—an effective and comprehensive but underutilized program to reduce cardiovascular risk in patients with CVD. US Cardiology. 2006;3(2):14-16.
12. DeBusk RF, Miller NH, Superko HR, et al. A case-management system for coronary risk factor modification after acute myocardial infarction. Ann Intern Med. 1994;120(9):721-729.
13. Buckingham SA, Taylor RS, Jolly K, et al. Home-based versus centre-based cardiac rehabilitation: abridged Cochrane systematic review and meta-analysis. Open Heart. 2016;3(2):e000463.
14. Taylor RS, Watt A, Dalal HM, et al. Home-based cardiac rehabilitation versus hospital-based rehabilitation: a cost effectiveness analysis. Int J Cardiol. 2007;119(2):196-201.
15. Kotb A, Hsieh S, Wells GA. The effect of telephone support interventions on coronary artery disease (CAD) patient outcomes during cardiac rehabilitation: a systematic review and meta-analysis. PLoS One. 2014;9(5):e96581.
16. Grace SL, McDonald J, Fishman D, Caruso V. Patient preferences for home-based versus hospital-based cardiac rehabilitation. J Cardiopulm Rehabil. 2005;25(1):24-29.
17. Wakefield B, Drwal K, Scherubel M, Klobucar T, Johnson S, Kaboli P. Feasibility and effectiveness of remote, telephone-based delivery of cardiac rehabilitation. Telemed J E Health. 2014;20(1):32-38.
18. Smith SC, Benjamin EJ, Bonow RO, et al; World Heart Federation and the Preventive Cardiovascular Nurses Association. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124(22):2458-2473.
19. Doran GT. There’s a S.M.A.R.T. way to write management’s goals and objectives. Manage Rev. 1981;70(11):35-36.
20. Dullaghan L, Lusk L, Donnelly P, McGeough M, Fitzsimons D. Communicating with people who have experienced heart attack. Emerg Nurse. 2013;21(6):33-36.
21. Campeau L. Letter: grading of angina pectoris. Circulation. 1976;54(3):522-523.
22. Fletcher GF, Balady GJ, Armstrong EA, et al. Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation. 2001;104(14):1694-1740.
23. Gibbons RJ, Balady GJ, Bricker JT, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. Committee to Update the 1997 Exercise Testing Guidelines. ACC/AHA 2002 guideline update for exercise testing: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing). J Am Coll Cardiol. 2002;40(8):1531-1540.
24. Borg G. Borg’s Perceived Exertion and Pain Scales. Champaign, IL: Human Kinetics; 1998.
25. Seki E, Watanabe Y, Sunayama S, et al. Effects of phase III cardiac rehabilitation programs on health-related quality of life in elderly patients with coronary artery disease: Juntendo Cardiac Rehabilitation Program (J-CARP). Circ J. 2003;67(1):73-77.
26. The transtheoretical model. Pro-Change Behavior Systems, Inc. http://www.prochange.com/transtheoretical-model-of-behavior-change. Published 2016. Accessed April 6, 2017.
27. Prochaska JO, Ever KE, Castle PH, et al. Enhancing multiple domains of well-being by decreasing multiple health risk behaviors: a randomized clinical trial. Popul Health Manag. 2012;15(5):276-286.
28. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
Veterans as Caregivers:Those Who Continue to Serve
More than 20% of the U.S. population will be aged ≥ 65 years by 2030, an increase from 13% in 2012.1 The likelihood of needing assistance with activities of daily living (ADLs) increases with age.2 People who need such assistance often depend on informal and unpaid assistance from friends and family. In 2009, about 65.7 million Americans (28.5%) provided informal care for people with an illness or disability, and that number only is expected to rise.3 These informal caregivers provide up to 80% of the total care hours needed by community-dwelling older adults—an estimated economic value of $450 billion in unpaid contributions in 2009.4,5
Caregiving can lead to significant physical, psychological, social, and financial burdens.6 The caregiving burden is associated with a host of adverse health behaviors and outcomes such as poor diet, lack of exercise and sleep, smoking, decreased participation in preventive health care, anxiety, depression, relationship difficulties, employment disruption, financial hardship, suicide, and higher mortality compared with that of noncaregivers.6-10 Additionally, care recipients are at increased risk for abuse or neglect when the caregiver is experiencing a significant burden.11 Therefore, efforts to improve caregiver support are important for both partners in the caregiver/care recipient dyad.
Caregiver support is beneficial to the health of caregivers and care recipients.10,12 For example, the Resources for Enhancing Alzheimer’s Caregiver Health (REACH) program has been shown to reduce the stress of informal caregiving and the risk of depression in caregivers.13,14 This program showed similar effects when implemented within the VHA.14 In the Partners in Dementia Care project, the VHA and Alzheimer’s Association coordinated care and support for veterans with dementia and their family and friends. This intervention resulted in lower caregiver strain and depression scores among participants.15
With a growing medical literature that shows the benefits of caregiver support interventions, the VHA developed a robust support program for informal caregivers of veterans. The VA caregiver support website (www.caregiver.va.gov) provides information and resources targeted to caregivers for veterans, including psychosocial and functional support for caregivers. The psychosocial support provided by the VA includes caregiver education, counseling, access to caregiver support coordinators, a caregiver support line, support groups, and referral to community support organizations.16 Functional support on the site includes financial assistance toward skilled home care, home hospice care, adult day care, home-based primary care, homemaker and home health aide services, telehealth, and respite care.16 Veterans who are caregiving for nonveterans have access to VHA psychosocial support but not to functional support services. For these veterans, functional caregiver support must come from family or referral to community organizations.
Background
In the U.S., about 11% of caregivers are veterans, but the availability of data about these caregivers is limited to veteran subgroups.3 For example, a 2011 study reported that 20% of veterans aged ≥ 60 years are caregivers.17 However, this estimate included child care for unimpaired children, which is not commonly included in other caregiving estimates. In another study, 30% of middle-aged active-duty officers reported helping their parents with instrumental ADLs (IADLs).18 These data suggest a significant proportion of veterans may be caregivers; however, the estimates do not identify prevalence of caregiving among a population of VHA enrolled veterans.
Likewise, few studies discuss the burden veterans experience from caregiving. A study of the 2009/2010 CDC Behavioral Risk Factor Surveillance System data found that female caregivers were more likely to report problems with sleep and mental health if they were veterans vs nonveterans.19 In a second study, caregiving veterans frequently reported physical (39%) and emotional (53%) strain, with emotional strain relating to depressive symptoms. The study of active-duty officers noted that worry was prevalent among military officers caregiving for parents from a distance.18 In contrast to the negative outcomes of caregiving, Monin and colleagues found that many veterans perceived caregiving as rewarding. Since caregiving may be a positive experience, veterans may benefit and be a potential resource for care to elderly and disabled citizens.17
Project Rationale and Goals
Social workers are the cornerstone of caregiver support at the George E. Wahlen VA Salt Lake City Health Care System (VASLCHCS) in Utah. They educate veterans and caregivers about VA resources to support caregivers of veterans. For those veterans who are caregiving for a nonveteran, the VASLCHCS social workers provide psychosocial support and help veterans connect to a local area agency on aging (AAA) for access to functional support. In practice, primary care clinic (PCC) providers have observed that directing a veteran to call the AAA does not usually result in a phone call. Therefore, an aim of this quality improvement (QI) project was to determine the most effective means of completing a successful AAA referral.
The VASLCHCS Geriatric Research Education and Clinical Center collaborates with the Utah Aging and Disability Resource Connection (ADRC) to improve awareness of available resources for veterans. Building on this collaborative project, the authors created a formal referral process for veterans needing local AAA services. This QI project had 3 aims: (1) estimate the prevalence of caregiving among veterans in the VASLCHCS primary care clinic; (2) identify perceived caregiving difficulties and resource use difficulty in caregiving tasks; and (3) test different strategies to connect veterans with a referral to community resources through the AAA.
The authors hypothesized that a veteran would be more likely to connect with the AAA if contact was initiated by the AAA rather than the standard practice of asking the veteran to make the call. However, the authors also hypothesized that a veteran who took the time to make the call would be more likely to use AAA resources compared with veterans who were called by the AAA.
Methods
The VASLCHCS Research and Development Office reviewed this project and determined that it met the definition of QI. Therefore, it did not require IRB approval.
The study drew from a convenience sample of veterans who were waiting for appointments in the PCC and who were referred by their health care provider (HCP). To identify caregivers, veterans were asked: “People may provide regular care or assistance to a friend or family member who has a health problem, long-term illness, or disability. During the past month, did you provide any such care or assistance to a friend or family member?” Referrals from HCPs were included in all calculations except the prevalence estimate.
The authors interviewed veterans over a 3-month period in 2015. As of November 2014, the clinic was serving about 11,000 veterans, of which 6,589 lived in Salt Lake County. The clinic also serves veterans who live in other counties in Utah, Nevada, Wyoming, Idaho, and Colorado.
Intervention and Partnering With Community Resources
All willing caregivers were provided a referral to a local AAA (Figure). Salt Lake County veterans interested in referral to the AAA were randomized to 1 of 2 referral methods: veteran-initiated referral (VIR), in which the veteran was given a handout with the phone number of the Salt Lake County caregiver support program (CSP), or provider-initiated referral (PIR), in which the veteran’s phone number was given to the CSP. Caregiving veterans living outside Salt Lake County were provided the AAA phone number in their area and instructed to call for information.
The interview form was randomized using an even or odd number before the interview. Some veterans who were randomized to a PIR needed to be moved into the VIR intervention arm because of the following reasons: the veteran’s care recipient was aged < 18 years (3); the veteran lived outside of Salt Lake County (20); the veteran did not want his/her name given to an outside agency (5); or the interviewer mistakenly gave the veteran the AAA contact information (4).
The primary author called caregivers in the PIR and VIR groups 2 to 4 weeks after the referral to determine whether they had contacted or were contacted by the AAA. Ten call attempts were made before participants were considered lost to follow-up. Caregivers that had been in contact with the AAA reported in open-ended fashion the resources to which they had been referred and whether those resources had been helpful.
Analysis
In this evaluation, the primary outcome of interest was whether contact between the veteran and AAA occurred. For the VIR group, contact was defined as the veteran having called the AAA, regardless of whether he or she actually spoke to someone. For the PIR group, contact occurred if the veteran reported receiving a phone call from AAA regardless of whether he or she had actually spoken with someone (eg, if the veteran reported that the AAA had left a voice mail, this was considered contact). Veterans also were asked whether connecting with the AAA led to resource referrals and whether these referrals were useful.
To achieve a power of 80% with a 95% confidence interval, 20 people were needed in each intervention group to detect a 40% difference in the rate of contact between the 2 groups. STATA12 (College Station, TX) was used to calculate Fisher exact and chi-square values to evaluate differences between groups.
Results
For the study, 433 PCC veterans were interviewed, and 157 (36%) self-identified as a caregiver. An additional 22 referrals were included for a total of 179 caregivers. Caregiver and care recipient characteristics, caregiver burden, and resource utilization were calculated for all 179 caregivers; however, all caregivers did not answer every question. Ninety-eight percent (176) of caregivers were men; 64% (109/170) were from Salt Lake County, and 5% were from outside Utah (8). Twelve percent (21) of the 179 caregivers were providing care for > 1 person. Of 177 caregivers, 3% (5) were caring for both a veteran and a nonveteran, 69% (122) were caring for a nonveteran only, and 28% (49) were caring for another veteran only (Table 1).
The most common burden reported by caregivers was stress (63%); 70% endorsed family/friends as a resource (Table 2). Just 6% (10) of caregivers used the AAA, whereas 26% (45) received VHA support. Of the 54 veterans who were caring for a veteran, 40 reported using the VHA as a resource. Five people caring for nonveterans reported using the VHA as a resource; however, data about which resources those caregivers were accessing were not collected (Table 3).
AAA Referral and Randomization
Sixty-five percent of caregivers accepted AAA referrals. Of 109 Salt Lake County caregivers, 70% accepted referral to the AAA. There was no statistically significant difference in referral acceptance rates when comparing Salt Lake County residents with nonresidents (P = .09).The authors were unable to obtain the phone number for 1 caregiver who had accepted a referral, and 1 caregiver who accepted referral did not want a follow-up. This left 111 caregivers available for follow-up, 75 in Salt Lake County. Fifty Salt Lake County veterans were randomly assigned to the VIR group and 25 to the PIR group. The 36 caregivers who accepted referrals outside Salt Lake County also were placed in the VIR group, for a total of 86 caregivers.
Follow-up
Ninety-eight percent of caregivers were reached for follow-up. Both people lost to follow-up were in Salt Lake County (1 in each group).
In Salt Lake County, 12% (6) of the VIR group and 64% (16) of the PIR group had connected with the AAA (P < .01). Although 64% of those in the PIR group reported having been called by the AAA, the AAA representative reported all 25 had been called. The AAA records showed 9 of those called were reached by voice mail, 6 were provided information about caregiving resources, 2 formally joined the support program, 5 declined help, 1 was no longer caregiving, 1 was too busy to talk, and 1 was the wrong phone number (and was lost to follow-up as well).
Outside of Salt Lake County 19% (7) reported calling the local AAA. There was no difference in referral completion between the Salt Lake County/non-Salt Lake County VIR groups (P = .4).
Fifteen percent of all VIR caregivers reported calling the AAA. There were no statistical differences between Salt Lake County VIR and non-Salt Lake County VIR for reasons why the veteran had not called the AAA (Table 4).
Of 28 people who connected with the AAA, 16 (57%) said they had received access to a needed resource as a result of the phone call. Seven caregivers (25%) said they had not been referred to other resources as a result of the call. The VIR group was more likely to be referred to other resources after contacting the AAA than was the PIR group, although this difference did not reach significance (69% vs 47%, P = .28).
Discussion
More than one-third (36%) of veterans seen in the VASLCHCS PCC are caregivers. This prevalence is higher than that reported for the general U.S. population and higher than that reported in other veteran groups.5,17,18 Most caregivers in this project were caring for nonveterans and only had access to VHA psychosocial caregiver support programs because VHA functional caregiver support (eg, respite, homemaker services) is not available to veterans who care for nonveterans. A majority (78%) of caregiving veterans reported some caregiver burden. Despite the burden, most are not using community resources. However when offered, more than half the caregivers were interested in an AAA referral.
Although the VHA does not provide functional caregiver support resources to veterans caring for nonveterans, there are other agencies that can assist veterans: AAAs for care recipients aged ≥ 60 years and the ADRCs for younger veterans. Through AAAs, caregivers can access a variety of support services, including transportation, adult day care, caregiver support, and health promotion programs. Partnership between agencies such as the VHA and the AAAs could benefit caregiving veterans. This QI project suggests ways to strengthen interagency cooperation.
This study also suggests that a provider or clinic-initiated referral is more likely to connect veterans with information and resources than the current practice of recommending that the veteran initiate the referral. Once in contact with the AAA, most caregivers were referred to needed resources. The next step will be to establish an efficient way for clinic staff to identify caregiving veterans and make referrals to community programs. Referrals could be made by any member of the patient aligned care team (PACT) to further standardize and streamline the process.
Thirty-one percent of veterans in this project were eligible for the VHA caregiver support program because they cared for a veteran. However, 25% of these caregiving veterans were not accessing this resource. Increasing awareness of the VHA caregiver support program among veterans caring for other veterans would improve caregiver support to both caregiving and care recipient veterans.
Limitations
One limitation of this project was the intentional exclusion of the women’s clinic from the sampling process. For consistency, the authors wanted to limit the intervention to 1 PCC and so they chose the clinic that serves the majority of the veterans who receive primary care at VASLCHCS. Additionally, the literature showed that male caregivers compared with women caregivers20,21 have different characteristics in regards to caregiver burden, and a well-designed study of women caregivers already has been published.19
Also, this study did not obtain data on age, health problems, or socioeconomic status of the caregivers to avoid identifying information. Last, the authors did not ask about time spent caregiving or type of care provided. These questions may be important for future studies. Future investigations should evaluate health care use and health of caregivers vs noncaregivers in the veteran population. It also could be important to determine methods for building bridges between the VHA, AAAs, and other community services.
Conclusion
To minimize the disruption that a research study might have caused to normal clinical workflow, the primary author played the role that a medical social worker or other PACT member might play in the future. This project sheds light on how to improve outcomes for community referrals and an important future step in this research would be to develop and test a process that would integrate the PACT into the referral process.
More than one-third of veterans seen in the VASLCHCS PCC are caregivers. To the authors’ knowledge, this is the first estimate of prevalence of caregiving in veterans who receive primary care from the VHA. About 63% of caregiving veterans perceived some burden due to caregiving, and 66% accepted referral to community resources. However, only 12% who were asked to self-refer made contact with the AAA compared with 64% when a provider made the referral for them. Provider referral is more effective in connecting caregiving veterans with resources. Development of interagency partnerships should be fostered to help veterans decrease caregiving burden.
This project is one of the few studies looking at this special group of caregivers: veterans who serve as caregivers. It highlights the need for the VHA to establish policies and partnerships to improve caregiver support to this valuable group of veterans.
1. Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States: population estimates and projections. http://www.census .gov/prod/2014pubs/p25-1140.pdf. Published May 2014. Accessed March 9, 2017.
2. Smith AK, Walter LC, Miao Y, Boscardin WJ, Covinsky KE. Disability during the last two years of life. JAMA Intern Med. 2013;173(16):1506-1513.
3. National Alliance for Caregiving, American Association of Retired Persons. Caregiving in the U.S. 2009 executive summary. http://assets.aarp.org/rgcenter/il/caregiving_09_es.pdf. Published November 2009. Accessed March 9, 2017.
4. Spillman BC, Wolff J, Freedman VA, Kasper JD; Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Informal caregiving for older Americans: an analysis of the 2011 national study of caregiving. https://aspe.hhs.gov/report/informal-caregiving-older-americans-analysis-2011-national-study-caregiving. Published April 1, 2014. Accessed March 9, 2017.
5. Feinberg L, Reinhard SC, Houser A, Choula R; AARP Public Policy Institute. Valuing the invaluable: 2011 update. The growing contributions and costs of family caregiving. https://assets.aarp .org/rgcenter/ppi/ltc/i51-caregiving.pdf. Published June 2011. Accessed March 9, 2017.
6. Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. JAMA. 2014;311(10):1052-1059.
7. Burton LC, Newsom JT, Schulz R, Hirsch CH, German PS. Preventive health behaviors among spousal caregivers. Prev Med. 1997;26(2):162-169.
8. Talley RC, Crews JE. Framing the public health of caregiving. Am J Public Health. 2007;97(2):224-228.
9. Hoffman GJ, Lee J, Mendez-Luck CA. Health behaviors among baby boomer informal caregivers. Gerontologist. 2012;52(2):219-230.
10. National Alliance for Caregiving. Caregivers of veterans—serving on the homefront: report of study findings. http://www.caregiving.org/data/2010 _Caregivers_of_Veterans_FULLREPORT_WEB_FINAL.pdf. Published November 2010. Accessed March 9, 2017.
11. Johannesen M, LoGuidice D. Elder abuse: a systematic review of risk factors in community dwelling elders. Age Ageing. 2013;42(3):292-298.
12. Goy E, Kansagara D, Freeman M;Department of Veterans Affairs, Health Services Research & Development Service. A systematic evidence review of interventions for non-professional caregivers of individuals with dementia. http://www.hsrd.research .va.gov/publications/esp/DementiaCaregivers-EXEC .pdf. Published October 2010. Accessed March 9, 2017.
13. Belle SH, Burgio L, Burns R, et al; Resources for Enhancing Alzheimer’s Caregiver Health (REACH) II Investigators. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized controlled trial. Ann Intern Med. 2006;145(10):727-738.
14. Nichols LO, Martindale-Adams J, Burns R, Graney MJ, Zuber J. Translation of a dementia caregiver support program in a health care system—REACH VA. Arch Intern Med. 2011;171(4):353-359.
15. Bass DM, Judge KS, Snow AL, et al. Caregiver outcomes of partners in dementia care: effect of a care coordination program for veterans with dementia and their family members and friends. J Am Geriatr Soc. 2013;61(8):1377-1386.
16. U.S. Department of Veteran Affairs. VA caregiver support: caregiver services. http://www.caregiver .va.gov/support/support_services.asp. Updated June 3, 2015. Accessed March 9, 2017.
17. Monin JK, Levy BR, Pietrzak RH. From serving in the military to serving loved ones: unique experiences of older veteran caregivers. Am J Geriatr Psychiatry. 2014;22(6):570-579.
18. Parker MW, Call VR, Dunkle R, Vaitkus M. “Out of sight” but not “out of mind”: parent contact and worry among senior ranking male officers in the military who live long distances from parents. Milit Psychol. 2002;14(4):257-277.
19. Lavela SL, Etingen B, Louise-Bender Pape T. Caregiving experiences and health conditions of women veteran and non-veteran caregivers. Womens Health Issues. 2013;23(4):e225-e232.
20. Yee JL, Schultz RS. Gender differences in psychiatric morbidity among family caregivers: a review and analysis. Gerontologist. 2000;40(2):147-164.
21. Collins CR. Men as caregivers of the elderly: support for the contributions of sons. J Multidiscip Healthc. 2014;7:525-531.
More than 20% of the U.S. population will be aged ≥ 65 years by 2030, an increase from 13% in 2012.1 The likelihood of needing assistance with activities of daily living (ADLs) increases with age.2 People who need such assistance often depend on informal and unpaid assistance from friends and family. In 2009, about 65.7 million Americans (28.5%) provided informal care for people with an illness or disability, and that number only is expected to rise.3 These informal caregivers provide up to 80% of the total care hours needed by community-dwelling older adults—an estimated economic value of $450 billion in unpaid contributions in 2009.4,5
Caregiving can lead to significant physical, psychological, social, and financial burdens.6 The caregiving burden is associated with a host of adverse health behaviors and outcomes such as poor diet, lack of exercise and sleep, smoking, decreased participation in preventive health care, anxiety, depression, relationship difficulties, employment disruption, financial hardship, suicide, and higher mortality compared with that of noncaregivers.6-10 Additionally, care recipients are at increased risk for abuse or neglect when the caregiver is experiencing a significant burden.11 Therefore, efforts to improve caregiver support are important for both partners in the caregiver/care recipient dyad.
Caregiver support is beneficial to the health of caregivers and care recipients.10,12 For example, the Resources for Enhancing Alzheimer’s Caregiver Health (REACH) program has been shown to reduce the stress of informal caregiving and the risk of depression in caregivers.13,14 This program showed similar effects when implemented within the VHA.14 In the Partners in Dementia Care project, the VHA and Alzheimer’s Association coordinated care and support for veterans with dementia and their family and friends. This intervention resulted in lower caregiver strain and depression scores among participants.15
With a growing medical literature that shows the benefits of caregiver support interventions, the VHA developed a robust support program for informal caregivers of veterans. The VA caregiver support website (www.caregiver.va.gov) provides information and resources targeted to caregivers for veterans, including psychosocial and functional support for caregivers. The psychosocial support provided by the VA includes caregiver education, counseling, access to caregiver support coordinators, a caregiver support line, support groups, and referral to community support organizations.16 Functional support on the site includes financial assistance toward skilled home care, home hospice care, adult day care, home-based primary care, homemaker and home health aide services, telehealth, and respite care.16 Veterans who are caregiving for nonveterans have access to VHA psychosocial support but not to functional support services. For these veterans, functional caregiver support must come from family or referral to community organizations.
Background
In the U.S., about 11% of caregivers are veterans, but the availability of data about these caregivers is limited to veteran subgroups.3 For example, a 2011 study reported that 20% of veterans aged ≥ 60 years are caregivers.17 However, this estimate included child care for unimpaired children, which is not commonly included in other caregiving estimates. In another study, 30% of middle-aged active-duty officers reported helping their parents with instrumental ADLs (IADLs).18 These data suggest a significant proportion of veterans may be caregivers; however, the estimates do not identify prevalence of caregiving among a population of VHA enrolled veterans.
Likewise, few studies discuss the burden veterans experience from caregiving. A study of the 2009/2010 CDC Behavioral Risk Factor Surveillance System data found that female caregivers were more likely to report problems with sleep and mental health if they were veterans vs nonveterans.19 In a second study, caregiving veterans frequently reported physical (39%) and emotional (53%) strain, with emotional strain relating to depressive symptoms. The study of active-duty officers noted that worry was prevalent among military officers caregiving for parents from a distance.18 In contrast to the negative outcomes of caregiving, Monin and colleagues found that many veterans perceived caregiving as rewarding. Since caregiving may be a positive experience, veterans may benefit and be a potential resource for care to elderly and disabled citizens.17
Project Rationale and Goals
Social workers are the cornerstone of caregiver support at the George E. Wahlen VA Salt Lake City Health Care System (VASLCHCS) in Utah. They educate veterans and caregivers about VA resources to support caregivers of veterans. For those veterans who are caregiving for a nonveteran, the VASLCHCS social workers provide psychosocial support and help veterans connect to a local area agency on aging (AAA) for access to functional support. In practice, primary care clinic (PCC) providers have observed that directing a veteran to call the AAA does not usually result in a phone call. Therefore, an aim of this quality improvement (QI) project was to determine the most effective means of completing a successful AAA referral.
The VASLCHCS Geriatric Research Education and Clinical Center collaborates with the Utah Aging and Disability Resource Connection (ADRC) to improve awareness of available resources for veterans. Building on this collaborative project, the authors created a formal referral process for veterans needing local AAA services. This QI project had 3 aims: (1) estimate the prevalence of caregiving among veterans in the VASLCHCS primary care clinic; (2) identify perceived caregiving difficulties and resource use difficulty in caregiving tasks; and (3) test different strategies to connect veterans with a referral to community resources through the AAA.
The authors hypothesized that a veteran would be more likely to connect with the AAA if contact was initiated by the AAA rather than the standard practice of asking the veteran to make the call. However, the authors also hypothesized that a veteran who took the time to make the call would be more likely to use AAA resources compared with veterans who were called by the AAA.
Methods
The VASLCHCS Research and Development Office reviewed this project and determined that it met the definition of QI. Therefore, it did not require IRB approval.
The study drew from a convenience sample of veterans who were waiting for appointments in the PCC and who were referred by their health care provider (HCP). To identify caregivers, veterans were asked: “People may provide regular care or assistance to a friend or family member who has a health problem, long-term illness, or disability. During the past month, did you provide any such care or assistance to a friend or family member?” Referrals from HCPs were included in all calculations except the prevalence estimate.
The authors interviewed veterans over a 3-month period in 2015. As of November 2014, the clinic was serving about 11,000 veterans, of which 6,589 lived in Salt Lake County. The clinic also serves veterans who live in other counties in Utah, Nevada, Wyoming, Idaho, and Colorado.
Intervention and Partnering With Community Resources
All willing caregivers were provided a referral to a local AAA (Figure). Salt Lake County veterans interested in referral to the AAA were randomized to 1 of 2 referral methods: veteran-initiated referral (VIR), in which the veteran was given a handout with the phone number of the Salt Lake County caregiver support program (CSP), or provider-initiated referral (PIR), in which the veteran’s phone number was given to the CSP. Caregiving veterans living outside Salt Lake County were provided the AAA phone number in their area and instructed to call for information.
The interview form was randomized using an even or odd number before the interview. Some veterans who were randomized to a PIR needed to be moved into the VIR intervention arm because of the following reasons: the veteran’s care recipient was aged < 18 years (3); the veteran lived outside of Salt Lake County (20); the veteran did not want his/her name given to an outside agency (5); or the interviewer mistakenly gave the veteran the AAA contact information (4).
The primary author called caregivers in the PIR and VIR groups 2 to 4 weeks after the referral to determine whether they had contacted or were contacted by the AAA. Ten call attempts were made before participants were considered lost to follow-up. Caregivers that had been in contact with the AAA reported in open-ended fashion the resources to which they had been referred and whether those resources had been helpful.
Analysis
In this evaluation, the primary outcome of interest was whether contact between the veteran and AAA occurred. For the VIR group, contact was defined as the veteran having called the AAA, regardless of whether he or she actually spoke to someone. For the PIR group, contact occurred if the veteran reported receiving a phone call from AAA regardless of whether he or she had actually spoken with someone (eg, if the veteran reported that the AAA had left a voice mail, this was considered contact). Veterans also were asked whether connecting with the AAA led to resource referrals and whether these referrals were useful.
To achieve a power of 80% with a 95% confidence interval, 20 people were needed in each intervention group to detect a 40% difference in the rate of contact between the 2 groups. STATA12 (College Station, TX) was used to calculate Fisher exact and chi-square values to evaluate differences between groups.
Results
For the study, 433 PCC veterans were interviewed, and 157 (36%) self-identified as a caregiver. An additional 22 referrals were included for a total of 179 caregivers. Caregiver and care recipient characteristics, caregiver burden, and resource utilization were calculated for all 179 caregivers; however, all caregivers did not answer every question. Ninety-eight percent (176) of caregivers were men; 64% (109/170) were from Salt Lake County, and 5% were from outside Utah (8). Twelve percent (21) of the 179 caregivers were providing care for > 1 person. Of 177 caregivers, 3% (5) were caring for both a veteran and a nonveteran, 69% (122) were caring for a nonveteran only, and 28% (49) were caring for another veteran only (Table 1).
The most common burden reported by caregivers was stress (63%); 70% endorsed family/friends as a resource (Table 2). Just 6% (10) of caregivers used the AAA, whereas 26% (45) received VHA support. Of the 54 veterans who were caring for a veteran, 40 reported using the VHA as a resource. Five people caring for nonveterans reported using the VHA as a resource; however, data about which resources those caregivers were accessing were not collected (Table 3).
AAA Referral and Randomization
Sixty-five percent of caregivers accepted AAA referrals. Of 109 Salt Lake County caregivers, 70% accepted referral to the AAA. There was no statistically significant difference in referral acceptance rates when comparing Salt Lake County residents with nonresidents (P = .09).The authors were unable to obtain the phone number for 1 caregiver who had accepted a referral, and 1 caregiver who accepted referral did not want a follow-up. This left 111 caregivers available for follow-up, 75 in Salt Lake County. Fifty Salt Lake County veterans were randomly assigned to the VIR group and 25 to the PIR group. The 36 caregivers who accepted referrals outside Salt Lake County also were placed in the VIR group, for a total of 86 caregivers.
Follow-up
Ninety-eight percent of caregivers were reached for follow-up. Both people lost to follow-up were in Salt Lake County (1 in each group).
In Salt Lake County, 12% (6) of the VIR group and 64% (16) of the PIR group had connected with the AAA (P < .01). Although 64% of those in the PIR group reported having been called by the AAA, the AAA representative reported all 25 had been called. The AAA records showed 9 of those called were reached by voice mail, 6 were provided information about caregiving resources, 2 formally joined the support program, 5 declined help, 1 was no longer caregiving, 1 was too busy to talk, and 1 was the wrong phone number (and was lost to follow-up as well).
Outside of Salt Lake County 19% (7) reported calling the local AAA. There was no difference in referral completion between the Salt Lake County/non-Salt Lake County VIR groups (P = .4).
Fifteen percent of all VIR caregivers reported calling the AAA. There were no statistical differences between Salt Lake County VIR and non-Salt Lake County VIR for reasons why the veteran had not called the AAA (Table 4).
Of 28 people who connected with the AAA, 16 (57%) said they had received access to a needed resource as a result of the phone call. Seven caregivers (25%) said they had not been referred to other resources as a result of the call. The VIR group was more likely to be referred to other resources after contacting the AAA than was the PIR group, although this difference did not reach significance (69% vs 47%, P = .28).
Discussion
More than one-third (36%) of veterans seen in the VASLCHCS PCC are caregivers. This prevalence is higher than that reported for the general U.S. population and higher than that reported in other veteran groups.5,17,18 Most caregivers in this project were caring for nonveterans and only had access to VHA psychosocial caregiver support programs because VHA functional caregiver support (eg, respite, homemaker services) is not available to veterans who care for nonveterans. A majority (78%) of caregiving veterans reported some caregiver burden. Despite the burden, most are not using community resources. However when offered, more than half the caregivers were interested in an AAA referral.
Although the VHA does not provide functional caregiver support resources to veterans caring for nonveterans, there are other agencies that can assist veterans: AAAs for care recipients aged ≥ 60 years and the ADRCs for younger veterans. Through AAAs, caregivers can access a variety of support services, including transportation, adult day care, caregiver support, and health promotion programs. Partnership between agencies such as the VHA and the AAAs could benefit caregiving veterans. This QI project suggests ways to strengthen interagency cooperation.
This study also suggests that a provider or clinic-initiated referral is more likely to connect veterans with information and resources than the current practice of recommending that the veteran initiate the referral. Once in contact with the AAA, most caregivers were referred to needed resources. The next step will be to establish an efficient way for clinic staff to identify caregiving veterans and make referrals to community programs. Referrals could be made by any member of the patient aligned care team (PACT) to further standardize and streamline the process.
Thirty-one percent of veterans in this project were eligible for the VHA caregiver support program because they cared for a veteran. However, 25% of these caregiving veterans were not accessing this resource. Increasing awareness of the VHA caregiver support program among veterans caring for other veterans would improve caregiver support to both caregiving and care recipient veterans.
Limitations
One limitation of this project was the intentional exclusion of the women’s clinic from the sampling process. For consistency, the authors wanted to limit the intervention to 1 PCC and so they chose the clinic that serves the majority of the veterans who receive primary care at VASLCHCS. Additionally, the literature showed that male caregivers compared with women caregivers20,21 have different characteristics in regards to caregiver burden, and a well-designed study of women caregivers already has been published.19
Also, this study did not obtain data on age, health problems, or socioeconomic status of the caregivers to avoid identifying information. Last, the authors did not ask about time spent caregiving or type of care provided. These questions may be important for future studies. Future investigations should evaluate health care use and health of caregivers vs noncaregivers in the veteran population. It also could be important to determine methods for building bridges between the VHA, AAAs, and other community services.
Conclusion
To minimize the disruption that a research study might have caused to normal clinical workflow, the primary author played the role that a medical social worker or other PACT member might play in the future. This project sheds light on how to improve outcomes for community referrals and an important future step in this research would be to develop and test a process that would integrate the PACT into the referral process.
More than one-third of veterans seen in the VASLCHCS PCC are caregivers. To the authors’ knowledge, this is the first estimate of prevalence of caregiving in veterans who receive primary care from the VHA. About 63% of caregiving veterans perceived some burden due to caregiving, and 66% accepted referral to community resources. However, only 12% who were asked to self-refer made contact with the AAA compared with 64% when a provider made the referral for them. Provider referral is more effective in connecting caregiving veterans with resources. Development of interagency partnerships should be fostered to help veterans decrease caregiving burden.
This project is one of the few studies looking at this special group of caregivers: veterans who serve as caregivers. It highlights the need for the VHA to establish policies and partnerships to improve caregiver support to this valuable group of veterans.
More than 20% of the U.S. population will be aged ≥ 65 years by 2030, an increase from 13% in 2012.1 The likelihood of needing assistance with activities of daily living (ADLs) increases with age.2 People who need such assistance often depend on informal and unpaid assistance from friends and family. In 2009, about 65.7 million Americans (28.5%) provided informal care for people with an illness or disability, and that number only is expected to rise.3 These informal caregivers provide up to 80% of the total care hours needed by community-dwelling older adults—an estimated economic value of $450 billion in unpaid contributions in 2009.4,5
Caregiving can lead to significant physical, psychological, social, and financial burdens.6 The caregiving burden is associated with a host of adverse health behaviors and outcomes such as poor diet, lack of exercise and sleep, smoking, decreased participation in preventive health care, anxiety, depression, relationship difficulties, employment disruption, financial hardship, suicide, and higher mortality compared with that of noncaregivers.6-10 Additionally, care recipients are at increased risk for abuse or neglect when the caregiver is experiencing a significant burden.11 Therefore, efforts to improve caregiver support are important for both partners in the caregiver/care recipient dyad.
Caregiver support is beneficial to the health of caregivers and care recipients.10,12 For example, the Resources for Enhancing Alzheimer’s Caregiver Health (REACH) program has been shown to reduce the stress of informal caregiving and the risk of depression in caregivers.13,14 This program showed similar effects when implemented within the VHA.14 In the Partners in Dementia Care project, the VHA and Alzheimer’s Association coordinated care and support for veterans with dementia and their family and friends. This intervention resulted in lower caregiver strain and depression scores among participants.15
With a growing medical literature that shows the benefits of caregiver support interventions, the VHA developed a robust support program for informal caregivers of veterans. The VA caregiver support website (www.caregiver.va.gov) provides information and resources targeted to caregivers for veterans, including psychosocial and functional support for caregivers. The psychosocial support provided by the VA includes caregiver education, counseling, access to caregiver support coordinators, a caregiver support line, support groups, and referral to community support organizations.16 Functional support on the site includes financial assistance toward skilled home care, home hospice care, adult day care, home-based primary care, homemaker and home health aide services, telehealth, and respite care.16 Veterans who are caregiving for nonveterans have access to VHA psychosocial support but not to functional support services. For these veterans, functional caregiver support must come from family or referral to community organizations.
Background
In the U.S., about 11% of caregivers are veterans, but the availability of data about these caregivers is limited to veteran subgroups.3 For example, a 2011 study reported that 20% of veterans aged ≥ 60 years are caregivers.17 However, this estimate included child care for unimpaired children, which is not commonly included in other caregiving estimates. In another study, 30% of middle-aged active-duty officers reported helping their parents with instrumental ADLs (IADLs).18 These data suggest a significant proportion of veterans may be caregivers; however, the estimates do not identify prevalence of caregiving among a population of VHA enrolled veterans.
Likewise, few studies discuss the burden veterans experience from caregiving. A study of the 2009/2010 CDC Behavioral Risk Factor Surveillance System data found that female caregivers were more likely to report problems with sleep and mental health if they were veterans vs nonveterans.19 In a second study, caregiving veterans frequently reported physical (39%) and emotional (53%) strain, with emotional strain relating to depressive symptoms. The study of active-duty officers noted that worry was prevalent among military officers caregiving for parents from a distance.18 In contrast to the negative outcomes of caregiving, Monin and colleagues found that many veterans perceived caregiving as rewarding. Since caregiving may be a positive experience, veterans may benefit and be a potential resource for care to elderly and disabled citizens.17
Project Rationale and Goals
Social workers are the cornerstone of caregiver support at the George E. Wahlen VA Salt Lake City Health Care System (VASLCHCS) in Utah. They educate veterans and caregivers about VA resources to support caregivers of veterans. For those veterans who are caregiving for a nonveteran, the VASLCHCS social workers provide psychosocial support and help veterans connect to a local area agency on aging (AAA) for access to functional support. In practice, primary care clinic (PCC) providers have observed that directing a veteran to call the AAA does not usually result in a phone call. Therefore, an aim of this quality improvement (QI) project was to determine the most effective means of completing a successful AAA referral.
The VASLCHCS Geriatric Research Education and Clinical Center collaborates with the Utah Aging and Disability Resource Connection (ADRC) to improve awareness of available resources for veterans. Building on this collaborative project, the authors created a formal referral process for veterans needing local AAA services. This QI project had 3 aims: (1) estimate the prevalence of caregiving among veterans in the VASLCHCS primary care clinic; (2) identify perceived caregiving difficulties and resource use difficulty in caregiving tasks; and (3) test different strategies to connect veterans with a referral to community resources through the AAA.
The authors hypothesized that a veteran would be more likely to connect with the AAA if contact was initiated by the AAA rather than the standard practice of asking the veteran to make the call. However, the authors also hypothesized that a veteran who took the time to make the call would be more likely to use AAA resources compared with veterans who were called by the AAA.
Methods
The VASLCHCS Research and Development Office reviewed this project and determined that it met the definition of QI. Therefore, it did not require IRB approval.
The study drew from a convenience sample of veterans who were waiting for appointments in the PCC and who were referred by their health care provider (HCP). To identify caregivers, veterans were asked: “People may provide regular care or assistance to a friend or family member who has a health problem, long-term illness, or disability. During the past month, did you provide any such care or assistance to a friend or family member?” Referrals from HCPs were included in all calculations except the prevalence estimate.
The authors interviewed veterans over a 3-month period in 2015. As of November 2014, the clinic was serving about 11,000 veterans, of which 6,589 lived in Salt Lake County. The clinic also serves veterans who live in other counties in Utah, Nevada, Wyoming, Idaho, and Colorado.
Intervention and Partnering With Community Resources
All willing caregivers were provided a referral to a local AAA (Figure). Salt Lake County veterans interested in referral to the AAA were randomized to 1 of 2 referral methods: veteran-initiated referral (VIR), in which the veteran was given a handout with the phone number of the Salt Lake County caregiver support program (CSP), or provider-initiated referral (PIR), in which the veteran’s phone number was given to the CSP. Caregiving veterans living outside Salt Lake County were provided the AAA phone number in their area and instructed to call for information.
The interview form was randomized using an even or odd number before the interview. Some veterans who were randomized to a PIR needed to be moved into the VIR intervention arm because of the following reasons: the veteran’s care recipient was aged < 18 years (3); the veteran lived outside of Salt Lake County (20); the veteran did not want his/her name given to an outside agency (5); or the interviewer mistakenly gave the veteran the AAA contact information (4).
The primary author called caregivers in the PIR and VIR groups 2 to 4 weeks after the referral to determine whether they had contacted or were contacted by the AAA. Ten call attempts were made before participants were considered lost to follow-up. Caregivers that had been in contact with the AAA reported in open-ended fashion the resources to which they had been referred and whether those resources had been helpful.
Analysis
In this evaluation, the primary outcome of interest was whether contact between the veteran and AAA occurred. For the VIR group, contact was defined as the veteran having called the AAA, regardless of whether he or she actually spoke to someone. For the PIR group, contact occurred if the veteran reported receiving a phone call from AAA regardless of whether he or she had actually spoken with someone (eg, if the veteran reported that the AAA had left a voice mail, this was considered contact). Veterans also were asked whether connecting with the AAA led to resource referrals and whether these referrals were useful.
To achieve a power of 80% with a 95% confidence interval, 20 people were needed in each intervention group to detect a 40% difference in the rate of contact between the 2 groups. STATA12 (College Station, TX) was used to calculate Fisher exact and chi-square values to evaluate differences between groups.
Results
For the study, 433 PCC veterans were interviewed, and 157 (36%) self-identified as a caregiver. An additional 22 referrals were included for a total of 179 caregivers. Caregiver and care recipient characteristics, caregiver burden, and resource utilization were calculated for all 179 caregivers; however, all caregivers did not answer every question. Ninety-eight percent (176) of caregivers were men; 64% (109/170) were from Salt Lake County, and 5% were from outside Utah (8). Twelve percent (21) of the 179 caregivers were providing care for > 1 person. Of 177 caregivers, 3% (5) were caring for both a veteran and a nonveteran, 69% (122) were caring for a nonveteran only, and 28% (49) were caring for another veteran only (Table 1).
The most common burden reported by caregivers was stress (63%); 70% endorsed family/friends as a resource (Table 2). Just 6% (10) of caregivers used the AAA, whereas 26% (45) received VHA support. Of the 54 veterans who were caring for a veteran, 40 reported using the VHA as a resource. Five people caring for nonveterans reported using the VHA as a resource; however, data about which resources those caregivers were accessing were not collected (Table 3).
AAA Referral and Randomization
Sixty-five percent of caregivers accepted AAA referrals. Of 109 Salt Lake County caregivers, 70% accepted referral to the AAA. There was no statistically significant difference in referral acceptance rates when comparing Salt Lake County residents with nonresidents (P = .09).The authors were unable to obtain the phone number for 1 caregiver who had accepted a referral, and 1 caregiver who accepted referral did not want a follow-up. This left 111 caregivers available for follow-up, 75 in Salt Lake County. Fifty Salt Lake County veterans were randomly assigned to the VIR group and 25 to the PIR group. The 36 caregivers who accepted referrals outside Salt Lake County also were placed in the VIR group, for a total of 86 caregivers.
Follow-up
Ninety-eight percent of caregivers were reached for follow-up. Both people lost to follow-up were in Salt Lake County (1 in each group).
In Salt Lake County, 12% (6) of the VIR group and 64% (16) of the PIR group had connected with the AAA (P < .01). Although 64% of those in the PIR group reported having been called by the AAA, the AAA representative reported all 25 had been called. The AAA records showed 9 of those called were reached by voice mail, 6 were provided information about caregiving resources, 2 formally joined the support program, 5 declined help, 1 was no longer caregiving, 1 was too busy to talk, and 1 was the wrong phone number (and was lost to follow-up as well).
Outside of Salt Lake County 19% (7) reported calling the local AAA. There was no difference in referral completion between the Salt Lake County/non-Salt Lake County VIR groups (P = .4).
Fifteen percent of all VIR caregivers reported calling the AAA. There were no statistical differences between Salt Lake County VIR and non-Salt Lake County VIR for reasons why the veteran had not called the AAA (Table 4).
Of 28 people who connected with the AAA, 16 (57%) said they had received access to a needed resource as a result of the phone call. Seven caregivers (25%) said they had not been referred to other resources as a result of the call. The VIR group was more likely to be referred to other resources after contacting the AAA than was the PIR group, although this difference did not reach significance (69% vs 47%, P = .28).
Discussion
More than one-third (36%) of veterans seen in the VASLCHCS PCC are caregivers. This prevalence is higher than that reported for the general U.S. population and higher than that reported in other veteran groups.5,17,18 Most caregivers in this project were caring for nonveterans and only had access to VHA psychosocial caregiver support programs because VHA functional caregiver support (eg, respite, homemaker services) is not available to veterans who care for nonveterans. A majority (78%) of caregiving veterans reported some caregiver burden. Despite the burden, most are not using community resources. However when offered, more than half the caregivers were interested in an AAA referral.
Although the VHA does not provide functional caregiver support resources to veterans caring for nonveterans, there are other agencies that can assist veterans: AAAs for care recipients aged ≥ 60 years and the ADRCs for younger veterans. Through AAAs, caregivers can access a variety of support services, including transportation, adult day care, caregiver support, and health promotion programs. Partnership between agencies such as the VHA and the AAAs could benefit caregiving veterans. This QI project suggests ways to strengthen interagency cooperation.
This study also suggests that a provider or clinic-initiated referral is more likely to connect veterans with information and resources than the current practice of recommending that the veteran initiate the referral. Once in contact with the AAA, most caregivers were referred to needed resources. The next step will be to establish an efficient way for clinic staff to identify caregiving veterans and make referrals to community programs. Referrals could be made by any member of the patient aligned care team (PACT) to further standardize and streamline the process.
Thirty-one percent of veterans in this project were eligible for the VHA caregiver support program because they cared for a veteran. However, 25% of these caregiving veterans were not accessing this resource. Increasing awareness of the VHA caregiver support program among veterans caring for other veterans would improve caregiver support to both caregiving and care recipient veterans.
Limitations
One limitation of this project was the intentional exclusion of the women’s clinic from the sampling process. For consistency, the authors wanted to limit the intervention to 1 PCC and so they chose the clinic that serves the majority of the veterans who receive primary care at VASLCHCS. Additionally, the literature showed that male caregivers compared with women caregivers20,21 have different characteristics in regards to caregiver burden, and a well-designed study of women caregivers already has been published.19
Also, this study did not obtain data on age, health problems, or socioeconomic status of the caregivers to avoid identifying information. Last, the authors did not ask about time spent caregiving or type of care provided. These questions may be important for future studies. Future investigations should evaluate health care use and health of caregivers vs noncaregivers in the veteran population. It also could be important to determine methods for building bridges between the VHA, AAAs, and other community services.
Conclusion
To minimize the disruption that a research study might have caused to normal clinical workflow, the primary author played the role that a medical social worker or other PACT member might play in the future. This project sheds light on how to improve outcomes for community referrals and an important future step in this research would be to develop and test a process that would integrate the PACT into the referral process.
More than one-third of veterans seen in the VASLCHCS PCC are caregivers. To the authors’ knowledge, this is the first estimate of prevalence of caregiving in veterans who receive primary care from the VHA. About 63% of caregiving veterans perceived some burden due to caregiving, and 66% accepted referral to community resources. However, only 12% who were asked to self-refer made contact with the AAA compared with 64% when a provider made the referral for them. Provider referral is more effective in connecting caregiving veterans with resources. Development of interagency partnerships should be fostered to help veterans decrease caregiving burden.
This project is one of the few studies looking at this special group of caregivers: veterans who serve as caregivers. It highlights the need for the VHA to establish policies and partnerships to improve caregiver support to this valuable group of veterans.
1. Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States: population estimates and projections. http://www.census .gov/prod/2014pubs/p25-1140.pdf. Published May 2014. Accessed March 9, 2017.
2. Smith AK, Walter LC, Miao Y, Boscardin WJ, Covinsky KE. Disability during the last two years of life. JAMA Intern Med. 2013;173(16):1506-1513.
3. National Alliance for Caregiving, American Association of Retired Persons. Caregiving in the U.S. 2009 executive summary. http://assets.aarp.org/rgcenter/il/caregiving_09_es.pdf. Published November 2009. Accessed March 9, 2017.
4. Spillman BC, Wolff J, Freedman VA, Kasper JD; Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Informal caregiving for older Americans: an analysis of the 2011 national study of caregiving. https://aspe.hhs.gov/report/informal-caregiving-older-americans-analysis-2011-national-study-caregiving. Published April 1, 2014. Accessed March 9, 2017.
5. Feinberg L, Reinhard SC, Houser A, Choula R; AARP Public Policy Institute. Valuing the invaluable: 2011 update. The growing contributions and costs of family caregiving. https://assets.aarp .org/rgcenter/ppi/ltc/i51-caregiving.pdf. Published June 2011. Accessed March 9, 2017.
6. Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. JAMA. 2014;311(10):1052-1059.
7. Burton LC, Newsom JT, Schulz R, Hirsch CH, German PS. Preventive health behaviors among spousal caregivers. Prev Med. 1997;26(2):162-169.
8. Talley RC, Crews JE. Framing the public health of caregiving. Am J Public Health. 2007;97(2):224-228.
9. Hoffman GJ, Lee J, Mendez-Luck CA. Health behaviors among baby boomer informal caregivers. Gerontologist. 2012;52(2):219-230.
10. National Alliance for Caregiving. Caregivers of veterans—serving on the homefront: report of study findings. http://www.caregiving.org/data/2010 _Caregivers_of_Veterans_FULLREPORT_WEB_FINAL.pdf. Published November 2010. Accessed March 9, 2017.
11. Johannesen M, LoGuidice D. Elder abuse: a systematic review of risk factors in community dwelling elders. Age Ageing. 2013;42(3):292-298.
12. Goy E, Kansagara D, Freeman M;Department of Veterans Affairs, Health Services Research & Development Service. A systematic evidence review of interventions for non-professional caregivers of individuals with dementia. http://www.hsrd.research .va.gov/publications/esp/DementiaCaregivers-EXEC .pdf. Published October 2010. Accessed March 9, 2017.
13. Belle SH, Burgio L, Burns R, et al; Resources for Enhancing Alzheimer’s Caregiver Health (REACH) II Investigators. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized controlled trial. Ann Intern Med. 2006;145(10):727-738.
14. Nichols LO, Martindale-Adams J, Burns R, Graney MJ, Zuber J. Translation of a dementia caregiver support program in a health care system—REACH VA. Arch Intern Med. 2011;171(4):353-359.
15. Bass DM, Judge KS, Snow AL, et al. Caregiver outcomes of partners in dementia care: effect of a care coordination program for veterans with dementia and their family members and friends. J Am Geriatr Soc. 2013;61(8):1377-1386.
16. U.S. Department of Veteran Affairs. VA caregiver support: caregiver services. http://www.caregiver .va.gov/support/support_services.asp. Updated June 3, 2015. Accessed March 9, 2017.
17. Monin JK, Levy BR, Pietrzak RH. From serving in the military to serving loved ones: unique experiences of older veteran caregivers. Am J Geriatr Psychiatry. 2014;22(6):570-579.
18. Parker MW, Call VR, Dunkle R, Vaitkus M. “Out of sight” but not “out of mind”: parent contact and worry among senior ranking male officers in the military who live long distances from parents. Milit Psychol. 2002;14(4):257-277.
19. Lavela SL, Etingen B, Louise-Bender Pape T. Caregiving experiences and health conditions of women veteran and non-veteran caregivers. Womens Health Issues. 2013;23(4):e225-e232.
20. Yee JL, Schultz RS. Gender differences in psychiatric morbidity among family caregivers: a review and analysis. Gerontologist. 2000;40(2):147-164.
21. Collins CR. Men as caregivers of the elderly: support for the contributions of sons. J Multidiscip Healthc. 2014;7:525-531.
1. Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States: population estimates and projections. http://www.census .gov/prod/2014pubs/p25-1140.pdf. Published May 2014. Accessed March 9, 2017.
2. Smith AK, Walter LC, Miao Y, Boscardin WJ, Covinsky KE. Disability during the last two years of life. JAMA Intern Med. 2013;173(16):1506-1513.
3. National Alliance for Caregiving, American Association of Retired Persons. Caregiving in the U.S. 2009 executive summary. http://assets.aarp.org/rgcenter/il/caregiving_09_es.pdf. Published November 2009. Accessed March 9, 2017.
4. Spillman BC, Wolff J, Freedman VA, Kasper JD; Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Informal caregiving for older Americans: an analysis of the 2011 national study of caregiving. https://aspe.hhs.gov/report/informal-caregiving-older-americans-analysis-2011-national-study-caregiving. Published April 1, 2014. Accessed March 9, 2017.
5. Feinberg L, Reinhard SC, Houser A, Choula R; AARP Public Policy Institute. Valuing the invaluable: 2011 update. The growing contributions and costs of family caregiving. https://assets.aarp .org/rgcenter/ppi/ltc/i51-caregiving.pdf. Published June 2011. Accessed March 9, 2017.
6. Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. JAMA. 2014;311(10):1052-1059.
7. Burton LC, Newsom JT, Schulz R, Hirsch CH, German PS. Preventive health behaviors among spousal caregivers. Prev Med. 1997;26(2):162-169.
8. Talley RC, Crews JE. Framing the public health of caregiving. Am J Public Health. 2007;97(2):224-228.
9. Hoffman GJ, Lee J, Mendez-Luck CA. Health behaviors among baby boomer informal caregivers. Gerontologist. 2012;52(2):219-230.
10. National Alliance for Caregiving. Caregivers of veterans—serving on the homefront: report of study findings. http://www.caregiving.org/data/2010 _Caregivers_of_Veterans_FULLREPORT_WEB_FINAL.pdf. Published November 2010. Accessed March 9, 2017.
11. Johannesen M, LoGuidice D. Elder abuse: a systematic review of risk factors in community dwelling elders. Age Ageing. 2013;42(3):292-298.
12. Goy E, Kansagara D, Freeman M;Department of Veterans Affairs, Health Services Research & Development Service. A systematic evidence review of interventions for non-professional caregivers of individuals with dementia. http://www.hsrd.research .va.gov/publications/esp/DementiaCaregivers-EXEC .pdf. Published October 2010. Accessed March 9, 2017.
13. Belle SH, Burgio L, Burns R, et al; Resources for Enhancing Alzheimer’s Caregiver Health (REACH) II Investigators. Enhancing the quality of life of dementia caregivers from different ethnic or racial groups: a randomized controlled trial. Ann Intern Med. 2006;145(10):727-738.
14. Nichols LO, Martindale-Adams J, Burns R, Graney MJ, Zuber J. Translation of a dementia caregiver support program in a health care system—REACH VA. Arch Intern Med. 2011;171(4):353-359.
15. Bass DM, Judge KS, Snow AL, et al. Caregiver outcomes of partners in dementia care: effect of a care coordination program for veterans with dementia and their family members and friends. J Am Geriatr Soc. 2013;61(8):1377-1386.
16. U.S. Department of Veteran Affairs. VA caregiver support: caregiver services. http://www.caregiver .va.gov/support/support_services.asp. Updated June 3, 2015. Accessed March 9, 2017.
17. Monin JK, Levy BR, Pietrzak RH. From serving in the military to serving loved ones: unique experiences of older veteran caregivers. Am J Geriatr Psychiatry. 2014;22(6):570-579.
18. Parker MW, Call VR, Dunkle R, Vaitkus M. “Out of sight” but not “out of mind”: parent contact and worry among senior ranking male officers in the military who live long distances from parents. Milit Psychol. 2002;14(4):257-277.
19. Lavela SL, Etingen B, Louise-Bender Pape T. Caregiving experiences and health conditions of women veteran and non-veteran caregivers. Womens Health Issues. 2013;23(4):e225-e232.
20. Yee JL, Schultz RS. Gender differences in psychiatric morbidity among family caregivers: a review and analysis. Gerontologist. 2000;40(2):147-164.
21. Collins CR. Men as caregivers of the elderly: support for the contributions of sons. J Multidiscip Healthc. 2014;7:525-531.
Applying a Time-Out and Standardized Report Form in Anesthesia Handoffs
Improving health care safety is one of the top priorities of the U.S. health care system. A key element for health care safety is the elimination of sentinel events—unexpected occurrences involving death or serious physical or psychological injury, such as loss of limb or function—or even the risk.1 Problems in communication, continuity of care, and planning have been identified as the root cause in more than 80% of documented sentinel events.2 As a direct result, The Joint Commission (JC) added National Patient Safety Goal 2E, which instructs each organization to implement a standardized approach to patient handoff.1 According to the JC, the objective of a handoff is to “provide accurate information about a patient’s care, treatment, and services, current condition, and any recent or anticipated changesand must include open communication and opportunities for questions.”1,3 The JC identified the patient handoff from anesthesia providers to the Surgical Intensive Care Unit (SICU) and Postanesthesia Care Unit (PACU) an opportunity for an improvement.1,3
At the Memphis VAMC in Tennessee, there was no established protocol for patient handoff from anesthesia providers to the SICU and PACU. The Anesthesia and SICU staffs were frustrated by inconsistent and incomplete postsurgical handoffs. Issues identified by the anesthesia team included difficulty contacting SICU staff to give a report and inconsistent availability of staff on first arrival to SICU. The SICU staff felt communication was rushed and there were inconsistencies in length and quality of the reports, resulting in incomplete postsurgical handoffs.
A baseline survey showed only 75% of staff felt the handoff report was thorough, and 67% “felt like a team.” In response, a multidisciplinary safe patient handoff committee (SPHOC) was formed by representatives from the involved units to discuss issues and offer solutions. The SPHOC efforts were aided by the VA National Center for Patient Safety (NCPS).
This quality improvement project was implemented as part of the U.S. Army Graduate Program in Anesthesia Nursing (USAGPAN) and the Northeastern University doctorate of nursing practice curriculum. The goal was to develop a simple, reliable, easily trainable handoff protocol for implemententation. This goal aligned with the priorites of the Memphis VAMC, USAGPAN, and VA to establish a culture based on patient safety and continuity of care.4
Methods
Standardization of handoffs began with JC National Patient Safety Goal 2E. There has been a wealth of medical literature on the need for standardization of handoffs and the implementation of specific handoff protocols in the postoperative setting. The SPHOC completed a review of the literature supporting standardization of handoff protocols. After completion, a second literature search was completed to identify the concepts for the implementation phase of the project. A critical appraisal of the evidence was completed using the method described by Melnyk and Fineout-Overholt.5 Literature from January 2005 through March 2015 was obtained via the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Google Scholar. The search methods included the keywords handover, handoff, transfer, and safety combined with anesthesia, PACU, surgery, operating room, and intensive care. Articles about handoffs not originating in the operating room (OR) were excluded.
The 13 articles found in the literature review established an overall need for standardization of handoffs outside the OR. Four articles identified a correlation between adverse events and poor or incomplete handoffs.3,6-8 Multiple articles discussed the need to develop a standardized handoff protocol in order to increase team work and quality of care.3,6,7,9,10 Petrovic and colleagues reported a 10% decrease in missed information and a boost in staff satisfaction from 61% to 81% with a standardized handoff.9 Additionally, a decrease in handoff time by > 1 minute was noted.8 Two articles identified an increase in quality of care after the implementation of a standardized handoff protocol.8,10
The second phase of the literature review examined relevant handoff information, best practices for participation in the handoff, and established staff buy-in for the process. Segall and colleagues created a table with handoff strategies consistently identified in the literature.10 The most relevant of these were using a structured written checklist to guide communication, using protocols to standardize the process, and providing formal team training.10
Six articles identified a written checklist and standardized handoff process as successful strategies used to improve patient safety.11-16 Zavalkoff and colleagues discussed the use of a template sheet filled out by the anesthesia provider prior to the handoff for consistency and accuracy of report.16 Catchpole and colleagues drew correlations between a Formula 1 pit stop and anesthesia handoffs and discussed the teamwork portion of the handoff protocol relating to staff buy-in.14 After delegating roles and making a set protocol for the handoff process, the study group was able to meet their objectives of efficient and safe handoff.14
With the information provided from the literature review, the SPHOC established a standardized handoff for the postsurgical patient. The committee created a handoff sheet for the anesthesia provider to use for report. This also included standardizing the handoff process and delineating specific roles for each provider.
After completing a NCPS training workshop, goals were identified at a SPHOC meeting. The SPHOC discussed current barriers to safe patient transfer and suggestions to overcome the barriers. Initial interventions planned by SPHOC focused on the problems of unsafe handoffs and delays in transfer. First, SICU identified the best phone number to call, which was distributed to the anesthesia and OR staffs. Additionally, the committee began tracking the number of attempted calls to reach SICU and availability of the nurse to take the report.
Implementation
A standardized handoff form was created by SPHOC, and anesthesia providers began to call time-out after the patient was deemed stable. After time-out was called, the SICU nurse provided his or her undivided attention and received the report. When SPHOC deemed the process successful, it was implemented in PACU as well. The entire OR, PACU, and anesthesia staffs were updated regarding the progress of the SPHOC on a monthly basis.
The implementation phase involved SPHOC tracking compliance of handoff sheets and time-outs. Compliance was tracked by counting the number of handoff sheets collected at the end of the day vs the total number of cases on the OR schedule. Tracking compliance with SICU transfers was monitored by the SICU members of the SPHOC through a tracking form. Initially a high level of SICU weekly compliance (93%) was noted.
Building on this success, SPHOC extended use of the handoff sheets and time-out to the PACU. Student registered nurse anesthetists (SRNAs) were tasked with education of the anesthesia and PACU staffs. Education continued via individual teaching, presentation at staff meetings, and e-mail reminders. To prevent confusion, no additional changes were made to the handoff sheet for an extended trial.
Despite these interventions, PACU compliance began to lag, averaging 33% over 3 weeks. Encouraging staff buy-in and a change in culture were identified as strategies to improve compliance. The third month of the trial started with 71% compliance. Interventions regarding staff buy-in emphasized individual accountability. Names were attached to handoff sheets, and those found with < 80% of sheets completed were provided with additional education. Those participants with ≥ 80% compliance were praised for their efforts.
Fostering a culture change proved to be more challenging. Interviews and discussions with anesthesia staff identified forgetting to fill out the sheet as the most common reason for noncompliance. Laminated copies of the handoff sheet were affixed to all anesthesia machines as a visual reminder. A sign denoting where to place the completed handoff sheets was placed in the PACU as a visual cue. The SPHOC stocked each anesthesia machine with handoff sheets on a daily basis.
To strengthen the culture of change, the PACU and SICU RNs were encouraged to ask for a time-out from the anesthesia provider. Handoff sheets were printed on yellow card stock to encourage anesthesia staff to “slow down for patient safety.” With these interventions, compliance increased to 98% by the end of the month.
Survey
An anonymous and voluntary survey was created and distributed to all staff involved in the handoff process. The 5-question survey was based on a 5-point Likert scale from 1 for strongly disagree to 5 for strongly disagree. The survey included the following questions: The new surgery report is very thorough; I feel more comfortable when assuming care of the postoperative patient; staff is more attentive when listening to the surgery report when a time-out is called; I feel the new surgery report is more effective and efficient; I feel I am more of a team with the OR with our changes in handoff of care process.
The survey was used as a baseline and to evaluate further changes in the process. Medical literature has shown that improper handoff communication was the leading cause of adverse events in the postsurgical patient.3,6
Results
Surgery to SICU transfers using the Handoff card increased from 33% in the first month to an average of 98% after interventions. In the 10-month intervention period,
After compliance initially increased, SPHOC focused on the more complex aspects of the handoff process—staff satisfaction, which was chosen based on an area of weakness identified in the initial survey results. Overall, staff was satisfied with the handoff sheets; however, only 67% of SICU staff reported that they felt part of the team with the OR as a result of the handoff of care process.
To address this issue, the team delineated roles for providers when a new surgical patient arrived in the SICU. This was dubbed the ABCs of safe handoff with roles for the anesthesia provider or respiratory therapist, the circulating nurse and SICU nurse, and the anesthesia provider. A graphic representation explains the mnemonic, the roles created, and laminated copies were distributed throughout the OR and SICU (Figure). Subsequent surveys showed 80% of staff felt more like a team with the new process.
Conclusion
The overall impact of the project has been to further promote a culture of patient safety at the Memphis VAMC and establish continuity of care as an institutional priority. The existing handoff sheet, time-out, and cross-check have been adapted to all hospital-wide transfers. With the SPHOC guidance and expertise, PACU began using a handoff sheet and time-out when transferring patients to the medical/surgical floors. The handoff sheet has also been adapted to fit the needs of transfers from the emergency department to the medical/surgical floors.
The framework of a standardized handoff is adaptable for other units to customize and has been adopted hospital-wide. The project is sustainable as it requires almost no money to create and sustain. The primary weakness of the process is the requirement of sustained staff participation and buy-in. Each unit and hospital invariably comes with a different culture and priorities; therefore, the process developed at Memphis VAMC may not meet the needs of other facilities. ˜
Acknowledgments
Special thanks to Susan Baldwin, RN; Bianca Mathews, MSN, RN; Wendy Regel, RN; Armance White, CRNA; Reginald Witt, MD; Odie Powell, RN; Alma Farris, RN; Clarisa Reed, RN; Susan Baily, RN; Linda Sueing, RN; Teresa Nguyen, RN; and John Craig, DNP, CRNA.
1. The Joint Commission. Topic library item: sentinel event policy and procedures.. http://www .jointcommission.org/Sentinel_Event_Policy _and_Procedures. Updated October 14, 2016. Accessed February 14, 2017.
2. Streitenberger K, Breen-Reid K, Harris C. Handoffs in care—can we make them safer? Pediatr Clin N Am. 2006;53(6):1185-1195.
3. Petrovic MA, Aboumatar H, Baumgartner WA, et al. Pilot implementation of a perioperative protocol to guide operating room-to-intensive care unit patient handoffs. J Cardiothorac Vasc Anesth. 2012;26(1):11-16.
4. U.S. Department of Veteran Affairs. VA national center for patient safety. http://www.patientsafety .va.gov/about/index.asp . Updated June 3, 2015. Accessed February 14, 2017.
5. Melnyk BM, Fineout-Overholt E. Evidence-Based Practice in Nursing & Healthcare: A Guide to Best Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
6. Hudson CC, McDonald B, Hudson JK, Tran D, Boodhwani M. Impact of anesthetic handover on mortality and morbidity in cardiac surgery: a cohort study. J Cardiothorac Vasc Anesth. 2015;29(1):11-16.
7. Lane-Fall MB, Beidas RS, Pascual JL, et al. Handoffs and transitions in critical care (HATRICC): protocol for a mixed methods study of operating room to intensive care unit handoffs. BMC Surg. 2012;14:96.
8. Nagpal K, Arora S, Abboudi M, et al. Postoperative handover: problems, pitfalls, and prevention of error. Ann Surg. 2010;252(1):171-176.
9. Petrovic MA, Martinez EA, Aboumatar H. Implementing a perioperative handoff tool to improve postprocedural patient transfers. Jt Comm J Qual Patient Saf. 2012;38(3):135-142.
10. Segall N, Bonifacio AS, Schroeder RA, et al; Durham VA Patient Safety Center of Inquiry. Can we make postoperative patient handovers safer? A systematic review of the literature. Anesth Analg. 2012;115(1):102-115.
11. Riesenberg LA, Leitzsch J, Little BW. Systematic review of handoff mnemonics literature. Am J Med Qual. 2009;24(3):196-204.
12. Arora V, Johnson J. A model for building a standardized hand-off protocol. Jt Comm J Qual Patient Saf. 206;32(11):646-655.
13. Riesenberg LA, Leitzsch J, Cunningham JM. Nursing handoffs: a systematic review of the literature. Am J Nurse. 2010;110(4):24-34.
14. Catchpole KR, de Leval MR, McEwan A, et al. Patient handover from surgery to intensive care: using Formula 1 pit‐stop and aviation models to improve safety and quality. Paediatr Anesth. 2007;17(5):470-478.
15. Wahr JA, Prager RL, Abernathy JH III, et al; American Heart Association Council on Cardiovascular Surgery and Anesthesia, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Patient safety in the cardiac operating room: human factors and teamwork: a scientific statement from the American Heart Association. Circulation. 2013;128(10):1139-1169.
16. Zavalkoff SR, Razack SI, Lavoie J, Dancea AB. Handover after pediatric heart surgery: a simple tool improves information exchange. Pediatr Crit Care Med. 2011;12(3):309-313.
Improving health care safety is one of the top priorities of the U.S. health care system. A key element for health care safety is the elimination of sentinel events—unexpected occurrences involving death or serious physical or psychological injury, such as loss of limb or function—or even the risk.1 Problems in communication, continuity of care, and planning have been identified as the root cause in more than 80% of documented sentinel events.2 As a direct result, The Joint Commission (JC) added National Patient Safety Goal 2E, which instructs each organization to implement a standardized approach to patient handoff.1 According to the JC, the objective of a handoff is to “provide accurate information about a patient’s care, treatment, and services, current condition, and any recent or anticipated changesand must include open communication and opportunities for questions.”1,3 The JC identified the patient handoff from anesthesia providers to the Surgical Intensive Care Unit (SICU) and Postanesthesia Care Unit (PACU) an opportunity for an improvement.1,3
At the Memphis VAMC in Tennessee, there was no established protocol for patient handoff from anesthesia providers to the SICU and PACU. The Anesthesia and SICU staffs were frustrated by inconsistent and incomplete postsurgical handoffs. Issues identified by the anesthesia team included difficulty contacting SICU staff to give a report and inconsistent availability of staff on first arrival to SICU. The SICU staff felt communication was rushed and there were inconsistencies in length and quality of the reports, resulting in incomplete postsurgical handoffs.
A baseline survey showed only 75% of staff felt the handoff report was thorough, and 67% “felt like a team.” In response, a multidisciplinary safe patient handoff committee (SPHOC) was formed by representatives from the involved units to discuss issues and offer solutions. The SPHOC efforts were aided by the VA National Center for Patient Safety (NCPS).
This quality improvement project was implemented as part of the U.S. Army Graduate Program in Anesthesia Nursing (USAGPAN) and the Northeastern University doctorate of nursing practice curriculum. The goal was to develop a simple, reliable, easily trainable handoff protocol for implemententation. This goal aligned with the priorites of the Memphis VAMC, USAGPAN, and VA to establish a culture based on patient safety and continuity of care.4
Methods
Standardization of handoffs began with JC National Patient Safety Goal 2E. There has been a wealth of medical literature on the need for standardization of handoffs and the implementation of specific handoff protocols in the postoperative setting. The SPHOC completed a review of the literature supporting standardization of handoff protocols. After completion, a second literature search was completed to identify the concepts for the implementation phase of the project. A critical appraisal of the evidence was completed using the method described by Melnyk and Fineout-Overholt.5 Literature from January 2005 through March 2015 was obtained via the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Google Scholar. The search methods included the keywords handover, handoff, transfer, and safety combined with anesthesia, PACU, surgery, operating room, and intensive care. Articles about handoffs not originating in the operating room (OR) were excluded.
The 13 articles found in the literature review established an overall need for standardization of handoffs outside the OR. Four articles identified a correlation between adverse events and poor or incomplete handoffs.3,6-8 Multiple articles discussed the need to develop a standardized handoff protocol in order to increase team work and quality of care.3,6,7,9,10 Petrovic and colleagues reported a 10% decrease in missed information and a boost in staff satisfaction from 61% to 81% with a standardized handoff.9 Additionally, a decrease in handoff time by > 1 minute was noted.8 Two articles identified an increase in quality of care after the implementation of a standardized handoff protocol.8,10
The second phase of the literature review examined relevant handoff information, best practices for participation in the handoff, and established staff buy-in for the process. Segall and colleagues created a table with handoff strategies consistently identified in the literature.10 The most relevant of these were using a structured written checklist to guide communication, using protocols to standardize the process, and providing formal team training.10
Six articles identified a written checklist and standardized handoff process as successful strategies used to improve patient safety.11-16 Zavalkoff and colleagues discussed the use of a template sheet filled out by the anesthesia provider prior to the handoff for consistency and accuracy of report.16 Catchpole and colleagues drew correlations between a Formula 1 pit stop and anesthesia handoffs and discussed the teamwork portion of the handoff protocol relating to staff buy-in.14 After delegating roles and making a set protocol for the handoff process, the study group was able to meet their objectives of efficient and safe handoff.14
With the information provided from the literature review, the SPHOC established a standardized handoff for the postsurgical patient. The committee created a handoff sheet for the anesthesia provider to use for report. This also included standardizing the handoff process and delineating specific roles for each provider.
After completing a NCPS training workshop, goals were identified at a SPHOC meeting. The SPHOC discussed current barriers to safe patient transfer and suggestions to overcome the barriers. Initial interventions planned by SPHOC focused on the problems of unsafe handoffs and delays in transfer. First, SICU identified the best phone number to call, which was distributed to the anesthesia and OR staffs. Additionally, the committee began tracking the number of attempted calls to reach SICU and availability of the nurse to take the report.
Implementation
A standardized handoff form was created by SPHOC, and anesthesia providers began to call time-out after the patient was deemed stable. After time-out was called, the SICU nurse provided his or her undivided attention and received the report. When SPHOC deemed the process successful, it was implemented in PACU as well. The entire OR, PACU, and anesthesia staffs were updated regarding the progress of the SPHOC on a monthly basis.
The implementation phase involved SPHOC tracking compliance of handoff sheets and time-outs. Compliance was tracked by counting the number of handoff sheets collected at the end of the day vs the total number of cases on the OR schedule. Tracking compliance with SICU transfers was monitored by the SICU members of the SPHOC through a tracking form. Initially a high level of SICU weekly compliance (93%) was noted.
Building on this success, SPHOC extended use of the handoff sheets and time-out to the PACU. Student registered nurse anesthetists (SRNAs) were tasked with education of the anesthesia and PACU staffs. Education continued via individual teaching, presentation at staff meetings, and e-mail reminders. To prevent confusion, no additional changes were made to the handoff sheet for an extended trial.
Despite these interventions, PACU compliance began to lag, averaging 33% over 3 weeks. Encouraging staff buy-in and a change in culture were identified as strategies to improve compliance. The third month of the trial started with 71% compliance. Interventions regarding staff buy-in emphasized individual accountability. Names were attached to handoff sheets, and those found with < 80% of sheets completed were provided with additional education. Those participants with ≥ 80% compliance were praised for their efforts.
Fostering a culture change proved to be more challenging. Interviews and discussions with anesthesia staff identified forgetting to fill out the sheet as the most common reason for noncompliance. Laminated copies of the handoff sheet were affixed to all anesthesia machines as a visual reminder. A sign denoting where to place the completed handoff sheets was placed in the PACU as a visual cue. The SPHOC stocked each anesthesia machine with handoff sheets on a daily basis.
To strengthen the culture of change, the PACU and SICU RNs were encouraged to ask for a time-out from the anesthesia provider. Handoff sheets were printed on yellow card stock to encourage anesthesia staff to “slow down for patient safety.” With these interventions, compliance increased to 98% by the end of the month.
Survey
An anonymous and voluntary survey was created and distributed to all staff involved in the handoff process. The 5-question survey was based on a 5-point Likert scale from 1 for strongly disagree to 5 for strongly disagree. The survey included the following questions: The new surgery report is very thorough; I feel more comfortable when assuming care of the postoperative patient; staff is more attentive when listening to the surgery report when a time-out is called; I feel the new surgery report is more effective and efficient; I feel I am more of a team with the OR with our changes in handoff of care process.
The survey was used as a baseline and to evaluate further changes in the process. Medical literature has shown that improper handoff communication was the leading cause of adverse events in the postsurgical patient.3,6
Results
Surgery to SICU transfers using the Handoff card increased from 33% in the first month to an average of 98% after interventions. In the 10-month intervention period,
After compliance initially increased, SPHOC focused on the more complex aspects of the handoff process—staff satisfaction, which was chosen based on an area of weakness identified in the initial survey results. Overall, staff was satisfied with the handoff sheets; however, only 67% of SICU staff reported that they felt part of the team with the OR as a result of the handoff of care process.
To address this issue, the team delineated roles for providers when a new surgical patient arrived in the SICU. This was dubbed the ABCs of safe handoff with roles for the anesthesia provider or respiratory therapist, the circulating nurse and SICU nurse, and the anesthesia provider. A graphic representation explains the mnemonic, the roles created, and laminated copies were distributed throughout the OR and SICU (Figure). Subsequent surveys showed 80% of staff felt more like a team with the new process.
Conclusion
The overall impact of the project has been to further promote a culture of patient safety at the Memphis VAMC and establish continuity of care as an institutional priority. The existing handoff sheet, time-out, and cross-check have been adapted to all hospital-wide transfers. With the SPHOC guidance and expertise, PACU began using a handoff sheet and time-out when transferring patients to the medical/surgical floors. The handoff sheet has also been adapted to fit the needs of transfers from the emergency department to the medical/surgical floors.
The framework of a standardized handoff is adaptable for other units to customize and has been adopted hospital-wide. The project is sustainable as it requires almost no money to create and sustain. The primary weakness of the process is the requirement of sustained staff participation and buy-in. Each unit and hospital invariably comes with a different culture and priorities; therefore, the process developed at Memphis VAMC may not meet the needs of other facilities. ˜
Acknowledgments
Special thanks to Susan Baldwin, RN; Bianca Mathews, MSN, RN; Wendy Regel, RN; Armance White, CRNA; Reginald Witt, MD; Odie Powell, RN; Alma Farris, RN; Clarisa Reed, RN; Susan Baily, RN; Linda Sueing, RN; Teresa Nguyen, RN; and John Craig, DNP, CRNA.
Improving health care safety is one of the top priorities of the U.S. health care system. A key element for health care safety is the elimination of sentinel events—unexpected occurrences involving death or serious physical or psychological injury, such as loss of limb or function—or even the risk.1 Problems in communication, continuity of care, and planning have been identified as the root cause in more than 80% of documented sentinel events.2 As a direct result, The Joint Commission (JC) added National Patient Safety Goal 2E, which instructs each organization to implement a standardized approach to patient handoff.1 According to the JC, the objective of a handoff is to “provide accurate information about a patient’s care, treatment, and services, current condition, and any recent or anticipated changesand must include open communication and opportunities for questions.”1,3 The JC identified the patient handoff from anesthesia providers to the Surgical Intensive Care Unit (SICU) and Postanesthesia Care Unit (PACU) an opportunity for an improvement.1,3
At the Memphis VAMC in Tennessee, there was no established protocol for patient handoff from anesthesia providers to the SICU and PACU. The Anesthesia and SICU staffs were frustrated by inconsistent and incomplete postsurgical handoffs. Issues identified by the anesthesia team included difficulty contacting SICU staff to give a report and inconsistent availability of staff on first arrival to SICU. The SICU staff felt communication was rushed and there were inconsistencies in length and quality of the reports, resulting in incomplete postsurgical handoffs.
A baseline survey showed only 75% of staff felt the handoff report was thorough, and 67% “felt like a team.” In response, a multidisciplinary safe patient handoff committee (SPHOC) was formed by representatives from the involved units to discuss issues and offer solutions. The SPHOC efforts were aided by the VA National Center for Patient Safety (NCPS).
This quality improvement project was implemented as part of the U.S. Army Graduate Program in Anesthesia Nursing (USAGPAN) and the Northeastern University doctorate of nursing practice curriculum. The goal was to develop a simple, reliable, easily trainable handoff protocol for implemententation. This goal aligned with the priorites of the Memphis VAMC, USAGPAN, and VA to establish a culture based on patient safety and continuity of care.4
Methods
Standardization of handoffs began with JC National Patient Safety Goal 2E. There has been a wealth of medical literature on the need for standardization of handoffs and the implementation of specific handoff protocols in the postoperative setting. The SPHOC completed a review of the literature supporting standardization of handoff protocols. After completion, a second literature search was completed to identify the concepts for the implementation phase of the project. A critical appraisal of the evidence was completed using the method described by Melnyk and Fineout-Overholt.5 Literature from January 2005 through March 2015 was obtained via the Cumulative Index to Nursing and Allied Health Literature (CINAHL) and Google Scholar. The search methods included the keywords handover, handoff, transfer, and safety combined with anesthesia, PACU, surgery, operating room, and intensive care. Articles about handoffs not originating in the operating room (OR) were excluded.
The 13 articles found in the literature review established an overall need for standardization of handoffs outside the OR. Four articles identified a correlation between adverse events and poor or incomplete handoffs.3,6-8 Multiple articles discussed the need to develop a standardized handoff protocol in order to increase team work and quality of care.3,6,7,9,10 Petrovic and colleagues reported a 10% decrease in missed information and a boost in staff satisfaction from 61% to 81% with a standardized handoff.9 Additionally, a decrease in handoff time by > 1 minute was noted.8 Two articles identified an increase in quality of care after the implementation of a standardized handoff protocol.8,10
The second phase of the literature review examined relevant handoff information, best practices for participation in the handoff, and established staff buy-in for the process. Segall and colleagues created a table with handoff strategies consistently identified in the literature.10 The most relevant of these were using a structured written checklist to guide communication, using protocols to standardize the process, and providing formal team training.10
Six articles identified a written checklist and standardized handoff process as successful strategies used to improve patient safety.11-16 Zavalkoff and colleagues discussed the use of a template sheet filled out by the anesthesia provider prior to the handoff for consistency and accuracy of report.16 Catchpole and colleagues drew correlations between a Formula 1 pit stop and anesthesia handoffs and discussed the teamwork portion of the handoff protocol relating to staff buy-in.14 After delegating roles and making a set protocol for the handoff process, the study group was able to meet their objectives of efficient and safe handoff.14
With the information provided from the literature review, the SPHOC established a standardized handoff for the postsurgical patient. The committee created a handoff sheet for the anesthesia provider to use for report. This also included standardizing the handoff process and delineating specific roles for each provider.
After completing a NCPS training workshop, goals were identified at a SPHOC meeting. The SPHOC discussed current barriers to safe patient transfer and suggestions to overcome the barriers. Initial interventions planned by SPHOC focused on the problems of unsafe handoffs and delays in transfer. First, SICU identified the best phone number to call, which was distributed to the anesthesia and OR staffs. Additionally, the committee began tracking the number of attempted calls to reach SICU and availability of the nurse to take the report.
Implementation
A standardized handoff form was created by SPHOC, and anesthesia providers began to call time-out after the patient was deemed stable. After time-out was called, the SICU nurse provided his or her undivided attention and received the report. When SPHOC deemed the process successful, it was implemented in PACU as well. The entire OR, PACU, and anesthesia staffs were updated regarding the progress of the SPHOC on a monthly basis.
The implementation phase involved SPHOC tracking compliance of handoff sheets and time-outs. Compliance was tracked by counting the number of handoff sheets collected at the end of the day vs the total number of cases on the OR schedule. Tracking compliance with SICU transfers was monitored by the SICU members of the SPHOC through a tracking form. Initially a high level of SICU weekly compliance (93%) was noted.
Building on this success, SPHOC extended use of the handoff sheets and time-out to the PACU. Student registered nurse anesthetists (SRNAs) were tasked with education of the anesthesia and PACU staffs. Education continued via individual teaching, presentation at staff meetings, and e-mail reminders. To prevent confusion, no additional changes were made to the handoff sheet for an extended trial.
Despite these interventions, PACU compliance began to lag, averaging 33% over 3 weeks. Encouraging staff buy-in and a change in culture were identified as strategies to improve compliance. The third month of the trial started with 71% compliance. Interventions regarding staff buy-in emphasized individual accountability. Names were attached to handoff sheets, and those found with < 80% of sheets completed were provided with additional education. Those participants with ≥ 80% compliance were praised for their efforts.
Fostering a culture change proved to be more challenging. Interviews and discussions with anesthesia staff identified forgetting to fill out the sheet as the most common reason for noncompliance. Laminated copies of the handoff sheet were affixed to all anesthesia machines as a visual reminder. A sign denoting where to place the completed handoff sheets was placed in the PACU as a visual cue. The SPHOC stocked each anesthesia machine with handoff sheets on a daily basis.
To strengthen the culture of change, the PACU and SICU RNs were encouraged to ask for a time-out from the anesthesia provider. Handoff sheets were printed on yellow card stock to encourage anesthesia staff to “slow down for patient safety.” With these interventions, compliance increased to 98% by the end of the month.
Survey
An anonymous and voluntary survey was created and distributed to all staff involved in the handoff process. The 5-question survey was based on a 5-point Likert scale from 1 for strongly disagree to 5 for strongly disagree. The survey included the following questions: The new surgery report is very thorough; I feel more comfortable when assuming care of the postoperative patient; staff is more attentive when listening to the surgery report when a time-out is called; I feel the new surgery report is more effective and efficient; I feel I am more of a team with the OR with our changes in handoff of care process.
The survey was used as a baseline and to evaluate further changes in the process. Medical literature has shown that improper handoff communication was the leading cause of adverse events in the postsurgical patient.3,6
Results
Surgery to SICU transfers using the Handoff card increased from 33% in the first month to an average of 98% after interventions. In the 10-month intervention period,
After compliance initially increased, SPHOC focused on the more complex aspects of the handoff process—staff satisfaction, which was chosen based on an area of weakness identified in the initial survey results. Overall, staff was satisfied with the handoff sheets; however, only 67% of SICU staff reported that they felt part of the team with the OR as a result of the handoff of care process.
To address this issue, the team delineated roles for providers when a new surgical patient arrived in the SICU. This was dubbed the ABCs of safe handoff with roles for the anesthesia provider or respiratory therapist, the circulating nurse and SICU nurse, and the anesthesia provider. A graphic representation explains the mnemonic, the roles created, and laminated copies were distributed throughout the OR and SICU (Figure). Subsequent surveys showed 80% of staff felt more like a team with the new process.
Conclusion
The overall impact of the project has been to further promote a culture of patient safety at the Memphis VAMC and establish continuity of care as an institutional priority. The existing handoff sheet, time-out, and cross-check have been adapted to all hospital-wide transfers. With the SPHOC guidance and expertise, PACU began using a handoff sheet and time-out when transferring patients to the medical/surgical floors. The handoff sheet has also been adapted to fit the needs of transfers from the emergency department to the medical/surgical floors.
The framework of a standardized handoff is adaptable for other units to customize and has been adopted hospital-wide. The project is sustainable as it requires almost no money to create and sustain. The primary weakness of the process is the requirement of sustained staff participation and buy-in. Each unit and hospital invariably comes with a different culture and priorities; therefore, the process developed at Memphis VAMC may not meet the needs of other facilities. ˜
Acknowledgments
Special thanks to Susan Baldwin, RN; Bianca Mathews, MSN, RN; Wendy Regel, RN; Armance White, CRNA; Reginald Witt, MD; Odie Powell, RN; Alma Farris, RN; Clarisa Reed, RN; Susan Baily, RN; Linda Sueing, RN; Teresa Nguyen, RN; and John Craig, DNP, CRNA.
1. The Joint Commission. Topic library item: sentinel event policy and procedures.. http://www .jointcommission.org/Sentinel_Event_Policy _and_Procedures. Updated October 14, 2016. Accessed February 14, 2017.
2. Streitenberger K, Breen-Reid K, Harris C. Handoffs in care—can we make them safer? Pediatr Clin N Am. 2006;53(6):1185-1195.
3. Petrovic MA, Aboumatar H, Baumgartner WA, et al. Pilot implementation of a perioperative protocol to guide operating room-to-intensive care unit patient handoffs. J Cardiothorac Vasc Anesth. 2012;26(1):11-16.
4. U.S. Department of Veteran Affairs. VA national center for patient safety. http://www.patientsafety .va.gov/about/index.asp . Updated June 3, 2015. Accessed February 14, 2017.
5. Melnyk BM, Fineout-Overholt E. Evidence-Based Practice in Nursing & Healthcare: A Guide to Best Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
6. Hudson CC, McDonald B, Hudson JK, Tran D, Boodhwani M. Impact of anesthetic handover on mortality and morbidity in cardiac surgery: a cohort study. J Cardiothorac Vasc Anesth. 2015;29(1):11-16.
7. Lane-Fall MB, Beidas RS, Pascual JL, et al. Handoffs and transitions in critical care (HATRICC): protocol for a mixed methods study of operating room to intensive care unit handoffs. BMC Surg. 2012;14:96.
8. Nagpal K, Arora S, Abboudi M, et al. Postoperative handover: problems, pitfalls, and prevention of error. Ann Surg. 2010;252(1):171-176.
9. Petrovic MA, Martinez EA, Aboumatar H. Implementing a perioperative handoff tool to improve postprocedural patient transfers. Jt Comm J Qual Patient Saf. 2012;38(3):135-142.
10. Segall N, Bonifacio AS, Schroeder RA, et al; Durham VA Patient Safety Center of Inquiry. Can we make postoperative patient handovers safer? A systematic review of the literature. Anesth Analg. 2012;115(1):102-115.
11. Riesenberg LA, Leitzsch J, Little BW. Systematic review of handoff mnemonics literature. Am J Med Qual. 2009;24(3):196-204.
12. Arora V, Johnson J. A model for building a standardized hand-off protocol. Jt Comm J Qual Patient Saf. 206;32(11):646-655.
13. Riesenberg LA, Leitzsch J, Cunningham JM. Nursing handoffs: a systematic review of the literature. Am J Nurse. 2010;110(4):24-34.
14. Catchpole KR, de Leval MR, McEwan A, et al. Patient handover from surgery to intensive care: using Formula 1 pit‐stop and aviation models to improve safety and quality. Paediatr Anesth. 2007;17(5):470-478.
15. Wahr JA, Prager RL, Abernathy JH III, et al; American Heart Association Council on Cardiovascular Surgery and Anesthesia, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Patient safety in the cardiac operating room: human factors and teamwork: a scientific statement from the American Heart Association. Circulation. 2013;128(10):1139-1169.
16. Zavalkoff SR, Razack SI, Lavoie J, Dancea AB. Handover after pediatric heart surgery: a simple tool improves information exchange. Pediatr Crit Care Med. 2011;12(3):309-313.
1. The Joint Commission. Topic library item: sentinel event policy and procedures.. http://www .jointcommission.org/Sentinel_Event_Policy _and_Procedures. Updated October 14, 2016. Accessed February 14, 2017.
2. Streitenberger K, Breen-Reid K, Harris C. Handoffs in care—can we make them safer? Pediatr Clin N Am. 2006;53(6):1185-1195.
3. Petrovic MA, Aboumatar H, Baumgartner WA, et al. Pilot implementation of a perioperative protocol to guide operating room-to-intensive care unit patient handoffs. J Cardiothorac Vasc Anesth. 2012;26(1):11-16.
4. U.S. Department of Veteran Affairs. VA national center for patient safety. http://www.patientsafety .va.gov/about/index.asp . Updated June 3, 2015. Accessed February 14, 2017.
5. Melnyk BM, Fineout-Overholt E. Evidence-Based Practice in Nursing & Healthcare: A Guide to Best Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
6. Hudson CC, McDonald B, Hudson JK, Tran D, Boodhwani M. Impact of anesthetic handover on mortality and morbidity in cardiac surgery: a cohort study. J Cardiothorac Vasc Anesth. 2015;29(1):11-16.
7. Lane-Fall MB, Beidas RS, Pascual JL, et al. Handoffs and transitions in critical care (HATRICC): protocol for a mixed methods study of operating room to intensive care unit handoffs. BMC Surg. 2012;14:96.
8. Nagpal K, Arora S, Abboudi M, et al. Postoperative handover: problems, pitfalls, and prevention of error. Ann Surg. 2010;252(1):171-176.
9. Petrovic MA, Martinez EA, Aboumatar H. Implementing a perioperative handoff tool to improve postprocedural patient transfers. Jt Comm J Qual Patient Saf. 2012;38(3):135-142.
10. Segall N, Bonifacio AS, Schroeder RA, et al; Durham VA Patient Safety Center of Inquiry. Can we make postoperative patient handovers safer? A systematic review of the literature. Anesth Analg. 2012;115(1):102-115.
11. Riesenberg LA, Leitzsch J, Little BW. Systematic review of handoff mnemonics literature. Am J Med Qual. 2009;24(3):196-204.
12. Arora V, Johnson J. A model for building a standardized hand-off protocol. Jt Comm J Qual Patient Saf. 206;32(11):646-655.
13. Riesenberg LA, Leitzsch J, Cunningham JM. Nursing handoffs: a systematic review of the literature. Am J Nurse. 2010;110(4):24-34.
14. Catchpole KR, de Leval MR, McEwan A, et al. Patient handover from surgery to intensive care: using Formula 1 pit‐stop and aviation models to improve safety and quality. Paediatr Anesth. 2007;17(5):470-478.
15. Wahr JA, Prager RL, Abernathy JH III, et al; American Heart Association Council on Cardiovascular Surgery and Anesthesia, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research. Patient safety in the cardiac operating room: human factors and teamwork: a scientific statement from the American Heart Association. Circulation. 2013;128(10):1139-1169.
16. Zavalkoff SR, Razack SI, Lavoie J, Dancea AB. Handover after pediatric heart surgery: a simple tool improves information exchange. Pediatr Crit Care Med. 2011;12(3):309-313.
Dementia Evaluation, Management, and Outreach
Dementia is a common, multifaceted problem with significant implications for function and quality of life among older individuals. Current demographic shifts are magnifying this problem. Particularly troubling are neuropsychiatric symptoms, which, in addition to creating caregiver distress, also are linked to functional decline, institutionalization, higher health care costs, and mortality (even after controlling for other potential confounders and severity of cognitive impairment). Furthermore, dementia is often unrecognized and underdiagnosed, and patients with dementia historically have poor access to care, particularly those living in rural areas.
The Geriatrics/Dementia Clinic at the Baltimore Veterans Affairs Medical Center (BVAMC) is a referral resource that provides extensive, multidisciplinary evaluations as well as coordinated subspecialist and interprofessional case review. A diverse group of clinicians (representing geriatrics, geriatric psychiatry, neuropsychology, clinical pharmacy, nursing, and social work) perform a half-day evaluation, followed by a meeting of the interdisciplinary team, and feedback session where patients and their families are given the results of the testing and diagnostic impressions as well as plans for further evaluation and treatment. Finally, in addition to the benefits it provides to veterans and their families, this clinic has proven to be an important resource for professional trainees.
Yet this model can be difficult to access, and those living in more remote regions had challenges availing themselves of this resource. Often, they would have to wake before dawn to drive 2 to 4 hours to the medical center. Furthermore, despite the many obvious benefits of this comprehensive approach, veterans and their families often left the clinic with a staggering amount of information and numerous recommendations for future care but without the assurance of integrated follow-up (a burden borne particularly by those living remotely).
The DEMO Program
In response to the dual challenges of access to and coordination of care, existing resources were leveraged with about $250,000 of VA T21 funds (adding both a full-time geriatric nurse practitioner and a psychology technician to the multidisciplinary team) to design and implement the novel DEMO (Dementia Evaluation, Management and Outreach) program. This program aimed to (1) extend dementia evaluations to regional community-based outpatient clinics (CBOCs) that serve veterans in outlying regions; and (2) improve the management and the follow-up that these veterans receive, with a focus on containing costs, while improving both the quality of and veterans’ (and their families’) satisfaction with health care.
Methods
Dementia evaluations were conducted by a geriatric nurse practitioner and psychology technician teamlet at the CBOCs. In addition to neuropsychological testing, medical records were reviewed, caregivers were interviewed, and the patients were examined. The data were then brought back to the full BVAMC Geriatrics/Dementia Clinic multidisciplinary team for discussion. The team reached a consensus diagnosis and then made a comprehensive plan for the further evaluation and management of these complex patients. The plan was entered into the Computerized Patient Record System and communicated to the patient and caregiver during a follow-up CBOC visit.
The teamlet frequently provided informal education during CBOC visits in addition to formal lectures given by experts from the BVAMC and the University of Maryland. The DEMO program was introduced to providers at the CBOCs by e-mail with follow-up information sessions provided on site.
Rather than having patients simply return to their CBOC primary care providers (PCPs) and exposing patients to the risks of poor communication/coordination of care, services were expanded to include regular phone follow-up calls with case management services that augmented those of their PCPs. The goal was to improve outcomes for these patients and provide alternatives to institutionalization.
Standardized instruments were used to gauge patient and caregiver satisfaction, obtain cost data from the VA and the Centers for Medicare & Medicaid Services, and medication data from the Pharmacy Benefits files.
The institutional review board provided approval to collect data on participants to assess the program’s clinical and economic impacts. Since all patients were suspected to have dementia, the informed consent procedures included additional protections. The patient’s understanding of the pertinent information related to participation in the study was assessed to help ensure that participants with dementia truly understood the conditions to which they were consenting. If the potential participant could not provide informed consent, it was obtained from a surrogate with durable power of attorney (the person recognized by Maryland law as the substitute decision maker or the veteran’s legal guardian). Consent was assessed on an ongoing basis regardless of the patient’s capacity to give informed consent, and those willing to have their data collected were enrolled in a “research” arm. These participants were compared with veterans in the dementia clinic during the enrollment period but who did not consent to participate in the research arm, controlling for sociodemographic characteristics and prior health care utilization.
Data Analysis
Patterns of health care utilization may fluctuate with time, and enrollment may identify potential problems that otherwise would not have been found. The authors looked at health care use over 6-month and 1-year intervals before and after enrollment, examining Occupational Physical Assessment Test (OPAT) data and fee-based outpatient data for both inpatient events (including nursing home utilization and hospitalization events) and outpatient visits (including primary, specialty and mental health care services; home care visits, and emergency department [ED]). The total cost incurred by outpatient visits and inpatient care was then adjusted to 2011 dollars. The relationship between program enrollment and health care use and costs was examined with a multivariate regression analyses, controlling for age and health care use in the prior year among veterans who were in the “consent” group or “nonconsent” group.
Outcome variables included the number of primary care, ED, specialty care, home health care, mental health care clinic, and inpatient visits; total inpatient bed days; and the total costs of all the events. The authors fit different multivariate models for these events according to their distributions. Specifically, the Poisson model was used if the distribution of the outcome variable was not overdispersed (eg, ED, primary care).
A negative binomial was used if the distribution of these events was overdispersed (eg, specialty care visits). Further, because the occurrence of inpatient events is relatively rare, a logit model was used to examine the relationship between enrollment status and probability of any inpatient events, regardless of the number of events. Generalized estimating equation (GEE) model with gamma distribution and log link function was used to examine the relationship between cost and program enrollment. The authors also examined the program’s effect on medication use, focusing on high-risk medications in older adults, comparing both the number of unique medications, as well as frequency such medications prescribed 1 year before and after the enrollment.1
Results
Two hundred ninety-eight (298) veterans were referred to DEMO from a 150-mile radius of Baltimore. Of these veterans, 132 consented to participate in this study. The study participants largely were representative of the total group as well as both the overall veteran population and the more general population of community-dwelling individuals with dementia (Table).
Veterans in the DEMO program largely had mild-to-moderate cognitive impairment with mean Mini-Mental State Examination (MMSE) score of 22 and significant functional limitations (Table). Only 3% displayed a pattern of “pure” dementia typical of Alzheimer disease, and 11% of those referred did not have significant abnormalities on neurocognitive testing.
The team averaged 10.3 recommendations (range 3-22), which focused on a diverse set of issues related to additional diagnostic and therapeutic concerns. Although screening data, including basic laboratory results and imaging, was requested in the referral form, in 71% of cases further diagnostic investigations were suggested. With regard to therapeutic suggestions, not surprisingly, medicines were cited as targets in a majority of cases (eg, discontinuing high-risk medications, initiating/titrating medications to minimize cardiovascular risk). While remaining mindful of the time to benefit and competing morbidities, measures to modify cardiovascular risk factors were suggested in more than half and treatment of depression in 15% of cases. Similarly, addressing poor sensory input was suggested in 38% of cases, with other common recommendations focusing on multiple environmental and social interventions (> 50%) as well as supports/outlets/respite for the caregivers.
The full multidisciplinary DEMO group met only weekly to review cases, and due to travel and scheduling difficulties, feedback to the patients and their families often was delayed for weeks. Although initial plans included regularly scheduled follow-up phone calls, demand quickly outstripped program resources. Nonetheless, chart reviews and abstracted adherence and utilization data revealed that PCPs successfully implemented 52% of recommendations within 2 weeks, rising to > 60% by 3 months. When patients were reevaluated at 1 year, they were remarkably stable: Mini–Mental State Examination (baseline 22.2 ± 5.0 → 22.3 ± 5.7 at follow-up) and Instrumental Activities of Daily Living scores (15.5 ± 10.6 → 17.7 ± 11.4).
Feedback
This program was enthusiastically received by both patients and their caregivers—100% and 98%, respectively—reporting overall satisfaction with the services received and 93% of caregivers indicating satisfaction with how the program met their needs. Caregivers were happy with the amount of time the provider took to answer questions (100% satisfied with the amount of time the DEMO provider spent and that they explained “what they wanted to know,” with 98% responding “good” or “great” for both), as well as with services and amount of help received (83% and 77% “very satisfied,” respectively).
In the survey, 98% of caregivers and patients felt that the program helped them deal more effectively with their problems, 97% would recommend the program to a friend in need of similar help, and 100% would come back if they were to seek help again. In keeping with DEMO’s initial aim of increasing access, there was favorable feedback on the ability to get in and be seen and convenience of location. In addition, referring providers universally expressed satisfaction with the referral process (referrals increased linearly); timeliness of scheduling; usefulness of the recommendations; and they planned on continuing to refer patients.
Although there was great variability, controlling for age and prior utilization, veterans in DEMO had statistically significant (all P < .05) fewer ED and specialty care visits and more mental health care clinic visits 183 to 365 daysafter referral dates compared with those in the nonconsented group. Veterans in the consented group also were less likely to use inpatient care than were veterans in the nonconsented group 183 to 365 days after referral dates. These trends were similarly reflected after controlling for age and prior utilization as well as when examining health care costs (data not shown). Nonetheless, DEMO did not seem to have any effect on overall inpatient bed days, primary/home-based care visits, or total costs. In fact, utilization of mental health care resources increased (Figures 1, 2, and 3).
Discussion
Cognitive issues in patients within the general population are common, and the patients cared for by the VA are no exception. Dementia is more common in rural compared with urban areas, and those living in more remote locations have reduced access to specialized evaluation, management, and support services.2 The authors describe a novel program that dramatically increased patient access, bringing the normally tertiary referral services to geographically remote CBOCs at a minimal investment. These services were well received by patients, caregivers, and PCPs. As anticipated, patients and their caregivers especially appreciated the ease and convenience of access. Considering the already significant burden(s) borne every day by those caring for patients with dementia, the benefit of this approach is evident.
Clinicians often feel uncomfortable in evaluating and managing patients with cognitive deficits. Nonetheless, the role of specialized clinics in diagnosing dementia has been demonstrated previously, and the present results are in agreement with previous studies.3 The novelty here is the provision of specialized care usually found only in large, academic medical centers to local CBOCs. By bringing specialized services to geographically isolated patients, the DEMO program was able to increase both access and utilization. Furthermore, providing coordination and ongoing, focused follow-up provided increases in satisfaction and efficiency.
An additional benefit of this approach is the opportunity for PCP education. The authors even found anecdotal reductions in ED usage as well as acute hospitalization and long-term placement—although it was not a statistical significant difference. The relatively high use of mental health care services in this population is in line with previous reports in similar populations, and greater utilization of mental health care services may be one explanation why overall costs did not differ between the 2 groups.4 Nonetheless, this intimates that such a program may yield savings over a longer term, as has been demonstrated in patients with a variety of other psychiatric diagnoses cared for in the community rather than in institutions.5,6
The prevalence of dementia and its associated costs are nearly $50,000 per year per person—suggesting a total cost in the hundreds of billions of dollars.7 Similarly, the importance of caregiver support (including psychosocial interventions, such as the one piloted here) has been demonstrated in a variety of settings (even without improvement in caregiver burden itself).8
There were a number of challenges in the rollout and delivery of DEMO. Although CBOC PCPs
Unfortunately, DEMO was underresourced to provide either real-time feedback true or first responder services. Misunderstandings concerning this were an early challenge to PCP acceptance. However, the longitudinal presence and close working relationships of the DEMO teamlet in each CBOC allowed their use as an adjunct to primary care, and increased the efficacy of both.
Limitations
A number of additional caveats must be made. First, this study had a relatively small number of participants, and there was great variability in health care utilization. This is particularly germane in this population of patients with dementia, which typically has an asymmetrically high use of health care resources. Additionally, the relatively limited follow-up period may have blunted the programs true effect(s). Further, although veterans in the nonconsented group were not officially enrolled in the program, there was likely spillover of the effects of the program on practice patterns, leading to an underestimation of the program’s impact.
Conclusion
With minimal resources, DEMO successfully brought expert evaluation (usually tertiary referral) services, and provided specialized case management in coordination with existing primary care to remote patients. Although there were a number of features rather unique to this setting (eg, infrastructural support; close working interdisciplinary and interprofessional relationships, buy-in at all levels, relative geographic density/demographic homogeneity of participants), specialized case management is increasingly being adopted throughout the VA (and elsewhere). Although the value of collaborative, interdisciplinary interventions has been shown in a variety of settings and conditions—nursing homes,9 chronic low back pain,10 safety among hospital inpatients11—its utility for dementia care is relatively underexplored.
Yet the effectiveness of team-based care for individuals has been demonstrated in a number of settings, including Alzheimer disease.12,13 In addition to involving a number of disciplines, collaborative care is marked by coordination. A number of recent systematic reviews have found that behavioral and multicomponent interventions directed towards the caregiver as well as case management were beneficial in improving some outcomes, although there is considerable heterogeneity in the effects.14,15 Future work will focus on examining methods to focus/optimize interventions based on individual patient characteristics.
Given the epidemiologic trends, care for patients with dementia is expected to grow. Novel interventions, like DEMO, are a particularly promising option to meet this challenge. In fact, just such a collaborative practice-ready workforce has been identified by the World Health Organization as crucial to meeting the challenges of the health needs in the 21st century.16 With the feasibility of such an approach in this population now evident, further studies (including larger sample sizes, across greater geographic regions, as well as among more diverse populations) should be undertaken. These results, if replicated, suggest a novel approach to the particularly vexing problem of caring for patients with dementia with potentially far-reaching public health implications.
Acknowledgments
Supported with T21 funds from VA to expand noninstitutional alternatives to institutional extended care for veterans, as well as the Geriatrics Research and Clinical Center (GRECC) at the Baltimore VAMC.
1. National Committee for Quality Assurance. 2011 HEDIS List. http://www.ncqa.org/tabid/1274/Default.aspx. Accessed December 16, 2016.
2. Russ TC, Batty GD, Hearnshaw GF, Fenton C, Starr JM. Geographical variation in dementia: systemic review with meta-analysis. Int J Epidemiol. 2012;41(4):1012-1032.
3. Wolfs CA, Kessels A, Dirksen CD, Severens JL, Verhey FR. Integrated multidisciplinary diagnostic approach for dementia care: randomized controlled trial. Br J Psychiatry. 2008;192(4):300-305.
4. King PR, Vair CL, Wade M, et al. Outpatient health care utilization in a sample of cognitively impaired veterans receiving care in VHA geriatric evaluation and management clinics. Psychol Serv. 2015;12(1):66-72.
5. Tam-Tham H, Cepoiu-Martin M, Ronksley PE, Maxwell CJ, Hemmelgarn BR. Dementia case management and risk of long-term care placement: a systemic review and meta-analysis. Int J Geriatr Psychiatr. 2013;28(9):889-902.
6. Rothbard AB, Kuno E, Schinnar AP, Hadley TR, Turk R. Service utilization and cost of community care for discharged state hospital patients: a 3-year follow-up study. Am J Psychiatry. 1999;156(6):920-927.
7. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326-1334.
8. Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. JAMA. 2014;311(10):1052-1060.
9. Nazir A, Unroe K, Tegeler M, Khan B, Azar J, Boustani M. Systematic review of interdisciplinary interventions in nursing homes. J Am Med Dir Assoc. 2013;14(7):471-478.
10. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ. 2015;350:h444.
11. O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678-684.
12. Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623-2633.
13. Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148-2157.
14. Health Quality Ontario. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8(4):1-98.
15. Reilly S, Miranda-Castillo C, Malouf R, et al. Case management approaches to home support for people with dementia. Cochrane Database Syst Rev. 2015;1:CD008345.
16. World Health Organization. Framework for action on interprofessional education and collaborative practice. http://apps.who.int/iris/bitstream/10665/70185/1/WHO_HRH_HPN_10.3_eng.pdf?ua=1. Published 2010. Accessed November 17, 2016.
Dementia is a common, multifaceted problem with significant implications for function and quality of life among older individuals. Current demographic shifts are magnifying this problem. Particularly troubling are neuropsychiatric symptoms, which, in addition to creating caregiver distress, also are linked to functional decline, institutionalization, higher health care costs, and mortality (even after controlling for other potential confounders and severity of cognitive impairment). Furthermore, dementia is often unrecognized and underdiagnosed, and patients with dementia historically have poor access to care, particularly those living in rural areas.
The Geriatrics/Dementia Clinic at the Baltimore Veterans Affairs Medical Center (BVAMC) is a referral resource that provides extensive, multidisciplinary evaluations as well as coordinated subspecialist and interprofessional case review. A diverse group of clinicians (representing geriatrics, geriatric psychiatry, neuropsychology, clinical pharmacy, nursing, and social work) perform a half-day evaluation, followed by a meeting of the interdisciplinary team, and feedback session where patients and their families are given the results of the testing and diagnostic impressions as well as plans for further evaluation and treatment. Finally, in addition to the benefits it provides to veterans and their families, this clinic has proven to be an important resource for professional trainees.
Yet this model can be difficult to access, and those living in more remote regions had challenges availing themselves of this resource. Often, they would have to wake before dawn to drive 2 to 4 hours to the medical center. Furthermore, despite the many obvious benefits of this comprehensive approach, veterans and their families often left the clinic with a staggering amount of information and numerous recommendations for future care but without the assurance of integrated follow-up (a burden borne particularly by those living remotely).
The DEMO Program
In response to the dual challenges of access to and coordination of care, existing resources were leveraged with about $250,000 of VA T21 funds (adding both a full-time geriatric nurse practitioner and a psychology technician to the multidisciplinary team) to design and implement the novel DEMO (Dementia Evaluation, Management and Outreach) program. This program aimed to (1) extend dementia evaluations to regional community-based outpatient clinics (CBOCs) that serve veterans in outlying regions; and (2) improve the management and the follow-up that these veterans receive, with a focus on containing costs, while improving both the quality of and veterans’ (and their families’) satisfaction with health care.
Methods
Dementia evaluations were conducted by a geriatric nurse practitioner and psychology technician teamlet at the CBOCs. In addition to neuropsychological testing, medical records were reviewed, caregivers were interviewed, and the patients were examined. The data were then brought back to the full BVAMC Geriatrics/Dementia Clinic multidisciplinary team for discussion. The team reached a consensus diagnosis and then made a comprehensive plan for the further evaluation and management of these complex patients. The plan was entered into the Computerized Patient Record System and communicated to the patient and caregiver during a follow-up CBOC visit.
The teamlet frequently provided informal education during CBOC visits in addition to formal lectures given by experts from the BVAMC and the University of Maryland. The DEMO program was introduced to providers at the CBOCs by e-mail with follow-up information sessions provided on site.
Rather than having patients simply return to their CBOC primary care providers (PCPs) and exposing patients to the risks of poor communication/coordination of care, services were expanded to include regular phone follow-up calls with case management services that augmented those of their PCPs. The goal was to improve outcomes for these patients and provide alternatives to institutionalization.
Standardized instruments were used to gauge patient and caregiver satisfaction, obtain cost data from the VA and the Centers for Medicare & Medicaid Services, and medication data from the Pharmacy Benefits files.
The institutional review board provided approval to collect data on participants to assess the program’s clinical and economic impacts. Since all patients were suspected to have dementia, the informed consent procedures included additional protections. The patient’s understanding of the pertinent information related to participation in the study was assessed to help ensure that participants with dementia truly understood the conditions to which they were consenting. If the potential participant could not provide informed consent, it was obtained from a surrogate with durable power of attorney (the person recognized by Maryland law as the substitute decision maker or the veteran’s legal guardian). Consent was assessed on an ongoing basis regardless of the patient’s capacity to give informed consent, and those willing to have their data collected were enrolled in a “research” arm. These participants were compared with veterans in the dementia clinic during the enrollment period but who did not consent to participate in the research arm, controlling for sociodemographic characteristics and prior health care utilization.
Data Analysis
Patterns of health care utilization may fluctuate with time, and enrollment may identify potential problems that otherwise would not have been found. The authors looked at health care use over 6-month and 1-year intervals before and after enrollment, examining Occupational Physical Assessment Test (OPAT) data and fee-based outpatient data for both inpatient events (including nursing home utilization and hospitalization events) and outpatient visits (including primary, specialty and mental health care services; home care visits, and emergency department [ED]). The total cost incurred by outpatient visits and inpatient care was then adjusted to 2011 dollars. The relationship between program enrollment and health care use and costs was examined with a multivariate regression analyses, controlling for age and health care use in the prior year among veterans who were in the “consent” group or “nonconsent” group.
Outcome variables included the number of primary care, ED, specialty care, home health care, mental health care clinic, and inpatient visits; total inpatient bed days; and the total costs of all the events. The authors fit different multivariate models for these events according to their distributions. Specifically, the Poisson model was used if the distribution of the outcome variable was not overdispersed (eg, ED, primary care).
A negative binomial was used if the distribution of these events was overdispersed (eg, specialty care visits). Further, because the occurrence of inpatient events is relatively rare, a logit model was used to examine the relationship between enrollment status and probability of any inpatient events, regardless of the number of events. Generalized estimating equation (GEE) model with gamma distribution and log link function was used to examine the relationship between cost and program enrollment. The authors also examined the program’s effect on medication use, focusing on high-risk medications in older adults, comparing both the number of unique medications, as well as frequency such medications prescribed 1 year before and after the enrollment.1
Results
Two hundred ninety-eight (298) veterans were referred to DEMO from a 150-mile radius of Baltimore. Of these veterans, 132 consented to participate in this study. The study participants largely were representative of the total group as well as both the overall veteran population and the more general population of community-dwelling individuals with dementia (Table).
Veterans in the DEMO program largely had mild-to-moderate cognitive impairment with mean Mini-Mental State Examination (MMSE) score of 22 and significant functional limitations (Table). Only 3% displayed a pattern of “pure” dementia typical of Alzheimer disease, and 11% of those referred did not have significant abnormalities on neurocognitive testing.
The team averaged 10.3 recommendations (range 3-22), which focused on a diverse set of issues related to additional diagnostic and therapeutic concerns. Although screening data, including basic laboratory results and imaging, was requested in the referral form, in 71% of cases further diagnostic investigations were suggested. With regard to therapeutic suggestions, not surprisingly, medicines were cited as targets in a majority of cases (eg, discontinuing high-risk medications, initiating/titrating medications to minimize cardiovascular risk). While remaining mindful of the time to benefit and competing morbidities, measures to modify cardiovascular risk factors were suggested in more than half and treatment of depression in 15% of cases. Similarly, addressing poor sensory input was suggested in 38% of cases, with other common recommendations focusing on multiple environmental and social interventions (> 50%) as well as supports/outlets/respite for the caregivers.
The full multidisciplinary DEMO group met only weekly to review cases, and due to travel and scheduling difficulties, feedback to the patients and their families often was delayed for weeks. Although initial plans included regularly scheduled follow-up phone calls, demand quickly outstripped program resources. Nonetheless, chart reviews and abstracted adherence and utilization data revealed that PCPs successfully implemented 52% of recommendations within 2 weeks, rising to > 60% by 3 months. When patients were reevaluated at 1 year, they were remarkably stable: Mini–Mental State Examination (baseline 22.2 ± 5.0 → 22.3 ± 5.7 at follow-up) and Instrumental Activities of Daily Living scores (15.5 ± 10.6 → 17.7 ± 11.4).
Feedback
This program was enthusiastically received by both patients and their caregivers—100% and 98%, respectively—reporting overall satisfaction with the services received and 93% of caregivers indicating satisfaction with how the program met their needs. Caregivers were happy with the amount of time the provider took to answer questions (100% satisfied with the amount of time the DEMO provider spent and that they explained “what they wanted to know,” with 98% responding “good” or “great” for both), as well as with services and amount of help received (83% and 77% “very satisfied,” respectively).
In the survey, 98% of caregivers and patients felt that the program helped them deal more effectively with their problems, 97% would recommend the program to a friend in need of similar help, and 100% would come back if they were to seek help again. In keeping with DEMO’s initial aim of increasing access, there was favorable feedback on the ability to get in and be seen and convenience of location. In addition, referring providers universally expressed satisfaction with the referral process (referrals increased linearly); timeliness of scheduling; usefulness of the recommendations; and they planned on continuing to refer patients.
Although there was great variability, controlling for age and prior utilization, veterans in DEMO had statistically significant (all P < .05) fewer ED and specialty care visits and more mental health care clinic visits 183 to 365 daysafter referral dates compared with those in the nonconsented group. Veterans in the consented group also were less likely to use inpatient care than were veterans in the nonconsented group 183 to 365 days after referral dates. These trends were similarly reflected after controlling for age and prior utilization as well as when examining health care costs (data not shown). Nonetheless, DEMO did not seem to have any effect on overall inpatient bed days, primary/home-based care visits, or total costs. In fact, utilization of mental health care resources increased (Figures 1, 2, and 3).
Discussion
Cognitive issues in patients within the general population are common, and the patients cared for by the VA are no exception. Dementia is more common in rural compared with urban areas, and those living in more remote locations have reduced access to specialized evaluation, management, and support services.2 The authors describe a novel program that dramatically increased patient access, bringing the normally tertiary referral services to geographically remote CBOCs at a minimal investment. These services were well received by patients, caregivers, and PCPs. As anticipated, patients and their caregivers especially appreciated the ease and convenience of access. Considering the already significant burden(s) borne every day by those caring for patients with dementia, the benefit of this approach is evident.
Clinicians often feel uncomfortable in evaluating and managing patients with cognitive deficits. Nonetheless, the role of specialized clinics in diagnosing dementia has been demonstrated previously, and the present results are in agreement with previous studies.3 The novelty here is the provision of specialized care usually found only in large, academic medical centers to local CBOCs. By bringing specialized services to geographically isolated patients, the DEMO program was able to increase both access and utilization. Furthermore, providing coordination and ongoing, focused follow-up provided increases in satisfaction and efficiency.
An additional benefit of this approach is the opportunity for PCP education. The authors even found anecdotal reductions in ED usage as well as acute hospitalization and long-term placement—although it was not a statistical significant difference. The relatively high use of mental health care services in this population is in line with previous reports in similar populations, and greater utilization of mental health care services may be one explanation why overall costs did not differ between the 2 groups.4 Nonetheless, this intimates that such a program may yield savings over a longer term, as has been demonstrated in patients with a variety of other psychiatric diagnoses cared for in the community rather than in institutions.5,6
The prevalence of dementia and its associated costs are nearly $50,000 per year per person—suggesting a total cost in the hundreds of billions of dollars.7 Similarly, the importance of caregiver support (including psychosocial interventions, such as the one piloted here) has been demonstrated in a variety of settings (even without improvement in caregiver burden itself).8
There were a number of challenges in the rollout and delivery of DEMO. Although CBOC PCPs
Unfortunately, DEMO was underresourced to provide either real-time feedback true or first responder services. Misunderstandings concerning this were an early challenge to PCP acceptance. However, the longitudinal presence and close working relationships of the DEMO teamlet in each CBOC allowed their use as an adjunct to primary care, and increased the efficacy of both.
Limitations
A number of additional caveats must be made. First, this study had a relatively small number of participants, and there was great variability in health care utilization. This is particularly germane in this population of patients with dementia, which typically has an asymmetrically high use of health care resources. Additionally, the relatively limited follow-up period may have blunted the programs true effect(s). Further, although veterans in the nonconsented group were not officially enrolled in the program, there was likely spillover of the effects of the program on practice patterns, leading to an underestimation of the program’s impact.
Conclusion
With minimal resources, DEMO successfully brought expert evaluation (usually tertiary referral) services, and provided specialized case management in coordination with existing primary care to remote patients. Although there were a number of features rather unique to this setting (eg, infrastructural support; close working interdisciplinary and interprofessional relationships, buy-in at all levels, relative geographic density/demographic homogeneity of participants), specialized case management is increasingly being adopted throughout the VA (and elsewhere). Although the value of collaborative, interdisciplinary interventions has been shown in a variety of settings and conditions—nursing homes,9 chronic low back pain,10 safety among hospital inpatients11—its utility for dementia care is relatively underexplored.
Yet the effectiveness of team-based care for individuals has been demonstrated in a number of settings, including Alzheimer disease.12,13 In addition to involving a number of disciplines, collaborative care is marked by coordination. A number of recent systematic reviews have found that behavioral and multicomponent interventions directed towards the caregiver as well as case management were beneficial in improving some outcomes, although there is considerable heterogeneity in the effects.14,15 Future work will focus on examining methods to focus/optimize interventions based on individual patient characteristics.
Given the epidemiologic trends, care for patients with dementia is expected to grow. Novel interventions, like DEMO, are a particularly promising option to meet this challenge. In fact, just such a collaborative practice-ready workforce has been identified by the World Health Organization as crucial to meeting the challenges of the health needs in the 21st century.16 With the feasibility of such an approach in this population now evident, further studies (including larger sample sizes, across greater geographic regions, as well as among more diverse populations) should be undertaken. These results, if replicated, suggest a novel approach to the particularly vexing problem of caring for patients with dementia with potentially far-reaching public health implications.
Acknowledgments
Supported with T21 funds from VA to expand noninstitutional alternatives to institutional extended care for veterans, as well as the Geriatrics Research and Clinical Center (GRECC) at the Baltimore VAMC.
Dementia is a common, multifaceted problem with significant implications for function and quality of life among older individuals. Current demographic shifts are magnifying this problem. Particularly troubling are neuropsychiatric symptoms, which, in addition to creating caregiver distress, also are linked to functional decline, institutionalization, higher health care costs, and mortality (even after controlling for other potential confounders and severity of cognitive impairment). Furthermore, dementia is often unrecognized and underdiagnosed, and patients with dementia historically have poor access to care, particularly those living in rural areas.
The Geriatrics/Dementia Clinic at the Baltimore Veterans Affairs Medical Center (BVAMC) is a referral resource that provides extensive, multidisciplinary evaluations as well as coordinated subspecialist and interprofessional case review. A diverse group of clinicians (representing geriatrics, geriatric psychiatry, neuropsychology, clinical pharmacy, nursing, and social work) perform a half-day evaluation, followed by a meeting of the interdisciplinary team, and feedback session where patients and their families are given the results of the testing and diagnostic impressions as well as plans for further evaluation and treatment. Finally, in addition to the benefits it provides to veterans and their families, this clinic has proven to be an important resource for professional trainees.
Yet this model can be difficult to access, and those living in more remote regions had challenges availing themselves of this resource. Often, they would have to wake before dawn to drive 2 to 4 hours to the medical center. Furthermore, despite the many obvious benefits of this comprehensive approach, veterans and their families often left the clinic with a staggering amount of information and numerous recommendations for future care but without the assurance of integrated follow-up (a burden borne particularly by those living remotely).
The DEMO Program
In response to the dual challenges of access to and coordination of care, existing resources were leveraged with about $250,000 of VA T21 funds (adding both a full-time geriatric nurse practitioner and a psychology technician to the multidisciplinary team) to design and implement the novel DEMO (Dementia Evaluation, Management and Outreach) program. This program aimed to (1) extend dementia evaluations to regional community-based outpatient clinics (CBOCs) that serve veterans in outlying regions; and (2) improve the management and the follow-up that these veterans receive, with a focus on containing costs, while improving both the quality of and veterans’ (and their families’) satisfaction with health care.
Methods
Dementia evaluations were conducted by a geriatric nurse practitioner and psychology technician teamlet at the CBOCs. In addition to neuropsychological testing, medical records were reviewed, caregivers were interviewed, and the patients were examined. The data were then brought back to the full BVAMC Geriatrics/Dementia Clinic multidisciplinary team for discussion. The team reached a consensus diagnosis and then made a comprehensive plan for the further evaluation and management of these complex patients. The plan was entered into the Computerized Patient Record System and communicated to the patient and caregiver during a follow-up CBOC visit.
The teamlet frequently provided informal education during CBOC visits in addition to formal lectures given by experts from the BVAMC and the University of Maryland. The DEMO program was introduced to providers at the CBOCs by e-mail with follow-up information sessions provided on site.
Rather than having patients simply return to their CBOC primary care providers (PCPs) and exposing patients to the risks of poor communication/coordination of care, services were expanded to include regular phone follow-up calls with case management services that augmented those of their PCPs. The goal was to improve outcomes for these patients and provide alternatives to institutionalization.
Standardized instruments were used to gauge patient and caregiver satisfaction, obtain cost data from the VA and the Centers for Medicare & Medicaid Services, and medication data from the Pharmacy Benefits files.
The institutional review board provided approval to collect data on participants to assess the program’s clinical and economic impacts. Since all patients were suspected to have dementia, the informed consent procedures included additional protections. The patient’s understanding of the pertinent information related to participation in the study was assessed to help ensure that participants with dementia truly understood the conditions to which they were consenting. If the potential participant could not provide informed consent, it was obtained from a surrogate with durable power of attorney (the person recognized by Maryland law as the substitute decision maker or the veteran’s legal guardian). Consent was assessed on an ongoing basis regardless of the patient’s capacity to give informed consent, and those willing to have their data collected were enrolled in a “research” arm. These participants were compared with veterans in the dementia clinic during the enrollment period but who did not consent to participate in the research arm, controlling for sociodemographic characteristics and prior health care utilization.
Data Analysis
Patterns of health care utilization may fluctuate with time, and enrollment may identify potential problems that otherwise would not have been found. The authors looked at health care use over 6-month and 1-year intervals before and after enrollment, examining Occupational Physical Assessment Test (OPAT) data and fee-based outpatient data for both inpatient events (including nursing home utilization and hospitalization events) and outpatient visits (including primary, specialty and mental health care services; home care visits, and emergency department [ED]). The total cost incurred by outpatient visits and inpatient care was then adjusted to 2011 dollars. The relationship between program enrollment and health care use and costs was examined with a multivariate regression analyses, controlling for age and health care use in the prior year among veterans who were in the “consent” group or “nonconsent” group.
Outcome variables included the number of primary care, ED, specialty care, home health care, mental health care clinic, and inpatient visits; total inpatient bed days; and the total costs of all the events. The authors fit different multivariate models for these events according to their distributions. Specifically, the Poisson model was used if the distribution of the outcome variable was not overdispersed (eg, ED, primary care).
A negative binomial was used if the distribution of these events was overdispersed (eg, specialty care visits). Further, because the occurrence of inpatient events is relatively rare, a logit model was used to examine the relationship between enrollment status and probability of any inpatient events, regardless of the number of events. Generalized estimating equation (GEE) model with gamma distribution and log link function was used to examine the relationship between cost and program enrollment. The authors also examined the program’s effect on medication use, focusing on high-risk medications in older adults, comparing both the number of unique medications, as well as frequency such medications prescribed 1 year before and after the enrollment.1
Results
Two hundred ninety-eight (298) veterans were referred to DEMO from a 150-mile radius of Baltimore. Of these veterans, 132 consented to participate in this study. The study participants largely were representative of the total group as well as both the overall veteran population and the more general population of community-dwelling individuals with dementia (Table).
Veterans in the DEMO program largely had mild-to-moderate cognitive impairment with mean Mini-Mental State Examination (MMSE) score of 22 and significant functional limitations (Table). Only 3% displayed a pattern of “pure” dementia typical of Alzheimer disease, and 11% of those referred did not have significant abnormalities on neurocognitive testing.
The team averaged 10.3 recommendations (range 3-22), which focused on a diverse set of issues related to additional diagnostic and therapeutic concerns. Although screening data, including basic laboratory results and imaging, was requested in the referral form, in 71% of cases further diagnostic investigations were suggested. With regard to therapeutic suggestions, not surprisingly, medicines were cited as targets in a majority of cases (eg, discontinuing high-risk medications, initiating/titrating medications to minimize cardiovascular risk). While remaining mindful of the time to benefit and competing morbidities, measures to modify cardiovascular risk factors were suggested in more than half and treatment of depression in 15% of cases. Similarly, addressing poor sensory input was suggested in 38% of cases, with other common recommendations focusing on multiple environmental and social interventions (> 50%) as well as supports/outlets/respite for the caregivers.
The full multidisciplinary DEMO group met only weekly to review cases, and due to travel and scheduling difficulties, feedback to the patients and their families often was delayed for weeks. Although initial plans included regularly scheduled follow-up phone calls, demand quickly outstripped program resources. Nonetheless, chart reviews and abstracted adherence and utilization data revealed that PCPs successfully implemented 52% of recommendations within 2 weeks, rising to > 60% by 3 months. When patients were reevaluated at 1 year, they were remarkably stable: Mini–Mental State Examination (baseline 22.2 ± 5.0 → 22.3 ± 5.7 at follow-up) and Instrumental Activities of Daily Living scores (15.5 ± 10.6 → 17.7 ± 11.4).
Feedback
This program was enthusiastically received by both patients and their caregivers—100% and 98%, respectively—reporting overall satisfaction with the services received and 93% of caregivers indicating satisfaction with how the program met their needs. Caregivers were happy with the amount of time the provider took to answer questions (100% satisfied with the amount of time the DEMO provider spent and that they explained “what they wanted to know,” with 98% responding “good” or “great” for both), as well as with services and amount of help received (83% and 77% “very satisfied,” respectively).
In the survey, 98% of caregivers and patients felt that the program helped them deal more effectively with their problems, 97% would recommend the program to a friend in need of similar help, and 100% would come back if they were to seek help again. In keeping with DEMO’s initial aim of increasing access, there was favorable feedback on the ability to get in and be seen and convenience of location. In addition, referring providers universally expressed satisfaction with the referral process (referrals increased linearly); timeliness of scheduling; usefulness of the recommendations; and they planned on continuing to refer patients.
Although there was great variability, controlling for age and prior utilization, veterans in DEMO had statistically significant (all P < .05) fewer ED and specialty care visits and more mental health care clinic visits 183 to 365 daysafter referral dates compared with those in the nonconsented group. Veterans in the consented group also were less likely to use inpatient care than were veterans in the nonconsented group 183 to 365 days after referral dates. These trends were similarly reflected after controlling for age and prior utilization as well as when examining health care costs (data not shown). Nonetheless, DEMO did not seem to have any effect on overall inpatient bed days, primary/home-based care visits, or total costs. In fact, utilization of mental health care resources increased (Figures 1, 2, and 3).
Discussion
Cognitive issues in patients within the general population are common, and the patients cared for by the VA are no exception. Dementia is more common in rural compared with urban areas, and those living in more remote locations have reduced access to specialized evaluation, management, and support services.2 The authors describe a novel program that dramatically increased patient access, bringing the normally tertiary referral services to geographically remote CBOCs at a minimal investment. These services were well received by patients, caregivers, and PCPs. As anticipated, patients and their caregivers especially appreciated the ease and convenience of access. Considering the already significant burden(s) borne every day by those caring for patients with dementia, the benefit of this approach is evident.
Clinicians often feel uncomfortable in evaluating and managing patients with cognitive deficits. Nonetheless, the role of specialized clinics in diagnosing dementia has been demonstrated previously, and the present results are in agreement with previous studies.3 The novelty here is the provision of specialized care usually found only in large, academic medical centers to local CBOCs. By bringing specialized services to geographically isolated patients, the DEMO program was able to increase both access and utilization. Furthermore, providing coordination and ongoing, focused follow-up provided increases in satisfaction and efficiency.
An additional benefit of this approach is the opportunity for PCP education. The authors even found anecdotal reductions in ED usage as well as acute hospitalization and long-term placement—although it was not a statistical significant difference. The relatively high use of mental health care services in this population is in line with previous reports in similar populations, and greater utilization of mental health care services may be one explanation why overall costs did not differ between the 2 groups.4 Nonetheless, this intimates that such a program may yield savings over a longer term, as has been demonstrated in patients with a variety of other psychiatric diagnoses cared for in the community rather than in institutions.5,6
The prevalence of dementia and its associated costs are nearly $50,000 per year per person—suggesting a total cost in the hundreds of billions of dollars.7 Similarly, the importance of caregiver support (including psychosocial interventions, such as the one piloted here) has been demonstrated in a variety of settings (even without improvement in caregiver burden itself).8
There were a number of challenges in the rollout and delivery of DEMO. Although CBOC PCPs
Unfortunately, DEMO was underresourced to provide either real-time feedback true or first responder services. Misunderstandings concerning this were an early challenge to PCP acceptance. However, the longitudinal presence and close working relationships of the DEMO teamlet in each CBOC allowed their use as an adjunct to primary care, and increased the efficacy of both.
Limitations
A number of additional caveats must be made. First, this study had a relatively small number of participants, and there was great variability in health care utilization. This is particularly germane in this population of patients with dementia, which typically has an asymmetrically high use of health care resources. Additionally, the relatively limited follow-up period may have blunted the programs true effect(s). Further, although veterans in the nonconsented group were not officially enrolled in the program, there was likely spillover of the effects of the program on practice patterns, leading to an underestimation of the program’s impact.
Conclusion
With minimal resources, DEMO successfully brought expert evaluation (usually tertiary referral) services, and provided specialized case management in coordination with existing primary care to remote patients. Although there were a number of features rather unique to this setting (eg, infrastructural support; close working interdisciplinary and interprofessional relationships, buy-in at all levels, relative geographic density/demographic homogeneity of participants), specialized case management is increasingly being adopted throughout the VA (and elsewhere). Although the value of collaborative, interdisciplinary interventions has been shown in a variety of settings and conditions—nursing homes,9 chronic low back pain,10 safety among hospital inpatients11—its utility for dementia care is relatively underexplored.
Yet the effectiveness of team-based care for individuals has been demonstrated in a number of settings, including Alzheimer disease.12,13 In addition to involving a number of disciplines, collaborative care is marked by coordination. A number of recent systematic reviews have found that behavioral and multicomponent interventions directed towards the caregiver as well as case management were beneficial in improving some outcomes, although there is considerable heterogeneity in the effects.14,15 Future work will focus on examining methods to focus/optimize interventions based on individual patient characteristics.
Given the epidemiologic trends, care for patients with dementia is expected to grow. Novel interventions, like DEMO, are a particularly promising option to meet this challenge. In fact, just such a collaborative practice-ready workforce has been identified by the World Health Organization as crucial to meeting the challenges of the health needs in the 21st century.16 With the feasibility of such an approach in this population now evident, further studies (including larger sample sizes, across greater geographic regions, as well as among more diverse populations) should be undertaken. These results, if replicated, suggest a novel approach to the particularly vexing problem of caring for patients with dementia with potentially far-reaching public health implications.
Acknowledgments
Supported with T21 funds from VA to expand noninstitutional alternatives to institutional extended care for veterans, as well as the Geriatrics Research and Clinical Center (GRECC) at the Baltimore VAMC.
1. National Committee for Quality Assurance. 2011 HEDIS List. http://www.ncqa.org/tabid/1274/Default.aspx. Accessed December 16, 2016.
2. Russ TC, Batty GD, Hearnshaw GF, Fenton C, Starr JM. Geographical variation in dementia: systemic review with meta-analysis. Int J Epidemiol. 2012;41(4):1012-1032.
3. Wolfs CA, Kessels A, Dirksen CD, Severens JL, Verhey FR. Integrated multidisciplinary diagnostic approach for dementia care: randomized controlled trial. Br J Psychiatry. 2008;192(4):300-305.
4. King PR, Vair CL, Wade M, et al. Outpatient health care utilization in a sample of cognitively impaired veterans receiving care in VHA geriatric evaluation and management clinics. Psychol Serv. 2015;12(1):66-72.
5. Tam-Tham H, Cepoiu-Martin M, Ronksley PE, Maxwell CJ, Hemmelgarn BR. Dementia case management and risk of long-term care placement: a systemic review and meta-analysis. Int J Geriatr Psychiatr. 2013;28(9):889-902.
6. Rothbard AB, Kuno E, Schinnar AP, Hadley TR, Turk R. Service utilization and cost of community care for discharged state hospital patients: a 3-year follow-up study. Am J Psychiatry. 1999;156(6):920-927.
7. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326-1334.
8. Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. JAMA. 2014;311(10):1052-1060.
9. Nazir A, Unroe K, Tegeler M, Khan B, Azar J, Boustani M. Systematic review of interdisciplinary interventions in nursing homes. J Am Med Dir Assoc. 2013;14(7):471-478.
10. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ. 2015;350:h444.
11. O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678-684.
12. Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623-2633.
13. Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148-2157.
14. Health Quality Ontario. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8(4):1-98.
15. Reilly S, Miranda-Castillo C, Malouf R, et al. Case management approaches to home support for people with dementia. Cochrane Database Syst Rev. 2015;1:CD008345.
16. World Health Organization. Framework for action on interprofessional education and collaborative practice. http://apps.who.int/iris/bitstream/10665/70185/1/WHO_HRH_HPN_10.3_eng.pdf?ua=1. Published 2010. Accessed November 17, 2016.
1. National Committee for Quality Assurance. 2011 HEDIS List. http://www.ncqa.org/tabid/1274/Default.aspx. Accessed December 16, 2016.
2. Russ TC, Batty GD, Hearnshaw GF, Fenton C, Starr JM. Geographical variation in dementia: systemic review with meta-analysis. Int J Epidemiol. 2012;41(4):1012-1032.
3. Wolfs CA, Kessels A, Dirksen CD, Severens JL, Verhey FR. Integrated multidisciplinary diagnostic approach for dementia care: randomized controlled trial. Br J Psychiatry. 2008;192(4):300-305.
4. King PR, Vair CL, Wade M, et al. Outpatient health care utilization in a sample of cognitively impaired veterans receiving care in VHA geriatric evaluation and management clinics. Psychol Serv. 2015;12(1):66-72.
5. Tam-Tham H, Cepoiu-Martin M, Ronksley PE, Maxwell CJ, Hemmelgarn BR. Dementia case management and risk of long-term care placement: a systemic review and meta-analysis. Int J Geriatr Psychiatr. 2013;28(9):889-902.
6. Rothbard AB, Kuno E, Schinnar AP, Hadley TR, Turk R. Service utilization and cost of community care for discharged state hospital patients: a 3-year follow-up study. Am J Psychiatry. 1999;156(6):920-927.
7. Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM. Monetary costs of dementia in the United States. N Engl J Med. 2013;368(14):1326-1334.
8. Adelman RD, Tmanova LL, Delgado D, Dion S, Lachs MS. Caregiver burden: a clinical review. JAMA. 2014;311(10):1052-1060.
9. Nazir A, Unroe K, Tegeler M, Khan B, Azar J, Boustani M. Systematic review of interdisciplinary interventions in nursing homes. J Am Med Dir Assoc. 2013;14(7):471-478.
10. Kamper SJ, Apeldoorn AT, Chiarotto A, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain: Cochrane systematic review and meta-analysis. BMJ. 2015;350:h444.
11. O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med. 2011;171(7):678-684.
12. Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623-2633.
13. Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148-2157.
14. Health Quality Ontario. Caregiver- and patient-directed interventions for dementia: an evidence-based analysis. Ont Health Technol Assess Ser. 2008;8(4):1-98.
15. Reilly S, Miranda-Castillo C, Malouf R, et al. Case management approaches to home support for people with dementia. Cochrane Database Syst Rev. 2015;1:CD008345.
16. World Health Organization. Framework for action on interprofessional education and collaborative practice. http://apps.who.int/iris/bitstream/10665/70185/1/WHO_HRH_HPN_10.3_eng.pdf?ua=1. Published 2010. Accessed November 17, 2016.
A Primary Care Approach to Managing Chronic Noncancer Pain
The Primary Care Chronic Pain Program (PC-CPP) of the Women’s Primary Care Clinics at the VA Salt Lake City Health Care System (VASLCHCS) in Utah was the first VA primary care clinical service to incorporate patient participation in obtaining chronic opioid medications in the treatment of chronic noncancer pain. In addition, the program used a multimodality approach for chronic pain treatment and veteran education about the relationship between physical and mental health issues.
Treatment Complexity
Chronic, noncancer pain is a complex issue in the primary care setting. Diagnosis is difficult, patient education is time consuming, goals and expectations are often unclear, and the experience can be unsatisfying for the patient and the provider.1 These issues, combined with an estimated prevalence rate of 71% for moderate pain among veterans seen in primary care, present a unique challenge for the primary care provider (PCP), given the limited time available to spend with these complex patients.2 Comorbidity rates with mental health issues (eg, depression, anxiety, substance use disorders, etc), which range from 18% to 44%, add to the management challenges for PCPs.3
Veterans also pose unique challenges in pain care as they have a 2-fold greater risk of death from opioid overdose compared with that of the general population, and Utah has been shown to have the highest rate of veteran overdoses.4 Developing programs to help PCPs efficiently manage patients with chronic noncancer pain and mental health comorbidities was vital at VASLCHCS.
Before VASLCHCS established the PC-CPP, the treatment for chronic noncancer pain and related mental health comorbidities followed a biomedical model that separated physical and mental health with the treatment focus on pharmacologic management of symptoms by separate services. Consistent with the biomedical model, management of chronic noncancer pain commonly included long-term use of opioids.
Over the past 2 decades, the use of opioids for treating chronic noncancer pain has significantly increased, with more than 62 million opioid prescriptions dispensed in 2012.5 There are no longitudinal follow-up studies, however, beyond 16 weeks on the use of opioids.6 Further, patients who are prescribed increased opioids continue to report high levels of pain, poor quality of life, and functional disability.7 High-dose opioids also are associated with overdose deaths.
Likewise, PCPs in the Women’s Primary Care Clinics at the VASLCHCS struggled with decreasing opioid use, often because other interventions for managing pain and related mental health conditions in primary care were not readily available. Although the VASLCHCS has an effective specialty pain service caring for patients with complex pain issues, opioid morphine equivalent doses > 200 mg/d, and palliative care, patients with chronic noncancer pain treated in the primary care setting did not have a consistent treatment approach.
A chart review of women veterans seen in Women’s Primary Care Clinic (N = 122) revealed that the majority of patients lacked timely urine drug screening, state database queries, signed medication management agreements, and documentation consistent with state and national guidelines. Additionally, many patients lacked provider follow-through regarding alternative and adjunctive therapy consults, which were often discontinued after failed contact attempts or no-shows to scheduled appointments.
There also was a general consensus among the Women’s Primary Care Clinic PCPs that caring for patients with chronic noncancer pain was exhausting, time consuming, ineffective, and often straining on the patient-provider relationship, as evidenced in many patients’ request to change providers secondary to pain management. The PC-CPP was developed to help systematically facilitate safe opioid prescribing, manage chronic pain issues, and document evidence-based care among women veterans receiving treatment for chronic noncancer pain at the Women’s Primary Care Clinics at VASLCHCS while coordinating and following through with nonpharmacologic interventions.
Program Development
National, state, VA, and professional licensure guidelines for chronic noncancer pain treatment standards were reviewed with the goal of creating a program that was evidence based, would benefit the patient in terms of opioid prescribing and pain control, and improve function while identifying key elements of care and documentation that adequately covered the prescriber of retribution.1,8-10
Concurrent to a review of the guidelines was a review of the literature with the goal of identifying useful patient education and alternative interventions and chronic pain programs that were already established and might meet the clinic’s needs.10,11 These reviews provided direction for a generalized approach to caring for patients with chronic nonmalignant pain. They also clarified that although pain education programs existed nationally, a program that offered a holistic, reproducible, adherence-driven yet patient-centered approach to the patient prescribed opioids chronically in a primary care setting was lacking.
Guideline recommendations included but were not limited to the following1,8-10:
- Patient education about chronic pain and opioids
- Evaluation of pain, function, opioid misuse risk at least twice yearly
- Patient-centered and driven treatment plans
- A holistic approach to chronic pain interventions
- Review of treatment plan efficacy at least twice yearly
- Enzyme multiplied immunoassay technique urine drug screening (UDS) 2 times per year
- State prescription monitoring program query annually
- Signed iMedConsent for treatment of chronic pain
- Plan for safe discontinuation of opioids
- Documentation that the above has been performed with patient understanding
The literature suggested a multimodality approach to chronic nonmalignant pain by minimizing the use of opioids over time while emphasizing nonpharmacologic therapies, such as cognitive behavioral therapy (CBT), mindfulness, meditation, yoga, and spiritual growth, to name a few.10,11 These findings are based on several studies, which suggested that passive coping strategies (eg, use of medication for immediate relief, depending on others, restricting medications) result in an increase in subjective pain among chronic nonmalignant pain patients.12 Helping patients reduce frequent use of passive coping strategies is believed to decrease pain.12 Active coping strategies (eg, engaging in therapies, staying busy or active, distracting attention from pain) have been found to decrease pain.12 The PC-CPP program shifted health care outcomes and responsibilities away from the hierarchal PCP-patient relationship toward a collaborative relationship that encourages patient-driven, patient-centered care outcomes and shared responsibilities.
Program Overview
The PC-CPP was shaped by the following hypotheses: (1) Transparent expectations and consequences would increase functional scores and decrease chronic opioid doses; (2) Treatment plans consisting of chronic opioid prescriptions linked with interactive nonpharmacologic interventions led to decreased pain and increased functional scores; (3) Transparent expectations combined with a streamlined approach to the chronic nonmalignant pain patient would improve patient and PCP satisfaction scores.
The PC-CPP was developed to provide an efficient, effective, and evidence-based approach to managing chronic nonmalignant pain and opioid therapy issues in the primary care setting. Referred patients attend 1 shared medical appointment (SMA) every 6 months with up to 19 other female veterans also referred to the PC-CPP. The group was composed of only female veterans as the pilot study for this SMA occurred in the Women’s Clinic. At each 6-month SMA, patients received education from the Taking Opioids Responsibly for Your Safety and the Safety of Others (TORYSSO) guide13 and signed the corresponding long-term opioid therapy for pain informed consent form (iMedConsent).
The patient and a staff member developed a treatment plan that was patient driven and included at least 1 nonpharmacologic treatment option. The 1-hour nonpharmacologic sessions were either group or individual and occurred weekly for 6 to 8 weeks. These options included CBT for chronic pain, Living Well With Chronic Conditions, trauma-sensitive yoga, smoking cessation, mindfulness for stress and anxiety, MOVE! weight management, Walk With Ease, and a self-help option (VA-issued Manage Stress Workbook, 2014). The workbook was included as an option for those who lived far away, were limited by work schedules, or were unable to afford the copays for a 6- to 8-session program.
Inclusion and Exclusion Criteria
Any female veteran patient enrolled in the VASLCHCS with a chronic nonmalignant pain diagnosis who received daily opioids for 3 or more consecutive months from a PCP was included. Excluded individuals were those with cognitive decline/dementia, serious mental illness, psychosis, active suicidality, disruptive behavior flag, or those excluded by PCP discretion if it was determined that the patient would do better in a one-on-one setting with the PCP (Table 1). Patients taking > 200 MED/d of opioids who were seen in the VASLCHCS specialty pain clinic were also excluded.
Patient and PCP Responsibilities
The patient was responsible for timely attendance and full participation in all SMA group classes as determined in the veteran’s Treatment Plan Agreement (TPA). In addition, the patient had to provide UDS when requested (a minimum of twice yearly) and communicate with the PCP if having a procedure requiring additional opioids. This was in line with the current standards set forth by the VA Opioid Safety Initiative (OSI) Taskforce.12
The PC-CPP provided education, evaluation, documentation, and referral and follow-up with the nonpharmacologic treatment options discussed but did not provide prescription medications. The PCP reviewed the medical documents completed in the PC-CPP, and the PCP was strongly encouraged to follow its recommendations. The expectation was that the PCP would support the PC-CPP when the care recommendation was for a pharmacist-guided opioid taper.
Lack of attendance was defined as a no-show or a reschedule. Patients were considered adherent if they missed fewer than 2 SMA appointments and 2 nonpharmacologic treatment appointments every 6 months. The patient was required to attend the SMA and nonpharmacologic treatment on the third appointment to remain adherent with PC-CPP expectations and agreements. Adherence was acknowledged after 12 and 24 months by a reduction in PC-CPP requirements.
Shared Medical Appointment
Patients referred to the program and who met inclusion criteria received letters explaining the importance of SMA attendance and follow-up reminder calls. At least 30 minutes before the SMA, the patient provided an UDS sample at the laboratory. Next, the patient received an individualized program packet that included the TORYSSO guide, iMedConsent, a TPA specific to the program, a brief pain inventory (BPI) and opioid risk tool, a list of medication disposal sites, and short descriptions of available nonpharmacologic therapies.
Each SMA began with a presentation delivered by a pharmacist, a psychologist, and a medical provider, discussing TORYSSO, program expectations, and holistic approaches to pain. Each SMA also included a rotating chronic pain information topic (eg, nutrition and pain, the physiology of addiction, and the value of multiple modalities in pain treatment). Together, the staff and patients reviewed and completed the blank forms enclosed in the individualized
Each visit was entered into a Computerized Patient Record System (CPRS) template, which included a pain diagnosis, Opioid Risk Tool score, pain and functional scores, opioid fill history, last comprehensive metabolic panel, last electrocardiogram if on methadone, dates of signed agreements, patient adherence with SMA and optional therapies, and follow-up (eFigure).
Every patient enrolled in the PC-CPP had to attend a SMA every 6 months. Patients continued this indefinitely while receiving opioids, and requirements were lessened for patients who had a history of meeting program requirements. For those fully adherent after the first year, only 1 nonpharmacologic intervention was needed (instead of 1 every 6 months) yearly. After 2 years of full adherence, nonpharmacologic interventions were no longer necessary as the expectation was that the patient would continue to use the strategies that they had learned over the previous 2 years. Patients left the PC-CPP if they chose to discontinue opioids, met any of the exclusion criteria, or were nonadherent. Tapering opioid medication was recommended for patients who missed a SMA meeting or 2 nonpharmacologic treatment meetings in a 6-month period; received opioids from more than 1 provider; test positive on a UDS for substances that should not be present; consistently testing negative on a UDS for substances that should be present (indicating diversion); or exhibiting other aberrant behavior (frequent requests for early refills, medications often lost/stolen, etc).
Program Barriers
The PC-CPP took about 2 years to set up, and several barriers were encountered. A thorough understanding of the following factors is necessary for establishing a similar program.
Initially, consults were placed by a designee (someone other than the PCP currently caring for the patient). The designee was usually a member of the PC-CPP who placed consults for all patients who had opioids listed on the CPRS profile. Further, patients who had any opioids within the past 3 months were initially included as were patients who wanted pain education but were not taking opioids. After 12 months, it became apparent that the focus of the PC-CPP should center on patients taking opioids for a minimum of 3 months consecutively. Patients who wanted only education could attend other hospital education opportunities, which helped keep the patient load manageable for PC-CPP staff. Further, to lessen patient confusion and improve adherence, the PCP placed the consult and discussed the program with the patient. Class sizes of 5 to 10 patients seemed to be ideal for patient participation and provider workload.
Patient Education
Initially, the SMA did not follow a standard curriculum, but the current format is more consistent, reproducible, streamlined, and organized. This adjustment improved SMA attendance as well as patient satisfaction, as the class started and finished on time. The SMA also started with numerous handouts, including brochures for nonpharmacologic programs offered at this facility. This led to patients feeling overwhelmed, missing the important forms, and wasted paper. Handouts were simplified to 2 color-coded forms (TPA and BPI).
The take-home assessment was streamlined to a single general assessment. This assessment consisted of 2 questions that asked patients to write a summary of what they learned and then write a summary of how they applied what they learned to their pain management. The VA Manage Stress Workbook also was added to the take-home materials. There are currently 5 different take-home options, which are necessary for those who live more than 50 miles from any VA facility or for those who have transportation issues.
Patient Distress
The SMA could be stressful for patients who felt they were being “punished” or who showed up more than 15 minutes late and had to reschedule the SMA. Having a mental health provider available was crucial for these situations.
Therapeutic Option Development
A cornerstone of the program was getting patients to participate in nonpharmacologic treatment options, which required a robust selection of programs. The VASLCHCS was fortunate to have many programs already available (Table 2), but this was not always the case for the VA community-based outpatient clinics (CBOCs).
Stakeholder Support
Before its start, PC-CPP was presented to the Pentad (a group of 5 individuals in the local facility who hold executive leadership positions) for approval. Tapering opioids can lead to feelings of hostility, frustration, or sadness for patients, so having the Pentad support for the program was crucial to address complaints made to patient advocates or senators. Provider support also was important to reinforce program rules. The PC-CPP inclusion criteria included only those patients whose PCP was agreeable to a taper when the patient did not comply with program expectations. This strategy helped to improve patient adherence with the PC-CPP and decrease patient arguments with clinic staff, as all patients are held to the same standards.
Staff
Finding willing staff can be a challenge. It is estimated that each site needed a program leader who can champion the program objectives and drive organization of staff, space, documentation, and consistency for the patients consulted to the PC-CPP. The goal is that the consistent, reproducible expectations for both the PCP and the patient will reduce overall workload for a clinic. Patients may test the firmness and conviction of the staff to the PC-CPP. Having staff who are able and willing to be firm on relaying information for adherence to the patient is vital.
Administrative Support
At a minimum, a medical support assistant was required to help with scheduling, reminder calls and letters, CPRS check-in/check-out, ensuring necessary forms are ready for the SMA, tracking adherence, and following-up on no-shows and rescheduling.
Documentation
The CPRS consult and note template titles required the approval of the template committee. Although the template is helpful, there is still a great deal that needs to be manually entered in the note, such as BPI scores, opioid risk scores, and chosen nonpharmacologic interventions scores of pain, function, and opioid risk as well individual comorbidities, diagnosis, and follow-up dates. Documentation is geared toward easy review for the PCP who should scan the document prior to renewing opioid medications. The PC-CPP consult became a message board. Once the patient attends the SMA, the designated staff will add a comment to the message board, identifying all dates attended, complete history of the patient’s intervention choices and rate of adherence, as well a follow-up SMA date and whether the patient should bring materials such as take-home tests.
Time Commitment
Program development carries a heavy time burden. One full-time equivalent clinician for 6 weeks for program development is needed. Time allotment is estimated to be the following:
- Medical provider—30 minutes per patient (chart review, documentation, consult resolution). With training, these duties could be completed by support staff
- Pharmacist—30 minutes per patient (chart review, UDS, Utah Division of Occupational and Professional Licensing, fill history). Additional time is needed for writing opioid tapers for qualifying patients
- Primary care mental health integration—a PhD spent 1 to 2 hours per SMA visit assisting patients who became distressed during the visit. Only once has a patient needed to be escorted to the emergency department for active suicidality. A PhD also spent 10+ hours per week running and managing the CBT for Chronic Pain Group
- Support staff—a registered nurse spent 4 hours each month preparing for the SMA (entering consults, ordering EMITs, purchasing snacks)
Conclusion
In this descriptive report, the authors presented an overview of a newly developed program to manage chronic nonmalignant pain and safe opioid prescribing in a primary care setting. A final report is pending. The intent with this interim report was to describe the PC-CPP at the VASLCHCS, its methods and protocols, and logistic considerations for other providers who are working with patients with chronic pain in a primary care model. Standard operating procedure and inclusion/exclusion criteria were included to help with clinical decision making for patients chronic pain for whom aberrant opioid-related behavior presents a problem.
The authors expect that the PC-CPP will provide more comprehensive assisted care, lending to decreased complications associated with accidental overdose, because since patients have been educated about risks for accidental overdose from chronic opioids and have the responsibility for their outcomes. The authors also anticipated that functional scores (as measured by the BPI) will increase despite lowering opioid doses because patients will use ancillary treatments for pain. The desired outcome is that patients will come to understand that pain control is best approached holistically rather than through opioid monotherapy.
There have been several recent initiatives within the VA to decrease opioid prescribing and increase patient safety. With this in mind, continued expansion of this program to CBOCs and male patients could be useful to providers. Also, this program was conducted in a small setting (Women’s Clinic), and there are many challenges with rolling out such a program in a larger clinic (eg, greater chance for provider disagreement, greater need for administrative staff support). Nonetheless, the benefits of close monitoring of prescription opioids and active encouragement to engage in nonpharmacologic therapies are substantial and deserve further advancement.
1. Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. http://www.fsmb.org/Media/Default /PDF/FSMB/Advocacy/pain_policy_july2013.pdf. Accessed November 4, 2016.
2. Buse D, Loder E, McAlary P. Chronic pain rehabilitation. Pain Management Rounds. 2005;6:355-360.
3. Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O’Conner PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17(3):173-179.
4. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241-248.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends in regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-6012.
6. Busse JW, Guyatt GH. Optimizing the use of patient data to improve outcomes for patients: narcotics for chronic noncancer pain. Expert Rev Pharmacoecon Outcomes Res. 2009;9(2):171-179.
7. Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125(1-2):172-179.
8. Agency Medical Directors Group. Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid therapy 2010 update. http://www.agen cymeddirectors.wa.gov/files/opioidgdline.pdf. Accessed November 4, 2016.
9. Utah Department of Health. Utah clinical guidelines on prescribing opioids for treatment of pain. http://health.utah.gov/prescription/pdf/guidelines/final.04.09opioidGuidlines.pdf. Published February 2009. Accessed November 4, 2016.
10. U.S. Department of Veterans Affairs, VA Academic Detailing Service. Pain management, opioid safety. VA educational guide (2014).http://www.va.gov/PAINMANAGEMENT/docs/OSI_1 _Tookit_Provider_AD_Educational_Guide_7_17.pdf. Published July 2014. Accessed November 2016.
11. Dobscha SK, Corson K, Leibowitz RQ, Sullivan MD, Gerrity MS. Rational, design, and baseline findings from a randomized trial of collaborative care for chronic musculoskeletal pain in primary care. Pain Med. 2008;9(8):1050-1064.
12. Mercado AC, Carroll LJ, Cassidy JD, Côté P. Passive coping is a risk factor for disabling neck or low back pain. Pain. 2005;117(1-2):51-57.
13. U.S. Department of Veterans Affairs, VA National Pain Management Program. Taking opioid responsibly for your safety and the safety of others: patient information guide on long-term opioid therapy for pain. http://www.veteranshealthlibrary.org/DiseasesConditions/ChronicPain/142,OpioidsIntro_VA. Updated December 9, 2015. Accessed November 17, 2016.
The Primary Care Chronic Pain Program (PC-CPP) of the Women’s Primary Care Clinics at the VA Salt Lake City Health Care System (VASLCHCS) in Utah was the first VA primary care clinical service to incorporate patient participation in obtaining chronic opioid medications in the treatment of chronic noncancer pain. In addition, the program used a multimodality approach for chronic pain treatment and veteran education about the relationship between physical and mental health issues.
Treatment Complexity
Chronic, noncancer pain is a complex issue in the primary care setting. Diagnosis is difficult, patient education is time consuming, goals and expectations are often unclear, and the experience can be unsatisfying for the patient and the provider.1 These issues, combined with an estimated prevalence rate of 71% for moderate pain among veterans seen in primary care, present a unique challenge for the primary care provider (PCP), given the limited time available to spend with these complex patients.2 Comorbidity rates with mental health issues (eg, depression, anxiety, substance use disorders, etc), which range from 18% to 44%, add to the management challenges for PCPs.3
Veterans also pose unique challenges in pain care as they have a 2-fold greater risk of death from opioid overdose compared with that of the general population, and Utah has been shown to have the highest rate of veteran overdoses.4 Developing programs to help PCPs efficiently manage patients with chronic noncancer pain and mental health comorbidities was vital at VASLCHCS.
Before VASLCHCS established the PC-CPP, the treatment for chronic noncancer pain and related mental health comorbidities followed a biomedical model that separated physical and mental health with the treatment focus on pharmacologic management of symptoms by separate services. Consistent with the biomedical model, management of chronic noncancer pain commonly included long-term use of opioids.
Over the past 2 decades, the use of opioids for treating chronic noncancer pain has significantly increased, with more than 62 million opioid prescriptions dispensed in 2012.5 There are no longitudinal follow-up studies, however, beyond 16 weeks on the use of opioids.6 Further, patients who are prescribed increased opioids continue to report high levels of pain, poor quality of life, and functional disability.7 High-dose opioids also are associated with overdose deaths.
Likewise, PCPs in the Women’s Primary Care Clinics at the VASLCHCS struggled with decreasing opioid use, often because other interventions for managing pain and related mental health conditions in primary care were not readily available. Although the VASLCHCS has an effective specialty pain service caring for patients with complex pain issues, opioid morphine equivalent doses > 200 mg/d, and palliative care, patients with chronic noncancer pain treated in the primary care setting did not have a consistent treatment approach.
A chart review of women veterans seen in Women’s Primary Care Clinic (N = 122) revealed that the majority of patients lacked timely urine drug screening, state database queries, signed medication management agreements, and documentation consistent with state and national guidelines. Additionally, many patients lacked provider follow-through regarding alternative and adjunctive therapy consults, which were often discontinued after failed contact attempts or no-shows to scheduled appointments.
There also was a general consensus among the Women’s Primary Care Clinic PCPs that caring for patients with chronic noncancer pain was exhausting, time consuming, ineffective, and often straining on the patient-provider relationship, as evidenced in many patients’ request to change providers secondary to pain management. The PC-CPP was developed to help systematically facilitate safe opioid prescribing, manage chronic pain issues, and document evidence-based care among women veterans receiving treatment for chronic noncancer pain at the Women’s Primary Care Clinics at VASLCHCS while coordinating and following through with nonpharmacologic interventions.
Program Development
National, state, VA, and professional licensure guidelines for chronic noncancer pain treatment standards were reviewed with the goal of creating a program that was evidence based, would benefit the patient in terms of opioid prescribing and pain control, and improve function while identifying key elements of care and documentation that adequately covered the prescriber of retribution.1,8-10
Concurrent to a review of the guidelines was a review of the literature with the goal of identifying useful patient education and alternative interventions and chronic pain programs that were already established and might meet the clinic’s needs.10,11 These reviews provided direction for a generalized approach to caring for patients with chronic nonmalignant pain. They also clarified that although pain education programs existed nationally, a program that offered a holistic, reproducible, adherence-driven yet patient-centered approach to the patient prescribed opioids chronically in a primary care setting was lacking.
Guideline recommendations included but were not limited to the following1,8-10:
- Patient education about chronic pain and opioids
- Evaluation of pain, function, opioid misuse risk at least twice yearly
- Patient-centered and driven treatment plans
- A holistic approach to chronic pain interventions
- Review of treatment plan efficacy at least twice yearly
- Enzyme multiplied immunoassay technique urine drug screening (UDS) 2 times per year
- State prescription monitoring program query annually
- Signed iMedConsent for treatment of chronic pain
- Plan for safe discontinuation of opioids
- Documentation that the above has been performed with patient understanding
The literature suggested a multimodality approach to chronic nonmalignant pain by minimizing the use of opioids over time while emphasizing nonpharmacologic therapies, such as cognitive behavioral therapy (CBT), mindfulness, meditation, yoga, and spiritual growth, to name a few.10,11 These findings are based on several studies, which suggested that passive coping strategies (eg, use of medication for immediate relief, depending on others, restricting medications) result in an increase in subjective pain among chronic nonmalignant pain patients.12 Helping patients reduce frequent use of passive coping strategies is believed to decrease pain.12 Active coping strategies (eg, engaging in therapies, staying busy or active, distracting attention from pain) have been found to decrease pain.12 The PC-CPP program shifted health care outcomes and responsibilities away from the hierarchal PCP-patient relationship toward a collaborative relationship that encourages patient-driven, patient-centered care outcomes and shared responsibilities.
Program Overview
The PC-CPP was shaped by the following hypotheses: (1) Transparent expectations and consequences would increase functional scores and decrease chronic opioid doses; (2) Treatment plans consisting of chronic opioid prescriptions linked with interactive nonpharmacologic interventions led to decreased pain and increased functional scores; (3) Transparent expectations combined with a streamlined approach to the chronic nonmalignant pain patient would improve patient and PCP satisfaction scores.
The PC-CPP was developed to provide an efficient, effective, and evidence-based approach to managing chronic nonmalignant pain and opioid therapy issues in the primary care setting. Referred patients attend 1 shared medical appointment (SMA) every 6 months with up to 19 other female veterans also referred to the PC-CPP. The group was composed of only female veterans as the pilot study for this SMA occurred in the Women’s Clinic. At each 6-month SMA, patients received education from the Taking Opioids Responsibly for Your Safety and the Safety of Others (TORYSSO) guide13 and signed the corresponding long-term opioid therapy for pain informed consent form (iMedConsent).
The patient and a staff member developed a treatment plan that was patient driven and included at least 1 nonpharmacologic treatment option. The 1-hour nonpharmacologic sessions were either group or individual and occurred weekly for 6 to 8 weeks. These options included CBT for chronic pain, Living Well With Chronic Conditions, trauma-sensitive yoga, smoking cessation, mindfulness for stress and anxiety, MOVE! weight management, Walk With Ease, and a self-help option (VA-issued Manage Stress Workbook, 2014). The workbook was included as an option for those who lived far away, were limited by work schedules, or were unable to afford the copays for a 6- to 8-session program.
Inclusion and Exclusion Criteria
Any female veteran patient enrolled in the VASLCHCS with a chronic nonmalignant pain diagnosis who received daily opioids for 3 or more consecutive months from a PCP was included. Excluded individuals were those with cognitive decline/dementia, serious mental illness, psychosis, active suicidality, disruptive behavior flag, or those excluded by PCP discretion if it was determined that the patient would do better in a one-on-one setting with the PCP (Table 1). Patients taking > 200 MED/d of opioids who were seen in the VASLCHCS specialty pain clinic were also excluded.
Patient and PCP Responsibilities
The patient was responsible for timely attendance and full participation in all SMA group classes as determined in the veteran’s Treatment Plan Agreement (TPA). In addition, the patient had to provide UDS when requested (a minimum of twice yearly) and communicate with the PCP if having a procedure requiring additional opioids. This was in line with the current standards set forth by the VA Opioid Safety Initiative (OSI) Taskforce.12
The PC-CPP provided education, evaluation, documentation, and referral and follow-up with the nonpharmacologic treatment options discussed but did not provide prescription medications. The PCP reviewed the medical documents completed in the PC-CPP, and the PCP was strongly encouraged to follow its recommendations. The expectation was that the PCP would support the PC-CPP when the care recommendation was for a pharmacist-guided opioid taper.
Lack of attendance was defined as a no-show or a reschedule. Patients were considered adherent if they missed fewer than 2 SMA appointments and 2 nonpharmacologic treatment appointments every 6 months. The patient was required to attend the SMA and nonpharmacologic treatment on the third appointment to remain adherent with PC-CPP expectations and agreements. Adherence was acknowledged after 12 and 24 months by a reduction in PC-CPP requirements.
Shared Medical Appointment
Patients referred to the program and who met inclusion criteria received letters explaining the importance of SMA attendance and follow-up reminder calls. At least 30 minutes before the SMA, the patient provided an UDS sample at the laboratory. Next, the patient received an individualized program packet that included the TORYSSO guide, iMedConsent, a TPA specific to the program, a brief pain inventory (BPI) and opioid risk tool, a list of medication disposal sites, and short descriptions of available nonpharmacologic therapies.
Each SMA began with a presentation delivered by a pharmacist, a psychologist, and a medical provider, discussing TORYSSO, program expectations, and holistic approaches to pain. Each SMA also included a rotating chronic pain information topic (eg, nutrition and pain, the physiology of addiction, and the value of multiple modalities in pain treatment). Together, the staff and patients reviewed and completed the blank forms enclosed in the individualized
Each visit was entered into a Computerized Patient Record System (CPRS) template, which included a pain diagnosis, Opioid Risk Tool score, pain and functional scores, opioid fill history, last comprehensive metabolic panel, last electrocardiogram if on methadone, dates of signed agreements, patient adherence with SMA and optional therapies, and follow-up (eFigure).
Every patient enrolled in the PC-CPP had to attend a SMA every 6 months. Patients continued this indefinitely while receiving opioids, and requirements were lessened for patients who had a history of meeting program requirements. For those fully adherent after the first year, only 1 nonpharmacologic intervention was needed (instead of 1 every 6 months) yearly. After 2 years of full adherence, nonpharmacologic interventions were no longer necessary as the expectation was that the patient would continue to use the strategies that they had learned over the previous 2 years. Patients left the PC-CPP if they chose to discontinue opioids, met any of the exclusion criteria, or were nonadherent. Tapering opioid medication was recommended for patients who missed a SMA meeting or 2 nonpharmacologic treatment meetings in a 6-month period; received opioids from more than 1 provider; test positive on a UDS for substances that should not be present; consistently testing negative on a UDS for substances that should be present (indicating diversion); or exhibiting other aberrant behavior (frequent requests for early refills, medications often lost/stolen, etc).
Program Barriers
The PC-CPP took about 2 years to set up, and several barriers were encountered. A thorough understanding of the following factors is necessary for establishing a similar program.
Initially, consults were placed by a designee (someone other than the PCP currently caring for the patient). The designee was usually a member of the PC-CPP who placed consults for all patients who had opioids listed on the CPRS profile. Further, patients who had any opioids within the past 3 months were initially included as were patients who wanted pain education but were not taking opioids. After 12 months, it became apparent that the focus of the PC-CPP should center on patients taking opioids for a minimum of 3 months consecutively. Patients who wanted only education could attend other hospital education opportunities, which helped keep the patient load manageable for PC-CPP staff. Further, to lessen patient confusion and improve adherence, the PCP placed the consult and discussed the program with the patient. Class sizes of 5 to 10 patients seemed to be ideal for patient participation and provider workload.
Patient Education
Initially, the SMA did not follow a standard curriculum, but the current format is more consistent, reproducible, streamlined, and organized. This adjustment improved SMA attendance as well as patient satisfaction, as the class started and finished on time. The SMA also started with numerous handouts, including brochures for nonpharmacologic programs offered at this facility. This led to patients feeling overwhelmed, missing the important forms, and wasted paper. Handouts were simplified to 2 color-coded forms (TPA and BPI).
The take-home assessment was streamlined to a single general assessment. This assessment consisted of 2 questions that asked patients to write a summary of what they learned and then write a summary of how they applied what they learned to their pain management. The VA Manage Stress Workbook also was added to the take-home materials. There are currently 5 different take-home options, which are necessary for those who live more than 50 miles from any VA facility or for those who have transportation issues.
Patient Distress
The SMA could be stressful for patients who felt they were being “punished” or who showed up more than 15 minutes late and had to reschedule the SMA. Having a mental health provider available was crucial for these situations.
Therapeutic Option Development
A cornerstone of the program was getting patients to participate in nonpharmacologic treatment options, which required a robust selection of programs. The VASLCHCS was fortunate to have many programs already available (Table 2), but this was not always the case for the VA community-based outpatient clinics (CBOCs).
Stakeholder Support
Before its start, PC-CPP was presented to the Pentad (a group of 5 individuals in the local facility who hold executive leadership positions) for approval. Tapering opioids can lead to feelings of hostility, frustration, or sadness for patients, so having the Pentad support for the program was crucial to address complaints made to patient advocates or senators. Provider support also was important to reinforce program rules. The PC-CPP inclusion criteria included only those patients whose PCP was agreeable to a taper when the patient did not comply with program expectations. This strategy helped to improve patient adherence with the PC-CPP and decrease patient arguments with clinic staff, as all patients are held to the same standards.
Staff
Finding willing staff can be a challenge. It is estimated that each site needed a program leader who can champion the program objectives and drive organization of staff, space, documentation, and consistency for the patients consulted to the PC-CPP. The goal is that the consistent, reproducible expectations for both the PCP and the patient will reduce overall workload for a clinic. Patients may test the firmness and conviction of the staff to the PC-CPP. Having staff who are able and willing to be firm on relaying information for adherence to the patient is vital.
Administrative Support
At a minimum, a medical support assistant was required to help with scheduling, reminder calls and letters, CPRS check-in/check-out, ensuring necessary forms are ready for the SMA, tracking adherence, and following-up on no-shows and rescheduling.
Documentation
The CPRS consult and note template titles required the approval of the template committee. Although the template is helpful, there is still a great deal that needs to be manually entered in the note, such as BPI scores, opioid risk scores, and chosen nonpharmacologic interventions scores of pain, function, and opioid risk as well individual comorbidities, diagnosis, and follow-up dates. Documentation is geared toward easy review for the PCP who should scan the document prior to renewing opioid medications. The PC-CPP consult became a message board. Once the patient attends the SMA, the designated staff will add a comment to the message board, identifying all dates attended, complete history of the patient’s intervention choices and rate of adherence, as well a follow-up SMA date and whether the patient should bring materials such as take-home tests.
Time Commitment
Program development carries a heavy time burden. One full-time equivalent clinician for 6 weeks for program development is needed. Time allotment is estimated to be the following:
- Medical provider—30 minutes per patient (chart review, documentation, consult resolution). With training, these duties could be completed by support staff
- Pharmacist—30 minutes per patient (chart review, UDS, Utah Division of Occupational and Professional Licensing, fill history). Additional time is needed for writing opioid tapers for qualifying patients
- Primary care mental health integration—a PhD spent 1 to 2 hours per SMA visit assisting patients who became distressed during the visit. Only once has a patient needed to be escorted to the emergency department for active suicidality. A PhD also spent 10+ hours per week running and managing the CBT for Chronic Pain Group
- Support staff—a registered nurse spent 4 hours each month preparing for the SMA (entering consults, ordering EMITs, purchasing snacks)
Conclusion
In this descriptive report, the authors presented an overview of a newly developed program to manage chronic nonmalignant pain and safe opioid prescribing in a primary care setting. A final report is pending. The intent with this interim report was to describe the PC-CPP at the VASLCHCS, its methods and protocols, and logistic considerations for other providers who are working with patients with chronic pain in a primary care model. Standard operating procedure and inclusion/exclusion criteria were included to help with clinical decision making for patients chronic pain for whom aberrant opioid-related behavior presents a problem.
The authors expect that the PC-CPP will provide more comprehensive assisted care, lending to decreased complications associated with accidental overdose, because since patients have been educated about risks for accidental overdose from chronic opioids and have the responsibility for their outcomes. The authors also anticipated that functional scores (as measured by the BPI) will increase despite lowering opioid doses because patients will use ancillary treatments for pain. The desired outcome is that patients will come to understand that pain control is best approached holistically rather than through opioid monotherapy.
There have been several recent initiatives within the VA to decrease opioid prescribing and increase patient safety. With this in mind, continued expansion of this program to CBOCs and male patients could be useful to providers. Also, this program was conducted in a small setting (Women’s Clinic), and there are many challenges with rolling out such a program in a larger clinic (eg, greater chance for provider disagreement, greater need for administrative staff support). Nonetheless, the benefits of close monitoring of prescription opioids and active encouragement to engage in nonpharmacologic therapies are substantial and deserve further advancement.
The Primary Care Chronic Pain Program (PC-CPP) of the Women’s Primary Care Clinics at the VA Salt Lake City Health Care System (VASLCHCS) in Utah was the first VA primary care clinical service to incorporate patient participation in obtaining chronic opioid medications in the treatment of chronic noncancer pain. In addition, the program used a multimodality approach for chronic pain treatment and veteran education about the relationship between physical and mental health issues.
Treatment Complexity
Chronic, noncancer pain is a complex issue in the primary care setting. Diagnosis is difficult, patient education is time consuming, goals and expectations are often unclear, and the experience can be unsatisfying for the patient and the provider.1 These issues, combined with an estimated prevalence rate of 71% for moderate pain among veterans seen in primary care, present a unique challenge for the primary care provider (PCP), given the limited time available to spend with these complex patients.2 Comorbidity rates with mental health issues (eg, depression, anxiety, substance use disorders, etc), which range from 18% to 44%, add to the management challenges for PCPs.3
Veterans also pose unique challenges in pain care as they have a 2-fold greater risk of death from opioid overdose compared with that of the general population, and Utah has been shown to have the highest rate of veteran overdoses.4 Developing programs to help PCPs efficiently manage patients with chronic noncancer pain and mental health comorbidities was vital at VASLCHCS.
Before VASLCHCS established the PC-CPP, the treatment for chronic noncancer pain and related mental health comorbidities followed a biomedical model that separated physical and mental health with the treatment focus on pharmacologic management of symptoms by separate services. Consistent with the biomedical model, management of chronic noncancer pain commonly included long-term use of opioids.
Over the past 2 decades, the use of opioids for treating chronic noncancer pain has significantly increased, with more than 62 million opioid prescriptions dispensed in 2012.5 There are no longitudinal follow-up studies, however, beyond 16 weeks on the use of opioids.6 Further, patients who are prescribed increased opioids continue to report high levels of pain, poor quality of life, and functional disability.7 High-dose opioids also are associated with overdose deaths.
Likewise, PCPs in the Women’s Primary Care Clinics at the VASLCHCS struggled with decreasing opioid use, often because other interventions for managing pain and related mental health conditions in primary care were not readily available. Although the VASLCHCS has an effective specialty pain service caring for patients with complex pain issues, opioid morphine equivalent doses > 200 mg/d, and palliative care, patients with chronic noncancer pain treated in the primary care setting did not have a consistent treatment approach.
A chart review of women veterans seen in Women’s Primary Care Clinic (N = 122) revealed that the majority of patients lacked timely urine drug screening, state database queries, signed medication management agreements, and documentation consistent with state and national guidelines. Additionally, many patients lacked provider follow-through regarding alternative and adjunctive therapy consults, which were often discontinued after failed contact attempts or no-shows to scheduled appointments.
There also was a general consensus among the Women’s Primary Care Clinic PCPs that caring for patients with chronic noncancer pain was exhausting, time consuming, ineffective, and often straining on the patient-provider relationship, as evidenced in many patients’ request to change providers secondary to pain management. The PC-CPP was developed to help systematically facilitate safe opioid prescribing, manage chronic pain issues, and document evidence-based care among women veterans receiving treatment for chronic noncancer pain at the Women’s Primary Care Clinics at VASLCHCS while coordinating and following through with nonpharmacologic interventions.
Program Development
National, state, VA, and professional licensure guidelines for chronic noncancer pain treatment standards were reviewed with the goal of creating a program that was evidence based, would benefit the patient in terms of opioid prescribing and pain control, and improve function while identifying key elements of care and documentation that adequately covered the prescriber of retribution.1,8-10
Concurrent to a review of the guidelines was a review of the literature with the goal of identifying useful patient education and alternative interventions and chronic pain programs that were already established and might meet the clinic’s needs.10,11 These reviews provided direction for a generalized approach to caring for patients with chronic nonmalignant pain. They also clarified that although pain education programs existed nationally, a program that offered a holistic, reproducible, adherence-driven yet patient-centered approach to the patient prescribed opioids chronically in a primary care setting was lacking.
Guideline recommendations included but were not limited to the following1,8-10:
- Patient education about chronic pain and opioids
- Evaluation of pain, function, opioid misuse risk at least twice yearly
- Patient-centered and driven treatment plans
- A holistic approach to chronic pain interventions
- Review of treatment plan efficacy at least twice yearly
- Enzyme multiplied immunoassay technique urine drug screening (UDS) 2 times per year
- State prescription monitoring program query annually
- Signed iMedConsent for treatment of chronic pain
- Plan for safe discontinuation of opioids
- Documentation that the above has been performed with patient understanding
The literature suggested a multimodality approach to chronic nonmalignant pain by minimizing the use of opioids over time while emphasizing nonpharmacologic therapies, such as cognitive behavioral therapy (CBT), mindfulness, meditation, yoga, and spiritual growth, to name a few.10,11 These findings are based on several studies, which suggested that passive coping strategies (eg, use of medication for immediate relief, depending on others, restricting medications) result in an increase in subjective pain among chronic nonmalignant pain patients.12 Helping patients reduce frequent use of passive coping strategies is believed to decrease pain.12 Active coping strategies (eg, engaging in therapies, staying busy or active, distracting attention from pain) have been found to decrease pain.12 The PC-CPP program shifted health care outcomes and responsibilities away from the hierarchal PCP-patient relationship toward a collaborative relationship that encourages patient-driven, patient-centered care outcomes and shared responsibilities.
Program Overview
The PC-CPP was shaped by the following hypotheses: (1) Transparent expectations and consequences would increase functional scores and decrease chronic opioid doses; (2) Treatment plans consisting of chronic opioid prescriptions linked with interactive nonpharmacologic interventions led to decreased pain and increased functional scores; (3) Transparent expectations combined with a streamlined approach to the chronic nonmalignant pain patient would improve patient and PCP satisfaction scores.
The PC-CPP was developed to provide an efficient, effective, and evidence-based approach to managing chronic nonmalignant pain and opioid therapy issues in the primary care setting. Referred patients attend 1 shared medical appointment (SMA) every 6 months with up to 19 other female veterans also referred to the PC-CPP. The group was composed of only female veterans as the pilot study for this SMA occurred in the Women’s Clinic. At each 6-month SMA, patients received education from the Taking Opioids Responsibly for Your Safety and the Safety of Others (TORYSSO) guide13 and signed the corresponding long-term opioid therapy for pain informed consent form (iMedConsent).
The patient and a staff member developed a treatment plan that was patient driven and included at least 1 nonpharmacologic treatment option. The 1-hour nonpharmacologic sessions were either group or individual and occurred weekly for 6 to 8 weeks. These options included CBT for chronic pain, Living Well With Chronic Conditions, trauma-sensitive yoga, smoking cessation, mindfulness for stress and anxiety, MOVE! weight management, Walk With Ease, and a self-help option (VA-issued Manage Stress Workbook, 2014). The workbook was included as an option for those who lived far away, were limited by work schedules, or were unable to afford the copays for a 6- to 8-session program.
Inclusion and Exclusion Criteria
Any female veteran patient enrolled in the VASLCHCS with a chronic nonmalignant pain diagnosis who received daily opioids for 3 or more consecutive months from a PCP was included. Excluded individuals were those with cognitive decline/dementia, serious mental illness, psychosis, active suicidality, disruptive behavior flag, or those excluded by PCP discretion if it was determined that the patient would do better in a one-on-one setting with the PCP (Table 1). Patients taking > 200 MED/d of opioids who were seen in the VASLCHCS specialty pain clinic were also excluded.
Patient and PCP Responsibilities
The patient was responsible for timely attendance and full participation in all SMA group classes as determined in the veteran’s Treatment Plan Agreement (TPA). In addition, the patient had to provide UDS when requested (a minimum of twice yearly) and communicate with the PCP if having a procedure requiring additional opioids. This was in line with the current standards set forth by the VA Opioid Safety Initiative (OSI) Taskforce.12
The PC-CPP provided education, evaluation, documentation, and referral and follow-up with the nonpharmacologic treatment options discussed but did not provide prescription medications. The PCP reviewed the medical documents completed in the PC-CPP, and the PCP was strongly encouraged to follow its recommendations. The expectation was that the PCP would support the PC-CPP when the care recommendation was for a pharmacist-guided opioid taper.
Lack of attendance was defined as a no-show or a reschedule. Patients were considered adherent if they missed fewer than 2 SMA appointments and 2 nonpharmacologic treatment appointments every 6 months. The patient was required to attend the SMA and nonpharmacologic treatment on the third appointment to remain adherent with PC-CPP expectations and agreements. Adherence was acknowledged after 12 and 24 months by a reduction in PC-CPP requirements.
Shared Medical Appointment
Patients referred to the program and who met inclusion criteria received letters explaining the importance of SMA attendance and follow-up reminder calls. At least 30 minutes before the SMA, the patient provided an UDS sample at the laboratory. Next, the patient received an individualized program packet that included the TORYSSO guide, iMedConsent, a TPA specific to the program, a brief pain inventory (BPI) and opioid risk tool, a list of medication disposal sites, and short descriptions of available nonpharmacologic therapies.
Each SMA began with a presentation delivered by a pharmacist, a psychologist, and a medical provider, discussing TORYSSO, program expectations, and holistic approaches to pain. Each SMA also included a rotating chronic pain information topic (eg, nutrition and pain, the physiology of addiction, and the value of multiple modalities in pain treatment). Together, the staff and patients reviewed and completed the blank forms enclosed in the individualized
Each visit was entered into a Computerized Patient Record System (CPRS) template, which included a pain diagnosis, Opioid Risk Tool score, pain and functional scores, opioid fill history, last comprehensive metabolic panel, last electrocardiogram if on methadone, dates of signed agreements, patient adherence with SMA and optional therapies, and follow-up (eFigure).
Every patient enrolled in the PC-CPP had to attend a SMA every 6 months. Patients continued this indefinitely while receiving opioids, and requirements were lessened for patients who had a history of meeting program requirements. For those fully adherent after the first year, only 1 nonpharmacologic intervention was needed (instead of 1 every 6 months) yearly. After 2 years of full adherence, nonpharmacologic interventions were no longer necessary as the expectation was that the patient would continue to use the strategies that they had learned over the previous 2 years. Patients left the PC-CPP if they chose to discontinue opioids, met any of the exclusion criteria, or were nonadherent. Tapering opioid medication was recommended for patients who missed a SMA meeting or 2 nonpharmacologic treatment meetings in a 6-month period; received opioids from more than 1 provider; test positive on a UDS for substances that should not be present; consistently testing negative on a UDS for substances that should be present (indicating diversion); or exhibiting other aberrant behavior (frequent requests for early refills, medications often lost/stolen, etc).
Program Barriers
The PC-CPP took about 2 years to set up, and several barriers were encountered. A thorough understanding of the following factors is necessary for establishing a similar program.
Initially, consults were placed by a designee (someone other than the PCP currently caring for the patient). The designee was usually a member of the PC-CPP who placed consults for all patients who had opioids listed on the CPRS profile. Further, patients who had any opioids within the past 3 months were initially included as were patients who wanted pain education but were not taking opioids. After 12 months, it became apparent that the focus of the PC-CPP should center on patients taking opioids for a minimum of 3 months consecutively. Patients who wanted only education could attend other hospital education opportunities, which helped keep the patient load manageable for PC-CPP staff. Further, to lessen patient confusion and improve adherence, the PCP placed the consult and discussed the program with the patient. Class sizes of 5 to 10 patients seemed to be ideal for patient participation and provider workload.
Patient Education
Initially, the SMA did not follow a standard curriculum, but the current format is more consistent, reproducible, streamlined, and organized. This adjustment improved SMA attendance as well as patient satisfaction, as the class started and finished on time. The SMA also started with numerous handouts, including brochures for nonpharmacologic programs offered at this facility. This led to patients feeling overwhelmed, missing the important forms, and wasted paper. Handouts were simplified to 2 color-coded forms (TPA and BPI).
The take-home assessment was streamlined to a single general assessment. This assessment consisted of 2 questions that asked patients to write a summary of what they learned and then write a summary of how they applied what they learned to their pain management. The VA Manage Stress Workbook also was added to the take-home materials. There are currently 5 different take-home options, which are necessary for those who live more than 50 miles from any VA facility or for those who have transportation issues.
Patient Distress
The SMA could be stressful for patients who felt they were being “punished” or who showed up more than 15 minutes late and had to reschedule the SMA. Having a mental health provider available was crucial for these situations.
Therapeutic Option Development
A cornerstone of the program was getting patients to participate in nonpharmacologic treatment options, which required a robust selection of programs. The VASLCHCS was fortunate to have many programs already available (Table 2), but this was not always the case for the VA community-based outpatient clinics (CBOCs).
Stakeholder Support
Before its start, PC-CPP was presented to the Pentad (a group of 5 individuals in the local facility who hold executive leadership positions) for approval. Tapering opioids can lead to feelings of hostility, frustration, or sadness for patients, so having the Pentad support for the program was crucial to address complaints made to patient advocates or senators. Provider support also was important to reinforce program rules. The PC-CPP inclusion criteria included only those patients whose PCP was agreeable to a taper when the patient did not comply with program expectations. This strategy helped to improve patient adherence with the PC-CPP and decrease patient arguments with clinic staff, as all patients are held to the same standards.
Staff
Finding willing staff can be a challenge. It is estimated that each site needed a program leader who can champion the program objectives and drive organization of staff, space, documentation, and consistency for the patients consulted to the PC-CPP. The goal is that the consistent, reproducible expectations for both the PCP and the patient will reduce overall workload for a clinic. Patients may test the firmness and conviction of the staff to the PC-CPP. Having staff who are able and willing to be firm on relaying information for adherence to the patient is vital.
Administrative Support
At a minimum, a medical support assistant was required to help with scheduling, reminder calls and letters, CPRS check-in/check-out, ensuring necessary forms are ready for the SMA, tracking adherence, and following-up on no-shows and rescheduling.
Documentation
The CPRS consult and note template titles required the approval of the template committee. Although the template is helpful, there is still a great deal that needs to be manually entered in the note, such as BPI scores, opioid risk scores, and chosen nonpharmacologic interventions scores of pain, function, and opioid risk as well individual comorbidities, diagnosis, and follow-up dates. Documentation is geared toward easy review for the PCP who should scan the document prior to renewing opioid medications. The PC-CPP consult became a message board. Once the patient attends the SMA, the designated staff will add a comment to the message board, identifying all dates attended, complete history of the patient’s intervention choices and rate of adherence, as well a follow-up SMA date and whether the patient should bring materials such as take-home tests.
Time Commitment
Program development carries a heavy time burden. One full-time equivalent clinician for 6 weeks for program development is needed. Time allotment is estimated to be the following:
- Medical provider—30 minutes per patient (chart review, documentation, consult resolution). With training, these duties could be completed by support staff
- Pharmacist—30 minutes per patient (chart review, UDS, Utah Division of Occupational and Professional Licensing, fill history). Additional time is needed for writing opioid tapers for qualifying patients
- Primary care mental health integration—a PhD spent 1 to 2 hours per SMA visit assisting patients who became distressed during the visit. Only once has a patient needed to be escorted to the emergency department for active suicidality. A PhD also spent 10+ hours per week running and managing the CBT for Chronic Pain Group
- Support staff—a registered nurse spent 4 hours each month preparing for the SMA (entering consults, ordering EMITs, purchasing snacks)
Conclusion
In this descriptive report, the authors presented an overview of a newly developed program to manage chronic nonmalignant pain and safe opioid prescribing in a primary care setting. A final report is pending. The intent with this interim report was to describe the PC-CPP at the VASLCHCS, its methods and protocols, and logistic considerations for other providers who are working with patients with chronic pain in a primary care model. Standard operating procedure and inclusion/exclusion criteria were included to help with clinical decision making for patients chronic pain for whom aberrant opioid-related behavior presents a problem.
The authors expect that the PC-CPP will provide more comprehensive assisted care, lending to decreased complications associated with accidental overdose, because since patients have been educated about risks for accidental overdose from chronic opioids and have the responsibility for their outcomes. The authors also anticipated that functional scores (as measured by the BPI) will increase despite lowering opioid doses because patients will use ancillary treatments for pain. The desired outcome is that patients will come to understand that pain control is best approached holistically rather than through opioid monotherapy.
There have been several recent initiatives within the VA to decrease opioid prescribing and increase patient safety. With this in mind, continued expansion of this program to CBOCs and male patients could be useful to providers. Also, this program was conducted in a small setting (Women’s Clinic), and there are many challenges with rolling out such a program in a larger clinic (eg, greater chance for provider disagreement, greater need for administrative staff support). Nonetheless, the benefits of close monitoring of prescription opioids and active encouragement to engage in nonpharmacologic therapies are substantial and deserve further advancement.
1. Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. http://www.fsmb.org/Media/Default /PDF/FSMB/Advocacy/pain_policy_july2013.pdf. Accessed November 4, 2016.
2. Buse D, Loder E, McAlary P. Chronic pain rehabilitation. Pain Management Rounds. 2005;6:355-360.
3. Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O’Conner PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17(3):173-179.
4. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241-248.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends in regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-6012.
6. Busse JW, Guyatt GH. Optimizing the use of patient data to improve outcomes for patients: narcotics for chronic noncancer pain. Expert Rev Pharmacoecon Outcomes Res. 2009;9(2):171-179.
7. Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125(1-2):172-179.
8. Agency Medical Directors Group. Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid therapy 2010 update. http://www.agen cymeddirectors.wa.gov/files/opioidgdline.pdf. Accessed November 4, 2016.
9. Utah Department of Health. Utah clinical guidelines on prescribing opioids for treatment of pain. http://health.utah.gov/prescription/pdf/guidelines/final.04.09opioidGuidlines.pdf. Published February 2009. Accessed November 4, 2016.
10. U.S. Department of Veterans Affairs, VA Academic Detailing Service. Pain management, opioid safety. VA educational guide (2014).http://www.va.gov/PAINMANAGEMENT/docs/OSI_1 _Tookit_Provider_AD_Educational_Guide_7_17.pdf. Published July 2014. Accessed November 2016.
11. Dobscha SK, Corson K, Leibowitz RQ, Sullivan MD, Gerrity MS. Rational, design, and baseline findings from a randomized trial of collaborative care for chronic musculoskeletal pain in primary care. Pain Med. 2008;9(8):1050-1064.
12. Mercado AC, Carroll LJ, Cassidy JD, Côté P. Passive coping is a risk factor for disabling neck or low back pain. Pain. 2005;117(1-2):51-57.
13. U.S. Department of Veterans Affairs, VA National Pain Management Program. Taking opioid responsibly for your safety and the safety of others: patient information guide on long-term opioid therapy for pain. http://www.veteranshealthlibrary.org/DiseasesConditions/ChronicPain/142,OpioidsIntro_VA. Updated December 9, 2015. Accessed November 17, 2016.
1. Federation of State Medical Boards. Model policy on the use of opioid analgesics in the treatment of chronic pain. http://www.fsmb.org/Media/Default /PDF/FSMB/Advocacy/pain_policy_july2013.pdf. Accessed November 4, 2016.
2. Buse D, Loder E, McAlary P. Chronic pain rehabilitation. Pain Management Rounds. 2005;6:355-360.
3. Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O’Conner PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17(3):173-179.
4. Dart RC, Surratt HL, Cicero TJ, et al. Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241-248.
5. Bohnert AS, Ilgen MA, Trafton JA, et al. Trends in regional variation in opioid overdose mortality among Veterans Health Administration patients, fiscal year 2001 to 2009. Clin J Pain. 2014;30(7):605-6012.
6. Busse JW, Guyatt GH. Optimizing the use of patient data to improve outcomes for patients: narcotics for chronic noncancer pain. Expert Rev Pharmacoecon Outcomes Res. 2009;9(2):171-179.
7. Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125(1-2):172-179.
8. Agency Medical Directors Group. Interagency guideline on opioid dosing for chronic non-cancer pain: an educational aid to improve care and safety with opioid therapy 2010 update. http://www.agen cymeddirectors.wa.gov/files/opioidgdline.pdf. Accessed November 4, 2016.
9. Utah Department of Health. Utah clinical guidelines on prescribing opioids for treatment of pain. http://health.utah.gov/prescription/pdf/guidelines/final.04.09opioidGuidlines.pdf. Published February 2009. Accessed November 4, 2016.
10. U.S. Department of Veterans Affairs, VA Academic Detailing Service. Pain management, opioid safety. VA educational guide (2014).http://www.va.gov/PAINMANAGEMENT/docs/OSI_1 _Tookit_Provider_AD_Educational_Guide_7_17.pdf. Published July 2014. Accessed November 2016.
11. Dobscha SK, Corson K, Leibowitz RQ, Sullivan MD, Gerrity MS. Rational, design, and baseline findings from a randomized trial of collaborative care for chronic musculoskeletal pain in primary care. Pain Med. 2008;9(8):1050-1064.
12. Mercado AC, Carroll LJ, Cassidy JD, Côté P. Passive coping is a risk factor for disabling neck or low back pain. Pain. 2005;117(1-2):51-57.
13. U.S. Department of Veterans Affairs, VA National Pain Management Program. Taking opioid responsibly for your safety and the safety of others: patient information guide on long-term opioid therapy for pain. http://www.veteranshealthlibrary.org/DiseasesConditions/ChronicPain/142,OpioidsIntro_VA. Updated December 9, 2015. Accessed November 17, 2016.
An Electronic Template to Improve Psychotropic Medication Review and Gradual Dose-Reduction Documentation
One in 5 Americans are taking at least 1 psychotropic medication.1 Elderly dementia patients in extended care facilities are the most likely population to be prescribed psychotropic medication: 87% of these patients are on at least 1 psychotropic medication, 66% on at least 2, 36% on at least 3, and 11% on 4 or more.2 Psychotropic medications alleviate the symptoms of mental illness, such as depression, anxiety, and psychosis. Unfortunately, these medications often have adverse effects (AEs), including, but not limited to, excessive sedation, cardiac abnormalities, and tardive dyskinesia.
In 1987, Congress passed the Nursing Home Reform Act (NHRA) as part of the Omnibus Budget Reconciliation Act. The NHRA mandated that residents must remain free of “physical or chemical restraints imposed for the purpose of discipline or convenience.”3
In 1991, in order to meet the NHRA requirements, the Centers for Medicare and Medicaid Services (CMS) issued a guideline that nursing homes should use antipsychotic drug therapy only to treat a specific condition as diagnosed and documented in the clinical record. In 2006, CMS published guidance on the reduction of psychotropic medication usage. These CMS guidelines are the community-accepted standards for prescribing psychotropic medications, and accrediting bodies expect compliance with the guidelines. The guidelines also recommend that antipsychotics should be prescribed at the lowest possible dose, used for the shortest period, and continually undergo gradual dose reduction (GDR).4 To ensure these standards are met, a review of the use of psychotropic medications should be performed regularly.
The purpose of this project was to improve documentation of GDR and review of psychotropic medication based on CMS guidelines in the community living centers (CLCs) at the Carl Vinson Veterans Affairs Medical Center (CVVAMC) in Dublin, Georgia.
Background
The CVVAMC provides long-term care to veterans living in CLCs in a comfortable, homelike environment with a person-centered, nursing-home level of care. During a patient’s stay, clinical pharmacy services reviews patient medication use regularly. Additionally, an interdisciplinary team (IDT) of physicians, pharmacists, nurses, recreation therapists, dieticians, and chaplains meet to discuss the medical, psychosocial, and spiritual needs of the residents at the time of admission and quarterly thereafter. Family and caregivers are invited to attend as well.
During the meeting, the IDT care plan is updated and reviewed with the veteran. Currently at the CVVAMC, care plans include discussion of pain management; however, the plan does not address psychotropic medication use. To be compliant with CMS guidelines, during IDT meetings discussion of psychotropic medication use would be advantageous, because physicians could receive input and feedback from other health care team members in determining whether psychotropic medication changes are needed.
Methods
All documentation and chart notes are recorded and stored in the Computerized Patient Record System (CPRS). The purpose of an electronic template within CPRS is to provide standardized, guided documentation in an easy-to-use format that can be integrated smoothly into the existing system. Template utilization has been shown to improve documentation of medication reconciliation, health records, and coding in the VA health system.5-7 A review of CPRS showed that no template existed for documenting psychotropic medication initiation and GDR.
Objectives
The authors’ primary objective was to improve patient record documentation in CPRS through implementation of staff education and an electronic template for physicians to document the management of psychotropic medications and patients’ GDRs. The secondary objective was to implement a discussion involving the physician and other members of the team during IDT meetings about patient safety and effectiveness of psychotropic medications.
Template
To improve the suboptimal compliance with the CMS guidelines, an electronic template was created to evaluate GDR attempts and facilitate review of prescribed psychotropic medications. The pharmacy and therapeutics committee approved the template, and the clinical applications coordinator implemented the electronic template. The template was then revised as necessary for ease of use, based on health care provider (HCP) and pharmacist feedback.
The use of the electronic template was phased in over a 1-month period. Complete implementation was achieved in January 2014. An in-service was provided for nurses, HCPs, and directors on the importance of psychotropic medication review and GDRs. A second in-service was conducted for the HCPs to learn how to properly complete the electronic template. Providers were instructed to use the template during IDT meetings when completing monthly chart reviews and changing psychotropic medications.
Additionally, resident assessment coordinators who attend all IDT meetings were requested to remind the team to discuss psychotropic medication use if appropriate in an effort to add a psychotropic medication review to the IDT care plan.
To determine whether the electronic template improved documentation, a retrospective chart review of CPRS was conducted February 2014. The study included veterans in the CLC who were prescribed psychotropic medications since January 2014. The subjects who screened positive for psychotropic medication use were further evaluated by chart review. Before and after implementation, information was collected on the number and percentage of patients who had documentation in their CPRS records of a psychotropic medication review and on whether a GDR evaluation was recorded in CPRS. Additionally, a clinical pharmacist specialist attending the IDT meeting monitored the incidence of psychotropic medication review or discussion that took place if the veteran was on an included agent.
Results
A total of 67 patients on psychotropic medication on the CLC wards since January 2014 were included in the program. Before implementation of the program, 35 patients had documentation of psychotropic medication review. Following implementation, this increased to 54 patients. Hence, 80% of the patients had appropriate documentation.
Before implementation, no patients had documentation regarding evaluation of a GDR in CPRS. After the program implementation, 54 patients had documentation of GDR. Of the 54 patients, 50 had documentation that a GDR could not be completed, and 4 patients had undergone a trial of GDR with appropriate documentation in CPRS (Figure).
The secondary objective evaluated how often a review of patient’s psychotropic medication occurred during IDT meetings. During March 2014, 21 patients with scheduled IDT meetings were prescribed psychotropic medications. For 14 of the 21 patients (67%) appropriateness of psychotropic medications was reviewed with the team and the resident’s families.
Discussion
Documentation is fundamental to improving quality of care, patient outcomes, and reimbursement for the facility. Effective since July 1, 2014, the Joint Commission standards for Nursing Care Center accreditation require the physician and consulting pharmacist to review the patients’ or residents’ medication list. The review should verify the following:
- Clinical indication for the antipsychotic medication;
- Necessity for ongoing use of the antipsychotic medication;
- Consideration of GDR of the antipsychotic medication;
- Consideration of an alternative to antipsychotic medication use.8
When used correctly, the psychotropic medication review and GDR template ensures veterans’ psychotropic medications are continually reviewed. Furthermore, better psychotropic medication management allows veterans to be adequately treated while minimizing the risk for potential AEs.
At CVVAMC, the electronic template helped improve documentation and evaluation of GDR. The CVVAMC success can be attributed partly to physicians who were willing to use the template.
New standards from the Joint Commission provided an additional incentive to use the template. The Joint Commission also required that patients and their family be involved in decisions about placing the resident on antipsychotic medication. The optimal time for this discussion is during IDT meetings. At CVVAMC, discussion of psychotropic medications took place in IDT meetings for 67% of the patients prescribed psychotropic medication.
It is important to note that tools, such as this template, are successful only if they are used by HCPs. The CPRS does not have any clinical reminders to prompt physicians to complete the task. Instead, HCPs had to develop a routine of entering information into the template. Additionally, if the resident assessment coordinator was not at the IDT meeting, discussion of psychotropic medication was not always completed. After completion of this project, psychotropic medication review has become a permanent component of the IDT care plan.
Conclusion
This project demonstrated that template development and use have the potential to improve documentation, patient care, and survey scores. The electronic template could potentially benefit any long-term care facility that uses an electronic recording system. Further research should focus on ease of use and on ensuring that a psychotropic medication review is added to all CLC IDT care plans.
Acknowledgments
The authors acknowledge Brooke Butler, clinical pharmacy specialist and project mentor for her assistance and guidance; and Deborah Hobbs, residency director, both at the Carl Vinson VA Medical Center for her input and leadership, and Freddie Miles, for her work on development of the electronic order set.
1. Medco Health Solutions. America’s state of mind. 2011. http://apps.who.int/medicinedocs/documents/s19032en/s19032en.pdf. Accessed August 31, 2016.
2. Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Behavioral symptoms and the administration of psychotropic drugs to aged patients with dementia in nursing homes and in acute geriatric wards. Int Psychogeriatr. 2004;16(1):61-74.
3. Rehnquist J. Psychotropic drug use in nursing homes. Office of Inspector General. https://oig.hhs.gov/oei/reports/oei-02-00-00491.pdf. Published November 2001. Accessed June 24, 2016.
4. Centers for Medicare and Medicaid Services. CMS Manual System. Pub 100-07 State Operations Provider Certification. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs-Items/CMS1201984.html. Published December 15, 2006. Accessed August 29, 2016.
5. Rose EA, Deshikachar AM, Schwartz KL, Severson RK. Use of a template to improve documentation and coding. Fam Med. 2001;33(7):516-521.
6. Fielstein EM, Brown SH, McBrine CS, Clark TK, Hardenbrook SP, Speroff T. The effect of standardized, computer-guided templates on quality of VA disability exams. AMIA Annu Symp Proc. 2006:249-253.
7. Edwards LB, Powers JB. Electronic medication reconciliation: a pilot demonstration on an inpatient geriatrics unit. Fed Pract. 2007;24(9):49-50, 53, 62.
8. Approved: memory care requirements for nursing center accreditation. http://www.jointcommission.org/ASSETS/1/18/JCP0114_MEMORY_CARE_NCC.PDF. Published January 2014. Accessed August 24, 2016.
One in 5 Americans are taking at least 1 psychotropic medication.1 Elderly dementia patients in extended care facilities are the most likely population to be prescribed psychotropic medication: 87% of these patients are on at least 1 psychotropic medication, 66% on at least 2, 36% on at least 3, and 11% on 4 or more.2 Psychotropic medications alleviate the symptoms of mental illness, such as depression, anxiety, and psychosis. Unfortunately, these medications often have adverse effects (AEs), including, but not limited to, excessive sedation, cardiac abnormalities, and tardive dyskinesia.
In 1987, Congress passed the Nursing Home Reform Act (NHRA) as part of the Omnibus Budget Reconciliation Act. The NHRA mandated that residents must remain free of “physical or chemical restraints imposed for the purpose of discipline or convenience.”3
In 1991, in order to meet the NHRA requirements, the Centers for Medicare and Medicaid Services (CMS) issued a guideline that nursing homes should use antipsychotic drug therapy only to treat a specific condition as diagnosed and documented in the clinical record. In 2006, CMS published guidance on the reduction of psychotropic medication usage. These CMS guidelines are the community-accepted standards for prescribing psychotropic medications, and accrediting bodies expect compliance with the guidelines. The guidelines also recommend that antipsychotics should be prescribed at the lowest possible dose, used for the shortest period, and continually undergo gradual dose reduction (GDR).4 To ensure these standards are met, a review of the use of psychotropic medications should be performed regularly.
The purpose of this project was to improve documentation of GDR and review of psychotropic medication based on CMS guidelines in the community living centers (CLCs) at the Carl Vinson Veterans Affairs Medical Center (CVVAMC) in Dublin, Georgia.
Background
The CVVAMC provides long-term care to veterans living in CLCs in a comfortable, homelike environment with a person-centered, nursing-home level of care. During a patient’s stay, clinical pharmacy services reviews patient medication use regularly. Additionally, an interdisciplinary team (IDT) of physicians, pharmacists, nurses, recreation therapists, dieticians, and chaplains meet to discuss the medical, psychosocial, and spiritual needs of the residents at the time of admission and quarterly thereafter. Family and caregivers are invited to attend as well.
During the meeting, the IDT care plan is updated and reviewed with the veteran. Currently at the CVVAMC, care plans include discussion of pain management; however, the plan does not address psychotropic medication use. To be compliant with CMS guidelines, during IDT meetings discussion of psychotropic medication use would be advantageous, because physicians could receive input and feedback from other health care team members in determining whether psychotropic medication changes are needed.
Methods
All documentation and chart notes are recorded and stored in the Computerized Patient Record System (CPRS). The purpose of an electronic template within CPRS is to provide standardized, guided documentation in an easy-to-use format that can be integrated smoothly into the existing system. Template utilization has been shown to improve documentation of medication reconciliation, health records, and coding in the VA health system.5-7 A review of CPRS showed that no template existed for documenting psychotropic medication initiation and GDR.
Objectives
The authors’ primary objective was to improve patient record documentation in CPRS through implementation of staff education and an electronic template for physicians to document the management of psychotropic medications and patients’ GDRs. The secondary objective was to implement a discussion involving the physician and other members of the team during IDT meetings about patient safety and effectiveness of psychotropic medications.
Template
To improve the suboptimal compliance with the CMS guidelines, an electronic template was created to evaluate GDR attempts and facilitate review of prescribed psychotropic medications. The pharmacy and therapeutics committee approved the template, and the clinical applications coordinator implemented the electronic template. The template was then revised as necessary for ease of use, based on health care provider (HCP) and pharmacist feedback.
The use of the electronic template was phased in over a 1-month period. Complete implementation was achieved in January 2014. An in-service was provided for nurses, HCPs, and directors on the importance of psychotropic medication review and GDRs. A second in-service was conducted for the HCPs to learn how to properly complete the electronic template. Providers were instructed to use the template during IDT meetings when completing monthly chart reviews and changing psychotropic medications.
Additionally, resident assessment coordinators who attend all IDT meetings were requested to remind the team to discuss psychotropic medication use if appropriate in an effort to add a psychotropic medication review to the IDT care plan.
To determine whether the electronic template improved documentation, a retrospective chart review of CPRS was conducted February 2014. The study included veterans in the CLC who were prescribed psychotropic medications since January 2014. The subjects who screened positive for psychotropic medication use were further evaluated by chart review. Before and after implementation, information was collected on the number and percentage of patients who had documentation in their CPRS records of a psychotropic medication review and on whether a GDR evaluation was recorded in CPRS. Additionally, a clinical pharmacist specialist attending the IDT meeting monitored the incidence of psychotropic medication review or discussion that took place if the veteran was on an included agent.
Results
A total of 67 patients on psychotropic medication on the CLC wards since January 2014 were included in the program. Before implementation of the program, 35 patients had documentation of psychotropic medication review. Following implementation, this increased to 54 patients. Hence, 80% of the patients had appropriate documentation.
Before implementation, no patients had documentation regarding evaluation of a GDR in CPRS. After the program implementation, 54 patients had documentation of GDR. Of the 54 patients, 50 had documentation that a GDR could not be completed, and 4 patients had undergone a trial of GDR with appropriate documentation in CPRS (Figure).
The secondary objective evaluated how often a review of patient’s psychotropic medication occurred during IDT meetings. During March 2014, 21 patients with scheduled IDT meetings were prescribed psychotropic medications. For 14 of the 21 patients (67%) appropriateness of psychotropic medications was reviewed with the team and the resident’s families.
Discussion
Documentation is fundamental to improving quality of care, patient outcomes, and reimbursement for the facility. Effective since July 1, 2014, the Joint Commission standards for Nursing Care Center accreditation require the physician and consulting pharmacist to review the patients’ or residents’ medication list. The review should verify the following:
- Clinical indication for the antipsychotic medication;
- Necessity for ongoing use of the antipsychotic medication;
- Consideration of GDR of the antipsychotic medication;
- Consideration of an alternative to antipsychotic medication use.8
When used correctly, the psychotropic medication review and GDR template ensures veterans’ psychotropic medications are continually reviewed. Furthermore, better psychotropic medication management allows veterans to be adequately treated while minimizing the risk for potential AEs.
At CVVAMC, the electronic template helped improve documentation and evaluation of GDR. The CVVAMC success can be attributed partly to physicians who were willing to use the template.
New standards from the Joint Commission provided an additional incentive to use the template. The Joint Commission also required that patients and their family be involved in decisions about placing the resident on antipsychotic medication. The optimal time for this discussion is during IDT meetings. At CVVAMC, discussion of psychotropic medications took place in IDT meetings for 67% of the patients prescribed psychotropic medication.
It is important to note that tools, such as this template, are successful only if they are used by HCPs. The CPRS does not have any clinical reminders to prompt physicians to complete the task. Instead, HCPs had to develop a routine of entering information into the template. Additionally, if the resident assessment coordinator was not at the IDT meeting, discussion of psychotropic medication was not always completed. After completion of this project, psychotropic medication review has become a permanent component of the IDT care plan.
Conclusion
This project demonstrated that template development and use have the potential to improve documentation, patient care, and survey scores. The electronic template could potentially benefit any long-term care facility that uses an electronic recording system. Further research should focus on ease of use and on ensuring that a psychotropic medication review is added to all CLC IDT care plans.
Acknowledgments
The authors acknowledge Brooke Butler, clinical pharmacy specialist and project mentor for her assistance and guidance; and Deborah Hobbs, residency director, both at the Carl Vinson VA Medical Center for her input and leadership, and Freddie Miles, for her work on development of the electronic order set.
One in 5 Americans are taking at least 1 psychotropic medication.1 Elderly dementia patients in extended care facilities are the most likely population to be prescribed psychotropic medication: 87% of these patients are on at least 1 psychotropic medication, 66% on at least 2, 36% on at least 3, and 11% on 4 or more.2 Psychotropic medications alleviate the symptoms of mental illness, such as depression, anxiety, and psychosis. Unfortunately, these medications often have adverse effects (AEs), including, but not limited to, excessive sedation, cardiac abnormalities, and tardive dyskinesia.
In 1987, Congress passed the Nursing Home Reform Act (NHRA) as part of the Omnibus Budget Reconciliation Act. The NHRA mandated that residents must remain free of “physical or chemical restraints imposed for the purpose of discipline or convenience.”3
In 1991, in order to meet the NHRA requirements, the Centers for Medicare and Medicaid Services (CMS) issued a guideline that nursing homes should use antipsychotic drug therapy only to treat a specific condition as diagnosed and documented in the clinical record. In 2006, CMS published guidance on the reduction of psychotropic medication usage. These CMS guidelines are the community-accepted standards for prescribing psychotropic medications, and accrediting bodies expect compliance with the guidelines. The guidelines also recommend that antipsychotics should be prescribed at the lowest possible dose, used for the shortest period, and continually undergo gradual dose reduction (GDR).4 To ensure these standards are met, a review of the use of psychotropic medications should be performed regularly.
The purpose of this project was to improve documentation of GDR and review of psychotropic medication based on CMS guidelines in the community living centers (CLCs) at the Carl Vinson Veterans Affairs Medical Center (CVVAMC) in Dublin, Georgia.
Background
The CVVAMC provides long-term care to veterans living in CLCs in a comfortable, homelike environment with a person-centered, nursing-home level of care. During a patient’s stay, clinical pharmacy services reviews patient medication use regularly. Additionally, an interdisciplinary team (IDT) of physicians, pharmacists, nurses, recreation therapists, dieticians, and chaplains meet to discuss the medical, psychosocial, and spiritual needs of the residents at the time of admission and quarterly thereafter. Family and caregivers are invited to attend as well.
During the meeting, the IDT care plan is updated and reviewed with the veteran. Currently at the CVVAMC, care plans include discussion of pain management; however, the plan does not address psychotropic medication use. To be compliant with CMS guidelines, during IDT meetings discussion of psychotropic medication use would be advantageous, because physicians could receive input and feedback from other health care team members in determining whether psychotropic medication changes are needed.
Methods
All documentation and chart notes are recorded and stored in the Computerized Patient Record System (CPRS). The purpose of an electronic template within CPRS is to provide standardized, guided documentation in an easy-to-use format that can be integrated smoothly into the existing system. Template utilization has been shown to improve documentation of medication reconciliation, health records, and coding in the VA health system.5-7 A review of CPRS showed that no template existed for documenting psychotropic medication initiation and GDR.
Objectives
The authors’ primary objective was to improve patient record documentation in CPRS through implementation of staff education and an electronic template for physicians to document the management of psychotropic medications and patients’ GDRs. The secondary objective was to implement a discussion involving the physician and other members of the team during IDT meetings about patient safety and effectiveness of psychotropic medications.
Template
To improve the suboptimal compliance with the CMS guidelines, an electronic template was created to evaluate GDR attempts and facilitate review of prescribed psychotropic medications. The pharmacy and therapeutics committee approved the template, and the clinical applications coordinator implemented the electronic template. The template was then revised as necessary for ease of use, based on health care provider (HCP) and pharmacist feedback.
The use of the electronic template was phased in over a 1-month period. Complete implementation was achieved in January 2014. An in-service was provided for nurses, HCPs, and directors on the importance of psychotropic medication review and GDRs. A second in-service was conducted for the HCPs to learn how to properly complete the electronic template. Providers were instructed to use the template during IDT meetings when completing monthly chart reviews and changing psychotropic medications.
Additionally, resident assessment coordinators who attend all IDT meetings were requested to remind the team to discuss psychotropic medication use if appropriate in an effort to add a psychotropic medication review to the IDT care plan.
To determine whether the electronic template improved documentation, a retrospective chart review of CPRS was conducted February 2014. The study included veterans in the CLC who were prescribed psychotropic medications since January 2014. The subjects who screened positive for psychotropic medication use were further evaluated by chart review. Before and after implementation, information was collected on the number and percentage of patients who had documentation in their CPRS records of a psychotropic medication review and on whether a GDR evaluation was recorded in CPRS. Additionally, a clinical pharmacist specialist attending the IDT meeting monitored the incidence of psychotropic medication review or discussion that took place if the veteran was on an included agent.
Results
A total of 67 patients on psychotropic medication on the CLC wards since January 2014 were included in the program. Before implementation of the program, 35 patients had documentation of psychotropic medication review. Following implementation, this increased to 54 patients. Hence, 80% of the patients had appropriate documentation.
Before implementation, no patients had documentation regarding evaluation of a GDR in CPRS. After the program implementation, 54 patients had documentation of GDR. Of the 54 patients, 50 had documentation that a GDR could not be completed, and 4 patients had undergone a trial of GDR with appropriate documentation in CPRS (Figure).
The secondary objective evaluated how often a review of patient’s psychotropic medication occurred during IDT meetings. During March 2014, 21 patients with scheduled IDT meetings were prescribed psychotropic medications. For 14 of the 21 patients (67%) appropriateness of psychotropic medications was reviewed with the team and the resident’s families.
Discussion
Documentation is fundamental to improving quality of care, patient outcomes, and reimbursement for the facility. Effective since July 1, 2014, the Joint Commission standards for Nursing Care Center accreditation require the physician and consulting pharmacist to review the patients’ or residents’ medication list. The review should verify the following:
- Clinical indication for the antipsychotic medication;
- Necessity for ongoing use of the antipsychotic medication;
- Consideration of GDR of the antipsychotic medication;
- Consideration of an alternative to antipsychotic medication use.8
When used correctly, the psychotropic medication review and GDR template ensures veterans’ psychotropic medications are continually reviewed. Furthermore, better psychotropic medication management allows veterans to be adequately treated while minimizing the risk for potential AEs.
At CVVAMC, the electronic template helped improve documentation and evaluation of GDR. The CVVAMC success can be attributed partly to physicians who were willing to use the template.
New standards from the Joint Commission provided an additional incentive to use the template. The Joint Commission also required that patients and their family be involved in decisions about placing the resident on antipsychotic medication. The optimal time for this discussion is during IDT meetings. At CVVAMC, discussion of psychotropic medications took place in IDT meetings for 67% of the patients prescribed psychotropic medication.
It is important to note that tools, such as this template, are successful only if they are used by HCPs. The CPRS does not have any clinical reminders to prompt physicians to complete the task. Instead, HCPs had to develop a routine of entering information into the template. Additionally, if the resident assessment coordinator was not at the IDT meeting, discussion of psychotropic medication was not always completed. After completion of this project, psychotropic medication review has become a permanent component of the IDT care plan.
Conclusion
This project demonstrated that template development and use have the potential to improve documentation, patient care, and survey scores. The electronic template could potentially benefit any long-term care facility that uses an electronic recording system. Further research should focus on ease of use and on ensuring that a psychotropic medication review is added to all CLC IDT care plans.
Acknowledgments
The authors acknowledge Brooke Butler, clinical pharmacy specialist and project mentor for her assistance and guidance; and Deborah Hobbs, residency director, both at the Carl Vinson VA Medical Center for her input and leadership, and Freddie Miles, for her work on development of the electronic order set.
1. Medco Health Solutions. America’s state of mind. 2011. http://apps.who.int/medicinedocs/documents/s19032en/s19032en.pdf. Accessed August 31, 2016.
2. Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Behavioral symptoms and the administration of psychotropic drugs to aged patients with dementia in nursing homes and in acute geriatric wards. Int Psychogeriatr. 2004;16(1):61-74.
3. Rehnquist J. Psychotropic drug use in nursing homes. Office of Inspector General. https://oig.hhs.gov/oei/reports/oei-02-00-00491.pdf. Published November 2001. Accessed June 24, 2016.
4. Centers for Medicare and Medicaid Services. CMS Manual System. Pub 100-07 State Operations Provider Certification. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs-Items/CMS1201984.html. Published December 15, 2006. Accessed August 29, 2016.
5. Rose EA, Deshikachar AM, Schwartz KL, Severson RK. Use of a template to improve documentation and coding. Fam Med. 2001;33(7):516-521.
6. Fielstein EM, Brown SH, McBrine CS, Clark TK, Hardenbrook SP, Speroff T. The effect of standardized, computer-guided templates on quality of VA disability exams. AMIA Annu Symp Proc. 2006:249-253.
7. Edwards LB, Powers JB. Electronic medication reconciliation: a pilot demonstration on an inpatient geriatrics unit. Fed Pract. 2007;24(9):49-50, 53, 62.
8. Approved: memory care requirements for nursing center accreditation. http://www.jointcommission.org/ASSETS/1/18/JCP0114_MEMORY_CARE_NCC.PDF. Published January 2014. Accessed August 24, 2016.
1. Medco Health Solutions. America’s state of mind. 2011. http://apps.who.int/medicinedocs/documents/s19032en/s19032en.pdf. Accessed August 31, 2016.
2. Pitkala KH, Laurila JV, Strandberg TE, Tilvis RS. Behavioral symptoms and the administration of psychotropic drugs to aged patients with dementia in nursing homes and in acute geriatric wards. Int Psychogeriatr. 2004;16(1):61-74.
3. Rehnquist J. Psychotropic drug use in nursing homes. Office of Inspector General. https://oig.hhs.gov/oei/reports/oei-02-00-00491.pdf. Published November 2001. Accessed June 24, 2016.
4. Centers for Medicare and Medicaid Services. CMS Manual System. Pub 100-07 State Operations Provider Certification. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Internet-Only-Manuals-IOMs-Items/CMS1201984.html. Published December 15, 2006. Accessed August 29, 2016.
5. Rose EA, Deshikachar AM, Schwartz KL, Severson RK. Use of a template to improve documentation and coding. Fam Med. 2001;33(7):516-521.
6. Fielstein EM, Brown SH, McBrine CS, Clark TK, Hardenbrook SP, Speroff T. The effect of standardized, computer-guided templates on quality of VA disability exams. AMIA Annu Symp Proc. 2006:249-253.
7. Edwards LB, Powers JB. Electronic medication reconciliation: a pilot demonstration on an inpatient geriatrics unit. Fed Pract. 2007;24(9):49-50, 53, 62.
8. Approved: memory care requirements for nursing center accreditation. http://www.jointcommission.org/ASSETS/1/18/JCP0114_MEMORY_CARE_NCC.PDF. Published January 2014. Accessed August 24, 2016.
Development and Implementation of a Geriatric Walking Clinic
Inactivity and increased sedentary time are major public health problems, particularly among older adults.1-3 Inactivity increases with age and produces deleterious effects on physical health, mental health, and quality of life and leads to increased health care costs.4 The high prevalence of a sedentary lifestyle among older veterans may be due to multiple factors, including misconceptions about the health benefits of exercise, lack of motivation, or associating exercise with discomfort or pain. Older veterans living in rural areas are at high risk because they are more sedentary than are urban-dwelling veterans.5 Of veterans aged ≥ 65 years who use health care services in VISN 16, 59% live in rural or highly rural areas.
Given the large number of older veterans and their at-risk status, addressing inactivity among this population is critical. Until recently, few programs existed within the VHA that addressed this need. Despite strong evidence that physical activity helps maintain functional independence and avoids institutionalization of frail elderly veterans, the VHA had no established procedures or guidelines for assessment and counseling.
To address this void, a Geriatric Walking Clinic (GWC) was established at the Central Arkansas Veterans Healthcare System (CAVHS) in March 2013. The GWC developed a patient-centric, home-based program that implements a comprehensive approach to assess, educate, motivate, and activate older veterans to commit to, engage in, and adhere to, a long-term program of regular physical activity primarily in the form of walking. The program uses proven strategies, such as motivational counseling, follow-up phone calls from a nurse, and self-monitoring using pedometers.6-8 Funding for the GWC project was provided by the VHA Office of Geriatrics and Extended Care as part of its Transition to the 21st Century (T21) initiative and by the VHA Office of Rural Health.
Methods
Quality improvement (QI) principles were used to develop the program, which received a nonresearch determination status from the local institutional review board. The GWC is staffed by a registered nurse, health technician, and physician. Both the nurse and the health technician were trained on the use of various assessments. Several tactics were developed to promote patient recruitment to the GWC, including systemwide in-services, an easy-to-use consultation request within the electronic medical record, patient and provider brochures, and informational booths and kiosks. Collaborations were developed with various clinical services to promote referrals. Several other services, such as Primary Care, Geriatrics, the Move! Weight Management Program, Cardiology (including Congestive Heart Failure), Hematology/Oncology, and Mental Health referred patients to the GWC.
The GWC targets sedentary, community-dwelling veterans aged ≥ 60 years who are able to ambulate in their home without an assistive device, are willing to walk for exercise, and are willing to receive phone calls. All-comers are included in the program. Although most of these veterans have multiple chronic medical problems, only those with absolute contraindications to exercise per the American College of Sports Medicine guidelines and those with any medical condition that is likely to compromise their ability to safely participate in the walking program are excluded (Figure).
First Visit
At the first visit, veterans receive a brief education about GWC, highlighting its potential health benefits. If veterans want to join, they are evaluated using a 3-tier screening assessment to determine the safety of starting a new walking regimen. Veterans who fail the 3-tier safety screening are referred to their primary care physician (PCP) for further assessment (eg, cardiac stress testing) to better define eligibility status. Veterans who pass the screen complete brief tests of physical performance, including gait speed, 6-Minute Walk Test (6MWT), Timed Up and Go test, and Berg Balance Scale.9-12 Participants also complete short surveys that provide useful information about their social support, barriers to exercise, response to physical activity, and usual activity level as measured by Community Healthy Activities Model Program for Seniors.13,14 This information is used to help develop an individualized walking prescription.
After completing the baseline assessments, the GWC staff members help the veteran set realistic goals, using motivational counseling techniques. The veteran receives a walking prescription to walk indoors or outdoors, based on current physical condition, self-identified goals, perceived barriers, and strength of support system; educational material about safe walking; a log for recording daily step count; information on follow-up calls; and an invitation to return for follow-up visits. The veteran also receives a pedometer and is instructed to continue his or her usual routine for the first week. The average daily step count is recorded as the baseline. The veteran is instructed to start the walking program after the baseline week with goals tailored to the personal activity level. For example: Some patients are asked to simply add an extra minute to their walking, whereas others savvy with pedometer numbers are asked to increase their step count.
Follow-up
Veterans are followed closely between their clinic appointments via phone calls from a nurse who provides encouragement and helps set new goals. The nurse collects the step count data to determine progress and set new walking goals. Those unable to adhere to their walking prescription are reassessed for their barriers. The nurse also helps participants identify ways to overcome individual challenges. The PCP is consulted when barriers include medical problems, such as pain or poor blood sugar control.
At the 6-week follow-up visit, the health care provider reviews the pedometer log and repeats all outcome assessments, including the physical performance testing and the participant surveys. Veterans receive feedback from these outcome assessments. To assess participant satisfaction, CAVHS GRECC developed a satisfaction questionnaire, which was given to participants.
Results
A total of 249 older veterans participated in the GWC program. The mean age was 67 (±6) years; 92% were male, 60% were white, and 39% were African American. Most participants lived in a rural location (60%) and were obese (69%); consistent with national standards, obesity was defined as a body mass index (BMI) ≥ 30 kg/m2. Several barriers to exercise were endorsed by the veterans. Most commonly endorsed barriers included bad weather, lack of motivation, feeling tired, and fear of pain. Most participants (93%) were actively engaged via regular phone follow-ups visits; 121 (49%) participants returned to the clinic for the 6-week reassessment. Repeat performance testing at the 6-week visit showed a clinically significant average 14% improvement in the 6MWT, 6% improvement in the Timed Up and Go test, and a 27% improvement in gait speed. Of those veterans who were obese, 64% lost weight. On entry into the program, 32 participants (13%) had poorly controlled diabetes mellitus (DM), defined as hemoglobin A1c (HbA1c) ≥ 8. Among this group, HbA1c improved by an average of 1.5% by the 6-week visit. The GWC program may have contributed to the improved glycemic control as a generally accepted frequency of monitoring HbA1c is at least 3 months.
At the 6-week clinic visit, 94% of those surveyed completed a program evaluation. The GWC scored high on satisfaction; over 80% strongly agreed that they were satisfied with the GWC program as a whole, 80% strongly agreed that the program increased their awareness about need for exercise, 82% strongly agreed that the clinician’s advice was applicable to them, and 77% strongly agreed that the program improved their motivation to walk regularly (Table 1).
Program Economics
An analysis of the clinic costs and benefits was performed to determine whether costs could potentially be offset by the savings realized from improved health outcomes of participating veterans. For this simplified analysis, costs of maintaining the GWC were set equal to the costs of the full-time equivalent employee hours, equipment, and educational materials. Based on the authors’ experience, they projected that for each 1,000 older veterans enrolled in the GWC, there is a requirement for 0.5 medical support assistant (GS-6 pay scale), 1.0 registered nurse grade 2 (RN), 1.0 health science specialist (GS-7), and 0.25 physician. At the host facility, the annual personnel costs are estimated at $205,149. The total annual cost of the GWC, including the equipment and educational materials, is estimated at $240,149.
Although full financial return on investment has yet to be determined, the authors estimated potential cost savings resulting if patients enrolled in a GWC achieved and maintained the types of improvements observed in the first cohort of patients. These estimates were based on identified improvements in 3 patient outcome measures cited in the medical literature that are associated with reductions in subsequent health care costs. These measures include gait speed, weight loss, and HbA1c. It is estimated that the cost savings associated with improvement of gait speed by 0.1 m/s (a clinically relevant change) is $1,200 annually.15
On average, patients enrolled in the GWC program improved their gait speed by 0.22 m/s. Cost savings related to gait speed improvement for 1,000 participants could reach $1,200,000. Conservative estimates of cost savings per 1% reduction of HbA1c is $950/year.16 Among those with poorly controlled DM (ie, HbA1c of ≥ 8), average HbA1c declined by 1.5%. Provided that 13% of the patients have poorly controlled DM, the total cost saving for 1,000 participants could be $209,950 annually.
It also is estimated that a 1% weight loss in obese patients is associated with a $256 decrease in subsequent total health care costs.17 In the GWC, the obese participants lost an average of 1.3% of their baseline weight. Assuming that about 60% of all older veterans participating in the clinic program are obese, annual cost savings per 1,000 participants related to weight loss is estimated to be $199,680. After accounting for the costs of operating the clinic, the total cost savings for a GWC with 1,000 enrolled older veterans is estimated to be as much as $1.4 million annually. Such a favorable cost assessment suggests that the program should be evaluated for widespread dissemination throughout the entire VHA system. Other potential benefits associated with GWC participation, such as improved quality of life and greater functional independence, may be of even greater importance to veterans.
Limitations
Results of this QI project need to be considered in light of its limitations. One of the most important limitations is the design of the project. Since this was a clinical initiative and not a research study, there was no control group or randomization. There were also limitations on data availability. HbA1c tests were not ordered as part of this QI project. Instead, baseline HbA1c was set equal to the most recent of any value obtained clinically within 2 months before the participant’s GWC enrollment; the 6-week follow-up value was set to any HbA1c obtained within 2 months after the 6-week visit. It is also recognized that factors other than the veterans’ participation in the GWC (eg, alterations in their DM medication regimen) may have contributed to the changes noted in some participant’s HbA1c. Although not necessarily a limitation, 140 of the 247 participants (57%) were enrolled in MOVE! as well. MOVE! is a widely popular weight management program in the VA focusing on diet control.18 The authors, however, have no information about the veterans’ adherence to MOVE!
Five immediate next steps to disseminate the program have been identified (Table 2).
Conclusion
The GWC was successfully developed and implemented as a QI project at CAVHS and was met with much satisfaction by older veterans. Participants experienced clinically significant improvements in physical performance and other health indicators, suggesting that these benefits could potentially offset clinic costs.
1. Jefferis BJ, Sartini C, Ash S, et al. Trajectories of objectively measured physical activity in free-living older men. Med Sci Sports Exerc. 2015;47(2):343-349.
2. Sun F, Norman IJ, While AE. Physical activity in older people: a systematic review. BMC Public Health. 2013;13:449.
3. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188.
4. Vogel T, Brechat PH, Lepretre PM, Kaltenbach G, Berthel M, Lonsdorfer J. Health benefits of physical activity in older patients: a review. Int J Clin Pract. 2009;63(2):303-320.
5. Patterson PD, Moore CG, Probst JC, Shinogle JA. Obesity and physical inactivity in rural America. J Rural Health. 2004;20(2):151-159.
6. Dubbert PM, Cooper KM, Kirchner KA, Meydrech EF, Bilbrew D. Effects of nurse counseling on walking for exercise in elderly primary care patients. J Gerontol A Biol Sci Med Sci. 2002;57(11):M733-M740.
7. Dubbert PM, Morey MC, Kirchner KA, Meydrech EF, Grothe K. Counseling for home-based walking and strength exercise in older primary care patients. Arch Intern Med. 2008;168(9):979-986.
8. Newton RL Jr, HH M, Dubbert PM, et al. Pedometer determined physical activity tracks in African American adults: the Jackson Heart Study. Int J Behav Nutr Phys Act. 2012;9:44.
9. Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19.
10. Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919-923.
11. Hiengkaew V, Jitaree K, Chaiyawat P. Minimal detectable changes of the Berg Balance Scale, Fugl-Meyer Assessment Scale, Timed “Up & Go” Test, gait speeds, and 2-minute walk test in individuals with chronic stroke with different degrees of ankle plantarflexor tone. Arch Phys Med Rehabil. 2012;93(7):1201-1208.
12. Muir SW, Berg K, Chesworth B, Speechley M. Use of the Berg Balance Scale for predicting multiple falls in community-dwelling elderly people: a prospective study. Phys Ther. 2008;88(4):449-459.
13. Clark DO, Nothwehr F. Exercise self-efficacy and its correlates among socioeconomically disadvantaged older adults. Health Educ Behav. 1999;26(4):535-546.
14. Stewart AL, Verboncoeur CJ, McLellan BY, et al. Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001;56(8):M465-M470.
15. Purser JL, Weinberger M, Cohen HJ, et al. Walking speed predicts health status and hospital costs for frail elderly male Veterans. J Rehabil Res Dev. 2005;42(4):535-546.
16. Wagner EH, Sandhu N, Newton KM, McCulloch DK, Ramsey SD, Grothaus LC. Effect of improved glycemic control on health care costs and utilization. JAMA. 2001;285(2):182-189.
17. Yu AP, Wu EQ, Birnbaum HG, et al. Short-term economic impact of body weight change among patients with type 2 diabetes treated with antidiabetic agents: analysis using claims, laboratory, and medical record data. Curr Med Res Opin. 2007;23(9):2157-2169.
18. Romanova M, Liang LJ, Deng ML, Li Z, Heber D. Effectiveness of the MOVE! multidisciplinary weight loss program for veterans in Los Angeles. Prev Chronic Dis. 2013;10:E112.
Inactivity and increased sedentary time are major public health problems, particularly among older adults.1-3 Inactivity increases with age and produces deleterious effects on physical health, mental health, and quality of life and leads to increased health care costs.4 The high prevalence of a sedentary lifestyle among older veterans may be due to multiple factors, including misconceptions about the health benefits of exercise, lack of motivation, or associating exercise with discomfort or pain. Older veterans living in rural areas are at high risk because they are more sedentary than are urban-dwelling veterans.5 Of veterans aged ≥ 65 years who use health care services in VISN 16, 59% live in rural or highly rural areas.
Given the large number of older veterans and their at-risk status, addressing inactivity among this population is critical. Until recently, few programs existed within the VHA that addressed this need. Despite strong evidence that physical activity helps maintain functional independence and avoids institutionalization of frail elderly veterans, the VHA had no established procedures or guidelines for assessment and counseling.
To address this void, a Geriatric Walking Clinic (GWC) was established at the Central Arkansas Veterans Healthcare System (CAVHS) in March 2013. The GWC developed a patient-centric, home-based program that implements a comprehensive approach to assess, educate, motivate, and activate older veterans to commit to, engage in, and adhere to, a long-term program of regular physical activity primarily in the form of walking. The program uses proven strategies, such as motivational counseling, follow-up phone calls from a nurse, and self-monitoring using pedometers.6-8 Funding for the GWC project was provided by the VHA Office of Geriatrics and Extended Care as part of its Transition to the 21st Century (T21) initiative and by the VHA Office of Rural Health.
Methods
Quality improvement (QI) principles were used to develop the program, which received a nonresearch determination status from the local institutional review board. The GWC is staffed by a registered nurse, health technician, and physician. Both the nurse and the health technician were trained on the use of various assessments. Several tactics were developed to promote patient recruitment to the GWC, including systemwide in-services, an easy-to-use consultation request within the electronic medical record, patient and provider brochures, and informational booths and kiosks. Collaborations were developed with various clinical services to promote referrals. Several other services, such as Primary Care, Geriatrics, the Move! Weight Management Program, Cardiology (including Congestive Heart Failure), Hematology/Oncology, and Mental Health referred patients to the GWC.
The GWC targets sedentary, community-dwelling veterans aged ≥ 60 years who are able to ambulate in their home without an assistive device, are willing to walk for exercise, and are willing to receive phone calls. All-comers are included in the program. Although most of these veterans have multiple chronic medical problems, only those with absolute contraindications to exercise per the American College of Sports Medicine guidelines and those with any medical condition that is likely to compromise their ability to safely participate in the walking program are excluded (Figure).
First Visit
At the first visit, veterans receive a brief education about GWC, highlighting its potential health benefits. If veterans want to join, they are evaluated using a 3-tier screening assessment to determine the safety of starting a new walking regimen. Veterans who fail the 3-tier safety screening are referred to their primary care physician (PCP) for further assessment (eg, cardiac stress testing) to better define eligibility status. Veterans who pass the screen complete brief tests of physical performance, including gait speed, 6-Minute Walk Test (6MWT), Timed Up and Go test, and Berg Balance Scale.9-12 Participants also complete short surveys that provide useful information about their social support, barriers to exercise, response to physical activity, and usual activity level as measured by Community Healthy Activities Model Program for Seniors.13,14 This information is used to help develop an individualized walking prescription.
After completing the baseline assessments, the GWC staff members help the veteran set realistic goals, using motivational counseling techniques. The veteran receives a walking prescription to walk indoors or outdoors, based on current physical condition, self-identified goals, perceived barriers, and strength of support system; educational material about safe walking; a log for recording daily step count; information on follow-up calls; and an invitation to return for follow-up visits. The veteran also receives a pedometer and is instructed to continue his or her usual routine for the first week. The average daily step count is recorded as the baseline. The veteran is instructed to start the walking program after the baseline week with goals tailored to the personal activity level. For example: Some patients are asked to simply add an extra minute to their walking, whereas others savvy with pedometer numbers are asked to increase their step count.
Follow-up
Veterans are followed closely between their clinic appointments via phone calls from a nurse who provides encouragement and helps set new goals. The nurse collects the step count data to determine progress and set new walking goals. Those unable to adhere to their walking prescription are reassessed for their barriers. The nurse also helps participants identify ways to overcome individual challenges. The PCP is consulted when barriers include medical problems, such as pain or poor blood sugar control.
At the 6-week follow-up visit, the health care provider reviews the pedometer log and repeats all outcome assessments, including the physical performance testing and the participant surveys. Veterans receive feedback from these outcome assessments. To assess participant satisfaction, CAVHS GRECC developed a satisfaction questionnaire, which was given to participants.
Results
A total of 249 older veterans participated in the GWC program. The mean age was 67 (±6) years; 92% were male, 60% were white, and 39% were African American. Most participants lived in a rural location (60%) and were obese (69%); consistent with national standards, obesity was defined as a body mass index (BMI) ≥ 30 kg/m2. Several barriers to exercise were endorsed by the veterans. Most commonly endorsed barriers included bad weather, lack of motivation, feeling tired, and fear of pain. Most participants (93%) were actively engaged via regular phone follow-ups visits; 121 (49%) participants returned to the clinic for the 6-week reassessment. Repeat performance testing at the 6-week visit showed a clinically significant average 14% improvement in the 6MWT, 6% improvement in the Timed Up and Go test, and a 27% improvement in gait speed. Of those veterans who were obese, 64% lost weight. On entry into the program, 32 participants (13%) had poorly controlled diabetes mellitus (DM), defined as hemoglobin A1c (HbA1c) ≥ 8. Among this group, HbA1c improved by an average of 1.5% by the 6-week visit. The GWC program may have contributed to the improved glycemic control as a generally accepted frequency of monitoring HbA1c is at least 3 months.
At the 6-week clinic visit, 94% of those surveyed completed a program evaluation. The GWC scored high on satisfaction; over 80% strongly agreed that they were satisfied with the GWC program as a whole, 80% strongly agreed that the program increased their awareness about need for exercise, 82% strongly agreed that the clinician’s advice was applicable to them, and 77% strongly agreed that the program improved their motivation to walk regularly (Table 1).
Program Economics
An analysis of the clinic costs and benefits was performed to determine whether costs could potentially be offset by the savings realized from improved health outcomes of participating veterans. For this simplified analysis, costs of maintaining the GWC were set equal to the costs of the full-time equivalent employee hours, equipment, and educational materials. Based on the authors’ experience, they projected that for each 1,000 older veterans enrolled in the GWC, there is a requirement for 0.5 medical support assistant (GS-6 pay scale), 1.0 registered nurse grade 2 (RN), 1.0 health science specialist (GS-7), and 0.25 physician. At the host facility, the annual personnel costs are estimated at $205,149. The total annual cost of the GWC, including the equipment and educational materials, is estimated at $240,149.
Although full financial return on investment has yet to be determined, the authors estimated potential cost savings resulting if patients enrolled in a GWC achieved and maintained the types of improvements observed in the first cohort of patients. These estimates were based on identified improvements in 3 patient outcome measures cited in the medical literature that are associated with reductions in subsequent health care costs. These measures include gait speed, weight loss, and HbA1c. It is estimated that the cost savings associated with improvement of gait speed by 0.1 m/s (a clinically relevant change) is $1,200 annually.15
On average, patients enrolled in the GWC program improved their gait speed by 0.22 m/s. Cost savings related to gait speed improvement for 1,000 participants could reach $1,200,000. Conservative estimates of cost savings per 1% reduction of HbA1c is $950/year.16 Among those with poorly controlled DM (ie, HbA1c of ≥ 8), average HbA1c declined by 1.5%. Provided that 13% of the patients have poorly controlled DM, the total cost saving for 1,000 participants could be $209,950 annually.
It also is estimated that a 1% weight loss in obese patients is associated with a $256 decrease in subsequent total health care costs.17 In the GWC, the obese participants lost an average of 1.3% of their baseline weight. Assuming that about 60% of all older veterans participating in the clinic program are obese, annual cost savings per 1,000 participants related to weight loss is estimated to be $199,680. After accounting for the costs of operating the clinic, the total cost savings for a GWC with 1,000 enrolled older veterans is estimated to be as much as $1.4 million annually. Such a favorable cost assessment suggests that the program should be evaluated for widespread dissemination throughout the entire VHA system. Other potential benefits associated with GWC participation, such as improved quality of life and greater functional independence, may be of even greater importance to veterans.
Limitations
Results of this QI project need to be considered in light of its limitations. One of the most important limitations is the design of the project. Since this was a clinical initiative and not a research study, there was no control group or randomization. There were also limitations on data availability. HbA1c tests were not ordered as part of this QI project. Instead, baseline HbA1c was set equal to the most recent of any value obtained clinically within 2 months before the participant’s GWC enrollment; the 6-week follow-up value was set to any HbA1c obtained within 2 months after the 6-week visit. It is also recognized that factors other than the veterans’ participation in the GWC (eg, alterations in their DM medication regimen) may have contributed to the changes noted in some participant’s HbA1c. Although not necessarily a limitation, 140 of the 247 participants (57%) were enrolled in MOVE! as well. MOVE! is a widely popular weight management program in the VA focusing on diet control.18 The authors, however, have no information about the veterans’ adherence to MOVE!
Five immediate next steps to disseminate the program have been identified (Table 2).
Conclusion
The GWC was successfully developed and implemented as a QI project at CAVHS and was met with much satisfaction by older veterans. Participants experienced clinically significant improvements in physical performance and other health indicators, suggesting that these benefits could potentially offset clinic costs.
Inactivity and increased sedentary time are major public health problems, particularly among older adults.1-3 Inactivity increases with age and produces deleterious effects on physical health, mental health, and quality of life and leads to increased health care costs.4 The high prevalence of a sedentary lifestyle among older veterans may be due to multiple factors, including misconceptions about the health benefits of exercise, lack of motivation, or associating exercise with discomfort or pain. Older veterans living in rural areas are at high risk because they are more sedentary than are urban-dwelling veterans.5 Of veterans aged ≥ 65 years who use health care services in VISN 16, 59% live in rural or highly rural areas.
Given the large number of older veterans and their at-risk status, addressing inactivity among this population is critical. Until recently, few programs existed within the VHA that addressed this need. Despite strong evidence that physical activity helps maintain functional independence and avoids institutionalization of frail elderly veterans, the VHA had no established procedures or guidelines for assessment and counseling.
To address this void, a Geriatric Walking Clinic (GWC) was established at the Central Arkansas Veterans Healthcare System (CAVHS) in March 2013. The GWC developed a patient-centric, home-based program that implements a comprehensive approach to assess, educate, motivate, and activate older veterans to commit to, engage in, and adhere to, a long-term program of regular physical activity primarily in the form of walking. The program uses proven strategies, such as motivational counseling, follow-up phone calls from a nurse, and self-monitoring using pedometers.6-8 Funding for the GWC project was provided by the VHA Office of Geriatrics and Extended Care as part of its Transition to the 21st Century (T21) initiative and by the VHA Office of Rural Health.
Methods
Quality improvement (QI) principles were used to develop the program, which received a nonresearch determination status from the local institutional review board. The GWC is staffed by a registered nurse, health technician, and physician. Both the nurse and the health technician were trained on the use of various assessments. Several tactics were developed to promote patient recruitment to the GWC, including systemwide in-services, an easy-to-use consultation request within the electronic medical record, patient and provider brochures, and informational booths and kiosks. Collaborations were developed with various clinical services to promote referrals. Several other services, such as Primary Care, Geriatrics, the Move! Weight Management Program, Cardiology (including Congestive Heart Failure), Hematology/Oncology, and Mental Health referred patients to the GWC.
The GWC targets sedentary, community-dwelling veterans aged ≥ 60 years who are able to ambulate in their home without an assistive device, are willing to walk for exercise, and are willing to receive phone calls. All-comers are included in the program. Although most of these veterans have multiple chronic medical problems, only those with absolute contraindications to exercise per the American College of Sports Medicine guidelines and those with any medical condition that is likely to compromise their ability to safely participate in the walking program are excluded (Figure).
First Visit
At the first visit, veterans receive a brief education about GWC, highlighting its potential health benefits. If veterans want to join, they are evaluated using a 3-tier screening assessment to determine the safety of starting a new walking regimen. Veterans who fail the 3-tier safety screening are referred to their primary care physician (PCP) for further assessment (eg, cardiac stress testing) to better define eligibility status. Veterans who pass the screen complete brief tests of physical performance, including gait speed, 6-Minute Walk Test (6MWT), Timed Up and Go test, and Berg Balance Scale.9-12 Participants also complete short surveys that provide useful information about their social support, barriers to exercise, response to physical activity, and usual activity level as measured by Community Healthy Activities Model Program for Seniors.13,14 This information is used to help develop an individualized walking prescription.
After completing the baseline assessments, the GWC staff members help the veteran set realistic goals, using motivational counseling techniques. The veteran receives a walking prescription to walk indoors or outdoors, based on current physical condition, self-identified goals, perceived barriers, and strength of support system; educational material about safe walking; a log for recording daily step count; information on follow-up calls; and an invitation to return for follow-up visits. The veteran also receives a pedometer and is instructed to continue his or her usual routine for the first week. The average daily step count is recorded as the baseline. The veteran is instructed to start the walking program after the baseline week with goals tailored to the personal activity level. For example: Some patients are asked to simply add an extra minute to their walking, whereas others savvy with pedometer numbers are asked to increase their step count.
Follow-up
Veterans are followed closely between their clinic appointments via phone calls from a nurse who provides encouragement and helps set new goals. The nurse collects the step count data to determine progress and set new walking goals. Those unable to adhere to their walking prescription are reassessed for their barriers. The nurse also helps participants identify ways to overcome individual challenges. The PCP is consulted when barriers include medical problems, such as pain or poor blood sugar control.
At the 6-week follow-up visit, the health care provider reviews the pedometer log and repeats all outcome assessments, including the physical performance testing and the participant surveys. Veterans receive feedback from these outcome assessments. To assess participant satisfaction, CAVHS GRECC developed a satisfaction questionnaire, which was given to participants.
Results
A total of 249 older veterans participated in the GWC program. The mean age was 67 (±6) years; 92% were male, 60% were white, and 39% were African American. Most participants lived in a rural location (60%) and were obese (69%); consistent with national standards, obesity was defined as a body mass index (BMI) ≥ 30 kg/m2. Several barriers to exercise were endorsed by the veterans. Most commonly endorsed barriers included bad weather, lack of motivation, feeling tired, and fear of pain. Most participants (93%) were actively engaged via regular phone follow-ups visits; 121 (49%) participants returned to the clinic for the 6-week reassessment. Repeat performance testing at the 6-week visit showed a clinically significant average 14% improvement in the 6MWT, 6% improvement in the Timed Up and Go test, and a 27% improvement in gait speed. Of those veterans who were obese, 64% lost weight. On entry into the program, 32 participants (13%) had poorly controlled diabetes mellitus (DM), defined as hemoglobin A1c (HbA1c) ≥ 8. Among this group, HbA1c improved by an average of 1.5% by the 6-week visit. The GWC program may have contributed to the improved glycemic control as a generally accepted frequency of monitoring HbA1c is at least 3 months.
At the 6-week clinic visit, 94% of those surveyed completed a program evaluation. The GWC scored high on satisfaction; over 80% strongly agreed that they were satisfied with the GWC program as a whole, 80% strongly agreed that the program increased their awareness about need for exercise, 82% strongly agreed that the clinician’s advice was applicable to them, and 77% strongly agreed that the program improved their motivation to walk regularly (Table 1).
Program Economics
An analysis of the clinic costs and benefits was performed to determine whether costs could potentially be offset by the savings realized from improved health outcomes of participating veterans. For this simplified analysis, costs of maintaining the GWC were set equal to the costs of the full-time equivalent employee hours, equipment, and educational materials. Based on the authors’ experience, they projected that for each 1,000 older veterans enrolled in the GWC, there is a requirement for 0.5 medical support assistant (GS-6 pay scale), 1.0 registered nurse grade 2 (RN), 1.0 health science specialist (GS-7), and 0.25 physician. At the host facility, the annual personnel costs are estimated at $205,149. The total annual cost of the GWC, including the equipment and educational materials, is estimated at $240,149.
Although full financial return on investment has yet to be determined, the authors estimated potential cost savings resulting if patients enrolled in a GWC achieved and maintained the types of improvements observed in the first cohort of patients. These estimates were based on identified improvements in 3 patient outcome measures cited in the medical literature that are associated with reductions in subsequent health care costs. These measures include gait speed, weight loss, and HbA1c. It is estimated that the cost savings associated with improvement of gait speed by 0.1 m/s (a clinically relevant change) is $1,200 annually.15
On average, patients enrolled in the GWC program improved their gait speed by 0.22 m/s. Cost savings related to gait speed improvement for 1,000 participants could reach $1,200,000. Conservative estimates of cost savings per 1% reduction of HbA1c is $950/year.16 Among those with poorly controlled DM (ie, HbA1c of ≥ 8), average HbA1c declined by 1.5%. Provided that 13% of the patients have poorly controlled DM, the total cost saving for 1,000 participants could be $209,950 annually.
It also is estimated that a 1% weight loss in obese patients is associated with a $256 decrease in subsequent total health care costs.17 In the GWC, the obese participants lost an average of 1.3% of their baseline weight. Assuming that about 60% of all older veterans participating in the clinic program are obese, annual cost savings per 1,000 participants related to weight loss is estimated to be $199,680. After accounting for the costs of operating the clinic, the total cost savings for a GWC with 1,000 enrolled older veterans is estimated to be as much as $1.4 million annually. Such a favorable cost assessment suggests that the program should be evaluated for widespread dissemination throughout the entire VHA system. Other potential benefits associated with GWC participation, such as improved quality of life and greater functional independence, may be of even greater importance to veterans.
Limitations
Results of this QI project need to be considered in light of its limitations. One of the most important limitations is the design of the project. Since this was a clinical initiative and not a research study, there was no control group or randomization. There were also limitations on data availability. HbA1c tests were not ordered as part of this QI project. Instead, baseline HbA1c was set equal to the most recent of any value obtained clinically within 2 months before the participant’s GWC enrollment; the 6-week follow-up value was set to any HbA1c obtained within 2 months after the 6-week visit. It is also recognized that factors other than the veterans’ participation in the GWC (eg, alterations in their DM medication regimen) may have contributed to the changes noted in some participant’s HbA1c. Although not necessarily a limitation, 140 of the 247 participants (57%) were enrolled in MOVE! as well. MOVE! is a widely popular weight management program in the VA focusing on diet control.18 The authors, however, have no information about the veterans’ adherence to MOVE!
Five immediate next steps to disseminate the program have been identified (Table 2).
Conclusion
The GWC was successfully developed and implemented as a QI project at CAVHS and was met with much satisfaction by older veterans. Participants experienced clinically significant improvements in physical performance and other health indicators, suggesting that these benefits could potentially offset clinic costs.
1. Jefferis BJ, Sartini C, Ash S, et al. Trajectories of objectively measured physical activity in free-living older men. Med Sci Sports Exerc. 2015;47(2):343-349.
2. Sun F, Norman IJ, While AE. Physical activity in older people: a systematic review. BMC Public Health. 2013;13:449.
3. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188.
4. Vogel T, Brechat PH, Lepretre PM, Kaltenbach G, Berthel M, Lonsdorfer J. Health benefits of physical activity in older patients: a review. Int J Clin Pract. 2009;63(2):303-320.
5. Patterson PD, Moore CG, Probst JC, Shinogle JA. Obesity and physical inactivity in rural America. J Rural Health. 2004;20(2):151-159.
6. Dubbert PM, Cooper KM, Kirchner KA, Meydrech EF, Bilbrew D. Effects of nurse counseling on walking for exercise in elderly primary care patients. J Gerontol A Biol Sci Med Sci. 2002;57(11):M733-M740.
7. Dubbert PM, Morey MC, Kirchner KA, Meydrech EF, Grothe K. Counseling for home-based walking and strength exercise in older primary care patients. Arch Intern Med. 2008;168(9):979-986.
8. Newton RL Jr, HH M, Dubbert PM, et al. Pedometer determined physical activity tracks in African American adults: the Jackson Heart Study. Int J Behav Nutr Phys Act. 2012;9:44.
9. Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19.
10. Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919-923.
11. Hiengkaew V, Jitaree K, Chaiyawat P. Minimal detectable changes of the Berg Balance Scale, Fugl-Meyer Assessment Scale, Timed “Up & Go” Test, gait speeds, and 2-minute walk test in individuals with chronic stroke with different degrees of ankle plantarflexor tone. Arch Phys Med Rehabil. 2012;93(7):1201-1208.
12. Muir SW, Berg K, Chesworth B, Speechley M. Use of the Berg Balance Scale for predicting multiple falls in community-dwelling elderly people: a prospective study. Phys Ther. 2008;88(4):449-459.
13. Clark DO, Nothwehr F. Exercise self-efficacy and its correlates among socioeconomically disadvantaged older adults. Health Educ Behav. 1999;26(4):535-546.
14. Stewart AL, Verboncoeur CJ, McLellan BY, et al. Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001;56(8):M465-M470.
15. Purser JL, Weinberger M, Cohen HJ, et al. Walking speed predicts health status and hospital costs for frail elderly male Veterans. J Rehabil Res Dev. 2005;42(4):535-546.
16. Wagner EH, Sandhu N, Newton KM, McCulloch DK, Ramsey SD, Grothaus LC. Effect of improved glycemic control on health care costs and utilization. JAMA. 2001;285(2):182-189.
17. Yu AP, Wu EQ, Birnbaum HG, et al. Short-term economic impact of body weight change among patients with type 2 diabetes treated with antidiabetic agents: analysis using claims, laboratory, and medical record data. Curr Med Res Opin. 2007;23(9):2157-2169.
18. Romanova M, Liang LJ, Deng ML, Li Z, Heber D. Effectiveness of the MOVE! multidisciplinary weight loss program for veterans in Los Angeles. Prev Chronic Dis. 2013;10:E112.
1. Jefferis BJ, Sartini C, Ash S, et al. Trajectories of objectively measured physical activity in free-living older men. Med Sci Sports Exerc. 2015;47(2):343-349.
2. Sun F, Norman IJ, While AE. Physical activity in older people: a systematic review. BMC Public Health. 2013;13:449.
3. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181-188.
4. Vogel T, Brechat PH, Lepretre PM, Kaltenbach G, Berthel M, Lonsdorfer J. Health benefits of physical activity in older patients: a review. Int J Clin Pract. 2009;63(2):303-320.
5. Patterson PD, Moore CG, Probst JC, Shinogle JA. Obesity and physical inactivity in rural America. J Rural Health. 2004;20(2):151-159.
6. Dubbert PM, Cooper KM, Kirchner KA, Meydrech EF, Bilbrew D. Effects of nurse counseling on walking for exercise in elderly primary care patients. J Gerontol A Biol Sci Med Sci. 2002;57(11):M733-M740.
7. Dubbert PM, Morey MC, Kirchner KA, Meydrech EF, Grothe K. Counseling for home-based walking and strength exercise in older primary care patients. Arch Intern Med. 2008;168(9):979-986.
8. Newton RL Jr, HH M, Dubbert PM, et al. Pedometer determined physical activity tracks in African American adults: the Jackson Heart Study. Int J Behav Nutr Phys Act. 2012;9:44.
9. Bohannon RW. Comfortable and maximum walking speed of adults aged 20-79 years: reference values and determinants. Age Ageing. 1997;26(1):15-19.
10. Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919-923.
11. Hiengkaew V, Jitaree K, Chaiyawat P. Minimal detectable changes of the Berg Balance Scale, Fugl-Meyer Assessment Scale, Timed “Up & Go” Test, gait speeds, and 2-minute walk test in individuals with chronic stroke with different degrees of ankle plantarflexor tone. Arch Phys Med Rehabil. 2012;93(7):1201-1208.
12. Muir SW, Berg K, Chesworth B, Speechley M. Use of the Berg Balance Scale for predicting multiple falls in community-dwelling elderly people: a prospective study. Phys Ther. 2008;88(4):449-459.
13. Clark DO, Nothwehr F. Exercise self-efficacy and its correlates among socioeconomically disadvantaged older adults. Health Educ Behav. 1999;26(4):535-546.
14. Stewart AL, Verboncoeur CJ, McLellan BY, et al. Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001;56(8):M465-M470.
15. Purser JL, Weinberger M, Cohen HJ, et al. Walking speed predicts health status and hospital costs for frail elderly male Veterans. J Rehabil Res Dev. 2005;42(4):535-546.
16. Wagner EH, Sandhu N, Newton KM, McCulloch DK, Ramsey SD, Grothaus LC. Effect of improved glycemic control on health care costs and utilization. JAMA. 2001;285(2):182-189.
17. Yu AP, Wu EQ, Birnbaum HG, et al. Short-term economic impact of body weight change among patients with type 2 diabetes treated with antidiabetic agents: analysis using claims, laboratory, and medical record data. Curr Med Res Opin. 2007;23(9):2157-2169.
18. Romanova M, Liang LJ, Deng ML, Li Z, Heber D. Effectiveness of the MOVE! multidisciplinary weight loss program for veterans in Los Angeles. Prev Chronic Dis. 2013;10:E112.
The Unique Value of Externships to Nursing Education and Health Care Organizations
New nurse graduates often have difficulty transitioning to the role of registered nurse (RN).1 Given the complexity of the health care environment, the need is growing to prepare nursing students for nursing practice. Although nursing education provides students with a basis for practice, school alone cannot prepare them for actual practice in the hospital setting.2 Compared with nurse residency programs, which provide extended postlicensure training, the national Veterans Affairs Learning Opportunity Residency (VALOR) program provides externships independent of nursing school. Externships allow students to train in a hospital setting (generally during the summer months) before becoming a licensed RN. Nursing students who are entering their senior year of coursework in a bachelor of science nursing program and who have a minimum 3.0 grade point average can apply for this competitive national scholarship offered at VAMCs. The VALOR program is a paid learning opportunity, and students gain hands-on clinical experience under the guidance of preceptors.
Little externship research exists in the nursing literature.3,4 The authors conducted the present study to help fill the gaps in the literature and to add to the only other study findings on VALOR.3 This program, started in 1990 to aid in nursing recruitment and retention, offers students early exposure to the complexities of nursing practice.
The authors investigated RNs’ experience in the VALOR prelicensure externship during the nurses’ senior year of coursework and the impact of this experience on their nursing practice. The program offers 800 hours of hospital-based experience outside the classroom. New nurses who gained only limited clinical exposure in nursing school may feel insecure about their clinical skills.5 Casey and colleagues found that students want more clinical experience than offered by nursing school practicums.6 The VALOR participants obtain additional clinical time, which contributes to their self-confidence when transitioning to the RN role.7
Literature Review
New graduate nurses work in complex health care environments with unfamiliar technologies, shift hours, heavy patient loads, psychological and professional stressors, socialization problems, and patient safety issues.8 They often are unable to connect their educational experience with the realities of practice and find the work environment incongruent with their nursing school education.9 Although new nurses’ difficulty in transitioning to their professional role has been addressed in the literature, transitional experience has not improved.10 Studies have found that new graduate nurses want more support than is given and have suggested that unfamiliar workplace dynamics create stress for new nurses.11
Anxiety, insecurity, and fear of failure are associated with the transition from student to practicing nurse.10 Because of the additional clinical experience gained in an externship, students likely are more self-confident when they assume the RN role.12 White suggested self-confident students see themselves as nurses and feel capable of caring for patients.13 Externship experience makes the transition to professional nursing less stressful, because externship students obtain an inside view of nursing culture.14 Students increase their understanding of nurses’ multiple roles and responsibilities, because these programs focus on increasing clinical skills and competency.15 To perform successfully as RNs, new graduates need competencies and knowledge beyond those obtained in nursing school.16
In the nursing profession, an association between job satisfaction and turnover exists.17,18 Of new graduate nurses, 35% to 69% leave their position within the first year of employment.19 Replacing nurses reduces hospital productivity and efficiency and increases cost.20 New graduate nurses leave because they are dissatisfied with and overwhelmed by the complexity of the work environment.21 Prelicensure nurse externships can aid in recruiting and retaining new graduate nurses for the hospitals that host these programs.22 For host facilities, recruitment rates of 50% to 79% have been reported.23,24
In a quantitative study, Nuttall surveyed 133 RNs about job satisfaction, role socialization, professionalism, and sense of belonging.3 Of these RNs, 34 had participated in VALOR and 99 had not. There was no evidence that the RNs with VALOR experience had a higher degree of professionalism, job satisfaction, or role socialization; only sense of belonging (age-adjusted) was higher for the VALOR group. The conflicting data on prelicensure externship outcomes call for further analysis of these programs.3 Nuttall noted that her study “was the first... to evaluate the VALOR program and future research [using a qualitative approach] is needed to identify additional outcomes related to this program.”3
Methods
This study using hermeneutic phenomenology was approved by the Salem VAMC in Virginia and by the institutional review board at Nova Southeastern University.24 Study participants provided written informed consent before being interviewed.
Interviewees
Data Collection and Analysis
Data collection began in March 2013 with a pilot test of the interview questions for appropriateness. Open-ended, semistructured questions were used to elicit nurses’ descriptions of their experience. Field notes were written, and all interviews were tape-recorded and professionally transcribed verbatim. Data saturation was reached after 12 nurses were interviewed. Transcripts were analyzed and interpreted using van Manen’s line-by-line approach.26 All 12 interviewees were invited to review the findings of the data analysis. Eleven of the 12 interviewees verified and validated the study findings.
Rigor using Lincoln and Guba’s criteria of credibility, dependability, transferability, and confirmability added trustworthiness to the study findings. Bracketing helped eliminate potential bias.27 Credibility was achieved with prolonged engagement and triangulation. To further enhance credibility, the authors invited qualitative research experts to validate the emerging themes and create an audit trail. For dependability, a flow chart was created for use by researchers who might want to replicate the study. Rich, lengthy descriptions and interviewees’ quotations were provided so researchers could judge the study’s transferability to other settings. Maintaining an audit trail and having a doctor of nursing practice independently code the data aided with confirmability. This study used findings from the literature, audio recordings, member checking, and field notes to assess data accuracy.
Results
The overarching theme discovered in this study was confidence. Subthemes were transitioning to the RN role, making decisions, and interacting with interprofessional staff.
Confidence
Interviewees felt confident in transitioning to the RN role, making decisions, and interacting with interprofessional staff. They shared that they had applied to the externship to gain additional clinical experience and that the program increased their self-confidence with respect to transitioning to the new role as RN. However, it is possible that these interviewees were highly motivated and would not have had difficulty transitioning to the RN role—this is addressed in the Limitations section of this article.
Interviewees said they initially approached VALOR with fear and apprehension but completed it feeling confident about becoming an RN. One interviewee stated, “The VALOR program gave me the confidence in my own abilities, so I was not scared and had confidence that, yes, I could do this job.” Another said, “Honestly, the entire externship program, regardless of which question you ask, my answer is going to always fall back on confidence. I became prepared for the RN job, I feel, before I graduated because of the [externship] experiences.”
Transitioning to RN Role
Transitioning involved understanding the RN’s scope of practice and feeling clinically competent. Students worked 40 hours a week over the summer and gained firsthand insight into working as an RN daily. Interviewees believed completing the externship made it easy to transition to the RN role because they knew what to expect.
Working side-by-side with nurses, students gained insight into RNs’ responsibilities and scope of practice. Interviewees reported that, after the externship, they had a better understanding of their patient care and licensure legal responsibilities.
Students began to feel clinically competent during the externship. Interviewees shared that they had had several opportunities to practice basic skills, such as giving injections. One interviewee said, “I don’t think I tried to stick a single IV when I was in school or in clinical [training].” Interviewees also commented that repeatedly practicing skills increased their self-confidence.
Students also gained firsthand insight into working with veterans and their families. During the externship, they learned about communicating therapeutically, providing education for caregivers, and advocating for patients and their families. Before the externship, they felt apprehensive about communicating with patients. One interviewee said that after the program, “Eventually you had to talk to patients, and eventually figured it out that it wasn’t so scary.”
Students found that patients were not always happy with their care, and procedures did not always go as planned. They also discovered that education did not end with the patient; family members needed education as well. The externship experience heightened students’ awareness of the RN’s role as patient advocate. One interviewee explained it is the RN’s responsibility to intervene on the patient’s behalf. Interviewees were surprised that patients would not tell their health care provider that they did not understand what was said or that they wanted another course of treatment.
The externship helped decrease learning-related stress. Interviewees indicated they had learned without fear of reprisal. One described feeling free to learn: “Uninhibited learning...you can ask what you need to without fear of not graduating.” Externship students were able to focus on learning the RN role without worrying about the next test or grade. They felt free to ask questions without fear of failing their clinical rotation.
The supportive and nurturing relationships that students developed in VALOR also increased their confidence when transitioning to the RN role. One interviewee said, “There was never the sense of, no, you learn my way, or I don’t want you here.” Interviewees shared that they felt comfortable and supported.
Decision Making
Interviewees reported that after VALOR, it was easy to make decisions regarding nursing practice, delegation, care prioritization, and career choice. As students, they found the school clinical setting did not provide the decision-making opportunities VALOR did, and they quickly realized nursing practice involved more than making patient-care decisions. One interviewee said, “In a classroom, a picture is painted of an idealistic environment that may not truly mimic the hospital unit.”
Students became familiar with the practice of delegating care to the appropriate staff and the next shift. One interviewee said VALOR “provided me with a better understanding of delegation in my RN role.” VALOR participants discovered that, as new nurses, they were less anxious when delegating to others.
Before RN licensure, VALOR participants learned about prioritizing patient care. One interviewee said, “It’s like everybody has to be charted on, and all the medications have to be passed out, but it’s a matter of getting everything done while doing the more important and more dire things first.” Students learned that all aspects of nursing are important, but they had to make rational decisions.
Interacting With Professionals
Interviewees who had been in VALOR said interacting with interprofessional (different disciplines) staff contributed to their working comfortably in teams and collaborating with others. Their collaborative relationships with physicians would help them later, when as new graduate nurses they again needed to work together with doctors. Typical comments were, “When I started as an RN, I felt I was not new at it because I had communicated with doctors in the externship program.”
Discussion
The present study found that nursing students who had been in the VALOR externship felt confident in their clinical skills when they were transitioning to the RN role. Other studies similarly have found that externship students were self-confident assuming the RN role, owing to their additional clinical experience.12,28 The VALOR program allows students to work alongside nurses and receive hands-on experience while interacting with interprofessional health care teams. Findings of Nuttall’s quantitative study contradict those of the present, qualitative study. Nuttall used surveys and a control group, whereas this phenomenologic study captured the essence of study participants’ experiences through interviews.
The RNs interviewed in the present study discovered that, unlike nursing school, VALOR provided a realistic view of full-time work as an RN. This finding aligns with Starr and Conley’s finding that, before participating in an externship, most students were unaware of the extent of RNs’ roles and responsibilities, whereas after the program they understood these roles and responsibilities better.28
The interviewees in this study thought VALOR improved their skills in communicating with patients, families, and interprofessional team members. Interviewees shared that they learned patient advocacy skills and that, through firsthand experience, realized nurses provide patients with a voice. Externships can help new graduate nurses become better communicators and can teach students the importance of patient communication and advocacy.12
This study also found that students wanted more exposure to realistic nursing environments, additional nursing skills practice, and more interaction with interprofessional team members. VALOR helped bridge the theory–practice gap by providing real-world nursing experience outside the academic environment and extra time for nursing skills development. In a study by Casey and colleagues, students indicated that the time allowed for nursing skills practice during school was inadequate.6
The VALOR program helped students learn about delegating work, whereas nursing school did not provide the opportunity to practice delegation. Other studies have corroborated that students do not practice delegation during nursing school clinical time.29,30
Study respondents noted they could focus on learning without the fear of passing their clinical rotation. They felt supported by staff and were comfortable asking questions. White suggested that externship students who feel supported by nursing staff are able to focus on patients instead of on their discomfort.13 Rush and colleagues found that constraints on the student experience in traditional academic clinical rotations were replaced with “freedom and fearlessness in learning” in externships.31
Ten of the 12 study participants applied for a new graduate nurse position at the VAMC where they had their externship. A potential benefit to organizations that sponsor a nursing externship is the recruitment of new graduate nurses.14 Before applying for a full-time position, VALOR students had the opportunity to become familiar with the work environment and assess their fit with the employer. One student found staff nurse work “scary” and “stressful” and decided against it. She said the VALOR externship helped her realize exactly what nursing entailed: “Until this experience, I did not realize I would not like the hospital environment. This was a reality check for me.” Another student decided that working different shifts and working holidays would be difficult for her. These 2 students’ externship experience convinced them to seek other nursing positions.
Limitations
All participants in this study were nurses with excellent academic grades. It is possible they were highly motivated and might not have had any difficulty transitioning to the RN role. The principal investigator in this study was a VALOR program coordinator who knew 3 of the study participants—a potential source of bias. It is possible participants did not want to speak negatively about the program for several reasons: the interviewer was their coordinator, they received a salary during the externship, and several worked for the VA at the time of the survey.
Researchers have acknowledged the likelihood that not all VALOR students have positive experiences. It is possible that students with negative experiences did not discuss them or did not participate in the study. Increasing the size of the study sample may have brought in students with negative experiences. There is also the possibility of researcher misinterpretation and bias. Although bracketing was used, it was not possible to eliminate all potential sources of bias from this qualitative study.
Future Research
This is 1 of only 2 studies on the VALOR externship. Given the contradictory findings of these studies—Nuttall reported VALOR experience did not affect students’ transition to the RN role,3 whereas in the present study VALOR students thought the program positively affected their successful transition—additional quantitative and qualitative research is needed.
In addition, the low recruitment rate of VALOR students should be compared with other studies’ recruitment rates. The 38% VALOR extern recruitment rate for the period 2007 to 2012 is lower than the rate for other programs (G. Fuller, August 27, 2014, e-mail communication). The VALOR program does not track retention of participants after employment. Longitudinal studies should compare VALOR participants’ length of employment with that of nonparticipants’.
Conclusion
Externships provide clinical experience outside the classroom, expose students to the realities of nursing practice before graduation, and serve as a recruitment tool for hospitals. These programs, in conjunction with school-based practicums, increase exposure to the clinical environment. Before graduation, students have the opportunity to practice skills, interact with interprofessional staff, and experience different hospital units, all of which contribute to career decisions. The present study found that the VALOR externship helped new graduate nurses with their transition to the workplace. However, it is important to recognize the limitations of this study.
Interviewees indicated they were confident when they were transitioning to their new nurse role and caring for patients before receiving their RN licensure. New graduate nurses discovered they acclimated to the hospital environment quicker. The reality of working day-to-day in a hospital setting allowed students to select a compatible work environment and understand the daily challenges health care professionals encounter. Interviewees shared that they felt “like the RN” during the externship, which lessened the shock of actually assuming the RN role.
Van Manen asserted there is no conclusion or ending to a phenomenological study.26 Continued research on hospital-based externships will demonstrate how these programs can assist in the development of new graduate nurses, ease their transition to practice, and benefit nursing education, practice, research, and public policy.
1. Clark CM, Springer PJ. Nurse residents' first-hand accounts on transition to practice. Nurs Outlook. 2012;60(4):e2-e8.
2. Myers S, Reidy P, French B, McHale J, Chisholm M, Griffin M. Safety concerns of hospital-based new-to-practice registered nurses and their preceptors. J Contin Educ Nurs. 2010;41(4):163-171.
3. Nuttall CM. A Comparative Study Evaluating the Impact of Participation in a VALOR Nurse Externship on Job Satisfaction, Sense of Belonging, Role Socialization and Sense of Professionalism: Transition From Graduate to Registered Nurse [dissertation]. Albuquerque: University of New Mexico; 2010.
4. Steen JE, Gould EW, Raingruber B, Hill J. Effect of student nurse intern position on ease of transition from student nurse to registered nurse. J Nurs Staff Dev. 2011;27(4):181-186.
5. Ulrich B, Krozek C, Early S, Ashlock CH, Africa LM, Carman ML. Improving retention, confidence, and competence of new graduate nurses: results from a 10-year longitudinal database. Nurs Econ. 2010;28(6):363-375.
6. Casey K, Fink R, Jaynes C, Campbell L, Cook P, Wilson V. Readiness for practice: the senior practicum experience. J Nurs Educ. 2011;50(11):646-652.
7. Shipman D, Hooten J, Stanley S. The VALOR program: preparing nursing students to care for our veterans. Fed Pract. 2014;31(9):35-38.
8. Walker A, Earl C, Costa B, Cuddihy L. Graduate nurses' transition and integration into the workplace: a qualitative comparison of graduate nurses' and nurse unit managers' perspectives. Nurs Educ Today. 2013;33(3):291-296.
9. Welding NM. Creating a nursing residency: decrease turnover and increase clinical competence. Medsurg Nurs. 2011;20(1):37-40.
10. Morrow S. New graduate transitions: leaving the nest, joining the flight. J Nurs Manag. 2009;17(3):278-287.
11. Parker V, Giles M, Lantry G, McMillan M. New graduate nurses' experience in their first year of practice. Nurs Educ Today. 2014;34(1):150-156.
12. Ruth-Sahd LA, Beck J, McCall C. Transformative learning during a nursing externship program: the reflections of senior nursing students. Nurs Educ Perspect. 2010;31(2):78-83.
13. White AH. Clinical decision making among fourth-year nursing students: an interpretive study. J Nurs Educ. 2003;42(3):113-121.
14. Kropkowski LR, Most R. Set for success: nurse "externs." Nurs Manag. 2008;39(7):8-9.
15. Rhoads J, Sensenig K, Ruth-Sahd L, Thompson E. Nursing externship: a collaborative endeavor between nursing education and nursing administration. Dimens Crit Care Nurs. 2003;22(6):255-258.
16. Hillman L, Foster RR. The impact of a nursing transitions programme on retention and cost savings. J Nurs Manag. 2011;19(1):50-56.
17. Baernholdt M, Mark BA. The nurse work environment, job satisfaction and turnover rates in rural and urban nursing units. J Nurs Manag. 2009;17(8):994-1001.
18. Jones CB. Revisiting nurse turnover costs: adjusting for inflation. J Nurs Adm. 2008;38(1):11-18.
19. Pine R, Tart K. Return on investment: benefits and challenges of baccalaureate nurse residency program. Nurs Econ. 2007;25(1):13-18, 39.
20. Beecroft PC, Dorey F, Wenten M. Turnover intention in new graduate nurses: a multivariate analysis. J Adv Nurs, 2008;62(1):41-52.
21. Phillips C, Esterman A, Kenny A. The theory of organisational socialisation and its potential for improving transition experiences for new graduate nurses. Nurs Educ Today. 2015;35(1):118-124.
22. Diefenbeck CA, Plowfield LA, Herrman JW. Clinical immersion: a residency model for nursing education. Nurs Educ Perspect. 2006;27(2):72-79.
23. Cantrell MA, Browne AM. The impact of a nurse externship on the transition process from graduate to registered nurse: part III. Recruitment and retention effects. J Nurs Staff Dev. 2006;22(1):11-14.
24. Kilpatrick K, Frunchak V. The nursing extern program: innovative strategies for students in transition. Health Care Manag (Frederick). 2006;25(3):236-242.
25. Kovner CT, Brewer CS, Fairchild S, Poornima S, Kim H, Djukic CM. Newly licensed RN's characteristics, work attitudes, and intentions to work. Am J Nurs. 2007;107(9):58-70
26. van Manen M. Researching Lived Experience: Human Science for an Action Sensitive Pedagogy. Albany: State University of New York Press; 1990.
27. Lincoln YS, Guba EG. Naturalistic Inquiry. Beverly Hills, CA: Sage; 1985.
28. Starr K, Conley VM. Becoming a registered nurse: the nurse extern experience. J Contin Educ Nurs. 2006;37(2):86-92.
29. Hasson F, McKenna HP, Keeney S. Delegating and supervising unregistered professionals: the student nurse experience. Nurs Educ Today. 2013;33(3):229-235.
30. Kramer M, Maguire P, Halfer D, et al. The organizational transformative power of nurse residency programs. Nurs Adm Q. 2012;36(2):155-168.
31. Rush KL, Peel K, McCracken B. Empowered learning on the inside: an externship experience. Nurs Educ Perspect. 2004;25(6):284-291
New nurse graduates often have difficulty transitioning to the role of registered nurse (RN).1 Given the complexity of the health care environment, the need is growing to prepare nursing students for nursing practice. Although nursing education provides students with a basis for practice, school alone cannot prepare them for actual practice in the hospital setting.2 Compared with nurse residency programs, which provide extended postlicensure training, the national Veterans Affairs Learning Opportunity Residency (VALOR) program provides externships independent of nursing school. Externships allow students to train in a hospital setting (generally during the summer months) before becoming a licensed RN. Nursing students who are entering their senior year of coursework in a bachelor of science nursing program and who have a minimum 3.0 grade point average can apply for this competitive national scholarship offered at VAMCs. The VALOR program is a paid learning opportunity, and students gain hands-on clinical experience under the guidance of preceptors.
Little externship research exists in the nursing literature.3,4 The authors conducted the present study to help fill the gaps in the literature and to add to the only other study findings on VALOR.3 This program, started in 1990 to aid in nursing recruitment and retention, offers students early exposure to the complexities of nursing practice.
The authors investigated RNs’ experience in the VALOR prelicensure externship during the nurses’ senior year of coursework and the impact of this experience on their nursing practice. The program offers 800 hours of hospital-based experience outside the classroom. New nurses who gained only limited clinical exposure in nursing school may feel insecure about their clinical skills.5 Casey and colleagues found that students want more clinical experience than offered by nursing school practicums.6 The VALOR participants obtain additional clinical time, which contributes to their self-confidence when transitioning to the RN role.7
Literature Review
New graduate nurses work in complex health care environments with unfamiliar technologies, shift hours, heavy patient loads, psychological and professional stressors, socialization problems, and patient safety issues.8 They often are unable to connect their educational experience with the realities of practice and find the work environment incongruent with their nursing school education.9 Although new nurses’ difficulty in transitioning to their professional role has been addressed in the literature, transitional experience has not improved.10 Studies have found that new graduate nurses want more support than is given and have suggested that unfamiliar workplace dynamics create stress for new nurses.11
Anxiety, insecurity, and fear of failure are associated with the transition from student to practicing nurse.10 Because of the additional clinical experience gained in an externship, students likely are more self-confident when they assume the RN role.12 White suggested self-confident students see themselves as nurses and feel capable of caring for patients.13 Externship experience makes the transition to professional nursing less stressful, because externship students obtain an inside view of nursing culture.14 Students increase their understanding of nurses’ multiple roles and responsibilities, because these programs focus on increasing clinical skills and competency.15 To perform successfully as RNs, new graduates need competencies and knowledge beyond those obtained in nursing school.16
In the nursing profession, an association between job satisfaction and turnover exists.17,18 Of new graduate nurses, 35% to 69% leave their position within the first year of employment.19 Replacing nurses reduces hospital productivity and efficiency and increases cost.20 New graduate nurses leave because they are dissatisfied with and overwhelmed by the complexity of the work environment.21 Prelicensure nurse externships can aid in recruiting and retaining new graduate nurses for the hospitals that host these programs.22 For host facilities, recruitment rates of 50% to 79% have been reported.23,24
In a quantitative study, Nuttall surveyed 133 RNs about job satisfaction, role socialization, professionalism, and sense of belonging.3 Of these RNs, 34 had participated in VALOR and 99 had not. There was no evidence that the RNs with VALOR experience had a higher degree of professionalism, job satisfaction, or role socialization; only sense of belonging (age-adjusted) was higher for the VALOR group. The conflicting data on prelicensure externship outcomes call for further analysis of these programs.3 Nuttall noted that her study “was the first... to evaluate the VALOR program and future research [using a qualitative approach] is needed to identify additional outcomes related to this program.”3
Methods
This study using hermeneutic phenomenology was approved by the Salem VAMC in Virginia and by the institutional review board at Nova Southeastern University.24 Study participants provided written informed consent before being interviewed.
Interviewees
Data Collection and Analysis
Data collection began in March 2013 with a pilot test of the interview questions for appropriateness. Open-ended, semistructured questions were used to elicit nurses’ descriptions of their experience. Field notes were written, and all interviews were tape-recorded and professionally transcribed verbatim. Data saturation was reached after 12 nurses were interviewed. Transcripts were analyzed and interpreted using van Manen’s line-by-line approach.26 All 12 interviewees were invited to review the findings of the data analysis. Eleven of the 12 interviewees verified and validated the study findings.
Rigor using Lincoln and Guba’s criteria of credibility, dependability, transferability, and confirmability added trustworthiness to the study findings. Bracketing helped eliminate potential bias.27 Credibility was achieved with prolonged engagement and triangulation. To further enhance credibility, the authors invited qualitative research experts to validate the emerging themes and create an audit trail. For dependability, a flow chart was created for use by researchers who might want to replicate the study. Rich, lengthy descriptions and interviewees’ quotations were provided so researchers could judge the study’s transferability to other settings. Maintaining an audit trail and having a doctor of nursing practice independently code the data aided with confirmability. This study used findings from the literature, audio recordings, member checking, and field notes to assess data accuracy.
Results
The overarching theme discovered in this study was confidence. Subthemes were transitioning to the RN role, making decisions, and interacting with interprofessional staff.
Confidence
Interviewees felt confident in transitioning to the RN role, making decisions, and interacting with interprofessional staff. They shared that they had applied to the externship to gain additional clinical experience and that the program increased their self-confidence with respect to transitioning to the new role as RN. However, it is possible that these interviewees were highly motivated and would not have had difficulty transitioning to the RN role—this is addressed in the Limitations section of this article.
Interviewees said they initially approached VALOR with fear and apprehension but completed it feeling confident about becoming an RN. One interviewee stated, “The VALOR program gave me the confidence in my own abilities, so I was not scared and had confidence that, yes, I could do this job.” Another said, “Honestly, the entire externship program, regardless of which question you ask, my answer is going to always fall back on confidence. I became prepared for the RN job, I feel, before I graduated because of the [externship] experiences.”
Transitioning to RN Role
Transitioning involved understanding the RN’s scope of practice and feeling clinically competent. Students worked 40 hours a week over the summer and gained firsthand insight into working as an RN daily. Interviewees believed completing the externship made it easy to transition to the RN role because they knew what to expect.
Working side-by-side with nurses, students gained insight into RNs’ responsibilities and scope of practice. Interviewees reported that, after the externship, they had a better understanding of their patient care and licensure legal responsibilities.
Students began to feel clinically competent during the externship. Interviewees shared that they had had several opportunities to practice basic skills, such as giving injections. One interviewee said, “I don’t think I tried to stick a single IV when I was in school or in clinical [training].” Interviewees also commented that repeatedly practicing skills increased their self-confidence.
Students also gained firsthand insight into working with veterans and their families. During the externship, they learned about communicating therapeutically, providing education for caregivers, and advocating for patients and their families. Before the externship, they felt apprehensive about communicating with patients. One interviewee said that after the program, “Eventually you had to talk to patients, and eventually figured it out that it wasn’t so scary.”
Students found that patients were not always happy with their care, and procedures did not always go as planned. They also discovered that education did not end with the patient; family members needed education as well. The externship experience heightened students’ awareness of the RN’s role as patient advocate. One interviewee explained it is the RN’s responsibility to intervene on the patient’s behalf. Interviewees were surprised that patients would not tell their health care provider that they did not understand what was said or that they wanted another course of treatment.
The externship helped decrease learning-related stress. Interviewees indicated they had learned without fear of reprisal. One described feeling free to learn: “Uninhibited learning...you can ask what you need to without fear of not graduating.” Externship students were able to focus on learning the RN role without worrying about the next test or grade. They felt free to ask questions without fear of failing their clinical rotation.
The supportive and nurturing relationships that students developed in VALOR also increased their confidence when transitioning to the RN role. One interviewee said, “There was never the sense of, no, you learn my way, or I don’t want you here.” Interviewees shared that they felt comfortable and supported.
Decision Making
Interviewees reported that after VALOR, it was easy to make decisions regarding nursing practice, delegation, care prioritization, and career choice. As students, they found the school clinical setting did not provide the decision-making opportunities VALOR did, and they quickly realized nursing practice involved more than making patient-care decisions. One interviewee said, “In a classroom, a picture is painted of an idealistic environment that may not truly mimic the hospital unit.”
Students became familiar with the practice of delegating care to the appropriate staff and the next shift. One interviewee said VALOR “provided me with a better understanding of delegation in my RN role.” VALOR participants discovered that, as new nurses, they were less anxious when delegating to others.
Before RN licensure, VALOR participants learned about prioritizing patient care. One interviewee said, “It’s like everybody has to be charted on, and all the medications have to be passed out, but it’s a matter of getting everything done while doing the more important and more dire things first.” Students learned that all aspects of nursing are important, but they had to make rational decisions.
Interacting With Professionals
Interviewees who had been in VALOR said interacting with interprofessional (different disciplines) staff contributed to their working comfortably in teams and collaborating with others. Their collaborative relationships with physicians would help them later, when as new graduate nurses they again needed to work together with doctors. Typical comments were, “When I started as an RN, I felt I was not new at it because I had communicated with doctors in the externship program.”
Discussion
The present study found that nursing students who had been in the VALOR externship felt confident in their clinical skills when they were transitioning to the RN role. Other studies similarly have found that externship students were self-confident assuming the RN role, owing to their additional clinical experience.12,28 The VALOR program allows students to work alongside nurses and receive hands-on experience while interacting with interprofessional health care teams. Findings of Nuttall’s quantitative study contradict those of the present, qualitative study. Nuttall used surveys and a control group, whereas this phenomenologic study captured the essence of study participants’ experiences through interviews.
The RNs interviewed in the present study discovered that, unlike nursing school, VALOR provided a realistic view of full-time work as an RN. This finding aligns with Starr and Conley’s finding that, before participating in an externship, most students were unaware of the extent of RNs’ roles and responsibilities, whereas after the program they understood these roles and responsibilities better.28
The interviewees in this study thought VALOR improved their skills in communicating with patients, families, and interprofessional team members. Interviewees shared that they learned patient advocacy skills and that, through firsthand experience, realized nurses provide patients with a voice. Externships can help new graduate nurses become better communicators and can teach students the importance of patient communication and advocacy.12
This study also found that students wanted more exposure to realistic nursing environments, additional nursing skills practice, and more interaction with interprofessional team members. VALOR helped bridge the theory–practice gap by providing real-world nursing experience outside the academic environment and extra time for nursing skills development. In a study by Casey and colleagues, students indicated that the time allowed for nursing skills practice during school was inadequate.6
The VALOR program helped students learn about delegating work, whereas nursing school did not provide the opportunity to practice delegation. Other studies have corroborated that students do not practice delegation during nursing school clinical time.29,30
Study respondents noted they could focus on learning without the fear of passing their clinical rotation. They felt supported by staff and were comfortable asking questions. White suggested that externship students who feel supported by nursing staff are able to focus on patients instead of on their discomfort.13 Rush and colleagues found that constraints on the student experience in traditional academic clinical rotations were replaced with “freedom and fearlessness in learning” in externships.31
Ten of the 12 study participants applied for a new graduate nurse position at the VAMC where they had their externship. A potential benefit to organizations that sponsor a nursing externship is the recruitment of new graduate nurses.14 Before applying for a full-time position, VALOR students had the opportunity to become familiar with the work environment and assess their fit with the employer. One student found staff nurse work “scary” and “stressful” and decided against it. She said the VALOR externship helped her realize exactly what nursing entailed: “Until this experience, I did not realize I would not like the hospital environment. This was a reality check for me.” Another student decided that working different shifts and working holidays would be difficult for her. These 2 students’ externship experience convinced them to seek other nursing positions.
Limitations
All participants in this study were nurses with excellent academic grades. It is possible they were highly motivated and might not have had any difficulty transitioning to the RN role. The principal investigator in this study was a VALOR program coordinator who knew 3 of the study participants—a potential source of bias. It is possible participants did not want to speak negatively about the program for several reasons: the interviewer was their coordinator, they received a salary during the externship, and several worked for the VA at the time of the survey.
Researchers have acknowledged the likelihood that not all VALOR students have positive experiences. It is possible that students with negative experiences did not discuss them or did not participate in the study. Increasing the size of the study sample may have brought in students with negative experiences. There is also the possibility of researcher misinterpretation and bias. Although bracketing was used, it was not possible to eliminate all potential sources of bias from this qualitative study.
Future Research
This is 1 of only 2 studies on the VALOR externship. Given the contradictory findings of these studies—Nuttall reported VALOR experience did not affect students’ transition to the RN role,3 whereas in the present study VALOR students thought the program positively affected their successful transition—additional quantitative and qualitative research is needed.
In addition, the low recruitment rate of VALOR students should be compared with other studies’ recruitment rates. The 38% VALOR extern recruitment rate for the period 2007 to 2012 is lower than the rate for other programs (G. Fuller, August 27, 2014, e-mail communication). The VALOR program does not track retention of participants after employment. Longitudinal studies should compare VALOR participants’ length of employment with that of nonparticipants’.
Conclusion
Externships provide clinical experience outside the classroom, expose students to the realities of nursing practice before graduation, and serve as a recruitment tool for hospitals. These programs, in conjunction with school-based practicums, increase exposure to the clinical environment. Before graduation, students have the opportunity to practice skills, interact with interprofessional staff, and experience different hospital units, all of which contribute to career decisions. The present study found that the VALOR externship helped new graduate nurses with their transition to the workplace. However, it is important to recognize the limitations of this study.
Interviewees indicated they were confident when they were transitioning to their new nurse role and caring for patients before receiving their RN licensure. New graduate nurses discovered they acclimated to the hospital environment quicker. The reality of working day-to-day in a hospital setting allowed students to select a compatible work environment and understand the daily challenges health care professionals encounter. Interviewees shared that they felt “like the RN” during the externship, which lessened the shock of actually assuming the RN role.
Van Manen asserted there is no conclusion or ending to a phenomenological study.26 Continued research on hospital-based externships will demonstrate how these programs can assist in the development of new graduate nurses, ease their transition to practice, and benefit nursing education, practice, research, and public policy.
New nurse graduates often have difficulty transitioning to the role of registered nurse (RN).1 Given the complexity of the health care environment, the need is growing to prepare nursing students for nursing practice. Although nursing education provides students with a basis for practice, school alone cannot prepare them for actual practice in the hospital setting.2 Compared with nurse residency programs, which provide extended postlicensure training, the national Veterans Affairs Learning Opportunity Residency (VALOR) program provides externships independent of nursing school. Externships allow students to train in a hospital setting (generally during the summer months) before becoming a licensed RN. Nursing students who are entering their senior year of coursework in a bachelor of science nursing program and who have a minimum 3.0 grade point average can apply for this competitive national scholarship offered at VAMCs. The VALOR program is a paid learning opportunity, and students gain hands-on clinical experience under the guidance of preceptors.
Little externship research exists in the nursing literature.3,4 The authors conducted the present study to help fill the gaps in the literature and to add to the only other study findings on VALOR.3 This program, started in 1990 to aid in nursing recruitment and retention, offers students early exposure to the complexities of nursing practice.
The authors investigated RNs’ experience in the VALOR prelicensure externship during the nurses’ senior year of coursework and the impact of this experience on their nursing practice. The program offers 800 hours of hospital-based experience outside the classroom. New nurses who gained only limited clinical exposure in nursing school may feel insecure about their clinical skills.5 Casey and colleagues found that students want more clinical experience than offered by nursing school practicums.6 The VALOR participants obtain additional clinical time, which contributes to their self-confidence when transitioning to the RN role.7
Literature Review
New graduate nurses work in complex health care environments with unfamiliar technologies, shift hours, heavy patient loads, psychological and professional stressors, socialization problems, and patient safety issues.8 They often are unable to connect their educational experience with the realities of practice and find the work environment incongruent with their nursing school education.9 Although new nurses’ difficulty in transitioning to their professional role has been addressed in the literature, transitional experience has not improved.10 Studies have found that new graduate nurses want more support than is given and have suggested that unfamiliar workplace dynamics create stress for new nurses.11
Anxiety, insecurity, and fear of failure are associated with the transition from student to practicing nurse.10 Because of the additional clinical experience gained in an externship, students likely are more self-confident when they assume the RN role.12 White suggested self-confident students see themselves as nurses and feel capable of caring for patients.13 Externship experience makes the transition to professional nursing less stressful, because externship students obtain an inside view of nursing culture.14 Students increase their understanding of nurses’ multiple roles and responsibilities, because these programs focus on increasing clinical skills and competency.15 To perform successfully as RNs, new graduates need competencies and knowledge beyond those obtained in nursing school.16
In the nursing profession, an association between job satisfaction and turnover exists.17,18 Of new graduate nurses, 35% to 69% leave their position within the first year of employment.19 Replacing nurses reduces hospital productivity and efficiency and increases cost.20 New graduate nurses leave because they are dissatisfied with and overwhelmed by the complexity of the work environment.21 Prelicensure nurse externships can aid in recruiting and retaining new graduate nurses for the hospitals that host these programs.22 For host facilities, recruitment rates of 50% to 79% have been reported.23,24
In a quantitative study, Nuttall surveyed 133 RNs about job satisfaction, role socialization, professionalism, and sense of belonging.3 Of these RNs, 34 had participated in VALOR and 99 had not. There was no evidence that the RNs with VALOR experience had a higher degree of professionalism, job satisfaction, or role socialization; only sense of belonging (age-adjusted) was higher for the VALOR group. The conflicting data on prelicensure externship outcomes call for further analysis of these programs.3 Nuttall noted that her study “was the first... to evaluate the VALOR program and future research [using a qualitative approach] is needed to identify additional outcomes related to this program.”3
Methods
This study using hermeneutic phenomenology was approved by the Salem VAMC in Virginia and by the institutional review board at Nova Southeastern University.24 Study participants provided written informed consent before being interviewed.
Interviewees
Data Collection and Analysis
Data collection began in March 2013 with a pilot test of the interview questions for appropriateness. Open-ended, semistructured questions were used to elicit nurses’ descriptions of their experience. Field notes were written, and all interviews were tape-recorded and professionally transcribed verbatim. Data saturation was reached after 12 nurses were interviewed. Transcripts were analyzed and interpreted using van Manen’s line-by-line approach.26 All 12 interviewees were invited to review the findings of the data analysis. Eleven of the 12 interviewees verified and validated the study findings.
Rigor using Lincoln and Guba’s criteria of credibility, dependability, transferability, and confirmability added trustworthiness to the study findings. Bracketing helped eliminate potential bias.27 Credibility was achieved with prolonged engagement and triangulation. To further enhance credibility, the authors invited qualitative research experts to validate the emerging themes and create an audit trail. For dependability, a flow chart was created for use by researchers who might want to replicate the study. Rich, lengthy descriptions and interviewees’ quotations were provided so researchers could judge the study’s transferability to other settings. Maintaining an audit trail and having a doctor of nursing practice independently code the data aided with confirmability. This study used findings from the literature, audio recordings, member checking, and field notes to assess data accuracy.
Results
The overarching theme discovered in this study was confidence. Subthemes were transitioning to the RN role, making decisions, and interacting with interprofessional staff.
Confidence
Interviewees felt confident in transitioning to the RN role, making decisions, and interacting with interprofessional staff. They shared that they had applied to the externship to gain additional clinical experience and that the program increased their self-confidence with respect to transitioning to the new role as RN. However, it is possible that these interviewees were highly motivated and would not have had difficulty transitioning to the RN role—this is addressed in the Limitations section of this article.
Interviewees said they initially approached VALOR with fear and apprehension but completed it feeling confident about becoming an RN. One interviewee stated, “The VALOR program gave me the confidence in my own abilities, so I was not scared and had confidence that, yes, I could do this job.” Another said, “Honestly, the entire externship program, regardless of which question you ask, my answer is going to always fall back on confidence. I became prepared for the RN job, I feel, before I graduated because of the [externship] experiences.”
Transitioning to RN Role
Transitioning involved understanding the RN’s scope of practice and feeling clinically competent. Students worked 40 hours a week over the summer and gained firsthand insight into working as an RN daily. Interviewees believed completing the externship made it easy to transition to the RN role because they knew what to expect.
Working side-by-side with nurses, students gained insight into RNs’ responsibilities and scope of practice. Interviewees reported that, after the externship, they had a better understanding of their patient care and licensure legal responsibilities.
Students began to feel clinically competent during the externship. Interviewees shared that they had had several opportunities to practice basic skills, such as giving injections. One interviewee said, “I don’t think I tried to stick a single IV when I was in school or in clinical [training].” Interviewees also commented that repeatedly practicing skills increased their self-confidence.
Students also gained firsthand insight into working with veterans and their families. During the externship, they learned about communicating therapeutically, providing education for caregivers, and advocating for patients and their families. Before the externship, they felt apprehensive about communicating with patients. One interviewee said that after the program, “Eventually you had to talk to patients, and eventually figured it out that it wasn’t so scary.”
Students found that patients were not always happy with their care, and procedures did not always go as planned. They also discovered that education did not end with the patient; family members needed education as well. The externship experience heightened students’ awareness of the RN’s role as patient advocate. One interviewee explained it is the RN’s responsibility to intervene on the patient’s behalf. Interviewees were surprised that patients would not tell their health care provider that they did not understand what was said or that they wanted another course of treatment.
The externship helped decrease learning-related stress. Interviewees indicated they had learned without fear of reprisal. One described feeling free to learn: “Uninhibited learning...you can ask what you need to without fear of not graduating.” Externship students were able to focus on learning the RN role without worrying about the next test or grade. They felt free to ask questions without fear of failing their clinical rotation.
The supportive and nurturing relationships that students developed in VALOR also increased their confidence when transitioning to the RN role. One interviewee said, “There was never the sense of, no, you learn my way, or I don’t want you here.” Interviewees shared that they felt comfortable and supported.
Decision Making
Interviewees reported that after VALOR, it was easy to make decisions regarding nursing practice, delegation, care prioritization, and career choice. As students, they found the school clinical setting did not provide the decision-making opportunities VALOR did, and they quickly realized nursing practice involved more than making patient-care decisions. One interviewee said, “In a classroom, a picture is painted of an idealistic environment that may not truly mimic the hospital unit.”
Students became familiar with the practice of delegating care to the appropriate staff and the next shift. One interviewee said VALOR “provided me with a better understanding of delegation in my RN role.” VALOR participants discovered that, as new nurses, they were less anxious when delegating to others.
Before RN licensure, VALOR participants learned about prioritizing patient care. One interviewee said, “It’s like everybody has to be charted on, and all the medications have to be passed out, but it’s a matter of getting everything done while doing the more important and more dire things first.” Students learned that all aspects of nursing are important, but they had to make rational decisions.
Interacting With Professionals
Interviewees who had been in VALOR said interacting with interprofessional (different disciplines) staff contributed to their working comfortably in teams and collaborating with others. Their collaborative relationships with physicians would help them later, when as new graduate nurses they again needed to work together with doctors. Typical comments were, “When I started as an RN, I felt I was not new at it because I had communicated with doctors in the externship program.”
Discussion
The present study found that nursing students who had been in the VALOR externship felt confident in their clinical skills when they were transitioning to the RN role. Other studies similarly have found that externship students were self-confident assuming the RN role, owing to their additional clinical experience.12,28 The VALOR program allows students to work alongside nurses and receive hands-on experience while interacting with interprofessional health care teams. Findings of Nuttall’s quantitative study contradict those of the present, qualitative study. Nuttall used surveys and a control group, whereas this phenomenologic study captured the essence of study participants’ experiences through interviews.
The RNs interviewed in the present study discovered that, unlike nursing school, VALOR provided a realistic view of full-time work as an RN. This finding aligns with Starr and Conley’s finding that, before participating in an externship, most students were unaware of the extent of RNs’ roles and responsibilities, whereas after the program they understood these roles and responsibilities better.28
The interviewees in this study thought VALOR improved their skills in communicating with patients, families, and interprofessional team members. Interviewees shared that they learned patient advocacy skills and that, through firsthand experience, realized nurses provide patients with a voice. Externships can help new graduate nurses become better communicators and can teach students the importance of patient communication and advocacy.12
This study also found that students wanted more exposure to realistic nursing environments, additional nursing skills practice, and more interaction with interprofessional team members. VALOR helped bridge the theory–practice gap by providing real-world nursing experience outside the academic environment and extra time for nursing skills development. In a study by Casey and colleagues, students indicated that the time allowed for nursing skills practice during school was inadequate.6
The VALOR program helped students learn about delegating work, whereas nursing school did not provide the opportunity to practice delegation. Other studies have corroborated that students do not practice delegation during nursing school clinical time.29,30
Study respondents noted they could focus on learning without the fear of passing their clinical rotation. They felt supported by staff and were comfortable asking questions. White suggested that externship students who feel supported by nursing staff are able to focus on patients instead of on their discomfort.13 Rush and colleagues found that constraints on the student experience in traditional academic clinical rotations were replaced with “freedom and fearlessness in learning” in externships.31
Ten of the 12 study participants applied for a new graduate nurse position at the VAMC where they had their externship. A potential benefit to organizations that sponsor a nursing externship is the recruitment of new graduate nurses.14 Before applying for a full-time position, VALOR students had the opportunity to become familiar with the work environment and assess their fit with the employer. One student found staff nurse work “scary” and “stressful” and decided against it. She said the VALOR externship helped her realize exactly what nursing entailed: “Until this experience, I did not realize I would not like the hospital environment. This was a reality check for me.” Another student decided that working different shifts and working holidays would be difficult for her. These 2 students’ externship experience convinced them to seek other nursing positions.
Limitations
All participants in this study were nurses with excellent academic grades. It is possible they were highly motivated and might not have had any difficulty transitioning to the RN role. The principal investigator in this study was a VALOR program coordinator who knew 3 of the study participants—a potential source of bias. It is possible participants did not want to speak negatively about the program for several reasons: the interviewer was their coordinator, they received a salary during the externship, and several worked for the VA at the time of the survey.
Researchers have acknowledged the likelihood that not all VALOR students have positive experiences. It is possible that students with negative experiences did not discuss them or did not participate in the study. Increasing the size of the study sample may have brought in students with negative experiences. There is also the possibility of researcher misinterpretation and bias. Although bracketing was used, it was not possible to eliminate all potential sources of bias from this qualitative study.
Future Research
This is 1 of only 2 studies on the VALOR externship. Given the contradictory findings of these studies—Nuttall reported VALOR experience did not affect students’ transition to the RN role,3 whereas in the present study VALOR students thought the program positively affected their successful transition—additional quantitative and qualitative research is needed.
In addition, the low recruitment rate of VALOR students should be compared with other studies’ recruitment rates. The 38% VALOR extern recruitment rate for the period 2007 to 2012 is lower than the rate for other programs (G. Fuller, August 27, 2014, e-mail communication). The VALOR program does not track retention of participants after employment. Longitudinal studies should compare VALOR participants’ length of employment with that of nonparticipants’.
Conclusion
Externships provide clinical experience outside the classroom, expose students to the realities of nursing practice before graduation, and serve as a recruitment tool for hospitals. These programs, in conjunction with school-based practicums, increase exposure to the clinical environment. Before graduation, students have the opportunity to practice skills, interact with interprofessional staff, and experience different hospital units, all of which contribute to career decisions. The present study found that the VALOR externship helped new graduate nurses with their transition to the workplace. However, it is important to recognize the limitations of this study.
Interviewees indicated they were confident when they were transitioning to their new nurse role and caring for patients before receiving their RN licensure. New graduate nurses discovered they acclimated to the hospital environment quicker. The reality of working day-to-day in a hospital setting allowed students to select a compatible work environment and understand the daily challenges health care professionals encounter. Interviewees shared that they felt “like the RN” during the externship, which lessened the shock of actually assuming the RN role.
Van Manen asserted there is no conclusion or ending to a phenomenological study.26 Continued research on hospital-based externships will demonstrate how these programs can assist in the development of new graduate nurses, ease their transition to practice, and benefit nursing education, practice, research, and public policy.
1. Clark CM, Springer PJ. Nurse residents' first-hand accounts on transition to practice. Nurs Outlook. 2012;60(4):e2-e8.
2. Myers S, Reidy P, French B, McHale J, Chisholm M, Griffin M. Safety concerns of hospital-based new-to-practice registered nurses and their preceptors. J Contin Educ Nurs. 2010;41(4):163-171.
3. Nuttall CM. A Comparative Study Evaluating the Impact of Participation in a VALOR Nurse Externship on Job Satisfaction, Sense of Belonging, Role Socialization and Sense of Professionalism: Transition From Graduate to Registered Nurse [dissertation]. Albuquerque: University of New Mexico; 2010.
4. Steen JE, Gould EW, Raingruber B, Hill J. Effect of student nurse intern position on ease of transition from student nurse to registered nurse. J Nurs Staff Dev. 2011;27(4):181-186.
5. Ulrich B, Krozek C, Early S, Ashlock CH, Africa LM, Carman ML. Improving retention, confidence, and competence of new graduate nurses: results from a 10-year longitudinal database. Nurs Econ. 2010;28(6):363-375.
6. Casey K, Fink R, Jaynes C, Campbell L, Cook P, Wilson V. Readiness for practice: the senior practicum experience. J Nurs Educ. 2011;50(11):646-652.
7. Shipman D, Hooten J, Stanley S. The VALOR program: preparing nursing students to care for our veterans. Fed Pract. 2014;31(9):35-38.
8. Walker A, Earl C, Costa B, Cuddihy L. Graduate nurses' transition and integration into the workplace: a qualitative comparison of graduate nurses' and nurse unit managers' perspectives. Nurs Educ Today. 2013;33(3):291-296.
9. Welding NM. Creating a nursing residency: decrease turnover and increase clinical competence. Medsurg Nurs. 2011;20(1):37-40.
10. Morrow S. New graduate transitions: leaving the nest, joining the flight. J Nurs Manag. 2009;17(3):278-287.
11. Parker V, Giles M, Lantry G, McMillan M. New graduate nurses' experience in their first year of practice. Nurs Educ Today. 2014;34(1):150-156.
12. Ruth-Sahd LA, Beck J, McCall C. Transformative learning during a nursing externship program: the reflections of senior nursing students. Nurs Educ Perspect. 2010;31(2):78-83.
13. White AH. Clinical decision making among fourth-year nursing students: an interpretive study. J Nurs Educ. 2003;42(3):113-121.
14. Kropkowski LR, Most R. Set for success: nurse "externs." Nurs Manag. 2008;39(7):8-9.
15. Rhoads J, Sensenig K, Ruth-Sahd L, Thompson E. Nursing externship: a collaborative endeavor between nursing education and nursing administration. Dimens Crit Care Nurs. 2003;22(6):255-258.
16. Hillman L, Foster RR. The impact of a nursing transitions programme on retention and cost savings. J Nurs Manag. 2011;19(1):50-56.
17. Baernholdt M, Mark BA. The nurse work environment, job satisfaction and turnover rates in rural and urban nursing units. J Nurs Manag. 2009;17(8):994-1001.
18. Jones CB. Revisiting nurse turnover costs: adjusting for inflation. J Nurs Adm. 2008;38(1):11-18.
19. Pine R, Tart K. Return on investment: benefits and challenges of baccalaureate nurse residency program. Nurs Econ. 2007;25(1):13-18, 39.
20. Beecroft PC, Dorey F, Wenten M. Turnover intention in new graduate nurses: a multivariate analysis. J Adv Nurs, 2008;62(1):41-52.
21. Phillips C, Esterman A, Kenny A. The theory of organisational socialisation and its potential for improving transition experiences for new graduate nurses. Nurs Educ Today. 2015;35(1):118-124.
22. Diefenbeck CA, Plowfield LA, Herrman JW. Clinical immersion: a residency model for nursing education. Nurs Educ Perspect. 2006;27(2):72-79.
23. Cantrell MA, Browne AM. The impact of a nurse externship on the transition process from graduate to registered nurse: part III. Recruitment and retention effects. J Nurs Staff Dev. 2006;22(1):11-14.
24. Kilpatrick K, Frunchak V. The nursing extern program: innovative strategies for students in transition. Health Care Manag (Frederick). 2006;25(3):236-242.
25. Kovner CT, Brewer CS, Fairchild S, Poornima S, Kim H, Djukic CM. Newly licensed RN's characteristics, work attitudes, and intentions to work. Am J Nurs. 2007;107(9):58-70
26. van Manen M. Researching Lived Experience: Human Science for an Action Sensitive Pedagogy. Albany: State University of New York Press; 1990.
27. Lincoln YS, Guba EG. Naturalistic Inquiry. Beverly Hills, CA: Sage; 1985.
28. Starr K, Conley VM. Becoming a registered nurse: the nurse extern experience. J Contin Educ Nurs. 2006;37(2):86-92.
29. Hasson F, McKenna HP, Keeney S. Delegating and supervising unregistered professionals: the student nurse experience. Nurs Educ Today. 2013;33(3):229-235.
30. Kramer M, Maguire P, Halfer D, et al. The organizational transformative power of nurse residency programs. Nurs Adm Q. 2012;36(2):155-168.
31. Rush KL, Peel K, McCracken B. Empowered learning on the inside: an externship experience. Nurs Educ Perspect. 2004;25(6):284-291
1. Clark CM, Springer PJ. Nurse residents' first-hand accounts on transition to practice. Nurs Outlook. 2012;60(4):e2-e8.
2. Myers S, Reidy P, French B, McHale J, Chisholm M, Griffin M. Safety concerns of hospital-based new-to-practice registered nurses and their preceptors. J Contin Educ Nurs. 2010;41(4):163-171.
3. Nuttall CM. A Comparative Study Evaluating the Impact of Participation in a VALOR Nurse Externship on Job Satisfaction, Sense of Belonging, Role Socialization and Sense of Professionalism: Transition From Graduate to Registered Nurse [dissertation]. Albuquerque: University of New Mexico; 2010.
4. Steen JE, Gould EW, Raingruber B, Hill J. Effect of student nurse intern position on ease of transition from student nurse to registered nurse. J Nurs Staff Dev. 2011;27(4):181-186.
5. Ulrich B, Krozek C, Early S, Ashlock CH, Africa LM, Carman ML. Improving retention, confidence, and competence of new graduate nurses: results from a 10-year longitudinal database. Nurs Econ. 2010;28(6):363-375.
6. Casey K, Fink R, Jaynes C, Campbell L, Cook P, Wilson V. Readiness for practice: the senior practicum experience. J Nurs Educ. 2011;50(11):646-652.
7. Shipman D, Hooten J, Stanley S. The VALOR program: preparing nursing students to care for our veterans. Fed Pract. 2014;31(9):35-38.
8. Walker A, Earl C, Costa B, Cuddihy L. Graduate nurses' transition and integration into the workplace: a qualitative comparison of graduate nurses' and nurse unit managers' perspectives. Nurs Educ Today. 2013;33(3):291-296.
9. Welding NM. Creating a nursing residency: decrease turnover and increase clinical competence. Medsurg Nurs. 2011;20(1):37-40.
10. Morrow S. New graduate transitions: leaving the nest, joining the flight. J Nurs Manag. 2009;17(3):278-287.
11. Parker V, Giles M, Lantry G, McMillan M. New graduate nurses' experience in their first year of practice. Nurs Educ Today. 2014;34(1):150-156.
12. Ruth-Sahd LA, Beck J, McCall C. Transformative learning during a nursing externship program: the reflections of senior nursing students. Nurs Educ Perspect. 2010;31(2):78-83.
13. White AH. Clinical decision making among fourth-year nursing students: an interpretive study. J Nurs Educ. 2003;42(3):113-121.
14. Kropkowski LR, Most R. Set for success: nurse "externs." Nurs Manag. 2008;39(7):8-9.
15. Rhoads J, Sensenig K, Ruth-Sahd L, Thompson E. Nursing externship: a collaborative endeavor between nursing education and nursing administration. Dimens Crit Care Nurs. 2003;22(6):255-258.
16. Hillman L, Foster RR. The impact of a nursing transitions programme on retention and cost savings. J Nurs Manag. 2011;19(1):50-56.
17. Baernholdt M, Mark BA. The nurse work environment, job satisfaction and turnover rates in rural and urban nursing units. J Nurs Manag. 2009;17(8):994-1001.
18. Jones CB. Revisiting nurse turnover costs: adjusting for inflation. J Nurs Adm. 2008;38(1):11-18.
19. Pine R, Tart K. Return on investment: benefits and challenges of baccalaureate nurse residency program. Nurs Econ. 2007;25(1):13-18, 39.
20. Beecroft PC, Dorey F, Wenten M. Turnover intention in new graduate nurses: a multivariate analysis. J Adv Nurs, 2008;62(1):41-52.
21. Phillips C, Esterman A, Kenny A. The theory of organisational socialisation and its potential for improving transition experiences for new graduate nurses. Nurs Educ Today. 2015;35(1):118-124.
22. Diefenbeck CA, Plowfield LA, Herrman JW. Clinical immersion: a residency model for nursing education. Nurs Educ Perspect. 2006;27(2):72-79.
23. Cantrell MA, Browne AM. The impact of a nurse externship on the transition process from graduate to registered nurse: part III. Recruitment and retention effects. J Nurs Staff Dev. 2006;22(1):11-14.
24. Kilpatrick K, Frunchak V. The nursing extern program: innovative strategies for students in transition. Health Care Manag (Frederick). 2006;25(3):236-242.
25. Kovner CT, Brewer CS, Fairchild S, Poornima S, Kim H, Djukic CM. Newly licensed RN's characteristics, work attitudes, and intentions to work. Am J Nurs. 2007;107(9):58-70
26. van Manen M. Researching Lived Experience: Human Science for an Action Sensitive Pedagogy. Albany: State University of New York Press; 1990.
27. Lincoln YS, Guba EG. Naturalistic Inquiry. Beverly Hills, CA: Sage; 1985.
28. Starr K, Conley VM. Becoming a registered nurse: the nurse extern experience. J Contin Educ Nurs. 2006;37(2):86-92.
29. Hasson F, McKenna HP, Keeney S. Delegating and supervising unregistered professionals: the student nurse experience. Nurs Educ Today. 2013;33(3):229-235.
30. Kramer M, Maguire P, Halfer D, et al. The organizational transformative power of nurse residency programs. Nurs Adm Q. 2012;36(2):155-168.
31. Rush KL, Peel K, McCracken B. Empowered learning on the inside: an externship experience. Nurs Educ Perspect. 2004;25(6):284-291