User login
Nonopioid Alternatives to Addressing Pain Intensity: A Retrospective Look at 2 Noninvasive Pain Treatment Devices
Chronic pain is common among veterans treated in Veterans Health Administration (VHA) facilities, and optimal management remains challenging in the context of the national opioid misuse epidemic. The Eastern Oklahoma VA Health Care System (EOVAHCS) Pain Program offers a range of services that allow clinicians to tailor multimodal treatment strategies to a veteran’s needs. In 2014, a Modality Clinic was established to assess the utility of adding noninvasive treatment devices to the pain program’s armamentarium. This article addresses the context for introducing these devices and describes the EOVAHCS Pain Program and Modality Clinic. Also discussed are procedures and findings from an initial quality improvement evaluation designed to inform decision making regarding retention, expansion, or elimination of the EOVAHCS noninvasive, pain treatment device program.
Opioid prescriptions increased from 76 million in 1991 to 219 million in 2011. In 2011, the annual cost of chronic pain in the US was estimated at $635 billion.1-6 The confluence of an increasing concern about undertreatment of pain and overconfidence for the safety of opioids led to what former US Surgeon General Vivek H. Murthy, MD, called the opioid crisis.7 As awareness of its unintended consequences of opioid prescribing increased, the VHA began looking for nonopioid treatments that would decrease pain intensity. The 1993 article by Kehlet and Dahl was one of the first discussions of a multimodal nonpharmacologic strategy for addressing acute postoperative pain.8 Their pivotal literature review concluded that nonpharmacologic modalities, such as acupuncture, cranial manipulation, cranial electrostimulation treatment (CES), and low-level light technologies (LLLT), carried less risk and produced equal or greater clinical effects than those of drug therapies.8
Multimodal treatment approaches increasingly are encouraged, and nonopioid pain control has become more common across medical disciplines from physical therapy to anesthesiology.8-10 Innovative, noninvasive devices designed for self-use have appeared on the market. Many of these devices incorporate microcurrent electrical therapy (MET), CES, and/or LLLT (also known as cold laser).11-16 LLLT is a light modality that seems to lead to increased ATP production, resulting in improved healing and decreased inflammation.13-16 Although CES has been studied in a variety of patient populations, its effectiveness is not well understood.16 Research on the effects of CES on neurotransmitter levels as well as activation of parts of the brain involved in pain reception and transmission should clarify these mechanisms. Research has shown improvements in sleep and mood as well as overall pain reduction.11,16 Research has focused primarily on individual modalities rather than on combination devices and has been conducted on populations unlike the veteran population (eg, women with fibromyalgia).
Most of the devices that use electrical or LLLT cannot be used safely by patients who have implantable electrical devices or have medical conditions such as unstable seizures, pregnancy, and active malignancies.
The most common adverse effects (AEs) of CES—dizziness and headaches—are minimal compared with the AEs of pain medications. MET and LLLT AEs generally are limited to skin irritation and muscle soreness.11 Most devices require a prescription, and manufacturers provide training for purchase.
The Pain Program
EOVAHCS initially established its consultative pain program in 2013 to provide support, recommendations, and education about managing pain in veterans to primary care providers (PCPs). Veterans are referred to the pain program for a face-to-face assessment and set of recommendations to assist in developing a comprehensive pain treatment plan. Consistent with its multimodal, biopsychosocial rehabilitation model approach, the program also offers several chronic pain treatment services, including patient education courses, cognitive behavioral therapy (CBT) for chronic pain, chiropractic care, biofeedback, relaxation training, steroid injections, pain coaching, and a pain modality (noninvasive device) clinic. During their assessment, veterans are evaluated for the appropriateness of these programs, including treatment through the Pain Modality Clinic.
Pain Modality Clinic
The EOVAHCS Pain Modality Clinic was created in 2014 as a treatment and device-trial program to provide veterans access to newer noninvasive, patient-driven treatment devices as part of an active chronic pain self-management plan. A crucial innovation is that these devices are designed to be used by patients in their homes. These devices can be expensive, and not every patient will benefit from their use; therefore, clinic leaders recommended a trial before a device is issued to a veteran for home use.
The Pain Modality Clinic coordinator trains clinic facilitators on the device according to manufacturer’s guidelines. Each participating veteran takes part in a device trial to confirm that he or she is able to use the recommended device independently and is likely to benefit from its use. When appropriate, veterans who do not respond to the initial device trial could test the potential benefit of another device. Although data from these device trials are collected primarily to inform clinical decision making, this information also is useful in guiding local policy regarding continued support for each of the modalities.
Veterans who have chronic or persistent pain (≥ 3 months) that interferes with function or quality of life are considered good candidates for a device trial if they are actively involved in pain self-care, logistically able to participate, able to use a device long-term, and have no contraindications. “Active involvement” could be met by participation in any pain management effort, whether a specific exercise program, CBT, or other treatment.
The Modality Clinic currently offers device trials for persistent pain with Alpha-Stim-M (AS-M; Electromedical Products International, Mineral Wells, TX), Laser Touch One (LTO; Renewal Technologies, LLC, Phoenix, AZ), and Neurolumen (Oklahoma City, OK). Neurolumen devices were not available in the clinic initially and will not be discussed further in this article.
The first Alpha-Stim machine using MET and CES technology was created in 1981 for in-office pain management. In 2012, the currently used AS-M became available.11 AS-M is FDA approved for treating pain, anxiety, depression, and sleep problems and is the device used in the EOVAHCS Modality Clinic. AS-M uses probes or electrodes to send a MET waveform through the body area in pain. The device uses ear clips to provide CES, which is thought to increase alpha waves in the brain.11 The LTO is a device that combines LLLT and MET technologies in a home-use design.14 LTO is FDA approved for treating painand is a portable personal pain-relief device applied to the area of pain using electroconductive gel.
Both devices are designed for long-term, self-use, making them viable parts of a multimodal, chronic pain treatment plan. Contraindications for AS-M and LTO include having a pacemaker or an implantable defibrillator, pregnancy, current malignancy, or seizures. Eligible veterans with persistent pain and high levels of depression, anxiety, and/or sleep problems generally are triaged to AS-M, whereas those who have only pain intensity issues usually are assigned to LTO. Referral to the Modality Clinic is not limited to a specific type of pain; common pain conditions seen in the clinic are spine and joint pain, arthritis pain, myofascial pain, headaches, and neuropathy.
Training and Device Trials
Eligible veterans are educated about the device and complete clinical informed consent, which is documented in the electronic health record. The veterans’ primary care and/or specialist providers are contacted for concurrence regarding veterans’ participation in the treatment.
Protocols for the device trials are based on the manufacturers’ recommendations, adjusted to what is feasible in the clinic (manufacturers approved the changes). The number of treatments per trial varies by device. For AS-M, veterans come to the clinic 5 days a week for 2 weeks. For LTO, veterans attend the clinic 5 days a week for 1 week.
At the beginning of a device trial, a trained facilitator teaches each veteran and caregiver to use the device, sets functional goals for the trial, and provides education on the trial questionnaires and daily pain logs. The veteran then follows the device protocol in the clinic where the facilitator can respond to questions and address any issues. With support from their caregivers, veterans are expected to become independent on their device use by the end of the trial. Clinic staff or the veteran can stop the device trial at any point, without affecting the veteran’s participation in or eligibility for other EOVAHCS pain programs.
This project was submitted to the University of Oklahoma Health Sciences Center Institutional Review Board and was exempted from institutional review board oversight as a retrospective, quality improvement effort. Before data analysis, the EOVAHCS Coordinator for Research and Development reviewed the procedures to ensure that all policies were being followed.
Methods
Data for veterans who completed valid treatments of AS-M or LTO from May 9, 2014 to August 20, 2016, were included in the analyses. For an AS-M treatment to be considered valid, the veteran must have attended at least 8 sessions and completed assessment instruments at baseline (preintervention) and following completion (postintervention). For an LTO treatment to be considered valid, the veteran must have attended at least 4 sessions and completed assessment measures at baseline and after completion.
Measures
Veterans completed the following measures at baseline and after trial completion:
The Beck Depression Inventory (BDI-II) is a 21-item measure designed to assess depressive symptoms. Each item assesses intensity on a 0-to-3 scale. Scores from 0 to 13 indicate minimum depression; 14 to 19, mild depression; 20 to 28, moderate depression, and 29 to 63, severe depression.17
The Beck Anxiety Inventory (BAI) is a 21-item measure of anxiety symptoms that uses a 0-to-3 scale to assess severity of subjective, somatic, or panic-related symptoms of anxiety. Scores ranging from 0 to 9 indicate minimal anxiety; 10 to 16, mild anxiety; 17 to 29, moderate anxiety, and 30 to 63, severe anxiety.18
The Pain Catastrophizing Scale (PCS) is a 13-item measure of pain catastrophizing, a crucial marker of how individuals experience pain. Items are scored on a 0-to-4 scale; scores of ≥ 30 indicate a clinically relevant level of catastrophizing.19
The Subjective Units of Distress Scale (SUD) is a single-item measure of the subjective intensity of disturbance or distress currently being experienced. It is scored from 0 to 10; 1 to 4 is mild, 5 to 6 is moderate, and 7 to 10 is severe.20
The Brief Pain Inventory (BPI) measures pain intensity and the impact of pain on functioning. Four items assess pain intensity at its worst, least, and average over the previous 24 hours and at the time of assessment; responses are on a 0-to-10 scale with 10 being most severe. The pain intensity measure is the average of scores on these 4 items. Pain interference is measured with respect to 7 daily activities; general activity, walking, work, mood, enjoyment of life, relations with others, and sleep. Each of these items is scored on a 0-to-10 scale with 10 being the most severe. The pain interference measure is the average of scores on these 7 items.21
Participants completed a daily pain log and recorded self-ratings (0-to-10 scale) of pain and relaxation levels before and after using the device. These scores were primarily used to assist in determining whether goals, set collaboratively by the clinician and the veteran at the first session, had been met.
Analysis
Descriptive statistics were used to characterize the sample overall and by modality. Paired t tests were used to assess changes on each assessment measure over time and for each device separately. The significance of change was assessed for 8 outcomes for each device. In this context, using a conservative Bonferroni correction, significance was set at P < .006. Because AS-M is designed to address depression, anxiety, and sleep as well as pain, whereas LTO is not, device assignments were based on clinical considerations rather than randomization. Therefore, no comparisons were made between devices, and outcomes were assessed independently for the 2 devices. Analyses were performed using SAS 9.4 (Cary, NC).
Results
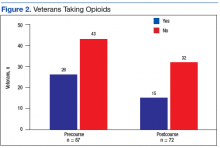
Device trials were initiated for 161 veterans (LTO, 70; AS-M, 91). Distribution of devices was unequal because veterans are assigned to 1 device or the other based on clinical presentation. Failure to complete a trial (n = 46; 28.6%) typically was because of travel barriers, lack of interest in continuing, and for 3 veterans, reports of headaches that they attributed to the AS-M treatment. Of the 115 participants who completed valid trials, 88 (76.5%) also completed assessment measures at pre- and postintervention (LTO = 38; AS-M = 50). None of the participants in this study completed trials with both the AS-M and LTO devices.
Most participants were male (84.1%) and rural residents (85.5%) (Table 1).
Pain Reduction
Treatment with AS-M or LTO was associated with statistically significant reductions in pain severity (BPI), pain interference (BPI), daily pain intensity scores (daily pain log), and pain catastrophizing (PCS) (Tables 2 and 3).
Impact on Mood
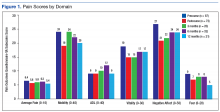
Use of AS-M was associated with statistically significant improvements in depression (BDI-II), anxiety (BAI), and distress (SUD) scores. In addition, veterans completing AS-M treatment showed a statistically significant improvement in self-reported relaxation scores. Interestingly, use of LTO also resulted in a statistically significant decrease in anxiety (BAI) and a nonstatistically significant decrease in depression (BDI-II).
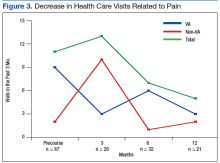
Figure 1 and 2 illustrates the clinical impact of each device in shifting participants from 1 level of symptom severity to another.
Discussion
Use of both AS-M and LTO at EOVAHC was associated with reduced pain intensity. The devices also had positive effects beyond pain in areas such as depression, anxiety, and distress. Remission of depression and anxiety symptoms has been associated with significant decline in pain symptoms, suggesting that pain is best treated through multimodal approaches.22
In the context of the opioid crisis, the availability of effective nonopioid, nonpharmacologic, noninvasive treatments for chronic pain is needed. The Joint Commission recently expanded its pain management guidelines to support hospitals offering nonpharmacologic pain treatments.23 Integrating AS-M, LTO, or similar products into standard pain management practices allows for other treatment pathways with positive outcomes for providers and patients. The Joint Commission also recommends an interdisciplinary approach, defined as a process whereby health care professionals from different disciplines collaborate to diagnose and treat patients experiencing difficult pain conditions. This approach facilitates multimodal management because these disciplines contribute knowledge about a variety of treatment options. Devices such AS-M and LTO are well suited to interdisciplinary pain management because they are not seen as being under the purview of a specific health care specialty.
Limitations
Our findings are limited because they are derived from a retrospective, quality improvement evaluation of outcomes from a single clinic. Findings must be considered in the context of the relatively small samples of veterans. Because analyses were conducted as part of a quality improvement effort, veterans were offered a specific device based on clinical indications, there were no comparisons between devices, and there was no comparison group. Although most participants were using medication and other treatments as part of their pain treatment plan, all reported continued pain intensity before use of a device. Analyses did not control for variation in treatments received concurrently. Last, the logs used to collect self-report data on daily pain and relaxation levels were not validated.
The data highlight a clear need for research to better understand the long-term effects of these devices as well as the characteristics of patients who respond best to each device. Noninvasive treatments for pain often are dismissed as placebos. Rigorously designed, controlled studies will help demonstrate that these devices offer a statistically significant benefit beyond any placebo effect.
Conclusion
Understanding of chronic pain and its treatment will continue to evolve. It is clear that each person dealing with chronic pain requires a tailored combination of treatments and multimodal approaches, which is more effective than any single treatment. Nonpharmacologic, noninvasive devices pose fewer risks and seem to be more effective in reducing pain intensity than traditional treatments, including medications or surgical intervention. In light of the current emphasis on evidence-based health care and as the evidence for the effectiveness of noninvasive pain devices modalities grows, it is likely that treatments incorporating modalities such as MET, CES, and LLLT will become common options for managing chronic pain.
1. US Department of Veterans Affairs. Pain as the 5th Vital Sign Toolkit. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Published October 2000. Accessed February 11, 2019.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
3. Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405-416.
4. Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5-12.
5. Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Weg MW, Lund BC. Trends in prevalent and incident opioid receipt: an observational study in Veterans Health Administration 2004-2012. J Gen Intern Med. 2015;30(5):597-604.
6. Reuben DB, Alvanzo AAH, Ashikaga T, et al. National Institutes of Health Pathways to Prevention Workshop: The role of opioids in the treatment of chronic pain. Ann Intern Med. 2015;162(4):295-300.
7. Murthy VH. Opioid epidemic: we all have a role in turning the tide. https://obamawhitehouse.archives.gov/blog/2016/10/05/opioid-epidemic-we-all-have-role-turning-tide. Published October 5, 2016. Accessed February 12, 2019.
8. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
9. Crane P, Feinberg L, Morris J. A multimodal physical therapy approach to the management of a patient with temporomandibular dysfunction and head and neck lymphedema: a case report. J Man Manip Ther. 2015;23(1): 37-42.
10. Arnstein P. Multimodal approaches to pain management. Nurs. 2011;41(3): 60-61.
11. Alpha-Stim. http://www.alpha-stim.com. Accessed March 22, 2019
12. Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia. Ann Intern Med. 2018;168(6):414-421.
13. Chow RT, Heller GZ, Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006;124(1):201-210.
14. Kulkarni AD, Smith RB. The use of microcurrent electrical therapy and cranial electrotherapy stimulation in pain control. Clin Pract Alternative Med. 2001;2(2):99-102.
15. Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897-1908.
16. Taylor AG, Anderson JG, Riedel SL, et al. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Manag Nurs. 2013;14(4):327-335.
17. Beck, AT, Steer, RA, Brown, GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
18. Beck AT, Steer RA. Beck Anxiety Inventory: Manual. San Antonio, TX: Psychological Corporation; 1993.
19. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524-532.
20. Wolpe J. The Practice of Behavior Therapy. 4th ed. Elmsford, NY: Pergamon; 1990.
21. Cleeland CS. The Brief Pain Inventory User Manual. https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/brief-pain-inventory.html. Published 2009. Accessed February 12, 2019.
22. Gerrits MM, van Marwijk HW, van Oppen P, Horst HVD, Penninx BW. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64-70.
23. The Joint Commission. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. https://www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Published July 2017. Accessed March 21, 2019.
Chronic pain is common among veterans treated in Veterans Health Administration (VHA) facilities, and optimal management remains challenging in the context of the national opioid misuse epidemic. The Eastern Oklahoma VA Health Care System (EOVAHCS) Pain Program offers a range of services that allow clinicians to tailor multimodal treatment strategies to a veteran’s needs. In 2014, a Modality Clinic was established to assess the utility of adding noninvasive treatment devices to the pain program’s armamentarium. This article addresses the context for introducing these devices and describes the EOVAHCS Pain Program and Modality Clinic. Also discussed are procedures and findings from an initial quality improvement evaluation designed to inform decision making regarding retention, expansion, or elimination of the EOVAHCS noninvasive, pain treatment device program.
Opioid prescriptions increased from 76 million in 1991 to 219 million in 2011. In 2011, the annual cost of chronic pain in the US was estimated at $635 billion.1-6 The confluence of an increasing concern about undertreatment of pain and overconfidence for the safety of opioids led to what former US Surgeon General Vivek H. Murthy, MD, called the opioid crisis.7 As awareness of its unintended consequences of opioid prescribing increased, the VHA began looking for nonopioid treatments that would decrease pain intensity. The 1993 article by Kehlet and Dahl was one of the first discussions of a multimodal nonpharmacologic strategy for addressing acute postoperative pain.8 Their pivotal literature review concluded that nonpharmacologic modalities, such as acupuncture, cranial manipulation, cranial electrostimulation treatment (CES), and low-level light technologies (LLLT), carried less risk and produced equal or greater clinical effects than those of drug therapies.8
Multimodal treatment approaches increasingly are encouraged, and nonopioid pain control has become more common across medical disciplines from physical therapy to anesthesiology.8-10 Innovative, noninvasive devices designed for self-use have appeared on the market. Many of these devices incorporate microcurrent electrical therapy (MET), CES, and/or LLLT (also known as cold laser).11-16 LLLT is a light modality that seems to lead to increased ATP production, resulting in improved healing and decreased inflammation.13-16 Although CES has been studied in a variety of patient populations, its effectiveness is not well understood.16 Research on the effects of CES on neurotransmitter levels as well as activation of parts of the brain involved in pain reception and transmission should clarify these mechanisms. Research has shown improvements in sleep and mood as well as overall pain reduction.11,16 Research has focused primarily on individual modalities rather than on combination devices and has been conducted on populations unlike the veteran population (eg, women with fibromyalgia).
Most of the devices that use electrical or LLLT cannot be used safely by patients who have implantable electrical devices or have medical conditions such as unstable seizures, pregnancy, and active malignancies.
The most common adverse effects (AEs) of CES—dizziness and headaches—are minimal compared with the AEs of pain medications. MET and LLLT AEs generally are limited to skin irritation and muscle soreness.11 Most devices require a prescription, and manufacturers provide training for purchase.
The Pain Program
EOVAHCS initially established its consultative pain program in 2013 to provide support, recommendations, and education about managing pain in veterans to primary care providers (PCPs). Veterans are referred to the pain program for a face-to-face assessment and set of recommendations to assist in developing a comprehensive pain treatment plan. Consistent with its multimodal, biopsychosocial rehabilitation model approach, the program also offers several chronic pain treatment services, including patient education courses, cognitive behavioral therapy (CBT) for chronic pain, chiropractic care, biofeedback, relaxation training, steroid injections, pain coaching, and a pain modality (noninvasive device) clinic. During their assessment, veterans are evaluated for the appropriateness of these programs, including treatment through the Pain Modality Clinic.
Pain Modality Clinic
The EOVAHCS Pain Modality Clinic was created in 2014 as a treatment and device-trial program to provide veterans access to newer noninvasive, patient-driven treatment devices as part of an active chronic pain self-management plan. A crucial innovation is that these devices are designed to be used by patients in their homes. These devices can be expensive, and not every patient will benefit from their use; therefore, clinic leaders recommended a trial before a device is issued to a veteran for home use.
The Pain Modality Clinic coordinator trains clinic facilitators on the device according to manufacturer’s guidelines. Each participating veteran takes part in a device trial to confirm that he or she is able to use the recommended device independently and is likely to benefit from its use. When appropriate, veterans who do not respond to the initial device trial could test the potential benefit of another device. Although data from these device trials are collected primarily to inform clinical decision making, this information also is useful in guiding local policy regarding continued support for each of the modalities.
Veterans who have chronic or persistent pain (≥ 3 months) that interferes with function or quality of life are considered good candidates for a device trial if they are actively involved in pain self-care, logistically able to participate, able to use a device long-term, and have no contraindications. “Active involvement” could be met by participation in any pain management effort, whether a specific exercise program, CBT, or other treatment.
The Modality Clinic currently offers device trials for persistent pain with Alpha-Stim-M (AS-M; Electromedical Products International, Mineral Wells, TX), Laser Touch One (LTO; Renewal Technologies, LLC, Phoenix, AZ), and Neurolumen (Oklahoma City, OK). Neurolumen devices were not available in the clinic initially and will not be discussed further in this article.
The first Alpha-Stim machine using MET and CES technology was created in 1981 for in-office pain management. In 2012, the currently used AS-M became available.11 AS-M is FDA approved for treating pain, anxiety, depression, and sleep problems and is the device used in the EOVAHCS Modality Clinic. AS-M uses probes or electrodes to send a MET waveform through the body area in pain. The device uses ear clips to provide CES, which is thought to increase alpha waves in the brain.11 The LTO is a device that combines LLLT and MET technologies in a home-use design.14 LTO is FDA approved for treating painand is a portable personal pain-relief device applied to the area of pain using electroconductive gel.
Both devices are designed for long-term, self-use, making them viable parts of a multimodal, chronic pain treatment plan. Contraindications for AS-M and LTO include having a pacemaker or an implantable defibrillator, pregnancy, current malignancy, or seizures. Eligible veterans with persistent pain and high levels of depression, anxiety, and/or sleep problems generally are triaged to AS-M, whereas those who have only pain intensity issues usually are assigned to LTO. Referral to the Modality Clinic is not limited to a specific type of pain; common pain conditions seen in the clinic are spine and joint pain, arthritis pain, myofascial pain, headaches, and neuropathy.
Training and Device Trials
Eligible veterans are educated about the device and complete clinical informed consent, which is documented in the electronic health record. The veterans’ primary care and/or specialist providers are contacted for concurrence regarding veterans’ participation in the treatment.
Protocols for the device trials are based on the manufacturers’ recommendations, adjusted to what is feasible in the clinic (manufacturers approved the changes). The number of treatments per trial varies by device. For AS-M, veterans come to the clinic 5 days a week for 2 weeks. For LTO, veterans attend the clinic 5 days a week for 1 week.
At the beginning of a device trial, a trained facilitator teaches each veteran and caregiver to use the device, sets functional goals for the trial, and provides education on the trial questionnaires and daily pain logs. The veteran then follows the device protocol in the clinic where the facilitator can respond to questions and address any issues. With support from their caregivers, veterans are expected to become independent on their device use by the end of the trial. Clinic staff or the veteran can stop the device trial at any point, without affecting the veteran’s participation in or eligibility for other EOVAHCS pain programs.
This project was submitted to the University of Oklahoma Health Sciences Center Institutional Review Board and was exempted from institutional review board oversight as a retrospective, quality improvement effort. Before data analysis, the EOVAHCS Coordinator for Research and Development reviewed the procedures to ensure that all policies were being followed.
Methods
Data for veterans who completed valid treatments of AS-M or LTO from May 9, 2014 to August 20, 2016, were included in the analyses. For an AS-M treatment to be considered valid, the veteran must have attended at least 8 sessions and completed assessment instruments at baseline (preintervention) and following completion (postintervention). For an LTO treatment to be considered valid, the veteran must have attended at least 4 sessions and completed assessment measures at baseline and after completion.
Measures
Veterans completed the following measures at baseline and after trial completion:
The Beck Depression Inventory (BDI-II) is a 21-item measure designed to assess depressive symptoms. Each item assesses intensity on a 0-to-3 scale. Scores from 0 to 13 indicate minimum depression; 14 to 19, mild depression; 20 to 28, moderate depression, and 29 to 63, severe depression.17
The Beck Anxiety Inventory (BAI) is a 21-item measure of anxiety symptoms that uses a 0-to-3 scale to assess severity of subjective, somatic, or panic-related symptoms of anxiety. Scores ranging from 0 to 9 indicate minimal anxiety; 10 to 16, mild anxiety; 17 to 29, moderate anxiety, and 30 to 63, severe anxiety.18
The Pain Catastrophizing Scale (PCS) is a 13-item measure of pain catastrophizing, a crucial marker of how individuals experience pain. Items are scored on a 0-to-4 scale; scores of ≥ 30 indicate a clinically relevant level of catastrophizing.19
The Subjective Units of Distress Scale (SUD) is a single-item measure of the subjective intensity of disturbance or distress currently being experienced. It is scored from 0 to 10; 1 to 4 is mild, 5 to 6 is moderate, and 7 to 10 is severe.20
The Brief Pain Inventory (BPI) measures pain intensity and the impact of pain on functioning. Four items assess pain intensity at its worst, least, and average over the previous 24 hours and at the time of assessment; responses are on a 0-to-10 scale with 10 being most severe. The pain intensity measure is the average of scores on these 4 items. Pain interference is measured with respect to 7 daily activities; general activity, walking, work, mood, enjoyment of life, relations with others, and sleep. Each of these items is scored on a 0-to-10 scale with 10 being the most severe. The pain interference measure is the average of scores on these 7 items.21
Participants completed a daily pain log and recorded self-ratings (0-to-10 scale) of pain and relaxation levels before and after using the device. These scores were primarily used to assist in determining whether goals, set collaboratively by the clinician and the veteran at the first session, had been met.
Analysis
Descriptive statistics were used to characterize the sample overall and by modality. Paired t tests were used to assess changes on each assessment measure over time and for each device separately. The significance of change was assessed for 8 outcomes for each device. In this context, using a conservative Bonferroni correction, significance was set at P < .006. Because AS-M is designed to address depression, anxiety, and sleep as well as pain, whereas LTO is not, device assignments were based on clinical considerations rather than randomization. Therefore, no comparisons were made between devices, and outcomes were assessed independently for the 2 devices. Analyses were performed using SAS 9.4 (Cary, NC).
Results
Device trials were initiated for 161 veterans (LTO, 70; AS-M, 91). Distribution of devices was unequal because veterans are assigned to 1 device or the other based on clinical presentation. Failure to complete a trial (n = 46; 28.6%) typically was because of travel barriers, lack of interest in continuing, and for 3 veterans, reports of headaches that they attributed to the AS-M treatment. Of the 115 participants who completed valid trials, 88 (76.5%) also completed assessment measures at pre- and postintervention (LTO = 38; AS-M = 50). None of the participants in this study completed trials with both the AS-M and LTO devices.
Most participants were male (84.1%) and rural residents (85.5%) (Table 1).
Pain Reduction
Treatment with AS-M or LTO was associated with statistically significant reductions in pain severity (BPI), pain interference (BPI), daily pain intensity scores (daily pain log), and pain catastrophizing (PCS) (Tables 2 and 3).
Impact on Mood
Use of AS-M was associated with statistically significant improvements in depression (BDI-II), anxiety (BAI), and distress (SUD) scores. In addition, veterans completing AS-M treatment showed a statistically significant improvement in self-reported relaxation scores. Interestingly, use of LTO also resulted in a statistically significant decrease in anxiety (BAI) and a nonstatistically significant decrease in depression (BDI-II).
Figure 1 and 2 illustrates the clinical impact of each device in shifting participants from 1 level of symptom severity to another.
Discussion
Use of both AS-M and LTO at EOVAHC was associated with reduced pain intensity. The devices also had positive effects beyond pain in areas such as depression, anxiety, and distress. Remission of depression and anxiety symptoms has been associated with significant decline in pain symptoms, suggesting that pain is best treated through multimodal approaches.22
In the context of the opioid crisis, the availability of effective nonopioid, nonpharmacologic, noninvasive treatments for chronic pain is needed. The Joint Commission recently expanded its pain management guidelines to support hospitals offering nonpharmacologic pain treatments.23 Integrating AS-M, LTO, or similar products into standard pain management practices allows for other treatment pathways with positive outcomes for providers and patients. The Joint Commission also recommends an interdisciplinary approach, defined as a process whereby health care professionals from different disciplines collaborate to diagnose and treat patients experiencing difficult pain conditions. This approach facilitates multimodal management because these disciplines contribute knowledge about a variety of treatment options. Devices such AS-M and LTO are well suited to interdisciplinary pain management because they are not seen as being under the purview of a specific health care specialty.
Limitations
Our findings are limited because they are derived from a retrospective, quality improvement evaluation of outcomes from a single clinic. Findings must be considered in the context of the relatively small samples of veterans. Because analyses were conducted as part of a quality improvement effort, veterans were offered a specific device based on clinical indications, there were no comparisons between devices, and there was no comparison group. Although most participants were using medication and other treatments as part of their pain treatment plan, all reported continued pain intensity before use of a device. Analyses did not control for variation in treatments received concurrently. Last, the logs used to collect self-report data on daily pain and relaxation levels were not validated.
The data highlight a clear need for research to better understand the long-term effects of these devices as well as the characteristics of patients who respond best to each device. Noninvasive treatments for pain often are dismissed as placebos. Rigorously designed, controlled studies will help demonstrate that these devices offer a statistically significant benefit beyond any placebo effect.
Conclusion
Understanding of chronic pain and its treatment will continue to evolve. It is clear that each person dealing with chronic pain requires a tailored combination of treatments and multimodal approaches, which is more effective than any single treatment. Nonpharmacologic, noninvasive devices pose fewer risks and seem to be more effective in reducing pain intensity than traditional treatments, including medications or surgical intervention. In light of the current emphasis on evidence-based health care and as the evidence for the effectiveness of noninvasive pain devices modalities grows, it is likely that treatments incorporating modalities such as MET, CES, and LLLT will become common options for managing chronic pain.
Chronic pain is common among veterans treated in Veterans Health Administration (VHA) facilities, and optimal management remains challenging in the context of the national opioid misuse epidemic. The Eastern Oklahoma VA Health Care System (EOVAHCS) Pain Program offers a range of services that allow clinicians to tailor multimodal treatment strategies to a veteran’s needs. In 2014, a Modality Clinic was established to assess the utility of adding noninvasive treatment devices to the pain program’s armamentarium. This article addresses the context for introducing these devices and describes the EOVAHCS Pain Program and Modality Clinic. Also discussed are procedures and findings from an initial quality improvement evaluation designed to inform decision making regarding retention, expansion, or elimination of the EOVAHCS noninvasive, pain treatment device program.
Opioid prescriptions increased from 76 million in 1991 to 219 million in 2011. In 2011, the annual cost of chronic pain in the US was estimated at $635 billion.1-6 The confluence of an increasing concern about undertreatment of pain and overconfidence for the safety of opioids led to what former US Surgeon General Vivek H. Murthy, MD, called the opioid crisis.7 As awareness of its unintended consequences of opioid prescribing increased, the VHA began looking for nonopioid treatments that would decrease pain intensity. The 1993 article by Kehlet and Dahl was one of the first discussions of a multimodal nonpharmacologic strategy for addressing acute postoperative pain.8 Their pivotal literature review concluded that nonpharmacologic modalities, such as acupuncture, cranial manipulation, cranial electrostimulation treatment (CES), and low-level light technologies (LLLT), carried less risk and produced equal or greater clinical effects than those of drug therapies.8
Multimodal treatment approaches increasingly are encouraged, and nonopioid pain control has become more common across medical disciplines from physical therapy to anesthesiology.8-10 Innovative, noninvasive devices designed for self-use have appeared on the market. Many of these devices incorporate microcurrent electrical therapy (MET), CES, and/or LLLT (also known as cold laser).11-16 LLLT is a light modality that seems to lead to increased ATP production, resulting in improved healing and decreased inflammation.13-16 Although CES has been studied in a variety of patient populations, its effectiveness is not well understood.16 Research on the effects of CES on neurotransmitter levels as well as activation of parts of the brain involved in pain reception and transmission should clarify these mechanisms. Research has shown improvements in sleep and mood as well as overall pain reduction.11,16 Research has focused primarily on individual modalities rather than on combination devices and has been conducted on populations unlike the veteran population (eg, women with fibromyalgia).
Most of the devices that use electrical or LLLT cannot be used safely by patients who have implantable electrical devices or have medical conditions such as unstable seizures, pregnancy, and active malignancies.
The most common adverse effects (AEs) of CES—dizziness and headaches—are minimal compared with the AEs of pain medications. MET and LLLT AEs generally are limited to skin irritation and muscle soreness.11 Most devices require a prescription, and manufacturers provide training for purchase.
The Pain Program
EOVAHCS initially established its consultative pain program in 2013 to provide support, recommendations, and education about managing pain in veterans to primary care providers (PCPs). Veterans are referred to the pain program for a face-to-face assessment and set of recommendations to assist in developing a comprehensive pain treatment plan. Consistent with its multimodal, biopsychosocial rehabilitation model approach, the program also offers several chronic pain treatment services, including patient education courses, cognitive behavioral therapy (CBT) for chronic pain, chiropractic care, biofeedback, relaxation training, steroid injections, pain coaching, and a pain modality (noninvasive device) clinic. During their assessment, veterans are evaluated for the appropriateness of these programs, including treatment through the Pain Modality Clinic.
Pain Modality Clinic
The EOVAHCS Pain Modality Clinic was created in 2014 as a treatment and device-trial program to provide veterans access to newer noninvasive, patient-driven treatment devices as part of an active chronic pain self-management plan. A crucial innovation is that these devices are designed to be used by patients in their homes. These devices can be expensive, and not every patient will benefit from their use; therefore, clinic leaders recommended a trial before a device is issued to a veteran for home use.
The Pain Modality Clinic coordinator trains clinic facilitators on the device according to manufacturer’s guidelines. Each participating veteran takes part in a device trial to confirm that he or she is able to use the recommended device independently and is likely to benefit from its use. When appropriate, veterans who do not respond to the initial device trial could test the potential benefit of another device. Although data from these device trials are collected primarily to inform clinical decision making, this information also is useful in guiding local policy regarding continued support for each of the modalities.
Veterans who have chronic or persistent pain (≥ 3 months) that interferes with function or quality of life are considered good candidates for a device trial if they are actively involved in pain self-care, logistically able to participate, able to use a device long-term, and have no contraindications. “Active involvement” could be met by participation in any pain management effort, whether a specific exercise program, CBT, or other treatment.
The Modality Clinic currently offers device trials for persistent pain with Alpha-Stim-M (AS-M; Electromedical Products International, Mineral Wells, TX), Laser Touch One (LTO; Renewal Technologies, LLC, Phoenix, AZ), and Neurolumen (Oklahoma City, OK). Neurolumen devices were not available in the clinic initially and will not be discussed further in this article.
The first Alpha-Stim machine using MET and CES technology was created in 1981 for in-office pain management. In 2012, the currently used AS-M became available.11 AS-M is FDA approved for treating pain, anxiety, depression, and sleep problems and is the device used in the EOVAHCS Modality Clinic. AS-M uses probes or electrodes to send a MET waveform through the body area in pain. The device uses ear clips to provide CES, which is thought to increase alpha waves in the brain.11 The LTO is a device that combines LLLT and MET technologies in a home-use design.14 LTO is FDA approved for treating painand is a portable personal pain-relief device applied to the area of pain using electroconductive gel.
Both devices are designed for long-term, self-use, making them viable parts of a multimodal, chronic pain treatment plan. Contraindications for AS-M and LTO include having a pacemaker or an implantable defibrillator, pregnancy, current malignancy, or seizures. Eligible veterans with persistent pain and high levels of depression, anxiety, and/or sleep problems generally are triaged to AS-M, whereas those who have only pain intensity issues usually are assigned to LTO. Referral to the Modality Clinic is not limited to a specific type of pain; common pain conditions seen in the clinic are spine and joint pain, arthritis pain, myofascial pain, headaches, and neuropathy.
Training and Device Trials
Eligible veterans are educated about the device and complete clinical informed consent, which is documented in the electronic health record. The veterans’ primary care and/or specialist providers are contacted for concurrence regarding veterans’ participation in the treatment.
Protocols for the device trials are based on the manufacturers’ recommendations, adjusted to what is feasible in the clinic (manufacturers approved the changes). The number of treatments per trial varies by device. For AS-M, veterans come to the clinic 5 days a week for 2 weeks. For LTO, veterans attend the clinic 5 days a week for 1 week.
At the beginning of a device trial, a trained facilitator teaches each veteran and caregiver to use the device, sets functional goals for the trial, and provides education on the trial questionnaires and daily pain logs. The veteran then follows the device protocol in the clinic where the facilitator can respond to questions and address any issues. With support from their caregivers, veterans are expected to become independent on their device use by the end of the trial. Clinic staff or the veteran can stop the device trial at any point, without affecting the veteran’s participation in or eligibility for other EOVAHCS pain programs.
This project was submitted to the University of Oklahoma Health Sciences Center Institutional Review Board and was exempted from institutional review board oversight as a retrospective, quality improvement effort. Before data analysis, the EOVAHCS Coordinator for Research and Development reviewed the procedures to ensure that all policies were being followed.
Methods
Data for veterans who completed valid treatments of AS-M or LTO from May 9, 2014 to August 20, 2016, were included in the analyses. For an AS-M treatment to be considered valid, the veteran must have attended at least 8 sessions and completed assessment instruments at baseline (preintervention) and following completion (postintervention). For an LTO treatment to be considered valid, the veteran must have attended at least 4 sessions and completed assessment measures at baseline and after completion.
Measures
Veterans completed the following measures at baseline and after trial completion:
The Beck Depression Inventory (BDI-II) is a 21-item measure designed to assess depressive symptoms. Each item assesses intensity on a 0-to-3 scale. Scores from 0 to 13 indicate minimum depression; 14 to 19, mild depression; 20 to 28, moderate depression, and 29 to 63, severe depression.17
The Beck Anxiety Inventory (BAI) is a 21-item measure of anxiety symptoms that uses a 0-to-3 scale to assess severity of subjective, somatic, or panic-related symptoms of anxiety. Scores ranging from 0 to 9 indicate minimal anxiety; 10 to 16, mild anxiety; 17 to 29, moderate anxiety, and 30 to 63, severe anxiety.18
The Pain Catastrophizing Scale (PCS) is a 13-item measure of pain catastrophizing, a crucial marker of how individuals experience pain. Items are scored on a 0-to-4 scale; scores of ≥ 30 indicate a clinically relevant level of catastrophizing.19
The Subjective Units of Distress Scale (SUD) is a single-item measure of the subjective intensity of disturbance or distress currently being experienced. It is scored from 0 to 10; 1 to 4 is mild, 5 to 6 is moderate, and 7 to 10 is severe.20
The Brief Pain Inventory (BPI) measures pain intensity and the impact of pain on functioning. Four items assess pain intensity at its worst, least, and average over the previous 24 hours and at the time of assessment; responses are on a 0-to-10 scale with 10 being most severe. The pain intensity measure is the average of scores on these 4 items. Pain interference is measured with respect to 7 daily activities; general activity, walking, work, mood, enjoyment of life, relations with others, and sleep. Each of these items is scored on a 0-to-10 scale with 10 being the most severe. The pain interference measure is the average of scores on these 7 items.21
Participants completed a daily pain log and recorded self-ratings (0-to-10 scale) of pain and relaxation levels before and after using the device. These scores were primarily used to assist in determining whether goals, set collaboratively by the clinician and the veteran at the first session, had been met.
Analysis
Descriptive statistics were used to characterize the sample overall and by modality. Paired t tests were used to assess changes on each assessment measure over time and for each device separately. The significance of change was assessed for 8 outcomes for each device. In this context, using a conservative Bonferroni correction, significance was set at P < .006. Because AS-M is designed to address depression, anxiety, and sleep as well as pain, whereas LTO is not, device assignments were based on clinical considerations rather than randomization. Therefore, no comparisons were made between devices, and outcomes were assessed independently for the 2 devices. Analyses were performed using SAS 9.4 (Cary, NC).
Results
Device trials were initiated for 161 veterans (LTO, 70; AS-M, 91). Distribution of devices was unequal because veterans are assigned to 1 device or the other based on clinical presentation. Failure to complete a trial (n = 46; 28.6%) typically was because of travel barriers, lack of interest in continuing, and for 3 veterans, reports of headaches that they attributed to the AS-M treatment. Of the 115 participants who completed valid trials, 88 (76.5%) also completed assessment measures at pre- and postintervention (LTO = 38; AS-M = 50). None of the participants in this study completed trials with both the AS-M and LTO devices.
Most participants were male (84.1%) and rural residents (85.5%) (Table 1).
Pain Reduction
Treatment with AS-M or LTO was associated with statistically significant reductions in pain severity (BPI), pain interference (BPI), daily pain intensity scores (daily pain log), and pain catastrophizing (PCS) (Tables 2 and 3).
Impact on Mood
Use of AS-M was associated with statistically significant improvements in depression (BDI-II), anxiety (BAI), and distress (SUD) scores. In addition, veterans completing AS-M treatment showed a statistically significant improvement in self-reported relaxation scores. Interestingly, use of LTO also resulted in a statistically significant decrease in anxiety (BAI) and a nonstatistically significant decrease in depression (BDI-II).
Figure 1 and 2 illustrates the clinical impact of each device in shifting participants from 1 level of symptom severity to another.
Discussion
Use of both AS-M and LTO at EOVAHC was associated with reduced pain intensity. The devices also had positive effects beyond pain in areas such as depression, anxiety, and distress. Remission of depression and anxiety symptoms has been associated with significant decline in pain symptoms, suggesting that pain is best treated through multimodal approaches.22
In the context of the opioid crisis, the availability of effective nonopioid, nonpharmacologic, noninvasive treatments for chronic pain is needed. The Joint Commission recently expanded its pain management guidelines to support hospitals offering nonpharmacologic pain treatments.23 Integrating AS-M, LTO, or similar products into standard pain management practices allows for other treatment pathways with positive outcomes for providers and patients. The Joint Commission also recommends an interdisciplinary approach, defined as a process whereby health care professionals from different disciplines collaborate to diagnose and treat patients experiencing difficult pain conditions. This approach facilitates multimodal management because these disciplines contribute knowledge about a variety of treatment options. Devices such AS-M and LTO are well suited to interdisciplinary pain management because they are not seen as being under the purview of a specific health care specialty.
Limitations
Our findings are limited because they are derived from a retrospective, quality improvement evaluation of outcomes from a single clinic. Findings must be considered in the context of the relatively small samples of veterans. Because analyses were conducted as part of a quality improvement effort, veterans were offered a specific device based on clinical indications, there were no comparisons between devices, and there was no comparison group. Although most participants were using medication and other treatments as part of their pain treatment plan, all reported continued pain intensity before use of a device. Analyses did not control for variation in treatments received concurrently. Last, the logs used to collect self-report data on daily pain and relaxation levels were not validated.
The data highlight a clear need for research to better understand the long-term effects of these devices as well as the characteristics of patients who respond best to each device. Noninvasive treatments for pain often are dismissed as placebos. Rigorously designed, controlled studies will help demonstrate that these devices offer a statistically significant benefit beyond any placebo effect.
Conclusion
Understanding of chronic pain and its treatment will continue to evolve. It is clear that each person dealing with chronic pain requires a tailored combination of treatments and multimodal approaches, which is more effective than any single treatment. Nonpharmacologic, noninvasive devices pose fewer risks and seem to be more effective in reducing pain intensity than traditional treatments, including medications or surgical intervention. In light of the current emphasis on evidence-based health care and as the evidence for the effectiveness of noninvasive pain devices modalities grows, it is likely that treatments incorporating modalities such as MET, CES, and LLLT will become common options for managing chronic pain.
1. US Department of Veterans Affairs. Pain as the 5th Vital Sign Toolkit. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Published October 2000. Accessed February 11, 2019.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
3. Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405-416.
4. Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5-12.
5. Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Weg MW, Lund BC. Trends in prevalent and incident opioid receipt: an observational study in Veterans Health Administration 2004-2012. J Gen Intern Med. 2015;30(5):597-604.
6. Reuben DB, Alvanzo AAH, Ashikaga T, et al. National Institutes of Health Pathways to Prevention Workshop: The role of opioids in the treatment of chronic pain. Ann Intern Med. 2015;162(4):295-300.
7. Murthy VH. Opioid epidemic: we all have a role in turning the tide. https://obamawhitehouse.archives.gov/blog/2016/10/05/opioid-epidemic-we-all-have-role-turning-tide. Published October 5, 2016. Accessed February 12, 2019.
8. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
9. Crane P, Feinberg L, Morris J. A multimodal physical therapy approach to the management of a patient with temporomandibular dysfunction and head and neck lymphedema: a case report. J Man Manip Ther. 2015;23(1): 37-42.
10. Arnstein P. Multimodal approaches to pain management. Nurs. 2011;41(3): 60-61.
11. Alpha-Stim. http://www.alpha-stim.com. Accessed March 22, 2019
12. Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia. Ann Intern Med. 2018;168(6):414-421.
13. Chow RT, Heller GZ, Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006;124(1):201-210.
14. Kulkarni AD, Smith RB. The use of microcurrent electrical therapy and cranial electrotherapy stimulation in pain control. Clin Pract Alternative Med. 2001;2(2):99-102.
15. Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897-1908.
16. Taylor AG, Anderson JG, Riedel SL, et al. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Manag Nurs. 2013;14(4):327-335.
17. Beck, AT, Steer, RA, Brown, GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
18. Beck AT, Steer RA. Beck Anxiety Inventory: Manual. San Antonio, TX: Psychological Corporation; 1993.
19. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524-532.
20. Wolpe J. The Practice of Behavior Therapy. 4th ed. Elmsford, NY: Pergamon; 1990.
21. Cleeland CS. The Brief Pain Inventory User Manual. https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/brief-pain-inventory.html. Published 2009. Accessed February 12, 2019.
22. Gerrits MM, van Marwijk HW, van Oppen P, Horst HVD, Penninx BW. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64-70.
23. The Joint Commission. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. https://www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Published July 2017. Accessed March 21, 2019.
1. US Department of Veterans Affairs. Pain as the 5th Vital Sign Toolkit. https://www.va.gov/PAINMANAGEMENT/docs/Pain_As_the_5th_Vital_Sign_Toolkit.pdf. Published October 2000. Accessed February 11, 2019.
2. Institute of Medicine (US) Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011.
3. Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: Controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405-416.
4. Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5-12.
5. Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Weg MW, Lund BC. Trends in prevalent and incident opioid receipt: an observational study in Veterans Health Administration 2004-2012. J Gen Intern Med. 2015;30(5):597-604.
6. Reuben DB, Alvanzo AAH, Ashikaga T, et al. National Institutes of Health Pathways to Prevention Workshop: The role of opioids in the treatment of chronic pain. Ann Intern Med. 2015;162(4):295-300.
7. Murthy VH. Opioid epidemic: we all have a role in turning the tide. https://obamawhitehouse.archives.gov/blog/2016/10/05/opioid-epidemic-we-all-have-role-turning-tide. Published October 5, 2016. Accessed February 12, 2019.
8. Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77(5):1048-1056.
9. Crane P, Feinberg L, Morris J. A multimodal physical therapy approach to the management of a patient with temporomandibular dysfunction and head and neck lymphedema: a case report. J Man Manip Ther. 2015;23(1): 37-42.
10. Arnstein P. Multimodal approaches to pain management. Nurs. 2011;41(3): 60-61.
11. Alpha-Stim. http://www.alpha-stim.com. Accessed March 22, 2019
12. Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia. Ann Intern Med. 2018;168(6):414-421.
13. Chow RT, Heller GZ, Barnsley L. The effect of 300 mW, 830 nm laser on chronic neck pain: a double-blind, randomized, placebo-controlled study. Pain. 2006;124(1):201-210.
14. Kulkarni AD, Smith RB. The use of microcurrent electrical therapy and cranial electrotherapy stimulation in pain control. Clin Pract Alternative Med. 2001;2(2):99-102.
15. Chow RT, Johnson MI, Lopes-Martins RA, Bjordal JM. Efficacy of low-level laser therapy in the management of neck pain: a systematic review and meta-analysis of randomised placebo or active-treatment controlled trials. Lancet. 2009;374(9705):1897-1908.
16. Taylor AG, Anderson JG, Riedel SL, et al. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Manag Nurs. 2013;14(4):327-335.
17. Beck, AT, Steer, RA, Brown, GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996.
18. Beck AT, Steer RA. Beck Anxiety Inventory: Manual. San Antonio, TX: Psychological Corporation; 1993.
19. Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524-532.
20. Wolpe J. The Practice of Behavior Therapy. 4th ed. Elmsford, NY: Pergamon; 1990.
21. Cleeland CS. The Brief Pain Inventory User Manual. https://www.mdanderson.org/research/departments-labs-institutes/departments-divisions/symptom-research/symptom-assessment-tools/brief-pain-inventory.html. Published 2009. Accessed February 12, 2019.
22. Gerrits MM, van Marwijk HW, van Oppen P, Horst HVD, Penninx BW. Longitudinal association between pain, and depression and anxiety over four years. J Psychosom Res. 2015;78(1):64-70.
23. The Joint Commission. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. https://www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Published July 2017. Accessed March 21, 2019.
Providing Rural Veterans With Access to Exercise Through Gerofit
Clinical video telehealth can be used to deliver functional circuit exercise training to older veterans in remote locations.
Exercise increases endurance, muscle strength, and functional performance with corresponding gains in mobility, survival, and quality of life.1 However, even with these benefits and improvements in clinical outcomes, only 15% of adults aged ≥ 65 years follow current guidelines for exercise.2 Despite their prior military training, the majority of veterans do not meet physical activity recommendations.3 Time, travel, and support are common barriers to exercise participation and adherence—barriers that are further amplified among older adults.
The Veterans Health Administration (VHA) is recognized as a world leader in telehealth service development. Currently, 677,000 veterans have received telehealth services, which represents 12% of the 5.6 million veterans under VHA care.4 Clinical video telehealth (CVT) is widely used within the VHA system to deliver health care that otherwise would not be available to veterans. Veterans who have difficulty traveling to the nearest US Department of Veteran Affairs (VA) medical center (VAMC) can access CVT programs at a participating VHA community-based outpatient clinic (CBOC). The VA has more than 45 CVT programs, including programs for mental health, weight management, cardiology, and dermatology. Outside the VA, cardiac exercise rehabilitation provided by CVT has been shown to be as effective as center-based programs in improving cardiovascular risk factors and functional capacity.5 A VHA exercise program that leveraged CVT resources and was dedicated to older adults with a wide range of comorbid conditions would have a high impact on the health and well-being of older veterans.
Gerofit is a VHA clinical demonstration program of supervised center-based exercise for veterans aged ≥ 65 years. Developed at the Durham VAMC Geriatric Research, Education, and Clinical Center (GRECC) in North Carolina, it has demonstrated improved clinical outcomes, including physical function, mobility, quality of life, and survival.6-10 The program offers veterans individualized exercise in a group setting that focuses on improving endurance, strength, and balance. The exercise prescription is based on the patient’s physical limitations as identified in a physical performance assessment.
With support from VHA Geriatric Extended Care (GEC) and the Office of Rural Health (ORH), Gerofit was implemented in 10 VAMCs across 8 VISNs. However, barriers such as travel time, distance, and transportation limit participation. Previously, we found that rural veterans lack access to exercise programs.11,12 Although some do aerobic exercise (AEX), most do not do resistance training (RT), though they are willing to learn. Access to Gerofit for rural veterans is expanding with recent support from the ORH Enterprise Wide Initiative. Rural program expansion includes several different Gerofit initiatives, many involving CBOCs.
The Salem VAMC Gerofit program sought to adapt the facility-based assessment and exercise procedures into a self-reliant CVT class for its CBOCs. This article describes the development of the Salem VAMC Gerofit CVT program, hereafter referred to as Tele-Gerofit.
Related: Expanding the Scope of Telemedicine in Gastroenterology
Program Design
Gerofit was established in 1986 at the Durham GRECC as an exercise and health promotion program for veterans aged ≥ 65 years.13 Its goal is to prevent or improve functional decline from physical inactivity and age-related conditions. Gerofit targets the geriatric patient population and thus extends beyond cardiac and pulmonary rehabilitation or weight loss programs. The primary exclusion criteria are based on safety issues in the context of a group exercise setting of older adults and include oxygen dependency, unstable cardiac disease, and moderate-to-severe cognitive impairment.
To participate in Gerofit, veterans must be able to perform activities of daily living and self-manage an exercise prescription developed by the exercise instructor based on physical performance testing. These physical performance tests include measures that are independent predictors of disability, loss of independent living, and death, as well as surrogate measures of exercise capacity (eg, strength, endurance, balance).14,15 A novel aspect of Gerofit is that the physical performance assessment is used not only to determine physical limitations, but also to individualize the exercise prescription based on the observed deficits in strength, endurance, or balance. These assessments are performed at initial enrollment; 3 months, 6 months, and 1 year later; and annually after that. Currently, the center-based Gerofit programs administer 5 items of the Senior Fitness Test: 6-minute walk, 10-meter walk (10-MWT), 30-second 1-arm curl, 30-second chair-stand test, and 8-foot up-and-go.15 The side-by-side, semitandem, and tandem standing balance tests from the short physical performance battery also are performed.16 In addition, participants complete a questionnaire that includes items from the physical functioning scale of the 12-Item Short Form Health Survey (SF-12).
After each assessment, the Gerofit exercise instructor reviews the results with the veteran and formulates an individualized exercise prescription along with goals for improvement. Veterans are encouraged to attend supervised center-based exercise sessions 3 times weekly. Classes are offered in a gym or fitness center at the VAMC or in leased space. Each patient uses a cue card that lists an exercise plan personalized for intensity and duration for aerobic exercise (AEX; eg, treadmill walking, stationary bicycling, arm ergometry), RT using dumbbells and weight equipment, and functional exercises for flexibility and balance. Some medical centers also offer yoga, tai chi, or dancing Gerofit classes.
For participants in the Durham Gerofit program, mortality decreased 25% over a decade (hazard ratio, 0.75; 95% CI, 0.61-0.91).9 A substudy that included the Psychological General Well-Being Index found that 81% of participants significantly increased their score after 1 year.7 Observed initial improvement in physical performance has been sustained over 5 years.10,17 One-year results from the recent Gerofit expansion to 6 other VAMCs showed clinically and statistically significantly improved physical performance from baseline to 3-, 6-, and 12-month follow-up.18
Adaptation of Gerofit to CVT Delivery
Initial work. The Greater Los Angeles VAMC Gerofit program conducted a pilot CVT exercise class of 6 veterans at the rural Bakersfield CBOC in California.19 Each week, an exercise instructor broadcast a 60-minute exercise class that included warm-up, RT with bands, progressive balance training, and flexibility. Trained student volunteers from California State University in Bakersfield kinesiology program were on site at the Bakersfield CBOC to perform the assessments and aid in exercises during the CVT sessions. Despite the lack of AEX per se, veterans showed significant improvement in endurance as measured by an increase in the number of steps completed in 2 minutes at the 3-month assessment (P = .049). Although exercises were not delivered in a circuit format, the improved endurance supported the potential for cardiovascular benefit from RT in older adults.
This pilot project also demonstrated that key components of the Gerofit program could be delivered safely by telehealth with onsite supervision. The Miami VA Healthcare System also offers CVT Gerofit exercise classes broadcast to the rural Florida CBOCs of Key Largo and Homestead.11 The exercise activities offered for the Miami CVT participants incorporate components of AEX (calisthenics) and RT (resistance bands). Veterans enjoyed the classes, and adherence was good. However, availability of staff and space are an ongoing challenge.
In Key Largo, 5 veterans participated before the CVT classes were placed on hold owing to the demands of other CVT programs and limited availability of the telehealth clinical technician (TCT). The Homestead CBOC continues to offer CVT Gerofit exercise classes and has 6 regular participants. Notably, the physical space at the Homestead CBOC is smaller than that at the Key Largo CBOC; the Homestead CBOC has adjusted by shifting to exercises performed while standing or sitting, ensuring participants’ safety and satisfaction.
The Baltimore, Maryland VAMC Gerofit program offers other innovative CVT exercise classes, including a tai chi class, and a class with exercise performed while sitting in a chair. Although the Baltimore VAMC CVT exercise classes do not have the scope of the center-based exercise prescriptions, they are unique in that they are broadcast not only to their affiliated CBOCs, but also other Gerofit programs in different VISNs.
Related: Telehealth for Rural Veterans With Neurologic Disorders
Salem VAMC Gerofit Program. The center-based Salem VAMC Gerofit program was established in July 2015. In fiscal year 2017, its dedicated exercise facility had more than 5,000 patient visits. Despite the program’s success, we prioritized establishing Tele-Gerofit because of the medical center’s rural location in southwest Virginia and the large number of veterans who receive care at CBOCs. Therefore, much as with the pilot CVT Gerofit classes in Los Angeles and Miami, the target setting was rural CBOCs. The goal for Salem VAMC Tele-Gerofit was to modify Gerofit delivery to the CVT format and a CBOC setting with minimal modification of the content and personnel requirements of both physical performance testing and exercise training procedures.
Adjustments for CBOC Setting. The enrollment process for Tele-Gerofit is the same as that for the center-based program. To start, a veteran’s primary care provider reviews the list of eligibility criteria and, if the veteran qualifies, places a consult. A Gerofit team member then contacts the veteran by phone to describe the program and schedule an assessment. At the baseline physical performance assessment, American College of Sports Medicine guidelines on exercise participation, health screening, and exercise intensity are used to evaluate veterans and rank them by their cardiovascular risk.20 All new program participants start with low-intensity exercise and gradually progress to recommended levels of exercise. Before starting an exercise class, participants are instructed on use of the 10-point rating of perceived exertion (RPE).
Each CBOC site is supplied with an RPE poster that is displayed for participants’ use. During a Tele-Gerofit class, the exercise instructor asks participants to periodically report their RPE. This class differs slightly from the center-based exercise sessions in which RPE is primarily assessed when a different exercise is introduced or the duration or intensity of an exercise is increased. The Gerofit instructor monitors exercise and treatment fidelity, but the onsite TCT observes for safety during class. The TCT also takes initial vital signs and sets up the room for the class. Emergency contacts and procedures are posted in each CBOC CVT room and are available to the center-based exercise instructor. Because the CBOCs are not inside medical facilities, some CBOC directors have asked that heart rate monitors be used as an extra safety precaution to ensure that high-risk participants do not exceed a heart rate limit that may be set by their cardiologists.
Modifications to Physical Performance Assessment. Physical performance testing had to be adapted to the small rooms available at the CBOCs. For measuring normal gait speed, the 10-MWT was replaced with the 4-meter walk test (4-MWT). The 4-MWT has excellent test–retest reliability with an intraclass correlation coefficient (ICC) of 0.93, but the discrepancy in gait speed between the 4-MWT and the 10-MWT is such that the tests cannot be used interchangeably.21 For measuring endurance, the 6-minute walk test was replaced with the 2-minute step test (2-MST). In older adults, the 2-MST has a moderate correlation with 6-minute walk distance (r = 0.36; P = .04) and high reliability (ICC, 0.90).15,22 The 30-second 1-arm curl, the 30-second chair-stand test, and the 8-foot up-and-go test are performed without modification and require only dumbbells, a chair without wheels, and a stopwatch.
The exercise instructor at the Salem VAMC conducts physical performance testing by 2-way videoconferencing with the veteran in a room at the CBOC. The TCT at the CBOC assists by measuring and demarcating 4 meters on the floor and a designated height on the wall for knee elevation for 4-MWT and 2-MST, respectively. The TCT remains in the room during the assessment visit. Except for taking vital signs before and after the physical performance assessment, the TCT does not participate in the testing. To date, more than 20 physical performance assessments have been conducted without difficulty at Salem-affiliated CBOCs. The primary challenge has been scheduling the room with CVT equipment (ie, camera and screen) for the 30-minute individual assessment session, which occurs on a rolling basis as individuals are enrolled and followed.
After the assessment is completed, the exercise instructor reviews the results with the participant and provides feedback on areas in need of improvement. However, these education sessions can be lengthy and are best supported by giving the patient a personalized handout.
Functional Circuit Exercise. In Tele-Gerofit, exercise training is delivered by CVT broadcast from the Salem VAMC to veterans in a room (equipped with steps, dumbbells, chairs, and bands) at the CBOC. This type of exercise training, which uses only mobile equipment and plyometric (weight-bearing) exercises, is referred to as functional exercise. The AEX includes marching in place, moving on and off a raised step, and body-weight exercises, while RT uses dumbbells, resistance bands, and plyometric exercises (Table 2).
Progression of intensity is achieved by increasing the rate of stepping and the size of the steps (AEX) or the number of repetitions and the weight of the dumbbells or bands (RT). Each veteran exercises at an intensity level that is appropriate for his or her baseline limitations and medical conditions. The exercise instructor uses different forms of the same equipment (eg, heavier dumbbells, higher steps) to vary intensity among individuals while having them perform the same exercises as a group. The challenge is to adjust the pace of the AEX or the timing of the RT repetitions for individuals new to the class.
Delivery of exercise training in the form of circuits allows for a diverse exercise program in a setting with limited space. Circuit training is an exercise modality that consists of a series of different exercises, each usually completed in 30 to 60 seconds, with minimal rest between each type of exercise. Each Tele-Gerofit circuit has a mix of AEX and RT exercises performed for 3 minutes consecutively (Figure).
The design of the circuit training can be adjusted based on the number of individuals in the class. Larger classes can be split into 2 groups that alternate between exercise sets, while smaller classes have 1 group performing the same exercise set and then rotating to either the AEX or RT set. Total exercise time to complete the circuit depends on the number of different exercises, number of repetitions, and the rest between repetitions and the different exercises. In this way, total exercise time can be made shorter or longer depending on the veteran’s capacity.
Frequency. Tele-Gerofit exercise classes are currently offered twice weekly and last about 1 hour, which includes warm-up (8-10 minutes), functional circuit training (40 minutes), and cooldown/stretching (8-10 minutes). A challenge for the exercise instructor is the need to provide ongoing clear instructions both to the class and to individuals as needed. As the exercise prescription for each patient is based on physical performance testing, the exercise instructor for the training must be familiar with the test results. Derivation of the exercise prescription in Tele-Gerofit follows the same process as center-based Gerofit.
Each patient is given an exercise prescription written to address any impairments noted in the different domains of the physical performance assessment, scored using age and sex percentiles. For instance, individuals scoring poorly on lower body strength are given specific lower body strengthening exercises. Participants are given an exercise program that guides them toward achieving recommended physical activity guidelines using their RPE to modulate each exercise. Duration and intensity of each type of planned exercise are formally discussed after initial and follow-up assessments. In addition, exercise training is informally progressed throughout the program. For Tele-Gerofit, instructors must design each class with the group in mind while being prepared for modifications and specific changes for individuals.
Discussion
Tele-Gerofit adapts the well-established center-based Gerofit program to be executed without an exercise facility while maintaining the content of the evidence-based procedures. Physical performance testing and exercise training were modified, adding elements necessary for CVT assessments and classes to be broadcast from the Salem VAMC to its affiliated CBOCs. Tele-Gerofit exercises are performed in a circuit style that allows a veteran or small structured groups of veterans to move among exercises and requires less space than traditional group exercise does. Safety and monitoring concerns are addressed with a safety procedure that includes emergency plans for each site, prescreening of enrolled participants, and monitoring of exercise intensity in accordance with national guidelines.1 Similar to the center-based Gerofit program, the exercise prescription is tailored to each veteran’s physical limitations based on initial and ongoing assessment of physical performance. Tele-Gerofit physical performance testing fulfills the same need with only a few modifications using validated measures. Tele-Gerofit assessments are administered by CVT without the need for additional staff on site.
Adaptation of center-based Gerofit exercise classes to Tele-Gerofit is a major innovation. Use of a circuit exercise design was supported by findings in older adults that RT alone, when performed quickly with minimal rest between each set and exercise station, increases both aerobic capacity and strength.23,24 Older adult RT trials that compared circuit RT with traditional RT found that strength gains are comparable between circuit and traditional RT.24-26 Working with adults aged > 60 years, Takeshima and colleagues conducted a trial of circuit exercise with added callisthenic exercises performed in place between RT on exercise machines.27 This dual-modality (AEX+RT) circuit approach was well tolerated and effective, increasing aerobic capacity and strength. Unfortunately, the resistance exercise machines used in those circuit exercise studies and in the center-based Gerofit program are not an option for Tele-Gerofit.
The requirement for an exercise facility was removed by designing Tele-Gerofit exercise to include only functional exercises that rely on body weight or small mobile exercise equipment. Although popular among young adults, functional circuit exercise is understudied in older adults. Recently, a 12-week functional circuit exercise intervention in frail elderly adults demonstrated significant improvements in gait speed and the timed chair-stand test.28 A pilot observational study of Gerofit participants at the Canandaigua VAMC offered 27 veterans functional circuit exercise instead of their traditional exercise facility class and found larger increases in the timed chair-stand test and 6-minute walk distance compared with 11 Gerofit participants in the traditional program.29
This Tele-Gerofit exercise training combines functional and circuit exercise strategies into telehealth delivery. However, its effect on physical performance remains to be demonstrated. To address this question, we are conducting a single-arm pilot study of Tele-Gerofit with CVT broadcast to 3 Salem CBOC affiliates (Wytheville, Staunton, and Danville, Virginia). The goal is to determine the effect on physical performance and collect feasibility data, including attendance rate and patient satisfaction with the video broadcast. In addition, we are planning an effectiveness trial to compare the impact of functional circuit exercise delivered in person (center based, not CVT) with the parent Gerofit exercise program on direct measures of endurance and strength, in addition to physical performance.
Related: Setting and Method of Measurement Affect Blood Pressure Readings in Older Veterans
Implementation research is needed to determine how Tele-Gerofit can be disseminated to other VAMCs and community-based centers beyond CBOCs. Although the cost of the equipment used to implement Tele-Gerofit is minimal, the program requires dedicated and experienced exercise instructors, and the sharing of telehealth resources with other clinical programs. The authors expect that a diverse group of stakeholders is needed across service lines of primary care, geriatrics and extended care, physical medicine and rehabilitation, and telehealth. Of note, this multidisciplinary collaboration is a hallmark of the Gerofit program. The recent success of the implementation of center-based Gerofit in VAMCs across the US demonstrates the program’s flexibility and robust results.18
Plans also include refining strategies for physical performance testing and exercise monitoring. For instance, we would like to adapt telehealth technology for heart rate monitors that can be worn by high-risk veterans at the CBOC and viewed in real time by the exercise instructor.
Conclusion
Gerofit, which is designed to help older veterans maintain independent living and prevent disability, has been demonstrated to improve quality of life and survival. Our goal has been to adapt Gerofit to CVT and provide a supervised, individualized exercise program in a group setting—a program that can be widely disseminated. Salem VAMC Tele-Gerofit is an innovative and prescriptive program that delivers CVT functional circuit exercise training to remote locations without the need for stationary exercise equipment. This approach has the potential to become an effective and feasible exercise strategy for preventing and minimizing disability in the increasing population of older veterans. Work is needed to determine whether Tele-Gerofit provides a rapid translation of Gerofit to clinical practice and improved outcomes with substantial cost savings from reduced hospitalization and institutionalization.
Acknowledgments
Gerofit has been funded by the Veterans Health Affairs Office of Geriatrics and Extended Care Non-Institutional Long-Term Care Funding and Mentored Partnership Program, and the Veterans Health Affairs Office of Rural Health Rural Enterprise-Wide Initiative.
The authors thank Kim Birkett, MPH, for assistance in editing, references, and graphics and the staff at the Wytheville, Staunton, and Danville community-based outpatient clinics for their support.
1. American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510-1530.
2. Centers for Disease Control and Prevention. Adult participation in aerobic and muscle-strengthening physical activities—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(17):326-330.
3. Littman AJ, Forsberg CW, Koepsell TD. Physical activity in a national sample of veterans. Med Sci Sports Exerc. 2009;41(5):1006-1013.
4. US Department of Veterans Affairs, Office of Rural Health. Annual Report: Thrive 2015. https://www.ruralhealth.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed July 16, 2018.
5. Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102(15):1183-1192.
6. Morey MC. Celebrating 20 years of excellence in exercise for the older veteran. Fed Pract. 2007;24(10):49-65.
7. Cowper PA, Morey MC, Bearon LB, et al. The impact of supervised exercise on the psychological well-being and health status of older veterans. J Appl Gerontol. 1991;10(4):469-485.
8. Morey MC, Cowper PA, Feussner JR, et al. Evaluation of a supervised exercise program in a geriatric population. J Am Geriatr Soc. 1989;37(4):348-354.
9. Morey MC, Pieper CF, Crowley GM, Sullivan RJ, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50(12):1929-1933.
10. Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44(10):1226-1231.
11. Valencia WM, Botros D, Pendlebury D, et al. Proactive reach and telehealth monitoring (Gerofit) enhance resistance exercise at rural setting. Innovat Aging. 2017;1(suppl 1):225.12. Pendlebury D, Botros D VW. Proactive Reach: an innovative access approach to identify & deliver GEROFIT exercise telehealth counseling to rural veterans & enhance CBOC services. J Am Geriatr Soc. 2017(suppl 1):S208. Poster presented at: Annual Scientific Meeting of the American Geriatrics Society; May 18, 2017; San Antonio, TX.
13. Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit program: a VA innovation. South Med J. 1994;87(5):S83-S87.
14. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467.
15. Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7(2):129-161.
16. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94.
17. Morey MC, Cowper PA, Feussner JR, et al. Two-year trends in physical performance following supervised exercise among community-dwelling older veterans. J Am Geriatr Soc. 1991;39(10):986-992.
18. Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit program. J Am Geriatr Soc. 2018;66(5):1009-1016.
19. Blanchard E, Castle S, Ines E, et al. Delivering a clinical exercise program to rural veterans via video telehealth. Poster C167 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
20. Riebe D, Ehrman JK, Liguori G, Magal M, eds; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia, PA: Wolters Kluwer Health; 2018.
21. Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36(1):24-30.
22. Pedrosa R, Holanda G. Correlation between the walk, 2-minute step and TUG tests among hypertensive older women. Rev Bras Fisioter. 2009;13(3):252-256.
23. Romero-Arenas S, Blazevich AJ, Martinez-Pascual M, et al. Effects of high-resistance circuit training in an elderly population. Exp Gerontol. 2013;48(3):334-340.
24. Brentano MA, Cadore EL, Da Silva EM, et al. Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J Strength Cond Res. 2008;22(6):1816-1825.
25. Romero-Arenas S, Martinez-Pascual M, Alcaraz PE. Impact of resistance circuit training on neuromuscular, cardiorespiratory and body composition adaptations in the elderly. Aging Dis. 2013;4(5):256-263.
26. Paoli A, Pacelli F, Bargossi AM, et al. Effects of three distinct protocols of fitness training on body composition, strength and blood lactate. J Sports Med Phys Fitness. 2010;50(1):43-51.
27. Takeshima N, Rogers ME, Islam MM, Yamauchi T, Watanabe E, Okada A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur J Appl Physiol. 2004;93(1-2):173-182.
28. Giné-Garriga M, Guerra M, Pagés E, Manini TM, Jiménez R, Unnithan VB. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. J Aging Phys Act. 2010;18(4):401-424.
29. Biddle ED, Reynolds P, Kopp T, Cammarata H, Conway P. Implementation of functional training tools elicits improvements in aerobic fitness and lower body strength in older veterans. Poster C169 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
Clinical video telehealth can be used to deliver functional circuit exercise training to older veterans in remote locations.
Clinical video telehealth can be used to deliver functional circuit exercise training to older veterans in remote locations.
Exercise increases endurance, muscle strength, and functional performance with corresponding gains in mobility, survival, and quality of life.1 However, even with these benefits and improvements in clinical outcomes, only 15% of adults aged ≥ 65 years follow current guidelines for exercise.2 Despite their prior military training, the majority of veterans do not meet physical activity recommendations.3 Time, travel, and support are common barriers to exercise participation and adherence—barriers that are further amplified among older adults.
The Veterans Health Administration (VHA) is recognized as a world leader in telehealth service development. Currently, 677,000 veterans have received telehealth services, which represents 12% of the 5.6 million veterans under VHA care.4 Clinical video telehealth (CVT) is widely used within the VHA system to deliver health care that otherwise would not be available to veterans. Veterans who have difficulty traveling to the nearest US Department of Veteran Affairs (VA) medical center (VAMC) can access CVT programs at a participating VHA community-based outpatient clinic (CBOC). The VA has more than 45 CVT programs, including programs for mental health, weight management, cardiology, and dermatology. Outside the VA, cardiac exercise rehabilitation provided by CVT has been shown to be as effective as center-based programs in improving cardiovascular risk factors and functional capacity.5 A VHA exercise program that leveraged CVT resources and was dedicated to older adults with a wide range of comorbid conditions would have a high impact on the health and well-being of older veterans.
Gerofit is a VHA clinical demonstration program of supervised center-based exercise for veterans aged ≥ 65 years. Developed at the Durham VAMC Geriatric Research, Education, and Clinical Center (GRECC) in North Carolina, it has demonstrated improved clinical outcomes, including physical function, mobility, quality of life, and survival.6-10 The program offers veterans individualized exercise in a group setting that focuses on improving endurance, strength, and balance. The exercise prescription is based on the patient’s physical limitations as identified in a physical performance assessment.
With support from VHA Geriatric Extended Care (GEC) and the Office of Rural Health (ORH), Gerofit was implemented in 10 VAMCs across 8 VISNs. However, barriers such as travel time, distance, and transportation limit participation. Previously, we found that rural veterans lack access to exercise programs.11,12 Although some do aerobic exercise (AEX), most do not do resistance training (RT), though they are willing to learn. Access to Gerofit for rural veterans is expanding with recent support from the ORH Enterprise Wide Initiative. Rural program expansion includes several different Gerofit initiatives, many involving CBOCs.
The Salem VAMC Gerofit program sought to adapt the facility-based assessment and exercise procedures into a self-reliant CVT class for its CBOCs. This article describes the development of the Salem VAMC Gerofit CVT program, hereafter referred to as Tele-Gerofit.
Related: Expanding the Scope of Telemedicine in Gastroenterology
Program Design
Gerofit was established in 1986 at the Durham GRECC as an exercise and health promotion program for veterans aged ≥ 65 years.13 Its goal is to prevent or improve functional decline from physical inactivity and age-related conditions. Gerofit targets the geriatric patient population and thus extends beyond cardiac and pulmonary rehabilitation or weight loss programs. The primary exclusion criteria are based on safety issues in the context of a group exercise setting of older adults and include oxygen dependency, unstable cardiac disease, and moderate-to-severe cognitive impairment.
To participate in Gerofit, veterans must be able to perform activities of daily living and self-manage an exercise prescription developed by the exercise instructor based on physical performance testing. These physical performance tests include measures that are independent predictors of disability, loss of independent living, and death, as well as surrogate measures of exercise capacity (eg, strength, endurance, balance).14,15 A novel aspect of Gerofit is that the physical performance assessment is used not only to determine physical limitations, but also to individualize the exercise prescription based on the observed deficits in strength, endurance, or balance. These assessments are performed at initial enrollment; 3 months, 6 months, and 1 year later; and annually after that. Currently, the center-based Gerofit programs administer 5 items of the Senior Fitness Test: 6-minute walk, 10-meter walk (10-MWT), 30-second 1-arm curl, 30-second chair-stand test, and 8-foot up-and-go.15 The side-by-side, semitandem, and tandem standing balance tests from the short physical performance battery also are performed.16 In addition, participants complete a questionnaire that includes items from the physical functioning scale of the 12-Item Short Form Health Survey (SF-12).
After each assessment, the Gerofit exercise instructor reviews the results with the veteran and formulates an individualized exercise prescription along with goals for improvement. Veterans are encouraged to attend supervised center-based exercise sessions 3 times weekly. Classes are offered in a gym or fitness center at the VAMC or in leased space. Each patient uses a cue card that lists an exercise plan personalized for intensity and duration for aerobic exercise (AEX; eg, treadmill walking, stationary bicycling, arm ergometry), RT using dumbbells and weight equipment, and functional exercises for flexibility and balance. Some medical centers also offer yoga, tai chi, or dancing Gerofit classes.
For participants in the Durham Gerofit program, mortality decreased 25% over a decade (hazard ratio, 0.75; 95% CI, 0.61-0.91).9 A substudy that included the Psychological General Well-Being Index found that 81% of participants significantly increased their score after 1 year.7 Observed initial improvement in physical performance has been sustained over 5 years.10,17 One-year results from the recent Gerofit expansion to 6 other VAMCs showed clinically and statistically significantly improved physical performance from baseline to 3-, 6-, and 12-month follow-up.18
Adaptation of Gerofit to CVT Delivery
Initial work. The Greater Los Angeles VAMC Gerofit program conducted a pilot CVT exercise class of 6 veterans at the rural Bakersfield CBOC in California.19 Each week, an exercise instructor broadcast a 60-minute exercise class that included warm-up, RT with bands, progressive balance training, and flexibility. Trained student volunteers from California State University in Bakersfield kinesiology program were on site at the Bakersfield CBOC to perform the assessments and aid in exercises during the CVT sessions. Despite the lack of AEX per se, veterans showed significant improvement in endurance as measured by an increase in the number of steps completed in 2 minutes at the 3-month assessment (P = .049). Although exercises were not delivered in a circuit format, the improved endurance supported the potential for cardiovascular benefit from RT in older adults.
This pilot project also demonstrated that key components of the Gerofit program could be delivered safely by telehealth with onsite supervision. The Miami VA Healthcare System also offers CVT Gerofit exercise classes broadcast to the rural Florida CBOCs of Key Largo and Homestead.11 The exercise activities offered for the Miami CVT participants incorporate components of AEX (calisthenics) and RT (resistance bands). Veterans enjoyed the classes, and adherence was good. However, availability of staff and space are an ongoing challenge.
In Key Largo, 5 veterans participated before the CVT classes were placed on hold owing to the demands of other CVT programs and limited availability of the telehealth clinical technician (TCT). The Homestead CBOC continues to offer CVT Gerofit exercise classes and has 6 regular participants. Notably, the physical space at the Homestead CBOC is smaller than that at the Key Largo CBOC; the Homestead CBOC has adjusted by shifting to exercises performed while standing or sitting, ensuring participants’ safety and satisfaction.
The Baltimore, Maryland VAMC Gerofit program offers other innovative CVT exercise classes, including a tai chi class, and a class with exercise performed while sitting in a chair. Although the Baltimore VAMC CVT exercise classes do not have the scope of the center-based exercise prescriptions, they are unique in that they are broadcast not only to their affiliated CBOCs, but also other Gerofit programs in different VISNs.
Related: Telehealth for Rural Veterans With Neurologic Disorders
Salem VAMC Gerofit Program. The center-based Salem VAMC Gerofit program was established in July 2015. In fiscal year 2017, its dedicated exercise facility had more than 5,000 patient visits. Despite the program’s success, we prioritized establishing Tele-Gerofit because of the medical center’s rural location in southwest Virginia and the large number of veterans who receive care at CBOCs. Therefore, much as with the pilot CVT Gerofit classes in Los Angeles and Miami, the target setting was rural CBOCs. The goal for Salem VAMC Tele-Gerofit was to modify Gerofit delivery to the CVT format and a CBOC setting with minimal modification of the content and personnel requirements of both physical performance testing and exercise training procedures.
Adjustments for CBOC Setting. The enrollment process for Tele-Gerofit is the same as that for the center-based program. To start, a veteran’s primary care provider reviews the list of eligibility criteria and, if the veteran qualifies, places a consult. A Gerofit team member then contacts the veteran by phone to describe the program and schedule an assessment. At the baseline physical performance assessment, American College of Sports Medicine guidelines on exercise participation, health screening, and exercise intensity are used to evaluate veterans and rank them by their cardiovascular risk.20 All new program participants start with low-intensity exercise and gradually progress to recommended levels of exercise. Before starting an exercise class, participants are instructed on use of the 10-point rating of perceived exertion (RPE).
Each CBOC site is supplied with an RPE poster that is displayed for participants’ use. During a Tele-Gerofit class, the exercise instructor asks participants to periodically report their RPE. This class differs slightly from the center-based exercise sessions in which RPE is primarily assessed when a different exercise is introduced or the duration or intensity of an exercise is increased. The Gerofit instructor monitors exercise and treatment fidelity, but the onsite TCT observes for safety during class. The TCT also takes initial vital signs and sets up the room for the class. Emergency contacts and procedures are posted in each CBOC CVT room and are available to the center-based exercise instructor. Because the CBOCs are not inside medical facilities, some CBOC directors have asked that heart rate monitors be used as an extra safety precaution to ensure that high-risk participants do not exceed a heart rate limit that may be set by their cardiologists.
Modifications to Physical Performance Assessment. Physical performance testing had to be adapted to the small rooms available at the CBOCs. For measuring normal gait speed, the 10-MWT was replaced with the 4-meter walk test (4-MWT). The 4-MWT has excellent test–retest reliability with an intraclass correlation coefficient (ICC) of 0.93, but the discrepancy in gait speed between the 4-MWT and the 10-MWT is such that the tests cannot be used interchangeably.21 For measuring endurance, the 6-minute walk test was replaced with the 2-minute step test (2-MST). In older adults, the 2-MST has a moderate correlation with 6-minute walk distance (r = 0.36; P = .04) and high reliability (ICC, 0.90).15,22 The 30-second 1-arm curl, the 30-second chair-stand test, and the 8-foot up-and-go test are performed without modification and require only dumbbells, a chair without wheels, and a stopwatch.
The exercise instructor at the Salem VAMC conducts physical performance testing by 2-way videoconferencing with the veteran in a room at the CBOC. The TCT at the CBOC assists by measuring and demarcating 4 meters on the floor and a designated height on the wall for knee elevation for 4-MWT and 2-MST, respectively. The TCT remains in the room during the assessment visit. Except for taking vital signs before and after the physical performance assessment, the TCT does not participate in the testing. To date, more than 20 physical performance assessments have been conducted without difficulty at Salem-affiliated CBOCs. The primary challenge has been scheduling the room with CVT equipment (ie, camera and screen) for the 30-minute individual assessment session, which occurs on a rolling basis as individuals are enrolled and followed.
After the assessment is completed, the exercise instructor reviews the results with the participant and provides feedback on areas in need of improvement. However, these education sessions can be lengthy and are best supported by giving the patient a personalized handout.
Functional Circuit Exercise. In Tele-Gerofit, exercise training is delivered by CVT broadcast from the Salem VAMC to veterans in a room (equipped with steps, dumbbells, chairs, and bands) at the CBOC. This type of exercise training, which uses only mobile equipment and plyometric (weight-bearing) exercises, is referred to as functional exercise. The AEX includes marching in place, moving on and off a raised step, and body-weight exercises, while RT uses dumbbells, resistance bands, and plyometric exercises (Table 2).
Progression of intensity is achieved by increasing the rate of stepping and the size of the steps (AEX) or the number of repetitions and the weight of the dumbbells or bands (RT). Each veteran exercises at an intensity level that is appropriate for his or her baseline limitations and medical conditions. The exercise instructor uses different forms of the same equipment (eg, heavier dumbbells, higher steps) to vary intensity among individuals while having them perform the same exercises as a group. The challenge is to adjust the pace of the AEX or the timing of the RT repetitions for individuals new to the class.
Delivery of exercise training in the form of circuits allows for a diverse exercise program in a setting with limited space. Circuit training is an exercise modality that consists of a series of different exercises, each usually completed in 30 to 60 seconds, with minimal rest between each type of exercise. Each Tele-Gerofit circuit has a mix of AEX and RT exercises performed for 3 minutes consecutively (Figure).
The design of the circuit training can be adjusted based on the number of individuals in the class. Larger classes can be split into 2 groups that alternate between exercise sets, while smaller classes have 1 group performing the same exercise set and then rotating to either the AEX or RT set. Total exercise time to complete the circuit depends on the number of different exercises, number of repetitions, and the rest between repetitions and the different exercises. In this way, total exercise time can be made shorter or longer depending on the veteran’s capacity.
Frequency. Tele-Gerofit exercise classes are currently offered twice weekly and last about 1 hour, which includes warm-up (8-10 minutes), functional circuit training (40 minutes), and cooldown/stretching (8-10 minutes). A challenge for the exercise instructor is the need to provide ongoing clear instructions both to the class and to individuals as needed. As the exercise prescription for each patient is based on physical performance testing, the exercise instructor for the training must be familiar with the test results. Derivation of the exercise prescription in Tele-Gerofit follows the same process as center-based Gerofit.
Each patient is given an exercise prescription written to address any impairments noted in the different domains of the physical performance assessment, scored using age and sex percentiles. For instance, individuals scoring poorly on lower body strength are given specific lower body strengthening exercises. Participants are given an exercise program that guides them toward achieving recommended physical activity guidelines using their RPE to modulate each exercise. Duration and intensity of each type of planned exercise are formally discussed after initial and follow-up assessments. In addition, exercise training is informally progressed throughout the program. For Tele-Gerofit, instructors must design each class with the group in mind while being prepared for modifications and specific changes for individuals.
Discussion
Tele-Gerofit adapts the well-established center-based Gerofit program to be executed without an exercise facility while maintaining the content of the evidence-based procedures. Physical performance testing and exercise training were modified, adding elements necessary for CVT assessments and classes to be broadcast from the Salem VAMC to its affiliated CBOCs. Tele-Gerofit exercises are performed in a circuit style that allows a veteran or small structured groups of veterans to move among exercises and requires less space than traditional group exercise does. Safety and monitoring concerns are addressed with a safety procedure that includes emergency plans for each site, prescreening of enrolled participants, and monitoring of exercise intensity in accordance with national guidelines.1 Similar to the center-based Gerofit program, the exercise prescription is tailored to each veteran’s physical limitations based on initial and ongoing assessment of physical performance. Tele-Gerofit physical performance testing fulfills the same need with only a few modifications using validated measures. Tele-Gerofit assessments are administered by CVT without the need for additional staff on site.
Adaptation of center-based Gerofit exercise classes to Tele-Gerofit is a major innovation. Use of a circuit exercise design was supported by findings in older adults that RT alone, when performed quickly with minimal rest between each set and exercise station, increases both aerobic capacity and strength.23,24 Older adult RT trials that compared circuit RT with traditional RT found that strength gains are comparable between circuit and traditional RT.24-26 Working with adults aged > 60 years, Takeshima and colleagues conducted a trial of circuit exercise with added callisthenic exercises performed in place between RT on exercise machines.27 This dual-modality (AEX+RT) circuit approach was well tolerated and effective, increasing aerobic capacity and strength. Unfortunately, the resistance exercise machines used in those circuit exercise studies and in the center-based Gerofit program are not an option for Tele-Gerofit.
The requirement for an exercise facility was removed by designing Tele-Gerofit exercise to include only functional exercises that rely on body weight or small mobile exercise equipment. Although popular among young adults, functional circuit exercise is understudied in older adults. Recently, a 12-week functional circuit exercise intervention in frail elderly adults demonstrated significant improvements in gait speed and the timed chair-stand test.28 A pilot observational study of Gerofit participants at the Canandaigua VAMC offered 27 veterans functional circuit exercise instead of their traditional exercise facility class and found larger increases in the timed chair-stand test and 6-minute walk distance compared with 11 Gerofit participants in the traditional program.29
This Tele-Gerofit exercise training combines functional and circuit exercise strategies into telehealth delivery. However, its effect on physical performance remains to be demonstrated. To address this question, we are conducting a single-arm pilot study of Tele-Gerofit with CVT broadcast to 3 Salem CBOC affiliates (Wytheville, Staunton, and Danville, Virginia). The goal is to determine the effect on physical performance and collect feasibility data, including attendance rate and patient satisfaction with the video broadcast. In addition, we are planning an effectiveness trial to compare the impact of functional circuit exercise delivered in person (center based, not CVT) with the parent Gerofit exercise program on direct measures of endurance and strength, in addition to physical performance.
Related: Setting and Method of Measurement Affect Blood Pressure Readings in Older Veterans
Implementation research is needed to determine how Tele-Gerofit can be disseminated to other VAMCs and community-based centers beyond CBOCs. Although the cost of the equipment used to implement Tele-Gerofit is minimal, the program requires dedicated and experienced exercise instructors, and the sharing of telehealth resources with other clinical programs. The authors expect that a diverse group of stakeholders is needed across service lines of primary care, geriatrics and extended care, physical medicine and rehabilitation, and telehealth. Of note, this multidisciplinary collaboration is a hallmark of the Gerofit program. The recent success of the implementation of center-based Gerofit in VAMCs across the US demonstrates the program’s flexibility and robust results.18
Plans also include refining strategies for physical performance testing and exercise monitoring. For instance, we would like to adapt telehealth technology for heart rate monitors that can be worn by high-risk veterans at the CBOC and viewed in real time by the exercise instructor.
Conclusion
Gerofit, which is designed to help older veterans maintain independent living and prevent disability, has been demonstrated to improve quality of life and survival. Our goal has been to adapt Gerofit to CVT and provide a supervised, individualized exercise program in a group setting—a program that can be widely disseminated. Salem VAMC Tele-Gerofit is an innovative and prescriptive program that delivers CVT functional circuit exercise training to remote locations without the need for stationary exercise equipment. This approach has the potential to become an effective and feasible exercise strategy for preventing and minimizing disability in the increasing population of older veterans. Work is needed to determine whether Tele-Gerofit provides a rapid translation of Gerofit to clinical practice and improved outcomes with substantial cost savings from reduced hospitalization and institutionalization.
Acknowledgments
Gerofit has been funded by the Veterans Health Affairs Office of Geriatrics and Extended Care Non-Institutional Long-Term Care Funding and Mentored Partnership Program, and the Veterans Health Affairs Office of Rural Health Rural Enterprise-Wide Initiative.
The authors thank Kim Birkett, MPH, for assistance in editing, references, and graphics and the staff at the Wytheville, Staunton, and Danville community-based outpatient clinics for their support.
Exercise increases endurance, muscle strength, and functional performance with corresponding gains in mobility, survival, and quality of life.1 However, even with these benefits and improvements in clinical outcomes, only 15% of adults aged ≥ 65 years follow current guidelines for exercise.2 Despite their prior military training, the majority of veterans do not meet physical activity recommendations.3 Time, travel, and support are common barriers to exercise participation and adherence—barriers that are further amplified among older adults.
The Veterans Health Administration (VHA) is recognized as a world leader in telehealth service development. Currently, 677,000 veterans have received telehealth services, which represents 12% of the 5.6 million veterans under VHA care.4 Clinical video telehealth (CVT) is widely used within the VHA system to deliver health care that otherwise would not be available to veterans. Veterans who have difficulty traveling to the nearest US Department of Veteran Affairs (VA) medical center (VAMC) can access CVT programs at a participating VHA community-based outpatient clinic (CBOC). The VA has more than 45 CVT programs, including programs for mental health, weight management, cardiology, and dermatology. Outside the VA, cardiac exercise rehabilitation provided by CVT has been shown to be as effective as center-based programs in improving cardiovascular risk factors and functional capacity.5 A VHA exercise program that leveraged CVT resources and was dedicated to older adults with a wide range of comorbid conditions would have a high impact on the health and well-being of older veterans.
Gerofit is a VHA clinical demonstration program of supervised center-based exercise for veterans aged ≥ 65 years. Developed at the Durham VAMC Geriatric Research, Education, and Clinical Center (GRECC) in North Carolina, it has demonstrated improved clinical outcomes, including physical function, mobility, quality of life, and survival.6-10 The program offers veterans individualized exercise in a group setting that focuses on improving endurance, strength, and balance. The exercise prescription is based on the patient’s physical limitations as identified in a physical performance assessment.
With support from VHA Geriatric Extended Care (GEC) and the Office of Rural Health (ORH), Gerofit was implemented in 10 VAMCs across 8 VISNs. However, barriers such as travel time, distance, and transportation limit participation. Previously, we found that rural veterans lack access to exercise programs.11,12 Although some do aerobic exercise (AEX), most do not do resistance training (RT), though they are willing to learn. Access to Gerofit for rural veterans is expanding with recent support from the ORH Enterprise Wide Initiative. Rural program expansion includes several different Gerofit initiatives, many involving CBOCs.
The Salem VAMC Gerofit program sought to adapt the facility-based assessment and exercise procedures into a self-reliant CVT class for its CBOCs. This article describes the development of the Salem VAMC Gerofit CVT program, hereafter referred to as Tele-Gerofit.
Related: Expanding the Scope of Telemedicine in Gastroenterology
Program Design
Gerofit was established in 1986 at the Durham GRECC as an exercise and health promotion program for veterans aged ≥ 65 years.13 Its goal is to prevent or improve functional decline from physical inactivity and age-related conditions. Gerofit targets the geriatric patient population and thus extends beyond cardiac and pulmonary rehabilitation or weight loss programs. The primary exclusion criteria are based on safety issues in the context of a group exercise setting of older adults and include oxygen dependency, unstable cardiac disease, and moderate-to-severe cognitive impairment.
To participate in Gerofit, veterans must be able to perform activities of daily living and self-manage an exercise prescription developed by the exercise instructor based on physical performance testing. These physical performance tests include measures that are independent predictors of disability, loss of independent living, and death, as well as surrogate measures of exercise capacity (eg, strength, endurance, balance).14,15 A novel aspect of Gerofit is that the physical performance assessment is used not only to determine physical limitations, but also to individualize the exercise prescription based on the observed deficits in strength, endurance, or balance. These assessments are performed at initial enrollment; 3 months, 6 months, and 1 year later; and annually after that. Currently, the center-based Gerofit programs administer 5 items of the Senior Fitness Test: 6-minute walk, 10-meter walk (10-MWT), 30-second 1-arm curl, 30-second chair-stand test, and 8-foot up-and-go.15 The side-by-side, semitandem, and tandem standing balance tests from the short physical performance battery also are performed.16 In addition, participants complete a questionnaire that includes items from the physical functioning scale of the 12-Item Short Form Health Survey (SF-12).
After each assessment, the Gerofit exercise instructor reviews the results with the veteran and formulates an individualized exercise prescription along with goals for improvement. Veterans are encouraged to attend supervised center-based exercise sessions 3 times weekly. Classes are offered in a gym or fitness center at the VAMC or in leased space. Each patient uses a cue card that lists an exercise plan personalized for intensity and duration for aerobic exercise (AEX; eg, treadmill walking, stationary bicycling, arm ergometry), RT using dumbbells and weight equipment, and functional exercises for flexibility and balance. Some medical centers also offer yoga, tai chi, or dancing Gerofit classes.
For participants in the Durham Gerofit program, mortality decreased 25% over a decade (hazard ratio, 0.75; 95% CI, 0.61-0.91).9 A substudy that included the Psychological General Well-Being Index found that 81% of participants significantly increased their score after 1 year.7 Observed initial improvement in physical performance has been sustained over 5 years.10,17 One-year results from the recent Gerofit expansion to 6 other VAMCs showed clinically and statistically significantly improved physical performance from baseline to 3-, 6-, and 12-month follow-up.18
Adaptation of Gerofit to CVT Delivery
Initial work. The Greater Los Angeles VAMC Gerofit program conducted a pilot CVT exercise class of 6 veterans at the rural Bakersfield CBOC in California.19 Each week, an exercise instructor broadcast a 60-minute exercise class that included warm-up, RT with bands, progressive balance training, and flexibility. Trained student volunteers from California State University in Bakersfield kinesiology program were on site at the Bakersfield CBOC to perform the assessments and aid in exercises during the CVT sessions. Despite the lack of AEX per se, veterans showed significant improvement in endurance as measured by an increase in the number of steps completed in 2 minutes at the 3-month assessment (P = .049). Although exercises were not delivered in a circuit format, the improved endurance supported the potential for cardiovascular benefit from RT in older adults.
This pilot project also demonstrated that key components of the Gerofit program could be delivered safely by telehealth with onsite supervision. The Miami VA Healthcare System also offers CVT Gerofit exercise classes broadcast to the rural Florida CBOCs of Key Largo and Homestead.11 The exercise activities offered for the Miami CVT participants incorporate components of AEX (calisthenics) and RT (resistance bands). Veterans enjoyed the classes, and adherence was good. However, availability of staff and space are an ongoing challenge.
In Key Largo, 5 veterans participated before the CVT classes were placed on hold owing to the demands of other CVT programs and limited availability of the telehealth clinical technician (TCT). The Homestead CBOC continues to offer CVT Gerofit exercise classes and has 6 regular participants. Notably, the physical space at the Homestead CBOC is smaller than that at the Key Largo CBOC; the Homestead CBOC has adjusted by shifting to exercises performed while standing or sitting, ensuring participants’ safety and satisfaction.
The Baltimore, Maryland VAMC Gerofit program offers other innovative CVT exercise classes, including a tai chi class, and a class with exercise performed while sitting in a chair. Although the Baltimore VAMC CVT exercise classes do not have the scope of the center-based exercise prescriptions, they are unique in that they are broadcast not only to their affiliated CBOCs, but also other Gerofit programs in different VISNs.
Related: Telehealth for Rural Veterans With Neurologic Disorders
Salem VAMC Gerofit Program. The center-based Salem VAMC Gerofit program was established in July 2015. In fiscal year 2017, its dedicated exercise facility had more than 5,000 patient visits. Despite the program’s success, we prioritized establishing Tele-Gerofit because of the medical center’s rural location in southwest Virginia and the large number of veterans who receive care at CBOCs. Therefore, much as with the pilot CVT Gerofit classes in Los Angeles and Miami, the target setting was rural CBOCs. The goal for Salem VAMC Tele-Gerofit was to modify Gerofit delivery to the CVT format and a CBOC setting with minimal modification of the content and personnel requirements of both physical performance testing and exercise training procedures.
Adjustments for CBOC Setting. The enrollment process for Tele-Gerofit is the same as that for the center-based program. To start, a veteran’s primary care provider reviews the list of eligibility criteria and, if the veteran qualifies, places a consult. A Gerofit team member then contacts the veteran by phone to describe the program and schedule an assessment. At the baseline physical performance assessment, American College of Sports Medicine guidelines on exercise participation, health screening, and exercise intensity are used to evaluate veterans and rank them by their cardiovascular risk.20 All new program participants start with low-intensity exercise and gradually progress to recommended levels of exercise. Before starting an exercise class, participants are instructed on use of the 10-point rating of perceived exertion (RPE).
Each CBOC site is supplied with an RPE poster that is displayed for participants’ use. During a Tele-Gerofit class, the exercise instructor asks participants to periodically report their RPE. This class differs slightly from the center-based exercise sessions in which RPE is primarily assessed when a different exercise is introduced or the duration or intensity of an exercise is increased. The Gerofit instructor monitors exercise and treatment fidelity, but the onsite TCT observes for safety during class. The TCT also takes initial vital signs and sets up the room for the class. Emergency contacts and procedures are posted in each CBOC CVT room and are available to the center-based exercise instructor. Because the CBOCs are not inside medical facilities, some CBOC directors have asked that heart rate monitors be used as an extra safety precaution to ensure that high-risk participants do not exceed a heart rate limit that may be set by their cardiologists.
Modifications to Physical Performance Assessment. Physical performance testing had to be adapted to the small rooms available at the CBOCs. For measuring normal gait speed, the 10-MWT was replaced with the 4-meter walk test (4-MWT). The 4-MWT has excellent test–retest reliability with an intraclass correlation coefficient (ICC) of 0.93, but the discrepancy in gait speed between the 4-MWT and the 10-MWT is such that the tests cannot be used interchangeably.21 For measuring endurance, the 6-minute walk test was replaced with the 2-minute step test (2-MST). In older adults, the 2-MST has a moderate correlation with 6-minute walk distance (r = 0.36; P = .04) and high reliability (ICC, 0.90).15,22 The 30-second 1-arm curl, the 30-second chair-stand test, and the 8-foot up-and-go test are performed without modification and require only dumbbells, a chair without wheels, and a stopwatch.
The exercise instructor at the Salem VAMC conducts physical performance testing by 2-way videoconferencing with the veteran in a room at the CBOC. The TCT at the CBOC assists by measuring and demarcating 4 meters on the floor and a designated height on the wall for knee elevation for 4-MWT and 2-MST, respectively. The TCT remains in the room during the assessment visit. Except for taking vital signs before and after the physical performance assessment, the TCT does not participate in the testing. To date, more than 20 physical performance assessments have been conducted without difficulty at Salem-affiliated CBOCs. The primary challenge has been scheduling the room with CVT equipment (ie, camera and screen) for the 30-minute individual assessment session, which occurs on a rolling basis as individuals are enrolled and followed.
After the assessment is completed, the exercise instructor reviews the results with the participant and provides feedback on areas in need of improvement. However, these education sessions can be lengthy and are best supported by giving the patient a personalized handout.
Functional Circuit Exercise. In Tele-Gerofit, exercise training is delivered by CVT broadcast from the Salem VAMC to veterans in a room (equipped with steps, dumbbells, chairs, and bands) at the CBOC. This type of exercise training, which uses only mobile equipment and plyometric (weight-bearing) exercises, is referred to as functional exercise. The AEX includes marching in place, moving on and off a raised step, and body-weight exercises, while RT uses dumbbells, resistance bands, and plyometric exercises (Table 2).
Progression of intensity is achieved by increasing the rate of stepping and the size of the steps (AEX) or the number of repetitions and the weight of the dumbbells or bands (RT). Each veteran exercises at an intensity level that is appropriate for his or her baseline limitations and medical conditions. The exercise instructor uses different forms of the same equipment (eg, heavier dumbbells, higher steps) to vary intensity among individuals while having them perform the same exercises as a group. The challenge is to adjust the pace of the AEX or the timing of the RT repetitions for individuals new to the class.
Delivery of exercise training in the form of circuits allows for a diverse exercise program in a setting with limited space. Circuit training is an exercise modality that consists of a series of different exercises, each usually completed in 30 to 60 seconds, with minimal rest between each type of exercise. Each Tele-Gerofit circuit has a mix of AEX and RT exercises performed for 3 minutes consecutively (Figure).
The design of the circuit training can be adjusted based on the number of individuals in the class. Larger classes can be split into 2 groups that alternate between exercise sets, while smaller classes have 1 group performing the same exercise set and then rotating to either the AEX or RT set. Total exercise time to complete the circuit depends on the number of different exercises, number of repetitions, and the rest between repetitions and the different exercises. In this way, total exercise time can be made shorter or longer depending on the veteran’s capacity.
Frequency. Tele-Gerofit exercise classes are currently offered twice weekly and last about 1 hour, which includes warm-up (8-10 minutes), functional circuit training (40 minutes), and cooldown/stretching (8-10 minutes). A challenge for the exercise instructor is the need to provide ongoing clear instructions both to the class and to individuals as needed. As the exercise prescription for each patient is based on physical performance testing, the exercise instructor for the training must be familiar with the test results. Derivation of the exercise prescription in Tele-Gerofit follows the same process as center-based Gerofit.
Each patient is given an exercise prescription written to address any impairments noted in the different domains of the physical performance assessment, scored using age and sex percentiles. For instance, individuals scoring poorly on lower body strength are given specific lower body strengthening exercises. Participants are given an exercise program that guides them toward achieving recommended physical activity guidelines using their RPE to modulate each exercise. Duration and intensity of each type of planned exercise are formally discussed after initial and follow-up assessments. In addition, exercise training is informally progressed throughout the program. For Tele-Gerofit, instructors must design each class with the group in mind while being prepared for modifications and specific changes for individuals.
Discussion
Tele-Gerofit adapts the well-established center-based Gerofit program to be executed without an exercise facility while maintaining the content of the evidence-based procedures. Physical performance testing and exercise training were modified, adding elements necessary for CVT assessments and classes to be broadcast from the Salem VAMC to its affiliated CBOCs. Tele-Gerofit exercises are performed in a circuit style that allows a veteran or small structured groups of veterans to move among exercises and requires less space than traditional group exercise does. Safety and monitoring concerns are addressed with a safety procedure that includes emergency plans for each site, prescreening of enrolled participants, and monitoring of exercise intensity in accordance with national guidelines.1 Similar to the center-based Gerofit program, the exercise prescription is tailored to each veteran’s physical limitations based on initial and ongoing assessment of physical performance. Tele-Gerofit physical performance testing fulfills the same need with only a few modifications using validated measures. Tele-Gerofit assessments are administered by CVT without the need for additional staff on site.
Adaptation of center-based Gerofit exercise classes to Tele-Gerofit is a major innovation. Use of a circuit exercise design was supported by findings in older adults that RT alone, when performed quickly with minimal rest between each set and exercise station, increases both aerobic capacity and strength.23,24 Older adult RT trials that compared circuit RT with traditional RT found that strength gains are comparable between circuit and traditional RT.24-26 Working with adults aged > 60 years, Takeshima and colleagues conducted a trial of circuit exercise with added callisthenic exercises performed in place between RT on exercise machines.27 This dual-modality (AEX+RT) circuit approach was well tolerated and effective, increasing aerobic capacity and strength. Unfortunately, the resistance exercise machines used in those circuit exercise studies and in the center-based Gerofit program are not an option for Tele-Gerofit.
The requirement for an exercise facility was removed by designing Tele-Gerofit exercise to include only functional exercises that rely on body weight or small mobile exercise equipment. Although popular among young adults, functional circuit exercise is understudied in older adults. Recently, a 12-week functional circuit exercise intervention in frail elderly adults demonstrated significant improvements in gait speed and the timed chair-stand test.28 A pilot observational study of Gerofit participants at the Canandaigua VAMC offered 27 veterans functional circuit exercise instead of their traditional exercise facility class and found larger increases in the timed chair-stand test and 6-minute walk distance compared with 11 Gerofit participants in the traditional program.29
This Tele-Gerofit exercise training combines functional and circuit exercise strategies into telehealth delivery. However, its effect on physical performance remains to be demonstrated. To address this question, we are conducting a single-arm pilot study of Tele-Gerofit with CVT broadcast to 3 Salem CBOC affiliates (Wytheville, Staunton, and Danville, Virginia). The goal is to determine the effect on physical performance and collect feasibility data, including attendance rate and patient satisfaction with the video broadcast. In addition, we are planning an effectiveness trial to compare the impact of functional circuit exercise delivered in person (center based, not CVT) with the parent Gerofit exercise program on direct measures of endurance and strength, in addition to physical performance.
Related: Setting and Method of Measurement Affect Blood Pressure Readings in Older Veterans
Implementation research is needed to determine how Tele-Gerofit can be disseminated to other VAMCs and community-based centers beyond CBOCs. Although the cost of the equipment used to implement Tele-Gerofit is minimal, the program requires dedicated and experienced exercise instructors, and the sharing of telehealth resources with other clinical programs. The authors expect that a diverse group of stakeholders is needed across service lines of primary care, geriatrics and extended care, physical medicine and rehabilitation, and telehealth. Of note, this multidisciplinary collaboration is a hallmark of the Gerofit program. The recent success of the implementation of center-based Gerofit in VAMCs across the US demonstrates the program’s flexibility and robust results.18
Plans also include refining strategies for physical performance testing and exercise monitoring. For instance, we would like to adapt telehealth technology for heart rate monitors that can be worn by high-risk veterans at the CBOC and viewed in real time by the exercise instructor.
Conclusion
Gerofit, which is designed to help older veterans maintain independent living and prevent disability, has been demonstrated to improve quality of life and survival. Our goal has been to adapt Gerofit to CVT and provide a supervised, individualized exercise program in a group setting—a program that can be widely disseminated. Salem VAMC Tele-Gerofit is an innovative and prescriptive program that delivers CVT functional circuit exercise training to remote locations without the need for stationary exercise equipment. This approach has the potential to become an effective and feasible exercise strategy for preventing and minimizing disability in the increasing population of older veterans. Work is needed to determine whether Tele-Gerofit provides a rapid translation of Gerofit to clinical practice and improved outcomes with substantial cost savings from reduced hospitalization and institutionalization.
Acknowledgments
Gerofit has been funded by the Veterans Health Affairs Office of Geriatrics and Extended Care Non-Institutional Long-Term Care Funding and Mentored Partnership Program, and the Veterans Health Affairs Office of Rural Health Rural Enterprise-Wide Initiative.
The authors thank Kim Birkett, MPH, for assistance in editing, references, and graphics and the staff at the Wytheville, Staunton, and Danville community-based outpatient clinics for their support.
1. American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510-1530.
2. Centers for Disease Control and Prevention. Adult participation in aerobic and muscle-strengthening physical activities—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(17):326-330.
3. Littman AJ, Forsberg CW, Koepsell TD. Physical activity in a national sample of veterans. Med Sci Sports Exerc. 2009;41(5):1006-1013.
4. US Department of Veterans Affairs, Office of Rural Health. Annual Report: Thrive 2015. https://www.ruralhealth.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed July 16, 2018.
5. Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102(15):1183-1192.
6. Morey MC. Celebrating 20 years of excellence in exercise for the older veteran. Fed Pract. 2007;24(10):49-65.
7. Cowper PA, Morey MC, Bearon LB, et al. The impact of supervised exercise on the psychological well-being and health status of older veterans. J Appl Gerontol. 1991;10(4):469-485.
8. Morey MC, Cowper PA, Feussner JR, et al. Evaluation of a supervised exercise program in a geriatric population. J Am Geriatr Soc. 1989;37(4):348-354.
9. Morey MC, Pieper CF, Crowley GM, Sullivan RJ, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50(12):1929-1933.
10. Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44(10):1226-1231.
11. Valencia WM, Botros D, Pendlebury D, et al. Proactive reach and telehealth monitoring (Gerofit) enhance resistance exercise at rural setting. Innovat Aging. 2017;1(suppl 1):225.12. Pendlebury D, Botros D VW. Proactive Reach: an innovative access approach to identify & deliver GEROFIT exercise telehealth counseling to rural veterans & enhance CBOC services. J Am Geriatr Soc. 2017(suppl 1):S208. Poster presented at: Annual Scientific Meeting of the American Geriatrics Society; May 18, 2017; San Antonio, TX.
13. Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit program: a VA innovation. South Med J. 1994;87(5):S83-S87.
14. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467.
15. Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7(2):129-161.
16. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94.
17. Morey MC, Cowper PA, Feussner JR, et al. Two-year trends in physical performance following supervised exercise among community-dwelling older veterans. J Am Geriatr Soc. 1991;39(10):986-992.
18. Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit program. J Am Geriatr Soc. 2018;66(5):1009-1016.
19. Blanchard E, Castle S, Ines E, et al. Delivering a clinical exercise program to rural veterans via video telehealth. Poster C167 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
20. Riebe D, Ehrman JK, Liguori G, Magal M, eds; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia, PA: Wolters Kluwer Health; 2018.
21. Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36(1):24-30.
22. Pedrosa R, Holanda G. Correlation between the walk, 2-minute step and TUG tests among hypertensive older women. Rev Bras Fisioter. 2009;13(3):252-256.
23. Romero-Arenas S, Blazevich AJ, Martinez-Pascual M, et al. Effects of high-resistance circuit training in an elderly population. Exp Gerontol. 2013;48(3):334-340.
24. Brentano MA, Cadore EL, Da Silva EM, et al. Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J Strength Cond Res. 2008;22(6):1816-1825.
25. Romero-Arenas S, Martinez-Pascual M, Alcaraz PE. Impact of resistance circuit training on neuromuscular, cardiorespiratory and body composition adaptations in the elderly. Aging Dis. 2013;4(5):256-263.
26. Paoli A, Pacelli F, Bargossi AM, et al. Effects of three distinct protocols of fitness training on body composition, strength and blood lactate. J Sports Med Phys Fitness. 2010;50(1):43-51.
27. Takeshima N, Rogers ME, Islam MM, Yamauchi T, Watanabe E, Okada A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur J Appl Physiol. 2004;93(1-2):173-182.
28. Giné-Garriga M, Guerra M, Pagés E, Manini TM, Jiménez R, Unnithan VB. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. J Aging Phys Act. 2010;18(4):401-424.
29. Biddle ED, Reynolds P, Kopp T, Cammarata H, Conway P. Implementation of functional training tools elicits improvements in aerobic fitness and lower body strength in older veterans. Poster C169 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
1. American College of Sports Medicine, Chodzko-Zajko WJ, Proctor DN, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41(7):1510-1530.
2. Centers for Disease Control and Prevention. Adult participation in aerobic and muscle-strengthening physical activities—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(17):326-330.
3. Littman AJ, Forsberg CW, Koepsell TD. Physical activity in a national sample of veterans. Med Sci Sports Exerc. 2009;41(5):1006-1013.
4. US Department of Veterans Affairs, Office of Rural Health. Annual Report: Thrive 2015. https://www.ruralhealth.va.gov/docs/ORH_Annual_Report_2015_FINAL.pdf. Published 2015. Accessed July 16, 2018.
5. Rawstorn JC, Gant N, Direito A, Beckmann C, Maddison R. Telehealth exercise-based cardiac rehabilitation: a systematic review and meta-analysis. Heart. 2016;102(15):1183-1192.
6. Morey MC. Celebrating 20 years of excellence in exercise for the older veteran. Fed Pract. 2007;24(10):49-65.
7. Cowper PA, Morey MC, Bearon LB, et al. The impact of supervised exercise on the psychological well-being and health status of older veterans. J Appl Gerontol. 1991;10(4):469-485.
8. Morey MC, Cowper PA, Feussner JR, et al. Evaluation of a supervised exercise program in a geriatric population. J Am Geriatr Soc. 1989;37(4):348-354.
9. Morey MC, Pieper CF, Crowley GM, Sullivan RJ, Puglisi CM. Exercise adherence and 10-year mortality in chronically ill older adults. J Am Geriatr Soc. 2002;50(12):1929-1933.
10. Morey MC, Pieper CF, Sullivan RJ Jr, Crowley GM, Cowper PA, Robbins MS. Five-year performance trends for older exercisers: a hierarchical model of endurance, strength, and flexibility. J Am Geriatr Soc. 1996;44(10):1226-1231.
11. Valencia WM, Botros D, Pendlebury D, et al. Proactive reach and telehealth monitoring (Gerofit) enhance resistance exercise at rural setting. Innovat Aging. 2017;1(suppl 1):225.12. Pendlebury D, Botros D VW. Proactive Reach: an innovative access approach to identify & deliver GEROFIT exercise telehealth counseling to rural veterans & enhance CBOC services. J Am Geriatr Soc. 2017(suppl 1):S208. Poster presented at: Annual Scientific Meeting of the American Geriatrics Society; May 18, 2017; San Antonio, TX.
13. Morey MC, Crowley GM, Robbins MS, Cowper PA, Sullivan RJ Jr. The Gerofit program: a VA innovation. South Med J. 1994;87(5):S83-S87.
14. Cooper R, Kuh D, Hardy R; Mortality Review Group; FALCon and HALCyon Study Teams. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467.
15. Rikli RE, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7(2):129-161.
16. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85-M94.
17. Morey MC, Cowper PA, Feussner JR, et al. Two-year trends in physical performance following supervised exercise among community-dwelling older veterans. J Am Geriatr Soc. 1991;39(10):986-992.
18. Morey MC, Lee CC, Castle S, et al. Should structured exercise be promoted as a model of care? Dissemination of the Department of Veterans Affairs Gerofit program. J Am Geriatr Soc. 2018;66(5):1009-1016.
19. Blanchard E, Castle S, Ines E, et al. Delivering a clinical exercise program to rural veterans via video telehealth. Poster C167 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
20. Riebe D, Ehrman JK, Liguori G, Magal M, eds; American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 10th ed. Philadelphia, PA: Wolters Kluwer Health; 2018.
21. Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36(1):24-30.
22. Pedrosa R, Holanda G. Correlation between the walk, 2-minute step and TUG tests among hypertensive older women. Rev Bras Fisioter. 2009;13(3):252-256.
23. Romero-Arenas S, Blazevich AJ, Martinez-Pascual M, et al. Effects of high-resistance circuit training in an elderly population. Exp Gerontol. 2013;48(3):334-340.
24. Brentano MA, Cadore EL, Da Silva EM, et al. Physiological adaptations to strength and circuit training in postmenopausal women with bone loss. J Strength Cond Res. 2008;22(6):1816-1825.
25. Romero-Arenas S, Martinez-Pascual M, Alcaraz PE. Impact of resistance circuit training on neuromuscular, cardiorespiratory and body composition adaptations in the elderly. Aging Dis. 2013;4(5):256-263.
26. Paoli A, Pacelli F, Bargossi AM, et al. Effects of three distinct protocols of fitness training on body composition, strength and blood lactate. J Sports Med Phys Fitness. 2010;50(1):43-51.
27. Takeshima N, Rogers ME, Islam MM, Yamauchi T, Watanabe E, Okada A. Effect of concurrent aerobic and resistance circuit exercise training on fitness in older adults. Eur J Appl Physiol. 2004;93(1-2):173-182.
28. Giné-Garriga M, Guerra M, Pagés E, Manini TM, Jiménez R, Unnithan VB. The effect of functional circuit training on physical frailty in frail older adults: a randomized controlled trial. J Aging Phys Act. 2010;18(4):401-424.
29. Biddle ED, Reynolds P, Kopp T, Cammarata H, Conway P. Implementation of functional training tools elicits improvements in aerobic fitness and lower body strength in older veterans. Poster C169 presented at: Annual Scientific Meeting of the American Geriatrics Society; May 19-21, 2016; Long Beach, CA.
Expanding the Scope of Telemedicine in Gastroenterology
Access to specialized services has been a consistently complex problem for many integrated health care systems, including the Veterans Health Administration (VHA). About two-thirds of veterans experience significant barriers when trying to obtain medical care.1 While these problems partly mirror difficulties that nonveterans face as well, there is a unique obligation toward those who put life and health at risk during their military service.2
To better meet demands, the VHA expanded personnel and clinic infrastructure with more providers and a network of community-based outpatient clinics (CBOC) that created more openings for clinic visits.3 Yet regional variability remains a significant problem for primary and even more so for specialty medical services.
Recent data show that more than one-fifth of all veterans live in areas with low population density and shortages of health care providers.4 The data point at a special problem in this context because these veterans often face long travel times to centers offering specialty services. The introduction of electronic consults functions as an alternative venue to obtain expert input but amounts to only 2% of total consult volume.5 A more interactive approach with face-to-face teleconferencing, case discussions, and special training led by expert clinicians has further improved access in such underserved areas and played a key role in the success of the VHA hepatitis C treatment initiative.6
Despite its clearly proven role and success, these e-consults come with some conceptual shortcomings. A key caveat is the lack of direct patient involvement. Obtaining information from the source rather than relying on symptoms documented by a third person can be essential in approaching medical problems. Experts may be able to tease out the often essential details of a history when making a diagnosis. A direct contact adds an additional, perhaps less tangible, component to the interaction that relies on verbal and nonverbal components of personal interactions and plays an important role in treatment success. Prior studies strongly link credibility of and trust in a provider as well as the related treatment success to such aspects of communication.7,8
Gastroenterology Telemedicine Services
The George E. Wahlen VA Medical Center in Salt Lake City, Utah, draws from a large catchment area that extends from the southern border of Utah to the neighboring states of Idaho, Wyoming, Nevada, and Montana. Large stretches of this territory are remote with population densities well below 5 persons per square mile. The authors therefore devised a specialty outreach program relying on telemedicine for patients with gastrointestinal and liver diseases and present the initial experience with the implementation of this program.
Phase 1: Finding the Champions
Prior studies clearly emphasized that most successful telemedicine clinics relied on key persons (“champions”) promoting the idea and carrying the additional logistic and time issues required to start and maintain the new program.9,10 Thus we created a small team that defined and refined goals, identified target groups, and worked out the logistics. Based on prior experiences, we focused initially on veterans with more chronic and likely functional disorders, such as diarrhea, constipation, dyspepsia, or nausea. The team also planned to accept patients with chronic liver disorders or abnormal test results that required further clarification. By consensus, the group excluded acute problems and bleeding as well as disorders with pain as primary manifestations. The underlying assumption was that a direct physical examination was less critical in most of these cases.
Phase 2: Outreach
Clinic managers and medical directors of the affiliated CBOC were informed of the planned telemedicine clinic. Also, we identified local champions who could function as point persons and assist in the organization of visits. One member of the team personally visited key sites to discuss needs and opportunities with CBOC personnel during a routine staff meeting. The goal was to introduce the program, the key personnel, to explain criteria for appropriate candidates that may benefit from telemedicine consults, and to agree on a referral pathway. Finally, we emphasized that the consultant would always defer to the referring provider or patient and honor their requests.
Phase 3: Identifying Appropriate Patients
The team planned for and has since used 4 different pathways to identify possible candidates for telemedicine visits. The consult triaging process with telemedicine is an option that is brought up with patients if their travel to the facility exceeds 100 miles. Similarly, the team reviews procedural requests to optimize diagnostic yields and limit patient burden. For example, if endoscopic testing is requested to address chronic abdominal pain or other concerns that had already prompted a similar request with negative results, then the team will ask for feedback and recommend a telemedicine consultation prior to performing the procedure. Telemedicine also is offered for follow-up encounters to veterans seen in the facility for clinical or procedural evaluations if they live ≥ 40 miles away. The 2 other pathways are requests from referring providers or patients that specifically ask for telemedicine visits.
Phase 4: Implementation
Since rolling out the program in November 2016, video visits have been used for more than 150 clinic encounters. Within the first 12 months, 124 patients were seen at least once using telemedicine links. Of 144 visits, 54 (38%) were follow-up visits; the rest constituted initial consultations. Focusing on initial encounters only, veterans specifically asked for a telemedicine visit in 16 cases (17.8%). One-third of these referrals was specifically marked as a telemedicine visit by the primary care provider. In the remaining cases, the triaging personnel brought up the possibility of a telemedicine interaction and requested feedback from the referring provider.
Veterans resided in many different areas within and outside of the facility’s immediate referral area (Figure).
Abnormal bowel patterns, gastroesophageal reflux, and dyspepsia accounted for most concerns (Table 1).
Beyond obtaining contextual data and information about the specific clinical manifestations, the rationale for these encounters was a detailed discussion of the problem and treatment options available. Ablative therapy in Barrett esophagus best exemplifies the potential relevance of such an encounter: Although conceptually appealing to decrease cancer risk, the approach requires a significant commitment typically involving repeated sessions of radiofrequency ablation followed by intense endoscopic surveillance. With travel distances of several hundred miles in these cases, these encounters provide relevant information to patients and the opportunity to make informed decisions without the burden and cost of a long trip.
A shift in telemedicine encounters will likely occur that will increasingly rely on access from home computers or handheld devices. However, the initial phase of this program relied on connections through a CBOC. Coordination between 2 sites adds a level of complexity to ensure availability of space and videoconferencing equipment. To limit the logistic burden and improve cost-effectiveness, the authors did not expect or request the presence of the primary or another independent provider. Instead, the team communicated with a locally designated point person who coordinated the remote encounters and assisted in implementing some of the suggested next steps. Prior site visits and communications with referring providers had established channels of communication to define concerns or highlight findings. The same channels also allowed the team to direct its attention to specific aspects of the physical examination to support or rule out a presumptive diagnosis.
If additional testing was suggested, Telemedicine Services generally ordered the appropriate assessments unless veterans requested relying on local resources better known to personnel at the remote site. The most common diagnostic steps recommended were laboratory tests (n = 21; 14.6%), endoscopic procedures (n = 18; 12.5%), and radiologic studies (n = 17; 11.8%) (Table 2).
Most of the treatment changes focused on medication and dietary management, followed by lifestyle modifications and behavioral or psychological interventions. Some treatments, such as ablation of dysplastic epithelium in patients with Barrett esophagus or pneumatic dilation of achalasia required traveling to the George E. Wahlen VAMC. Nonetheless, the number of trips were limited as the team could assess appropriateness, explain approaches, and evaluate symptomatic outcomes with the initial or subsequent remote encounters. Most of the follow-up involved the primary care providers (n = 62; 43.1%), while repeat remote encounters were suggested in 31 visits (21.5%) and an in-person clinic follow-up in 7 cases (n = 4.9%). In the remaining cases, veterans were asked to contact the team directly or through their primary care provider if additional input was needed.
Discussion
The initial implementation of a specialty telemedicine clinic taught us several lessons that will not only guide this program expansion, but also may be relevant for others introducing telemedicine into their specialty clinics. At first glance, videoconferencing with patients resembles more conventional clinic encounters. However, it adds another angle as many steps from scheduling a visit to implementing recommendations rely on different members at the remote site. Thus, the success of such a program depends on establishing a true partnership with the teams at the various satellite sites. It also requires ongoing feedback from all team members and fine-tuning to effectively integrate it into the routine operations of both sites.
Feedback about the program has been very positive with comments often asking for an expansion beyond gastroenterology. Concerns largely were limited to scheduling problems that may become less relevant if the new telehealth initiative moves forward and enables health care providers to directly connect with computers or handheld devices at the patient’s home. Prior studies demonstrated that most individuals have access to such technology and accept it as a viable or even attractive option for medical encounters.11,12
For some, remaining in the comfort of their own home is not only more convenient, but also adds a sense of security, further adding to its appeal.13 As suggested by the economist Richard Thaler, simple nudges may be required to increase use and perhaps utility of telemedicine or e-consults.14 At this stage, it is the active choice of the referral or triaging provider to consider telemedicine as an option. To facilitate deviation from the established routine, we plan to revise the consult requests by using a drop-down menu option that brings up e-consult, telemedicine, or clinic visit as alternatives and requires an active choice rather than defaulting to conventional face-to-face visits.
Despite an overall successful launch of the specialty telemedicine clinic, several conceptual questions require additional in-depth assessments. While video visits indeed include the literal face time that characterizes normal clinic visits, does this translate into the “face value” that may contribute to treatment success? If detailed information about physical findings is needed, remote encounters require a third person at the distant site to complete this step, which may not only be a logistic burden, but also could influence the perceived utility and affect outcomes.
Previously published studies have demonstrated the effectiveness of video-based interactions and allow providers to address these points to some degree. Remote encounters have established roles in mental healthcare that is less dependent on physical findings.15 Distance monitoring of devices or biomarkers, such as blood sugar levels or blood pressure, are becoming routine and often are combined with corrective interventions.16-18
Recently completed trials showed satisfaction did not differ from conventional clinic encounters when telemedicine encounters were used to manage chronic headaches or provide postoperative follow-up after urologic surgery.19,20 For gastroenterology, telemedicine outreach after hospitalizations not only improved care, but also lowered rates of testing after discharge.21 In patients with inflammatory bowel disease, a group that was not targeted during this initial phase, proactive and close follow-up with remote technology can decrease the need for hospitalization.22
These data are consistent with encouraging feedback received. Nonetheless, it is important to assess whether this approach is superior to established and cheaper alternatives, most notably simple telephone interactions. Video-linkage obviously allows nonverbal elements of communication, which play an important role in patient preference and satisfaction, treatment implementation, and impact.7,8,23-25 Providers described patients as more focused and engaged compared with telephone interactions and valued the ability to incorporate body language in their assessment.26
Telemedicine clinics offered by specialty providers may not improve access as defined by wait times only, which would require adding more clinical time and personnel. However, it can lower barriers to care imposed by long distances between rural areas and facilities with specialized expertise. Even if a remote encounter concludes with the recommendation to visit the clinic for more detailed testing or treatment, explaining the need for such steps and involving the patient in the decision-making process may affect adherence.
Conclusion
Although the experiences of the team at George E. Wahlen VA Medical Center support the use of telemedicine in specialty clinics, the next phase of the project needs to address the utility of this approach and define the perceived value and potential problems of telemedicine. Obtaining this insight will require complex data sets with feedback from patients and referring and consulting providers. As trade-offs will likely vary between different diseases or symptoms, such studies will provide a better definition of clinical scenarios best suited for remote encounters. In addition, they may provide approximate values for distance or efforts that may make the cost of a direct clinic visit worth it, thereby defining boundary-condition.
1. Elnitsky CA, Andresen EM, Clark ME, McGarity S, Hall CG, Kerns RD. Access to the US Department of Veterans Affairs health system: self-reported barriers to care among returnees of Operations Enduring Freedom and Iraqi Freedom. BMC Health Serv Res. 2013;13:498.
2. Woolhandler S, Himmelstein DU, Distajo R, et al. America’s neglected veterans: 1.7 million who served have no health coverage. Int J Health Serv. 2005;35(2):313-323.
3. Rosenheck R. Primary care satellite clinics and improved access to general and mental health services. Health Serv Res. 2000;35:777-790.
4. Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52(suppl 1):459-480.
5. Kirsh S, Carey E, Aron DC, et al. Impact of a national specialty e-consultation implementation project on access. Am J Manag Care. 2015;21(12):e648-e654.
6. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C virus infection: best practices from the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504.
7. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;333(7651):999-1003.
8. Weinland SR, Morris CB, Dalton C, et al. Cognitive factors affect treatment response to medical and psychological treatments in functional bowel disorders. Am J Gastroenterol. 2010;105(6):1397-1406.
9. Wade V, Eliott J. The role of the champion in telehealth service development: a qualitative analysis. J Telemed Telecare. 2012;18(8):490-492.
10. Postema TR, Peeters JM, Friele RD. Key factors influencing the implementation success of a home telecare application. Int J Med Inform. 2012;81(6):415-423.
11. Tahir D. Trump and VA unveil telehealth initiative. https://www.politico.com/tipsheets/morning-ehealth/2017/08/04/trump-and-va-unveil-telehealth-initiative-221706. Published August 4, 2017. Accessed July 11, 2018.
12. Gardner MR, Jenkins SM, O’Neil DA, Gardner MR, Jenkins SM, O’Neil DA. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21(4):281-285.
13. Powell RE, Henstenburg JM, Cooper G, Hollander JE, Rising KL. Patient perceptions of telehealth primary care video visits. Ann Fam Med. 2017;15(3):225-229.
14. Benartzi S, Beshears J, Milkman KL, et al. Should governments invest more in nudging? Psychol Sci. 2017;28(8):1041-1055.
15. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2017:1357633x17730443.
16. Dalouk K, Gandhi N, Jessel P, et al. Outcomes of telemedicine video-conferencing clinic versus in-person clinic follow-up for implantable cardioverter-defibrillator recipients. Circ Arrhythm Electrophysiol. 2017;10(9) pii: e005217.
17. Warren R, Carlisle K, Mihala G, Scuffham PA. Effects of telemonitoring on glycaemic control and healthcare costs in type 2 diabetes: a randomised controlled trial. J Telemed Telecare. 2017:1357633x17723943.
18. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14(9):e1002389.
19. Müller KI, Alstadhaug KB, Bekkelund SI. Headache patients’ satisfaction with telemedicine: a 12-month follow-up randomized non-inferiority trial. Eur J Neurol. 2017;24(6):807-815.
20. Viers BR, Lightner DJ, Rivera ME, et al. Efficiency, satisfaction, and costs for remote video visits following radical prostatectomy: a randomized controlled trial. Eur Urol. 2015;68:729-735.
21. Wallace P, Barber J, Clayton W, et al. Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations. Health Technol Assess. 2004;8(50):1-106, iii-iv.
22. de Jong MJ, van der Meulen-de Jong AE, Romberg-Camps MJ, et al. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet. 2017;390(10098):959-968.
23. Czerniak E, Biegon A, Ziv A, et al. Manipulating the placebo response in experimental pain by altering doctor’s performance style. Front Psychol. 2016;7:874.
24. Moffet HH, Parker MM, Sarkar U, et al. Adherence to laboratory test requests by patients with diabetes: the Diabetes Study of Northern California (DISTANCE). Am J Manag Care. 2011;17(5):339-344.
25. Richter KP, Shireman TI, Ellerbeck EF, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. J Med Internet Res. 2015;17(5):e113.
26. Voils CI, Venne VL, Weidenbacher H, Sperber N, Datta S. Comparison of telephone and televideo modes for delivery of genetic counseling: a randomized trial. J Genet Couns. 2018;27(2):339-348.
Access to specialized services has been a consistently complex problem for many integrated health care systems, including the Veterans Health Administration (VHA). About two-thirds of veterans experience significant barriers when trying to obtain medical care.1 While these problems partly mirror difficulties that nonveterans face as well, there is a unique obligation toward those who put life and health at risk during their military service.2
To better meet demands, the VHA expanded personnel and clinic infrastructure with more providers and a network of community-based outpatient clinics (CBOC) that created more openings for clinic visits.3 Yet regional variability remains a significant problem for primary and even more so for specialty medical services.
Recent data show that more than one-fifth of all veterans live in areas with low population density and shortages of health care providers.4 The data point at a special problem in this context because these veterans often face long travel times to centers offering specialty services. The introduction of electronic consults functions as an alternative venue to obtain expert input but amounts to only 2% of total consult volume.5 A more interactive approach with face-to-face teleconferencing, case discussions, and special training led by expert clinicians has further improved access in such underserved areas and played a key role in the success of the VHA hepatitis C treatment initiative.6
Despite its clearly proven role and success, these e-consults come with some conceptual shortcomings. A key caveat is the lack of direct patient involvement. Obtaining information from the source rather than relying on symptoms documented by a third person can be essential in approaching medical problems. Experts may be able to tease out the often essential details of a history when making a diagnosis. A direct contact adds an additional, perhaps less tangible, component to the interaction that relies on verbal and nonverbal components of personal interactions and plays an important role in treatment success. Prior studies strongly link credibility of and trust in a provider as well as the related treatment success to such aspects of communication.7,8
Gastroenterology Telemedicine Services
The George E. Wahlen VA Medical Center in Salt Lake City, Utah, draws from a large catchment area that extends from the southern border of Utah to the neighboring states of Idaho, Wyoming, Nevada, and Montana. Large stretches of this territory are remote with population densities well below 5 persons per square mile. The authors therefore devised a specialty outreach program relying on telemedicine for patients with gastrointestinal and liver diseases and present the initial experience with the implementation of this program.
Phase 1: Finding the Champions
Prior studies clearly emphasized that most successful telemedicine clinics relied on key persons (“champions”) promoting the idea and carrying the additional logistic and time issues required to start and maintain the new program.9,10 Thus we created a small team that defined and refined goals, identified target groups, and worked out the logistics. Based on prior experiences, we focused initially on veterans with more chronic and likely functional disorders, such as diarrhea, constipation, dyspepsia, or nausea. The team also planned to accept patients with chronic liver disorders or abnormal test results that required further clarification. By consensus, the group excluded acute problems and bleeding as well as disorders with pain as primary manifestations. The underlying assumption was that a direct physical examination was less critical in most of these cases.
Phase 2: Outreach
Clinic managers and medical directors of the affiliated CBOC were informed of the planned telemedicine clinic. Also, we identified local champions who could function as point persons and assist in the organization of visits. One member of the team personally visited key sites to discuss needs and opportunities with CBOC personnel during a routine staff meeting. The goal was to introduce the program, the key personnel, to explain criteria for appropriate candidates that may benefit from telemedicine consults, and to agree on a referral pathway. Finally, we emphasized that the consultant would always defer to the referring provider or patient and honor their requests.
Phase 3: Identifying Appropriate Patients
The team planned for and has since used 4 different pathways to identify possible candidates for telemedicine visits. The consult triaging process with telemedicine is an option that is brought up with patients if their travel to the facility exceeds 100 miles. Similarly, the team reviews procedural requests to optimize diagnostic yields and limit patient burden. For example, if endoscopic testing is requested to address chronic abdominal pain or other concerns that had already prompted a similar request with negative results, then the team will ask for feedback and recommend a telemedicine consultation prior to performing the procedure. Telemedicine also is offered for follow-up encounters to veterans seen in the facility for clinical or procedural evaluations if they live ≥ 40 miles away. The 2 other pathways are requests from referring providers or patients that specifically ask for telemedicine visits.
Phase 4: Implementation
Since rolling out the program in November 2016, video visits have been used for more than 150 clinic encounters. Within the first 12 months, 124 patients were seen at least once using telemedicine links. Of 144 visits, 54 (38%) were follow-up visits; the rest constituted initial consultations. Focusing on initial encounters only, veterans specifically asked for a telemedicine visit in 16 cases (17.8%). One-third of these referrals was specifically marked as a telemedicine visit by the primary care provider. In the remaining cases, the triaging personnel brought up the possibility of a telemedicine interaction and requested feedback from the referring provider.
Veterans resided in many different areas within and outside of the facility’s immediate referral area (Figure).
Abnormal bowel patterns, gastroesophageal reflux, and dyspepsia accounted for most concerns (Table 1).
Beyond obtaining contextual data and information about the specific clinical manifestations, the rationale for these encounters was a detailed discussion of the problem and treatment options available. Ablative therapy in Barrett esophagus best exemplifies the potential relevance of such an encounter: Although conceptually appealing to decrease cancer risk, the approach requires a significant commitment typically involving repeated sessions of radiofrequency ablation followed by intense endoscopic surveillance. With travel distances of several hundred miles in these cases, these encounters provide relevant information to patients and the opportunity to make informed decisions without the burden and cost of a long trip.
A shift in telemedicine encounters will likely occur that will increasingly rely on access from home computers or handheld devices. However, the initial phase of this program relied on connections through a CBOC. Coordination between 2 sites adds a level of complexity to ensure availability of space and videoconferencing equipment. To limit the logistic burden and improve cost-effectiveness, the authors did not expect or request the presence of the primary or another independent provider. Instead, the team communicated with a locally designated point person who coordinated the remote encounters and assisted in implementing some of the suggested next steps. Prior site visits and communications with referring providers had established channels of communication to define concerns or highlight findings. The same channels also allowed the team to direct its attention to specific aspects of the physical examination to support or rule out a presumptive diagnosis.
If additional testing was suggested, Telemedicine Services generally ordered the appropriate assessments unless veterans requested relying on local resources better known to personnel at the remote site. The most common diagnostic steps recommended were laboratory tests (n = 21; 14.6%), endoscopic procedures (n = 18; 12.5%), and radiologic studies (n = 17; 11.8%) (Table 2).
Most of the treatment changes focused on medication and dietary management, followed by lifestyle modifications and behavioral or psychological interventions. Some treatments, such as ablation of dysplastic epithelium in patients with Barrett esophagus or pneumatic dilation of achalasia required traveling to the George E. Wahlen VAMC. Nonetheless, the number of trips were limited as the team could assess appropriateness, explain approaches, and evaluate symptomatic outcomes with the initial or subsequent remote encounters. Most of the follow-up involved the primary care providers (n = 62; 43.1%), while repeat remote encounters were suggested in 31 visits (21.5%) and an in-person clinic follow-up in 7 cases (n = 4.9%). In the remaining cases, veterans were asked to contact the team directly or through their primary care provider if additional input was needed.
Discussion
The initial implementation of a specialty telemedicine clinic taught us several lessons that will not only guide this program expansion, but also may be relevant for others introducing telemedicine into their specialty clinics. At first glance, videoconferencing with patients resembles more conventional clinic encounters. However, it adds another angle as many steps from scheduling a visit to implementing recommendations rely on different members at the remote site. Thus, the success of such a program depends on establishing a true partnership with the teams at the various satellite sites. It also requires ongoing feedback from all team members and fine-tuning to effectively integrate it into the routine operations of both sites.
Feedback about the program has been very positive with comments often asking for an expansion beyond gastroenterology. Concerns largely were limited to scheduling problems that may become less relevant if the new telehealth initiative moves forward and enables health care providers to directly connect with computers or handheld devices at the patient’s home. Prior studies demonstrated that most individuals have access to such technology and accept it as a viable or even attractive option for medical encounters.11,12
For some, remaining in the comfort of their own home is not only more convenient, but also adds a sense of security, further adding to its appeal.13 As suggested by the economist Richard Thaler, simple nudges may be required to increase use and perhaps utility of telemedicine or e-consults.14 At this stage, it is the active choice of the referral or triaging provider to consider telemedicine as an option. To facilitate deviation from the established routine, we plan to revise the consult requests by using a drop-down menu option that brings up e-consult, telemedicine, or clinic visit as alternatives and requires an active choice rather than defaulting to conventional face-to-face visits.
Despite an overall successful launch of the specialty telemedicine clinic, several conceptual questions require additional in-depth assessments. While video visits indeed include the literal face time that characterizes normal clinic visits, does this translate into the “face value” that may contribute to treatment success? If detailed information about physical findings is needed, remote encounters require a third person at the distant site to complete this step, which may not only be a logistic burden, but also could influence the perceived utility and affect outcomes.
Previously published studies have demonstrated the effectiveness of video-based interactions and allow providers to address these points to some degree. Remote encounters have established roles in mental healthcare that is less dependent on physical findings.15 Distance monitoring of devices or biomarkers, such as blood sugar levels or blood pressure, are becoming routine and often are combined with corrective interventions.16-18
Recently completed trials showed satisfaction did not differ from conventional clinic encounters when telemedicine encounters were used to manage chronic headaches or provide postoperative follow-up after urologic surgery.19,20 For gastroenterology, telemedicine outreach after hospitalizations not only improved care, but also lowered rates of testing after discharge.21 In patients with inflammatory bowel disease, a group that was not targeted during this initial phase, proactive and close follow-up with remote technology can decrease the need for hospitalization.22
These data are consistent with encouraging feedback received. Nonetheless, it is important to assess whether this approach is superior to established and cheaper alternatives, most notably simple telephone interactions. Video-linkage obviously allows nonverbal elements of communication, which play an important role in patient preference and satisfaction, treatment implementation, and impact.7,8,23-25 Providers described patients as more focused and engaged compared with telephone interactions and valued the ability to incorporate body language in their assessment.26
Telemedicine clinics offered by specialty providers may not improve access as defined by wait times only, which would require adding more clinical time and personnel. However, it can lower barriers to care imposed by long distances between rural areas and facilities with specialized expertise. Even if a remote encounter concludes with the recommendation to visit the clinic for more detailed testing or treatment, explaining the need for such steps and involving the patient in the decision-making process may affect adherence.
Conclusion
Although the experiences of the team at George E. Wahlen VA Medical Center support the use of telemedicine in specialty clinics, the next phase of the project needs to address the utility of this approach and define the perceived value and potential problems of telemedicine. Obtaining this insight will require complex data sets with feedback from patients and referring and consulting providers. As trade-offs will likely vary between different diseases or symptoms, such studies will provide a better definition of clinical scenarios best suited for remote encounters. In addition, they may provide approximate values for distance or efforts that may make the cost of a direct clinic visit worth it, thereby defining boundary-condition.
Access to specialized services has been a consistently complex problem for many integrated health care systems, including the Veterans Health Administration (VHA). About two-thirds of veterans experience significant barriers when trying to obtain medical care.1 While these problems partly mirror difficulties that nonveterans face as well, there is a unique obligation toward those who put life and health at risk during their military service.2
To better meet demands, the VHA expanded personnel and clinic infrastructure with more providers and a network of community-based outpatient clinics (CBOC) that created more openings for clinic visits.3 Yet regional variability remains a significant problem for primary and even more so for specialty medical services.
Recent data show that more than one-fifth of all veterans live in areas with low population density and shortages of health care providers.4 The data point at a special problem in this context because these veterans often face long travel times to centers offering specialty services. The introduction of electronic consults functions as an alternative venue to obtain expert input but amounts to only 2% of total consult volume.5 A more interactive approach with face-to-face teleconferencing, case discussions, and special training led by expert clinicians has further improved access in such underserved areas and played a key role in the success of the VHA hepatitis C treatment initiative.6
Despite its clearly proven role and success, these e-consults come with some conceptual shortcomings. A key caveat is the lack of direct patient involvement. Obtaining information from the source rather than relying on symptoms documented by a third person can be essential in approaching medical problems. Experts may be able to tease out the often essential details of a history when making a diagnosis. A direct contact adds an additional, perhaps less tangible, component to the interaction that relies on verbal and nonverbal components of personal interactions and plays an important role in treatment success. Prior studies strongly link credibility of and trust in a provider as well as the related treatment success to such aspects of communication.7,8
Gastroenterology Telemedicine Services
The George E. Wahlen VA Medical Center in Salt Lake City, Utah, draws from a large catchment area that extends from the southern border of Utah to the neighboring states of Idaho, Wyoming, Nevada, and Montana. Large stretches of this territory are remote with population densities well below 5 persons per square mile. The authors therefore devised a specialty outreach program relying on telemedicine for patients with gastrointestinal and liver diseases and present the initial experience with the implementation of this program.
Phase 1: Finding the Champions
Prior studies clearly emphasized that most successful telemedicine clinics relied on key persons (“champions”) promoting the idea and carrying the additional logistic and time issues required to start and maintain the new program.9,10 Thus we created a small team that defined and refined goals, identified target groups, and worked out the logistics. Based on prior experiences, we focused initially on veterans with more chronic and likely functional disorders, such as diarrhea, constipation, dyspepsia, or nausea. The team also planned to accept patients with chronic liver disorders or abnormal test results that required further clarification. By consensus, the group excluded acute problems and bleeding as well as disorders with pain as primary manifestations. The underlying assumption was that a direct physical examination was less critical in most of these cases.
Phase 2: Outreach
Clinic managers and medical directors of the affiliated CBOC were informed of the planned telemedicine clinic. Also, we identified local champions who could function as point persons and assist in the organization of visits. One member of the team personally visited key sites to discuss needs and opportunities with CBOC personnel during a routine staff meeting. The goal was to introduce the program, the key personnel, to explain criteria for appropriate candidates that may benefit from telemedicine consults, and to agree on a referral pathway. Finally, we emphasized that the consultant would always defer to the referring provider or patient and honor their requests.
Phase 3: Identifying Appropriate Patients
The team planned for and has since used 4 different pathways to identify possible candidates for telemedicine visits. The consult triaging process with telemedicine is an option that is brought up with patients if their travel to the facility exceeds 100 miles. Similarly, the team reviews procedural requests to optimize diagnostic yields and limit patient burden. For example, if endoscopic testing is requested to address chronic abdominal pain or other concerns that had already prompted a similar request with negative results, then the team will ask for feedback and recommend a telemedicine consultation prior to performing the procedure. Telemedicine also is offered for follow-up encounters to veterans seen in the facility for clinical or procedural evaluations if they live ≥ 40 miles away. The 2 other pathways are requests from referring providers or patients that specifically ask for telemedicine visits.
Phase 4: Implementation
Since rolling out the program in November 2016, video visits have been used for more than 150 clinic encounters. Within the first 12 months, 124 patients were seen at least once using telemedicine links. Of 144 visits, 54 (38%) were follow-up visits; the rest constituted initial consultations. Focusing on initial encounters only, veterans specifically asked for a telemedicine visit in 16 cases (17.8%). One-third of these referrals was specifically marked as a telemedicine visit by the primary care provider. In the remaining cases, the triaging personnel brought up the possibility of a telemedicine interaction and requested feedback from the referring provider.
Veterans resided in many different areas within and outside of the facility’s immediate referral area (Figure).
Abnormal bowel patterns, gastroesophageal reflux, and dyspepsia accounted for most concerns (Table 1).
Beyond obtaining contextual data and information about the specific clinical manifestations, the rationale for these encounters was a detailed discussion of the problem and treatment options available. Ablative therapy in Barrett esophagus best exemplifies the potential relevance of such an encounter: Although conceptually appealing to decrease cancer risk, the approach requires a significant commitment typically involving repeated sessions of radiofrequency ablation followed by intense endoscopic surveillance. With travel distances of several hundred miles in these cases, these encounters provide relevant information to patients and the opportunity to make informed decisions without the burden and cost of a long trip.
A shift in telemedicine encounters will likely occur that will increasingly rely on access from home computers or handheld devices. However, the initial phase of this program relied on connections through a CBOC. Coordination between 2 sites adds a level of complexity to ensure availability of space and videoconferencing equipment. To limit the logistic burden and improve cost-effectiveness, the authors did not expect or request the presence of the primary or another independent provider. Instead, the team communicated with a locally designated point person who coordinated the remote encounters and assisted in implementing some of the suggested next steps. Prior site visits and communications with referring providers had established channels of communication to define concerns or highlight findings. The same channels also allowed the team to direct its attention to specific aspects of the physical examination to support or rule out a presumptive diagnosis.
If additional testing was suggested, Telemedicine Services generally ordered the appropriate assessments unless veterans requested relying on local resources better known to personnel at the remote site. The most common diagnostic steps recommended were laboratory tests (n = 21; 14.6%), endoscopic procedures (n = 18; 12.5%), and radiologic studies (n = 17; 11.8%) (Table 2).
Most of the treatment changes focused on medication and dietary management, followed by lifestyle modifications and behavioral or psychological interventions. Some treatments, such as ablation of dysplastic epithelium in patients with Barrett esophagus or pneumatic dilation of achalasia required traveling to the George E. Wahlen VAMC. Nonetheless, the number of trips were limited as the team could assess appropriateness, explain approaches, and evaluate symptomatic outcomes with the initial or subsequent remote encounters. Most of the follow-up involved the primary care providers (n = 62; 43.1%), while repeat remote encounters were suggested in 31 visits (21.5%) and an in-person clinic follow-up in 7 cases (n = 4.9%). In the remaining cases, veterans were asked to contact the team directly or through their primary care provider if additional input was needed.
Discussion
The initial implementation of a specialty telemedicine clinic taught us several lessons that will not only guide this program expansion, but also may be relevant for others introducing telemedicine into their specialty clinics. At first glance, videoconferencing with patients resembles more conventional clinic encounters. However, it adds another angle as many steps from scheduling a visit to implementing recommendations rely on different members at the remote site. Thus, the success of such a program depends on establishing a true partnership with the teams at the various satellite sites. It also requires ongoing feedback from all team members and fine-tuning to effectively integrate it into the routine operations of both sites.
Feedback about the program has been very positive with comments often asking for an expansion beyond gastroenterology. Concerns largely were limited to scheduling problems that may become less relevant if the new telehealth initiative moves forward and enables health care providers to directly connect with computers or handheld devices at the patient’s home. Prior studies demonstrated that most individuals have access to such technology and accept it as a viable or even attractive option for medical encounters.11,12
For some, remaining in the comfort of their own home is not only more convenient, but also adds a sense of security, further adding to its appeal.13 As suggested by the economist Richard Thaler, simple nudges may be required to increase use and perhaps utility of telemedicine or e-consults.14 At this stage, it is the active choice of the referral or triaging provider to consider telemedicine as an option. To facilitate deviation from the established routine, we plan to revise the consult requests by using a drop-down menu option that brings up e-consult, telemedicine, or clinic visit as alternatives and requires an active choice rather than defaulting to conventional face-to-face visits.
Despite an overall successful launch of the specialty telemedicine clinic, several conceptual questions require additional in-depth assessments. While video visits indeed include the literal face time that characterizes normal clinic visits, does this translate into the “face value” that may contribute to treatment success? If detailed information about physical findings is needed, remote encounters require a third person at the distant site to complete this step, which may not only be a logistic burden, but also could influence the perceived utility and affect outcomes.
Previously published studies have demonstrated the effectiveness of video-based interactions and allow providers to address these points to some degree. Remote encounters have established roles in mental healthcare that is less dependent on physical findings.15 Distance monitoring of devices or biomarkers, such as blood sugar levels or blood pressure, are becoming routine and often are combined with corrective interventions.16-18
Recently completed trials showed satisfaction did not differ from conventional clinic encounters when telemedicine encounters were used to manage chronic headaches or provide postoperative follow-up after urologic surgery.19,20 For gastroenterology, telemedicine outreach after hospitalizations not only improved care, but also lowered rates of testing after discharge.21 In patients with inflammatory bowel disease, a group that was not targeted during this initial phase, proactive and close follow-up with remote technology can decrease the need for hospitalization.22
These data are consistent with encouraging feedback received. Nonetheless, it is important to assess whether this approach is superior to established and cheaper alternatives, most notably simple telephone interactions. Video-linkage obviously allows nonverbal elements of communication, which play an important role in patient preference and satisfaction, treatment implementation, and impact.7,8,23-25 Providers described patients as more focused and engaged compared with telephone interactions and valued the ability to incorporate body language in their assessment.26
Telemedicine clinics offered by specialty providers may not improve access as defined by wait times only, which would require adding more clinical time and personnel. However, it can lower barriers to care imposed by long distances between rural areas and facilities with specialized expertise. Even if a remote encounter concludes with the recommendation to visit the clinic for more detailed testing or treatment, explaining the need for such steps and involving the patient in the decision-making process may affect adherence.
Conclusion
Although the experiences of the team at George E. Wahlen VA Medical Center support the use of telemedicine in specialty clinics, the next phase of the project needs to address the utility of this approach and define the perceived value and potential problems of telemedicine. Obtaining this insight will require complex data sets with feedback from patients and referring and consulting providers. As trade-offs will likely vary between different diseases or symptoms, such studies will provide a better definition of clinical scenarios best suited for remote encounters. In addition, they may provide approximate values for distance or efforts that may make the cost of a direct clinic visit worth it, thereby defining boundary-condition.
1. Elnitsky CA, Andresen EM, Clark ME, McGarity S, Hall CG, Kerns RD. Access to the US Department of Veterans Affairs health system: self-reported barriers to care among returnees of Operations Enduring Freedom and Iraqi Freedom. BMC Health Serv Res. 2013;13:498.
2. Woolhandler S, Himmelstein DU, Distajo R, et al. America’s neglected veterans: 1.7 million who served have no health coverage. Int J Health Serv. 2005;35(2):313-323.
3. Rosenheck R. Primary care satellite clinics and improved access to general and mental health services. Health Serv Res. 2000;35:777-790.
4. Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52(suppl 1):459-480.
5. Kirsh S, Carey E, Aron DC, et al. Impact of a national specialty e-consultation implementation project on access. Am J Manag Care. 2015;21(12):e648-e654.
6. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C virus infection: best practices from the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504.
7. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;333(7651):999-1003.
8. Weinland SR, Morris CB, Dalton C, et al. Cognitive factors affect treatment response to medical and psychological treatments in functional bowel disorders. Am J Gastroenterol. 2010;105(6):1397-1406.
9. Wade V, Eliott J. The role of the champion in telehealth service development: a qualitative analysis. J Telemed Telecare. 2012;18(8):490-492.
10. Postema TR, Peeters JM, Friele RD. Key factors influencing the implementation success of a home telecare application. Int J Med Inform. 2012;81(6):415-423.
11. Tahir D. Trump and VA unveil telehealth initiative. https://www.politico.com/tipsheets/morning-ehealth/2017/08/04/trump-and-va-unveil-telehealth-initiative-221706. Published August 4, 2017. Accessed July 11, 2018.
12. Gardner MR, Jenkins SM, O’Neil DA, Gardner MR, Jenkins SM, O’Neil DA. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21(4):281-285.
13. Powell RE, Henstenburg JM, Cooper G, Hollander JE, Rising KL. Patient perceptions of telehealth primary care video visits. Ann Fam Med. 2017;15(3):225-229.
14. Benartzi S, Beshears J, Milkman KL, et al. Should governments invest more in nudging? Psychol Sci. 2017;28(8):1041-1055.
15. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2017:1357633x17730443.
16. Dalouk K, Gandhi N, Jessel P, et al. Outcomes of telemedicine video-conferencing clinic versus in-person clinic follow-up for implantable cardioverter-defibrillator recipients. Circ Arrhythm Electrophysiol. 2017;10(9) pii: e005217.
17. Warren R, Carlisle K, Mihala G, Scuffham PA. Effects of telemonitoring on glycaemic control and healthcare costs in type 2 diabetes: a randomised controlled trial. J Telemed Telecare. 2017:1357633x17723943.
18. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14(9):e1002389.
19. Müller KI, Alstadhaug KB, Bekkelund SI. Headache patients’ satisfaction with telemedicine: a 12-month follow-up randomized non-inferiority trial. Eur J Neurol. 2017;24(6):807-815.
20. Viers BR, Lightner DJ, Rivera ME, et al. Efficiency, satisfaction, and costs for remote video visits following radical prostatectomy: a randomized controlled trial. Eur Urol. 2015;68:729-735.
21. Wallace P, Barber J, Clayton W, et al. Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations. Health Technol Assess. 2004;8(50):1-106, iii-iv.
22. de Jong MJ, van der Meulen-de Jong AE, Romberg-Camps MJ, et al. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet. 2017;390(10098):959-968.
23. Czerniak E, Biegon A, Ziv A, et al. Manipulating the placebo response in experimental pain by altering doctor’s performance style. Front Psychol. 2016;7:874.
24. Moffet HH, Parker MM, Sarkar U, et al. Adherence to laboratory test requests by patients with diabetes: the Diabetes Study of Northern California (DISTANCE). Am J Manag Care. 2011;17(5):339-344.
25. Richter KP, Shireman TI, Ellerbeck EF, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. J Med Internet Res. 2015;17(5):e113.
26. Voils CI, Venne VL, Weidenbacher H, Sperber N, Datta S. Comparison of telephone and televideo modes for delivery of genetic counseling: a randomized trial. J Genet Couns. 2018;27(2):339-348.
1. Elnitsky CA, Andresen EM, Clark ME, McGarity S, Hall CG, Kerns RD. Access to the US Department of Veterans Affairs health system: self-reported barriers to care among returnees of Operations Enduring Freedom and Iraqi Freedom. BMC Health Serv Res. 2013;13:498.
2. Woolhandler S, Himmelstein DU, Distajo R, et al. America’s neglected veterans: 1.7 million who served have no health coverage. Int J Health Serv. 2005;35(2):313-323.
3. Rosenheck R. Primary care satellite clinics and improved access to general and mental health services. Health Serv Res. 2000;35:777-790.
4. Doyle JM, Streeter RA. Veterans’ location in health professional shortage areas: implications for access to care and workforce supply. Health Serv Res. 2017;52(suppl 1):459-480.
5. Kirsh S, Carey E, Aron DC, et al. Impact of a national specialty e-consultation implementation project on access. Am J Manag Care. 2015;21(12):e648-e654.
6. Belperio PS, Chartier M, Ross DB, Alaigh P, Shulkin D. Curing hepatitis C virus infection: best practices from the U.S. Department of Veterans Affairs. Ann Intern Med. 2017;167(7):499-504.
7. Kaptchuk TJ, Kelley JM, Conboy LA, et al. Components of placebo effect: randomised controlled trial in patients with irritable bowel syndrome. BMJ. 2008;333(7651):999-1003.
8. Weinland SR, Morris CB, Dalton C, et al. Cognitive factors affect treatment response to medical and psychological treatments in functional bowel disorders. Am J Gastroenterol. 2010;105(6):1397-1406.
9. Wade V, Eliott J. The role of the champion in telehealth service development: a qualitative analysis. J Telemed Telecare. 2012;18(8):490-492.
10. Postema TR, Peeters JM, Friele RD. Key factors influencing the implementation success of a home telecare application. Int J Med Inform. 2012;81(6):415-423.
11. Tahir D. Trump and VA unveil telehealth initiative. https://www.politico.com/tipsheets/morning-ehealth/2017/08/04/trump-and-va-unveil-telehealth-initiative-221706. Published August 4, 2017. Accessed July 11, 2018.
12. Gardner MR, Jenkins SM, O’Neil DA, Gardner MR, Jenkins SM, O’Neil DA. Perceptions of video-based appointments from the patient’s home: a patient survey. Telemed J E Health. 2015;21(4):281-285.
13. Powell RE, Henstenburg JM, Cooper G, Hollander JE, Rising KL. Patient perceptions of telehealth primary care video visits. Ann Fam Med. 2017;15(3):225-229.
14. Benartzi S, Beshears J, Milkman KL, et al. Should governments invest more in nudging? Psychol Sci. 2017;28(8):1041-1055.
15. Turgoose D, Ashwick R, Murphy D. Systematic review of lessons learned from delivering tele-therapy to veterans with post-traumatic stress disorder. J Telemed Telecare. 2017:1357633x17730443.
16. Dalouk K, Gandhi N, Jessel P, et al. Outcomes of telemedicine video-conferencing clinic versus in-person clinic follow-up for implantable cardioverter-defibrillator recipients. Circ Arrhythm Electrophysiol. 2017;10(9) pii: e005217.
17. Warren R, Carlisle K, Mihala G, Scuffham PA. Effects of telemonitoring on glycaemic control and healthcare costs in type 2 diabetes: a randomised controlled trial. J Telemed Telecare. 2017:1357633x17723943.
18. Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14(9):e1002389.
19. Müller KI, Alstadhaug KB, Bekkelund SI. Headache patients’ satisfaction with telemedicine: a 12-month follow-up randomized non-inferiority trial. Eur J Neurol. 2017;24(6):807-815.
20. Viers BR, Lightner DJ, Rivera ME, et al. Efficiency, satisfaction, and costs for remote video visits following radical prostatectomy: a randomized controlled trial. Eur Urol. 2015;68:729-735.
21. Wallace P, Barber J, Clayton W, et al. Virtual outreach: a randomised controlled trial and economic evaluation of joint teleconferenced medical consultations. Health Technol Assess. 2004;8(50):1-106, iii-iv.
22. de Jong MJ, van der Meulen-de Jong AE, Romberg-Camps MJ, et al. Telemedicine for management of inflammatory bowel disease (myIBDcoach): a pragmatic, multicentre, randomised controlled trial. Lancet. 2017;390(10098):959-968.
23. Czerniak E, Biegon A, Ziv A, et al. Manipulating the placebo response in experimental pain by altering doctor’s performance style. Front Psychol. 2016;7:874.
24. Moffet HH, Parker MM, Sarkar U, et al. Adherence to laboratory test requests by patients with diabetes: the Diabetes Study of Northern California (DISTANCE). Am J Manag Care. 2011;17(5):339-344.
25. Richter KP, Shireman TI, Ellerbeck EF, et al. Comparative and cost effectiveness of telemedicine versus telephone counseling for smoking cessation. J Med Internet Res. 2015;17(5):e113.
26. Voils CI, Venne VL, Weidenbacher H, Sperber N, Datta S. Comparison of telephone and televideo modes for delivery of genetic counseling: a randomized trial. J Genet Couns. 2018;27(2):339-348.
Using Stroke Order Sets to Improve Compliance With Quality Measures for Ischemic Stroke Admissions
Stroke and cardiovascular disease (CVD) create a heavy economic burden on the health care system in the US.1 About 795,000 people have a stroke in the US each year. In 2013, stroke was the cause of 1 in every 20 deaths in the US.2 On average, someone in the US has a stroke every 40 seconds, and someone dies of one about every 4 minutes.3 Stroke also accounts for 889,000 hospitalizations per year.4,5
Stroke has been studied widely, and evidence-based guidelines have been created for the management of stroke. Despite these published guidelines for stroke care, inconsistencies in stroke management of veterans still exist. These inconsistencies led to the creation of guidelines that include quality measurements for the care of veterans with stroke.
Several campaigns have been mounted to bolster quality care for veterans with ischemic stroke. These include the Primary Stroke Center Certification by The Joint Commission (JC),6 Get With the Guidelines by the American Stroke Association,7 the Paul Coverdell Registry by the Centers for Disease Control and Prevention,8 and other efforts by the National Quality Forum (NQF) and the Centers for Medicare and Medicaid Services.9 These organizations have independently and collaboratively established quality metrics associated with health care delivery for the care of veterans with stroke. Some of these metrics have been distinguished as performance measures, or metrics that are suitable for public reporting, and may be used for comparing institutions and rewarding those who meet specific thresholds (ie, pay for performance).10
The aim of this project was to increase compliance at the Atlanta VA Medical Center (VAMC) in Decatur, Georgia, with JC National Quality Measures for the care of veterans with ischemic stroke, thus providing optimal care for veterans admitted for ischemic stroke management.
There are 3 phases in the management of a patient with a stroke: stroke presentation, admission/management, and discharge. This project focused on the admission/management phase. The stroke presentation phase is completed in the emergency department (ED), and the discharge phase has a check list for stroke, including atrial fibrillation (AF) and counseling prior to discharge. Data from the check list and counseling were not included in this project.
Specific attention was given to the following JC measures: stroke (STK) 1, STK 5, and STK 10 because the Atlanta VAMC was below the national average for these core measures for fiscal year 2015. Compliance was accomplished by creating order sets for the admission and subsequent care of veterans with ischemic stroke, tracking order set usage, and reporting regularly to the medicine/admitting team members on use rates and meeting quality measures. This project underwent the quality vs research review process and was determined to be a quality improvement (QI) project, so the project did not require institutional review board approval.
Methods
At the Atlanta VAMC, all patients admitted for stroke workup or management are admitted to the medicine service. The medicine admitting teams are composed of an attending physician, a medicine resident, a nurse practitioner (NP), a pharmacist, and 2 interns; and the hospitalist team composed of a hospitalist. The project began January 1, 2016, and ended December 31, 2016.
The hospitalist created evidence-based admission orders for all patients admitted for stroke or transient ischemic attack (TIA).The measures used were from the JC Specification Manual for Joint Commission National Quality as well as The American College of Cardiology/American Heart Association classification of care metrics.5
The order sets were reviewed and confirmed by a neurologist. The JC quality measures required for the care of patients admitted for stroke management were embedded in these order sets. These order sets were placed directly under the general admission orders in the Computerized Patient Record System (CPRS)
The quality measures included:
- STK 1: Veteran admitted for stroke received venous thromboembolic (VTE) prophylaxis in a timely manner. Pharmacologic management for VTE prophylaxis with subcutaneous low-molecular weight heparin and/or application of bilateral sequential compression devices were tracked.
- STK 5: Veteran admitted for stroke administered antithrombotic therapy by end of hospital day 2. Aspirin, aspirin/dipyridamole, and ticlopidine were tracked.
- STK 10: Veteran admitted for stroke assessed for rehabilitation services during admission. Physical therapy and occupational therapy consult placements were tracked. Quality measures, such as administration of tissue plasminogen activator (tPA), were not embedded in the order set because veterans who met the criteria for tPA were immediately administered tPA in the ED or transferred to the closest stroke center.
In this QI project, only quality measures that had to be completed in the inpatient setting were included. Quality measures such as tPA administration, National Institutes of Health (NIH) Stroke Scale timely documentation, swallow screen prior to po intake, and stroke transfers were completed in the ED prior to clearance for admission, so these were not included in the project. The Atlanta VAMC ED has protocols to care for these patients, but they do not have order sets with markers that could trace their usage.
All admission orders placed were reviewed by a QI team to check whether the stroke order set had been used. The ability to determine order set use was accomplished by adding the unique identifier Stroke Order Set Marker, which allowed for querying using structured query language (SQL) within the Corporate Data Warehouse.
Next, all admissions were checked through chart review for compliance with quality measures. Admissions that had not been completed for all quality measures were identified, and the physicians or NPs caring for those veterans were alerted. These order sets were supposed to be used during admission of all patients admitted for stroke management or workup; however, some patients were admitted without the use of the order sets.
The successful completion of the quality measures were then compared between the groups of patients admitted using the order set and the group of patients in which the order set was not used at their admission. The physicians were provided acceptable reasons, including contraindications to certain medications such as patient history of allergy. The admitting physician made decisions on the antiplatelet medications to use or on neurology recommendations. The neurology department was consulted on all patients who had acute or subacute ischemic stroke findings on magnetic resonance imaging (MRI).
At the beginning of the month, internal medicine residents from Emory University and Morehouse School of Medicine received orientation on the use of the stroke order set from the team NP and chief resident. Tips on how to use the CPRS and how to access the stroke order sets also were created.
One challenge the project faced was the continuous change in the admitting team pool: Some residents did not remember to use the stroke order sets.
Results
Of 323 admitted patients with stroke, 93 admissions were entered using the stroke order set. Out of these completed orders, 85 (91%) veterans admitted for ischemic stroke or TIA management received timely VTE prophylaxis, and 8 (9%) veterans did not. Of the 230 admissions completed without using the stroke order set, 167 (73%) veterans received timely VTE prophylaxis, and 63 (28%) veterans did not. Additionally out of the 93 veterans admitted using the stroke order set, 74 (80%) veterans admitted for the management of ischemic stroke received antithrombotic therapy by end of hospital day 2, whereas 19 (20%) veterans did not, and there were no clear contraindications documented as to why.
For veterans admitted without using the order set, 167 (73%) veterans admitted for the management of ischemic stroke received antithrombotic therapy by the end of hospital day 2, whereas 76 (33%) veterans did not. Last, 90 (97%) of the 93 veterans admitted for stroke workup using the order set were assessed for rehabilitation services during admission, whereas 3 (3%) were not. For the veterans who were admitted without using the stroke order set, 162 (70%) were assessed for rehabilitation services during admission, whereas 68 (30%) were not.
Out of 969 compliance measures looked at, 237 measures were missed and 732 measures were appropriately completed irrespective of whether the stroke order set was used. Out of the 279 admissions where the stroke order set was used, 249 (89%) quality measures were met.
The study threshold for meeting the standards was the national average for 2015, which was 91.1% for the administration of VTE prophylaxis in a timely manner, 97.9% for administering antithrombotic therapy by end of hospital day 2, and 94.2% for assessment of the patient by rehabilitation services during the admission.
Discussion
Despite the repeated training and orientation, compliance to the order set usage was not optimal, likely secondary to a frequent change in the pool of admitting physicians using the order set. Also, the order set was new to staff, thus, admitting physicians sometimes forgot to use them. The next step in this project will be to create an order set for the ED with markers for tracing usage. These order sets will include all quality measures that need to be completed in the ED, such as the NIH Stroke Scale timely documentation, tPA administration data, swallow screen prior to po intake, and stroke transfers.
This QI project also streamlined the process for stroke admissions. With the creation of the order set, all orders needed for stroke were available to the admitting physician, resulting in less need for searching the order individually from a large pool of orders (Figures 3 and 4).
Several reputable institutions have quality metrics and performance measures typically focused on processes of care based on specific clinical guidelines recommendations. Clinical guidelines are usually based on sufficient evidence that failure to provide the recommended care is likely to result in suboptimal clinical outcomes. Stroke quality measure compliance is part of the Reporting Hospital Quality Data for Annual Payment Update (RHQDAPU) initiative, and most hospitals will be required to report these measures in order to receive full Medicare payments.11
Limitations
Limitations of this study relate to CPRS functions, which must be specifically activated at different VA sites in order to enable the use of these functions. Also, the successful creation of these order sets depended on the information specialist’s knowledge of the capabilities of the CPRS.
Conclusion
Gaps in practice and recommended guidelines can be bridged by creating standardized admission orders embedded with required quality measures. The Atlanta VAMC project showed that the use of a standardized stroke admission order set significantly improved compliance to quality measures for veterans admitted for ischemic stroke management. This is consistent with a study completed in the ED, which showed that for veterans hospitalized for acute ischemic stroke, electronic order set use was associated with increased use of IV tPA.12 Creating order sets can be challenging, but if these barriers can be overcome, with the first order set, similar templates can be used to create order sets for other clinical conditions, such as heart failure, sepsis, and chronic obstructive pulmonary disease exacerbation.
1. Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447-454.
2. Centers for Disease Control and Prevention. Vital signs: recent trends in stroke death rates–United States, 2000-2015. https://www.cdc.gov/mmwr/volumes/66/wr/mm6635e1.htm. Published September 8, 2017. Accessed June 14, 2018.
3. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e229-e445.
4. Lloyd-Jones D, Adams R, Carnethon M, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2009;119(3):480-486.
5. Poisson SN, Josephson SA. Quality measures in stroke. Neurohospitalist. 2011;1(2):71-77.
6. The Joint Commission. Primary Stroke Centers—Stroke Performance Measurement. https://www.jointcommission.org/performance_ measurement.aspx. Accessed June 14, 2018.
7. American Stroke Association. Get with the guidelines–stroke. http://www.heart.org/HEARTORG/Professional/GetWithTheGuidelines/GetWithTheGuidelines-Stroke/Get-With-The-Guidelines-Stroke-Overview_UCM_308021_Article.jsp#.WyKre1VKiUk. Accessed June 14, 2018.
8. Centers for Disease Control and Prevention. The Paul Coverdell National Acute Stroke Registry. www.cdc.gov/DHDSP/stroke_registry.htm. Published March 13, 2008.
9. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
10. American College of Cardiology/American Heart Association Task Force on Performance Measures, Bonow RO, Masoudi FA, et al. ACC/AHA classification of care metrics: performance measures and quality metrics: a report of the American College of Cardiology/American Heart Association Task Force on performance measures. Circulation. 2008;118(24):2662-2666.
11. Centers for Medicare and Medicaid Services. Reporting Hospital Quality Data for Annual Payment Update https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/downloads/HospitalFactSheetAP.pdf. Published November 2004. Accessed June 14, 2018.
12. Ballard DW, Kim AS, Huang J, et al; KP CREST Network Investigators. Implementation of computerized physician order entry is associated with increased thrombolytic administration for emergency department veterans with acute ischemic stroke. Ann Emerg Med. 2015;66(6):601-610.
Stroke and cardiovascular disease (CVD) create a heavy economic burden on the health care system in the US.1 About 795,000 people have a stroke in the US each year. In 2013, stroke was the cause of 1 in every 20 deaths in the US.2 On average, someone in the US has a stroke every 40 seconds, and someone dies of one about every 4 minutes.3 Stroke also accounts for 889,000 hospitalizations per year.4,5
Stroke has been studied widely, and evidence-based guidelines have been created for the management of stroke. Despite these published guidelines for stroke care, inconsistencies in stroke management of veterans still exist. These inconsistencies led to the creation of guidelines that include quality measurements for the care of veterans with stroke.
Several campaigns have been mounted to bolster quality care for veterans with ischemic stroke. These include the Primary Stroke Center Certification by The Joint Commission (JC),6 Get With the Guidelines by the American Stroke Association,7 the Paul Coverdell Registry by the Centers for Disease Control and Prevention,8 and other efforts by the National Quality Forum (NQF) and the Centers for Medicare and Medicaid Services.9 These organizations have independently and collaboratively established quality metrics associated with health care delivery for the care of veterans with stroke. Some of these metrics have been distinguished as performance measures, or metrics that are suitable for public reporting, and may be used for comparing institutions and rewarding those who meet specific thresholds (ie, pay for performance).10
The aim of this project was to increase compliance at the Atlanta VA Medical Center (VAMC) in Decatur, Georgia, with JC National Quality Measures for the care of veterans with ischemic stroke, thus providing optimal care for veterans admitted for ischemic stroke management.
There are 3 phases in the management of a patient with a stroke: stroke presentation, admission/management, and discharge. This project focused on the admission/management phase. The stroke presentation phase is completed in the emergency department (ED), and the discharge phase has a check list for stroke, including atrial fibrillation (AF) and counseling prior to discharge. Data from the check list and counseling were not included in this project.
Specific attention was given to the following JC measures: stroke (STK) 1, STK 5, and STK 10 because the Atlanta VAMC was below the national average for these core measures for fiscal year 2015. Compliance was accomplished by creating order sets for the admission and subsequent care of veterans with ischemic stroke, tracking order set usage, and reporting regularly to the medicine/admitting team members on use rates and meeting quality measures. This project underwent the quality vs research review process and was determined to be a quality improvement (QI) project, so the project did not require institutional review board approval.
Methods
At the Atlanta VAMC, all patients admitted for stroke workup or management are admitted to the medicine service. The medicine admitting teams are composed of an attending physician, a medicine resident, a nurse practitioner (NP), a pharmacist, and 2 interns; and the hospitalist team composed of a hospitalist. The project began January 1, 2016, and ended December 31, 2016.
The hospitalist created evidence-based admission orders for all patients admitted for stroke or transient ischemic attack (TIA).The measures used were from the JC Specification Manual for Joint Commission National Quality as well as The American College of Cardiology/American Heart Association classification of care metrics.5
The order sets were reviewed and confirmed by a neurologist. The JC quality measures required for the care of patients admitted for stroke management were embedded in these order sets. These order sets were placed directly under the general admission orders in the Computerized Patient Record System (CPRS)
The quality measures included:
- STK 1: Veteran admitted for stroke received venous thromboembolic (VTE) prophylaxis in a timely manner. Pharmacologic management for VTE prophylaxis with subcutaneous low-molecular weight heparin and/or application of bilateral sequential compression devices were tracked.
- STK 5: Veteran admitted for stroke administered antithrombotic therapy by end of hospital day 2. Aspirin, aspirin/dipyridamole, and ticlopidine were tracked.
- STK 10: Veteran admitted for stroke assessed for rehabilitation services during admission. Physical therapy and occupational therapy consult placements were tracked. Quality measures, such as administration of tissue plasminogen activator (tPA), were not embedded in the order set because veterans who met the criteria for tPA were immediately administered tPA in the ED or transferred to the closest stroke center.
In this QI project, only quality measures that had to be completed in the inpatient setting were included. Quality measures such as tPA administration, National Institutes of Health (NIH) Stroke Scale timely documentation, swallow screen prior to po intake, and stroke transfers were completed in the ED prior to clearance for admission, so these were not included in the project. The Atlanta VAMC ED has protocols to care for these patients, but they do not have order sets with markers that could trace their usage.
All admission orders placed were reviewed by a QI team to check whether the stroke order set had been used. The ability to determine order set use was accomplished by adding the unique identifier Stroke Order Set Marker, which allowed for querying using structured query language (SQL) within the Corporate Data Warehouse.
Next, all admissions were checked through chart review for compliance with quality measures. Admissions that had not been completed for all quality measures were identified, and the physicians or NPs caring for those veterans were alerted. These order sets were supposed to be used during admission of all patients admitted for stroke management or workup; however, some patients were admitted without the use of the order sets.
The successful completion of the quality measures were then compared between the groups of patients admitted using the order set and the group of patients in which the order set was not used at their admission. The physicians were provided acceptable reasons, including contraindications to certain medications such as patient history of allergy. The admitting physician made decisions on the antiplatelet medications to use or on neurology recommendations. The neurology department was consulted on all patients who had acute or subacute ischemic stroke findings on magnetic resonance imaging (MRI).
At the beginning of the month, internal medicine residents from Emory University and Morehouse School of Medicine received orientation on the use of the stroke order set from the team NP and chief resident. Tips on how to use the CPRS and how to access the stroke order sets also were created.
One challenge the project faced was the continuous change in the admitting team pool: Some residents did not remember to use the stroke order sets.
Results
Of 323 admitted patients with stroke, 93 admissions were entered using the stroke order set. Out of these completed orders, 85 (91%) veterans admitted for ischemic stroke or TIA management received timely VTE prophylaxis, and 8 (9%) veterans did not. Of the 230 admissions completed without using the stroke order set, 167 (73%) veterans received timely VTE prophylaxis, and 63 (28%) veterans did not. Additionally out of the 93 veterans admitted using the stroke order set, 74 (80%) veterans admitted for the management of ischemic stroke received antithrombotic therapy by end of hospital day 2, whereas 19 (20%) veterans did not, and there were no clear contraindications documented as to why.
For veterans admitted without using the order set, 167 (73%) veterans admitted for the management of ischemic stroke received antithrombotic therapy by the end of hospital day 2, whereas 76 (33%) veterans did not. Last, 90 (97%) of the 93 veterans admitted for stroke workup using the order set were assessed for rehabilitation services during admission, whereas 3 (3%) were not. For the veterans who were admitted without using the stroke order set, 162 (70%) were assessed for rehabilitation services during admission, whereas 68 (30%) were not.
Out of 969 compliance measures looked at, 237 measures were missed and 732 measures were appropriately completed irrespective of whether the stroke order set was used. Out of the 279 admissions where the stroke order set was used, 249 (89%) quality measures were met.
The study threshold for meeting the standards was the national average for 2015, which was 91.1% for the administration of VTE prophylaxis in a timely manner, 97.9% for administering antithrombotic therapy by end of hospital day 2, and 94.2% for assessment of the patient by rehabilitation services during the admission.
Discussion
Despite the repeated training and orientation, compliance to the order set usage was not optimal, likely secondary to a frequent change in the pool of admitting physicians using the order set. Also, the order set was new to staff, thus, admitting physicians sometimes forgot to use them. The next step in this project will be to create an order set for the ED with markers for tracing usage. These order sets will include all quality measures that need to be completed in the ED, such as the NIH Stroke Scale timely documentation, tPA administration data, swallow screen prior to po intake, and stroke transfers.
This QI project also streamlined the process for stroke admissions. With the creation of the order set, all orders needed for stroke were available to the admitting physician, resulting in less need for searching the order individually from a large pool of orders (Figures 3 and 4).
Several reputable institutions have quality metrics and performance measures typically focused on processes of care based on specific clinical guidelines recommendations. Clinical guidelines are usually based on sufficient evidence that failure to provide the recommended care is likely to result in suboptimal clinical outcomes. Stroke quality measure compliance is part of the Reporting Hospital Quality Data for Annual Payment Update (RHQDAPU) initiative, and most hospitals will be required to report these measures in order to receive full Medicare payments.11
Limitations
Limitations of this study relate to CPRS functions, which must be specifically activated at different VA sites in order to enable the use of these functions. Also, the successful creation of these order sets depended on the information specialist’s knowledge of the capabilities of the CPRS.
Conclusion
Gaps in practice and recommended guidelines can be bridged by creating standardized admission orders embedded with required quality measures. The Atlanta VAMC project showed that the use of a standardized stroke admission order set significantly improved compliance to quality measures for veterans admitted for ischemic stroke management. This is consistent with a study completed in the ED, which showed that for veterans hospitalized for acute ischemic stroke, electronic order set use was associated with increased use of IV tPA.12 Creating order sets can be challenging, but if these barriers can be overcome, with the first order set, similar templates can be used to create order sets for other clinical conditions, such as heart failure, sepsis, and chronic obstructive pulmonary disease exacerbation.
Stroke and cardiovascular disease (CVD) create a heavy economic burden on the health care system in the US.1 About 795,000 people have a stroke in the US each year. In 2013, stroke was the cause of 1 in every 20 deaths in the US.2 On average, someone in the US has a stroke every 40 seconds, and someone dies of one about every 4 minutes.3 Stroke also accounts for 889,000 hospitalizations per year.4,5
Stroke has been studied widely, and evidence-based guidelines have been created for the management of stroke. Despite these published guidelines for stroke care, inconsistencies in stroke management of veterans still exist. These inconsistencies led to the creation of guidelines that include quality measurements for the care of veterans with stroke.
Several campaigns have been mounted to bolster quality care for veterans with ischemic stroke. These include the Primary Stroke Center Certification by The Joint Commission (JC),6 Get With the Guidelines by the American Stroke Association,7 the Paul Coverdell Registry by the Centers for Disease Control and Prevention,8 and other efforts by the National Quality Forum (NQF) and the Centers for Medicare and Medicaid Services.9 These organizations have independently and collaboratively established quality metrics associated with health care delivery for the care of veterans with stroke. Some of these metrics have been distinguished as performance measures, or metrics that are suitable for public reporting, and may be used for comparing institutions and rewarding those who meet specific thresholds (ie, pay for performance).10
The aim of this project was to increase compliance at the Atlanta VA Medical Center (VAMC) in Decatur, Georgia, with JC National Quality Measures for the care of veterans with ischemic stroke, thus providing optimal care for veterans admitted for ischemic stroke management.
There are 3 phases in the management of a patient with a stroke: stroke presentation, admission/management, and discharge. This project focused on the admission/management phase. The stroke presentation phase is completed in the emergency department (ED), and the discharge phase has a check list for stroke, including atrial fibrillation (AF) and counseling prior to discharge. Data from the check list and counseling were not included in this project.
Specific attention was given to the following JC measures: stroke (STK) 1, STK 5, and STK 10 because the Atlanta VAMC was below the national average for these core measures for fiscal year 2015. Compliance was accomplished by creating order sets for the admission and subsequent care of veterans with ischemic stroke, tracking order set usage, and reporting regularly to the medicine/admitting team members on use rates and meeting quality measures. This project underwent the quality vs research review process and was determined to be a quality improvement (QI) project, so the project did not require institutional review board approval.
Methods
At the Atlanta VAMC, all patients admitted for stroke workup or management are admitted to the medicine service. The medicine admitting teams are composed of an attending physician, a medicine resident, a nurse practitioner (NP), a pharmacist, and 2 interns; and the hospitalist team composed of a hospitalist. The project began January 1, 2016, and ended December 31, 2016.
The hospitalist created evidence-based admission orders for all patients admitted for stroke or transient ischemic attack (TIA).The measures used were from the JC Specification Manual for Joint Commission National Quality as well as The American College of Cardiology/American Heart Association classification of care metrics.5
The order sets were reviewed and confirmed by a neurologist. The JC quality measures required for the care of patients admitted for stroke management were embedded in these order sets. These order sets were placed directly under the general admission orders in the Computerized Patient Record System (CPRS)
The quality measures included:
- STK 1: Veteran admitted for stroke received venous thromboembolic (VTE) prophylaxis in a timely manner. Pharmacologic management for VTE prophylaxis with subcutaneous low-molecular weight heparin and/or application of bilateral sequential compression devices were tracked.
- STK 5: Veteran admitted for stroke administered antithrombotic therapy by end of hospital day 2. Aspirin, aspirin/dipyridamole, and ticlopidine were tracked.
- STK 10: Veteran admitted for stroke assessed for rehabilitation services during admission. Physical therapy and occupational therapy consult placements were tracked. Quality measures, such as administration of tissue plasminogen activator (tPA), were not embedded in the order set because veterans who met the criteria for tPA were immediately administered tPA in the ED or transferred to the closest stroke center.
In this QI project, only quality measures that had to be completed in the inpatient setting were included. Quality measures such as tPA administration, National Institutes of Health (NIH) Stroke Scale timely documentation, swallow screen prior to po intake, and stroke transfers were completed in the ED prior to clearance for admission, so these were not included in the project. The Atlanta VAMC ED has protocols to care for these patients, but they do not have order sets with markers that could trace their usage.
All admission orders placed were reviewed by a QI team to check whether the stroke order set had been used. The ability to determine order set use was accomplished by adding the unique identifier Stroke Order Set Marker, which allowed for querying using structured query language (SQL) within the Corporate Data Warehouse.
Next, all admissions were checked through chart review for compliance with quality measures. Admissions that had not been completed for all quality measures were identified, and the physicians or NPs caring for those veterans were alerted. These order sets were supposed to be used during admission of all patients admitted for stroke management or workup; however, some patients were admitted without the use of the order sets.
The successful completion of the quality measures were then compared between the groups of patients admitted using the order set and the group of patients in which the order set was not used at their admission. The physicians were provided acceptable reasons, including contraindications to certain medications such as patient history of allergy. The admitting physician made decisions on the antiplatelet medications to use or on neurology recommendations. The neurology department was consulted on all patients who had acute or subacute ischemic stroke findings on magnetic resonance imaging (MRI).
At the beginning of the month, internal medicine residents from Emory University and Morehouse School of Medicine received orientation on the use of the stroke order set from the team NP and chief resident. Tips on how to use the CPRS and how to access the stroke order sets also were created.
One challenge the project faced was the continuous change in the admitting team pool: Some residents did not remember to use the stroke order sets.
Results
Of 323 admitted patients with stroke, 93 admissions were entered using the stroke order set. Out of these completed orders, 85 (91%) veterans admitted for ischemic stroke or TIA management received timely VTE prophylaxis, and 8 (9%) veterans did not. Of the 230 admissions completed without using the stroke order set, 167 (73%) veterans received timely VTE prophylaxis, and 63 (28%) veterans did not. Additionally out of the 93 veterans admitted using the stroke order set, 74 (80%) veterans admitted for the management of ischemic stroke received antithrombotic therapy by end of hospital day 2, whereas 19 (20%) veterans did not, and there were no clear contraindications documented as to why.
For veterans admitted without using the order set, 167 (73%) veterans admitted for the management of ischemic stroke received antithrombotic therapy by the end of hospital day 2, whereas 76 (33%) veterans did not. Last, 90 (97%) of the 93 veterans admitted for stroke workup using the order set were assessed for rehabilitation services during admission, whereas 3 (3%) were not. For the veterans who were admitted without using the stroke order set, 162 (70%) were assessed for rehabilitation services during admission, whereas 68 (30%) were not.
Out of 969 compliance measures looked at, 237 measures were missed and 732 measures were appropriately completed irrespective of whether the stroke order set was used. Out of the 279 admissions where the stroke order set was used, 249 (89%) quality measures were met.
The study threshold for meeting the standards was the national average for 2015, which was 91.1% for the administration of VTE prophylaxis in a timely manner, 97.9% for administering antithrombotic therapy by end of hospital day 2, and 94.2% for assessment of the patient by rehabilitation services during the admission.
Discussion
Despite the repeated training and orientation, compliance to the order set usage was not optimal, likely secondary to a frequent change in the pool of admitting physicians using the order set. Also, the order set was new to staff, thus, admitting physicians sometimes forgot to use them. The next step in this project will be to create an order set for the ED with markers for tracing usage. These order sets will include all quality measures that need to be completed in the ED, such as the NIH Stroke Scale timely documentation, tPA administration data, swallow screen prior to po intake, and stroke transfers.
This QI project also streamlined the process for stroke admissions. With the creation of the order set, all orders needed for stroke were available to the admitting physician, resulting in less need for searching the order individually from a large pool of orders (Figures 3 and 4).
Several reputable institutions have quality metrics and performance measures typically focused on processes of care based on specific clinical guidelines recommendations. Clinical guidelines are usually based on sufficient evidence that failure to provide the recommended care is likely to result in suboptimal clinical outcomes. Stroke quality measure compliance is part of the Reporting Hospital Quality Data for Annual Payment Update (RHQDAPU) initiative, and most hospitals will be required to report these measures in order to receive full Medicare payments.11
Limitations
Limitations of this study relate to CPRS functions, which must be specifically activated at different VA sites in order to enable the use of these functions. Also, the successful creation of these order sets depended on the information specialist’s knowledge of the capabilities of the CPRS.
Conclusion
Gaps in practice and recommended guidelines can be bridged by creating standardized admission orders embedded with required quality measures. The Atlanta VAMC project showed that the use of a standardized stroke admission order set significantly improved compliance to quality measures for veterans admitted for ischemic stroke management. This is consistent with a study completed in the ED, which showed that for veterans hospitalized for acute ischemic stroke, electronic order set use was associated with increased use of IV tPA.12 Creating order sets can be challenging, but if these barriers can be overcome, with the first order set, similar templates can be used to create order sets for other clinical conditions, such as heart failure, sepsis, and chronic obstructive pulmonary disease exacerbation.
1. Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447-454.
2. Centers for Disease Control and Prevention. Vital signs: recent trends in stroke death rates–United States, 2000-2015. https://www.cdc.gov/mmwr/volumes/66/wr/mm6635e1.htm. Published September 8, 2017. Accessed June 14, 2018.
3. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e229-e445.
4. Lloyd-Jones D, Adams R, Carnethon M, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2009;119(3):480-486.
5. Poisson SN, Josephson SA. Quality measures in stroke. Neurohospitalist. 2011;1(2):71-77.
6. The Joint Commission. Primary Stroke Centers—Stroke Performance Measurement. https://www.jointcommission.org/performance_ measurement.aspx. Accessed June 14, 2018.
7. American Stroke Association. Get with the guidelines–stroke. http://www.heart.org/HEARTORG/Professional/GetWithTheGuidelines/GetWithTheGuidelines-Stroke/Get-With-The-Guidelines-Stroke-Overview_UCM_308021_Article.jsp#.WyKre1VKiUk. Accessed June 14, 2018.
8. Centers for Disease Control and Prevention. The Paul Coverdell National Acute Stroke Registry. www.cdc.gov/DHDSP/stroke_registry.htm. Published March 13, 2008.
9. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
10. American College of Cardiology/American Heart Association Task Force on Performance Measures, Bonow RO, Masoudi FA, et al. ACC/AHA classification of care metrics: performance measures and quality metrics: a report of the American College of Cardiology/American Heart Association Task Force on performance measures. Circulation. 2008;118(24):2662-2666.
11. Centers for Medicare and Medicaid Services. Reporting Hospital Quality Data for Annual Payment Update https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/downloads/HospitalFactSheetAP.pdf. Published November 2004. Accessed June 14, 2018.
12. Ballard DW, Kim AS, Huang J, et al; KP CREST Network Investigators. Implementation of computerized physician order entry is associated with increased thrombolytic administration for emergency department veterans with acute ischemic stroke. Ann Emerg Med. 2015;66(6):601-610.
1. Writing Group Members, Mozaffarian D, Benjamin EJ, Go AS, et al; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133(4):447-454.
2. Centers for Disease Control and Prevention. Vital signs: recent trends in stroke death rates–United States, 2000-2015. https://www.cdc.gov/mmwr/volumes/66/wr/mm6635e1.htm. Published September 8, 2017. Accessed June 14, 2018.
3. Benjamin EJ, Blaha MJ, Chiuve SE, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e229-e445.
4. Lloyd-Jones D, Adams R, Carnethon M, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2009;119(3):480-486.
5. Poisson SN, Josephson SA. Quality measures in stroke. Neurohospitalist. 2011;1(2):71-77.
6. The Joint Commission. Primary Stroke Centers—Stroke Performance Measurement. https://www.jointcommission.org/performance_ measurement.aspx. Accessed June 14, 2018.
7. American Stroke Association. Get with the guidelines–stroke. http://www.heart.org/HEARTORG/Professional/GetWithTheGuidelines/GetWithTheGuidelines-Stroke/Get-With-The-Guidelines-Stroke-Overview_UCM_308021_Article.jsp#.WyKre1VKiUk. Accessed June 14, 2018.
8. Centers for Disease Control and Prevention. The Paul Coverdell National Acute Stroke Registry. www.cdc.gov/DHDSP/stroke_registry.htm. Published March 13, 2008.
9. Reeves MJ, Parker C, Fonarow GC, Smith EE, Schwamm LH. Development of stroke performance measures: definitions, methods, and current measures. Stroke. 2010;41(7):1573-1578.
10. American College of Cardiology/American Heart Association Task Force on Performance Measures, Bonow RO, Masoudi FA, et al. ACC/AHA classification of care metrics: performance measures and quality metrics: a report of the American College of Cardiology/American Heart Association Task Force on performance measures. Circulation. 2008;118(24):2662-2666.
11. Centers for Medicare and Medicaid Services. Reporting Hospital Quality Data for Annual Payment Update https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/downloads/HospitalFactSheetAP.pdf. Published November 2004. Accessed June 14, 2018.
12. Ballard DW, Kim AS, Huang J, et al; KP CREST Network Investigators. Implementation of computerized physician order entry is associated with increased thrombolytic administration for emergency department veterans with acute ischemic stroke. Ann Emerg Med. 2015;66(6):601-610.
Using Simulation to Enhance Care of Older Veterans
Enhancing patient-centered care is an important priority for caregivers in all settings but particularly in long-term care (LTC) where patients also are residents. Simulation is a potential strategy to effect cultural change for health care providers at LTC facilities. In a clinical setting, simulation is an educational model that allows staff to practice behaviors or skills without putting patients at risk. Due to limited time, staffing, and budget resources, the use of simulation for training is not common at LTC facilities.
Background
The simulation model is considered an effective teaching and learning strategy for replicating experiences in nursing practice.1,2 The interactive experience provides the learner with opportunities to engage with patients through psychomotor participation, critical thinking, reflection, and debriefing. Prelicensure nursing education programs and acute care hospitals are the most common users of simulation learning. However, there is a paucity of literature addressing the use of simulation in LTC.
One study was conducted in 2002 by P.K. Beville called the Virtual Dementia Tour, a program using evidence-based simulation. Beville found that study participants using the simulation model had a heightened awareness of the challenges of confused elderly as well as unrealistic expectations by caregivers.3 Although the Virtual Dementia Tour is available for a fee for training professional caregivers, lay people, family, and first responders, many LTC facilities do not have sufficient funding for simulation and simulation equipment, and many do not have dedicated staff for nursing education. Most staff in LTC facilities are unlicensed and may not have had simulation training experience. Additionally, due to staffing and budget constraints, staff education may be limited.
This article will describe the successful implementation of a simulation-based quality improvement project created by and used at the Louis Stokes Cleveland VA Medical Center (LSCVAMC) LTC facility. The LSCVAMC has acute care beds and is adjacent to its LTC facility. Also, a previously successful simulation educational program to improve delirium care was conducted at this acute care hospital.4
Experiential Learning Opportunity
Many residents in a LTC facility have been diagnosed with dementia. As part of a cultural transformation at LSCVAMC, a simulation program was used to help sensitize LTC caregivers to the many sensory changes that occur in older veterans with dementia. The program was guided by the Kolb model.5
The Kolb model of experiential learning includes 4 elements: abstract conceptualization (knowledge), active experimentation (application), concrete experience (engagement), and reflective observation (self-evaluation).5 A simulation program can touch all 4 elements of the Kolb model by providing an educational experience for all learning styles as well as facilitating critical thinking. Nursing homes that provide care to residents with dementia are required to include dementia education annually to staff.6 Long-term care facilities can take advantage of using simulation education along with their traditional educational programs to provide staff with exposure to realistic resident care conditions.7
Methods
The simulation learning experience was provided to all nursing, recreation therapy, and rehabilitation services staff at the LTC facility. The goal was to create an affective, psychomotor learning experience that refreshed, reminded, and sensitized the staff to the challenges that many residents face. The LSCVAMC LTC leadership was supportive of this simulation model because it was not time intensive for direct care staff, and the materials needed were inexpensive. The only equipment that was purchased were several eyeglass readers, popcorn kernels, and Vaseline, resulting in a budget of about $10. The simulation program was scheduled on all shifts. In addition to the simulation experience, the model consists of pre- and postsurveys and a dementia review handout. Staff were able to complete a pre- and postsurvey as well as a debriefing all within a 30-minute time slot.
The pre- and posteducation surveys used were designed to measure learnings and provide data for future education planning. The surveys required only a yes/no and short answers. To reduce the total time of participants’ involvement in the simulation program, an online survey was used. Presurvey questions were designed to identify basic knowledge and experience with dementia, both at work and in personal life. The postsurvey questions sought to identify affective feelings about the participants experience as well as lessons learned and how that could impact future care.
The dementia review handout that was provided to staff 2 weeks before the simulation provided an overview of dementia. It included communication techniques and care planning suggestions.8 The time spent in the simulation room was about 10 to 15 minutes but depended on the activity. The total in-service time was about 30 minutes, depending on the time allotted for debriefing. Room choice was influenced by the number of participants performing the simulation at the same time. Activity stations/tables generally provided 1 experience at a time.
The room that was used had adjustable lighting with the ability to provide a low light setting. Activities were chosen based on the goals for the physical and cognitive disabilities to be simulated. Table 1 identifies equipment used with success and chosen with consideration for ease and expense in describing the disability.
Simulation activities were based on the staff learning needs determined by the presimulation survey. Simulated deficits impacted activities of daily living, mood, and cognition. Neuropathy, arthritis, paralysis, dementia, glaucoma, cataracts, and hearing loss are conditions that are easily represented in a simulation.
Participants also gained additional knowledge of dementia through the Kolb process, which was included in the debriefing. The survey followed the completion of the simulation session to identify knowledge deficits for general remediation and program development and expansion.
Discussion
During the dementia simulation, active experimentation or application learning may be counterintuitive. Staff do not apply their knowledge of dementia directly as in other education settings where they can practice or demonstrate a skill. Instead, participants experience care from the perspective of residents. This learning transitions well into reflective observation as the participants begin to understand the challenges of the cognitively impaired resident, which are manifested in the residents’ behaviors.
Debriefing and a postsimulation survey provide a guided reflection to assimilate new knowledge and revise presimulation attitudes about dementia.9 Reflective observation or self-evaluation is a learning activity that is not a routine part of staff education but can be a powerful learning tool. The postsimulation survey incorporated Bloom’s taxonomy: the affective domain of learning by challenging staff to organize their values with the experience and resolving in their mind any conflicts.10 The goal of the process is to help internalize the education by encouraging changes in behavior (in this case dementia care) and considering the new experience.
Survey Results
The 30-minute program allowed 155 staff to experience cognitive and physical impairment while completing tasks. The pre- and postsurveys were analyzed by 2 learning and dementia survey content experts. The survey questions were open-ended with the intention of eliciting affective behavior responses and staff could provide comments (See eTables 1 and 2 at www.mdedge.com/fedprac). All participants indicated they had knowledge of dementia before the simulation, but 70% acknowledged in the postsimulation survey that they did not have the dementia knowledge that they thought they had. Patience and understanding were most commonly reported in the reflective observation/affective domain (values are internalized leading to changes in behavior).
Participants also described success in closing the loop of experiential learning as a result of the simulation. Some participants verbalized experiencing emotional distress when they realized that their temporary, frustrating impairment was a permanent condition for the residents. Postexperience comments supported the success of the Kolb model experiential learning activity.
Conclusion
Dementia simulation can augment didactic education for improving the quality of dementia care. The virtual dementia simulation was an inexpensive educational program that did not adversely impact scheduling or patient care in a LTC facility. Care providers provided anecdotal feedback that suggested that the program increased their awareness of the difficulty of performing activities of daily living for patients with dementia. The simulation touched all 4 elements of the Kolb Model. The participants had gained new knowledge or reinforced existing knowledge. The simulation activities addressed the application and engagement parts of the model. Self-evaluation resulted from the debriefing time and postsurvey questions. The virtual dementia simulation will be repeated with additional debrief time and a long-term follow-up survey to identify additional learning needs and changes in professional practice.
Acknowledgments
The author thanks Nurse Educator Lisa Weber, MSN, RN-BC, for her contribution to the manuscript.
1. Aebersold M, Tschannen D. Simulation in nursing practice: the impact on patient care. Online J Issues Nurs. 2013;18(2):6.
2. Mariani B, Doolen J. Nursing simulation research: what are the perceived gaps? Clin Simulation in Nurs. 2016;12(1):30-36.
3. Beville PK. Virtual Dementia Tour helps sensitize health care providers. Am J Alzheimers Dis Other Demen. 2002;17(3):183-190.
4. Kresevic D, Heath B, Fine-Smilovich E, et al. Simulation training, coaching, and cue cards improve delirium care. Fed Pract. 2016;33(12):22-28.
5. Chmil JV, Turk M, Adamson K, Larew C. Effects of an experiential learning simulation design on clinical nursing judgment development. Nurse Educ. 2015;40(5):228-232.
6. Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; reform of requirements for long-term care facilities, final rule. https://www.federalregister.gov/documents/2016/10/04/2016-23503/medicare-and-medicaid-programs-reform-of-requirements-for-long-term-care-facilities. Published October 4, 2016. Accessed May 22, 2018.
7. Donahoe J, Moon L, VanCleave K. Increasing student empathy toward older adults using the virtual dementia tour. J Baccalaureate Soc Work. 2014;19(1):S23-S40.
8. Coggins MD. Behavioral expressions in dementia patients. http://www.todaysgeriatricmedicine.com/archive/0115p6.shtml. Published 2015. Accessed May 10, 2018.
9. Al Sabei SD, Lasater K. Simulation debriefing for clinical judgment development: a concept analysis. Nurse Educ Today. 2016;45:42-47.
10. Anderson LW, Krathwohl DR, Bloom BS, eds. A Taxonomy for Learning, Teaching, and Assessing: A revision of Bloom’s Taxonomy of Educational Objectives. New York: Longman; 2001.
Enhancing patient-centered care is an important priority for caregivers in all settings but particularly in long-term care (LTC) where patients also are residents. Simulation is a potential strategy to effect cultural change for health care providers at LTC facilities. In a clinical setting, simulation is an educational model that allows staff to practice behaviors or skills without putting patients at risk. Due to limited time, staffing, and budget resources, the use of simulation for training is not common at LTC facilities.
Background
The simulation model is considered an effective teaching and learning strategy for replicating experiences in nursing practice.1,2 The interactive experience provides the learner with opportunities to engage with patients through psychomotor participation, critical thinking, reflection, and debriefing. Prelicensure nursing education programs and acute care hospitals are the most common users of simulation learning. However, there is a paucity of literature addressing the use of simulation in LTC.
One study was conducted in 2002 by P.K. Beville called the Virtual Dementia Tour, a program using evidence-based simulation. Beville found that study participants using the simulation model had a heightened awareness of the challenges of confused elderly as well as unrealistic expectations by caregivers.3 Although the Virtual Dementia Tour is available for a fee for training professional caregivers, lay people, family, and first responders, many LTC facilities do not have sufficient funding for simulation and simulation equipment, and many do not have dedicated staff for nursing education. Most staff in LTC facilities are unlicensed and may not have had simulation training experience. Additionally, due to staffing and budget constraints, staff education may be limited.
This article will describe the successful implementation of a simulation-based quality improvement project created by and used at the Louis Stokes Cleveland VA Medical Center (LSCVAMC) LTC facility. The LSCVAMC has acute care beds and is adjacent to its LTC facility. Also, a previously successful simulation educational program to improve delirium care was conducted at this acute care hospital.4
Experiential Learning Opportunity
Many residents in a LTC facility have been diagnosed with dementia. As part of a cultural transformation at LSCVAMC, a simulation program was used to help sensitize LTC caregivers to the many sensory changes that occur in older veterans with dementia. The program was guided by the Kolb model.5
The Kolb model of experiential learning includes 4 elements: abstract conceptualization (knowledge), active experimentation (application), concrete experience (engagement), and reflective observation (self-evaluation).5 A simulation program can touch all 4 elements of the Kolb model by providing an educational experience for all learning styles as well as facilitating critical thinking. Nursing homes that provide care to residents with dementia are required to include dementia education annually to staff.6 Long-term care facilities can take advantage of using simulation education along with their traditional educational programs to provide staff with exposure to realistic resident care conditions.7
Methods
The simulation learning experience was provided to all nursing, recreation therapy, and rehabilitation services staff at the LTC facility. The goal was to create an affective, psychomotor learning experience that refreshed, reminded, and sensitized the staff to the challenges that many residents face. The LSCVAMC LTC leadership was supportive of this simulation model because it was not time intensive for direct care staff, and the materials needed were inexpensive. The only equipment that was purchased were several eyeglass readers, popcorn kernels, and Vaseline, resulting in a budget of about $10. The simulation program was scheduled on all shifts. In addition to the simulation experience, the model consists of pre- and postsurveys and a dementia review handout. Staff were able to complete a pre- and postsurvey as well as a debriefing all within a 30-minute time slot.
The pre- and posteducation surveys used were designed to measure learnings and provide data for future education planning. The surveys required only a yes/no and short answers. To reduce the total time of participants’ involvement in the simulation program, an online survey was used. Presurvey questions were designed to identify basic knowledge and experience with dementia, both at work and in personal life. The postsurvey questions sought to identify affective feelings about the participants experience as well as lessons learned and how that could impact future care.
The dementia review handout that was provided to staff 2 weeks before the simulation provided an overview of dementia. It included communication techniques and care planning suggestions.8 The time spent in the simulation room was about 10 to 15 minutes but depended on the activity. The total in-service time was about 30 minutes, depending on the time allotted for debriefing. Room choice was influenced by the number of participants performing the simulation at the same time. Activity stations/tables generally provided 1 experience at a time.
The room that was used had adjustable lighting with the ability to provide a low light setting. Activities were chosen based on the goals for the physical and cognitive disabilities to be simulated. Table 1 identifies equipment used with success and chosen with consideration for ease and expense in describing the disability.
Simulation activities were based on the staff learning needs determined by the presimulation survey. Simulated deficits impacted activities of daily living, mood, and cognition. Neuropathy, arthritis, paralysis, dementia, glaucoma, cataracts, and hearing loss are conditions that are easily represented in a simulation.
Participants also gained additional knowledge of dementia through the Kolb process, which was included in the debriefing. The survey followed the completion of the simulation session to identify knowledge deficits for general remediation and program development and expansion.
Discussion
During the dementia simulation, active experimentation or application learning may be counterintuitive. Staff do not apply their knowledge of dementia directly as in other education settings where they can practice or demonstrate a skill. Instead, participants experience care from the perspective of residents. This learning transitions well into reflective observation as the participants begin to understand the challenges of the cognitively impaired resident, which are manifested in the residents’ behaviors.
Debriefing and a postsimulation survey provide a guided reflection to assimilate new knowledge and revise presimulation attitudes about dementia.9 Reflective observation or self-evaluation is a learning activity that is not a routine part of staff education but can be a powerful learning tool. The postsimulation survey incorporated Bloom’s taxonomy: the affective domain of learning by challenging staff to organize their values with the experience and resolving in their mind any conflicts.10 The goal of the process is to help internalize the education by encouraging changes in behavior (in this case dementia care) and considering the new experience.
Survey Results
The 30-minute program allowed 155 staff to experience cognitive and physical impairment while completing tasks. The pre- and postsurveys were analyzed by 2 learning and dementia survey content experts. The survey questions were open-ended with the intention of eliciting affective behavior responses and staff could provide comments (See eTables 1 and 2 at www.mdedge.com/fedprac). All participants indicated they had knowledge of dementia before the simulation, but 70% acknowledged in the postsimulation survey that they did not have the dementia knowledge that they thought they had. Patience and understanding were most commonly reported in the reflective observation/affective domain (values are internalized leading to changes in behavior).
Participants also described success in closing the loop of experiential learning as a result of the simulation. Some participants verbalized experiencing emotional distress when they realized that their temporary, frustrating impairment was a permanent condition for the residents. Postexperience comments supported the success of the Kolb model experiential learning activity.
Conclusion
Dementia simulation can augment didactic education for improving the quality of dementia care. The virtual dementia simulation was an inexpensive educational program that did not adversely impact scheduling or patient care in a LTC facility. Care providers provided anecdotal feedback that suggested that the program increased their awareness of the difficulty of performing activities of daily living for patients with dementia. The simulation touched all 4 elements of the Kolb Model. The participants had gained new knowledge or reinforced existing knowledge. The simulation activities addressed the application and engagement parts of the model. Self-evaluation resulted from the debriefing time and postsurvey questions. The virtual dementia simulation will be repeated with additional debrief time and a long-term follow-up survey to identify additional learning needs and changes in professional practice.
Acknowledgments
The author thanks Nurse Educator Lisa Weber, MSN, RN-BC, for her contribution to the manuscript.
Enhancing patient-centered care is an important priority for caregivers in all settings but particularly in long-term care (LTC) where patients also are residents. Simulation is a potential strategy to effect cultural change for health care providers at LTC facilities. In a clinical setting, simulation is an educational model that allows staff to practice behaviors or skills without putting patients at risk. Due to limited time, staffing, and budget resources, the use of simulation for training is not common at LTC facilities.
Background
The simulation model is considered an effective teaching and learning strategy for replicating experiences in nursing practice.1,2 The interactive experience provides the learner with opportunities to engage with patients through psychomotor participation, critical thinking, reflection, and debriefing. Prelicensure nursing education programs and acute care hospitals are the most common users of simulation learning. However, there is a paucity of literature addressing the use of simulation in LTC.
One study was conducted in 2002 by P.K. Beville called the Virtual Dementia Tour, a program using evidence-based simulation. Beville found that study participants using the simulation model had a heightened awareness of the challenges of confused elderly as well as unrealistic expectations by caregivers.3 Although the Virtual Dementia Tour is available for a fee for training professional caregivers, lay people, family, and first responders, many LTC facilities do not have sufficient funding for simulation and simulation equipment, and many do not have dedicated staff for nursing education. Most staff in LTC facilities are unlicensed and may not have had simulation training experience. Additionally, due to staffing and budget constraints, staff education may be limited.
This article will describe the successful implementation of a simulation-based quality improvement project created by and used at the Louis Stokes Cleveland VA Medical Center (LSCVAMC) LTC facility. The LSCVAMC has acute care beds and is adjacent to its LTC facility. Also, a previously successful simulation educational program to improve delirium care was conducted at this acute care hospital.4
Experiential Learning Opportunity
Many residents in a LTC facility have been diagnosed with dementia. As part of a cultural transformation at LSCVAMC, a simulation program was used to help sensitize LTC caregivers to the many sensory changes that occur in older veterans with dementia. The program was guided by the Kolb model.5
The Kolb model of experiential learning includes 4 elements: abstract conceptualization (knowledge), active experimentation (application), concrete experience (engagement), and reflective observation (self-evaluation).5 A simulation program can touch all 4 elements of the Kolb model by providing an educational experience for all learning styles as well as facilitating critical thinking. Nursing homes that provide care to residents with dementia are required to include dementia education annually to staff.6 Long-term care facilities can take advantage of using simulation education along with their traditional educational programs to provide staff with exposure to realistic resident care conditions.7
Methods
The simulation learning experience was provided to all nursing, recreation therapy, and rehabilitation services staff at the LTC facility. The goal was to create an affective, psychomotor learning experience that refreshed, reminded, and sensitized the staff to the challenges that many residents face. The LSCVAMC LTC leadership was supportive of this simulation model because it was not time intensive for direct care staff, and the materials needed were inexpensive. The only equipment that was purchased were several eyeglass readers, popcorn kernels, and Vaseline, resulting in a budget of about $10. The simulation program was scheduled on all shifts. In addition to the simulation experience, the model consists of pre- and postsurveys and a dementia review handout. Staff were able to complete a pre- and postsurvey as well as a debriefing all within a 30-minute time slot.
The pre- and posteducation surveys used were designed to measure learnings and provide data for future education planning. The surveys required only a yes/no and short answers. To reduce the total time of participants’ involvement in the simulation program, an online survey was used. Presurvey questions were designed to identify basic knowledge and experience with dementia, both at work and in personal life. The postsurvey questions sought to identify affective feelings about the participants experience as well as lessons learned and how that could impact future care.
The dementia review handout that was provided to staff 2 weeks before the simulation provided an overview of dementia. It included communication techniques and care planning suggestions.8 The time spent in the simulation room was about 10 to 15 minutes but depended on the activity. The total in-service time was about 30 minutes, depending on the time allotted for debriefing. Room choice was influenced by the number of participants performing the simulation at the same time. Activity stations/tables generally provided 1 experience at a time.
The room that was used had adjustable lighting with the ability to provide a low light setting. Activities were chosen based on the goals for the physical and cognitive disabilities to be simulated. Table 1 identifies equipment used with success and chosen with consideration for ease and expense in describing the disability.
Simulation activities were based on the staff learning needs determined by the presimulation survey. Simulated deficits impacted activities of daily living, mood, and cognition. Neuropathy, arthritis, paralysis, dementia, glaucoma, cataracts, and hearing loss are conditions that are easily represented in a simulation.
Participants also gained additional knowledge of dementia through the Kolb process, which was included in the debriefing. The survey followed the completion of the simulation session to identify knowledge deficits for general remediation and program development and expansion.
Discussion
During the dementia simulation, active experimentation or application learning may be counterintuitive. Staff do not apply their knowledge of dementia directly as in other education settings where they can practice or demonstrate a skill. Instead, participants experience care from the perspective of residents. This learning transitions well into reflective observation as the participants begin to understand the challenges of the cognitively impaired resident, which are manifested in the residents’ behaviors.
Debriefing and a postsimulation survey provide a guided reflection to assimilate new knowledge and revise presimulation attitudes about dementia.9 Reflective observation or self-evaluation is a learning activity that is not a routine part of staff education but can be a powerful learning tool. The postsimulation survey incorporated Bloom’s taxonomy: the affective domain of learning by challenging staff to organize their values with the experience and resolving in their mind any conflicts.10 The goal of the process is to help internalize the education by encouraging changes in behavior (in this case dementia care) and considering the new experience.
Survey Results
The 30-minute program allowed 155 staff to experience cognitive and physical impairment while completing tasks. The pre- and postsurveys were analyzed by 2 learning and dementia survey content experts. The survey questions were open-ended with the intention of eliciting affective behavior responses and staff could provide comments (See eTables 1 and 2 at www.mdedge.com/fedprac). All participants indicated they had knowledge of dementia before the simulation, but 70% acknowledged in the postsimulation survey that they did not have the dementia knowledge that they thought they had. Patience and understanding were most commonly reported in the reflective observation/affective domain (values are internalized leading to changes in behavior).
Participants also described success in closing the loop of experiential learning as a result of the simulation. Some participants verbalized experiencing emotional distress when they realized that their temporary, frustrating impairment was a permanent condition for the residents. Postexperience comments supported the success of the Kolb model experiential learning activity.
Conclusion
Dementia simulation can augment didactic education for improving the quality of dementia care. The virtual dementia simulation was an inexpensive educational program that did not adversely impact scheduling or patient care in a LTC facility. Care providers provided anecdotal feedback that suggested that the program increased their awareness of the difficulty of performing activities of daily living for patients with dementia. The simulation touched all 4 elements of the Kolb Model. The participants had gained new knowledge or reinforced existing knowledge. The simulation activities addressed the application and engagement parts of the model. Self-evaluation resulted from the debriefing time and postsurvey questions. The virtual dementia simulation will be repeated with additional debrief time and a long-term follow-up survey to identify additional learning needs and changes in professional practice.
Acknowledgments
The author thanks Nurse Educator Lisa Weber, MSN, RN-BC, for her contribution to the manuscript.
1. Aebersold M, Tschannen D. Simulation in nursing practice: the impact on patient care. Online J Issues Nurs. 2013;18(2):6.
2. Mariani B, Doolen J. Nursing simulation research: what are the perceived gaps? Clin Simulation in Nurs. 2016;12(1):30-36.
3. Beville PK. Virtual Dementia Tour helps sensitize health care providers. Am J Alzheimers Dis Other Demen. 2002;17(3):183-190.
4. Kresevic D, Heath B, Fine-Smilovich E, et al. Simulation training, coaching, and cue cards improve delirium care. Fed Pract. 2016;33(12):22-28.
5. Chmil JV, Turk M, Adamson K, Larew C. Effects of an experiential learning simulation design on clinical nursing judgment development. Nurse Educ. 2015;40(5):228-232.
6. Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; reform of requirements for long-term care facilities, final rule. https://www.federalregister.gov/documents/2016/10/04/2016-23503/medicare-and-medicaid-programs-reform-of-requirements-for-long-term-care-facilities. Published October 4, 2016. Accessed May 22, 2018.
7. Donahoe J, Moon L, VanCleave K. Increasing student empathy toward older adults using the virtual dementia tour. J Baccalaureate Soc Work. 2014;19(1):S23-S40.
8. Coggins MD. Behavioral expressions in dementia patients. http://www.todaysgeriatricmedicine.com/archive/0115p6.shtml. Published 2015. Accessed May 10, 2018.
9. Al Sabei SD, Lasater K. Simulation debriefing for clinical judgment development: a concept analysis. Nurse Educ Today. 2016;45:42-47.
10. Anderson LW, Krathwohl DR, Bloom BS, eds. A Taxonomy for Learning, Teaching, and Assessing: A revision of Bloom’s Taxonomy of Educational Objectives. New York: Longman; 2001.
1. Aebersold M, Tschannen D. Simulation in nursing practice: the impact on patient care. Online J Issues Nurs. 2013;18(2):6.
2. Mariani B, Doolen J. Nursing simulation research: what are the perceived gaps? Clin Simulation in Nurs. 2016;12(1):30-36.
3. Beville PK. Virtual Dementia Tour helps sensitize health care providers. Am J Alzheimers Dis Other Demen. 2002;17(3):183-190.
4. Kresevic D, Heath B, Fine-Smilovich E, et al. Simulation training, coaching, and cue cards improve delirium care. Fed Pract. 2016;33(12):22-28.
5. Chmil JV, Turk M, Adamson K, Larew C. Effects of an experiential learning simulation design on clinical nursing judgment development. Nurse Educ. 2015;40(5):228-232.
6. Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; reform of requirements for long-term care facilities, final rule. https://www.federalregister.gov/documents/2016/10/04/2016-23503/medicare-and-medicaid-programs-reform-of-requirements-for-long-term-care-facilities. Published October 4, 2016. Accessed May 22, 2018.
7. Donahoe J, Moon L, VanCleave K. Increasing student empathy toward older adults using the virtual dementia tour. J Baccalaureate Soc Work. 2014;19(1):S23-S40.
8. Coggins MD. Behavioral expressions in dementia patients. http://www.todaysgeriatricmedicine.com/archive/0115p6.shtml. Published 2015. Accessed May 10, 2018.
9. Al Sabei SD, Lasater K. Simulation debriefing for clinical judgment development: a concept analysis. Nurse Educ Today. 2016;45:42-47.
10. Anderson LW, Krathwohl DR, Bloom BS, eds. A Taxonomy for Learning, Teaching, and Assessing: A revision of Bloom’s Taxonomy of Educational Objectives. New York: Longman; 2001.
Workforce Assessment of VA Home-Based Primary Care Pharmacists
Home-Based Primary Care (HBPC) is a unique interdisciplinary program within the Veteran’s Health Administration (VHA) that specifically targets veterans with complex, chronic disabling diseases who have difficulty traveling to a VHA facility.1 Veterans are provided comprehensive longitudinal primary care in their homes, with the goal of maximizing the veteran’s independence. Clinical pharmacists are known as medication experts and have an essential role within interdisciplinary teams, including HBPC, improving medication safety, and decreasing inappropriate prescribing practices.2,3 Clinical pharmacy specialists (CPSs) within the VHA work collaboratively but autonomously as advanced practice providers assisting with the pharmacologic management of many diseases and chronic conditions. The remainder of this article will refer to the HBPC pharmacist as a CPS.
The CPS is actively involved in providing comprehensive medication management (CMM) services across VHA and has the expertise to effectively assist veterans in achieving targeted clinical outcomes. While the value and role of CPSs in the primary care setting are described extensively in the literature, data regarding the CPS in HBPC are limited.4-6 Therefore, the purpose of the assessment was to evaluate the status of the HBPC pharmacy workforce, identify current pharmacist activities and strong practices, and clarify national variations among programs. Future use of this analysis may assist with standardization of the HBPC CPS role and development of business rules in combination with a workload-based staffing model tool.
Background
The role of the pharmacist in the HBPC setting has evolved from providing basic medication therapy reviews to an advanced role providing CMM services under a VHA scope of practice (SOP), which outlines 8 functions that may be authorized, including medication prescriptive authority.7 The SOP may be disease specific (limited) but is increasingly transitioning to have a practice-area scope (global), which is consistent with other VHA advanced practice providers.7 Effective use of a CPS in this role allows for optimization of CMM and increasing veteran access to VHA care.
The VHA employed 7,285 pharmacists in 2014.8 Many were considered CPSs with prescriptive authority. These pharmacists were responsible for ordering more than 1.7 million distinct prescriptions across the VHA in fiscal year 2014, which represented 2.6% of the total prescriptions that year.7 A 2007 VHA study also demonstrated both an increase in appropriate prescribing practices and improved medication use when CPSs worked in collaboration with the HBPC team.9 With this evolution of VHA pharmacists, there has been an increase in the use of CPSs in HBPC and changes in staffing ratios to allow for additional clinical activities and comprehensive patient care provision.1
The HBPC model serves a complex population in which each veteran has about 8 chronic conditions.1,10 An interdisciplinary team consisting of various health care professionals, such as physicians, nurse practitioners, nurses, social workers, registered dietitians, psychologists, rehabilitation therapists, pharmacists, etc, work collaboratively to care for these veterans in the patient’s home. This team is a type of patient-centered medical home (PCMH) that focuses on providing primary care services to an at-risk veteran population who have difficulty leaving the home.1 Home-based primary care has been shown to be cost-effective, reducing average annual cost of health care by up to 24%.10 Another study showed that patients using HBPC had a 27% reduction in hospital admissions and 69% reduction in inpatient hospital days when compared with patients who were not using HBPC.11
The interdisciplinary team meets at least once weekly to discuss and design individualized care plans for veterans enrolled in the program. It is desirable for pharmacists on these teams to have special expertise and certification in geriatric pharmacotherapy and chronic disease management (eg, board-certified geriatric pharmacist [BCGP], board-certified pharmacotherapy specialist [BCPS], or board-certified ambulatory care pharmacist [BCACP]) due to the complexity of comorbidities of these veterans.12 Additional education such as postgraduate pharmacy residency training also is beneficial for CPSs in this setting.
The CPS proactively performs CMM that is often greater in scope than a targeted disease review due to multiple comorbid conditions that are often present within veteran patients.1 These comprehensive medication reviews are considered a core function and must be performed on enrollment in HBPC, quarterly, and when clinically indicated or requested by the team.13 Sufficient time must be allocated to the CPS in order to provide these high-quality medication reviews. Additional core functions of the CPS are outlined in the functional statement and/or SOP, but responsibilities include CMM and disease management. This typically consists of prescribing and/or adjusting medications, as well as providing patient and caregiver education, which can be performed either face-to-face or via telehealth visits (eg, telephone and video). A CPS also may make home visits to assess the veteran, either independently or with other disciplines of the HBPC team.
The HBPC Subject Matter Expert (SME) workgroup was chartered by the Veterans Affairs Central Office (VACO) Pharmacy Benefits Management Service (PBM) Clinical Pharmacy Practice Office (CPPO) to explore pharmacy practice changes in the HBPC setting. This workgroup serves as clinical practice leadership within the HBPC setting to provide expertise and lead initiatives supporting the advanced practice role of the HBPC CPS.
As HBPC programs expanded throughout VHA, it was paramount to determine the current state of HBPC pharmacy practice by collecting necessary data points to assess uniformity and better understand opportunities for practice standardization. The SME workgroup developed a voluntary yet comprehensive survey assessment that served to proactively assess the future of HBPC pharmacy.
Methods
The HBPC SME workgroup, in conjunction with CPPO, developed the assessment. Questions were designed and tested within a small group of CPSs and then distributed electronically. In August 2014, the assessment was e-mailed to all 21 VHA service areas with an active HBPC program, and responses were collected through a Microsoft SharePoint (Redmond, WA) survey. A response was requested from chiefs of pharmacy, clinical pharmacy leadership, or a representative.
This voluntary assessment contained 24 multipart questions related to background information of HBPC programs and clinical pharmacy services. Duplicate responses were consolidated and clarified with individual sites post hoc.
Descriptive statistics were used to analyze responses. To standardize the comparison across sites with a variety of full-time equivalent employees (FTEEs), the average patient census was divided by the CPS FTEE allocated to the programs at that site. For example, if a site reported 316 patients with 0.25 CPS FTEE, a standardized ratio for this site was 1,264 patients per FTEE. If a patient census range was reported, the median number would be used.
Results
The team received responses from 130 of 141 VHA facilities (92%), encompassing 270 CPSs. A total of 168.75 FTEEs were officially designated as HBPC CPSs. All 21 VHA service areas at the time were represented. The majority of responding programs (67%) had < 1 CPS FTEE allocated to HBPC; many of these CPSs were working in other pharmacy areas but were only dedicated to HBPC part-time.
Nearly 90% of CPSs completed postgraduate year 1 residency training. Fifty-seven percent of CPSs held advanced certifications, such as BCGP, BCACP, or BCPS. Sixty-two percent of CPSs with these specialized board certifications had residency training. Use of a SOP was reported by 76% of CPSs, and 66% of these had a global practice-area scope. Table 1 outlines the functions authorized by a global or limited SOP.6
Overall, 52% of sites reported CPS involvement in CMM of primarily anticoagulation, diabetes mellitus (DM), anemia, hyperlipidemia, and hypertension. The reported average time spent for each disease encounter is delineated in Table 2.
Thirty-five percent of sites reported CPS participation in home visits, and the majority of those completed between 1 and 10 home visits per month. The types of interventions provided often included medication education, assessment of medication adherence, and CMM for DM, hyperlipidemia, hypertension, etc. Multiple interventions often were made during each home visit.
The workload of medication reviews was divided among multiple CPSs in 55% of the programs. The majority of programs completed fewer than 20 initial medication reviews per month and between 21 and 80 quarterly medication reviews per month (81% and 62%, respectively). The average time for a CPS to complete initial medication reviews was 78 minutes and 42 minutes for quarterly medication reviews.
Sites with CPSs that held a SOP (76%) took an average of 83 minutes to complete an initial medication review and 48 minutes to complete a quarterly review. Sites with CPSs without a SOP (24%) took an average of 72 minutes to complete an initial medication review and 36 minutes to complete a quarterly review. Many CPSs allocate ≤ 20 hours per month on routine pharmacy functions (eg, prescription verification, dispensing activities, nonformulary medication requests) and ≤ 20 hours per month on nonpatient care activities (eg, education, medication use evaluations, training, projects), 67% and 82%, respectively.
Ninety-seven percent of CPSs actively attended weekly HBPC program interdisciplinary team (IDT) meetings, with 67% attending 1 weekly IDT meeting and 30% attending 2 to 5 weekly IDT meetings. Time spent attending IDT and roundtable discussions averaged 3.5 hours per week. Multiple programs noted growth within the 12 months preceding the survey, as 37 sites were granted approval for a total of 29.75 additional CPS FTEEs, and an additional 10 sites had a total of 9.25 FTEEs pending approval.
Discussion
Analysis of this assessment allowed the HBPC CPS SME workgroup to identify strong practices and variations in individual HBPC pharmacy programs. The majority of CPSs (66%) are using global, practice area-based SOPs, which allows more autonomy via direct patient care to veterans through CMM and home visits. This trend suggests the focus of the HBPC CPS role has expanded beyond traditional pharmacist activities. These global SOPs result in a higher yield of CPS functions, such as developing, documenting, and executing therapeutic plans and prescribing medications (Table 1). A higher percentage of CPSs with a practice area-based SOP were authorized to perform all 8 functions. Therefore, increased use of practice area-based SOPs and the expansion of the HBPC CPS role can support the team and increase clinical services available to veterans.
Clinical pharmacy specialists using SOPs take longer to complete medication reviews compared with those not using SOPs. Although the assessment was not designed to evaluate the reasons for these time differences, post-hoc follow-up clarification with individual sites determined CPS use of a SOP can lend to a more time-intensive and comprehensive medication review. This may lead to more optimized and safe medication regimens and elimination of unnecessary and/or inappropriate medications for HBPC veterans.
While only 35% of pharmacists were participating in the home visits at the time of this assessment, this is another area to explore as an opportunity to expand CMM. Although the assessment showed the majority of these home visits addressed medication education and adherence, programs may find it advantageous to provide CPS home visits for veterans identified as high risk or requiring specialized CMM. Additional data are needed regarding the ideal population to target for CPS home visits, as well as the estimated benefits of conducting home visits, such as outcomes and efficiency.
With growth noted in multiple programs, HBPC leadership should continue to encourage expanded pharmacist roles at an advanced practice level with SOPs, to provide veteran-centered care. This practice allows the team to concentrate efforts on patient acuity while increasing veteran access to VHA care. Additional CPS FTEEs are necessary to allow for expansion of the HBPC CPS role. The data also demonstrate HBPC often uses a part-time workforce where pharmacists are assigned to HBPC < 40 hours per week, and multiple pharmacists may be used to fulfill 1 CPS FTEE position. Home-Based Primary Care programs are encouraged to consolidate the number of CPSs involved as core individuals to promote continuity and avoid fragmented care.
Limitations
One limitation of the assessment is that the questions were designed and tested by a small group of CPSs, which may have led to response bias and potential misinterpretation of some questions. Time spent on medication reviews may have been underestimated, as some sites reported the maximum allowable workload credit time rather than actual time spent. Recall bias also is a limitation because the assessment relied on the recollection of the CPS or chief of pharmacy. Additionally, while the assessment focused on quantity and time spent on medication reviews, it was not designed to evaluate quality. Examination of what constitutes a high-quality medication review would be helpful to provide guidance and standardize care across the VHA.
Conclusion
Clinical pharmacy specialists practicing in the VHA HBPC setting are highly trained clinicians. A significant percentage of CPSs practice with a SOP that includes prescriptive privileges. However, variations in practice and function exist in the system. This presents an excellent opportunity for future standardization and promotion of the highest and best use of the CPS to improve quality of care for HBPC. With the expansion of the CPS role, there is potential for pharmacists to increase clinical activities and improve care for home-based veterans. The CPPO HBPC SME workgroup will continue to examine and explore the CPS role in this practice setting, develop staffing and practice guidance documents, and assess the benefit of CPS home visits.
1. US Department of Veterans Affairs. VHA Directive 1141.01: home-based primary care special population aligned care team program. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5417. Updated September 20, 2017. Accessed April 3, 2018.
2. Brahmbhatt M, Palla K, Kossifologos A, Mitchell D, Lee T. Appropriateness of medication prescribing using the STOPP/START criteria in veterans receiving home-based primary care. Consult Pharm. 2013;28(6):361-369.
3. Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428-437.
4. Rose AJ, McCullough MB, Carter BL, Rudin RS. The clinical pharmacy specialist: part of the solution. J Gen Intern Med. 2017;32(4):375–377.
5. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice: a report to the U.S. Surgeon General 2011. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed April 3, 2018.
6. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070–2077.
7. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
8. US Department of Veteran Affairs, Veterans Health Administration, Healthcare Talent Management Workforce Management & Consulting Office. VHA workforce planning report 2015.https://www.vacareers.va.gov/assets/common/print/2015_VHA_Workforce_Succession_Strategic_Plan.pdf. Published 2015. Accessed April 3, 2018.
9. Davis RG, Hepfinger CA, Sauer KA, Wilhardt MS. Retrospective evaluation of medication appropriateness and clinical pharmacist drug therapy recommendations for home-based primary care veterans. Am J Geriatr Pharmacother. 2007;5(1):40-47.
10. Beales JL, Edes T. Veteran’s Affairs home based primary care. Clin Geriatr Med. 2009;25(1):149-154.
11. Cooper DF, Granadillo OR, Stacey CM. Home-based primary care: the care of the veteran at home. Home Healthc Nurse. 2007;25(5):315-322.
12. Pradel FG, Palumbo FB, Flowers L, et al. White paper: value of specialty certification in pharmacy. J Am Pharm Assoc. 2004;44(5):612-620.
13. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(1). Clinical pharmacy services. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120. Updated June 29, 2017. Accessed April 3, 2018
Home-Based Primary Care (HBPC) is a unique interdisciplinary program within the Veteran’s Health Administration (VHA) that specifically targets veterans with complex, chronic disabling diseases who have difficulty traveling to a VHA facility.1 Veterans are provided comprehensive longitudinal primary care in their homes, with the goal of maximizing the veteran’s independence. Clinical pharmacists are known as medication experts and have an essential role within interdisciplinary teams, including HBPC, improving medication safety, and decreasing inappropriate prescribing practices.2,3 Clinical pharmacy specialists (CPSs) within the VHA work collaboratively but autonomously as advanced practice providers assisting with the pharmacologic management of many diseases and chronic conditions. The remainder of this article will refer to the HBPC pharmacist as a CPS.
The CPS is actively involved in providing comprehensive medication management (CMM) services across VHA and has the expertise to effectively assist veterans in achieving targeted clinical outcomes. While the value and role of CPSs in the primary care setting are described extensively in the literature, data regarding the CPS in HBPC are limited.4-6 Therefore, the purpose of the assessment was to evaluate the status of the HBPC pharmacy workforce, identify current pharmacist activities and strong practices, and clarify national variations among programs. Future use of this analysis may assist with standardization of the HBPC CPS role and development of business rules in combination with a workload-based staffing model tool.
Background
The role of the pharmacist in the HBPC setting has evolved from providing basic medication therapy reviews to an advanced role providing CMM services under a VHA scope of practice (SOP), which outlines 8 functions that may be authorized, including medication prescriptive authority.7 The SOP may be disease specific (limited) but is increasingly transitioning to have a practice-area scope (global), which is consistent with other VHA advanced practice providers.7 Effective use of a CPS in this role allows for optimization of CMM and increasing veteran access to VHA care.
The VHA employed 7,285 pharmacists in 2014.8 Many were considered CPSs with prescriptive authority. These pharmacists were responsible for ordering more than 1.7 million distinct prescriptions across the VHA in fiscal year 2014, which represented 2.6% of the total prescriptions that year.7 A 2007 VHA study also demonstrated both an increase in appropriate prescribing practices and improved medication use when CPSs worked in collaboration with the HBPC team.9 With this evolution of VHA pharmacists, there has been an increase in the use of CPSs in HBPC and changes in staffing ratios to allow for additional clinical activities and comprehensive patient care provision.1
The HBPC model serves a complex population in which each veteran has about 8 chronic conditions.1,10 An interdisciplinary team consisting of various health care professionals, such as physicians, nurse practitioners, nurses, social workers, registered dietitians, psychologists, rehabilitation therapists, pharmacists, etc, work collaboratively to care for these veterans in the patient’s home. This team is a type of patient-centered medical home (PCMH) that focuses on providing primary care services to an at-risk veteran population who have difficulty leaving the home.1 Home-based primary care has been shown to be cost-effective, reducing average annual cost of health care by up to 24%.10 Another study showed that patients using HBPC had a 27% reduction in hospital admissions and 69% reduction in inpatient hospital days when compared with patients who were not using HBPC.11
The interdisciplinary team meets at least once weekly to discuss and design individualized care plans for veterans enrolled in the program. It is desirable for pharmacists on these teams to have special expertise and certification in geriatric pharmacotherapy and chronic disease management (eg, board-certified geriatric pharmacist [BCGP], board-certified pharmacotherapy specialist [BCPS], or board-certified ambulatory care pharmacist [BCACP]) due to the complexity of comorbidities of these veterans.12 Additional education such as postgraduate pharmacy residency training also is beneficial for CPSs in this setting.
The CPS proactively performs CMM that is often greater in scope than a targeted disease review due to multiple comorbid conditions that are often present within veteran patients.1 These comprehensive medication reviews are considered a core function and must be performed on enrollment in HBPC, quarterly, and when clinically indicated or requested by the team.13 Sufficient time must be allocated to the CPS in order to provide these high-quality medication reviews. Additional core functions of the CPS are outlined in the functional statement and/or SOP, but responsibilities include CMM and disease management. This typically consists of prescribing and/or adjusting medications, as well as providing patient and caregiver education, which can be performed either face-to-face or via telehealth visits (eg, telephone and video). A CPS also may make home visits to assess the veteran, either independently or with other disciplines of the HBPC team.
The HBPC Subject Matter Expert (SME) workgroup was chartered by the Veterans Affairs Central Office (VACO) Pharmacy Benefits Management Service (PBM) Clinical Pharmacy Practice Office (CPPO) to explore pharmacy practice changes in the HBPC setting. This workgroup serves as clinical practice leadership within the HBPC setting to provide expertise and lead initiatives supporting the advanced practice role of the HBPC CPS.
As HBPC programs expanded throughout VHA, it was paramount to determine the current state of HBPC pharmacy practice by collecting necessary data points to assess uniformity and better understand opportunities for practice standardization. The SME workgroup developed a voluntary yet comprehensive survey assessment that served to proactively assess the future of HBPC pharmacy.
Methods
The HBPC SME workgroup, in conjunction with CPPO, developed the assessment. Questions were designed and tested within a small group of CPSs and then distributed electronically. In August 2014, the assessment was e-mailed to all 21 VHA service areas with an active HBPC program, and responses were collected through a Microsoft SharePoint (Redmond, WA) survey. A response was requested from chiefs of pharmacy, clinical pharmacy leadership, or a representative.
This voluntary assessment contained 24 multipart questions related to background information of HBPC programs and clinical pharmacy services. Duplicate responses were consolidated and clarified with individual sites post hoc.
Descriptive statistics were used to analyze responses. To standardize the comparison across sites with a variety of full-time equivalent employees (FTEEs), the average patient census was divided by the CPS FTEE allocated to the programs at that site. For example, if a site reported 316 patients with 0.25 CPS FTEE, a standardized ratio for this site was 1,264 patients per FTEE. If a patient census range was reported, the median number would be used.
Results
The team received responses from 130 of 141 VHA facilities (92%), encompassing 270 CPSs. A total of 168.75 FTEEs were officially designated as HBPC CPSs. All 21 VHA service areas at the time were represented. The majority of responding programs (67%) had < 1 CPS FTEE allocated to HBPC; many of these CPSs were working in other pharmacy areas but were only dedicated to HBPC part-time.
Nearly 90% of CPSs completed postgraduate year 1 residency training. Fifty-seven percent of CPSs held advanced certifications, such as BCGP, BCACP, or BCPS. Sixty-two percent of CPSs with these specialized board certifications had residency training. Use of a SOP was reported by 76% of CPSs, and 66% of these had a global practice-area scope. Table 1 outlines the functions authorized by a global or limited SOP.6
Overall, 52% of sites reported CPS involvement in CMM of primarily anticoagulation, diabetes mellitus (DM), anemia, hyperlipidemia, and hypertension. The reported average time spent for each disease encounter is delineated in Table 2.
Thirty-five percent of sites reported CPS participation in home visits, and the majority of those completed between 1 and 10 home visits per month. The types of interventions provided often included medication education, assessment of medication adherence, and CMM for DM, hyperlipidemia, hypertension, etc. Multiple interventions often were made during each home visit.
The workload of medication reviews was divided among multiple CPSs in 55% of the programs. The majority of programs completed fewer than 20 initial medication reviews per month and between 21 and 80 quarterly medication reviews per month (81% and 62%, respectively). The average time for a CPS to complete initial medication reviews was 78 minutes and 42 minutes for quarterly medication reviews.
Sites with CPSs that held a SOP (76%) took an average of 83 minutes to complete an initial medication review and 48 minutes to complete a quarterly review. Sites with CPSs without a SOP (24%) took an average of 72 minutes to complete an initial medication review and 36 minutes to complete a quarterly review. Many CPSs allocate ≤ 20 hours per month on routine pharmacy functions (eg, prescription verification, dispensing activities, nonformulary medication requests) and ≤ 20 hours per month on nonpatient care activities (eg, education, medication use evaluations, training, projects), 67% and 82%, respectively.
Ninety-seven percent of CPSs actively attended weekly HBPC program interdisciplinary team (IDT) meetings, with 67% attending 1 weekly IDT meeting and 30% attending 2 to 5 weekly IDT meetings. Time spent attending IDT and roundtable discussions averaged 3.5 hours per week. Multiple programs noted growth within the 12 months preceding the survey, as 37 sites were granted approval for a total of 29.75 additional CPS FTEEs, and an additional 10 sites had a total of 9.25 FTEEs pending approval.
Discussion
Analysis of this assessment allowed the HBPC CPS SME workgroup to identify strong practices and variations in individual HBPC pharmacy programs. The majority of CPSs (66%) are using global, practice area-based SOPs, which allows more autonomy via direct patient care to veterans through CMM and home visits. This trend suggests the focus of the HBPC CPS role has expanded beyond traditional pharmacist activities. These global SOPs result in a higher yield of CPS functions, such as developing, documenting, and executing therapeutic plans and prescribing medications (Table 1). A higher percentage of CPSs with a practice area-based SOP were authorized to perform all 8 functions. Therefore, increased use of practice area-based SOPs and the expansion of the HBPC CPS role can support the team and increase clinical services available to veterans.
Clinical pharmacy specialists using SOPs take longer to complete medication reviews compared with those not using SOPs. Although the assessment was not designed to evaluate the reasons for these time differences, post-hoc follow-up clarification with individual sites determined CPS use of a SOP can lend to a more time-intensive and comprehensive medication review. This may lead to more optimized and safe medication regimens and elimination of unnecessary and/or inappropriate medications for HBPC veterans.
While only 35% of pharmacists were participating in the home visits at the time of this assessment, this is another area to explore as an opportunity to expand CMM. Although the assessment showed the majority of these home visits addressed medication education and adherence, programs may find it advantageous to provide CPS home visits for veterans identified as high risk or requiring specialized CMM. Additional data are needed regarding the ideal population to target for CPS home visits, as well as the estimated benefits of conducting home visits, such as outcomes and efficiency.
With growth noted in multiple programs, HBPC leadership should continue to encourage expanded pharmacist roles at an advanced practice level with SOPs, to provide veteran-centered care. This practice allows the team to concentrate efforts on patient acuity while increasing veteran access to VHA care. Additional CPS FTEEs are necessary to allow for expansion of the HBPC CPS role. The data also demonstrate HBPC often uses a part-time workforce where pharmacists are assigned to HBPC < 40 hours per week, and multiple pharmacists may be used to fulfill 1 CPS FTEE position. Home-Based Primary Care programs are encouraged to consolidate the number of CPSs involved as core individuals to promote continuity and avoid fragmented care.
Limitations
One limitation of the assessment is that the questions were designed and tested by a small group of CPSs, which may have led to response bias and potential misinterpretation of some questions. Time spent on medication reviews may have been underestimated, as some sites reported the maximum allowable workload credit time rather than actual time spent. Recall bias also is a limitation because the assessment relied on the recollection of the CPS or chief of pharmacy. Additionally, while the assessment focused on quantity and time spent on medication reviews, it was not designed to evaluate quality. Examination of what constitutes a high-quality medication review would be helpful to provide guidance and standardize care across the VHA.
Conclusion
Clinical pharmacy specialists practicing in the VHA HBPC setting are highly trained clinicians. A significant percentage of CPSs practice with a SOP that includes prescriptive privileges. However, variations in practice and function exist in the system. This presents an excellent opportunity for future standardization and promotion of the highest and best use of the CPS to improve quality of care for HBPC. With the expansion of the CPS role, there is potential for pharmacists to increase clinical activities and improve care for home-based veterans. The CPPO HBPC SME workgroup will continue to examine and explore the CPS role in this practice setting, develop staffing and practice guidance documents, and assess the benefit of CPS home visits.
Home-Based Primary Care (HBPC) is a unique interdisciplinary program within the Veteran’s Health Administration (VHA) that specifically targets veterans with complex, chronic disabling diseases who have difficulty traveling to a VHA facility.1 Veterans are provided comprehensive longitudinal primary care in their homes, with the goal of maximizing the veteran’s independence. Clinical pharmacists are known as medication experts and have an essential role within interdisciplinary teams, including HBPC, improving medication safety, and decreasing inappropriate prescribing practices.2,3 Clinical pharmacy specialists (CPSs) within the VHA work collaboratively but autonomously as advanced practice providers assisting with the pharmacologic management of many diseases and chronic conditions. The remainder of this article will refer to the HBPC pharmacist as a CPS.
The CPS is actively involved in providing comprehensive medication management (CMM) services across VHA and has the expertise to effectively assist veterans in achieving targeted clinical outcomes. While the value and role of CPSs in the primary care setting are described extensively in the literature, data regarding the CPS in HBPC are limited.4-6 Therefore, the purpose of the assessment was to evaluate the status of the HBPC pharmacy workforce, identify current pharmacist activities and strong practices, and clarify national variations among programs. Future use of this analysis may assist with standardization of the HBPC CPS role and development of business rules in combination with a workload-based staffing model tool.
Background
The role of the pharmacist in the HBPC setting has evolved from providing basic medication therapy reviews to an advanced role providing CMM services under a VHA scope of practice (SOP), which outlines 8 functions that may be authorized, including medication prescriptive authority.7 The SOP may be disease specific (limited) but is increasingly transitioning to have a practice-area scope (global), which is consistent with other VHA advanced practice providers.7 Effective use of a CPS in this role allows for optimization of CMM and increasing veteran access to VHA care.
The VHA employed 7,285 pharmacists in 2014.8 Many were considered CPSs with prescriptive authority. These pharmacists were responsible for ordering more than 1.7 million distinct prescriptions across the VHA in fiscal year 2014, which represented 2.6% of the total prescriptions that year.7 A 2007 VHA study also demonstrated both an increase in appropriate prescribing practices and improved medication use when CPSs worked in collaboration with the HBPC team.9 With this evolution of VHA pharmacists, there has been an increase in the use of CPSs in HBPC and changes in staffing ratios to allow for additional clinical activities and comprehensive patient care provision.1
The HBPC model serves a complex population in which each veteran has about 8 chronic conditions.1,10 An interdisciplinary team consisting of various health care professionals, such as physicians, nurse practitioners, nurses, social workers, registered dietitians, psychologists, rehabilitation therapists, pharmacists, etc, work collaboratively to care for these veterans in the patient’s home. This team is a type of patient-centered medical home (PCMH) that focuses on providing primary care services to an at-risk veteran population who have difficulty leaving the home.1 Home-based primary care has been shown to be cost-effective, reducing average annual cost of health care by up to 24%.10 Another study showed that patients using HBPC had a 27% reduction in hospital admissions and 69% reduction in inpatient hospital days when compared with patients who were not using HBPC.11
The interdisciplinary team meets at least once weekly to discuss and design individualized care plans for veterans enrolled in the program. It is desirable for pharmacists on these teams to have special expertise and certification in geriatric pharmacotherapy and chronic disease management (eg, board-certified geriatric pharmacist [BCGP], board-certified pharmacotherapy specialist [BCPS], or board-certified ambulatory care pharmacist [BCACP]) due to the complexity of comorbidities of these veterans.12 Additional education such as postgraduate pharmacy residency training also is beneficial for CPSs in this setting.
The CPS proactively performs CMM that is often greater in scope than a targeted disease review due to multiple comorbid conditions that are often present within veteran patients.1 These comprehensive medication reviews are considered a core function and must be performed on enrollment in HBPC, quarterly, and when clinically indicated or requested by the team.13 Sufficient time must be allocated to the CPS in order to provide these high-quality medication reviews. Additional core functions of the CPS are outlined in the functional statement and/or SOP, but responsibilities include CMM and disease management. This typically consists of prescribing and/or adjusting medications, as well as providing patient and caregiver education, which can be performed either face-to-face or via telehealth visits (eg, telephone and video). A CPS also may make home visits to assess the veteran, either independently or with other disciplines of the HBPC team.
The HBPC Subject Matter Expert (SME) workgroup was chartered by the Veterans Affairs Central Office (VACO) Pharmacy Benefits Management Service (PBM) Clinical Pharmacy Practice Office (CPPO) to explore pharmacy practice changes in the HBPC setting. This workgroup serves as clinical practice leadership within the HBPC setting to provide expertise and lead initiatives supporting the advanced practice role of the HBPC CPS.
As HBPC programs expanded throughout VHA, it was paramount to determine the current state of HBPC pharmacy practice by collecting necessary data points to assess uniformity and better understand opportunities for practice standardization. The SME workgroup developed a voluntary yet comprehensive survey assessment that served to proactively assess the future of HBPC pharmacy.
Methods
The HBPC SME workgroup, in conjunction with CPPO, developed the assessment. Questions were designed and tested within a small group of CPSs and then distributed electronically. In August 2014, the assessment was e-mailed to all 21 VHA service areas with an active HBPC program, and responses were collected through a Microsoft SharePoint (Redmond, WA) survey. A response was requested from chiefs of pharmacy, clinical pharmacy leadership, or a representative.
This voluntary assessment contained 24 multipart questions related to background information of HBPC programs and clinical pharmacy services. Duplicate responses were consolidated and clarified with individual sites post hoc.
Descriptive statistics were used to analyze responses. To standardize the comparison across sites with a variety of full-time equivalent employees (FTEEs), the average patient census was divided by the CPS FTEE allocated to the programs at that site. For example, if a site reported 316 patients with 0.25 CPS FTEE, a standardized ratio for this site was 1,264 patients per FTEE. If a patient census range was reported, the median number would be used.
Results
The team received responses from 130 of 141 VHA facilities (92%), encompassing 270 CPSs. A total of 168.75 FTEEs were officially designated as HBPC CPSs. All 21 VHA service areas at the time were represented. The majority of responding programs (67%) had < 1 CPS FTEE allocated to HBPC; many of these CPSs were working in other pharmacy areas but were only dedicated to HBPC part-time.
Nearly 90% of CPSs completed postgraduate year 1 residency training. Fifty-seven percent of CPSs held advanced certifications, such as BCGP, BCACP, or BCPS. Sixty-two percent of CPSs with these specialized board certifications had residency training. Use of a SOP was reported by 76% of CPSs, and 66% of these had a global practice-area scope. Table 1 outlines the functions authorized by a global or limited SOP.6
Overall, 52% of sites reported CPS involvement in CMM of primarily anticoagulation, diabetes mellitus (DM), anemia, hyperlipidemia, and hypertension. The reported average time spent for each disease encounter is delineated in Table 2.
Thirty-five percent of sites reported CPS participation in home visits, and the majority of those completed between 1 and 10 home visits per month. The types of interventions provided often included medication education, assessment of medication adherence, and CMM for DM, hyperlipidemia, hypertension, etc. Multiple interventions often were made during each home visit.
The workload of medication reviews was divided among multiple CPSs in 55% of the programs. The majority of programs completed fewer than 20 initial medication reviews per month and between 21 and 80 quarterly medication reviews per month (81% and 62%, respectively). The average time for a CPS to complete initial medication reviews was 78 minutes and 42 minutes for quarterly medication reviews.
Sites with CPSs that held a SOP (76%) took an average of 83 minutes to complete an initial medication review and 48 minutes to complete a quarterly review. Sites with CPSs without a SOP (24%) took an average of 72 minutes to complete an initial medication review and 36 minutes to complete a quarterly review. Many CPSs allocate ≤ 20 hours per month on routine pharmacy functions (eg, prescription verification, dispensing activities, nonformulary medication requests) and ≤ 20 hours per month on nonpatient care activities (eg, education, medication use evaluations, training, projects), 67% and 82%, respectively.
Ninety-seven percent of CPSs actively attended weekly HBPC program interdisciplinary team (IDT) meetings, with 67% attending 1 weekly IDT meeting and 30% attending 2 to 5 weekly IDT meetings. Time spent attending IDT and roundtable discussions averaged 3.5 hours per week. Multiple programs noted growth within the 12 months preceding the survey, as 37 sites were granted approval for a total of 29.75 additional CPS FTEEs, and an additional 10 sites had a total of 9.25 FTEEs pending approval.
Discussion
Analysis of this assessment allowed the HBPC CPS SME workgroup to identify strong practices and variations in individual HBPC pharmacy programs. The majority of CPSs (66%) are using global, practice area-based SOPs, which allows more autonomy via direct patient care to veterans through CMM and home visits. This trend suggests the focus of the HBPC CPS role has expanded beyond traditional pharmacist activities. These global SOPs result in a higher yield of CPS functions, such as developing, documenting, and executing therapeutic plans and prescribing medications (Table 1). A higher percentage of CPSs with a practice area-based SOP were authorized to perform all 8 functions. Therefore, increased use of practice area-based SOPs and the expansion of the HBPC CPS role can support the team and increase clinical services available to veterans.
Clinical pharmacy specialists using SOPs take longer to complete medication reviews compared with those not using SOPs. Although the assessment was not designed to evaluate the reasons for these time differences, post-hoc follow-up clarification with individual sites determined CPS use of a SOP can lend to a more time-intensive and comprehensive medication review. This may lead to more optimized and safe medication regimens and elimination of unnecessary and/or inappropriate medications for HBPC veterans.
While only 35% of pharmacists were participating in the home visits at the time of this assessment, this is another area to explore as an opportunity to expand CMM. Although the assessment showed the majority of these home visits addressed medication education and adherence, programs may find it advantageous to provide CPS home visits for veterans identified as high risk or requiring specialized CMM. Additional data are needed regarding the ideal population to target for CPS home visits, as well as the estimated benefits of conducting home visits, such as outcomes and efficiency.
With growth noted in multiple programs, HBPC leadership should continue to encourage expanded pharmacist roles at an advanced practice level with SOPs, to provide veteran-centered care. This practice allows the team to concentrate efforts on patient acuity while increasing veteran access to VHA care. Additional CPS FTEEs are necessary to allow for expansion of the HBPC CPS role. The data also demonstrate HBPC often uses a part-time workforce where pharmacists are assigned to HBPC < 40 hours per week, and multiple pharmacists may be used to fulfill 1 CPS FTEE position. Home-Based Primary Care programs are encouraged to consolidate the number of CPSs involved as core individuals to promote continuity and avoid fragmented care.
Limitations
One limitation of the assessment is that the questions were designed and tested by a small group of CPSs, which may have led to response bias and potential misinterpretation of some questions. Time spent on medication reviews may have been underestimated, as some sites reported the maximum allowable workload credit time rather than actual time spent. Recall bias also is a limitation because the assessment relied on the recollection of the CPS or chief of pharmacy. Additionally, while the assessment focused on quantity and time spent on medication reviews, it was not designed to evaluate quality. Examination of what constitutes a high-quality medication review would be helpful to provide guidance and standardize care across the VHA.
Conclusion
Clinical pharmacy specialists practicing in the VHA HBPC setting are highly trained clinicians. A significant percentage of CPSs practice with a SOP that includes prescriptive privileges. However, variations in practice and function exist in the system. This presents an excellent opportunity for future standardization and promotion of the highest and best use of the CPS to improve quality of care for HBPC. With the expansion of the CPS role, there is potential for pharmacists to increase clinical activities and improve care for home-based veterans. The CPPO HBPC SME workgroup will continue to examine and explore the CPS role in this practice setting, develop staffing and practice guidance documents, and assess the benefit of CPS home visits.
1. US Department of Veterans Affairs. VHA Directive 1141.01: home-based primary care special population aligned care team program. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5417. Updated September 20, 2017. Accessed April 3, 2018.
2. Brahmbhatt M, Palla K, Kossifologos A, Mitchell D, Lee T. Appropriateness of medication prescribing using the STOPP/START criteria in veterans receiving home-based primary care. Consult Pharm. 2013;28(6):361-369.
3. Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428-437.
4. Rose AJ, McCullough MB, Carter BL, Rudin RS. The clinical pharmacy specialist: part of the solution. J Gen Intern Med. 2017;32(4):375–377.
5. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice: a report to the U.S. Surgeon General 2011. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed April 3, 2018.
6. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070–2077.
7. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
8. US Department of Veteran Affairs, Veterans Health Administration, Healthcare Talent Management Workforce Management & Consulting Office. VHA workforce planning report 2015.https://www.vacareers.va.gov/assets/common/print/2015_VHA_Workforce_Succession_Strategic_Plan.pdf. Published 2015. Accessed April 3, 2018.
9. Davis RG, Hepfinger CA, Sauer KA, Wilhardt MS. Retrospective evaluation of medication appropriateness and clinical pharmacist drug therapy recommendations for home-based primary care veterans. Am J Geriatr Pharmacother. 2007;5(1):40-47.
10. Beales JL, Edes T. Veteran’s Affairs home based primary care. Clin Geriatr Med. 2009;25(1):149-154.
11. Cooper DF, Granadillo OR, Stacey CM. Home-based primary care: the care of the veteran at home. Home Healthc Nurse. 2007;25(5):315-322.
12. Pradel FG, Palumbo FB, Flowers L, et al. White paper: value of specialty certification in pharmacy. J Am Pharm Assoc. 2004;44(5):612-620.
13. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(1). Clinical pharmacy services. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120. Updated June 29, 2017. Accessed April 3, 2018
1. US Department of Veterans Affairs. VHA Directive 1141.01: home-based primary care special population aligned care team program. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=5417. Updated September 20, 2017. Accessed April 3, 2018.
2. Brahmbhatt M, Palla K, Kossifologos A, Mitchell D, Lee T. Appropriateness of medication prescribing using the STOPP/START criteria in veterans receiving home-based primary care. Consult Pharm. 2013;28(6):361-369.
3. Hanlon JT, Weinberger M, Samsa GP, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100(4):428-437.
4. Rose AJ, McCullough MB, Carter BL, Rudin RS. The clinical pharmacy specialist: part of the solution. J Gen Intern Med. 2017;32(4):375–377.
5. Giberson S, Yoder S, Lee MP. Improving patient and health system outcomes through advanced pharmacy practice: a report to the U.S. Surgeon General 2011. https://www.accp.com/docs/positions/misc/Improving_Patient_and_Health_System_Outcomes.pdf. Published December 2011. Accessed April 3, 2018.
6. Lee AJ, Boro MS, Knapp KK, Meier JL, Korman NE. Clinical and economic outcomes of pharmacist recommendations in a Veterans Affairs medical center. Am J Health Syst Pharm. 2002;59(21):2070–2077.
7. Ourth H, Groppi J, Morreale AP, Quicci-Roberts K. Clinical pharmacist prescribing activities in the Veterans Health Administration. Am J Health Syst Pharm. 2016;73(18):1406-1415.
8. US Department of Veteran Affairs, Veterans Health Administration, Healthcare Talent Management Workforce Management & Consulting Office. VHA workforce planning report 2015.https://www.vacareers.va.gov/assets/common/print/2015_VHA_Workforce_Succession_Strategic_Plan.pdf. Published 2015. Accessed April 3, 2018.
9. Davis RG, Hepfinger CA, Sauer KA, Wilhardt MS. Retrospective evaluation of medication appropriateness and clinical pharmacist drug therapy recommendations for home-based primary care veterans. Am J Geriatr Pharmacother. 2007;5(1):40-47.
10. Beales JL, Edes T. Veteran’s Affairs home based primary care. Clin Geriatr Med. 2009;25(1):149-154.
11. Cooper DF, Granadillo OR, Stacey CM. Home-based primary care: the care of the veteran at home. Home Healthc Nurse. 2007;25(5):315-322.
12. Pradel FG, Palumbo FB, Flowers L, et al. White paper: value of specialty certification in pharmacy. J Am Pharm Assoc. 2004;44(5):612-620.
13. US Department of Veterans Affairs, Veterans Health Administration. VHA Handbook 1108.11(1). Clinical pharmacy services. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=3120. Updated June 29, 2017. Accessed April 3, 2018
Pilot Inpatient Pain Pharmacist Consult Service at the West Palm Beach VA Medical Center
The term pain refers to unpleasant sensory, emotional experiences associated with actual or potential tissue damage and is described in terms of such damage.1 Acute pain from neurophysiologic responses to noxious stimuli resolves upon tissue healing or stimuli removal (eg, 3-6 months); however, chronic pain lingers beyond the expected time course of the acute injury and repair process.1,2 Despite pain management advances, the under- or overtreatment of pain for different patient populations (eg, cancer and noncancer) remains an important concern.3,4
Hospitals have focused on optimizing inpatient pain management, because uncontrolled pain remains the most common reason for readmissions the first week postsurgery.5 The safe use of opioids (prescription, illicit synthetic, or heroin) poses major challenges and raises significant concerns. Rates of opioid-related hospital admissions have increased by 42% since 2009, and total overdose deaths reached a new high of 47,055 in 2014, including 28,647 (61%) from opioids.6
Opioids rank among the medications most often associated with serious adverse events (AEs), including respiratory depression and death.7 In response, the Joint Commission recommends patient assessments for opioid-related AEs, technology to monitor opioid prescribing, pharmacist consultation for opioid conversions and route of administration changes, provider education about risks of opioids, and risk screening tools for opioid-related oversedation and respiratory depression.7 Treatment guidelines strive to minimize the impact of acute pain by offering a scientific basis for practice, but evidence suggests a lack of suitable pain programs.7 Increases in opioid prescribing along with clinical guidelines and state laws recommending specialty pain service referrals for patients on high-dose opioids, have increased demand for competent pain clinicians.8
The expertise of a clinical pharmacy specialists (CPS) can help refine ineffective and potentially harmful pain medication therapy in complex patient cases. Existing literature outlines the various benefits of pharmacist participation in collaborative pain services for cancer and noncancer pain, as well as cases involving substance abuse.9-13 Various articles support pharmacists’ role as an educator and team member who can add valuable, trustworthy clinical knowledge that enhances clinical encounters and guides protocol/policy development.12,13 These efforts have improved patient satisfaction and encouraged physicians and nurses to proactively seek pharmacists’ advice for difficult cases.9
Frequent communication with interdisciplinary teams have helped considerably in establishing clinical pharmacy services that benefit patient care and offer sources of professional accomplishment.10,11 For example, pharmacists at Kaweah Delta Medical Center in Visalia, CA launched an innovative pain program that encompassed consultations and opioid stewardship, which demonstrated that pharmacists can improve patient outcomes in the front lines of pain management.14 Pain CPSs have advanced knowledge of pharmacokinetics, pharmacodynamics, and therapeutics to promote safe and effective analgesic use, as well as to identify opioid use disorders. Evidence suggests that pharmacists’ presence on interdisciplinary pain teams improves outcomes by optimizing medication selection, improving adherence, and preventing AEs.8
Following the plan-do-study-act model for quality improvement (QI), this project hoped to expand current pain programs at the West Palm Beach VAMC (WPBVAMC) by evaluating the feasibility of an inpatient pain pharmacist consult service (IPPCS) at the 301-bed teaching facility, which includes 130 acute/intensive care and 120 nursing/domiciliary beds.15 Staff provide primary- and secondary-level care to veterans in 7 counties along Florida’s southeastern coast.
In 2009, the WPBVAMC PGY-2 Pain Management and Palliative Care Program became the VA’s first accredited pain pharmacy residency. Residents train with the Physical Medicine & Rehabilitation (PMR) and Chronic Pain Management departments, which provide outpatient services from 7 pain physicians, a pain psychologist, registered nurse, chiropractor, acupuncturist, physical/occupational therapy (PT/OT), and 3 pain/palliative care CPSs.
The WPBVAMC had established interdisciplinary outpatient chronic pain clinic (OCPC) physician- and pharmacist-run services along with a pain CPS electronic consult (e-consult) program. However, no formal mechanisms for inpatient pain consultations existed. Prior to the IPPCS outlined in this study, OCPC practitioners, including 2 pain CPSs, managed impromptu inpatient pain issues as “curbside consultations” along with usual day-to-day clinic duties.
The OCPC, PMR, and Clinical Pharmacy administration recognized the need for more clinical support to manage complex analgesic issues in inpatient veterans, as these patients often have acute pain with underlying chronic pain syndromes. In a national survey, veterans were significantly more likely than were nonveterans to report painful health conditions (65.5% vs 56.4%) and to classify their pain as severe (9.1% vs 6.3%).16 The WPBVAMC administration concluded that an IPPCS would offer a more efficient means of handling such cases. The IPPCS would formally streamline inpatient pain consults, enabling CPSs to thoroughly evaluate pain-related issues to propose evidence-based recommendations.
The primary objective of this QI project was to assess the IPPCS implementation as part of multimodal care to satisfy unmet patient care needs at the WPBVAMC. Secondary objectives for program feasibility included identifying the volume and type of pain consults, categorizing pharmacist interventions, classifying providers’ satisfaction, and determining types of responses to pharmacists’ medication recommendations.
Methods
This QI project ascertained the feasibility of the IPPCS by evaluating all consults obtained during the pilot period from November 2, 2015 through May 6, 2016. The IPPCS was accessible Monday through Friday during normal business hours. Goal turnaround time for consult completion was 24 to 72 hours, given the lack of coverage on holidays and weekends. The target population included veterans hospitalized at the WPBVAMC inpatient ward or nursing home with uncontrolled pain on IV and/or oral analgesic medications. All IPPCS consults submitted during the pilot period were included in the sample.
The WPBVAMC Scientific Advisory Committee (SAC) approved this QI program prior to initiation. Following supervisory support from the OCPC, PMR, and Clinical Pharmacy departments, hospital technologists assisted in creating a consult link in the Computerized Patient Record System (CPRS), which allowed providers to submit IPPCS requests for specific patients efficiently. Consults were categorized as postoperative pain, acute or chronic pain, malignant pain, or end-of-life pain. Inpatient providers could enter requests for assistance with 1 or more of the following: opioid dose conversions, opioid taper/titration schedules, general opioid treatment recommendations, or nonopioid/adjuvant recommendations.
The Medical Records Committee approved a customized CPRS subjective-objective-assessment-recommendations (SOAR) note template, which helped standardize the pain CPS documentation. To promote consult requests and interdisciplinary collaboration, inpatient clinicians received education about the IPPCS at respective meetings (eg, General Medicine staff and Clinical Pharmacy meetings).
All CPSs involved in this project were residency-trained in direct patient care, including pain and palliative care, and maintained national board certification as pharmacotherapy specialists. Their role included reviewing patients’ electronic medical records, conducting face-to-face pain assessments, completing opioid risk assessments, evaluating analgesic regimen appropriateness, reviewing medication adherence, completing pain medication reconciliation, querying the Florida Prescription Drug Monitoring Program (PDMP), interpreting urine drug testing (UDT), and delivering provider/patient/caregiver education. Parameters used to determine the appropriateness of analgesic regimens included, but were not limited to:
- Use of oral instead of IV medications if oral dosing was feasible/possible/appropriate;
- Dose and adjustments per renal/hepatic function;
- Adequate treatment duration and titration;
- No therapeutic drug class duplications;
- Medication tolerability (eg, allergies, AEs, drug interactions); and
- Opioid risk assessment per Opioid Risk Tool (ORT) score and medical history.
Consulting providers clarified patients’ pain diagnoses prior to pharmacy consultations.
After face-to-face patient interviews, the inpatient pain CPS prepared pain management recommendations, including nonopioid/adjuvant pain medications and/or opioid dose adjustments. The IPPCS also collaborated with pain physicians for intervention procedures, nonpharmacologic recommendations, and for more complex patients who may have required additional imaging or detailed physical evaluations. Pain CPSs documented CPRS notes with the SOAR template and discussed all recommendations with appropriate inpatient teams.
Respective providers received questionnaires hosted on SurveyMonkey.com (San Mateo, CA) to gauge their satisfaction with the IPPCS at the end of the pilot period, which helped determine program utility. Data collected for the pilot included patient demographics; patient admission diagnosis; consulting inpatient service; type of pain and reason for IPPCS request; total morphine equivalent daily dose (MEDD); pertinent past medical history (ie, sleep apnea, psychiatric comorbidities, or substance use disorder [SUD]); ORT score; patients’ reported average pain severity on the 10-point Numeric Pain Rating Scale (NPRS); number of requests submitted; medications discontinued, initiated, or dose increased/decreased; and number of pharmacist recommendations, including number accepted by providers. The ORT is a 5-item questionnaire used to determine risk of opioid-related aberrant behaviors in adults to help discriminate between low-risk and high-risk individuals (Table 1).17
Descriptive statistics were used to evaluate the results, and a Likert scale was used to evaluate responses the from provider satisfaction questionnaires. The IPPCS collected and organized the data using Microsoft Excel (Redmond, WA).
Results
By the end of the pilot period in May 2016, the IPPCS had received 100 consult requests and completed 81% (Figure). The remaining consults included 11% forwarded to other disciplines. The service discontinued 8% of the requests, given patients’ hospital discharge prior to IPPCS review.
Baseline patient data are outlined in Tables 2, 3, 4, and 5. For each of the 100 consults, providers could select more than 1 reason for the request. The nonopioid/adjuvant treatment recommendations were the most common at 49% (62/128). Patients could have more than 1 pertinent medical comorbidity, with psychiatric illnesses the most prevalent at 68% (133/197). A mean ORT score of 8.1 indicated a high risk for opioid-related aberrant behavior. Overall, half the patients (37/73) were high risk, 25% (18/73) were medium risk (ORT 4-7), and 25% (18/73) were low risk (ORT 0-3). Patients’ reported average pain was often severe (NPRS 7-10) at 54% (40/74) or moderate (NPRS 4-6) at 39% (29/74).
The IPPCS recommended various medications for initiation, discontinuation, or dosage changes (Table 6). For example, the IPPCS recommended initiation of topical agents in 38% (48/128) of cases. The inpatient pain CPS offered opioid initiation in 17% (22/128) of cases, with immediate-release oral morphine as the most predominant. Notably, opioids remained the most common medications suggested for discontinuation at 74% (38/51), including 47% (18/38) for IV hydromorphone. Dose titration recommendations mainly included anticonvulsants at 33% (16/48), and most dose reductions involved opioids at 78% (7/9), namely, oxycodone/acetaminophen and IV hydromorphone.
Providers accepted 76% (179/234) of IPPCS pharmacist medication recommendations. The most common included initiation/optimization of adjuvant therapy (eg, anticonvulsants, serotonin-norepinephrine reuptake inhibitors [SNRIs], and topical agents) at 46% (83/179), followed by opioid discontinuation (eg, IV hydromorphone) at 22% (40/179). Although this project primarily tracked medication interventions, examples of accepted nonpharmacologic recommendations included UDT and referrals to other programs (eg, pain psychology, substance abuse, mental health, acupuncture, chiropractor, PT/OT/PMR, and interventional pain management), which received support from each respective discipline. Declined pharmacologic recommendations mostly included topicals (eg, lidocaine, trolamine, and capsaicin cream) at 35% (19/55). However, findings also show that providers implemented 100% of medication recommendations in whole for 58% (47/81) of consults.
Likert scale satisfaction questionnaires offered insight into providers’ perception of the IPPCS (Table 7). One provider felt “neutral” about the consult submission process, given the time needed to complete the CPRS requests, but all other providers “agreed” or “strongly agreed” that the IPPCS was user-friendly. More importantly, 100% (15/15) “agreed” or “strongly agreed” that the inpatient pain CPS answered consults promptly with reasonable, evidence-based recommendations. All respondents declared that they would recommend the IPPCS to other practitioners and felt comfortable entering requests for future patients.
Discussion
The IPPCS achieved a total of 100 consults, which served as the sample for the pilot program. With support from the OCPC, PMR, and Clinical Pharmacy Department Administration, the IPPCS operated from November 2, 2015 through May 6, 2016. Results suggested that this new service could assist in managing inpatient pain issues in collaboration with inpatient multidisciplinary teams.
The most popular reason for IPPCS consults was acute on chronic pain. Given national efforts to improve opioid prescribing through the VA Opioid Safety Initiative (OSI) and the 2016 CDC Guideline for Opioid Prescribing, most pain consults requested nonopioid/adjuvant recommendations.18,19 Despite the wide MEDD range in this sample, the median/mean generally remained below recommended limits per current guidelines.18,19 However, the small sample size and lack of patient diversity (mostly white male veterans) limited the generalizability to non-VA medical facilities. Veterans often experienced both chronic pain and psychiatric disturbances, which explained the significant number of underlying mental health comorbidities observed. This affirmed the close interrelationship between pain and psychiatric issues described in the literature.20
Providers’ acceptance of pharmacologic and nonpharmacologic treatment modalities supported a comprehensive, multidisciplinary, and biopsychosocial approach to effective analgesic management. During this pilot, the most common pharmacy medication recommendations, namely, discontinuation of inappropriate opioids (eg, IV hydromorphone in patients who are controlled on and/or able to tolerate oral medications) and dose titration of adjuvant medications (eg, anticonvulsants for neuropathic pain), revealed that the IPPCS provided needed expertise and alternatives for complex pain patients. The IPPCS was well received, as inpatient providers accepted and implemented a large proportion of pharmacist recommendations. Despite risks of bias with a nonvalidated questionnaire, providers offered positive feedback. In the future, distributing satisfaction evaluations to patients also would provide more insight into how others perceived the IPPCS.
Limitations
Reasons for unaccepted recommendations included perceived limited effectiveness and/or feasibility of topical agents for acute pain, as providers seemed to favor systemic therapy for supposedly more immediate analgesia. Prescriber preference may explain why inpatient teams sometimes declined adjuvant therapy recommendations. However, the 2016 American Pain Society Guidelines on the Management of Postoperative Pain support a multimodal approach and confirm that adjuvants can reduce patients’ opioid requirements.21
Consulting teams did not execute some opioid recommendations, which may be due to various factors, including patient-related or provider-related factors in the inpatient vs outpatient setting. Lack of retrospective analysis for comparison of results pre- and post-IPPCS implementation also limited the outcomes. However, this project was piloted as a QI initiative after providers identified significant needs for inpatient pain management at the WPBVAMC. No retrospective analysis was undertaken, as this project analyzed only responses during the pilot program.
Other obstacles of the IPPCS included request appropriateness and triaging. The inpatient pain CPS deferred management of some consults to other disciplines (eg, gastroenterology) for more appropriate care. The IPPCS deferred certain cases of acute pancreatic pain or generalized abdominal pain for further workup to address patients’ underlying issues. The inpatient pain CPS relayed pertinent information regarding appropriate consults to inpatient teams. In the future, developing more specific inclusion/exclusion criteria and delivering provider education about proper IPPCS requests may resolve this issue.
Challenges with pain consults from inpatient psychiatry stemmed from patients’ skepticism and unwillingness to accept nonopioid/adjuvant therapies. Additionally, comorbid psychiatric disorders are often associated with SUDs and potentially opioid-related aberrant behavior. More than 40% of opioid-dependent individuals have comorbid psychiatric disorders, especially depression, anxiety, and bipolar disorder.22 Poorly-managed pain also drives SUD, as 80% of these patients illegally obtain prescription opioids. Thus, undertreatment of pain may push individuals to secure pain medications from illegal/illicit sources to achieve analgesia.23 Following pain physician consultation, the IPPCS continued inpatient opioids for 12% (10/81) of patients with a SUD history, including 5 with postoperative pain or other acute processes, since patients were kept in a monitored health care environment. The remaining included 4 with malignant pain and 1 with end-of-life pain. Overall, the IPPCS recommended that inpatient teams discharge these patients on as little opioids as possible, as well as to make referrals to substance abuse programs when necessary. Effective pain management of patients with aberrant behavior requires a comprehensive interdisciplinary team approach. To mitigate risk, effectively treat pain, and maintain patient safety, clinicians must recognize biologic, chemical, social, and psychiatric aspects of substance abuse.21
Another limitation during this pilot was an inability to promptly assess the impact of recommendations, given limited opportunities to reevaluate patients. In the future, more dedicated time for the inpatient pain CPSs to respond to consults may allow for better follow-up rather than initial consults only. Providers sometimes discharged patients within 24 hours of submitting consults as well, which left no time for the inpatient pain CPS consultation. However, the IPPCS forwarded appropriate requests to pain CPS e-consult services for chart review recommendations. Encouraging providers to submit consults earlier in patients’ hospital admissions may help reduce the number of incomplete IPPCS requests. Although expanding service hours would require more dedicated CPS staffing resources, it is another option for quicker consult completion and prompt follow-up.
Future Directions
Future efforts to expand this project include ensuring patient safety through judicious opioid use. Smooth transitions of care will particularly help to improve the quality of pain management. Current WPBVAMC policies stated that the primary care provider (PCP) alone must agree to continue prescribing outpatient analgesic medications, including opioids, prescribed from the OCPC once patients return to Primary Care. Continued provider education would ideally promote efficient utilization of the IPPCS and OCPC.
The pain pharmacy SOAR note template also could undergo additional edits/revisions, including the addition of opioid overdose risk assessments. For improved documentation and standardization, the template could autopopulate patient-specific information when the inpatient pain CPS chooses the designated note title. The IPPCS also hoped to streamline the CPRS consult link for more convenience and ease of use. Ultimately, the IPPCS wished to provide ongoing provider education, inpatient opioid therapy, and other topics upon request.
Conclusion
The IPPCS received positive provider feedback and collected 100 consults (averaging 4 per week) during the 6-month pilot QI project. Most consults were for acute or chronic pain and requested nonopioid/adjuvant recommendations. The new service intended to fulfill unmet needs at the WPBVAMC by expanding the facility’s current pain programs. Prescribers reported a high level of satisfaction and a willingness to not only refer other clinicians to the program, but also continue using the consult. Providers unanimously agreed that the pain CPS provided reasonable, evidence-based recommendations. This project demonstrated that the IPPCS can aid in meeting new demands amid the challenging landscape of pain practice.
1. D’Arcy Y. Treating acute pain in the hospitalized patient. Nurse Pract. 2012;37(8):22-30.
2. Marks AD, Rodgers PE. Diagnosis and management of acute pain in the hospitalized patient. Am J Med. 2014;3(3):e396-e408.
3. Paice JA, Von Roenn JH. Under- or overtreatment of pain in the patient with cancer: how to achieve proper balance. J Clin Oncol. 2014;32(16):1721-1726.
4. Mafi JN, McCarthy EP, Davis RB, Landon BE. Worsening trends in the management and treatment of back pain. JAMA Intern Med. 2013;173(17):1573-1581.
5. Palomano RC, Rathmell JP, Krenzischek DA, Dunwoody CJ. Emerging trends and new approaches to acute pain management. J Perianesth Nurs. 2008;23(suppl 1):S43-S53.
6. U.S. Department of Health & Human Services, Office of the Surgeon General. Facing addiction in America: the surgeon general’s report on alcohol, drugs, and health, executive summary. https://addiction.surgeongeneral.gov/executive-summary.pdf. Published November 2016. Accessed November 1, 2017.
7. Bagian JP, Cohen M, Barnsteiner JH, et al. Safe use of opioids in hospitals. Sentinel Event Alert. 2012;49:1-5.
8. Atkinson TJ, Gulum AH, Forkum WG. The future of pain pharmacy: directed by need. Integrated Pharm Res Pract. 2016;2016(5):33-42.
9. Lothian ST, Fotis MA, Von Gutten CF, et al. Cancer pain management through a pharmacist-based analgesic dosing service. Am J Health Syst Pharm. 1999;56:1119-1125.
10. Lynn MA. Pharmacist interventions in pain management. Am J Health Syst Pharm. 2004;61(14):1487-1489.
11. Strickland JM, Huskey A, Brushwood DB. Pharmacist-physician collaboration in pain management practice. J Opioid Manag. 2007;3(6):295-301.
12. Fan T and Elgourt T. Pain management pharmacy service in a community hospital. Am J Health Syst Pharm. 2008;65(16):1560-1565.
13. Andrews LB, Bridgeman MB, Dalal KS, et al. Implementation of a pharmacist-directed pain management consultation service for hospitalised adults with a history of substance abuse. Int J Clin Pract. 2013;67(12):1342-1349.
14. Poirier RH, Brown CS, Garcia YT, Gann NY, Sandoval RA, McNulty JR. Implementation of a pharmacist directed pain management service in the inpatient setting. http://www.ashpadvantage.com/bestpractices/2014_papers/Kaweah-Delta.htm. Published 2014. Accessed November 1, 2017.
15. Langley GL, Moen R, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nded. San Francisco, CA: Jossey-Bass; 2009.
16. Nahin, RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254.
17. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med. 2005;6(6):432-442.
18. Nazario M. Opioid therapy risk management: the VA opioid safety and naloxone distribution initiatives. http://jfpsmeeting.pharmacist.com/sites/default/files/slides/Opioid%20Therapy%20Risk%20Management.pdf. Published October 15, 2015. Accessed November 1, 2017.
19. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. MMWR Rep. 2016;65(1):1-49.
20. Outcalt SD, Kroenke K, Krebs EE, et al. Chronic pain and comorbid mental health conditions: independent associations of posttraumatic stress disorder and depression with pain, disability, and quality of life. J Behav Med. 2015;38:535.
21. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
22. NIDA/SAMHSA Blending Initiative. https://www.drugabuse.gov/nidasamhsa-blending-initiative. Updated November 2015. Accessed November 1, 2017.
23. Alford DP, German JS, Samet JH, Cheng DM, Lloyd-Travaglini CA, Saitz R. Primary care patients with drug use report chronic pain and self-medicate with alcohol and other drugs. J Gen Intern Med. 2016;31(5):486-491.
The term pain refers to unpleasant sensory, emotional experiences associated with actual or potential tissue damage and is described in terms of such damage.1 Acute pain from neurophysiologic responses to noxious stimuli resolves upon tissue healing or stimuli removal (eg, 3-6 months); however, chronic pain lingers beyond the expected time course of the acute injury and repair process.1,2 Despite pain management advances, the under- or overtreatment of pain for different patient populations (eg, cancer and noncancer) remains an important concern.3,4
Hospitals have focused on optimizing inpatient pain management, because uncontrolled pain remains the most common reason for readmissions the first week postsurgery.5 The safe use of opioids (prescription, illicit synthetic, or heroin) poses major challenges and raises significant concerns. Rates of opioid-related hospital admissions have increased by 42% since 2009, and total overdose deaths reached a new high of 47,055 in 2014, including 28,647 (61%) from opioids.6
Opioids rank among the medications most often associated with serious adverse events (AEs), including respiratory depression and death.7 In response, the Joint Commission recommends patient assessments for opioid-related AEs, technology to monitor opioid prescribing, pharmacist consultation for opioid conversions and route of administration changes, provider education about risks of opioids, and risk screening tools for opioid-related oversedation and respiratory depression.7 Treatment guidelines strive to minimize the impact of acute pain by offering a scientific basis for practice, but evidence suggests a lack of suitable pain programs.7 Increases in opioid prescribing along with clinical guidelines and state laws recommending specialty pain service referrals for patients on high-dose opioids, have increased demand for competent pain clinicians.8
The expertise of a clinical pharmacy specialists (CPS) can help refine ineffective and potentially harmful pain medication therapy in complex patient cases. Existing literature outlines the various benefits of pharmacist participation in collaborative pain services for cancer and noncancer pain, as well as cases involving substance abuse.9-13 Various articles support pharmacists’ role as an educator and team member who can add valuable, trustworthy clinical knowledge that enhances clinical encounters and guides protocol/policy development.12,13 These efforts have improved patient satisfaction and encouraged physicians and nurses to proactively seek pharmacists’ advice for difficult cases.9
Frequent communication with interdisciplinary teams have helped considerably in establishing clinical pharmacy services that benefit patient care and offer sources of professional accomplishment.10,11 For example, pharmacists at Kaweah Delta Medical Center in Visalia, CA launched an innovative pain program that encompassed consultations and opioid stewardship, which demonstrated that pharmacists can improve patient outcomes in the front lines of pain management.14 Pain CPSs have advanced knowledge of pharmacokinetics, pharmacodynamics, and therapeutics to promote safe and effective analgesic use, as well as to identify opioid use disorders. Evidence suggests that pharmacists’ presence on interdisciplinary pain teams improves outcomes by optimizing medication selection, improving adherence, and preventing AEs.8
Following the plan-do-study-act model for quality improvement (QI), this project hoped to expand current pain programs at the West Palm Beach VAMC (WPBVAMC) by evaluating the feasibility of an inpatient pain pharmacist consult service (IPPCS) at the 301-bed teaching facility, which includes 130 acute/intensive care and 120 nursing/domiciliary beds.15 Staff provide primary- and secondary-level care to veterans in 7 counties along Florida’s southeastern coast.
In 2009, the WPBVAMC PGY-2 Pain Management and Palliative Care Program became the VA’s first accredited pain pharmacy residency. Residents train with the Physical Medicine & Rehabilitation (PMR) and Chronic Pain Management departments, which provide outpatient services from 7 pain physicians, a pain psychologist, registered nurse, chiropractor, acupuncturist, physical/occupational therapy (PT/OT), and 3 pain/palliative care CPSs.
The WPBVAMC had established interdisciplinary outpatient chronic pain clinic (OCPC) physician- and pharmacist-run services along with a pain CPS electronic consult (e-consult) program. However, no formal mechanisms for inpatient pain consultations existed. Prior to the IPPCS outlined in this study, OCPC practitioners, including 2 pain CPSs, managed impromptu inpatient pain issues as “curbside consultations” along with usual day-to-day clinic duties.
The OCPC, PMR, and Clinical Pharmacy administration recognized the need for more clinical support to manage complex analgesic issues in inpatient veterans, as these patients often have acute pain with underlying chronic pain syndromes. In a national survey, veterans were significantly more likely than were nonveterans to report painful health conditions (65.5% vs 56.4%) and to classify their pain as severe (9.1% vs 6.3%).16 The WPBVAMC administration concluded that an IPPCS would offer a more efficient means of handling such cases. The IPPCS would formally streamline inpatient pain consults, enabling CPSs to thoroughly evaluate pain-related issues to propose evidence-based recommendations.
The primary objective of this QI project was to assess the IPPCS implementation as part of multimodal care to satisfy unmet patient care needs at the WPBVAMC. Secondary objectives for program feasibility included identifying the volume and type of pain consults, categorizing pharmacist interventions, classifying providers’ satisfaction, and determining types of responses to pharmacists’ medication recommendations.
Methods
This QI project ascertained the feasibility of the IPPCS by evaluating all consults obtained during the pilot period from November 2, 2015 through May 6, 2016. The IPPCS was accessible Monday through Friday during normal business hours. Goal turnaround time for consult completion was 24 to 72 hours, given the lack of coverage on holidays and weekends. The target population included veterans hospitalized at the WPBVAMC inpatient ward or nursing home with uncontrolled pain on IV and/or oral analgesic medications. All IPPCS consults submitted during the pilot period were included in the sample.
The WPBVAMC Scientific Advisory Committee (SAC) approved this QI program prior to initiation. Following supervisory support from the OCPC, PMR, and Clinical Pharmacy departments, hospital technologists assisted in creating a consult link in the Computerized Patient Record System (CPRS), which allowed providers to submit IPPCS requests for specific patients efficiently. Consults were categorized as postoperative pain, acute or chronic pain, malignant pain, or end-of-life pain. Inpatient providers could enter requests for assistance with 1 or more of the following: opioid dose conversions, opioid taper/titration schedules, general opioid treatment recommendations, or nonopioid/adjuvant recommendations.
The Medical Records Committee approved a customized CPRS subjective-objective-assessment-recommendations (SOAR) note template, which helped standardize the pain CPS documentation. To promote consult requests and interdisciplinary collaboration, inpatient clinicians received education about the IPPCS at respective meetings (eg, General Medicine staff and Clinical Pharmacy meetings).
All CPSs involved in this project were residency-trained in direct patient care, including pain and palliative care, and maintained national board certification as pharmacotherapy specialists. Their role included reviewing patients’ electronic medical records, conducting face-to-face pain assessments, completing opioid risk assessments, evaluating analgesic regimen appropriateness, reviewing medication adherence, completing pain medication reconciliation, querying the Florida Prescription Drug Monitoring Program (PDMP), interpreting urine drug testing (UDT), and delivering provider/patient/caregiver education. Parameters used to determine the appropriateness of analgesic regimens included, but were not limited to:
- Use of oral instead of IV medications if oral dosing was feasible/possible/appropriate;
- Dose and adjustments per renal/hepatic function;
- Adequate treatment duration and titration;
- No therapeutic drug class duplications;
- Medication tolerability (eg, allergies, AEs, drug interactions); and
- Opioid risk assessment per Opioid Risk Tool (ORT) score and medical history.
Consulting providers clarified patients’ pain diagnoses prior to pharmacy consultations.
After face-to-face patient interviews, the inpatient pain CPS prepared pain management recommendations, including nonopioid/adjuvant pain medications and/or opioid dose adjustments. The IPPCS also collaborated with pain physicians for intervention procedures, nonpharmacologic recommendations, and for more complex patients who may have required additional imaging or detailed physical evaluations. Pain CPSs documented CPRS notes with the SOAR template and discussed all recommendations with appropriate inpatient teams.
Respective providers received questionnaires hosted on SurveyMonkey.com (San Mateo, CA) to gauge their satisfaction with the IPPCS at the end of the pilot period, which helped determine program utility. Data collected for the pilot included patient demographics; patient admission diagnosis; consulting inpatient service; type of pain and reason for IPPCS request; total morphine equivalent daily dose (MEDD); pertinent past medical history (ie, sleep apnea, psychiatric comorbidities, or substance use disorder [SUD]); ORT score; patients’ reported average pain severity on the 10-point Numeric Pain Rating Scale (NPRS); number of requests submitted; medications discontinued, initiated, or dose increased/decreased; and number of pharmacist recommendations, including number accepted by providers. The ORT is a 5-item questionnaire used to determine risk of opioid-related aberrant behaviors in adults to help discriminate between low-risk and high-risk individuals (Table 1).17
Descriptive statistics were used to evaluate the results, and a Likert scale was used to evaluate responses the from provider satisfaction questionnaires. The IPPCS collected and organized the data using Microsoft Excel (Redmond, WA).
Results
By the end of the pilot period in May 2016, the IPPCS had received 100 consult requests and completed 81% (Figure). The remaining consults included 11% forwarded to other disciplines. The service discontinued 8% of the requests, given patients’ hospital discharge prior to IPPCS review.
Baseline patient data are outlined in Tables 2, 3, 4, and 5. For each of the 100 consults, providers could select more than 1 reason for the request. The nonopioid/adjuvant treatment recommendations were the most common at 49% (62/128). Patients could have more than 1 pertinent medical comorbidity, with psychiatric illnesses the most prevalent at 68% (133/197). A mean ORT score of 8.1 indicated a high risk for opioid-related aberrant behavior. Overall, half the patients (37/73) were high risk, 25% (18/73) were medium risk (ORT 4-7), and 25% (18/73) were low risk (ORT 0-3). Patients’ reported average pain was often severe (NPRS 7-10) at 54% (40/74) or moderate (NPRS 4-6) at 39% (29/74).
The IPPCS recommended various medications for initiation, discontinuation, or dosage changes (Table 6). For example, the IPPCS recommended initiation of topical agents in 38% (48/128) of cases. The inpatient pain CPS offered opioid initiation in 17% (22/128) of cases, with immediate-release oral morphine as the most predominant. Notably, opioids remained the most common medications suggested for discontinuation at 74% (38/51), including 47% (18/38) for IV hydromorphone. Dose titration recommendations mainly included anticonvulsants at 33% (16/48), and most dose reductions involved opioids at 78% (7/9), namely, oxycodone/acetaminophen and IV hydromorphone.
Providers accepted 76% (179/234) of IPPCS pharmacist medication recommendations. The most common included initiation/optimization of adjuvant therapy (eg, anticonvulsants, serotonin-norepinephrine reuptake inhibitors [SNRIs], and topical agents) at 46% (83/179), followed by opioid discontinuation (eg, IV hydromorphone) at 22% (40/179). Although this project primarily tracked medication interventions, examples of accepted nonpharmacologic recommendations included UDT and referrals to other programs (eg, pain psychology, substance abuse, mental health, acupuncture, chiropractor, PT/OT/PMR, and interventional pain management), which received support from each respective discipline. Declined pharmacologic recommendations mostly included topicals (eg, lidocaine, trolamine, and capsaicin cream) at 35% (19/55). However, findings also show that providers implemented 100% of medication recommendations in whole for 58% (47/81) of consults.
Likert scale satisfaction questionnaires offered insight into providers’ perception of the IPPCS (Table 7). One provider felt “neutral” about the consult submission process, given the time needed to complete the CPRS requests, but all other providers “agreed” or “strongly agreed” that the IPPCS was user-friendly. More importantly, 100% (15/15) “agreed” or “strongly agreed” that the inpatient pain CPS answered consults promptly with reasonable, evidence-based recommendations. All respondents declared that they would recommend the IPPCS to other practitioners and felt comfortable entering requests for future patients.
Discussion
The IPPCS achieved a total of 100 consults, which served as the sample for the pilot program. With support from the OCPC, PMR, and Clinical Pharmacy Department Administration, the IPPCS operated from November 2, 2015 through May 6, 2016. Results suggested that this new service could assist in managing inpatient pain issues in collaboration with inpatient multidisciplinary teams.
The most popular reason for IPPCS consults was acute on chronic pain. Given national efforts to improve opioid prescribing through the VA Opioid Safety Initiative (OSI) and the 2016 CDC Guideline for Opioid Prescribing, most pain consults requested nonopioid/adjuvant recommendations.18,19 Despite the wide MEDD range in this sample, the median/mean generally remained below recommended limits per current guidelines.18,19 However, the small sample size and lack of patient diversity (mostly white male veterans) limited the generalizability to non-VA medical facilities. Veterans often experienced both chronic pain and psychiatric disturbances, which explained the significant number of underlying mental health comorbidities observed. This affirmed the close interrelationship between pain and psychiatric issues described in the literature.20
Providers’ acceptance of pharmacologic and nonpharmacologic treatment modalities supported a comprehensive, multidisciplinary, and biopsychosocial approach to effective analgesic management. During this pilot, the most common pharmacy medication recommendations, namely, discontinuation of inappropriate opioids (eg, IV hydromorphone in patients who are controlled on and/or able to tolerate oral medications) and dose titration of adjuvant medications (eg, anticonvulsants for neuropathic pain), revealed that the IPPCS provided needed expertise and alternatives for complex pain patients. The IPPCS was well received, as inpatient providers accepted and implemented a large proportion of pharmacist recommendations. Despite risks of bias with a nonvalidated questionnaire, providers offered positive feedback. In the future, distributing satisfaction evaluations to patients also would provide more insight into how others perceived the IPPCS.
Limitations
Reasons for unaccepted recommendations included perceived limited effectiveness and/or feasibility of topical agents for acute pain, as providers seemed to favor systemic therapy for supposedly more immediate analgesia. Prescriber preference may explain why inpatient teams sometimes declined adjuvant therapy recommendations. However, the 2016 American Pain Society Guidelines on the Management of Postoperative Pain support a multimodal approach and confirm that adjuvants can reduce patients’ opioid requirements.21
Consulting teams did not execute some opioid recommendations, which may be due to various factors, including patient-related or provider-related factors in the inpatient vs outpatient setting. Lack of retrospective analysis for comparison of results pre- and post-IPPCS implementation also limited the outcomes. However, this project was piloted as a QI initiative after providers identified significant needs for inpatient pain management at the WPBVAMC. No retrospective analysis was undertaken, as this project analyzed only responses during the pilot program.
Other obstacles of the IPPCS included request appropriateness and triaging. The inpatient pain CPS deferred management of some consults to other disciplines (eg, gastroenterology) for more appropriate care. The IPPCS deferred certain cases of acute pancreatic pain or generalized abdominal pain for further workup to address patients’ underlying issues. The inpatient pain CPS relayed pertinent information regarding appropriate consults to inpatient teams. In the future, developing more specific inclusion/exclusion criteria and delivering provider education about proper IPPCS requests may resolve this issue.
Challenges with pain consults from inpatient psychiatry stemmed from patients’ skepticism and unwillingness to accept nonopioid/adjuvant therapies. Additionally, comorbid psychiatric disorders are often associated with SUDs and potentially opioid-related aberrant behavior. More than 40% of opioid-dependent individuals have comorbid psychiatric disorders, especially depression, anxiety, and bipolar disorder.22 Poorly-managed pain also drives SUD, as 80% of these patients illegally obtain prescription opioids. Thus, undertreatment of pain may push individuals to secure pain medications from illegal/illicit sources to achieve analgesia.23 Following pain physician consultation, the IPPCS continued inpatient opioids for 12% (10/81) of patients with a SUD history, including 5 with postoperative pain or other acute processes, since patients were kept in a monitored health care environment. The remaining included 4 with malignant pain and 1 with end-of-life pain. Overall, the IPPCS recommended that inpatient teams discharge these patients on as little opioids as possible, as well as to make referrals to substance abuse programs when necessary. Effective pain management of patients with aberrant behavior requires a comprehensive interdisciplinary team approach. To mitigate risk, effectively treat pain, and maintain patient safety, clinicians must recognize biologic, chemical, social, and psychiatric aspects of substance abuse.21
Another limitation during this pilot was an inability to promptly assess the impact of recommendations, given limited opportunities to reevaluate patients. In the future, more dedicated time for the inpatient pain CPSs to respond to consults may allow for better follow-up rather than initial consults only. Providers sometimes discharged patients within 24 hours of submitting consults as well, which left no time for the inpatient pain CPS consultation. However, the IPPCS forwarded appropriate requests to pain CPS e-consult services for chart review recommendations. Encouraging providers to submit consults earlier in patients’ hospital admissions may help reduce the number of incomplete IPPCS requests. Although expanding service hours would require more dedicated CPS staffing resources, it is another option for quicker consult completion and prompt follow-up.
Future Directions
Future efforts to expand this project include ensuring patient safety through judicious opioid use. Smooth transitions of care will particularly help to improve the quality of pain management. Current WPBVAMC policies stated that the primary care provider (PCP) alone must agree to continue prescribing outpatient analgesic medications, including opioids, prescribed from the OCPC once patients return to Primary Care. Continued provider education would ideally promote efficient utilization of the IPPCS and OCPC.
The pain pharmacy SOAR note template also could undergo additional edits/revisions, including the addition of opioid overdose risk assessments. For improved documentation and standardization, the template could autopopulate patient-specific information when the inpatient pain CPS chooses the designated note title. The IPPCS also hoped to streamline the CPRS consult link for more convenience and ease of use. Ultimately, the IPPCS wished to provide ongoing provider education, inpatient opioid therapy, and other topics upon request.
Conclusion
The IPPCS received positive provider feedback and collected 100 consults (averaging 4 per week) during the 6-month pilot QI project. Most consults were for acute or chronic pain and requested nonopioid/adjuvant recommendations. The new service intended to fulfill unmet needs at the WPBVAMC by expanding the facility’s current pain programs. Prescribers reported a high level of satisfaction and a willingness to not only refer other clinicians to the program, but also continue using the consult. Providers unanimously agreed that the pain CPS provided reasonable, evidence-based recommendations. This project demonstrated that the IPPCS can aid in meeting new demands amid the challenging landscape of pain practice.
The term pain refers to unpleasant sensory, emotional experiences associated with actual or potential tissue damage and is described in terms of such damage.1 Acute pain from neurophysiologic responses to noxious stimuli resolves upon tissue healing or stimuli removal (eg, 3-6 months); however, chronic pain lingers beyond the expected time course of the acute injury and repair process.1,2 Despite pain management advances, the under- or overtreatment of pain for different patient populations (eg, cancer and noncancer) remains an important concern.3,4
Hospitals have focused on optimizing inpatient pain management, because uncontrolled pain remains the most common reason for readmissions the first week postsurgery.5 The safe use of opioids (prescription, illicit synthetic, or heroin) poses major challenges and raises significant concerns. Rates of opioid-related hospital admissions have increased by 42% since 2009, and total overdose deaths reached a new high of 47,055 in 2014, including 28,647 (61%) from opioids.6
Opioids rank among the medications most often associated with serious adverse events (AEs), including respiratory depression and death.7 In response, the Joint Commission recommends patient assessments for opioid-related AEs, technology to monitor opioid prescribing, pharmacist consultation for opioid conversions and route of administration changes, provider education about risks of opioids, and risk screening tools for opioid-related oversedation and respiratory depression.7 Treatment guidelines strive to minimize the impact of acute pain by offering a scientific basis for practice, but evidence suggests a lack of suitable pain programs.7 Increases in opioid prescribing along with clinical guidelines and state laws recommending specialty pain service referrals for patients on high-dose opioids, have increased demand for competent pain clinicians.8
The expertise of a clinical pharmacy specialists (CPS) can help refine ineffective and potentially harmful pain medication therapy in complex patient cases. Existing literature outlines the various benefits of pharmacist participation in collaborative pain services for cancer and noncancer pain, as well as cases involving substance abuse.9-13 Various articles support pharmacists’ role as an educator and team member who can add valuable, trustworthy clinical knowledge that enhances clinical encounters and guides protocol/policy development.12,13 These efforts have improved patient satisfaction and encouraged physicians and nurses to proactively seek pharmacists’ advice for difficult cases.9
Frequent communication with interdisciplinary teams have helped considerably in establishing clinical pharmacy services that benefit patient care and offer sources of professional accomplishment.10,11 For example, pharmacists at Kaweah Delta Medical Center in Visalia, CA launched an innovative pain program that encompassed consultations and opioid stewardship, which demonstrated that pharmacists can improve patient outcomes in the front lines of pain management.14 Pain CPSs have advanced knowledge of pharmacokinetics, pharmacodynamics, and therapeutics to promote safe and effective analgesic use, as well as to identify opioid use disorders. Evidence suggests that pharmacists’ presence on interdisciplinary pain teams improves outcomes by optimizing medication selection, improving adherence, and preventing AEs.8
Following the plan-do-study-act model for quality improvement (QI), this project hoped to expand current pain programs at the West Palm Beach VAMC (WPBVAMC) by evaluating the feasibility of an inpatient pain pharmacist consult service (IPPCS) at the 301-bed teaching facility, which includes 130 acute/intensive care and 120 nursing/domiciliary beds.15 Staff provide primary- and secondary-level care to veterans in 7 counties along Florida’s southeastern coast.
In 2009, the WPBVAMC PGY-2 Pain Management and Palliative Care Program became the VA’s first accredited pain pharmacy residency. Residents train with the Physical Medicine & Rehabilitation (PMR) and Chronic Pain Management departments, which provide outpatient services from 7 pain physicians, a pain psychologist, registered nurse, chiropractor, acupuncturist, physical/occupational therapy (PT/OT), and 3 pain/palliative care CPSs.
The WPBVAMC had established interdisciplinary outpatient chronic pain clinic (OCPC) physician- and pharmacist-run services along with a pain CPS electronic consult (e-consult) program. However, no formal mechanisms for inpatient pain consultations existed. Prior to the IPPCS outlined in this study, OCPC practitioners, including 2 pain CPSs, managed impromptu inpatient pain issues as “curbside consultations” along with usual day-to-day clinic duties.
The OCPC, PMR, and Clinical Pharmacy administration recognized the need for more clinical support to manage complex analgesic issues in inpatient veterans, as these patients often have acute pain with underlying chronic pain syndromes. In a national survey, veterans were significantly more likely than were nonveterans to report painful health conditions (65.5% vs 56.4%) and to classify their pain as severe (9.1% vs 6.3%).16 The WPBVAMC administration concluded that an IPPCS would offer a more efficient means of handling such cases. The IPPCS would formally streamline inpatient pain consults, enabling CPSs to thoroughly evaluate pain-related issues to propose evidence-based recommendations.
The primary objective of this QI project was to assess the IPPCS implementation as part of multimodal care to satisfy unmet patient care needs at the WPBVAMC. Secondary objectives for program feasibility included identifying the volume and type of pain consults, categorizing pharmacist interventions, classifying providers’ satisfaction, and determining types of responses to pharmacists’ medication recommendations.
Methods
This QI project ascertained the feasibility of the IPPCS by evaluating all consults obtained during the pilot period from November 2, 2015 through May 6, 2016. The IPPCS was accessible Monday through Friday during normal business hours. Goal turnaround time for consult completion was 24 to 72 hours, given the lack of coverage on holidays and weekends. The target population included veterans hospitalized at the WPBVAMC inpatient ward or nursing home with uncontrolled pain on IV and/or oral analgesic medications. All IPPCS consults submitted during the pilot period were included in the sample.
The WPBVAMC Scientific Advisory Committee (SAC) approved this QI program prior to initiation. Following supervisory support from the OCPC, PMR, and Clinical Pharmacy departments, hospital technologists assisted in creating a consult link in the Computerized Patient Record System (CPRS), which allowed providers to submit IPPCS requests for specific patients efficiently. Consults were categorized as postoperative pain, acute or chronic pain, malignant pain, or end-of-life pain. Inpatient providers could enter requests for assistance with 1 or more of the following: opioid dose conversions, opioid taper/titration schedules, general opioid treatment recommendations, or nonopioid/adjuvant recommendations.
The Medical Records Committee approved a customized CPRS subjective-objective-assessment-recommendations (SOAR) note template, which helped standardize the pain CPS documentation. To promote consult requests and interdisciplinary collaboration, inpatient clinicians received education about the IPPCS at respective meetings (eg, General Medicine staff and Clinical Pharmacy meetings).
All CPSs involved in this project were residency-trained in direct patient care, including pain and palliative care, and maintained national board certification as pharmacotherapy specialists. Their role included reviewing patients’ electronic medical records, conducting face-to-face pain assessments, completing opioid risk assessments, evaluating analgesic regimen appropriateness, reviewing medication adherence, completing pain medication reconciliation, querying the Florida Prescription Drug Monitoring Program (PDMP), interpreting urine drug testing (UDT), and delivering provider/patient/caregiver education. Parameters used to determine the appropriateness of analgesic regimens included, but were not limited to:
- Use of oral instead of IV medications if oral dosing was feasible/possible/appropriate;
- Dose and adjustments per renal/hepatic function;
- Adequate treatment duration and titration;
- No therapeutic drug class duplications;
- Medication tolerability (eg, allergies, AEs, drug interactions); and
- Opioid risk assessment per Opioid Risk Tool (ORT) score and medical history.
Consulting providers clarified patients’ pain diagnoses prior to pharmacy consultations.
After face-to-face patient interviews, the inpatient pain CPS prepared pain management recommendations, including nonopioid/adjuvant pain medications and/or opioid dose adjustments. The IPPCS also collaborated with pain physicians for intervention procedures, nonpharmacologic recommendations, and for more complex patients who may have required additional imaging or detailed physical evaluations. Pain CPSs documented CPRS notes with the SOAR template and discussed all recommendations with appropriate inpatient teams.
Respective providers received questionnaires hosted on SurveyMonkey.com (San Mateo, CA) to gauge their satisfaction with the IPPCS at the end of the pilot period, which helped determine program utility. Data collected for the pilot included patient demographics; patient admission diagnosis; consulting inpatient service; type of pain and reason for IPPCS request; total morphine equivalent daily dose (MEDD); pertinent past medical history (ie, sleep apnea, psychiatric comorbidities, or substance use disorder [SUD]); ORT score; patients’ reported average pain severity on the 10-point Numeric Pain Rating Scale (NPRS); number of requests submitted; medications discontinued, initiated, or dose increased/decreased; and number of pharmacist recommendations, including number accepted by providers. The ORT is a 5-item questionnaire used to determine risk of opioid-related aberrant behaviors in adults to help discriminate between low-risk and high-risk individuals (Table 1).17
Descriptive statistics were used to evaluate the results, and a Likert scale was used to evaluate responses the from provider satisfaction questionnaires. The IPPCS collected and organized the data using Microsoft Excel (Redmond, WA).
Results
By the end of the pilot period in May 2016, the IPPCS had received 100 consult requests and completed 81% (Figure). The remaining consults included 11% forwarded to other disciplines. The service discontinued 8% of the requests, given patients’ hospital discharge prior to IPPCS review.
Baseline patient data are outlined in Tables 2, 3, 4, and 5. For each of the 100 consults, providers could select more than 1 reason for the request. The nonopioid/adjuvant treatment recommendations were the most common at 49% (62/128). Patients could have more than 1 pertinent medical comorbidity, with psychiatric illnesses the most prevalent at 68% (133/197). A mean ORT score of 8.1 indicated a high risk for opioid-related aberrant behavior. Overall, half the patients (37/73) were high risk, 25% (18/73) were medium risk (ORT 4-7), and 25% (18/73) were low risk (ORT 0-3). Patients’ reported average pain was often severe (NPRS 7-10) at 54% (40/74) or moderate (NPRS 4-6) at 39% (29/74).
The IPPCS recommended various medications for initiation, discontinuation, or dosage changes (Table 6). For example, the IPPCS recommended initiation of topical agents in 38% (48/128) of cases. The inpatient pain CPS offered opioid initiation in 17% (22/128) of cases, with immediate-release oral morphine as the most predominant. Notably, opioids remained the most common medications suggested for discontinuation at 74% (38/51), including 47% (18/38) for IV hydromorphone. Dose titration recommendations mainly included anticonvulsants at 33% (16/48), and most dose reductions involved opioids at 78% (7/9), namely, oxycodone/acetaminophen and IV hydromorphone.
Providers accepted 76% (179/234) of IPPCS pharmacist medication recommendations. The most common included initiation/optimization of adjuvant therapy (eg, anticonvulsants, serotonin-norepinephrine reuptake inhibitors [SNRIs], and topical agents) at 46% (83/179), followed by opioid discontinuation (eg, IV hydromorphone) at 22% (40/179). Although this project primarily tracked medication interventions, examples of accepted nonpharmacologic recommendations included UDT and referrals to other programs (eg, pain psychology, substance abuse, mental health, acupuncture, chiropractor, PT/OT/PMR, and interventional pain management), which received support from each respective discipline. Declined pharmacologic recommendations mostly included topicals (eg, lidocaine, trolamine, and capsaicin cream) at 35% (19/55). However, findings also show that providers implemented 100% of medication recommendations in whole for 58% (47/81) of consults.
Likert scale satisfaction questionnaires offered insight into providers’ perception of the IPPCS (Table 7). One provider felt “neutral” about the consult submission process, given the time needed to complete the CPRS requests, but all other providers “agreed” or “strongly agreed” that the IPPCS was user-friendly. More importantly, 100% (15/15) “agreed” or “strongly agreed” that the inpatient pain CPS answered consults promptly with reasonable, evidence-based recommendations. All respondents declared that they would recommend the IPPCS to other practitioners and felt comfortable entering requests for future patients.
Discussion
The IPPCS achieved a total of 100 consults, which served as the sample for the pilot program. With support from the OCPC, PMR, and Clinical Pharmacy Department Administration, the IPPCS operated from November 2, 2015 through May 6, 2016. Results suggested that this new service could assist in managing inpatient pain issues in collaboration with inpatient multidisciplinary teams.
The most popular reason for IPPCS consults was acute on chronic pain. Given national efforts to improve opioid prescribing through the VA Opioid Safety Initiative (OSI) and the 2016 CDC Guideline for Opioid Prescribing, most pain consults requested nonopioid/adjuvant recommendations.18,19 Despite the wide MEDD range in this sample, the median/mean generally remained below recommended limits per current guidelines.18,19 However, the small sample size and lack of patient diversity (mostly white male veterans) limited the generalizability to non-VA medical facilities. Veterans often experienced both chronic pain and psychiatric disturbances, which explained the significant number of underlying mental health comorbidities observed. This affirmed the close interrelationship between pain and psychiatric issues described in the literature.20
Providers’ acceptance of pharmacologic and nonpharmacologic treatment modalities supported a comprehensive, multidisciplinary, and biopsychosocial approach to effective analgesic management. During this pilot, the most common pharmacy medication recommendations, namely, discontinuation of inappropriate opioids (eg, IV hydromorphone in patients who are controlled on and/or able to tolerate oral medications) and dose titration of adjuvant medications (eg, anticonvulsants for neuropathic pain), revealed that the IPPCS provided needed expertise and alternatives for complex pain patients. The IPPCS was well received, as inpatient providers accepted and implemented a large proportion of pharmacist recommendations. Despite risks of bias with a nonvalidated questionnaire, providers offered positive feedback. In the future, distributing satisfaction evaluations to patients also would provide more insight into how others perceived the IPPCS.
Limitations
Reasons for unaccepted recommendations included perceived limited effectiveness and/or feasibility of topical agents for acute pain, as providers seemed to favor systemic therapy for supposedly more immediate analgesia. Prescriber preference may explain why inpatient teams sometimes declined adjuvant therapy recommendations. However, the 2016 American Pain Society Guidelines on the Management of Postoperative Pain support a multimodal approach and confirm that adjuvants can reduce patients’ opioid requirements.21
Consulting teams did not execute some opioid recommendations, which may be due to various factors, including patient-related or provider-related factors in the inpatient vs outpatient setting. Lack of retrospective analysis for comparison of results pre- and post-IPPCS implementation also limited the outcomes. However, this project was piloted as a QI initiative after providers identified significant needs for inpatient pain management at the WPBVAMC. No retrospective analysis was undertaken, as this project analyzed only responses during the pilot program.
Other obstacles of the IPPCS included request appropriateness and triaging. The inpatient pain CPS deferred management of some consults to other disciplines (eg, gastroenterology) for more appropriate care. The IPPCS deferred certain cases of acute pancreatic pain or generalized abdominal pain for further workup to address patients’ underlying issues. The inpatient pain CPS relayed pertinent information regarding appropriate consults to inpatient teams. In the future, developing more specific inclusion/exclusion criteria and delivering provider education about proper IPPCS requests may resolve this issue.
Challenges with pain consults from inpatient psychiatry stemmed from patients’ skepticism and unwillingness to accept nonopioid/adjuvant therapies. Additionally, comorbid psychiatric disorders are often associated with SUDs and potentially opioid-related aberrant behavior. More than 40% of opioid-dependent individuals have comorbid psychiatric disorders, especially depression, anxiety, and bipolar disorder.22 Poorly-managed pain also drives SUD, as 80% of these patients illegally obtain prescription opioids. Thus, undertreatment of pain may push individuals to secure pain medications from illegal/illicit sources to achieve analgesia.23 Following pain physician consultation, the IPPCS continued inpatient opioids for 12% (10/81) of patients with a SUD history, including 5 with postoperative pain or other acute processes, since patients were kept in a monitored health care environment. The remaining included 4 with malignant pain and 1 with end-of-life pain. Overall, the IPPCS recommended that inpatient teams discharge these patients on as little opioids as possible, as well as to make referrals to substance abuse programs when necessary. Effective pain management of patients with aberrant behavior requires a comprehensive interdisciplinary team approach. To mitigate risk, effectively treat pain, and maintain patient safety, clinicians must recognize biologic, chemical, social, and psychiatric aspects of substance abuse.21
Another limitation during this pilot was an inability to promptly assess the impact of recommendations, given limited opportunities to reevaluate patients. In the future, more dedicated time for the inpatient pain CPSs to respond to consults may allow for better follow-up rather than initial consults only. Providers sometimes discharged patients within 24 hours of submitting consults as well, which left no time for the inpatient pain CPS consultation. However, the IPPCS forwarded appropriate requests to pain CPS e-consult services for chart review recommendations. Encouraging providers to submit consults earlier in patients’ hospital admissions may help reduce the number of incomplete IPPCS requests. Although expanding service hours would require more dedicated CPS staffing resources, it is another option for quicker consult completion and prompt follow-up.
Future Directions
Future efforts to expand this project include ensuring patient safety through judicious opioid use. Smooth transitions of care will particularly help to improve the quality of pain management. Current WPBVAMC policies stated that the primary care provider (PCP) alone must agree to continue prescribing outpatient analgesic medications, including opioids, prescribed from the OCPC once patients return to Primary Care. Continued provider education would ideally promote efficient utilization of the IPPCS and OCPC.
The pain pharmacy SOAR note template also could undergo additional edits/revisions, including the addition of opioid overdose risk assessments. For improved documentation and standardization, the template could autopopulate patient-specific information when the inpatient pain CPS chooses the designated note title. The IPPCS also hoped to streamline the CPRS consult link for more convenience and ease of use. Ultimately, the IPPCS wished to provide ongoing provider education, inpatient opioid therapy, and other topics upon request.
Conclusion
The IPPCS received positive provider feedback and collected 100 consults (averaging 4 per week) during the 6-month pilot QI project. Most consults were for acute or chronic pain and requested nonopioid/adjuvant recommendations. The new service intended to fulfill unmet needs at the WPBVAMC by expanding the facility’s current pain programs. Prescribers reported a high level of satisfaction and a willingness to not only refer other clinicians to the program, but also continue using the consult. Providers unanimously agreed that the pain CPS provided reasonable, evidence-based recommendations. This project demonstrated that the IPPCS can aid in meeting new demands amid the challenging landscape of pain practice.
1. D’Arcy Y. Treating acute pain in the hospitalized patient. Nurse Pract. 2012;37(8):22-30.
2. Marks AD, Rodgers PE. Diagnosis and management of acute pain in the hospitalized patient. Am J Med. 2014;3(3):e396-e408.
3. Paice JA, Von Roenn JH. Under- or overtreatment of pain in the patient with cancer: how to achieve proper balance. J Clin Oncol. 2014;32(16):1721-1726.
4. Mafi JN, McCarthy EP, Davis RB, Landon BE. Worsening trends in the management and treatment of back pain. JAMA Intern Med. 2013;173(17):1573-1581.
5. Palomano RC, Rathmell JP, Krenzischek DA, Dunwoody CJ. Emerging trends and new approaches to acute pain management. J Perianesth Nurs. 2008;23(suppl 1):S43-S53.
6. U.S. Department of Health & Human Services, Office of the Surgeon General. Facing addiction in America: the surgeon general’s report on alcohol, drugs, and health, executive summary. https://addiction.surgeongeneral.gov/executive-summary.pdf. Published November 2016. Accessed November 1, 2017.
7. Bagian JP, Cohen M, Barnsteiner JH, et al. Safe use of opioids in hospitals. Sentinel Event Alert. 2012;49:1-5.
8. Atkinson TJ, Gulum AH, Forkum WG. The future of pain pharmacy: directed by need. Integrated Pharm Res Pract. 2016;2016(5):33-42.
9. Lothian ST, Fotis MA, Von Gutten CF, et al. Cancer pain management through a pharmacist-based analgesic dosing service. Am J Health Syst Pharm. 1999;56:1119-1125.
10. Lynn MA. Pharmacist interventions in pain management. Am J Health Syst Pharm. 2004;61(14):1487-1489.
11. Strickland JM, Huskey A, Brushwood DB. Pharmacist-physician collaboration in pain management practice. J Opioid Manag. 2007;3(6):295-301.
12. Fan T and Elgourt T. Pain management pharmacy service in a community hospital. Am J Health Syst Pharm. 2008;65(16):1560-1565.
13. Andrews LB, Bridgeman MB, Dalal KS, et al. Implementation of a pharmacist-directed pain management consultation service for hospitalised adults with a history of substance abuse. Int J Clin Pract. 2013;67(12):1342-1349.
14. Poirier RH, Brown CS, Garcia YT, Gann NY, Sandoval RA, McNulty JR. Implementation of a pharmacist directed pain management service in the inpatient setting. http://www.ashpadvantage.com/bestpractices/2014_papers/Kaweah-Delta.htm. Published 2014. Accessed November 1, 2017.
15. Langley GL, Moen R, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nded. San Francisco, CA: Jossey-Bass; 2009.
16. Nahin, RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254.
17. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med. 2005;6(6):432-442.
18. Nazario M. Opioid therapy risk management: the VA opioid safety and naloxone distribution initiatives. http://jfpsmeeting.pharmacist.com/sites/default/files/slides/Opioid%20Therapy%20Risk%20Management.pdf. Published October 15, 2015. Accessed November 1, 2017.
19. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. MMWR Rep. 2016;65(1):1-49.
20. Outcalt SD, Kroenke K, Krebs EE, et al. Chronic pain and comorbid mental health conditions: independent associations of posttraumatic stress disorder and depression with pain, disability, and quality of life. J Behav Med. 2015;38:535.
21. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
22. NIDA/SAMHSA Blending Initiative. https://www.drugabuse.gov/nidasamhsa-blending-initiative. Updated November 2015. Accessed November 1, 2017.
23. Alford DP, German JS, Samet JH, Cheng DM, Lloyd-Travaglini CA, Saitz R. Primary care patients with drug use report chronic pain and self-medicate with alcohol and other drugs. J Gen Intern Med. 2016;31(5):486-491.
1. D’Arcy Y. Treating acute pain in the hospitalized patient. Nurse Pract. 2012;37(8):22-30.
2. Marks AD, Rodgers PE. Diagnosis and management of acute pain in the hospitalized patient. Am J Med. 2014;3(3):e396-e408.
3. Paice JA, Von Roenn JH. Under- or overtreatment of pain in the patient with cancer: how to achieve proper balance. J Clin Oncol. 2014;32(16):1721-1726.
4. Mafi JN, McCarthy EP, Davis RB, Landon BE. Worsening trends in the management and treatment of back pain. JAMA Intern Med. 2013;173(17):1573-1581.
5. Palomano RC, Rathmell JP, Krenzischek DA, Dunwoody CJ. Emerging trends and new approaches to acute pain management. J Perianesth Nurs. 2008;23(suppl 1):S43-S53.
6. U.S. Department of Health & Human Services, Office of the Surgeon General. Facing addiction in America: the surgeon general’s report on alcohol, drugs, and health, executive summary. https://addiction.surgeongeneral.gov/executive-summary.pdf. Published November 2016. Accessed November 1, 2017.
7. Bagian JP, Cohen M, Barnsteiner JH, et al. Safe use of opioids in hospitals. Sentinel Event Alert. 2012;49:1-5.
8. Atkinson TJ, Gulum AH, Forkum WG. The future of pain pharmacy: directed by need. Integrated Pharm Res Pract. 2016;2016(5):33-42.
9. Lothian ST, Fotis MA, Von Gutten CF, et al. Cancer pain management through a pharmacist-based analgesic dosing service. Am J Health Syst Pharm. 1999;56:1119-1125.
10. Lynn MA. Pharmacist interventions in pain management. Am J Health Syst Pharm. 2004;61(14):1487-1489.
11. Strickland JM, Huskey A, Brushwood DB. Pharmacist-physician collaboration in pain management practice. J Opioid Manag. 2007;3(6):295-301.
12. Fan T and Elgourt T. Pain management pharmacy service in a community hospital. Am J Health Syst Pharm. 2008;65(16):1560-1565.
13. Andrews LB, Bridgeman MB, Dalal KS, et al. Implementation of a pharmacist-directed pain management consultation service for hospitalised adults with a history of substance abuse. Int J Clin Pract. 2013;67(12):1342-1349.
14. Poirier RH, Brown CS, Garcia YT, Gann NY, Sandoval RA, McNulty JR. Implementation of a pharmacist directed pain management service in the inpatient setting. http://www.ashpadvantage.com/bestpractices/2014_papers/Kaweah-Delta.htm. Published 2014. Accessed November 1, 2017.
15. Langley GL, Moen R, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2nded. San Francisco, CA: Jossey-Bass; 2009.
16. Nahin, RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254.
17. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med. 2005;6(6):432-442.
18. Nazario M. Opioid therapy risk management: the VA opioid safety and naloxone distribution initiatives. http://jfpsmeeting.pharmacist.com/sites/default/files/slides/Opioid%20Therapy%20Risk%20Management.pdf. Published October 15, 2015. Accessed November 1, 2017.
19. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain – United States, 2016. MMWR Rep. 2016;65(1):1-49.
20. Outcalt SD, Kroenke K, Krebs EE, et al. Chronic pain and comorbid mental health conditions: independent associations of posttraumatic stress disorder and depression with pain, disability, and quality of life. J Behav Med. 2015;38:535.
21. Chou R, Gordon DB, de Leon-Casasola OA, et al. Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17(2):131-157.
22. NIDA/SAMHSA Blending Initiative. https://www.drugabuse.gov/nidasamhsa-blending-initiative. Updated November 2015. Accessed November 1, 2017.
23. Alford DP, German JS, Samet JH, Cheng DM, Lloyd-Travaglini CA, Saitz R. Primary care patients with drug use report chronic pain and self-medicate with alcohol and other drugs. J Gen Intern Med. 2016;31(5):486-491.
Responding to the Opioid Crisis: An Indian Health Service Pharmacist-Led Pain Management Clinic
According to the National Academy of Medicine (NAM), about 100 million people live with chronic pain in the U.S.1 There is evidence for the use of opioids to treat acute pain lasting 12 weeks or less. However, high-quality studies that analyze the benefit and safety of long-term opioid therapy are not yet available.2 In 2013, 249 million opioid prescriptions were written, equivalent to about 1 prescription per adult living in the U.S.3
Between 1999 and 2008, nonmedical use of prescription pain killers in the American Indian and Alaska Native populations was 2 to 3 times the frequency found in the white and black populations, respectively.1 These clinically contradictory practices created an environment conducive to opioid abuse and overdose. According to the Centers for Disease Control and Prevention, 1 in 4 people on chronic opioid therapy struggle with addiction.4 In 2014, more than 14,000 people in the U.S. died from overdoses involving prescription opioids.
The Comprehensive Addiction and Recovery Act of 2016 authorized prescription drug monitoring programs (PMPs) and a task force to create optimal pain treatment practices.5 The American Pharmacists Association (APhA), a major proponent of this law, argued that pharmacists are an underutilized resource despite having valuable clinical knowledge in the initiation, monitoring, and discontinuation of opioids. Additionally, pharmacists are able to refer patients to nonpharmacologic forms of pain management and dispense naloxone for emergent opioid overdose reversal.
Former Surgeon General Vivek Murthy developed the Turn the Tide Rx campaign to curb and reverse the opioid crisis in the U.S.3 The turnthetiderx.org website offers a pledge for clinicians who agree to be educated about pain management. It encourages open communication among prescribers and contains guideline-based resources on assessing pain and addiction risk, appropriate opioid prescribing, and how to manage opioid overdose. As required by law for prescribers in most states, there are instructions on how to access and analyze PMP opioid usage. The website also provides fact sheets about opioid treatments, safe disposal of medications, and helpline information.
Local Opioid Misuse Initiatives
New Mexico (NM) has one of the nation’s highest opioid and heroin overdose death rates.6 In January 2015, the U.S. Attorney’s Office and the University of New Mexico Health and Sciences Center partnered to launch the Heroin and Opioid Prevention and Education Initiative. The partners recognized that joint action between medical sciences and law enforcement was crucial to address the consequences of the opioid epidemic on public health and safety.
In 2017, the IHS established the National Committee on Heroin, Opioids, and Pain Efforts. Comprised of a variety of pharmacy and other subject-matter experts, this committee has a multipronged strategy to address the opioid epidemic from training to expanding medication-assisted treatment.7
Pharmacists provide clinical services in a variety of interdisciplinary ambulatory care clinics at Gallup Indian Medical Center (GIMC) in NM. The GIMC is located outside reservation boundaries but is centrally located to serve Navajo, Zuni, and a variety of other native populations. Pharmacist-run clinics include diabetes mellitus, anticoagulation, asthma, anemia, infectious diseases, and chronic pain. At GIMC, pain management pharmacists use a collective approach to curb opioid misuse. This article describes the establishment and impact of a pharmacist-led pain management clinic (PMC) at GIMC.
Pain Management Clinic
The understaffed urgent care clinic (UCC), emergency department (ED), primary care, and specialty practices plus a growing burden of complicated pain patients incentivized the development of the GIMC PMC. Under a collaborative practice agreement, pain management pharmacists were tasked with assessing, treating, and controlling noncancer chronic pain while improving quality of care and patient satisfaction (eAppendix 1, available at www.fedprac.com). The PMC goal was to improve functionality and pain scores and to reduce patient visits to the UCC and ED.
Originally, the PMC only performed medication titration for patients. In 2012, a former pharmacy resident became the PMC coordinator and has since helped to transform and expand its services. Currently, the coordinator dedicates about 20 hours per week managing pain patients in various capacities. Over time, other pain management pharmacists joined the PMC and support activities for 5 to 10 hours per week. There are now 4 pain management pharmacists who rotate through the PMC.
Pain management clinic visits generally are held once weekly for 3 hours and are occasionally expanded to full days based on patient schedule load. The initial 2 PMC appointments for each patient are 1 hour and are held within a 2-week period. Subsequent visits are each 30 minutes at 1 to 2 month intervals, depending on patient pain level and medication titration requirements. This standardized follow-up ensures new patients receive the close monitoring of slow dosage titrations necessary to achieve maximum therapeutic benefit.
Referrals
Patients only are admitted into the PMC via consults from primary care providers (PCPs). The consultations generally involve patients with complicated medical histories or who require complex therapies. The patients are contacted by telephone prior to scheduling. If a patient is not interested in PMC services, the consult is amended and the PCP is notified. This policy has been newly implemented to reduce the no-show rates. Pain management pharmacists then conduct PMC visits. The PMC activities are reported to the Pharmacy and Therapeutics committee annually. The PMC pharmacists also are available for telephone consultations from any internal hospital system department during weekday business hours.
The PMC patient population is fluid. New patients are accepted and clinically stable patients are discharged from PMC on a regular basis. The stable patients are released back to the PCP for further follow-up. Patients are considered stable if they have reached pain-related goals, are on maintenance doses of opioids, and/or are regularly participating in applicable interventional and referred therapies. If a patient requires reentry into PMC care, a new referral can be placed.
Interventional Programs
Since its inception, PMC has been a referral service to interventional programs. Physicians in the family medicine clinic provide twice weekly pain/palliative care clinic visits. These PCPs typically perform trigger point injections with lidocaine to relax muscles, which may disrupt nerve fibers. Ideally, this treatment reduces the use of opioids, non-steroidal anti-inflammatory drugs (NSAIDs), steroids, and epidural medications. Three acupuncturists were hired, which has reduced scheduling bottlenecks and wait times for patients to return for follow-up, especially in a treatment modality requiring frequent visits for effectiveness.
Pain Committee
As a part of an expansion of pain management services, GIMC established a pain committee (PC). The PC includes the GIMC medical director, PMC coordinator, pain management pharmacists, palliative care providers, PCPs, and specialty care providers. The PC created a detailed policy and procedures on management of chronic non-cancer pain for the Gallup Service Unit (eAppendix 2, available at www.fedprac.com). The PC offers guidance and completes consultations, performs internal review of prescribing patterns, and provides an appeals process for patients who have broken pain agreements. Physician and administrative champions have been instrumental to ensure proper pain management at GIMC.
Many VA facilities have deployed pain management clinics. At the VA Boston Healthcare System (VABHS), a pain management center is staffed by a multidisciplinary team that consists of anesthesiologists, neurologists, psychiatrists, nurses, and pharmacists.8 This pain clinic operates multiple days per week to accommodate demand, and patients are followed at least once a month. The VABHS often synchronized clinic visit dates with medication refill dates. Pharmacists offer an e-consult pain service to provide immediate recommendations to PCPs to bridge those patients awaiting appointments with pain clinic specialists at some VA facilities in Florida.9 Insufficient funding has prevented GIMC from increased PMC clinic hours.
Clinic Scope
Currently, the PMC sees the majority of PC cases. Pain management pharmacists are selected to conduct pain management visits based on interest and competency. Qualifications to work as a PMC pharmacist include on-the-job training, at least 6 annual pain management continuing medical education (CME) credits, participation in the NM naloxone training webinar, and completion of the physical assessment portion of the NM pharmacist clinician training course. It is highly recommended that pharmacists attend the PAINWeek conference in Las Vegas, Nevada, to obtain the necessary CME credits. In addition, pharmacists are requested to obtain the IHS National Clinical Pharmacy Specialist (NCPS) qualification within a year of practice.
Pain management pharmacists in the PMC review the indications for pain management, monitor medication therapy and adherence, adjust doses, manage adverse effects, study trends in pain and mood screening tools, and assess changes in functionality. These pharmacists are able to prescribe, discontinue, or titrate noncontrolled substance adjunctive therapies without a PCP cosignature. Adjunctive medication therapies can be NSAIDs, anticonvulsants, neuropathic pain relievers, muscle relaxers, and topical analgesics.
If adjustments to controlled substances are warranted, pain management pharmacists present the case to the PCP via electronic health record (EHR) notification or telephone conversation. These pharmacists ensure hard copies of controlled substance prescriptions are retrieved and provide refill coordination if assistance is requested by the patient. Pharmacists provide 28-day (not 30-day) prescriptions for opioid and controlled substance prescriptions to reduce weekend refill requests. Pain management pharmacists also order a variety of laboratory tests (eg, liver and renal function tests, and complete blood counts) related to the safe use of medications. If a patient is deemed unfit for PMC management, such as due to pain agreement violations, the PMC coordinator formally presents the case during PC meetings.
The PMC often recommends a multitude of nonpharmacologic treatments, including ice, hot rice socks, an anti-inflammatory diet, massage therapy, tennis ball massage for muscle tension and pinched nerves, chair exercises, transcutaneous electrical nerve stimulation therapy, aquatic therapy, and distraction therapy. Pain management pharmacists also can coordinate referrals to specialists and interventional therapies (eg, physical therapy; occupational therapy; acupuncture; trigger point injections; podiatry; orthopedics; ear, nose and throat; and diabetes mellitus).
Pain management pharmacists use a variety of established tools in the pain management clinic. The PMC EHR template and interview process are consistent with the universal precautions approach to unified pain management.10 Many of the questionnaires, tools, and laboratory tests are repeated periodically based on PMC policy and patient-specific need. These tools include a controlled substance pain agreement, consent for chronic opioid therapy, Opioid Risk Tool (ORT), Current Opioid Misuse Measure (COMM) for opioid abuse risk assessment, and the Patient Health Questionnaire (PHQ-9) for concurrent depression (Table and eAppendices 1, 3, and 4, available at www.fedprac.com). The ORT recently was added to the patient assessment packet to provide a stronger assessment of opioid misuse and abuse risk. Patient goals also are discussed with an emphasis on realistic changes, the level of control that would satisfy the patient and is feasible, what activities of daily living or hobbies the patient would like to regain, and what relationships the patient would like to improve.
PMC Patients
New patients are required to complete urine drug tests (UDTs), which can be performed in house or sent out if specific drug levels are required. Federally, marijuana is an illicit substance and is not prescribed or dispensed. Unpredictable effects of traditional medicine on the UDT have been observed. If a UDT shows positive for illicit substances, a discussion with the patient on toxicity and risks is initiated allowing them to choose to continue to use other substances for pain or use only the pain medication(s) appropriately prescribed by GIMC providers. Patients can be deemed ineligible for PMC management if they do not discontinue the use of illicit substances.
Health care providers and pharmacists involved in the prescribing or dispensing of controlled substances also complete a review of the patient’s PMP profile. It is mandatory in NM to complete PMP surveillance prior to prescribing controlled substances in quantities greater than 12 units within a 72-hour period and every 3 months for refills of chronic opioids.11 The PMP Interconnect service allows registered providers in NM to search for controlled substance usage in 25 states. All but 1 state in the U.S. has a PMP in development or in place. Federal providers who do not have a NM professional license also may apply for a NM PMP login to take advantage of the PMP Interconnect service. If the patient’s PMP is negative for prescribed opioids or positive for nonprescribed substances, the providers will conduct more research to reassess the risks and benefits of ongoing opioid therapy.
Random pill counts for tracked patients also have been incorporated into the PMC policy. These pill counts can be requested regardless of clinic appointment dates and can show whether patients are taking too many pills or diverting pills. Pill counts also may show that the patient may not need as many tablets per prescription if they consistently have more than expected based on dosing frequency. If a patient has adhered to prescribing recommendations, he or she may be allowed an early refill of the chronic opioid to cover them during a vacation or other unexpected event. However, if the pill counts are not consistent with instructions, then prescribing may be restricted to a 5-day, 7-day, or 14-day supply only. If patients do not present for pill count within 24 hours of the request, they can have their opioid use privileges at GIMC revoked as consented in the pain agreement. Patients are educated on proper storage and security of opioids medication at home and funding for lock boxes is expected in the near future. Patients may be referred to other services if opioid-use disorders are identified and confirmed.
Other administrative components of the pain management pharmacist responsibilities include clinical chart reviews before appointments or telephone consultations, sending appointment letters, contacting patients about and conducting random pill counts, and documenting PMC visit notes and updating flowsheets (eAppendices 4 and 5, available at www.fedprac.com). Currently under development is an EHR function that can quickly provide a summary of a patient’s pain management without a tedious search through the chart. Pain management pharmacists regularly instruct pharmacists and providers on changes in regulatory requirements of pain management as it pertains to prescription fills or clinical indications.
Ancillary Pharmacy Services
All pain patients on opioids therapies receive an annual pain evaluation. This evaluation provides a holistic description of the patient’s pain, a second opinion, and a collective review of the safety and efficacy of treatment. Components of the pain evaluation include review of medication toxicities and adverse effects, reconciliation of the treatment with current pain diagnosis and intensity, and coordination of opioid tapering schedules. Physical assessments and pill counts also are performed. Issues that have been uncovered include incidences of opioid-induced hyperalgesia and patients filling opioids prescriptions without taking the medications because they did not want to tell the provider the medication did not work.
Naloxone overdose prevention training and dispensing has been critical for ensuring maximum safety and life-saving methods in the realm of opioid therapy. Providers, patients, and rescue buddies are trained in the indication for, the administration of, and directions after naloxone use. Patients then are provided with naloxone nasal spray after the conclusion of the training and with instruction on refill procedures. Naloxone can be administered without legal penalty by anyone in NM. When refills for naloxone are requested, a naloxone-specific refill note is filed in the EHR to collect data on naloxone usage. In collaboration with a local suicide and substance abuse prevention network, 2 pain management pharmacists have created a Pills Can Kill booklet for dispersion to patients in waiting rooms, offices, schools, and throughout the county.
MedSafe receptacles (Houston, Texas) for collection of unused or unwanted medications, including controlled substances, are available in the pharmacy. These receptacles give patients a convenient and free outlet to remove circulating opioids from general access in the community.
Clinic Impact
Previously at GIMC, clinical pain conditions were managed by providers with minimal pain management expertise. Providers in the UCC and ED treated both acute and chronic pain with minimally enforced opioid prescribing limits. Often, patients were prescribed the dangerous drug trio of opioids, benzodiazepines, and muscle relaxers. In addition, there were inconsistent PMP checks, scheduled or random UDTs, or pill counts.
Currently, providers adhere more strongly to their scope of practice, which has resulted in less erratic opioid prescribing, from initiating therapy to prescribing large quantities. Providers in the UCC and ED more often triage chronic opioid pain patients to the PCP rather than obliging patients’ requests for chronic pain medication refills. Since UCC and ED providers now focus on the treatment of acute pain, there have been fewer drug seekers frequenting those departments, which has reduced the drug-seeking burden on PCPs and the outpatient pharmacy.
Increased utilization and study of the PMP profiles at GIMC has helped uncover important information, including incidences of theft and diversion. For example, a patient or caregiver who claimed stolen opioids was discovered to be diverting opioids from patients under her care and acutely drugging her patients in order for their UDTs to appear positive for opioids. Patients have been found drug and doctor shopping under maiden and married names, under multiple birthdays, in other states, or under a different gender altogether. Some patients have been selling immense amounts of opioids and others consuming immense amounts.
Statewide statistics from the NM Board of Pharmacy from 2016 describes a 20.1% increase in PMP usage over the previous year.12 Data from 3 million prescriptions are uploaded to the PMP annually, and more than 100,000 search requests are processed monthly.11 There was a 16% decrease in opioid prescriptions per patient from multiple providers, a 14.2% decline in concurrent opioid and benzodiazepine prescriptions, and a 7.2% reduction in total opioid prescriptions.12 Fortunately, opioid overdose death rates are down 7% from the previous year.
There is an assumption of mutual trust between patient and provider. However, patients are given more responsibility for pain management through the use of a variety of objective tools and labs. Only a few people have requested refills on the naloxone kits other than for replacement of expired product. Based on experience, many patients and rescue buddies have inquired why they did not know of opioid-related risks earlier and stated that if they had naloxone available at home earlier, they could have saved a life. Further research is needed on the true impact of naloxone and its expanding access in the community.
The PMC has shown clinical success beyond simply helping to curb inappropriate opioid prescribing and overdose deaths. Investigative work such as initiating the right drug for the pain level or defining twice daily dosing as every 12 hours and not any 2 times per day has improved pain and function greatly. Many cases of hyperalgesia have been reduced by careful reductions in daily opioid doses.
Conclusion
The PMC and partners will continue to sustain efforts and transform now that the opioid crisis is exposed in the media, the IHS, and the PHS. The ongoing goals of the pain committee are to lift the dependence on opioids as long-term treatment, identify alternative solutions to pain conditions, and execute appropriate coordination of treatment. Optimization of pain management will depend on a variety of administrative and supportive service changes. Expansion of more PMC days is being considered to reduce no-show rates and the delay to first scheduled appointment. Recruitment and maintenance of specialists in behavioral health are crucial.
Securing avenues for medication-assisted therapies with buprenorphine and methadone also will help to reverse the prevalence of the opioid use disorders. The public hospital in Gallup, NM, has begun to mirror GIMC policies for consistent community actions toward improved pain management. Additionally, the GIMC experiences have been shared with area IHS facility pharmacists who are becoming increasingly involved in the national efforts to improve pain care. The pharmacy pain management clinic services at GIMC have emphasized judicious opioid prescribing, reduced overdose risk in the community, and improved patient functionality and quality of care through close pharmacotherapy monitoring.
1. The American Academy of Pain Medicine. AAPM Facts and Figures in Pain. http://www.painmed.org/patientcenter/facts_on_pain.aspx. Accessed September 18, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain---United States, 2016. JAMA. 2016;315(15):1624-1645.
3. U.S. Department of Health and Human Services. The surgeon general’s call to end the opioid crisis. https://turnthetiderx.org/. Accessed September 18, 2017.
4. Centers for Disease Control and Prevention. Prescription opioid overdose data. http://www.cdc.gov/drugoverdose/data/overdose.html. Updated August 1, 2017. Accessed September 18, 2017.
5. American Pharmacists Association. United States House of Representatives approves opioid bill package. http://www.pharmacist.com/united-states-house-representatives-approves-opioid-bill-package. Published May 13, 2016. Accessed September 18, 2017.
6. U.S. Attorney’s Office, District of New MexicoHeroin and opioid prevention and education (HOPE) initiative. http://www.hopeinitiativenm.org/. Ac cessed May 6, 2017.
7. U.S. Department of Health and Human Services,National Committee on Heroin, Opioids, and Pain Efforts (HOPE). Indian Health Service Circular No. 17-04. https://www.ihs.gov/ihm/index.cfm?module=dsp_ihm_circ_main&circ=ihm_circ_1704. Published March 24, 2017. Accessed May 6, 2017.
8. Rapoport A, Akbik H. Pharmacist-managed pain clinic at a Veterans Affairs Medical Center. Am J Health Syst Pharm. 2004;61(13):1341-1343.
9. Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015 July;32(7):14-19.
10. Zacharoff, KL, Menefee Pujol, L, Corsini, E. PainEDU.org Manual. A Pocket Guide to Pain Management. 4th ed. Newton, MA : Inflexxion, Inc; 2010.
11. New Mexico Board of Pharmacy. Prescription monitoring program. http://nmpmp.org/. Accessed May 6, 2017.
12. New Mexico Department of Health Reports on Improved Opioid Prescribing Practices, Reduced Drug Overdose Death Rates. NABP e-News. http://nabp.bmetrack.com/c/v?e=A90B1D&c=8AB9&t= 0&l=193A4F8E. Accessed January 16, 2017.
According to the National Academy of Medicine (NAM), about 100 million people live with chronic pain in the U.S.1 There is evidence for the use of opioids to treat acute pain lasting 12 weeks or less. However, high-quality studies that analyze the benefit and safety of long-term opioid therapy are not yet available.2 In 2013, 249 million opioid prescriptions were written, equivalent to about 1 prescription per adult living in the U.S.3
Between 1999 and 2008, nonmedical use of prescription pain killers in the American Indian and Alaska Native populations was 2 to 3 times the frequency found in the white and black populations, respectively.1 These clinically contradictory practices created an environment conducive to opioid abuse and overdose. According to the Centers for Disease Control and Prevention, 1 in 4 people on chronic opioid therapy struggle with addiction.4 In 2014, more than 14,000 people in the U.S. died from overdoses involving prescription opioids.
The Comprehensive Addiction and Recovery Act of 2016 authorized prescription drug monitoring programs (PMPs) and a task force to create optimal pain treatment practices.5 The American Pharmacists Association (APhA), a major proponent of this law, argued that pharmacists are an underutilized resource despite having valuable clinical knowledge in the initiation, monitoring, and discontinuation of opioids. Additionally, pharmacists are able to refer patients to nonpharmacologic forms of pain management and dispense naloxone for emergent opioid overdose reversal.
Former Surgeon General Vivek Murthy developed the Turn the Tide Rx campaign to curb and reverse the opioid crisis in the U.S.3 The turnthetiderx.org website offers a pledge for clinicians who agree to be educated about pain management. It encourages open communication among prescribers and contains guideline-based resources on assessing pain and addiction risk, appropriate opioid prescribing, and how to manage opioid overdose. As required by law for prescribers in most states, there are instructions on how to access and analyze PMP opioid usage. The website also provides fact sheets about opioid treatments, safe disposal of medications, and helpline information.
Local Opioid Misuse Initiatives
New Mexico (NM) has one of the nation’s highest opioid and heroin overdose death rates.6 In January 2015, the U.S. Attorney’s Office and the University of New Mexico Health and Sciences Center partnered to launch the Heroin and Opioid Prevention and Education Initiative. The partners recognized that joint action between medical sciences and law enforcement was crucial to address the consequences of the opioid epidemic on public health and safety.
In 2017, the IHS established the National Committee on Heroin, Opioids, and Pain Efforts. Comprised of a variety of pharmacy and other subject-matter experts, this committee has a multipronged strategy to address the opioid epidemic from training to expanding medication-assisted treatment.7
Pharmacists provide clinical services in a variety of interdisciplinary ambulatory care clinics at Gallup Indian Medical Center (GIMC) in NM. The GIMC is located outside reservation boundaries but is centrally located to serve Navajo, Zuni, and a variety of other native populations. Pharmacist-run clinics include diabetes mellitus, anticoagulation, asthma, anemia, infectious diseases, and chronic pain. At GIMC, pain management pharmacists use a collective approach to curb opioid misuse. This article describes the establishment and impact of a pharmacist-led pain management clinic (PMC) at GIMC.
Pain Management Clinic
The understaffed urgent care clinic (UCC), emergency department (ED), primary care, and specialty practices plus a growing burden of complicated pain patients incentivized the development of the GIMC PMC. Under a collaborative practice agreement, pain management pharmacists were tasked with assessing, treating, and controlling noncancer chronic pain while improving quality of care and patient satisfaction (eAppendix 1, available at www.fedprac.com). The PMC goal was to improve functionality and pain scores and to reduce patient visits to the UCC and ED.
Originally, the PMC only performed medication titration for patients. In 2012, a former pharmacy resident became the PMC coordinator and has since helped to transform and expand its services. Currently, the coordinator dedicates about 20 hours per week managing pain patients in various capacities. Over time, other pain management pharmacists joined the PMC and support activities for 5 to 10 hours per week. There are now 4 pain management pharmacists who rotate through the PMC.
Pain management clinic visits generally are held once weekly for 3 hours and are occasionally expanded to full days based on patient schedule load. The initial 2 PMC appointments for each patient are 1 hour and are held within a 2-week period. Subsequent visits are each 30 minutes at 1 to 2 month intervals, depending on patient pain level and medication titration requirements. This standardized follow-up ensures new patients receive the close monitoring of slow dosage titrations necessary to achieve maximum therapeutic benefit.
Referrals
Patients only are admitted into the PMC via consults from primary care providers (PCPs). The consultations generally involve patients with complicated medical histories or who require complex therapies. The patients are contacted by telephone prior to scheduling. If a patient is not interested in PMC services, the consult is amended and the PCP is notified. This policy has been newly implemented to reduce the no-show rates. Pain management pharmacists then conduct PMC visits. The PMC activities are reported to the Pharmacy and Therapeutics committee annually. The PMC pharmacists also are available for telephone consultations from any internal hospital system department during weekday business hours.
The PMC patient population is fluid. New patients are accepted and clinically stable patients are discharged from PMC on a regular basis. The stable patients are released back to the PCP for further follow-up. Patients are considered stable if they have reached pain-related goals, are on maintenance doses of opioids, and/or are regularly participating in applicable interventional and referred therapies. If a patient requires reentry into PMC care, a new referral can be placed.
Interventional Programs
Since its inception, PMC has been a referral service to interventional programs. Physicians in the family medicine clinic provide twice weekly pain/palliative care clinic visits. These PCPs typically perform trigger point injections with lidocaine to relax muscles, which may disrupt nerve fibers. Ideally, this treatment reduces the use of opioids, non-steroidal anti-inflammatory drugs (NSAIDs), steroids, and epidural medications. Three acupuncturists were hired, which has reduced scheduling bottlenecks and wait times for patients to return for follow-up, especially in a treatment modality requiring frequent visits for effectiveness.
Pain Committee
As a part of an expansion of pain management services, GIMC established a pain committee (PC). The PC includes the GIMC medical director, PMC coordinator, pain management pharmacists, palliative care providers, PCPs, and specialty care providers. The PC created a detailed policy and procedures on management of chronic non-cancer pain for the Gallup Service Unit (eAppendix 2, available at www.fedprac.com). The PC offers guidance and completes consultations, performs internal review of prescribing patterns, and provides an appeals process for patients who have broken pain agreements. Physician and administrative champions have been instrumental to ensure proper pain management at GIMC.
Many VA facilities have deployed pain management clinics. At the VA Boston Healthcare System (VABHS), a pain management center is staffed by a multidisciplinary team that consists of anesthesiologists, neurologists, psychiatrists, nurses, and pharmacists.8 This pain clinic operates multiple days per week to accommodate demand, and patients are followed at least once a month. The VABHS often synchronized clinic visit dates with medication refill dates. Pharmacists offer an e-consult pain service to provide immediate recommendations to PCPs to bridge those patients awaiting appointments with pain clinic specialists at some VA facilities in Florida.9 Insufficient funding has prevented GIMC from increased PMC clinic hours.
Clinic Scope
Currently, the PMC sees the majority of PC cases. Pain management pharmacists are selected to conduct pain management visits based on interest and competency. Qualifications to work as a PMC pharmacist include on-the-job training, at least 6 annual pain management continuing medical education (CME) credits, participation in the NM naloxone training webinar, and completion of the physical assessment portion of the NM pharmacist clinician training course. It is highly recommended that pharmacists attend the PAINWeek conference in Las Vegas, Nevada, to obtain the necessary CME credits. In addition, pharmacists are requested to obtain the IHS National Clinical Pharmacy Specialist (NCPS) qualification within a year of practice.
Pain management pharmacists in the PMC review the indications for pain management, monitor medication therapy and adherence, adjust doses, manage adverse effects, study trends in pain and mood screening tools, and assess changes in functionality. These pharmacists are able to prescribe, discontinue, or titrate noncontrolled substance adjunctive therapies without a PCP cosignature. Adjunctive medication therapies can be NSAIDs, anticonvulsants, neuropathic pain relievers, muscle relaxers, and topical analgesics.
If adjustments to controlled substances are warranted, pain management pharmacists present the case to the PCP via electronic health record (EHR) notification or telephone conversation. These pharmacists ensure hard copies of controlled substance prescriptions are retrieved and provide refill coordination if assistance is requested by the patient. Pharmacists provide 28-day (not 30-day) prescriptions for opioid and controlled substance prescriptions to reduce weekend refill requests. Pain management pharmacists also order a variety of laboratory tests (eg, liver and renal function tests, and complete blood counts) related to the safe use of medications. If a patient is deemed unfit for PMC management, such as due to pain agreement violations, the PMC coordinator formally presents the case during PC meetings.
The PMC often recommends a multitude of nonpharmacologic treatments, including ice, hot rice socks, an anti-inflammatory diet, massage therapy, tennis ball massage for muscle tension and pinched nerves, chair exercises, transcutaneous electrical nerve stimulation therapy, aquatic therapy, and distraction therapy. Pain management pharmacists also can coordinate referrals to specialists and interventional therapies (eg, physical therapy; occupational therapy; acupuncture; trigger point injections; podiatry; orthopedics; ear, nose and throat; and diabetes mellitus).
Pain management pharmacists use a variety of established tools in the pain management clinic. The PMC EHR template and interview process are consistent with the universal precautions approach to unified pain management.10 Many of the questionnaires, tools, and laboratory tests are repeated periodically based on PMC policy and patient-specific need. These tools include a controlled substance pain agreement, consent for chronic opioid therapy, Opioid Risk Tool (ORT), Current Opioid Misuse Measure (COMM) for opioid abuse risk assessment, and the Patient Health Questionnaire (PHQ-9) for concurrent depression (Table and eAppendices 1, 3, and 4, available at www.fedprac.com). The ORT recently was added to the patient assessment packet to provide a stronger assessment of opioid misuse and abuse risk. Patient goals also are discussed with an emphasis on realistic changes, the level of control that would satisfy the patient and is feasible, what activities of daily living or hobbies the patient would like to regain, and what relationships the patient would like to improve.
PMC Patients
New patients are required to complete urine drug tests (UDTs), which can be performed in house or sent out if specific drug levels are required. Federally, marijuana is an illicit substance and is not prescribed or dispensed. Unpredictable effects of traditional medicine on the UDT have been observed. If a UDT shows positive for illicit substances, a discussion with the patient on toxicity and risks is initiated allowing them to choose to continue to use other substances for pain or use only the pain medication(s) appropriately prescribed by GIMC providers. Patients can be deemed ineligible for PMC management if they do not discontinue the use of illicit substances.
Health care providers and pharmacists involved in the prescribing or dispensing of controlled substances also complete a review of the patient’s PMP profile. It is mandatory in NM to complete PMP surveillance prior to prescribing controlled substances in quantities greater than 12 units within a 72-hour period and every 3 months for refills of chronic opioids.11 The PMP Interconnect service allows registered providers in NM to search for controlled substance usage in 25 states. All but 1 state in the U.S. has a PMP in development or in place. Federal providers who do not have a NM professional license also may apply for a NM PMP login to take advantage of the PMP Interconnect service. If the patient’s PMP is negative for prescribed opioids or positive for nonprescribed substances, the providers will conduct more research to reassess the risks and benefits of ongoing opioid therapy.
Random pill counts for tracked patients also have been incorporated into the PMC policy. These pill counts can be requested regardless of clinic appointment dates and can show whether patients are taking too many pills or diverting pills. Pill counts also may show that the patient may not need as many tablets per prescription if they consistently have more than expected based on dosing frequency. If a patient has adhered to prescribing recommendations, he or she may be allowed an early refill of the chronic opioid to cover them during a vacation or other unexpected event. However, if the pill counts are not consistent with instructions, then prescribing may be restricted to a 5-day, 7-day, or 14-day supply only. If patients do not present for pill count within 24 hours of the request, they can have their opioid use privileges at GIMC revoked as consented in the pain agreement. Patients are educated on proper storage and security of opioids medication at home and funding for lock boxes is expected in the near future. Patients may be referred to other services if opioid-use disorders are identified and confirmed.
Other administrative components of the pain management pharmacist responsibilities include clinical chart reviews before appointments or telephone consultations, sending appointment letters, contacting patients about and conducting random pill counts, and documenting PMC visit notes and updating flowsheets (eAppendices 4 and 5, available at www.fedprac.com). Currently under development is an EHR function that can quickly provide a summary of a patient’s pain management without a tedious search through the chart. Pain management pharmacists regularly instruct pharmacists and providers on changes in regulatory requirements of pain management as it pertains to prescription fills or clinical indications.
Ancillary Pharmacy Services
All pain patients on opioids therapies receive an annual pain evaluation. This evaluation provides a holistic description of the patient’s pain, a second opinion, and a collective review of the safety and efficacy of treatment. Components of the pain evaluation include review of medication toxicities and adverse effects, reconciliation of the treatment with current pain diagnosis and intensity, and coordination of opioid tapering schedules. Physical assessments and pill counts also are performed. Issues that have been uncovered include incidences of opioid-induced hyperalgesia and patients filling opioids prescriptions without taking the medications because they did not want to tell the provider the medication did not work.
Naloxone overdose prevention training and dispensing has been critical for ensuring maximum safety and life-saving methods in the realm of opioid therapy. Providers, patients, and rescue buddies are trained in the indication for, the administration of, and directions after naloxone use. Patients then are provided with naloxone nasal spray after the conclusion of the training and with instruction on refill procedures. Naloxone can be administered without legal penalty by anyone in NM. When refills for naloxone are requested, a naloxone-specific refill note is filed in the EHR to collect data on naloxone usage. In collaboration with a local suicide and substance abuse prevention network, 2 pain management pharmacists have created a Pills Can Kill booklet for dispersion to patients in waiting rooms, offices, schools, and throughout the county.
MedSafe receptacles (Houston, Texas) for collection of unused or unwanted medications, including controlled substances, are available in the pharmacy. These receptacles give patients a convenient and free outlet to remove circulating opioids from general access in the community.
Clinic Impact
Previously at GIMC, clinical pain conditions were managed by providers with minimal pain management expertise. Providers in the UCC and ED treated both acute and chronic pain with minimally enforced opioid prescribing limits. Often, patients were prescribed the dangerous drug trio of opioids, benzodiazepines, and muscle relaxers. In addition, there were inconsistent PMP checks, scheduled or random UDTs, or pill counts.
Currently, providers adhere more strongly to their scope of practice, which has resulted in less erratic opioid prescribing, from initiating therapy to prescribing large quantities. Providers in the UCC and ED more often triage chronic opioid pain patients to the PCP rather than obliging patients’ requests for chronic pain medication refills. Since UCC and ED providers now focus on the treatment of acute pain, there have been fewer drug seekers frequenting those departments, which has reduced the drug-seeking burden on PCPs and the outpatient pharmacy.
Increased utilization and study of the PMP profiles at GIMC has helped uncover important information, including incidences of theft and diversion. For example, a patient or caregiver who claimed stolen opioids was discovered to be diverting opioids from patients under her care and acutely drugging her patients in order for their UDTs to appear positive for opioids. Patients have been found drug and doctor shopping under maiden and married names, under multiple birthdays, in other states, or under a different gender altogether. Some patients have been selling immense amounts of opioids and others consuming immense amounts.
Statewide statistics from the NM Board of Pharmacy from 2016 describes a 20.1% increase in PMP usage over the previous year.12 Data from 3 million prescriptions are uploaded to the PMP annually, and more than 100,000 search requests are processed monthly.11 There was a 16% decrease in opioid prescriptions per patient from multiple providers, a 14.2% decline in concurrent opioid and benzodiazepine prescriptions, and a 7.2% reduction in total opioid prescriptions.12 Fortunately, opioid overdose death rates are down 7% from the previous year.
There is an assumption of mutual trust between patient and provider. However, patients are given more responsibility for pain management through the use of a variety of objective tools and labs. Only a few people have requested refills on the naloxone kits other than for replacement of expired product. Based on experience, many patients and rescue buddies have inquired why they did not know of opioid-related risks earlier and stated that if they had naloxone available at home earlier, they could have saved a life. Further research is needed on the true impact of naloxone and its expanding access in the community.
The PMC has shown clinical success beyond simply helping to curb inappropriate opioid prescribing and overdose deaths. Investigative work such as initiating the right drug for the pain level or defining twice daily dosing as every 12 hours and not any 2 times per day has improved pain and function greatly. Many cases of hyperalgesia have been reduced by careful reductions in daily opioid doses.
Conclusion
The PMC and partners will continue to sustain efforts and transform now that the opioid crisis is exposed in the media, the IHS, and the PHS. The ongoing goals of the pain committee are to lift the dependence on opioids as long-term treatment, identify alternative solutions to pain conditions, and execute appropriate coordination of treatment. Optimization of pain management will depend on a variety of administrative and supportive service changes. Expansion of more PMC days is being considered to reduce no-show rates and the delay to first scheduled appointment. Recruitment and maintenance of specialists in behavioral health are crucial.
Securing avenues for medication-assisted therapies with buprenorphine and methadone also will help to reverse the prevalence of the opioid use disorders. The public hospital in Gallup, NM, has begun to mirror GIMC policies for consistent community actions toward improved pain management. Additionally, the GIMC experiences have been shared with area IHS facility pharmacists who are becoming increasingly involved in the national efforts to improve pain care. The pharmacy pain management clinic services at GIMC have emphasized judicious opioid prescribing, reduced overdose risk in the community, and improved patient functionality and quality of care through close pharmacotherapy monitoring.
According to the National Academy of Medicine (NAM), about 100 million people live with chronic pain in the U.S.1 There is evidence for the use of opioids to treat acute pain lasting 12 weeks or less. However, high-quality studies that analyze the benefit and safety of long-term opioid therapy are not yet available.2 In 2013, 249 million opioid prescriptions were written, equivalent to about 1 prescription per adult living in the U.S.3
Between 1999 and 2008, nonmedical use of prescription pain killers in the American Indian and Alaska Native populations was 2 to 3 times the frequency found in the white and black populations, respectively.1 These clinically contradictory practices created an environment conducive to opioid abuse and overdose. According to the Centers for Disease Control and Prevention, 1 in 4 people on chronic opioid therapy struggle with addiction.4 In 2014, more than 14,000 people in the U.S. died from overdoses involving prescription opioids.
The Comprehensive Addiction and Recovery Act of 2016 authorized prescription drug monitoring programs (PMPs) and a task force to create optimal pain treatment practices.5 The American Pharmacists Association (APhA), a major proponent of this law, argued that pharmacists are an underutilized resource despite having valuable clinical knowledge in the initiation, monitoring, and discontinuation of opioids. Additionally, pharmacists are able to refer patients to nonpharmacologic forms of pain management and dispense naloxone for emergent opioid overdose reversal.
Former Surgeon General Vivek Murthy developed the Turn the Tide Rx campaign to curb and reverse the opioid crisis in the U.S.3 The turnthetiderx.org website offers a pledge for clinicians who agree to be educated about pain management. It encourages open communication among prescribers and contains guideline-based resources on assessing pain and addiction risk, appropriate opioid prescribing, and how to manage opioid overdose. As required by law for prescribers in most states, there are instructions on how to access and analyze PMP opioid usage. The website also provides fact sheets about opioid treatments, safe disposal of medications, and helpline information.
Local Opioid Misuse Initiatives
New Mexico (NM) has one of the nation’s highest opioid and heroin overdose death rates.6 In January 2015, the U.S. Attorney’s Office and the University of New Mexico Health and Sciences Center partnered to launch the Heroin and Opioid Prevention and Education Initiative. The partners recognized that joint action between medical sciences and law enforcement was crucial to address the consequences of the opioid epidemic on public health and safety.
In 2017, the IHS established the National Committee on Heroin, Opioids, and Pain Efforts. Comprised of a variety of pharmacy and other subject-matter experts, this committee has a multipronged strategy to address the opioid epidemic from training to expanding medication-assisted treatment.7
Pharmacists provide clinical services in a variety of interdisciplinary ambulatory care clinics at Gallup Indian Medical Center (GIMC) in NM. The GIMC is located outside reservation boundaries but is centrally located to serve Navajo, Zuni, and a variety of other native populations. Pharmacist-run clinics include diabetes mellitus, anticoagulation, asthma, anemia, infectious diseases, and chronic pain. At GIMC, pain management pharmacists use a collective approach to curb opioid misuse. This article describes the establishment and impact of a pharmacist-led pain management clinic (PMC) at GIMC.
Pain Management Clinic
The understaffed urgent care clinic (UCC), emergency department (ED), primary care, and specialty practices plus a growing burden of complicated pain patients incentivized the development of the GIMC PMC. Under a collaborative practice agreement, pain management pharmacists were tasked with assessing, treating, and controlling noncancer chronic pain while improving quality of care and patient satisfaction (eAppendix 1, available at www.fedprac.com). The PMC goal was to improve functionality and pain scores and to reduce patient visits to the UCC and ED.
Originally, the PMC only performed medication titration for patients. In 2012, a former pharmacy resident became the PMC coordinator and has since helped to transform and expand its services. Currently, the coordinator dedicates about 20 hours per week managing pain patients in various capacities. Over time, other pain management pharmacists joined the PMC and support activities for 5 to 10 hours per week. There are now 4 pain management pharmacists who rotate through the PMC.
Pain management clinic visits generally are held once weekly for 3 hours and are occasionally expanded to full days based on patient schedule load. The initial 2 PMC appointments for each patient are 1 hour and are held within a 2-week period. Subsequent visits are each 30 minutes at 1 to 2 month intervals, depending on patient pain level and medication titration requirements. This standardized follow-up ensures new patients receive the close monitoring of slow dosage titrations necessary to achieve maximum therapeutic benefit.
Referrals
Patients only are admitted into the PMC via consults from primary care providers (PCPs). The consultations generally involve patients with complicated medical histories or who require complex therapies. The patients are contacted by telephone prior to scheduling. If a patient is not interested in PMC services, the consult is amended and the PCP is notified. This policy has been newly implemented to reduce the no-show rates. Pain management pharmacists then conduct PMC visits. The PMC activities are reported to the Pharmacy and Therapeutics committee annually. The PMC pharmacists also are available for telephone consultations from any internal hospital system department during weekday business hours.
The PMC patient population is fluid. New patients are accepted and clinically stable patients are discharged from PMC on a regular basis. The stable patients are released back to the PCP for further follow-up. Patients are considered stable if they have reached pain-related goals, are on maintenance doses of opioids, and/or are regularly participating in applicable interventional and referred therapies. If a patient requires reentry into PMC care, a new referral can be placed.
Interventional Programs
Since its inception, PMC has been a referral service to interventional programs. Physicians in the family medicine clinic provide twice weekly pain/palliative care clinic visits. These PCPs typically perform trigger point injections with lidocaine to relax muscles, which may disrupt nerve fibers. Ideally, this treatment reduces the use of opioids, non-steroidal anti-inflammatory drugs (NSAIDs), steroids, and epidural medications. Three acupuncturists were hired, which has reduced scheduling bottlenecks and wait times for patients to return for follow-up, especially in a treatment modality requiring frequent visits for effectiveness.
Pain Committee
As a part of an expansion of pain management services, GIMC established a pain committee (PC). The PC includes the GIMC medical director, PMC coordinator, pain management pharmacists, palliative care providers, PCPs, and specialty care providers. The PC created a detailed policy and procedures on management of chronic non-cancer pain for the Gallup Service Unit (eAppendix 2, available at www.fedprac.com). The PC offers guidance and completes consultations, performs internal review of prescribing patterns, and provides an appeals process for patients who have broken pain agreements. Physician and administrative champions have been instrumental to ensure proper pain management at GIMC.
Many VA facilities have deployed pain management clinics. At the VA Boston Healthcare System (VABHS), a pain management center is staffed by a multidisciplinary team that consists of anesthesiologists, neurologists, psychiatrists, nurses, and pharmacists.8 This pain clinic operates multiple days per week to accommodate demand, and patients are followed at least once a month. The VABHS often synchronized clinic visit dates with medication refill dates. Pharmacists offer an e-consult pain service to provide immediate recommendations to PCPs to bridge those patients awaiting appointments with pain clinic specialists at some VA facilities in Florida.9 Insufficient funding has prevented GIMC from increased PMC clinic hours.
Clinic Scope
Currently, the PMC sees the majority of PC cases. Pain management pharmacists are selected to conduct pain management visits based on interest and competency. Qualifications to work as a PMC pharmacist include on-the-job training, at least 6 annual pain management continuing medical education (CME) credits, participation in the NM naloxone training webinar, and completion of the physical assessment portion of the NM pharmacist clinician training course. It is highly recommended that pharmacists attend the PAINWeek conference in Las Vegas, Nevada, to obtain the necessary CME credits. In addition, pharmacists are requested to obtain the IHS National Clinical Pharmacy Specialist (NCPS) qualification within a year of practice.
Pain management pharmacists in the PMC review the indications for pain management, monitor medication therapy and adherence, adjust doses, manage adverse effects, study trends in pain and mood screening tools, and assess changes in functionality. These pharmacists are able to prescribe, discontinue, or titrate noncontrolled substance adjunctive therapies without a PCP cosignature. Adjunctive medication therapies can be NSAIDs, anticonvulsants, neuropathic pain relievers, muscle relaxers, and topical analgesics.
If adjustments to controlled substances are warranted, pain management pharmacists present the case to the PCP via electronic health record (EHR) notification or telephone conversation. These pharmacists ensure hard copies of controlled substance prescriptions are retrieved and provide refill coordination if assistance is requested by the patient. Pharmacists provide 28-day (not 30-day) prescriptions for opioid and controlled substance prescriptions to reduce weekend refill requests. Pain management pharmacists also order a variety of laboratory tests (eg, liver and renal function tests, and complete blood counts) related to the safe use of medications. If a patient is deemed unfit for PMC management, such as due to pain agreement violations, the PMC coordinator formally presents the case during PC meetings.
The PMC often recommends a multitude of nonpharmacologic treatments, including ice, hot rice socks, an anti-inflammatory diet, massage therapy, tennis ball massage for muscle tension and pinched nerves, chair exercises, transcutaneous electrical nerve stimulation therapy, aquatic therapy, and distraction therapy. Pain management pharmacists also can coordinate referrals to specialists and interventional therapies (eg, physical therapy; occupational therapy; acupuncture; trigger point injections; podiatry; orthopedics; ear, nose and throat; and diabetes mellitus).
Pain management pharmacists use a variety of established tools in the pain management clinic. The PMC EHR template and interview process are consistent with the universal precautions approach to unified pain management.10 Many of the questionnaires, tools, and laboratory tests are repeated periodically based on PMC policy and patient-specific need. These tools include a controlled substance pain agreement, consent for chronic opioid therapy, Opioid Risk Tool (ORT), Current Opioid Misuse Measure (COMM) for opioid abuse risk assessment, and the Patient Health Questionnaire (PHQ-9) for concurrent depression (Table and eAppendices 1, 3, and 4, available at www.fedprac.com). The ORT recently was added to the patient assessment packet to provide a stronger assessment of opioid misuse and abuse risk. Patient goals also are discussed with an emphasis on realistic changes, the level of control that would satisfy the patient and is feasible, what activities of daily living or hobbies the patient would like to regain, and what relationships the patient would like to improve.
PMC Patients
New patients are required to complete urine drug tests (UDTs), which can be performed in house or sent out if specific drug levels are required. Federally, marijuana is an illicit substance and is not prescribed or dispensed. Unpredictable effects of traditional medicine on the UDT have been observed. If a UDT shows positive for illicit substances, a discussion with the patient on toxicity and risks is initiated allowing them to choose to continue to use other substances for pain or use only the pain medication(s) appropriately prescribed by GIMC providers. Patients can be deemed ineligible for PMC management if they do not discontinue the use of illicit substances.
Health care providers and pharmacists involved in the prescribing or dispensing of controlled substances also complete a review of the patient’s PMP profile. It is mandatory in NM to complete PMP surveillance prior to prescribing controlled substances in quantities greater than 12 units within a 72-hour period and every 3 months for refills of chronic opioids.11 The PMP Interconnect service allows registered providers in NM to search for controlled substance usage in 25 states. All but 1 state in the U.S. has a PMP in development or in place. Federal providers who do not have a NM professional license also may apply for a NM PMP login to take advantage of the PMP Interconnect service. If the patient’s PMP is negative for prescribed opioids or positive for nonprescribed substances, the providers will conduct more research to reassess the risks and benefits of ongoing opioid therapy.
Random pill counts for tracked patients also have been incorporated into the PMC policy. These pill counts can be requested regardless of clinic appointment dates and can show whether patients are taking too many pills or diverting pills. Pill counts also may show that the patient may not need as many tablets per prescription if they consistently have more than expected based on dosing frequency. If a patient has adhered to prescribing recommendations, he or she may be allowed an early refill of the chronic opioid to cover them during a vacation or other unexpected event. However, if the pill counts are not consistent with instructions, then prescribing may be restricted to a 5-day, 7-day, or 14-day supply only. If patients do not present for pill count within 24 hours of the request, they can have their opioid use privileges at GIMC revoked as consented in the pain agreement. Patients are educated on proper storage and security of opioids medication at home and funding for lock boxes is expected in the near future. Patients may be referred to other services if opioid-use disorders are identified and confirmed.
Other administrative components of the pain management pharmacist responsibilities include clinical chart reviews before appointments or telephone consultations, sending appointment letters, contacting patients about and conducting random pill counts, and documenting PMC visit notes and updating flowsheets (eAppendices 4 and 5, available at www.fedprac.com). Currently under development is an EHR function that can quickly provide a summary of a patient’s pain management without a tedious search through the chart. Pain management pharmacists regularly instruct pharmacists and providers on changes in regulatory requirements of pain management as it pertains to prescription fills or clinical indications.
Ancillary Pharmacy Services
All pain patients on opioids therapies receive an annual pain evaluation. This evaluation provides a holistic description of the patient’s pain, a second opinion, and a collective review of the safety and efficacy of treatment. Components of the pain evaluation include review of medication toxicities and adverse effects, reconciliation of the treatment with current pain diagnosis and intensity, and coordination of opioid tapering schedules. Physical assessments and pill counts also are performed. Issues that have been uncovered include incidences of opioid-induced hyperalgesia and patients filling opioids prescriptions without taking the medications because they did not want to tell the provider the medication did not work.
Naloxone overdose prevention training and dispensing has been critical for ensuring maximum safety and life-saving methods in the realm of opioid therapy. Providers, patients, and rescue buddies are trained in the indication for, the administration of, and directions after naloxone use. Patients then are provided with naloxone nasal spray after the conclusion of the training and with instruction on refill procedures. Naloxone can be administered without legal penalty by anyone in NM. When refills for naloxone are requested, a naloxone-specific refill note is filed in the EHR to collect data on naloxone usage. In collaboration with a local suicide and substance abuse prevention network, 2 pain management pharmacists have created a Pills Can Kill booklet for dispersion to patients in waiting rooms, offices, schools, and throughout the county.
MedSafe receptacles (Houston, Texas) for collection of unused or unwanted medications, including controlled substances, are available in the pharmacy. These receptacles give patients a convenient and free outlet to remove circulating opioids from general access in the community.
Clinic Impact
Previously at GIMC, clinical pain conditions were managed by providers with minimal pain management expertise. Providers in the UCC and ED treated both acute and chronic pain with minimally enforced opioid prescribing limits. Often, patients were prescribed the dangerous drug trio of opioids, benzodiazepines, and muscle relaxers. In addition, there were inconsistent PMP checks, scheduled or random UDTs, or pill counts.
Currently, providers adhere more strongly to their scope of practice, which has resulted in less erratic opioid prescribing, from initiating therapy to prescribing large quantities. Providers in the UCC and ED more often triage chronic opioid pain patients to the PCP rather than obliging patients’ requests for chronic pain medication refills. Since UCC and ED providers now focus on the treatment of acute pain, there have been fewer drug seekers frequenting those departments, which has reduced the drug-seeking burden on PCPs and the outpatient pharmacy.
Increased utilization and study of the PMP profiles at GIMC has helped uncover important information, including incidences of theft and diversion. For example, a patient or caregiver who claimed stolen opioids was discovered to be diverting opioids from patients under her care and acutely drugging her patients in order for their UDTs to appear positive for opioids. Patients have been found drug and doctor shopping under maiden and married names, under multiple birthdays, in other states, or under a different gender altogether. Some patients have been selling immense amounts of opioids and others consuming immense amounts.
Statewide statistics from the NM Board of Pharmacy from 2016 describes a 20.1% increase in PMP usage over the previous year.12 Data from 3 million prescriptions are uploaded to the PMP annually, and more than 100,000 search requests are processed monthly.11 There was a 16% decrease in opioid prescriptions per patient from multiple providers, a 14.2% decline in concurrent opioid and benzodiazepine prescriptions, and a 7.2% reduction in total opioid prescriptions.12 Fortunately, opioid overdose death rates are down 7% from the previous year.
There is an assumption of mutual trust between patient and provider. However, patients are given more responsibility for pain management through the use of a variety of objective tools and labs. Only a few people have requested refills on the naloxone kits other than for replacement of expired product. Based on experience, many patients and rescue buddies have inquired why they did not know of opioid-related risks earlier and stated that if they had naloxone available at home earlier, they could have saved a life. Further research is needed on the true impact of naloxone and its expanding access in the community.
The PMC has shown clinical success beyond simply helping to curb inappropriate opioid prescribing and overdose deaths. Investigative work such as initiating the right drug for the pain level or defining twice daily dosing as every 12 hours and not any 2 times per day has improved pain and function greatly. Many cases of hyperalgesia have been reduced by careful reductions in daily opioid doses.
Conclusion
The PMC and partners will continue to sustain efforts and transform now that the opioid crisis is exposed in the media, the IHS, and the PHS. The ongoing goals of the pain committee are to lift the dependence on opioids as long-term treatment, identify alternative solutions to pain conditions, and execute appropriate coordination of treatment. Optimization of pain management will depend on a variety of administrative and supportive service changes. Expansion of more PMC days is being considered to reduce no-show rates and the delay to first scheduled appointment. Recruitment and maintenance of specialists in behavioral health are crucial.
Securing avenues for medication-assisted therapies with buprenorphine and methadone also will help to reverse the prevalence of the opioid use disorders. The public hospital in Gallup, NM, has begun to mirror GIMC policies for consistent community actions toward improved pain management. Additionally, the GIMC experiences have been shared with area IHS facility pharmacists who are becoming increasingly involved in the national efforts to improve pain care. The pharmacy pain management clinic services at GIMC have emphasized judicious opioid prescribing, reduced overdose risk in the community, and improved patient functionality and quality of care through close pharmacotherapy monitoring.
1. The American Academy of Pain Medicine. AAPM Facts and Figures in Pain. http://www.painmed.org/patientcenter/facts_on_pain.aspx. Accessed September 18, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain---United States, 2016. JAMA. 2016;315(15):1624-1645.
3. U.S. Department of Health and Human Services. The surgeon general’s call to end the opioid crisis. https://turnthetiderx.org/. Accessed September 18, 2017.
4. Centers for Disease Control and Prevention. Prescription opioid overdose data. http://www.cdc.gov/drugoverdose/data/overdose.html. Updated August 1, 2017. Accessed September 18, 2017.
5. American Pharmacists Association. United States House of Representatives approves opioid bill package. http://www.pharmacist.com/united-states-house-representatives-approves-opioid-bill-package. Published May 13, 2016. Accessed September 18, 2017.
6. U.S. Attorney’s Office, District of New MexicoHeroin and opioid prevention and education (HOPE) initiative. http://www.hopeinitiativenm.org/. Ac cessed May 6, 2017.
7. U.S. Department of Health and Human Services,National Committee on Heroin, Opioids, and Pain Efforts (HOPE). Indian Health Service Circular No. 17-04. https://www.ihs.gov/ihm/index.cfm?module=dsp_ihm_circ_main&circ=ihm_circ_1704. Published March 24, 2017. Accessed May 6, 2017.
8. Rapoport A, Akbik H. Pharmacist-managed pain clinic at a Veterans Affairs Medical Center. Am J Health Syst Pharm. 2004;61(13):1341-1343.
9. Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015 July;32(7):14-19.
10. Zacharoff, KL, Menefee Pujol, L, Corsini, E. PainEDU.org Manual. A Pocket Guide to Pain Management. 4th ed. Newton, MA : Inflexxion, Inc; 2010.
11. New Mexico Board of Pharmacy. Prescription monitoring program. http://nmpmp.org/. Accessed May 6, 2017.
12. New Mexico Department of Health Reports on Improved Opioid Prescribing Practices, Reduced Drug Overdose Death Rates. NABP e-News. http://nabp.bmetrack.com/c/v?e=A90B1D&c=8AB9&t= 0&l=193A4F8E. Accessed January 16, 2017.
1. The American Academy of Pain Medicine. AAPM Facts and Figures in Pain. http://www.painmed.org/patientcenter/facts_on_pain.aspx. Accessed September 18, 2017.
2. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain---United States, 2016. JAMA. 2016;315(15):1624-1645.
3. U.S. Department of Health and Human Services. The surgeon general’s call to end the opioid crisis. https://turnthetiderx.org/. Accessed September 18, 2017.
4. Centers for Disease Control and Prevention. Prescription opioid overdose data. http://www.cdc.gov/drugoverdose/data/overdose.html. Updated August 1, 2017. Accessed September 18, 2017.
5. American Pharmacists Association. United States House of Representatives approves opioid bill package. http://www.pharmacist.com/united-states-house-representatives-approves-opioid-bill-package. Published May 13, 2016. Accessed September 18, 2017.
6. U.S. Attorney’s Office, District of New MexicoHeroin and opioid prevention and education (HOPE) initiative. http://www.hopeinitiativenm.org/. Ac cessed May 6, 2017.
7. U.S. Department of Health and Human Services,National Committee on Heroin, Opioids, and Pain Efforts (HOPE). Indian Health Service Circular No. 17-04. https://www.ihs.gov/ihm/index.cfm?module=dsp_ihm_circ_main&circ=ihm_circ_1704. Published March 24, 2017. Accessed May 6, 2017.
8. Rapoport A, Akbik H. Pharmacist-managed pain clinic at a Veterans Affairs Medical Center. Am J Health Syst Pharm. 2004;61(13):1341-1343.
9. Miller DM, Harvey TL. Pharmacist pain e-consults that result in a therapy change. Fed Pract. 2015 July;32(7):14-19.
10. Zacharoff, KL, Menefee Pujol, L, Corsini, E. PainEDU.org Manual. A Pocket Guide to Pain Management. 4th ed. Newton, MA : Inflexxion, Inc; 2010.
11. New Mexico Board of Pharmacy. Prescription monitoring program. http://nmpmp.org/. Accessed May 6, 2017.
12. New Mexico Department of Health Reports on Improved Opioid Prescribing Practices, Reduced Drug Overdose Death Rates. NABP e-News. http://nabp.bmetrack.com/c/v?e=A90B1D&c=8AB9&t= 0&l=193A4F8E. Accessed January 16, 2017.
Restoring Function in Veterans With Complex Chronic Pain
According to the International Association for the Study of Pain (IASP), pain is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”1 Chronic pain (pain lasting more than 3 months) has a high prevalence in the U.S. veteran population. In a recently published article by Richard Nahin, PhD, of the National Institutes of Health, 65.5% of U.S. veterans reported pain in the previous 3 months with 9.1% classified as having severe pain (defined as “which occurs most days or every day and bothers the individual a lot”) compared with 6.4% among nonveterans.2 In addition, male veterans were more likely to report severe pain, 9%, compared with male nonveterans, 4.7%.2 Veterans make up about 6.2% of the U.S. population; therefore, the number of veterans negatively impacted by pain is substantial.3,4 Compared with individuals with other chronic diseases, such as heart disease, chronic obstructive pulmonary disease, or diabetes mellitus, a recent population-based, matched cohort study reported that only patients with Alzheimer disease have a poorer quality of life (QOL) than do those with chronic pain.5
Background
When comparing veterans to nonveterans, Nahin also reported that younger veterans aged 18 to 39 years had significantly higher rates for severe pain, compared with similarly aged nonveterans, 7.8% vs 3.2%, respectively. The prevalence of severe pain was significantly higher among veterans than it was for nonveterans experiencing the following: back pain, 21.6% vs 16.7% among nonveterans; jaw pain, 37.5% vs 22.9%, respectively; severe migraine and headaches, 26.4% vs 15.9%, respectively; and neck pain, 27.7% vs 21.9%, respectively. The veterans also were more likely than were nonveterans to have joint pain, 43.6% vs 31.5% , respectively.2
A study by Kerns and colleagues noted that almost 50% of older veterans (mean age 65.6 years) experience chronic pain regularly.6 Based on responses of 685 veterans to the Health-Risk Behavior Screening Questionnaire (HRBSQ), this study also found that the presence of pain was strongly associated with patient reports of worsening health and emotional distress. Rollin Gallagher, MD, of the Philadelphia VAMC, reported that veterans who experienced pain tended to have more personal problems due to higher rates of psychiatric and social comorbidities, such as substance abuse, depression, posttraumatic stress syndrome, and early work disabilities.7 Gallagher also has noted that the number of veterans seeking pain treatment has grown steadily over the past 2 decades due to the aging veteran population retiring and seeking VA care for chronic illness management.
In January 2017, the VA released an analysis of health care use among recent Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), and Operation New Dawn (OND) veterans from October 2001 through June 2015.8 The VA noted that 1,965,534 veterans have become eligible for VA health care since fiscal year 2002. Of the 1,218,857 OIF/OEF/OND veterans treated during this period, 62.3% (759,850) were treated for diseases of the musculoskeletal system and connective tissue, 58.1% (708,062) were treated for mental disorders, and 58.7% (715,263) were treated for “symptoms, signs and ill-defined conditions.”
According to the VA, “the ICD-9-CM diagnostic category ‘Symptoms, Signs and Ill-Defined Conditions’ is a diverse, catch-all category that consists of 160 sub-categories and includes primarily symptoms that do not yet have an identified cause and clinical findings that are not coded elsewhere.” The most frequently reported codes in this category, in order of magnitude are General Symptoms (ICD-9-CM 780), Symptoms Involving Respiratory System and Other Chest Symptoms (ICD-9-CM 786), and Symptoms Involving Head and Neck (ICD-9-CM 784).
Musculoskeletal ailments (ie, joint and back disorders), mental health disorders and symptoms, signs, and ill-defined conditions are the 3 most frequently coded diagnoses related to medical treatment in OEF/OIF/OND veterans. This demonstrates the high rate of pain-related conditions with comorbid mental health diagnoses.
Public Health Challenge
Recognizing that pain is a public health challenge, the National Academy of Sciences published the landmark study Relieving Pain in America.9 The study reported that pain affects at least 100 million Americans, greatly reducing quality of life. In addition, annual financial costs to society are estimated at $560 to $635 billion, with federal and state costs almost $100 billion annually. Given the challenges of addressing chronic pain, especially in the U.S. veteran population, the VHA has likewise outlined 6 recommendations for transforming VA pain care:
- Educate veterans/families to promote self-efficacy and shared decision making, provide access to all relevant sources;
- Educate/train all team members to their discipline-specific competencies, including team-based care;
- Develop and integrate nonpharmacologic modalities into care plans;
- Institute evidence-based medication prescribing, use of pain procedures, and safe opioid use (universal precautions);
- Implement approaches for bringing the veteran’s whole team together, such as virtual pain consulting (SCAN-ECHO, e-consults, telehealth, clinical video teleconsultation and education) and for maintaining ongoing communication between team members; and
- Establish metrics to monitor pain care and outcomes at both the individual level and population level.10
The American Pain Society (APS) differentiates multidisciplinary care vs interdisciplinary pain care.11 Multidisciplinary pain care is provided by several disciplines that may not be coordinated. Treatment may occur with different goals and in parallel rather than with an integrated approach. The APS suggests that professional identities are clearly defined, team membership is a secondary consideration in multidisciplinary care, and the leadership is typically hierarchical with a physician in charge. In this model of care, each team member has a “clearly defined place in the overall care of the patient, contributing their expertise in relative isolation from one another.”11
In contrast, according to APS, interdisciplinary teams have complementary roles that enhance patient care. Each discipline has valuable knowledge and a set of skills that complement other team members who are collaborative partners. The interdisciplinary approach encourages complementary roles and responsibilities, conjoint problem solving, and shared accountability. Treatment decisions are consensus based.
Pain Programs
In a review of 4 interdisciplinary pain programs (Mayo Clinic Pain Rehabilitation Center, the Brooks Rehabilitation Pain Rehabilitation Program, the Rehabilitation Institute of Chicago Center for Pain Management, and the Cleveland Clinic Foundation Chronic Pain Rehabilitation Program), Stanos found that the compositions of the staff were similar.12 In general, staff consisted of pain management physicians, pain psychologists, physical and occupational therapists, and nurse coordinators. The Mayo Clinic had more personnel, including a clinical pharmacist, the Brooks program had an additional biofeedback specialist, and the Cleveland Clinic had a tai chi instructor. The programs ranged from 3 to 5 weeks of daily programming. The duration of services provided were dependent on the payers. Stanos concluded that functional status, as measured by the Pain Disability Index, improved on discharge, 6 months, and 1 year after treatment at the Cleveland Clinic.
Cosio and Lin described their experience in a multidisciplinary outpatient pain clinic at Jesse Brown VAMC in Chicago.13 Their study noted that the number of veterans in their multidisciplinary pain clinic on chronic opioids significantly decreased, the degree of pain relief increased, and veterans reported improvements in mobility and ability to complete activities of daily living (ADLs). Overall veteran satisfaction with this pain program was reportedly high.
Cosio and Lin also published a study of the effect of complementary alternative medicine (CAM) utilization at a VAMC, which included a 12-week pain education school that was offered to all veterans and families.14 They noted that veterans began using at least 1 more CAM modality before the completion of the pain education program. However, it is unclear from the 2 studies whether the pain education program was incorporated into their multidisciplinary pain clinic.
Outpatient Functional Restoration Program
Given the challenges of addressing chronic pain and at the same time fostering an interdisciplinary approach to management, the VA Puget Sound Health Care System (VAPSHCS) team initiated a program development and quality improvement process for addressing pain and restoring function for veteran patients.
The VA Northwest Health Network (VISN 20) offers health care services for veterans located in the states of Alaska, Idaho, Oregon, Washington, and parts of California and Montana. VISN 20 has 8 parent facilities, which include the Seattle and American Lake divisions of the VAPSHCS. The VAPSHCS has established a comprehensive, interdisciplinary functional restoration pain program that integrates medical, psychosocial, and complementary alternative medicine.
The Outpatient Functional Restoration Program (OFRPP) pain team consists of a chief who is board certified in pain medicine and addiction medicine; a board-certified pain medicine physician; 2 physician assistants, one of whom has formal training in acupuncture and another who is trained in tai chi, qigong, hypnosis, and mindfulness; nurse care coordinators; a pain psychologist with training in acceptance and commitment therapy, cognitive behavioral therapy, yoga nidra, and hypnosis; a second pain psychologist who has a background in rehabilitation psychology; a physical therapist; and a pain clinical pharmacy specialist.
Prior to participation in OFRPP, veterans were required to attend 4 weekly pain education classes for 4 consecutive weeks. The classes educate veterans and their families on the complexity of managing chronic pain. Topics cover medical, pharmacologic and nonpharmacologic approaches to pain, including CAM and psychological modalities (Table 1). The pain orientation classes introduce veterans to available treatment options, and in some cases, veterans decide committing to a more intensive pain rehabilitation program is a good fit.
The program is based on the biopsychosocial model of pain care and Commission on Accreditation for Rehabilitation Facilities (CARF) interdisciplinary pain rehabilitation program standards. The length of the program was determined after reviewing data from existing VA outpatient pain rehabilitation programs; Pain Clinic staff availability, training and experience; and survey responses from veterans completing the 4-week education. This survey asked veterans whether they would be interested in an outpatient pain rehabilitation program and their preference for length of the program and treatment modalities.
Since its inception, OFRPP has earned a 3-year CARF accreditation. Veterans participate in VAPSHCS American Lake division OFRPP education twice weekly for 4 hours for a total of 8 weeks (Table 2). Each week of programming includes 2 hours of physical therapy didactics, 2 hours of physical therapy (eg, paced cardio exercise, stretching, and core strength and conditioning), 2 hours of mind-body medicine (eg, mantram repetition and neuroplasticity education), and 2 hours of psychology education (behavioral interventions and psychological strategies for pain self-management of pain).
There is also 1 hour of pharmacotherapy education regarding commonly prescribed pain medications and how to take medications safely to avoid common adverse events. The nurse is responsible for care coordination and analysis of outcome measures, data collection, and quality improvement.
Program Effectiveness
Program effectiveness is measured using the POQ-VA (Pain Outcomes Questionnaire-VA). The POQ results and participant feedback are used to ensure ongoing program evaluation and improvement. This outcome measure was selected as the POQ-VA evaluates intervention effectiveness of all the major pain outcomes domains. This questionnaire was developed and validated by the VA.
The sample size was 957 veterans.15 The POQ-VA is reverse scored, meaning lower scores indicate improvement. Eighty-seven veterans have completed the program with 20 participants completing the 3-month outcome measures, 31 participants completing 6-month outcome measures, and 17 participants completing 12-month outcome measures.
The pain score decreased close to 1 point at 12 months. The mobility gains were maintained at 12 months. The ADL did not improve much after 1 year (Figure 1).
In the other POQ-VA subscales, vitality improved somewhat.
Limitations
Only a small sample size of veterans with chronic pain participated in the functional restoration pain program. Long-term follow-up of participants who successfully completed the program also is desired.
Conclusion
Veterans experiencing complex chronic noncancer pain present a challenge for the VA health care system. Successful management of this requires cooperation among different disciplines and fostering a multimodal and interdisciplinary approach. Functional restoration pain programs have existed for a while and have shown clear evidence of their superiority over monotherapies for patients with chronic noncancer pain.
This functional restoration pain program incorporated various evidence-based medical, rehabilitative, psychological interventions with mind body medicine, mindfulness and integrative pain modalities. The authors continually meet and assess the success of the program. Although the initial outcome measures are encouraging, increased veteran participation in answering their post program completion surveys is desired. The goal is to improve veterans’ self-management of their chronic pain, leading to reductions in pain symptoms, medication, and health care provider use, as well as improve veterans’ function and overall QOL.
1. International Association for the Study of Pain. IASP taxonomy. https://www.iasp-pain.org/Taxonomy#Pain. Updated May 22, 2012. Accessed August 31, 2017.
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254.
3. U.S. Census Bureau. 2011-2015 American community services 5-year estimates. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_15_5YRB21002&prodType=table. Accessed August 31, 2017.
4. U.S. Census Bureau, population division. Annual estimates of the resident population: April 1, 2010 to July 1, 2016. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview .xhtml?pid=PEP_2016_PEPANNRES&SPC=pt. Accessed August 31, 2017.
5. Hogan ME, Taddio A, Katz J, Shah V, Krahn M. Health utilities in people with chronic pain using a population level survey and linked health care administrative data. Pain. 2017;158(3):408-416.
6. Kerns RD, Otis J, Rosenberg R, Reid MC. Veterans reports of pain and associations with ratings of health, health risk-behaviors, affective distress and use of the healthcare system. J Rehabil Res Dev. 2003;40(5):371-379.
7. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378.
8. U.S. Department of Veterans Affairs. Analysis of VA health care utilization among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) veterans. https://www.publichealth.va.gov/docs/epidemiol ogy/healthcare-utilization-report-fy2015-qtr3.pdf. Published January 2017. Accessed August 31, 2017.
9. National Academies of Science. Institute of Medicine: Relieving pain in America: a blueprint for transforming prevention care, education, and research. https://iprcc.nih.gov/docs/032712_mtg _presentations/iom_pain_report_508comp.pdf. Published June 29, 2011. Accessed August 31, 2017.
10. U.S. Department of Veterans Affairs. Transforming VA pain care. https://www.va.gov/painmanagement/Updated August 17, 2017. Accessed August 31, 2017.
11. American Pain Society. Interdisciplinary pain management. http://americanpainsociety.org/uploads/about/position-statements/interdisciplinary-white -paper.pdf. Accessed August 31, 2017.
12. Stanos S. Focused review of interdisciplinary pain rehabilitation programs for chronic pain management. Curr Pain Headache Rep. 2012;16(2):147-152.
13. Cosio D, Lin EH. (538) Efficacy of an outpatient, multidisciplinary VA pain management clinic: findings from a one-year outcome study. Pain. 2014;15(4):S110.
14. Cosio D, Lin EH. Effects of a pain education program in complementary and alternative medicine treatment utilization at a VA medical center. Complement Ther Med. 2015;23(3):413-422.
15. Clark ME, Gironda RJ, Young RW. Development and validation of the pain outcomes questionnaire-VA. J Rehabil Res Dev. 2003;40(5)-381-395.
According to the International Association for the Study of Pain (IASP), pain is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”1 Chronic pain (pain lasting more than 3 months) has a high prevalence in the U.S. veteran population. In a recently published article by Richard Nahin, PhD, of the National Institutes of Health, 65.5% of U.S. veterans reported pain in the previous 3 months with 9.1% classified as having severe pain (defined as “which occurs most days or every day and bothers the individual a lot”) compared with 6.4% among nonveterans.2 In addition, male veterans were more likely to report severe pain, 9%, compared with male nonveterans, 4.7%.2 Veterans make up about 6.2% of the U.S. population; therefore, the number of veterans negatively impacted by pain is substantial.3,4 Compared with individuals with other chronic diseases, such as heart disease, chronic obstructive pulmonary disease, or diabetes mellitus, a recent population-based, matched cohort study reported that only patients with Alzheimer disease have a poorer quality of life (QOL) than do those with chronic pain.5
Background
When comparing veterans to nonveterans, Nahin also reported that younger veterans aged 18 to 39 years had significantly higher rates for severe pain, compared with similarly aged nonveterans, 7.8% vs 3.2%, respectively. The prevalence of severe pain was significantly higher among veterans than it was for nonveterans experiencing the following: back pain, 21.6% vs 16.7% among nonveterans; jaw pain, 37.5% vs 22.9%, respectively; severe migraine and headaches, 26.4% vs 15.9%, respectively; and neck pain, 27.7% vs 21.9%, respectively. The veterans also were more likely than were nonveterans to have joint pain, 43.6% vs 31.5% , respectively.2
A study by Kerns and colleagues noted that almost 50% of older veterans (mean age 65.6 years) experience chronic pain regularly.6 Based on responses of 685 veterans to the Health-Risk Behavior Screening Questionnaire (HRBSQ), this study also found that the presence of pain was strongly associated with patient reports of worsening health and emotional distress. Rollin Gallagher, MD, of the Philadelphia VAMC, reported that veterans who experienced pain tended to have more personal problems due to higher rates of psychiatric and social comorbidities, such as substance abuse, depression, posttraumatic stress syndrome, and early work disabilities.7 Gallagher also has noted that the number of veterans seeking pain treatment has grown steadily over the past 2 decades due to the aging veteran population retiring and seeking VA care for chronic illness management.
In January 2017, the VA released an analysis of health care use among recent Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), and Operation New Dawn (OND) veterans from October 2001 through June 2015.8 The VA noted that 1,965,534 veterans have become eligible for VA health care since fiscal year 2002. Of the 1,218,857 OIF/OEF/OND veterans treated during this period, 62.3% (759,850) were treated for diseases of the musculoskeletal system and connective tissue, 58.1% (708,062) were treated for mental disorders, and 58.7% (715,263) were treated for “symptoms, signs and ill-defined conditions.”
According to the VA, “the ICD-9-CM diagnostic category ‘Symptoms, Signs and Ill-Defined Conditions’ is a diverse, catch-all category that consists of 160 sub-categories and includes primarily symptoms that do not yet have an identified cause and clinical findings that are not coded elsewhere.” The most frequently reported codes in this category, in order of magnitude are General Symptoms (ICD-9-CM 780), Symptoms Involving Respiratory System and Other Chest Symptoms (ICD-9-CM 786), and Symptoms Involving Head and Neck (ICD-9-CM 784).
Musculoskeletal ailments (ie, joint and back disorders), mental health disorders and symptoms, signs, and ill-defined conditions are the 3 most frequently coded diagnoses related to medical treatment in OEF/OIF/OND veterans. This demonstrates the high rate of pain-related conditions with comorbid mental health diagnoses.
Public Health Challenge
Recognizing that pain is a public health challenge, the National Academy of Sciences published the landmark study Relieving Pain in America.9 The study reported that pain affects at least 100 million Americans, greatly reducing quality of life. In addition, annual financial costs to society are estimated at $560 to $635 billion, with federal and state costs almost $100 billion annually. Given the challenges of addressing chronic pain, especially in the U.S. veteran population, the VHA has likewise outlined 6 recommendations for transforming VA pain care:
- Educate veterans/families to promote self-efficacy and shared decision making, provide access to all relevant sources;
- Educate/train all team members to their discipline-specific competencies, including team-based care;
- Develop and integrate nonpharmacologic modalities into care plans;
- Institute evidence-based medication prescribing, use of pain procedures, and safe opioid use (universal precautions);
- Implement approaches for bringing the veteran’s whole team together, such as virtual pain consulting (SCAN-ECHO, e-consults, telehealth, clinical video teleconsultation and education) and for maintaining ongoing communication between team members; and
- Establish metrics to monitor pain care and outcomes at both the individual level and population level.10
The American Pain Society (APS) differentiates multidisciplinary care vs interdisciplinary pain care.11 Multidisciplinary pain care is provided by several disciplines that may not be coordinated. Treatment may occur with different goals and in parallel rather than with an integrated approach. The APS suggests that professional identities are clearly defined, team membership is a secondary consideration in multidisciplinary care, and the leadership is typically hierarchical with a physician in charge. In this model of care, each team member has a “clearly defined place in the overall care of the patient, contributing their expertise in relative isolation from one another.”11
In contrast, according to APS, interdisciplinary teams have complementary roles that enhance patient care. Each discipline has valuable knowledge and a set of skills that complement other team members who are collaborative partners. The interdisciplinary approach encourages complementary roles and responsibilities, conjoint problem solving, and shared accountability. Treatment decisions are consensus based.
Pain Programs
In a review of 4 interdisciplinary pain programs (Mayo Clinic Pain Rehabilitation Center, the Brooks Rehabilitation Pain Rehabilitation Program, the Rehabilitation Institute of Chicago Center for Pain Management, and the Cleveland Clinic Foundation Chronic Pain Rehabilitation Program), Stanos found that the compositions of the staff were similar.12 In general, staff consisted of pain management physicians, pain psychologists, physical and occupational therapists, and nurse coordinators. The Mayo Clinic had more personnel, including a clinical pharmacist, the Brooks program had an additional biofeedback specialist, and the Cleveland Clinic had a tai chi instructor. The programs ranged from 3 to 5 weeks of daily programming. The duration of services provided were dependent on the payers. Stanos concluded that functional status, as measured by the Pain Disability Index, improved on discharge, 6 months, and 1 year after treatment at the Cleveland Clinic.
Cosio and Lin described their experience in a multidisciplinary outpatient pain clinic at Jesse Brown VAMC in Chicago.13 Their study noted that the number of veterans in their multidisciplinary pain clinic on chronic opioids significantly decreased, the degree of pain relief increased, and veterans reported improvements in mobility and ability to complete activities of daily living (ADLs). Overall veteran satisfaction with this pain program was reportedly high.
Cosio and Lin also published a study of the effect of complementary alternative medicine (CAM) utilization at a VAMC, which included a 12-week pain education school that was offered to all veterans and families.14 They noted that veterans began using at least 1 more CAM modality before the completion of the pain education program. However, it is unclear from the 2 studies whether the pain education program was incorporated into their multidisciplinary pain clinic.
Outpatient Functional Restoration Program
Given the challenges of addressing chronic pain and at the same time fostering an interdisciplinary approach to management, the VA Puget Sound Health Care System (VAPSHCS) team initiated a program development and quality improvement process for addressing pain and restoring function for veteran patients.
The VA Northwest Health Network (VISN 20) offers health care services for veterans located in the states of Alaska, Idaho, Oregon, Washington, and parts of California and Montana. VISN 20 has 8 parent facilities, which include the Seattle and American Lake divisions of the VAPSHCS. The VAPSHCS has established a comprehensive, interdisciplinary functional restoration pain program that integrates medical, psychosocial, and complementary alternative medicine.
The Outpatient Functional Restoration Program (OFRPP) pain team consists of a chief who is board certified in pain medicine and addiction medicine; a board-certified pain medicine physician; 2 physician assistants, one of whom has formal training in acupuncture and another who is trained in tai chi, qigong, hypnosis, and mindfulness; nurse care coordinators; a pain psychologist with training in acceptance and commitment therapy, cognitive behavioral therapy, yoga nidra, and hypnosis; a second pain psychologist who has a background in rehabilitation psychology; a physical therapist; and a pain clinical pharmacy specialist.
Prior to participation in OFRPP, veterans were required to attend 4 weekly pain education classes for 4 consecutive weeks. The classes educate veterans and their families on the complexity of managing chronic pain. Topics cover medical, pharmacologic and nonpharmacologic approaches to pain, including CAM and psychological modalities (Table 1). The pain orientation classes introduce veterans to available treatment options, and in some cases, veterans decide committing to a more intensive pain rehabilitation program is a good fit.
The program is based on the biopsychosocial model of pain care and Commission on Accreditation for Rehabilitation Facilities (CARF) interdisciplinary pain rehabilitation program standards. The length of the program was determined after reviewing data from existing VA outpatient pain rehabilitation programs; Pain Clinic staff availability, training and experience; and survey responses from veterans completing the 4-week education. This survey asked veterans whether they would be interested in an outpatient pain rehabilitation program and their preference for length of the program and treatment modalities.
Since its inception, OFRPP has earned a 3-year CARF accreditation. Veterans participate in VAPSHCS American Lake division OFRPP education twice weekly for 4 hours for a total of 8 weeks (Table 2). Each week of programming includes 2 hours of physical therapy didactics, 2 hours of physical therapy (eg, paced cardio exercise, stretching, and core strength and conditioning), 2 hours of mind-body medicine (eg, mantram repetition and neuroplasticity education), and 2 hours of psychology education (behavioral interventions and psychological strategies for pain self-management of pain).
There is also 1 hour of pharmacotherapy education regarding commonly prescribed pain medications and how to take medications safely to avoid common adverse events. The nurse is responsible for care coordination and analysis of outcome measures, data collection, and quality improvement.
Program Effectiveness
Program effectiveness is measured using the POQ-VA (Pain Outcomes Questionnaire-VA). The POQ results and participant feedback are used to ensure ongoing program evaluation and improvement. This outcome measure was selected as the POQ-VA evaluates intervention effectiveness of all the major pain outcomes domains. This questionnaire was developed and validated by the VA.
The sample size was 957 veterans.15 The POQ-VA is reverse scored, meaning lower scores indicate improvement. Eighty-seven veterans have completed the program with 20 participants completing the 3-month outcome measures, 31 participants completing 6-month outcome measures, and 17 participants completing 12-month outcome measures.
The pain score decreased close to 1 point at 12 months. The mobility gains were maintained at 12 months. The ADL did not improve much after 1 year (Figure 1).
In the other POQ-VA subscales, vitality improved somewhat.
Limitations
Only a small sample size of veterans with chronic pain participated in the functional restoration pain program. Long-term follow-up of participants who successfully completed the program also is desired.
Conclusion
Veterans experiencing complex chronic noncancer pain present a challenge for the VA health care system. Successful management of this requires cooperation among different disciplines and fostering a multimodal and interdisciplinary approach. Functional restoration pain programs have existed for a while and have shown clear evidence of their superiority over monotherapies for patients with chronic noncancer pain.
This functional restoration pain program incorporated various evidence-based medical, rehabilitative, psychological interventions with mind body medicine, mindfulness and integrative pain modalities. The authors continually meet and assess the success of the program. Although the initial outcome measures are encouraging, increased veteran participation in answering their post program completion surveys is desired. The goal is to improve veterans’ self-management of their chronic pain, leading to reductions in pain symptoms, medication, and health care provider use, as well as improve veterans’ function and overall QOL.
According to the International Association for the Study of Pain (IASP), pain is “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”1 Chronic pain (pain lasting more than 3 months) has a high prevalence in the U.S. veteran population. In a recently published article by Richard Nahin, PhD, of the National Institutes of Health, 65.5% of U.S. veterans reported pain in the previous 3 months with 9.1% classified as having severe pain (defined as “which occurs most days or every day and bothers the individual a lot”) compared with 6.4% among nonveterans.2 In addition, male veterans were more likely to report severe pain, 9%, compared with male nonveterans, 4.7%.2 Veterans make up about 6.2% of the U.S. population; therefore, the number of veterans negatively impacted by pain is substantial.3,4 Compared with individuals with other chronic diseases, such as heart disease, chronic obstructive pulmonary disease, or diabetes mellitus, a recent population-based, matched cohort study reported that only patients with Alzheimer disease have a poorer quality of life (QOL) than do those with chronic pain.5
Background
When comparing veterans to nonveterans, Nahin also reported that younger veterans aged 18 to 39 years had significantly higher rates for severe pain, compared with similarly aged nonveterans, 7.8% vs 3.2%, respectively. The prevalence of severe pain was significantly higher among veterans than it was for nonveterans experiencing the following: back pain, 21.6% vs 16.7% among nonveterans; jaw pain, 37.5% vs 22.9%, respectively; severe migraine and headaches, 26.4% vs 15.9%, respectively; and neck pain, 27.7% vs 21.9%, respectively. The veterans also were more likely than were nonveterans to have joint pain, 43.6% vs 31.5% , respectively.2
A study by Kerns and colleagues noted that almost 50% of older veterans (mean age 65.6 years) experience chronic pain regularly.6 Based on responses of 685 veterans to the Health-Risk Behavior Screening Questionnaire (HRBSQ), this study also found that the presence of pain was strongly associated with patient reports of worsening health and emotional distress. Rollin Gallagher, MD, of the Philadelphia VAMC, reported that veterans who experienced pain tended to have more personal problems due to higher rates of psychiatric and social comorbidities, such as substance abuse, depression, posttraumatic stress syndrome, and early work disabilities.7 Gallagher also has noted that the number of veterans seeking pain treatment has grown steadily over the past 2 decades due to the aging veteran population retiring and seeking VA care for chronic illness management.
In January 2017, the VA released an analysis of health care use among recent Operation Iraqi Freedom (OIF), Operation Enduring Freedom (OEF), and Operation New Dawn (OND) veterans from October 2001 through June 2015.8 The VA noted that 1,965,534 veterans have become eligible for VA health care since fiscal year 2002. Of the 1,218,857 OIF/OEF/OND veterans treated during this period, 62.3% (759,850) were treated for diseases of the musculoskeletal system and connective tissue, 58.1% (708,062) were treated for mental disorders, and 58.7% (715,263) were treated for “symptoms, signs and ill-defined conditions.”
According to the VA, “the ICD-9-CM diagnostic category ‘Symptoms, Signs and Ill-Defined Conditions’ is a diverse, catch-all category that consists of 160 sub-categories and includes primarily symptoms that do not yet have an identified cause and clinical findings that are not coded elsewhere.” The most frequently reported codes in this category, in order of magnitude are General Symptoms (ICD-9-CM 780), Symptoms Involving Respiratory System and Other Chest Symptoms (ICD-9-CM 786), and Symptoms Involving Head and Neck (ICD-9-CM 784).
Musculoskeletal ailments (ie, joint and back disorders), mental health disorders and symptoms, signs, and ill-defined conditions are the 3 most frequently coded diagnoses related to medical treatment in OEF/OIF/OND veterans. This demonstrates the high rate of pain-related conditions with comorbid mental health diagnoses.
Public Health Challenge
Recognizing that pain is a public health challenge, the National Academy of Sciences published the landmark study Relieving Pain in America.9 The study reported that pain affects at least 100 million Americans, greatly reducing quality of life. In addition, annual financial costs to society are estimated at $560 to $635 billion, with federal and state costs almost $100 billion annually. Given the challenges of addressing chronic pain, especially in the U.S. veteran population, the VHA has likewise outlined 6 recommendations for transforming VA pain care:
- Educate veterans/families to promote self-efficacy and shared decision making, provide access to all relevant sources;
- Educate/train all team members to their discipline-specific competencies, including team-based care;
- Develop and integrate nonpharmacologic modalities into care plans;
- Institute evidence-based medication prescribing, use of pain procedures, and safe opioid use (universal precautions);
- Implement approaches for bringing the veteran’s whole team together, such as virtual pain consulting (SCAN-ECHO, e-consults, telehealth, clinical video teleconsultation and education) and for maintaining ongoing communication between team members; and
- Establish metrics to monitor pain care and outcomes at both the individual level and population level.10
The American Pain Society (APS) differentiates multidisciplinary care vs interdisciplinary pain care.11 Multidisciplinary pain care is provided by several disciplines that may not be coordinated. Treatment may occur with different goals and in parallel rather than with an integrated approach. The APS suggests that professional identities are clearly defined, team membership is a secondary consideration in multidisciplinary care, and the leadership is typically hierarchical with a physician in charge. In this model of care, each team member has a “clearly defined place in the overall care of the patient, contributing their expertise in relative isolation from one another.”11
In contrast, according to APS, interdisciplinary teams have complementary roles that enhance patient care. Each discipline has valuable knowledge and a set of skills that complement other team members who are collaborative partners. The interdisciplinary approach encourages complementary roles and responsibilities, conjoint problem solving, and shared accountability. Treatment decisions are consensus based.
Pain Programs
In a review of 4 interdisciplinary pain programs (Mayo Clinic Pain Rehabilitation Center, the Brooks Rehabilitation Pain Rehabilitation Program, the Rehabilitation Institute of Chicago Center for Pain Management, and the Cleveland Clinic Foundation Chronic Pain Rehabilitation Program), Stanos found that the compositions of the staff were similar.12 In general, staff consisted of pain management physicians, pain psychologists, physical and occupational therapists, and nurse coordinators. The Mayo Clinic had more personnel, including a clinical pharmacist, the Brooks program had an additional biofeedback specialist, and the Cleveland Clinic had a tai chi instructor. The programs ranged from 3 to 5 weeks of daily programming. The duration of services provided were dependent on the payers. Stanos concluded that functional status, as measured by the Pain Disability Index, improved on discharge, 6 months, and 1 year after treatment at the Cleveland Clinic.
Cosio and Lin described their experience in a multidisciplinary outpatient pain clinic at Jesse Brown VAMC in Chicago.13 Their study noted that the number of veterans in their multidisciplinary pain clinic on chronic opioids significantly decreased, the degree of pain relief increased, and veterans reported improvements in mobility and ability to complete activities of daily living (ADLs). Overall veteran satisfaction with this pain program was reportedly high.
Cosio and Lin also published a study of the effect of complementary alternative medicine (CAM) utilization at a VAMC, which included a 12-week pain education school that was offered to all veterans and families.14 They noted that veterans began using at least 1 more CAM modality before the completion of the pain education program. However, it is unclear from the 2 studies whether the pain education program was incorporated into their multidisciplinary pain clinic.
Outpatient Functional Restoration Program
Given the challenges of addressing chronic pain and at the same time fostering an interdisciplinary approach to management, the VA Puget Sound Health Care System (VAPSHCS) team initiated a program development and quality improvement process for addressing pain and restoring function for veteran patients.
The VA Northwest Health Network (VISN 20) offers health care services for veterans located in the states of Alaska, Idaho, Oregon, Washington, and parts of California and Montana. VISN 20 has 8 parent facilities, which include the Seattle and American Lake divisions of the VAPSHCS. The VAPSHCS has established a comprehensive, interdisciplinary functional restoration pain program that integrates medical, psychosocial, and complementary alternative medicine.
The Outpatient Functional Restoration Program (OFRPP) pain team consists of a chief who is board certified in pain medicine and addiction medicine; a board-certified pain medicine physician; 2 physician assistants, one of whom has formal training in acupuncture and another who is trained in tai chi, qigong, hypnosis, and mindfulness; nurse care coordinators; a pain psychologist with training in acceptance and commitment therapy, cognitive behavioral therapy, yoga nidra, and hypnosis; a second pain psychologist who has a background in rehabilitation psychology; a physical therapist; and a pain clinical pharmacy specialist.
Prior to participation in OFRPP, veterans were required to attend 4 weekly pain education classes for 4 consecutive weeks. The classes educate veterans and their families on the complexity of managing chronic pain. Topics cover medical, pharmacologic and nonpharmacologic approaches to pain, including CAM and psychological modalities (Table 1). The pain orientation classes introduce veterans to available treatment options, and in some cases, veterans decide committing to a more intensive pain rehabilitation program is a good fit.
The program is based on the biopsychosocial model of pain care and Commission on Accreditation for Rehabilitation Facilities (CARF) interdisciplinary pain rehabilitation program standards. The length of the program was determined after reviewing data from existing VA outpatient pain rehabilitation programs; Pain Clinic staff availability, training and experience; and survey responses from veterans completing the 4-week education. This survey asked veterans whether they would be interested in an outpatient pain rehabilitation program and their preference for length of the program and treatment modalities.
Since its inception, OFRPP has earned a 3-year CARF accreditation. Veterans participate in VAPSHCS American Lake division OFRPP education twice weekly for 4 hours for a total of 8 weeks (Table 2). Each week of programming includes 2 hours of physical therapy didactics, 2 hours of physical therapy (eg, paced cardio exercise, stretching, and core strength and conditioning), 2 hours of mind-body medicine (eg, mantram repetition and neuroplasticity education), and 2 hours of psychology education (behavioral interventions and psychological strategies for pain self-management of pain).
There is also 1 hour of pharmacotherapy education regarding commonly prescribed pain medications and how to take medications safely to avoid common adverse events. The nurse is responsible for care coordination and analysis of outcome measures, data collection, and quality improvement.
Program Effectiveness
Program effectiveness is measured using the POQ-VA (Pain Outcomes Questionnaire-VA). The POQ results and participant feedback are used to ensure ongoing program evaluation and improvement. This outcome measure was selected as the POQ-VA evaluates intervention effectiveness of all the major pain outcomes domains. This questionnaire was developed and validated by the VA.
The sample size was 957 veterans.15 The POQ-VA is reverse scored, meaning lower scores indicate improvement. Eighty-seven veterans have completed the program with 20 participants completing the 3-month outcome measures, 31 participants completing 6-month outcome measures, and 17 participants completing 12-month outcome measures.
The pain score decreased close to 1 point at 12 months. The mobility gains were maintained at 12 months. The ADL did not improve much after 1 year (Figure 1).
In the other POQ-VA subscales, vitality improved somewhat.
Limitations
Only a small sample size of veterans with chronic pain participated in the functional restoration pain program. Long-term follow-up of participants who successfully completed the program also is desired.
Conclusion
Veterans experiencing complex chronic noncancer pain present a challenge for the VA health care system. Successful management of this requires cooperation among different disciplines and fostering a multimodal and interdisciplinary approach. Functional restoration pain programs have existed for a while and have shown clear evidence of their superiority over monotherapies for patients with chronic noncancer pain.
This functional restoration pain program incorporated various evidence-based medical, rehabilitative, psychological interventions with mind body medicine, mindfulness and integrative pain modalities. The authors continually meet and assess the success of the program. Although the initial outcome measures are encouraging, increased veteran participation in answering their post program completion surveys is desired. The goal is to improve veterans’ self-management of their chronic pain, leading to reductions in pain symptoms, medication, and health care provider use, as well as improve veterans’ function and overall QOL.
1. International Association for the Study of Pain. IASP taxonomy. https://www.iasp-pain.org/Taxonomy#Pain. Updated May 22, 2012. Accessed August 31, 2017.
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254.
3. U.S. Census Bureau. 2011-2015 American community services 5-year estimates. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_15_5YRB21002&prodType=table. Accessed August 31, 2017.
4. U.S. Census Bureau, population division. Annual estimates of the resident population: April 1, 2010 to July 1, 2016. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview .xhtml?pid=PEP_2016_PEPANNRES&SPC=pt. Accessed August 31, 2017.
5. Hogan ME, Taddio A, Katz J, Shah V, Krahn M. Health utilities in people with chronic pain using a population level survey and linked health care administrative data. Pain. 2017;158(3):408-416.
6. Kerns RD, Otis J, Rosenberg R, Reid MC. Veterans reports of pain and associations with ratings of health, health risk-behaviors, affective distress and use of the healthcare system. J Rehabil Res Dev. 2003;40(5):371-379.
7. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378.
8. U.S. Department of Veterans Affairs. Analysis of VA health care utilization among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) veterans. https://www.publichealth.va.gov/docs/epidemiol ogy/healthcare-utilization-report-fy2015-qtr3.pdf. Published January 2017. Accessed August 31, 2017.
9. National Academies of Science. Institute of Medicine: Relieving pain in America: a blueprint for transforming prevention care, education, and research. https://iprcc.nih.gov/docs/032712_mtg _presentations/iom_pain_report_508comp.pdf. Published June 29, 2011. Accessed August 31, 2017.
10. U.S. Department of Veterans Affairs. Transforming VA pain care. https://www.va.gov/painmanagement/Updated August 17, 2017. Accessed August 31, 2017.
11. American Pain Society. Interdisciplinary pain management. http://americanpainsociety.org/uploads/about/position-statements/interdisciplinary-white -paper.pdf. Accessed August 31, 2017.
12. Stanos S. Focused review of interdisciplinary pain rehabilitation programs for chronic pain management. Curr Pain Headache Rep. 2012;16(2):147-152.
13. Cosio D, Lin EH. (538) Efficacy of an outpatient, multidisciplinary VA pain management clinic: findings from a one-year outcome study. Pain. 2014;15(4):S110.
14. Cosio D, Lin EH. Effects of a pain education program in complementary and alternative medicine treatment utilization at a VA medical center. Complement Ther Med. 2015;23(3):413-422.
15. Clark ME, Gironda RJ, Young RW. Development and validation of the pain outcomes questionnaire-VA. J Rehabil Res Dev. 2003;40(5)-381-395.
1. International Association for the Study of Pain. IASP taxonomy. https://www.iasp-pain.org/Taxonomy#Pain. Updated May 22, 2012. Accessed August 31, 2017.
2. Nahin RL. Severe pain in veterans: the effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254.
3. U.S. Census Bureau. 2011-2015 American community services 5-year estimates. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=ACS_15_5YRB21002&prodType=table. Accessed August 31, 2017.
4. U.S. Census Bureau, population division. Annual estimates of the resident population: April 1, 2010 to July 1, 2016. https://factfinder.census.gov/faces/tableservices/jsf/pages/productview .xhtml?pid=PEP_2016_PEPANNRES&SPC=pt. Accessed August 31, 2017.
5. Hogan ME, Taddio A, Katz J, Shah V, Krahn M. Health utilities in people with chronic pain using a population level survey and linked health care administrative data. Pain. 2017;158(3):408-416.
6. Kerns RD, Otis J, Rosenberg R, Reid MC. Veterans reports of pain and associations with ratings of health, health risk-behaviors, affective distress and use of the healthcare system. J Rehabil Res Dev. 2003;40(5):371-379.
7. Gallagher RM. Advancing the pain agenda in the veteran population. Anesthesiol Clin. 2016;34(2):357-378.
8. U.S. Department of Veterans Affairs. Analysis of VA health care utilization among Operation Enduring Freedom (OEF), Operation Iraqi Freedom (OIF), and Operation New Dawn (OND) veterans. https://www.publichealth.va.gov/docs/epidemiol ogy/healthcare-utilization-report-fy2015-qtr3.pdf. Published January 2017. Accessed August 31, 2017.
9. National Academies of Science. Institute of Medicine: Relieving pain in America: a blueprint for transforming prevention care, education, and research. https://iprcc.nih.gov/docs/032712_mtg _presentations/iom_pain_report_508comp.pdf. Published June 29, 2011. Accessed August 31, 2017.
10. U.S. Department of Veterans Affairs. Transforming VA pain care. https://www.va.gov/painmanagement/Updated August 17, 2017. Accessed August 31, 2017.
11. American Pain Society. Interdisciplinary pain management. http://americanpainsociety.org/uploads/about/position-statements/interdisciplinary-white -paper.pdf. Accessed August 31, 2017.
12. Stanos S. Focused review of interdisciplinary pain rehabilitation programs for chronic pain management. Curr Pain Headache Rep. 2012;16(2):147-152.
13. Cosio D, Lin EH. (538) Efficacy of an outpatient, multidisciplinary VA pain management clinic: findings from a one-year outcome study. Pain. 2014;15(4):S110.
14. Cosio D, Lin EH. Effects of a pain education program in complementary and alternative medicine treatment utilization at a VA medical center. Complement Ther Med. 2015;23(3):413-422.
15. Clark ME, Gironda RJ, Young RW. Development and validation of the pain outcomes questionnaire-VA. J Rehabil Res Dev. 2003;40(5)-381-395.
Development and Implementation of a Homeless Mobile Medical/Mental Veteran Intervention
Research has consistently identified remarkably high rates of addiction, mental illness, and health problems in the homeless population.1-9 Yet in spite of extensive service needs for these problems, abundant evidence exists of consistent underuse of health care services by homeless populations.10-12 Most of the homeless population reside in emergency shelters or in transitional or supportive housing, but many remain in places not meant for human habitation.
Homelessness is significantly overrepresented among military veterans.13 The January 2016 national point-in-time count identified 39,471 veterans experiencing homelessness.13 Iraq and Afghanistan veterans seem to have an especially high risk for homelessness.13-15 Disheartening statistics such as these prompted former VA Secretary Eric Shinseki to pledge to end veteran homelessness by December 2015.16 He argued in support of this mission that 85% of veteran homeless resources go to health care—implying that homelessness among veterans is primarily a health care issue, which is heavily burdened by substance abuse and other psychiatric and medical illnesses.17
Health care service use has been associated with improved health, mental health, and outcomes among homeless populations.12,18 Unfortunately, access to these services is limited by barriers associated with homelessness, such as transportation or lack of proper identification.19,20 Veterans experiencing homelessness also face these common barriers to health care, and unsheltered veterans especially underutilize VA health care services.21
Housing First—a successful model that places individuals into housing without prerequisites for sobriety, active participation in treatment, or other behavioral accomplishments, such as gainful employment—has not managed yet to place all the disengaged homeless veteran population into stable housing.22 However, the Housing First model, which is based on the individual’s priorities, is consistent with the approach of a new program at the VA North Texas Health Care System (VANTHCS).
The VHA, similar to other health care systems, is engaged in a cultural transformation to convert its health care approach from a traditional medical model to patient-centered care (PCC).23 In this priority area, a strategic objective is for the VHA to partner with each veteran to create a personalized, proactive strategy to optimize health and well-being and when needed provide state-of-the-art disease management. Patient-centered care is designed to address veterans’ specific needs in spiritual, environmental, physical, mental, and social domains and empower veterans to become active participants in their care. Patient-centered care differs from the traditional medical model in that patients are active participants in their treatment, partnering and collaborating with their providers on care that is quality-of-life centered instead of disease centered.23 This model is based on both respect for patients as unique individuals and on the obligation to care for them on their own terms, focused on their self-identified goals and aspirations.24
At VANTHCS, the Homeless Mobile Medical/Mental Veteran (HMMM-V) pilot program was designed to deliver effective health care services to a homeless subpopulation of veterans who historically have been the most difficult to serve—those living in unsheltered environments, such as under bridges and in encampments. The purpose of the HMMM-V program was to contact and serve veterans not currently being reached by the VA system of care, using a PCC model.
This pilot program was initially funded in January 2013 by a 2-year grant from the Office of Patient Centered Care and Cultural Transformation to apply the PCC approach to engage veteran participation. For this project, the VA Personal Health Inventory tool—originally designed for use with the general veteran population—was adapted for use with the homeless veteran population. The grant funding period covered the design, development, and implementation of the HMMM-V program; thereafter, VANTHCS provided resources through its Comprehensive Homeless Center Programs to assure its sustainability and continued use of the clinical assessment tool created for this project.
This article describes the development and implementation of this novel program with sufficient detail to inform the development of similar programs in other sites. Descriptions of the program and staffing, creation of community partnerships, and modification of an assessment instrument are provided. It also illustrates the original implementation period of the HMMM-V program through presentation of self-reported data on the first homeless veterans it served.
Equipment and Staffing
A custom 28-foot mobile outreach vehicle was assembled according to specifications identified by the HMMM-V team as necessary to conduct the program’s interventions. The van became fully operational on April 8, 2015, after it underwent all the required reviews and inspections (eg, safety, infection control, etc) and was accredited in 2015 by the Commission on Accreditation of Rehabilitation Facilities.
The HMMM-V van has a driver compartment that is separate from its services rooms, which include a patient registration area, a fully equipped examination room, a laboratory area, and a bathroom. The vehicle is equipped with a wheelchair lift and an awning to shade outdoor areas where tables and chairs are set up for patient/staff waiting and rest areas. The vehicle is stocked with essential equipment and supplies needed to conduct work in off-street locations, vacant lots, under bridges, fields, unpaved paths, etc. It also is equipped with telemedicine capabilities to provide clinical supervision and access to attending physicians and specialists at VANTHCS. Personnel carry cell phones and laptop computers with secure Internet connections using a commercially available mobile wireless Wi-Fi hotspot to facilitate documentation of medical records and communication from the field.
This reliable type of equipment is routine for use in VA field operations; the only hurdle using these technologies for the program was acquiring funding and purchasing the equipment. The vehicle is further equipped with a refrigerator solely for secure storage of pharmaceutical supplies, a second refrigerator for specimens, and wall-mounted blood pressure and otoscope/ophthalmoscope units. The vehicle is supplied with thermometers, scales, phlebotomy supplies, and first-aid kits and is stocked with vaccines and medications, including antibiotic, hypertensive, diabetic, allergy, and over-the-counter pain medications. A more comprehensive list of supplies for the vehicle is available from the authors on request.
Medication provisions supplied to the HMMM-V mobile clinic conform to the Texas State Board of Pharmacy compliance regulations. Because the vehicle is designated as federal property and has U.S. government license plates, it is considered an extension of VANTHCS Pharmacy Service and falls under its pharmacy license. A medication formulary was created with input from HMMM-V prescribers and Dallas VAMC Pharmacy Service pharmacists. To safeguard the integrity of these pharmaceutical agents, the HMMM-V physician assistant picks up the medications before field deployment and returns the unused medications to the Dallas VAMC at the end of the day. The medications are transported in locked containers and placed either in a locked medication refrigerator or cabinet on the mobile unit.
For medication prescriptions that need laboratory testing before prescribing them, HMMM-V prescribers can check the VA electronic medical record from the field to determine whether these tests have been completed recently. If not, then HMMM-V team has an agreement with Dallas VA Pathology and Laboratory Medicine Service for testing samples obtained in the field.
The program was designed for staffing of the vehicle by 2 professional teams, each includes medical (physician’s assistant or registered nurse), mental health (psychiatrist, residents), and social work providers (licensed social workers, clinical social workers); trainees of these disciplines; a peer support specialist; and an administrative clerk. The staffing varies daily, depending on available personnel. When personnel deploy to the field, they go in pairs or groups to address potential safety issues. Cell phones are available to summon police or ambulance services in an emergency. Systematic safety training was conducted with all field personnel before their first deployment to guard against vulnerability to danger in these settings.
Once in the field, personnel engage unsheltered homeless individuals to assess eligibility for VA services. Veterans found ineligible are assisted with application for military discharge upgrade, service-connected compensation, or appeal for health care coverage. Veterans eligible for VA care receive physical examinations, vital and glucose checks, influenza and pneumonia vaccinations, first-aid skin and wound care, medication management with limited medications provided at point of care, blood and urine testing, peer support services, suicide assessments, clinical mental health evaluations, and social work services through the HMMM-V program.
Social work assistance provided includes psychosocial assessment and care coordination for psychosocial needs such as mental health, substance abuse, vision, dental, housing, employment, legal aid, transportation, food, income, hygiene, and weather-appropriate provision needs.
Community Partnerships
The HMMM-V program benefitted from a number of partnerships with community agencies. During development of the program, HMMM-V personnel accompanied the Dallas Police Department’s Crisis Intervention Unit on typical homeless crisis services deployments into the field to learn about the locations and nature of encampments and homeless peregrination patterns in the Dallas area.
To aid in the design and selection of features for the mobile outreach vehicle, team members toured Homeless Outreach Medical Service mobile clinics from 2 local county hospitals, Parkland Hospital and John Peter Smith Hospital. The staff for these mobile clinics were interviewed about their experience with various components of their programs and their recommendations for optimal design of the mobile medical clinic for service delivery.
Numerous agencies in the Dallas area that serve the homeless population assisted with locating and connecting homeless veterans to HMMM-V programs. These partnering agencies also serve homeless individuals who do not qualify for the HMMM-V program, such as veterans with other-than-honorable military discharges.
The HMMM-V mobile outreach vehicle travels to partnering agencies and provides services on a recurring basis. These agencies are the Dallas International Street Church, a church and faith-based agency aiding the recovery of people with “broken lives”; Cornerstone Ministries, a church-based ministry serving people with adverse circumstances; and City Square’s Opportunity Center, human services and community development programs for low-income city residents. The mobile clinic also travels regularly to other areas to serve homeless veterans residing in unsheltered locations, such as homeless encampments and under bridges.
Clinical Assessment
The project used a modification of the VA Personalized Health Inventory (PHI) for general veteran populations, which assesses 8 areas of self-identified needs to address the specific concerns of homeless veterans served by a mobile clinic.25 Version 19 of the PHI (revised September 18, 2012), the version of the instrument available to the team at the inception of the project, was deployed with the HMMM-V personnel into the field. It imposed a heavy interview time burden (several hours), and its content areas did not seem appropriate to address the immediate concerns of homeless populations (eg, sections pertaining to personal development through hobbies, recreation, or volunteering; healthy living spaces with plenty of lighting and color; “eating healthy, balanced meals with plenty of fruits and vegetables each day”).25
Based on HMMM-V personnel feedback, the team modified this tool and developed a patient-centered health inventory (P-CHI) for homeless veterans that was acceptable in length and applicable to the situational characteristics of homeless existence. The tool’s 10 “current and desired states” were revised to remove domains of exercise and flexibility, sleep and relaxation, and mind-body techniques. The intervention and prevention domains were combined. A material needs (clothing, furniture, transportation, financial benefits) domain was added, and a new domain on reducing alcohol/drug use was created by moving this material from the food and drink domain.
The remaining domains were modified to fit the homeless living situation (Food and Drink = Nutrition; Personal Development = Employment/Vocation; Family, Friends, and Co-Workers = Family/Social/Legal Support; Spirit and Soul = Personal/Spiritual Fulfillment; Surroundings = Housing). Current state ratings were revised to reflect level of satisfaction, and ratings of Desired State were replaced with level of importance.
The modifications resulted in 9 domains, which were assembled into a grid for efficient rating of both satisfaction and importance for each domain (rated 1 to 10, lowest to highest, respectively), followed by an instruction to mark an X in a designated space in all the domains with which the individual would like help (Table). The intent was to reduce the burden of the instrument by having the participant complete sections providing detailed information about only the domains selected by the participant.
The details of each domain in the original VA PHI tool were captured through open-ended questions in text responses provided by the veteran. Because open-ended text responses are not conducive for summarizing characteristics of the population served or for evaluating program activities, the detailed sections covering the domains were revised completely to capture data within categoric and numeric variables. Items from the validated Homeless Supplement Interview were added to collect information not provided in the Homeless Operations Management and Evaluation System interview that is routinely administered to all veterans accessing homeless VA services.26-28
The information collected in these domains cover duration of current homeless episode, lifetime number of homeless episodes, current living arrangements and dissatisfactions with these arrangements, frequency and source of meals, employment history and current work status, sources of income, special material needs, medical and dental problems and sources of care, current medications, mental health problems and sources of care, urgent mental health concerns, current amount and frequency of alcohol and drug use, substance abuse treatment history, relationships with family and intimate partners, legal assistance needs, and self-identified needs for spiritual and personal fulfillment. This instrument is available on request to the authors.
Veterans Served
The project began with 1 team of professionals deploying with the HMMM-V vehicle while a second team was being assembled. Currently, the 2 HMMM-V teams deploy the mobile clinic 4 days per week. The mobile clinic visits agencies that serve the homeless, including emergency shelters and food ministries, as well as homeless encampments. To date, 195 homeless veterans have been served by the mobile clinic, 111 were currently enrolled with the VA, 8 were not enrolled but eligible for services, and 77 were not eligible for VA services. Of the unenrolled veterans, those eligible for services were offered VA enrollment assistance; those ineligible for VA services were offered a community referral.
For the veterans encountered in the field, the following interventions were provided: 49 housing placement referrals, 4 rental assistance referrals, 4 legal referrals, 27 medical care interventions, 13 dental referrals, 11 vision/hearing referrals, 12 mental health interventions, 9 substance abuse treatment referrals, 14 employment assistance referrals, 13 disability benefit applications, 18 transportation assists, 23 goods delivered, and 159 information assists. The HMMM-V mobile clinic also is deployed to participate in various educational and outreach events. At the time this article was written, the mobile clinic has reached nearly 2,000 veterans and community partners in at least 25 such events.
Of the veterans served to date, 73 completed the P-CHI. These veterans were predominantly male (77%), and the majority (60%) were black. The median age of the sample was 58 years, and typically they had a high school level of education (12.7; SD, 2.1 mean years of education). About half (49%) the sample were separated or divorced, and only a minority were currently married (8%). Half (50%) the sample served in the U.S. Army, with the post-Vietnam era being the era of service most represented (19%). Few (21%) veterans reported exposure to hostile or friendly fire during their service. More than three-fourths (80%) of the sample had experienced a homeless episode prior to their current one. On average, members of the sample had experienced a median of 3 lifetime homeless episodes. They had a mean 4.1 (SD, 5.8) lifetime number of years of homelessness, and 3.0 (SD, 5.2) years in their current homeless episode. Nearly one-third (31%) reported that they were currently staying in a homeless shelter, and nearly one-sixth (16%) were currently unsheltered in street settings, such as under bridges or in outdoor encampments at the time of the initial visit.
The mean number of minutes spent completing the P-CHI was 18.5 (SD, 9.4). The veterans indicated that they would like assistance with a mean 3.2 (SD, 2.2) number of domains. The domains with the highest average importance ratings were housing (mean, 9.4; SD, 1.7) and medical/dental care (mean, 8.9; SD, 2.2); the domains with the lowest average importance rating were reducing alcohol/drug use (mean, 6.4; SD, 4.1) and employment/vocation (mean, 6.3; SD, 4.2). The domains with the highest average satisfaction ratings were personal/spiritual fulfillment (mean, 7.3; SD, 2.9) and reducing substance use (mean, 5.9; SD, 4.0), and the domains with the lowest average satisfaction ratings were housing (mean, 2.9; SD, 2.9), material needs (mean, 4.2; SD, 3.3), and employment/vocation (mean, 4.2; SD, 3.2). The domain with the greatest indication of desire for help was housing, endorsed by more than four-fifths (84%) of the sample. This highly endorsed housing domain also was one of the lowest in satisfaction. The domains with the least expressed interest in obtaining help were reducing substance use (18%) and personal/spiritual fulfillment (15%).Reducing substance abuse also was one of the lowest domains of importance and the least for dissatisfaction.
Challenges and Barriers
As anticipated from its inception, this project encountered many challenges and barriers. The first was with the design, construction, and delivery of the mobile clinic unit. The vehicle took more than 2 years to be delivered. There were delays in progress necessitated by required selection of an approved vendor to build the vehicle, extensive specification of details and features, and stocking it with equipment and supplies. The weight of the unit had to be < 26,000 pounds to avoid the requirement of a commercial driver’s license, which limited the size of the vehicle to 28 feet. Stocking the unit with equipment and supplies required attention to a myriad of specifications and decisions. For example, separate refrigerators were needed for specimens, medications, and food; pharmaceutical regulations governing medications in mobile clinics required strict adherence; and difficulties were encountered in attempting to establish adequate and secure connectivity for communications devices in the field.
Once the mobile unit was delivered and prepared for deployment, the next set of challenges pertained to learning all of the instructions required to operate and drive the vehicle and learning how to maneuver the vehicle in the field. Specific challenges for driving the vehicle encountered included unexpectedly low overpasses that prohibited passage, narrow spaces for passage, rough and uneven terrain in off-road settings, and lateral and vertical tilt of roads creating potential for sideswipes and undercarriage scrapes. Maintenance schedules needed to be developed and implemented for cleaning the unit, inspection compliance, repairs, refueling, and emptying waste materials.
Staffing the vehicle required the development of unique job specifications addressing special expertise in accessing VA databases for veteran verification and registration and for driving the mobile clinic vehicle. Schedules and deployment plans for 2 teams that shared the same vehicle had to be established and followed. Locating veterans in unsheltered settings, such as under bridges and in encampments, required community intelligence facilitated through partnerships with knowledgeable members of the Dallas police crisis unit and by gaining field experience to locate where the usual homeless gathering places are, especially those inhabited by veterans. Safety of team members and equipment/supplies in the field was paramount from the start, and additional steps beyond safety training required extra measures, such as special care in navigating known dangerous areas. Provision of services necessitated completion of everything needed in a single visit due to the likelihood of loss to follow-up and acceptance of the limited types of service that could be provided in a mobile clinic. Special procedures were needed to provide referrals to sources of available care for non-VA-qualifying veterans.
Discussion
The HMMM-V program for delivery of PCC to homeless veterans is an innovative pilot program designed to connect with difficult-to-reach homeless veterans and engage them in care. The deliverables provided by this project are (1) A mobile outreach vehicle to deliver care to homeless veterans and outreach to other veterans and community agencies in North Texas; (2) The P-CHI assessment tool for homeless veterans modified and adapted for use with this special population; and (3) pilot data on its first cohort of homeless veterans served, describing their baseline characteristics and their stated satisfaction and preferences about their goals and aspirations for their physical, emotional, and mental health and well-being.
The HMMM-V program successfully identified homeless veterans in need of services, and more than one-third of these veterans were not previously engaged in VA services. Compared with the “typical” veterans served at VANTHCS homeless programs, veterans served by the HMMM-V comprised a greater proportion of minorities and a higher proportion who had been exposed to combat.29 Age and gender characteristics were similar.29 When compared with veterans who access care at VANTHCS and have not experienced homelessness, those served by the HMMM-V were younger and more likely to belong to a minority group; however, they were similar in combat exposure and gender.1 The veterans served by the HMMM-V program also were considerably older and had more homeless chronicity than did nonveteran homeless populations, consistent with other research.4,29,30
The veterans served by the HMMM-V program not surprisingly made housing their top priority in need of help, consistent with the Housing First model.22,31 They also indicated that employment/vocation and reducing substance use were of lower importance. Need for assistance with reducing substance use and social support were the domains least often identified as areas where help was needed, which seems inconsistent with the higher established rates of substance abuse problems among homeless veterans.1
With additional fieldwork, the HMMM-V program is expected to allow refinement of procedures for identifying and serving veterans from a patient-centered care perspective. The P-CHI will be further tested and developed, and the next step will be to create and pilot intervention templates for a Patient-Centered Health Improvement Plan, based on the P-CHI results. This process parallels the original development treatment plans for the VA’s Personalized Health Plan based on the PHI.25 Once the HMMM-V program is fully established in Dallas, the plans are for an expansion that will cover a broader geographic area in North Texas that includes rural areas.
The HMMM-V program was designed to address the barriers to health care that are encountered by homeless veterans. It is unique in homeless veteran care due to its patient-centered approach that partners with homeless veterans to prioritize their needs as determined by them rather than based solely on policies or provider conceptualizations of their needs. Access to services, engagement in care, and successful utilization of needed services may lead to measurable improvements in health care outcomes among homeless populations of veterans. Desired goals include remission of illness through appropriate medical intervention, preventing morbidity, achieving healthy lifestyles, recovery from addiction, stabilization of psychiatric illness, and attainment of stable housing.
The first hurdle for implementing this type of program in other settings is the identification of resources needed for these efforts. Need of additional staffing resources, however, may be circumvented by allowing employees working in other areas to rotate in community outreach shifts in the mobile unit. Another hurdle encountered in implementation of the HMMM-V initiative was the initial difficulty finding homeless veterans in community settings, especially those in unsheltered locations. The HMMM-V program addressed this issue by partnering with other agencies serving the homeless in the community. Therefore, a general recommendation for other entities seeking to implement this type of program is to reach out to these community partners from the outset.
Conclusion
The HMMM-V has the potential to engage the most difficult-to-reach homeless veterans in need of health services by delivering care and providing resources in challenging environments. Further work is needed to validate the P-CHI for use with this program and to conduct well-designed and implemented research to demonstrate effectiveness of this intervention on veteran outcomes, especially quality of life. Once this additional work is accomplished, this innovative program can potentially be implemented by VAMCs across the nation, and potentially in more general community care settings, to more effectively reach out and deliver services to homeless members of the community.
Acknowledgments
Grant support was received from the Department of Veterans Affairs, Office of Patient Centered Care. The authors would like to acknowledge all the clinicians, trainees, and support staff who have contributed to the success of the HMMM-V program: Tara Ayala, Jose Cabrera, Tony Castillo, Rachael Lynn David, Teresa DeShazo, Sylvia Figueroa, Steven Fisher, Eric Gary, Evelyn Gibbs, Kevin Hosey, JoAnn Joseph, Taly Drimer Kagan, Miranda Kelly, Michelle King-Thompson, Sharon Marcus, Shiji Mathew, Moneeza Matin, John Moreno, Joseph Neifert, Joel Price, Tiffany Price, Natalie Qualls, Reginald Robertson, Kristine Rodrigues, Jon Saffelder, Jill Stokes, Scott Stone, and John Smith.
1. LePage JP, Bradshaw LD, Cipher DJ, Crawford AM, Hooshyar D. The effects of homelessness on veterans’ health care service use: an evaluation of independence from comorbidities. Public Health. 2014;128(11):985-992.
2. Fischer PJ, Breakey WR. The epidemiology of alcohol, drug, and mental disorders among homeless persons. Am Psychol. 1991;46(11):1115-1128.
3. Robertson MJ, Zlotnick C, Westerfelt A. Drug use disorders and treatment contact among homeless adults in Alameda County, California. Am J Public Health. 1997;87(2):221-228.
4. North CS, Eyrich KM, Pollio DE, Spitznagel EL. Are rates of psychiatric disorders in the homeless population changing? Am J Public Health. 2004;94(1):103-108.
5. Fazel S, Khosla V, Doll H, Geddes J. The prevalence of mental disorders among the homeless in western countries: systematic review and meta-regression analysis. PLoS Med. 2008;5(12):e225.
6. Harpaz-Rotem I, Rosenheck RA, Desai R. The mental health of children exposed to maternal mental illness and homelessness. Community Ment Health J. 2006;42(5):437-448.
7. Pollio DE, Eyrich-Garg KM, North CS. The homeless. In: Johnson BA, ed. Addiction Medicine: Science and Practice. New York, NY: Springer; 2011:1487-1504.
8. Padgett D, Struening EL, Andrews H. Factors affecting the use of medical, mental health, alcohol, and drug treatment services by homeless adults. Med Care. 1990;28(9):805-821.
9. Baggett TP, Singer DE, Rao SR, O’Connell JJ, Bharel M, Rigotti NA. Food insufficiency and health services utilization in a national sample of homeless adults. J Gen Intern Med. 2011;26(6):627-634.
10. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162(2):370-376.
11. Fuehrlein BS, Cowell AJ, Pollio D, Cupps L, Balfour ME, North CS. A prospective study of the associations among housing status and costs of services in a homeless population. Psychiatr Serv. 2015;66(1):27-32.
12. Pollio DE, North CS, Eyrich KM, Foster DA, Spitznagel E. Modeling service access in a homeless population. J Psychoactive Drugs. 2003;35(4):487-495.
13. U.S. Department of Housing and Urban Development Office of Community Planning and Development. The 2016 Annual Homeless Assessment Report (AHAR) to Congress. Part 1: point-in-time estimates of homelessness. https://www.hudexchange.info/resources/documents/2016-AHAR-Part-1.pdf. Published 2016. Accessed August 7, 2017.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among U.S. veterans. Epidemiol Rev. 2015;37:177-195.
15. Williamson V, Mulhall E. Coming home: the housing crisis and homelessness threaten new veterans. Iraq and Afghanistan Veterans of America, January, 2009. http://media.iava.org/IAVA_coming_home_2009%20The%20Housing%20Crisis%20and%20Homelessness%20Threaten%20New%20Veterans.pdf. Accessed August 10, 2017
16. Shinseki EK. Remarks by Secretary Eric K. Shinseki. National Summit on Homeless Veterans; November 3, 2009; Washington, DC. https://www.va.gov/opa/speeches/2009/09_1103.asp. Updated August 8, 2016. Accessed August 7, 2017.
17. Shinseki EK. Remarks by Secretary Eric K. Shinseki. 2014 National Coalition for Homeless Veterans Annual Meeting; May 30, 2014; Arlington, VA. https://www.va.gov/opa/speeches/2014/05_30_2014.asp. Updated April 21, 2015. Accessed August 7, 2017.
18. Pollio DE, Spitznagel EL, North CS, Thompson S, Foster DA. Service use over time and achievement of stable housing in a mentally ill homeless population. Psychiatr Serv. 2000;51(12):1536-1543.
19. Page J. Barriers to transferring care of homeless people with serious mental illnesses to community mental health organizations: perspectives of street-based programs. Best Practices in Mental Health: An International Journal. 2007;3(1):26.
20. Young AS, Chinman MJ, Cradock-O’Leary JA, et al. Characteristics of individuals with severe mental illness who use emergency services. Community Ment Health J. 2005;41(2):159-168.
21. Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and low-income veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461.
22. Tsemberis S, Gulcur L, Nakae M. Housing First, consumer choice, and harm reduction for homeless individuals with a dual diagnosis. Am J Public Health. 2004;94(4):651-656.
23. U.S. Department of Veterans Affairs, Veterans Health Administration. VA Patient Centered Care. http://www.va.gov/patientcenteredcare. Updated July 24,2017. Accessed August 7, 2017.
24. Epstein RM, Street RL Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100-103.
25. U.S. Department of Veterans Affairs, Office of Patient Centered Care and Cultural Transformation. My story: personal health inventory. https://www.va.gov/PATIENTCENTEREDCARE/docs/VA-OPCC-Personal-Health-Inventory-final-508.pdf. Published October 7, 2013. Accessed August 7, 2017
26. North CS, Smith EM, Pollio DE. The Homeless Supplement to the Diagnostic Interview Schedule (DIS/HS). St. Louis: Washington University, 2004.
27. North CS, Eyrich KM, Pollio DE, Foster DA, Cottler LB, Spitznagel EL. The homeless supplement to the diagnostic interview schedule: test-retest analyses. Int J Methods Psychiatr Res. 2004;13(3):184-191.
28. LaSalle JL. Homeless Operations Management and Evaluation System (HOMES) user manual-phase 1. http://www.vfwsc.org/homes.pdf. Published April 19, 2011. Accessed August 7, 2017.
29. Petrovich JC, Pollio DE, North CS. Characteristics and service use of homeless veterans and nonveterans residing in a low-demand emergency shelter. Psychiatr Serv. 2014;65(6):751-757.
30. North CS, Smith EM. A comparison of homeless men and women: different populations, different needs. Community Ment Health J. 1993;29(5):423-431.
31. Kertesz SG, Austin EL, Holmes SK, et al. Making housing first happen: organizational leadership in VA’s expansion of permanent supportive housing. J Gen Intern Med. 2014;29(suppl 4):835-844.
Research has consistently identified remarkably high rates of addiction, mental illness, and health problems in the homeless population.1-9 Yet in spite of extensive service needs for these problems, abundant evidence exists of consistent underuse of health care services by homeless populations.10-12 Most of the homeless population reside in emergency shelters or in transitional or supportive housing, but many remain in places not meant for human habitation.
Homelessness is significantly overrepresented among military veterans.13 The January 2016 national point-in-time count identified 39,471 veterans experiencing homelessness.13 Iraq and Afghanistan veterans seem to have an especially high risk for homelessness.13-15 Disheartening statistics such as these prompted former VA Secretary Eric Shinseki to pledge to end veteran homelessness by December 2015.16 He argued in support of this mission that 85% of veteran homeless resources go to health care—implying that homelessness among veterans is primarily a health care issue, which is heavily burdened by substance abuse and other psychiatric and medical illnesses.17
Health care service use has been associated with improved health, mental health, and outcomes among homeless populations.12,18 Unfortunately, access to these services is limited by barriers associated with homelessness, such as transportation or lack of proper identification.19,20 Veterans experiencing homelessness also face these common barriers to health care, and unsheltered veterans especially underutilize VA health care services.21
Housing First—a successful model that places individuals into housing without prerequisites for sobriety, active participation in treatment, or other behavioral accomplishments, such as gainful employment—has not managed yet to place all the disengaged homeless veteran population into stable housing.22 However, the Housing First model, which is based on the individual’s priorities, is consistent with the approach of a new program at the VA North Texas Health Care System (VANTHCS).
The VHA, similar to other health care systems, is engaged in a cultural transformation to convert its health care approach from a traditional medical model to patient-centered care (PCC).23 In this priority area, a strategic objective is for the VHA to partner with each veteran to create a personalized, proactive strategy to optimize health and well-being and when needed provide state-of-the-art disease management. Patient-centered care is designed to address veterans’ specific needs in spiritual, environmental, physical, mental, and social domains and empower veterans to become active participants in their care. Patient-centered care differs from the traditional medical model in that patients are active participants in their treatment, partnering and collaborating with their providers on care that is quality-of-life centered instead of disease centered.23 This model is based on both respect for patients as unique individuals and on the obligation to care for them on their own terms, focused on their self-identified goals and aspirations.24
At VANTHCS, the Homeless Mobile Medical/Mental Veteran (HMMM-V) pilot program was designed to deliver effective health care services to a homeless subpopulation of veterans who historically have been the most difficult to serve—those living in unsheltered environments, such as under bridges and in encampments. The purpose of the HMMM-V program was to contact and serve veterans not currently being reached by the VA system of care, using a PCC model.
This pilot program was initially funded in January 2013 by a 2-year grant from the Office of Patient Centered Care and Cultural Transformation to apply the PCC approach to engage veteran participation. For this project, the VA Personal Health Inventory tool—originally designed for use with the general veteran population—was adapted for use with the homeless veteran population. The grant funding period covered the design, development, and implementation of the HMMM-V program; thereafter, VANTHCS provided resources through its Comprehensive Homeless Center Programs to assure its sustainability and continued use of the clinical assessment tool created for this project.
This article describes the development and implementation of this novel program with sufficient detail to inform the development of similar programs in other sites. Descriptions of the program and staffing, creation of community partnerships, and modification of an assessment instrument are provided. It also illustrates the original implementation period of the HMMM-V program through presentation of self-reported data on the first homeless veterans it served.
Equipment and Staffing
A custom 28-foot mobile outreach vehicle was assembled according to specifications identified by the HMMM-V team as necessary to conduct the program’s interventions. The van became fully operational on April 8, 2015, after it underwent all the required reviews and inspections (eg, safety, infection control, etc) and was accredited in 2015 by the Commission on Accreditation of Rehabilitation Facilities.
The HMMM-V van has a driver compartment that is separate from its services rooms, which include a patient registration area, a fully equipped examination room, a laboratory area, and a bathroom. The vehicle is equipped with a wheelchair lift and an awning to shade outdoor areas where tables and chairs are set up for patient/staff waiting and rest areas. The vehicle is stocked with essential equipment and supplies needed to conduct work in off-street locations, vacant lots, under bridges, fields, unpaved paths, etc. It also is equipped with telemedicine capabilities to provide clinical supervision and access to attending physicians and specialists at VANTHCS. Personnel carry cell phones and laptop computers with secure Internet connections using a commercially available mobile wireless Wi-Fi hotspot to facilitate documentation of medical records and communication from the field.
This reliable type of equipment is routine for use in VA field operations; the only hurdle using these technologies for the program was acquiring funding and purchasing the equipment. The vehicle is further equipped with a refrigerator solely for secure storage of pharmaceutical supplies, a second refrigerator for specimens, and wall-mounted blood pressure and otoscope/ophthalmoscope units. The vehicle is supplied with thermometers, scales, phlebotomy supplies, and first-aid kits and is stocked with vaccines and medications, including antibiotic, hypertensive, diabetic, allergy, and over-the-counter pain medications. A more comprehensive list of supplies for the vehicle is available from the authors on request.
Medication provisions supplied to the HMMM-V mobile clinic conform to the Texas State Board of Pharmacy compliance regulations. Because the vehicle is designated as federal property and has U.S. government license plates, it is considered an extension of VANTHCS Pharmacy Service and falls under its pharmacy license. A medication formulary was created with input from HMMM-V prescribers and Dallas VAMC Pharmacy Service pharmacists. To safeguard the integrity of these pharmaceutical agents, the HMMM-V physician assistant picks up the medications before field deployment and returns the unused medications to the Dallas VAMC at the end of the day. The medications are transported in locked containers and placed either in a locked medication refrigerator or cabinet on the mobile unit.
For medication prescriptions that need laboratory testing before prescribing them, HMMM-V prescribers can check the VA electronic medical record from the field to determine whether these tests have been completed recently. If not, then HMMM-V team has an agreement with Dallas VA Pathology and Laboratory Medicine Service for testing samples obtained in the field.
The program was designed for staffing of the vehicle by 2 professional teams, each includes medical (physician’s assistant or registered nurse), mental health (psychiatrist, residents), and social work providers (licensed social workers, clinical social workers); trainees of these disciplines; a peer support specialist; and an administrative clerk. The staffing varies daily, depending on available personnel. When personnel deploy to the field, they go in pairs or groups to address potential safety issues. Cell phones are available to summon police or ambulance services in an emergency. Systematic safety training was conducted with all field personnel before their first deployment to guard against vulnerability to danger in these settings.
Once in the field, personnel engage unsheltered homeless individuals to assess eligibility for VA services. Veterans found ineligible are assisted with application for military discharge upgrade, service-connected compensation, or appeal for health care coverage. Veterans eligible for VA care receive physical examinations, vital and glucose checks, influenza and pneumonia vaccinations, first-aid skin and wound care, medication management with limited medications provided at point of care, blood and urine testing, peer support services, suicide assessments, clinical mental health evaluations, and social work services through the HMMM-V program.
Social work assistance provided includes psychosocial assessment and care coordination for psychosocial needs such as mental health, substance abuse, vision, dental, housing, employment, legal aid, transportation, food, income, hygiene, and weather-appropriate provision needs.
Community Partnerships
The HMMM-V program benefitted from a number of partnerships with community agencies. During development of the program, HMMM-V personnel accompanied the Dallas Police Department’s Crisis Intervention Unit on typical homeless crisis services deployments into the field to learn about the locations and nature of encampments and homeless peregrination patterns in the Dallas area.
To aid in the design and selection of features for the mobile outreach vehicle, team members toured Homeless Outreach Medical Service mobile clinics from 2 local county hospitals, Parkland Hospital and John Peter Smith Hospital. The staff for these mobile clinics were interviewed about their experience with various components of their programs and their recommendations for optimal design of the mobile medical clinic for service delivery.
Numerous agencies in the Dallas area that serve the homeless population assisted with locating and connecting homeless veterans to HMMM-V programs. These partnering agencies also serve homeless individuals who do not qualify for the HMMM-V program, such as veterans with other-than-honorable military discharges.
The HMMM-V mobile outreach vehicle travels to partnering agencies and provides services on a recurring basis. These agencies are the Dallas International Street Church, a church and faith-based agency aiding the recovery of people with “broken lives”; Cornerstone Ministries, a church-based ministry serving people with adverse circumstances; and City Square’s Opportunity Center, human services and community development programs for low-income city residents. The mobile clinic also travels regularly to other areas to serve homeless veterans residing in unsheltered locations, such as homeless encampments and under bridges.
Clinical Assessment
The project used a modification of the VA Personalized Health Inventory (PHI) for general veteran populations, which assesses 8 areas of self-identified needs to address the specific concerns of homeless veterans served by a mobile clinic.25 Version 19 of the PHI (revised September 18, 2012), the version of the instrument available to the team at the inception of the project, was deployed with the HMMM-V personnel into the field. It imposed a heavy interview time burden (several hours), and its content areas did not seem appropriate to address the immediate concerns of homeless populations (eg, sections pertaining to personal development through hobbies, recreation, or volunteering; healthy living spaces with plenty of lighting and color; “eating healthy, balanced meals with plenty of fruits and vegetables each day”).25
Based on HMMM-V personnel feedback, the team modified this tool and developed a patient-centered health inventory (P-CHI) for homeless veterans that was acceptable in length and applicable to the situational characteristics of homeless existence. The tool’s 10 “current and desired states” were revised to remove domains of exercise and flexibility, sleep and relaxation, and mind-body techniques. The intervention and prevention domains were combined. A material needs (clothing, furniture, transportation, financial benefits) domain was added, and a new domain on reducing alcohol/drug use was created by moving this material from the food and drink domain.
The remaining domains were modified to fit the homeless living situation (Food and Drink = Nutrition; Personal Development = Employment/Vocation; Family, Friends, and Co-Workers = Family/Social/Legal Support; Spirit and Soul = Personal/Spiritual Fulfillment; Surroundings = Housing). Current state ratings were revised to reflect level of satisfaction, and ratings of Desired State were replaced with level of importance.
The modifications resulted in 9 domains, which were assembled into a grid for efficient rating of both satisfaction and importance for each domain (rated 1 to 10, lowest to highest, respectively), followed by an instruction to mark an X in a designated space in all the domains with which the individual would like help (Table). The intent was to reduce the burden of the instrument by having the participant complete sections providing detailed information about only the domains selected by the participant.
The details of each domain in the original VA PHI tool were captured through open-ended questions in text responses provided by the veteran. Because open-ended text responses are not conducive for summarizing characteristics of the population served or for evaluating program activities, the detailed sections covering the domains were revised completely to capture data within categoric and numeric variables. Items from the validated Homeless Supplement Interview were added to collect information not provided in the Homeless Operations Management and Evaluation System interview that is routinely administered to all veterans accessing homeless VA services.26-28
The information collected in these domains cover duration of current homeless episode, lifetime number of homeless episodes, current living arrangements and dissatisfactions with these arrangements, frequency and source of meals, employment history and current work status, sources of income, special material needs, medical and dental problems and sources of care, current medications, mental health problems and sources of care, urgent mental health concerns, current amount and frequency of alcohol and drug use, substance abuse treatment history, relationships with family and intimate partners, legal assistance needs, and self-identified needs for spiritual and personal fulfillment. This instrument is available on request to the authors.
Veterans Served
The project began with 1 team of professionals deploying with the HMMM-V vehicle while a second team was being assembled. Currently, the 2 HMMM-V teams deploy the mobile clinic 4 days per week. The mobile clinic visits agencies that serve the homeless, including emergency shelters and food ministries, as well as homeless encampments. To date, 195 homeless veterans have been served by the mobile clinic, 111 were currently enrolled with the VA, 8 were not enrolled but eligible for services, and 77 were not eligible for VA services. Of the unenrolled veterans, those eligible for services were offered VA enrollment assistance; those ineligible for VA services were offered a community referral.
For the veterans encountered in the field, the following interventions were provided: 49 housing placement referrals, 4 rental assistance referrals, 4 legal referrals, 27 medical care interventions, 13 dental referrals, 11 vision/hearing referrals, 12 mental health interventions, 9 substance abuse treatment referrals, 14 employment assistance referrals, 13 disability benefit applications, 18 transportation assists, 23 goods delivered, and 159 information assists. The HMMM-V mobile clinic also is deployed to participate in various educational and outreach events. At the time this article was written, the mobile clinic has reached nearly 2,000 veterans and community partners in at least 25 such events.
Of the veterans served to date, 73 completed the P-CHI. These veterans were predominantly male (77%), and the majority (60%) were black. The median age of the sample was 58 years, and typically they had a high school level of education (12.7; SD, 2.1 mean years of education). About half (49%) the sample were separated or divorced, and only a minority were currently married (8%). Half (50%) the sample served in the U.S. Army, with the post-Vietnam era being the era of service most represented (19%). Few (21%) veterans reported exposure to hostile or friendly fire during their service. More than three-fourths (80%) of the sample had experienced a homeless episode prior to their current one. On average, members of the sample had experienced a median of 3 lifetime homeless episodes. They had a mean 4.1 (SD, 5.8) lifetime number of years of homelessness, and 3.0 (SD, 5.2) years in their current homeless episode. Nearly one-third (31%) reported that they were currently staying in a homeless shelter, and nearly one-sixth (16%) were currently unsheltered in street settings, such as under bridges or in outdoor encampments at the time of the initial visit.
The mean number of minutes spent completing the P-CHI was 18.5 (SD, 9.4). The veterans indicated that they would like assistance with a mean 3.2 (SD, 2.2) number of domains. The domains with the highest average importance ratings were housing (mean, 9.4; SD, 1.7) and medical/dental care (mean, 8.9; SD, 2.2); the domains with the lowest average importance rating were reducing alcohol/drug use (mean, 6.4; SD, 4.1) and employment/vocation (mean, 6.3; SD, 4.2). The domains with the highest average satisfaction ratings were personal/spiritual fulfillment (mean, 7.3; SD, 2.9) and reducing substance use (mean, 5.9; SD, 4.0), and the domains with the lowest average satisfaction ratings were housing (mean, 2.9; SD, 2.9), material needs (mean, 4.2; SD, 3.3), and employment/vocation (mean, 4.2; SD, 3.2). The domain with the greatest indication of desire for help was housing, endorsed by more than four-fifths (84%) of the sample. This highly endorsed housing domain also was one of the lowest in satisfaction. The domains with the least expressed interest in obtaining help were reducing substance use (18%) and personal/spiritual fulfillment (15%).Reducing substance abuse also was one of the lowest domains of importance and the least for dissatisfaction.
Challenges and Barriers
As anticipated from its inception, this project encountered many challenges and barriers. The first was with the design, construction, and delivery of the mobile clinic unit. The vehicle took more than 2 years to be delivered. There were delays in progress necessitated by required selection of an approved vendor to build the vehicle, extensive specification of details and features, and stocking it with equipment and supplies. The weight of the unit had to be < 26,000 pounds to avoid the requirement of a commercial driver’s license, which limited the size of the vehicle to 28 feet. Stocking the unit with equipment and supplies required attention to a myriad of specifications and decisions. For example, separate refrigerators were needed for specimens, medications, and food; pharmaceutical regulations governing medications in mobile clinics required strict adherence; and difficulties were encountered in attempting to establish adequate and secure connectivity for communications devices in the field.
Once the mobile unit was delivered and prepared for deployment, the next set of challenges pertained to learning all of the instructions required to operate and drive the vehicle and learning how to maneuver the vehicle in the field. Specific challenges for driving the vehicle encountered included unexpectedly low overpasses that prohibited passage, narrow spaces for passage, rough and uneven terrain in off-road settings, and lateral and vertical tilt of roads creating potential for sideswipes and undercarriage scrapes. Maintenance schedules needed to be developed and implemented for cleaning the unit, inspection compliance, repairs, refueling, and emptying waste materials.
Staffing the vehicle required the development of unique job specifications addressing special expertise in accessing VA databases for veteran verification and registration and for driving the mobile clinic vehicle. Schedules and deployment plans for 2 teams that shared the same vehicle had to be established and followed. Locating veterans in unsheltered settings, such as under bridges and in encampments, required community intelligence facilitated through partnerships with knowledgeable members of the Dallas police crisis unit and by gaining field experience to locate where the usual homeless gathering places are, especially those inhabited by veterans. Safety of team members and equipment/supplies in the field was paramount from the start, and additional steps beyond safety training required extra measures, such as special care in navigating known dangerous areas. Provision of services necessitated completion of everything needed in a single visit due to the likelihood of loss to follow-up and acceptance of the limited types of service that could be provided in a mobile clinic. Special procedures were needed to provide referrals to sources of available care for non-VA-qualifying veterans.
Discussion
The HMMM-V program for delivery of PCC to homeless veterans is an innovative pilot program designed to connect with difficult-to-reach homeless veterans and engage them in care. The deliverables provided by this project are (1) A mobile outreach vehicle to deliver care to homeless veterans and outreach to other veterans and community agencies in North Texas; (2) The P-CHI assessment tool for homeless veterans modified and adapted for use with this special population; and (3) pilot data on its first cohort of homeless veterans served, describing their baseline characteristics and their stated satisfaction and preferences about their goals and aspirations for their physical, emotional, and mental health and well-being.
The HMMM-V program successfully identified homeless veterans in need of services, and more than one-third of these veterans were not previously engaged in VA services. Compared with the “typical” veterans served at VANTHCS homeless programs, veterans served by the HMMM-V comprised a greater proportion of minorities and a higher proportion who had been exposed to combat.29 Age and gender characteristics were similar.29 When compared with veterans who access care at VANTHCS and have not experienced homelessness, those served by the HMMM-V were younger and more likely to belong to a minority group; however, they were similar in combat exposure and gender.1 The veterans served by the HMMM-V program also were considerably older and had more homeless chronicity than did nonveteran homeless populations, consistent with other research.4,29,30
The veterans served by the HMMM-V program not surprisingly made housing their top priority in need of help, consistent with the Housing First model.22,31 They also indicated that employment/vocation and reducing substance use were of lower importance. Need for assistance with reducing substance use and social support were the domains least often identified as areas where help was needed, which seems inconsistent with the higher established rates of substance abuse problems among homeless veterans.1
With additional fieldwork, the HMMM-V program is expected to allow refinement of procedures for identifying and serving veterans from a patient-centered care perspective. The P-CHI will be further tested and developed, and the next step will be to create and pilot intervention templates for a Patient-Centered Health Improvement Plan, based on the P-CHI results. This process parallels the original development treatment plans for the VA’s Personalized Health Plan based on the PHI.25 Once the HMMM-V program is fully established in Dallas, the plans are for an expansion that will cover a broader geographic area in North Texas that includes rural areas.
The HMMM-V program was designed to address the barriers to health care that are encountered by homeless veterans. It is unique in homeless veteran care due to its patient-centered approach that partners with homeless veterans to prioritize their needs as determined by them rather than based solely on policies or provider conceptualizations of their needs. Access to services, engagement in care, and successful utilization of needed services may lead to measurable improvements in health care outcomes among homeless populations of veterans. Desired goals include remission of illness through appropriate medical intervention, preventing morbidity, achieving healthy lifestyles, recovery from addiction, stabilization of psychiatric illness, and attainment of stable housing.
The first hurdle for implementing this type of program in other settings is the identification of resources needed for these efforts. Need of additional staffing resources, however, may be circumvented by allowing employees working in other areas to rotate in community outreach shifts in the mobile unit. Another hurdle encountered in implementation of the HMMM-V initiative was the initial difficulty finding homeless veterans in community settings, especially those in unsheltered locations. The HMMM-V program addressed this issue by partnering with other agencies serving the homeless in the community. Therefore, a general recommendation for other entities seeking to implement this type of program is to reach out to these community partners from the outset.
Conclusion
The HMMM-V has the potential to engage the most difficult-to-reach homeless veterans in need of health services by delivering care and providing resources in challenging environments. Further work is needed to validate the P-CHI for use with this program and to conduct well-designed and implemented research to demonstrate effectiveness of this intervention on veteran outcomes, especially quality of life. Once this additional work is accomplished, this innovative program can potentially be implemented by VAMCs across the nation, and potentially in more general community care settings, to more effectively reach out and deliver services to homeless members of the community.
Acknowledgments
Grant support was received from the Department of Veterans Affairs, Office of Patient Centered Care. The authors would like to acknowledge all the clinicians, trainees, and support staff who have contributed to the success of the HMMM-V program: Tara Ayala, Jose Cabrera, Tony Castillo, Rachael Lynn David, Teresa DeShazo, Sylvia Figueroa, Steven Fisher, Eric Gary, Evelyn Gibbs, Kevin Hosey, JoAnn Joseph, Taly Drimer Kagan, Miranda Kelly, Michelle King-Thompson, Sharon Marcus, Shiji Mathew, Moneeza Matin, John Moreno, Joseph Neifert, Joel Price, Tiffany Price, Natalie Qualls, Reginald Robertson, Kristine Rodrigues, Jon Saffelder, Jill Stokes, Scott Stone, and John Smith.
Research has consistently identified remarkably high rates of addiction, mental illness, and health problems in the homeless population.1-9 Yet in spite of extensive service needs for these problems, abundant evidence exists of consistent underuse of health care services by homeless populations.10-12 Most of the homeless population reside in emergency shelters or in transitional or supportive housing, but many remain in places not meant for human habitation.
Homelessness is significantly overrepresented among military veterans.13 The January 2016 national point-in-time count identified 39,471 veterans experiencing homelessness.13 Iraq and Afghanistan veterans seem to have an especially high risk for homelessness.13-15 Disheartening statistics such as these prompted former VA Secretary Eric Shinseki to pledge to end veteran homelessness by December 2015.16 He argued in support of this mission that 85% of veteran homeless resources go to health care—implying that homelessness among veterans is primarily a health care issue, which is heavily burdened by substance abuse and other psychiatric and medical illnesses.17
Health care service use has been associated with improved health, mental health, and outcomes among homeless populations.12,18 Unfortunately, access to these services is limited by barriers associated with homelessness, such as transportation or lack of proper identification.19,20 Veterans experiencing homelessness also face these common barriers to health care, and unsheltered veterans especially underutilize VA health care services.21
Housing First—a successful model that places individuals into housing without prerequisites for sobriety, active participation in treatment, or other behavioral accomplishments, such as gainful employment—has not managed yet to place all the disengaged homeless veteran population into stable housing.22 However, the Housing First model, which is based on the individual’s priorities, is consistent with the approach of a new program at the VA North Texas Health Care System (VANTHCS).
The VHA, similar to other health care systems, is engaged in a cultural transformation to convert its health care approach from a traditional medical model to patient-centered care (PCC).23 In this priority area, a strategic objective is for the VHA to partner with each veteran to create a personalized, proactive strategy to optimize health and well-being and when needed provide state-of-the-art disease management. Patient-centered care is designed to address veterans’ specific needs in spiritual, environmental, physical, mental, and social domains and empower veterans to become active participants in their care. Patient-centered care differs from the traditional medical model in that patients are active participants in their treatment, partnering and collaborating with their providers on care that is quality-of-life centered instead of disease centered.23 This model is based on both respect for patients as unique individuals and on the obligation to care for them on their own terms, focused on their self-identified goals and aspirations.24
At VANTHCS, the Homeless Mobile Medical/Mental Veteran (HMMM-V) pilot program was designed to deliver effective health care services to a homeless subpopulation of veterans who historically have been the most difficult to serve—those living in unsheltered environments, such as under bridges and in encampments. The purpose of the HMMM-V program was to contact and serve veterans not currently being reached by the VA system of care, using a PCC model.
This pilot program was initially funded in January 2013 by a 2-year grant from the Office of Patient Centered Care and Cultural Transformation to apply the PCC approach to engage veteran participation. For this project, the VA Personal Health Inventory tool—originally designed for use with the general veteran population—was adapted for use with the homeless veteran population. The grant funding period covered the design, development, and implementation of the HMMM-V program; thereafter, VANTHCS provided resources through its Comprehensive Homeless Center Programs to assure its sustainability and continued use of the clinical assessment tool created for this project.
This article describes the development and implementation of this novel program with sufficient detail to inform the development of similar programs in other sites. Descriptions of the program and staffing, creation of community partnerships, and modification of an assessment instrument are provided. It also illustrates the original implementation period of the HMMM-V program through presentation of self-reported data on the first homeless veterans it served.
Equipment and Staffing
A custom 28-foot mobile outreach vehicle was assembled according to specifications identified by the HMMM-V team as necessary to conduct the program’s interventions. The van became fully operational on April 8, 2015, after it underwent all the required reviews and inspections (eg, safety, infection control, etc) and was accredited in 2015 by the Commission on Accreditation of Rehabilitation Facilities.
The HMMM-V van has a driver compartment that is separate from its services rooms, which include a patient registration area, a fully equipped examination room, a laboratory area, and a bathroom. The vehicle is equipped with a wheelchair lift and an awning to shade outdoor areas where tables and chairs are set up for patient/staff waiting and rest areas. The vehicle is stocked with essential equipment and supplies needed to conduct work in off-street locations, vacant lots, under bridges, fields, unpaved paths, etc. It also is equipped with telemedicine capabilities to provide clinical supervision and access to attending physicians and specialists at VANTHCS. Personnel carry cell phones and laptop computers with secure Internet connections using a commercially available mobile wireless Wi-Fi hotspot to facilitate documentation of medical records and communication from the field.
This reliable type of equipment is routine for use in VA field operations; the only hurdle using these technologies for the program was acquiring funding and purchasing the equipment. The vehicle is further equipped with a refrigerator solely for secure storage of pharmaceutical supplies, a second refrigerator for specimens, and wall-mounted blood pressure and otoscope/ophthalmoscope units. The vehicle is supplied with thermometers, scales, phlebotomy supplies, and first-aid kits and is stocked with vaccines and medications, including antibiotic, hypertensive, diabetic, allergy, and over-the-counter pain medications. A more comprehensive list of supplies for the vehicle is available from the authors on request.
Medication provisions supplied to the HMMM-V mobile clinic conform to the Texas State Board of Pharmacy compliance regulations. Because the vehicle is designated as federal property and has U.S. government license plates, it is considered an extension of VANTHCS Pharmacy Service and falls under its pharmacy license. A medication formulary was created with input from HMMM-V prescribers and Dallas VAMC Pharmacy Service pharmacists. To safeguard the integrity of these pharmaceutical agents, the HMMM-V physician assistant picks up the medications before field deployment and returns the unused medications to the Dallas VAMC at the end of the day. The medications are transported in locked containers and placed either in a locked medication refrigerator or cabinet on the mobile unit.
For medication prescriptions that need laboratory testing before prescribing them, HMMM-V prescribers can check the VA electronic medical record from the field to determine whether these tests have been completed recently. If not, then HMMM-V team has an agreement with Dallas VA Pathology and Laboratory Medicine Service for testing samples obtained in the field.
The program was designed for staffing of the vehicle by 2 professional teams, each includes medical (physician’s assistant or registered nurse), mental health (psychiatrist, residents), and social work providers (licensed social workers, clinical social workers); trainees of these disciplines; a peer support specialist; and an administrative clerk. The staffing varies daily, depending on available personnel. When personnel deploy to the field, they go in pairs or groups to address potential safety issues. Cell phones are available to summon police or ambulance services in an emergency. Systematic safety training was conducted with all field personnel before their first deployment to guard against vulnerability to danger in these settings.
Once in the field, personnel engage unsheltered homeless individuals to assess eligibility for VA services. Veterans found ineligible are assisted with application for military discharge upgrade, service-connected compensation, or appeal for health care coverage. Veterans eligible for VA care receive physical examinations, vital and glucose checks, influenza and pneumonia vaccinations, first-aid skin and wound care, medication management with limited medications provided at point of care, blood and urine testing, peer support services, suicide assessments, clinical mental health evaluations, and social work services through the HMMM-V program.
Social work assistance provided includes psychosocial assessment and care coordination for psychosocial needs such as mental health, substance abuse, vision, dental, housing, employment, legal aid, transportation, food, income, hygiene, and weather-appropriate provision needs.
Community Partnerships
The HMMM-V program benefitted from a number of partnerships with community agencies. During development of the program, HMMM-V personnel accompanied the Dallas Police Department’s Crisis Intervention Unit on typical homeless crisis services deployments into the field to learn about the locations and nature of encampments and homeless peregrination patterns in the Dallas area.
To aid in the design and selection of features for the mobile outreach vehicle, team members toured Homeless Outreach Medical Service mobile clinics from 2 local county hospitals, Parkland Hospital and John Peter Smith Hospital. The staff for these mobile clinics were interviewed about their experience with various components of their programs and their recommendations for optimal design of the mobile medical clinic for service delivery.
Numerous agencies in the Dallas area that serve the homeless population assisted with locating and connecting homeless veterans to HMMM-V programs. These partnering agencies also serve homeless individuals who do not qualify for the HMMM-V program, such as veterans with other-than-honorable military discharges.
The HMMM-V mobile outreach vehicle travels to partnering agencies and provides services on a recurring basis. These agencies are the Dallas International Street Church, a church and faith-based agency aiding the recovery of people with “broken lives”; Cornerstone Ministries, a church-based ministry serving people with adverse circumstances; and City Square’s Opportunity Center, human services and community development programs for low-income city residents. The mobile clinic also travels regularly to other areas to serve homeless veterans residing in unsheltered locations, such as homeless encampments and under bridges.
Clinical Assessment
The project used a modification of the VA Personalized Health Inventory (PHI) for general veteran populations, which assesses 8 areas of self-identified needs to address the specific concerns of homeless veterans served by a mobile clinic.25 Version 19 of the PHI (revised September 18, 2012), the version of the instrument available to the team at the inception of the project, was deployed with the HMMM-V personnel into the field. It imposed a heavy interview time burden (several hours), and its content areas did not seem appropriate to address the immediate concerns of homeless populations (eg, sections pertaining to personal development through hobbies, recreation, or volunteering; healthy living spaces with plenty of lighting and color; “eating healthy, balanced meals with plenty of fruits and vegetables each day”).25
Based on HMMM-V personnel feedback, the team modified this tool and developed a patient-centered health inventory (P-CHI) for homeless veterans that was acceptable in length and applicable to the situational characteristics of homeless existence. The tool’s 10 “current and desired states” were revised to remove domains of exercise and flexibility, sleep and relaxation, and mind-body techniques. The intervention and prevention domains were combined. A material needs (clothing, furniture, transportation, financial benefits) domain was added, and a new domain on reducing alcohol/drug use was created by moving this material from the food and drink domain.
The remaining domains were modified to fit the homeless living situation (Food and Drink = Nutrition; Personal Development = Employment/Vocation; Family, Friends, and Co-Workers = Family/Social/Legal Support; Spirit and Soul = Personal/Spiritual Fulfillment; Surroundings = Housing). Current state ratings were revised to reflect level of satisfaction, and ratings of Desired State were replaced with level of importance.
The modifications resulted in 9 domains, which were assembled into a grid for efficient rating of both satisfaction and importance for each domain (rated 1 to 10, lowest to highest, respectively), followed by an instruction to mark an X in a designated space in all the domains with which the individual would like help (Table). The intent was to reduce the burden of the instrument by having the participant complete sections providing detailed information about only the domains selected by the participant.
The details of each domain in the original VA PHI tool were captured through open-ended questions in text responses provided by the veteran. Because open-ended text responses are not conducive for summarizing characteristics of the population served or for evaluating program activities, the detailed sections covering the domains were revised completely to capture data within categoric and numeric variables. Items from the validated Homeless Supplement Interview were added to collect information not provided in the Homeless Operations Management and Evaluation System interview that is routinely administered to all veterans accessing homeless VA services.26-28
The information collected in these domains cover duration of current homeless episode, lifetime number of homeless episodes, current living arrangements and dissatisfactions with these arrangements, frequency and source of meals, employment history and current work status, sources of income, special material needs, medical and dental problems and sources of care, current medications, mental health problems and sources of care, urgent mental health concerns, current amount and frequency of alcohol and drug use, substance abuse treatment history, relationships with family and intimate partners, legal assistance needs, and self-identified needs for spiritual and personal fulfillment. This instrument is available on request to the authors.
Veterans Served
The project began with 1 team of professionals deploying with the HMMM-V vehicle while a second team was being assembled. Currently, the 2 HMMM-V teams deploy the mobile clinic 4 days per week. The mobile clinic visits agencies that serve the homeless, including emergency shelters and food ministries, as well as homeless encampments. To date, 195 homeless veterans have been served by the mobile clinic, 111 were currently enrolled with the VA, 8 were not enrolled but eligible for services, and 77 were not eligible for VA services. Of the unenrolled veterans, those eligible for services were offered VA enrollment assistance; those ineligible for VA services were offered a community referral.
For the veterans encountered in the field, the following interventions were provided: 49 housing placement referrals, 4 rental assistance referrals, 4 legal referrals, 27 medical care interventions, 13 dental referrals, 11 vision/hearing referrals, 12 mental health interventions, 9 substance abuse treatment referrals, 14 employment assistance referrals, 13 disability benefit applications, 18 transportation assists, 23 goods delivered, and 159 information assists. The HMMM-V mobile clinic also is deployed to participate in various educational and outreach events. At the time this article was written, the mobile clinic has reached nearly 2,000 veterans and community partners in at least 25 such events.
Of the veterans served to date, 73 completed the P-CHI. These veterans were predominantly male (77%), and the majority (60%) were black. The median age of the sample was 58 years, and typically they had a high school level of education (12.7; SD, 2.1 mean years of education). About half (49%) the sample were separated or divorced, and only a minority were currently married (8%). Half (50%) the sample served in the U.S. Army, with the post-Vietnam era being the era of service most represented (19%). Few (21%) veterans reported exposure to hostile or friendly fire during their service. More than three-fourths (80%) of the sample had experienced a homeless episode prior to their current one. On average, members of the sample had experienced a median of 3 lifetime homeless episodes. They had a mean 4.1 (SD, 5.8) lifetime number of years of homelessness, and 3.0 (SD, 5.2) years in their current homeless episode. Nearly one-third (31%) reported that they were currently staying in a homeless shelter, and nearly one-sixth (16%) were currently unsheltered in street settings, such as under bridges or in outdoor encampments at the time of the initial visit.
The mean number of minutes spent completing the P-CHI was 18.5 (SD, 9.4). The veterans indicated that they would like assistance with a mean 3.2 (SD, 2.2) number of domains. The domains with the highest average importance ratings were housing (mean, 9.4; SD, 1.7) and medical/dental care (mean, 8.9; SD, 2.2); the domains with the lowest average importance rating were reducing alcohol/drug use (mean, 6.4; SD, 4.1) and employment/vocation (mean, 6.3; SD, 4.2). The domains with the highest average satisfaction ratings were personal/spiritual fulfillment (mean, 7.3; SD, 2.9) and reducing substance use (mean, 5.9; SD, 4.0), and the domains with the lowest average satisfaction ratings were housing (mean, 2.9; SD, 2.9), material needs (mean, 4.2; SD, 3.3), and employment/vocation (mean, 4.2; SD, 3.2). The domain with the greatest indication of desire for help was housing, endorsed by more than four-fifths (84%) of the sample. This highly endorsed housing domain also was one of the lowest in satisfaction. The domains with the least expressed interest in obtaining help were reducing substance use (18%) and personal/spiritual fulfillment (15%).Reducing substance abuse also was one of the lowest domains of importance and the least for dissatisfaction.
Challenges and Barriers
As anticipated from its inception, this project encountered many challenges and barriers. The first was with the design, construction, and delivery of the mobile clinic unit. The vehicle took more than 2 years to be delivered. There were delays in progress necessitated by required selection of an approved vendor to build the vehicle, extensive specification of details and features, and stocking it with equipment and supplies. The weight of the unit had to be < 26,000 pounds to avoid the requirement of a commercial driver’s license, which limited the size of the vehicle to 28 feet. Stocking the unit with equipment and supplies required attention to a myriad of specifications and decisions. For example, separate refrigerators were needed for specimens, medications, and food; pharmaceutical regulations governing medications in mobile clinics required strict adherence; and difficulties were encountered in attempting to establish adequate and secure connectivity for communications devices in the field.
Once the mobile unit was delivered and prepared for deployment, the next set of challenges pertained to learning all of the instructions required to operate and drive the vehicle and learning how to maneuver the vehicle in the field. Specific challenges for driving the vehicle encountered included unexpectedly low overpasses that prohibited passage, narrow spaces for passage, rough and uneven terrain in off-road settings, and lateral and vertical tilt of roads creating potential for sideswipes and undercarriage scrapes. Maintenance schedules needed to be developed and implemented for cleaning the unit, inspection compliance, repairs, refueling, and emptying waste materials.
Staffing the vehicle required the development of unique job specifications addressing special expertise in accessing VA databases for veteran verification and registration and for driving the mobile clinic vehicle. Schedules and deployment plans for 2 teams that shared the same vehicle had to be established and followed. Locating veterans in unsheltered settings, such as under bridges and in encampments, required community intelligence facilitated through partnerships with knowledgeable members of the Dallas police crisis unit and by gaining field experience to locate where the usual homeless gathering places are, especially those inhabited by veterans. Safety of team members and equipment/supplies in the field was paramount from the start, and additional steps beyond safety training required extra measures, such as special care in navigating known dangerous areas. Provision of services necessitated completion of everything needed in a single visit due to the likelihood of loss to follow-up and acceptance of the limited types of service that could be provided in a mobile clinic. Special procedures were needed to provide referrals to sources of available care for non-VA-qualifying veterans.
Discussion
The HMMM-V program for delivery of PCC to homeless veterans is an innovative pilot program designed to connect with difficult-to-reach homeless veterans and engage them in care. The deliverables provided by this project are (1) A mobile outreach vehicle to deliver care to homeless veterans and outreach to other veterans and community agencies in North Texas; (2) The P-CHI assessment tool for homeless veterans modified and adapted for use with this special population; and (3) pilot data on its first cohort of homeless veterans served, describing their baseline characteristics and their stated satisfaction and preferences about their goals and aspirations for their physical, emotional, and mental health and well-being.
The HMMM-V program successfully identified homeless veterans in need of services, and more than one-third of these veterans were not previously engaged in VA services. Compared with the “typical” veterans served at VANTHCS homeless programs, veterans served by the HMMM-V comprised a greater proportion of minorities and a higher proportion who had been exposed to combat.29 Age and gender characteristics were similar.29 When compared with veterans who access care at VANTHCS and have not experienced homelessness, those served by the HMMM-V were younger and more likely to belong to a minority group; however, they were similar in combat exposure and gender.1 The veterans served by the HMMM-V program also were considerably older and had more homeless chronicity than did nonveteran homeless populations, consistent with other research.4,29,30
The veterans served by the HMMM-V program not surprisingly made housing their top priority in need of help, consistent with the Housing First model.22,31 They also indicated that employment/vocation and reducing substance use were of lower importance. Need for assistance with reducing substance use and social support were the domains least often identified as areas where help was needed, which seems inconsistent with the higher established rates of substance abuse problems among homeless veterans.1
With additional fieldwork, the HMMM-V program is expected to allow refinement of procedures for identifying and serving veterans from a patient-centered care perspective. The P-CHI will be further tested and developed, and the next step will be to create and pilot intervention templates for a Patient-Centered Health Improvement Plan, based on the P-CHI results. This process parallels the original development treatment plans for the VA’s Personalized Health Plan based on the PHI.25 Once the HMMM-V program is fully established in Dallas, the plans are for an expansion that will cover a broader geographic area in North Texas that includes rural areas.
The HMMM-V program was designed to address the barriers to health care that are encountered by homeless veterans. It is unique in homeless veteran care due to its patient-centered approach that partners with homeless veterans to prioritize their needs as determined by them rather than based solely on policies or provider conceptualizations of their needs. Access to services, engagement in care, and successful utilization of needed services may lead to measurable improvements in health care outcomes among homeless populations of veterans. Desired goals include remission of illness through appropriate medical intervention, preventing morbidity, achieving healthy lifestyles, recovery from addiction, stabilization of psychiatric illness, and attainment of stable housing.
The first hurdle for implementing this type of program in other settings is the identification of resources needed for these efforts. Need of additional staffing resources, however, may be circumvented by allowing employees working in other areas to rotate in community outreach shifts in the mobile unit. Another hurdle encountered in implementation of the HMMM-V initiative was the initial difficulty finding homeless veterans in community settings, especially those in unsheltered locations. The HMMM-V program addressed this issue by partnering with other agencies serving the homeless in the community. Therefore, a general recommendation for other entities seeking to implement this type of program is to reach out to these community partners from the outset.
Conclusion
The HMMM-V has the potential to engage the most difficult-to-reach homeless veterans in need of health services by delivering care and providing resources in challenging environments. Further work is needed to validate the P-CHI for use with this program and to conduct well-designed and implemented research to demonstrate effectiveness of this intervention on veteran outcomes, especially quality of life. Once this additional work is accomplished, this innovative program can potentially be implemented by VAMCs across the nation, and potentially in more general community care settings, to more effectively reach out and deliver services to homeless members of the community.
Acknowledgments
Grant support was received from the Department of Veterans Affairs, Office of Patient Centered Care. The authors would like to acknowledge all the clinicians, trainees, and support staff who have contributed to the success of the HMMM-V program: Tara Ayala, Jose Cabrera, Tony Castillo, Rachael Lynn David, Teresa DeShazo, Sylvia Figueroa, Steven Fisher, Eric Gary, Evelyn Gibbs, Kevin Hosey, JoAnn Joseph, Taly Drimer Kagan, Miranda Kelly, Michelle King-Thompson, Sharon Marcus, Shiji Mathew, Moneeza Matin, John Moreno, Joseph Neifert, Joel Price, Tiffany Price, Natalie Qualls, Reginald Robertson, Kristine Rodrigues, Jon Saffelder, Jill Stokes, Scott Stone, and John Smith.
1. LePage JP, Bradshaw LD, Cipher DJ, Crawford AM, Hooshyar D. The effects of homelessness on veterans’ health care service use: an evaluation of independence from comorbidities. Public Health. 2014;128(11):985-992.
2. Fischer PJ, Breakey WR. The epidemiology of alcohol, drug, and mental disorders among homeless persons. Am Psychol. 1991;46(11):1115-1128.
3. Robertson MJ, Zlotnick C, Westerfelt A. Drug use disorders and treatment contact among homeless adults in Alameda County, California. Am J Public Health. 1997;87(2):221-228.
4. North CS, Eyrich KM, Pollio DE, Spitznagel EL. Are rates of psychiatric disorders in the homeless population changing? Am J Public Health. 2004;94(1):103-108.
5. Fazel S, Khosla V, Doll H, Geddes J. The prevalence of mental disorders among the homeless in western countries: systematic review and meta-regression analysis. PLoS Med. 2008;5(12):e225.
6. Harpaz-Rotem I, Rosenheck RA, Desai R. The mental health of children exposed to maternal mental illness and homelessness. Community Ment Health J. 2006;42(5):437-448.
7. Pollio DE, Eyrich-Garg KM, North CS. The homeless. In: Johnson BA, ed. Addiction Medicine: Science and Practice. New York, NY: Springer; 2011:1487-1504.
8. Padgett D, Struening EL, Andrews H. Factors affecting the use of medical, mental health, alcohol, and drug treatment services by homeless adults. Med Care. 1990;28(9):805-821.
9. Baggett TP, Singer DE, Rao SR, O’Connell JJ, Bharel M, Rigotti NA. Food insufficiency and health services utilization in a national sample of homeless adults. J Gen Intern Med. 2011;26(6):627-634.
10. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162(2):370-376.
11. Fuehrlein BS, Cowell AJ, Pollio D, Cupps L, Balfour ME, North CS. A prospective study of the associations among housing status and costs of services in a homeless population. Psychiatr Serv. 2015;66(1):27-32.
12. Pollio DE, North CS, Eyrich KM, Foster DA, Spitznagel E. Modeling service access in a homeless population. J Psychoactive Drugs. 2003;35(4):487-495.
13. U.S. Department of Housing and Urban Development Office of Community Planning and Development. The 2016 Annual Homeless Assessment Report (AHAR) to Congress. Part 1: point-in-time estimates of homelessness. https://www.hudexchange.info/resources/documents/2016-AHAR-Part-1.pdf. Published 2016. Accessed August 7, 2017.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among U.S. veterans. Epidemiol Rev. 2015;37:177-195.
15. Williamson V, Mulhall E. Coming home: the housing crisis and homelessness threaten new veterans. Iraq and Afghanistan Veterans of America, January, 2009. http://media.iava.org/IAVA_coming_home_2009%20The%20Housing%20Crisis%20and%20Homelessness%20Threaten%20New%20Veterans.pdf. Accessed August 10, 2017
16. Shinseki EK. Remarks by Secretary Eric K. Shinseki. National Summit on Homeless Veterans; November 3, 2009; Washington, DC. https://www.va.gov/opa/speeches/2009/09_1103.asp. Updated August 8, 2016. Accessed August 7, 2017.
17. Shinseki EK. Remarks by Secretary Eric K. Shinseki. 2014 National Coalition for Homeless Veterans Annual Meeting; May 30, 2014; Arlington, VA. https://www.va.gov/opa/speeches/2014/05_30_2014.asp. Updated April 21, 2015. Accessed August 7, 2017.
18. Pollio DE, Spitznagel EL, North CS, Thompson S, Foster DA. Service use over time and achievement of stable housing in a mentally ill homeless population. Psychiatr Serv. 2000;51(12):1536-1543.
19. Page J. Barriers to transferring care of homeless people with serious mental illnesses to community mental health organizations: perspectives of street-based programs. Best Practices in Mental Health: An International Journal. 2007;3(1):26.
20. Young AS, Chinman MJ, Cradock-O’Leary JA, et al. Characteristics of individuals with severe mental illness who use emergency services. Community Ment Health J. 2005;41(2):159-168.
21. Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and low-income veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461.
22. Tsemberis S, Gulcur L, Nakae M. Housing First, consumer choice, and harm reduction for homeless individuals with a dual diagnosis. Am J Public Health. 2004;94(4):651-656.
23. U.S. Department of Veterans Affairs, Veterans Health Administration. VA Patient Centered Care. http://www.va.gov/patientcenteredcare. Updated July 24,2017. Accessed August 7, 2017.
24. Epstein RM, Street RL Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100-103.
25. U.S. Department of Veterans Affairs, Office of Patient Centered Care and Cultural Transformation. My story: personal health inventory. https://www.va.gov/PATIENTCENTEREDCARE/docs/VA-OPCC-Personal-Health-Inventory-final-508.pdf. Published October 7, 2013. Accessed August 7, 2017
26. North CS, Smith EM, Pollio DE. The Homeless Supplement to the Diagnostic Interview Schedule (DIS/HS). St. Louis: Washington University, 2004.
27. North CS, Eyrich KM, Pollio DE, Foster DA, Cottler LB, Spitznagel EL. The homeless supplement to the diagnostic interview schedule: test-retest analyses. Int J Methods Psychiatr Res. 2004;13(3):184-191.
28. LaSalle JL. Homeless Operations Management and Evaluation System (HOMES) user manual-phase 1. http://www.vfwsc.org/homes.pdf. Published April 19, 2011. Accessed August 7, 2017.
29. Petrovich JC, Pollio DE, North CS. Characteristics and service use of homeless veterans and nonveterans residing in a low-demand emergency shelter. Psychiatr Serv. 2014;65(6):751-757.
30. North CS, Smith EM. A comparison of homeless men and women: different populations, different needs. Community Ment Health J. 1993;29(5):423-431.
31. Kertesz SG, Austin EL, Holmes SK, et al. Making housing first happen: organizational leadership in VA’s expansion of permanent supportive housing. J Gen Intern Med. 2014;29(suppl 4):835-844.
1. LePage JP, Bradshaw LD, Cipher DJ, Crawford AM, Hooshyar D. The effects of homelessness on veterans’ health care service use: an evaluation of independence from comorbidities. Public Health. 2014;128(11):985-992.
2. Fischer PJ, Breakey WR. The epidemiology of alcohol, drug, and mental disorders among homeless persons. Am Psychol. 1991;46(11):1115-1128.
3. Robertson MJ, Zlotnick C, Westerfelt A. Drug use disorders and treatment contact among homeless adults in Alameda County, California. Am J Public Health. 1997;87(2):221-228.
4. North CS, Eyrich KM, Pollio DE, Spitznagel EL. Are rates of psychiatric disorders in the homeless population changing? Am J Public Health. 2004;94(1):103-108.
5. Fazel S, Khosla V, Doll H, Geddes J. The prevalence of mental disorders among the homeless in western countries: systematic review and meta-regression analysis. PLoS Med. 2008;5(12):e225.
6. Harpaz-Rotem I, Rosenheck RA, Desai R. The mental health of children exposed to maternal mental illness and homelessness. Community Ment Health J. 2006;42(5):437-448.
7. Pollio DE, Eyrich-Garg KM, North CS. The homeless. In: Johnson BA, ed. Addiction Medicine: Science and Practice. New York, NY: Springer; 2011:1487-1504.
8. Padgett D, Struening EL, Andrews H. Factors affecting the use of medical, mental health, alcohol, and drug treatment services by homeless adults. Med Care. 1990;28(9):805-821.
9. Baggett TP, Singer DE, Rao SR, O’Connell JJ, Bharel M, Rigotti NA. Food insufficiency and health services utilization in a national sample of homeless adults. J Gen Intern Med. 2011;26(6):627-634.
10. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162(2):370-376.
11. Fuehrlein BS, Cowell AJ, Pollio D, Cupps L, Balfour ME, North CS. A prospective study of the associations among housing status and costs of services in a homeless population. Psychiatr Serv. 2015;66(1):27-32.
12. Pollio DE, North CS, Eyrich KM, Foster DA, Spitznagel E. Modeling service access in a homeless population. J Psychoactive Drugs. 2003;35(4):487-495.
13. U.S. Department of Housing and Urban Development Office of Community Planning and Development. The 2016 Annual Homeless Assessment Report (AHAR) to Congress. Part 1: point-in-time estimates of homelessness. https://www.hudexchange.info/resources/documents/2016-AHAR-Part-1.pdf. Published 2016. Accessed August 7, 2017.
14. Tsai J, Rosenheck RA. Risk factors for homelessness among U.S. veterans. Epidemiol Rev. 2015;37:177-195.
15. Williamson V, Mulhall E. Coming home: the housing crisis and homelessness threaten new veterans. Iraq and Afghanistan Veterans of America, January, 2009. http://media.iava.org/IAVA_coming_home_2009%20The%20Housing%20Crisis%20and%20Homelessness%20Threaten%20New%20Veterans.pdf. Accessed August 10, 2017
16. Shinseki EK. Remarks by Secretary Eric K. Shinseki. National Summit on Homeless Veterans; November 3, 2009; Washington, DC. https://www.va.gov/opa/speeches/2009/09_1103.asp. Updated August 8, 2016. Accessed August 7, 2017.
17. Shinseki EK. Remarks by Secretary Eric K. Shinseki. 2014 National Coalition for Homeless Veterans Annual Meeting; May 30, 2014; Arlington, VA. https://www.va.gov/opa/speeches/2014/05_30_2014.asp. Updated April 21, 2015. Accessed August 7, 2017.
18. Pollio DE, Spitznagel EL, North CS, Thompson S, Foster DA. Service use over time and achievement of stable housing in a mentally ill homeless population. Psychiatr Serv. 2000;51(12):1536-1543.
19. Page J. Barriers to transferring care of homeless people with serious mental illnesses to community mental health organizations: perspectives of street-based programs. Best Practices in Mental Health: An International Journal. 2007;3(1):26.
20. Young AS, Chinman MJ, Cradock-O’Leary JA, et al. Characteristics of individuals with severe mental illness who use emergency services. Community Ment Health J. 2005;41(2):159-168.
21. Gabrielian S, Yuan AH, Andersen RM, Rubenstein LV, Gelberg L. VA health service utilization for homeless and low-income veterans: a spotlight on the VA Supportive Housing (VASH) program in greater Los Angeles. Med Care. 2014;52(5):454-461.
22. Tsemberis S, Gulcur L, Nakae M. Housing First, consumer choice, and harm reduction for homeless individuals with a dual diagnosis. Am J Public Health. 2004;94(4):651-656.
23. U.S. Department of Veterans Affairs, Veterans Health Administration. VA Patient Centered Care. http://www.va.gov/patientcenteredcare. Updated July 24,2017. Accessed August 7, 2017.
24. Epstein RM, Street RL Jr. The values and value of patient-centered care. Ann Fam Med. 2011;9(2):100-103.
25. U.S. Department of Veterans Affairs, Office of Patient Centered Care and Cultural Transformation. My story: personal health inventory. https://www.va.gov/PATIENTCENTEREDCARE/docs/VA-OPCC-Personal-Health-Inventory-final-508.pdf. Published October 7, 2013. Accessed August 7, 2017
26. North CS, Smith EM, Pollio DE. The Homeless Supplement to the Diagnostic Interview Schedule (DIS/HS). St. Louis: Washington University, 2004.
27. North CS, Eyrich KM, Pollio DE, Foster DA, Cottler LB, Spitznagel EL. The homeless supplement to the diagnostic interview schedule: test-retest analyses. Int J Methods Psychiatr Res. 2004;13(3):184-191.
28. LaSalle JL. Homeless Operations Management and Evaluation System (HOMES) user manual-phase 1. http://www.vfwsc.org/homes.pdf. Published April 19, 2011. Accessed August 7, 2017.
29. Petrovich JC, Pollio DE, North CS. Characteristics and service use of homeless veterans and nonveterans residing in a low-demand emergency shelter. Psychiatr Serv. 2014;65(6):751-757.
30. North CS, Smith EM. A comparison of homeless men and women: different populations, different needs. Community Ment Health J. 1993;29(5):423-431.
31. Kertesz SG, Austin EL, Holmes SK, et al. Making housing first happen: organizational leadership in VA’s expansion of permanent supportive housing. J Gen Intern Med. 2014;29(suppl 4):835-844.
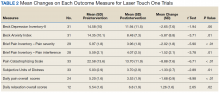
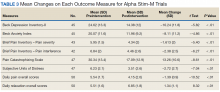
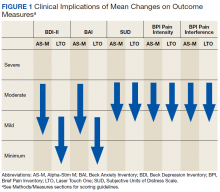
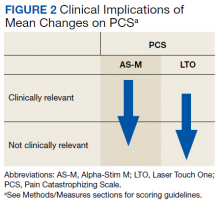
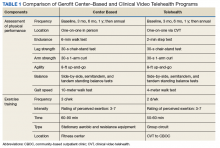
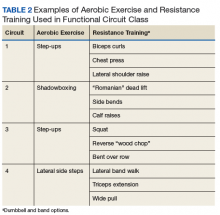
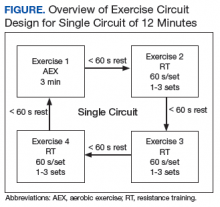
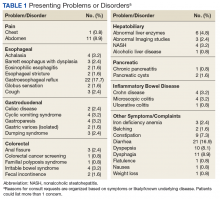
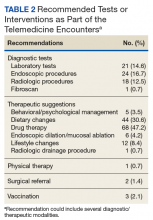


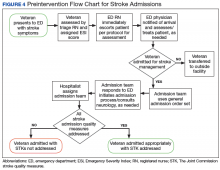
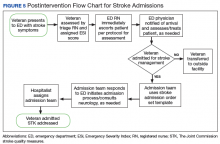
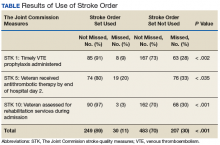

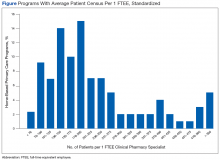
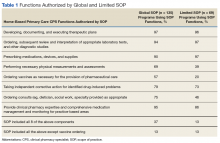
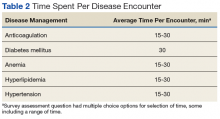

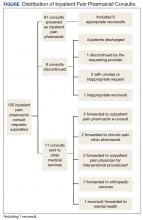
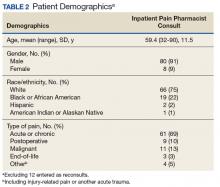
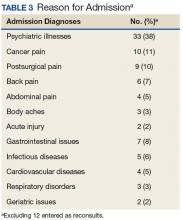
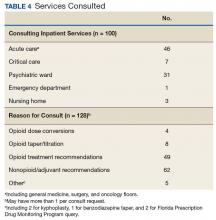

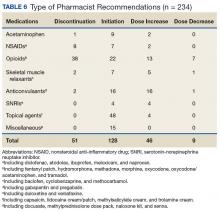
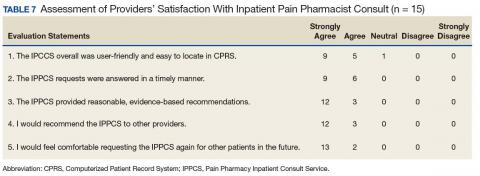
 Disclaimer
Disclaimer